User login
First-of-its kind guideline on lipid monitoring in endocrine diseases
Endocrine diseases of any type – not just diabetes – can represent a cardiovascular risk and patients with those disorders should be screened for high cholesterol, according to a new clinical practice guideline from the Endocrine Society.
“The simple recommendation to check a lipid panel in patients with endocrine diseases and calculate cardiovascular risk may be practice changing because that is not done routinely,” Connie Newman, MD, chair of the Endocrine Society committee that developed the guideline, said in an interview.
“Usually the focus is on assessment and treatment of the endocrine disease, rather than on assessment and treatment of atherosclerotic cardiovascular disease risk,” said Newman, an adjunct professor of medicine in the department of medicine, division of endocrinology, diabetes & metabolism, at New York University.
Whereas diabetes, well-known for its increased cardiovascular risk profile, is commonly addressed in other cardiovascular and cholesterol practice management guidelines, the array of other endocrine diseases are not typically included.
“This guideline is the first of its kind,” Dr. Newman said. “The Endocrine Society has not previously issued a guideline on lipid management in endocrine disorders [and] other organizations have not written guidelines on this topic.
“Rather, guidelines have been written on cholesterol management, but these do not describe cholesterol management in patients with endocrine diseases such as thyroid disease [hypothyroidism and hyperthyroidism], Cushing’s syndrome, acromegaly, growth hormone deficiency, menopause, male hypogonadism, and obesity,” she noted.
But these conditions carry a host of cardiovascular risk factors that may require careful monitoring and management.
“Although endocrine hormones, such as thyroid hormone, cortisol, estrogen, testosterone, growth hormone, and insulin, affect pathways for lipid metabolism, physicians lack guidance on lipid abnormalities, cardiovascular risk, and treatment to reduce lipids and cardiovascular risk in patients with endocrine diseases,” she explained.
Vinaya Simha, MD, an internal medicine specialist at the Mayo Clinic in Rochester, Minn., agrees that the guideline is notable in addressing an unmet need.
Recommendations that stand out to Dr. Simha include the suggestion of adding eicosapentaenoic acid (EPA) ethyl ester to reduce the risk of cardiovascular disease in adults with diabetes or atherosclerotic cardiovascular disease who have elevated triglyceride levels despite statin treatment.
James L. Rosenzweig, MD, an endocrinologist at Hebrew SeniorLife in Boston, agreed that this is an important addition to an area that needs more guidance.
“Many of these clinical situations can exacerbate dyslipidemia and some also increase the cardiovascular risk to a greater extent in combination with elevated cholesterol and/or triglycerides,” he said in an interview.
“In many cases, treatment of the underlying disorder appropriately can have an important impact in resolving the lipid disorder. In others, more aggressive pharmacological treatment is indicated,” he said.
“I think that this will be a valuable resource, especially for endocrinologists, but it can be used as well by providers in other disciplines.”
Key recommendations for different endocrine conditions
The guideline, published in the Journal of Clinical Endocrinology & Metabolism, details those risks and provides evidence-based recommendations on their management and treatment.
Key recommendations include:
- Obtain a lipid panel and evaluate cardiovascular risk factors in all adults with endocrine disorders.
- In patients with and risk factors for cardiovascular disease, start statin therapy in addition to lifestyle modification to reduce cardiovascular risk. “This could mean earlier treatment because other guidelines recommend consideration of therapy at age 40,” Dr. Newman said.
- Statin therapy is also recommended for adults over 40 with with a duration of diabetes of more than 20 years and/or microvascular complications, regardless of their cardiovascular risk score. “This means earlier treatment of patients with type 1 diabetes with statins in order to reduce cardiovascular disease risk,” Dr. Newman noted.
- In patients with hyperlipidemia, rule out as the cause before treating with lipid-lowering medications. And among patients who are found to have hypothyroidism, reevaluate the lipid profile when the patient has thyroid hormone levels in the normal range.
- Adults with persistent endogenous Cushing’s syndrome should have their lipid profile monitored. Statin therapy should be considered in addition to lifestyle modifications, irrespective of the cardiovascular risk score.
- In postmenopausal women, high cholesterol or triglycerides should be treated with statins rather than hormone therapy.
- Evaluate and treat lipids and other cardiovascular risk factors in women who enter menopause early (before the age of 40-45 years).
Nice summary of ‘risk-enhancing’ endocrine disorders
Dr. Simha said in an interview that the new guideline is “probably the first comprehensive statement addressing lipid treatment in patients with a broad range of endocrine disorders besides diabetes.”
“Most of the treatment recommendations are congruent with other current guidelines such as the American College of Cardiology/American Heart Association [guidelines], but there is specific mention of which endocrine disorders represent enhanced cardiovascular risk,” she explained.
The new recommendations are notable for including “a nice summary of how different endocrine disorders affect lipid values, and also which endocrine disorders need to be considered as ‘risk-enhancing factors,’ ” Dr. Simha noted.
“The use of EPA in patients with hypertriglyceridemia is novel, compared to the ACC/AHA recommendation. This reflects new data which is now available,” she added.
The American Association of Clinical Endocrinologists also just issued a new algorithm on lipid management and prevention of cardiovascular disease in which treatment of hypertriglyceridemia is emphasized.
In addition, the new Endocrine Society guideline “also mentions an LDL [cholesterol] treatment threshold of 70 mg/dL, and 55 mg/dL in some patient categories, which previous guidelines have not,” Dr. Simha noted.
Overall, Dr. Newman added that the goal of the guideline is to increase awareness of key issues with endocrine diseases that may not necessarily be on clinicians’ radars.
“We hope that it will make a lipid panel and cardiovascular risk evaluation routine in adults with endocrine diseases and cause a greater focus on therapies to reduce heart disease and stroke,” she said.
Dr. Newman, Dr. Simha, and Dr. Rosenzweig reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Endocrine diseases of any type – not just diabetes – can represent a cardiovascular risk and patients with those disorders should be screened for high cholesterol, according to a new clinical practice guideline from the Endocrine Society.
“The simple recommendation to check a lipid panel in patients with endocrine diseases and calculate cardiovascular risk may be practice changing because that is not done routinely,” Connie Newman, MD, chair of the Endocrine Society committee that developed the guideline, said in an interview.
“Usually the focus is on assessment and treatment of the endocrine disease, rather than on assessment and treatment of atherosclerotic cardiovascular disease risk,” said Newman, an adjunct professor of medicine in the department of medicine, division of endocrinology, diabetes & metabolism, at New York University.
Whereas diabetes, well-known for its increased cardiovascular risk profile, is commonly addressed in other cardiovascular and cholesterol practice management guidelines, the array of other endocrine diseases are not typically included.
“This guideline is the first of its kind,” Dr. Newman said. “The Endocrine Society has not previously issued a guideline on lipid management in endocrine disorders [and] other organizations have not written guidelines on this topic.
“Rather, guidelines have been written on cholesterol management, but these do not describe cholesterol management in patients with endocrine diseases such as thyroid disease [hypothyroidism and hyperthyroidism], Cushing’s syndrome, acromegaly, growth hormone deficiency, menopause, male hypogonadism, and obesity,” she noted.
But these conditions carry a host of cardiovascular risk factors that may require careful monitoring and management.
“Although endocrine hormones, such as thyroid hormone, cortisol, estrogen, testosterone, growth hormone, and insulin, affect pathways for lipid metabolism, physicians lack guidance on lipid abnormalities, cardiovascular risk, and treatment to reduce lipids and cardiovascular risk in patients with endocrine diseases,” she explained.
Vinaya Simha, MD, an internal medicine specialist at the Mayo Clinic in Rochester, Minn., agrees that the guideline is notable in addressing an unmet need.
Recommendations that stand out to Dr. Simha include the suggestion of adding eicosapentaenoic acid (EPA) ethyl ester to reduce the risk of cardiovascular disease in adults with diabetes or atherosclerotic cardiovascular disease who have elevated triglyceride levels despite statin treatment.
James L. Rosenzweig, MD, an endocrinologist at Hebrew SeniorLife in Boston, agreed that this is an important addition to an area that needs more guidance.
“Many of these clinical situations can exacerbate dyslipidemia and some also increase the cardiovascular risk to a greater extent in combination with elevated cholesterol and/or triglycerides,” he said in an interview.
“In many cases, treatment of the underlying disorder appropriately can have an important impact in resolving the lipid disorder. In others, more aggressive pharmacological treatment is indicated,” he said.
“I think that this will be a valuable resource, especially for endocrinologists, but it can be used as well by providers in other disciplines.”
Key recommendations for different endocrine conditions
The guideline, published in the Journal of Clinical Endocrinology & Metabolism, details those risks and provides evidence-based recommendations on their management and treatment.
Key recommendations include:
- Obtain a lipid panel and evaluate cardiovascular risk factors in all adults with endocrine disorders.
- In patients with and risk factors for cardiovascular disease, start statin therapy in addition to lifestyle modification to reduce cardiovascular risk. “This could mean earlier treatment because other guidelines recommend consideration of therapy at age 40,” Dr. Newman said.
- Statin therapy is also recommended for adults over 40 with with a duration of diabetes of more than 20 years and/or microvascular complications, regardless of their cardiovascular risk score. “This means earlier treatment of patients with type 1 diabetes with statins in order to reduce cardiovascular disease risk,” Dr. Newman noted.
- In patients with hyperlipidemia, rule out as the cause before treating with lipid-lowering medications. And among patients who are found to have hypothyroidism, reevaluate the lipid profile when the patient has thyroid hormone levels in the normal range.
- Adults with persistent endogenous Cushing’s syndrome should have their lipid profile monitored. Statin therapy should be considered in addition to lifestyle modifications, irrespective of the cardiovascular risk score.
- In postmenopausal women, high cholesterol or triglycerides should be treated with statins rather than hormone therapy.
- Evaluate and treat lipids and other cardiovascular risk factors in women who enter menopause early (before the age of 40-45 years).
Nice summary of ‘risk-enhancing’ endocrine disorders
Dr. Simha said in an interview that the new guideline is “probably the first comprehensive statement addressing lipid treatment in patients with a broad range of endocrine disorders besides diabetes.”
“Most of the treatment recommendations are congruent with other current guidelines such as the American College of Cardiology/American Heart Association [guidelines], but there is specific mention of which endocrine disorders represent enhanced cardiovascular risk,” she explained.
The new recommendations are notable for including “a nice summary of how different endocrine disorders affect lipid values, and also which endocrine disorders need to be considered as ‘risk-enhancing factors,’ ” Dr. Simha noted.
“The use of EPA in patients with hypertriglyceridemia is novel, compared to the ACC/AHA recommendation. This reflects new data which is now available,” she added.
The American Association of Clinical Endocrinologists also just issued a new algorithm on lipid management and prevention of cardiovascular disease in which treatment of hypertriglyceridemia is emphasized.
In addition, the new Endocrine Society guideline “also mentions an LDL [cholesterol] treatment threshold of 70 mg/dL, and 55 mg/dL in some patient categories, which previous guidelines have not,” Dr. Simha noted.
Overall, Dr. Newman added that the goal of the guideline is to increase awareness of key issues with endocrine diseases that may not necessarily be on clinicians’ radars.
“We hope that it will make a lipid panel and cardiovascular risk evaluation routine in adults with endocrine diseases and cause a greater focus on therapies to reduce heart disease and stroke,” she said.
Dr. Newman, Dr. Simha, and Dr. Rosenzweig reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Endocrine diseases of any type – not just diabetes – can represent a cardiovascular risk and patients with those disorders should be screened for high cholesterol, according to a new clinical practice guideline from the Endocrine Society.
“The simple recommendation to check a lipid panel in patients with endocrine diseases and calculate cardiovascular risk may be practice changing because that is not done routinely,” Connie Newman, MD, chair of the Endocrine Society committee that developed the guideline, said in an interview.
“Usually the focus is on assessment and treatment of the endocrine disease, rather than on assessment and treatment of atherosclerotic cardiovascular disease risk,” said Newman, an adjunct professor of medicine in the department of medicine, division of endocrinology, diabetes & metabolism, at New York University.
Whereas diabetes, well-known for its increased cardiovascular risk profile, is commonly addressed in other cardiovascular and cholesterol practice management guidelines, the array of other endocrine diseases are not typically included.
“This guideline is the first of its kind,” Dr. Newman said. “The Endocrine Society has not previously issued a guideline on lipid management in endocrine disorders [and] other organizations have not written guidelines on this topic.
“Rather, guidelines have been written on cholesterol management, but these do not describe cholesterol management in patients with endocrine diseases such as thyroid disease [hypothyroidism and hyperthyroidism], Cushing’s syndrome, acromegaly, growth hormone deficiency, menopause, male hypogonadism, and obesity,” she noted.
But these conditions carry a host of cardiovascular risk factors that may require careful monitoring and management.
“Although endocrine hormones, such as thyroid hormone, cortisol, estrogen, testosterone, growth hormone, and insulin, affect pathways for lipid metabolism, physicians lack guidance on lipid abnormalities, cardiovascular risk, and treatment to reduce lipids and cardiovascular risk in patients with endocrine diseases,” she explained.
Vinaya Simha, MD, an internal medicine specialist at the Mayo Clinic in Rochester, Minn., agrees that the guideline is notable in addressing an unmet need.
Recommendations that stand out to Dr. Simha include the suggestion of adding eicosapentaenoic acid (EPA) ethyl ester to reduce the risk of cardiovascular disease in adults with diabetes or atherosclerotic cardiovascular disease who have elevated triglyceride levels despite statin treatment.
James L. Rosenzweig, MD, an endocrinologist at Hebrew SeniorLife in Boston, agreed that this is an important addition to an area that needs more guidance.
“Many of these clinical situations can exacerbate dyslipidemia and some also increase the cardiovascular risk to a greater extent in combination with elevated cholesterol and/or triglycerides,” he said in an interview.
“In many cases, treatment of the underlying disorder appropriately can have an important impact in resolving the lipid disorder. In others, more aggressive pharmacological treatment is indicated,” he said.
“I think that this will be a valuable resource, especially for endocrinologists, but it can be used as well by providers in other disciplines.”
Key recommendations for different endocrine conditions
The guideline, published in the Journal of Clinical Endocrinology & Metabolism, details those risks and provides evidence-based recommendations on their management and treatment.
Key recommendations include:
- Obtain a lipid panel and evaluate cardiovascular risk factors in all adults with endocrine disorders.
- In patients with and risk factors for cardiovascular disease, start statin therapy in addition to lifestyle modification to reduce cardiovascular risk. “This could mean earlier treatment because other guidelines recommend consideration of therapy at age 40,” Dr. Newman said.
- Statin therapy is also recommended for adults over 40 with with a duration of diabetes of more than 20 years and/or microvascular complications, regardless of their cardiovascular risk score. “This means earlier treatment of patients with type 1 diabetes with statins in order to reduce cardiovascular disease risk,” Dr. Newman noted.
- In patients with hyperlipidemia, rule out as the cause before treating with lipid-lowering medications. And among patients who are found to have hypothyroidism, reevaluate the lipid profile when the patient has thyroid hormone levels in the normal range.
- Adults with persistent endogenous Cushing’s syndrome should have their lipid profile monitored. Statin therapy should be considered in addition to lifestyle modifications, irrespective of the cardiovascular risk score.
- In postmenopausal women, high cholesterol or triglycerides should be treated with statins rather than hormone therapy.
- Evaluate and treat lipids and other cardiovascular risk factors in women who enter menopause early (before the age of 40-45 years).
Nice summary of ‘risk-enhancing’ endocrine disorders
Dr. Simha said in an interview that the new guideline is “probably the first comprehensive statement addressing lipid treatment in patients with a broad range of endocrine disorders besides diabetes.”
“Most of the treatment recommendations are congruent with other current guidelines such as the American College of Cardiology/American Heart Association [guidelines], but there is specific mention of which endocrine disorders represent enhanced cardiovascular risk,” she explained.
The new recommendations are notable for including “a nice summary of how different endocrine disorders affect lipid values, and also which endocrine disorders need to be considered as ‘risk-enhancing factors,’ ” Dr. Simha noted.
“The use of EPA in patients with hypertriglyceridemia is novel, compared to the ACC/AHA recommendation. This reflects new data which is now available,” she added.
The American Association of Clinical Endocrinologists also just issued a new algorithm on lipid management and prevention of cardiovascular disease in which treatment of hypertriglyceridemia is emphasized.
In addition, the new Endocrine Society guideline “also mentions an LDL [cholesterol] treatment threshold of 70 mg/dL, and 55 mg/dL in some patient categories, which previous guidelines have not,” Dr. Simha noted.
Overall, Dr. Newman added that the goal of the guideline is to increase awareness of key issues with endocrine diseases that may not necessarily be on clinicians’ radars.
“We hope that it will make a lipid panel and cardiovascular risk evaluation routine in adults with endocrine diseases and cause a greater focus on therapies to reduce heart disease and stroke,” she said.
Dr. Newman, Dr. Simha, and Dr. Rosenzweig reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
New return-to-play recommendations for athletes with COVID-19
The latest recommendations from sports cardiologists on getting athletes with COVID-19 back on the playing field safely emphasize a more judicious approach to screening for cardiac injury.
The new recommendations, made by the American College of Cardiology’s Sports and Exercise Cardiology Section, are for adult athletes in competitive sports and also for two important groups: younger athletes taking part in competitive high school sports and older athletes aged 35 and older, the Masters athletes, who continue to be active throughout their lives. The document was published online in JAMA Cardiology.
Because of the evolving nature of knowledge about COVID-19, updates on recommendations for safe return to play for athletes of all ages will continue to be made, senior author Aaron L. Baggish, MD, director of the cardiovascular performance program at Massachusetts General Hospital, Boston, said.
“The recommendations we released in May were entirely based on our experience taking care of hospitalized patients with COVID-19; we had no athletes in this population. We used a lot of conservative guesswork around how this would apply to otherwise healthy athletes,” Dr. Baggish said in an interview.
“But as sports started to open up, and we started to see large numbers of first professional and then college athletes come back into training, we realized that we needed to stop and ask whether the recommendations we put forward back in May were still appropriate,” Dr. Baggish said.
“Once we started to actually get into the trenches with these athletes, literally hundreds of them, and applying the testing strategies that we had initially recommended in everybody, we realized that we probably had some room for improvement, and that’s why we reconvened, to make these revisions,” he said.
Essentially, the recommendations now urge less cardiac testing. “Cardiac injury is not as common as we may have originally thought,” said Dr. Baggish.
“In the early days of COVID, people who were hospitalized had evidence of heart injury, and so we wondered if that prevalence would also be applicable to otherwise young, healthy people who got COVID. If that had been the case, we would have been in big trouble with respect to getting people back into sports. So this is why we started with a conservative screening approach and a lot of testing in order to not miss a huge burden of disease,” he said.
“But what we’ve learned over the past few months is that young people who get either asymptomatic or mild infection appear to have very, very low risk of having associated heart injury, so the need for testing in that population, when people who have infections recover fully, is almost certainly not going to be high yield,” Dr. Baggish said.
First iteration of the recommendations
Published in May in the early weeks of the pandemic, the first recommendations for safe return to play said that all athletes should stop training for at least 2 weeks after their symptoms resolve, then undergo “careful, clinical cardiovascular evaluation in combination with cardiac biomarkers and imaging.”
Additional testing with cardiac MRI, exercise testing, or ambulatory rhythm monitoring was to be done “based on the clinical course and initial testing.”
But experts caution that monitoring on such a scale in everyone is unnecessary and could even be counterproductive.
“Sending young athletes for extensive testing is not warranted and could send them to unnecessary testing, cardiac imaging, and so on,” Dr. Baggish said.
Only those athletes who continue to have symptoms or whose symptoms return when they get back to their athletic activities should go on for more screening.
“There, in essence, is the single main change from May, and that is a move away from screening with testing everyone, [and instead] confining that to the people who had moderate or greater severity disease,” he said.
