User login
FDA approves Nivestym, second biosimilar to Neupogen
Nivestym (filgrastim-aafi), a biosimilar to Neupogen (filgrastim) was approved July 20 by the Food and Drug Administration, according to a statement provided by the agency. Nivestym is the second biosimilar to Neupogen to be approved in the United States.
- Patients with cancer receiving myelosuppressive chemotherapy.
- Patients with acute myeloid leukemia receiving induction or consolidation chemotherapy.
- Patients with cancer undergoing bone marrow transplantation.
- Patients undergoing autologous peripheral blood progenitor cell collection and therapy.
- Patients with severe chronic neutropenia.
According to a press release from Pfizer, the manufacturer of the biosimilar, Nivestym is expected to be available in the United States at a significant discount to the current wholesale acquisition cost of Neupogen, which is not inclusive of discounts to payers, providers, distributors, and other purchasing organizations.
The FDA statement notes that a biosimilar is approved based on a showing that it is highly similar to an already approved biologic product, known as a reference product. The biosimilar also must be shown to have no clinically meaningful differences in terms of safety and effectiveness from the reference product. Only minor differences in clinically inactive components are allowable in biosimilar products.
Prescribing information is available here.
Nivestym (filgrastim-aafi), a biosimilar to Neupogen (filgrastim) was approved July 20 by the Food and Drug Administration, according to a statement provided by the agency. Nivestym is the second biosimilar to Neupogen to be approved in the United States.
- Patients with cancer receiving myelosuppressive chemotherapy.
- Patients with acute myeloid leukemia receiving induction or consolidation chemotherapy.
- Patients with cancer undergoing bone marrow transplantation.
- Patients undergoing autologous peripheral blood progenitor cell collection and therapy.
- Patients with severe chronic neutropenia.
According to a press release from Pfizer, the manufacturer of the biosimilar, Nivestym is expected to be available in the United States at a significant discount to the current wholesale acquisition cost of Neupogen, which is not inclusive of discounts to payers, providers, distributors, and other purchasing organizations.
The FDA statement notes that a biosimilar is approved based on a showing that it is highly similar to an already approved biologic product, known as a reference product. The biosimilar also must be shown to have no clinically meaningful differences in terms of safety and effectiveness from the reference product. Only minor differences in clinically inactive components are allowable in biosimilar products.
Prescribing information is available here.
Nivestym (filgrastim-aafi), a biosimilar to Neupogen (filgrastim) was approved July 20 by the Food and Drug Administration, according to a statement provided by the agency. Nivestym is the second biosimilar to Neupogen to be approved in the United States.
- Patients with cancer receiving myelosuppressive chemotherapy.
- Patients with acute myeloid leukemia receiving induction or consolidation chemotherapy.
- Patients with cancer undergoing bone marrow transplantation.
- Patients undergoing autologous peripheral blood progenitor cell collection and therapy.
- Patients with severe chronic neutropenia.
According to a press release from Pfizer, the manufacturer of the biosimilar, Nivestym is expected to be available in the United States at a significant discount to the current wholesale acquisition cost of Neupogen, which is not inclusive of discounts to payers, providers, distributors, and other purchasing organizations.
The FDA statement notes that a biosimilar is approved based on a showing that it is highly similar to an already approved biologic product, known as a reference product. The biosimilar also must be shown to have no clinically meaningful differences in terms of safety and effectiveness from the reference product. Only minor differences in clinically inactive components are allowable in biosimilar products.
Prescribing information is available here.
FDA approves IDH1 inhibitor for relapsed/refractory AML
The Food and Drug Administration has approved ivosidenib (Tibsovo) as the first treatment of adult patients with relapsed/refractory acute myeloid leukemia (AML) and an isocitrate dehydrogenase-1 (IDH1) mutation.
More specifically, the oral treatment has been approved for patients whose mutations have been identified by the Abbott RealTime IDH1 assay, a companion diagnostic test.
The approval was based on results from a phase 1, open-label, single-arm, multicenter, dose-escalation and expansion trial of adult patients in this AML population. The primary end point was combined complete remission and complete remission with partial hematologic improvement; this combined rate was 32.8%, and the median duration of this remission was 8.2 months.
The most serious adverse events included differentiation syndrome, QTc prolongation, and Guillain-Barré syndrome. Other adverse reactions included fatigue, leukocytosis, arthralgia, diarrhea, dyspnea, edema, and constipation.
Ivosidenib is marketed as Tibsovo by Agios Pharmaceuticals. The RealTime IDH1 Assay is marketed by Abbott Laboratories.
The Food and Drug Administration has approved ivosidenib (Tibsovo) as the first treatment of adult patients with relapsed/refractory acute myeloid leukemia (AML) and an isocitrate dehydrogenase-1 (IDH1) mutation.
More specifically, the oral treatment has been approved for patients whose mutations have been identified by the Abbott RealTime IDH1 assay, a companion diagnostic test.
The approval was based on results from a phase 1, open-label, single-arm, multicenter, dose-escalation and expansion trial of adult patients in this AML population. The primary end point was combined complete remission and complete remission with partial hematologic improvement; this combined rate was 32.8%, and the median duration of this remission was 8.2 months.
The most serious adverse events included differentiation syndrome, QTc prolongation, and Guillain-Barré syndrome. Other adverse reactions included fatigue, leukocytosis, arthralgia, diarrhea, dyspnea, edema, and constipation.
Ivosidenib is marketed as Tibsovo by Agios Pharmaceuticals. The RealTime IDH1 Assay is marketed by Abbott Laboratories.
The Food and Drug Administration has approved ivosidenib (Tibsovo) as the first treatment of adult patients with relapsed/refractory acute myeloid leukemia (AML) and an isocitrate dehydrogenase-1 (IDH1) mutation.
More specifically, the oral treatment has been approved for patients whose mutations have been identified by the Abbott RealTime IDH1 assay, a companion diagnostic test.
The approval was based on results from a phase 1, open-label, single-arm, multicenter, dose-escalation and expansion trial of adult patients in this AML population. The primary end point was combined complete remission and complete remission with partial hematologic improvement; this combined rate was 32.8%, and the median duration of this remission was 8.2 months.
The most serious adverse events included differentiation syndrome, QTc prolongation, and Guillain-Barré syndrome. Other adverse reactions included fatigue, leukocytosis, arthralgia, diarrhea, dyspnea, edema, and constipation.
Ivosidenib is marketed as Tibsovo by Agios Pharmaceuticals. The RealTime IDH1 Assay is marketed by Abbott Laboratories.
Ibrutinib stacks up well on safety in pooled analysis
in the treatment of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and mantle cell lymphoma (MCL), according to findings from a pooled analysis.
Susan M. O’Brien, MD, of the University of California, Irvine, and her colleagues reported pooled data from four randomized, controlled trials that included a 756 patients treated with ibrutinib and 749 patients who received a comparator drug. Patients were treated for either CLL/SLL or MCL, and safety was assessed by comparing crude and exposure-adjusted incidence rates of reported adverse events (AEs).
The comparator drugs included intravenous ofatumumab, oral chlorambucil, intravenous bendamustine plus rituximab, and intravenous temsirolimus.
While adverse event data have been published for each study analyzed, the researchers noted that the pooled analysis allows for an “in-depth assessment of the frequency and severity of both common AEs as well as additional AEs of clinical interest.”
Ibrutinib-treated patients had low rates of treatment discontinuation, compared with comparator-treatment patients (27% vs. 85%), the researchers reported in Clinical Lymphoma, Myeloma & Leukemia. Most discontinuations were caused by disease progression.
In terms of AEs, the types of events reported were similar among the drugs, with the three most common being infections, gastrointestinal disorders, and general disorders/administration-site conditions.
Diarrhea, muscle spasms, and arthralgia were reported more often among ibrutinib-treated patients. The prevalence of the most common all-grade AEs generally decreased over time with ibrutinib, peaking in the first 3 months of treatment. For serious AEs, only atrial fibrillation was higher with ibrutinib than comparator drugs when adjusted for exposure.
SOURCE: O’Brien SM et al. Clin Lymphoma Myeloma Leuk. 2018 Jun 27. doi: 10.1016/j.clml.2018.06.016.
in the treatment of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and mantle cell lymphoma (MCL), according to findings from a pooled analysis.
Susan M. O’Brien, MD, of the University of California, Irvine, and her colleagues reported pooled data from four randomized, controlled trials that included a 756 patients treated with ibrutinib and 749 patients who received a comparator drug. Patients were treated for either CLL/SLL or MCL, and safety was assessed by comparing crude and exposure-adjusted incidence rates of reported adverse events (AEs).
The comparator drugs included intravenous ofatumumab, oral chlorambucil, intravenous bendamustine plus rituximab, and intravenous temsirolimus.
While adverse event data have been published for each study analyzed, the researchers noted that the pooled analysis allows for an “in-depth assessment of the frequency and severity of both common AEs as well as additional AEs of clinical interest.”
Ibrutinib-treated patients had low rates of treatment discontinuation, compared with comparator-treatment patients (27% vs. 85%), the researchers reported in Clinical Lymphoma, Myeloma & Leukemia. Most discontinuations were caused by disease progression.
