User login
COVID-19–associated ocular mucormycosis outbreak case study reveals high-risk group for deadly complication
Earlier this year, hospitals in India were dealing not only with the coronavirus pandemic but also with a surge in a potentially lethal fungal infection in patients previously treated for COVID-19. Mucormycosis, also known as black fungus, is typically a rare infection, but India had recorded more than 45,000 cases as of July 2021.
Now, a recent report suggests that patients with COVID-19–associated rhino-orbital cerebral mucormycosis (CAM) may have a higher mortality rate than previously estimated. The study was published Dec. 9 in JAMA Ophthalmology.
“The mortality indicators we observed, such as assisted ventilation and presence of severe orbital manifestations, can help physicians triage patients for emergency procedures, such as functional endoscopic sinus surgery (FESS), and administer systemic antifungal agents when in short supply,” the study authors wrote.
Mucormycosis usually infects immunocompromised patients. Previous research has found that poorly controlled diabetes – an epidemic in India – and use of high-dose systemic corticosteroids are two main risk factors for developing CAM. Even before COVID-19, India had a high incidence of mucormycosis compared to other countries, but cases exist around the world. In fact, on Dec. 17, the Centers for Disease Control and Prevention reported 10 isolated cases of COVID-19–associated mucormycosis identified in Arkansas hospitals between July and September 2021.
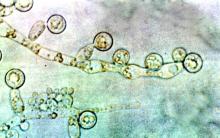
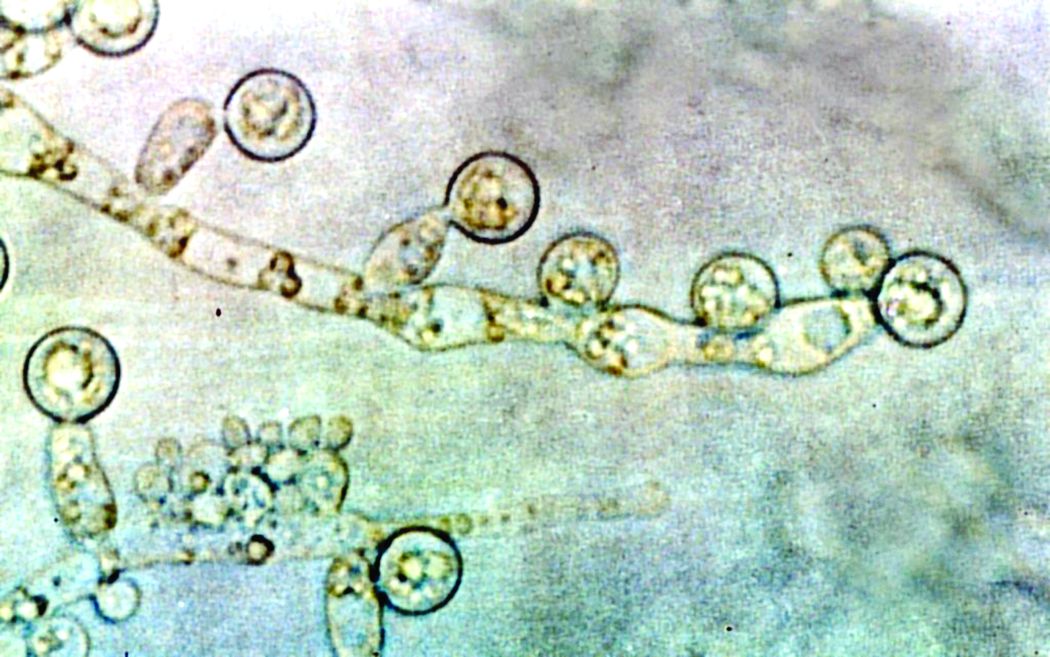
The disease can cause blurred vision, black lesions on the nose or inside of the mouth, and facial swelling. In rhino-orbital cerebral mucormycosis, extensive infection can necessitate orbital exenteration surgery, a disfiguring procedure that typically involves removal of the entire contents of the bony eye socket, as well as removal of the sinuses. Estimates for the mortality rate for this disease range from 14% to nearly 80%.
To better understand the cumulative morality rates for CAM and to identify additional risk factors, researchers reviewed the medical records of patients diagnosed and treated for CAM at a tertiary care multispecialty government hospital in Maharashtra, a state in the west-central region of India. The analysis included patients who died after admission or who had at minimum 30 days of documented follow-up. All diagnoses occurred between March 1 and May 30, 2021. All patients underwent comprehensive ophthalmic exams and routine blood workups.
Seventy-three patients were included in the study, with the average age of 53.5 years; 66% of the patients were male, and 74% of all patients had diabetes. Of the 47 individuals with available COVID-19 vaccination information, 89% had not had either shot of the vaccine, and 11% had the first dose. No patients in the cohort had received both doses of the vaccine; 87% of the patients were previously hospitalized for COVID-19, with 43 needing supplemental oxygen, 14 receiving noninvasive ventilation and ventilator support (NIV), and three requiring mechanical ventilation.
Patients developed CAM a median of 28 days after being discharged from the hospital for COVID-19 treatment; 26 patients died, 18 patients underwent FESS, and five underwent orbital exenteration. While 36% of patients died overall, the researchers found the cumulative probability of death from CAM rose from 26% at day 7 to 53% at day 21. They also found that the patients who died had more severe COVID-19, indicated by more days spent on supplemental oxygen (P = .003) and increased need for NIV or mechanical ventilation (P = .02) compared to patients who survived CAM. Those who died also had poorer visual acuity, with 35% of the group having no light perception during examination compared to 6% of surviving CAM patients (P = .02).
These findings are largely “confirmatory to what we previously knew, which is that [CAM] is a very bad disease with high morbidity and high mortality,” Ilan Schwartz, MD, PHD, an infectious disease physician at the University of Alberta, Edmonton, who researches emerging fungal infections, said in an interview. He was not involved with the research.
While larger studies looking at similar questions have been published, the new report has longer patient follow-up and is “better positioned to be able to estimate the mortality rate,” Dr. Schwartz noted. Even with 30 days of follow-up, “patients can have ongoing problems for many months, and so it’s possible that the true mortality rate is even higher, once you get beyond that period,” he added.
But Santosh G. Honavar, MD, the director of medical services at the Centre for Sight Eye Hospital in Hyderabad, India, also unaffiliated with the study, noted that the subset of patients included in the latest report may have had much more severe infection – and subsequently higher mortality rates – than a more generalized study in a broader patient population.
For example, a study by Mrittika Sen, PhD, Dr. Honavar, and their coauthors, published in the Indian Journal of Ophthalmology earlier this year, found a mortality rate of 14% when they examined the records of more than 2,800 patients across 102 treatment centers.
Taking that into account, “we believe that the actual mortality may be somewhere between the 14% reported by Sen et al. from the large Indian series and the 53% that we report at 3 weeks,” the JAMA Ophthalmology authors wrote.
Dr. Honavar also noted that the new report of severe infection outcomes identifies subgroups at higher risk of death due to CAM: those with severe COVID-19 infection or orbital disease. These groups “would need higher surveillance for mucormycosis, thus enabling early diagnosis and prompt initiation of amphotericin B upon diagnosis of mucormycosis,” he said in an interview. “These measures can possibly minimize the risk of death.”
Ongoing research on CAM cases will continue to inform knowledge and treatment of the disease, but there are still unanswered questions. “We still have a fairly unsatisfactory understanding of exactly why this [CAM] epidemic occurred and why it was so bad,” Dr. Schwartz noted. And while mucormycosis cases have seemed to drop off since the surge earlier this year, “I don’t think we’re out of the woods,” he added. “There’s a lot more awareness in India and around the world about this disease now, but we’re still quite vulnerable to seeing it again.”
Dr. Honavar is the editor-in-chief of the Indian Journal of Ophthalmology. Dr. Schwartz reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Earlier this year, hospitals in India were dealing not only with the coronavirus pandemic but also with a surge in a potentially lethal fungal infection in patients previously treated for COVID-19. Mucormycosis, also known as black fungus, is typically a rare infection, but India had recorded more than 45,000 cases as of July 2021.
Now, a recent report suggests that patients with COVID-19–associated rhino-orbital cerebral mucormycosis (CAM) may have a higher mortality rate than previously estimated. The study was published Dec. 9 in JAMA Ophthalmology.
“The mortality indicators we observed, such as assisted ventilation and presence of severe orbital manifestations, can help physicians triage patients for emergency procedures, such as functional endoscopic sinus surgery (FESS), and administer systemic antifungal agents when in short supply,” the study authors wrote.
Mucormycosis usually infects immunocompromised patients. Previous research has found that poorly controlled diabetes – an epidemic in India – and use of high-dose systemic corticosteroids are two main risk factors for developing CAM. Even before COVID-19, India had a high incidence of mucormycosis compared to other countries, but cases exist around the world. In fact, on Dec. 17, the Centers for Disease Control and Prevention reported 10 isolated cases of COVID-19–associated mucormycosis identified in Arkansas hospitals between July and September 2021.
The disease can cause blurred vision, black lesions on the nose or inside of the mouth, and facial swelling. In rhino-orbital cerebral mucormycosis, extensive infection can necessitate orbital exenteration surgery, a disfiguring procedure that typically involves removal of the entire contents of the bony eye socket, as well as removal of the sinuses. Estimates for the mortality rate for this disease range from 14% to nearly 80%.
To better understand the cumulative morality rates for CAM and to identify additional risk factors, researchers reviewed the medical records of patients diagnosed and treated for CAM at a tertiary care multispecialty government hospital in Maharashtra, a state in the west-central region of India. The analysis included patients who died after admission or who had at minimum 30 days of documented follow-up. All diagnoses occurred between March 1 and May 30, 2021. All patients underwent comprehensive ophthalmic exams and routine blood workups.
Seventy-three patients were included in the study, with the average age of 53.5 years; 66% of the patients were male, and 74% of all patients had diabetes. Of the 47 individuals with available COVID-19 vaccination information, 89% had not had either shot of the vaccine, and 11% had the first dose. No patients in the cohort had received both doses of the vaccine; 87% of the patients were previously hospitalized for COVID-19, with 43 needing supplemental oxygen, 14 receiving noninvasive ventilation and ventilator support (NIV), and three requiring mechanical ventilation.
Patients developed CAM a median of 28 days after being discharged from the hospital for COVID-19 treatment; 26 patients died, 18 patients underwent FESS, and five underwent orbital exenteration. While 36% of patients died overall, the researchers found the cumulative probability of death from CAM rose from 26% at day 7 to 53% at day 21. They also found that the patients who died had more severe COVID-19, indicated by more days spent on supplemental oxygen (P = .003) and increased need for NIV or mechanical ventilation (P = .02) compared to patients who survived CAM. Those who died also had poorer visual acuity, with 35% of the group having no light perception during examination compared to 6% of surviving CAM patients (P = .02).
These findings are largely “confirmatory to what we previously knew, which is that [CAM] is a very bad disease with high morbidity and high mortality,” Ilan Schwartz, MD, PHD, an infectious disease physician at the University of Alberta, Edmonton, who researches emerging fungal infections, said in an interview. He was not involved with the research.
While larger studies looking at similar questions have been published, the new report has longer patient follow-up and is “better positioned to be able to estimate the mortality rate,” Dr. Schwartz noted. Even with 30 days of follow-up, “patients can have ongoing problems for many months, and so it’s possible that the true mortality rate is even higher, once you get beyond that period,” he added.
But Santosh G. Honavar, MD, the director of medical services at the Centre for Sight Eye Hospital in Hyderabad, India, also unaffiliated with the study, noted that the subset of patients included in the latest report may have had much more severe infection – and subsequently higher mortality rates – than a more generalized study in a broader patient population.
For example, a study by Mrittika Sen, PhD, Dr. Honavar, and their coauthors, published in the Indian Journal of Ophthalmology earlier this year, found a mortality rate of 14% when they examined the records of more than 2,800 patients across 102 treatment centers.
Taking that into account, “we believe that the actual mortality may be somewhere between the 14% reported by Sen et al. from the large Indian series and the 53% that we report at 3 weeks,” the JAMA Ophthalmology authors wrote.
Dr. Honavar also noted that the new report of severe infection outcomes identifies subgroups at higher risk of death due to CAM: those with severe COVID-19 infection or orbital disease. These groups “would need higher surveillance for mucormycosis, thus enabling early diagnosis and prompt initiation of amphotericin B upon diagnosis of mucormycosis,” he said in an interview. “These measures can possibly minimize the risk of death.”
Ongoing research on CAM cases will continue to inform knowledge and treatment of the disease, but there are still unanswered questions. “We still have a fairly unsatisfactory understanding of exactly why this [CAM] epidemic occurred and why it was so bad,” Dr. Schwartz noted. And while mucormycosis cases have seemed to drop off since the surge earlier this year, “I don’t think we’re out of the woods,” he added. “There’s a lot more awareness in India and around the world about this disease now, but we’re still quite vulnerable to seeing it again.”
Dr. Honavar is the editor-in-chief of the Indian Journal of Ophthalmology. Dr. Schwartz reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Earlier this year, hospitals in India were dealing not only with the coronavirus pandemic but also with a surge in a potentially lethal fungal infection in patients previously treated for COVID-19. Mucormycosis, also known as black fungus, is typically a rare infection, but India had recorded more than 45,000 cases as of July 2021.
