User login
CHMP backs 2 biosimilar pegfilgrastim products
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for 2 pegfilgrastim biosimilar candidates—Udenyca and Pelgraz.
Both products have been deemed highly similar to the reference product, Neulasta, a growth-colony-stimulating factor intended to reduce the duration of neutropenia and the incidence of febrile neutropenia due to chemotherapy.
The CHMP’s recommendations for Pelgraz and Udenyca will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
If approved, Udenyca and Pelgraz will be available as 6 mg solutions for injection.
The full indication for both products will be to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults receiving cytotoxic chemotherapy for malignancies, except chronic myeloid leukemia and myelodysplastic syndromes.
The CHMP said data have shown that Pelgraz and Udenyca both have comparable quality, safety, and efficacy to Neulasta.
Pelgraz’s marketing authorization application is supported by data from a phase 1 pharmacokinetic (PK) and pharmacodynamic (PD) study in healthy volunteers and a phase 3 study of breast cancer patients receiving docetaxel, doxorubicin, and cyclophosphamide.
Results from the phase 1 study were published in Clinical Pharmacology in Drug Development in 2016.
Udenyca’s marketing authorization application is supported by data from an immunogenicity study as well as a PK/PD study comparing Udenyca (formerly CHS-1701) and Neulasta in healthy subjects.
Results from the PK/PD trial were presented at the 2017 ASCO Annual Meeting.
The applicant for Udenyca is ERA Consulting GmbH. The applicant for Pelgraz is Accord Healthcare Limited (the international arm of Intas Pharmaceuticals Ltd).
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for 2 pegfilgrastim biosimilar candidates—Udenyca and Pelgraz.
Both products have been deemed highly similar to the reference product, Neulasta, a growth-colony-stimulating factor intended to reduce the duration of neutropenia and the incidence of febrile neutropenia due to chemotherapy.
The CHMP’s recommendations for Pelgraz and Udenyca will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
If approved, Udenyca and Pelgraz will be available as 6 mg solutions for injection.
The full indication for both products will be to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults receiving cytotoxic chemotherapy for malignancies, except chronic myeloid leukemia and myelodysplastic syndromes.
The CHMP said data have shown that Pelgraz and Udenyca both have comparable quality, safety, and efficacy to Neulasta.
Pelgraz’s marketing authorization application is supported by data from a phase 1 pharmacokinetic (PK) and pharmacodynamic (PD) study in healthy volunteers and a phase 3 study of breast cancer patients receiving docetaxel, doxorubicin, and cyclophosphamide.
Results from the phase 1 study were published in Clinical Pharmacology in Drug Development in 2016.
Udenyca’s marketing authorization application is supported by data from an immunogenicity study as well as a PK/PD study comparing Udenyca (formerly CHS-1701) and Neulasta in healthy subjects.
Results from the PK/PD trial were presented at the 2017 ASCO Annual Meeting.
The applicant for Udenyca is ERA Consulting GmbH. The applicant for Pelgraz is Accord Healthcare Limited (the international arm of Intas Pharmaceuticals Ltd).
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for 2 pegfilgrastim biosimilar candidates—Udenyca and Pelgraz.
Both products have been deemed highly similar to the reference product, Neulasta, a growth-colony-stimulating factor intended to reduce the duration of neutropenia and the incidence of febrile neutropenia due to chemotherapy.
The CHMP’s recommendations for Pelgraz and Udenyca will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
If approved, Udenyca and Pelgraz will be available as 6 mg solutions for injection.
The full indication for both products will be to reduce the duration of neutropenia and the incidence of febrile neutropenia in adults receiving cytotoxic chemotherapy for malignancies, except chronic myeloid leukemia and myelodysplastic syndromes.
The CHMP said data have shown that Pelgraz and Udenyca both have comparable quality, safety, and efficacy to Neulasta.
Pelgraz’s marketing authorization application is supported by data from a phase 1 pharmacokinetic (PK) and pharmacodynamic (PD) study in healthy volunteers and a phase 3 study of breast cancer patients receiving docetaxel, doxorubicin, and cyclophosphamide.
Results from the phase 1 study were published in Clinical Pharmacology in Drug Development in 2016.
Udenyca’s marketing authorization application is supported by data from an immunogenicity study as well as a PK/PD study comparing Udenyca (formerly CHS-1701) and Neulasta in healthy subjects.
Results from the PK/PD trial were presented at the 2017 ASCO Annual Meeting.
The applicant for Udenyca is ERA Consulting GmbH. The applicant for Pelgraz is Accord Healthcare Limited (the international arm of Intas Pharmaceuticals Ltd).
Global burden of hematologic malignancies
Research has shown an increase in the global incidence of leukemia and non-Hodgkin lymphoma (NHL) in recent years.
The Global Burden of Disease (GBD) study showed that, from 2006 to 2016, the incidence of NHL increased 45%, and the incidence of leukemia increased 26%.
These increases were largely due to population growth and aging.
Results from the GDB study were published in JAMA Oncology.
The study indicated that, in 2016, there were 17.2 million cases of cancer worldwide and 8.9 million cancer deaths.
One in 3 men were likely to get cancer during their lifetime, as were 1 in 5 women. Cancer was associated with 213.2 million disability-adjusted life years (DALYs).
The following table lists the 2016 global incidence and mortality figures for all cancers combined and for individual hematologic malignancies.
| Cancer type | Cases, thousands | Deaths, thousands |
| All cancers | 17,228 | 8927 |
| Leukemias | 467 | 310 |
| Acute lymphoid leukemia | 76 | 51 |
| Chronic lymphoid leukemia | 105 | 35 |
| Acute myeloid leukemia | 103 | 85 |
| Chronic myeloid leukemia | 32 | 22 |
| Other leukemias | 150 | 117 |
| Hodgkin lymphoma | 73 | 29 |
| NHL | 461 | 240 |
| Multiple myeloma | 139 | 98 |
Leukemia
In 2016, there were 467,000 new cases of leukemia and 310,000 leukemia deaths. Leukemia was responsible for 10.2 million DALYs. Leukemia developed in 1 in 118 men and 1 in 194 women worldwide.
Between 2006 and 2016, the global leukemia incidence increased by 26%—from 370,482 to 466,802 cases.
The researchers said the factors contributing to this increase were population growth (12%), population aging (10%), and an increase in age-specific incidence rates (3%).
NHL
In 2016, there were 461,000 new cases of NHL and 240,000 NHL deaths. NHL was responsible for 6.8 million DALYs. NHL developed in 1 in 110 men and 1 in 161 women worldwide.
Between 2006 and 2016, NHL increased by 45%, from 319,078 to 461,164 cases.
The factors contributing to this increase were increasing age-specific incidence rates (17%), changing population age structure (15%), and population growth (12%).
“A large proportion of the increase in cancer incidence can be explained by improving life expectancy and population growth—a development that can at least partially be attributed to a reduced burden from other common diseases,” the study authors wrote.
The authors also pointed out that prevention efforts are less effective for hematologic malignancies than for other cancers.
Research has shown an increase in the global incidence of leukemia and non-Hodgkin lymphoma (NHL) in recent years.
The Global Burden of Disease (GBD) study showed that, from 2006 to 2016, the incidence of NHL increased 45%, and the incidence of leukemia increased 26%.
These increases were largely due to population growth and aging.
Results from the GDB study were published in JAMA Oncology.
The study indicated that, in 2016, there were 17.2 million cases of cancer worldwide and 8.9 million cancer deaths.
One in 3 men were likely to get cancer during their lifetime, as were 1 in 5 women. Cancer was associated with 213.2 million disability-adjusted life years (DALYs).
The following table lists the 2016 global incidence and mortality figures for all cancers combined and for individual hematologic malignancies.
| Cancer type | Cases, thousands | Deaths, thousands |
| All cancers | 17,228 | 8927 |
| Leukemias | 467 | 310 |
| Acute lymphoid leukemia | 76 | 51 |
| Chronic lymphoid leukemia | 105 | 35 |
| Acute myeloid leukemia | 103 | 85 |
| Chronic myeloid leukemia | 32 | 22 |
| Other leukemias | 150 | 117 |
| Hodgkin lymphoma | 73 | 29 |
| NHL | 461 | 240 |
| Multiple myeloma | 139 | 98 |
Leukemia
In 2016, there were 467,000 new cases of leukemia and 310,000 leukemia deaths. Leukemia was responsible for 10.2 million DALYs. Leukemia developed in 1 in 118 men and 1 in 194 women worldwide.
Between 2006 and 2016, the global leukemia incidence increased by 26%—from 370,482 to 466,802 cases.
The researchers said the factors contributing to this increase were population growth (12%), population aging (10%), and an increase in age-specific incidence rates (3%).
NHL
In 2016, there were 461,000 new cases of NHL and 240,000 NHL deaths. NHL was responsible for 6.8 million DALYs. NHL developed in 1 in 110 men and 1 in 161 women worldwide.
Between 2006 and 2016, NHL increased by 45%, from 319,078 to 461,164 cases.
The factors contributing to this increase were increasing age-specific incidence rates (17%), changing population age structure (15%), and population growth (12%).
“A large proportion of the increase in cancer incidence can be explained by improving life expectancy and population growth—a development that can at least partially be attributed to a reduced burden from other common diseases,” the study authors wrote.
The authors also pointed out that prevention efforts are less effective for hematologic malignancies than for other cancers.
Research has shown an increase in the global incidence of leukemia and non-Hodgkin lymphoma (NHL) in recent years.
The Global Burden of Disease (GBD) study showed that, from 2006 to 2016, the incidence of NHL increased 45%, and the incidence of leukemia increased 26%.
These increases were largely due to population growth and aging.
Results from the GDB study were published in JAMA Oncology.
The study indicated that, in 2016, there were 17.2 million cases of cancer worldwide and 8.9 million cancer deaths.
One in 3 men were likely to get cancer during their lifetime, as were 1 in 5 women. Cancer was associated with 213.2 million disability-adjusted life years (DALYs).
The following table lists the 2016 global incidence and mortality figures for all cancers combined and for individual hematologic malignancies.
| Cancer type | Cases, thousands | Deaths, thousands |
| All cancers | 17,228 | 8927 |
| Leukemias | 467 | 310 |
| Acute lymphoid leukemia | 76 | 51 |
| Chronic lymphoid leukemia | 105 | 35 |
| Acute myeloid leukemia | 103 | 85 |
| Chronic myeloid leukemia | 32 | 22 |
| Other leukemias | 150 | 117 |
| Hodgkin lymphoma | 73 | 29 |
| NHL | 461 | 240 |
| Multiple myeloma | 139 | 98 |
Leukemia
In 2016, there were 467,000 new cases of leukemia and 310,000 leukemia deaths. Leukemia was responsible for 10.2 million DALYs. Leukemia developed in 1 in 118 men and 1 in 194 women worldwide.
Between 2006 and 2016, the global leukemia incidence increased by 26%—from 370,482 to 466,802 cases.
The researchers said the factors contributing to this increase were population growth (12%), population aging (10%), and an increase in age-specific incidence rates (3%).
NHL
In 2016, there were 461,000 new cases of NHL and 240,000 NHL deaths. NHL was responsible for 6.8 million DALYs. NHL developed in 1 in 110 men and 1 in 161 women worldwide.
Between 2006 and 2016, NHL increased by 45%, from 319,078 to 461,164 cases.
The factors contributing to this increase were increasing age-specific incidence rates (17%), changing population age structure (15%), and population growth (12%).
