User login
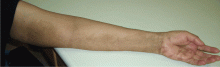
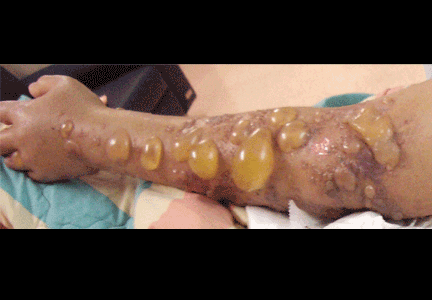
Multiple huge bullae after renal transplant
Q: What is the most likely diagnosis?
- Contact dermatitis
- Herpes zoster
- Herpes simplex
- Pemphigus
- Bullous pemphigoid
- Graft-vs-host disease
A: The correct answer is herpes zoster (shingles), which represents reactivation of varicella-zoster virus.
The diagnosis of herpes zoster is usually based solely on the clinical presentation. It is typically characterized in immunocompetent patients by a unilateral vesicular eruption with a well-defined dermatomal distribution. But occasionally, as in this patient on immunosuppressant drugs, it presents with atypical skin lesions such as multiple huge bullae involving multiple dermatomes.1,2
Patients treated with immunosuppressive agents after organ transplantation are at high risk of herpes zoster. A recent published retrospective study of adult kidney transplant recipients showed an average incidence of approximately 28 per 1,000 person-years.3
Treatment involves analgesics and sometimes antiviral drugs, and the decisions should take into account the patient’s age and immune status.1
- Nagel MA, Gilden DH. The protean neurologic manifestations of varicella-zoster virus infection. Cleve Clin J Med 2007; 74:489–504.
- Albrecht MA. Clinical manifestations of varicella-zoster virus infection: Herpes zoster. InRose BD, editor: UpToDate. Waltham, MA: UpToDate, 2008.
- Arness T, Pedersen R, Dierkhising R, Kremers W, Patel R. Varicella zoster virus-associated disease in adult kidney transplant recipients: incidence and risk-factor analysis. Transpl Infect Dis 2008; 10:260–268.
Q: What is the most likely diagnosis?
- Contact dermatitis
- Herpes zoster
- Herpes simplex
- Pemphigus
- Bullous pemphigoid
- Graft-vs-host disease
A: The correct answer is herpes zoster (shingles), which represents reactivation of varicella-zoster virus.
The diagnosis of herpes zoster is usually based solely on the clinical presentation. It is typically characterized in immunocompetent patients by a unilateral vesicular eruption with a well-defined dermatomal distribution. But occasionally, as in this patient on immunosuppressant drugs, it presents with atypical skin lesions such as multiple huge bullae involving multiple dermatomes.1,2
Patients treated with immunosuppressive agents after organ transplantation are at high risk of herpes zoster. A recent published retrospective study of adult kidney transplant recipients showed an average incidence of approximately 28 per 1,000 person-years.3
Treatment involves analgesics and sometimes antiviral drugs, and the decisions should take into account the patient’s age and immune status.1
Q: What is the most likely diagnosis?
- Contact dermatitis
- Herpes zoster
- Herpes simplex
- Pemphigus
- Bullous pemphigoid
- Graft-vs-host disease
A: The correct answer is herpes zoster (shingles), which represents reactivation of varicella-zoster virus.
The diagnosis of herpes zoster is usually based solely on the clinical presentation. It is typically characterized in immunocompetent patients by a unilateral vesicular eruption with a well-defined dermatomal distribution. But occasionally, as in this patient on immunosuppressant drugs, it presents with atypical skin lesions such as multiple huge bullae involving multiple dermatomes.1,2
Patients treated with immunosuppressive agents after organ transplantation are at high risk of herpes zoster. A recent published retrospective study of adult kidney transplant recipients showed an average incidence of approximately 28 per 1,000 person-years.3
Treatment involves analgesics and sometimes antiviral drugs, and the decisions should take into account the patient’s age and immune status.1
- Nagel MA, Gilden DH. The protean neurologic manifestations of varicella-zoster virus infection. Cleve Clin J Med 2007; 74:489–504.
- Albrecht MA. Clinical manifestations of varicella-zoster virus infection: Herpes zoster. InRose BD, editor: UpToDate. Waltham, MA: UpToDate, 2008.
- Arness T, Pedersen R, Dierkhising R, Kremers W, Patel R. Varicella zoster virus-associated disease in adult kidney transplant recipients: incidence and risk-factor analysis. Transpl Infect Dis 2008; 10:260–268.
- Nagel MA, Gilden DH. The protean neurologic manifestations of varicella-zoster virus infection. Cleve Clin J Med 2007; 74:489–504.
- Albrecht MA. Clinical manifestations of varicella-zoster virus infection: Herpes zoster. InRose BD, editor: UpToDate. Waltham, MA: UpToDate, 2008.
- Arness T, Pedersen R, Dierkhising R, Kremers W, Patel R. Varicella zoster virus-associated disease in adult kidney transplant recipients: incidence and risk-factor analysis. Transpl Infect Dis 2008; 10:260–268.
Volume Cuts Renal Artery Bypass Deaths in Low-Risk Patients
TUCSON, ARIZ. — In-hospital mortality for renal artery bypass in low-surgical-risk patients was more than fourfold greater at low-volume than at high-volume hospitals across the nation during a recent 6-year period.
However, among patients at intermediate or high surgical risk, in-hospital mortality for renal artery bypass (RAB) did not vary significantly based on a hospital's RAB volume, Dr. J. Gregory Modrall reported at the annual meeting of the Southern Association for Vascular Surgery.
This latter finding came as a surprise. The study hypothesis was that a high hospital RAB volume is a proxy for greater surgical team expertise, which was expected to translate into improved outcomes, explained Dr. Modrall of the department of surgery, University of Texas at Dallas.
He and his coinvestigators analyzed the Healthcare Cost and Utilization Project Nationwide Inpatient Sample, the nation's largest all-payer inpatient database, with more than 1,000 participating U.S. hospitals. During the years 2000–2005, they identified 7,413 patients who underwent RAB. The frequency of the operation was 3.2 cases per 100,000 discharges.
Dividing the hospitals into tertiles based on annual RAB volume, the investigators defined low-volume centers as those doing fewer than two per year, medium-volume hospitals as those doing two to five annually, and high-volume hospitals as those doing more than five.
For decades RAB has been a treatment mainstay for renal artery stenosis with presumed renovascular hypertension or ischemic nephropathy. However, according to Dr. Modrall's Nationwide Inpatient Sample analysis, the annual number of RABs performed plummeted by 49% between 2000 and 2005.
Overall national in-hospital mortality for RAB was 9.6%. There was no significant difference in crude rates based on hospital volume: 9.9% in low-volume, 10.5% in medium-volume, and 8.2% in high-volume centers.
However, it was a different story after adjustment for patient risk profile. In an earlier study, Dr. Modrall and his colleagues identified five independent risk factors for in-hospital mortality with RAB: advanced age, female sex, and a history of chronic renal failure, heart failure, or chronic lung disease.
When the investigators adjusted for risk factor level, patients in the lowest-risk quartile had an in-hospital mortality of 6.4% in low-volume hospitals, 3.7% in medium-volume hospitals, and 1.5% in high-volume hospitals.
In the remaining quartiles of patient risk, however, there was no significant difference in mortality based on hospital volume. For example, patients in the second-highest risk quartile had an in-hospital mortality of 9.9% in low-, 7.8% in medium-, and 11.9% in high-volume hospitals. Among those in the highest-risk quartile, the rates were 15.2%, 20.2%, and 16.3%, respectively.
In a separate multivariate analysis with hospital RAB volume as a continuous variable, there was a 2% decrease in the risk of in-hospital mortality for each additional RAB done per year at a hospital.
“If you have two hospitals operating on precisely the same patient population and one does six cases per year and the other does five, the hospital that does six bypasses per year will have a 2% lower risk of in-hospital mortality,” Dr. Modrall said.
These findings have important implications for patient management, the surgeon stressed. “Surgeons who practice in a low-volume hospital may want to consider lower-risk alternatives: transferring the patient to a high-volume referral center with documented expertise in renovascular surgery, or performing renal artery stenting, or even medical management. And certainly if you do contemplate doing renal artery bypass operations, I would strongly urge you to consider the five components of patient risk,” he said.
Dr. Bruce A. Perler, a study discussant, observed that Dr. Modrall's “somewhat sobering study” illustrates a key point: “There's no area of vascular surgery where percutaneous angioplasty has had a more profound impact than in renal artery occlusive disease.”
It's counterintuitive that high-volume hospitals, which do so much better with low-risk patients, don't also achieve better results with higher-risk patients, noted Dr. Perler, professor of surgery and chief of vascular surgery at Johns Hopkins University, Baltimore.
Dr. Modrall said he thinks patient risk factors are the major determinant of in-hospital mortality. In low-risk patients, surgical expertise as reflected in hospital RAB volume can make a big impact. Not so in heavily risk-laden patients.
“Patients who are high risk have so many risk factors that it really doesn't matter where they have their operation—they're probably going to have relatively poor outcomes,” he explained.
The lowest-risk patients had a mortality of 6.4% in low-volume hospitals and 1.5% in high-volume hospitals. DR. MODRALL
ELSEVIER GLOBAL MEDICAL NEWS
TUCSON, ARIZ. — In-hospital mortality for renal artery bypass in low-surgical-risk patients was more than fourfold greater at low-volume than at high-volume hospitals across the nation during a recent 6-year period.
However, among patients at intermediate or high surgical risk, in-hospital mortality for renal artery bypass (RAB) did not vary significantly based on a hospital's RAB volume, Dr. J. Gregory Modrall reported at the annual meeting of the Southern Association for Vascular Surgery.
This latter finding came as a surprise. The study hypothesis was that a high hospital RAB volume is a proxy for greater surgical team expertise, which was expected to translate into improved outcomes, explained Dr. Modrall of the department of surgery, University of Texas at Dallas.
He and his coinvestigators analyzed the Healthcare Cost and Utilization Project Nationwide Inpatient Sample, the nation's largest all-payer inpatient database, with more than 1,000 participating U.S. hospitals. During the years 2000–2005, they identified 7,413 patients who underwent RAB. The frequency of the operation was 3.2 cases per 100,000 discharges.
Dividing the hospitals into tertiles based on annual RAB volume, the investigators defined low-volume centers as those doing fewer than two per year, medium-volume hospitals as those doing two to five annually, and high-volume hospitals as those doing more than five.
For decades RAB has been a treatment mainstay for renal artery stenosis with presumed renovascular hypertension or ischemic nephropathy. However, according to Dr. Modrall's Nationwide Inpatient Sample analysis, the annual number of RABs performed plummeted by 49% between 2000 and 2005.
Overall national in-hospital mortality for RAB was 9.6%. There was no significant difference in crude rates based on hospital volume: 9.9% in low-volume, 10.5% in medium-volume, and 8.2% in high-volume centers.
However, it was a different story after adjustment for patient risk profile. In an earlier study, Dr. Modrall and his colleagues identified five independent risk factors for in-hospital mortality with RAB: advanced age, female sex, and a history of chronic renal failure, heart failure, or chronic lung disease.
When the investigators adjusted for risk factor level, patients in the lowest-risk quartile had an in-hospital mortality of 6.4% in low-volume hospitals, 3.7% in medium-volume hospitals, and 1.5% in high-volume hospitals.
In the remaining quartiles of patient risk, however, there was no significant difference in mortality based on hospital volume. For example, patients in the second-highest risk quartile had an in-hospital mortality of 9.9% in low-, 7.8% in medium-, and 11.9% in high-volume hospitals. Among those in the highest-risk quartile, the rates were 15.2%, 20.2%, and 16.3%, respectively.
In a separate multivariate analysis with hospital RAB volume as a continuous variable, there was a 2% decrease in the risk of in-hospital mortality for each additional RAB done per year at a hospital.
“If you have two hospitals operating on precisely the same patient population and one does six cases per year and the other does five, the hospital that does six bypasses per year will have a 2% lower risk of in-hospital mortality,” Dr. Modrall said.
These findings have important implications for patient management, the surgeon stressed. “Surgeons who practice in a low-volume hospital may want to consider lower-risk alternatives: transferring the patient to a high-volume referral center with documented expertise in renovascular surgery, or performing renal artery stenting, or even medical management. And certainly if you do contemplate doing renal artery bypass operations, I would strongly urge you to consider the five components of patient risk,” he said.
Dr. Bruce A. Perler, a study discussant, observed that Dr. Modrall's “somewhat sobering study” illustrates a key point: “There's no area of vascular surgery where percutaneous angioplasty has had a more profound impact than in renal artery occlusive disease.”
It's counterintuitive that high-volume hospitals, which do so much better with low-risk patients, don't also achieve better results with higher-risk patients, noted Dr. Perler, professor of surgery and chief of vascular surgery at Johns Hopkins University, Baltimore.
Dr. Modrall said he thinks patient risk factors are the major determinant of in-hospital mortality. In low-risk patients, surgical expertise as reflected in hospital RAB volume can make a big impact. Not so in heavily risk-laden patients.
“Patients who are high risk have so many risk factors that it really doesn't matter where they have their operation—they're probably going to have relatively poor outcomes,” he explained.
The lowest-risk patients had a mortality of 6.4% in low-volume hospitals and 1.5% in high-volume hospitals. DR. MODRALL
ELSEVIER GLOBAL MEDICAL NEWS
TUCSON, ARIZ. — In-hospital mortality for renal artery bypass in low-surgical-risk patients was more than fourfold greater at low-volume than at high-volume hospitals across the nation during a recent 6-year period.
However, among patients at intermediate or high surgical risk, in-hospital mortality for renal artery bypass (RAB) did not vary significantly based on a hospital's RAB volume, Dr. J. Gregory Modrall reported at the annual meeting of the Southern Association for Vascular Surgery.
This latter finding came as a surprise. The study hypothesis was that a high hospital RAB volume is a proxy for greater surgical team expertise, which was expected to translate into improved outcomes, explained Dr. Modrall of the department of surgery, University of Texas at Dallas.
He and his coinvestigators analyzed the Healthcare Cost and Utilization Project Nationwide Inpatient Sample, the nation's largest all-payer inpatient database, with more than 1,000 participating U.S. hospitals. During the years 2000–2005, they identified 7,413 patients who underwent RAB. The frequency of the operation was 3.2 cases per 100,000 discharges.
Dividing the hospitals into tertiles based on annual RAB volume, the investigators defined low-volume centers as those doing fewer than two per year, medium-volume hospitals as those doing two to five annually, and high-volume hospitals as those doing more than five.
For decades RAB has been a treatment mainstay for renal artery stenosis with presumed renovascular hypertension or ischemic nephropathy. However, according to Dr. Modrall's Nationwide Inpatient Sample analysis, the annual number of RABs performed plummeted by 49% between 2000 and 2005.
Overall national in-hospital mortality for RAB was 9.6%. There was no significant difference in crude rates based on hospital volume: 9.9% in low-volume, 10.5% in medium-volume, and 8.2% in high-volume centers.
However, it was a different story after adjustment for patient risk profile. In an earlier study, Dr. Modrall and his colleagues identified five independent risk factors for in-hospital mortality with RAB: advanced age, female sex, and a history of chronic renal failure, heart failure, or chronic lung disease.
When the investigators adjusted for risk factor level, patients in the lowest-risk quartile had an in-hospital mortality of 6.4% in low-volume hospitals, 3.7% in medium-volume hospitals, and 1.5% in high-volume hospitals.
In the remaining quartiles of patient risk, however, there was no significant difference in mortality based on hospital volume. For example, patients in the second-highest risk quartile had an in-hospital mortality of 9.9% in low-, 7.8% in medium-, and 11.9% in high-volume hospitals. Among those in the highest-risk quartile, the rates were 15.2%, 20.2%, and 16.3%, respectively.
In a separate multivariate analysis with hospital RAB volume as a continuous variable, there was a 2% decrease in the risk of in-hospital mortality for each additional RAB done per year at a hospital.
“If you have two hospitals operating on precisely the same patient population and one does six cases per year and the other does five, the hospital that does six bypasses per year will have a 2% lower risk of in-hospital mortality,” Dr. Modrall said.
These findings have important implications for patient management, the surgeon stressed. “Surgeons who practice in a low-volume hospital may want to consider lower-risk alternatives: transferring the patient to a high-volume referral center with documented expertise in renovascular surgery, or performing renal artery stenting, or even medical management. And certainly if you do contemplate doing renal artery bypass operations, I would strongly urge you to consider the five components of patient risk,” he said.
Dr. Bruce A. Perler, a study discussant, observed that Dr. Modrall's “somewhat sobering study” illustrates a key point: “There's no area of vascular surgery where percutaneous angioplasty has had a more profound impact than in renal artery occlusive disease.”
It's counterintuitive that high-volume hospitals, which do so much better with low-risk patients, don't also achieve better results with higher-risk patients, noted Dr. Perler, professor of surgery and chief of vascular surgery at Johns Hopkins University, Baltimore.
Dr. Modrall said he thinks patient risk factors are the major determinant of in-hospital mortality. In low-risk patients, surgical expertise as reflected in hospital RAB volume can make a big impact. Not so in heavily risk-laden patients.
“Patients who are high risk have so many risk factors that it really doesn't matter where they have their operation—they're probably going to have relatively poor outcomes,” he explained.
The lowest-risk patients had a mortality of 6.4% in low-volume hospitals and 1.5% in high-volume hospitals. DR. MODRALL
ELSEVIER GLOBAL MEDICAL NEWS
Intrarenal Fenoldopam May Help Protect Kidneys
HOLLYWOOD, FLA. — Targeted renal therapy with the vasodilating drug fenoldopam was effective for treating acute kidney injury and for preventing contrast-induced nephropathy in results from a pair of studies.
By infusing the drug directly into patients' renal arteries with a specially designed catheter, targeted therapy allows the use of a substantial dose of fenoldopam mesylate while avoiding systemic adverse effects such as hypotension, Dr. James A. Tumlin said at ISET 2009, an international symposium on endovascular therapy.
He reported treating a series of 28 patients with oliguria and diuretic-unresponsive acute kidney injury. Their diuretic unresponsiveness was defined as a failure to double their urine output after a single bolus dose of 80–120 mg furosemide. Their average serum creatinine level at entry into the study was about 1.7 mg/dL. Many of the patients had one or more comorbidities, with 57% having respiratory distress, 46% on mechanical ventilation, 43% having sepsis, and 39% with a left ventricular ejection fraction less than 35%.
All patients received intrarenal fenoldopam via a Benephit peripheral vascular catheter made by FlowMedica Inc. The catheter is designed to infuse both renal arteries with a single device, and was approved by the Food and Drug Administration in late 2008 for targeted renal therapy in patients at risk for developing acute kidney injury. Dr. Tumlin is a consultant to and has received grant support from FlowMedica.