Now, a recent report suggests that patients with COVID-19–associated rhino-orbital cerebral mucormycosis (CAM) may have a higher mortality rate than previously estimated. The study was published Dec. 9 in JAMA Ophthalmology.
“The mortality indicators we observed, such as assisted ventilation and presence of severe orbital manifestations, can help physicians triage patients for emergency procedures, such as functional endoscopic sinus surgery (FESS), and administer systemic antifungal agents when in short supply,” the study authors wrote.
Mucormycosis usually infects immunocompromised patients. Previous research has found that poorly controlled diabetes – an epidemic in India – and use of high-dose systemic corticosteroids are two main risk factors for developing CAM. Even before COVID-19, India had a high incidence of mucormycosis compared to other countries, but cases exist around the world. In fact, on Dec. 17, the Centers for Disease Control and Prevention reported 10 isolated cases of COVID-19–associated mucormycosis identified in Arkansas hospitals between July and September 2021.
The disease can cause blurred vision, black lesions on the nose or inside of the mouth, and facial swelling. In rhino-orbital cerebral mucormycosis, extensive infection can necessitate orbital exenteration surgery, a disfiguring procedure that typically involves removal of the entire contents of the bony eye socket, as well as removal of the sinuses. Estimates for the mortality rate for this disease range from 14% to nearly 80%.
To better understand the cumulative morality rates for CAM and to identify additional risk factors, researchers reviewed the medical records of patients diagnosed and treated for CAM at a tertiary care multispecialty government hospital in Maharashtra, a state in the west-central region of India. The analysis included patients who died after admission or who had at minimum 30 days of documented follow-up. All diagnoses occurred between March 1 and May 30, 2021. All patients underwent comprehensive ophthalmic exams and routine blood workups.
Seventy-three patients were included in the study, with the average age of 53.5 years; 66% of the patients were male, and 74% of all patients had diabetes. Of the 47 individuals with available COVID-19 vaccination information, 89% had not had either shot of the vaccine, and 11% had the first dose. No patients in the cohort had received both doses of the vaccine; 87% of the patients were previously hospitalized for COVID-19, with 43 needing supplemental oxygen, 14 receiving noninvasive ventilation and ventilator support (NIV), and three requiring mechanical ventilation.
Patients developed CAM a median of 28 days after being discharged from the hospital for COVID-19 treatment; 26 patients died, 18 patients underwent FESS, and five underwent orbital exenteration. While 36% of patients died overall, the researchers found the cumulative probability of death from CAM rose from 26% at day 7 to 53% at day 21. They also found that the patients who died had more severe COVID-19, indicated by more days spent on supplemental oxygen (P = .003) and increased need for NIV or mechanical ventilation (P = .02) compared to patients who survived CAM. Those who died also had poorer visual acuity, with 35% of the group having no light perception during examination compared to 6% of surviving CAM patients (P = .02).
These findings are largely “confirmatory to what we previously knew, which is that [CAM] is a very bad disease with high morbidity and high mortality,” Ilan Schwartz, MD, PHD, an infectious disease physician at the University of Alberta, Edmonton, who researches emerging fungal infections, said in an interview. He was not involved with the research.
While larger studies looking at similar questions have been published, the new report has longer patient follow-up and is “better positioned to be able to estimate the mortality rate,” Dr. Schwartz noted. Even with 30 days of follow-up, “patients can have ongoing problems for many months, and so it’s possible that the true mortality rate is even higher, once you get beyond that period,” he added.
But Santosh G. Honavar, MD, the director of medical services at the Centre for Sight Eye Hospital in Hyderabad, India, also unaffiliated with the study, noted that the subset of patients included in the latest report may have had much more severe infection – and subsequently higher mortality rates – than a more generalized study in a broader patient population.
For example, a study by Mrittika Sen, PhD, Dr. Honavar, and their coauthors, published in the Indian Journal of Ophthalmology earlier this year, found a mortality rate of 14% when they examined the records of more than 2,800 patients across 102 treatment centers.
Taking that into account, “we believe that the actual mortality may be somewhere between the 14% reported by Sen et al. from the large Indian series and the 53% that we report at 3 weeks,” the JAMA Ophthalmology authors wrote.
Dr. Honavar also noted that the new report of severe infection outcomes identifies subgroups at higher risk of death due to CAM: those with severe COVID-19 infection or orbital disease. These groups “would need higher surveillance for mucormycosis, thus enabling early diagnosis and prompt initiation of amphotericin B upon diagnosis of mucormycosis,” he said in an interview. “These measures can possibly minimize the risk of death.”
Ongoing research on CAM cases will continue to inform knowledge and treatment of the disease, but there are still unanswered questions. “We still have a fairly unsatisfactory understanding of exactly why this [CAM] epidemic occurred and why it was so bad,” Dr. Schwartz noted. And while mucormycosis cases have seemed to drop off since the surge earlier this year, “I don’t think we’re out of the woods,” he added. “There’s a lot more awareness in India and around the world about this disease now, but we’re still quite vulnerable to seeing it again.”
Dr. Honavar is the editor-in-chief of the Indian Journal of Ophthalmology. Dr. Schwartz reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA OPHTHALMOLOGY
Fungal infection can mimic lung cancer metastases
A fungal infection typically seen in the lungs may have a variety of unusual clinical presentations elsewhere in the body, even raising suspicion of cancer in some cases, a medical resident reported at the annual meeting of the American College of Chest Physicians.
In one recent and unusual presentation, a 58-year-old woman with persistent headaches had skull lesions on computed tomography (CT) was eventually diagnosed with disseminated coccidioidomycosis (Valley fever), a fungal infection endemic to the Southwestern U.S.
The imaging pattern of her head CT was initially concerning for cancer metastasis, according to Sharjeel Israr, MD, a third-year internal medicine resident at Creighton University in Phoenix, Ariz.
However, the subsequent chest CT revealed a suspicious chest mass. A biopsy of that mass led to the correct diagnosis of disseminated coccidioidomycosis, according to Dr. Israr, who presented the case report in an e-poster at the CHEST meeting, which was held virtually this year.
Mistaken identity
Coccidioidomycosis, caused by the fungus Coccidioides, usually affects the lungs, according to the Centers for Disease Control and Prevention. However, in severe cases it can spread to other parts of the body. In those cases, it’s referred to as disseminated coccidioidomycosis.
Arizona accounted for about 10,000 out of 18,000 reported Valley fever cases in 2019, according to the latest statistics from the CDC.
Coccidioidomycosis is frequently mistaken not only for cancer, but also for rheumatic conditions and bacterial infections, according to Valley fever specialist John Galgiani, MD, director of the Valley Fever Center for Excellence at the University of Arizona in Tucson.
“Where Valley fever is common, it should very frequently be in the differential for masses that are thought to be cancer,” Dr. Galgiani said in an interview. “This case is a good example of that.”
Challenging case
In an interview, Dr. Israr said the case was challenging to crack despite the fact that Valley fever is very common in Phoenix.
“It was definitely on the differential from the get-go, but it was very, very low our differential, just based on the presentation that she had,” said Dr. Israr.
The patient had history of diabetes and presented with headaches for 4 weeks. However, she had no pulmonary symptoms or meningeal signs, according to Dr. Israr.
A head CT revealed multiple osseous skull lesions and a left temporal lobe lesion.
“The fact that this patient had lesions in the skull, specifically, is something that raised our initial red flags for cancer – especially since she presented with just a headache as her only complaint,” he said.
The imaging pattern was concerning for metastasis, according to Dr. Israr, particularly since a subsequent CT of the chest showed multiple pulmonary nodules plus a 7.7-cm mass in the right lower lobe.
Once the biopsy confirmed coccidioidomycosis, the patient was started on fluconazole 600 mg twice daily, according to Dr. Israr.
Although severe disseminated coccidioidomycosis can be difficult to treat, the lung lesion had decreased in size from 7.7 cm to 4.2 cm about 3 months later, Dr. Israr said.
“At the end of the day, she didn’t have cancer, and it’s something that we’re treating and she’s actually doing better right now,” Dr. Israr said in the interview.
Dr. Israr and coauthors of the case reported they had no relevant relationships to disclose.
A fungal infection typically seen in the lungs may have a variety of unusual clinical presentations elsewhere in the body, even raising suspicion of cancer in some cases, a medical resident reported at the annual meeting of the American College of Chest Physicians.
In one recent and unusual presentation, a 58-year-old woman with persistent headaches had skull lesions on computed tomography (CT) was eventually diagnosed with disseminated coccidioidomycosis (Valley fever), a fungal infection endemic to the Southwestern U.S.
The imaging pattern of her head CT was initially concerning for cancer metastasis, according to Sharjeel Israr, MD, a third-year internal medicine resident at Creighton University in Phoenix, Ariz.
However, the subsequent chest CT revealed a suspicious chest mass. A biopsy of that mass led to the correct diagnosis of disseminated coccidioidomycosis, according to Dr. Israr, who presented the case report in an e-poster at the CHEST meeting, which was held virtually this year.
Mistaken identity
Coccidioidomycosis, caused by the fungus Coccidioides, usually affects the lungs, according to the Centers for Disease Control and Prevention. However, in severe cases it can spread to other parts of the body. In those cases, it’s referred to as disseminated coccidioidomycosis.
Arizona accounted for about 10,000 out of 18,000 reported Valley fever cases in 2019, according to the latest statistics from the CDC.
Coccidioidomycosis is frequently mistaken not only for cancer, but also for rheumatic conditions and bacterial infections, according to Valley fever specialist John Galgiani, MD, director of the Valley Fever Center for Excellence at the University of Arizona in Tucson.
“Where Valley fever is common, it should very frequently be in the differential for masses that are thought to be cancer,” Dr. Galgiani said in an interview. “This case is a good example of that.”
Challenging case
In an interview, Dr. Israr said the case was challenging to crack despite the fact that Valley fever is very common in Phoenix.
“It was definitely on the differential from the get-go, but it was very, very low our differential, just based on the presentation that she had,” said Dr. Israr.
The patient had history of diabetes and presented with headaches for 4 weeks. However, she had no pulmonary symptoms or meningeal signs, according to Dr. Israr.
A head CT revealed multiple osseous skull lesions and a left temporal lobe lesion.
“The fact that this patient had lesions in the skull, specifically, is something that raised our initial red flags for cancer – especially since she presented with just a headache as her only complaint,” he said.
The imaging pattern was concerning for metastasis, according to Dr. Israr, particularly since a subsequent CT of the chest showed multiple pulmonary nodules plus a 7.7-cm mass in the right lower lobe.
Once the biopsy confirmed coccidioidomycosis, the patient was started on fluconazole 600 mg twice daily, according to Dr. Israr.
Although severe disseminated coccidioidomycosis can be difficult to treat, the lung lesion had decreased in size from 7.7 cm to 4.2 cm about 3 months later, Dr. Israr said.
“At the end of the day, she didn’t have cancer, and it’s something that we’re treating and she’s actually doing better right now,” Dr. Israr said in the interview.
Dr. Israr and coauthors of the case reported they had no relevant relationships to disclose.
A fungal infection typically seen in the lungs may have a variety of unusual clinical presentations elsewhere in the body, even raising suspicion of cancer in some cases, a medical resident reported at the annual meeting of the American College of Chest Physicians.
In one recent and unusual presentation, a 58-year-old woman with persistent headaches had skull lesions on computed tomography (CT) was eventually diagnosed with disseminated coccidioidomycosis (Valley fever), a fungal infection endemic to the Southwestern U.S.
The imaging pattern of her head CT was initially concerning for cancer metastasis, according to Sharjeel Israr, MD, a third-year internal medicine resident at Creighton University in Phoenix, Ariz.
However, the subsequent chest CT revealed a suspicious chest mass. A biopsy of that mass led to the correct diagnosis of disseminated coccidioidomycosis, according to Dr. Israr, who presented the case report in an e-poster at the CHEST meeting, which was held virtually this year.
Mistaken identity
Coccidioidomycosis, caused by the fungus Coccidioides, usually affects the lungs, according to the Centers for Disease Control and Prevention. However, in severe cases it can spread to other parts of the body. In those cases, it’s referred to as disseminated coccidioidomycosis.
Arizona accounted for about 10,000 out of 18,000 reported Valley fever cases in 2019, according to the latest statistics from the CDC.
Coccidioidomycosis is frequently mistaken not only for cancer, but also for rheumatic conditions and bacterial infections, according to Valley fever specialist John Galgiani, MD, director of the Valley Fever Center for Excellence at the University of Arizona in Tucson.
“Where Valley fever is common, it should very frequently be in the differential for masses that are thought to be cancer,” Dr. Galgiani said in an interview. “This case is a good example of that.”
Challenging case
In an interview, Dr. Israr said the case was challenging to crack despite the fact that Valley fever is very common in Phoenix.
“It was definitely on the differential from the get-go, but it was very, very low our differential, just based on the presentation that she had,” said Dr. Israr.
The patient had history of diabetes and presented with headaches for 4 weeks. However, she had no pulmonary symptoms or meningeal signs, according to Dr. Israr.
A head CT revealed multiple osseous skull lesions and a left temporal lobe lesion.
“The fact that this patient had lesions in the skull, specifically, is something that raised our initial red flags for cancer – especially since she presented with just a headache as her only complaint,” he said.
