User login
What are the signs of post–acute infection syndromes?
The long-term health consequences of COVID-19 have refocused our attention on post–acute infection syndromes (PAIS), starting a discussion on the need for a complete understanding of multisystemic pathophysiology, clinical indicators, and the epidemiology of these syndromes, representing a significant blind spot in the field of medicine. A better understanding of these persistent symptom profiles, not only for post-acute sequelae of SARS-CoV-2 infection (PASC), better known as long COVID, but also for other diseases with unexplainable post-acute sequelae, would allow doctors to fine tune the diagnostic criteria. Having a clear definition and better understanding of post–acute infection symptoms is a necessary step toward developing an evidence-based, multidisciplinary management approach.
PAIS, PASC, or long COVID
The observation of unexplained chronic sequelae after SARS-CoV-2 is known as PASC or long COVID.
Long COVID has been reported as a syndrome in survivors of serious and critical disease, but the effects also persist over time for subjects who experienced a mild infection that did not require admission to hospital. This means that PASC, especially when occurring after a mild or moderate COVID-19 infection, shares many of the same characteristics as chronic diseases triggered by other pathogenic organisms, many of which have not been sufficiently clarified.
PAIS are characterized by a set of core symptoms centering on the following:
- Exertion intolerance
- Disproportionate levels of fatigue
- Neurocognitive and sensory impairment
- Flu-like symptoms
- Unrefreshing sleep
- Myalgia/arthralgia
A plethora of nonspecific symptoms are often present to various degrees.
These similarities suggest a unifying pathophysiology that needs to be elucidated to properly understand and manage postinfectious chronic disability.
Overview of PAIS
A detailed revision on what is currently known about PAIS was published in Nature Medicine. It provided various useful pieces of information to assist with the poor recognition of these conditions in clinical practice, a result of which is that patients might experience delayed or a complete lack of clinical care.
The following consolidated postinfection sequelae are mentioned:
- Q fever fatigue syndrome, which follows infection by the intracellular bacterium Coxiella burnetii
- Post-dengue fatigue syndrome, which can follow infection by the mosquito-borne dengue virus
- Fatiguing and rheumatic symptoms in a subset of individuals infected with chikungunya virus, a mosquito-borne virus that causes fever and joint pain in the acute phase
- Post-polio syndrome, which can emerge as many as 15-40 years after an initial poliomyelitis attack (similarly, some other neurotropic microbes, such as West Nile virus, might lead to persistent effects)
- Prolonged, debilitating, chronic symptoms have long been reported in a subset of patients after common and typically nonserious infections. For example, after mononucleosis, a condition generally caused by Epstein-Barr virus (EBV), and after an outbreak of Giardia lamblia, an intestinal parasite that usually causes acute intestinal illness. In fact, several studies identified the association of this outbreak of giardiasis with chronic fatigue, irritable bowel syndrome (IBS), and fibromyalgia persisting for many years.
- Views expressed in the literature regarding the frequency and the validity of posttreatment Lyme disease syndrome are divided. Although substantial evidence points to persistence of arthralgia, fatigue, and subjective neurocognitive impairments in a minority of patients with Lyme disease after the recommended antibiotic treatment, some of the early studies have failed to characterize the initial Lyme disease episode with sufficient rigor.
Symptoms and signs
The symptoms and signs which, based on the evidence available, are seen more frequently in health care checks may be characterized as the following:
- Exertion intolerance, fatigue
- Flu-like and ‘sickness behavior’ symptoms: fever, feverishness, muscle pain, feeling sick, malaise, sweating, irritability
- Neurological/neurocognitive symptoms: brain fog, impaired concentration or memory, trouble finding words
- Rheumatologic symptoms: chronic or recurrent joint pain
- Trigger-specific symptoms: for example, eye problems post Ebola, IBS post Giardia, anosmia and ageusia post COVID-19, motor disturbances post polio and post West Nile virus
Myalgic encephalomyelitis/chronic fatigue syndrome
Patients with this disorder experience worsening of symptoms following physical, cognitive, or emotional exertion above their (very low) tolerated limit. Other prominent features frequently observed in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) are neurocognitive impairments (colloquially referred to as brain fog), unrefreshing sleep, pain, sensory disturbances, gastrointestinal issues, and various forms of dysautonomia. Up to 75% of ME/CFS cases report an infection-like episode preceding the onset of their illness. Postinfectious and postviral fatigue syndromes were originally postulated as subsets of chronic fatigue syndrome. However, there appears to be no clear consensus at present about whether these terms should be considered synonymous to the ME/CFS label or any of its subsets, or include a wider range of postinfectious fatigue conditions.
Practical diagnostic criteria
From a revision of the available criteria, it emerges that the diagnostic criteria for a PAIS should include not only the presence of symptoms, but ideally also the intensity, course, and constellation of symptoms within an individual, as the individual symptoms and symptom trajectories of PAIS vary over time, rendering a mere comparison of symptom presence at a single time point misleading. Furthermore, when a diagnosis of ME/CFS is made, attention should be given to the choice of diagnostic criteria, with preference given to the more conservative criteria, so as not to run the risk of overestimating the syndrome.
Asthenia is the cornerstone symptom for most epidemiological studies on PAIS, but it would be reductive to concentrate only on this rather than the other characteristics, such as the exacerbation of symptoms following exertion, together with other characteristic symptoms and signs that may allow for better identification of the overall, observable clinical picture in these postinfection syndromes, which have significant impacts on a patient’s quality of life.
This article was translated from Univadis Italy. A version of this article appeared on Medscape.com.
The long-term health consequences of COVID-19 have refocused our attention on post–acute infection syndromes (PAIS), starting a discussion on the need for a complete understanding of multisystemic pathophysiology, clinical indicators, and the epidemiology of these syndromes, representing a significant blind spot in the field of medicine. A better understanding of these persistent symptom profiles, not only for post-acute sequelae of SARS-CoV-2 infection (PASC), better known as long COVID, but also for other diseases with unexplainable post-acute sequelae, would allow doctors to fine tune the diagnostic criteria. Having a clear definition and better understanding of post–acute infection symptoms is a necessary step toward developing an evidence-based, multidisciplinary management approach.
PAIS, PASC, or long COVID
The observation of unexplained chronic sequelae after SARS-CoV-2 is known as PASC or long COVID.
Long COVID has been reported as a syndrome in survivors of serious and critical disease, but the effects also persist over time for subjects who experienced a mild infection that did not require admission to hospital. This means that PASC, especially when occurring after a mild or moderate COVID-19 infection, shares many of the same characteristics as chronic diseases triggered by other pathogenic organisms, many of which have not been sufficiently clarified.
PAIS are characterized by a set of core symptoms centering on the following:
- Exertion intolerance
- Disproportionate levels of fatigue
- Neurocognitive and sensory impairment
- Flu-like symptoms
- Unrefreshing sleep
- Myalgia/arthralgia
A plethora of nonspecific symptoms are often present to various degrees.
These similarities suggest a unifying pathophysiology that needs to be elucidated to properly understand and manage postinfectious chronic disability.
Overview of PAIS
A detailed revision on what is currently known about PAIS was published in Nature Medicine. It provided various useful pieces of information to assist with the poor recognition of these conditions in clinical practice, a result of which is that patients might experience delayed or a complete lack of clinical care.
The following consolidated postinfection sequelae are mentioned:
- Q fever fatigue syndrome, which follows infection by the intracellular bacterium Coxiella burnetii
- Post-dengue fatigue syndrome, which can follow infection by the mosquito-borne dengue virus
- Fatiguing and rheumatic symptoms in a subset of individuals infected with chikungunya virus, a mosquito-borne virus that causes fever and joint pain in the acute phase
- Post-polio syndrome, which can emerge as many as 15-40 years after an initial poliomyelitis attack (similarly, some other neurotropic microbes, such as West Nile virus, might lead to persistent effects)
- Prolonged, debilitating, chronic symptoms have long been reported in a subset of patients after common and typically nonserious infections. For example, after mononucleosis, a condition generally caused by Epstein-Barr virus (EBV), and after an outbreak of Giardia lamblia, an intestinal parasite that usually causes acute intestinal illness. In fact, several studies identified the association of this outbreak of giardiasis with chronic fatigue, irritable bowel syndrome (IBS), and fibromyalgia persisting for many years.
- Views expressed in the literature regarding the frequency and the validity of posttreatment Lyme disease syndrome are divided. Although substantial evidence points to persistence of arthralgia, fatigue, and subjective neurocognitive impairments in a minority of patients with Lyme disease after the recommended antibiotic treatment, some of the early studies have failed to characterize the initial Lyme disease episode with sufficient rigor.
Symptoms and signs
The symptoms and signs which, based on the evidence available, are seen more frequently in health care checks may be characterized as the following:
- Exertion intolerance, fatigue
- Flu-like and ‘sickness behavior’ symptoms: fever, feverishness, muscle pain, feeling sick, malaise, sweating, irritability
- Neurological/neurocognitive symptoms: brain fog, impaired concentration or memory, trouble finding words
- Rheumatologic symptoms: chronic or recurrent joint pain
- Trigger-specific symptoms: for example, eye problems post Ebola, IBS post Giardia, anosmia and ageusia post COVID-19, motor disturbances post polio and post West Nile virus
Myalgic encephalomyelitis/chronic fatigue syndrome
Patients with this disorder experience worsening of symptoms following physical, cognitive, or emotional exertion above their (very low) tolerated limit. Other prominent features frequently observed in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) are neurocognitive impairments (colloquially referred to as brain fog), unrefreshing sleep, pain, sensory disturbances, gastrointestinal issues, and various forms of dysautonomia. Up to 75% of ME/CFS cases report an infection-like episode preceding the onset of their illness. Postinfectious and postviral fatigue syndromes were originally postulated as subsets of chronic fatigue syndrome. However, there appears to be no clear consensus at present about whether these terms should be considered synonymous to the ME/CFS label or any of its subsets, or include a wider range of postinfectious fatigue conditions.
Practical diagnostic criteria
From a revision of the available criteria, it emerges that the diagnostic criteria for a PAIS should include not only the presence of symptoms, but ideally also the intensity, course, and constellation of symptoms within an individual, as the individual symptoms and symptom trajectories of PAIS vary over time, rendering a mere comparison of symptom presence at a single time point misleading. Furthermore, when a diagnosis of ME/CFS is made, attention should be given to the choice of diagnostic criteria, with preference given to the more conservative criteria, so as not to run the risk of overestimating the syndrome.
Asthenia is the cornerstone symptom for most epidemiological studies on PAIS, but it would be reductive to concentrate only on this rather than the other characteristics, such as the exacerbation of symptoms following exertion, together with other characteristic symptoms and signs that may allow for better identification of the overall, observable clinical picture in these postinfection syndromes, which have significant impacts on a patient’s quality of life.
This article was translated from Univadis Italy. A version of this article appeared on Medscape.com.
The long-term health consequences of COVID-19 have refocused our attention on post–acute infection syndromes (PAIS), starting a discussion on the need for a complete understanding of multisystemic pathophysiology, clinical indicators, and the epidemiology of these syndromes, representing a significant blind spot in the field of medicine. A better understanding of these persistent symptom profiles, not only for post-acute sequelae of SARS-CoV-2 infection (PASC), better known as long COVID, but also for other diseases with unexplainable post-acute sequelae, would allow doctors to fine tune the diagnostic criteria. Having a clear definition and better understanding of post–acute infection symptoms is a necessary step toward developing an evidence-based, multidisciplinary management approach.
PAIS, PASC, or long COVID
The observation of unexplained chronic sequelae after SARS-CoV-2 is known as PASC or long COVID.
Long COVID has been reported as a syndrome in survivors of serious and critical disease, but the effects also persist over time for subjects who experienced a mild infection that did not require admission to hospital. This means that PASC, especially when occurring after a mild or moderate COVID-19 infection, shares many of the same characteristics as chronic diseases triggered by other pathogenic organisms, many of which have not been sufficiently clarified.
PAIS are characterized by a set of core symptoms centering on the following:
- Exertion intolerance
- Disproportionate levels of fatigue
- Neurocognitive and sensory impairment
- Flu-like symptoms
- Unrefreshing sleep
- Myalgia/arthralgia
A plethora of nonspecific symptoms are often present to various degrees.
These similarities suggest a unifying pathophysiology that needs to be elucidated to properly understand and manage postinfectious chronic disability.
Overview of PAIS
A detailed revision on what is currently known about PAIS was published in Nature Medicine. It provided various useful pieces of information to assist with the poor recognition of these conditions in clinical practice, a result of which is that patients might experience delayed or a complete lack of clinical care.
The following consolidated postinfection sequelae are mentioned:
- Q fever fatigue syndrome, which follows infection by the intracellular bacterium Coxiella burnetii
- Post-dengue fatigue syndrome, which can follow infection by the mosquito-borne dengue virus
- Fatiguing and rheumatic symptoms in a subset of individuals infected with chikungunya virus, a mosquito-borne virus that causes fever and joint pain in the acute phase
- Post-polio syndrome, which can emerge as many as 15-40 years after an initial poliomyelitis attack (similarly, some other neurotropic microbes, such as West Nile virus, might lead to persistent effects)
- Prolonged, debilitating, chronic symptoms have long been reported in a subset of patients after common and typically nonserious infections. For example, after mononucleosis, a condition generally caused by Epstein-Barr virus (EBV), and after an outbreak of Giardia lamblia, an intestinal parasite that usually causes acute intestinal illness. In fact, several studies identified the association of this outbreak of giardiasis with chronic fatigue, irritable bowel syndrome (IBS), and fibromyalgia persisting for many years.
- Views expressed in the literature regarding the frequency and the validity of posttreatment Lyme disease syndrome are divided. Although substantial evidence points to persistence of arthralgia, fatigue, and subjective neurocognitive impairments in a minority of patients with Lyme disease after the recommended antibiotic treatment, some of the early studies have failed to characterize the initial Lyme disease episode with sufficient rigor.
Symptoms and signs
The symptoms and signs which, based on the evidence available, are seen more frequently in health care checks may be characterized as the following:
- Exertion intolerance, fatigue
- Flu-like and ‘sickness behavior’ symptoms: fever, feverishness, muscle pain, feeling sick, malaise, sweating, irritability
- Neurological/neurocognitive symptoms: brain fog, impaired concentration or memory, trouble finding words
- Rheumatologic symptoms: chronic or recurrent joint pain
- Trigger-specific symptoms: for example, eye problems post Ebola, IBS post Giardia, anosmia and ageusia post COVID-19, motor disturbances post polio and post West Nile virus
Myalgic encephalomyelitis/chronic fatigue syndrome
Patients with this disorder experience worsening of symptoms following physical, cognitive, or emotional exertion above their (very low) tolerated limit. Other prominent features frequently observed in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) are neurocognitive impairments (colloquially referred to as brain fog), unrefreshing sleep, pain, sensory disturbances, gastrointestinal issues, and various forms of dysautonomia. Up to 75% of ME/CFS cases report an infection-like episode preceding the onset of their illness. Postinfectious and postviral fatigue syndromes were originally postulated as subsets of chronic fatigue syndrome. However, there appears to be no clear consensus at present about whether these terms should be considered synonymous to the ME/CFS label or any of its subsets, or include a wider range of postinfectious fatigue conditions.
Practical diagnostic criteria
From a revision of the available criteria, it emerges that the diagnostic criteria for a PAIS should include not only the presence of symptoms, but ideally also the intensity, course, and constellation of symptoms within an individual, as the individual symptoms and symptom trajectories of PAIS vary over time, rendering a mere comparison of symptom presence at a single time point misleading. Furthermore, when a diagnosis of ME/CFS is made, attention should be given to the choice of diagnostic criteria, with preference given to the more conservative criteria, so as not to run the risk of overestimating the syndrome.
Asthenia is the cornerstone symptom for most epidemiological studies on PAIS, but it would be reductive to concentrate only on this rather than the other characteristics, such as the exacerbation of symptoms following exertion, together with other characteristic symptoms and signs that may allow for better identification of the overall, observable clinical picture in these postinfection syndromes, which have significant impacts on a patient’s quality of life.