In terms of AEs, the types of events reported were similar among the drugs, with the three most common being infections, gastrointestinal disorders, and general disorders/administration-site conditions.
Diarrhea, muscle spasms, and arthralgia were reported more often among ibrutinib-treated patients. The prevalence of the most common all-grade AEs generally decreased over time with ibrutinib, peaking in the first 3 months of treatment. For serious AEs, only atrial fibrillation was higher with ibrutinib than comparator drugs when adjusted for exposure.
SOURCE: O’Brien SM et al. Clin Lymphoma Myeloma Leuk. 2018 Jun 27. doi: 10.1016/j.clml.2018.06.016.
in the treatment of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and mantle cell lymphoma (MCL), according to findings from a pooled analysis.
Susan M. O’Brien, MD, of the University of California, Irvine, and her colleagues reported pooled data from four randomized, controlled trials that included a 756 patients treated with ibrutinib and 749 patients who received a comparator drug. Patients were treated for either CLL/SLL or MCL, and safety was assessed by comparing crude and exposure-adjusted incidence rates of reported adverse events (AEs).
The comparator drugs included intravenous ofatumumab, oral chlorambucil, intravenous bendamustine plus rituximab, and intravenous temsirolimus.
While adverse event data have been published for each study analyzed, the researchers noted that the pooled analysis allows for an “in-depth assessment of the frequency and severity of both common AEs as well as additional AEs of clinical interest.”
Ibrutinib-treated patients had low rates of treatment discontinuation, compared with comparator-treatment patients (27% vs. 85%), the researchers reported in Clinical Lymphoma, Myeloma & Leukemia. Most discontinuations were caused by disease progression.
In terms of AEs, the types of events reported were similar among the drugs, with the three most common being infections, gastrointestinal disorders, and general disorders/administration-site conditions.
Diarrhea, muscle spasms, and arthralgia were reported more often among ibrutinib-treated patients. The prevalence of the most common all-grade AEs generally decreased over time with ibrutinib, peaking in the first 3 months of treatment. For serious AEs, only atrial fibrillation was higher with ibrutinib than comparator drugs when adjusted for exposure.
SOURCE: O’Brien SM et al. Clin Lymphoma Myeloma Leuk. 2018 Jun 27. doi: 10.1016/j.clml.2018.06.016.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Diabetics have higher risk of hematologic, other cancers
A review of data from more than 19 million people indicates that diabetes significantly raises a person’s risk of developing cancer.
When researchers compared patients with diabetes and without, both male and female diabetics had an increased risk of leukemias and lymphomas as well as certain solid tumors.
Researchers also found that diabetes conferred a higher cancer risk for women than men, both for all cancers combined and for some specific cancers, including leukemia.
“The link between diabetes and the risk of developing cancer is now firmly established,” said Toshiaki Ohkuma, PhD, of The George Institute for Global Health at the University of New South Wales in Australia.
“We have also demonstrated, for the first time, that women with diabetes are more likely to develop any form of cancer and have a significantly higher chance of developing kidney, oral, and stomach cancers and leukemia.”
Dr Ohkuma and his colleagues reported these findings in Diabetologia.
The researchers conducted a systematic search in PubMed MEDLINE to identify reports on the links between diabetes and cancer. Additional reports were identified from the reference lists of the relevant studies.
Only those cohort studies providing relative risks (RRs) for the association between diabetes and cancer for both women and men were included. In total, 107 relevant articles were identified, along with 36 cohorts of individual participant data.
RRs for cancer were obtained for patients with diabetes (types 1 and 2 combined) versus those without diabetes, for both men and women. The women-to-men ratios of these relative risk ratios (RRRs) were then calculated to determine the excess risk in women if present.
Data on all-site cancer was available from 47 studies, involving 121 cohorts and 19,239,302 individuals.
Diabetics vs non-diabetics
Women with diabetes had a 27% higher risk of all-site cancer compared to women without diabetes (RR=1.27; 95% CI 1.21, 1.32; P<0.001).
For men, the risk of all-site cancer was 19% higher among those with diabetes than those without (RR=1.19; 95% CI 1.13, 1.25; P<0.001).
There were several hematologic malignancies for which diabetics had an increased risk, as shown in the following table.
| Cancer type | RR for women (99% CI) | RR for men (99% CI) |
| Lymphatic and hematopoietic tissue | 1.24 (1.05, 1.46)* | 1.21 (0.98, 1.48) |
| Leukemia | 1.53 (1.00, 2.33) | 1.22 (0.80, 1.85) |
| Myeloid leukemia | 0.83 (0.39, 1.76) | 1.12 (0.77, 1.62) |
| Acute myeloid leukemia | 1.33 (1.12, 1.57)* | 1.14 (0.56, 2.33) |
| Chronic myeloid leukemia | 1.67 (1.27, 2.20)* | 1.62 (1.32, 1.98)* |
| Lymphoid leukemia | 1.74 (0.31, 9.79) | 1.20 (0.86, 1.68) |
| Lymphoma | 2.31 (0.57, 9.30) | 1.80 (0.68, 4.75) |
| Non-Hodgkin lymphoma | 1.16 (1.02, 1.32)* | 1.20 (1.08, 1.34)* |
| Hodgkin lymphoma | 1.20 (0.61, 2.38) | 1.36 (1.05, 1.77)* |
| Multiple myeloma | 1.19 (0.97, 1.47) | 1.12 (0.90, 1.41) |
| *denotes statistical significance with a P value < 0.01 | ||
Sex comparison
Calculation of the women-to-men ratio revealed that women with diabetes had a 6% greater excess risk of all-site cancer compared to men with diabetes (RRR=1.06; 95% CI 1.03, 1.09; P<0.001).
The women-to-men ratios also showed significantly higher risks for female diabetics for:
- Kidney cancer—RRR=1.11 (99% CI 1.04, 1.18; P<0.001)
- Oral cancer—RRR=1.13 (99% CI 1.00, 1.28; P=0.009)
- Stomach cancer—RRR=1.14 (99% CI 1.07, 1.22; P<0.001)
- Leukemia—RRR=1.15 (99% CI 1.02, 1.28; P=0.002).
However, women had a significantly lower risk of liver cancer (RRR=0.88; 99% CI 0.79, 0.99; P=0.005).
There are several possible reasons for the excess cancer risk observed in women, according to study author Sanne Peters, PhD, of The George Institute for Global Health at the University of Oxford in the UK.
For example, on average, women are in the pre-diabetic state of impaired glucose tolerance 2 years longer than men.
“Historically, we know that women are often under-treated when they first present with symptoms of diabetes, are less likely to receive intensive care, and are not taking the same levels of medications as men,” Dr Peters said. “All of these could go some way into explaining why women are at greater risk of developing cancer, but, without more research, we can’t be certain.”
A review of data from more than 19 million people indicates that diabetes significantly raises a person’s risk of developing cancer.
When researchers compared patients with diabetes and without, both male and female diabetics had an increased risk of leukemias and lymphomas as well as certain solid tumors.
Researchers also found that diabetes conferred a higher cancer risk for women than men, both for all cancers combined and for some specific cancers, including leukemia.
“The link between diabetes and the risk of developing cancer is now firmly established,” said Toshiaki Ohkuma, PhD, of The George Institute for Global Health at the University of New South Wales in Australia.
“We have also demonstrated, for the first time, that women with diabetes are more likely to develop any form of cancer and have a significantly higher chance of developing kidney, oral, and stomach cancers and leukemia.”
Dr Ohkuma and his colleagues reported these findings in Diabetologia.
The researchers conducted a systematic search in PubMed MEDLINE to identify reports on the links between diabetes and cancer. Additional reports were identified from the reference lists of the relevant studies.
Only those cohort studies providing relative risks (RRs) for the association between diabetes and cancer for both women and men were included. In total, 107 relevant articles were identified, along with 36 cohorts of individual participant data.
RRs for cancer were obtained for patients with diabetes (types 1 and 2 combined) versus those without diabetes, for both men and women. The women-to-men ratios of these relative risk ratios (RRRs) were then calculated to determine the excess risk in women if present.
Data on all-site cancer was available from 47 studies, involving 121 cohorts and 19,239,302 individuals.
Diabetics vs non-diabetics
Women with diabetes had a 27% higher risk of all-site cancer compared to women without diabetes (RR=1.27; 95% CI 1.21, 1.32; P<0.001).
For men, the risk of all-site cancer was 19% higher among those with diabetes than those without (RR=1.19; 95% CI 1.13, 1.25; P<0.001).
There were several hematologic malignancies for which diabetics had an increased risk, as shown in the following table.
| Cancer type | RR for women (99% CI) | RR for men (99% CI) |
| Lymphatic and hematopoietic tissue | 1.24 (1.05, 1.46)* | 1.21 (0.98, 1.48) |
| Leukemia | 1.53 (1.00, 2.33) | 1.22 (0.80, 1.85) |
| Myeloid leukemia | 0.83 (0.39, 1.76) | 1.12 (0.77, 1.62) |
| Acute myeloid leukemia | 1.33 (1.12, 1.57)* | 1.14 (0.56, 2.33) |
| Chronic myeloid leukemia | 1.67 (1.27, 2.20)* | 1.62 (1.32, 1.98)* |
| Lymphoid leukemia | 1.74 (0.31, 9.79) | 1.20 (0.86, 1.68) |
| Lymphoma | 2.31 (0.57, 9.30) | 1.80 (0.68, 4.75) |
| Non-Hodgkin lymphoma | 1.16 (1.02, 1.32)* | 1.20 (1.08, 1.34)* |
| Hodgkin lymphoma | 1.20 (0.61, 2.38) | 1.36 (1.05, 1.77)* |
| Multiple myeloma | 1.19 (0.97, 1.47) | 1.12 (0.90, 1.41) |
| *denotes statistical significance with a P value < 0.01 | ||
Sex comparison
Calculation of the women-to-men ratio revealed that women with diabetes had a 6% greater excess risk of all-site cancer compared to men with diabetes (RRR=1.06; 95% CI 1.03, 1.09; P<0.001).
