User login
Treating Patients With Multiple Myeloma in the VA
The following is a lightly edited transcript of a portion of a teleconference discussion on treating patients with multiple myeloma in the VHA. The conclusion will be published in the August special issue. For more information and to listen to the conversation, visit FedPrac.com/AVAHOupdates.
Biology of Multiple Myeloma
Dr. Munshi. There are many new advances in understanding of basic molecular and genomic changes in multiple myeloma (MM) that involve signaling pathways that drive MM. We have many drugs that target signaling pathways and the number of newer mutational changes that are identified could have therapeutic as well as prognostic significance.
One of the important findings is the lack of specific myeloma-related mutations. Unlike Waldenström macroglobulinemia, which has about 90% patients with Myd88 mutation, in myeloma we do not see that. The mutation frequency, at the maximum, is in the range of 20% for any one gene, and the 3 to 4 most common genes mutated are KRAS, NRAS, BRAF, and P53.
Importantly, if we look at RAS and RAF combined, they target the MEK pathway. So, almost 45% patients have a mutation affecting the MEK pathway, and potentially we can use drugs in the future to see if MEK inhibitors will provide some benefit. And there are anecdotal reports and 1 medium-sized study that has used either a pure MEK inhibitor or a BRAF inhibitor with responses in MM patients. This is a very exciting new area of development.
The second important biological feature in myeloma is clonal shifts. You see multiple clones even at the time of diagnosis. The clonal complexity increases and different clones shift over time with treatment, various other interventions, and changes in myeloma growth patterns. In the future, we cannot just do genomic or cytogenetic analysis one time and sit on it. Over the course of the disease, we may have to repeat it to see if a new clone has evolved. Low-grade or low-risk disease can become a high-grade disease over time.
What is becoming apparent is that sometimes a clone that has almost disappeared reappears after 3, 4, or 5 years. The patient can become resistant to a drug that he or she previously was responsive to with the emergenceof a resistant clone. Three or 4 years later the old sensitive clone can reappear and again be sensitive to the drug that it was not responsive to. The patient may be able to use the same drug again down the line and/or consider similar pathways for targeting. This is one of the major advances that is happening now and is going to inform how we treat patients and how we evaluate patients.
Dr. Mehta. Dr. Munshi, is this going to become “big data?” Are we going to get a lot of information about the molecular changes and findings in our patients not only over time with the evolution of the disease, but also with treatment with various combinations and single agents? Do you think that we at the VA would be able to contribute to some type of banking of material with reference labs that can help to interpret all of the data that we’re going to be able to generate?
Tissue Banking
Dr. Munshi. I think that’s a very important and interesting question. Some mechanisms should be developed so we could not only bank, but also study these genomic patterns. From the VA point of view, there would be some peculiarities that we should understand. One is the age of the patient population—veterans usually end up being older. Number 2, we know the disease is more common in the African American population, and we need to understand why. Tissue banking may help us compare the genomic differences and similarities to understand who may be predisposed to increased frequency of myeloma.
Finally, we still have to keep Agent Orange in mind. Although it is becoming quite an old exposure, a lot of times myeloma occurs. There is a recent paper in JAMA Oncology that showed that incidence of MGUS [monoclonal gammopathy of undetermined significance] is higher in people who are exposed to Agent Orange. Tissue banking to understand that also would be extremely important, and the banked tissue should be processed to understand what’s happening at the molecular level.
Dr. Ascensão. What’s interesting is that there are some DoD specimens (most of it is serology is my understanding), but we may be able to get other material. Having predisease tissue and watching as the patient develops MGUS, smoldering MM, or MM would allow us to see whether there were already mutational changes in the individual even before Agent Orange exposure or, perhaps, was a result of the exposure.
Dr. Mehta. If we could do that, we could even develop protocols to prevent progression of disease. We could imagine a day when we can see the first event occurring and do something to prevent the progression to MGUS and then to smoldering and overt myeloma. Now, we’re in a particularly good position having a national network to propose this type of national bank.
Dr. Ascensão. We have some advantages as a group with a high prevalence of African American patients, as Dr. Munshi mentioned. And we have the biologic components of pathogenesis with Agent Orange. I think it’s something we can afford. Dr. Roodman, what do people in the field need to know about the biology of myeloma that’s going to help them?
Dr. Roodman. People still don’t understand some of the mechanisms underlying support of myeloma growth by the microenvironment. There are multiple targets that have been examined, and none of them work especially well except for what we already use, proteasome antagonists and immunomodulatory agents. In terms of the biology of myeloma bone disease, healing myeloma bone lesions is still a major issue that needs to be addressed. The question is how to do that. I have a VA grant to look at that question and other groups are actively studying the problem.
How particular myeloma clones become dominant is still a wide-open question. Some researchers are pursuing how the microenvironment selectively allows more aggressive clones to become dominant. Currently, the major research focus is on intrinsic changes in the myeloma cell but the microenvironment may also be contributing to the process.
Relapse
Dr. Ascensão. Years ago, people who relapsed, relapsed with bone disease, which may not be necessarily how people are relapsing these days. We are seeing testicular relapses, hepatic relapses, or pulmonary relapses in individuals who are exposed to some of the new agents. There may be interesting developments in terms of interactions in the hepatocellular microenvironment component and the myeloma cells at that level.
Dr. Roodman. These types of relapses are by myeloma cells that can grow independent of the bone marrow micro-environment and these myeloma cells are behaving more like a lymphoma than a myeloma. Several groups have been studying these types of relapses and are examining the expression of adhesion molecules and loss of expression of adhesion molecules to understand why the myeloma cells aren’t anchored in the marrow. This is just my opinion, but we really need to decide on something that could be done within the VA and ask questions similar to the 2 VA clinical trials Dr. Munshi developed. Those were doable in the VA, and we were able to get support for these trials. I think we have to ask questions that allow us to take advantage of the unique features of the VA patient population.
Dr. Chauncey. I would offer a comment from the clinical perspective. You mentioned that this is an observation with newer therapies, and it’s certainly been an observation in the marrow transplantation setting that the pattern of relapse changes. As treatments become more effective, the pattern of relapse can change. When we first started performing autologous transplantation, the pattern of relapse changed from the chemotherapy used at the time. When we started performing allogeneic transplantation, and to the extent that we use that option, we see a different pattern of relapse with substantially more extramedullary disease. This is really a polyclonal or oligoclonal disease, and as different clones evolve over time, whether it’s immunologically mediated or cytotoxic suppression of the initially dominant clone, you see clonal evolution with a different clinical presentation.
Immune System
Dr. Ascensão. Dr. Munshi, what do you think about the immunologic aspects of the disease in terms of its evolution?
Dr. Munshi. They are both aspects of the impact of myeloma on the immune system as Dr. Chauncey mentioned with progression similar to what Dr. Roodman described in the bone, but with greater impact on immune functions. With both pro- and antifunctions you get more TH17 responses, increased T regulatory cell responses, but also more microenvironmental immune cells change.
The second effect is that the immune cell also affects the myeloma growth. For example, proinflammatory cytokine produce interleukin (IL)6, IL17, IL21, and IL23 that affect myeloma or provide myeloma cell growth and signaling mechanism. Also, the PDC (plasmacytoid dendritic cell) is one of the best bone marrow components that induces and supports myeloma growth. With progression, some of these microenvironmental elements actually play a greater role in having the disease function or progress growth more aggressively than otherwise.
The second important aspect that comes into the picture in the immune and bone marrow microenvironment is the role of selecting the clone. There are literally hundreds of clones in a given patient. Certain clones would be supported preferentially by the immune cells, and in some cases, these aggressive clones become independent and grow without the need of support. That’s when they end up becoming extramedullary disease, which also determines how the myeloma cell is growing with these molecular changes.
Dr. Mehta. Another point of evidence for the immune system dysregulation in allowing growth of myeloma is the impact of some of the new trials. So the KEYSTONE 023 trial, for example, showed that pembrolizumab, the programmed death 1 protein (PD1) inhibitor, helped to prevent progression of disease and actually increased survival. The CAR T [chimeric antigen receptor T-cell] studies, some of which were presented at ASH [American Society of Hematology annual meeting] last December, also are beginning to show promise, almost as well as in chronic lymphocytic leukemia. We could look at the therapeutic response as an indicator of biologic pathogenesis and say, “likely the immune system is a major determinant.”
Standard of Therapy
Dr. Ascensão. The Spanish group published some interesting data on high-risk MGUS and smoldering MM. What do you as investigators and clinicians look at to make a diagnosis of MGUS, and what kind of test would you do in order to separate these different groups? How would you define current standard of therapy, and do you assign specific therapies for specific groups of patients?
Dr. Munshi. The current standard is a 3-drug regimen in the U.S. The most common 3-drug regimen that we all usually use, definitely in younger people, but also in older people with some dose modification, was a proteasome inhibitor and immunomodulator with dexamethasone—commonly lenalidomide, bortezomib, and dexamethasone (RVD). One can use carfilzomib also. Now we are beginning to switch to ixazomib, an oral proteasome inhibitor, and it can become an all-oral regimen that could be very convenient. We will have a VA study utilizing a similar agent to make an all-oral regimen for treatment.
An alternative people use that has a financial difference is to use a proteasome inhibitor with cyclophosphamide and dexamethasone, a VCD-like regimen. The study is going to use ixazomib, cyclophosphamide, and dexamethasone followed by ixazomib maintenance. That’s the usual induction regimen.
The question is do we do this differently whether the patient is high risk or low risk? My personal bias in the answer is, not really. At the beginning, we will present the best treatment for the patient’s health. If they are older patients with a lot of comorbidities, which is more important, we can use weekly bortezomib instead of using the full dose. We can use subcutaneous weekly bortezomib or a similar 3-drug regimen. And so high risk or low risk doesn’t change how we’re doing the beginning.
The place where the risk stratification comes into the picture is if patients get a transplant. We are beginning to do posttransplant consolidation, and that’s where we would add 2 cycles of consolidation. If consolidation is not done, for high-risk patients one may do maintenance with 2 drugs like bortezomib and lenalidomide alone; whereas in a low-risk patient, you could do lenalidomide only and not use bortezomib with it. This is the impact of risk stratification as far as what we do. It comes more at the later time rather than earlier time point.
Dr. Mehta. There are still a lot of unanswered questions. One area where I think the VA is very good at becoming involved, if we choose to do so, would be some of the drug dosings. For example, dexamethasone used to be used in high dose and the ECOG study showed that you can use it in lower dose weekly rather than 4 days on, 4 days off. The Myeloma In VA (MIVA) group led by Dr. Munshi did a study looking at different doses of lenalidomide. There’s still unanswered questions even in this standard of care regimen that we use where we could try to make the regimen more tolerable for patients.
Dr. Roodman. RVd (modified RVD) has been piloted by Dr. Noopur Raje at Mass General, but I don’t think it’s a large study. She uses it in older patients. To follow-up on what you said, which I think is a really great idea, we could look at RVd as well as 2 doses of dexamethasone, or use oral proteasome antagonists instead.
Dr. Mehta. Yes, to reduce the neurotoxicity.
Dr. Roodman. That is correct. But I think the VA is set up to look at those kinds of questions because most of our patients are older.
Dr. Mehta. They have all the comorbidities.
Dr. Chauncey. We have what we think is an optimal regimen for an optimal patient, and then we have what we often see in the clinic. I’m not sure of the quantitative VA demographics, but if you look at the U.S. myeloma population, the median age is 70 years. While a triplet like
RVd or a carfilzomib-based triplet is probably optimal based on depth of response and the theoretical aspect of suppression of all subclones, it’s not really an accessible regimen for many patients that I see in clinic who are not transplant eligible.
For the nontransplant patients and more frail patients, the doublets or the cyclophosphamide/bortezomib/dexamethasone are reasonable options. I know there are proponents saying that everybody should get RVd or dose-attenuated RVd; I don’t think that’s practical for many of the patients that we see in our clinics.
Dr. Mehta. Why is it not practical in most of your patients? Because they have to come once a week to the clinic?
Dr. Chauncey. That’s part of it, but I find that it’s often too intense with excessive toxicity. The patients are older with comorbidities and they have more limited physiologic reserve. Part of it is coming to clinic, and that’s where all oral regimens such as Rd are useful. I don’t know that if you have an older patient and you see a good response that you’re tracking in terms of their Mspike (monoclonal protein) and their CRAB criteria, that everybody requires the aggressive approach of RVd or a similar triplet with a proteasome inhibitor, an IMiD [immunomodulator drug], and a steroid as induction therapy.
This is true even for some of the older patients who come to autologous transplantation. The data we talk about are in a younger group of patients who can tolerate the full dose of those regimens. The all-oral regimens are attractive, but it’s also quite a financial burden, maybe not to an individual patient, but certainly to the whole health care system. I’m not sure if there is a depth or durability of response advantage for the oral proteasome inhibitor over those available as IV or subcutaneous dosing.
Pricing
Dr. Ascensão. It’s interesting you bring that up because at the Washington DC VAMC, ixazomib may be priced similarly to bortezomib.
Dr. Chauncey. Well that would be very good and very interesting. It’s possible the federal pricing would make it a lot more attractive. It’s not the case outside of the VA, but there the cost burden for oral medication is often shifted to the patient.
Dr. Cosgriff. The price of carfilzomib is about $7,000 to $9,000 per month or per cycle. When we priced ixazomib here in Portland, it actually was more expensive than bortezomib, but it was cheaper than carfilzomib.
Dr. Mehta. If we can do a drug company-sponsored study, the ixazomib people would surely be interested in its usefulness as a triplet in VA patients. It also would be good for the VA patient because you don’t have to come once a week and it has much less neurotoxicity compared to the other proteasome inhibitors, which is a big problem.
Dr. Chauncey. That would be a great study to offer the right patient. I see a lot of myeloma patients in clinic that aren’t really eligible for transplantation. Many of those patients do well on doublet therapy, though they will often require dose adjustments, both down and up, as well as tracking disease status.
Dr. Cosgriff. In Portland, we’re using a lenalidomidebased regimen as a first-line therapy. We’re starting to see more and more individuals starting RVD upfront. There are some select individuals who have diabetic neuropathy or something like that, who may go on just
a lenalidomide plus dexamethasone, leaving out the bortezomib because of neuropathy issues.
Our second-line choice at this point in time has been carfilzomib, but with ixazomib being a cheaper option, we may actually consider switching over to that as a second-line choice. When you look at the clinical trials they cite in the product literature, the grade 1 neuropathy is about 18% with ixazomib and 14% with the placebo arm, so it does add some peripheral neuropathy. Maybe not as much as we would think.
While these oral agents and all-oral regimens are nice to have, we still bring in the patients for clinic and for monitoring of white blood count. It doesn’t necessarily decrease the burden of clinic visits. We still have to get lab draws. Yes, the patient is not sitting in a chair waiting for the bortezomib infusion to be mixed by the pharmacy, but we still have considerations for travel and those types of things.
We treat the entire state of Oregon and parts of southwest Washington. Fortunately, we have community-based outpatient clinics (CBOCs) and VAMCs in other areas of the state, and we also utilize places like Walla Walla and Spokane, Washington, where we can draw and check labs remotely, which decreases the travel burden, but it still is a burden to the patient to actually go in for lab tests. We’re not looking at ixazomib as a first line currently.
We’re going to wait for data to be published on ixazomib in the first line. In a couple of presentations, researchers have suggested that if you tease the data, ixazomib may be inferior to bortezomib in the first-line setting. It will be interesting to see what the data say for that as far as a first-line setting choice.
Dr. Chauncey. I second what Dr. Cosgriff said. You can’t just give patients 3 bottles of pills and say, “See you in 3 months.” Here in the Northwest we cover large rural areas, and there are many CBOCs whose labs feed directly into our CPRS (Computerized Patient Record System) so it’s very accessible and easy to follow patients that come in less frequently, but that doesn’t change the need for regular clinical follow-up. The other thing I’d say is the noncomparative data of carfilzomib/lenalidomide/dexamethasone (CRD) is quite compelling in terms of depth and duration of response. We usually don’t want to accept convenience over efficacy. Whether that durability translates long term, we don’t really know, but I think right now, CRD is the best available regimen, again, with the caveat that it’s not really accessible to patients of all ages and performance status.
Dr. Ascensão. At the Washington DC VAMC in our first cycle it is day 1, 4, 8, and 11 with bortezomib, but after that we go pretty much to weekly bortezomib. We also tend to use what I would call lower dose 20 mg dexamethasone in patients over the age of 70 years. And we feel that it is a lot less toxic. We use subcutaneous bortezomib for pretty much everybody else.
Managing Adverse Effects
Dr. Chauncey. I think we’re all using subcutaneous bortezomib at this point. Dexamethasone doesn’t get a lot of independent attention, but there’s no question that, as Dr. Mehta mentioned, the older regimen that we used, the dose-dense dexamethasone of the VAD regimen, was
quite disabling. In addition to obvious hyperglycemia, there were psychiatric problems and, ultimately, profound steroid myopathy that seemed to affect patients in variable fashion. Different patients seem to be more or less susceptible, but after a couple of cycles, it starts to kick in and is progressive.
So we’ve since abandoned those massive doses. But when you look at the ECOG study (E4A03) that really defined the lower dose (40 mg weekly), there’s no question the higher dose was more toxic but also more effective in terms of disease response. While there are many older patients that I would start with a lower dose of dexamethasone, whether it’s with lenalidomide or bortezomib, I keep in mind there can be a steep dose response curve for dexamethasone. If you’re giving 20 mg and you’re not getting the response you need, then you increase the dose. There is definitely a dose response, but the higher doses are just not as well tolerated.
Dr. Cosgriff. I don’t know if other institutions are doing it, but instead of doing 40 mg as a single dose because of patient performance status, providers in Portland will prescribe 20 mg on days 1, 2, 8, 9, 15, and 16. They’ll break up that 40 mg dose and give it that way.
Dr. Chauncey. I don’t know if that strategy is biologically equivalent in terms of antimyeloma activity or less toxic in terms of myopathy. There’s almost always some disease marker to track, so that whether you’re using the serum free-light chain assay or serum protein electrophoresis, you can see if the strategy you’re using is working in real time.
Dr. Cosgriff. I’ve never known whether it’s been shown to be more efficacious or if it’s just a way of getting around some of the adverse effects. However, it does pose some alternate challenges. With higher doses of steroids, you’re looking at 2 days where the patient can become hyperglycemic, if not, a little bit longer.
The other thing with it is that adding on that extra day of dexamethasone can interfere with some other drugs and some other therapies. In individuals who have had a deep vein thrombosis for whatever reason and they’re on warfarin, now we have an agent that really screws up our warfarin monitoring. We would have to consider switching them to another agent.
Thrombosis
Dr. Mehta. It’s also prothrombotic.
Dr. Chauncey. If you actually ask the patient, independent of the hyperglycemia, independent of the myopathy, independent of psychosis, but just quality of life, they’ll typically tell you that the on-and-off of steroids is the worst part of the regimen. It’s often the roller coaster ride of short-term hypomania followed by dysphoria.
Dr. Mehta. And the lack of sleep. They describe it as being out of their skin.
Dr. Chauncey. They are. And as soon as they stop, often there is a depression.
Dr. Mehta. It is very, very difficult. And some actually develop psychosis.
Dr. Ascensão. We all use some form of acyclovir or its derivative for the prevention of shingles in patients exposed to the proteasome inhibitors. We use aspirin, usually low dose (81 mg), for deep vein thrombosis prophylaxis. But is anybody using other anticoagulants or putting everybody prophylactically on proton-pump inhibitors (PPIs) or just seeing how people do first and then adjusting?
Dr. Chauncey. I typically use conventional dose aspirin, and if there’s breakthrough thrombosis, the first response should be that it is not the best regimen for this patient. Sometimes you have to go back to it, and if someone’s an anticoagulation candidate, then full anticoagulation is
needed if that’s the best regimen. Usually if there’s a breakthrough thrombosis, it is a deal breaker, and you’re ready to move on to a nonthrombogenic regimen.
There has been an observation (there is some biological basis to back this up) that if you give bortezomib with an IMiD, the regimen became less thrombogenic than with the IMiD and dexamethasone alone.
Dr. Ascensão. On aspirin, even if they’re on an IMiD plus a proteasome inhibitor, I just don’t know that the data are good enough for us to avoid it at this point in time. And I don’t necessarily put people on a PPI unless they’ve got added gastrointestinal problems and unless they have associated heartburn or dyspepsia symptoms.
Dr. Mehta. I use low-dose aspirin in every patient. And if they breakthrough, they go on full anticoagulation usually with a new oral anticoagulant. I use PPIs only if needed, although most of them do need it, and, of course, bisphosphonates so the bone protective aspect.
Click to read the digital edition.
The following is a lightly edited transcript of a portion of a teleconference discussion on treating patients with multiple myeloma in the VHA. The conclusion will be published in the August special issue. For more information and to listen to the conversation, visit FedPrac.com/AVAHOupdates.
Biology of Multiple Myeloma
Dr. Munshi. There are many new advances in understanding of basic molecular and genomic changes in multiple myeloma (MM) that involve signaling pathways that drive MM. We have many drugs that target signaling pathways and the number of newer mutational changes that are identified could have therapeutic as well as prognostic significance.
One of the important findings is the lack of specific myeloma-related mutations. Unlike Waldenström macroglobulinemia, which has about 90% patients with Myd88 mutation, in myeloma we do not see that. The mutation frequency, at the maximum, is in the range of 20% for any one gene, and the 3 to 4 most common genes mutated are KRAS, NRAS, BRAF, and P53.
