User login
CAR T-cell therapy demonstrates efficacy in mice with MM
WASHINGTON, DC—A chimeric antigen receptor (CAR) T-cell therapy is active against multiple myeloma (MM), according to preclinical research.
The therapy, known as P-BCMA-101, demonstrated persistent anti-tumor activity in a mouse model of MM.
Treatment with P-BCMA-101 eliminated tumors after relapse and prolonged survival in the mice, when compared to other CAR-T cell therapies.
These results were presented in a poster at the AACR Annual Meeting 2017 (abstract 3759).
The research was conducted by employees of Poseida Therapeutics Inc., the company developing P-BCMA-101, as well as others.
About P-BCMA-101
The researchers explained that P-BCMA-101 employs a B-cell maturation antigen (BCMA)-specific Centyrin™ rather than a single-chain variable fragment (scFv) for antigen detection.
Centyrins™ are fully human and have similar binding affinities as scFvs. However, Centyrins are smaller, more thermostable, and predicted to be less immunogenic.
P-BCMA-101 is engineered using the PiggyBac™ DNA modification system. PiggyBac eliminates the need to use lentivirus or gamma-retrovirus as a gene delivery mechanism, resulting in improved manufacturing and cost savings.
In addition, the increased cargo capacity of PiggyBac allows for the incorporation of a safety switch and a selectable gene. The safety switch can be “flipped” to enable depletion in case adverse events occur. And the selectable gene allows for enrichment of CARTyrin+ cells using a non-genotoxic drug.
Findings
The researchers found that more than 70% of P-BCMA-101 cells possessed a stem cell memory phenotype, creating a significant population of self-renewing, multipotent progenitors capable of reconstituting the entire spectrum of memory and effector T-cell subsets required to prevent cancer relapse. Similar competitor products typically report 0% to 20% stem cell memory phenotype.
In addition, P-BCMA-101 was enriched with more than 95% of T cells successfully modified, which compares favorably to the roughly 30% to 50% commonly expected with clinical manufacture using lentivirus.
P-BCMA-101 did not exhibit effects of CAR-mediated tonic signaling, a common cause of T-cell exhaustion that leads to poor durability. Tonic signaling is caused by oligomerization of unstable binding domains commonly seen with traditional scFv CARs.
The researchers tested P-BCMA-101 in NSG mice bearing luciferase+ MM.1S cells. The mice received a single administration of either 4 x 106 or 12 x 106 P-BCMA-101 cells.
P-BCMA-101 treatment reduced tumor burden to the limit of detection within 7 days. Conversely, all untreated control mice succumbed to MM within 4 weeks.
P-BCMA-101 expanded and persisted in the treated mice, eliminated tumors following relapse, and prolonged survival.
Most treated mice survived 110 days, and none of them died from tumor burden during the study. This compares favorably to lentivirus-based products that have shown roughly 50-day survival in the same model.
Based on these results, Poseida plans to initiate a phase 1 trial of P-BCMA-101 in patients with relapsed or refractory MM. ![]()
WASHINGTON, DC—A chimeric antigen receptor (CAR) T-cell therapy is active against multiple myeloma (MM), according to preclinical research.
The therapy, known as P-BCMA-101, demonstrated persistent anti-tumor activity in a mouse model of MM.
Treatment with P-BCMA-101 eliminated tumors after relapse and prolonged survival in the mice, when compared to other CAR-T cell therapies.
These results were presented in a poster at the AACR Annual Meeting 2017 (abstract 3759).
The research was conducted by employees of Poseida Therapeutics Inc., the company developing P-BCMA-101, as well as others.
About P-BCMA-101
The researchers explained that P-BCMA-101 employs a B-cell maturation antigen (BCMA)-specific Centyrin™ rather than a single-chain variable fragment (scFv) for antigen detection.
Centyrins™ are fully human and have similar binding affinities as scFvs. However, Centyrins are smaller, more thermostable, and predicted to be less immunogenic.
P-BCMA-101 is engineered using the PiggyBac™ DNA modification system. PiggyBac eliminates the need to use lentivirus or gamma-retrovirus as a gene delivery mechanism, resulting in improved manufacturing and cost savings.
In addition, the increased cargo capacity of PiggyBac allows for the incorporation of a safety switch and a selectable gene. The safety switch can be “flipped” to enable depletion in case adverse events occur. And the selectable gene allows for enrichment of CARTyrin+ cells using a non-genotoxic drug.
Findings
The researchers found that more than 70% of P-BCMA-101 cells possessed a stem cell memory phenotype, creating a significant population of self-renewing, multipotent progenitors capable of reconstituting the entire spectrum of memory and effector T-cell subsets required to prevent cancer relapse. Similar competitor products typically report 0% to 20% stem cell memory phenotype.
In addition, P-BCMA-101 was enriched with more than 95% of T cells successfully modified, which compares favorably to the roughly 30% to 50% commonly expected with clinical manufacture using lentivirus.
P-BCMA-101 did not exhibit effects of CAR-mediated tonic signaling, a common cause of T-cell exhaustion that leads to poor durability. Tonic signaling is caused by oligomerization of unstable binding domains commonly seen with traditional scFv CARs.
The researchers tested P-BCMA-101 in NSG mice bearing luciferase+ MM.1S cells. The mice received a single administration of either 4 x 106 or 12 x 106 P-BCMA-101 cells.
P-BCMA-101 treatment reduced tumor burden to the limit of detection within 7 days. Conversely, all untreated control mice succumbed to MM within 4 weeks.
P-BCMA-101 expanded and persisted in the treated mice, eliminated tumors following relapse, and prolonged survival.
Most treated mice survived 110 days, and none of them died from tumor burden during the study. This compares favorably to lentivirus-based products that have shown roughly 50-day survival in the same model.
Based on these results, Poseida plans to initiate a phase 1 trial of P-BCMA-101 in patients with relapsed or refractory MM. ![]()
WASHINGTON, DC—A chimeric antigen receptor (CAR) T-cell therapy is active against multiple myeloma (MM), according to preclinical research.
The therapy, known as P-BCMA-101, demonstrated persistent anti-tumor activity in a mouse model of MM.
Treatment with P-BCMA-101 eliminated tumors after relapse and prolonged survival in the mice, when compared to other CAR-T cell therapies.
These results were presented in a poster at the AACR Annual Meeting 2017 (abstract 3759).
The research was conducted by employees of Poseida Therapeutics Inc., the company developing P-BCMA-101, as well as others.
About P-BCMA-101
The researchers explained that P-BCMA-101 employs a B-cell maturation antigen (BCMA)-specific Centyrin™ rather than a single-chain variable fragment (scFv) for antigen detection.
Centyrins™ are fully human and have similar binding affinities as scFvs. However, Centyrins are smaller, more thermostable, and predicted to be less immunogenic.
P-BCMA-101 is engineered using the PiggyBac™ DNA modification system. PiggyBac eliminates the need to use lentivirus or gamma-retrovirus as a gene delivery mechanism, resulting in improved manufacturing and cost savings.
In addition, the increased cargo capacity of PiggyBac allows for the incorporation of a safety switch and a selectable gene. The safety switch can be “flipped” to enable depletion in case adverse events occur. And the selectable gene allows for enrichment of CARTyrin+ cells using a non-genotoxic drug.
Findings
The researchers found that more than 70% of P-BCMA-101 cells possessed a stem cell memory phenotype, creating a significant population of self-renewing, multipotent progenitors capable of reconstituting the entire spectrum of memory and effector T-cell subsets required to prevent cancer relapse. Similar competitor products typically report 0% to 20% stem cell memory phenotype.
In addition, P-BCMA-101 was enriched with more than 95% of T cells successfully modified, which compares favorably to the roughly 30% to 50% commonly expected with clinical manufacture using lentivirus.
P-BCMA-101 did not exhibit effects of CAR-mediated tonic signaling, a common cause of T-cell exhaustion that leads to poor durability. Tonic signaling is caused by oligomerization of unstable binding domains commonly seen with traditional scFv CARs.
The researchers tested P-BCMA-101 in NSG mice bearing luciferase+ MM.1S cells. The mice received a single administration of either 4 x 106 or 12 x 106 P-BCMA-101 cells.
P-BCMA-101 treatment reduced tumor burden to the limit of detection within 7 days. Conversely, all untreated control mice succumbed to MM within 4 weeks.
P-BCMA-101 expanded and persisted in the treated mice, eliminated tumors following relapse, and prolonged survival.
Most treated mice survived 110 days, and none of them died from tumor burden during the study. This compares favorably to lentivirus-based products that have shown roughly 50-day survival in the same model.
Based on these results, Poseida plans to initiate a phase 1 trial of P-BCMA-101 in patients with relapsed or refractory MM. ![]()
Drug granted orphan designation for MM
The US Food and Drug Administration (FDA) has granted orphan drug designation for tasquinimod as a treatment for multiple myeloma (MM).
Tasquinimod is an immunomodulatory, anti-metastatic, and anti-angiogenic compound being developed by Active Biotech AB.
The company says tasquinimod works by inhibiting the function of S100A9, a pro-inflammatory protein that is elevated in MM and other malignancies.
S100A9 is believed to aid cancer development by recruiting and activating immune cells such as myeloid-derived suppressor cells.
Active Biotech AB says that, by targeting the S100A9 pathway, tasquinimod interferes with the accumulation and activation of myeloid-derived suppressor cells in the tumor microenvironment, which decreases immune suppression and angiogenesis.
Tasquinimod also inhibits the hypoxic response in the tumor by binding to HDAC4, according to Active Biotech AB.
The company says tasquinimod has produced “robust results” in animal models of MM, and research presented at the AACR Annual Meeting 2015 supports this statement.
Investigators found that tasquinimod reduced tumor growth and improved survival in mouse models of MM. And these effects were associated with reduced angiogenesis in the bone marrow.
Tasquinimod was previously under development as a treatment for prostate cancer, but research suggested the drug did not have a favorable risk-benefit ratio in this patient population.
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent rare diseases/disorders affecting fewer than 200,000 people in the US.
Orphan designation provides companies with certain incentives to develop products for rare diseases. This includes a 50% tax break on research and development, a fee waiver, access to federal grants, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation for tasquinimod as a treatment for multiple myeloma (MM).
Tasquinimod is an immunomodulatory, anti-metastatic, and anti-angiogenic compound being developed by Active Biotech AB.
The company says tasquinimod works by inhibiting the function of S100A9, a pro-inflammatory protein that is elevated in MM and other malignancies.
S100A9 is believed to aid cancer development by recruiting and activating immune cells such as myeloid-derived suppressor cells.
Active Biotech AB says that, by targeting the S100A9 pathway, tasquinimod interferes with the accumulation and activation of myeloid-derived suppressor cells in the tumor microenvironment, which decreases immune suppression and angiogenesis.
Tasquinimod also inhibits the hypoxic response in the tumor by binding to HDAC4, according to Active Biotech AB.
The company says tasquinimod has produced “robust results” in animal models of MM, and research presented at the AACR Annual Meeting 2015 supports this statement.
Investigators found that tasquinimod reduced tumor growth and improved survival in mouse models of MM. And these effects were associated with reduced angiogenesis in the bone marrow.
Tasquinimod was previously under development as a treatment for prostate cancer, but research suggested the drug did not have a favorable risk-benefit ratio in this patient population.
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent rare diseases/disorders affecting fewer than 200,000 people in the US.
Orphan designation provides companies with certain incentives to develop products for rare diseases. This includes a 50% tax break on research and development, a fee waiver, access to federal grants, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation for tasquinimod as a treatment for multiple myeloma (MM).
Tasquinimod is an immunomodulatory, anti-metastatic, and anti-angiogenic compound being developed by Active Biotech AB.
The company says tasquinimod works by inhibiting the function of S100A9, a pro-inflammatory protein that is elevated in MM and other malignancies.
S100A9 is believed to aid cancer development by recruiting and activating immune cells such as myeloid-derived suppressor cells.
Active Biotech AB says that, by targeting the S100A9 pathway, tasquinimod interferes with the accumulation and activation of myeloid-derived suppressor cells in the tumor microenvironment, which decreases immune suppression and angiogenesis.
Tasquinimod also inhibits the hypoxic response in the tumor by binding to HDAC4, according to Active Biotech AB.
The company says tasquinimod has produced “robust results” in animal models of MM, and research presented at the AACR Annual Meeting 2015 supports this statement.
Investigators found that tasquinimod reduced tumor growth and improved survival in mouse models of MM. And these effects were associated with reduced angiogenesis in the bone marrow.
Tasquinimod was previously under development as a treatment for prostate cancer, but research suggested the drug did not have a favorable risk-benefit ratio in this patient population.
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent rare diseases/disorders affecting fewer than 200,000 people in the US.
Orphan designation provides companies with certain incentives to develop products for rare diseases. This includes a 50% tax break on research and development, a fee waiver, access to federal grants, and 7 years of market exclusivity if the product is approved. ![]()
Multiple Myeloma: Updates on Diagnosis and Management
Multiple myeloma (MM) is a disease that is primarily treated by hematologists; however, it is important for primary care providers (PCPs) to be aware of the presentation and diagnosis of this disease. Multiple myeloma often is seen in the veteran population, and VA providers should be familiar with its diagnosis and treatment so that an appropriate referral can be made. Often, the initial signs and symptoms of the disease are subtle and require an astute eye by the PCP to diagnose and initiate a workup.
Once a veteran has an established diagnosis of MM or one of its precursor syndromes, the PCP will invariably be alerted to an adverse event (AE) of treatment or complication of the disease and should be aware of such complications to assist in management or referral. Patients with MM may achieve long-term remission; therefore, it is likely that the PCP will see an evolution in their treatment and care. Last, PCPs and patients often have a close relationship, and patients expect the PCP to understand their diagnosis and treatment plan.
Presentation
Multiple myeloma is a disease in which a neoplastic proliferation of plasma cells produces a monoclonal immunoglobulin. It is almost invariably preceded by premalignant stages of monoclonal gammopathy of undetermined significance (MGUS) and smoldering MM (SMM), although not all cases of MGUS will eventually progress to MM.1 Common signs and symptoms include anemia, bone pain or lytic lesions on X-ray, kidney injury, fatigue, hypercalcemia, and weight loss.2 Anemia is usually a normocytic, normochromic anemia and can be due to involvement of the bone marrow, secondary to renal disease, or it may be dilutional, related to a high monoclonal protein (M protein) level.
There are several identifiable causes for renal disease in patients with MM, including light chain cast nephropathy, hypercalcemia, light chain amyloidosis, and light chain deposition disease. Without intervention, progressive renal damage may occur.3
Diagnosis
All patients with a suspected diagnosis of MM should undergo a basic workup, including complete blood count; peripheral blood smear; complete chemistry panel, including calcium and albumin; serum free light chain analysis (FLC); serum protein electrophoresis (SPEP) and immunofixation; urinalysis; 24-hour urine collection for electrophoresis (UPEP) and immunofixation; serum B2-microglobulin; and lactate dehydrogenase.4 A FLC analysis is particularly useful for the diagnosis and monitoring of MM, when only small amounts of M protein are secreted into the serum/urine or for nonsecretory myeloma, as well as for light-chainonly myeloma.5
A bone marrow biopsy and aspirate should be performed in the diagnosis of MM to evaluate the bone marrow involvement and genetic abnormality of myeloma cells with fluorescence in situ hybridization (FISH) and cytogenetics, both of which are very important in risk stratification and for treatment planning. A skeletal survey is also typically performed to look for bone lesions.4 Magnetic resonance imaging (MRI) can also be useful to evaluate for possible soft tissue lesions when a bone survey is negative, or to evaluate for spinal cord compression.5 Additionally, an MRI should be performed in patients with SMM at the initial assessment, because focal lesions in the setting of SMM are associated with an increased risk to progression.6 Since plain radiographs are usually abnormal only after ≥ 30% of the bone is destroyed, an MRI offers a more sensitive image.
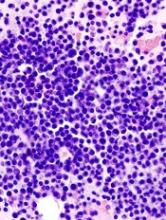
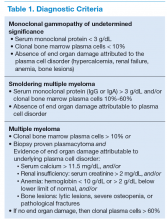
Two MM precursor syndromes are worth noting: MGUS and SMM. In evaluating a patient for possible MM, it is important to differentiate between MGUS, asymptomatic SMM, and MM that requires treatment.4 Monoclonal gammopathy of undetermined significance is diagnosed when a patient has a serum M protein that is < 3 g/dL, clonal bone marrow plasma cells < 10%, and no identifiable end organ damage.5 Smoldering MM is diagnosed when either the serum M protein is > 3 g/dL or bone marrow clonal plasma cells are > 10% in the absence of end organ damage.
Symptomatic MM is characterized by > 10% clonal bone marrow involvement with end organ damage that includes hypercalcemia, renal failure, anemia, or bone lesions. The diagnostic criteria are summarized in Table 1. The International Myeloma Working Group produced updated guidelines in 2014, which now include patients with > 60% bone marrow involvement of plasma cells, serum FLC ratio of > 100, and > 1 focal lesions on an MRI study as symptomatic MM.5,6
Most patients with MM will have a M protein produced by the malignant plasma cells detected on an SPEP or UPEP. The majority of immunoglobulins were IgG and IgA, whereas IgD and IgM were much less common.2 A minority of patients will not have a detectable M protein on SPEP or UPEP. Some patients will produce only light chains and are designated as light-chain-only myeloma. For these patients, the FLC assay is useful for diagnosis and disease monitoring. Patients who have an absence of M protein on SPEP/UPEP and normal FLC assay ratios are considered to have nonsecretory myeloma.7
Staging and Risk Stratification
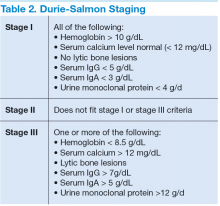
Two staging systems are used to evaluate a patient’s prognosis: the Durie-Salmon staging system, which is based on tumor burden (Table 2); and the International Staging System (ISS), which uses a combination of serum beta 2 microglobulin (B2M) and serum albumin levels to produce a powerful and reproducible 3-stage classification and is more commonly used by hematologists due to its simplicity to use and reliable reproducibility (Table 3).
In the Durie-Salmon staging system, patients with stage I disease have a lower tumor burden, defined as hemoglobin > 10 g/dL, normal calcium level, no evidence of lytic bone lesions, and low amounts of protein produced (IgG < 5 g/dL; IgA < 3 g/dL; urine protein < 4 g/d). Patients are classified as stage III if they have any of the following: hemoglobin < 8.5 g/dL, hypercalcemia with level > 12 mg/dL, bony lytic lesions, or high amounts of protein produced (IgG > 7 g/dL; IgA > 5 g/dL; or urine protein > 12 g/d). Patients with stage II disease do not fall into either of these categories. Stage III disease can be further differentiated into stage IIIA or stage IIIB disease if renal involvement is present.8
In the ISS system, patients with stage I disease have B2M levels that are < 3.5 mg/dL and albumin levels > 3.5 g/dL and have a median overall survival (OS) of 62 months. In this classification, stage III patients have B2M levels that are > 5.5 mg/dL and median OS was 29 months. Stage II patients do not meet either of these criteria and OS was 44 months.9 In a study by Mayo Clinic, OS has improved over the past decade, with OS for ISS stage III patients increasing to 4.2 years. Overall survival for both ISS stage I and stage III disease seems to have increased as well, although the end point has not been reached.10
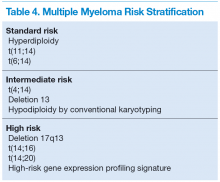
All myeloma patients are risk stratified at initial diagnosis based on their cytogenetic abnormalities identified mainly by FISH studies and conventional cytogenetics, which can serve as an alternative if FISH is unavailable. Genetic abnormalities of MM are the major predictor for the outcome and will affect treatment choice. Three risk groups have been identified: high-risk, intermediate-risk, and standard-risk MM (Table 4).11
Management of MGUS and SMM
Patients with MGUS progress to malignant conditions at a rate of 1% per year.12 Those individuals who are diagnosed with MGUS or SMM typically do not require therapy. According to the International Myeloma Working Group guidelines, patients should be monitored based on risk stratification. Those with low-risk MGUS (IgG M protein < 1.5 g/dL and no abnormal FLC ratio) can be monitored every 6 months for 2 to
3 years. Those who are intermediate to high risk need a baseline bone marrow biopsy in addition to skeletal survey and should check urine and serum levels for protein every 6 months for the first year and then annually thereafter.
Patients with SMM are at an increased risk of progression to symptomatic MM compared with patients with MGUS (10% per year for the first 5 years, 3% per year for the next 5 years).13 Therefore, experts recommend physician visits and laboratory testing for M proteins every 2 to 3 months for the first year and then an evaluation every 6 to 12 months if the patient remains clinically stable.14 Additionally, there are new
data to suggest that early therapy with lenalidomide plus dexamethasone for SMM can prolong time to disease progression as well as increase OS in individuals with SMM at high risk for progression.15
Patients With MM
All patients with a diagnosis of MM require immediate treatment. Initial choice of therapy is driven by whether a patient is eligible for an autologous stem cell transplant (ASCT), because certain agents, such as alkylating agents, should typically be avoided in those who are transplant eligible. Initial therapy for patients with MM is also based on genetic risk stratification of the disease. Patients with high-risk disease require a complete response (CR) treatment for long-term OS and thus benefit from an aggressive treatment strategy. Standard-risk patients have similar OS regardless of whether or not CR is achieved and thus can either be treated with an aggressive approach or a sequential therapy approach.16
Transplant-Eligible Patients
All patients should be evaluated for transplant eligibility, because it results in superior progression-free survival (PFS) and OS in patients with MM compared with standard chemotherapy. Transplant eligibility requirements differ, depending on the transplant center. There is no strict age limit in the U.S. for determining transplant eligibility. Physiological age and factors such as functional status and liver function are often considered before making a transplant decision.