Their goal dosage was a fenoldopam infusion of 0.4 mcg/kg per minute, and the actual average dose used was 0.39 mcg/kg per minute, with a maximum dose given to any patient of 0.8 mcg/kg per minute. The target duration of treatment was 48 hours, and the actual average duration was 42 hours, with a maximum of 72 hours.
Renal recovery, defined as a fall in serum creatinine, occurred in 17 patients (61%) by the fourth day after treatment, and in 27 (96%) of patients by a week after treatment. Three of the patients (11%) died during follow-up, and another four (14%) required dialysis during follow-up. (None of the dialysis patients died.) “This was an unusually low mortality rate,” compared with the historic experience with similar patients who did not undergo renal infusion with fenoldopam, said Dr. Tumlin, a nephrologist and professor of medicine at the University of Tennessee in Chattanooga.
The second experience using intrarenal fenoldopam that was presented at the meeting included data from 593 patients who were enrolled in a targeted renal therapy registry. The series included 340 patients who were treated to prevent contrast-induced nephropathy and another 40 patients who were treated for acute kidney injury that they developed following coronary artery bypass grafting. The patients had been treated by 38 different physicians at 19 medical centers.
Intrarenal fenoldopam was given to 94% of the registry's patients, at a median dosage of 0.4 mcg/kg per minute, with a range of 0.05–0.8 mcg/kg per minute. The remaining patients received another drug, such as sodium bicarbonate, reported Dr. John H. Rundback in a separate talk at the meeting. The median duration of the fenoldopam infusion was 180 minutes.
Bilateral renal-artery catheterization was performed successfully in 95% of the registry patients, a procedure that took an average of 2 minutes. Five of the 593 patients in the registry (0.8%) had a complication from renal-artery catheterization: Three had groin complications (the catheter is often inserted through the femoral artery), one had a renal-artery dissection, and one developed hypotension.
Registry outcomes showed that the 0.4-mcg/kg per minute dosage was much more effective than was a 0.2- mcg/kg per minute dosage for preventing contrast-induced nephropathy, and that treatment for at least an hour was more effective than a briefer infusion, said Dr. Rundback, an interventional radiologist and director of the Interventional Institute at Holy Name Hospital, Teaneck, N.J. Dr. Rundback has been a consultant to and a member of the scientific advisory board of FlowMedica.
The predicted incidence of contrast-induced nephropathy was about 27%, yet the rate among 268 patients who were treated with at least 0.4 mcg/kg per minute of fenoldopam for at least 1 hour was less than 1%, Dr. Rundback said.
Patients who received fenoldopam had 'an unusually low mortality rate.' DR. TUMLIN
HOLLYWOOD, FLA. — Targeted renal therapy with the vasodilating drug fenoldopam was effective for treating acute kidney injury and for preventing contrast-induced nephropathy in results from a pair of studies.
By infusing the drug directly into patients' renal arteries with a specially designed catheter, targeted therapy allows the use of a substantial dose of fenoldopam mesylate while avoiding systemic adverse effects such as hypotension, Dr. James A. Tumlin said at ISET 2009, an international symposium on endovascular therapy.
He reported treating a series of 28 patients with oliguria and diuretic-unresponsive acute kidney injury. Their diuretic unresponsiveness was defined as a failure to double their urine output after a single bolus dose of 80–120 mg furosemide. Their average serum creatinine level at entry into the study was about 1.7 mg/dL. Many of the patients had one or more comorbidities, with 57% having respiratory distress, 46% on mechanical ventilation, 43% having sepsis, and 39% with a left ventricular ejection fraction less than 35%.
All patients received intrarenal fenoldopam via a Benephit peripheral vascular catheter made by FlowMedica Inc. The catheter is designed to infuse both renal arteries with a single device, and was approved by the Food and Drug Administration in late 2008 for targeted renal therapy in patients at risk for developing acute kidney injury. Dr. Tumlin is a consultant to and has received grant support from FlowMedica.
Their goal dosage was a fenoldopam infusion of 0.4 mcg/kg per minute, and the actual average dose used was 0.39 mcg/kg per minute, with a maximum dose given to any patient of 0.8 mcg/kg per minute. The target duration of treatment was 48 hours, and the actual average duration was 42 hours, with a maximum of 72 hours.
Renal recovery, defined as a fall in serum creatinine, occurred in 17 patients (61%) by the fourth day after treatment, and in 27 (96%) of patients by a week after treatment. Three of the patients (11%) died during follow-up, and another four (14%) required dialysis during follow-up. (None of the dialysis patients died.) “This was an unusually low mortality rate,” compared with the historic experience with similar patients who did not undergo renal infusion with fenoldopam, said Dr. Tumlin, a nephrologist and professor of medicine at the University of Tennessee in Chattanooga.
The second experience using intrarenal fenoldopam that was presented at the meeting included data from 593 patients who were enrolled in a targeted renal therapy registry. The series included 340 patients who were treated to prevent contrast-induced nephropathy and another 40 patients who were treated for acute kidney injury that they developed following coronary artery bypass grafting. The patients had been treated by 38 different physicians at 19 medical centers.
Intrarenal fenoldopam was given to 94% of the registry's patients, at a median dosage of 0.4 mcg/kg per minute, with a range of 0.05–0.8 mcg/kg per minute. The remaining patients received another drug, such as sodium bicarbonate, reported Dr. John H. Rundback in a separate talk at the meeting. The median duration of the fenoldopam infusion was 180 minutes.
Bilateral renal-artery catheterization was performed successfully in 95% of the registry patients, a procedure that took an average of 2 minutes. Five of the 593 patients in the registry (0.8%) had a complication from renal-artery catheterization: Three had groin complications (the catheter is often inserted through the femoral artery), one had a renal-artery dissection, and one developed hypotension.
Registry outcomes showed that the 0.4-mcg/kg per minute dosage was much more effective than was a 0.2- mcg/kg per minute dosage for preventing contrast-induced nephropathy, and that treatment for at least an hour was more effective than a briefer infusion, said Dr. Rundback, an interventional radiologist and director of the Interventional Institute at Holy Name Hospital, Teaneck, N.J. Dr. Rundback has been a consultant to and a member of the scientific advisory board of FlowMedica.
The predicted incidence of contrast-induced nephropathy was about 27%, yet the rate among 268 patients who were treated with at least 0.4 mcg/kg per minute of fenoldopam for at least 1 hour was less than 1%, Dr. Rundback said.
Patients who received fenoldopam had 'an unusually low mortality rate.' DR. TUMLIN
HOLLYWOOD, FLA. — Targeted renal therapy with the vasodilating drug fenoldopam was effective for treating acute kidney injury and for preventing contrast-induced nephropathy in results from a pair of studies.
By infusing the drug directly into patients' renal arteries with a specially designed catheter, targeted therapy allows the use of a substantial dose of fenoldopam mesylate while avoiding systemic adverse effects such as hypotension, Dr. James A. Tumlin said at ISET 2009, an international symposium on endovascular therapy.
He reported treating a series of 28 patients with oliguria and diuretic-unresponsive acute kidney injury. Their diuretic unresponsiveness was defined as a failure to double their urine output after a single bolus dose of 80–120 mg furosemide. Their average serum creatinine level at entry into the study was about 1.7 mg/dL. Many of the patients had one or more comorbidities, with 57% having respiratory distress, 46% on mechanical ventilation, 43% having sepsis, and 39% with a left ventricular ejection fraction less than 35%.
All patients received intrarenal fenoldopam via a Benephit peripheral vascular catheter made by FlowMedica Inc. The catheter is designed to infuse both renal arteries with a single device, and was approved by the Food and Drug Administration in late 2008 for targeted renal therapy in patients at risk for developing acute kidney injury. Dr. Tumlin is a consultant to and has received grant support from FlowMedica.
Their goal dosage was a fenoldopam infusion of 0.4 mcg/kg per minute, and the actual average dose used was 0.39 mcg/kg per minute, with a maximum dose given to any patient of 0.8 mcg/kg per minute. The target duration of treatment was 48 hours, and the actual average duration was 42 hours, with a maximum of 72 hours.
Renal recovery, defined as a fall in serum creatinine, occurred in 17 patients (61%) by the fourth day after treatment, and in 27 (96%) of patients by a week after treatment. Three of the patients (11%) died during follow-up, and another four (14%) required dialysis during follow-up. (None of the dialysis patients died.) “This was an unusually low mortality rate,” compared with the historic experience with similar patients who did not undergo renal infusion with fenoldopam, said Dr. Tumlin, a nephrologist and professor of medicine at the University of Tennessee in Chattanooga.
The second experience using intrarenal fenoldopam that was presented at the meeting included data from 593 patients who were enrolled in a targeted renal therapy registry. The series included 340 patients who were treated to prevent contrast-induced nephropathy and another 40 patients who were treated for acute kidney injury that they developed following coronary artery bypass grafting. The patients had been treated by 38 different physicians at 19 medical centers.
Intrarenal fenoldopam was given to 94% of the registry's patients, at a median dosage of 0.4 mcg/kg per minute, with a range of 0.05–0.8 mcg/kg per minute. The remaining patients received another drug, such as sodium bicarbonate, reported Dr. John H. Rundback in a separate talk at the meeting. The median duration of the fenoldopam infusion was 180 minutes.
Bilateral renal-artery catheterization was performed successfully in 95% of the registry patients, a procedure that took an average of 2 minutes. Five of the 593 patients in the registry (0.8%) had a complication from renal-artery catheterization: Three had groin complications (the catheter is often inserted through the femoral artery), one had a renal-artery dissection, and one developed hypotension.
Registry outcomes showed that the 0.4-mcg/kg per minute dosage was much more effective than was a 0.2- mcg/kg per minute dosage for preventing contrast-induced nephropathy, and that treatment for at least an hour was more effective than a briefer infusion, said Dr. Rundback, an interventional radiologist and director of the Interventional Institute at Holy Name Hospital, Teaneck, N.J. Dr. Rundback has been a consultant to and a member of the scientific advisory board of FlowMedica.
The predicted incidence of contrast-induced nephropathy was about 27%, yet the rate among 268 patients who were treated with at least 0.4 mcg/kg per minute of fenoldopam for at least 1 hour was less than 1%, Dr. Rundback said.
Patients who received fenoldopam had 'an unusually low mortality rate.' DR. TUMLIN
Guidelines Back Prostate Ca Chemoprevention
Sherry Boschert contributed to this story.
Healthy men with a prostate-specific antigen score of 3.0 or lower should consider taking a 5-alpha reductase inhibitor to prevent prostate cancer, according to newly released clinical practice guidelines.
The guidelines, issued by the American Urological Association and the American Society of Clinical Oncology, apply to men who plan to get yearly PSA tests or regular prostate cancer screening and who have no signs of prostate cancer. In addition, for men who already are taking the drugs (to treat benign prostatic hyperplasia or male-pattern baldness, for example), physicians should discuss continuing the drug specifically to prevent prostate cancer (J. Urol. 2009;181:1642–57; J. Clin Oncol. 2009 Feb. 24 [doi:10.1200/JCO.2008.16.9599]; www.asco.org/guidelines
The guidelines are based on evidence from nine randomized, controlled clinical trials, including the Prostate Cancer Prevention Trial (PCPT). “That body of evidence provided proof” that the 5-alpha reductase inhibitors will decrease the risk of prostate cancer in men being regularly screened for the disease, Dr. Barnett S. Kramer said at a press conference held in conjunction with a symposium on genitourinary cancers.
All nine trials have enrolled men undergoing regular screening for prostate cancer. This fact is important, because it has been well-established that screening doubles the risk of being diagnosed with prostate cancer, added Dr. Kramer, cochair of the panel that produced the guidelines.
The PCPT found an overall 25% relative risk reduction for prostate cancer in men who took the 5-alpha reductase inhibitor finasteride for 1–7 years. Because most of the men in this study were white, the results “principally apply only to white men,” he noted, adding that subanalyses found no substantive differences among racial or ethnic groups.
It's not clear whether preventive treatment will affect mortality from prostate cancer or whether doses lower than those currently used would be effective in prevention, said Dr. Kramer, associate director of disease prevention at the National Institutes of Health. In addition, “we can't be confident of its effect in men who choose not to be screened.”
The 5-alpha reductase inhibitors are known to lower levels of dihydrotestosterone, which can contribute to prostate cancer growth. Their most common side effects include erectile dysfunction, decreased libido, and ejaculatory dysfunction, with rarer cases of gynecomastia.
When the PCPT was published, there was concern over what appeared to be an increase in the rate of high-grade tumors diagnosed among those randomized to finasteride (37% of the prostate cancers in the finasteride arm were high grade, vs. 22% of those in the placebo arm). But a subsequent analysis determined this increase was likely to be spurious, Dr. Kramer said, adding that his comments represented those of ASCO and the AUA, not the U.S. government or NIH.
Although Dr. Kramer said he believed that cost needs to be part of this discussion, the available data were not strong enough to calculate cost-effectiveness.
Currently, approximately one in six U.S. men gets diagnosed with prostate cancer, the second-leading cause of death from cancer in men and, after skin cancer, the most common cancer diagnosed in men in the United States.
He pointed out that prostate cancer rates in the United States are among the highest in the world. In addition, since the principal driver of prostate cancer risk is age, the recommendations target “healthy men who fulfill risk criteria by virtue of age.”
The guidelines could apply to millions of men. During the briefing, Dr. Howard Sandler, a prostate cancer specialist at Cedars-Sinai Medical Center in Los Angeles, said he expects there will be many different attitudes about taking a pill every day “for preventing a condition that may not occur.”
He said he personally might consider a trial of over a month, and if he developed side effects to the drug, it would not be worth the potential benefits. But if he did not develop adverse effects, “ultimately, I would become reassured that taking one pill per day … might decrease my risk for prostate cancer,” he said, adding that he had not yet decided whether to pursue such preventive treatment.
The ASCO Web site offers physicians a “Decision Aid Tool,” composed of charts and diagrams to help explain the risks and benefits of 5-alpha reductase inhibitors to patients and their families. ASCO also has produced a corresponding patient guide, which is available at www.cancer.net
Dr. Schellhammer has received research funds or support from GlaxoSmithKline, which markets the 5-alpha reductase inhibitor dutasteride, and from other pharmaceutical companies. Dr. Kramer has no conflicts of interest related to the guidelines.
The annual Genitourinary Cancers Symposium is sponsored by the American Society of Clinical Oncology, American Society for Therapeutic Radiology and Oncology, and Society of Urologic Oncology.
Sherry Boschert contributed to this story.
Healthy men with a prostate-specific antigen score of 3.0 or lower should consider taking a 5-alpha reductase inhibitor to prevent prostate cancer, according to newly released clinical practice guidelines.
The guidelines, issued by the American Urological Association and the American Society of Clinical Oncology, apply to men who plan to get yearly PSA tests or regular prostate cancer screening and who have no signs of prostate cancer. In addition, for men who already are taking the drugs (to treat benign prostatic hyperplasia or male-pattern baldness, for example), physicians should discuss continuing the drug specifically to prevent prostate cancer (J. Urol. 2009;181:1642–57; J. Clin Oncol. 2009 Feb. 24 [doi:10.1200/JCO.2008.16.9599]; www.asco.org/guidelines
The guidelines are based on evidence from nine randomized, controlled clinical trials, including the Prostate Cancer Prevention Trial (PCPT). “That body of evidence provided proof” that the 5-alpha reductase inhibitors will decrease the risk of prostate cancer in men being regularly screened for the disease, Dr. Barnett S. Kramer said at a press conference held in conjunction with a symposium on genitourinary cancers.
All nine trials have enrolled men undergoing regular screening for prostate cancer. This fact is important, because it has been well-established that screening doubles the risk of being diagnosed with prostate cancer, added Dr. Kramer, cochair of the panel that produced the guidelines.
The PCPT found an overall 25% relative risk reduction for prostate cancer in men who took the 5-alpha reductase inhibitor finasteride for 1–7 years. Because most of the men in this study were white, the results “principally apply only to white men,” he noted, adding that subanalyses found no substantive differences among racial or ethnic groups.
It's not clear whether preventive treatment will affect mortality from prostate cancer or whether doses lower than those currently used would be effective in prevention, said Dr. Kramer, associate director of disease prevention at the National Institutes of Health. In addition, “we can't be confident of its effect in men who choose not to be screened.”
The 5-alpha reductase inhibitors are known to lower levels of dihydrotestosterone, which can contribute to prostate cancer growth. Their most common side effects include erectile dysfunction, decreased libido, and ejaculatory dysfunction, with rarer cases of gynecomastia.
When the PCPT was published, there was concern over what appeared to be an increase in the rate of high-grade tumors diagnosed among those randomized to finasteride (37% of the prostate cancers in the finasteride arm were high grade, vs. 22% of those in the placebo arm). But a subsequent analysis determined this increase was likely to be spurious, Dr. Kramer said, adding that his comments represented those of ASCO and the AUA, not the U.S. government or NIH.
Although Dr. Kramer said he believed that cost needs to be part of this discussion, the available data were not strong enough to calculate cost-effectiveness.
Currently, approximately one in six U.S. men gets diagnosed with prostate cancer, the second-leading cause of death from cancer in men and, after skin cancer, the most common cancer diagnosed in men in the United States.
He pointed out that prostate cancer rates in the United States are among the highest in the world. In addition, since the principal driver of prostate cancer risk is age, the recommendations target “healthy men who fulfill risk criteria by virtue of age.”
The guidelines could apply to millions of men. During the briefing, Dr. Howard Sandler, a prostate cancer specialist at Cedars-Sinai Medical Center in Los Angeles, said he expects there will be many different attitudes about taking a pill every day “for preventing a condition that may not occur.”
He said he personally might consider a trial of over a month, and if he developed side effects to the drug, it would not be worth the potential benefits. But if he did not develop adverse effects, “ultimately, I would become reassured that taking one pill per day … might decrease my risk for prostate cancer,” he said, adding that he had not yet decided whether to pursue such preventive treatment.
The ASCO Web site offers physicians a “Decision Aid Tool,” composed of charts and diagrams to help explain the risks and benefits of 5-alpha reductase inhibitors to patients and their families. ASCO also has produced a corresponding patient guide, which is available at www.cancer.net
Dr. Schellhammer has received research funds or support from GlaxoSmithKline, which markets the 5-alpha reductase inhibitor dutasteride, and from other pharmaceutical companies. Dr. Kramer has no conflicts of interest related to the guidelines.
The annual Genitourinary Cancers Symposium is sponsored by the American Society of Clinical Oncology, American Society for Therapeutic Radiology and Oncology, and Society of Urologic Oncology.
Sherry Boschert contributed to this story.
Healthy men with a prostate-specific antigen score of 3.0 or lower should consider taking a 5-alpha reductase inhibitor to prevent prostate cancer, according to newly released clinical practice guidelines.