“A large proportion of the increase in cancer incidence can be explained by improving life expectancy and population growth—a development that can at least partially be attributed to a reduced burden from other common diseases,” the study authors wrote.
The authors also pointed out that prevention efforts are less effective for hematologic malignancies than for other cancers.
Study could change treatment of MLSM7
New findings could help improve treatment of an inherited bone marrow disorder known as myelodysplasia and leukemia syndrome with monosomy 7 (MLSM7), according to researchers.
While studying families affected by MLSM7, researchers identified germline mutations in SAMD9L or SAMD9 in patients who had hematologic abnormalities, myelodysplastic syndromes (MDS), or acute myeloid leukemia (AML).
However, these mutations were also present in apparently healthy family members, and the researchers found that bone marrow monosomy 7 sometimes resolved without treatment.
The team recounted these findings in JCI Insight.
The researchers analyzed blood samples from 16 siblings in 5 families affected by MLSM7 and found they all carried germline mutations in SAMD9 or SAMD9L. In 3 of the 5 families, there were apparently healthy parents who also carried the mutations.
“Surprisingly, the health consequences of these mutations varied tremendously for reasons that must still be determined, but the findings are already affecting how we may choose to manage these patients,” said study author Jeffery Klco, MD, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Three of the 16 siblings developed AML and died of the disease or related complications. Two other siblings were diagnosed with MDS.
The remaining 11 siblings with the mutations were apparently healthy, although several had been treated for anemia and other conditions associated with low blood counts.
Some of these patients had a previous history of bone marrow monosomy 7 that spontaneously corrected over time. These patients, despite no therapy, appeared to have normal bone marrow function.
“This was an even greater surprise,” Dr Klco said. “The spontaneous recovery experienced by some children with the germline mutations suggests some patients with SAMD9 and SAMD9L mutations who were previously considered candidates for bone marrow transplantation may recover hematologic function on their own.”
Dr Klco and his colleagues have a theory that could explain the spontaneous correction. The team noted that SAMD9 and SAMD9L are activated in response to viral infections. While the normal function of both proteins is poorly understood, abnormally activated SAMD9 and SAMD9L are known to inhibit cell growth.
In this study, deep sequencing showed that selective pressure on developing blood cells favors cells without the SAMD9 or SAMD9L mutations. That may increase pressure for cells to selectively jettison chromosome 7 with the gene alteration or take other molecular measures to counteract the mutant protein.
Implications for treatment
This research also showed that, in patients who developed AML, loss of chromosome 7 was associated with the development of mutations in additional genes, including ETV6, KRAS, SETBP1, and RUNX1.
These same mutations are broadly associated with monosomy 7 in AML, which suggests that understanding how SAMD9 and SAMD9L mutations contribute to leukemia has implications beyond familial cases.
The presence of secondary mutations may also help clinicians identify which patients will benefit from immediate treatment, including chemotherapy or transplant to prevent or treat AML or myelodysplasia, Dr Klco said.
For patients without the mutations or significant symptoms due to low blood cell counts, watchful waiting with careful follow-up may sometimes be an option.
“Now that we know this disease can resolve without treatment in some patients, we need to focus on developing screening and treatment guidelines,” Dr Klco said. “We want to reserve hematopoietic bone marrow transplantation for those who truly need the procedure. These findings will help to point the way.”
“So little is known about SAMD9 and SAMD9L that we need to continue working in the lab to better understand how these mutations impact blood cell development and how they are activated in response to infections and other types of stress.”
New findings could help improve treatment of an inherited bone marrow disorder known as myelodysplasia and leukemia syndrome with monosomy 7 (MLSM7), according to researchers.
While studying families affected by MLSM7, researchers identified germline mutations in SAMD9L or SAMD9 in patients who had hematologic abnormalities, myelodysplastic syndromes (MDS), or acute myeloid leukemia (AML).
However, these mutations were also present in apparently healthy family members, and the researchers found that bone marrow monosomy 7 sometimes resolved without treatment.
The team recounted these findings in JCI Insight.
The researchers analyzed blood samples from 16 siblings in 5 families affected by MLSM7 and found they all carried germline mutations in SAMD9 or SAMD9L. In 3 of the 5 families, there were apparently healthy parents who also carried the mutations.
“Surprisingly, the health consequences of these mutations varied tremendously for reasons that must still be determined, but the findings are already affecting how we may choose to manage these patients,” said study author Jeffery Klco, MD, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Three of the 16 siblings developed AML and died of the disease or related complications. Two other siblings were diagnosed with MDS.
The remaining 11 siblings with the mutations were apparently healthy, although several had been treated for anemia and other conditions associated with low blood counts.
Some of these patients had a previous history of bone marrow monosomy 7 that spontaneously corrected over time. These patients, despite no therapy, appeared to have normal bone marrow function.
“This was an even greater surprise,” Dr Klco said. “The spontaneous recovery experienced by some children with the germline mutations suggests some patients with SAMD9 and SAMD9L mutations who were previously considered candidates for bone marrow transplantation may recover hematologic function on their own.”
Dr Klco and his colleagues have a theory that could explain the spontaneous correction. The team noted that SAMD9 and SAMD9L are activated in response to viral infections. While the normal function of both proteins is poorly understood, abnormally activated SAMD9 and SAMD9L are known to inhibit cell growth.
In this study, deep sequencing showed that selective pressure on developing blood cells favors cells without the SAMD9 or SAMD9L mutations. That may increase pressure for cells to selectively jettison chromosome 7 with the gene alteration or take other molecular measures to counteract the mutant protein.
Implications for treatment
This research also showed that, in patients who developed AML, loss of chromosome 7 was associated with the development of mutations in additional genes, including ETV6, KRAS, SETBP1, and RUNX1.
These same mutations are broadly associated with monosomy 7 in AML, which suggests that understanding how SAMD9 and SAMD9L mutations contribute to leukemia has implications beyond familial cases.
The presence of secondary mutations may also help clinicians identify which patients will benefit from immediate treatment, including chemotherapy or transplant to prevent or treat AML or myelodysplasia, Dr Klco said.
For patients without the mutations or significant symptoms due to low blood cell counts, watchful waiting with careful follow-up may sometimes be an option.
“Now that we know this disease can resolve without treatment in some patients, we need to focus on developing screening and treatment guidelines,” Dr Klco said. “We want to reserve hematopoietic bone marrow transplantation for those who truly need the procedure. These findings will help to point the way.”
“So little is known about SAMD9 and SAMD9L that we need to continue working in the lab to better understand how these mutations impact blood cell development and how they are activated in response to infections and other types of stress.”
New findings could help improve treatment of an inherited bone marrow disorder known as myelodysplasia and leukemia syndrome with monosomy 7 (MLSM7), according to researchers.
While studying families affected by MLSM7, researchers identified germline mutations in SAMD9L or SAMD9 in patients who had hematologic abnormalities, myelodysplastic syndromes (MDS), or acute myeloid leukemia (AML).
However, these mutations were also present in apparently healthy family members, and the researchers found that bone marrow monosomy 7 sometimes resolved without treatment.
The team recounted these findings in JCI Insight.
The researchers analyzed blood samples from 16 siblings in 5 families affected by MLSM7 and found they all carried germline mutations in SAMD9 or SAMD9L. In 3 of the 5 families, there were apparently healthy parents who also carried the mutations.
“Surprisingly, the health consequences of these mutations varied tremendously for reasons that must still be determined, but the findings are already affecting how we may choose to manage these patients,” said study author Jeffery Klco, MD, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Three of the 16 siblings developed AML and died of the disease or related complications. Two other siblings were diagnosed with MDS.
The remaining 11 siblings with the mutations were apparently healthy, although several had been treated for anemia and other conditions associated with low blood counts.
Some of these patients had a previous history of bone marrow monosomy 7 that spontaneously corrected over time. These patients, despite no therapy, appeared to have normal bone marrow function.
“This was an even greater surprise,” Dr Klco said. “The spontaneous recovery experienced by some children with the germline mutations suggests some patients with SAMD9 and SAMD9L mutations who were previously considered candidates for bone marrow transplantation may recover hematologic function on their own.”
Dr Klco and his colleagues have a theory that could explain the spontaneous correction. The team noted that SAMD9 and SAMD9L are activated in response to viral infections. While the normal function of both proteins is poorly understood, abnormally activated SAMD9 and SAMD9L are known to inhibit cell growth.
In this study, deep sequencing showed that selective pressure on developing blood cells favors cells without the SAMD9 or SAMD9L mutations. That may increase pressure for cells to selectively jettison chromosome 7 with the gene alteration or take other molecular measures to counteract the mutant protein.
Implications for treatment
This research also showed that, in patients who developed AML, loss of chromosome 7 was associated with the development of mutations in additional genes, including ETV6, KRAS, SETBP1, and RUNX1.
These same mutations are broadly associated with monosomy 7 in AML, which suggests that understanding how SAMD9 and SAMD9L mutations contribute to leukemia has implications beyond familial cases.
The presence of secondary mutations may also help clinicians identify which patients will benefit from immediate treatment, including chemotherapy or transplant to prevent or treat AML or myelodysplasia, Dr Klco said.
For patients without the mutations or significant symptoms due to low blood cell counts, watchful waiting with careful follow-up may sometimes be an option.
“Now that we know this disease can resolve without treatment in some patients, we need to focus on developing screening and treatment guidelines,” Dr Klco said. “We want to reserve hematopoietic bone marrow transplantation for those who truly need the procedure. These findings will help to point the way.”
“So little is known about SAMD9 and SAMD9L that we need to continue working in the lab to better understand how these mutations impact blood cell development and how they are activated in response to infections and other types of stress.”
Intrathecal methotrexate dosing in acute leukemia falls short
STOCKHOLM – Although intrathecal chemoprophylaxis for prevention of central nervous system involvement is an essential component of modern regimens to treat acute lymphoblastic leukemia (ALL), patients don’t always receive the recommended number of doses, potentially compromising remissions.
But as a large-scale audit of health care delivery in the United Kingdom has suggested, many of the possible causes for suboptimal delivery of intrathecal methotrexate (IT MTX) appear to be modifiable, reported Sven A. Sommerfeld, MD, and his colleagues from the Christie Hospital in Manchester, England.
“In our clinical observations, and confirmed in this audit, patients on intensive chemotherapy, including induction and consolidation protocols for acute lymphoblastic leukemia, can develop a number of issues and toxicities, which can interfere with the administration of IT MTX,” they wrote in a poster presented at the annual congress of the European Hematology Association.
Reasons for canceling or postponing scheduled doses of IT MTX range from “fairly compelling clinical reasons affecting patient safety or tolerance” to more mundane issues, such as scheduling and staffing problems, the investigators found.
“I think there are a number of factors that can improve compliance, including having your cancellation rate as low as possible. You have already prescribed the treatment, the patient is supposed to be attending [clinic], yet for some reason you cannot go ahead,” Dr. Sommerfeld said in an interview. “I think blood product support is important, but there are capacity issues. Organization of complex treatment protocols that involve systemic chemotherapy and concurrent intrathecal administration can be difficult as different people oversee both of these treatments.”
For example, protocols specify delivery of intrathecal chemoprophylaxis on specific days and induction chemotherapy on other days, and it may be difficult to coordinate the care so that the doses don’t overlap, he said.