The women-to-men ratios also showed significantly higher risks for female diabetics for:
- Kidney cancer—RRR=1.11 (99% CI 1.04, 1.18; P<0.001)
- Oral cancer—RRR=1.13 (99% CI 1.00, 1.28; P=0.009)
- Stomach cancer—RRR=1.14 (99% CI 1.07, 1.22; P<0.001)
- Leukemia—RRR=1.15 (99% CI 1.02, 1.28; P=0.002).
However, women had a significantly lower risk of liver cancer (RRR=0.88; 99% CI 0.79, 0.99; P=0.005).
There are several possible reasons for the excess cancer risk observed in women, according to study author Sanne Peters, PhD, of The George Institute for Global Health at the University of Oxford in the UK.
For example, on average, women are in the pre-diabetic state of impaired glucose tolerance 2 years longer than men.
“Historically, we know that women are often under-treated when they first present with symptoms of diabetes, are less likely to receive intensive care, and are not taking the same levels of medications as men,” Dr Peters said. “All of these could go some way into explaining why women are at greater risk of developing cancer, but, without more research, we can’t be certain.”
A review of data from more than 19 million people indicates that diabetes significantly raises a person’s risk of developing cancer.
When researchers compared patients with diabetes and without, both male and female diabetics had an increased risk of leukemias and lymphomas as well as certain solid tumors.
Researchers also found that diabetes conferred a higher cancer risk for women than men, both for all cancers combined and for some specific cancers, including leukemia.
“The link between diabetes and the risk of developing cancer is now firmly established,” said Toshiaki Ohkuma, PhD, of The George Institute for Global Health at the University of New South Wales in Australia.
“We have also demonstrated, for the first time, that women with diabetes are more likely to develop any form of cancer and have a significantly higher chance of developing kidney, oral, and stomach cancers and leukemia.”
Dr Ohkuma and his colleagues reported these findings in Diabetologia.
The researchers conducted a systematic search in PubMed MEDLINE to identify reports on the links between diabetes and cancer. Additional reports were identified from the reference lists of the relevant studies.
Only those cohort studies providing relative risks (RRs) for the association between diabetes and cancer for both women and men were included. In total, 107 relevant articles were identified, along with 36 cohorts of individual participant data.
RRs for cancer were obtained for patients with diabetes (types 1 and 2 combined) versus those without diabetes, for both men and women. The women-to-men ratios of these relative risk ratios (RRRs) were then calculated to determine the excess risk in women if present.
Data on all-site cancer was available from 47 studies, involving 121 cohorts and 19,239,302 individuals.
Diabetics vs non-diabetics
Women with diabetes had a 27% higher risk of all-site cancer compared to women without diabetes (RR=1.27; 95% CI 1.21, 1.32; P<0.001).
For men, the risk of all-site cancer was 19% higher among those with diabetes than those without (RR=1.19; 95% CI 1.13, 1.25; P<0.001).
There were several hematologic malignancies for which diabetics had an increased risk, as shown in the following table.
| Cancer type | RR for women (99% CI) | RR for men (99% CI) |
| Lymphatic and hematopoietic tissue | 1.24 (1.05, 1.46)* | 1.21 (0.98, 1.48) |
| Leukemia | 1.53 (1.00, 2.33) | 1.22 (0.80, 1.85) |
| Myeloid leukemia | 0.83 (0.39, 1.76) | 1.12 (0.77, 1.62) |
| Acute myeloid leukemia | 1.33 (1.12, 1.57)* | 1.14 (0.56, 2.33) |
| Chronic myeloid leukemia | 1.67 (1.27, 2.20)* | 1.62 (1.32, 1.98)* |
| Lymphoid leukemia | 1.74 (0.31, 9.79) | 1.20 (0.86, 1.68) |
| Lymphoma | 2.31 (0.57, 9.30) | 1.80 (0.68, 4.75) |
| Non-Hodgkin lymphoma | 1.16 (1.02, 1.32)* | 1.20 (1.08, 1.34)* |
| Hodgkin lymphoma | 1.20 (0.61, 2.38) | 1.36 (1.05, 1.77)* |
| Multiple myeloma | 1.19 (0.97, 1.47) | 1.12 (0.90, 1.41) |
| *denotes statistical significance with a P value < 0.01 | ||
Sex comparison
Calculation of the women-to-men ratio revealed that women with diabetes had a 6% greater excess risk of all-site cancer compared to men with diabetes (RRR=1.06; 95% CI 1.03, 1.09; P<0.001).
The women-to-men ratios also showed significantly higher risks for female diabetics for:
- Kidney cancer—RRR=1.11 (99% CI 1.04, 1.18; P<0.001)
- Oral cancer—RRR=1.13 (99% CI 1.00, 1.28; P=0.009)
- Stomach cancer—RRR=1.14 (99% CI 1.07, 1.22; P<0.001)
- Leukemia—RRR=1.15 (99% CI 1.02, 1.28; P=0.002).
However, women had a significantly lower risk of liver cancer (RRR=0.88; 99% CI 0.79, 0.99; P=0.005).
There are several possible reasons for the excess cancer risk observed in women, according to study author Sanne Peters, PhD, of The George Institute for Global Health at the University of Oxford in the UK.
For example, on average, women are in the pre-diabetic state of impaired glucose tolerance 2 years longer than men.
“Historically, we know that women are often under-treated when they first present with symptoms of diabetes, are less likely to receive intensive care, and are not taking the same levels of medications as men,” Dr Peters said. “All of these could go some way into explaining why women are at greater risk of developing cancer, but, without more research, we can’t be certain.”
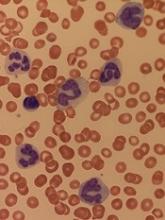
How ALL invades the CNS
Researchers believe they have solved the mystery of how acute lymphoblastic leukemia (ALL) infiltrates the central nervous system (CNS).
Experiments in mice suggested that ALL enters the CNS not by breaching the blood-brain barrier but by evading it.
The researchers said they found that expression of the laminin receptor α6 integrin, which is common in ALL, allows cells to use neural migratory pathways to invade the CNS.
“It’s a very unexpected way for cells to travel into the central nervous system,” said Dorothy Sipkins, MD, PhD, of Duke University in Durham, North Carolina.
She and her colleagues described the cells’ journey in Nature.
The researchers said they found that α6 integrin–laminin interactions mediate the migration of ALL cells toward the cerebrospinal fluid.
The team noted that α6 integrin is expressed in most cases of ALL, and laminin surrounds blood vessels that pass directly through the vertebrae to the meninges tissue that lines the spinal cord and brain.
Experiments indicated that ALL cells latch onto the laminin surrounding these blood vessels and travel down into the meninges region where cerebral spinal fluid circulates.
“Understanding how ALL gets into the central nervous system arms us with new ways to target this pathway and hopefully shut it down,” Dr Sipkins noted.
She and her colleagues found that treatment with a PI3Kδ inhibitor may be one way to do that.
The team tested the PI3Kδ inhibitor GS-649443 in a mouse model of CNS ALL (Nalm-6) and found the drug decreased α6 integrin expression on ALL cells.
Mice treated with the inhibitor had a 50% decrease in CNS disease burden compared to vehicle-treated controls. However, there was no significant difference between treated mice and controls when it came to bone marrow or splenic Nalm-6 disease burden or peripheral blood cell counts.
The researchers observed similar results in another model of CNS disease (RCH-ACV ALL).
The team also tested α6 integrin-neutralizing antibodies in Nalm-6-engrafted mice. There was no difference in peripheral disease burden between targeted and isotype control antibody-treated mice. However, anti-α6 integrin-treated mice had a reduction in cerebrospinal fluid blast counts.
This research was supported by the Duke Cancer Institute and Gilead Sciences, Inc., which provided the PI3Kδ inhibitor.
Researchers believe they have solved the mystery of how acute lymphoblastic leukemia (ALL) infiltrates the central nervous system (CNS).
Experiments in mice suggested that ALL enters the CNS not by breaching the blood-brain barrier but by evading it.
The researchers said they found that expression of the laminin receptor α6 integrin, which is common in ALL, allows cells to use neural migratory pathways to invade the CNS.
“It’s a very unexpected way for cells to travel into the central nervous system,” said Dorothy Sipkins, MD, PhD, of Duke University in Durham, North Carolina.
She and her colleagues described the cells’ journey in Nature.
The researchers said they found that α6 integrin–laminin interactions mediate the migration of ALL cells toward the cerebrospinal fluid.