This article was translated from Univadis Italy. A version of this article appeared on Medscape.com.
Diabetes tied to risk of long COVID, too
Individuals with diabetes who experience COVID-19 are at increased risk for long COVID compared to individuals without diabetes, according to data from a literature review of seven studies.
Diabetes remains a risk factor for severe COVID-19, but whether it is a risk factor for postacute sequelae of COVID-19 (PASC), also known as long COVID, remains unclear, Jessica L. Harding, PhD, of Emory University, said in a late-breaking poster session at the annual scientific sessions of the American Diabetes Association.
Long COVID is generally defined as “sequelae that extend beyond the 4 weeks after initial infection” and may include a range of symptoms that affect multiple organs, Dr. Harding said. A study conducted in January of 2022 suggested that type 2 diabetes was one of several strong risk factors for long COVID, she noted.
Dr. Harding and colleagues reviewed data from seven studies published from Jan. 1, 2020, to Jan. 27, 2022, on the risk of PASC in people with and without diabetes. The studies included patients with a minimum of 4 weeks’ follow-up after COVID-19 diagnosis. All seven studies had a longitudinal cohort design, and included adults from high-income countries, with study populations ranging from 104 to 4,182.
Across the studies, long COVID definitions varied, but included ongoing symptoms of fatigue, cough, and dyspnea, with follow-up periods of 4 weeks to 7 months.
Overall, three of the seven studies indicated that diabetes was a risk factor for long COVID (odds ratio [OR] greater than 4 for all) and four studies indicated that diabetes was not a risk factor for long COVID (OR, 0.5-2.2).
One of the three studies showing increased risk included 2,334 individuals hospitalized with COVID-19; of these about 5% had diabetes. The odds ratio for PASC for individuals with diabetes was 4.18. In another study of 209 persons with COVID-19, of whom 22% had diabetes, diabetes was significantly correlated with respiratory viral disease (meaning at least two respiratory symptoms). The third study showing an increased risk of long COVID in diabetes patients included 104 kidney transplant patients, of whom 20% had diabetes; the odds ratio for PASC was 4.42.
The findings were limited by several factors, including the relatively small number of studies and the heterogeneity of studies regarding definitions of long COVID, specific populations at risk, follow-up times, and risk adjustment, Dr. Harding noted.
More high-quality studies across multiple populations and settings are needed to determine if diabetes is indeed a risk factor for long COVID, she said.
In the meantime, “careful monitoring of people with diabetes for development of PASC may be advised,” Dr. Harding concluded.
Findings support need for screening
“Given the devastating impact of COVID on people with diabetes, it’s important to know what data has been accumulated on long COVID for future research and discoveries in this area,” Robert A. Gabbay, MD, chief science and medical officer for the American Diabetes Association, said in an interview. “The more information we have, the better we can understand the implications.”
Dr. Gabbay said he was surprised by the current study findings. “We know very little on this subject, so yes, I am surprised to see just how significant the risk of long COVID for people with diabetes seems to be, but clearly, more research needs to be done to understand long COVID,” he emphasized.
The take-home message for clinicians is the importance of screening patients for PASC; also “ask your patients if they had COVID, to better understand any symptoms they might have that could be related to PACS,” he noted.
“It is crucial that we confirm these results and then look at risk factors in people with diabetes that might explain who is at highest risk and ultimately understand the causes and potential cure,” Dr. Gabbay added.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Harding and Dr. Gabbay had no financial conflicts to disclose.
Individuals with diabetes who experience COVID-19 are at increased risk for long COVID compared to individuals without diabetes, according to data from a literature review of seven studies.
Diabetes remains a risk factor for severe COVID-19, but whether it is a risk factor for postacute sequelae of COVID-19 (PASC), also known as long COVID, remains unclear, Jessica L. Harding, PhD, of Emory University, said in a late-breaking poster session at the annual scientific sessions of the American Diabetes Association.
Long COVID is generally defined as “sequelae that extend beyond the 4 weeks after initial infection” and may include a range of symptoms that affect multiple organs, Dr. Harding said. A study conducted in January of 2022 suggested that type 2 diabetes was one of several strong risk factors for long COVID, she noted.
Dr. Harding and colleagues reviewed data from seven studies published from Jan. 1, 2020, to Jan. 27, 2022, on the risk of PASC in people with and without diabetes. The studies included patients with a minimum of 4 weeks’ follow-up after COVID-19 diagnosis. All seven studies had a longitudinal cohort design, and included adults from high-income countries, with study populations ranging from 104 to 4,182.
Across the studies, long COVID definitions varied, but included ongoing symptoms of fatigue, cough, and dyspnea, with follow-up periods of 4 weeks to 7 months.
Overall, three of the seven studies indicated that diabetes was a risk factor for long COVID (odds ratio [OR] greater than 4 for all) and four studies indicated that diabetes was not a risk factor for long COVID (OR, 0.5-2.2).
One of the three studies showing increased risk included 2,334 individuals hospitalized with COVID-19; of these about 5% had diabetes. The odds ratio for PASC for individuals with diabetes was 4.18. In another study of 209 persons with COVID-19, of whom 22% had diabetes, diabetes was significantly correlated with respiratory viral disease (meaning at least two respiratory symptoms). The third study showing an increased risk of long COVID in diabetes patients included 104 kidney transplant patients, of whom 20% had diabetes; the odds ratio for PASC was 4.42.
The findings were limited by several factors, including the relatively small number of studies and the heterogeneity of studies regarding definitions of long COVID, specific populations at risk, follow-up times, and risk adjustment, Dr. Harding noted.
More high-quality studies across multiple populations and settings are needed to determine if diabetes is indeed a risk factor for long COVID, she said.
In the meantime, “careful monitoring of people with diabetes for development of PASC may be advised,” Dr. Harding concluded.
Findings support need for screening
“Given the devastating impact of COVID on people with diabetes, it’s important to know what data has been accumulated on long COVID for future research and discoveries in this area,” Robert A. Gabbay, MD, chief science and medical officer for the American Diabetes Association, said in an interview. “The more information we have, the better we can understand the implications.”
Dr. Gabbay said he was surprised by the current study findings. “We know very little on this subject, so yes, I am surprised to see just how significant the risk of long COVID for people with diabetes seems to be, but clearly, more research needs to be done to understand long COVID,” he emphasized.
The take-home message for clinicians is the importance of screening patients for PASC; also “ask your patients if they had COVID, to better understand any symptoms they might have that could be related to PACS,” he noted.
“It is crucial that we confirm these results and then look at risk factors in people with diabetes that might explain who is at highest risk and ultimately understand the causes and potential cure,” Dr. Gabbay added.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Harding and Dr. Gabbay had no financial conflicts to disclose.
Individuals with diabetes who experience COVID-19 are at increased risk for long COVID compared to individuals without diabetes, according to data from a literature review of seven studies.
Diabetes remains a risk factor for severe COVID-19, but whether it is a risk factor for postacute sequelae of COVID-19 (PASC), also known as long COVID, remains unclear, Jessica L. Harding, PhD, of Emory University, said in a late-breaking poster session at the annual scientific sessions of the American Diabetes Association.
Long COVID is generally defined as “sequelae that extend beyond the 4 weeks after initial infection” and may include a range of symptoms that affect multiple organs, Dr. Harding said. A study conducted in January of 2022 suggested that type 2 diabetes was one of several strong risk factors for long COVID, she noted.
Dr. Harding and colleagues reviewed data from seven studies published from Jan. 1, 2020, to Jan. 27, 2022, on the risk of PASC in people with and without diabetes. The studies included patients with a minimum of 4 weeks’ follow-up after COVID-19 diagnosis. All seven studies had a longitudinal cohort design, and included adults from high-income countries, with study populations ranging from 104 to 4,182.
Across the studies, long COVID definitions varied, but included ongoing symptoms of fatigue, cough, and dyspnea, with follow-up periods of 4 weeks to 7 months.
Overall, three of the seven studies indicated that diabetes was a risk factor for long COVID (odds ratio [OR] greater than 4 for all) and four studies indicated that diabetes was not a risk factor for long COVID (OR, 0.5-2.2).
One of the three studies showing increased risk included 2,334 individuals hospitalized with COVID-19; of these about 5% had diabetes. The odds ratio for PASC for individuals with diabetes was 4.18. In another study of 209 persons with COVID-19, of whom 22% had diabetes, diabetes was significantly correlated with respiratory viral disease (meaning at least two respiratory symptoms). The third study showing an increased risk of long COVID in diabetes patients included 104 kidney transplant patients, of whom 20% had diabetes; the odds ratio for PASC was 4.42.
The findings were limited by several factors, including the relatively small number of studies and the heterogeneity of studies regarding definitions of long COVID, specific populations at risk, follow-up times, and risk adjustment, Dr. Harding noted.
More high-quality studies across multiple populations and settings are needed to determine if diabetes is indeed a risk factor for long COVID, she said.
In the meantime, “careful monitoring of people with diabetes for development of PASC may be advised,” Dr. Harding concluded.
Findings support need for screening
“Given the devastating impact of COVID on people with diabetes, it’s important to know what data has been accumulated on long COVID for future research and discoveries in this area,” Robert A. Gabbay, MD, chief science and medical officer for the American Diabetes Association, said in an interview. “The more information we have, the better we can understand the implications.”
Dr. Gabbay said he was surprised by the current study findings. “We know very little on this subject, so yes, I am surprised to see just how significant the risk of long COVID for people with diabetes seems to be, but clearly, more research needs to be done to understand long COVID,” he emphasized.
The take-home message for clinicians is the importance of screening patients for PASC; also “ask your patients if they had COVID, to better understand any symptoms they might have that could be related to PACS,” he noted.
“It is crucial that we confirm these results and then look at risk factors in people with diabetes that might explain who is at highest risk and ultimately understand the causes and potential cure,” Dr. Gabbay added.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Harding and Dr. Gabbay had no financial conflicts to disclose.
FROM ADA 2022
‘Alarming’ new data on disordered sleep after COVID-19
Such disturbances are especially common among Black people, new research shows.
The “high” prevalence of moderate to severe sleep disturbances is “alarming,” study investigator Cinthya Pena Orbea, MD, sleep specialist at the Cleveland Clinic, said in an interview.
The findings were presented at the annual meeting of the Associated Professional Sleep Societies.
Dr. Pena and colleagues analyzed data on 962 patients with PASC seen at the Cleveland Clinic ReCOVer Clinic between February 2021 and April 2022.
More than two-thirds of patients (67.2%) reported at least moderate fatigue, while 21.8% reported severe fatigue, Dr. Pena reported.
In addition, 41.3% reported at least moderate sleep disturbances, while 8% of patients reported severe sleep disturbances, including insomnia, “which may impair quality of life,” Dr. Pena said.
Obesity, mood disorders, and Black race emerged as contributors to problems with sleep and fatigue after COVID.
Notably, after adjusting for demographics, Black race conferred threefold higher odds of moderate to severe sleep disturbances.
“We don’t know why this is, and one of our next steps is to better understand race-specific determinants of sleep disturbances after COVID and create targeted interventions,” Dr. Pena said.
How long after COVID the fatigue and sleep problems last “remains uncertain,” Dr. Pena acknowledged. However, in her clinical experience with therapy, patients’ sleep and fatigue may improve after 6 or 8 months.
Ruth Benca, MD, PhD, cochair of the Alliance for Sleep, is not surprised by the Cleveland Clinic findings.
“Sleep disturbances and fatigue are part of the sequelae of COVID,” Dr. Benca, who was not involved in the study, said in an interview.
“We know that people who have had COVID have more trouble sleeping afterwards. There is the COVID insomnia created in all of us just out of our worries, fears, isolation, and stress. And then there’s an actual impact of having the infection itself on worsening sleep,” said Dr. Benca, with Wake Forest University and Atrium Health Wake Forest Baptist, both in Winston-Salem, N.C.
The study had no specific funding. The authors have disclosed no relevant financial relationships. Dr. Benca is a consultant for Idorsia Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Such disturbances are especially common among Black people, new research shows.
The “high” prevalence of moderate to severe sleep disturbances is “alarming,” study investigator Cinthya Pena Orbea, MD, sleep specialist at the Cleveland Clinic, said in an interview.
The findings were presented at the annual meeting of the Associated Professional Sleep Societies.
Dr. Pena and colleagues analyzed data on 962 patients with PASC seen at the Cleveland Clinic ReCOVer Clinic between February 2021 and April 2022.
More than two-thirds of patients (67.2%) reported at least moderate fatigue, while 21.8% reported severe fatigue, Dr. Pena reported.
In addition, 41.3% reported at least moderate sleep disturbances, while 8% of patients reported severe sleep disturbances, including insomnia, “which may impair quality of life,” Dr. Pena said.
Obesity, mood disorders, and Black race emerged as contributors to problems with sleep and fatigue after COVID.
Notably, after adjusting for demographics, Black race conferred threefold higher odds of moderate to severe sleep disturbances.
“We don’t know why this is, and one of our next steps is to better understand race-specific determinants of sleep disturbances after COVID and create targeted interventions,” Dr. Pena said.
How long after COVID the fatigue and sleep problems last “remains uncertain,” Dr. Pena acknowledged. However, in her clinical experience with therapy, patients’ sleep and fatigue may improve after 6 or 8 months.
Ruth Benca, MD, PhD, cochair of the Alliance for Sleep, is not surprised by the Cleveland Clinic findings.
“Sleep disturbances and fatigue are part of the sequelae of COVID,” Dr. Benca, who was not involved in the study, said in an interview.
“We know that people who have had COVID have more trouble sleeping afterwards. There is the COVID insomnia created in all of us just out of our worries, fears, isolation, and stress. And then there’s an actual impact of having the infection itself on worsening sleep,” said Dr. Benca, with Wake Forest University and Atrium Health Wake Forest Baptist, both in Winston-Salem, N.C.
The study had no specific funding. The authors have disclosed no relevant financial relationships. Dr. Benca is a consultant for Idorsia Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Such disturbances are especially common among Black people, new research shows.
The “high” prevalence of moderate to severe sleep disturbances is “alarming,” study investigator Cinthya Pena Orbea, MD, sleep specialist at the Cleveland Clinic, said in an interview.
The findings were presented at the annual meeting of the Associated Professional Sleep Societies.
Dr. Pena and colleagues analyzed data on 962 patients with PASC seen at the Cleveland Clinic ReCOVer Clinic between February 2021 and April 2022.
More than two-thirds of patients (67.2%) reported at least moderate fatigue, while 21.8% reported severe fatigue, Dr. Pena reported.
In addition, 41.3% reported at least moderate sleep disturbances, while 8% of patients reported severe sleep disturbances, including insomnia, “which may impair quality of life,” Dr. Pena said.
Obesity, mood disorders, and Black race emerged as contributors to problems with sleep and fatigue after COVID.
Notably, after adjusting for demographics, Black race conferred threefold higher odds of moderate to severe sleep disturbances.
“We don’t know why this is, and one of our next steps is to better understand race-specific determinants of sleep disturbances after COVID and create targeted interventions,” Dr. Pena said.
How long after COVID the fatigue and sleep problems last “remains uncertain,” Dr. Pena acknowledged. However, in her clinical experience with therapy, patients’ sleep and fatigue may improve after 6 or 8 months.
Ruth Benca, MD, PhD, cochair of the Alliance for Sleep, is not surprised by the Cleveland Clinic findings.
“Sleep disturbances and fatigue are part of the sequelae of COVID,” Dr. Benca, who was not involved in the study, said in an interview.
“We know that people who have had COVID have more trouble sleeping afterwards. There is the COVID insomnia created in all of us just out of our worries, fears, isolation, and stress. And then there’s an actual impact of having the infection itself on worsening sleep,” said Dr. Benca, with Wake Forest University and Atrium Health Wake Forest Baptist, both in Winston-Salem, N.C.
The study had no specific funding. The authors have disclosed no relevant financial relationships. Dr. Benca is a consultant for Idorsia Pharmaceuticals.
A version of this article first appeared on Medscape.com.
FROM SLEEP 2022
CDC says about 20% get long COVID. New models try to define it
As the number of people reporting persistent, and sometimes debilitating, symptoms from COVID-19 increases, researchers have struggled to pinpoint exactly how common so-called “long COVID” is, as well as how to clearly define exactly who has it or who is likely to get it.
