User login
Obesity Management in the Era of GLP-1 RAs: The Role of GLP-1 RAs
Obesity Management in the Era of GLP-1 RAs: The Role of GLP-1 RAs
Click to view more from Gastroenterology Data Trends 2025.
- Lin K, Mehrotra A, Tsai TC. Metabolic Bariatric Surgery in the Era of GLP-1 Receptor Agonists for Obesity Management. JAMA Netw Open. 2024;7(10):e2441380. doi:10.1001/jamanetworkopen.2024.41380
- Camilleri M, El-Omar EM. Ten reasons gastroenterologists and hepatologists should be treating obesity. Gut. 2023;72(6):1033-1038. doi:10.1136/gutjnl-2023-329639
- Camilleri M. Definite benefits of GLP-1 receptor agonists: what is the risk of gastroparesis and lung aspiration? Gut. 2024. doi:10.1136/gutjnl-2024-333036
- Camilleri M, Carlson P, Dilmaghani S. Letter to the Editor. Prevalence and variations in gastric emptying delay in response to GLP-1 receptor agonist liraglutide. Obesity (Silver Spring). 2024;32(2):232-233. doi:10.1002/oby.23941
- Camilleri, M. Incretin impact on gastric function in obesity: physiology, and pharmacological, surgical and endoscopic treatments. J Physiol. 2024.doi:10.1113/JP287535
- Kindel TL, Wang AY, Wadhwa A, et al; American Gastroenterological Association; American Society for Metabolic and Bariatric Surgery; American Society of Anesthesiologists; International Society of Perioperative Care of Patients with Obesity;
Society of American Gastrointestinal and Endoscopic Surgeons. Multisociety Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2024:S1542-3565(24)00910-8. doi:10.1016/j.cgh.2024.10.003
Click to view more from Gastroenterology Data Trends 2025.
Click to view more from Gastroenterology Data Trends 2025.
- Lin K, Mehrotra A, Tsai TC. Metabolic Bariatric Surgery in the Era of GLP-1 Receptor Agonists for Obesity Management. JAMA Netw Open. 2024;7(10):e2441380. doi:10.1001/jamanetworkopen.2024.41380
- Camilleri M, El-Omar EM. Ten reasons gastroenterologists and hepatologists should be treating obesity. Gut. 2023;72(6):1033-1038. doi:10.1136/gutjnl-2023-329639
- Camilleri M. Definite benefits of GLP-1 receptor agonists: what is the risk of gastroparesis and lung aspiration? Gut. 2024. doi:10.1136/gutjnl-2024-333036
- Camilleri M, Carlson P, Dilmaghani S. Letter to the Editor. Prevalence and variations in gastric emptying delay in response to GLP-1 receptor agonist liraglutide. Obesity (Silver Spring). 2024;32(2):232-233. doi:10.1002/oby.23941
- Camilleri, M. Incretin impact on gastric function in obesity: physiology, and pharmacological, surgical and endoscopic treatments. J Physiol. 2024.doi:10.1113/JP287535
- Kindel TL, Wang AY, Wadhwa A, et al; American Gastroenterological Association; American Society for Metabolic and Bariatric Surgery; American Society of Anesthesiologists; International Society of Perioperative Care of Patients with Obesity;
Society of American Gastrointestinal and Endoscopic Surgeons. Multisociety Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2024:S1542-3565(24)00910-8. doi:10.1016/j.cgh.2024.10.003
- Lin K, Mehrotra A, Tsai TC. Metabolic Bariatric Surgery in the Era of GLP-1 Receptor Agonists for Obesity Management. JAMA Netw Open. 2024;7(10):e2441380. doi:10.1001/jamanetworkopen.2024.41380
- Camilleri M, El-Omar EM. Ten reasons gastroenterologists and hepatologists should be treating obesity. Gut. 2023;72(6):1033-1038. doi:10.1136/gutjnl-2023-329639
- Camilleri M. Definite benefits of GLP-1 receptor agonists: what is the risk of gastroparesis and lung aspiration? Gut. 2024. doi:10.1136/gutjnl-2024-333036
- Camilleri M, Carlson P, Dilmaghani S. Letter to the Editor. Prevalence and variations in gastric emptying delay in response to GLP-1 receptor agonist liraglutide. Obesity (Silver Spring). 2024;32(2):232-233. doi:10.1002/oby.23941
- Camilleri, M. Incretin impact on gastric function in obesity: physiology, and pharmacological, surgical and endoscopic treatments. J Physiol. 2024.doi:10.1113/JP287535
- Kindel TL, Wang AY, Wadhwa A, et al; American Gastroenterological Association; American Society for Metabolic and Bariatric Surgery; American Society of Anesthesiologists; International Society of Perioperative Care of Patients with Obesity;
Society of American Gastrointestinal and Endoscopic Surgeons. Multisociety Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2024:S1542-3565(24)00910-8. doi:10.1016/j.cgh.2024.10.003
Obesity Management in the Era of GLP-1 RAs: The Role of GLP-1 RAs
Obesity Management in the Era of GLP-1 RAs: The Role of GLP-1 RAs
Intermittent Fasting Outperforms Daily Calorie Cutting for Weight Loss
a randomized study found.
A 4:3 IMF program produced modestly superior weight loss than DCR of 2.89 kg over 12 months in the context of a guidelines-based, high-intensity, comprehensive behavioral weight loss program, according to Danielle M. Ostendorf, PhD, MS, co–lead author and an assistant professor at the University of Tennessee, Knoxville, and Victoria Catenacci, MD, study principal investigator, co–lead author, and an associate professor located at the University of Colorado Anschutz Medical Campus, Aurora.
The study, published in Annals of Internal Medicine, found that objectively measured percentage caloric restriction was greater in the 4:3 IMF group, whereas there was no between-group difference in change in total moderate to vigorous physical activity, suggesting that differences in weight loss may have been caused by greater adherence to 4:3 IMF. The 4:3 IMF program was well tolerated and attrition was lower in this group: 19% for IMF group vs 30% for DCR group.
The authors noted that alternative patterns for restricting dietary energy intake are gaining attention owing to the difficulty of adhering to a reduced-calorie diet daily, with most adults who lose weight through DCR showing significant weight regain a year later.
According to Ostendorf and Catenacci, fasting strategies “come in two different flavors and oftentimes get confused in the lay press and by patients and researchers. And there is a difference between IMF and time-restricted eating (TRE),” they said in an interview. “TRE involves limiting the daily window of food intake to 8-10 hours or less on most days of the week — for example, 16:8 or 14:10 strategies. TRE is done every day, consistently and involves eating in the predefined window, and fasting outside of that window.”
IMF is a more periodic and significant fast and involves cycling between complete or near-complete (> 75%) energy restriction on fast days and ad libitum energy intake on nonfast days.
An appealing feature of IMF is that dieters do not have to focus on counting calories and restricting intake every day as they do with DCR, the authors wrote. Furthermore, the periodic nature of fasting is simpler and may mitigate the constant hunger associated with DCR.
Some said the diet was dreadful, but many said it was the easiest diet they had ever been on. “But it did take time for people to adjust to this strategy,” Catenacci said. “It was reassuring to see no evidence of increased binge-eating behaviors.”
Although objectively measured adherence to the targeted energy deficit (percentage caloric restriction from baseline) was below the target of 34.3% in both groups, the 4:3 IMF group showed greater percentage caloric restriction over 12 months. This suggests that, on average, the 4:3 IMF group may be more sustainable over a year than the DCR group. However, weight loss varied in both groups. Future studies should evaluate biological and behavioral predictors of response to both 4:3 IMF and DCR groups in order to personalize recommendations for weight loss.
Study Details
The investigators randomized 165 patients at the University of Colorado Anschutz Medical Campus, with a mean age of 42 years (18-60), a mean baseline weight of 97.4 kg, and a mean baseline body mass index (BMI) of 34.1 to IMF (n = 84) or DCR (n = 81). Of these, 74% were women and 86% were White individuals, and 125 (76%) completed the trial.
The 4:3 IMF group restricted energy intake by 80% on 3 nonconsecutive fast days per week, with ad libitum intake on the other 4 days (4:3 IMF). The 80% calorie reduction fasting corresponded to about 400-600 kcals/d for women and 500-700 kcals/d for men.
“Participants were only required to count calories on their fast days, which is part of the appeal,” Ostendorf said. Although permitted to eat what they wanted on nonfast days, participants were encouraged to make healthy food choices and consume healthy portion sizes.
For its part, the DCR group reduced daily energy intake by 34% to match the weekly energy deficit of 4:3 IMF.
Both groups participated in a high-intensity comprehensive weight loss program with group-based behavioral support and a recommended increase in moderate-intensity physical activity to 300 min/wk.
On the primary endpoint, the 4:3 IMF group showed a weight loss of 7.7 kg (95% CI, –9.6 to –5.9 kg) compared with 4.8 kg (95% CI, –6.8 to –2.8 kg, P =.040) in the DCR group at 12 months. The percentage change in body weight from baseline was –7.6% (95% CI, –9.5% to –5.7%) in the 4:3 IMF group and –5% (95% CI, –6.9% to –3.1%) in the DCR group.
At 12 months, 58% (n = 50) of participants in the 4:3 IMF group achieved weight loss of at least 5% vs 47% (n = 27) of those in the DCR group. In addition, 38% (n = 26) of participants in the 4:3 IMF group achieved weight loss of at least 10% at 12 months vs 16% (n = 9) of those in the DCR group. Changes in body composition, BMI, and waist circumference also tended to favor the 4:3 IMF group.
On other 12-month measures, point estimates of change in systolic blood pressure, total and low-density lipoprotein cholesterol levels, triglyceride level, homeostasis model assessment of insulin resistance, fasting glucose level, and hemoglobin A1c level favored 4:3 IMF. Point estimates of change in diastolic blood pressure and high-density lipoprotein cholesterol level favored DCR.
Currently lacking, the authors said, are data on safety in children and older adults, and adults affected by a long list of conditions: Diabetes, cardiovascular disease, kidney disease (stage 4 or 5), cancer, and eating disorders. Also, people of normal weight or only mild overweight, and pregnant or lactating women. “There have been concerns about IMF causing eating disorders, so we did not include people with eating disorders in our study,” Ostendorf and Catenacci said.
Offering an outside perspective on the findings, James O. Hill, PhD, director of the Nutrition Obesity Research Center and a professor at the University of Alabama at Birmingham believes IMF is a viable option for people trying to lose weight and has prescribed this approach for some in his practice. “But there is no one strategy that works for everyone,” he said in an interview. “I recommend IMF as a science-based strategy that can be effective for some people, and I think it should be on the list of science-based tools that people can consider using.” But as it won’t work for everyone, “we need to consider both metabolic success and behavioral success. In other words, would it be more effective if people could do it and how easy or hard is it for people to do?”
Audra Wilson, MS, RD, a bariatric dietitian at Northwestern Medicine Delnor Hospital in Geneva, Illinois, who was not involved in the study, expressed more reservations. “We do not specifically recommend intermittent fasting at Northwestern Medicine. There is no set protocol for this diet, and it can vary in ways that can limit nutrition to the point where we are not meeting needs on a regular basis,” she said in an interview.
Moreover, this study did not specify exact nutritional recommendations for participants but merely reduced overall caloric intake. “Although intermittent fasting may be helpful to some, in my nearly 10 years of experience I have not seen it be effective for many and especially not long term,” Wilson added.
Concerningly, IMF can foster disordered eating patterns of restriction followed by binging. “Although a balanced diet is more difficult to achieve, guidance from professionals like dietitians can give patients the tools to achieve balance, meet all nutrient needs, achieve satiety, and maybe most importantly, have a better relationship with food,” she said.
As for the influence of metabolic factors that may be associated with better weight loss, Ostendorf said, “be on the lookout for future publications in this area. We are analyzing data around changes in energy expenditure and changes in hunger-related hormones, among others.” A colleague is collecting biological samples to study genetics in this context. “However, in general, it appeared that the difference in weight loss was due to a greater caloric deficit in the 4:3 IMF group.”
Ostendorf and Catenacci are currently conducting a pilot study testing 4:3 IMF in breast cancer survivors. “We think this is a promising strategy for weight loss in breast cancer survivors who struggle with overweight/obesity in addition to their cancer diagnosis,” Ostendorf said.
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Ostendorf, Catenacci, Hill, and Wilson disclosed no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
a randomized study found.
A 4:3 IMF program produced modestly superior weight loss than DCR of 2.89 kg over 12 months in the context of a guidelines-based, high-intensity, comprehensive behavioral weight loss program, according to Danielle M. Ostendorf, PhD, MS, co–lead author and an assistant professor at the University of Tennessee, Knoxville, and Victoria Catenacci, MD, study principal investigator, co–lead author, and an associate professor located at the University of Colorado Anschutz Medical Campus, Aurora.
The study, published in Annals of Internal Medicine, found that objectively measured percentage caloric restriction was greater in the 4:3 IMF group, whereas there was no between-group difference in change in total moderate to vigorous physical activity, suggesting that differences in weight loss may have been caused by greater adherence to 4:3 IMF. The 4:3 IMF program was well tolerated and attrition was lower in this group: 19% for IMF group vs 30% for DCR group.
The authors noted that alternative patterns for restricting dietary energy intake are gaining attention owing to the difficulty of adhering to a reduced-calorie diet daily, with most adults who lose weight through DCR showing significant weight regain a year later.
According to Ostendorf and Catenacci, fasting strategies “come in two different flavors and oftentimes get confused in the lay press and by patients and researchers. And there is a difference between IMF and time-restricted eating (TRE),” they said in an interview. “TRE involves limiting the daily window of food intake to 8-10 hours or less on most days of the week — for example, 16:8 or 14:10 strategies. TRE is done every day, consistently and involves eating in the predefined window, and fasting outside of that window.”
IMF is a more periodic and significant fast and involves cycling between complete or near-complete (> 75%) energy restriction on fast days and ad libitum energy intake on nonfast days.
An appealing feature of IMF is that dieters do not have to focus on counting calories and restricting intake every day as they do with DCR, the authors wrote. Furthermore, the periodic nature of fasting is simpler and may mitigate the constant hunger associated with DCR.
Some said the diet was dreadful, but many said it was the easiest diet they had ever been on. “But it did take time for people to adjust to this strategy,” Catenacci said. “It was reassuring to see no evidence of increased binge-eating behaviors.”
Although objectively measured adherence to the targeted energy deficit (percentage caloric restriction from baseline) was below the target of 34.3% in both groups, the 4:3 IMF group showed greater percentage caloric restriction over 12 months. This suggests that, on average, the 4:3 IMF group may be more sustainable over a year than the DCR group. However, weight loss varied in both groups. Future studies should evaluate biological and behavioral predictors of response to both 4:3 IMF and DCR groups in order to personalize recommendations for weight loss.
Study Details
The investigators randomized 165 patients at the University of Colorado Anschutz Medical Campus, with a mean age of 42 years (18-60), a mean baseline weight of 97.4 kg, and a mean baseline body mass index (BMI) of 34.1 to IMF (n = 84) or DCR (n = 81). Of these, 74% were women and 86% were White individuals, and 125 (76%) completed the trial.
The 4:3 IMF group restricted energy intake by 80% on 3 nonconsecutive fast days per week, with ad libitum intake on the other 4 days (4:3 IMF). The 80% calorie reduction fasting corresponded to about 400-600 kcals/d for women and 500-700 kcals/d for men.
“Participants were only required to count calories on their fast days, which is part of the appeal,” Ostendorf said. Although permitted to eat what they wanted on nonfast days, participants were encouraged to make healthy food choices and consume healthy portion sizes.
For its part, the DCR group reduced daily energy intake by 34% to match the weekly energy deficit of 4:3 IMF.
Both groups participated in a high-intensity comprehensive weight loss program with group-based behavioral support and a recommended increase in moderate-intensity physical activity to 300 min/wk.
On the primary endpoint, the 4:3 IMF group showed a weight loss of 7.7 kg (95% CI, –9.6 to –5.9 kg) compared with 4.8 kg (95% CI, –6.8 to –2.8 kg, P =.040) in the DCR group at 12 months. The percentage change in body weight from baseline was –7.6% (95% CI, –9.5% to –5.7%) in the 4:3 IMF group and –5% (95% CI, –6.9% to –3.1%) in the DCR group.
At 12 months, 58% (n = 50) of participants in the 4:3 IMF group achieved weight loss of at least 5% vs 47% (n = 27) of those in the DCR group. In addition, 38% (n = 26) of participants in the 4:3 IMF group achieved weight loss of at least 10% at 12 months vs 16% (n = 9) of those in the DCR group. Changes in body composition, BMI, and waist circumference also tended to favor the 4:3 IMF group.
On other 12-month measures, point estimates of change in systolic blood pressure, total and low-density lipoprotein cholesterol levels, triglyceride level, homeostasis model assessment of insulin resistance, fasting glucose level, and hemoglobin A1c level favored 4:3 IMF. Point estimates of change in diastolic blood pressure and high-density lipoprotein cholesterol level favored DCR.
Currently lacking, the authors said, are data on safety in children and older adults, and adults affected by a long list of conditions: Diabetes, cardiovascular disease, kidney disease (stage 4 or 5), cancer, and eating disorders. Also, people of normal weight or only mild overweight, and pregnant or lactating women. “There have been concerns about IMF causing eating disorders, so we did not include people with eating disorders in our study,” Ostendorf and Catenacci said.
Offering an outside perspective on the findings, James O. Hill, PhD, director of the Nutrition Obesity Research Center and a professor at the University of Alabama at Birmingham believes IMF is a viable option for people trying to lose weight and has prescribed this approach for some in his practice. “But there is no one strategy that works for everyone,” he said in an interview. “I recommend IMF as a science-based strategy that can be effective for some people, and I think it should be on the list of science-based tools that people can consider using.” But as it won’t work for everyone, “we need to consider both metabolic success and behavioral success. In other words, would it be more effective if people could do it and how easy or hard is it for people to do?”
Audra Wilson, MS, RD, a bariatric dietitian at Northwestern Medicine Delnor Hospital in Geneva, Illinois, who was not involved in the study, expressed more reservations. “We do not specifically recommend intermittent fasting at Northwestern Medicine. There is no set protocol for this diet, and it can vary in ways that can limit nutrition to the point where we are not meeting needs on a regular basis,” she said in an interview.
Moreover, this study did not specify exact nutritional recommendations for participants but merely reduced overall caloric intake. “Although intermittent fasting may be helpful to some, in my nearly 10 years of experience I have not seen it be effective for many and especially not long term,” Wilson added.
Concerningly, IMF can foster disordered eating patterns of restriction followed by binging. “Although a balanced diet is more difficult to achieve, guidance from professionals like dietitians can give patients the tools to achieve balance, meet all nutrient needs, achieve satiety, and maybe most importantly, have a better relationship with food,” she said.
As for the influence of metabolic factors that may be associated with better weight loss, Ostendorf said, “be on the lookout for future publications in this area. We are analyzing data around changes in energy expenditure and changes in hunger-related hormones, among others.” A colleague is collecting biological samples to study genetics in this context. “However, in general, it appeared that the difference in weight loss was due to a greater caloric deficit in the 4:3 IMF group.”
Ostendorf and Catenacci are currently conducting a pilot study testing 4:3 IMF in breast cancer survivors. “We think this is a promising strategy for weight loss in breast cancer survivors who struggle with overweight/obesity in addition to their cancer diagnosis,” Ostendorf said.
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Ostendorf, Catenacci, Hill, and Wilson disclosed no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
a randomized study found.
A 4:3 IMF program produced modestly superior weight loss than DCR of 2.89 kg over 12 months in the context of a guidelines-based, high-intensity, comprehensive behavioral weight loss program, according to Danielle M. Ostendorf, PhD, MS, co–lead author and an assistant professor at the University of Tennessee, Knoxville, and Victoria Catenacci, MD, study principal investigator, co–lead author, and an associate professor located at the University of Colorado Anschutz Medical Campus, Aurora.