The imaging pattern was concerning for metastasis, according to Dr. Israr, particularly since a subsequent CT of the chest showed multiple pulmonary nodules plus a 7.7-cm mass in the right lower lobe.
Once the biopsy confirmed coccidioidomycosis, the patient was started on fluconazole 600 mg twice daily, according to Dr. Israr.
Although severe disseminated coccidioidomycosis can be difficult to treat, the lung lesion had decreased in size from 7.7 cm to 4.2 cm about 3 months later, Dr. Israr said.
“At the end of the day, she didn’t have cancer, and it’s something that we’re treating and she’s actually doing better right now,” Dr. Israr said in the interview.
Dr. Israr and coauthors of the case reported they had no relevant relationships to disclose.
REPORTING FROM CHEST 2021
Oteseconazole promising for recurrent yeast infections
A phase 3, randomized, double-blind, controlled trial has shown that oteseconazole (Mycovia Pharmaceuticals), an oral antifungal agent, is safe and effective in treating acute and recurrent yeast infections (vulvovaginal candidiasis [VVC]) and in preventing recurrence of acute VVC episodes.
Findings of the ultraVIOLET trial, which compared oteseconazole with the standard fluconazole, were presented at IDWeek 2021, an annual scientific meeting on infectious diseases, by lead author Mark G. Martens, MD, a professor in the department of obstetrics and gynecology at Drexel University College of Medicine in Philadelphia.
About 75% of all women will have a yeast infection in their lifetime, Dr. Martens noted. About 138 million women worldwide have recurring episodes (at least three acute episodes in the last year) of the debilitating condition.
“Recurrent vulvovaginal candidiasis typically requires treatment of the acute episode followed by long-term suppressive therapy with either weekly or biweekly fluconazole,” Dr. Martens said. However, when therapy stops, more than 50% of patients with recurrent VVC experience an infection within the next 6 months, which takes a significant toll on daily life.
Additionally, fluconazole has been linked with safety issues concerning chronic dosing, he said, citing liver toxicity, drug-drug interactions and “increased risk of miscarriage and birth defects when used during pregnancy.”
Topical treatments have been associated with messy application and burning, he noted.
For this study, researchers enrolled 219 women with a history of recurrent VVC at 51 U.S. sites. Participants were randomized either to 600 mg oteseconazole on day 1, 450 mg oteseconazole on day 2 or placebo capsules; or three sequential 150 mg doses (every 72 hours) of fluconazole together with matching placebo capsules.
In the maintenance phase, 185 women with resolved acute VVC (clinical signs and symptoms were scored below 3) on day 14 received 150 mg oteseconazole or placebo weekly for 11 weeks.
Oteseconazole was superior to fluconazole/placebo in the proportion of subjects with at least one culture-verified acute VVC episode through week 50 in the intent-to-treat population (P < .001) which included subjects who failed to clear their infection in the induction phase.
The average percentage of participants with at least one culture-verified acute VVC episode through week 50 was lower in the oteseconazole group (5.1%), compared with the fluconazole/placebo group (42.2%).
Oteseconazole was noninferior to fluconazole in the proportion of subjects with resolved acute VVC infections at day 14 – 93.2% for the oteseconazole group vs. 95.8% for the fluconazole/placebo group.
The percentages of women who had at least one treatment-emergent adverse event (TEAE) were similar – 54% in the oteseconazole group and 64% in the fluconazole/placebo group. Most TEAEs were mild or moderate and there were no drug-related SAEs or adverse effects on liver function.
“There was no difference in the two groups in he baseline characteristics of age, race, and history of diabetes,” he said.
Oluwatosin Goje, MD, an ob.gyn. with the Cleveland Clinic told this news organization that the drug may offer another option for women who don’t respond to azoles.
“The CDC guidelines say, and I agree, that most episodes of recurrent VVC that are caused by Candida albicans will respond to topical azoles, to oral azoles, to the known drugs that are available. You just may have to use them for a prolonged period of time,” Dr. Goje said. But some patients won’t respond to azoles, the currently available drugs, and topical treatments – so new options are welcome for them, she noted.
She pointed out that the U.S. Food and Drug Administration in June approved ibrexafungerp (Brexafemme), the first oral nonazole treatment for vaginal yeast infections. It was the first approved medicine in a novel antifungal class in more than 2 decades.
Dr. Goje, who runs a large clinic with substantial numbers of women with recurrent yeast infections, said the psychosocial problems women with recurrent yeast infections face – and the time off work and money spent trying to get temporary relief from over-the-counter medications – is underestimated.
“Women have long suffered vaginitis. It can be a lot of social and economic burden. So anything in the toolbox to help women is welcome,” Dr. Goje said.
The study was sponsored by Mycovia Pharmaceuticals. Dr. Martens reports no relevant financial relationships. Several coauthors are either employees of Mycovia or receive support from the company. Dr. Goje has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A phase 3, randomized, double-blind, controlled trial has shown that oteseconazole (Mycovia Pharmaceuticals), an oral antifungal agent, is safe and effective in treating acute and recurrent yeast infections (vulvovaginal candidiasis [VVC]) and in preventing recurrence of acute VVC episodes.
Findings of the ultraVIOLET trial, which compared oteseconazole with the standard fluconazole, were presented at IDWeek 2021, an annual scientific meeting on infectious diseases, by lead author Mark G. Martens, MD, a professor in the department of obstetrics and gynecology at Drexel University College of Medicine in Philadelphia.
About 75% of all women will have a yeast infection in their lifetime, Dr. Martens noted. About 138 million women worldwide have recurring episodes (at least three acute episodes in the last year) of the debilitating condition.
“Recurrent vulvovaginal candidiasis typically requires treatment of the acute episode followed by long-term suppressive therapy with either weekly or biweekly fluconazole,” Dr. Martens said. However, when therapy stops, more than 50% of patients with recurrent VVC experience an infection within the next 6 months, which takes a significant toll on daily life.
Additionally, fluconazole has been linked with safety issues concerning chronic dosing, he said, citing liver toxicity, drug-drug interactions and “increased risk of miscarriage and birth defects when used during pregnancy.”
Topical treatments have been associated with messy application and burning, he noted.
For this study, researchers enrolled 219 women with a history of recurrent VVC at 51 U.S. sites. Participants were randomized either to 600 mg oteseconazole on day 1, 450 mg oteseconazole on day 2 or placebo capsules; or three sequential 150 mg doses (every 72 hours) of fluconazole together with matching placebo capsules.
In the maintenance phase, 185 women with resolved acute VVC (clinical signs and symptoms were scored below 3) on day 14 received 150 mg oteseconazole or placebo weekly for 11 weeks.
Oteseconazole was superior to fluconazole/placebo in the proportion of subjects with at least one culture-verified acute VVC episode through week 50 in the intent-to-treat population (P < .001) which included subjects who failed to clear their infection in the induction phase.
The average percentage of participants with at least one culture-verified acute VVC episode through week 50 was lower in the oteseconazole group (5.1%), compared with the fluconazole/placebo group (42.2%).
Oteseconazole was noninferior to fluconazole in the proportion of subjects with resolved acute VVC infections at day 14 – 93.2% for the oteseconazole group vs. 95.8% for the fluconazole/placebo group.
The percentages of women who had at least one treatment-emergent adverse event (TEAE) were similar – 54% in the oteseconazole group and 64% in the fluconazole/placebo group. Most TEAEs were mild or moderate and there were no drug-related SAEs or adverse effects on liver function.
“There was no difference in the two groups in he baseline characteristics of age, race, and history of diabetes,” he said.
Oluwatosin Goje, MD, an ob.gyn. with the Cleveland Clinic told this news organization that the drug may offer another option for women who don’t respond to azoles.
“The CDC guidelines say, and I agree, that most episodes of recurrent VVC that are caused by Candida albicans will respond to topical azoles, to oral azoles, to the known drugs that are available. You just may have to use them for a prolonged period of time,” Dr. Goje said. But some patients won’t respond to azoles, the currently available drugs, and topical treatments – so new options are welcome for them, she noted.
She pointed out that the U.S. Food and Drug Administration in June approved ibrexafungerp (Brexafemme), the first oral nonazole treatment for vaginal yeast infections. It was the first approved medicine in a novel antifungal class in more than 2 decades.
Dr. Goje, who runs a large clinic with substantial numbers of women with recurrent yeast infections, said the psychosocial problems women with recurrent yeast infections face – and the time off work and money spent trying to get temporary relief from over-the-counter medications – is underestimated.
“Women have long suffered vaginitis. It can be a lot of social and economic burden. So anything in the toolbox to help women is welcome,” Dr. Goje said.
The study was sponsored by Mycovia Pharmaceuticals. Dr. Martens reports no relevant financial relationships. Several coauthors are either employees of Mycovia or receive support from the company. Dr. Goje has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A phase 3, randomized, double-blind, controlled trial has shown that oteseconazole (Mycovia Pharmaceuticals), an oral antifungal agent, is safe and effective in treating acute and recurrent yeast infections (vulvovaginal candidiasis [VVC]) and in preventing recurrence of acute VVC episodes.
Findings of the ultraVIOLET trial, which compared oteseconazole with the standard fluconazole, were presented at IDWeek 2021, an annual scientific meeting on infectious diseases, by lead author Mark G. Martens, MD, a professor in the department of obstetrics and gynecology at Drexel University College of Medicine in Philadelphia.
About 75% of all women will have a yeast infection in their lifetime, Dr. Martens noted. About 138 million women worldwide have recurring episodes (at least three acute episodes in the last year) of the debilitating condition.
“Recurrent vulvovaginal candidiasis typically requires treatment of the acute episode followed by long-term suppressive therapy with either weekly or biweekly fluconazole,” Dr. Martens said. However, when therapy stops, more than 50% of patients with recurrent VVC experience an infection within the next 6 months, which takes a significant toll on daily life.
Additionally, fluconazole has been linked with safety issues concerning chronic dosing, he said, citing liver toxicity, drug-drug interactions and “increased risk of miscarriage and birth defects when used during pregnancy.”
Topical treatments have been associated with messy application and burning, he noted.
For this study, researchers enrolled 219 women with a history of recurrent VVC at 51 U.S. sites. Participants were randomized either to 600 mg oteseconazole on day 1, 450 mg oteseconazole on day 2 or placebo capsules; or three sequential 150 mg doses (every 72 hours) of fluconazole together with matching placebo capsules.
In the maintenance phase, 185 women with resolved acute VVC (clinical signs and symptoms were scored below 3) on day 14 received 150 mg oteseconazole or placebo weekly for 11 weeks.
Oteseconazole was superior to fluconazole/placebo in the proportion of subjects with at least one culture-verified acute VVC episode through week 50 in the intent-to-treat population (P < .001) which included subjects who failed to clear their infection in the induction phase.
The average percentage of participants with at least one culture-verified acute VVC episode through week 50 was lower in the oteseconazole group (5.1%), compared with the fluconazole/placebo group (42.2%).
Oteseconazole was noninferior to fluconazole in the proportion of subjects with resolved acute VVC infections at day 14 – 93.2% for the oteseconazole group vs. 95.8% for the fluconazole/placebo group.
The percentages of women who had at least one treatment-emergent adverse event (TEAE) were similar – 54% in the oteseconazole group and 64% in the fluconazole/placebo group. Most TEAEs were mild or moderate and there were no drug-related SAEs or adverse effects on liver function.
“There was no difference in the two groups in he baseline characteristics of age, race, and history of diabetes,” he said.
Oluwatosin Goje, MD, an ob.gyn. with the Cleveland Clinic told this news organization that the drug may offer another option for women who don’t respond to azoles.
“The CDC guidelines say, and I agree, that most episodes of recurrent VVC that are caused by Candida albicans will respond to topical azoles, to oral azoles, to the known drugs that are available. You just may have to use them for a prolonged period of time,” Dr. Goje said. But some patients won’t respond to azoles, the currently available drugs, and topical treatments – so new options are welcome for them, she noted.
She pointed out that the U.S. Food and Drug Administration in June approved ibrexafungerp (Brexafemme), the first oral nonazole treatment for vaginal yeast infections. It was the first approved medicine in a novel antifungal class in more than 2 decades.
Dr. Goje, who runs a large clinic with substantial numbers of women with recurrent yeast infections, said the psychosocial problems women with recurrent yeast infections face – and the time off work and money spent trying to get temporary relief from over-the-counter medications – is underestimated.
“Women have long suffered vaginitis. It can be a lot of social and economic burden. So anything in the toolbox to help women is welcome,” Dr. Goje said.
The study was sponsored by Mycovia Pharmaceuticals. Dr. Martens reports no relevant financial relationships. Several coauthors are either employees of Mycovia or receive support from the company. Dr. Goje has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Candida auris transmission can be contained in postacute care settings
A new study from Orange County, California, shows how Candida auris, an emerging pathogen, was successfully identified and contained in long-term acute care hospitals (LTACHs) and ventilator-capable skilled-nursing facilities (vSNFs).
Lead author Ellora Karmarkar, MD, MSc, formerly an epidemic intelligence service officer with the Centers for Disease Control and Prevention and currently with the California Department of Public Health, said in an interview that the prospective surveillance of urine cultures for C. auris was prompted by “seeing what was happening in New York, New Jersey, and Illinois [being] pretty alarming for a lot of the health officials in California, [who] know that LTACHs are high-risk facilities because they take care of really sick people. Some of those people are there for a very long time.”
