User login
1 in 4 Unresponsive Coma Patients May Retain Some Awareness
“We found that at least 1 in 4 patients who are unresponsive to commands might actually be quite present and highly cognitive,” said study investigator Nicholas D. Schiff, MD, Feil Family Brain & Mind Research Institute and Department of Neurology, Weill Cornell Medicine, Rockefeller University Hospital, New York.
“In other words, if you go to the bedside and carefully examine someone with a severe brain injury and find no evidence of responsiveness, no one has been able to give you an a priori number to say how likely you are to be wrong in thinking this person is actually unaware, not processing language, and not capable of high-level cognitive work. And the answer to that now is at least 1 in 4 times.”
The findings were published online in The New England Journal of Medicine.
Clinical Implications?
Cognitive motor dissociation (CMD) is a condition whereby patients with a severe brain injury who are unresponsive to commands at the bedside show brain activity on functional MRI (fMRI) or electroencephalography (EEG) when presented with selective motor imagery commands, such as “imagine playing tennis,” or “ imagine opening and closing your hand.”
Previous research shows that CMD is present in 10%-20% of people with a disorder of consciousness, a rate similar to that in patients with acute or chronic brain injury.
Understanding that a patient who appears unconscious has signs of cognitive processing could change the way clinicians and family interact with such individuals. Unresponsive patients who are aware may eventually be able to harness emerging communication technologies such as brain-computer interfaces.
In addition, knowing an individual’s CMD status could aid in prognosis. “We know from one study that there’s a four times increased likelihood that patients will be independent in a year in their function if they have cognitive motor dissociation,” said Dr. Schiff.
Unlike most previous studies of CMD, which were conducted at single sites and had relatively small cohorts, this new study included 353 adults with a disorder of consciousness (mean age, 37.9 years; 64% male) at six multinational sites.
Participants were recruited using a variety of methods, including consecutive enrollment of critically ill patients in the intensive care unit and enrollment of those with chronic illness or injury who were in the postacute phase of brain injury.
Response to Commands
Study participants were at different stages of recovery from an acute brain injury that had occurred an average of 8 months before the study started.
To determine the presence or absence of an observable response to commands among participants, trained staff used the Coma Recovery Scale–Revised (CRS-R); scores on this instrument range from 0 to 23, and higher scores indicate better neurobehavioral function.
About 40% of individuals were diagnosed with coma or vegetative state, 29% with minimally conscious state–minus, and 22% with minimally conscious state–plus. In all, 10% had emerged from a minimally conscious state.
Researchers assessed response to timed and repeated commands using fMRI or EEG in participants without an observable response to verbal commands, including those with a behavioral diagnosis of coma, vegetative state, or minimally conscious state–minus, and in participants with an observable response to verbal commands.
Of the 353 study participants, 61% underwent at least one fMRI assessment and 74% at least one EEG assessment. Both fMRI and EEG were performed in 35% of participants.
Dr. Schiff explained the two assessment types provide slightly different information, in that they measuring different types of brain signals. He also noted that although “every medical center in the world” has EEG, many do not have fMRI.
The brain imaging assessments captured brain activity within the motor area of the frontal cortex when tasked with motor imagery.
Of the 241 participants deemed to be in a coma or vegetative state or minimally conscious state–minus on the basis of CRS-R score, 60 (25%) had a response to commands on task-based fMRI, task-based EEG, or both.
The percentage of participants with CMD varied across study sites, from 2% to 45%, but Dr. Schiff said the reason for this is unclear.
The proportion of participants with CMD may have been even higher if all individuals had been assessed with both imaging techniques, he said.
Higher Rate of Awareness Than in Previous Research
The investigators noted that the percentage of participants with CMD in their study was up to 10 percentage points higher than in previous studies. This may be due to the multimodal approach that classified participants undergoing assessment with both fMRI and EEG on the basis of responses on either technique, they said.
The median age was lower among participants with CMD than those without CMD (30.5 years vs 45.3 years).
Compared with participants without CMD, a higher percentage of those with such dissociation had brain trauma as an etiologic factor (65% vs 38%) and a diagnosis of minimally conscious state–minus on the CRS-R (53% vs 38%).
Among people with CMD, 18% were assessed with fMRI only, 22% with EEG only, and 60% with both fMRI and EEG.
Dr. Schiff noted that the use of both fMRI and EEG appears to be more sensitive in detecting brain activity during tasks compared with use of one of these techniques alone.
Of the 112 participants with a diagnosis of minimally conscious state–plus or who had emerged from the minimally conscious state, 38% had a response to commands on task-based fMRI, task-based EEG, or both. Among these participants, 23% were assessed with fMRI only, 19% with EEG only, and 58% with both fMRI and EEG.
Research shows “it’s very clear that people with severe brain injury continue to get better over time,” noted Dr. Schiff. “Every month and week matters, and so it probably is the case that a lot of these patients are picking up the level of recovery, and the later we go out to measure them, the more likely we are to find people who are CMD than not.”
These new results should prompt further study to explore whether detection of CMD can lead to improved outcomes, the investigators noted. “In addition, the standardization, validation, and simplification of task-based fMRI and EEG methods that are used to detect cognitive motor dissociation are needed to prompt widespread clinical integration of these techniques and investigation of the bioethical implications of the findings.”
All study participants with chronic brain injury had survived their initial illness or injury and had access to a research facility with advanced fMRI and EEG capabilities. “This survival bias may reflect greater cognitive reserve and resilience over time among the participants. As such, the results of our study may not be generalizable to the overall population of patients with cognitive motor dissociation,” the investigators wrote.
Another study limitation was that participating sites used heterogeneous strategies to acquire, analyze, and interpret data, which led to differences in the number, type, and ordering of the cognitive tasks assessed on fMRI and EEG.
“These differences, along with variations in recruitment strategies and participant characteristics, may have contributed to the unequal percentage of participants with cognitive motor dissociation observed at each site. Our findings may therefore not be generalizable across all centers,” the researchers wrote.
Only a few academic medical centers have the specially trained personnel and techniques needed to assess patients for CMD — which, the researchers noted, limits the feasibility of performing these assessments in general practice.
Challenging Research
Commenting on the research, Aarti Sarwal, MD, professor of neurology and section chief, Neurocritical Care, Virginia Commonwealth University, Richmond, Virginia, noted that this was a “very challenging” study to perform, given that only a few academic centers are equipped to perform both fMRI and quantitative EEG analysis.
“In general, finding patients this far out, who have access to clinical, radiological, and electrophysiological testing and were provided good care enough to receive these, is a mammoth task in itself.”
Dr. Sarwal said the study builds on efforts of the Curing Coma campaign , a clinical, scientific, and public health effort of the Neurocritical Care Society to tackle the concept of coma as a treatable medical entity.
“It continues to highlight the challenges of prognostication in acute brain injured patients by showing a higher presence of cognitive function than previously perceived,” she said.
Dr. Sarwal believes that the study’s largest impact is underscoring the need for more research into understanding the degree and quality of cognitive processing in patients with a disorder of consciousness. But it also underlines the need for a “healthy debate” on the cost/benefit analysis of pursuing such research, given the limited number of patients with access to resources.
“This debate needs to include the caregivers and families outside the traditional realms of stakeholders overseeing the science.”
Although communication with comatose patients is still “a ways away,” this research is “a step in the right direction,” said Dr. Sarwal.
The study was funded by the James S. McDonnell Foundation and others. Dr. Schiff and Dr. Sarwal report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
“We found that at least 1 in 4 patients who are unresponsive to commands might actually be quite present and highly cognitive,” said study investigator Nicholas D. Schiff, MD, Feil Family Brain & Mind Research Institute and Department of Neurology, Weill Cornell Medicine, Rockefeller University Hospital, New York.
“In other words, if you go to the bedside and carefully examine someone with a severe brain injury and find no evidence of responsiveness, no one has been able to give you an a priori number to say how likely you are to be wrong in thinking this person is actually unaware, not processing language, and not capable of high-level cognitive work. And the answer to that now is at least 1 in 4 times.”
The findings were published online in The New England Journal of Medicine.
Clinical Implications?
Cognitive motor dissociation (CMD) is a condition whereby patients with a severe brain injury who are unresponsive to commands at the bedside show brain activity on functional MRI (fMRI) or electroencephalography (EEG) when presented with selective motor imagery commands, such as “imagine playing tennis,” or “ imagine opening and closing your hand.”
Previous research shows that CMD is present in 10%-20% of people with a disorder of consciousness, a rate similar to that in patients with acute or chronic brain injury.
Understanding that a patient who appears unconscious has signs of cognitive processing could change the way clinicians and family interact with such individuals. Unresponsive patients who are aware may eventually be able to harness emerging communication technologies such as brain-computer interfaces.
In addition, knowing an individual’s CMD status could aid in prognosis. “We know from one study that there’s a four times increased likelihood that patients will be independent in a year in their function if they have cognitive motor dissociation,” said Dr. Schiff.
Unlike most previous studies of CMD, which were conducted at single sites and had relatively small cohorts, this new study included 353 adults with a disorder of consciousness (mean age, 37.9 years; 64% male) at six multinational sites.
Participants were recruited using a variety of methods, including consecutive enrollment of critically ill patients in the intensive care unit and enrollment of those with chronic illness or injury who were in the postacute phase of brain injury.
Response to Commands
Study participants were at different stages of recovery from an acute brain injury that had occurred an average of 8 months before the study started.
To determine the presence or absence of an observable response to commands among participants, trained staff used the Coma Recovery Scale–Revised (CRS-R); scores on this instrument range from 0 to 23, and higher scores indicate better neurobehavioral function.
About 40% of individuals were diagnosed with coma or vegetative state, 29% with minimally conscious state–minus, and 22% with minimally conscious state–plus. In all, 10% had emerged from a minimally conscious state.
Researchers assessed response to timed and repeated commands using fMRI or EEG in participants without an observable response to verbal commands, including those with a behavioral diagnosis of coma, vegetative state, or minimally conscious state–minus, and in participants with an observable response to verbal commands.
Of the 353 study participants, 61% underwent at least one fMRI assessment and 74% at least one EEG assessment. Both fMRI and EEG were performed in 35% of participants.
Dr. Schiff explained the two assessment types provide slightly different information, in that they measuring different types of brain signals. He also noted that although “every medical center in the world” has EEG, many do not have fMRI.
The brain imaging assessments captured brain activity within the motor area of the frontal cortex when tasked with motor imagery.
Of the 241 participants deemed to be in a coma or vegetative state or minimally conscious state–minus on the basis of CRS-R score, 60 (25%) had a response to commands on task-based fMRI, task-based EEG, or both.
The percentage of participants with CMD varied across study sites, from 2% to 45%, but Dr. Schiff said the reason for this is unclear.
The proportion of participants with CMD may have been even higher if all individuals had been assessed with both imaging techniques, he said.
Higher Rate of Awareness Than in Previous Research
The investigators noted that the percentage of participants with CMD in their study was up to 10 percentage points higher than in previous studies. This may be due to the multimodal approach that classified participants undergoing assessment with both fMRI and EEG on the basis of responses on either technique, they said.
The median age was lower among participants with CMD than those without CMD (30.5 years vs 45.3 years).
Compared with participants without CMD, a higher percentage of those with such dissociation had brain trauma as an etiologic factor (65% vs 38%) and a diagnosis of minimally conscious state–minus on the CRS-R (53% vs 38%).
Among people with CMD, 18% were assessed with fMRI only, 22% with EEG only, and 60% with both fMRI and EEG.
Dr. Schiff noted that the use of both fMRI and EEG appears to be more sensitive in detecting brain activity during tasks compared with use of one of these techniques alone.
Of the 112 participants with a diagnosis of minimally conscious state–plus or who had emerged from the minimally conscious state, 38% had a response to commands on task-based fMRI, task-based EEG, or both. Among these participants, 23% were assessed with fMRI only, 19% with EEG only, and 58% with both fMRI and EEG.
Research shows “it’s very clear that people with severe brain injury continue to get better over time,” noted Dr. Schiff. “Every month and week matters, and so it probably is the case that a lot of these patients are picking up the level of recovery, and the later we go out to measure them, the more likely we are to find people who are CMD than not.”
These new results should prompt further study to explore whether detection of CMD can lead to improved outcomes, the investigators noted. “In addition, the standardization, validation, and simplification of task-based fMRI and EEG methods that are used to detect cognitive motor dissociation are needed to prompt widespread clinical integration of these techniques and investigation of the bioethical implications of the findings.”
All study participants with chronic brain injury had survived their initial illness or injury and had access to a research facility with advanced fMRI and EEG capabilities. “This survival bias may reflect greater cognitive reserve and resilience over time among the participants. As such, the results of our study may not be generalizable to the overall population of patients with cognitive motor dissociation,” the investigators wrote.
Another study limitation was that participating sites used heterogeneous strategies to acquire, analyze, and interpret data, which led to differences in the number, type, and ordering of the cognitive tasks assessed on fMRI and EEG.
“These differences, along with variations in recruitment strategies and participant characteristics, may have contributed to the unequal percentage of participants with cognitive motor dissociation observed at each site. Our findings may therefore not be generalizable across all centers,” the researchers wrote.
Only a few academic medical centers have the specially trained personnel and techniques needed to assess patients for CMD — which, the researchers noted, limits the feasibility of performing these assessments in general practice.
Challenging Research
Commenting on the research, Aarti Sarwal, MD, professor of neurology and section chief, Neurocritical Care, Virginia Commonwealth University, Richmond, Virginia, noted that this was a “very challenging” study to perform, given that only a few academic centers are equipped to perform both fMRI and quantitative EEG analysis.
“In general, finding patients this far out, who have access to clinical, radiological, and electrophysiological testing and were provided good care enough to receive these, is a mammoth task in itself.”
Dr. Sarwal said the study builds on efforts of the Curing Coma campaign , a clinical, scientific, and public health effort of the Neurocritical Care Society to tackle the concept of coma as a treatable medical entity.
“It continues to highlight the challenges of prognostication in acute brain injured patients by showing a higher presence of cognitive function than previously perceived,” she said.
Dr. Sarwal believes that the study’s largest impact is underscoring the need for more research into understanding the degree and quality of cognitive processing in patients with a disorder of consciousness. But it also underlines the need for a “healthy debate” on the cost/benefit analysis of pursuing such research, given the limited number of patients with access to resources.
“This debate needs to include the caregivers and families outside the traditional realms of stakeholders overseeing the science.”
Although communication with comatose patients is still “a ways away,” this research is “a step in the right direction,” said Dr. Sarwal.
The study was funded by the James S. McDonnell Foundation and others. Dr. Schiff and Dr. Sarwal report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
“We found that at least 1 in 4 patients who are unresponsive to commands might actually be quite present and highly cognitive,” said study investigator Nicholas D. Schiff, MD, Feil Family Brain & Mind Research Institute and Department of Neurology, Weill Cornell Medicine, Rockefeller University Hospital, New York.
“In other words, if you go to the bedside and carefully examine someone with a severe brain injury and find no evidence of responsiveness, no one has been able to give you an a priori number to say how likely you are to be wrong in thinking this person is actually unaware, not processing language, and not capable of high-level cognitive work. And the answer to that now is at least 1 in 4 times.”
The findings were published online in The New England Journal of Medicine.
Clinical Implications?
Cognitive motor dissociation (CMD) is a condition whereby patients with a severe brain injury who are unresponsive to commands at the bedside show brain activity on functional MRI (fMRI) or electroencephalography (EEG) when presented with selective motor imagery commands, such as “imagine playing tennis,” or “ imagine opening and closing your hand.”
Previous research shows that CMD is present in 10%-20% of people with a disorder of consciousness, a rate similar to that in patients with acute or chronic brain injury.
Understanding that a patient who appears unconscious has signs of cognitive processing could change the way clinicians and family interact with such individuals. Unresponsive patients who are aware may eventually be able to harness emerging communication technologies such as brain-computer interfaces.