The study, published in Annals of Internal Medicine, found that objectively measured percentage caloric restriction was greater in the 4:3 IMF group, whereas there was no between-group difference in change in total moderate to vigorous physical activity, suggesting that differences in weight loss may have been caused by greater adherence to 4:3 IMF. The 4:3 IMF program was well tolerated and attrition was lower in this group: 19% for IMF group vs 30% for DCR group.
The authors noted that alternative patterns for restricting dietary energy intake are gaining attention owing to the difficulty of adhering to a reduced-calorie diet daily, with most adults who lose weight through DCR showing significant weight regain a year later.
According to Ostendorf and Catenacci, fasting strategies “come in two different flavors and oftentimes get confused in the lay press and by patients and researchers. And there is a difference between IMF and time-restricted eating (TRE),” they said in an interview. “TRE involves limiting the daily window of food intake to 8-10 hours or less on most days of the week — for example, 16:8 or 14:10 strategies. TRE is done every day, consistently and involves eating in the predefined window, and fasting outside of that window.”
IMF is a more periodic and significant fast and involves cycling between complete or near-complete (> 75%) energy restriction on fast days and ad libitum energy intake on nonfast days.
An appealing feature of IMF is that dieters do not have to focus on counting calories and restricting intake every day as they do with DCR, the authors wrote. Furthermore, the periodic nature of fasting is simpler and may mitigate the constant hunger associated with DCR.
Some said the diet was dreadful, but many said it was the easiest diet they had ever been on. “But it did take time for people to adjust to this strategy,” Catenacci said. “It was reassuring to see no evidence of increased binge-eating behaviors.”
Although objectively measured adherence to the targeted energy deficit (percentage caloric restriction from baseline) was below the target of 34.3% in both groups, the 4:3 IMF group showed greater percentage caloric restriction over 12 months. This suggests that, on average, the 4:3 IMF group may be more sustainable over a year than the DCR group. However, weight loss varied in both groups. Future studies should evaluate biological and behavioral predictors of response to both 4:3 IMF and DCR groups in order to personalize recommendations for weight loss.
Study Details
The investigators randomized 165 patients at the University of Colorado Anschutz Medical Campus, with a mean age of 42 years (18-60), a mean baseline weight of 97.4 kg, and a mean baseline body mass index (BMI) of 34.1 to IMF (n = 84) or DCR (n = 81). Of these, 74% were women and 86% were White individuals, and 125 (76%) completed the trial.
The 4:3 IMF group restricted energy intake by 80% on 3 nonconsecutive fast days per week, with ad libitum intake on the other 4 days (4:3 IMF). The 80% calorie reduction fasting corresponded to about 400-600 kcals/d for women and 500-700 kcals/d for men.
“Participants were only required to count calories on their fast days, which is part of the appeal,” Ostendorf said. Although permitted to eat what they wanted on nonfast days, participants were encouraged to make healthy food choices and consume healthy portion sizes.
For its part, the DCR group reduced daily energy intake by 34% to match the weekly energy deficit of 4:3 IMF.
Both groups participated in a high-intensity comprehensive weight loss program with group-based behavioral support and a recommended increase in moderate-intensity physical activity to 300 min/wk.
On the primary endpoint, the 4:3 IMF group showed a weight loss of 7.7 kg (95% CI, –9.6 to –5.9 kg) compared with 4.8 kg (95% CI, –6.8 to –2.8 kg, P =.040) in the DCR group at 12 months. The percentage change in body weight from baseline was –7.6% (95% CI, –9.5% to –5.7%) in the 4:3 IMF group and –5% (95% CI, –6.9% to –3.1%) in the DCR group.
At 12 months, 58% (n = 50) of participants in the 4:3 IMF group achieved weight loss of at least 5% vs 47% (n = 27) of those in the DCR group. In addition, 38% (n = 26) of participants in the 4:3 IMF group achieved weight loss of at least 10% at 12 months vs 16% (n = 9) of those in the DCR group. Changes in body composition, BMI, and waist circumference also tended to favor the 4:3 IMF group.
On other 12-month measures, point estimates of change in systolic blood pressure, total and low-density lipoprotein cholesterol levels, triglyceride level, homeostasis model assessment of insulin resistance, fasting glucose level, and hemoglobin A1c level favored 4:3 IMF. Point estimates of change in diastolic blood pressure and high-density lipoprotein cholesterol level favored DCR.
Currently lacking, the authors said, are data on safety in children and older adults, and adults affected by a long list of conditions: Diabetes, cardiovascular disease, kidney disease (stage 4 or 5), cancer, and eating disorders. Also, people of normal weight or only mild overweight, and pregnant or lactating women. “There have been concerns about IMF causing eating disorders, so we did not include people with eating disorders in our study,” Ostendorf and Catenacci said.
Offering an outside perspective on the findings, James O. Hill, PhD, director of the Nutrition Obesity Research Center and a professor at the University of Alabama at Birmingham believes IMF is a viable option for people trying to lose weight and has prescribed this approach for some in his practice. “But there is no one strategy that works for everyone,” he said in an interview. “I recommend IMF as a science-based strategy that can be effective for some people, and I think it should be on the list of science-based tools that people can consider using.” But as it won’t work for everyone, “we need to consider both metabolic success and behavioral success. In other words, would it be more effective if people could do it and how easy or hard is it for people to do?”
Audra Wilson, MS, RD, a bariatric dietitian at Northwestern Medicine Delnor Hospital in Geneva, Illinois, who was not involved in the study, expressed more reservations. “We do not specifically recommend intermittent fasting at Northwestern Medicine. There is no set protocol for this diet, and it can vary in ways that can limit nutrition to the point where we are not meeting needs on a regular basis,” she said in an interview.
Moreover, this study did not specify exact nutritional recommendations for participants but merely reduced overall caloric intake. “Although intermittent fasting may be helpful to some, in my nearly 10 years of experience I have not seen it be effective for many and especially not long term,” Wilson added.
Concerningly, IMF can foster disordered eating patterns of restriction followed by binging. “Although a balanced diet is more difficult to achieve, guidance from professionals like dietitians can give patients the tools to achieve balance, meet all nutrient needs, achieve satiety, and maybe most importantly, have a better relationship with food,” she said.
As for the influence of metabolic factors that may be associated with better weight loss, Ostendorf said, “be on the lookout for future publications in this area. We are analyzing data around changes in energy expenditure and changes in hunger-related hormones, among others.” A colleague is collecting biological samples to study genetics in this context. “However, in general, it appeared that the difference in weight loss was due to a greater caloric deficit in the 4:3 IMF group.”
Ostendorf and Catenacci are currently conducting a pilot study testing 4:3 IMF in breast cancer survivors. “We think this is a promising strategy for weight loss in breast cancer survivors who struggle with overweight/obesity in addition to their cancer diagnosis,” Ostendorf said.
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Ostendorf, Catenacci, Hill, and Wilson disclosed no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Safety Profile of GLP-1s ‘Reassuring’ in Upper Endoscopy
according to a meta-analysis of more than 80,000 patients.
Safety profiles, however, were comparable across groups, suggesting that prolonged fasting may be a sufficient management strategy, instead of withholding GLP-1RAs, lead author Antonio Facciorusso, MD, PhD, of the University of Foggia, Italy, and colleagues reported.
“The impact of GLP-1RAs on slowing gastric motility has raised concerns in patients undergoing endoscopic procedures, particularly upper endoscopies,” the investigators wrote in Clinical Gastroenterology and Hepatology. “This is due to the perceived risk of aspiration of retained gastric contents in sedated patients and the decreased visibility of the gastric mucosa, which can reduce the diagnostic yield of the examination.”
The American Society of Anesthesiologists (ASA) recommends withholding GLP-1RAs before procedures or surgery, whereas AGA suggests an individualized approach, citing limited supporting data.
A previous meta-analysis reported that GLP-1RAs mildly delayed gastric emptying, but clinical relevance remained unclear.
The present meta-analysis aimed to clarify this uncertainty by analyzing 13 retrospective studies that involved 84,065 patients undergoing upper endoscopy. Outcomes were compared among GLP-1RA users vs non-users, including rates of retained gastric contents, aborted procedures, and adverse events.
Patients on GLP-1RAs had significantly higher rates of retained gastric contents than non-users (odds ratio [OR], 5.56), a finding that held steady (OR, 4.20) after adjusting for age, sex, diabetes, body mass index, and other therapies.
GLP-1RAs were also associated with an increased likelihood of aborted procedures (OR, 5.13; 1% vs. 0.3%) and a higher need for repeat endoscopies (OR, 2.19; 1% vs 2%); however, Facciorusso and colleagues noted that these events, in absolute terms, were relatively uncommon.
“The rate of aborted and repeat procedures in the included studies was low,” the investigators wrote. “This meant that only for every 110 patients undergoing upper endoscopy while in GLP-1RA therapy would we observe an aborted procedure and only for every 120 patients would we need to repeat the procedure.”
The overall safety profile of GLP-1RAs in the context of upper endoscopy remained largely reassuring, they added. Specifically, rates of bronchial aspiration were not significantly different between users and non-users. What’s more, no single study reported a statistically significant increase in major complications, including pulmonary adverse events, among GLP-1RA users.
According to Facciorusso and colleagues, these findings suggest that retained gastric contents do not appear to substantially heighten the risk of serious harm, though further prospective studies are needed.
“Our comprehensive analysis indicates that, while the use of GLP-1RA results in higher rates of [retained gastric contents], the actual clinical impact appears to be limited,” they wrote. “Therefore, there is no strong evidence to support the routine discontinuation of the drug before upper endoscopy procedures.”
Instead, they supported the AGA task force’s recommendation for an individualized approach, and not withholding GLP-1RAs unnecessarily, calling this “the best compromise.”
“Prolonging the duration of fasting for solids could represent the optimal approach in these patients, although this strategy requires further evaluation,” the investigators concluded.
The investigators disclosed no conflicts of interest.
according to a meta-analysis of more than 80,000 patients.
Safety profiles, however, were comparable across groups, suggesting that prolonged fasting may be a sufficient management strategy, instead of withholding GLP-1RAs, lead author Antonio Facciorusso, MD, PhD, of the University of Foggia, Italy, and colleagues reported.
“The impact of GLP-1RAs on slowing gastric motility has raised concerns in patients undergoing endoscopic procedures, particularly upper endoscopies,” the investigators wrote in Clinical Gastroenterology and Hepatology. “This is due to the perceived risk of aspiration of retained gastric contents in sedated patients and the decreased visibility of the gastric mucosa, which can reduce the diagnostic yield of the examination.”
The American Society of Anesthesiologists (ASA) recommends withholding GLP-1RAs before procedures or surgery, whereas AGA suggests an individualized approach, citing limited supporting data.
A previous meta-analysis reported that GLP-1RAs mildly delayed gastric emptying, but clinical relevance remained unclear.
The present meta-analysis aimed to clarify this uncertainty by analyzing 13 retrospective studies that involved 84,065 patients undergoing upper endoscopy. Outcomes were compared among GLP-1RA users vs non-users, including rates of retained gastric contents, aborted procedures, and adverse events.
Patients on GLP-1RAs had significantly higher rates of retained gastric contents than non-users (odds ratio [OR], 5.56), a finding that held steady (OR, 4.20) after adjusting for age, sex, diabetes, body mass index, and other therapies.
GLP-1RAs were also associated with an increased likelihood of aborted procedures (OR, 5.13; 1% vs. 0.3%) and a higher need for repeat endoscopies (OR, 2.19; 1% vs 2%); however, Facciorusso and colleagues noted that these events, in absolute terms, were relatively uncommon.
“The rate of aborted and repeat procedures in the included studies was low,” the investigators wrote. “This meant that only for every 110 patients undergoing upper endoscopy while in GLP-1RA therapy would we observe an aborted procedure and only for every 120 patients would we need to repeat the procedure.”
The overall safety profile of GLP-1RAs in the context of upper endoscopy remained largely reassuring, they added. Specifically, rates of bronchial aspiration were not significantly different between users and non-users. What’s more, no single study reported a statistically significant increase in major complications, including pulmonary adverse events, among GLP-1RA users.
According to Facciorusso and colleagues, these findings suggest that retained gastric contents do not appear to substantially heighten the risk of serious harm, though further prospective studies are needed.
“Our comprehensive analysis indicates that, while the use of GLP-1RA results in higher rates of [retained gastric contents], the actual clinical impact appears to be limited,” they wrote. “Therefore, there is no strong evidence to support the routine discontinuation of the drug before upper endoscopy procedures.”
Instead, they supported the AGA task force’s recommendation for an individualized approach, and not withholding GLP-1RAs unnecessarily, calling this “the best compromise.”
“Prolonging the duration of fasting for solids could represent the optimal approach in these patients, although this strategy requires further evaluation,” the investigators concluded.
The investigators disclosed no conflicts of interest.
according to a meta-analysis of more than 80,000 patients.
Safety profiles, however, were comparable across groups, suggesting that prolonged fasting may be a sufficient management strategy, instead of withholding GLP-1RAs, lead author Antonio Facciorusso, MD, PhD, of the University of Foggia, Italy, and colleagues reported.
“The impact of GLP-1RAs on slowing gastric motility has raised concerns in patients undergoing endoscopic procedures, particularly upper endoscopies,” the investigators wrote in Clinical Gastroenterology and Hepatology. “This is due to the perceived risk of aspiration of retained gastric contents in sedated patients and the decreased visibility of the gastric mucosa, which can reduce the diagnostic yield of the examination.”
The American Society of Anesthesiologists (ASA) recommends withholding GLP-1RAs before procedures or surgery, whereas AGA suggests an individualized approach, citing limited supporting data.
A previous meta-analysis reported that GLP-1RAs mildly delayed gastric emptying, but clinical relevance remained unclear.
The present meta-analysis aimed to clarify this uncertainty by analyzing 13 retrospective studies that involved 84,065 patients undergoing upper endoscopy. Outcomes were compared among GLP-1RA users vs non-users, including rates of retained gastric contents, aborted procedures, and adverse events.
Patients on GLP-1RAs had significantly higher rates of retained gastric contents than non-users (odds ratio [OR], 5.56), a finding that held steady (OR, 4.20) after adjusting for age, sex, diabetes, body mass index, and other therapies.
GLP-1RAs were also associated with an increased likelihood of aborted procedures (OR, 5.13; 1% vs. 0.3%) and a higher need for repeat endoscopies (OR, 2.19; 1% vs 2%); however, Facciorusso and colleagues noted that these events, in absolute terms, were relatively uncommon.
“The rate of aborted and repeat procedures in the included studies was low,” the investigators wrote. “This meant that only for every 110 patients undergoing upper endoscopy while in GLP-1RA therapy would we observe an aborted procedure and only for every 120 patients would we need to repeat the procedure.”
The overall safety profile of GLP-1RAs in the context of upper endoscopy remained largely reassuring, they added. Specifically, rates of bronchial aspiration were not significantly different between users and non-users. What’s more, no single study reported a statistically significant increase in major complications, including pulmonary adverse events, among GLP-1RA users.
According to Facciorusso and colleagues, these findings suggest that retained gastric contents do not appear to substantially heighten the risk of serious harm, though further prospective studies are needed.
“Our comprehensive analysis indicates that, while the use of GLP-1RA results in higher rates of [retained gastric contents], the actual clinical impact appears to be limited,” they wrote. “Therefore, there is no strong evidence to support the routine discontinuation of the drug before upper endoscopy procedures.”
Instead, they supported the AGA task force’s recommendation for an individualized approach, and not withholding GLP-1RAs unnecessarily, calling this “the best compromise.”
“Prolonging the duration of fasting for solids could represent the optimal approach in these patients, although this strategy requires further evaluation,” the investigators concluded.
The investigators disclosed no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Bariatric Surgery: Nutrition’s Role in Patient Outcomes
, according to an updated clinical practice statement from the Obesity Medicine Association (OMA).
The update offers guidance on how to manage metabolic and bariatric surgery patients’ nutrition, from preoperative nutritional assessments through identification and treatment of the most common nutritional problems associated with bariatric procedures.
“The main takeaway really is that obesity is a complex and chronic disease. It requires the same model of care as diabetes or other chronic conditions,” said Rutuja Patel, DO, senior author and an obesity medicine specialist at Northwestern Medicine Regional Medical Group in Winfield, Illinois.
The development of an interdisciplinary team of medical providers with evidence-based nutrition knowledge and consistent information improves the quality of nutrition care provided to bariatric surgery patients, the authors wrote.
“Collaborative multidisciplinary care that takes into consideration the whole patient in a biopsychosocial way and uses multiple modalities — including medical, behavioral, nutritional, and others — leads to the best outcomes in these complex patients,” Patel said.
The updated statement, published online in Obesity Pillars, offers a variety of tools and checklists to aid clinicians, especially those who may not have access to a multidisciplinary team or dietitian knowledgeable about bariatric nutrition.
It is a follow-up to the OMA’s 2022 clinical practice statement, which provided an overview of bariatric surgery, gastrointestinal hormones, and the microbiome in patients with obesity.
Presurgical Guidance
The new guidance lays out the various components of preoperative nutrition screenings, among which is a medication review to determine if the patient is taking drugs that may affect weight and calorie intake. These include antihypertensives, diabetes agents, hormonal contraceptives, antidepressants, migraine medications, and antipsychotics.
In taking a patient’s history, clinicians should ask about major events associated with weight changes, such as medication changes, illness, pregnancy, divorce, stressful employment, food insecurity, and periods of disordered eating.
The fundamental approach to conducting a nutrition assessment is an understanding of the role that various sections of the gastrointestinal tract play in micronutrient absorption, the authors wrote. As an educational tool, the update includes a diagram that indicates the areas of the stomach, duodenum, jejunum, and ileum that may be altered by bariatric surgery and how they factor into micronutrient absorption.
“It makes it easier to see why certain surgical procedures are more likely to cause certain deficiencies,” Patel said.
Postsurgery Patient Management
Post surgery, clinicians should monitor patients for other problems that could affect nutrient absorption, including food intolerances, drug-nutrient interactions, and increased gastrointestinal transit time.
Patel and coauthors discussed the pros and cons of multivitamin mineral supplement formulations as well as specific vitamin and mineral recommendations for patients undergoing certain metabolic or bariatric surgery procedures. They included three supplemental cases in the appendix to illustrate supplementation recommendations and long-term maintenance suggestions.
“It’s important to remember that most of these deficiencies present without many clinical symptoms, so it becomes essential to screen for them and repeat as needed,” Patel said.
The update also tackles postoperative nutritional assessments and diet progression. No evidence supports following one postsurgical diet progression protocol over another, but they generally proceed from a clear liquid diet to foods with normal textures, the authors noted. Clinicians should adapt them according to the procedure type, they added.
In addition, clinicians must troubleshoot any nutrition-related concerns, including constipation, dehydration, nausea, heartburn, and fatigue, for up to a year after surgery, they wrote.
Metabolic and bariatric surgery patients should be evaluated annually at a minimum, if not more frequently, to gauge nutritional health, the authors wrote. Treating obesity as a disease involves more than weight loss — instead, it’s about improving the quality of life of patients through procedures, medications, and lifestyle modifications, they added.
Track New Developments
With ongoing changes in the field of metabolic and bariatric surgery, it’s helpful for clinicians to remain updated about new approaches across various disciplines linked to obesity management and treatment, said Christina Poa-Li, MD, a surgeon at Huntington Health Medical Center, affiliated with Cedars-Sinai Health System, in Pasadena, California, who was not involved in developing the updated practice statement.
“For example, the rapidly growing prescription of anti-obesity medications and their use in both preoperative and postoperative surgical patients drastically affect their nutrition,” she said. “Providers of various backgrounds and specialties will benefit from the most updated guidance on evaluating patient nutrition.”
Clinicians should consider expanding their patient population to include those with metabolic dysfunction–associated steatohepatitis or metabolic dysfunction–associated steatotic liver disease, Poa-Li said.
“These patient subpopulations may not have been considered for bariatric surgery or even referral to a bariatric surgeon for consultation previously,” she said. “It is important to increase awareness among clinicians of the potential benefits for metabolic and bariatric surgery for these patients.”
The report didn’t receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Patel and Poa-Li reported no relevant disclosures.
