User login
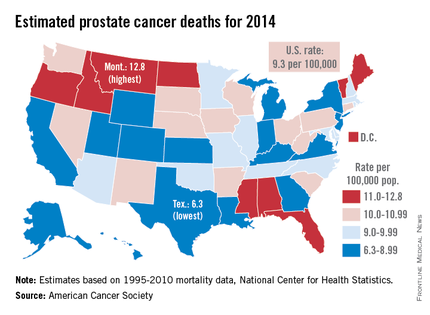
Prostate cancer death rate highest in Montana
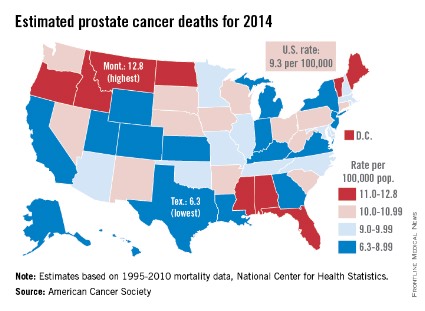
Montana is expected to have the highest rate of prostate cancer deaths in the United States for 2014, and it will be more than twice as high as that of Texas, which should have the lowest rate, the American Cancer Society reported.
Based on mortality data for 1995-2010 from the National Center for Health Statistics, the ACS estimates put the death rate at 12.8 per 100,000 population for Montana and 6.3 per 100,000 for Texas.
After Texas, Wyoming has the lowest estimated prostate cancer rate for 2014 at 6.9 per 100,000, followed by Utah at 7.2. The District of Columbia has the second-highest rate at 12.4 per 100,000, with Maine third at 12.0, according to the ACS estimates.
Although the number of prostate cancer deaths has been decreasing – dropping by an average of 3.1% per year from 2006 to 2010 – it is still the second-leading cause of cancer death in men. With almost 29,500 deaths expected in the United States this year, the national death rate from prostate cancer for 2014 is estimated to be 9.3 per 100,000, according to the ACS report.
Montana is expected to have the highest rate of prostate cancer deaths in the United States for 2014, and it will be more than twice as high as that of Texas, which should have the lowest rate, the American Cancer Society reported.
Based on mortality data for 1995-2010 from the National Center for Health Statistics, the ACS estimates put the death rate at 12.8 per 100,000 population for Montana and 6.3 per 100,000 for Texas.
After Texas, Wyoming has the lowest estimated prostate cancer rate for 2014 at 6.9 per 100,000, followed by Utah at 7.2. The District of Columbia has the second-highest rate at 12.4 per 100,000, with Maine third at 12.0, according to the ACS estimates.
Although the number of prostate cancer deaths has been decreasing – dropping by an average of 3.1% per year from 2006 to 2010 – it is still the second-leading cause of cancer death in men. With almost 29,500 deaths expected in the United States this year, the national death rate from prostate cancer for 2014 is estimated to be 9.3 per 100,000, according to the ACS report.

Montana is expected to have the highest rate of prostate cancer deaths in the United States for 2014, and it will be more than twice as high as that of Texas, which should have the lowest rate, the American Cancer Society reported.
Based on mortality data for 1995-2010 from the National Center for Health Statistics, the ACS estimates put the death rate at 12.8 per 100,000 population for Montana and 6.3 per 100,000 for Texas.
After Texas, Wyoming has the lowest estimated prostate cancer rate for 2014 at 6.9 per 100,000, followed by Utah at 7.2. The District of Columbia has the second-highest rate at 12.4 per 100,000, with Maine third at 12.0, according to the ACS estimates.
Although the number of prostate cancer deaths has been decreasing – dropping by an average of 3.1% per year from 2006 to 2010 – it is still the second-leading cause of cancer death in men. With almost 29,500 deaths expected in the United States this year, the national death rate from prostate cancer for 2014 is estimated to be 9.3 per 100,000, according to the ACS report.

Acute kidney injury elevates death risk long after RRT
Acute kidney injury survivors treated with renal replacement therapy in the ICU have high long-term mortality risk, regardless of the type of RRT they received, according to a recent study.
Intensity of RRT performed in intensive care units, researchers also found, made no difference in the likelihood of a patient needing maintenance dialysis or having protein in the urine within 4 years after the intervention.
The results, published in PLoS Medicine (2014 Feb. 11 [doi:10.1371/journal.pmed.1001601]), come from POST-RENAL, an extended follow-up in a trial of 1,464 adult AKI patients in ICUs who were randomized to receive RRT of higher or lower intensity. Dr. Martin Gallagher of the George Institute for Global Health in Sydney, Australia, led the study. Patients were treated in 35 centers in Australia or New Zealand between December 2005 and August 2008.
The researchers did not see high-intensity RRT associated with any improvements in long-term survival: At a median of 43.9 months after randomization, 63% of patients in the lower-intensity group and 62% of patients in the higher-intensity group had died (risk ratio, 1.04; 95% confidence interval, 0.96-1.12; P = .49).
Of the 810 patients who survived more than 90 days past randomization, rates of maintenance dialysis were similarly low: 5.1% for the lower-intensity RRT group and 5.8% for the higher-intensity group (RR, 1.12; 95% CI, 0.63-2.00; P = .69). Both groups, however, saw significantly high rates of albuminuria: 40% and 44%, respectively (P = .48). Chronic proteinuria is an established risk factor for death, cardiovascular disease, and additional dialysis.
"Only one-third of randomized patients were alive 3.5 years later, a lower survival than that seen in recognized high mortality conditions such as the acute respiratory distress syndrome. Although, in our patients the risk of subsequent maintenance dialysis dependence is low, almost half have evidence of significant proteinuria, portending further risk in the years to come," Dr. Gallagher and his colleagues said in their analysis.
The findings "support the view that survivors of AKI are at increased risk and that closer surveillance may be justified. In addition, our findings suggest that chronic proteinuria reduction strategies, which have shown benefit in some patient groups with proteinuria, may warrant investigation as a therapeutic intervention," they wrote.
The study was supported by the Australian government. One coauthor, Dr. Rinaldo Bellomo, disclosed receiving financial support from Eli Lilly, Cardinal Health, and CSL Bioplasma. The George Institute for Global Health, Dr. Gallagher’s institution, has received research funding from Servier, Novartis, and other companies.

|
|
This data showing a relatively low rate of chronic renal replacement therapy will be helpful in counseling critically ill patients with acute kidney injury. The high rate of mortality highlights the importance of further studies into appropriate risk-reduction strategies and interventions for these individuals.
Dr. Seema Chandra is a hospitalist at West Kendall Baptist Hospital and is on the advisory board of Hospitalist News.

|
|
This data showing a relatively low rate of chronic renal replacement therapy will be helpful in counseling critically ill patients with acute kidney injury. The high rate of mortality highlights the importance of further studies into appropriate risk-reduction strategies and interventions for these individuals.
Dr. Seema Chandra is a hospitalist at West Kendall Baptist Hospital and is on the advisory board of Hospitalist News.

|
|
This data showing a relatively low rate of chronic renal replacement therapy will be helpful in counseling critically ill patients with acute kidney injury. The high rate of mortality highlights the importance of further studies into appropriate risk-reduction strategies and interventions for these individuals.
Dr. Seema Chandra is a hospitalist at West Kendall Baptist Hospital and is on the advisory board of Hospitalist News.
Acute kidney injury survivors treated with renal replacement therapy in the ICU have high long-term mortality risk, regardless of the type of RRT they received, according to a recent study.
Intensity of RRT performed in intensive care units, researchers also found, made no difference in the likelihood of a patient needing maintenance dialysis or having protein in the urine within 4 years after the intervention.
The results, published in PLoS Medicine (2014 Feb. 11 [doi:10.1371/journal.pmed.1001601]), come from POST-RENAL, an extended follow-up in a trial of 1,464 adult AKI patients in ICUs who were randomized to receive RRT of higher or lower intensity. Dr. Martin Gallagher of the George Institute for Global Health in Sydney, Australia, led the study. Patients were treated in 35 centers in Australia or New Zealand between December 2005 and August 2008.
The researchers did not see high-intensity RRT associated with any improvements in long-term survival: At a median of 43.9 months after randomization, 63% of patients in the lower-intensity group and 62% of patients in the higher-intensity group had died (risk ratio, 1.04; 95% confidence interval, 0.96-1.12; P = .49).
Of the 810 patients who survived more than 90 days past randomization, rates of maintenance dialysis were similarly low: 5.1% for the lower-intensity RRT group and 5.8% for the higher-intensity group (RR, 1.12; 95% CI, 0.63-2.00; P = .69). Both groups, however, saw significantly high rates of albuminuria: 40% and 44%, respectively (P = .48). Chronic proteinuria is an established risk factor for death, cardiovascular disease, and additional dialysis.
"Only one-third of randomized patients were alive 3.5 years later, a lower survival than that seen in recognized high mortality conditions such as the acute respiratory distress syndrome. Although, in our patients the risk of subsequent maintenance dialysis dependence is low, almost half have evidence of significant proteinuria, portending further risk in the years to come," Dr. Gallagher and his colleagues said in their analysis.
The findings "support the view that survivors of AKI are at increased risk and that closer surveillance may be justified. In addition, our findings suggest that chronic proteinuria reduction strategies, which have shown benefit in some patient groups with proteinuria, may warrant investigation as a therapeutic intervention," they wrote.
The study was supported by the Australian government. One coauthor, Dr. Rinaldo Bellomo, disclosed receiving financial support from Eli Lilly, Cardinal Health, and CSL Bioplasma. The George Institute for Global Health, Dr. Gallagher’s institution, has received research funding from Servier, Novartis, and other companies.
Acute kidney injury survivors treated with renal replacement therapy in the ICU have high long-term mortality risk, regardless of the type of RRT they received, according to a recent study.
Intensity of RRT performed in intensive care units, researchers also found, made no difference in the likelihood of a patient needing maintenance dialysis or having protein in the urine within 4 years after the intervention.
The results, published in PLoS Medicine (2014 Feb. 11 [doi:10.1371/journal.pmed.1001601]), come from POST-RENAL, an extended follow-up in a trial of 1,464 adult AKI patients in ICUs who were randomized to receive RRT of higher or lower intensity. Dr. Martin Gallagher of the George Institute for Global Health in Sydney, Australia, led the study. Patients were treated in 35 centers in Australia or New Zealand between December 2005 and August 2008.
The researchers did not see high-intensity RRT associated with any improvements in long-term survival: At a median of 43.9 months after randomization, 63% of patients in the lower-intensity group and 62% of patients in the higher-intensity group had died (risk ratio, 1.04; 95% confidence interval, 0.96-1.12; P = .49).
Of the 810 patients who survived more than 90 days past randomization, rates of maintenance dialysis were similarly low: 5.1% for the lower-intensity RRT group and 5.8% for the higher-intensity group (RR, 1.12; 95% CI, 0.63-2.00; P = .69). Both groups, however, saw significantly high rates of albuminuria: 40% and 44%, respectively (P = .48). Chronic proteinuria is an established risk factor for death, cardiovascular disease, and additional dialysis.
"Only one-third of randomized patients were alive 3.5 years later, a lower survival than that seen in recognized high mortality conditions such as the acute respiratory distress syndrome. Although, in our patients the risk of subsequent maintenance dialysis dependence is low, almost half have evidence of significant proteinuria, portending further risk in the years to come," Dr. Gallagher and his colleagues said in their analysis.
The findings "support the view that survivors of AKI are at increased risk and that closer surveillance may be justified. In addition, our findings suggest that chronic proteinuria reduction strategies, which have shown benefit in some patient groups with proteinuria, may warrant investigation as a therapeutic intervention," they wrote.
The study was supported by the Australian government. One coauthor, Dr. Rinaldo Bellomo, disclosed receiving financial support from Eli Lilly, Cardinal Health, and CSL Bioplasma. The George Institute for Global Health, Dr. Gallagher’s institution, has received research funding from Servier, Novartis, and other companies.
FROM PLOS MEDICINE
Acute kidney injury elevates death risk long after RRT
People with acute kidney injury receiving renal replacement therapy have an increased long-term risk of dying regardless of the type of RRT they receive, according to a new study.
Intensity of RRT performed in intensive care units, researchers also found, made no difference in the likelihood of a patient needing maintenance dialysis or having protein in the urine within 4 years after the intervention.
The results, published Feb. 11 in PLoS Medicine (2014 Feb. 11 [doi: 10.1371/journal.pmed.1001601]), come from extended follow-up in a trial of 1,464 AKI patients in ICUs who were randomized to receive RRT of higher or lower intensity. Dr. Martin Gallagher of the George Institute for Global Health in Sydney, Australia, led the study.
The researchers did not see high-intensity RRT associated with any improvements in long-term survival: At a median of 43.9 months after randomization, 63% of patients in the lower-intensity group and 62% of patients in the higher-intensity group had died (risk ratio, 1.04; 95% CI, 0.96-1.12; P = .49).
Of the 810 patients who survived more than 90 days past randomization, rates of maintenance dialysis were similarly low: 5.1% for the lower-intensity RRT group and 5.8% for the higher-intensity group (RR, 1.12; 95% CI, 0.63-2.00; P = .69). Both groups, however, saw high rates of albuminuria: 40% and 44%, respectively (P = .48). Chronic proteinuria is an established risk factor for death, cardiovascular disease, and additional dialysis.
Dr. Gallagher and his colleagues wrote in their analysis that the findings "support the view that survivors of AKI are at increased risk and that closer surveillance may be justified. In addition, our findings suggest that chronic proteinuria reduction strategies, which have shown benefit in some patient groups with proteinuria, may warrant investigation as a therapeutic intervention."
The study was supported by the Australian government. One coauthor, Dr. Rinaldo Bellomo, disclosed receiving financial support from Eli Lilly, Cardinal Health, and CSL Bioplasma. The George Institute for Global Health, Dr. Gallagher’s institution, has received research funding from Servier, Novartis, and other companies.
People with acute kidney injury receiving renal replacement therapy have an increased long-term risk of dying regardless of the type of RRT they receive, according to a new study.
Intensity of RRT performed in intensive care units, researchers also found, made no difference in the likelihood of a patient needing maintenance dialysis or having protein in the urine within 4 years after the intervention.
The results, published Feb. 11 in PLoS Medicine (2014 Feb. 11 [doi: 10.1371/journal.pmed.1001601]), come from extended follow-up in a trial of 1,464 AKI patients in ICUs who were randomized to receive RRT of higher or lower intensity. Dr. Martin Gallagher of the George Institute for Global Health in Sydney, Australia, led the study.
The researchers did not see high-intensity RRT associated with any improvements in long-term survival: At a median of 43.9 months after randomization, 63% of patients in the lower-intensity group and 62% of patients in the higher-intensity group had died (risk ratio, 1.04; 95% CI, 0.96-1.12; P = .49).
Of the 810 patients who survived more than 90 days past randomization, rates of maintenance dialysis were similarly low: 5.1% for the lower-intensity RRT group and 5.8% for the higher-intensity group (RR, 1.12; 95% CI, 0.63-2.00; P = .69). Both groups, however, saw high rates of albuminuria: 40% and 44%, respectively (P = .48). Chronic proteinuria is an established risk factor for death, cardiovascular disease, and additional dialysis.
Dr. Gallagher and his colleagues wrote in their analysis that the findings "support the view that survivors of AKI are at increased risk and that closer surveillance may be justified. In addition, our findings suggest that chronic proteinuria reduction strategies, which have shown benefit in some patient groups with proteinuria, may warrant investigation as a therapeutic intervention."
The study was supported by the Australian government. One coauthor, Dr. Rinaldo Bellomo, disclosed receiving financial support from Eli Lilly, Cardinal Health, and CSL Bioplasma. The George Institute for Global Health, Dr. Gallagher’s institution, has received research funding from Servier, Novartis, and other companies.
People with acute kidney injury receiving renal replacement therapy have an increased long-term risk of dying regardless of the type of RRT they receive, according to a new study.
Intensity of RRT performed in intensive care units, researchers also found, made no difference in the likelihood of a patient needing maintenance dialysis or having protein in the urine within 4 years after the intervention.
The results, published Feb. 11 in PLoS Medicine (2014 Feb. 11 [doi: 10.1371/journal.pmed.1001601]), come from extended follow-up in a trial of 1,464 AKI patients in ICUs who were randomized to receive RRT of higher or lower intensity. Dr. Martin Gallagher of the George Institute for Global Health in Sydney, Australia, led the study.
The researchers did not see high-intensity RRT associated with any improvements in long-term survival: At a median of 43.9 months after randomization, 63% of patients in the lower-intensity group and 62% of patients in the higher-intensity group had died (risk ratio, 1.04; 95% CI, 0.96-1.12; P = .49).
Of the 810 patients who survived more than 90 days past randomization, rates of maintenance dialysis were similarly low: 5.1% for the lower-intensity RRT group and 5.8% for the higher-intensity group (RR, 1.12; 95% CI, 0.63-2.00; P = .69). Both groups, however, saw high rates of albuminuria: 40% and 44%, respectively (P = .48). Chronic proteinuria is an established risk factor for death, cardiovascular disease, and additional dialysis.
Dr. Gallagher and his colleagues wrote in their analysis that the findings "support the view that survivors of AKI are at increased risk and that closer surveillance may be justified. In addition, our findings suggest that chronic proteinuria reduction strategies, which have shown benefit in some patient groups with proteinuria, may warrant investigation as a therapeutic intervention."
The study was supported by the Australian government. One coauthor, Dr. Rinaldo Bellomo, disclosed receiving financial support from Eli Lilly, Cardinal Health, and CSL Bioplasma. The George Institute for Global Health, Dr. Gallagher’s institution, has received research funding from Servier, Novartis, and other companies.
FROM PLOS MEDICINE
Preoperative organ dysfunction worsens SAVR outcomes
SNOWMASS, COLO. – The presence of preoperative dysfunction in more than any one of four key organ systems profoundly reduces survival in patients undergoing surgical aortic valve replacement, a study showed.
"If you have two or more dysfunctional organ systems, you really need to think about what you’re doing for this patient. At 5 years, only about 40% of these patients are alive. It makes a lot of sense to me to say that if you have a patient with severe COPD [chronic obstructive pulmonary disease] and renal dysfunction, that patient should probably never get a surgical valve," Dr. Vinod H. Thourani said at the Annual Cardiovascular Conference at Snowmass.
In a retrospective analysis of a registry with prospectively entered data, 29% of 1,759 patients who underwent surgical aortic valve replacement (SAVR) with or without coronary artery bypass grafting at Emory University during 2002-2010 had preoperative dysfunction of one or more of four organ systems under scrutiny. Eighty-five patients had severe COPD, as defined by a forced expiratory volume in 1 second (FEV1) that was less than 50% of predicted, 140 had chronic renal failure, 149 had a prior stroke, and 241 had heart failure with a left ventricular ejection less than 35%.
Patients with chronic renal failure had far and away the worst 30-day and long-term outcomes. Half were dead within 3 years. The 7-year survival rate was just 11.7%.
The second-worst outcomes were seen in patients with severe COPD preoperatively. Their 7-year survival rate was 30.8%.
"Anyone with an FEV1 below about 40% becomes a higher-risk surgical candidate; think instead of TAVR [transcatheter aortic valve replacement],"advised Dr. Thourani of the division of cardiothoracic surgery at Emory University, Atlanta.
In contrast, outcomes in patients with either heart failure or prior stroke "were not that bad," he said, pointing to 7-year survival rates of 55.9% and 48.6%, respectively.
Ninety-five patients (5.4%) in this recently published study (Ann. Thorac. Surg. 2013;95:838-45) had more than one dysfunctional organ system prior to SAVR. Median survival in patients without dysfunction in any of the four organ systems was 8.2 years and counting. With one dysfunctional organ, it was still good at 7.2 years. However, with two dysfunctional organ systems, the median survival dropped precipitously to 4.1 years. With three dysfunctional organ systems, it was 5.9 years.
Dr. Thourini serves as a consultant to Edwards Lifesciences, Sorin, and St. Jude Medical.
SNOWMASS, COLO. – The presence of preoperative dysfunction in more than any one of four key organ systems profoundly reduces survival in patients undergoing surgical aortic valve replacement, a study showed.
"If you have two or more dysfunctional organ systems, you really need to think about what you’re doing for this patient. At 5 years, only about 40% of these patients are alive. It makes a lot of sense to me to say that if you have a patient with severe COPD [chronic obstructive pulmonary disease] and renal dysfunction, that patient should probably never get a surgical valve," Dr. Vinod H. Thourani said at the Annual Cardiovascular Conference at Snowmass.
In a retrospective analysis of a registry with prospectively entered data, 29% of 1,759 patients who underwent surgical aortic valve replacement (SAVR) with or without coronary artery bypass grafting at Emory University during 2002-2010 had preoperative dysfunction of one or more of four organ systems under scrutiny. Eighty-five patients had severe COPD, as defined by a forced expiratory volume in 1 second (FEV1) that was less than 50% of predicted, 140 had chronic renal failure, 149 had a prior stroke, and 241 had heart failure with a left ventricular ejection less than 35%.
Patients with chronic renal failure had far and away the worst 30-day and long-term outcomes. Half were dead within 3 years. The 7-year survival rate was just 11.7%.
The second-worst outcomes were seen in patients with severe COPD preoperatively. Their 7-year survival rate was 30.8%.
"Anyone with an FEV1 below about 40% becomes a higher-risk surgical candidate; think instead of TAVR [transcatheter aortic valve replacement],"advised Dr. Thourani of the division of cardiothoracic surgery at Emory University, Atlanta.
In contrast, outcomes in patients with either heart failure or prior stroke "were not that bad," he said, pointing to 7-year survival rates of 55.9% and 48.6%, respectively.
Ninety-five patients (5.4%) in this recently published study (Ann. Thorac. Surg. 2013;95:838-45) had more than one dysfunctional organ system prior to SAVR. Median survival in patients without dysfunction in any of the four organ systems was 8.2 years and counting. With one dysfunctional organ, it was still good at 7.2 years. However, with two dysfunctional organ systems, the median survival dropped precipitously to 4.1 years. With three dysfunctional organ systems, it was 5.9 years.
Dr. Thourini serves as a consultant to Edwards Lifesciences, Sorin, and St. Jude Medical.
SNOWMASS, COLO. – The presence of preoperative dysfunction in more than any one of four key organ systems profoundly reduces survival in patients undergoing surgical aortic valve replacement, a study showed.
"If you have two or more dysfunctional organ systems, you really need to think about what you’re doing for this patient. At 5 years, only about 40% of these patients are alive. It makes a lot of sense to me to say that if you have a patient with severe COPD [chronic obstructive pulmonary disease] and renal dysfunction, that patient should probably never get a surgical valve," Dr. Vinod H. Thourani said at the Annual Cardiovascular Conference at Snowmass.
In a retrospective analysis of a registry with prospectively entered data, 29% of 1,759 patients who underwent surgical aortic valve replacement (SAVR) with or without coronary artery bypass grafting at Emory University during 2002-2010 had preoperative dysfunction of one or more of four organ systems under scrutiny. Eighty-five patients had severe COPD, as defined by a forced expiratory volume in 1 second (FEV1) that was less than 50% of predicted, 140 had chronic renal failure, 149 had a prior stroke, and 241 had heart failure with a left ventricular ejection less than 35%.
Patients with chronic renal failure had far and away the worst 30-day and long-term outcomes. Half were dead within 3 years. The 7-year survival rate was just 11.7%.
The second-worst outcomes were seen in patients with severe COPD preoperatively. Their 7-year survival rate was 30.8%.
"Anyone with an FEV1 below about 40% becomes a higher-risk surgical candidate; think instead of TAVR [transcatheter aortic valve replacement],"advised Dr. Thourani of the division of cardiothoracic surgery at Emory University, Atlanta.
In contrast, outcomes in patients with either heart failure or prior stroke "were not that bad," he said, pointing to 7-year survival rates of 55.9% and 48.6%, respectively.
Ninety-five patients (5.4%) in this recently published study (Ann. Thorac. Surg. 2013;95:838-45) had more than one dysfunctional organ system prior to SAVR. Median survival in patients without dysfunction in any of the four organ systems was 8.2 years and counting. With one dysfunctional organ, it was still good at 7.2 years. However, with two dysfunctional organ systems, the median survival dropped precipitously to 4.1 years. With three dysfunctional organ systems, it was 5.9 years.
Dr. Thourini serves as a consultant to Edwards Lifesciences, Sorin, and St. Jude Medical.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Kidney Patients With Diabetes: Managing Their Medication
CE/CME No: CR-1402
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the incidence and staging of chronic kidney disease (CKD).
• Enumerate the treatment goals for the CKD patient with diabetes.
• Describe the hypoglycemic medications that can be used at each stage of CKD.
FACULTY
Cheryl Gilmartin is a Clinical Pharmacist in Nephrology and a Clinical Assistant Professor in the College of Pharmacy at the University of Illinois at Chicago. Jane S. Davis, a member of the Clinician Reviews editorial board, is a nurse practitioner in the Division of Nephrology at the University of Alabama at Birmingham and is the communications chairperson for the National Kidney Foundation’s Council of Advanced Practitioners (NKF-CAP). Kim Zuber, past chair of the NKF-CAP, is a physician assistant with Metropolitan Nephrology in Alexandria, Virginia, and Clinton, Maryland.
ACCREDITATION STATEMENT
Article begins on next page >>
Because the vast majority of patients with chronic kidney disease (CKD) are diabetic and thus take hypoglycemic medications, knowledge of renal dosing for these medications, their mechanisms of action, and their safety profiles, as well as consideration of A1C goals, is vital for the practicing clinician. Management of the diabetic CKD patient, identified by stage of kidney disease, is outlined, with dosing regimens as determined by the glomerular filtration rate. Special attention is given to insulin management.
Diabetes is the most common cause of chronic kidney disease (CKD) throughout the world and leaves in its wake huge financial and social burdens.1 Diabetes has been the main cause of kidney failure in the United States for a number of years; as of 2012, diabetes became the most common cause of kidney failure in the world.2
The cost of caring for the rapidly increasing diabetes population in the US has been enormous. In 2012, the price tag for treating diabetes was $245 billion for medical care alone.3 The societal cost—loss in life years, loss of productivity caused by an increase in early retirement and disability, and burden of caregiving on families—is immeasurable.
In addition to CKD, diabetes is a major risk factor for heart disease and can lead to blindness and amputations. The rising incidence of diabetes has resulted in a new sense of urgency and has prompted the health care community to find new ways to reduce the burden on the patient, as well as encourage more aggressive research on halting progression of the disease, preventing end-stage kidney failure, and avoiding the need for dialysis.
Nearly two out of three (59%) adults in the US will be diagnosed with CKD stages 3 to 5 during their lifetimes.4 Some of this can be attributed to normal loss of kidney function associated with aging. However, much is due to the double burden of diabetes and hypertension.5 Patients with CKD require ongoing medical care, and much of it will be provided in primary care practices.6 This article discusses the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 staging guidelines and explains which oral and injectable diabetes medications are acceptable at each stage of CKD (as defined by the glomerular filtration rate [GFR]) and how to simplify dosing.
On the next page: Staging CKD >>
STAGING CHRONIC KIDNEY DISEASE
The increasing incidence of CKD was first recognized in the 1990s. The need to care for the patient with CKD was understood, but there was a dearth of standardized management practices. Research was hindered by the many names used to identify kidney disease in the literature: from mildly elevated serum creatinine to low clearance to renal insufficiency.7 To rectify this, the National Kidney Foundation (NKF) convened an expert panel to develop a standard language and units of measurement to define kidney disease. The panel was tasked with developing classifications and an evaluation system for renal disease; identifying ways to attain more timely referrals to nephrology; clarifying research objectives; and delineating how to improve medication dosing. Ultimately, the main objective was to improve management of the patient with CKD.