Importantly, if we look at RAS and RAF combined, they target the MEK pathway. So, almost 45% patients have a mutation affecting the MEK pathway, and potentially we can use drugs in the future to see if MEK inhibitors will provide some benefit. And there are anecdotal reports and 1 medium-sized study that has used either a pure MEK inhibitor or a BRAF inhibitor with responses in MM patients. This is a very exciting new area of development.
The second important biological feature in myeloma is clonal shifts. You see multiple clones even at the time of diagnosis. The clonal complexity increases and different clones shift over time with treatment, various other interventions, and changes in myeloma growth patterns. In the future, we cannot just do genomic or cytogenetic analysis one time and sit on it. Over the course of the disease, we may have to repeat it to see if a new clone has evolved. Low-grade or low-risk disease can become a high-grade disease over time.
What is becoming apparent is that sometimes a clone that has almost disappeared reappears after 3, 4, or 5 years. The patient can become resistant to a drug that he or she previously was responsive to with the emergenceof a resistant clone. Three or 4 years later the old sensitive clone can reappear and again be sensitive to the drug that it was not responsive to. The patient may be able to use the same drug again down the line and/or consider similar pathways for targeting. This is one of the major advances that is happening now and is going to inform how we treat patients and how we evaluate patients.
Dr. Mehta. Dr. Munshi, is this going to become “big data?” Are we going to get a lot of information about the molecular changes and findings in our patients not only over time with the evolution of the disease, but also with treatment with various combinations and single agents? Do you think that we at the VA would be able to contribute to some type of banking of material with reference labs that can help to interpret all of the data that we’re going to be able to generate?
Tissue Banking
Dr. Munshi. I think that’s a very important and interesting question. Some mechanisms should be developed so we could not only bank, but also study these genomic patterns. From the VA point of view, there would be some peculiarities that we should understand. One is the age of the patient population—veterans usually end up being older. Number 2, we know the disease is more common in the African American population, and we need to understand why. Tissue banking may help us compare the genomic differences and similarities to understand who may be predisposed to increased frequency of myeloma.
Finally, we still have to keep Agent Orange in mind. Although it is becoming quite an old exposure, a lot of times myeloma occurs. There is a recent paper in JAMA Oncology that showed that incidence of MGUS [monoclonal gammopathy of undetermined significance] is higher in people who are exposed to Agent Orange. Tissue banking to understand that also would be extremely important, and the banked tissue should be processed to understand what’s happening at the molecular level.
Dr. Ascensão. What’s interesting is that there are some DoD specimens (most of it is serology is my understanding), but we may be able to get other material. Having predisease tissue and watching as the patient develops MGUS, smoldering MM, or MM would allow us to see whether there were already mutational changes in the individual even before Agent Orange exposure or, perhaps, was a result of the exposure.
Dr. Mehta. If we could do that, we could even develop protocols to prevent progression of disease. We could imagine a day when we can see the first event occurring and do something to prevent the progression to MGUS and then to smoldering and overt myeloma. Now, we’re in a particularly good position having a national network to propose this type of national bank.
Dr. Ascensão. We have some advantages as a group with a high prevalence of African American patients, as Dr. Munshi mentioned. And we have the biologic components of pathogenesis with Agent Orange. I think it’s something we can afford. Dr. Roodman, what do people in the field need to know about the biology of myeloma that’s going to help them?
Dr. Roodman. People still don’t understand some of the mechanisms underlying support of myeloma growth by the microenvironment. There are multiple targets that have been examined, and none of them work especially well except for what we already use, proteasome antagonists and immunomodulatory agents. In terms of the biology of myeloma bone disease, healing myeloma bone lesions is still a major issue that needs to be addressed. The question is how to do that. I have a VA grant to look at that question and other groups are actively studying the problem.
How particular myeloma clones become dominant is still a wide-open question. Some researchers are pursuing how the microenvironment selectively allows more aggressive clones to become dominant. Currently, the major research focus is on intrinsic changes in the myeloma cell but the microenvironment may also be contributing to the process.
Relapse
Dr. Ascensão. Years ago, people who relapsed, relapsed with bone disease, which may not be necessarily how people are relapsing these days. We are seeing testicular relapses, hepatic relapses, or pulmonary relapses in individuals who are exposed to some of the new agents. There may be interesting developments in terms of interactions in the hepatocellular microenvironment component and the myeloma cells at that level.
Dr. Roodman. These types of relapses are by myeloma cells that can grow independent of the bone marrow micro-environment and these myeloma cells are behaving more like a lymphoma than a myeloma. Several groups have been studying these types of relapses and are examining the expression of adhesion molecules and loss of expression of adhesion molecules to understand why the myeloma cells aren’t anchored in the marrow. This is just my opinion, but we really need to decide on something that could be done within the VA and ask questions similar to the 2 VA clinical trials Dr. Munshi developed. Those were doable in the VA, and we were able to get support for these trials. I think we have to ask questions that allow us to take advantage of the unique features of the VA patient population.
Dr. Chauncey. I would offer a comment from the clinical perspective. You mentioned that this is an observation with newer therapies, and it’s certainly been an observation in the marrow transplantation setting that the pattern of relapse changes. As treatments become more effective, the pattern of relapse can change. When we first started performing autologous transplantation, the pattern of relapse changed from the chemotherapy used at the time. When we started performing allogeneic transplantation, and to the extent that we use that option, we see a different pattern of relapse with substantially more extramedullary disease. This is really a polyclonal or oligoclonal disease, and as different clones evolve over time, whether it’s immunologically mediated or cytotoxic suppression of the initially dominant clone, you see clonal evolution with a different clinical presentation.
Immune System
Dr. Ascensão. Dr. Munshi, what do you think about the immunologic aspects of the disease in terms of its evolution?
Dr. Munshi. They are both aspects of the impact of myeloma on the immune system as Dr. Chauncey mentioned with progression similar to what Dr. Roodman described in the bone, but with greater impact on immune functions. With both pro- and antifunctions you get more TH17 responses, increased T regulatory cell responses, but also more microenvironmental immune cells change.
The second effect is that the immune cell also affects the myeloma growth. For example, proinflammatory cytokine produce interleukin (IL)6, IL17, IL21, and IL23 that affect myeloma or provide myeloma cell growth and signaling mechanism. Also, the PDC (plasmacytoid dendritic cell) is one of the best bone marrow components that induces and supports myeloma growth. With progression, some of these microenvironmental elements actually play a greater role in having the disease function or progress growth more aggressively than otherwise.
The second important aspect that comes into the picture in the immune and bone marrow microenvironment is the role of selecting the clone. There are literally hundreds of clones in a given patient. Certain clones would be supported preferentially by the immune cells, and in some cases, these aggressive clones become independent and grow without the need of support. That’s when they end up becoming extramedullary disease, which also determines how the myeloma cell is growing with these molecular changes.
Dr. Mehta. Another point of evidence for the immune system dysregulation in allowing growth of myeloma is the impact of some of the new trials. So the KEYSTONE 023 trial, for example, showed that pembrolizumab, the programmed death 1 protein (PD1) inhibitor, helped to prevent progression of disease and actually increased survival. The CAR T [chimeric antigen receptor T-cell] studies, some of which were presented at ASH [American Society of Hematology annual meeting] last December, also are beginning to show promise, almost as well as in chronic lymphocytic leukemia. We could look at the therapeutic response as an indicator of biologic pathogenesis and say, “likely the immune system is a major determinant.”
Standard of Therapy
Dr. Ascensão. The Spanish group published some interesting data on high-risk MGUS and smoldering MM. What do you as investigators and clinicians look at to make a diagnosis of MGUS, and what kind of test would you do in order to separate these different groups? How would you define current standard of therapy, and do you assign specific therapies for specific groups of patients?
Dr. Munshi. The current standard is a 3-drug regimen in the U.S. The most common 3-drug regimen that we all usually use, definitely in younger people, but also in older people with some dose modification, was a proteasome inhibitor and immunomodulator with dexamethasone—commonly lenalidomide, bortezomib, and dexamethasone (RVD). One can use carfilzomib also. Now we are beginning to switch to ixazomib, an oral proteasome inhibitor, and it can become an all-oral regimen that could be very convenient. We will have a VA study utilizing a similar agent to make an all-oral regimen for treatment.
An alternative people use that has a financial difference is to use a proteasome inhibitor with cyclophosphamide and dexamethasone, a VCD-like regimen. The study is going to use ixazomib, cyclophosphamide, and dexamethasone followed by ixazomib maintenance. That’s the usual induction regimen.
The question is do we do this differently whether the patient is high risk or low risk? My personal bias in the answer is, not really. At the beginning, we will present the best treatment for the patient’s health. If they are older patients with a lot of comorbidities, which is more important, we can use weekly bortezomib instead of using the full dose. We can use subcutaneous weekly bortezomib or a similar 3-drug regimen. And so high risk or low risk doesn’t change how we’re doing the beginning.
The place where the risk stratification comes into the picture is if patients get a transplant. We are beginning to do posttransplant consolidation, and that’s where we would add 2 cycles of consolidation. If consolidation is not done, for high-risk patients one may do maintenance with 2 drugs like bortezomib and lenalidomide alone; whereas in a low-risk patient, you could do lenalidomide only and not use bortezomib with it. This is the impact of risk stratification as far as what we do. It comes more at the later time rather than earlier time point.
Dr. Mehta. There are still a lot of unanswered questions. One area where I think the VA is very good at becoming involved, if we choose to do so, would be some of the drug dosings. For example, dexamethasone used to be used in high dose and the ECOG study showed that you can use it in lower dose weekly rather than 4 days on, 4 days off. The Myeloma In VA (MIVA) group led by Dr. Munshi did a study looking at different doses of lenalidomide. There’s still unanswered questions even in this standard of care regimen that we use where we could try to make the regimen more tolerable for patients.
Dr. Roodman. RVd (modified RVD) has been piloted by Dr. Noopur Raje at Mass General, but I don’t think it’s a large study. She uses it in older patients. To follow-up on what you said, which I think is a really great idea, we could look at RVd as well as 2 doses of dexamethasone, or use oral proteasome antagonists instead.
Dr. Mehta. Yes, to reduce the neurotoxicity.
Dr. Roodman. That is correct. But I think the VA is set up to look at those kinds of questions because most of our patients are older.
Dr. Mehta. They have all the comorbidities.
Dr. Chauncey. We have what we think is an optimal regimen for an optimal patient, and then we have what we often see in the clinic. I’m not sure of the quantitative VA demographics, but if you look at the U.S. myeloma population, the median age is 70 years. While a triplet like
RVd or a carfilzomib-based triplet is probably optimal based on depth of response and the theoretical aspect of suppression of all subclones, it’s not really an accessible regimen for many patients that I see in clinic who are not transplant eligible.
For the nontransplant patients and more frail patients, the doublets or the cyclophosphamide/bortezomib/dexamethasone are reasonable options. I know there are proponents saying that everybody should get RVd or dose-attenuated RVd; I don’t think that’s practical for many of the patients that we see in our clinics.
Dr. Mehta. Why is it not practical in most of your patients? Because they have to come once a week to the clinic?
Dr. Chauncey. That’s part of it, but I find that it’s often too intense with excessive toxicity. The patients are older with comorbidities and they have more limited physiologic reserve. Part of it is coming to clinic, and that’s where all oral regimens such as Rd are useful. I don’t know that if you have an older patient and you see a good response that you’re tracking in terms of their Mspike (monoclonal protein) and their CRAB criteria, that everybody requires the aggressive approach of RVd or a similar triplet with a proteasome inhibitor, an IMiD [immunomodulator drug], and a steroid as induction therapy.
This is true even for some of the older patients who come to autologous transplantation. The data we talk about are in a younger group of patients who can tolerate the full dose of those regimens. The all-oral regimens are attractive, but it’s also quite a financial burden, maybe not to an individual patient, but certainly to the whole health care system. I’m not sure if there is a depth or durability of response advantage for the oral proteasome inhibitor over those available as IV or subcutaneous dosing.
Pricing
Dr. Ascensão. It’s interesting you bring that up because at the Washington DC VAMC, ixazomib may be priced similarly to bortezomib.
Dr. Chauncey. Well that would be very good and very interesting. It’s possible the federal pricing would make it a lot more attractive. It’s not the case outside of the VA, but there the cost burden for oral medication is often shifted to the patient.
Dr. Cosgriff. The price of carfilzomib is about $7,000 to $9,000 per month or per cycle. When we priced ixazomib here in Portland, it actually was more expensive than bortezomib, but it was cheaper than carfilzomib.
Dr. Mehta. If we can do a drug company-sponsored study, the ixazomib people would surely be interested in its usefulness as a triplet in VA patients. It also would be good for the VA patient because you don’t have to come once a week and it has much less neurotoxicity compared to the other proteasome inhibitors, which is a big problem.
Dr. Chauncey. That would be a great study to offer the right patient. I see a lot of myeloma patients in clinic that aren’t really eligible for transplantation. Many of those patients do well on doublet therapy, though they will often require dose adjustments, both down and up, as well as tracking disease status.
Dr. Cosgriff. In Portland, we’re using a lenalidomidebased regimen as a first-line therapy. We’re starting to see more and more individuals starting RVD upfront. There are some select individuals who have diabetic neuropathy or something like that, who may go on just
a lenalidomide plus dexamethasone, leaving out the bortezomib because of neuropathy issues.
Our second-line choice at this point in time has been carfilzomib, but with ixazomib being a cheaper option, we may actually consider switching over to that as a second-line choice. When you look at the clinical trials they cite in the product literature, the grade 1 neuropathy is about 18% with ixazomib and 14% with the placebo arm, so it does add some peripheral neuropathy. Maybe not as much as we would think.
While these oral agents and all-oral regimens are nice to have, we still bring in the patients for clinic and for monitoring of white blood count. It doesn’t necessarily decrease the burden of clinic visits. We still have to get lab draws. Yes, the patient is not sitting in a chair waiting for the bortezomib infusion to be mixed by the pharmacy, but we still have considerations for travel and those types of things.
We treat the entire state of Oregon and parts of southwest Washington. Fortunately, we have community-based outpatient clinics (CBOCs) and VAMCs in other areas of the state, and we also utilize places like Walla Walla and Spokane, Washington, where we can draw and check labs remotely, which decreases the travel burden, but it still is a burden to the patient to actually go in for lab tests. We’re not looking at ixazomib as a first line currently.
We’re going to wait for data to be published on ixazomib in the first line. In a couple of presentations, researchers have suggested that if you tease the data, ixazomib may be inferior to bortezomib in the first-line setting. It will be interesting to see what the data say for that as far as a first-line setting choice.
Dr. Chauncey. I second what Dr. Cosgriff said. You can’t just give patients 3 bottles of pills and say, “See you in 3 months.” Here in the Northwest we cover large rural areas, and there are many CBOCs whose labs feed directly into our CPRS (Computerized Patient Record System) so it’s very accessible and easy to follow patients that come in less frequently, but that doesn’t change the need for regular clinical follow-up. The other thing I’d say is the noncomparative data of carfilzomib/lenalidomide/dexamethasone (CRD) is quite compelling in terms of depth and duration of response. We usually don’t want to accept convenience over efficacy. Whether that durability translates long term, we don’t really know, but I think right now, CRD is the best available regimen, again, with the caveat that it’s not really accessible to patients of all ages and performance status.
Dr. Ascensão. At the Washington DC VAMC in our first cycle it is day 1, 4, 8, and 11 with bortezomib, but after that we go pretty much to weekly bortezomib. We also tend to use what I would call lower dose 20 mg dexamethasone in patients over the age of 70 years. And we feel that it is a lot less toxic. We use subcutaneous bortezomib for pretty much everybody else.
Managing Adverse Effects
Dr. Chauncey. I think we’re all using subcutaneous bortezomib at this point. Dexamethasone doesn’t get a lot of independent attention, but there’s no question that, as Dr. Mehta mentioned, the older regimen that we used, the dose-dense dexamethasone of the VAD regimen, was
quite disabling. In addition to obvious hyperglycemia, there were psychiatric problems and, ultimately, profound steroid myopathy that seemed to affect patients in variable fashion. Different patients seem to be more or less susceptible, but after a couple of cycles, it starts to kick in and is progressive.
So we’ve since abandoned those massive doses. But when you look at the ECOG study (E4A03) that really defined the lower dose (40 mg weekly), there’s no question the higher dose was more toxic but also more effective in terms of disease response. While there are many older patients that I would start with a lower dose of dexamethasone, whether it’s with lenalidomide or bortezomib, I keep in mind there can be a steep dose response curve for dexamethasone. If you’re giving 20 mg and you’re not getting the response you need, then you increase the dose. There is definitely a dose response, but the higher doses are just not as well tolerated.
Dr. Cosgriff. I don’t know if other institutions are doing it, but instead of doing 40 mg as a single dose because of patient performance status, providers in Portland will prescribe 20 mg on days 1, 2, 8, 9, 15, and 16. They’ll break up that 40 mg dose and give it that way.
Dr. Chauncey. I don’t know if that strategy is biologically equivalent in terms of antimyeloma activity or less toxic in terms of myopathy. There’s almost always some disease marker to track, so that whether you’re using the serum free-light chain assay or serum protein electrophoresis, you can see if the strategy you’re using is working in real time.
Dr. Cosgriff. I’ve never known whether it’s been shown to be more efficacious or if it’s just a way of getting around some of the adverse effects. However, it does pose some alternate challenges. With higher doses of steroids, you’re looking at 2 days where the patient can become hyperglycemic, if not, a little bit longer.
The other thing with it is that adding on that extra day of dexamethasone can interfere with some other drugs and some other therapies. In individuals who have had a deep vein thrombosis for whatever reason and they’re on warfarin, now we have an agent that really screws up our warfarin monitoring. We would have to consider switching them to another agent.
Thrombosis
Dr. Mehta. It’s also prothrombotic.
Dr. Chauncey. If you actually ask the patient, independent of the hyperglycemia, independent of the myopathy, independent of psychosis, but just quality of life, they’ll typically tell you that the on-and-off of steroids is the worst part of the regimen. It’s often the roller coaster ride of short-term hypomania followed by dysphoria.
Dr. Mehta. And the lack of sleep. They describe it as being out of their skin.
Dr. Chauncey. They are. And as soon as they stop, often there is a depression.
Dr. Mehta. It is very, very difficult. And some actually develop psychosis.
Dr. Ascensão. We all use some form of acyclovir or its derivative for the prevention of shingles in patients exposed to the proteasome inhibitors. We use aspirin, usually low dose (81 mg), for deep vein thrombosis prophylaxis. But is anybody using other anticoagulants or putting everybody prophylactically on proton-pump inhibitors (PPIs) or just seeing how people do first and then adjusting?
Dr. Chauncey. I typically use conventional dose aspirin, and if there’s breakthrough thrombosis, the first response should be that it is not the best regimen for this patient. Sometimes you have to go back to it, and if someone’s an anticoagulation candidate, then full anticoagulation is
needed if that’s the best regimen. Usually if there’s a breakthrough thrombosis, it is a deal breaker, and you’re ready to move on to a nonthrombogenic regimen.
There has been an observation (there is some biological basis to back this up) that if you give bortezomib with an IMiD, the regimen became less thrombogenic than with the IMiD and dexamethasone alone.
Dr. Ascensão. On aspirin, even if they’re on an IMiD plus a proteasome inhibitor, I just don’t know that the data are good enough for us to avoid it at this point in time. And I don’t necessarily put people on a PPI unless they’ve got added gastrointestinal problems and unless they have associated heartburn or dyspepsia symptoms.
Dr. Mehta. I use low-dose aspirin in every patient. And if they breakthrough, they go on full anticoagulation usually with a new oral anticoagulant. I use PPIs only if needed, although most of them do need it, and, of course, bisphosphonates so the bone protective aspect.
Click to read the digital edition.
The following is a lightly edited transcript of a portion of a teleconference discussion on treating patients with multiple myeloma in the VHA. The conclusion will be published in the August special issue. For more information and to listen to the conversation, visit FedPrac.com/AVAHOupdates.
Biology of Multiple Myeloma
Dr. Munshi. There are many new advances in understanding of basic molecular and genomic changes in multiple myeloma (MM) that involve signaling pathways that drive MM. We have many drugs that target signaling pathways and the number of newer mutational changes that are identified could have therapeutic as well as prognostic significance.
One of the important findings is the lack of specific myeloma-related mutations. Unlike Waldenström macroglobulinemia, which has about 90% patients with Myd88 mutation, in myeloma we do not see that. The mutation frequency, at the maximum, is in the range of 20% for any one gene, and the 3 to 4 most common genes mutated are KRAS, NRAS, BRAF, and P53.
Importantly, if we look at RAS and RAF combined, they target the MEK pathway. So, almost 45% patients have a mutation affecting the MEK pathway, and potentially we can use drugs in the future to see if MEK inhibitors will provide some benefit. And there are anecdotal reports and 1 medium-sized study that has used either a pure MEK inhibitor or a BRAF inhibitor with responses in MM patients. This is a very exciting new area of development.
The second important biological feature in myeloma is clonal shifts. You see multiple clones even at the time of diagnosis. The clonal complexity increases and different clones shift over time with treatment, various other interventions, and changes in myeloma growth patterns. In the future, we cannot just do genomic or cytogenetic analysis one time and sit on it. Over the course of the disease, we may have to repeat it to see if a new clone has evolved. Low-grade or low-risk disease can become a high-grade disease over time.