A version of this article appeared on Medscape.com.
, according to an updated clinical practice statement from the Obesity Medicine Association (OMA).
The update offers guidance on how to manage metabolic and bariatric surgery patients’ nutrition, from preoperative nutritional assessments through identification and treatment of the most common nutritional problems associated with bariatric procedures.
“The main takeaway really is that obesity is a complex and chronic disease. It requires the same model of care as diabetes or other chronic conditions,” said Rutuja Patel, DO, senior author and an obesity medicine specialist at Northwestern Medicine Regional Medical Group in Winfield, Illinois.
The development of an interdisciplinary team of medical providers with evidence-based nutrition knowledge and consistent information improves the quality of nutrition care provided to bariatric surgery patients, the authors wrote.
“Collaborative multidisciplinary care that takes into consideration the whole patient in a biopsychosocial way and uses multiple modalities — including medical, behavioral, nutritional, and others — leads to the best outcomes in these complex patients,” Patel said.
The updated statement, published online in Obesity Pillars, offers a variety of tools and checklists to aid clinicians, especially those who may not have access to a multidisciplinary team or dietitian knowledgeable about bariatric nutrition.
It is a follow-up to the OMA’s 2022 clinical practice statement, which provided an overview of bariatric surgery, gastrointestinal hormones, and the microbiome in patients with obesity.
Presurgical Guidance
The new guidance lays out the various components of preoperative nutrition screenings, among which is a medication review to determine if the patient is taking drugs that may affect weight and calorie intake. These include antihypertensives, diabetes agents, hormonal contraceptives, antidepressants, migraine medications, and antipsychotics.
In taking a patient’s history, clinicians should ask about major events associated with weight changes, such as medication changes, illness, pregnancy, divorce, stressful employment, food insecurity, and periods of disordered eating.
The fundamental approach to conducting a nutrition assessment is an understanding of the role that various sections of the gastrointestinal tract play in micronutrient absorption, the authors wrote. As an educational tool, the update includes a diagram that indicates the areas of the stomach, duodenum, jejunum, and ileum that may be altered by bariatric surgery and how they factor into micronutrient absorption.
“It makes it easier to see why certain surgical procedures are more likely to cause certain deficiencies,” Patel said.
Postsurgery Patient Management
Post surgery, clinicians should monitor patients for other problems that could affect nutrient absorption, including food intolerances, drug-nutrient interactions, and increased gastrointestinal transit time.
Patel and coauthors discussed the pros and cons of multivitamin mineral supplement formulations as well as specific vitamin and mineral recommendations for patients undergoing certain metabolic or bariatric surgery procedures. They included three supplemental cases in the appendix to illustrate supplementation recommendations and long-term maintenance suggestions.
“It’s important to remember that most of these deficiencies present without many clinical symptoms, so it becomes essential to screen for them and repeat as needed,” Patel said.
The update also tackles postoperative nutritional assessments and diet progression. No evidence supports following one postsurgical diet progression protocol over another, but they generally proceed from a clear liquid diet to foods with normal textures, the authors noted. Clinicians should adapt them according to the procedure type, they added.
In addition, clinicians must troubleshoot any nutrition-related concerns, including constipation, dehydration, nausea, heartburn, and fatigue, for up to a year after surgery, they wrote.
Metabolic and bariatric surgery patients should be evaluated annually at a minimum, if not more frequently, to gauge nutritional health, the authors wrote. Treating obesity as a disease involves more than weight loss — instead, it’s about improving the quality of life of patients through procedures, medications, and lifestyle modifications, they added.
Track New Developments
With ongoing changes in the field of metabolic and bariatric surgery, it’s helpful for clinicians to remain updated about new approaches across various disciplines linked to obesity management and treatment, said Christina Poa-Li, MD, a surgeon at Huntington Health Medical Center, affiliated with Cedars-Sinai Health System, in Pasadena, California, who was not involved in developing the updated practice statement.
“For example, the rapidly growing prescription of anti-obesity medications and their use in both preoperative and postoperative surgical patients drastically affect their nutrition,” she said. “Providers of various backgrounds and specialties will benefit from the most updated guidance on evaluating patient nutrition.”
Clinicians should consider expanding their patient population to include those with metabolic dysfunction–associated steatohepatitis or metabolic dysfunction–associated steatotic liver disease, Poa-Li said.
“These patient subpopulations may not have been considered for bariatric surgery or even referral to a bariatric surgeon for consultation previously,” she said. “It is important to increase awareness among clinicians of the potential benefits for metabolic and bariatric surgery for these patients.”
The report didn’t receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Patel and Poa-Li reported no relevant disclosures.
A version of this article appeared on Medscape.com.
, according to an updated clinical practice statement from the Obesity Medicine Association (OMA).
The update offers guidance on how to manage metabolic and bariatric surgery patients’ nutrition, from preoperative nutritional assessments through identification and treatment of the most common nutritional problems associated with bariatric procedures.
“The main takeaway really is that obesity is a complex and chronic disease. It requires the same model of care as diabetes or other chronic conditions,” said Rutuja Patel, DO, senior author and an obesity medicine specialist at Northwestern Medicine Regional Medical Group in Winfield, Illinois.
The development of an interdisciplinary team of medical providers with evidence-based nutrition knowledge and consistent information improves the quality of nutrition care provided to bariatric surgery patients, the authors wrote.
“Collaborative multidisciplinary care that takes into consideration the whole patient in a biopsychosocial way and uses multiple modalities — including medical, behavioral, nutritional, and others — leads to the best outcomes in these complex patients,” Patel said.
The updated statement, published online in Obesity Pillars, offers a variety of tools and checklists to aid clinicians, especially those who may not have access to a multidisciplinary team or dietitian knowledgeable about bariatric nutrition.
It is a follow-up to the OMA’s 2022 clinical practice statement, which provided an overview of bariatric surgery, gastrointestinal hormones, and the microbiome in patients with obesity.
Presurgical Guidance
The new guidance lays out the various components of preoperative nutrition screenings, among which is a medication review to determine if the patient is taking drugs that may affect weight and calorie intake. These include antihypertensives, diabetes agents, hormonal contraceptives, antidepressants, migraine medications, and antipsychotics.
In taking a patient’s history, clinicians should ask about major events associated with weight changes, such as medication changes, illness, pregnancy, divorce, stressful employment, food insecurity, and periods of disordered eating.
The fundamental approach to conducting a nutrition assessment is an understanding of the role that various sections of the gastrointestinal tract play in micronutrient absorption, the authors wrote. As an educational tool, the update includes a diagram that indicates the areas of the stomach, duodenum, jejunum, and ileum that may be altered by bariatric surgery and how they factor into micronutrient absorption.
“It makes it easier to see why certain surgical procedures are more likely to cause certain deficiencies,” Patel said.
Postsurgery Patient Management
Post surgery, clinicians should monitor patients for other problems that could affect nutrient absorption, including food intolerances, drug-nutrient interactions, and increased gastrointestinal transit time.
Patel and coauthors discussed the pros and cons of multivitamin mineral supplement formulations as well as specific vitamin and mineral recommendations for patients undergoing certain metabolic or bariatric surgery procedures. They included three supplemental cases in the appendix to illustrate supplementation recommendations and long-term maintenance suggestions.
“It’s important to remember that most of these deficiencies present without many clinical symptoms, so it becomes essential to screen for them and repeat as needed,” Patel said.
The update also tackles postoperative nutritional assessments and diet progression. No evidence supports following one postsurgical diet progression protocol over another, but they generally proceed from a clear liquid diet to foods with normal textures, the authors noted. Clinicians should adapt them according to the procedure type, they added.
In addition, clinicians must troubleshoot any nutrition-related concerns, including constipation, dehydration, nausea, heartburn, and fatigue, for up to a year after surgery, they wrote.
Metabolic and bariatric surgery patients should be evaluated annually at a minimum, if not more frequently, to gauge nutritional health, the authors wrote. Treating obesity as a disease involves more than weight loss — instead, it’s about improving the quality of life of patients through procedures, medications, and lifestyle modifications, they added.
Track New Developments
With ongoing changes in the field of metabolic and bariatric surgery, it’s helpful for clinicians to remain updated about new approaches across various disciplines linked to obesity management and treatment, said Christina Poa-Li, MD, a surgeon at Huntington Health Medical Center, affiliated with Cedars-Sinai Health System, in Pasadena, California, who was not involved in developing the updated practice statement.
“For example, the rapidly growing prescription of anti-obesity medications and their use in both preoperative and postoperative surgical patients drastically affect their nutrition,” she said. “Providers of various backgrounds and specialties will benefit from the most updated guidance on evaluating patient nutrition.”
Clinicians should consider expanding their patient population to include those with metabolic dysfunction–associated steatohepatitis or metabolic dysfunction–associated steatotic liver disease, Poa-Li said.
“These patient subpopulations may not have been considered for bariatric surgery or even referral to a bariatric surgeon for consultation previously,” she said. “It is important to increase awareness among clinicians of the potential benefits for metabolic and bariatric surgery for these patients.”
The report didn’t receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Patel and Poa-Li reported no relevant disclosures.
A version of this article appeared on Medscape.com.
Bariatric Surgery Lowers Risk for Long-Term Liver Complications in MASH-Related Cirrhosis
according to a recent study by Cleveland Clinic researchers.
Compared with patients who received medical therapy alone, those who underwent bariatric surgery had a 72% lower risk of developing serious complications of liver disease and an 80% lower risk for progression to decompensated cirrhosis.
The results could have major implications for patients with metabolic dysfunction–associated steatohepatitis (MASH), particularly given that about 20% of patients with MASH progress to cirrhosis, the researchers said.
“Currently, lifestyle intervention is the only therapeutic recommendation for compensated MASH-related cirrhosis,” said Steven Nissen, MD, the senior author and chief academic officer of the Miller Family Heart, Vascular, and Thoracic Institute, Cleveland Clinic, Ohio.
“However, lifestyle changes alone rarely provide the weight loss and metabolic changes needed to reduce the risk of liver complications in this patient population,” he said. “This study shows that bariatric surgery is an effective treatment that can influence the trajectory of cirrhosis progression in select patients.”
The study was published online in Nature Medicine.
Significantly Reduced Risks
As part of the Surgical Procedures Eliminate Compensated Cirrhosis in Advancing Long-Term (SPECCIAL) observational study, Nissen and colleagues compared the long-term effects of metabolic surgery and medical treatment in patients with obesity and compensated, biopsy-proven MASH-related cirrhosis. They looked for six major adverse liver outcomes (MALO): ascites, variceal hemorrhage, hepatic encephalopathy, hepatocellular carcinoma, liver transplantation, and all-cause mortality.
Among nearly 37,000 patients who underwent liver biopsy at the Cleveland Clinic Health System between 1995 and 2020, the research team identified 168 patients (69.6% women) with MASH-related cirrhosis, Child-Pugh class A, and model for end-stage liver disease scores ≤ 10. Among those, 62 underwent metabolic surgery (37 Roux-en-Y gastric bypass and 25 sleeve gastrectomy) and 106 had nonsurgical treatment.
After overlap weighting, the groups had balanced baseline characteristics, including mean body mass index of 42.7, Fibrosis-4 score of 2.1, albumin of 4.1 g/dL, bilirubin of 0.6 mg/dL, and Elixhauser comorbidity index of 9. In each group, 84.5% had type 2 diabetes and 79.1% had Ishak fibrosis stage 6.
Overall, the 15-year cumulative incidence of MALO was 20.9% in the surgical group and 46.4% in the nonsurgical group (adjusted hazard ratio [aHR], 0.28; P = .003), with MALO occurring in 10 surgical patients and 42 nonsurgical patients.
Assuming a causal effect, the number needed to treat with metabolic surgery to avoid one incidence of MALO over a 15-year timespan was 4.5.
The 15-year incidence of MALO was similar between surgical methods, with 20.1% for Roux-en-Y gastric bypass and 19.9% for sleeve gastrectomy.
In addition, the 15-year cumulative incidence of progression to decompensated cirrhosis was 15.6% in the surgical group and 30.7% in the nonsurgical group (aHR, 0.2; P = .01), with decompensation occurring in four surgical patients and 33 nonsurgical patients.
At 15 years, patients in the surgical group lost 31.6 kg or about 26.6% of their weight, and those in the nonsurgical group lost 10.7 kg or 9.8%.
Among patients with type 2 diabetes at baseline, metabolic surgery was associated with a reduction in hemoglobin A1c levels, as well as diabetes remission for some patients.
Potential to Fill an Unmet Need
Previous studies have indicated that bariatric surgery can effectively treat noncirrhotic MASH and lead to histologic resolution of MASH. In a 2021 study, Nissen and colleagues found bariatric surgery was associated with a lower risk for MALO and major adverse cardiovascular events in patients with biopsy-proven MASH without cirrhosis. Now, Nissen said, the SPECCIAL study indicates surgery may be a good option for MASH-related cirrhosis as well.
The study authors also noted that similar findings are theoretically possible from medical therapies, given the emergence of a new generation of anti-obesity medications. In this study, 16.8% of the surgical group and 14.3% of the nonsurgical group took semaglutide or tirzepatide at some point during the follow-up period.
“Patients with MASH-related cirrhosis have extremely limited treatment options,” said Sobia Laique, MD, study coauthor and a transplant hepatologist at the Cleveland Clinic who specializes in MASH-related cirrhosis. She cofounded the Cleveland Clinic’s task force on metabolic dysfunction–associated steatotic liver disease (MASLD) to improve screening, management, and patient outcomes for MASLD and related comorbidities.
“No therapeutic interventions have demonstrated efficacy in mitigating the risk of severe liver complications for these patients,” she said. “This underscores a critical unmet need for the development of effective therapies specifically targeting patients with compensated MASH-related cirrhosis.”
No funding was reported for this study. Several authors reported receiving grant funding, consultant fees, and advisory roles for various pharmaceutical companies.
A version of this article appeared on Medscape.com.
according to a recent study by Cleveland Clinic researchers.
Compared with patients who received medical therapy alone, those who underwent bariatric surgery had a 72% lower risk of developing serious complications of liver disease and an 80% lower risk for progression to decompensated cirrhosis.
The results could have major implications for patients with metabolic dysfunction–associated steatohepatitis (MASH), particularly given that about 20% of patients with MASH progress to cirrhosis, the researchers said.
“Currently, lifestyle intervention is the only therapeutic recommendation for compensated MASH-related cirrhosis,” said Steven Nissen, MD, the senior author and chief academic officer of the Miller Family Heart, Vascular, and Thoracic Institute, Cleveland Clinic, Ohio.
“However, lifestyle changes alone rarely provide the weight loss and metabolic changes needed to reduce the risk of liver complications in this patient population,” he said. “This study shows that bariatric surgery is an effective treatment that can influence the trajectory of cirrhosis progression in select patients.”
The study was published online in Nature Medicine.
Significantly Reduced Risks
As part of the Surgical Procedures Eliminate Compensated Cirrhosis in Advancing Long-Term (SPECCIAL) observational study, Nissen and colleagues compared the long-term effects of metabolic surgery and medical treatment in patients with obesity and compensated, biopsy-proven MASH-related cirrhosis. They looked for six major adverse liver outcomes (MALO): ascites, variceal hemorrhage, hepatic encephalopathy, hepatocellular carcinoma, liver transplantation, and all-cause mortality.
Among nearly 37,000 patients who underwent liver biopsy at the Cleveland Clinic Health System between 1995 and 2020, the research team identified 168 patients (69.6% women) with MASH-related cirrhosis, Child-Pugh class A, and model for end-stage liver disease scores ≤ 10. Among those, 62 underwent metabolic surgery (37 Roux-en-Y gastric bypass and 25 sleeve gastrectomy) and 106 had nonsurgical treatment.
After overlap weighting, the groups had balanced baseline characteristics, including mean body mass index of 42.7, Fibrosis-4 score of 2.1, albumin of 4.1 g/dL, bilirubin of 0.6 mg/dL, and Elixhauser comorbidity index of 9. In each group, 84.5% had type 2 diabetes and 79.1% had Ishak fibrosis stage 6.
Overall, the 15-year cumulative incidence of MALO was 20.9% in the surgical group and 46.4% in the nonsurgical group (adjusted hazard ratio [aHR], 0.28; P = .003), with MALO occurring in 10 surgical patients and 42 nonsurgical patients.
Assuming a causal effect, the number needed to treat with metabolic surgery to avoid one incidence of MALO over a 15-year timespan was 4.5.
The 15-year incidence of MALO was similar between surgical methods, with 20.1% for Roux-en-Y gastric bypass and 19.9% for sleeve gastrectomy.
In addition, the 15-year cumulative incidence of progression to decompensated cirrhosis was 15.6% in the surgical group and 30.7% in the nonsurgical group (aHR, 0.2; P = .01), with decompensation occurring in four surgical patients and 33 nonsurgical patients.
At 15 years, patients in the surgical group lost 31.6 kg or about 26.6% of their weight, and those in the nonsurgical group lost 10.7 kg or 9.8%.
Among patients with type 2 diabetes at baseline, metabolic surgery was associated with a reduction in hemoglobin A1c levels, as well as diabetes remission for some patients.
Potential to Fill an Unmet Need
Previous studies have indicated that bariatric surgery can effectively treat noncirrhotic MASH and lead to histologic resolution of MASH. In a 2021 study, Nissen and colleagues found bariatric surgery was associated with a lower risk for MALO and major adverse cardiovascular events in patients with biopsy-proven MASH without cirrhosis. Now, Nissen said, the SPECCIAL study indicates surgery may be a good option for MASH-related cirrhosis as well.
The study authors also noted that similar findings are theoretically possible from medical therapies, given the emergence of a new generation of anti-obesity medications. In this study, 16.8% of the surgical group and 14.3% of the nonsurgical group took semaglutide or tirzepatide at some point during the follow-up period.
“Patients with MASH-related cirrhosis have extremely limited treatment options,” said Sobia Laique, MD, study coauthor and a transplant hepatologist at the Cleveland Clinic who specializes in MASH-related cirrhosis. She cofounded the Cleveland Clinic’s task force on metabolic dysfunction–associated steatotic liver disease (MASLD) to improve screening, management, and patient outcomes for MASLD and related comorbidities.
“No therapeutic interventions have demonstrated efficacy in mitigating the risk of severe liver complications for these patients,” she said. “This underscores a critical unmet need for the development of effective therapies specifically targeting patients with compensated MASH-related cirrhosis.”
No funding was reported for this study. Several authors reported receiving grant funding, consultant fees, and advisory roles for various pharmaceutical companies.
A version of this article appeared on Medscape.com.
according to a recent study by Cleveland Clinic researchers.
Compared with patients who received medical therapy alone, those who underwent bariatric surgery had a 72% lower risk of developing serious complications of liver disease and an 80% lower risk for progression to decompensated cirrhosis.
The results could have major implications for patients with metabolic dysfunction–associated steatohepatitis (MASH), particularly given that about 20% of patients with MASH progress to cirrhosis, the researchers said.
“Currently, lifestyle intervention is the only therapeutic recommendation for compensated MASH-related cirrhosis,” said Steven Nissen, MD, the senior author and chief academic officer of the Miller Family Heart, Vascular, and Thoracic Institute, Cleveland Clinic, Ohio.
“However, lifestyle changes alone rarely provide the weight loss and metabolic changes needed to reduce the risk of liver complications in this patient population,” he said. “This study shows that bariatric surgery is an effective treatment that can influence the trajectory of cirrhosis progression in select patients.”
The study was published online in Nature Medicine.
Significantly Reduced Risks
As part of the Surgical Procedures Eliminate Compensated Cirrhosis in Advancing Long-Term (SPECCIAL) observational study, Nissen and colleagues compared the long-term effects of metabolic surgery and medical treatment in patients with obesity and compensated, biopsy-proven MASH-related cirrhosis. They looked for six major adverse liver outcomes (MALO): ascites, variceal hemorrhage, hepatic encephalopathy, hepatocellular carcinoma, liver transplantation, and all-cause mortality.