Dr. Sommerfeld and his colleagues conducted an audit of standard of care for patients aged 16-60 years who were diagnosed with B-cell or T-cell ALL and received IT MTX under one of two clinical protocols: UKALL 2011 or UKALL 14.
The investigators examined data on IT MTX compliance and assigned each patient a compliance score calculated by the number of administered doses divided by protocol-defined number of doses. They also examined pharmacy records of cancellations of protocol-scheduled IT MTX from January 2016 through July 2017.
They found that the total number of IT MTX prescriptions delivered as a proportion of the number prescribed ranged from a low of 61.8% in 2009 to a high of 84.2% in 2007.
When the investigators looked at records for individual patients, including 29 adolescent and young adults receiving IT MTX under the UKALL 2011 protocol and 27 adults receiving it under the UKALL 14 protocol, they found that failure to maintain coagulation levels within parameters accounted for 23% of cancellations. The next most common reasons for cancellation were rescheduling for administrative or clinical reasons in 20% of canceled doses, low platelet levels in 19% of cases, and patient nonattendance or communication failure in 16% of cases. Other factors included problems with lumbar access, vincristine schedule for the same day, recent anticoagulation, and delay of blood results.
“Notable is the variable approach to IT MTX in patients requiring therapeutic dose anticoagulation, reflecting clinical decisions of different physicians on a case-by-case basis. Whilst a full discussion of this topic and management of asparaginase-associated [venous thromboembolism] is beyond the scope of this audit, we generally recognize today that many affected patients can be managed to continue therapy as per protocol,” the investigators wrote.
Integrating relevant prescriptions into a single information system, monitoring clinics for treatment backlogs, and improving clinical resources, such as staffing, could help to improve the efficacy of IT MTX therapy, they suggested.
The study was supported by the National Health Service Foundation Trust. Dr. Sommerfeld reported having no relevant financial disclosures.
SOURCE: Sommerfeld SA et al. EHA Congress, Abstract PS930.
STOCKHOLM – Although intrathecal chemoprophylaxis for prevention of central nervous system involvement is an essential component of modern regimens to treat acute lymphoblastic leukemia (ALL), patients don’t always receive the recommended number of doses, potentially compromising remissions.
But as a large-scale audit of health care delivery in the United Kingdom has suggested, many of the possible causes for suboptimal delivery of intrathecal methotrexate (IT MTX) appear to be modifiable, reported Sven A. Sommerfeld, MD, and his colleagues from the Christie Hospital in Manchester, England.
“In our clinical observations, and confirmed in this audit, patients on intensive chemotherapy, including induction and consolidation protocols for acute lymphoblastic leukemia, can develop a number of issues and toxicities, which can interfere with the administration of IT MTX,” they wrote in a poster presented at the annual congress of the European Hematology Association.
Reasons for canceling or postponing scheduled doses of IT MTX range from “fairly compelling clinical reasons affecting patient safety or tolerance” to more mundane issues, such as scheduling and staffing problems, the investigators found.
“I think there are a number of factors that can improve compliance, including having your cancellation rate as low as possible. You have already prescribed the treatment, the patient is supposed to be attending [clinic], yet for some reason you cannot go ahead,” Dr. Sommerfeld said in an interview. “I think blood product support is important, but there are capacity issues. Organization of complex treatment protocols that involve systemic chemotherapy and concurrent intrathecal administration can be difficult as different people oversee both of these treatments.”
For example, protocols specify delivery of intrathecal chemoprophylaxis on specific days and induction chemotherapy on other days, and it may be difficult to coordinate the care so that the doses don’t overlap, he said.
Dr. Sommerfeld and his colleagues conducted an audit of standard of care for patients aged 16-60 years who were diagnosed with B-cell or T-cell ALL and received IT MTX under one of two clinical protocols: UKALL 2011 or UKALL 14.
The investigators examined data on IT MTX compliance and assigned each patient a compliance score calculated by the number of administered doses divided by protocol-defined number of doses. They also examined pharmacy records of cancellations of protocol-scheduled IT MTX from January 2016 through July 2017.
They found that the total number of IT MTX prescriptions delivered as a proportion of the number prescribed ranged from a low of 61.8% in 2009 to a high of 84.2% in 2007.
When the investigators looked at records for individual patients, including 29 adolescent and young adults receiving IT MTX under the UKALL 2011 protocol and 27 adults receiving it under the UKALL 14 protocol, they found that failure to maintain coagulation levels within parameters accounted for 23% of cancellations. The next most common reasons for cancellation were rescheduling for administrative or clinical reasons in 20% of canceled doses, low platelet levels in 19% of cases, and patient nonattendance or communication failure in 16% of cases. Other factors included problems with lumbar access, vincristine schedule for the same day, recent anticoagulation, and delay of blood results.
“Notable is the variable approach to IT MTX in patients requiring therapeutic dose anticoagulation, reflecting clinical decisions of different physicians on a case-by-case basis. Whilst a full discussion of this topic and management of asparaginase-associated [venous thromboembolism] is beyond the scope of this audit, we generally recognize today that many affected patients can be managed to continue therapy as per protocol,” the investigators wrote.
Integrating relevant prescriptions into a single information system, monitoring clinics for treatment backlogs, and improving clinical resources, such as staffing, could help to improve the efficacy of IT MTX therapy, they suggested.
The study was supported by the National Health Service Foundation Trust. Dr. Sommerfeld reported having no relevant financial disclosures.
SOURCE: Sommerfeld SA et al. EHA Congress, Abstract PS930.
STOCKHOLM – Although intrathecal chemoprophylaxis for prevention of central nervous system involvement is an essential component of modern regimens to treat acute lymphoblastic leukemia (ALL), patients don’t always receive the recommended number of doses, potentially compromising remissions.
But as a large-scale audit of health care delivery in the United Kingdom has suggested, many of the possible causes for suboptimal delivery of intrathecal methotrexate (IT MTX) appear to be modifiable, reported Sven A. Sommerfeld, MD, and his colleagues from the Christie Hospital in Manchester, England.
“In our clinical observations, and confirmed in this audit, patients on intensive chemotherapy, including induction and consolidation protocols for acute lymphoblastic leukemia, can develop a number of issues and toxicities, which can interfere with the administration of IT MTX,” they wrote in a poster presented at the annual congress of the European Hematology Association.
Reasons for canceling or postponing scheduled doses of IT MTX range from “fairly compelling clinical reasons affecting patient safety or tolerance” to more mundane issues, such as scheduling and staffing problems, the investigators found.
“I think there are a number of factors that can improve compliance, including having your cancellation rate as low as possible. You have already prescribed the treatment, the patient is supposed to be attending [clinic], yet for some reason you cannot go ahead,” Dr. Sommerfeld said in an interview. “I think blood product support is important, but there are capacity issues. Organization of complex treatment protocols that involve systemic chemotherapy and concurrent intrathecal administration can be difficult as different people oversee both of these treatments.”
For example, protocols specify delivery of intrathecal chemoprophylaxis on specific days and induction chemotherapy on other days, and it may be difficult to coordinate the care so that the doses don’t overlap, he said.
Dr. Sommerfeld and his colleagues conducted an audit of standard of care for patients aged 16-60 years who were diagnosed with B-cell or T-cell ALL and received IT MTX under one of two clinical protocols: UKALL 2011 or UKALL 14.
The investigators examined data on IT MTX compliance and assigned each patient a compliance score calculated by the number of administered doses divided by protocol-defined number of doses. They also examined pharmacy records of cancellations of protocol-scheduled IT MTX from January 2016 through July 2017.
They found that the total number of IT MTX prescriptions delivered as a proportion of the number prescribed ranged from a low of 61.8% in 2009 to a high of 84.2% in 2007.
When the investigators looked at records for individual patients, including 29 adolescent and young adults receiving IT MTX under the UKALL 2011 protocol and 27 adults receiving it under the UKALL 14 protocol, they found that failure to maintain coagulation levels within parameters accounted for 23% of cancellations. The next most common reasons for cancellation were rescheduling for administrative or clinical reasons in 20% of canceled doses, low platelet levels in 19% of cases, and patient nonattendance or communication failure in 16% of cases. Other factors included problems with lumbar access, vincristine schedule for the same day, recent anticoagulation, and delay of blood results.
“Notable is the variable approach to IT MTX in patients requiring therapeutic dose anticoagulation, reflecting clinical decisions of different physicians on a case-by-case basis. Whilst a full discussion of this topic and management of asparaginase-associated [venous thromboembolism] is beyond the scope of this audit, we generally recognize today that many affected patients can be managed to continue therapy as per protocol,” the investigators wrote.
Integrating relevant prescriptions into a single information system, monitoring clinics for treatment backlogs, and improving clinical resources, such as staffing, could help to improve the efficacy of IT MTX therapy, they suggested.
The study was supported by the National Health Service Foundation Trust. Dr. Sommerfeld reported having no relevant financial disclosures.
SOURCE: Sommerfeld SA et al. EHA Congress, Abstract PS930.
REPORTING FROM THE EHA CONGRESS
Key clinical point:
Major finding: The total number of intrathecal methotrexate prescriptions delivered as a proportion of the number prescribed ranged from a low of 61.8% in 2009 to a high of 84.2% in 2007.
Study details: Audit of standard of care delivery of intrathecal methotrexate in the United Kingdom.
Disclosures: The study was supported by the National Health Service Foundation Trust. Dr. Sommerfeld reported having no relevant financial disclosures.
Source: Sommerfeld SA et al. EHA Congress, Abstract PS930.
Psoriasis, Etanercept, and Myelodysplasia: Looking for Connections
Physicians from Menoufia University and Cairo University in Egypt, and Al Hada Armed Forces Hospital in Saudi Arabia report on a patient who developed myelodysplasia with excess blasts 1 year after he started on the tumor necrosis factor-alpha blocker etanercept for psoriasis. The patient, a 76-year-old man, arrived at the emergency department (ED) with ecchymosis and recurrent epistaxis. He had a critically low platelet count, anemia, and normal leukocyte count. The reticulocyte index, serum ferritin, and folate levels indicated ineffective erythropoiesis. Bone marrow aspirate and biopsy confirmed a diagnosis of myelodysplastic syndrome.
The physicians stopped the etanercept and administered 2 cycles of azacitidine and folic acid supplementation, but the response was minima,l and the patient platelet count worsened. While waiting for the third cycle, the patient was readmitted to the ED with lower gastrointestinal bleeding, epistaxis, and shock. He died of cardiopulmonary arrest.
The physicians note that immune dysregulation and altered T-cell hemostasis are essential to the development of myelodysplastic syndrome. They also note that nonspecific activation and proliferation of T lymphocytes has been documented as promoting epidermal growth in genetically susceptible psoriasis patients.
Myelodysplastic syndrome has been associated with psoriasis in about 7% of cases, and researchers have found a higher incidence of leukemia and laryngeal cancer in families of psoriasis patients. There also have been reports of leukemia in psoriasis patients on systemic immunosuppressives. Etanercept has various hematologic adverse effects, including pancytopenia and aplastic anemia.
However, only 4 cases (including this one) have been reported of myelodysplastic syndrome in psoriasis patients. Taken together, the cases add to the growing evidence that suggests a link between myelodysplastic syndrome and etanercept treatment for psoriasis. Those patients, the physicians caution, should be considered at dual risk from treatment and disease. The physicians also recommend regular routine blood counts and discontinuing etanercept at onset of any cytopenias.
