User login
Triglyceride puzzle: Do TG metabolites better predict risk?
Triglyceride levels are a measure of cardiovascular risk and a target for therapy, but a focus on TG levels as a bad guy in CV risk assessments may be missing the mark, a population-based cohort study suggests.
The analysis, based on 30,000 participants in the Copenhagen General Population Study, saw sharply increased risks for all-cause mortality, CV mortality, and cancer mortality over 10 years among those with robust TG metabolism.
Those significant risks, gauged by concentrations of two molecules considered markers of TG metabolic rate, were independent of body mass index (BMI) and a range of other TG-linked risk factors, including plasma TG levels themselves.
All-cause mortality jumped 31% for plasma levels of glycerol in the highest versus lowest quartiles and rose 18% for highest-quartile levels of beta-hydroxybutyrate. In parallel, CV mortality climbed 37% for glycerol and 18% for beta-hydroxybutyrate in the study, published in the European Heart Journal.
The findings “implicate triglyceride metabolic rate as a risk factor for mortality not explained by high plasma triglycerides or high BMI,” the report states. The study, it continues, may be the first to link increased mortality to more active TG metabolism – according to levels of the two biomarkers – in the general population.
The results were “really, really surprising,” senior author Børge G. Nordestgaard, MD, DMSc, said in an interview. They are “completely novel” and “may make people think differently” about TG and mortality risk.
Given their unexpected findings, the group conducted further analyses for evidence that the metabolite-mortality associations weren’t independent. “We tried to stratify them away, but they stayed,” said Dr. Nordestgaard, of the University of Copenhagen.
In a weight-stratified analysis, for example, findings were similar in people with normal weight and with overweight and who were obese, Dr. Nordestgaard observed. “Even in the ones with normal weight by World Health Organization criteria, we saw the same and maybe even stronger relationships” between TG metabolism and mortality.
The study authors were is careful to note the retrospective cohort study’s limitations, but its findings “at most support an association, not causation,” Michael Miller, MD, Hospital of the University of Pennsylvania, Philadelphia, observed in an interview. Therefore, it can’t answer “whether and to what extent glycerol and/or beta-hydroxybutyrate independently contribute to mortality beyond triglyceride levels per se.”
Assessing levels of the two biomarkers “was an interesting way to indirectly assess whole-body TG metabolism,” but they were not fasting levels, said Dr. Miller, who wasn’t part of the study.
Also, the analysis doesn’t account for heparinization and other factors “that artificially raise glycerol levels” and suffers in other ways “from the inherent limitations of residual confounding,” said Dr. Miller, who is also chief of medicine at Corporal Michael J Crescenz VA Medical Center, Philadelphia.
The analysis tracked 30,000 men and women, participants in the much larger Copenhagen General Population Study cohort, for a median of 10.7 years. During that time, 9,897 of them died.
Plasma levels of glycerol and beta-hydroxybutyrate, the study authors noted, were measured using high-throughput nuclear magnetic resonance spectroscopy.
Glycerol levels greater than 80 mcmol/L represented the highest quartile and those less than 52 mcmol/L the lowest quartile. The corresponding beta-hydroxybutyrate quartiles were greater than 154 mcmol/L and less than 91 mcmol/L, respectively.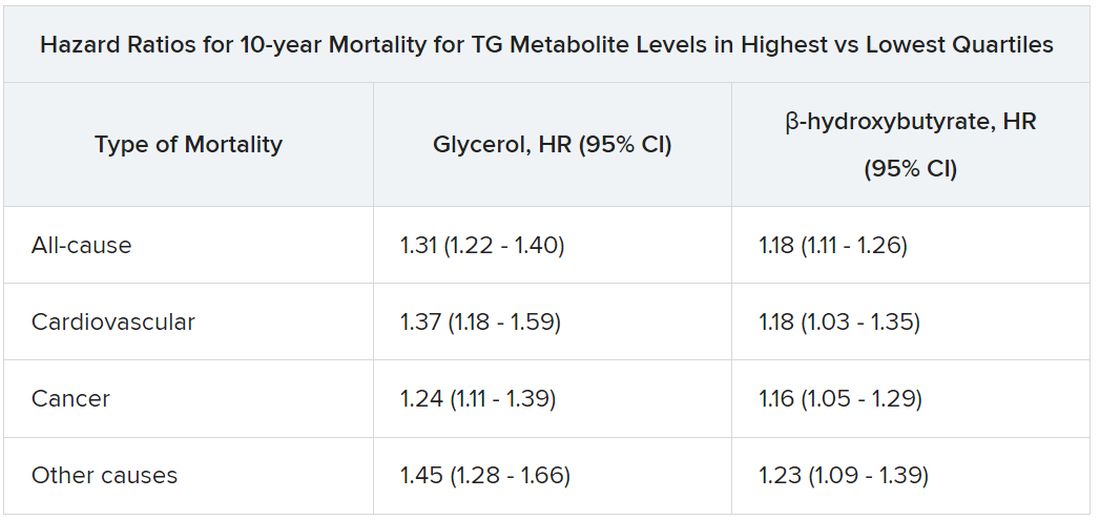
Mortality risks were independent not only of BMI and TG levels but also of age, greater waist circumference, many other standard CV risk factors, chronic obstructive pulmonary disease, diabetes, insulin use, and CV comorbidities and medications.
Dr. Nordestgaard, who also stressed that the findings are only hypothesis generating, speculated that glycerol and beta-hydroxybutyrate could potentially serve as biomarkers for predicting risk or guiding therapy and, indeed, might be amenable to risk-factor modification. “But I have absolutely no data to support that.”
The study was funded by the Independent Research Fund, and by Johan Boserup and Lise Boserups Grant. Dr. Nordestgaard reported consulting for or giving talks sponsored by AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Esperion, and Silence Therapeutics. The other authors reported no conflicts. Dr. Miller disclosed serving as a scientific adviser for Amarin and 89bio.
A version of this article first appeared on Medscape.com.
Triglyceride levels are a measure of cardiovascular risk and a target for therapy, but a focus on TG levels as a bad guy in CV risk assessments may be missing the mark, a population-based cohort study suggests.
The analysis, based on 30,000 participants in the Copenhagen General Population Study, saw sharply increased risks for all-cause mortality, CV mortality, and cancer mortality over 10 years among those with robust TG metabolism.
Those significant risks, gauged by concentrations of two molecules considered markers of TG metabolic rate, were independent of body mass index (BMI) and a range of other TG-linked risk factors, including plasma TG levels themselves.
All-cause mortality jumped 31% for plasma levels of glycerol in the highest versus lowest quartiles and rose 18% for highest-quartile levels of beta-hydroxybutyrate. In parallel, CV mortality climbed 37% for glycerol and 18% for beta-hydroxybutyrate in the study, published in the European Heart Journal.
The findings “implicate triglyceride metabolic rate as a risk factor for mortality not explained by high plasma triglycerides or high BMI,” the report states. The study, it continues, may be the first to link increased mortality to more active TG metabolism – according to levels of the two biomarkers – in the general population.
The results were “really, really surprising,” senior author Børge G. Nordestgaard, MD, DMSc, said in an interview. They are “completely novel” and “may make people think differently” about TG and mortality risk.
Given their unexpected findings, the group conducted further analyses for evidence that the metabolite-mortality associations weren’t independent. “We tried to stratify them away, but they stayed,” said Dr. Nordestgaard, of the University of Copenhagen.
In a weight-stratified analysis, for example, findings were similar in people with normal weight and with overweight and who were obese, Dr. Nordestgaard observed. “Even in the ones with normal weight by World Health Organization criteria, we saw the same and maybe even stronger relationships” between TG metabolism and mortality.
The study authors were is careful to note the retrospective cohort study’s limitations, but its findings “at most support an association, not causation,” Michael Miller, MD, Hospital of the University of Pennsylvania, Philadelphia, observed in an interview. Therefore, it can’t answer “whether and to what extent glycerol and/or beta-hydroxybutyrate independently contribute to mortality beyond triglyceride levels per se.”
Assessing levels of the two biomarkers “was an interesting way to indirectly assess whole-body TG metabolism,” but they were not fasting levels, said Dr. Miller, who wasn’t part of the study.
Also, the analysis doesn’t account for heparinization and other factors “that artificially raise glycerol levels” and suffers in other ways “from the inherent limitations of residual confounding,” said Dr. Miller, who is also chief of medicine at Corporal Michael J Crescenz VA Medical Center, Philadelphia.
The analysis tracked 30,000 men and women, participants in the much larger Copenhagen General Population Study cohort, for a median of 10.7 years. During that time, 9,897 of them died.
Plasma levels of glycerol and beta-hydroxybutyrate, the study authors noted, were measured using high-throughput nuclear magnetic resonance spectroscopy.
Glycerol levels greater than 80 mcmol/L represented the highest quartile and those less than 52 mcmol/L the lowest quartile. The corresponding beta-hydroxybutyrate quartiles were greater than 154 mcmol/L and less than 91 mcmol/L, respectively.
Mortality risks were independent not only of BMI and TG levels but also of age, greater waist circumference, many other standard CV risk factors, chronic obstructive pulmonary disease, diabetes, insulin use, and CV comorbidities and medications.
Dr. Nordestgaard, who also stressed that the findings are only hypothesis generating, speculated that glycerol and beta-hydroxybutyrate could potentially serve as biomarkers for predicting risk or guiding therapy and, indeed, might be amenable to risk-factor modification. “But I have absolutely no data to support that.”
The study was funded by the Independent Research Fund, and by Johan Boserup and Lise Boserups Grant. Dr. Nordestgaard reported consulting for or giving talks sponsored by AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Esperion, and Silence Therapeutics. The other authors reported no conflicts. Dr. Miller disclosed serving as a scientific adviser for Amarin and 89bio.
A version of this article first appeared on Medscape.com.
Triglyceride levels are a measure of cardiovascular risk and a target for therapy, but a focus on TG levels as a bad guy in CV risk assessments may be missing the mark, a population-based cohort study suggests.
The analysis, based on 30,000 participants in the Copenhagen General Population Study, saw sharply increased risks for all-cause mortality, CV mortality, and cancer mortality over 10 years among those with robust TG metabolism.
Those significant risks, gauged by concentrations of two molecules considered markers of TG metabolic rate, were independent of body mass index (BMI) and a range of other TG-linked risk factors, including plasma TG levels themselves.
All-cause mortality jumped 31% for plasma levels of glycerol in the highest versus lowest quartiles and rose 18% for highest-quartile levels of beta-hydroxybutyrate. In parallel, CV mortality climbed 37% for glycerol and 18% for beta-hydroxybutyrate in the study, published in the European Heart Journal.
The findings “implicate triglyceride metabolic rate as a risk factor for mortality not explained by high plasma triglycerides or high BMI,” the report states. The study, it continues, may be the first to link increased mortality to more active TG metabolism – according to levels of the two biomarkers – in the general population.
The results were “really, really surprising,” senior author Børge G. Nordestgaard, MD, DMSc, said in an interview. They are “completely novel” and “may make people think differently” about TG and mortality risk.
Given their unexpected findings, the group conducted further analyses for evidence that the metabolite-mortality associations weren’t independent. “We tried to stratify them away, but they stayed,” said Dr. Nordestgaard, of the University of Copenhagen.
In a weight-stratified analysis, for example, findings were similar in people with normal weight and with overweight and who were obese, Dr. Nordestgaard observed. “Even in the ones with normal weight by World Health Organization criteria, we saw the same and maybe even stronger relationships” between TG metabolism and mortality.
The study authors were is careful to note the retrospective cohort study’s limitations, but its findings “at most support an association, not causation,” Michael Miller, MD, Hospital of the University of Pennsylvania, Philadelphia, observed in an interview. Therefore, it can’t answer “whether and to what extent glycerol and/or beta-hydroxybutyrate independently contribute to mortality beyond triglyceride levels per se.”
Assessing levels of the two biomarkers “was an interesting way to indirectly assess whole-body TG metabolism,” but they were not fasting levels, said Dr. Miller, who wasn’t part of the study.
Also, the analysis doesn’t account for heparinization and other factors “that artificially raise glycerol levels” and suffers in other ways “from the inherent limitations of residual confounding,” said Dr. Miller, who is also chief of medicine at Corporal Michael J Crescenz VA Medical Center, Philadelphia.
The analysis tracked 30,000 men and women, participants in the much larger Copenhagen General Population Study cohort, for a median of 10.7 years. During that time, 9,897 of them died.
Plasma levels of glycerol and beta-hydroxybutyrate, the study authors noted, were measured using high-throughput nuclear magnetic resonance spectroscopy.
Glycerol levels greater than 80 mcmol/L represented the highest quartile and those less than 52 mcmol/L the lowest quartile. The corresponding beta-hydroxybutyrate quartiles were greater than 154 mcmol/L and less than 91 mcmol/L, respectively.
Mortality risks were independent not only of BMI and TG levels but also of age, greater waist circumference, many other standard CV risk factors, chronic obstructive pulmonary disease, diabetes, insulin use, and CV comorbidities and medications.
Dr. Nordestgaard, who also stressed that the findings are only hypothesis generating, speculated that glycerol and beta-hydroxybutyrate could potentially serve as biomarkers for predicting risk or guiding therapy and, indeed, might be amenable to risk-factor modification. “But I have absolutely no data to support that.”
The study was funded by the Independent Research Fund, and by Johan Boserup and Lise Boserups Grant. Dr. Nordestgaard reported consulting for or giving talks sponsored by AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Esperion, and Silence Therapeutics. The other authors reported no conflicts. Dr. Miller disclosed serving as a scientific adviser for Amarin and 89bio.
A version of this article first appeared on Medscape.com.
FROM THE EUROPEAN HEART JOURNAL
‘Water fasting’ benefits don’t last
Health benefits of prolonged “water fasting” (zero calories) or Buchinger fasting (200-300 calories/day) don’t last, according to authors of a review of eight studies.
Five days of fasting lowered weight by about 6%, but this weight was regained after 3 months of regular eating, the investigators found. The article was published in Nutrition Reviews.
“Water fasting led to improvements in blood pressure, cholesterol, and blood sugar levels, but these were short-lived,” senior author Krista A. Varady, PhD, told this news organization.
“Levels returned to baseline ... quickly after participants started eating. Most benefits disappeared in 3-4 months,” said Dr. Varady, professor of nutrition at the University of Illinois, Chicago.
“My overall conclusion,” she said, “is that I guess you could try it, but it just seems like a lot of work, and all those metabolic benefits disappear. I would encourage someone hoping to lose weight to try intermittent fasting instead of water fasting, because there’s a lot more data to show it can help with weight management.
“People should consult their doctor if they have diabetes or any other major obesity-related conditions before doing water fasting,” Dr. Varady cautioned.
“Healthy people with obesity can probably fast safely for 5 days on their own (if they don’t have any other conditions). However, no one should undertake one of these fasts for more than 5 days without medical supervision,” she stressed.
Eight studies of water and Buchinger fasting
Although several favorable effects of prolonged fasting have been observed, benefits must be weighed against risks, Dr. Varady and her coauthors wrote.
Most medically supervised fasting programs have reported only minor adverse events, which included hunger, headaches, nausea, vomiting, dry mouth, and fatigue. However, more severe events have been documented, including edema, abnormal results on liver function tests, decreased bone density, and metabolic acidosis.
The researchers aimed to determine the effect of prolonged fasting on weight, blood pressure, lipid levels, and glycemic control, as well as safety and the effects of refeeding.
They examined two types of prolonged fasting: water fasting and Buchinger fasting, which involves consuming 250 mL of fruit or vegetable juice for lunch and 250 mL of soup for dinner every day of the 5- to 20-day fast.
Buchinger fasting is popular in Central Europe. Water fasting “institutes” exist in the United States, such as one in California, Dr. Varady noted.
The researchers excluded fasting during Ramadan or fasting practiced by Seventh Day Adventists.
They identified four studies of water fasting and four studies of Buchinger fasting (of which one study of 1,422 participants assessed fasting for 5, 10, 15, and 20 days).
The review showed that prolonged fasting for 5-20 days produced large increases in circulating ketones, weight loss of 2%-10%, and decreases in systolic and diastolic blood pressure.
People who fasted 5 days typically lost 4%-6% of their weight; those who fasted 7-10 days lost 2%-10% of their weight; and those who fasted 15-20 days lost 7%-10% of their weight.
LDL cholesterol and triglyceride levels decreased in some trials.
Fasting glucose levels, fasting insulin levels, insulin resistance, and A1c decreased in adults without diabetes but remained unchanged in patients with type 1 or type 2 diabetes.
Some participants experienced metabolic acidosis, headaches, insomnia, or hunger.
About two-thirds of the weight lost was of lean mass, and one-third was of fat mass. The loss of lean mass loss suggests that prolonged fasting may increase the breakdown of muscle proteins, which is a concern, the researchers noted.
Few of the trials examined the effects of refeeding. In one study, normal-weight adults lost 6% of their weight after 5 days of water-only fasting but then gained it all back after 3 months of eating regularly.
In three trials, participants regained 1%-2% of their weight 2-4 months after fasting; however, those trials instructed participants to follow a calorie-restricted diet during the refeeding period.
Three to 4 months after the fast was completed, none of the metabolic benefits were maintained, even when weight loss was maintained.
The study did not receive external funding. Dr. Varady has received author fees from Hachette Book Group for “The Every Other Day Diet” and from Pan Macmillan Press for “The Fastest Diet.” The other authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Health benefits of prolonged “water fasting” (zero calories) or Buchinger fasting (200-300 calories/day) don’t last, according to authors of a review of eight studies.
Five days of fasting lowered weight by about 6%, but this weight was regained after 3 months of regular eating, the investigators found. The article was published in Nutrition Reviews.
“Water fasting led to improvements in blood pressure, cholesterol, and blood sugar levels, but these were short-lived,” senior author Krista A. Varady, PhD, told this news organization.
“Levels returned to baseline ... quickly after participants started eating. Most benefits disappeared in 3-4 months,” said Dr. Varady, professor of nutrition at the University of Illinois, Chicago.
“My overall conclusion,” she said, “is that I guess you could try it, but it just seems like a lot of work, and all those metabolic benefits disappear. I would encourage someone hoping to lose weight to try intermittent fasting instead of water fasting, because there’s a lot more data to show it can help with weight management.
“People should consult their doctor if they have diabetes or any other major obesity-related conditions before doing water fasting,” Dr. Varady cautioned.
“Healthy people with obesity can probably fast safely for 5 days on their own (if they don’t have any other conditions). However, no one should undertake one of these fasts for more than 5 days without medical supervision,” she stressed.
Eight studies of water and Buchinger fasting
Although several favorable effects of prolonged fasting have been observed, benefits must be weighed against risks, Dr. Varady and her coauthors wrote.