Among nearly 37,000 patients who underwent liver biopsy at the Cleveland Clinic Health System between 1995 and 2020, the research team identified 168 patients (69.6% women) with MASH-related cirrhosis, Child-Pugh class A, and model for end-stage liver disease scores ≤ 10. Among those, 62 underwent metabolic surgery (37 Roux-en-Y gastric bypass and 25 sleeve gastrectomy) and 106 had nonsurgical treatment.
After overlap weighting, the groups had balanced baseline characteristics, including mean body mass index of 42.7, Fibrosis-4 score of 2.1, albumin of 4.1 g/dL, bilirubin of 0.6 mg/dL, and Elixhauser comorbidity index of 9. In each group, 84.5% had type 2 diabetes and 79.1% had Ishak fibrosis stage 6.
Overall, the 15-year cumulative incidence of MALO was 20.9% in the surgical group and 46.4% in the nonsurgical group (adjusted hazard ratio [aHR], 0.28; P = .003), with MALO occurring in 10 surgical patients and 42 nonsurgical patients.
Assuming a causal effect, the number needed to treat with metabolic surgery to avoid one incidence of MALO over a 15-year timespan was 4.5.
The 15-year incidence of MALO was similar between surgical methods, with 20.1% for Roux-en-Y gastric bypass and 19.9% for sleeve gastrectomy.
In addition, the 15-year cumulative incidence of progression to decompensated cirrhosis was 15.6% in the surgical group and 30.7% in the nonsurgical group (aHR, 0.2; P = .01), with decompensation occurring in four surgical patients and 33 nonsurgical patients.
At 15 years, patients in the surgical group lost 31.6 kg or about 26.6% of their weight, and those in the nonsurgical group lost 10.7 kg or 9.8%.
Among patients with type 2 diabetes at baseline, metabolic surgery was associated with a reduction in hemoglobin A1c levels, as well as diabetes remission for some patients.
Potential to Fill an Unmet Need
Previous studies have indicated that bariatric surgery can effectively treat noncirrhotic MASH and lead to histologic resolution of MASH. In a 2021 study, Nissen and colleagues found bariatric surgery was associated with a lower risk for MALO and major adverse cardiovascular events in patients with biopsy-proven MASH without cirrhosis. Now, Nissen said, the SPECCIAL study indicates surgery may be a good option for MASH-related cirrhosis as well.
The study authors also noted that similar findings are theoretically possible from medical therapies, given the emergence of a new generation of anti-obesity medications. In this study, 16.8% of the surgical group and 14.3% of the nonsurgical group took semaglutide or tirzepatide at some point during the follow-up period.
“Patients with MASH-related cirrhosis have extremely limited treatment options,” said Sobia Laique, MD, study coauthor and a transplant hepatologist at the Cleveland Clinic who specializes in MASH-related cirrhosis. She cofounded the Cleveland Clinic’s task force on metabolic dysfunction–associated steatotic liver disease (MASLD) to improve screening, management, and patient outcomes for MASLD and related comorbidities.
“No therapeutic interventions have demonstrated efficacy in mitigating the risk of severe liver complications for these patients,” she said. “This underscores a critical unmet need for the development of effective therapies specifically targeting patients with compensated MASH-related cirrhosis.”
No funding was reported for this study. Several authors reported receiving grant funding, consultant fees, and advisory roles for various pharmaceutical companies.
A version of this article appeared on Medscape.com.
FROM NATURE MEDICINE
Impact of 3 Months of Supervised Exercise on Function by Arthritis Status
Impact of 3 Months of Supervised Exercise on Function by Arthritis Status
About half of US adults aged ≥ 65 years report arthritis, and of those, 44% have an arthritis-attributable activity limitation.1,2 Arthritis is a significant health issue for veterans, with veterans reporting higher rates of disability compared with the civilian population.3
Osteoarthritis (OA) is the most common type of arthritis.4 Among individuals aged ≥ 40 years, the incidence of OA is nearly twice as high among veterans compared with civilians and is a leading cause of separation from military service and disability.5,6 OA pain and disability have been shown to be associated with increases in health care and medication use, including opioids, nonsteroidal anti-inflammatory medications, and muscle relaxants.7,8 Because OA is chronic and has no cure, safe and effective management strategies—such as exercise— are critical to minimize pain and maintain physical function.9
Exercise can reduce pain and disability associated with OA and is a first-line recommendation in guidelines for the treatment of knee and hip OA.9 Given the limited exercise and high levels of physical inactivity among veterans with OA, there is a need to identify opportunities that support veterans with OA engaging in regular exercise.
Gerofit, an outpatient clinical exercise program available at 30 Veterans Health Administration (VHA) sites, may provide an opportunity for older veterans with arthritis to engage in exercise.10 Gerofit is specifically designed for veterans aged ≥ 65 years. It is not disease-specific and supports older veterans with multiple chronic conditions, including OA. Veterans aged ≥ 65 years with a referral from a VA clinician are eligible for Gerofit. Those who are unable to perform activities of daily living; unable to independently function without assistance; have a history of unstable angina, proliferative diabetic retinopathy, oxygen dependence, volatile behavioral issues, or are unable to work successfully in a group environment/setting; experience active substance abuse, homelessness, or uncontrolled incontinence; and have open wounds that cannot be appropriately dressed are excluded from Gerofit. Exercise sessions are held 3 times per week and last from 60 to 90 minutes. Sessions are supervised by Gerofit staff and include personalized exercise prescriptions based on functional assessments. Exercise prescriptions include aerobic, resistance, and balance/flexibility components and are modified by the Gerofit program staff as needed. Gerofit adopts a functional fitness approach and includes individual progression as appropriate according to evidence-based guidelines, using the Borg ratings of perceived exertion. 11 Assessments are performed at baseline, 3 months, 6 months, and annually thereafter. Clinical staff conduct all assessments, including physical function testing, and record them in a database. Assessments are reviewed with the veteran to chart progress and identify future goals or needs. Veterans perform personalized self-paced exercises in the Gerofit group setting. Exercise prescriptions are continuously modified to meet individualized needs and goals. Veterans may participate continuously with no end date.
Participation in supervised exercise is associated with improved physical function and individuals with arthritis can improve function even though their baseline functional status is lower than individuals without arthritis. 12 In this analysis, we examine the impact of exercise on the status and location of arthritis (upper body, lower body, or both). Lower body arthritis is more common than upper body arthritis and lower extremity function is associated with increased ability to perform activities of daily living, resulting in independence among older adults.13,14 We also include upper body strength measures to capture important functional movements such as reaching and pulling.15 Among those who participate in Gerofit, the greatest gains in physical function occur during the initial 3 months, which tend to be sustained over 12 months.16 For this reason, this study focused on the initial 3 months of the program.
Older adults with arthritis may have pain and functional limitations that exceed those of the general older adult population. Exercise programs for older adults that do not specifically target arthritis but are able to improve physical function among those with arthritis could potentially increase access to exercise for older adults living with arthritis. Therefore, the purpose of this study was to determine whether change in physical function with participation in Gerofit for 3 months varies by arthritis status, including no arthritis, any arthritis, lower body arthritis, or both upper and lower body arthritis compared with no arthritis.
Methods
This is a secondary analysis of previously collected data from 10 VHA Gerofit sites (Ann Arbor, Baltimore, Greater Los Angeles, Canandaigua, Cincinnati, Miami, Honolulu, Denver, Durham, and Pittsburgh) from 2002 to 2019. Implementation data regarding the consistency of the program delivery at Gerofit expansion sites have been previously published.16 Although the delivery of Gerofit transitioned to telehealth due to COVID-19, data for this analysis were collected from in-person exercise sessions prior to the pandemic.17 Data were collected for clinical purposes. This project was part of the Gerofit quality improvement initiative and was reviewed and approved by the Durham Institutional Review Board as quality improvement.
Participants in Gerofit who completed baseline and 3-month assessments were included to analyze the effects of exercise on physical function. At each of the time points, physical functional assessments included: (1) usual gait speed (> 10 meters [m/s], or 10- meter walk test [10MWT]); (2) lower body strength (chair stands [number completed in 30 seconds]); (3) upper body strength (number of arm curls [5-lb for females/8-lb for males] completed in 30 seconds); and (4) 6-minute walk distance [6MWD] in meters to measure aerobic endurance). These measures have been validated in older adults.18-21 Arm curls were added to the physical function assessments after the 10MWT, chair stands, and 6MWD; therefore, fewer participants had data for this measure. Participants self-reported at baseline on 45 common medical conditions, including arthritis or rheumatism (both upper body and lower body were offered as choices). Self-reporting has been shown to be an acceptable method of identifying arthritis in adults.22
Descriptive statistics at baseline were calculated for all participants. One-way analysis of variance and X2 tests were used to determine differences in baseline characteristics across arthritis status. The primary outcomes were changes in physical function measures from baseline to 3 months by arthritis status. Arthritis status was defined as: any arthritis, which includes individuals who reported upper body arthritis, lower body arthritis, or both; and arthritis status individuals reporting either upper body arthritis, lower body arthritis, or both. Categories of arthritis for arthritis status were mutually exclusive. Two separate linear models were constructed for each of the 4 physical function measures, with change from baseline to 3 months as the outcome (dependent variable) and arthritis status, age, and body mass index (BMI) as predictors (independent variables). The first model compared any arthritis with no arthritis and the second model compared arthritis status (both upper and lower body arthritis vs lower body arthritis) with no arthritis. These models were used to obtain mean changes and 95% CIs in physical function and to test for differences in the change in physical function measures by arthritis status. Statistical analyses were performed using R software, version 4.0.3.
Results
Baseline and 3-month data were available for 737 Gerofit participants and included in the analysis. The mean (SD) age was 73.5 (7.1) years. A total of 707 participants were male (95.9%) and 322 (43.6%) reported some arthritis, with arthritis in both the upper and lower body being reported by 168 participants (52.2%) (Table 1). There were no differences in age, sex, or race for those with any arthritis compared with those with no arthritis, but BMI was significantly higher in those reporting any arthritis compared with no arthritis. For the baseline functional measures, statistically significant differences were observed between those with no arthritis and those reporting any arthritis for the 10MWT (P = .001), chair stands (P = .046), and 6MWD (P = .001), but not for arm curls (P = .77), with those with no arthritis performing better.
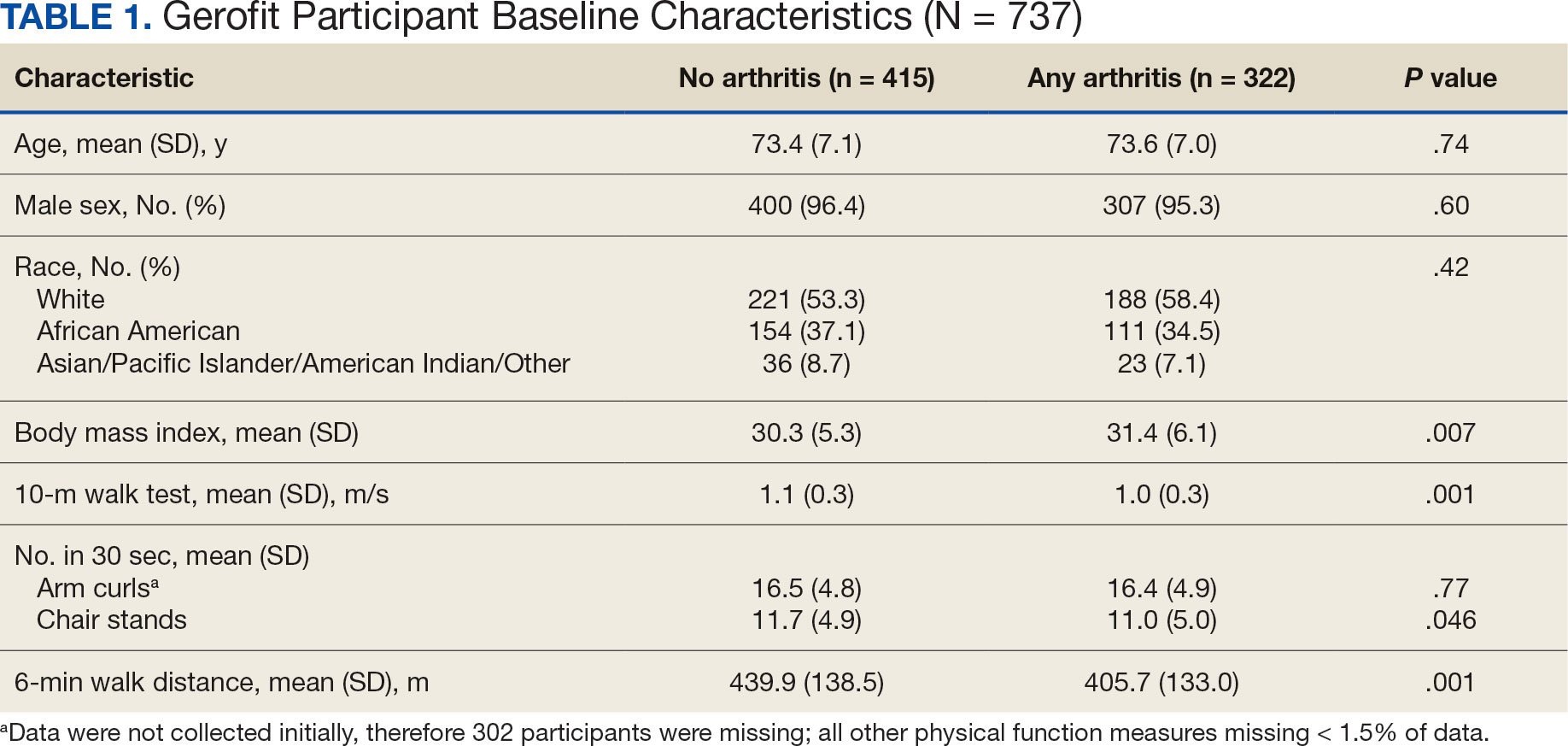
All 4 arthritis status groups showed improvements in each of the physical function measures over 3 months. For the 10MWT the mean change (95% CI) in gait speed (m/s) was 0.06 (0.04-0.08) for patients with no arthritis, 0.07 (0.05- 0.08) for any arthritis, 0.07 (0.04-0.11) for lower body arthritis, and 0.07 (0.04- 0.09) for both lower and upper body arthritis. For the number of arm curls in 30 seconds the mean change (95% CI) was 2.3 (1.8-2.8) for patients with no arthritis, 2.1 (1.5-2.6) for any arthritis, 2.0 (1.1-3.0) for lower body arthritis, and 1.9 (1.1-2.7) for both lower and upper body arthritis. For the number of chair stands in 30 seconds the mean change (95% CI) was 2.1 (1.7-2.4) for patients with no arthritis, 2.2 (1.8-2.6) for any arthritis, 2.3 (1.6-2.9), for lower body arthritis, and 2.0 (1.5-2.5) for both lower and upper body arthritis. For the 6MWD distance in meters the mean change (95% CI) was 21.5 (15.5-27.4) for patients with no arthritis, 28.6 (21.9-35.3) for any arthritis, 30.4 (19.5-41.3) for lower body arthritis, and 28.6 (19.2-38.0) for both lower and upper body arthritis (Figure).
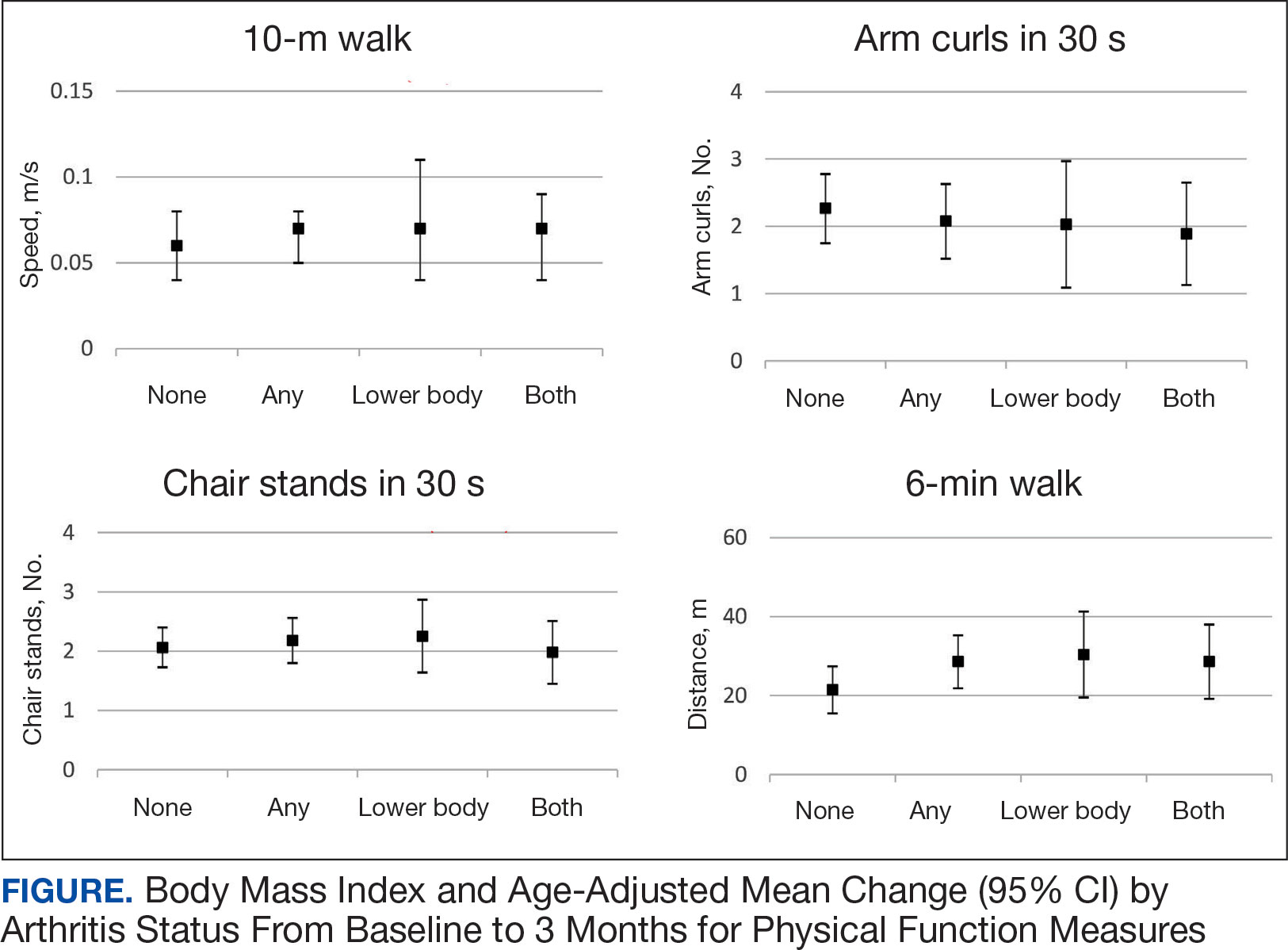
We used 2 models to measure the change from baseline to 3 months for each of the arthritis groups. Model 1 compared any arthritis vs no arthritis and model 2 compared lower body arthritis and both upper and lower body arthritis vs no arthritis for each physical function measure (Table 2). There were no statistically significant differences in 3-month change in physical function for any of the physical function measures between arthritis groups after adjusting for age and BMI.