For VA patients, transplants are generally considered in those aged < 65 years, and patients are referred to 1 of 3 transplant centers: VA Puget Sound Healthcare System in Seattle, Washington; Tennessee Valley Healthcare System in Nashville; or South Texas Veterans Healthcare System in San Antonio.17 All patients who are transplant eligible should receive induction therapy for 2 to 4 months before stem cell collection. This is to reduce tumor burden, for symptomatic management, as well as to lessen end organ damage. After stem cell collection, patients undergo either upfront ASCT or resume induction therapy and undergo a transplant after first relapse.
Bortezomib Regimens
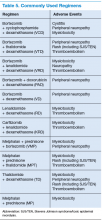
Bortezomib is a proteasome inhibitor (PI) and has been used as upfront chemotherapy for transplant-eligible patients, traditionally to avoid alkylating agents that could affect stem cell harvest. It is highly efficacious in the treatment of patients with MM. Two- or 3-drug regimens have been used. Common regimens include bortezomib, cyclophosphamide, dexamethasone; bortezomib, thalidomide, dexamethasone (VTD); bortezomib, lenalidomide, dexamethasone (VRD); bortezomib, doxorubicin, dexamethasone; as well as bortezomib, dexamethasone.18 Dexamethasone is less expensive than VTD or VRD, well tolerated, and efficacious. It is often used upfront for newly diagnosed MM.19 Threedrug regimens have shown to be more efficacious than 2-drug regimens in clinical trials (Table 5).20
Of note, bortezomib is not cleared through the kidney, which makes it an ideal choice for patients with renal function impairment. A significant potential AE with bortezomib is the onset of peripheral neuropathy. Bortezomib can be administered once or twice weekly. Twice-weekly administration of bortezomib is preferred when rapid results are needed, such as light chain cast nephropathy causing acute renal failure.21
Lenalidomide Plus Dexamethasone
Lenalidomide is a second-generation immunomodulating agent that is being increasingly used as initial therapy for MM. There is currently no data showing superiority of bortezomib-based regimens to lenalidomide plus dexamethasone in reference to OS. Bortezomib-based regimens seem to overcome the poor prognosis associated with t(4;14) translocation and thus should be considered in choosing initial chemotherapy treatment.22
Lenalidomide can affect stem cell collection; therefore, it is important to collect stem cells in transplanteligible patients who are aged < 65 years or for those who have received more than 4 cycles of treatment with this regimen.23,24 A major AE to lenalidomidecontaining regimens is the increased risk of thrombosis. All patients on lenalidomide require treatment with aspirin at a minimum; however, those at higher risk for thrombosis may require low-molecular weight heparin or warfarin.25
Carfilzomib Plus Lenalidomide Plus Dexamethasone
Carfilzomib is a recently approved PI that has shown promise in combination with lenalidomide and dexamethasone as initial therapy for MM. Several phase 2 trials have reported favorable results with carfilzomib in combination with lenalidomide and dexamethasone in MM.26,27 More studies are needed to establish efficacy and safety before this regimen is routinely used as upfront therapy.11
Thalidomide Plus Dexamethasone
Although there are no randomized controlled trials comparing lenalidomide plus dexamethasone with thalidomide plus dexamethasone, these regimens have been compared in retrospective studies. In these studies, lenalidomide plus dexamethasone showed both a higher response rate as well as an increased PFS and OS compared with thalidomide plus dexamethasone. Additionally, lenalidomide’s AE profile was more favorable than that of thalidomide. In light of this, lenalidomide plus dexamethasone is preferred to thalidomide plus dexamethasone in the management of MM, although the latter can be considered when lenalidomide is not available or when a patient does not tolerate lenalidomide.28
VDT-PACE
A multidrug combination that should be considered in select populations is the VDT-PACE regimen, which includes bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide. This regimen can be considered in those patients who have aggressive disease, such as those with plasma cell leukemia or with multiple extramedullary plasmacytomas.11
Autologous Stem Cell Transplant
Previous data suggest that ASCT improves OS in MM by 12 months.29 A more recent open-label, randomized trial comparing melphalan and ASCT to melphalanprednisone-lenalidomide showed significant prolonged PFS and OS among patients with MM.30 Although the role of ASCT may change as new drugs are integrated into initial therapy of MM, ASCT is still the preferred approach in transplant-eligible patients. As such, all patients who are eligible should be considered to receive a transplant.
There remains debate about whether ASCT should be performed early, after 2 to 4 cycles of induction therapy, or late after first relapse. Several randomized trials failed to show a difference in survival for early vs delayed ASCT approach.31 Generally, transplant can be delayed for patients with standard-risk MM who have responded well to therapy.11 Those patients who do not achieve a CR with their first ASCT may benefit from a second (tandem) ASCT.32 An allogeneic transplant is occasionally used in select populations and is the only potentially curative
therapy for these patients. However, its high mortality rate precludes its everyday use.
Transplant-Ineligible Patients
For patients with newly diagnosed MM who are ineligible for ASCT due to age or other comorbidities, chemotherapy is the only option. Many patients will benefit not only in survival, but also in quality of life. Immunomodulatory agents, such as lenalidomide and thalidomide, and PIs, such as bortezomib, are highly effective and well tolerated. There has been a general shift to using these agents upfront in transplant ineligible patients.
All previously mentioned regimens can also be used in transplant-ineligible patients. Although no longer the preferred treatment, melphalan can be considered in resource-poor settings.11 Patients who are not transplant eligible are treated for a fixed period of 9 to 18 months, although lenalidomide plus dexamethasone is often continued until relapse.11,33
Melphalan Plus Prednisone Plus Bortezomib
The addition of bortezomib to melphalan and prednisone results in improved OS compared with that of melphalan and dexamethasone alone.34 Peripheral neuropathy is a significant AE and can be minimized by giving bortezomib once weekly.
Melphalan Plus Prednisone Plus Thalidomide
Melphalan plus prednisone plus thalidomide has shown an OS benefit compared with that of melphalan and prednisone alone. The regimen has a high toxicity rate (> 50%) and a deep vein thrombosis rate of 20%, so patients undergoing treatment with this regimen require thromboprophylaxis.35,36
Melphalan Plus Prednisone
Although melphalan plus prednisone has fallen out of favor due to the existence of more efficacious regimens, it may be useful in an elderly patient population who lack access to newer agents, such as lenalidomide, thalidomide, and bortezomib.
Assessing Treatment Response
The International Myeloma Working Group has established criteria for assessing disease response. Patient’s response to therapy should be assessed with a FLC assay before each cycle with SPEP and UPEP and in those without measurable M protein levels. A bone marrow biopsy can be helpful in patients with immeasurable M protein levels and low FLC levels, as well as to establish that a CR is present.
A CR is defined as negative SPEP/UPEP, disappearance of soft tissue plamacytomas, and < 5% plasma cells in bone marrow. A very good partial response is defined as serum/urine M protein being present on immunofixation but not electrophoresis or reduction in serum M protein by 90% and urine M protein < 100 mg/d. For those without measurable M protein, a reduction in FLC ratio by 90% is required. A partial response is defined as > 50% reduction of the serum monoclonal protein and/or < 200 mg urinary M protein per 24 hours or > 90% reduction in urinary M protein. For those without M protein present, they should have > 50% decrease in FLC ratio.5
Maintenance Therapy
There is currently considerable debate about whether patients should be treated with maintenance therapy following induction chemotherapy or transplant. In patients treated with transplant, there have been several studies to investigate the use of maintenance therapy. Lenalidomide
has been evaluated for maintenance therapy following stem cell transplant and has shown superior PFS with dexamethasone as post-ASCT maintenance; however, this is at the cost of increased secondary cancers.37
Thalidomide has also been studied as maintenance therapy and seems to have a modest improvement in PFS and OS but at the cost of increased toxicities, such as neuropathy and thromboembolism.38,39 Still other studies compared bortezomib maintenance with thalidomide
maintenance in posttransplant patients and was able to show improved OS. As a result, certain patients with intermediate- or high-risk disease may be eligible for bortezomib for maintenance following transplant.11 For transplant-ineligible patients, there is no clear role for maintenance therapy.
Refractory/Relapsed Disease Treatments
Nearly all patients with MM will relapse at some point in their disease. Relapse is usually detected during surveillance and should be considered when the patient develops new bone lesions, hypercalcemia, anemia, renal failure, or rapid rise in M protein levels. For these patients, treatment options that should be considered are an ASCT if one has not been done before; an additional ASCT, providing the patient has not relapsed within 12 months of the first; repeating the initial chemotherapy regimen; or choosing an alternative chemotherapy regimen.5,11 For those who are not transplant candidates, chemotherapy remains the only option.
Single-agent bortezomib has shown promise in relapsed/refractory MM, with more than one-third of patients having partial response or CR.40,41 A study of bortezomib combined with liposomal doxorubicin has also shown a modest improvement in time to progression compared with single-agent bortezomib and can also be considered for relapsed/refractory disease.42 An additional option is an immunonomodulatory drug, such as lenalidomide or thalidomide. Unfortunately, poorer outcomes have been reported for patients who are refractory to both lenalidomide or bortezomib.43
Pomalidomide is a thalidomide analog and has activity in MM that is relapsed or refractory with acceptable response rates when used with dexamethasone.44 Carfilzomib is a PI approved for treatment of relapsed or refractory MM in patients who have failed lenalidomide and bortezomib.45 The FDA recently approved panobinostat, a histone deacetylase inhibitor, for patients with MM who have failed 2 prior therapies, and it has shown improved PFS.46 Patients should be treated in a clinical trial whenever possible, and emerging new options include second generation PIs, such as ixazomib, other histone deacetlyase inhibitors, AKT/P13K/mTOR inhibitors, heat-shock-protein inhibitors, and monoclonal antibodies.
Conclusions
Despite significant advances in the management of MM, the disease remains incurable. Virtually all patients will develop relapsed disease, although strides in the field have provided opportunities for longer term remissions. There are a multitude of strategies that are under investigation, and further studies are needed to establish their role in the treatment of patients with MM.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Landgren O, Kyle R, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113(22):5412-5417.
2. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
3. Hutchison CA, Batuman V, Behrens J, et al; International Kidney and Monoclonal Gammopathy Research Group. The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat Review Nephrol. 2011;8(1):43-51.
4. Dimopoulous M, Kyle R, Fermand JP, et al; International Myeloma Workshop Consensus Panel 3. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705.
5. Palumbo A, Rajkumar S, San Miguel JF, et al. International Melanoma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol. 2014;32(6):587-600.
6. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548.
7. Dimopoulos MA, Kastritis E, Terpo E. Non-secretory myeloma: one, two, or more entities? Oncology (Williston Park). 2013;27(9):930-932.
8. Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36(3):842-854.
9. Griepp P, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412-3420.
10. Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014; 28(5):1122-1128.
11. Rajkumar SV. Multiple myeloma: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014;89(10):999-1009.
12. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564-569.
13. Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356(25):2582-2590.
14. Landgren O. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma: biological insights and early treatment strategies. Hematology Am Soc Hematol Educ Program. 2013;2013(1):478-487.
15. Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N Engl J Med. 2013;369(5):438-447.
16. Haessler K, Shaughnessy JD Jr, Zhan F, et al. Benefit of complete response in multiple myeloma limited to high-risk subgroup identified by gene expression profiling. Clin Cancer Res. 2007;13(23):7073-7079.
17. Xiang Z, Mehta P. Management of multiple myeloma and its precursor syndromes. Fed Pract. 2014;31(suppl 3):6S-13S.
18. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: multiple myeloma. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/PDF/myeloma.pdf. Updated March 10, 2015. Accessed July 8, 2015.
19. Kumar S, Flinn I, Richardson P, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclosphosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119(19):4375-4382.
20. Moreau P, Avet-Loiseau H, Facon T, et al. Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood. 2011;118(22):5752-5758.
21. Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomized, phase 3, noninferiority study. Lancet Oncol. 2011;12(5):431-440.
22. Pineda-Roman M, Zangari M, Haessler J, et al. Sustained complete remissions in multiple myeloma linked to bortezomib in total therapy 3: comparison with total therapy 2. Br J Haematol. 2008;140(6):624-634.
23. Kumar S, Dispenzieri A, Lacy MQ, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21(9):2035-2042.
24. Kumar S, Giralt S, Stadtmauer EA, et al; International Myeloma Working Group. Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell collection following initial therapy with thalidomide-, lenalidomide-, or bortezomibcontaining regimens. Blood. 2009;114(9):1729-1735.
25. Larocca A, Cavallo F, Bringhen S, et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma patients treated with lenalidomide. Blood. 2012;119(4):933-939.
26. Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120(9):1801-1809.
27. Korde N, Zingone A, Kwok M, et al. Phase II clinical and correlative study of carfilzomib, lenalidomide, and dexamethasone followed by lenalidomide extended dosing (CRD-R) induces high rates of MRD negativity in newly diagnosed multiple myeloma patients [Abstract]. Blood. 2013;122(21):538.
28. Gay F, Hayman SR, Lacy MQ, et al. Lenalidomide plus dexamethasone versus thalidomide plus dexamethasone in newly diagnosed multiple myeloma: a comparative analysis of 411 patients. Blood. 2010;115(7):1343-1350.
29. Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med. 1996;335(2):91-97.
30. Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371(10):895-905.
31. Fermand JP, Ravaud P, Chevret S, et al. High-dose therapy and autologous stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998;92(9):3131-3136.
32. Elice F, Raimondi R, Tosetto A, et al. Prolonged overall survival with second on-demand autologous stem cell transplant in multiple myeloma. Am J Hematol. 2006;81(6):426-431.
33. Facon T, Dimopoulos MA, Dispenzieri A, et al. Initial phase 3 results of the FIRST (frontline investigation of lenalidomide + dexamethasone versus standard thalidomide) trial (MM-020/IFM 07 01) in newly diagnosed multiple myeloma (NDMM) patients (pts) ineligible for stem cell transplantation (SCT). Blood. 2013;122(21):2.
34. San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906-917.
35. Facon T, Mary JY, Hulin C, et al; Intergroupe Français du Myélome. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370(9594):1209-1218.
36. Hulin C, Facon T, Rodon P, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma. IFM 01/01 trial. J Clin Oncol. 2009;27(22):3664-3670.
37. Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1782-1791.
38. Attal M., Harousseau JL, Leyvraz S, et al; Inter-Groupe Francophone du Myélome (IFM). Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108(10):3289-3294.
39. Spencer A, Prince HM, Roberts AW, et al. Consolidation therapy with low-dose thalidomide and prednisolone prolongs the survival of multiple myeloma patients undergoing a single autologous stem-cell transplantation procedure. J Clin Oncol. 2009;27(11):1788-1793.
40. Sonneveld P, Schmidt-Wolf IG, van der Holt B, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/GMMG-HD4 trial. J Clin Oncol. 2012;30(24):2946-2955.
41. Richardson PG, Sonneveld P, Schuster MW, et al; Assessment of Proteasome Inhibition for Extending Remissions (APEX) Investigators. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487-2498.
42. Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25(25):3892-3901.
43. Kumar SK, Lee JH, Lahuerta JJ, et al; International Myeloma Working Group. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26(1):149-157.
44. Lacy MQ, Hayman SR, Gertz MA, et al. Pomalidomide (CC4047) plus low-dose dexamethasone as therapy for relapsed multiple myeloma. J Clin Oncol. 2009;27(30):5008-5014.
45. Siegel DS, Martin T, Wang M, et al. A phase 2 study of single agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120(14):2817-2825.
46. San-Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15(11):1195-1206.
Multiple myeloma (MM) is a disease that is primarily treated by hematologists; however, it is important for primary care providers (PCPs) to be aware of the presentation and diagnosis of this disease. Multiple myeloma often is seen in the veteran population, and VA providers should be familiar with its diagnosis and treatment so that an appropriate referral can be made. Often, the initial signs and symptoms of the disease are subtle and require an astute eye by the PCP to diagnose and initiate a workup.
Once a veteran has an established diagnosis of MM or one of its precursor syndromes, the PCP will invariably be alerted to an adverse event (AE) of treatment or complication of the disease and should be aware of such complications to assist in management or referral. Patients with MM may achieve long-term remission; therefore, it is likely that the PCP will see an evolution in their treatment and care. Last, PCPs and patients often have a close relationship, and patients expect the PCP to understand their diagnosis and treatment plan.
Presentation
Multiple myeloma is a disease in which a neoplastic proliferation of plasma cells produces a monoclonal immunoglobulin. It is almost invariably preceded by premalignant stages of monoclonal gammopathy of undetermined significance (MGUS) and smoldering MM (SMM), although not all cases of MGUS will eventually progress to MM.1 Common signs and symptoms include anemia, bone pain or lytic lesions on X-ray, kidney injury, fatigue, hypercalcemia, and weight loss.2 Anemia is usually a normocytic, normochromic anemia and can be due to involvement of the bone marrow, secondary to renal disease, or it may be dilutional, related to a high monoclonal protein (M protein) level.
There are several identifiable causes for renal disease in patients with MM, including light chain cast nephropathy, hypercalcemia, light chain amyloidosis, and light chain deposition disease. Without intervention, progressive renal damage may occur.3
Diagnosis
All patients with a suspected diagnosis of MM should undergo a basic workup, including complete blood count; peripheral blood smear; complete chemistry panel, including calcium and albumin; serum free light chain analysis (FLC); serum protein electrophoresis (SPEP) and immunofixation; urinalysis; 24-hour urine collection for electrophoresis (UPEP) and immunofixation; serum B2-microglobulin; and lactate dehydrogenase.4 A FLC analysis is particularly useful for the diagnosis and monitoring of MM, when only small amounts of M protein are secreted into the serum/urine or for nonsecretory myeloma, as well as for light-chainonly myeloma.5
A bone marrow biopsy and aspirate should be performed in the diagnosis of MM to evaluate the bone marrow involvement and genetic abnormality of myeloma cells with fluorescence in situ hybridization (FISH) and cytogenetics, both of which are very important in risk stratification and for treatment planning. A skeletal survey is also typically performed to look for bone lesions.4 Magnetic resonance imaging (MRI) can also be useful to evaluate for possible soft tissue lesions when a bone survey is negative, or to evaluate for spinal cord compression.5 Additionally, an MRI should be performed in patients with SMM at the initial assessment, because focal lesions in the setting of SMM are associated with an increased risk to progression.6 Since plain radiographs are usually abnormal only after ≥ 30% of the bone is destroyed, an MRI offers a more sensitive image.
Two MM precursor syndromes are worth noting: MGUS and SMM. In evaluating a patient for possible MM, it is important to differentiate between MGUS, asymptomatic SMM, and MM that requires treatment.4 Monoclonal gammopathy of undetermined significance is diagnosed when a patient has a serum M protein that is < 3 g/dL, clonal bone marrow plasma cells < 10%, and no identifiable end organ damage.5 Smoldering MM is diagnosed when either the serum M protein is > 3 g/dL or bone marrow clonal plasma cells are > 10% in the absence of end organ damage.
Symptomatic MM is characterized by > 10% clonal bone marrow involvement with end organ damage that includes hypercalcemia, renal failure, anemia, or bone lesions. The diagnostic criteria are summarized in Table 1. The International Myeloma Working Group produced updated guidelines in 2014, which now include patients with > 60% bone marrow involvement of plasma cells, serum FLC ratio of > 100, and > 1 focal lesions on an MRI study as symptomatic MM.5,6
Most patients with MM will have a M protein produced by the malignant plasma cells detected on an SPEP or UPEP. The majority of immunoglobulins were IgG and IgA, whereas IgD and IgM were much less common.2 A minority of patients will not have a detectable M protein on SPEP or UPEP. Some patients will produce only light chains and are designated as light-chain-only myeloma. For these patients, the FLC assay is useful for diagnosis and disease monitoring. Patients who have an absence of M protein on SPEP/UPEP and normal FLC assay ratios are considered to have nonsecretory myeloma.7
Staging and Risk Stratification
Two staging systems are used to evaluate a patient’s prognosis: the Durie-Salmon staging system, which is based on tumor burden (Table 2); and the International Staging System (ISS), which uses a combination of serum beta 2 microglobulin (B2M) and serum albumin levels to produce a powerful and reproducible 3-stage classification and is more commonly used by hematologists due to its simplicity to use and reliable reproducibility (Table 3).
