User login
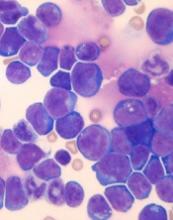
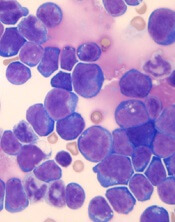
Stress linked to disease markers in CLL
New research has linked stress levels to markers of progressive disease in patients with chronic lymphocytic leukemia (CLL).
Researchers found that CLL patients who reported more stress also had higher absolute lymphocyte counts and elevated levels of three other markers of more advanced disease—tumor necrosis factor-α (TNFα), interleukin 16 (IL-16), and chemokine ligand 3 (CCL3).
“All four variables we measured are related to prognosis in CLL patients, so they have a lot of relevance,” said study author Barbara L. Andersen, PhD, of The Ohio State University in Columbus.
She and her colleagues described this research in Cancer.
The study involved 96 patients with relapsed/refractory CLL who were entering a phase 2 trial of ibrutinib (NCT01589302). Data collection for this study was done before patients received their first dose of ibrutinib.
All patients completed a survey that measured CLL-related stress. They were asked questions like how often they had intrusive thoughts about their disease, how often they tried to avoid thinking about it, and how often they felt jumpy and easily startled.
The researchers used blood samples to determine patients’ absolute lymphocyte counts and to measure levels of eight cytokines known to promote unhealthy levels of inflammation—IL-6, IL-10, IL-16, TNFα, a proliferation‐inducing ligand (APRIL), B‐cell activating factor (BAFF), vascular endothelial growth factor (VEGF), and CCL3.
In an analysis controlling for demographic characteristics, comorbidities, the presence of 17p deletion, and correlates of inflammation, higher stress was significantly associated with higher:
- Absolute lymphocyte counts (P<0.05)
- Levels of TNFα (P<0.05)
- Levels of IL‐16 (P<0.01)
- Levels of CCL3 (P<0.05).
“The fact that stress shows an effect on CLL even after we controlled for other factors suggests it may be relevant to the course of CLL,” Dr. Andersen said.
She added that the researchers are still following these patients and will examine the relationship between stress and disease markers throughout treatment.
This study was supported by the National Cancer Institute, Pharmacylics (the company developing ibrutinib), and a Pelotonia Idea Award from The Ohio State University Comprehensive Cancer Center.
New research has linked stress levels to markers of progressive disease in patients with chronic lymphocytic leukemia (CLL).
Researchers found that CLL patients who reported more stress also had higher absolute lymphocyte counts and elevated levels of three other markers of more advanced disease—tumor necrosis factor-α (TNFα), interleukin 16 (IL-16), and chemokine ligand 3 (CCL3).
“All four variables we measured are related to prognosis in CLL patients, so they have a lot of relevance,” said study author Barbara L. Andersen, PhD, of The Ohio State University in Columbus.
She and her colleagues described this research in Cancer.
The study involved 96 patients with relapsed/refractory CLL who were entering a phase 2 trial of ibrutinib (NCT01589302). Data collection for this study was done before patients received their first dose of ibrutinib.
All patients completed a survey that measured CLL-related stress. They were asked questions like how often they had intrusive thoughts about their disease, how often they tried to avoid thinking about it, and how often they felt jumpy and easily startled.
The researchers used blood samples to determine patients’ absolute lymphocyte counts and to measure levels of eight cytokines known to promote unhealthy levels of inflammation—IL-6, IL-10, IL-16, TNFα, a proliferation‐inducing ligand (APRIL), B‐cell activating factor (BAFF), vascular endothelial growth factor (VEGF), and CCL3.
In an analysis controlling for demographic characteristics, comorbidities, the presence of 17p deletion, and correlates of inflammation, higher stress was significantly associated with higher:
- Absolute lymphocyte counts (P<0.05)
- Levels of TNFα (P<0.05)
- Levels of IL‐16 (P<0.01)
- Levels of CCL3 (P<0.05).
“The fact that stress shows an effect on CLL even after we controlled for other factors suggests it may be relevant to the course of CLL,” Dr. Andersen said.
She added that the researchers are still following these patients and will examine the relationship between stress and disease markers throughout treatment.
This study was supported by the National Cancer Institute, Pharmacylics (the company developing ibrutinib), and a Pelotonia Idea Award from The Ohio State University Comprehensive Cancer Center.
New research has linked stress levels to markers of progressive disease in patients with chronic lymphocytic leukemia (CLL).
Researchers found that CLL patients who reported more stress also had higher absolute lymphocyte counts and elevated levels of three other markers of more advanced disease—tumor necrosis factor-α (TNFα), interleukin 16 (IL-16), and chemokine ligand 3 (CCL3).
“All four variables we measured are related to prognosis in CLL patients, so they have a lot of relevance,” said study author Barbara L. Andersen, PhD, of The Ohio State University in Columbus.
She and her colleagues described this research in Cancer.
The study involved 96 patients with relapsed/refractory CLL who were entering a phase 2 trial of ibrutinib (NCT01589302). Data collection for this study was done before patients received their first dose of ibrutinib.
All patients completed a survey that measured CLL-related stress. They were asked questions like how often they had intrusive thoughts about their disease, how often they tried to avoid thinking about it, and how often they felt jumpy and easily startled.
The researchers used blood samples to determine patients’ absolute lymphocyte counts and to measure levels of eight cytokines known to promote unhealthy levels of inflammation—IL-6, IL-10, IL-16, TNFα, a proliferation‐inducing ligand (APRIL), B‐cell activating factor (BAFF), vascular endothelial growth factor (VEGF), and CCL3.
In an analysis controlling for demographic characteristics, comorbidities, the presence of 17p deletion, and correlates of inflammation, higher stress was significantly associated with higher:
- Absolute lymphocyte counts (P<0.05)
- Levels of TNFα (P<0.05)
- Levels of IL‐16 (P<0.01)
- Levels of CCL3 (P<0.05).
“The fact that stress shows an effect on CLL even after we controlled for other factors suggests it may be relevant to the course of CLL,” Dr. Andersen said.
She added that the researchers are still following these patients and will examine the relationship between stress and disease markers throughout treatment.
This study was supported by the National Cancer Institute, Pharmacylics (the company developing ibrutinib), and a Pelotonia Idea Award from The Ohio State University Comprehensive Cancer Center.
OBI-3424 receives orphan designation for ALL
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to OBI-3424 for the treatment of acute lymphoblastic leukemia (ALL).
OBI-3424 is a small-molecule prodrug that targets cancers overexpressing aldo-keto reductase 1C3 (AKR1C3) and selectively releases a DNA alkylating agent in the presence of the AKR1C3 enzyme.
AKR1C3 overexpression has been observed in ALL, particularly T-cell ALL.
OBI-3424 demonstrated activity against T-ALL in preclinical research presented as a poster at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics in October 2017.
Researchers reported that OBI-3424 “exerted profound in vivo efficacy” against T-ALL xenografts derived mainly from patients with aggressive and fatal T-ALL.
The researchers said OBI-3424 significantly reduced leukemia bone marrow infiltration in 4 of 6 evaluable T-ALL xenografts, and OBI-3424 was considered well tolerated.
The poster presentation describing this research is available for download from the website of OBI Pharma, the company developing OBI-3424 in cooperation with Ascenta Pharma.
OBI-3424 also has orphan drug designation from the FDA as a treatment for hepatocellular carcinoma.
Enrollment has begun in a phase 1/2 trial (NCT03592264) of OBI-3424 in patients with hepatocellular carcinoma and castrate-resistant prostate cancer.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to OBI-3424 for the treatment of acute lymphoblastic leukemia (ALL).
OBI-3424 is a small-molecule prodrug that targets cancers overexpressing aldo-keto reductase 1C3 (AKR1C3) and selectively releases a DNA alkylating agent in the presence of the AKR1C3 enzyme.
AKR1C3 overexpression has been observed in ALL, particularly T-cell ALL.
OBI-3424 demonstrated activity against T-ALL in preclinical research presented as a poster at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics in October 2017.
Researchers reported that OBI-3424 “exerted profound in vivo efficacy” against T-ALL xenografts derived mainly from patients with aggressive and fatal T-ALL.
The researchers said OBI-3424 significantly reduced leukemia bone marrow infiltration in 4 of 6 evaluable T-ALL xenografts, and OBI-3424 was considered well tolerated.
The poster presentation describing this research is available for download from the website of OBI Pharma, the company developing OBI-3424 in cooperation with Ascenta Pharma.
OBI-3424 also has orphan drug designation from the FDA as a treatment for hepatocellular carcinoma.
Enrollment has begun in a phase 1/2 trial (NCT03592264) of OBI-3424 in patients with hepatocellular carcinoma and castrate-resistant prostate cancer.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to OBI-3424 for the treatment of acute lymphoblastic leukemia (ALL).
OBI-3424 is a small-molecule prodrug that targets cancers overexpressing aldo-keto reductase 1C3 (AKR1C3) and selectively releases a DNA alkylating agent in the presence of the AKR1C3 enzyme.
AKR1C3 overexpression has been observed in ALL, particularly T-cell ALL.
OBI-3424 demonstrated activity against T-ALL in preclinical research presented as a poster at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics in October 2017.
Researchers reported that OBI-3424 “exerted profound in vivo efficacy” against T-ALL xenografts derived mainly from patients with aggressive and fatal T-ALL.
The researchers said OBI-3424 significantly reduced leukemia bone marrow infiltration in 4 of 6 evaluable T-ALL xenografts, and OBI-3424 was considered well tolerated.
The poster presentation describing this research is available for download from the website of OBI Pharma, the company developing OBI-3424 in cooperation with Ascenta Pharma.