What is becoming apparent is that sometimes a clone that has almost disappeared reappears after 3, 4, or 5 years. The patient can become resistant to a drug that he or she previously was responsive to with the emergenceof a resistant clone. Three or 4 years later the old sensitive clone can reappear and again be sensitive to the drug that it was not responsive to. The patient may be able to use the same drug again down the line and/or consider similar pathways for targeting. This is one of the major advances that is happening now and is going to inform how we treat patients and how we evaluate patients.
Dr. Mehta. Dr. Munshi, is this going to become “big data?” Are we going to get a lot of information about the molecular changes and findings in our patients not only over time with the evolution of the disease, but also with treatment with various combinations and single agents? Do you think that we at the VA would be able to contribute to some type of banking of material with reference labs that can help to interpret all of the data that we’re going to be able to generate?
Tissue Banking
Dr. Munshi. I think that’s a very important and interesting question. Some mechanisms should be developed so we could not only bank, but also study these genomic patterns. From the VA point of view, there would be some peculiarities that we should understand. One is the age of the patient population—veterans usually end up being older. Number 2, we know the disease is more common in the African American population, and we need to understand why. Tissue banking may help us compare the genomic differences and similarities to understand who may be predisposed to increased frequency of myeloma.
Finally, we still have to keep Agent Orange in mind. Although it is becoming quite an old exposure, a lot of times myeloma occurs. There is a recent paper in JAMA Oncology that showed that incidence of MGUS [monoclonal gammopathy of undetermined significance] is higher in people who are exposed to Agent Orange. Tissue banking to understand that also would be extremely important, and the banked tissue should be processed to understand what’s happening at the molecular level.
Dr. Ascensão. What’s interesting is that there are some DoD specimens (most of it is serology is my understanding), but we may be able to get other material. Having predisease tissue and watching as the patient develops MGUS, smoldering MM, or MM would allow us to see whether there were already mutational changes in the individual even before Agent Orange exposure or, perhaps, was a result of the exposure.
Dr. Mehta. If we could do that, we could even develop protocols to prevent progression of disease. We could imagine a day when we can see the first event occurring and do something to prevent the progression to MGUS and then to smoldering and overt myeloma. Now, we’re in a particularly good position having a national network to propose this type of national bank.
Dr. Ascensão. We have some advantages as a group with a high prevalence of African American patients, as Dr. Munshi mentioned. And we have the biologic components of pathogenesis with Agent Orange. I think it’s something we can afford. Dr. Roodman, what do people in the field need to know about the biology of myeloma that’s going to help them?
Dr. Roodman. People still don’t understand some of the mechanisms underlying support of myeloma growth by the microenvironment. There are multiple targets that have been examined, and none of them work especially well except for what we already use, proteasome antagonists and immunomodulatory agents. In terms of the biology of myeloma bone disease, healing myeloma bone lesions is still a major issue that needs to be addressed. The question is how to do that. I have a VA grant to look at that question and other groups are actively studying the problem.
How particular myeloma clones become dominant is still a wide-open question. Some researchers are pursuing how the microenvironment selectively allows more aggressive clones to become dominant. Currently, the major research focus is on intrinsic changes in the myeloma cell but the microenvironment may also be contributing to the process.
Relapse
Dr. Ascensão. Years ago, people who relapsed, relapsed with bone disease, which may not be necessarily how people are relapsing these days. We are seeing testicular relapses, hepatic relapses, or pulmonary relapses in individuals who are exposed to some of the new agents. There may be interesting developments in terms of interactions in the hepatocellular microenvironment component and the myeloma cells at that level.
Dr. Roodman. These types of relapses are by myeloma cells that can grow independent of the bone marrow micro-environment and these myeloma cells are behaving more like a lymphoma than a myeloma. Several groups have been studying these types of relapses and are examining the expression of adhesion molecules and loss of expression of adhesion molecules to understand why the myeloma cells aren’t anchored in the marrow. This is just my opinion, but we really need to decide on something that could be done within the VA and ask questions similar to the 2 VA clinical trials Dr. Munshi developed. Those were doable in the VA, and we were able to get support for these trials. I think we have to ask questions that allow us to take advantage of the unique features of the VA patient population.
Dr. Chauncey. I would offer a comment from the clinical perspective. You mentioned that this is an observation with newer therapies, and it’s certainly been an observation in the marrow transplantation setting that the pattern of relapse changes. As treatments become more effective, the pattern of relapse can change. When we first started performing autologous transplantation, the pattern of relapse changed from the chemotherapy used at the time. When we started performing allogeneic transplantation, and to the extent that we use that option, we see a different pattern of relapse with substantially more extramedullary disease. This is really a polyclonal or oligoclonal disease, and as different clones evolve over time, whether it’s immunologically mediated or cytotoxic suppression of the initially dominant clone, you see clonal evolution with a different clinical presentation.
Immune System
Dr. Ascensão. Dr. Munshi, what do you think about the immunologic aspects of the disease in terms of its evolution?
Dr. Munshi. They are both aspects of the impact of myeloma on the immune system as Dr. Chauncey mentioned with progression similar to what Dr. Roodman described in the bone, but with greater impact on immune functions. With both pro- and antifunctions you get more TH17 responses, increased T regulatory cell responses, but also more microenvironmental immune cells change.
The second effect is that the immune cell also affects the myeloma growth. For example, proinflammatory cytokine produce interleukin (IL)6, IL17, IL21, and IL23 that affect myeloma or provide myeloma cell growth and signaling mechanism. Also, the PDC (plasmacytoid dendritic cell) is one of the best bone marrow components that induces and supports myeloma growth. With progression, some of these microenvironmental elements actually play a greater role in having the disease function or progress growth more aggressively than otherwise.
The second important aspect that comes into the picture in the immune and bone marrow microenvironment is the role of selecting the clone. There are literally hundreds of clones in a given patient. Certain clones would be supported preferentially by the immune cells, and in some cases, these aggressive clones become independent and grow without the need of support. That’s when they end up becoming extramedullary disease, which also determines how the myeloma cell is growing with these molecular changes.
Dr. Mehta. Another point of evidence for the immune system dysregulation in allowing growth of myeloma is the impact of some of the new trials. So the KEYSTONE 023 trial, for example, showed that pembrolizumab, the programmed death 1 protein (PD1) inhibitor, helped to prevent progression of disease and actually increased survival. The CAR T [chimeric antigen receptor T-cell] studies, some of which were presented at ASH [American Society of Hematology annual meeting] last December, also are beginning to show promise, almost as well as in chronic lymphocytic leukemia. We could look at the therapeutic response as an indicator of biologic pathogenesis and say, “likely the immune system is a major determinant.”
Standard of Therapy
Dr. Ascensão. The Spanish group published some interesting data on high-risk MGUS and smoldering MM. What do you as investigators and clinicians look at to make a diagnosis of MGUS, and what kind of test would you do in order to separate these different groups? How would you define current standard of therapy, and do you assign specific therapies for specific groups of patients?
Dr. Munshi. The current standard is a 3-drug regimen in the U.S. The most common 3-drug regimen that we all usually use, definitely in younger people, but also in older people with some dose modification, was a proteasome inhibitor and immunomodulator with dexamethasone—commonly lenalidomide, bortezomib, and dexamethasone (RVD). One can use carfilzomib also. Now we are beginning to switch to ixazomib, an oral proteasome inhibitor, and it can become an all-oral regimen that could be very convenient. We will have a VA study utilizing a similar agent to make an all-oral regimen for treatment.
An alternative people use that has a financial difference is to use a proteasome inhibitor with cyclophosphamide and dexamethasone, a VCD-like regimen. The study is going to use ixazomib, cyclophosphamide, and dexamethasone followed by ixazomib maintenance. That’s the usual induction regimen.
The question is do we do this differently whether the patient is high risk or low risk? My personal bias in the answer is, not really. At the beginning, we will present the best treatment for the patient’s health. If they are older patients with a lot of comorbidities, which is more important, we can use weekly bortezomib instead of using the full dose. We can use subcutaneous weekly bortezomib or a similar 3-drug regimen. And so high risk or low risk doesn’t change how we’re doing the beginning.
The place where the risk stratification comes into the picture is if patients get a transplant. We are beginning to do posttransplant consolidation, and that’s where we would add 2 cycles of consolidation. If consolidation is not done, for high-risk patients one may do maintenance with 2 drugs like bortezomib and lenalidomide alone; whereas in a low-risk patient, you could do lenalidomide only and not use bortezomib with it. This is the impact of risk stratification as far as what we do. It comes more at the later time rather than earlier time point.
Dr. Mehta. There are still a lot of unanswered questions. One area where I think the VA is very good at becoming involved, if we choose to do so, would be some of the drug dosings. For example, dexamethasone used to be used in high dose and the ECOG study showed that you can use it in lower dose weekly rather than 4 days on, 4 days off. The Myeloma In VA (MIVA) group led by Dr. Munshi did a study looking at different doses of lenalidomide. There’s still unanswered questions even in this standard of care regimen that we use where we could try to make the regimen more tolerable for patients.
Dr. Roodman. RVd (modified RVD) has been piloted by Dr. Noopur Raje at Mass General, but I don’t think it’s a large study. She uses it in older patients. To follow-up on what you said, which I think is a really great idea, we could look at RVd as well as 2 doses of dexamethasone, or use oral proteasome antagonists instead.
Dr. Mehta. Yes, to reduce the neurotoxicity.
Dr. Roodman. That is correct. But I think the VA is set up to look at those kinds of questions because most of our patients are older.
Dr. Mehta. They have all the comorbidities.
Dr. Chauncey. We have what we think is an optimal regimen for an optimal patient, and then we have what we often see in the clinic. I’m not sure of the quantitative VA demographics, but if you look at the U.S. myeloma population, the median age is 70 years. While a triplet like
RVd or a carfilzomib-based triplet is probably optimal based on depth of response and the theoretical aspect of suppression of all subclones, it’s not really an accessible regimen for many patients that I see in clinic who are not transplant eligible.
For the nontransplant patients and more frail patients, the doublets or the cyclophosphamide/bortezomib/dexamethasone are reasonable options. I know there are proponents saying that everybody should get RVd or dose-attenuated RVd; I don’t think that’s practical for many of the patients that we see in our clinics.
Dr. Mehta. Why is it not practical in most of your patients? Because they have to come once a week to the clinic?
Dr. Chauncey. That’s part of it, but I find that it’s often too intense with excessive toxicity. The patients are older with comorbidities and they have more limited physiologic reserve. Part of it is coming to clinic, and that’s where all oral regimens such as Rd are useful. I don’t know that if you have an older patient and you see a good response that you’re tracking in terms of their Mspike (monoclonal protein) and their CRAB criteria, that everybody requires the aggressive approach of RVd or a similar triplet with a proteasome inhibitor, an IMiD [immunomodulator drug], and a steroid as induction therapy.
This is true even for some of the older patients who come to autologous transplantation. The data we talk about are in a younger group of patients who can tolerate the full dose of those regimens. The all-oral regimens are attractive, but it’s also quite a financial burden, maybe not to an individual patient, but certainly to the whole health care system. I’m not sure if there is a depth or durability of response advantage for the oral proteasome inhibitor over those available as IV or subcutaneous dosing.
Pricing
Dr. Ascensão. It’s interesting you bring that up because at the Washington DC VAMC, ixazomib may be priced similarly to bortezomib.
Dr. Chauncey. Well that would be very good and very interesting. It’s possible the federal pricing would make it a lot more attractive. It’s not the case outside of the VA, but there the cost burden for oral medication is often shifted to the patient.
Dr. Cosgriff. The price of carfilzomib is about $7,000 to $9,000 per month or per cycle. When we priced ixazomib here in Portland, it actually was more expensive than bortezomib, but it was cheaper than carfilzomib.
Dr. Mehta. If we can do a drug company-sponsored study, the ixazomib people would surely be interested in its usefulness as a triplet in VA patients. It also would be good for the VA patient because you don’t have to come once a week and it has much less neurotoxicity compared to the other proteasome inhibitors, which is a big problem.
Dr. Chauncey. That would be a great study to offer the right patient. I see a lot of myeloma patients in clinic that aren’t really eligible for transplantation. Many of those patients do well on doublet therapy, though they will often require dose adjustments, both down and up, as well as tracking disease status.
Dr. Cosgriff. In Portland, we’re using a lenalidomidebased regimen as a first-line therapy. We’re starting to see more and more individuals starting RVD upfront. There are some select individuals who have diabetic neuropathy or something like that, who may go on just
a lenalidomide plus dexamethasone, leaving out the bortezomib because of neuropathy issues.
Our second-line choice at this point in time has been carfilzomib, but with ixazomib being a cheaper option, we may actually consider switching over to that as a second-line choice. When you look at the clinical trials they cite in the product literature, the grade 1 neuropathy is about 18% with ixazomib and 14% with the placebo arm, so it does add some peripheral neuropathy. Maybe not as much as we would think.
While these oral agents and all-oral regimens are nice to have, we still bring in the patients for clinic and for monitoring of white blood count. It doesn’t necessarily decrease the burden of clinic visits. We still have to get lab draws. Yes, the patient is not sitting in a chair waiting for the bortezomib infusion to be mixed by the pharmacy, but we still have considerations for travel and those types of things.
We treat the entire state of Oregon and parts of southwest Washington. Fortunately, we have community-based outpatient clinics (CBOCs) and VAMCs in other areas of the state, and we also utilize places like Walla Walla and Spokane, Washington, where we can draw and check labs remotely, which decreases the travel burden, but it still is a burden to the patient to actually go in for lab tests. We’re not looking at ixazomib as a first line currently.
We’re going to wait for data to be published on ixazomib in the first line. In a couple of presentations, researchers have suggested that if you tease the data, ixazomib may be inferior to bortezomib in the first-line setting. It will be interesting to see what the data say for that as far as a first-line setting choice.
Dr. Chauncey. I second what Dr. Cosgriff said. You can’t just give patients 3 bottles of pills and say, “See you in 3 months.” Here in the Northwest we cover large rural areas, and there are many CBOCs whose labs feed directly into our CPRS (Computerized Patient Record System) so it’s very accessible and easy to follow patients that come in less frequently, but that doesn’t change the need for regular clinical follow-up. The other thing I’d say is the noncomparative data of carfilzomib/lenalidomide/dexamethasone (CRD) is quite compelling in terms of depth and duration of response. We usually don’t want to accept convenience over efficacy. Whether that durability translates long term, we don’t really know, but I think right now, CRD is the best available regimen, again, with the caveat that it’s not really accessible to patients of all ages and performance status.
Dr. Ascensão. At the Washington DC VAMC in our first cycle it is day 1, 4, 8, and 11 with bortezomib, but after that we go pretty much to weekly bortezomib. We also tend to use what I would call lower dose 20 mg dexamethasone in patients over the age of 70 years. And we feel that it is a lot less toxic. We use subcutaneous bortezomib for pretty much everybody else.
Managing Adverse Effects
Dr. Chauncey. I think we’re all using subcutaneous bortezomib at this point. Dexamethasone doesn’t get a lot of independent attention, but there’s no question that, as Dr. Mehta mentioned, the older regimen that we used, the dose-dense dexamethasone of the VAD regimen, was
quite disabling. In addition to obvious hyperglycemia, there were psychiatric problems and, ultimately, profound steroid myopathy that seemed to affect patients in variable fashion. Different patients seem to be more or less susceptible, but after a couple of cycles, it starts to kick in and is progressive.
So we’ve since abandoned those massive doses. But when you look at the ECOG study (E4A03) that really defined the lower dose (40 mg weekly), there’s no question the higher dose was more toxic but also more effective in terms of disease response. While there are many older patients that I would start with a lower dose of dexamethasone, whether it’s with lenalidomide or bortezomib, I keep in mind there can be a steep dose response curve for dexamethasone. If you’re giving 20 mg and you’re not getting the response you need, then you increase the dose. There is definitely a dose response, but the higher doses are just not as well tolerated.
Dr. Cosgriff. I don’t know if other institutions are doing it, but instead of doing 40 mg as a single dose because of patient performance status, providers in Portland will prescribe 20 mg on days 1, 2, 8, 9, 15, and 16. They’ll break up that 40 mg dose and give it that way.
Dr. Chauncey. I don’t know if that strategy is biologically equivalent in terms of antimyeloma activity or less toxic in terms of myopathy. There’s almost always some disease marker to track, so that whether you’re using the serum free-light chain assay or serum protein electrophoresis, you can see if the strategy you’re using is working in real time.
Dr. Cosgriff. I’ve never known whether it’s been shown to be more efficacious or if it’s just a way of getting around some of the adverse effects. However, it does pose some alternate challenges. With higher doses of steroids, you’re looking at 2 days where the patient can become hyperglycemic, if not, a little bit longer.
The other thing with it is that adding on that extra day of dexamethasone can interfere with some other drugs and some other therapies. In individuals who have had a deep vein thrombosis for whatever reason and they’re on warfarin, now we have an agent that really screws up our warfarin monitoring. We would have to consider switching them to another agent.
Thrombosis
Dr. Mehta. It’s also prothrombotic.
Dr. Chauncey. If you actually ask the patient, independent of the hyperglycemia, independent of the myopathy, independent of psychosis, but just quality of life, they’ll typically tell you that the on-and-off of steroids is the worst part of the regimen. It’s often the roller coaster ride of short-term hypomania followed by dysphoria.
Dr. Mehta. And the lack of sleep. They describe it as being out of their skin.
Dr. Chauncey. They are. And as soon as they stop, often there is a depression.
Dr. Mehta. It is very, very difficult. And some actually develop psychosis.
Dr. Ascensão. We all use some form of acyclovir or its derivative for the prevention of shingles in patients exposed to the proteasome inhibitors. We use aspirin, usually low dose (81 mg), for deep vein thrombosis prophylaxis. But is anybody using other anticoagulants or putting everybody prophylactically on proton-pump inhibitors (PPIs) or just seeing how people do first and then adjusting?
Dr. Chauncey. I typically use conventional dose aspirin, and if there’s breakthrough thrombosis, the first response should be that it is not the best regimen for this patient. Sometimes you have to go back to it, and if someone’s an anticoagulation candidate, then full anticoagulation is
needed if that’s the best regimen. Usually if there’s a breakthrough thrombosis, it is a deal breaker, and you’re ready to move on to a nonthrombogenic regimen.
There has been an observation (there is some biological basis to back this up) that if you give bortezomib with an IMiD, the regimen became less thrombogenic than with the IMiD and dexamethasone alone.
Dr. Ascensão. On aspirin, even if they’re on an IMiD plus a proteasome inhibitor, I just don’t know that the data are good enough for us to avoid it at this point in time. And I don’t necessarily put people on a PPI unless they’ve got added gastrointestinal problems and unless they have associated heartburn or dyspepsia symptoms.
Dr. Mehta. I use low-dose aspirin in every patient. And if they breakthrough, they go on full anticoagulation usually with a new oral anticoagulant. I use PPIs only if needed, although most of them do need it, and, of course, bisphosphonates so the bone protective aspect.
Click to read the digital edition.
Consensus Statement Supporting the Recommendation for Single-Fraction Palliative Radiotherapy for Uncomplicated, Painful Bone Metastases
The authors would like to acknowledge Tony Quang, MD, JD, for the advice given on this project.
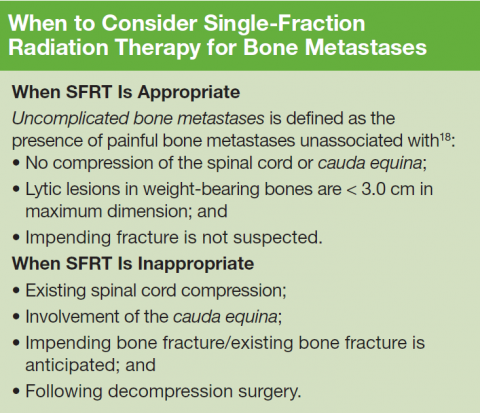
Palliative radiotherapy for bone metastases is typically delivered either as a short course of 1 to 5 fractions or protracted over longer courses of up to 20 treatments. These longer courses can be burdensome and discourage its utilization, despite a 50% to 80% likelihood of meaningful pain relief from only a single fraction of radiation therapy. Meanwhile, there are multiple randomized studies that have demonstrated that shorter course(s) are equivalent for pain control.
Although the VHA currently has 143 medical facilities that have cancer diagnostic and treatment capabilities, only 40 have radiation oncology services on-site.1 Thus, access to palliative radiotherapy may be limited for veterans who do not live close by, and many may seek care outside the VHA. At VHA radiation oncology centers, single-fraction radiation therapy (SFRT) is routinely offered by the majority of radiation oncologists.2,3 However, the longer course is commonly preferred outside the VA, and a recent SEER-Medicare analysis of more than 3,000 patients demonstrated that the majority of patients treated outside the VA actually receive more than 10 treatments.4 For this reason, the VA National Palliative Radiotherapy Task Force prepared this document to provide guidance for clinicians within and outside the VA to increase awareness of the appropriateness, effectiveness, and convenience of SFRT as opposed to longer courses of treatment that increase the burden of care at the end of life and often are unnecessary.
