User login
Kidney stones on the rise: Where are the specialists?
While increasing the number of nephrologists who specialize in kidney stones is necessary, nonspecialists need to play a larger role in recognizing and preventing kidney stones.
Primary care and emergency department physicians can be the front lines of counseling patients who do not have underlying genetic causes of kidney stones on how to prevent a recurrence, according to Irina Jaeger, MD, a urologist at University Hospitals and an assistant professor of urology at Case Western Reserve University, both in Cleveland.
“A lot of this care can be implemented by our primary care physicians, such as counseling on decreasing sodium in the diet and increasing fluid intake, which benefits so many different health conditions as well as stones,” said Gregory E. Tasian, MD, MSCE, an attending pediatric urologist at Children’s Hospital of Philadelphia. “If we can think about this holistically, we can really make strides.”
Focus on prevention
Taking a holistic approach, Dr. Tasian added, will require rethinking how health teams approach patient care and manage kidney stones.
“We think of stones as episodic events that are painful, and then pass,” he said. “But it’s really a disorder of mineral metabolism.”
Understanding these episodes as a chronic disease can also explain why nephrolithiasis often goes hand in hand with higher instances of heart attack and stroke, hypertension, and bone breaks, he added.
Simple measures such as staying hydrated and consuming citrate in the form of lemon water or lemonade can help patients prevent recurring kidney stones, Dr. Jaeger said.
But patients who have had a stone also need to see a specialist to rule out any underlying causes. Kidney stones are routinely viewed as episodic events that don’t pose much of a health threat, but between 30% and 50% of people diagnosed with stones will experience a recurrence within 5 years. Educating patients on how they can prevent future episodes is a crucial part of care.
“Even if they are passing the stones on their own without surgery, they should really be evaluated by a urologist or a nephrologist,” Dr. Jaeger said.
David S. Goldfarb, MD, clinical director of the division of nephrology at NYU Langone Health, New York, said that access to nephrologists who specialize in kidney stones is a critical piece of prevention. While urologists can treat stones, nephrologists get to the bottom of why the stones occurred in the first place and help patients prevent further stones from forming.
“The majority of urologists in the U.S. don’t do much in regard to prevention,” he said. “There needs to be more nephrologists.”
Kidney stones now appear to be increasingly common in patient populations that previously did not have the condition.
A study published in 2016 in the Clinical Journal of the American Society of Nephrology found that the annual incidence of kidney stones increased 16% from 1997 to 2012, with the biggest increase seen among teenagers. Stones were 52% more common among girls and women than among men, but the condition is also becoming more common in men starting at age 25. Meanwhile, Black Americans of all ages saw greater rates of kidney stone development than their White counterparts.
Fewer residents are choosing to specialize in nephrology, with a decrease in the choice of fellowship of 50% from 2009 to 2019, according to a 2023 report by the American Society of Nephrology.
A 2019 survey of nearly 4,200 residents found that only 60% of nephrology fellowship positions were filled in 2018, and the majority of those residents reported a lack of interest in the kidney as being the most critical factor in not selecting the specialty. Others reported lack of exposure to nephrology overall.
Diagnosing the root cause
Getting to the root cause of how further kidney stones can be prevented usually requires a nephrologist, according to Dr. Jaeger.
“As a urologist, 90% of what we do is surgery,” she said.
Although urologists are trained in analyzing 24-hour urine tests, which can reveal risks that can be addressed by preventive changes, many urologists tap a specialized nephrologist, who may analyze the samples with a keener eye.
“When individuals pass a stone, fewer than 10% seek care with a specialist after that and that’s a missed opportunity to prevent future stones,” Dr. Tasian said.
Not all nephrologists specialize in stones, but they may be better equipped to recognize when a patient needs to see someone who does. Failing to involve a nephrologist who specializes in kidney stones can have grave consequences for patient health.
Dr. Goldfarb is currently caring for a patient with a kidney transplant that had begun to lose function. Clinicians who originally cared for the patient took a kidney biopsy, which showed fragments of calcium oxalate, a common type of kidney stone, in her native kidneys.
After receiving a kidney transplant, her health began to decline again and a second biopsy found that the new kidney was forming the same type of stones. Her nephrologist knew this meant she likely had a genetic disorder and referred her to Dr. Goldfarb, who specializes in underlying genetic causes of kidney stones. A genetic test revealed that the patient had primary hyperoxaluria.
“She would have been treated completely differently if that had been recognized as the cause of her original kidney disease,” Dr. Goldfarb said. “Now her kidney transplant is getting kidney stones and I’m working with her to prevent that.”
Under Dr. Goldfarb, the patient will have access to a new experimental drug, called nedosiran, currently in clinical trials. It is specifically for primary hyperoxaluria.
“The kidney doctor that made the diagnosis correctly and referred her to me isn’t a kidney stone specialist; he is a general nephrologist who has taken an interest in the topic of kidney stones, recognizing there is sometimes some nuance and specialty of issues related to this,” Dr. Goldfarb said.
A version of this article appeared on Medscape.com.
While increasing the number of nephrologists who specialize in kidney stones is necessary, nonspecialists need to play a larger role in recognizing and preventing kidney stones.
Primary care and emergency department physicians can be the front lines of counseling patients who do not have underlying genetic causes of kidney stones on how to prevent a recurrence, according to Irina Jaeger, MD, a urologist at University Hospitals and an assistant professor of urology at Case Western Reserve University, both in Cleveland.
“A lot of this care can be implemented by our primary care physicians, such as counseling on decreasing sodium in the diet and increasing fluid intake, which benefits so many different health conditions as well as stones,” said Gregory E. Tasian, MD, MSCE, an attending pediatric urologist at Children’s Hospital of Philadelphia. “If we can think about this holistically, we can really make strides.”
Focus on prevention
Taking a holistic approach, Dr. Tasian added, will require rethinking how health teams approach patient care and manage kidney stones.
“We think of stones as episodic events that are painful, and then pass,” he said. “But it’s really a disorder of mineral metabolism.”
Understanding these episodes as a chronic disease can also explain why nephrolithiasis often goes hand in hand with higher instances of heart attack and stroke, hypertension, and bone breaks, he added.
Simple measures such as staying hydrated and consuming citrate in the form of lemon water or lemonade can help patients prevent recurring kidney stones, Dr. Jaeger said.
But patients who have had a stone also need to see a specialist to rule out any underlying causes. Kidney stones are routinely viewed as episodic events that don’t pose much of a health threat, but between 30% and 50% of people diagnosed with stones will experience a recurrence within 5 years. Educating patients on how they can prevent future episodes is a crucial part of care.
“Even if they are passing the stones on their own without surgery, they should really be evaluated by a urologist or a nephrologist,” Dr. Jaeger said.
David S. Goldfarb, MD, clinical director of the division of nephrology at NYU Langone Health, New York, said that access to nephrologists who specialize in kidney stones is a critical piece of prevention. While urologists can treat stones, nephrologists get to the bottom of why the stones occurred in the first place and help patients prevent further stones from forming.
“The majority of urologists in the U.S. don’t do much in regard to prevention,” he said. “There needs to be more nephrologists.”
Kidney stones now appear to be increasingly common in patient populations that previously did not have the condition.
A study published in 2016 in the Clinical Journal of the American Society of Nephrology found that the annual incidence of kidney stones increased 16% from 1997 to 2012, with the biggest increase seen among teenagers. Stones were 52% more common among girls and women than among men, but the condition is also becoming more common in men starting at age 25. Meanwhile, Black Americans of all ages saw greater rates of kidney stone development than their White counterparts.
Fewer residents are choosing to specialize in nephrology, with a decrease in the choice of fellowship of 50% from 2009 to 2019, according to a 2023 report by the American Society of Nephrology.
A 2019 survey of nearly 4,200 residents found that only 60% of nephrology fellowship positions were filled in 2018, and the majority of those residents reported a lack of interest in the kidney as being the most critical factor in not selecting the specialty. Others reported lack of exposure to nephrology overall.
Diagnosing the root cause
Getting to the root cause of how further kidney stones can be prevented usually requires a nephrologist, according to Dr. Jaeger.
“As a urologist, 90% of what we do is surgery,” she said.
Although urologists are trained in analyzing 24-hour urine tests, which can reveal risks that can be addressed by preventive changes, many urologists tap a specialized nephrologist, who may analyze the samples with a keener eye.
“When individuals pass a stone, fewer than 10% seek care with a specialist after that and that’s a missed opportunity to prevent future stones,” Dr. Tasian said.
Not all nephrologists specialize in stones, but they may be better equipped to recognize when a patient needs to see someone who does. Failing to involve a nephrologist who specializes in kidney stones can have grave consequences for patient health.
Dr. Goldfarb is currently caring for a patient with a kidney transplant that had begun to lose function. Clinicians who originally cared for the patient took a kidney biopsy, which showed fragments of calcium oxalate, a common type of kidney stone, in her native kidneys.
After receiving a kidney transplant, her health began to decline again and a second biopsy found that the new kidney was forming the same type of stones. Her nephrologist knew this meant she likely had a genetic disorder and referred her to Dr. Goldfarb, who specializes in underlying genetic causes of kidney stones. A genetic test revealed that the patient had primary hyperoxaluria.
“She would have been treated completely differently if that had been recognized as the cause of her original kidney disease,” Dr. Goldfarb said. “Now her kidney transplant is getting kidney stones and I’m working with her to prevent that.”
Under Dr. Goldfarb, the patient will have access to a new experimental drug, called nedosiran, currently in clinical trials. It is specifically for primary hyperoxaluria.
“The kidney doctor that made the diagnosis correctly and referred her to me isn’t a kidney stone specialist; he is a general nephrologist who has taken an interest in the topic of kidney stones, recognizing there is sometimes some nuance and specialty of issues related to this,” Dr. Goldfarb said.
A version of this article appeared on Medscape.com.
While increasing the number of nephrologists who specialize in kidney stones is necessary, nonspecialists need to play a larger role in recognizing and preventing kidney stones.
Primary care and emergency department physicians can be the front lines of counseling patients who do not have underlying genetic causes of kidney stones on how to prevent a recurrence, according to Irina Jaeger, MD, a urologist at University Hospitals and an assistant professor of urology at Case Western Reserve University, both in Cleveland.
“A lot of this care can be implemented by our primary care physicians, such as counseling on decreasing sodium in the diet and increasing fluid intake, which benefits so many different health conditions as well as stones,” said Gregory E. Tasian, MD, MSCE, an attending pediatric urologist at Children’s Hospital of Philadelphia. “If we can think about this holistically, we can really make strides.”
Focus on prevention
Taking a holistic approach, Dr. Tasian added, will require rethinking how health teams approach patient care and manage kidney stones.
“We think of stones as episodic events that are painful, and then pass,” he said. “But it’s really a disorder of mineral metabolism.”
Understanding these episodes as a chronic disease can also explain why nephrolithiasis often goes hand in hand with higher instances of heart attack and stroke, hypertension, and bone breaks, he added.
Simple measures such as staying hydrated and consuming citrate in the form of lemon water or lemonade can help patients prevent recurring kidney stones, Dr. Jaeger said.
But patients who have had a stone also need to see a specialist to rule out any underlying causes. Kidney stones are routinely viewed as episodic events that don’t pose much of a health threat, but between 30% and 50% of people diagnosed with stones will experience a recurrence within 5 years. Educating patients on how they can prevent future episodes is a crucial part of care.
“Even if they are passing the stones on their own without surgery, they should really be evaluated by a urologist or a nephrologist,” Dr. Jaeger said.
David S. Goldfarb, MD, clinical director of the division of nephrology at NYU Langone Health, New York, said that access to nephrologists who specialize in kidney stones is a critical piece of prevention. While urologists can treat stones, nephrologists get to the bottom of why the stones occurred in the first place and help patients prevent further stones from forming.
“The majority of urologists in the U.S. don’t do much in regard to prevention,” he said. “There needs to be more nephrologists.”
Kidney stones now appear to be increasingly common in patient populations that previously did not have the condition.
A study published in 2016 in the Clinical Journal of the American Society of Nephrology found that the annual incidence of kidney stones increased 16% from 1997 to 2012, with the biggest increase seen among teenagers. Stones were 52% more common among girls and women than among men, but the condition is also becoming more common in men starting at age 25. Meanwhile, Black Americans of all ages saw greater rates of kidney stone development than their White counterparts.
Fewer residents are choosing to specialize in nephrology, with a decrease in the choice of fellowship of 50% from 2009 to 2019, according to a 2023 report by the American Society of Nephrology.
A 2019 survey of nearly 4,200 residents found that only 60% of nephrology fellowship positions were filled in 2018, and the majority of those residents reported a lack of interest in the kidney as being the most critical factor in not selecting the specialty. Others reported lack of exposure to nephrology overall.
Diagnosing the root cause
Getting to the root cause of how further kidney stones can be prevented usually requires a nephrologist, according to Dr. Jaeger.
“As a urologist, 90% of what we do is surgery,” she said.
Although urologists are trained in analyzing 24-hour urine tests, which can reveal risks that can be addressed by preventive changes, many urologists tap a specialized nephrologist, who may analyze the samples with a keener eye.
“When individuals pass a stone, fewer than 10% seek care with a specialist after that and that’s a missed opportunity to prevent future stones,” Dr. Tasian said.
Not all nephrologists specialize in stones, but they may be better equipped to recognize when a patient needs to see someone who does. Failing to involve a nephrologist who specializes in kidney stones can have grave consequences for patient health.
Dr. Goldfarb is currently caring for a patient with a kidney transplant that had begun to lose function. Clinicians who originally cared for the patient took a kidney biopsy, which showed fragments of calcium oxalate, a common type of kidney stone, in her native kidneys.
After receiving a kidney transplant, her health began to decline again and a second biopsy found that the new kidney was forming the same type of stones. Her nephrologist knew this meant she likely had a genetic disorder and referred her to Dr. Goldfarb, who specializes in underlying genetic causes of kidney stones. A genetic test revealed that the patient had primary hyperoxaluria.
“She would have been treated completely differently if that had been recognized as the cause of her original kidney disease,” Dr. Goldfarb said. “Now her kidney transplant is getting kidney stones and I’m working with her to prevent that.”
Under Dr. Goldfarb, the patient will have access to a new experimental drug, called nedosiran, currently in clinical trials. It is specifically for primary hyperoxaluria.
“The kidney doctor that made the diagnosis correctly and referred her to me isn’t a kidney stone specialist; he is a general nephrologist who has taken an interest in the topic of kidney stones, recognizing there is sometimes some nuance and specialty of issues related to this,” Dr. Goldfarb said.
A version of this article appeared on Medscape.com.
Simple blood test may predict heart and kidney risk in T2D
, suggests an analysis of the CREDENCE trial.
The research, published online in the journal Circulation, also revealed that patients treated with the sodium-glucose cotransporter-2 inhibitor canagliflozin (Invokana, Invokamet) had lower levels of the biomarkers after 1 year compared with those given placebo.
Examination of biomarker levels in more than 2,600 patients from CREDENCE showed that high baseline concentrations of the individual biomarkers were able to predict the future risk for a composite endpoint of renal and heart outcomes.
The combination of all four biomarkers into a single panel revealed that patients with the highest levels were more than four times as likely to experience the composite endpoint than were those with the lowest levels.
As two of the biomarkers used in the study have yet to have established prognostic thresholds, the results remain exploratory.
Lead author James L. Januzzi, MD, director of the Heart Failure and Biomarker Trials at the Baim Institute for Clinical Research, Boston, said that further study will help refine the predictive value of the panel.
“Given that the American Heart Association/American College of Cardiology and the American Diabetes Association now all recommend measurement of biomarkers to enhance the ability to predict risk in persons with type 2 diabetes, these results may considerably extend the reach of biomarker-based testing, refining accuracy even further,” he said in a press release.
In an interview, Dr. Januzzi said that “three out of the four biomarkers are already clinically and commercially available,” while the fourth, for insulin-like growth factor binding protein 7 (IGFBP7), is “on the near horizon.”
He stressed that the “future for multiple biomarker testing, however, will be less about ordering each individual test, and ultimately will revolve around panels of blood work that are ordered as a single test.”
Dr. Januzzi added that “rather than using the rather primitive approach that we took” of looking at the individual biomarkers in adjusted models, the next stage “will be to utilize algorithms to combine the results into a single value.
“A clinician will not have to struggle with looking at individual results but will just receive one aggregated test result that informs them whether a patient is at low, medium or higher risk,” he explained.
However, this will require determining the relative importance of each biomarker and weighting them in the final model.
Consequently, the current results “set the foundation for identifying some very powerful individual tests that may ultimately, in aggregate, help us to help our patients with diabetes avoid a major complication,” Dr. Januzzi said.
By revealing that some individuals with both type 2 diabetes and kidney disease are at higher risk than others, he also hopes the findings can be leveraged to treat patients with “varying degrees of intensity with proven therapies, including weight loss, dietary adjustment, and pharmacologic intervention.”
Dr. Januzzi added: “Diabetes affects a dramatic, and growing, percentage of our population, and this type of personalized strategy to reduce the major complications of this rather common disease is an important step forward.”
The authors noted that there is a “bidirectional relationship” between cardiovascular disease and chronic kidney disease (CKD), such that either diagnosis may increase the risk of, or exacerbate, the other.
Individuals with type 2 diabetes and CKD albuminuria, they added, are at particularly high risk for major cardiovascular events, and studies have shown that several circulating cardiorenal stress biomarkers may predict the onset and progression of CKD in type 2 diabetes, as well as predict cardiovascular events.
Several biomarkers associated with myocardial stress and necrosis
The recent CANVAS trial revealed that, among individuals with type 2 diabetes with and without CKD, several biomarkers were associated with myocardial stress and necrosis, and renal tubular injury, predicting the progression of CKD with albuminuria, and the risk for heart failure events.
Taking inspiration from those findings, the current researchers studied a panel of similar cardiac and renal biomarkers among participants from the CREDENCE trial, for which 4,401 patients with type 2 diabetes and CKD at high risk of progression were randomly assigned to canagliflozin or placebo.
The current analysis involved 2,627 participants who had baseline plasma samples available for analysis of four circulating biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP), high-sensitivity cardiac troponin T (hs-cTnT), growth differentiation factor-15 (GDF-15), and IGFBP7.
Among those, 2,385 participants also had year 1 plasma samples available for analysis, while year 3 plasma samples were available for 895 individuals.
The results showed that, in general, median baseline concentrations of each biomarker in both treatment groups were elevated compared with healthy reference populations.
Baseline log-transformed concentrations of each biomarker were also strongly predictive of cardiac and renal outcomes, including heart failure and progression of CKD.
For example, each unit increase in baseline NT-proBNP concentrations was associated with a hazard ratio of 1.35 for the primary composite endpoint of end-stage kidney disease, doubling of serum creatinine levels, renal death, or cardiovascular disease (P < .001).
For each unit increase in hs-cTnT levels, the hazard ratio for the primary composite was 1.73 (P < .001), for GDF-15 it was 1.84 (P < .0001), and for IGFBP7 the hazard ratio was 3.14 (P < .001).
Combining the four biomarkers into a single multimarker panel revealed that, compared with individuals with a low-risk score, those with a high-risk score had a hazard ratio for the primary outcome of 4.01, whereas those with a moderate risk score had a hazard ratio of 2.39 (P < .001 for both).
For the individual outcome of heart failure hospitalization, the effect was even greater. A high-risk score was associated with a hazard ratio vs. a low-risk score of 6.04 (P < .001), whereas patients with a moderate risk score had a hazard ratio of 2.45 (P = .04).
The researchers also reported that, between baseline and year 1, concentrations of all four biomarkers rose from 6% to 29% in the placebo group, but from 3% to just 10% in those treated with canagliflozin.
“It was reassuring to discover that canagliflozin helped reduce risks the most in people with the highest chances for complications,” said Dr. Januzzi.
The CREDENCE trial and the current analysis were funded by Janssen Research & Development LLC. NT-proBNP, hs-cTnT, GDF-15, and IGFBP7 reagents were provided by Roche Diagnostics. Dr. Januzzi is funded in part by the Hutter Family Professorship. Dr. Januzzi declared relationships with Imbria Pharmaceuticals, Jana Care, Abbott, Applied Therapeutics, HeartFlow, Innolife, Roche Diagnostics, Beckman, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Merck, Novartis, Pfizer, Siemens, Abbott, AbbVie, CVRx, Intercept, and Takeda.
A version of this article first appeared on Medscape.com.
, suggests an analysis of the CREDENCE trial.
The research, published online in the journal Circulation, also revealed that patients treated with the sodium-glucose cotransporter-2 inhibitor canagliflozin (Invokana, Invokamet) had lower levels of the biomarkers after 1 year compared with those given placebo.
Examination of biomarker levels in more than 2,600 patients from CREDENCE showed that high baseline concentrations of the individual biomarkers were able to predict the future risk for a composite endpoint of renal and heart outcomes.
The combination of all four biomarkers into a single panel revealed that patients with the highest levels were more than four times as likely to experience the composite endpoint than were those with the lowest levels.