Therefore, the study authors decided to focus their investigations there, rather than in acute care hospitals, which were believed to be at lower risk for C. auris outbreaks.
The Orange County Health Department, working with the California Department of Health and the CDC, asked labs to prospectively identify all Candida isolates in urines from LTACHs between September 2018 and February 2019. Normally, labs do not speciate Candida from nonsterile body sites.
Dan Diekema, MD, an epidemiologist and clinical microbiologist at the University of Iowa, Iowa City, who was not involved in the study, told this news organization, “Acute care hospitals really ought to be moving toward doing species identification of Candida from nonsterile sites if they really want to have a better chance of detecting this early.”
The OCHD also screened LTACH and vSNF patients with composite cultures from the axilla-groin or nasal swabs. Screening was undertaken because 5%-10% of colonized patients later develop invasive infections, and 30%-60% die.
The first bloodstream infection was detected in May 2019. Per the report, published online Sept. 7 in Annals of Internal Medicine, “As of 1 January 2020, of 182 patients, 22 (12%) died within 30 days of C. auris identification; 47 (26%) died within 90 days. One of 47 deaths was attributed to C. auris.” Whole-genome sequencing showed that the isolates were all closely related in clade III.
Experts conducted extensive education in infection control at the LTACHs, and communication among the LTACHs and between the long-term facilities and acute care hospitals was improved. As a result, receiving facilities accepting transfers began culturing their newly admitted patients and quickly identified 4 of 99 patients with C. auris who had no known history of colonization. By October 2019, the outbreak was contained in two facilities, down from the nine where C. auris was initially found.
Dr. Diekema noted, “The challenge, of course, for a new emerging MDRO [multidrug-resistant organism] like Candida auris, is that the initial approach, in general, has to be almost passive, when you have not seen the organism. ... Passive surveillance means that you just carefully monitor your clinical cultures, and the first time you detect the MDRO of concern, then you begin doing the point prevalence surveys. ... This [prospective] kind of approach is really good for how we should move forward with both initial detection and containment of MDRO spread.”
Many outbreak studies are confined to a particular institution. Authors of an accompanying editorial commented that this study “underlines the importance of proactive protocols for outbreak investigations and containment measures across the entirety of the health care network serving at-risk patients.”
In her research, Dr. Karmarkar observed that, “some of these facilities don’t have the same infrastructure and infection prevention and control that an acute care hospital might.”
She said in an interview that, “one of the challenges was that people were so focused on COVID that they forgot about the MDROs. ... Some of the things that we recommend to help control Candida auris are also excellent practices for every other organism including COVID care. ... What I appreciated about this investigation is that every facility that we went to was so open to learning, so happy to have us there. They’re very interested in learning about Candida auris and understanding what they could do to control it.”
While recent attention has been on the frightening levels of multidrug resistance in C. auris, Dr. Karmarkar concluded that the “central message in our investigation is that with the right effort, the right approach, and the right team this is an intervenable issue. It’s not inevitable if the attention is focused on it to pick it up early and then try to contain it.”
Dr. Karmarkar reports no relevant financial relationships. Dr. Diekema reports research funding from bioMerieux and consulting fees from Opgen.
A version of this article first appeared on Medscape.com.
A new study from Orange County, California, shows how Candida auris, an emerging pathogen, was successfully identified and contained in long-term acute care hospitals (LTACHs) and ventilator-capable skilled-nursing facilities (vSNFs).
Lead author Ellora Karmarkar, MD, MSc, formerly an epidemic intelligence service officer with the Centers for Disease Control and Prevention and currently with the California Department of Public Health, said in an interview that the prospective surveillance of urine cultures for C. auris was prompted by “seeing what was happening in New York, New Jersey, and Illinois [being] pretty alarming for a lot of the health officials in California, [who] know that LTACHs are high-risk facilities because they take care of really sick people. Some of those people are there for a very long time.”
Therefore, the study authors decided to focus their investigations there, rather than in acute care hospitals, which were believed to be at lower risk for C. auris outbreaks.
The Orange County Health Department, working with the California Department of Health and the CDC, asked labs to prospectively identify all Candida isolates in urines from LTACHs between September 2018 and February 2019. Normally, labs do not speciate Candida from nonsterile body sites.
Dan Diekema, MD, an epidemiologist and clinical microbiologist at the University of Iowa, Iowa City, who was not involved in the study, told this news organization, “Acute care hospitals really ought to be moving toward doing species identification of Candida from nonsterile sites if they really want to have a better chance of detecting this early.”
The OCHD also screened LTACH and vSNF patients with composite cultures from the axilla-groin or nasal swabs. Screening was undertaken because 5%-10% of colonized patients later develop invasive infections, and 30%-60% die.
The first bloodstream infection was detected in May 2019. Per the report, published online Sept. 7 in Annals of Internal Medicine, “As of 1 January 2020, of 182 patients, 22 (12%) died within 30 days of C. auris identification; 47 (26%) died within 90 days. One of 47 deaths was attributed to C. auris.” Whole-genome sequencing showed that the isolates were all closely related in clade III.
Experts conducted extensive education in infection control at the LTACHs, and communication among the LTACHs and between the long-term facilities and acute care hospitals was improved. As a result, receiving facilities accepting transfers began culturing their newly admitted patients and quickly identified 4 of 99 patients with C. auris who had no known history of colonization. By October 2019, the outbreak was contained in two facilities, down from the nine where C. auris was initially found.
Dr. Diekema noted, “The challenge, of course, for a new emerging MDRO [multidrug-resistant organism] like Candida auris, is that the initial approach, in general, has to be almost passive, when you have not seen the organism. ... Passive surveillance means that you just carefully monitor your clinical cultures, and the first time you detect the MDRO of concern, then you begin doing the point prevalence surveys. ... This [prospective] kind of approach is really good for how we should move forward with both initial detection and containment of MDRO spread.”
Many outbreak studies are confined to a particular institution. Authors of an accompanying editorial commented that this study “underlines the importance of proactive protocols for outbreak investigations and containment measures across the entirety of the health care network serving at-risk patients.”
In her research, Dr. Karmarkar observed that, “some of these facilities don’t have the same infrastructure and infection prevention and control that an acute care hospital might.”
She said in an interview that, “one of the challenges was that people were so focused on COVID that they forgot about the MDROs. ... Some of the things that we recommend to help control Candida auris are also excellent practices for every other organism including COVID care. ... What I appreciated about this investigation is that every facility that we went to was so open to learning, so happy to have us there. They’re very interested in learning about Candida auris and understanding what they could do to control it.”
While recent attention has been on the frightening levels of multidrug resistance in C. auris, Dr. Karmarkar concluded that the “central message in our investigation is that with the right effort, the right approach, and the right team this is an intervenable issue. It’s not inevitable if the attention is focused on it to pick it up early and then try to contain it.”
Dr. Karmarkar reports no relevant financial relationships. Dr. Diekema reports research funding from bioMerieux and consulting fees from Opgen.
A version of this article first appeared on Medscape.com.
A new study from Orange County, California, shows how Candida auris, an emerging pathogen, was successfully identified and contained in long-term acute care hospitals (LTACHs) and ventilator-capable skilled-nursing facilities (vSNFs).
Lead author Ellora Karmarkar, MD, MSc, formerly an epidemic intelligence service officer with the Centers for Disease Control and Prevention and currently with the California Department of Public Health, said in an interview that the prospective surveillance of urine cultures for C. auris was prompted by “seeing what was happening in New York, New Jersey, and Illinois [being] pretty alarming for a lot of the health officials in California, [who] know that LTACHs are high-risk facilities because they take care of really sick people. Some of those people are there for a very long time.”
Therefore, the study authors decided to focus their investigations there, rather than in acute care hospitals, which were believed to be at lower risk for C. auris outbreaks.
The Orange County Health Department, working with the California Department of Health and the CDC, asked labs to prospectively identify all Candida isolates in urines from LTACHs between September 2018 and February 2019. Normally, labs do not speciate Candida from nonsterile body sites.
Dan Diekema, MD, an epidemiologist and clinical microbiologist at the University of Iowa, Iowa City, who was not involved in the study, told this news organization, “Acute care hospitals really ought to be moving toward doing species identification of Candida from nonsterile sites if they really want to have a better chance of detecting this early.”
The OCHD also screened LTACH and vSNF patients with composite cultures from the axilla-groin or nasal swabs. Screening was undertaken because 5%-10% of colonized patients later develop invasive infections, and 30%-60% die.
The first bloodstream infection was detected in May 2019. Per the report, published online Sept. 7 in Annals of Internal Medicine, “As of 1 January 2020, of 182 patients, 22 (12%) died within 30 days of C. auris identification; 47 (26%) died within 90 days. One of 47 deaths was attributed to C. auris.” Whole-genome sequencing showed that the isolates were all closely related in clade III.
Experts conducted extensive education in infection control at the LTACHs, and communication among the LTACHs and between the long-term facilities and acute care hospitals was improved. As a result, receiving facilities accepting transfers began culturing their newly admitted patients and quickly identified 4 of 99 patients with C. auris who had no known history of colonization. By October 2019, the outbreak was contained in two facilities, down from the nine where C. auris was initially found.
Dr. Diekema noted, “The challenge, of course, for a new emerging MDRO [multidrug-resistant organism] like Candida auris, is that the initial approach, in general, has to be almost passive, when you have not seen the organism. ... Passive surveillance means that you just carefully monitor your clinical cultures, and the first time you detect the MDRO of concern, then you begin doing the point prevalence surveys. ... This [prospective] kind of approach is really good for how we should move forward with both initial detection and containment of MDRO spread.”
Many outbreak studies are confined to a particular institution. Authors of an accompanying editorial commented that this study “underlines the importance of proactive protocols for outbreak investigations and containment measures across the entirety of the health care network serving at-risk patients.”
In her research, Dr. Karmarkar observed that, “some of these facilities don’t have the same infrastructure and infection prevention and control that an acute care hospital might.”
She said in an interview that, “one of the challenges was that people were so focused on COVID that they forgot about the MDROs. ... Some of the things that we recommend to help control Candida auris are also excellent practices for every other organism including COVID care. ... What I appreciated about this investigation is that every facility that we went to was so open to learning, so happy to have us there. They’re very interested in learning about Candida auris and understanding what they could do to control it.”
While recent attention has been on the frightening levels of multidrug resistance in C. auris, Dr. Karmarkar concluded that the “central message in our investigation is that with the right effort, the right approach, and the right team this is an intervenable issue. It’s not inevitable if the attention is focused on it to pick it up early and then try to contain it.”
Dr. Karmarkar reports no relevant financial relationships. Dr. Diekema reports research funding from bioMerieux and consulting fees from Opgen.
A version of this article first appeared on Medscape.com.
Untreatable, drug-resistant fungus found in Texas and Washington, D.C.
The CDC has reported two clusters of Candida auris infections resistant to all antifungal medications in long-term care facilities in 2021. Because these panresistant infections occurred without any exposure to antifungal drugs, the cases are even more worrisome. These clusters are the first time such nosocomial transmission has been detected.
In the District of Columbia, three panresistant isolates were discovered through screening for skin colonization with resistant organisms at a long-term acute care facility (LTAC) that cares for patients who are seriously ill, often on mechanical ventilation.
In Texas, the resistant organisms were found both by screening and in specimens from ill patients at an LTAC and a short-term acute care hospital that share patients. Two were panresistant, and five others were resistant to fluconazole and echinocandins.
These clusters occurred simultaneously and independently of each other; there were no links between the two institutions.
Colonization of skin with C. auris can lead to invasive infections in 5%-10% of affected patients. Routine skin surveillance cultures are not commonly done for Candida, although perirectal cultures for vancomycin-resistant enterococci and nasal swabs for MRSA have been done for years. Some areas, like Los Angeles, have recommended screening for C. auris in high-risk patients – defined as those who were on a ventilator or had a tracheostomy admitted from an LTAC or skilled nursing facility in Los Angeles County, New York, New Jersey, or Illinois.
In the past, about 85% of C. auris isolates in the United States have been resistant to azoles (for example, fluconazole), 33% to amphotericin B, and 1% to echinocandins. Because of generally strong susceptibility, an echinocandin such as micafungin or caspofungin has been the drug of choice for an invasive Candida infection.
C. auris is particularly difficult to deal with for several reasons. First, it can continue to live in the environment, on both dry or moist surfaces, for up to 2 weeks. Outbreaks have occurred both from hand (person-to-person) transmission or via inanimate surfaces that have become contaminated. Equally troublesome is that people become colonized with the yeast indefinitely.
Meghan Lyman, MD, of the fungal diseases branch of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, said in an interview that facilities might be slow in recognizing the problem and in identifying the organism. “We encounter problems in noninvasive specimens, especially urine,” Dr. Lyman added.
“Sometimes ... they consider Candida [to represent] colonization so they will often not speciate it.” She emphasized the need for facilities that care for ventilated patients to consider screening. “Higher priority ... are places in areas where there’s a lot of C. auris transmission or in nearby areas that are likely to get introductions.” Even those that do speciate may have difficulty identifying C. auris.
Further, Dr. Lyman stressed “the importance of antifungal susceptibility testing and testing for resistance. Because that’s also something that’s not widely available at all hospitals and clinical labs ... you can send it to the [CDC’s] antimicrobial resistance lab network” for testing.