Most medically supervised fasting programs have reported only minor adverse events, which included hunger, headaches, nausea, vomiting, dry mouth, and fatigue. However, more severe events have been documented, including edema, abnormal results on liver function tests, decreased bone density, and metabolic acidosis.
The researchers aimed to determine the effect of prolonged fasting on weight, blood pressure, lipid levels, and glycemic control, as well as safety and the effects of refeeding.
They examined two types of prolonged fasting: water fasting and Buchinger fasting, which involves consuming 250 mL of fruit or vegetable juice for lunch and 250 mL of soup for dinner every day of the 5- to 20-day fast.
Buchinger fasting is popular in Central Europe. Water fasting “institutes” exist in the United States, such as one in California, Dr. Varady noted.
The researchers excluded fasting during Ramadan or fasting practiced by Seventh Day Adventists.
They identified four studies of water fasting and four studies of Buchinger fasting (of which one study of 1,422 participants assessed fasting for 5, 10, 15, and 20 days).
The review showed that prolonged fasting for 5-20 days produced large increases in circulating ketones, weight loss of 2%-10%, and decreases in systolic and diastolic blood pressure.
People who fasted 5 days typically lost 4%-6% of their weight; those who fasted 7-10 days lost 2%-10% of their weight; and those who fasted 15-20 days lost 7%-10% of their weight.
LDL cholesterol and triglyceride levels decreased in some trials.
Fasting glucose levels, fasting insulin levels, insulin resistance, and A1c decreased in adults without diabetes but remained unchanged in patients with type 1 or type 2 diabetes.
Some participants experienced metabolic acidosis, headaches, insomnia, or hunger.
About two-thirds of the weight lost was of lean mass, and one-third was of fat mass. The loss of lean mass loss suggests that prolonged fasting may increase the breakdown of muscle proteins, which is a concern, the researchers noted.
Few of the trials examined the effects of refeeding. In one study, normal-weight adults lost 6% of their weight after 5 days of water-only fasting but then gained it all back after 3 months of eating regularly.
In three trials, participants regained 1%-2% of their weight 2-4 months after fasting; however, those trials instructed participants to follow a calorie-restricted diet during the refeeding period.
Three to 4 months after the fast was completed, none of the metabolic benefits were maintained, even when weight loss was maintained.
The study did not receive external funding. Dr. Varady has received author fees from Hachette Book Group for “The Every Other Day Diet” and from Pan Macmillan Press for “The Fastest Diet.” The other authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Health benefits of prolonged “water fasting” (zero calories) or Buchinger fasting (200-300 calories/day) don’t last, according to authors of a review of eight studies.
Five days of fasting lowered weight by about 6%, but this weight was regained after 3 months of regular eating, the investigators found. The article was published in Nutrition Reviews.
“Water fasting led to improvements in blood pressure, cholesterol, and blood sugar levels, but these were short-lived,” senior author Krista A. Varady, PhD, told this news organization.
“Levels returned to baseline ... quickly after participants started eating. Most benefits disappeared in 3-4 months,” said Dr. Varady, professor of nutrition at the University of Illinois, Chicago.
“My overall conclusion,” she said, “is that I guess you could try it, but it just seems like a lot of work, and all those metabolic benefits disappear. I would encourage someone hoping to lose weight to try intermittent fasting instead of water fasting, because there’s a lot more data to show it can help with weight management.
“People should consult their doctor if they have diabetes or any other major obesity-related conditions before doing water fasting,” Dr. Varady cautioned.
“Healthy people with obesity can probably fast safely for 5 days on their own (if they don’t have any other conditions). However, no one should undertake one of these fasts for more than 5 days without medical supervision,” she stressed.
Eight studies of water and Buchinger fasting
Although several favorable effects of prolonged fasting have been observed, benefits must be weighed against risks, Dr. Varady and her coauthors wrote.
Most medically supervised fasting programs have reported only minor adverse events, which included hunger, headaches, nausea, vomiting, dry mouth, and fatigue. However, more severe events have been documented, including edema, abnormal results on liver function tests, decreased bone density, and metabolic acidosis.
The researchers aimed to determine the effect of prolonged fasting on weight, blood pressure, lipid levels, and glycemic control, as well as safety and the effects of refeeding.
They examined two types of prolonged fasting: water fasting and Buchinger fasting, which involves consuming 250 mL of fruit or vegetable juice for lunch and 250 mL of soup for dinner every day of the 5- to 20-day fast.
Buchinger fasting is popular in Central Europe. Water fasting “institutes” exist in the United States, such as one in California, Dr. Varady noted.
The researchers excluded fasting during Ramadan or fasting practiced by Seventh Day Adventists.
They identified four studies of water fasting and four studies of Buchinger fasting (of which one study of 1,422 participants assessed fasting for 5, 10, 15, and 20 days).
The review showed that prolonged fasting for 5-20 days produced large increases in circulating ketones, weight loss of 2%-10%, and decreases in systolic and diastolic blood pressure.
People who fasted 5 days typically lost 4%-6% of their weight; those who fasted 7-10 days lost 2%-10% of their weight; and those who fasted 15-20 days lost 7%-10% of their weight.
LDL cholesterol and triglyceride levels decreased in some trials.
Fasting glucose levels, fasting insulin levels, insulin resistance, and A1c decreased in adults without diabetes but remained unchanged in patients with type 1 or type 2 diabetes.
Some participants experienced metabolic acidosis, headaches, insomnia, or hunger.
About two-thirds of the weight lost was of lean mass, and one-third was of fat mass. The loss of lean mass loss suggests that prolonged fasting may increase the breakdown of muscle proteins, which is a concern, the researchers noted.
Few of the trials examined the effects of refeeding. In one study, normal-weight adults lost 6% of their weight after 5 days of water-only fasting but then gained it all back after 3 months of eating regularly.
In three trials, participants regained 1%-2% of their weight 2-4 months after fasting; however, those trials instructed participants to follow a calorie-restricted diet during the refeeding period.
Three to 4 months after the fast was completed, none of the metabolic benefits were maintained, even when weight loss was maintained.
The study did not receive external funding. Dr. Varady has received author fees from Hachette Book Group for “The Every Other Day Diet” and from Pan Macmillan Press for “The Fastest Diet.” The other authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Low-dose colchicine for ASCVD: Your questions answered
This transcript has been edited for clarity.
Dr. O’Donoghue: We’re going to discuss a very important and emerging topic, which is the use of low-dose colchicine. I think there’s much interest in the use of this drug, which now has a Food and Drug Administration indication, which we’ll talk about further, and it’s also been written into both European and American guidelines that have been recently released.
who’s been at the forefront of research into anti-inflammatory therapeutics.
Lifestyle lipid-lowering paramount
Dr. O’Donoghue: As we think about the concept behind the use of colchicine, we’ve obviously done a large amount of research into lipid-lowering drugs, but where does colchicine now fit in?
Dr. Ridker: Let’s make sure we get the basics down. Anti-inflammatory therapy is going to be added on top of quality other care. This is not a replacement for lipids; it’s not a change in diet, exercise, and smoking cessation. The new data are really telling us that a patient who’s aggressively treated to guideline-recommended levels can still do much better in terms of preventing heart attack, stroke, cardiovascular death, and revascularization by adding low-dose colchicine as the first proven anti-inflammatory therapy for atherosclerotic disease.
I have to say, Michelle, for me, it’s been a wonderful end of a journey in many ways. This story starts almost 30 years ago for quite a few of us, thinking about inflammation and atherosclerosis. The whole C-reactive protein (CRP) story is still an ongoing one. We recently showed, for example, that residual inflammatory risk in some 30,000 patients, all taking a statin, was a far better predictor of the likelihood of more cardiovascular events, in particular cardiovascular death, than was residual cholesterol risk.
Think about that. We’re all aggressively giving second lipid-lowering drugs in our very sick patients, but that means inflammation is really the untapped piece of this.
The two clinical trials we have in front of us, the COLCOT trial and the LoDoCo2 trial – both New England Journal of Medicine papers, both with roughly 5,000 patients – provide very clear evidence that following a relatively recent myocardial infarction (that’s COLCOT) in chronic stable atherosclerosis (that’s LoDoCo2), we’re getting 25%-30% relative risk reductions in major adverse cardiovascular events (MACEs) on top of aggressive statin therapy. That’s a big deal. It’s safe, it works, and it’s fully consistent with all the information we have about inflammation being part and parcel of atherosclerosis. It’s a pretty exciting time.
Inflammatory pathway
Dr. O’Donoghue: It beautifully proves the inflammatory hypothesis in many ways. You led CANTOS, and that was a much more specific target. Here, in terms of the effects of colchicine, what do we know about how it may work on the inflammatory cascade?
Dr. Ridker: Our CANTOS trial was proof of principle that you could directly target, with a very specific monoclonal antibody, a specific piece of this innate immune cascade and lower cardiovascular event rates.
Colchicine is a more broad-spectrum drug. It does have a number of antineutrophil effects – that’s important, by the way. Neutrophils are really becoming very important in atherosclerotic disease progression. It’s an indirect inhibitor of the so-called NLRP3 inflammasome, which is where both interleukin-1 (that’s the target for canakinumab) and IL-6 are up-regulated. As you know, it’s been used to treat gout and pericarditis in high doses in short, little bursts.
The change here is this use of low-dose colchicine, that’s 0.5 mg once a day for years to treat chronic, stable atherosclerosis. It is very much like using a statin. The idea here is to prevent the progression of the disease by slowing down and maybe stabilizing the plaque so we have fewer heart attacks and strokes down the road.
It’s entering the armamentarium – at least my armamentarium – as chronic, stable secondary prevention. That’s where the new American College of Cardiology/American Heart Association guidelines also put it. It’s really in as a treatment for chronic, stable atherosclerosis. I think that’s where it belongs.
When to start colchicine, and in whom?
Dr. O’Donoghue: To that point, as we think about the efficacy, I think it’s nice, as you outlined, that we have two complementary trials that are both showing a consistent reduction in MACEs, one in the post–acute coronary syndrome (ACS) state and one for more chronic patients.
At what point do you think would be the appropriate time to start therapy, and who would you be starting it for?
Dr. Ridker: Michelle, that’s a great question. There’s a very interesting analysis that just came out from the LoDoCo2 investigators. It’s kind of a landmark analysis. What they show is that 1 year, 2 years, 3 years, and 4 years since the initiating myocardial infarction, the drug is very effective.
In fact, you could think about starting this drug at your clinic in patients with chronic, stable atherosclerotic disease. That’s just like we would start a statin in people who had a heart attack some time ago, and that’s absolutely fine.
I’m using it for what I call my frequent fliers, those patients who just keep coming back. They’re already on aggressive lipid-lowering therapy. I have them on beta-blockers, aspirin, and all the usual things. I say, look, I can get a large risk reduction by starting them on this drug.
There are a few caveats, Michelle. Like all drugs, colchicine comes with some adverse effects. Most of them are pretty rare, but there are some patients I would not give this drug to, just to be very clear. Colchicine is cleared by the kidney and by the liver. Patients who have severe chronic kidney disease and severe liver disease – this is a no-go for those patients. We should talk about where patients in that realm might want to go.
Then there are some unusual drugs. Colchicine is metabolized by the CYP3A4 and the P-glycoprotein pathway. There are a few drugs, such as ketoconazole, fluconazole, and cyclosporine, that if your primary care doctor or internist is going to start for a short term, you probably want to stop your colchicine for a week or two.
In people with familial Mediterranean fever, for whom colchicine is lifesaving and life-changing and who take it for 20, 30, or 40 years, there’s been no increase in risk for cancer. There have been very few adverse effects. I think it’s interesting that we, who practice in North America, basically never see familial Mediterranean fever. If we were practicing in Lebanon, Israel, or North Africa, this would be a very common therapy that we’d all be extremely familiar with.
Dr. O’Donoghue: To that point, it’s interesting to hear that colchicine was even used by the ancient Greeks and ancient Egyptians. It’s a drug that’s been around for a long time.
In terms of its safety, some people have been talking about the fact that an increase in noncardiovascular death was seen in LoDoCo2. What are your thoughts on that? Is that anything that we should be concerned about?
Colchicine safety and contraindications
Dr. Ridker: First, to set the record straight, a meta-analysis has been done of all-cause mortality in the various colchicine trials, and the hazard ratio is 1.04. I’ll remind you, and all of us know, that the hazard ratios for all-cause mortality in the PCSK9 trials, the bempedoic acid trials, and the ezetimibe trials are also essentially neutral. We’re in a state where we don’t let these trials roll long enough to see benefits necessarily on all-cause mortality. Some of us think we probably should, but that’s just the reality of trials.
One of most interesting things that was part of the FDA review, I suspect, was that there was no specific cause of any of this. It was not like there was a set of particular issues. I suspect that most people think this is probably the play of chance and with time, things will get better.
Again, I do want to emphasize this is not a drug for severe chronic kidney disease and severe liver disease, because those patients will get in trouble with this. The other thing that’s worth knowing is when you start a patient on low-dose colchicine – that’s 0.5 mg/d – there will be some patients who get some short-term gastrointestinal upset. That’s very common when you start colchicine at the much higher doses you might use to treat acute gout or pericarditis. In these trials, the vast majority of patients treated through that, and there were very few episodes long-term. I think it’s generally safe. That’s where we’re at.
Dr. O’Donoghue: Paul, you’ve been a leader, certainly, at looking at CRP as a marker of inflammation. Do you, in your practice, consider CRP levels when making a decision about who is appropriate for this therapy?
Dr. Ridker: That’s another terrific question. I do, because I’m trying to distinguish in my own mind patients who have residual inflammatory risk, in whom the high-sensitivity CRP (hsCRP) level remains high despite being on statins versus those with residual cholesterol risk, in whom I’m really predominantly worried about LDL cholesterol, that I haven’t brought it down far enough.
I do measure it, and if the CRP remains high and the LDL cholesterol is low, to me, that’s residual inflammatory risk and that’s the patient I would target this to. Conversely, if the LDL cholesterol was still, say, above some threshold of 75-100 and I’m worried about that, even if the CRP is low, I’ll probably add a second lipid-lowering drug.
The complexity of this, however, is that CRP was not measured in either LoDoCo2 or COLCOT. That’s mostly because they didn’t have much funding. These trials were done really on a shoestring. They were not sponsored by major pharma at all. We know that the median hsCRP in these trials was probably around 3.5-4 mg/L so I’m pretty comfortable doing that. Others have just advocated giving it to many patients. I must say I like to use biomarkers to think through the biology and who might have the best benefit-to-risk ratio. In my practice, I am doing it that way.
Inpatient vs. outpatient initiation
Dr. O’Donoghue: This is perhaps my last question for you before we wrap up. I know you talked about use of low-dose colchicine for patients with more chronic, stable coronary disease. Now obviously, COLCOT studied patients who were early post ACS, and there we certainly think about the anti-inflammatory effects as potentially having more benefit. What are your thoughts about early initiation of colchicine in that setting, the acute hospitalized setting? Do you think it’s more appropriate for an outpatient start?
Dr. Ridker: Today, I think this is all about chronic, stable atherosclerosis. Yes, COLCOT enrolled their patients within 30 days of a recent myocardial infarction, but as we all know, that’s a pretty stable phase. The vast majority were enrolled after 15 days. There were a small number enrolled within 3 days or something like that, but the benefit is about the same in all these patients.
Conversely, there’s been a small number of trials looking at colchicine in acute coronary ischemia and they’ve not been terribly promising. That makes some sense, though, right? We want to get an artery open. In acute ischemia, that’s about revascularization. It’s about oxygenation. It’s about reperfusion injury. My guess is that 3, 4, 5, or 6 days later, when it becomes a stable situation, is when the drug is probably effective.
Again, there will be some ongoing true intervention trials with large sample sizes for acute coronary ischemia. We don’t have those yet. Right now, I think it’s a therapy for chronic, stable angina. That’s many of our patients.
I would say that if you compare the relative benefit in these trials of adding ezetimibe to a statin, that’s a 5% or 6% benefit. For PCSK9 inhibitors – we all use them – it’s about a 15% benefit. These are 25%-30% risk reductions. If we’re going to think about what’s the next drug to give on top of the statin, serious consideration should be given to low-dose colchicine.
Let me also emphasize that this is not an either/or situation. This is about the fact that we now understand atherosclerosis to be a disorder both of lipid accumulation and a proinflammatory systemic response. We can give these drugs together. I suspect that the best patient care is going to be very aggressive lipid-lowering combined with pretty aggressive inflammation inhibition. I suspect that, down the road, that’s where all of us are going to be.
Dr. O’Donoghue: Thank you so much, Paul, for walking us through that today. I think it was a very nice, succinct review of the evidence, and then also just getting our minds more accustomed to the concept that we can now start to target more orthogonal axes that really get at the pathobiology of what’s going on in the atherosclerotic plaque. I think it’s an important topic.
Dr. O’Donoghue is an associate professor of medicine at Harvard Medical School and an associate physician at Brigham and Women’s Hospital, both in Boston. Dr. Ridker is director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital. Both Dr. O’Donoghue and Dr. Ridker reported numerous conflicts of interest.
This transcript has been edited for clarity.
Dr. O’Donoghue: We’re going to discuss a very important and emerging topic, which is the use of low-dose colchicine. I think there’s much interest in the use of this drug, which now has a Food and Drug Administration indication, which we’ll talk about further, and it’s also been written into both European and American guidelines that have been recently released.
who’s been at the forefront of research into anti-inflammatory therapeutics.
Lifestyle lipid-lowering paramount
Dr. O’Donoghue: As we think about the concept behind the use of colchicine, we’ve obviously done a large amount of research into lipid-lowering drugs, but where does colchicine now fit in?
Dr. Ridker: Let’s make sure we get the basics down. Anti-inflammatory therapy is going to be added on top of quality other care. This is not a replacement for lipids; it’s not a change in diet, exercise, and smoking cessation. The new data are really telling us that a patient who’s aggressively treated to guideline-recommended levels can still do much better in terms of preventing heart attack, stroke, cardiovascular death, and revascularization by adding low-dose colchicine as the first proven anti-inflammatory therapy for atherosclerotic disease.
I have to say, Michelle, for me, it’s been a wonderful end of a journey in many ways. This story starts almost 30 years ago for quite a few of us, thinking about inflammation and atherosclerosis. The whole C-reactive protein (CRP) story is still an ongoing one. We recently showed, for example, that residual inflammatory risk in some 30,000 patients, all taking a statin, was a far better predictor of the likelihood of more cardiovascular events, in particular cardiovascular death, than was residual cholesterol risk.
Think about that. We’re all aggressively giving second lipid-lowering drugs in our very sick patients, but that means inflammation is really the untapped piece of this.