The team noted that α6 integrin is expressed in most cases of ALL, and laminin surrounds blood vessels that pass directly through the vertebrae to the meninges tissue that lines the spinal cord and brain.
Experiments indicated that ALL cells latch onto the laminin surrounding these blood vessels and travel down into the meninges region where cerebral spinal fluid circulates.
“Understanding how ALL gets into the central nervous system arms us with new ways to target this pathway and hopefully shut it down,” Dr Sipkins noted.
She and her colleagues found that treatment with a PI3Kδ inhibitor may be one way to do that.
The team tested the PI3Kδ inhibitor GS-649443 in a mouse model of CNS ALL (Nalm-6) and found the drug decreased α6 integrin expression on ALL cells.
Mice treated with the inhibitor had a 50% decrease in CNS disease burden compared to vehicle-treated controls. However, there was no significant difference between treated mice and controls when it came to bone marrow or splenic Nalm-6 disease burden or peripheral blood cell counts.
The researchers observed similar results in another model of CNS disease (RCH-ACV ALL).
The team also tested α6 integrin-neutralizing antibodies in Nalm-6-engrafted mice. There was no difference in peripheral disease burden between targeted and isotype control antibody-treated mice. However, anti-α6 integrin-treated mice had a reduction in cerebrospinal fluid blast counts.
This research was supported by the Duke Cancer Institute and Gilead Sciences, Inc., which provided the PI3Kδ inhibitor.
Researchers believe they have solved the mystery of how acute lymphoblastic leukemia (ALL) infiltrates the central nervous system (CNS).
Experiments in mice suggested that ALL enters the CNS not by breaching the blood-brain barrier but by evading it.
The researchers said they found that expression of the laminin receptor α6 integrin, which is common in ALL, allows cells to use neural migratory pathways to invade the CNS.
“It’s a very unexpected way for cells to travel into the central nervous system,” said Dorothy Sipkins, MD, PhD, of Duke University in Durham, North Carolina.
She and her colleagues described the cells’ journey in Nature.
The researchers said they found that α6 integrin–laminin interactions mediate the migration of ALL cells toward the cerebrospinal fluid.
The team noted that α6 integrin is expressed in most cases of ALL, and laminin surrounds blood vessels that pass directly through the vertebrae to the meninges tissue that lines the spinal cord and brain.
Experiments indicated that ALL cells latch onto the laminin surrounding these blood vessels and travel down into the meninges region where cerebral spinal fluid circulates.
“Understanding how ALL gets into the central nervous system arms us with new ways to target this pathway and hopefully shut it down,” Dr Sipkins noted.
She and her colleagues found that treatment with a PI3Kδ inhibitor may be one way to do that.
The team tested the PI3Kδ inhibitor GS-649443 in a mouse model of CNS ALL (Nalm-6) and found the drug decreased α6 integrin expression on ALL cells.
Mice treated with the inhibitor had a 50% decrease in CNS disease burden compared to vehicle-treated controls. However, there was no significant difference between treated mice and controls when it came to bone marrow or splenic Nalm-6 disease burden or peripheral blood cell counts.
The researchers observed similar results in another model of CNS disease (RCH-ACV ALL).
The team also tested α6 integrin-neutralizing antibodies in Nalm-6-engrafted mice. There was no difference in peripheral disease burden between targeted and isotype control antibody-treated mice. However, anti-α6 integrin-treated mice had a reduction in cerebrospinal fluid blast counts.
This research was supported by the Duke Cancer Institute and Gilead Sciences, Inc., which provided the PI3Kδ inhibitor.
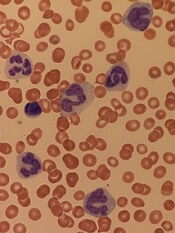
Study suggests dasatinib could treat AML, JMML
New research suggests dasatinib could treat certain patients with juvenile myelomonocytic leukemia (JMML) or acute myeloid leukemia (AML).
The study showed that TNK2 inhibition has a negative effect on PTPN11-mutant leukemias.
PTPN11-mutant JMML and AML cells were sensitive to treatment with dasatinib, which inhibits TNK2.
Dasatinib also induced hematologic remission in a patient with PTPN11-mutant JMML.
Investigators reported these results in Science Signaling.
Past research showed that mutations in PTPN11 result in excessive cell proliferation and drive tumor growth in some cases of JMML and AML.
In the current study, investigators analyzed PTPN11-mutated leukemia cells and found that PTPN11 is activated by TNK2.
The investigators said TNK2 phosphorylates PTPN11, which then dephosphorylates TNK2 in a negative feedback loop. They also found that coexpression of TNK2 and mutant PTPN11 results in “robust” MAPK pathway activation.
Inhibiting TNK2 with dasatinib blocked MAPK signaling and colony formation in vitro.
Additional experiments showed that PTPN11-mutant AML samples were significantly more sensitive to dasatinib than wild-type AML samples.
Investigators also tested dasatinib in a sample from a JMML patient carrying a PTPN11 G60R mutation.
This patient’s cells were 10 times more sensitive to dasatinib than the average sample from a cohort of 151 patients who had AML, acute lymphoblastic leukemia, myeloproliferative neoplasms, or chronic lymphocytic leukemia.
Because the JMML patient’s cells were so responsive to dasatinib, the investigators decided to administer the drug to the patient.
The patient achieved sustained hematologic remission with dasatinib, and this allowed him to receive a stem cell transplant using an unrelated cord blood donor. The patient had previously failed 2 transplants (with myeloablative conditioning) from a matched sibling donor.
The third transplant prolonged the patient’s life by a year, but he eventually died of relapsed disease.
The investigators said this case study and the in vitro results support further investigation into the efficacy of dasatinib and other TNK2 inhibitors in PTPN11-mutant leukemias.
New research suggests dasatinib could treat certain patients with juvenile myelomonocytic leukemia (JMML) or acute myeloid leukemia (AML).
The study showed that TNK2 inhibition has a negative effect on PTPN11-mutant leukemias.
PTPN11-mutant JMML and AML cells were sensitive to treatment with dasatinib, which inhibits TNK2.
Dasatinib also induced hematologic remission in a patient with PTPN11-mutant JMML.
Investigators reported these results in Science Signaling.
Past research showed that mutations in PTPN11 result in excessive cell proliferation and drive tumor growth in some cases of JMML and AML.
In the current study, investigators analyzed PTPN11-mutated leukemia cells and found that PTPN11 is activated by TNK2.
The investigators said TNK2 phosphorylates PTPN11, which then dephosphorylates TNK2 in a negative feedback loop. They also found that coexpression of TNK2 and mutant PTPN11 results in “robust” MAPK pathway activation.
Inhibiting TNK2 with dasatinib blocked MAPK signaling and colony formation in vitro.
Additional experiments showed that PTPN11-mutant AML samples were significantly more sensitive to dasatinib than wild-type AML samples.
Investigators also tested dasatinib in a sample from a JMML patient carrying a PTPN11 G60R mutation.
This patient’s cells were 10 times more sensitive to dasatinib than the average sample from a cohort of 151 patients who had AML, acute lymphoblastic leukemia, myeloproliferative neoplasms, or chronic lymphocytic leukemia.
Because the JMML patient’s cells were so responsive to dasatinib, the investigators decided to administer the drug to the patient.
The patient achieved sustained hematologic remission with dasatinib, and this allowed him to receive a stem cell transplant using an unrelated cord blood donor. The patient had previously failed 2 transplants (with myeloablative conditioning) from a matched sibling donor.
The third transplant prolonged the patient’s life by a year, but he eventually died of relapsed disease.
The investigators said this case study and the in vitro results support further investigation into the efficacy of dasatinib and other TNK2 inhibitors in PTPN11-mutant leukemias.
New research suggests dasatinib could treat certain patients with juvenile myelomonocytic leukemia (JMML) or acute myeloid leukemia (AML).
The study showed that TNK2 inhibition has a negative effect on PTPN11-mutant leukemias.
PTPN11-mutant JMML and AML cells were sensitive to treatment with dasatinib, which inhibits TNK2.
Dasatinib also induced hematologic remission in a patient with PTPN11-mutant JMML.
Investigators reported these results in Science Signaling.
Past research showed that mutations in PTPN11 result in excessive cell proliferation and drive tumor growth in some cases of JMML and AML.
In the current study, investigators analyzed PTPN11-mutated leukemia cells and found that PTPN11 is activated by TNK2.
The investigators said TNK2 phosphorylates PTPN11, which then dephosphorylates TNK2 in a negative feedback loop. They also found that coexpression of TNK2 and mutant PTPN11 results in “robust” MAPK pathway activation.
Inhibiting TNK2 with dasatinib blocked MAPK signaling and colony formation in vitro.
Additional experiments showed that PTPN11-mutant AML samples were significantly more sensitive to dasatinib than wild-type AML samples.
Investigators also tested dasatinib in a sample from a JMML patient carrying a PTPN11 G60R mutation.
This patient’s cells were 10 times more sensitive to dasatinib than the average sample from a cohort of 151 patients who had AML, acute lymphoblastic leukemia, myeloproliferative neoplasms, or chronic lymphocytic leukemia.
Because the JMML patient’s cells were so responsive to dasatinib, the investigators decided to administer the drug to the patient.