In 2002, the NKF’s Dialysis Outcomes Quality Initiative (K/DOQI) defined the stages of kidney disease based on assessment of GFR and other kidney disease markers.8 GFR is a mathematical calculation for determining kidney function that corrects for loss of kidney function and incorporates the patient’s gender, race, age, and body size—the nonmodifiable risk factors known in 2001. GFR is expressed in mL/min/1.73 m2. Classifying patients with CKD according to the stage of kidney disease by GFR provides a mechanism for studying the efficacy and adverse reactions of medications while correlating them to each stage. This is of particular importance when treating patients with diabetes and kidney disease, as it can help to avoid adverse effects associated with inappropriate renal dosing. Many medications are renally eliminated; as kidney function diminishes, the pharmacokinetics of drugs are altered, potentially causing toxicity and an increased risk for drug interactions. As the GFR falls, even drugs not eliminated by the kidney may have altered pharmacokinetics and pharmacodynamics that may cause renal or systemic toxicity.1
Determining the precise method for renally dosing medication has been one of the most vigorously debated topics in CKD. Many medications were prescribed using the patient’s serum creatinine (SCr) concentration, a marker but not an exact measure of kidney disease.8 The Cockcroft-Gault equation, which provides an estimate of creatinine clearance (eCrCl; an alternate GFR measurement), was primarily used to determine renal drug dosing. 9
In 1999, a multisite study group worked to develop an equation to measure loss of kidney function in the patient with CKD.10,11 The new formula, known as the MDRD (Modification of Diet in Renal Disease) equation, standardized the measurement of loss of kidney function and provided an estimated GFR (eGFR) that was more accurate then eCrCl. At that time, the National Kidney Disease Education Program (NKDEP) decided to use the MDRD equation to assess renal function and use the eCrCl for dosing medications. The latter was chosen because most drugs were analyzed using eCrCl.9 More recently, the NKDEP stated that either the eCrCl or the eGFR is acceptable for renal drug dosing. Hudson and Nyman9 advise that when discrepancies occur between eGFR and eCrCl, clinical judgment should be applied in the elderly and in those with extremes in body weight. In those cases, therapeutic outcomes and risk versus benefit need to be considered when determining renal dosing.
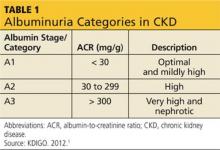
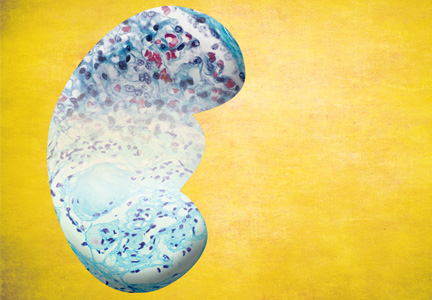
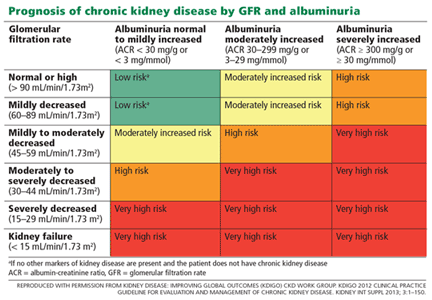
The publication of CKD stages by K/DOQI in 2002 sparked research that has uncovered additional criteria that might be useful in slowing the progression and improving the management of CKD. Thus, in 2013, new guidelines by KDIGO incorporating the latest international findings were published.1 KDIGO expands the definition of CKD to include albuminuria (ALB) as an added risk factor (see Figure) and has modified K/DOQI stages 3 and 5. ALB was included because it has predictive value for progression of CKD to kidney failure. In the new classification, there are three ranges defined for ALB measured in mg/g (see Table 1). The KDIGO guidelines, like K/DOQI, classify kidney disease using a 1-to-5 staging system; however, the stage numbers in the KDIGO classification are prefaced by a G (ie, G1, G2). Also, as mentioned above, stages G3 and G5 in the KDIGO classification have been further categorized.
CKD stage 3 was divided for practical reasons. It was found that the original CKD stage 3 was too large and diverse a classification to use for research studies, tracking of hospitalizations, and precise dosing of medications.12 K/DOQI stage 3 encompassed a GFR from 30 to 59 mL/min/1.73 m2. The KDIGO guideline divides K/DOQI stage 3 into G3a (GFR of 45 to 59 mL/min/1.73 m2) and G3b (GFR of 30 to 44 mL/min/1.73 m2). The staging is then further classified by the presence or absence of ALB. The patient with a GFR of 56 mL/min/1.73 m2 with ALB is in CKD stage 3aA3 (very high and nephrotic ALB) and has a greater risk for disease progression than the patient with a GFR of 56 mL/min/1.73 m2 without ALB. The latter patient is in CKD stage 3aA1(optimal and mildly high ALB). Finally, many medication dosages that were acceptable at a GFR of 56 mL/min/1.73 m2 had to be further adjusted for patients with a GFR of 30 mL/min/1.73 m2 to avoid toxicity. This further justified the KDIGO reclassification of stage 3.
Stage 5 from K/DOQI only reflected a GFR < 15 mL/min/1.73 m2. KDIGO utilizes the same GFR but splits the stage into patients not receiving dialysis and those receiving dialysis, G5 and G5D, respectively. The dosing and timing of medications due to drug dialyzability—including renal-specific medications—may be quite different for patients on dialysis as compared to those with a GFR < 15 mL/min/1.73 m2 not on dialysis, making the distinction beneficial.
On the next page: Diabetes management >>
DIABETES MANAGEMENT
The treatment of patients early in diabetic CKD is often the responsibility of primary care providers, who are faced with the daunting task of addressing the renal dosing of medications. The eGFR or eCrCl needs to be utilized to adjust diabetic medications, largely to avoid hypoglycemia. To alleviate some of the difficulty, the KDIGO CKD stage and the eCrCl and eGFR for diabetic medications are delineated in Table 2.1
Note that the guidelines are not meant to be strictly adhered to but rather are intended as a tool for managing the patient with CKD. Clinically significant patient factors need to be considered to adequately adjust drugs in CKD. Also, as new data become available from postmarketing reports, dosing adjustments, added precautions, or updated risk factors in these fragile patients may need to be incorporated into the guidelines. As new diabetic medications are approved by the FDA, kidney-specific dosing will continue to be a moving target.
While making lifestyle changes is very important early in diabetes, by the time diabetic nephropathy manifests, more aggressive action is warranted.12 A part of the diabetic microvascular triad (along with retinopathy and neuropathy), nephropathy signals existing and irreversible organ damage. Before instituting treatment to delay diabetic nephropathy progression, an A1C goal needs to be established. The long-standing goal has been an A1C < 7%, according to guidelines established by the American Diabetes Association, or 6.5%, as recognized by the American Association of Clinical Endocrinologists.13 The Diabetes Control and Complications Trial (DCCT) demonstrated microvascular benefit of tight glycemic control in type 1 diabetic patients when the A1C was maintained at an average of 7.2% (versus 9.1% in conventional therapy).14 The United Kingdom Prospective Diabetes Study similarly demonstrated microvascular benefit with intensive blood glucose control in type 2 diabetic patients who were maintained at a median A1C of 7% compared to the conventional treatment group maintained at an A1C of 7.9%.15
In January 2013, Perkovic and colleagues, in a post hoc analysis of the ADVANCE Trial, showed that maintaining an A1C between 6.5% and 7% in a patient with diabetes and macroalbuminuria (UACR > 300 μg/mg) slows progression to kidney failure.16 The time needed to treat with intensive glucose control was five years, and the number needed to treat was 41. Thus, for every 41 patients with diabetes, CKD, and ALB whose A1C is below 7%, one will not progress to kidney failure in five years of treatment.
The five-year lead time is troublesome, however. It discourages many patients from taking steps to achieve strict glycemic control; a significant number fail to follow their diabetic diets or take their medications until the damage is done. For the elderly kidney patient with diabetes and multiple other comorbidities, aggressively managing diabetes may result in hypoglycemia.12 On balance, the slight loss of kidney function five years hence is less problematic than the present risk for a fall and resulting hip fracture due to hypoglycemia. For this reason, the KDOQI glycemic guidelines suggest an A1C between 7% to 7.5% as the goal for those with significant comorbidities.12
On the next page: Diabetic medication dosing for CKD >>
DIABETIC MEDICATION DOSING FOR CKD
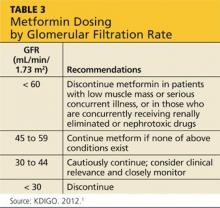
Glycemic control is important in delaying the progression of kidney failure in the patient with CKD. Hypoglycemic medications by definition may cause low blood glucose levels. This is of particular concern in patients with diminishing renal function, particularly the elderly and CKD patients. Antidiabetes drugs for patients in CKD stages 1 and 2 have few renal precautions, although care must be taken with metformin (see Table 3). Metformin is the drug of choice for the patient newly diagnosed with diabetes. It is inexpensive and effective, causes hypoglycemia only with intensive exercise, is taken orally, and is generally well tolerated. However, the package insert (PI) indicates stopping it in patients whose kidney disease has progressed to a GFR < 60 mL/min/1.73 m2 because it causes an increased risk for lactic acidosis.17,18
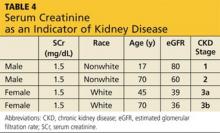
Metformin was developed prior to 1998, when the approval of the dosage regimen was tied to SCr levels rather than the presently accepted eGFR.19 As a result, the PI states that metformin should not be used in women with an SCr > 1.4 mg/dL or in men with an SCr > 1.5 mg/dL.17 However, as noted, SCr concentration alone is a very poor indicator of kidney disease (see Table 4).
Currently, the KDIGO guidelines recommend assessing the GFR for metformin dosing. When the GFR is < 60 mL/min/1.73 m2, KDIGO recommends metformin be discontinued in patients with low muscle mass or serious concurrent illness, or those who are concurrently receiving renally eliminated or nephrotoxic drugs (this includes, but is not limited to, renin-angiotensin-aldosterone system [RAAS] inhibitors, NSAIDs, and diuretics).1 In those patients who do not have the exclusions delineated above, KDIGO recommends that metformin may be continued when the GFR is > 45 mL/min/1.73 m2 (stages G1 to G3a), closely monitored and reconsidered when the GFR is 30 to 44 mL/min/1.73 m2 (stage G3b), and discontinued when the GFR is < 30 mL/min/1.73 m2 (stages G4 to G5). The KDIGO differs from other sources that recommend metformin be stopped when the GFR is < 50 to 70 ml/min/1.73 m2.20-22 The metformin PI also recommends stopping metformin the day of or day before and two days after procedures in which radiographic iodinated contrast dye is administered, to avoid acute kidney failure. Finally, it is important to note that any combination medication formulated with metformin should be stopped when the GFR is 50 mL/min/1.73 m2 because lactic acidosis has also been seen in patients taking these medications.21
At stage 3a (GFR, 45 to 60 mL/min/1.73 m2), the sulfonylureas require dosing changes (see Table 2). Glipizide is often the oral medication of choice during CKD, and it may be used in all stages, including dialysis, but does require renal dosing and monitoring.23 Although the insulins are more effective than glipizide in glycemic control, many practitioners prescribe it for patients who will not accept an injectable medication. Glyburide is not used at this stage, as it can lead to complications in patients with a GFR < 60 mL/min/1.73 m2, particularly hypoglycemia.
Like the sulfonylureas, the meglitinides enhance insulin secretion.13,18 Repaglinide and nateglinide are fast-acting and need to be taken with meals. Because it is recommended that the dose of the meglitinides be omitted when a meal is skipped, they are good for patients who eat meals irregularly. The meglitinides require initiation at a low dose in severe renal insufficiency.
The thiazolidinediones (TDZs) are used with caution in patients with CKD, not only because of controversial adverse cardiac effects, but also for peripheral edema that interferes with CKD management.18 The TDZs (rosiglitazone and pioglitazone) require no dose adjustment and appear to have a beneficial effect in obese patients and in patients who experienced weight gain with other hypoglycemic agents.
The newest of the diabetic drugs, canagliflozin, requires a working kidney to be effective.24,25 It is used with precaution when GFR is 45 to 59 mL/min/1.73 m2 at stage G3a and is contraindicated when GFR is < 45 mL/min/1.73 m2 in stage G3b. Canagliflozin's effects are felt in early segment of proximal convoluted tubule where it blocks sodium-glucose co-transporter 2, thereby lowering reabsorption of glucose and increasing excretion of glucose in the urine. In practical terms, it can affect kidney function and, due to the inhibition of the glucose-sodium pathway, induce hyperkalemia.25 Monitoring of both the GFR and potassium is vital when administering this drug. As data from postmarketing reports come in and practitioners gain experience, use of canaglifozin in the patient with CKD will be further delineated.
At a GFR between 30 and 45 mL/min/1.73 m2, CKD stage 3b, alpha-glucosidase inhibitors acarbose and miglitol are contraindicated. The GLP-1 mimetic liraglutide needs to be avoided when the GFR is < 60 mL/min/1.73 m2 (stage G3a). While exenatide requires close monitoring and caution when the GFR is < 50 mL/min/1.73 m2, it needs to be discontinued at a GFR
< 30 mL/min/1.73 m2 (stage 4). DPP-4 inhibitors become problematic at a GFR < 60 mL/min/
1.73 m2 beginning at stage G3a. All except the DPP-4 inhibitor linagliptin require dosing changes as the loss of GFR continues. Exact dosing adjustments for the drug categories are found in Table 2.
In stage 4 CKD (GFR, 15 to 30 mL/min/1.73 m2), more medications are eliminated from consideration. The amylinomimetic pramlintide cannot be used for a patient with a GFR < 15 mL/min/1.73 m2 (see Table 2). Both GLP-1 mimetics, exenatide and liraglutide, must be stopped when the GFR drops to 30 mL/min/1.73 m2. DDP-4 inhibitors need to be dosed according to the GFR but are still acceptable at CKD stage 4. Ideally, patients with diabetes should be referred to nephrology at stage 3, but referral is vital by stage 4.
Although oral medications are not as commonly prescribed as insulins at CKD stages 4 and 5, in stage 5 CKD (GFR, < 15 mL/min/1.73 m2), a limited number of oral diabetic agents are available (see Table 2). Glipizide is inexpensive and generic; therefore, it is the most commonly used oral medication in this stage. A recent study found linagliptin was safe to use throughout all stages of kidney disease without adjustment for GFR.26 While nateglinide is still acceptable in stage 5, it is used less frequently than glipizide.
As the kidney fails, the need for diabetic medications also decreases and dosing needs to take into account any residual renal function. Because glucose is produced in the proximal tubules of the kidney (known as gluconeogenesis), the level of glucose falls as the kidney fails. Excretion of insulin and other diabetes medications decreases, and thus the amount of insulin remaining in circulation is increased. This, in combination with the reduced glucose, increases the patient’s risk for hypoglycemia.13 Although protocols for medication adjustment are tied to GFR levels, close follow-up by the practitioner is required because GFR is not a reliable measure of kidney function when creatinine levels change rapidly.27
On the next page: Insulins >>
INSULINS
Many patients start insulin prior to reaching CKD stage 4 or 5 because it is the best choice for managing diabetes through all stages of CKD.28 Insulin can slow the progression to kidney failure by providing better A1C control, and all patients should be encouraged to start insulin as soon as it is appropriate.29 Many patients are very reluctant to use injectables, but by stage 5 they are willing to use insulin if it will slow progression of disease and help prevent the need for dialysis.2
The long-acting basal insulins (detemir and glargine) or a combination of basal and oral medication can work quite well at stage 5. Many patients will require a low dose of the long-acting basal insulins and short-acting insulin with meals. As the kidney fails, the short-acting doses can often be discontinued.29 A protocol that mixes long- and short-acting insulin with the largest meal is very effective at this stage. The long-acting insulin dose will need to be decreased as the kidney continues to fail. The American College of Physicians protocol for insulin suggests a 25% decrease at stage 4 and a 50% decrease at stage 5. Each patient is different, however, and dosing must be determined individually.23
On the next page: Patient education >>
PATIENT EDUCATION
There is a correlation between patients who participate in kidney disease education programs and a decrease in the progression of kidney disease (see “Case Study: The Recalcitrant Patient With Diabetes"). These classes can be taught by a physician, physician assistant, nurse practitioner, or clinical nurse specialist. To alleviate the burden of end-stage kidney disease in the US, Medicare Part B pays for six outpatient kidney disease patient education classes per lifetime of the beneficiary.30
Since 2010, when kidney disease education programs were rolled out, over 10,000 classes have been taught.31 Many practitioners report that patients are more compliant and understand their disease better when they attend classes.32 Data are being gathered to determine the effectiveness of the classes on patient outcomes.
On the next page: Conclusion >>
CONCLUSION
Knowing how to manage the patient with both diabetes and kidney disease is increasingly vital as this patient population grows. Much of the management of these patients will fall to the primary care practitioner.6 The most effective way to start treatment is to identify which CKD stage the patient fits into.
Once the patient is properly categorized, safe and effective diabetic medications can be selected and dosed according to stage. Although the new classification system may be difficult to incorporate into some electronic medical record systems and practitioner behavior, ultimately, it will allow safer management of the patient with CKD and a better predictive power of outcome.
1. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International Suppl. 2013;3:1-150.
2. Brazie M. Finding the sweet spot: trouble-shooting diabetic dilemmas. Oral presentation at NKF Spring Clinical Meetings; May 2012, Washington, DC.
3. Estimated cost of diabetes care $245 billion in U.S. in 2012. Renal & Urology News. March 9, 2013. www.renalandurologynews.com/estimated-cost-of-diabetes-245-billion-in-us-in-2012/article/283616/. Accessed January 17, 2013.
4. Grams ME, Chow EK, Segev DL, Coresh J. Lifetime incidence of CKD stages 3-5 in the United States. Am J Kidney Dis. 2013;62(2):245-252.
5. Greenberg A, ed. National Kidney Foundation Primer of Kidney Diseases. 5th ed. Philadelphia, PA: Saunders Elsevier; 2009.
6. Spann SJ, Nutting PA, Galliher JM, et al. Management of type 2 diabetes in the primary care setting: a practice-based research study. Ann Fam Med. 2006;4(1):23-31.
7. Hsu CY, Chertow GM. Chronic renal confusion: insufficiency, failure, dysfunction, or disease. Am J Kidney Dis. 2000;49(3):482-496.
8. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;39(suppl 1):S1-S266.
9. Hudson JQ, Nyman HA. Use of the estimated glomerular filtration rate for drug dosing in the chronic kidney disease patient. Curr Opin Nephrol Hypertens. 2011;20:482-491.
10. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461-470.
11. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612.
12. NKF-KDOQI clinical practice guideline for diabetes and CKD, guideline 2: management of hyperglycemia and general diabetes care in CKD. Am J Kidney Dis. 2012;60(5):850-886. www.kidney.org/professionals/kdoqi/guideline_diabetes/guide2.htm. Accessed January 2, 2014.
13. Abe M, Okada K, Soma M. Antidiabetic agents in patients with chronic kidney disease and end-stage renal disease on dialysis: metabolism and clinical practice. Curr Drug Metab. 2011;12(1):57-69.
14. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381-389.
15. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405-411.
16. Perkovic V, Heerspink HL, Chalmers J, et al. Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int. 2013;83(3):517-23.
17. Metformin [package insert]. US Department of Health and Human Services, US Food and Drug Administration. www.fda.gov/ohrms/dockets/dailys/02/May02/053102/800471e6.pdf. Accessed January 17, 2013.
18. Triplitt CL, Reasner CA. Chapter 83. Diabetes Mellitus. In: Wells BG, ed. Pharmacotherapy: A Pathophysiologic Approach. 8th ed. New York, NY: McGraw-Hill; 2011.
19. FDA. Guidance for industry: pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulato ryInformation/Guidances/ucm072127.pdf. Accessed January 17, 2013.
20. Lacy CF, Armstrong LL, Goldman MP, Lance LL. Drug Information Handbook. 20th ed. Hudson, OH: Lexi-Comp, Inc.; 2011.
21. Rocha A, Almeida M, Santos J, Carvalho A. Metformin in patients with chronic kidney disease: strengths and weaknesses. J Nephrol. 2013; 26(10):55-60.
22. Gilbert SJ, Weiner DE, eds. National Kidney Foundation Primer on Kidney Diseases. 6th ed. Philadelphia, PA: Elsevier Saunders; 2014.
23. Aronoff GR, Bennett WM, Berns JS, et al. Drug Prescribing in Renal Failure: Dosing Guidelines for Adults and Children. 5th ed. Philadelphia, PA: American College of Physicians; 2007.
24. Valentine V. The role of the kidney and the sodium-glucose co-transporter inhibition in diabetes management. Clin Diabetes. 2012;30(4):151-155.
25. Invokana [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2013.
26. McGill JB, Sloan L, Newman J, et al. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment. Diabetes Care. 2013;36(2):237-244.
27. National Kidney Disease and Education Program (NKDEP). Estimated glomerular filtration rate (eGFR) info sheet. NIH publication No.10-6286, March 2010.
28. Thummel K, Shen D, Isoherranen N, et al. Design and optimization of dosage regimens: pharmacokinetic data. In: Hardman J, Limbird L, Goodman G (eds). Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill; 2006.
29. Snyder RW, Berns JS. Use of insulin and oral hypoglycemic medications in patients with diabetes mellitus and advanced kidney disease. Semin Dial. 2004;17(5):365-70.
30. Centers for Medicare and Medicaid Services. Medicare program: revisions to payment policies under the Physician Fee Schedule, Clinical Laboratory Fee Schedule & other revisions to Part B for CY 2014; Final Rule. Federal Register. 2009;74(226):61738-62188. www.gpo.gov/fdsys/pkg/FR-2009-11-25/html/E9-26502.htm. Accessed January 17, 2013.
31. Zuber K, Davis J. Kidney disease education: a niche for PAs and NPs. JAAPA. 2013;26(7):42-47.
32. Zuber K, Davis J. Stories from the trenches: the first year experience with kidney disease education. Nephrol News Issues. 2012;26(2):20-21.
CE/CME No: CR-1402
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the incidence and staging of chronic kidney disease (CKD).
• Enumerate the treatment goals for the CKD patient with diabetes.
• Describe the hypoglycemic medications that can be used at each stage of CKD.
FACULTY
Cheryl Gilmartin is a Clinical Pharmacist in Nephrology and a Clinical Assistant Professor in the College of Pharmacy at the University of Illinois at Chicago. Jane S. Davis, a member of the Clinician Reviews editorial board, is a nurse practitioner in the Division of Nephrology at the University of Alabama at Birmingham and is the communications chairperson for the National Kidney Foundation’s Council of Advanced Practitioners (NKF-CAP). Kim Zuber, past chair of the NKF-CAP, is a physician assistant with Metropolitan Nephrology in Alexandria, Virginia, and Clinton, Maryland.
ACCREDITATION STATEMENT
Article begins on next page >>
Because the vast majority of patients with chronic kidney disease (CKD) are diabetic and thus take hypoglycemic medications, knowledge of renal dosing for these medications, their mechanisms of action, and their safety profiles, as well as consideration of A1C goals, is vital for the practicing clinician. Management of the diabetic CKD patient, identified by stage of kidney disease, is outlined, with dosing regimens as determined by the glomerular filtration rate. Special attention is given to insulin management.
Diabetes is the most common cause of chronic kidney disease (CKD) throughout the world and leaves in its wake huge financial and social burdens.1 Diabetes has been the main cause of kidney failure in the United States for a number of years; as of 2012, diabetes became the most common cause of kidney failure in the world.2
The cost of caring for the rapidly increasing diabetes population in the US has been enormous. In 2012, the price tag for treating diabetes was $245 billion for medical care alone.3 The societal cost—loss in life years, loss of productivity caused by an increase in early retirement and disability, and burden of caregiving on families—is immeasurable.
In addition to CKD, diabetes is a major risk factor for heart disease and can lead to blindness and amputations. The rising incidence of diabetes has resulted in a new sense of urgency and has prompted the health care community to find new ways to reduce the burden on the patient, as well as encourage more aggressive research on halting progression of the disease, preventing end-stage kidney failure, and avoiding the need for dialysis.
Nearly two out of three (59%) adults in the US will be diagnosed with CKD stages 3 to 5 during their lifetimes.4 Some of this can be attributed to normal loss of kidney function associated with aging. However, much is due to the double burden of diabetes and hypertension.5 Patients with CKD require ongoing medical care, and much of it will be provided in primary care practices.6 This article discusses the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 staging guidelines and explains which oral and injectable diabetes medications are acceptable at each stage of CKD (as defined by the glomerular filtration rate [GFR]) and how to simplify dosing.
On the next page: Staging CKD >>
STAGING CHRONIC KIDNEY DISEASE
The increasing incidence of CKD was first recognized in the 1990s. The need to care for the patient with CKD was understood, but there was a dearth of standardized management practices. Research was hindered by the many names used to identify kidney disease in the literature: from mildly elevated serum creatinine to low clearance to renal insufficiency.7 To rectify this, the National Kidney Foundation (NKF) convened an expert panel to develop a standard language and units of measurement to define kidney disease. The panel was tasked with developing classifications and an evaluation system for renal disease; identifying ways to attain more timely referrals to nephrology; clarifying research objectives; and delineating how to improve medication dosing. Ultimately, the main objective was to improve management of the patient with CKD.
In 2002, the NKF’s Dialysis Outcomes Quality Initiative (K/DOQI) defined the stages of kidney disease based on assessment of GFR and other kidney disease markers.8 GFR is a mathematical calculation for determining kidney function that corrects for loss of kidney function and incorporates the patient’s gender, race, age, and body size—the nonmodifiable risk factors known in 2001. GFR is expressed in mL/min/1.73 m2. Classifying patients with CKD according to the stage of kidney disease by GFR provides a mechanism for studying the efficacy and adverse reactions of medications while correlating them to each stage. This is of particular importance when treating patients with diabetes and kidney disease, as it can help to avoid adverse effects associated with inappropriate renal dosing. Many medications are renally eliminated; as kidney function diminishes, the pharmacokinetics of drugs are altered, potentially causing toxicity and an increased risk for drug interactions. As the GFR falls, even drugs not eliminated by the kidney may have altered pharmacokinetics and pharmacodynamics that may cause renal or systemic toxicity.1
Determining the precise method for renally dosing medication has been one of the most vigorously debated topics in CKD. Many medications were prescribed using the patient’s serum creatinine (SCr) concentration, a marker but not an exact measure of kidney disease.8 The Cockcroft-Gault equation, which provides an estimate of creatinine clearance (eCrCl; an alternate GFR measurement), was primarily used to determine renal drug dosing. 9
In 1999, a multisite study group worked to develop an equation to measure loss of kidney function in the patient with CKD.10,11 The new formula, known as the MDRD (Modification of Diet in Renal Disease) equation, standardized the measurement of loss of kidney function and provided an estimated GFR (eGFR) that was more accurate then eCrCl. At that time, the National Kidney Disease Education Program (NKDEP) decided to use the MDRD equation to assess renal function and use the eCrCl for dosing medications. The latter was chosen because most drugs were analyzed using eCrCl.9 More recently, the NKDEP stated that either the eCrCl or the eGFR is acceptable for renal drug dosing. Hudson and Nyman9 advise that when discrepancies occur between eGFR and eCrCl, clinical judgment should be applied in the elderly and in those with extremes in body weight. In those cases, therapeutic outcomes and risk versus benefit need to be considered when determining renal dosing.
The publication of CKD stages by K/DOQI in 2002 sparked research that has uncovered additional criteria that might be useful in slowing the progression and improving the management of CKD. Thus, in 2013, new guidelines by KDIGO incorporating the latest international findings were published.1 KDIGO expands the definition of CKD to include albuminuria (ALB) as an added risk factor (see Figure) and has modified K/DOQI stages 3 and 5. ALB was included because it has predictive value for progression of CKD to kidney failure. In the new classification, there are three ranges defined for ALB measured in mg/g (see Table 1). The KDIGO guidelines, like K/DOQI, classify kidney disease using a 1-to-5 staging system; however, the stage numbers in the KDIGO classification are prefaced by a G (ie, G1, G2). Also, as mentioned above, stages G3 and G5 in the KDIGO classification have been further categorized.