The two clinical trials we have in front of us, the COLCOT trial and the LoDoCo2 trial – both New England Journal of Medicine papers, both with roughly 5,000 patients – provide very clear evidence that following a relatively recent myocardial infarction (that’s COLCOT) in chronic stable atherosclerosis (that’s LoDoCo2), we’re getting 25%-30% relative risk reductions in major adverse cardiovascular events (MACEs) on top of aggressive statin therapy. That’s a big deal. It’s safe, it works, and it’s fully consistent with all the information we have about inflammation being part and parcel of atherosclerosis. It’s a pretty exciting time.
Inflammatory pathway
Dr. O’Donoghue: It beautifully proves the inflammatory hypothesis in many ways. You led CANTOS, and that was a much more specific target. Here, in terms of the effects of colchicine, what do we know about how it may work on the inflammatory cascade?
Dr. Ridker: Our CANTOS trial was proof of principle that you could directly target, with a very specific monoclonal antibody, a specific piece of this innate immune cascade and lower cardiovascular event rates.
Colchicine is a more broad-spectrum drug. It does have a number of antineutrophil effects – that’s important, by the way. Neutrophils are really becoming very important in atherosclerotic disease progression. It’s an indirect inhibitor of the so-called NLRP3 inflammasome, which is where both interleukin-1 (that’s the target for canakinumab) and IL-6 are up-regulated. As you know, it’s been used to treat gout and pericarditis in high doses in short, little bursts.
The change here is this use of low-dose colchicine, that’s 0.5 mg once a day for years to treat chronic, stable atherosclerosis. It is very much like using a statin. The idea here is to prevent the progression of the disease by slowing down and maybe stabilizing the plaque so we have fewer heart attacks and strokes down the road.
It’s entering the armamentarium – at least my armamentarium – as chronic, stable secondary prevention. That’s where the new American College of Cardiology/American Heart Association guidelines also put it. It’s really in as a treatment for chronic, stable atherosclerosis. I think that’s where it belongs.
When to start colchicine, and in whom?
Dr. O’Donoghue: To that point, as we think about the efficacy, I think it’s nice, as you outlined, that we have two complementary trials that are both showing a consistent reduction in MACEs, one in the post–acute coronary syndrome (ACS) state and one for more chronic patients.
At what point do you think would be the appropriate time to start therapy, and who would you be starting it for?
Dr. Ridker: Michelle, that’s a great question. There’s a very interesting analysis that just came out from the LoDoCo2 investigators. It’s kind of a landmark analysis. What they show is that 1 year, 2 years, 3 years, and 4 years since the initiating myocardial infarction, the drug is very effective.
In fact, you could think about starting this drug at your clinic in patients with chronic, stable atherosclerotic disease. That’s just like we would start a statin in people who had a heart attack some time ago, and that’s absolutely fine.
I’m using it for what I call my frequent fliers, those patients who just keep coming back. They’re already on aggressive lipid-lowering therapy. I have them on beta-blockers, aspirin, and all the usual things. I say, look, I can get a large risk reduction by starting them on this drug.
There are a few caveats, Michelle. Like all drugs, colchicine comes with some adverse effects. Most of them are pretty rare, but there are some patients I would not give this drug to, just to be very clear. Colchicine is cleared by the kidney and by the liver. Patients who have severe chronic kidney disease and severe liver disease – this is a no-go for those patients. We should talk about where patients in that realm might want to go.
Then there are some unusual drugs. Colchicine is metabolized by the CYP3A4 and the P-glycoprotein pathway. There are a few drugs, such as ketoconazole, fluconazole, and cyclosporine, that if your primary care doctor or internist is going to start for a short term, you probably want to stop your colchicine for a week or two.
In people with familial Mediterranean fever, for whom colchicine is lifesaving and life-changing and who take it for 20, 30, or 40 years, there’s been no increase in risk for cancer. There have been very few adverse effects. I think it’s interesting that we, who practice in North America, basically never see familial Mediterranean fever. If we were practicing in Lebanon, Israel, or North Africa, this would be a very common therapy that we’d all be extremely familiar with.
Dr. O’Donoghue: To that point, it’s interesting to hear that colchicine was even used by the ancient Greeks and ancient Egyptians. It’s a drug that’s been around for a long time.
In terms of its safety, some people have been talking about the fact that an increase in noncardiovascular death was seen in LoDoCo2. What are your thoughts on that? Is that anything that we should be concerned about?
Colchicine safety and contraindications
Dr. Ridker: First, to set the record straight, a meta-analysis has been done of all-cause mortality in the various colchicine trials, and the hazard ratio is 1.04. I’ll remind you, and all of us know, that the hazard ratios for all-cause mortality in the PCSK9 trials, the bempedoic acid trials, and the ezetimibe trials are also essentially neutral. We’re in a state where we don’t let these trials roll long enough to see benefits necessarily on all-cause mortality. Some of us think we probably should, but that’s just the reality of trials.
One of most interesting things that was part of the FDA review, I suspect, was that there was no specific cause of any of this. It was not like there was a set of particular issues. I suspect that most people think this is probably the play of chance and with time, things will get better.
Again, I do want to emphasize this is not a drug for severe chronic kidney disease and severe liver disease, because those patients will get in trouble with this. The other thing that’s worth knowing is when you start a patient on low-dose colchicine – that’s 0.5 mg/d – there will be some patients who get some short-term gastrointestinal upset. That’s very common when you start colchicine at the much higher doses you might use to treat acute gout or pericarditis. In these trials, the vast majority of patients treated through that, and there were very few episodes long-term. I think it’s generally safe. That’s where we’re at.
Dr. O’Donoghue: Paul, you’ve been a leader, certainly, at looking at CRP as a marker of inflammation. Do you, in your practice, consider CRP levels when making a decision about who is appropriate for this therapy?
Dr. Ridker: That’s another terrific question. I do, because I’m trying to distinguish in my own mind patients who have residual inflammatory risk, in whom the high-sensitivity CRP (hsCRP) level remains high despite being on statins versus those with residual cholesterol risk, in whom I’m really predominantly worried about LDL cholesterol, that I haven’t brought it down far enough.
I do measure it, and if the CRP remains high and the LDL cholesterol is low, to me, that’s residual inflammatory risk and that’s the patient I would target this to. Conversely, if the LDL cholesterol was still, say, above some threshold of 75-100 and I’m worried about that, even if the CRP is low, I’ll probably add a second lipid-lowering drug.
The complexity of this, however, is that CRP was not measured in either LoDoCo2 or COLCOT. That’s mostly because they didn’t have much funding. These trials were done really on a shoestring. They were not sponsored by major pharma at all. We know that the median hsCRP in these trials was probably around 3.5-4 mg/L so I’m pretty comfortable doing that. Others have just advocated giving it to many patients. I must say I like to use biomarkers to think through the biology and who might have the best benefit-to-risk ratio. In my practice, I am doing it that way.
Inpatient vs. outpatient initiation
Dr. O’Donoghue: This is perhaps my last question for you before we wrap up. I know you talked about use of low-dose colchicine for patients with more chronic, stable coronary disease. Now obviously, COLCOT studied patients who were early post ACS, and there we certainly think about the anti-inflammatory effects as potentially having more benefit. What are your thoughts about early initiation of colchicine in that setting, the acute hospitalized setting? Do you think it’s more appropriate for an outpatient start?
Dr. Ridker: Today, I think this is all about chronic, stable atherosclerosis. Yes, COLCOT enrolled their patients within 30 days of a recent myocardial infarction, but as we all know, that’s a pretty stable phase. The vast majority were enrolled after 15 days. There were a small number enrolled within 3 days or something like that, but the benefit is about the same in all these patients.
Conversely, there’s been a small number of trials looking at colchicine in acute coronary ischemia and they’ve not been terribly promising. That makes some sense, though, right? We want to get an artery open. In acute ischemia, that’s about revascularization. It’s about oxygenation. It’s about reperfusion injury. My guess is that 3, 4, 5, or 6 days later, when it becomes a stable situation, is when the drug is probably effective.
Again, there will be some ongoing true intervention trials with large sample sizes for acute coronary ischemia. We don’t have those yet. Right now, I think it’s a therapy for chronic, stable angina. That’s many of our patients.
I would say that if you compare the relative benefit in these trials of adding ezetimibe to a statin, that’s a 5% or 6% benefit. For PCSK9 inhibitors – we all use them – it’s about a 15% benefit. These are 25%-30% risk reductions. If we’re going to think about what’s the next drug to give on top of the statin, serious consideration should be given to low-dose colchicine.
Let me also emphasize that this is not an either/or situation. This is about the fact that we now understand atherosclerosis to be a disorder both of lipid accumulation and a proinflammatory systemic response. We can give these drugs together. I suspect that the best patient care is going to be very aggressive lipid-lowering combined with pretty aggressive inflammation inhibition. I suspect that, down the road, that’s where all of us are going to be.
Dr. O’Donoghue: Thank you so much, Paul, for walking us through that today. I think it was a very nice, succinct review of the evidence, and then also just getting our minds more accustomed to the concept that we can now start to target more orthogonal axes that really get at the pathobiology of what’s going on in the atherosclerotic plaque. I think it’s an important topic.
Dr. O’Donoghue is an associate professor of medicine at Harvard Medical School and an associate physician at Brigham and Women’s Hospital, both in Boston. Dr. Ridker is director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital. Both Dr. O’Donoghue and Dr. Ridker reported numerous conflicts of interest.
This transcript has been edited for clarity.
Dr. O’Donoghue: We’re going to discuss a very important and emerging topic, which is the use of low-dose colchicine. I think there’s much interest in the use of this drug, which now has a Food and Drug Administration indication, which we’ll talk about further, and it’s also been written into both European and American guidelines that have been recently released.
who’s been at the forefront of research into anti-inflammatory therapeutics.
Lifestyle lipid-lowering paramount
Dr. O’Donoghue: As we think about the concept behind the use of colchicine, we’ve obviously done a large amount of research into lipid-lowering drugs, but where does colchicine now fit in?
Dr. Ridker: Let’s make sure we get the basics down. Anti-inflammatory therapy is going to be added on top of quality other care. This is not a replacement for lipids; it’s not a change in diet, exercise, and smoking cessation. The new data are really telling us that a patient who’s aggressively treated to guideline-recommended levels can still do much better in terms of preventing heart attack, stroke, cardiovascular death, and revascularization by adding low-dose colchicine as the first proven anti-inflammatory therapy for atherosclerotic disease.
I have to say, Michelle, for me, it’s been a wonderful end of a journey in many ways. This story starts almost 30 years ago for quite a few of us, thinking about inflammation and atherosclerosis. The whole C-reactive protein (CRP) story is still an ongoing one. We recently showed, for example, that residual inflammatory risk in some 30,000 patients, all taking a statin, was a far better predictor of the likelihood of more cardiovascular events, in particular cardiovascular death, than was residual cholesterol risk.
Think about that. We’re all aggressively giving second lipid-lowering drugs in our very sick patients, but that means inflammation is really the untapped piece of this.
The two clinical trials we have in front of us, the COLCOT trial and the LoDoCo2 trial – both New England Journal of Medicine papers, both with roughly 5,000 patients – provide very clear evidence that following a relatively recent myocardial infarction (that’s COLCOT) in chronic stable atherosclerosis (that’s LoDoCo2), we’re getting 25%-30% relative risk reductions in major adverse cardiovascular events (MACEs) on top of aggressive statin therapy. That’s a big deal. It’s safe, it works, and it’s fully consistent with all the information we have about inflammation being part and parcel of atherosclerosis. It’s a pretty exciting time.
Inflammatory pathway
Dr. O’Donoghue: It beautifully proves the inflammatory hypothesis in many ways. You led CANTOS, and that was a much more specific target. Here, in terms of the effects of colchicine, what do we know about how it may work on the inflammatory cascade?
Dr. Ridker: Our CANTOS trial was proof of principle that you could directly target, with a very specific monoclonal antibody, a specific piece of this innate immune cascade and lower cardiovascular event rates.
Colchicine is a more broad-spectrum drug. It does have a number of antineutrophil effects – that’s important, by the way. Neutrophils are really becoming very important in atherosclerotic disease progression. It’s an indirect inhibitor of the so-called NLRP3 inflammasome, which is where both interleukin-1 (that’s the target for canakinumab) and IL-6 are up-regulated. As you know, it’s been used to treat gout and pericarditis in high doses in short, little bursts.
The change here is this use of low-dose colchicine, that’s 0.5 mg once a day for years to treat chronic, stable atherosclerosis. It is very much like using a statin. The idea here is to prevent the progression of the disease by slowing down and maybe stabilizing the plaque so we have fewer heart attacks and strokes down the road.
It’s entering the armamentarium – at least my armamentarium – as chronic, stable secondary prevention. That’s where the new American College of Cardiology/American Heart Association guidelines also put it. It’s really in as a treatment for chronic, stable atherosclerosis. I think that’s where it belongs.
When to start colchicine, and in whom?
Dr. O’Donoghue: To that point, as we think about the efficacy, I think it’s nice, as you outlined, that we have two complementary trials that are both showing a consistent reduction in MACEs, one in the post–acute coronary syndrome (ACS) state and one for more chronic patients.
At what point do you think would be the appropriate time to start therapy, and who would you be starting it for?
Dr. Ridker: Michelle, that’s a great question. There’s a very interesting analysis that just came out from the LoDoCo2 investigators. It’s kind of a landmark analysis. What they show is that 1 year, 2 years, 3 years, and 4 years since the initiating myocardial infarction, the drug is very effective.
In fact, you could think about starting this drug at your clinic in patients with chronic, stable atherosclerotic disease. That’s just like we would start a statin in people who had a heart attack some time ago, and that’s absolutely fine.
I’m using it for what I call my frequent fliers, those patients who just keep coming back. They’re already on aggressive lipid-lowering therapy. I have them on beta-blockers, aspirin, and all the usual things. I say, look, I can get a large risk reduction by starting them on this drug.
There are a few caveats, Michelle. Like all drugs, colchicine comes with some adverse effects. Most of them are pretty rare, but there are some patients I would not give this drug to, just to be very clear. Colchicine is cleared by the kidney and by the liver. Patients who have severe chronic kidney disease and severe liver disease – this is a no-go for those patients. We should talk about where patients in that realm might want to go.
Then there are some unusual drugs. Colchicine is metabolized by the CYP3A4 and the P-glycoprotein pathway. There are a few drugs, such as ketoconazole, fluconazole, and cyclosporine, that if your primary care doctor or internist is going to start for a short term, you probably want to stop your colchicine for a week or two.
In people with familial Mediterranean fever, for whom colchicine is lifesaving and life-changing and who take it for 20, 30, or 40 years, there’s been no increase in risk for cancer. There have been very few adverse effects. I think it’s interesting that we, who practice in North America, basically never see familial Mediterranean fever. If we were practicing in Lebanon, Israel, or North Africa, this would be a very common therapy that we’d all be extremely familiar with.
Dr. O’Donoghue: To that point, it’s interesting to hear that colchicine was even used by the ancient Greeks and ancient Egyptians. It’s a drug that’s been around for a long time.
In terms of its safety, some people have been talking about the fact that an increase in noncardiovascular death was seen in LoDoCo2. What are your thoughts on that? Is that anything that we should be concerned about?
Colchicine safety and contraindications
Dr. Ridker: First, to set the record straight, a meta-analysis has been done of all-cause mortality in the various colchicine trials, and the hazard ratio is 1.04. I’ll remind you, and all of us know, that the hazard ratios for all-cause mortality in the PCSK9 trials, the bempedoic acid trials, and the ezetimibe trials are also essentially neutral. We’re in a state where we don’t let these trials roll long enough to see benefits necessarily on all-cause mortality. Some of us think we probably should, but that’s just the reality of trials.
One of most interesting things that was part of the FDA review, I suspect, was that there was no specific cause of any of this. It was not like there was a set of particular issues. I suspect that most people think this is probably the play of chance and with time, things will get better.
Again, I do want to emphasize this is not a drug for severe chronic kidney disease and severe liver disease, because those patients will get in trouble with this. The other thing that’s worth knowing is when you start a patient on low-dose colchicine – that’s 0.5 mg/d – there will be some patients who get some short-term gastrointestinal upset. That’s very common when you start colchicine at the much higher doses you might use to treat acute gout or pericarditis. In these trials, the vast majority of patients treated through that, and there were very few episodes long-term. I think it’s generally safe. That’s where we’re at.
Dr. O’Donoghue: Paul, you’ve been a leader, certainly, at looking at CRP as a marker of inflammation. Do you, in your practice, consider CRP levels when making a decision about who is appropriate for this therapy?
Dr. Ridker: That’s another terrific question. I do, because I’m trying to distinguish in my own mind patients who have residual inflammatory risk, in whom the high-sensitivity CRP (hsCRP) level remains high despite being on statins versus those with residual cholesterol risk, in whom I’m really predominantly worried about LDL cholesterol, that I haven’t brought it down far enough.
I do measure it, and if the CRP remains high and the LDL cholesterol is low, to me, that’s residual inflammatory risk and that’s the patient I would target this to. Conversely, if the LDL cholesterol was still, say, above some threshold of 75-100 and I’m worried about that, even if the CRP is low, I’ll probably add a second lipid-lowering drug.
The complexity of this, however, is that CRP was not measured in either LoDoCo2 or COLCOT. That’s mostly because they didn’t have much funding. These trials were done really on a shoestring. They were not sponsored by major pharma at all. We know that the median hsCRP in these trials was probably around 3.5-4 mg/L so I’m pretty comfortable doing that. Others have just advocated giving it to many patients. I must say I like to use biomarkers to think through the biology and who might have the best benefit-to-risk ratio. In my practice, I am doing it that way.
Inpatient vs. outpatient initiation
Dr. O’Donoghue: This is perhaps my last question for you before we wrap up. I know you talked about use of low-dose colchicine for patients with more chronic, stable coronary disease. Now obviously, COLCOT studied patients who were early post ACS, and there we certainly think about the anti-inflammatory effects as potentially having more benefit. What are your thoughts about early initiation of colchicine in that setting, the acute hospitalized setting? Do you think it’s more appropriate for an outpatient start?
Dr. Ridker: Today, I think this is all about chronic, stable atherosclerosis. Yes, COLCOT enrolled their patients within 30 days of a recent myocardial infarction, but as we all know, that’s a pretty stable phase. The vast majority were enrolled after 15 days. There were a small number enrolled within 3 days or something like that, but the benefit is about the same in all these patients.
Conversely, there’s been a small number of trials looking at colchicine in acute coronary ischemia and they’ve not been terribly promising. That makes some sense, though, right? We want to get an artery open. In acute ischemia, that’s about revascularization. It’s about oxygenation. It’s about reperfusion injury. My guess is that 3, 4, 5, or 6 days later, when it becomes a stable situation, is when the drug is probably effective.
Again, there will be some ongoing true intervention trials with large sample sizes for acute coronary ischemia. We don’t have those yet. Right now, I think it’s a therapy for chronic, stable angina. That’s many of our patients.