As two of the biomarkers used in the study have yet to have established prognostic thresholds, the results remain exploratory.
Lead author James L. Januzzi, MD, director of the Heart Failure and Biomarker Trials at the Baim Institute for Clinical Research, Boston, said that further study will help refine the predictive value of the panel.
“Given that the American Heart Association/American College of Cardiology and the American Diabetes Association now all recommend measurement of biomarkers to enhance the ability to predict risk in persons with type 2 diabetes, these results may considerably extend the reach of biomarker-based testing, refining accuracy even further,” he said in a press release.
In an interview, Dr. Januzzi said that “three out of the four biomarkers are already clinically and commercially available,” while the fourth, for insulin-like growth factor binding protein 7 (IGFBP7), is “on the near horizon.”
He stressed that the “future for multiple biomarker testing, however, will be less about ordering each individual test, and ultimately will revolve around panels of blood work that are ordered as a single test.”
Dr. Januzzi added that “rather than using the rather primitive approach that we took” of looking at the individual biomarkers in adjusted models, the next stage “will be to utilize algorithms to combine the results into a single value.
“A clinician will not have to struggle with looking at individual results but will just receive one aggregated test result that informs them whether a patient is at low, medium or higher risk,” he explained.
However, this will require determining the relative importance of each biomarker and weighting them in the final model.
Consequently, the current results “set the foundation for identifying some very powerful individual tests that may ultimately, in aggregate, help us to help our patients with diabetes avoid a major complication,” Dr. Januzzi said.
By revealing that some individuals with both type 2 diabetes and kidney disease are at higher risk than others, he also hopes the findings can be leveraged to treat patients with “varying degrees of intensity with proven therapies, including weight loss, dietary adjustment, and pharmacologic intervention.”
Dr. Januzzi added: “Diabetes affects a dramatic, and growing, percentage of our population, and this type of personalized strategy to reduce the major complications of this rather common disease is an important step forward.”
The authors noted that there is a “bidirectional relationship” between cardiovascular disease and chronic kidney disease (CKD), such that either diagnosis may increase the risk of, or exacerbate, the other.
Individuals with type 2 diabetes and CKD albuminuria, they added, are at particularly high risk for major cardiovascular events, and studies have shown that several circulating cardiorenal stress biomarkers may predict the onset and progression of CKD in type 2 diabetes, as well as predict cardiovascular events.
Several biomarkers associated with myocardial stress and necrosis
The recent CANVAS trial revealed that, among individuals with type 2 diabetes with and without CKD, several biomarkers were associated with myocardial stress and necrosis, and renal tubular injury, predicting the progression of CKD with albuminuria, and the risk for heart failure events.
Taking inspiration from those findings, the current researchers studied a panel of similar cardiac and renal biomarkers among participants from the CREDENCE trial, for which 4,401 patients with type 2 diabetes and CKD at high risk of progression were randomly assigned to canagliflozin or placebo.
The current analysis involved 2,627 participants who had baseline plasma samples available for analysis of four circulating biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP), high-sensitivity cardiac troponin T (hs-cTnT), growth differentiation factor-15 (GDF-15), and IGFBP7.
Among those, 2,385 participants also had year 1 plasma samples available for analysis, while year 3 plasma samples were available for 895 individuals.
The results showed that, in general, median baseline concentrations of each biomarker in both treatment groups were elevated compared with healthy reference populations.
Baseline log-transformed concentrations of each biomarker were also strongly predictive of cardiac and renal outcomes, including heart failure and progression of CKD.
For example, each unit increase in baseline NT-proBNP concentrations was associated with a hazard ratio of 1.35 for the primary composite endpoint of end-stage kidney disease, doubling of serum creatinine levels, renal death, or cardiovascular disease (P < .001).
For each unit increase in hs-cTnT levels, the hazard ratio for the primary composite was 1.73 (P < .001), for GDF-15 it was 1.84 (P < .0001), and for IGFBP7 the hazard ratio was 3.14 (P < .001).
Combining the four biomarkers into a single multimarker panel revealed that, compared with individuals with a low-risk score, those with a high-risk score had a hazard ratio for the primary outcome of 4.01, whereas those with a moderate risk score had a hazard ratio of 2.39 (P < .001 for both).
For the individual outcome of heart failure hospitalization, the effect was even greater. A high-risk score was associated with a hazard ratio vs. a low-risk score of 6.04 (P < .001), whereas patients with a moderate risk score had a hazard ratio of 2.45 (P = .04).
The researchers also reported that, between baseline and year 1, concentrations of all four biomarkers rose from 6% to 29% in the placebo group, but from 3% to just 10% in those treated with canagliflozin.
“It was reassuring to discover that canagliflozin helped reduce risks the most in people with the highest chances for complications,” said Dr. Januzzi.
The CREDENCE trial and the current analysis were funded by Janssen Research & Development LLC. NT-proBNP, hs-cTnT, GDF-15, and IGFBP7 reagents were provided by Roche Diagnostics. Dr. Januzzi is funded in part by the Hutter Family Professorship. Dr. Januzzi declared relationships with Imbria Pharmaceuticals, Jana Care, Abbott, Applied Therapeutics, HeartFlow, Innolife, Roche Diagnostics, Beckman, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Merck, Novartis, Pfizer, Siemens, Abbott, AbbVie, CVRx, Intercept, and Takeda.
A version of this article first appeared on Medscape.com.
, suggests an analysis of the CREDENCE trial.
The research, published online in the journal Circulation, also revealed that patients treated with the sodium-glucose cotransporter-2 inhibitor canagliflozin (Invokana, Invokamet) had lower levels of the biomarkers after 1 year compared with those given placebo.
Examination of biomarker levels in more than 2,600 patients from CREDENCE showed that high baseline concentrations of the individual biomarkers were able to predict the future risk for a composite endpoint of renal and heart outcomes.
The combination of all four biomarkers into a single panel revealed that patients with the highest levels were more than four times as likely to experience the composite endpoint than were those with the lowest levels.
As two of the biomarkers used in the study have yet to have established prognostic thresholds, the results remain exploratory.
Lead author James L. Januzzi, MD, director of the Heart Failure and Biomarker Trials at the Baim Institute for Clinical Research, Boston, said that further study will help refine the predictive value of the panel.
“Given that the American Heart Association/American College of Cardiology and the American Diabetes Association now all recommend measurement of biomarkers to enhance the ability to predict risk in persons with type 2 diabetes, these results may considerably extend the reach of biomarker-based testing, refining accuracy even further,” he said in a press release.
In an interview, Dr. Januzzi said that “three out of the four biomarkers are already clinically and commercially available,” while the fourth, for insulin-like growth factor binding protein 7 (IGFBP7), is “on the near horizon.”
He stressed that the “future for multiple biomarker testing, however, will be less about ordering each individual test, and ultimately will revolve around panels of blood work that are ordered as a single test.”
Dr. Januzzi added that “rather than using the rather primitive approach that we took” of looking at the individual biomarkers in adjusted models, the next stage “will be to utilize algorithms to combine the results into a single value.
“A clinician will not have to struggle with looking at individual results but will just receive one aggregated test result that informs them whether a patient is at low, medium or higher risk,” he explained.
However, this will require determining the relative importance of each biomarker and weighting them in the final model.
Consequently, the current results “set the foundation for identifying some very powerful individual tests that may ultimately, in aggregate, help us to help our patients with diabetes avoid a major complication,” Dr. Januzzi said.
By revealing that some individuals with both type 2 diabetes and kidney disease are at higher risk than others, he also hopes the findings can be leveraged to treat patients with “varying degrees of intensity with proven therapies, including weight loss, dietary adjustment, and pharmacologic intervention.”
Dr. Januzzi added: “Diabetes affects a dramatic, and growing, percentage of our population, and this type of personalized strategy to reduce the major complications of this rather common disease is an important step forward.”
The authors noted that there is a “bidirectional relationship” between cardiovascular disease and chronic kidney disease (CKD), such that either diagnosis may increase the risk of, or exacerbate, the other.
Individuals with type 2 diabetes and CKD albuminuria, they added, are at particularly high risk for major cardiovascular events, and studies have shown that several circulating cardiorenal stress biomarkers may predict the onset and progression of CKD in type 2 diabetes, as well as predict cardiovascular events.
Several biomarkers associated with myocardial stress and necrosis
The recent CANVAS trial revealed that, among individuals with type 2 diabetes with and without CKD, several biomarkers were associated with myocardial stress and necrosis, and renal tubular injury, predicting the progression of CKD with albuminuria, and the risk for heart failure events.
Taking inspiration from those findings, the current researchers studied a panel of similar cardiac and renal biomarkers among participants from the CREDENCE trial, for which 4,401 patients with type 2 diabetes and CKD at high risk of progression were randomly assigned to canagliflozin or placebo.
The current analysis involved 2,627 participants who had baseline plasma samples available for analysis of four circulating biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP), high-sensitivity cardiac troponin T (hs-cTnT), growth differentiation factor-15 (GDF-15), and IGFBP7.
Among those, 2,385 participants also had year 1 plasma samples available for analysis, while year 3 plasma samples were available for 895 individuals.
The results showed that, in general, median baseline concentrations of each biomarker in both treatment groups were elevated compared with healthy reference populations.
Baseline log-transformed concentrations of each biomarker were also strongly predictive of cardiac and renal outcomes, including heart failure and progression of CKD.
For example, each unit increase in baseline NT-proBNP concentrations was associated with a hazard ratio of 1.35 for the primary composite endpoint of end-stage kidney disease, doubling of serum creatinine levels, renal death, or cardiovascular disease (P < .001).
For each unit increase in hs-cTnT levels, the hazard ratio for the primary composite was 1.73 (P < .001), for GDF-15 it was 1.84 (P < .0001), and for IGFBP7 the hazard ratio was 3.14 (P < .001).
Combining the four biomarkers into a single multimarker panel revealed that, compared with individuals with a low-risk score, those with a high-risk score had a hazard ratio for the primary outcome of 4.01, whereas those with a moderate risk score had a hazard ratio of 2.39 (P < .001 for both).
For the individual outcome of heart failure hospitalization, the effect was even greater. A high-risk score was associated with a hazard ratio vs. a low-risk score of 6.04 (P < .001), whereas patients with a moderate risk score had a hazard ratio of 2.45 (P = .04).
The researchers also reported that, between baseline and year 1, concentrations of all four biomarkers rose from 6% to 29% in the placebo group, but from 3% to just 10% in those treated with canagliflozin.
“It was reassuring to discover that canagliflozin helped reduce risks the most in people with the highest chances for complications,” said Dr. Januzzi.
The CREDENCE trial and the current analysis were funded by Janssen Research & Development LLC. NT-proBNP, hs-cTnT, GDF-15, and IGFBP7 reagents were provided by Roche Diagnostics. Dr. Januzzi is funded in part by the Hutter Family Professorship. Dr. Januzzi declared relationships with Imbria Pharmaceuticals, Jana Care, Abbott, Applied Therapeutics, HeartFlow, Innolife, Roche Diagnostics, Beckman, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Merck, Novartis, Pfizer, Siemens, Abbott, AbbVie, CVRx, Intercept, and Takeda.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
Pig kidneys show ‘life-sustaining’ function in human
– marking another important step toward opening up a new supply of much-needed organs for those with end-stage kidney disease.
A team of researchers in Alabama removed a brain-dead person’s kidneys and transplanted two kidneys that had been taken from a genetically modified pig. The researchers monitored the patient’s response to the organs and tracked the kidneys’ function over a 7-day period. The findings were published in JAMA Surgery.
During the first 24 hours after transplantation, the pig kidneys made more than 37 liters of urine. “It was really a remarkable thing to see,” lead investigator Jayme Locke, MD, professor of surgery and the Arnold G. Diethelm Endowed Chair in Transplantation Surgery, University of Alabama at Birmingham, said in a press release.
The recipient was given standard maintenance immunosuppression - tacrolimus, mycophenolate mofetil, and prednisone. The target tacrolimus level (8-10 ng/dL) was reached by postoperative day 2 and was maintained through study completion.
At the end of the study, the serum creatinine level was 0.9 mg/dL, and creatinine clearance was 200 mL/min. Creatinine levels are an indicator of kidney function and demonstrate the organ’s ability to filter waste from blood, according to Roger Lord, PhD, senior lecturer (medical sciences) in the School of Behavioural and Health Sciences, Australian Catholic University, who was not involved in the research.
This is the first time that it has been demonstrated that a standard immunosuppression regimen may be sufficient to support xenotransplantation with pig kidneys and in which creatinine clearance was achieved.
The finding comes less than 2 years after the same team published results from a similar experiment. In that transplant, the investigators didn’t observe significant creatinine excretion into the urine.
In the team’s previous attempts, kidney function was delayed because the brain-dead recipients had deteriorated physiologically. This time, the subject was stable, and the team was able to observe urine production within 4 minutes of restoration of blood flow to the transplanted pig organs.
“This new work firmly establishes that the xenografts not only make urine but provide life-sustaining kidney function by clearing serum creatinine,” Locke said in an interview. “This is the first time in history this has been shown.”
The investigators are hoping animal-sourced organs could become an alternative for human transplantations, potentially solving the serious shortage of human organs available for patients on transplant waiting lists.
Organ transplantation can treat patients with advanced kidney disease and kidney failure, but there are not enough human organs available to meet the need. More than 92,000 people in the United States are waiting for a kidney, according to the American Kidney Fund.
Organ rejection is a risk with xenotransplants – animal-to-human organ transplants. Investigators in this study used kidneys from pigs with 10 gene modifications. The modifications were intended to decrease the likelihood of the organs being rejected by a human host.
The kidneys were still viable at the end of the 7-day period. In addition, there was no microscopic blood clot formation, another indicator of normal kidney function, according to Dr. Lord, who provided comments to the UK Science Media Centre.
The long-term outcomes of animal-to-human organ transplantation remain unclear. Dr. Lord describes the operation as a “first step” to demonstrate that genetically modified, transplanted pig kidneys can function normally so as to remove creatinine over a 7-day period.
Dr. Locke and colleagues said: “Future research in living human recipients is necessary to determine long-term xenograft kidney function and whether xenografts could serve as a bridge or destination therapy for end-stage kidney disease.
“Because our study represents a single case, generalizability of the findings is limited. This study showcases xenotransplant as a viable potential solution to an organ shortage crisis responsible for thousands of preventable deaths annually,” they concluded.
A version of this article first appeared on Medscape.com .
– marking another important step toward opening up a new supply of much-needed organs for those with end-stage kidney disease.
A team of researchers in Alabama removed a brain-dead person’s kidneys and transplanted two kidneys that had been taken from a genetically modified pig. The researchers monitored the patient’s response to the organs and tracked the kidneys’ function over a 7-day period. The findings were published in JAMA Surgery.
During the first 24 hours after transplantation, the pig kidneys made more than 37 liters of urine. “It was really a remarkable thing to see,” lead investigator Jayme Locke, MD, professor of surgery and the Arnold G. Diethelm Endowed Chair in Transplantation Surgery, University of Alabama at Birmingham, said in a press release.
The recipient was given standard maintenance immunosuppression - tacrolimus, mycophenolate mofetil, and prednisone. The target tacrolimus level (8-10 ng/dL) was reached by postoperative day 2 and was maintained through study completion.
At the end of the study, the serum creatinine level was 0.9 mg/dL, and creatinine clearance was 200 mL/min. Creatinine levels are an indicator of kidney function and demonstrate the organ’s ability to filter waste from blood, according to Roger Lord, PhD, senior lecturer (medical sciences) in the School of Behavioural and Health Sciences, Australian Catholic University, who was not involved in the research.
This is the first time that it has been demonstrated that a standard immunosuppression regimen may be sufficient to support xenotransplantation with pig kidneys and in which creatinine clearance was achieved.
The finding comes less than 2 years after the same team published results from a similar experiment. In that transplant, the investigators didn’t observe significant creatinine excretion into the urine.
In the team’s previous attempts, kidney function was delayed because the brain-dead recipients had deteriorated physiologically. This time, the subject was stable, and the team was able to observe urine production within 4 minutes of restoration of blood flow to the transplanted pig organs.
“This new work firmly establishes that the xenografts not only make urine but provide life-sustaining kidney function by clearing serum creatinine,” Locke said in an interview. “This is the first time in history this has been shown.”
The investigators are hoping animal-sourced organs could become an alternative for human transplantations, potentially solving the serious shortage of human organs available for patients on transplant waiting lists.
Organ transplantation can treat patients with advanced kidney disease and kidney failure, but there are not enough human organs available to meet the need. More than 92,000 people in the United States are waiting for a kidney, according to the American Kidney Fund.
Organ rejection is a risk with xenotransplants – animal-to-human organ transplants. Investigators in this study used kidneys from pigs with 10 gene modifications. The modifications were intended to decrease the likelihood of the organs being rejected by a human host.
The kidneys were still viable at the end of the 7-day period. In addition, there was no microscopic blood clot formation, another indicator of normal kidney function, according to Dr. Lord, who provided comments to the UK Science Media Centre.
The long-term outcomes of animal-to-human organ transplantation remain unclear. Dr. Lord describes the operation as a “first step” to demonstrate that genetically modified, transplanted pig kidneys can function normally so as to remove creatinine over a 7-day period.
Dr. Locke and colleagues said: “Future research in living human recipients is necessary to determine long-term xenograft kidney function and whether xenografts could serve as a bridge or destination therapy for end-stage kidney disease.
“Because our study represents a single case, generalizability of the findings is limited. This study showcases xenotransplant as a viable potential solution to an organ shortage crisis responsible for thousands of preventable deaths annually,” they concluded.
A version of this article first appeared on Medscape.com .
– marking another important step toward opening up a new supply of much-needed organs for those with end-stage kidney disease.
A team of researchers in Alabama removed a brain-dead person’s kidneys and transplanted two kidneys that had been taken from a genetically modified pig. The researchers monitored the patient’s response to the organs and tracked the kidneys’ function over a 7-day period. The findings were published in JAMA Surgery.
During the first 24 hours after transplantation, the pig kidneys made more than 37 liters of urine. “It was really a remarkable thing to see,” lead investigator Jayme Locke, MD, professor of surgery and the Arnold G. Diethelm Endowed Chair in Transplantation Surgery, University of Alabama at Birmingham, said in a press release.
The recipient was given standard maintenance immunosuppression - tacrolimus, mycophenolate mofetil, and prednisone. The target tacrolimus level (8-10 ng/dL) was reached by postoperative day 2 and was maintained through study completion.
At the end of the study, the serum creatinine level was 0.9 mg/dL, and creatinine clearance was 200 mL/min. Creatinine levels are an indicator of kidney function and demonstrate the organ’s ability to filter waste from blood, according to Roger Lord, PhD, senior lecturer (medical sciences) in the School of Behavioural and Health Sciences, Australian Catholic University, who was not involved in the research.
This is the first time that it has been demonstrated that a standard immunosuppression regimen may be sufficient to support xenotransplantation with pig kidneys and in which creatinine clearance was achieved.
The finding comes less than 2 years after the same team published results from a similar experiment. In that transplant, the investigators didn’t observe significant creatinine excretion into the urine.
In the team’s previous attempts, kidney function was delayed because the brain-dead recipients had deteriorated physiologically. This time, the subject was stable, and the team was able to observe urine production within 4 minutes of restoration of blood flow to the transplanted pig organs.
“This new work firmly establishes that the xenografts not only make urine but provide life-sustaining kidney function by clearing serum creatinine,” Locke said in an interview. “This is the first time in history this has been shown.”
The investigators are hoping animal-sourced organs could become an alternative for human transplantations, potentially solving the serious shortage of human organs available for patients on transplant waiting lists.
Organ transplantation can treat patients with advanced kidney disease and kidney failure, but there are not enough human organs available to meet the need. More than 92,000 people in the United States are waiting for a kidney, according to the American Kidney Fund.
Organ rejection is a risk with xenotransplants – animal-to-human organ transplants. Investigators in this study used kidneys from pigs with 10 gene modifications. The modifications were intended to decrease the likelihood of the organs being rejected by a human host.
The kidneys were still viable at the end of the 7-day period. In addition, there was no microscopic blood clot formation, another indicator of normal kidney function, according to Dr. Lord, who provided comments to the UK Science Media Centre.
The long-term outcomes of animal-to-human organ transplantation remain unclear. Dr. Lord describes the operation as a “first step” to demonstrate that genetically modified, transplanted pig kidneys can function normally so as to remove creatinine over a 7-day period.
Dr. Locke and colleagues said: “Future research in living human recipients is necessary to determine long-term xenograft kidney function and whether xenografts could serve as a bridge or destination therapy for end-stage kidney disease.
“Because our study represents a single case, generalizability of the findings is limited. This study showcases xenotransplant as a viable potential solution to an organ shortage crisis responsible for thousands of preventable deaths annually,” they concluded.
A version of this article first appeared on Medscape.com .
FROM JAMA SURGERY
Better than dialysis? Artificial kidney could be the future
Nearly 90,000 patients in the United States are waiting for a lifesaving kidney transplant, yet only about 25,000 kidney transplants were performed last year. Thousands die each year while they wait. Others are not suitable transplant candidates.