COVID-19 has brought particular challenges. Rodney E. Rohde, PhD, MS, professor and chair, clinical lab science program, Texas State University, San Marcos, said in an interview that he is worried about all the steroids and broad-spectrum antibiotics patients receive.
They’re “being given medical interventions, whether it’s ventilators or [extracorporeal membrane oxygenation] or IVs or central lines or catheters for UTIs and you’re creating highways, right for something that may be right there,” said Dr. Rohde, who was not involved in the CDC study. “It’s a perfect storm, not just for C. auris, but I worry about bacterial resistance agents, too, like MRSA and so forth, having kind of a spike in those types of infections with COVID. So, it’s kind of a doubly dangerous time, I think.”
Multiresistant bacteria are a major health problem, causing illnesses in 2.8 million people annually in the United States, and causing about 35,000 deaths.
Dr. Rohde raised another, rarely mentioned concern. “We’re in crisis mode. People are leaving our field more than they ever had before. The medical laboratory is being decimated because people have burned out after these past 14 months. And so I worry just about competent medical laboratory professionals that are on board to deal with these types of other crises that are popping up within hospitals and long-term care facilities. It kind of keeps me awake.”
Dr. Rohde and Dr. Lyman shared their concern that COVID caused a decrease in screening for other infections and drug-resistant organisms. Bare-bones staffing and shortages of personal protective equipment have likely fueled the spread of these infections as well.
In an outbreak of C. auris in a Florida hospital’s COVID unit in 2020, 35 of 67 patients became colonized, and 6 became ill. The epidemiologists investigating thought that contaminated gowns or gloves, computers, and other equipment were likely sources of transmission.
Low pay, especially in nursing homes, is another problem Dr. Rohde mentioned. It’s an additional problem in both acute and long-term care that “some of the lowest-paid people are the environmental services people, and so the turnover is crazy.” Yet, we rely on them to keep everyone safe. He added that, in addition to pay, he “tries to give them the appreciation and the recognition that they really deserve.”
There are a few specific measures that can be taken to protect patients. Dr. Lyman concluded. “The best way is identifying cases and really ensuring good infection control to prevent the spread.” It’s back to basics – limiting broad-spectrum antibiotics and invasive medical devices, and especially good handwashing and thorough cleaning.
Dr. Lyman and Dr. Rohde have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The CDC has reported two clusters of Candida auris infections resistant to all antifungal medications in long-term care facilities in 2021. Because these panresistant infections occurred without any exposure to antifungal drugs, the cases are even more worrisome. These clusters are the first time such nosocomial transmission has been detected.
In the District of Columbia, three panresistant isolates were discovered through screening for skin colonization with resistant organisms at a long-term acute care facility (LTAC) that cares for patients who are seriously ill, often on mechanical ventilation.
In Texas, the resistant organisms were found both by screening and in specimens from ill patients at an LTAC and a short-term acute care hospital that share patients. Two were panresistant, and five others were resistant to fluconazole and echinocandins.
These clusters occurred simultaneously and independently of each other; there were no links between the two institutions.
Colonization of skin with C. auris can lead to invasive infections in 5%-10% of affected patients. Routine skin surveillance cultures are not commonly done for Candida, although perirectal cultures for vancomycin-resistant enterococci and nasal swabs for MRSA have been done for years. Some areas, like Los Angeles, have recommended screening for C. auris in high-risk patients – defined as those who were on a ventilator or had a tracheostomy admitted from an LTAC or skilled nursing facility in Los Angeles County, New York, New Jersey, or Illinois.
In the past, about 85% of C. auris isolates in the United States have been resistant to azoles (for example, fluconazole), 33% to amphotericin B, and 1% to echinocandins. Because of generally strong susceptibility, an echinocandin such as micafungin or caspofungin has been the drug of choice for an invasive Candida infection.
C. auris is particularly difficult to deal with for several reasons. First, it can continue to live in the environment, on both dry or moist surfaces, for up to 2 weeks. Outbreaks have occurred both from hand (person-to-person) transmission or via inanimate surfaces that have become contaminated. Equally troublesome is that people become colonized with the yeast indefinitely.
Meghan Lyman, MD, of the fungal diseases branch of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, said in an interview that facilities might be slow in recognizing the problem and in identifying the organism. “We encounter problems in noninvasive specimens, especially urine,” Dr. Lyman added.
“Sometimes ... they consider Candida [to represent] colonization so they will often not speciate it.” She emphasized the need for facilities that care for ventilated patients to consider screening. “Higher priority ... are places in areas where there’s a lot of C. auris transmission or in nearby areas that are likely to get introductions.” Even those that do speciate may have difficulty identifying C. auris.
Further, Dr. Lyman stressed “the importance of antifungal susceptibility testing and testing for resistance. Because that’s also something that’s not widely available at all hospitals and clinical labs ... you can send it to the [CDC’s] antimicrobial resistance lab network” for testing.
COVID-19 has brought particular challenges. Rodney E. Rohde, PhD, MS, professor and chair, clinical lab science program, Texas State University, San Marcos, said in an interview that he is worried about all the steroids and broad-spectrum antibiotics patients receive.
They’re “being given medical interventions, whether it’s ventilators or [extracorporeal membrane oxygenation] or IVs or central lines or catheters for UTIs and you’re creating highways, right for something that may be right there,” said Dr. Rohde, who was not involved in the CDC study. “It’s a perfect storm, not just for C. auris, but I worry about bacterial resistance agents, too, like MRSA and so forth, having kind of a spike in those types of infections with COVID. So, it’s kind of a doubly dangerous time, I think.”
Multiresistant bacteria are a major health problem, causing illnesses in 2.8 million people annually in the United States, and causing about 35,000 deaths.
Dr. Rohde raised another, rarely mentioned concern. “We’re in crisis mode. People are leaving our field more than they ever had before. The medical laboratory is being decimated because people have burned out after these past 14 months. And so I worry just about competent medical laboratory professionals that are on board to deal with these types of other crises that are popping up within hospitals and long-term care facilities. It kind of keeps me awake.”
Dr. Rohde and Dr. Lyman shared their concern that COVID caused a decrease in screening for other infections and drug-resistant organisms. Bare-bones staffing and shortages of personal protective equipment have likely fueled the spread of these infections as well.
In an outbreak of C. auris in a Florida hospital’s COVID unit in 2020, 35 of 67 patients became colonized, and 6 became ill. The epidemiologists investigating thought that contaminated gowns or gloves, computers, and other equipment were likely sources of transmission.
Low pay, especially in nursing homes, is another problem Dr. Rohde mentioned. It’s an additional problem in both acute and long-term care that “some of the lowest-paid people are the environmental services people, and so the turnover is crazy.” Yet, we rely on them to keep everyone safe. He added that, in addition to pay, he “tries to give them the appreciation and the recognition that they really deserve.”
There are a few specific measures that can be taken to protect patients. Dr. Lyman concluded. “The best way is identifying cases and really ensuring good infection control to prevent the spread.” It’s back to basics – limiting broad-spectrum antibiotics and invasive medical devices, and especially good handwashing and thorough cleaning.
Dr. Lyman and Dr. Rohde have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The CDC has reported two clusters of Candida auris infections resistant to all antifungal medications in long-term care facilities in 2021. Because these panresistant infections occurred without any exposure to antifungal drugs, the cases are even more worrisome. These clusters are the first time such nosocomial transmission has been detected.
In the District of Columbia, three panresistant isolates were discovered through screening for skin colonization with resistant organisms at a long-term acute care facility (LTAC) that cares for patients who are seriously ill, often on mechanical ventilation.
In Texas, the resistant organisms were found both by screening and in specimens from ill patients at an LTAC and a short-term acute care hospital that share patients. Two were panresistant, and five others were resistant to fluconazole and echinocandins.
These clusters occurred simultaneously and independently of each other; there were no links between the two institutions.
Colonization of skin with C. auris can lead to invasive infections in 5%-10% of affected patients. Routine skin surveillance cultures are not commonly done for Candida, although perirectal cultures for vancomycin-resistant enterococci and nasal swabs for MRSA have been done for years. Some areas, like Los Angeles, have recommended screening for C. auris in high-risk patients – defined as those who were on a ventilator or had a tracheostomy admitted from an LTAC or skilled nursing facility in Los Angeles County, New York, New Jersey, or Illinois.
In the past, about 85% of C. auris isolates in the United States have been resistant to azoles (for example, fluconazole), 33% to amphotericin B, and 1% to echinocandins. Because of generally strong susceptibility, an echinocandin such as micafungin or caspofungin has been the drug of choice for an invasive Candida infection.
C. auris is particularly difficult to deal with for several reasons. First, it can continue to live in the environment, on both dry or moist surfaces, for up to 2 weeks. Outbreaks have occurred both from hand (person-to-person) transmission or via inanimate surfaces that have become contaminated. Equally troublesome is that people become colonized with the yeast indefinitely.
Meghan Lyman, MD, of the fungal diseases branch of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, said in an interview that facilities might be slow in recognizing the problem and in identifying the organism. “We encounter problems in noninvasive specimens, especially urine,” Dr. Lyman added.
“Sometimes ... they consider Candida [to represent] colonization so they will often not speciate it.” She emphasized the need for facilities that care for ventilated patients to consider screening. “Higher priority ... are places in areas where there’s a lot of C. auris transmission or in nearby areas that are likely to get introductions.” Even those that do speciate may have difficulty identifying C. auris.
Further, Dr. Lyman stressed “the importance of antifungal susceptibility testing and testing for resistance. Because that’s also something that’s not widely available at all hospitals and clinical labs ... you can send it to the [CDC’s] antimicrobial resistance lab network” for testing.
COVID-19 has brought particular challenges. Rodney E. Rohde, PhD, MS, professor and chair, clinical lab science program, Texas State University, San Marcos, said in an interview that he is worried about all the steroids and broad-spectrum antibiotics patients receive.
They’re “being given medical interventions, whether it’s ventilators or [extracorporeal membrane oxygenation] or IVs or central lines or catheters for UTIs and you’re creating highways, right for something that may be right there,” said Dr. Rohde, who was not involved in the CDC study. “It’s a perfect storm, not just for C. auris, but I worry about bacterial resistance agents, too, like MRSA and so forth, having kind of a spike in those types of infections with COVID. So, it’s kind of a doubly dangerous time, I think.”
Multiresistant bacteria are a major health problem, causing illnesses in 2.8 million people annually in the United States, and causing about 35,000 deaths.
Dr. Rohde raised another, rarely mentioned concern. “We’re in crisis mode. People are leaving our field more than they ever had before. The medical laboratory is being decimated because people have burned out after these past 14 months. And so I worry just about competent medical laboratory professionals that are on board to deal with these types of other crises that are popping up within hospitals and long-term care facilities. It kind of keeps me awake.”
Dr. Rohde and Dr. Lyman shared their concern that COVID caused a decrease in screening for other infections and drug-resistant organisms. Bare-bones staffing and shortages of personal protective equipment have likely fueled the spread of these infections as well.
In an outbreak of C. auris in a Florida hospital’s COVID unit in 2020, 35 of 67 patients became colonized, and 6 became ill. The epidemiologists investigating thought that contaminated gowns or gloves, computers, and other equipment were likely sources of transmission.
Low pay, especially in nursing homes, is another problem Dr. Rohde mentioned. It’s an additional problem in both acute and long-term care that “some of the lowest-paid people are the environmental services people, and so the turnover is crazy.” Yet, we rely on them to keep everyone safe. He added that, in addition to pay, he “tries to give them the appreciation and the recognition that they really deserve.”
There are a few specific measures that can be taken to protect patients. Dr. Lyman concluded. “The best way is identifying cases and really ensuring good infection control to prevent the spread.” It’s back to basics – limiting broad-spectrum antibiotics and invasive medical devices, and especially good handwashing and thorough cleaning.
Dr. Lyman and Dr. Rohde have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fungemia, other fungal infections associated with s. Boulardii probiotics
Life-threatening fungal bloodstream infections associated with probiotic supplements have been reported in the journal Emerging Infectious Diseases by a group of researchers in Finland. While individuals consume these mixtures of bacteria and yeast in the hopes of “balancing” their microbiome or preventing diarrhea from antibiotic use, some died or developed yeast infections requiring prolonged antifungal treatment.
In a retrospective registry study at five university hospitals in Finland, the researchers found 46 patients between 2009 and 2018 with Saccharomyces sp. of yeast in their blood associated with ingesting probiotics. At least 20 (43%) had been using S. cerevisiae var. boulardii as a probiotic, with the organism then causing a bloodstream infection. Overall, 37% of the fungemic patients died.
Juha Rannikko, MD, lead author and infectious disease faculty member at Tampere University Hospital, Finland, said in an interview that there were an additional 1,153 nonblood isolates of Saccharomyces. He expressed surprise at the large number of nonblood isolates, saying: “If extrapolated ... it is about 10 nonblood Saccharomyces boulardii–associated findings for each Saccharomyces boulardii–associated fungemia.”
Most of the yeast infections (59%) occurred in patients with underlying gastrointestinal disease. Prior studies suggested that patients receiving enteral nutrition might become ill from translocation of the yeast from the inflamed GI tract.
If there were positive cultures for yeast from sites other than blood, physicians changed the antibiotics in 38% of patients.