Veterans, Cancer, and Metastases
Within the VA, an estimated 40,000 new cancer cases are diagnosed each year, and 175,000 veterans undergo cancer care within the VHA annually.1 Unfortunately, the majority will develop bone metastases with postmortem examinations, suggesting that the rate can be as high as 90% at the end of life.5-7 For many, including veterans with cancer, pain control can be difficult, and access to palliative radiotherapy is critical.8
Single-Fraction Palliatiev Radiation Therapy
Historically, patients with painful bone metastases have been treated with courses of palliative radiotherapy ranging between 2 and 4 weeks of daily treatments. However, several large randomized clinical trials comparing a single treatment with multiple treatments have established that SFRT provides equivalent rates of pain relief even when it may be required for a second time.9-12 Recommendations based on these trials have been incorporated into various treatment guidelines that widely acknowledge the efficacy of SFRT.13-15
For this reason, SFRT is often preferred at many centers because it is substantially more convenient for patients with cancer. It reduces travel time for daily radiation clinic visits, which allows for more time with loved ones outside the medical establishment. Furthermore, SFRT improves patient access to radiotherapy and reduces costs. The benefits can be direct as well as indirect to those who have to take time for numerous visits.
Longer courses of palliative radiotherapy can be burdensome for patients and primary care providers. Unnecessarily protracted courses of palliative radiotherapy also delay the receipt of systemic therapies because they are typically considered unsafe to administer concurrently. Moreover, when SFRT is unavailable, the burden of long-course palliation is known to discourage health care providers from referring patients since opioid therapy is more convenient, even though it exchanges lucidity for analgesia.16,17
For this reason, the authors believe that it is in the best interest for veterans with terminal cancers and their providers to be aware of the shorter SFRT for effective, convenient pain relief. This treatment option is particularly relevant for patients with a poor performance status, patients already in hospice care, or patient who travel long distances.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
2. Moghanaki D, Cheuk AV, Fosmire H, et al; U.S. Veterans Healthcare Administration National Palliative Radiotherapy Taskforce. Availability of single fraction palliative radiotherapy for cancer patients receiving end-of-life care within the Veterans Healthcare Administration. J Palliat Med. 2014;17(11):1221-1225.
3. Dawson GA, Glushko I, Hagan MP. A cross-sectional view of radiation dose fractionation schemes used for painful bone metastases cases within Veterans Health Administration Radiation Oncology Centers. J Clin Oncol. 2015;33(29 suppl):abstract 177.
4. Bekelman JE, Epstein AJ, Emanuel EJ. Single- vs multiple-fraction radiotherapy for bone metastases from prostate cancer. JAMA. 2013;310(14):1501-1502.
5. Galasko CSB. The anatomy and pathways of skeletal metastases. In: Weiss L, Gilbert AH, eds. Bone Metastasis. Boston, MA: GK Hall; 1981:49-63.
6. Bubendorf L, Schöpfer A, Wagner U, et al. Metastatic patterns in prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578-583.
7. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20, pt 2):6243s-6249s.
8. Geriatrics and Extended Care Strategic Healthcare Group, National Pain Management Coordinating Committee, Veterans Health Administration. Pain as the 5th Vital Sign Toolkit. Rev. ed. Washington, DC: National Pain Management Coordinating Committee; 2000.
9. Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798-804.
10. Chow E, Hoskins PJ, Wu J, et al. A phase III international randomised trial comparing single with multiple fractions for re-irradiation of painful bone metastases: National Cancer Institute of Canada Clinical Trials Group (NCTC CTG) SC 20. Clin Oncol (R Coll Radiol). 2006;18(2):125-128.
11. Fairchild A, Barnes E, Ghosh S, et al. International patterns of practice in palliative radiotherapy for painful bone metastases: evidence-based practice? Int J Radiat Oncol Biol Phys. 2009;75(5):1501-1510.
12. Chow E, van der Linden YM, Roos D, et al. Single fraction versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncol. 2014;15(2):164-171.
13. Lutz ST, Berk L, Chang E, et al; American Society for Radiation Oncology (ASTRO). Palliative radiotherapy for bone metastases: an ASTRO evidencebased guideline. Int J Radiat Oncol, Biol, Phys. 2011;79(4):965-976.
14. Expert Panel on Radiation Oncology-Bone Metastases, Lo SS, Lutz ST, Chang EL, et al. ACR Appropriateness Criteria® spinal bone metastases. J Palliat Med. 2013;16(1):9-19.
15. Expert Panel on Radiation Oncology-Bone Metastases, Lutz ST, Lo SS, Chang EL, et al. ACR Appropriateness Criteria® non-spinal bone metastases. J Palliative Med. 2012;15(5):521-526.
16. Guadagnolo BA, Liao KP, Elting L, Giordano S, Buchholz TA, Shih YC. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol. 2013;31(1):80-87.
17. Schuster J, Han T, Anscher M, Moghanaki D. Hospice providers awareness of the benefits and availability of single-fraction palliative radiotherapy. J Hospice Palliat Care Nurs. 2014;16(2):67-72.
18. Cheon PM, Wong E, Thavarajah N, et al. A definition of “uncomplicated bone metastases” based on previous bone metastases trials comparing single-fraction and multi-fraction radiation therapy. J Bone Oncol. 2015;4(1):13-17.
The authors would like to acknowledge Tony Quang, MD, JD, for the advice given on this project.
Palliative radiotherapy for bone metastases is typically delivered either as a short course of 1 to 5 fractions or protracted over longer courses of up to 20 treatments. These longer courses can be burdensome and discourage its utilization, despite a 50% to 80% likelihood of meaningful pain relief from only a single fraction of radiation therapy. Meanwhile, there are multiple randomized studies that have demonstrated that shorter course(s) are equivalent for pain control.
Although the VHA currently has 143 medical facilities that have cancer diagnostic and treatment capabilities, only 40 have radiation oncology services on-site.1 Thus, access to palliative radiotherapy may be limited for veterans who do not live close by, and many may seek care outside the VHA. At VHA radiation oncology centers, single-fraction radiation therapy (SFRT) is routinely offered by the majority of radiation oncologists.2,3 However, the longer course is commonly preferred outside the VA, and a recent SEER-Medicare analysis of more than 3,000 patients demonstrated that the majority of patients treated outside the VA actually receive more than 10 treatments.4 For this reason, the VA National Palliative Radiotherapy Task Force prepared this document to provide guidance for clinicians within and outside the VA to increase awareness of the appropriateness, effectiveness, and convenience of SFRT as opposed to longer courses of treatment that increase the burden of care at the end of life and often are unnecessary.
Veterans, Cancer, and Metastases
Within the VA, an estimated 40,000 new cancer cases are diagnosed each year, and 175,000 veterans undergo cancer care within the VHA annually.1 Unfortunately, the majority will develop bone metastases with postmortem examinations, suggesting that the rate can be as high as 90% at the end of life.5-7 For many, including veterans with cancer, pain control can be difficult, and access to palliative radiotherapy is critical.8
Single-Fraction Palliatiev Radiation Therapy
Historically, patients with painful bone metastases have been treated with courses of palliative radiotherapy ranging between 2 and 4 weeks of daily treatments. However, several large randomized clinical trials comparing a single treatment with multiple treatments have established that SFRT provides equivalent rates of pain relief even when it may be required for a second time.9-12 Recommendations based on these trials have been incorporated into various treatment guidelines that widely acknowledge the efficacy of SFRT.13-15
For this reason, SFRT is often preferred at many centers because it is substantially more convenient for patients with cancer. It reduces travel time for daily radiation clinic visits, which allows for more time with loved ones outside the medical establishment. Furthermore, SFRT improves patient access to radiotherapy and reduces costs. The benefits can be direct as well as indirect to those who have to take time for numerous visits.
Longer courses of palliative radiotherapy can be burdensome for patients and primary care providers. Unnecessarily protracted courses of palliative radiotherapy also delay the receipt of systemic therapies because they are typically considered unsafe to administer concurrently. Moreover, when SFRT is unavailable, the burden of long-course palliation is known to discourage health care providers from referring patients since opioid therapy is more convenient, even though it exchanges lucidity for analgesia.16,17
For this reason, the authors believe that it is in the best interest for veterans with terminal cancers and their providers to be aware of the shorter SFRT for effective, convenient pain relief. This treatment option is particularly relevant for patients with a poor performance status, patients already in hospice care, or patient who travel long distances.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
The authors would like to acknowledge Tony Quang, MD, JD, for the advice given on this project.
Palliative radiotherapy for bone metastases is typically delivered either as a short course of 1 to 5 fractions or protracted over longer courses of up to 20 treatments. These longer courses can be burdensome and discourage its utilization, despite a 50% to 80% likelihood of meaningful pain relief from only a single fraction of radiation therapy. Meanwhile, there are multiple randomized studies that have demonstrated that shorter course(s) are equivalent for pain control.
Although the VHA currently has 143 medical facilities that have cancer diagnostic and treatment capabilities, only 40 have radiation oncology services on-site.1 Thus, access to palliative radiotherapy may be limited for veterans who do not live close by, and many may seek care outside the VHA. At VHA radiation oncology centers, single-fraction radiation therapy (SFRT) is routinely offered by the majority of radiation oncologists.2,3 However, the longer course is commonly preferred outside the VA, and a recent SEER-Medicare analysis of more than 3,000 patients demonstrated that the majority of patients treated outside the VA actually receive more than 10 treatments.4 For this reason, the VA National Palliative Radiotherapy Task Force prepared this document to provide guidance for clinicians within and outside the VA to increase awareness of the appropriateness, effectiveness, and convenience of SFRT as opposed to longer courses of treatment that increase the burden of care at the end of life and often are unnecessary.
Veterans, Cancer, and Metastases
Within the VA, an estimated 40,000 new cancer cases are diagnosed each year, and 175,000 veterans undergo cancer care within the VHA annually.1 Unfortunately, the majority will develop bone metastases with postmortem examinations, suggesting that the rate can be as high as 90% at the end of life.5-7 For many, including veterans with cancer, pain control can be difficult, and access to palliative radiotherapy is critical.8
Single-Fraction Palliatiev Radiation Therapy
Historically, patients with painful bone metastases have been treated with courses of palliative radiotherapy ranging between 2 and 4 weeks of daily treatments. However, several large randomized clinical trials comparing a single treatment with multiple treatments have established that SFRT provides equivalent rates of pain relief even when it may be required for a second time.9-12 Recommendations based on these trials have been incorporated into various treatment guidelines that widely acknowledge the efficacy of SFRT.13-15
For this reason, SFRT is often preferred at many centers because it is substantially more convenient for patients with cancer. It reduces travel time for daily radiation clinic visits, which allows for more time with loved ones outside the medical establishment. Furthermore, SFRT improves patient access to radiotherapy and reduces costs. The benefits can be direct as well as indirect to those who have to take time for numerous visits.
Longer courses of palliative radiotherapy can be burdensome for patients and primary care providers. Unnecessarily protracted courses of palliative radiotherapy also delay the receipt of systemic therapies because they are typically considered unsafe to administer concurrently. Moreover, when SFRT is unavailable, the burden of long-course palliation is known to discourage health care providers from referring patients since opioid therapy is more convenient, even though it exchanges lucidity for analgesia.16,17
For this reason, the authors believe that it is in the best interest for veterans with terminal cancers and their providers to be aware of the shorter SFRT for effective, convenient pain relief. This treatment option is particularly relevant for patients with a poor performance status, patients already in hospice care, or patient who travel long distances.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
2. Moghanaki D, Cheuk AV, Fosmire H, et al; U.S. Veterans Healthcare Administration National Palliative Radiotherapy Taskforce. Availability of single fraction palliative radiotherapy for cancer patients receiving end-of-life care within the Veterans Healthcare Administration. J Palliat Med. 2014;17(11):1221-1225.
3. Dawson GA, Glushko I, Hagan MP. A cross-sectional view of radiation dose fractionation schemes used for painful bone metastases cases within Veterans Health Administration Radiation Oncology Centers. J Clin Oncol. 2015;33(29 suppl):abstract 177.
4. Bekelman JE, Epstein AJ, Emanuel EJ. Single- vs multiple-fraction radiotherapy for bone metastases from prostate cancer. JAMA. 2013;310(14):1501-1502.
5. Galasko CSB. The anatomy and pathways of skeletal metastases. In: Weiss L, Gilbert AH, eds. Bone Metastasis. Boston, MA: GK Hall; 1981:49-63.
6. Bubendorf L, Schöpfer A, Wagner U, et al. Metastatic patterns in prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578-583.
7. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20, pt 2):6243s-6249s.
8. Geriatrics and Extended Care Strategic Healthcare Group, National Pain Management Coordinating Committee, Veterans Health Administration. Pain as the 5th Vital Sign Toolkit. Rev. ed. Washington, DC: National Pain Management Coordinating Committee; 2000.
9. Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798-804.
10. Chow E, Hoskins PJ, Wu J, et al. A phase III international randomised trial comparing single with multiple fractions for re-irradiation of painful bone metastases: National Cancer Institute of Canada Clinical Trials Group (NCTC CTG) SC 20. Clin Oncol (R Coll Radiol). 2006;18(2):125-128.
11. Fairchild A, Barnes E, Ghosh S, et al. International patterns of practice in palliative radiotherapy for painful bone metastases: evidence-based practice? Int J Radiat Oncol Biol Phys. 2009;75(5):1501-1510.
12. Chow E, van der Linden YM, Roos D, et al. Single fraction versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncol. 2014;15(2):164-171.
13. Lutz ST, Berk L, Chang E, et al; American Society for Radiation Oncology (ASTRO). Palliative radiotherapy for bone metastases: an ASTRO evidencebased guideline. Int J Radiat Oncol, Biol, Phys. 2011;79(4):965-976.
14. Expert Panel on Radiation Oncology-Bone Metastases, Lo SS, Lutz ST, Chang EL, et al. ACR Appropriateness Criteria® spinal bone metastases. J Palliat Med. 2013;16(1):9-19.
15. Expert Panel on Radiation Oncology-Bone Metastases, Lutz ST, Lo SS, Chang EL, et al. ACR Appropriateness Criteria® non-spinal bone metastases. J Palliative Med. 2012;15(5):521-526.
16. Guadagnolo BA, Liao KP, Elting L, Giordano S, Buchholz TA, Shih YC. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol. 2013;31(1):80-87.
17. Schuster J, Han T, Anscher M, Moghanaki D. Hospice providers awareness of the benefits and availability of single-fraction palliative radiotherapy. J Hospice Palliat Care Nurs. 2014;16(2):67-72.
18. Cheon PM, Wong E, Thavarajah N, et al. A definition of “uncomplicated bone metastases” based on previous bone metastases trials comparing single-fraction and multi-fraction radiation therapy. J Bone Oncol. 2015;4(1):13-17.
1. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
2. Moghanaki D, Cheuk AV, Fosmire H, et al; U.S. Veterans Healthcare Administration National Palliative Radiotherapy Taskforce. Availability of single fraction palliative radiotherapy for cancer patients receiving end-of-life care within the Veterans Healthcare Administration. J Palliat Med. 2014;17(11):1221-1225.
3. Dawson GA, Glushko I, Hagan MP. A cross-sectional view of radiation dose fractionation schemes used for painful bone metastases cases within Veterans Health Administration Radiation Oncology Centers. J Clin Oncol. 2015;33(29 suppl):abstract 177.
4. Bekelman JE, Epstein AJ, Emanuel EJ. Single- vs multiple-fraction radiotherapy for bone metastases from prostate cancer. JAMA. 2013;310(14):1501-1502.
5. Galasko CSB. The anatomy and pathways of skeletal metastases. In: Weiss L, Gilbert AH, eds. Bone Metastasis. Boston, MA: GK Hall; 1981:49-63.
6. Bubendorf L, Schöpfer A, Wagner U, et al. Metastatic patterns in prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578-583.
7. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20, pt 2):6243s-6249s.
8. Geriatrics and Extended Care Strategic Healthcare Group, National Pain Management Coordinating Committee, Veterans Health Administration. Pain as the 5th Vital Sign Toolkit. Rev. ed. Washington, DC: National Pain Management Coordinating Committee; 2000.
9. Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798-804.
10. Chow E, Hoskins PJ, Wu J, et al. A phase III international randomised trial comparing single with multiple fractions for re-irradiation of painful bone metastases: National Cancer Institute of Canada Clinical Trials Group (NCTC CTG) SC 20. Clin Oncol (R Coll Radiol). 2006;18(2):125-128.
11. Fairchild A, Barnes E, Ghosh S, et al. International patterns of practice in palliative radiotherapy for painful bone metastases: evidence-based practice? Int J Radiat Oncol Biol Phys. 2009;75(5):1501-1510.
12. Chow E, van der Linden YM, Roos D, et al. Single fraction versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncol. 2014;15(2):164-171.
13. Lutz ST, Berk L, Chang E, et al; American Society for Radiation Oncology (ASTRO). Palliative radiotherapy for bone metastases: an ASTRO evidencebased guideline. Int J Radiat Oncol, Biol, Phys. 2011;79(4):965-976.
14. Expert Panel on Radiation Oncology-Bone Metastases, Lo SS, Lutz ST, Chang EL, et al. ACR Appropriateness Criteria® spinal bone metastases. J Palliat Med. 2013;16(1):9-19.
15. Expert Panel on Radiation Oncology-Bone Metastases, Lutz ST, Lo SS, Chang EL, et al. ACR Appropriateness Criteria® non-spinal bone metastases. J Palliative Med. 2012;15(5):521-526.
16. Guadagnolo BA, Liao KP, Elting L, Giordano S, Buchholz TA, Shih YC. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol. 2013;31(1):80-87.
17. Schuster J, Han T, Anscher M, Moghanaki D. Hospice providers awareness of the benefits and availability of single-fraction palliative radiotherapy. J Hospice Palliat Care Nurs. 2014;16(2):67-72.
18. Cheon PM, Wong E, Thavarajah N, et al. A definition of “uncomplicated bone metastases” based on previous bone metastases trials comparing single-fraction and multi-fraction radiation therapy. J Bone Oncol. 2015;4(1):13-17.
Exercise better than meds to reduce fatigue in cancer patients
Exercise and/or psychological therapy work better than medications to reduce cancer-related fatigue, according to research published in JAMA Oncology.
Researchers conducted a review and meta-analysis of more than 113 studies and found that exercise and psychological interventions, as well as a combination of both, were associated with reduced fatigue during and after cancer treatment.
However, pharmaceutical interventions were not associated with the same magnitude of improvement.
The researchers therefore concluded that exercise and psychological therapy should be recommended over medications.
“If a cancer patient is having trouble with fatigue, rather than looking for extra cups of coffee, a nap, or a pharmaceutical solution, consider a 15-minute walk,” said study author Karen Mustian, PhD, of the University of Rochester Medical Center in Rochester, New York.
“It’s a really simple concept, but it’s very hard for patients and the medical community to wrap their heads around it because these interventions have not been front-and-center in the past. Our research gives clinicians a valuable asset to alleviate cancer-related fatigue.”
Dr Mustian and her colleagues reached their conclusions after analyzing data from 113 randomized clinical trials testing various treatments for cancer-related fatigue.
There were 11,525 patients enrolled in these studies. Nearly half (46.9%) were women with breast cancer. Ten studies focused on other types of cancer and enrolled only men.
Dr Mustian and her colleagues performed a meta-analysis to establish and compare the mean weighted effect sizes (WESs) of the fatigue treatments.
The team found that exercise alone—whether aerobic or anaerobic—reduced cancer-related fatigue most significantly. The WES was 0.30 (95% CI, 0.25-0.36; P<0.001).
Psychological interventions—such as therapy designed to provide education, change personal behavior, and adapt the way a person thinks about his or her circumstances—also improved fatigue. The WES was 0.27 (95% CI, 0.21-0.330.30; P<0.001).
A combination of psychological interventions and exercise had a significant improvement on fatigue as well. The WES was 0.26 (95% CI, 0.13-0.38; P<0.001).
However, the drugs tested for treating cancer-related fatigue—paroxetine hydrochloride, modafinil, armodafinil, methylphenidate hydrochloride, dexymethylphenidate, dexamphetamine, and methylprednisolone—were not as effective as the other interventions. The WES was 0.09 (95% CI, 0.00-0.19; P=0.05).
“The literature bears out that these drugs don’t work very well, although they are continually prescribed,” Dr Mustian said. “Cancer patients already take a lot of medications, and they all come with risks and side effects. So any time you can subtract a pharmaceutical from the picture it usually benefits patients.” ![]()
Exercise and/or psychological therapy work better than medications to reduce cancer-related fatigue, according to research published in JAMA Oncology.
Researchers conducted a review and meta-analysis of more than 113 studies and found that exercise and psychological interventions, as well as a combination of both, were associated with reduced fatigue during and after cancer treatment.
However, pharmaceutical interventions were not associated with the same magnitude of improvement.
The researchers therefore concluded that exercise and psychological therapy should be recommended over medications.
“If a cancer patient is having trouble with fatigue, rather than looking for extra cups of coffee, a nap, or a pharmaceutical solution, consider a 15-minute walk,” said study author Karen Mustian, PhD, of the University of Rochester Medical Center in Rochester, New York.
“It’s a really simple concept, but it’s very hard for patients and the medical community to wrap their heads around it because these interventions have not been front-and-center in the past. Our research gives clinicians a valuable asset to alleviate cancer-related fatigue.”
Dr Mustian and her colleagues reached their conclusions after analyzing data from 113 randomized clinical trials testing various treatments for cancer-related fatigue.
There were 11,525 patients enrolled in these studies. Nearly half (46.9%) were women with breast cancer. Ten studies focused on other types of cancer and enrolled only men.
Dr Mustian and her colleagues performed a meta-analysis to establish and compare the mean weighted effect sizes (WESs) of the fatigue treatments.
The team found that exercise alone—whether aerobic or anaerobic—reduced cancer-related fatigue most significantly. The WES was 0.30 (95% CI, 0.25-0.36; P<0.001).