Half a million people are on dialysis, the only transplant alternative for those with kidney failure. This greatly impacts their work, relationships, and quality of life.
Researchers from The Kidney Project hope to solve this public health crisis with a futuristic approach: an implantable bioartificial kidney. That hope is slowly approaching reality. Early prototypes have been tested successfully in preclinical research and clinical trials could lie ahead.
This news organization spoke with two researchers who came up with the idea: nephrologist William Dr. Fissell, MD, of Vanderbilt University in Nashville, Tenn., and Shuvo Dr. Roy, PhD, a biomedical engineer at the University of California, San Francisco. This interview has been edited for length and clarity.
Question: Could you summarize the clinical problem with chronic kidney disease?
Dr. Fissell: Dialysis treatment, although lifesaving, is incomplete. Healthy kidneys do a variety of things that dialysis cannot provide. Transplant is absolutely the best remedy, but donor organs are vanishingly scarce.
Do you envision your implantable, bioartificial kidney as a bridge to transplantation? Or can it be even more, like a bionic organ, as good as a natural organ and thus better than a transplant?
Dr. Roy: We see it initially as a bridge to transplantation or as a better option than dialysis for those who will never get a transplant. We’re not trying to create the “Six Million Dollar Man.” The goal is to keep patients off dialysis – to deliver some, but probably not all, of the benefits of a kidney transplant in a mass-produced device that anybody can receive.
Dr. Fissell: The technology is aimed at people in stage 5 renal disease, the final stage, when kidneys are failing, and dialysis is the only option to maintain life. We want to make dialysis a thing of the past, put dialysis machines in museums like the iron lung, which was so vital to keeping people alive several decades ago but is mostly obsolete today.
How did you two come up with this idea? How did you get started working together?
Dr. Roy: I had just begun my career as a research biomedical engineer when I met Dr. William Fissell, who was then contemplating a career in nephrology. He opened my eyes to the problems faced by patients affected by kidney failure. Through our discussions, we quickly realized that while we could improve dialysis machines, patients needed and deserved something better – a treatment that improves their health while also allowing them to keep a job, travel readily, and consume food and drink without restrictions. Basically, something that works more like a kidney transplant.
How does the artificial kidney differ from dialysis?
Dr. Fissell: Dialysis is an intermittent stop-and-start treatment. The artificial kidney is continuous, around-the-clock treatment. There are a couple of advantages to that. The first is that you can maintain your body’s fluid balance. In dialysis, you get rid of 2-3 days’ worth of fluid in a couple of hours, and that’s very stressful to the heart and maybe to the brain as well. Second advantage is that patients will be able to eat a normal diet. Some waste products that are byproducts of our nutritional intake are slow to leave the body. So in dialysis, we restrict the diet severely and add medicines to soak up extra phosphorus. With a continuous treatment, you can balance excretion and intake.
The other aspect is that dialysis requires an immense amount of disposables. Hundreds of liters of water per patient per treatment, hundreds of thousands of dialysis cartridges and IV bags every year. The artificial kidney doesn’t need a water supply, disposable sorbent, or cartridges.
How does the artificial kidney work?
Dr. Fissell: Just like a healthy kidney. We have a unit that filters the blood so that red blood cells, white blood cells, platelets, antibodies, albumin – all the good stuff that your body worked hard to synthesize – stays in the blood, but a watery soup of toxins and waste is separated out. In a second unit, called the bioreactor, kidney cells concentrate those wastes and toxins into urine.
Dr. Roy: We used a technology called silicon micro-machining to invent an entirely new membrane that mimics a healthy kidney’s filters. It filters the blood just using the patient’s heart as a pump. No electric motors, no batteries, no wires. This lets us have something that’s completely implanted.
We also developed a cell culture of kidney cells that function in an artificial kidney. Normally, cells in a dish don’t fully adopt the features of a cell in the body. We looked at the literature around 3-D printing of organs. We learned that, in addition to fluid flow, stiff scaffolds, like cell culture dishes, trigger specific signals that keep the cells from functioning. We overcame that by looking at the physical microenvironment of the cells – not the hormones and proteins, but instead the fundamentals of the laboratory environment. For example, most organs are soft, yet plastic lab dishes are hard. By using tools that replicated the softness and fluid flow of a healthy kidney, remarkably, these cells functioned better than on a plastic dish.
Would patients need immunosuppressive or anticoagulation medication?
Dr. Fissell: They wouldn’t need either. The structure and chemistry of the device prevents blood from clotting. And the membranes in the device are a physical barrier between the host immune system and the donor cells, so the body won’t reject the device.
What is the state of the technology now?
Dr. Fissell: We have shown the function of the filters and the function of the cells, both separately and together, in preclinical in vivo testing. What we now need to do is construct clinical-grade devices and complete sterility and biocompatibility testing to initiate a human trial. That’s going to take between $12 million and $15 million in device manufacturing.
So it’s more a matter of money than time until the first clinical trials?
Dr. Roy: Yes, exactly. We don’t like to say that a clinical trial will start by such-and-such year. From the very start of the project, we have been resource limited.
A version of this article first appeared on Medscape.com.
Nearly 90,000 patients in the United States are waiting for a lifesaving kidney transplant, yet only about 25,000 kidney transplants were performed last year. Thousands die each year while they wait. Others are not suitable transplant candidates.
Half a million people are on dialysis, the only transplant alternative for those with kidney failure. This greatly impacts their work, relationships, and quality of life.
Researchers from The Kidney Project hope to solve this public health crisis with a futuristic approach: an implantable bioartificial kidney. That hope is slowly approaching reality. Early prototypes have been tested successfully in preclinical research and clinical trials could lie ahead.
This news organization spoke with two researchers who came up with the idea: nephrologist William Dr. Fissell, MD, of Vanderbilt University in Nashville, Tenn., and Shuvo Dr. Roy, PhD, a biomedical engineer at the University of California, San Francisco. This interview has been edited for length and clarity.
Question: Could you summarize the clinical problem with chronic kidney disease?
Dr. Fissell: Dialysis treatment, although lifesaving, is incomplete. Healthy kidneys do a variety of things that dialysis cannot provide. Transplant is absolutely the best remedy, but donor organs are vanishingly scarce.
Do you envision your implantable, bioartificial kidney as a bridge to transplantation? Or can it be even more, like a bionic organ, as good as a natural organ and thus better than a transplant?
Dr. Roy: We see it initially as a bridge to transplantation or as a better option than dialysis for those who will never get a transplant. We’re not trying to create the “Six Million Dollar Man.” The goal is to keep patients off dialysis – to deliver some, but probably not all, of the benefits of a kidney transplant in a mass-produced device that anybody can receive.
Dr. Fissell: The technology is aimed at people in stage 5 renal disease, the final stage, when kidneys are failing, and dialysis is the only option to maintain life. We want to make dialysis a thing of the past, put dialysis machines in museums like the iron lung, which was so vital to keeping people alive several decades ago but is mostly obsolete today.
How did you two come up with this idea? How did you get started working together?
Dr. Roy: I had just begun my career as a research biomedical engineer when I met Dr. William Fissell, who was then contemplating a career in nephrology. He opened my eyes to the problems faced by patients affected by kidney failure. Through our discussions, we quickly realized that while we could improve dialysis machines, patients needed and deserved something better – a treatment that improves their health while also allowing them to keep a job, travel readily, and consume food and drink without restrictions. Basically, something that works more like a kidney transplant.
How does the artificial kidney differ from dialysis?
Dr. Fissell: Dialysis is an intermittent stop-and-start treatment. The artificial kidney is continuous, around-the-clock treatment. There are a couple of advantages to that. The first is that you can maintain your body’s fluid balance. In dialysis, you get rid of 2-3 days’ worth of fluid in a couple of hours, and that’s very stressful to the heart and maybe to the brain as well. Second advantage is that patients will be able to eat a normal diet. Some waste products that are byproducts of our nutritional intake are slow to leave the body. So in dialysis, we restrict the diet severely and add medicines to soak up extra phosphorus. With a continuous treatment, you can balance excretion and intake.
The other aspect is that dialysis requires an immense amount of disposables. Hundreds of liters of water per patient per treatment, hundreds of thousands of dialysis cartridges and IV bags every year. The artificial kidney doesn’t need a water supply, disposable sorbent, or cartridges.
How does the artificial kidney work?
Dr. Fissell: Just like a healthy kidney. We have a unit that filters the blood so that red blood cells, white blood cells, platelets, antibodies, albumin – all the good stuff that your body worked hard to synthesize – stays in the blood, but a watery soup of toxins and waste is separated out. In a second unit, called the bioreactor, kidney cells concentrate those wastes and toxins into urine.
Dr. Roy: We used a technology called silicon micro-machining to invent an entirely new membrane that mimics a healthy kidney’s filters. It filters the blood just using the patient’s heart as a pump. No electric motors, no batteries, no wires. This lets us have something that’s completely implanted.
We also developed a cell culture of kidney cells that function in an artificial kidney. Normally, cells in a dish don’t fully adopt the features of a cell in the body. We looked at the literature around 3-D printing of organs. We learned that, in addition to fluid flow, stiff scaffolds, like cell culture dishes, trigger specific signals that keep the cells from functioning. We overcame that by looking at the physical microenvironment of the cells – not the hormones and proteins, but instead the fundamentals of the laboratory environment. For example, most organs are soft, yet plastic lab dishes are hard. By using tools that replicated the softness and fluid flow of a healthy kidney, remarkably, these cells functioned better than on a plastic dish.
Would patients need immunosuppressive or anticoagulation medication?
Dr. Fissell: They wouldn’t need either. The structure and chemistry of the device prevents blood from clotting. And the membranes in the device are a physical barrier between the host immune system and the donor cells, so the body won’t reject the device.
What is the state of the technology now?
Dr. Fissell: We have shown the function of the filters and the function of the cells, both separately and together, in preclinical in vivo testing. What we now need to do is construct clinical-grade devices and complete sterility and biocompatibility testing to initiate a human trial. That’s going to take between $12 million and $15 million in device manufacturing.
So it’s more a matter of money than time until the first clinical trials?
Dr. Roy: Yes, exactly. We don’t like to say that a clinical trial will start by such-and-such year. From the very start of the project, we have been resource limited.
A version of this article first appeared on Medscape.com.
Nearly 90,000 patients in the United States are waiting for a lifesaving kidney transplant, yet only about 25,000 kidney transplants were performed last year. Thousands die each year while they wait. Others are not suitable transplant candidates.
Half a million people are on dialysis, the only transplant alternative for those with kidney failure. This greatly impacts their work, relationships, and quality of life.
Researchers from The Kidney Project hope to solve this public health crisis with a futuristic approach: an implantable bioartificial kidney. That hope is slowly approaching reality. Early prototypes have been tested successfully in preclinical research and clinical trials could lie ahead.
This news organization spoke with two researchers who came up with the idea: nephrologist William Dr. Fissell, MD, of Vanderbilt University in Nashville, Tenn., and Shuvo Dr. Roy, PhD, a biomedical engineer at the University of California, San Francisco. This interview has been edited for length and clarity.
Question: Could you summarize the clinical problem with chronic kidney disease?
Dr. Fissell: Dialysis treatment, although lifesaving, is incomplete. Healthy kidneys do a variety of things that dialysis cannot provide. Transplant is absolutely the best remedy, but donor organs are vanishingly scarce.
Do you envision your implantable, bioartificial kidney as a bridge to transplantation? Or can it be even more, like a bionic organ, as good as a natural organ and thus better than a transplant?
Dr. Roy: We see it initially as a bridge to transplantation or as a better option than dialysis for those who will never get a transplant. We’re not trying to create the “Six Million Dollar Man.” The goal is to keep patients off dialysis – to deliver some, but probably not all, of the benefits of a kidney transplant in a mass-produced device that anybody can receive.
Dr. Fissell: The technology is aimed at people in stage 5 renal disease, the final stage, when kidneys are failing, and dialysis is the only option to maintain life. We want to make dialysis a thing of the past, put dialysis machines in museums like the iron lung, which was so vital to keeping people alive several decades ago but is mostly obsolete today.
How did you two come up with this idea? How did you get started working together?
Dr. Roy: I had just begun my career as a research biomedical engineer when I met Dr. William Fissell, who was then contemplating a career in nephrology. He opened my eyes to the problems faced by patients affected by kidney failure. Through our discussions, we quickly realized that while we could improve dialysis machines, patients needed and deserved something better – a treatment that improves their health while also allowing them to keep a job, travel readily, and consume food and drink without restrictions. Basically, something that works more like a kidney transplant.
How does the artificial kidney differ from dialysis?
Dr. Fissell: Dialysis is an intermittent stop-and-start treatment. The artificial kidney is continuous, around-the-clock treatment. There are a couple of advantages to that. The first is that you can maintain your body’s fluid balance. In dialysis, you get rid of 2-3 days’ worth of fluid in a couple of hours, and that’s very stressful to the heart and maybe to the brain as well. Second advantage is that patients will be able to eat a normal diet. Some waste products that are byproducts of our nutritional intake are slow to leave the body. So in dialysis, we restrict the diet severely and add medicines to soak up extra phosphorus. With a continuous treatment, you can balance excretion and intake.
The other aspect is that dialysis requires an immense amount of disposables. Hundreds of liters of water per patient per treatment, hundreds of thousands of dialysis cartridges and IV bags every year. The artificial kidney doesn’t need a water supply, disposable sorbent, or cartridges.
How does the artificial kidney work?
Dr. Fissell: Just like a healthy kidney. We have a unit that filters the blood so that red blood cells, white blood cells, platelets, antibodies, albumin – all the good stuff that your body worked hard to synthesize – stays in the blood, but a watery soup of toxins and waste is separated out. In a second unit, called the bioreactor, kidney cells concentrate those wastes and toxins into urine.
Dr. Roy: We used a technology called silicon micro-machining to invent an entirely new membrane that mimics a healthy kidney’s filters. It filters the blood just using the patient’s heart as a pump. No electric motors, no batteries, no wires. This lets us have something that’s completely implanted.
We also developed a cell culture of kidney cells that function in an artificial kidney. Normally, cells in a dish don’t fully adopt the features of a cell in the body. We looked at the literature around 3-D printing of organs. We learned that, in addition to fluid flow, stiff scaffolds, like cell culture dishes, trigger specific signals that keep the cells from functioning. We overcame that by looking at the physical microenvironment of the cells – not the hormones and proteins, but instead the fundamentals of the laboratory environment. For example, most organs are soft, yet plastic lab dishes are hard. By using tools that replicated the softness and fluid flow of a healthy kidney, remarkably, these cells functioned better than on a plastic dish.
Would patients need immunosuppressive or anticoagulation medication?
Dr. Fissell: They wouldn’t need either. The structure and chemistry of the device prevents blood from clotting. And the membranes in the device are a physical barrier between the host immune system and the donor cells, so the body won’t reject the device.
What is the state of the technology now?
Dr. Fissell: We have shown the function of the filters and the function of the cells, both separately and together, in preclinical in vivo testing. What we now need to do is construct clinical-grade devices and complete sterility and biocompatibility testing to initiate a human trial. That’s going to take between $12 million and $15 million in device manufacturing.
So it’s more a matter of money than time until the first clinical trials?
Dr. Roy: Yes, exactly. We don’t like to say that a clinical trial will start by such-and-such year. From the very start of the project, we have been resource limited.
A version of this article first appeared on Medscape.com.
Higher occurrence of kidney stones with more added sugar
Consuming a higher percentage of calories from added sugars is linked with a higher prevalence of kidney stones, new research suggests.
Though added sugars have been linked with multiple poor health outcomes, their link with kidney stones has been unclear.
Added sugars are sugars or caloric sweeteners added to foods or drinks during processing or preparation to add flavor or shelf life. They do not include natural sugars such as lactose in milk and fructose in fruits.
Researchers, led by Shan Yin, a urologist at Affiliated Hospital of North Sichuan Medical College, in Nanchong, China, compared the added-sugar intake by quartiles in the U.S. National Health and Nutrition Examination Survey 2007-2018.
A total of 28,303 adults were included in this study, with an average age of 48. Women who consumed less than 600 or more than 3,500 kcal or men who consumed less than 800 or more than 4,200 kcal were excluded.
Researchers adjusted for factors including age, race, education, income, physical activity, and marital, employment, and smoking status.
Compared with the first quartile of percentage added-sugar calorie intake, the population in the fourth quartile, with the highest added sugar intake, had a higher prevalence of kidney stones (odds ratio, 1.39; 95% confidence interval, 1.17-1.65).
Compared with the group with fewer than 5% of calories from added sugar, the group that consumed at least 25% of calories from added sugar had nearly twice the prevalence of kidney stones (OR, 1.88; 95% CI, 1.52-2.32).
Findings were published online in Frontiers in Nutrition.
“By identifying this association, policymakers and health professionals can emphasize the need for public health initiatives to reduce added sugar consumption and promote healthy dietary habits,” the authors write.
Added sugar in the U.S. diet
Sugar-sweetened beverages such as soft drinks and energy and sports drinks account for 34.4% of added sugars in the American diet. Previous studies have shown the relationship between consuming sugar-sweetened beverages and a higher risk of obesity, diabetes, and cardiovascular disease, diseases that often co-occur with kidney stones.
Researchers note that even though most added sugars in the United States come from sugar-sweetened beverages, it’s unclear whether the association between added sugars and kidney stones is caused by the beverages or other sources. For instance, fructose intake has been found to be independently associated with kidney stones.
How much is too much?
The recommended upper limit on added sugar is controversial and varies widely by health organization. The American Heart Association says daily average intake from added sugars should be no more than 150 kcal for adult males (about 9 teaspoons) and no more than 100 kcal for women (about 6 teaspoons). The Institute of Medicine allows up to 25% of calories to be consumed from added sugars. The 2020 Dietary Guidelines for Americans and World Health Organization set 10% of calories as the recommended upper limit.
Further investigating what causes kidney stones is critical as kidney stones are common worldwide, affecting about 1 in 10 people in the United States alone, and occurrence is increasing. Kidney stones have a high recurrence rate – about half of people who get them have a second episode within 10 years, the authors note.
The researchers acknowledge that because participants self-reported food intake, there is the potential for recall bias. Additionally, because of the cross-sectional design, the researchers were not able to determine whether sugar intake or kidney stone occurrence came first.
This work was supported by the Doctoral Fund Project of North Sichuan Medical College. The authors declare no relevant financial relationships.
Consuming a higher percentage of calories from added sugars is linked with a higher prevalence of kidney stones, new research suggests.
Though added sugars have been linked with multiple poor health outcomes, their link with kidney stones has been unclear.
Added sugars are sugars or caloric sweeteners added to foods or drinks during processing or preparation to add flavor or shelf life. They do not include natural sugars such as lactose in milk and fructose in fruits.
Researchers, led by Shan Yin, a urologist at Affiliated Hospital of North Sichuan Medical College, in Nanchong, China, compared the added-sugar intake by quartiles in the U.S. National Health and Nutrition Examination Survey 2007-2018.
A total of 28,303 adults were included in this study, with an average age of 48. Women who consumed less than 600 or more than 3,500 kcal or men who consumed less than 800 or more than 4,200 kcal were excluded.
Researchers adjusted for factors including age, race, education, income, physical activity, and marital, employment, and smoking status.
Compared with the first quartile of percentage added-sugar calorie intake, the population in the fourth quartile, with the highest added sugar intake, had a higher prevalence of kidney stones (odds ratio, 1.39; 95% confidence interval, 1.17-1.65).
Compared with the group with fewer than 5% of calories from added sugar, the group that consumed at least 25% of calories from added sugar had nearly twice the prevalence of kidney stones (OR, 1.88; 95% CI, 1.52-2.32).
Findings were published online in Frontiers in Nutrition.
“By identifying this association, policymakers and health professionals can emphasize the need for public health initiatives to reduce added sugar consumption and promote healthy dietary habits,” the authors write.
Added sugar in the U.S. diet
Sugar-sweetened beverages such as soft drinks and energy and sports drinks account for 34.4% of added sugars in the American diet. Previous studies have shown the relationship between consuming sugar-sweetened beverages and a higher risk of obesity, diabetes, and cardiovascular disease, diseases that often co-occur with kidney stones.
Researchers note that even though most added sugars in the United States come from sugar-sweetened beverages, it’s unclear whether the association between added sugars and kidney stones is caused by the beverages or other sources. For instance, fructose intake has been found to be independently associated with kidney stones.
How much is too much?
The recommended upper limit on added sugar is controversial and varies widely by health organization. The American Heart Association says daily average intake from added sugars should be no more than 150 kcal for adult males (about 9 teaspoons) and no more than 100 kcal for women (about 6 teaspoons). The Institute of Medicine allows up to 25% of calories to be consumed from added sugars. The 2020 Dietary Guidelines for Americans and World Health Organization set 10% of calories as the recommended upper limit.