Both iterations of the recommendations end with the same message.
“We are at the beginning of our knowledge about the cardiotoxic effects of COVID-19 but we are gathering evidence every day,” said Dr. Baggish. “Just as they did earlier, we acknowledge that our approaches are subject to change when we learn more about how COVID affects the heart, and specifically the hearts of athletes. This will be an ongoing process.”
Something to lean on
The recommendations are welcome, said James E. Udelson, MD, chief of the division of cardiology at Tufts Medical Center, Boston, coauthor of an accompanying editorial.
“It was a bit of the wild west out there, because each university, each college, all with good intentions, had been all struggling to figure out what to do, and how much to do. Probably the most important message from this new paper is the fact that now there is something out there that all coaches, athletes, families, schools, trainers can get some guidance from,” Dr. Udelson said in an interview.
Refining the cardiac screening criteria was a necessary step, Dr. Udelson said.
“How much cardiac imaging do you do? That is a matter of controversy,” said Dr. Udelson, who coauthored the commentary with Tufts cardiologist Ethan Rowin, MD, and Michael A. Curtis, MEd, a certified strength and conditioning specialist at the University of Virginia, Charlottesville. “The problem is that if you use a very sensitive imaging test on a lot of people, sometimes you find things that you really didn’t need to know about. They’re really not important. And now, the athlete is told he or she cannot play for 3 months because they might have myocarditis.
“Should we be too sensitive, meaning do we want to pick up anything no matter whether it’s important or not?” he added. “There will be a lot of false positives, and we are going to disqualify a lot of people. Or do you tune it a different way?”
Dr. Udelson said he would like to see commercial sports donate money to support research into the potential cardiotoxicity of COVID-19.
“If the organizations that benefit from these athletes, like the National Collegiate Athletic Association and professional sports leagues, can fund some of this research, that would be a huge help,” Dr. Udelson said.
“These are the top sports cardiologists in the country, and they have to start somewhere, and these are all based on what we know right now, as well as their own extensive experience. We all know that we are just at the beginning of our knowledge of this. But we have to have something to guide this huge community out there that is really thirsty for help.”
Dr. Baggish reports receiving research funding for the study of athletes in competitive sports from the National Heart, Lung, and Blood Institute; the National Football League Players Association; and the American Heart Association and receiving compensation for his role as team cardiologist from the US Olympic Committee/US Olympic Training Centers, US Soccer, US Rowing, the New England Patriots, the Boston Bruins, the New England Revolution, and Harvard University. Dr. Udelson has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The latest recommendations from sports cardiologists on getting athletes with COVID-19 back on the playing field safely emphasize a more judicious approach to screening for cardiac injury.
The new recommendations, made by the American College of Cardiology’s Sports and Exercise Cardiology Section, are for adult athletes in competitive sports and also for two important groups: younger athletes taking part in competitive high school sports and older athletes aged 35 and older, the Masters athletes, who continue to be active throughout their lives. The document was published online in JAMA Cardiology.
Because of the evolving nature of knowledge about COVID-19, updates on recommendations for safe return to play for athletes of all ages will continue to be made, senior author Aaron L. Baggish, MD, director of the cardiovascular performance program at Massachusetts General Hospital, Boston, said.
“The recommendations we released in May were entirely based on our experience taking care of hospitalized patients with COVID-19; we had no athletes in this population. We used a lot of conservative guesswork around how this would apply to otherwise healthy athletes,” Dr. Baggish said in an interview.
“But as sports started to open up, and we started to see large numbers of first professional and then college athletes come back into training, we realized that we needed to stop and ask whether the recommendations we put forward back in May were still appropriate,” Dr. Baggish said.
“Once we started to actually get into the trenches with these athletes, literally hundreds of them, and applying the testing strategies that we had initially recommended in everybody, we realized that we probably had some room for improvement, and that’s why we reconvened, to make these revisions,” he said.
Essentially, the recommendations now urge less cardiac testing. “Cardiac injury is not as common as we may have originally thought,” said Dr. Baggish.
“In the early days of COVID, people who were hospitalized had evidence of heart injury, and so we wondered if that prevalence would also be applicable to otherwise young, healthy people who got COVID. If that had been the case, we would have been in big trouble with respect to getting people back into sports. So this is why we started with a conservative screening approach and a lot of testing in order to not miss a huge burden of disease,” he said.
“But what we’ve learned over the past few months is that young people who get either asymptomatic or mild infection appear to have very, very low risk of having associated heart injury, so the need for testing in that population, when people who have infections recover fully, is almost certainly not going to be high yield,” Dr. Baggish said.
First iteration of the recommendations
Published in May in the early weeks of the pandemic, the first recommendations for safe return to play said that all athletes should stop training for at least 2 weeks after their symptoms resolve, then undergo “careful, clinical cardiovascular evaluation in combination with cardiac biomarkers and imaging.”
Additional testing with cardiac MRI, exercise testing, or ambulatory rhythm monitoring was to be done “based on the clinical course and initial testing.”
But experts caution that monitoring on such a scale in everyone is unnecessary and could even be counterproductive.
“Sending young athletes for extensive testing is not warranted and could send them to unnecessary testing, cardiac imaging, and so on,” Dr. Baggish said.
Only those athletes who continue to have symptoms or whose symptoms return when they get back to their athletic activities should go on for more screening.
“There, in essence, is the single main change from May, and that is a move away from screening with testing everyone, [and instead] confining that to the people who had moderate or greater severity disease,” he said.
Both iterations of the recommendations end with the same message.
“We are at the beginning of our knowledge about the cardiotoxic effects of COVID-19 but we are gathering evidence every day,” said Dr. Baggish. “Just as they did earlier, we acknowledge that our approaches are subject to change when we learn more about how COVID affects the heart, and specifically the hearts of athletes. This will be an ongoing process.”
Something to lean on
The recommendations are welcome, said James E. Udelson, MD, chief of the division of cardiology at Tufts Medical Center, Boston, coauthor of an accompanying editorial.
“It was a bit of the wild west out there, because each university, each college, all with good intentions, had been all struggling to figure out what to do, and how much to do. Probably the most important message from this new paper is the fact that now there is something out there that all coaches, athletes, families, schools, trainers can get some guidance from,” Dr. Udelson said in an interview.
Refining the cardiac screening criteria was a necessary step, Dr. Udelson said.
“How much cardiac imaging do you do? That is a matter of controversy,” said Dr. Udelson, who coauthored the commentary with Tufts cardiologist Ethan Rowin, MD, and Michael A. Curtis, MEd, a certified strength and conditioning specialist at the University of Virginia, Charlottesville. “The problem is that if you use a very sensitive imaging test on a lot of people, sometimes you find things that you really didn’t need to know about. They’re really not important. And now, the athlete is told he or she cannot play for 3 months because they might have myocarditis.
“Should we be too sensitive, meaning do we want to pick up anything no matter whether it’s important or not?” he added. “There will be a lot of false positives, and we are going to disqualify a lot of people. Or do you tune it a different way?”
Dr. Udelson said he would like to see commercial sports donate money to support research into the potential cardiotoxicity of COVID-19.
“If the organizations that benefit from these athletes, like the National Collegiate Athletic Association and professional sports leagues, can fund some of this research, that would be a huge help,” Dr. Udelson said.
“These are the top sports cardiologists in the country, and they have to start somewhere, and these are all based on what we know right now, as well as their own extensive experience. We all know that we are just at the beginning of our knowledge of this. But we have to have something to guide this huge community out there that is really thirsty for help.”
Dr. Baggish reports receiving research funding for the study of athletes in competitive sports from the National Heart, Lung, and Blood Institute; the National Football League Players Association; and the American Heart Association and receiving compensation for his role as team cardiologist from the US Olympic Committee/US Olympic Training Centers, US Soccer, US Rowing, the New England Patriots, the Boston Bruins, the New England Revolution, and Harvard University. Dr. Udelson has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The latest recommendations from sports cardiologists on getting athletes with COVID-19 back on the playing field safely emphasize a more judicious approach to screening for cardiac injury.
The new recommendations, made by the American College of Cardiology’s Sports and Exercise Cardiology Section, are for adult athletes in competitive sports and also for two important groups: younger athletes taking part in competitive high school sports and older athletes aged 35 and older, the Masters athletes, who continue to be active throughout their lives. The document was published online in JAMA Cardiology.
Because of the evolving nature of knowledge about COVID-19, updates on recommendations for safe return to play for athletes of all ages will continue to be made, senior author Aaron L. Baggish, MD, director of the cardiovascular performance program at Massachusetts General Hospital, Boston, said.
“The recommendations we released in May were entirely based on our experience taking care of hospitalized patients with COVID-19; we had no athletes in this population. We used a lot of conservative guesswork around how this would apply to otherwise healthy athletes,” Dr. Baggish said in an interview.
“But as sports started to open up, and we started to see large numbers of first professional and then college athletes come back into training, we realized that we needed to stop and ask whether the recommendations we put forward back in May were still appropriate,” Dr. Baggish said.
“Once we started to actually get into the trenches with these athletes, literally hundreds of them, and applying the testing strategies that we had initially recommended in everybody, we realized that we probably had some room for improvement, and that’s why we reconvened, to make these revisions,” he said.
Essentially, the recommendations now urge less cardiac testing. “Cardiac injury is not as common as we may have originally thought,” said Dr. Baggish.
“In the early days of COVID, people who were hospitalized had evidence of heart injury, and so we wondered if that prevalence would also be applicable to otherwise young, healthy people who got COVID. If that had been the case, we would have been in big trouble with respect to getting people back into sports. So this is why we started with a conservative screening approach and a lot of testing in order to not miss a huge burden of disease,” he said.
“But what we’ve learned over the past few months is that young people who get either asymptomatic or mild infection appear to have very, very low risk of having associated heart injury, so the need for testing in that population, when people who have infections recover fully, is almost certainly not going to be high yield,” Dr. Baggish said.
First iteration of the recommendations
Published in May in the early weeks of the pandemic, the first recommendations for safe return to play said that all athletes should stop training for at least 2 weeks after their symptoms resolve, then undergo “careful, clinical cardiovascular evaluation in combination with cardiac biomarkers and imaging.”
Additional testing with cardiac MRI, exercise testing, or ambulatory rhythm monitoring was to be done “based on the clinical course and initial testing.”
But experts caution that monitoring on such a scale in everyone is unnecessary and could even be counterproductive.
“Sending young athletes for extensive testing is not warranted and could send them to unnecessary testing, cardiac imaging, and so on,” Dr. Baggish said.
Only those athletes who continue to have symptoms or whose symptoms return when they get back to their athletic activities should go on for more screening.
“There, in essence, is the single main change from May, and that is a move away from screening with testing everyone, [and instead] confining that to the people who had moderate or greater severity disease,” he said.
Both iterations of the recommendations end with the same message.
“We are at the beginning of our knowledge about the cardiotoxic effects of COVID-19 but we are gathering evidence every day,” said Dr. Baggish. “Just as they did earlier, we acknowledge that our approaches are subject to change when we learn more about how COVID affects the heart, and specifically the hearts of athletes. This will be an ongoing process.”
Something to lean on
The recommendations are welcome, said James E. Udelson, MD, chief of the division of cardiology at Tufts Medical Center, Boston, coauthor of an accompanying editorial.
“It was a bit of the wild west out there, because each university, each college, all with good intentions, had been all struggling to figure out what to do, and how much to do. Probably the most important message from this new paper is the fact that now there is something out there that all coaches, athletes, families, schools, trainers can get some guidance from,” Dr. Udelson said in an interview.
Refining the cardiac screening criteria was a necessary step, Dr. Udelson said.
“How much cardiac imaging do you do? That is a matter of controversy,” said Dr. Udelson, who coauthored the commentary with Tufts cardiologist Ethan Rowin, MD, and Michael A. Curtis, MEd, a certified strength and conditioning specialist at the University of Virginia, Charlottesville. “The problem is that if you use a very sensitive imaging test on a lot of people, sometimes you find things that you really didn’t need to know about. They’re really not important. And now, the athlete is told he or she cannot play for 3 months because they might have myocarditis.
“Should we be too sensitive, meaning do we want to pick up anything no matter whether it’s important or not?” he added. “There will be a lot of false positives, and we are going to disqualify a lot of people. Or do you tune it a different way?”
Dr. Udelson said he would like to see commercial sports donate money to support research into the potential cardiotoxicity of COVID-19.
“If the organizations that benefit from these athletes, like the National Collegiate Athletic Association and professional sports leagues, can fund some of this research, that would be a huge help,” Dr. Udelson said.
“These are the top sports cardiologists in the country, and they have to start somewhere, and these are all based on what we know right now, as well as their own extensive experience. We all know that we are just at the beginning of our knowledge of this. But we have to have something to guide this huge community out there that is really thirsty for help.”
Dr. Baggish reports receiving research funding for the study of athletes in competitive sports from the National Heart, Lung, and Blood Institute; the National Football League Players Association; and the American Heart Association and receiving compensation for his role as team cardiologist from the US Olympic Committee/US Olympic Training Centers, US Soccer, US Rowing, the New England Patriots, the Boston Bruins, the New England Revolution, and Harvard University. Dr. Udelson has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
AACE issues ‘cookbook’ algorithm to manage dyslipidemia
A new algorithm on lipid management and prevention of cardiovascular disease from the American Association of Clinical Endocrinologists* (AACE) and the American College of Endocrinology (ACE) is “a nice cookbook” that many clinicians, especially those who are not lipid experts, will find useful, according to writing committee chair Yehuda Handelsman, MD.
The algorithm, published Oct. 10 in Endocrine Practice as 10 slides, or as part of a more detailed consensus statement, is a companion to the 2017 AACE/ACE guidelines for lipid management and includes more recent information about new therapies.
“What we’re trying to do here is to say, ‘focus on LDL-C, triglycerides, high-risk patients, and lifestyle. Understand all the medications available to you to reduce LDL-C and reduce triglycerides,’ ” Dr. Handelsman, of the Metabolic Institute of America, Tarzana, Calif., explained in an interview.
“We touch on lipoprotein(a), which we still don’t have medication for, but it identifies people at high risk, and we need that.”
Clinicians also need to know “that we’ve got some newer drugs in the market that can manage people who have statin intolerance,” Dr. Handelsman added.
“We introduced new therapies like icosapent ethyl” (Vascepa, Amarin) for hypertriglyceridemia, “when to use it, and how to use it. Even though it was not part of the 2017 guideline, we gave recommendations based on current data in the algorithm.”
Although there is no good evidence that lowering triglycerides reduces heart disease, he continued, many experts believe that the target triglyceride level should be less than 150 mg/dL, and the algorithm explains how to treat to this goal.
“Last, and most importantly, I cannot fail to underscore the fact that lifestyle is very important,” he emphasized.
Robert H. Eckel, MD, of the University of Colorado at Denver, Aurora, and president of medicine and science at the American Diabetes Association, who was not involved with this algorithm, said in an interview that the algorithm is important since it offers “the clinician or health care practitioner an approach, a kind of a cookbook or application of the guidelines, for how to manage lipid disorders in patients at risk ... It’s geared for the nonexperts too,” he said.
Dyslipidemia treatment summarized in 10 slides
The AACE/ACE algorithm comprises 10 slides, one each for dyslipidemic states, secondary causes of lipid disorders, screening for and assessing lipid disorders and atherosclerotic CVD (ASCVD) risk, ASCVD risk categories and treatment goals, lifestyle recommendations, treating LDL-C to goal, managing statin intolerance and safety, management of hypertriglyceridemia and the role of icosapent ethyl, assessment and management of elevated lipoprotein(a), and profiles of medications for dyslipidemia.
The algorithm defines five ASCVD risk categories and recommends increasingly lower LDL-C, non–HDL-C, and apo B target levels with increasing risk, but the same triglyceride target for all.
First, “treatment of lipid disorders begins with lifestyle therapy to improve nutrition, physical activity, weight, and other factors that affect lipids,” the consensus statement authors stress.
Next, “LDL-C has been, and remains, the main focus of efforts to improve lipid profiles in individuals at risk for ASCVD” (see table).
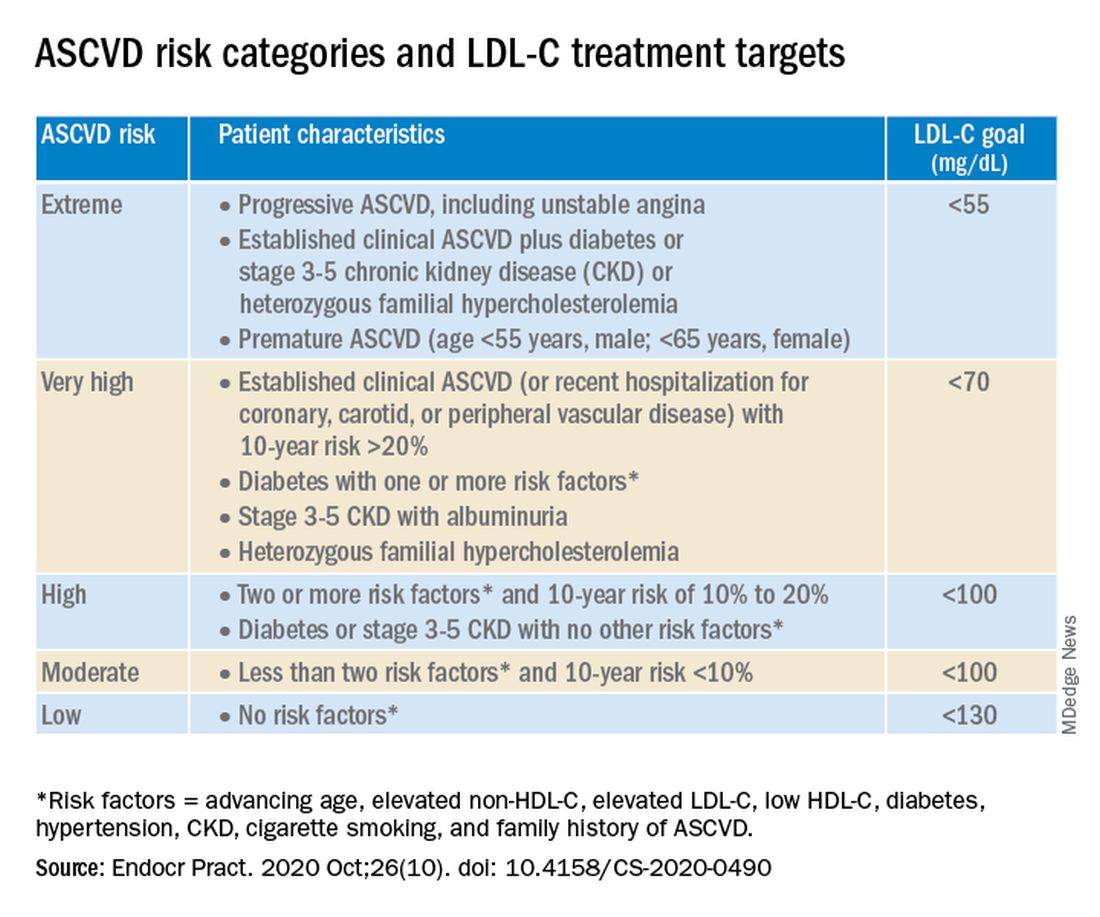
“We stratify [LDL-C] not as a one-treatment-target-for-all,” but rather as extreme, very high, high, moderate, and low ASCVD risk, Dr. Handelsman explained, with different treatment pathways (specified in another slide) to reach different risk-dependent goals.
“Unlike the ACC [American College of Cardiology] guideline, which shows if you want to further reduce LDL after statin give ezetimibe first, we say ‘no’,” he noted. “If somebody has an extreme risk, and you don’t think ezetimibe will get to a goal below 55 mg/dL, you should go first with a PCSK9 [proprotein convertase subtilisin/kexin type 9] inhibitor, and only then add ezetimibe or [colesevelam] or other drugs,” he said.