In the Durie-Salmon staging system, patients with stage I disease have a lower tumor burden, defined as hemoglobin > 10 g/dL, normal calcium level, no evidence of lytic bone lesions, and low amounts of protein produced (IgG < 5 g/dL; IgA < 3 g/dL; urine protein < 4 g/d). Patients are classified as stage III if they have any of the following: hemoglobin < 8.5 g/dL, hypercalcemia with level > 12 mg/dL, bony lytic lesions, or high amounts of protein produced (IgG > 7 g/dL; IgA > 5 g/dL; or urine protein > 12 g/d). Patients with stage II disease do not fall into either of these categories. Stage III disease can be further differentiated into stage IIIA or stage IIIB disease if renal involvement is present.8
In the ISS system, patients with stage I disease have B2M levels that are < 3.5 mg/dL and albumin levels > 3.5 g/dL and have a median overall survival (OS) of 62 months. In this classification, stage III patients have B2M levels that are > 5.5 mg/dL and median OS was 29 months. Stage II patients do not meet either of these criteria and OS was 44 months.9 In a study by Mayo Clinic, OS has improved over the past decade, with OS for ISS stage III patients increasing to 4.2 years. Overall survival for both ISS stage I and stage III disease seems to have increased as well, although the end point has not been reached.10
All myeloma patients are risk stratified at initial diagnosis based on their cytogenetic abnormalities identified mainly by FISH studies and conventional cytogenetics, which can serve as an alternative if FISH is unavailable. Genetic abnormalities of MM are the major predictor for the outcome and will affect treatment choice. Three risk groups have been identified: high-risk, intermediate-risk, and standard-risk MM (Table 4).11
Management of MGUS and SMM
Patients with MGUS progress to malignant conditions at a rate of 1% per year.12 Those individuals who are diagnosed with MGUS or SMM typically do not require therapy. According to the International Myeloma Working Group guidelines, patients should be monitored based on risk stratification. Those with low-risk MGUS (IgG M protein < 1.5 g/dL and no abnormal FLC ratio) can be monitored every 6 months for 2 to
3 years. Those who are intermediate to high risk need a baseline bone marrow biopsy in addition to skeletal survey and should check urine and serum levels for protein every 6 months for the first year and then annually thereafter.
Patients with SMM are at an increased risk of progression to symptomatic MM compared with patients with MGUS (10% per year for the first 5 years, 3% per year for the next 5 years).13 Therefore, experts recommend physician visits and laboratory testing for M proteins every 2 to 3 months for the first year and then an evaluation every 6 to 12 months if the patient remains clinically stable.14 Additionally, there are new
data to suggest that early therapy with lenalidomide plus dexamethasone for SMM can prolong time to disease progression as well as increase OS in individuals with SMM at high risk for progression.15
Patients With MM
All patients with a diagnosis of MM require immediate treatment. Initial choice of therapy is driven by whether a patient is eligible for an autologous stem cell transplant (ASCT), because certain agents, such as alkylating agents, should typically be avoided in those who are transplant eligible. Initial therapy for patients with MM is also based on genetic risk stratification of the disease. Patients with high-risk disease require a complete response (CR) treatment for long-term OS and thus benefit from an aggressive treatment strategy. Standard-risk patients have similar OS regardless of whether or not CR is achieved and thus can either be treated with an aggressive approach or a sequential therapy approach.16
Transplant-Eligible Patients
All patients should be evaluated for transplant eligibility, because it results in superior progression-free survival (PFS) and OS in patients with MM compared with standard chemotherapy. Transplant eligibility requirements differ, depending on the transplant center. There is no strict age limit in the U.S. for determining transplant eligibility. Physiological age and factors such as functional status and liver function are often considered before making a transplant decision.
For VA patients, transplants are generally considered in those aged < 65 years, and patients are referred to 1 of 3 transplant centers: VA Puget Sound Healthcare System in Seattle, Washington; Tennessee Valley Healthcare System in Nashville; or South Texas Veterans Healthcare System in San Antonio.17 All patients who are transplant eligible should receive induction therapy for 2 to 4 months before stem cell collection. This is to reduce tumor burden, for symptomatic management, as well as to lessen end organ damage. After stem cell collection, patients undergo either upfront ASCT or resume induction therapy and undergo a transplant after first relapse.
Bortezomib Regimens
Bortezomib is a proteasome inhibitor (PI) and has been used as upfront chemotherapy for transplant-eligible patients, traditionally to avoid alkylating agents that could affect stem cell harvest. It is highly efficacious in the treatment of patients with MM. Two- or 3-drug regimens have been used. Common regimens include bortezomib, cyclophosphamide, dexamethasone; bortezomib, thalidomide, dexamethasone (VTD); bortezomib, lenalidomide, dexamethasone (VRD); bortezomib, doxorubicin, dexamethasone; as well as bortezomib, dexamethasone.18 Dexamethasone is less expensive than VTD or VRD, well tolerated, and efficacious. It is often used upfront for newly diagnosed MM.19 Threedrug regimens have shown to be more efficacious than 2-drug regimens in clinical trials (Table 5).20
Of note, bortezomib is not cleared through the kidney, which makes it an ideal choice for patients with renal function impairment. A significant potential AE with bortezomib is the onset of peripheral neuropathy. Bortezomib can be administered once or twice weekly. Twice-weekly administration of bortezomib is preferred when rapid results are needed, such as light chain cast nephropathy causing acute renal failure.21
Lenalidomide Plus Dexamethasone
Lenalidomide is a second-generation immunomodulating agent that is being increasingly used as initial therapy for MM. There is currently no data showing superiority of bortezomib-based regimens to lenalidomide plus dexamethasone in reference to OS. Bortezomib-based regimens seem to overcome the poor prognosis associated with t(4;14) translocation and thus should be considered in choosing initial chemotherapy treatment.22
Lenalidomide can affect stem cell collection; therefore, it is important to collect stem cells in transplanteligible patients who are aged < 65 years or for those who have received more than 4 cycles of treatment with this regimen.23,24 A major AE to lenalidomidecontaining regimens is the increased risk of thrombosis. All patients on lenalidomide require treatment with aspirin at a minimum; however, those at higher risk for thrombosis may require low-molecular weight heparin or warfarin.25
Carfilzomib Plus Lenalidomide Plus Dexamethasone
Carfilzomib is a recently approved PI that has shown promise in combination with lenalidomide and dexamethasone as initial therapy for MM. Several phase 2 trials have reported favorable results with carfilzomib in combination with lenalidomide and dexamethasone in MM.26,27 More studies are needed to establish efficacy and safety before this regimen is routinely used as upfront therapy.11
Thalidomide Plus Dexamethasone
Although there are no randomized controlled trials comparing lenalidomide plus dexamethasone with thalidomide plus dexamethasone, these regimens have been compared in retrospective studies. In these studies, lenalidomide plus dexamethasone showed both a higher response rate as well as an increased PFS and OS compared with thalidomide plus dexamethasone. Additionally, lenalidomide’s AE profile was more favorable than that of thalidomide. In light of this, lenalidomide plus dexamethasone is preferred to thalidomide plus dexamethasone in the management of MM, although the latter can be considered when lenalidomide is not available or when a patient does not tolerate lenalidomide.28
VDT-PACE
A multidrug combination that should be considered in select populations is the VDT-PACE regimen, which includes bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide. This regimen can be considered in those patients who have aggressive disease, such as those with plasma cell leukemia or with multiple extramedullary plasmacytomas.11
Autologous Stem Cell Transplant
Previous data suggest that ASCT improves OS in MM by 12 months.29 A more recent open-label, randomized trial comparing melphalan and ASCT to melphalanprednisone-lenalidomide showed significant prolonged PFS and OS among patients with MM.30 Although the role of ASCT may change as new drugs are integrated into initial therapy of MM, ASCT is still the preferred approach in transplant-eligible patients. As such, all patients who are eligible should be considered to receive a transplant.
There remains debate about whether ASCT should be performed early, after 2 to 4 cycles of induction therapy, or late after first relapse. Several randomized trials failed to show a difference in survival for early vs delayed ASCT approach.31 Generally, transplant can be delayed for patients with standard-risk MM who have responded well to therapy.11 Those patients who do not achieve a CR with their first ASCT may benefit from a second (tandem) ASCT.32 An allogeneic transplant is occasionally used in select populations and is the only potentially curative
therapy for these patients. However, its high mortality rate precludes its everyday use.
Transplant-Ineligible Patients
For patients with newly diagnosed MM who are ineligible for ASCT due to age or other comorbidities, chemotherapy is the only option. Many patients will benefit not only in survival, but also in quality of life. Immunomodulatory agents, such as lenalidomide and thalidomide, and PIs, such as bortezomib, are highly effective and well tolerated. There has been a general shift to using these agents upfront in transplant ineligible patients.
All previously mentioned regimens can also be used in transplant-ineligible patients. Although no longer the preferred treatment, melphalan can be considered in resource-poor settings.11 Patients who are not transplant eligible are treated for a fixed period of 9 to 18 months, although lenalidomide plus dexamethasone is often continued until relapse.11,33
Melphalan Plus Prednisone Plus Bortezomib
The addition of bortezomib to melphalan and prednisone results in improved OS compared with that of melphalan and dexamethasone alone.34 Peripheral neuropathy is a significant AE and can be minimized by giving bortezomib once weekly.
Melphalan Plus Prednisone Plus Thalidomide
Melphalan plus prednisone plus thalidomide has shown an OS benefit compared with that of melphalan and prednisone alone. The regimen has a high toxicity rate (> 50%) and a deep vein thrombosis rate of 20%, so patients undergoing treatment with this regimen require thromboprophylaxis.35,36
Melphalan Plus Prednisone
Although melphalan plus prednisone has fallen out of favor due to the existence of more efficacious regimens, it may be useful in an elderly patient population who lack access to newer agents, such as lenalidomide, thalidomide, and bortezomib.
Assessing Treatment Response
The International Myeloma Working Group has established criteria for assessing disease response. Patient’s response to therapy should be assessed with a FLC assay before each cycle with SPEP and UPEP and in those without measurable M protein levels. A bone marrow biopsy can be helpful in patients with immeasurable M protein levels and low FLC levels, as well as to establish that a CR is present.
A CR is defined as negative SPEP/UPEP, disappearance of soft tissue plamacytomas, and < 5% plasma cells in bone marrow. A very good partial response is defined as serum/urine M protein being present on immunofixation but not electrophoresis or reduction in serum M protein by 90% and urine M protein < 100 mg/d. For those without measurable M protein, a reduction in FLC ratio by 90% is required. A partial response is defined as > 50% reduction of the serum monoclonal protein and/or < 200 mg urinary M protein per 24 hours or > 90% reduction in urinary M protein. For those without M protein present, they should have > 50% decrease in FLC ratio.5
Maintenance Therapy
There is currently considerable debate about whether patients should be treated with maintenance therapy following induction chemotherapy or transplant. In patients treated with transplant, there have been several studies to investigate the use of maintenance therapy. Lenalidomide
has been evaluated for maintenance therapy following stem cell transplant and has shown superior PFS with dexamethasone as post-ASCT maintenance; however, this is at the cost of increased secondary cancers.37
Thalidomide has also been studied as maintenance therapy and seems to have a modest improvement in PFS and OS but at the cost of increased toxicities, such as neuropathy and thromboembolism.38,39 Still other studies compared bortezomib maintenance with thalidomide
maintenance in posttransplant patients and was able to show improved OS. As a result, certain patients with intermediate- or high-risk disease may be eligible for bortezomib for maintenance following transplant.11 For transplant-ineligible patients, there is no clear role for maintenance therapy.
Refractory/Relapsed Disease Treatments
Nearly all patients with MM will relapse at some point in their disease. Relapse is usually detected during surveillance and should be considered when the patient develops new bone lesions, hypercalcemia, anemia, renal failure, or rapid rise in M protein levels. For these patients, treatment options that should be considered are an ASCT if one has not been done before; an additional ASCT, providing the patient has not relapsed within 12 months of the first; repeating the initial chemotherapy regimen; or choosing an alternative chemotherapy regimen.5,11 For those who are not transplant candidates, chemotherapy remains the only option.
Single-agent bortezomib has shown promise in relapsed/refractory MM, with more than one-third of patients having partial response or CR.40,41 A study of bortezomib combined with liposomal doxorubicin has also shown a modest improvement in time to progression compared with single-agent bortezomib and can also be considered for relapsed/refractory disease.42 An additional option is an immunonomodulatory drug, such as lenalidomide or thalidomide. Unfortunately, poorer outcomes have been reported for patients who are refractory to both lenalidomide or bortezomib.43
Pomalidomide is a thalidomide analog and has activity in MM that is relapsed or refractory with acceptable response rates when used with dexamethasone.44 Carfilzomib is a PI approved for treatment of relapsed or refractory MM in patients who have failed lenalidomide and bortezomib.45 The FDA recently approved panobinostat, a histone deacetylase inhibitor, for patients with MM who have failed 2 prior therapies, and it has shown improved PFS.46 Patients should be treated in a clinical trial whenever possible, and emerging new options include second generation PIs, such as ixazomib, other histone deacetlyase inhibitors, AKT/P13K/mTOR inhibitors, heat-shock-protein inhibitors, and monoclonal antibodies.
Conclusions
Despite significant advances in the management of MM, the disease remains incurable. Virtually all patients will develop relapsed disease, although strides in the field have provided opportunities for longer term remissions. There are a multitude of strategies that are under investigation, and further studies are needed to establish their role in the treatment of patients with MM.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Multiple myeloma (MM) is a disease that is primarily treated by hematologists; however, it is important for primary care providers (PCPs) to be aware of the presentation and diagnosis of this disease. Multiple myeloma often is seen in the veteran population, and VA providers should be familiar with its diagnosis and treatment so that an appropriate referral can be made. Often, the initial signs and symptoms of the disease are subtle and require an astute eye by the PCP to diagnose and initiate a workup.
Once a veteran has an established diagnosis of MM or one of its precursor syndromes, the PCP will invariably be alerted to an adverse event (AE) of treatment or complication of the disease and should be aware of such complications to assist in management or referral. Patients with MM may achieve long-term remission; therefore, it is likely that the PCP will see an evolution in their treatment and care. Last, PCPs and patients often have a close relationship, and patients expect the PCP to understand their diagnosis and treatment plan.
Presentation
Multiple myeloma is a disease in which a neoplastic proliferation of plasma cells produces a monoclonal immunoglobulin. It is almost invariably preceded by premalignant stages of monoclonal gammopathy of undetermined significance (MGUS) and smoldering MM (SMM), although not all cases of MGUS will eventually progress to MM.1 Common signs and symptoms include anemia, bone pain or lytic lesions on X-ray, kidney injury, fatigue, hypercalcemia, and weight loss.2 Anemia is usually a normocytic, normochromic anemia and can be due to involvement of the bone marrow, secondary to renal disease, or it may be dilutional, related to a high monoclonal protein (M protein) level.
There are several identifiable causes for renal disease in patients with MM, including light chain cast nephropathy, hypercalcemia, light chain amyloidosis, and light chain deposition disease. Without intervention, progressive renal damage may occur.3
Diagnosis
All patients with a suspected diagnosis of MM should undergo a basic workup, including complete blood count; peripheral blood smear; complete chemistry panel, including calcium and albumin; serum free light chain analysis (FLC); serum protein electrophoresis (SPEP) and immunofixation; urinalysis; 24-hour urine collection for electrophoresis (UPEP) and immunofixation; serum B2-microglobulin; and lactate dehydrogenase.4 A FLC analysis is particularly useful for the diagnosis and monitoring of MM, when only small amounts of M protein are secreted into the serum/urine or for nonsecretory myeloma, as well as for light-chainonly myeloma.5
A bone marrow biopsy and aspirate should be performed in the diagnosis of MM to evaluate the bone marrow involvement and genetic abnormality of myeloma cells with fluorescence in situ hybridization (FISH) and cytogenetics, both of which are very important in risk stratification and for treatment planning. A skeletal survey is also typically performed to look for bone lesions.4 Magnetic resonance imaging (MRI) can also be useful to evaluate for possible soft tissue lesions when a bone survey is negative, or to evaluate for spinal cord compression.5 Additionally, an MRI should be performed in patients with SMM at the initial assessment, because focal lesions in the setting of SMM are associated with an increased risk to progression.6 Since plain radiographs are usually abnormal only after ≥ 30% of the bone is destroyed, an MRI offers a more sensitive image.
Two MM precursor syndromes are worth noting: MGUS and SMM. In evaluating a patient for possible MM, it is important to differentiate between MGUS, asymptomatic SMM, and MM that requires treatment.4 Monoclonal gammopathy of undetermined significance is diagnosed when a patient has a serum M protein that is < 3 g/dL, clonal bone marrow plasma cells < 10%, and no identifiable end organ damage.5 Smoldering MM is diagnosed when either the serum M protein is > 3 g/dL or bone marrow clonal plasma cells are > 10% in the absence of end organ damage.
Symptomatic MM is characterized by > 10% clonal bone marrow involvement with end organ damage that includes hypercalcemia, renal failure, anemia, or bone lesions. The diagnostic criteria are summarized in Table 1. The International Myeloma Working Group produced updated guidelines in 2014, which now include patients with > 60% bone marrow involvement of plasma cells, serum FLC ratio of > 100, and > 1 focal lesions on an MRI study as symptomatic MM.5,6
Most patients with MM will have a M protein produced by the malignant plasma cells detected on an SPEP or UPEP. The majority of immunoglobulins were IgG and IgA, whereas IgD and IgM were much less common.2 A minority of patients will not have a detectable M protein on SPEP or UPEP. Some patients will produce only light chains and are designated as light-chain-only myeloma. For these patients, the FLC assay is useful for diagnosis and disease monitoring. Patients who have an absence of M protein on SPEP/UPEP and normal FLC assay ratios are considered to have nonsecretory myeloma.7
Staging and Risk Stratification
Two staging systems are used to evaluate a patient’s prognosis: the Durie-Salmon staging system, which is based on tumor burden (Table 2); and the International Staging System (ISS), which uses a combination of serum beta 2 microglobulin (B2M) and serum albumin levels to produce a powerful and reproducible 3-stage classification and is more commonly used by hematologists due to its simplicity to use and reliable reproducibility (Table 3).
In the Durie-Salmon staging system, patients with stage I disease have a lower tumor burden, defined as hemoglobin > 10 g/dL, normal calcium level, no evidence of lytic bone lesions, and low amounts of protein produced (IgG < 5 g/dL; IgA < 3 g/dL; urine protein < 4 g/d). Patients are classified as stage III if they have any of the following: hemoglobin < 8.5 g/dL, hypercalcemia with level > 12 mg/dL, bony lytic lesions, or high amounts of protein produced (IgG > 7 g/dL; IgA > 5 g/dL; or urine protein > 12 g/d). Patients with stage II disease do not fall into either of these categories. Stage III disease can be further differentiated into stage IIIA or stage IIIB disease if renal involvement is present.8
In the ISS system, patients with stage I disease have B2M levels that are < 3.5 mg/dL and albumin levels > 3.5 g/dL and have a median overall survival (OS) of 62 months. In this classification, stage III patients have B2M levels that are > 5.5 mg/dL and median OS was 29 months. Stage II patients do not meet either of these criteria and OS was 44 months.9 In a study by Mayo Clinic, OS has improved over the past decade, with OS for ISS stage III patients increasing to 4.2 years. Overall survival for both ISS stage I and stage III disease seems to have increased as well, although the end point has not been reached.10
All myeloma patients are risk stratified at initial diagnosis based on their cytogenetic abnormalities identified mainly by FISH studies and conventional cytogenetics, which can serve as an alternative if FISH is unavailable. Genetic abnormalities of MM are the major predictor for the outcome and will affect treatment choice. Three risk groups have been identified: high-risk, intermediate-risk, and standard-risk MM (Table 4).11
Management of MGUS and SMM
Patients with MGUS progress to malignant conditions at a rate of 1% per year.12 Those individuals who are diagnosed with MGUS or SMM typically do not require therapy. According to the International Myeloma Working Group guidelines, patients should be monitored based on risk stratification. Those with low-risk MGUS (IgG M protein < 1.5 g/dL and no abnormal FLC ratio) can be monitored every 6 months for 2 to
3 years. Those who are intermediate to high risk need a baseline bone marrow biopsy in addition to skeletal survey and should check urine and serum levels for protein every 6 months for the first year and then annually thereafter.
Patients with SMM are at an increased risk of progression to symptomatic MM compared with patients with MGUS (10% per year for the first 5 years, 3% per year for the next 5 years).13 Therefore, experts recommend physician visits and laboratory testing for M proteins every 2 to 3 months for the first year and then an evaluation every 6 to 12 months if the patient remains clinically stable.14 Additionally, there are new
data to suggest that early therapy with lenalidomide plus dexamethasone for SMM can prolong time to disease progression as well as increase OS in individuals with SMM at high risk for progression.15
Patients With MM
All patients with a diagnosis of MM require immediate treatment. Initial choice of therapy is driven by whether a patient is eligible for an autologous stem cell transplant (ASCT), because certain agents, such as alkylating agents, should typically be avoided in those who are transplant eligible. Initial therapy for patients with MM is also based on genetic risk stratification of the disease. Patients with high-risk disease require a complete response (CR) treatment for long-term OS and thus benefit from an aggressive treatment strategy. Standard-risk patients have similar OS regardless of whether or not CR is achieved and thus can either be treated with an aggressive approach or a sequential therapy approach.16
Transplant-Eligible Patients
All patients should be evaluated for transplant eligibility, because it results in superior progression-free survival (PFS) and OS in patients with MM compared with standard chemotherapy. Transplant eligibility requirements differ, depending on the transplant center. There is no strict age limit in the U.S. for determining transplant eligibility. Physiological age and factors such as functional status and liver function are often considered before making a transplant decision.
For VA patients, transplants are generally considered in those aged < 65 years, and patients are referred to 1 of 3 transplant centers: VA Puget Sound Healthcare System in Seattle, Washington; Tennessee Valley Healthcare System in Nashville; or South Texas Veterans Healthcare System in San Antonio.17 All patients who are transplant eligible should receive induction therapy for 2 to 4 months before stem cell collection. This is to reduce tumor burden, for symptomatic management, as well as to lessen end organ damage. After stem cell collection, patients undergo either upfront ASCT or resume induction therapy and undergo a transplant after first relapse.