Source:
Dawoud NM, Ayoub OH, Essa ES, Dawoud DM. Indian J Dermatol Venereol Leprol. 2018;84(4):463-465.
doi: 10.4103/ijdvl.IJDVL_463_17
Physicians from Menoufia University and Cairo University in Egypt, and Al Hada Armed Forces Hospital in Saudi Arabia report on a patient who developed myelodysplasia with excess blasts 1 year after he started on the tumor necrosis factor-alpha blocker etanercept for psoriasis. The patient, a 76-year-old man, arrived at the emergency department (ED) with ecchymosis and recurrent epistaxis. He had a critically low platelet count, anemia, and normal leukocyte count. The reticulocyte index, serum ferritin, and folate levels indicated ineffective erythropoiesis. Bone marrow aspirate and biopsy confirmed a diagnosis of myelodysplastic syndrome.
The physicians stopped the etanercept and administered 2 cycles of azacitidine and folic acid supplementation, but the response was minima,l and the patient platelet count worsened. While waiting for the third cycle, the patient was readmitted to the ED with lower gastrointestinal bleeding, epistaxis, and shock. He died of cardiopulmonary arrest.
The physicians note that immune dysregulation and altered T-cell hemostasis are essential to the development of myelodysplastic syndrome. They also note that nonspecific activation and proliferation of T lymphocytes has been documented as promoting epidermal growth in genetically susceptible psoriasis patients.
Myelodysplastic syndrome has been associated with psoriasis in about 7% of cases, and researchers have found a higher incidence of leukemia and laryngeal cancer in families of psoriasis patients. There also have been reports of leukemia in psoriasis patients on systemic immunosuppressives. Etanercept has various hematologic adverse effects, including pancytopenia and aplastic anemia.
However, only 4 cases (including this one) have been reported of myelodysplastic syndrome in psoriasis patients. Taken together, the cases add to the growing evidence that suggests a link between myelodysplastic syndrome and etanercept treatment for psoriasis. Those patients, the physicians caution, should be considered at dual risk from treatment and disease. The physicians also recommend regular routine blood counts and discontinuing etanercept at onset of any cytopenias.
Source:
Dawoud NM, Ayoub OH, Essa ES, Dawoud DM. Indian J Dermatol Venereol Leprol. 2018;84(4):463-465.
doi: 10.4103/ijdvl.IJDVL_463_17
Physicians from Menoufia University and Cairo University in Egypt, and Al Hada Armed Forces Hospital in Saudi Arabia report on a patient who developed myelodysplasia with excess blasts 1 year after he started on the tumor necrosis factor-alpha blocker etanercept for psoriasis. The patient, a 76-year-old man, arrived at the emergency department (ED) with ecchymosis and recurrent epistaxis. He had a critically low platelet count, anemia, and normal leukocyte count. The reticulocyte index, serum ferritin, and folate levels indicated ineffective erythropoiesis. Bone marrow aspirate and biopsy confirmed a diagnosis of myelodysplastic syndrome.
The physicians stopped the etanercept and administered 2 cycles of azacitidine and folic acid supplementation, but the response was minima,l and the patient platelet count worsened. While waiting for the third cycle, the patient was readmitted to the ED with lower gastrointestinal bleeding, epistaxis, and shock. He died of cardiopulmonary arrest.
The physicians note that immune dysregulation and altered T-cell hemostasis are essential to the development of myelodysplastic syndrome. They also note that nonspecific activation and proliferation of T lymphocytes has been documented as promoting epidermal growth in genetically susceptible psoriasis patients.
Myelodysplastic syndrome has been associated with psoriasis in about 7% of cases, and researchers have found a higher incidence of leukemia and laryngeal cancer in families of psoriasis patients. There also have been reports of leukemia in psoriasis patients on systemic immunosuppressives. Etanercept has various hematologic adverse effects, including pancytopenia and aplastic anemia.
However, only 4 cases (including this one) have been reported of myelodysplastic syndrome in psoriasis patients. Taken together, the cases add to the growing evidence that suggests a link between myelodysplastic syndrome and etanercept treatment for psoriasis. Those patients, the physicians caution, should be considered at dual risk from treatment and disease. The physicians also recommend regular routine blood counts and discontinuing etanercept at onset of any cytopenias.
Source:
Dawoud NM, Ayoub OH, Essa ES, Dawoud DM. Indian J Dermatol Venereol Leprol. 2018;84(4):463-465.
doi: 10.4103/ijdvl.IJDVL_463_17
Myeloma frailty index predicts survival based on biological age
A new index of frailty predicts survival in older patients with multiple myeloma based on accumulation of aging-associated deficits, rather than chronological age alone, investigators report. A 16% increased risk of death was seen for each 10% increase in the deficit-accumulation frailty index (DAFI), which includes 25 variables related health, function, and activities of daily living.
There was only a weak correlation between chronological age and increase in deficits tracked by the index, in contrast to a cohort without cancer, in which age and frailty were strongly correlated, the investigators reported in JCO Clinical Cancer Informatics.
“Our results demonstrate that, for patients with multiple myeloma, chronological age alone is not a good measure for assessing overall health,” study author Tanya M. Wildes, MD, of Washington University, St. Louis, said in a news release from the American Society of Clinical Oncology.
Existing tools to assess frailty include an index proposed by the International Myeloma Working Group that looks at age plus other indexes related to comorbidities and activities of daily living, and the revised Myeloma Comorbidity Index that incorporates age with other prognostic factors.
“Although both tools provide prognostic information, chronological age automatically increases frailty without taking biologic or functional age into account,” Dr. Wildes and her coauthors wrote in their report.
By contrast, the DAFI is based on the concept of biologic age, in which the health status of an individual is measured based on the proportion of aging-associated deficits they have accumulated, according to the authors.
To create the DAFI, Dr. Wildes and her colleagues analyzed nearly 2.7 million records of noncancer patients aged 66 years or older in the SEER Medicare Health Outcomes Survey (MHOS) database. They identified 25 variables in the database representing chronic health conditions, activities of daily living, functioning, mental health, and general health.
An individual’s DAFI score was calculated as the sum of scores for each of the 25 variables as 0 for absent, 0.5 for limited, and 1 for present. Predicted DAFI means were calculated for each year of age and used to create age-specific cut points to determine whether an individual would be considered frail or not versus others of the same age.
“In other words, the same frailty score may qualify an 80-year-old individual as fit and a 70-year-old as frail, depending on the cutoff for their respective age group,” investigators explained in their report.
They applied the index to 305 patients with newly diagnosed myeloma in the SEER-MHOS database who were 66 years of age or older (median age, 76 years) and had completed the survey within 1 year of diagnosis.
The DAFI classified 52% of the myeloma patients as frail, and for that group, median overall survival was 26.8 months, versus 43.7 months for nonfrail patients (P = .015), according to the reported data. For each 10% increase in score, the risk of death increased by 16% (P less than .001).
Notably, advancing age was very weakly correlated with increased age-related deficits in the myeloma cohort (r2 = 0.15; P = .010), according to investigators, but very strongly correlated with deficits in the cohort of noncancer patients (r2 = 0.98; P less than .001).
“This suggests that, in patients with multiple myeloma, the prevalence of impairments across domains of function, chronic comorbidities, general health, and mental health are more related to the overall burden of myeloma rather than chronological age alone,” the investigators wrote.
The information used to calculate a DAFI score is easily obtainable during a clinic visit, according to the authors, who provided an overview of all 25 variables in the journal article.
Further development of a computerized program would further enhance usability in the clinic, allowing for real-time calculation during a patient visit, they said.
Survivorship expert Merry Jennifer Markham, MD, said in the ASCO news release that this frailty index is notable because it accounts for more than just chronological age. “Knowing this information can help oncologists have more informed discussions with patients about their prognosis, which in turn can empower patients and families as they weigh treatment options,” she said.
The research was supported by National Cancer Institute. Dr. Wildes reported honoraria from Carevive Systems and research funding from Janssen Oncology. Another coauthor reported honoraria from Celgene and Janssen, and a consulting or advisory role with Amgen and Takeda.
SOURCE: Mian HS et al. JCO Clin Cancer Inform. 2018 Jul 25. 2018 Jul 25. doi: 10.1200/CCI.18.00043.
A new index of frailty predicts survival in older patients with multiple myeloma based on accumulation of aging-associated deficits, rather than chronological age alone, investigators report. A 16% increased risk of death was seen for each 10% increase in the deficit-accumulation frailty index (DAFI), which includes 25 variables related health, function, and activities of daily living.
There was only a weak correlation between chronological age and increase in deficits tracked by the index, in contrast to a cohort without cancer, in which age and frailty were strongly correlated, the investigators reported in JCO Clinical Cancer Informatics.
“Our results demonstrate that, for patients with multiple myeloma, chronological age alone is not a good measure for assessing overall health,” study author Tanya M. Wildes, MD, of Washington University, St. Louis, said in a news release from the American Society of Clinical Oncology.
Existing tools to assess frailty include an index proposed by the International Myeloma Working Group that looks at age plus other indexes related to comorbidities and activities of daily living, and the revised Myeloma Comorbidity Index that incorporates age with other prognostic factors.
“Although both tools provide prognostic information, chronological age automatically increases frailty without taking biologic or functional age into account,” Dr. Wildes and her coauthors wrote in their report.
By contrast, the DAFI is based on the concept of biologic age, in which the health status of an individual is measured based on the proportion of aging-associated deficits they have accumulated, according to the authors.
To create the DAFI, Dr. Wildes and her colleagues analyzed nearly 2.7 million records of noncancer patients aged 66 years or older in the SEER Medicare Health Outcomes Survey (MHOS) database. They identified 25 variables in the database representing chronic health conditions, activities of daily living, functioning, mental health, and general health.
An individual’s DAFI score was calculated as the sum of scores for each of the 25 variables as 0 for absent, 0.5 for limited, and 1 for present. Predicted DAFI means were calculated for each year of age and used to create age-specific cut points to determine whether an individual would be considered frail or not versus others of the same age.
“In other words, the same frailty score may qualify an 80-year-old individual as fit and a 70-year-old as frail, depending on the cutoff for their respective age group,” investigators explained in their report.
They applied the index to 305 patients with newly diagnosed myeloma in the SEER-MHOS database who were 66 years of age or older (median age, 76 years) and had completed the survey within 1 year of diagnosis.
The DAFI classified 52% of the myeloma patients as frail, and for that group, median overall survival was 26.8 months, versus 43.7 months for nonfrail patients (P = .015), according to the reported data. For each 10% increase in score, the risk of death increased by 16% (P less than .001).
Notably, advancing age was very weakly correlated with increased age-related deficits in the myeloma cohort (r2 = 0.15; P = .010), according to investigators, but very strongly correlated with deficits in the cohort of noncancer patients (r2 = 0.98; P less than .001).
“This suggests that, in patients with multiple myeloma, the prevalence of impairments across domains of function, chronic comorbidities, general health, and mental health are more related to the overall burden of myeloma rather than chronological age alone,” the investigators wrote.
The information used to calculate a DAFI score is easily obtainable during a clinic visit, according to the authors, who provided an overview of all 25 variables in the journal article.
Further development of a computerized program would further enhance usability in the clinic, allowing for real-time calculation during a patient visit, they said.
Survivorship expert Merry Jennifer Markham, MD, said in the ASCO news release that this frailty index is notable because it accounts for more than just chronological age. “Knowing this information can help oncologists have more informed discussions with patients about their prognosis, which in turn can empower patients and families as they weigh treatment options,” she said.