The guidelines, issued by the American Urological Association and the American Society of Clinical Oncology, apply to men who plan to get yearly PSA tests or regular prostate cancer screening and who have no signs of prostate cancer. In addition, for men who already are taking the drugs (to treat benign prostatic hyperplasia or male-pattern baldness, for example), physicians should discuss continuing the drug specifically to prevent prostate cancer (J. Urol. 2009;181:1642–57; J. Clin Oncol. 2009 Feb. 24 [doi:10.1200/JCO.2008.16.9599]; www.asco.org/guidelines
The guidelines are based on evidence from nine randomized, controlled clinical trials, including the Prostate Cancer Prevention Trial (PCPT). “That body of evidence provided proof” that the 5-alpha reductase inhibitors will decrease the risk of prostate cancer in men being regularly screened for the disease, Dr. Barnett S. Kramer said at a press conference held in conjunction with a symposium on genitourinary cancers.
All nine trials have enrolled men undergoing regular screening for prostate cancer. This fact is important, because it has been well-established that screening doubles the risk of being diagnosed with prostate cancer, added Dr. Kramer, cochair of the panel that produced the guidelines.
The PCPT found an overall 25% relative risk reduction for prostate cancer in men who took the 5-alpha reductase inhibitor finasteride for 1–7 years. Because most of the men in this study were white, the results “principally apply only to white men,” he noted, adding that subanalyses found no substantive differences among racial or ethnic groups.
It's not clear whether preventive treatment will affect mortality from prostate cancer or whether doses lower than those currently used would be effective in prevention, said Dr. Kramer, associate director of disease prevention at the National Institutes of Health. In addition, “we can't be confident of its effect in men who choose not to be screened.”
The 5-alpha reductase inhibitors are known to lower levels of dihydrotestosterone, which can contribute to prostate cancer growth. Their most common side effects include erectile dysfunction, decreased libido, and ejaculatory dysfunction, with rarer cases of gynecomastia.
When the PCPT was published, there was concern over what appeared to be an increase in the rate of high-grade tumors diagnosed among those randomized to finasteride (37% of the prostate cancers in the finasteride arm were high grade, vs. 22% of those in the placebo arm). But a subsequent analysis determined this increase was likely to be spurious, Dr. Kramer said, adding that his comments represented those of ASCO and the AUA, not the U.S. government or NIH.
Although Dr. Kramer said he believed that cost needs to be part of this discussion, the available data were not strong enough to calculate cost-effectiveness.
Currently, approximately one in six U.S. men gets diagnosed with prostate cancer, the second-leading cause of death from cancer in men and, after skin cancer, the most common cancer diagnosed in men in the United States.
He pointed out that prostate cancer rates in the United States are among the highest in the world. In addition, since the principal driver of prostate cancer risk is age, the recommendations target “healthy men who fulfill risk criteria by virtue of age.”
The guidelines could apply to millions of men. During the briefing, Dr. Howard Sandler, a prostate cancer specialist at Cedars-Sinai Medical Center in Los Angeles, said he expects there will be many different attitudes about taking a pill every day “for preventing a condition that may not occur.”
He said he personally might consider a trial of over a month, and if he developed side effects to the drug, it would not be worth the potential benefits. But if he did not develop adverse effects, “ultimately, I would become reassured that taking one pill per day … might decrease my risk for prostate cancer,” he said, adding that he had not yet decided whether to pursue such preventive treatment.
The ASCO Web site offers physicians a “Decision Aid Tool,” composed of charts and diagrams to help explain the risks and benefits of 5-alpha reductase inhibitors to patients and their families. ASCO also has produced a corresponding patient guide, which is available at www.cancer.net
Dr. Schellhammer has received research funds or support from GlaxoSmithKline, which markets the 5-alpha reductase inhibitor dutasteride, and from other pharmaceutical companies. Dr. Kramer has no conflicts of interest related to the guidelines.
The annual Genitourinary Cancers Symposium is sponsored by the American Society of Clinical Oncology, American Society for Therapeutic Radiology and Oncology, and Society of Urologic Oncology.
Urine Test May Detect Aggressive Prostate Ca
A molecular urine test that detects the fusion of two genes associated with more aggressive prostate cancers was highly specific for prostate cancer in a study in men undergoing prostate biopsies, investigators report based on an interim review.
The test, which is not available commercially, detects fusions between TMPRSS2 (T2), an androgen-responsive gene, and the oncogenic transcription factor ERG. This fusion is found in about half of all prostate tumors, and has been associated with adverse clinical outcomes.
Initially reported at the end of 2005, this fusion was the first specific chromosomal rearrangement identified in prostate tumors, and appears to be “an ideal target for a diagnostic test because of the high specificity for prostate cancer,” according to Jack Groskopf, Ph.D., director of research and development in the cancer diagnostics division of Gen-Probe Inc., the San Diego-based company developing the test.
The test, known as the T2:ERG test, may eventually be useful in determining prognosis and selecting treatment in men diagnosed with prostate cancer, Dr. Groskopf said at a press briefing in advance of the study's presentation at a symposium on genitourinary cancers. He cited the need for a test that can help determine which prostate cancers require aggressive treatment and which could be managed conservatively.
To date, the test has been used on urine specimens collected in 556 men at three medical centers following a digital rectal exam and before prostate biopsy. The test predicted the presence of prostate cancer on biopsy with a specificity of 84%, compared with a specificity of 27% for serum prostate-specific antigen (PSA), with similar results at all three sites, he said.
The gene fusion has been present in about 42% of all positive biopsies to date, an indication that the test is “doing pretty well” in terms of sensitivity, as this correlates to the prevalence of the gene fusion in about half of all prostate cancers, he noted.
In addition, there have been significant correlations between a positive test and indicators of cancer aggressiveness, Gleason score, percent of prostate cancer involvement, and percent positive core, providing preliminary evidence indicating that T2:ERG status correlates with the criteria for aggressive cancers, he said.
The next step is to follow up and confirm these findings, and “perhaps more importantly,” to start studying the correlation between the urine test and pathologic features in prostatectomy tissue, such as tumor volume, stage, and grade, Dr. Groskopf said.
Describing this work as an “important first step,” Dr. Howard Sandler, moderator of the briefing, remarked that it represents “an amazingly short interval between the basic fundamental discovery and potential clinical utility of a diagnostic test.”
If it turns out that gene fusion is related to progression of prostate cancer in half of all men who develop prostate cancer, the molecular change will have major implications for diagnosis and possibly treatment as well, because it may also be a therapeutic target, said Dr. Sandler, chairman of radiation oncology at the Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles. “There is interest in targeting our diagnostic strategies towards patients who will have cancers that really need to be treated, not those that are overdiagnosed and are never destined to cause clinical harm,” he added.
The annual Genitourinary Cancers Symposium is sponsored by the American Society of Clinical Oncology, American Society for Therapeutic Radiology and Oncology, and Society of Urologic Oncology.
A molecular urine test that detects the fusion of two genes associated with more aggressive prostate cancers was highly specific for prostate cancer in a study in men undergoing prostate biopsies, investigators report based on an interim review.
The test, which is not available commercially, detects fusions between TMPRSS2 (T2), an androgen-responsive gene, and the oncogenic transcription factor ERG. This fusion is found in about half of all prostate tumors, and has been associated with adverse clinical outcomes.
Initially reported at the end of 2005, this fusion was the first specific chromosomal rearrangement identified in prostate tumors, and appears to be “an ideal target for a diagnostic test because of the high specificity for prostate cancer,” according to Jack Groskopf, Ph.D., director of research and development in the cancer diagnostics division of Gen-Probe Inc., the San Diego-based company developing the test.
The test, known as the T2:ERG test, may eventually be useful in determining prognosis and selecting treatment in men diagnosed with prostate cancer, Dr. Groskopf said at a press briefing in advance of the study's presentation at a symposium on genitourinary cancers. He cited the need for a test that can help determine which prostate cancers require aggressive treatment and which could be managed conservatively.
To date, the test has been used on urine specimens collected in 556 men at three medical centers following a digital rectal exam and before prostate biopsy. The test predicted the presence of prostate cancer on biopsy with a specificity of 84%, compared with a specificity of 27% for serum prostate-specific antigen (PSA), with similar results at all three sites, he said.
The gene fusion has been present in about 42% of all positive biopsies to date, an indication that the test is “doing pretty well” in terms of sensitivity, as this correlates to the prevalence of the gene fusion in about half of all prostate cancers, he noted.
In addition, there have been significant correlations between a positive test and indicators of cancer aggressiveness, Gleason score, percent of prostate cancer involvement, and percent positive core, providing preliminary evidence indicating that T2:ERG status correlates with the criteria for aggressive cancers, he said.
The next step is to follow up and confirm these findings, and “perhaps more importantly,” to start studying the correlation between the urine test and pathologic features in prostatectomy tissue, such as tumor volume, stage, and grade, Dr. Groskopf said.
Describing this work as an “important first step,” Dr. Howard Sandler, moderator of the briefing, remarked that it represents “an amazingly short interval between the basic fundamental discovery and potential clinical utility of a diagnostic test.”
If it turns out that gene fusion is related to progression of prostate cancer in half of all men who develop prostate cancer, the molecular change will have major implications for diagnosis and possibly treatment as well, because it may also be a therapeutic target, said Dr. Sandler, chairman of radiation oncology at the Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles. “There is interest in targeting our diagnostic strategies towards patients who will have cancers that really need to be treated, not those that are overdiagnosed and are never destined to cause clinical harm,” he added.
The annual Genitourinary Cancers Symposium is sponsored by the American Society of Clinical Oncology, American Society for Therapeutic Radiology and Oncology, and Society of Urologic Oncology.
A molecular urine test that detects the fusion of two genes associated with more aggressive prostate cancers was highly specific for prostate cancer in a study in men undergoing prostate biopsies, investigators report based on an interim review.
The test, which is not available commercially, detects fusions between TMPRSS2 (T2), an androgen-responsive gene, and the oncogenic transcription factor ERG. This fusion is found in about half of all prostate tumors, and has been associated with adverse clinical outcomes.
Initially reported at the end of 2005, this fusion was the first specific chromosomal rearrangement identified in prostate tumors, and appears to be “an ideal target for a diagnostic test because of the high specificity for prostate cancer,” according to Jack Groskopf, Ph.D., director of research and development in the cancer diagnostics division of Gen-Probe Inc., the San Diego-based company developing the test.
The test, known as the T2:ERG test, may eventually be useful in determining prognosis and selecting treatment in men diagnosed with prostate cancer, Dr. Groskopf said at a press briefing in advance of the study's presentation at a symposium on genitourinary cancers. He cited the need for a test that can help determine which prostate cancers require aggressive treatment and which could be managed conservatively.
To date, the test has been used on urine specimens collected in 556 men at three medical centers following a digital rectal exam and before prostate biopsy. The test predicted the presence of prostate cancer on biopsy with a specificity of 84%, compared with a specificity of 27% for serum prostate-specific antigen (PSA), with similar results at all three sites, he said.
The gene fusion has been present in about 42% of all positive biopsies to date, an indication that the test is “doing pretty well” in terms of sensitivity, as this correlates to the prevalence of the gene fusion in about half of all prostate cancers, he noted.
In addition, there have been significant correlations between a positive test and indicators of cancer aggressiveness, Gleason score, percent of prostate cancer involvement, and percent positive core, providing preliminary evidence indicating that T2:ERG status correlates with the criteria for aggressive cancers, he said.
The next step is to follow up and confirm these findings, and “perhaps more importantly,” to start studying the correlation between the urine test and pathologic features in prostatectomy tissue, such as tumor volume, stage, and grade, Dr. Groskopf said.
Describing this work as an “important first step,” Dr. Howard Sandler, moderator of the briefing, remarked that it represents “an amazingly short interval between the basic fundamental discovery and potential clinical utility of a diagnostic test.”
If it turns out that gene fusion is related to progression of prostate cancer in half of all men who develop prostate cancer, the molecular change will have major implications for diagnosis and possibly treatment as well, because it may also be a therapeutic target, said Dr. Sandler, chairman of radiation oncology at the Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles. “There is interest in targeting our diagnostic strategies towards patients who will have cancers that really need to be treated, not those that are overdiagnosed and are never destined to cause clinical harm,” he added.
The annual Genitourinary Cancers Symposium is sponsored by the American Society of Clinical Oncology, American Society for Therapeutic Radiology and Oncology, and Society of Urologic Oncology.
Herbal Options Exist for Erectile Dysfunction
SAN DIEGO — Before you recommend a treatment for erectile dysfunction, make sure to rule out underlying factors that may contribute to the condition.
“Treat the whole person. Try to take care of other medical problems they might have,” Dr. Edward (Lev) Linkner advised at a meeting sponsored by the Scripps Center for Integrative Medicine and the American Board of Integrative Holistic Medicine.
Medical conditions that should be ruled out include arteriosclerosis, diabetes, Syndrome X (cardiometabolic syndrome), hypothyroidism, and hypogonadism. Emotional etiologies such as depression and relationship difficulties should also be investigated, as should stress and lifestyle habits, especially smoking and alcohol consumption.
“Even bicycling can put abnormal pressure on pelvic nerves and result in erectile dysfunction,” said Dr. Linkner, a founder of the American Board of Holistic Medicine and current member of their board of directors. He is in private practice in Ann Arbor, Mich.
If erectile dysfunction is secondary to a treatable disease or condition, treatment of that disease or condition may be all that's necessary. If not, consider one of the following herbs:
▸ Yohimbine. This herb is derived from bark shavings of a West African tree, Pausinystalia yohimbe. “Some studies show positive results, some don't,” Dr. Linkner said. “The major side effects are hypertension, anxiety, nausea, trembling, and insomnia.” Yohimbine is contraindicated for use by those with liver and kidney disease. It is available in tablet form as the prescription drug Yocon (yohimbine hydrochloride), an alpha2-adrenergic blocker that increases blood flow to the penis. “It may be especially helpful for erectile dysfunction induced by the use of selective serotonin reuptake inhibitors,” Dr. Linkner said. He recommends a dose of one-half to one whole 5.4-mg tablet t.i.d.
▸ Ginkgo biloba. A German study showed that this herb increased blood flow to the penis within 8 weeks of starting treatment, and half of the participants regained normal potency (J. Sex Educ. Ther. 1991;17:53–61). The recommended dose is 60–120 mg b.i.d.
▸ Damiana (Turnera diffusa). Although poorly studied, this herb has been used as an aphrodisiac for centuries, especially in Mexico. Its leaves also are used to make a tea. The recommended dose is 300–450 mg once daily.
▸ Ginseng. Panax ginseng appears to works best for erectile dysfunction, but Siberian ginseng also can be used. Ginsenoside, a triterpenoid saponin, increases nitric oxide and “works like a natural Viagra,” Dr. Linkner said. The recommended dose is 200–1,000 mg.
▸ Muria puama. In European studies, this extract of a shrub (Ptychopetalum olacoides) from the Amazon has been shown to increase erections and libido. It is listed in the British Herbal Pharmacopoeia as a treatment for erectile dysfunction and dysentery.
▸ Maca (Lepidum meyenii). This radishlike plant native to the Peruvian Andes is thought to increase sexual function and stamina. The recommended dose is 1.5–3 g daily.
Adaptogens—herbs that are restorative or enhance physical performance—also may have a role in the treatment of erectile dysfunction, said Dr. Linkner, also of the University of Michigan, Ann Arbor. They “increase energy and resistance to all types of stress, thereby preventing fatigue, enhancing memory, concentration, and improving work performance.” Some adaptogens are cordyceps, ashwagandha, and rhodiola.
Other herbs with historical use in treating erectile dysfunction include oats, which increase stamina and decrease irritability; rosemary, which may help the adrenal gland produce more sex hormones; and catuba, a Brazilian herb that may increase libido.
Arginine, a biologic precursor to nitric oxide, also is used as a treatment for erectile dysfunction. However, it may reduce blood pressure, so precautions should be taken with patients on antihypertensive medications. The recommended dose of arginine is 500–1,500 mg b.i.d., or 1,000 mg 30 minutes before sex.
Dr. Linkner had no conflicts of interest to disclose.
SAN DIEGO — Before you recommend a treatment for erectile dysfunction, make sure to rule out underlying factors that may contribute to the condition.
“Treat the whole person. Try to take care of other medical problems they might have,” Dr. Edward (Lev) Linkner advised at a meeting sponsored by the Scripps Center for Integrative Medicine and the American Board of Integrative Holistic Medicine.
Medical conditions that should be ruled out include arteriosclerosis, diabetes, Syndrome X (cardiometabolic syndrome), hypothyroidism, and hypogonadism. Emotional etiologies such as depression and relationship difficulties should also be investigated, as should stress and lifestyle habits, especially smoking and alcohol consumption.
“Even bicycling can put abnormal pressure on pelvic nerves and result in erectile dysfunction,” said Dr. Linkner, a founder of the American Board of Holistic Medicine and current member of their board of directors. He is in private practice in Ann Arbor, Mich.
If erectile dysfunction is secondary to a treatable disease or condition, treatment of that disease or condition may be all that's necessary. If not, consider one of the following herbs:
▸ Yohimbine. This herb is derived from bark shavings of a West African tree, Pausinystalia yohimbe. “Some studies show positive results, some don't,” Dr. Linkner said. “The major side effects are hypertension, anxiety, nausea, trembling, and insomnia.” Yohimbine is contraindicated for use by those with liver and kidney disease. It is available in tablet form as the prescription drug Yocon (yohimbine hydrochloride), an alpha2-adrenergic blocker that increases blood flow to the penis. “It may be especially helpful for erectile dysfunction induced by the use of selective serotonin reuptake inhibitors,” Dr. Linkner said. He recommends a dose of one-half to one whole 5.4-mg tablet t.i.d.
▸ Ginkgo biloba. A German study showed that this herb increased blood flow to the penis within 8 weeks of starting treatment, and half of the participants regained normal potency (J. Sex Educ. Ther. 1991;17:53–61). The recommended dose is 60–120 mg b.i.d.
▸ Damiana (Turnera diffusa). Although poorly studied, this herb has been used as an aphrodisiac for centuries, especially in Mexico. Its leaves also are used to make a tea. The recommended dose is 300–450 mg once daily.
▸ Ginseng. Panax ginseng appears to works best for erectile dysfunction, but Siberian ginseng also can be used. Ginsenoside, a triterpenoid saponin, increases nitric oxide and “works like a natural Viagra,” Dr. Linkner said. The recommended dose is 200–1,000 mg.
▸ Muria puama. In European studies, this extract of a shrub (Ptychopetalum olacoides) from the Amazon has been shown to increase erections and libido. It is listed in the British Herbal Pharmacopoeia as a treatment for erectile dysfunction and dysentery.
▸ Maca (Lepidum meyenii). This radishlike plant native to the Peruvian Andes is thought to increase sexual function and stamina. The recommended dose is 1.5–3 g daily.
Adaptogens—herbs that are restorative or enhance physical performance—also may have a role in the treatment of erectile dysfunction, said Dr. Linkner, also of the University of Michigan, Ann Arbor. They “increase energy and resistance to all types of stress, thereby preventing fatigue, enhancing memory, concentration, and improving work performance.” Some adaptogens are cordyceps, ashwagandha, and rhodiola.