The consensus statement authors expand on this scenario. “Treatment for patients at extreme risk should begin with lifestyle therapy plus a high-intensity statin (atorvastatin 40 to 80 mg or rosuvastatin 20 to 40 mg, or the highest tolerated statin dose) to achieve an LDL-C goal of less than 55 mg/dL.”
“If LDL-C remains above goal after 3 months,” a PCSK9 inhibitor (evolocumab [Repatha, Amgen] or alirocumab [Praluent, Sanofi/Regeneron]), the cholesterol absorption inhibitor ezetimibe, or the bile acid sequestrant colesevelam (Welchol, Daiichi Sankyo) or the adenosine triphosphate-citrate lyase (ACL) inhibitor bempedoic acid (Nexletol, Esperion) “should be added, depending on required LDL-C lowering, and a third agent should be added if the combination fails to achieve the goal.”
However, “because the cost of ezetimibe is low, it may be preferred over PCSK9 inhibitors as second-line therapy to achieve an LDL-C below 70 mg/dL for patients who require no more than 15%-20% further reduction to reach goals.”
For patients at moderate or high risk, lipid management should begin with a moderate-intensity statin and be increased to a high-intensity statin before adding a second lipid-lowering medication to reach an LDL-C below 100 mg/dL.
According to the consensus statement, the desirable goal for triglycerides is less than 150 mg/dL.
In all patients with triglyceride levels of at least 500 mg/dL, statin therapy should be combined with a fibrate, prescription-grade omega-3 fatty acid, and/or niacin to reduce triglycerides.
In any patient with established ASCVD or diabetes with at least 2 ASCVD risk factors and triglycerides of 135-499 mg/dL, icosapent ethyl should be added to a statin to prevent ASCVD events.
Statement aligns with major guidelines
In general, the 2017 AACE/ACE guidelines and algorithm are “pretty similar” to other guidelines such as the 2018 ACC/American Heart Association (AHA) guidelines for cholesterol management, the 2019 ACC/AHA guidelines for primary prevention of CVD, and the 2019 European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) guidelines for the management of dyslipidemia, according to Dr. Eckel.
They have “all have now taken into consideration the evidence behind PCSK9 inhibitors,” he noted. “That’s important because those drugs have proven to be effective.”
Two differences, he pointed out, are that the 2019 ESC/EAS guidelines suggest that lipoprotein(a) measurement be considered at least once in every adult’s lifetime, and they recommend apo B analysis in people with high triglycerides but normal LDL (or no higher than 100 mg/dL), to identify additional risk.
*AACE changes its name, broadens focus
Shortly after its algorithm was published, AACE announced that it has a new organization name and brand, the American Association of Clinical Endocrinology, which “more clearly defines AACE as a community of individuals who work together to elevate the practice of clinical endocrinology,” according to an Oct. 20 statement.
The change is meant to acknowledge AACE’s “more modern, inclusive approach to endocrinology that supports multidisciplinary care teams – with endocrinologists leading the way.”
Along with the name change is a new global website. The statement notes that “health care professionals and community members can access all of the valuable clinical content such as guidelines, disease state networks and important education by visiting the pro portal in the top right corner of the site, or by going directly to pro.aace.com.”
Dr. Handelsman discloses that he receives research grant support from Amgen, Applied Therapeutics, AstraZeneca, BMS, Gan & Lee, Novo Nordisk, and Sanofi, and he is a consultant and/or speaker for Amarin, BI-Lilly, and Sanofi.
Dr. Eckel has received consultant/advisory board fees from Kowa, Novo Nordisk, and Provention Bio.
A new algorithm on lipid management and prevention of cardiovascular disease from the American Association of Clinical Endocrinologists* (AACE) and the American College of Endocrinology (ACE) is “a nice cookbook” that many clinicians, especially those who are not lipid experts, will find useful, according to writing committee chair Yehuda Handelsman, MD.
The algorithm, published Oct. 10 in Endocrine Practice as 10 slides, or as part of a more detailed consensus statement, is a companion to the 2017 AACE/ACE guidelines for lipid management and includes more recent information about new therapies.
“What we’re trying to do here is to say, ‘focus on LDL-C, triglycerides, high-risk patients, and lifestyle. Understand all the medications available to you to reduce LDL-C and reduce triglycerides,’ ” Dr. Handelsman, of the Metabolic Institute of America, Tarzana, Calif., explained in an interview.
“We touch on lipoprotein(a), which we still don’t have medication for, but it identifies people at high risk, and we need that.”
Clinicians also need to know “that we’ve got some newer drugs in the market that can manage people who have statin intolerance,” Dr. Handelsman added.
“We introduced new therapies like icosapent ethyl” (Vascepa, Amarin) for hypertriglyceridemia, “when to use it, and how to use it. Even though it was not part of the 2017 guideline, we gave recommendations based on current data in the algorithm.”
Although there is no good evidence that lowering triglycerides reduces heart disease, he continued, many experts believe that the target triglyceride level should be less than 150 mg/dL, and the algorithm explains how to treat to this goal.
“Last, and most importantly, I cannot fail to underscore the fact that lifestyle is very important,” he emphasized.
Robert H. Eckel, MD, of the University of Colorado at Denver, Aurora, and president of medicine and science at the American Diabetes Association, who was not involved with this algorithm, said in an interview that the algorithm is important since it offers “the clinician or health care practitioner an approach, a kind of a cookbook or application of the guidelines, for how to manage lipid disorders in patients at risk ... It’s geared for the nonexperts too,” he said.
Dyslipidemia treatment summarized in 10 slides
The AACE/ACE algorithm comprises 10 slides, one each for dyslipidemic states, secondary causes of lipid disorders, screening for and assessing lipid disorders and atherosclerotic CVD (ASCVD) risk, ASCVD risk categories and treatment goals, lifestyle recommendations, treating LDL-C to goal, managing statin intolerance and safety, management of hypertriglyceridemia and the role of icosapent ethyl, assessment and management of elevated lipoprotein(a), and profiles of medications for dyslipidemia.
The algorithm defines five ASCVD risk categories and recommends increasingly lower LDL-C, non–HDL-C, and apo B target levels with increasing risk, but the same triglyceride target for all.
First, “treatment of lipid disorders begins with lifestyle therapy to improve nutrition, physical activity, weight, and other factors that affect lipids,” the consensus statement authors stress.
Next, “LDL-C has been, and remains, the main focus of efforts to improve lipid profiles in individuals at risk for ASCVD” (see table).

“We stratify [LDL-C] not as a one-treatment-target-for-all,” but rather as extreme, very high, high, moderate, and low ASCVD risk, Dr. Handelsman explained, with different treatment pathways (specified in another slide) to reach different risk-dependent goals.
“Unlike the ACC [American College of Cardiology] guideline, which shows if you want to further reduce LDL after statin give ezetimibe first, we say ‘no’,” he noted. “If somebody has an extreme risk, and you don’t think ezetimibe will get to a goal below 55 mg/dL, you should go first with a PCSK9 [proprotein convertase subtilisin/kexin type 9] inhibitor, and only then add ezetimibe or [colesevelam] or other drugs,” he said.
The consensus statement authors expand on this scenario. “Treatment for patients at extreme risk should begin with lifestyle therapy plus a high-intensity statin (atorvastatin 40 to 80 mg or rosuvastatin 20 to 40 mg, or the highest tolerated statin dose) to achieve an LDL-C goal of less than 55 mg/dL.”
“If LDL-C remains above goal after 3 months,” a PCSK9 inhibitor (evolocumab [Repatha, Amgen] or alirocumab [Praluent, Sanofi/Regeneron]), the cholesterol absorption inhibitor ezetimibe, or the bile acid sequestrant colesevelam (Welchol, Daiichi Sankyo) or the adenosine triphosphate-citrate lyase (ACL) inhibitor bempedoic acid (Nexletol, Esperion) “should be added, depending on required LDL-C lowering, and a third agent should be added if the combination fails to achieve the goal.”
However, “because the cost of ezetimibe is low, it may be preferred over PCSK9 inhibitors as second-line therapy to achieve an LDL-C below 70 mg/dL for patients who require no more than 15%-20% further reduction to reach goals.”
For patients at moderate or high risk, lipid management should begin with a moderate-intensity statin and be increased to a high-intensity statin before adding a second lipid-lowering medication to reach an LDL-C below 100 mg/dL.
According to the consensus statement, the desirable goal for triglycerides is less than 150 mg/dL.
In all patients with triglyceride levels of at least 500 mg/dL, statin therapy should be combined with a fibrate, prescription-grade omega-3 fatty acid, and/or niacin to reduce triglycerides.
In any patient with established ASCVD or diabetes with at least 2 ASCVD risk factors and triglycerides of 135-499 mg/dL, icosapent ethyl should be added to a statin to prevent ASCVD events.
Statement aligns with major guidelines
In general, the 2017 AACE/ACE guidelines and algorithm are “pretty similar” to other guidelines such as the 2018 ACC/American Heart Association (AHA) guidelines for cholesterol management, the 2019 ACC/AHA guidelines for primary prevention of CVD, and the 2019 European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) guidelines for the management of dyslipidemia, according to Dr. Eckel.
They have “all have now taken into consideration the evidence behind PCSK9 inhibitors,” he noted. “That’s important because those drugs have proven to be effective.”
Two differences, he pointed out, are that the 2019 ESC/EAS guidelines suggest that lipoprotein(a) measurement be considered at least once in every adult’s lifetime, and they recommend apo B analysis in people with high triglycerides but normal LDL (or no higher than 100 mg/dL), to identify additional risk.
*AACE changes its name, broadens focus
Shortly after its algorithm was published, AACE announced that it has a new organization name and brand, the American Association of Clinical Endocrinology, which “more clearly defines AACE as a community of individuals who work together to elevate the practice of clinical endocrinology,” according to an Oct. 20 statement.
The change is meant to acknowledge AACE’s “more modern, inclusive approach to endocrinology that supports multidisciplinary care teams – with endocrinologists leading the way.”
Along with the name change is a new global website. The statement notes that “health care professionals and community members can access all of the valuable clinical content such as guidelines, disease state networks and important education by visiting the pro portal in the top right corner of the site, or by going directly to pro.aace.com.”
Dr. Handelsman discloses that he receives research grant support from Amgen, Applied Therapeutics, AstraZeneca, BMS, Gan & Lee, Novo Nordisk, and Sanofi, and he is a consultant and/or speaker for Amarin, BI-Lilly, and Sanofi.
Dr. Eckel has received consultant/advisory board fees from Kowa, Novo Nordisk, and Provention Bio.
A new algorithm on lipid management and prevention of cardiovascular disease from the American Association of Clinical Endocrinologists* (AACE) and the American College of Endocrinology (ACE) is “a nice cookbook” that many clinicians, especially those who are not lipid experts, will find useful, according to writing committee chair Yehuda Handelsman, MD.
The algorithm, published Oct. 10 in Endocrine Practice as 10 slides, or as part of a more detailed consensus statement, is a companion to the 2017 AACE/ACE guidelines for lipid management and includes more recent information about new therapies.
“What we’re trying to do here is to say, ‘focus on LDL-C, triglycerides, high-risk patients, and lifestyle. Understand all the medications available to you to reduce LDL-C and reduce triglycerides,’ ” Dr. Handelsman, of the Metabolic Institute of America, Tarzana, Calif., explained in an interview.
“We touch on lipoprotein(a), which we still don’t have medication for, but it identifies people at high risk, and we need that.”
Clinicians also need to know “that we’ve got some newer drugs in the market that can manage people who have statin intolerance,” Dr. Handelsman added.
“We introduced new therapies like icosapent ethyl” (Vascepa, Amarin) for hypertriglyceridemia, “when to use it, and how to use it. Even though it was not part of the 2017 guideline, we gave recommendations based on current data in the algorithm.”
Although there is no good evidence that lowering triglycerides reduces heart disease, he continued, many experts believe that the target triglyceride level should be less than 150 mg/dL, and the algorithm explains how to treat to this goal.
“Last, and most importantly, I cannot fail to underscore the fact that lifestyle is very important,” he emphasized.
Robert H. Eckel, MD, of the University of Colorado at Denver, Aurora, and president of medicine and science at the American Diabetes Association, who was not involved with this algorithm, said in an interview that the algorithm is important since it offers “the clinician or health care practitioner an approach, a kind of a cookbook or application of the guidelines, for how to manage lipid disorders in patients at risk ... It’s geared for the nonexperts too,” he said.
Dyslipidemia treatment summarized in 10 slides
The AACE/ACE algorithm comprises 10 slides, one each for dyslipidemic states, secondary causes of lipid disorders, screening for and assessing lipid disorders and atherosclerotic CVD (ASCVD) risk, ASCVD risk categories and treatment goals, lifestyle recommendations, treating LDL-C to goal, managing statin intolerance and safety, management of hypertriglyceridemia and the role of icosapent ethyl, assessment and management of elevated lipoprotein(a), and profiles of medications for dyslipidemia.
The algorithm defines five ASCVD risk categories and recommends increasingly lower LDL-C, non–HDL-C, and apo B target levels with increasing risk, but the same triglyceride target for all.
First, “treatment of lipid disorders begins with lifestyle therapy to improve nutrition, physical activity, weight, and other factors that affect lipids,” the consensus statement authors stress.
Next, “LDL-C has been, and remains, the main focus of efforts to improve lipid profiles in individuals at risk for ASCVD” (see table).

“We stratify [LDL-C] not as a one-treatment-target-for-all,” but rather as extreme, very high, high, moderate, and low ASCVD risk, Dr. Handelsman explained, with different treatment pathways (specified in another slide) to reach different risk-dependent goals.
“Unlike the ACC [American College of Cardiology] guideline, which shows if you want to further reduce LDL after statin give ezetimibe first, we say ‘no’,” he noted. “If somebody has an extreme risk, and you don’t think ezetimibe will get to a goal below 55 mg/dL, you should go first with a PCSK9 [proprotein convertase subtilisin/kexin type 9] inhibitor, and only then add ezetimibe or [colesevelam] or other drugs,” he said.
The consensus statement authors expand on this scenario. “Treatment for patients at extreme risk should begin with lifestyle therapy plus a high-intensity statin (atorvastatin 40 to 80 mg or rosuvastatin 20 to 40 mg, or the highest tolerated statin dose) to achieve an LDL-C goal of less than 55 mg/dL.”
“If LDL-C remains above goal after 3 months,” a PCSK9 inhibitor (evolocumab [Repatha, Amgen] or alirocumab [Praluent, Sanofi/Regeneron]), the cholesterol absorption inhibitor ezetimibe, or the bile acid sequestrant colesevelam (Welchol, Daiichi Sankyo) or the adenosine triphosphate-citrate lyase (ACL) inhibitor bempedoic acid (Nexletol, Esperion) “should be added, depending on required LDL-C lowering, and a third agent should be added if the combination fails to achieve the goal.”
However, “because the cost of ezetimibe is low, it may be preferred over PCSK9 inhibitors as second-line therapy to achieve an LDL-C below 70 mg/dL for patients who require no more than 15%-20% further reduction to reach goals.”
For patients at moderate or high risk, lipid management should begin with a moderate-intensity statin and be increased to a high-intensity statin before adding a second lipid-lowering medication to reach an LDL-C below 100 mg/dL.
According to the consensus statement, the desirable goal for triglycerides is less than 150 mg/dL.
In all patients with triglyceride levels of at least 500 mg/dL, statin therapy should be combined with a fibrate, prescription-grade omega-3 fatty acid, and/or niacin to reduce triglycerides.
In any patient with established ASCVD or diabetes with at least 2 ASCVD risk factors and triglycerides of 135-499 mg/dL, icosapent ethyl should be added to a statin to prevent ASCVD events.
Statement aligns with major guidelines
In general, the 2017 AACE/ACE guidelines and algorithm are “pretty similar” to other guidelines such as the 2018 ACC/American Heart Association (AHA) guidelines for cholesterol management, the 2019 ACC/AHA guidelines for primary prevention of CVD, and the 2019 European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) guidelines for the management of dyslipidemia, according to Dr. Eckel.
They have “all have now taken into consideration the evidence behind PCSK9 inhibitors,” he noted. “That’s important because those drugs have proven to be effective.”
Two differences, he pointed out, are that the 2019 ESC/EAS guidelines suggest that lipoprotein(a) measurement be considered at least once in every adult’s lifetime, and they recommend apo B analysis in people with high triglycerides but normal LDL (or no higher than 100 mg/dL), to identify additional risk.
*AACE changes its name, broadens focus
Shortly after its algorithm was published, AACE announced that it has a new organization name and brand, the American Association of Clinical Endocrinology, which “more clearly defines AACE as a community of individuals who work together to elevate the practice of clinical endocrinology,” according to an Oct. 20 statement.
The change is meant to acknowledge AACE’s “more modern, inclusive approach to endocrinology that supports multidisciplinary care teams – with endocrinologists leading the way.”
Along with the name change is a new global website. The statement notes that “health care professionals and community members can access all of the valuable clinical content such as guidelines, disease state networks and important education by visiting the pro portal in the top right corner of the site, or by going directly to pro.aace.com.”
Dr. Handelsman discloses that he receives research grant support from Amgen, Applied Therapeutics, AstraZeneca, BMS, Gan & Lee, Novo Nordisk, and Sanofi, and he is a consultant and/or speaker for Amarin, BI-Lilly, and Sanofi.
Dr. Eckel has received consultant/advisory board fees from Kowa, Novo Nordisk, and Provention Bio.
Higher serum omega-3 tied to better outcome after STEMI
Regular consumption of foods rich in omega-3 fatty acids was associated with improved prognosis after ST-segment myocardial infarction (STEMI) in a new observational study.
The prospective study, which involved 944 patients with STEMI who underwent primary percutaneous coronary intervention (PCI), showed that plasma levels of fatty acids at the time of the STEMI were inversely associated with both incident major adverse cardiovascular events (MACE) and cardiovascular readmissions (adjusted hazard ratio, 0.76 and 0.74 for 1-SD increase; for both, P < .05).
No association was seen for the endpoint of all-cause mortality.
“What we showed is that your consumption of fish and other sources of omega-3 fatty acids before the heart attack impacts your prognosis after the heart attack. It’s a novel approach because it’s not primary prevention or secondary prevention,” said Aleix Sala-Vila, PharmD, PhD, from the Institut Hospital del Mar d’Investigacions Mèdiques (IMIM) in Barcelona, Spain.
Sala-Vila, co–senior author Antoni Bayés-Genís, MD, PhD, Heart Universitari Germans Trias I Pujol, Barcelona, and first author Iolanda Lázaro, PhD, also from IMIM, reported their findings online Oct. 26 in the Journal of the American College of Cardiology.
It has been established that dietary omega-3 eicosapentaenoic acid (EPA) has cardioprotective properties, but observational studies and randomized trials of EPA intake have yielded disparate findings.
This study avoided the usual traps of nutritional epidemiology research – self-reported food diaries and intake questionnaires. For this study, the researchers measured tissue levels of EPA and alpha-linolenic acid (ALA) by measuring serum phosphatidylcholine (PC) levels, which reflect dietary intake during the previous 3 or 4 weeks.
This technique, said Sala-Vila, not only provides a more reliable measure of fatty acid intake over time but also avoids measurement errors related to fatty acid content variation.
For example, “The EPA content of a piece of fish eaten in January could be very different from one eaten in June,” explained Sala-Vila.
That said, he acknowledged that this technique, which uses gas chromatography, does not at present have a clear clinical application. “It’s quite difficult just to convert levels of serum-PC EPA into consumption of fatty fish. We feel that the best advice at this point is that given by the American Heart Association to eat two servings of fatty fish a week.”
EPA and ALA: Partners in prevention?