In addition, knowing an individual’s CMD status could aid in prognosis. “We know from one study that there’s a four times increased likelihood that patients will be independent in a year in their function if they have cognitive motor dissociation,” said Dr. Schiff.
Unlike most previous studies of CMD, which were conducted at single sites and had relatively small cohorts, this new study included 353 adults with a disorder of consciousness (mean age, 37.9 years; 64% male) at six multinational sites.
Participants were recruited using a variety of methods, including consecutive enrollment of critically ill patients in the intensive care unit and enrollment of those with chronic illness or injury who were in the postacute phase of brain injury.
Response to Commands
Study participants were at different stages of recovery from an acute brain injury that had occurred an average of 8 months before the study started.
To determine the presence or absence of an observable response to commands among participants, trained staff used the Coma Recovery Scale–Revised (CRS-R); scores on this instrument range from 0 to 23, and higher scores indicate better neurobehavioral function.
About 40% of individuals were diagnosed with coma or vegetative state, 29% with minimally conscious state–minus, and 22% with minimally conscious state–plus. In all, 10% had emerged from a minimally conscious state.
Researchers assessed response to timed and repeated commands using fMRI or EEG in participants without an observable response to verbal commands, including those with a behavioral diagnosis of coma, vegetative state, or minimally conscious state–minus, and in participants with an observable response to verbal commands.
Of the 353 study participants, 61% underwent at least one fMRI assessment and 74% at least one EEG assessment. Both fMRI and EEG were performed in 35% of participants.
Dr. Schiff explained the two assessment types provide slightly different information, in that they measuring different types of brain signals. He also noted that although “every medical center in the world” has EEG, many do not have fMRI.
The brain imaging assessments captured brain activity within the motor area of the frontal cortex when tasked with motor imagery.
Of the 241 participants deemed to be in a coma or vegetative state or minimally conscious state–minus on the basis of CRS-R score, 60 (25%) had a response to commands on task-based fMRI, task-based EEG, or both.
The percentage of participants with CMD varied across study sites, from 2% to 45%, but Dr. Schiff said the reason for this is unclear.
The proportion of participants with CMD may have been even higher if all individuals had been assessed with both imaging techniques, he said.
Higher Rate of Awareness Than in Previous Research
The investigators noted that the percentage of participants with CMD in their study was up to 10 percentage points higher than in previous studies. This may be due to the multimodal approach that classified participants undergoing assessment with both fMRI and EEG on the basis of responses on either technique, they said.
The median age was lower among participants with CMD than those without CMD (30.5 years vs 45.3 years).
Compared with participants without CMD, a higher percentage of those with such dissociation had brain trauma as an etiologic factor (65% vs 38%) and a diagnosis of minimally conscious state–minus on the CRS-R (53% vs 38%).
Among people with CMD, 18% were assessed with fMRI only, 22% with EEG only, and 60% with both fMRI and EEG.
Dr. Schiff noted that the use of both fMRI and EEG appears to be more sensitive in detecting brain activity during tasks compared with use of one of these techniques alone.
Of the 112 participants with a diagnosis of minimally conscious state–plus or who had emerged from the minimally conscious state, 38% had a response to commands on task-based fMRI, task-based EEG, or both. Among these participants, 23% were assessed with fMRI only, 19% with EEG only, and 58% with both fMRI and EEG.
Research shows “it’s very clear that people with severe brain injury continue to get better over time,” noted Dr. Schiff. “Every month and week matters, and so it probably is the case that a lot of these patients are picking up the level of recovery, and the later we go out to measure them, the more likely we are to find people who are CMD than not.”
These new results should prompt further study to explore whether detection of CMD can lead to improved outcomes, the investigators noted. “In addition, the standardization, validation, and simplification of task-based fMRI and EEG methods that are used to detect cognitive motor dissociation are needed to prompt widespread clinical integration of these techniques and investigation of the bioethical implications of the findings.”
All study participants with chronic brain injury had survived their initial illness or injury and had access to a research facility with advanced fMRI and EEG capabilities. “This survival bias may reflect greater cognitive reserve and resilience over time among the participants. As such, the results of our study may not be generalizable to the overall population of patients with cognitive motor dissociation,” the investigators wrote.
Another study limitation was that participating sites used heterogeneous strategies to acquire, analyze, and interpret data, which led to differences in the number, type, and ordering of the cognitive tasks assessed on fMRI and EEG.
“These differences, along with variations in recruitment strategies and participant characteristics, may have contributed to the unequal percentage of participants with cognitive motor dissociation observed at each site. Our findings may therefore not be generalizable across all centers,” the researchers wrote.
Only a few academic medical centers have the specially trained personnel and techniques needed to assess patients for CMD — which, the researchers noted, limits the feasibility of performing these assessments in general practice.
Challenging Research
Commenting on the research, Aarti Sarwal, MD, professor of neurology and section chief, Neurocritical Care, Virginia Commonwealth University, Richmond, Virginia, noted that this was a “very challenging” study to perform, given that only a few academic centers are equipped to perform both fMRI and quantitative EEG analysis.
“In general, finding patients this far out, who have access to clinical, radiological, and electrophysiological testing and were provided good care enough to receive these, is a mammoth task in itself.”
Dr. Sarwal said the study builds on efforts of the Curing Coma campaign , a clinical, scientific, and public health effort of the Neurocritical Care Society to tackle the concept of coma as a treatable medical entity.
“It continues to highlight the challenges of prognostication in acute brain injured patients by showing a higher presence of cognitive function than previously perceived,” she said.
Dr. Sarwal believes that the study’s largest impact is underscoring the need for more research into understanding the degree and quality of cognitive processing in patients with a disorder of consciousness. But it also underlines the need for a “healthy debate” on the cost/benefit analysis of pursuing such research, given the limited number of patients with access to resources.
“This debate needs to include the caregivers and families outside the traditional realms of stakeholders overseeing the science.”
Although communication with comatose patients is still “a ways away,” this research is “a step in the right direction,” said Dr. Sarwal.
The study was funded by the James S. McDonnell Foundation and others. Dr. Schiff and Dr. Sarwal report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Dementia Deemed Highly Preventable: Here’s How
A new report on the preventability of dementia is both exciting and paradigm-shifting. The new study, published in The Lancet by the Lancet Commission on Dementia, estimates that .
This is paradigm-shifting because dementia is often perceived as an inevitable consequence of the aging process, with a major genetic component. But this study suggests that modifying these risk factors can benefit everyone, irrespective of genetic risk, and that it’s important to have a life-course approach. It’s never too early or too late to start to modify these factors.
We’ve known for a long time that many chronic diseases are highly preventable and modifiable. Some that come to mind are type 2 diabetes, coronary heart disease, and even certain forms of cancer. Modifiable risk factors include cigarette smoking, diet, physical activity, and maintaining a healthy weight. This study suggests that many of the same risk factors and more are relevant to reducing risk for dementia.
Let’s go through the risk factors, many of which are behavioral. These risk factors include lifestyle factors such as lack of physical activity, cigarette smoking, excessive alcohol consumption, and obesity. The cardiovascular or vascular-specific risk factors include not only those behavioral factors but also hypertension, high LDL cholesterol, and diabetes. Cognitive engagement–specific risk factors include social isolation, which is a major risk factor for dementia, as well as untreated hearing or vision loss, which can exacerbate social isolation and depression, and low educational attainment, which can be related to less cognitive engagement.
They also mention traumatic brain injury from an accident or contact sports without head protection as a risk factor, and the environmental risk factor of air pollution or poor air quality.
Two of these risk factors are new since the previous report in 2020: elevated LDL cholesterol and untreated vision loss, both of which are quite treatable. Overall, these findings suggest that a lot can be done to lower dementia risk, but it requires individual behavior modifications as well as a comprehensive approach with involvement of the healthcare system for improved screening, access, and public policy to reduce air pollution.
Some of these risk factors are more relevant to women, especially the social isolation that is so common later in life in women. In the United States, close to two out of three patients with dementia are women.
So, informing our patients about these risk factors and what can be done in terms of behavior modification, increased screening, and treatment for these conditions can go a long way in helping our patients reduce their risk for dementia.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
A new report on the preventability of dementia is both exciting and paradigm-shifting. The new study, published in The Lancet by the Lancet Commission on Dementia, estimates that .
This is paradigm-shifting because dementia is often perceived as an inevitable consequence of the aging process, with a major genetic component. But this study suggests that modifying these risk factors can benefit everyone, irrespective of genetic risk, and that it’s important to have a life-course approach. It’s never too early or too late to start to modify these factors.
We’ve known for a long time that many chronic diseases are highly preventable and modifiable. Some that come to mind are type 2 diabetes, coronary heart disease, and even certain forms of cancer. Modifiable risk factors include cigarette smoking, diet, physical activity, and maintaining a healthy weight. This study suggests that many of the same risk factors and more are relevant to reducing risk for dementia.
Let’s go through the risk factors, many of which are behavioral. These risk factors include lifestyle factors such as lack of physical activity, cigarette smoking, excessive alcohol consumption, and obesity. The cardiovascular or vascular-specific risk factors include not only those behavioral factors but also hypertension, high LDL cholesterol, and diabetes. Cognitive engagement–specific risk factors include social isolation, which is a major risk factor for dementia, as well as untreated hearing or vision loss, which can exacerbate social isolation and depression, and low educational attainment, which can be related to less cognitive engagement.
They also mention traumatic brain injury from an accident or contact sports without head protection as a risk factor, and the environmental risk factor of air pollution or poor air quality.
Two of these risk factors are new since the previous report in 2020: elevated LDL cholesterol and untreated vision loss, both of which are quite treatable. Overall, these findings suggest that a lot can be done to lower dementia risk, but it requires individual behavior modifications as well as a comprehensive approach with involvement of the healthcare system for improved screening, access, and public policy to reduce air pollution.
Some of these risk factors are more relevant to women, especially the social isolation that is so common later in life in women. In the United States, close to two out of three patients with dementia are women.
So, informing our patients about these risk factors and what can be done in terms of behavior modification, increased screening, and treatment for these conditions can go a long way in helping our patients reduce their risk for dementia.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
A new report on the preventability of dementia is both exciting and paradigm-shifting. The new study, published in The Lancet by the Lancet Commission on Dementia, estimates that .
This is paradigm-shifting because dementia is often perceived as an inevitable consequence of the aging process, with a major genetic component. But this study suggests that modifying these risk factors can benefit everyone, irrespective of genetic risk, and that it’s important to have a life-course approach. It’s never too early or too late to start to modify these factors.
We’ve known for a long time that many chronic diseases are highly preventable and modifiable. Some that come to mind are type 2 diabetes, coronary heart disease, and even certain forms of cancer. Modifiable risk factors include cigarette smoking, diet, physical activity, and maintaining a healthy weight. This study suggests that many of the same risk factors and more are relevant to reducing risk for dementia.
Let’s go through the risk factors, many of which are behavioral. These risk factors include lifestyle factors such as lack of physical activity, cigarette smoking, excessive alcohol consumption, and obesity. The cardiovascular or vascular-specific risk factors include not only those behavioral factors but also hypertension, high LDL cholesterol, and diabetes. Cognitive engagement–specific risk factors include social isolation, which is a major risk factor for dementia, as well as untreated hearing or vision loss, which can exacerbate social isolation and depression, and low educational attainment, which can be related to less cognitive engagement.
They also mention traumatic brain injury from an accident or contact sports without head protection as a risk factor, and the environmental risk factor of air pollution or poor air quality.
Two of these risk factors are new since the previous report in 2020: elevated LDL cholesterol and untreated vision loss, both of which are quite treatable. Overall, these findings suggest that a lot can be done to lower dementia risk, but it requires individual behavior modifications as well as a comprehensive approach with involvement of the healthcare system for improved screening, access, and public policy to reduce air pollution.
Some of these risk factors are more relevant to women, especially the social isolation that is so common later in life in women. In the United States, close to two out of three patients with dementia are women.