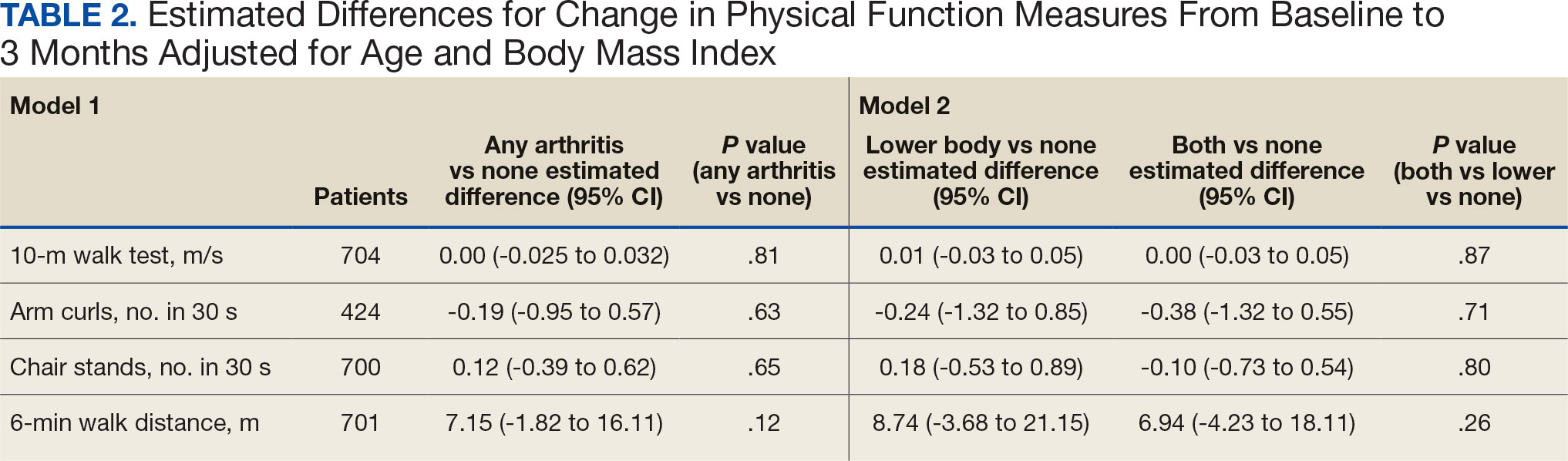
Discussion
Participation in Gerofit was associated with functional gains among all participants over 3 months, regardless of arthritis status. Older veterans reporting any arthritis had significantly lower physical function scores upon enrollment into Gerofit compared with those veterans reporting no arthritis. However, compared with individuals who reported no arthritis, individuals who reported arthritis (any arthritis, lower body arthritis only, or both lower and upper body arthritis) experienced similar improvements (ie, no statistically significant differences in mean change from baseline to follow-up among those with and without arthritis). This study suggests that progressive, multicomponent exercise programs for older adults may be beneficial for those with arthritis.
Involvement of multiple sites of arthritis is associated with moderate to severe functional limitations as well as lower healthrelated quality of life.23 While it has been found that individuals with arthritis can improve function with supervised exercise, even though their baseline functional status is lower than individuals without arthritis, it was not clear whether individuals with multiple joint involvement also would benefit.12 The results of this study suggest that these individuals can improve across various domains of physical function despite variation in arthritis location and status. As incidence of arthritis increases with age, targeting older adults for exercise programs such as Gerofit may improve functional limitations and health-related quality of life associated with arthritis.2
We evaluated physical function using multiple measures to assess upper (arm curls) and lower (chair stands, 10MWT) extremity physical function and aerobic endurance (6MWD). Participants in this study reached clinically meaningful changes with 3 months of participation in Gerofit for most of the physical function measures. Gerofit participants had a mean gait speed improvement of 0.05 to 0.07 m/s compared with 0.10 to 0.30 m/s, which was reported previously. 24,25 In this study, nearly all groups achieved the clinically important improvements in the chair stand in 30 seconds (2.0 to 2.6) and the 6MWD (21.8 to 59.1 m) that have been reported in the literature.24-26
The Osteoarthritis Research Society International recommends the chair stand and 6MWD performance-based tests for individuals with hip and knee arthritis because they align with patient-reported outcomes and represent the types of activities relevant to this population.27 The findings of this study suggest that improvement in these physical function measures with participation in exercise align with data from arthritis-specific exercise programs designed for wide implementation. Hughes and colleagues reported improvements in the 6MWD after the 8-week Fit and Strong exercise intervention, which included walking and lower body resistance training.28 The Arthritis Foundation’s Walk With Ease program is a 6-week walking program that has shown improvements in chair stands and gait speed.29 Another Arthritis Foundation program, People with Arthritis Can Exercise, is an 8-week course consisting of a variety of resistance, aerobic, and balance activities. This program has been associated with increases in chair stands but not gait speed or 6MWD.30,31
This study found that participation in a VHA outpatient clinical supervised exercise program results in improvements in physical function that can be realized by older adults regardless of arthritis burden. Gerofit programs typically require 1.5 to 2.0 dedicated full-time equivalent employees to run the program effectively and additional administrative support, depending on size of the program.32 The cost savings generated by the program include reductions in hospitalization rates, emergency department visits, days in hospital, and medication use and provide a compelling argument for the program’s financial viability to health care systems through long-term savings and improved health outcomes for older adults.33-36
While evidenced-based arthritis programs exist, this study illustrates that an exercise program without a focus on arthritis also improves physical function, potentially reducing the risk of disability related to arthritis. The clinical implication for these findings is that arthritis-specific exercise programs may not be needed to achieve functional improvements in individuals with arthritis. This is critical for under-resourced or exercise- limited health care systems or communities. Therefore, if exercise programming is limited, or arthritis-specific programs and interventions are not available, nonspecific exercise programs will also be beneficial to individuals with arthritis. Thus, individuals with arthritis should be encouraged to participate in any available exercise programming to achieve improvements in physical function. In addition, many older adults have multiple comorbidities, most of which improve with participation in exercise. 37 Disease-specific exercise programs can offer tailored exercises and coaching related to common barriers in participation, such as joint pain for arthritis.31 It is unclear whether these additional programmatic components are associated with greater improvements in outcomes, such as physical function. More research is needed to explore the benefits of disease-specific tailored exercise programs compared with general exercise programs.
Strengths and Limitations
This study demonstrated the effect of participation in a clinical, supervised exercise program in a real-world setting. It suggests that even exercise programs not specifically targeted for arthritis populations can improve physical function among those with arthritis.
As a VHA clinical supervised exercise program, Gerofit may not be generalizable to all older adults or other exercise programs. In addition, this analysis only included a veteran population that was > 95% male and may not be generalizable to other populations. Arthritis status was defined by self-report and not verified in the health record. However, this approach has been shown to be acceptable in this setting and the most common type of arthritis in this population (OA) is a painful musculoskeletal condition associated with functional limitations.4,22,38,39 Self-reported arthritis or rheumatism is associated with functional limitations.1 Therefore, it is unlikely that the results would differ for physician-diagnosed or radiographically defined OA. Additionally, the study did not have data on the total number of joints with arthritis or arthritis severity but rather used upper body, lower body, and both upper and lower body arthritis as a proxy for arthritis status. While our models were adjusted for age and BMI, 2 known confounding factors for the association between arthritis and physical function, there are other potential confounding factors that were not included in the models. 40,41 Finally, this study only included individuals with completed baseline and 3-month follow-up assessments, and the individuals who participated for longer or shorter periods may have had different physical function outcomes than individuals included in this study.
Conclusions
Participation in 3 months VHA Gerofit outpatient supervised exercise programs can improve physical function for all older adults, regardless of arthritis status. These programs may increase access to exercise programming that is beneficial for common conditions affecting older adults, such as arthritis.
- Centers for Disease Control and Prevention. Prevalence and most common causes of disability among adults- -United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58:421-426.
- Theis KA, Murphy LB, Guglielmo D, et al. Prevalence of arthritis and arthritis-attributable activity limitation—United States, 2016–2018. MMWR Morb Mortal Wkly Rep. 2021;70:1401-1407. doi:10.15585/mmwr.mm7040a2
- Murphy LB, Helmick CG, Allen KD, et al. Arthritis among veterans—United States, 2011–2013. MMWR Morb Mortal Wkly Rep. 2014;63:999-1003.
- Park J, Mendy A, Vieira ER. Various types of arthritis in the United States: prevalence and age-related trends from 1999 to 2014. Am J Public Health. 2018;108:256-258.
- Cameron KL, Hsiao MS, Owens BD, Burks R, Svoboda SJ. Incidence of physician-diagnosed osteoarthritis among active duty United States military service members. Arthritis Rheum. 2011;63:2974-2982. doi:10.1002/art.30498
- Patzkowski JC, Rivera JC, Ficke JR, Wenke JC. The changing face of disability in the US Army: the Operation Enduring Freedom and Operation Iraqi Freedom effect. J Am Acad Orthop Surg. 2012;20(suppl 1):S23-S30. doi:10.5435/JAAOS-20-08-S23
- Rivera JC, Amuan ME, Morris RM, Johnson AE, Pugh MJ. Arthritis, comorbidities, and care utilization in veterans of Operations Enduring and Iraqi Freedom. J Orthop Res. 2017;35:682-687. doi:10.1002/jor.23323
- Singh JA, Nelson DB, Fink HA, Nichol KL. Health-related quality of life predicts future health care utilization and mortality in veterans with self-reported physician-diagnosed arthritis: the Veterans Arthritis Quality of Life Study. Semin Arthritis Rheum. 2005;34:755- 765. doi:10.1016/j.semarthrit.2004.08.001
- Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: the Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Semin Arthritis Rheum. 2014;43:701-712. doi:10.1016/j.semarthrit.2013.11.012
- Morey MC, Crowley GM, Robbins MS, Cowper PA, Sullivan RJ Jr. The Gerofit Program: a VA innovation. South Med J. 1994;87:S83-S87.
- Chen MJ, Fan X, Moe ST. Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: a meta-analysis. J Sports Sci. 2002;20:873-899. doi:10.1080/026404102320761787
- Morey MC, Pieper CF, Sullivan RJ Jr, Crowley GM, Cowper PA, Robbins MS. Five-year performance trends for older exercisers: a hierarchical model of endurance, strength, and flexibility. J Am Geriatr Soc. 1996;44:1226-1231. doi:10.1111/j.1532-5415.1996.tb01374.x
- Allen KD, Gol ight ly YM. State of the evidence. Curr Opin Rheumatol. 2015;27:276-283. doi:10.1097/BOR.0000000000000161
- den Ouden MEM, Schuurmans MJ, Arts IEMA, van der Schouw YT. Association between physical performance characteristics and independence in activities of daily living in middle-aged and elderly men. Geriatr Gerontol Int. 2013;13:274-280. doi:10.1111/j.1447-0594.2012.00890.x
- Daly M, Vidt ME, Eggebeen JD, et al. Upper extremity muscle volumes and functional strength after resistance training in older adults. J Aging Phys Act. 2013;21:186-207. doi:10.1123/japa.21.2.186
- Morey MC, Lee CC, Castle S, et al. Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit Program. J Am Geriatr Soc. 2018;66:1009-1016. doi:10.1111/jgs.15276
- Jennings SC, Manning KM, Bettger JP, et al. Rapid transition to telehealth group exercise and functional assessments in response to COVID-19. Gerontol Geriatr Med. 2020;6:2333721420980313. doi:10.1177/ 2333721420980313
- Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314-322. doi:10.1046/j.1532-5415.2003.51104.x
- Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community residing older adults. Res Q Exerc Sport. 1999;70:113- 119. doi:10.1080/02701367.1999.10608028
- Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7:129-161. doi:10.1123/japa.7.2.129
- Harada ND, Chiu V, Stewart AL. Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil. 1999;80:837-841. doi:10.1016/s0003-9993(99)90236-8
- Peeters GGME, Alshurafa M, Schaap L, de Vet HCW. Diagnostic accuracy of self-reported arthritis in the general adult population is acceptable. J Clin Epidemiol. 2015;68:452-459. doi:10.1016/j.jclinepi.2014.09.019
- Cuperus N, Vliet Vlieland TPM, Mahler EAM, Kersten CC, Hoogeboom TJ, van den Ende CHM. The clinical burden of generalized osteoarthritis represented by self-reported health-related quality of life and activity limitations: a cross-sectional study. Rheumatol Int. 2015;35:871-877. doi:10.1007/s00296-014-3149-1
- Coleman G, Dobson F, Hinman RS, Bennell K, White DK. Measures of physical performance. Arthritis Care Res (Hoboken). 2020;72(suppl 10):452-485. doi:10.1002/acr.24373
- Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743-749. doi:10.1111/j.1532-5415.2006.00701.x
- Wright AA, Cook CE, Baxter GD, Dockerty JD, Abbott JH. A comparison of 3 methodological approaches to defining major clinically important improvement of 4 performance measures in patients with hip osteoarthritis. J Orthop Sports Phys Ther. 2011;41:319-327. doi:10.2519/jospt.2011.3515
- Dobson F, Hinman R, Roos EM, et al. OARSI recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:1042- 1052. doi:10.1016/j.joca.2013.05.002
- Hughes SL, Seymour RB, Campbell R, Pollak N, Huber G, Sharma L. Impact of the fit and strong intervention on older adults with osteoarthritis. Gerontologist. 2004;44:217-228. doi:10.1093/geront/44.2.217
- Callahan LF, Shreffler JH, Altpeter M, et al. Evaluation of group and self-directed formats of the Arthritis Foundation's Walk With Ease Program. Arthritis Care Res (Hoboken). 2011;63:1098-1107. doi:10.1002/acr.20490
- Boutaugh ML. Arthritis Foundation community-based physical activity programs: effectiveness and implementation issues. Arthritis Rheum. 2003;49:463-470. doi:10.1002/art.11050
- Callahan LF, Mielenz T, Freburger J, et al. A randomized controlled trial of the People with Arthritis Can Exercise Program: symptoms, function, physical activity, and psychosocial outcomes. Arthritis Rheum. 2008;59:92-101. doi:10.1002/art.23239
- Hall KS, Jennings SC, Pearson MP. Outpatient care models: the Gerofit model of care for exercise promotion in older adults. In: Malone ML, Boltz M, Macias Tejada J, White H, eds. Geriatrics Models of Care. Springer; 2024:205-213. doi:10.1007/978-3-031-56204-4_21
- Pepin MJ, Valencia WM, Bettger JP, et al. Impact of supervised exercise on one-year medication use in older veterans with multiple morbidities. Gerontol Geriatr Med. 2020;6:2333721420956751. doi:10.1177/ 2333721420956751
- Abbate L, Li J, Veazie P, et al. Does Gerofit exercise reduce veterans’ use of emergency department and inpatient care? Innov Aging. 2020;4(suppl 1):771. doi:10.1093/geroni/igaa057.2786
- Morey MC, Pieper CF, Crowley GM, Sullivan RJ Jr, Puglisi CM. Exercise adherence and 10-year mortality in chronically ill older adults. J Am Geriatr Soc. 2002;50:1929-1933. doi:10.1046/j.1532-5415.2002.50602.x
- Manning KM, Hall KS, Sloane R, et al. Longitudinal analysis of physical function in older adults: the effects of physical inactivity and exercise training. Aging Cell. 2024;23:e13987. doi:10.1111/acel.13987
- Bean JF, Vora A, Frontera WR. Benefits of exercise for community-dwelling older adults. Arch Phys Med Rehabil. 2004;85(7 suppl 3):S31-S42; quiz S3-S4. doi:10.1016/j.apmr.2004.03.010
- Covinsky KE, Lindquist K, Dunlop DD, Yelin E. Pain, functional limitations, and aging. J Am Geriatr Soc. 2009; 57:1556-1561. doi:10.1111/j.1532-5415.2009.02388.x
- Katz JN, Wright EA, Baron JA, Losina E. Development and validation of an index of musculoskeletal functional limitations. BMC Musculoskelet Disord. 2009;10:62. doi:10.1186/1471-2474-10-62
- Allen KD, Thoma LM, Golightly YM. Epidemiology of osteoarthritis. Osteoarthritis Cartilage. 2022;30:184-195. doi:10.1016/j.joca.2021.04.020
- Riebe D, Blissmer BJ, Greaney ML, Ewing Garber C, Lees FD, Clark PG. The relationship between obesity, physical activity, and physical function in older adults. J Aging Health. 2009;21:1159-1178. doi:10.1177/0898264309350076
About half of US adults aged ≥ 65 years report arthritis, and of those, 44% have an arthritis-attributable activity limitation.1,2 Arthritis is a significant health issue for veterans, with veterans reporting higher rates of disability compared with the civilian population.3
Osteoarthritis (OA) is the most common type of arthritis.4 Among individuals aged ≥ 40 years, the incidence of OA is nearly twice as high among veterans compared with civilians and is a leading cause of separation from military service and disability.5,6 OA pain and disability have been shown to be associated with increases in health care and medication use, including opioids, nonsteroidal anti-inflammatory medications, and muscle relaxants.7,8 Because OA is chronic and has no cure, safe and effective management strategies—such as exercise— are critical to minimize pain and maintain physical function.9
Exercise can reduce pain and disability associated with OA and is a first-line recommendation in guidelines for the treatment of knee and hip OA.9 Given the limited exercise and high levels of physical inactivity among veterans with OA, there is a need to identify opportunities that support veterans with OA engaging in regular exercise.
Gerofit, an outpatient clinical exercise program available at 30 Veterans Health Administration (VHA) sites, may provide an opportunity for older veterans with arthritis to engage in exercise.10 Gerofit is specifically designed for veterans aged ≥ 65 years. It is not disease-specific and supports older veterans with multiple chronic conditions, including OA. Veterans aged ≥ 65 years with a referral from a VA clinician are eligible for Gerofit. Those who are unable to perform activities of daily living; unable to independently function without assistance; have a history of unstable angina, proliferative diabetic retinopathy, oxygen dependence, volatile behavioral issues, or are unable to work successfully in a group environment/setting; experience active substance abuse, homelessness, or uncontrolled incontinence; and have open wounds that cannot be appropriately dressed are excluded from Gerofit. Exercise sessions are held 3 times per week and last from 60 to 90 minutes. Sessions are supervised by Gerofit staff and include personalized exercise prescriptions based on functional assessments. Exercise prescriptions include aerobic, resistance, and balance/flexibility components and are modified by the Gerofit program staff as needed. Gerofit adopts a functional fitness approach and includes individual progression as appropriate according to evidence-based guidelines, using the Borg ratings of perceived exertion. 11 Assessments are performed at baseline, 3 months, 6 months, and annually thereafter. Clinical staff conduct all assessments, including physical function testing, and record them in a database. Assessments are reviewed with the veteran to chart progress and identify future goals or needs. Veterans perform personalized self-paced exercises in the Gerofit group setting. Exercise prescriptions are continuously modified to meet individualized needs and goals. Veterans may participate continuously with no end date.
Participation in supervised exercise is associated with improved physical function and individuals with arthritis can improve function even though their baseline functional status is lower than individuals without arthritis. 12 In this analysis, we examine the impact of exercise on the status and location of arthritis (upper body, lower body, or both). Lower body arthritis is more common than upper body arthritis and lower extremity function is associated with increased ability to perform activities of daily living, resulting in independence among older adults.13,14 We also include upper body strength measures to capture important functional movements such as reaching and pulling.15 Among those who participate in Gerofit, the greatest gains in physical function occur during the initial 3 months, which tend to be sustained over 12 months.16 For this reason, this study focused on the initial 3 months of the program.
Older adults with arthritis may have pain and functional limitations that exceed those of the general older adult population. Exercise programs for older adults that do not specifically target arthritis but are able to improve physical function among those with arthritis could potentially increase access to exercise for older adults living with arthritis. Therefore, the purpose of this study was to determine whether change in physical function with participation in Gerofit for 3 months varies by arthritis status, including no arthritis, any arthritis, lower body arthritis, or both upper and lower body arthritis compared with no arthritis.
Methods
This is a secondary analysis of previously collected data from 10 VHA Gerofit sites (Ann Arbor, Baltimore, Greater Los Angeles, Canandaigua, Cincinnati, Miami, Honolulu, Denver, Durham, and Pittsburgh) from 2002 to 2019. Implementation data regarding the consistency of the program delivery at Gerofit expansion sites have been previously published.16 Although the delivery of Gerofit transitioned to telehealth due to COVID-19, data for this analysis were collected from in-person exercise sessions prior to the pandemic.17 Data were collected for clinical purposes. This project was part of the Gerofit quality improvement initiative and was reviewed and approved by the Durham Institutional Review Board as quality improvement.