In addition to the findings regarding EPA, the researchers also found that serum-PC ALA was inversely related to all-cause mortality after STEMI (HR, 0.65 for 1-SD increase; P < .05).
A trend was seen for an association between ALA and lower risk for incident MACE (P = .093).
ALA is readily available from inexpensive plant sources (eg, chia seeds, flax seeds, walnuts, soy beans) and has been associated with lower all-cause mortality in high-risk individuals.
This omega-3 fatty acid is often given short shrift in the fatty acid world because of the seven-step enzymatic process needed to convert it into more beneficial forms.
“We know that the conversion of ALA to EPA or DHA [docohexaenoic acid] is marginal, but we decided to include it in the study because we feel that this fatty acid is becoming more important because there are some issues with fish consumption – people are concerned about pollutants and sustainability, and some just don’t like it,” explained Sala-Vila.
“We were shocked to see that the marine-derived and vegetable-derived fatty acids don’t appear to compete, but rather they act synergistically,” said Sala-Villa. The researchers suggested that marine and vegetable omega-3 fatty acids may act as “partners in prevention.”
“We are not metabolically adapted to converting ALA to EPA, but despite this, there is a large body of evidence showing that one way to increase the status of EPA and DHA in our membranes is by eating these sources of fatty acids,” said Sala-Vila.
For almost 20 years, Sala-Vila has been studying how the consumption of foods rich in omega-3 affects disease. Two of his current projects involve studying levels of ALA in red blood cell membranes as a risk factor for ischemic stroke and omega-3 status in individuals with cognitive impairment who are at high risk for Alzheimer’s disease.
Applicable to all patients with atherosclerosis
In comments to theheart.org | Medscape Cardiology, Deepak Bhatt, MD, called the study “terrific,” adding that the effort is “as good as it gets” for observational nutrition research.
“I think one has to view these findings in the larger universe of what is really a revolution in omega-3 fatty acid research,” said Bhatt.
This universe, he said, includes a wealth of observational research showing the benefits of omega-3s, two outcome trials – JELIS and REDUCE-IT – that showed the benefits of EPA supplementation, and two imaging studies – EVAPORATE and CHERRY – that showed favorable effects of EPA on the vasculature.
REDUCE-IT, for which Bhatt served as principal investigator, showed that treatment with icosapent ethyl (Vascepa), a high-dose purified form of EPA, led to a 25% relative risk reduction in MACE in an at-risk Western population.
The results, said Bhatt, who co-wrote an editorial that accompanies the current Sala-Vila article, “likely apply to all patients with atherosclerosis or who are at high risk for it” and supports the practice of counseling patients to increase their intake of food rich in omega-3 fatty acids.
The field may be due for a shake-up, he noted. At next month’s American Heart Association meeting, the results of another trial of another prescription-grade EPA/DHA supplement will be presented, and they are expected to be negative.
AstraZeneca announced in January 2020 the early closure of the STRENGTH trial of Epanova after an interim analysis showed a low likelihood of their product demonstrating benefit in the enrolled population.
Epanova is a fish-oil derived mixture of free fatty acids, primarily EPA and DHA. It is approved in the United States and is indicated as an adjunct to diet to reduce triglyceride levels in adults with severe (≥500 mg/dL) hypertriglyceridemia. This indication is not affected by the data from the STRENGTH trial, according to a company press release.
Sala-Vila has received grants and support from the California Walnut Commission, including a grant to support part of this study. Bayés-Genís and Bhatt have relationships with a number of companies.
This article first appeared on Medscape.com.
Regular consumption of foods rich in omega-3 fatty acids was associated with improved prognosis after ST-segment myocardial infarction (STEMI) in a new observational study.
The prospective study, which involved 944 patients with STEMI who underwent primary percutaneous coronary intervention (PCI), showed that plasma levels of fatty acids at the time of the STEMI were inversely associated with both incident major adverse cardiovascular events (MACE) and cardiovascular readmissions (adjusted hazard ratio, 0.76 and 0.74 for 1-SD increase; for both, P < .05).
No association was seen for the endpoint of all-cause mortality.
“What we showed is that your consumption of fish and other sources of omega-3 fatty acids before the heart attack impacts your prognosis after the heart attack. It’s a novel approach because it’s not primary prevention or secondary prevention,” said Aleix Sala-Vila, PharmD, PhD, from the Institut Hospital del Mar d’Investigacions Mèdiques (IMIM) in Barcelona, Spain.
Sala-Vila, co–senior author Antoni Bayés-Genís, MD, PhD, Heart Universitari Germans Trias I Pujol, Barcelona, and first author Iolanda Lázaro, PhD, also from IMIM, reported their findings online Oct. 26 in the Journal of the American College of Cardiology.
It has been established that dietary omega-3 eicosapentaenoic acid (EPA) has cardioprotective properties, but observational studies and randomized trials of EPA intake have yielded disparate findings.
This study avoided the usual traps of nutritional epidemiology research – self-reported food diaries and intake questionnaires. For this study, the researchers measured tissue levels of EPA and alpha-linolenic acid (ALA) by measuring serum phosphatidylcholine (PC) levels, which reflect dietary intake during the previous 3 or 4 weeks.
This technique, said Sala-Vila, not only provides a more reliable measure of fatty acid intake over time but also avoids measurement errors related to fatty acid content variation.
For example, “The EPA content of a piece of fish eaten in January could be very different from one eaten in June,” explained Sala-Vila.
That said, he acknowledged that this technique, which uses gas chromatography, does not at present have a clear clinical application. “It’s quite difficult just to convert levels of serum-PC EPA into consumption of fatty fish. We feel that the best advice at this point is that given by the American Heart Association to eat two servings of fatty fish a week.”
EPA and ALA: Partners in prevention?
In addition to the findings regarding EPA, the researchers also found that serum-PC ALA was inversely related to all-cause mortality after STEMI (HR, 0.65 for 1-SD increase; P < .05).
A trend was seen for an association between ALA and lower risk for incident MACE (P = .093).
ALA is readily available from inexpensive plant sources (eg, chia seeds, flax seeds, walnuts, soy beans) and has been associated with lower all-cause mortality in high-risk individuals.
This omega-3 fatty acid is often given short shrift in the fatty acid world because of the seven-step enzymatic process needed to convert it into more beneficial forms.
“We know that the conversion of ALA to EPA or DHA [docohexaenoic acid] is marginal, but we decided to include it in the study because we feel that this fatty acid is becoming more important because there are some issues with fish consumption – people are concerned about pollutants and sustainability, and some just don’t like it,” explained Sala-Vila.
“We were shocked to see that the marine-derived and vegetable-derived fatty acids don’t appear to compete, but rather they act synergistically,” said Sala-Villa. The researchers suggested that marine and vegetable omega-3 fatty acids may act as “partners in prevention.”
“We are not metabolically adapted to converting ALA to EPA, but despite this, there is a large body of evidence showing that one way to increase the status of EPA and DHA in our membranes is by eating these sources of fatty acids,” said Sala-Vila.
For almost 20 years, Sala-Vila has been studying how the consumption of foods rich in omega-3 affects disease. Two of his current projects involve studying levels of ALA in red blood cell membranes as a risk factor for ischemic stroke and omega-3 status in individuals with cognitive impairment who are at high risk for Alzheimer’s disease.
Applicable to all patients with atherosclerosis
In comments to theheart.org | Medscape Cardiology, Deepak Bhatt, MD, called the study “terrific,” adding that the effort is “as good as it gets” for observational nutrition research.
“I think one has to view these findings in the larger universe of what is really a revolution in omega-3 fatty acid research,” said Bhatt.
This universe, he said, includes a wealth of observational research showing the benefits of omega-3s, two outcome trials – JELIS and REDUCE-IT – that showed the benefits of EPA supplementation, and two imaging studies – EVAPORATE and CHERRY – that showed favorable effects of EPA on the vasculature.
REDUCE-IT, for which Bhatt served as principal investigator, showed that treatment with icosapent ethyl (Vascepa), a high-dose purified form of EPA, led to a 25% relative risk reduction in MACE in an at-risk Western population.
The results, said Bhatt, who co-wrote an editorial that accompanies the current Sala-Vila article, “likely apply to all patients with atherosclerosis or who are at high risk for it” and supports the practice of counseling patients to increase their intake of food rich in omega-3 fatty acids.
The field may be due for a shake-up, he noted. At next month’s American Heart Association meeting, the results of another trial of another prescription-grade EPA/DHA supplement will be presented, and they are expected to be negative.
AstraZeneca announced in January 2020 the early closure of the STRENGTH trial of Epanova after an interim analysis showed a low likelihood of their product demonstrating benefit in the enrolled population.
Epanova is a fish-oil derived mixture of free fatty acids, primarily EPA and DHA. It is approved in the United States and is indicated as an adjunct to diet to reduce triglyceride levels in adults with severe (≥500 mg/dL) hypertriglyceridemia. This indication is not affected by the data from the STRENGTH trial, according to a company press release.
Sala-Vila has received grants and support from the California Walnut Commission, including a grant to support part of this study. Bayés-Genís and Bhatt have relationships with a number of companies.
This article first appeared on Medscape.com.
Regular consumption of foods rich in omega-3 fatty acids was associated with improved prognosis after ST-segment myocardial infarction (STEMI) in a new observational study.
The prospective study, which involved 944 patients with STEMI who underwent primary percutaneous coronary intervention (PCI), showed that plasma levels of fatty acids at the time of the STEMI were inversely associated with both incident major adverse cardiovascular events (MACE) and cardiovascular readmissions (adjusted hazard ratio, 0.76 and 0.74 for 1-SD increase; for both, P < .05).
No association was seen for the endpoint of all-cause mortality.
“What we showed is that your consumption of fish and other sources of omega-3 fatty acids before the heart attack impacts your prognosis after the heart attack. It’s a novel approach because it’s not primary prevention or secondary prevention,” said Aleix Sala-Vila, PharmD, PhD, from the Institut Hospital del Mar d’Investigacions Mèdiques (IMIM) in Barcelona, Spain.
Sala-Vila, co–senior author Antoni Bayés-Genís, MD, PhD, Heart Universitari Germans Trias I Pujol, Barcelona, and first author Iolanda Lázaro, PhD, also from IMIM, reported their findings online Oct. 26 in the Journal of the American College of Cardiology.
It has been established that dietary omega-3 eicosapentaenoic acid (EPA) has cardioprotective properties, but observational studies and randomized trials of EPA intake have yielded disparate findings.
This study avoided the usual traps of nutritional epidemiology research – self-reported food diaries and intake questionnaires. For this study, the researchers measured tissue levels of EPA and alpha-linolenic acid (ALA) by measuring serum phosphatidylcholine (PC) levels, which reflect dietary intake during the previous 3 or 4 weeks.
This technique, said Sala-Vila, not only provides a more reliable measure of fatty acid intake over time but also avoids measurement errors related to fatty acid content variation.
For example, “The EPA content of a piece of fish eaten in January could be very different from one eaten in June,” explained Sala-Vila.
That said, he acknowledged that this technique, which uses gas chromatography, does not at present have a clear clinical application. “It’s quite difficult just to convert levels of serum-PC EPA into consumption of fatty fish. We feel that the best advice at this point is that given by the American Heart Association to eat two servings of fatty fish a week.”
EPA and ALA: Partners in prevention?
In addition to the findings regarding EPA, the researchers also found that serum-PC ALA was inversely related to all-cause mortality after STEMI (HR, 0.65 for 1-SD increase; P < .05).
A trend was seen for an association between ALA and lower risk for incident MACE (P = .093).
ALA is readily available from inexpensive plant sources (eg, chia seeds, flax seeds, walnuts, soy beans) and has been associated with lower all-cause mortality in high-risk individuals.
This omega-3 fatty acid is often given short shrift in the fatty acid world because of the seven-step enzymatic process needed to convert it into more beneficial forms.
“We know that the conversion of ALA to EPA or DHA [docohexaenoic acid] is marginal, but we decided to include it in the study because we feel that this fatty acid is becoming more important because there are some issues with fish consumption – people are concerned about pollutants and sustainability, and some just don’t like it,” explained Sala-Vila.
“We were shocked to see that the marine-derived and vegetable-derived fatty acids don’t appear to compete, but rather they act synergistically,” said Sala-Villa. The researchers suggested that marine and vegetable omega-3 fatty acids may act as “partners in prevention.”
“We are not metabolically adapted to converting ALA to EPA, but despite this, there is a large body of evidence showing that one way to increase the status of EPA and DHA in our membranes is by eating these sources of fatty acids,” said Sala-Vila.
For almost 20 years, Sala-Vila has been studying how the consumption of foods rich in omega-3 affects disease. Two of his current projects involve studying levels of ALA in red blood cell membranes as a risk factor for ischemic stroke and omega-3 status in individuals with cognitive impairment who are at high risk for Alzheimer’s disease.
Applicable to all patients with atherosclerosis
In comments to theheart.org | Medscape Cardiology, Deepak Bhatt, MD, called the study “terrific,” adding that the effort is “as good as it gets” for observational nutrition research.
“I think one has to view these findings in the larger universe of what is really a revolution in omega-3 fatty acid research,” said Bhatt.
This universe, he said, includes a wealth of observational research showing the benefits of omega-3s, two outcome trials – JELIS and REDUCE-IT – that showed the benefits of EPA supplementation, and two imaging studies – EVAPORATE and CHERRY – that showed favorable effects of EPA on the vasculature.
REDUCE-IT, for which Bhatt served as principal investigator, showed that treatment with icosapent ethyl (Vascepa), a high-dose purified form of EPA, led to a 25% relative risk reduction in MACE in an at-risk Western population.
The results, said Bhatt, who co-wrote an editorial that accompanies the current Sala-Vila article, “likely apply to all patients with atherosclerosis or who are at high risk for it” and supports the practice of counseling patients to increase their intake of food rich in omega-3 fatty acids.
The field may be due for a shake-up, he noted. At next month’s American Heart Association meeting, the results of another trial of another prescription-grade EPA/DHA supplement will be presented, and they are expected to be negative.
AstraZeneca announced in January 2020 the early closure of the STRENGTH trial of Epanova after an interim analysis showed a low likelihood of their product demonstrating benefit in the enrolled population.
Epanova is a fish-oil derived mixture of free fatty acids, primarily EPA and DHA. It is approved in the United States and is indicated as an adjunct to diet to reduce triglyceride levels in adults with severe (≥500 mg/dL) hypertriglyceridemia. This indication is not affected by the data from the STRENGTH trial, according to a company press release.
Sala-Vila has received grants and support from the California Walnut Commission, including a grant to support part of this study. Bayés-Genís and Bhatt have relationships with a number of companies.
This article first appeared on Medscape.com.
Cannabis may improve liver function in patients with obesity
Cannabis use is associated with a decrease in the prevalence of steatohepatitis and a slowing of its progression in patients with obesity, results from a retrospective cohort study show.
This suggests “that the anti-inflammatory effects of cannabis may be leading to reduced prevalence of steatohepatitis in cannabis users,” said Ikechukwu Achebe, MD, from the John H. Stroger, Jr. Hospital of Cook County in Chicago.
Liver injuries such as nonalcoholic steatohepatitis are characterized by hepatocellular injury and inflammation, which combine to contribute to an increase in the risk for liver failure, cirrhosis, and hepatocellular carcinoma.
“This is where cannabis comes in,” said Dr. Achebe, who presented the study results at the virtual annual meeting of the American College of Gastroenterology. “It is the most commonly used psychoactive substance worldwide and has been shown to reduce hepatic myofibroblast and stellate cell injury. Studies using mouse models have demonstrated reduced liver fibrosis and cirrhosis as a consequence of cannabis exposure.”
Given this possible connection, Dr. Achebe and colleagues set out to determine whether cannabis use affects the prevalence and progression of nonalcoholic fatty liver disease (NAFLD) in obese patients.
To do so, they analyzed the discharge records of 879,952 obese adults in the 2016 Healthcare Cost and Utilization Project National Inpatient Sample. The primary outcome was the prevalence of the four presentations of NAFLD: steatosis, steatohepatitis, cirrhosis, and hepatocellular carcinoma.
The researchers compared disease stages in cannabis users and nonusers. In the study cohort of 14,236 patients, 1.6% used cannabis. Steatohepatitis was less common among cannabis users than among nonusers (0.4% vs. 0.7%; P < .001), as was cirrhosis (1.1% vs. 1.5%; P < .001).
After propensity matching, the association between cannabis use and lower rates of steatohepatitis remained significant (0.4% vs. 0.5%; P = .035), but the association between cannabis use and the prevalence of nonalcoholic fatty liver, cirrhosis, and hepatocellular carcinoma did not.
These results might be partly explained by the protective effect of cannabis on hepatocytes regulated by the endocannabinoid system, the researchers concluded.
More studies are needed to explore this relation, said Dr. Achebe.
The challenge of self-reported use
The study is “incredibly interesting,” said Nancy S. Reau, MD, from Rush Medical College, Chicago. However, the association between cannabis and nonalcoholic fatty liver needs to be further investigated before clinicians can counsel their patients to use the agent to prevent progression.
It is difficult in a study such as this to tease out other lifestyle factors that might be linked to cannabis use, she explained. For example, “is it possible that the cannabis users exercise more, drink more coffee, or eat differently?”
And “self-reported use is challenging,” Dr. Reau said in an interview. “This cannot differentiate someone who occasionally uses from someone who is a heavy daily user. There must be some minimum level of exposure needed for it to have protective effects, if they exist.”
This study was honored at the meeting as an ACG Newsworthy Abstract and an ACG Outstanding Poster Presenter.
Dr. Achebe disclosed no relevant financial relationships. Dr. Reau reported receiving research support from Genfit and having a consultant relationship with Intercept Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
Cannabis use is associated with a decrease in the prevalence of steatohepatitis and a slowing of its progression in patients with obesity, results from a retrospective cohort study show.
This suggests “that the anti-inflammatory effects of cannabis may be leading to reduced prevalence of steatohepatitis in cannabis users,” said Ikechukwu Achebe, MD, from the John H. Stroger, Jr. Hospital of Cook County in Chicago.
Liver injuries such as nonalcoholic steatohepatitis are characterized by hepatocellular injury and inflammation, which combine to contribute to an increase in the risk for liver failure, cirrhosis, and hepatocellular carcinoma.
“This is where cannabis comes in,” said Dr. Achebe, who presented the study results at the virtual annual meeting of the American College of Gastroenterology. “It is the most commonly used psychoactive substance worldwide and has been shown to reduce hepatic myofibroblast and stellate cell injury. Studies using mouse models have demonstrated reduced liver fibrosis and cirrhosis as a consequence of cannabis exposure.”
Given this possible connection, Dr. Achebe and colleagues set out to determine whether cannabis use affects the prevalence and progression of nonalcoholic fatty liver disease (NAFLD) in obese patients.
To do so, they analyzed the discharge records of 879,952 obese adults in the 2016 Healthcare Cost and Utilization Project National Inpatient Sample. The primary outcome was the prevalence of the four presentations of NAFLD: steatosis, steatohepatitis, cirrhosis, and hepatocellular carcinoma.
The researchers compared disease stages in cannabis users and nonusers. In the study cohort of 14,236 patients, 1.6% used cannabis. Steatohepatitis was less common among cannabis users than among nonusers (0.4% vs. 0.7%; P < .001), as was cirrhosis (1.1% vs. 1.5%; P < .001).
After propensity matching, the association between cannabis use and lower rates of steatohepatitis remained significant (0.4% vs. 0.5%; P = .035), but the association between cannabis use and the prevalence of nonalcoholic fatty liver, cirrhosis, and hepatocellular carcinoma did not.
These results might be partly explained by the protective effect of cannabis on hepatocytes regulated by the endocannabinoid system, the researchers concluded.
More studies are needed to explore this relation, said Dr. Achebe.