The research was supported by National Cancer Institute. Dr. Wildes reported honoraria from Carevive Systems and research funding from Janssen Oncology. Another coauthor reported honoraria from Celgene and Janssen, and a consulting or advisory role with Amgen and Takeda.
SOURCE: Mian HS et al. JCO Clin Cancer Inform. 2018 Jul 25. 2018 Jul 25. doi: 10.1200/CCI.18.00043.
A new index of frailty predicts survival in older patients with multiple myeloma based on accumulation of aging-associated deficits, rather than chronological age alone, investigators report. A 16% increased risk of death was seen for each 10% increase in the deficit-accumulation frailty index (DAFI), which includes 25 variables related health, function, and activities of daily living.
There was only a weak correlation between chronological age and increase in deficits tracked by the index, in contrast to a cohort without cancer, in which age and frailty were strongly correlated, the investigators reported in JCO Clinical Cancer Informatics.
“Our results demonstrate that, for patients with multiple myeloma, chronological age alone is not a good measure for assessing overall health,” study author Tanya M. Wildes, MD, of Washington University, St. Louis, said in a news release from the American Society of Clinical Oncology.
Existing tools to assess frailty include an index proposed by the International Myeloma Working Group that looks at age plus other indexes related to comorbidities and activities of daily living, and the revised Myeloma Comorbidity Index that incorporates age with other prognostic factors.
“Although both tools provide prognostic information, chronological age automatically increases frailty without taking biologic or functional age into account,” Dr. Wildes and her coauthors wrote in their report.
By contrast, the DAFI is based on the concept of biologic age, in which the health status of an individual is measured based on the proportion of aging-associated deficits they have accumulated, according to the authors.
To create the DAFI, Dr. Wildes and her colleagues analyzed nearly 2.7 million records of noncancer patients aged 66 years or older in the SEER Medicare Health Outcomes Survey (MHOS) database. They identified 25 variables in the database representing chronic health conditions, activities of daily living, functioning, mental health, and general health.
An individual’s DAFI score was calculated as the sum of scores for each of the 25 variables as 0 for absent, 0.5 for limited, and 1 for present. Predicted DAFI means were calculated for each year of age and used to create age-specific cut points to determine whether an individual would be considered frail or not versus others of the same age.
“In other words, the same frailty score may qualify an 80-year-old individual as fit and a 70-year-old as frail, depending on the cutoff for their respective age group,” investigators explained in their report.
They applied the index to 305 patients with newly diagnosed myeloma in the SEER-MHOS database who were 66 years of age or older (median age, 76 years) and had completed the survey within 1 year of diagnosis.
The DAFI classified 52% of the myeloma patients as frail, and for that group, median overall survival was 26.8 months, versus 43.7 months for nonfrail patients (P = .015), according to the reported data. For each 10% increase in score, the risk of death increased by 16% (P less than .001).
Notably, advancing age was very weakly correlated with increased age-related deficits in the myeloma cohort (r2 = 0.15; P = .010), according to investigators, but very strongly correlated with deficits in the cohort of noncancer patients (r2 = 0.98; P less than .001).
“This suggests that, in patients with multiple myeloma, the prevalence of impairments across domains of function, chronic comorbidities, general health, and mental health are more related to the overall burden of myeloma rather than chronological age alone,” the investigators wrote.
The information used to calculate a DAFI score is easily obtainable during a clinic visit, according to the authors, who provided an overview of all 25 variables in the journal article.
Further development of a computerized program would further enhance usability in the clinic, allowing for real-time calculation during a patient visit, they said.
Survivorship expert Merry Jennifer Markham, MD, said in the ASCO news release that this frailty index is notable because it accounts for more than just chronological age. “Knowing this information can help oncologists have more informed discussions with patients about their prognosis, which in turn can empower patients and families as they weigh treatment options,” she said.
The research was supported by National Cancer Institute. Dr. Wildes reported honoraria from Carevive Systems and research funding from Janssen Oncology. Another coauthor reported honoraria from Celgene and Janssen, and a consulting or advisory role with Amgen and Takeda.
SOURCE: Mian HS et al. JCO Clin Cancer Inform. 2018 Jul 25. 2018 Jul 25. doi: 10.1200/CCI.18.00043.
FROM JCO CLINICAL CANCER INFORMATICS
Key clinical point: A new index of frailty predicts survival in older patients with multiple myeloma based on accumulation of aging-associated deficits, rather than on chronological age alone.
Major finding: Median overall survival was 26.8 months for patients classified as frail, vs. 43.7 months for nonfrail patients (P = .015).
Study details: Retrospective analysis of 2.7 million records of noncancer patients to create an index subsequently validated in records for 305 patients with newly diagnosed multiple myeloma (aged 66 years and older).
Disclosures: The research was supported by National Cancer Institute. Authors reported disclosures related to Celgene, Janssen, Amgen, Takeda, and Carevive Systems.
Source: Mian HS et al. JCO Clin Cancer Inform. 2018 Jul 25. doi: 10.1200/CCI.18.00043.
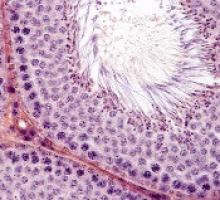
Treatments, disease affect spermatogonia in boys
Alkylating agents, hydroxyurea (HU), and certain non-malignant diseases can significantly deplete spermatogonial cell counts in young boys, according to research published in Human Reproduction.
Boys who received alkylating agents to treat cancer had significantly lower spermatogonial cell counts than control subjects or boys with malignant/nonmalignant diseases treated with non-alkylating agents.
Five of 6 SCD patients treated with HU had a totally depleted spermatogonial pool, and the remaining patient had a low spermatogonial cell count.
Five boys with non-malignant diseases who were not exposed to chemotherapy had significantly lower spermatogonial cell counts than controls.
“Our findings of a dramatic decrease in germ cell numbers in boys treated with alkylating agents and in sickle cell disease patients treated with hydroxyurea suggest that storing frozen testicular tissue from these boys should be performed before these treatments are initiated,” said study author Cecilia Petersen, MD, PhD, of Karolinska Institutet and University Hospital in Stockholm, Sweden.
“This needs to be communicated to physicians as well as patients and their parents or carers. However, until sperm that are able to fertilize eggs are produced from stored testicular tissue, we cannot confirm that germ cell quantity might determine the success of transplantation of the tissue in adulthood. Further research on this is needed to establish a realistic fertility preservation technique.”
Dr Petersen and her colleagues also noted that preserving testicular tissue may not be a viable option for boys who have low spermatogonial cell counts prior to treatment.
Patients and controls
For this study, the researchers analyzed testicular tissue from 32 boys facing treatments that carried a high risk of infertility—testicular irradiation, chemotherapy, or radiotherapy in advance of stem cell transplant.
Twenty boys had the tissue taken after initial chemotherapy, and 12 had it taken before starting any treatment.1
Eight patients had received chemotherapy with non-alkylating agents, 6 (all with malignancies) had received alkylating agents, and 6 (all with SCD) had received HU.
Diseases included acute lymphoblastic leukemia (n=6), SCD (n=6), acute myeloid leukemia (n=3), thalassemia major (n=3), neuroblastoma (n=2), juvenile myelomonocytic leukemia (n=2), myelodysplastic syndromes (n=2), primary immunodeficiency (n=2), Wilms tumor (n=1), adrenoleukodystrophy (n=1), hepatoblastoma (n=1), primitive neuroectodermal tumor (n=1), severe aplastic anemia (n=1), and Fanconi anemia (n=1).
The researchers compared samples from these 32 patients to 14 healthy testicular tissue samples stored in the biobank at the Karolinska University Hospital.
For both sample types, the team counted the number of spermatogonial cells found in a cross-section of seminiferous tubules.
“We could compare the number of spermatogonia with those found in the healthy boys as a way to estimate the effect of medical treatment or the disease itself on the future fertility of a patient,” explained study author Jan-Bernd Stukenborg, PhD, of Karolinska Institutet and University Hospital.
Impact of treatment
There was no significant difference in the mean quantity of spermatogonia per transverse tubular cross-section (S/T) between patients exposed to non-alkylating agents (1.7 ± 1.0, n=8) and biobank controls (4.1 ± 4.6, n=14).
However, samples from patients who received alkylating agents had a significantly lower mean S/T value (0.2 ± 0.3, n=6) than samples from patients treated with non-alkylating agents (P=0.003) and biobank controls (P<0.001).
“We found that the numbers of germ cells present in the cross-sections of the seminiferous tubules were significantly depleted and close to 0 in patients treated with alkylating agents,” Dr Stukenborg said.
Samples from the SCD patients also had a significantly lower mean S/T value (0.3 ± 0.6, n=6) than biobank controls (P=0.003).
Dr Stukenborg noted that the germ cell pool was totally depleted in 5 of the boys with SCD, and the pool was “very low” in the sixth SCD patient.
“This was not seen in patients who had not started treatment or were treated with non-alkylating agents or in the biobank tissues,” Dr Stukenborg said.2
He and his colleagues noted that it is possible for germ cells to recover to normal levels after treatment that is highly toxic to the testes, but high doses of alkylating agents and radiotherapy to the testicles are strongly associated with permanent or long-term infertility.
“The first group of boys who received bone marrow transplants are now reaching their thirties,” said study author Kirsi Jahnukainen, MD, PhD, of Helsinki University Central Hospital in Finland.
“Recent data suggest they may have a high chance of their sperm production recovering, even if they received high-dose alkylating therapies, so long as they had no testicular irradiation.”
Impact of disease
The researchers also found evidence to suggest that, for some boys, their disease may have affected spermatogonial cell counts before any treatment began.
Five patients with non-malignant disease who had not been exposed to chemotherapy (3 with thalassemia major, 1 with Fanconi anemia, and 1 with primary immunodeficiency) had a significantly lower mean S/T value (0.4 ± 0.5) than controls (P=0.006).
“Among patients who had not been treated previously with chemotherapy, there were several boys with a low number of germ cells for their age,” Dr Jahnukainen said.
“This suggests that some non-malignant diseases that require bone marrow transplants may affect the fertility of young boys even before exposure to therapy that is toxic for the testes.”
The researchers noted that a limitation of this study was that biobank samples had no detailed information regarding previous medical treatments and testicular volumes.
1. Testicular tissue is taken from patients under general anesthesia. The surgeon removes approximately 20% of the tissue from the testicular capsule in one of the testicles. For this study, a third of the tissue was taken to the Karolinska Institutet for analysis.
2. A recent meta-analysis showed that normal testicular tissue samples of newborns contain approximately 2.5 germ cells per tubular cross-section. This number decreases to approximately 1.2 within the first 3 years of age, followed by an increase up to 2.6 germ cells per tubular cross-section at 6 to 7 years, reaching a plateau until the age of 11. At the onset of puberty, an increase of up to 7 spermatogonia per tubular cross-section could be observed.
Alkylating agents, hydroxyurea (HU), and certain non-malignant diseases can significantly deplete spermatogonial cell counts in young boys, according to research published in Human Reproduction.
Boys who received alkylating agents to treat cancer had significantly lower spermatogonial cell counts than control subjects or boys with malignant/nonmalignant diseases treated with non-alkylating agents.
Five of 6 SCD patients treated with HU had a totally depleted spermatogonial pool, and the remaining patient had a low spermatogonial cell count.