Conventional wisdom has been that patients receiving broad-spectrum antibiotics should also receive an S. cerevisiae var. boulardii probiotic to prevent Clostridioides difficile infections. Dr. Rannikko and coauthors questioned this, noting results of such studies of prophylaxis were equivocal. “There is not enough evidence that clinicians should use Saccharomyces (probiotics) alongside antibiotics,” Dr. Rannikko concluded.
Laila Woc-Colburn, MD, associate professor at the Emory University School of Medicine, Atlanta, told this news organization that although the study was well done and was published in Emerging Infectious Diseases, the findings do not represent an “emerging” infectious disease. “We have known this for a while – since the 1990s,” she said. Warnings about probiotics are part of the standard advice Dr. Woc-Colburn gives transplant, chemotherapy, or immunosuppressed patients. “Don’t do these probiotics, because this is what’s going to happen,” she tells them. And she told this news organization, “If I see this in the blood, the first question I’m going to ask my patients is ... what probiotic were you drinking?”
Dr. Woc-Colburn said the Finnish researchers “did their due diligence” when conducting the study. “They were clear on their limitations. And they came out to the same conclusion as the 2005 Muñoz paper: That if we have some GI disruption, we should not be taking probiotics.”
She acknowledged that the Emerging Infectious Diseases study adds a substantial number of cases to those previously reported in the literature and confirms previous findings and recommendations to avoid probiotics if immunosuppressed or acutely ill.
Dr. Rannikko and Dr. Woc-Coburn have reported no relevant financial relationships. Dr. Rannikko has received a lecture fee from Novo Nordisk and a virtual congress attendance fee from Roche.
A version of this article first appeared on Medscape.com.
Life-threatening fungal bloodstream infections associated with probiotic supplements have been reported in the journal Emerging Infectious Diseases by a group of researchers in Finland. While individuals consume these mixtures of bacteria and yeast in the hopes of “balancing” their microbiome or preventing diarrhea from antibiotic use, some died or developed yeast infections requiring prolonged antifungal treatment.
In a retrospective registry study at five university hospitals in Finland, the researchers found 46 patients between 2009 and 2018 with Saccharomyces sp. of yeast in their blood associated with ingesting probiotics. At least 20 (43%) had been using S. cerevisiae var. boulardii as a probiotic, with the organism then causing a bloodstream infection. Overall, 37% of the fungemic patients died.
Juha Rannikko, MD, lead author and infectious disease faculty member at Tampere University Hospital, Finland, said in an interview that there were an additional 1,153 nonblood isolates of Saccharomyces. He expressed surprise at the large number of nonblood isolates, saying: “If extrapolated ... it is about 10 nonblood Saccharomyces boulardii–associated findings for each Saccharomyces boulardii–associated fungemia.”
Most of the yeast infections (59%) occurred in patients with underlying gastrointestinal disease. Prior studies suggested that patients receiving enteral nutrition might become ill from translocation of the yeast from the inflamed GI tract.
If there were positive cultures for yeast from sites other than blood, physicians changed the antibiotics in 38% of patients.
Conventional wisdom has been that patients receiving broad-spectrum antibiotics should also receive an S. cerevisiae var. boulardii probiotic to prevent Clostridioides difficile infections. Dr. Rannikko and coauthors questioned this, noting results of such studies of prophylaxis were equivocal. “There is not enough evidence that clinicians should use Saccharomyces (probiotics) alongside antibiotics,” Dr. Rannikko concluded.
Laila Woc-Colburn, MD, associate professor at the Emory University School of Medicine, Atlanta, told this news organization that although the study was well done and was published in Emerging Infectious Diseases, the findings do not represent an “emerging” infectious disease. “We have known this for a while – since the 1990s,” she said. Warnings about probiotics are part of the standard advice Dr. Woc-Colburn gives transplant, chemotherapy, or immunosuppressed patients. “Don’t do these probiotics, because this is what’s going to happen,” she tells them. And she told this news organization, “If I see this in the blood, the first question I’m going to ask my patients is ... what probiotic were you drinking?”
Dr. Woc-Colburn said the Finnish researchers “did their due diligence” when conducting the study. “They were clear on their limitations. And they came out to the same conclusion as the 2005 Muñoz paper: That if we have some GI disruption, we should not be taking probiotics.”
She acknowledged that the Emerging Infectious Diseases study adds a substantial number of cases to those previously reported in the literature and confirms previous findings and recommendations to avoid probiotics if immunosuppressed or acutely ill.
Dr. Rannikko and Dr. Woc-Coburn have reported no relevant financial relationships. Dr. Rannikko has received a lecture fee from Novo Nordisk and a virtual congress attendance fee from Roche.
A version of this article first appeared on Medscape.com.
Life-threatening fungal bloodstream infections associated with probiotic supplements have been reported in the journal Emerging Infectious Diseases by a group of researchers in Finland. While individuals consume these mixtures of bacteria and yeast in the hopes of “balancing” their microbiome or preventing diarrhea from antibiotic use, some died or developed yeast infections requiring prolonged antifungal treatment.
In a retrospective registry study at five university hospitals in Finland, the researchers found 46 patients between 2009 and 2018 with Saccharomyces sp. of yeast in their blood associated with ingesting probiotics. At least 20 (43%) had been using S. cerevisiae var. boulardii as a probiotic, with the organism then causing a bloodstream infection. Overall, 37% of the fungemic patients died.
Juha Rannikko, MD, lead author and infectious disease faculty member at Tampere University Hospital, Finland, said in an interview that there were an additional 1,153 nonblood isolates of Saccharomyces. He expressed surprise at the large number of nonblood isolates, saying: “If extrapolated ... it is about 10 nonblood Saccharomyces boulardii–associated findings for each Saccharomyces boulardii–associated fungemia.”
Most of the yeast infections (59%) occurred in patients with underlying gastrointestinal disease. Prior studies suggested that patients receiving enteral nutrition might become ill from translocation of the yeast from the inflamed GI tract.
If there were positive cultures for yeast from sites other than blood, physicians changed the antibiotics in 38% of patients.
Conventional wisdom has been that patients receiving broad-spectrum antibiotics should also receive an S. cerevisiae var. boulardii probiotic to prevent Clostridioides difficile infections. Dr. Rannikko and coauthors questioned this, noting results of such studies of prophylaxis were equivocal. “There is not enough evidence that clinicians should use Saccharomyces (probiotics) alongside antibiotics,” Dr. Rannikko concluded.
Laila Woc-Colburn, MD, associate professor at the Emory University School of Medicine, Atlanta, told this news organization that although the study was well done and was published in Emerging Infectious Diseases, the findings do not represent an “emerging” infectious disease. “We have known this for a while – since the 1990s,” she said. Warnings about probiotics are part of the standard advice Dr. Woc-Colburn gives transplant, chemotherapy, or immunosuppressed patients. “Don’t do these probiotics, because this is what’s going to happen,” she tells them. And she told this news organization, “If I see this in the blood, the first question I’m going to ask my patients is ... what probiotic were you drinking?”
Dr. Woc-Colburn said the Finnish researchers “did their due diligence” when conducting the study. “They were clear on their limitations. And they came out to the same conclusion as the 2005 Muñoz paper: That if we have some GI disruption, we should not be taking probiotics.”
She acknowledged that the Emerging Infectious Diseases study adds a substantial number of cases to those previously reported in the literature and confirms previous findings and recommendations to avoid probiotics if immunosuppressed or acutely ill.
Dr. Rannikko and Dr. Woc-Coburn have reported no relevant financial relationships. Dr. Rannikko has received a lecture fee from Novo Nordisk and a virtual congress attendance fee from Roche.
A version of this article first appeared on Medscape.com.
FDA approves ibrexafungerp for vaginal yeast infection
Ibrexafungerp is the first drug approved in a new antifungal class for vulvovaginal candidiasis (VVC) in more than 20 years, the drug’s manufacturer Scynexis said in a press release. It becomes the first and only nonazole treatment for vaginal yeast infections.
The biotechnology company said approval came after positive results from two phase 3 studies in which oral ibrexafungerp demonstrated efficacy and tolerability. The most common reactions observed in clinical trials were diarrhea, nausea, abdominal pain, dizziness, and vomiting.
There are few other treatments for vaginal yeast infections, which is the second most common cause of vaginitis. Those previously approved agents include several topical azole antifungals and oral fluconazole (Diflucan), which, Scynexis said, is the only other orally administered antifungal approved for the treatment of VVC in the United States and has accounted for over more than 90% of prescriptions written for the condition each year.
However, the company noted, oral fluconazole reports a 55% therapeutic cure rate on its label, which now also includes warnings of potential fetal harm, demonstrating the need for new oral options.
The new drug may not fill that need for pregnant women, however, as the company noted that ibrexafungerp should not be used during pregnancy, and administration during pregnancy “may cause fetal harm based on animal studies.”
Because of possible teratogenic effects, the company advised clinicians to verify pregnancy status in females of reproductive potential before prescribing ibrexafungerp and advises effective contraception during treatment.
VVC can come with substantial morbidity, including genital pain, itching and burning, reduced sexual pleasure, and psychological distress.
David Angulo, MD, chief medical officer for Scynexis, said in a statement the tablets brings new benefits.
Dr. Angulo said the drug “has a differentiated fungicidal mechanism of action that kills a broad range of Candida species, including azole-resistant strains. We are working on completing our CANDLE study investigating ibrexafungerp for the prevention of recurrent VVC and expect we will be submitting a supplemental NDA [new drug application] in the first half of 2022.”
Scynexis said it partnered with Amplity Health, a Pennsylvania-based pharmaceutical company, to support U.S. marketing of the drug. The commercial launch will follow the approval.
Ibrexafungerp was granted approval through both the FDA’s Qualified Infectious Disease Product and Fast Track designations. It is expected to be marketed exclusively in the United States for 10 years.
A version of this article first appeared on Medscape.com.
Ibrexafungerp is the first drug approved in a new antifungal class for vulvovaginal candidiasis (VVC) in more than 20 years, the drug’s manufacturer Scynexis said in a press release. It becomes the first and only nonazole treatment for vaginal yeast infections.
The biotechnology company said approval came after positive results from two phase 3 studies in which oral ibrexafungerp demonstrated efficacy and tolerability. The most common reactions observed in clinical trials were diarrhea, nausea, abdominal pain, dizziness, and vomiting.
There are few other treatments for vaginal yeast infections, which is the second most common cause of vaginitis. Those previously approved agents include several topical azole antifungals and oral fluconazole (Diflucan), which, Scynexis said, is the only other orally administered antifungal approved for the treatment of VVC in the United States and has accounted for over more than 90% of prescriptions written for the condition each year.
However, the company noted, oral fluconazole reports a 55% therapeutic cure rate on its label, which now also includes warnings of potential fetal harm, demonstrating the need for new oral options.
The new drug may not fill that need for pregnant women, however, as the company noted that ibrexafungerp should not be used during pregnancy, and administration during pregnancy “may cause fetal harm based on animal studies.”
Because of possible teratogenic effects, the company advised clinicians to verify pregnancy status in females of reproductive potential before prescribing ibrexafungerp and advises effective contraception during treatment.
VVC can come with substantial morbidity, including genital pain, itching and burning, reduced sexual pleasure, and psychological distress.
David Angulo, MD, chief medical officer for Scynexis, said in a statement the tablets brings new benefits.
Dr. Angulo said the drug “has a differentiated fungicidal mechanism of action that kills a broad range of Candida species, including azole-resistant strains. We are working on completing our CANDLE study investigating ibrexafungerp for the prevention of recurrent VVC and expect we will be submitting a supplemental NDA [new drug application] in the first half of 2022.”
Scynexis said it partnered with Amplity Health, a Pennsylvania-based pharmaceutical company, to support U.S. marketing of the drug. The commercial launch will follow the approval.
Ibrexafungerp was granted approval through both the FDA’s Qualified Infectious Disease Product and Fast Track designations. It is expected to be marketed exclusively in the United States for 10 years.
A version of this article first appeared on Medscape.com.
Ibrexafungerp is the first drug approved in a new antifungal class for vulvovaginal candidiasis (VVC) in more than 20 years, the drug’s manufacturer Scynexis said in a press release. It becomes the first and only nonazole treatment for vaginal yeast infections.
The biotechnology company said approval came after positive results from two phase 3 studies in which oral ibrexafungerp demonstrated efficacy and tolerability. The most common reactions observed in clinical trials were diarrhea, nausea, abdominal pain, dizziness, and vomiting.
There are few other treatments for vaginal yeast infections, which is the second most common cause of vaginitis. Those previously approved agents include several topical azole antifungals and oral fluconazole (Diflucan), which, Scynexis said, is the only other orally administered antifungal approved for the treatment of VVC in the United States and has accounted for over more than 90% of prescriptions written for the condition each year.
However, the company noted, oral fluconazole reports a 55% therapeutic cure rate on its label, which now also includes warnings of potential fetal harm, demonstrating the need for new oral options.
The new drug may not fill that need for pregnant women, however, as the company noted that ibrexafungerp should not be used during pregnancy, and administration during pregnancy “may cause fetal harm based on animal studies.”
Because of possible teratogenic effects, the company advised clinicians to verify pregnancy status in females of reproductive potential before prescribing ibrexafungerp and advises effective contraception during treatment.