So, informing our patients about these risk factors and what can be done in terms of behavior modification, increased screening, and treatment for these conditions can go a long way in helping our patients reduce their risk for dementia.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
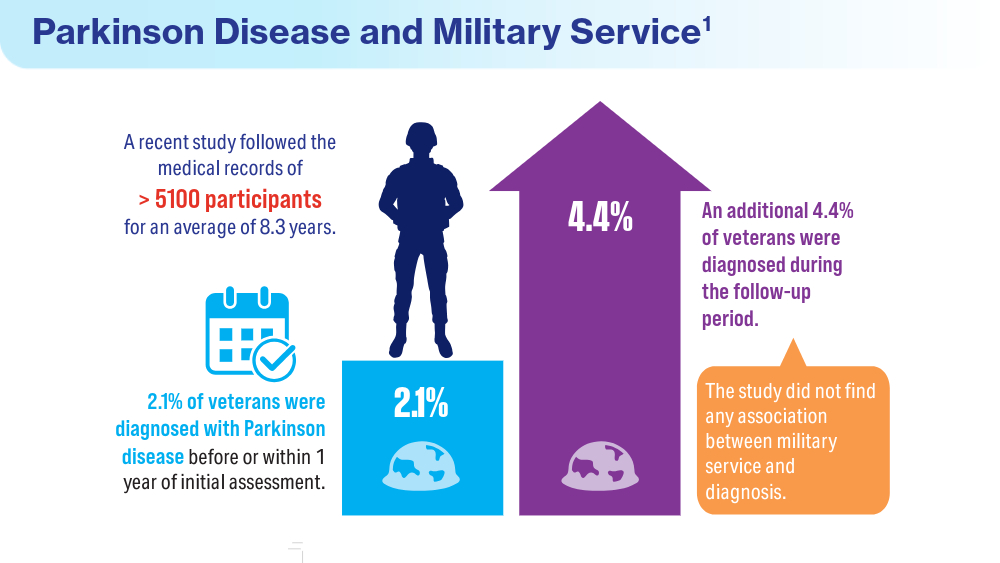
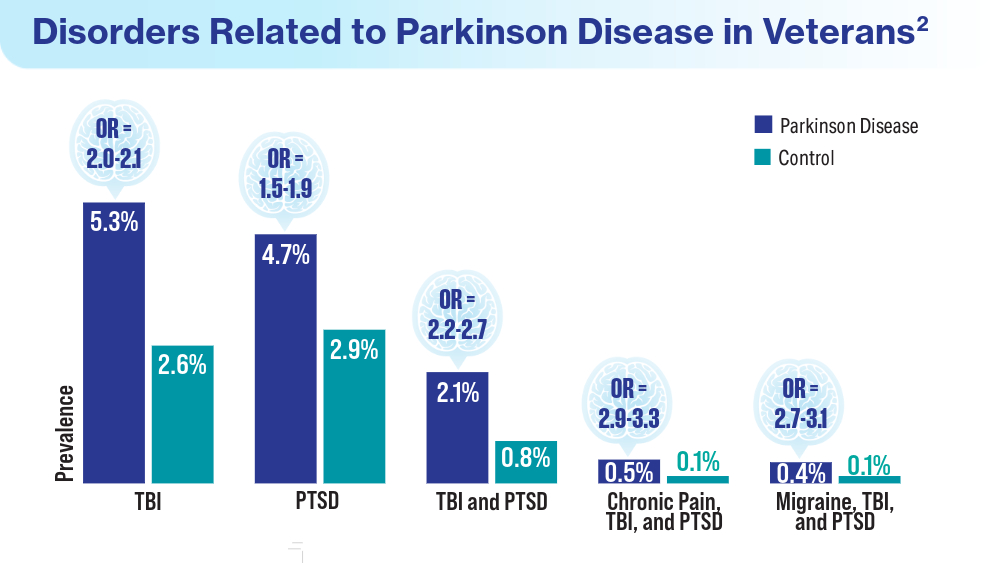
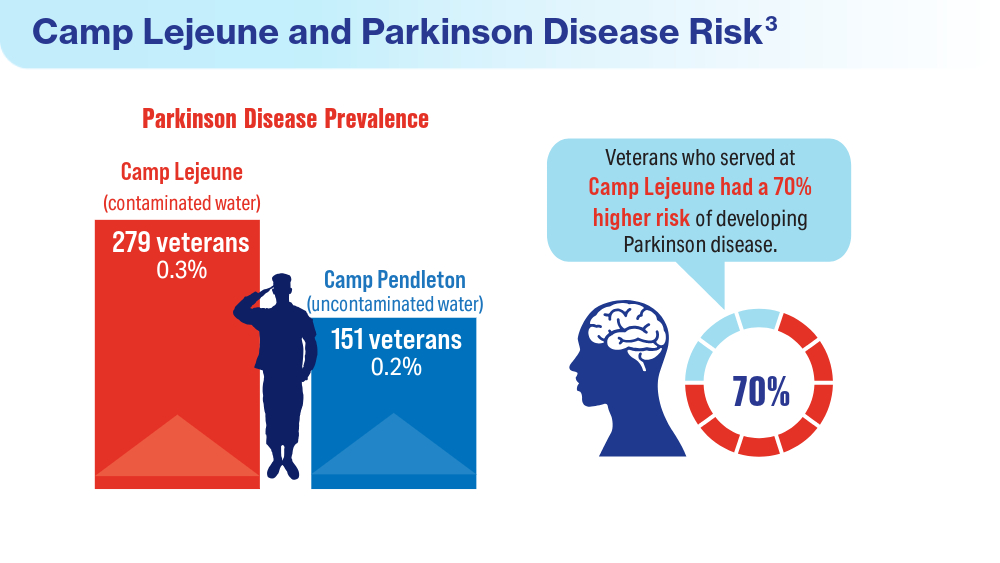
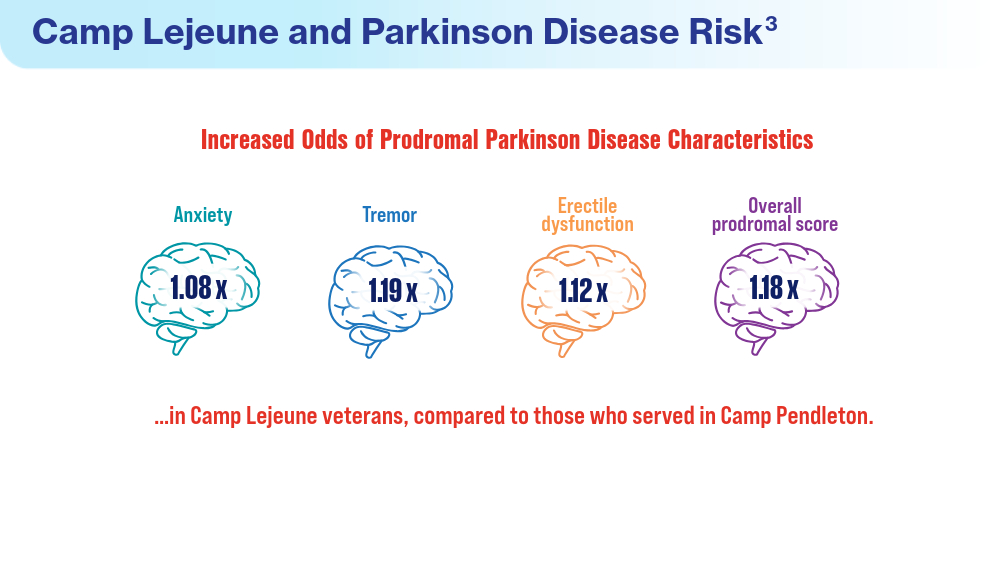
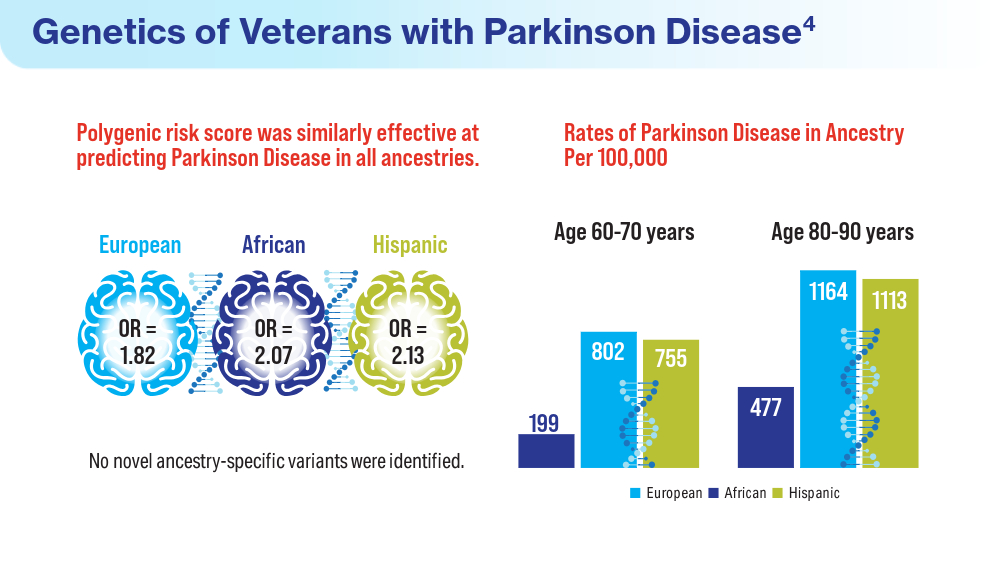
Data Trends 2024: Parkinson Disease
- Power MC, Parthasarathy V, Gianattasio KZ, et al. Investigation of the association of military employment and Parkinson’s disease with a validated Parkinson’s disease case-finding strategy. Brain Inj. 2023;37(5):383-387. doi:10.1080/02699052.2022.2158234
- Scott GD, Neilson LE, Woltjer R, Quinn JF, Lim MM. Lifelong association of disorders related to military trauma with subsequent Parkinson’s disease. Mov Disord. 2023;38(8):1483-1492. doi:10.1002/mds.29457
- Goldman SM, Weaver FM, Stroupe KT, et al. Risk of Parkinson disease among service members at Marine Corps Base Camp Lejeune. JAMA Neurol. 2023;80(7):673-681. doi:10.1001/jamaneurol.2023.1168
- Pankratz N, Cole BR, Beutel KM, Liao KP, Ashe J. Parkinson disease genetics extended to African and Hispanic ancestries in the VA Million Veteran Program. Neurol Genet. 2023;10(1):e200110. doi:10.1212/NXG.0000000000200110
- Power MC, Parthasarathy V, Gianattasio KZ, et al. Investigation of the association of military employment and Parkinson’s disease with a validated Parkinson’s disease case-finding strategy. Brain Inj. 2023;37(5):383-387. doi:10.1080/02699052.2022.2158234
- Scott GD, Neilson LE, Woltjer R, Quinn JF, Lim MM. Lifelong association of disorders related to military trauma with subsequent Parkinson’s disease. Mov Disord. 2023;38(8):1483-1492. doi:10.1002/mds.29457
- Goldman SM, Weaver FM, Stroupe KT, et al. Risk of Parkinson disease among service members at Marine Corps Base Camp Lejeune. JAMA Neurol. 2023;80(7):673-681. doi:10.1001/jamaneurol.2023.1168
- Pankratz N, Cole BR, Beutel KM, Liao KP, Ashe J. Parkinson disease genetics extended to African and Hispanic ancestries in the VA Million Veteran Program. Neurol Genet. 2023;10(1):e200110. doi:10.1212/NXG.0000000000200110
- Power MC, Parthasarathy V, Gianattasio KZ, et al. Investigation of the association of military employment and Parkinson’s disease with a validated Parkinson’s disease case-finding strategy. Brain Inj. 2023;37(5):383-387. doi:10.1080/02699052.2022.2158234
- Scott GD, Neilson LE, Woltjer R, Quinn JF, Lim MM. Lifelong association of disorders related to military trauma with subsequent Parkinson’s disease. Mov Disord. 2023;38(8):1483-1492. doi:10.1002/mds.29457
- Goldman SM, Weaver FM, Stroupe KT, et al. Risk of Parkinson disease among service members at Marine Corps Base Camp Lejeune. JAMA Neurol. 2023;80(7):673-681. doi:10.1001/jamaneurol.2023.1168
- Pankratz N, Cole BR, Beutel KM, Liao KP, Ashe J. Parkinson disease genetics extended to African and Hispanic ancestries in the VA Million Veteran Program. Neurol Genet. 2023;10(1):e200110. doi:10.1212/NXG.0000000000200110
Federal Health Care Data Trends 2024
Federal Health Care Data Trends is a special supplement to Federal Practitioner, showcasing the latest research in health care for veterans and active-duty military members via compelling infographics. Click below to view highlights from the issue:
Federal Health Care Data Trends is a special supplement to Federal Practitioner, showcasing the latest research in health care for veterans and active-duty military members via compelling infographics. Click below to view highlights from the issue:
Federal Health Care Data Trends is a special supplement to Federal Practitioner, showcasing the latest research in health care for veterans and active-duty military members via compelling infographics. Click below to view highlights from the issue:
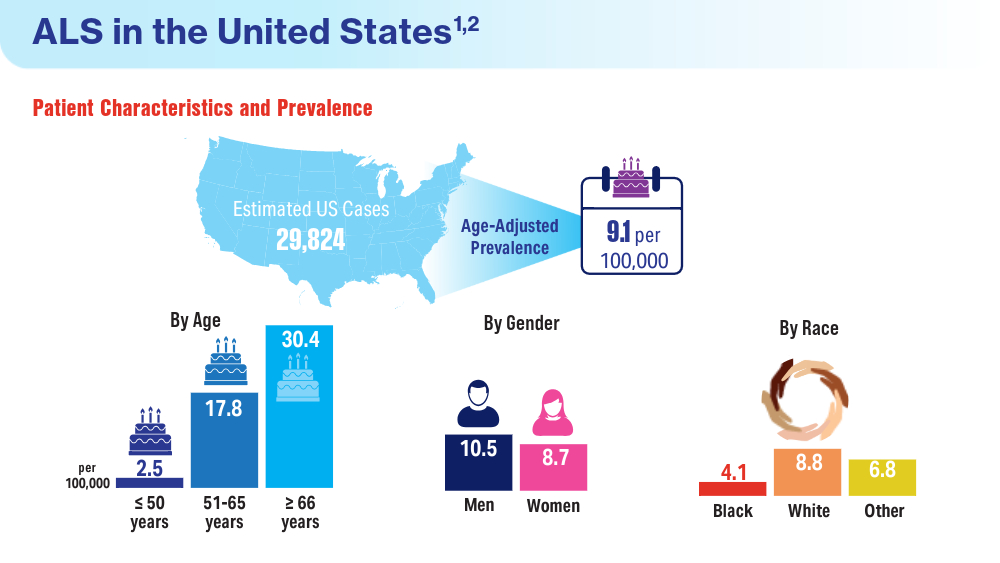
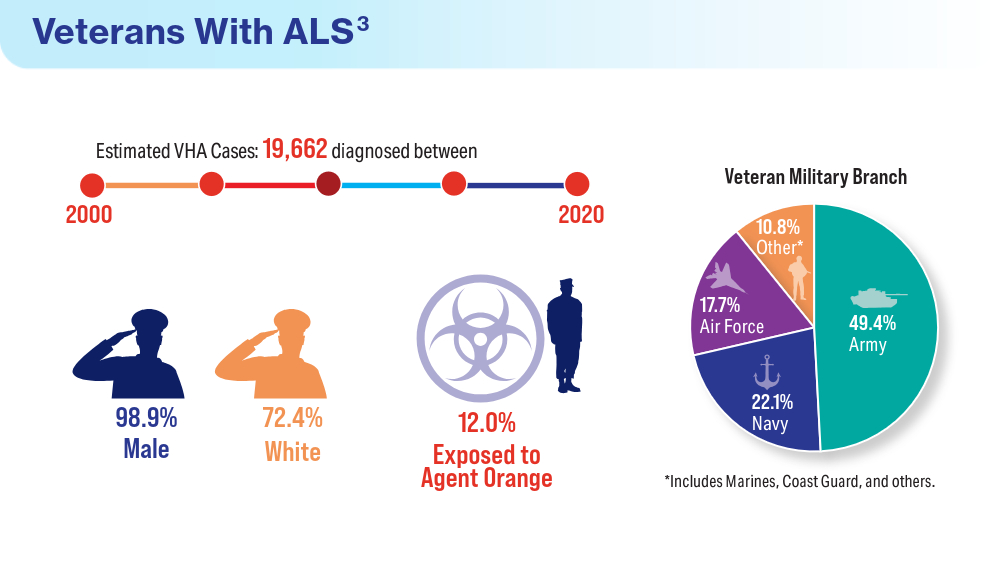
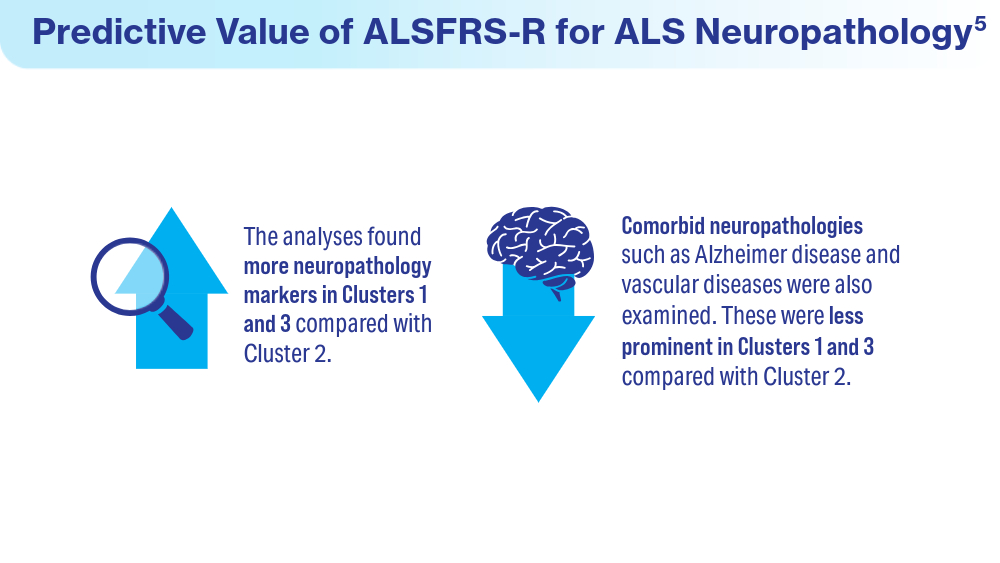
Data Trends 2024: Amyotrophic Lateral Sclerosis (ALS)
- Mehta P, Raymond J, Zhang Y, et al. Prevalence of amyotrophic lateral sclerosis in the United States, 2018. Amyotroph Lateral Scler Frontotemporal Degener. Published online August 21, 2023. doi:10.1080/21678421.2023.2245858
- What is amyotrophic lateral sclerosis (ALS)? Centers for Disease Control and Prevention. Updated May 13, 2022. Accessed April 15, 2024. https://www.cdc.gov/als/WhatisAmyotrophiclateralsclerosis.html
- Reimer RJ, Goncalves A, Soper B, et al. An electronic health record cohort of veterans with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. Published online August 9, 2023. doi:10.1080/21678421.2023.2239300
- Kudritzki V, Howard IM. Telehealth-based exercise in amyotrophic lateral sclerosis. Front Neurol. 2023;14:1238916. doi:10.3389/fneur.2023.1238916
- Colvin LE, Foster ZW, Stein TD, et al. Utility of the ALSFRS-R for predicting ALS and comorbid disease neuropathology: the Veterans Affairs Biorepository Brain Bank. Muscle Nerve. 2022;66(2):167-174. doi:10.1002/mus.27635
- Rabadi MH, Russell KC, Xu C. Predictors of mortality in veterans with amyotrophic lateral sclerosis: respiratory status and speech disorder at presentation. Med Sci Monit. 2024;30:e943288. doi:10.12659/MSM.943288
- Mehta P, Raymond J, Zhang Y, et al. Prevalence of amyotrophic lateral sclerosis in the United States, 2018. Amyotroph Lateral Scler Frontotemporal Degener. Published online August 21, 2023. doi:10.1080/21678421.2023.2245858
- What is amyotrophic lateral sclerosis (ALS)? Centers for Disease Control and Prevention. Updated May 13, 2022. Accessed April 15, 2024. https://www.cdc.gov/als/WhatisAmyotrophiclateralsclerosis.html
- Reimer RJ, Goncalves A, Soper B, et al. An electronic health record cohort of veterans with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. Published online August 9, 2023. doi:10.1080/21678421.2023.2239300
- Kudritzki V, Howard IM. Telehealth-based exercise in amyotrophic lateral sclerosis. Front Neurol. 2023;14:1238916. doi:10.3389/fneur.2023.1238916
- Colvin LE, Foster ZW, Stein TD, et al. Utility of the ALSFRS-R for predicting ALS and comorbid disease neuropathology: the Veterans Affairs Biorepository Brain Bank. Muscle Nerve. 2022;66(2):167-174. doi:10.1002/mus.27635
- Rabadi MH, Russell KC, Xu C. Predictors of mortality in veterans with amyotrophic lateral sclerosis: respiratory status and speech disorder at presentation. Med Sci Monit. 2024;30:e943288. doi:10.12659/MSM.943288
- Mehta P, Raymond J, Zhang Y, et al. Prevalence of amyotrophic lateral sclerosis in the United States, 2018. Amyotroph Lateral Scler Frontotemporal Degener. Published online August 21, 2023. doi:10.1080/21678421.2023.2245858
- What is amyotrophic lateral sclerosis (ALS)? Centers for Disease Control and Prevention. Updated May 13, 2022. Accessed April 15, 2024. https://www.cdc.gov/als/WhatisAmyotrophiclateralsclerosis.html
- Reimer RJ, Goncalves A, Soper B, et al. An electronic health record cohort of veterans with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. Published online August 9, 2023. doi:10.1080/21678421.2023.2239300
- Kudritzki V, Howard IM. Telehealth-based exercise in amyotrophic lateral sclerosis. Front Neurol. 2023;14:1238916. doi:10.3389/fneur.2023.1238916
- Colvin LE, Foster ZW, Stein TD, et al. Utility of the ALSFRS-R for predicting ALS and comorbid disease neuropathology: the Veterans Affairs Biorepository Brain Bank. Muscle Nerve. 2022;66(2):167-174. doi:10.1002/mus.27635
- Rabadi MH, Russell KC, Xu C. Predictors of mortality in veterans with amyotrophic lateral sclerosis: respiratory status and speech disorder at presentation. Med Sci Monit. 2024;30:e943288. doi:10.12659/MSM.943288
AHS White Paper Guides Treatment of Posttraumatic Headache in Youth
The guidance document, the first of its kind, covers risk factors for prolonged recovery, along with pharmacologic and nonpharmacologic management strategies, and supports an emphasis on multidisciplinary care, lead author Carlyn Patterson Gentile, MD, PhD, attending physician in the Division of Neurology at Children’s Hospital of Philadelphia in Pennsylvania, and colleagues reported.