CKD stage 3 was divided for practical reasons. It was found that the original CKD stage 3 was too large and diverse a classification to use for research studies, tracking of hospitalizations, and precise dosing of medications.12 K/DOQI stage 3 encompassed a GFR from 30 to 59 mL/min/1.73 m2. The KDIGO guideline divides K/DOQI stage 3 into G3a (GFR of 45 to 59 mL/min/1.73 m2) and G3b (GFR of 30 to 44 mL/min/1.73 m2). The staging is then further classified by the presence or absence of ALB. The patient with a GFR of 56 mL/min/1.73 m2 with ALB is in CKD stage 3aA3 (very high and nephrotic ALB) and has a greater risk for disease progression than the patient with a GFR of 56 mL/min/1.73 m2 without ALB. The latter patient is in CKD stage 3aA1(optimal and mildly high ALB). Finally, many medication dosages that were acceptable at a GFR of 56 mL/min/1.73 m2 had to be further adjusted for patients with a GFR of 30 mL/min/1.73 m2 to avoid toxicity. This further justified the KDIGO reclassification of stage 3.
Stage 5 from K/DOQI only reflected a GFR < 15 mL/min/1.73 m2. KDIGO utilizes the same GFR but splits the stage into patients not receiving dialysis and those receiving dialysis, G5 and G5D, respectively. The dosing and timing of medications due to drug dialyzability—including renal-specific medications—may be quite different for patients on dialysis as compared to those with a GFR < 15 mL/min/1.73 m2 not on dialysis, making the distinction beneficial.
On the next page: Diabetes management >>
DIABETES MANAGEMENT
The treatment of patients early in diabetic CKD is often the responsibility of primary care providers, who are faced with the daunting task of addressing the renal dosing of medications. The eGFR or eCrCl needs to be utilized to adjust diabetic medications, largely to avoid hypoglycemia. To alleviate some of the difficulty, the KDIGO CKD stage and the eCrCl and eGFR for diabetic medications are delineated in Table 2.1
Note that the guidelines are not meant to be strictly adhered to but rather are intended as a tool for managing the patient with CKD. Clinically significant patient factors need to be considered to adequately adjust drugs in CKD. Also, as new data become available from postmarketing reports, dosing adjustments, added precautions, or updated risk factors in these fragile patients may need to be incorporated into the guidelines. As new diabetic medications are approved by the FDA, kidney-specific dosing will continue to be a moving target.
While making lifestyle changes is very important early in diabetes, by the time diabetic nephropathy manifests, more aggressive action is warranted.12 A part of the diabetic microvascular triad (along with retinopathy and neuropathy), nephropathy signals existing and irreversible organ damage. Before instituting treatment to delay diabetic nephropathy progression, an A1C goal needs to be established. The long-standing goal has been an A1C < 7%, according to guidelines established by the American Diabetes Association, or 6.5%, as recognized by the American Association of Clinical Endocrinologists.13 The Diabetes Control and Complications Trial (DCCT) demonstrated microvascular benefit of tight glycemic control in type 1 diabetic patients when the A1C was maintained at an average of 7.2% (versus 9.1% in conventional therapy).14 The United Kingdom Prospective Diabetes Study similarly demonstrated microvascular benefit with intensive blood glucose control in type 2 diabetic patients who were maintained at a median A1C of 7% compared to the conventional treatment group maintained at an A1C of 7.9%.15
In January 2013, Perkovic and colleagues, in a post hoc analysis of the ADVANCE Trial, showed that maintaining an A1C between 6.5% and 7% in a patient with diabetes and macroalbuminuria (UACR > 300 μg/mg) slows progression to kidney failure.16 The time needed to treat with intensive glucose control was five years, and the number needed to treat was 41. Thus, for every 41 patients with diabetes, CKD, and ALB whose A1C is below 7%, one will not progress to kidney failure in five years of treatment.
The five-year lead time is troublesome, however. It discourages many patients from taking steps to achieve strict glycemic control; a significant number fail to follow their diabetic diets or take their medications until the damage is done. For the elderly kidney patient with diabetes and multiple other comorbidities, aggressively managing diabetes may result in hypoglycemia.12 On balance, the slight loss of kidney function five years hence is less problematic than the present risk for a fall and resulting hip fracture due to hypoglycemia. For this reason, the KDOQI glycemic guidelines suggest an A1C between 7% to 7.5% as the goal for those with significant comorbidities.12
On the next page: Diabetic medication dosing for CKD >>
DIABETIC MEDICATION DOSING FOR CKD
Glycemic control is important in delaying the progression of kidney failure in the patient with CKD. Hypoglycemic medications by definition may cause low blood glucose levels. This is of particular concern in patients with diminishing renal function, particularly the elderly and CKD patients. Antidiabetes drugs for patients in CKD stages 1 and 2 have few renal precautions, although care must be taken with metformin (see Table 3). Metformin is the drug of choice for the patient newly diagnosed with diabetes. It is inexpensive and effective, causes hypoglycemia only with intensive exercise, is taken orally, and is generally well tolerated. However, the package insert (PI) indicates stopping it in patients whose kidney disease has progressed to a GFR < 60 mL/min/1.73 m2 because it causes an increased risk for lactic acidosis.17,18
Metformin was developed prior to 1998, when the approval of the dosage regimen was tied to SCr levels rather than the presently accepted eGFR.19 As a result, the PI states that metformin should not be used in women with an SCr > 1.4 mg/dL or in men with an SCr > 1.5 mg/dL.17 However, as noted, SCr concentration alone is a very poor indicator of kidney disease (see Table 4).
Currently, the KDIGO guidelines recommend assessing the GFR for metformin dosing. When the GFR is < 60 mL/min/1.73 m2, KDIGO recommends metformin be discontinued in patients with low muscle mass or serious concurrent illness, or those who are concurrently receiving renally eliminated or nephrotoxic drugs (this includes, but is not limited to, renin-angiotensin-aldosterone system [RAAS] inhibitors, NSAIDs, and diuretics).1 In those patients who do not have the exclusions delineated above, KDIGO recommends that metformin may be continued when the GFR is > 45 mL/min/1.73 m2 (stages G1 to G3a), closely monitored and reconsidered when the GFR is 30 to 44 mL/min/1.73 m2 (stage G3b), and discontinued when the GFR is < 30 mL/min/1.73 m2 (stages G4 to G5). The KDIGO differs from other sources that recommend metformin be stopped when the GFR is < 50 to 70 ml/min/1.73 m2.20-22 The metformin PI also recommends stopping metformin the day of or day before and two days after procedures in which radiographic iodinated contrast dye is administered, to avoid acute kidney failure. Finally, it is important to note that any combination medication formulated with metformin should be stopped when the GFR is 50 mL/min/1.73 m2 because lactic acidosis has also been seen in patients taking these medications.21
At stage 3a (GFR, 45 to 60 mL/min/1.73 m2), the sulfonylureas require dosing changes (see Table 2). Glipizide is often the oral medication of choice during CKD, and it may be used in all stages, including dialysis, but does require renal dosing and monitoring.23 Although the insulins are more effective than glipizide in glycemic control, many practitioners prescribe it for patients who will not accept an injectable medication. Glyburide is not used at this stage, as it can lead to complications in patients with a GFR < 60 mL/min/1.73 m2, particularly hypoglycemia.
Like the sulfonylureas, the meglitinides enhance insulin secretion.13,18 Repaglinide and nateglinide are fast-acting and need to be taken with meals. Because it is recommended that the dose of the meglitinides be omitted when a meal is skipped, they are good for patients who eat meals irregularly. The meglitinides require initiation at a low dose in severe renal insufficiency.
The thiazolidinediones (TDZs) are used with caution in patients with CKD, not only because of controversial adverse cardiac effects, but also for peripheral edema that interferes with CKD management.18 The TDZs (rosiglitazone and pioglitazone) require no dose adjustment and appear to have a beneficial effect in obese patients and in patients who experienced weight gain with other hypoglycemic agents.
The newest of the diabetic drugs, canagliflozin, requires a working kidney to be effective.24,25 It is used with precaution when GFR is 45 to 59 mL/min/1.73 m2 at stage G3a and is contraindicated when GFR is < 45 mL/min/1.73 m2 in stage G3b. Canagliflozin's effects are felt in early segment of proximal convoluted tubule where it blocks sodium-glucose co-transporter 2, thereby lowering reabsorption of glucose and increasing excretion of glucose in the urine. In practical terms, it can affect kidney function and, due to the inhibition of the glucose-sodium pathway, induce hyperkalemia.25 Monitoring of both the GFR and potassium is vital when administering this drug. As data from postmarketing reports come in and practitioners gain experience, use of canaglifozin in the patient with CKD will be further delineated.
At a GFR between 30 and 45 mL/min/1.73 m2, CKD stage 3b, alpha-glucosidase inhibitors acarbose and miglitol are contraindicated. The GLP-1 mimetic liraglutide needs to be avoided when the GFR is < 60 mL/min/1.73 m2 (stage G3a). While exenatide requires close monitoring and caution when the GFR is < 50 mL/min/1.73 m2, it needs to be discontinued at a GFR
< 30 mL/min/1.73 m2 (stage 4). DPP-4 inhibitors become problematic at a GFR < 60 mL/min/
1.73 m2 beginning at stage G3a. All except the DPP-4 inhibitor linagliptin require dosing changes as the loss of GFR continues. Exact dosing adjustments for the drug categories are found in Table 2.
In stage 4 CKD (GFR, 15 to 30 mL/min/1.73 m2), more medications are eliminated from consideration. The amylinomimetic pramlintide cannot be used for a patient with a GFR < 15 mL/min/1.73 m2 (see Table 2). Both GLP-1 mimetics, exenatide and liraglutide, must be stopped when the GFR drops to 30 mL/min/1.73 m2. DDP-4 inhibitors need to be dosed according to the GFR but are still acceptable at CKD stage 4. Ideally, patients with diabetes should be referred to nephrology at stage 3, but referral is vital by stage 4.
Although oral medications are not as commonly prescribed as insulins at CKD stages 4 and 5, in stage 5 CKD (GFR, < 15 mL/min/1.73 m2), a limited number of oral diabetic agents are available (see Table 2). Glipizide is inexpensive and generic; therefore, it is the most commonly used oral medication in this stage. A recent study found linagliptin was safe to use throughout all stages of kidney disease without adjustment for GFR.26 While nateglinide is still acceptable in stage 5, it is used less frequently than glipizide.
As the kidney fails, the need for diabetic medications also decreases and dosing needs to take into account any residual renal function. Because glucose is produced in the proximal tubules of the kidney (known as gluconeogenesis), the level of glucose falls as the kidney fails. Excretion of insulin and other diabetes medications decreases, and thus the amount of insulin remaining in circulation is increased. This, in combination with the reduced glucose, increases the patient’s risk for hypoglycemia.13 Although protocols for medication adjustment are tied to GFR levels, close follow-up by the practitioner is required because GFR is not a reliable measure of kidney function when creatinine levels change rapidly.27
On the next page: Insulins >>
INSULINS
Many patients start insulin prior to reaching CKD stage 4 or 5 because it is the best choice for managing diabetes through all stages of CKD.28 Insulin can slow the progression to kidney failure by providing better A1C control, and all patients should be encouraged to start insulin as soon as it is appropriate.29 Many patients are very reluctant to use injectables, but by stage 5 they are willing to use insulin if it will slow progression of disease and help prevent the need for dialysis.2
The long-acting basal insulins (detemir and glargine) or a combination of basal and oral medication can work quite well at stage 5. Many patients will require a low dose of the long-acting basal insulins and short-acting insulin with meals. As the kidney fails, the short-acting doses can often be discontinued.29 A protocol that mixes long- and short-acting insulin with the largest meal is very effective at this stage. The long-acting insulin dose will need to be decreased as the kidney continues to fail. The American College of Physicians protocol for insulin suggests a 25% decrease at stage 4 and a 50% decrease at stage 5. Each patient is different, however, and dosing must be determined individually.23
On the next page: Patient education >>
PATIENT EDUCATION
There is a correlation between patients who participate in kidney disease education programs and a decrease in the progression of kidney disease (see “Case Study: The Recalcitrant Patient With Diabetes"). These classes can be taught by a physician, physician assistant, nurse practitioner, or clinical nurse specialist. To alleviate the burden of end-stage kidney disease in the US, Medicare Part B pays for six outpatient kidney disease patient education classes per lifetime of the beneficiary.30
Since 2010, when kidney disease education programs were rolled out, over 10,000 classes have been taught.31 Many practitioners report that patients are more compliant and understand their disease better when they attend classes.32 Data are being gathered to determine the effectiveness of the classes on patient outcomes.
On the next page: Conclusion >>
CONCLUSION
Knowing how to manage the patient with both diabetes and kidney disease is increasingly vital as this patient population grows. Much of the management of these patients will fall to the primary care practitioner.6 The most effective way to start treatment is to identify which CKD stage the patient fits into.
Once the patient is properly categorized, safe and effective diabetic medications can be selected and dosed according to stage. Although the new classification system may be difficult to incorporate into some electronic medical record systems and practitioner behavior, ultimately, it will allow safer management of the patient with CKD and a better predictive power of outcome.
CE/CME No: CR-1402
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the incidence and staging of chronic kidney disease (CKD).
• Enumerate the treatment goals for the CKD patient with diabetes.
• Describe the hypoglycemic medications that can be used at each stage of CKD.
FACULTY
Cheryl Gilmartin is a Clinical Pharmacist in Nephrology and a Clinical Assistant Professor in the College of Pharmacy at the University of Illinois at Chicago. Jane S. Davis, a member of the Clinician Reviews editorial board, is a nurse practitioner in the Division of Nephrology at the University of Alabama at Birmingham and is the communications chairperson for the National Kidney Foundation’s Council of Advanced Practitioners (NKF-CAP). Kim Zuber, past chair of the NKF-CAP, is a physician assistant with Metropolitan Nephrology in Alexandria, Virginia, and Clinton, Maryland.
ACCREDITATION STATEMENT
Article begins on next page >>
Because the vast majority of patients with chronic kidney disease (CKD) are diabetic and thus take hypoglycemic medications, knowledge of renal dosing for these medications, their mechanisms of action, and their safety profiles, as well as consideration of A1C goals, is vital for the practicing clinician. Management of the diabetic CKD patient, identified by stage of kidney disease, is outlined, with dosing regimens as determined by the glomerular filtration rate. Special attention is given to insulin management.
Diabetes is the most common cause of chronic kidney disease (CKD) throughout the world and leaves in its wake huge financial and social burdens.1 Diabetes has been the main cause of kidney failure in the United States for a number of years; as of 2012, diabetes became the most common cause of kidney failure in the world.2
The cost of caring for the rapidly increasing diabetes population in the US has been enormous. In 2012, the price tag for treating diabetes was $245 billion for medical care alone.3 The societal cost—loss in life years, loss of productivity caused by an increase in early retirement and disability, and burden of caregiving on families—is immeasurable.
In addition to CKD, diabetes is a major risk factor for heart disease and can lead to blindness and amputations. The rising incidence of diabetes has resulted in a new sense of urgency and has prompted the health care community to find new ways to reduce the burden on the patient, as well as encourage more aggressive research on halting progression of the disease, preventing end-stage kidney failure, and avoiding the need for dialysis.
Nearly two out of three (59%) adults in the US will be diagnosed with CKD stages 3 to 5 during their lifetimes.4 Some of this can be attributed to normal loss of kidney function associated with aging. However, much is due to the double burden of diabetes and hypertension.5 Patients with CKD require ongoing medical care, and much of it will be provided in primary care practices.6 This article discusses the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 staging guidelines and explains which oral and injectable diabetes medications are acceptable at each stage of CKD (as defined by the glomerular filtration rate [GFR]) and how to simplify dosing.
On the next page: Staging CKD >>
STAGING CHRONIC KIDNEY DISEASE
The increasing incidence of CKD was first recognized in the 1990s. The need to care for the patient with CKD was understood, but there was a dearth of standardized management practices. Research was hindered by the many names used to identify kidney disease in the literature: from mildly elevated serum creatinine to low clearance to renal insufficiency.7 To rectify this, the National Kidney Foundation (NKF) convened an expert panel to develop a standard language and units of measurement to define kidney disease. The panel was tasked with developing classifications and an evaluation system for renal disease; identifying ways to attain more timely referrals to nephrology; clarifying research objectives; and delineating how to improve medication dosing. Ultimately, the main objective was to improve management of the patient with CKD.
In 2002, the NKF’s Dialysis Outcomes Quality Initiative (K/DOQI) defined the stages of kidney disease based on assessment of GFR and other kidney disease markers.8 GFR is a mathematical calculation for determining kidney function that corrects for loss of kidney function and incorporates the patient’s gender, race, age, and body size—the nonmodifiable risk factors known in 2001. GFR is expressed in mL/min/1.73 m2. Classifying patients with CKD according to the stage of kidney disease by GFR provides a mechanism for studying the efficacy and adverse reactions of medications while correlating them to each stage. This is of particular importance when treating patients with diabetes and kidney disease, as it can help to avoid adverse effects associated with inappropriate renal dosing. Many medications are renally eliminated; as kidney function diminishes, the pharmacokinetics of drugs are altered, potentially causing toxicity and an increased risk for drug interactions. As the GFR falls, even drugs not eliminated by the kidney may have altered pharmacokinetics and pharmacodynamics that may cause renal or systemic toxicity.1
Determining the precise method for renally dosing medication has been one of the most vigorously debated topics in CKD. Many medications were prescribed using the patient’s serum creatinine (SCr) concentration, a marker but not an exact measure of kidney disease.8 The Cockcroft-Gault equation, which provides an estimate of creatinine clearance (eCrCl; an alternate GFR measurement), was primarily used to determine renal drug dosing. 9
In 1999, a multisite study group worked to develop an equation to measure loss of kidney function in the patient with CKD.10,11 The new formula, known as the MDRD (Modification of Diet in Renal Disease) equation, standardized the measurement of loss of kidney function and provided an estimated GFR (eGFR) that was more accurate then eCrCl. At that time, the National Kidney Disease Education Program (NKDEP) decided to use the MDRD equation to assess renal function and use the eCrCl for dosing medications. The latter was chosen because most drugs were analyzed using eCrCl.9 More recently, the NKDEP stated that either the eCrCl or the eGFR is acceptable for renal drug dosing. Hudson and Nyman9 advise that when discrepancies occur between eGFR and eCrCl, clinical judgment should be applied in the elderly and in those with extremes in body weight. In those cases, therapeutic outcomes and risk versus benefit need to be considered when determining renal dosing.
The publication of CKD stages by K/DOQI in 2002 sparked research that has uncovered additional criteria that might be useful in slowing the progression and improving the management of CKD. Thus, in 2013, new guidelines by KDIGO incorporating the latest international findings were published.1 KDIGO expands the definition of CKD to include albuminuria (ALB) as an added risk factor (see Figure) and has modified K/DOQI stages 3 and 5. ALB was included because it has predictive value for progression of CKD to kidney failure. In the new classification, there are three ranges defined for ALB measured in mg/g (see Table 1). The KDIGO guidelines, like K/DOQI, classify kidney disease using a 1-to-5 staging system; however, the stage numbers in the KDIGO classification are prefaced by a G (ie, G1, G2). Also, as mentioned above, stages G3 and G5 in the KDIGO classification have been further categorized.
CKD stage 3 was divided for practical reasons. It was found that the original CKD stage 3 was too large and diverse a classification to use for research studies, tracking of hospitalizations, and precise dosing of medications.12 K/DOQI stage 3 encompassed a GFR from 30 to 59 mL/min/1.73 m2. The KDIGO guideline divides K/DOQI stage 3 into G3a (GFR of 45 to 59 mL/min/1.73 m2) and G3b (GFR of 30 to 44 mL/min/1.73 m2). The staging is then further classified by the presence or absence of ALB. The patient with a GFR of 56 mL/min/1.73 m2 with ALB is in CKD stage 3aA3 (very high and nephrotic ALB) and has a greater risk for disease progression than the patient with a GFR of 56 mL/min/1.73 m2 without ALB. The latter patient is in CKD stage 3aA1(optimal and mildly high ALB). Finally, many medication dosages that were acceptable at a GFR of 56 mL/min/1.73 m2 had to be further adjusted for patients with a GFR of 30 mL/min/1.73 m2 to avoid toxicity. This further justified the KDIGO reclassification of stage 3.
Stage 5 from K/DOQI only reflected a GFR < 15 mL/min/1.73 m2. KDIGO utilizes the same GFR but splits the stage into patients not receiving dialysis and those receiving dialysis, G5 and G5D, respectively. The dosing and timing of medications due to drug dialyzability—including renal-specific medications—may be quite different for patients on dialysis as compared to those with a GFR < 15 mL/min/1.73 m2 not on dialysis, making the distinction beneficial.
On the next page: Diabetes management >>
DIABETES MANAGEMENT
The treatment of patients early in diabetic CKD is often the responsibility of primary care providers, who are faced with the daunting task of addressing the renal dosing of medications. The eGFR or eCrCl needs to be utilized to adjust diabetic medications, largely to avoid hypoglycemia. To alleviate some of the difficulty, the KDIGO CKD stage and the eCrCl and eGFR for diabetic medications are delineated in Table 2.1
Note that the guidelines are not meant to be strictly adhered to but rather are intended as a tool for managing the patient with CKD. Clinically significant patient factors need to be considered to adequately adjust drugs in CKD. Also, as new data become available from postmarketing reports, dosing adjustments, added precautions, or updated risk factors in these fragile patients may need to be incorporated into the guidelines. As new diabetic medications are approved by the FDA, kidney-specific dosing will continue to be a moving target.
While making lifestyle changes is very important early in diabetes, by the time diabetic nephropathy manifests, more aggressive action is warranted.12 A part of the diabetic microvascular triad (along with retinopathy and neuropathy), nephropathy signals existing and irreversible organ damage. Before instituting treatment to delay diabetic nephropathy progression, an A1C goal needs to be established. The long-standing goal has been an A1C < 7%, according to guidelines established by the American Diabetes Association, or 6.5%, as recognized by the American Association of Clinical Endocrinologists.13 The Diabetes Control and Complications Trial (DCCT) demonstrated microvascular benefit of tight glycemic control in type 1 diabetic patients when the A1C was maintained at an average of 7.2% (versus 9.1% in conventional therapy).14 The United Kingdom Prospective Diabetes Study similarly demonstrated microvascular benefit with intensive blood glucose control in type 2 diabetic patients who were maintained at a median A1C of 7% compared to the conventional treatment group maintained at an A1C of 7.9%.15
In January 2013, Perkovic and colleagues, in a post hoc analysis of the ADVANCE Trial, showed that maintaining an A1C between 6.5% and 7% in a patient with diabetes and macroalbuminuria (UACR > 300 μg/mg) slows progression to kidney failure.16 The time needed to treat with intensive glucose control was five years, and the number needed to treat was 41. Thus, for every 41 patients with diabetes, CKD, and ALB whose A1C is below 7%, one will not progress to kidney failure in five years of treatment.
The five-year lead time is troublesome, however. It discourages many patients from taking steps to achieve strict glycemic control; a significant number fail to follow their diabetic diets or take their medications until the damage is done. For the elderly kidney patient with diabetes and multiple other comorbidities, aggressively managing diabetes may result in hypoglycemia.12 On balance, the slight loss of kidney function five years hence is less problematic than the present risk for a fall and resulting hip fracture due to hypoglycemia. For this reason, the KDOQI glycemic guidelines suggest an A1C between 7% to 7.5% as the goal for those with significant comorbidities.12
On the next page: Diabetic medication dosing for CKD >>
DIABETIC MEDICATION DOSING FOR CKD
Glycemic control is important in delaying the progression of kidney failure in the patient with CKD. Hypoglycemic medications by definition may cause low blood glucose levels. This is of particular concern in patients with diminishing renal function, particularly the elderly and CKD patients. Antidiabetes drugs for patients in CKD stages 1 and 2 have few renal precautions, although care must be taken with metformin (see Table 3). Metformin is the drug of choice for the patient newly diagnosed with diabetes. It is inexpensive and effective, causes hypoglycemia only with intensive exercise, is taken orally, and is generally well tolerated. However, the package insert (PI) indicates stopping it in patients whose kidney disease has progressed to a GFR < 60 mL/min/1.73 m2 because it causes an increased risk for lactic acidosis.17,18
Metformin was developed prior to 1998, when the approval of the dosage regimen was tied to SCr levels rather than the presently accepted eGFR.19 As a result, the PI states that metformin should not be used in women with an SCr > 1.4 mg/dL or in men with an SCr > 1.5 mg/dL.17 However, as noted, SCr concentration alone is a very poor indicator of kidney disease (see Table 4).
Currently, the KDIGO guidelines recommend assessing the GFR for metformin dosing. When the GFR is < 60 mL/min/1.73 m2, KDIGO recommends metformin be discontinued in patients with low muscle mass or serious concurrent illness, or those who are concurrently receiving renally eliminated or nephrotoxic drugs (this includes, but is not limited to, renin-angiotensin-aldosterone system [RAAS] inhibitors, NSAIDs, and diuretics).1 In those patients who do not have the exclusions delineated above, KDIGO recommends that metformin may be continued when the GFR is > 45 mL/min/1.73 m2 (stages G1 to G3a), closely monitored and reconsidered when the GFR is 30 to 44 mL/min/1.73 m2 (stage G3b), and discontinued when the GFR is < 30 mL/min/1.73 m2 (stages G4 to G5). The KDIGO differs from other sources that recommend metformin be stopped when the GFR is < 50 to 70 ml/min/1.73 m2.20-22 The metformin PI also recommends stopping metformin the day of or day before and two days after procedures in which radiographic iodinated contrast dye is administered, to avoid acute kidney failure. Finally, it is important to note that any combination medication formulated with metformin should be stopped when the GFR is 50 mL/min/1.73 m2 because lactic acidosis has also been seen in patients taking these medications.21
At stage 3a (GFR, 45 to 60 mL/min/1.73 m2), the sulfonylureas require dosing changes (see Table 2). Glipizide is often the oral medication of choice during CKD, and it may be used in all stages, including dialysis, but does require renal dosing and monitoring.23 Although the insulins are more effective than glipizide in glycemic control, many practitioners prescribe it for patients who will not accept an injectable medication. Glyburide is not used at this stage, as it can lead to complications in patients with a GFR < 60 mL/min/1.73 m2, particularly hypoglycemia.
Like the sulfonylureas, the meglitinides enhance insulin secretion.13,18 Repaglinide and nateglinide are fast-acting and need to be taken with meals. Because it is recommended that the dose of the meglitinides be omitted when a meal is skipped, they are good for patients who eat meals irregularly. The meglitinides require initiation at a low dose in severe renal insufficiency.
The thiazolidinediones (TDZs) are used with caution in patients with CKD, not only because of controversial adverse cardiac effects, but also for peripheral edema that interferes with CKD management.18 The TDZs (rosiglitazone and pioglitazone) require no dose adjustment and appear to have a beneficial effect in obese patients and in patients who experienced weight gain with other hypoglycemic agents.
The newest of the diabetic drugs, canagliflozin, requires a working kidney to be effective.24,25 It is used with precaution when GFR is 45 to 59 mL/min/1.73 m2 at stage G3a and is contraindicated when GFR is < 45 mL/min/1.73 m2 in stage G3b. Canagliflozin's effects are felt in early segment of proximal convoluted tubule where it blocks sodium-glucose co-transporter 2, thereby lowering reabsorption of glucose and increasing excretion of glucose in the urine. In practical terms, it can affect kidney function and, due to the inhibition of the glucose-sodium pathway, induce hyperkalemia.25 Monitoring of both the GFR and potassium is vital when administering this drug. As data from postmarketing reports come in and practitioners gain experience, use of canaglifozin in the patient with CKD will be further delineated.
At a GFR between 30 and 45 mL/min/1.73 m2, CKD stage 3b, alpha-glucosidase inhibitors acarbose and miglitol are contraindicated. The GLP-1 mimetic liraglutide needs to be avoided when the GFR is < 60 mL/min/1.73 m2 (stage G3a). While exenatide requires close monitoring and caution when the GFR is < 50 mL/min/1.73 m2, it needs to be discontinued at a GFR
< 30 mL/min/1.73 m2 (stage 4). DPP-4 inhibitors become problematic at a GFR < 60 mL/min/
1.73 m2 beginning at stage G3a. All except the DPP-4 inhibitor linagliptin require dosing changes as the loss of GFR continues. Exact dosing adjustments for the drug categories are found in Table 2.