VVC can come with substantial morbidity, including genital pain, itching and burning, reduced sexual pleasure, and psychological distress.
David Angulo, MD, chief medical officer for Scynexis, said in a statement the tablets brings new benefits.
Dr. Angulo said the drug “has a differentiated fungicidal mechanism of action that kills a broad range of Candida species, including azole-resistant strains. We are working on completing our CANDLE study investigating ibrexafungerp for the prevention of recurrent VVC and expect we will be submitting a supplemental NDA [new drug application] in the first half of 2022.”
Scynexis said it partnered with Amplity Health, a Pennsylvania-based pharmaceutical company, to support U.S. marketing of the drug. The commercial launch will follow the approval.
Ibrexafungerp was granted approval through both the FDA’s Qualified Infectious Disease Product and Fast Track designations. It is expected to be marketed exclusively in the United States for 10 years.
A version of this article first appeared on Medscape.com.
Infective endocarditis from IV drug use tied to hemorrhagic stroke
One consequence of the ongoing opioid epidemic in the United States may be an increase in the number of hemorrhagic strokes caused by infective endocarditis, research suggests.
Intravenous drug use (IVDU) can cause this bacterial infection of the heart. In a single-center study, infective endocarditis was associated with an increase in the risk for hemorrhagic stroke as well as an increase in health care use and costs.
“Patients who are known IV drug users who have endocarditis should be more carefully screened for symptoms of cardiovascular disease,” Shahid M. Nimjee, MD, PhD, associate professor of neurosurgery and surgical director of the Comprehensive Stroke Center at the Ohio State University Wexner Medical Center, Columbus, said in a press release.
The findings were presented at the International Stroke Conference sponsored by the American Heart Association.
In the United States, 47,000 patients are treated in the hospital for endocarditis each year. Endocarditis increases the risk for stroke, which can entail significant morbidity and mortality, the authors noted.
IVDU is a risk factor for endocarditis. In the context of the opioid epidemic, Dr. Nimjee and colleagues sought to compare the risk for stroke among patients with endocarditis from IVDU with the risk among patients with endocarditis from other causes.
They retrospectively studied patients who had undergone treatment for infective endocarditis at Wexner Medical Center between Jan. 1, 2014, and July 1, 2018. They examined patients’ concomitant intravenous drug abuse and evaluated demographics, risk factors, and associated costs.
Dramatic increase
In all, 351 patients met the study’s inclusion criteria, and 170 (48%) had a history of IVDU-associated endocarditis. The incidence of patients with IVDU-associated endocarditis increased 630% from 2014 to 2018.
The prevalence of overall intracranial hemorrhage was increased among patients with IVDU, compared with those without (25.9% vs. 13.9%; P = .005).
This increase in prevalence included increases in intraparenchymal hemorrhage (12.4% vs. 5.1%; P = .012), subarachnoid hemorrhage (17.6% vs. 4.4%; P = .0001), and cerebral microbleeds (14.1% vs. 7.2%; P = .022).
IVDU also was associated with an increase in prevalence of infectious intracranial aneurysm (10.6% vs. 1.8%; P = .0001) and brain abscess (4.7% vs. 1.1%; P = .025).
Compared with patients with endocarditis from other causes, significantly higher numbers of patients with IVDU-associated endocarditis were homeless (5.9% vs. 1.1%; P = .014), uninsured (10.0% vs. 2.8%; P = .005), and unemployed (75.9% vs. 31.7%; P = .0001).
Medical costs were more than twice as high among patients with endocarditis from IVDU than among those with endocarditis from other causes. The difference in health care costs during admission per patient was more than $100,000.
“The wider societal impact of the opioid epidemic is not well understood,” Dr. Nimjee said in the press release. “Our research suggests that the impact of the opioid epidemic is far-reaching and contributes to increased costs in the criminal justice, health care systems, and the workplace. The increased costs can be particularly substantial for stroke care.”
Nationwide data desirable
“Past publications from the U.S. have shown an increase in incidence of IVDU-related endocarditis, and the current publication emphasizes this worrying trend,” Manuel Bolognese, MD, head of the stroke center at the Lucerne (Switzerland) Cantonal Hospital, said in an interview. “The higher degree of hemorrhagic strokes and brain abscesses as further complications is alarming as well and shows that IVDU-related endocarditis is becoming a more and more relevant medical problem in the U.S., with high morbidity and mortality.”
The study period is long enough to show a clear trend of increasing incidence of IVDU-related endocarditis, Dr. Bolognese said. The study’s biggest weaknesses are its retrospective design and restriction to a single center.
“Without knowing the prevalence of drug abuse and the socioeconomical situation in Columbus, it is difficult to generalize these findings to other regions in the U.S.A. or even abroad,” he said.
Also, the abstract does not provide some essential information, said Dr. Bolognese. It would be important to know which valve was affected in each patient, which bacteria were identified, whether patients also used nonopioid drugs, and what each patient’s immune status was.
A lack of sterile material such as syringes could explain the apparent association between IVDU-associated endocarditis and low socioeconomic status, said Dr. Bolognese. Delayed presentation to medical institutions because of a lack of insurance could have led to a more complicated course.
“It would be interesting to see numbers from a broader spectrum in a nationwide registry,” said Dr. Bolognese. “It might be worth studying interventions to improve the hygienic aspects (like supply of sterile material, especially in the most vulnerable groups, like homeless people) or to provide easier access to emergency health care despite lack of insurance, which could decrease the incidence of IVDU.”
Dr. Nimjee and Dr. Bolognese disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One consequence of the ongoing opioid epidemic in the United States may be an increase in the number of hemorrhagic strokes caused by infective endocarditis, research suggests.
Intravenous drug use (IVDU) can cause this bacterial infection of the heart. In a single-center study, infective endocarditis was associated with an increase in the risk for hemorrhagic stroke as well as an increase in health care use and costs.
“Patients who are known IV drug users who have endocarditis should be more carefully screened for symptoms of cardiovascular disease,” Shahid M. Nimjee, MD, PhD, associate professor of neurosurgery and surgical director of the Comprehensive Stroke Center at the Ohio State University Wexner Medical Center, Columbus, said in a press release.
The findings were presented at the International Stroke Conference sponsored by the American Heart Association.
In the United States, 47,000 patients are treated in the hospital for endocarditis each year. Endocarditis increases the risk for stroke, which can entail significant morbidity and mortality, the authors noted.
IVDU is a risk factor for endocarditis. In the context of the opioid epidemic, Dr. Nimjee and colleagues sought to compare the risk for stroke among patients with endocarditis from IVDU with the risk among patients with endocarditis from other causes.
They retrospectively studied patients who had undergone treatment for infective endocarditis at Wexner Medical Center between Jan. 1, 2014, and July 1, 2018. They examined patients’ concomitant intravenous drug abuse and evaluated demographics, risk factors, and associated costs.
Dramatic increase
In all, 351 patients met the study’s inclusion criteria, and 170 (48%) had a history of IVDU-associated endocarditis. The incidence of patients with IVDU-associated endocarditis increased 630% from 2014 to 2018.
The prevalence of overall intracranial hemorrhage was increased among patients with IVDU, compared with those without (25.9% vs. 13.9%; P = .005).
This increase in prevalence included increases in intraparenchymal hemorrhage (12.4% vs. 5.1%; P = .012), subarachnoid hemorrhage (17.6% vs. 4.4%; P = .0001), and cerebral microbleeds (14.1% vs. 7.2%; P = .022).
IVDU also was associated with an increase in prevalence of infectious intracranial aneurysm (10.6% vs. 1.8%; P = .0001) and brain abscess (4.7% vs. 1.1%; P = .025).
Compared with patients with endocarditis from other causes, significantly higher numbers of patients with IVDU-associated endocarditis were homeless (5.9% vs. 1.1%; P = .014), uninsured (10.0% vs. 2.8%; P = .005), and unemployed (75.9% vs. 31.7%; P = .0001).
Medical costs were more than twice as high among patients with endocarditis from IVDU than among those with endocarditis from other causes. The difference in health care costs during admission per patient was more than $100,000.
“The wider societal impact of the opioid epidemic is not well understood,” Dr. Nimjee said in the press release. “Our research suggests that the impact of the opioid epidemic is far-reaching and contributes to increased costs in the criminal justice, health care systems, and the workplace. The increased costs can be particularly substantial for stroke care.”
Nationwide data desirable
“Past publications from the U.S. have shown an increase in incidence of IVDU-related endocarditis, and the current publication emphasizes this worrying trend,” Manuel Bolognese, MD, head of the stroke center at the Lucerne (Switzerland) Cantonal Hospital, said in an interview. “The higher degree of hemorrhagic strokes and brain abscesses as further complications is alarming as well and shows that IVDU-related endocarditis is becoming a more and more relevant medical problem in the U.S., with high morbidity and mortality.”
The study period is long enough to show a clear trend of increasing incidence of IVDU-related endocarditis, Dr. Bolognese said. The study’s biggest weaknesses are its retrospective design and restriction to a single center.
“Without knowing the prevalence of drug abuse and the socioeconomical situation in Columbus, it is difficult to generalize these findings to other regions in the U.S.A. or even abroad,” he said.
Also, the abstract does not provide some essential information, said Dr. Bolognese. It would be important to know which valve was affected in each patient, which bacteria were identified, whether patients also used nonopioid drugs, and what each patient’s immune status was.
A lack of sterile material such as syringes could explain the apparent association between IVDU-associated endocarditis and low socioeconomic status, said Dr. Bolognese. Delayed presentation to medical institutions because of a lack of insurance could have led to a more complicated course.
“It would be interesting to see numbers from a broader spectrum in a nationwide registry,” said Dr. Bolognese. “It might be worth studying interventions to improve the hygienic aspects (like supply of sterile material, especially in the most vulnerable groups, like homeless people) or to provide easier access to emergency health care despite lack of insurance, which could decrease the incidence of IVDU.”
Dr. Nimjee and Dr. Bolognese disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One consequence of the ongoing opioid epidemic in the United States may be an increase in the number of hemorrhagic strokes caused by infective endocarditis, research suggests.
Intravenous drug use (IVDU) can cause this bacterial infection of the heart. In a single-center study, infective endocarditis was associated with an increase in the risk for hemorrhagic stroke as well as an increase in health care use and costs.
“Patients who are known IV drug users who have endocarditis should be more carefully screened for symptoms of cardiovascular disease,” Shahid M. Nimjee, MD, PhD, associate professor of neurosurgery and surgical director of the Comprehensive Stroke Center at the Ohio State University Wexner Medical Center, Columbus, said in a press release.
The findings were presented at the International Stroke Conference sponsored by the American Heart Association.
In the United States, 47,000 patients are treated in the hospital for endocarditis each year. Endocarditis increases the risk for stroke, which can entail significant morbidity and mortality, the authors noted.
IVDU is a risk factor for endocarditis. In the context of the opioid epidemic, Dr. Nimjee and colleagues sought to compare the risk for stroke among patients with endocarditis from IVDU with the risk among patients with endocarditis from other causes.
They retrospectively studied patients who had undergone treatment for infective endocarditis at Wexner Medical Center between Jan. 1, 2014, and July 1, 2018. They examined patients’ concomitant intravenous drug abuse and evaluated demographics, risk factors, and associated costs.
Dramatic increase
In all, 351 patients met the study’s inclusion criteria, and 170 (48%) had a history of IVDU-associated endocarditis. The incidence of patients with IVDU-associated endocarditis increased 630% from 2014 to 2018.
The prevalence of overall intracranial hemorrhage was increased among patients with IVDU, compared with those without (25.9% vs. 13.9%; P = .005).
This increase in prevalence included increases in intraparenchymal hemorrhage (12.4% vs. 5.1%; P = .012), subarachnoid hemorrhage (17.6% vs. 4.4%; P = .0001), and cerebral microbleeds (14.1% vs. 7.2%; P = .022).
IVDU also was associated with an increase in prevalence of infectious intracranial aneurysm (10.6% vs. 1.8%; P = .0001) and brain abscess (4.7% vs. 1.1%; P = .025).
Compared with patients with endocarditis from other causes, significantly higher numbers of patients with IVDU-associated endocarditis were homeless (5.9% vs. 1.1%; P = .014), uninsured (10.0% vs. 2.8%; P = .005), and unemployed (75.9% vs. 31.7%; P = .0001).
Medical costs were more than twice as high among patients with endocarditis from IVDU than among those with endocarditis from other causes. The difference in health care costs during admission per patient was more than $100,000.
“The wider societal impact of the opioid epidemic is not well understood,” Dr. Nimjee said in the press release. “Our research suggests that the impact of the opioid epidemic is far-reaching and contributes to increased costs in the criminal justice, health care systems, and the workplace. The increased costs can be particularly substantial for stroke care.”
Nationwide data desirable
“Past publications from the U.S. have shown an increase in incidence of IVDU-related endocarditis, and the current publication emphasizes this worrying trend,” Manuel Bolognese, MD, head of the stroke center at the Lucerne (Switzerland) Cantonal Hospital, said in an interview. “The higher degree of hemorrhagic strokes and brain abscesses as further complications is alarming as well and shows that IVDU-related endocarditis is becoming a more and more relevant medical problem in the U.S., with high morbidity and mortality.”
The study period is long enough to show a clear trend of increasing incidence of IVDU-related endocarditis, Dr. Bolognese said. The study’s biggest weaknesses are its retrospective design and restriction to a single center.