OBI-3424 also has orphan drug designation from the FDA as a treatment for hepatocellular carcinoma.
Enrollment has begun in a phase 1/2 trial (NCT03592264) of OBI-3424 in patients with hepatocellular carcinoma and castrate-resistant prostate cancer.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
Researchers propose new acute leukemia subtypes
An extensive analysis of mixed phenotype acute leukemia (MPAL) has led to new insights that may have implications for disease classification and treatment.
Researchers believe they have identified new subtypes of MPAL that should be included in the World Health Organization classification for acute leukemia.
Each of these subtypes share genomic characteristics with other acute leukemias, which suggests they might respond to treatments that are already in use.
This research also has shed light on how MPAL evolves and appears to provide an explanation for why MPAL displays characteristics of both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
“ALL and AML have very different treatments, but MPAL has features of both, so the question of how best to treat patients with MPAL has been challenging the leukemia community worldwide, and long-term survival of patients has been poor,” said study author Charles G. Mullighan, MD, of St. Jude Children’s Research Hospital in Memphis, Tenn.
In the current study, published in Nature, Dr. Mullighan and his colleagues used whole-genome, whole-exome, and RNA sequencing to analyze 115 samples from pediatric patients with MPAL.
The analysis revealed mutations that define the two most common subtypes of MPAL – B/myeloid and T/myeloid – and suggested these subtypes share similarities with other leukemia subtypes.
The researchers found that 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL. In fact, the team said the gene expression profiles of ZNF384r B-ALL and ZNF384r MPAL were indistinguishable.
“That is biologically and clinically important,” Dr. Mullighan said. “The findings suggest the ZNF384 rearrangement defines a distinct leukemia subtype, and the alteration should be used to guide treatment.”
The researchers noted that patients with ZNF384r exhibited higher FLT3 expression than that of patients with other types of B/myeloid or T/myeloid MPAL, so patients with ZNF384r MPAL might respond well to treatment with a FLT3 inhibitor.
This study also showed that cases of B/myeloid MPAL without ZNF384r shared genomic features with other B-ALL subtypes, such as Ph-like B-ALL.
In addition, the analysis showed that T/myeloid MPAL and early T-cell precursor ALL have similar gene expression profiles.
The team identified several genes that were mutated at similar frequencies in T/myeloid MPAL and early T-cell precursor ALL, including WT1, ETV6, EZH2, and FLT3.
WT1 was the most frequently mutated transcription factor gene in T/myeloid MPAL.
Based on these findings, the researchers said the WHO classification of acute leukemia should be updated to include: ZNF384r acute leukemia (either B-ALL or MPAL), WT1-mutant T/myeloid MPAL, and Ph-like B/myeloid MPAL.
This research was supported by the National Cancer Institute, the National Institutes of Health, Cookies for Kids’ Cancer, and other organizations. The researchers reported having no competing interests.
SOURCE: Alexander TB et al. Nature. 2018 Sep 12. doi: 10.1038/s41586-018-0436-0.
An extensive analysis of mixed phenotype acute leukemia (MPAL) has led to new insights that may have implications for disease classification and treatment.
Researchers believe they have identified new subtypes of MPAL that should be included in the World Health Organization classification for acute leukemia.
Each of these subtypes share genomic characteristics with other acute leukemias, which suggests they might respond to treatments that are already in use.
This research also has shed light on how MPAL evolves and appears to provide an explanation for why MPAL displays characteristics of both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
“ALL and AML have very different treatments, but MPAL has features of both, so the question of how best to treat patients with MPAL has been challenging the leukemia community worldwide, and long-term survival of patients has been poor,” said study author Charles G. Mullighan, MD, of St. Jude Children’s Research Hospital in Memphis, Tenn.
In the current study, published in Nature, Dr. Mullighan and his colleagues used whole-genome, whole-exome, and RNA sequencing to analyze 115 samples from pediatric patients with MPAL.
The analysis revealed mutations that define the two most common subtypes of MPAL – B/myeloid and T/myeloid – and suggested these subtypes share similarities with other leukemia subtypes.
The researchers found that 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL. In fact, the team said the gene expression profiles of ZNF384r B-ALL and ZNF384r MPAL were indistinguishable.
“That is biologically and clinically important,” Dr. Mullighan said. “The findings suggest the ZNF384 rearrangement defines a distinct leukemia subtype, and the alteration should be used to guide treatment.”
The researchers noted that patients with ZNF384r exhibited higher FLT3 expression than that of patients with other types of B/myeloid or T/myeloid MPAL, so patients with ZNF384r MPAL might respond well to treatment with a FLT3 inhibitor.
This study also showed that cases of B/myeloid MPAL without ZNF384r shared genomic features with other B-ALL subtypes, such as Ph-like B-ALL.
In addition, the analysis showed that T/myeloid MPAL and early T-cell precursor ALL have similar gene expression profiles.
The team identified several genes that were mutated at similar frequencies in T/myeloid MPAL and early T-cell precursor ALL, including WT1, ETV6, EZH2, and FLT3.
WT1 was the most frequently mutated transcription factor gene in T/myeloid MPAL.
Based on these findings, the researchers said the WHO classification of acute leukemia should be updated to include: ZNF384r acute leukemia (either B-ALL or MPAL), WT1-mutant T/myeloid MPAL, and Ph-like B/myeloid MPAL.
This research was supported by the National Cancer Institute, the National Institutes of Health, Cookies for Kids’ Cancer, and other organizations. The researchers reported having no competing interests.
SOURCE: Alexander TB et al. Nature. 2018 Sep 12. doi: 10.1038/s41586-018-0436-0.
An extensive analysis of mixed phenotype acute leukemia (MPAL) has led to new insights that may have implications for disease classification and treatment.
Researchers believe they have identified new subtypes of MPAL that should be included in the World Health Organization classification for acute leukemia.
Each of these subtypes share genomic characteristics with other acute leukemias, which suggests they might respond to treatments that are already in use.
This research also has shed light on how MPAL evolves and appears to provide an explanation for why MPAL displays characteristics of both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
“ALL and AML have very different treatments, but MPAL has features of both, so the question of how best to treat patients with MPAL has been challenging the leukemia community worldwide, and long-term survival of patients has been poor,” said study author Charles G. Mullighan, MD, of St. Jude Children’s Research Hospital in Memphis, Tenn.
In the current study, published in Nature, Dr. Mullighan and his colleagues used whole-genome, whole-exome, and RNA sequencing to analyze 115 samples from pediatric patients with MPAL.
The analysis revealed mutations that define the two most common subtypes of MPAL – B/myeloid and T/myeloid – and suggested these subtypes share similarities with other leukemia subtypes.
The researchers found that 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL. In fact, the team said the gene expression profiles of ZNF384r B-ALL and ZNF384r MPAL were indistinguishable.
“That is biologically and clinically important,” Dr. Mullighan said. “The findings suggest the ZNF384 rearrangement defines a distinct leukemia subtype, and the alteration should be used to guide treatment.”
The researchers noted that patients with ZNF384r exhibited higher FLT3 expression than that of patients with other types of B/myeloid or T/myeloid MPAL, so patients with ZNF384r MPAL might respond well to treatment with a FLT3 inhibitor.
This study also showed that cases of B/myeloid MPAL without ZNF384r shared genomic features with other B-ALL subtypes, such as Ph-like B-ALL.
In addition, the analysis showed that T/myeloid MPAL and early T-cell precursor ALL have similar gene expression profiles.
The team identified several genes that were mutated at similar frequencies in T/myeloid MPAL and early T-cell precursor ALL, including WT1, ETV6, EZH2, and FLT3.
WT1 was the most frequently mutated transcription factor gene in T/myeloid MPAL.
Based on these findings, the researchers said the WHO classification of acute leukemia should be updated to include: ZNF384r acute leukemia (either B-ALL or MPAL), WT1-mutant T/myeloid MPAL, and Ph-like B/myeloid MPAL.
This research was supported by the National Cancer Institute, the National Institutes of Health, Cookies for Kids’ Cancer, and other organizations. The researchers reported having no competing interests.
SOURCE: Alexander TB et al. Nature. 2018 Sep 12. doi: 10.1038/s41586-018-0436-0.
FROM NATURE
Key clinical point:
Major finding: In total, 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL.
Study details: Whole-genome, -exome, and RNA sequencing of 115 samples from pediatric patients with MPAL.
Disclosures: This research was supported by the National Cancer Institute and other organizations. The researchers reported having no competing interests.
Source: Alexander TB et al. Nature. 2018 Sep 12. doi: 10.1038/s41586-018-0436-0.
ASCO addresses financial barriers to cancer clinical trials
The American Society of Clinical Oncology (ASCO) has issued a policy statement addressing financial barriers to patient participation in cancer clinical trials.
ASCO’s policy statement outlines a series of recommendations designed to address multiple financial barriers that impede access to clinical trials, including patient costs that aren’t covered consistently by health insurance; a lack of information provided to patients about clinical trial costs; and limited available research data on financial hardships related to participation in clinical trials.
“Clinical trials are essential for evaluating the safety and efficacy of new cancer treatments, but cancer researchers have seen consistently low patient participation levels—especially among underserved patient populations—in part, due to the financial burdens facing many patients with cancer,” said ASCO President Monica M. Bertagnolli, MD.
“Addressing financial barriers will help improve the enrollment rate and the efficiency, quality, and applicability of cancer research. By including more—and more diverse—participants in our research studies, we expand our ability to care for all patients.”
The recommendations in ASCO’s policy statement include:
- Improve payer clinical trial coverage policies. Payment policies should be revised to be made consistent, streamlined, and transparent to all stakeholders.
- Payers should have clear definitions of “routine costs.”
- Payers should streamline prior authorization processes and facilitate trial enrollment through provider reimbursement of clinical trial-related services.