Five boys with non-malignant diseases who were not exposed to chemotherapy had significantly lower spermatogonial cell counts than controls.
“Our findings of a dramatic decrease in germ cell numbers in boys treated with alkylating agents and in sickle cell disease patients treated with hydroxyurea suggest that storing frozen testicular tissue from these boys should be performed before these treatments are initiated,” said study author Cecilia Petersen, MD, PhD, of Karolinska Institutet and University Hospital in Stockholm, Sweden.
“This needs to be communicated to physicians as well as patients and their parents or carers. However, until sperm that are able to fertilize eggs are produced from stored testicular tissue, we cannot confirm that germ cell quantity might determine the success of transplantation of the tissue in adulthood. Further research on this is needed to establish a realistic fertility preservation technique.”
Dr Petersen and her colleagues also noted that preserving testicular tissue may not be a viable option for boys who have low spermatogonial cell counts prior to treatment.
Patients and controls
For this study, the researchers analyzed testicular tissue from 32 boys facing treatments that carried a high risk of infertility—testicular irradiation, chemotherapy, or radiotherapy in advance of stem cell transplant.
Twenty boys had the tissue taken after initial chemotherapy, and 12 had it taken before starting any treatment.1
Eight patients had received chemotherapy with non-alkylating agents, 6 (all with malignancies) had received alkylating agents, and 6 (all with SCD) had received HU.
Diseases included acute lymphoblastic leukemia (n=6), SCD (n=6), acute myeloid leukemia (n=3), thalassemia major (n=3), neuroblastoma (n=2), juvenile myelomonocytic leukemia (n=2), myelodysplastic syndromes (n=2), primary immunodeficiency (n=2), Wilms tumor (n=1), adrenoleukodystrophy (n=1), hepatoblastoma (n=1), primitive neuroectodermal tumor (n=1), severe aplastic anemia (n=1), and Fanconi anemia (n=1).
The researchers compared samples from these 32 patients to 14 healthy testicular tissue samples stored in the biobank at the Karolinska University Hospital.
For both sample types, the team counted the number of spermatogonial cells found in a cross-section of seminiferous tubules.
“We could compare the number of spermatogonia with those found in the healthy boys as a way to estimate the effect of medical treatment or the disease itself on the future fertility of a patient,” explained study author Jan-Bernd Stukenborg, PhD, of Karolinska Institutet and University Hospital.
Impact of treatment
There was no significant difference in the mean quantity of spermatogonia per transverse tubular cross-section (S/T) between patients exposed to non-alkylating agents (1.7 ± 1.0, n=8) and biobank controls (4.1 ± 4.6, n=14).
However, samples from patients who received alkylating agents had a significantly lower mean S/T value (0.2 ± 0.3, n=6) than samples from patients treated with non-alkylating agents (P=0.003) and biobank controls (P<0.001).
“We found that the numbers of germ cells present in the cross-sections of the seminiferous tubules were significantly depleted and close to 0 in patients treated with alkylating agents,” Dr Stukenborg said.
Samples from the SCD patients also had a significantly lower mean S/T value (0.3 ± 0.6, n=6) than biobank controls (P=0.003).
Dr Stukenborg noted that the germ cell pool was totally depleted in 5 of the boys with SCD, and the pool was “very low” in the sixth SCD patient.
“This was not seen in patients who had not started treatment or were treated with non-alkylating agents or in the biobank tissues,” Dr Stukenborg said.2
He and his colleagues noted that it is possible for germ cells to recover to normal levels after treatment that is highly toxic to the testes, but high doses of alkylating agents and radiotherapy to the testicles are strongly associated with permanent or long-term infertility.
“The first group of boys who received bone marrow transplants are now reaching their thirties,” said study author Kirsi Jahnukainen, MD, PhD, of Helsinki University Central Hospital in Finland.
“Recent data suggest they may have a high chance of their sperm production recovering, even if they received high-dose alkylating therapies, so long as they had no testicular irradiation.”
Impact of disease
The researchers also found evidence to suggest that, for some boys, their disease may have affected spermatogonial cell counts before any treatment began.
Five patients with non-malignant disease who had not been exposed to chemotherapy (3 with thalassemia major, 1 with Fanconi anemia, and 1 with primary immunodeficiency) had a significantly lower mean S/T value (0.4 ± 0.5) than controls (P=0.006).
“Among patients who had not been treated previously with chemotherapy, there were several boys with a low number of germ cells for their age,” Dr Jahnukainen said.
“This suggests that some non-malignant diseases that require bone marrow transplants may affect the fertility of young boys even before exposure to therapy that is toxic for the testes.”
The researchers noted that a limitation of this study was that biobank samples had no detailed information regarding previous medical treatments and testicular volumes.
1. Testicular tissue is taken from patients under general anesthesia. The surgeon removes approximately 20% of the tissue from the testicular capsule in one of the testicles. For this study, a third of the tissue was taken to the Karolinska Institutet for analysis.
2. A recent meta-analysis showed that normal testicular tissue samples of newborns contain approximately 2.5 germ cells per tubular cross-section. This number decreases to approximately 1.2 within the first 3 years of age, followed by an increase up to 2.6 germ cells per tubular cross-section at 6 to 7 years, reaching a plateau until the age of 11. At the onset of puberty, an increase of up to 7 spermatogonia per tubular cross-section could be observed.
Alkylating agents, hydroxyurea (HU), and certain non-malignant diseases can significantly deplete spermatogonial cell counts in young boys, according to research published in Human Reproduction.
Boys who received alkylating agents to treat cancer had significantly lower spermatogonial cell counts than control subjects or boys with malignant/nonmalignant diseases treated with non-alkylating agents.
Five of 6 SCD patients treated with HU had a totally depleted spermatogonial pool, and the remaining patient had a low spermatogonial cell count.
Five boys with non-malignant diseases who were not exposed to chemotherapy had significantly lower spermatogonial cell counts than controls.
“Our findings of a dramatic decrease in germ cell numbers in boys treated with alkylating agents and in sickle cell disease patients treated with hydroxyurea suggest that storing frozen testicular tissue from these boys should be performed before these treatments are initiated,” said study author Cecilia Petersen, MD, PhD, of Karolinska Institutet and University Hospital in Stockholm, Sweden.
“This needs to be communicated to physicians as well as patients and their parents or carers. However, until sperm that are able to fertilize eggs are produced from stored testicular tissue, we cannot confirm that germ cell quantity might determine the success of transplantation of the tissue in adulthood. Further research on this is needed to establish a realistic fertility preservation technique.”
Dr Petersen and her colleagues also noted that preserving testicular tissue may not be a viable option for boys who have low spermatogonial cell counts prior to treatment.
Patients and controls
For this study, the researchers analyzed testicular tissue from 32 boys facing treatments that carried a high risk of infertility—testicular irradiation, chemotherapy, or radiotherapy in advance of stem cell transplant.
Twenty boys had the tissue taken after initial chemotherapy, and 12 had it taken before starting any treatment.1
Eight patients had received chemotherapy with non-alkylating agents, 6 (all with malignancies) had received alkylating agents, and 6 (all with SCD) had received HU.
Diseases included acute lymphoblastic leukemia (n=6), SCD (n=6), acute myeloid leukemia (n=3), thalassemia major (n=3), neuroblastoma (n=2), juvenile myelomonocytic leukemia (n=2), myelodysplastic syndromes (n=2), primary immunodeficiency (n=2), Wilms tumor (n=1), adrenoleukodystrophy (n=1), hepatoblastoma (n=1), primitive neuroectodermal tumor (n=1), severe aplastic anemia (n=1), and Fanconi anemia (n=1).
The researchers compared samples from these 32 patients to 14 healthy testicular tissue samples stored in the biobank at the Karolinska University Hospital.
For both sample types, the team counted the number of spermatogonial cells found in a cross-section of seminiferous tubules.
“We could compare the number of spermatogonia with those found in the healthy boys as a way to estimate the effect of medical treatment or the disease itself on the future fertility of a patient,” explained study author Jan-Bernd Stukenborg, PhD, of Karolinska Institutet and University Hospital.
Impact of treatment
There was no significant difference in the mean quantity of spermatogonia per transverse tubular cross-section (S/T) between patients exposed to non-alkylating agents (1.7 ± 1.0, n=8) and biobank controls (4.1 ± 4.6, n=14).
However, samples from patients who received alkylating agents had a significantly lower mean S/T value (0.2 ± 0.3, n=6) than samples from patients treated with non-alkylating agents (P=0.003) and biobank controls (P<0.001).
“We found that the numbers of germ cells present in the cross-sections of the seminiferous tubules were significantly depleted and close to 0 in patients treated with alkylating agents,” Dr Stukenborg said.
Samples from the SCD patients also had a significantly lower mean S/T value (0.3 ± 0.6, n=6) than biobank controls (P=0.003).
Dr Stukenborg noted that the germ cell pool was totally depleted in 5 of the boys with SCD, and the pool was “very low” in the sixth SCD patient.
“This was not seen in patients who had not started treatment or were treated with non-alkylating agents or in the biobank tissues,” Dr Stukenborg said.2
He and his colleagues noted that it is possible for germ cells to recover to normal levels after treatment that is highly toxic to the testes, but high doses of alkylating agents and radiotherapy to the testicles are strongly associated with permanent or long-term infertility.
“The first group of boys who received bone marrow transplants are now reaching their thirties,” said study author Kirsi Jahnukainen, MD, PhD, of Helsinki University Central Hospital in Finland.
“Recent data suggest they may have a high chance of their sperm production recovering, even if they received high-dose alkylating therapies, so long as they had no testicular irradiation.”
Impact of disease
The researchers also found evidence to suggest that, for some boys, their disease may have affected spermatogonial cell counts before any treatment began.
Five patients with non-malignant disease who had not been exposed to chemotherapy (3 with thalassemia major, 1 with Fanconi anemia, and 1 with primary immunodeficiency) had a significantly lower mean S/T value (0.4 ± 0.5) than controls (P=0.006).
“Among patients who had not been treated previously with chemotherapy, there were several boys with a low number of germ cells for their age,” Dr Jahnukainen said.
“This suggests that some non-malignant diseases that require bone marrow transplants may affect the fertility of young boys even before exposure to therapy that is toxic for the testes.”
The researchers noted that a limitation of this study was that biobank samples had no detailed information regarding previous medical treatments and testicular volumes.
1. Testicular tissue is taken from patients under general anesthesia. The surgeon removes approximately 20% of the tissue from the testicular capsule in one of the testicles. For this study, a third of the tissue was taken to the Karolinska Institutet for analysis.
2. A recent meta-analysis showed that normal testicular tissue samples of newborns contain approximately 2.5 germ cells per tubular cross-section. This number decreases to approximately 1.2 within the first 3 years of age, followed by an increase up to 2.6 germ cells per tubular cross-section at 6 to 7 years, reaching a plateau until the age of 11. At the onset of puberty, an increase of up to 7 spermatogonia per tubular cross-section could be observed.
Team finds potential therapeutic targets for T-ALL
Researchers have found the NOTCH1 pathway “hijacks” heat shock transcription factor 1 (HSF1) signaling in T-cell acute lymphoblastic leukemia (T-ALL).
Therefore, blocking one or more genes in the HSF1 pathway could represent a new approach to treating T-ALL.
An experimental drug, PU-H71, is already in development against one of these targets, heat shock protein 90 (HSP90).
The researchers found that PU-H71 was active against T-ALL in vitro and in vivo.