Participants in Gerofit who completed baseline and 3-month assessments were included to analyze the effects of exercise on physical function. At each of the time points, physical functional assessments included: (1) usual gait speed (> 10 meters [m/s], or 10- meter walk test [10MWT]); (2) lower body strength (chair stands [number completed in 30 seconds]); (3) upper body strength (number of arm curls [5-lb for females/8-lb for males] completed in 30 seconds); and (4) 6-minute walk distance [6MWD] in meters to measure aerobic endurance). These measures have been validated in older adults.18-21 Arm curls were added to the physical function assessments after the 10MWT, chair stands, and 6MWD; therefore, fewer participants had data for this measure. Participants self-reported at baseline on 45 common medical conditions, including arthritis or rheumatism (both upper body and lower body were offered as choices). Self-reporting has been shown to be an acceptable method of identifying arthritis in adults.22
Descriptive statistics at baseline were calculated for all participants. One-way analysis of variance and X2 tests were used to determine differences in baseline characteristics across arthritis status. The primary outcomes were changes in physical function measures from baseline to 3 months by arthritis status. Arthritis status was defined as: any arthritis, which includes individuals who reported upper body arthritis, lower body arthritis, or both; and arthritis status individuals reporting either upper body arthritis, lower body arthritis, or both. Categories of arthritis for arthritis status were mutually exclusive. Two separate linear models were constructed for each of the 4 physical function measures, with change from baseline to 3 months as the outcome (dependent variable) and arthritis status, age, and body mass index (BMI) as predictors (independent variables). The first model compared any arthritis with no arthritis and the second model compared arthritis status (both upper and lower body arthritis vs lower body arthritis) with no arthritis. These models were used to obtain mean changes and 95% CIs in physical function and to test for differences in the change in physical function measures by arthritis status. Statistical analyses were performed using R software, version 4.0.3.
Results
Baseline and 3-month data were available for 737 Gerofit participants and included in the analysis. The mean (SD) age was 73.5 (7.1) years. A total of 707 participants were male (95.9%) and 322 (43.6%) reported some arthritis, with arthritis in both the upper and lower body being reported by 168 participants (52.2%) (Table 1). There were no differences in age, sex, or race for those with any arthritis compared with those with no arthritis, but BMI was significantly higher in those reporting any arthritis compared with no arthritis. For the baseline functional measures, statistically significant differences were observed between those with no arthritis and those reporting any arthritis for the 10MWT (P = .001), chair stands (P = .046), and 6MWD (P = .001), but not for arm curls (P = .77), with those with no arthritis performing better.

All 4 arthritis status groups showed improvements in each of the physical function measures over 3 months. For the 10MWT the mean change (95% CI) in gait speed (m/s) was 0.06 (0.04-0.08) for patients with no arthritis, 0.07 (0.05- 0.08) for any arthritis, 0.07 (0.04-0.11) for lower body arthritis, and 0.07 (0.04- 0.09) for both lower and upper body arthritis. For the number of arm curls in 30 seconds the mean change (95% CI) was 2.3 (1.8-2.8) for patients with no arthritis, 2.1 (1.5-2.6) for any arthritis, 2.0 (1.1-3.0) for lower body arthritis, and 1.9 (1.1-2.7) for both lower and upper body arthritis. For the number of chair stands in 30 seconds the mean change (95% CI) was 2.1 (1.7-2.4) for patients with no arthritis, 2.2 (1.8-2.6) for any arthritis, 2.3 (1.6-2.9), for lower body arthritis, and 2.0 (1.5-2.5) for both lower and upper body arthritis. For the 6MWD distance in meters the mean change (95% CI) was 21.5 (15.5-27.4) for patients with no arthritis, 28.6 (21.9-35.3) for any arthritis, 30.4 (19.5-41.3) for lower body arthritis, and 28.6 (19.2-38.0) for both lower and upper body arthritis (Figure).

We used 2 models to measure the change from baseline to 3 months for each of the arthritis groups. Model 1 compared any arthritis vs no arthritis and model 2 compared lower body arthritis and both upper and lower body arthritis vs no arthritis for each physical function measure (Table 2). There were no statistically significant differences in 3-month change in physical function for any of the physical function measures between arthritis groups after adjusting for age and BMI.

Discussion
Participation in Gerofit was associated with functional gains among all participants over 3 months, regardless of arthritis status. Older veterans reporting any arthritis had significantly lower physical function scores upon enrollment into Gerofit compared with those veterans reporting no arthritis. However, compared with individuals who reported no arthritis, individuals who reported arthritis (any arthritis, lower body arthritis only, or both lower and upper body arthritis) experienced similar improvements (ie, no statistically significant differences in mean change from baseline to follow-up among those with and without arthritis). This study suggests that progressive, multicomponent exercise programs for older adults may be beneficial for those with arthritis.
Involvement of multiple sites of arthritis is associated with moderate to severe functional limitations as well as lower healthrelated quality of life.23 While it has been found that individuals with arthritis can improve function with supervised exercise, even though their baseline functional status is lower than individuals without arthritis, it was not clear whether individuals with multiple joint involvement also would benefit.12 The results of this study suggest that these individuals can improve across various domains of physical function despite variation in arthritis location and status. As incidence of arthritis increases with age, targeting older adults for exercise programs such as Gerofit may improve functional limitations and health-related quality of life associated with arthritis.2
We evaluated physical function using multiple measures to assess upper (arm curls) and lower (chair stands, 10MWT) extremity physical function and aerobic endurance (6MWD). Participants in this study reached clinically meaningful changes with 3 months of participation in Gerofit for most of the physical function measures. Gerofit participants had a mean gait speed improvement of 0.05 to 0.07 m/s compared with 0.10 to 0.30 m/s, which was reported previously. 24,25 In this study, nearly all groups achieved the clinically important improvements in the chair stand in 30 seconds (2.0 to 2.6) and the 6MWD (21.8 to 59.1 m) that have been reported in the literature.24-26
The Osteoarthritis Research Society International recommends the chair stand and 6MWD performance-based tests for individuals with hip and knee arthritis because they align with patient-reported outcomes and represent the types of activities relevant to this population.27 The findings of this study suggest that improvement in these physical function measures with participation in exercise align with data from arthritis-specific exercise programs designed for wide implementation. Hughes and colleagues reported improvements in the 6MWD after the 8-week Fit and Strong exercise intervention, which included walking and lower body resistance training.28 The Arthritis Foundation’s Walk With Ease program is a 6-week walking program that has shown improvements in chair stands and gait speed.29 Another Arthritis Foundation program, People with Arthritis Can Exercise, is an 8-week course consisting of a variety of resistance, aerobic, and balance activities. This program has been associated with increases in chair stands but not gait speed or 6MWD.30,31
This study found that participation in a VHA outpatient clinical supervised exercise program results in improvements in physical function that can be realized by older adults regardless of arthritis burden. Gerofit programs typically require 1.5 to 2.0 dedicated full-time equivalent employees to run the program effectively and additional administrative support, depending on size of the program.32 The cost savings generated by the program include reductions in hospitalization rates, emergency department visits, days in hospital, and medication use and provide a compelling argument for the program’s financial viability to health care systems through long-term savings and improved health outcomes for older adults.33-36
While evidenced-based arthritis programs exist, this study illustrates that an exercise program without a focus on arthritis also improves physical function, potentially reducing the risk of disability related to arthritis. The clinical implication for these findings is that arthritis-specific exercise programs may not be needed to achieve functional improvements in individuals with arthritis. This is critical for under-resourced or exercise- limited health care systems or communities. Therefore, if exercise programming is limited, or arthritis-specific programs and interventions are not available, nonspecific exercise programs will also be beneficial to individuals with arthritis. Thus, individuals with arthritis should be encouraged to participate in any available exercise programming to achieve improvements in physical function. In addition, many older adults have multiple comorbidities, most of which improve with participation in exercise. 37 Disease-specific exercise programs can offer tailored exercises and coaching related to common barriers in participation, such as joint pain for arthritis.31 It is unclear whether these additional programmatic components are associated with greater improvements in outcomes, such as physical function. More research is needed to explore the benefits of disease-specific tailored exercise programs compared with general exercise programs.
Strengths and Limitations
This study demonstrated the effect of participation in a clinical, supervised exercise program in a real-world setting. It suggests that even exercise programs not specifically targeted for arthritis populations can improve physical function among those with arthritis.
As a VHA clinical supervised exercise program, Gerofit may not be generalizable to all older adults or other exercise programs. In addition, this analysis only included a veteran population that was > 95% male and may not be generalizable to other populations. Arthritis status was defined by self-report and not verified in the health record. However, this approach has been shown to be acceptable in this setting and the most common type of arthritis in this population (OA) is a painful musculoskeletal condition associated with functional limitations.4,22,38,39 Self-reported arthritis or rheumatism is associated with functional limitations.1 Therefore, it is unlikely that the results would differ for physician-diagnosed or radiographically defined OA. Additionally, the study did not have data on the total number of joints with arthritis or arthritis severity but rather used upper body, lower body, and both upper and lower body arthritis as a proxy for arthritis status. While our models were adjusted for age and BMI, 2 known confounding factors for the association between arthritis and physical function, there are other potential confounding factors that were not included in the models. 40,41 Finally, this study only included individuals with completed baseline and 3-month follow-up assessments, and the individuals who participated for longer or shorter periods may have had different physical function outcomes than individuals included in this study.
Conclusions
Participation in 3 months VHA Gerofit outpatient supervised exercise programs can improve physical function for all older adults, regardless of arthritis status. These programs may increase access to exercise programming that is beneficial for common conditions affecting older adults, such as arthritis.
About half of US adults aged ≥ 65 years report arthritis, and of those, 44% have an arthritis-attributable activity limitation.1,2 Arthritis is a significant health issue for veterans, with veterans reporting higher rates of disability compared with the civilian population.3
Osteoarthritis (OA) is the most common type of arthritis.4 Among individuals aged ≥ 40 years, the incidence of OA is nearly twice as high among veterans compared with civilians and is a leading cause of separation from military service and disability.5,6 OA pain and disability have been shown to be associated with increases in health care and medication use, including opioids, nonsteroidal anti-inflammatory medications, and muscle relaxants.7,8 Because OA is chronic and has no cure, safe and effective management strategies—such as exercise— are critical to minimize pain and maintain physical function.9
Exercise can reduce pain and disability associated with OA and is a first-line recommendation in guidelines for the treatment of knee and hip OA.9 Given the limited exercise and high levels of physical inactivity among veterans with OA, there is a need to identify opportunities that support veterans with OA engaging in regular exercise.
Gerofit, an outpatient clinical exercise program available at 30 Veterans Health Administration (VHA) sites, may provide an opportunity for older veterans with arthritis to engage in exercise.10 Gerofit is specifically designed for veterans aged ≥ 65 years. It is not disease-specific and supports older veterans with multiple chronic conditions, including OA. Veterans aged ≥ 65 years with a referral from a VA clinician are eligible for Gerofit. Those who are unable to perform activities of daily living; unable to independently function without assistance; have a history of unstable angina, proliferative diabetic retinopathy, oxygen dependence, volatile behavioral issues, or are unable to work successfully in a group environment/setting; experience active substance abuse, homelessness, or uncontrolled incontinence; and have open wounds that cannot be appropriately dressed are excluded from Gerofit. Exercise sessions are held 3 times per week and last from 60 to 90 minutes. Sessions are supervised by Gerofit staff and include personalized exercise prescriptions based on functional assessments. Exercise prescriptions include aerobic, resistance, and balance/flexibility components and are modified by the Gerofit program staff as needed. Gerofit adopts a functional fitness approach and includes individual progression as appropriate according to evidence-based guidelines, using the Borg ratings of perceived exertion. 11 Assessments are performed at baseline, 3 months, 6 months, and annually thereafter. Clinical staff conduct all assessments, including physical function testing, and record them in a database. Assessments are reviewed with the veteran to chart progress and identify future goals or needs. Veterans perform personalized self-paced exercises in the Gerofit group setting. Exercise prescriptions are continuously modified to meet individualized needs and goals. Veterans may participate continuously with no end date.
Participation in supervised exercise is associated with improved physical function and individuals with arthritis can improve function even though their baseline functional status is lower than individuals without arthritis. 12 In this analysis, we examine the impact of exercise on the status and location of arthritis (upper body, lower body, or both). Lower body arthritis is more common than upper body arthritis and lower extremity function is associated with increased ability to perform activities of daily living, resulting in independence among older adults.13,14 We also include upper body strength measures to capture important functional movements such as reaching and pulling.15 Among those who participate in Gerofit, the greatest gains in physical function occur during the initial 3 months, which tend to be sustained over 12 months.16 For this reason, this study focused on the initial 3 months of the program.
Older adults with arthritis may have pain and functional limitations that exceed those of the general older adult population. Exercise programs for older adults that do not specifically target arthritis but are able to improve physical function among those with arthritis could potentially increase access to exercise for older adults living with arthritis. Therefore, the purpose of this study was to determine whether change in physical function with participation in Gerofit for 3 months varies by arthritis status, including no arthritis, any arthritis, lower body arthritis, or both upper and lower body arthritis compared with no arthritis.
Methods
This is a secondary analysis of previously collected data from 10 VHA Gerofit sites (Ann Arbor, Baltimore, Greater Los Angeles, Canandaigua, Cincinnati, Miami, Honolulu, Denver, Durham, and Pittsburgh) from 2002 to 2019. Implementation data regarding the consistency of the program delivery at Gerofit expansion sites have been previously published.16 Although the delivery of Gerofit transitioned to telehealth due to COVID-19, data for this analysis were collected from in-person exercise sessions prior to the pandemic.17 Data were collected for clinical purposes. This project was part of the Gerofit quality improvement initiative and was reviewed and approved by the Durham Institutional Review Board as quality improvement.
Participants in Gerofit who completed baseline and 3-month assessments were included to analyze the effects of exercise on physical function. At each of the time points, physical functional assessments included: (1) usual gait speed (> 10 meters [m/s], or 10- meter walk test [10MWT]); (2) lower body strength (chair stands [number completed in 30 seconds]); (3) upper body strength (number of arm curls [5-lb for females/8-lb for males] completed in 30 seconds); and (4) 6-minute walk distance [6MWD] in meters to measure aerobic endurance). These measures have been validated in older adults.18-21 Arm curls were added to the physical function assessments after the 10MWT, chair stands, and 6MWD; therefore, fewer participants had data for this measure. Participants self-reported at baseline on 45 common medical conditions, including arthritis or rheumatism (both upper body and lower body were offered as choices). Self-reporting has been shown to be an acceptable method of identifying arthritis in adults.22
Descriptive statistics at baseline were calculated for all participants. One-way analysis of variance and X2 tests were used to determine differences in baseline characteristics across arthritis status. The primary outcomes were changes in physical function measures from baseline to 3 months by arthritis status. Arthritis status was defined as: any arthritis, which includes individuals who reported upper body arthritis, lower body arthritis, or both; and arthritis status individuals reporting either upper body arthritis, lower body arthritis, or both. Categories of arthritis for arthritis status were mutually exclusive. Two separate linear models were constructed for each of the 4 physical function measures, with change from baseline to 3 months as the outcome (dependent variable) and arthritis status, age, and body mass index (BMI) as predictors (independent variables). The first model compared any arthritis with no arthritis and the second model compared arthritis status (both upper and lower body arthritis vs lower body arthritis) with no arthritis. These models were used to obtain mean changes and 95% CIs in physical function and to test for differences in the change in physical function measures by arthritis status. Statistical analyses were performed using R software, version 4.0.3.
Results
Baseline and 3-month data were available for 737 Gerofit participants and included in the analysis. The mean (SD) age was 73.5 (7.1) years. A total of 707 participants were male (95.9%) and 322 (43.6%) reported some arthritis, with arthritis in both the upper and lower body being reported by 168 participants (52.2%) (Table 1). There were no differences in age, sex, or race for those with any arthritis compared with those with no arthritis, but BMI was significantly higher in those reporting any arthritis compared with no arthritis. For the baseline functional measures, statistically significant differences were observed between those with no arthritis and those reporting any arthritis for the 10MWT (P = .001), chair stands (P = .046), and 6MWD (P = .001), but not for arm curls (P = .77), with those with no arthritis performing better.

All 4 arthritis status groups showed improvements in each of the physical function measures over 3 months. For the 10MWT the mean change (95% CI) in gait speed (m/s) was 0.06 (0.04-0.08) for patients with no arthritis, 0.07 (0.05- 0.08) for any arthritis, 0.07 (0.04-0.11) for lower body arthritis, and 0.07 (0.04- 0.09) for both lower and upper body arthritis. For the number of arm curls in 30 seconds the mean change (95% CI) was 2.3 (1.8-2.8) for patients with no arthritis, 2.1 (1.5-2.6) for any arthritis, 2.0 (1.1-3.0) for lower body arthritis, and 1.9 (1.1-2.7) for both lower and upper body arthritis. For the number of chair stands in 30 seconds the mean change (95% CI) was 2.1 (1.7-2.4) for patients with no arthritis, 2.2 (1.8-2.6) for any arthritis, 2.3 (1.6-2.9), for lower body arthritis, and 2.0 (1.5-2.5) for both lower and upper body arthritis. For the 6MWD distance in meters the mean change (95% CI) was 21.5 (15.5-27.4) for patients with no arthritis, 28.6 (21.9-35.3) for any arthritis, 30.4 (19.5-41.3) for lower body arthritis, and 28.6 (19.2-38.0) for both lower and upper body arthritis (Figure).

We used 2 models to measure the change from baseline to 3 months for each of the arthritis groups. Model 1 compared any arthritis vs no arthritis and model 2 compared lower body arthritis and both upper and lower body arthritis vs no arthritis for each physical function measure (Table 2). There were no statistically significant differences in 3-month change in physical function for any of the physical function measures between arthritis groups after adjusting for age and BMI.

Discussion
Participation in Gerofit was associated with functional gains among all participants over 3 months, regardless of arthritis status. Older veterans reporting any arthritis had significantly lower physical function scores upon enrollment into Gerofit compared with those veterans reporting no arthritis. However, compared with individuals who reported no arthritis, individuals who reported arthritis (any arthritis, lower body arthritis only, or both lower and upper body arthritis) experienced similar improvements (ie, no statistically significant differences in mean change from baseline to follow-up among those with and without arthritis). This study suggests that progressive, multicomponent exercise programs for older adults may be beneficial for those with arthritis.
Involvement of multiple sites of arthritis is associated with moderate to severe functional limitations as well as lower healthrelated quality of life.23 While it has been found that individuals with arthritis can improve function with supervised exercise, even though their baseline functional status is lower than individuals without arthritis, it was not clear whether individuals with multiple joint involvement also would benefit.12 The results of this study suggest that these individuals can improve across various domains of physical function despite variation in arthritis location and status. As incidence of arthritis increases with age, targeting older adults for exercise programs such as Gerofit may improve functional limitations and health-related quality of life associated with arthritis.2
We evaluated physical function using multiple measures to assess upper (arm curls) and lower (chair stands, 10MWT) extremity physical function and aerobic endurance (6MWD). Participants in this study reached clinically meaningful changes with 3 months of participation in Gerofit for most of the physical function measures. Gerofit participants had a mean gait speed improvement of 0.05 to 0.07 m/s compared with 0.10 to 0.30 m/s, which was reported previously. 24,25 In this study, nearly all groups achieved the clinically important improvements in the chair stand in 30 seconds (2.0 to 2.6) and the 6MWD (21.8 to 59.1 m) that have been reported in the literature.24-26
The Osteoarthritis Research Society International recommends the chair stand and 6MWD performance-based tests for individuals with hip and knee arthritis because they align with patient-reported outcomes and represent the types of activities relevant to this population.27 The findings of this study suggest that improvement in these physical function measures with participation in exercise align with data from arthritis-specific exercise programs designed for wide implementation. Hughes and colleagues reported improvements in the 6MWD after the 8-week Fit and Strong exercise intervention, which included walking and lower body resistance training.28 The Arthritis Foundation’s Walk With Ease program is a 6-week walking program that has shown improvements in chair stands and gait speed.29 Another Arthritis Foundation program, People with Arthritis Can Exercise, is an 8-week course consisting of a variety of resistance, aerobic, and balance activities. This program has been associated with increases in chair stands but not gait speed or 6MWD.30,31
This study found that participation in a VHA outpatient clinical supervised exercise program results in improvements in physical function that can be realized by older adults regardless of arthritis burden. Gerofit programs typically require 1.5 to 2.0 dedicated full-time equivalent employees to run the program effectively and additional administrative support, depending on size of the program.32 The cost savings generated by the program include reductions in hospitalization rates, emergency department visits, days in hospital, and medication use and provide a compelling argument for the program’s financial viability to health care systems through long-term savings and improved health outcomes for older adults.33-36
While evidenced-based arthritis programs exist, this study illustrates that an exercise program without a focus on arthritis also improves physical function, potentially reducing the risk of disability related to arthritis. The clinical implication for these findings is that arthritis-specific exercise programs may not be needed to achieve functional improvements in individuals with arthritis. This is critical for under-resourced or exercise- limited health care systems or communities. Therefore, if exercise programming is limited, or arthritis-specific programs and interventions are not available, nonspecific exercise programs will also be beneficial to individuals with arthritis. Thus, individuals with arthritis should be encouraged to participate in any available exercise programming to achieve improvements in physical function. In addition, many older adults have multiple comorbidities, most of which improve with participation in exercise. 37 Disease-specific exercise programs can offer tailored exercises and coaching related to common barriers in participation, such as joint pain for arthritis.31 It is unclear whether these additional programmatic components are associated with greater improvements in outcomes, such as physical function. More research is needed to explore the benefits of disease-specific tailored exercise programs compared with general exercise programs.