In stage 4 CKD (GFR, 15 to 30 mL/min/1.73 m2), more medications are eliminated from consideration. The amylinomimetic pramlintide cannot be used for a patient with a GFR < 15 mL/min/1.73 m2 (see Table 2). Both GLP-1 mimetics, exenatide and liraglutide, must be stopped when the GFR drops to 30 mL/min/1.73 m2. DDP-4 inhibitors need to be dosed according to the GFR but are still acceptable at CKD stage 4. Ideally, patients with diabetes should be referred to nephrology at stage 3, but referral is vital by stage 4.
Although oral medications are not as commonly prescribed as insulins at CKD stages 4 and 5, in stage 5 CKD (GFR, < 15 mL/min/1.73 m2), a limited number of oral diabetic agents are available (see Table 2). Glipizide is inexpensive and generic; therefore, it is the most commonly used oral medication in this stage. A recent study found linagliptin was safe to use throughout all stages of kidney disease without adjustment for GFR.26 While nateglinide is still acceptable in stage 5, it is used less frequently than glipizide.
As the kidney fails, the need for diabetic medications also decreases and dosing needs to take into account any residual renal function. Because glucose is produced in the proximal tubules of the kidney (known as gluconeogenesis), the level of glucose falls as the kidney fails. Excretion of insulin and other diabetes medications decreases, and thus the amount of insulin remaining in circulation is increased. This, in combination with the reduced glucose, increases the patient’s risk for hypoglycemia.13 Although protocols for medication adjustment are tied to GFR levels, close follow-up by the practitioner is required because GFR is not a reliable measure of kidney function when creatinine levels change rapidly.27
On the next page: Insulins >>
INSULINS
Many patients start insulin prior to reaching CKD stage 4 or 5 because it is the best choice for managing diabetes through all stages of CKD.28 Insulin can slow the progression to kidney failure by providing better A1C control, and all patients should be encouraged to start insulin as soon as it is appropriate.29 Many patients are very reluctant to use injectables, but by stage 5 they are willing to use insulin if it will slow progression of disease and help prevent the need for dialysis.2
The long-acting basal insulins (detemir and glargine) or a combination of basal and oral medication can work quite well at stage 5. Many patients will require a low dose of the long-acting basal insulins and short-acting insulin with meals. As the kidney fails, the short-acting doses can often be discontinued.29 A protocol that mixes long- and short-acting insulin with the largest meal is very effective at this stage. The long-acting insulin dose will need to be decreased as the kidney continues to fail. The American College of Physicians protocol for insulin suggests a 25% decrease at stage 4 and a 50% decrease at stage 5. Each patient is different, however, and dosing must be determined individually.23
On the next page: Patient education >>
PATIENT EDUCATION
There is a correlation between patients who participate in kidney disease education programs and a decrease in the progression of kidney disease (see “Case Study: The Recalcitrant Patient With Diabetes"). These classes can be taught by a physician, physician assistant, nurse practitioner, or clinical nurse specialist. To alleviate the burden of end-stage kidney disease in the US, Medicare Part B pays for six outpatient kidney disease patient education classes per lifetime of the beneficiary.30
Since 2010, when kidney disease education programs were rolled out, over 10,000 classes have been taught.31 Many practitioners report that patients are more compliant and understand their disease better when they attend classes.32 Data are being gathered to determine the effectiveness of the classes on patient outcomes.
On the next page: Conclusion >>
CONCLUSION
Knowing how to manage the patient with both diabetes and kidney disease is increasingly vital as this patient population grows. Much of the management of these patients will fall to the primary care practitioner.6 The most effective way to start treatment is to identify which CKD stage the patient fits into.
Once the patient is properly categorized, safe and effective diabetic medications can be selected and dosed according to stage. Although the new classification system may be difficult to incorporate into some electronic medical record systems and practitioner behavior, ultimately, it will allow safer management of the patient with CKD and a better predictive power of outcome.
1. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International Suppl. 2013;3:1-150.
2. Brazie M. Finding the sweet spot: trouble-shooting diabetic dilemmas. Oral presentation at NKF Spring Clinical Meetings; May 2012, Washington, DC.
3. Estimated cost of diabetes care $245 billion in U.S. in 2012. Renal & Urology News. March 9, 2013. www.renalandurologynews.com/estimated-cost-of-diabetes-245-billion-in-us-in-2012/article/283616/. Accessed January 17, 2013.
4. Grams ME, Chow EK, Segev DL, Coresh J. Lifetime incidence of CKD stages 3-5 in the United States. Am J Kidney Dis. 2013;62(2):245-252.
5. Greenberg A, ed. National Kidney Foundation Primer of Kidney Diseases. 5th ed. Philadelphia, PA: Saunders Elsevier; 2009.
6. Spann SJ, Nutting PA, Galliher JM, et al. Management of type 2 diabetes in the primary care setting: a practice-based research study. Ann Fam Med. 2006;4(1):23-31.
7. Hsu CY, Chertow GM. Chronic renal confusion: insufficiency, failure, dysfunction, or disease. Am J Kidney Dis. 2000;49(3):482-496.
8. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;39(suppl 1):S1-S266.
9. Hudson JQ, Nyman HA. Use of the estimated glomerular filtration rate for drug dosing in the chronic kidney disease patient. Curr Opin Nephrol Hypertens. 2011;20:482-491.
10. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461-470.
11. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612.
12. NKF-KDOQI clinical practice guideline for diabetes and CKD, guideline 2: management of hyperglycemia and general diabetes care in CKD. Am J Kidney Dis. 2012;60(5):850-886. www.kidney.org/professionals/kdoqi/guideline_diabetes/guide2.htm. Accessed January 2, 2014.
13. Abe M, Okada K, Soma M. Antidiabetic agents in patients with chronic kidney disease and end-stage renal disease on dialysis: metabolism and clinical practice. Curr Drug Metab. 2011;12(1):57-69.
14. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381-389.
15. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405-411.
16. Perkovic V, Heerspink HL, Chalmers J, et al. Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int. 2013;83(3):517-23.
17. Metformin [package insert]. US Department of Health and Human Services, US Food and Drug Administration. www.fda.gov/ohrms/dockets/dailys/02/May02/053102/800471e6.pdf. Accessed January 17, 2013.
18. Triplitt CL, Reasner CA. Chapter 83. Diabetes Mellitus. In: Wells BG, ed. Pharmacotherapy: A Pathophysiologic Approach. 8th ed. New York, NY: McGraw-Hill; 2011.
19. FDA. Guidance for industry: pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulato ryInformation/Guidances/ucm072127.pdf. Accessed January 17, 2013.
20. Lacy CF, Armstrong LL, Goldman MP, Lance LL. Drug Information Handbook. 20th ed. Hudson, OH: Lexi-Comp, Inc.; 2011.
21. Rocha A, Almeida M, Santos J, Carvalho A. Metformin in patients with chronic kidney disease: strengths and weaknesses. J Nephrol. 2013; 26(10):55-60.
22. Gilbert SJ, Weiner DE, eds. National Kidney Foundation Primer on Kidney Diseases. 6th ed. Philadelphia, PA: Elsevier Saunders; 2014.
23. Aronoff GR, Bennett WM, Berns JS, et al. Drug Prescribing in Renal Failure: Dosing Guidelines for Adults and Children. 5th ed. Philadelphia, PA: American College of Physicians; 2007.
24. Valentine V. The role of the kidney and the sodium-glucose co-transporter inhibition in diabetes management. Clin Diabetes. 2012;30(4):151-155.
25. Invokana [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2013.
26. McGill JB, Sloan L, Newman J, et al. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment. Diabetes Care. 2013;36(2):237-244.
27. National Kidney Disease and Education Program (NKDEP). Estimated glomerular filtration rate (eGFR) info sheet. NIH publication No.10-6286, March 2010.
28. Thummel K, Shen D, Isoherranen N, et al. Design and optimization of dosage regimens: pharmacokinetic data. In: Hardman J, Limbird L, Goodman G (eds). Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill; 2006.
29. Snyder RW, Berns JS. Use of insulin and oral hypoglycemic medications in patients with diabetes mellitus and advanced kidney disease. Semin Dial. 2004;17(5):365-70.
30. Centers for Medicare and Medicaid Services. Medicare program: revisions to payment policies under the Physician Fee Schedule, Clinical Laboratory Fee Schedule & other revisions to Part B for CY 2014; Final Rule. Federal Register. 2009;74(226):61738-62188. www.gpo.gov/fdsys/pkg/FR-2009-11-25/html/E9-26502.htm. Accessed January 17, 2013.
31. Zuber K, Davis J. Kidney disease education: a niche for PAs and NPs. JAAPA. 2013;26(7):42-47.
32. Zuber K, Davis J. Stories from the trenches: the first year experience with kidney disease education. Nephrol News Issues. 2012;26(2):20-21.
1. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International Suppl. 2013;3:1-150.
2. Brazie M. Finding the sweet spot: trouble-shooting diabetic dilemmas. Oral presentation at NKF Spring Clinical Meetings; May 2012, Washington, DC.
3. Estimated cost of diabetes care $245 billion in U.S. in 2012. Renal & Urology News. March 9, 2013. www.renalandurologynews.com/estimated-cost-of-diabetes-245-billion-in-us-in-2012/article/283616/. Accessed January 17, 2013.
4. Grams ME, Chow EK, Segev DL, Coresh J. Lifetime incidence of CKD stages 3-5 in the United States. Am J Kidney Dis. 2013;62(2):245-252.
5. Greenberg A, ed. National Kidney Foundation Primer of Kidney Diseases. 5th ed. Philadelphia, PA: Saunders Elsevier; 2009.
6. Spann SJ, Nutting PA, Galliher JM, et al. Management of type 2 diabetes in the primary care setting: a practice-based research study. Ann Fam Med. 2006;4(1):23-31.
7. Hsu CY, Chertow GM. Chronic renal confusion: insufficiency, failure, dysfunction, or disease. Am J Kidney Dis. 2000;49(3):482-496.
8. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;39(suppl 1):S1-S266.
9. Hudson JQ, Nyman HA. Use of the estimated glomerular filtration rate for drug dosing in the chronic kidney disease patient. Curr Opin Nephrol Hypertens. 2011;20:482-491.
10. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461-470.
11. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612.
12. NKF-KDOQI clinical practice guideline for diabetes and CKD, guideline 2: management of hyperglycemia and general diabetes care in CKD. Am J Kidney Dis. 2012;60(5):850-886. www.kidney.org/professionals/kdoqi/guideline_diabetes/guide2.htm. Accessed January 2, 2014.
13. Abe M, Okada K, Soma M. Antidiabetic agents in patients with chronic kidney disease and end-stage renal disease on dialysis: metabolism and clinical practice. Curr Drug Metab. 2011;12(1):57-69.
14. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381-389.
15. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405-411.
16. Perkovic V, Heerspink HL, Chalmers J, et al. Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int. 2013;83(3):517-23.
17. Metformin [package insert]. US Department of Health and Human Services, US Food and Drug Administration. www.fda.gov/ohrms/dockets/dailys/02/May02/053102/800471e6.pdf. Accessed January 17, 2013.
18. Triplitt CL, Reasner CA. Chapter 83. Diabetes Mellitus. In: Wells BG, ed. Pharmacotherapy: A Pathophysiologic Approach. 8th ed. New York, NY: McGraw-Hill; 2011.
19. FDA. Guidance for industry: pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulato ryInformation/Guidances/ucm072127.pdf. Accessed January 17, 2013.
20. Lacy CF, Armstrong LL, Goldman MP, Lance LL. Drug Information Handbook. 20th ed. Hudson, OH: Lexi-Comp, Inc.; 2011.
21. Rocha A, Almeida M, Santos J, Carvalho A. Metformin in patients with chronic kidney disease: strengths and weaknesses. J Nephrol. 2013; 26(10):55-60.
22. Gilbert SJ, Weiner DE, eds. National Kidney Foundation Primer on Kidney Diseases. 6th ed. Philadelphia, PA: Elsevier Saunders; 2014.
23. Aronoff GR, Bennett WM, Berns JS, et al. Drug Prescribing in Renal Failure: Dosing Guidelines for Adults and Children. 5th ed. Philadelphia, PA: American College of Physicians; 2007.
24. Valentine V. The role of the kidney and the sodium-glucose co-transporter inhibition in diabetes management. Clin Diabetes. 2012;30(4):151-155.
25. Invokana [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2013.
26. McGill JB, Sloan L, Newman J, et al. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment. Diabetes Care. 2013;36(2):237-244.
27. National Kidney Disease and Education Program (NKDEP). Estimated glomerular filtration rate (eGFR) info sheet. NIH publication No.10-6286, March 2010.
28. Thummel K, Shen D, Isoherranen N, et al. Design and optimization of dosage regimens: pharmacokinetic data. In: Hardman J, Limbird L, Goodman G (eds). Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill; 2006.
29. Snyder RW, Berns JS. Use of insulin and oral hypoglycemic medications in patients with diabetes mellitus and advanced kidney disease. Semin Dial. 2004;17(5):365-70.
30. Centers for Medicare and Medicaid Services. Medicare program: revisions to payment policies under the Physician Fee Schedule, Clinical Laboratory Fee Schedule & other revisions to Part B for CY 2014; Final Rule. Federal Register. 2009;74(226):61738-62188. www.gpo.gov/fdsys/pkg/FR-2009-11-25/html/E9-26502.htm. Accessed January 17, 2013.
31. Zuber K, Davis J. Kidney disease education: a niche for PAs and NPs. JAAPA. 2013;26(7):42-47.
32. Zuber K, Davis J. Stories from the trenches: the first year experience with kidney disease education. Nephrol News Issues. 2012;26(2):20-21.
Should patients with gout avoid thiazides for hypertension?
The decision should be individualized, taking into consideration the degree to which the thiazide increases the serum urate level, whether this increase can be managed without overly complicating the patient’s hypouricemic therapy, and, most importantly, what effect switching to another drug will have on the control of the patient’s hypertension. No study has directly addressed this issue.
My practice in most patients, for reasons I explain below, is to use a thiazide if it helps to control the blood pressure and to adjust the dose of the hypouricemic therapy as needed to reduce the serum urate to the desired level.
THIAZIDES REMAIN IMPORTANT IN ANTIHYPERTENSIVE THERAPY
Many patients with gout also have hypertension, perhaps due in part to the same hyperuricemia that caused their gouty arthritis. It is well documented that thiazide diuretics can raise the serum urate level.1 In some studies2 (but not all3), patients using thiazides had a higher incidence of gouty arthritis. Thus, it is reasonable to ask if we should avoid thiazides in patients with coexistent gout and hypertension.
Many hypertensive patients fail to reach their target blood pressures (although with the “looser” recommendations in the latest guidelines,4 we may appear to be doing a better job). The reasons for failing to reach target pressures are complex and many: physicians may simply not be aggressive enough in pursuing a target blood pressure; patients cannot tolerate the drugs or cannot afford the drugs; and many patients need two or more antihypertensive drugs to achieve adequate control. Thiazides are cheap and effective5 and work synergistically with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers.6
Thus, in many patients, avoiding or discontinuing a thiazide may inhibit our ability to control their hypertension, which is a key contributor to cardiovascular events and chronic kidney injury in patients with gout. Since other diuretics (eg, loop diuretics, which can lower blood pressure but often require split doses) also raise the serum urate level, switching to one of them will not eliminate concern over hyperuricemia.
Thiazides and serum urate
Thiazides slightly increase the serum urate level and in a dose-dependent manner. At the doses commonly used in treating hypertension (12.5 or 25 mg once a day), hydrochlorothiazide increases the serum urate level by 0.8 mg/dL or less in patients with normal renal function, as shown in a number of older hypertension treatment trials and in a recent prospective study.1 The effect of chlorthalidone is similar.
In patients with chronic gout treated with a xanthine oxidase inhibitor (allopurinol or febuxostat) to lower the serum urate to the American College of Rheumatology’s recommended target level7 of less than 6.0 mg/dL (or < 5 mg/dL in the British Rheumatology guidelines), this small elevation in serum urate is unlikely to negate the clinical efficacy of these drugs when dosing is optimized. Small studies have demonstrated a clinically insignificant pharmacodynamic interaction between thiazides and xanthine oxidase inhibitors.8,9 When I add a thiazide to a patient’s regimen, I do not usually need to increase the dose of allopurinol significantly to keep the serum urate level below the desired target.
Switch antihypertensive therapy
Occasionally, in a patient with chronic gout and mild hypertension who has a serum urate level marginally above the estimated precipitation threshold of 6.7 mg/dL, it is reasonable to simply switch the thiazide to another antihypertensive, such as losartan. Losartan is a weak uricosuric and can lower the serum urate level slightly, possibly making the addition of another hypouricemic agent unnecessary, while still controlling the blood pressure with a single pill. This decision must be individualized, taking into consideration the efficacy and cost of the alternative antihypertensive drug, as well as the potential but as yet unproven cardiovascular and renal benefits of lowering the serum urate with a more potent hypouricemic to a degree not likely to be attained with losartan alone.
Continue thiazide, adjust gout therapy
Discontinuing a thiazide or switching to another antihypertensive drug may increase the cost and decrease the efficacy of hypertensive therapy. Continuing thiazide therapy and, if necessary, adjusting hypouricemic therapy will not worsen the control of the serum urate level or gouty arthritis, and in most patients will not complicate the management of gout.
ASPIRIN AND HYPERURICEMIA
In answer to a separate but related question, aspirin in low doses for cardioprotection (81 mg daily) also need not be stopped in patients with hyperuricemia or gout in an effort to better control the serum urate level. Low-dose aspirin increases the serum urate level by about 0.3 mg/dL. Since patients with gout have a higher risk of having cardiovascular disease, metabolic syndrome, and chronic kidney disease, many will benefit from low-dose aspirin therapy.
- McAdams DeMarco MA, Maynard JW, Baer AN, et al. Diuretic use, increased serum urate levels, and risk of incident gout in a population-based study of adults with hypertension: the Atherosclerosis Risk in Communities cohort study. Arthritis Rheum 2012; 64:121–129.
- Choi HK, Soriano LC, Zhang Y, Rodríguez LA. Antihypertensive drugs and risk of incident gout among patients with hypertension: population based case-control study. BMJ 2012; 344:d8190.
- Hueskes BA, Roovers EA, Mantel-Teeuwisse AK, Janssens HJ, van de Lisdonk EH, Janssen M. Use of diuretics and the risk of gouty arthritis: a systematic review. Semin Arthritis Rheum 2012; 41:879–889.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2013; doi: 10.1001/jama2013.284427
- Fuchs FD. Diuretics: still essential drugs for the management of hypertension. Expert Rev Cardiovasc Ther 2009; 7:591–598.
- Sood N, Reinhart KM, Baker WL. Combination therapy for the management of hypertension: a review of the evidence. Am J Health Syst Pharm 2010; 67:885–894.
- Khanna D, Fitzgerald JD, Khanna PP, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 2012; 64:1431–1446.
- Löffler W, Landthaler R, de Vries JX, et al. Interaction of allopurinol and hydrochlorothiazide during prolonged oral administration of both drugs in normal subjects. I. Uric acid kinetics. Clin Investig 1994; 72:1071–1075.
- Grabowski B, Khosravan R, Wu JT, Vernillet L, Lademacher C. Effect of hydrochlorothiazide on the pharmacokinetics and pharmacodynamics of febuxostat, a non-purine selective inhibitor of xanthine oxidase. Br J Clin Pharmacol 2010; 70:57–64.
The decision should be individualized, taking into consideration the degree to which the thiazide increases the serum urate level, whether this increase can be managed without overly complicating the patient’s hypouricemic therapy, and, most importantly, what effect switching to another drug will have on the control of the patient’s hypertension. No study has directly addressed this issue.
My practice in most patients, for reasons I explain below, is to use a thiazide if it helps to control the blood pressure and to adjust the dose of the hypouricemic therapy as needed to reduce the serum urate to the desired level.
THIAZIDES REMAIN IMPORTANT IN ANTIHYPERTENSIVE THERAPY
Many patients with gout also have hypertension, perhaps due in part to the same hyperuricemia that caused their gouty arthritis. It is well documented that thiazide diuretics can raise the serum urate level.1 In some studies2 (but not all3), patients using thiazides had a higher incidence of gouty arthritis. Thus, it is reasonable to ask if we should avoid thiazides in patients with coexistent gout and hypertension.
Many hypertensive patients fail to reach their target blood pressures (although with the “looser” recommendations in the latest guidelines,4 we may appear to be doing a better job). The reasons for failing to reach target pressures are complex and many: physicians may simply not be aggressive enough in pursuing a target blood pressure; patients cannot tolerate the drugs or cannot afford the drugs; and many patients need two or more antihypertensive drugs to achieve adequate control. Thiazides are cheap and effective5 and work synergistically with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers.6
Thus, in many patients, avoiding or discontinuing a thiazide may inhibit our ability to control their hypertension, which is a key contributor to cardiovascular events and chronic kidney injury in patients with gout. Since other diuretics (eg, loop diuretics, which can lower blood pressure but often require split doses) also raise the serum urate level, switching to one of them will not eliminate concern over hyperuricemia.
Thiazides and serum urate
Thiazides slightly increase the serum urate level and in a dose-dependent manner. At the doses commonly used in treating hypertension (12.5 or 25 mg once a day), hydrochlorothiazide increases the serum urate level by 0.8 mg/dL or less in patients with normal renal function, as shown in a number of older hypertension treatment trials and in a recent prospective study.1 The effect of chlorthalidone is similar.
In patients with chronic gout treated with a xanthine oxidase inhibitor (allopurinol or febuxostat) to lower the serum urate to the American College of Rheumatology’s recommended target level7 of less than 6.0 mg/dL (or < 5 mg/dL in the British Rheumatology guidelines), this small elevation in serum urate is unlikely to negate the clinical efficacy of these drugs when dosing is optimized. Small studies have demonstrated a clinically insignificant pharmacodynamic interaction between thiazides and xanthine oxidase inhibitors.8,9 When I add a thiazide to a patient’s regimen, I do not usually need to increase the dose of allopurinol significantly to keep the serum urate level below the desired target.
Switch antihypertensive therapy
Occasionally, in a patient with chronic gout and mild hypertension who has a serum urate level marginally above the estimated precipitation threshold of 6.7 mg/dL, it is reasonable to simply switch the thiazide to another antihypertensive, such as losartan. Losartan is a weak uricosuric and can lower the serum urate level slightly, possibly making the addition of another hypouricemic agent unnecessary, while still controlling the blood pressure with a single pill. This decision must be individualized, taking into consideration the efficacy and cost of the alternative antihypertensive drug, as well as the potential but as yet unproven cardiovascular and renal benefits of lowering the serum urate with a more potent hypouricemic to a degree not likely to be attained with losartan alone.
Continue thiazide, adjust gout therapy
Discontinuing a thiazide or switching to another antihypertensive drug may increase the cost and decrease the efficacy of hypertensive therapy. Continuing thiazide therapy and, if necessary, adjusting hypouricemic therapy will not worsen the control of the serum urate level or gouty arthritis, and in most patients will not complicate the management of gout.
ASPIRIN AND HYPERURICEMIA
In answer to a separate but related question, aspirin in low doses for cardioprotection (81 mg daily) also need not be stopped in patients with hyperuricemia or gout in an effort to better control the serum urate level. Low-dose aspirin increases the serum urate level by about 0.3 mg/dL. Since patients with gout have a higher risk of having cardiovascular disease, metabolic syndrome, and chronic kidney disease, many will benefit from low-dose aspirin therapy.
The decision should be individualized, taking into consideration the degree to which the thiazide increases the serum urate level, whether this increase can be managed without overly complicating the patient’s hypouricemic therapy, and, most importantly, what effect switching to another drug will have on the control of the patient’s hypertension. No study has directly addressed this issue.
My practice in most patients, for reasons I explain below, is to use a thiazide if it helps to control the blood pressure and to adjust the dose of the hypouricemic therapy as needed to reduce the serum urate to the desired level.
THIAZIDES REMAIN IMPORTANT IN ANTIHYPERTENSIVE THERAPY
Many patients with gout also have hypertension, perhaps due in part to the same hyperuricemia that caused their gouty arthritis. It is well documented that thiazide diuretics can raise the serum urate level.1 In some studies2 (but not all3), patients using thiazides had a higher incidence of gouty arthritis. Thus, it is reasonable to ask if we should avoid thiazides in patients with coexistent gout and hypertension.
Many hypertensive patients fail to reach their target blood pressures (although with the “looser” recommendations in the latest guidelines,4 we may appear to be doing a better job). The reasons for failing to reach target pressures are complex and many: physicians may simply not be aggressive enough in pursuing a target blood pressure; patients cannot tolerate the drugs or cannot afford the drugs; and many patients need two or more antihypertensive drugs to achieve adequate control. Thiazides are cheap and effective5 and work synergistically with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers.6
Thus, in many patients, avoiding or discontinuing a thiazide may inhibit our ability to control their hypertension, which is a key contributor to cardiovascular events and chronic kidney injury in patients with gout. Since other diuretics (eg, loop diuretics, which can lower blood pressure but often require split doses) also raise the serum urate level, switching to one of them will not eliminate concern over hyperuricemia.
Thiazides and serum urate
Thiazides slightly increase the serum urate level and in a dose-dependent manner. At the doses commonly used in treating hypertension (12.5 or 25 mg once a day), hydrochlorothiazide increases the serum urate level by 0.8 mg/dL or less in patients with normal renal function, as shown in a number of older hypertension treatment trials and in a recent prospective study.1 The effect of chlorthalidone is similar.
In patients with chronic gout treated with a xanthine oxidase inhibitor (allopurinol or febuxostat) to lower the serum urate to the American College of Rheumatology’s recommended target level7 of less than 6.0 mg/dL (or < 5 mg/dL in the British Rheumatology guidelines), this small elevation in serum urate is unlikely to negate the clinical efficacy of these drugs when dosing is optimized. Small studies have demonstrated a clinically insignificant pharmacodynamic interaction between thiazides and xanthine oxidase inhibitors.8,9 When I add a thiazide to a patient’s regimen, I do not usually need to increase the dose of allopurinol significantly to keep the serum urate level below the desired target.
Switch antihypertensive therapy
Occasionally, in a patient with chronic gout and mild hypertension who has a serum urate level marginally above the estimated precipitation threshold of 6.7 mg/dL, it is reasonable to simply switch the thiazide to another antihypertensive, such as losartan. Losartan is a weak uricosuric and can lower the serum urate level slightly, possibly making the addition of another hypouricemic agent unnecessary, while still controlling the blood pressure with a single pill. This decision must be individualized, taking into consideration the efficacy and cost of the alternative antihypertensive drug, as well as the potential but as yet unproven cardiovascular and renal benefits of lowering the serum urate with a more potent hypouricemic to a degree not likely to be attained with losartan alone.
Continue thiazide, adjust gout therapy
Discontinuing a thiazide or switching to another antihypertensive drug may increase the cost and decrease the efficacy of hypertensive therapy. Continuing thiazide therapy and, if necessary, adjusting hypouricemic therapy will not worsen the control of the serum urate level or gouty arthritis, and in most patients will not complicate the management of gout.
ASPIRIN AND HYPERURICEMIA
In answer to a separate but related question, aspirin in low doses for cardioprotection (81 mg daily) also need not be stopped in patients with hyperuricemia or gout in an effort to better control the serum urate level. Low-dose aspirin increases the serum urate level by about 0.3 mg/dL. Since patients with gout have a higher risk of having cardiovascular disease, metabolic syndrome, and chronic kidney disease, many will benefit from low-dose aspirin therapy.
- McAdams DeMarco MA, Maynard JW, Baer AN, et al. Diuretic use, increased serum urate levels, and risk of incident gout in a population-based study of adults with hypertension: the Atherosclerosis Risk in Communities cohort study. Arthritis Rheum 2012; 64:121–129.
- Choi HK, Soriano LC, Zhang Y, Rodríguez LA. Antihypertensive drugs and risk of incident gout among patients with hypertension: population based case-control study. BMJ 2012; 344:d8190.