Other herbs with historical use in treating erectile dysfunction include oats, which increase stamina and decrease irritability; rosemary, which may help the adrenal gland produce more sex hormones; and catuba, a Brazilian herb that may increase libido.
Arginine, a biologic precursor to nitric oxide, also is used as a treatment for erectile dysfunction. However, it may reduce blood pressure, so precautions should be taken with patients on antihypertensive medications. The recommended dose of arginine is 500–1,500 mg b.i.d., or 1,000 mg 30 minutes before sex.
Dr. Linkner had no conflicts of interest to disclose.
SAN DIEGO — Before you recommend a treatment for erectile dysfunction, make sure to rule out underlying factors that may contribute to the condition.
“Treat the whole person. Try to take care of other medical problems they might have,” Dr. Edward (Lev) Linkner advised at a meeting sponsored by the Scripps Center for Integrative Medicine and the American Board of Integrative Holistic Medicine.
Medical conditions that should be ruled out include arteriosclerosis, diabetes, Syndrome X (cardiometabolic syndrome), hypothyroidism, and hypogonadism. Emotional etiologies such as depression and relationship difficulties should also be investigated, as should stress and lifestyle habits, especially smoking and alcohol consumption.
“Even bicycling can put abnormal pressure on pelvic nerves and result in erectile dysfunction,” said Dr. Linkner, a founder of the American Board of Holistic Medicine and current member of their board of directors. He is in private practice in Ann Arbor, Mich.
If erectile dysfunction is secondary to a treatable disease or condition, treatment of that disease or condition may be all that's necessary. If not, consider one of the following herbs:
▸ Yohimbine. This herb is derived from bark shavings of a West African tree, Pausinystalia yohimbe. “Some studies show positive results, some don't,” Dr. Linkner said. “The major side effects are hypertension, anxiety, nausea, trembling, and insomnia.” Yohimbine is contraindicated for use by those with liver and kidney disease. It is available in tablet form as the prescription drug Yocon (yohimbine hydrochloride), an alpha2-adrenergic blocker that increases blood flow to the penis. “It may be especially helpful for erectile dysfunction induced by the use of selective serotonin reuptake inhibitors,” Dr. Linkner said. He recommends a dose of one-half to one whole 5.4-mg tablet t.i.d.
▸ Ginkgo biloba. A German study showed that this herb increased blood flow to the penis within 8 weeks of starting treatment, and half of the participants regained normal potency (J. Sex Educ. Ther. 1991;17:53–61). The recommended dose is 60–120 mg b.i.d.
▸ Damiana (Turnera diffusa). Although poorly studied, this herb has been used as an aphrodisiac for centuries, especially in Mexico. Its leaves also are used to make a tea. The recommended dose is 300–450 mg once daily.
▸ Ginseng. Panax ginseng appears to works best for erectile dysfunction, but Siberian ginseng also can be used. Ginsenoside, a triterpenoid saponin, increases nitric oxide and “works like a natural Viagra,” Dr. Linkner said. The recommended dose is 200–1,000 mg.
▸ Muria puama. In European studies, this extract of a shrub (Ptychopetalum olacoides) from the Amazon has been shown to increase erections and libido. It is listed in the British Herbal Pharmacopoeia as a treatment for erectile dysfunction and dysentery.
▸ Maca (Lepidum meyenii). This radishlike plant native to the Peruvian Andes is thought to increase sexual function and stamina. The recommended dose is 1.5–3 g daily.
Adaptogens—herbs that are restorative or enhance physical performance—also may have a role in the treatment of erectile dysfunction, said Dr. Linkner, also of the University of Michigan, Ann Arbor. They “increase energy and resistance to all types of stress, thereby preventing fatigue, enhancing memory, concentration, and improving work performance.” Some adaptogens are cordyceps, ashwagandha, and rhodiola.
Other herbs with historical use in treating erectile dysfunction include oats, which increase stamina and decrease irritability; rosemary, which may help the adrenal gland produce more sex hormones; and catuba, a Brazilian herb that may increase libido.
Arginine, a biologic precursor to nitric oxide, also is used as a treatment for erectile dysfunction. However, it may reduce blood pressure, so precautions should be taken with patients on antihypertensive medications. The recommended dose of arginine is 500–1,500 mg b.i.d., or 1,000 mg 30 minutes before sex.
Dr. Linkner had no conflicts of interest to disclose.
Four Factors Help Predict Prostate Cancer Risk
A Dutch study of 5,176 men found four factors to be helpful in predicting a man's risk of developing prostate cancer: serum prostate-specific antigen, a previous negative biopsy of the prostate, family history of prostate cancer, and prostate volume.
“PSA assessments combined with the other common predictive factors may offer a personalized assessment of a man's risk of future prostate cancer,” coauthor Monique J. Roobol, Ph.D., said at a briefing before the study's presentation at a symposium on genitourinary cancers.
The men, aged 55–75 years at baseline, were from the Rotterdam section of the European Randomized Study of Screening for Prostate Cancer. They were screened every 4 years for prostate cancer.
Using multivariate modeling, with data on factors at baseline and prostate cancer 4 years later, the investigators determined that PSA “is still the most significant predictor … but there are other factors that also contribute to risk estimation,” said Dr. Roobol, an epidemiologist in urologic oncology at Erasmus Medical Center, Rotterdam, the Netherlands.
The average risk of developing biopsy-detectable prostate cancer over 4 years was 5.1% at an average PSA level of 1.5 ng/mL in the study, so men with a serum PSA level above 1.5 ng/mL are at an above-average risk of developing prostate cancer over 4 years—and are at a sevenfold greater risk than are those with levels below this level, Dr. Roobol said.
Other risk factors were found to modify this threshold level: The risk of prostate cancer increases if there is a positive family history of prostate cancer, but decreases with an increasing prostate volume at the same PSA level. Risk also decreases if a man ever had a negative prostate biopsy, she said.
For example, a man with a high serum PSA, a positive family history, a small prostate, and no previous negative biopsy was at a higher than average risk, while a man with a low serum PSA and no additional risk factors was at a very low risk, which increased if he had a positive family history, she explained.
To illustrate the effect of prostate volume on PSA level (with a volume under 40 cc considered a small prostate), she said a man with a high PSA, no previous negative biopsy, and a small prostate was at a higher than average risk, which would decrease if he had a larger prostate.
Based on these findings, she and her associates concluded that a man with a PSA of 1.3 ng/mL, with no previous negative biopsy, a positive family history, and a prostate volume under 40 cc has a 5% risk of developing prostate cancer within 4 years. However, a man with a previous negative biopsy, no family history, and a large prostate can have a PSA up to 4 ng/mL “before reaching the similar threshold of a 5% risk of prostate cancer within 4 years,” Dr. Roobol added.
Using these individualized predictive factors to identify men above certain risk thresholds could make it possible to identify men who “may warrant more frequent screening and vice versa,” she said, adding that “active risk-reduction strategies can be applied, if available.”
The moderator of the press briefing, Dr. Howard Sandler, chairman of radiation oncology at the Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, described this approach as “a very sensible method of integrating other factors besides PSA into the complex problem of prostate cancer screening and detection.”
The study is in press at the Journal of Urology. Dr. Roobol had no disclosures to report. The annual Genitourinary Cancers Symposium is sponsored by the American Society of Clinical Oncology, American Society for Therapeutic Radiology and Oncology, and Society of Urologic Oncology.
A Dutch study of 5,176 men found four factors to be helpful in predicting a man's risk of developing prostate cancer: serum prostate-specific antigen, a previous negative biopsy of the prostate, family history of prostate cancer, and prostate volume.
“PSA assessments combined with the other common predictive factors may offer a personalized assessment of a man's risk of future prostate cancer,” coauthor Monique J. Roobol, Ph.D., said at a briefing before the study's presentation at a symposium on genitourinary cancers.
The men, aged 55–75 years at baseline, were from the Rotterdam section of the European Randomized Study of Screening for Prostate Cancer. They were screened every 4 years for prostate cancer.
Using multivariate modeling, with data on factors at baseline and prostate cancer 4 years later, the investigators determined that PSA “is still the most significant predictor … but there are other factors that also contribute to risk estimation,” said Dr. Roobol, an epidemiologist in urologic oncology at Erasmus Medical Center, Rotterdam, the Netherlands.
The average risk of developing biopsy-detectable prostate cancer over 4 years was 5.1% at an average PSA level of 1.5 ng/mL in the study, so men with a serum PSA level above 1.5 ng/mL are at an above-average risk of developing prostate cancer over 4 years—and are at a sevenfold greater risk than are those with levels below this level, Dr. Roobol said.
Other risk factors were found to modify this threshold level: The risk of prostate cancer increases if there is a positive family history of prostate cancer, but decreases with an increasing prostate volume at the same PSA level. Risk also decreases if a man ever had a negative prostate biopsy, she said.
For example, a man with a high serum PSA, a positive family history, a small prostate, and no previous negative biopsy was at a higher than average risk, while a man with a low serum PSA and no additional risk factors was at a very low risk, which increased if he had a positive family history, she explained.
To illustrate the effect of prostate volume on PSA level (with a volume under 40 cc considered a small prostate), she said a man with a high PSA, no previous negative biopsy, and a small prostate was at a higher than average risk, which would decrease if he had a larger prostate.
Based on these findings, she and her associates concluded that a man with a PSA of 1.3 ng/mL, with no previous negative biopsy, a positive family history, and a prostate volume under 40 cc has a 5% risk of developing prostate cancer within 4 years. However, a man with a previous negative biopsy, no family history, and a large prostate can have a PSA up to 4 ng/mL “before reaching the similar threshold of a 5% risk of prostate cancer within 4 years,” Dr. Roobol added.
Using these individualized predictive factors to identify men above certain risk thresholds could make it possible to identify men who “may warrant more frequent screening and vice versa,” she said, adding that “active risk-reduction strategies can be applied, if available.”
The moderator of the press briefing, Dr. Howard Sandler, chairman of radiation oncology at the Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, described this approach as “a very sensible method of integrating other factors besides PSA into the complex problem of prostate cancer screening and detection.”
The study is in press at the Journal of Urology. Dr. Roobol had no disclosures to report. The annual Genitourinary Cancers Symposium is sponsored by the American Society of Clinical Oncology, American Society for Therapeutic Radiology and Oncology, and Society of Urologic Oncology.
A Dutch study of 5,176 men found four factors to be helpful in predicting a man's risk of developing prostate cancer: serum prostate-specific antigen, a previous negative biopsy of the prostate, family history of prostate cancer, and prostate volume.
“PSA assessments combined with the other common predictive factors may offer a personalized assessment of a man's risk of future prostate cancer,” coauthor Monique J. Roobol, Ph.D., said at a briefing before the study's presentation at a symposium on genitourinary cancers.
The men, aged 55–75 years at baseline, were from the Rotterdam section of the European Randomized Study of Screening for Prostate Cancer. They were screened every 4 years for prostate cancer.
Using multivariate modeling, with data on factors at baseline and prostate cancer 4 years later, the investigators determined that PSA “is still the most significant predictor … but there are other factors that also contribute to risk estimation,” said Dr. Roobol, an epidemiologist in urologic oncology at Erasmus Medical Center, Rotterdam, the Netherlands.
The average risk of developing biopsy-detectable prostate cancer over 4 years was 5.1% at an average PSA level of 1.5 ng/mL in the study, so men with a serum PSA level above 1.5 ng/mL are at an above-average risk of developing prostate cancer over 4 years—and are at a sevenfold greater risk than are those with levels below this level, Dr. Roobol said.
Other risk factors were found to modify this threshold level: The risk of prostate cancer increases if there is a positive family history of prostate cancer, but decreases with an increasing prostate volume at the same PSA level. Risk also decreases if a man ever had a negative prostate biopsy, she said.
For example, a man with a high serum PSA, a positive family history, a small prostate, and no previous negative biopsy was at a higher than average risk, while a man with a low serum PSA and no additional risk factors was at a very low risk, which increased if he had a positive family history, she explained.
To illustrate the effect of prostate volume on PSA level (with a volume under 40 cc considered a small prostate), she said a man with a high PSA, no previous negative biopsy, and a small prostate was at a higher than average risk, which would decrease if he had a larger prostate.
Based on these findings, she and her associates concluded that a man with a PSA of 1.3 ng/mL, with no previous negative biopsy, a positive family history, and a prostate volume under 40 cc has a 5% risk of developing prostate cancer within 4 years. However, a man with a previous negative biopsy, no family history, and a large prostate can have a PSA up to 4 ng/mL “before reaching the similar threshold of a 5% risk of prostate cancer within 4 years,” Dr. Roobol added.
Using these individualized predictive factors to identify men above certain risk thresholds could make it possible to identify men who “may warrant more frequent screening and vice versa,” she said, adding that “active risk-reduction strategies can be applied, if available.”
The moderator of the press briefing, Dr. Howard Sandler, chairman of radiation oncology at the Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, described this approach as “a very sensible method of integrating other factors besides PSA into the complex problem of prostate cancer screening and detection.”
The study is in press at the Journal of Urology. Dr. Roobol had no disclosures to report. The annual Genitourinary Cancers Symposium is sponsored by the American Society of Clinical Oncology, American Society for Therapeutic Radiology and Oncology, and Society of Urologic Oncology.
Autosomal dominant polycystic kidney disease: Emerging concepts of pathogenesis and new treatments
A 25-year-old married white woman presented to a clinic because of pelvic pain. A computed tomographic scan of her abdomen and pelvis without intravenous contrast showed two definite cysts in the right kidney (the larger measuring 2.5 cm) and a 1.5-cm cyst in the left kidney. It also showed several smaller (< 1 cm) areas of low density in both kidneys that suggested cysts. Renal ultrasonography also showed two cysts in the left kidney and one in the right kidney. The kidneys were normal-sized—the right one measured 12.5 cm and the left one 12.7 cm.
She had no family history of autosomal dominant polycystic kidney disease (ADPKD), and renal ultrasonography of her parents showed no cystic disease. She had no history of headache or heart murmur, and her blood pressure was normal. Her kidneys were barely palpable, her liver was not enlarged, and she had no cardiac murmur or click. She was not taking any medications. Her serum creatinine level was 0.7 mg/dL, hemoglobin 14.0 g/dL, and urinalysis normal.
Does this patient have ADPKD? Based on the studies done so far, would genetic testing be useful? If the genetic analysis does show a mutation, what additional information can be derived from the location of that mutation? Can she do anything to improve her prognosis?
ADPKD ACCOUNTS FOR ABOUT 3% OF END-STAGE RENAL DISEASE
ADPKD is the most common of all inherited renal diseases, with 600,000 to 700,000 cases in the United States and about 12.5 million cases worldwide. About 5,000 to 6,000 new cases are diagnosed yearly in the United States, about 40% of them by age 45. Typically, patients with ADPKD have a family history of the disease, but about 5% to 10% do not. In about 50% of cases, ADPKD progresses to end-stage renal disease by age 60, and it accounts for about 3% of cases of end-stage renal disease in the United States.1
CYSTS IN KIDNEYS AND OTHER ORGANS, AND NONCYSTIC FEATURES
In ADPKD, cysts in the kidneys increase in number and size over time, ultimately destroying normal renal tissue. However, renal function remains steady over many years until the kidneys have approximately quadrupled in volume to 1,500 cm3 (normal combined kidney volume is about 250 to 400 cm3), which defines a tipping point beyond which renal function can rapidly decline.2,3 Ultimately, the patient will need renal replacement therapy, ie, dialysis or renal transplantation.
The cysts (kidney and liver) cause discomfort and pain by putting pressure on the abdominal wall, flanks, and back, by impinging on neighboring organs, by bleeding into the cysts, and by the development of kidney stones or infected cysts (which are uncommon, though urinary tract infections themselves are more frequent). Kidney stones occur in about 20% of patients with ADPKD, and uric acid stones are almost as common as calcium oxalate stones. Compression of the iliac vein and inferior vena cava with possible thrombus formation and pulmonary embolism can be caused by enormous enlargement of the cystic kidneys, particularly the right.4 Interestingly, the patients at greatest risk of pulmonary embolism after renal transplantation are those with ADPKD.5
Cysts can also develop in other organs. Liver cysts develop in about 80% of patients. Usually, the cysts do not affect liver function, but because they are substantially estrogen-dependent they can be more of a clinical problem in women. About 10% of patients have cysts in the pancreas, but these are functionally insignificant. Other locations of cysts include the spleen, arachnoid membranes, and seminal vesicles in men.
Intracranial aneurysms are a key noncystic feature, and these are strongly influenced by family history. A patient with ADPKD who has a family member with ADPKD as well as an intracranial aneurysm or subarachnoid hemorrhage has about a 20% chance of having an intracranial aneurysm. A key clinical warning is a “sentinel” or “thunderclap” headache, which patients typically rate as at least a 10 on a scale of 10 in severity. In a patient with ADPKD, this type of headache can signal a leaking aneurysm causing irritation and edema of the surrounding brain tissue that temporarily tamponades the bleeding before the aneurysm actually ruptures. This is a critical period when a patient should immediately obtain emergency care.
Cardiac valve abnormalities occur in about one-third of patients. Most common is mitral valve prolapse, which is usually mild. Abnormalities can also occur in the aortic valve and the left ventricular outflow tract.
Hernias are the third general noncystic feature of ADPKD. Patients with ADPKD have an increased prevalence of umbilical, hiatal, and inguinal hernias, as well as diverticulae of the colon.
DOES THIS PATIENT HAVE ADPKD?
The Ravine ultrasonographic criteria for the diagnosis of ADPKD are based on the patient’s age, family history, and number of cysts (Table 1).6,7 Alternatively, Torres (Vincent E. Torres, personal communication, March 2008) recommends that, in the absence of a family history of ADPKD or other findings to suggest other cystic disease, the diagnosis of ADPKD can be made if the patient has a total of at least 20 renal cysts.
Our patient had only three definite cysts, was 25 years old, and had no family history of ADPKD and so did not technically meet the Ravine criteria of five cysts at this age, or the Torres criteria, for having ADPKD. Nevertheless, because she was concerned about overt disease possibly developing later and about passing on a genetic defect to her future offspring, she decided to undergo genetic testing.
CLINICAL GENETICS OF ADPKD: TWO MAJOR TYPES
There are two major genetic forms of ADPKD, caused by mutations in the genes PKD1 and PKD2.
PKD1 has been mapped to the short arm of the 16th chromosome. Its gene product is polycystin 1. Mutations in PKD1 account for about 85% of all cases of polycystic kidney disease. The cysts appear when patients are in their 20s, and the disease progresses relatively rapidly, so that most patients enter end-stage renal disease when they are in their 50s.
PKD2 has been mapped to the long arm of the fourth chromosome. Its product is polycystin 2. PKD2 mutations account for about 15% of all cases of ADPKD, and the disease progresses more slowly, usually with end-stage disease developing when the patients usually are in their 70s.
Screening for mutations by direct DNA sequencing in ADPKD
Genetic testing for PKD1 and PKD2 mutations is available (www.athenadiagnostics.com).8 The Human Gene Mutation Database lists at least 270 different PKD1 mutations and 70 different PKD2 mutations.8 Most are unique to a single family.