The challenge of self-reported use
The study is “incredibly interesting,” said Nancy S. Reau, MD, from Rush Medical College, Chicago. However, the association between cannabis and nonalcoholic fatty liver needs to be further investigated before clinicians can counsel their patients to use the agent to prevent progression.
It is difficult in a study such as this to tease out other lifestyle factors that might be linked to cannabis use, she explained. For example, “is it possible that the cannabis users exercise more, drink more coffee, or eat differently?”
And “self-reported use is challenging,” Dr. Reau said in an interview. “This cannot differentiate someone who occasionally uses from someone who is a heavy daily user. There must be some minimum level of exposure needed for it to have protective effects, if they exist.”
This study was honored at the meeting as an ACG Newsworthy Abstract and an ACG Outstanding Poster Presenter.
Dr. Achebe disclosed no relevant financial relationships. Dr. Reau reported receiving research support from Genfit and having a consultant relationship with Intercept Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
Cannabis use is associated with a decrease in the prevalence of steatohepatitis and a slowing of its progression in patients with obesity, results from a retrospective cohort study show.
This suggests “that the anti-inflammatory effects of cannabis may be leading to reduced prevalence of steatohepatitis in cannabis users,” said Ikechukwu Achebe, MD, from the John H. Stroger, Jr. Hospital of Cook County in Chicago.
Liver injuries such as nonalcoholic steatohepatitis are characterized by hepatocellular injury and inflammation, which combine to contribute to an increase in the risk for liver failure, cirrhosis, and hepatocellular carcinoma.
“This is where cannabis comes in,” said Dr. Achebe, who presented the study results at the virtual annual meeting of the American College of Gastroenterology. “It is the most commonly used psychoactive substance worldwide and has been shown to reduce hepatic myofibroblast and stellate cell injury. Studies using mouse models have demonstrated reduced liver fibrosis and cirrhosis as a consequence of cannabis exposure.”
Given this possible connection, Dr. Achebe and colleagues set out to determine whether cannabis use affects the prevalence and progression of nonalcoholic fatty liver disease (NAFLD) in obese patients.
To do so, they analyzed the discharge records of 879,952 obese adults in the 2016 Healthcare Cost and Utilization Project National Inpatient Sample. The primary outcome was the prevalence of the four presentations of NAFLD: steatosis, steatohepatitis, cirrhosis, and hepatocellular carcinoma.
The researchers compared disease stages in cannabis users and nonusers. In the study cohort of 14,236 patients, 1.6% used cannabis. Steatohepatitis was less common among cannabis users than among nonusers (0.4% vs. 0.7%; P < .001), as was cirrhosis (1.1% vs. 1.5%; P < .001).
After propensity matching, the association between cannabis use and lower rates of steatohepatitis remained significant (0.4% vs. 0.5%; P = .035), but the association between cannabis use and the prevalence of nonalcoholic fatty liver, cirrhosis, and hepatocellular carcinoma did not.
These results might be partly explained by the protective effect of cannabis on hepatocytes regulated by the endocannabinoid system, the researchers concluded.
More studies are needed to explore this relation, said Dr. Achebe.
The challenge of self-reported use
The study is “incredibly interesting,” said Nancy S. Reau, MD, from Rush Medical College, Chicago. However, the association between cannabis and nonalcoholic fatty liver needs to be further investigated before clinicians can counsel their patients to use the agent to prevent progression.
It is difficult in a study such as this to tease out other lifestyle factors that might be linked to cannabis use, she explained. For example, “is it possible that the cannabis users exercise more, drink more coffee, or eat differently?”
And “self-reported use is challenging,” Dr. Reau said in an interview. “This cannot differentiate someone who occasionally uses from someone who is a heavy daily user. There must be some minimum level of exposure needed for it to have protective effects, if they exist.”
This study was honored at the meeting as an ACG Newsworthy Abstract and an ACG Outstanding Poster Presenter.
Dr. Achebe disclosed no relevant financial relationships. Dr. Reau reported receiving research support from Genfit and having a consultant relationship with Intercept Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
FROM ACG 2020
Artificially sweetened drinks add to CVD risk
Sugary and artificially sweetened drinks are each associated with an increased risk of developing cardiovascular disease, according to results from a large prospective cohort study.
However, the design of that study fails to take into account other sources of dietary sugar, according to one expert.
In a research letter published online Oct. 26 in the Journal of the American College of Cardiology, Eloi Chazelas, a PhD candidate at Sorbonne Paris Nord University in Paris, and colleagues, shared results from nearly 105,000 subjects (79% women, mean age 43 at baseline, median follow up 6.6 years) enrolled in the NutriNet-Santé cohort study.
In this observational study, which began recruiting in 2009, dietary patterns are self-reported by subjects, while health outcomes are validated by investigators.
Mr. Chazelas and his colleagues identified 1,379 first incident cases of stroke, transient ischemic attack, myocardial infarction, acute coronary syndrome, and angioplasty in the cohort during 2009-2019. Cases that occurred during the first 3 years’ follow up were excluded from the analysis, to avoid potential reverse causality bias.
After adjustment for a wide range of dietary, demographic and health confounders, the investigators found that high consumers of sugary drinks or artificially sweetened drinks saw 20% and 32% higher risk of such events, respectively, compared with people who reported drinking neither beverage type (hazard ratio: 1.20; 95% confidence interval 1.04-1.40, P for trend < .0009 and HR: 1.32; 95% CI, 1.00-1.73, P for trend < .03).
Sugary drinks were defined as containing 5% or more of sugars, including natural fruit juices. The high consumers in the study had a median intake of 185 mL per day of sugary drinks, or 176 mL per day for artificially sweetened drinks. Natural noncaloric sweeteners such as Stevia were included in the artificially sweetened group.
The findings, Mr. Chazelas and colleagues wrote in their analysis, add to evidence that artificially sweetened beverages “might not be a healthy substitute for sugary drinks.” While research has suggested that artificial sweeteners induce glucose intolerance by disturbing gut microbiota, they noted, more and bigger studies are needed to understand the mechanisms by which they might bear on cardiovascular disease risk.
Robert A. Vogel, MD, of the University of Colorado Denver, urged caution in interpreting the researchers’ results. In an interview, Dr. Vogel, a preventive cardiologist, said that it is “notoriously difficult” to evaluate what a food or food group does to the body outside of a carefully controlled trial. What little randomized trial evidence exists comparing the health effects of artificially sweetened and sugary drinks includes a 2012 trial in children that found diet drinks associated with reductions in body fat – if anything a positive indication for heart health.
With adults enrolled in an observational study, things are much more easily confounded, Dr. Vogel said. “So subjects self-report that they’re not consuming one thing – sugary or sweetened beverages. What else are they putting into their diet? Maybe they’re eating dessert and consuming sugar that way. Try as you will to unconfound, to do a multivariate correction for all these factors is just very difficult.”
In addition, Dr. Vogel noted, the investigators made no attempt to discern among the different sweeteners consumed. “Stevia, saccharine, Sucralose – it’s highly unlikely that each of these agents has the same effect on gut microbiota.”
In 2019, researchers led by Mr. Chazelas looked at cancer risk in high consumers of the sugary and artificially sweetened drinks in some 107,000 patients from the cohort, and reported that sugary drinks were significantly associated with the risk of overall cancer. They saw no similar association for artificially sweetened drinks.
The NutriNet-Santé study is funded by the French government, and the investigators disclosed no financial support from commercial entities. Dr. Vogel has received research support from Sanofi and speaking fees from Regeneron.
Sugary and artificially sweetened drinks are each associated with an increased risk of developing cardiovascular disease, according to results from a large prospective cohort study.
However, the design of that study fails to take into account other sources of dietary sugar, according to one expert.
In a research letter published online Oct. 26 in the Journal of the American College of Cardiology, Eloi Chazelas, a PhD candidate at Sorbonne Paris Nord University in Paris, and colleagues, shared results from nearly 105,000 subjects (79% women, mean age 43 at baseline, median follow up 6.6 years) enrolled in the NutriNet-Santé cohort study.
In this observational study, which began recruiting in 2009, dietary patterns are self-reported by subjects, while health outcomes are validated by investigators.
Mr. Chazelas and his colleagues identified 1,379 first incident cases of stroke, transient ischemic attack, myocardial infarction, acute coronary syndrome, and angioplasty in the cohort during 2009-2019. Cases that occurred during the first 3 years’ follow up were excluded from the analysis, to avoid potential reverse causality bias.
After adjustment for a wide range of dietary, demographic and health confounders, the investigators found that high consumers of sugary drinks or artificially sweetened drinks saw 20% and 32% higher risk of such events, respectively, compared with people who reported drinking neither beverage type (hazard ratio: 1.20; 95% confidence interval 1.04-1.40, P for trend < .0009 and HR: 1.32; 95% CI, 1.00-1.73, P for trend < .03).
Sugary drinks were defined as containing 5% or more of sugars, including natural fruit juices. The high consumers in the study had a median intake of 185 mL per day of sugary drinks, or 176 mL per day for artificially sweetened drinks. Natural noncaloric sweeteners such as Stevia were included in the artificially sweetened group.
The findings, Mr. Chazelas and colleagues wrote in their analysis, add to evidence that artificially sweetened beverages “might not be a healthy substitute for sugary drinks.” While research has suggested that artificial sweeteners induce glucose intolerance by disturbing gut microbiota, they noted, more and bigger studies are needed to understand the mechanisms by which they might bear on cardiovascular disease risk.
Robert A. Vogel, MD, of the University of Colorado Denver, urged caution in interpreting the researchers’ results. In an interview, Dr. Vogel, a preventive cardiologist, said that it is “notoriously difficult” to evaluate what a food or food group does to the body outside of a carefully controlled trial. What little randomized trial evidence exists comparing the health effects of artificially sweetened and sugary drinks includes a 2012 trial in children that found diet drinks associated with reductions in body fat – if anything a positive indication for heart health.
With adults enrolled in an observational study, things are much more easily confounded, Dr. Vogel said. “So subjects self-report that they’re not consuming one thing – sugary or sweetened beverages. What else are they putting into their diet? Maybe they’re eating dessert and consuming sugar that way. Try as you will to unconfound, to do a multivariate correction for all these factors is just very difficult.”
In addition, Dr. Vogel noted, the investigators made no attempt to discern among the different sweeteners consumed. “Stevia, saccharine, Sucralose – it’s highly unlikely that each of these agents has the same effect on gut microbiota.”
In 2019, researchers led by Mr. Chazelas looked at cancer risk in high consumers of the sugary and artificially sweetened drinks in some 107,000 patients from the cohort, and reported that sugary drinks were significantly associated with the risk of overall cancer. They saw no similar association for artificially sweetened drinks.
The NutriNet-Santé study is funded by the French government, and the investigators disclosed no financial support from commercial entities. Dr. Vogel has received research support from Sanofi and speaking fees from Regeneron.
Sugary and artificially sweetened drinks are each associated with an increased risk of developing cardiovascular disease, according to results from a large prospective cohort study.
However, the design of that study fails to take into account other sources of dietary sugar, according to one expert.
In a research letter published online Oct. 26 in the Journal of the American College of Cardiology, Eloi Chazelas, a PhD candidate at Sorbonne Paris Nord University in Paris, and colleagues, shared results from nearly 105,000 subjects (79% women, mean age 43 at baseline, median follow up 6.6 years) enrolled in the NutriNet-Santé cohort study.
In this observational study, which began recruiting in 2009, dietary patterns are self-reported by subjects, while health outcomes are validated by investigators.
Mr. Chazelas and his colleagues identified 1,379 first incident cases of stroke, transient ischemic attack, myocardial infarction, acute coronary syndrome, and angioplasty in the cohort during 2009-2019. Cases that occurred during the first 3 years’ follow up were excluded from the analysis, to avoid potential reverse causality bias.
After adjustment for a wide range of dietary, demographic and health confounders, the investigators found that high consumers of sugary drinks or artificially sweetened drinks saw 20% and 32% higher risk of such events, respectively, compared with people who reported drinking neither beverage type (hazard ratio: 1.20; 95% confidence interval 1.04-1.40, P for trend < .0009 and HR: 1.32; 95% CI, 1.00-1.73, P for trend < .03).
Sugary drinks were defined as containing 5% or more of sugars, including natural fruit juices. The high consumers in the study had a median intake of 185 mL per day of sugary drinks, or 176 mL per day for artificially sweetened drinks. Natural noncaloric sweeteners such as Stevia were included in the artificially sweetened group.
The findings, Mr. Chazelas and colleagues wrote in their analysis, add to evidence that artificially sweetened beverages “might not be a healthy substitute for sugary drinks.” While research has suggested that artificial sweeteners induce glucose intolerance by disturbing gut microbiota, they noted, more and bigger studies are needed to understand the mechanisms by which they might bear on cardiovascular disease risk.
Robert A. Vogel, MD, of the University of Colorado Denver, urged caution in interpreting the researchers’ results. In an interview, Dr. Vogel, a preventive cardiologist, said that it is “notoriously difficult” to evaluate what a food or food group does to the body outside of a carefully controlled trial. What little randomized trial evidence exists comparing the health effects of artificially sweetened and sugary drinks includes a 2012 trial in children that found diet drinks associated with reductions in body fat – if anything a positive indication for heart health.
With adults enrolled in an observational study, things are much more easily confounded, Dr. Vogel said. “So subjects self-report that they’re not consuming one thing – sugary or sweetened beverages. What else are they putting into their diet? Maybe they’re eating dessert and consuming sugar that way. Try as you will to unconfound, to do a multivariate correction for all these factors is just very difficult.”
In addition, Dr. Vogel noted, the investigators made no attempt to discern among the different sweeteners consumed. “Stevia, saccharine, Sucralose – it’s highly unlikely that each of these agents has the same effect on gut microbiota.”
In 2019, researchers led by Mr. Chazelas looked at cancer risk in high consumers of the sugary and artificially sweetened drinks in some 107,000 patients from the cohort, and reported that sugary drinks were significantly associated with the risk of overall cancer. They saw no similar association for artificially sweetened drinks.
The NutriNet-Santé study is funded by the French government, and the investigators disclosed no financial support from commercial entities. Dr. Vogel has received research support from Sanofi and speaking fees from Regeneron.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Certain statins linked to lower mortality risk in patients admitted for sepsis
Among individuals admitted to hospitals with sepsis, statin users had a lower mortality, compared with nonstatin users, according to a recent analysis focused on a large and diverse cohort of patients in California.
Mortality hazard ratios at 30 and 90 days were lower by about 20% for statin users admitted for sepsis, compared with nonstatin users, according to results of the retrospective cohort study.
Hydrophilic and synthetic statins had more favorable mortality outcomes, compared with lipophilic and fungal-derived statins, respectively, added investigator Brannen Liang, MD, a third-year internal medicine resident at Kaiser Permanente Los Angeles Medical Center.
These findings suggest a potential benefit of statins in patients with sepsis, with certain types of statins having a greater protective effect than others, according to Dr. Liang, who presented the original research in a presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
“I think there’s potential for extending the use of statins to other indications, such as sepsis,” Dr. Liang said in an interview, though he also cautioned that the present study is hypothesis generating and more research is necessary.
Using a certain statin type over another (i.e., a hydrophilic, synthetic statin) might be a consideration for populations who are at greater risk for sepsis, such as the immunocompromised, patients with diabetes, or elderly and who also require a statin for an indication such as hyperlipidemia, he added.
While the link between statin use and sepsis mortality outcomes is not new, this study is unique in that it replicates results of earlier studies in a large and diverse real-world population, Dr. Liang said.
“Numerous studies seem to suggest that statins may play a role in attenuating the mortality of patients admitted to the hospital with sepsis, for whatever reason – whether this is due to their anti-inflammatory effects, their lipid-lowering effects, or if they truly have an antimicrobial effect, which has been studied in vitro and in animal studies,” he said in an interview.
It’s impossible to definitively conclude from retrospective studies such as this whether statins reduce sepsis-related mortality risk, but the present study at least makes the case for using certain types of statins when they are indicated in high-risk patients, said Steven Q. Simpson, MD, FCCP, professor of medicine in the division of pulmonary and critical care medicine at the University of Kansas, Kansas City.
“If you have patients at high risk for sepsis and they need a statin, you could give consideration to using a hydrophilic and synthetic statin, rather than either of the other choices,” said Dr. Simpson, CHEST president-elect and senior advisor to the Solving Sepsis initiative of the Biomedical Advanced Research and Development Authority of the Department of Health & Human Services.
The retrospective cohort study by Dr. Liang and colleagues included a total of 137,019 individuals admitted for sepsis within the Kaiser Permanente Southern California health system between 2008 and 2018. Of that group, 36,908 were taking a statin.
Overall, the mean age of patients admitted for sepsis was 66.9 years, and 50.4% were female. Nearly 50% were White, about 12% were Black, 28% were Hispanic, and 8% were Asian. A diagnosis of ischemic heart disease was reported for 43% of statin users and 23% of nonusers, while diabetes mellitus was reported for 60% of statin users and 37% of nonusers (P < .0001 for both comparisons).
Differences in mortality favored statin users, compared with nonusers, with hazard ratios of 0.79 (95% confidence interval, 0.77-0.82) at 30 days and similarly, 0.79 (95% CI, 0.77-0.81) at 90 days, Dr. Liang reported, noting that the models were adjusted for age, race, sex, and comorbidities.
Further analysis suggested a mortality advantage of lipophilic, compared with hydrophilic statins, and an advantage of fungal-derived statins over synthetic-derived statins, the investigator added.
In the comparison of lipophilic statin users and hydrophilic statin users, the 30- and 90-day mortality HRs were 1.13 (95% CI, 1.02-1.26) and 1.17 (95% CI, 1.07-1.28), respectively, the data show. For fungal-derived statin users, compared with synthetic derived statin users, 30- and 90-day mortality HRs were 1.12 (95% CI, 1.06-1.19) and 1.14 (95% CI, 1.09-1.20), respectively.
Dr. Liang and coauthors disclosed no relevant relationships with respect to the work presented at the CHEST meeting.
SOURCE: Liang B et al. CHEST 2020, Abstract A589.
Among individuals admitted to hospitals with sepsis, statin users had a lower mortality, compared with nonstatin users, according to a recent analysis focused on a large and diverse cohort of patients in California.
Mortality hazard ratios at 30 and 90 days were lower by about 20% for statin users admitted for sepsis, compared with nonstatin users, according to results of the retrospective cohort study.
Hydrophilic and synthetic statins had more favorable mortality outcomes, compared with lipophilic and fungal-derived statins, respectively, added investigator Brannen Liang, MD, a third-year internal medicine resident at Kaiser Permanente Los Angeles Medical Center.
These findings suggest a potential benefit of statins in patients with sepsis, with certain types of statins having a greater protective effect than others, according to Dr. Liang, who presented the original research in a presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
“I think there’s potential for extending the use of statins to other indications, such as sepsis,” Dr. Liang said in an interview, though he also cautioned that the present study is hypothesis generating and more research is necessary.
Using a certain statin type over another (i.e., a hydrophilic, synthetic statin) might be a consideration for populations who are at greater risk for sepsis, such as the immunocompromised, patients with diabetes, or elderly and who also require a statin for an indication such as hyperlipidemia, he added.
While the link between statin use and sepsis mortality outcomes is not new, this study is unique in that it replicates results of earlier studies in a large and diverse real-world population, Dr. Liang said.
“Numerous studies seem to suggest that statins may play a role in attenuating the mortality of patients admitted to the hospital with sepsis, for whatever reason – whether this is due to their anti-inflammatory effects, their lipid-lowering effects, or if they truly have an antimicrobial effect, which has been studied in vitro and in animal studies,” he said in an interview.
It’s impossible to definitively conclude from retrospective studies such as this whether statins reduce sepsis-related mortality risk, but the present study at least makes the case for using certain types of statins when they are indicated in high-risk patients, said Steven Q. Simpson, MD, FCCP, professor of medicine in the division of pulmonary and critical care medicine at the University of Kansas, Kansas City.