- Hueskes BA, Roovers EA, Mantel-Teeuwisse AK, Janssens HJ, van de Lisdonk EH, Janssen M. Use of diuretics and the risk of gouty arthritis: a systematic review. Semin Arthritis Rheum 2012; 41:879–889.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2013; doi: 10.1001/jama2013.284427
- Fuchs FD. Diuretics: still essential drugs for the management of hypertension. Expert Rev Cardiovasc Ther 2009; 7:591–598.
- Sood N, Reinhart KM, Baker WL. Combination therapy for the management of hypertension: a review of the evidence. Am J Health Syst Pharm 2010; 67:885–894.
- Khanna D, Fitzgerald JD, Khanna PP, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 2012; 64:1431–1446.
- Löffler W, Landthaler R, de Vries JX, et al. Interaction of allopurinol and hydrochlorothiazide during prolonged oral administration of both drugs in normal subjects. I. Uric acid kinetics. Clin Investig 1994; 72:1071–1075.
- Grabowski B, Khosravan R, Wu JT, Vernillet L, Lademacher C. Effect of hydrochlorothiazide on the pharmacokinetics and pharmacodynamics of febuxostat, a non-purine selective inhibitor of xanthine oxidase. Br J Clin Pharmacol 2010; 70:57–64.
- McAdams DeMarco MA, Maynard JW, Baer AN, et al. Diuretic use, increased serum urate levels, and risk of incident gout in a population-based study of adults with hypertension: the Atherosclerosis Risk in Communities cohort study. Arthritis Rheum 2012; 64:121–129.
- Choi HK, Soriano LC, Zhang Y, Rodríguez LA. Antihypertensive drugs and risk of incident gout among patients with hypertension: population based case-control study. BMJ 2012; 344:d8190.
- Hueskes BA, Roovers EA, Mantel-Teeuwisse AK, Janssens HJ, van de Lisdonk EH, Janssen M. Use of diuretics and the risk of gouty arthritis: a systematic review. Semin Arthritis Rheum 2012; 41:879–889.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2013; doi: 10.1001/jama2013.284427
- Fuchs FD. Diuretics: still essential drugs for the management of hypertension. Expert Rev Cardiovasc Ther 2009; 7:591–598.
- Sood N, Reinhart KM, Baker WL. Combination therapy for the management of hypertension: a review of the evidence. Am J Health Syst Pharm 2010; 67:885–894.
- Khanna D, Fitzgerald JD, Khanna PP, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 2012; 64:1431–1446.
- Löffler W, Landthaler R, de Vries JX, et al. Interaction of allopurinol and hydrochlorothiazide during prolonged oral administration of both drugs in normal subjects. I. Uric acid kinetics. Clin Investig 1994; 72:1071–1075.
- Grabowski B, Khosravan R, Wu JT, Vernillet L, Lademacher C. Effect of hydrochlorothiazide on the pharmacokinetics and pharmacodynamics of febuxostat, a non-purine selective inhibitor of xanthine oxidase. Br J Clin Pharmacol 2010; 70:57–64.
Better prostate cancer screening approach needed
SAN DIEGO – Over the past 20 years, death rates from prostate cancer in the United States have declined by 39%, due largely to early detection and/or improved treatment, according to Dr. Peter R. Carroll.
"It’s important to realize that this accounts for 20% of the decrease in cancer-specific deaths in men," said Dr. Carroll, professor and chair of the department of urology at the University of California, San Francisco. "However, the Achilles’ heel of PSA [prostate-specific antigen] testing is that it does so at the risk of overdetection – detecting disease that would not have become clinically apparent over a patient’s lifetime if left untreated. In this country, detection and treatment are too tightly linked."
In addition, widespread use of serum PSA has resulted in a "dramatic stage and grade shift, with most cancers currently being detected of limited cancer grade and stage," he said. Data from his colleagues at UCSF found that between 1990 and 2012, age-adjusted death rates from prostate cancer decreased 3.96% in North America yet increased 41% worldwide. In the United States, men with nonpalpable PSA-driven cancer comprise the largest segment of prostate cancer patients. "We think that we’ve seen a leveling of overdetection, but I think we’ll see another round of overdetection, because of a lowering PSA threshold to prompt biopsy, aggressive rescreening, the use of PSA velocity at low PSA values to prompt biopsy, and the use of saturation biopsies," he said during a conference sponsored by the American Association for Cancer Research and the Prostate Cancer Foundation.
In 2012 the U.S. Preventive Services Task Force came out against prostate cancer screening, classifying it as a grade D recommendation (Ann. Intern. Med. 2012;157[2]:120-34). The magnitude of overdetection varies with time period, age, comorbidities, region, definition, and screening practices and is thought to range between 2% and 67%, Dr. Carroll said. "I think a good number is somewhere between 35% and 40%." A recently published nomogram for predicting overdiagnosis found that depending on a man’s age, Gleason score, and PSA level, the likelihood that his tumor has been overdiagnosed ranges from 2.9% to 88.1% (J. Natl. Cancer Inst. 2014 [doi:10.1093/jnci.djt367]).
If prostate cancer screening is to be undertaken, "it should only be done recognizing that selective, rather than indiscriminate, treatment should follow," Dr. Carroll said. "Such an approach has been shown to reduce mortality while managing many with active surveillance in lieu of immediate treatment" (Lancet Oncol. 2010;11[8]:725-32). At UCSF, where more than 1,000 men are on active surveillance, the 5-year treatment-free survival is 65%, the 5-year overall survival is 97%, and the 5-year prostate cancer–specific survival is 100%. "The window of opportunity for treatment appears to be open for a long period of time," he said.
Potential solutions Dr. Carroll proposed to decrease the rates of overdetection include:
• Reducing the treatment of low-risk tumors.
• Identifying high-risk populations and targeting prevention and screening efforts to those populations.
• Developing new screening markers.
• Developing clinical and patient tools to support informed decision making about prevention, screening, biopsy, and treatment.
• Changing screening guidelines.
"The single biggest predictor of risk is a baseline PSA. It trumps ethnicity and family history," Dr. Carroll said. "I think there’s a strong rationale for a baseline screening between ages 45 and 55. If you screen beyond age 70, you increase the risk of overdetection. But if you stop screening you also increase the mortality. So beyond age 70 you want to individualize, consider screening only in those with a long life expectancy, and perhaps change the rationale for biopsy. Digital rectal examination in my mind is optional as a primary screening maneuver. Screening can be done at 1- to 2-year intervals. One thing we need to get away from is using PSA velocity at low PSA levels. That drives overdetection quite a bit."
Dr. Carroll disclosed that he has received honoraria, research support, and/or consulting fees from Genomic Health, Intuitive, Janssen, and Myriad.
SAN DIEGO – Over the past 20 years, death rates from prostate cancer in the United States have declined by 39%, due largely to early detection and/or improved treatment, according to Dr. Peter R. Carroll.
"It’s important to realize that this accounts for 20% of the decrease in cancer-specific deaths in men," said Dr. Carroll, professor and chair of the department of urology at the University of California, San Francisco. "However, the Achilles’ heel of PSA [prostate-specific antigen] testing is that it does so at the risk of overdetection – detecting disease that would not have become clinically apparent over a patient’s lifetime if left untreated. In this country, detection and treatment are too tightly linked."
In addition, widespread use of serum PSA has resulted in a "dramatic stage and grade shift, with most cancers currently being detected of limited cancer grade and stage," he said. Data from his colleagues at UCSF found that between 1990 and 2012, age-adjusted death rates from prostate cancer decreased 3.96% in North America yet increased 41% worldwide. In the United States, men with nonpalpable PSA-driven cancer comprise the largest segment of prostate cancer patients. "We think that we’ve seen a leveling of overdetection, but I think we’ll see another round of overdetection, because of a lowering PSA threshold to prompt biopsy, aggressive rescreening, the use of PSA velocity at low PSA values to prompt biopsy, and the use of saturation biopsies," he said during a conference sponsored by the American Association for Cancer Research and the Prostate Cancer Foundation.
In 2012 the U.S. Preventive Services Task Force came out against prostate cancer screening, classifying it as a grade D recommendation (Ann. Intern. Med. 2012;157[2]:120-34). The magnitude of overdetection varies with time period, age, comorbidities, region, definition, and screening practices and is thought to range between 2% and 67%, Dr. Carroll said. "I think a good number is somewhere between 35% and 40%." A recently published nomogram for predicting overdiagnosis found that depending on a man’s age, Gleason score, and PSA level, the likelihood that his tumor has been overdiagnosed ranges from 2.9% to 88.1% (J. Natl. Cancer Inst. 2014 [doi:10.1093/jnci.djt367]).
If prostate cancer screening is to be undertaken, "it should only be done recognizing that selective, rather than indiscriminate, treatment should follow," Dr. Carroll said. "Such an approach has been shown to reduce mortality while managing many with active surveillance in lieu of immediate treatment" (Lancet Oncol. 2010;11[8]:725-32). At UCSF, where more than 1,000 men are on active surveillance, the 5-year treatment-free survival is 65%, the 5-year overall survival is 97%, and the 5-year prostate cancer–specific survival is 100%. "The window of opportunity for treatment appears to be open for a long period of time," he said.
Potential solutions Dr. Carroll proposed to decrease the rates of overdetection include:
• Reducing the treatment of low-risk tumors.
• Identifying high-risk populations and targeting prevention and screening efforts to those populations.
• Developing new screening markers.
• Developing clinical and patient tools to support informed decision making about prevention, screening, biopsy, and treatment.
• Changing screening guidelines.
"The single biggest predictor of risk is a baseline PSA. It trumps ethnicity and family history," Dr. Carroll said. "I think there’s a strong rationale for a baseline screening between ages 45 and 55. If you screen beyond age 70, you increase the risk of overdetection. But if you stop screening you also increase the mortality. So beyond age 70 you want to individualize, consider screening only in those with a long life expectancy, and perhaps change the rationale for biopsy. Digital rectal examination in my mind is optional as a primary screening maneuver. Screening can be done at 1- to 2-year intervals. One thing we need to get away from is using PSA velocity at low PSA levels. That drives overdetection quite a bit."
Dr. Carroll disclosed that he has received honoraria, research support, and/or consulting fees from Genomic Health, Intuitive, Janssen, and Myriad.
SAN DIEGO – Over the past 20 years, death rates from prostate cancer in the United States have declined by 39%, due largely to early detection and/or improved treatment, according to Dr. Peter R. Carroll.
"It’s important to realize that this accounts for 20% of the decrease in cancer-specific deaths in men," said Dr. Carroll, professor and chair of the department of urology at the University of California, San Francisco. "However, the Achilles’ heel of PSA [prostate-specific antigen] testing is that it does so at the risk of overdetection – detecting disease that would not have become clinically apparent over a patient’s lifetime if left untreated. In this country, detection and treatment are too tightly linked."
In addition, widespread use of serum PSA has resulted in a "dramatic stage and grade shift, with most cancers currently being detected of limited cancer grade and stage," he said. Data from his colleagues at UCSF found that between 1990 and 2012, age-adjusted death rates from prostate cancer decreased 3.96% in North America yet increased 41% worldwide. In the United States, men with nonpalpable PSA-driven cancer comprise the largest segment of prostate cancer patients. "We think that we’ve seen a leveling of overdetection, but I think we’ll see another round of overdetection, because of a lowering PSA threshold to prompt biopsy, aggressive rescreening, the use of PSA velocity at low PSA values to prompt biopsy, and the use of saturation biopsies," he said during a conference sponsored by the American Association for Cancer Research and the Prostate Cancer Foundation.
In 2012 the U.S. Preventive Services Task Force came out against prostate cancer screening, classifying it as a grade D recommendation (Ann. Intern. Med. 2012;157[2]:120-34). The magnitude of overdetection varies with time period, age, comorbidities, region, definition, and screening practices and is thought to range between 2% and 67%, Dr. Carroll said. "I think a good number is somewhere between 35% and 40%." A recently published nomogram for predicting overdiagnosis found that depending on a man’s age, Gleason score, and PSA level, the likelihood that his tumor has been overdiagnosed ranges from 2.9% to 88.1% (J. Natl. Cancer Inst. 2014 [doi:10.1093/jnci.djt367]).
If prostate cancer screening is to be undertaken, "it should only be done recognizing that selective, rather than indiscriminate, treatment should follow," Dr. Carroll said. "Such an approach has been shown to reduce mortality while managing many with active surveillance in lieu of immediate treatment" (Lancet Oncol. 2010;11[8]:725-32). At UCSF, where more than 1,000 men are on active surveillance, the 5-year treatment-free survival is 65%, the 5-year overall survival is 97%, and the 5-year prostate cancer–specific survival is 100%. "The window of opportunity for treatment appears to be open for a long period of time," he said.
Potential solutions Dr. Carroll proposed to decrease the rates of overdetection include:
• Reducing the treatment of low-risk tumors.
• Identifying high-risk populations and targeting prevention and screening efforts to those populations.
• Developing new screening markers.
• Developing clinical and patient tools to support informed decision making about prevention, screening, biopsy, and treatment.
• Changing screening guidelines.
"The single biggest predictor of risk is a baseline PSA. It trumps ethnicity and family history," Dr. Carroll said. "I think there’s a strong rationale for a baseline screening between ages 45 and 55. If you screen beyond age 70, you increase the risk of overdetection. But if you stop screening you also increase the mortality. So beyond age 70 you want to individualize, consider screening only in those with a long life expectancy, and perhaps change the rationale for biopsy. Digital rectal examination in my mind is optional as a primary screening maneuver. Screening can be done at 1- to 2-year intervals. One thing we need to get away from is using PSA velocity at low PSA levels. That drives overdetection quite a bit."
Dr. Carroll disclosed that he has received honoraria, research support, and/or consulting fees from Genomic Health, Intuitive, Janssen, and Myriad.
EXPERT ANALYSIS FROM ADVANCES IN PROSTATE CANCER RESEARCH
Possible target in prostate cancer prevention research: 5-alpha reductase
SAN DIEGO – Now is the time for researchers and clinicians to examine strategies and interventions for preventing prostate cancer, in the opinion of Dr. Peter H. Gann.
"In the last 12 years or so we have seen a litany of failure with regard to prostate cancer prevention in trials with clinical endpoints," Dr. Gann, professor and director of pathology research at the University of Illinois at Chicago, said during a conference sponsored by the American Association for Cancer Research and the Prostate Cancer Foundation.
"With vitamin E supplementation, for example, we see a possible increased risk of prostate cancer, no effect with selenium and possibly and increased risk of diabetes, no effect from soy, and the potential impact of green tea polyphenols is unresolved."
Moreover, it’s conceivable that you might need to screen 1,500 average-risk patients to prevent one prostate cancer from occurring. "That means that preventive agents don’t have to be safe; they have to be incredibly safe if they’re going to be used in this way," Dr. Gann said.
He proposed five ways to advance prostate cancer prevention efforts:
• Develop better preclinical models. Canadian investigators have reported success with generating high fidelity patient-derived xenografts for accelerating prostate cancer discovery and drug development (Cancer Res. 2013 Dec. 19 [doi:10.1158/0008-5472.CAN-13-2921-T]). "One of the incredible things about this is that the transplantable cells can be obtained from needle biopsies," said Dr. Gann, who was not involved with the study. "Architecture and protein marker expression is preserved in these xenografts, as are other molecular characteristics of the tumor, which is fantastic."
An unrelated Australian study demonstrated that it’s possible to generate xenografts of the earlier low-to-moderate grade, localized tumors (Nat. Protoc. 2013;8:836-48). "Why is this exciting for us in prevention? More and more we’re thinking about not how to prevent things from the initiation stage but rather in terms of progression," Dr. Gann explained. "Being able to have individualized samples that we can study versus agents that may inhibit progression is a major opportunity, I think."
• Improve clinical trial design and infrastructure. The existing networks for prostate cancer prevention trials are largely undeveloped, unlike cooperative group networks available for therapeutic trials, according to Dr. Gann. "Many investigators with promising ideas do not have access to the clinical infrastructure or funding sources they need for translational research," he noted. "Moreover, the pros and cons of various available designs for phase II and III trials have not been adequately debated, amidst a shifting landscape in which some designs become less feasible as others become more feasible."
• Develop better risk stratification and patient targeting. What if clinicians could do a better job of sorting out who is truly at risk for prostate cancer? "Active surveillance cohorts are the most promising opportunity we have for prevention trials involving low-risk interventions," Dr. Gann said. Cumulative results from genome-wide association studies and the expected results from sequencing studies capable of identifying rare genetic variants with high penetrance hold promise for identifying populations for whom preventive strategies would have the most benefit, he added.
• Develop better interventional agents. To date, "I think we’ve taken a haphazard approach to identifying agents for prostate cancer prevention," Dr. Gann said. "With preclinical models lacking, sometimes they’re not vetted very well, either. We’re not going to be able to develop a rational approach to preventing prostate cancer until we have a better idea of how it all comes about. One idea is to do high throughput cell assay based drug screening, which we do for therapeutics but not for chemopreventive agents. The question is, do we have the right libraries of compounds and do we have the right readouts? I suggest that we can start with compounds, especially dietary agents that could inhibit 5-alpha reductase. We also need to think beyond a pharmacologic approach: studying the effects of diet and physical activity, for example."
• Establish better intermediate endpoints for phase II trials. A lack of intermediate endpoint biomarkers (IEBs) for phase II trials is creating a "phase II bottleneck," he said. "There is an ongoing explosion of opportunities provided by new biotechnology and computational methods, but converting these opportunities into validated IEBs for trials will take thought and planning."
Dr. Gann disclosed that he has received grant support from GlaxoSmithKline.
SAN DIEGO – Now is the time for researchers and clinicians to examine strategies and interventions for preventing prostate cancer, in the opinion of Dr. Peter H. Gann.
"In the last 12 years or so we have seen a litany of failure with regard to prostate cancer prevention in trials with clinical endpoints," Dr. Gann, professor and director of pathology research at the University of Illinois at Chicago, said during a conference sponsored by the American Association for Cancer Research and the Prostate Cancer Foundation.
"With vitamin E supplementation, for example, we see a possible increased risk of prostate cancer, no effect with selenium and possibly and increased risk of diabetes, no effect from soy, and the potential impact of green tea polyphenols is unresolved."
Moreover, it’s conceivable that you might need to screen 1,500 average-risk patients to prevent one prostate cancer from occurring. "That means that preventive agents don’t have to be safe; they have to be incredibly safe if they’re going to be used in this way," Dr. Gann said.
He proposed five ways to advance prostate cancer prevention efforts:
• Develop better preclinical models. Canadian investigators have reported success with generating high fidelity patient-derived xenografts for accelerating prostate cancer discovery and drug development (Cancer Res. 2013 Dec. 19 [doi:10.1158/0008-5472.CAN-13-2921-T]). "One of the incredible things about this is that the transplantable cells can be obtained from needle biopsies," said Dr. Gann, who was not involved with the study. "Architecture and protein marker expression is preserved in these xenografts, as are other molecular characteristics of the tumor, which is fantastic."
An unrelated Australian study demonstrated that it’s possible to generate xenografts of the earlier low-to-moderate grade, localized tumors (Nat. Protoc. 2013;8:836-48). "Why is this exciting for us in prevention? More and more we’re thinking about not how to prevent things from the initiation stage but rather in terms of progression," Dr. Gann explained. "Being able to have individualized samples that we can study versus agents that may inhibit progression is a major opportunity, I think."
• Improve clinical trial design and infrastructure. The existing networks for prostate cancer prevention trials are largely undeveloped, unlike cooperative group networks available for therapeutic trials, according to Dr. Gann. "Many investigators with promising ideas do not have access to the clinical infrastructure or funding sources they need for translational research," he noted. "Moreover, the pros and cons of various available designs for phase II and III trials have not been adequately debated, amidst a shifting landscape in which some designs become less feasible as others become more feasible."
• Develop better risk stratification and patient targeting. What if clinicians could do a better job of sorting out who is truly at risk for prostate cancer? "Active surveillance cohorts are the most promising opportunity we have for prevention trials involving low-risk interventions," Dr. Gann said. Cumulative results from genome-wide association studies and the expected results from sequencing studies capable of identifying rare genetic variants with high penetrance hold promise for identifying populations for whom preventive strategies would have the most benefit, he added.
• Develop better interventional agents. To date, "I think we’ve taken a haphazard approach to identifying agents for prostate cancer prevention," Dr. Gann said. "With preclinical models lacking, sometimes they’re not vetted very well, either. We’re not going to be able to develop a rational approach to preventing prostate cancer until we have a better idea of how it all comes about. One idea is to do high throughput cell assay based drug screening, which we do for therapeutics but not for chemopreventive agents. The question is, do we have the right libraries of compounds and do we have the right readouts? I suggest that we can start with compounds, especially dietary agents that could inhibit 5-alpha reductase. We also need to think beyond a pharmacologic approach: studying the effects of diet and physical activity, for example."
• Establish better intermediate endpoints for phase II trials. A lack of intermediate endpoint biomarkers (IEBs) for phase II trials is creating a "phase II bottleneck," he said. "There is an ongoing explosion of opportunities provided by new biotechnology and computational methods, but converting these opportunities into validated IEBs for trials will take thought and planning."
Dr. Gann disclosed that he has received grant support from GlaxoSmithKline.
SAN DIEGO – Now is the time for researchers and clinicians to examine strategies and interventions for preventing prostate cancer, in the opinion of Dr. Peter H. Gann.
"In the last 12 years or so we have seen a litany of failure with regard to prostate cancer prevention in trials with clinical endpoints," Dr. Gann, professor and director of pathology research at the University of Illinois at Chicago, said during a conference sponsored by the American Association for Cancer Research and the Prostate Cancer Foundation.
"With vitamin E supplementation, for example, we see a possible increased risk of prostate cancer, no effect with selenium and possibly and increased risk of diabetes, no effect from soy, and the potential impact of green tea polyphenols is unresolved."
Moreover, it’s conceivable that you might need to screen 1,500 average-risk patients to prevent one prostate cancer from occurring. "That means that preventive agents don’t have to be safe; they have to be incredibly safe if they’re going to be used in this way," Dr. Gann said.
He proposed five ways to advance prostate cancer prevention efforts:
• Develop better preclinical models. Canadian investigators have reported success with generating high fidelity patient-derived xenografts for accelerating prostate cancer discovery and drug development (Cancer Res. 2013 Dec. 19 [doi:10.1158/0008-5472.CAN-13-2921-T]). "One of the incredible things about this is that the transplantable cells can be obtained from needle biopsies," said Dr. Gann, who was not involved with the study. "Architecture and protein marker expression is preserved in these xenografts, as are other molecular characteristics of the tumor, which is fantastic."
An unrelated Australian study demonstrated that it’s possible to generate xenografts of the earlier low-to-moderate grade, localized tumors (Nat. Protoc. 2013;8:836-48). "Why is this exciting for us in prevention? More and more we’re thinking about not how to prevent things from the initiation stage but rather in terms of progression," Dr. Gann explained. "Being able to have individualized samples that we can study versus agents that may inhibit progression is a major opportunity, I think."
• Improve clinical trial design and infrastructure. The existing networks for prostate cancer prevention trials are largely undeveloped, unlike cooperative group networks available for therapeutic trials, according to Dr. Gann. "Many investigators with promising ideas do not have access to the clinical infrastructure or funding sources they need for translational research," he noted. "Moreover, the pros and cons of various available designs for phase II and III trials have not been adequately debated, amidst a shifting landscape in which some designs become less feasible as others become more feasible."
• Develop better risk stratification and patient targeting. What if clinicians could do a better job of sorting out who is truly at risk for prostate cancer? "Active surveillance cohorts are the most promising opportunity we have for prevention trials involving low-risk interventions," Dr. Gann said. Cumulative results from genome-wide association studies and the expected results from sequencing studies capable of identifying rare genetic variants with high penetrance hold promise for identifying populations for whom preventive strategies would have the most benefit, he added.
• Develop better interventional agents. To date, "I think we’ve taken a haphazard approach to identifying agents for prostate cancer prevention," Dr. Gann said. "With preclinical models lacking, sometimes they’re not vetted very well, either. We’re not going to be able to develop a rational approach to preventing prostate cancer until we have a better idea of how it all comes about. One idea is to do high throughput cell assay based drug screening, which we do for therapeutics but not for chemopreventive agents. The question is, do we have the right libraries of compounds and do we have the right readouts? I suggest that we can start with compounds, especially dietary agents that could inhibit 5-alpha reductase. We also need to think beyond a pharmacologic approach: studying the effects of diet and physical activity, for example."
• Establish better intermediate endpoints for phase II trials. A lack of intermediate endpoint biomarkers (IEBs) for phase II trials is creating a "phase II bottleneck," he said. "There is an ongoing explosion of opportunities provided by new biotechnology and computational methods, but converting these opportunities into validated IEBs for trials will take thought and planning."
Dr. Gann disclosed that he has received grant support from GlaxoSmithKline.
EXPERT ANALYSIS AT ADVANCES IN PROSTATE CANCER RESEARCH
Return of the 'pisse-mongers,' this time with data
Great effort has been spent on identifying easily measured biomarkers to predict the progression of coronary disease and chronic kidney disease (CKD). Interestingly, these two disease processes seem to share some biomarkers and perhaps some pathogenic mechanisms. An ultimate hope is that some of these markers will be found to also contribute directly to organ dysfunction and be amenable to therapy. Blood pressure and (in many people’s minds) low-density lipoprotein cholesterol fulfill this hope. The jury remains out on C-reactive protein and serum urate. There are others.
In this issue of the Journal, Stephen et al review the data indicating that albuminuria helps predict the progression of CKD, coronary disease, ventricular remodeling, and, in some studies, all-cause mortality. Proteinuria has generally been assumed to be a marker of renal injury, but, the authors point out, albumin can under some circumstances initiate inflammatory mechanisms and stimulate mediators of fibrosis.
Although not mentioned by Stephen et al, albumin (like hemoglobin) is susceptible to nonenzymatic glycosylation in patients with diabetes. There is a hint in the literature that glycosylated albumin may be preferentially excreted. Its effects on various tissues are incompletely studied, but it strikes me that perhaps this molecule plays a unique pathogenic role in diabetic renal and vascular disease, even more than native albumin. Further evaluation of this specific marker may lead to even stronger associations (although in a select population of patients with poorly controlled diabetes).
The focus on urine as a fluid with diagnostic and predictive characteristics is certainly not new. Both Hippocrates and Galen recognized the value of inspecting urine. Uroscopy (now urinalysis) may be the oldest surviving laboratory test. Recently, my friend Joe Nally, a coauthor with Stephen et al, shared with me a paper detailing the romantic yet checkered history of urinalysis.1
Gilles de Corbeil in the 12th century wrote a poem on judging urine, intending it as an aid for remembering the supposed 20 different diagnostic colors of urine and describing in detail the use of the urine flask, a bladder-shaped container for studying the partitioning of the urine colors and substance as representative of the diseased parts of the body. A urine flask was even illustrated in a version of Chaucer’s Canterbury Tales as a recognized accoutrement of the stylish physician (Figure 1). The “art” of uroscopy grew so successful over the centuries as a component of rampant medical charlatanry (casting no aspersions, of course, on current nephrologists) that the Royal College of Physicians in 1601 felt pressed to attack the “pisse-mongers” by stating, “It is ridiculous and foolish to divine the…course of disease…from the inspection of urine.”1 This dictate was apparently ignored then, but seemingly is too frequently followed by clinicians today, contributing to the oft-delayed diagnosis of glomerulonephritis and other renal diseases.
In 1637, Thomas Brian published The Pisse-Prophet or Certaine Pisse Pot Lectures, in which he railed against the witchcraft of uroscopy, which he said should only be performed by university-trained physicians. Jump forward to 1827, when Richard Bright elegantly described acute glomerulonephritis, although not the microscopic findings that would be illustrated in accurate detail by Golding Bird in his 1844 treatise, Urinary Deposits. Sitting on the bookshelf behind my desk is a copy of Richard W. Lippman’s Urine and Urinary Sediment: A Practical Manual and Atlas (1957). I have no urine flask—rheumatologists know their limitations.