Our patient was tested for mutations of the PKD1 and PKD2 genes by polymerase chain reaction amplification and direct DNA sequencing. She was found to possess a DNA sequence variant at a nucleotide position in the PKD1 gene previously reported as a disease-associated mutation. She is therefore likely to be affected with or predisposed to developing ADPKD.
Furthermore, the position of her mutation means she has a worse prognosis. Rossetti et al,9 in a study of 324 PKD1 patients, found that only 19% of those who had mutations in the 5′ region of the gene (ie, at positions below 7,812) still had adequate renal function at 60 years of age, compared with 40% of those with mutations in the 3′ region (P = .025).
Other risk factors for more rapid kidney failure in ADPKD include male sex, onset of hypertension before age 35, gross hematuria before age 30 in men, and, in women, having had three or more pregnancies.
THE ‘TWO-HIT’ HYPOTHESIS
The time of onset and the rate of progression of ADPKD can vary from patient to patient, even in the same family. Besides the factors mentioned above, another reason may be that second mutations (“second hits”) have to occur before the cysts develop.
The first mutation exists in all the kidney tubular cells and is the germline mutation in the PKD gene inherited from the affected parent. This is necessary but not sufficient for cyst formation.
The second hit is a somatic mutation in an individual tubular cell that inactivates to varying degrees the unaffected gene from the normal parent. It is these second hits that allow abnormal focal (monoclonal) proliferation of renal tubular cells and cyst formation (reviewed by Arnaout10 and by Pei11). There is no way to predict these second hits, and their identity is unknown.
Other genetic variations may occur, such as transheterozygous mutations, in which a person may have a mutation of PKD1 as well as PKD2.
Germline mutations of PKD1 or PKD2 combined with somatic mutations of the normal paired chromosome depress levels of their normal gene products (polycystin 1 and polycystin 2) to the point that cysts develop.
The timing and frequency of these second hits blur the distinction between the time course for the progression of PKD1 and PKD2 disease, and can accelerate the course of both.
BASIC RESEARCH POINTS THE WAY TO TREATMENTS FOR ADPKD
Polycystin 1 and polycystin 2 are the normal gene products of the genes which, when mutated, are responsible for PKD1 and PKD2, respectively. Research into the structure and function of the polycystin 1 and polycystin 2 proteins—and what goes wrong when they are not produced in sufficient quantity or accurately—is pointing the way to possible treatments for ADPKD.
When the polycystins are not functioning, as in ADPKD, these proliferative pathways are unopposed. However, proliferation can be countered in other ways. One of the prime movers of cell proliferation, acting through adenylyl cyclase and cAMP, is vasopressin. In genetically produced polycystic animals, two antagonists of the vasopressin V2 receptor (VPV2R), OPC31260 and OPC41061 (tolvaptan), decreased cAMP and ERK, prevented or reduced renal cysts, and preserved renal function.15,16 Not surprisingly, simply increasing water intake decreases vasopressin production and the development of polycystic kidney disease in rats.17 Definitive proof of the role of vasopressin in causing cyst formation was achieved by crossing PCK rats (genetically destined to develop polycystic kidneys) with Brattleboro rats (totally lacking vasopressin) in order to generate rats with polycystic kidneys and varying amounts of vasopressin.18 PCK animals with no vasopressin had virtually no cAMP or renal cysts, whereas PCK animals with increasing amounts of vasopressin had progressively larger kidneys with more numerous cysts. Administration of synthetic vasopressin to PCK rats that totally lacked vasopressin re-created the full cystic disease.
Normally, cAMP is broken down by phosphodiesterases. Caffeine and methylxanthine products such as theophylline interfere with phosphodiesterase activity, raise cAMP in epithelial cell cultures from patients with ADPKD,19 and increase cyst formation in canine kidney cell cultures.20 One could infer that caffeine-containing drinks and foods would be undesirable for ADPKD patients.
The absence of polycystin permits excessive kinase activity in the mTOR pathway and the development of renal cysts.14 The mTOR system can be blocked by rapamycin (sirolimus, Rapamune). Wahl et al21 found that inhibition of mTOR with rapamycin slows PKD progression in rats. In a prospective study in humans, rapamycin reduced polycystic liver volumes in ADPKD renal transplant recipients.22
Rapamycin, however, can have significant side effects that include hypertriglyceridemia, hypercholesterolemia, thrombocytopenia, anemia, leukopenia, oral ulcers, impaired wound healing, proteinuria, thrombotic thrombocytopenic purpura, interstitial pneumonia, infection, and venous thrombosis. Many of these appear to be dose-related and can generally be reversed by stopping or reducing the dose. However, this drug is not approved by the US Food and Drug Administration for the treatment of ADPKD, and we absolutely do not advocate using it “off-label.”
What does this mean for our patient?
Although these results were derived primarily from animal experiments, they do provide a substantial rationale for advising our patient to:
Drink approximately 3 L of water throughout the day right up to bedtime in order to suppress vasopressin secretion and the stimulation of cAMP. This should be done under a doctor’s direction and with regular monitoring.15,17,18,23
Avoid caffeine and methylxanthines because they block phosphodiesterase, thereby leaving more cAMP to stimulate cyst formation.19,20
Follow a low-sodium diet (< 2,300 mg/day), which, while helping to control hypertension and kidney stone formation, may also help to maintain smaller cysts and kidneys. Keith et al,24 in an experiment in rats, found that the greater the sodium content of the rats’ diet, the greater the cyst sizes and kidney volumes by the end of 3 months.
Consider participating in a study. Several clinical treatment studies in ADPKD are currently enrolling patients who qualify:
- The Halt Progression of Polycystic Kidney Disease (HALT PKD) study, funded by the National Institutes of Health, is comparing the combination of an angiotensin-converting enzyme (ACE) inhibitor and an angiotensin receptor blocker (ARB) vs an ACE inhibitor plus placebo. Participating centers are Beth Israel Deaconess Medical Center, Cleveland Clinic, Emory University, Mayo Clinic, Tufts-New England Medical Center, University of Colorado Health Sciences Center, and University of Kansas Medical Center. This study involves approximately 1,020 patients nationwide.
- The Tolvaptan Efficacy and Safety in Management of Polycystic Disease and its Outcomes (TEMPO) study plans to enroll approximately 1,500 patients.
- Rapamycin is being studied in a pilot study at Cleveland Clinic and in another study in Zurich, Switzerland.
- A study of everolimus, a shorter-acting mTOR inhibitor, is beginning.
- A study of somatostatin is under way in Italy.
HYPERTENSION AND ADPKD
Uncontrolled hypertension is a key factor in the rate of progression of kidney disease in general and ADPKD in particular. It needs to be effectively treated. The target blood pressure should be in the range of 110 to 130 mm Hg systolic and 70 to 80 mm Hg diastolic.
Hypertension develops at least in part because the renin-angiotensin-aldosterone system (RAAS) is up-regulated in ADPKD due to renal cysts compressing and stretching blood vessels.25 Synthesis of immunoreactive renin, which normally takes place in the juxtaglomerular apparatus, shifts to the walls of the arterioles. There is also ectopic renin synthesis in the epithelium of dilated tubules and cysts. Greater renin production causes increases in angiotensin II and vasoconstriction, in aldosterone and sodium retention, and both angiotensin II and aldosterone can cause fibrosis and mitogenesis, which enhance cyst formation.
ACE inhibitors partially reverse the decrease in renal blood flow, renal vascular resistance, and the increase in filtration fraction. However, because some angiotensin II is also produced by an ACE-independent pathway via a chymase-like enzyme, ARBs may have a broader role in treating ADPKD.
In experimental rats with polycystic kidney disease, Keith et al24 found that blood pressure, kidney weight, plasma creatinine, and histology score (reflecting the volume of cysts as a percentage of the cortex) were all lower in animals receiving the ACE inhibitor enalapril (Vasotec) or the ARB losartan (Cozaar) than in controls or those receiving hydralazine. They also reported that the number of cysts and the size of the kidneys increased as the amount of sodium in the animals’ drinking water increased.
The potential benefits of giving ACE inhibitors or ARBs to interrupt the RAAS in polycystic disease include reduced intraglomerular pressure, reduced renal vasoconstriction (and consequently, increased renal blood flow), less proteinuria, and decreased production of transforming growth factor beta with less fibrosis. In addition, Schrier et al26 found that “rigorous blood pressure control” (goal < 120/80 mm Hg) led to a greater reduction in left ventricular mass index over time than did standard blood pressure control (goal 135–140/85–90 mm Hg) in patients with ADPKD, and that treatment with enalapril led to a greater reduction than with amlodipine (Norvasc), a calcium channel blocker.
The renal risks of ACE inhibitors include ischemia from further reduction in renal blood flow (which is already compromised by expanding cysts), hyperkalemia, and reversible renal failure that can typically be avoided by judicious dosing and monitoring.27 In addition, these drugs have the well-known side effects of cough and angioedema, and they should be avoided in pregnancy.
If diuretics are used, hypokalemia should be avoided because of both clinical and experimental evidence that it promotes cyst development. In patients who have hyperaldosteronism and hypokalemia, the degree of cyst formation in their kidneys is much greater than in other forms of hypertension. Hypokalemia has also been shown to increase cyst formation in rat models.
What does this mean for our patient?
When hypertension develops in an ADPKD patient, it would probably be best treated with an ACE inhibitor or an ARB. However, should our patient become pregnant, these drugs are to be avoided. Children of a parent with ADPKD have a 50:50 chance of having ADPKD. Genetic counseling may be advisable.
Chapman et al28 found that pregnant women with ADPKD have a significantly higher frequency of maternal complications (particularly hypertension, edema, and preeclampsia) than patients without ADPKD (35% vs 19%, P < .001). Normotensive women with ADPKD and serum creatinine levels of 1.2 mg/dL or less typically had successful, uncomplicated pregnancies. However, 16% of normotensive ADPKD women developed new-onset hypertension in pregnancy and 11% developed preeclampsia; these patients were more likely to develop chronic hypertension. Preeclampsia developed in 7 (54%) of 13 hypertensive women with ADPKD vs 13 (8%) of 157 normotensive ADPKD women. Moreover, 4 (80%) of 5 women with ADPKD who had prepregnancy serum creatinine levels higher than 1.2 mg/dL developed end-stage renal disease 15 years earlier than the general ADPKD population. Overall fetal complication rates were similar in those with or without ADPKD (32.6% vs 26.2%), but fetal prematurity due to preeclampsia was increased significantly (28% vs 10%, P < .01).28
The authors concluded that hypertensive ADPKD women are at high risk of fetal and maternal complications and measures should be taken to prevent the development of preeclampsia in these women.
In conclusion, the patient with ADPKD can present many therapeutic challenges. Fortunately, new treatment approaches combined with established ones should begin to have a favorable impact on outcomes.
- US Renal Data Services. Table A.1, Incident counts of reported ESRD: all patients. USRDS 2008 Annual Data Report, Vol. 3, page 7.
- Grantham JJ, Torres VE, Chapman AB, et al; CRISP Investigators. Volume progression in polycystic kidney disease. N Engl J Med 2006; 354:2122–2130.
- Grantham JJ, Cook LT, Torres VE, et al. Determinants of renal volume in autosomal-dominant polycystic kidney disease. Kidney Int 2008; 73:108–116.
- O’Sullivan DA, Torres VE, Heit JA, Liggett S, King BF. Compression of the inferior vena cava by right renal cysts: an unusual cause of IVC and/or iliofemoral thrombosis with pulmonary embolism in autosomal dominant polycystic kidney disease. Clin Nephrol 1998; 49:332–334.
- Tveit DP, Hypolite I, Bucci J, et al. Risk factors for hospitalizations resulting from pulmonary embolism after renal transplantation in the United States. J Nephrol 2001; 14:361–368.
- Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 1994; 343:824–827.
- Rizk D, Chapman AB. Cystic and inherited kidney disease. Am J Kidney Dis 2004; 42:1305–1317.
- Rossetti S, Consugar MB, Chapman AB, et al. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2007; 18:2143–2160.
- Rossetti S, Burton S, Strmecki L, et al. The position of the polycystic kidney disease 1 (PKD1) gene mutation correlates with the severity of renal disease. J Am Soc Nephrol 2002; 13:1230–1237.
- Arnaout MA. Molecular genetics and pathogenesis of autosomal dominant polycystic kidney disease. Annu Rev Med 2001; 52:93–123.
- Pei Y. A “two-hit” model of cystogenesis in autosomal dominant polycystic kidney disease? Trends Mol Med 2001; 7:151–156.
- Nauli S, Alenghat FJ, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 2003; 33:129–137.
- Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem 2004; 279:40419–40430.
- Shillingford JM, Murcia NS, Larson CH, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA 2006; 103:5466–5471.
- Wang X, Gattone V, Harris PC, Torres VE. Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J Am Soc Nephrol 2005; 16:846–851.
- Gattone VH, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 2003; 9:1323–1326.
- Nagao S, Nishii K, Katsuvama M, et al. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol 2006; 17:2220–2227.
- Wang W, Wu Y, Ward CJ, Harris PC, Torres VE. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 2008; 19:102–108.
- Belibi FA, Wallace DP, Yamaguchi T, Christensen M, Reif G, Grantham JJ. The effect of caffeine on renal epithelial cells from patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2002; 13:2723–2729.
- Mangoo-Karim R, Uchich M, Lechene C, Grantham JJ. Renal epithelial cyst formation and enlargement in vitro: dependence on cAMP. Proc Natl Acad Sci U S A 1989; 86:6007–6011.
- Wahl PR, Serra AL, Le Hir M, Molle KD, Hall MN, Wuthrich RP. Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD). Nephrol Dial Transplant 2006; 21:598–604.
- Qian Q, Du H, King BF, Kumar S, Dean PG, Cosio FG, Torres VE. Sirolimus reduces polycystic liver volume in ADPKD patients. J Am Soc Nephrol 2008; 19:631–638.
- Grantham JJ. Therapy for polycystic kidney disease? It’s water, stupid! J Am Soc Nephrol 2008: 12:1–2.
- Keith DS, Torres VE, Johnson CM, Holley KE. Effect of sodium chloride, enalapril, and losartan on the development of polycystic kidney disease in Han:SPRD rats. Am J Kidney Dis 1994; 24:491–498.
- Ecder T, Schrier RW. Hypertension in autosomal dominant polycystic kidney disease: early occurrence and unique aspects. J Am Soc Nephrol 2001; 12:194–200.
- Schrier R, McFann K, Johnson A, et al. Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal-dominant polycystic kidney disease: results of a seven-year prospective randomized study. J Am Soc Nephrol 2002; 13:1733–1739.
- Chapman AB, Gabow PA, Schrier RW. Reversible renal failure associated with angiotensin-converting enzyme inhibitors in polycystic kidney disease. Ann Intern Med 1991; 115:769–773.
- Chapman AB, Johnson AM, Gabow PA. Pregnancy outcome and its relationship to progression of renal failure in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 1994; 5:1178–1185.
A 25-year-old married white woman presented to a clinic because of pelvic pain. A computed tomographic scan of her abdomen and pelvis without intravenous contrast showed two definite cysts in the right kidney (the larger measuring 2.5 cm) and a 1.5-cm cyst in the left kidney. It also showed several smaller (< 1 cm) areas of low density in both kidneys that suggested cysts. Renal ultrasonography also showed two cysts in the left kidney and one in the right kidney. The kidneys were normal-sized—the right one measured 12.5 cm and the left one 12.7 cm.
She had no family history of autosomal dominant polycystic kidney disease (ADPKD), and renal ultrasonography of her parents showed no cystic disease. She had no history of headache or heart murmur, and her blood pressure was normal. Her kidneys were barely palpable, her liver was not enlarged, and she had no cardiac murmur or click. She was not taking any medications. Her serum creatinine level was 0.7 mg/dL, hemoglobin 14.0 g/dL, and urinalysis normal.
Does this patient have ADPKD? Based on the studies done so far, would genetic testing be useful? If the genetic analysis does show a mutation, what additional information can be derived from the location of that mutation? Can she do anything to improve her prognosis?
ADPKD ACCOUNTS FOR ABOUT 3% OF END-STAGE RENAL DISEASE
ADPKD is the most common of all inherited renal diseases, with 600,000 to 700,000 cases in the United States and about 12.5 million cases worldwide. About 5,000 to 6,000 new cases are diagnosed yearly in the United States, about 40% of them by age 45. Typically, patients with ADPKD have a family history of the disease, but about 5% to 10% do not. In about 50% of cases, ADPKD progresses to end-stage renal disease by age 60, and it accounts for about 3% of cases of end-stage renal disease in the United States.1
CYSTS IN KIDNEYS AND OTHER ORGANS, AND NONCYSTIC FEATURES
In ADPKD, cysts in the kidneys increase in number and size over time, ultimately destroying normal renal tissue. However, renal function remains steady over many years until the kidneys have approximately quadrupled in volume to 1,500 cm3 (normal combined kidney volume is about 250 to 400 cm3), which defines a tipping point beyond which renal function can rapidly decline.2,3 Ultimately, the patient will need renal replacement therapy, ie, dialysis or renal transplantation.
The cysts (kidney and liver) cause discomfort and pain by putting pressure on the abdominal wall, flanks, and back, by impinging on neighboring organs, by bleeding into the cysts, and by the development of kidney stones or infected cysts (which are uncommon, though urinary tract infections themselves are more frequent). Kidney stones occur in about 20% of patients with ADPKD, and uric acid stones are almost as common as calcium oxalate stones. Compression of the iliac vein and inferior vena cava with possible thrombus formation and pulmonary embolism can be caused by enormous enlargement of the cystic kidneys, particularly the right.4 Interestingly, the patients at greatest risk of pulmonary embolism after renal transplantation are those with ADPKD.5
Cysts can also develop in other organs. Liver cysts develop in about 80% of patients. Usually, the cysts do not affect liver function, but because they are substantially estrogen-dependent they can be more of a clinical problem in women. About 10% of patients have cysts in the pancreas, but these are functionally insignificant. Other locations of cysts include the spleen, arachnoid membranes, and seminal vesicles in men.
Intracranial aneurysms are a key noncystic feature, and these are strongly influenced by family history. A patient with ADPKD who has a family member with ADPKD as well as an intracranial aneurysm or subarachnoid hemorrhage has about a 20% chance of having an intracranial aneurysm. A key clinical warning is a “sentinel” or “thunderclap” headache, which patients typically rate as at least a 10 on a scale of 10 in severity. In a patient with ADPKD, this type of headache can signal a leaking aneurysm causing irritation and edema of the surrounding brain tissue that temporarily tamponades the bleeding before the aneurysm actually ruptures. This is a critical period when a patient should immediately obtain emergency care.
Cardiac valve abnormalities occur in about one-third of patients. Most common is mitral valve prolapse, which is usually mild. Abnormalities can also occur in the aortic valve and the left ventricular outflow tract.