“If you have patients at high risk for sepsis and they need a statin, you could give consideration to using a hydrophilic and synthetic statin, rather than either of the other choices,” said Dr. Simpson, CHEST president-elect and senior advisor to the Solving Sepsis initiative of the Biomedical Advanced Research and Development Authority of the Department of Health & Human Services.
The retrospective cohort study by Dr. Liang and colleagues included a total of 137,019 individuals admitted for sepsis within the Kaiser Permanente Southern California health system between 2008 and 2018. Of that group, 36,908 were taking a statin.
Overall, the mean age of patients admitted for sepsis was 66.9 years, and 50.4% were female. Nearly 50% were White, about 12% were Black, 28% were Hispanic, and 8% were Asian. A diagnosis of ischemic heart disease was reported for 43% of statin users and 23% of nonusers, while diabetes mellitus was reported for 60% of statin users and 37% of nonusers (P < .0001 for both comparisons).
Differences in mortality favored statin users, compared with nonusers, with hazard ratios of 0.79 (95% confidence interval, 0.77-0.82) at 30 days and similarly, 0.79 (95% CI, 0.77-0.81) at 90 days, Dr. Liang reported, noting that the models were adjusted for age, race, sex, and comorbidities.
Further analysis suggested a mortality advantage of lipophilic, compared with hydrophilic statins, and an advantage of fungal-derived statins over synthetic-derived statins, the investigator added.
In the comparison of lipophilic statin users and hydrophilic statin users, the 30- and 90-day mortality HRs were 1.13 (95% CI, 1.02-1.26) and 1.17 (95% CI, 1.07-1.28), respectively, the data show. For fungal-derived statin users, compared with synthetic derived statin users, 30- and 90-day mortality HRs were 1.12 (95% CI, 1.06-1.19) and 1.14 (95% CI, 1.09-1.20), respectively.
Dr. Liang and coauthors disclosed no relevant relationships with respect to the work presented at the CHEST meeting.
SOURCE: Liang B et al. CHEST 2020, Abstract A589.
Among individuals admitted to hospitals with sepsis, statin users had a lower mortality, compared with nonstatin users, according to a recent analysis focused on a large and diverse cohort of patients in California.
Mortality hazard ratios at 30 and 90 days were lower by about 20% for statin users admitted for sepsis, compared with nonstatin users, according to results of the retrospective cohort study.
Hydrophilic and synthetic statins had more favorable mortality outcomes, compared with lipophilic and fungal-derived statins, respectively, added investigator Brannen Liang, MD, a third-year internal medicine resident at Kaiser Permanente Los Angeles Medical Center.
These findings suggest a potential benefit of statins in patients with sepsis, with certain types of statins having a greater protective effect than others, according to Dr. Liang, who presented the original research in a presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
“I think there’s potential for extending the use of statins to other indications, such as sepsis,” Dr. Liang said in an interview, though he also cautioned that the present study is hypothesis generating and more research is necessary.
Using a certain statin type over another (i.e., a hydrophilic, synthetic statin) might be a consideration for populations who are at greater risk for sepsis, such as the immunocompromised, patients with diabetes, or elderly and who also require a statin for an indication such as hyperlipidemia, he added.
While the link between statin use and sepsis mortality outcomes is not new, this study is unique in that it replicates results of earlier studies in a large and diverse real-world population, Dr. Liang said.
“Numerous studies seem to suggest that statins may play a role in attenuating the mortality of patients admitted to the hospital with sepsis, for whatever reason – whether this is due to their anti-inflammatory effects, their lipid-lowering effects, or if they truly have an antimicrobial effect, which has been studied in vitro and in animal studies,” he said in an interview.
It’s impossible to definitively conclude from retrospective studies such as this whether statins reduce sepsis-related mortality risk, but the present study at least makes the case for using certain types of statins when they are indicated in high-risk patients, said Steven Q. Simpson, MD, FCCP, professor of medicine in the division of pulmonary and critical care medicine at the University of Kansas, Kansas City.
“If you have patients at high risk for sepsis and they need a statin, you could give consideration to using a hydrophilic and synthetic statin, rather than either of the other choices,” said Dr. Simpson, CHEST president-elect and senior advisor to the Solving Sepsis initiative of the Biomedical Advanced Research and Development Authority of the Department of Health & Human Services.
The retrospective cohort study by Dr. Liang and colleagues included a total of 137,019 individuals admitted for sepsis within the Kaiser Permanente Southern California health system between 2008 and 2018. Of that group, 36,908 were taking a statin.
Overall, the mean age of patients admitted for sepsis was 66.9 years, and 50.4% were female. Nearly 50% were White, about 12% were Black, 28% were Hispanic, and 8% were Asian. A diagnosis of ischemic heart disease was reported for 43% of statin users and 23% of nonusers, while diabetes mellitus was reported for 60% of statin users and 37% of nonusers (P < .0001 for both comparisons).
Differences in mortality favored statin users, compared with nonusers, with hazard ratios of 0.79 (95% confidence interval, 0.77-0.82) at 30 days and similarly, 0.79 (95% CI, 0.77-0.81) at 90 days, Dr. Liang reported, noting that the models were adjusted for age, race, sex, and comorbidities.
Further analysis suggested a mortality advantage of lipophilic, compared with hydrophilic statins, and an advantage of fungal-derived statins over synthetic-derived statins, the investigator added.
In the comparison of lipophilic statin users and hydrophilic statin users, the 30- and 90-day mortality HRs were 1.13 (95% CI, 1.02-1.26) and 1.17 (95% CI, 1.07-1.28), respectively, the data show. For fungal-derived statin users, compared with synthetic derived statin users, 30- and 90-day mortality HRs were 1.12 (95% CI, 1.06-1.19) and 1.14 (95% CI, 1.09-1.20), respectively.
Dr. Liang and coauthors disclosed no relevant relationships with respect to the work presented at the CHEST meeting.
SOURCE: Liang B et al. CHEST 2020, Abstract A589.
FROM CHEST 2020
Bariatric surgery linked to longer life
A new analysis of the Swedish Obese Subjects (SOS) study shows that bariatric surgery is associated with about a 3-year increase in lifespan, compared with obese patients who do not undergo surgery. Still, surgery did not restore normal lifespan: Surgical patients’ lifespan remained less than that of a sample from the general Swedish population. The study follows other reports suggesting reduced mortality after bariatric surgery, but with a longer follow-up.
“These data add even more evidence to the growing literature showing that patients who undergo bariatric surgery experience a reduction in all-cause long-term mortality. In making decisions around bariatric surgical procedures and care, patients and their health care providers need to understand the trade-offs between improved weight, health, and longer-term survival versus the surgical risks and problems over time,” said Anita P. Courcoulas, MD, MPH, chief of minimally invasive bariatric and general surgery at the University of Pittsburgh Medical Center, said in an interview. Dr. Courcoulas was not involved in the study.
The results appeared in the New England Journal of Medicine.
The SOS study drew from 25 surgical departments and 480 primary health care centers in Sweden. The researchers examined data from 2,007 patients who underwent bariatric surgery between 1987 and 2001, and compared their outcomes to 2,040 matched controls. All were between age 37 and 60 years, with a body mass index (BMI) of at least 34 kg/m2 for men and 38 for women. They also compared outcomes with 1,135 randomly sampled from the Swedish population registry.
Procedures included banding (18%), vertical banded gastroplasty (69%), and gastric bypass (13%). After an initial BMI reduction of about 11, the surgery group stabilized by year 8 at a BMI about 7 lower than baseline, and there was little change in BMI among controls.
After a mean follow-up of 24 years (interquartile range, 22-27 years), there were 10.7 deaths per 1,000 person-years in the surgery group, 13.2 among obese controls, and 5.2 in the general population (hazard ratio, 0.77 for surgery versus no surgery; P < .001). The general population had a lower mortality than nonsurgical controls (HR, 0.44; P < .001).
The surgery group had a higher median life expectancy than controls (median, 2.4 years; adjusted difference, 3.0 years; P < .001). The general population group had a median life expectancy that was 7.4 years higher than the control group (adjusted difference, 8.5 years; P < .001). The surgery group’s median life expectancy was still shorter than the general population reference (adjusted difference, 5.5 years; P < .001).
Cardiovascular disease risk was lower in the surgery group (HR, 0.70; 95% confidence interval, 0.57-0.85), as was risk of MI (HR, 0.51; 95% CI, 0.33-0.79), heart failure (HR, 0.52; 95% CI, 0.31-0.88), and stroke (HR, 0.45; 95% CI, 0.24-0.84). Cancer mortality was also lower (HR, 0.77; 95% CI, 0.61-0.96).
In the surgery group, causes of death that were elevated over the general population included cardiovascular causes (HR, 2.64; 95% CI, 1.78-3.91) and noncardiovascular causes, mainly infections; postsurgical complications; and factors such as alcoholism, suicide, or trauma (HR, 1.50; 95% CI, 1.18-1.91).
The study is limited by its retrospective nature, and because the surgical techniques used at the time are less effective than those used today, and could lead to weight gain over time. As a result, many patients who underwent surgery remained heavier than the general population. It’s also possible that negative health effects accumulated before surgery and persisted afterwards, according to Dr. Courcoulas.
The findings are likely generalizable to people with obesity, many of whom choose not to undergo bariatric surgery despite the potential benefits. “The population studied in SOS had a similar profile of underlying medical diseases to those groups who undergo bariatric surgery today and in the U.S. and around the world,” said Dr. Courcoulas.
The study was funded by the Swedish Research Council and others. Dr. Courcoulas has no relevant financial disclosures
SOURCE: Carlsson L et al. N Engl J Med. 2020 Oct 15. doi: 10.1056/NEJMoa2002449.
A new analysis of the Swedish Obese Subjects (SOS) study shows that bariatric surgery is associated with about a 3-year increase in lifespan, compared with obese patients who do not undergo surgery. Still, surgery did not restore normal lifespan: Surgical patients’ lifespan remained less than that of a sample from the general Swedish population. The study follows other reports suggesting reduced mortality after bariatric surgery, but with a longer follow-up.
“These data add even more evidence to the growing literature showing that patients who undergo bariatric surgery experience a reduction in all-cause long-term mortality. In making decisions around bariatric surgical procedures and care, patients and their health care providers need to understand the trade-offs between improved weight, health, and longer-term survival versus the surgical risks and problems over time,” said Anita P. Courcoulas, MD, MPH, chief of minimally invasive bariatric and general surgery at the University of Pittsburgh Medical Center, said in an interview. Dr. Courcoulas was not involved in the study.
The results appeared in the New England Journal of Medicine.
The SOS study drew from 25 surgical departments and 480 primary health care centers in Sweden. The researchers examined data from 2,007 patients who underwent bariatric surgery between 1987 and 2001, and compared their outcomes to 2,040 matched controls. All were between age 37 and 60 years, with a body mass index (BMI) of at least 34 kg/m2 for men and 38 for women. They also compared outcomes with 1,135 randomly sampled from the Swedish population registry.
Procedures included banding (18%), vertical banded gastroplasty (69%), and gastric bypass (13%). After an initial BMI reduction of about 11, the surgery group stabilized by year 8 at a BMI about 7 lower than baseline, and there was little change in BMI among controls.
After a mean follow-up of 24 years (interquartile range, 22-27 years), there were 10.7 deaths per 1,000 person-years in the surgery group, 13.2 among obese controls, and 5.2 in the general population (hazard ratio, 0.77 for surgery versus no surgery; P < .001). The general population had a lower mortality than nonsurgical controls (HR, 0.44; P < .001).
The surgery group had a higher median life expectancy than controls (median, 2.4 years; adjusted difference, 3.0 years; P < .001). The general population group had a median life expectancy that was 7.4 years higher than the control group (adjusted difference, 8.5 years; P < .001). The surgery group’s median life expectancy was still shorter than the general population reference (adjusted difference, 5.5 years; P < .001).
Cardiovascular disease risk was lower in the surgery group (HR, 0.70; 95% confidence interval, 0.57-0.85), as was risk of MI (HR, 0.51; 95% CI, 0.33-0.79), heart failure (HR, 0.52; 95% CI, 0.31-0.88), and stroke (HR, 0.45; 95% CI, 0.24-0.84). Cancer mortality was also lower (HR, 0.77; 95% CI, 0.61-0.96).
In the surgery group, causes of death that were elevated over the general population included cardiovascular causes (HR, 2.64; 95% CI, 1.78-3.91) and noncardiovascular causes, mainly infections; postsurgical complications; and factors such as alcoholism, suicide, or trauma (HR, 1.50; 95% CI, 1.18-1.91).
The study is limited by its retrospective nature, and because the surgical techniques used at the time are less effective than those used today, and could lead to weight gain over time. As a result, many patients who underwent surgery remained heavier than the general population. It’s also possible that negative health effects accumulated before surgery and persisted afterwards, according to Dr. Courcoulas.
The findings are likely generalizable to people with obesity, many of whom choose not to undergo bariatric surgery despite the potential benefits. “The population studied in SOS had a similar profile of underlying medical diseases to those groups who undergo bariatric surgery today and in the U.S. and around the world,” said Dr. Courcoulas.
The study was funded by the Swedish Research Council and others. Dr. Courcoulas has no relevant financial disclosures
SOURCE: Carlsson L et al. N Engl J Med. 2020 Oct 15. doi: 10.1056/NEJMoa2002449.
A new analysis of the Swedish Obese Subjects (SOS) study shows that bariatric surgery is associated with about a 3-year increase in lifespan, compared with obese patients who do not undergo surgery. Still, surgery did not restore normal lifespan: Surgical patients’ lifespan remained less than that of a sample from the general Swedish population. The study follows other reports suggesting reduced mortality after bariatric surgery, but with a longer follow-up.
“These data add even more evidence to the growing literature showing that patients who undergo bariatric surgery experience a reduction in all-cause long-term mortality. In making decisions around bariatric surgical procedures and care, patients and their health care providers need to understand the trade-offs between improved weight, health, and longer-term survival versus the surgical risks and problems over time,” said Anita P. Courcoulas, MD, MPH, chief of minimally invasive bariatric and general surgery at the University of Pittsburgh Medical Center, said in an interview. Dr. Courcoulas was not involved in the study.
The results appeared in the New England Journal of Medicine.
The SOS study drew from 25 surgical departments and 480 primary health care centers in Sweden. The researchers examined data from 2,007 patients who underwent bariatric surgery between 1987 and 2001, and compared their outcomes to 2,040 matched controls. All were between age 37 and 60 years, with a body mass index (BMI) of at least 34 kg/m2 for men and 38 for women. They also compared outcomes with 1,135 randomly sampled from the Swedish population registry.
Procedures included banding (18%), vertical banded gastroplasty (69%), and gastric bypass (13%). After an initial BMI reduction of about 11, the surgery group stabilized by year 8 at a BMI about 7 lower than baseline, and there was little change in BMI among controls.
After a mean follow-up of 24 years (interquartile range, 22-27 years), there were 10.7 deaths per 1,000 person-years in the surgery group, 13.2 among obese controls, and 5.2 in the general population (hazard ratio, 0.77 for surgery versus no surgery; P < .001). The general population had a lower mortality than nonsurgical controls (HR, 0.44; P < .001).
The surgery group had a higher median life expectancy than controls (median, 2.4 years; adjusted difference, 3.0 years; P < .001). The general population group had a median life expectancy that was 7.4 years higher than the control group (adjusted difference, 8.5 years; P < .001). The surgery group’s median life expectancy was still shorter than the general population reference (adjusted difference, 5.5 years; P < .001).
Cardiovascular disease risk was lower in the surgery group (HR, 0.70; 95% confidence interval, 0.57-0.85), as was risk of MI (HR, 0.51; 95% CI, 0.33-0.79), heart failure (HR, 0.52; 95% CI, 0.31-0.88), and stroke (HR, 0.45; 95% CI, 0.24-0.84). Cancer mortality was also lower (HR, 0.77; 95% CI, 0.61-0.96).
In the surgery group, causes of death that were elevated over the general population included cardiovascular causes (HR, 2.64; 95% CI, 1.78-3.91) and noncardiovascular causes, mainly infections; postsurgical complications; and factors such as alcoholism, suicide, or trauma (HR, 1.50; 95% CI, 1.18-1.91).
The study is limited by its retrospective nature, and because the surgical techniques used at the time are less effective than those used today, and could lead to weight gain over time. As a result, many patients who underwent surgery remained heavier than the general population. It’s also possible that negative health effects accumulated before surgery and persisted afterwards, according to Dr. Courcoulas.
The findings are likely generalizable to people with obesity, many of whom choose not to undergo bariatric surgery despite the potential benefits. “The population studied in SOS had a similar profile of underlying medical diseases to those groups who undergo bariatric surgery today and in the U.S. and around the world,” said Dr. Courcoulas.
The study was funded by the Swedish Research Council and others. Dr. Courcoulas has no relevant financial disclosures
SOURCE: Carlsson L et al. N Engl J Med. 2020 Oct 15. doi: 10.1056/NEJMoa2002449.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
MI recurrences drop, but women underestimate disease risk
The number of heart attack survivors in the United States who experienced repeat attacks within a year decreased between 2008 and 2017, especially among women, yet women’s awareness of their risk of death from heart disease also decreased, according to data from a pair of studies published in Circulation.
Recurrent MI rates drop, but not enough
Although the overall morbidity and mortality from coronary heart disease (CHD) in the United States has been on the decline for decades, CHD remains the leading cause of death and disability in both sexes, wrote Sanne A.E. Peters, PhD, of Imperial College London, and colleagues.
To better assess the rates of recurrent CHD by sex, the researchers reviewed data from 770,408 women and 700,477 men younger than 65 years with commercial health insurance or aged 66 years and older with Medicare who were hospitalized for myocardial infarction between 2008 and 2017. The patients were followed for 1 year for recurrent MIs, recurrent CHD events, heart failure hospitalization, and all-cause mortality.
In the study of recurrent heart disease, the rate of recurrent heart attacks per 1,000 person-years declined from 89.2 to 72.3 in women and from 94.2 to 81.3 in men. In addition, the rate of recurrent heart disease events (defined as either an MI or an artery-opening procedure), dropped per 1,000 person-years from 166.3 to 133.3 in women and from 198.1 to 176.8 in men. The reduction was significantly greater among women compared with men (P < .001 for both recurrent MIs and recurrent CHD events) and the differences by sex were consistent throughout the study period.
However, no significant difference occurred in recurrent MI rates among younger women (aged 21-54 years), or men aged 55-79 years, the researchers noted.
Heart failure rates per 1,000 person-years decreased from 177.4 to 158.1 in women and from 162.9 to 156.1 in men during the study period, and all-cause mortality decreased per 1,000 person-years from 403.2 to 389.5 for women and from 436.1 to 417.9 in men.
Potential contributing factors to the reductions in rates of recurrent events after a heart attack may include improved acute cardiac procedures, in-hospital therapy, and secondary prevention, the researchers noted. In addition, “changes in the type and definition of MI may also have contributed to the decline in recurrent events,” they said. “Also, the introduction and increasing sensitivity of cardiac biomarkers assays, especially cardiac troponin, may have contributed to an increased detection of less severe MIs over time, which, in turn, could have resulted in artifactual reductions in the consequences of MI,” they said.
The study findings were limited by several factors including the use of claims data, lack of information on the severity of heart attacks, and inability to analyze population subgroups, but the results were strengthened by the use of a large, multicultural database.
Despite the decline seen in this study, overall rates of recurrent MI, recurrent CHD events, heart failure hospitalization, and mortality remain high, the researchers said, and the results “highlight the need for interventions to ensure men and women receive guideline recommended treatment to lower the risk for recurrent MI, recurrent CHD, heart failure, and mortality after hospital discharge for MI,” they concluded.