The team recounted these findings in Nature Medicine.
“Our study shows how the NOTCH1 pathway hijacks the heat shock transcription factor 1 pathway to promote tumor growth,” said study author Iannis Aifantis, PhD, of NYU School of Medicine in New York, New York.
“The cancer cells are sending into overdrive a system that helps healthy cells respond to stress.”
Dr Aifantis and his colleagues found that HSF1 is involved in the pathogenesis of T-ALL. When they knocked down HSF1 in T-ALL cell lines, the researchers observed an increase in apoptosis, defective proteostasis, and a decrease in the growth of leukemic cells.
Similarly, HSF1 was deemed necessary for disease progression in mouse models of T-ALL. When the researchers deleted HSF1, mice experienced “striking” reductions in leukemic burden and “dramatic” improvements in survival. However, HSF1 deletion did not affect normal hematopoiesis.
Dr Aifantis and his colleagues also showed that NOTCH1 regulates the epichaperome in T-ALL. The team said previous studies have shown that, in the presence of oncogenic stress, heat shock proteins participate in a network nucleated by HSP90 and HSP70 chaperones—the epichaperome.
The researchers found that an intact epichaperome was critical for T-ALL by showing that pharmacologic inhibition of HSP90 and HSP70 significantly hindered the growth of human T-ALL in vitro. The team also found the HSP90 inhibitor PU-H71 reduced leukemic burden and extended survival in a NOTCH1-inducible T-ALL mouse model.
Then, the researchers found NOTCH1 levels could predict response to HSP90 inhibition in vitro. T-ALL patient samples expressing high levels of nuclear NOTCH1 and high levels of epichaperome were significantly more sensitive to treatment with PU-H71.
PU-H71 is already in early clinical trials of patients with breast cancer. If further testing proves successful, PU-H71 could be quickly adapted for trials in T-ALL patients, according to Dr Aifantis.
In the meantime, he and his colleagues plan to evaluate the effects of another 8 proteins produced by genes active in the HSF1 pathway to see if any show promising anticancer activity in T-ALL.
Researchers have found the NOTCH1 pathway “hijacks” heat shock transcription factor 1 (HSF1) signaling in T-cell acute lymphoblastic leukemia (T-ALL).
Therefore, blocking one or more genes in the HSF1 pathway could represent a new approach to treating T-ALL.
An experimental drug, PU-H71, is already in development against one of these targets, heat shock protein 90 (HSP90).
The researchers found that PU-H71 was active against T-ALL in vitro and in vivo.
The team recounted these findings in Nature Medicine.
“Our study shows how the NOTCH1 pathway hijacks the heat shock transcription factor 1 pathway to promote tumor growth,” said study author Iannis Aifantis, PhD, of NYU School of Medicine in New York, New York.
“The cancer cells are sending into overdrive a system that helps healthy cells respond to stress.”
Dr Aifantis and his colleagues found that HSF1 is involved in the pathogenesis of T-ALL. When they knocked down HSF1 in T-ALL cell lines, the researchers observed an increase in apoptosis, defective proteostasis, and a decrease in the growth of leukemic cells.
Similarly, HSF1 was deemed necessary for disease progression in mouse models of T-ALL. When the researchers deleted HSF1, mice experienced “striking” reductions in leukemic burden and “dramatic” improvements in survival. However, HSF1 deletion did not affect normal hematopoiesis.
Dr Aifantis and his colleagues also showed that NOTCH1 regulates the epichaperome in T-ALL. The team said previous studies have shown that, in the presence of oncogenic stress, heat shock proteins participate in a network nucleated by HSP90 and HSP70 chaperones—the epichaperome.
The researchers found that an intact epichaperome was critical for T-ALL by showing that pharmacologic inhibition of HSP90 and HSP70 significantly hindered the growth of human T-ALL in vitro. The team also found the HSP90 inhibitor PU-H71 reduced leukemic burden and extended survival in a NOTCH1-inducible T-ALL mouse model.
Then, the researchers found NOTCH1 levels could predict response to HSP90 inhibition in vitro. T-ALL patient samples expressing high levels of nuclear NOTCH1 and high levels of epichaperome were significantly more sensitive to treatment with PU-H71.
PU-H71 is already in early clinical trials of patients with breast cancer. If further testing proves successful, PU-H71 could be quickly adapted for trials in T-ALL patients, according to Dr Aifantis.
In the meantime, he and his colleagues plan to evaluate the effects of another 8 proteins produced by genes active in the HSF1 pathway to see if any show promising anticancer activity in T-ALL.
Researchers have found the NOTCH1 pathway “hijacks” heat shock transcription factor 1 (HSF1) signaling in T-cell acute lymphoblastic leukemia (T-ALL).
Therefore, blocking one or more genes in the HSF1 pathway could represent a new approach to treating T-ALL.
An experimental drug, PU-H71, is already in development against one of these targets, heat shock protein 90 (HSP90).
The researchers found that PU-H71 was active against T-ALL in vitro and in vivo.
The team recounted these findings in Nature Medicine.
“Our study shows how the NOTCH1 pathway hijacks the heat shock transcription factor 1 pathway to promote tumor growth,” said study author Iannis Aifantis, PhD, of NYU School of Medicine in New York, New York.
“The cancer cells are sending into overdrive a system that helps healthy cells respond to stress.”
Dr Aifantis and his colleagues found that HSF1 is involved in the pathogenesis of T-ALL. When they knocked down HSF1 in T-ALL cell lines, the researchers observed an increase in apoptosis, defective proteostasis, and a decrease in the growth of leukemic cells.
Similarly, HSF1 was deemed necessary for disease progression in mouse models of T-ALL. When the researchers deleted HSF1, mice experienced “striking” reductions in leukemic burden and “dramatic” improvements in survival. However, HSF1 deletion did not affect normal hematopoiesis.
Dr Aifantis and his colleagues also showed that NOTCH1 regulates the epichaperome in T-ALL. The team said previous studies have shown that, in the presence of oncogenic stress, heat shock proteins participate in a network nucleated by HSP90 and HSP70 chaperones—the epichaperome.
The researchers found that an intact epichaperome was critical for T-ALL by showing that pharmacologic inhibition of HSP90 and HSP70 significantly hindered the growth of human T-ALL in vitro. The team also found the HSP90 inhibitor PU-H71 reduced leukemic burden and extended survival in a NOTCH1-inducible T-ALL mouse model.
Then, the researchers found NOTCH1 levels could predict response to HSP90 inhibition in vitro. T-ALL patient samples expressing high levels of nuclear NOTCH1 and high levels of epichaperome were significantly more sensitive to treatment with PU-H71.
PU-H71 is already in early clinical trials of patients with breast cancer. If further testing proves successful, PU-H71 could be quickly adapted for trials in T-ALL patients, according to Dr Aifantis.
In the meantime, he and his colleagues plan to evaluate the effects of another 8 proteins produced by genes active in the HSF1 pathway to see if any show promising anticancer activity in T-ALL.
FDA approves drug for IDH1-mutated AML
The US Food and Drug Administration (FDA) has granted full approval for the isocitrate dehydrogenase-1 (IDH1) inhibitor ivosidenib (Tibsovo®).
The drug is approved to treat adults with relapsed or refractory acute myeloid leukemia (AML) who have an IDH1 mutation, as detected by an FDA-approved test.
Ivosidenib was approved concurrently with the RealTime IDH1 Assay, a companion diagnostic that can detect IDH1 mutation.
The FDA granted approval of ivosidenib to Agios Pharmaceuticals, Inc., and approval of the RealTime IDH1 Assay to Abbott Laboratories.
The FDA’s approval of ivosidenib was based on data from a single-arm, phase 1 study. Agios received fast track, priority review, and orphan drug designations for ivosidenib.
Safety risks
Ivosidenib must be dispensed with a patient medication guide that describes important information about the drug’s uses and risks.
The prescribing information for ivosidenib includes a Boxed Warning noting that patients treated with ivosidenib have experienced symptoms of differentiation syndrome, which can be fatal if not treated.
Signs and symptoms of differentiation syndrome may include fever, dyspnea, acute respiratory distress, radiographic pulmonary infiltrates, pleural or pericardial effusions, rapid weight gain, peripheral edema, or hepatic, renal, or multi-organ dysfunction.
At first suspicion of symptoms, doctors should treat patients with corticosteroids and monitor them closely until symptoms subside.
Ivosidenib also poses a risk of life-threatening QT prolongation and Guillain-Barré syndrome, so patients should be monitored for these adverse events (AEs) as well.
Phase 1 trial
Results from the phase 1 trial of ivosidenib were presented at the 2018 ASCO Annual Meeting and published simultaneously in NEJM. The following data were pulled from the drug’s prescribing information.
Efficacy results were available for 174 adults with relapsed/refractory AML and an IDH1 mutation identified or confirmed by the Abbott RealTime™ IDH1 assay. The patients received ivosidenib at a starting dose of 500 mg daily until disease progression, unacceptable toxicity, or hematopoietic stem cell transplant.
The patients had a median age of 67 (range, 18 to 87) and had received a median of 2 prior therapies (range, 1 to 6). Sixty-three percent were refractory to previous therapy, and 33% had secondary AML.
The study’s primary endpoint was the combined rate of complete remission (CR) rate and CR with partial hematologic improvement (CRh).
The CR+CRh rate was 32.8% (57/174), the CR rate was 24.7% (43/174), and the CRh rate was 8% (14/174).
The median duration of CR+CRh was 8.2 months (95% CI 5.6, 12). The median time to best response of CR or CRh was 2.0 months (range, 0.9 to 5.6 months).
Twelve percent of patients (21/174) went on to transplant.
The researchers evaluated the safety of ivosidenib in 179 patients treated with a dose of 500 mg daily. The median duration of exposure to ivosidenib was 3.9 months (range, 0.1 to 39.5 months).
Nineteen percent of patients (34/179) experienced differentiation syndrome. Seventy-nine percent of these patients (27/34) recovered after treatment or ivosidenib dose interruption.
The most frequent serious AEs (>5%) were differentiation syndrome (10%), leukocytosis (10%), and electrocardiogram QT prolongation (7%). There was one case of progressive multifocal leukoencephalopathy.
The most common AEs leading to dose interruption were QT prolongation (7%), differentiation syndrome (3%), leukocytosis (3%), and dyspnea (3%). AEs leading to a dose reduction included QT prolongation (1%), diarrhea (1%), nausea (1%), decreased hemoglobin (1%), and increased transaminases (1%).
AEs leading to permanent discontinuation of ivosidenib included Guillain-Barré syndrome (1%), rash (1%), stomatitis (1%), and creatinine increase (1%).
For additional data and more details on ivosidenib, see the full prescribing information or visit Tibsovo.com.
The US Food and Drug Administration (FDA) has granted full approval for the isocitrate dehydrogenase-1 (IDH1) inhibitor ivosidenib (Tibsovo®).
The drug is approved to treat adults with relapsed or refractory acute myeloid leukemia (AML) who have an IDH1 mutation, as detected by an FDA-approved test.
Ivosidenib was approved concurrently with the RealTime IDH1 Assay, a companion diagnostic that can detect IDH1 mutation.
The FDA granted approval of ivosidenib to Agios Pharmaceuticals, Inc., and approval of the RealTime IDH1 Assay to Abbott Laboratories.
The FDA’s approval of ivosidenib was based on data from a single-arm, phase 1 study. Agios received fast track, priority review, and orphan drug designations for ivosidenib.