As we enter 2014, all of us at the Journal offer you our sincere wishes for a personally healthy and universally peaceful new year.
- Haber MH. Pisse prophecy: a brief history of urinalysis. Clin Lab Med 1988; 8:415–430.
Great effort has been spent on identifying easily measured biomarkers to predict the progression of coronary disease and chronic kidney disease (CKD). Interestingly, these two disease processes seem to share some biomarkers and perhaps some pathogenic mechanisms. An ultimate hope is that some of these markers will be found to also contribute directly to organ dysfunction and be amenable to therapy. Blood pressure and (in many people’s minds) low-density lipoprotein cholesterol fulfill this hope. The jury remains out on C-reactive protein and serum urate. There are others.
In this issue of the Journal, Stephen et al review the data indicating that albuminuria helps predict the progression of CKD, coronary disease, ventricular remodeling, and, in some studies, all-cause mortality. Proteinuria has generally been assumed to be a marker of renal injury, but, the authors point out, albumin can under some circumstances initiate inflammatory mechanisms and stimulate mediators of fibrosis.
Although not mentioned by Stephen et al, albumin (like hemoglobin) is susceptible to nonenzymatic glycosylation in patients with diabetes. There is a hint in the literature that glycosylated albumin may be preferentially excreted. Its effects on various tissues are incompletely studied, but it strikes me that perhaps this molecule plays a unique pathogenic role in diabetic renal and vascular disease, even more than native albumin. Further evaluation of this specific marker may lead to even stronger associations (although in a select population of patients with poorly controlled diabetes).
The focus on urine as a fluid with diagnostic and predictive characteristics is certainly not new. Both Hippocrates and Galen recognized the value of inspecting urine. Uroscopy (now urinalysis) may be the oldest surviving laboratory test. Recently, my friend Joe Nally, a coauthor with Stephen et al, shared with me a paper detailing the romantic yet checkered history of urinalysis.1
Gilles de Corbeil in the 12th century wrote a poem on judging urine, intending it as an aid for remembering the supposed 20 different diagnostic colors of urine and describing in detail the use of the urine flask, a bladder-shaped container for studying the partitioning of the urine colors and substance as representative of the diseased parts of the body. A urine flask was even illustrated in a version of Chaucer’s Canterbury Tales as a recognized accoutrement of the stylish physician (Figure 1). The “art” of uroscopy grew so successful over the centuries as a component of rampant medical charlatanry (casting no aspersions, of course, on current nephrologists) that the Royal College of Physicians in 1601 felt pressed to attack the “pisse-mongers” by stating, “It is ridiculous and foolish to divine the…course of disease…from the inspection of urine.”1 This dictate was apparently ignored then, but seemingly is too frequently followed by clinicians today, contributing to the oft-delayed diagnosis of glomerulonephritis and other renal diseases.
In 1637, Thomas Brian published The Pisse-Prophet or Certaine Pisse Pot Lectures, in which he railed against the witchcraft of uroscopy, which he said should only be performed by university-trained physicians. Jump forward to 1827, when Richard Bright elegantly described acute glomerulonephritis, although not the microscopic findings that would be illustrated in accurate detail by Golding Bird in his 1844 treatise, Urinary Deposits. Sitting on the bookshelf behind my desk is a copy of Richard W. Lippman’s Urine and Urinary Sediment: A Practical Manual and Atlas (1957). I have no urine flask—rheumatologists know their limitations.
As we enter 2014, all of us at the Journal offer you our sincere wishes for a personally healthy and universally peaceful new year.
Great effort has been spent on identifying easily measured biomarkers to predict the progression of coronary disease and chronic kidney disease (CKD). Interestingly, these two disease processes seem to share some biomarkers and perhaps some pathogenic mechanisms. An ultimate hope is that some of these markers will be found to also contribute directly to organ dysfunction and be amenable to therapy. Blood pressure and (in many people’s minds) low-density lipoprotein cholesterol fulfill this hope. The jury remains out on C-reactive protein and serum urate. There are others.
In this issue of the Journal, Stephen et al review the data indicating that albuminuria helps predict the progression of CKD, coronary disease, ventricular remodeling, and, in some studies, all-cause mortality. Proteinuria has generally been assumed to be a marker of renal injury, but, the authors point out, albumin can under some circumstances initiate inflammatory mechanisms and stimulate mediators of fibrosis.
Although not mentioned by Stephen et al, albumin (like hemoglobin) is susceptible to nonenzymatic glycosylation in patients with diabetes. There is a hint in the literature that glycosylated albumin may be preferentially excreted. Its effects on various tissues are incompletely studied, but it strikes me that perhaps this molecule plays a unique pathogenic role in diabetic renal and vascular disease, even more than native albumin. Further evaluation of this specific marker may lead to even stronger associations (although in a select population of patients with poorly controlled diabetes).
The focus on urine as a fluid with diagnostic and predictive characteristics is certainly not new. Both Hippocrates and Galen recognized the value of inspecting urine. Uroscopy (now urinalysis) may be the oldest surviving laboratory test. Recently, my friend Joe Nally, a coauthor with Stephen et al, shared with me a paper detailing the romantic yet checkered history of urinalysis.1
Gilles de Corbeil in the 12th century wrote a poem on judging urine, intending it as an aid for remembering the supposed 20 different diagnostic colors of urine and describing in detail the use of the urine flask, a bladder-shaped container for studying the partitioning of the urine colors and substance as representative of the diseased parts of the body. A urine flask was even illustrated in a version of Chaucer’s Canterbury Tales as a recognized accoutrement of the stylish physician (Figure 1). The “art” of uroscopy grew so successful over the centuries as a component of rampant medical charlatanry (casting no aspersions, of course, on current nephrologists) that the Royal College of Physicians in 1601 felt pressed to attack the “pisse-mongers” by stating, “It is ridiculous and foolish to divine the…course of disease…from the inspection of urine.”1 This dictate was apparently ignored then, but seemingly is too frequently followed by clinicians today, contributing to the oft-delayed diagnosis of glomerulonephritis and other renal diseases.
In 1637, Thomas Brian published The Pisse-Prophet or Certaine Pisse Pot Lectures, in which he railed against the witchcraft of uroscopy, which he said should only be performed by university-trained physicians. Jump forward to 1827, when Richard Bright elegantly described acute glomerulonephritis, although not the microscopic findings that would be illustrated in accurate detail by Golding Bird in his 1844 treatise, Urinary Deposits. Sitting on the bookshelf behind my desk is a copy of Richard W. Lippman’s Urine and Urinary Sediment: A Practical Manual and Atlas (1957). I have no urine flask—rheumatologists know their limitations.
As we enter 2014, all of us at the Journal offer you our sincere wishes for a personally healthy and universally peaceful new year.
- Haber MH. Pisse prophecy: a brief history of urinalysis. Clin Lab Med 1988; 8:415–430.
- Haber MH. Pisse prophecy: a brief history of urinalysis. Clin Lab Med 1988; 8:415–430.
Albuminuria: When urine predicts kidney and cardiovascular disease
“One can obtain considerable information concerning the general health by examining the urine.”
—Hippocrates (460?–355? BCE)
Chronic kidney disease is a notable public health concern because it is an important risk factor for end-stage renal disease, cardiovascular disease, and death. Its prevalence1 exceeds 10% and is considerably higher in high-risk groups, such as those with diabetes or hypertension, which are growing in the United States.
While high levels of total protein in the urine have always been recognized as pathologic, a growing body of evidence links excretion of the protein albumin to adverse cardiovascular outcomes, and most international guidelines now recommend measuring albumin specifically. Albuminuria is a predictor of declining renal function and is independently associated with adverse cardiovascular outcomes. Thus, clinicians need to detect it early, manage it effectively, and reduce concurrent risk factors for cardiovascular disease.
Therefore, this review will focus on albuminuria. However, because the traditional standard for urinary protein measurement was total protein, and because a few guidelines still recommend measuring total protein rather than albumin, we will also briefly discuss total urinary protein.
MOST URINARY PROTEIN IS ALBUMIN
Most of the protein in the urine is albumin filtered from the plasma. Less than half of the rest is derived from the distal renal tubules (uromodulin or Tamm-Horsfall mucoprotein), 2 and urine also contains a small and varying proportion of immunoglobulins, low-molecular-weight proteins, and light chains.
Normal healthy people lose less than 30 mg of albumin in the urine per day. In greater amounts, albumin is the major urinary protein in most kidney diseases. Other proteins in urine can be specific markers of less-common illnesses such as plasma cell dyscrasia, glomerulopathy, and renal tubular disease.
MEASURING PROTEINURIA AND ALBUMINURIA
Albumin is not a homogeneous molecule in urine. It undergoes changes to its molecular configuration in the presence of certain ions, peptides, hormones, and drugs, and as a result of proteolytic fragmentation both in the plasma and in renal tubules.3 Consequently, measuring urinary albumin involves a trade-off between convenience and accuracy.
A 24-hour timed urine sample has long been the gold standard for measuring albuminuria, but the collection is cumbersome and time-consuming, and the test is prone to laboratory error.
Dipstick measurements are more convenient and are better at detecting albumin than other proteins in urine, but they have low sensitivity and high interobserver variation.3–5
The albumin-to-creatinine ratio (ACR). As the quantity of protein in the urine changes with time of day, exertion, stress level, and posture, spot-checking of urine samples is not as good as timed collection. However, a simultaneous measurement of creatinine in a spot urine sample adjusts for protein concentration, which can vary with a person’s hydration status. The ACR so obtained is consistent with the 24-hour timed collection (the gold standard) and is the recommended method of assessing albuminuria.3 An early morning urine sample is favored, as it avoids orthostatic variations and varies less in the same individual.
In a study in the general population comparing the ACR in a random sample and in an early morning sample, only 44% of those who had an ACR of 30 mg/g or higher in the random sample had one this high in the early morning sample.6 However, getting an early morning sample is not always feasible in clinical practice. If you are going to measure albuminuria, the Kidney Disease Outcomes and Quality Initiative7 suggests checking the ACR in a random sample and then, if the test is positive, following up and confirming it within 3 months with an early morning sample.
Also, since creatinine excretion differs with race, diet, and muscle mass, if the 24-hour creatinine excretion is not close to 1 g, the ACR will give an erroneous estimate of the 24-hour excretion rate.3
Table 1 compares the various methods of measuring protein in the urine.3,5,8,9 Of note, methods of measuring albumin and total protein vary considerably in their precision and accuracy, making it impossible to reliably translate values from one to the other.5
National and international guidelines (Table 2)7,10–13 agree that albuminuria should be tested in diabetic patients, as it is a surrogate marker for early diabetic nephropathy.3,13 Most guidelines also recommend measuring albuminuria by a urine ACR test as the preferred measure, even in people without diabetes.
Also, no single cutoff is universally accepted for distinguishing pathologic albuminuria from physiologic albuminuria, nor is there a universally accepted unit of measure.14 Because approximately 1 g of creatinine is lost in the urine per day, the ACR has the convenient property of numerically matching the albumin excretory rate expressed in milligrams per 24 hours. The other commonly used unit is milligrams of albumin per millimole of creatinine; 30 mg/g is roughly equal to 3 mg/mmol.
The term microalbuminuria was traditionally used to refer to albumin excretion of 30 to 299 mg per 24 hours, and macroalbuminuria to 300 mg or more per 24 hours. However, as there is no pathophysiologic basis to these thresholds (see outcomes data below), the current Kidney Disease Improving Global Outcomes (KDIGO) guidelines do not recommend using these terms.13,15
RENAL COMPLICATIONS OF ALBUMINURIA
A failure of the glomerular filtration barrier or of proximal tubular reabsorption accounts for most cases of pathologic albuminuria.16 Processes affecting the glomerular filtration of albumin include endothelial cell dysfunction and abnormalities with the glomerular basement membrane, podocytes, or the slit diaphragms among the podocytic processes.17
Ultrafiltrated albumin has been directly implicated in tubulointerstitial damage and glomerulosclerosis through diverse pathways. In the proximal tubule, albumin up-regulates interleukin 8 (a chemoattractant for lymphocytes and neutrophils), induces synthesis of endothelin 1 (which stimulates renal cell proliferation, extracellular matrix production, and monocyte attraction), and causes apoptosis of tubular cells.18 In response to albumin, proximal tubular cells also stimulate interstitial fibroblasts via paracrine release of transforming growth factor beta, either directly or by activating complement or macrophages.18,19
Studies linking albuminuria to kidney disease
Albuminuria has traditionally been associated with diabetes mellitus as a predictor of overt diabetic nephropathy,20,21 although in type 1 diabetes, established albuminuria can spontaneously regress.22
Albuminuria is also a strong predictor of progression in chronic kidney disease.23 In fact, in the last decade, albuminuria has become an independent criterion in the definition of chronic kidney disease; excretion of more than 30 mg of albumin per day, sustained for at least 3 months, qualifies as chronic kidney disease, with independent prognostic implications (Table 3).13
Astor et al,24 in a meta-analysis of 13 studies with more than 21,000 patients with chronic kidney disease, found that the risk of end-stage renal disease was three times higher in those with albuminuria.
Gansevoort et al,23 in a meta-analysis of nine studies with nearly 850,000 participants from the general population, found that the risk of end-stage renal disease increased continuously as albumin excretion increased. They also found that as albuminuria increased, so did the risk of progression of chronic kidney disease and the incidence of acute kidney injury.
Hemmelgarn et al,25 in a large pooled cohort study with more than 1.5 million participants from the general population, showed that increasing albuminuria was associated with a decline in the estimated glomerular filtration rate (GFR) and with progression to end-stage renal disease across all strata of baseline renal function. For example, in persons with an estimated GFR of 60 mL/min/1.73 m2
- 1 per 1,000 person-years for those with no proteinuria
- 2.8 per 1,000 person-years for those with mild proteinuria (trace or 1+ by dipstick or ACR 30–300 mg/g)
- 13.4 per 1,000 person-years for those with heavy proteinuria (2+ or ACR > 300 mg/g).
Rates of progression to end-stage renal disease were:
- 0.03 per 1,000 person-years with no proteinuria
- 0.05 per 1,000 person-years with mild proteinuria
- 1 per 1,000 person-years with heavy proteinuria.25
CARDIOVASCULAR CONSEQUENCES OF ALBUMINURIA
The exact pathophysiologic link between albuminuria and cardiovascular disease is unknown, but several mechanisms have been proposed.
One is that generalized endothelial dysfunction causes both albuminuria and cardiovascular disease.26 Endothelium-derived nitric oxide has vasodilator, antiplatelet, antiproliferative, antiadhesive, permeability-decreasing, and anti-inflammatory properties. Impaired endothelial synthesis of nitric oxide has been independently associated with both microalbuminuria and diabetes.27
Low levels of heparan sulfate (which has antithrombogenic effects and decreases vessel permeability) in the glycocalyx lining vessel walls may also account for albuminuria and for the other cardiovascular effects.28–30 These changes may be the consequence of chronic low-grade inflammation, which precedes the occurrence and progression of both albuminuria and atherothrombotic disease. The resulting abnormalities in the endothelial glycocalyx could lead to increased glomerular permeability to albumin and may also be implicated in the pathogenesis of atherosclerosis.26
In an atherosclerotic aorta and coronary arteries, the endothelial dysfunction may cause increased leakage of cholesterol and glycated end-products into the myocardium, resulting in increasing wall stiffness and left ventricular mass. A similar atherosclerotic process may account for coronary artery microthrombi, resulting in subendocardial ischemia that could lead to systolic and diastolic heart dysfunction.31
Studies linking albuminuria to heart disease
There is convincing evidence that albuminuria is associated with cardiovascular disease. An ACR between 30 and 300 mg/g was independently associated with myocardial infarction and ischemia.32 People with albuminuria have more than twice the risk of severe coronary artery disease, and albuminuria is also associated with increased intimal thickening in the carotid arteries.33,34 An ACR in the same range has been associated with increased incidence and progression of coronary artery calcification.35 Higher brachial-ankle pulse-wave velocity has also been demonstrated with albuminuria in a dose-dependent fashion.36,37
An ACR of 30 to 300 mg/g has been linked to left ventricular hypertrophy independently of other risk factors,38 and functionally with diastolic dysfunction and abnormal midwall shortening.39 In a study of a subgroup of patients with diabetes from a population-based cohort of Native American patients (the Strong Heart Study),39 the prevalence of diastolic dysfunction was:
- 16% in those with no albuminuria
- 26% in those with an ACR of 30 to 300 mg/g
- 31% in those with an ACR greater than 300 mg/g.
The association persisted even after controlling for age, sex, hypertension, and other covariates.
Those pathologic associations have been directly linked to clinical outcomes. For patients with heart failure (New York Heart Association class II–IV), a study found that albuminuria (an ACR > 30 mg/g) conferred a 41% higher risk of admission for heart failure, and an ACR greater than 300 mg/g was associated with an 88% higher risk.40
In an analysis of a prospective cohort from the general population with albuminuria and a low prevalence of renal dysfunction (the Prevention of Renal and Vascular Endstage Disease study),41 albuminuria was associated with a modest increase in the incidence of the composite end point of myocardial infarction, stroke, ischemic heart disease, revascularization procedures, and all-cause mortality per doubling of the urine albumin excretion (hazard ratio 1.08, range 1.04 –1.12).
The relationship to cardiovascular outcomes extends below traditional lower-limit thresholds of albuminuria (corresponding to an ACR > 30 mg/g). A subgroup of patients from the Framingham Offspring Study without prevalent cardiovascular disease, hypertension, diabetes, or kidney disease, and thus with a low to intermediate probability of cardiovascular events, were found to have thresholds for albuminuria as low as 5.3 mg/g in men and 10.8 mg/g in women to discriminate between incident coronary artery disease, heart failure, cerebrovascular disease, other peripheral vascular disease, or death.42
In a meta-analysis including more than 1 million patients in the general population, increasing albuminuria was associated with an increase in deaths from all causes in a continuous manner, with no threshold effect.43 In patients with an ACR of 30 mg/g, the hazard ratio for death was 1.63, increasing to 2.22 for those with more than 300 mg/g compared with those with no albuminuria. A similar increase in the risk of myocardial infarction, heart failure, stroke, or sudden cardiac death was noted with higher ACR.43
Important prospective cohort studies and meta-analyses related to albuminuria and kidney and cardiovascular disease and death are summarized in the eTable.23,39–50
THE CASE FOR TREATING ALBUMINURIA
Reduced progression of renal disease
Treating patients who have proteinuric chronic kidney disease with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) can reduce the risk of progression of renal failure. However, it is unclear whether this benefit is the result of treating concomitant risk factors independent of the reduction in albuminuria, and there is no consistent treatment effect in improving renal outcomes across studies.
Fink et al,51 in a meta-analysis, found that chronic kidney disease patients with diabetes, hypertension, and macroalbuminuria had a 40% lower risk of progression to end-stage renal disease if they received an ACE inhibitor (relative risk [RR] 0.60, 95% confidence interval [CI] 0.43–0.83). In the same meta-analysis, ARBs also reduced the risk of progression to end-stage renal disease (RR 0.77, 95% CI 0.66–0.90).
Jafar et al,52 in an analysis of pooled patient-level data including only nondiabetic patients on ACE inhibitor therapy (n = 1,860), found that the risk of progression of renal failure, defined as a doubling of serum creatinine or end-stage renal disease, was reduced (RR 0.70, 95% CI 0.55–0.88). Patients with higher levels of albuminuria showed the most benefit, but the effect was not conclusive for albuminuria below 500 mg/day at baseline.
Maione et al,53 in a meta-analysis that included patients with albuminuria who were treated with ACE inhibitors vs placebo (n = 8,231), found a similar reduction in risk of:
- Progression to end-stage renal disease (RR 0.67, 95% CI 0.54–0.84)
- Doubling of serum creatinine (RR 0.62, 95% CI 0.46–0.84)
- Progression of albuminuria (RR 0.49, 95% CI 0.36–0.65)
- Normalization of pathologic albuminuria (as defined by the trialists in the individual studies) (RR 2.99, 95% CI 1.82–4.91).
Similar results were obtained for patients with albuminuria who were treated with ARBs.53
ONTARGET.54 In contrast, in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial, the combination of an ACE inhibitor and an ARB showed no benefit in reducing the progression of renal failure, and in those patients with chronic kidney disease there was a higher risk of a doubling of serum creatinine or of the development of end-stage renal disease and hyperkalemia.
Also, in a pooled analysis of the ONTARGET and Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease (TRANSCEND) trials, a 50% reduction in baseline albuminuria was associated with reduced progression of renal failure in those with a baseline ACR less than 10 mg/g.55
Improved cardiovascular outcomes
There is also evidence of better cardiovascular outcomes with treatment of albuminuria. Again, it is uncertain whether this is a result of treating risk factors other than albuminuria with ACE inhibitors or ARBs, and there is no parallel benefit demonstrated across all studies.
LIFE.47,48 In the Losartan Intervention for Endpoint Reduction in Hypertension trial, survival analyses suggested a decrease in risk of cardiovascular adverse events as the degree of proteinuria improved with ARB therapy.
Maione et al,53 in a meta-analysis including 8,231 patients with albuminuria and at least one other risk factor, found a significant reduction in the rate of nonfatal cardiovascular outcomes (angina, myocardial infarction, revascularization, stroke, transient ischemic attack, or heart failure) with ACE inhibitors vs placebo (RR 0.88, CI 0.82–0.94) and also in 3,888 patients treated with ARBs vs placebo (RR 0.77, CI 0.61–0.98). However, the meta-analysis did not show that ACE inhibitor or ARB therapy reduced rate of cardiovascular or all-cause mortality.
Fink et al,51 in their meta-analysis of 18 trials of ACE inhibitors and four trials of ARBs, also found no evidence that ACE inhibitor or ARB therapy reduced cardiovascular mortality rates.38
The ONTARGET trial evaluated the combination of an ACE inhibitor and ARB therapy in patients with diabetes or preexisting peripheral vascular disease. Reductions in the rate of cardiovascular disease or death were not observed, and in those with chronic kidney disease, there was a higher risk of doubling of serum creatinine or development of end-stage renal disease and adverse events of hyperkalemia.56 And although an increase in baseline albuminuria was associated with worse cardiovascular outcomes, its reduction in the ONTARGET and TRANSCEND trials did not demonstrate better outcomes when the baseline ACR was greater than 10 mg/g.55
WHO SHOULD BE TESTED?
The benefit of adding albuminuria to conventional cardiovascular risk stratification such as Framingham risk scoring is not conclusive. However, today’s clinician may view albuminuria as a biomarker for renal and cardiovascular disease, as albuminuria might be a surrogate marker for endothelial dysfunction in the glomerular capillaries or other vital vascular beds.
High-risk populations and chronic kidney disease patients
Nearly all the current guidelines recommend annual screening for albuminuria in patients with diabetes and hypertension (Table 2).7,10–13 Other high-risk populations include people with cardiovascular disease, a family history of end-stage renal disease, and metabolic syndrome. Additionally, chronic kidney disease patients whose estimated GFR defines them as being in stage 3 or higher (ie, GFR < 60 mL/min/1.73m2), regardless of other comorbidities, should be tested for albuminuria, as it is an important risk predictor.
Most experts prefer that albuminuria be measured by urine ACR in a first morning voided sample, though this is not the only option.
Screening the general population
Given that albuminuria has been shown to be such an important prognosticator for patients at high risk and those with chronic kidney disease, the question arises whether screening for albuminuria in the asymptomatic low-risk general population would foster earlier detection and therefore enable earlier intervention and result in improved outcomes. However, a systematic review done for the United States Preventive Services Task Force and for an American College of Physicians clinical practice guideline did not find robust evidence to support this.51
OUR RECOMMENDATIONS
Who should be tested?
- Patients with chronic kidney disease stage 3, 4, or 5 (GFR < 60 mL/min/1.73m2) who are not on dialysis
- Patients who are considered at higher risk of adverse outcomes, such as those with diabetes, hypertension, a family history of end-stage renal disease, or cardiovascular disease. Testing is useful for recognizing increased renal and cardiovascular risk and may lead clinicians to prescribe or titrate a renin-angiotensin system antagonist, a statin, or both, or to modify other cardiovascular risk factors.
- Not recommended: routine screening in the general population who are asymptomatic or are considered at low risk.
Which test should be used?
Based on current evidence and most guidelines, we recommend the urine ACR test as the screening test for people with diabetes and others deemed to be at high risk.
What should be done about albuminuria?
- Controlling blood pressure is important, and though there is debate about the target blood pressure, an individualized plan should be developed with the patient based on age, comorbidities, and goals of care.
- An ACE inhibitor or ARB, if not contraindicated, is recommended for patients with diabetes whose ACR is greater than 30 mg/g and for patients with chronic kidney disease and an ACR greater than 300 mg/g.
- Current evidence does not support the combined use of an ACE inhibitor and an ARB, as proof of benefit is lacking and the risk of adverse events is higher.
- Refer patients with high or unexplained albuminuria to a nephrologist or clinic specializing in chronic kidney disease.
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298:2038–2047.
- Hoyer JR, Seiler MW. Pathophysiology of Tamm-Horsfall protein. Kidney Int 1979; 16:279–289.
- Viswanathan G, Upadhyay A. Assessment of proteinuria. Adv Chronic Kidney Dis 2011; 18:243–248.
- Guh JY. Proteinuria versus albuminuria in chronic kidney disease. Nephrology (Carlton) 2010; 15(suppl 2):53–56.
- Lamb EJ, MacKenzie F, Stevens PE. How should proteinuria be detected and measured? Ann Clin Biochem 2009; 46:205–217.
- Saydah SH, Pavkov ME, Zhang C, et al. Albuminuria prevalence in first morning void compared with previous random urine from adults in the National Health and Nutrition Examination Survey, 2009-2010. Clin Chem 2013; 59:675–683.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
- Younes N, Cleary PA, Steffes MW, et al; DCCT/EDIC Research Group. Comparison of urinary albumin-creatinine ratio and albumin excretion rate in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Clin J Am Soc Nephrol 2010; 5:1235–1242.
- Brinkman JW, de Zeeuw D, Duker JJ, et al. Falsely low urinary albumin concentrations after prolonged frozen storage of urine samples. Clin Chem 2005; 51:2181–2183.
- National Collaborating Centre for Chronic Conditions (UK). Chronic Kidney Disease: National Clinical Guideline for Early Identification and Management in Adults in Primary and Secondary Care. London: Royal College of Physicians (UK) 2008.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3:1–150.
- Johnson DW. Global proteinuria guidelines: are we nearly there yet? Clin Biochem Rev 2011; 32:89–95.
- Ruggenenti P, Remuzzi G. Time to abandon microalbuminuria? Kidney Int 2006; 70:1214–1222.
- Glassock RJ. Is the presence of microalbuminuria a relevant marker of kidney disease? Curr Hypertens Rep 2010; 12:364–368.
- Zhang A, Huang S. Progress in pathogenesis of proteinuria. Int J Nephrol 2012; 2012:314251.
- Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol 2006; 17:2974–2984.
- Karalliedde J, Viberti G. Proteinuria in diabetes: bystander or pathway to cardiorenal disease? J Am Soc Nephrol 2010; 21:2020–2027.
- Svendsen PA, Oxenbøll B, Christiansen JS. Microalbuminuria in diabetic patients—a longitudinal study. Acta Endocrinol Suppl (Copenh) 1981; 242:53–54.
- Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1982; 1:1430–1432.
- Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003; 348:2285–2293.
- Gansevoort RT, Matsushita K, van der Velde M, et al; Chronic Kidney Disease Prognosis Consortium. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011; 80:93–104.
- Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 2011; 79:1331–1340.
- Hemmelgarn BR, Manns BJ, Lloyd A, et al; Alberta Kidney Disease Network. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010; 303:423–429.
- Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol 2006; 17:2106–2111.
- Stehouwer CD, Henry RM, Dekker JM, Nijpels G, Heine RJ, Bouter LM. Microalbuminuria is associated with impaired brachial artery, flow-mediated vasodilation in elderly individuals without and with diabetes: further evidence for a link between microalbuminuria and endothelial dysfunction—the Hoorn Study. Kidney Int Suppl 2004; 92:S42–S44.