Now, Centers for Disease Control and Prevention researchers have concluded that one in five adults aged 18 and older have at least one health condition that might be related to their previous COVID-19 illness; that number goes up to one in four among those 65 and older. Their data was published in the CDC’s Morbidity and Mortality Weekly Report.
The conditions associated with what’s been officially termed postacute sequelae of COVID-19, or PASC, include kidney failure, blood clots, other vascular issues, respiratory issues, heart problems, mental health or neurologic problems, and musculoskeletal conditions. But none of those conditions is unique to long COVID.
Another new study, published in The Lancet Digital Health, is trying to help better characterize what long COVID is, and what it isn’t.
that could help identify those likely to develop it.
CDC data
The CDC team came to its conclusions by evaluating the EHRs of more than 353,000 adults who were diagnosed with COVID-19 or got a positive test result, then comparing those records with 1.6 million patients who had a medical visit in the same month without a positive test result or a COVID-19 diagnosis.
They looked at data from March 2020 to November 2021, tagging 26 conditions often linked to post-COVID issues.
Overall, more than 38% of the COVID patients and 16% of those without COVID had at least one of these 26 conditions. They assessed the absolute risk difference between the patients and the non-COVID patients who developed one of the conditions, finding a 20.8–percentage point difference for those 18-64, yielding the one in five figure, and a 26.9–percentage point difference for those 65 and above, translating to about one in four.
“These findings suggest the need for increased awareness for post-COVID conditions so that improved post-COVID care and management of patients who survived COVID-19 can be developed and implemented,” said study author Lara Bull-Otterson, PhD, MPH, colead of data analytics at the Healthcare Data Advisory Unit of the CDC.
Pinpointing long COVID characteristics
Long COVID is difficult to identify, because many of its symptoms are similar to those of other conditions, so researchers are looking for better ways to characterize it to help improve both diagnosis and treatment.
Researchers on the Lancet study evaluated data from the National COVID Cohort Collaborative, N3C, a national NIH database that includes information from more than 8 million people. The team looked at the health records of 98,000 adult COVID patients and used that information, along with data from about nearly 600 long-COVID patients treated at three long-COVID clinics, to create three machine learning models for identifying long-COVID patients.
The models aimed to identify long-COVID patients in three groups: all patients, those hospitalized with COVID, and those with COVID but not hospitalized. The models were judged by the researchers to be accurate because those identified at risk for long COVID from the database were similar to those actually treated for long COVID at the clinics.
“Our algorithm is not intended to diagnose long COVID,” said lead author Emily Pfaff, PhD, research assistant professor of medicine at the University of North Carolina at Chapel Hill. “Rather, it is intended to identify patients in EHR data who ‘look like’ patients seen by physicians for long COVID.’’
Next, the researchers say, they will incorporate the new patterns they found with a diagnosis code for COVID and include it in the models to further test their accuracy. The models could also be used to help recruit patients for clinical trials, the researchers say.
Perspective and caveats
The figures of one in five and one in four found by the CDC researchers don’t surprise David Putrino, PT, PhD, director of rehabilitation innovation for Mount Sinai Health System in New York and director of its Abilities Research Center, which cares for long-COVID patients.
“Those numbers are high and it’s alarming,” he said. “But we’ve been sounding the alarm for quite some time, and we’ve been assuming that about one in five end up with long COVID.”
He does see a limitation to the CDC research – that some symptoms could have emerged later, and some in the control group could have had an undiagnosed COVID infection and gone on to develop long COVID.
As for machine learning, “this is something we need to approach with caution,” Dr. Putrino said. “There are a lot of variables we don’t understand about long COVID,’’ and that could result in spurious conclusions.
“Although I am supportive of this work going on, I am saying, ‘Scrutinize the tools with a grain of salt.’ Electronic records, Dr. Putrino points out, include information that the doctors enter, not what the patient says.
Dr. Pfaff responds: “It is entirely appropriate to approach both machine learning and EHR data with relevant caveats in mind. There are many clinical factors that are not recorded in the EHR, and the EHR is not representative of all persons with long COVID.” Those data can only reflect those who seek care for a condition, a natural limitation.
When it comes to algorithms, they are limited by data they have access to, such as the electronic health records in this research. However, the immense size and diversity in the data used “does allow us to make some assertations with much more confidence than if we were using data from a single or small number of health care systems,” she said.
A version of this article first appeared on Medscape.com.
As the number of people reporting persistent, and sometimes debilitating, symptoms from COVID-19 increases, researchers have struggled to pinpoint exactly how common so-called “long COVID” is, as well as how to clearly define exactly who has it or who is likely to get it.
Now, Centers for Disease Control and Prevention researchers have concluded that one in five adults aged 18 and older have at least one health condition that might be related to their previous COVID-19 illness; that number goes up to one in four among those 65 and older. Their data was published in the CDC’s Morbidity and Mortality Weekly Report.
The conditions associated with what’s been officially termed postacute sequelae of COVID-19, or PASC, include kidney failure, blood clots, other vascular issues, respiratory issues, heart problems, mental health or neurologic problems, and musculoskeletal conditions. But none of those conditions is unique to long COVID.
Another new study, published in The Lancet Digital Health, is trying to help better characterize what long COVID is, and what it isn’t.
that could help identify those likely to develop it.
CDC data
The CDC team came to its conclusions by evaluating the EHRs of more than 353,000 adults who were diagnosed with COVID-19 or got a positive test result, then comparing those records with 1.6 million patients who had a medical visit in the same month without a positive test result or a COVID-19 diagnosis.
They looked at data from March 2020 to November 2021, tagging 26 conditions often linked to post-COVID issues.
Overall, more than 38% of the COVID patients and 16% of those without COVID had at least one of these 26 conditions. They assessed the absolute risk difference between the patients and the non-COVID patients who developed one of the conditions, finding a 20.8–percentage point difference for those 18-64, yielding the one in five figure, and a 26.9–percentage point difference for those 65 and above, translating to about one in four.
“These findings suggest the need for increased awareness for post-COVID conditions so that improved post-COVID care and management of patients who survived COVID-19 can be developed and implemented,” said study author Lara Bull-Otterson, PhD, MPH, colead of data analytics at the Healthcare Data Advisory Unit of the CDC.
Pinpointing long COVID characteristics
Long COVID is difficult to identify, because many of its symptoms are similar to those of other conditions, so researchers are looking for better ways to characterize it to help improve both diagnosis and treatment.
Researchers on the Lancet study evaluated data from the National COVID Cohort Collaborative, N3C, a national NIH database that includes information from more than 8 million people. The team looked at the health records of 98,000 adult COVID patients and used that information, along with data from about nearly 600 long-COVID patients treated at three long-COVID clinics, to create three machine learning models for identifying long-COVID patients.
The models aimed to identify long-COVID patients in three groups: all patients, those hospitalized with COVID, and those with COVID but not hospitalized. The models were judged by the researchers to be accurate because those identified at risk for long COVID from the database were similar to those actually treated for long COVID at the clinics.
“Our algorithm is not intended to diagnose long COVID,” said lead author Emily Pfaff, PhD, research assistant professor of medicine at the University of North Carolina at Chapel Hill. “Rather, it is intended to identify patients in EHR data who ‘look like’ patients seen by physicians for long COVID.’’
Next, the researchers say, they will incorporate the new patterns they found with a diagnosis code for COVID and include it in the models to further test their accuracy. The models could also be used to help recruit patients for clinical trials, the researchers say.
Perspective and caveats
The figures of one in five and one in four found by the CDC researchers don’t surprise David Putrino, PT, PhD, director of rehabilitation innovation for Mount Sinai Health System in New York and director of its Abilities Research Center, which cares for long-COVID patients.
“Those numbers are high and it’s alarming,” he said. “But we’ve been sounding the alarm for quite some time, and we’ve been assuming that about one in five end up with long COVID.”
He does see a limitation to the CDC research – that some symptoms could have emerged later, and some in the control group could have had an undiagnosed COVID infection and gone on to develop long COVID.
As for machine learning, “this is something we need to approach with caution,” Dr. Putrino said. “There are a lot of variables we don’t understand about long COVID,’’ and that could result in spurious conclusions.
“Although I am supportive of this work going on, I am saying, ‘Scrutinize the tools with a grain of salt.’ Electronic records, Dr. Putrino points out, include information that the doctors enter, not what the patient says.
Dr. Pfaff responds: “It is entirely appropriate to approach both machine learning and EHR data with relevant caveats in mind. There are many clinical factors that are not recorded in the EHR, and the EHR is not representative of all persons with long COVID.” Those data can only reflect those who seek care for a condition, a natural limitation.
When it comes to algorithms, they are limited by data they have access to, such as the electronic health records in this research. However, the immense size and diversity in the data used “does allow us to make some assertations with much more confidence than if we were using data from a single or small number of health care systems,” she said.
A version of this article first appeared on Medscape.com.
As the number of people reporting persistent, and sometimes debilitating, symptoms from COVID-19 increases, researchers have struggled to pinpoint exactly how common so-called “long COVID” is, as well as how to clearly define exactly who has it or who is likely to get it.
Now, Centers for Disease Control and Prevention researchers have concluded that one in five adults aged 18 and older have at least one health condition that might be related to their previous COVID-19 illness; that number goes up to one in four among those 65 and older. Their data was published in the CDC’s Morbidity and Mortality Weekly Report.
The conditions associated with what’s been officially termed postacute sequelae of COVID-19, or PASC, include kidney failure, blood clots, other vascular issues, respiratory issues, heart problems, mental health or neurologic problems, and musculoskeletal conditions. But none of those conditions is unique to long COVID.
Another new study, published in The Lancet Digital Health, is trying to help better characterize what long COVID is, and what it isn’t.
that could help identify those likely to develop it.
CDC data
The CDC team came to its conclusions by evaluating the EHRs of more than 353,000 adults who were diagnosed with COVID-19 or got a positive test result, then comparing those records with 1.6 million patients who had a medical visit in the same month without a positive test result or a COVID-19 diagnosis.
They looked at data from March 2020 to November 2021, tagging 26 conditions often linked to post-COVID issues.
Overall, more than 38% of the COVID patients and 16% of those without COVID had at least one of these 26 conditions. They assessed the absolute risk difference between the patients and the non-COVID patients who developed one of the conditions, finding a 20.8–percentage point difference for those 18-64, yielding the one in five figure, and a 26.9–percentage point difference for those 65 and above, translating to about one in four.
“These findings suggest the need for increased awareness for post-COVID conditions so that improved post-COVID care and management of patients who survived COVID-19 can be developed and implemented,” said study author Lara Bull-Otterson, PhD, MPH, colead of data analytics at the Healthcare Data Advisory Unit of the CDC.
Pinpointing long COVID characteristics
Long COVID is difficult to identify, because many of its symptoms are similar to those of other conditions, so researchers are looking for better ways to characterize it to help improve both diagnosis and treatment.
Researchers on the Lancet study evaluated data from the National COVID Cohort Collaborative, N3C, a national NIH database that includes information from more than 8 million people. The team looked at the health records of 98,000 adult COVID patients and used that information, along with data from about nearly 600 long-COVID patients treated at three long-COVID clinics, to create three machine learning models for identifying long-COVID patients.
The models aimed to identify long-COVID patients in three groups: all patients, those hospitalized with COVID, and those with COVID but not hospitalized. The models were judged by the researchers to be accurate because those identified at risk for long COVID from the database were similar to those actually treated for long COVID at the clinics.
“Our algorithm is not intended to diagnose long COVID,” said lead author Emily Pfaff, PhD, research assistant professor of medicine at the University of North Carolina at Chapel Hill. “Rather, it is intended to identify patients in EHR data who ‘look like’ patients seen by physicians for long COVID.’’
Next, the researchers say, they will incorporate the new patterns they found with a diagnosis code for COVID and include it in the models to further test their accuracy. The models could also be used to help recruit patients for clinical trials, the researchers say.
Perspective and caveats
The figures of one in five and one in four found by the CDC researchers don’t surprise David Putrino, PT, PhD, director of rehabilitation innovation for Mount Sinai Health System in New York and director of its Abilities Research Center, which cares for long-COVID patients.
“Those numbers are high and it’s alarming,” he said. “But we’ve been sounding the alarm for quite some time, and we’ve been assuming that about one in five end up with long COVID.”
He does see a limitation to the CDC research – that some symptoms could have emerged later, and some in the control group could have had an undiagnosed COVID infection and gone on to develop long COVID.
As for machine learning, “this is something we need to approach with caution,” Dr. Putrino said. “There are a lot of variables we don’t understand about long COVID,’’ and that could result in spurious conclusions.
“Although I am supportive of this work going on, I am saying, ‘Scrutinize the tools with a grain of salt.’ Electronic records, Dr. Putrino points out, include information that the doctors enter, not what the patient says.
Dr. Pfaff responds: “It is entirely appropriate to approach both machine learning and EHR data with relevant caveats in mind. There are many clinical factors that are not recorded in the EHR, and the EHR is not representative of all persons with long COVID.” Those data can only reflect those who seek care for a condition, a natural limitation.
When it comes to algorithms, they are limited by data they have access to, such as the electronic health records in this research. However, the immense size and diversity in the data used “does allow us to make some assertations with much more confidence than if we were using data from a single or small number of health care systems,” she said.
A version of this article first appeared on Medscape.com.
Long COVID neuropsychiatric deficits greater than expected
NEW ORLEANS – , adding to mounting evidence of the significant toll the chronic condition can have on mental health.
“Many clinicians have observed the symptoms we describe in this study, however this report is among the first which identify the specific deficits using neuropsychological testing to better characterize the syndrome,” Sean T. Lynch, MD, first author of a study on the issue presented at the annual meeting of the American Psychiatric Association, said in an interview.
Dr. Lynch, of the department of psychiatry, Westchester Medical Center Health System, Valhalla, N.Y., and his colleagues enrolled 60 participants who had experienced acute COVID-19 disease 6-8 months earlier and had undergone neuropsychological, psychiatric, medical, functional, and quality-of-life assessments. Results from the study were published online in the Journal of the Academy of Consultation–Liaison Psychiatry (2022 Jan 25. doi: 10.1016/j.jaclp.2022.01.003).
Among the study participants, 32 were seeking treatment for brain fog in a clinical program for survivors of COVID-19, while the remaining 28 were part of an ongoing longitudinal investigation of neuropsychological, medical, and psychiatric sequelae of COVID-19, but were not seeking care for the persistent symptoms.
Assessments for neurocognitive impairment included a battery of tests used in infectious and other diseases, including the Test of Premorbid Function, the Patient Assessment of Own Function, the Trail Making Test parts A and B, the Stroop Color and Word Test, and others.
Overall, the battery of assessments showed that 37 (62%) of participants had neuropsychological test impairment, with results below the 16th percentile in two tests, while 16 (27%) showed scores indicative of severe impairment (below the second percentile in at least one test and below the 16th percentile in one test).
Those reporting brain fog had scores that were even lower than expected on tests of attention, processing speed, memory, and executive function. And among those reporting brain fog, significantly more had scores reflecting severe impairment compared with the controls (38% vs. 14%; P < .04).
“Based on what we’ve observed in our patients and what others have previously reported, we did expect to find some impairment in this study sample,” Dr. Lynch noted.
“However, we were surprised to find that 27% of the study sample had extremely low neuropsychological test scores, meaning that they scored at least two standard deviations below the expected score on at least one neuropsychological test based on their age and level of education.”
The brain fog group also reported significantly higher levels of depression, fatigue, PTSD, and functional difficulties, and lower quality of life.
Severe impairment on the neuropsychological tests correlated with the extent of acute COVID-19 symptoms, as well as depression scores, number of medical comorbidities, and subjective cognitive complaints.
An analysis of serum levels of the inflammatory markers among 50 of the 60 participants showed that 45% of the patients had an elevated IL-6, 20% had elevated TNF-alpha, and 41% had elevated CRP, compared with reference ranges.