Strengths and Limitations
This study demonstrated the effect of participation in a clinical, supervised exercise program in a real-world setting. It suggests that even exercise programs not specifically targeted for arthritis populations can improve physical function among those with arthritis.
As a VHA clinical supervised exercise program, Gerofit may not be generalizable to all older adults or other exercise programs. In addition, this analysis only included a veteran population that was > 95% male and may not be generalizable to other populations. Arthritis status was defined by self-report and not verified in the health record. However, this approach has been shown to be acceptable in this setting and the most common type of arthritis in this population (OA) is a painful musculoskeletal condition associated with functional limitations.4,22,38,39 Self-reported arthritis or rheumatism is associated with functional limitations.1 Therefore, it is unlikely that the results would differ for physician-diagnosed or radiographically defined OA. Additionally, the study did not have data on the total number of joints with arthritis or arthritis severity but rather used upper body, lower body, and both upper and lower body arthritis as a proxy for arthritis status. While our models were adjusted for age and BMI, 2 known confounding factors for the association between arthritis and physical function, there are other potential confounding factors that were not included in the models. 40,41 Finally, this study only included individuals with completed baseline and 3-month follow-up assessments, and the individuals who participated for longer or shorter periods may have had different physical function outcomes than individuals included in this study.
Conclusions
Participation in 3 months VHA Gerofit outpatient supervised exercise programs can improve physical function for all older adults, regardless of arthritis status. These programs may increase access to exercise programming that is beneficial for common conditions affecting older adults, such as arthritis.
- Centers for Disease Control and Prevention. Prevalence and most common causes of disability among adults- -United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58:421-426.
- Theis KA, Murphy LB, Guglielmo D, et al. Prevalence of arthritis and arthritis-attributable activity limitation—United States, 2016–2018. MMWR Morb Mortal Wkly Rep. 2021;70:1401-1407. doi:10.15585/mmwr.mm7040a2
- Murphy LB, Helmick CG, Allen KD, et al. Arthritis among veterans—United States, 2011–2013. MMWR Morb Mortal Wkly Rep. 2014;63:999-1003.
- Park J, Mendy A, Vieira ER. Various types of arthritis in the United States: prevalence and age-related trends from 1999 to 2014. Am J Public Health. 2018;108:256-258.
- Cameron KL, Hsiao MS, Owens BD, Burks R, Svoboda SJ. Incidence of physician-diagnosed osteoarthritis among active duty United States military service members. Arthritis Rheum. 2011;63:2974-2982. doi:10.1002/art.30498
- Patzkowski JC, Rivera JC, Ficke JR, Wenke JC. The changing face of disability in the US Army: the Operation Enduring Freedom and Operation Iraqi Freedom effect. J Am Acad Orthop Surg. 2012;20(suppl 1):S23-S30. doi:10.5435/JAAOS-20-08-S23
- Rivera JC, Amuan ME, Morris RM, Johnson AE, Pugh MJ. Arthritis, comorbidities, and care utilization in veterans of Operations Enduring and Iraqi Freedom. J Orthop Res. 2017;35:682-687. doi:10.1002/jor.23323
- Singh JA, Nelson DB, Fink HA, Nichol KL. Health-related quality of life predicts future health care utilization and mortality in veterans with self-reported physician-diagnosed arthritis: the Veterans Arthritis Quality of Life Study. Semin Arthritis Rheum. 2005;34:755- 765. doi:10.1016/j.semarthrit.2004.08.001
- Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: the Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Semin Arthritis Rheum. 2014;43:701-712. doi:10.1016/j.semarthrit.2013.11.012
- Morey MC, Crowley GM, Robbins MS, Cowper PA, Sullivan RJ Jr. The Gerofit Program: a VA innovation. South Med J. 1994;87:S83-S87.
- Chen MJ, Fan X, Moe ST. Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: a meta-analysis. J Sports Sci. 2002;20:873-899. doi:10.1080/026404102320761787
- Morey MC, Pieper CF, Sullivan RJ Jr, Crowley GM, Cowper PA, Robbins MS. Five-year performance trends for older exercisers: a hierarchical model of endurance, strength, and flexibility. J Am Geriatr Soc. 1996;44:1226-1231. doi:10.1111/j.1532-5415.1996.tb01374.x
- Allen KD, Gol ight ly YM. State of the evidence. Curr Opin Rheumatol. 2015;27:276-283. doi:10.1097/BOR.0000000000000161
- den Ouden MEM, Schuurmans MJ, Arts IEMA, van der Schouw YT. Association between physical performance characteristics and independence in activities of daily living in middle-aged and elderly men. Geriatr Gerontol Int. 2013;13:274-280. doi:10.1111/j.1447-0594.2012.00890.x
- Daly M, Vidt ME, Eggebeen JD, et al. Upper extremity muscle volumes and functional strength after resistance training in older adults. J Aging Phys Act. 2013;21:186-207. doi:10.1123/japa.21.2.186
- Morey MC, Lee CC, Castle S, et al. Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit Program. J Am Geriatr Soc. 2018;66:1009-1016. doi:10.1111/jgs.15276
- Jennings SC, Manning KM, Bettger JP, et al. Rapid transition to telehealth group exercise and functional assessments in response to COVID-19. Gerontol Geriatr Med. 2020;6:2333721420980313. doi:10.1177/ 2333721420980313
- Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314-322. doi:10.1046/j.1532-5415.2003.51104.x
- Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community residing older adults. Res Q Exerc Sport. 1999;70:113- 119. doi:10.1080/02701367.1999.10608028
- Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7:129-161. doi:10.1123/japa.7.2.129
- Harada ND, Chiu V, Stewart AL. Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil. 1999;80:837-841. doi:10.1016/s0003-9993(99)90236-8
- Peeters GGME, Alshurafa M, Schaap L, de Vet HCW. Diagnostic accuracy of self-reported arthritis in the general adult population is acceptable. J Clin Epidemiol. 2015;68:452-459. doi:10.1016/j.jclinepi.2014.09.019
- Cuperus N, Vliet Vlieland TPM, Mahler EAM, Kersten CC, Hoogeboom TJ, van den Ende CHM. The clinical burden of generalized osteoarthritis represented by self-reported health-related quality of life and activity limitations: a cross-sectional study. Rheumatol Int. 2015;35:871-877. doi:10.1007/s00296-014-3149-1
- Coleman G, Dobson F, Hinman RS, Bennell K, White DK. Measures of physical performance. Arthritis Care Res (Hoboken). 2020;72(suppl 10):452-485. doi:10.1002/acr.24373
- Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743-749. doi:10.1111/j.1532-5415.2006.00701.x
- Wright AA, Cook CE, Baxter GD, Dockerty JD, Abbott JH. A comparison of 3 methodological approaches to defining major clinically important improvement of 4 performance measures in patients with hip osteoarthritis. J Orthop Sports Phys Ther. 2011;41:319-327. doi:10.2519/jospt.2011.3515
- Dobson F, Hinman R, Roos EM, et al. OARSI recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:1042- 1052. doi:10.1016/j.joca.2013.05.002
- Hughes SL, Seymour RB, Campbell R, Pollak N, Huber G, Sharma L. Impact of the fit and strong intervention on older adults with osteoarthritis. Gerontologist. 2004;44:217-228. doi:10.1093/geront/44.2.217
- Callahan LF, Shreffler JH, Altpeter M, et al. Evaluation of group and self-directed formats of the Arthritis Foundation's Walk With Ease Program. Arthritis Care Res (Hoboken). 2011;63:1098-1107. doi:10.1002/acr.20490
- Boutaugh ML. Arthritis Foundation community-based physical activity programs: effectiveness and implementation issues. Arthritis Rheum. 2003;49:463-470. doi:10.1002/art.11050
- Callahan LF, Mielenz T, Freburger J, et al. A randomized controlled trial of the People with Arthritis Can Exercise Program: symptoms, function, physical activity, and psychosocial outcomes. Arthritis Rheum. 2008;59:92-101. doi:10.1002/art.23239
- Hall KS, Jennings SC, Pearson MP. Outpatient care models: the Gerofit model of care for exercise promotion in older adults. In: Malone ML, Boltz M, Macias Tejada J, White H, eds. Geriatrics Models of Care. Springer; 2024:205-213. doi:10.1007/978-3-031-56204-4_21
- Pepin MJ, Valencia WM, Bettger JP, et al. Impact of supervised exercise on one-year medication use in older veterans with multiple morbidities. Gerontol Geriatr Med. 2020;6:2333721420956751. doi:10.1177/ 2333721420956751
- Abbate L, Li J, Veazie P, et al. Does Gerofit exercise reduce veterans’ use of emergency department and inpatient care? Innov Aging. 2020;4(suppl 1):771. doi:10.1093/geroni/igaa057.2786
- Morey MC, Pieper CF, Crowley GM, Sullivan RJ Jr, Puglisi CM. Exercise adherence and 10-year mortality in chronically ill older adults. J Am Geriatr Soc. 2002;50:1929-1933. doi:10.1046/j.1532-5415.2002.50602.x
- Manning KM, Hall KS, Sloane R, et al. Longitudinal analysis of physical function in older adults: the effects of physical inactivity and exercise training. Aging Cell. 2024;23:e13987. doi:10.1111/acel.13987
- Bean JF, Vora A, Frontera WR. Benefits of exercise for community-dwelling older adults. Arch Phys Med Rehabil. 2004;85(7 suppl 3):S31-S42; quiz S3-S4. doi:10.1016/j.apmr.2004.03.010
- Covinsky KE, Lindquist K, Dunlop DD, Yelin E. Pain, functional limitations, and aging. J Am Geriatr Soc. 2009; 57:1556-1561. doi:10.1111/j.1532-5415.2009.02388.x
- Katz JN, Wright EA, Baron JA, Losina E. Development and validation of an index of musculoskeletal functional limitations. BMC Musculoskelet Disord. 2009;10:62. doi:10.1186/1471-2474-10-62
- Allen KD, Thoma LM, Golightly YM. Epidemiology of osteoarthritis. Osteoarthritis Cartilage. 2022;30:184-195. doi:10.1016/j.joca.2021.04.020
- Riebe D, Blissmer BJ, Greaney ML, Ewing Garber C, Lees FD, Clark PG. The relationship between obesity, physical activity, and physical function in older adults. J Aging Health. 2009;21:1159-1178. doi:10.1177/0898264309350076
- Centers for Disease Control and Prevention. Prevalence and most common causes of disability among adults- -United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58:421-426.
- Theis KA, Murphy LB, Guglielmo D, et al. Prevalence of arthritis and arthritis-attributable activity limitation—United States, 2016–2018. MMWR Morb Mortal Wkly Rep. 2021;70:1401-1407. doi:10.15585/mmwr.mm7040a2
- Murphy LB, Helmick CG, Allen KD, et al. Arthritis among veterans—United States, 2011–2013. MMWR Morb Mortal Wkly Rep. 2014;63:999-1003.
- Park J, Mendy A, Vieira ER. Various types of arthritis in the United States: prevalence and age-related trends from 1999 to 2014. Am J Public Health. 2018;108:256-258.
- Cameron KL, Hsiao MS, Owens BD, Burks R, Svoboda SJ. Incidence of physician-diagnosed osteoarthritis among active duty United States military service members. Arthritis Rheum. 2011;63:2974-2982. doi:10.1002/art.30498
- Patzkowski JC, Rivera JC, Ficke JR, Wenke JC. The changing face of disability in the US Army: the Operation Enduring Freedom and Operation Iraqi Freedom effect. J Am Acad Orthop Surg. 2012;20(suppl 1):S23-S30. doi:10.5435/JAAOS-20-08-S23
- Rivera JC, Amuan ME, Morris RM, Johnson AE, Pugh MJ. Arthritis, comorbidities, and care utilization in veterans of Operations Enduring and Iraqi Freedom. J Orthop Res. 2017;35:682-687. doi:10.1002/jor.23323
- Singh JA, Nelson DB, Fink HA, Nichol KL. Health-related quality of life predicts future health care utilization and mortality in veterans with self-reported physician-diagnosed arthritis: the Veterans Arthritis Quality of Life Study. Semin Arthritis Rheum. 2005;34:755- 765. doi:10.1016/j.semarthrit.2004.08.001
- Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: the Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Semin Arthritis Rheum. 2014;43:701-712. doi:10.1016/j.semarthrit.2013.11.012
- Morey MC, Crowley GM, Robbins MS, Cowper PA, Sullivan RJ Jr. The Gerofit Program: a VA innovation. South Med J. 1994;87:S83-S87.
- Chen MJ, Fan X, Moe ST. Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: a meta-analysis. J Sports Sci. 2002;20:873-899. doi:10.1080/026404102320761787
- Morey MC, Pieper CF, Sullivan RJ Jr, Crowley GM, Cowper PA, Robbins MS. Five-year performance trends for older exercisers: a hierarchical model of endurance, strength, and flexibility. J Am Geriatr Soc. 1996;44:1226-1231. doi:10.1111/j.1532-5415.1996.tb01374.x
- Allen KD, Gol ight ly YM. State of the evidence. Curr Opin Rheumatol. 2015;27:276-283. doi:10.1097/BOR.0000000000000161
- den Ouden MEM, Schuurmans MJ, Arts IEMA, van der Schouw YT. Association between physical performance characteristics and independence in activities of daily living in middle-aged and elderly men. Geriatr Gerontol Int. 2013;13:274-280. doi:10.1111/j.1447-0594.2012.00890.x
- Daly M, Vidt ME, Eggebeen JD, et al. Upper extremity muscle volumes and functional strength after resistance training in older adults. J Aging Phys Act. 2013;21:186-207. doi:10.1123/japa.21.2.186
- Morey MC, Lee CC, Castle S, et al. Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit Program. J Am Geriatr Soc. 2018;66:1009-1016. doi:10.1111/jgs.15276
- Jennings SC, Manning KM, Bettger JP, et al. Rapid transition to telehealth group exercise and functional assessments in response to COVID-19. Gerontol Geriatr Med. 2020;6:2333721420980313. doi:10.1177/ 2333721420980313
- Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314-322. doi:10.1046/j.1532-5415.2003.51104.x
- Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community residing older adults. Res Q Exerc Sport. 1999;70:113- 119. doi:10.1080/02701367.1999.10608028
- Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7:129-161. doi:10.1123/japa.7.2.129
- Harada ND, Chiu V, Stewart AL. Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil. 1999;80:837-841. doi:10.1016/s0003-9993(99)90236-8
- Peeters GGME, Alshurafa M, Schaap L, de Vet HCW. Diagnostic accuracy of self-reported arthritis in the general adult population is acceptable. J Clin Epidemiol. 2015;68:452-459. doi:10.1016/j.jclinepi.2014.09.019
- Cuperus N, Vliet Vlieland TPM, Mahler EAM, Kersten CC, Hoogeboom TJ, van den Ende CHM. The clinical burden of generalized osteoarthritis represented by self-reported health-related quality of life and activity limitations: a cross-sectional study. Rheumatol Int. 2015;35:871-877. doi:10.1007/s00296-014-3149-1
- Coleman G, Dobson F, Hinman RS, Bennell K, White DK. Measures of physical performance. Arthritis Care Res (Hoboken). 2020;72(suppl 10):452-485. doi:10.1002/acr.24373
- Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743-749. doi:10.1111/j.1532-5415.2006.00701.x
- Wright AA, Cook CE, Baxter GD, Dockerty JD, Abbott JH. A comparison of 3 methodological approaches to defining major clinically important improvement of 4 performance measures in patients with hip osteoarthritis. J Orthop Sports Phys Ther. 2011;41:319-327. doi:10.2519/jospt.2011.3515
- Dobson F, Hinman R, Roos EM, et al. OARSI recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:1042- 1052. doi:10.1016/j.joca.2013.05.002
- Hughes SL, Seymour RB, Campbell R, Pollak N, Huber G, Sharma L. Impact of the fit and strong intervention on older adults with osteoarthritis. Gerontologist. 2004;44:217-228. doi:10.1093/geront/44.2.217
- Callahan LF, Shreffler JH, Altpeter M, et al. Evaluation of group and self-directed formats of the Arthritis Foundation's Walk With Ease Program. Arthritis Care Res (Hoboken). 2011;63:1098-1107. doi:10.1002/acr.20490
- Boutaugh ML. Arthritis Foundation community-based physical activity programs: effectiveness and implementation issues. Arthritis Rheum. 2003;49:463-470. doi:10.1002/art.11050
- Callahan LF, Mielenz T, Freburger J, et al. A randomized controlled trial of the People with Arthritis Can Exercise Program: symptoms, function, physical activity, and psychosocial outcomes. Arthritis Rheum. 2008;59:92-101. doi:10.1002/art.23239
- Hall KS, Jennings SC, Pearson MP. Outpatient care models: the Gerofit model of care for exercise promotion in older adults. In: Malone ML, Boltz M, Macias Tejada J, White H, eds. Geriatrics Models of Care. Springer; 2024:205-213. doi:10.1007/978-3-031-56204-4_21
- Pepin MJ, Valencia WM, Bettger JP, et al. Impact of supervised exercise on one-year medication use in older veterans with multiple morbidities. Gerontol Geriatr Med. 2020;6:2333721420956751. doi:10.1177/ 2333721420956751
- Abbate L, Li J, Veazie P, et al. Does Gerofit exercise reduce veterans’ use of emergency department and inpatient care? Innov Aging. 2020;4(suppl 1):771. doi:10.1093/geroni/igaa057.2786
- Morey MC, Pieper CF, Crowley GM, Sullivan RJ Jr, Puglisi CM. Exercise adherence and 10-year mortality in chronically ill older adults. J Am Geriatr Soc. 2002;50:1929-1933. doi:10.1046/j.1532-5415.2002.50602.x
- Manning KM, Hall KS, Sloane R, et al. Longitudinal analysis of physical function in older adults: the effects of physical inactivity and exercise training. Aging Cell. 2024;23:e13987. doi:10.1111/acel.13987
- Bean JF, Vora A, Frontera WR. Benefits of exercise for community-dwelling older adults. Arch Phys Med Rehabil. 2004;85(7 suppl 3):S31-S42; quiz S3-S4. doi:10.1016/j.apmr.2004.03.010
- Covinsky KE, Lindquist K, Dunlop DD, Yelin E. Pain, functional limitations, and aging. J Am Geriatr Soc. 2009; 57:1556-1561. doi:10.1111/j.1532-5415.2009.02388.x
- Katz JN, Wright EA, Baron JA, Losina E. Development and validation of an index of musculoskeletal functional limitations. BMC Musculoskelet Disord. 2009;10:62. doi:10.1186/1471-2474-10-62
- Allen KD, Thoma LM, Golightly YM. Epidemiology of osteoarthritis. Osteoarthritis Cartilage. 2022;30:184-195. doi:10.1016/j.joca.2021.04.020
- Riebe D, Blissmer BJ, Greaney ML, Ewing Garber C, Lees FD, Clark PG. The relationship between obesity, physical activity, and physical function in older adults. J Aging Health. 2009;21:1159-1178. doi:10.1177/0898264309350076
Impact of 3 Months of Supervised Exercise on Function by Arthritis Status
Impact of 3 Months of Supervised Exercise on Function by Arthritis Status
Efficacy of Anti-Obesity Medications in Adult and Older Adult Veteran Populations
Efficacy of Anti-Obesity Medications in Adult and Older Adult Veteran Populations
The impact of obesity in the United States is significant. Between August 2021 and August 2023, the prevalence of obesity (body mass index ≥ 30) in US adults was 40.3%.1 The prevalence of obesity in adults aged 40 to 59 years was 46.4%, higher than the prevalence in adults aged 20 to 39 years (35.5%) and those aged ≥ 60 years (38.9%).1 The excess annual medical costs associated with obesity in the US are estimated at nearly $173 billion.2
The first-line treatment for obesity is lifestyle modifications, including a healthy diet and exercise. When lifestyle modifications are not enough to achieve weight-loss goals, bariatric surgery and anti-obesity medications (AOMs) are often considered. Five medications were approved for the long-term tretament of obesity by the US Food and Drug Administration (FDA) between 2021 and 2023, when this study was conducted: semaglutide (Wegovy), liraglutide (Saxenda), phentermine and topiramate, naltrexone and bupropion, and orlistat. The clinically meaningful (and commonly accepted) weight-loss target for these medications is ≥ 5% from baseline by week 12 of the maximally tolerated dose of therapy. A 5% weight loss has been shown to be clinically significant in improving cardiometabolic risk factors.3,4 These medications are intended to be used as an adjunct to healthy diet and exercise. Of note, semaglutide and liraglutide carry brand names, which are associated with different dosing for the treatment of type 2 diabetes mellitus (T2DM).