“There are no guidelines to inform the management of posttraumatic headache in youth, but multiple studies have been conducted over the past 2 decades,” the authors wrote in Headache. “This white paper aims to provide a thorough review of the current literature, identify gaps in knowledge, and provide a road map for [posttraumatic headache] management in youth based on available evidence and expert opinion.”
Clarity for an Underrecognized Issue
According to Russell Lonser, MD, professor and chair of neurological surgery at Ohio State University, Columbus, the white paper is important because it offers concrete guidance for health care providers who may be less familiar with posttraumatic headache in youth.
“It brings together all of the previous literature ... in a very well-written way,” Dr. Lonser said in an interview. “More than anything, it could reassure [providers] that they shouldn’t be hunting down potentially magical cures, and reassure them in symptomatic management.”
Meeryo C. Choe, MD, associate clinical professor of pediatric neurology at UCLA Health in Calabasas, California, said the paper also helps shine a light on what may be a more common condition than the public suspects.
“While the media focuses on the effects of concussion in professional sports athletes, the biggest population of athletes is in our youth population,” Dr. Choe said in a written comment. “Almost 25 million children participate in sports throughout the country, and yet we lack guidelines on how to treat posttraumatic headache which can often develop into persistent postconcussive symptoms.”
This white paper, she noted, builds on Dr. Gentile’s 2021 systematic review, introduces new management recommendations, and aligns with the latest consensus statement from the Concussion in Sport Group.
Risk Factors
The white paper first emphasizes the importance of early identification of youth at high risk for prolonged recovery from posttraumatic headache. Risk factors include female sex, adolescent age, a high number of acute symptoms following the initial injury, and social determinants of health.
“I agree that it is important to identify these patients early to improve the recovery trajectory,” Dr. Choe said.
Identifying these individuals quickly allows for timely intervention with both pharmacologic and nonpharmacologic therapies, Dr. Gentile and colleagues noted, potentially mitigating persistent symptoms. Clinicians are encouraged to perform thorough initial assessments to identify these risk factors and initiate early, personalized management plans.
Initial Management of Acute Posttraumatic Headache
For the initial management of acute posttraumatic headache, the white paper recommends a scheduled dosing regimen of simple analgesics. Ibuprofen at a dosage of 10 mg/kg every 6-8 hours (up to a maximum of 600 mg per dose) combined with acetaminophen has shown the best evidence for efficacy. Provided the patient is clinically stable, this regimen should be initiated within 48 hours of the injury and maintained with scheduled dosing for 3-10 days.
If effective, these medications can subsequently be used on an as-needed basis. Careful usage of analgesics is crucial, the white paper cautions, as overadministration can lead to medication-overuse headaches, complicating the recovery process.
Secondary Treatment Options
In cases where first-line oral medications are ineffective, the AHS white paper outlines several secondary treatment options. These include acute intravenous therapies such as ketorolac, dopamine receptor antagonists, and intravenous fluids. Nerve blocks and oral corticosteroid bridges may also be considered.
The white paper stresses the importance of individualized treatment plans that consider the specific needs and responses of each patient, noting that the evidence supporting these approaches is primarily derived from retrospective studies and case reports.
“Patient preferences should be factored in,” said Sean Rose, MD, pediatric neurologist and codirector of the Complex Concussion Clinic at Nationwide Children’s Hospital, Columbus, Ohio.
Supplements and Preventive Measures
For adolescents and young adults at high risk of prolonged posttraumatic headache, the white paper suggests the use of riboflavin and magnesium supplements. Small randomized clinical trials suggest that these supplements may aid in speeding recovery when administered for 1-2 weeks within 48 hours of injury.
If significant headache persists after 2 weeks, a regimen of riboflavin 400 mg daily and magnesium 400-500 mg nightly can be trialed for 6-8 weeks, in line with recommendations for migraine prevention. Additionally, melatonin at a dose of 3-5 mg nightly for an 8-week course may be considered for patients experiencing comorbid sleep disturbances.
Targeted Preventative Therapy
The white paper emphasizes the importance of targeting preventative therapy to the primary headache phenotype.
For instance, patients presenting with a migraine phenotype, or those with a personal or family history of migraines, may be most likely to respond to medications proven effective in migraine prevention, such as amitriptyline, topiramate, and propranolol.
“Most research evidence [for treating posttraumatic headache in youth] is still based on the treatment of migraine,” Dr. Rose pointed out in a written comment.
Dr. Gentile and colleagues recommend initiating preventive therapies 4-6 weeks post injury if headaches are not improving, occur more than 1-2 days per week, or significantly impact daily functioning.
Specialist Referrals and Physical Activity
Referral to a headache specialist is advised for patients who do not respond to first-line acute and preventive therapies. Specialists can offer advanced diagnostic and therapeutic options, the authors noted, ensuring a comprehensive approach to managing posttraumatic headache.
The white paper also recommends noncontact, sub–symptom threshold aerobic physical activity and activities of daily living after an initial 24-48 hour period of symptom-limited cognitive and physical rest. Engaging in these activities may promote faster recovery and help patients gradually return to their normal routines.
“This has been a shift in the concussion treatment approach over the last decade, and is one of the most important interventions we can recommend as physicians,” Dr. Choe noted. “This is where pediatricians and emergency department physicians seeing children acutely can really make a difference in the recovery trajectory for a child after a concussion. ‘Cocoon therapy’ has been proven not only to not work, but be detrimental to recovery.”
Nonpharmacologic Interventions
Based on clinical assessment, nonpharmacologic interventions may also be considered, according to the white paper. These interventions include cervico-vestibular therapy, which addresses neck and balance issues, and cognitive-behavioral therapy, which helps manage the psychological aspects of chronic headache. Dr. Gentile and colleagues highlighted the potential benefits of a collaborative care model that incorporates these nonpharmacologic interventions alongside pharmacologic treatments, providing a holistic approach to posttraumatic headache management.
“Persisting headaches after concussion are often driven by multiple factors,” Dr. Rose said. “Multidisciplinary concussion clinics can offer multiple treatment approaches such as behavioral, physical therapy, exercise, and medication options.”
Unmet Needs
The white paper concludes by calling for high-quality prospective cohort studies and placebo-controlled, randomized, controlled trials to further advance the understanding and treatment of posttraumatic headache in children.
Dr. Lonser, Dr. Choe, and Dr. Rose all agreed.
“More focused treatment trials are needed to gauge efficacy in children with headache after concussion,” Dr. Rose said.
Specifically, Dr. Gentile and colleagues underscored the need to standardize data collection via common elements, which could improve the ability to compare results across studies and develop more effective treatments. In addition, research into the underlying pathophysiology of posttraumatic headache is crucial for identifying new therapeutic targets and clinical and biological markers that can personalize patient care.
They also stressed the importance of exploring the impact of health disparities and social determinants on posttraumatic headache outcomes, aiming to develop interventions that are equitable and accessible to all patient populations.The white paper was approved by the AHS, and supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke K23 NS124986. The authors disclosed relationships with Eli Lilly, Pfizer, Amgen, and others. The interviewees disclosed no conflicts of interest.
The guidance document, the first of its kind, covers risk factors for prolonged recovery, along with pharmacologic and nonpharmacologic management strategies, and supports an emphasis on multidisciplinary care, lead author Carlyn Patterson Gentile, MD, PhD, attending physician in the Division of Neurology at Children’s Hospital of Philadelphia in Pennsylvania, and colleagues reported.
“There are no guidelines to inform the management of posttraumatic headache in youth, but multiple studies have been conducted over the past 2 decades,” the authors wrote in Headache. “This white paper aims to provide a thorough review of the current literature, identify gaps in knowledge, and provide a road map for [posttraumatic headache] management in youth based on available evidence and expert opinion.”
Clarity for an Underrecognized Issue
According to Russell Lonser, MD, professor and chair of neurological surgery at Ohio State University, Columbus, the white paper is important because it offers concrete guidance for health care providers who may be less familiar with posttraumatic headache in youth.
“It brings together all of the previous literature ... in a very well-written way,” Dr. Lonser said in an interview. “More than anything, it could reassure [providers] that they shouldn’t be hunting down potentially magical cures, and reassure them in symptomatic management.”
Meeryo C. Choe, MD, associate clinical professor of pediatric neurology at UCLA Health in Calabasas, California, said the paper also helps shine a light on what may be a more common condition than the public suspects.
“While the media focuses on the effects of concussion in professional sports athletes, the biggest population of athletes is in our youth population,” Dr. Choe said in a written comment. “Almost 25 million children participate in sports throughout the country, and yet we lack guidelines on how to treat posttraumatic headache which can often develop into persistent postconcussive symptoms.”
This white paper, she noted, builds on Dr. Gentile’s 2021 systematic review, introduces new management recommendations, and aligns with the latest consensus statement from the Concussion in Sport Group.
Risk Factors
The white paper first emphasizes the importance of early identification of youth at high risk for prolonged recovery from posttraumatic headache. Risk factors include female sex, adolescent age, a high number of acute symptoms following the initial injury, and social determinants of health.
“I agree that it is important to identify these patients early to improve the recovery trajectory,” Dr. Choe said.
Identifying these individuals quickly allows for timely intervention with both pharmacologic and nonpharmacologic therapies, Dr. Gentile and colleagues noted, potentially mitigating persistent symptoms. Clinicians are encouraged to perform thorough initial assessments to identify these risk factors and initiate early, personalized management plans.
Initial Management of Acute Posttraumatic Headache
For the initial management of acute posttraumatic headache, the white paper recommends a scheduled dosing regimen of simple analgesics. Ibuprofen at a dosage of 10 mg/kg every 6-8 hours (up to a maximum of 600 mg per dose) combined with acetaminophen has shown the best evidence for efficacy. Provided the patient is clinically stable, this regimen should be initiated within 48 hours of the injury and maintained with scheduled dosing for 3-10 days.
If effective, these medications can subsequently be used on an as-needed basis. Careful usage of analgesics is crucial, the white paper cautions, as overadministration can lead to medication-overuse headaches, complicating the recovery process.
Secondary Treatment Options
In cases where first-line oral medications are ineffective, the AHS white paper outlines several secondary treatment options. These include acute intravenous therapies such as ketorolac, dopamine receptor antagonists, and intravenous fluids. Nerve blocks and oral corticosteroid bridges may also be considered.
The white paper stresses the importance of individualized treatment plans that consider the specific needs and responses of each patient, noting that the evidence supporting these approaches is primarily derived from retrospective studies and case reports.
“Patient preferences should be factored in,” said Sean Rose, MD, pediatric neurologist and codirector of the Complex Concussion Clinic at Nationwide Children’s Hospital, Columbus, Ohio.
Supplements and Preventive Measures
For adolescents and young adults at high risk of prolonged posttraumatic headache, the white paper suggests the use of riboflavin and magnesium supplements. Small randomized clinical trials suggest that these supplements may aid in speeding recovery when administered for 1-2 weeks within 48 hours of injury.
If significant headache persists after 2 weeks, a regimen of riboflavin 400 mg daily and magnesium 400-500 mg nightly can be trialed for 6-8 weeks, in line with recommendations for migraine prevention. Additionally, melatonin at a dose of 3-5 mg nightly for an 8-week course may be considered for patients experiencing comorbid sleep disturbances.
Targeted Preventative Therapy
The white paper emphasizes the importance of targeting preventative therapy to the primary headache phenotype.
For instance, patients presenting with a migraine phenotype, or those with a personal or family history of migraines, may be most likely to respond to medications proven effective in migraine prevention, such as amitriptyline, topiramate, and propranolol.
“Most research evidence [for treating posttraumatic headache in youth] is still based on the treatment of migraine,” Dr. Rose pointed out in a written comment.
Dr. Gentile and colleagues recommend initiating preventive therapies 4-6 weeks post injury if headaches are not improving, occur more than 1-2 days per week, or significantly impact daily functioning.
Specialist Referrals and Physical Activity
Referral to a headache specialist is advised for patients who do not respond to first-line acute and preventive therapies. Specialists can offer advanced diagnostic and therapeutic options, the authors noted, ensuring a comprehensive approach to managing posttraumatic headache.
The white paper also recommends noncontact, sub–symptom threshold aerobic physical activity and activities of daily living after an initial 24-48 hour period of symptom-limited cognitive and physical rest. Engaging in these activities may promote faster recovery and help patients gradually return to their normal routines.
“This has been a shift in the concussion treatment approach over the last decade, and is one of the most important interventions we can recommend as physicians,” Dr. Choe noted. “This is where pediatricians and emergency department physicians seeing children acutely can really make a difference in the recovery trajectory for a child after a concussion. ‘Cocoon therapy’ has been proven not only to not work, but be detrimental to recovery.”