The patient achieved sustained hematologic remission with dasatinib, and this allowed him to receive a stem cell transplant using an unrelated cord blood donor. The patient had previously failed 2 transplants (with myeloablative conditioning) from a matched sibling donor.
The third transplant prolonged the patient’s life by a year, but he eventually died of relapsed disease.
The investigators said this case study and the in vitro results support further investigation into the efficacy of dasatinib and other TNK2 inhibitors in PTPN11-mutant leukemias.
Lab results may help predict complications in ALL treatment
(ALL) who were treated with four-drug induction therapy.
Kasper Warrick, MD, and his colleagues at Indiana University in Indianapolis reported findings from a retrospective study of 73 ALL patients at their hospital. They performed chart reviews comparing a cohort of 42 patients who were discharged on day 4 of their induction treatment with 31 similar patients who had a longer hospital stay or admission to the intensive care unit. The report was published in Leukemia Research.
Univariate analysis found that patients with a longer length of stay were more likely to have a fever, pretransfusion hemoglobin of less than 8 g/dL, lower serum bicarbonate values, abnormal serum calcium, and abnormal serum phosphate. Multivariate stepwise logistic regression found that low serum bicarbonate and a lower platelet count on day 4 of admission was predictive of a prolonged hospital stay. About a third of patients from each group had an unplanned readmission within 30 days.
The researchers concluded that early discharge is safe in only a subgroup of high-risk ALL patients undergoing induction chemotherapy. “Treating physicians could opt for a discharge only after normalization of electrolyte abnormalities and renal functions, and when no transfusion support is needed (stable hematocrit and platelet count),” they wrote. Even in those cases, they recommended “aggressive and close outpatient follow” since patients are vulnerable to complications and readmissions.
SOURCE: Warrick K et al. Leuk Res. 2018 Jun 30:71:36-42.
(ALL) who were treated with four-drug induction therapy.
Kasper Warrick, MD, and his colleagues at Indiana University in Indianapolis reported findings from a retrospective study of 73 ALL patients at their hospital. They performed chart reviews comparing a cohort of 42 patients who were discharged on day 4 of their induction treatment with 31 similar patients who had a longer hospital stay or admission to the intensive care unit. The report was published in Leukemia Research.
Univariate analysis found that patients with a longer length of stay were more likely to have a fever, pretransfusion hemoglobin of less than 8 g/dL, lower serum bicarbonate values, abnormal serum calcium, and abnormal serum phosphate. Multivariate stepwise logistic regression found that low serum bicarbonate and a lower platelet count on day 4 of admission was predictive of a prolonged hospital stay. About a third of patients from each group had an unplanned readmission within 30 days.
The researchers concluded that early discharge is safe in only a subgroup of high-risk ALL patients undergoing induction chemotherapy. “Treating physicians could opt for a discharge only after normalization of electrolyte abnormalities and renal functions, and when no transfusion support is needed (stable hematocrit and platelet count),” they wrote. Even in those cases, they recommended “aggressive and close outpatient follow” since patients are vulnerable to complications and readmissions.
SOURCE: Warrick K et al. Leuk Res. 2018 Jun 30:71:36-42.
(ALL) who were treated with four-drug induction therapy.
Kasper Warrick, MD, and his colleagues at Indiana University in Indianapolis reported findings from a retrospective study of 73 ALL patients at their hospital. They performed chart reviews comparing a cohort of 42 patients who were discharged on day 4 of their induction treatment with 31 similar patients who had a longer hospital stay or admission to the intensive care unit. The report was published in Leukemia Research.
Univariate analysis found that patients with a longer length of stay were more likely to have a fever, pretransfusion hemoglobin of less than 8 g/dL, lower serum bicarbonate values, abnormal serum calcium, and abnormal serum phosphate. Multivariate stepwise logistic regression found that low serum bicarbonate and a lower platelet count on day 4 of admission was predictive of a prolonged hospital stay. About a third of patients from each group had an unplanned readmission within 30 days.
The researchers concluded that early discharge is safe in only a subgroup of high-risk ALL patients undergoing induction chemotherapy. “Treating physicians could opt for a discharge only after normalization of electrolyte abnormalities and renal functions, and when no transfusion support is needed (stable hematocrit and platelet count),” they wrote. Even in those cases, they recommended “aggressive and close outpatient follow” since patients are vulnerable to complications and readmissions.
SOURCE: Warrick K et al. Leuk Res. 2018 Jun 30:71:36-42.
FROM LEUKEMIA RESEARCH
Explaining enasidenib resistance in AML
New research helps explain enasidenib resistance among patients with IDH2-mutant acute myeloid leukemia (AML).
Researchers found that leukemic cells stop responding to enasidenib when IDH2 clones develop additional mutations.
This may mean that enasidenib will have to be combined with other drugs to prevent AML relapse, the researchers said.
They reported their findings in Nature Medicine.
Previous research indicated that enasidenib prompts differentiation to induce responses in AML. In a phase 1/2 trial, enasidenib produced responses in about 40% of patients with relapsed/refractory, IDH2-mutated AML. However, most patients eventually relapsed.
“[T]he initial studies did not show which AML cells responded to enasidenib and started to differentiate again,” said Stéphane de Botton, MD, PhD, of Institut Gustave Roussy in Villejuif, France.
“It was also unclear how the cells become resistant to therapy. We wanted to answer these questions.”
To do so, Dr de Botton and his colleagues analyzed sequential samples from 37 AML patients treated with enasidenib on the phase 1/2 trial. Thirty of these patients had initially responded to the drug.
“We used techniques to study genetic mutations on a cell-by-cell basis and reconstructed the ‘family tree’ of a patient’s AML,” said Lynn Quek, MD, of the University of Oxford in the UK.
“We then tracked changes in the family of AML cells as they responded to enasidenib and as patients lost response to the drug. This is the first time that anyone has done such a detailed study at a single-cell level.”
The researchers said they observed variable differentiation arrest in IDH2-mutant clones before enasidenib treatment.
Overall, treatment promoted hematopoietic differentiation from either terminal or ancestral mutant clones. However, enasidenib also promoted differentiation of nonmutant cells in a minority of patients.
When the researchers compared samples taken at diagnosis and relapse, they did not find second-site mutations in IDH2 at relapse.
The team said relapse was the result of clonal evolution or selection of terminal or ancestral clones, which suggests there are multiple pathways that could potentially be targeted to restore differentiation arrest.
“We have provided genetic proof that enasidenib was able to differentiate cancer cells so that some of their normal functions were restored, even though they still contained the IDH2 mutation,” said Virginie Penard-Lacronique, of Gustave Roussy.
“This is important because, unless we can track these clones, we don’t know whether the mature cells in a patient are coming from normal cells after all the cancer cells have been killed or from leukemic cells that are now able to mature. In this paper, we show that, in 4 out of 5 cases, the mature cells are coming from leukemic bone marrow cells that can be induced to differentiate by this new drug.”
The researchers said these results suggest enasidenib must be combined with other drugs to prevent AML relapse.
“Now that we have shown that [enasidenib] needs to be combined with other drugs to stop the cancer returning, we think that it’s important that the combination therapy should be given to AML patients early on in their disease,” Dr de Botton said. “International trials are now taking place comparing combinations of enasidenib and other drugs with the normal standard of care.”
New research helps explain enasidenib resistance among patients with IDH2-mutant acute myeloid leukemia (AML).
Researchers found that leukemic cells stop responding to enasidenib when IDH2 clones develop additional mutations.
This may mean that enasidenib will have to be combined with other drugs to prevent AML relapse, the researchers said.
They reported their findings in Nature Medicine.
Previous research indicated that enasidenib prompts differentiation to induce responses in AML. In a phase 1/2 trial, enasidenib produced responses in about 40% of patients with relapsed/refractory, IDH2-mutated AML. However, most patients eventually relapsed.
“[T]he initial studies did not show which AML cells responded to enasidenib and started to differentiate again,” said Stéphane de Botton, MD, PhD, of Institut Gustave Roussy in Villejuif, France.
“It was also unclear how the cells become resistant to therapy. We wanted to answer these questions.”
To do so, Dr de Botton and his colleagues analyzed sequential samples from 37 AML patients treated with enasidenib on the phase 1/2 trial. Thirty of these patients had initially responded to the drug.
“We used techniques to study genetic mutations on a cell-by-cell basis and reconstructed the ‘family tree’ of a patient’s AML,” said Lynn Quek, MD, of the University of Oxford in the UK.
“We then tracked changes in the family of AML cells as they responded to enasidenib and as patients lost response to the drug. This is the first time that anyone has done such a detailed study at a single-cell level.”
The researchers said they observed variable differentiation arrest in IDH2-mutant clones before enasidenib treatment.
Overall, treatment promoted hematopoietic differentiation from either terminal or ancestral mutant clones. However, enasidenib also promoted differentiation of nonmutant cells in a minority of patients.
When the researchers compared samples taken at diagnosis and relapse, they did not find second-site mutations in IDH2 at relapse.
The team said relapse was the result of clonal evolution or selection of terminal or ancestral clones, which suggests there are multiple pathways that could potentially be targeted to restore differentiation arrest.