Further investigating what causes kidney stones is critical as kidney stones are common worldwide, affecting about 1 in 10 people in the United States alone, and occurrence is increasing. Kidney stones have a high recurrence rate – about half of people who get them have a second episode within 10 years, the authors note.
The researchers acknowledge that because participants self-reported food intake, there is the potential for recall bias. Additionally, because of the cross-sectional design, the researchers were not able to determine whether sugar intake or kidney stone occurrence came first.
This work was supported by the Doctoral Fund Project of North Sichuan Medical College. The authors declare no relevant financial relationships.
Consuming a higher percentage of calories from added sugars is linked with a higher prevalence of kidney stones, new research suggests.
Though added sugars have been linked with multiple poor health outcomes, their link with kidney stones has been unclear.
Added sugars are sugars or caloric sweeteners added to foods or drinks during processing or preparation to add flavor or shelf life. They do not include natural sugars such as lactose in milk and fructose in fruits.
Researchers, led by Shan Yin, a urologist at Affiliated Hospital of North Sichuan Medical College, in Nanchong, China, compared the added-sugar intake by quartiles in the U.S. National Health and Nutrition Examination Survey 2007-2018.
A total of 28,303 adults were included in this study, with an average age of 48. Women who consumed less than 600 or more than 3,500 kcal or men who consumed less than 800 or more than 4,200 kcal were excluded.
Researchers adjusted for factors including age, race, education, income, physical activity, and marital, employment, and smoking status.
Compared with the first quartile of percentage added-sugar calorie intake, the population in the fourth quartile, with the highest added sugar intake, had a higher prevalence of kidney stones (odds ratio, 1.39; 95% confidence interval, 1.17-1.65).
Compared with the group with fewer than 5% of calories from added sugar, the group that consumed at least 25% of calories from added sugar had nearly twice the prevalence of kidney stones (OR, 1.88; 95% CI, 1.52-2.32).
Findings were published online in Frontiers in Nutrition.
“By identifying this association, policymakers and health professionals can emphasize the need for public health initiatives to reduce added sugar consumption and promote healthy dietary habits,” the authors write.
Added sugar in the U.S. diet
Sugar-sweetened beverages such as soft drinks and energy and sports drinks account for 34.4% of added sugars in the American diet. Previous studies have shown the relationship between consuming sugar-sweetened beverages and a higher risk of obesity, diabetes, and cardiovascular disease, diseases that often co-occur with kidney stones.
Researchers note that even though most added sugars in the United States come from sugar-sweetened beverages, it’s unclear whether the association between added sugars and kidney stones is caused by the beverages or other sources. For instance, fructose intake has been found to be independently associated with kidney stones.
How much is too much?
The recommended upper limit on added sugar is controversial and varies widely by health organization. The American Heart Association says daily average intake from added sugars should be no more than 150 kcal for adult males (about 9 teaspoons) and no more than 100 kcal for women (about 6 teaspoons). The Institute of Medicine allows up to 25% of calories to be consumed from added sugars. The 2020 Dietary Guidelines for Americans and World Health Organization set 10% of calories as the recommended upper limit.
Further investigating what causes kidney stones is critical as kidney stones are common worldwide, affecting about 1 in 10 people in the United States alone, and occurrence is increasing. Kidney stones have a high recurrence rate – about half of people who get them have a second episode within 10 years, the authors note.
The researchers acknowledge that because participants self-reported food intake, there is the potential for recall bias. Additionally, because of the cross-sectional design, the researchers were not able to determine whether sugar intake or kidney stone occurrence came first.
This work was supported by the Doctoral Fund Project of North Sichuan Medical College. The authors declare no relevant financial relationships.
FROM FRONTIERS IN NUTRITION
Experts call for early screening for chronic kidney disease
MADRID – A late diagnosis of chronic kidney disease is cause for concern. Scientific societies are therefore advocating for screening at younger ages to reverse this trend and slow the progression of the disease. Nearly all patients seen in primary care are candidates for screening because of their risk factors for kidney disease.
During the 29th National Conference of General and Family Medicine of the Spanish Society for General and Family Physicians, Teresa Benedito, MD, family doctor and member of the society’s cardiovascular group, and Roberto Alcázar, MD, nephrologist at the Infanta Leonor University Hospital, Madrid, presented a clinical case encountered in primary care. They used this case to frame a strong argument for the importance of early screening for chronic kidney disease, and they discussed how to properly manage such screening.
The presentation followed the guidelines in the SEMG publication regarding the management and referral of patients with type 2 diabetes. Dr. Benedito explained that the first thing to ask oneself during a patient visit is “whether they present risk factors for kidney disease. If so, we can’t let them leave before we do a kidney screening.” She then listed the factors in question: age older than 60 years, African heritage, family history of chronic kidney disease, decreased kidney mass, weight loss at birth, hypertension, diabetes, smoking, obesity, and low socioeconomic status.
For his part, Dr. Alcázar mentioned how these factors are similar to cardiovascular risk factors, because “the kidneys are a ball of vessels with double capillarization for purifying blood. They’re the organs with the most arteries per unit of weight, so anything that can damage the arteries can damage the kidneys.”
Candidates for screening
“Chronic kidney disease develops in 15% of the adult population in Spain. So, it’s worth asking how many patients have been diagnosed and who should we should be screening.” To the factors listed above, Dr. Alcázar added treatment with nephrotoxic drugs (including nonsteroidal anti-inflammatory drugs) for patients with obstructive urinary tract disease, and a history of acute kidney injury for patients with chronic autoimmune disease or neoplasms. “Thus, nearly all patients seen in primary care would need to be screened.”
Another fundamental question raised was whether patients should be screened before age 60 years. “As a nephrologist, I feel that we have been diagnosing chronic kidney disease late, even though we’ve been doing everything by the book,” said Dr. Alcázar. In his opinion, “the answer to whether we should be screening earlier ... is yes, for two reasons: first, because it’s cost-effective, and second, because it’s very inexpensive.”
Dr. Benedito explained in detail the process for diagnosing this disease. She began by defining the disease as changes in kidney structure and function that last longer than 3 months. These changes are identified by use of two criteria: glomerular filtration rate less than 60 mL/min and kidney injury or lesions with or without reduced filtration rate (renal biopsy, albumin/creatinine ratio greater than 30 mg/g, proteinuria, alterations in urinary sediment or in imaging tests). Thus, “if one of these two criteria persists for more than 3 months, the diagnosis is chronic kidney disease. Also, high creatinine levels are not diagnostic for the disease,” she emphasized.
Two related parameters
Glomerular filtration and albuminuria “are highly relevant, because screening for chronic kidney disease is based on these two parameters,” said Dr. Benedito. Glomerular filtration rate varies with age, sex, ethnicity, and body mass. It is useful for identifying the stage of the disease and for monitoring disease progression. Albuminuria, on the other hand, is an indication of the severity of the disease. It’s an early marker for kidney injury and systemic disease and is more sensitive than proteinuria. Therefore, “this factor, together with glomerular filtration rate, allows us to detect, classify, and monitor the progression of chronic kidney disease.”
On this point, Dr. Alcázar emphasized the importance of trends, since variation in glomerular filtration depends on serum creatinine, which can vary by nearly 9%. He explained that glomerular filtration rate is related to the number of nephrons remaining. A glomerular filtration rate of less than 60 mL/min implies that more than half of the nephrons in each kidney have been lost. Albuminuria informs about structural damage (that is, the condition of the remaining nephrons). It’s therefore essential to test for both parameters. “We need to be actively monitoring and then making our decisions based on trends and not on isolated results. We need to be aware of albuminuria when we make our decisions,” said Dr. Alcázar. Some studies have shown the importance of testing for albuminuria whenever creatinine level is assessed. “We need to buy into this. If we don’t do this, we’ll only ever have half the information we need.”
Reducing late diagnosis
According to the IBERICAN study, 14% of patients seen in primary care in Spain have chronic kidney disease. “This statistic should make us stop and think, own our responsibility, and ask ourselves why this screening isn’t taking place [earlier],” said Dr. Benedito. She added, “We need to head off this trend toward late diagnosis. As the disease progresses, it significantly increases cardiovascular risk and leads to higher mortality, going on dialysis, transplants, et cetera.”
Dr. Alcázar noted that 80% of nephrology cases that are referred to him come from primary care. He explained the need to understand that “these patients have a sevenfold greater risk of suffering a serious cardiovascular event within the next year than people without kidney problems.” Most of these patients will experience an event, even if they don’t undergo dialysis (stage 3 and those near stage 4).
Correct staging
Also fundamental is having a detailed understanding of how staging is performed. Dr. Benedito explained that a chart that pairs glomerular filtration rate (six categories) with the level of albuminuria (three categories) should be used during the visit. For example, a case might be classified as G3a-A2. However, the simplified form of the chart may prove more practical. It classifies chronic kidney disease as being associated with mild, moderate, and severe risk, using different colors to aid comprehension.
Dr. Alcázar noted that the latest guidelines from the European Society of Hypertension for 2023 include albuminuria as an important parameter. The guidelines indicate that for a patient with moderate or severe risk, it is not necessary to calculate their score. “It’s considered high cardiovascular risk, and steps would need to be taken for intervention.”
He then listed the tools available for reversing albuminuria. The process begins by reducing salt consumption and involves the use of medications (angiotensin-converting enzyme inhibitors/angiotensin II receptor antagonists, aldosterone receptor antagonists, glucagon-like peptide-1 analogues, and sodium-glucose cotransporter-2 inhibitors, which slow kidney damage regardless of other measures) and strict management of cardiovascular risk factors (smoking, weight management, blood glucose, hypertension, and moderate physical activity).
Reducing cardiovascular risk
Dr. Alcázar highlighted important factors to keep in mind when managing each of the cardiovascular risk factors. For hypertension, the aim is to achieve levels less than 130/80 mm Hg, although recommendations vary, depending on the guidelines consulted. “KDIGO (Kidney Disease: Improving Global Outcomes) 2021 states that there is no evidence for monitoring diastolic blood pressure, only systolic blood pressure. If we measure it according to the standardized form, SBP should be less than 120 mm Hg, and if not, we would fall back on readings of 130/80 mm Hg.”
For lipid control (specifically, low-density lipoprotein cholesterol), the staging chart indicates that for patients at mild risk, levels should be less than 100 mg/dL; for those at moderate risk, less than 70 mg/dL; and for those at severe risk, less than 55 mg/dL. Hypertriglyceridemia “should only be treated with fibrates if it comes in over 1,000 mg/dL. Also, care must be taken, because these drugs interfere with creatinine excretion, increasing it,” said Dr. Alcázar.
Guidelines from the KDIGO and the American Diabetes Association state that anyone with diabetes and chronic kidney disease should receive a sodium-glucose cotransporter-2 inhibitor if their glomerular filtration rate exceeds 20 mL/min, “which may contradict slightly what it says on the label. Also, if they have hypertension, they should take an angiotensin-converting enzyme inhibitor,” said Dr. Alcázar. He added that “oral antidiabetics, including metformin, must be adjusted based on renal function if glomerular filtration rate is under 30 mL/min.”
Act immediately
When asked whether the course of chronic kidney disease can be changed, Dr. Alcázar responded with an emphatic yes and added that cardiovascular risk can also be substantially reduced. “As nephrologists, we don’t have access to patients in early stages. But family doctors do. Hence the importance of early screening, because going on dialysis at age 60 isn’t the same as at 80.” Currently, “scientific societies are encouraging authorities to screen for chronic kidney disease at earlier ages.”
Regarding drug-based therapy, Dr. Alcázar said that “empagliflozin is not currently indicated for chronic kidney disease in adults.” This sodium-glucose cotransporter-2 inhibitor delays kidney disease and reduces morbidity. Both benefits were highlighted in two recent studies (DAPA-CKD and CREDENCE). Published in January, EMPA-KIDNEY presents a new twist on nephroprotection for patients with chronic kidney disease (diabetic or not) whose glomerular filtration rates are between 20 and 40 mL/min without albuminuria or whose glomerular filtration rates are between 45 and 90 mL/min with albuminuria. For more than 6,000 patients, empagliflozin was observed “to clearly reduce kidney disease progression, cardiovascular mortality and all-cause mortality, and the need to go on dialysis,” stated Dr. Alcázar.
What professionals expect
Dr. Benedito also explained the criteria for referral to a specialist: glomerular filtration rate less than 30 mL/min (unless the patient is older than 80 years and does not have progressively worsening renal function), albumin/creatinine ratio greater than 300 mg/g, acute worsening of renal function, progressive worsening of renal function of greater than 5 mL/min/yr, chronic kidney disease, hypertension treated with triple therapy (including a diuretic) at maximum doses, anemia of less than 10 g/dL, and nonurologic hematuria, especially in combination with albuminuria.
Dr. Benedito explained what nephrologists expect from family doctors in the management of chronic kidney disease: “screening for early detection, identifying and treating risk factors for chronic kidney disease, detecting progression and complications, adjusting drugs based on glomerular filtration rate, and ensuring that our patients are benefiting from sodium-glucose cotransporter-2 inhibitors. These are among the most important steps to be taken.”
Dr. Alcázar mentioned what family doctors expect from nephrologists: “two-way communication, accessibility, coordination of actions to be taken, and using shared and mutually agreed-upon protocols.”
This article was translated from the Medscape Spanish Edition and a version appeared on Medscape.com.
MADRID – A late diagnosis of chronic kidney disease is cause for concern. Scientific societies are therefore advocating for screening at younger ages to reverse this trend and slow the progression of the disease. Nearly all patients seen in primary care are candidates for screening because of their risk factors for kidney disease.
During the 29th National Conference of General and Family Medicine of the Spanish Society for General and Family Physicians, Teresa Benedito, MD, family doctor and member of the society’s cardiovascular group, and Roberto Alcázar, MD, nephrologist at the Infanta Leonor University Hospital, Madrid, presented a clinical case encountered in primary care. They used this case to frame a strong argument for the importance of early screening for chronic kidney disease, and they discussed how to properly manage such screening.
The presentation followed the guidelines in the SEMG publication regarding the management and referral of patients with type 2 diabetes. Dr. Benedito explained that the first thing to ask oneself during a patient visit is “whether they present risk factors for kidney disease. If so, we can’t let them leave before we do a kidney screening.” She then listed the factors in question: age older than 60 years, African heritage, family history of chronic kidney disease, decreased kidney mass, weight loss at birth, hypertension, diabetes, smoking, obesity, and low socioeconomic status.
For his part, Dr. Alcázar mentioned how these factors are similar to cardiovascular risk factors, because “the kidneys are a ball of vessels with double capillarization for purifying blood. They’re the organs with the most arteries per unit of weight, so anything that can damage the arteries can damage the kidneys.”
Candidates for screening
“Chronic kidney disease develops in 15% of the adult population in Spain. So, it’s worth asking how many patients have been diagnosed and who should we should be screening.” To the factors listed above, Dr. Alcázar added treatment with nephrotoxic drugs (including nonsteroidal anti-inflammatory drugs) for patients with obstructive urinary tract disease, and a history of acute kidney injury for patients with chronic autoimmune disease or neoplasms. “Thus, nearly all patients seen in primary care would need to be screened.”
Another fundamental question raised was whether patients should be screened before age 60 years. “As a nephrologist, I feel that we have been diagnosing chronic kidney disease late, even though we’ve been doing everything by the book,” said Dr. Alcázar. In his opinion, “the answer to whether we should be screening earlier ... is yes, for two reasons: first, because it’s cost-effective, and second, because it’s very inexpensive.”
Dr. Benedito explained in detail the process for diagnosing this disease. She began by defining the disease as changes in kidney structure and function that last longer than 3 months. These changes are identified by use of two criteria: glomerular filtration rate less than 60 mL/min and kidney injury or lesions with or without reduced filtration rate (renal biopsy, albumin/creatinine ratio greater than 30 mg/g, proteinuria, alterations in urinary sediment or in imaging tests). Thus, “if one of these two criteria persists for more than 3 months, the diagnosis is chronic kidney disease. Also, high creatinine levels are not diagnostic for the disease,” she emphasized.
Two related parameters
Glomerular filtration and albuminuria “are highly relevant, because screening for chronic kidney disease is based on these two parameters,” said Dr. Benedito. Glomerular filtration rate varies with age, sex, ethnicity, and body mass. It is useful for identifying the stage of the disease and for monitoring disease progression. Albuminuria, on the other hand, is an indication of the severity of the disease. It’s an early marker for kidney injury and systemic disease and is more sensitive than proteinuria. Therefore, “this factor, together with glomerular filtration rate, allows us to detect, classify, and monitor the progression of chronic kidney disease.”
On this point, Dr. Alcázar emphasized the importance of trends, since variation in glomerular filtration depends on serum creatinine, which can vary by nearly 9%. He explained that glomerular filtration rate is related to the number of nephrons remaining. A glomerular filtration rate of less than 60 mL/min implies that more than half of the nephrons in each kidney have been lost. Albuminuria informs about structural damage (that is, the condition of the remaining nephrons). It’s therefore essential to test for both parameters. “We need to be actively monitoring and then making our decisions based on trends and not on isolated results. We need to be aware of albuminuria when we make our decisions,” said Dr. Alcázar. Some studies have shown the importance of testing for albuminuria whenever creatinine level is assessed. “We need to buy into this. If we don’t do this, we’ll only ever have half the information we need.”
Reducing late diagnosis
According to the IBERICAN study, 14% of patients seen in primary care in Spain have chronic kidney disease. “This statistic should make us stop and think, own our responsibility, and ask ourselves why this screening isn’t taking place [earlier],” said Dr. Benedito. She added, “We need to head off this trend toward late diagnosis. As the disease progresses, it significantly increases cardiovascular risk and leads to higher mortality, going on dialysis, transplants, et cetera.”
Dr. Alcázar noted that 80% of nephrology cases that are referred to him come from primary care. He explained the need to understand that “these patients have a sevenfold greater risk of suffering a serious cardiovascular event within the next year than people without kidney problems.” Most of these patients will experience an event, even if they don’t undergo dialysis (stage 3 and those near stage 4).
Correct staging
Also fundamental is having a detailed understanding of how staging is performed. Dr. Benedito explained that a chart that pairs glomerular filtration rate (six categories) with the level of albuminuria (three categories) should be used during the visit. For example, a case might be classified as G3a-A2. However, the simplified form of the chart may prove more practical. It classifies chronic kidney disease as being associated with mild, moderate, and severe risk, using different colors to aid comprehension.
Dr. Alcázar noted that the latest guidelines from the European Society of Hypertension for 2023 include albuminuria as an important parameter. The guidelines indicate that for a patient with moderate or severe risk, it is not necessary to calculate their score. “It’s considered high cardiovascular risk, and steps would need to be taken for intervention.”
He then listed the tools available for reversing albuminuria. The process begins by reducing salt consumption and involves the use of medications (angiotensin-converting enzyme inhibitors/angiotensin II receptor antagonists, aldosterone receptor antagonists, glucagon-like peptide-1 analogues, and sodium-glucose cotransporter-2 inhibitors, which slow kidney damage regardless of other measures) and strict management of cardiovascular risk factors (smoking, weight management, blood glucose, hypertension, and moderate physical activity).
Reducing cardiovascular risk
Dr. Alcázar highlighted important factors to keep in mind when managing each of the cardiovascular risk factors. For hypertension, the aim is to achieve levels less than 130/80 mm Hg, although recommendations vary, depending on the guidelines consulted. “KDIGO (Kidney Disease: Improving Global Outcomes) 2021 states that there is no evidence for monitoring diastolic blood pressure, only systolic blood pressure. If we measure it according to the standardized form, SBP should be less than 120 mm Hg, and if not, we would fall back on readings of 130/80 mm Hg.”
For lipid control (specifically, low-density lipoprotein cholesterol), the staging chart indicates that for patients at mild risk, levels should be less than 100 mg/dL; for those at moderate risk, less than 70 mg/dL; and for those at severe risk, less than 55 mg/dL. Hypertriglyceridemia “should only be treated with fibrates if it comes in over 1,000 mg/dL. Also, care must be taken, because these drugs interfere with creatinine excretion, increasing it,” said Dr. Alcázar.
Guidelines from the KDIGO and the American Diabetes Association state that anyone with diabetes and chronic kidney disease should receive a sodium-glucose cotransporter-2 inhibitor if their glomerular filtration rate exceeds 20 mL/min, “which may contradict slightly what it says on the label. Also, if they have hypertension, they should take an angiotensin-converting enzyme inhibitor,” said Dr. Alcázar. He added that “oral antidiabetics, including metformin, must be adjusted based on renal function if glomerular filtration rate is under 30 mL/min.”
Act immediately
When asked whether the course of chronic kidney disease can be changed, Dr. Alcázar responded with an emphatic yes and added that cardiovascular risk can also be substantially reduced. “As nephrologists, we don’t have access to patients in early stages. But family doctors do. Hence the importance of early screening, because going on dialysis at age 60 isn’t the same as at 80.” Currently, “scientific societies are encouraging authorities to screen for chronic kidney disease at earlier ages.”