- State Medicaid programs should universally guarantee coverage of routine care costs of clinical trials for their beneficiaries.
- The U.S. Centers for Medicare & Medicaid Services (CMS) should revise current policy that requires Medicare Advantage beneficiaries to revert to fee-for-service coverage during clinical trials.
- CMS’s Innovation Center should explore the effectiveness of alternative payment models in support of clinical trial accrual.
- During the clinical trials development and enrollment process, provide patients with clear, transparent information about potential trial-related out-of-pocket costs and include mechanisms to support patient financial/health literacy.
- Clinical trial sponsors should perform—and make available to enrolling institutions—comprehensive, prospective coverage analyses.
- Research sites should consider offering in-house financial navigation/counseling to patients or consider partnering with organizations that provide such services.
- Design clinical trials to minimize incremental costs, consistent with scientific objectives and participant safety.
- Remove impediments to ethically appropriate financial compensation for trial-related out-of-pocket costs. Provision of such financial support should not be considered undue inducement.
- Office for Human Research Protections should develop guidance on targeted financial support.
- Incentivize research that will better characterize patient costs incurred for participating in cancer clinical trials and support the longer-term development of tools to identify and mitigate the risk of trial-associated financial hardship.
“Continued progress against cancer depends on improving patient access to participation in clinical research,” Dr. Bertagnolli said.
“The recommendations in ASCO’s statement aim to ensure that no patient is denied access to a clinical trial for financial reasons and that patients are not harmed financially because of their contributions to advancing science. Ultimately, this is about strengthening the nation’s cancer research enterprise as a whole.”
The American Society of Clinical Oncology (ASCO) has issued a policy statement addressing financial barriers to patient participation in cancer clinical trials.
ASCO’s policy statement outlines a series of recommendations designed to address multiple financial barriers that impede access to clinical trials, including patient costs that aren’t covered consistently by health insurance; a lack of information provided to patients about clinical trial costs; and limited available research data on financial hardships related to participation in clinical trials.
“Clinical trials are essential for evaluating the safety and efficacy of new cancer treatments, but cancer researchers have seen consistently low patient participation levels—especially among underserved patient populations—in part, due to the financial burdens facing many patients with cancer,” said ASCO President Monica M. Bertagnolli, MD.
“Addressing financial barriers will help improve the enrollment rate and the efficiency, quality, and applicability of cancer research. By including more—and more diverse—participants in our research studies, we expand our ability to care for all patients.”
The recommendations in ASCO’s policy statement include:
- Improve payer clinical trial coverage policies. Payment policies should be revised to be made consistent, streamlined, and transparent to all stakeholders.
- Payers should have clear definitions of “routine costs.”
- Payers should streamline prior authorization processes and facilitate trial enrollment through provider reimbursement of clinical trial-related services.
- State Medicaid programs should universally guarantee coverage of routine care costs of clinical trials for their beneficiaries.
- The U.S. Centers for Medicare & Medicaid Services (CMS) should revise current policy that requires Medicare Advantage beneficiaries to revert to fee-for-service coverage during clinical trials.
- CMS’s Innovation Center should explore the effectiveness of alternative payment models in support of clinical trial accrual.
- During the clinical trials development and enrollment process, provide patients with clear, transparent information about potential trial-related out-of-pocket costs and include mechanisms to support patient financial/health literacy.
- Clinical trial sponsors should perform—and make available to enrolling institutions—comprehensive, prospective coverage analyses.
- Research sites should consider offering in-house financial navigation/counseling to patients or consider partnering with organizations that provide such services.
- Design clinical trials to minimize incremental costs, consistent with scientific objectives and participant safety.
- Remove impediments to ethically appropriate financial compensation for trial-related out-of-pocket costs. Provision of such financial support should not be considered undue inducement.
- Office for Human Research Protections should develop guidance on targeted financial support.
- Incentivize research that will better characterize patient costs incurred for participating in cancer clinical trials and support the longer-term development of tools to identify and mitigate the risk of trial-associated financial hardship.
“Continued progress against cancer depends on improving patient access to participation in clinical research,” Dr. Bertagnolli said.
“The recommendations in ASCO’s statement aim to ensure that no patient is denied access to a clinical trial for financial reasons and that patients are not harmed financially because of their contributions to advancing science. Ultimately, this is about strengthening the nation’s cancer research enterprise as a whole.”
The American Society of Clinical Oncology (ASCO) has issued a policy statement addressing financial barriers to patient participation in cancer clinical trials.
ASCO’s policy statement outlines a series of recommendations designed to address multiple financial barriers that impede access to clinical trials, including patient costs that aren’t covered consistently by health insurance; a lack of information provided to patients about clinical trial costs; and limited available research data on financial hardships related to participation in clinical trials.
“Clinical trials are essential for evaluating the safety and efficacy of new cancer treatments, but cancer researchers have seen consistently low patient participation levels—especially among underserved patient populations—in part, due to the financial burdens facing many patients with cancer,” said ASCO President Monica M. Bertagnolli, MD.
“Addressing financial barriers will help improve the enrollment rate and the efficiency, quality, and applicability of cancer research. By including more—and more diverse—participants in our research studies, we expand our ability to care for all patients.”
The recommendations in ASCO’s policy statement include:
- Improve payer clinical trial coverage policies. Payment policies should be revised to be made consistent, streamlined, and transparent to all stakeholders.
- Payers should have clear definitions of “routine costs.”
- Payers should streamline prior authorization processes and facilitate trial enrollment through provider reimbursement of clinical trial-related services.
- State Medicaid programs should universally guarantee coverage of routine care costs of clinical trials for their beneficiaries.
- The U.S. Centers for Medicare & Medicaid Services (CMS) should revise current policy that requires Medicare Advantage beneficiaries to revert to fee-for-service coverage during clinical trials.
- CMS’s Innovation Center should explore the effectiveness of alternative payment models in support of clinical trial accrual.
- During the clinical trials development and enrollment process, provide patients with clear, transparent information about potential trial-related out-of-pocket costs and include mechanisms to support patient financial/health literacy.
- Clinical trial sponsors should perform—and make available to enrolling institutions—comprehensive, prospective coverage analyses.
- Research sites should consider offering in-house financial navigation/counseling to patients or consider partnering with organizations that provide such services.
- Design clinical trials to minimize incremental costs, consistent with scientific objectives and participant safety.
- Remove impediments to ethically appropriate financial compensation for trial-related out-of-pocket costs. Provision of such financial support should not be considered undue inducement.
- Office for Human Research Protections should develop guidance on targeted financial support.
- Incentivize research that will better characterize patient costs incurred for participating in cancer clinical trials and support the longer-term development of tools to identify and mitigate the risk of trial-associated financial hardship.
“Continued progress against cancer depends on improving patient access to participation in clinical research,” Dr. Bertagnolli said.
“The recommendations in ASCO’s statement aim to ensure that no patient is denied access to a clinical trial for financial reasons and that patients are not harmed financially because of their contributions to advancing science. Ultimately, this is about strengthening the nation’s cancer research enterprise as a whole.”
Children with BCP-ALL show inflammatory marker differences at birth
Patients who develop B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in childhood may have dysregulated immune function at birth, according to a study published in Cancer Research.
Investigators evaluated neonatal concentrations of inflammatory markers and found significant differences between children who were later diagnosed with BCP-ALL and leukemia-free control subjects.
“Our findings suggest that children who develop ALL are immunologically disparate already at birth,” said study author Signe Holst Søegaard, PhD, of Statens Serum Institut in Copenhagen. “This may link to other observations suggesting that children who develop ALL respond differently to infections in early childhood, potentially promoting subsequent genetic events required for transformation to ALL, or speculations that they are unable to eliminate preleukemic cells.”
She noted that the study could not determine if the associations shown are causal or consequential so further studies will be needed both to confirm the findings and identify the underlying mechanisms.
For this study, Dr. Søegaard and her colleagues measured concentrations of 10 inflammatory markers on neonatal dried blood spots from 178 patients with BCP-ALL and 178 matched controls. The patients were diagnosed with BCP-ALL at ages 1-9 years.
Compared with controls, children who later developed BCP-ALL had significantly different neonatal concentrations of eight inflammatory markers.
Concentrations of interleukin (IL)–8, soluble receptor sIL-6R alpha, transforming growth factor (TGF)–beta 1, monocyte chemotactic protein (MCP)–1, and C-reactive protein (CRP) were significantly lower among the BCP-ALL patients.
On the other hand, concentrations of IL-6, IL-17, and IL-18 were significantly higher among BCP-ALL patients than controls.
The investigators noted that IL-10 concentrations were too low for accurate measurement in all patients and controls. Additionally, a “large proportion” of patients and controls had IL-6 and IL-17 concentrations that were below the limit of detection.
“We also demonstrated that several previously shown ALL risk factors – namely, birth order, gestational age, and sex – were associated with the neonatal concentrations of inflammatory markers,” Dr. Søegaard said. “These findings raise the interesting possibility that the effects of some known ALL risk factors partly act through prenatal programming of immune function.”
The investigators found that increasing birth order was associated with significantly higher IL-18 and lower CRP concentrations.
Increasing gestational age was associated with significantly lower sIL-6R alpha and TGF-beta 1 concentrations and higher CRP concentrations. And boys had significantly lower sIL-6R alpha and IL-8 concentrations and higher CRP concentrations than girls.
However, none of the following factors were significantly associated with concentrations of inflammatory biomarkers: maternal age at delivery, maternal hospital contact attributable to infection during pregnancy, maternal prescription for antimicrobials during pregnancy, birth weight, and mode of delivery.
“Our findings underline the role the child’s baseline immune characteristics may play in the development of ALL,” Dr. Søegaard said. “However, we cannot yet use our research results to predict who will develop childhood ALL.”