Many women don’t recognize heart disease risk
Although women showed a greater reduction in recurrent MI and recurrent CHD events compared with men, the awareness of heart disease as the No. 1 killer of women has declined, according to a special report from the American Heart Association.
Based on survey data from 2009, 65% of women were aware that heart disease was their leading cause of death (LCOD); by 2019 the number dropped to 44%. The 10-year decline occurred across all races and ethnicities, as well as ages, with the exception of women aged 65 years and older.
The American Heart Association has conducted national surveys since 1997 to monitor awareness of cardiovascular disease among U.S. women. Data from earlier surveys showed increased awareness of heart disease as LCOD and increased awareness of heart attack symptoms between 1997 and 2012, wrote Mary Cushman, MD, of the University of Vermont, Burlington, chair of the writing group for the statement, and colleagues.
However, overall awareness and knowledge of heart disease among women remains poor, they wrote.
“Awareness programs designed to educate the public about CVD among women in the United States include Go Red for Women by the American Heart Association; The Heart Truth by the National Heart, Lung, and Blood Institute; and Make the Call, Don’t Miss a Beat by the U.S. Department of Health and Human Services,” the researchers noted. To determine the change in awareness of heart disease as the LCOD among women, the researchers conducted a multivariate analysis of 1,158 women who completed the 2009 survey and 1,345 who completed the 2019 survey. The average age was 50 years; roughly 70% of the participants in the 2009 survey and 62% in the 2019 survey were non-Hispanic White.
The greatest declines in awareness of heart disease as LCOD occurred among Hispanics and non-Hispanic Blacks and among all respondents aged 25-34 years.
Awareness of heart disease as LCOD was 30% lower among women with high blood pressure compared with women overall, the researchers noted.
“In both surveys, higher educational attainment was strongly related to awareness that heart disease is the LCOD,” the researchers said. However, the results highlight the need for renewed efforts to educate younger women, Hispanic women, and non-Hispanic Black women, they emphasized. Unpublished data from the AHA survey showed that “younger women were less likely to report leading a heart-healthy lifestyle and were more likely to identify multiple barriers to leading a heart-healthy lifestyle, including lack of time, stress, and lack of confidence,” they wrote.
In addition, awareness of heart attack warning signs declined overall and within each ethnic group between 2009 and 2019.
The survey results were limited by several factors including the use of an online-only model that might limit generalizability to populations without online access, and was conducted only in English, the researchers wrote.
Heart disease needs new PR plan
The study of heart disease risk awareness among women was an important update to understand how well the message about women’s risk is getting out, said Martha Gulati, MD, president-elect of the American Society of Preventive Cardiology, in an interview.
The issue remains that heart disease is the No. 1 killer of women, and the decrease in awareness “means we need to amplify our message,” she said.
“I also question whether the symbol of the red dress [for women’s heart disease] is working, and it seems that now is the time to change this symbol,” she emphasized. “I wear a red dress pin on my lab coat and every day someone asks what it means, and no one recognizes it,” she said. “I think ‘Go Red for Women’ is great and part of our outward campaign, but our symbol needs to change to increase the connection and awareness in women,” she said.
What might be a better symbol? Simply, a heart, said Dr. Gulati. But “we need to study whatever is next to really connect with women and make them understand their risk for heart disease,” she added.
“Additionally, we really need to get to minority women,” she said. “We are lagging there, and the survey was conducted in English so it missed many people,” she noted.
Dr. Gulati said she was shocked at how much awareness of heart disease risk has fallen among women, even in those with risk factors such as hypertension, who were 30% less likely to be aware that heart disease remains their leading cause of death. “Younger women as well as very unaware; what this means to me is that our public education efforts need to be amplified,” Dr. Gulati said.
Barriers to educating women about heart disease risk include language and access to affordable screening, Dr. Gulati emphasized. “We need to ensure screening for heart disease is always included as a covered cost for a preventive service,” she said.
“Research needs to be done to identify what works toward educating women about cardiac risk. We need to identify a marketing tool to increase awareness in women. It might be something different for one race versus another,” Dr. Gulati said. “Our messaging needs to improve, but how we improve it needs more than just health care professionals,” she said.
Focus on prevention to reduce MI recurrence
“The study regarding recurrent events after MI is important because we really don’t know much about recurrent coronary heart disease after a MI over time,” said Dr. Gulati. These data can be helpful in managing surviving patients and understanding future risk, she said. “But I was surprised to see fewer recurrent events in women, as women still have more heart failure than men even if it has declined with time,” she noted.
Dr. Gulati questioned several aspects of the study and highlighted some of the limitations. “These are claims data, so do they accurately reflect the U.S. population?” she asked. “Remember, this is a study of people who survived a heart attack; those who didn’t survive aren’t included, and that group is more likely to be women, especially women younger than 55 years,” she said.
In addition, Dr. Gulati noted the lack of data on type of heart attack and on treatment adherence or referral to cardiac rehab, as well as lack of data on long-term medication adherence or follow-up care.
Prevention is the key take-home message from both studies, “whether we are talking primary prevention for the heart disease awareness study or secondary prevention for the recurrent heart attack study,” Dr. Gulati said.
The recurrent heart disease study was supported in part by Amgen and the University of Alabama at Birmingham. Lead author Dr. Peters disclosed support from a UK Medical Research Council Skills Development Fellowship with no financial conflicts. Dr. Cushman had no financial conflicts to disclose; several coauthors on the writing committee disclosed relationships with companies including Amarin and Boehringer Ingelheim. Dr. Gulati had no financial conflicts to disclose.
SOURCE: Peters SAE et al. Circulation. 2020 Sep 21. doi: 10.1161/CIRCULATIONAHA.120.047065; Cushman M et al. Circulation. 2020 Sep 21. doi: 10.1161/CIR.0000000000000907.
The number of heart attack survivors in the United States who experienced repeat attacks within a year decreased between 2008 and 2017, especially among women, yet women’s awareness of their risk of death from heart disease also decreased, according to data from a pair of studies published in Circulation.
Recurrent MI rates drop, but not enough
Although the overall morbidity and mortality from coronary heart disease (CHD) in the United States has been on the decline for decades, CHD remains the leading cause of death and disability in both sexes, wrote Sanne A.E. Peters, PhD, of Imperial College London, and colleagues.
To better assess the rates of recurrent CHD by sex, the researchers reviewed data from 770,408 women and 700,477 men younger than 65 years with commercial health insurance or aged 66 years and older with Medicare who were hospitalized for myocardial infarction between 2008 and 2017. The patients were followed for 1 year for recurrent MIs, recurrent CHD events, heart failure hospitalization, and all-cause mortality.
In the study of recurrent heart disease, the rate of recurrent heart attacks per 1,000 person-years declined from 89.2 to 72.3 in women and from 94.2 to 81.3 in men. In addition, the rate of recurrent heart disease events (defined as either an MI or an artery-opening procedure), dropped per 1,000 person-years from 166.3 to 133.3 in women and from 198.1 to 176.8 in men. The reduction was significantly greater among women compared with men (P < .001 for both recurrent MIs and recurrent CHD events) and the differences by sex were consistent throughout the study period.
However, no significant difference occurred in recurrent MI rates among younger women (aged 21-54 years), or men aged 55-79 years, the researchers noted.
Heart failure rates per 1,000 person-years decreased from 177.4 to 158.1 in women and from 162.9 to 156.1 in men during the study period, and all-cause mortality decreased per 1,000 person-years from 403.2 to 389.5 for women and from 436.1 to 417.9 in men.
Potential contributing factors to the reductions in rates of recurrent events after a heart attack may include improved acute cardiac procedures, in-hospital therapy, and secondary prevention, the researchers noted. In addition, “changes in the type and definition of MI may also have contributed to the decline in recurrent events,” they said. “Also, the introduction and increasing sensitivity of cardiac biomarkers assays, especially cardiac troponin, may have contributed to an increased detection of less severe MIs over time, which, in turn, could have resulted in artifactual reductions in the consequences of MI,” they said.
The study findings were limited by several factors including the use of claims data, lack of information on the severity of heart attacks, and inability to analyze population subgroups, but the results were strengthened by the use of a large, multicultural database.
Despite the decline seen in this study, overall rates of recurrent MI, recurrent CHD events, heart failure hospitalization, and mortality remain high, the researchers said, and the results “highlight the need for interventions to ensure men and women receive guideline recommended treatment to lower the risk for recurrent MI, recurrent CHD, heart failure, and mortality after hospital discharge for MI,” they concluded.
Many women don’t recognize heart disease risk
Although women showed a greater reduction in recurrent MI and recurrent CHD events compared with men, the awareness of heart disease as the No. 1 killer of women has declined, according to a special report from the American Heart Association.
Based on survey data from 2009, 65% of women were aware that heart disease was their leading cause of death (LCOD); by 2019 the number dropped to 44%. The 10-year decline occurred across all races and ethnicities, as well as ages, with the exception of women aged 65 years and older.
The American Heart Association has conducted national surveys since 1997 to monitor awareness of cardiovascular disease among U.S. women. Data from earlier surveys showed increased awareness of heart disease as LCOD and increased awareness of heart attack symptoms between 1997 and 2012, wrote Mary Cushman, MD, of the University of Vermont, Burlington, chair of the writing group for the statement, and colleagues.
However, overall awareness and knowledge of heart disease among women remains poor, they wrote.
“Awareness programs designed to educate the public about CVD among women in the United States include Go Red for Women by the American Heart Association; The Heart Truth by the National Heart, Lung, and Blood Institute; and Make the Call, Don’t Miss a Beat by the U.S. Department of Health and Human Services,” the researchers noted. To determine the change in awareness of heart disease as the LCOD among women, the researchers conducted a multivariate analysis of 1,158 women who completed the 2009 survey and 1,345 who completed the 2019 survey. The average age was 50 years; roughly 70% of the participants in the 2009 survey and 62% in the 2019 survey were non-Hispanic White.
The greatest declines in awareness of heart disease as LCOD occurred among Hispanics and non-Hispanic Blacks and among all respondents aged 25-34 years.
Awareness of heart disease as LCOD was 30% lower among women with high blood pressure compared with women overall, the researchers noted.
“In both surveys, higher educational attainment was strongly related to awareness that heart disease is the LCOD,” the researchers said. However, the results highlight the need for renewed efforts to educate younger women, Hispanic women, and non-Hispanic Black women, they emphasized. Unpublished data from the AHA survey showed that “younger women were less likely to report leading a heart-healthy lifestyle and were more likely to identify multiple barriers to leading a heart-healthy lifestyle, including lack of time, stress, and lack of confidence,” they wrote.
In addition, awareness of heart attack warning signs declined overall and within each ethnic group between 2009 and 2019.
The survey results were limited by several factors including the use of an online-only model that might limit generalizability to populations without online access, and was conducted only in English, the researchers wrote.
Heart disease needs new PR plan
The study of heart disease risk awareness among women was an important update to understand how well the message about women’s risk is getting out, said Martha Gulati, MD, president-elect of the American Society of Preventive Cardiology, in an interview.
The issue remains that heart disease is the No. 1 killer of women, and the decrease in awareness “means we need to amplify our message,” she said.
“I also question whether the symbol of the red dress [for women’s heart disease] is working, and it seems that now is the time to change this symbol,” she emphasized. “I wear a red dress pin on my lab coat and every day someone asks what it means, and no one recognizes it,” she said. “I think ‘Go Red for Women’ is great and part of our outward campaign, but our symbol needs to change to increase the connection and awareness in women,” she said.
What might be a better symbol? Simply, a heart, said Dr. Gulati. But “we need to study whatever is next to really connect with women and make them understand their risk for heart disease,” she added.
“Additionally, we really need to get to minority women,” she said. “We are lagging there, and the survey was conducted in English so it missed many people,” she noted.
Dr. Gulati said she was shocked at how much awareness of heart disease risk has fallen among women, even in those with risk factors such as hypertension, who were 30% less likely to be aware that heart disease remains their leading cause of death. “Younger women as well as very unaware; what this means to me is that our public education efforts need to be amplified,” Dr. Gulati said.
Barriers to educating women about heart disease risk include language and access to affordable screening, Dr. Gulati emphasized. “We need to ensure screening for heart disease is always included as a covered cost for a preventive service,” she said.
“Research needs to be done to identify what works toward educating women about cardiac risk. We need to identify a marketing tool to increase awareness in women. It might be something different for one race versus another,” Dr. Gulati said. “Our messaging needs to improve, but how we improve it needs more than just health care professionals,” she said.
Focus on prevention to reduce MI recurrence
“The study regarding recurrent events after MI is important because we really don’t know much about recurrent coronary heart disease after a MI over time,” said Dr. Gulati. These data can be helpful in managing surviving patients and understanding future risk, she said. “But I was surprised to see fewer recurrent events in women, as women still have more heart failure than men even if it has declined with time,” she noted.
Dr. Gulati questioned several aspects of the study and highlighted some of the limitations. “These are claims data, so do they accurately reflect the U.S. population?” she asked. “Remember, this is a study of people who survived a heart attack; those who didn’t survive aren’t included, and that group is more likely to be women, especially women younger than 55 years,” she said.
In addition, Dr. Gulati noted the lack of data on type of heart attack and on treatment adherence or referral to cardiac rehab, as well as lack of data on long-term medication adherence or follow-up care.
Prevention is the key take-home message from both studies, “whether we are talking primary prevention for the heart disease awareness study or secondary prevention for the recurrent heart attack study,” Dr. Gulati said.
The recurrent heart disease study was supported in part by Amgen and the University of Alabama at Birmingham. Lead author Dr. Peters disclosed support from a UK Medical Research Council Skills Development Fellowship with no financial conflicts. Dr. Cushman had no financial conflicts to disclose; several coauthors on the writing committee disclosed relationships with companies including Amarin and Boehringer Ingelheim. Dr. Gulati had no financial conflicts to disclose.
SOURCE: Peters SAE et al. Circulation. 2020 Sep 21. doi: 10.1161/CIRCULATIONAHA.120.047065; Cushman M et al. Circulation. 2020 Sep 21. doi: 10.1161/CIR.0000000000000907.
The number of heart attack survivors in the United States who experienced repeat attacks within a year decreased between 2008 and 2017, especially among women, yet women’s awareness of their risk of death from heart disease also decreased, according to data from a pair of studies published in Circulation.
Recurrent MI rates drop, but not enough
Although the overall morbidity and mortality from coronary heart disease (CHD) in the United States has been on the decline for decades, CHD remains the leading cause of death and disability in both sexes, wrote Sanne A.E. Peters, PhD, of Imperial College London, and colleagues.
To better assess the rates of recurrent CHD by sex, the researchers reviewed data from 770,408 women and 700,477 men younger than 65 years with commercial health insurance or aged 66 years and older with Medicare who were hospitalized for myocardial infarction between 2008 and 2017. The patients were followed for 1 year for recurrent MIs, recurrent CHD events, heart failure hospitalization, and all-cause mortality.
In the study of recurrent heart disease, the rate of recurrent heart attacks per 1,000 person-years declined from 89.2 to 72.3 in women and from 94.2 to 81.3 in men. In addition, the rate of recurrent heart disease events (defined as either an MI or an artery-opening procedure), dropped per 1,000 person-years from 166.3 to 133.3 in women and from 198.1 to 176.8 in men. The reduction was significantly greater among women compared with men (P < .001 for both recurrent MIs and recurrent CHD events) and the differences by sex were consistent throughout the study period.
However, no significant difference occurred in recurrent MI rates among younger women (aged 21-54 years), or men aged 55-79 years, the researchers noted.
Heart failure rates per 1,000 person-years decreased from 177.4 to 158.1 in women and from 162.9 to 156.1 in men during the study period, and all-cause mortality decreased per 1,000 person-years from 403.2 to 389.5 for women and from 436.1 to 417.9 in men.
Potential contributing factors to the reductions in rates of recurrent events after a heart attack may include improved acute cardiac procedures, in-hospital therapy, and secondary prevention, the researchers noted. In addition, “changes in the type and definition of MI may also have contributed to the decline in recurrent events,” they said. “Also, the introduction and increasing sensitivity of cardiac biomarkers assays, especially cardiac troponin, may have contributed to an increased detection of less severe MIs over time, which, in turn, could have resulted in artifactual reductions in the consequences of MI,” they said.
The study findings were limited by several factors including the use of claims data, lack of information on the severity of heart attacks, and inability to analyze population subgroups, but the results were strengthened by the use of a large, multicultural database.
Despite the decline seen in this study, overall rates of recurrent MI, recurrent CHD events, heart failure hospitalization, and mortality remain high, the researchers said, and the results “highlight the need for interventions to ensure men and women receive guideline recommended treatment to lower the risk for recurrent MI, recurrent CHD, heart failure, and mortality after hospital discharge for MI,” they concluded.
Many women don’t recognize heart disease risk
Although women showed a greater reduction in recurrent MI and recurrent CHD events compared with men, the awareness of heart disease as the No. 1 killer of women has declined, according to a special report from the American Heart Association.
Based on survey data from 2009, 65% of women were aware that heart disease was their leading cause of death (LCOD); by 2019 the number dropped to 44%. The 10-year decline occurred across all races and ethnicities, as well as ages, with the exception of women aged 65 years and older.
The American Heart Association has conducted national surveys since 1997 to monitor awareness of cardiovascular disease among U.S. women. Data from earlier surveys showed increased awareness of heart disease as LCOD and increased awareness of heart attack symptoms between 1997 and 2012, wrote Mary Cushman, MD, of the University of Vermont, Burlington, chair of the writing group for the statement, and colleagues.
However, overall awareness and knowledge of heart disease among women remains poor, they wrote.
“Awareness programs designed to educate the public about CVD among women in the United States include Go Red for Women by the American Heart Association; The Heart Truth by the National Heart, Lung, and Blood Institute; and Make the Call, Don’t Miss a Beat by the U.S. Department of Health and Human Services,” the researchers noted. To determine the change in awareness of heart disease as the LCOD among women, the researchers conducted a multivariate analysis of 1,158 women who completed the 2009 survey and 1,345 who completed the 2019 survey. The average age was 50 years; roughly 70% of the participants in the 2009 survey and 62% in the 2019 survey were non-Hispanic White.
The greatest declines in awareness of heart disease as LCOD occurred among Hispanics and non-Hispanic Blacks and among all respondents aged 25-34 years.
Awareness of heart disease as LCOD was 30% lower among women with high blood pressure compared with women overall, the researchers noted.
“In both surveys, higher educational attainment was strongly related to awareness that heart disease is the LCOD,” the researchers said. However, the results highlight the need for renewed efforts to educate younger women, Hispanic women, and non-Hispanic Black women, they emphasized. Unpublished data from the AHA survey showed that “younger women were less likely to report leading a heart-healthy lifestyle and were more likely to identify multiple barriers to leading a heart-healthy lifestyle, including lack of time, stress, and lack of confidence,” they wrote.
In addition, awareness of heart attack warning signs declined overall and within each ethnic group between 2009 and 2019.
The survey results were limited by several factors including the use of an online-only model that might limit generalizability to populations without online access, and was conducted only in English, the researchers wrote.
Heart disease needs new PR plan
The study of heart disease risk awareness among women was an important update to understand how well the message about women’s risk is getting out, said Martha Gulati, MD, president-elect of the American Society of Preventive Cardiology, in an interview.
The issue remains that heart disease is the No. 1 killer of women, and the decrease in awareness “means we need to amplify our message,” she said.
“I also question whether the symbol of the red dress [for women’s heart disease] is working, and it seems that now is the time to change this symbol,” she emphasized. “I wear a red dress pin on my lab coat and every day someone asks what it means, and no one recognizes it,” she said. “I think ‘Go Red for Women’ is great and part of our outward campaign, but our symbol needs to change to increase the connection and awareness in women,” she said.
What might be a better symbol? Simply, a heart, said Dr. Gulati. But “we need to study whatever is next to really connect with women and make them understand their risk for heart disease,” she added.