I would say that if you compare the relative benefit in these trials of adding ezetimibe to a statin, that’s a 5% or 6% benefit. For PCSK9 inhibitors – we all use them – it’s about a 15% benefit. These are 25%-30% risk reductions. If we’re going to think about what’s the next drug to give on top of the statin, serious consideration should be given to low-dose colchicine.
Let me also emphasize that this is not an either/or situation. This is about the fact that we now understand atherosclerosis to be a disorder both of lipid accumulation and a proinflammatory systemic response. We can give these drugs together. I suspect that the best patient care is going to be very aggressive lipid-lowering combined with pretty aggressive inflammation inhibition. I suspect that, down the road, that’s where all of us are going to be.
Dr. O’Donoghue: Thank you so much, Paul, for walking us through that today. I think it was a very nice, succinct review of the evidence, and then also just getting our minds more accustomed to the concept that we can now start to target more orthogonal axes that really get at the pathobiology of what’s going on in the atherosclerotic plaque. I think it’s an important topic.
Dr. O’Donoghue is an associate professor of medicine at Harvard Medical School and an associate physician at Brigham and Women’s Hospital, both in Boston. Dr. Ridker is director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital. Both Dr. O’Donoghue and Dr. Ridker reported numerous conflicts of interest.
Prioritize nutrients, limit ultraprocessed food in diabetes
In a large cohort of older adults with type 2 diabetes in Italy, those with the highest intake of ultraprocessed food and beverages (UPF) were more likely to die of all causes or cardiovascular disease (CVD) within a decade than those with the lowest intake – independent of adherence to a healthy Mediterranean diet.
Adults in the top quartile of UPF intake had a 64% increased risk of all-cause death and a 2.5-fold increased risk of CVD death during follow-up, compared with those in the lowest quartile, after adjusting for variables including Mediterranean diet score.
These findings from the Moli-sani study by Marialaura Bonaccio, PhD, from the Institute for Research, Hospitalization and Healthcare (IRCCS) Neuromed, in Pozzilli, Italy, and colleagues, were published online in the American Journal of Clinical Nutrition.
“Dietary recommendations for prevention and management of type 2 diabetes almost exclusively prioritize consumption of nutritionally balanced foods that are the source of fiber [and] healthy fats and [are] poor in free sugars, and promote dietary patterns – such as the Mediterranean diet and the DASH diet – that place a large emphasis on food groups (for example, whole grains, legumes, nuts, fruits, and vegetables) regardless of food processing,” the researchers note.
The research suggests that “besides prioritizing the adoption of a diet based on nutritional requirements, dietary guidelines for the management of type 2 diabetes should also recommend limiting UPF,” they conclude.
“In addition to the adoption of a diet based on well-known nutritional requirements, dietary recommendations should also suggest limiting the consumption of ultraprocessed foods as much as possible,” Giovanni de Gaetano, MD, PhD, president, IRCCS Neuromed, echoed, in a press release from the institute.
“In this context, and not only for people with diabetes, the front-of-pack nutrition labels should also include information on the degree of food processing,” he observed.
Caroline M. Apovian, MD, who was not involved with the study, agrees that it is wise to limit consumption of UPF.
However, we need more research to better understand which components of UPF are harmful and the biologic mechanisms, Dr. Apovian, who is codirector, Center for Weight Management and Wellness, Brigham and Women’s Hospital, and a professor of medicine at Harvard Medical School, both in Boston, told this news organization in an interview.
She noted that in a randomized crossover trial in 20 patients who were instructed to eat as much or as little as they wanted, people ate more and gained weight during 2 weeks of a diet high in UPF, compared with 2 weeks of an unprocessed diet matched for presented calories, carbohydrate, sugar, fat, sodium, and fiber.
Ultraprocessed foods classed according to Nova system
UPF is “made mostly or entirely from substances derived from foods and additives, using a series of processes and containing minimal whole foods,” and they “are usually nutrient-poor, high in calories, added sugar, sodium, and unhealthy fats,” the Italian researchers write.
High intake of UPF, they add, may exacerbate health risks in people with type 2 diabetes, who are already at higher risk of premature mortality, mainly due to diabetes-related complications.
The researchers analyzed data from a subset of patients in the Moli-sani study of environmental and genetic factors underlying disease, which enrolled 24,325 individuals aged 35 and older who lived in Molise, in central-southern Italy, in 2005-2010.
The current analysis included 1,065 participants in Moli-sani who had type 2 diabetes at baseline and completed a food frequency questionnaire by which participants reported their consumption of 188 foods and beverages in the previous 12 months.
Participants were a mean age of 65 years, and 60% were men.
Most UPF intake was from processed meat (22.4%), crispbread/rusks (16.6%), nonhomemade pizza (11.2%), and cakes, pies, pastries, and puddings (8.8%).
Researchers categorized foods and beverages into four groups with increasing degrees of processing, based on the Nova Food Classification System:
- Group 1: Fresh or minimally processed foods and beverages (for example, fruit, meat, milk).
- Group 2: Processed culinary ingredients (for example, oils, butter).
- Group 3: Processed foods and beverages (for example, canned fish, bread).
- Group 4: UPF (22 foods and beverages including carbonated drinks, processed meats, sweet or savory packaged snacks, margarine, and foods and beverages with artificial sweeteners).
Participants were divided into four quartiles based on UPF consumption.
The mean percentage of UPF consumption out of total food and beverage intake was 2.8%, 5.2%, 7.7%, and 14.4% for quartiles 1, 2, 3, and 4, respectively. By sex, these rates for quartile 1 were < 4.7% for women and < 3.7% for men, and for quartile 4 were ≥ 10.5% for women and ≥ 9% for men.
Participants with the highest UPF intake were younger (mean age, 63 vs. 67 years) but otherwise had similar characteristics as other participants.
During a median follow-up of 11.6 years, 308 participants died from all causes, including 129 who died from CVD.
Compared with participants with the lowest intake of UPF (quartile 1), those with the highest intake (quartile 4) had a higher risk of all-cause mortality (hazard ratio, 1.70) and CVD mortality (HR, 2.64) during follow-up, after multivariable adjustment. The analysis adjusted for sex, age, energy intake, residence, education, housing, smoking, body mass index, leisure-time physical activity, history of cancer or cardiovascular disease, hypertension, hyperlipidemia, aspirin use, years since type 2 diabetes diagnosis, and special diet for blood glucose control.
After further adjusting for Mediterranean diet score, the risk of all-cause and CVD mortality during follow-up for patients with the highest versus lowest intake of UPF remained similar (HR, 1.64 and 2.55, respectively).
There was a linear dose–response relationship between UPF and all-cause and CVD mortality.
Increasing intake of fruit drinks, carbonated drinks, and salty biscuits was associated with higher all-cause and CVD mortality rates, and consumption of stock cubes and margarine was further related to higher CVD death.
The researchers acknowledge that the study was observational, and therefore cannot determine cause and effect, and was not designed to specifically collect dietary data according to the Nova classification. The findings may not be generalizable to other populations.
The analysis was partly funded by grants from the AIRC and Italian Ministry of Health. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a large cohort of older adults with type 2 diabetes in Italy, those with the highest intake of ultraprocessed food and beverages (UPF) were more likely to die of all causes or cardiovascular disease (CVD) within a decade than those with the lowest intake – independent of adherence to a healthy Mediterranean diet.
Adults in the top quartile of UPF intake had a 64% increased risk of all-cause death and a 2.5-fold increased risk of CVD death during follow-up, compared with those in the lowest quartile, after adjusting for variables including Mediterranean diet score.
These findings from the Moli-sani study by Marialaura Bonaccio, PhD, from the Institute for Research, Hospitalization and Healthcare (IRCCS) Neuromed, in Pozzilli, Italy, and colleagues, were published online in the American Journal of Clinical Nutrition.
“Dietary recommendations for prevention and management of type 2 diabetes almost exclusively prioritize consumption of nutritionally balanced foods that are the source of fiber [and] healthy fats and [are] poor in free sugars, and promote dietary patterns – such as the Mediterranean diet and the DASH diet – that place a large emphasis on food groups (for example, whole grains, legumes, nuts, fruits, and vegetables) regardless of food processing,” the researchers note.
The research suggests that “besides prioritizing the adoption of a diet based on nutritional requirements, dietary guidelines for the management of type 2 diabetes should also recommend limiting UPF,” they conclude.
“In addition to the adoption of a diet based on well-known nutritional requirements, dietary recommendations should also suggest limiting the consumption of ultraprocessed foods as much as possible,” Giovanni de Gaetano, MD, PhD, president, IRCCS Neuromed, echoed, in a press release from the institute.
“In this context, and not only for people with diabetes, the front-of-pack nutrition labels should also include information on the degree of food processing,” he observed.
Caroline M. Apovian, MD, who was not involved with the study, agrees that it is wise to limit consumption of UPF.
However, we need more research to better understand which components of UPF are harmful and the biologic mechanisms, Dr. Apovian, who is codirector, Center for Weight Management and Wellness, Brigham and Women’s Hospital, and a professor of medicine at Harvard Medical School, both in Boston, told this news organization in an interview.
She noted that in a randomized crossover trial in 20 patients who were instructed to eat as much or as little as they wanted, people ate more and gained weight during 2 weeks of a diet high in UPF, compared with 2 weeks of an unprocessed diet matched for presented calories, carbohydrate, sugar, fat, sodium, and fiber.
Ultraprocessed foods classed according to Nova system
UPF is “made mostly or entirely from substances derived from foods and additives, using a series of processes and containing minimal whole foods,” and they “are usually nutrient-poor, high in calories, added sugar, sodium, and unhealthy fats,” the Italian researchers write.
High intake of UPF, they add, may exacerbate health risks in people with type 2 diabetes, who are already at higher risk of premature mortality, mainly due to diabetes-related complications.
The researchers analyzed data from a subset of patients in the Moli-sani study of environmental and genetic factors underlying disease, which enrolled 24,325 individuals aged 35 and older who lived in Molise, in central-southern Italy, in 2005-2010.
The current analysis included 1,065 participants in Moli-sani who had type 2 diabetes at baseline and completed a food frequency questionnaire by which participants reported their consumption of 188 foods and beverages in the previous 12 months.
Participants were a mean age of 65 years, and 60% were men.
Most UPF intake was from processed meat (22.4%), crispbread/rusks (16.6%), nonhomemade pizza (11.2%), and cakes, pies, pastries, and puddings (8.8%).
Researchers categorized foods and beverages into four groups with increasing degrees of processing, based on the Nova Food Classification System:
- Group 1: Fresh or minimally processed foods and beverages (for example, fruit, meat, milk).
- Group 2: Processed culinary ingredients (for example, oils, butter).
- Group 3: Processed foods and beverages (for example, canned fish, bread).
- Group 4: UPF (22 foods and beverages including carbonated drinks, processed meats, sweet or savory packaged snacks, margarine, and foods and beverages with artificial sweeteners).
Participants were divided into four quartiles based on UPF consumption.
The mean percentage of UPF consumption out of total food and beverage intake was 2.8%, 5.2%, 7.7%, and 14.4% for quartiles 1, 2, 3, and 4, respectively. By sex, these rates for quartile 1 were < 4.7% for women and < 3.7% for men, and for quartile 4 were ≥ 10.5% for women and ≥ 9% for men.
Participants with the highest UPF intake were younger (mean age, 63 vs. 67 years) but otherwise had similar characteristics as other participants.
During a median follow-up of 11.6 years, 308 participants died from all causes, including 129 who died from CVD.
Compared with participants with the lowest intake of UPF (quartile 1), those with the highest intake (quartile 4) had a higher risk of all-cause mortality (hazard ratio, 1.70) and CVD mortality (HR, 2.64) during follow-up, after multivariable adjustment. The analysis adjusted for sex, age, energy intake, residence, education, housing, smoking, body mass index, leisure-time physical activity, history of cancer or cardiovascular disease, hypertension, hyperlipidemia, aspirin use, years since type 2 diabetes diagnosis, and special diet for blood glucose control.
After further adjusting for Mediterranean diet score, the risk of all-cause and CVD mortality during follow-up for patients with the highest versus lowest intake of UPF remained similar (HR, 1.64 and 2.55, respectively).
There was a linear dose–response relationship between UPF and all-cause and CVD mortality.
Increasing intake of fruit drinks, carbonated drinks, and salty biscuits was associated with higher all-cause and CVD mortality rates, and consumption of stock cubes and margarine was further related to higher CVD death.
The researchers acknowledge that the study was observational, and therefore cannot determine cause and effect, and was not designed to specifically collect dietary data according to the Nova classification. The findings may not be generalizable to other populations.
The analysis was partly funded by grants from the AIRC and Italian Ministry of Health. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a large cohort of older adults with type 2 diabetes in Italy, those with the highest intake of ultraprocessed food and beverages (UPF) were more likely to die of all causes or cardiovascular disease (CVD) within a decade than those with the lowest intake – independent of adherence to a healthy Mediterranean diet.
Adults in the top quartile of UPF intake had a 64% increased risk of all-cause death and a 2.5-fold increased risk of CVD death during follow-up, compared with those in the lowest quartile, after adjusting for variables including Mediterranean diet score.
These findings from the Moli-sani study by Marialaura Bonaccio, PhD, from the Institute for Research, Hospitalization and Healthcare (IRCCS) Neuromed, in Pozzilli, Italy, and colleagues, were published online in the American Journal of Clinical Nutrition.
“Dietary recommendations for prevention and management of type 2 diabetes almost exclusively prioritize consumption of nutritionally balanced foods that are the source of fiber [and] healthy fats and [are] poor in free sugars, and promote dietary patterns – such as the Mediterranean diet and the DASH diet – that place a large emphasis on food groups (for example, whole grains, legumes, nuts, fruits, and vegetables) regardless of food processing,” the researchers note.
The research suggests that “besides prioritizing the adoption of a diet based on nutritional requirements, dietary guidelines for the management of type 2 diabetes should also recommend limiting UPF,” they conclude.
“In addition to the adoption of a diet based on well-known nutritional requirements, dietary recommendations should also suggest limiting the consumption of ultraprocessed foods as much as possible,” Giovanni de Gaetano, MD, PhD, president, IRCCS Neuromed, echoed, in a press release from the institute.
“In this context, and not only for people with diabetes, the front-of-pack nutrition labels should also include information on the degree of food processing,” he observed.
Caroline M. Apovian, MD, who was not involved with the study, agrees that it is wise to limit consumption of UPF.
However, we need more research to better understand which components of UPF are harmful and the biologic mechanisms, Dr. Apovian, who is codirector, Center for Weight Management and Wellness, Brigham and Women’s Hospital, and a professor of medicine at Harvard Medical School, both in Boston, told this news organization in an interview.
She noted that in a randomized crossover trial in 20 patients who were instructed to eat as much or as little as they wanted, people ate more and gained weight during 2 weeks of a diet high in UPF, compared with 2 weeks of an unprocessed diet matched for presented calories, carbohydrate, sugar, fat, sodium, and fiber.
Ultraprocessed foods classed according to Nova system
UPF is “made mostly or entirely from substances derived from foods and additives, using a series of processes and containing minimal whole foods,” and they “are usually nutrient-poor, high in calories, added sugar, sodium, and unhealthy fats,” the Italian researchers write.
High intake of UPF, they add, may exacerbate health risks in people with type 2 diabetes, who are already at higher risk of premature mortality, mainly due to diabetes-related complications.
The researchers analyzed data from a subset of patients in the Moli-sani study of environmental and genetic factors underlying disease, which enrolled 24,325 individuals aged 35 and older who lived in Molise, in central-southern Italy, in 2005-2010.
The current analysis included 1,065 participants in Moli-sani who had type 2 diabetes at baseline and completed a food frequency questionnaire by which participants reported their consumption of 188 foods and beverages in the previous 12 months.
Participants were a mean age of 65 years, and 60% were men.
Most UPF intake was from processed meat (22.4%), crispbread/rusks (16.6%), nonhomemade pizza (11.2%), and cakes, pies, pastries, and puddings (8.8%).
Researchers categorized foods and beverages into four groups with increasing degrees of processing, based on the Nova Food Classification System:
- Group 1: Fresh or minimally processed foods and beverages (for example, fruit, meat, milk).
- Group 2: Processed culinary ingredients (for example, oils, butter).
- Group 3: Processed foods and beverages (for example, canned fish, bread).
- Group 4: UPF (22 foods and beverages including carbonated drinks, processed meats, sweet or savory packaged snacks, margarine, and foods and beverages with artificial sweeteners).
Participants were divided into four quartiles based on UPF consumption.
The mean percentage of UPF consumption out of total food and beverage intake was 2.8%, 5.2%, 7.7%, and 14.4% for quartiles 1, 2, 3, and 4, respectively. By sex, these rates for quartile 1 were < 4.7% for women and < 3.7% for men, and for quartile 4 were ≥ 10.5% for women and ≥ 9% for men.
Participants with the highest UPF intake were younger (mean age, 63 vs. 67 years) but otherwise had similar characteristics as other participants.
During a median follow-up of 11.6 years, 308 participants died from all causes, including 129 who died from CVD.
Compared with participants with the lowest intake of UPF (quartile 1), those with the highest intake (quartile 4) had a higher risk of all-cause mortality (hazard ratio, 1.70) and CVD mortality (HR, 2.64) during follow-up, after multivariable adjustment. The analysis adjusted for sex, age, energy intake, residence, education, housing, smoking, body mass index, leisure-time physical activity, history of cancer or cardiovascular disease, hypertension, hyperlipidemia, aspirin use, years since type 2 diabetes diagnosis, and special diet for blood glucose control.
After further adjusting for Mediterranean diet score, the risk of all-cause and CVD mortality during follow-up for patients with the highest versus lowest intake of UPF remained similar (HR, 1.64 and 2.55, respectively).
There was a linear dose–response relationship between UPF and all-cause and CVD mortality.
Increasing intake of fruit drinks, carbonated drinks, and salty biscuits was associated with higher all-cause and CVD mortality rates, and consumption of stock cubes and margarine was further related to higher CVD death.
The researchers acknowledge that the study was observational, and therefore cannot determine cause and effect, and was not designed to specifically collect dietary data according to the Nova classification. The findings may not be generalizable to other populations.
The analysis was partly funded by grants from the AIRC and Italian Ministry of Health. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Exercise program boosted physical, but not mental, health in young children with overweight
A defined exercise program significantly improved cardiometabolic health and body composition in children with overweight and obesity, but no effect was seen on mental health, based on data from 92 children.
Childhood obesity is associated with negative health outcomes including type 2 diabetes, cardiovascular disease, and mental health disorders, and exercise is considered essential to treatment, wrote Jairo H. Migueles, PhD, of the University of Granada, Spain, and colleagues. However, the effect on children with obesity and overweight of an exercise program on physical and mental health, including within-individual changes, has not been well studied, they said.