Nonpharmacologic Interventions
Based on clinical assessment, nonpharmacologic interventions may also be considered, according to the white paper. These interventions include cervico-vestibular therapy, which addresses neck and balance issues, and cognitive-behavioral therapy, which helps manage the psychological aspects of chronic headache. Dr. Gentile and colleagues highlighted the potential benefits of a collaborative care model that incorporates these nonpharmacologic interventions alongside pharmacologic treatments, providing a holistic approach to posttraumatic headache management.
“Persisting headaches after concussion are often driven by multiple factors,” Dr. Rose said. “Multidisciplinary concussion clinics can offer multiple treatment approaches such as behavioral, physical therapy, exercise, and medication options.”
Unmet Needs
The white paper concludes by calling for high-quality prospective cohort studies and placebo-controlled, randomized, controlled trials to further advance the understanding and treatment of posttraumatic headache in children.
Dr. Lonser, Dr. Choe, and Dr. Rose all agreed.
“More focused treatment trials are needed to gauge efficacy in children with headache after concussion,” Dr. Rose said.
Specifically, Dr. Gentile and colleagues underscored the need to standardize data collection via common elements, which could improve the ability to compare results across studies and develop more effective treatments. In addition, research into the underlying pathophysiology of posttraumatic headache is crucial for identifying new therapeutic targets and clinical and biological markers that can personalize patient care.
They also stressed the importance of exploring the impact of health disparities and social determinants on posttraumatic headache outcomes, aiming to develop interventions that are equitable and accessible to all patient populations.The white paper was approved by the AHS, and supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke K23 NS124986. The authors disclosed relationships with Eli Lilly, Pfizer, Amgen, and others. The interviewees disclosed no conflicts of interest.
The guidance document, the first of its kind, covers risk factors for prolonged recovery, along with pharmacologic and nonpharmacologic management strategies, and supports an emphasis on multidisciplinary care, lead author Carlyn Patterson Gentile, MD, PhD, attending physician in the Division of Neurology at Children’s Hospital of Philadelphia in Pennsylvania, and colleagues reported.
“There are no guidelines to inform the management of posttraumatic headache in youth, but multiple studies have been conducted over the past 2 decades,” the authors wrote in Headache. “This white paper aims to provide a thorough review of the current literature, identify gaps in knowledge, and provide a road map for [posttraumatic headache] management in youth based on available evidence and expert opinion.”
Clarity for an Underrecognized Issue
According to Russell Lonser, MD, professor and chair of neurological surgery at Ohio State University, Columbus, the white paper is important because it offers concrete guidance for health care providers who may be less familiar with posttraumatic headache in youth.
“It brings together all of the previous literature ... in a very well-written way,” Dr. Lonser said in an interview. “More than anything, it could reassure [providers] that they shouldn’t be hunting down potentially magical cures, and reassure them in symptomatic management.”
Meeryo C. Choe, MD, associate clinical professor of pediatric neurology at UCLA Health in Calabasas, California, said the paper also helps shine a light on what may be a more common condition than the public suspects.
“While the media focuses on the effects of concussion in professional sports athletes, the biggest population of athletes is in our youth population,” Dr. Choe said in a written comment. “Almost 25 million children participate in sports throughout the country, and yet we lack guidelines on how to treat posttraumatic headache which can often develop into persistent postconcussive symptoms.”
This white paper, she noted, builds on Dr. Gentile’s 2021 systematic review, introduces new management recommendations, and aligns with the latest consensus statement from the Concussion in Sport Group.
Risk Factors
The white paper first emphasizes the importance of early identification of youth at high risk for prolonged recovery from posttraumatic headache. Risk factors include female sex, adolescent age, a high number of acute symptoms following the initial injury, and social determinants of health.
“I agree that it is important to identify these patients early to improve the recovery trajectory,” Dr. Choe said.
Identifying these individuals quickly allows for timely intervention with both pharmacologic and nonpharmacologic therapies, Dr. Gentile and colleagues noted, potentially mitigating persistent symptoms. Clinicians are encouraged to perform thorough initial assessments to identify these risk factors and initiate early, personalized management plans.
Initial Management of Acute Posttraumatic Headache
For the initial management of acute posttraumatic headache, the white paper recommends a scheduled dosing regimen of simple analgesics. Ibuprofen at a dosage of 10 mg/kg every 6-8 hours (up to a maximum of 600 mg per dose) combined with acetaminophen has shown the best evidence for efficacy. Provided the patient is clinically stable, this regimen should be initiated within 48 hours of the injury and maintained with scheduled dosing for 3-10 days.
If effective, these medications can subsequently be used on an as-needed basis. Careful usage of analgesics is crucial, the white paper cautions, as overadministration can lead to medication-overuse headaches, complicating the recovery process.
Secondary Treatment Options
In cases where first-line oral medications are ineffective, the AHS white paper outlines several secondary treatment options. These include acute intravenous therapies such as ketorolac, dopamine receptor antagonists, and intravenous fluids. Nerve blocks and oral corticosteroid bridges may also be considered.
The white paper stresses the importance of individualized treatment plans that consider the specific needs and responses of each patient, noting that the evidence supporting these approaches is primarily derived from retrospective studies and case reports.
“Patient preferences should be factored in,” said Sean Rose, MD, pediatric neurologist and codirector of the Complex Concussion Clinic at Nationwide Children’s Hospital, Columbus, Ohio.
Supplements and Preventive Measures
For adolescents and young adults at high risk of prolonged posttraumatic headache, the white paper suggests the use of riboflavin and magnesium supplements. Small randomized clinical trials suggest that these supplements may aid in speeding recovery when administered for 1-2 weeks within 48 hours of injury.
If significant headache persists after 2 weeks, a regimen of riboflavin 400 mg daily and magnesium 400-500 mg nightly can be trialed for 6-8 weeks, in line with recommendations for migraine prevention. Additionally, melatonin at a dose of 3-5 mg nightly for an 8-week course may be considered for patients experiencing comorbid sleep disturbances.
Targeted Preventative Therapy
The white paper emphasizes the importance of targeting preventative therapy to the primary headache phenotype.
For instance, patients presenting with a migraine phenotype, or those with a personal or family history of migraines, may be most likely to respond to medications proven effective in migraine prevention, such as amitriptyline, topiramate, and propranolol.
“Most research evidence [for treating posttraumatic headache in youth] is still based on the treatment of migraine,” Dr. Rose pointed out in a written comment.
Dr. Gentile and colleagues recommend initiating preventive therapies 4-6 weeks post injury if headaches are not improving, occur more than 1-2 days per week, or significantly impact daily functioning.
Specialist Referrals and Physical Activity
Referral to a headache specialist is advised for patients who do not respond to first-line acute and preventive therapies. Specialists can offer advanced diagnostic and therapeutic options, the authors noted, ensuring a comprehensive approach to managing posttraumatic headache.
The white paper also recommends noncontact, sub–symptom threshold aerobic physical activity and activities of daily living after an initial 24-48 hour period of symptom-limited cognitive and physical rest. Engaging in these activities may promote faster recovery and help patients gradually return to their normal routines.
“This has been a shift in the concussion treatment approach over the last decade, and is one of the most important interventions we can recommend as physicians,” Dr. Choe noted. “This is where pediatricians and emergency department physicians seeing children acutely can really make a difference in the recovery trajectory for a child after a concussion. ‘Cocoon therapy’ has been proven not only to not work, but be detrimental to recovery.”
Nonpharmacologic Interventions
Based on clinical assessment, nonpharmacologic interventions may also be considered, according to the white paper. These interventions include cervico-vestibular therapy, which addresses neck and balance issues, and cognitive-behavioral therapy, which helps manage the psychological aspects of chronic headache. Dr. Gentile and colleagues highlighted the potential benefits of a collaborative care model that incorporates these nonpharmacologic interventions alongside pharmacologic treatments, providing a holistic approach to posttraumatic headache management.
“Persisting headaches after concussion are often driven by multiple factors,” Dr. Rose said. “Multidisciplinary concussion clinics can offer multiple treatment approaches such as behavioral, physical therapy, exercise, and medication options.”
Unmet Needs
The white paper concludes by calling for high-quality prospective cohort studies and placebo-controlled, randomized, controlled trials to further advance the understanding and treatment of posttraumatic headache in children.
Dr. Lonser, Dr. Choe, and Dr. Rose all agreed.
“More focused treatment trials are needed to gauge efficacy in children with headache after concussion,” Dr. Rose said.
Specifically, Dr. Gentile and colleagues underscored the need to standardize data collection via common elements, which could improve the ability to compare results across studies and develop more effective treatments. In addition, research into the underlying pathophysiology of posttraumatic headache is crucial for identifying new therapeutic targets and clinical and biological markers that can personalize patient care.
They also stressed the importance of exploring the impact of health disparities and social determinants on posttraumatic headache outcomes, aiming to develop interventions that are equitable and accessible to all patient populations.The white paper was approved by the AHS, and supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke K23 NS124986. The authors disclosed relationships with Eli Lilly, Pfizer, Amgen, and others. The interviewees disclosed no conflicts of interest.
FROM HEADACHE
New First-Line Therapies for Migraine Prevention
This transcript has been edited for clarity.
Today I am going to talk about the position statement from the American Headache Society (AHS) “Calcitonin gene-related peptide [CGRP]–targeting therapies are a first-line option for the prevention of migraine”. This update is of critical importance because about three fourths of people with migraine get their care from a primary care clinician, not from a neurologist or a headache specialist. CGRP-targeting therapies have transformed migraine care at the specialty level, but many in primary care are not yet familiar with this class of medicines. Until this new statement was released, CGRPs were not viewed as first-line agents for migraine. That has now changed.
Two main types of therapy for people with migraine headache are: (1) acute or abortive therapy (when a headache develops, it is treated), and (2) preventive therapy. Preventive therapy is typically used when the patient has headaches on 4 or more days per month. Preventive therapy is aimed at reducing the frequency and severity of headaches. About 40% of patients with migraine qualify for preventive therapy, but only a minority are receiving it.
The armamentarium for preventive therapy of migraines had not changed in a long time — until now. First-line preventive therapy has traditionally consisted of three classes of agents: beta-blockers, tricyclic antidepressants, and topiramate. These medicines were developed for different therapeutic purposes, yet they work for migraines. These drugs may have off-target effects that can make them difficult to tolerate.
Based on new evidence, candesartan — an angiotensin receptor blocker (ARB) — is now also a first-line drug for migraine. This is good news, because ARBs are a drug class that we have a lot of experience with, are easy to use, and could be an excellent choice for people with concomitant hypertension or chronic kidney disease. The serotonin-norepinephrine reuptake inhibitors (venlafaxine and duloxetine) are also considered first-line agents for migraine treatment.
In the AHS’s new position statement, the two main drug classes are small-molecule CGRP receptor antagonists and monoclonal antibodies.
The role of the neuropeptide CGRP in migraine was originally discovered after finding that blood levels of CGRP were elevated during migraine attacks. This led to the discovery of agents that blocked CGRP, initially for acute treatment of migraine, and then for preventive therapy. Multiple clinical studies show the CGRP targeting therapies to be as or even more effective than traditional first-line agents at decreasing the number of migraine days per month.
The efficacy and safety of these agents have been demonstrated in both randomized trials and in real-world studies. Other important positive endpoints include fewer days of migraine, reduced acute medication use, and improvements in many quality-of-life outcomes. Studies also have shown that CGRP-targeting therapies are well tolerated and safe, with very few serious adverse events.
Furthermore, studies have shown the CGRP targeting therapies are effective in individuals who have failed multiple other first-line therapies. They fit now both as first-line agents and as agents that can be used in difficult-to-treat patients as well as in patients who struggle with acute medication overuse, which is often very challenging.
To quote from the AHS statement,
Side effects are uncommon and can include hypertension, constipation, and Raynaud phenomenon.
The position statement is strong and is based on a lot of evidence and clinical experience. CGRP-targeting therapies are now first-line agents for the prevention of migraine headache. We should learn more about and begin to feel comfortable using this class of agents because they stand to benefit our patients greatly. I’d suggest looking at the table below and picking one new agent to become familiar with so that you can add that agent to your toolbox. 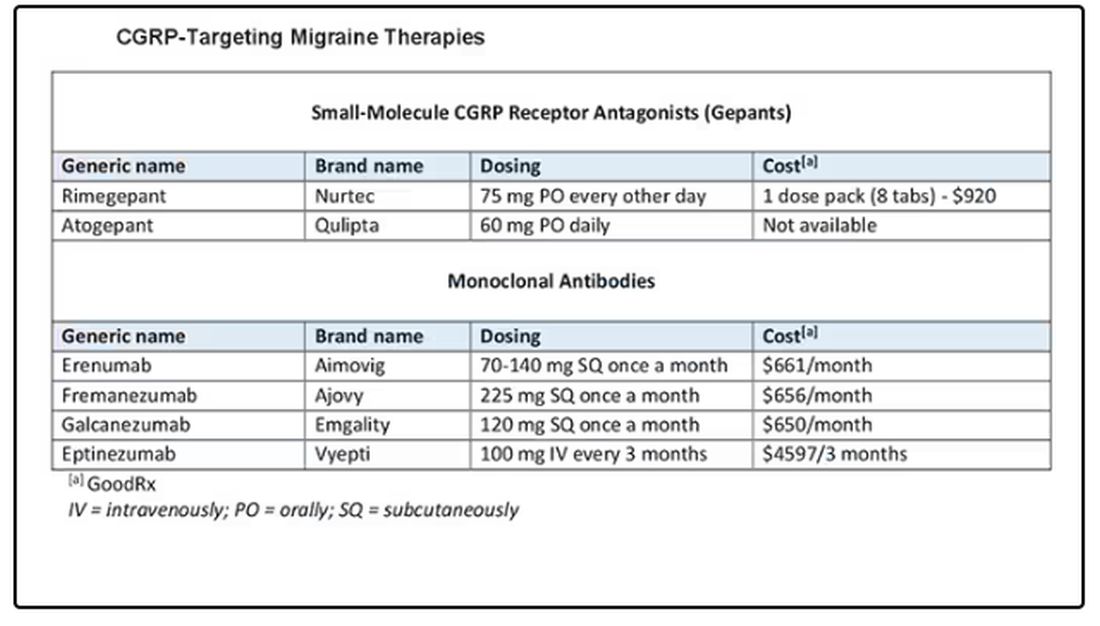
Dr. Skolnik, professor, Department of Family Medicine, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, Pennsylvania, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, Bayer, and Teva.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Today I am going to talk about the position statement from the American Headache Society (AHS) “Calcitonin gene-related peptide [CGRP]–targeting therapies are a first-line option for the prevention of migraine”. This update is of critical importance because about three fourths of people with migraine get their care from a primary care clinician, not from a neurologist or a headache specialist. CGRP-targeting therapies have transformed migraine care at the specialty level, but many in primary care are not yet familiar with this class of medicines. Until this new statement was released, CGRPs were not viewed as first-line agents for migraine. That has now changed.
Two main types of therapy for people with migraine headache are: (1) acute or abortive therapy (when a headache develops, it is treated), and (2) preventive therapy. Preventive therapy is typically used when the patient has headaches on 4 or more days per month. Preventive therapy is aimed at reducing the frequency and severity of headaches. About 40% of patients with migraine qualify for preventive therapy, but only a minority are receiving it.
The armamentarium for preventive therapy of migraines had not changed in a long time — until now. First-line preventive therapy has traditionally consisted of three classes of agents: beta-blockers, tricyclic antidepressants, and topiramate. These medicines were developed for different therapeutic purposes, yet they work for migraines. These drugs may have off-target effects that can make them difficult to tolerate.