Bortezomib Regimens
Bortezomib is a proteasome inhibitor (PI) and has been used as upfront chemotherapy for transplant-eligible patients, traditionally to avoid alkylating agents that could affect stem cell harvest. It is highly efficacious in the treatment of patients with MM. Two- or 3-drug regimens have been used. Common regimens include bortezomib, cyclophosphamide, dexamethasone; bortezomib, thalidomide, dexamethasone (VTD); bortezomib, lenalidomide, dexamethasone (VRD); bortezomib, doxorubicin, dexamethasone; as well as bortezomib, dexamethasone.18 Dexamethasone is less expensive than VTD or VRD, well tolerated, and efficacious. It is often used upfront for newly diagnosed MM.19 Threedrug regimens have shown to be more efficacious than 2-drug regimens in clinical trials (Table 5).20
Of note, bortezomib is not cleared through the kidney, which makes it an ideal choice for patients with renal function impairment. A significant potential AE with bortezomib is the onset of peripheral neuropathy. Bortezomib can be administered once or twice weekly. Twice-weekly administration of bortezomib is preferred when rapid results are needed, such as light chain cast nephropathy causing acute renal failure.21
Lenalidomide Plus Dexamethasone
Lenalidomide is a second-generation immunomodulating agent that is being increasingly used as initial therapy for MM. There is currently no data showing superiority of bortezomib-based regimens to lenalidomide plus dexamethasone in reference to OS. Bortezomib-based regimens seem to overcome the poor prognosis associated with t(4;14) translocation and thus should be considered in choosing initial chemotherapy treatment.22
Lenalidomide can affect stem cell collection; therefore, it is important to collect stem cells in transplanteligible patients who are aged < 65 years or for those who have received more than 4 cycles of treatment with this regimen.23,24 A major AE to lenalidomidecontaining regimens is the increased risk of thrombosis. All patients on lenalidomide require treatment with aspirin at a minimum; however, those at higher risk for thrombosis may require low-molecular weight heparin or warfarin.25
Carfilzomib Plus Lenalidomide Plus Dexamethasone
Carfilzomib is a recently approved PI that has shown promise in combination with lenalidomide and dexamethasone as initial therapy for MM. Several phase 2 trials have reported favorable results with carfilzomib in combination with lenalidomide and dexamethasone in MM.26,27 More studies are needed to establish efficacy and safety before this regimen is routinely used as upfront therapy.11
Thalidomide Plus Dexamethasone
Although there are no randomized controlled trials comparing lenalidomide plus dexamethasone with thalidomide plus dexamethasone, these regimens have been compared in retrospective studies. In these studies, lenalidomide plus dexamethasone showed both a higher response rate as well as an increased PFS and OS compared with thalidomide plus dexamethasone. Additionally, lenalidomide’s AE profile was more favorable than that of thalidomide. In light of this, lenalidomide plus dexamethasone is preferred to thalidomide plus dexamethasone in the management of MM, although the latter can be considered when lenalidomide is not available or when a patient does not tolerate lenalidomide.28
VDT-PACE
A multidrug combination that should be considered in select populations is the VDT-PACE regimen, which includes bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide. This regimen can be considered in those patients who have aggressive disease, such as those with plasma cell leukemia or with multiple extramedullary plasmacytomas.11
Autologous Stem Cell Transplant
Previous data suggest that ASCT improves OS in MM by 12 months.29 A more recent open-label, randomized trial comparing melphalan and ASCT to melphalanprednisone-lenalidomide showed significant prolonged PFS and OS among patients with MM.30 Although the role of ASCT may change as new drugs are integrated into initial therapy of MM, ASCT is still the preferred approach in transplant-eligible patients. As such, all patients who are eligible should be considered to receive a transplant.
There remains debate about whether ASCT should be performed early, after 2 to 4 cycles of induction therapy, or late after first relapse. Several randomized trials failed to show a difference in survival for early vs delayed ASCT approach.31 Generally, transplant can be delayed for patients with standard-risk MM who have responded well to therapy.11 Those patients who do not achieve a CR with their first ASCT may benefit from a second (tandem) ASCT.32 An allogeneic transplant is occasionally used in select populations and is the only potentially curative
therapy for these patients. However, its high mortality rate precludes its everyday use.
Transplant-Ineligible Patients
For patients with newly diagnosed MM who are ineligible for ASCT due to age or other comorbidities, chemotherapy is the only option. Many patients will benefit not only in survival, but also in quality of life. Immunomodulatory agents, such as lenalidomide and thalidomide, and PIs, such as bortezomib, are highly effective and well tolerated. There has been a general shift to using these agents upfront in transplant ineligible patients.
All previously mentioned regimens can also be used in transplant-ineligible patients. Although no longer the preferred treatment, melphalan can be considered in resource-poor settings.11 Patients who are not transplant eligible are treated for a fixed period of 9 to 18 months, although lenalidomide plus dexamethasone is often continued until relapse.11,33
Melphalan Plus Prednisone Plus Bortezomib
The addition of bortezomib to melphalan and prednisone results in improved OS compared with that of melphalan and dexamethasone alone.34 Peripheral neuropathy is a significant AE and can be minimized by giving bortezomib once weekly.
Melphalan Plus Prednisone Plus Thalidomide
Melphalan plus prednisone plus thalidomide has shown an OS benefit compared with that of melphalan and prednisone alone. The regimen has a high toxicity rate (> 50%) and a deep vein thrombosis rate of 20%, so patients undergoing treatment with this regimen require thromboprophylaxis.35,36
Melphalan Plus Prednisone
Although melphalan plus prednisone has fallen out of favor due to the existence of more efficacious regimens, it may be useful in an elderly patient population who lack access to newer agents, such as lenalidomide, thalidomide, and bortezomib.
Assessing Treatment Response
The International Myeloma Working Group has established criteria for assessing disease response. Patient’s response to therapy should be assessed with a FLC assay before each cycle with SPEP and UPEP and in those without measurable M protein levels. A bone marrow biopsy can be helpful in patients with immeasurable M protein levels and low FLC levels, as well as to establish that a CR is present.
A CR is defined as negative SPEP/UPEP, disappearance of soft tissue plamacytomas, and < 5% plasma cells in bone marrow. A very good partial response is defined as serum/urine M protein being present on immunofixation but not electrophoresis or reduction in serum M protein by 90% and urine M protein < 100 mg/d. For those without measurable M protein, a reduction in FLC ratio by 90% is required. A partial response is defined as > 50% reduction of the serum monoclonal protein and/or < 200 mg urinary M protein per 24 hours or > 90% reduction in urinary M protein. For those without M protein present, they should have > 50% decrease in FLC ratio.5
Maintenance Therapy
There is currently considerable debate about whether patients should be treated with maintenance therapy following induction chemotherapy or transplant. In patients treated with transplant, there have been several studies to investigate the use of maintenance therapy. Lenalidomide
has been evaluated for maintenance therapy following stem cell transplant and has shown superior PFS with dexamethasone as post-ASCT maintenance; however, this is at the cost of increased secondary cancers.37
Thalidomide has also been studied as maintenance therapy and seems to have a modest improvement in PFS and OS but at the cost of increased toxicities, such as neuropathy and thromboembolism.38,39 Still other studies compared bortezomib maintenance with thalidomide
maintenance in posttransplant patients and was able to show improved OS. As a result, certain patients with intermediate- or high-risk disease may be eligible for bortezomib for maintenance following transplant.11 For transplant-ineligible patients, there is no clear role for maintenance therapy.
Refractory/Relapsed Disease Treatments
Nearly all patients with MM will relapse at some point in their disease. Relapse is usually detected during surveillance and should be considered when the patient develops new bone lesions, hypercalcemia, anemia, renal failure, or rapid rise in M protein levels. For these patients, treatment options that should be considered are an ASCT if one has not been done before; an additional ASCT, providing the patient has not relapsed within 12 months of the first; repeating the initial chemotherapy regimen; or choosing an alternative chemotherapy regimen.5,11 For those who are not transplant candidates, chemotherapy remains the only option.
Single-agent bortezomib has shown promise in relapsed/refractory MM, with more than one-third of patients having partial response or CR.40,41 A study of bortezomib combined with liposomal doxorubicin has also shown a modest improvement in time to progression compared with single-agent bortezomib and can also be considered for relapsed/refractory disease.42 An additional option is an immunonomodulatory drug, such as lenalidomide or thalidomide. Unfortunately, poorer outcomes have been reported for patients who are refractory to both lenalidomide or bortezomib.43
Pomalidomide is a thalidomide analog and has activity in MM that is relapsed or refractory with acceptable response rates when used with dexamethasone.44 Carfilzomib is a PI approved for treatment of relapsed or refractory MM in patients who have failed lenalidomide and bortezomib.45 The FDA recently approved panobinostat, a histone deacetylase inhibitor, for patients with MM who have failed 2 prior therapies, and it has shown improved PFS.46 Patients should be treated in a clinical trial whenever possible, and emerging new options include second generation PIs, such as ixazomib, other histone deacetlyase inhibitors, AKT/P13K/mTOR inhibitors, heat-shock-protein inhibitors, and monoclonal antibodies.
Conclusions
Despite significant advances in the management of MM, the disease remains incurable. Virtually all patients will develop relapsed disease, although strides in the field have provided opportunities for longer term remissions. There are a multitude of strategies that are under investigation, and further studies are needed to establish their role in the treatment of patients with MM.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Landgren O, Kyle R, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113(22):5412-5417.
2. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
3. Hutchison CA, Batuman V, Behrens J, et al; International Kidney and Monoclonal Gammopathy Research Group. The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat Review Nephrol. 2011;8(1):43-51.
4. Dimopoulous M, Kyle R, Fermand JP, et al; International Myeloma Workshop Consensus Panel 3. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705.
5. Palumbo A, Rajkumar S, San Miguel JF, et al. International Melanoma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol. 2014;32(6):587-600.
6. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548.
7. Dimopoulos MA, Kastritis E, Terpo E. Non-secretory myeloma: one, two, or more entities? Oncology (Williston Park). 2013;27(9):930-932.
8. Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36(3):842-854.
9. Griepp P, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412-3420.
10. Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014; 28(5):1122-1128.
11. Rajkumar SV. Multiple myeloma: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014;89(10):999-1009.
12. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564-569.
13. Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356(25):2582-2590.
14. Landgren O. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma: biological insights and early treatment strategies. Hematology Am Soc Hematol Educ Program. 2013;2013(1):478-487.
15. Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N Engl J Med. 2013;369(5):438-447.
16. Haessler K, Shaughnessy JD Jr, Zhan F, et al. Benefit of complete response in multiple myeloma limited to high-risk subgroup identified by gene expression profiling. Clin Cancer Res. 2007;13(23):7073-7079.
17. Xiang Z, Mehta P. Management of multiple myeloma and its precursor syndromes. Fed Pract. 2014;31(suppl 3):6S-13S.
18. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: multiple myeloma. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/PDF/myeloma.pdf. Updated March 10, 2015. Accessed July 8, 2015.
19. Kumar S, Flinn I, Richardson P, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclosphosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119(19):4375-4382.
20. Moreau P, Avet-Loiseau H, Facon T, et al. Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood. 2011;118(22):5752-5758.
21. Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomized, phase 3, noninferiority study. Lancet Oncol. 2011;12(5):431-440.
22. Pineda-Roman M, Zangari M, Haessler J, et al. Sustained complete remissions in multiple myeloma linked to bortezomib in total therapy 3: comparison with total therapy 2. Br J Haematol. 2008;140(6):624-634.
23. Kumar S, Dispenzieri A, Lacy MQ, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21(9):2035-2042.
24. Kumar S, Giralt S, Stadtmauer EA, et al; International Myeloma Working Group. Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell collection following initial therapy with thalidomide-, lenalidomide-, or bortezomibcontaining regimens. Blood. 2009;114(9):1729-1735.
25. Larocca A, Cavallo F, Bringhen S, et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma patients treated with lenalidomide. Blood. 2012;119(4):933-939.
26. Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120(9):1801-1809.
27. Korde N, Zingone A, Kwok M, et al. Phase II clinical and correlative study of carfilzomib, lenalidomide, and dexamethasone followed by lenalidomide extended dosing (CRD-R) induces high rates of MRD negativity in newly diagnosed multiple myeloma patients [Abstract]. Blood. 2013;122(21):538.
28. Gay F, Hayman SR, Lacy MQ, et al. Lenalidomide plus dexamethasone versus thalidomide plus dexamethasone in newly diagnosed multiple myeloma: a comparative analysis of 411 patients. Blood. 2010;115(7):1343-1350.
29. Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med. 1996;335(2):91-97.
30. Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371(10):895-905.
31. Fermand JP, Ravaud P, Chevret S, et al. High-dose therapy and autologous stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998;92(9):3131-3136.
32. Elice F, Raimondi R, Tosetto A, et al. Prolonged overall survival with second on-demand autologous stem cell transplant in multiple myeloma. Am J Hematol. 2006;81(6):426-431.
33. Facon T, Dimopoulos MA, Dispenzieri A, et al. Initial phase 3 results of the FIRST (frontline investigation of lenalidomide + dexamethasone versus standard thalidomide) trial (MM-020/IFM 07 01) in newly diagnosed multiple myeloma (NDMM) patients (pts) ineligible for stem cell transplantation (SCT). Blood. 2013;122(21):2.
34. San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906-917.
35. Facon T, Mary JY, Hulin C, et al; Intergroupe Français du Myélome. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370(9594):1209-1218.
36. Hulin C, Facon T, Rodon P, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma. IFM 01/01 trial. J Clin Oncol. 2009;27(22):3664-3670.
37. Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1782-1791.
38. Attal M., Harousseau JL, Leyvraz S, et al; Inter-Groupe Francophone du Myélome (IFM). Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108(10):3289-3294.
39. Spencer A, Prince HM, Roberts AW, et al. Consolidation therapy with low-dose thalidomide and prednisolone prolongs the survival of multiple myeloma patients undergoing a single autologous stem-cell transplantation procedure. J Clin Oncol. 2009;27(11):1788-1793.
40. Sonneveld P, Schmidt-Wolf IG, van der Holt B, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/GMMG-HD4 trial. J Clin Oncol. 2012;30(24):2946-2955.
41. Richardson PG, Sonneveld P, Schuster MW, et al; Assessment of Proteasome Inhibition for Extending Remissions (APEX) Investigators. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487-2498.
42. Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25(25):3892-3901.
43. Kumar SK, Lee JH, Lahuerta JJ, et al; International Myeloma Working Group. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26(1):149-157.
44. Lacy MQ, Hayman SR, Gertz MA, et al. Pomalidomide (CC4047) plus low-dose dexamethasone as therapy for relapsed multiple myeloma. J Clin Oncol. 2009;27(30):5008-5014.
45. Siegel DS, Martin T, Wang M, et al. A phase 2 study of single agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120(14):2817-2825.
46. San-Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15(11):1195-1206.
1. Landgren O, Kyle R, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113(22):5412-5417.
2. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
3. Hutchison CA, Batuman V, Behrens J, et al; International Kidney and Monoclonal Gammopathy Research Group. The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat Review Nephrol. 2011;8(1):43-51.
4. Dimopoulous M, Kyle R, Fermand JP, et al; International Myeloma Workshop Consensus Panel 3. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705.
5. Palumbo A, Rajkumar S, San Miguel JF, et al. International Melanoma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol. 2014;32(6):587-600.
6. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548.
7. Dimopoulos MA, Kastritis E, Terpo E. Non-secretory myeloma: one, two, or more entities? Oncology (Williston Park). 2013;27(9):930-932.
8. Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36(3):842-854.
9. Griepp P, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412-3420.
10. Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014; 28(5):1122-1128.
11. Rajkumar SV. Multiple myeloma: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014;89(10):999-1009.
12. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564-569.
13. Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356(25):2582-2590.
14. Landgren O. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma: biological insights and early treatment strategies. Hematology Am Soc Hematol Educ Program. 2013;2013(1):478-487.
15. Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N Engl J Med. 2013;369(5):438-447.
16. Haessler K, Shaughnessy JD Jr, Zhan F, et al. Benefit of complete response in multiple myeloma limited to high-risk subgroup identified by gene expression profiling. Clin Cancer Res. 2007;13(23):7073-7079.
17. Xiang Z, Mehta P. Management of multiple myeloma and its precursor syndromes. Fed Pract. 2014;31(suppl 3):6S-13S.
18. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: multiple myeloma. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/PDF/myeloma.pdf. Updated March 10, 2015. Accessed July 8, 2015.
19. Kumar S, Flinn I, Richardson P, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclosphosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119(19):4375-4382.
20. Moreau P, Avet-Loiseau H, Facon T, et al. Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood. 2011;118(22):5752-5758.
21. Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomized, phase 3, noninferiority study. Lancet Oncol. 2011;12(5):431-440.
22. Pineda-Roman M, Zangari M, Haessler J, et al. Sustained complete remissions in multiple myeloma linked to bortezomib in total therapy 3: comparison with total therapy 2. Br J Haematol. 2008;140(6):624-634.
23. Kumar S, Dispenzieri A, Lacy MQ, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21(9):2035-2042.
24. Kumar S, Giralt S, Stadtmauer EA, et al; International Myeloma Working Group. Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell collection following initial therapy with thalidomide-, lenalidomide-, or bortezomibcontaining regimens. Blood. 2009;114(9):1729-1735.
25. Larocca A, Cavallo F, Bringhen S, et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma patients treated with lenalidomide. Blood. 2012;119(4):933-939.
26. Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120(9):1801-1809.
27. Korde N, Zingone A, Kwok M, et al. Phase II clinical and correlative study of carfilzomib, lenalidomide, and dexamethasone followed by lenalidomide extended dosing (CRD-R) induces high rates of MRD negativity in newly diagnosed multiple myeloma patients [Abstract]. Blood. 2013;122(21):538.
28. Gay F, Hayman SR, Lacy MQ, et al. Lenalidomide plus dexamethasone versus thalidomide plus dexamethasone in newly diagnosed multiple myeloma: a comparative analysis of 411 patients. Blood. 2010;115(7):1343-1350.
29. Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med. 1996;335(2):91-97.
30. Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371(10):895-905.
31. Fermand JP, Ravaud P, Chevret S, et al. High-dose therapy and autologous stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998;92(9):3131-3136.
32. Elice F, Raimondi R, Tosetto A, et al. Prolonged overall survival with second on-demand autologous stem cell transplant in multiple myeloma. Am J Hematol. 2006;81(6):426-431.
33. Facon T, Dimopoulos MA, Dispenzieri A, et al. Initial phase 3 results of the FIRST (frontline investigation of lenalidomide + dexamethasone versus standard thalidomide) trial (MM-020/IFM 07 01) in newly diagnosed multiple myeloma (NDMM) patients (pts) ineligible for stem cell transplantation (SCT). Blood. 2013;122(21):2.
34. San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906-917.
35. Facon T, Mary JY, Hulin C, et al; Intergroupe Français du Myélome. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370(9594):1209-1218.
36. Hulin C, Facon T, Rodon P, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma. IFM 01/01 trial. J Clin Oncol. 2009;27(22):3664-3670.
37. Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1782-1791.
38. Attal M., Harousseau JL, Leyvraz S, et al; Inter-Groupe Francophone du Myélome (IFM). Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108(10):3289-3294.
39. Spencer A, Prince HM, Roberts AW, et al. Consolidation therapy with low-dose thalidomide and prednisolone prolongs the survival of multiple myeloma patients undergoing a single autologous stem-cell transplantation procedure. J Clin Oncol. 2009;27(11):1788-1793.
40. Sonneveld P, Schmidt-Wolf IG, van der Holt B, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/GMMG-HD4 trial. J Clin Oncol. 2012;30(24):2946-2955.
41. Richardson PG, Sonneveld P, Schuster MW, et al; Assessment of Proteasome Inhibition for Extending Remissions (APEX) Investigators. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487-2498.
42. Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25(25):3892-3901.
43. Kumar SK, Lee JH, Lahuerta JJ, et al; International Myeloma Working Group. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26(1):149-157.
44. Lacy MQ, Hayman SR, Gertz MA, et al. Pomalidomide (CC4047) plus low-dose dexamethasone as therapy for relapsed multiple myeloma. J Clin Oncol. 2009;27(30):5008-5014.
45. Siegel DS, Martin T, Wang M, et al. A phase 2 study of single agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120(14):2817-2825.
46. San-Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15(11):1195-1206.
Weighing risks, benefits of autologous HSCT in MM
The use of autologous transplant after a 3-drug induction regimen for multiple myeloma (MM) prolongs progression-free survival (PFS) but not overall survival (OS), according to new research.
In a phase 3 trial, newly diagnosed MM patients who received lenalidomide, bortezomib, and dexamethasone (RVD) followed by an autologous hematopoietic stem cell transplant (HSCT) had significantly better PFS but similar OS when compared to patients who only received RVD.
In addition, HSCT recipients had significantly higher rates of high-grade blood and lymphatic system disorders, gastrointestinal events, and infections.
The study also showed that OS outcomes were similar for patients who received HSCT after completing treatment with RVD and patients who were in the RVD-only treatment arm but underwent HSCT later, as salvage therapy.
Researchers believe this suggests MM patients can potentially choose when to undergo a transplant.
The team reported their findings in NEJM. The study was supported by grants from Celgene and Janssen and by funds from the French Ministry of Health Programme Hospitalier de Recherche Clinique and from the French National Research Agency.
“Over the past decade, drugs that modulate the immune system and agents known as proteasome inhibitors have shown a great deal of promise in patients with multiple myeloma, when used in combination with chemotherapy,” said study author Paul Richardson, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
“This led us to propose that these combinations could be used selectively with established modalities such as transplant for patients with newly diagnosed myeloma, and this, in turn, raised questions about where and how transplant should be fit into the therapeutic paradigm. Our trial sought to comprehensively address these issues in a prospective fashion, and provide a foundation for future studies as the next generation of agents, such as monoclonal antibodies, impact the field.”