Regarding drug-based therapy, Dr. Alcázar said that “empagliflozin is not currently indicated for chronic kidney disease in adults.” This sodium-glucose cotransporter-2 inhibitor delays kidney disease and reduces morbidity. Both benefits were highlighted in two recent studies (DAPA-CKD and CREDENCE). Published in January, EMPA-KIDNEY presents a new twist on nephroprotection for patients with chronic kidney disease (diabetic or not) whose glomerular filtration rates are between 20 and 40 mL/min without albuminuria or whose glomerular filtration rates are between 45 and 90 mL/min with albuminuria. For more than 6,000 patients, empagliflozin was observed “to clearly reduce kidney disease progression, cardiovascular mortality and all-cause mortality, and the need to go on dialysis,” stated Dr. Alcázar.
What professionals expect
Dr. Benedito also explained the criteria for referral to a specialist: glomerular filtration rate less than 30 mL/min (unless the patient is older than 80 years and does not have progressively worsening renal function), albumin/creatinine ratio greater than 300 mg/g, acute worsening of renal function, progressive worsening of renal function of greater than 5 mL/min/yr, chronic kidney disease, hypertension treated with triple therapy (including a diuretic) at maximum doses, anemia of less than 10 g/dL, and nonurologic hematuria, especially in combination with albuminuria.
Dr. Benedito explained what nephrologists expect from family doctors in the management of chronic kidney disease: “screening for early detection, identifying and treating risk factors for chronic kidney disease, detecting progression and complications, adjusting drugs based on glomerular filtration rate, and ensuring that our patients are benefiting from sodium-glucose cotransporter-2 inhibitors. These are among the most important steps to be taken.”
Dr. Alcázar mentioned what family doctors expect from nephrologists: “two-way communication, accessibility, coordination of actions to be taken, and using shared and mutually agreed-upon protocols.”
This article was translated from the Medscape Spanish Edition and a version appeared on Medscape.com.
MADRID – A late diagnosis of chronic kidney disease is cause for concern. Scientific societies are therefore advocating for screening at younger ages to reverse this trend and slow the progression of the disease. Nearly all patients seen in primary care are candidates for screening because of their risk factors for kidney disease.
During the 29th National Conference of General and Family Medicine of the Spanish Society for General and Family Physicians, Teresa Benedito, MD, family doctor and member of the society’s cardiovascular group, and Roberto Alcázar, MD, nephrologist at the Infanta Leonor University Hospital, Madrid, presented a clinical case encountered in primary care. They used this case to frame a strong argument for the importance of early screening for chronic kidney disease, and they discussed how to properly manage such screening.
The presentation followed the guidelines in the SEMG publication regarding the management and referral of patients with type 2 diabetes. Dr. Benedito explained that the first thing to ask oneself during a patient visit is “whether they present risk factors for kidney disease. If so, we can’t let them leave before we do a kidney screening.” She then listed the factors in question: age older than 60 years, African heritage, family history of chronic kidney disease, decreased kidney mass, weight loss at birth, hypertension, diabetes, smoking, obesity, and low socioeconomic status.
For his part, Dr. Alcázar mentioned how these factors are similar to cardiovascular risk factors, because “the kidneys are a ball of vessels with double capillarization for purifying blood. They’re the organs with the most arteries per unit of weight, so anything that can damage the arteries can damage the kidneys.”
Candidates for screening
“Chronic kidney disease develops in 15% of the adult population in Spain. So, it’s worth asking how many patients have been diagnosed and who should we should be screening.” To the factors listed above, Dr. Alcázar added treatment with nephrotoxic drugs (including nonsteroidal anti-inflammatory drugs) for patients with obstructive urinary tract disease, and a history of acute kidney injury for patients with chronic autoimmune disease or neoplasms. “Thus, nearly all patients seen in primary care would need to be screened.”
Another fundamental question raised was whether patients should be screened before age 60 years. “As a nephrologist, I feel that we have been diagnosing chronic kidney disease late, even though we’ve been doing everything by the book,” said Dr. Alcázar. In his opinion, “the answer to whether we should be screening earlier ... is yes, for two reasons: first, because it’s cost-effective, and second, because it’s very inexpensive.”
Dr. Benedito explained in detail the process for diagnosing this disease. She began by defining the disease as changes in kidney structure and function that last longer than 3 months. These changes are identified by use of two criteria: glomerular filtration rate less than 60 mL/min and kidney injury or lesions with or without reduced filtration rate (renal biopsy, albumin/creatinine ratio greater than 30 mg/g, proteinuria, alterations in urinary sediment or in imaging tests). Thus, “if one of these two criteria persists for more than 3 months, the diagnosis is chronic kidney disease. Also, high creatinine levels are not diagnostic for the disease,” she emphasized.
Two related parameters
Glomerular filtration and albuminuria “are highly relevant, because screening for chronic kidney disease is based on these two parameters,” said Dr. Benedito. Glomerular filtration rate varies with age, sex, ethnicity, and body mass. It is useful for identifying the stage of the disease and for monitoring disease progression. Albuminuria, on the other hand, is an indication of the severity of the disease. It’s an early marker for kidney injury and systemic disease and is more sensitive than proteinuria. Therefore, “this factor, together with glomerular filtration rate, allows us to detect, classify, and monitor the progression of chronic kidney disease.”
On this point, Dr. Alcázar emphasized the importance of trends, since variation in glomerular filtration depends on serum creatinine, which can vary by nearly 9%. He explained that glomerular filtration rate is related to the number of nephrons remaining. A glomerular filtration rate of less than 60 mL/min implies that more than half of the nephrons in each kidney have been lost. Albuminuria informs about structural damage (that is, the condition of the remaining nephrons). It’s therefore essential to test for both parameters. “We need to be actively monitoring and then making our decisions based on trends and not on isolated results. We need to be aware of albuminuria when we make our decisions,” said Dr. Alcázar. Some studies have shown the importance of testing for albuminuria whenever creatinine level is assessed. “We need to buy into this. If we don’t do this, we’ll only ever have half the information we need.”
Reducing late diagnosis
According to the IBERICAN study, 14% of patients seen in primary care in Spain have chronic kidney disease. “This statistic should make us stop and think, own our responsibility, and ask ourselves why this screening isn’t taking place [earlier],” said Dr. Benedito. She added, “We need to head off this trend toward late diagnosis. As the disease progresses, it significantly increases cardiovascular risk and leads to higher mortality, going on dialysis, transplants, et cetera.”
Dr. Alcázar noted that 80% of nephrology cases that are referred to him come from primary care. He explained the need to understand that “these patients have a sevenfold greater risk of suffering a serious cardiovascular event within the next year than people without kidney problems.” Most of these patients will experience an event, even if they don’t undergo dialysis (stage 3 and those near stage 4).
Correct staging
Also fundamental is having a detailed understanding of how staging is performed. Dr. Benedito explained that a chart that pairs glomerular filtration rate (six categories) with the level of albuminuria (three categories) should be used during the visit. For example, a case might be classified as G3a-A2. However, the simplified form of the chart may prove more practical. It classifies chronic kidney disease as being associated with mild, moderate, and severe risk, using different colors to aid comprehension.
Dr. Alcázar noted that the latest guidelines from the European Society of Hypertension for 2023 include albuminuria as an important parameter. The guidelines indicate that for a patient with moderate or severe risk, it is not necessary to calculate their score. “It’s considered high cardiovascular risk, and steps would need to be taken for intervention.”
He then listed the tools available for reversing albuminuria. The process begins by reducing salt consumption and involves the use of medications (angiotensin-converting enzyme inhibitors/angiotensin II receptor antagonists, aldosterone receptor antagonists, glucagon-like peptide-1 analogues, and sodium-glucose cotransporter-2 inhibitors, which slow kidney damage regardless of other measures) and strict management of cardiovascular risk factors (smoking, weight management, blood glucose, hypertension, and moderate physical activity).
Reducing cardiovascular risk
Dr. Alcázar highlighted important factors to keep in mind when managing each of the cardiovascular risk factors. For hypertension, the aim is to achieve levels less than 130/80 mm Hg, although recommendations vary, depending on the guidelines consulted. “KDIGO (Kidney Disease: Improving Global Outcomes) 2021 states that there is no evidence for monitoring diastolic blood pressure, only systolic blood pressure. If we measure it according to the standardized form, SBP should be less than 120 mm Hg, and if not, we would fall back on readings of 130/80 mm Hg.”
For lipid control (specifically, low-density lipoprotein cholesterol), the staging chart indicates that for patients at mild risk, levels should be less than 100 mg/dL; for those at moderate risk, less than 70 mg/dL; and for those at severe risk, less than 55 mg/dL. Hypertriglyceridemia “should only be treated with fibrates if it comes in over 1,000 mg/dL. Also, care must be taken, because these drugs interfere with creatinine excretion, increasing it,” said Dr. Alcázar.
Guidelines from the KDIGO and the American Diabetes Association state that anyone with diabetes and chronic kidney disease should receive a sodium-glucose cotransporter-2 inhibitor if their glomerular filtration rate exceeds 20 mL/min, “which may contradict slightly what it says on the label. Also, if they have hypertension, they should take an angiotensin-converting enzyme inhibitor,” said Dr. Alcázar. He added that “oral antidiabetics, including metformin, must be adjusted based on renal function if glomerular filtration rate is under 30 mL/min.”
Act immediately
When asked whether the course of chronic kidney disease can be changed, Dr. Alcázar responded with an emphatic yes and added that cardiovascular risk can also be substantially reduced. “As nephrologists, we don’t have access to patients in early stages. But family doctors do. Hence the importance of early screening, because going on dialysis at age 60 isn’t the same as at 80.” Currently, “scientific societies are encouraging authorities to screen for chronic kidney disease at earlier ages.”
Regarding drug-based therapy, Dr. Alcázar said that “empagliflozin is not currently indicated for chronic kidney disease in adults.” This sodium-glucose cotransporter-2 inhibitor delays kidney disease and reduces morbidity. Both benefits were highlighted in two recent studies (DAPA-CKD and CREDENCE). Published in January, EMPA-KIDNEY presents a new twist on nephroprotection for patients with chronic kidney disease (diabetic or not) whose glomerular filtration rates are between 20 and 40 mL/min without albuminuria or whose glomerular filtration rates are between 45 and 90 mL/min with albuminuria. For more than 6,000 patients, empagliflozin was observed “to clearly reduce kidney disease progression, cardiovascular mortality and all-cause mortality, and the need to go on dialysis,” stated Dr. Alcázar.
What professionals expect
Dr. Benedito also explained the criteria for referral to a specialist: glomerular filtration rate less than 30 mL/min (unless the patient is older than 80 years and does not have progressively worsening renal function), albumin/creatinine ratio greater than 300 mg/g, acute worsening of renal function, progressive worsening of renal function of greater than 5 mL/min/yr, chronic kidney disease, hypertension treated with triple therapy (including a diuretic) at maximum doses, anemia of less than 10 g/dL, and nonurologic hematuria, especially in combination with albuminuria.
Dr. Benedito explained what nephrologists expect from family doctors in the management of chronic kidney disease: “screening for early detection, identifying and treating risk factors for chronic kidney disease, detecting progression and complications, adjusting drugs based on glomerular filtration rate, and ensuring that our patients are benefiting from sodium-glucose cotransporter-2 inhibitors. These are among the most important steps to be taken.”
Dr. Alcázar mentioned what family doctors expect from nephrologists: “two-way communication, accessibility, coordination of actions to be taken, and using shared and mutually agreed-upon protocols.”
This article was translated from the Medscape Spanish Edition and a version appeared on Medscape.com.
Strategies for complete B-cell depletion evolve for patients with lupus nephritis
SEOUL, SOUTH KOREA – B cell–depleting therapies in patients with lupus nephritis have a higher likelihood of complete response if B cells are almost completely depleted, and strategies for achieving more complete B-cell depletion continue to be tested, according to evidence presented by Richard A. Furie, MD, at an international congress on systemic lupus erythematosus (SLE).
“If you go back about 20 years ago or so, when we designed the LUNAR and EXPLORER trials, we were scared to death of rituximab [Rituxan and biosimilars], about what would happen when you deplete B cells,” said Dr. Furie, chief of the division of rheumatology at Northwell Health in New York.
The LUNAR trial, which compared rituximab with placebo in patients with lupus nephritis, did not show a statistically significant difference in renal outcomes at 1 year. However, a post hoc analysis done several years later told a different story. It looked at patients who achieved complete peripheral depletion of B cells, defined as zero cells per microliter in peripheral blood. “You can see about a fourfold increase in complete response rates in those who were complete B-cell depleters at 1 year,” Dr. Furie told the conference.
It therefore raises the question of how to achieve greater B-cell depletion rates in patients. Dr. Furie said one strategy might be to first mobilize memory B cells and neutralize B cell–activating factor using belimumab (Benlysta), and then treat with rituximab to eliminate B cells. This strategy of sequential belimumab-rituximab treatment has been taken in several clinical trials.
More potent B-cell depletion with obinutuzumab
Another approach is to choose more potent B cell–depleting therapies, such as obinutuzumab (Gazyva), which is an anti-CD20 monoclonal antibody that was approved in 2013 for the treatment of chronic lymphocytic leukemia.
The NOBILITY trial compared obinutuzumab with placebo in 125 patients with lupus nephritis who were on background treatment with mycophenolate and corticosteroids. At 1 year, significantly more patients achieved B-cell thresholds either below 5 cells per microliter or even zero cells per microliter than had been seen previously with rituximab.
That also translated into clinical benefit, Dr. Furie said. By week 76, half the patients who had sustained depletion of B cells below 0.4 cells per microliter had a complete response, compared with 35% of those who still had detectable B cells and 18% of the placebo group. Treatment with obinutuzumab did not show any link to higher rates of serious adverse events, serious infections, or deaths.
“I think we’re all pretty much convinced more is better, without introducing safety issues,” Dr. Furie said in an interview.
Joan Merrill, MD, professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, said the data did suggest that renal outcomes were better with more complete depletion, but raised the question of whether this might increase the risk of infections or infectious severity.
Dr. Furie noted that complete response not only required improvement in proteinuria, complement levels, and anti–double-stranded DNA antibodies, but also in serum creatinine, “because maintenance of eGFR [estimated glomerular filtration rate] is the name of the game with lupus nephritis.”
However, he also pointed out that there may be a ceiling for response rates in patients with lupus nephritis when using stricter endpoints for serum creatinine. The NOBILITY trial required patients to achieve a serum creatinine that did not increase by more than 15% from baseline. But when researchers did an analysis that instead only required patients to achieve a reduction in proteinuria and maintain normal creatinine, the complete response rate in complete B-cell depleters increased to 72%, compared with 50% in partial depleters and 37% in the placebo group.
Newer strategies for greater B-cell depletion
A third strategy for achieving greater B-cell depletion is bispecific T-cell engagers, or BiTEs. “I called it a ‘frenemy,’ where it’s taking the activated T cell and introducing it to the B cell, and it can kill it via direct T-cell killing,” Dr. Furie said in an interview. Mosunetuzumab (Lunsumio) is one example, and is currently in a phase 1 clinical trial of patients with SLE.
And the fourth strategy, which has proved so successful in lymphoma, is chimeric antigen receptor T-cell therapy (CAR T). Dr. Furie cited the recent publication of data from a CAR T clinical trial in five patients with refractory SLE. He said the data were impressive but the question for this treatment approach will be which patients are most likely to benefit and whether CAR T will experience the same ceiling effect because of pre-existing kidney damage.
“We won’t be seeing 100% response rates,” he said. “What we’ll be seeing, as a maximum, might be about 70%.” The big question for B-cell depletion in lupus was therefore how best to achieve it. “Is the future a potent monoclonal antibody, or is it in fact CAR T?”
Dr. Merrill said the analyses from B-cell depletion trials, showing greater response rates among more complete depleters, highlighted the importance of a personalized approach to treating lupus.
“One size fits all is never optimal in any disease, but it will prove a nonstarter in lupus, where we ought to be trying to find the optimal treatment regimen for each patient guided by biomarkers,” she said in an interview.
Dr. Furie reported having financial relationships with Genentech/Roche, which manufactures obinutuzumab and rituximab, as well as GlaxoSmithKline, Kezar Life Sciences, Kyverna Therapeutics, and Takeda. Dr. Merrill reported consulting for and receiving research support from a range of pharmaceutical companies including Genentech/Roche, GlaxoSmithKline, Pfizer, Janssen, Bristol-Myers Squibb, AbbVie, and AstraZeneca.
SEOUL, SOUTH KOREA – B cell–depleting therapies in patients with lupus nephritis have a higher likelihood of complete response if B cells are almost completely depleted, and strategies for achieving more complete B-cell depletion continue to be tested, according to evidence presented by Richard A. Furie, MD, at an international congress on systemic lupus erythematosus (SLE).
“If you go back about 20 years ago or so, when we designed the LUNAR and EXPLORER trials, we were scared to death of rituximab [Rituxan and biosimilars], about what would happen when you deplete B cells,” said Dr. Furie, chief of the division of rheumatology at Northwell Health in New York.
The LUNAR trial, which compared rituximab with placebo in patients with lupus nephritis, did not show a statistically significant difference in renal outcomes at 1 year. However, a post hoc analysis done several years later told a different story. It looked at patients who achieved complete peripheral depletion of B cells, defined as zero cells per microliter in peripheral blood. “You can see about a fourfold increase in complete response rates in those who were complete B-cell depleters at 1 year,” Dr. Furie told the conference.
It therefore raises the question of how to achieve greater B-cell depletion rates in patients. Dr. Furie said one strategy might be to first mobilize memory B cells and neutralize B cell–activating factor using belimumab (Benlysta), and then treat with rituximab to eliminate B cells. This strategy of sequential belimumab-rituximab treatment has been taken in several clinical trials.
More potent B-cell depletion with obinutuzumab
Another approach is to choose more potent B cell–depleting therapies, such as obinutuzumab (Gazyva), which is an anti-CD20 monoclonal antibody that was approved in 2013 for the treatment of chronic lymphocytic leukemia.
The NOBILITY trial compared obinutuzumab with placebo in 125 patients with lupus nephritis who were on background treatment with mycophenolate and corticosteroids. At 1 year, significantly more patients achieved B-cell thresholds either below 5 cells per microliter or even zero cells per microliter than had been seen previously with rituximab.
That also translated into clinical benefit, Dr. Furie said. By week 76, half the patients who had sustained depletion of B cells below 0.4 cells per microliter had a complete response, compared with 35% of those who still had detectable B cells and 18% of the placebo group. Treatment with obinutuzumab did not show any link to higher rates of serious adverse events, serious infections, or deaths.
“I think we’re all pretty much convinced more is better, without introducing safety issues,” Dr. Furie said in an interview.
Joan Merrill, MD, professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, said the data did suggest that renal outcomes were better with more complete depletion, but raised the question of whether this might increase the risk of infections or infectious severity.
Dr. Furie noted that complete response not only required improvement in proteinuria, complement levels, and anti–double-stranded DNA antibodies, but also in serum creatinine, “because maintenance of eGFR [estimated glomerular filtration rate] is the name of the game with lupus nephritis.”
However, he also pointed out that there may be a ceiling for response rates in patients with lupus nephritis when using stricter endpoints for serum creatinine. The NOBILITY trial required patients to achieve a serum creatinine that did not increase by more than 15% from baseline. But when researchers did an analysis that instead only required patients to achieve a reduction in proteinuria and maintain normal creatinine, the complete response rate in complete B-cell depleters increased to 72%, compared with 50% in partial depleters and 37% in the placebo group.
Newer strategies for greater B-cell depletion
A third strategy for achieving greater B-cell depletion is bispecific T-cell engagers, or BiTEs. “I called it a ‘frenemy,’ where it’s taking the activated T cell and introducing it to the B cell, and it can kill it via direct T-cell killing,” Dr. Furie said in an interview. Mosunetuzumab (Lunsumio) is one example, and is currently in a phase 1 clinical trial of patients with SLE.
And the fourth strategy, which has proved so successful in lymphoma, is chimeric antigen receptor T-cell therapy (CAR T). Dr. Furie cited the recent publication of data from a CAR T clinical trial in five patients with refractory SLE. He said the data were impressive but the question for this treatment approach will be which patients are most likely to benefit and whether CAR T will experience the same ceiling effect because of pre-existing kidney damage.
“We won’t be seeing 100% response rates,” he said. “What we’ll be seeing, as a maximum, might be about 70%.” The big question for B-cell depletion in lupus was therefore how best to achieve it. “Is the future a potent monoclonal antibody, or is it in fact CAR T?”
Dr. Merrill said the analyses from B-cell depletion trials, showing greater response rates among more complete depleters, highlighted the importance of a personalized approach to treating lupus.
“One size fits all is never optimal in any disease, but it will prove a nonstarter in lupus, where we ought to be trying to find the optimal treatment regimen for each patient guided by biomarkers,” she said in an interview.