“We have provided genetic proof that enasidenib was able to differentiate cancer cells so that some of their normal functions were restored, even though they still contained the IDH2 mutation,” said Virginie Penard-Lacronique, of Gustave Roussy.
“This is important because, unless we can track these clones, we don’t know whether the mature cells in a patient are coming from normal cells after all the cancer cells have been killed or from leukemic cells that are now able to mature. In this paper, we show that, in 4 out of 5 cases, the mature cells are coming from leukemic bone marrow cells that can be induced to differentiate by this new drug.”
The researchers said these results suggest enasidenib must be combined with other drugs to prevent AML relapse.
“Now that we have shown that [enasidenib] needs to be combined with other drugs to stop the cancer returning, we think that it’s important that the combination therapy should be given to AML patients early on in their disease,” Dr de Botton said. “International trials are now taking place comparing combinations of enasidenib and other drugs with the normal standard of care.”
New research helps explain enasidenib resistance among patients with IDH2-mutant acute myeloid leukemia (AML).
Researchers found that leukemic cells stop responding to enasidenib when IDH2 clones develop additional mutations.
This may mean that enasidenib will have to be combined with other drugs to prevent AML relapse, the researchers said.
They reported their findings in Nature Medicine.
Previous research indicated that enasidenib prompts differentiation to induce responses in AML. In a phase 1/2 trial, enasidenib produced responses in about 40% of patients with relapsed/refractory, IDH2-mutated AML. However, most patients eventually relapsed.
“[T]he initial studies did not show which AML cells responded to enasidenib and started to differentiate again,” said Stéphane de Botton, MD, PhD, of Institut Gustave Roussy in Villejuif, France.
“It was also unclear how the cells become resistant to therapy. We wanted to answer these questions.”
To do so, Dr de Botton and his colleagues analyzed sequential samples from 37 AML patients treated with enasidenib on the phase 1/2 trial. Thirty of these patients had initially responded to the drug.
“We used techniques to study genetic mutations on a cell-by-cell basis and reconstructed the ‘family tree’ of a patient’s AML,” said Lynn Quek, MD, of the University of Oxford in the UK.
“We then tracked changes in the family of AML cells as they responded to enasidenib and as patients lost response to the drug. This is the first time that anyone has done such a detailed study at a single-cell level.”
The researchers said they observed variable differentiation arrest in IDH2-mutant clones before enasidenib treatment.
Overall, treatment promoted hematopoietic differentiation from either terminal or ancestral mutant clones. However, enasidenib also promoted differentiation of nonmutant cells in a minority of patients.
When the researchers compared samples taken at diagnosis and relapse, they did not find second-site mutations in IDH2 at relapse.
The team said relapse was the result of clonal evolution or selection of terminal or ancestral clones, which suggests there are multiple pathways that could potentially be targeted to restore differentiation arrest.
“We have provided genetic proof that enasidenib was able to differentiate cancer cells so that some of their normal functions were restored, even though they still contained the IDH2 mutation,” said Virginie Penard-Lacronique, of Gustave Roussy.
“This is important because, unless we can track these clones, we don’t know whether the mature cells in a patient are coming from normal cells after all the cancer cells have been killed or from leukemic cells that are now able to mature. In this paper, we show that, in 4 out of 5 cases, the mature cells are coming from leukemic bone marrow cells that can be induced to differentiate by this new drug.”
The researchers said these results suggest enasidenib must be combined with other drugs to prevent AML relapse.
“Now that we have shown that [enasidenib] needs to be combined with other drugs to stop the cancer returning, we think that it’s important that the combination therapy should be given to AML patients early on in their disease,” Dr de Botton said. “International trials are now taking place comparing combinations of enasidenib and other drugs with the normal standard of care.”
Mutations linked to higher risk of SNs in CCSs
New research has shown that childhood cancer survivors (CCSs) with certain germline mutations have higher relative rates (RRs) of secondary neoplasms (SNs) later in life.
Mutation carriers had significantly higher rates of breast cancer and sarcoma if they had received radiation to treat their initial cancer.
Among CCSs who did not receive radiation, the mutations were associated with increased rates of any SN, breast cancer, nonmelanoma skin cancer, and 2 or more histologically distinct SNs.
These findings were reported in the Journal of Clinical Oncology.
Researchers sequenced samples from 3006 CCSs who were at least 5 years from their initial cancer diagnosis as well as 341 samples from cancer-free control subjects.
All subjects were participants in the St. Jude Lifetime Cohort Study, a retrospective study with prospective clinical follow-up.
Thirty-five percent of the CCSs had survived leukemia, and 19% had survived lymphoma.
The CCS’s median age at childhood cancer diagnosis was 7.1 years, and the median follow-up was 28 years. The controls had a median age of 36.4 at follow-up.
Results
There were 1120 SNs diagnosed in 439 CCSs (14.6%). Ninety-one CCSs developed 2 or more histologically distinct SNs. The median time to SN diagnosis was 25.6 years
Non-melanoma skin cancer (580 in 159 CCSs), meningiomas (233 in 102 CCSs), thyroid cancer (67 in 67 CCSs), and breast cancer (60 in 53 CCSs) were among the SNs reported.
There were 15 neoplasms recorded in the control group—14 cases of non-melanoma skin cancer and 1 meningioma.
Pathogenic or likely pathogenic (P/LP) mutations in 32 genes were reported in 175 CCSs. The prevalence in CCSs (5.8%) was nearly 10-fold higher than in controls (0.6%).
The most commonly mutated genes in CCSs were RB1 (n=43), NF1 (n=22), BRCA2 (n=14), BRCA1 (n=12), and TP53 (n=10).
In a multivariable analysis adjusted for sex, age at primary cancer diagnosis, and treatment, P/LP mutation carriers had a significantly higher rate of any SN (RR=1.8).
The rate of subsequent breast cancer was significantly increased among females with a P/LP mutation (RR= 9.4), recipients of chest radiation (RR=7.9), and those with higher anthracycline exposure (RR=2.4).
The rate of subsequent sarcoma was significantly increased for mutation carriers (RR=10.9) and CCSs with greater exposure to alkylating agents (RR=3.8).
Among irradiated CCSs, P/LP mutations were associated with significantly increased rates of breast cancer (RR=13.9) and sarcoma (RR=10.6)
Among non-irradiated CCSs, P/LP mutations were associated with significantly increased rates of any SN (RR=4.7), breast cancer (RR=7.7), nonmelanoma skin cancer (RR=11.0), and 2 or more histologically distinct SNs (RR=18.6).
The researchers said the higher risk of SNs in CCSs with P/LP mutations suggests all CCSs should be referred for genetic counseling.
New research has shown that childhood cancer survivors (CCSs) with certain germline mutations have higher relative rates (RRs) of secondary neoplasms (SNs) later in life.
Mutation carriers had significantly higher rates of breast cancer and sarcoma if they had received radiation to treat their initial cancer.
Among CCSs who did not receive radiation, the mutations were associated with increased rates of any SN, breast cancer, nonmelanoma skin cancer, and 2 or more histologically distinct SNs.
These findings were reported in the Journal of Clinical Oncology.
Researchers sequenced samples from 3006 CCSs who were at least 5 years from their initial cancer diagnosis as well as 341 samples from cancer-free control subjects.
All subjects were participants in the St. Jude Lifetime Cohort Study, a retrospective study with prospective clinical follow-up.
Thirty-five percent of the CCSs had survived leukemia, and 19% had survived lymphoma.
The CCS’s median age at childhood cancer diagnosis was 7.1 years, and the median follow-up was 28 years. The controls had a median age of 36.4 at follow-up.
Results
There were 1120 SNs diagnosed in 439 CCSs (14.6%). Ninety-one CCSs developed 2 or more histologically distinct SNs. The median time to SN diagnosis was 25.6 years
Non-melanoma skin cancer (580 in 159 CCSs), meningiomas (233 in 102 CCSs), thyroid cancer (67 in 67 CCSs), and breast cancer (60 in 53 CCSs) were among the SNs reported.
There were 15 neoplasms recorded in the control group—14 cases of non-melanoma skin cancer and 1 meningioma.
Pathogenic or likely pathogenic (P/LP) mutations in 32 genes were reported in 175 CCSs. The prevalence in CCSs (5.8%) was nearly 10-fold higher than in controls (0.6%).
The most commonly mutated genes in CCSs were RB1 (n=43), NF1 (n=22), BRCA2 (n=14), BRCA1 (n=12), and TP53 (n=10).
In a multivariable analysis adjusted for sex, age at primary cancer diagnosis, and treatment, P/LP mutation carriers had a significantly higher rate of any SN (RR=1.8).
The rate of subsequent breast cancer was significantly increased among females with a P/LP mutation (RR= 9.4), recipients of chest radiation (RR=7.9), and those with higher anthracycline exposure (RR=2.4).
The rate of subsequent sarcoma was significantly increased for mutation carriers (RR=10.9) and CCSs with greater exposure to alkylating agents (RR=3.8).
Among irradiated CCSs, P/LP mutations were associated with significantly increased rates of breast cancer (RR=13.9) and sarcoma (RR=10.6)
Among non-irradiated CCSs, P/LP mutations were associated with significantly increased rates of any SN (RR=4.7), breast cancer (RR=7.7), nonmelanoma skin cancer (RR=11.0), and 2 or more histologically distinct SNs (RR=18.6).