Hernias are the third general noncystic feature of ADPKD. Patients with ADPKD have an increased prevalence of umbilical, hiatal, and inguinal hernias, as well as diverticulae of the colon.
DOES THIS PATIENT HAVE ADPKD?
The Ravine ultrasonographic criteria for the diagnosis of ADPKD are based on the patient’s age, family history, and number of cysts (Table 1).6,7 Alternatively, Torres (Vincent E. Torres, personal communication, March 2008) recommends that, in the absence of a family history of ADPKD or other findings to suggest other cystic disease, the diagnosis of ADPKD can be made if the patient has a total of at least 20 renal cysts.
Our patient had only three definite cysts, was 25 years old, and had no family history of ADPKD and so did not technically meet the Ravine criteria of five cysts at this age, or the Torres criteria, for having ADPKD. Nevertheless, because she was concerned about overt disease possibly developing later and about passing on a genetic defect to her future offspring, she decided to undergo genetic testing.
CLINICAL GENETICS OF ADPKD: TWO MAJOR TYPES
There are two major genetic forms of ADPKD, caused by mutations in the genes PKD1 and PKD2.
PKD1 has been mapped to the short arm of the 16th chromosome. Its gene product is polycystin 1. Mutations in PKD1 account for about 85% of all cases of polycystic kidney disease. The cysts appear when patients are in their 20s, and the disease progresses relatively rapidly, so that most patients enter end-stage renal disease when they are in their 50s.
PKD2 has been mapped to the long arm of the fourth chromosome. Its product is polycystin 2. PKD2 mutations account for about 15% of all cases of ADPKD, and the disease progresses more slowly, usually with end-stage disease developing when the patients usually are in their 70s.
Screening for mutations by direct DNA sequencing in ADPKD
Genetic testing for PKD1 and PKD2 mutations is available (www.athenadiagnostics.com).8 The Human Gene Mutation Database lists at least 270 different PKD1 mutations and 70 different PKD2 mutations.8 Most are unique to a single family.
Our patient was tested for mutations of the PKD1 and PKD2 genes by polymerase chain reaction amplification and direct DNA sequencing. She was found to possess a DNA sequence variant at a nucleotide position in the PKD1 gene previously reported as a disease-associated mutation. She is therefore likely to be affected with or predisposed to developing ADPKD.
Furthermore, the position of her mutation means she has a worse prognosis. Rossetti et al,9 in a study of 324 PKD1 patients, found that only 19% of those who had mutations in the 5′ region of the gene (ie, at positions below 7,812) still had adequate renal function at 60 years of age, compared with 40% of those with mutations in the 3′ region (P = .025).
Other risk factors for more rapid kidney failure in ADPKD include male sex, onset of hypertension before age 35, gross hematuria before age 30 in men, and, in women, having had three or more pregnancies.
THE ‘TWO-HIT’ HYPOTHESIS
The time of onset and the rate of progression of ADPKD can vary from patient to patient, even in the same family. Besides the factors mentioned above, another reason may be that second mutations (“second hits”) have to occur before the cysts develop.
The first mutation exists in all the kidney tubular cells and is the germline mutation in the PKD gene inherited from the affected parent. This is necessary but not sufficient for cyst formation.
The second hit is a somatic mutation in an individual tubular cell that inactivates to varying degrees the unaffected gene from the normal parent. It is these second hits that allow abnormal focal (monoclonal) proliferation of renal tubular cells and cyst formation (reviewed by Arnaout10 and by Pei11). There is no way to predict these second hits, and their identity is unknown.
Other genetic variations may occur, such as transheterozygous mutations, in which a person may have a mutation of PKD1 as well as PKD2.
Germline mutations of PKD1 or PKD2 combined with somatic mutations of the normal paired chromosome depress levels of their normal gene products (polycystin 1 and polycystin 2) to the point that cysts develop.
The timing and frequency of these second hits blur the distinction between the time course for the progression of PKD1 and PKD2 disease, and can accelerate the course of both.
BASIC RESEARCH POINTS THE WAY TO TREATMENTS FOR ADPKD
Polycystin 1 and polycystin 2 are the normal gene products of the genes which, when mutated, are responsible for PKD1 and PKD2, respectively. Research into the structure and function of the polycystin 1 and polycystin 2 proteins—and what goes wrong when they are not produced in sufficient quantity or accurately—is pointing the way to possible treatments for ADPKD.
When the polycystins are not functioning, as in ADPKD, these proliferative pathways are unopposed. However, proliferation can be countered in other ways. One of the prime movers of cell proliferation, acting through adenylyl cyclase and cAMP, is vasopressin. In genetically produced polycystic animals, two antagonists of the vasopressin V2 receptor (VPV2R), OPC31260 and OPC41061 (tolvaptan), decreased cAMP and ERK, prevented or reduced renal cysts, and preserved renal function.15,16 Not surprisingly, simply increasing water intake decreases vasopressin production and the development of polycystic kidney disease in rats.17 Definitive proof of the role of vasopressin in causing cyst formation was achieved by crossing PCK rats (genetically destined to develop polycystic kidneys) with Brattleboro rats (totally lacking vasopressin) in order to generate rats with polycystic kidneys and varying amounts of vasopressin.18 PCK animals with no vasopressin had virtually no cAMP or renal cysts, whereas PCK animals with increasing amounts of vasopressin had progressively larger kidneys with more numerous cysts. Administration of synthetic vasopressin to PCK rats that totally lacked vasopressin re-created the full cystic disease.
Normally, cAMP is broken down by phosphodiesterases. Caffeine and methylxanthine products such as theophylline interfere with phosphodiesterase activity, raise cAMP in epithelial cell cultures from patients with ADPKD,19 and increase cyst formation in canine kidney cell cultures.20 One could infer that caffeine-containing drinks and foods would be undesirable for ADPKD patients.
The absence of polycystin permits excessive kinase activity in the mTOR pathway and the development of renal cysts.14 The mTOR system can be blocked by rapamycin (sirolimus, Rapamune). Wahl et al21 found that inhibition of mTOR with rapamycin slows PKD progression in rats. In a prospective study in humans, rapamycin reduced polycystic liver volumes in ADPKD renal transplant recipients.22
Rapamycin, however, can have significant side effects that include hypertriglyceridemia, hypercholesterolemia, thrombocytopenia, anemia, leukopenia, oral ulcers, impaired wound healing, proteinuria, thrombotic thrombocytopenic purpura, interstitial pneumonia, infection, and venous thrombosis. Many of these appear to be dose-related and can generally be reversed by stopping or reducing the dose. However, this drug is not approved by the US Food and Drug Administration for the treatment of ADPKD, and we absolutely do not advocate using it “off-label.”
What does this mean for our patient?
Although these results were derived primarily from animal experiments, they do provide a substantial rationale for advising our patient to:
Drink approximately 3 L of water throughout the day right up to bedtime in order to suppress vasopressin secretion and the stimulation of cAMP. This should be done under a doctor’s direction and with regular monitoring.15,17,18,23
Avoid caffeine and methylxanthines because they block phosphodiesterase, thereby leaving more cAMP to stimulate cyst formation.19,20
Follow a low-sodium diet (< 2,300 mg/day), which, while helping to control hypertension and kidney stone formation, may also help to maintain smaller cysts and kidneys. Keith et al,24 in an experiment in rats, found that the greater the sodium content of the rats’ diet, the greater the cyst sizes and kidney volumes by the end of 3 months.
Consider participating in a study. Several clinical treatment studies in ADPKD are currently enrolling patients who qualify:
- The Halt Progression of Polycystic Kidney Disease (HALT PKD) study, funded by the National Institutes of Health, is comparing the combination of an angiotensin-converting enzyme (ACE) inhibitor and an angiotensin receptor blocker (ARB) vs an ACE inhibitor plus placebo. Participating centers are Beth Israel Deaconess Medical Center, Cleveland Clinic, Emory University, Mayo Clinic, Tufts-New England Medical Center, University of Colorado Health Sciences Center, and University of Kansas Medical Center. This study involves approximately 1,020 patients nationwide.
- The Tolvaptan Efficacy and Safety in Management of Polycystic Disease and its Outcomes (TEMPO) study plans to enroll approximately 1,500 patients.
- Rapamycin is being studied in a pilot study at Cleveland Clinic and in another study in Zurich, Switzerland.
- A study of everolimus, a shorter-acting mTOR inhibitor, is beginning.
- A study of somatostatin is under way in Italy.
HYPERTENSION AND ADPKD
Uncontrolled hypertension is a key factor in the rate of progression of kidney disease in general and ADPKD in particular. It needs to be effectively treated. The target blood pressure should be in the range of 110 to 130 mm Hg systolic and 70 to 80 mm Hg diastolic.
Hypertension develops at least in part because the renin-angiotensin-aldosterone system (RAAS) is up-regulated in ADPKD due to renal cysts compressing and stretching blood vessels.25 Synthesis of immunoreactive renin, which normally takes place in the juxtaglomerular apparatus, shifts to the walls of the arterioles. There is also ectopic renin synthesis in the epithelium of dilated tubules and cysts. Greater renin production causes increases in angiotensin II and vasoconstriction, in aldosterone and sodium retention, and both angiotensin II and aldosterone can cause fibrosis and mitogenesis, which enhance cyst formation.
ACE inhibitors partially reverse the decrease in renal blood flow, renal vascular resistance, and the increase in filtration fraction. However, because some angiotensin II is also produced by an ACE-independent pathway via a chymase-like enzyme, ARBs may have a broader role in treating ADPKD.
In experimental rats with polycystic kidney disease, Keith et al24 found that blood pressure, kidney weight, plasma creatinine, and histology score (reflecting the volume of cysts as a percentage of the cortex) were all lower in animals receiving the ACE inhibitor enalapril (Vasotec) or the ARB losartan (Cozaar) than in controls or those receiving hydralazine. They also reported that the number of cysts and the size of the kidneys increased as the amount of sodium in the animals’ drinking water increased.
The potential benefits of giving ACE inhibitors or ARBs to interrupt the RAAS in polycystic disease include reduced intraglomerular pressure, reduced renal vasoconstriction (and consequently, increased renal blood flow), less proteinuria, and decreased production of transforming growth factor beta with less fibrosis. In addition, Schrier et al26 found that “rigorous blood pressure control” (goal < 120/80 mm Hg) led to a greater reduction in left ventricular mass index over time than did standard blood pressure control (goal 135–140/85–90 mm Hg) in patients with ADPKD, and that treatment with enalapril led to a greater reduction than with amlodipine (Norvasc), a calcium channel blocker.
The renal risks of ACE inhibitors include ischemia from further reduction in renal blood flow (which is already compromised by expanding cysts), hyperkalemia, and reversible renal failure that can typically be avoided by judicious dosing and monitoring.27 In addition, these drugs have the well-known side effects of cough and angioedema, and they should be avoided in pregnancy.
If diuretics are used, hypokalemia should be avoided because of both clinical and experimental evidence that it promotes cyst development. In patients who have hyperaldosteronism and hypokalemia, the degree of cyst formation in their kidneys is much greater than in other forms of hypertension. Hypokalemia has also been shown to increase cyst formation in rat models.
What does this mean for our patient?
When hypertension develops in an ADPKD patient, it would probably be best treated with an ACE inhibitor or an ARB. However, should our patient become pregnant, these drugs are to be avoided. Children of a parent with ADPKD have a 50:50 chance of having ADPKD. Genetic counseling may be advisable.
Chapman et al28 found that pregnant women with ADPKD have a significantly higher frequency of maternal complications (particularly hypertension, edema, and preeclampsia) than patients without ADPKD (35% vs 19%, P < .001). Normotensive women with ADPKD and serum creatinine levels of 1.2 mg/dL or less typically had successful, uncomplicated pregnancies. However, 16% of normotensive ADPKD women developed new-onset hypertension in pregnancy and 11% developed preeclampsia; these patients were more likely to develop chronic hypertension. Preeclampsia developed in 7 (54%) of 13 hypertensive women with ADPKD vs 13 (8%) of 157 normotensive ADPKD women. Moreover, 4 (80%) of 5 women with ADPKD who had prepregnancy serum creatinine levels higher than 1.2 mg/dL developed end-stage renal disease 15 years earlier than the general ADPKD population. Overall fetal complication rates were similar in those with or without ADPKD (32.6% vs 26.2%), but fetal prematurity due to preeclampsia was increased significantly (28% vs 10%, P < .01).28
The authors concluded that hypertensive ADPKD women are at high risk of fetal and maternal complications and measures should be taken to prevent the development of preeclampsia in these women.
In conclusion, the patient with ADPKD can present many therapeutic challenges. Fortunately, new treatment approaches combined with established ones should begin to have a favorable impact on outcomes.
A 25-year-old married white woman presented to a clinic because of pelvic pain. A computed tomographic scan of her abdomen and pelvis without intravenous contrast showed two definite cysts in the right kidney (the larger measuring 2.5 cm) and a 1.5-cm cyst in the left kidney. It also showed several smaller (< 1 cm) areas of low density in both kidneys that suggested cysts. Renal ultrasonography also showed two cysts in the left kidney and one in the right kidney. The kidneys were normal-sized—the right one measured 12.5 cm and the left one 12.7 cm.
She had no family history of autosomal dominant polycystic kidney disease (ADPKD), and renal ultrasonography of her parents showed no cystic disease. She had no history of headache or heart murmur, and her blood pressure was normal. Her kidneys were barely palpable, her liver was not enlarged, and she had no cardiac murmur or click. She was not taking any medications. Her serum creatinine level was 0.7 mg/dL, hemoglobin 14.0 g/dL, and urinalysis normal.
Does this patient have ADPKD? Based on the studies done so far, would genetic testing be useful? If the genetic analysis does show a mutation, what additional information can be derived from the location of that mutation? Can she do anything to improve her prognosis?
ADPKD ACCOUNTS FOR ABOUT 3% OF END-STAGE RENAL DISEASE
ADPKD is the most common of all inherited renal diseases, with 600,000 to 700,000 cases in the United States and about 12.5 million cases worldwide. About 5,000 to 6,000 new cases are diagnosed yearly in the United States, about 40% of them by age 45. Typically, patients with ADPKD have a family history of the disease, but about 5% to 10% do not. In about 50% of cases, ADPKD progresses to end-stage renal disease by age 60, and it accounts for about 3% of cases of end-stage renal disease in the United States.1
CYSTS IN KIDNEYS AND OTHER ORGANS, AND NONCYSTIC FEATURES
In ADPKD, cysts in the kidneys increase in number and size over time, ultimately destroying normal renal tissue. However, renal function remains steady over many years until the kidneys have approximately quadrupled in volume to 1,500 cm3 (normal combined kidney volume is about 250 to 400 cm3), which defines a tipping point beyond which renal function can rapidly decline.2,3 Ultimately, the patient will need renal replacement therapy, ie, dialysis or renal transplantation.
The cysts (kidney and liver) cause discomfort and pain by putting pressure on the abdominal wall, flanks, and back, by impinging on neighboring organs, by bleeding into the cysts, and by the development of kidney stones or infected cysts (which are uncommon, though urinary tract infections themselves are more frequent). Kidney stones occur in about 20% of patients with ADPKD, and uric acid stones are almost as common as calcium oxalate stones. Compression of the iliac vein and inferior vena cava with possible thrombus formation and pulmonary embolism can be caused by enormous enlargement of the cystic kidneys, particularly the right.4 Interestingly, the patients at greatest risk of pulmonary embolism after renal transplantation are those with ADPKD.5
Cysts can also develop in other organs. Liver cysts develop in about 80% of patients. Usually, the cysts do not affect liver function, but because they are substantially estrogen-dependent they can be more of a clinical problem in women. About 10% of patients have cysts in the pancreas, but these are functionally insignificant. Other locations of cysts include the spleen, arachnoid membranes, and seminal vesicles in men.
Intracranial aneurysms are a key noncystic feature, and these are strongly influenced by family history. A patient with ADPKD who has a family member with ADPKD as well as an intracranial aneurysm or subarachnoid hemorrhage has about a 20% chance of having an intracranial aneurysm. A key clinical warning is a “sentinel” or “thunderclap” headache, which patients typically rate as at least a 10 on a scale of 10 in severity. In a patient with ADPKD, this type of headache can signal a leaking aneurysm causing irritation and edema of the surrounding brain tissue that temporarily tamponades the bleeding before the aneurysm actually ruptures. This is a critical period when a patient should immediately obtain emergency care.
Cardiac valve abnormalities occur in about one-third of patients. Most common is mitral valve prolapse, which is usually mild. Abnormalities can also occur in the aortic valve and the left ventricular outflow tract.
Hernias are the third general noncystic feature of ADPKD. Patients with ADPKD have an increased prevalence of umbilical, hiatal, and inguinal hernias, as well as diverticulae of the colon.
DOES THIS PATIENT HAVE ADPKD?
The Ravine ultrasonographic criteria for the diagnosis of ADPKD are based on the patient’s age, family history, and number of cysts (Table 1).6,7 Alternatively, Torres (Vincent E. Torres, personal communication, March 2008) recommends that, in the absence of a family history of ADPKD or other findings to suggest other cystic disease, the diagnosis of ADPKD can be made if the patient has a total of at least 20 renal cysts.
Our patient had only three definite cysts, was 25 years old, and had no family history of ADPKD and so did not technically meet the Ravine criteria of five cysts at this age, or the Torres criteria, for having ADPKD. Nevertheless, because she was concerned about overt disease possibly developing later and about passing on a genetic defect to her future offspring, she decided to undergo genetic testing.
CLINICAL GENETICS OF ADPKD: TWO MAJOR TYPES
There are two major genetic forms of ADPKD, caused by mutations in the genes PKD1 and PKD2.
PKD1 has been mapped to the short arm of the 16th chromosome. Its gene product is polycystin 1. Mutations in PKD1 account for about 85% of all cases of polycystic kidney disease. The cysts appear when patients are in their 20s, and the disease progresses relatively rapidly, so that most patients enter end-stage renal disease when they are in their 50s.
PKD2 has been mapped to the long arm of the fourth chromosome. Its product is polycystin 2. PKD2 mutations account for about 15% of all cases of ADPKD, and the disease progresses more slowly, usually with end-stage disease developing when the patients usually are in their 70s.
Screening for mutations by direct DNA sequencing in ADPKD
Genetic testing for PKD1 and PKD2 mutations is available (www.athenadiagnostics.com).8 The Human Gene Mutation Database lists at least 270 different PKD1 mutations and 70 different PKD2 mutations.8 Most are unique to a single family.
Our patient was tested for mutations of the PKD1 and PKD2 genes by polymerase chain reaction amplification and direct DNA sequencing. She was found to possess a DNA sequence variant at a nucleotide position in the PKD1 gene previously reported as a disease-associated mutation. She is therefore likely to be affected with or predisposed to developing ADPKD.
Furthermore, the position of her mutation means she has a worse prognosis. Rossetti et al,9 in a study of 324 PKD1 patients, found that only 19% of those who had mutations in the 5′ region of the gene (ie, at positions below 7,812) still had adequate renal function at 60 years of age, compared with 40% of those with mutations in the 3′ region (P = .025).