Dr. Furie reported having financial relationships with Genentech/Roche, which manufactures obinutuzumab and rituximab, as well as GlaxoSmithKline, Kezar Life Sciences, Kyverna Therapeutics, and Takeda. Dr. Merrill reported consulting for and receiving research support from a range of pharmaceutical companies including Genentech/Roche, GlaxoSmithKline, Pfizer, Janssen, Bristol-Myers Squibb, AbbVie, and AstraZeneca.
SEOUL, SOUTH KOREA – B cell–depleting therapies in patients with lupus nephritis have a higher likelihood of complete response if B cells are almost completely depleted, and strategies for achieving more complete B-cell depletion continue to be tested, according to evidence presented by Richard A. Furie, MD, at an international congress on systemic lupus erythematosus (SLE).
“If you go back about 20 years ago or so, when we designed the LUNAR and EXPLORER trials, we were scared to death of rituximab [Rituxan and biosimilars], about what would happen when you deplete B cells,” said Dr. Furie, chief of the division of rheumatology at Northwell Health in New York.
The LUNAR trial, which compared rituximab with placebo in patients with lupus nephritis, did not show a statistically significant difference in renal outcomes at 1 year. However, a post hoc analysis done several years later told a different story. It looked at patients who achieved complete peripheral depletion of B cells, defined as zero cells per microliter in peripheral blood. “You can see about a fourfold increase in complete response rates in those who were complete B-cell depleters at 1 year,” Dr. Furie told the conference.
It therefore raises the question of how to achieve greater B-cell depletion rates in patients. Dr. Furie said one strategy might be to first mobilize memory B cells and neutralize B cell–activating factor using belimumab (Benlysta), and then treat with rituximab to eliminate B cells. This strategy of sequential belimumab-rituximab treatment has been taken in several clinical trials.
More potent B-cell depletion with obinutuzumab
Another approach is to choose more potent B cell–depleting therapies, such as obinutuzumab (Gazyva), which is an anti-CD20 monoclonal antibody that was approved in 2013 for the treatment of chronic lymphocytic leukemia.
The NOBILITY trial compared obinutuzumab with placebo in 125 patients with lupus nephritis who were on background treatment with mycophenolate and corticosteroids. At 1 year, significantly more patients achieved B-cell thresholds either below 5 cells per microliter or even zero cells per microliter than had been seen previously with rituximab.
That also translated into clinical benefit, Dr. Furie said. By week 76, half the patients who had sustained depletion of B cells below 0.4 cells per microliter had a complete response, compared with 35% of those who still had detectable B cells and 18% of the placebo group. Treatment with obinutuzumab did not show any link to higher rates of serious adverse events, serious infections, or deaths.
“I think we’re all pretty much convinced more is better, without introducing safety issues,” Dr. Furie said in an interview.
Joan Merrill, MD, professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, said the data did suggest that renal outcomes were better with more complete depletion, but raised the question of whether this might increase the risk of infections or infectious severity.
Dr. Furie noted that complete response not only required improvement in proteinuria, complement levels, and anti–double-stranded DNA antibodies, but also in serum creatinine, “because maintenance of eGFR [estimated glomerular filtration rate] is the name of the game with lupus nephritis.”
However, he also pointed out that there may be a ceiling for response rates in patients with lupus nephritis when using stricter endpoints for serum creatinine. The NOBILITY trial required patients to achieve a serum creatinine that did not increase by more than 15% from baseline. But when researchers did an analysis that instead only required patients to achieve a reduction in proteinuria and maintain normal creatinine, the complete response rate in complete B-cell depleters increased to 72%, compared with 50% in partial depleters and 37% in the placebo group.
Newer strategies for greater B-cell depletion
A third strategy for achieving greater B-cell depletion is bispecific T-cell engagers, or BiTEs. “I called it a ‘frenemy,’ where it’s taking the activated T cell and introducing it to the B cell, and it can kill it via direct T-cell killing,” Dr. Furie said in an interview. Mosunetuzumab (Lunsumio) is one example, and is currently in a phase 1 clinical trial of patients with SLE.
And the fourth strategy, which has proved so successful in lymphoma, is chimeric antigen receptor T-cell therapy (CAR T). Dr. Furie cited the recent publication of data from a CAR T clinical trial in five patients with refractory SLE. He said the data were impressive but the question for this treatment approach will be which patients are most likely to benefit and whether CAR T will experience the same ceiling effect because of pre-existing kidney damage.
“We won’t be seeing 100% response rates,” he said. “What we’ll be seeing, as a maximum, might be about 70%.” The big question for B-cell depletion in lupus was therefore how best to achieve it. “Is the future a potent monoclonal antibody, or is it in fact CAR T?”
Dr. Merrill said the analyses from B-cell depletion trials, showing greater response rates among more complete depleters, highlighted the importance of a personalized approach to treating lupus.
“One size fits all is never optimal in any disease, but it will prove a nonstarter in lupus, where we ought to be trying to find the optimal treatment regimen for each patient guided by biomarkers,” she said in an interview.
Dr. Furie reported having financial relationships with Genentech/Roche, which manufactures obinutuzumab and rituximab, as well as GlaxoSmithKline, Kezar Life Sciences, Kyverna Therapeutics, and Takeda. Dr. Merrill reported consulting for and receiving research support from a range of pharmaceutical companies including Genentech/Roche, GlaxoSmithKline, Pfizer, Janssen, Bristol-Myers Squibb, AbbVie, and AstraZeneca.
AT LUPUS 2023
Transplant centers often skip the top spot on the kidney waitlist
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
The idea of rationing medical care is anathema to most doctors. Sure, we acknowledge that the realities of health care costs and insurance companies might limit our options, but there is always a sense that when something is truly, truly needed, we can get it done.
Except in one very particular situation, a situation where rationing of care is the norm. That situation? Organ transplantation.
There is no way around this: More patients need organ transplants than there are organs available to transplant. It is cold, hard arithmetic. No amount of negotiating with an insurance company or engaging in prior authorization can change that.
As a kidney doctor, this issue is close to my heart. There are around 100,000 people on the kidney transplant waiting list in the U.S., with 3,000 new patients being added per month. There are only 25,000 kidney transplants per year. And each year, around 5,000 people die while waiting for a transplant.
A world of scarcity, like the world of kidney transplant, is ripe for bias at best and abuse at worst. It is in part for that reason that the Kidney Allocation System exists. It answers the cold, hard arithmetic of transplant scarcity with the cold, hard arithmetic of a computer algorithm, ranking individuals on the waitlist on a variety of factors to ensure that those who will benefit most from a transplant get it first.
This area is a bit complex but I’ll try to break it down into what you need to know. There are 56 organ procurement organizations (OPOs) in the United States. These are nonprofits with the responsibility to recover organs from deceased donors in their area.
Each of those OPOs maintains a ranked list of those waiting for a kidney transplant. Depending on the OPO, the list may range from a couple hundred people to a couple thousand, but one thing is the same, no matter what: If you are at the top of the list, you should be the next to get a transplant.
Most OPOs have multiple transplant centers in them, and each center is going to prioritize its own patients. If a Yale patient is No. 1 on the list and a kidney offer comes in, it would be a good idea for us to accept, because if we reject the offer, the organ may go to a competing center whose patients is ranked No. 2.
But 11 OPOs around the country are served by only one center. This gives that center huge flexibility to determine who gets what kidney, because if they refuse an offer for whoever is at the top of their list, they can still give the kidney to the second person on their list, or third, or 30th, theoretically.
But in practice, does this phenomenon, known colloquially as “list diving,” actually happen? This manuscript from Sumit Mohan and colleagues suggests that it does, and at rates that are, frankly, eye-popping.
The Columbia team used data from the Scientific Registry of Transplant Recipients to conduct the analysis. The database tracks all aspects of the transplant process, from listing to ranking to, eventually, the transplant itself. With that data, they could determine how often, across these 11 OPOs, the No. 1 person on the list did not get the available kidney.
The answer? Out of 4,668 transplants conducted from 2015 to 2019, the transplant centers skipped their highest-ranked person 3,169 times – 68% of the time.
This graph shows the distribution of where on the list these kidneys went. You can see some centers diving down 100 or 200 places.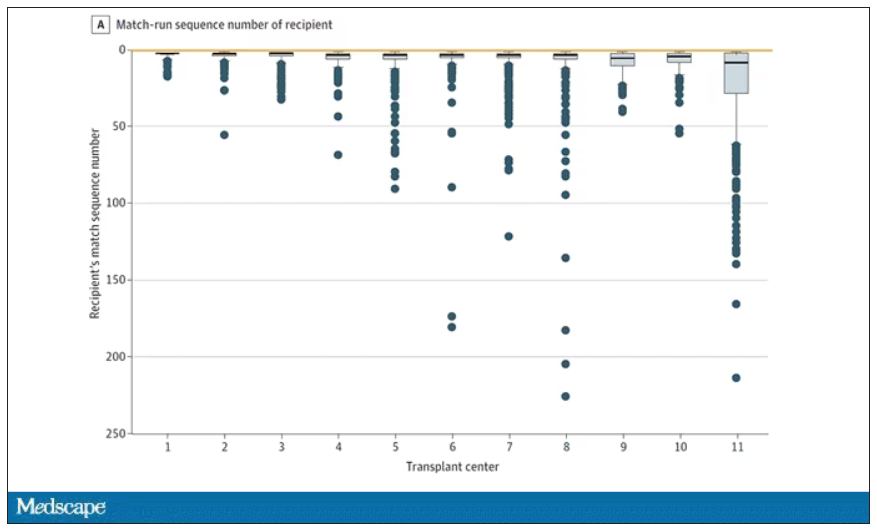
Transplant centers have lists of different lengths, so this graph shows you how far down on the percentage scale the centers dived. You can see centers skipping right to the bottom of their list in some cases.
Now, I should make it clear that transplant centers do have legitimate discretion here. Transplant centers may pass up a less-than-perfect kidney for their No. 1 spot, knowing that that individual will get more offers soon, in favor of someone further down the list who will not see an offer for a while. It’s gaming the system a bit, but not, you know, for evil. And the data support this. Top-ranked people who got skipped had received a lower-quality kidney offer than those who did not get skipped. But I will also note that those who were skipped were less likely to be White, less likely to be Hispanic, and more likely to be male. That should raise your eyebrows.
Interestingly, this practice may not be limited to those cases where the OPO has only one transplant center. Conducting the same analysis across all 231 kidney transplant centers in the U.S., the authors found that the top candidate was skipped 76% of the time.
So, what’s going on here? I’m sure that some of this list-skipping is for legitimate medical reasons. And it should be pointed out that recipients have a right to refuse an offer as well – and might be more picky if they know they are at the top of the list. But patient preference was listed as the reason for list diving in only about 14% of cases. The vast majority (65%) of reasons given were based on donor quality. The problem is that donor quality can be quite subjective. And remember, these organs were transplanted eventually so they couldn’t have been that bad.
Putting the data together, though, I can’t shake the sense that centers are using the list more for guidance than as a real mechanism to ensure an equitable allocation system. With all the flexibility that centers have to bypass individuals on the list, the list loses its meaning and its power.
I spoke to one transplant nephrologist who suggested that these data should prompt an investigation by the United Network for Organ Sharing, the body that governs all these OPOs. That may be a necessary step.
I hope there comes a day when this issue is moot, when growing kidneys in the lab – or regenerating one’s own kidneys – is a possibility. But that day is not yet here and we must deal with the scarcity we have. In this world, we need the list to prevent abuse. But the list only works if the list is followed.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Conn. He reported having no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
The idea of rationing medical care is anathema to most doctors. Sure, we acknowledge that the realities of health care costs and insurance companies might limit our options, but there is always a sense that when something is truly, truly needed, we can get it done.
Except in one very particular situation, a situation where rationing of care is the norm. That situation? Organ transplantation.
There is no way around this: More patients need organ transplants than there are organs available to transplant. It is cold, hard arithmetic. No amount of negotiating with an insurance company or engaging in prior authorization can change that.
As a kidney doctor, this issue is close to my heart. There are around 100,000 people on the kidney transplant waiting list in the U.S., with 3,000 new patients being added per month. There are only 25,000 kidney transplants per year. And each year, around 5,000 people die while waiting for a transplant.
A world of scarcity, like the world of kidney transplant, is ripe for bias at best and abuse at worst. It is in part for that reason that the Kidney Allocation System exists. It answers the cold, hard arithmetic of transplant scarcity with the cold, hard arithmetic of a computer algorithm, ranking individuals on the waitlist on a variety of factors to ensure that those who will benefit most from a transplant get it first.
This area is a bit complex but I’ll try to break it down into what you need to know. There are 56 organ procurement organizations (OPOs) in the United States. These are nonprofits with the responsibility to recover organs from deceased donors in their area.
Each of those OPOs maintains a ranked list of those waiting for a kidney transplant. Depending on the OPO, the list may range from a couple hundred people to a couple thousand, but one thing is the same, no matter what: If you are at the top of the list, you should be the next to get a transplant.
Most OPOs have multiple transplant centers in them, and each center is going to prioritize its own patients. If a Yale patient is No. 1 on the list and a kidney offer comes in, it would be a good idea for us to accept, because if we reject the offer, the organ may go to a competing center whose patients is ranked No. 2.
But 11 OPOs around the country are served by only one center. This gives that center huge flexibility to determine who gets what kidney, because if they refuse an offer for whoever is at the top of their list, they can still give the kidney to the second person on their list, or third, or 30th, theoretically.
But in practice, does this phenomenon, known colloquially as “list diving,” actually happen? This manuscript from Sumit Mohan and colleagues suggests that it does, and at rates that are, frankly, eye-popping.
The Columbia team used data from the Scientific Registry of Transplant Recipients to conduct the analysis. The database tracks all aspects of the transplant process, from listing to ranking to, eventually, the transplant itself. With that data, they could determine how often, across these 11 OPOs, the No. 1 person on the list did not get the available kidney.
The answer? Out of 4,668 transplants conducted from 2015 to 2019, the transplant centers skipped their highest-ranked person 3,169 times – 68% of the time.
This graph shows the distribution of where on the list these kidneys went. You can see some centers diving down 100 or 200 places.
Transplant centers have lists of different lengths, so this graph shows you how far down on the percentage scale the centers dived. You can see centers skipping right to the bottom of their list in some cases.
Now, I should make it clear that transplant centers do have legitimate discretion here. Transplant centers may pass up a less-than-perfect kidney for their No. 1 spot, knowing that that individual will get more offers soon, in favor of someone further down the list who will not see an offer for a while. It’s gaming the system a bit, but not, you know, for evil. And the data support this. Top-ranked people who got skipped had received a lower-quality kidney offer than those who did not get skipped. But I will also note that those who were skipped were less likely to be White, less likely to be Hispanic, and more likely to be male. That should raise your eyebrows.
Interestingly, this practice may not be limited to those cases where the OPO has only one transplant center. Conducting the same analysis across all 231 kidney transplant centers in the U.S., the authors found that the top candidate was skipped 76% of the time.
So, what’s going on here? I’m sure that some of this list-skipping is for legitimate medical reasons. And it should be pointed out that recipients have a right to refuse an offer as well – and might be more picky if they know they are at the top of the list. But patient preference was listed as the reason for list diving in only about 14% of cases. The vast majority (65%) of reasons given were based on donor quality. The problem is that donor quality can be quite subjective. And remember, these organs were transplanted eventually so they couldn’t have been that bad.
Putting the data together, though, I can’t shake the sense that centers are using the list more for guidance than as a real mechanism to ensure an equitable allocation system. With all the flexibility that centers have to bypass individuals on the list, the list loses its meaning and its power.
I spoke to one transplant nephrologist who suggested that these data should prompt an investigation by the United Network for Organ Sharing, the body that governs all these OPOs. That may be a necessary step.
I hope there comes a day when this issue is moot, when growing kidneys in the lab – or regenerating one’s own kidneys – is a possibility. But that day is not yet here and we must deal with the scarcity we have. In this world, we need the list to prevent abuse. But the list only works if the list is followed.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Conn. He reported having no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
The idea of rationing medical care is anathema to most doctors. Sure, we acknowledge that the realities of health care costs and insurance companies might limit our options, but there is always a sense that when something is truly, truly needed, we can get it done.
Except in one very particular situation, a situation where rationing of care is the norm. That situation? Organ transplantation.
There is no way around this: More patients need organ transplants than there are organs available to transplant. It is cold, hard arithmetic. No amount of negotiating with an insurance company or engaging in prior authorization can change that.
As a kidney doctor, this issue is close to my heart. There are around 100,000 people on the kidney transplant waiting list in the U.S., with 3,000 new patients being added per month. There are only 25,000 kidney transplants per year. And each year, around 5,000 people die while waiting for a transplant.
A world of scarcity, like the world of kidney transplant, is ripe for bias at best and abuse at worst. It is in part for that reason that the Kidney Allocation System exists. It answers the cold, hard arithmetic of transplant scarcity with the cold, hard arithmetic of a computer algorithm, ranking individuals on the waitlist on a variety of factors to ensure that those who will benefit most from a transplant get it first.
This area is a bit complex but I’ll try to break it down into what you need to know. There are 56 organ procurement organizations (OPOs) in the United States. These are nonprofits with the responsibility to recover organs from deceased donors in their area.
Each of those OPOs maintains a ranked list of those waiting for a kidney transplant. Depending on the OPO, the list may range from a couple hundred people to a couple thousand, but one thing is the same, no matter what: If you are at the top of the list, you should be the next to get a transplant.
Most OPOs have multiple transplant centers in them, and each center is going to prioritize its own patients. If a Yale patient is No. 1 on the list and a kidney offer comes in, it would be a good idea for us to accept, because if we reject the offer, the organ may go to a competing center whose patients is ranked No. 2.
But 11 OPOs around the country are served by only one center. This gives that center huge flexibility to determine who gets what kidney, because if they refuse an offer for whoever is at the top of their list, they can still give the kidney to the second person on their list, or third, or 30th, theoretically.
But in practice, does this phenomenon, known colloquially as “list diving,” actually happen? This manuscript from Sumit Mohan and colleagues suggests that it does, and at rates that are, frankly, eye-popping.
The Columbia team used data from the Scientific Registry of Transplant Recipients to conduct the analysis. The database tracks all aspects of the transplant process, from listing to ranking to, eventually, the transplant itself. With that data, they could determine how often, across these 11 OPOs, the No. 1 person on the list did not get the available kidney.
The answer? Out of 4,668 transplants conducted from 2015 to 2019, the transplant centers skipped their highest-ranked person 3,169 times – 68% of the time.
This graph shows the distribution of where on the list these kidneys went. You can see some centers diving down 100 or 200 places.
Transplant centers have lists of different lengths, so this graph shows you how far down on the percentage scale the centers dived. You can see centers skipping right to the bottom of their list in some cases.
Now, I should make it clear that transplant centers do have legitimate discretion here. Transplant centers may pass up a less-than-perfect kidney for their No. 1 spot, knowing that that individual will get more offers soon, in favor of someone further down the list who will not see an offer for a while. It’s gaming the system a bit, but not, you know, for evil. And the data support this. Top-ranked people who got skipped had received a lower-quality kidney offer than those who did not get skipped. But I will also note that those who were skipped were less likely to be White, less likely to be Hispanic, and more likely to be male. That should raise your eyebrows.
Interestingly, this practice may not be limited to those cases where the OPO has only one transplant center. Conducting the same analysis across all 231 kidney transplant centers in the U.S., the authors found that the top candidate was skipped 76% of the time.
So, what’s going on here? I’m sure that some of this list-skipping is for legitimate medical reasons. And it should be pointed out that recipients have a right to refuse an offer as well – and might be more picky if they know they are at the top of the list. But patient preference was listed as the reason for list diving in only about 14% of cases. The vast majority (65%) of reasons given were based on donor quality. The problem is that donor quality can be quite subjective. And remember, these organs were transplanted eventually so they couldn’t have been that bad.
Putting the data together, though, I can’t shake the sense that centers are using the list more for guidance than as a real mechanism to ensure an equitable allocation system. With all the flexibility that centers have to bypass individuals on the list, the list loses its meaning and its power.
I spoke to one transplant nephrologist who suggested that these data should prompt an investigation by the United Network for Organ Sharing, the body that governs all these OPOs. That may be a necessary step.
I hope there comes a day when this issue is moot, when growing kidneys in the lab – or regenerating one’s own kidneys – is a possibility. But that day is not yet here and we must deal with the scarcity we have. In this world, we need the list to prevent abuse. But the list only works if the list is followed.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Conn. He reported having no conflicts of interest.
A version of this article first appeared on Medscape.com.
Lupus nephritis: Hopes, questions arise for baricitinib
MILAN – The oral Janus kinase (JAK) 1/2 inhibitor baricitinib (Olumiant) demonstrated significantly better efficacy than cyclophosphamide infusions in the treatment of lupus nephritis in a small, independently funded, phase 3, double-blind clinical trial, Manal Hassanien, MD, reported at the annual European Congress of Rheumatology.