The study enrolled 700 adult patients under the age of 65 who were newly diagnosed with MM. They were treated at 69 centers in France, Belgium, and Switzerland.
The patients were randomized to 2 treatment arms. Both groups received 3 initial cycles of RVD.
One group then received 5 more cycles of RVD. The other received high-dose chemotherapy (melphalan) followed by an autologous HSCT and 2 additional cycles of RVD.
Patients in both groups then received lenalidomide as maintenance therapy for 1 year or until they progressed, experienced unacceptable adverse events (AEs), or withdrew consent.
Response and survival
The complete response rate was significantly higher in the HSCT arm than the RVD-alone arm—59% and 48%, respectively (P=0.03).
And there was a significantly higher percentage of patients who were negative for minimal residual disease in the HSCT arm than in the RVD arm—79% and 65%, respectively (P<0.001).
The median PFS was significantly longer in the HSCT arm than the RVD arm—50 months and 36 months, respectively (P<0.001). The researchers said this benefit was observed across all patient subgroups, including those stratified according to International Staging System stage and cytogenetic risk.
There was no significant between-group difference in the rate of OS at 4 years, which was 81% in the HSCT arm and 82% in the RVD arm.
Subsequent therapy
In the RVD arm, 207 patients progressed, and 172 received second-line therapy, which was followed by salvage HSCT in 136 patients (79%).
In the HSCT arm, 149 patients progressed, and 123 received second-line therapy, which was followed by a second HSCT in 21 patients (17%).
Safety
Nine percent of patients in the RVD arm and 11% in the HSCT arm discontinued treatment due to AEs. There were 2 treatment-related deaths in the RVD arm and 6 in the HSCT arm.
Grade 3/4 AEs with a significantly higher incidence in the HSCT arm than the RVD arm were blood and lymphatic system disorders (95% and 64%, respectively, P<0.001), gastrointestinal disorders (28% and 7%, respectively, P<0.001), and infections (20% and 9%, respectively, P<0.001).
Thirteen patients in the RVD arm and 17 in the HSCT arm had at least 1 invasive second primary malignancy (P=0.36). Acute myeloid leukemia occurred in 1 patient in the RVD arm and 4 in the HSCT arm (P=0.21).
The researchers said these results suggest the benefits of HSCT plus RVD must be weighed against the increased risk of toxicity associated with high-dose chemotherapy plus HSCT, particularly since HSCT after progression might be as effective as early HSCT for ensuring long-term OS. ![]()
The use of autologous transplant after a 3-drug induction regimen for multiple myeloma (MM) prolongs progression-free survival (PFS) but not overall survival (OS), according to new research.
In a phase 3 trial, newly diagnosed MM patients who received lenalidomide, bortezomib, and dexamethasone (RVD) followed by an autologous hematopoietic stem cell transplant (HSCT) had significantly better PFS but similar OS when compared to patients who only received RVD.
In addition, HSCT recipients had significantly higher rates of high-grade blood and lymphatic system disorders, gastrointestinal events, and infections.
The study also showed that OS outcomes were similar for patients who received HSCT after completing treatment with RVD and patients who were in the RVD-only treatment arm but underwent HSCT later, as salvage therapy.
Researchers believe this suggests MM patients can potentially choose when to undergo a transplant.
The team reported their findings in NEJM. The study was supported by grants from Celgene and Janssen and by funds from the French Ministry of Health Programme Hospitalier de Recherche Clinique and from the French National Research Agency.
“Over the past decade, drugs that modulate the immune system and agents known as proteasome inhibitors have shown a great deal of promise in patients with multiple myeloma, when used in combination with chemotherapy,” said study author Paul Richardson, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
“This led us to propose that these combinations could be used selectively with established modalities such as transplant for patients with newly diagnosed myeloma, and this, in turn, raised questions about where and how transplant should be fit into the therapeutic paradigm. Our trial sought to comprehensively address these issues in a prospective fashion, and provide a foundation for future studies as the next generation of agents, such as monoclonal antibodies, impact the field.”
The study enrolled 700 adult patients under the age of 65 who were newly diagnosed with MM. They were treated at 69 centers in France, Belgium, and Switzerland.
The patients were randomized to 2 treatment arms. Both groups received 3 initial cycles of RVD.
One group then received 5 more cycles of RVD. The other received high-dose chemotherapy (melphalan) followed by an autologous HSCT and 2 additional cycles of RVD.
Patients in both groups then received lenalidomide as maintenance therapy for 1 year or until they progressed, experienced unacceptable adverse events (AEs), or withdrew consent.
Response and survival
The complete response rate was significantly higher in the HSCT arm than the RVD-alone arm—59% and 48%, respectively (P=0.03).
And there was a significantly higher percentage of patients who were negative for minimal residual disease in the HSCT arm than in the RVD arm—79% and 65%, respectively (P<0.001).
The median PFS was significantly longer in the HSCT arm than the RVD arm—50 months and 36 months, respectively (P<0.001). The researchers said this benefit was observed across all patient subgroups, including those stratified according to International Staging System stage and cytogenetic risk.
There was no significant between-group difference in the rate of OS at 4 years, which was 81% in the HSCT arm and 82% in the RVD arm.
Subsequent therapy
In the RVD arm, 207 patients progressed, and 172 received second-line therapy, which was followed by salvage HSCT in 136 patients (79%).
In the HSCT arm, 149 patients progressed, and 123 received second-line therapy, which was followed by a second HSCT in 21 patients (17%).
Safety
Nine percent of patients in the RVD arm and 11% in the HSCT arm discontinued treatment due to AEs. There were 2 treatment-related deaths in the RVD arm and 6 in the HSCT arm.
Grade 3/4 AEs with a significantly higher incidence in the HSCT arm than the RVD arm were blood and lymphatic system disorders (95% and 64%, respectively, P<0.001), gastrointestinal disorders (28% and 7%, respectively, P<0.001), and infections (20% and 9%, respectively, P<0.001).
Thirteen patients in the RVD arm and 17 in the HSCT arm had at least 1 invasive second primary malignancy (P=0.36). Acute myeloid leukemia occurred in 1 patient in the RVD arm and 4 in the HSCT arm (P=0.21).
The researchers said these results suggest the benefits of HSCT plus RVD must be weighed against the increased risk of toxicity associated with high-dose chemotherapy plus HSCT, particularly since HSCT after progression might be as effective as early HSCT for ensuring long-term OS. ![]()
The use of autologous transplant after a 3-drug induction regimen for multiple myeloma (MM) prolongs progression-free survival (PFS) but not overall survival (OS), according to new research.
In a phase 3 trial, newly diagnosed MM patients who received lenalidomide, bortezomib, and dexamethasone (RVD) followed by an autologous hematopoietic stem cell transplant (HSCT) had significantly better PFS but similar OS when compared to patients who only received RVD.
In addition, HSCT recipients had significantly higher rates of high-grade blood and lymphatic system disorders, gastrointestinal events, and infections.
The study also showed that OS outcomes were similar for patients who received HSCT after completing treatment with RVD and patients who were in the RVD-only treatment arm but underwent HSCT later, as salvage therapy.
Researchers believe this suggests MM patients can potentially choose when to undergo a transplant.
The team reported their findings in NEJM. The study was supported by grants from Celgene and Janssen and by funds from the French Ministry of Health Programme Hospitalier de Recherche Clinique and from the French National Research Agency.
“Over the past decade, drugs that modulate the immune system and agents known as proteasome inhibitors have shown a great deal of promise in patients with multiple myeloma, when used in combination with chemotherapy,” said study author Paul Richardson, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
“This led us to propose that these combinations could be used selectively with established modalities such as transplant for patients with newly diagnosed myeloma, and this, in turn, raised questions about where and how transplant should be fit into the therapeutic paradigm. Our trial sought to comprehensively address these issues in a prospective fashion, and provide a foundation for future studies as the next generation of agents, such as monoclonal antibodies, impact the field.”
The study enrolled 700 adult patients under the age of 65 who were newly diagnosed with MM. They were treated at 69 centers in France, Belgium, and Switzerland.
The patients were randomized to 2 treatment arms. Both groups received 3 initial cycles of RVD.
One group then received 5 more cycles of RVD. The other received high-dose chemotherapy (melphalan) followed by an autologous HSCT and 2 additional cycles of RVD.
Patients in both groups then received lenalidomide as maintenance therapy for 1 year or until they progressed, experienced unacceptable adverse events (AEs), or withdrew consent.
Response and survival
The complete response rate was significantly higher in the HSCT arm than the RVD-alone arm—59% and 48%, respectively (P=0.03).
And there was a significantly higher percentage of patients who were negative for minimal residual disease in the HSCT arm than in the RVD arm—79% and 65%, respectively (P<0.001).
The median PFS was significantly longer in the HSCT arm than the RVD arm—50 months and 36 months, respectively (P<0.001). The researchers said this benefit was observed across all patient subgroups, including those stratified according to International Staging System stage and cytogenetic risk.
There was no significant between-group difference in the rate of OS at 4 years, which was 81% in the HSCT arm and 82% in the RVD arm.
Subsequent therapy
In the RVD arm, 207 patients progressed, and 172 received second-line therapy, which was followed by salvage HSCT in 136 patients (79%).
In the HSCT arm, 149 patients progressed, and 123 received second-line therapy, which was followed by a second HSCT in 21 patients (17%).
Safety
Nine percent of patients in the RVD arm and 11% in the HSCT arm discontinued treatment due to AEs. There were 2 treatment-related deaths in the RVD arm and 6 in the HSCT arm.
Grade 3/4 AEs with a significantly higher incidence in the HSCT arm than the RVD arm were blood and lymphatic system disorders (95% and 64%, respectively, P<0.001), gastrointestinal disorders (28% and 7%, respectively, P<0.001), and infections (20% and 9%, respectively, P<0.001).
Thirteen patients in the RVD arm and 17 in the HSCT arm had at least 1 invasive second primary malignancy (P=0.36). Acute myeloid leukemia occurred in 1 patient in the RVD arm and 4 in the HSCT arm (P=0.21).
The researchers said these results suggest the benefits of HSCT plus RVD must be weighed against the increased risk of toxicity associated with high-dose chemotherapy plus HSCT, particularly since HSCT after progression might be as effective as early HSCT for ensuring long-term OS. ![]()
Method may help MM patients avoid bone marrow biopsies
Engineers say they have devised a microfluidic technique to capture and count circulating plasma cells from small samples of blood.
They believe the technique, described in Scientific Reports, may allow patients with multiple myeloma (MM) to avoid bone marrow biopsies in favor of conventional blood draws.
“Procedures of the traditional tissue biopsy are painful, associated with complications such as potential infections, and often available only in central hospitals, which require patients to travel long distances,” said Mohammad Qasaimeh, PhD, of New York University Abu Dhabi in United Arab Emirates.
“Capturing plasma cells from blood samples can serve as a liquid biopsy, which can be performed in clinics as often as required, and serve as a diagnostic and prognostic test during and after chemotherapy treatment. Moreover, captured cells can be used for drug testing and, thus, serve as a tool for personalized medicine.”
About the technique
The current microfluidic technique builds on a design that was developed by George Whitesides, PhD, of Harvard University in Cambridge, Massachusetts.
Dr Whitesides and his colleagues fabricated a microchip, the channel of which they etched with repeating, V-shaped grooves, similar to a herringbone pattern.
The grooves cause any fluid flowing through the microchip to swirl about in eddies, rather passing straight through. The cells within the fluid therefore have a higher chance of making contact with the floor of the device.
Researchers have since reproduced this microfluidic design, coating the microchip’s floor with certain molecules to attract cells of interest.
Qasaimeh and colleagues used the microfluidic herringbone design to capture circulating plasma cells. They coated the channels of a microchip with CD138, an antibody expressed on the membranes of plasma cells.
The team then flowed 1 mL samples of blood through the device. The herringbone grooves circulated the blood in the microfluidic channels, where the antibodies grabbed onto any passing plasma cells and let the rest of the blood flow out of the device.
Once the cells were isolated in the microchip, the researchers could count the cells, as well as determine the kinds of antibodies each cell secretes.
Capturing cells
The researchers tested the device using blood samples from healthy donors as well as MM patients.
The team observed very low numbers of circulating plasma cells in healthy samples—2 to 5 cells per mL of blood—and substantially higher counts in MM patients—45 to 184 cells per mL.
The researchers noted that MM patients in remission also exhibited higher counts of circulating plasma cells—20 to 24 cells per mL—than healthy donors.
Rahit Karnik, PhD, of Massachusetts Institute of Technology in Cambridge, said this device may reveal more subtle information about a patient’s state, even in remission.
“When patients go into remission, their antibody levels can look normal,” Dr Karnik said. “But we detect a level of circulating plasma cells that is above the baseline. It’s hard to tell whether these cells are cancerous, but at least this technique is giving us more information. With the ease of a blood draw, this may enable us to track cancer in a much better way.”
Analyzing antibodies
The researchers also looked at antibodies produced by the plasma cells captured in the device.
The team determined the ratio of plasma cells producing kappa- and lambda-type antibodies and compared them to conventional blood tests for the same antibodies, for both healthy subjects and MM patients.
Both sets of results matched, which validates the microfluidic device’s accuracy.
Next steps
Dr Karnik said, in the future, researchers may use this design to perform genetic tests on the captured cells or look for mutations in the cells that may further characterize MM.
“We can capture and stain these cells in the device, which opens the possibility of studying whether there are new mutations in the cells,” Dr Karnik said.
“With cancers like multiple myeloma, even for patients in remission, cancer can recur. Detecting the level or mutation of plasma cells in blood might provide an early detection method for these patients.” ![]()
Engineers say they have devised a microfluidic technique to capture and count circulating plasma cells from small samples of blood.
They believe the technique, described in Scientific Reports, may allow patients with multiple myeloma (MM) to avoid bone marrow biopsies in favor of conventional blood draws.
“Procedures of the traditional tissue biopsy are painful, associated with complications such as potential infections, and often available only in central hospitals, which require patients to travel long distances,” said Mohammad Qasaimeh, PhD, of New York University Abu Dhabi in United Arab Emirates.
“Capturing plasma cells from blood samples can serve as a liquid biopsy, which can be performed in clinics as often as required, and serve as a diagnostic and prognostic test during and after chemotherapy treatment. Moreover, captured cells can be used for drug testing and, thus, serve as a tool for personalized medicine.”
About the technique
The current microfluidic technique builds on a design that was developed by George Whitesides, PhD, of Harvard University in Cambridge, Massachusetts.
Dr Whitesides and his colleagues fabricated a microchip, the channel of which they etched with repeating, V-shaped grooves, similar to a herringbone pattern.
The grooves cause any fluid flowing through the microchip to swirl about in eddies, rather passing straight through. The cells within the fluid therefore have a higher chance of making contact with the floor of the device.
Researchers have since reproduced this microfluidic design, coating the microchip’s floor with certain molecules to attract cells of interest.
Qasaimeh and colleagues used the microfluidic herringbone design to capture circulating plasma cells. They coated the channels of a microchip with CD138, an antibody expressed on the membranes of plasma cells.
The team then flowed 1 mL samples of blood through the device. The herringbone grooves circulated the blood in the microfluidic channels, where the antibodies grabbed onto any passing plasma cells and let the rest of the blood flow out of the device.
Once the cells were isolated in the microchip, the researchers could count the cells, as well as determine the kinds of antibodies each cell secretes.
Capturing cells
The researchers tested the device using blood samples from healthy donors as well as MM patients.
The team observed very low numbers of circulating plasma cells in healthy samples—2 to 5 cells per mL of blood—and substantially higher counts in MM patients—45 to 184 cells per mL.
The researchers noted that MM patients in remission also exhibited higher counts of circulating plasma cells—20 to 24 cells per mL—than healthy donors.
Rahit Karnik, PhD, of Massachusetts Institute of Technology in Cambridge, said this device may reveal more subtle information about a patient’s state, even in remission.
“When patients go into remission, their antibody levels can look normal,” Dr Karnik said. “But we detect a level of circulating plasma cells that is above the baseline. It’s hard to tell whether these cells are cancerous, but at least this technique is giving us more information. With the ease of a blood draw, this may enable us to track cancer in a much better way.”
Analyzing antibodies
The researchers also looked at antibodies produced by the plasma cells captured in the device.
The team determined the ratio of plasma cells producing kappa- and lambda-type antibodies and compared them to conventional blood tests for the same antibodies, for both healthy subjects and MM patients.
Both sets of results matched, which validates the microfluidic device’s accuracy.
Next steps
Dr Karnik said, in the future, researchers may use this design to perform genetic tests on the captured cells or look for mutations in the cells that may further characterize MM.
“We can capture and stain these cells in the device, which opens the possibility of studying whether there are new mutations in the cells,” Dr Karnik said.
“With cancers like multiple myeloma, even for patients in remission, cancer can recur. Detecting the level or mutation of plasma cells in blood might provide an early detection method for these patients.” ![]()
Engineers say they have devised a microfluidic technique to capture and count circulating plasma cells from small samples of blood.
They believe the technique, described in Scientific Reports, may allow patients with multiple myeloma (MM) to avoid bone marrow biopsies in favor of conventional blood draws.
“Procedures of the traditional tissue biopsy are painful, associated with complications such as potential infections, and often available only in central hospitals, which require patients to travel long distances,” said Mohammad Qasaimeh, PhD, of New York University Abu Dhabi in United Arab Emirates.
“Capturing plasma cells from blood samples can serve as a liquid biopsy, which can be performed in clinics as often as required, and serve as a diagnostic and prognostic test during and after chemotherapy treatment. Moreover, captured cells can be used for drug testing and, thus, serve as a tool for personalized medicine.”
About the technique
The current microfluidic technique builds on a design that was developed by George Whitesides, PhD, of Harvard University in Cambridge, Massachusetts.
Dr Whitesides and his colleagues fabricated a microchip, the channel of which they etched with repeating, V-shaped grooves, similar to a herringbone pattern.
The grooves cause any fluid flowing through the microchip to swirl about in eddies, rather passing straight through. The cells within the fluid therefore have a higher chance of making contact with the floor of the device.
Researchers have since reproduced this microfluidic design, coating the microchip’s floor with certain molecules to attract cells of interest.
Qasaimeh and colleagues used the microfluidic herringbone design to capture circulating plasma cells. They coated the channels of a microchip with CD138, an antibody expressed on the membranes of plasma cells.
The team then flowed 1 mL samples of blood through the device. The herringbone grooves circulated the blood in the microfluidic channels, where the antibodies grabbed onto any passing plasma cells and let the rest of the blood flow out of the device.
Once the cells were isolated in the microchip, the researchers could count the cells, as well as determine the kinds of antibodies each cell secretes.
Capturing cells
The researchers tested the device using blood samples from healthy donors as well as MM patients.
The team observed very low numbers of circulating plasma cells in healthy samples—2 to 5 cells per mL of blood—and substantially higher counts in MM patients—45 to 184 cells per mL.
The researchers noted that MM patients in remission also exhibited higher counts of circulating plasma cells—20 to 24 cells per mL—than healthy donors.
Rahit Karnik, PhD, of Massachusetts Institute of Technology in Cambridge, said this device may reveal more subtle information about a patient’s state, even in remission.
“When patients go into remission, their antibody levels can look normal,” Dr Karnik said. “But we detect a level of circulating plasma cells that is above the baseline. It’s hard to tell whether these cells are cancerous, but at least this technique is giving us more information. With the ease of a blood draw, this may enable us to track cancer in a much better way.”
Analyzing antibodies
The researchers also looked at antibodies produced by the plasma cells captured in the device.
The team determined the ratio of plasma cells producing kappa- and lambda-type antibodies and compared them to conventional blood tests for the same antibodies, for both healthy subjects and MM patients.
Both sets of results matched, which validates the microfluidic device’s accuracy.
Next steps
Dr Karnik said, in the future, researchers may use this design to perform genetic tests on the captured cells or look for mutations in the cells that may further characterize MM.
“We can capture and stain these cells in the device, which opens the possibility of studying whether there are new mutations in the cells,” Dr Karnik said.
“With cancers like multiple myeloma, even for patients in remission, cancer can recur. Detecting the level or mutation of plasma cells in blood might provide an early detection method for these patients.” ![]()
Transplantation plus VRD tops VRD alone in myeloma
Adding high-dose chemotherapy and autologous stem cell transplantation to two cycles of bortezomib, lenalidomide, and dexamethasone (VRD) significantly improved progression-free survival in adults with newly diagnosed multiple myeloma, according to new analyses from the multicenter, randomized, phase III IFM 2009 Study.
Median PFS was 50 months in the transplantation group, versus 36 months when patients only received five cycles of VRD for consolidation (adjusted hazard ratio for disease progression or death, 0.65; 95% confidence interval, 0.53 to 0.80; P less than .001), reported Michel Attal, MD, of Institut Universitaire du Cancer de Toulouse-Oncopole in Toulouse, France, and his associates. “Transplantation was also associated with a higher rate of complete response, a lower rate of minimal residual disease detection, and a longer median time to progression,” they wrote (N Engl J Med. 2017 April 6. doi: 10.1056/NEJMoa1611750).
Transplantation did not improve 4-year overall survival (81% with transplantation plus VRD and 82% with VRD only), perhaps because VRD-only patients had the option of salvage transplantation if their disease progressed, the investigators said. In addition, grade 3 or 4 neutropenias were significantly more common with transplantation than otherwise (92% versus 47%), as were grade 3 or 4 gastrointestinal disorders (28% versus 7%) and infections (20% versus 9%). However, the groups did not significantly differ in terms of treatment-related deaths, second primary cancers, thromboembolic events, or peripheral neuropathy.