Based on new evidence, candesartan — an angiotensin receptor blocker (ARB) — is now also a first-line drug for migraine. This is good news, because ARBs are a drug class that we have a lot of experience with, are easy to use, and could be an excellent choice for people with concomitant hypertension or chronic kidney disease. The serotonin-norepinephrine reuptake inhibitors (venlafaxine and duloxetine) are also considered first-line agents for migraine treatment.
In the AHS’s new position statement, the two main drug classes are small-molecule CGRP receptor antagonists and monoclonal antibodies.
The role of the neuropeptide CGRP in migraine was originally discovered after finding that blood levels of CGRP were elevated during migraine attacks. This led to the discovery of agents that blocked CGRP, initially for acute treatment of migraine, and then for preventive therapy. Multiple clinical studies show the CGRP targeting therapies to be as or even more effective than traditional first-line agents at decreasing the number of migraine days per month.
The efficacy and safety of these agents have been demonstrated in both randomized trials and in real-world studies. Other important positive endpoints include fewer days of migraine, reduced acute medication use, and improvements in many quality-of-life outcomes. Studies also have shown that CGRP-targeting therapies are well tolerated and safe, with very few serious adverse events.
Furthermore, studies have shown the CGRP targeting therapies are effective in individuals who have failed multiple other first-line therapies. They fit now both as first-line agents and as agents that can be used in difficult-to-treat patients as well as in patients who struggle with acute medication overuse, which is often very challenging.
To quote from the AHS statement,
Side effects are uncommon and can include hypertension, constipation, and Raynaud phenomenon.
The position statement is strong and is based on a lot of evidence and clinical experience. CGRP-targeting therapies are now first-line agents for the prevention of migraine headache. We should learn more about and begin to feel comfortable using this class of agents because they stand to benefit our patients greatly. I’d suggest looking at the table below and picking one new agent to become familiar with so that you can add that agent to your toolbox. 
Dr. Skolnik, professor, Department of Family Medicine, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, Pennsylvania, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, Bayer, and Teva.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Today I am going to talk about the position statement from the American Headache Society (AHS) “Calcitonin gene-related peptide [CGRP]–targeting therapies are a first-line option for the prevention of migraine”. This update is of critical importance because about three fourths of people with migraine get their care from a primary care clinician, not from a neurologist or a headache specialist. CGRP-targeting therapies have transformed migraine care at the specialty level, but many in primary care are not yet familiar with this class of medicines. Until this new statement was released, CGRPs were not viewed as first-line agents for migraine. That has now changed.
Two main types of therapy for people with migraine headache are: (1) acute or abortive therapy (when a headache develops, it is treated), and (2) preventive therapy. Preventive therapy is typically used when the patient has headaches on 4 or more days per month. Preventive therapy is aimed at reducing the frequency and severity of headaches. About 40% of patients with migraine qualify for preventive therapy, but only a minority are receiving it.
The armamentarium for preventive therapy of migraines had not changed in a long time — until now. First-line preventive therapy has traditionally consisted of three classes of agents: beta-blockers, tricyclic antidepressants, and topiramate. These medicines were developed for different therapeutic purposes, yet they work for migraines. These drugs may have off-target effects that can make them difficult to tolerate.
Based on new evidence, candesartan — an angiotensin receptor blocker (ARB) — is now also a first-line drug for migraine. This is good news, because ARBs are a drug class that we have a lot of experience with, are easy to use, and could be an excellent choice for people with concomitant hypertension or chronic kidney disease. The serotonin-norepinephrine reuptake inhibitors (venlafaxine and duloxetine) are also considered first-line agents for migraine treatment.
In the AHS’s new position statement, the two main drug classes are small-molecule CGRP receptor antagonists and monoclonal antibodies.
The role of the neuropeptide CGRP in migraine was originally discovered after finding that blood levels of CGRP were elevated during migraine attacks. This led to the discovery of agents that blocked CGRP, initially for acute treatment of migraine, and then for preventive therapy. Multiple clinical studies show the CGRP targeting therapies to be as or even more effective than traditional first-line agents at decreasing the number of migraine days per month.
The efficacy and safety of these agents have been demonstrated in both randomized trials and in real-world studies. Other important positive endpoints include fewer days of migraine, reduced acute medication use, and improvements in many quality-of-life outcomes. Studies also have shown that CGRP-targeting therapies are well tolerated and safe, with very few serious adverse events.
Furthermore, studies have shown the CGRP targeting therapies are effective in individuals who have failed multiple other first-line therapies. They fit now both as first-line agents and as agents that can be used in difficult-to-treat patients as well as in patients who struggle with acute medication overuse, which is often very challenging.
To quote from the AHS statement,
Side effects are uncommon and can include hypertension, constipation, and Raynaud phenomenon.
The position statement is strong and is based on a lot of evidence and clinical experience. CGRP-targeting therapies are now first-line agents for the prevention of migraine headache. We should learn more about and begin to feel comfortable using this class of agents because they stand to benefit our patients greatly. I’d suggest looking at the table below and picking one new agent to become familiar with so that you can add that agent to your toolbox. 
Dr. Skolnik, professor, Department of Family Medicine, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, Pennsylvania, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, Bayer, and Teva.
A version of this article appeared on Medscape.com.
How Clinicians Can Help Patients Navigate Psychedelics/Microdosing
Peter Grinspoon, MD, has some advice for clinicians when patients ask questions about microdosing of psychedelics: Keep the lines of communication open — and don’t be judgmental.
“If you’re dismissive or critical or sound like you’re judging them, then the patients just clam up,” said Dr. Grinspoon, a professor of medicine at Harvard Medical School and a primary care physician at Massachusetts General Hospital, both in Boston.
Psychedelic drugs are still illegal in the majority of states despite the growth of public interest in and use of these substances. That growth is evidenced by a flurry of workshops, reports, law enforcement seizures, and pressure by Congressional members for the Food and Drug Administration to approve new psychedelic drugs, just in the past year.
A recent study in JAMA Health Forum showed a nearly 14-fold increase in Google searches — from 7.9 to 105.6 per 10 million nationwide — for the term “microdosing” and related wording, between 2015 and 2023.
Two states — Oregon and Colorado — have decriminalized certain psychedelic drugs and are in various stages of establishing regulations and centers for prospective clients. Almost two dozen localities, like Ann Arbor, Michigan, have decriminalized psychedelic drugs. A handful of states have active legislation to decriminalize use, while others have bills that never made it out of committee.
But no definitive studies have reported that microdosing produces positive mental effects at a higher rate than placebo, according to Dr. Grinspoon. So
“We’re in this renaissance where everybody is idealizing these medications, as opposed to 20 years ago when we were in the war on drugs and everybody was dismissing them,” Dr. Grinspoon said. “The truth is somewhere in between.”
The Science
Microdosing is defined as taking doses of 1/5 to 1/20 of the conventional recreational amount, which might include a dried psilocybin mushroom, lysergic acid diethylamide, or 3,4-methylenedioxymethamphetamine. But even that much may be neither effective nor safe.
Dr. Grinspoon said clinicians should tell patients that psychedelics may cause harm, although the drugs are relatively nontoxic and are not addictive. An illegally obtained psilocybin could cause negative reactions, especially if the drug has been adulterated with other substances and if the actual dose is higher than what was indicated by the seller.
He noted that people have different reactions to psychedelics, just as they have to prescription medications. He cited one example of a woman who microdosed and could not sleep for 2 weeks afterward. Only recently have randomized, double-blinded studies begun on benefits and harms.
Researchers have also begun investigating whether long-term microdosing of psilocybin could lead to valvular heart disease (VHD), said Kevin Yang, MD, a psychiatry resident at the University of California San Diego School of Medicine. A recent review of evidence concluded that microdosing various psychedelics over a period of months can lead to drug-induced VHD.
“It’s extremely important to emphasize with patients that not only do we not know if it works or not, we also don’t really know how safe it is,” Dr. Yang said.
Dr. Yang also said clinicians should consider referring patients to a mental health professional, and especially those that may have expertise in psychedelic therapies.
One of those experts is Rachel Yehuda, PhD, director of the Center for Psychedelic Psychotherapy and Trauma Research at Icahn School of Medicine at Mount Sinai in New York City. She said therapists should be able to assess the patient’s perceived need for microdosing and “invite reflections about why current approaches are falling short.”
“I would also not actively discourage it either but remain curious until both of you have a better understanding of the reasons for seeking this out and potential alternative strategies for obtaining more therapeutic benefits,” she said. “I think it is really important to study the effects of both micro- and macrodosing of psychedelics but not move in advance of the data.”
Navigating Legality
Recent ballot measures in Oregon and Colorado directed the states to develop regulated and licensed psilocybin-assisted therapy centers for legal “trips.” Oregon’s first center was opened in 2023, and Colorado is now developing its own licensing model.
According to the Oregon Health Authority, the centers are not medical facilities, and prescription or referral from a medical professional is not required.
The Oregon Academy of Family Physicians (OAFP) has yet to release guidance to clinicians on how to talk to their patients about these drugs or potential interest in visiting a licensed therapy center.
However, Betsy Boyd-Flynn, executive director of OAFP, said the organization is working on continuing medical education for what the average family physician needs to know if a patient asks about use.
“We suspect that many of our members have interest and want to learn more,” she said.
Dr. Grinspoon said clinicians should talk with patients about legality during these conversations.
“The big question I get is: ‘I really want to try microdosing, but how do I obtain the mushrooms?’ ” he said. “You can’t really as a physician tell them to do anything illegal. So you tell them to be safe, be careful, and to use their judgment.”
Patients who want to pursue microdosing who do not live in Oregon have two legal and safe options, Dr. Grinspoon said: Enroll in a clinical study or find a facility in a state or country — such as Oregon or Jamaica — that offers microdosing with psilocybin.
Clinicians also should warn their patients that the consequences of obtaining illicit psilocybin could exacerbate the mental health stresses they are seeking to alleviate.
“It’s going to get worse if they get tangled up with law enforcement or take something that’s contaminated and they get real sick,” he said.
Lisa Gillespie contributed reporting to this story. A version of this article appeared on Medscape.com.
Peter Grinspoon, MD, has some advice for clinicians when patients ask questions about microdosing of psychedelics: Keep the lines of communication open — and don’t be judgmental.
“If you’re dismissive or critical or sound like you’re judging them, then the patients just clam up,” said Dr. Grinspoon, a professor of medicine at Harvard Medical School and a primary care physician at Massachusetts General Hospital, both in Boston.
Psychedelic drugs are still illegal in the majority of states despite the growth of public interest in and use of these substances. That growth is evidenced by a flurry of workshops, reports, law enforcement seizures, and pressure by Congressional members for the Food and Drug Administration to approve new psychedelic drugs, just in the past year.
A recent study in JAMA Health Forum showed a nearly 14-fold increase in Google searches — from 7.9 to 105.6 per 10 million nationwide — for the term “microdosing” and related wording, between 2015 and 2023.
Two states — Oregon and Colorado — have decriminalized certain psychedelic drugs and are in various stages of establishing regulations and centers for prospective clients. Almost two dozen localities, like Ann Arbor, Michigan, have decriminalized psychedelic drugs. A handful of states have active legislation to decriminalize use, while others have bills that never made it out of committee.
But no definitive studies have reported that microdosing produces positive mental effects at a higher rate than placebo, according to Dr. Grinspoon. So
“We’re in this renaissance where everybody is idealizing these medications, as opposed to 20 years ago when we were in the war on drugs and everybody was dismissing them,” Dr. Grinspoon said. “The truth is somewhere in between.”
The Science
Microdosing is defined as taking doses of 1/5 to 1/20 of the conventional recreational amount, which might include a dried psilocybin mushroom, lysergic acid diethylamide, or 3,4-methylenedioxymethamphetamine. But even that much may be neither effective nor safe.
Dr. Grinspoon said clinicians should tell patients that psychedelics may cause harm, although the drugs are relatively nontoxic and are not addictive. An illegally obtained psilocybin could cause negative reactions, especially if the drug has been adulterated with other substances and if the actual dose is higher than what was indicated by the seller.
He noted that people have different reactions to psychedelics, just as they have to prescription medications. He cited one example of a woman who microdosed and could not sleep for 2 weeks afterward. Only recently have randomized, double-blinded studies begun on benefits and harms.
Researchers have also begun investigating whether long-term microdosing of psilocybin could lead to valvular heart disease (VHD), said Kevin Yang, MD, a psychiatry resident at the University of California San Diego School of Medicine. A recent review of evidence concluded that microdosing various psychedelics over a period of months can lead to drug-induced VHD.
“It’s extremely important to emphasize with patients that not only do we not know if it works or not, we also don’t really know how safe it is,” Dr. Yang said.
Dr. Yang also said clinicians should consider referring patients to a mental health professional, and especially those that may have expertise in psychedelic therapies.
One of those experts is Rachel Yehuda, PhD, director of the Center for Psychedelic Psychotherapy and Trauma Research at Icahn School of Medicine at Mount Sinai in New York City. She said therapists should be able to assess the patient’s perceived need for microdosing and “invite reflections about why current approaches are falling short.”
“I would also not actively discourage it either but remain curious until both of you have a better understanding of the reasons for seeking this out and potential alternative strategies for obtaining more therapeutic benefits,” she said. “I think it is really important to study the effects of both micro- and macrodosing of psychedelics but not move in advance of the data.”
Navigating Legality
Recent ballot measures in Oregon and Colorado directed the states to develop regulated and licensed psilocybin-assisted therapy centers for legal “trips.” Oregon’s first center was opened in 2023, and Colorado is now developing its own licensing model.
According to the Oregon Health Authority, the centers are not medical facilities, and prescription or referral from a medical professional is not required.
The Oregon Academy of Family Physicians (OAFP) has yet to release guidance to clinicians on how to talk to their patients about these drugs or potential interest in visiting a licensed therapy center.
However, Betsy Boyd-Flynn, executive director of OAFP, said the organization is working on continuing medical education for what the average family physician needs to know if a patient asks about use.
“We suspect that many of our members have interest and want to learn more,” she said.
Dr. Grinspoon said clinicians should talk with patients about legality during these conversations.
“The big question I get is: ‘I really want to try microdosing, but how do I obtain the mushrooms?’ ” he said. “You can’t really as a physician tell them to do anything illegal. So you tell them to be safe, be careful, and to use their judgment.”
Patients who want to pursue microdosing who do not live in Oregon have two legal and safe options, Dr. Grinspoon said: Enroll in a clinical study or find a facility in a state or country — such as Oregon or Jamaica — that offers microdosing with psilocybin.
Clinicians also should warn their patients that the consequences of obtaining illicit psilocybin could exacerbate the mental health stresses they are seeking to alleviate.
“It’s going to get worse if they get tangled up with law enforcement or take something that’s contaminated and they get real sick,” he said.
Lisa Gillespie contributed reporting to this story. A version of this article appeared on Medscape.com.
Peter Grinspoon, MD, has some advice for clinicians when patients ask questions about microdosing of psychedelics: Keep the lines of communication open — and don’t be judgmental.
“If you’re dismissive or critical or sound like you’re judging them, then the patients just clam up,” said Dr. Grinspoon, a professor of medicine at Harvard Medical School and a primary care physician at Massachusetts General Hospital, both in Boston.
Psychedelic drugs are still illegal in the majority of states despite the growth of public interest in and use of these substances. That growth is evidenced by a flurry of workshops, reports, law enforcement seizures, and pressure by Congressional members for the Food and Drug Administration to approve new psychedelic drugs, just in the past year.
A recent study in JAMA Health Forum showed a nearly 14-fold increase in Google searches — from 7.9 to 105.6 per 10 million nationwide — for the term “microdosing” and related wording, between 2015 and 2023.
Two states — Oregon and Colorado — have decriminalized certain psychedelic drugs and are in various stages of establishing regulations and centers for prospective clients. Almost two dozen localities, like Ann Arbor, Michigan, have decriminalized psychedelic drugs. A handful of states have active legislation to decriminalize use, while others have bills that never made it out of committee.