The researchers said the higher risk of SNs in CCSs with P/LP mutations suggests all CCSs should be referred for genetic counseling.
New research has shown that childhood cancer survivors (CCSs) with certain germline mutations have higher relative rates (RRs) of secondary neoplasms (SNs) later in life.
Mutation carriers had significantly higher rates of breast cancer and sarcoma if they had received radiation to treat their initial cancer.
Among CCSs who did not receive radiation, the mutations were associated with increased rates of any SN, breast cancer, nonmelanoma skin cancer, and 2 or more histologically distinct SNs.
These findings were reported in the Journal of Clinical Oncology.
Researchers sequenced samples from 3006 CCSs who were at least 5 years from their initial cancer diagnosis as well as 341 samples from cancer-free control subjects.
All subjects were participants in the St. Jude Lifetime Cohort Study, a retrospective study with prospective clinical follow-up.
Thirty-five percent of the CCSs had survived leukemia, and 19% had survived lymphoma.
The CCS’s median age at childhood cancer diagnosis was 7.1 years, and the median follow-up was 28 years. The controls had a median age of 36.4 at follow-up.
Results
There were 1120 SNs diagnosed in 439 CCSs (14.6%). Ninety-one CCSs developed 2 or more histologically distinct SNs. The median time to SN diagnosis was 25.6 years
Non-melanoma skin cancer (580 in 159 CCSs), meningiomas (233 in 102 CCSs), thyroid cancer (67 in 67 CCSs), and breast cancer (60 in 53 CCSs) were among the SNs reported.
There were 15 neoplasms recorded in the control group—14 cases of non-melanoma skin cancer and 1 meningioma.
Pathogenic or likely pathogenic (P/LP) mutations in 32 genes were reported in 175 CCSs. The prevalence in CCSs (5.8%) was nearly 10-fold higher than in controls (0.6%).
The most commonly mutated genes in CCSs were RB1 (n=43), NF1 (n=22), BRCA2 (n=14), BRCA1 (n=12), and TP53 (n=10).
In a multivariable analysis adjusted for sex, age at primary cancer diagnosis, and treatment, P/LP mutation carriers had a significantly higher rate of any SN (RR=1.8).
The rate of subsequent breast cancer was significantly increased among females with a P/LP mutation (RR= 9.4), recipients of chest radiation (RR=7.9), and those with higher anthracycline exposure (RR=2.4).
The rate of subsequent sarcoma was significantly increased for mutation carriers (RR=10.9) and CCSs with greater exposure to alkylating agents (RR=3.8).
Among irradiated CCSs, P/LP mutations were associated with significantly increased rates of breast cancer (RR=13.9) and sarcoma (RR=10.6)
Among non-irradiated CCSs, P/LP mutations were associated with significantly increased rates of any SN (RR=4.7), breast cancer (RR=7.7), nonmelanoma skin cancer (RR=11.0), and 2 or more histologically distinct SNs (RR=18.6).
The researchers said the higher risk of SNs in CCSs with P/LP mutations suggests all CCSs should be referred for genetic counseling.
CAR T Therapy: From Bench to Bedside and Back
Release Date: July 15, 2018
Expiration Date: July 14, 2019
Note: This activity is no longer available for credit
Introductory Comments: (Duration: 9 minutes)
Aaron P. Rapoport, MD
Bone Marrow Transplant Program
University of Maryland School of Medicine
Baltimore, MD
Presentation: (Duration: 39 minutes)
Carl H. June, MD
Richard W. Vague Professor in Immunotherapy
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA
Provided by:

Learning Objectives
• Review clinical data and individual case studies to determine where CAR T-cell therapy might be appropriate in the treatment of adult and pediatric patients with leukemia, lymphoma, and multiple myeloma.
• Discuss the management of cytotoxicity of CAR T-cell therapy.
Target Audience
Hematologists, oncologists, and other members of the healthcare team who treat or manage patients with hematologic malignancies.
Statement of Need
It is critical that clinicians managing patients with acute leukemia and other hematologic malignancies are cognizant of exciting breakthroughs and are also able to integrate recent progress into practice. However, given the overwhelming influx of data, it is no surprise that many hematology professionals face difficulties in identifying the most relevant findings for clinical practice. Hematologists are unable to stay abreast of the latest evidence on investigational agents. Educational programs are thus crucial to address this important professional practice gap.
Faculty
Carl H. June, MD
Richard W. Vague Professor in Immunotherapy
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA
Disclosures: Consultant: Novartis; Grant/Research support and royalties/IPR: Novartis
Stockholder: Tmunity Therapeutics, Inc.
Aaron P. Rapoport, MD
Bone Marrow Transplant Program
University of Maryland School of Medicine
Baltimore, Maryland
Disclosures: No relevant financial relationships with a commercial supporter
Permissions
- Slide 3: Complex tumor, host and environmental factors govern the strength and timing of anti-cancer immune responses
- Reprinted from Immunity, Vol 39/No 1, Chen DS, Mellman I, Oncology meets immunology: the cancer-immunity cycle, pp 1-10, 2013, with permission from Elsevier
- Slide 9: Genes differentially expressed in CART19 cellular infusion products from CLL patients
- From Fraietta JA, Lacey SF, Orlando EJ, . . . June CH, Melenhorst JJ. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 2018; 24:563-571
- Slide 10: Characterization of CLL CAR T cells in NSG CLL model
- Same as slide 9
- Slide 15: First adult ALL patient
- Photos originally published in Kaiser Health News/Photo courtesy of Dr Keith Eaton. Available at: https://khn.org/news/cascade-of-costs-could-push-new-gene-therapy-above-1-million-per-patient/
- Slide 21: Efficient trafficking of CTL019 T Cells to CNS in ALL
- From N Engl J Med, Grupp SA, Kalos M, Barrett D, . . V. June CH, Chimeric antigen receptor-modified T cells for acute lymphoid leukemia, Volume No 368, pp 1509-1518. Copyright © 2013 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
- Slide 26: Long-term persistence and expression of CTL019 is associated with durable remission in leukemia: Predictive Biomarker
- From Porter DL, Hwang WT, Frey NV . . . June CH. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015; 7(303):303ra139. Reprinted with permission from AAAS.
- Slide 28: Rapid massive expansion of clonal CART cell population in patient #10
- Initially published in Fraietta JA, Nobles CL, Sammons MA, . . . June CH, Melenhors JJ. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature 2018; 558(7709):307-312
- Slide 29: Mapping CAR integration site in Pt #10
- Same as slide 28.
- Slide 31: Long-term stable persistence of TET2-deficient CAR T cells in Pt #10
- Same as slide 28
- Slide 32: Epigenetic and genetic changes uncovered by ATAC-seq in Pt #10
- Same as slide 28.
- Slide 33: TET2 knock down in healthy donor T cells
- Same as slide 28.
- Slide 34: TET2 knock down in healthy donor T cells
- Same as slide 28.
- Slide 36: CAR T for myeloma: BCMA
- From Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev 2011; 244(1):115-33. Reprinted with permission from John Wiley and Sons.
- Slide 38: CAR T for myeloma: Patient #1
- Photo originally published by UT Southwestern Medical Center. Available at: https://www.utsouthwestern.edu/newsroom/articles/year-2018/wright-car-t.html
- Slide 39: Autoimmunity is the “Achilles’ Heel” of immunotherapy
- First published in June CH, Warshauer JT, and Bluestone JA. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med 2017;23(5):540-7
- Slide 41: Multiplex CRISPR /Cas9 editing: Universal T cells TCR, HLA, PD-1, CTLA-4 and Fas
- From Ren J, Zhang X, Liu X, Fang C, Jiang S, June CH, Zhao Y. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget 2017; 8:17002-17011.
- Slide 45: CAR T-cell trials for cancer are now global
- From June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science 2018; 359:1361-1365. Reprinted with permission from AAAS.
Disclaimer
The content and views presented in this educational activity are those of the author and do not necessarily reflect those of Hemedicus or Frontline Medical Communications. This material is prepared based upon a review of multiple sources of information, but it is not exhaustive of the subject matter. Therefore, healthcare professionals and other individuals should review and consider other publications and materials on the subject matter before relying solely upon the information contained within this educational activity.
Release Date: July 15, 2018
Expiration Date: July 14, 2019
Note: This activity is no longer available for credit
Introductory Comments: (Duration: 9 minutes)
Aaron P. Rapoport, MD
Bone Marrow Transplant Program
University of Maryland School of Medicine
Baltimore, MD
Presentation: (Duration: 39 minutes)
Carl H. June, MD
Richard W. Vague Professor in Immunotherapy
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA
Provided by:

Learning Objectives
• Review clinical data and individual case studies to determine where CAR T-cell therapy might be appropriate in the treatment of adult and pediatric patients with leukemia, lymphoma, and multiple myeloma.
• Discuss the management of cytotoxicity of CAR T-cell therapy.
Target Audience
Hematologists, oncologists, and other members of the healthcare team who treat or manage patients with hematologic malignancies.
Statement of Need
It is critical that clinicians managing patients with acute leukemia and other hematologic malignancies are cognizant of exciting breakthroughs and are also able to integrate recent progress into practice. However, given the overwhelming influx of data, it is no surprise that many hematology professionals face difficulties in identifying the most relevant findings for clinical practice. Hematologists are unable to stay abreast of the latest evidence on investigational agents. Educational programs are thus crucial to address this important professional practice gap.