“Additionally, we really need to get to minority women,” she said. “We are lagging there, and the survey was conducted in English so it missed many people,” she noted.
Dr. Gulati said she was shocked at how much awareness of heart disease risk has fallen among women, even in those with risk factors such as hypertension, who were 30% less likely to be aware that heart disease remains their leading cause of death. “Younger women as well as very unaware; what this means to me is that our public education efforts need to be amplified,” Dr. Gulati said.
Barriers to educating women about heart disease risk include language and access to affordable screening, Dr. Gulati emphasized. “We need to ensure screening for heart disease is always included as a covered cost for a preventive service,” she said.
“Research needs to be done to identify what works toward educating women about cardiac risk. We need to identify a marketing tool to increase awareness in women. It might be something different for one race versus another,” Dr. Gulati said. “Our messaging needs to improve, but how we improve it needs more than just health care professionals,” she said.
Focus on prevention to reduce MI recurrence
“The study regarding recurrent events after MI is important because we really don’t know much about recurrent coronary heart disease after a MI over time,” said Dr. Gulati. These data can be helpful in managing surviving patients and understanding future risk, she said. “But I was surprised to see fewer recurrent events in women, as women still have more heart failure than men even if it has declined with time,” she noted.
Dr. Gulati questioned several aspects of the study and highlighted some of the limitations. “These are claims data, so do they accurately reflect the U.S. population?” she asked. “Remember, this is a study of people who survived a heart attack; those who didn’t survive aren’t included, and that group is more likely to be women, especially women younger than 55 years,” she said.
In addition, Dr. Gulati noted the lack of data on type of heart attack and on treatment adherence or referral to cardiac rehab, as well as lack of data on long-term medication adherence or follow-up care.
Prevention is the key take-home message from both studies, “whether we are talking primary prevention for the heart disease awareness study or secondary prevention for the recurrent heart attack study,” Dr. Gulati said.
The recurrent heart disease study was supported in part by Amgen and the University of Alabama at Birmingham. Lead author Dr. Peters disclosed support from a UK Medical Research Council Skills Development Fellowship with no financial conflicts. Dr. Cushman had no financial conflicts to disclose; several coauthors on the writing committee disclosed relationships with companies including Amarin and Boehringer Ingelheim. Dr. Gulati had no financial conflicts to disclose.
SOURCE: Peters SAE et al. Circulation. 2020 Sep 21. doi: 10.1161/CIRCULATIONAHA.120.047065; Cushman M et al. Circulation. 2020 Sep 21. doi: 10.1161/CIR.0000000000000907.
FROM CIRCULATION
Time-restricted eating shows no weight-loss benefit in RCT
The popular new weight-loss approach of eating within a restricted window of time during the day, allowing for an extended period of fasting – also known as intermittent fasting – does not result in greater weight loss, compared with nonrestricted meal timing, results from a randomized clinical trial show.
“I was very surprised by all of [the results],” senior author Ethan J. Weiss, MD, said in an interview.
“Part of the reason we did the study was because I had been doing time-restricted eating myself for years and even recommending it to friends and patients as an effective weight-loss tool,” said Dr. Weiss, of the Cardiovascular Research Institute, University of California, San Francisco.
“But no matter how you slice it, prescription of time-restricted eating – at least this version –is not a very effective weight-loss strategy,” Dr. Weiss said.
The study, published online in JAMA Internal Medicine by Dylan A. Lowe, PhD, also of the University of California, San Francisco, involved 116 participants who were randomized to a 12-week regimen of either three structured meals per day or time-restricted eating, with instructions to eat only between 12:00 p.m. and 8:00 p.m. and to completely abstain from eating at other times.
The participants were not given any specific instructions regarding caloric or macronutrient intake “so as to offer a simple, real-world recommendation to free-living individuals,” the authors wrote.
Although some prior research has shown improvements in measures such as glucose tolerance with time-restricted eating, studies showing weight loss with the approach, including one recently reported by Medscape Medical News, have been small and lacked control groups.
“To my knowledge this is the first randomized, controlled trial and definitely the biggest,” Dr. Weiss. “I think it is the most comprehensive dataset available in people, at least for this intervention.”
Participants used app to log details
At baseline, participants had a mean weight of 99.2 kg (approximately 219 lb). Their mean age was 46.5 years and 60.3% were men. They were drawn from anywhere in the United States and received study surveys through a custom mobile study application on the Eureka Research Platform. They were given a Bluetooth weight scale to use daily, which was connected with the app, and randomized to one of the two interventions. A subset of 50 participants living near San Francisco underwent in-person testing.
At the end of the 12 weeks, those in the time-restricted eating group (n = 59) did have a significant decrease in weight, compared with baseline (−0.94 kg; P = .01), while weight loss in the consistent-meal group (n = 57) was not significant (−0.68 kg; P = .07).
But importantly, the difference in weight loss between the groups was not significant (−0.26 kg; P = .63).
There were no significant differences in secondary outcomes of fasting insulin, glucose, hemoglobin A1c, or blood lipids within or between the time-restricted eating and consistent-meal group either. Nor were there any significant differences in resting metabolic rate.
Although participants did not self-report their caloric intake, the authors estimated that the differences were not significant using mathematical modeling developed at the National Institutes of Health.
Rates of adherence to the diets were 92.1% in the consistent-meal group versus 83.5% in the time-restricted group.
Not all diets are equal: Time-restricted eating group lost more lean mass
In a subset analysis, loss of lean mass was significantly greater in the time-restricted eating group, compared with the consistent-meals group, in terms of both appendicular lean mass (P = .009) and the appendicular lean mass index (P = .005).
In fact, as much as 65% of the weight lost (1.10 kg of the average 1.70 kg) in the time-restricted eating group consisted of lean mass, while much less was fat mass (0.51 kg).
“The proportion of lean mass loss in this study (approximately 65%) far exceeds the normal range of 20%-30%,” the authors wrote. “In addition, there was a highly significant between-group difference in appendicular lean mass.”
Appendicular lean mass correlates with nutritional and physical status, and its reduction can lead to weakness, disability, and impaired quality of life.
“This serves as a caution for patient populations at risk for sarcopenia because time-restricted eating could exacerbate muscle loss,” the authors asserted.
Furthermore, previous studies suggest that the loss of lean mass in such studies is positively linked with weight regain.
While a limitation of the work is that self-reported measures of energy or macronutrient or protein intake were not obtained, the authors speculated that the role of protein intake could be linked to the greater loss of lean mass.
“Given the loss of appendicular lean mass in participants in the time-restricted eating arm and previous reports of decreased protein consumption from time-restricted eating, it is possible that protein intake was altered by time-restricted eating in this cohort, and this clearly warrants future study,” they wrote.
Dr. Weiss said the findings underscore that not all weight loss in dieting is beneficial.
“Losing 1 kg of lean mass (is not equal) to a kilogram of fat,” he said. “Indeed, if one loses 0.65 kg of lean mass and only 0.35 kg of fat mass, that is an intervention I’d probably pass on.”
Time-restricted eating is popular, perhaps because it’s easy?
Time-restricted eating has gained popularity in recent years.
The approach “is attractive as a weight-loss option in that it does not require tedious and time-consuming methods such as calorie counting or adherence to complicated diets,” the authors noted. “Indeed, we found that self-reported adherence to the time-restricted eating schedule was high; however, in contrast to our hypothesis, there was no greater weight loss with time-restricted eating compared with the consistent meal timing.”
They explain that the 12 p.m. to 8 p.m. window for eating was chosen because they thought people might find it easier culturally to skip breakfast than dinner, the more social meal.
However, an 8 p.m. cutoff is somewhat late given there is some suggestion that fasting several hours before bedtime is most beneficial, Dr. Weiss noted. So it may be worth examining different time windows.
“I am very intrigued about looking at early time-restricted eating – 6 a.m. to 2 p.m.,” for example, he said. “It is on our list.”
Meanwhile, the study results support previous research showing no effect on weight outcomes in relation to skipping breakfast.
The study received funding from the UCSF cardiology division’s Cardiology Innovations Award Program and the National Institute of Diabetes and Digestive and Kidney Diseases, with additional support from the James Peter Read Foundation. Dr. Weiss has reported nonfinancial support from Mocacare and nonfinancial support from iHealth Labs during the conduct of the study. He also is a cofounder and equity stakeholder of Keyto, and owns stock and was formerly on the board of Virta.
A version of this article originally appeared on Medscape.com.
The popular new weight-loss approach of eating within a restricted window of time during the day, allowing for an extended period of fasting – also known as intermittent fasting – does not result in greater weight loss, compared with nonrestricted meal timing, results from a randomized clinical trial show.
“I was very surprised by all of [the results],” senior author Ethan J. Weiss, MD, said in an interview.
“Part of the reason we did the study was because I had been doing time-restricted eating myself for years and even recommending it to friends and patients as an effective weight-loss tool,” said Dr. Weiss, of the Cardiovascular Research Institute, University of California, San Francisco.
“But no matter how you slice it, prescription of time-restricted eating – at least this version –is not a very effective weight-loss strategy,” Dr. Weiss said.
The study, published online in JAMA Internal Medicine by Dylan A. Lowe, PhD, also of the University of California, San Francisco, involved 116 participants who were randomized to a 12-week regimen of either three structured meals per day or time-restricted eating, with instructions to eat only between 12:00 p.m. and 8:00 p.m. and to completely abstain from eating at other times.
The participants were not given any specific instructions regarding caloric or macronutrient intake “so as to offer a simple, real-world recommendation to free-living individuals,” the authors wrote.
Although some prior research has shown improvements in measures such as glucose tolerance with time-restricted eating, studies showing weight loss with the approach, including one recently reported by Medscape Medical News, have been small and lacked control groups.
“To my knowledge this is the first randomized, controlled trial and definitely the biggest,” Dr. Weiss. “I think it is the most comprehensive dataset available in people, at least for this intervention.”
Participants used app to log details
At baseline, participants had a mean weight of 99.2 kg (approximately 219 lb). Their mean age was 46.5 years and 60.3% were men. They were drawn from anywhere in the United States and received study surveys through a custom mobile study application on the Eureka Research Platform. They were given a Bluetooth weight scale to use daily, which was connected with the app, and randomized to one of the two interventions. A subset of 50 participants living near San Francisco underwent in-person testing.
At the end of the 12 weeks, those in the time-restricted eating group (n = 59) did have a significant decrease in weight, compared with baseline (−0.94 kg; P = .01), while weight loss in the consistent-meal group (n = 57) was not significant (−0.68 kg; P = .07).
But importantly, the difference in weight loss between the groups was not significant (−0.26 kg; P = .63).
There were no significant differences in secondary outcomes of fasting insulin, glucose, hemoglobin A1c, or blood lipids within or between the time-restricted eating and consistent-meal group either. Nor were there any significant differences in resting metabolic rate.
Although participants did not self-report their caloric intake, the authors estimated that the differences were not significant using mathematical modeling developed at the National Institutes of Health.
Rates of adherence to the diets were 92.1% in the consistent-meal group versus 83.5% in the time-restricted group.
Not all diets are equal: Time-restricted eating group lost more lean mass
In a subset analysis, loss of lean mass was significantly greater in the time-restricted eating group, compared with the consistent-meals group, in terms of both appendicular lean mass (P = .009) and the appendicular lean mass index (P = .005).
In fact, as much as 65% of the weight lost (1.10 kg of the average 1.70 kg) in the time-restricted eating group consisted of lean mass, while much less was fat mass (0.51 kg).
“The proportion of lean mass loss in this study (approximately 65%) far exceeds the normal range of 20%-30%,” the authors wrote. “In addition, there was a highly significant between-group difference in appendicular lean mass.”
Appendicular lean mass correlates with nutritional and physical status, and its reduction can lead to weakness, disability, and impaired quality of life.
“This serves as a caution for patient populations at risk for sarcopenia because time-restricted eating could exacerbate muscle loss,” the authors asserted.
Furthermore, previous studies suggest that the loss of lean mass in such studies is positively linked with weight regain.
While a limitation of the work is that self-reported measures of energy or macronutrient or protein intake were not obtained, the authors speculated that the role of protein intake could be linked to the greater loss of lean mass.
“Given the loss of appendicular lean mass in participants in the time-restricted eating arm and previous reports of decreased protein consumption from time-restricted eating, it is possible that protein intake was altered by time-restricted eating in this cohort, and this clearly warrants future study,” they wrote.
Dr. Weiss said the findings underscore that not all weight loss in dieting is beneficial.
“Losing 1 kg of lean mass (is not equal) to a kilogram of fat,” he said. “Indeed, if one loses 0.65 kg of lean mass and only 0.35 kg of fat mass, that is an intervention I’d probably pass on.”
Time-restricted eating is popular, perhaps because it’s easy?
Time-restricted eating has gained popularity in recent years.
The approach “is attractive as a weight-loss option in that it does not require tedious and time-consuming methods such as calorie counting or adherence to complicated diets,” the authors noted. “Indeed, we found that self-reported adherence to the time-restricted eating schedule was high; however, in contrast to our hypothesis, there was no greater weight loss with time-restricted eating compared with the consistent meal timing.”
They explain that the 12 p.m. to 8 p.m. window for eating was chosen because they thought people might find it easier culturally to skip breakfast than dinner, the more social meal.
However, an 8 p.m. cutoff is somewhat late given there is some suggestion that fasting several hours before bedtime is most beneficial, Dr. Weiss noted. So it may be worth examining different time windows.
“I am very intrigued about looking at early time-restricted eating – 6 a.m. to 2 p.m.,” for example, he said. “It is on our list.”
Meanwhile, the study results support previous research showing no effect on weight outcomes in relation to skipping breakfast.
The study received funding from the UCSF cardiology division’s Cardiology Innovations Award Program and the National Institute of Diabetes and Digestive and Kidney Diseases, with additional support from the James Peter Read Foundation. Dr. Weiss has reported nonfinancial support from Mocacare and nonfinancial support from iHealth Labs during the conduct of the study. He also is a cofounder and equity stakeholder of Keyto, and owns stock and was formerly on the board of Virta.
A version of this article originally appeared on Medscape.com.
The popular new weight-loss approach of eating within a restricted window of time during the day, allowing for an extended period of fasting – also known as intermittent fasting – does not result in greater weight loss, compared with nonrestricted meal timing, results from a randomized clinical trial show.
“I was very surprised by all of [the results],” senior author Ethan J. Weiss, MD, said in an interview.
“Part of the reason we did the study was because I had been doing time-restricted eating myself for years and even recommending it to friends and patients as an effective weight-loss tool,” said Dr. Weiss, of the Cardiovascular Research Institute, University of California, San Francisco.
“But no matter how you slice it, prescription of time-restricted eating – at least this version –is not a very effective weight-loss strategy,” Dr. Weiss said.
The study, published online in JAMA Internal Medicine by Dylan A. Lowe, PhD, also of the University of California, San Francisco, involved 116 participants who were randomized to a 12-week regimen of either three structured meals per day or time-restricted eating, with instructions to eat only between 12:00 p.m. and 8:00 p.m. and to completely abstain from eating at other times.
The participants were not given any specific instructions regarding caloric or macronutrient intake “so as to offer a simple, real-world recommendation to free-living individuals,” the authors wrote.
Although some prior research has shown improvements in measures such as glucose tolerance with time-restricted eating, studies showing weight loss with the approach, including one recently reported by Medscape Medical News, have been small and lacked control groups.
“To my knowledge this is the first randomized, controlled trial and definitely the biggest,” Dr. Weiss. “I think it is the most comprehensive dataset available in people, at least for this intervention.”
Participants used app to log details
At baseline, participants had a mean weight of 99.2 kg (approximately 219 lb). Their mean age was 46.5 years and 60.3% were men. They were drawn from anywhere in the United States and received study surveys through a custom mobile study application on the Eureka Research Platform. They were given a Bluetooth weight scale to use daily, which was connected with the app, and randomized to one of the two interventions. A subset of 50 participants living near San Francisco underwent in-person testing.
At the end of the 12 weeks, those in the time-restricted eating group (n = 59) did have a significant decrease in weight, compared with baseline (−0.94 kg; P = .01), while weight loss in the consistent-meal group (n = 57) was not significant (−0.68 kg; P = .07).
But importantly, the difference in weight loss between the groups was not significant (−0.26 kg; P = .63).
There were no significant differences in secondary outcomes of fasting insulin, glucose, hemoglobin A1c, or blood lipids within or between the time-restricted eating and consistent-meal group either. Nor were there any significant differences in resting metabolic rate.
Although participants did not self-report their caloric intake, the authors estimated that the differences were not significant using mathematical modeling developed at the National Institutes of Health.
Rates of adherence to the diets were 92.1% in the consistent-meal group versus 83.5% in the time-restricted group.
Not all diets are equal: Time-restricted eating group lost more lean mass
In a subset analysis, loss of lean mass was significantly greater in the time-restricted eating group, compared with the consistent-meals group, in terms of both appendicular lean mass (P = .009) and the appendicular lean mass index (P = .005).
In fact, as much as 65% of the weight lost (1.10 kg of the average 1.70 kg) in the time-restricted eating group consisted of lean mass, while much less was fat mass (0.51 kg).
“The proportion of lean mass loss in this study (approximately 65%) far exceeds the normal range of 20%-30%,” the authors wrote. “In addition, there was a highly significant between-group difference in appendicular lean mass.”
Appendicular lean mass correlates with nutritional and physical status, and its reduction can lead to weakness, disability, and impaired quality of life.
“This serves as a caution for patient populations at risk for sarcopenia because time-restricted eating could exacerbate muscle loss,” the authors asserted.
Furthermore, previous studies suggest that the loss of lean mass in such studies is positively linked with weight regain.
While a limitation of the work is that self-reported measures of energy or macronutrient or protein intake were not obtained, the authors speculated that the role of protein intake could be linked to the greater loss of lean mass.
“Given the loss of appendicular lean mass in participants in the time-restricted eating arm and previous reports of decreased protein consumption from time-restricted eating, it is possible that protein intake was altered by time-restricted eating in this cohort, and this clearly warrants future study,” they wrote.
Dr. Weiss said the findings underscore that not all weight loss in dieting is beneficial.
“Losing 1 kg of lean mass (is not equal) to a kilogram of fat,” he said. “Indeed, if one loses 0.65 kg of lean mass and only 0.35 kg of fat mass, that is an intervention I’d probably pass on.”
Time-restricted eating is popular, perhaps because it’s easy?
Time-restricted eating has gained popularity in recent years.
The approach “is attractive as a weight-loss option in that it does not require tedious and time-consuming methods such as calorie counting or adherence to complicated diets,” the authors noted. “Indeed, we found that self-reported adherence to the time-restricted eating schedule was high; however, in contrast to our hypothesis, there was no greater weight loss with time-restricted eating compared with the consistent meal timing.”
They explain that the 12 p.m. to 8 p.m. window for eating was chosen because they thought people might find it easier culturally to skip breakfast than dinner, the more social meal.
However, an 8 p.m. cutoff is somewhat late given there is some suggestion that fasting several hours before bedtime is most beneficial, Dr. Weiss noted. So it may be worth examining different time windows.
“I am very intrigued about looking at early time-restricted eating – 6 a.m. to 2 p.m.,” for example, he said. “It is on our list.”
Meanwhile, the study results support previous research showing no effect on weight outcomes in relation to skipping breakfast.
The study received funding from the UCSF cardiology division’s Cardiology Innovations Award Program and the National Institute of Diabetes and Digestive and Kidney Diseases, with additional support from the James Peter Read Foundation. Dr. Weiss has reported nonfinancial support from Mocacare and nonfinancial support from iHealth Labs during the conduct of the study. He also is a cofounder and equity stakeholder of Keyto, and owns stock and was formerly on the board of Virta.
A version of this article originally appeared on Medscape.com.