IL-6 levels were found to correlate with acute COVID-19 symptoms, the number of medical comorbidities, fatigue, and measures of executive function, while C-reactive protein (CRP) correlated with current COVID-19 symptoms and depression scores.
In terms of clinical factors that might predict low neuropsychological test scores, Dr. Lynch noted that the “markers that we found to be significant included severity of acute COVID-19 illness, current post-COVID-19 symptoms, measures of depression and anxiety, level of fatigue, and number of medical comorbidities.”
Dr. Lynch noted that the ongoing study will include up to 18-month follow-ups that are currently underway. “The [follow-ups] will examine if symptoms improve over time and evaluate if any intervention that took place was successful,” he said.
Survey supports findings
The detrimental effects of mental health symptoms in long COVID were further supported in another study at the APA meeting, an online survey of 787 survivors of acute COVID-19.
In the community survey, presented by Michael Van Ameringen, MD, a professor in the department of psychiatry and behavioral neurosciences at McMaster University, in Hamilton, Ont., all respondents (100%) reported having persistent symptoms of the virus, and as many as 68% indicated that they had not returned to normal functioning, despite only 15% of the respondents having been hospitalized with COVID-19.
A large proportion showed significant depression, anxiety, and posttraumatic stress disorder (PTSD), and the most commonly reported persistent symptoms were fatigue in 75.9% of respondents, brain fog in 67.9%, concentration difficulties in 61.1%, and weakness in 51.2%.
As many as 88.2% of patients said they experienced persistent neurocognitive symptoms, with poor memory and concentration; 56% reported problems with word finding; and 54.1% had slowed thinking.
The respondents showed high rates of anxiety (41.7%) as well as depression (61.4%) as determined by scores above 9 on the Generalized Anxiety Disorder–7 (GAD-7) and Patient Health Questionnaires (PHQ-9).
As many as 40.5% of respondents showed probable PTSD, with scores above 30 on the PTSD checklist (PCL-5). Their mean resilience score on the Brief Resilient Coping Scale was 13.5, suggesting low resilience.
Among the respondents, 43.3% said they had received past treatment for mental health, while 33.5% were currently receiving mental health treatment.
Dr. Van Ameringen noted the important limitation of the study being an online survey with no control group, but said the responses nevertheless raise the question of the role of prior psychiatric disorders in long COVID.
“In our sample, 40% of respondents had a past psychiatric history, so you wonder if that also makes you vulnerable to long COVID,” he said in an interview.
“About a third were getting psychiatric help, but I think the more impaired you are, the more likely you are to seek help.”
Those who were hospitalized with COVID-19 were at a higher risk of PTSD compared with those not hospitalized (P < .001), as were those under the age of 30 (P < .05) or between 31 and 50 vs. over 50 (P < .01).
Dr. Van Ameringen noted that the survey’s high rate of subjects who had not returned to normal functioning was especially striking.
“This is not a minor issue – these are people who are no longer functioning in society,” he said.
In pandemics, the brain tends to be ‘overlooked’
Further addressing the neurological effects of COVID-19 at the APA meeting, Avindra Nath, MD, clinical director of the National Institutes of Neurologic Disorders and Stroke in Bethesda, Md., noted that the persisting cognitive and psychiatric symptoms after illness, such as brain fog and depression and anxiety, are not necessarily unique to COVID-19.
“We have seen this before,” he said. “There have been at least seven or eight human coronaviruses, and the interesting thing is each one affects the brain and causes neurological complications.”
The effects are classified differently and have slightly different receptors, “but the consequences are the same.”
Of note, however, research published in The Lancet Psychiatry (2021 May. doi: 10.1016/S2215-0366[21]00084-5) revealed that symptoms such as dementia, mood, and anxiety are significantly higher after COVID-19 compared with other respiratory infections, with the differences increasing at 180 days since the index event.
Dr. Nath noted that, over the decades, he has observed that in pandemics “the brain tends to get overlooked.” He explained that “what can be most important in the end is what happened in the brain, because those are the things that really cause the long-term consequences.”
“These patients are depressed; they have dementia, they have brain fog, and even now that we recognize these issues, we haven’t done a very good job of studying them,” he said. “There’s so much we still don’t know, and a lot of patients are left with these symptoms and nowhere to go.”
Dr. Lynch, Dr. Van Ameringen, and Dr. Nath had no disclosures to report.
NEW ORLEANS – , adding to mounting evidence of the significant toll the chronic condition can have on mental health.
“Many clinicians have observed the symptoms we describe in this study, however this report is among the first which identify the specific deficits using neuropsychological testing to better characterize the syndrome,” Sean T. Lynch, MD, first author of a study on the issue presented at the annual meeting of the American Psychiatric Association, said in an interview.
Dr. Lynch, of the department of psychiatry, Westchester Medical Center Health System, Valhalla, N.Y., and his colleagues enrolled 60 participants who had experienced acute COVID-19 disease 6-8 months earlier and had undergone neuropsychological, psychiatric, medical, functional, and quality-of-life assessments. Results from the study were published online in the Journal of the Academy of Consultation–Liaison Psychiatry (2022 Jan 25. doi: 10.1016/j.jaclp.2022.01.003).
Among the study participants, 32 were seeking treatment for brain fog in a clinical program for survivors of COVID-19, while the remaining 28 were part of an ongoing longitudinal investigation of neuropsychological, medical, and psychiatric sequelae of COVID-19, but were not seeking care for the persistent symptoms.
Assessments for neurocognitive impairment included a battery of tests used in infectious and other diseases, including the Test of Premorbid Function, the Patient Assessment of Own Function, the Trail Making Test parts A and B, the Stroop Color and Word Test, and others.
Overall, the battery of assessments showed that 37 (62%) of participants had neuropsychological test impairment, with results below the 16th percentile in two tests, while 16 (27%) showed scores indicative of severe impairment (below the second percentile in at least one test and below the 16th percentile in one test).
Those reporting brain fog had scores that were even lower than expected on tests of attention, processing speed, memory, and executive function. And among those reporting brain fog, significantly more had scores reflecting severe impairment compared with the controls (38% vs. 14%; P < .04).
“Based on what we’ve observed in our patients and what others have previously reported, we did expect to find some impairment in this study sample,” Dr. Lynch noted.
“However, we were surprised to find that 27% of the study sample had extremely low neuropsychological test scores, meaning that they scored at least two standard deviations below the expected score on at least one neuropsychological test based on their age and level of education.”
The brain fog group also reported significantly higher levels of depression, fatigue, PTSD, and functional difficulties, and lower quality of life.
Severe impairment on the neuropsychological tests correlated with the extent of acute COVID-19 symptoms, as well as depression scores, number of medical comorbidities, and subjective cognitive complaints.
An analysis of serum levels of the inflammatory markers among 50 of the 60 participants showed that 45% of the patients had an elevated IL-6, 20% had elevated TNF-alpha, and 41% had elevated CRP, compared with reference ranges.
IL-6 levels were found to correlate with acute COVID-19 symptoms, the number of medical comorbidities, fatigue, and measures of executive function, while C-reactive protein (CRP) correlated with current COVID-19 symptoms and depression scores.
In terms of clinical factors that might predict low neuropsychological test scores, Dr. Lynch noted that the “markers that we found to be significant included severity of acute COVID-19 illness, current post-COVID-19 symptoms, measures of depression and anxiety, level of fatigue, and number of medical comorbidities.”
Dr. Lynch noted that the ongoing study will include up to 18-month follow-ups that are currently underway. “The [follow-ups] will examine if symptoms improve over time and evaluate if any intervention that took place was successful,” he said.
Survey supports findings
The detrimental effects of mental health symptoms in long COVID were further supported in another study at the APA meeting, an online survey of 787 survivors of acute COVID-19.
In the community survey, presented by Michael Van Ameringen, MD, a professor in the department of psychiatry and behavioral neurosciences at McMaster University, in Hamilton, Ont., all respondents (100%) reported having persistent symptoms of the virus, and as many as 68% indicated that they had not returned to normal functioning, despite only 15% of the respondents having been hospitalized with COVID-19.
A large proportion showed significant depression, anxiety, and posttraumatic stress disorder (PTSD), and the most commonly reported persistent symptoms were fatigue in 75.9% of respondents, brain fog in 67.9%, concentration difficulties in 61.1%, and weakness in 51.2%.
As many as 88.2% of patients said they experienced persistent neurocognitive symptoms, with poor memory and concentration; 56% reported problems with word finding; and 54.1% had slowed thinking.
The respondents showed high rates of anxiety (41.7%) as well as depression (61.4%) as determined by scores above 9 on the Generalized Anxiety Disorder–7 (GAD-7) and Patient Health Questionnaires (PHQ-9).
As many as 40.5% of respondents showed probable PTSD, with scores above 30 on the PTSD checklist (PCL-5). Their mean resilience score on the Brief Resilient Coping Scale was 13.5, suggesting low resilience.
Among the respondents, 43.3% said they had received past treatment for mental health, while 33.5% were currently receiving mental health treatment.
Dr. Van Ameringen noted the important limitation of the study being an online survey with no control group, but said the responses nevertheless raise the question of the role of prior psychiatric disorders in long COVID.
“In our sample, 40% of respondents had a past psychiatric history, so you wonder if that also makes you vulnerable to long COVID,” he said in an interview.
“About a third were getting psychiatric help, but I think the more impaired you are, the more likely you are to seek help.”
Those who were hospitalized with COVID-19 were at a higher risk of PTSD compared with those not hospitalized (P < .001), as were those under the age of 30 (P < .05) or between 31 and 50 vs. over 50 (P < .01).
Dr. Van Ameringen noted that the survey’s high rate of subjects who had not returned to normal functioning was especially striking.
“This is not a minor issue – these are people who are no longer functioning in society,” he said.
In pandemics, the brain tends to be ‘overlooked’
Further addressing the neurological effects of COVID-19 at the APA meeting, Avindra Nath, MD, clinical director of the National Institutes of Neurologic Disorders and Stroke in Bethesda, Md., noted that the persisting cognitive and psychiatric symptoms after illness, such as brain fog and depression and anxiety, are not necessarily unique to COVID-19.
“We have seen this before,” he said. “There have been at least seven or eight human coronaviruses, and the interesting thing is each one affects the brain and causes neurological complications.”
The effects are classified differently and have slightly different receptors, “but the consequences are the same.”
Of note, however, research published in The Lancet Psychiatry (2021 May. doi: 10.1016/S2215-0366[21]00084-5) revealed that symptoms such as dementia, mood, and anxiety are significantly higher after COVID-19 compared with other respiratory infections, with the differences increasing at 180 days since the index event.
Dr. Nath noted that, over the decades, he has observed that in pandemics “the brain tends to get overlooked.” He explained that “what can be most important in the end is what happened in the brain, because those are the things that really cause the long-term consequences.”
“These patients are depressed; they have dementia, they have brain fog, and even now that we recognize these issues, we haven’t done a very good job of studying them,” he said. “There’s so much we still don’t know, and a lot of patients are left with these symptoms and nowhere to go.”
Dr. Lynch, Dr. Van Ameringen, and Dr. Nath had no disclosures to report.
NEW ORLEANS – , adding to mounting evidence of the significant toll the chronic condition can have on mental health.
“Many clinicians have observed the symptoms we describe in this study, however this report is among the first which identify the specific deficits using neuropsychological testing to better characterize the syndrome,” Sean T. Lynch, MD, first author of a study on the issue presented at the annual meeting of the American Psychiatric Association, said in an interview.
Dr. Lynch, of the department of psychiatry, Westchester Medical Center Health System, Valhalla, N.Y., and his colleagues enrolled 60 participants who had experienced acute COVID-19 disease 6-8 months earlier and had undergone neuropsychological, psychiatric, medical, functional, and quality-of-life assessments. Results from the study were published online in the Journal of the Academy of Consultation–Liaison Psychiatry (2022 Jan 25. doi: 10.1016/j.jaclp.2022.01.003).
Among the study participants, 32 were seeking treatment for brain fog in a clinical program for survivors of COVID-19, while the remaining 28 were part of an ongoing longitudinal investigation of neuropsychological, medical, and psychiatric sequelae of COVID-19, but were not seeking care for the persistent symptoms.
Assessments for neurocognitive impairment included a battery of tests used in infectious and other diseases, including the Test of Premorbid Function, the Patient Assessment of Own Function, the Trail Making Test parts A and B, the Stroop Color and Word Test, and others.
Overall, the battery of assessments showed that 37 (62%) of participants had neuropsychological test impairment, with results below the 16th percentile in two tests, while 16 (27%) showed scores indicative of severe impairment (below the second percentile in at least one test and below the 16th percentile in one test).
Those reporting brain fog had scores that were even lower than expected on tests of attention, processing speed, memory, and executive function. And among those reporting brain fog, significantly more had scores reflecting severe impairment compared with the controls (38% vs. 14%; P < .04).
“Based on what we’ve observed in our patients and what others have previously reported, we did expect to find some impairment in this study sample,” Dr. Lynch noted.
“However, we were surprised to find that 27% of the study sample had extremely low neuropsychological test scores, meaning that they scored at least two standard deviations below the expected score on at least one neuropsychological test based on their age and level of education.”
The brain fog group also reported significantly higher levels of depression, fatigue, PTSD, and functional difficulties, and lower quality of life.
Severe impairment on the neuropsychological tests correlated with the extent of acute COVID-19 symptoms, as well as depression scores, number of medical comorbidities, and subjective cognitive complaints.
An analysis of serum levels of the inflammatory markers among 50 of the 60 participants showed that 45% of the patients had an elevated IL-6, 20% had elevated TNF-alpha, and 41% had elevated CRP, compared with reference ranges.
IL-6 levels were found to correlate with acute COVID-19 symptoms, the number of medical comorbidities, fatigue, and measures of executive function, while C-reactive protein (CRP) correlated with current COVID-19 symptoms and depression scores.
In terms of clinical factors that might predict low neuropsychological test scores, Dr. Lynch noted that the “markers that we found to be significant included severity of acute COVID-19 illness, current post-COVID-19 symptoms, measures of depression and anxiety, level of fatigue, and number of medical comorbidities.”
Dr. Lynch noted that the ongoing study will include up to 18-month follow-ups that are currently underway. “The [follow-ups] will examine if symptoms improve over time and evaluate if any intervention that took place was successful,” he said.
Survey supports findings
The detrimental effects of mental health symptoms in long COVID were further supported in another study at the APA meeting, an online survey of 787 survivors of acute COVID-19.
In the community survey, presented by Michael Van Ameringen, MD, a professor in the department of psychiatry and behavioral neurosciences at McMaster University, in Hamilton, Ont., all respondents (100%) reported having persistent symptoms of the virus, and as many as 68% indicated that they had not returned to normal functioning, despite only 15% of the respondents having been hospitalized with COVID-19.
A large proportion showed significant depression, anxiety, and posttraumatic stress disorder (PTSD), and the most commonly reported persistent symptoms were fatigue in 75.9% of respondents, brain fog in 67.9%, concentration difficulties in 61.1%, and weakness in 51.2%.
As many as 88.2% of patients said they experienced persistent neurocognitive symptoms, with poor memory and concentration; 56% reported problems with word finding; and 54.1% had slowed thinking.
The respondents showed high rates of anxiety (41.7%) as well as depression (61.4%) as determined by scores above 9 on the Generalized Anxiety Disorder–7 (GAD-7) and Patient Health Questionnaires (PHQ-9).
As many as 40.5% of respondents showed probable PTSD, with scores above 30 on the PTSD checklist (PCL-5). Their mean resilience score on the Brief Resilient Coping Scale was 13.5, suggesting low resilience.
Among the respondents, 43.3% said they had received past treatment for mental health, while 33.5% were currently receiving mental health treatment.
Dr. Van Ameringen noted the important limitation of the study being an online survey with no control group, but said the responses nevertheless raise the question of the role of prior psychiatric disorders in long COVID.
“In our sample, 40% of respondents had a past psychiatric history, so you wonder if that also makes you vulnerable to long COVID,” he said in an interview.
“About a third were getting psychiatric help, but I think the more impaired you are, the more likely you are to seek help.”