Baricitinib, licensed by Eli Lilly, has been recognized as a potential therapeutic option in systemic lupus, and is approved in the United States to treat RA, alopecia areata, and COVID-19 in certain hospitalized adults. It is also approved to treat atopic dermatitis in Europe. However, it previously yielded disappointing results in phase 3 clinical trials SLE-BRAVE-I and SLE-BRAVE-II for systemic lupus erythematosus. The trial results presented at EULAR suggest that baricitinib could be beneficial in the treatment of lupus nephritis, further establishing the role of JAK inhibitors in autoimmune disease therapy.
“Lupus nephritis typically develops within 5 years of initial lupus symptoms,” said Dr. Hassanien, of the rheumatology research and advanced therapeutics department at Assiut (Egypt) University. “Research has shown that up to 60% of lupus patients will eventually develop lupus nephritis. The management of proliferative lupus nephritis usually involves an initial phase focused on preventing the development of irreversible damage, followed by a maintenance phase to control lupus activity. Despite significant progress, lupus nephritis still carries an increased risk of end-stage renal disease and mortality.”
The study’s primary endpoint of 24-hour proteinuria response rate (≥ 50% reduction from baseline) at week 12 was significantly greater with baricitinib 4 mg daily, compared with monthly cyclophosphamide infusions at 0.7 mg/m2 (70% vs. 43%; P < .0001). At week 24, 76.6% of the baricitinib group met the primary endpoint, compared with 50% in the cyclophosphamide group. Two multiplicity-controlled secondary endpoints, C3 serum level and the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K), also showed statistical significance at 12 weeks (P < .01).
The 6-month trial included 60 adult patients (age 18 years and older) with a clinical diagnosis of lupus nephritis fulfilling classification criteria for LN grade III and IV. Patients needed to demonstrate objective signs of active nephritis consistent with persistent proteinuria greater than 0.5 g/day and/or cellular casts at screening to be included. Additional inclusion criteria were SLEDAI-2K greater than 4 and assessment of anti–double-stranded DNA and C3 serum levels at study entry. The patients were randomly assigned to two equal-sized groups, with one group receiving baricitinib 4 mg daily and a monthly placebo saline infusion, and the other group receiving monthly cyclophosphamide infusions and oral placebo tablets.
The incidence of adverse events was comparable between the two treatment groups, with 48% of patients in the baricitinib group and 46% in the cyclophosphamide group experiencing adverse events. Only three serious adverse events, specifically serious infection or herpes zoster, were recorded, leading to treatment discontinuation.
Two patients (6.6%) in the baricitinib group and one patient (3.3%) in the cyclophosphamide group were affected. The researchers recorded no major adverse cardiovascular or venous thromboembolic events, which are known to occur at higher rates among some users of baricitinib and other JAK inhibitors. The safety profile of baricitinib was consistent with observations made in other inflammatory musculoskeletal diseases, and no new risks were identified.
However, there were some concerns expressed by audience members during the presentation.
“The primary endpoint is limited at proteinuria, while biopsy is considered the gold standard for measuring efficacy,” said Eric F. Morand, MD, head of the Monash Health rheumatology unit, Melbourne. This was not the only critical comment regarding the study that emerged during the discussion. The use of a 4-mg dosage regimen throughout the entire study duration (despite official recommendations suggesting a 2-mg dosage in the long run) and the positive outcomes observed in the control group treated with cyclophosphamide were also mentioned.
Dr. Hassanien acknowledged that this is a small and relatively short study and disclosed plans to extend the follow-up period to 1 year and conduct a renal biopsy.
Dr. Hassanien reported no relevant financial relationships. Assiut University funded the trial.
A version of this article first appeared on Medscape.com.
MILAN – The oral Janus kinase (JAK) 1/2 inhibitor baricitinib (Olumiant) demonstrated significantly better efficacy than cyclophosphamide infusions in the treatment of lupus nephritis in a small, independently funded, phase 3, double-blind clinical trial, Manal Hassanien, MD, reported at the annual European Congress of Rheumatology.
Baricitinib, licensed by Eli Lilly, has been recognized as a potential therapeutic option in systemic lupus, and is approved in the United States to treat RA, alopecia areata, and COVID-19 in certain hospitalized adults. It is also approved to treat atopic dermatitis in Europe. However, it previously yielded disappointing results in phase 3 clinical trials SLE-BRAVE-I and SLE-BRAVE-II for systemic lupus erythematosus. The trial results presented at EULAR suggest that baricitinib could be beneficial in the treatment of lupus nephritis, further establishing the role of JAK inhibitors in autoimmune disease therapy.
“Lupus nephritis typically develops within 5 years of initial lupus symptoms,” said Dr. Hassanien, of the rheumatology research and advanced therapeutics department at Assiut (Egypt) University. “Research has shown that up to 60% of lupus patients will eventually develop lupus nephritis. The management of proliferative lupus nephritis usually involves an initial phase focused on preventing the development of irreversible damage, followed by a maintenance phase to control lupus activity. Despite significant progress, lupus nephritis still carries an increased risk of end-stage renal disease and mortality.”
The study’s primary endpoint of 24-hour proteinuria response rate (≥ 50% reduction from baseline) at week 12 was significantly greater with baricitinib 4 mg daily, compared with monthly cyclophosphamide infusions at 0.7 mg/m2 (70% vs. 43%; P < .0001). At week 24, 76.6% of the baricitinib group met the primary endpoint, compared with 50% in the cyclophosphamide group. Two multiplicity-controlled secondary endpoints, C3 serum level and the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K), also showed statistical significance at 12 weeks (P < .01).
The 6-month trial included 60 adult patients (age 18 years and older) with a clinical diagnosis of lupus nephritis fulfilling classification criteria for LN grade III and IV. Patients needed to demonstrate objective signs of active nephritis consistent with persistent proteinuria greater than 0.5 g/day and/or cellular casts at screening to be included. Additional inclusion criteria were SLEDAI-2K greater than 4 and assessment of anti–double-stranded DNA and C3 serum levels at study entry. The patients were randomly assigned to two equal-sized groups, with one group receiving baricitinib 4 mg daily and a monthly placebo saline infusion, and the other group receiving monthly cyclophosphamide infusions and oral placebo tablets.
The incidence of adverse events was comparable between the two treatment groups, with 48% of patients in the baricitinib group and 46% in the cyclophosphamide group experiencing adverse events. Only three serious adverse events, specifically serious infection or herpes zoster, were recorded, leading to treatment discontinuation.
Two patients (6.6%) in the baricitinib group and one patient (3.3%) in the cyclophosphamide group were affected. The researchers recorded no major adverse cardiovascular or venous thromboembolic events, which are known to occur at higher rates among some users of baricitinib and other JAK inhibitors. The safety profile of baricitinib was consistent with observations made in other inflammatory musculoskeletal diseases, and no new risks were identified.
However, there were some concerns expressed by audience members during the presentation.
“The primary endpoint is limited at proteinuria, while biopsy is considered the gold standard for measuring efficacy,” said Eric F. Morand, MD, head of the Monash Health rheumatology unit, Melbourne. This was not the only critical comment regarding the study that emerged during the discussion. The use of a 4-mg dosage regimen throughout the entire study duration (despite official recommendations suggesting a 2-mg dosage in the long run) and the positive outcomes observed in the control group treated with cyclophosphamide were also mentioned.
Dr. Hassanien acknowledged that this is a small and relatively short study and disclosed plans to extend the follow-up period to 1 year and conduct a renal biopsy.
Dr. Hassanien reported no relevant financial relationships. Assiut University funded the trial.
A version of this article first appeared on Medscape.com.
MILAN – The oral Janus kinase (JAK) 1/2 inhibitor baricitinib (Olumiant) demonstrated significantly better efficacy than cyclophosphamide infusions in the treatment of lupus nephritis in a small, independently funded, phase 3, double-blind clinical trial, Manal Hassanien, MD, reported at the annual European Congress of Rheumatology.
Baricitinib, licensed by Eli Lilly, has been recognized as a potential therapeutic option in systemic lupus, and is approved in the United States to treat RA, alopecia areata, and COVID-19 in certain hospitalized adults. It is also approved to treat atopic dermatitis in Europe. However, it previously yielded disappointing results in phase 3 clinical trials SLE-BRAVE-I and SLE-BRAVE-II for systemic lupus erythematosus. The trial results presented at EULAR suggest that baricitinib could be beneficial in the treatment of lupus nephritis, further establishing the role of JAK inhibitors in autoimmune disease therapy.
“Lupus nephritis typically develops within 5 years of initial lupus symptoms,” said Dr. Hassanien, of the rheumatology research and advanced therapeutics department at Assiut (Egypt) University. “Research has shown that up to 60% of lupus patients will eventually develop lupus nephritis. The management of proliferative lupus nephritis usually involves an initial phase focused on preventing the development of irreversible damage, followed by a maintenance phase to control lupus activity. Despite significant progress, lupus nephritis still carries an increased risk of end-stage renal disease and mortality.”
The study’s primary endpoint of 24-hour proteinuria response rate (≥ 50% reduction from baseline) at week 12 was significantly greater with baricitinib 4 mg daily, compared with monthly cyclophosphamide infusions at 0.7 mg/m2 (70% vs. 43%; P < .0001). At week 24, 76.6% of the baricitinib group met the primary endpoint, compared with 50% in the cyclophosphamide group. Two multiplicity-controlled secondary endpoints, C3 serum level and the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K), also showed statistical significance at 12 weeks (P < .01).
The 6-month trial included 60 adult patients (age 18 years and older) with a clinical diagnosis of lupus nephritis fulfilling classification criteria for LN grade III and IV. Patients needed to demonstrate objective signs of active nephritis consistent with persistent proteinuria greater than 0.5 g/day and/or cellular casts at screening to be included. Additional inclusion criteria were SLEDAI-2K greater than 4 and assessment of anti–double-stranded DNA and C3 serum levels at study entry. The patients were randomly assigned to two equal-sized groups, with one group receiving baricitinib 4 mg daily and a monthly placebo saline infusion, and the other group receiving monthly cyclophosphamide infusions and oral placebo tablets.
The incidence of adverse events was comparable between the two treatment groups, with 48% of patients in the baricitinib group and 46% in the cyclophosphamide group experiencing adverse events. Only three serious adverse events, specifically serious infection or herpes zoster, were recorded, leading to treatment discontinuation.
Two patients (6.6%) in the baricitinib group and one patient (3.3%) in the cyclophosphamide group were affected. The researchers recorded no major adverse cardiovascular or venous thromboembolic events, which are known to occur at higher rates among some users of baricitinib and other JAK inhibitors. The safety profile of baricitinib was consistent with observations made in other inflammatory musculoskeletal diseases, and no new risks were identified.
However, there were some concerns expressed by audience members during the presentation.
“The primary endpoint is limited at proteinuria, while biopsy is considered the gold standard for measuring efficacy,” said Eric F. Morand, MD, head of the Monash Health rheumatology unit, Melbourne. This was not the only critical comment regarding the study that emerged during the discussion. The use of a 4-mg dosage regimen throughout the entire study duration (despite official recommendations suggesting a 2-mg dosage in the long run) and the positive outcomes observed in the control group treated with cyclophosphamide were also mentioned.
Dr. Hassanien acknowledged that this is a small and relatively short study and disclosed plans to extend the follow-up period to 1 year and conduct a renal biopsy.
Dr. Hassanien reported no relevant financial relationships. Assiut University funded the trial.
A version of this article first appeared on Medscape.com.
AT EULAR 2023
FDA approves new drug, sotagliflozin, for heart failure
Sotagliflozin, a novel agent that inhibits sodium-glucose cotransporter 1 as well as SGLT2, has received marketing approval from the Food and Drug Administration for reducing the risk for cardiovascular death, hospitalization for heart failure, and urgent heart failure visits in patients with heart failure, and also for preventing these same events in patients with type 2 diabetes, chronic kidney disease (CKD), and other cardiovascular disease risk factors.
This puts sotagliflozin in direct competition with two SGLT2 inhibitors, dapagliflozin (Farxiga) and empagliflozin (Jardiance), that already have indications for preventing heart failure hospitalizations in patients with heart failure as well as approvals for type 2 diabetes and preservation of renal function.
Officials at Lexicon Pharmaceuticals, the company that developed and will market sotagliflozin under the trade name Inpefa, said in a press release that they expect U.S. sales of the agent to begin before the end of June 2023. The release also highlighted that the approval broadly covered use in patients with heart failure across the full range of both reduced and preserved left ventricular ejection fractions.
They base this niche target for sotagliflozin on results from the SOLOIST-WHF trial, which randomized 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure and showed a significant 33% reduction in the rate of deaths from cardiovascular causes and hospitalizations and urgent visits for heart failure, compared with control patients during a median 9 months of follow-up. Nearly half of the enrolled patients received their first dose while still hospitalized, while the other half received their first dose a median of 2 days after hospital discharge. The drug appeared safe.
Cutting heart failure rehospitalizations in half
An exploratory post hoc analysis of SOLOIST-WHF showed that treatment with sotagliflozin cut the rate of rehospitalizations roughly in half after both 30 and 90 days compared with control patients, according to an abstract presented at the 2022 annual scientific sessions of the AHA that has not yet been published in a peer-reviewed journal.
The only SGLT2 inhibitor tested so far when initiated in patients during hospitalization for heart failure is empagliflozin, in the EMPULSE trial, which randomized 530 patients. EMPULSE also showed that starting an SGLT2 inhibitor in this setting was safe and resulted in significant clinical benefit, the study’s primary endpoint, defined as a composite of death from any cause, number of heart failure events, and time to first heart failure event, or a 5-point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score at 90 days.
In the DELIVER trial, which tested dapagliflozin in patients with heart failure with preserved ejection fraction, roughly 10% of patients started study treatment during or within 30 days of heart failure hospitalization, and in this subgroup, dapagliflozin appeared as effective as it was in the other 90% of patients who did not start the drug during an acute or subacute phase.
Despite the SOLOIST-WHF evidence for sotagliflozin’s safety and efficacy in this economically important clinical setting, some experts say the drug faces an uphill path as it contends for market share against two solidly established, albeit dramatically underused, SGLT2 inhibitors. (Recent data document that 20% or fewer of U.S. patients eligible for treatment with an SGLT2 inhibitor receive it, such as a review of 49,000 patients hospitalized during 2021-2022 with heart failure with reduced ejection fraction.)
Others foresee a clear role for sotagliflozin, particularly because of additional facets of the drug’s performance in trials that they perceive give it an edge over dapagliflozin and empagliflozin. This includes evidence that sotagliflozin treatment uniquely (within the SGLT2 inhibitor class) cuts the rate of strokes and myocardial infarctions, as well as evidence of its apparent ability to lower hemoglobin A1c levels in patients with type 2 diabetes and with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a property likely linked to inhibition of SGLT1 in the gut that dampens intestinal glucose absorption.
Sotagliflozin uptake ‘will be a challenge’
“It will be a challenge” for sotagliflozin uptake, given the head start that both dapagliflozin and empagliflozin have had as well-documented agents for patients with heart failure, commented Javed Butler, MD, a heart failure clinician and trialist who is president of the Baylor Scott & White Research Institute in Dallas.
Given the position dapagliflozin and empagliflozin currently have in U.S. heart failure management – with the SGLT2 inhibitor class called out in guidelines as foundational for treating patients with heart failure with reduced ejection fraction and likely soon for heart failure with preserved ejection fraction as well – “I can’t imagine [sotagliflozin] will be considered a preferred option,” Dr. Butler said in an interview.
Another expert was even more dismissive of sotagliflozin’s role.
“There is no persuasive evidence that sotagliflozin has any advantages, compared with the SGLT2 inhibitors, for the treatment of heart failure,” said Milton Packer, MD, a heart failure specialist and trialist at Baylor University Medical Center, Dallas. “I do not see why U.S. physicians might pivot from established SGLT2 inhibitors to sotagliflozin,” unless it was priced “at a very meaningful discount to available SGLT2 inhibitors.”
At the time it announced the FDA’s approval, Lexicon did not provide details on how it would price sotagliflozin. Existing retail prices for dapagliflozin and empagliflozin run about $550-$600/month, a price point that has contributed to slow U.S. uptake of the drug class. But experts anticipate a dramatic shake-up of the U.S. market for SGLT2 inhibitors with expected introduction of a generic SGLT2 inhibitor formulation by 2025, a development that could further dampen sotagliflozin’s prospects.
Other experts are more optimistic about the new agent’s uptake, perhaps none more than Deepak L. Bhatt, MD, MPH, who led both pivotal trials that provide the bulk of sotagliflozin’s evidence package.
In addition to SOLOIST-WHF, Dr. Bhatt also headed the SCORED trial, with 10,584 patients with type 2 diabetes, CKD, and risks for cardiovascular disease randomized to sotagliflozin or placebo and followed for a median of 16 months. The primary result showed that sotagliflozin treatment cut the combined rate of deaths from cardiovascular causes, hospitalizations for heart failure, and urgent visits for heart failure by a significant 26% relative to control patients.
A clear MACE benefit
“The data from SOLOIST-WHF and SCORED look at least as good as the data for the SGLT2 inhibitors for heart failure, and what appears to be different are the rates for MI and stroke in SCORED,” said Dr. Bhatt, director of Mount Sinai Heart, New York.
“I believe the rate of major adverse cardiovascular events [MACE] were reduced [in SCORED], and this is different from the SGLT2 inhibitors,” he said in an interview.
In 2022, Dr. Bhatt reported results from a prespecified secondary analysis of SCORED that showed that treatment with sotagliflozin cut the rate of MACE by a significant 21%-26%, compared with placebo. This finding was, in part, driven by the first data to show a substantial benefit from an SGLT inhibitor on stroke rates.
And while SCORED did not report a significant benefit for slowing progression of CKD, subsequent post hoc analyses have suggested this advantage also in as-yet-unpublished findings, Dr. Bhatt added.
But he said he doubted nephrologists will see it as a first-line agent for slowing CKD progression – an indication already held by dapagliflozin, pending for empagliflozin, and also in place for a third SGLT2 inhibitor, canagliflozin (Invokana) – because sotagliflozin lacks clear significant and prespecified evidence for this effect.
Dr. Bhatt also acknowledged the limitation of sotagliflozin compared with the SGLT2 inhibitors as an agent for glucose control, again because of no evidence for this effect from a prospective analysis and no pending indication for type 2 diabetes treatment. But the SCORED data showed a clear A1c benefit, even in patients with severely reduced renal function.
Mostly for cardiologists? ‘Compelling’ reductions in MIs and strokes
That may mean sotagliflozin “won’t get much use by endocrinologists nor by primary care physicians,” commented Carol L. Wysham, MD, an endocrinologist with MultiCare in Spokane, Wash.
Sotagliflozin “will be a cardiology drug,” and will “have a hard time” competing with the SGLT2 inhibitors, she predicted.
Dr. Bhatt agreed that sotagliflozin “will be perceived as a drug for cardiologists to prescribe. I don’t see endocrinologists, nephrologists, and primary care physicians reaching for this drug if it has a heart failure label.” But, he added, “my hope is that the company files for additional indications. It deserves an indication for glycemic control.”
The evidence for a heart failure benefit from sotagliflozin is “valid and compelling,” and “having this option is great,” commented Mikhail N. Kosiborod, MD, a cardiologist, vice president of research at Saint Luke’s Health System, and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo. But, he added, “it will be a reasonably tall task for sotagliflozin to come from behind and be disruptive in a space where there are already two well-established SGLT2 inhibitors” approved for preventing heart failure hospitalizations, “with a lot of data to back them up,”
The feature that sets sotagliflozin apart from the approved SGLT2 inhibitors is the “really compelling decrease” it produced in rates of MIs and strokes “that we simply do not see with SGLT2 inhibitors,” Dr. Kosiborod said in an interview.
He also cited results from SCORED that suggest “a meaningful reduction in A1c” when indirectly compared with SGLT2 inhibitors, especially in patients with more severe CKD. The lack of a dedicated A1c-lowering trial or an approved type 2 diabetes indication “will not be a problem for cardiologists,” he predicted, but also agreed that it is less likely to be used by primary care physicians in low-risk patients.
“I can see myself prescribing sotagliflozin,” said Dr. Kosiborod, a SCORED coinvestigator, especially for patients with coexisting type 2 diabetes, heart failure, CKD, and atherosclerotic cardiovascular disease. These patients may get “more bang for the buck” because of a reduced risk for MI and stroke, making sotagliflozin “a solid consideration in these patients if the economic factors align.”
Like others, Dr. Kosiborod cited the big impact pricing will have, especially if, as expected, a generic SGLT2 inhibitor soon comes onto the U.S. market. “Access and affordability are very important.”
SOLOIST-WHF and SCORED were sponsored initially by Sanofi and later by Lexicon after Sanofi pulled out of sotagliflozin development. Dr. Butler has been a consultant for Lexicon as well as for AstraZeneca (which markets Farxiga), Boehringer Ingelheim and Lilly (which jointly market Jardiance), and Janssen (which markets Invokana), as well as for numerous other companies. Dr. Packer has been a consultant for AstraZeneca, Boehringer Ingelheim, Lilly, and numerous other companies. Dr. Bhatt was lead investigator for SOLOIST-WHF and SCORED and has been an adviser for Boehringer Ingelheim and Janssen and numerous other companies. Dr. Wysham has been an adviser, speaker, and consultant for AstraZeneca, Boehringer Ingelheim, Lilly, Janssen, Novo Nordisk, and Sanofi, an adviser for Abbott, and a speaker for Insulet. Dr. Kosiborod was a member of the SCORED Steering Committee and has been a consultant for Lexicon, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Novo Nordisk, and numerous other companies.