Before the advent of immunomodulatory drugs and proteasome inhibitors, several randomized trials showed that the benefits of high-dose chemotherapy with autologous stem cell transplantation outperformed conventional chemotherapy in multiple myeloma. For IFM 2009, 700 newly diagnosed patients aged up to 65 years received three 21-day cycles of VRD induction: bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11; lenalidomide 25 mg on days 1-14; and dexamethasone 20 mg on days 1, 2, 4, 5, 8, 9, 11, and 12. Consolidation consisted of either five more VRD cycles with dexamethasone reduced to 10 mg/day, or high-dose (200 mg/m2) melphalan plus transplantation followed by two VRD cycles with the reduced dexamethasone dose. Both arms received 1 year of lenalidomide maintenance (10 mg/day for 3 months, followed by 15/mg day, depending on side effects).
Transplantation was associated with a significantly higher likelihood of complete response (59% versus 48%; P = .03) and undetectable minimal residual disease (79% versus 65%; P less than .001). Undetectable minimal residual disease, in turn, predicted longer PFS (adjusted hazard ratio, 0.30; P = .001). In contrast, age, sex, isotype of the monoclonal component, International Staging System disease stage, and cytogenetic risk profile did not significantly affect PFS among transplant patients.
The PFS benefit of transplantation “must be weighed against the increased risk of toxic effects associated with high-dose chemotherapy plus transplantation, especially since we found that later transplantation might be as effective as early transplantation in securing long- term survival,” the researchers said. “Our results suggest that the use of a combination therapy that incorporates newer proteasome inhibitors, next-generation immunomodulatory drugs, and potent monoclonal antibodies along with transplantation tailored according to minimal residual disease detection could further improve outcomes among adults up to 65 years of age who have multiple myeloma.”
Celgene, Janssen, the French Ministry of Health Programme Hospitalier de Recherche Clinique, and the French National Research Agency funded the study. Dr. Attal had no conflicts. Nine coinvestigators disclosed ties to Celgene, Janssen, and several other pharmaceutical companies. Two coinvestigators disclosed ownership or founder roles in OncoPep, one of whom disclosed patent licensure to OncoPep for a multipeptide vaccine. The other investigators had no conflicts.
In the IFM 2009 trial, the planned treatments were realistic. The combination of immunomodulatory drugs and proteasome inhibitors is associated with high response rates similar to those achieved with transplantation, whereas previous trials had used less effective chemotherapy regimens as comparators.
[Transplantation] resulted in a deeper and longer initial treatment response than did nontransplantation. However, the benefits of transplantation were more modest than some might have hoped, and it did not appear to be curative.
A question remains as to whether all patients with multiple myeloma should undergo immediate autologous stem-cell transplantation, since the use of delayed transplantation resulted in similar overall survival, with some patients not needing transplantation to date. The observed lack of a survival benefit for early transplantation was consistent with previous results that suggested salvage transplantation is an equalizer in this regard.
Charles A. Schiffer, MD, and Jeffrey A. Zonder, MD, are with Karmanos Cancer Institute, Wayne State University, Detroit, Mich. Dr. Zonder is a member of the editorial advisory board of Hematology News. Their comments were taken from an editorial (N Engl J Med. 2017 April 6. doi: 10.1056/NEJMe1700453).
In the IFM 2009 trial, the planned treatments were realistic. The combination of immunomodulatory drugs and proteasome inhibitors is associated with high response rates similar to those achieved with transplantation, whereas previous trials had used less effective chemotherapy regimens as comparators.
[Transplantation] resulted in a deeper and longer initial treatment response than did nontransplantation. However, the benefits of transplantation were more modest than some might have hoped, and it did not appear to be curative.
A question remains as to whether all patients with multiple myeloma should undergo immediate autologous stem-cell transplantation, since the use of delayed transplantation resulted in similar overall survival, with some patients not needing transplantation to date. The observed lack of a survival benefit for early transplantation was consistent with previous results that suggested salvage transplantation is an equalizer in this regard.
Charles A. Schiffer, MD, and Jeffrey A. Zonder, MD, are with Karmanos Cancer Institute, Wayne State University, Detroit, Mich. Dr. Zonder is a member of the editorial advisory board of Hematology News. Their comments were taken from an editorial (N Engl J Med. 2017 April 6. doi: 10.1056/NEJMe1700453).
In the IFM 2009 trial, the planned treatments were realistic. The combination of immunomodulatory drugs and proteasome inhibitors is associated with high response rates similar to those achieved with transplantation, whereas previous trials had used less effective chemotherapy regimens as comparators.
[Transplantation] resulted in a deeper and longer initial treatment response than did nontransplantation. However, the benefits of transplantation were more modest than some might have hoped, and it did not appear to be curative.
A question remains as to whether all patients with multiple myeloma should undergo immediate autologous stem-cell transplantation, since the use of delayed transplantation resulted in similar overall survival, with some patients not needing transplantation to date. The observed lack of a survival benefit for early transplantation was consistent with previous results that suggested salvage transplantation is an equalizer in this regard.
Charles A. Schiffer, MD, and Jeffrey A. Zonder, MD, are with Karmanos Cancer Institute, Wayne State University, Detroit, Mich. Dr. Zonder is a member of the editorial advisory board of Hematology News. Their comments were taken from an editorial (N Engl J Med. 2017 April 6. doi: 10.1056/NEJMe1700453).
Adding high-dose chemotherapy and autologous stem cell transplantation to two cycles of bortezomib, lenalidomide, and dexamethasone (VRD) significantly improved progression-free survival in adults with newly diagnosed multiple myeloma, according to new analyses from the multicenter, randomized, phase III IFM 2009 Study.
Median PFS was 50 months in the transplantation group, versus 36 months when patients only received five cycles of VRD for consolidation (adjusted hazard ratio for disease progression or death, 0.65; 95% confidence interval, 0.53 to 0.80; P less than .001), reported Michel Attal, MD, of Institut Universitaire du Cancer de Toulouse-Oncopole in Toulouse, France, and his associates. “Transplantation was also associated with a higher rate of complete response, a lower rate of minimal residual disease detection, and a longer median time to progression,” they wrote (N Engl J Med. 2017 April 6. doi: 10.1056/NEJMoa1611750).
Transplantation did not improve 4-year overall survival (81% with transplantation plus VRD and 82% with VRD only), perhaps because VRD-only patients had the option of salvage transplantation if their disease progressed, the investigators said. In addition, grade 3 or 4 neutropenias were significantly more common with transplantation than otherwise (92% versus 47%), as were grade 3 or 4 gastrointestinal disorders (28% versus 7%) and infections (20% versus 9%). However, the groups did not significantly differ in terms of treatment-related deaths, second primary cancers, thromboembolic events, or peripheral neuropathy.
Before the advent of immunomodulatory drugs and proteasome inhibitors, several randomized trials showed that the benefits of high-dose chemotherapy with autologous stem cell transplantation outperformed conventional chemotherapy in multiple myeloma. For IFM 2009, 700 newly diagnosed patients aged up to 65 years received three 21-day cycles of VRD induction: bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11; lenalidomide 25 mg on days 1-14; and dexamethasone 20 mg on days 1, 2, 4, 5, 8, 9, 11, and 12. Consolidation consisted of either five more VRD cycles with dexamethasone reduced to 10 mg/day, or high-dose (200 mg/m2) melphalan plus transplantation followed by two VRD cycles with the reduced dexamethasone dose. Both arms received 1 year of lenalidomide maintenance (10 mg/day for 3 months, followed by 15/mg day, depending on side effects).
Transplantation was associated with a significantly higher likelihood of complete response (59% versus 48%; P = .03) and undetectable minimal residual disease (79% versus 65%; P less than .001). Undetectable minimal residual disease, in turn, predicted longer PFS (adjusted hazard ratio, 0.30; P = .001). In contrast, age, sex, isotype of the monoclonal component, International Staging System disease stage, and cytogenetic risk profile did not significantly affect PFS among transplant patients.
The PFS benefit of transplantation “must be weighed against the increased risk of toxic effects associated with high-dose chemotherapy plus transplantation, especially since we found that later transplantation might be as effective as early transplantation in securing long- term survival,” the researchers said. “Our results suggest that the use of a combination therapy that incorporates newer proteasome inhibitors, next-generation immunomodulatory drugs, and potent monoclonal antibodies along with transplantation tailored according to minimal residual disease detection could further improve outcomes among adults up to 65 years of age who have multiple myeloma.”
Celgene, Janssen, the French Ministry of Health Programme Hospitalier de Recherche Clinique, and the French National Research Agency funded the study. Dr. Attal had no conflicts. Nine coinvestigators disclosed ties to Celgene, Janssen, and several other pharmaceutical companies. Two coinvestigators disclosed ownership or founder roles in OncoPep, one of whom disclosed patent licensure to OncoPep for a multipeptide vaccine. The other investigators had no conflicts.
Adding high-dose chemotherapy and autologous stem cell transplantation to two cycles of bortezomib, lenalidomide, and dexamethasone (VRD) significantly improved progression-free survival in adults with newly diagnosed multiple myeloma, according to new analyses from the multicenter, randomized, phase III IFM 2009 Study.
Median PFS was 50 months in the transplantation group, versus 36 months when patients only received five cycles of VRD for consolidation (adjusted hazard ratio for disease progression or death, 0.65; 95% confidence interval, 0.53 to 0.80; P less than .001), reported Michel Attal, MD, of Institut Universitaire du Cancer de Toulouse-Oncopole in Toulouse, France, and his associates. “Transplantation was also associated with a higher rate of complete response, a lower rate of minimal residual disease detection, and a longer median time to progression,” they wrote (N Engl J Med. 2017 April 6. doi: 10.1056/NEJMoa1611750).
Transplantation did not improve 4-year overall survival (81% with transplantation plus VRD and 82% with VRD only), perhaps because VRD-only patients had the option of salvage transplantation if their disease progressed, the investigators said. In addition, grade 3 or 4 neutropenias were significantly more common with transplantation than otherwise (92% versus 47%), as were grade 3 or 4 gastrointestinal disorders (28% versus 7%) and infections (20% versus 9%). However, the groups did not significantly differ in terms of treatment-related deaths, second primary cancers, thromboembolic events, or peripheral neuropathy.
Before the advent of immunomodulatory drugs and proteasome inhibitors, several randomized trials showed that the benefits of high-dose chemotherapy with autologous stem cell transplantation outperformed conventional chemotherapy in multiple myeloma. For IFM 2009, 700 newly diagnosed patients aged up to 65 years received three 21-day cycles of VRD induction: bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11; lenalidomide 25 mg on days 1-14; and dexamethasone 20 mg on days 1, 2, 4, 5, 8, 9, 11, and 12. Consolidation consisted of either five more VRD cycles with dexamethasone reduced to 10 mg/day, or high-dose (200 mg/m2) melphalan plus transplantation followed by two VRD cycles with the reduced dexamethasone dose. Both arms received 1 year of lenalidomide maintenance (10 mg/day for 3 months, followed by 15/mg day, depending on side effects).
Transplantation was associated with a significantly higher likelihood of complete response (59% versus 48%; P = .03) and undetectable minimal residual disease (79% versus 65%; P less than .001). Undetectable minimal residual disease, in turn, predicted longer PFS (adjusted hazard ratio, 0.30; P = .001). In contrast, age, sex, isotype of the monoclonal component, International Staging System disease stage, and cytogenetic risk profile did not significantly affect PFS among transplant patients.
The PFS benefit of transplantation “must be weighed against the increased risk of toxic effects associated with high-dose chemotherapy plus transplantation, especially since we found that later transplantation might be as effective as early transplantation in securing long- term survival,” the researchers said. “Our results suggest that the use of a combination therapy that incorporates newer proteasome inhibitors, next-generation immunomodulatory drugs, and potent monoclonal antibodies along with transplantation tailored according to minimal residual disease detection could further improve outcomes among adults up to 65 years of age who have multiple myeloma.”
Celgene, Janssen, the French Ministry of Health Programme Hospitalier de Recherche Clinique, and the French National Research Agency funded the study. Dr. Attal had no conflicts. Nine coinvestigators disclosed ties to Celgene, Janssen, and several other pharmaceutical companies. Two coinvestigators disclosed ownership or founder roles in OncoPep, one of whom disclosed patent licensure to OncoPep for a multipeptide vaccine. The other investigators had no conflicts.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: High-dose chemotherapy and autologous stem cell transplantation plus bortezomib, lenalidomide, and dexamethasone (VRD) outperformed consolidation with VRD only in adults with newly diagnosed multiple myeloma.
Major finding: Median progression-free survival was 50 months with transplantation plus VRD and 36 months with VRD only (adjusted hazard ratio for disease progression or death, 0.65; P less than .001).
Data source: A randomized, multicenter, open-label, phase III trial of 700 patients with multiple myeloma.
Disclosures: Celgene, Janssen, the French Ministry of Health Programme Hospitalier de Recherche Clinique, and the French National Research Agency funded the study. Dr. Attal had no conflicts. Nine coinvestigators disclosed ties to Celgene, Janssen, and several other pharmaceutical companies. Two coinvestigators disclosed ownership or founder roles in OncoPep, one of whom disclosed patent licensure to OncoPep for a multipeptide vaccine. The other investigators had no conflicts.
Diagnosing Gastric Plasmacytoma
Extramedullary plasmacytomas are rare ( > 5% of all plasma cell neoplasms), and the gastric version is even more rare. That rarity combined with unspecific symptoms that mimic other tumors “can mislead the clinician into a diagnosis of a more aggressive tumor,” say clinicians from University Hospital Center of Coimbra in Portugal. They reported on a patient who presented that diagnostic puzzle.
The patient arrived in the emergency department with symptoms of upper gastrointestinal bleeding. He had a history of tongue carcinoma, cirrhosis, diabetes mellitus, chronic gastritis, and obesity, among other health conditions, and was taking a panoply of medications. The patient’s family history included a grandmother with breast cancer and an aunt with head and neck cancer.
Related: Treatment and Management of Multiple Myeloma
The clinicians embarked on an extensive diagnostic process of elimination. Endoscopy, immunohistochemistry, and other tests helped rule out Helicobacter pylori infection, lymphoma, carcinoma, melanoma, and neuroendocrine carcinoma. “Rethinking our possibilities,” the clinicians say, and taking into account the presence of mature and immature plasmocytes in the periphery, they next considered plasmacytoma/multiple myeloma (MM). On the third immunohistochemical panel, they got the evidence they needed for the diagnosis: gastric plasmacytoma (GP)/ involvement by MM.
Gastric plasmacytomas have a good prognosis. Surgical excision normally is the treatment of choice with good results, the clinicians say. After endoscopic polypectomy without radiation, the patient was discharged with no further therapy. Although diagnosed with prostate cancer in the 6 years following, the patient has had no signs of plasmacytic relapse.
Related: Rare Cancer Gets Timely Right Treatment
Due to their cellular similarity, GP needs to be differentiated from MM. But GP may be the initial manifestation of a MM, the clinicians note. Therefore, the patient must undergo systemic evaluation to exclude bone marrow involvement and to make sure there is no clinical or laboratory evidence of myeloma.
Sources:
1. Oliveira RC, Amaro P, Julião MJ, Cipriano MA. BMJ Case Rep. 2017. pii: bcr2016218967.
doi: 10.1136/bcr-2016-218967.
2. International Myeloma Working Group. Br J Haematol. 2003;12(5):749-57.
doi: 10.1046/j.1365-2141.2003.04355.x
3. Kilciksiz S, Karakoyun-Celik O, Agaoglu FY, Haydaroglu A. A review for solitary
plasmacytoma of bone and extramedullary plasmacytoma. Sci World J. 2012;2012:895765.
doi: 10.1100/2012/895765.
Extramedullary plasmacytomas are rare ( > 5% of all plasma cell neoplasms), and the gastric version is even more rare. That rarity combined with unspecific symptoms that mimic other tumors “can mislead the clinician into a diagnosis of a more aggressive tumor,” say clinicians from University Hospital Center of Coimbra in Portugal. They reported on a patient who presented that diagnostic puzzle.
The patient arrived in the emergency department with symptoms of upper gastrointestinal bleeding. He had a history of tongue carcinoma, cirrhosis, diabetes mellitus, chronic gastritis, and obesity, among other health conditions, and was taking a panoply of medications. The patient’s family history included a grandmother with breast cancer and an aunt with head and neck cancer.
Related: Treatment and Management of Multiple Myeloma
The clinicians embarked on an extensive diagnostic process of elimination. Endoscopy, immunohistochemistry, and other tests helped rule out Helicobacter pylori infection, lymphoma, carcinoma, melanoma, and neuroendocrine carcinoma. “Rethinking our possibilities,” the clinicians say, and taking into account the presence of mature and immature plasmocytes in the periphery, they next considered plasmacytoma/multiple myeloma (MM). On the third immunohistochemical panel, they got the evidence they needed for the diagnosis: gastric plasmacytoma (GP)/ involvement by MM.
Gastric plasmacytomas have a good prognosis. Surgical excision normally is the treatment of choice with good results, the clinicians say. After endoscopic polypectomy without radiation, the patient was discharged with no further therapy. Although diagnosed with prostate cancer in the 6 years following, the patient has had no signs of plasmacytic relapse.
Related: Rare Cancer Gets Timely Right Treatment
Due to their cellular similarity, GP needs to be differentiated from MM. But GP may be the initial manifestation of a MM, the clinicians note. Therefore, the patient must undergo systemic evaluation to exclude bone marrow involvement and to make sure there is no clinical or laboratory evidence of myeloma.
Sources:
1. Oliveira RC, Amaro P, Julião MJ, Cipriano MA. BMJ Case Rep. 2017. pii: bcr2016218967.
doi: 10.1136/bcr-2016-218967.
2. International Myeloma Working Group. Br J Haematol. 2003;12(5):749-57.
doi: 10.1046/j.1365-2141.2003.04355.x
3. Kilciksiz S, Karakoyun-Celik O, Agaoglu FY, Haydaroglu A. A review for solitary
plasmacytoma of bone and extramedullary plasmacytoma. Sci World J. 2012;2012:895765.
doi: 10.1100/2012/895765.
Extramedullary plasmacytomas are rare ( > 5% of all plasma cell neoplasms), and the gastric version is even more rare. That rarity combined with unspecific symptoms that mimic other tumors “can mislead the clinician into a diagnosis of a more aggressive tumor,” say clinicians from University Hospital Center of Coimbra in Portugal. They reported on a patient who presented that diagnostic puzzle.
The patient arrived in the emergency department with symptoms of upper gastrointestinal bleeding. He had a history of tongue carcinoma, cirrhosis, diabetes mellitus, chronic gastritis, and obesity, among other health conditions, and was taking a panoply of medications. The patient’s family history included a grandmother with breast cancer and an aunt with head and neck cancer.
Related: Treatment and Management of Multiple Myeloma
The clinicians embarked on an extensive diagnostic process of elimination. Endoscopy, immunohistochemistry, and other tests helped rule out Helicobacter pylori infection, lymphoma, carcinoma, melanoma, and neuroendocrine carcinoma. “Rethinking our possibilities,” the clinicians say, and taking into account the presence of mature and immature plasmocytes in the periphery, they next considered plasmacytoma/multiple myeloma (MM). On the third immunohistochemical panel, they got the evidence they needed for the diagnosis: gastric plasmacytoma (GP)/ involvement by MM.
Gastric plasmacytomas have a good prognosis. Surgical excision normally is the treatment of choice with good results, the clinicians say. After endoscopic polypectomy without radiation, the patient was discharged with no further therapy. Although diagnosed with prostate cancer in the 6 years following, the patient has had no signs of plasmacytic relapse.
Related: Rare Cancer Gets Timely Right Treatment
Due to their cellular similarity, GP needs to be differentiated from MM. But GP may be the initial manifestation of a MM, the clinicians note. Therefore, the patient must undergo systemic evaluation to exclude bone marrow involvement and to make sure there is no clinical or laboratory evidence of myeloma.
Sources:
1. Oliveira RC, Amaro P, Julião MJ, Cipriano MA. BMJ Case Rep. 2017. pii: bcr2016218967.
doi: 10.1136/bcr-2016-218967.
2. International Myeloma Working Group. Br J Haematol. 2003;12(5):749-57.
doi: 10.1046/j.1365-2141.2003.04355.x
3. Kilciksiz S, Karakoyun-Celik O, Agaoglu FY, Haydaroglu A. A review for solitary
plasmacytoma of bone and extramedullary plasmacytoma. Sci World J. 2012;2012:895765.
doi: 10.1100/2012/895765.
ASCO addresses needs of SGMs with cancer
The American Society of Clinical Oncology (ASCO) has issued recommendations addressing the needs of sexual and gender minority (SGM) populations with cancer.
The recommendations are designed to focus attention on the challenges facing the SGM community—including discrimination and greater risk of anxiety and depression, resulting in disparate care—and concrete steps that can help minimize health disparities among SGM individuals.
The recommendations were published in a policy statement in the Journal of Clinical Oncology.
“Sexual and gender minorities face unique challenges related to cancer risk, discrimination, and other psychosocial issues,” said ASCO President Daniel F. Hayes, MD.
“Compounding these challenges is the fact that providers may have a lack of knowledge and sensitivity about the health risks and health needs facing their SGM patients.”
SGMs include individuals who are lesbian, gay, bisexual, transgender, and intersex (also referred to as those with differences in sex development).