Other risk factors for more rapid kidney failure in ADPKD include male sex, onset of hypertension before age 35, gross hematuria before age 30 in men, and, in women, having had three or more pregnancies.
THE ‘TWO-HIT’ HYPOTHESIS
The time of onset and the rate of progression of ADPKD can vary from patient to patient, even in the same family. Besides the factors mentioned above, another reason may be that second mutations (“second hits”) have to occur before the cysts develop.
The first mutation exists in all the kidney tubular cells and is the germline mutation in the PKD gene inherited from the affected parent. This is necessary but not sufficient for cyst formation.
The second hit is a somatic mutation in an individual tubular cell that inactivates to varying degrees the unaffected gene from the normal parent. It is these second hits that allow abnormal focal (monoclonal) proliferation of renal tubular cells and cyst formation (reviewed by Arnaout10 and by Pei11). There is no way to predict these second hits, and their identity is unknown.
Other genetic variations may occur, such as transheterozygous mutations, in which a person may have a mutation of PKD1 as well as PKD2.
Germline mutations of PKD1 or PKD2 combined with somatic mutations of the normal paired chromosome depress levels of their normal gene products (polycystin 1 and polycystin 2) to the point that cysts develop.
The timing and frequency of these second hits blur the distinction between the time course for the progression of PKD1 and PKD2 disease, and can accelerate the course of both.
BASIC RESEARCH POINTS THE WAY TO TREATMENTS FOR ADPKD
Polycystin 1 and polycystin 2 are the normal gene products of the genes which, when mutated, are responsible for PKD1 and PKD2, respectively. Research into the structure and function of the polycystin 1 and polycystin 2 proteins—and what goes wrong when they are not produced in sufficient quantity or accurately—is pointing the way to possible treatments for ADPKD.
When the polycystins are not functioning, as in ADPKD, these proliferative pathways are unopposed. However, proliferation can be countered in other ways. One of the prime movers of cell proliferation, acting through adenylyl cyclase and cAMP, is vasopressin. In genetically produced polycystic animals, two antagonists of the vasopressin V2 receptor (VPV2R), OPC31260 and OPC41061 (tolvaptan), decreased cAMP and ERK, prevented or reduced renal cysts, and preserved renal function.15,16 Not surprisingly, simply increasing water intake decreases vasopressin production and the development of polycystic kidney disease in rats.17 Definitive proof of the role of vasopressin in causing cyst formation was achieved by crossing PCK rats (genetically destined to develop polycystic kidneys) with Brattleboro rats (totally lacking vasopressin) in order to generate rats with polycystic kidneys and varying amounts of vasopressin.18 PCK animals with no vasopressin had virtually no cAMP or renal cysts, whereas PCK animals with increasing amounts of vasopressin had progressively larger kidneys with more numerous cysts. Administration of synthetic vasopressin to PCK rats that totally lacked vasopressin re-created the full cystic disease.
Normally, cAMP is broken down by phosphodiesterases. Caffeine and methylxanthine products such as theophylline interfere with phosphodiesterase activity, raise cAMP in epithelial cell cultures from patients with ADPKD,19 and increase cyst formation in canine kidney cell cultures.20 One could infer that caffeine-containing drinks and foods would be undesirable for ADPKD patients.
The absence of polycystin permits excessive kinase activity in the mTOR pathway and the development of renal cysts.14 The mTOR system can be blocked by rapamycin (sirolimus, Rapamune). Wahl et al21 found that inhibition of mTOR with rapamycin slows PKD progression in rats. In a prospective study in humans, rapamycin reduced polycystic liver volumes in ADPKD renal transplant recipients.22
Rapamycin, however, can have significant side effects that include hypertriglyceridemia, hypercholesterolemia, thrombocytopenia, anemia, leukopenia, oral ulcers, impaired wound healing, proteinuria, thrombotic thrombocytopenic purpura, interstitial pneumonia, infection, and venous thrombosis. Many of these appear to be dose-related and can generally be reversed by stopping or reducing the dose. However, this drug is not approved by the US Food and Drug Administration for the treatment of ADPKD, and we absolutely do not advocate using it “off-label.”
What does this mean for our patient?
Although these results were derived primarily from animal experiments, they do provide a substantial rationale for advising our patient to:
Drink approximately 3 L of water throughout the day right up to bedtime in order to suppress vasopressin secretion and the stimulation of cAMP. This should be done under a doctor’s direction and with regular monitoring.15,17,18,23
Avoid caffeine and methylxanthines because they block phosphodiesterase, thereby leaving more cAMP to stimulate cyst formation.19,20
Follow a low-sodium diet (< 2,300 mg/day), which, while helping to control hypertension and kidney stone formation, may also help to maintain smaller cysts and kidneys. Keith et al,24 in an experiment in rats, found that the greater the sodium content of the rats’ diet, the greater the cyst sizes and kidney volumes by the end of 3 months.
Consider participating in a study. Several clinical treatment studies in ADPKD are currently enrolling patients who qualify:
- The Halt Progression of Polycystic Kidney Disease (HALT PKD) study, funded by the National Institutes of Health, is comparing the combination of an angiotensin-converting enzyme (ACE) inhibitor and an angiotensin receptor blocker (ARB) vs an ACE inhibitor plus placebo. Participating centers are Beth Israel Deaconess Medical Center, Cleveland Clinic, Emory University, Mayo Clinic, Tufts-New England Medical Center, University of Colorado Health Sciences Center, and University of Kansas Medical Center. This study involves approximately 1,020 patients nationwide.
- The Tolvaptan Efficacy and Safety in Management of Polycystic Disease and its Outcomes (TEMPO) study plans to enroll approximately 1,500 patients.
- Rapamycin is being studied in a pilot study at Cleveland Clinic and in another study in Zurich, Switzerland.
- A study of everolimus, a shorter-acting mTOR inhibitor, is beginning.
- A study of somatostatin is under way in Italy.
HYPERTENSION AND ADPKD
Uncontrolled hypertension is a key factor in the rate of progression of kidney disease in general and ADPKD in particular. It needs to be effectively treated. The target blood pressure should be in the range of 110 to 130 mm Hg systolic and 70 to 80 mm Hg diastolic.
Hypertension develops at least in part because the renin-angiotensin-aldosterone system (RAAS) is up-regulated in ADPKD due to renal cysts compressing and stretching blood vessels.25 Synthesis of immunoreactive renin, which normally takes place in the juxtaglomerular apparatus, shifts to the walls of the arterioles. There is also ectopic renin synthesis in the epithelium of dilated tubules and cysts. Greater renin production causes increases in angiotensin II and vasoconstriction, in aldosterone and sodium retention, and both angiotensin II and aldosterone can cause fibrosis and mitogenesis, which enhance cyst formation.
ACE inhibitors partially reverse the decrease in renal blood flow, renal vascular resistance, and the increase in filtration fraction. However, because some angiotensin II is also produced by an ACE-independent pathway via a chymase-like enzyme, ARBs may have a broader role in treating ADPKD.
In experimental rats with polycystic kidney disease, Keith et al24 found that blood pressure, kidney weight, plasma creatinine, and histology score (reflecting the volume of cysts as a percentage of the cortex) were all lower in animals receiving the ACE inhibitor enalapril (Vasotec) or the ARB losartan (Cozaar) than in controls or those receiving hydralazine. They also reported that the number of cysts and the size of the kidneys increased as the amount of sodium in the animals’ drinking water increased.
The potential benefits of giving ACE inhibitors or ARBs to interrupt the RAAS in polycystic disease include reduced intraglomerular pressure, reduced renal vasoconstriction (and consequently, increased renal blood flow), less proteinuria, and decreased production of transforming growth factor beta with less fibrosis. In addition, Schrier et al26 found that “rigorous blood pressure control” (goal < 120/80 mm Hg) led to a greater reduction in left ventricular mass index over time than did standard blood pressure control (goal 135–140/85–90 mm Hg) in patients with ADPKD, and that treatment with enalapril led to a greater reduction than with amlodipine (Norvasc), a calcium channel blocker.
The renal risks of ACE inhibitors include ischemia from further reduction in renal blood flow (which is already compromised by expanding cysts), hyperkalemia, and reversible renal failure that can typically be avoided by judicious dosing and monitoring.27 In addition, these drugs have the well-known side effects of cough and angioedema, and they should be avoided in pregnancy.
If diuretics are used, hypokalemia should be avoided because of both clinical and experimental evidence that it promotes cyst development. In patients who have hyperaldosteronism and hypokalemia, the degree of cyst formation in their kidneys is much greater than in other forms of hypertension. Hypokalemia has also been shown to increase cyst formation in rat models.
What does this mean for our patient?
When hypertension develops in an ADPKD patient, it would probably be best treated with an ACE inhibitor or an ARB. However, should our patient become pregnant, these drugs are to be avoided. Children of a parent with ADPKD have a 50:50 chance of having ADPKD. Genetic counseling may be advisable.
Chapman et al28 found that pregnant women with ADPKD have a significantly higher frequency of maternal complications (particularly hypertension, edema, and preeclampsia) than patients without ADPKD (35% vs 19%, P < .001). Normotensive women with ADPKD and serum creatinine levels of 1.2 mg/dL or less typically had successful, uncomplicated pregnancies. However, 16% of normotensive ADPKD women developed new-onset hypertension in pregnancy and 11% developed preeclampsia; these patients were more likely to develop chronic hypertension. Preeclampsia developed in 7 (54%) of 13 hypertensive women with ADPKD vs 13 (8%) of 157 normotensive ADPKD women. Moreover, 4 (80%) of 5 women with ADPKD who had prepregnancy serum creatinine levels higher than 1.2 mg/dL developed end-stage renal disease 15 years earlier than the general ADPKD population. Overall fetal complication rates were similar in those with or without ADPKD (32.6% vs 26.2%), but fetal prematurity due to preeclampsia was increased significantly (28% vs 10%, P < .01).28
The authors concluded that hypertensive ADPKD women are at high risk of fetal and maternal complications and measures should be taken to prevent the development of preeclampsia in these women.
In conclusion, the patient with ADPKD can present many therapeutic challenges. Fortunately, new treatment approaches combined with established ones should begin to have a favorable impact on outcomes.
- US Renal Data Services. Table A.1, Incident counts of reported ESRD: all patients. USRDS 2008 Annual Data Report, Vol. 3, page 7.
- Grantham JJ, Torres VE, Chapman AB, et al; CRISP Investigators. Volume progression in polycystic kidney disease. N Engl J Med 2006; 354:2122–2130.
- Grantham JJ, Cook LT, Torres VE, et al. Determinants of renal volume in autosomal-dominant polycystic kidney disease. Kidney Int 2008; 73:108–116.
- O’Sullivan DA, Torres VE, Heit JA, Liggett S, King BF. Compression of the inferior vena cava by right renal cysts: an unusual cause of IVC and/or iliofemoral thrombosis with pulmonary embolism in autosomal dominant polycystic kidney disease. Clin Nephrol 1998; 49:332–334.
- Tveit DP, Hypolite I, Bucci J, et al. Risk factors for hospitalizations resulting from pulmonary embolism after renal transplantation in the United States. J Nephrol 2001; 14:361–368.
- Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 1994; 343:824–827.
- Rizk D, Chapman AB. Cystic and inherited kidney disease. Am J Kidney Dis 2004; 42:1305–1317.
- Rossetti S, Consugar MB, Chapman AB, et al. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2007; 18:2143–2160.
- Rossetti S, Burton S, Strmecki L, et al. The position of the polycystic kidney disease 1 (PKD1) gene mutation correlates with the severity of renal disease. J Am Soc Nephrol 2002; 13:1230–1237.
- Arnaout MA. Molecular genetics and pathogenesis of autosomal dominant polycystic kidney disease. Annu Rev Med 2001; 52:93–123.
- Pei Y. A “two-hit” model of cystogenesis in autosomal dominant polycystic kidney disease? Trends Mol Med 2001; 7:151–156.
- Nauli S, Alenghat FJ, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 2003; 33:129–137.
- Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem 2004; 279:40419–40430.
- Shillingford JM, Murcia NS, Larson CH, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA 2006; 103:5466–5471.
- Wang X, Gattone V, Harris PC, Torres VE. Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J Am Soc Nephrol 2005; 16:846–851.
- Gattone VH, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 2003; 9:1323–1326.
- Nagao S, Nishii K, Katsuvama M, et al. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol 2006; 17:2220–2227.
- Wang W, Wu Y, Ward CJ, Harris PC, Torres VE. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 2008; 19:102–108.
- Belibi FA, Wallace DP, Yamaguchi T, Christensen M, Reif G, Grantham JJ. The effect of caffeine on renal epithelial cells from patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2002; 13:2723–2729.
- Mangoo-Karim R, Uchich M, Lechene C, Grantham JJ. Renal epithelial cyst formation and enlargement in vitro: dependence on cAMP. Proc Natl Acad Sci U S A 1989; 86:6007–6011.
- Wahl PR, Serra AL, Le Hir M, Molle KD, Hall MN, Wuthrich RP. Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD). Nephrol Dial Transplant 2006; 21:598–604.
- Qian Q, Du H, King BF, Kumar S, Dean PG, Cosio FG, Torres VE. Sirolimus reduces polycystic liver volume in ADPKD patients. J Am Soc Nephrol 2008; 19:631–638.
- Grantham JJ. Therapy for polycystic kidney disease? It’s water, stupid! J Am Soc Nephrol 2008: 12:1–2.
- Keith DS, Torres VE, Johnson CM, Holley KE. Effect of sodium chloride, enalapril, and losartan on the development of polycystic kidney disease in Han:SPRD rats. Am J Kidney Dis 1994; 24:491–498.
- Ecder T, Schrier RW. Hypertension in autosomal dominant polycystic kidney disease: early occurrence and unique aspects. J Am Soc Nephrol 2001; 12:194–200.
- Schrier R, McFann K, Johnson A, et al. Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal-dominant polycystic kidney disease: results of a seven-year prospective randomized study. J Am Soc Nephrol 2002; 13:1733–1739.
- Chapman AB, Gabow PA, Schrier RW. Reversible renal failure associated with angiotensin-converting enzyme inhibitors in polycystic kidney disease. Ann Intern Med 1991; 115:769–773.
- Chapman AB, Johnson AM, Gabow PA. Pregnancy outcome and its relationship to progression of renal failure in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 1994; 5:1178–1185.
- US Renal Data Services. Table A.1, Incident counts of reported ESRD: all patients. USRDS 2008 Annual Data Report, Vol. 3, page 7.
- Grantham JJ, Torres VE, Chapman AB, et al; CRISP Investigators. Volume progression in polycystic kidney disease. N Engl J Med 2006; 354:2122–2130.
- Grantham JJ, Cook LT, Torres VE, et al. Determinants of renal volume in autosomal-dominant polycystic kidney disease. Kidney Int 2008; 73:108–116.
- O’Sullivan DA, Torres VE, Heit JA, Liggett S, King BF. Compression of the inferior vena cava by right renal cysts: an unusual cause of IVC and/or iliofemoral thrombosis with pulmonary embolism in autosomal dominant polycystic kidney disease. Clin Nephrol 1998; 49:332–334.
- Tveit DP, Hypolite I, Bucci J, et al. Risk factors for hospitalizations resulting from pulmonary embolism after renal transplantation in the United States. J Nephrol 2001; 14:361–368.
- Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 1994; 343:824–827.
- Rizk D, Chapman AB. Cystic and inherited kidney disease. Am J Kidney Dis 2004; 42:1305–1317.
- Rossetti S, Consugar MB, Chapman AB, et al. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2007; 18:2143–2160.
- Rossetti S, Burton S, Strmecki L, et al. The position of the polycystic kidney disease 1 (PKD1) gene mutation correlates with the severity of renal disease. J Am Soc Nephrol 2002; 13:1230–1237.
- Arnaout MA. Molecular genetics and pathogenesis of autosomal dominant polycystic kidney disease. Annu Rev Med 2001; 52:93–123.
- Pei Y. A “two-hit” model of cystogenesis in autosomal dominant polycystic kidney disease? Trends Mol Med 2001; 7:151–156.
- Nauli S, Alenghat FJ, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 2003; 33:129–137.
- Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem 2004; 279:40419–40430.
- Shillingford JM, Murcia NS, Larson CH, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA 2006; 103:5466–5471.
- Wang X, Gattone V, Harris PC, Torres VE. Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J Am Soc Nephrol 2005; 16:846–851.
- Gattone VH, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 2003; 9:1323–1326.
- Nagao S, Nishii K, Katsuvama M, et al. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol 2006; 17:2220–2227.
- Wang W, Wu Y, Ward CJ, Harris PC, Torres VE. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 2008; 19:102–108.
- Belibi FA, Wallace DP, Yamaguchi T, Christensen M, Reif G, Grantham JJ. The effect of caffeine on renal epithelial cells from patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2002; 13:2723–2729.
- Mangoo-Karim R, Uchich M, Lechene C, Grantham JJ. Renal epithelial cyst formation and enlargement in vitro: dependence on cAMP. Proc Natl Acad Sci U S A 1989; 86:6007–6011.
- Wahl PR, Serra AL, Le Hir M, Molle KD, Hall MN, Wuthrich RP. Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD). Nephrol Dial Transplant 2006; 21:598–604.
- Qian Q, Du H, King BF, Kumar S, Dean PG, Cosio FG, Torres VE. Sirolimus reduces polycystic liver volume in ADPKD patients. J Am Soc Nephrol 2008; 19:631–638.
- Grantham JJ. Therapy for polycystic kidney disease? It’s water, stupid! J Am Soc Nephrol 2008: 12:1–2.
- Keith DS, Torres VE, Johnson CM, Holley KE. Effect of sodium chloride, enalapril, and losartan on the development of polycystic kidney disease in Han:SPRD rats. Am J Kidney Dis 1994; 24:491–498.
- Ecder T, Schrier RW. Hypertension in autosomal dominant polycystic kidney disease: early occurrence and unique aspects. J Am Soc Nephrol 2001; 12:194–200.
- Schrier R, McFann K, Johnson A, et al. Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal-dominant polycystic kidney disease: results of a seven-year prospective randomized study. J Am Soc Nephrol 2002; 13:1733–1739.
- Chapman AB, Gabow PA, Schrier RW. Reversible renal failure associated with angiotensin-converting enzyme inhibitors in polycystic kidney disease. Ann Intern Med 1991; 115:769–773.
- Chapman AB, Johnson AM, Gabow PA. Pregnancy outcome and its relationship to progression of renal failure in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 1994; 5:1178–1185.
KEY POINTS
- In ADPKD the expanding cysts destroy normally functioning kidney tissue, causing hypertension, pain, and other complications, but renal function remains relatively stable until kidney volumes reach a critical size.
- Testing for genetic defects that cause ADPKD is available. The specific mutation involved (PKD1 or PKD2) affects the age of onset and therefore the rate of disease progression as well as the likelihood of cardiovascular complications. Other factors include somatic mutations (“second hits”) of the normal paired chromosome.