The study was sponsored by the Dagmar Marshall Foundation, the A.P. Møller Foundation, the Danish Childhood Cancer Foundation, the Arvid Nilsson Foundation, and the Danish Cancer Research Foundation. The investigators reported having no conflicts of interest.
SOURCE: Søegaard SH et al. Cancer Res. 2018;78(18);5458-63.
Patients who develop B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in childhood may have dysregulated immune function at birth, according to a study published in Cancer Research.
Investigators evaluated neonatal concentrations of inflammatory markers and found significant differences between children who were later diagnosed with BCP-ALL and leukemia-free control subjects.
“Our findings suggest that children who develop ALL are immunologically disparate already at birth,” said study author Signe Holst Søegaard, PhD, of Statens Serum Institut in Copenhagen. “This may link to other observations suggesting that children who develop ALL respond differently to infections in early childhood, potentially promoting subsequent genetic events required for transformation to ALL, or speculations that they are unable to eliminate preleukemic cells.”
She noted that the study could not determine if the associations shown are causal or consequential so further studies will be needed both to confirm the findings and identify the underlying mechanisms.
For this study, Dr. Søegaard and her colleagues measured concentrations of 10 inflammatory markers on neonatal dried blood spots from 178 patients with BCP-ALL and 178 matched controls. The patients were diagnosed with BCP-ALL at ages 1-9 years.
Compared with controls, children who later developed BCP-ALL had significantly different neonatal concentrations of eight inflammatory markers.
Concentrations of interleukin (IL)–8, soluble receptor sIL-6R alpha, transforming growth factor (TGF)–beta 1, monocyte chemotactic protein (MCP)–1, and C-reactive protein (CRP) were significantly lower among the BCP-ALL patients.
On the other hand, concentrations of IL-6, IL-17, and IL-18 were significantly higher among BCP-ALL patients than controls.
The investigators noted that IL-10 concentrations were too low for accurate measurement in all patients and controls. Additionally, a “large proportion” of patients and controls had IL-6 and IL-17 concentrations that were below the limit of detection.
“We also demonstrated that several previously shown ALL risk factors – namely, birth order, gestational age, and sex – were associated with the neonatal concentrations of inflammatory markers,” Dr. Søegaard said. “These findings raise the interesting possibility that the effects of some known ALL risk factors partly act through prenatal programming of immune function.”
The investigators found that increasing birth order was associated with significantly higher IL-18 and lower CRP concentrations.
Increasing gestational age was associated with significantly lower sIL-6R alpha and TGF-beta 1 concentrations and higher CRP concentrations. And boys had significantly lower sIL-6R alpha and IL-8 concentrations and higher CRP concentrations than girls.
However, none of the following factors were significantly associated with concentrations of inflammatory biomarkers: maternal age at delivery, maternal hospital contact attributable to infection during pregnancy, maternal prescription for antimicrobials during pregnancy, birth weight, and mode of delivery.
“Our findings underline the role the child’s baseline immune characteristics may play in the development of ALL,” Dr. Søegaard said. “However, we cannot yet use our research results to predict who will develop childhood ALL.”
The study was sponsored by the Dagmar Marshall Foundation, the A.P. Møller Foundation, the Danish Childhood Cancer Foundation, the Arvid Nilsson Foundation, and the Danish Cancer Research Foundation. The investigators reported having no conflicts of interest.
SOURCE: Søegaard SH et al. Cancer Res. 2018;78(18);5458-63.
Patients who develop B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in childhood may have dysregulated immune function at birth, according to a study published in Cancer Research.
Investigators evaluated neonatal concentrations of inflammatory markers and found significant differences between children who were later diagnosed with BCP-ALL and leukemia-free control subjects.
“Our findings suggest that children who develop ALL are immunologically disparate already at birth,” said study author Signe Holst Søegaard, PhD, of Statens Serum Institut in Copenhagen. “This may link to other observations suggesting that children who develop ALL respond differently to infections in early childhood, potentially promoting subsequent genetic events required for transformation to ALL, or speculations that they are unable to eliminate preleukemic cells.”
She noted that the study could not determine if the associations shown are causal or consequential so further studies will be needed both to confirm the findings and identify the underlying mechanisms.
For this study, Dr. Søegaard and her colleagues measured concentrations of 10 inflammatory markers on neonatal dried blood spots from 178 patients with BCP-ALL and 178 matched controls. The patients were diagnosed with BCP-ALL at ages 1-9 years.
Compared with controls, children who later developed BCP-ALL had significantly different neonatal concentrations of eight inflammatory markers.
Concentrations of interleukin (IL)–8, soluble receptor sIL-6R alpha, transforming growth factor (TGF)–beta 1, monocyte chemotactic protein (MCP)–1, and C-reactive protein (CRP) were significantly lower among the BCP-ALL patients.
On the other hand, concentrations of IL-6, IL-17, and IL-18 were significantly higher among BCP-ALL patients than controls.
The investigators noted that IL-10 concentrations were too low for accurate measurement in all patients and controls. Additionally, a “large proportion” of patients and controls had IL-6 and IL-17 concentrations that were below the limit of detection.
“We also demonstrated that several previously shown ALL risk factors – namely, birth order, gestational age, and sex – were associated with the neonatal concentrations of inflammatory markers,” Dr. Søegaard said. “These findings raise the interesting possibility that the effects of some known ALL risk factors partly act through prenatal programming of immune function.”
The investigators found that increasing birth order was associated with significantly higher IL-18 and lower CRP concentrations.
Increasing gestational age was associated with significantly lower sIL-6R alpha and TGF-beta 1 concentrations and higher CRP concentrations. And boys had significantly lower sIL-6R alpha and IL-8 concentrations and higher CRP concentrations than girls.
However, none of the following factors were significantly associated with concentrations of inflammatory biomarkers: maternal age at delivery, maternal hospital contact attributable to infection during pregnancy, maternal prescription for antimicrobials during pregnancy, birth weight, and mode of delivery.
“Our findings underline the role the child’s baseline immune characteristics may play in the development of ALL,” Dr. Søegaard said. “However, we cannot yet use our research results to predict who will develop childhood ALL.”
The study was sponsored by the Dagmar Marshall Foundation, the A.P. Møller Foundation, the Danish Childhood Cancer Foundation, the Arvid Nilsson Foundation, and the Danish Cancer Research Foundation. The investigators reported having no conflicts of interest.
SOURCE: Søegaard SH et al. Cancer Res. 2018;78(18);5458-63.
FROM CANCER RESEARCH
Key clinical point:
Major finding: Neonatal concentrations of some inflammatory markers were significantly different between BCP-ALL patients and controls.
Study details: Ten markers were measured in 178 patients with BCP-ALL and 178 matched controls.
Disclosures: The study was sponsored by the Dagmar Marshall Foundation, the A.P. Møller Foundation, the Danish Childhood Cancer Foundation, the Arvid Nilsson Foundation, and the Danish Cancer Research Foundation. The investigators reported having no conflicts of interest.
Source: Søegaard SH et al. Cancer Res. 2018;78(18);5458-63.
Pruritus linked to wide variety of cancers
A wide variety of hematologic, dermatologic, and solid organ malignancies are associated with pruritus, a large, single-center, retrospective study suggests.
Blacks with pruritus had a higher odds ratio of hematologic malignancies, among others, while whites had higher likelihood of liver, gastrointestinal, respiratory and gynecologic cancers, results of the study show.
The results by race help address a gap in the literature, according to Shawn G. Kwatra, MD, of Johns Hopkins University, Baltimore, and his coinvestigators.
“Little is known about the association between pruritus and malignancy among different ethnic groups,” Dr. Kwatra and his coauthors wrote in the Journal of the American Academy of Dermatology.
The study shows a stronger association with more types of malignancies than has been reported previously, according to the investigators.
“The main difference is that prior studies focused on diagnosis of malignancy after the onset of pruritus, while our study includes malignancies diagnosed on or after pruritus onset,” they wrote.
Retrospective data for the study, which came from the Johns Hopkins Health System, included 16,925 patients aged 18 years or older who presented with itching or pruritus between April 4, 2013 and Dec. 31, 2017.
Of those 16,925 patients, 2,903 were also diagnosed with a concomitant malignancy during that time period. Compared with patients with no itching diagnosis during that time period, the pruritus patients more likely to have a concomitant malignancy, with an OR of 5.76 (95% confidence interval, 5.53-6.00), Dr. Kwatra and his colleagues found.
Malignancies most strongly associated with pruritus included those of the skin, liver, gallbladder and biliary tract, and hematopoietic system.
Among hematologic malignancies, pruritus was most strongly linked to myeloid leukemia and primary cutaneous lymphoma, while among skin cancers, squamous cell carcinoma was most strongly linked.
Whites had higher odds of any malignancy versus blacks, according to investigators, with ORs of 6.12 (95% CI, 5.81-6.46) and 5.61 (95% CI, 5.21-6.04), respectively.
Blacks with pruritus had higher ORs for hematologic and soft tissue malignancies including those of the muscle, fat, and peripheral nerve, investigators said, while whites had higher ORs for skin and liver malignancies.
The investigators also looked at the prevalence of skin eruptions in patients with pruritus and malignancy. “Eruption is variable by malignancy type and points to differing underlying mechanisms of pruritus,” they reported.
The highest rates of skin eruption were in patients with myeloid leukemia at 66%, followed by bone cancers at 58%, lymphocytic leukemia at 57%, multiple myeloma at 53%, and bronchus at 53%. The lowest rates of skin eruption were in patients with gallbladder and biliary tract, colon, pancreas, and liver malignancies.
Dr. Kwatra reported that he is an advisory board member for Menlo Therapeutics and Trevi Therapeutics.