Those who were hospitalized with COVID-19 were at a higher risk of PTSD compared with those not hospitalized (P < .001), as were those under the age of 30 (P < .05) or between 31 and 50 vs. over 50 (P < .01).
Dr. Van Ameringen noted that the survey’s high rate of subjects who had not returned to normal functioning was especially striking.
“This is not a minor issue – these are people who are no longer functioning in society,” he said.
In pandemics, the brain tends to be ‘overlooked’
Further addressing the neurological effects of COVID-19 at the APA meeting, Avindra Nath, MD, clinical director of the National Institutes of Neurologic Disorders and Stroke in Bethesda, Md., noted that the persisting cognitive and psychiatric symptoms after illness, such as brain fog and depression and anxiety, are not necessarily unique to COVID-19.
“We have seen this before,” he said. “There have been at least seven or eight human coronaviruses, and the interesting thing is each one affects the brain and causes neurological complications.”
The effects are classified differently and have slightly different receptors, “but the consequences are the same.”
Of note, however, research published in The Lancet Psychiatry (2021 May. doi: 10.1016/S2215-0366[21]00084-5) revealed that symptoms such as dementia, mood, and anxiety are significantly higher after COVID-19 compared with other respiratory infections, with the differences increasing at 180 days since the index event.
Dr. Nath noted that, over the decades, he has observed that in pandemics “the brain tends to get overlooked.” He explained that “what can be most important in the end is what happened in the brain, because those are the things that really cause the long-term consequences.”
“These patients are depressed; they have dementia, they have brain fog, and even now that we recognize these issues, we haven’t done a very good job of studying them,” he said. “There’s so much we still don’t know, and a lot of patients are left with these symptoms and nowhere to go.”
Dr. Lynch, Dr. Van Ameringen, and Dr. Nath had no disclosures to report.
AT APA 2022
Most COVID long-haulers suffer long-term debilitating neurologic symptoms
Most COVID-19 long-haulers continue to have brain fog, fatigue, and compromised quality of life more than a year after the initial infection, results from the most extensive follow-up to date of a group of long COVID patients show.
Most patients continue to experience debilitating neurologic symptoms an average of 15 months from symptom onset, Igor Koralnik, MD, who oversees the Neuro COVID-19 Clinic at Northwestern Medicine in Chicago, said during a press briefing.
Surprisingly, in some cases, new symptoms appear that didn’t exist before, including variation of heart rate and blood pressure, and gastrointestinal symptoms, indicating there may be a late appearance in dysfunction of the autonomic nervous system in those patients, Dr. Koralnik said.
The study was published online in Annals of Clinical and Translational Neurology.
Evolving symptoms
The investigators evaluated the evolution of neurologic symptoms in 52 adults who had mild COVID-19 symptoms and were not admitted to the hospital.
Their mean age was 43 years, 73% were women and 77% had received a COVID-19 vaccine. These patients have now been followed for between 11 and 18 months since their initial infection.
Overall, between first and follow-up evaluations, there was no significant change in the frequency of most neurologic symptoms, including brain fog (81% vs. 71%), numbness/tingling (69% vs. 65%), headache (67% vs. 54%), dizziness (50% vs. 54%), blurred vision (34% vs. 44%), tinnitus (33% vs. 42%), and fatigue (87% vs. 81%).
The only neurologic symptoms that decreased over time were loss of taste (63% vs. 27%) and smell (58% vs. 21%).
Conversely, heart rate and blood pressure variation (35% vs. 56%) and gastrointestinal symptoms (27% vs. 48%; P = .04) increased at follow-up evaluations.
Patients reported subjective improvements in their recovery, cognitive function and fatigue, but quality of life measures remained lower than the average population of the United States.
There was a neutral effect of COVID vaccination on long COVID symptoms – it didn’t cure long COVID or make long COVID worse, which is a reason given by some long-haulers for not getting vaccinated, Dr. Koralnik told the briefing.
Therefore, “we continue to encourage our patients to get vaccinated and boosted according to the Centers for Disease Control and Prevention recommendation,” he said.
Escape from the ‘pit of despair’
To date, the Northwestern Medicine Neuro COVID-19 Clinic has treated nearly 1,400 COVID long-haulers from across the United States.
Emily Caffee, a physical therapist from Wheaton, Ill., is one of them.
Speaking at the briefing, the 36-year-old described her saga and roller coaster of recovering from long COVID in three acts: her initial infection, followed by a descent into a pit of physical and emotional despair, followed by her eventual escape from that pit more than two years later.
Following a fairly mild case of COVID, Ms. Caffee said worsening neurologic symptoms forced her to take medical leave from her very physical and cognitively demanding job.
Ms. Caffee said she experienced crushing fatigue and brain fog, as well as rapid heart rate and blood pressure changes going from sitting to standing position.
She went from being a competitive athlete to someone who could barely get off the couch or empty the dishwasher.
With the ongoing help of her medical team, she slowly returned to daily activities and eventually to work on a limited basis.
Today, Ms. Caffee says she’s 90%-95% better but still she has some lingering symptoms and does not yet feel like her pre-COVID self.
It’s been a very slow climb out of the pit, Ms. Caffee said.
This study has no specific funding. The authors disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Most COVID-19 long-haulers continue to have brain fog, fatigue, and compromised quality of life more than a year after the initial infection, results from the most extensive follow-up to date of a group of long COVID patients show.
Most patients continue to experience debilitating neurologic symptoms an average of 15 months from symptom onset, Igor Koralnik, MD, who oversees the Neuro COVID-19 Clinic at Northwestern Medicine in Chicago, said during a press briefing.
Surprisingly, in some cases, new symptoms appear that didn’t exist before, including variation of heart rate and blood pressure, and gastrointestinal symptoms, indicating there may be a late appearance in dysfunction of the autonomic nervous system in those patients, Dr. Koralnik said.
The study was published online in Annals of Clinical and Translational Neurology.
Evolving symptoms
The investigators evaluated the evolution of neurologic symptoms in 52 adults who had mild COVID-19 symptoms and were not admitted to the hospital.
Their mean age was 43 years, 73% were women and 77% had received a COVID-19 vaccine. These patients have now been followed for between 11 and 18 months since their initial infection.
Overall, between first and follow-up evaluations, there was no significant change in the frequency of most neurologic symptoms, including brain fog (81% vs. 71%), numbness/tingling (69% vs. 65%), headache (67% vs. 54%), dizziness (50% vs. 54%), blurred vision (34% vs. 44%), tinnitus (33% vs. 42%), and fatigue (87% vs. 81%).
The only neurologic symptoms that decreased over time were loss of taste (63% vs. 27%) and smell (58% vs. 21%).
Conversely, heart rate and blood pressure variation (35% vs. 56%) and gastrointestinal symptoms (27% vs. 48%; P = .04) increased at follow-up evaluations.
Patients reported subjective improvements in their recovery, cognitive function and fatigue, but quality of life measures remained lower than the average population of the United States.
There was a neutral effect of COVID vaccination on long COVID symptoms – it didn’t cure long COVID or make long COVID worse, which is a reason given by some long-haulers for not getting vaccinated, Dr. Koralnik told the briefing.
Therefore, “we continue to encourage our patients to get vaccinated and boosted according to the Centers for Disease Control and Prevention recommendation,” he said.
Escape from the ‘pit of despair’
To date, the Northwestern Medicine Neuro COVID-19 Clinic has treated nearly 1,400 COVID long-haulers from across the United States.
Emily Caffee, a physical therapist from Wheaton, Ill., is one of them.
Speaking at the briefing, the 36-year-old described her saga and roller coaster of recovering from long COVID in three acts: her initial infection, followed by a descent into a pit of physical and emotional despair, followed by her eventual escape from that pit more than two years later.
Following a fairly mild case of COVID, Ms. Caffee said worsening neurologic symptoms forced her to take medical leave from her very physical and cognitively demanding job.
Ms. Caffee said she experienced crushing fatigue and brain fog, as well as rapid heart rate and blood pressure changes going from sitting to standing position.
She went from being a competitive athlete to someone who could barely get off the couch or empty the dishwasher.
With the ongoing help of her medical team, she slowly returned to daily activities and eventually to work on a limited basis.
Today, Ms. Caffee says she’s 90%-95% better but still she has some lingering symptoms and does not yet feel like her pre-COVID self.
It’s been a very slow climb out of the pit, Ms. Caffee said.
This study has no specific funding. The authors disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Most COVID-19 long-haulers continue to have brain fog, fatigue, and compromised quality of life more than a year after the initial infection, results from the most extensive follow-up to date of a group of long COVID patients show.
Most patients continue to experience debilitating neurologic symptoms an average of 15 months from symptom onset, Igor Koralnik, MD, who oversees the Neuro COVID-19 Clinic at Northwestern Medicine in Chicago, said during a press briefing.
Surprisingly, in some cases, new symptoms appear that didn’t exist before, including variation of heart rate and blood pressure, and gastrointestinal symptoms, indicating there may be a late appearance in dysfunction of the autonomic nervous system in those patients, Dr. Koralnik said.
The study was published online in Annals of Clinical and Translational Neurology.
Evolving symptoms
The investigators evaluated the evolution of neurologic symptoms in 52 adults who had mild COVID-19 symptoms and were not admitted to the hospital.
Their mean age was 43 years, 73% were women and 77% had received a COVID-19 vaccine. These patients have now been followed for between 11 and 18 months since their initial infection.
Overall, between first and follow-up evaluations, there was no significant change in the frequency of most neurologic symptoms, including brain fog (81% vs. 71%), numbness/tingling (69% vs. 65%), headache (67% vs. 54%), dizziness (50% vs. 54%), blurred vision (34% vs. 44%), tinnitus (33% vs. 42%), and fatigue (87% vs. 81%).
The only neurologic symptoms that decreased over time were loss of taste (63% vs. 27%) and smell (58% vs. 21%).
Conversely, heart rate and blood pressure variation (35% vs. 56%) and gastrointestinal symptoms (27% vs. 48%; P = .04) increased at follow-up evaluations.
Patients reported subjective improvements in their recovery, cognitive function and fatigue, but quality of life measures remained lower than the average population of the United States.
There was a neutral effect of COVID vaccination on long COVID symptoms – it didn’t cure long COVID or make long COVID worse, which is a reason given by some long-haulers for not getting vaccinated, Dr. Koralnik told the briefing.
Therefore, “we continue to encourage our patients to get vaccinated and boosted according to the Centers for Disease Control and Prevention recommendation,” he said.
Escape from the ‘pit of despair’
To date, the Northwestern Medicine Neuro COVID-19 Clinic has treated nearly 1,400 COVID long-haulers from across the United States.
Emily Caffee, a physical therapist from Wheaton, Ill., is one of them.
Speaking at the briefing, the 36-year-old described her saga and roller coaster of recovering from long COVID in three acts: her initial infection, followed by a descent into a pit of physical and emotional despair, followed by her eventual escape from that pit more than two years later.
Following a fairly mild case of COVID, Ms. Caffee said worsening neurologic symptoms forced her to take medical leave from her very physical and cognitively demanding job.
Ms. Caffee said she experienced crushing fatigue and brain fog, as well as rapid heart rate and blood pressure changes going from sitting to standing position.
She went from being a competitive athlete to someone who could barely get off the couch or empty the dishwasher.
With the ongoing help of her medical team, she slowly returned to daily activities and eventually to work on a limited basis.
Today, Ms. Caffee says she’s 90%-95% better but still she has some lingering symptoms and does not yet feel like her pre-COVID self.
It’s been a very slow climb out of the pit, Ms. Caffee said.
This study has no specific funding. The authors disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF CLINICAL AND TRANSLATIONAL NEUROLOGY
Neuropsychiatric risks of COVID-19: New data
The neuropsychiatric ramifications of severe COVID-19 infection appear to be no different than for other severe acute respiratory infections (SARI).
This suggests that disease severity, rather than pathogen, is the most relevant factor in new-onset neuropsychiatric illness, the investigators note.
The risk of new-onset neuropsychological illness after severe COVID-19 infection are “substantial, but similar to those after other severe respiratory infections,” study investigator Peter Watkinson, MD, Nuffield Department of Clinical Neurosciences, University of Oxford, and John Radcliffe Hospital, Oxford, England, told this news organization.
The study was published online in JAMA Psychiatry.
Significant mental health burden
Research has shown a significant burden of neuropsychological illness after severe COVID-19 infection. However, it’s unclear how this risk compares to SARI.
To investigate, Dr. Watkinson and colleagues evaluated electronic health record data on more than 8.3 million adults, including 16,679 (0.02%) who survived a hospital admission for SARI and 32,525 (0.03%) who survived a hospital stay for COVID-19.
Compared with the remaining population, risks of new anxiety disorder, dementia, psychotic disorder, depression, and bipolar disorder diagnoses were significantly and similarly increased in adults surviving hospitalization for either COVID-19 or SARI.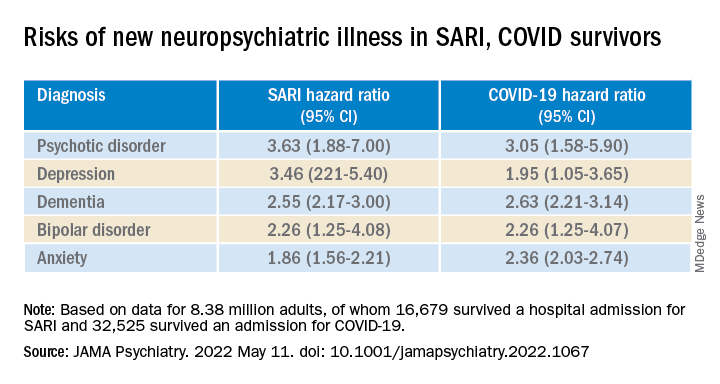
Compared with the wider population, survivors of severe SARI or COVID-19 were also at increased risk of starting treatment with antidepressants, hypnotics/anxiolytics, or antipsychotics.
When comparing survivors of SARI hospitalization to survivors of COVID-19 hospitalization, no significant differences were observed in the postdischarge rates of new-onset anxiety disorder, dementia, depression, or bipolar affective disorder.
The SARI and COVID groups also did not differ in terms of their postdischarge risks of antidepressant or hypnotic/anxiolytic use, but the COVID survivors had a 20% lower risk of starting an antipsychotic.
“In this cohort study, SARI were found to be associated with significant postacute neuropsychiatric morbidity, for which COVID-19 is not distinctly different,” Dr. Watkinson and colleagues write.
“These results may help refine our understanding of the post–severe COVID-19 phenotype and may inform post-discharge support for patients requiring hospital-based and intensive care for SARI regardless of causative pathogen,” they write.
Caveats, cautionary notes
Kevin McConway, PhD, emeritus professor of applied statistics at the Open University in Milton Keynes, England, described the study as “impressive.” However, he pointed out that the study’s observational design is a limitation.
“One can never be absolutely certain about the interpretation of findings of an observational study. What the research can’t tell us is what caused the increased psychiatric risks for people hospitalized with COVID-19 or some other serious respiratory disease,” Dr. McConway said.
“It can’t tell us what might happen in the future, when, we all hope, many fewer are being hospitalized with COVID-19 than was the case in those first two waves, and the current backlog of provision of some health services has decreased,” he added.
“So we can’t just say that, in general, serious COVID-19 has much the same neuropsychiatric consequences as other very serious respiratory illness. Maybe it does, maybe it doesn’t,” Dr. McConway cautioned.
Max Taquet, PhD, with the University of Oxford, noted that the study is limited to hospitalized adult patients, leaving open the question of risk in nonhospitalized individuals – which is the overwhelming majority of patients with COVID-19 – or in children.
Whether the neuropsychiatric risks have remained the same since the emergence of the Omicron variant also remains “an open question since all patients in this study were diagnosed before July 2021,” Dr. Taquet said in statement.
The study was funded by the Wellcome Trust, the John Fell Oxford University Press Research Fund, the Oxford Wellcome Institutional Strategic Support Fund and Cancer Research UK, through the Cancer Research UK Oxford Centre. Dr. Watkinson disclosed grants from the National Institute for Health Research and Sensyne Health outside the submitted work; and serving as chief medical officer for Sensyne Health prior to this work, as well as holding shares in the company. Dr. McConway is a trustee of the UK Science Media Centre and a member of its advisory committee. His comments were provided in his capacity as an independent professional statistician. Dr. Taquet has worked on similar studies trying to identify, quantify, and specify the neurological and psychiatric consequences of COVID-19.