Psychological interventions—such as therapy designed to provide education, change personal behavior, and adapt the way a person thinks about his or her circumstances—also improved fatigue. The WES was 0.27 (95% CI, 0.21-0.330.30; P<0.001).
A combination of psychological interventions and exercise had a significant improvement on fatigue as well. The WES was 0.26 (95% CI, 0.13-0.38; P<0.001).
However, the drugs tested for treating cancer-related fatigue—paroxetine hydrochloride, modafinil, armodafinil, methylphenidate hydrochloride, dexymethylphenidate, dexamphetamine, and methylprednisolone—were not as effective as the other interventions. The WES was 0.09 (95% CI, 0.00-0.19; P=0.05).
“The literature bears out that these drugs don’t work very well, although they are continually prescribed,” Dr Mustian said. “Cancer patients already take a lot of medications, and they all come with risks and side effects. So any time you can subtract a pharmaceutical from the picture it usually benefits patients.” ![]()
Exercise and/or psychological therapy work better than medications to reduce cancer-related fatigue, according to research published in JAMA Oncology.
Researchers conducted a review and meta-analysis of more than 113 studies and found that exercise and psychological interventions, as well as a combination of both, were associated with reduced fatigue during and after cancer treatment.
However, pharmaceutical interventions were not associated with the same magnitude of improvement.
The researchers therefore concluded that exercise and psychological therapy should be recommended over medications.
“If a cancer patient is having trouble with fatigue, rather than looking for extra cups of coffee, a nap, or a pharmaceutical solution, consider a 15-minute walk,” said study author Karen Mustian, PhD, of the University of Rochester Medical Center in Rochester, New York.
“It’s a really simple concept, but it’s very hard for patients and the medical community to wrap their heads around it because these interventions have not been front-and-center in the past. Our research gives clinicians a valuable asset to alleviate cancer-related fatigue.”
Dr Mustian and her colleagues reached their conclusions after analyzing data from 113 randomized clinical trials testing various treatments for cancer-related fatigue.
There were 11,525 patients enrolled in these studies. Nearly half (46.9%) were women with breast cancer. Ten studies focused on other types of cancer and enrolled only men.
Dr Mustian and her colleagues performed a meta-analysis to establish and compare the mean weighted effect sizes (WESs) of the fatigue treatments.
The team found that exercise alone—whether aerobic or anaerobic—reduced cancer-related fatigue most significantly. The WES was 0.30 (95% CI, 0.25-0.36; P<0.001).
Psychological interventions—such as therapy designed to provide education, change personal behavior, and adapt the way a person thinks about his or her circumstances—also improved fatigue. The WES was 0.27 (95% CI, 0.21-0.330.30; P<0.001).
A combination of psychological interventions and exercise had a significant improvement on fatigue as well. The WES was 0.26 (95% CI, 0.13-0.38; P<0.001).
However, the drugs tested for treating cancer-related fatigue—paroxetine hydrochloride, modafinil, armodafinil, methylphenidate hydrochloride, dexymethylphenidate, dexamphetamine, and methylprednisolone—were not as effective as the other interventions. The WES was 0.09 (95% CI, 0.00-0.19; P=0.05).
“The literature bears out that these drugs don’t work very well, although they are continually prescribed,” Dr Mustian said. “Cancer patients already take a lot of medications, and they all come with risks and side effects. So any time you can subtract a pharmaceutical from the picture it usually benefits patients.” ![]()
‘Strong evidence’ links obesity to cancers
There is strong evidence linking adiposity to esophageal adenocarcinoma, multiple myeloma, and cancer of the colon, rectum, biliary tract, pancreas, endometrium, kidney, and postmenopausal breast, according to the authors of an umbrella review published in the Feb. 28 edition of the BMJ.
“Several meta-analyses support the link between obesity and cancer, but substantial heterogeneity exists between studies,” wrote Maria Kyrgiou, MD, of Imperial College London and her coauthors. “The reported associations may be causal, but they may also be flawed, as inherent study biases such as residual confounding and selective reporting of positive results may exaggerate the effect of obesity on cancer.”
In this umbrella review, researchers analyzed 49 papers that included a total of 204 meta-analyses, which in turn summarized 2,179 individual study estimates from 507 unique cohort or case-control studies.
When researchers applied a threshold for significance of P less than .000001, the summary random effects were significant in 35 meta-analyses; 31 of these found increased risk with adiposity of esophageal adenocarcinoma, multiple myeloma, and cancers of the colon, rectum, liver, biliary tract system (cancers of gallbladder, extrahepatic bile duct, and ampulla of Vater), pancreas, postmenopausal breast, endometrium, and kidney.
“The effect of obesity on the incidence and mortality of cancer is well recognized and was evident in our umbrella review, with approximately 77% of the included meta-analyses reporting a nominally statistically significant summary random effects estimate,” the authors reported.
Overall, the summary estimates were similar between men and women for esophageal adenocarcinoma, esophageal squamous cell carcinoma, multiple myeloma, leukemia, and gastric, lung, kidney, and thyroid cancers.
However, men had a 30% higher risk of colon cancer per 5-kg/m2 increase of body mass index, compared with a 9% increase in risk in women for the same rise in BMI. Men also showed an increased risk of melanoma with increasing BMI, whereas women did not.
Women who had never used hormone therapy showed an 11% increase in the risk of postmenopausal breast cancer with each 5 kg of weight gained. Similarly, each 0.1 increase in waist-to-hip ratio in these women was associated with a 21% increase in the risk of endometrial cancer.
The analysis also revealed an inverse relationship in four meta-analyses for esophageal squamous cell carcinoma and lung cancer.
The authors said their findings agree with those of the World Cancer Research Fund, which currently states there is a convincing causal relationship with obesity for esophageal adenocarcinoma and cancers of the pancreas, colorectum, postmenopausal breast, endometrium, kidney, and liver.
While this umbrella analysis did not find strong evidence for an association with liver cancer, the authors said the evidence was “highly suggestive” but suffered from small study effects, excess significance bias, and substantial heterogeneity between studies.
“To draw firmer conclusions, we need prospective studies and large consortiums with better assessment of the changing nature of body fatness and with comprehensive standardized reporting of analyses,” they wrote. “As obesity becomes one of the greatest public health problems worldwide, evidence of the strength of the associations between obesity and cancer may allow finer selection of people at high risk, who could be selected for personalized primary and secondary prevention strategies.”
The study was supported by the Genesis Research Trust, Sigrid Jusélius Fellowship, the World Cancer Research Fund International Regular Grant Programme, Ovarian Cancer Action, the Imperial Experimental Cancer Medicine Centre, the Cancer Research UK Imperial Centre, Imperial Healthcare NHS Trust NIHR BRC. No relevant conflicts of interest were declared.
There is strong evidence linking adiposity to esophageal adenocarcinoma, multiple myeloma, and cancer of the colon, rectum, biliary tract, pancreas, endometrium, kidney, and postmenopausal breast, according to the authors of an umbrella review published in the Feb. 28 edition of the BMJ.
“Several meta-analyses support the link between obesity and cancer, but substantial heterogeneity exists between studies,” wrote Maria Kyrgiou, MD, of Imperial College London and her coauthors. “The reported associations may be causal, but they may also be flawed, as inherent study biases such as residual confounding and selective reporting of positive results may exaggerate the effect of obesity on cancer.”
In this umbrella review, researchers analyzed 49 papers that included a total of 204 meta-analyses, which in turn summarized 2,179 individual study estimates from 507 unique cohort or case-control studies.
When researchers applied a threshold for significance of P less than .000001, the summary random effects were significant in 35 meta-analyses; 31 of these found increased risk with adiposity of esophageal adenocarcinoma, multiple myeloma, and cancers of the colon, rectum, liver, biliary tract system (cancers of gallbladder, extrahepatic bile duct, and ampulla of Vater), pancreas, postmenopausal breast, endometrium, and kidney.
“The effect of obesity on the incidence and mortality of cancer is well recognized and was evident in our umbrella review, with approximately 77% of the included meta-analyses reporting a nominally statistically significant summary random effects estimate,” the authors reported.
Overall, the summary estimates were similar between men and women for esophageal adenocarcinoma, esophageal squamous cell carcinoma, multiple myeloma, leukemia, and gastric, lung, kidney, and thyroid cancers.
However, men had a 30% higher risk of colon cancer per 5-kg/m2 increase of body mass index, compared with a 9% increase in risk in women for the same rise in BMI. Men also showed an increased risk of melanoma with increasing BMI, whereas women did not.
Women who had never used hormone therapy showed an 11% increase in the risk of postmenopausal breast cancer with each 5 kg of weight gained. Similarly, each 0.1 increase in waist-to-hip ratio in these women was associated with a 21% increase in the risk of endometrial cancer.
The analysis also revealed an inverse relationship in four meta-analyses for esophageal squamous cell carcinoma and lung cancer.
The authors said their findings agree with those of the World Cancer Research Fund, which currently states there is a convincing causal relationship with obesity for esophageal adenocarcinoma and cancers of the pancreas, colorectum, postmenopausal breast, endometrium, kidney, and liver.
While this umbrella analysis did not find strong evidence for an association with liver cancer, the authors said the evidence was “highly suggestive” but suffered from small study effects, excess significance bias, and substantial heterogeneity between studies.
“To draw firmer conclusions, we need prospective studies and large consortiums with better assessment of the changing nature of body fatness and with comprehensive standardized reporting of analyses,” they wrote. “As obesity becomes one of the greatest public health problems worldwide, evidence of the strength of the associations between obesity and cancer may allow finer selection of people at high risk, who could be selected for personalized primary and secondary prevention strategies.”
The study was supported by the Genesis Research Trust, Sigrid Jusélius Fellowship, the World Cancer Research Fund International Regular Grant Programme, Ovarian Cancer Action, the Imperial Experimental Cancer Medicine Centre, the Cancer Research UK Imperial Centre, Imperial Healthcare NHS Trust NIHR BRC. No relevant conflicts of interest were declared.
There is strong evidence linking adiposity to esophageal adenocarcinoma, multiple myeloma, and cancer of the colon, rectum, biliary tract, pancreas, endometrium, kidney, and postmenopausal breast, according to the authors of an umbrella review published in the Feb. 28 edition of the BMJ.
“Several meta-analyses support the link between obesity and cancer, but substantial heterogeneity exists between studies,” wrote Maria Kyrgiou, MD, of Imperial College London and her coauthors. “The reported associations may be causal, but they may also be flawed, as inherent study biases such as residual confounding and selective reporting of positive results may exaggerate the effect of obesity on cancer.”
In this umbrella review, researchers analyzed 49 papers that included a total of 204 meta-analyses, which in turn summarized 2,179 individual study estimates from 507 unique cohort or case-control studies.
When researchers applied a threshold for significance of P less than .000001, the summary random effects were significant in 35 meta-analyses; 31 of these found increased risk with adiposity of esophageal adenocarcinoma, multiple myeloma, and cancers of the colon, rectum, liver, biliary tract system (cancers of gallbladder, extrahepatic bile duct, and ampulla of Vater), pancreas, postmenopausal breast, endometrium, and kidney.
“The effect of obesity on the incidence and mortality of cancer is well recognized and was evident in our umbrella review, with approximately 77% of the included meta-analyses reporting a nominally statistically significant summary random effects estimate,” the authors reported.
Overall, the summary estimates were similar between men and women for esophageal adenocarcinoma, esophageal squamous cell carcinoma, multiple myeloma, leukemia, and gastric, lung, kidney, and thyroid cancers.
However, men had a 30% higher risk of colon cancer per 5-kg/m2 increase of body mass index, compared with a 9% increase in risk in women for the same rise in BMI. Men also showed an increased risk of melanoma with increasing BMI, whereas women did not.
Women who had never used hormone therapy showed an 11% increase in the risk of postmenopausal breast cancer with each 5 kg of weight gained. Similarly, each 0.1 increase in waist-to-hip ratio in these women was associated with a 21% increase in the risk of endometrial cancer.
The analysis also revealed an inverse relationship in four meta-analyses for esophageal squamous cell carcinoma and lung cancer.
The authors said their findings agree with those of the World Cancer Research Fund, which currently states there is a convincing causal relationship with obesity for esophageal adenocarcinoma and cancers of the pancreas, colorectum, postmenopausal breast, endometrium, kidney, and liver.
While this umbrella analysis did not find strong evidence for an association with liver cancer, the authors said the evidence was “highly suggestive” but suffered from small study effects, excess significance bias, and substantial heterogeneity between studies.
“To draw firmer conclusions, we need prospective studies and large consortiums with better assessment of the changing nature of body fatness and with comprehensive standardized reporting of analyses,” they wrote. “As obesity becomes one of the greatest public health problems worldwide, evidence of the strength of the associations between obesity and cancer may allow finer selection of people at high risk, who could be selected for personalized primary and secondary prevention strategies.”
The study was supported by the Genesis Research Trust, Sigrid Jusélius Fellowship, the World Cancer Research Fund International Regular Grant Programme, Ovarian Cancer Action, the Imperial Experimental Cancer Medicine Centre, the Cancer Research UK Imperial Centre, Imperial Healthcare NHS Trust NIHR BRC. No relevant conflicts of interest were declared.
FROM BMJ
Key clinical point: An umbrella analysis of systematic reviews has found strong evidence linking adiposity to a range of cancers including esophageal adenocarcinoma, and cancer of the colon, kidney, and pancreas.
Major finding: Adiposity is significantly associated with cancers of the esophagus, colon, rectum, biliary tract, pancreas, endometrium, kidney, postmenopausal breast, and to multiple myeloma.
Data source: An umbrella review of 204 meta-analyses.
Disclosures: The study was supported by the Genesis Research Trust, Sigrid Jusélius Fellowship, the World Cancer Research Fund International Regular Grant Programme, Ovarian Cancer Action, the Imperial Experimental Cancer Medicine Centre, the Cancer Research UK Imperial Centre, Imperial Healthcare NHS Trust NIHR BRC. No relevant conflicts of interest were declared.
FDA expands approved indication for lenalidomide
The US Food and Drug Administration (FDA) has approved a new indication for lenalidomide (Revlimid).
The drug is now approved for use as maintenance therapy after autologous hematopoietic stem cell transplant (auto-HSCT) in patients with multiple myeloma (MM).
The expanded indication makes lenalidomide the first treatment to receive FDA approval for maintenance following auto-HSCT.
The drug was previously FDA-approved for use in combination with dexamethasone to treat patients with MM.
Lenalidomide is also FDA-approved to treat patients with transfusion-dependent anemia due to low-or intermediate-1-risk myelodysplastic syndromes associated with deletion 5q, with or without additional cytogenetic abnormalities.
And lenalidomide is FDA-approved to treat patients with mantle cell lymphoma who have relapsed or progressed after 2 prior therapies, one of which included bortezomib.
Lenalidomide is a product of Celgene.
Studies: Lenalidomide maintenance
The latest approval for lenalidomide was based on results of 2 cooperative group-led studies, CALGB 10010410 and IFM 2005-0211.
Results from both studies were published in NEJM in May 2012 (CALGB 100104, IFM 2005-02). The updated data reported here are included in the prescribing information for lenalidomide.
CALGB 100104 was a phase 3, double-blind study of 460 patients with newly diagnosed MM undergoing auto-HSCT. The patients received continuous daily treatment with lenalidomide or placebo until relapse.
IFM 2005-02 was a phase 3, double-blind study of 614 patients newly diagnosed with MM. The patients were randomized to receive a 2-month consolidation regimen after auto-HSCT, which consisted of lenalidomide monotherapy followed by continuous daily treatment with lenalidomide or placebo until relapse.
Survival
In both studies, the primary efficacy endpoint was progression-free survival (PFS). The PFS data for both studies were updated to reflect results as of March 2015.
In the CALGB study, the median PFS was 5.7 years in the lenalidomide arm and 1.9 years in the placebo arm (hazard ratio [HR]=0.38 [95% CI: 0.28-0.50]).
In the IFM study, the median PFS was 3.9 years in the lenalidomide arm and 2 years in the placebo arm (HR=0.53 [95% CI: 0.44-0.64]).
These studies were not powered for an overall survival (OS) endpoint. However, OS was recorded, and the OS data for both studies were updated to reflect results as of February 2016.
The median OS in the CALGB study was 9.3 years in the lenalidomide arm and 7 years in the placebo arm (HR=0.59 [95% CI: 0.44-0.78]).
In the IFM study, the median OS was 8.8 years in the lenalidomide arm and 7.3 years in the placebo arm (HR=0.90 [95% CI: 0.72-1.13]).
Adverse events
The most frequently reported adverse events in ≥20% of patients in the lenalidomide arm across both studies (CALGB and IFM, respectively) were neutropenia (79%, 61%), thrombocytopenia (72%, 24%), leukopenia (23%, 32%), anemia (21%, 9%), upper respiratory tract infection (27%, 11%), bronchitis (5%, 47%), nasopharyngitis (2%, 35%), cough (10%, 27%), gastroenteritis (0%, 23%), diarrhea (55%, 39%), rash (32%, 8%), fatigue (23%, 11%), asthenia (0%, 30%), muscle spasm (0%, 33%), and pyrexia (8%, 21%).
The most frequently reported grade 3/4 events (more than 20% in the lenalidomide arm) were neutropenia, thrombocytopenia, and leukopenia.
Hematologic second primary malignancies (SPM) occurred in 7.5% of patients receiving lenalidomide maintenance and 3.3% of controls.
The incidence of hematologic plus solid tumor SPM (excluding squamous cell carcinoma and basal cell carcinoma) was 14.9% in the lenalidomide group and 8.8% in the control group, with a median follow-up of 91.5 months.
Non-melanoma skin cancer SPM, including squamous cell carcinoma and basal cell carcinoma, occurred in 3.9% of patients receiving lenalidomide maintenance and 2.6% of controls. ![]()
The US Food and Drug Administration (FDA) has approved a new indication for lenalidomide (Revlimid).
The drug is now approved for use as maintenance therapy after autologous hematopoietic stem cell transplant (auto-HSCT) in patients with multiple myeloma (MM).
The expanded indication makes lenalidomide the first treatment to receive FDA approval for maintenance following auto-HSCT.
The drug was previously FDA-approved for use in combination with dexamethasone to treat patients with MM.
Lenalidomide is also FDA-approved to treat patients with transfusion-dependent anemia due to low-or intermediate-1-risk myelodysplastic syndromes associated with deletion 5q, with or without additional cytogenetic abnormalities.
And lenalidomide is FDA-approved to treat patients with mantle cell lymphoma who have relapsed or progressed after 2 prior therapies, one of which included bortezomib.
Lenalidomide is a product of Celgene.
Studies: Lenalidomide maintenance
The latest approval for lenalidomide was based on results of 2 cooperative group-led studies, CALGB 10010410 and IFM 2005-0211.
Results from both studies were published in NEJM in May 2012 (CALGB 100104, IFM 2005-02). The updated data reported here are included in the prescribing information for lenalidomide.
CALGB 100104 was a phase 3, double-blind study of 460 patients with newly diagnosed MM undergoing auto-HSCT. The patients received continuous daily treatment with lenalidomide or placebo until relapse.
IFM 2005-02 was a phase 3, double-blind study of 614 patients newly diagnosed with MM. The patients were randomized to receive a 2-month consolidation regimen after auto-HSCT, which consisted of lenalidomide monotherapy followed by continuous daily treatment with lenalidomide or placebo until relapse.
Survival
In both studies, the primary efficacy endpoint was progression-free survival (PFS). The PFS data for both studies were updated to reflect results as of March 2015.
In the CALGB study, the median PFS was 5.7 years in the lenalidomide arm and 1.9 years in the placebo arm (hazard ratio [HR]=0.38 [95% CI: 0.28-0.50]).
In the IFM study, the median PFS was 3.9 years in the lenalidomide arm and 2 years in the placebo arm (HR=0.53 [95% CI: 0.44-0.64]).
These studies were not powered for an overall survival (OS) endpoint. However, OS was recorded, and the OS data for both studies were updated to reflect results as of February 2016.
The median OS in the CALGB study was 9.3 years in the lenalidomide arm and 7 years in the placebo arm (HR=0.59 [95% CI: 0.44-0.78]).
In the IFM study, the median OS was 8.8 years in the lenalidomide arm and 7.3 years in the placebo arm (HR=0.90 [95% CI: 0.72-1.13]).
Adverse events
The most frequently reported adverse events in ≥20% of patients in the lenalidomide arm across both studies (CALGB and IFM, respectively) were neutropenia (79%, 61%), thrombocytopenia (72%, 24%), leukopenia (23%, 32%), anemia (21%, 9%), upper respiratory tract infection (27%, 11%), bronchitis (5%, 47%), nasopharyngitis (2%, 35%), cough (10%, 27%), gastroenteritis (0%, 23%), diarrhea (55%, 39%), rash (32%, 8%), fatigue (23%, 11%), asthenia (0%, 30%), muscle spasm (0%, 33%), and pyrexia (8%, 21%).
The most frequently reported grade 3/4 events (more than 20% in the lenalidomide arm) were neutropenia, thrombocytopenia, and leukopenia.
Hematologic second primary malignancies (SPM) occurred in 7.5% of patients receiving lenalidomide maintenance and 3.3% of controls.
The incidence of hematologic plus solid tumor SPM (excluding squamous cell carcinoma and basal cell carcinoma) was 14.9% in the lenalidomide group and 8.8% in the control group, with a median follow-up of 91.5 months.