SOURCE: Kwatra SG et al. J Am Acad Dermatol. 2018 Sep 11. doi: 10.1016/j.jaad.2018.08.044.
A wide variety of hematologic, dermatologic, and solid organ malignancies are associated with pruritus, a large, single-center, retrospective study suggests.
Blacks with pruritus had a higher odds ratio of hematologic malignancies, among others, while whites had higher likelihood of liver, gastrointestinal, respiratory and gynecologic cancers, results of the study show.
The results by race help address a gap in the literature, according to Shawn G. Kwatra, MD, of Johns Hopkins University, Baltimore, and his coinvestigators.
“Little is known about the association between pruritus and malignancy among different ethnic groups,” Dr. Kwatra and his coauthors wrote in the Journal of the American Academy of Dermatology.
The study shows a stronger association with more types of malignancies than has been reported previously, according to the investigators.
“The main difference is that prior studies focused on diagnosis of malignancy after the onset of pruritus, while our study includes malignancies diagnosed on or after pruritus onset,” they wrote.
Retrospective data for the study, which came from the Johns Hopkins Health System, included 16,925 patients aged 18 years or older who presented with itching or pruritus between April 4, 2013 and Dec. 31, 2017.
Of those 16,925 patients, 2,903 were also diagnosed with a concomitant malignancy during that time period. Compared with patients with no itching diagnosis during that time period, the pruritus patients more likely to have a concomitant malignancy, with an OR of 5.76 (95% confidence interval, 5.53-6.00), Dr. Kwatra and his colleagues found.
Malignancies most strongly associated with pruritus included those of the skin, liver, gallbladder and biliary tract, and hematopoietic system.
Among hematologic malignancies, pruritus was most strongly linked to myeloid leukemia and primary cutaneous lymphoma, while among skin cancers, squamous cell carcinoma was most strongly linked.
Whites had higher odds of any malignancy versus blacks, according to investigators, with ORs of 6.12 (95% CI, 5.81-6.46) and 5.61 (95% CI, 5.21-6.04), respectively.
Blacks with pruritus had higher ORs for hematologic and soft tissue malignancies including those of the muscle, fat, and peripheral nerve, investigators said, while whites had higher ORs for skin and liver malignancies.
The investigators also looked at the prevalence of skin eruptions in patients with pruritus and malignancy. “Eruption is variable by malignancy type and points to differing underlying mechanisms of pruritus,” they reported.
The highest rates of skin eruption were in patients with myeloid leukemia at 66%, followed by bone cancers at 58%, lymphocytic leukemia at 57%, multiple myeloma at 53%, and bronchus at 53%. The lowest rates of skin eruption were in patients with gallbladder and biliary tract, colon, pancreas, and liver malignancies.
Dr. Kwatra reported that he is an advisory board member for Menlo Therapeutics and Trevi Therapeutics.
SOURCE: Kwatra SG et al. J Am Acad Dermatol. 2018 Sep 11. doi: 10.1016/j.jaad.2018.08.044.
A wide variety of hematologic, dermatologic, and solid organ malignancies are associated with pruritus, a large, single-center, retrospective study suggests.
Blacks with pruritus had a higher odds ratio of hematologic malignancies, among others, while whites had higher likelihood of liver, gastrointestinal, respiratory and gynecologic cancers, results of the study show.
The results by race help address a gap in the literature, according to Shawn G. Kwatra, MD, of Johns Hopkins University, Baltimore, and his coinvestigators.
“Little is known about the association between pruritus and malignancy among different ethnic groups,” Dr. Kwatra and his coauthors wrote in the Journal of the American Academy of Dermatology.
The study shows a stronger association with more types of malignancies than has been reported previously, according to the investigators.
“The main difference is that prior studies focused on diagnosis of malignancy after the onset of pruritus, while our study includes malignancies diagnosed on or after pruritus onset,” they wrote.
Retrospective data for the study, which came from the Johns Hopkins Health System, included 16,925 patients aged 18 years or older who presented with itching or pruritus between April 4, 2013 and Dec. 31, 2017.
Of those 16,925 patients, 2,903 were also diagnosed with a concomitant malignancy during that time period. Compared with patients with no itching diagnosis during that time period, the pruritus patients more likely to have a concomitant malignancy, with an OR of 5.76 (95% confidence interval, 5.53-6.00), Dr. Kwatra and his colleagues found.
Malignancies most strongly associated with pruritus included those of the skin, liver, gallbladder and biliary tract, and hematopoietic system.
Among hematologic malignancies, pruritus was most strongly linked to myeloid leukemia and primary cutaneous lymphoma, while among skin cancers, squamous cell carcinoma was most strongly linked.
Whites had higher odds of any malignancy versus blacks, according to investigators, with ORs of 6.12 (95% CI, 5.81-6.46) and 5.61 (95% CI, 5.21-6.04), respectively.
Blacks with pruritus had higher ORs for hematologic and soft tissue malignancies including those of the muscle, fat, and peripheral nerve, investigators said, while whites had higher ORs for skin and liver malignancies.
The investigators also looked at the prevalence of skin eruptions in patients with pruritus and malignancy. “Eruption is variable by malignancy type and points to differing underlying mechanisms of pruritus,” they reported.
The highest rates of skin eruption were in patients with myeloid leukemia at 66%, followed by bone cancers at 58%, lymphocytic leukemia at 57%, multiple myeloma at 53%, and bronchus at 53%. The lowest rates of skin eruption were in patients with gallbladder and biliary tract, colon, pancreas, and liver malignancies.
Dr. Kwatra reported that he is an advisory board member for Menlo Therapeutics and Trevi Therapeutics.
SOURCE: Kwatra SG et al. J Am Acad Dermatol. 2018 Sep 11. doi: 10.1016/j.jaad.2018.08.044.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point:
Major finding: Blacks with pruritus had higher odds ratios for hematologic and soft tissue malignancies, while whites had higher ORs for skin and liver malignancies.
Study details: A retrospective study of 16,925 adults with itching or pruritus seen at a tertiary care center.
Disclosures: Dr. Kwatra reported serving as an advisory board member for Menlo Therapeutics and Trevi Therapeutics.
Source: Kwatra SG et al. J Am Acad Dermatol. 2018 Sep 11. doi: 10.1016/j.jaad.2018.08.044.
Kids with BCP-ALL exhibit immunological disparities at birth
Patients who develop B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in childhood may have dysregulated immune function at birth, according to a study published in Cancer Research.
Investigators evaluated neonatal concentrations of inflammatory markers and found significant differences between children who were later diagnosed with BCP-ALL and leukemia-free control subjects.
“Our findings suggest that children who develop ALL are immunologically disparate already at birth,” said study author Signe Holst Søegaard, a PhD student at Statens Serum Institut in Copenhagen, Denmark.
“This may link to other observations suggesting that children who develop ALL respond differently to infections in early childhood, potentially promoting subsequent genetic events required for transformation to ALL, or speculations that they are unable to eliminate preleukemic cells.”
“Importantly, our study does not inform about the nature of the associations observed—i.e., whether they are causal or consequential. Accordingly, further studies are needed both to confirm the findings and to identify the underlying mechanisms.”
For this study, Søegaard and her colleagues measured concentrations of 10 inflammatory markers on neonatal dried blood spots from 178 patients with BCP-ALL and 178 matched controls. The patients were diagnosed with BCP-ALL at ages 1 to 9.
The inflammatory markers assessed were interleukin (IL)-6, its soluble receptor sIL-6Rα, IL-8, IL-10, IL-12, IL-17, IL-18, transforming growth factor (TGF)-β1, monocyte chemotactic protein (MCP)-1, and C-reactive protein (CRP).
Results
Compared to controls, children who later developed BCP-ALL had significantly different neonatal concentrations of eight inflammatory markers.
Concentrations of sIL-6Rα, IL-8, TGF-β1, MCP-1, and CRP were significantly lower among the BCP-ALL patients. The adjusted odds ratios (adjusted for birth weight and maternal age) of BCP-ALL were 0.82 for sIL-6Rα, 0.84 for IL-8, 0.83 for TGF-β1, 0.68 for MCP-1, and 0.83 for CRP.
On the other hand, concentrations of IL-6, IL-17, and IL-18 were significantly higher among BCP-ALL patients than controls. The adjusted odds ratios were 1.19 for IL-6, 1.12 for IL-17, and 1.08 for IL-18.
The investigators noted that IL-10 concentrations were too low for accurate measurement in all patients and controls. Additionally, a “large proportion” of patients and controls (31% to 61%) had IL-6 and IL-17 concentrations that were below the limit of detection.
“We also demonstrated that several previously shown ALL risk factors—namely, birth order, gestational age, and sex—were associated with the neonatal concentrations of inflammatory markers,” Søegaard said. “These findings raise the interesting possibility that the effects of some known ALL risk factors partly act through prenatal programming of immune function.”
The investigators found that increasing birth order was associated with significantly higher IL-18 and lower CRP concentrations.
Increasing gestational age was associated with significantly lower sIL-6Rα and TGF-β1 concentrations and higher CRP concentrations. And males had significantly lower sIL-6Rα and IL-8 concentrations and higher CRP concentrations than females.
However, none of the following factors were significantly associated with concentrations of inflammatory biomarkers: maternal age at delivery, maternal hospital contact due to infection during pregnancy, maternal prescription for antimicrobials during pregnancy, birth weight, and mode of delivery.
“Our findings underline the role the child’s baseline immune characteristics may play in the development of ALL,” Søegaard said. “However, we cannot yet use our research results to predict who will develop childhood ALL. In future studies, we will further characterize the relation between immune constitution at birth and risk of childhood ALL with the ultimate goal of developing preventive strategies targeting predisposed children.”
Søegaard noted that this study had its limitations, including the small number of inflammatory markers studied. In addition, the limited sample size made it impossible to detect potential differences between BCP-ALL subtypes.