- Intracranial aneurysms are a key noncystic feature and may present with a very severe (“sentinel” or “thunderclap”) headache requiring immediate medical attention. Their occurrence is strongly influenced by family history.
- Basic research indicates that patients may be advised to increase their water intake, limit their sodium intake, and avoid caffeine and methylxanthine derivatives.
Renal, Heart Failure Respond to Same Therapies
VANCOUVER, B.C. — Chronic kidney disease and heart failure often go hand in hand, and the treatment strategy is similar for both. But there are some finer points to treating patients who have both conditions, according to Dr. Michael Copland, a nephrologist at Vancouver General Hospital.
In patients with cardiorenal syndrome, cardiac and renal dysfunction synergistically amplify each other. The end result is a sharply elevated rate of cardiovascular events. In general, 44% of deaths among patients with chronic kidney disease are due to cardiovascular causes, Dr. Copland said at the annual Canadian Hospitalist Conference.
For patients with kidney disease and heart failure, focus on diet and lifestyle changes, and control of hypertension, diabetes, and lipids. “These are all very cardiovascular-sounding items, but each of these items carries with it a survival benefit in terms of kidney protection for this group of people,” he said.
Renoprotective measures include angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers (ARBs). “We should be doing our best to get all of our patients on ACE inhibitors and ARBs if they have impaired renal function—particularly if they are diabetic, particularly if they have protein in their urine—because that … is associated with preservation of their renal function,” Dr. Copland said at the meeting, which was sponsored by the University of British Columbia.
Renal function should be monitored closely after starting these agents. “We will accept a 25% loss of renal function up front,” he noted, because this short-term trade-off is acceptable for the long-term gain in renal protection. But “if renal function continues to worsen, that's the one group of people in whom I would have to say I would abandon the therapy.”
Development of hyperkalemia is not a reason to discontinue ACE inhibitors and ARBs. “I add on other therapies for their hyperkalemia,” Dr. Copland said. Calcium resonium is typically preferred over sodium polystyrene, because the sodium in the latter will worsen heart failure.
In treating blood pressure, “our initial target would be 130/80 mm Hg, particularly for people who have protein in their urine,” he said. If they still have proteinuria at that target, “we would just keep going as low as they tolerate.”
Recent studies of epoetin alfa have found no cardiovascular benefit for patients with chronic kidney disease, and a trend toward an increased risk of death. “People do feel better, so they stay off of dialysis for longer. So from a cost point of view, which is not a clinical parameter, we use this therapy,” Dr. Copland said.
“Sometimes treating the heart failure actually treats the kidney disease,” he noted. For challenging patients who have both high cardiac output and volume overload, treatments include loop diuretics, nitrates, positive airway pressure, nesiritide, and possibly ultrafiltration, which may offer an alternative to diuresis.
Trials of ultrafiltration have had conflicting results, Dr. Copland said. In the largest one to date—the UNLOAD trial (Ultrafiltration vs. IV Diuretics for Patients Hospitalized for Acute Decompensated CHF)—patients given ultrafiltration had a greater weight loss than patients given diuretics, with a difference of 5 vs. 3 kg (J. Am. Coll. Cardiol. 2007;49:675–83). Subjective dyspnea scores did not differ. But at 90 days, patients given ultrafiltration were less likely to have been rehospitalized for heart failure (18% vs. 32%).
“The problem with all of these trials is that they excluded the sickest groups of people, so I think the jury is still a bit out,” he said.
Dr. Copland said that he sits on the advisory board of Baxter Healthcare.
'Sometimes treating the heart failure actually treats the kidney disease.' DR. COPLAND
VANCOUVER, B.C. — Chronic kidney disease and heart failure often go hand in hand, and the treatment strategy is similar for both. But there are some finer points to treating patients who have both conditions, according to Dr. Michael Copland, a nephrologist at Vancouver General Hospital.
In patients with cardiorenal syndrome, cardiac and renal dysfunction synergistically amplify each other. The end result is a sharply elevated rate of cardiovascular events. In general, 44% of deaths among patients with chronic kidney disease are due to cardiovascular causes, Dr. Copland said at the annual Canadian Hospitalist Conference.
For patients with kidney disease and heart failure, focus on diet and lifestyle changes, and control of hypertension, diabetes, and lipids. “These are all very cardiovascular-sounding items, but each of these items carries with it a survival benefit in terms of kidney protection for this group of people,” he said.
Renoprotective measures include angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers (ARBs). “We should be doing our best to get all of our patients on ACE inhibitors and ARBs if they have impaired renal function—particularly if they are diabetic, particularly if they have protein in their urine—because that … is associated with preservation of their renal function,” Dr. Copland said at the meeting, which was sponsored by the University of British Columbia.
Renal function should be monitored closely after starting these agents. “We will accept a 25% loss of renal function up front,” he noted, because this short-term trade-off is acceptable for the long-term gain in renal protection. But “if renal function continues to worsen, that's the one group of people in whom I would have to say I would abandon the therapy.”
Development of hyperkalemia is not a reason to discontinue ACE inhibitors and ARBs. “I add on other therapies for their hyperkalemia,” Dr. Copland said. Calcium resonium is typically preferred over sodium polystyrene, because the sodium in the latter will worsen heart failure.
In treating blood pressure, “our initial target would be 130/80 mm Hg, particularly for people who have protein in their urine,” he said. If they still have proteinuria at that target, “we would just keep going as low as they tolerate.”
Recent studies of epoetin alfa have found no cardiovascular benefit for patients with chronic kidney disease, and a trend toward an increased risk of death. “People do feel better, so they stay off of dialysis for longer. So from a cost point of view, which is not a clinical parameter, we use this therapy,” Dr. Copland said.
“Sometimes treating the heart failure actually treats the kidney disease,” he noted. For challenging patients who have both high cardiac output and volume overload, treatments include loop diuretics, nitrates, positive airway pressure, nesiritide, and possibly ultrafiltration, which may offer an alternative to diuresis.
Trials of ultrafiltration have had conflicting results, Dr. Copland said. In the largest one to date—the UNLOAD trial (Ultrafiltration vs. IV Diuretics for Patients Hospitalized for Acute Decompensated CHF)—patients given ultrafiltration had a greater weight loss than patients given diuretics, with a difference of 5 vs. 3 kg (J. Am. Coll. Cardiol. 2007;49:675–83). Subjective dyspnea scores did not differ. But at 90 days, patients given ultrafiltration were less likely to have been rehospitalized for heart failure (18% vs. 32%).
“The problem with all of these trials is that they excluded the sickest groups of people, so I think the jury is still a bit out,” he said.
Dr. Copland said that he sits on the advisory board of Baxter Healthcare.
'Sometimes treating the heart failure actually treats the kidney disease.' DR. COPLAND
VANCOUVER, B.C. — Chronic kidney disease and heart failure often go hand in hand, and the treatment strategy is similar for both. But there are some finer points to treating patients who have both conditions, according to Dr. Michael Copland, a nephrologist at Vancouver General Hospital.
In patients with cardiorenal syndrome, cardiac and renal dysfunction synergistically amplify each other. The end result is a sharply elevated rate of cardiovascular events. In general, 44% of deaths among patients with chronic kidney disease are due to cardiovascular causes, Dr. Copland said at the annual Canadian Hospitalist Conference.
For patients with kidney disease and heart failure, focus on diet and lifestyle changes, and control of hypertension, diabetes, and lipids. “These are all very cardiovascular-sounding items, but each of these items carries with it a survival benefit in terms of kidney protection for this group of people,” he said.
Renoprotective measures include angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers (ARBs). “We should be doing our best to get all of our patients on ACE inhibitors and ARBs if they have impaired renal function—particularly if they are diabetic, particularly if they have protein in their urine—because that … is associated with preservation of their renal function,” Dr. Copland said at the meeting, which was sponsored by the University of British Columbia.
Renal function should be monitored closely after starting these agents. “We will accept a 25% loss of renal function up front,” he noted, because this short-term trade-off is acceptable for the long-term gain in renal protection. But “if renal function continues to worsen, that's the one group of people in whom I would have to say I would abandon the therapy.”
Development of hyperkalemia is not a reason to discontinue ACE inhibitors and ARBs. “I add on other therapies for their hyperkalemia,” Dr. Copland said. Calcium resonium is typically preferred over sodium polystyrene, because the sodium in the latter will worsen heart failure.
In treating blood pressure, “our initial target would be 130/80 mm Hg, particularly for people who have protein in their urine,” he said. If they still have proteinuria at that target, “we would just keep going as low as they tolerate.”
Recent studies of epoetin alfa have found no cardiovascular benefit for patients with chronic kidney disease, and a trend toward an increased risk of death. “People do feel better, so they stay off of dialysis for longer. So from a cost point of view, which is not a clinical parameter, we use this therapy,” Dr. Copland said.
“Sometimes treating the heart failure actually treats the kidney disease,” he noted. For challenging patients who have both high cardiac output and volume overload, treatments include loop diuretics, nitrates, positive airway pressure, nesiritide, and possibly ultrafiltration, which may offer an alternative to diuresis.
Trials of ultrafiltration have had conflicting results, Dr. Copland said. In the largest one to date—the UNLOAD trial (Ultrafiltration vs. IV Diuretics for Patients Hospitalized for Acute Decompensated CHF)—patients given ultrafiltration had a greater weight loss than patients given diuretics, with a difference of 5 vs. 3 kg (J. Am. Coll. Cardiol. 2007;49:675–83). Subjective dyspnea scores did not differ. But at 90 days, patients given ultrafiltration were less likely to have been rehospitalized for heart failure (18% vs. 32%).
“The problem with all of these trials is that they excluded the sickest groups of people, so I think the jury is still a bit out,” he said.
Dr. Copland said that he sits on the advisory board of Baxter Healthcare.
'Sometimes treating the heart failure actually treats the kidney disease.' DR. COPLAND
Intensive Renal Support Doesn't Lower Mortality
PHILADELPHIA — Intensive renal support in critically ill patients with acute kidney injury did not decrease mortality, accelerate recovery of renal function, or alter the rate of nonrenal organ failure in the randomized Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network study.
Results of previous smaller studies evaluating different strategies of renal support have been inconsistent, and while some studies favored more intensive, higher-dose approaches, such a strategy has not been adopted widely in the United States.
To address the uncertainty as to the optimal approach for acute kidney injury, a large trial was undertaken comparing conventional with intensive renal support. An integrative approach was used, in which hemodynamically stable patients were on standard intermittent therapy and hemodynamically unstable patients were on continuous therapy or sustained low-efficiency dialysis, according to the lead investigator, Dr. Paul M. Palevsky.
“Patients could move between modalities of therapy as their hemodynamic status changed, but they remained within a dosing schedule which was five to six times per week for a total effluent flow rate of 35 mL/kg per hour in the intensive group, and three times per week for a total effluent flow rate of 20 mL/kg per hour in the less-intensive group,” said Dr. Palevsky of the renal section, VA Pittsburgh Healthcare System, and the department of medicine at the University of Pittsburgh.
To be eligible, patients had to be 18 years or older, critically ill, have acute kidney injury consistent with acute tubular necrosis, and have failure of one or more nonrenal organs, or sepsis.
A total of 563 patients were randomized to the intensive group; 561 were randomized to the conventional group.
The populations were well matched at baseline in terms of age, at a mean of 60 years, as well as in race, sex, and ethnicity and in baseline severity of illness and renal function, Dr. Palevsky said at the annual meeting of the American Society of Nephrology.
Mean serum creatinine was 1.1 mg/dL in both groups at baseline. A total of 88% of patients had an estimated glomerular filtration rate of at least 45 mL/min per 1.73 m
Therapy continued for 28 days or until the patient regained renal function, was withdrawn from life-sustaining care, was discharged from the acute care hospital, or died.
Patients in the intensive therapy group received an average of 5.4 sessions per week, with an interval between treatments of 1.1 days, while those in the less-intensive group averaged 3 sessions per week with an interval of 2 days between treatments.
Prescribed and delivered flow rates of venovenous hemodiafiltration were approximately 36 mL/kg per hour for the intensive group and just over 20 mL/kg per hour for the less-intensive group (N. Engl. J. Med. 2008;359:7–20).
“We observed no difference between the two groups on our primary outcome measure of 60-day all-cause mortality, with 53.6% in the intensive arm and 51.5% in the less-intensive arm,” he said.
Complete recovery of kidney function by day 28 was seen in 15% of the intensive therapy group, and in 18% of those in the less-intensive group; there was no difference between the groups in number of days free of organ failure.
“We then looked at 1-year mortality and again there was no difference, with a total mortality of 34% in each group. Among patients who survived to day 60 there was an additional 20% mortality at 1 year,” he said. Not only was mortality not decreased with the intensive therapy, but a greater percentage of patients undergoing intensive therapy experienced treatment-related hypotension and had more hypocalcemia and hypophosphatemia, he noted.
Dr. Palevsky disclosed no conflicts of interest.
PHILADELPHIA — Intensive renal support in critically ill patients with acute kidney injury did not decrease mortality, accelerate recovery of renal function, or alter the rate of nonrenal organ failure in the randomized Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network study.
Results of previous smaller studies evaluating different strategies of renal support have been inconsistent, and while some studies favored more intensive, higher-dose approaches, such a strategy has not been adopted widely in the United States.
To address the uncertainty as to the optimal approach for acute kidney injury, a large trial was undertaken comparing conventional with intensive renal support. An integrative approach was used, in which hemodynamically stable patients were on standard intermittent therapy and hemodynamically unstable patients were on continuous therapy or sustained low-efficiency dialysis, according to the lead investigator, Dr. Paul M. Palevsky.
“Patients could move between modalities of therapy as their hemodynamic status changed, but they remained within a dosing schedule which was five to six times per week for a total effluent flow rate of 35 mL/kg per hour in the intensive group, and three times per week for a total effluent flow rate of 20 mL/kg per hour in the less-intensive group,” said Dr. Palevsky of the renal section, VA Pittsburgh Healthcare System, and the department of medicine at the University of Pittsburgh.
To be eligible, patients had to be 18 years or older, critically ill, have acute kidney injury consistent with acute tubular necrosis, and have failure of one or more nonrenal organs, or sepsis.
A total of 563 patients were randomized to the intensive group; 561 were randomized to the conventional group.
The populations were well matched at baseline in terms of age, at a mean of 60 years, as well as in race, sex, and ethnicity and in baseline severity of illness and renal function, Dr. Palevsky said at the annual meeting of the American Society of Nephrology.
Mean serum creatinine was 1.1 mg/dL in both groups at baseline. A total of 88% of patients had an estimated glomerular filtration rate of at least 45 mL/min per 1.73 m
Therapy continued for 28 days or until the patient regained renal function, was withdrawn from life-sustaining care, was discharged from the acute care hospital, or died.
Patients in the intensive therapy group received an average of 5.4 sessions per week, with an interval between treatments of 1.1 days, while those in the less-intensive group averaged 3 sessions per week with an interval of 2 days between treatments.
Prescribed and delivered flow rates of venovenous hemodiafiltration were approximately 36 mL/kg per hour for the intensive group and just over 20 mL/kg per hour for the less-intensive group (N. Engl. J. Med. 2008;359:7–20).
“We observed no difference between the two groups on our primary outcome measure of 60-day all-cause mortality, with 53.6% in the intensive arm and 51.5% in the less-intensive arm,” he said.
Complete recovery of kidney function by day 28 was seen in 15% of the intensive therapy group, and in 18% of those in the less-intensive group; there was no difference between the groups in number of days free of organ failure.
“We then looked at 1-year mortality and again there was no difference, with a total mortality of 34% in each group. Among patients who survived to day 60 there was an additional 20% mortality at 1 year,” he said. Not only was mortality not decreased with the intensive therapy, but a greater percentage of patients undergoing intensive therapy experienced treatment-related hypotension and had more hypocalcemia and hypophosphatemia, he noted.
Dr. Palevsky disclosed no conflicts of interest.
PHILADELPHIA — Intensive renal support in critically ill patients with acute kidney injury did not decrease mortality, accelerate recovery of renal function, or alter the rate of nonrenal organ failure in the randomized Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network study.
Results of previous smaller studies evaluating different strategies of renal support have been inconsistent, and while some studies favored more intensive, higher-dose approaches, such a strategy has not been adopted widely in the United States.
To address the uncertainty as to the optimal approach for acute kidney injury, a large trial was undertaken comparing conventional with intensive renal support. An integrative approach was used, in which hemodynamically stable patients were on standard intermittent therapy and hemodynamically unstable patients were on continuous therapy or sustained low-efficiency dialysis, according to the lead investigator, Dr. Paul M. Palevsky.
“Patients could move between modalities of therapy as their hemodynamic status changed, but they remained within a dosing schedule which was five to six times per week for a total effluent flow rate of 35 mL/kg per hour in the intensive group, and three times per week for a total effluent flow rate of 20 mL/kg per hour in the less-intensive group,” said Dr. Palevsky of the renal section, VA Pittsburgh Healthcare System, and the department of medicine at the University of Pittsburgh.
To be eligible, patients had to be 18 years or older, critically ill, have acute kidney injury consistent with acute tubular necrosis, and have failure of one or more nonrenal organs, or sepsis.
A total of 563 patients were randomized to the intensive group; 561 were randomized to the conventional group.
The populations were well matched at baseline in terms of age, at a mean of 60 years, as well as in race, sex, and ethnicity and in baseline severity of illness and renal function, Dr. Palevsky said at the annual meeting of the American Society of Nephrology.
Mean serum creatinine was 1.1 mg/dL in both groups at baseline. A total of 88% of patients had an estimated glomerular filtration rate of at least 45 mL/min per 1.73 m
Therapy continued for 28 days or until the patient regained renal function, was withdrawn from life-sustaining care, was discharged from the acute care hospital, or died.
Patients in the intensive therapy group received an average of 5.4 sessions per week, with an interval between treatments of 1.1 days, while those in the less-intensive group averaged 3 sessions per week with an interval of 2 days between treatments.
Prescribed and delivered flow rates of venovenous hemodiafiltration were approximately 36 mL/kg per hour for the intensive group and just over 20 mL/kg per hour for the less-intensive group (N. Engl. J. Med. 2008;359:7–20).
“We observed no difference between the two groups on our primary outcome measure of 60-day all-cause mortality, with 53.6% in the intensive arm and 51.5% in the less-intensive arm,” he said.
Complete recovery of kidney function by day 28 was seen in 15% of the intensive therapy group, and in 18% of those in the less-intensive group; there was no difference between the groups in number of days free of organ failure.
“We then looked at 1-year mortality and again there was no difference, with a total mortality of 34% in each group. Among patients who survived to day 60 there was an additional 20% mortality at 1 year,” he said. Not only was mortality not decreased with the intensive therapy, but a greater percentage of patients undergoing intensive therapy experienced treatment-related hypotension and had more hypocalcemia and hypophosphatemia, he noted.
Dr. Palevsky disclosed no conflicts of interest.