- Wasty F, Alavi MZ, Moore S. Distribution of glycosaminoglycans in the intima of human aortas: changes in atherosclerosis and diabetes mellitus. Diabetologia 1993; 36:316–322.
- Ylä-Herttuala S, Sumuvuori H, Karkola K, Möttönen M, Nikkari T. Glycosaminoglycans in normal and atherosclerotic human coronary arteries. Lab Invest 1986; 54:402–407.
- Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 1989; 32:219–226.
- van Hoeven KH, Factor SM. A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation 1990; 82:848–855.
- Diercks GF, van Boven AJ, Hillege HL, et al. Microalbuminuria is independently associated with ischaemic electrocardiographic abnormalities in a large non-diabetic population. The PREVEND (Prevention of REnal and Vascular ENdstage Disease) study. Eur Heart J 2000; 21:1922–1927.
- Bigazzi R, Bianchi S, Nenci R, Baldari D, Baldari G, Campese VM. Increased thickness of the carotid artery in patients with essential hypertension and microalbuminuria. J Hum Hypertens 1995; 9:827–833.
- Tuttle KR, Puhlman ME, Cooney SK, Short R. Urinary albumin and insulin as predictors of coronary artery disease: an angiographic study. Am J Kidney Dis 1999; 34:918–925.
- DeFilippis AP, Kramer HJ, Katz R, et al. Association between coronary artery calcification progression and microalbuminuria: the MESA study. JACC Cardiovasc Imaging 2010; 3:595–604.
- Liu CS, Pi-Sunyer FX, Li CI, et al. Albuminuria is strongly associated with arterial stiffness, especially in diabetic or hypertensive subjects—a population-based study (Taichung Community Health Study, TCHS). Atherosclerosis 2010; 211:315–321.
- Upadhyay A, Hwang SJ, Mitchell GF, et al. Arterial stiffness in mild-to-moderate CKD. J Am Soc Nephrol 2009; 20:2044–2053.
- Pontremoli R, Sofia A, Ravera M, et al. Prevalence and clinical correlates of microalbuminuria in essential hypertension: the MAGIC Study. Microalbuminuria: a Genoa Investigation on Complications. Hypertension 1997; 30:1135–1143.
- Liu JE, Robbins DC, Palmieri V, et al. Association of albuminuria with systolic and diastolic left ventricular dysfunction in type 2 diabetes: the Strong Heart Study. J Am Coll Cardiol 2003; 41:2022–2028.
- Jackson CE, Solomon SD, Gerstein HC, et al; CHARM Investigators and Committees. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet 2009; 374:543–550.
- Smink PA, Lambers Heerspink HJ, Gansevoort RT, et al. Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: the PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am J Kidney Dis 2012; 60:804–811.
- Arnlöv J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation 2005; 112:969–975.
- Chronic Kidney Disease Prognosis Consortium; Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375:2073–2081.
- van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011; 79:1341–1352.
- Ruggenenti P, Porrini E, Motterlini N, et al; BENEDICT Study Investigators. Measurable urinary albumin predicts cardiovascular risk among normoalbuminuric patients with type 2 diabetes. J Am Soc Nephrol 2012; 23:1717–1724.
- Hallan S, Astor B, Romundstad S, Aasarød K, Kvenild K, Coresh J. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: the HUNT II Study. Arch Intern Med 2007; 167:2490–2496.
- Ibsen H, Wachtell K, Olsen MH, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE Study. Kidney Int Suppl 2004; 92:S56–S58.
- Olsen MH, Wachtell K, Bella JN, et al. Albuminuria predicts cardiovascular events independently of left ventricular mass in hypertension: a LIFE substudy. J Hum Hypertens 2004; 18:453–459.
- Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation 2004; 110:32–35.
- Gerstein HC, Mann JF, Yi Q, et al; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426.
- Fink HA, Ishani A, Taylor BC, et al. Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: a systematic review for the US Preventive Services Task Force and for an American College of Physicians Clinical Practice Guideline. Ann Intern Med 2012; 156:570–581.
- Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 2001; 135:73–87.
- Maione A, Navaneethan SD, Graziano G, et al. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and combined therapy in patients with micro- and macroalbuminuria and other cardiovascular risk factors: a systematic review of randomized controlled trials. Nephrol Dial Transplant 2011; 26:2827–2847.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Schmieder RE, Mann JF, Schumacher H, et al; ONTARGET Investigators. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol 2011; 22:1353–1364.
- Tobe SW, Clase CM, Gao P, et al; ONTARGET and TRANSCEND Investigators. Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation 2011; 123:1098–1107.
“One can obtain considerable information concerning the general health by examining the urine.”
—Hippocrates (460?–355? BCE)
Chronic kidney disease is a notable public health concern because it is an important risk factor for end-stage renal disease, cardiovascular disease, and death. Its prevalence1 exceeds 10% and is considerably higher in high-risk groups, such as those with diabetes or hypertension, which are growing in the United States.
While high levels of total protein in the urine have always been recognized as pathologic, a growing body of evidence links excretion of the protein albumin to adverse cardiovascular outcomes, and most international guidelines now recommend measuring albumin specifically. Albuminuria is a predictor of declining renal function and is independently associated with adverse cardiovascular outcomes. Thus, clinicians need to detect it early, manage it effectively, and reduce concurrent risk factors for cardiovascular disease.
Therefore, this review will focus on albuminuria. However, because the traditional standard for urinary protein measurement was total protein, and because a few guidelines still recommend measuring total protein rather than albumin, we will also briefly discuss total urinary protein.
MOST URINARY PROTEIN IS ALBUMIN
Most of the protein in the urine is albumin filtered from the plasma. Less than half of the rest is derived from the distal renal tubules (uromodulin or Tamm-Horsfall mucoprotein), 2 and urine also contains a small and varying proportion of immunoglobulins, low-molecular-weight proteins, and light chains.
Normal healthy people lose less than 30 mg of albumin in the urine per day. In greater amounts, albumin is the major urinary protein in most kidney diseases. Other proteins in urine can be specific markers of less-common illnesses such as plasma cell dyscrasia, glomerulopathy, and renal tubular disease.
MEASURING PROTEINURIA AND ALBUMINURIA
Albumin is not a homogeneous molecule in urine. It undergoes changes to its molecular configuration in the presence of certain ions, peptides, hormones, and drugs, and as a result of proteolytic fragmentation both in the plasma and in renal tubules.3 Consequently, measuring urinary albumin involves a trade-off between convenience and accuracy.
A 24-hour timed urine sample has long been the gold standard for measuring albuminuria, but the collection is cumbersome and time-consuming, and the test is prone to laboratory error.
Dipstick measurements are more convenient and are better at detecting albumin than other proteins in urine, but they have low sensitivity and high interobserver variation.3–5
The albumin-to-creatinine ratio (ACR). As the quantity of protein in the urine changes with time of day, exertion, stress level, and posture, spot-checking of urine samples is not as good as timed collection. However, a simultaneous measurement of creatinine in a spot urine sample adjusts for protein concentration, which can vary with a person’s hydration status. The ACR so obtained is consistent with the 24-hour timed collection (the gold standard) and is the recommended method of assessing albuminuria.3 An early morning urine sample is favored, as it avoids orthostatic variations and varies less in the same individual.
In a study in the general population comparing the ACR in a random sample and in an early morning sample, only 44% of those who had an ACR of 30 mg/g or higher in the random sample had one this high in the early morning sample.6 However, getting an early morning sample is not always feasible in clinical practice. If you are going to measure albuminuria, the Kidney Disease Outcomes and Quality Initiative7 suggests checking the ACR in a random sample and then, if the test is positive, following up and confirming it within 3 months with an early morning sample.
Also, since creatinine excretion differs with race, diet, and muscle mass, if the 24-hour creatinine excretion is not close to 1 g, the ACR will give an erroneous estimate of the 24-hour excretion rate.3
Table 1 compares the various methods of measuring protein in the urine.3,5,8,9 Of note, methods of measuring albumin and total protein vary considerably in their precision and accuracy, making it impossible to reliably translate values from one to the other.5
National and international guidelines (Table 2)7,10–13 agree that albuminuria should be tested in diabetic patients, as it is a surrogate marker for early diabetic nephropathy.3,13 Most guidelines also recommend measuring albuminuria by a urine ACR test as the preferred measure, even in people without diabetes.
Also, no single cutoff is universally accepted for distinguishing pathologic albuminuria from physiologic albuminuria, nor is there a universally accepted unit of measure.14 Because approximately 1 g of creatinine is lost in the urine per day, the ACR has the convenient property of numerically matching the albumin excretory rate expressed in milligrams per 24 hours. The other commonly used unit is milligrams of albumin per millimole of creatinine; 30 mg/g is roughly equal to 3 mg/mmol.
The term microalbuminuria was traditionally used to refer to albumin excretion of 30 to 299 mg per 24 hours, and macroalbuminuria to 300 mg or more per 24 hours. However, as there is no pathophysiologic basis to these thresholds (see outcomes data below), the current Kidney Disease Improving Global Outcomes (KDIGO) guidelines do not recommend using these terms.13,15
RENAL COMPLICATIONS OF ALBUMINURIA
A failure of the glomerular filtration barrier or of proximal tubular reabsorption accounts for most cases of pathologic albuminuria.16 Processes affecting the glomerular filtration of albumin include endothelial cell dysfunction and abnormalities with the glomerular basement membrane, podocytes, or the slit diaphragms among the podocytic processes.17
Ultrafiltrated albumin has been directly implicated in tubulointerstitial damage and glomerulosclerosis through diverse pathways. In the proximal tubule, albumin up-regulates interleukin 8 (a chemoattractant for lymphocytes and neutrophils), induces synthesis of endothelin 1 (which stimulates renal cell proliferation, extracellular matrix production, and monocyte attraction), and causes apoptosis of tubular cells.18 In response to albumin, proximal tubular cells also stimulate interstitial fibroblasts via paracrine release of transforming growth factor beta, either directly or by activating complement or macrophages.18,19
Studies linking albuminuria to kidney disease
Albuminuria has traditionally been associated with diabetes mellitus as a predictor of overt diabetic nephropathy,20,21 although in type 1 diabetes, established albuminuria can spontaneously regress.22
Albuminuria is also a strong predictor of progression in chronic kidney disease.23 In fact, in the last decade, albuminuria has become an independent criterion in the definition of chronic kidney disease; excretion of more than 30 mg of albumin per day, sustained for at least 3 months, qualifies as chronic kidney disease, with independent prognostic implications (Table 3).13
Astor et al,24 in a meta-analysis of 13 studies with more than 21,000 patients with chronic kidney disease, found that the risk of end-stage renal disease was three times higher in those with albuminuria.
Gansevoort et al,23 in a meta-analysis of nine studies with nearly 850,000 participants from the general population, found that the risk of end-stage renal disease increased continuously as albumin excretion increased. They also found that as albuminuria increased, so did the risk of progression of chronic kidney disease and the incidence of acute kidney injury.
Hemmelgarn et al,25 in a large pooled cohort study with more than 1.5 million participants from the general population, showed that increasing albuminuria was associated with a decline in the estimated glomerular filtration rate (GFR) and with progression to end-stage renal disease across all strata of baseline renal function. For example, in persons with an estimated GFR of 60 mL/min/1.73 m2
- 1 per 1,000 person-years for those with no proteinuria
- 2.8 per 1,000 person-years for those with mild proteinuria (trace or 1+ by dipstick or ACR 30–300 mg/g)
- 13.4 per 1,000 person-years for those with heavy proteinuria (2+ or ACR > 300 mg/g).
Rates of progression to end-stage renal disease were:
- 0.03 per 1,000 person-years with no proteinuria
- 0.05 per 1,000 person-years with mild proteinuria
- 1 per 1,000 person-years with heavy proteinuria.25
CARDIOVASCULAR CONSEQUENCES OF ALBUMINURIA
The exact pathophysiologic link between albuminuria and cardiovascular disease is unknown, but several mechanisms have been proposed.
One is that generalized endothelial dysfunction causes both albuminuria and cardiovascular disease.26 Endothelium-derived nitric oxide has vasodilator, antiplatelet, antiproliferative, antiadhesive, permeability-decreasing, and anti-inflammatory properties. Impaired endothelial synthesis of nitric oxide has been independently associated with both microalbuminuria and diabetes.27
Low levels of heparan sulfate (which has antithrombogenic effects and decreases vessel permeability) in the glycocalyx lining vessel walls may also account for albuminuria and for the other cardiovascular effects.28–30 These changes may be the consequence of chronic low-grade inflammation, which precedes the occurrence and progression of both albuminuria and atherothrombotic disease. The resulting abnormalities in the endothelial glycocalyx could lead to increased glomerular permeability to albumin and may also be implicated in the pathogenesis of atherosclerosis.26
In an atherosclerotic aorta and coronary arteries, the endothelial dysfunction may cause increased leakage of cholesterol and glycated end-products into the myocardium, resulting in increasing wall stiffness and left ventricular mass. A similar atherosclerotic process may account for coronary artery microthrombi, resulting in subendocardial ischemia that could lead to systolic and diastolic heart dysfunction.31
Studies linking albuminuria to heart disease
There is convincing evidence that albuminuria is associated with cardiovascular disease. An ACR between 30 and 300 mg/g was independently associated with myocardial infarction and ischemia.32 People with albuminuria have more than twice the risk of severe coronary artery disease, and albuminuria is also associated with increased intimal thickening in the carotid arteries.33,34 An ACR in the same range has been associated with increased incidence and progression of coronary artery calcification.35 Higher brachial-ankle pulse-wave velocity has also been demonstrated with albuminuria in a dose-dependent fashion.36,37
An ACR of 30 to 300 mg/g has been linked to left ventricular hypertrophy independently of other risk factors,38 and functionally with diastolic dysfunction and abnormal midwall shortening.39 In a study of a subgroup of patients with diabetes from a population-based cohort of Native American patients (the Strong Heart Study),39 the prevalence of diastolic dysfunction was:
- 16% in those with no albuminuria
- 26% in those with an ACR of 30 to 300 mg/g
- 31% in those with an ACR greater than 300 mg/g.
The association persisted even after controlling for age, sex, hypertension, and other covariates.
Those pathologic associations have been directly linked to clinical outcomes. For patients with heart failure (New York Heart Association class II–IV), a study found that albuminuria (an ACR > 30 mg/g) conferred a 41% higher risk of admission for heart failure, and an ACR greater than 300 mg/g was associated with an 88% higher risk.40
In an analysis of a prospective cohort from the general population with albuminuria and a low prevalence of renal dysfunction (the Prevention of Renal and Vascular Endstage Disease study),41 albuminuria was associated with a modest increase in the incidence of the composite end point of myocardial infarction, stroke, ischemic heart disease, revascularization procedures, and all-cause mortality per doubling of the urine albumin excretion (hazard ratio 1.08, range 1.04 –1.12).
The relationship to cardiovascular outcomes extends below traditional lower-limit thresholds of albuminuria (corresponding to an ACR > 30 mg/g). A subgroup of patients from the Framingham Offspring Study without prevalent cardiovascular disease, hypertension, diabetes, or kidney disease, and thus with a low to intermediate probability of cardiovascular events, were found to have thresholds for albuminuria as low as 5.3 mg/g in men and 10.8 mg/g in women to discriminate between incident coronary artery disease, heart failure, cerebrovascular disease, other peripheral vascular disease, or death.42
In a meta-analysis including more than 1 million patients in the general population, increasing albuminuria was associated with an increase in deaths from all causes in a continuous manner, with no threshold effect.43 In patients with an ACR of 30 mg/g, the hazard ratio for death was 1.63, increasing to 2.22 for those with more than 300 mg/g compared with those with no albuminuria. A similar increase in the risk of myocardial infarction, heart failure, stroke, or sudden cardiac death was noted with higher ACR.43
Important prospective cohort studies and meta-analyses related to albuminuria and kidney and cardiovascular disease and death are summarized in the eTable.23,39–50
THE CASE FOR TREATING ALBUMINURIA
Reduced progression of renal disease
Treating patients who have proteinuric chronic kidney disease with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) can reduce the risk of progression of renal failure. However, it is unclear whether this benefit is the result of treating concomitant risk factors independent of the reduction in albuminuria, and there is no consistent treatment effect in improving renal outcomes across studies.
Fink et al,51 in a meta-analysis, found that chronic kidney disease patients with diabetes, hypertension, and macroalbuminuria had a 40% lower risk of progression to end-stage renal disease if they received an ACE inhibitor (relative risk [RR] 0.60, 95% confidence interval [CI] 0.43–0.83). In the same meta-analysis, ARBs also reduced the risk of progression to end-stage renal disease (RR 0.77, 95% CI 0.66–0.90).
Jafar et al,52 in an analysis of pooled patient-level data including only nondiabetic patients on ACE inhibitor therapy (n = 1,860), found that the risk of progression of renal failure, defined as a doubling of serum creatinine or end-stage renal disease, was reduced (RR 0.70, 95% CI 0.55–0.88). Patients with higher levels of albuminuria showed the most benefit, but the effect was not conclusive for albuminuria below 500 mg/day at baseline.
Maione et al,53 in a meta-analysis that included patients with albuminuria who were treated with ACE inhibitors vs placebo (n = 8,231), found a similar reduction in risk of:
- Progression to end-stage renal disease (RR 0.67, 95% CI 0.54–0.84)
- Doubling of serum creatinine (RR 0.62, 95% CI 0.46–0.84)
- Progression of albuminuria (RR 0.49, 95% CI 0.36–0.65)
- Normalization of pathologic albuminuria (as defined by the trialists in the individual studies) (RR 2.99, 95% CI 1.82–4.91).
Similar results were obtained for patients with albuminuria who were treated with ARBs.53
ONTARGET.54 In contrast, in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial, the combination of an ACE inhibitor and an ARB showed no benefit in reducing the progression of renal failure, and in those patients with chronic kidney disease there was a higher risk of a doubling of serum creatinine or of the development of end-stage renal disease and hyperkalemia.
Also, in a pooled analysis of the ONTARGET and Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease (TRANSCEND) trials, a 50% reduction in baseline albuminuria was associated with reduced progression of renal failure in those with a baseline ACR less than 10 mg/g.55
Improved cardiovascular outcomes
There is also evidence of better cardiovascular outcomes with treatment of albuminuria. Again, it is uncertain whether this is a result of treating risk factors other than albuminuria with ACE inhibitors or ARBs, and there is no parallel benefit demonstrated across all studies.
LIFE.47,48 In the Losartan Intervention for Endpoint Reduction in Hypertension trial, survival analyses suggested a decrease in risk of cardiovascular adverse events as the degree of proteinuria improved with ARB therapy.
Maione et al,53 in a meta-analysis including 8,231 patients with albuminuria and at least one other risk factor, found a significant reduction in the rate of nonfatal cardiovascular outcomes (angina, myocardial infarction, revascularization, stroke, transient ischemic attack, or heart failure) with ACE inhibitors vs placebo (RR 0.88, CI 0.82–0.94) and also in 3,888 patients treated with ARBs vs placebo (RR 0.77, CI 0.61–0.98). However, the meta-analysis did not show that ACE inhibitor or ARB therapy reduced rate of cardiovascular or all-cause mortality.
Fink et al,51 in their meta-analysis of 18 trials of ACE inhibitors and four trials of ARBs, also found no evidence that ACE inhibitor or ARB therapy reduced cardiovascular mortality rates.38
The ONTARGET trial evaluated the combination of an ACE inhibitor and ARB therapy in patients with diabetes or preexisting peripheral vascular disease. Reductions in the rate of cardiovascular disease or death were not observed, and in those with chronic kidney disease, there was a higher risk of doubling of serum creatinine or development of end-stage renal disease and adverse events of hyperkalemia.56 And although an increase in baseline albuminuria was associated with worse cardiovascular outcomes, its reduction in the ONTARGET and TRANSCEND trials did not demonstrate better outcomes when the baseline ACR was greater than 10 mg/g.55
WHO SHOULD BE TESTED?
The benefit of adding albuminuria to conventional cardiovascular risk stratification such as Framingham risk scoring is not conclusive. However, today’s clinician may view albuminuria as a biomarker for renal and cardiovascular disease, as albuminuria might be a surrogate marker for endothelial dysfunction in the glomerular capillaries or other vital vascular beds.
High-risk populations and chronic kidney disease patients
Nearly all the current guidelines recommend annual screening for albuminuria in patients with diabetes and hypertension (Table 2).7,10–13 Other high-risk populations include people with cardiovascular disease, a family history of end-stage renal disease, and metabolic syndrome. Additionally, chronic kidney disease patients whose estimated GFR defines them as being in stage 3 or higher (ie, GFR < 60 mL/min/1.73m2), regardless of other comorbidities, should be tested for albuminuria, as it is an important risk predictor.
Most experts prefer that albuminuria be measured by urine ACR in a first morning voided sample, though this is not the only option.
Screening the general population
Given that albuminuria has been shown to be such an important prognosticator for patients at high risk and those with chronic kidney disease, the question arises whether screening for albuminuria in the asymptomatic low-risk general population would foster earlier detection and therefore enable earlier intervention and result in improved outcomes. However, a systematic review done for the United States Preventive Services Task Force and for an American College of Physicians clinical practice guideline did not find robust evidence to support this.51
OUR RECOMMENDATIONS
Who should be tested?
- Patients with chronic kidney disease stage 3, 4, or 5 (GFR < 60 mL/min/1.73m2) who are not on dialysis
- Patients who are considered at higher risk of adverse outcomes, such as those with diabetes, hypertension, a family history of end-stage renal disease, or cardiovascular disease. Testing is useful for recognizing increased renal and cardiovascular risk and may lead clinicians to prescribe or titrate a renin-angiotensin system antagonist, a statin, or both, or to modify other cardiovascular risk factors.
- Not recommended: routine screening in the general population who are asymptomatic or are considered at low risk.
Which test should be used?
Based on current evidence and most guidelines, we recommend the urine ACR test as the screening test for people with diabetes and others deemed to be at high risk.
What should be done about albuminuria?
- Controlling blood pressure is important, and though there is debate about the target blood pressure, an individualized plan should be developed with the patient based on age, comorbidities, and goals of care.
- An ACE inhibitor or ARB, if not contraindicated, is recommended for patients with diabetes whose ACR is greater than 30 mg/g and for patients with chronic kidney disease and an ACR greater than 300 mg/g.
- Current evidence does not support the combined use of an ACE inhibitor and an ARB, as proof of benefit is lacking and the risk of adverse events is higher.
- Refer patients with high or unexplained albuminuria to a nephrologist or clinic specializing in chronic kidney disease.
“One can obtain considerable information concerning the general health by examining the urine.”
—Hippocrates (460?–355? BCE)
Chronic kidney disease is a notable public health concern because it is an important risk factor for end-stage renal disease, cardiovascular disease, and death. Its prevalence1 exceeds 10% and is considerably higher in high-risk groups, such as those with diabetes or hypertension, which are growing in the United States.
While high levels of total protein in the urine have always been recognized as pathologic, a growing body of evidence links excretion of the protein albumin to adverse cardiovascular outcomes, and most international guidelines now recommend measuring albumin specifically. Albuminuria is a predictor of declining renal function and is independently associated with adverse cardiovascular outcomes. Thus, clinicians need to detect it early, manage it effectively, and reduce concurrent risk factors for cardiovascular disease.
Therefore, this review will focus on albuminuria. However, because the traditional standard for urinary protein measurement was total protein, and because a few guidelines still recommend measuring total protein rather than albumin, we will also briefly discuss total urinary protein.
MOST URINARY PROTEIN IS ALBUMIN
Most of the protein in the urine is albumin filtered from the plasma. Less than half of the rest is derived from the distal renal tubules (uromodulin or Tamm-Horsfall mucoprotein), 2 and urine also contains a small and varying proportion of immunoglobulins, low-molecular-weight proteins, and light chains.
Normal healthy people lose less than 30 mg of albumin in the urine per day. In greater amounts, albumin is the major urinary protein in most kidney diseases. Other proteins in urine can be specific markers of less-common illnesses such as plasma cell dyscrasia, glomerulopathy, and renal tubular disease.
MEASURING PROTEINURIA AND ALBUMINURIA
Albumin is not a homogeneous molecule in urine. It undergoes changes to its molecular configuration in the presence of certain ions, peptides, hormones, and drugs, and as a result of proteolytic fragmentation both in the plasma and in renal tubules.3 Consequently, measuring urinary albumin involves a trade-off between convenience and accuracy.
A 24-hour timed urine sample has long been the gold standard for measuring albuminuria, but the collection is cumbersome and time-consuming, and the test is prone to laboratory error.
Dipstick measurements are more convenient and are better at detecting albumin than other proteins in urine, but they have low sensitivity and high interobserver variation.3–5
The albumin-to-creatinine ratio (ACR). As the quantity of protein in the urine changes with time of day, exertion, stress level, and posture, spot-checking of urine samples is not as good as timed collection. However, a simultaneous measurement of creatinine in a spot urine sample adjusts for protein concentration, which can vary with a person’s hydration status. The ACR so obtained is consistent with the 24-hour timed collection (the gold standard) and is the recommended method of assessing albuminuria.3 An early morning urine sample is favored, as it avoids orthostatic variations and varies less in the same individual.
In a study in the general population comparing the ACR in a random sample and in an early morning sample, only 44% of those who had an ACR of 30 mg/g or higher in the random sample had one this high in the early morning sample.6 However, getting an early morning sample is not always feasible in clinical practice. If you are going to measure albuminuria, the Kidney Disease Outcomes and Quality Initiative7 suggests checking the ACR in a random sample and then, if the test is positive, following up and confirming it within 3 months with an early morning sample.
Also, since creatinine excretion differs with race, diet, and muscle mass, if the 24-hour creatinine excretion is not close to 1 g, the ACR will give an erroneous estimate of the 24-hour excretion rate.3
Table 1 compares the various methods of measuring protein in the urine.3,5,8,9 Of note, methods of measuring albumin and total protein vary considerably in their precision and accuracy, making it impossible to reliably translate values from one to the other.5
National and international guidelines (Table 2)7,10–13 agree that albuminuria should be tested in diabetic patients, as it is a surrogate marker for early diabetic nephropathy.3,13 Most guidelines also recommend measuring albuminuria by a urine ACR test as the preferred measure, even in people without diabetes.
Also, no single cutoff is universally accepted for distinguishing pathologic albuminuria from physiologic albuminuria, nor is there a universally accepted unit of measure.14 Because approximately 1 g of creatinine is lost in the urine per day, the ACR has the convenient property of numerically matching the albumin excretory rate expressed in milligrams per 24 hours. The other commonly used unit is milligrams of albumin per millimole of creatinine; 30 mg/g is roughly equal to 3 mg/mmol.
The term microalbuminuria was traditionally used to refer to albumin excretion of 30 to 299 mg per 24 hours, and macroalbuminuria to 300 mg or more per 24 hours. However, as there is no pathophysiologic basis to these thresholds (see outcomes data below), the current Kidney Disease Improving Global Outcomes (KDIGO) guidelines do not recommend using these terms.13,15
RENAL COMPLICATIONS OF ALBUMINURIA
A failure of the glomerular filtration barrier or of proximal tubular reabsorption accounts for most cases of pathologic albuminuria.16 Processes affecting the glomerular filtration of albumin include endothelial cell dysfunction and abnormalities with the glomerular basement membrane, podocytes, or the slit diaphragms among the podocytic processes.17
Ultrafiltrated albumin has been directly implicated in tubulointerstitial damage and glomerulosclerosis through diverse pathways. In the proximal tubule, albumin up-regulates interleukin 8 (a chemoattractant for lymphocytes and neutrophils), induces synthesis of endothelin 1 (which stimulates renal cell proliferation, extracellular matrix production, and monocyte attraction), and causes apoptosis of tubular cells.18 In response to albumin, proximal tubular cells also stimulate interstitial fibroblasts via paracrine release of transforming growth factor beta, either directly or by activating complement or macrophages.18,19
Studies linking albuminuria to kidney disease
Albuminuria has traditionally been associated with diabetes mellitus as a predictor of overt diabetic nephropathy,20,21 although in type 1 diabetes, established albuminuria can spontaneously regress.22
Albuminuria is also a strong predictor of progression in chronic kidney disease.23 In fact, in the last decade, albuminuria has become an independent criterion in the definition of chronic kidney disease; excretion of more than 30 mg of albumin per day, sustained for at least 3 months, qualifies as chronic kidney disease, with independent prognostic implications (Table 3).13
Astor et al,24 in a meta-analysis of 13 studies with more than 21,000 patients with chronic kidney disease, found that the risk of end-stage renal disease was three times higher in those with albuminuria.