In a study published in JAMA Network Open, the researchers reviewed data from 36 girls and 56 boys with overweight or obesity who were randomized to a 20-week exercise program with aerobic and resistance elements, or waitlisted to serve as controls. The participants ranged in age from 8 to 11 years with a mean age of 10 years. The data were collected between Nov. 1, 2014, and June 30, 2016, as part of a parallel-group randomized clinical trial. The exercise program consisted of three to five 90-minute exercise sessions per week for 20 weeks, and the control children continued their usual routines.
The main cardiometabolic outcomes measured in the study were divided into three categories: body composition, physical fitness, and traditional risk factors (waist circumference, blood lipid levels, glucose levels, insulin levels, and blood pressure).
A cardiometabolic risk score was defined by z score. The researchers also added cardiorespiratory fitness (CRF) to the cardiometabolic risk score. Mental health was assessed using composite standardized scores for psychological well-being and poor mental health.
After 20 weeks, cardiometabolic risk scores decreased by approximately 0.38 standard deviations in the exercise group compared with the control group. In addition, specific measures of cardiometabolic health improved significantly from baseline in the exercise group compared with control children for low-density lipoprotein (change of –7.00 mg/dL), body mass index (–5.9 kg/m2), fat mass index (−0.67), and visceral adipose tissue (31.44 g).
Cardiorespiratory fitness improved by 2.75 laps in the exercise group compared with control children. In addition, significantly more children in the exercise group showed meaningful changes (defined as individual changes of at least 0.2 SDs) compared with control children in measures of fat mass index (37 vs. 17, P < .001) and CRF performance (30 vs. 17, P = .03).
However, no significant effects appeared on mental health outcomes in exercisers, the researchers noted.
The reduction in cardiometabolic score was attributable mainly to improvements in cardiovascular fitness, blood lipid levels, and total and visceral adiposity, the researchers wrote in their discussion. The lack of changes in mental health measures may be a result of the healthy mental state of the children at the study outset, they said. “The null effect on mental health outcomes needs to be further investigated, including, among other things, whether the instruments are sensitive enough to detect changes and whether there is a ceiling effect in young children who might be mentally healthy overall,” they wrote.
The findings were limited by several factors, including the relatively small sample size and lack of blinding for some evaluators. However, the results show the potential of exercise programs to affect meaningful change and improve cardiometabolic health in overweight and obese children, although more research is needed to explore the effects of larger-scale and longer-lasting public health interventions combining exercise and other health behaviors such as diet, the researchers concluded.
Bottom line: Exercise works
The increasing rates of overweight and obesity in children in the United States have “significant downstream consequences that include increased risk of metabolic disease, including diabetes and hypertension, as well as increased rates of anxiety and depression,” Neil Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, said in an interview.
Therefore, the effect of interventions such as exercise training on outcomes is important, he said.
The current study findings are “what you would hope for and expect – improvement in cardiometabolic parameters and fitness,” said Dr. Skolnik. “It was encouraging to see the effect of this relatively short duration of intervention has a clear positive effect on weight, BMI, and cardiometabolic parameters,” he said. “The real benefit, of course, comes from sustaining these habits over a long period of time.”
The lack of improvement in mental health is not surprising given the small study population “who did not have a high rate of mental health problems to begin with,” Dr. Skolnik added.
Barriers to promoting exercise programs for obese and overweight children in primary care are many, Dr. Skolnik said, including “having the motivation and funding to create programs like this so they are readily available to youth.”
However, the key message from the current study is simple and straightforward, according to Dr. Skolnik. “Exercise works! It works to improve fitness, cardiometabolic parameters, and weight control,” he said.
“There is always room for more research,” Dr. Skolnik added. The questions now are not about whether exercise benefits health; they are about figuring out how to implement the known benefits of exercise into daily living for all children, athletes and nonathletes alike, he said. “We need to find nonjudgmental ways to encourage exercise as a part of routine daily healthy living, up there with brushing teeth every day,” he emphasized.
The study was supported by grants from the Spanish Ministry of Economy and Competitiveness and El Fondo Europeo de Desarrollo Regional (FEDER) and by the MCIN (Ministerio de Ciencia e Innovación) / AEI (Agencia Estatal de Investigación. The researchers and Dr. Skolnik had no financial conflicts to disclose. Dr. Skolnik serves on the editorial advisory board of Family Practice News.
A defined exercise program significantly improved cardiometabolic health and body composition in children with overweight and obesity, but no effect was seen on mental health, based on data from 92 children.
Childhood obesity is associated with negative health outcomes including type 2 diabetes, cardiovascular disease, and mental health disorders, and exercise is considered essential to treatment, wrote Jairo H. Migueles, PhD, of the University of Granada, Spain, and colleagues. However, the effect on children with obesity and overweight of an exercise program on physical and mental health, including within-individual changes, has not been well studied, they said.
In a study published in JAMA Network Open, the researchers reviewed data from 36 girls and 56 boys with overweight or obesity who were randomized to a 20-week exercise program with aerobic and resistance elements, or waitlisted to serve as controls. The participants ranged in age from 8 to 11 years with a mean age of 10 years. The data were collected between Nov. 1, 2014, and June 30, 2016, as part of a parallel-group randomized clinical trial. The exercise program consisted of three to five 90-minute exercise sessions per week for 20 weeks, and the control children continued their usual routines.
The main cardiometabolic outcomes measured in the study were divided into three categories: body composition, physical fitness, and traditional risk factors (waist circumference, blood lipid levels, glucose levels, insulin levels, and blood pressure).
A cardiometabolic risk score was defined by z score. The researchers also added cardiorespiratory fitness (CRF) to the cardiometabolic risk score. Mental health was assessed using composite standardized scores for psychological well-being and poor mental health.
After 20 weeks, cardiometabolic risk scores decreased by approximately 0.38 standard deviations in the exercise group compared with the control group. In addition, specific measures of cardiometabolic health improved significantly from baseline in the exercise group compared with control children for low-density lipoprotein (change of –7.00 mg/dL), body mass index (–5.9 kg/m2), fat mass index (−0.67), and visceral adipose tissue (31.44 g).
Cardiorespiratory fitness improved by 2.75 laps in the exercise group compared with control children. In addition, significantly more children in the exercise group showed meaningful changes (defined as individual changes of at least 0.2 SDs) compared with control children in measures of fat mass index (37 vs. 17, P < .001) and CRF performance (30 vs. 17, P = .03).
However, no significant effects appeared on mental health outcomes in exercisers, the researchers noted.
The reduction in cardiometabolic score was attributable mainly to improvements in cardiovascular fitness, blood lipid levels, and total and visceral adiposity, the researchers wrote in their discussion. The lack of changes in mental health measures may be a result of the healthy mental state of the children at the study outset, they said. “The null effect on mental health outcomes needs to be further investigated, including, among other things, whether the instruments are sensitive enough to detect changes and whether there is a ceiling effect in young children who might be mentally healthy overall,” they wrote.
The findings were limited by several factors, including the relatively small sample size and lack of blinding for some evaluators. However, the results show the potential of exercise programs to affect meaningful change and improve cardiometabolic health in overweight and obese children, although more research is needed to explore the effects of larger-scale and longer-lasting public health interventions combining exercise and other health behaviors such as diet, the researchers concluded.
Bottom line: Exercise works
The increasing rates of overweight and obesity in children in the United States have “significant downstream consequences that include increased risk of metabolic disease, including diabetes and hypertension, as well as increased rates of anxiety and depression,” Neil Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, said in an interview.
Therefore, the effect of interventions such as exercise training on outcomes is important, he said.
The current study findings are “what you would hope for and expect – improvement in cardiometabolic parameters and fitness,” said Dr. Skolnik. “It was encouraging to see the effect of this relatively short duration of intervention has a clear positive effect on weight, BMI, and cardiometabolic parameters,” he said. “The real benefit, of course, comes from sustaining these habits over a long period of time.”
The lack of improvement in mental health is not surprising given the small study population “who did not have a high rate of mental health problems to begin with,” Dr. Skolnik added.
Barriers to promoting exercise programs for obese and overweight children in primary care are many, Dr. Skolnik said, including “having the motivation and funding to create programs like this so they are readily available to youth.”
However, the key message from the current study is simple and straightforward, according to Dr. Skolnik. “Exercise works! It works to improve fitness, cardiometabolic parameters, and weight control,” he said.
“There is always room for more research,” Dr. Skolnik added. The questions now are not about whether exercise benefits health; they are about figuring out how to implement the known benefits of exercise into daily living for all children, athletes and nonathletes alike, he said. “We need to find nonjudgmental ways to encourage exercise as a part of routine daily healthy living, up there with brushing teeth every day,” he emphasized.
The study was supported by grants from the Spanish Ministry of Economy and Competitiveness and El Fondo Europeo de Desarrollo Regional (FEDER) and by the MCIN (Ministerio de Ciencia e Innovación) / AEI (Agencia Estatal de Investigación. The researchers and Dr. Skolnik had no financial conflicts to disclose. Dr. Skolnik serves on the editorial advisory board of Family Practice News.
A defined exercise program significantly improved cardiometabolic health and body composition in children with overweight and obesity, but no effect was seen on mental health, based on data from 92 children.
Childhood obesity is associated with negative health outcomes including type 2 diabetes, cardiovascular disease, and mental health disorders, and exercise is considered essential to treatment, wrote Jairo H. Migueles, PhD, of the University of Granada, Spain, and colleagues. However, the effect on children with obesity and overweight of an exercise program on physical and mental health, including within-individual changes, has not been well studied, they said.
In a study published in JAMA Network Open, the researchers reviewed data from 36 girls and 56 boys with overweight or obesity who were randomized to a 20-week exercise program with aerobic and resistance elements, or waitlisted to serve as controls. The participants ranged in age from 8 to 11 years with a mean age of 10 years. The data were collected between Nov. 1, 2014, and June 30, 2016, as part of a parallel-group randomized clinical trial. The exercise program consisted of three to five 90-minute exercise sessions per week for 20 weeks, and the control children continued their usual routines.
The main cardiometabolic outcomes measured in the study were divided into three categories: body composition, physical fitness, and traditional risk factors (waist circumference, blood lipid levels, glucose levels, insulin levels, and blood pressure).
A cardiometabolic risk score was defined by z score. The researchers also added cardiorespiratory fitness (CRF) to the cardiometabolic risk score. Mental health was assessed using composite standardized scores for psychological well-being and poor mental health.
After 20 weeks, cardiometabolic risk scores decreased by approximately 0.38 standard deviations in the exercise group compared with the control group. In addition, specific measures of cardiometabolic health improved significantly from baseline in the exercise group compared with control children for low-density lipoprotein (change of –7.00 mg/dL), body mass index (–5.9 kg/m2), fat mass index (−0.67), and visceral adipose tissue (31.44 g).
Cardiorespiratory fitness improved by 2.75 laps in the exercise group compared with control children. In addition, significantly more children in the exercise group showed meaningful changes (defined as individual changes of at least 0.2 SDs) compared with control children in measures of fat mass index (37 vs. 17, P < .001) and CRF performance (30 vs. 17, P = .03).
However, no significant effects appeared on mental health outcomes in exercisers, the researchers noted.
The reduction in cardiometabolic score was attributable mainly to improvements in cardiovascular fitness, blood lipid levels, and total and visceral adiposity, the researchers wrote in their discussion. The lack of changes in mental health measures may be a result of the healthy mental state of the children at the study outset, they said. “The null effect on mental health outcomes needs to be further investigated, including, among other things, whether the instruments are sensitive enough to detect changes and whether there is a ceiling effect in young children who might be mentally healthy overall,” they wrote.
The findings were limited by several factors, including the relatively small sample size and lack of blinding for some evaluators. However, the results show the potential of exercise programs to affect meaningful change and improve cardiometabolic health in overweight and obese children, although more research is needed to explore the effects of larger-scale and longer-lasting public health interventions combining exercise and other health behaviors such as diet, the researchers concluded.
Bottom line: Exercise works
The increasing rates of overweight and obesity in children in the United States have “significant downstream consequences that include increased risk of metabolic disease, including diabetes and hypertension, as well as increased rates of anxiety and depression,” Neil Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, said in an interview.
Therefore, the effect of interventions such as exercise training on outcomes is important, he said.
The current study findings are “what you would hope for and expect – improvement in cardiometabolic parameters and fitness,” said Dr. Skolnik. “It was encouraging to see the effect of this relatively short duration of intervention has a clear positive effect on weight, BMI, and cardiometabolic parameters,” he said. “The real benefit, of course, comes from sustaining these habits over a long period of time.”
The lack of improvement in mental health is not surprising given the small study population “who did not have a high rate of mental health problems to begin with,” Dr. Skolnik added.
Barriers to promoting exercise programs for obese and overweight children in primary care are many, Dr. Skolnik said, including “having the motivation and funding to create programs like this so they are readily available to youth.”
However, the key message from the current study is simple and straightforward, according to Dr. Skolnik. “Exercise works! It works to improve fitness, cardiometabolic parameters, and weight control,” he said.
“There is always room for more research,” Dr. Skolnik added. The questions now are not about whether exercise benefits health; they are about figuring out how to implement the known benefits of exercise into daily living for all children, athletes and nonathletes alike, he said. “We need to find nonjudgmental ways to encourage exercise as a part of routine daily healthy living, up there with brushing teeth every day,” he emphasized.
The study was supported by grants from the Spanish Ministry of Economy and Competitiveness and El Fondo Europeo de Desarrollo Regional (FEDER) and by the MCIN (Ministerio de Ciencia e Innovación) / AEI (Agencia Estatal de Investigación. The researchers and Dr. Skolnik had no financial conflicts to disclose. Dr. Skolnik serves on the editorial advisory board of Family Practice News.
FROM JAMA NETWORK OPEN
Statins post PCI: Moderate intensity plus ezetimibe may be preferable
, suggests a “real-world” cohort study that is consistent with trial evidence.
In the observational study with more than 273,000 patients who received percutaneous coronary intervention (PCI) with drug-eluting stents (DES), risk for a broad composite clinical primary endpoint fell by one-fourth (P < .001) among those put on the two-drug regimen with a moderate-intensity statin, compared with those getting a high-intensity statin alone.
The dual-agent approach was also associated with a 15% drop in statin discontinuation and a 20% reduced risk for new-onset diabetes requiring medication (P < .001 for both benefits), reported investigators in the Journal of the American College of Cardiology.
The study’s primary endpoint – a composite of cardiovascular (CV) death, myocardial infarction (MI), coronary revascularization, heart failure (HF) hospitalization, or nonfatal stroke at 3 years – replicated that of the randomized RACING trial conducted by many of the same researchers and published about a year ago in The Lancet.
RACING demonstrated that ezetimibe plus a moderate-intensity statin could be as effective as a high-intensity statin in patients with CV disease, “but have fewer side effects and better compliance,” Myeong-Ki Hong, MD, PhD, Severance Hospital, Yonsei University, Seoul, South Korea, said in an interview.
Dr. Hong is senior author on the current observational study based on the CONNECT-DES registry, which compared rosuvastatin 10 mg/day plus ezetimibe 10 mg/day – used in RACING – with rosuvastatin 20 mg/day in a nationwide cohort of 72,050 patients.
“As we know, populations who are enrolled in randomized studies do not sufficiently represent real patients in practice,” he observed, “so we wanted to evaluate the generalizability of the RACING results in daily clinical practice.”
Deepak L. Bhatt, MD, said he likes studies that look at whether clinical trial results “play out in the real world,” as this one did. “They have largely replicated the results of the RACING trial,” suggesting the approach using a moderate-intensity statin “is the way to go,” Dr. Bhatt of Mount Sinai Health System, New York, who was not affiliated with the current report, said in an interview. “In fact, the moderate-intensity combination regimen was actually better in this study.”
He said the observed reduction in new-onset diabetes with the moderate-intensity statin approach is also important. “There is a link between high-dose statins and diabetes. So, if given the choice, if you can get the benefits from a cardiovascular perspective with a lower risk of diabetes, it makes sense to use the combination therapy.”
Dr. Bhatt said he had been using high-intensity statin monotherapy in his high-risk patients, but RACING made him reconsider the value of moderate-dose statin combination therapy. “Going with lower doses of two drugs instead of high doses of one drug minimizes side effects and, in some cases, can even enhance efficacy – so this is not an unreasonable paradigm.”
In the current cohort study of patients prescribed rosuvastatin after DES implantation, 10,794 received rosuvastatin 10 mg/day plus ezetimibe 10 mg/day, and 61,256 were put on rosuvastatin 20 mg/day.
Hazard ratio risk reductions with the dual-agent lipid-lowering therapy approach, compared with high-intensity statin monotherapy, were more favorable for the primary composite clinical endpoint and important secondary events:
- HR, 0.75; 95% confidence interval, 0.70-0.79; P < .001) for CV death, MI, coronary artery revascularization, HF, or stroke at 3 years.
- HR, 0.85; 95% CI, 0.78-0.94; P = .001) for statin discontinuation.
- HR, 0.80; 95% CI, 0.72-0.88; P < .001) for new-onset diabetes requiring medication.
But HRs for rhabdomyolysis, cholecystectomy, or a new cancer diagnosis did not indicate significant differences between the two groups.
“Now that there is evidence to support the favorable clinical outcomes of combination lipid-lowering therapy with moderate-intensity statin plus ezetimibe” for secondary prevention from both RACING and a study reflecting daily clinical practice, Dr. Hong said, “physicians may feel more comfortable with this approach.”
The registry analysis “is remarkable not only for validating the results of the RACING trial in routine clinical practice in a high-risk secondary prevention population, but also for its innovative methodology,” states an accompanying editorial by Ori Ben-Yehuda, MD, Sulpizio Cardiovascular Center, University of California, San Diego.
Use of such a large single-payer database in their study “affords even greater external validity to the findings, complementing the internal validity of the randomized RACING trial,” Dr. Ben-Yehuda writes.
The rationale for combination therapy is strong, but additional data would be helpful, particularly for informing guidelines, he continues. “A pragmatic trial randomizing a broad racial and ethnic group of patients to low-dose statin,” such as a starting dose of 10 mg/day atorvastatin or 5 mg/day rosuvastatin “plus ezetimibe vs. high-intensity statin alone would provide much needed data to help guide lipid-lowering therapy for millions of patients and hopefully increase persistence on therapy.”
The study was supported by the Cardiovascular Research Center, Seoul, South Korea. Dr. Hong and Dr. Ben-Yehuda have disclosed no relevant financial relationships. Dr. Bhatt has previously disclosed grants and/or personal fees from many companies; personal fees from WebMD and other publications or organizations; and having other relationships with Medscape Cardiology and other publications or organizations.
A version of this article appeared on Medscape.com.
, suggests a “real-world” cohort study that is consistent with trial evidence.