Safety risks
Ivosidenib must be dispensed with a patient medication guide that describes important information about the drug’s uses and risks.
The prescribing information for ivosidenib includes a Boxed Warning noting that patients treated with ivosidenib have experienced symptoms of differentiation syndrome, which can be fatal if not treated.
Signs and symptoms of differentiation syndrome may include fever, dyspnea, acute respiratory distress, radiographic pulmonary infiltrates, pleural or pericardial effusions, rapid weight gain, peripheral edema, or hepatic, renal, or multi-organ dysfunction.
At first suspicion of symptoms, doctors should treat patients with corticosteroids and monitor them closely until symptoms subside.
Ivosidenib also poses a risk of life-threatening QT prolongation and Guillain-Barré syndrome, so patients should be monitored for these adverse events (AEs) as well.
Phase 1 trial
Results from the phase 1 trial of ivosidenib were presented at the 2018 ASCO Annual Meeting and published simultaneously in NEJM. The following data were pulled from the drug’s prescribing information.
Efficacy results were available for 174 adults with relapsed/refractory AML and an IDH1 mutation identified or confirmed by the Abbott RealTime™ IDH1 assay. The patients received ivosidenib at a starting dose of 500 mg daily until disease progression, unacceptable toxicity, or hematopoietic stem cell transplant.
The patients had a median age of 67 (range, 18 to 87) and had received a median of 2 prior therapies (range, 1 to 6). Sixty-three percent were refractory to previous therapy, and 33% had secondary AML.
The study’s primary endpoint was the combined rate of complete remission (CR) rate and CR with partial hematologic improvement (CRh).
The CR+CRh rate was 32.8% (57/174), the CR rate was 24.7% (43/174), and the CRh rate was 8% (14/174).
The median duration of CR+CRh was 8.2 months (95% CI 5.6, 12). The median time to best response of CR or CRh was 2.0 months (range, 0.9 to 5.6 months).
Twelve percent of patients (21/174) went on to transplant.
The researchers evaluated the safety of ivosidenib in 179 patients treated with a dose of 500 mg daily. The median duration of exposure to ivosidenib was 3.9 months (range, 0.1 to 39.5 months).
Nineteen percent of patients (34/179) experienced differentiation syndrome. Seventy-nine percent of these patients (27/34) recovered after treatment or ivosidenib dose interruption.
The most frequent serious AEs (>5%) were differentiation syndrome (10%), leukocytosis (10%), and electrocardiogram QT prolongation (7%). There was one case of progressive multifocal leukoencephalopathy.
The most common AEs leading to dose interruption were QT prolongation (7%), differentiation syndrome (3%), leukocytosis (3%), and dyspnea (3%). AEs leading to a dose reduction included QT prolongation (1%), diarrhea (1%), nausea (1%), decreased hemoglobin (1%), and increased transaminases (1%).
AEs leading to permanent discontinuation of ivosidenib included Guillain-Barré syndrome (1%), rash (1%), stomatitis (1%), and creatinine increase (1%).
For additional data and more details on ivosidenib, see the full prescribing information or visit Tibsovo.com.
The US Food and Drug Administration (FDA) has granted full approval for the isocitrate dehydrogenase-1 (IDH1) inhibitor ivosidenib (Tibsovo®).
The drug is approved to treat adults with relapsed or refractory acute myeloid leukemia (AML) who have an IDH1 mutation, as detected by an FDA-approved test.
Ivosidenib was approved concurrently with the RealTime IDH1 Assay, a companion diagnostic that can detect IDH1 mutation.
The FDA granted approval of ivosidenib to Agios Pharmaceuticals, Inc., and approval of the RealTime IDH1 Assay to Abbott Laboratories.
The FDA’s approval of ivosidenib was based on data from a single-arm, phase 1 study. Agios received fast track, priority review, and orphan drug designations for ivosidenib.
Safety risks
Ivosidenib must be dispensed with a patient medication guide that describes important information about the drug’s uses and risks.
The prescribing information for ivosidenib includes a Boxed Warning noting that patients treated with ivosidenib have experienced symptoms of differentiation syndrome, which can be fatal if not treated.
Signs and symptoms of differentiation syndrome may include fever, dyspnea, acute respiratory distress, radiographic pulmonary infiltrates, pleural or pericardial effusions, rapid weight gain, peripheral edema, or hepatic, renal, or multi-organ dysfunction.
At first suspicion of symptoms, doctors should treat patients with corticosteroids and monitor them closely until symptoms subside.
Ivosidenib also poses a risk of life-threatening QT prolongation and Guillain-Barré syndrome, so patients should be monitored for these adverse events (AEs) as well.
Phase 1 trial
Results from the phase 1 trial of ivosidenib were presented at the 2018 ASCO Annual Meeting and published simultaneously in NEJM. The following data were pulled from the drug’s prescribing information.
Efficacy results were available for 174 adults with relapsed/refractory AML and an IDH1 mutation identified or confirmed by the Abbott RealTime™ IDH1 assay. The patients received ivosidenib at a starting dose of 500 mg daily until disease progression, unacceptable toxicity, or hematopoietic stem cell transplant.
The patients had a median age of 67 (range, 18 to 87) and had received a median of 2 prior therapies (range, 1 to 6). Sixty-three percent were refractory to previous therapy, and 33% had secondary AML.
The study’s primary endpoint was the combined rate of complete remission (CR) rate and CR with partial hematologic improvement (CRh).
The CR+CRh rate was 32.8% (57/174), the CR rate was 24.7% (43/174), and the CRh rate was 8% (14/174).
The median duration of CR+CRh was 8.2 months (95% CI 5.6, 12). The median time to best response of CR or CRh was 2.0 months (range, 0.9 to 5.6 months).
Twelve percent of patients (21/174) went on to transplant.
The researchers evaluated the safety of ivosidenib in 179 patients treated with a dose of 500 mg daily. The median duration of exposure to ivosidenib was 3.9 months (range, 0.1 to 39.5 months).
Nineteen percent of patients (34/179) experienced differentiation syndrome. Seventy-nine percent of these patients (27/34) recovered after treatment or ivosidenib dose interruption.
The most frequent serious AEs (>5%) were differentiation syndrome (10%), leukocytosis (10%), and electrocardiogram QT prolongation (7%). There was one case of progressive multifocal leukoencephalopathy.
The most common AEs leading to dose interruption were QT prolongation (7%), differentiation syndrome (3%), leukocytosis (3%), and dyspnea (3%). AEs leading to a dose reduction included QT prolongation (1%), diarrhea (1%), nausea (1%), decreased hemoglobin (1%), and increased transaminases (1%).
AEs leading to permanent discontinuation of ivosidenib included Guillain-Barré syndrome (1%), rash (1%), stomatitis (1%), and creatinine increase (1%).
For additional data and more details on ivosidenib, see the full prescribing information or visit Tibsovo.com.
FDA approves biosimilar filgrastim
The US Food and Drug Administration (FDA) has approved the leukocyte growth factor Nivestym™ (filgrastim-aafi), a biosimilar to Neupogen (filgrastim).
Nivestym is approved to treat patients with nonmyeloid malignancies who are receiving myelosuppressive chemotherapy or undergoing bone marrow transplant, acute myeloid leukemia patients receiving induction or consolidation chemotherapy, patients undergoing autologous peripheral blood progenitor cell collection, and patients with severe chronic neutropenia.
The FDA’s approval of Nivestym was based on a review of evidence suggesting the drug is highly similar to Neupogen, according to Pfizer, the company developing Nivestym.
The full approved indication for Nivestym is as follows:
- To decrease the incidence of infection, as manifested by febrile neutropenia, in patients with nonmyeloid malignancies receiving myelosuppressive anticancer drugs associated with a significant incidence of severe neutropenia with fever
- To reduce the time to neutrophil recovery and the duration of fever following induction or consolidation chemotherapy in patients with acute myeloid leukemia
- To reduce the duration of neutropenia and neutropenia-related clinical sequelae (eg, febrile neutropenia) in patients with nonmyeloid malignancies undergoing myeloablative chemotherapy followed by bone marrow transplant
- For the mobilization of autologous hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis
- For chronic administration to reduce the incidence and duration of sequelae of severe neutropenia (eg, fever, infections, oropharyngeal ulcers) in symptomatic patients with congenital neutropenia, cyclic neutropenia, or idiopathic neutropenia.
For more details on Nivestym, see the full prescribing information.
The US Food and Drug Administration (FDA) has approved the leukocyte growth factor Nivestym™ (filgrastim-aafi), a biosimilar to Neupogen (filgrastim).
Nivestym is approved to treat patients with nonmyeloid malignancies who are receiving myelosuppressive chemotherapy or undergoing bone marrow transplant, acute myeloid leukemia patients receiving induction or consolidation chemotherapy, patients undergoing autologous peripheral blood progenitor cell collection, and patients with severe chronic neutropenia.
The FDA’s approval of Nivestym was based on a review of evidence suggesting the drug is highly similar to Neupogen, according to Pfizer, the company developing Nivestym.
The full approved indication for Nivestym is as follows:
- To decrease the incidence of infection, as manifested by febrile neutropenia, in patients with nonmyeloid malignancies receiving myelosuppressive anticancer drugs associated with a significant incidence of severe neutropenia with fever
- To reduce the time to neutrophil recovery and the duration of fever following induction or consolidation chemotherapy in patients with acute myeloid leukemia
- To reduce the duration of neutropenia and neutropenia-related clinical sequelae (eg, febrile neutropenia) in patients with nonmyeloid malignancies undergoing myeloablative chemotherapy followed by bone marrow transplant
- For the mobilization of autologous hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis
- For chronic administration to reduce the incidence and duration of sequelae of severe neutropenia (eg, fever, infections, oropharyngeal ulcers) in symptomatic patients with congenital neutropenia, cyclic neutropenia, or idiopathic neutropenia.
For more details on Nivestym, see the full prescribing information.
The US Food and Drug Administration (FDA) has approved the leukocyte growth factor Nivestym™ (filgrastim-aafi), a biosimilar to Neupogen (filgrastim).
Nivestym is approved to treat patients with nonmyeloid malignancies who are receiving myelosuppressive chemotherapy or undergoing bone marrow transplant, acute myeloid leukemia patients receiving induction or consolidation chemotherapy, patients undergoing autologous peripheral blood progenitor cell collection, and patients with severe chronic neutropenia.
The FDA’s approval of Nivestym was based on a review of evidence suggesting the drug is highly similar to Neupogen, according to Pfizer, the company developing Nivestym.
The full approved indication for Nivestym is as follows:
- To decrease the incidence of infection, as manifested by febrile neutropenia, in patients with nonmyeloid malignancies receiving myelosuppressive anticancer drugs associated with a significant incidence of severe neutropenia with fever
- To reduce the time to neutrophil recovery and the duration of fever following induction or consolidation chemotherapy in patients with acute myeloid leukemia
- To reduce the duration of neutropenia and neutropenia-related clinical sequelae (eg, febrile neutropenia) in patients with nonmyeloid malignancies undergoing myeloablative chemotherapy followed by bone marrow transplant
- For the mobilization of autologous hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis
- For chronic administration to reduce the incidence and duration of sequelae of severe neutropenia (eg, fever, infections, oropharyngeal ulcers) in symptomatic patients with congenital neutropenia, cyclic neutropenia, or idiopathic neutropenia.
For more details on Nivestym, see the full prescribing information.