But no definitive studies have reported that microdosing produces positive mental effects at a higher rate than placebo, according to Dr. Grinspoon. So
“We’re in this renaissance where everybody is idealizing these medications, as opposed to 20 years ago when we were in the war on drugs and everybody was dismissing them,” Dr. Grinspoon said. “The truth is somewhere in between.”
The Science
Microdosing is defined as taking doses of 1/5 to 1/20 of the conventional recreational amount, which might include a dried psilocybin mushroom, lysergic acid diethylamide, or 3,4-methylenedioxymethamphetamine. But even that much may be neither effective nor safe.
Dr. Grinspoon said clinicians should tell patients that psychedelics may cause harm, although the drugs are relatively nontoxic and are not addictive. An illegally obtained psilocybin could cause negative reactions, especially if the drug has been adulterated with other substances and if the actual dose is higher than what was indicated by the seller.
He noted that people have different reactions to psychedelics, just as they have to prescription medications. He cited one example of a woman who microdosed and could not sleep for 2 weeks afterward. Only recently have randomized, double-blinded studies begun on benefits and harms.
Researchers have also begun investigating whether long-term microdosing of psilocybin could lead to valvular heart disease (VHD), said Kevin Yang, MD, a psychiatry resident at the University of California San Diego School of Medicine. A recent review of evidence concluded that microdosing various psychedelics over a period of months can lead to drug-induced VHD.
“It’s extremely important to emphasize with patients that not only do we not know if it works or not, we also don’t really know how safe it is,” Dr. Yang said.
Dr. Yang also said clinicians should consider referring patients to a mental health professional, and especially those that may have expertise in psychedelic therapies.
One of those experts is Rachel Yehuda, PhD, director of the Center for Psychedelic Psychotherapy and Trauma Research at Icahn School of Medicine at Mount Sinai in New York City. She said therapists should be able to assess the patient’s perceived need for microdosing and “invite reflections about why current approaches are falling short.”
“I would also not actively discourage it either but remain curious until both of you have a better understanding of the reasons for seeking this out and potential alternative strategies for obtaining more therapeutic benefits,” she said. “I think it is really important to study the effects of both micro- and macrodosing of psychedelics but not move in advance of the data.”
Navigating Legality
Recent ballot measures in Oregon and Colorado directed the states to develop regulated and licensed psilocybin-assisted therapy centers for legal “trips.” Oregon’s first center was opened in 2023, and Colorado is now developing its own licensing model.
According to the Oregon Health Authority, the centers are not medical facilities, and prescription or referral from a medical professional is not required.
The Oregon Academy of Family Physicians (OAFP) has yet to release guidance to clinicians on how to talk to their patients about these drugs or potential interest in visiting a licensed therapy center.
However, Betsy Boyd-Flynn, executive director of OAFP, said the organization is working on continuing medical education for what the average family physician needs to know if a patient asks about use.
“We suspect that many of our members have interest and want to learn more,” she said.
Dr. Grinspoon said clinicians should talk with patients about legality during these conversations.
“The big question I get is: ‘I really want to try microdosing, but how do I obtain the mushrooms?’ ” he said. “You can’t really as a physician tell them to do anything illegal. So you tell them to be safe, be careful, and to use their judgment.”
Patients who want to pursue microdosing who do not live in Oregon have two legal and safe options, Dr. Grinspoon said: Enroll in a clinical study or find a facility in a state or country — such as Oregon or Jamaica — that offers microdosing with psilocybin.
Clinicians also should warn their patients that the consequences of obtaining illicit psilocybin could exacerbate the mental health stresses they are seeking to alleviate.
“It’s going to get worse if they get tangled up with law enforcement or take something that’s contaminated and they get real sick,” he said.
Lisa Gillespie contributed reporting to this story. A version of this article appeared on Medscape.com.
Anxiety Linked to a Threefold Increased Risk for Dementia
TOPLINE:
, new research shows.
METHODOLOGY:
- A total of 2132 participants aged 55-85 years (mean age, 76 years) were recruited from the Hunter Community Study. Of these, 53% were women.
- Participants were assessed over three different waves, 5 years apart. Demographic and health-related data were captured at wave 1.
- Researchers used the Kessler Psychological Distress Scale (K10) to measure anxiety at two points: Baseline (wave 1) and first follow-up (wave 2), with a 5-year interval between them. Anxiety was classified as chronic if present during both waves, resolved if only present at wave 1, and new if only appearing at wave 2.
- The primary outcome, incident all-cause dementia, during the follow-up period (maximum 13 years after baseline) was identified using the International Classification of Disease-10 codes.
TAKEAWAY:
- Out of 2132 cognitively healthy participants, 64 developed dementia, with an average time to diagnosis of 10 years. Chronic anxiety was linked to a 2.8-fold increased risk for dementia, while new-onset anxiety was associated with a 3.2-fold increased risk (P = .01).
- Participants younger than 70 years with chronic anxiety had a 4.6-fold increased risk for dementia (P = .03), and those with new-onset anxiety had a 7.2 times higher risk for dementia (P = .004).
- There was no significant risk for dementia in participants with anxiety that had resolved.
- Investigators speculated that individuals with anxiety were more likely to engage in unhealthy lifestyle behaviors, such as poor diet and smoking, which can lead to cardiovascular disease — a condition strongly associated with dementia.
IN PRACTICE:
“This prospective cohort study used causal inference methods to explore the role of anxiety in promoting the development of dementia,” lead author Kay Khaing, MMed, The University of Newcastle, Australia, wrote in a press release. “The findings suggest that anxiety may be a new risk factor to target in the prevention of dementia and also indicate that treating anxiety may reduce this risk.”
SOURCE:
Kay Khaing, MMed, of The University of Newcastle, Australia, led the study, which was published online in the Journal of the American Geriatrics Society.
LIMITATIONS:
Anxiety was measured using K10, which assessed symptoms experienced in the most recent 4 weeks, raising concerns about its accuracy over the entire observation period. The authors acknowledged that despite using a combination of the total K10 score and the anxiety subscale, the overlap of anxiety and depression might not be fully disentangled, leading to residual confounding by depression. Additionally, 33% of participants were lost to follow-up, and those lost had higher anxiety rates at baseline, potentially leading to missing cases of dementia and affecting the effect estimate.
DISCLOSURES:
This study did not report any funding or conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
, new research shows.
METHODOLOGY:
- A total of 2132 participants aged 55-85 years (mean age, 76 years) were recruited from the Hunter Community Study. Of these, 53% were women.
- Participants were assessed over three different waves, 5 years apart. Demographic and health-related data were captured at wave 1.
- Researchers used the Kessler Psychological Distress Scale (K10) to measure anxiety at two points: Baseline (wave 1) and first follow-up (wave 2), with a 5-year interval between them. Anxiety was classified as chronic if present during both waves, resolved if only present at wave 1, and new if only appearing at wave 2.
- The primary outcome, incident all-cause dementia, during the follow-up period (maximum 13 years after baseline) was identified using the International Classification of Disease-10 codes.
TAKEAWAY:
- Out of 2132 cognitively healthy participants, 64 developed dementia, with an average time to diagnosis of 10 years. Chronic anxiety was linked to a 2.8-fold increased risk for dementia, while new-onset anxiety was associated with a 3.2-fold increased risk (P = .01).
- Participants younger than 70 years with chronic anxiety had a 4.6-fold increased risk for dementia (P = .03), and those with new-onset anxiety had a 7.2 times higher risk for dementia (P = .004).
- There was no significant risk for dementia in participants with anxiety that had resolved.
- Investigators speculated that individuals with anxiety were more likely to engage in unhealthy lifestyle behaviors, such as poor diet and smoking, which can lead to cardiovascular disease — a condition strongly associated with dementia.
IN PRACTICE:
“This prospective cohort study used causal inference methods to explore the role of anxiety in promoting the development of dementia,” lead author Kay Khaing, MMed, The University of Newcastle, Australia, wrote in a press release. “The findings suggest that anxiety may be a new risk factor to target in the prevention of dementia and also indicate that treating anxiety may reduce this risk.”
SOURCE:
Kay Khaing, MMed, of The University of Newcastle, Australia, led the study, which was published online in the Journal of the American Geriatrics Society.
LIMITATIONS:
Anxiety was measured using K10, which assessed symptoms experienced in the most recent 4 weeks, raising concerns about its accuracy over the entire observation period. The authors acknowledged that despite using a combination of the total K10 score and the anxiety subscale, the overlap of anxiety and depression might not be fully disentangled, leading to residual confounding by depression. Additionally, 33% of participants were lost to follow-up, and those lost had higher anxiety rates at baseline, potentially leading to missing cases of dementia and affecting the effect estimate.
DISCLOSURES:
This study did not report any funding or conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
, new research shows.
METHODOLOGY:
- A total of 2132 participants aged 55-85 years (mean age, 76 years) were recruited from the Hunter Community Study. Of these, 53% were women.
- Participants were assessed over three different waves, 5 years apart. Demographic and health-related data were captured at wave 1.
- Researchers used the Kessler Psychological Distress Scale (K10) to measure anxiety at two points: Baseline (wave 1) and first follow-up (wave 2), with a 5-year interval between them. Anxiety was classified as chronic if present during both waves, resolved if only present at wave 1, and new if only appearing at wave 2.
- The primary outcome, incident all-cause dementia, during the follow-up period (maximum 13 years after baseline) was identified using the International Classification of Disease-10 codes.
TAKEAWAY:
- Out of 2132 cognitively healthy participants, 64 developed dementia, with an average time to diagnosis of 10 years. Chronic anxiety was linked to a 2.8-fold increased risk for dementia, while new-onset anxiety was associated with a 3.2-fold increased risk (P = .01).
- Participants younger than 70 years with chronic anxiety had a 4.6-fold increased risk for dementia (P = .03), and those with new-onset anxiety had a 7.2 times higher risk for dementia (P = .004).
- There was no significant risk for dementia in participants with anxiety that had resolved.
- Investigators speculated that individuals with anxiety were more likely to engage in unhealthy lifestyle behaviors, such as poor diet and smoking, which can lead to cardiovascular disease — a condition strongly associated with dementia.
IN PRACTICE:
“This prospective cohort study used causal inference methods to explore the role of anxiety in promoting the development of dementia,” lead author Kay Khaing, MMed, The University of Newcastle, Australia, wrote in a press release. “The findings suggest that anxiety may be a new risk factor to target in the prevention of dementia and also indicate that treating anxiety may reduce this risk.”
SOURCE:
Kay Khaing, MMed, of The University of Newcastle, Australia, led the study, which was published online in the Journal of the American Geriatrics Society.
LIMITATIONS:
Anxiety was measured using K10, which assessed symptoms experienced in the most recent 4 weeks, raising concerns about its accuracy over the entire observation period. The authors acknowledged that despite using a combination of the total K10 score and the anxiety subscale, the overlap of anxiety and depression might not be fully disentangled, leading to residual confounding by depression. Additionally, 33% of participants were lost to follow-up, and those lost had higher anxiety rates at baseline, potentially leading to missing cases of dementia and affecting the effect estimate.
DISCLOSURES:
This study did not report any funding or conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
Does Headache Surgery Really Work? Neurologists Remain Unconvinced
Jeffrey E. Janis, MD, is on a mission. The professor of plastic surgery, surgery, neurosurgery, and neurology at The Ohio State University Wexner Medical Center, Columbus, Ohio, wants to convince neurologists of the safety and efficacy of nerve decompression surgery for treatment-resistant headache. However, many neurologists remain unconvinced.
There’s 24 years of evidence behind this surgical technique across hundreds of different studies with different study designs,” Dr. Janis said.
Yet this treatment approach — surgery on peripheral nerves rather than the brain or spinal cord — hasn’t garnered much support from neurologists. A scan of the agenda of topics at the recently held 2024 annual meeting of the American Headache Society showed few if any studies or presentations on this topic. And neurologists this news organization spoke to said they believe the surgery is experimental and unproven.
Experts do agree drugs don’t work for all patients with migraines. Up to 30% of patients don’t respond to the “laundry list of medications” available to treat the condition, said Dr. Janis.
Many patients have also tried, and failed, alternative treatment approaches such as massage, acupuncture, craniosacral therapy, transdermal patches, electrical stimulation, cryoablation, neurostimulation, and radiofrequency ablation.
If nothing else works, is surgery for headaches the answer?
Long-Held Theory
The idea that pinched, irritated, or compressed peripheral nerves can trigger migraine attacks has been around for nearly 25 years. Studies suggest that in addition to migraine, nerve compression can lead to other headache conditions, including occipital neuralgia, supraorbital neuralgia , and post-traumatic headaches.
This has led to the development of surgical techniques to deactivate various compression trigger sites — what Dr. Janis calls “pinch points” — which could involve muscles, bone, fascia, blood vessels, or scar tissue from prior trauma or surgery.
The procedure is predominantly performed by plastic surgeons, but to a lesser degree by neurosurgeons and ear, nose, and throat specialists.
Target nerves in surgical interventions include those in the frontal region of the head above the eye, temporal region, neck region, and nasal region. Affected areas are usually identified either through patient self-reports or by using a nerve block agent such as lidocaine or Botox at specific points, Dr. Janis noted. If pain subsides after an injection, that location is marked as a target.
One of the barriers to referring complicated patients for surgery is that neurologists evaluating migraine treatments “speak a different language” than surgeons performing the procedure, said Dr. Janis.
Neurologists tend to focus on reduction in monthly migraine days (MMD), while surgeons typically use the Migraine Headache Index that incorporates the frequency, intensity, and duration of migraine attacks.
“Rather than try to convince somebody to speak a different language, we thought, why don’t we just learn their language so we can build bridges and take down barriers,” said Dr. Janis, coauthor of a systematic review and meta-analysis published online recently in Plastic and Reconstructive Surgery.
Investigators examined 19 studies in the review, including five randomized controlled trials (RCTs), published from January 2020 to September 2023, with a total of 1603 participants who were mostly female and ranged in age from 9 to 72 years. Study follow-ups extended from 6 to 38 months. All but three studies were carried out in the United States, and six different compression sites were addressed during surgery.
Investigators found that across studies and by a number of measures, migraine frequency and severity improved after surgery.
Monthly migraine days decreased by 36%-92% and the number of overall migraine attacks per month dropped 25%-87.5%. Patients also reported decreases in attack duration of 41%-75% and intensity of 28%-82% across studies.
“Even using the neurologist-standard language of monthly migraine days, this surgery works,” said Dr. Janis. “Now this is documented both in the surgical literature and the nonsurgical literature.”
The most common complications were ecchymosis, hair loss or thinning, itching, dryness, and rhinorrhea, all of which Dr. Janis described as “fairly minor.” Major complications such as intraoperative bleeding and wound dehiscence were rare, occurring in 1% or less of participants.
‘One And Done?’
These surgeries are usually done on an outpatient basis and generally offer long-term results, Dr. Janis said.
“The idea is one and done,” he said. “The literature around this type of surgery says that whatever type of effect you get at 1 year is likely to be permanent.”
The American Society of Plastic Surgeons agrees. A 2018 position paper developed by experts and commissioned by the society reports that the intervention is safe and effective for appropriate patients, based on a comprehensive literature search and review of a large body of peer-reviewed scientific evidence.
“There is substantial, extensively replicated clinical data that demonstrates a significant reduction in [migraine headache] symptoms and frequency (even complete elimination of headache pain) following trigger site surgery,” the authors noted.
Pamela Blake, MD, a neurologist, board-certified headache specialist, and medical director at the Headache Center of River Oaks, Houston, is a proponent of what she said can be “lifesaving” headache surgery.
“If a doctor told you that you can either treat this problem with medications that you’ll need to take for the rest of your life or you can have a surgical procedure as an outpatient that has extremely low risk and has, in my experience, a 75% chance of reducing or eliminating your pain, you probably would be interested in surgery,” she said.
Continued Skepticism
However, other neurologists and clinicians appear doubtful about this intervention, including Hans-Christoph Diener, MD, PhD, professor of neurology and director, Essen Headache Centre, University of Duisburg-Essen in Germany.