“Without knowing the prevalence of drug abuse and the socioeconomical situation in Columbus, it is difficult to generalize these findings to other regions in the U.S.A. or even abroad,” he said.
Also, the abstract does not provide some essential information, said Dr. Bolognese. It would be important to know which valve was affected in each patient, which bacteria were identified, whether patients also used nonopioid drugs, and what each patient’s immune status was.
A lack of sterile material such as syringes could explain the apparent association between IVDU-associated endocarditis and low socioeconomic status, said Dr. Bolognese. Delayed presentation to medical institutions because of a lack of insurance could have led to a more complicated course.
“It would be interesting to see numbers from a broader spectrum in a nationwide registry,” said Dr. Bolognese. “It might be worth studying interventions to improve the hygienic aspects (like supply of sterile material, especially in the most vulnerable groups, like homeless people) or to provide easier access to emergency health care despite lack of insurance, which could decrease the incidence of IVDU.”
Dr. Nimjee and Dr. Bolognese disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Posaconazole prophylaxis was effective in children with ALL undergoing chemotherapy
Targeted prophylaxis with posaconazole was more effective than fluconazole in children with acute lymphoblastic leukemia who were undergoing induction chemotherapy in order to prevent invasive fungal infection, according to a study by Tian Zhang of Xidian University, Xi’an, China, and colleagues.
The researchers performed a single-center, retrospective cohort study of 155 patients with newly diagnosed acute lymphoblastic leukemia, comparing invasive fungal infections in those who received no prophylaxis (60 patients), posaconazole prophylaxis (70), or fluconazole prophylaxis (55) during induction therapy, according to a report published in the Journal of Microbiology, Immunology and Infection.
Proven and probable invasive fungal infections occurred during the induction phase in 45% in the no-prophylaxis group, in 18% of the posaconazole group and in 72% of the fluconazole group. Posaconazole prophylaxis reduced the odds of invasive fungal infections by greater than 60%, prolonged infection-free survival significantly, and did not increase the risk of hepatotoxicity.
In addition, the researchers found that the combination of age at diagnosis, clinically documented bacterial infection in the first 15 days of induction therapy, and absolute neutrophil count curve enabled significant prediction of the susceptibility to infections after receiving posaconazole prophylaxis.
“In general, these findings may serve as a basis for developing screening protocols to identify children who are at high risk for infection despite posaconazole prophylaxis so that early intervention can be initiated to mitigate fungal infections,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Zhang T et al. J Microbiol Immunol Infect. 2020 Aug 1. doi: 10.1016/j.jmii.2020.07.008.
Targeted prophylaxis with posaconazole was more effective than fluconazole in children with acute lymphoblastic leukemia who were undergoing induction chemotherapy in order to prevent invasive fungal infection, according to a study by Tian Zhang of Xidian University, Xi’an, China, and colleagues.
The researchers performed a single-center, retrospective cohort study of 155 patients with newly diagnosed acute lymphoblastic leukemia, comparing invasive fungal infections in those who received no prophylaxis (60 patients), posaconazole prophylaxis (70), or fluconazole prophylaxis (55) during induction therapy, according to a report published in the Journal of Microbiology, Immunology and Infection.
Proven and probable invasive fungal infections occurred during the induction phase in 45% in the no-prophylaxis group, in 18% of the posaconazole group and in 72% of the fluconazole group. Posaconazole prophylaxis reduced the odds of invasive fungal infections by greater than 60%, prolonged infection-free survival significantly, and did not increase the risk of hepatotoxicity.
In addition, the researchers found that the combination of age at diagnosis, clinically documented bacterial infection in the first 15 days of induction therapy, and absolute neutrophil count curve enabled significant prediction of the susceptibility to infections after receiving posaconazole prophylaxis.
“In general, these findings may serve as a basis for developing screening protocols to identify children who are at high risk for infection despite posaconazole prophylaxis so that early intervention can be initiated to mitigate fungal infections,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Zhang T et al. J Microbiol Immunol Infect. 2020 Aug 1. doi: 10.1016/j.jmii.2020.07.008.
Targeted prophylaxis with posaconazole was more effective than fluconazole in children with acute lymphoblastic leukemia who were undergoing induction chemotherapy in order to prevent invasive fungal infection, according to a study by Tian Zhang of Xidian University, Xi’an, China, and colleagues.
The researchers performed a single-center, retrospective cohort study of 155 patients with newly diagnosed acute lymphoblastic leukemia, comparing invasive fungal infections in those who received no prophylaxis (60 patients), posaconazole prophylaxis (70), or fluconazole prophylaxis (55) during induction therapy, according to a report published in the Journal of Microbiology, Immunology and Infection.
Proven and probable invasive fungal infections occurred during the induction phase in 45% in the no-prophylaxis group, in 18% of the posaconazole group and in 72% of the fluconazole group. Posaconazole prophylaxis reduced the odds of invasive fungal infections by greater than 60%, prolonged infection-free survival significantly, and did not increase the risk of hepatotoxicity.
In addition, the researchers found that the combination of age at diagnosis, clinically documented bacterial infection in the first 15 days of induction therapy, and absolute neutrophil count curve enabled significant prediction of the susceptibility to infections after receiving posaconazole prophylaxis.
“In general, these findings may serve as a basis for developing screening protocols to identify children who are at high risk for infection despite posaconazole prophylaxis so that early intervention can be initiated to mitigate fungal infections,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Zhang T et al. J Microbiol Immunol Infect. 2020 Aug 1. doi: 10.1016/j.jmii.2020.07.008.
FROM THE JOURNAL OF MICROBIOLOGY, IMMUNOLOGY AND INFECTION
ID consult for Candida bloodstream infections can reduce mortality risk
findings from a large retrospective study suggest.
Mortality attributable to Candida bloodstream infection ranges between 15% and 47%, and delay in initiation of appropriate treatment has been associated with increased mortality. Previous small studies showed that ID consultation has conferred benefits to patients with Candida bloodstream infections. Carlos Mejia-Chew, MD, and colleagues from Washington University, St. Louis, sought to explore this further by performing a retrospective, single-center cohort study of 1,691 patients aged 18 years or older with Candida bloodstream infection from 2002 to 2015. They analyzed demographics, comorbidities, predisposing factors, all-cause mortality, antifungal use, central-line removal, and ophthalmological and echocardiographic evaluation in order to compare 90-day all-cause mortality between individuals with and without an ID consultation.
They found that those patients who received an ID consult for a Candida bloodstream infection had a significantly lower 90-day mortality rate than did those who did not (29% vs. 51%).
With a model using inverse weighting by the propensity score, they found that ID consultation was associated with a hazard ratio of 0.81 for mortality (95% confidence interval, 0.73-0.91; P less than .0001). In the ID consultation group, the median duration of antifungal therapy was significantly longer (18 vs. 14 days; P less than .0001); central-line removal was significantly more common (76% vs. 59%; P less than .0001); echocardiography use was more frequent (57% vs. 33%; P less than .0001); and ophthalmological examinations were performed more often (53% vs. 17%; P less than .0001). Importantly, fewer patients in the ID consultation group were untreated (2% vs. 14%; P less than .0001).
In an accompanying commentary, Katrien Lagrou, MD, and Eric Van Wijngaerden, MD, of the department of microbiology, immunology and transplantation, University Hospitals Leuven (Belgium) stated: “We think that the high proportion of patients (14%) with a Candida bloodstream infection who did not receive any antifungal treatment and did not have an infectious disease consultation is a particularly alarming finding. ... Ninety-day mortality in these untreated patients was high (67%).”
“We believe every hospital should have an expert management strategy addressing all individual cases of candidaemia. The need for such expert management should be incorporated in all future candidaemia management guidelines,” they concluded.
The study was funded by the Astellas Global Development Pharma, the Washington University Institute of Clinical and Translational Sciences, and the Agency for Healthcare Research and Quality. Several of the authors had financial connections to Astellas Global Development or other pharmaceutical companies. Dr. Lagrou and Dr. Van Wijngaerden both reported receiving personal fees and nonfinancial support from a number of pharmaceutical companies, but all outside the scope of the study.
SOURCE: Mejia-Chew C et al. Lancet Infect Dis. 2019;19:1336-44.
findings from a large retrospective study suggest.
Mortality attributable to Candida bloodstream infection ranges between 15% and 47%, and delay in initiation of appropriate treatment has been associated with increased mortality. Previous small studies showed that ID consultation has conferred benefits to patients with Candida bloodstream infections. Carlos Mejia-Chew, MD, and colleagues from Washington University, St. Louis, sought to explore this further by performing a retrospective, single-center cohort study of 1,691 patients aged 18 years or older with Candida bloodstream infection from 2002 to 2015. They analyzed demographics, comorbidities, predisposing factors, all-cause mortality, antifungal use, central-line removal, and ophthalmological and echocardiographic evaluation in order to compare 90-day all-cause mortality between individuals with and without an ID consultation.
They found that those patients who received an ID consult for a Candida bloodstream infection had a significantly lower 90-day mortality rate than did those who did not (29% vs. 51%).
With a model using inverse weighting by the propensity score, they found that ID consultation was associated with a hazard ratio of 0.81 for mortality (95% confidence interval, 0.73-0.91; P less than .0001). In the ID consultation group, the median duration of antifungal therapy was significantly longer (18 vs. 14 days; P less than .0001); central-line removal was significantly more common (76% vs. 59%; P less than .0001); echocardiography use was more frequent (57% vs. 33%; P less than .0001); and ophthalmological examinations were performed more often (53% vs. 17%; P less than .0001). Importantly, fewer patients in the ID consultation group were untreated (2% vs. 14%; P less than .0001).
In an accompanying commentary, Katrien Lagrou, MD, and Eric Van Wijngaerden, MD, of the department of microbiology, immunology and transplantation, University Hospitals Leuven (Belgium) stated: “We think that the high proportion of patients (14%) with a Candida bloodstream infection who did not receive any antifungal treatment and did not have an infectious disease consultation is a particularly alarming finding. ... Ninety-day mortality in these untreated patients was high (67%).”
“We believe every hospital should have an expert management strategy addressing all individual cases of candidaemia. The need for such expert management should be incorporated in all future candidaemia management guidelines,” they concluded.
The study was funded by the Astellas Global Development Pharma, the Washington University Institute of Clinical and Translational Sciences, and the Agency for Healthcare Research and Quality. Several of the authors had financial connections to Astellas Global Development or other pharmaceutical companies. Dr. Lagrou and Dr. Van Wijngaerden both reported receiving personal fees and nonfinancial support from a number of pharmaceutical companies, but all outside the scope of the study.
SOURCE: Mejia-Chew C et al. Lancet Infect Dis. 2019;19:1336-44.
findings from a large retrospective study suggest.
Mortality attributable to Candida bloodstream infection ranges between 15% and 47%, and delay in initiation of appropriate treatment has been associated with increased mortality. Previous small studies showed that ID consultation has conferred benefits to patients with Candida bloodstream infections. Carlos Mejia-Chew, MD, and colleagues from Washington University, St. Louis, sought to explore this further by performing a retrospective, single-center cohort study of 1,691 patients aged 18 years or older with Candida bloodstream infection from 2002 to 2015. They analyzed demographics, comorbidities, predisposing factors, all-cause mortality, antifungal use, central-line removal, and ophthalmological and echocardiographic evaluation in order to compare 90-day all-cause mortality between individuals with and without an ID consultation.
They found that those patients who received an ID consult for a Candida bloodstream infection had a significantly lower 90-day mortality rate than did those who did not (29% vs. 51%).
With a model using inverse weighting by the propensity score, they found that ID consultation was associated with a hazard ratio of 0.81 for mortality (95% confidence interval, 0.73-0.91; P less than .0001). In the ID consultation group, the median duration of antifungal therapy was significantly longer (18 vs. 14 days; P less than .0001); central-line removal was significantly more common (76% vs. 59%; P less than .0001); echocardiography use was more frequent (57% vs. 33%; P less than .0001); and ophthalmological examinations were performed more often (53% vs. 17%; P less than .0001). Importantly, fewer patients in the ID consultation group were untreated (2% vs. 14%; P less than .0001).
In an accompanying commentary, Katrien Lagrou, MD, and Eric Van Wijngaerden, MD, of the department of microbiology, immunology and transplantation, University Hospitals Leuven (Belgium) stated: “We think that the high proportion of patients (14%) with a Candida bloodstream infection who did not receive any antifungal treatment and did not have an infectious disease consultation is a particularly alarming finding. ... Ninety-day mortality in these untreated patients was high (67%).”
“We believe every hospital should have an expert management strategy addressing all individual cases of candidaemia. The need for such expert management should be incorporated in all future candidaemia management guidelines,” they concluded.
The study was funded by the Astellas Global Development Pharma, the Washington University Institute of Clinical and Translational Sciences, and the Agency for Healthcare Research and Quality. Several of the authors had financial connections to Astellas Global Development or other pharmaceutical companies. Dr. Lagrou and Dr. Van Wijngaerden both reported receiving personal fees and nonfinancial support from a number of pharmaceutical companies, but all outside the scope of the study.
SOURCE: Mejia-Chew C et al. Lancet Infect Dis. 2019;19:1336-44.
FROM LANCET: INFECTIOUS DISEASES