In the observational study with more than 273,000 patients who received percutaneous coronary intervention (PCI) with drug-eluting stents (DES), risk for a broad composite clinical primary endpoint fell by one-fourth (P < .001) among those put on the two-drug regimen with a moderate-intensity statin, compared with those getting a high-intensity statin alone.
The dual-agent approach was also associated with a 15% drop in statin discontinuation and a 20% reduced risk for new-onset diabetes requiring medication (P < .001 for both benefits), reported investigators in the Journal of the American College of Cardiology.
The study’s primary endpoint – a composite of cardiovascular (CV) death, myocardial infarction (MI), coronary revascularization, heart failure (HF) hospitalization, or nonfatal stroke at 3 years – replicated that of the randomized RACING trial conducted by many of the same researchers and published about a year ago in The Lancet.
RACING demonstrated that ezetimibe plus a moderate-intensity statin could be as effective as a high-intensity statin in patients with CV disease, “but have fewer side effects and better compliance,” Myeong-Ki Hong, MD, PhD, Severance Hospital, Yonsei University, Seoul, South Korea, said in an interview.
Dr. Hong is senior author on the current observational study based on the CONNECT-DES registry, which compared rosuvastatin 10 mg/day plus ezetimibe 10 mg/day – used in RACING – with rosuvastatin 20 mg/day in a nationwide cohort of 72,050 patients.
“As we know, populations who are enrolled in randomized studies do not sufficiently represent real patients in practice,” he observed, “so we wanted to evaluate the generalizability of the RACING results in daily clinical practice.”
Deepak L. Bhatt, MD, said he likes studies that look at whether clinical trial results “play out in the real world,” as this one did. “They have largely replicated the results of the RACING trial,” suggesting the approach using a moderate-intensity statin “is the way to go,” Dr. Bhatt of Mount Sinai Health System, New York, who was not affiliated with the current report, said in an interview. “In fact, the moderate-intensity combination regimen was actually better in this study.”
He said the observed reduction in new-onset diabetes with the moderate-intensity statin approach is also important. “There is a link between high-dose statins and diabetes. So, if given the choice, if you can get the benefits from a cardiovascular perspective with a lower risk of diabetes, it makes sense to use the combination therapy.”
Dr. Bhatt said he had been using high-intensity statin monotherapy in his high-risk patients, but RACING made him reconsider the value of moderate-dose statin combination therapy. “Going with lower doses of two drugs instead of high doses of one drug minimizes side effects and, in some cases, can even enhance efficacy – so this is not an unreasonable paradigm.”
In the current cohort study of patients prescribed rosuvastatin after DES implantation, 10,794 received rosuvastatin 10 mg/day plus ezetimibe 10 mg/day, and 61,256 were put on rosuvastatin 20 mg/day.
Hazard ratio risk reductions with the dual-agent lipid-lowering therapy approach, compared with high-intensity statin monotherapy, were more favorable for the primary composite clinical endpoint and important secondary events:
- HR, 0.75; 95% confidence interval, 0.70-0.79; P < .001) for CV death, MI, coronary artery revascularization, HF, or stroke at 3 years.
- HR, 0.85; 95% CI, 0.78-0.94; P = .001) for statin discontinuation.
- HR, 0.80; 95% CI, 0.72-0.88; P < .001) for new-onset diabetes requiring medication.
But HRs for rhabdomyolysis, cholecystectomy, or a new cancer diagnosis did not indicate significant differences between the two groups.
“Now that there is evidence to support the favorable clinical outcomes of combination lipid-lowering therapy with moderate-intensity statin plus ezetimibe” for secondary prevention from both RACING and a study reflecting daily clinical practice, Dr. Hong said, “physicians may feel more comfortable with this approach.”
The registry analysis “is remarkable not only for validating the results of the RACING trial in routine clinical practice in a high-risk secondary prevention population, but also for its innovative methodology,” states an accompanying editorial by Ori Ben-Yehuda, MD, Sulpizio Cardiovascular Center, University of California, San Diego.
Use of such a large single-payer database in their study “affords even greater external validity to the findings, complementing the internal validity of the randomized RACING trial,” Dr. Ben-Yehuda writes.
The rationale for combination therapy is strong, but additional data would be helpful, particularly for informing guidelines, he continues. “A pragmatic trial randomizing a broad racial and ethnic group of patients to low-dose statin,” such as a starting dose of 10 mg/day atorvastatin or 5 mg/day rosuvastatin “plus ezetimibe vs. high-intensity statin alone would provide much needed data to help guide lipid-lowering therapy for millions of patients and hopefully increase persistence on therapy.”
The study was supported by the Cardiovascular Research Center, Seoul, South Korea. Dr. Hong and Dr. Ben-Yehuda have disclosed no relevant financial relationships. Dr. Bhatt has previously disclosed grants and/or personal fees from many companies; personal fees from WebMD and other publications or organizations; and having other relationships with Medscape Cardiology and other publications or organizations.
A version of this article appeared on Medscape.com.
, suggests a “real-world” cohort study that is consistent with trial evidence.
In the observational study with more than 273,000 patients who received percutaneous coronary intervention (PCI) with drug-eluting stents (DES), risk for a broad composite clinical primary endpoint fell by one-fourth (P < .001) among those put on the two-drug regimen with a moderate-intensity statin, compared with those getting a high-intensity statin alone.
The dual-agent approach was also associated with a 15% drop in statin discontinuation and a 20% reduced risk for new-onset diabetes requiring medication (P < .001 for both benefits), reported investigators in the Journal of the American College of Cardiology.
The study’s primary endpoint – a composite of cardiovascular (CV) death, myocardial infarction (MI), coronary revascularization, heart failure (HF) hospitalization, or nonfatal stroke at 3 years – replicated that of the randomized RACING trial conducted by many of the same researchers and published about a year ago in The Lancet.
RACING demonstrated that ezetimibe plus a moderate-intensity statin could be as effective as a high-intensity statin in patients with CV disease, “but have fewer side effects and better compliance,” Myeong-Ki Hong, MD, PhD, Severance Hospital, Yonsei University, Seoul, South Korea, said in an interview.
Dr. Hong is senior author on the current observational study based on the CONNECT-DES registry, which compared rosuvastatin 10 mg/day plus ezetimibe 10 mg/day – used in RACING – with rosuvastatin 20 mg/day in a nationwide cohort of 72,050 patients.
“As we know, populations who are enrolled in randomized studies do not sufficiently represent real patients in practice,” he observed, “so we wanted to evaluate the generalizability of the RACING results in daily clinical practice.”
Deepak L. Bhatt, MD, said he likes studies that look at whether clinical trial results “play out in the real world,” as this one did. “They have largely replicated the results of the RACING trial,” suggesting the approach using a moderate-intensity statin “is the way to go,” Dr. Bhatt of Mount Sinai Health System, New York, who was not affiliated with the current report, said in an interview. “In fact, the moderate-intensity combination regimen was actually better in this study.”
He said the observed reduction in new-onset diabetes with the moderate-intensity statin approach is also important. “There is a link between high-dose statins and diabetes. So, if given the choice, if you can get the benefits from a cardiovascular perspective with a lower risk of diabetes, it makes sense to use the combination therapy.”
Dr. Bhatt said he had been using high-intensity statin monotherapy in his high-risk patients, but RACING made him reconsider the value of moderate-dose statin combination therapy. “Going with lower doses of two drugs instead of high doses of one drug minimizes side effects and, in some cases, can even enhance efficacy – so this is not an unreasonable paradigm.”
In the current cohort study of patients prescribed rosuvastatin after DES implantation, 10,794 received rosuvastatin 10 mg/day plus ezetimibe 10 mg/day, and 61,256 were put on rosuvastatin 20 mg/day.
Hazard ratio risk reductions with the dual-agent lipid-lowering therapy approach, compared with high-intensity statin monotherapy, were more favorable for the primary composite clinical endpoint and important secondary events:
- HR, 0.75; 95% confidence interval, 0.70-0.79; P < .001) for CV death, MI, coronary artery revascularization, HF, or stroke at 3 years.
- HR, 0.85; 95% CI, 0.78-0.94; P = .001) for statin discontinuation.
- HR, 0.80; 95% CI, 0.72-0.88; P < .001) for new-onset diabetes requiring medication.
But HRs for rhabdomyolysis, cholecystectomy, or a new cancer diagnosis did not indicate significant differences between the two groups.
“Now that there is evidence to support the favorable clinical outcomes of combination lipid-lowering therapy with moderate-intensity statin plus ezetimibe” for secondary prevention from both RACING and a study reflecting daily clinical practice, Dr. Hong said, “physicians may feel more comfortable with this approach.”
The registry analysis “is remarkable not only for validating the results of the RACING trial in routine clinical practice in a high-risk secondary prevention population, but also for its innovative methodology,” states an accompanying editorial by Ori Ben-Yehuda, MD, Sulpizio Cardiovascular Center, University of California, San Diego.
Use of such a large single-payer database in their study “affords even greater external validity to the findings, complementing the internal validity of the randomized RACING trial,” Dr. Ben-Yehuda writes.
The rationale for combination therapy is strong, but additional data would be helpful, particularly for informing guidelines, he continues. “A pragmatic trial randomizing a broad racial and ethnic group of patients to low-dose statin,” such as a starting dose of 10 mg/day atorvastatin or 5 mg/day rosuvastatin “plus ezetimibe vs. high-intensity statin alone would provide much needed data to help guide lipid-lowering therapy for millions of patients and hopefully increase persistence on therapy.”
The study was supported by the Cardiovascular Research Center, Seoul, South Korea. Dr. Hong and Dr. Ben-Yehuda have disclosed no relevant financial relationships. Dr. Bhatt has previously disclosed grants and/or personal fees from many companies; personal fees from WebMD and other publications or organizations; and having other relationships with Medscape Cardiology and other publications or organizations.
A version of this article appeared on Medscape.com.
FROM JACC
Daily statin cuts cardiovascular risk in HIV
BRISBANE, AUSTRALIA – that show pitavastatin therapy is associated with a significantly lower risk of cardiovascular events than placebo.
“There was a significant 35% lower risk of major adverse cardiovascular events after a median follow-up of 5.1 years “ said Steven Grinspoon, MD, from Massachusetts General Hospital and Harvard Medical School in Boston, who presented the final analysis of data from the REPRIEVE trial at the International AIDS Society Conference on HIV Science.
The results were simultaneously published in the New England Journal of Medicine. The primary endpoint of major adverse cardiovascular events included a composite of outcomes that included cardiovascular death, stroke, myocardial infarction, hospitalization for unstable angina, and transient ischemic attack among those treated with pitavastatin, compared with placebo (95% confidence interval, 0.48-0.90; P = .002).
The REPRIEVE trial was halted earlier this year for efficacy after an interim analysis pointed to a significantly lower rate of cardiovascular events in the treatment group.
The international double-blind, placebo-controlled trial randomly assigned 7,769 people with HIV infection, who were at low to moderate risk of cardiovascular disease, to either 4 mg daily of pitavastatin calcium or placebo.
The secondary outcome – a composite of major cardiovascular events and all-cause mortality – also showed a significant 21% reduction in risk with pitavastatin treatment, compared with placebo (95% CI, 0.65-0.96).
Cardiovascular events in HIV
HIV infection is an independent risk factor for cardiovascular disease, Dr. Grinspoon pointed out, and those living with HIV have about double the risk of myocardial infarction and stroke, compared with the general population.
“There’s an unmet need for people living with HIV who have low to moderate traditional risk, for whom HIV is even considered a risk equivalent but for whom no primary prevention strategy has been tested in a large trial,” Dr. Grinspoon said during an interview.
Those enrolled in the study had a 10-year Atherosclerotic Cardiovascular Disease risk score ranging from 2.1% to 7%, with a median of 4.5%. While LDL cholesterol levels at baseline ranged from 87 to 128 mg/dL, the study showed a similar reduction in cardiovascular risk regardless of LDL.
“These are types of people who, if they came to the doctor’s office right now before REPRIEVE, they would largely be told your risk score is not really making you eligible for a statin,” Dr. Grinspoon said.
He explained that what is most interesting about the reduction in risk is that it was nearly twice what would be expected with LDL lowering, based on what has previously been seen in statin trials in non–HIV-positive populations.
“I think the data are suggesting that it’s certainly in part due to the reduction in LDL – that is very important – but it’s also due to other factors beyond changes in LDL,” Dr. Grinspoon said. He speculated that the statin could be affecting anti-inflammatory and immune pathways, and that this could account for some of the reduction in cardiovascular risk, but “those data are cooking, and they’re being analyzed as we speak.”
In a substudy analysis of REPRIEVE, Markella Zanni, MD, associate professor of medicine at Harvard Medical School and Massachusetts General Hospital, focused on the women in the clinical trial.
Women’s risk
In REPRIEVE, 31.1% of the study population were women. Dr. Zanni and her team investigated whether there are differences in the way HIV affects the risk of developing atherosclerotic cardiovascular disease in women, compared with men.
They found that women have both higher levels of inflammatory markers, such as interleukin-6, C-reactive protein, and D-dimer, but a lower prevalence of coronary artery plaques than men.
“This finding represents an interesting paradox given that high levels of select inflammatory markers have been associated with coronary artery plaque, both among women living with HIV and among men living with HIV,” Dr. Zanni explained.
She says the researchers were hoping to further explore whether inflammation is fueling the increased risk for atherosclerotic disease, and particularly the higher risk evident in women living with HIV, compared with men.
“Women living with HIV should discuss with their treating clinicians heart risks and possible prevention strategies, including statin therapy coupled with healthy lifestyle changes addressing modifiable, traditional metabolic risk factors” she said.
Time for primary prevention?
All patients in the study were on antiretroviral therapy and investigators report that pitavastatin does not interact with these medications. The median CD4 cell count was 621 cells/mm3, and 87.5% of participants had an HIV viral load below the lower limit of quantification.
Participants were enrolled from 12 countries including the United States, Spain, Brazil, South Africa, and Thailand, and around two-thirds were non-White. Individuals of South Asian ethnicity showed the biggest reduction in cardiovascular risk with pitavastatin treatment.
There was a 74% higher rate of muscle pain and weakness in the pitavastatin group – affecting 91 people in the treatment arm and 53 in the placebo arm – but the majority were low grade. The rate of rhabdomyolysis of grade 3 or above was lower in the statin group, with three cases, compared with four cases in the placebo group.
Commenting on the findings, Laura Waters, MD, a genitourinary and HIV medicine consultant at Central and North West London NHS Foundation Trust’s Mortimer Market Centre, said that, while HIV infection was considered a risk factor for cardiovascular disease, risk calculators don’t specifically adjust for HIV infection.
“Now that we’ve got effective HIV drugs and people can enjoy normal life expectancy, cardiovascular disease is a particular issue for people with HIV,” she said.
Dr. Waters, who was not involved with the study, suggested that people living with HIV should discuss the use of statins with their doctor, but she acknowledged there are some barriers to treatment in people living with HIV. “It’s another pill, and when it’s a borderline [decision] it is easy to say, ‘I have to think about it,’ ” she said, with the result that statin treatment is often deferred.
The REPRIEVE study was supported by grants from the National Institutes of Health, Kowa Pharmaceuticals America, Gilead Sciences, and ViiV Healthcare. Dr. Grinspoon declared institutional grants from National Institutes of Health, Kowa Pharmaceuticals America, Gilead Sciences, and ViiV Healthcare and consultancies unrelated to the study. Dr. Zanni reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BRISBANE, AUSTRALIA – that show pitavastatin therapy is associated with a significantly lower risk of cardiovascular events than placebo.
“There was a significant 35% lower risk of major adverse cardiovascular events after a median follow-up of 5.1 years “ said Steven Grinspoon, MD, from Massachusetts General Hospital and Harvard Medical School in Boston, who presented the final analysis of data from the REPRIEVE trial at the International AIDS Society Conference on HIV Science.
The results were simultaneously published in the New England Journal of Medicine. The primary endpoint of major adverse cardiovascular events included a composite of outcomes that included cardiovascular death, stroke, myocardial infarction, hospitalization for unstable angina, and transient ischemic attack among those treated with pitavastatin, compared with placebo (95% confidence interval, 0.48-0.90; P = .002).
The REPRIEVE trial was halted earlier this year for efficacy after an interim analysis pointed to a significantly lower rate of cardiovascular events in the treatment group.
The international double-blind, placebo-controlled trial randomly assigned 7,769 people with HIV infection, who were at low to moderate risk of cardiovascular disease, to either 4 mg daily of pitavastatin calcium or placebo.
The secondary outcome – a composite of major cardiovascular events and all-cause mortality – also showed a significant 21% reduction in risk with pitavastatin treatment, compared with placebo (95% CI, 0.65-0.96).
Cardiovascular events in HIV
HIV infection is an independent risk factor for cardiovascular disease, Dr. Grinspoon pointed out, and those living with HIV have about double the risk of myocardial infarction and stroke, compared with the general population.
“There’s an unmet need for people living with HIV who have low to moderate traditional risk, for whom HIV is even considered a risk equivalent but for whom no primary prevention strategy has been tested in a large trial,” Dr. Grinspoon said during an interview.
Those enrolled in the study had a 10-year Atherosclerotic Cardiovascular Disease risk score ranging from 2.1% to 7%, with a median of 4.5%. While LDL cholesterol levels at baseline ranged from 87 to 128 mg/dL, the study showed a similar reduction in cardiovascular risk regardless of LDL.
“These are types of people who, if they came to the doctor’s office right now before REPRIEVE, they would largely be told your risk score is not really making you eligible for a statin,” Dr. Grinspoon said.
He explained that what is most interesting about the reduction in risk is that it was nearly twice what would be expected with LDL lowering, based on what has previously been seen in statin trials in non–HIV-positive populations.
“I think the data are suggesting that it’s certainly in part due to the reduction in LDL – that is very important – but it’s also due to other factors beyond changes in LDL,” Dr. Grinspoon said. He speculated that the statin could be affecting anti-inflammatory and immune pathways, and that this could account for some of the reduction in cardiovascular risk, but “those data are cooking, and they’re being analyzed as we speak.”
In a substudy analysis of REPRIEVE, Markella Zanni, MD, associate professor of medicine at Harvard Medical School and Massachusetts General Hospital, focused on the women in the clinical trial.
Women’s risk
In REPRIEVE, 31.1% of the study population were women. Dr. Zanni and her team investigated whether there are differences in the way HIV affects the risk of developing atherosclerotic cardiovascular disease in women, compared with men.
They found that women have both higher levels of inflammatory markers, such as interleukin-6, C-reactive protein, and D-dimer, but a lower prevalence of coronary artery plaques than men.
“This finding represents an interesting paradox given that high levels of select inflammatory markers have been associated with coronary artery plaque, both among women living with HIV and among men living with HIV,” Dr. Zanni explained.