ASCO’s policy statement notes that SGM populations bear a disproportionate cancer burden stemming from several factors, including:
- Lower rates of cancer screening, in part due to lower rates of insurance coverage, exclusion from traditional screening campaigns, and previous experience of discrimination in the healthcare system
- A hesitancy on the part of SGM patients to disclose their sexual orientation to providers due to a fear of stigmatization, which can create additional barriers to care.
ASCO’s statement calls for a coordinated effort to address health disparities among SGM populations, including:
- Increased patient access to culturally competent support services
- Expanded cancer prevention education for SGM individuals
- Robust policies prohibiting discrimination
- Adequate insurance coverage to meet the needs of SGM individuals affected by cancer
- Inclusion of SGM status as a required data element in cancer registries and clinical trials
- Increased focus on SGM populations in cancer research.
“Our objective was to raise awareness among oncology providers, patients, policy makers, and other stakeholders about the cancer care needs of SGM populations and the barriers that SGM individuals face in getting the highest-quality care,” said Jennifer J. Griggs, MD, lead author of the policy statement and a professor at the University of Michigan in Ann Arbor.
“To address these barriers, a coordinated effort is needed to enhance education for patients and providers, to improve outreach and support, and to encourage productive policy and legislative action.” ![]()
The American Society of Clinical Oncology (ASCO) has issued recommendations addressing the needs of sexual and gender minority (SGM) populations with cancer.
The recommendations are designed to focus attention on the challenges facing the SGM community—including discrimination and greater risk of anxiety and depression, resulting in disparate care—and concrete steps that can help minimize health disparities among SGM individuals.
The recommendations were published in a policy statement in the Journal of Clinical Oncology.
“Sexual and gender minorities face unique challenges related to cancer risk, discrimination, and other psychosocial issues,” said ASCO President Daniel F. Hayes, MD.
“Compounding these challenges is the fact that providers may have a lack of knowledge and sensitivity about the health risks and health needs facing their SGM patients.”
SGMs include individuals who are lesbian, gay, bisexual, transgender, and intersex (also referred to as those with differences in sex development).
ASCO’s policy statement notes that SGM populations bear a disproportionate cancer burden stemming from several factors, including:
- Lower rates of cancer screening, in part due to lower rates of insurance coverage, exclusion from traditional screening campaigns, and previous experience of discrimination in the healthcare system
- A hesitancy on the part of SGM patients to disclose their sexual orientation to providers due to a fear of stigmatization, which can create additional barriers to care.
ASCO’s statement calls for a coordinated effort to address health disparities among SGM populations, including:
- Increased patient access to culturally competent support services
- Expanded cancer prevention education for SGM individuals
- Robust policies prohibiting discrimination
- Adequate insurance coverage to meet the needs of SGM individuals affected by cancer
- Inclusion of SGM status as a required data element in cancer registries and clinical trials
- Increased focus on SGM populations in cancer research.
“Our objective was to raise awareness among oncology providers, patients, policy makers, and other stakeholders about the cancer care needs of SGM populations and the barriers that SGM individuals face in getting the highest-quality care,” said Jennifer J. Griggs, MD, lead author of the policy statement and a professor at the University of Michigan in Ann Arbor.
“To address these barriers, a coordinated effort is needed to enhance education for patients and providers, to improve outreach and support, and to encourage productive policy and legislative action.” ![]()
The American Society of Clinical Oncology (ASCO) has issued recommendations addressing the needs of sexual and gender minority (SGM) populations with cancer.
The recommendations are designed to focus attention on the challenges facing the SGM community—including discrimination and greater risk of anxiety and depression, resulting in disparate care—and concrete steps that can help minimize health disparities among SGM individuals.
The recommendations were published in a policy statement in the Journal of Clinical Oncology.
“Sexual and gender minorities face unique challenges related to cancer risk, discrimination, and other psychosocial issues,” said ASCO President Daniel F. Hayes, MD.
“Compounding these challenges is the fact that providers may have a lack of knowledge and sensitivity about the health risks and health needs facing their SGM patients.”
SGMs include individuals who are lesbian, gay, bisexual, transgender, and intersex (also referred to as those with differences in sex development).
ASCO’s policy statement notes that SGM populations bear a disproportionate cancer burden stemming from several factors, including:
- Lower rates of cancer screening, in part due to lower rates of insurance coverage, exclusion from traditional screening campaigns, and previous experience of discrimination in the healthcare system
- A hesitancy on the part of SGM patients to disclose their sexual orientation to providers due to a fear of stigmatization, which can create additional barriers to care.
ASCO’s statement calls for a coordinated effort to address health disparities among SGM populations, including:
- Increased patient access to culturally competent support services
- Expanded cancer prevention education for SGM individuals
- Robust policies prohibiting discrimination
- Adequate insurance coverage to meet the needs of SGM individuals affected by cancer
- Inclusion of SGM status as a required data element in cancer registries and clinical trials
- Increased focus on SGM populations in cancer research.
“Our objective was to raise awareness among oncology providers, patients, policy makers, and other stakeholders about the cancer care needs of SGM populations and the barriers that SGM individuals face in getting the highest-quality care,” said Jennifer J. Griggs, MD, lead author of the policy statement and a professor at the University of Michigan in Ann Arbor.
“To address these barriers, a coordinated effort is needed to enhance education for patients and providers, to improve outreach and support, and to encourage productive policy and legislative action.” ![]()
Get ready for cancer immunotherapy-induced rheumatic diseases
SNOWMASS, COLO. – Physicians can expect to encounter more and more patients with inflammatory arthritis and other rheumatic adverse events induced by immune checkpoint inhibitors as a result of anticipated exponential growth in the use of these drugs to treat an expanding list of cancers, Clifton O. Bingham III, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
These cancer immunotherapy–induced rheumatic diseases may superficially look like the classic forms of familiar autoimmune diseases, but they have highly atypical features that will affect treatment decisions.
“What we’ve seen consistently is that the normal doses of prednisone we would use to treat an inflammatory arthritis are really ineffective in most of these patients. We’ve had to use super doses – up to 120 mg/day – for initial control, and then 7.5-40 mg daily for maintenance of response,” according to Dr. Bingham, professor of medicine and director of the Johns Hopkins Arthritis Center in Baltimore.
To date, only limited data from case series are available on rheumatic IRAEs. There are no prospective patient registries logging accurate data on the incidence of these rheumatic adverse events among cancer patients treated with immune checkpoint inhibitors (ICIs). These IRAEs, which lie at the intersection of rheumatology and oncology, are of special interest to Dr. Bingham – he and his coinvestigators have published five articles on the topic over the course of a single year.
In a wide-ranging talk at the symposium, he touched on the phenotypic spectrum of rheumatologic IRAEs, his conviction that they are greatly underdiagnosed, why physicians can expect to encounter them much more frequently, rheumatologic IRAE treatment issues, and the risks of prescribing ICIs in patients with known preexisting rheumatologic disease.
Rheumatologic IRAE presentations
Inflammatory arthritis is the most common form of rheumatologic IRAE, followed by sicca syndrome. At the Johns Hopkins Arthritis Center, Dr. Bingham and his coworkers have 25 well-characterized patients with inflammatory arthritis resulting from an ICI, only 1 of whom is HLA-B27-positive.
“Also, just one is autoantibody-positive, even though they all look for all the world as though they have rheumatoid arthritis,” the rheumatologist observed.
This ICI-induced inflammatory arthritis initially presents most commonly in the midsize and large joints – knees, ankles, elbows – then expands to include small joints such as the wrists, proximal interphalangeal joints, and the metacarpophalangeal joints.
Notably, the Hopkins group also has three patients with classic reactive arthritis marked by conjunctivitis, urethritis, arthritis, and dactylitis.
“I don’t know about you, but, in our general rheumatology practice, we see maybe one case of reactive arthritis in several years, so this is something that has struck us as really quite interesting,” said Dr. Bingham, who is also director of research in the division of rheumatology at Johns Hopkins.
The arthritis center is also managing a group of patients with ICI-induced sicca syndrome, which is uniformly extremely severe and treatment resistant, as well as a couple of patients with myositis IRAE, one with polymyalgia rheumatica, and two with crystal disease that is highly inflammatory in nature, difficult to treat, and includes an inflammatory polyarthritis component not typical in patients with crystal arthritis.
Why physicians will see more rheumatologic IRAEs
ICIs have dramatically transformed the treatment of selected advanced-stage cancers. For example, whereas patients with metastatic melanoma historically had a 2-year survival rate of 5%, combination therapy with the ICIs ipilimumab (Yervoy) and nivolumab (Opdivo) resulted in a 60% rate of partial or complete remission in a landmark clinical trial.
The basis of cancer immunotherapy is the discovery that, in order for cancer cells to thrive, they emit blocking signals that downregulate the native ability of T cells to recognize and kill them. This is true for both solid tumors and hematologic malignancies. The ICIs inhibit these blocking signals, which include cytotoxic T-lymphocyte–associated protein 4 (CTLA4), programmed death-1 (PD-1), and programmed death ligand-1 (PDL-1), thereby freeing up the T cells for tumor fighting.
Because of the nonspecific mechanism of this T-cell activation, however, ICIs have, as their main toxicities, T-cell–mediated autoimmune inflammatory tissue damage, which gets lumped under the umbrella term IRAEs. It can affect almost every organ system. Skin rashes are the most common, colitis second. Other commonly encountered IRAEs include thyroiditis, hypophysitis, hepatitis, peripheral neuropathy, and pneumonitis.
In addition to the four currently approved ICIs – ipilimumab, nivolumab, pembrolizumab (Keytruda), and atezolizumab (Tecentriq) – investigational ICIs targeting CTLA4, PD-1, and/or PDL-1 are in development. Plus, new ICIs targeting other blocking signals, including lymphocyte activation gene-3, CD137, and T-cell immunoglobulin and mucin domain-3, are now in clinical trials.
Clinical trials aimed at expanding the indications of existing ICIs and using ICIs in earlier-stage cancers in an effort to improve rates of lasting remission are also underway.
All told, probably at least 400 clinical trials of ICIs are ongoing worldwide, the rheumatologist estimated.
“More people will be exposed to these drugs, and we’ll see more and more of these rheumatologic IRAEs,” Dr. Bingham predicted.
Rheumatologic IRAEs are seriously underdiagnosed
Back in the pre-ICI days, Dr. Bingham was coauthor of a major study which concluded that clinical trialists in oncology consistently downgrade the severity of rheumatologic adverse events, often by 1 or 2 grades (J Rheumatol. 2007 Jun;34[6]:1401-14).
Unpublished details of ICI clinical trials in melanoma that he obtained from Bristol-Myers Squibb suggest that the true rate of rheumatologic IRAEs is about 20%, or roughly double that reported in the studies. That’s because the adverse events–grading system used in oncology undercalls the severity of arthritis and autoimmune disorders.
Indeed, the National Cancer Institute’s Common Terminology Criteria for Adverse Events, used in oncology clinical trials, is confusing on the topic of musculoskeletal and connective tissue disorders as treatment-emergent adverse events, according to Dr. Bingham. He noted that an oncologist can code a swollen joint in three different ways – joint effusion, arthritis, or arthralgia – and it takes disabling interference with self-care in activities of daily living for that swollen joint to rise to the level of a Grade 3 adverse event. From a rheumatology trialist’s perspective, that would be a Grade 4 disability.
Plus, neither the product labeling nor the patient information guides for the approved immunotherapy drugs mention the importance of monitoring for rheumatologic IRAEs or their management.
“There is poor awareness of musculoskeletal and rheumatic IRAEs in the general oncology community,” Dr. Bingham asserted. “But, if you talk with any oncology nurses who work in a clinical trial, they will tell you they’re seeing these events with significant frequency and severity.”
Treatment and response
It’s critical to gain control of rheumatologic IRAEs quickly so that patients can get on with their cancer immunotherapy. Dr. Bingham uses intra-articular steroid injections for patients with oligoarthritis and high-dose oral prednisone for polyarticular disease. He starts methotrexate and/or leflunomide early because the conventional disease-modifying antirheumatic drugs have roughly a 2-month delay in onset of action. He has had several patients who are unable to taper steroids despite background methotrexate.
In the most severely affected patients, he has turned to biologic agents in consultation with their oncologists. Tumor necrosis factor (TNF) inhibitors are the ones he and other rheumatologists have used most often.
“Notably, we have not been able to taper down very well. We have patients who are out more than 2 years now who still require their TNF inhibitor to treat their inflammatory arthritis, and these are patients on conventional disease–modifying antirheumatic drugs as well. As soon as it’s tapered, the arthritis begins to come back,” according to Dr. Bingham.
In marked contrast, colitis as an IRAE typically clears in response to just one or two doses of a TNF inhibitor.
One audience member related that she’d encountered a roadblock in trying to get authorization for a TNF inhibitor for a patient with a rheumatologic IRAE secondary to ICI treatment for metastatic melanoma because the labeling states these agents are relatively contraindicated in melanoma patients. Dr. Bingham offered a tip: Collaborate with the patient’s oncologist.
“In most cases, oncologists can get infliximab for these patients and administer it in their infusion centers. They are able to get things authorized with very little trouble,” he said.
Besides, most of these patients with severe inflammatory arthritis meet conventional criteria for TNF inhibitor therapy, based on their number of infected joints and elevated acute phase reactants for longer than 6 weeks, Dr. Bingham noted.
“We’ve had some very interesting conversations with patients. It’s impressive to see the impact arthritis can have on people. A lot of patients have said, ‘I don’t care if I die. Get me functional right now.’ That’s pretty profound. Quality of life is still very important for people, even when dealing with life-threatening diseases,” he observed.
Oncologists are actually eager for their patients to get on steroid-sparing therapy because of concern that high doses of steroids may reduce the efficacy of cancer immunotherapy. That’s not an issue with the TNF inhibitors, the rheumatologist continued.
Turning to the utility of other classes of biologic agents, Dr. Bingham advised avoiding abatacept (Orencia) because its mechanism of action is likely to cause interference with the cancer immunotherapy. Rituximab (Rituxan) takes too long to act. Anakinra (Kineret), tofacitinib (Xeljanz), and tocilizumab (Actemra), on the other hand, are agents he is interested in using as alternatives to TNF inhibitors, although he hasn’t done so yet.
Use of ICIs in patients with preexisting autoimmune disease
The experience here is entirely anecdotal, since such patients have been excluded from ICI clinical trials, but the available evidence suggests physicians should be prepared for higher rheumatologic IRAE rates in this setting. Investigators at Vanderbilt University reported that 8 of 30 cancer patients with known preexisting autoimmune disease experienced flares of that disease when treated with ipilimumab, and 10 developed a new IRAE (Therap Adv Gastroenterol. 2016 Jul;9[4]:457-62).
The Hopkins group has three patients with preexisting rheumatoid arthritis and two with preexisting scleroderma who have received ICIs. All three rheumatoid arthritis patients flared. Rheumatologists are trying to manage these flares so the patients can continue on their ICI. One of the scleroderma patients experienced no change in that disease while on an ICI, while the other showed a definite improvement in scleroderma symptoms.
“I think the jury’s still out in terms of what you do about ICI therapy in patients with preexisting autoimmunity. The data would say that there’s maybe a 50-50 chance of the autoimmune disease becoming worse, but, if patients have an otherwise fatal cancer, I think it’s probably worth the chance,” Dr. Bingham said.
Anecdotal reports suggest that more severe IRAEs may be a favorable prognostic sign in terms of cancer eradication, but a lot more patient experience will be needed in order to be sure, the rheumatologist said.
Dr. Bingham reported serving as a consultant to Bristol-Myers Squibb.
SNOWMASS, COLO. – Physicians can expect to encounter more and more patients with inflammatory arthritis and other rheumatic adverse events induced by immune checkpoint inhibitors as a result of anticipated exponential growth in the use of these drugs to treat an expanding list of cancers, Clifton O. Bingham III, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
These cancer immunotherapy–induced rheumatic diseases may superficially look like the classic forms of familiar autoimmune diseases, but they have highly atypical features that will affect treatment decisions.
“What we’ve seen consistently is that the normal doses of prednisone we would use to treat an inflammatory arthritis are really ineffective in most of these patients. We’ve had to use super doses – up to 120 mg/day – for initial control, and then 7.5-40 mg daily for maintenance of response,” according to Dr. Bingham, professor of medicine and director of the Johns Hopkins Arthritis Center in Baltimore.
To date, only limited data from case series are available on rheumatic IRAEs. There are no prospective patient registries logging accurate data on the incidence of these rheumatic adverse events among cancer patients treated with immune checkpoint inhibitors (ICIs). These IRAEs, which lie at the intersection of rheumatology and oncology, are of special interest to Dr. Bingham – he and his coinvestigators have published five articles on the topic over the course of a single year.
In a wide-ranging talk at the symposium, he touched on the phenotypic spectrum of rheumatologic IRAEs, his conviction that they are greatly underdiagnosed, why physicians can expect to encounter them much more frequently, rheumatologic IRAE treatment issues, and the risks of prescribing ICIs in patients with known preexisting rheumatologic disease.
Rheumatologic IRAE presentations
Inflammatory arthritis is the most common form of rheumatologic IRAE, followed by sicca syndrome. At the Johns Hopkins Arthritis Center, Dr. Bingham and his coworkers have 25 well-characterized patients with inflammatory arthritis resulting from an ICI, only 1 of whom is HLA-B27-positive.
“Also, just one is autoantibody-positive, even though they all look for all the world as though they have rheumatoid arthritis,” the rheumatologist observed.
This ICI-induced inflammatory arthritis initially presents most commonly in the midsize and large joints – knees, ankles, elbows – then expands to include small joints such as the wrists, proximal interphalangeal joints, and the metacarpophalangeal joints.
Notably, the Hopkins group also has three patients with classic reactive arthritis marked by conjunctivitis, urethritis, arthritis, and dactylitis.
“I don’t know about you, but, in our general rheumatology practice, we see maybe one case of reactive arthritis in several years, so this is something that has struck us as really quite interesting,” said Dr. Bingham, who is also director of research in the division of rheumatology at Johns Hopkins.
The arthritis center is also managing a group of patients with ICI-induced sicca syndrome, which is uniformly extremely severe and treatment resistant, as well as a couple of patients with myositis IRAE, one with polymyalgia rheumatica, and two with crystal disease that is highly inflammatory in nature, difficult to treat, and includes an inflammatory polyarthritis component not typical in patients with crystal arthritis.
Why physicians will see more rheumatologic IRAEs
ICIs have dramatically transformed the treatment of selected advanced-stage cancers. For example, whereas patients with metastatic melanoma historically had a 2-year survival rate of 5%, combination therapy with the ICIs ipilimumab (Yervoy) and nivolumab (Opdivo) resulted in a 60% rate of partial or complete remission in a landmark clinical trial.
The basis of cancer immunotherapy is the discovery that, in order for cancer cells to thrive, they emit blocking signals that downregulate the native ability of T cells to recognize and kill them. This is true for both solid tumors and hematologic malignancies. The ICIs inhibit these blocking signals, which include cytotoxic T-lymphocyte–associated protein 4 (CTLA4), programmed death-1 (PD-1), and programmed death ligand-1 (PDL-1), thereby freeing up the T cells for tumor fighting.
Because of the nonspecific mechanism of this T-cell activation, however, ICIs have, as their main toxicities, T-cell–mediated autoimmune inflammatory tissue damage, which gets lumped under the umbrella term IRAEs. It can affect almost every organ system. Skin rashes are the most common, colitis second. Other commonly encountered IRAEs include thyroiditis, hypophysitis, hepatitis, peripheral neuropathy, and pneumonitis.
In addition to the four currently approved ICIs – ipilimumab, nivolumab, pembrolizumab (Keytruda), and atezolizumab (Tecentriq) – investigational ICIs targeting CTLA4, PD-1, and/or PDL-1 are in development. Plus, new ICIs targeting other blocking signals, including lymphocyte activation gene-3, CD137, and T-cell immunoglobulin and mucin domain-3, are now in clinical trials.
Clinical trials aimed at expanding the indications of existing ICIs and using ICIs in earlier-stage cancers in an effort to improve rates of lasting remission are also underway.
All told, probably at least 400 clinical trials of ICIs are ongoing worldwide, the rheumatologist estimated.
“More people will be exposed to these drugs, and we’ll see more and more of these rheumatologic IRAEs,” Dr. Bingham predicted.
Rheumatologic IRAEs are seriously underdiagnosed
Back in the pre-ICI days, Dr. Bingham was coauthor of a major study which concluded that clinical trialists in oncology consistently downgrade the severity of rheumatologic adverse events, often by 1 or 2 grades (J Rheumatol. 2007 Jun;34[6]:1401-14).
Unpublished details of ICI clinical trials in melanoma that he obtained from Bristol-Myers Squibb suggest that the true rate of rheumatologic IRAEs is about 20%, or roughly double that reported in the studies. That’s because the adverse events–grading system used in oncology undercalls the severity of arthritis and autoimmune disorders.
Indeed, the National Cancer Institute’s Common Terminology Criteria for Adverse Events, used in oncology clinical trials, is confusing on the topic of musculoskeletal and connective tissue disorders as treatment-emergent adverse events, according to Dr. Bingham. He noted that an oncologist can code a swollen joint in three different ways – joint effusion, arthritis, or arthralgia – and it takes disabling interference with self-care in activities of daily living for that swollen joint to rise to the level of a Grade 3 adverse event. From a rheumatology trialist’s perspective, that would be a Grade 4 disability.
Plus, neither the product labeling nor the patient information guides for the approved immunotherapy drugs mention the importance of monitoring for rheumatologic IRAEs or their management.