A version of this article first appeared on Medscape.com.
The neuropsychiatric ramifications of severe COVID-19 infection appear to be no different than for other severe acute respiratory infections (SARI).
This suggests that disease severity, rather than pathogen, is the most relevant factor in new-onset neuropsychiatric illness, the investigators note.
The risk of new-onset neuropsychological illness after severe COVID-19 infection are “substantial, but similar to those after other severe respiratory infections,” study investigator Peter Watkinson, MD, Nuffield Department of Clinical Neurosciences, University of Oxford, and John Radcliffe Hospital, Oxford, England, told this news organization.
The study was published online in JAMA Psychiatry.
Significant mental health burden
Research has shown a significant burden of neuropsychological illness after severe COVID-19 infection. However, it’s unclear how this risk compares to SARI.
To investigate, Dr. Watkinson and colleagues evaluated electronic health record data on more than 8.3 million adults, including 16,679 (0.02%) who survived a hospital admission for SARI and 32,525 (0.03%) who survived a hospital stay for COVID-19.
Compared with the remaining population, risks of new anxiety disorder, dementia, psychotic disorder, depression, and bipolar disorder diagnoses were significantly and similarly increased in adults surviving hospitalization for either COVID-19 or SARI.
Compared with the wider population, survivors of severe SARI or COVID-19 were also at increased risk of starting treatment with antidepressants, hypnotics/anxiolytics, or antipsychotics.
When comparing survivors of SARI hospitalization to survivors of COVID-19 hospitalization, no significant differences were observed in the postdischarge rates of new-onset anxiety disorder, dementia, depression, or bipolar affective disorder.
The SARI and COVID groups also did not differ in terms of their postdischarge risks of antidepressant or hypnotic/anxiolytic use, but the COVID survivors had a 20% lower risk of starting an antipsychotic.
“In this cohort study, SARI were found to be associated with significant postacute neuropsychiatric morbidity, for which COVID-19 is not distinctly different,” Dr. Watkinson and colleagues write.
“These results may help refine our understanding of the post–severe COVID-19 phenotype and may inform post-discharge support for patients requiring hospital-based and intensive care for SARI regardless of causative pathogen,” they write.
Caveats, cautionary notes
Kevin McConway, PhD, emeritus professor of applied statistics at the Open University in Milton Keynes, England, described the study as “impressive.” However, he pointed out that the study’s observational design is a limitation.
“One can never be absolutely certain about the interpretation of findings of an observational study. What the research can’t tell us is what caused the increased psychiatric risks for people hospitalized with COVID-19 or some other serious respiratory disease,” Dr. McConway said.
“It can’t tell us what might happen in the future, when, we all hope, many fewer are being hospitalized with COVID-19 than was the case in those first two waves, and the current backlog of provision of some health services has decreased,” he added.
“So we can’t just say that, in general, serious COVID-19 has much the same neuropsychiatric consequences as other very serious respiratory illness. Maybe it does, maybe it doesn’t,” Dr. McConway cautioned.
Max Taquet, PhD, with the University of Oxford, noted that the study is limited to hospitalized adult patients, leaving open the question of risk in nonhospitalized individuals – which is the overwhelming majority of patients with COVID-19 – or in children.
Whether the neuropsychiatric risks have remained the same since the emergence of the Omicron variant also remains “an open question since all patients in this study were diagnosed before July 2021,” Dr. Taquet said in statement.
The study was funded by the Wellcome Trust, the John Fell Oxford University Press Research Fund, the Oxford Wellcome Institutional Strategic Support Fund and Cancer Research UK, through the Cancer Research UK Oxford Centre. Dr. Watkinson disclosed grants from the National Institute for Health Research and Sensyne Health outside the submitted work; and serving as chief medical officer for Sensyne Health prior to this work, as well as holding shares in the company. Dr. McConway is a trustee of the UK Science Media Centre and a member of its advisory committee. His comments were provided in his capacity as an independent professional statistician. Dr. Taquet has worked on similar studies trying to identify, quantify, and specify the neurological and psychiatric consequences of COVID-19.
A version of this article first appeared on Medscape.com.
The neuropsychiatric ramifications of severe COVID-19 infection appear to be no different than for other severe acute respiratory infections (SARI).
This suggests that disease severity, rather than pathogen, is the most relevant factor in new-onset neuropsychiatric illness, the investigators note.
The risk of new-onset neuropsychological illness after severe COVID-19 infection are “substantial, but similar to those after other severe respiratory infections,” study investigator Peter Watkinson, MD, Nuffield Department of Clinical Neurosciences, University of Oxford, and John Radcliffe Hospital, Oxford, England, told this news organization.
The study was published online in JAMA Psychiatry.
Significant mental health burden
Research has shown a significant burden of neuropsychological illness after severe COVID-19 infection. However, it’s unclear how this risk compares to SARI.
To investigate, Dr. Watkinson and colleagues evaluated electronic health record data on more than 8.3 million adults, including 16,679 (0.02%) who survived a hospital admission for SARI and 32,525 (0.03%) who survived a hospital stay for COVID-19.
Compared with the remaining population, risks of new anxiety disorder, dementia, psychotic disorder, depression, and bipolar disorder diagnoses were significantly and similarly increased in adults surviving hospitalization for either COVID-19 or SARI.
Compared with the wider population, survivors of severe SARI or COVID-19 were also at increased risk of starting treatment with antidepressants, hypnotics/anxiolytics, or antipsychotics.
When comparing survivors of SARI hospitalization to survivors of COVID-19 hospitalization, no significant differences were observed in the postdischarge rates of new-onset anxiety disorder, dementia, depression, or bipolar affective disorder.
The SARI and COVID groups also did not differ in terms of their postdischarge risks of antidepressant or hypnotic/anxiolytic use, but the COVID survivors had a 20% lower risk of starting an antipsychotic.
“In this cohort study, SARI were found to be associated with significant postacute neuropsychiatric morbidity, for which COVID-19 is not distinctly different,” Dr. Watkinson and colleagues write.
“These results may help refine our understanding of the post–severe COVID-19 phenotype and may inform post-discharge support for patients requiring hospital-based and intensive care for SARI regardless of causative pathogen,” they write.
Caveats, cautionary notes
Kevin McConway, PhD, emeritus professor of applied statistics at the Open University in Milton Keynes, England, described the study as “impressive.” However, he pointed out that the study’s observational design is a limitation.
“One can never be absolutely certain about the interpretation of findings of an observational study. What the research can’t tell us is what caused the increased psychiatric risks for people hospitalized with COVID-19 or some other serious respiratory disease,” Dr. McConway said.
“It can’t tell us what might happen in the future, when, we all hope, many fewer are being hospitalized with COVID-19 than was the case in those first two waves, and the current backlog of provision of some health services has decreased,” he added.
“So we can’t just say that, in general, serious COVID-19 has much the same neuropsychiatric consequences as other very serious respiratory illness. Maybe it does, maybe it doesn’t,” Dr. McConway cautioned.
Max Taquet, PhD, with the University of Oxford, noted that the study is limited to hospitalized adult patients, leaving open the question of risk in nonhospitalized individuals – which is the overwhelming majority of patients with COVID-19 – or in children.
Whether the neuropsychiatric risks have remained the same since the emergence of the Omicron variant also remains “an open question since all patients in this study were diagnosed before July 2021,” Dr. Taquet said in statement.
The study was funded by the Wellcome Trust, the John Fell Oxford University Press Research Fund, the Oxford Wellcome Institutional Strategic Support Fund and Cancer Research UK, through the Cancer Research UK Oxford Centre. Dr. Watkinson disclosed grants from the National Institute for Health Research and Sensyne Health outside the submitted work; and serving as chief medical officer for Sensyne Health prior to this work, as well as holding shares in the company. Dr. McConway is a trustee of the UK Science Media Centre and a member of its advisory committee. His comments were provided in his capacity as an independent professional statistician. Dr. Taquet has worked on similar studies trying to identify, quantify, and specify the neurological and psychiatric consequences of COVID-19.
A version of this article first appeared on Medscape.com.
Most COVID-19 survivors return to work within 2 years
The burden of persistent COVID-19 symptoms appeared to improve over time, but a higher percentage of former patients reported poor health, compared with the general population. This suggests that some patients need more time to completely recover from COVID-19, wrote the authors of the new study, which was published in The Lancet Respiratory Medicine. Previous research has shown that the health effects of COVID-19 last for up to a year, but data from longer-term studies are limited, said Lixue Huang, MD, of Capital Medical University, Beijing, one of the study authors, and colleagues.
Methods and results
In the new study, the researchers reviewed data from 1,192 adult patients who were discharged from the hospital after surviving COVID-19 between Jan. 7, 2020, and May 29, 2020. The researchers measured the participants’ health outcomes at 6 months, 12 months, and 2 years after their onset of symptoms. A community-based dataset of 3,383 adults with no history of COVID-19 served as controls to measure the recovery of the COVID-19 patients. The median age of the patients at the time of hospital discharge was 57 years, and 46% were women. The median follow-up time after the onset of symptoms was 185 days, 349 days, and 685 days for the 6-month, 12-month, and 2-year visits, respectively. The researchers measured health outcomes using a 6-min walking distance (6MWD) test, laboratory tests, and questionnaires about symptoms, mental health, health-related quality of life, returning to work, and health care use since leaving the hospital.
Overall, the proportion of COVID-19 survivors with at least one symptom decreased from 68% at 6 months to 55% at 2 years (P < .0001). The most frequent symptoms were fatigue and muscle weakness, reported by approximately one-third of the patients (31%); sleep problems also were reported by 31% of the patients.
The proportion of individuals with poor results on the 6MWD decreased continuously over time, not only in COVID-19 survivors overall, but also in three subgroups of varying initial disease severity. Of the 494 survivors who reported working before becoming ill, 438 (89%) had returned to their original jobs 2 years later. The most common reasons for not returning to work were decreased physical function, unwillingness to return, and unemployment, the researchers noted.
However, at 2 years, COVID-19 survivors reported more pain and discomfort, as well as more anxiety and depression, compared with the controls (23% vs. 5% and 12% vs. 5%, respectively).
In addition, significantly more survivors who needed high levels of respiratory support while hospitalized had lung diffusion impairment (65%), reduced residual volume (62%), and total lung capacity (39%), compared with matched controls (36%, 20%, and 6%, respectively) at 2 years.
Long-COVID concerns
Approximately half of the survivors had symptoms of long COVID at 2 years. These individuals were more likely to report pain or discomfort or anxiety or depression, as well as mobility problems, compared to survivors without long COVID. Participants with long-COVID symptoms were more than twice as likely to have an outpatient clinic visit (odds ratio, 2.82), and not quite twice as likely to be rehospitalized (OR, 1.64).
“We found that [health-related quality of life], exercise capacity, and mental health continued to improve throughout the 2 years regardless of initial disease severity, but about half still had symptomatic sequelae at 2 years,” the researchers wrote in their paper.
Findings can inform doctor-patient discussions
“We are increasingly recognizing that the health effects of COVID-19 may persist beyond acute illness, therefore this is a timely study to assess the long-term impact of COVID-19 with a long follow-up period,” said Suman Pal, MD, an internal medicine physician at the University of New Mexico, Albuquerque, in an interview.
The findings are consistent with the existing literature, said Dr. Pal, who was not involved in the study. The data from the study “can help clinicians have discussions regarding expected recovery and long-term prognosis for patients with COVID-19,” he noted.
What patients should know is that “studies such as this can help COVID-19 survivors understand and monitor persistent symptoms they may experience, and bring them to the attention of their clinicians,” said Dr. Pal.
However, “As a single-center study with high attrition of subjects during the study period, the findings may not be generalizable,” Dr. Pal emphasized. “Larger-scale studies and patient registries distributed over different geographical areas and time periods will help obtain a better understanding of the nature and prevalence of long COVID,” he said.
The study findings were limited by several factors, including the lack of formerly hospitalized controls with respiratory infections other than COVID-19 to determine which outcomes are COVID-19 specific, the researchers noted. Other limitations included the use of data from only patients at a single center, and from the early stages of the pandemic, as well as the use of self-reports for comorbidities and health outcomes, they said.
However, the results represent the longest-known published longitudinal follow-up of patients who recovered from acute COVID-19, the researchers emphasized. Study strengths included the large sample size, longitudinal design, and long-term follow-up with non-COVID controls to determine outcomes. The researchers noted their plans to conduct annual follow-ups in the current study population. They added that more research is needed to explore rehabilitation programs to promote recovery for COVID-19 survivors and to reduce the effects of long COVID.
The study was supported by the Chinese Academy of Medical Sciences, National Natural Science Foundation of China, National Key Research and Development Program of China, National Administration of Traditional Chinese Medicine, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, China Evergrande Group, Jack Ma Foundation, Sino Biopharmaceutical, Ping An Insurance (Group), and New Sunshine Charity Foundation. The researchers and Dr. Pal had no financial conflicts to disclose.
This article was updated on 5/16/2022.
The burden of persistent COVID-19 symptoms appeared to improve over time, but a higher percentage of former patients reported poor health, compared with the general population. This suggests that some patients need more time to completely recover from COVID-19, wrote the authors of the new study, which was published in The Lancet Respiratory Medicine. Previous research has shown that the health effects of COVID-19 last for up to a year, but data from longer-term studies are limited, said Lixue Huang, MD, of Capital Medical University, Beijing, one of the study authors, and colleagues.
Methods and results
In the new study, the researchers reviewed data from 1,192 adult patients who were discharged from the hospital after surviving COVID-19 between Jan. 7, 2020, and May 29, 2020. The researchers measured the participants’ health outcomes at 6 months, 12 months, and 2 years after their onset of symptoms. A community-based dataset of 3,383 adults with no history of COVID-19 served as controls to measure the recovery of the COVID-19 patients. The median age of the patients at the time of hospital discharge was 57 years, and 46% were women. The median follow-up time after the onset of symptoms was 185 days, 349 days, and 685 days for the 6-month, 12-month, and 2-year visits, respectively. The researchers measured health outcomes using a 6-min walking distance (6MWD) test, laboratory tests, and questionnaires about symptoms, mental health, health-related quality of life, returning to work, and health care use since leaving the hospital.
Overall, the proportion of COVID-19 survivors with at least one symptom decreased from 68% at 6 months to 55% at 2 years (P < .0001). The most frequent symptoms were fatigue and muscle weakness, reported by approximately one-third of the patients (31%); sleep problems also were reported by 31% of the patients.
The proportion of individuals with poor results on the 6MWD decreased continuously over time, not only in COVID-19 survivors overall, but also in three subgroups of varying initial disease severity. Of the 494 survivors who reported working before becoming ill, 438 (89%) had returned to their original jobs 2 years later. The most common reasons for not returning to work were decreased physical function, unwillingness to return, and unemployment, the researchers noted.
However, at 2 years, COVID-19 survivors reported more pain and discomfort, as well as more anxiety and depression, compared with the controls (23% vs. 5% and 12% vs. 5%, respectively).
In addition, significantly more survivors who needed high levels of respiratory support while hospitalized had lung diffusion impairment (65%), reduced residual volume (62%), and total lung capacity (39%), compared with matched controls (36%, 20%, and 6%, respectively) at 2 years.
Long-COVID concerns
Approximately half of the survivors had symptoms of long COVID at 2 years. These individuals were more likely to report pain or discomfort or anxiety or depression, as well as mobility problems, compared to survivors without long COVID. Participants with long-COVID symptoms were more than twice as likely to have an outpatient clinic visit (odds ratio, 2.82), and not quite twice as likely to be rehospitalized (OR, 1.64).
“We found that [health-related quality of life], exercise capacity, and mental health continued to improve throughout the 2 years regardless of initial disease severity, but about half still had symptomatic sequelae at 2 years,” the researchers wrote in their paper.