She says the researchers were hoping to further explore whether inflammation is fueling the increased risk for atherosclerotic disease, and particularly the higher risk evident in women living with HIV, compared with men.
“Women living with HIV should discuss with their treating clinicians heart risks and possible prevention strategies, including statin therapy coupled with healthy lifestyle changes addressing modifiable, traditional metabolic risk factors” she said.
Time for primary prevention?
All patients in the study were on antiretroviral therapy and investigators report that pitavastatin does not interact with these medications. The median CD4 cell count was 621 cells/mm3, and 87.5% of participants had an HIV viral load below the lower limit of quantification.
Participants were enrolled from 12 countries including the United States, Spain, Brazil, South Africa, and Thailand, and around two-thirds were non-White. Individuals of South Asian ethnicity showed the biggest reduction in cardiovascular risk with pitavastatin treatment.
There was a 74% higher rate of muscle pain and weakness in the pitavastatin group – affecting 91 people in the treatment arm and 53 in the placebo arm – but the majority were low grade. The rate of rhabdomyolysis of grade 3 or above was lower in the statin group, with three cases, compared with four cases in the placebo group.
Commenting on the findings, Laura Waters, MD, a genitourinary and HIV medicine consultant at Central and North West London NHS Foundation Trust’s Mortimer Market Centre, said that, while HIV infection was considered a risk factor for cardiovascular disease, risk calculators don’t specifically adjust for HIV infection.
“Now that we’ve got effective HIV drugs and people can enjoy normal life expectancy, cardiovascular disease is a particular issue for people with HIV,” she said.
Dr. Waters, who was not involved with the study, suggested that people living with HIV should discuss the use of statins with their doctor, but she acknowledged there are some barriers to treatment in people living with HIV. “It’s another pill, and when it’s a borderline [decision] it is easy to say, ‘I have to think about it,’ ” she said, with the result that statin treatment is often deferred.
The REPRIEVE study was supported by grants from the National Institutes of Health, Kowa Pharmaceuticals America, Gilead Sciences, and ViiV Healthcare. Dr. Grinspoon declared institutional grants from National Institutes of Health, Kowa Pharmaceuticals America, Gilead Sciences, and ViiV Healthcare and consultancies unrelated to the study. Dr. Zanni reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BRISBANE, AUSTRALIA – that show pitavastatin therapy is associated with a significantly lower risk of cardiovascular events than placebo.
“There was a significant 35% lower risk of major adverse cardiovascular events after a median follow-up of 5.1 years “ said Steven Grinspoon, MD, from Massachusetts General Hospital and Harvard Medical School in Boston, who presented the final analysis of data from the REPRIEVE trial at the International AIDS Society Conference on HIV Science.
The results were simultaneously published in the New England Journal of Medicine. The primary endpoint of major adverse cardiovascular events included a composite of outcomes that included cardiovascular death, stroke, myocardial infarction, hospitalization for unstable angina, and transient ischemic attack among those treated with pitavastatin, compared with placebo (95% confidence interval, 0.48-0.90; P = .002).
The REPRIEVE trial was halted earlier this year for efficacy after an interim analysis pointed to a significantly lower rate of cardiovascular events in the treatment group.
The international double-blind, placebo-controlled trial randomly assigned 7,769 people with HIV infection, who were at low to moderate risk of cardiovascular disease, to either 4 mg daily of pitavastatin calcium or placebo.
The secondary outcome – a composite of major cardiovascular events and all-cause mortality – also showed a significant 21% reduction in risk with pitavastatin treatment, compared with placebo (95% CI, 0.65-0.96).
Cardiovascular events in HIV
HIV infection is an independent risk factor for cardiovascular disease, Dr. Grinspoon pointed out, and those living with HIV have about double the risk of myocardial infarction and stroke, compared with the general population.
“There’s an unmet need for people living with HIV who have low to moderate traditional risk, for whom HIV is even considered a risk equivalent but for whom no primary prevention strategy has been tested in a large trial,” Dr. Grinspoon said during an interview.
Those enrolled in the study had a 10-year Atherosclerotic Cardiovascular Disease risk score ranging from 2.1% to 7%, with a median of 4.5%. While LDL cholesterol levels at baseline ranged from 87 to 128 mg/dL, the study showed a similar reduction in cardiovascular risk regardless of LDL.
“These are types of people who, if they came to the doctor’s office right now before REPRIEVE, they would largely be told your risk score is not really making you eligible for a statin,” Dr. Grinspoon said.
He explained that what is most interesting about the reduction in risk is that it was nearly twice what would be expected with LDL lowering, based on what has previously been seen in statin trials in non–HIV-positive populations.
“I think the data are suggesting that it’s certainly in part due to the reduction in LDL – that is very important – but it’s also due to other factors beyond changes in LDL,” Dr. Grinspoon said. He speculated that the statin could be affecting anti-inflammatory and immune pathways, and that this could account for some of the reduction in cardiovascular risk, but “those data are cooking, and they’re being analyzed as we speak.”
In a substudy analysis of REPRIEVE, Markella Zanni, MD, associate professor of medicine at Harvard Medical School and Massachusetts General Hospital, focused on the women in the clinical trial.
Women’s risk
In REPRIEVE, 31.1% of the study population were women. Dr. Zanni and her team investigated whether there are differences in the way HIV affects the risk of developing atherosclerotic cardiovascular disease in women, compared with men.
They found that women have both higher levels of inflammatory markers, such as interleukin-6, C-reactive protein, and D-dimer, but a lower prevalence of coronary artery plaques than men.
“This finding represents an interesting paradox given that high levels of select inflammatory markers have been associated with coronary artery plaque, both among women living with HIV and among men living with HIV,” Dr. Zanni explained.
She says the researchers were hoping to further explore whether inflammation is fueling the increased risk for atherosclerotic disease, and particularly the higher risk evident in women living with HIV, compared with men.
“Women living with HIV should discuss with their treating clinicians heart risks and possible prevention strategies, including statin therapy coupled with healthy lifestyle changes addressing modifiable, traditional metabolic risk factors” she said.
Time for primary prevention?
All patients in the study were on antiretroviral therapy and investigators report that pitavastatin does not interact with these medications. The median CD4 cell count was 621 cells/mm3, and 87.5% of participants had an HIV viral load below the lower limit of quantification.
Participants were enrolled from 12 countries including the United States, Spain, Brazil, South Africa, and Thailand, and around two-thirds were non-White. Individuals of South Asian ethnicity showed the biggest reduction in cardiovascular risk with pitavastatin treatment.
There was a 74% higher rate of muscle pain and weakness in the pitavastatin group – affecting 91 people in the treatment arm and 53 in the placebo arm – but the majority were low grade. The rate of rhabdomyolysis of grade 3 or above was lower in the statin group, with three cases, compared with four cases in the placebo group.
Commenting on the findings, Laura Waters, MD, a genitourinary and HIV medicine consultant at Central and North West London NHS Foundation Trust’s Mortimer Market Centre, said that, while HIV infection was considered a risk factor for cardiovascular disease, risk calculators don’t specifically adjust for HIV infection.
“Now that we’ve got effective HIV drugs and people can enjoy normal life expectancy, cardiovascular disease is a particular issue for people with HIV,” she said.
Dr. Waters, who was not involved with the study, suggested that people living with HIV should discuss the use of statins with their doctor, but she acknowledged there are some barriers to treatment in people living with HIV. “It’s another pill, and when it’s a borderline [decision] it is easy to say, ‘I have to think about it,’ ” she said, with the result that statin treatment is often deferred.
The REPRIEVE study was supported by grants from the National Institutes of Health, Kowa Pharmaceuticals America, Gilead Sciences, and ViiV Healthcare. Dr. Grinspoon declared institutional grants from National Institutes of Health, Kowa Pharmaceuticals America, Gilead Sciences, and ViiV Healthcare and consultancies unrelated to the study. Dr. Zanni reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT IAS 2023
USPSTF maintains ‘insufficient evidence’ for lipid disorder screenings in kids and teens
The group’s final recommendation and corresponding evidence report were published in the Journal of the American Medical Association, following a draft recommendation in January.
The organization reached a similar conclusion following its evaluation in 2016.
“There’s just not enough evidence to determine whether or not screening all children for high cholesterol improves their heart health into adulthood,” said Katrina Donahue, MD, MPH, a USPSTF member and a professor in the department of family medicine at the University of North Carolina at Chapel Hill. “We’re calling for additional research on the effectiveness of screening for and treatment of high cholesterol in children and adolescents to prevent heart attacks, strokes, and death in adulthood.”
The task force recommended other evidence-based strategies to promote heart health, such as screening for obesity and interventions to prevent tobacco use.
The recommendation was the result of a review of 43 studies from MEDLINE and the Cochrane Central Register of Controlled Trials through May 16, 2022. No randomized controlled trial directly addressed the effectiveness or harms of lipid screening for children and adolescents. The task force continued to use article alerts and targeted journal searches through March 24, 2023.
Conditions such as familial hypercholesterolemia and multifactorial dyslipidemia can cause abnormally high lipid levels in children, potentially leading to premature cardiovascular events such as myocardial infarction, stroke, and death in adulthood. According to the USPSTF, the prevalence of FH in U.S. children and adolescents ranges from 0.2% to 0.4% (one in every 250-500 youth). Multifactorial dyslipidemia is more common – the prevalence in children and adolescents ranges from 7.1% to 9.4%.
In an editorial response to the task force’s statement, the authors, including Sarah D. de Ferranti, MD, department of pediatrics at Harvard Medical School, Boston, question the impact of not screening children to identify FH and other conditions and caution against the subsequent delay in treatment.
“Treating FH during childhood slows the progression of vascular finding in atherosclerosis,” the authors write.
They note that the recommendation “leaves a void for clinicians seeking to provide care for patients today” while additional research is conducted.
Sarah Nosal, MD, a member of the board of directors of the American Academy of Family Physicians, said that despite the lack of a recommendation, primary care clinicians can still encourage proper nutrition and physical activity for patients.
Dr. Nosal said that even without clear recommendations from the USPSTF, in the rare case of a patient with a family history of FH, she would order a lipid test and discuss treatment plans with the patient and family, if needed.
“We really don’t want to do tests that we don’t know what to do with the information,” she said.
One USPSTF member reported receiving grants from Healthwise, a nonprofit organization, outside the submitted work.
A version of this article first appeared on Medscape.com.
The group’s final recommendation and corresponding evidence report were published in the Journal of the American Medical Association, following a draft recommendation in January.
The organization reached a similar conclusion following its evaluation in 2016.
“There’s just not enough evidence to determine whether or not screening all children for high cholesterol improves their heart health into adulthood,” said Katrina Donahue, MD, MPH, a USPSTF member and a professor in the department of family medicine at the University of North Carolina at Chapel Hill. “We’re calling for additional research on the effectiveness of screening for and treatment of high cholesterol in children and adolescents to prevent heart attacks, strokes, and death in adulthood.”
The task force recommended other evidence-based strategies to promote heart health, such as screening for obesity and interventions to prevent tobacco use.
The recommendation was the result of a review of 43 studies from MEDLINE and the Cochrane Central Register of Controlled Trials through May 16, 2022. No randomized controlled trial directly addressed the effectiveness or harms of lipid screening for children and adolescents. The task force continued to use article alerts and targeted journal searches through March 24, 2023.
Conditions such as familial hypercholesterolemia and multifactorial dyslipidemia can cause abnormally high lipid levels in children, potentially leading to premature cardiovascular events such as myocardial infarction, stroke, and death in adulthood. According to the USPSTF, the prevalence of FH in U.S. children and adolescents ranges from 0.2% to 0.4% (one in every 250-500 youth). Multifactorial dyslipidemia is more common – the prevalence in children and adolescents ranges from 7.1% to 9.4%.
In an editorial response to the task force’s statement, the authors, including Sarah D. de Ferranti, MD, department of pediatrics at Harvard Medical School, Boston, question the impact of not screening children to identify FH and other conditions and caution against the subsequent delay in treatment.
“Treating FH during childhood slows the progression of vascular finding in atherosclerosis,” the authors write.
They note that the recommendation “leaves a void for clinicians seeking to provide care for patients today” while additional research is conducted.
Sarah Nosal, MD, a member of the board of directors of the American Academy of Family Physicians, said that despite the lack of a recommendation, primary care clinicians can still encourage proper nutrition and physical activity for patients.
Dr. Nosal said that even without clear recommendations from the USPSTF, in the rare case of a patient with a family history of FH, she would order a lipid test and discuss treatment plans with the patient and family, if needed.
“We really don’t want to do tests that we don’t know what to do with the information,” she said.
One USPSTF member reported receiving grants from Healthwise, a nonprofit organization, outside the submitted work.
A version of this article first appeared on Medscape.com.
The group’s final recommendation and corresponding evidence report were published in the Journal of the American Medical Association, following a draft recommendation in January.
The organization reached a similar conclusion following its evaluation in 2016.
“There’s just not enough evidence to determine whether or not screening all children for high cholesterol improves their heart health into adulthood,” said Katrina Donahue, MD, MPH, a USPSTF member and a professor in the department of family medicine at the University of North Carolina at Chapel Hill. “We’re calling for additional research on the effectiveness of screening for and treatment of high cholesterol in children and adolescents to prevent heart attacks, strokes, and death in adulthood.”
The task force recommended other evidence-based strategies to promote heart health, such as screening for obesity and interventions to prevent tobacco use.
The recommendation was the result of a review of 43 studies from MEDLINE and the Cochrane Central Register of Controlled Trials through May 16, 2022. No randomized controlled trial directly addressed the effectiveness or harms of lipid screening for children and adolescents. The task force continued to use article alerts and targeted journal searches through March 24, 2023.
Conditions such as familial hypercholesterolemia and multifactorial dyslipidemia can cause abnormally high lipid levels in children, potentially leading to premature cardiovascular events such as myocardial infarction, stroke, and death in adulthood. According to the USPSTF, the prevalence of FH in U.S. children and adolescents ranges from 0.2% to 0.4% (one in every 250-500 youth). Multifactorial dyslipidemia is more common – the prevalence in children and adolescents ranges from 7.1% to 9.4%.
In an editorial response to the task force’s statement, the authors, including Sarah D. de Ferranti, MD, department of pediatrics at Harvard Medical School, Boston, question the impact of not screening children to identify FH and other conditions and caution against the subsequent delay in treatment.
“Treating FH during childhood slows the progression of vascular finding in atherosclerosis,” the authors write.
They note that the recommendation “leaves a void for clinicians seeking to provide care for patients today” while additional research is conducted.
Sarah Nosal, MD, a member of the board of directors of the American Academy of Family Physicians, said that despite the lack of a recommendation, primary care clinicians can still encourage proper nutrition and physical activity for patients.
Dr. Nosal said that even without clear recommendations from the USPSTF, in the rare case of a patient with a family history of FH, she would order a lipid test and discuss treatment plans with the patient and family, if needed.
“We really don’t want to do tests that we don’t know what to do with the information,” she said.
One USPSTF member reported receiving grants from Healthwise, a nonprofit organization, outside the submitted work.
A version of this article first appeared on Medscape.com.
FDA expands inclisiran statin-adjunct indication to include primary prevention
Novartis has announced.
The first-in-class small interfering RNA (siRNA) agent was approved in 2021 as an adjunct to statins for patients with clinical cardiovascular disease or heterozygous familial hypercholesterolemia. The indications now include patients taking statins for primary dyslipidemia who have high-risk comorbidities such as diabetes but who do not have a history of cardiovascular events, the company said.
Inclisiran, with a mechanism of action unique among drugs for dyslipidemia, works by “silencing” RNA involved in the synthesis of proprotein convertase subtilisin/kexin type 9. The protein helps regulate the number of LDL cholesterol cell-surface receptors.
Novartis said it has “global rights to develop, manufacture and commercialize Leqvio under a license and collaboration agreement with Alnylam Pharmaceuticals.”
A version of this article first appeared on Medscape.com.
Novartis has announced.
The first-in-class small interfering RNA (siRNA) agent was approved in 2021 as an adjunct to statins for patients with clinical cardiovascular disease or heterozygous familial hypercholesterolemia. The indications now include patients taking statins for primary dyslipidemia who have high-risk comorbidities such as diabetes but who do not have a history of cardiovascular events, the company said.
Inclisiran, with a mechanism of action unique among drugs for dyslipidemia, works by “silencing” RNA involved in the synthesis of proprotein convertase subtilisin/kexin type 9. The protein helps regulate the number of LDL cholesterol cell-surface receptors.
Novartis said it has “global rights to develop, manufacture and commercialize Leqvio under a license and collaboration agreement with Alnylam Pharmaceuticals.”
A version of this article first appeared on Medscape.com.
Novartis has announced.
The first-in-class small interfering RNA (siRNA) agent was approved in 2021 as an adjunct to statins for patients with clinical cardiovascular disease or heterozygous familial hypercholesterolemia. The indications now include patients taking statins for primary dyslipidemia who have high-risk comorbidities such as diabetes but who do not have a history of cardiovascular events, the company said.
Inclisiran, with a mechanism of action unique among drugs for dyslipidemia, works by “silencing” RNA involved in the synthesis of proprotein convertase subtilisin/kexin type 9. The protein helps regulate the number of LDL cholesterol cell-surface receptors.
Novartis said it has “global rights to develop, manufacture and commercialize Leqvio under a license and collaboration agreement with Alnylam Pharmaceuticals.”
A version of this article first appeared on Medscape.com.
SAFE algorithm detects liver disease in general population
VIENNA – An algorithm, the Steatosis-Associated Fibrosis Estimator (SAFE), was developed to detect clinically significant fibrosis in patients with nonalcoholic fatty liver disease (NAFLD). It is effective at detecting chronic liver disease from all causes with or without NAFLD in the general population, according the results of a U.S. population-based study. The algorithm was designed for use in primary care to help slow the steep rise in liver disease burden.
On the basis of the SAFE score, 61.3% of participants were at low risk for clinically significant fibrosis; 11.2% were at high risk; and 27.5% were at intermediate risk. Upon validation, very few of the low-risk participants had liver fibrosis, while nearly a third of those with a high-risk score had clinically significant fibrosis. In addition, a high percentage of the patients with high-risk SAFE scores had viral hepatitis and elevations in ferritin level.
“This is the first time that there has been a test that provides a score to detect low-risk liver disease in primary care,” said Ray Kim, MD, from Stanford (Calif.) University, senior investigator, who was speaking to this news organization at the annual International Liver Congress sponsored by the European Association for the Study of the Liver
“Primary care doctors currently detect liver disease through a serendipitous abnormal finding on ultrasound or blood tests that detect elevated transaminases, and then the patient is referred to a hepatologist, who figures out what is really going on,” said Dr. Kim.