During a debate on the topic a decade ago at the International Headache Congress, Dr. Diener argued that, as migraine is a complex multigene-related disorder of the brain, it doesn’t make sense that surgery would affect the epigenetics of 22 different genes.
Recently, he said that his views have not changed.
The topic remains controversial, and some neurologists are uncomfortable even openly discussing the procedure. Two clinicians who previously commented on this article later asked not to be included.
One neurologist, who asked to remain anonymous, said that Dr. Janis’s review article is “merely a review collecting 19 studies over the previous 10-plus years.”
Other limitations cited by this neurologist are the lack of consistency in procedures among the various studies and the inclusion of only four RCTs, the most recent of which was published 8 years ago, suggesting “the study was probably done closer to 9 or 10 years ago,” the neurologist said.
Dr. Blake suggested some neurologists’ reluctance could be due to limited background on the procedure, which she said isn’t widely discussed at headache meetings and is covered mostly in plastic surgery journals, not neurology literature. Access to surgery is further limited by a lack of specialists who perform the procedure and inconsistent insurance coverage.
A closer collaboration between neurologists and surgeons who perform the procedure could benefit patients, Dr. Blake noted.
“The headache doctor’s role is to identify who’s a candidate for surgery, who meets the criteria for nerve compression, and then follow that patient postoperatively, managing their medications, although usually we get them off their medications,” she added.
From Dr. Janis’s perspective, things are starting to change.
“I’m definitely seeing a greater comfort level among neurologists who are understanding where this sits in the algorithm for treatment, especially for complicated patients,” he said.
Dr. Janis receives royalties from Thieme and Springer Publishing. Dr. Blake reported no relevant conflicts. Dr. Diener received research support from the German Research Council; serves on the editorial boards of Cephalalgia, Lancet Neurology, and Drugs; and has received honoraria for participation in clinical trials, contribution to advisory boards, or oral presentations from AbbVie, Lilly, Lundbeck, Novartis, Pfizer, Teva, Weber & Weber, and WebMD.
A version of this article appeared on Medscape.com.
Jeffrey E. Janis, MD, is on a mission. The professor of plastic surgery, surgery, neurosurgery, and neurology at The Ohio State University Wexner Medical Center, Columbus, Ohio, wants to convince neurologists of the safety and efficacy of nerve decompression surgery for treatment-resistant headache. However, many neurologists remain unconvinced.
There’s 24 years of evidence behind this surgical technique across hundreds of different studies with different study designs,” Dr. Janis said.
Yet this treatment approach — surgery on peripheral nerves rather than the brain or spinal cord — hasn’t garnered much support from neurologists. A scan of the agenda of topics at the recently held 2024 annual meeting of the American Headache Society showed few if any studies or presentations on this topic. And neurologists this news organization spoke to said they believe the surgery is experimental and unproven.
Experts do agree drugs don’t work for all patients with migraines. Up to 30% of patients don’t respond to the “laundry list of medications” available to treat the condition, said Dr. Janis.
Many patients have also tried, and failed, alternative treatment approaches such as massage, acupuncture, craniosacral therapy, transdermal patches, electrical stimulation, cryoablation, neurostimulation, and radiofrequency ablation.
If nothing else works, is surgery for headaches the answer?
Long-Held Theory
The idea that pinched, irritated, or compressed peripheral nerves can trigger migraine attacks has been around for nearly 25 years. Studies suggest that in addition to migraine, nerve compression can lead to other headache conditions, including occipital neuralgia, supraorbital neuralgia , and post-traumatic headaches.
This has led to the development of surgical techniques to deactivate various compression trigger sites — what Dr. Janis calls “pinch points” — which could involve muscles, bone, fascia, blood vessels, or scar tissue from prior trauma or surgery.
The procedure is predominantly performed by plastic surgeons, but to a lesser degree by neurosurgeons and ear, nose, and throat specialists.
Target nerves in surgical interventions include those in the frontal region of the head above the eye, temporal region, neck region, and nasal region. Affected areas are usually identified either through patient self-reports or by using a nerve block agent such as lidocaine or Botox at specific points, Dr. Janis noted. If pain subsides after an injection, that location is marked as a target.
One of the barriers to referring complicated patients for surgery is that neurologists evaluating migraine treatments “speak a different language” than surgeons performing the procedure, said Dr. Janis.
Neurologists tend to focus on reduction in monthly migraine days (MMD), while surgeons typically use the Migraine Headache Index that incorporates the frequency, intensity, and duration of migraine attacks.
“Rather than try to convince somebody to speak a different language, we thought, why don’t we just learn their language so we can build bridges and take down barriers,” said Dr. Janis, coauthor of a systematic review and meta-analysis published online recently in Plastic and Reconstructive Surgery.
Investigators examined 19 studies in the review, including five randomized controlled trials (RCTs), published from January 2020 to September 2023, with a total of 1603 participants who were mostly female and ranged in age from 9 to 72 years. Study follow-ups extended from 6 to 38 months. All but three studies were carried out in the United States, and six different compression sites were addressed during surgery.
Investigators found that across studies and by a number of measures, migraine frequency and severity improved after surgery.
Monthly migraine days decreased by 36%-92% and the number of overall migraine attacks per month dropped 25%-87.5%. Patients also reported decreases in attack duration of 41%-75% and intensity of 28%-82% across studies.
“Even using the neurologist-standard language of monthly migraine days, this surgery works,” said Dr. Janis. “Now this is documented both in the surgical literature and the nonsurgical literature.”
The most common complications were ecchymosis, hair loss or thinning, itching, dryness, and rhinorrhea, all of which Dr. Janis described as “fairly minor.” Major complications such as intraoperative bleeding and wound dehiscence were rare, occurring in 1% or less of participants.
‘One And Done?’
These surgeries are usually done on an outpatient basis and generally offer long-term results, Dr. Janis said.
“The idea is one and done,” he said. “The literature around this type of surgery says that whatever type of effect you get at 1 year is likely to be permanent.”
The American Society of Plastic Surgeons agrees. A 2018 position paper developed by experts and commissioned by the society reports that the intervention is safe and effective for appropriate patients, based on a comprehensive literature search and review of a large body of peer-reviewed scientific evidence.
“There is substantial, extensively replicated clinical data that demonstrates a significant reduction in [migraine headache] symptoms and frequency (even complete elimination of headache pain) following trigger site surgery,” the authors noted.
Pamela Blake, MD, a neurologist, board-certified headache specialist, and medical director at the Headache Center of River Oaks, Houston, is a proponent of what she said can be “lifesaving” headache surgery.
“If a doctor told you that you can either treat this problem with medications that you’ll need to take for the rest of your life or you can have a surgical procedure as an outpatient that has extremely low risk and has, in my experience, a 75% chance of reducing or eliminating your pain, you probably would be interested in surgery,” she said.
Continued Skepticism
However, other neurologists and clinicians appear doubtful about this intervention, including Hans-Christoph Diener, MD, PhD, professor of neurology and director, Essen Headache Centre, University of Duisburg-Essen in Germany.
During a debate on the topic a decade ago at the International Headache Congress, Dr. Diener argued that, as migraine is a complex multigene-related disorder of the brain, it doesn’t make sense that surgery would affect the epigenetics of 22 different genes.
Recently, he said that his views have not changed.
The topic remains controversial, and some neurologists are uncomfortable even openly discussing the procedure. Two clinicians who previously commented on this article later asked not to be included.
One neurologist, who asked to remain anonymous, said that Dr. Janis’s review article is “merely a review collecting 19 studies over the previous 10-plus years.”
Other limitations cited by this neurologist are the lack of consistency in procedures among the various studies and the inclusion of only four RCTs, the most recent of which was published 8 years ago, suggesting “the study was probably done closer to 9 or 10 years ago,” the neurologist said.
Dr. Blake suggested some neurologists’ reluctance could be due to limited background on the procedure, which she said isn’t widely discussed at headache meetings and is covered mostly in plastic surgery journals, not neurology literature. Access to surgery is further limited by a lack of specialists who perform the procedure and inconsistent insurance coverage.
A closer collaboration between neurologists and surgeons who perform the procedure could benefit patients, Dr. Blake noted.
“The headache doctor’s role is to identify who’s a candidate for surgery, who meets the criteria for nerve compression, and then follow that patient postoperatively, managing their medications, although usually we get them off their medications,” she added.
From Dr. Janis’s perspective, things are starting to change.
“I’m definitely seeing a greater comfort level among neurologists who are understanding where this sits in the algorithm for treatment, especially for complicated patients,” he said.
Dr. Janis receives royalties from Thieme and Springer Publishing. Dr. Blake reported no relevant conflicts. Dr. Diener received research support from the German Research Council; serves on the editorial boards of Cephalalgia, Lancet Neurology, and Drugs; and has received honoraria for participation in clinical trials, contribution to advisory boards, or oral presentations from AbbVie, Lilly, Lundbeck, Novartis, Pfizer, Teva, Weber & Weber, and WebMD.
A version of this article appeared on Medscape.com.
Jeffrey E. Janis, MD, is on a mission. The professor of plastic surgery, surgery, neurosurgery, and neurology at The Ohio State University Wexner Medical Center, Columbus, Ohio, wants to convince neurologists of the safety and efficacy of nerve decompression surgery for treatment-resistant headache. However, many neurologists remain unconvinced.
There’s 24 years of evidence behind this surgical technique across hundreds of different studies with different study designs,” Dr. Janis said.
Yet this treatment approach — surgery on peripheral nerves rather than the brain or spinal cord — hasn’t garnered much support from neurologists. A scan of the agenda of topics at the recently held 2024 annual meeting of the American Headache Society showed few if any studies or presentations on this topic. And neurologists this news organization spoke to said they believe the surgery is experimental and unproven.
Experts do agree drugs don’t work for all patients with migraines. Up to 30% of patients don’t respond to the “laundry list of medications” available to treat the condition, said Dr. Janis.
Many patients have also tried, and failed, alternative treatment approaches such as massage, acupuncture, craniosacral therapy, transdermal patches, electrical stimulation, cryoablation, neurostimulation, and radiofrequency ablation.
If nothing else works, is surgery for headaches the answer?
Long-Held Theory
The idea that pinched, irritated, or compressed peripheral nerves can trigger migraine attacks has been around for nearly 25 years. Studies suggest that in addition to migraine, nerve compression can lead to other headache conditions, including occipital neuralgia, supraorbital neuralgia , and post-traumatic headaches.
This has led to the development of surgical techniques to deactivate various compression trigger sites — what Dr. Janis calls “pinch points” — which could involve muscles, bone, fascia, blood vessels, or scar tissue from prior trauma or surgery.
The procedure is predominantly performed by plastic surgeons, but to a lesser degree by neurosurgeons and ear, nose, and throat specialists.
Target nerves in surgical interventions include those in the frontal region of the head above the eye, temporal region, neck region, and nasal region. Affected areas are usually identified either through patient self-reports or by using a nerve block agent such as lidocaine or Botox at specific points, Dr. Janis noted. If pain subsides after an injection, that location is marked as a target.
One of the barriers to referring complicated patients for surgery is that neurologists evaluating migraine treatments “speak a different language” than surgeons performing the procedure, said Dr. Janis.
Neurologists tend to focus on reduction in monthly migraine days (MMD), while surgeons typically use the Migraine Headache Index that incorporates the frequency, intensity, and duration of migraine attacks.
“Rather than try to convince somebody to speak a different language, we thought, why don’t we just learn their language so we can build bridges and take down barriers,” said Dr. Janis, coauthor of a systematic review and meta-analysis published online recently in Plastic and Reconstructive Surgery.
Investigators examined 19 studies in the review, including five randomized controlled trials (RCTs), published from January 2020 to September 2023, with a total of 1603 participants who were mostly female and ranged in age from 9 to 72 years. Study follow-ups extended from 6 to 38 months. All but three studies were carried out in the United States, and six different compression sites were addressed during surgery.
Investigators found that across studies and by a number of measures, migraine frequency and severity improved after surgery.
Monthly migraine days decreased by 36%-92% and the number of overall migraine attacks per month dropped 25%-87.5%. Patients also reported decreases in attack duration of 41%-75% and intensity of 28%-82% across studies.
“Even using the neurologist-standard language of monthly migraine days, this surgery works,” said Dr. Janis. “Now this is documented both in the surgical literature and the nonsurgical literature.”
The most common complications were ecchymosis, hair loss or thinning, itching, dryness, and rhinorrhea, all of which Dr. Janis described as “fairly minor.” Major complications such as intraoperative bleeding and wound dehiscence were rare, occurring in 1% or less of participants.
‘One And Done?’
These surgeries are usually done on an outpatient basis and generally offer long-term results, Dr. Janis said.
“The idea is one and done,” he said. “The literature around this type of surgery says that whatever type of effect you get at 1 year is likely to be permanent.”
The American Society of Plastic Surgeons agrees. A 2018 position paper developed by experts and commissioned by the society reports that the intervention is safe and effective for appropriate patients, based on a comprehensive literature search and review of a large body of peer-reviewed scientific evidence.
“There is substantial, extensively replicated clinical data that demonstrates a significant reduction in [migraine headache] symptoms and frequency (even complete elimination of headache pain) following trigger site surgery,” the authors noted.
Pamela Blake, MD, a neurologist, board-certified headache specialist, and medical director at the Headache Center of River Oaks, Houston, is a proponent of what she said can be “lifesaving” headache surgery.
“If a doctor told you that you can either treat this problem with medications that you’ll need to take for the rest of your life or you can have a surgical procedure as an outpatient that has extremely low risk and has, in my experience, a 75% chance of reducing or eliminating your pain, you probably would be interested in surgery,” she said.
Continued Skepticism
However, other neurologists and clinicians appear doubtful about this intervention, including Hans-Christoph Diener, MD, PhD, professor of neurology and director, Essen Headache Centre, University of Duisburg-Essen in Germany.
During a debate on the topic a decade ago at the International Headache Congress, Dr. Diener argued that, as migraine is a complex multigene-related disorder of the brain, it doesn’t make sense that surgery would affect the epigenetics of 22 different genes.
Recently, he said that his views have not changed.
The topic remains controversial, and some neurologists are uncomfortable even openly discussing the procedure. Two clinicians who previously commented on this article later asked not to be included.
One neurologist, who asked to remain anonymous, said that Dr. Janis’s review article is “merely a review collecting 19 studies over the previous 10-plus years.”
Other limitations cited by this neurologist are the lack of consistency in procedures among the various studies and the inclusion of only four RCTs, the most recent of which was published 8 years ago, suggesting “the study was probably done closer to 9 or 10 years ago,” the neurologist said.
Dr. Blake suggested some neurologists’ reluctance could be due to limited background on the procedure, which she said isn’t widely discussed at headache meetings and is covered mostly in plastic surgery journals, not neurology literature. Access to surgery is further limited by a lack of specialists who perform the procedure and inconsistent insurance coverage.
A closer collaboration between neurologists and surgeons who perform the procedure could benefit patients, Dr. Blake noted.
“The headache doctor’s role is to identify who’s a candidate for surgery, who meets the criteria for nerve compression, and then follow that patient postoperatively, managing their medications, although usually we get them off their medications,” she added.
From Dr. Janis’s perspective, things are starting to change.
“I’m definitely seeing a greater comfort level among neurologists who are understanding where this sits in the algorithm for treatment, especially for complicated patients,” he said.
Dr. Janis receives royalties from Thieme and Springer Publishing. Dr. Blake reported no relevant conflicts. Dr. Diener received research support from the German Research Council; serves on the editorial boards of Cephalalgia, Lancet Neurology, and Drugs; and has received honoraria for participation in clinical trials, contribution to advisory boards, or oral presentations from AbbVie, Lilly, Lundbeck, Novartis, Pfizer, Teva, Weber & Weber, and WebMD.
A version of this article appeared on Medscape.com.