Non-melanoma skin cancer SPM, including squamous cell carcinoma and basal cell carcinoma, occurred in 3.9% of patients receiving lenalidomide maintenance and 2.6% of controls. ![]()
The US Food and Drug Administration (FDA) has approved a new indication for lenalidomide (Revlimid).
The drug is now approved for use as maintenance therapy after autologous hematopoietic stem cell transplant (auto-HSCT) in patients with multiple myeloma (MM).
The expanded indication makes lenalidomide the first treatment to receive FDA approval for maintenance following auto-HSCT.
The drug was previously FDA-approved for use in combination with dexamethasone to treat patients with MM.
Lenalidomide is also FDA-approved to treat patients with transfusion-dependent anemia due to low-or intermediate-1-risk myelodysplastic syndromes associated with deletion 5q, with or without additional cytogenetic abnormalities.
And lenalidomide is FDA-approved to treat patients with mantle cell lymphoma who have relapsed or progressed after 2 prior therapies, one of which included bortezomib.
Lenalidomide is a product of Celgene.
Studies: Lenalidomide maintenance
The latest approval for lenalidomide was based on results of 2 cooperative group-led studies, CALGB 10010410 and IFM 2005-0211.
Results from both studies were published in NEJM in May 2012 (CALGB 100104, IFM 2005-02). The updated data reported here are included in the prescribing information for lenalidomide.
CALGB 100104 was a phase 3, double-blind study of 460 patients with newly diagnosed MM undergoing auto-HSCT. The patients received continuous daily treatment with lenalidomide or placebo until relapse.
IFM 2005-02 was a phase 3, double-blind study of 614 patients newly diagnosed with MM. The patients were randomized to receive a 2-month consolidation regimen after auto-HSCT, which consisted of lenalidomide monotherapy followed by continuous daily treatment with lenalidomide or placebo until relapse.
Survival
In both studies, the primary efficacy endpoint was progression-free survival (PFS). The PFS data for both studies were updated to reflect results as of March 2015.
In the CALGB study, the median PFS was 5.7 years in the lenalidomide arm and 1.9 years in the placebo arm (hazard ratio [HR]=0.38 [95% CI: 0.28-0.50]).
In the IFM study, the median PFS was 3.9 years in the lenalidomide arm and 2 years in the placebo arm (HR=0.53 [95% CI: 0.44-0.64]).
These studies were not powered for an overall survival (OS) endpoint. However, OS was recorded, and the OS data for both studies were updated to reflect results as of February 2016.
The median OS in the CALGB study was 9.3 years in the lenalidomide arm and 7 years in the placebo arm (HR=0.59 [95% CI: 0.44-0.78]).
In the IFM study, the median OS was 8.8 years in the lenalidomide arm and 7.3 years in the placebo arm (HR=0.90 [95% CI: 0.72-1.13]).
Adverse events
The most frequently reported adverse events in ≥20% of patients in the lenalidomide arm across both studies (CALGB and IFM, respectively) were neutropenia (79%, 61%), thrombocytopenia (72%, 24%), leukopenia (23%, 32%), anemia (21%, 9%), upper respiratory tract infection (27%, 11%), bronchitis (5%, 47%), nasopharyngitis (2%, 35%), cough (10%, 27%), gastroenteritis (0%, 23%), diarrhea (55%, 39%), rash (32%, 8%), fatigue (23%, 11%), asthenia (0%, 30%), muscle spasm (0%, 33%), and pyrexia (8%, 21%).
The most frequently reported grade 3/4 events (more than 20% in the lenalidomide arm) were neutropenia, thrombocytopenia, and leukopenia.
Hematologic second primary malignancies (SPM) occurred in 7.5% of patients receiving lenalidomide maintenance and 3.3% of controls.
The incidence of hematologic plus solid tumor SPM (excluding squamous cell carcinoma and basal cell carcinoma) was 14.9% in the lenalidomide group and 8.8% in the control group, with a median follow-up of 91.5 months.
Non-melanoma skin cancer SPM, including squamous cell carcinoma and basal cell carcinoma, occurred in 3.9% of patients receiving lenalidomide maintenance and 2.6% of controls. ![]()
VZV vaccine reduces HZ incidence after HSCT
ORLANDO, FL—Results of a phase 3 trial suggest an inactivated varicella zoster virus (VZV) vaccine known as V212 can reduce the risk of herpes zoster (HZ) in patients who have undergone autologous hematopoietic stem cell transplant (HSCT).
V212 reduced the hazard rate of HZ by an estimated 64% compared to placebo.
The vaccine also reduced the incidence of moderate-to-severe HZ pain and other HZ-related complications, such as hospitalization.
The overall incidence of adverse events (AEs) and the incidence of serious AEs were similar among vaccinated patients and those who received placebo.
Drew J. Winston, MD, of the University of California Los Angeles Medical Center, presented these results as one of the “Best Abstracts” at the 2017 BMT Tandem Meetings (abstract 6). The trial was sponsored by Merck, the company developing V212.
Treatment
This randomized, double-blind trial enrolled 1230 patients age 18 and older who were undergoing HSCT for any indication and had a history of varicella infection and/or were seropositive for VZV antibody.
The patients were randomized to receive:
- A 4-dose regimen of V212 (n=560) from a consistency lot (a lot having a targeted potency as required by regulators in order to demonstrate that the vaccine can be manufactured consistently)
- A 4-dose regimen of V212 (n=106) from a high-antigen lot (a lot having a higher antigen potency added to assess the safety profile of V212)
- Placebo (n=564).
Randomization was stratified by age (< 50 years vs ≥ 50 years) and by intended duration of post-transplant antiviral prophylaxis (≤3 months vs >3 to 6 months).
Dose 1 of V212 or placebo was given within approximately 30 days before HSCT, and doses 2, 3, and 4 were given approximately 30, 60, and 90 days after HSCT.
Patient characteristics
The median patient age was 57 (range, 19-76) for the consistency lot group, 56 (range, 21-75) for the high-antigen lot group, and 56 (range, 19-79) for the placebo group.
Underlying diseases were non-Hodgkin lymphoma (42%, 40%, and 44%, respectively), Hodgkin lymphoma (10%, 9%, and 9%, respectively), multiple myeloma (44%, 47% and 41%, respectively), acute leukemia (2%, 1%, and 2%, respectively), and “other” diseases (2%, 3% and 4%, respectively).
Roughly 30% of patients in each group received anti-viral agents for 3 months or less after HSCT. Twenty percent to 25% received antiviral therapy for more than 3 months to 6 months.
Thirty-seven percent to 40% received antiviral agents for more than 6 months. And 7% to 12% of patients did not receive any antiviral therapy.
HZ incidence
The average follow-up time for HZ surveillance was approximately 2.3 years (median: 2.6 years) post-vaccination.
Confirmed HZ occurred in 42 of the 538 patients who received V212 from a consistency lot and 113 of the 535 patients who received placebo. (Patients receiving V212 from a high-antigen lot were only included in the safety analysis.)
The estimated efficacy of V212 was 63.8% after adjustment for patient age and the duration of antiviral prophylaxis. Vaccine efficacy against HZ was defined as the relative reduction of hazard rate of HZ in vaccine recipients compared with placebo recipients.
The vaccine met the pre-specified criterion for success, as the lower bound of the 95% confidence interval (CI) was greater than 25%. The 95% CI was 48.4% to 74.6% (P<0.0001).
“The study demonstrates that the inactivated varicella vaccine is very effective for preventing herpes zoster after autologous stem cell transplantation,” Dr Winston said.
Pain, PHN, and other complications
V212 also reduced the incidence of moderate-to-severe HZ pain—according to the Zoster Brief Pain Inventory (ZBPI) score—by an estimated 69.5% (95% CI, 0.490-0.818).
Nineteen patients in the V212 consistency lot group had moderate-to-severe pain, as did 61 placebo-treated patients.
V212 conferred an estimated 83.7% (95% CI, 0.446-0.952) reduction in the incidence of post-herpetic neuralgia (PHN). Three patients in the V212 consistency lot group and 18 patients in the placebo group had PHN.
PHN was defined as pain in the area of the HZ rash with a “worst pain in the last 24 hours” score of 3 or greater (on a 0-10 scale) on the ZBPI that persists or appears 90 days or beyond after HZ rash onset following HSCT.
Patients who received V212 also saw an estimated 73.5% (95% CI, 0.498-0.860) reduction in “other” HZ complications. Twelve patients in the V212 consistency group and 44 in the placebo group had such complications.
“Other” complications included hospitalization or prolongation of hospitalization due to HZ, disseminated HZ (including disseminated HZ rash or VZV viremia), visceral HZ, ophthalmic HZ, neurological impairment due to HZ, and the administration of intravenous acyclovir therapy for the treatment of HZ post-HSCT.
Safety
All patients who received at least 1 dose of the vaccine or placebo and had follow-up data were included in the safety analysis. Patients were followed for AEs up to 28 days after the fourth vaccination dose.
AEs occurred in 97% of patients who received the vaccine (consistency and high-antigen groups assessed together) and 96.9% of placebo-treated patients. Vaccine-related AEs occurred in 32.6% and 12.6%, respectively.
“Of course, in this population of autologous stem cell transplant patients, adverse events of any type were very common in almost all patients,” Dr Winston said. “However, vaccine-related adverse events were greater in the vaccine recipients compared to the placebo patients, but this was primarily due to an increased incidence of injection-site adverse events in the vaccine recipients.”
Injection-site reactions occurred in 191 vaccinated patients and 36 placebo-treated patients.
The most common systemic AEs—in vaccinated and placebo-treated patients, respectively—were diarrhea (60.1% and 61.9%), nausea (56.5% and 57.8%), pyrexia (49.8% and 46.9%), mucosal inflammation (39.7% and 41.7%), thrombocytopenia (36.4% and 38.4%), febrile neutropenia (33.9% and 28.3%), vomiting (32.6% and 36.6%), anemia (26.6% and 24.4%), neutropenia (25.1% and 23.5%), decreased appetite (23.1% and 23.8%), fatigue (21.8% and 20.7%), hypokalemia (21.3% and 19.9%), and constipation (16.1% and 18.4%).
The incidence of serious AEs was 32.9% in vaccinated patients and 32.7% in the placebo group. The incidence of serious vaccine-related AEs was 0.8% and 0.9%, respectively.
The most common serious AEs—in vaccinated and placebo-treated patients, respectively—were infection (12.3% and 11.9%), relapsed malignancy (7.8% for both), febrile neutropenia (5.3% and 4.9%), pyrexia (3.2% and 4.0%), gastrointestinal disorders (3.2% and 3.6%), respiratory failure (2.7% and 2.2%), cardiac disorders (1.7% and 1.6%), and mucositis (1.2% and 0.9%).
Death occurred in 6.2% of vaccinated patients and 6.3% of placebo-treated patients. Three percent and 3.1%, respectively, discontinued the study due to AEs. ![]()
ORLANDO, FL—Results of a phase 3 trial suggest an inactivated varicella zoster virus (VZV) vaccine known as V212 can reduce the risk of herpes zoster (HZ) in patients who have undergone autologous hematopoietic stem cell transplant (HSCT).
V212 reduced the hazard rate of HZ by an estimated 64% compared to placebo.
The vaccine also reduced the incidence of moderate-to-severe HZ pain and other HZ-related complications, such as hospitalization.
The overall incidence of adverse events (AEs) and the incidence of serious AEs were similar among vaccinated patients and those who received placebo.
Drew J. Winston, MD, of the University of California Los Angeles Medical Center, presented these results as one of the “Best Abstracts” at the 2017 BMT Tandem Meetings (abstract 6). The trial was sponsored by Merck, the company developing V212.
Treatment
This randomized, double-blind trial enrolled 1230 patients age 18 and older who were undergoing HSCT for any indication and had a history of varicella infection and/or were seropositive for VZV antibody.
The patients were randomized to receive:
- A 4-dose regimen of V212 (n=560) from a consistency lot (a lot having a targeted potency as required by regulators in order to demonstrate that the vaccine can be manufactured consistently)
- A 4-dose regimen of V212 (n=106) from a high-antigen lot (a lot having a higher antigen potency added to assess the safety profile of V212)
- Placebo (n=564).
Randomization was stratified by age (< 50 years vs ≥ 50 years) and by intended duration of post-transplant antiviral prophylaxis (≤3 months vs >3 to 6 months).
Dose 1 of V212 or placebo was given within approximately 30 days before HSCT, and doses 2, 3, and 4 were given approximately 30, 60, and 90 days after HSCT.
Patient characteristics
The median patient age was 57 (range, 19-76) for the consistency lot group, 56 (range, 21-75) for the high-antigen lot group, and 56 (range, 19-79) for the placebo group.
Underlying diseases were non-Hodgkin lymphoma (42%, 40%, and 44%, respectively), Hodgkin lymphoma (10%, 9%, and 9%, respectively), multiple myeloma (44%, 47% and 41%, respectively), acute leukemia (2%, 1%, and 2%, respectively), and “other” diseases (2%, 3% and 4%, respectively).
Roughly 30% of patients in each group received anti-viral agents for 3 months or less after HSCT. Twenty percent to 25% received antiviral therapy for more than 3 months to 6 months.
Thirty-seven percent to 40% received antiviral agents for more than 6 months. And 7% to 12% of patients did not receive any antiviral therapy.
HZ incidence
The average follow-up time for HZ surveillance was approximately 2.3 years (median: 2.6 years) post-vaccination.
Confirmed HZ occurred in 42 of the 538 patients who received V212 from a consistency lot and 113 of the 535 patients who received placebo. (Patients receiving V212 from a high-antigen lot were only included in the safety analysis.)
The estimated efficacy of V212 was 63.8% after adjustment for patient age and the duration of antiviral prophylaxis. Vaccine efficacy against HZ was defined as the relative reduction of hazard rate of HZ in vaccine recipients compared with placebo recipients.
The vaccine met the pre-specified criterion for success, as the lower bound of the 95% confidence interval (CI) was greater than 25%. The 95% CI was 48.4% to 74.6% (P<0.0001).
“The study demonstrates that the inactivated varicella vaccine is very effective for preventing herpes zoster after autologous stem cell transplantation,” Dr Winston said.
Pain, PHN, and other complications
V212 also reduced the incidence of moderate-to-severe HZ pain—according to the Zoster Brief Pain Inventory (ZBPI) score—by an estimated 69.5% (95% CI, 0.490-0.818).
Nineteen patients in the V212 consistency lot group had moderate-to-severe pain, as did 61 placebo-treated patients.
V212 conferred an estimated 83.7% (95% CI, 0.446-0.952) reduction in the incidence of post-herpetic neuralgia (PHN). Three patients in the V212 consistency lot group and 18 patients in the placebo group had PHN.
PHN was defined as pain in the area of the HZ rash with a “worst pain in the last 24 hours” score of 3 or greater (on a 0-10 scale) on the ZBPI that persists or appears 90 days or beyond after HZ rash onset following HSCT.
Patients who received V212 also saw an estimated 73.5% (95% CI, 0.498-0.860) reduction in “other” HZ complications. Twelve patients in the V212 consistency group and 44 in the placebo group had such complications.
“Other” complications included hospitalization or prolongation of hospitalization due to HZ, disseminated HZ (including disseminated HZ rash or VZV viremia), visceral HZ, ophthalmic HZ, neurological impairment due to HZ, and the administration of intravenous acyclovir therapy for the treatment of HZ post-HSCT.
Safety
All patients who received at least 1 dose of the vaccine or placebo and had follow-up data were included in the safety analysis. Patients were followed for AEs up to 28 days after the fourth vaccination dose.
AEs occurred in 97% of patients who received the vaccine (consistency and high-antigen groups assessed together) and 96.9% of placebo-treated patients. Vaccine-related AEs occurred in 32.6% and 12.6%, respectively.
“Of course, in this population of autologous stem cell transplant patients, adverse events of any type were very common in almost all patients,” Dr Winston said. “However, vaccine-related adverse events were greater in the vaccine recipients compared to the placebo patients, but this was primarily due to an increased incidence of injection-site adverse events in the vaccine recipients.”
Injection-site reactions occurred in 191 vaccinated patients and 36 placebo-treated patients.
The most common systemic AEs—in vaccinated and placebo-treated patients, respectively—were diarrhea (60.1% and 61.9%), nausea (56.5% and 57.8%), pyrexia (49.8% and 46.9%), mucosal inflammation (39.7% and 41.7%), thrombocytopenia (36.4% and 38.4%), febrile neutropenia (33.9% and 28.3%), vomiting (32.6% and 36.6%), anemia (26.6% and 24.4%), neutropenia (25.1% and 23.5%), decreased appetite (23.1% and 23.8%), fatigue (21.8% and 20.7%), hypokalemia (21.3% and 19.9%), and constipation (16.1% and 18.4%).
The incidence of serious AEs was 32.9% in vaccinated patients and 32.7% in the placebo group. The incidence of serious vaccine-related AEs was 0.8% and 0.9%, respectively.
The most common serious AEs—in vaccinated and placebo-treated patients, respectively—were infection (12.3% and 11.9%), relapsed malignancy (7.8% for both), febrile neutropenia (5.3% and 4.9%), pyrexia (3.2% and 4.0%), gastrointestinal disorders (3.2% and 3.6%), respiratory failure (2.7% and 2.2%), cardiac disorders (1.7% and 1.6%), and mucositis (1.2% and 0.9%).
Death occurred in 6.2% of vaccinated patients and 6.3% of placebo-treated patients. Three percent and 3.1%, respectively, discontinued the study due to AEs. ![]()
ORLANDO, FL—Results of a phase 3 trial suggest an inactivated varicella zoster virus (VZV) vaccine known as V212 can reduce the risk of herpes zoster (HZ) in patients who have undergone autologous hematopoietic stem cell transplant (HSCT).
V212 reduced the hazard rate of HZ by an estimated 64% compared to placebo.
The vaccine also reduced the incidence of moderate-to-severe HZ pain and other HZ-related complications, such as hospitalization.
The overall incidence of adverse events (AEs) and the incidence of serious AEs were similar among vaccinated patients and those who received placebo.
Drew J. Winston, MD, of the University of California Los Angeles Medical Center, presented these results as one of the “Best Abstracts” at the 2017 BMT Tandem Meetings (abstract 6). The trial was sponsored by Merck, the company developing V212.
Treatment
This randomized, double-blind trial enrolled 1230 patients age 18 and older who were undergoing HSCT for any indication and had a history of varicella infection and/or were seropositive for VZV antibody.
The patients were randomized to receive:
- A 4-dose regimen of V212 (n=560) from a consistency lot (a lot having a targeted potency as required by regulators in order to demonstrate that the vaccine can be manufactured consistently)
- A 4-dose regimen of V212 (n=106) from a high-antigen lot (a lot having a higher antigen potency added to assess the safety profile of V212)
- Placebo (n=564).
Randomization was stratified by age (< 50 years vs ≥ 50 years) and by intended duration of post-transplant antiviral prophylaxis (≤3 months vs >3 to 6 months).
Dose 1 of V212 or placebo was given within approximately 30 days before HSCT, and doses 2, 3, and 4 were given approximately 30, 60, and 90 days after HSCT.
Patient characteristics
The median patient age was 57 (range, 19-76) for the consistency lot group, 56 (range, 21-75) for the high-antigen lot group, and 56 (range, 19-79) for the placebo group.
Underlying diseases were non-Hodgkin lymphoma (42%, 40%, and 44%, respectively), Hodgkin lymphoma (10%, 9%, and 9%, respectively), multiple myeloma (44%, 47% and 41%, respectively), acute leukemia (2%, 1%, and 2%, respectively), and “other” diseases (2%, 3% and 4%, respectively).
Roughly 30% of patients in each group received anti-viral agents for 3 months or less after HSCT. Twenty percent to 25% received antiviral therapy for more than 3 months to 6 months.
Thirty-seven percent to 40% received antiviral agents for more than 6 months. And 7% to 12% of patients did not receive any antiviral therapy.
HZ incidence
The average follow-up time for HZ surveillance was approximately 2.3 years (median: 2.6 years) post-vaccination.
Confirmed HZ occurred in 42 of the 538 patients who received V212 from a consistency lot and 113 of the 535 patients who received placebo. (Patients receiving V212 from a high-antigen lot were only included in the safety analysis.)
The estimated efficacy of V212 was 63.8% after adjustment for patient age and the duration of antiviral prophylaxis. Vaccine efficacy against HZ was defined as the relative reduction of hazard rate of HZ in vaccine recipients compared with placebo recipients.
The vaccine met the pre-specified criterion for success, as the lower bound of the 95% confidence interval (CI) was greater than 25%. The 95% CI was 48.4% to 74.6% (P<0.0001).
“The study demonstrates that the inactivated varicella vaccine is very effective for preventing herpes zoster after autologous stem cell transplantation,” Dr Winston said.
Pain, PHN, and other complications
V212 also reduced the incidence of moderate-to-severe HZ pain—according to the Zoster Brief Pain Inventory (ZBPI) score—by an estimated 69.5% (95% CI, 0.490-0.818).
Nineteen patients in the V212 consistency lot group had moderate-to-severe pain, as did 61 placebo-treated patients.
V212 conferred an estimated 83.7% (95% CI, 0.446-0.952) reduction in the incidence of post-herpetic neuralgia (PHN). Three patients in the V212 consistency lot group and 18 patients in the placebo group had PHN.
PHN was defined as pain in the area of the HZ rash with a “worst pain in the last 24 hours” score of 3 or greater (on a 0-10 scale) on the ZBPI that persists or appears 90 days or beyond after HZ rash onset following HSCT.