“This approach is limited, so we need to get SAFE into primary care so these doctors can automatically calculate their scores, and if the patient is over 100 [high risk of chronic liver disease], then they need help [referral to a hepatologist].”
Liver deaths sharply rising
Public health data show that more people are dying of liver disease today than previously. Deaths in the United States have doubled over the past 20 years, said Dr. Kim. “If our mission is to help these patients and prevent death, [things are] moving in the wrong direction.”
He stressed that in order to change the direction, “primary care doctors need to engage with the issue and have appropriate tools to identify people with liver disease.”
Most often, the reason for this rise in deaths is that cases are being diagnosed at advanced stages of disease in which reversibility is limited, he added. “We want to move upstream where people might have early-stage disease and where we can intervene and make a difference.”
In an effort to help earlier detection of liver disease, the SAFE score was developed and validated by Dr. Kim and his colleagues to detect clinically significant (greater than stage 2) fibrosis in patients with NAFLD in primary care. The score is based upon age, body mass index, diabetes, platelet level, aspartate and alanine aminotransferase levels, and globulin level. A score of less than zero signifies that a patient is at low risk for liver fibrosis, while a score greater than 100 signifies a high risk of fibrosis. A score between 0 and 99 denotes intermediate risk of fibrosis.
“Unlike other noninvasive tests that detect advanced fibrosis, this one detects early-stage fibrosis. We’ve shown that the SAFE estimator is better than all other blood-based markers,” explained Dr. Kim.
Applying SAFE to the general population
In the study presented here at EASL, Dr. Kim aimed to expand the horizon for SAFE testing to the U.S. general population and to assess whether SAFE was effective in screening for chronic liver disease regardless of steatosis of the liver.
Together with first author Nakia Chung, MD, also from Stanford University, Dr. Kim applied the SAFE score to data from 7,156 participants of the National Health and Nutrition Examination Survey (NHANES) for 2017-2020. NHANES is representative of the noninstitutionalized, civilian population of the United States. It includes broad demographic, clinical, and laboratory data, including transient elastography data. FibroScans were first used in 2017, so the investigators had 3 years of FibroScan data with which to validate the score.
The researchers extrapolated the NHANES sample data to the U.S. population. They found that the proportion of adults with steatosis (CAP score > 274 dB/m) and significant fibrosis (LSM > 8.0 kPa) was 42.7% (95% confidence interval, 41.0%- 44.3%) and 8.9% (7.6%-10.2%), respectively. In addition, 11.3% (10.2%-12.5%) of the adult U.S. population demonstrated a significant amount of alcohol use, 2.3% (1.4%-3.3%) showed evidence of hepatitis B or C, and 5.4% (4.6%-6.2%) had elevated serum ferritin levels.
The researchers then stratified the patients according to previously defined SAFE tiers of low, intermediate, and high risk and projected findings to the U.S. general population.
“When we applied our score to the general population, we found multiple abnormalities in the high-risk groups [SAFE >100] having Fibroscan data that are consistent with stage 2 or higher fibrosis regardless of etiology, “Dr. Kim pointed out.
Results also showed that very few patients with SAFE less than 0 had liver fibrosis (4% among those with liver stiffness measure [LSM] > 8kPa, and 0.8% with LSM > 12kPa). Among those with SAFE > 100, nearly a third (31.5%) had LSM of > 8kPa, and 16.5% had LSM > 12kPa.
In addition to fibrosis, liver abnormalities were common among patients with SAFE greater than 100, including steatosis (68.0%), viral hepatitis (7.0%), and abnormal ferritin levels (12.9%); 10.8% of these patients used alcohol.
“Right now, some patients are referred, but on examination and FibroScan, they might actually be okay, so it it’s a waste of time and money for everyone. We can preempt all of this by doing a blood test and focusing on those people who really need a scan,” said Dr. Kim.
The researcher is now working with primary care colleagues to help further develop and integrate SAFE into the primary care setting.
Fibrosis score in patients with metabolic dysfunction
Also presenting at the same session on population health was Willem Pieter Brouwer, MD, PhD, from Erasmus University Medical Center, Rotterdam, the Netherlands. He reported results of a validation study of a new risk score – the Metabolic Dysfunction-Associated Fibrosis–5 (MAF-5) score – for use for people with metabolic dysfunction who are recommended for screening for liver fibrosis.
“We believe the MAF-5 score may be a good alternative to the FIB-4 [a liver fibrosis biomarker] for use in the referral pathway for liver health evaluation,” remarked Dr. Brouwer. “The clinical practice guidelines recommend using FIB-4 scores, but these have a poor-moderate performance in the population setting.
“We developed and validated our score in a population of 5,500 from the NHANES 2017-2020 cycle and validated the score in populations from Rotterdam, which is a cohort of elderly participants, and in fibrosis among patients with biopsy-proven NAFLD from Colombia and Belgium,” he explained.
He also validated the score against different existing scoring systems and different methods of measuring liver stiffness and validated it for prognostic use to predict all-cause mortality in the NHANES III cohort.
Dr. Brouwer removed age as a factor of his new MAF-5 score; the score is thus stable for patients of all ages and is suitable for detecting liver disease in younger patients. “This is very important because these patients are currently underserved and have the most years of life to win.”
Referring to the SAFE score discussed by Dr. Kim, as well as other scores, he said, “The FIB-4, SAFE, and NFS [NAFLD fibrosis score] all include age in the scores, which causes problems and limitations in aging populations, as more and more patients will be referred due to an increasing score. Hence, the elderly are mostly all referred for liver checkups.”
Dr. Kim and Dr. Brouwer have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VIENNA – An algorithm, the Steatosis-Associated Fibrosis Estimator (SAFE), was developed to detect clinically significant fibrosis in patients with nonalcoholic fatty liver disease (NAFLD). It is effective at detecting chronic liver disease from all causes with or without NAFLD in the general population, according the results of a U.S. population-based study. The algorithm was designed for use in primary care to help slow the steep rise in liver disease burden.
On the basis of the SAFE score, 61.3% of participants were at low risk for clinically significant fibrosis; 11.2% were at high risk; and 27.5% were at intermediate risk. Upon validation, very few of the low-risk participants had liver fibrosis, while nearly a third of those with a high-risk score had clinically significant fibrosis. In addition, a high percentage of the patients with high-risk SAFE scores had viral hepatitis and elevations in ferritin level.
“This is the first time that there has been a test that provides a score to detect low-risk liver disease in primary care,” said Ray Kim, MD, from Stanford (Calif.) University, senior investigator, who was speaking to this news organization at the annual International Liver Congress sponsored by the European Association for the Study of the Liver
“Primary care doctors currently detect liver disease through a serendipitous abnormal finding on ultrasound or blood tests that detect elevated transaminases, and then the patient is referred to a hepatologist, who figures out what is really going on,” said Dr. Kim.
“This approach is limited, so we need to get SAFE into primary care so these doctors can automatically calculate their scores, and if the patient is over 100 [high risk of chronic liver disease], then they need help [referral to a hepatologist].”
Liver deaths sharply rising
Public health data show that more people are dying of liver disease today than previously. Deaths in the United States have doubled over the past 20 years, said Dr. Kim. “If our mission is to help these patients and prevent death, [things are] moving in the wrong direction.”
He stressed that in order to change the direction, “primary care doctors need to engage with the issue and have appropriate tools to identify people with liver disease.”
Most often, the reason for this rise in deaths is that cases are being diagnosed at advanced stages of disease in which reversibility is limited, he added. “We want to move upstream where people might have early-stage disease and where we can intervene and make a difference.”
In an effort to help earlier detection of liver disease, the SAFE score was developed and validated by Dr. Kim and his colleagues to detect clinically significant (greater than stage 2) fibrosis in patients with NAFLD in primary care. The score is based upon age, body mass index, diabetes, platelet level, aspartate and alanine aminotransferase levels, and globulin level. A score of less than zero signifies that a patient is at low risk for liver fibrosis, while a score greater than 100 signifies a high risk of fibrosis. A score between 0 and 99 denotes intermediate risk of fibrosis.
“Unlike other noninvasive tests that detect advanced fibrosis, this one detects early-stage fibrosis. We’ve shown that the SAFE estimator is better than all other blood-based markers,” explained Dr. Kim.
Applying SAFE to the general population
In the study presented here at EASL, Dr. Kim aimed to expand the horizon for SAFE testing to the U.S. general population and to assess whether SAFE was effective in screening for chronic liver disease regardless of steatosis of the liver.
Together with first author Nakia Chung, MD, also from Stanford University, Dr. Kim applied the SAFE score to data from 7,156 participants of the National Health and Nutrition Examination Survey (NHANES) for 2017-2020. NHANES is representative of the noninstitutionalized, civilian population of the United States. It includes broad demographic, clinical, and laboratory data, including transient elastography data. FibroScans were first used in 2017, so the investigators had 3 years of FibroScan data with which to validate the score.
The researchers extrapolated the NHANES sample data to the U.S. population. They found that the proportion of adults with steatosis (CAP score > 274 dB/m) and significant fibrosis (LSM > 8.0 kPa) was 42.7% (95% confidence interval, 41.0%- 44.3%) and 8.9% (7.6%-10.2%), respectively. In addition, 11.3% (10.2%-12.5%) of the adult U.S. population demonstrated a significant amount of alcohol use, 2.3% (1.4%-3.3%) showed evidence of hepatitis B or C, and 5.4% (4.6%-6.2%) had elevated serum ferritin levels.
The researchers then stratified the patients according to previously defined SAFE tiers of low, intermediate, and high risk and projected findings to the U.S. general population.
“When we applied our score to the general population, we found multiple abnormalities in the high-risk groups [SAFE >100] having Fibroscan data that are consistent with stage 2 or higher fibrosis regardless of etiology, “Dr. Kim pointed out.
Results also showed that very few patients with SAFE less than 0 had liver fibrosis (4% among those with liver stiffness measure [LSM] > 8kPa, and 0.8% with LSM > 12kPa). Among those with SAFE > 100, nearly a third (31.5%) had LSM of > 8kPa, and 16.5% had LSM > 12kPa.
In addition to fibrosis, liver abnormalities were common among patients with SAFE greater than 100, including steatosis (68.0%), viral hepatitis (7.0%), and abnormal ferritin levels (12.9%); 10.8% of these patients used alcohol.
“Right now, some patients are referred, but on examination and FibroScan, they might actually be okay, so it it’s a waste of time and money for everyone. We can preempt all of this by doing a blood test and focusing on those people who really need a scan,” said Dr. Kim.
The researcher is now working with primary care colleagues to help further develop and integrate SAFE into the primary care setting.
Fibrosis score in patients with metabolic dysfunction
Also presenting at the same session on population health was Willem Pieter Brouwer, MD, PhD, from Erasmus University Medical Center, Rotterdam, the Netherlands. He reported results of a validation study of a new risk score – the Metabolic Dysfunction-Associated Fibrosis–5 (MAF-5) score – for use for people with metabolic dysfunction who are recommended for screening for liver fibrosis.
“We believe the MAF-5 score may be a good alternative to the FIB-4 [a liver fibrosis biomarker] for use in the referral pathway for liver health evaluation,” remarked Dr. Brouwer. “The clinical practice guidelines recommend using FIB-4 scores, but these have a poor-moderate performance in the population setting.
“We developed and validated our score in a population of 5,500 from the NHANES 2017-2020 cycle and validated the score in populations from Rotterdam, which is a cohort of elderly participants, and in fibrosis among patients with biopsy-proven NAFLD from Colombia and Belgium,” he explained.
He also validated the score against different existing scoring systems and different methods of measuring liver stiffness and validated it for prognostic use to predict all-cause mortality in the NHANES III cohort.
Dr. Brouwer removed age as a factor of his new MAF-5 score; the score is thus stable for patients of all ages and is suitable for detecting liver disease in younger patients. “This is very important because these patients are currently underserved and have the most years of life to win.”
Referring to the SAFE score discussed by Dr. Kim, as well as other scores, he said, “The FIB-4, SAFE, and NFS [NAFLD fibrosis score] all include age in the scores, which causes problems and limitations in aging populations, as more and more patients will be referred due to an increasing score. Hence, the elderly are mostly all referred for liver checkups.”
Dr. Kim and Dr. Brouwer have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VIENNA – An algorithm, the Steatosis-Associated Fibrosis Estimator (SAFE), was developed to detect clinically significant fibrosis in patients with nonalcoholic fatty liver disease (NAFLD). It is effective at detecting chronic liver disease from all causes with or without NAFLD in the general population, according the results of a U.S. population-based study. The algorithm was designed for use in primary care to help slow the steep rise in liver disease burden.
On the basis of the SAFE score, 61.3% of participants were at low risk for clinically significant fibrosis; 11.2% were at high risk; and 27.5% were at intermediate risk. Upon validation, very few of the low-risk participants had liver fibrosis, while nearly a third of those with a high-risk score had clinically significant fibrosis. In addition, a high percentage of the patients with high-risk SAFE scores had viral hepatitis and elevations in ferritin level.
“This is the first time that there has been a test that provides a score to detect low-risk liver disease in primary care,” said Ray Kim, MD, from Stanford (Calif.) University, senior investigator, who was speaking to this news organization at the annual International Liver Congress sponsored by the European Association for the Study of the Liver
“Primary care doctors currently detect liver disease through a serendipitous abnormal finding on ultrasound or blood tests that detect elevated transaminases, and then the patient is referred to a hepatologist, who figures out what is really going on,” said Dr. Kim.
“This approach is limited, so we need to get SAFE into primary care so these doctors can automatically calculate their scores, and if the patient is over 100 [high risk of chronic liver disease], then they need help [referral to a hepatologist].”
Liver deaths sharply rising
Public health data show that more people are dying of liver disease today than previously. Deaths in the United States have doubled over the past 20 years, said Dr. Kim. “If our mission is to help these patients and prevent death, [things are] moving in the wrong direction.”
He stressed that in order to change the direction, “primary care doctors need to engage with the issue and have appropriate tools to identify people with liver disease.”
Most often, the reason for this rise in deaths is that cases are being diagnosed at advanced stages of disease in which reversibility is limited, he added. “We want to move upstream where people might have early-stage disease and where we can intervene and make a difference.”
In an effort to help earlier detection of liver disease, the SAFE score was developed and validated by Dr. Kim and his colleagues to detect clinically significant (greater than stage 2) fibrosis in patients with NAFLD in primary care. The score is based upon age, body mass index, diabetes, platelet level, aspartate and alanine aminotransferase levels, and globulin level. A score of less than zero signifies that a patient is at low risk for liver fibrosis, while a score greater than 100 signifies a high risk of fibrosis. A score between 0 and 99 denotes intermediate risk of fibrosis.
“Unlike other noninvasive tests that detect advanced fibrosis, this one detects early-stage fibrosis. We’ve shown that the SAFE estimator is better than all other blood-based markers,” explained Dr. Kim.
Applying SAFE to the general population
In the study presented here at EASL, Dr. Kim aimed to expand the horizon for SAFE testing to the U.S. general population and to assess whether SAFE was effective in screening for chronic liver disease regardless of steatosis of the liver.
Together with first author Nakia Chung, MD, also from Stanford University, Dr. Kim applied the SAFE score to data from 7,156 participants of the National Health and Nutrition Examination Survey (NHANES) for 2017-2020. NHANES is representative of the noninstitutionalized, civilian population of the United States. It includes broad demographic, clinical, and laboratory data, including transient elastography data. FibroScans were first used in 2017, so the investigators had 3 years of FibroScan data with which to validate the score.
The researchers extrapolated the NHANES sample data to the U.S. population. They found that the proportion of adults with steatosis (CAP score > 274 dB/m) and significant fibrosis (LSM > 8.0 kPa) was 42.7% (95% confidence interval, 41.0%- 44.3%) and 8.9% (7.6%-10.2%), respectively. In addition, 11.3% (10.2%-12.5%) of the adult U.S. population demonstrated a significant amount of alcohol use, 2.3% (1.4%-3.3%) showed evidence of hepatitis B or C, and 5.4% (4.6%-6.2%) had elevated serum ferritin levels.
The researchers then stratified the patients according to previously defined SAFE tiers of low, intermediate, and high risk and projected findings to the U.S. general population.
“When we applied our score to the general population, we found multiple abnormalities in the high-risk groups [SAFE >100] having Fibroscan data that are consistent with stage 2 or higher fibrosis regardless of etiology, “Dr. Kim pointed out.
Results also showed that very few patients with SAFE less than 0 had liver fibrosis (4% among those with liver stiffness measure [LSM] > 8kPa, and 0.8% with LSM > 12kPa). Among those with SAFE > 100, nearly a third (31.5%) had LSM of > 8kPa, and 16.5% had LSM > 12kPa.
In addition to fibrosis, liver abnormalities were common among patients with SAFE greater than 100, including steatosis (68.0%), viral hepatitis (7.0%), and abnormal ferritin levels (12.9%); 10.8% of these patients used alcohol.
“Right now, some patients are referred, but on examination and FibroScan, they might actually be okay, so it it’s a waste of time and money for everyone. We can preempt all of this by doing a blood test and focusing on those people who really need a scan,” said Dr. Kim.
The researcher is now working with primary care colleagues to help further develop and integrate SAFE into the primary care setting.
Fibrosis score in patients with metabolic dysfunction
Also presenting at the same session on population health was Willem Pieter Brouwer, MD, PhD, from Erasmus University Medical Center, Rotterdam, the Netherlands. He reported results of a validation study of a new risk score – the Metabolic Dysfunction-Associated Fibrosis–5 (MAF-5) score – for use for people with metabolic dysfunction who are recommended for screening for liver fibrosis.
“We believe the MAF-5 score may be a good alternative to the FIB-4 [a liver fibrosis biomarker] for use in the referral pathway for liver health evaluation,” remarked Dr. Brouwer. “The clinical practice guidelines recommend using FIB-4 scores, but these have a poor-moderate performance in the population setting.
“We developed and validated our score in a population of 5,500 from the NHANES 2017-2020 cycle and validated the score in populations from Rotterdam, which is a cohort of elderly participants, and in fibrosis among patients with biopsy-proven NAFLD from Colombia and Belgium,” he explained.
He also validated the score against different existing scoring systems and different methods of measuring liver stiffness and validated it for prognostic use to predict all-cause mortality in the NHANES III cohort.
Dr. Brouwer removed age as a factor of his new MAF-5 score; the score is thus stable for patients of all ages and is suitable for detecting liver disease in younger patients. “This is very important because these patients are currently underserved and have the most years of life to win.”
Referring to the SAFE score discussed by Dr. Kim, as well as other scores, he said, “The FIB-4, SAFE, and NFS [NAFLD fibrosis score] all include age in the scores, which causes problems and limitations in aging populations, as more and more patients will be referred due to an increasing score. Hence, the elderly are mostly all referred for liver checkups.”
Dr. Kim and Dr. Brouwer have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ILC 2023