Findings can inform doctor-patient discussions
“We are increasingly recognizing that the health effects of COVID-19 may persist beyond acute illness, therefore this is a timely study to assess the long-term impact of COVID-19 with a long follow-up period,” said Suman Pal, MD, an internal medicine physician at the University of New Mexico, Albuquerque, in an interview.
The findings are consistent with the existing literature, said Dr. Pal, who was not involved in the study. The data from the study “can help clinicians have discussions regarding expected recovery and long-term prognosis for patients with COVID-19,” he noted.
What patients should know is that “studies such as this can help COVID-19 survivors understand and monitor persistent symptoms they may experience, and bring them to the attention of their clinicians,” said Dr. Pal.
However, “As a single-center study with high attrition of subjects during the study period, the findings may not be generalizable,” Dr. Pal emphasized. “Larger-scale studies and patient registries distributed over different geographical areas and time periods will help obtain a better understanding of the nature and prevalence of long COVID,” he said.
The study findings were limited by several factors, including the lack of formerly hospitalized controls with respiratory infections other than COVID-19 to determine which outcomes are COVID-19 specific, the researchers noted. Other limitations included the use of data from only patients at a single center, and from the early stages of the pandemic, as well as the use of self-reports for comorbidities and health outcomes, they said.
However, the results represent the longest-known published longitudinal follow-up of patients who recovered from acute COVID-19, the researchers emphasized. Study strengths included the large sample size, longitudinal design, and long-term follow-up with non-COVID controls to determine outcomes. The researchers noted their plans to conduct annual follow-ups in the current study population. They added that more research is needed to explore rehabilitation programs to promote recovery for COVID-19 survivors and to reduce the effects of long COVID.
The study was supported by the Chinese Academy of Medical Sciences, National Natural Science Foundation of China, National Key Research and Development Program of China, National Administration of Traditional Chinese Medicine, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, China Evergrande Group, Jack Ma Foundation, Sino Biopharmaceutical, Ping An Insurance (Group), and New Sunshine Charity Foundation. The researchers and Dr. Pal had no financial conflicts to disclose.
This article was updated on 5/16/2022.
The burden of persistent COVID-19 symptoms appeared to improve over time, but a higher percentage of former patients reported poor health, compared with the general population. This suggests that some patients need more time to completely recover from COVID-19, wrote the authors of the new study, which was published in The Lancet Respiratory Medicine. Previous research has shown that the health effects of COVID-19 last for up to a year, but data from longer-term studies are limited, said Lixue Huang, MD, of Capital Medical University, Beijing, one of the study authors, and colleagues.
Methods and results
In the new study, the researchers reviewed data from 1,192 adult patients who were discharged from the hospital after surviving COVID-19 between Jan. 7, 2020, and May 29, 2020. The researchers measured the participants’ health outcomes at 6 months, 12 months, and 2 years after their onset of symptoms. A community-based dataset of 3,383 adults with no history of COVID-19 served as controls to measure the recovery of the COVID-19 patients. The median age of the patients at the time of hospital discharge was 57 years, and 46% were women. The median follow-up time after the onset of symptoms was 185 days, 349 days, and 685 days for the 6-month, 12-month, and 2-year visits, respectively. The researchers measured health outcomes using a 6-min walking distance (6MWD) test, laboratory tests, and questionnaires about symptoms, mental health, health-related quality of life, returning to work, and health care use since leaving the hospital.
Overall, the proportion of COVID-19 survivors with at least one symptom decreased from 68% at 6 months to 55% at 2 years (P < .0001). The most frequent symptoms were fatigue and muscle weakness, reported by approximately one-third of the patients (31%); sleep problems also were reported by 31% of the patients.
The proportion of individuals with poor results on the 6MWD decreased continuously over time, not only in COVID-19 survivors overall, but also in three subgroups of varying initial disease severity. Of the 494 survivors who reported working before becoming ill, 438 (89%) had returned to their original jobs 2 years later. The most common reasons for not returning to work were decreased physical function, unwillingness to return, and unemployment, the researchers noted.
However, at 2 years, COVID-19 survivors reported more pain and discomfort, as well as more anxiety and depression, compared with the controls (23% vs. 5% and 12% vs. 5%, respectively).
In addition, significantly more survivors who needed high levels of respiratory support while hospitalized had lung diffusion impairment (65%), reduced residual volume (62%), and total lung capacity (39%), compared with matched controls (36%, 20%, and 6%, respectively) at 2 years.
Long-COVID concerns
Approximately half of the survivors had symptoms of long COVID at 2 years. These individuals were more likely to report pain or discomfort or anxiety or depression, as well as mobility problems, compared to survivors without long COVID. Participants with long-COVID symptoms were more than twice as likely to have an outpatient clinic visit (odds ratio, 2.82), and not quite twice as likely to be rehospitalized (OR, 1.64).
“We found that [health-related quality of life], exercise capacity, and mental health continued to improve throughout the 2 years regardless of initial disease severity, but about half still had symptomatic sequelae at 2 years,” the researchers wrote in their paper.
Findings can inform doctor-patient discussions
“We are increasingly recognizing that the health effects of COVID-19 may persist beyond acute illness, therefore this is a timely study to assess the long-term impact of COVID-19 with a long follow-up period,” said Suman Pal, MD, an internal medicine physician at the University of New Mexico, Albuquerque, in an interview.
The findings are consistent with the existing literature, said Dr. Pal, who was not involved in the study. The data from the study “can help clinicians have discussions regarding expected recovery and long-term prognosis for patients with COVID-19,” he noted.
What patients should know is that “studies such as this can help COVID-19 survivors understand and monitor persistent symptoms they may experience, and bring them to the attention of their clinicians,” said Dr. Pal.
However, “As a single-center study with high attrition of subjects during the study period, the findings may not be generalizable,” Dr. Pal emphasized. “Larger-scale studies and patient registries distributed over different geographical areas and time periods will help obtain a better understanding of the nature and prevalence of long COVID,” he said.
The study findings were limited by several factors, including the lack of formerly hospitalized controls with respiratory infections other than COVID-19 to determine which outcomes are COVID-19 specific, the researchers noted. Other limitations included the use of data from only patients at a single center, and from the early stages of the pandemic, as well as the use of self-reports for comorbidities and health outcomes, they said.
However, the results represent the longest-known published longitudinal follow-up of patients who recovered from acute COVID-19, the researchers emphasized. Study strengths included the large sample size, longitudinal design, and long-term follow-up with non-COVID controls to determine outcomes. The researchers noted their plans to conduct annual follow-ups in the current study population. They added that more research is needed to explore rehabilitation programs to promote recovery for COVID-19 survivors and to reduce the effects of long COVID.
The study was supported by the Chinese Academy of Medical Sciences, National Natural Science Foundation of China, National Key Research and Development Program of China, National Administration of Traditional Chinese Medicine, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, China Evergrande Group, Jack Ma Foundation, Sino Biopharmaceutical, Ping An Insurance (Group), and New Sunshine Charity Foundation. The researchers and Dr. Pal had no financial conflicts to disclose.
This article was updated on 5/16/2022.
FROM THE LANCET RESPIRATORY MEDICINE
COVID-19 patients remain sedentary after hospital discharge
After hospitalization, COVID-19 patients 9 hours per day of sedentary time at 3-6 months after discharge, according to data from 37 individuals.
COVID-19 patients experience a wide range of clinical manifestations, and roughly half of those who were hospitalized for COVID-19 report persisting symptoms both physical and mental up to a year after discharge, Bram van Bakel, MD, of Radboud University Medical Center, Nijmegen, the Netherlands, said in a presentation at the presentation at the annual congress of the European Association of Preventive Cardiology.
However, data on physical activity patterns and the impact on recovery after postinfection hospital discharge are limited, he said. Dr. van Bakel and colleagues aimed to assess physical activity, sedentary behavior, and sleep duration in COVID-19 patients at 3-6 months after hospital discharge to explore the association with patient characteristics, disease severity and cardiac dysfunction.
“We hypothesized that COVID-19 survivors will demonstrate low volumes of physical activity and a high sedentary time, especially those with a more severe disease course,” such as longer hospital duration and admission to intensive care, cardiac dysfunction, and persistent symptoms at 3-6 months post discharge, he said.
Dr. van Bakel and colleagues enrolled 37 adult patients in a cross-sectional cohort study. They objectively assessed physical activity, sedentary behavior, and sleep duration for 24 hrs/day during 8 subsequent days in COVID-19 survivors at 3-6 months post hospitalization. The average age of the patients was 60 years, 78% were male, and the average assessment time was 125 days after hospital discharge.
The researchers compared activity patterns based on patient and disease characteristics, cardiac biomarker release during hospitalization, abnormal transthoracic echocardiogram regarding left and right ventricular function and volumes at 3-6 months of follow-up, and the persistence of symptoms after discharge.
Overall, patients spent a median of 4.2 hours per day in light-intensity physical activity, and 1 hour per day in moderate to vigorous physical activity. The overall median time spent sitting was 9.8 hours per day; this was accumulated in approximately 6 prolonged sitting periods of 30 minutes or more and 41.1 short sitting periods of less than 30 minutes.
The median sleep duration was 9.8 hours per day; sleep duration was significantly higher in women, compared with men (9.2 vs. 8.5 hours/day; P = .03), and in patients with persistent symptoms, compared with those without persistent symptoms (9.1 hrs/day vs. 8.3 hrs/day; P = .02). No other differences in activity or sitting patterns appeared among subgroups. Sedentary time of 10 hours or more per day overall puts individuals at increased risk for detrimental health effects, Dr. van Bakel said.
The study findings were limited by the small sample and cross-sectional design, he noted.
However, the results suggest that COVID-19 patients spent most of their time sedentary within the first 3-6 months after hospital discharge. The similar activity patterns across subgroups support a uniform approach to rehabilitation for these patients to target persisting symptoms and prevent long-term health consequences, said Dr. van Bakel. Further studies are warranted in a larger cohort with a prospective design and longitudinal follow-up.
The current study “highlights the need for ongoing rehabilitation in severe COVID-19 survivors after hospitalization to restore premorbid function and endurance,” Alba Miranda Azola, MD, of Johns Hopkins University, Baltimore, said in an interview.
“The findings regarding inactivity are not surprising,” said Dr. Azola. “Immobility during hospitalization results in muscle atrophy and marked decreased endurance. The need for prolonged use of sedation and paralytics during intensive care stays of severe COVID-19 patients is associated with critical illness myopathy. Also, many patients continue to experience hypoxia and dyspnea on exertion for several months after leaving the hospital. The functional impairments and limited activity tolerance often preclude patients from engaging on outpatient rehabilitation programs.
“I do think it surprising that the level of inactivity observed was independent of disease severity and patient factors, but it definitely speaks to the importance of establishing post hospitalization follow-up care that focuses on restoring function and mobility,” Dr. Azola noted.
The study findings may have long-term clinical implications, as COVID-19 survivors who experience functional decline that limits activity and who continue to lead a sedentary lifestyle may be at increased risk for health issues such as heart disease and type 2 diabetes, Dr. Azola said.
Rigorous research is needed to study the functional and health impact of rehabilitation interventions during and after hospitalization, she emphasized. “Additionally, studies are needed on innovative rehabilitation interventions that improve accessibility to services to patients.”
The study received no outside funding. The researchers and Dr. Azola had no financial conflicts to disclose. Dr. Azola had no financial conflicts to disclose.
After hospitalization, COVID-19 patients 9 hours per day of sedentary time at 3-6 months after discharge, according to data from 37 individuals.
COVID-19 patients experience a wide range of clinical manifestations, and roughly half of those who were hospitalized for COVID-19 report persisting symptoms both physical and mental up to a year after discharge, Bram van Bakel, MD, of Radboud University Medical Center, Nijmegen, the Netherlands, said in a presentation at the presentation at the annual congress of the European Association of Preventive Cardiology.
However, data on physical activity patterns and the impact on recovery after postinfection hospital discharge are limited, he said. Dr. van Bakel and colleagues aimed to assess physical activity, sedentary behavior, and sleep duration in COVID-19 patients at 3-6 months after hospital discharge to explore the association with patient characteristics, disease severity and cardiac dysfunction.
“We hypothesized that COVID-19 survivors will demonstrate low volumes of physical activity and a high sedentary time, especially those with a more severe disease course,” such as longer hospital duration and admission to intensive care, cardiac dysfunction, and persistent symptoms at 3-6 months post discharge, he said.
Dr. van Bakel and colleagues enrolled 37 adult patients in a cross-sectional cohort study. They objectively assessed physical activity, sedentary behavior, and sleep duration for 24 hrs/day during 8 subsequent days in COVID-19 survivors at 3-6 months post hospitalization. The average age of the patients was 60 years, 78% were male, and the average assessment time was 125 days after hospital discharge.
The researchers compared activity patterns based on patient and disease characteristics, cardiac biomarker release during hospitalization, abnormal transthoracic echocardiogram regarding left and right ventricular function and volumes at 3-6 months of follow-up, and the persistence of symptoms after discharge.
Overall, patients spent a median of 4.2 hours per day in light-intensity physical activity, and 1 hour per day in moderate to vigorous physical activity. The overall median time spent sitting was 9.8 hours per day; this was accumulated in approximately 6 prolonged sitting periods of 30 minutes or more and 41.1 short sitting periods of less than 30 minutes.
The median sleep duration was 9.8 hours per day; sleep duration was significantly higher in women, compared with men (9.2 vs. 8.5 hours/day; P = .03), and in patients with persistent symptoms, compared with those without persistent symptoms (9.1 hrs/day vs. 8.3 hrs/day; P = .02). No other differences in activity or sitting patterns appeared among subgroups. Sedentary time of 10 hours or more per day overall puts individuals at increased risk for detrimental health effects, Dr. van Bakel said.
The study findings were limited by the small sample and cross-sectional design, he noted.
However, the results suggest that COVID-19 patients spent most of their time sedentary within the first 3-6 months after hospital discharge. The similar activity patterns across subgroups support a uniform approach to rehabilitation for these patients to target persisting symptoms and prevent long-term health consequences, said Dr. van Bakel. Further studies are warranted in a larger cohort with a prospective design and longitudinal follow-up.
The current study “highlights the need for ongoing rehabilitation in severe COVID-19 survivors after hospitalization to restore premorbid function and endurance,” Alba Miranda Azola, MD, of Johns Hopkins University, Baltimore, said in an interview.
“The findings regarding inactivity are not surprising,” said Dr. Azola. “Immobility during hospitalization results in muscle atrophy and marked decreased endurance. The need for prolonged use of sedation and paralytics during intensive care stays of severe COVID-19 patients is associated with critical illness myopathy. Also, many patients continue to experience hypoxia and dyspnea on exertion for several months after leaving the hospital. The functional impairments and limited activity tolerance often preclude patients from engaging on outpatient rehabilitation programs.
“I do think it surprising that the level of inactivity observed was independent of disease severity and patient factors, but it definitely speaks to the importance of establishing post hospitalization follow-up care that focuses on restoring function and mobility,” Dr. Azola noted.
The study findings may have long-term clinical implications, as COVID-19 survivors who experience functional decline that limits activity and who continue to lead a sedentary lifestyle may be at increased risk for health issues such as heart disease and type 2 diabetes, Dr. Azola said.
Rigorous research is needed to study the functional and health impact of rehabilitation interventions during and after hospitalization, she emphasized. “Additionally, studies are needed on innovative rehabilitation interventions that improve accessibility to services to patients.”
The study received no outside funding. The researchers and Dr. Azola had no financial conflicts to disclose. Dr. Azola had no financial conflicts to disclose.
After hospitalization, COVID-19 patients 9 hours per day of sedentary time at 3-6 months after discharge, according to data from 37 individuals.
COVID-19 patients experience a wide range of clinical manifestations, and roughly half of those who were hospitalized for COVID-19 report persisting symptoms both physical and mental up to a year after discharge, Bram van Bakel, MD, of Radboud University Medical Center, Nijmegen, the Netherlands, said in a presentation at the presentation at the annual congress of the European Association of Preventive Cardiology.
However, data on physical activity patterns and the impact on recovery after postinfection hospital discharge are limited, he said. Dr. van Bakel and colleagues aimed to assess physical activity, sedentary behavior, and sleep duration in COVID-19 patients at 3-6 months after hospital discharge to explore the association with patient characteristics, disease severity and cardiac dysfunction.