Gansevoort et al,23 in a meta-analysis of nine studies with nearly 850,000 participants from the general population, found that the risk of end-stage renal disease increased continuously as albumin excretion increased. They also found that as albuminuria increased, so did the risk of progression of chronic kidney disease and the incidence of acute kidney injury.
Hemmelgarn et al,25 in a large pooled cohort study with more than 1.5 million participants from the general population, showed that increasing albuminuria was associated with a decline in the estimated glomerular filtration rate (GFR) and with progression to end-stage renal disease across all strata of baseline renal function. For example, in persons with an estimated GFR of 60 mL/min/1.73 m2
- 1 per 1,000 person-years for those with no proteinuria
- 2.8 per 1,000 person-years for those with mild proteinuria (trace or 1+ by dipstick or ACR 30–300 mg/g)
- 13.4 per 1,000 person-years for those with heavy proteinuria (2+ or ACR > 300 mg/g).
Rates of progression to end-stage renal disease were:
- 0.03 per 1,000 person-years with no proteinuria
- 0.05 per 1,000 person-years with mild proteinuria
- 1 per 1,000 person-years with heavy proteinuria.25
CARDIOVASCULAR CONSEQUENCES OF ALBUMINURIA
The exact pathophysiologic link between albuminuria and cardiovascular disease is unknown, but several mechanisms have been proposed.
One is that generalized endothelial dysfunction causes both albuminuria and cardiovascular disease.26 Endothelium-derived nitric oxide has vasodilator, antiplatelet, antiproliferative, antiadhesive, permeability-decreasing, and anti-inflammatory properties. Impaired endothelial synthesis of nitric oxide has been independently associated with both microalbuminuria and diabetes.27
Low levels of heparan sulfate (which has antithrombogenic effects and decreases vessel permeability) in the glycocalyx lining vessel walls may also account for albuminuria and for the other cardiovascular effects.28–30 These changes may be the consequence of chronic low-grade inflammation, which precedes the occurrence and progression of both albuminuria and atherothrombotic disease. The resulting abnormalities in the endothelial glycocalyx could lead to increased glomerular permeability to albumin and may also be implicated in the pathogenesis of atherosclerosis.26
In an atherosclerotic aorta and coronary arteries, the endothelial dysfunction may cause increased leakage of cholesterol and glycated end-products into the myocardium, resulting in increasing wall stiffness and left ventricular mass. A similar atherosclerotic process may account for coronary artery microthrombi, resulting in subendocardial ischemia that could lead to systolic and diastolic heart dysfunction.31
Studies linking albuminuria to heart disease
There is convincing evidence that albuminuria is associated with cardiovascular disease. An ACR between 30 and 300 mg/g was independently associated with myocardial infarction and ischemia.32 People with albuminuria have more than twice the risk of severe coronary artery disease, and albuminuria is also associated with increased intimal thickening in the carotid arteries.33,34 An ACR in the same range has been associated with increased incidence and progression of coronary artery calcification.35 Higher brachial-ankle pulse-wave velocity has also been demonstrated with albuminuria in a dose-dependent fashion.36,37
An ACR of 30 to 300 mg/g has been linked to left ventricular hypertrophy independently of other risk factors,38 and functionally with diastolic dysfunction and abnormal midwall shortening.39 In a study of a subgroup of patients with diabetes from a population-based cohort of Native American patients (the Strong Heart Study),39 the prevalence of diastolic dysfunction was:
- 16% in those with no albuminuria
- 26% in those with an ACR of 30 to 300 mg/g
- 31% in those with an ACR greater than 300 mg/g.
The association persisted even after controlling for age, sex, hypertension, and other covariates.
Those pathologic associations have been directly linked to clinical outcomes. For patients with heart failure (New York Heart Association class II–IV), a study found that albuminuria (an ACR > 30 mg/g) conferred a 41% higher risk of admission for heart failure, and an ACR greater than 300 mg/g was associated with an 88% higher risk.40
In an analysis of a prospective cohort from the general population with albuminuria and a low prevalence of renal dysfunction (the Prevention of Renal and Vascular Endstage Disease study),41 albuminuria was associated with a modest increase in the incidence of the composite end point of myocardial infarction, stroke, ischemic heart disease, revascularization procedures, and all-cause mortality per doubling of the urine albumin excretion (hazard ratio 1.08, range 1.04 –1.12).
The relationship to cardiovascular outcomes extends below traditional lower-limit thresholds of albuminuria (corresponding to an ACR > 30 mg/g). A subgroup of patients from the Framingham Offspring Study without prevalent cardiovascular disease, hypertension, diabetes, or kidney disease, and thus with a low to intermediate probability of cardiovascular events, were found to have thresholds for albuminuria as low as 5.3 mg/g in men and 10.8 mg/g in women to discriminate between incident coronary artery disease, heart failure, cerebrovascular disease, other peripheral vascular disease, or death.42
In a meta-analysis including more than 1 million patients in the general population, increasing albuminuria was associated with an increase in deaths from all causes in a continuous manner, with no threshold effect.43 In patients with an ACR of 30 mg/g, the hazard ratio for death was 1.63, increasing to 2.22 for those with more than 300 mg/g compared with those with no albuminuria. A similar increase in the risk of myocardial infarction, heart failure, stroke, or sudden cardiac death was noted with higher ACR.43
Important prospective cohort studies and meta-analyses related to albuminuria and kidney and cardiovascular disease and death are summarized in the eTable.23,39–50
THE CASE FOR TREATING ALBUMINURIA
Reduced progression of renal disease
Treating patients who have proteinuric chronic kidney disease with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) can reduce the risk of progression of renal failure. However, it is unclear whether this benefit is the result of treating concomitant risk factors independent of the reduction in albuminuria, and there is no consistent treatment effect in improving renal outcomes across studies.
Fink et al,51 in a meta-analysis, found that chronic kidney disease patients with diabetes, hypertension, and macroalbuminuria had a 40% lower risk of progression to end-stage renal disease if they received an ACE inhibitor (relative risk [RR] 0.60, 95% confidence interval [CI] 0.43–0.83). In the same meta-analysis, ARBs also reduced the risk of progression to end-stage renal disease (RR 0.77, 95% CI 0.66–0.90).
Jafar et al,52 in an analysis of pooled patient-level data including only nondiabetic patients on ACE inhibitor therapy (n = 1,860), found that the risk of progression of renal failure, defined as a doubling of serum creatinine or end-stage renal disease, was reduced (RR 0.70, 95% CI 0.55–0.88). Patients with higher levels of albuminuria showed the most benefit, but the effect was not conclusive for albuminuria below 500 mg/day at baseline.
Maione et al,53 in a meta-analysis that included patients with albuminuria who were treated with ACE inhibitors vs placebo (n = 8,231), found a similar reduction in risk of:
- Progression to end-stage renal disease (RR 0.67, 95% CI 0.54–0.84)
- Doubling of serum creatinine (RR 0.62, 95% CI 0.46–0.84)
- Progression of albuminuria (RR 0.49, 95% CI 0.36–0.65)
- Normalization of pathologic albuminuria (as defined by the trialists in the individual studies) (RR 2.99, 95% CI 1.82–4.91).
Similar results were obtained for patients with albuminuria who were treated with ARBs.53
ONTARGET.54 In contrast, in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial, the combination of an ACE inhibitor and an ARB showed no benefit in reducing the progression of renal failure, and in those patients with chronic kidney disease there was a higher risk of a doubling of serum creatinine or of the development of end-stage renal disease and hyperkalemia.
Also, in a pooled analysis of the ONTARGET and Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease (TRANSCEND) trials, a 50% reduction in baseline albuminuria was associated with reduced progression of renal failure in those with a baseline ACR less than 10 mg/g.55
Improved cardiovascular outcomes
There is also evidence of better cardiovascular outcomes with treatment of albuminuria. Again, it is uncertain whether this is a result of treating risk factors other than albuminuria with ACE inhibitors or ARBs, and there is no parallel benefit demonstrated across all studies.
LIFE.47,48 In the Losartan Intervention for Endpoint Reduction in Hypertension trial, survival analyses suggested a decrease in risk of cardiovascular adverse events as the degree of proteinuria improved with ARB therapy.
Maione et al,53 in a meta-analysis including 8,231 patients with albuminuria and at least one other risk factor, found a significant reduction in the rate of nonfatal cardiovascular outcomes (angina, myocardial infarction, revascularization, stroke, transient ischemic attack, or heart failure) with ACE inhibitors vs placebo (RR 0.88, CI 0.82–0.94) and also in 3,888 patients treated with ARBs vs placebo (RR 0.77, CI 0.61–0.98). However, the meta-analysis did not show that ACE inhibitor or ARB therapy reduced rate of cardiovascular or all-cause mortality.
Fink et al,51 in their meta-analysis of 18 trials of ACE inhibitors and four trials of ARBs, also found no evidence that ACE inhibitor or ARB therapy reduced cardiovascular mortality rates.38
The ONTARGET trial evaluated the combination of an ACE inhibitor and ARB therapy in patients with diabetes or preexisting peripheral vascular disease. Reductions in the rate of cardiovascular disease or death were not observed, and in those with chronic kidney disease, there was a higher risk of doubling of serum creatinine or development of end-stage renal disease and adverse events of hyperkalemia.56 And although an increase in baseline albuminuria was associated with worse cardiovascular outcomes, its reduction in the ONTARGET and TRANSCEND trials did not demonstrate better outcomes when the baseline ACR was greater than 10 mg/g.55
WHO SHOULD BE TESTED?
The benefit of adding albuminuria to conventional cardiovascular risk stratification such as Framingham risk scoring is not conclusive. However, today’s clinician may view albuminuria as a biomarker for renal and cardiovascular disease, as albuminuria might be a surrogate marker for endothelial dysfunction in the glomerular capillaries or other vital vascular beds.
High-risk populations and chronic kidney disease patients
Nearly all the current guidelines recommend annual screening for albuminuria in patients with diabetes and hypertension (Table 2).7,10–13 Other high-risk populations include people with cardiovascular disease, a family history of end-stage renal disease, and metabolic syndrome. Additionally, chronic kidney disease patients whose estimated GFR defines them as being in stage 3 or higher (ie, GFR < 60 mL/min/1.73m2), regardless of other comorbidities, should be tested for albuminuria, as it is an important risk predictor.
Most experts prefer that albuminuria be measured by urine ACR in a first morning voided sample, though this is not the only option.
Screening the general population
Given that albuminuria has been shown to be such an important prognosticator for patients at high risk and those with chronic kidney disease, the question arises whether screening for albuminuria in the asymptomatic low-risk general population would foster earlier detection and therefore enable earlier intervention and result in improved outcomes. However, a systematic review done for the United States Preventive Services Task Force and for an American College of Physicians clinical practice guideline did not find robust evidence to support this.51
OUR RECOMMENDATIONS
Who should be tested?
- Patients with chronic kidney disease stage 3, 4, or 5 (GFR < 60 mL/min/1.73m2) who are not on dialysis
- Patients who are considered at higher risk of adverse outcomes, such as those with diabetes, hypertension, a family history of end-stage renal disease, or cardiovascular disease. Testing is useful for recognizing increased renal and cardiovascular risk and may lead clinicians to prescribe or titrate a renin-angiotensin system antagonist, a statin, or both, or to modify other cardiovascular risk factors.
- Not recommended: routine screening in the general population who are asymptomatic or are considered at low risk.
Which test should be used?
Based on current evidence and most guidelines, we recommend the urine ACR test as the screening test for people with diabetes and others deemed to be at high risk.
What should be done about albuminuria?
- Controlling blood pressure is important, and though there is debate about the target blood pressure, an individualized plan should be developed with the patient based on age, comorbidities, and goals of care.
- An ACE inhibitor or ARB, if not contraindicated, is recommended for patients with diabetes whose ACR is greater than 30 mg/g and for patients with chronic kidney disease and an ACR greater than 300 mg/g.
- Current evidence does not support the combined use of an ACE inhibitor and an ARB, as proof of benefit is lacking and the risk of adverse events is higher.
- Refer patients with high or unexplained albuminuria to a nephrologist or clinic specializing in chronic kidney disease.
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298:2038–2047.
- Hoyer JR, Seiler MW. Pathophysiology of Tamm-Horsfall protein. Kidney Int 1979; 16:279–289.
- Viswanathan G, Upadhyay A. Assessment of proteinuria. Adv Chronic Kidney Dis 2011; 18:243–248.
- Guh JY. Proteinuria versus albuminuria in chronic kidney disease. Nephrology (Carlton) 2010; 15(suppl 2):53–56.
- Lamb EJ, MacKenzie F, Stevens PE. How should proteinuria be detected and measured? Ann Clin Biochem 2009; 46:205–217.
- Saydah SH, Pavkov ME, Zhang C, et al. Albuminuria prevalence in first morning void compared with previous random urine from adults in the National Health and Nutrition Examination Survey, 2009-2010. Clin Chem 2013; 59:675–683.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
- Younes N, Cleary PA, Steffes MW, et al; DCCT/EDIC Research Group. Comparison of urinary albumin-creatinine ratio and albumin excretion rate in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Clin J Am Soc Nephrol 2010; 5:1235–1242.
- Brinkman JW, de Zeeuw D, Duker JJ, et al. Falsely low urinary albumin concentrations after prolonged frozen storage of urine samples. Clin Chem 2005; 51:2181–2183.
- National Collaborating Centre for Chronic Conditions (UK). Chronic Kidney Disease: National Clinical Guideline for Early Identification and Management in Adults in Primary and Secondary Care. London: Royal College of Physicians (UK) 2008.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3:1–150.
- Johnson DW. Global proteinuria guidelines: are we nearly there yet? Clin Biochem Rev 2011; 32:89–95.
- Ruggenenti P, Remuzzi G. Time to abandon microalbuminuria? Kidney Int 2006; 70:1214–1222.
- Glassock RJ. Is the presence of microalbuminuria a relevant marker of kidney disease? Curr Hypertens Rep 2010; 12:364–368.
- Zhang A, Huang S. Progress in pathogenesis of proteinuria. Int J Nephrol 2012; 2012:314251.
- Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol 2006; 17:2974–2984.
- Karalliedde J, Viberti G. Proteinuria in diabetes: bystander or pathway to cardiorenal disease? J Am Soc Nephrol 2010; 21:2020–2027.
- Svendsen PA, Oxenbøll B, Christiansen JS. Microalbuminuria in diabetic patients—a longitudinal study. Acta Endocrinol Suppl (Copenh) 1981; 242:53–54.
- Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1982; 1:1430–1432.
- Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003; 348:2285–2293.
- Gansevoort RT, Matsushita K, van der Velde M, et al; Chronic Kidney Disease Prognosis Consortium. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011; 80:93–104.
- Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 2011; 79:1331–1340.
- Hemmelgarn BR, Manns BJ, Lloyd A, et al; Alberta Kidney Disease Network. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010; 303:423–429.
- Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol 2006; 17:2106–2111.
- Stehouwer CD, Henry RM, Dekker JM, Nijpels G, Heine RJ, Bouter LM. Microalbuminuria is associated with impaired brachial artery, flow-mediated vasodilation in elderly individuals without and with diabetes: further evidence for a link between microalbuminuria and endothelial dysfunction—the Hoorn Study. Kidney Int Suppl 2004; 92:S42–S44.
- Wasty F, Alavi MZ, Moore S. Distribution of glycosaminoglycans in the intima of human aortas: changes in atherosclerosis and diabetes mellitus. Diabetologia 1993; 36:316–322.
- Ylä-Herttuala S, Sumuvuori H, Karkola K, Möttönen M, Nikkari T. Glycosaminoglycans in normal and atherosclerotic human coronary arteries. Lab Invest 1986; 54:402–407.
- Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 1989; 32:219–226.
- van Hoeven KH, Factor SM. A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation 1990; 82:848–855.
- Diercks GF, van Boven AJ, Hillege HL, et al. Microalbuminuria is independently associated with ischaemic electrocardiographic abnormalities in a large non-diabetic population. The PREVEND (Prevention of REnal and Vascular ENdstage Disease) study. Eur Heart J 2000; 21:1922–1927.
- Bigazzi R, Bianchi S, Nenci R, Baldari D, Baldari G, Campese VM. Increased thickness of the carotid artery in patients with essential hypertension and microalbuminuria. J Hum Hypertens 1995; 9:827–833.
- Tuttle KR, Puhlman ME, Cooney SK, Short R. Urinary albumin and insulin as predictors of coronary artery disease: an angiographic study. Am J Kidney Dis 1999; 34:918–925.
- DeFilippis AP, Kramer HJ, Katz R, et al. Association between coronary artery calcification progression and microalbuminuria: the MESA study. JACC Cardiovasc Imaging 2010; 3:595–604.
- Liu CS, Pi-Sunyer FX, Li CI, et al. Albuminuria is strongly associated with arterial stiffness, especially in diabetic or hypertensive subjects—a population-based study (Taichung Community Health Study, TCHS). Atherosclerosis 2010; 211:315–321.
- Upadhyay A, Hwang SJ, Mitchell GF, et al. Arterial stiffness in mild-to-moderate CKD. J Am Soc Nephrol 2009; 20:2044–2053.
- Pontremoli R, Sofia A, Ravera M, et al. Prevalence and clinical correlates of microalbuminuria in essential hypertension: the MAGIC Study. Microalbuminuria: a Genoa Investigation on Complications. Hypertension 1997; 30:1135–1143.
- Liu JE, Robbins DC, Palmieri V, et al. Association of albuminuria with systolic and diastolic left ventricular dysfunction in type 2 diabetes: the Strong Heart Study. J Am Coll Cardiol 2003; 41:2022–2028.
- Jackson CE, Solomon SD, Gerstein HC, et al; CHARM Investigators and Committees. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet 2009; 374:543–550.
- Smink PA, Lambers Heerspink HJ, Gansevoort RT, et al. Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: the PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am J Kidney Dis 2012; 60:804–811.
- Arnlöv J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation 2005; 112:969–975.
- Chronic Kidney Disease Prognosis Consortium; Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375:2073–2081.
- van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011; 79:1341–1352.
- Ruggenenti P, Porrini E, Motterlini N, et al; BENEDICT Study Investigators. Measurable urinary albumin predicts cardiovascular risk among normoalbuminuric patients with type 2 diabetes. J Am Soc Nephrol 2012; 23:1717–1724.
- Hallan S, Astor B, Romundstad S, Aasarød K, Kvenild K, Coresh J. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: the HUNT II Study. Arch Intern Med 2007; 167:2490–2496.
- Ibsen H, Wachtell K, Olsen MH, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE Study. Kidney Int Suppl 2004; 92:S56–S58.
- Olsen MH, Wachtell K, Bella JN, et al. Albuminuria predicts cardiovascular events independently of left ventricular mass in hypertension: a LIFE substudy. J Hum Hypertens 2004; 18:453–459.
- Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation 2004; 110:32–35.
- Gerstein HC, Mann JF, Yi Q, et al; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426.
- Fink HA, Ishani A, Taylor BC, et al. Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: a systematic review for the US Preventive Services Task Force and for an American College of Physicians Clinical Practice Guideline. Ann Intern Med 2012; 156:570–581.
- Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 2001; 135:73–87.
- Maione A, Navaneethan SD, Graziano G, et al. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and combined therapy in patients with micro- and macroalbuminuria and other cardiovascular risk factors: a systematic review of randomized controlled trials. Nephrol Dial Transplant 2011; 26:2827–2847.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Schmieder RE, Mann JF, Schumacher H, et al; ONTARGET Investigators. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol 2011; 22:1353–1364.
- Tobe SW, Clase CM, Gao P, et al; ONTARGET and TRANSCEND Investigators. Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation 2011; 123:1098–1107.
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298:2038–2047.
- Hoyer JR, Seiler MW. Pathophysiology of Tamm-Horsfall protein. Kidney Int 1979; 16:279–289.
- Viswanathan G, Upadhyay A. Assessment of proteinuria. Adv Chronic Kidney Dis 2011; 18:243–248.
- Guh JY. Proteinuria versus albuminuria in chronic kidney disease. Nephrology (Carlton) 2010; 15(suppl 2):53–56.
- Lamb EJ, MacKenzie F, Stevens PE. How should proteinuria be detected and measured? Ann Clin Biochem 2009; 46:205–217.
- Saydah SH, Pavkov ME, Zhang C, et al. Albuminuria prevalence in first morning void compared with previous random urine from adults in the National Health and Nutrition Examination Survey, 2009-2010. Clin Chem 2013; 59:675–683.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
- Younes N, Cleary PA, Steffes MW, et al; DCCT/EDIC Research Group. Comparison of urinary albumin-creatinine ratio and albumin excretion rate in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Clin J Am Soc Nephrol 2010; 5:1235–1242.
- Brinkman JW, de Zeeuw D, Duker JJ, et al. Falsely low urinary albumin concentrations after prolonged frozen storage of urine samples. Clin Chem 2005; 51:2181–2183.
- National Collaborating Centre for Chronic Conditions (UK). Chronic Kidney Disease: National Clinical Guideline for Early Identification and Management in Adults in Primary and Secondary Care. London: Royal College of Physicians (UK) 2008.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3:1–150.
- Johnson DW. Global proteinuria guidelines: are we nearly there yet? Clin Biochem Rev 2011; 32:89–95.
- Ruggenenti P, Remuzzi G. Time to abandon microalbuminuria? Kidney Int 2006; 70:1214–1222.
- Glassock RJ. Is the presence of microalbuminuria a relevant marker of kidney disease? Curr Hypertens Rep 2010; 12:364–368.
- Zhang A, Huang S. Progress in pathogenesis of proteinuria. Int J Nephrol 2012; 2012:314251.
- Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol 2006; 17:2974–2984.
- Karalliedde J, Viberti G. Proteinuria in diabetes: bystander or pathway to cardiorenal disease? J Am Soc Nephrol 2010; 21:2020–2027.
- Svendsen PA, Oxenbøll B, Christiansen JS. Microalbuminuria in diabetic patients—a longitudinal study. Acta Endocrinol Suppl (Copenh) 1981; 242:53–54.
- Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1982; 1:1430–1432.
- Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003; 348:2285–2293.
- Gansevoort RT, Matsushita K, van der Velde M, et al; Chronic Kidney Disease Prognosis Consortium. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011; 80:93–104.
- Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 2011; 79:1331–1340.
- Hemmelgarn BR, Manns BJ, Lloyd A, et al; Alberta Kidney Disease Network. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010; 303:423–429.
- Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol 2006; 17:2106–2111.
- Stehouwer CD, Henry RM, Dekker JM, Nijpels G, Heine RJ, Bouter LM. Microalbuminuria is associated with impaired brachial artery, flow-mediated vasodilation in elderly individuals without and with diabetes: further evidence for a link between microalbuminuria and endothelial dysfunction—the Hoorn Study. Kidney Int Suppl 2004; 92:S42–S44.
- Wasty F, Alavi MZ, Moore S. Distribution of glycosaminoglycans in the intima of human aortas: changes in atherosclerosis and diabetes mellitus. Diabetologia 1993; 36:316–322.
- Ylä-Herttuala S, Sumuvuori H, Karkola K, Möttönen M, Nikkari T. Glycosaminoglycans in normal and atherosclerotic human coronary arteries. Lab Invest 1986; 54:402–407.
- Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 1989; 32:219–226.
- van Hoeven KH, Factor SM. A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation 1990; 82:848–855.
- Diercks GF, van Boven AJ, Hillege HL, et al. Microalbuminuria is independently associated with ischaemic electrocardiographic abnormalities in a large non-diabetic population. The PREVEND (Prevention of REnal and Vascular ENdstage Disease) study. Eur Heart J 2000; 21:1922–1927.
- Bigazzi R, Bianchi S, Nenci R, Baldari D, Baldari G, Campese VM. Increased thickness of the carotid artery in patients with essential hypertension and microalbuminuria. J Hum Hypertens 1995; 9:827–833.
- Tuttle KR, Puhlman ME, Cooney SK, Short R. Urinary albumin and insulin as predictors of coronary artery disease: an angiographic study. Am J Kidney Dis 1999; 34:918–925.
- DeFilippis AP, Kramer HJ, Katz R, et al. Association between coronary artery calcification progression and microalbuminuria: the MESA study. JACC Cardiovasc Imaging 2010; 3:595–604.
- Liu CS, Pi-Sunyer FX, Li CI, et al. Albuminuria is strongly associated with arterial stiffness, especially in diabetic or hypertensive subjects—a population-based study (Taichung Community Health Study, TCHS). Atherosclerosis 2010; 211:315–321.
- Upadhyay A, Hwang SJ, Mitchell GF, et al. Arterial stiffness in mild-to-moderate CKD. J Am Soc Nephrol 2009; 20:2044–2053.
- Pontremoli R, Sofia A, Ravera M, et al. Prevalence and clinical correlates of microalbuminuria in essential hypertension: the MAGIC Study. Microalbuminuria: a Genoa Investigation on Complications. Hypertension 1997; 30:1135–1143.
- Liu JE, Robbins DC, Palmieri V, et al. Association of albuminuria with systolic and diastolic left ventricular dysfunction in type 2 diabetes: the Strong Heart Study. J Am Coll Cardiol 2003; 41:2022–2028.
- Jackson CE, Solomon SD, Gerstein HC, et al; CHARM Investigators and Committees. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet 2009; 374:543–550.
- Smink PA, Lambers Heerspink HJ, Gansevoort RT, et al. Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: the PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am J Kidney Dis 2012; 60:804–811.
- Arnlöv J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation 2005; 112:969–975.
- Chronic Kidney Disease Prognosis Consortium; Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375:2073–2081.
- van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011; 79:1341–1352.
- Ruggenenti P, Porrini E, Motterlini N, et al; BENEDICT Study Investigators. Measurable urinary albumin predicts cardiovascular risk among normoalbuminuric patients with type 2 diabetes. J Am Soc Nephrol 2012; 23:1717–1724.
- Hallan S, Astor B, Romundstad S, Aasarød K, Kvenild K, Coresh J. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: the HUNT II Study. Arch Intern Med 2007; 167:2490–2496.
- Ibsen H, Wachtell K, Olsen MH, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE Study. Kidney Int Suppl 2004; 92:S56–S58.
- Olsen MH, Wachtell K, Bella JN, et al. Albuminuria predicts cardiovascular events independently of left ventricular mass in hypertension: a LIFE substudy. J Hum Hypertens 2004; 18:453–459.
- Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation 2004; 110:32–35.
- Gerstein HC, Mann JF, Yi Q, et al; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426.
- Fink HA, Ishani A, Taylor BC, et al. Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: a systematic review for the US Preventive Services Task Force and for an American College of Physicians Clinical Practice Guideline. Ann Intern Med 2012; 156:570–581.
- Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 2001; 135:73–87.
- Maione A, Navaneethan SD, Graziano G, et al. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and combined therapy in patients with micro- and macroalbuminuria and other cardiovascular risk factors: a systematic review of randomized controlled trials. Nephrol Dial Transplant 2011; 26:2827–2847.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Schmieder RE, Mann JF, Schumacher H, et al; ONTARGET Investigators. Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol 2011; 22:1353–1364.
- Tobe SW, Clase CM, Gao P, et al; ONTARGET and TRANSCEND Investigators. Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation 2011; 123:1098–1107.
KEY POINTS
- Albuminuria is best measured by the albumin-to-creatinine ratio.
- In several studies, albuminuria has been independently associated with a higher risk of death, cardiovascular events, heart failure, stroke, and progression of chronic kidney disease.
- Despite strong evidence linking albuminuria to adverse outcomes, evidence is limited in favor of routinely screening for it in the general population.
- Evaluating and managing albuminuria require understanding the limits of its clinical measures, controlling other risk factors for progression of renal disease, managing it medically, and referring to a specialist in certain situations.