The study was sponsored by the Dagmar Marshall Foundation, the A.P. Møller Foundation, the Danish Childhood Cancer Foundation, the Arvid Nilsson Foundation, and the Danish Cancer Research Foundation. There were no conflicts of interest disclosed.
Patients who develop B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in childhood may have dysregulated immune function at birth, according to a study published in Cancer Research.
Investigators evaluated neonatal concentrations of inflammatory markers and found significant differences between children who were later diagnosed with BCP-ALL and leukemia-free control subjects.
“Our findings suggest that children who develop ALL are immunologically disparate already at birth,” said study author Signe Holst Søegaard, a PhD student at Statens Serum Institut in Copenhagen, Denmark.
“This may link to other observations suggesting that children who develop ALL respond differently to infections in early childhood, potentially promoting subsequent genetic events required for transformation to ALL, or speculations that they are unable to eliminate preleukemic cells.”
“Importantly, our study does not inform about the nature of the associations observed—i.e., whether they are causal or consequential. Accordingly, further studies are needed both to confirm the findings and to identify the underlying mechanisms.”
For this study, Søegaard and her colleagues measured concentrations of 10 inflammatory markers on neonatal dried blood spots from 178 patients with BCP-ALL and 178 matched controls. The patients were diagnosed with BCP-ALL at ages 1 to 9.
The inflammatory markers assessed were interleukin (IL)-6, its soluble receptor sIL-6Rα, IL-8, IL-10, IL-12, IL-17, IL-18, transforming growth factor (TGF)-β1, monocyte chemotactic protein (MCP)-1, and C-reactive protein (CRP).
Results
Compared to controls, children who later developed BCP-ALL had significantly different neonatal concentrations of eight inflammatory markers.
Concentrations of sIL-6Rα, IL-8, TGF-β1, MCP-1, and CRP were significantly lower among the BCP-ALL patients. The adjusted odds ratios (adjusted for birth weight and maternal age) of BCP-ALL were 0.82 for sIL-6Rα, 0.84 for IL-8, 0.83 for TGF-β1, 0.68 for MCP-1, and 0.83 for CRP.
On the other hand, concentrations of IL-6, IL-17, and IL-18 were significantly higher among BCP-ALL patients than controls. The adjusted odds ratios were 1.19 for IL-6, 1.12 for IL-17, and 1.08 for IL-18.
The investigators noted that IL-10 concentrations were too low for accurate measurement in all patients and controls. Additionally, a “large proportion” of patients and controls (31% to 61%) had IL-6 and IL-17 concentrations that were below the limit of detection.
“We also demonstrated that several previously shown ALL risk factors—namely, birth order, gestational age, and sex—were associated with the neonatal concentrations of inflammatory markers,” Søegaard said. “These findings raise the interesting possibility that the effects of some known ALL risk factors partly act through prenatal programming of immune function.”
The investigators found that increasing birth order was associated with significantly higher IL-18 and lower CRP concentrations.
Increasing gestational age was associated with significantly lower sIL-6Rα and TGF-β1 concentrations and higher CRP concentrations. And males had significantly lower sIL-6Rα and IL-8 concentrations and higher CRP concentrations than females.
However, none of the following factors were significantly associated with concentrations of inflammatory biomarkers: maternal age at delivery, maternal hospital contact due to infection during pregnancy, maternal prescription for antimicrobials during pregnancy, birth weight, and mode of delivery.
“Our findings underline the role the child’s baseline immune characteristics may play in the development of ALL,” Søegaard said. “However, we cannot yet use our research results to predict who will develop childhood ALL. In future studies, we will further characterize the relation between immune constitution at birth and risk of childhood ALL with the ultimate goal of developing preventive strategies targeting predisposed children.”
Søegaard noted that this study had its limitations, including the small number of inflammatory markers studied. In addition, the limited sample size made it impossible to detect potential differences between BCP-ALL subtypes.
The study was sponsored by the Dagmar Marshall Foundation, the A.P. Møller Foundation, the Danish Childhood Cancer Foundation, the Arvid Nilsson Foundation, and the Danish Cancer Research Foundation. There were no conflicts of interest disclosed.
Patients who develop B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in childhood may have dysregulated immune function at birth, according to a study published in Cancer Research.
Investigators evaluated neonatal concentrations of inflammatory markers and found significant differences between children who were later diagnosed with BCP-ALL and leukemia-free control subjects.
“Our findings suggest that children who develop ALL are immunologically disparate already at birth,” said study author Signe Holst Søegaard, a PhD student at Statens Serum Institut in Copenhagen, Denmark.
“This may link to other observations suggesting that children who develop ALL respond differently to infections in early childhood, potentially promoting subsequent genetic events required for transformation to ALL, or speculations that they are unable to eliminate preleukemic cells.”
“Importantly, our study does not inform about the nature of the associations observed—i.e., whether they are causal or consequential. Accordingly, further studies are needed both to confirm the findings and to identify the underlying mechanisms.”
For this study, Søegaard and her colleagues measured concentrations of 10 inflammatory markers on neonatal dried blood spots from 178 patients with BCP-ALL and 178 matched controls. The patients were diagnosed with BCP-ALL at ages 1 to 9.
The inflammatory markers assessed were interleukin (IL)-6, its soluble receptor sIL-6Rα, IL-8, IL-10, IL-12, IL-17, IL-18, transforming growth factor (TGF)-β1, monocyte chemotactic protein (MCP)-1, and C-reactive protein (CRP).
Results
Compared to controls, children who later developed BCP-ALL had significantly different neonatal concentrations of eight inflammatory markers.
Concentrations of sIL-6Rα, IL-8, TGF-β1, MCP-1, and CRP were significantly lower among the BCP-ALL patients. The adjusted odds ratios (adjusted for birth weight and maternal age) of BCP-ALL were 0.82 for sIL-6Rα, 0.84 for IL-8, 0.83 for TGF-β1, 0.68 for MCP-1, and 0.83 for CRP.
On the other hand, concentrations of IL-6, IL-17, and IL-18 were significantly higher among BCP-ALL patients than controls. The adjusted odds ratios were 1.19 for IL-6, 1.12 for IL-17, and 1.08 for IL-18.
The investigators noted that IL-10 concentrations were too low for accurate measurement in all patients and controls. Additionally, a “large proportion” of patients and controls (31% to 61%) had IL-6 and IL-17 concentrations that were below the limit of detection.
“We also demonstrated that several previously shown ALL risk factors—namely, birth order, gestational age, and sex—were associated with the neonatal concentrations of inflammatory markers,” Søegaard said. “These findings raise the interesting possibility that the effects of some known ALL risk factors partly act through prenatal programming of immune function.”
The investigators found that increasing birth order was associated with significantly higher IL-18 and lower CRP concentrations.
Increasing gestational age was associated with significantly lower sIL-6Rα and TGF-β1 concentrations and higher CRP concentrations. And males had significantly lower sIL-6Rα and IL-8 concentrations and higher CRP concentrations than females.
However, none of the following factors were significantly associated with concentrations of inflammatory biomarkers: maternal age at delivery, maternal hospital contact due to infection during pregnancy, maternal prescription for antimicrobials during pregnancy, birth weight, and mode of delivery.
“Our findings underline the role the child’s baseline immune characteristics may play in the development of ALL,” Søegaard said. “However, we cannot yet use our research results to predict who will develop childhood ALL. In future studies, we will further characterize the relation between immune constitution at birth and risk of childhood ALL with the ultimate goal of developing preventive strategies targeting predisposed children.”
Søegaard noted that this study had its limitations, including the small number of inflammatory markers studied. In addition, the limited sample size made it impossible to detect potential differences between BCP-ALL subtypes.
The study was sponsored by the Dagmar Marshall Foundation, the A.P. Møller Foundation, the Danish Childhood Cancer Foundation, the Arvid Nilsson Foundation, and the Danish Cancer Research Foundation. There were no conflicts of interest disclosed.
FDA approves drug for hairy cell leukemia
The U.S. Food and Drug Administration (FDA) has approved moxetumomab pasudotox-tdfk (Lumoxiti), a CD22-directed cytotoxin, to treat hairy cell leukemia (HCL).
Moxetumomab pasudotox is approved to treat adults with relapsed or refractory HCL who have received at least two prior systemic therapies, including treatment with a purine nucleoside analog.
The prescribing information for moxetumomab pasudotox includes a Boxed Warning noting that the drug poses risks of capillary leak syndrome (CLS) and hemolytic uremic syndrome (HUS). Treatment with moxetumomab pasudotox should be delayed or discontinued in patients who develop CLS and discontinued in patients with HUS.
The FDA granted the application for moxetumomab pasudotox fast track and priority review designations, and the drug received orphan drug designation from the FDA.
The agency granted the approval of moxetumomab pasudotox to AstraZeneca Pharmaceuticals based on results from a phase 3 trial (NCT01829711).
Data from this study were presented at the 2018 ASCO Annual Meeting (abstract 7004).
The trial included 80 patients with relapsed or refractory HCL who had received at least two prior lines of therapy.
At a median of 16.7 months of follow-up, the objective response rate was 75% (60/80), the complete response (CR) rate was 41% (33/80), and the durable CR rate was 30% (24/80). Durable CR was defined as CR with hematologic remission for more than 180 days.
Most patients with a CR achieved minimal residual disease negativity (82%; 27/33).
The median duration of response was not reached, nor was the median progression-free survival.
The most frequent treatment-related adverse events (AEs) were nausea (28%), peripheral edema (26%), headache (21%), and pyrexia (20%). Other treatment-related AEs included infections (8%) and neutropenia (3%).
Treatment-related AEs that led to discontinuation included HUS (5%), CLS (3%), and increased blood creatinine (3%).