Patients who received V212 also saw an estimated 73.5% (95% CI, 0.498-0.860) reduction in “other” HZ complications. Twelve patients in the V212 consistency group and 44 in the placebo group had such complications.
“Other” complications included hospitalization or prolongation of hospitalization due to HZ, disseminated HZ (including disseminated HZ rash or VZV viremia), visceral HZ, ophthalmic HZ, neurological impairment due to HZ, and the administration of intravenous acyclovir therapy for the treatment of HZ post-HSCT.
Safety
All patients who received at least 1 dose of the vaccine or placebo and had follow-up data were included in the safety analysis. Patients were followed for AEs up to 28 days after the fourth vaccination dose.
AEs occurred in 97% of patients who received the vaccine (consistency and high-antigen groups assessed together) and 96.9% of placebo-treated patients. Vaccine-related AEs occurred in 32.6% and 12.6%, respectively.
“Of course, in this population of autologous stem cell transplant patients, adverse events of any type were very common in almost all patients,” Dr Winston said. “However, vaccine-related adverse events were greater in the vaccine recipients compared to the placebo patients, but this was primarily due to an increased incidence of injection-site adverse events in the vaccine recipients.”
Injection-site reactions occurred in 191 vaccinated patients and 36 placebo-treated patients.
The most common systemic AEs—in vaccinated and placebo-treated patients, respectively—were diarrhea (60.1% and 61.9%), nausea (56.5% and 57.8%), pyrexia (49.8% and 46.9%), mucosal inflammation (39.7% and 41.7%), thrombocytopenia (36.4% and 38.4%), febrile neutropenia (33.9% and 28.3%), vomiting (32.6% and 36.6%), anemia (26.6% and 24.4%), neutropenia (25.1% and 23.5%), decreased appetite (23.1% and 23.8%), fatigue (21.8% and 20.7%), hypokalemia (21.3% and 19.9%), and constipation (16.1% and 18.4%).
The incidence of serious AEs was 32.9% in vaccinated patients and 32.7% in the placebo group. The incidence of serious vaccine-related AEs was 0.8% and 0.9%, respectively.
The most common serious AEs—in vaccinated and placebo-treated patients, respectively—were infection (12.3% and 11.9%), relapsed malignancy (7.8% for both), febrile neutropenia (5.3% and 4.9%), pyrexia (3.2% and 4.0%), gastrointestinal disorders (3.2% and 3.6%), respiratory failure (2.7% and 2.2%), cardiac disorders (1.7% and 1.6%), and mucositis (1.2% and 0.9%).
Death occurred in 6.2% of vaccinated patients and 6.3% of placebo-treated patients. Three percent and 3.1%, respectively, discontinued the study due to AEs. ![]()
Inpatient palliative care improves QOL for HSCT patients
ORLANDO, FL—New research shows that patients who received inpatient palliative care while undergoing hematopoietic stem cell transplant (HSCT) experienced significant improvements in quality of life (QOL), decreases in depression, and reductions in symptom burden compared to patients who received transplant care alone.
Areej R. El-Jawahri, MD, of Harvard Medical School in Boston, Massachusetts, presented these results at the 2017 BMT Tandem Meetings (abstract 49).
She noted that palliative care is rarely used for patients with hematologic malignancies, “in part, because of misperceptions equating palliative care with just end-of-life care.”
So Dr El-Jawahri and her colleagues decided to evaluate palliative care in patients with hematologic malignancies who were scheduled to undergo HSCT.
The researchers enrolled 160 patients on the trial. Eighty-one were randomized to receive inpatient palliative care integrated with transplant care (intervention arm), and 79 were randomized to transplant care alone (control).
The latter group could request palliative care consultations, but only 2 patients did so, Dr El-Jawahri pointed out.
Patients receiving the intervention had at least twice-weekly visits with a palliative care clinician throughout their hospitalization.
“Importantly, palliative care only followed patients during their transplant hospitalization,” Dr El-Jawahri noted. “This was purely an inpatient palliative care intervention.”
Palliative care focused primarily on managing patients’ symptoms, establishing rapport with patients and families, and helping them cope with their illness. The predominant symptoms addressed included pain, nausea, diarrhea, constipation, insomnia, fatigue, depression, and anxiety.
Researchers assessed QOL, symptom burden, and mood at baseline, during hospitalization (Week 2), and at 3 and 6 months using well-validated scales.
They assessed QOL using the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale, mood using the Hospital Anxiety and Depression Scale (HADS) and Patient Health Questionnaire (PHQ-9), and symptom burden using the Edmonton Symptom Assessment Scale (ESAS).
They also measured post-traumatic stress (PTSD) at baseline as well as 3 and 6 months after HSCT using the PTSD checklist.
The primary endpoint of the study was patient-reported QOL at Week 2 of hospitalization. Researchers chose Week 2 because studies have shown the highest symptom burden and QOL deterioration during that period.
Demographics
Patients were a mean age of 57, and a little more than half were female. Most were white, had a college degree or higher, and were married.
Their diagnoses included, for the control and intervention arms, respectively: acute lymphoblastic leukemia (9%, 5%), acute myeloid leukemia/myelodysplastic syndromes (30%, 30%), myelofibrosis/chronic myeloid leukemia (9%, 10%), lymphoma (33%, 23%), and multiple myeloma (19%, 33%).
Results
At baseline, patients in each group had comparable QOL and mood scores.
However, at Week 2, after ANCOVA adjustment for baseline scores, patients in the intervention arm had a clinically and statistically significant effect of the intervention in all areas measured except for the PHQ-9 depression score.
In particular, the HADS depression and anxiety scores were significantly improved, at P=0.008 and P<0.001, respectively, compared to control.
At 3 months, the FACT-BMT (P=0.048), HADS-Depression (P=0.002), PHQ-9-Depression (P=0.002), and PTSD symptom (P=0.002) scores were significantly improved in the intervention group.
And at 6 months, the HADS-Depression assessment (P=0.024), the PHQ-9-Depression assessment (P=0.027), and the PTSD symptom assessment (P=0.013) remained significantly improved. However, there were no significant differences in anxiety between the 2 groups.
The researchers concluded that a relatively brief inpatient care intervention led to “remarkable sustained improvements” in depression and post-traumatic stress symptoms at 3 and 6 months after HSCT.
“This is the first study showing the benefits of palliative care for patients with hematologic malignancies undergoing stem cell transplant,” Dr El-Jawahri said.
“It’s also the first study showing the benefits of palliative care for patients with cancer pursuing curative therapy and extends the potential benefit of palliative care in a population of patients with serious illness. [T]he significant part of what palliative care does is helping patients and families cope with serious and potentially life-threatening illness.”
The researchers recommend future studies to evaluate the impact of early integration of palliative care for this patient population. ![]()
ORLANDO, FL—New research shows that patients who received inpatient palliative care while undergoing hematopoietic stem cell transplant (HSCT) experienced significant improvements in quality of life (QOL), decreases in depression, and reductions in symptom burden compared to patients who received transplant care alone.
Areej R. El-Jawahri, MD, of Harvard Medical School in Boston, Massachusetts, presented these results at the 2017 BMT Tandem Meetings (abstract 49).
She noted that palliative care is rarely used for patients with hematologic malignancies, “in part, because of misperceptions equating palliative care with just end-of-life care.”
So Dr El-Jawahri and her colleagues decided to evaluate palliative care in patients with hematologic malignancies who were scheduled to undergo HSCT.
The researchers enrolled 160 patients on the trial. Eighty-one were randomized to receive inpatient palliative care integrated with transplant care (intervention arm), and 79 were randomized to transplant care alone (control).
The latter group could request palliative care consultations, but only 2 patients did so, Dr El-Jawahri pointed out.
Patients receiving the intervention had at least twice-weekly visits with a palliative care clinician throughout their hospitalization.
“Importantly, palliative care only followed patients during their transplant hospitalization,” Dr El-Jawahri noted. “This was purely an inpatient palliative care intervention.”
Palliative care focused primarily on managing patients’ symptoms, establishing rapport with patients and families, and helping them cope with their illness. The predominant symptoms addressed included pain, nausea, diarrhea, constipation, insomnia, fatigue, depression, and anxiety.
Researchers assessed QOL, symptom burden, and mood at baseline, during hospitalization (Week 2), and at 3 and 6 months using well-validated scales.
They assessed QOL using the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale, mood using the Hospital Anxiety and Depression Scale (HADS) and Patient Health Questionnaire (PHQ-9), and symptom burden using the Edmonton Symptom Assessment Scale (ESAS).
They also measured post-traumatic stress (PTSD) at baseline as well as 3 and 6 months after HSCT using the PTSD checklist.
The primary endpoint of the study was patient-reported QOL at Week 2 of hospitalization. Researchers chose Week 2 because studies have shown the highest symptom burden and QOL deterioration during that period.
Demographics
Patients were a mean age of 57, and a little more than half were female. Most were white, had a college degree or higher, and were married.
Their diagnoses included, for the control and intervention arms, respectively: acute lymphoblastic leukemia (9%, 5%), acute myeloid leukemia/myelodysplastic syndromes (30%, 30%), myelofibrosis/chronic myeloid leukemia (9%, 10%), lymphoma (33%, 23%), and multiple myeloma (19%, 33%).
Results
At baseline, patients in each group had comparable QOL and mood scores.
However, at Week 2, after ANCOVA adjustment for baseline scores, patients in the intervention arm had a clinically and statistically significant effect of the intervention in all areas measured except for the PHQ-9 depression score.
In particular, the HADS depression and anxiety scores were significantly improved, at P=0.008 and P<0.001, respectively, compared to control.
At 3 months, the FACT-BMT (P=0.048), HADS-Depression (P=0.002), PHQ-9-Depression (P=0.002), and PTSD symptom (P=0.002) scores were significantly improved in the intervention group.
And at 6 months, the HADS-Depression assessment (P=0.024), the PHQ-9-Depression assessment (P=0.027), and the PTSD symptom assessment (P=0.013) remained significantly improved. However, there were no significant differences in anxiety between the 2 groups.
The researchers concluded that a relatively brief inpatient care intervention led to “remarkable sustained improvements” in depression and post-traumatic stress symptoms at 3 and 6 months after HSCT.
“This is the first study showing the benefits of palliative care for patients with hematologic malignancies undergoing stem cell transplant,” Dr El-Jawahri said.
“It’s also the first study showing the benefits of palliative care for patients with cancer pursuing curative therapy and extends the potential benefit of palliative care in a population of patients with serious illness. [T]he significant part of what palliative care does is helping patients and families cope with serious and potentially life-threatening illness.”
The researchers recommend future studies to evaluate the impact of early integration of palliative care for this patient population. ![]()
ORLANDO, FL—New research shows that patients who received inpatient palliative care while undergoing hematopoietic stem cell transplant (HSCT) experienced significant improvements in quality of life (QOL), decreases in depression, and reductions in symptom burden compared to patients who received transplant care alone.
Areej R. El-Jawahri, MD, of Harvard Medical School in Boston, Massachusetts, presented these results at the 2017 BMT Tandem Meetings (abstract 49).
She noted that palliative care is rarely used for patients with hematologic malignancies, “in part, because of misperceptions equating palliative care with just end-of-life care.”
So Dr El-Jawahri and her colleagues decided to evaluate palliative care in patients with hematologic malignancies who were scheduled to undergo HSCT.
The researchers enrolled 160 patients on the trial. Eighty-one were randomized to receive inpatient palliative care integrated with transplant care (intervention arm), and 79 were randomized to transplant care alone (control).
The latter group could request palliative care consultations, but only 2 patients did so, Dr El-Jawahri pointed out.
Patients receiving the intervention had at least twice-weekly visits with a palliative care clinician throughout their hospitalization.
“Importantly, palliative care only followed patients during their transplant hospitalization,” Dr El-Jawahri noted. “This was purely an inpatient palliative care intervention.”
Palliative care focused primarily on managing patients’ symptoms, establishing rapport with patients and families, and helping them cope with their illness. The predominant symptoms addressed included pain, nausea, diarrhea, constipation, insomnia, fatigue, depression, and anxiety.
Researchers assessed QOL, symptom burden, and mood at baseline, during hospitalization (Week 2), and at 3 and 6 months using well-validated scales.
They assessed QOL using the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale, mood using the Hospital Anxiety and Depression Scale (HADS) and Patient Health Questionnaire (PHQ-9), and symptom burden using the Edmonton Symptom Assessment Scale (ESAS).
They also measured post-traumatic stress (PTSD) at baseline as well as 3 and 6 months after HSCT using the PTSD checklist.
The primary endpoint of the study was patient-reported QOL at Week 2 of hospitalization. Researchers chose Week 2 because studies have shown the highest symptom burden and QOL deterioration during that period.
Demographics
Patients were a mean age of 57, and a little more than half were female. Most were white, had a college degree or higher, and were married.
Their diagnoses included, for the control and intervention arms, respectively: acute lymphoblastic leukemia (9%, 5%), acute myeloid leukemia/myelodysplastic syndromes (30%, 30%), myelofibrosis/chronic myeloid leukemia (9%, 10%), lymphoma (33%, 23%), and multiple myeloma (19%, 33%).
Results
At baseline, patients in each group had comparable QOL and mood scores.
However, at Week 2, after ANCOVA adjustment for baseline scores, patients in the intervention arm had a clinically and statistically significant effect of the intervention in all areas measured except for the PHQ-9 depression score.
In particular, the HADS depression and anxiety scores were significantly improved, at P=0.008 and P<0.001, respectively, compared to control.
At 3 months, the FACT-BMT (P=0.048), HADS-Depression (P=0.002), PHQ-9-Depression (P=0.002), and PTSD symptom (P=0.002) scores were significantly improved in the intervention group.
And at 6 months, the HADS-Depression assessment (P=0.024), the PHQ-9-Depression assessment (P=0.027), and the PTSD symptom assessment (P=0.013) remained significantly improved. However, there were no significant differences in anxiety between the 2 groups.
The researchers concluded that a relatively brief inpatient care intervention led to “remarkable sustained improvements” in depression and post-traumatic stress symptoms at 3 and 6 months after HSCT.
“This is the first study showing the benefits of palliative care for patients with hematologic malignancies undergoing stem cell transplant,” Dr El-Jawahri said.
“It’s also the first study showing the benefits of palliative care for patients with cancer pursuing curative therapy and extends the potential benefit of palliative care in a population of patients with serious illness. [T]he significant part of what palliative care does is helping patients and families cope with serious and potentially life-threatening illness.”
The researchers recommend future studies to evaluate the impact of early integration of palliative care for this patient population. ![]()
CHMP recommends authorization of antiemetic agent
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for the antiemetic agent rolapitant (Varuby) as a treatment for adults with cancer.
The drug is intended to be used in combination with other antiemetic agents to prevent delayed nausea and vomiting associated with highly and moderately emetogenic chemotherapy.
The CHMP’s recommendation regarding rolapitant has been forwarded to the European Commission, which is expected to make a decision about the drug within 2 months.
If the commission authorizes marketing of rolapitant, the drug will be available as 90 mg film-coated tablets.
The applicant for rolapitant is Tesaro UK Limited.
Rolapitant clinical trials
Results from three phase 3 trials suggested that rolapitant (at 180 mg) in combination with a 5-HT3 receptor antagonist and dexamethasone was more effective than the 5-HT3 receptor antagonist and dexamethasone on their own (active control).
The 3-drug combination demonstrated a significant reduction in episodes of vomiting or use of rescue medication during the 25- to 120-hour period following administration of highly emetogenic and moderately emetogenic chemotherapy regimens.
In addition, patients who received rolapitant reported experiencing less nausea that interfered with normal daily life and fewer episodes of vomiting or retching over multiple cycles of chemotherapy.
Highly emetogenic chemotherapy
The clinical profile of rolapitant in cisplatin-based, highly emetogenic chemotherapy (HEC) was confirmed in two phase 3 studies: HEC1 and HEC2. Results from these trials were published in The Lancet Oncology in August 2015.
Both trials met their primary endpoint of complete response (CR) and demonstrated statistical superiority of the rolapitant combination compared to active control.
In HEC1, 264 patients received the rolapitant combination, and 262 received active control. The proportion of patients achieving a CR was 72.7% and 58.4%, respectively (P<0.001).
In HEC2, 271 patients received the rolapitant combination, and 273 received active control. The proportion of patients achieving a CR was 70.1% and 61.9%, respectively (P=0.043).
The most common adverse events (in the rolapitant and control groups, respectively) were neutropenia (9% and 8%), hiccups (5% and 4%), and abdominal pain (3% and 2%).
Moderately emetogenic chemotherapy
Researchers conducted another phase 3 trial to compare the rolapitant combination with active control in 1332 patients receiving moderately emetogenic chemotherapy. Results from this trial were also published in The Lancet Oncology in August 2015.
This trial met its primary endpoint of CR and demonstrated statistical superiority of the rolapitant combination compared to active control. The proportion of patients achieving a CR was 71.3% and 61.6%, respectively (P<0.001).
The most common adverse events (in the rolapitant and control groups, respectively) were decreased appetite (9% and 7%), neutropenia (7% and 6%), dizziness (6% and 4%), dyspepsia (4% and 2%), urinary tract infection (4% and 3%), stomatitis (4% and 2%), and anemia (3% and 2%). ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for the antiemetic agent rolapitant (Varuby) as a treatment for adults with cancer.
The drug is intended to be used in combination with other antiemetic agents to prevent delayed nausea and vomiting associated with highly and moderately emetogenic chemotherapy.
The CHMP’s recommendation regarding rolapitant has been forwarded to the European Commission, which is expected to make a decision about the drug within 2 months.
If the commission authorizes marketing of rolapitant, the drug will be available as 90 mg film-coated tablets.
The applicant for rolapitant is Tesaro UK Limited.
Rolapitant clinical trials
Results from three phase 3 trials suggested that rolapitant (at 180 mg) in combination with a 5-HT3 receptor antagonist and dexamethasone was more effective than the 5-HT3 receptor antagonist and dexamethasone on their own (active control).
The 3-drug combination demonstrated a significant reduction in episodes of vomiting or use of rescue medication during the 25- to 120-hour period following administration of highly emetogenic and moderately emetogenic chemotherapy regimens.
In addition, patients who received rolapitant reported experiencing less nausea that interfered with normal daily life and fewer episodes of vomiting or retching over multiple cycles of chemotherapy.
Highly emetogenic chemotherapy
The clinical profile of rolapitant in cisplatin-based, highly emetogenic chemotherapy (HEC) was confirmed in two phase 3 studies: HEC1 and HEC2. Results from these trials were published in The Lancet Oncology in August 2015.
Both trials met their primary endpoint of complete response (CR) and demonstrated statistical superiority of the rolapitant combination compared to active control.
In HEC1, 264 patients received the rolapitant combination, and 262 received active control. The proportion of patients achieving a CR was 72.7% and 58.4%, respectively (P<0.001).
In HEC2, 271 patients received the rolapitant combination, and 273 received active control. The proportion of patients achieving a CR was 70.1% and 61.9%, respectively (P=0.043).
The most common adverse events (in the rolapitant and control groups, respectively) were neutropenia (9% and 8%), hiccups (5% and 4%), and abdominal pain (3% and 2%).
Moderately emetogenic chemotherapy
Researchers conducted another phase 3 trial to compare the rolapitant combination with active control in 1332 patients receiving moderately emetogenic chemotherapy. Results from this trial were also published in The Lancet Oncology in August 2015.
This trial met its primary endpoint of CR and demonstrated statistical superiority of the rolapitant combination compared to active control. The proportion of patients achieving a CR was 71.3% and 61.6%, respectively (P<0.001).
The most common adverse events (in the rolapitant and control groups, respectively) were decreased appetite (9% and 7%), neutropenia (7% and 6%), dizziness (6% and 4%), dyspepsia (4% and 2%), urinary tract infection (4% and 3%), stomatitis (4% and 2%), and anemia (3% and 2%). ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for the antiemetic agent rolapitant (Varuby) as a treatment for adults with cancer.
The drug is intended to be used in combination with other antiemetic agents to prevent delayed nausea and vomiting associated with highly and moderately emetogenic chemotherapy.
The CHMP’s recommendation regarding rolapitant has been forwarded to the European Commission, which is expected to make a decision about the drug within 2 months.
If the commission authorizes marketing of rolapitant, the drug will be available as 90 mg film-coated tablets.
The applicant for rolapitant is Tesaro UK Limited.
Rolapitant clinical trials
Results from three phase 3 trials suggested that rolapitant (at 180 mg) in combination with a 5-HT3 receptor antagonist and dexamethasone was more effective than the 5-HT3 receptor antagonist and dexamethasone on their own (active control).
The 3-drug combination demonstrated a significant reduction in episodes of vomiting or use of rescue medication during the 25- to 120-hour period following administration of highly emetogenic and moderately emetogenic chemotherapy regimens.
In addition, patients who received rolapitant reported experiencing less nausea that interfered with normal daily life and fewer episodes of vomiting or retching over multiple cycles of chemotherapy.
Highly emetogenic chemotherapy
The clinical profile of rolapitant in cisplatin-based, highly emetogenic chemotherapy (HEC) was confirmed in two phase 3 studies: HEC1 and HEC2. Results from these trials were published in The Lancet Oncology in August 2015.
Both trials met their primary endpoint of complete response (CR) and demonstrated statistical superiority of the rolapitant combination compared to active control.
In HEC1, 264 patients received the rolapitant combination, and 262 received active control. The proportion of patients achieving a CR was 72.7% and 58.4%, respectively (P<0.001).