“We hypothesized that COVID-19 survivors will demonstrate low volumes of physical activity and a high sedentary time, especially those with a more severe disease course,” such as longer hospital duration and admission to intensive care, cardiac dysfunction, and persistent symptoms at 3-6 months post discharge, he said.
Dr. van Bakel and colleagues enrolled 37 adult patients in a cross-sectional cohort study. They objectively assessed physical activity, sedentary behavior, and sleep duration for 24 hrs/day during 8 subsequent days in COVID-19 survivors at 3-6 months post hospitalization. The average age of the patients was 60 years, 78% were male, and the average assessment time was 125 days after hospital discharge.
The researchers compared activity patterns based on patient and disease characteristics, cardiac biomarker release during hospitalization, abnormal transthoracic echocardiogram regarding left and right ventricular function and volumes at 3-6 months of follow-up, and the persistence of symptoms after discharge.
Overall, patients spent a median of 4.2 hours per day in light-intensity physical activity, and 1 hour per day in moderate to vigorous physical activity. The overall median time spent sitting was 9.8 hours per day; this was accumulated in approximately 6 prolonged sitting periods of 30 minutes or more and 41.1 short sitting periods of less than 30 minutes.
The median sleep duration was 9.8 hours per day; sleep duration was significantly higher in women, compared with men (9.2 vs. 8.5 hours/day; P = .03), and in patients with persistent symptoms, compared with those without persistent symptoms (9.1 hrs/day vs. 8.3 hrs/day; P = .02). No other differences in activity or sitting patterns appeared among subgroups. Sedentary time of 10 hours or more per day overall puts individuals at increased risk for detrimental health effects, Dr. van Bakel said.
The study findings were limited by the small sample and cross-sectional design, he noted.
However, the results suggest that COVID-19 patients spent most of their time sedentary within the first 3-6 months after hospital discharge. The similar activity patterns across subgroups support a uniform approach to rehabilitation for these patients to target persisting symptoms and prevent long-term health consequences, said Dr. van Bakel. Further studies are warranted in a larger cohort with a prospective design and longitudinal follow-up.
The current study “highlights the need for ongoing rehabilitation in severe COVID-19 survivors after hospitalization to restore premorbid function and endurance,” Alba Miranda Azola, MD, of Johns Hopkins University, Baltimore, said in an interview.
“The findings regarding inactivity are not surprising,” said Dr. Azola. “Immobility during hospitalization results in muscle atrophy and marked decreased endurance. The need for prolonged use of sedation and paralytics during intensive care stays of severe COVID-19 patients is associated with critical illness myopathy. Also, many patients continue to experience hypoxia and dyspnea on exertion for several months after leaving the hospital. The functional impairments and limited activity tolerance often preclude patients from engaging on outpatient rehabilitation programs.
“I do think it surprising that the level of inactivity observed was independent of disease severity and patient factors, but it definitely speaks to the importance of establishing post hospitalization follow-up care that focuses on restoring function and mobility,” Dr. Azola noted.
The study findings may have long-term clinical implications, as COVID-19 survivors who experience functional decline that limits activity and who continue to lead a sedentary lifestyle may be at increased risk for health issues such as heart disease and type 2 diabetes, Dr. Azola said.
Rigorous research is needed to study the functional and health impact of rehabilitation interventions during and after hospitalization, she emphasized. “Additionally, studies are needed on innovative rehabilitation interventions that improve accessibility to services to patients.”
The study received no outside funding. The researchers and Dr. Azola had no financial conflicts to disclose. Dr. Azola had no financial conflicts to disclose.
FROM ESC PREVENTIVE CARDIOLOGY 2022
SARS-CoV-2 stays in GI tract long after it clears the lungs
New data present further evidence that SARS-CoV-2 infection can settle in the gastrointestinal tract and that it can persist long after the infection has cleared the lungs.
Infection of the GI tract may figure prominently in long COVID, the study authors suggested.
Led by Aravind Natarajan, PhD, with the departments of genetics and medicine at Stanford (Calif.) University, they analyzed fecal RNA shedding up to 10 months after a COVID-19 diagnosis in 673 stool samples from 113 patients with mild to moderate disease.
They found that, in the week after diagnosis, COVID RNA remnants were present in the stool of approximately half (49.2%) of the patients. Seven months later, about 4% of them shed fecal viral RNA.
The authors noted that there was no ongoing SARS-CoV-2 RNA shedding in respiratory samples of patients at the 4-month mark.
Using self-reported symptoms regularly collected by questionnaire, they also found a correlation of long-term fecal shedding of SARS-CoV-2 RNA with abdominal pain, nausea, and vomiting.
The findings were published online in Med.
Implications of long-term viral shedding
Previous studies have found SARS-CoV-2 RNA in respiratory and fecal samples and have documented viral replication in lung and intestinal tissue. But before the current study, little had been known about long-term shedding, especially in those who have mild COVID. Most studies of viral shedding have been with severe COVID cases.
The authors noted that most studies of this kind are cross-sectional. The few other longitudinal studies have focused on early time points just after diagnosis.
Senior author Ami S. Bhatt, MD, associate professor in the departments of medicine and hematology at Stanford University, said in an interview that, though the viral genetic material in the feces lingers, on the basis of available evidence, it is highly unlikely to be contagious in most cases.
She said that understanding the dynamics of fecal shedding of SARS-CoV-2 genetic material will help interpret wastewater-based studies that are trying to determine population prevalence of the virus.
“While we don’t know the exact clinical importance of the longer-term shedding of SARS-CoV-2 in individuals with COVID-19, some have speculated that those who have long-term shedding of SARS-CoV-2 may have ongoing infections that might benefit from treatment,” she said.
“Our data support the idea that the long-term GI-related symptoms in some people might be the consequence of an ongoing infection in the GI tract, even after the respiratory infection has cleared,” Dr. Bhatt said.
“Alternatively, the presence of ongoing viral genetic material in the gut might be a trigger for the immune system to continually be active against the virus, and our immune system reaction may be the reason for long COVID–type symptoms,” she added. “This area is ripe for additional studies.”
Dr. Bhatt and colleagues will continue studying viral shedding in fecal samples as part of the nationwide RECOVER Initiative.
When reached for comment, David A. Johnson, MD, professor of medicine and chief of gastroenterology, Eastern Virginia Medical School, Norfolk, said in an interview that previous studies have indicated that the virus may be detected in the stool for a month or more and for about 2 weeks on average. Whether the virus is infectious has been in question.
But it’s not so much that the virus is infectious in the GI tract and causing symptoms, he said. Rather, there are biomic changes related to COVID, including a loss of diversity in the gut bacteria, which disrupts the balance.
“This may actually in some way predispose some patients to impaired clearance of their symptoms,” Dr. Johnson explained. “There seems to be a growing recognition that this entity called long-haul COVID may be related to specific bacterial disruptions, and the more rapidly you can resolve these disruptions, the less likely you are to continue with long-haul symptoms.”
He said that, among people who have mild COVID, the virus typically clears and gut bacteria return to normal. With severe or persistent illness, gut dysbiosis persists, he said.
“People need to be aware that the GI tract is involved in a sizable percent of patients with COVID,” Dr. Johnson said. “The GI-tract testing may reflect that the virus is there, but persistence of the detectable test positivity is very unlikely to reflect active virus.”
The authors noted that they collected only six samples from the participants over the 10-month study period.
“Follow-up studies with more frequent sampling, especially in the first 2 months after diagnosis, may help build a more nuanced model of decline of fecal viral RNA concentration over time,” they wrote.
The study was supported by a Stanford ChemH-IMA grant, fellowships from the AACR and the National Science Foundation, and the National Institutes of Health. The authors and Dr. Johnson reported no relevant financial relationships. Dr. Johnson is a regular contributor to this news organization.
A version of this article first appeared on Medscape.com.
New data present further evidence that SARS-CoV-2 infection can settle in the gastrointestinal tract and that it can persist long after the infection has cleared the lungs.
Infection of the GI tract may figure prominently in long COVID, the study authors suggested.
Led by Aravind Natarajan, PhD, with the departments of genetics and medicine at Stanford (Calif.) University, they analyzed fecal RNA shedding up to 10 months after a COVID-19 diagnosis in 673 stool samples from 113 patients with mild to moderate disease.
They found that, in the week after diagnosis, COVID RNA remnants were present in the stool of approximately half (49.2%) of the patients. Seven months later, about 4% of them shed fecal viral RNA.
The authors noted that there was no ongoing SARS-CoV-2 RNA shedding in respiratory samples of patients at the 4-month mark.
Using self-reported symptoms regularly collected by questionnaire, they also found a correlation of long-term fecal shedding of SARS-CoV-2 RNA with abdominal pain, nausea, and vomiting.
The findings were published online in Med.
Implications of long-term viral shedding
Previous studies have found SARS-CoV-2 RNA in respiratory and fecal samples and have documented viral replication in lung and intestinal tissue. But before the current study, little had been known about long-term shedding, especially in those who have mild COVID. Most studies of viral shedding have been with severe COVID cases.
The authors noted that most studies of this kind are cross-sectional. The few other longitudinal studies have focused on early time points just after diagnosis.
Senior author Ami S. Bhatt, MD, associate professor in the departments of medicine and hematology at Stanford University, said in an interview that, though the viral genetic material in the feces lingers, on the basis of available evidence, it is highly unlikely to be contagious in most cases.
She said that understanding the dynamics of fecal shedding of SARS-CoV-2 genetic material will help interpret wastewater-based studies that are trying to determine population prevalence of the virus.
“While we don’t know the exact clinical importance of the longer-term shedding of SARS-CoV-2 in individuals with COVID-19, some have speculated that those who have long-term shedding of SARS-CoV-2 may have ongoing infections that might benefit from treatment,” she said.
“Our data support the idea that the long-term GI-related symptoms in some people might be the consequence of an ongoing infection in the GI tract, even after the respiratory infection has cleared,” Dr. Bhatt said.
“Alternatively, the presence of ongoing viral genetic material in the gut might be a trigger for the immune system to continually be active against the virus, and our immune system reaction may be the reason for long COVID–type symptoms,” she added. “This area is ripe for additional studies.”
Dr. Bhatt and colleagues will continue studying viral shedding in fecal samples as part of the nationwide RECOVER Initiative.
When reached for comment, David A. Johnson, MD, professor of medicine and chief of gastroenterology, Eastern Virginia Medical School, Norfolk, said in an interview that previous studies have indicated that the virus may be detected in the stool for a month or more and for about 2 weeks on average. Whether the virus is infectious has been in question.
But it’s not so much that the virus is infectious in the GI tract and causing symptoms, he said. Rather, there are biomic changes related to COVID, including a loss of diversity in the gut bacteria, which disrupts the balance.
“This may actually in some way predispose some patients to impaired clearance of their symptoms,” Dr. Johnson explained. “There seems to be a growing recognition that this entity called long-haul COVID may be related to specific bacterial disruptions, and the more rapidly you can resolve these disruptions, the less likely you are to continue with long-haul symptoms.”
He said that, among people who have mild COVID, the virus typically clears and gut bacteria return to normal. With severe or persistent illness, gut dysbiosis persists, he said.
“People need to be aware that the GI tract is involved in a sizable percent of patients with COVID,” Dr. Johnson said. “The GI-tract testing may reflect that the virus is there, but persistence of the detectable test positivity is very unlikely to reflect active virus.”
The authors noted that they collected only six samples from the participants over the 10-month study period.
“Follow-up studies with more frequent sampling, especially in the first 2 months after diagnosis, may help build a more nuanced model of decline of fecal viral RNA concentration over time,” they wrote.
The study was supported by a Stanford ChemH-IMA grant, fellowships from the AACR and the National Science Foundation, and the National Institutes of Health. The authors and Dr. Johnson reported no relevant financial relationships. Dr. Johnson is a regular contributor to this news organization.
A version of this article first appeared on Medscape.com.
New data present further evidence that SARS-CoV-2 infection can settle in the gastrointestinal tract and that it can persist long after the infection has cleared the lungs.
Infection of the GI tract may figure prominently in long COVID, the study authors suggested.
Led by Aravind Natarajan, PhD, with the departments of genetics and medicine at Stanford (Calif.) University, they analyzed fecal RNA shedding up to 10 months after a COVID-19 diagnosis in 673 stool samples from 113 patients with mild to moderate disease.
They found that, in the week after diagnosis, COVID RNA remnants were present in the stool of approximately half (49.2%) of the patients. Seven months later, about 4% of them shed fecal viral RNA.
The authors noted that there was no ongoing SARS-CoV-2 RNA shedding in respiratory samples of patients at the 4-month mark.
Using self-reported symptoms regularly collected by questionnaire, they also found a correlation of long-term fecal shedding of SARS-CoV-2 RNA with abdominal pain, nausea, and vomiting.
The findings were published online in Med.
Implications of long-term viral shedding
Previous studies have found SARS-CoV-2 RNA in respiratory and fecal samples and have documented viral replication in lung and intestinal tissue. But before the current study, little had been known about long-term shedding, especially in those who have mild COVID. Most studies of viral shedding have been with severe COVID cases.
The authors noted that most studies of this kind are cross-sectional. The few other longitudinal studies have focused on early time points just after diagnosis.
Senior author Ami S. Bhatt, MD, associate professor in the departments of medicine and hematology at Stanford University, said in an interview that, though the viral genetic material in the feces lingers, on the basis of available evidence, it is highly unlikely to be contagious in most cases.
She said that understanding the dynamics of fecal shedding of SARS-CoV-2 genetic material will help interpret wastewater-based studies that are trying to determine population prevalence of the virus.
“While we don’t know the exact clinical importance of the longer-term shedding of SARS-CoV-2 in individuals with COVID-19, some have speculated that those who have long-term shedding of SARS-CoV-2 may have ongoing infections that might benefit from treatment,” she said.
“Our data support the idea that the long-term GI-related symptoms in some people might be the consequence of an ongoing infection in the GI tract, even after the respiratory infection has cleared,” Dr. Bhatt said.
“Alternatively, the presence of ongoing viral genetic material in the gut might be a trigger for the immune system to continually be active against the virus, and our immune system reaction may be the reason for long COVID–type symptoms,” she added. “This area is ripe for additional studies.”
Dr. Bhatt and colleagues will continue studying viral shedding in fecal samples as part of the nationwide RECOVER Initiative.
When reached for comment, David A. Johnson, MD, professor of medicine and chief of gastroenterology, Eastern Virginia Medical School, Norfolk, said in an interview that previous studies have indicated that the virus may be detected in the stool for a month or more and for about 2 weeks on average. Whether the virus is infectious has been in question.
But it’s not so much that the virus is infectious in the GI tract and causing symptoms, he said. Rather, there are biomic changes related to COVID, including a loss of diversity in the gut bacteria, which disrupts the balance.
“This may actually in some way predispose some patients to impaired clearance of their symptoms,” Dr. Johnson explained. “There seems to be a growing recognition that this entity called long-haul COVID may be related to specific bacterial disruptions, and the more rapidly you can resolve these disruptions, the less likely you are to continue with long-haul symptoms.”
He said that, among people who have mild COVID, the virus typically clears and gut bacteria return to normal. With severe or persistent illness, gut dysbiosis persists, he said.
“People need to be aware that the GI tract is involved in a sizable percent of patients with COVID,” Dr. Johnson said. “The GI-tract testing may reflect that the virus is there, but persistence of the detectable test positivity is very unlikely to reflect active virus.”
The authors noted that they collected only six samples from the participants over the 10-month study period.
“Follow-up studies with more frequent sampling, especially in the first 2 months after diagnosis, may help build a more nuanced model of decline of fecal viral RNA concentration over time,” they wrote.
The study was supported by a Stanford ChemH-IMA grant, fellowships from the AACR and the National Science Foundation, and the National Institutes of Health. The authors and Dr. Johnson reported no relevant financial relationships. Dr. Johnson is a regular contributor to this news organization.
A version of this article first appeared on Medscape.com.
FROM MED