In all, seven patients (9%) had CLS, and seven (9%) had HUS. This includes four (5%) patients who had both. CLS and HUS proved manageable and reversible.
There were three deaths in this trial, but none of them were considered treatment-related.
The U.S. Food and Drug Administration (FDA) has approved moxetumomab pasudotox-tdfk (Lumoxiti), a CD22-directed cytotoxin, to treat hairy cell leukemia (HCL).
Moxetumomab pasudotox is approved to treat adults with relapsed or refractory HCL who have received at least two prior systemic therapies, including treatment with a purine nucleoside analog.
The prescribing information for moxetumomab pasudotox includes a Boxed Warning noting that the drug poses risks of capillary leak syndrome (CLS) and hemolytic uremic syndrome (HUS). Treatment with moxetumomab pasudotox should be delayed or discontinued in patients who develop CLS and discontinued in patients with HUS.
The FDA granted the application for moxetumomab pasudotox fast track and priority review designations, and the drug received orphan drug designation from the FDA.
The agency granted the approval of moxetumomab pasudotox to AstraZeneca Pharmaceuticals based on results from a phase 3 trial (NCT01829711).
Data from this study were presented at the 2018 ASCO Annual Meeting (abstract 7004).
The trial included 80 patients with relapsed or refractory HCL who had received at least two prior lines of therapy.
At a median of 16.7 months of follow-up, the objective response rate was 75% (60/80), the complete response (CR) rate was 41% (33/80), and the durable CR rate was 30% (24/80). Durable CR was defined as CR with hematologic remission for more than 180 days.
Most patients with a CR achieved minimal residual disease negativity (82%; 27/33).
The median duration of response was not reached, nor was the median progression-free survival.
The most frequent treatment-related adverse events (AEs) were nausea (28%), peripheral edema (26%), headache (21%), and pyrexia (20%). Other treatment-related AEs included infections (8%) and neutropenia (3%).
Treatment-related AEs that led to discontinuation included HUS (5%), CLS (3%), and increased blood creatinine (3%).
In all, seven patients (9%) had CLS, and seven (9%) had HUS. This includes four (5%) patients who had both. CLS and HUS proved manageable and reversible.
There were three deaths in this trial, but none of them were considered treatment-related.
The U.S. Food and Drug Administration (FDA) has approved moxetumomab pasudotox-tdfk (Lumoxiti), a CD22-directed cytotoxin, to treat hairy cell leukemia (HCL).
Moxetumomab pasudotox is approved to treat adults with relapsed or refractory HCL who have received at least two prior systemic therapies, including treatment with a purine nucleoside analog.
The prescribing information for moxetumomab pasudotox includes a Boxed Warning noting that the drug poses risks of capillary leak syndrome (CLS) and hemolytic uremic syndrome (HUS). Treatment with moxetumomab pasudotox should be delayed or discontinued in patients who develop CLS and discontinued in patients with HUS.
The FDA granted the application for moxetumomab pasudotox fast track and priority review designations, and the drug received orphan drug designation from the FDA.
The agency granted the approval of moxetumomab pasudotox to AstraZeneca Pharmaceuticals based on results from a phase 3 trial (NCT01829711).
Data from this study were presented at the 2018 ASCO Annual Meeting (abstract 7004).
The trial included 80 patients with relapsed or refractory HCL who had received at least two prior lines of therapy.
At a median of 16.7 months of follow-up, the objective response rate was 75% (60/80), the complete response (CR) rate was 41% (33/80), and the durable CR rate was 30% (24/80). Durable CR was defined as CR with hematologic remission for more than 180 days.
Most patients with a CR achieved minimal residual disease negativity (82%; 27/33).
The median duration of response was not reached, nor was the median progression-free survival.
The most frequent treatment-related adverse events (AEs) were nausea (28%), peripheral edema (26%), headache (21%), and pyrexia (20%). Other treatment-related AEs included infections (8%) and neutropenia (3%).
Treatment-related AEs that led to discontinuation included HUS (5%), CLS (3%), and increased blood creatinine (3%).
In all, seven patients (9%) had CLS, and seven (9%) had HUS. This includes four (5%) patients who had both. CLS and HUS proved manageable and reversible.
There were three deaths in this trial, but none of them were considered treatment-related.
FDA approves new hairy cell leukemia drug
The Food and Drug Administration (FDA) has approved moxetumomab pasudotox-tdfk (Lumoxiti), a CD22-directed cytotoxin, to treat hairy cell leukemia (HCL).
Moxetumomab pasudotox is approved to treat adults with relapsed or refractory HCL who have received at least two prior systemic therapies, including treatment with a purine nucleoside analog.
The prescribing information for moxetumomab pasudotox includes a boxed warning noting that the drug poses risks of capillary leak syndrome and hemolytic uremic syndrome. Other serious warnings include the risk of decreased renal function, infusion-related reactions, and electrolyte abnormalities.
The FDA granted the application for moxetumomab pasudotox fast track, priority review, and an orphan drug designation.
The agency approved AstraZeneca’s moxetumomab pasudotox based on results from a phase 3 trial (NCT01829711). Data from this study were presented at the 2018 annual meeting of the American Society of Clinical Oncology (abstract 7004).
The trial included 80 patients with relapsed or refractory HCL who had received at least two prior lines of therapy.
At a median of 16.7 months of follow-up, the objective response rate was 75%, the complete response (CR) rate was 41%, and the durable CR rate was 30%. Durable CR was defined as CR with hematologic remission for more than 180 days.
Most patients with a CR achieved minimal residual disease negativity (82%; 27/33).
The median duration of response was not reached, nor was the median progression-free survival.
The most common treatment-related adverse events (AEs) were nausea, peripheral edema, headache, and pyrexia. Other treatment-related AEs included infections and neutropenia.
Treatment-related AEs that led to discontinuation included capillary leak syndrome, hemolytic uremic syndrome, and increased blood creatinine.
There were three deaths in this trial, but none of them were considered treatment related.
The Food and Drug Administration (FDA) has approved moxetumomab pasudotox-tdfk (Lumoxiti), a CD22-directed cytotoxin, to treat hairy cell leukemia (HCL).
Moxetumomab pasudotox is approved to treat adults with relapsed or refractory HCL who have received at least two prior systemic therapies, including treatment with a purine nucleoside analog.
The prescribing information for moxetumomab pasudotox includes a boxed warning noting that the drug poses risks of capillary leak syndrome and hemolytic uremic syndrome. Other serious warnings include the risk of decreased renal function, infusion-related reactions, and electrolyte abnormalities.
The FDA granted the application for moxetumomab pasudotox fast track, priority review, and an orphan drug designation.
The agency approved AstraZeneca’s moxetumomab pasudotox based on results from a phase 3 trial (NCT01829711). Data from this study were presented at the 2018 annual meeting of the American Society of Clinical Oncology (abstract 7004).
The trial included 80 patients with relapsed or refractory HCL who had received at least two prior lines of therapy.
At a median of 16.7 months of follow-up, the objective response rate was 75%, the complete response (CR) rate was 41%, and the durable CR rate was 30%. Durable CR was defined as CR with hematologic remission for more than 180 days.
Most patients with a CR achieved minimal residual disease negativity (82%; 27/33).
The median duration of response was not reached, nor was the median progression-free survival.
The most common treatment-related adverse events (AEs) were nausea, peripheral edema, headache, and pyrexia. Other treatment-related AEs included infections and neutropenia.
Treatment-related AEs that led to discontinuation included capillary leak syndrome, hemolytic uremic syndrome, and increased blood creatinine.
There were three deaths in this trial, but none of them were considered treatment related.
The Food and Drug Administration (FDA) has approved moxetumomab pasudotox-tdfk (Lumoxiti), a CD22-directed cytotoxin, to treat hairy cell leukemia (HCL).
Moxetumomab pasudotox is approved to treat adults with relapsed or refractory HCL who have received at least two prior systemic therapies, including treatment with a purine nucleoside analog.
The prescribing information for moxetumomab pasudotox includes a boxed warning noting that the drug poses risks of capillary leak syndrome and hemolytic uremic syndrome. Other serious warnings include the risk of decreased renal function, infusion-related reactions, and electrolyte abnormalities.
The FDA granted the application for moxetumomab pasudotox fast track, priority review, and an orphan drug designation.
The agency approved AstraZeneca’s moxetumomab pasudotox based on results from a phase 3 trial (NCT01829711). Data from this study were presented at the 2018 annual meeting of the American Society of Clinical Oncology (abstract 7004).
The trial included 80 patients with relapsed or refractory HCL who had received at least two prior lines of therapy.
At a median of 16.7 months of follow-up, the objective response rate was 75%, the complete response (CR) rate was 41%, and the durable CR rate was 30%. Durable CR was defined as CR with hematologic remission for more than 180 days.
Most patients with a CR achieved minimal residual disease negativity (82%; 27/33).
The median duration of response was not reached, nor was the median progression-free survival.
The most common treatment-related adverse events (AEs) were nausea, peripheral edema, headache, and pyrexia. Other treatment-related AEs included infections and neutropenia.
Treatment-related AEs that led to discontinuation included capillary leak syndrome, hemolytic uremic syndrome, and increased blood creatinine.
There were three deaths in this trial, but none of them were considered treatment related.
Insights could change treatment, classification of MPAL
An extensive analysis of mixed phenotype acute leukemia (MPAL) has led to new insights that may have implications for disease classification and treatment.
Researchers believe they have identified new subtypes of MPAL that should be included in the World Health Organization (WHO) classification for acute leukemia.
Each of these subtypes shares genomic characteristics with other acute leukemias, which suggests the new subtypes might respond to treatments that are already in use.