In HEC2, 271 patients received the rolapitant combination, and 273 received active control. The proportion of patients achieving a CR was 70.1% and 61.9%, respectively (P=0.043).
The most common adverse events (in the rolapitant and control groups, respectively) were neutropenia (9% and 8%), hiccups (5% and 4%), and abdominal pain (3% and 2%).
Moderately emetogenic chemotherapy
Researchers conducted another phase 3 trial to compare the rolapitant combination with active control in 1332 patients receiving moderately emetogenic chemotherapy. Results from this trial were also published in The Lancet Oncology in August 2015.
This trial met its primary endpoint of CR and demonstrated statistical superiority of the rolapitant combination compared to active control. The proportion of patients achieving a CR was 71.3% and 61.6%, respectively (P<0.001).
The most common adverse events (in the rolapitant and control groups, respectively) were decreased appetite (9% and 7%), neutropenia (7% and 6%), dizziness (6% and 4%), dyspepsia (4% and 2%), urinary tract infection (4% and 3%), stomatitis (4% and 2%), and anemia (3% and 2%). ![]()
Immune recovery may predict survival in myeloma patients with stem cell transplants
Polyclonal immunoglobulin recovery 1 year after autologous stem cell transplantation (ASCT) in multiple myeloma patients may help to predict progression-free and overall survival, according to research published online Jan. 25 in Haematologica.
“Most multiple myeloma patients (85%-90%) exhibit immunoparesis at the time of diagnosis,” wrote Verónica González-Calle, MD, of the Instituto de Investigación Biomédica de Salamanca (Spain) and her coauthors. While the recovery of polyclonal immunoglobulins is expected after high doses of akylating agents like melphalan and the infusion of stem cells in the setting of ASCT, but whether the “persistence of immunoparesis after ASCT may predict worse progression or survival in patients with multiple myeloma” has not been studied.
In a retrospective cohort study, Dr. González-Calle and her colleagues evaluated 295 patients with symptomatic multiple myeloma who underwent ASCT at two referral centers in Spain.
One year after the transfer, 52% of the surviving 169 patients had experienced immunoglobulin recovery – defined as normalization of polyclonal immunoglobulin levels – and 48% had not (Haematologica. 2017 Jan 25. doi: 10.3324/haematol.2016.158345). Of the 88 patients who experienced immunoglobulin recovery, 36% had recovered by 100 days, 18% by 6 months, 17% by 9 months, and 26% by 1 year after stem cell transfer.
Immunoglobulin recovery significantly affected both progression-free survival (PFS) and overall survival. Median PFS was significantly longer in the 88 patients who experienced immunoglobulin recovery than in those who did not (60.4 vs. 27.9 months; hazard ratio, 0.45; 95% CI, 0.31-0.66; P less than .001). Similarly, median overall survival was 11.3 years in the group who experienced immunoglobulin recovery and 7.3 years in those with persistent immunoparesis from 1 year (P = .002).
There was also a significant interaction between the time to immunoglobulin recovery and the duration of PFS, with a shorter recovery time being associated with a significantly lower PFS.
“One possible explanation is that the prognostic significance of the polyclonal Ig recovery could be established only in those patients who lived enough to have experienced complete and uneventful B-cell reconstitution 1 year after ASCT,” the authors wrote. “Thus, if the polyclonal Igs have recovered by this time, our results would lead us to expect a positive outcome. By contrast, persistence of immunoparesis at this time was independently associated with shorter [progression-free survival] and worse [overall survival].”
The authors said polyclonal immunoglobulin recovery after 1 year could be considered an independent long-term marker for predicting PFS and overall survival. It is also a marker that could be easily assessed in clinical practice.
One author was supported by the Fundación AMIR and another was supported by the Fundación Española de Hematología y Hemoterapia and Janssen. No conflicts of interest were declared.
Polyclonal immunoglobulin recovery 1 year after autologous stem cell transplantation (ASCT) in multiple myeloma patients may help to predict progression-free and overall survival, according to research published online Jan. 25 in Haematologica.
“Most multiple myeloma patients (85%-90%) exhibit immunoparesis at the time of diagnosis,” wrote Verónica González-Calle, MD, of the Instituto de Investigación Biomédica de Salamanca (Spain) and her coauthors. While the recovery of polyclonal immunoglobulins is expected after high doses of akylating agents like melphalan and the infusion of stem cells in the setting of ASCT, but whether the “persistence of immunoparesis after ASCT may predict worse progression or survival in patients with multiple myeloma” has not been studied.
In a retrospective cohort study, Dr. González-Calle and her colleagues evaluated 295 patients with symptomatic multiple myeloma who underwent ASCT at two referral centers in Spain.
One year after the transfer, 52% of the surviving 169 patients had experienced immunoglobulin recovery – defined as normalization of polyclonal immunoglobulin levels – and 48% had not (Haematologica. 2017 Jan 25. doi: 10.3324/haematol.2016.158345). Of the 88 patients who experienced immunoglobulin recovery, 36% had recovered by 100 days, 18% by 6 months, 17% by 9 months, and 26% by 1 year after stem cell transfer.
Immunoglobulin recovery significantly affected both progression-free survival (PFS) and overall survival. Median PFS was significantly longer in the 88 patients who experienced immunoglobulin recovery than in those who did not (60.4 vs. 27.9 months; hazard ratio, 0.45; 95% CI, 0.31-0.66; P less than .001). Similarly, median overall survival was 11.3 years in the group who experienced immunoglobulin recovery and 7.3 years in those with persistent immunoparesis from 1 year (P = .002).
There was also a significant interaction between the time to immunoglobulin recovery and the duration of PFS, with a shorter recovery time being associated with a significantly lower PFS.
“One possible explanation is that the prognostic significance of the polyclonal Ig recovery could be established only in those patients who lived enough to have experienced complete and uneventful B-cell reconstitution 1 year after ASCT,” the authors wrote. “Thus, if the polyclonal Igs have recovered by this time, our results would lead us to expect a positive outcome. By contrast, persistence of immunoparesis at this time was independently associated with shorter [progression-free survival] and worse [overall survival].”
The authors said polyclonal immunoglobulin recovery after 1 year could be considered an independent long-term marker for predicting PFS and overall survival. It is also a marker that could be easily assessed in clinical practice.
One author was supported by the Fundación AMIR and another was supported by the Fundación Española de Hematología y Hemoterapia and Janssen. No conflicts of interest were declared.
Polyclonal immunoglobulin recovery 1 year after autologous stem cell transplantation (ASCT) in multiple myeloma patients may help to predict progression-free and overall survival, according to research published online Jan. 25 in Haematologica.
“Most multiple myeloma patients (85%-90%) exhibit immunoparesis at the time of diagnosis,” wrote Verónica González-Calle, MD, of the Instituto de Investigación Biomédica de Salamanca (Spain) and her coauthors. While the recovery of polyclonal immunoglobulins is expected after high doses of akylating agents like melphalan and the infusion of stem cells in the setting of ASCT, but whether the “persistence of immunoparesis after ASCT may predict worse progression or survival in patients with multiple myeloma” has not been studied.
In a retrospective cohort study, Dr. González-Calle and her colleagues evaluated 295 patients with symptomatic multiple myeloma who underwent ASCT at two referral centers in Spain.
One year after the transfer, 52% of the surviving 169 patients had experienced immunoglobulin recovery – defined as normalization of polyclonal immunoglobulin levels – and 48% had not (Haematologica. 2017 Jan 25. doi: 10.3324/haematol.2016.158345). Of the 88 patients who experienced immunoglobulin recovery, 36% had recovered by 100 days, 18% by 6 months, 17% by 9 months, and 26% by 1 year after stem cell transfer.
Immunoglobulin recovery significantly affected both progression-free survival (PFS) and overall survival. Median PFS was significantly longer in the 88 patients who experienced immunoglobulin recovery than in those who did not (60.4 vs. 27.9 months; hazard ratio, 0.45; 95% CI, 0.31-0.66; P less than .001). Similarly, median overall survival was 11.3 years in the group who experienced immunoglobulin recovery and 7.3 years in those with persistent immunoparesis from 1 year (P = .002).
There was also a significant interaction between the time to immunoglobulin recovery and the duration of PFS, with a shorter recovery time being associated with a significantly lower PFS.
“One possible explanation is that the prognostic significance of the polyclonal Ig recovery could be established only in those patients who lived enough to have experienced complete and uneventful B-cell reconstitution 1 year after ASCT,” the authors wrote. “Thus, if the polyclonal Igs have recovered by this time, our results would lead us to expect a positive outcome. By contrast, persistence of immunoparesis at this time was independently associated with shorter [progression-free survival] and worse [overall survival].”
The authors said polyclonal immunoglobulin recovery after 1 year could be considered an independent long-term marker for predicting PFS and overall survival. It is also a marker that could be easily assessed in clinical practice.
One author was supported by the Fundación AMIR and another was supported by the Fundación Española de Hematología y Hemoterapia and Janssen. No conflicts of interest were declared.
FROM HAEMATOLOGICA
Key clinical point:
Major finding: One year after ASCT, 52% of surviving patients had experienced immunoglobulin recovery.
Data source: A retrospective cohort study in 295 patients with symptomatic multiple myeloma who underwent autologous stem cell transfer.
Disclosures: One author was supported by the Fundación AMIR and another was supported by the Fundación Española de Hematología y Hemoterapia and Janssen. No conflicts of interest were declared.
Phase III trial: VZV protects auto-HCT patients
ORLANDO – An inactivated varicella zoster virus vaccine currently in development for adult patients undergoing autologous hematopoietic stem cell transplantation is efficacious and well tolerated, according to findings from a randomized, placebo-controlled, phase III trial.
During the course of the 2 1/2-year pivotal multicenter trial, confirmed herpes zoster infections occurred in 42 of 560 patients who were randomized to receive inactivated varicella zoster virus vaccine (ZVIN) consistency lot (overall incidence of 32.8 cases/1,000 patient-years), compared with 113 of 564 patients who received placebo (overall incidence of 91.8/1,000 patient-years). The estimated vaccine efficacy was 63.8% after adjusting for age and duration of antiviral prophylaxis, Drew J. Winston, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The vaccine also was effective for reducing moderate and severe herpes zoster pain (estimated vaccine efficacy, 69.5%), for preventing postherpetic neuralgia (estimated vaccine efficacy, 83.7%), and for prevention of herpes zoster–related complications (estimated vaccine efficacy, 73.5%), he noted.
Study subjects were adults aged 18 years or older who were undergoing autologous hematopoietic stem cell transplantation (auto-HCT) for a malignancy or other indication. The most common underlying diseases were lymphoma and multiple myeloma. All patients had a history of varicella infection or were seropositive for varicella zoster virus (VZV) antibody, and had no history of VZV vaccine or herpes zoster infection within the prior year.
They were randomized to receive a four-dose regimen of either ZVIN consistency lot, ZVIN high-antigen lot, or placebo. A group of 106 patients who received the ZVIN high-antigen lot were included in the safety analysis only. The first ZVIN dose was administered about a month before transplantation, and doses two through four were administered about 30, 60, and 90 days after transplantation. About 90% in each group received antiviral agents after transplantation, and the duration of the use of antivirals also was similar in the groups. All patients were followed for the duration of the study, and those who developed herpes zoster were followed for 6 months after onset.
Herpes zoster cases were confirmed by polymerase chain reaction or by blinded endpoint committee adjudication.
Serious adverse events and vaccine-related serious adverse events occurred in a similar proportion of patients in the treatment and placebo groups (32.9% and 32.7%, and 0.8% and 0.9%, respectively). Vaccine-related events were primarily injection-site reactions. Systemic adverse events that occurred up to 28 days after vaccination were mainly gastrointestinal side effects, such as diarrhea, nausea, and vomiting. Pyrexia, oral mucositis, thrombocytopenia, and febrile neutropenia also were reported.
The most common serious adverse events were infectious complications, such as febrile neutropenia and relapse of underlying disease.
The findings are notable, as patients undergoing auto-HCT have an increased risk of developing herpes zoster infection and its complications, including postherpetic neuralgia, secondary bacterial infections, and disseminated VZV infection, as well as an increased risk of hospitalization and mortality, Dr. Winston explained.
Herpes zoster infections are associated primarily with cell-mediated immunity, and in older studies done prior to the routine use of antiviral prophylaxis, the reported incidence in auto-HCT patients was between 16% and 25%. Because of this high risk, current guidelines call for antiviral prophylaxis during auto-HCT, but even in this current era of acyclovir or valacyclovir prophylaxis, infections occur at relatively high rates after auto-HCT, he noted.
“Now another approach to prevention of herpes zoster infection is vaccination,” he said.
The live attenuated vaccine currently on the market is generally contraindicated in immunocompromised patients – at least in early period after transplantation, but ZVIN showed promise with respect to safety in earlier studies, which led to the current trial.
“This study demonstrated that the inactivated varicella vaccine is very effective for preventing herpes zoster after autologous stem cell transplantation,” Dr. Winston said, noting that efficacy was observed both in those younger than age 50 years and in those aged 50 and older, and also in those who received prophylaxis for less than 3 months and for 3-6 months.
“Finally!” said one audience member, who noted during a discussion of the findings that there has long been a need for a vaccine to prevent herpes zoster in auto-HCT patients.
Dr. Winston reported receiving research funding from Oxford, and serving as a consultant to Merck and Chimerix.
ORLANDO – An inactivated varicella zoster virus vaccine currently in development for adult patients undergoing autologous hematopoietic stem cell transplantation is efficacious and well tolerated, according to findings from a randomized, placebo-controlled, phase III trial.
During the course of the 2 1/2-year pivotal multicenter trial, confirmed herpes zoster infections occurred in 42 of 560 patients who were randomized to receive inactivated varicella zoster virus vaccine (ZVIN) consistency lot (overall incidence of 32.8 cases/1,000 patient-years), compared with 113 of 564 patients who received placebo (overall incidence of 91.8/1,000 patient-years). The estimated vaccine efficacy was 63.8% after adjusting for age and duration of antiviral prophylaxis, Drew J. Winston, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The vaccine also was effective for reducing moderate and severe herpes zoster pain (estimated vaccine efficacy, 69.5%), for preventing postherpetic neuralgia (estimated vaccine efficacy, 83.7%), and for prevention of herpes zoster–related complications (estimated vaccine efficacy, 73.5%), he noted.
Study subjects were adults aged 18 years or older who were undergoing autologous hematopoietic stem cell transplantation (auto-HCT) for a malignancy or other indication. The most common underlying diseases were lymphoma and multiple myeloma. All patients had a history of varicella infection or were seropositive for varicella zoster virus (VZV) antibody, and had no history of VZV vaccine or herpes zoster infection within the prior year.
They were randomized to receive a four-dose regimen of either ZVIN consistency lot, ZVIN high-antigen lot, or placebo. A group of 106 patients who received the ZVIN high-antigen lot were included in the safety analysis only. The first ZVIN dose was administered about a month before transplantation, and doses two through four were administered about 30, 60, and 90 days after transplantation. About 90% in each group received antiviral agents after transplantation, and the duration of the use of antivirals also was similar in the groups. All patients were followed for the duration of the study, and those who developed herpes zoster were followed for 6 months after onset.
Herpes zoster cases were confirmed by polymerase chain reaction or by blinded endpoint committee adjudication.
Serious adverse events and vaccine-related serious adverse events occurred in a similar proportion of patients in the treatment and placebo groups (32.9% and 32.7%, and 0.8% and 0.9%, respectively). Vaccine-related events were primarily injection-site reactions. Systemic adverse events that occurred up to 28 days after vaccination were mainly gastrointestinal side effects, such as diarrhea, nausea, and vomiting. Pyrexia, oral mucositis, thrombocytopenia, and febrile neutropenia also were reported.
The most common serious adverse events were infectious complications, such as febrile neutropenia and relapse of underlying disease.
The findings are notable, as patients undergoing auto-HCT have an increased risk of developing herpes zoster infection and its complications, including postherpetic neuralgia, secondary bacterial infections, and disseminated VZV infection, as well as an increased risk of hospitalization and mortality, Dr. Winston explained.
Herpes zoster infections are associated primarily with cell-mediated immunity, and in older studies done prior to the routine use of antiviral prophylaxis, the reported incidence in auto-HCT patients was between 16% and 25%. Because of this high risk, current guidelines call for antiviral prophylaxis during auto-HCT, but even in this current era of acyclovir or valacyclovir prophylaxis, infections occur at relatively high rates after auto-HCT, he noted.
“Now another approach to prevention of herpes zoster infection is vaccination,” he said.
The live attenuated vaccine currently on the market is generally contraindicated in immunocompromised patients – at least in early period after transplantation, but ZVIN showed promise with respect to safety in earlier studies, which led to the current trial.
“This study demonstrated that the inactivated varicella vaccine is very effective for preventing herpes zoster after autologous stem cell transplantation,” Dr. Winston said, noting that efficacy was observed both in those younger than age 50 years and in those aged 50 and older, and also in those who received prophylaxis for less than 3 months and for 3-6 months.
“Finally!” said one audience member, who noted during a discussion of the findings that there has long been a need for a vaccine to prevent herpes zoster in auto-HCT patients.
Dr. Winston reported receiving research funding from Oxford, and serving as a consultant to Merck and Chimerix.
ORLANDO – An inactivated varicella zoster virus vaccine currently in development for adult patients undergoing autologous hematopoietic stem cell transplantation is efficacious and well tolerated, according to findings from a randomized, placebo-controlled, phase III trial.
During the course of the 2 1/2-year pivotal multicenter trial, confirmed herpes zoster infections occurred in 42 of 560 patients who were randomized to receive inactivated varicella zoster virus vaccine (ZVIN) consistency lot (overall incidence of 32.8 cases/1,000 patient-years), compared with 113 of 564 patients who received placebo (overall incidence of 91.8/1,000 patient-years). The estimated vaccine efficacy was 63.8% after adjusting for age and duration of antiviral prophylaxis, Drew J. Winston, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The vaccine also was effective for reducing moderate and severe herpes zoster pain (estimated vaccine efficacy, 69.5%), for preventing postherpetic neuralgia (estimated vaccine efficacy, 83.7%), and for prevention of herpes zoster–related complications (estimated vaccine efficacy, 73.5%), he noted.
Study subjects were adults aged 18 years or older who were undergoing autologous hematopoietic stem cell transplantation (auto-HCT) for a malignancy or other indication. The most common underlying diseases were lymphoma and multiple myeloma. All patients had a history of varicella infection or were seropositive for varicella zoster virus (VZV) antibody, and had no history of VZV vaccine or herpes zoster infection within the prior year.
They were randomized to receive a four-dose regimen of either ZVIN consistency lot, ZVIN high-antigen lot, or placebo. A group of 106 patients who received the ZVIN high-antigen lot were included in the safety analysis only. The first ZVIN dose was administered about a month before transplantation, and doses two through four were administered about 30, 60, and 90 days after transplantation. About 90% in each group received antiviral agents after transplantation, and the duration of the use of antivirals also was similar in the groups. All patients were followed for the duration of the study, and those who developed herpes zoster were followed for 6 months after onset.
Herpes zoster cases were confirmed by polymerase chain reaction or by blinded endpoint committee adjudication.
Serious adverse events and vaccine-related serious adverse events occurred in a similar proportion of patients in the treatment and placebo groups (32.9% and 32.7%, and 0.8% and 0.9%, respectively). Vaccine-related events were primarily injection-site reactions. Systemic adverse events that occurred up to 28 days after vaccination were mainly gastrointestinal side effects, such as diarrhea, nausea, and vomiting. Pyrexia, oral mucositis, thrombocytopenia, and febrile neutropenia also were reported.
The most common serious adverse events were infectious complications, such as febrile neutropenia and relapse of underlying disease.
The findings are notable, as patients undergoing auto-HCT have an increased risk of developing herpes zoster infection and its complications, including postherpetic neuralgia, secondary bacterial infections, and disseminated VZV infection, as well as an increased risk of hospitalization and mortality, Dr. Winston explained.
Herpes zoster infections are associated primarily with cell-mediated immunity, and in older studies done prior to the routine use of antiviral prophylaxis, the reported incidence in auto-HCT patients was between 16% and 25%. Because of this high risk, current guidelines call for antiviral prophylaxis during auto-HCT, but even in this current era of acyclovir or valacyclovir prophylaxis, infections occur at relatively high rates after auto-HCT, he noted.
“Now another approach to prevention of herpes zoster infection is vaccination,” he said.
The live attenuated vaccine currently on the market is generally contraindicated in immunocompromised patients – at least in early period after transplantation, but ZVIN showed promise with respect to safety in earlier studies, which led to the current trial.
“This study demonstrated that the inactivated varicella vaccine is very effective for preventing herpes zoster after autologous stem cell transplantation,” Dr. Winston said, noting that efficacy was observed both in those younger than age 50 years and in those aged 50 and older, and also in those who received prophylaxis for less than 3 months and for 3-6 months.
“Finally!” said one audience member, who noted during a discussion of the findings that there has long been a need for a vaccine to prevent herpes zoster in auto-HCT patients.
Dr. Winston reported receiving research funding from Oxford, and serving as a consultant to Merck and Chimerix.
AT THE 2017 BMT TANDEM MEETINGS
Key clinical point:
Major finding: Overall incidence of herpes zoster was 32.8 cases/1,000 patient-years vs. 91.8/1,000 patient-years in patients in the vaccine and placebo groups, respectively.
Data source: A randomized, placebo-controlled phase III trial involving 1,230 patients.
Disclosures: Dr. Winston reported receiving research funding from Oxford, and serving as a consultant to Merck and Chimerix.