A version of this article first appeared on Medscape.com.
Sotagliflozin, a novel agent that inhibits sodium-glucose cotransporter 1 as well as SGLT2, has received marketing approval from the Food and Drug Administration for reducing the risk for cardiovascular death, hospitalization for heart failure, and urgent heart failure visits in patients with heart failure, and also for preventing these same events in patients with type 2 diabetes, chronic kidney disease (CKD), and other cardiovascular disease risk factors.
This puts sotagliflozin in direct competition with two SGLT2 inhibitors, dapagliflozin (Farxiga) and empagliflozin (Jardiance), that already have indications for preventing heart failure hospitalizations in patients with heart failure as well as approvals for type 2 diabetes and preservation of renal function.
Officials at Lexicon Pharmaceuticals, the company that developed and will market sotagliflozin under the trade name Inpefa, said in a press release that they expect U.S. sales of the agent to begin before the end of June 2023. The release also highlighted that the approval broadly covered use in patients with heart failure across the full range of both reduced and preserved left ventricular ejection fractions.
They base this niche target for sotagliflozin on results from the SOLOIST-WHF trial, which randomized 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure and showed a significant 33% reduction in the rate of deaths from cardiovascular causes and hospitalizations and urgent visits for heart failure, compared with control patients during a median 9 months of follow-up. Nearly half of the enrolled patients received their first dose while still hospitalized, while the other half received their first dose a median of 2 days after hospital discharge. The drug appeared safe.
Cutting heart failure rehospitalizations in half
An exploratory post hoc analysis of SOLOIST-WHF showed that treatment with sotagliflozin cut the rate of rehospitalizations roughly in half after both 30 and 90 days compared with control patients, according to an abstract presented at the 2022 annual scientific sessions of the AHA that has not yet been published in a peer-reviewed journal.
The only SGLT2 inhibitor tested so far when initiated in patients during hospitalization for heart failure is empagliflozin, in the EMPULSE trial, which randomized 530 patients. EMPULSE also showed that starting an SGLT2 inhibitor in this setting was safe and resulted in significant clinical benefit, the study’s primary endpoint, defined as a composite of death from any cause, number of heart failure events, and time to first heart failure event, or a 5-point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score at 90 days.
In the DELIVER trial, which tested dapagliflozin in patients with heart failure with preserved ejection fraction, roughly 10% of patients started study treatment during or within 30 days of heart failure hospitalization, and in this subgroup, dapagliflozin appeared as effective as it was in the other 90% of patients who did not start the drug during an acute or subacute phase.
Despite the SOLOIST-WHF evidence for sotagliflozin’s safety and efficacy in this economically important clinical setting, some experts say the drug faces an uphill path as it contends for market share against two solidly established, albeit dramatically underused, SGLT2 inhibitors. (Recent data document that 20% or fewer of U.S. patients eligible for treatment with an SGLT2 inhibitor receive it, such as a review of 49,000 patients hospitalized during 2021-2022 with heart failure with reduced ejection fraction.)
Others foresee a clear role for sotagliflozin, particularly because of additional facets of the drug’s performance in trials that they perceive give it an edge over dapagliflozin and empagliflozin. This includes evidence that sotagliflozin treatment uniquely (within the SGLT2 inhibitor class) cuts the rate of strokes and myocardial infarctions, as well as evidence of its apparent ability to lower hemoglobin A1c levels in patients with type 2 diabetes and with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a property likely linked to inhibition of SGLT1 in the gut that dampens intestinal glucose absorption.
Sotagliflozin uptake ‘will be a challenge’
“It will be a challenge” for sotagliflozin uptake, given the head start that both dapagliflozin and empagliflozin have had as well-documented agents for patients with heart failure, commented Javed Butler, MD, a heart failure clinician and trialist who is president of the Baylor Scott & White Research Institute in Dallas.
Given the position dapagliflozin and empagliflozin currently have in U.S. heart failure management – with the SGLT2 inhibitor class called out in guidelines as foundational for treating patients with heart failure with reduced ejection fraction and likely soon for heart failure with preserved ejection fraction as well – “I can’t imagine [sotagliflozin] will be considered a preferred option,” Dr. Butler said in an interview.
Another expert was even more dismissive of sotagliflozin’s role.
“There is no persuasive evidence that sotagliflozin has any advantages, compared with the SGLT2 inhibitors, for the treatment of heart failure,” said Milton Packer, MD, a heart failure specialist and trialist at Baylor University Medical Center, Dallas. “I do not see why U.S. physicians might pivot from established SGLT2 inhibitors to sotagliflozin,” unless it was priced “at a very meaningful discount to available SGLT2 inhibitors.”
At the time it announced the FDA’s approval, Lexicon did not provide details on how it would price sotagliflozin. Existing retail prices for dapagliflozin and empagliflozin run about $550-$600/month, a price point that has contributed to slow U.S. uptake of the drug class. But experts anticipate a dramatic shake-up of the U.S. market for SGLT2 inhibitors with expected introduction of a generic SGLT2 inhibitor formulation by 2025, a development that could further dampen sotagliflozin’s prospects.
Other experts are more optimistic about the new agent’s uptake, perhaps none more than Deepak L. Bhatt, MD, MPH, who led both pivotal trials that provide the bulk of sotagliflozin’s evidence package.
In addition to SOLOIST-WHF, Dr. Bhatt also headed the SCORED trial, with 10,584 patients with type 2 diabetes, CKD, and risks for cardiovascular disease randomized to sotagliflozin or placebo and followed for a median of 16 months. The primary result showed that sotagliflozin treatment cut the combined rate of deaths from cardiovascular causes, hospitalizations for heart failure, and urgent visits for heart failure by a significant 26% relative to control patients.
A clear MACE benefit
“The data from SOLOIST-WHF and SCORED look at least as good as the data for the SGLT2 inhibitors for heart failure, and what appears to be different are the rates for MI and stroke in SCORED,” said Dr. Bhatt, director of Mount Sinai Heart, New York.
“I believe the rate of major adverse cardiovascular events [MACE] were reduced [in SCORED], and this is different from the SGLT2 inhibitors,” he said in an interview.
In 2022, Dr. Bhatt reported results from a prespecified secondary analysis of SCORED that showed that treatment with sotagliflozin cut the rate of MACE by a significant 21%-26%, compared with placebo. This finding was, in part, driven by the first data to show a substantial benefit from an SGLT inhibitor on stroke rates.
And while SCORED did not report a significant benefit for slowing progression of CKD, subsequent post hoc analyses have suggested this advantage also in as-yet-unpublished findings, Dr. Bhatt added.
But he said he doubted nephrologists will see it as a first-line agent for slowing CKD progression – an indication already held by dapagliflozin, pending for empagliflozin, and also in place for a third SGLT2 inhibitor, canagliflozin (Invokana) – because sotagliflozin lacks clear significant and prespecified evidence for this effect.
Dr. Bhatt also acknowledged the limitation of sotagliflozin compared with the SGLT2 inhibitors as an agent for glucose control, again because of no evidence for this effect from a prospective analysis and no pending indication for type 2 diabetes treatment. But the SCORED data showed a clear A1c benefit, even in patients with severely reduced renal function.
Mostly for cardiologists? ‘Compelling’ reductions in MIs and strokes
That may mean sotagliflozin “won’t get much use by endocrinologists nor by primary care physicians,” commented Carol L. Wysham, MD, an endocrinologist with MultiCare in Spokane, Wash.
Sotagliflozin “will be a cardiology drug,” and will “have a hard time” competing with the SGLT2 inhibitors, she predicted.
Dr. Bhatt agreed that sotagliflozin “will be perceived as a drug for cardiologists to prescribe. I don’t see endocrinologists, nephrologists, and primary care physicians reaching for this drug if it has a heart failure label.” But, he added, “my hope is that the company files for additional indications. It deserves an indication for glycemic control.”
The evidence for a heart failure benefit from sotagliflozin is “valid and compelling,” and “having this option is great,” commented Mikhail N. Kosiborod, MD, a cardiologist, vice president of research at Saint Luke’s Health System, and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo. But, he added, “it will be a reasonably tall task for sotagliflozin to come from behind and be disruptive in a space where there are already two well-established SGLT2 inhibitors” approved for preventing heart failure hospitalizations, “with a lot of data to back them up,”
The feature that sets sotagliflozin apart from the approved SGLT2 inhibitors is the “really compelling decrease” it produced in rates of MIs and strokes “that we simply do not see with SGLT2 inhibitors,” Dr. Kosiborod said in an interview.
He also cited results from SCORED that suggest “a meaningful reduction in A1c” when indirectly compared with SGLT2 inhibitors, especially in patients with more severe CKD. The lack of a dedicated A1c-lowering trial or an approved type 2 diabetes indication “will not be a problem for cardiologists,” he predicted, but also agreed that it is less likely to be used by primary care physicians in low-risk patients.
“I can see myself prescribing sotagliflozin,” said Dr. Kosiborod, a SCORED coinvestigator, especially for patients with coexisting type 2 diabetes, heart failure, CKD, and atherosclerotic cardiovascular disease. These patients may get “more bang for the buck” because of a reduced risk for MI and stroke, making sotagliflozin “a solid consideration in these patients if the economic factors align.”
Like others, Dr. Kosiborod cited the big impact pricing will have, especially if, as expected, a generic SGLT2 inhibitor soon comes onto the U.S. market. “Access and affordability are very important.”
SOLOIST-WHF and SCORED were sponsored initially by Sanofi and later by Lexicon after Sanofi pulled out of sotagliflozin development. Dr. Butler has been a consultant for Lexicon as well as for AstraZeneca (which markets Farxiga), Boehringer Ingelheim and Lilly (which jointly market Jardiance), and Janssen (which markets Invokana), as well as for numerous other companies. Dr. Packer has been a consultant for AstraZeneca, Boehringer Ingelheim, Lilly, and numerous other companies. Dr. Bhatt was lead investigator for SOLOIST-WHF and SCORED and has been an adviser for Boehringer Ingelheim and Janssen and numerous other companies. Dr. Wysham has been an adviser, speaker, and consultant for AstraZeneca, Boehringer Ingelheim, Lilly, Janssen, Novo Nordisk, and Sanofi, an adviser for Abbott, and a speaker for Insulet. Dr. Kosiborod was a member of the SCORED Steering Committee and has been a consultant for Lexicon, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Novo Nordisk, and numerous other companies.
A version of this article first appeared on Medscape.com.
Sotagliflozin, a novel agent that inhibits sodium-glucose cotransporter 1 as well as SGLT2, has received marketing approval from the Food and Drug Administration for reducing the risk for cardiovascular death, hospitalization for heart failure, and urgent heart failure visits in patients with heart failure, and also for preventing these same events in patients with type 2 diabetes, chronic kidney disease (CKD), and other cardiovascular disease risk factors.
This puts sotagliflozin in direct competition with two SGLT2 inhibitors, dapagliflozin (Farxiga) and empagliflozin (Jardiance), that already have indications for preventing heart failure hospitalizations in patients with heart failure as well as approvals for type 2 diabetes and preservation of renal function.
Officials at Lexicon Pharmaceuticals, the company that developed and will market sotagliflozin under the trade name Inpefa, said in a press release that they expect U.S. sales of the agent to begin before the end of June 2023. The release also highlighted that the approval broadly covered use in patients with heart failure across the full range of both reduced and preserved left ventricular ejection fractions.
They base this niche target for sotagliflozin on results from the SOLOIST-WHF trial, which randomized 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure and showed a significant 33% reduction in the rate of deaths from cardiovascular causes and hospitalizations and urgent visits for heart failure, compared with control patients during a median 9 months of follow-up. Nearly half of the enrolled patients received their first dose while still hospitalized, while the other half received their first dose a median of 2 days after hospital discharge. The drug appeared safe.
Cutting heart failure rehospitalizations in half
An exploratory post hoc analysis of SOLOIST-WHF showed that treatment with sotagliflozin cut the rate of rehospitalizations roughly in half after both 30 and 90 days compared with control patients, according to an abstract presented at the 2022 annual scientific sessions of the AHA that has not yet been published in a peer-reviewed journal.
The only SGLT2 inhibitor tested so far when initiated in patients during hospitalization for heart failure is empagliflozin, in the EMPULSE trial, which randomized 530 patients. EMPULSE also showed that starting an SGLT2 inhibitor in this setting was safe and resulted in significant clinical benefit, the study’s primary endpoint, defined as a composite of death from any cause, number of heart failure events, and time to first heart failure event, or a 5-point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score at 90 days.
In the DELIVER trial, which tested dapagliflozin in patients with heart failure with preserved ejection fraction, roughly 10% of patients started study treatment during or within 30 days of heart failure hospitalization, and in this subgroup, dapagliflozin appeared as effective as it was in the other 90% of patients who did not start the drug during an acute or subacute phase.
Despite the SOLOIST-WHF evidence for sotagliflozin’s safety and efficacy in this economically important clinical setting, some experts say the drug faces an uphill path as it contends for market share against two solidly established, albeit dramatically underused, SGLT2 inhibitors. (Recent data document that 20% or fewer of U.S. patients eligible for treatment with an SGLT2 inhibitor receive it, such as a review of 49,000 patients hospitalized during 2021-2022 with heart failure with reduced ejection fraction.)
Others foresee a clear role for sotagliflozin, particularly because of additional facets of the drug’s performance in trials that they perceive give it an edge over dapagliflozin and empagliflozin. This includes evidence that sotagliflozin treatment uniquely (within the SGLT2 inhibitor class) cuts the rate of strokes and myocardial infarctions, as well as evidence of its apparent ability to lower hemoglobin A1c levels in patients with type 2 diabetes and with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a property likely linked to inhibition of SGLT1 in the gut that dampens intestinal glucose absorption.
Sotagliflozin uptake ‘will be a challenge’
“It will be a challenge” for sotagliflozin uptake, given the head start that both dapagliflozin and empagliflozin have had as well-documented agents for patients with heart failure, commented Javed Butler, MD, a heart failure clinician and trialist who is president of the Baylor Scott & White Research Institute in Dallas.
Given the position dapagliflozin and empagliflozin currently have in U.S. heart failure management – with the SGLT2 inhibitor class called out in guidelines as foundational for treating patients with heart failure with reduced ejection fraction and likely soon for heart failure with preserved ejection fraction as well – “I can’t imagine [sotagliflozin] will be considered a preferred option,” Dr. Butler said in an interview.
Another expert was even more dismissive of sotagliflozin’s role.
“There is no persuasive evidence that sotagliflozin has any advantages, compared with the SGLT2 inhibitors, for the treatment of heart failure,” said Milton Packer, MD, a heart failure specialist and trialist at Baylor University Medical Center, Dallas. “I do not see why U.S. physicians might pivot from established SGLT2 inhibitors to sotagliflozin,” unless it was priced “at a very meaningful discount to available SGLT2 inhibitors.”
At the time it announced the FDA’s approval, Lexicon did not provide details on how it would price sotagliflozin. Existing retail prices for dapagliflozin and empagliflozin run about $550-$600/month, a price point that has contributed to slow U.S. uptake of the drug class. But experts anticipate a dramatic shake-up of the U.S. market for SGLT2 inhibitors with expected introduction of a generic SGLT2 inhibitor formulation by 2025, a development that could further dampen sotagliflozin’s prospects.
Other experts are more optimistic about the new agent’s uptake, perhaps none more than Deepak L. Bhatt, MD, MPH, who led both pivotal trials that provide the bulk of sotagliflozin’s evidence package.
In addition to SOLOIST-WHF, Dr. Bhatt also headed the SCORED trial, with 10,584 patients with type 2 diabetes, CKD, and risks for cardiovascular disease randomized to sotagliflozin or placebo and followed for a median of 16 months. The primary result showed that sotagliflozin treatment cut the combined rate of deaths from cardiovascular causes, hospitalizations for heart failure, and urgent visits for heart failure by a significant 26% relative to control patients.
A clear MACE benefit
“The data from SOLOIST-WHF and SCORED look at least as good as the data for the SGLT2 inhibitors for heart failure, and what appears to be different are the rates for MI and stroke in SCORED,” said Dr. Bhatt, director of Mount Sinai Heart, New York.
“I believe the rate of major adverse cardiovascular events [MACE] were reduced [in SCORED], and this is different from the SGLT2 inhibitors,” he said in an interview.
In 2022, Dr. Bhatt reported results from a prespecified secondary analysis of SCORED that showed that treatment with sotagliflozin cut the rate of MACE by a significant 21%-26%, compared with placebo. This finding was, in part, driven by the first data to show a substantial benefit from an SGLT inhibitor on stroke rates.
And while SCORED did not report a significant benefit for slowing progression of CKD, subsequent post hoc analyses have suggested this advantage also in as-yet-unpublished findings, Dr. Bhatt added.
But he said he doubted nephrologists will see it as a first-line agent for slowing CKD progression – an indication already held by dapagliflozin, pending for empagliflozin, and also in place for a third SGLT2 inhibitor, canagliflozin (Invokana) – because sotagliflozin lacks clear significant and prespecified evidence for this effect.
Dr. Bhatt also acknowledged the limitation of sotagliflozin compared with the SGLT2 inhibitors as an agent for glucose control, again because of no evidence for this effect from a prospective analysis and no pending indication for type 2 diabetes treatment. But the SCORED data showed a clear A1c benefit, even in patients with severely reduced renal function.
Mostly for cardiologists? ‘Compelling’ reductions in MIs and strokes
That may mean sotagliflozin “won’t get much use by endocrinologists nor by primary care physicians,” commented Carol L. Wysham, MD, an endocrinologist with MultiCare in Spokane, Wash.
Sotagliflozin “will be a cardiology drug,” and will “have a hard time” competing with the SGLT2 inhibitors, she predicted.
Dr. Bhatt agreed that sotagliflozin “will be perceived as a drug for cardiologists to prescribe. I don’t see endocrinologists, nephrologists, and primary care physicians reaching for this drug if it has a heart failure label.” But, he added, “my hope is that the company files for additional indications. It deserves an indication for glycemic control.”
The evidence for a heart failure benefit from sotagliflozin is “valid and compelling,” and “having this option is great,” commented Mikhail N. Kosiborod, MD, a cardiologist, vice president of research at Saint Luke’s Health System, and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo. But, he added, “it will be a reasonably tall task for sotagliflozin to come from behind and be disruptive in a space where there are already two well-established SGLT2 inhibitors” approved for preventing heart failure hospitalizations, “with a lot of data to back them up,”
The feature that sets sotagliflozin apart from the approved SGLT2 inhibitors is the “really compelling decrease” it produced in rates of MIs and strokes “that we simply do not see with SGLT2 inhibitors,” Dr. Kosiborod said in an interview.
He also cited results from SCORED that suggest “a meaningful reduction in A1c” when indirectly compared with SGLT2 inhibitors, especially in patients with more severe CKD. The lack of a dedicated A1c-lowering trial or an approved type 2 diabetes indication “will not be a problem for cardiologists,” he predicted, but also agreed that it is less likely to be used by primary care physicians in low-risk patients.
“I can see myself prescribing sotagliflozin,” said Dr. Kosiborod, a SCORED coinvestigator, especially for patients with coexisting type 2 diabetes, heart failure, CKD, and atherosclerotic cardiovascular disease. These patients may get “more bang for the buck” because of a reduced risk for MI and stroke, making sotagliflozin “a solid consideration in these patients if the economic factors align.”
Like others, Dr. Kosiborod cited the big impact pricing will have, especially if, as expected, a generic SGLT2 inhibitor soon comes onto the U.S. market. “Access and affordability are very important.”
SOLOIST-WHF and SCORED were sponsored initially by Sanofi and later by Lexicon after Sanofi pulled out of sotagliflozin development. Dr. Butler has been a consultant for Lexicon as well as for AstraZeneca (which markets Farxiga), Boehringer Ingelheim and Lilly (which jointly market Jardiance), and Janssen (which markets Invokana), as well as for numerous other companies. Dr. Packer has been a consultant for AstraZeneca, Boehringer Ingelheim, Lilly, and numerous other companies. Dr. Bhatt was lead investigator for SOLOIST-WHF and SCORED and has been an adviser for Boehringer Ingelheim and Janssen and numerous other companies. Dr. Wysham has been an adviser, speaker, and consultant for AstraZeneca, Boehringer Ingelheim, Lilly, Janssen, Novo Nordisk, and Sanofi, an adviser for Abbott, and a speaker for Insulet. Dr. Kosiborod was a member of the SCORED Steering Committee and has been a consultant for Lexicon, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Novo Nordisk, and numerous other companies.
A version of this article first appeared on Medscape.com.