Faculty
Carl H. June, MD
Richard W. Vague Professor in Immunotherapy
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA
Disclosures: Consultant: Novartis; Grant/Research support and royalties/IPR: Novartis
Stockholder: Tmunity Therapeutics, Inc.
Aaron P. Rapoport, MD
Bone Marrow Transplant Program
University of Maryland School of Medicine
Baltimore, Maryland
Disclosures: No relevant financial relationships with a commercial supporter
Permissions
- Slide 3: Complex tumor, host and environmental factors govern the strength and timing of anti-cancer immune responses
- Reprinted from Immunity, Vol 39/No 1, Chen DS, Mellman I, Oncology meets immunology: the cancer-immunity cycle, pp 1-10, 2013, with permission from Elsevier
- Slide 9: Genes differentially expressed in CART19 cellular infusion products from CLL patients
- From Fraietta JA, Lacey SF, Orlando EJ, . . . June CH, Melenhorst JJ. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 2018; 24:563-571
- Slide 10: Characterization of CLL CAR T cells in NSG CLL model
- Same as slide 9
- Slide 15: First adult ALL patient
- Photos originally published in Kaiser Health News/Photo courtesy of Dr Keith Eaton. Available at: https://khn.org/news/cascade-of-costs-could-push-new-gene-therapy-above-1-million-per-patient/
- Slide 21: Efficient trafficking of CTL019 T Cells to CNS in ALL
- From N Engl J Med, Grupp SA, Kalos M, Barrett D, . . V. June CH, Chimeric antigen receptor-modified T cells for acute lymphoid leukemia, Volume No 368, pp 1509-1518. Copyright © 2013 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
- Slide 26: Long-term persistence and expression of CTL019 is associated with durable remission in leukemia: Predictive Biomarker
- From Porter DL, Hwang WT, Frey NV . . . June CH. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015; 7(303):303ra139. Reprinted with permission from AAAS.
- Slide 28: Rapid massive expansion of clonal CART cell population in patient #10
- Initially published in Fraietta JA, Nobles CL, Sammons MA, . . . June CH, Melenhors JJ. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature 2018; 558(7709):307-312
- Slide 29: Mapping CAR integration site in Pt #10
- Same as slide 28.
- Slide 31: Long-term stable persistence of TET2-deficient CAR T cells in Pt #10
- Same as slide 28
- Slide 32: Epigenetic and genetic changes uncovered by ATAC-seq in Pt #10
- Same as slide 28.
- Slide 33: TET2 knock down in healthy donor T cells
- Same as slide 28.
- Slide 34: TET2 knock down in healthy donor T cells
- Same as slide 28.
- Slide 36: CAR T for myeloma: BCMA
- From Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev 2011; 244(1):115-33. Reprinted with permission from John Wiley and Sons.
- Slide 38: CAR T for myeloma: Patient #1
- Photo originally published by UT Southwestern Medical Center. Available at: https://www.utsouthwestern.edu/newsroom/articles/year-2018/wright-car-t.html
- Slide 39: Autoimmunity is the “Achilles’ Heel” of immunotherapy
- First published in June CH, Warshauer JT, and Bluestone JA. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med 2017;23(5):540-7
- Slide 41: Multiplex CRISPR /Cas9 editing: Universal T cells TCR, HLA, PD-1, CTLA-4 and Fas
- From Ren J, Zhang X, Liu X, Fang C, Jiang S, June CH, Zhao Y. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget 2017; 8:17002-17011.
- Slide 45: CAR T-cell trials for cancer are now global
- From June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science 2018; 359:1361-1365. Reprinted with permission from AAAS.
Disclaimer
The content and views presented in this educational activity are those of the author and do not necessarily reflect those of Hemedicus or Frontline Medical Communications. This material is prepared based upon a review of multiple sources of information, but it is not exhaustive of the subject matter. Therefore, healthcare professionals and other individuals should review and consider other publications and materials on the subject matter before relying solely upon the information contained within this educational activity.
Release Date: July 15, 2018
Expiration Date: July 14, 2019
Note: This activity is no longer available for credit
Introductory Comments: (Duration: 9 minutes)
Aaron P. Rapoport, MD
Bone Marrow Transplant Program
University of Maryland School of Medicine
Baltimore, MD
Presentation: (Duration: 39 minutes)
Carl H. June, MD
Richard W. Vague Professor in Immunotherapy
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA
Provided by:

Learning Objectives
• Review clinical data and individual case studies to determine where CAR T-cell therapy might be appropriate in the treatment of adult and pediatric patients with leukemia, lymphoma, and multiple myeloma.
• Discuss the management of cytotoxicity of CAR T-cell therapy.
Target Audience
Hematologists, oncologists, and other members of the healthcare team who treat or manage patients with hematologic malignancies.
Statement of Need
It is critical that clinicians managing patients with acute leukemia and other hematologic malignancies are cognizant of exciting breakthroughs and are also able to integrate recent progress into practice. However, given the overwhelming influx of data, it is no surprise that many hematology professionals face difficulties in identifying the most relevant findings for clinical practice. Hematologists are unable to stay abreast of the latest evidence on investigational agents. Educational programs are thus crucial to address this important professional practice gap.
Faculty
Carl H. June, MD
Richard W. Vague Professor in Immunotherapy
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA
Disclosures: Consultant: Novartis; Grant/Research support and royalties/IPR: Novartis
Stockholder: Tmunity Therapeutics, Inc.
Aaron P. Rapoport, MD
Bone Marrow Transplant Program
University of Maryland School of Medicine
Baltimore, Maryland
Disclosures: No relevant financial relationships with a commercial supporter
Permissions
- Slide 3: Complex tumor, host and environmental factors govern the strength and timing of anti-cancer immune responses
- Reprinted from Immunity, Vol 39/No 1, Chen DS, Mellman I, Oncology meets immunology: the cancer-immunity cycle, pp 1-10, 2013, with permission from Elsevier
- Slide 9: Genes differentially expressed in CART19 cellular infusion products from CLL patients
- From Fraietta JA, Lacey SF, Orlando EJ, . . . June CH, Melenhorst JJ. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 2018; 24:563-571
- Slide 10: Characterization of CLL CAR T cells in NSG CLL model
- Same as slide 9
- Slide 15: First adult ALL patient
- Photos originally published in Kaiser Health News/Photo courtesy of Dr Keith Eaton. Available at: https://khn.org/news/cascade-of-costs-could-push-new-gene-therapy-above-1-million-per-patient/
- Slide 21: Efficient trafficking of CTL019 T Cells to CNS in ALL
- From N Engl J Med, Grupp SA, Kalos M, Barrett D, . . V. June CH, Chimeric antigen receptor-modified T cells for acute lymphoid leukemia, Volume No 368, pp 1509-1518. Copyright © 2013 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
- Slide 26: Long-term persistence and expression of CTL019 is associated with durable remission in leukemia: Predictive Biomarker
- From Porter DL, Hwang WT, Frey NV . . . June CH. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015; 7(303):303ra139. Reprinted with permission from AAAS.
- Slide 28: Rapid massive expansion of clonal CART cell population in patient #10
- Initially published in Fraietta JA, Nobles CL, Sammons MA, . . . June CH, Melenhors JJ. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature 2018; 558(7709):307-312
- Slide 29: Mapping CAR integration site in Pt #10
- Same as slide 28.
- Slide 31: Long-term stable persistence of TET2-deficient CAR T cells in Pt #10
- Same as slide 28
- Slide 32: Epigenetic and genetic changes uncovered by ATAC-seq in Pt #10
- Same as slide 28.
- Slide 33: TET2 knock down in healthy donor T cells
- Same as slide 28.
- Slide 34: TET2 knock down in healthy donor T cells
- Same as slide 28.
- Slide 36: CAR T for myeloma: BCMA
- From Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev 2011; 244(1):115-33. Reprinted with permission from John Wiley and Sons.
- Slide 38: CAR T for myeloma: Patient #1
- Photo originally published by UT Southwestern Medical Center. Available at: https://www.utsouthwestern.edu/newsroom/articles/year-2018/wright-car-t.html
- Slide 39: Autoimmunity is the “Achilles’ Heel” of immunotherapy
- First published in June CH, Warshauer JT, and Bluestone JA. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med 2017;23(5):540-7
- Slide 41: Multiplex CRISPR /Cas9 editing: Universal T cells TCR, HLA, PD-1, CTLA-4 and Fas
- From Ren J, Zhang X, Liu X, Fang C, Jiang S, June CH, Zhao Y. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget 2017; 8:17002-17011.
- Slide 45: CAR T-cell trials for cancer are now global
- From June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science 2018; 359:1361-1365. Reprinted with permission from AAAS.
Disclaimer
The content and views presented in this educational activity are those of the author and do not necessarily reflect those of Hemedicus or Frontline Medical Communications. This material is prepared based upon a review of multiple sources of information, but it is not exhaustive of the subject matter. Therefore, healthcare professionals and other individuals should review and consider other publications and materials on the subject matter before relying solely upon the information contained within this educational activity.