“There is poor awareness of musculoskeletal and rheumatic IRAEs in the general oncology community,” Dr. Bingham asserted. “But, if you talk with any oncology nurses who work in a clinical trial, they will tell you they’re seeing these events with significant frequency and severity.”
Treatment and response
It’s critical to gain control of rheumatologic IRAEs quickly so that patients can get on with their cancer immunotherapy. Dr. Bingham uses intra-articular steroid injections for patients with oligoarthritis and high-dose oral prednisone for polyarticular disease. He starts methotrexate and/or leflunomide early because the conventional disease-modifying antirheumatic drugs have roughly a 2-month delay in onset of action. He has had several patients who are unable to taper steroids despite background methotrexate.
In the most severely affected patients, he has turned to biologic agents in consultation with their oncologists. Tumor necrosis factor (TNF) inhibitors are the ones he and other rheumatologists have used most often.
“Notably, we have not been able to taper down very well. We have patients who are out more than 2 years now who still require their TNF inhibitor to treat their inflammatory arthritis, and these are patients on conventional disease–modifying antirheumatic drugs as well. As soon as it’s tapered, the arthritis begins to come back,” according to Dr. Bingham.
In marked contrast, colitis as an IRAE typically clears in response to just one or two doses of a TNF inhibitor.
One audience member related that she’d encountered a roadblock in trying to get authorization for a TNF inhibitor for a patient with a rheumatologic IRAE secondary to ICI treatment for metastatic melanoma because the labeling states these agents are relatively contraindicated in melanoma patients. Dr. Bingham offered a tip: Collaborate with the patient’s oncologist.
“In most cases, oncologists can get infliximab for these patients and administer it in their infusion centers. They are able to get things authorized with very little trouble,” he said.
Besides, most of these patients with severe inflammatory arthritis meet conventional criteria for TNF inhibitor therapy, based on their number of infected joints and elevated acute phase reactants for longer than 6 weeks, Dr. Bingham noted.
“We’ve had some very interesting conversations with patients. It’s impressive to see the impact arthritis can have on people. A lot of patients have said, ‘I don’t care if I die. Get me functional right now.’ That’s pretty profound. Quality of life is still very important for people, even when dealing with life-threatening diseases,” he observed.
Oncologists are actually eager for their patients to get on steroid-sparing therapy because of concern that high doses of steroids may reduce the efficacy of cancer immunotherapy. That’s not an issue with the TNF inhibitors, the rheumatologist continued.
Turning to the utility of other classes of biologic agents, Dr. Bingham advised avoiding abatacept (Orencia) because its mechanism of action is likely to cause interference with the cancer immunotherapy. Rituximab (Rituxan) takes too long to act. Anakinra (Kineret), tofacitinib (Xeljanz), and tocilizumab (Actemra), on the other hand, are agents he is interested in using as alternatives to TNF inhibitors, although he hasn’t done so yet.
Use of ICIs in patients with preexisting autoimmune disease
The experience here is entirely anecdotal, since such patients have been excluded from ICI clinical trials, but the available evidence suggests physicians should be prepared for higher rheumatologic IRAE rates in this setting. Investigators at Vanderbilt University reported that 8 of 30 cancer patients with known preexisting autoimmune disease experienced flares of that disease when treated with ipilimumab, and 10 developed a new IRAE (Therap Adv Gastroenterol. 2016 Jul;9[4]:457-62).
The Hopkins group has three patients with preexisting rheumatoid arthritis and two with preexisting scleroderma who have received ICIs. All three rheumatoid arthritis patients flared. Rheumatologists are trying to manage these flares so the patients can continue on their ICI. One of the scleroderma patients experienced no change in that disease while on an ICI, while the other showed a definite improvement in scleroderma symptoms.
“I think the jury’s still out in terms of what you do about ICI therapy in patients with preexisting autoimmunity. The data would say that there’s maybe a 50-50 chance of the autoimmune disease becoming worse, but, if patients have an otherwise fatal cancer, I think it’s probably worth the chance,” Dr. Bingham said.
Anecdotal reports suggest that more severe IRAEs may be a favorable prognostic sign in terms of cancer eradication, but a lot more patient experience will be needed in order to be sure, the rheumatologist said.
Dr. Bingham reported serving as a consultant to Bristol-Myers Squibb.
SNOWMASS, COLO. – Physicians can expect to encounter more and more patients with inflammatory arthritis and other rheumatic adverse events induced by immune checkpoint inhibitors as a result of anticipated exponential growth in the use of these drugs to treat an expanding list of cancers, Clifton O. Bingham III, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
These cancer immunotherapy–induced rheumatic diseases may superficially look like the classic forms of familiar autoimmune diseases, but they have highly atypical features that will affect treatment decisions.
“What we’ve seen consistently is that the normal doses of prednisone we would use to treat an inflammatory arthritis are really ineffective in most of these patients. We’ve had to use super doses – up to 120 mg/day – for initial control, and then 7.5-40 mg daily for maintenance of response,” according to Dr. Bingham, professor of medicine and director of the Johns Hopkins Arthritis Center in Baltimore.
To date, only limited data from case series are available on rheumatic IRAEs. There are no prospective patient registries logging accurate data on the incidence of these rheumatic adverse events among cancer patients treated with immune checkpoint inhibitors (ICIs). These IRAEs, which lie at the intersection of rheumatology and oncology, are of special interest to Dr. Bingham – he and his coinvestigators have published five articles on the topic over the course of a single year.
In a wide-ranging talk at the symposium, he touched on the phenotypic spectrum of rheumatologic IRAEs, his conviction that they are greatly underdiagnosed, why physicians can expect to encounter them much more frequently, rheumatologic IRAE treatment issues, and the risks of prescribing ICIs in patients with known preexisting rheumatologic disease.
Rheumatologic IRAE presentations
Inflammatory arthritis is the most common form of rheumatologic IRAE, followed by sicca syndrome. At the Johns Hopkins Arthritis Center, Dr. Bingham and his coworkers have 25 well-characterized patients with inflammatory arthritis resulting from an ICI, only 1 of whom is HLA-B27-positive.
“Also, just one is autoantibody-positive, even though they all look for all the world as though they have rheumatoid arthritis,” the rheumatologist observed.
This ICI-induced inflammatory arthritis initially presents most commonly in the midsize and large joints – knees, ankles, elbows – then expands to include small joints such as the wrists, proximal interphalangeal joints, and the metacarpophalangeal joints.
Notably, the Hopkins group also has three patients with classic reactive arthritis marked by conjunctivitis, urethritis, arthritis, and dactylitis.
“I don’t know about you, but, in our general rheumatology practice, we see maybe one case of reactive arthritis in several years, so this is something that has struck us as really quite interesting,” said Dr. Bingham, who is also director of research in the division of rheumatology at Johns Hopkins.
The arthritis center is also managing a group of patients with ICI-induced sicca syndrome, which is uniformly extremely severe and treatment resistant, as well as a couple of patients with myositis IRAE, one with polymyalgia rheumatica, and two with crystal disease that is highly inflammatory in nature, difficult to treat, and includes an inflammatory polyarthritis component not typical in patients with crystal arthritis.
Why physicians will see more rheumatologic IRAEs
ICIs have dramatically transformed the treatment of selected advanced-stage cancers. For example, whereas patients with metastatic melanoma historically had a 2-year survival rate of 5%, combination therapy with the ICIs ipilimumab (Yervoy) and nivolumab (Opdivo) resulted in a 60% rate of partial or complete remission in a landmark clinical trial.
The basis of cancer immunotherapy is the discovery that, in order for cancer cells to thrive, they emit blocking signals that downregulate the native ability of T cells to recognize and kill them. This is true for both solid tumors and hematologic malignancies. The ICIs inhibit these blocking signals, which include cytotoxic T-lymphocyte–associated protein 4 (CTLA4), programmed death-1 (PD-1), and programmed death ligand-1 (PDL-1), thereby freeing up the T cells for tumor fighting.
Because of the nonspecific mechanism of this T-cell activation, however, ICIs have, as their main toxicities, T-cell–mediated autoimmune inflammatory tissue damage, which gets lumped under the umbrella term IRAEs. It can affect almost every organ system. Skin rashes are the most common, colitis second. Other commonly encountered IRAEs include thyroiditis, hypophysitis, hepatitis, peripheral neuropathy, and pneumonitis.
In addition to the four currently approved ICIs – ipilimumab, nivolumab, pembrolizumab (Keytruda), and atezolizumab (Tecentriq) – investigational ICIs targeting CTLA4, PD-1, and/or PDL-1 are in development. Plus, new ICIs targeting other blocking signals, including lymphocyte activation gene-3, CD137, and T-cell immunoglobulin and mucin domain-3, are now in clinical trials.
Clinical trials aimed at expanding the indications of existing ICIs and using ICIs in earlier-stage cancers in an effort to improve rates of lasting remission are also underway.
All told, probably at least 400 clinical trials of ICIs are ongoing worldwide, the rheumatologist estimated.
“More people will be exposed to these drugs, and we’ll see more and more of these rheumatologic IRAEs,” Dr. Bingham predicted.
Rheumatologic IRAEs are seriously underdiagnosed
Back in the pre-ICI days, Dr. Bingham was coauthor of a major study which concluded that clinical trialists in oncology consistently downgrade the severity of rheumatologic adverse events, often by 1 or 2 grades (J Rheumatol. 2007 Jun;34[6]:1401-14).
Unpublished details of ICI clinical trials in melanoma that he obtained from Bristol-Myers Squibb suggest that the true rate of rheumatologic IRAEs is about 20%, or roughly double that reported in the studies. That’s because the adverse events–grading system used in oncology undercalls the severity of arthritis and autoimmune disorders.
Indeed, the National Cancer Institute’s Common Terminology Criteria for Adverse Events, used in oncology clinical trials, is confusing on the topic of musculoskeletal and connective tissue disorders as treatment-emergent adverse events, according to Dr. Bingham. He noted that an oncologist can code a swollen joint in three different ways – joint effusion, arthritis, or arthralgia – and it takes disabling interference with self-care in activities of daily living for that swollen joint to rise to the level of a Grade 3 adverse event. From a rheumatology trialist’s perspective, that would be a Grade 4 disability.
Plus, neither the product labeling nor the patient information guides for the approved immunotherapy drugs mention the importance of monitoring for rheumatologic IRAEs or their management.
“There is poor awareness of musculoskeletal and rheumatic IRAEs in the general oncology community,” Dr. Bingham asserted. “But, if you talk with any oncology nurses who work in a clinical trial, they will tell you they’re seeing these events with significant frequency and severity.”
Treatment and response
It’s critical to gain control of rheumatologic IRAEs quickly so that patients can get on with their cancer immunotherapy. Dr. Bingham uses intra-articular steroid injections for patients with oligoarthritis and high-dose oral prednisone for polyarticular disease. He starts methotrexate and/or leflunomide early because the conventional disease-modifying antirheumatic drugs have roughly a 2-month delay in onset of action. He has had several patients who are unable to taper steroids despite background methotrexate.
In the most severely affected patients, he has turned to biologic agents in consultation with their oncologists. Tumor necrosis factor (TNF) inhibitors are the ones he and other rheumatologists have used most often.
“Notably, we have not been able to taper down very well. We have patients who are out more than 2 years now who still require their TNF inhibitor to treat their inflammatory arthritis, and these are patients on conventional disease–modifying antirheumatic drugs as well. As soon as it’s tapered, the arthritis begins to come back,” according to Dr. Bingham.
In marked contrast, colitis as an IRAE typically clears in response to just one or two doses of a TNF inhibitor.
One audience member related that she’d encountered a roadblock in trying to get authorization for a TNF inhibitor for a patient with a rheumatologic IRAE secondary to ICI treatment for metastatic melanoma because the labeling states these agents are relatively contraindicated in melanoma patients. Dr. Bingham offered a tip: Collaborate with the patient’s oncologist.
“In most cases, oncologists can get infliximab for these patients and administer it in their infusion centers. They are able to get things authorized with very little trouble,” he said.
Besides, most of these patients with severe inflammatory arthritis meet conventional criteria for TNF inhibitor therapy, based on their number of infected joints and elevated acute phase reactants for longer than 6 weeks, Dr. Bingham noted.
“We’ve had some very interesting conversations with patients. It’s impressive to see the impact arthritis can have on people. A lot of patients have said, ‘I don’t care if I die. Get me functional right now.’ That’s pretty profound. Quality of life is still very important for people, even when dealing with life-threatening diseases,” he observed.
Oncologists are actually eager for their patients to get on steroid-sparing therapy because of concern that high doses of steroids may reduce the efficacy of cancer immunotherapy. That’s not an issue with the TNF inhibitors, the rheumatologist continued.
Turning to the utility of other classes of biologic agents, Dr. Bingham advised avoiding abatacept (Orencia) because its mechanism of action is likely to cause interference with the cancer immunotherapy. Rituximab (Rituxan) takes too long to act. Anakinra (Kineret), tofacitinib (Xeljanz), and tocilizumab (Actemra), on the other hand, are agents he is interested in using as alternatives to TNF inhibitors, although he hasn’t done so yet.
Use of ICIs in patients with preexisting autoimmune disease
The experience here is entirely anecdotal, since such patients have been excluded from ICI clinical trials, but the available evidence suggests physicians should be prepared for higher rheumatologic IRAE rates in this setting. Investigators at Vanderbilt University reported that 8 of 30 cancer patients with known preexisting autoimmune disease experienced flares of that disease when treated with ipilimumab, and 10 developed a new IRAE (Therap Adv Gastroenterol. 2016 Jul;9[4]:457-62).
The Hopkins group has three patients with preexisting rheumatoid arthritis and two with preexisting scleroderma who have received ICIs. All three rheumatoid arthritis patients flared. Rheumatologists are trying to manage these flares so the patients can continue on their ICI. One of the scleroderma patients experienced no change in that disease while on an ICI, while the other showed a definite improvement in scleroderma symptoms.
“I think the jury’s still out in terms of what you do about ICI therapy in patients with preexisting autoimmunity. The data would say that there’s maybe a 50-50 chance of the autoimmune disease becoming worse, but, if patients have an otherwise fatal cancer, I think it’s probably worth the chance,” Dr. Bingham said.
Anecdotal reports suggest that more severe IRAEs may be a favorable prognostic sign in terms of cancer eradication, but a lot more patient experience will be needed in order to be sure, the rheumatologist said.
Dr. Bingham reported serving as a consultant to Bristol-Myers Squibb.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Report shows increase in blood cancer incidence and survival
A report on cancer in the US suggests the incidence of leukemia and myeloma has been on the rise in recent years, but the incidence of non-Hodgkin lymphoma (NHL) has been on the decline.
Meanwhile, annual death rates for leukemia and NHL have decreased, and annual death rates for myeloma have decreased in men but not in women.
Furthermore, patients with leukemia, NHL, and myeloma have seen a substantial improvement in 5-year survival rates in recent years relative to patients in the late 1970s.
These findings are part of the Annual Report to the Nation on the Status of Cancer, 1975-2014, which has been published in the Journal of the National Cancer Institute.
This report is released each year, but the current edition includes a special section focused on survival.
“While trends in death rates are the most commonly used measure to assess progress against cancer, survival trends are also an important measure to evaluate progress in improvement of cancer outcomes,” said Ahmedin Jemal, DVM, PhD, of the American Cancer Society.
“We last included a special section on cancer survival in 2004, and, as we found then, survival improved over time for almost all cancers at every stage of diagnosis.”
For the current report, researchers calculated the 5-year average annual percent changes (AAPCs) for 2009 to 2013 for cancer incidence and for 2010 to 2014 for cancer mortality.
Cancer incidence (2009-2013)
In women, the AAPC increased 1.5% for leukemia (P<0.05), decreased 0.5% for NHL (P<0.05), and increased 2.2% for myeloma (P<0.05).
In men, the AAPC increased 1.7% for leukemia (P<0.05), decreased 0.2% for NHL, and increased 2.8% for myeloma (P<0.05).
Cancer mortality (2010-2014)
In women, the AAPC decreased 1.2% for leukemia (P<0.05), decreased 2.2% for NHL (P<0.05), and increased 0.5% for myeloma.
In men, the AAPC decreased 1.0% for leukemia (P<0.05), decreased 2.0% for NHL (P<0.05), and decreased 0.9% for myeloma (P<0.05).
5-year survival
The researchers compared 5-year relative survival for cancers diagnosed from 1975 to 1977 and those diagnosed from 2006 to 2012.
The absolute percentage change over time (for both sexes combined) was 26.1% for NHL, 25.7% for myeloma, and 28.5% for leukemia.
Five-year survival for patients diagnosed in 1975-1977 was 46.5% for NHL, 24.6% for myeloma, and 34.2% for leukemia.
Five-year survival for patients diagnosed in 2006-2012 was 72.6% for NHL, 50.2% for myeloma, and 62.7% for leukemia. ![]()
A report on cancer in the US suggests the incidence of leukemia and myeloma has been on the rise in recent years, but the incidence of non-Hodgkin lymphoma (NHL) has been on the decline.
Meanwhile, annual death rates for leukemia and NHL have decreased, and annual death rates for myeloma have decreased in men but not in women.
Furthermore, patients with leukemia, NHL, and myeloma have seen a substantial improvement in 5-year survival rates in recent years relative to patients in the late 1970s.
These findings are part of the Annual Report to the Nation on the Status of Cancer, 1975-2014, which has been published in the Journal of the National Cancer Institute.
This report is released each year, but the current edition includes a special section focused on survival.
“While trends in death rates are the most commonly used measure to assess progress against cancer, survival trends are also an important measure to evaluate progress in improvement of cancer outcomes,” said Ahmedin Jemal, DVM, PhD, of the American Cancer Society.
“We last included a special section on cancer survival in 2004, and, as we found then, survival improved over time for almost all cancers at every stage of diagnosis.”
For the current report, researchers calculated the 5-year average annual percent changes (AAPCs) for 2009 to 2013 for cancer incidence and for 2010 to 2014 for cancer mortality.
Cancer incidence (2009-2013)
In women, the AAPC increased 1.5% for leukemia (P<0.05), decreased 0.5% for NHL (P<0.05), and increased 2.2% for myeloma (P<0.05).
In men, the AAPC increased 1.7% for leukemia (P<0.05), decreased 0.2% for NHL, and increased 2.8% for myeloma (P<0.05).
Cancer mortality (2010-2014)
In women, the AAPC decreased 1.2% for leukemia (P<0.05), decreased 2.2% for NHL (P<0.05), and increased 0.5% for myeloma.
In men, the AAPC decreased 1.0% for leukemia (P<0.05), decreased 2.0% for NHL (P<0.05), and decreased 0.9% for myeloma (P<0.05).
5-year survival
The researchers compared 5-year relative survival for cancers diagnosed from 1975 to 1977 and those diagnosed from 2006 to 2012.
The absolute percentage change over time (for both sexes combined) was 26.1% for NHL, 25.7% for myeloma, and 28.5% for leukemia.
Five-year survival for patients diagnosed in 1975-1977 was 46.5% for NHL, 24.6% for myeloma, and 34.2% for leukemia.
Five-year survival for patients diagnosed in 2006-2012 was 72.6% for NHL, 50.2% for myeloma, and 62.7% for leukemia. ![]()
A report on cancer in the US suggests the incidence of leukemia and myeloma has been on the rise in recent years, but the incidence of non-Hodgkin lymphoma (NHL) has been on the decline.
Meanwhile, annual death rates for leukemia and NHL have decreased, and annual death rates for myeloma have decreased in men but not in women.
Furthermore, patients with leukemia, NHL, and myeloma have seen a substantial improvement in 5-year survival rates in recent years relative to patients in the late 1970s.
These findings are part of the Annual Report to the Nation on the Status of Cancer, 1975-2014, which has been published in the Journal of the National Cancer Institute.
This report is released each year, but the current edition includes a special section focused on survival.
“While trends in death rates are the most commonly used measure to assess progress against cancer, survival trends are also an important measure to evaluate progress in improvement of cancer outcomes,” said Ahmedin Jemal, DVM, PhD, of the American Cancer Society.
“We last included a special section on cancer survival in 2004, and, as we found then, survival improved over time for almost all cancers at every stage of diagnosis.”
For the current report, researchers calculated the 5-year average annual percent changes (AAPCs) for 2009 to 2013 for cancer incidence and for 2010 to 2014 for cancer mortality.
Cancer incidence (2009-2013)
In women, the AAPC increased 1.5% for leukemia (P<0.05), decreased 0.5% for NHL (P<0.05), and increased 2.2% for myeloma (P<0.05).
In men, the AAPC increased 1.7% for leukemia (P<0.05), decreased 0.2% for NHL, and increased 2.8% for myeloma (P<0.05).
Cancer mortality (2010-2014)
In women, the AAPC decreased 1.2% for leukemia (P<0.05), decreased 2.2% for NHL (P<0.05), and increased 0.5% for myeloma.
In men, the AAPC decreased 1.0% for leukemia (P<0.05), decreased 2.0% for NHL (P<0.05), and decreased 0.9% for myeloma (P<0.05).
5-year survival
The researchers compared 5-year relative survival for cancers diagnosed from 1975 to 1977 and those diagnosed from 2006 to 2012.
The absolute percentage change over time (for both sexes combined) was 26.1% for NHL, 25.7% for myeloma, and 28.5% for leukemia.
Five-year survival for patients diagnosed in 1975-1977 was 46.5% for NHL, 24.6% for myeloma, and 34.2% for leukemia.
Five-year survival for patients diagnosed in 2006-2012 was 72.6% for NHL, 50.2% for myeloma, and 62.7% for leukemia. ![]()