This research has also shed light on how MPAL evolves and appears to provide an explanation for why MPAL displays characteristics of both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
“ALL and AML have very different treatments, but MPAL has features of both, so the question of how best to treat patients with MPAL has been challenging the leukemia community worldwide, and long-term survival of patients has been poor,” said Charles Mullighan, MBBS, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
With these issues in mind, Dr. Mullighan and his colleagues conducted their study of MPAL and described their findings in Nature.
New classifications
The researchers used whole-genome, whole-exome, and RNA sequencing to analyze 115 samples from pediatric patients with MPAL.
The analysis revealed mutations that define the two most common subtypes of MPAL—B/myeloid and T/myeloid—and suggested these subtypes share similarities with other leukemia subtypes.
The researchers found that 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL. In fact, the team said the gene expression profiles of ZNF384r B-ALL and ZNF384r MPAL were indistinguishable.
“That is biologically and clinically important,” Dr. Mullighan said. “The findings suggest the ZNF384 rearrangement defines a distinct leukemia subtype, and the alteration should be used to guide treatment.”
The researchers noted that patients with ZNF384r exhibited higher FLT3 expression than patients with other types of B/myeloid or T/myeloid MPAL, so patients with ZNF384r MPAL might respond well to treatment with a FLT3 inhibitor.
This study also showed that cases of B/myeloid MPAL without ZNF384r shared genomic features with other B-ALL subtypes, such as Ph-like B-ALL, which may have implications for treatment.
Another of the researchers’ discoveries was that T/myeloid MPAL and early T-cell precursor ALL have similar gene expression profiles.
The team identified several genes that were mutated at similar frequencies in T/myeloid MPAL and early T-cell precursor ALL, including WT1, ETV6, EZH2, and FLT3. WT1 was the most frequently mutated transcription factor gene in T/myeloid MPAL.
Based on these findings, the researchers said the WHO classification of acute leukemia should be updated to include:
- ZNF384r acute leukemia (either B-ALL or MPAL)
- WT1-mutant T/myeloid MPAL
- Ph-like B/myeloid MPAL.
Evolution of MPAL
The researchers’ analyses also revealed leukemia-initiating genetic alterations in early hematopoietic progenitors.
The team said this and other findings—including the common genomic features of ZNF384r MPAL and B-ALL—suggest the ambiguous phenotype of MPAL results from alterations in immature hematopoietic progenitors.
“These findings suggest that the founding mutation occurs early in blood cell development, in some cases in hematopoietic stem cells, and results in an acute leukemia with features of both myeloid and lymphoid cells,” said study author Thomas Alexander, MD, of the University of North Carolina at Chapel Hill.
“One previous theory was that the reason you have two different cancer types within the same patient is that they acquire different mutations that drive them to become AML or ALL, with genomically distinct tumors within the same patient. That doesn’t seem to be the case from our data. Our proposed model is that the mutations occur earlier in development in cells that retain the potential to acquire myeloid or lymphoid features.”
This research was supported by the National Cancer Institute, the National Institutes of Health, Cookies for Kids’ Cancer, and other organizations.
An extensive analysis of mixed phenotype acute leukemia (MPAL) has led to new insights that may have implications for disease classification and treatment.
Researchers believe they have identified new subtypes of MPAL that should be included in the World Health Organization (WHO) classification for acute leukemia.
Each of these subtypes shares genomic characteristics with other acute leukemias, which suggests the new subtypes might respond to treatments that are already in use.
This research has also shed light on how MPAL evolves and appears to provide an explanation for why MPAL displays characteristics of both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
“ALL and AML have very different treatments, but MPAL has features of both, so the question of how best to treat patients with MPAL has been challenging the leukemia community worldwide, and long-term survival of patients has been poor,” said Charles Mullighan, MBBS, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
With these issues in mind, Dr. Mullighan and his colleagues conducted their study of MPAL and described their findings in Nature.
New classifications
The researchers used whole-genome, whole-exome, and RNA sequencing to analyze 115 samples from pediatric patients with MPAL.
The analysis revealed mutations that define the two most common subtypes of MPAL—B/myeloid and T/myeloid—and suggested these subtypes share similarities with other leukemia subtypes.
The researchers found that 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL. In fact, the team said the gene expression profiles of ZNF384r B-ALL and ZNF384r MPAL were indistinguishable.
“That is biologically and clinically important,” Dr. Mullighan said. “The findings suggest the ZNF384 rearrangement defines a distinct leukemia subtype, and the alteration should be used to guide treatment.”
The researchers noted that patients with ZNF384r exhibited higher FLT3 expression than patients with other types of B/myeloid or T/myeloid MPAL, so patients with ZNF384r MPAL might respond well to treatment with a FLT3 inhibitor.
This study also showed that cases of B/myeloid MPAL without ZNF384r shared genomic features with other B-ALL subtypes, such as Ph-like B-ALL, which may have implications for treatment.
Another of the researchers’ discoveries was that T/myeloid MPAL and early T-cell precursor ALL have similar gene expression profiles.
The team identified several genes that were mutated at similar frequencies in T/myeloid MPAL and early T-cell precursor ALL, including WT1, ETV6, EZH2, and FLT3. WT1 was the most frequently mutated transcription factor gene in T/myeloid MPAL.
Based on these findings, the researchers said the WHO classification of acute leukemia should be updated to include:
- ZNF384r acute leukemia (either B-ALL or MPAL)
- WT1-mutant T/myeloid MPAL
- Ph-like B/myeloid MPAL.
Evolution of MPAL
The researchers’ analyses also revealed leukemia-initiating genetic alterations in early hematopoietic progenitors.
The team said this and other findings—including the common genomic features of ZNF384r MPAL and B-ALL—suggest the ambiguous phenotype of MPAL results from alterations in immature hematopoietic progenitors.
“These findings suggest that the founding mutation occurs early in blood cell development, in some cases in hematopoietic stem cells, and results in an acute leukemia with features of both myeloid and lymphoid cells,” said study author Thomas Alexander, MD, of the University of North Carolina at Chapel Hill.
“One previous theory was that the reason you have two different cancer types within the same patient is that they acquire different mutations that drive them to become AML or ALL, with genomically distinct tumors within the same patient. That doesn’t seem to be the case from our data. Our proposed model is that the mutations occur earlier in development in cells that retain the potential to acquire myeloid or lymphoid features.”
This research was supported by the National Cancer Institute, the National Institutes of Health, Cookies for Kids’ Cancer, and other organizations.
An extensive analysis of mixed phenotype acute leukemia (MPAL) has led to new insights that may have implications for disease classification and treatment.
Researchers believe they have identified new subtypes of MPAL that should be included in the World Health Organization (WHO) classification for acute leukemia.
Each of these subtypes shares genomic characteristics with other acute leukemias, which suggests the new subtypes might respond to treatments that are already in use.
This research has also shed light on how MPAL evolves and appears to provide an explanation for why MPAL displays characteristics of both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
“ALL and AML have very different treatments, but MPAL has features of both, so the question of how best to treat patients with MPAL has been challenging the leukemia community worldwide, and long-term survival of patients has been poor,” said Charles Mullighan, MBBS, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
With these issues in mind, Dr. Mullighan and his colleagues conducted their study of MPAL and described their findings in Nature.
New classifications
The researchers used whole-genome, whole-exome, and RNA sequencing to analyze 115 samples from pediatric patients with MPAL.
The analysis revealed mutations that define the two most common subtypes of MPAL—B/myeloid and T/myeloid—and suggested these subtypes share similarities with other leukemia subtypes.
The researchers found that 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL. In fact, the team said the gene expression profiles of ZNF384r B-ALL and ZNF384r MPAL were indistinguishable.
“That is biologically and clinically important,” Dr. Mullighan said. “The findings suggest the ZNF384 rearrangement defines a distinct leukemia subtype, and the alteration should be used to guide treatment.”
The researchers noted that patients with ZNF384r exhibited higher FLT3 expression than patients with other types of B/myeloid or T/myeloid MPAL, so patients with ZNF384r MPAL might respond well to treatment with a FLT3 inhibitor.
This study also showed that cases of B/myeloid MPAL without ZNF384r shared genomic features with other B-ALL subtypes, such as Ph-like B-ALL, which may have implications for treatment.
Another of the researchers’ discoveries was that T/myeloid MPAL and early T-cell precursor ALL have similar gene expression profiles.
The team identified several genes that were mutated at similar frequencies in T/myeloid MPAL and early T-cell precursor ALL, including WT1, ETV6, EZH2, and FLT3. WT1 was the most frequently mutated transcription factor gene in T/myeloid MPAL.
Based on these findings, the researchers said the WHO classification of acute leukemia should be updated to include:
- ZNF384r acute leukemia (either B-ALL or MPAL)
- WT1-mutant T/myeloid MPAL
- Ph-like B/myeloid MPAL.
Evolution of MPAL
The researchers’ analyses also revealed leukemia-initiating genetic alterations in early hematopoietic progenitors.
The team said this and other findings—including the common genomic features of ZNF384r MPAL and B-ALL—suggest the ambiguous phenotype of MPAL results from alterations in immature hematopoietic progenitors.
“These findings suggest that the founding mutation occurs early in blood cell development, in some cases in hematopoietic stem cells, and results in an acute leukemia with features of both myeloid and lymphoid cells,” said study author Thomas Alexander, MD, of the University of North Carolina at Chapel Hill.
“One previous theory was that the reason you have two different cancer types within the same patient is that they acquire different mutations that drive them to become AML or ALL, with genomically distinct tumors within the same patient. That doesn’t seem to be the case from our data. Our proposed model is that the mutations occur earlier in development in cells that retain the potential to acquire myeloid or lymphoid features.”
This research was supported by the National Cancer Institute, the National Institutes of Health, Cookies for Kids’ Cancer, and other organizations.