All 5 FDA-approved AOMs were available at the Veterans Affairs Sioux Falls Health Care System (VASFHCS) for the treatment of obesity at the time of the study. To qualify for an AOM, a veteran at VASFHCS must first work with a dietitian or be enrolled in the MOVE! clinic to participate in the weight management program, which focuses on dietary, exercise, and behavioral changes. At VASFHCS, AOMs are prescribed by primary care practitioners, clinical pharmacy providers, and advanced practitioners within the MOVE! program.
Ample data exist for the efficacy of AOMs. However, no published research has reported on AOM efficacy by age group (Appendix).5-11 While most of the AOM clinical trials included older adults, the average age of participants was typically between 40 and 50 years. It is well-known that pharmacokinetic and pharmacodynamic changes occur as age increases. Renal and hepatic clearance is reduced while the volume distribution and sensitivities to some medications may increase. 12 Although this study did not focus on specific pharmacokinetic and pharmacodynamic changes with respect to AOM, it is important to recognize that this may play a role in the efficacy and safety of AOMs in older adults.
Methods
This retrospective single-center chart review was performed using the VASFHCS Computerized Patient Record System to compare the efficacy of AOMs in older adults (aged ≥ 65 years) vs adults (aged < 65 years). The primary endpoint was the percent change in body weight from baseline to 6 and 12 months after initiation of AOM therapy in the older adult vs adult population. Secondary endpoints included changes in low-density lipoprotein (LDL), hemoglobin A1c (HbA1c), and blood pressure (BP) from baseline compared to 12 months on AOM therapy. HbA1c was assessed in patients with T2DM or prediabetes at the time of AOM initiation. Two safety endpoints were also explored to determine the incidence of medication adverse events (AEs) and subsequent discontinuation of AOM. A subset analysis was performed to determine whether there was a difference in percent change in body weight between patients in 3 age groups: 18 to 40 years, 41 to 64 years, and ≥ 65 years.
The study population included patients who were prescribed an AOM between January 1, 2021, and June 30, 2023. Patients were excluded if they did not continue AOM therapy for ≥ 6 months after initiation or if they underwent gastric bypass surgery while undergoing AOM therapy. Patients taking semaglutide (Ozempic) or liraglutide (Victoza) for both T2DM and weight loss who were eventually switched to the weight loss formulations (Wegovy or Saxenda) were included. Patients who switched between semaglutide and liraglutide for weight loss were also included. Those taking semaglutide or liraglutide solely for T2DM treatment were excluded because they are dosed differently.
Collected data included age, gender, race, weight (baseline, 6 and 12 months after initiation of AOM), metabolic laboratory values/vital signs (HbA1c, LDL, and BP at baseline and 12 months after initiation of AOM), diagnosis of T2DM or prediabetes, reported AEs associated with AOM therapy, and date of AOM initiation and discontinuation (if applicable). Baseline values were defined at the time of medication initiation or values documented within 6 months prior to medication initiation if true baseline data were not reported. If values were not recorded at months 6 and 12 after AOM initiation, values documented closest to those targets were used. Weights were used for baseline, 6-, and 12-month data unless they were unavailable due to use of virtual care modalities. In these cases, patient-reported weights were used. Patients were included in the 6-month data, but not the 12-month data, if they were taking AOMs for > 6 months but not for 12 months. If patients had been on multiple AOMs, baseline data were recorded at the start of the first medication that was used for 6 months or longer. Twelve-month data were recorded after subsequent medication change. Twelve-month metabolic laboratory values/vital signs were recorded for patients included in the study even if they did not complete ≥ 12 months of AOM therapy.
Statistical Analysis
Data from patients who were prescribed an AOM from January 2021 to June 2023 and who remained on the medication for ≥ 6 months were analyzed. Baseline characteristics were analyzed using descriptive statistics. The primary and secondary endpoints were evaluated using the t test. The safety endpoints were analyzed using descriptive statistics. An analysis of variance test was used for the subset analysis. Results with P < .05 were statistically significant.
Results
A total of 144 participants were included in this study, 116 in the adult group (aged < 65 years) and 28 in the older adult group (aged ≥ 65 years). Sixty-seven patients were excluded due to prespecified inclusion and exclusion criteria.
Other than the predetermined mean age differences (48 years vs 71 years), there were multiple differences in patient baseline characteristics. When comparing older adults and adults, average weight (283 lb vs 269 lb) and White race (89% vs 87%) were slightly higher in the older adult group. Also, a higher prevalence of T2DM (54% and 18%) and a lower prevalence of prediabetes (21% and 33%) was noted in the older adult group. HbA1c and BP were similar between both groups at baseline, while LDL was slightly lower in the older adult group (Table 1).
Patients in the adult group lost a mean 7.0% and 8.7% of body weight at 6 and 12 months, respectively, while the older adult group lost 5.0% and 6.6% body weight at 6 and 12 months, respectively. The difference in percent change in body weight was not statistically different at 6 (P = .08) or 12 (P = .26) months between patients in the adult group vs the older adult group or in the specific age groups (18-40 years, 41-64 years, ≥ 65 years) at 6 months (P = .24) or 12 months (P = .53) (Figure).
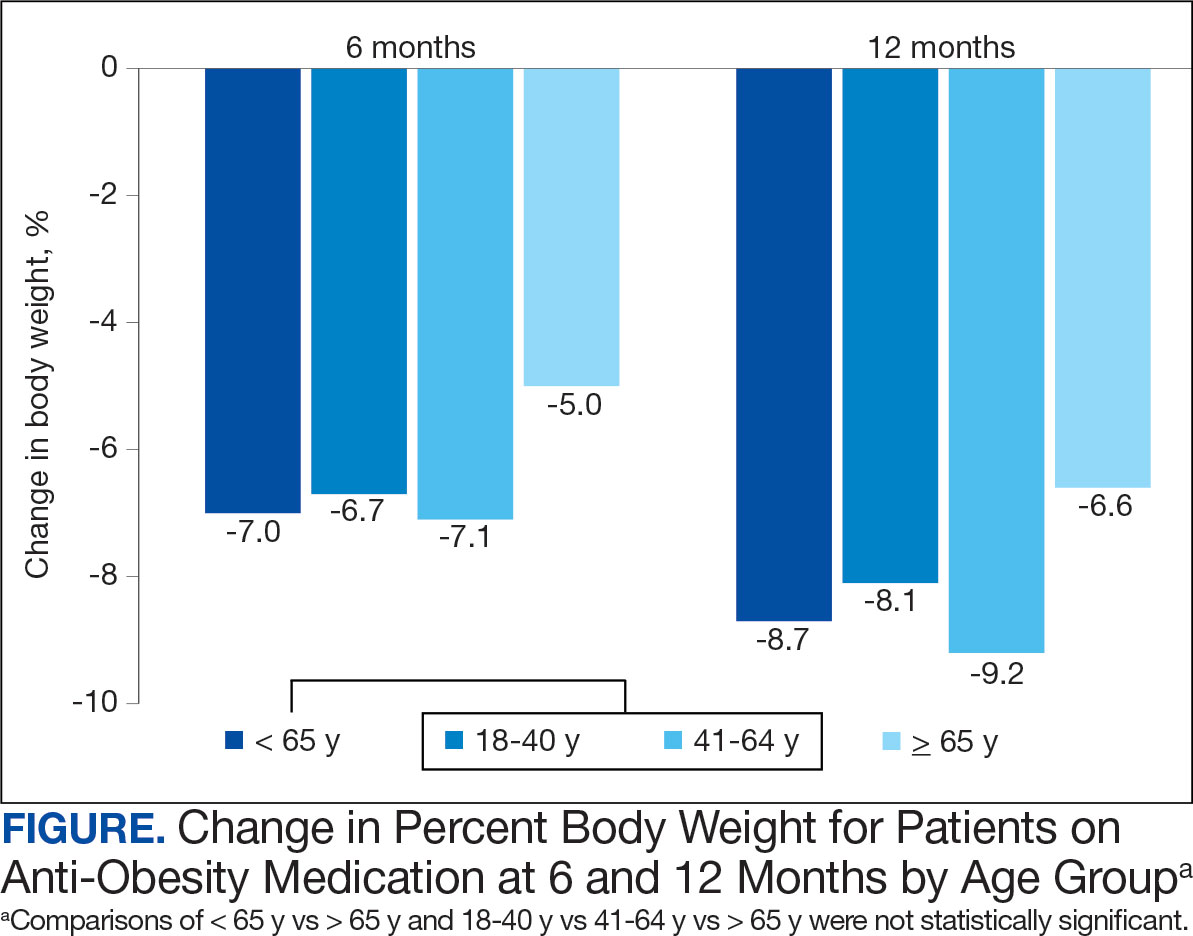
At 12 months, the difference between the adult group vs the older adult group was not statistically significant for HbA1c in patients with T2DM or prediabetes (P = .73), LDL (P = .95), systolic BP (P = .58), or diastolic BP (P = .51) (Table 2).
For the safety endpoint, the incidence of AEs was found to be different between groups. There were more reported AEs (61.2% vs 39.3%) and a greater increase in therapy discontinuation due to AEs (6.0% vs 0%) in the adult group compared to the older adult group (Table 3).
Discussion
Patients taking AOMs revealed no statistically significant difference in percent change in body weight at 6 or 12 months between adults aged < 65 years and older adults aged ≥ 65 years. The subset analysis also showed no statistically significant difference in change in percent body weight between more narrowly defined age groups of 18 to 40 years, 41 to 64 years, and ≥ 65 years. This suggests that AOM may have similar efficacy for weight loss in all ages of adults.
Secondary endpoint findings showed no statistically significant difference in HbA1c (in patients with T2DM or prediabetes), LDL, or BP at 12 months between the 2 groups. Although this study did not differentiate secondary outcomes based on the individual AOM, the change in HbA1c in both groups was expected, given that 70% of the patients included in this study were taking a glucagon-like peptide-1 agonist (liraglutide and semaglutide) at some point during the study. It’s also worth noting that secondary endpoints were collected for patients who discontinued the AOM between 6 and 12 months. Therefore, the patients’ HbA1c, LDL, and BP may not have accurately reflected the change that could have been expected if they had continued AOM therapy beyond the 12-month period.
Due to the different mechanisms and range in efficacy that AOMs have in regard to weight loss, changes in all outcomes, including weight, HbA1c, LDL, and BP were expected to vary as patients were included even after switching AOM (collection of data started after ≥ 6 months on a single AOM). Switching of AOM after the first 6 months of therapy was recorded in 25% of the patients in the ≥ 65 years group and 330% of the patients in the < 65 years group.
The incidence of AEs and subsequent discontinuation of AOMs in this study was higher in the adult group. This study excluded patients who did not continue taking an AOM for at least 6 months. As a result, the incidence of AEs between the 2 groups within the first 6 months of AOM therapy remains unknown. It is possible that during the first 6 months of therapy, patients aged < 65 years were more willing to tolerate or had fewer severe AEs compared with the older adult group. It’s also possible that the smaller number of patients in the older adult group was due to increased AEs that led them to discontinue early (before completion of 6 months of therapy) and/or prescriber discomfort in using AOMs in the older adult population. In addition, because the specific medication(s) taken by patients in each group were not detailed, it is unknown whether the adult group was taking AOMs associated with a greater number of AEs.
Limitations
This was a retrospective study with a relatively small sample size. A larger sample size may have shown more precise differences between age groups and may be more representative of the general population. Additionally, data were reliant on appropriate documentation, and adherence to AOM therapy was not assessed due to the retrospective nature of this study. At times, the study relied on patient reported data points, such as weight, if a clinic weight was not available. Also, this study did not account for many potential confounding factors such as other medications taken by the patient, which can affect outcomes including weight, HbA1c, LDL, blood pressure, and AEs.
Conclusions
This retrospective study of patients taking AOMs showed no statistically significant difference in weight loss at 6 or 12 months between adults aged < 65 years and older adults aged ≥ 65 years. A subset analysis found no statistically significant difference in change in body weight between specific age groups (18-40 years, 41-64 years, and ≥ 65 years). There was also no statistically significant difference in secondary outcomes, including change in HbA1c (in patients with T2DM or prediabetes), LDL or BP between age groups. The safety endpoints showed a higher incidence of medication AEs in the adult group, with more of these adults discontinuing therapy due to AEs. This study indicates that AOM may have similar outcomes for weight loss and metabolic laboratory values/vital sign changes between adults and older adults. Also, our findings suggest that patients aged < 65 years may experience more AEs than patients aged ≥ 65 years after ≥ 6 months of AOM therapy. Larger studies are needed to further evaluate these age-specific findings.
- Emmerich SD, Fryar CD, Stierman B, Ogden CL. Obesity and severe obesity prevalence in adults: United States, August 2021-August 2023. NCHS Data Brief No. 508. National Center for Health Statistics; 2024. Accessed December 11, 2024. https://www.cdc.gov/nchs/products/databriefs/db508.htm
- Ward ZJ, Bleich SN, Long MW, Gortmaker SL. Association of body mass index with health care expenditures in the United States by age and sex. PLoS One. 2021;16(3):e0247307. doi:10.1371/journal.pone.0247307
- Horn DB, Almandoz JP, Look M. What is clinically relevant weight loss for your patients and how can it be achieved? A narrative review. Postgrad Med. 2022;134(4):359-375. doi:10.1080/00325481.2022.2051366
- American Diabetes Association (ADA). Standards of care in diabetes–2023. Diabetes Care. 2023;46(suppl 1):S128- S2139. doi:10.2337/dc23-S008
- Wilding JPH, Batterham RL, Calanna S, et al. Onceweekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002. doi:10.1056/NEJMoa2032183
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11-22. doi:10.1056/NEJMoa1411892
- Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20(2):330-342. doi:10.1038/oby.2011.330
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341-1352. doi:10.1016/S0140-6736(11)60205-5
- Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297-308. doi:10.3945/ajcn.111.024927
- Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595-605. doi:10.1016/S0140-6736(10)60888-4
- Sjöström L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352(9123):167-172. doi:10.1016s0140-6736(97)11509-4
- Mangoni AA, Jackson SHD. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6-14. doi:10.1046/j.1365-2125.2003.02007.x
The impact of obesity in the United States is significant. Between August 2021 and August 2023, the prevalence of obesity (body mass index ≥ 30) in US adults was 40.3%.1 The prevalence of obesity in adults aged 40 to 59 years was 46.4%, higher than the prevalence in adults aged 20 to 39 years (35.5%) and those aged ≥ 60 years (38.9%).1 The excess annual medical costs associated with obesity in the US are estimated at nearly $173 billion.2
The first-line treatment for obesity is lifestyle modifications, including a healthy diet and exercise. When lifestyle modifications are not enough to achieve weight-loss goals, bariatric surgery and anti-obesity medications (AOMs) are often considered. Five medications were approved for the long-term tretament of obesity by the US Food and Drug Administration (FDA) between 2021 and 2023, when this study was conducted: semaglutide (Wegovy), liraglutide (Saxenda), phentermine and topiramate, naltrexone and bupropion, and orlistat. The clinically meaningful (and commonly accepted) weight-loss target for these medications is ≥ 5% from baseline by week 12 of the maximally tolerated dose of therapy. A 5% weight loss has been shown to be clinically significant in improving cardiometabolic risk factors.3,4 These medications are intended to be used as an adjunct to healthy diet and exercise. Of note, semaglutide and liraglutide carry brand names, which are associated with different dosing for the treatment of type 2 diabetes mellitus (T2DM).
All 5 FDA-approved AOMs were available at the Veterans Affairs Sioux Falls Health Care System (VASFHCS) for the treatment of obesity at the time of the study. To qualify for an AOM, a veteran at VASFHCS must first work with a dietitian or be enrolled in the MOVE! clinic to participate in the weight management program, which focuses on dietary, exercise, and behavioral changes. At VASFHCS, AOMs are prescribed by primary care practitioners, clinical pharmacy providers, and advanced practitioners within the MOVE! program.
Ample data exist for the efficacy of AOMs. However, no published research has reported on AOM efficacy by age group (Appendix).5-11 While most of the AOM clinical trials included older adults, the average age of participants was typically between 40 and 50 years. It is well-known that pharmacokinetic and pharmacodynamic changes occur as age increases. Renal and hepatic clearance is reduced while the volume distribution and sensitivities to some medications may increase. 12 Although this study did not focus on specific pharmacokinetic and pharmacodynamic changes with respect to AOM, it is important to recognize that this may play a role in the efficacy and safety of AOMs in older adults.

Methods
This retrospective single-center chart review was performed using the VASFHCS Computerized Patient Record System to compare the efficacy of AOMs in older adults (aged ≥ 65 years) vs adults (aged < 65 years). The primary endpoint was the percent change in body weight from baseline to 6 and 12 months after initiation of AOM therapy in the older adult vs adult population. Secondary endpoints included changes in low-density lipoprotein (LDL), hemoglobin A1c (HbA1c), and blood pressure (BP) from baseline compared to 12 months on AOM therapy. HbA1c was assessed in patients with T2DM or prediabetes at the time of AOM initiation. Two safety endpoints were also explored to determine the incidence of medication adverse events (AEs) and subsequent discontinuation of AOM. A subset analysis was performed to determine whether there was a difference in percent change in body weight between patients in 3 age groups: 18 to 40 years, 41 to 64 years, and ≥ 65 years.
The study population included patients who were prescribed an AOM between January 1, 2021, and June 30, 2023. Patients were excluded if they did not continue AOM therapy for ≥ 6 months after initiation or if they underwent gastric bypass surgery while undergoing AOM therapy. Patients taking semaglutide (Ozempic) or liraglutide (Victoza) for both T2DM and weight loss who were eventually switched to the weight loss formulations (Wegovy or Saxenda) were included. Patients who switched between semaglutide and liraglutide for weight loss were also included. Those taking semaglutide or liraglutide solely for T2DM treatment were excluded because they are dosed differently.
Collected data included age, gender, race, weight (baseline, 6 and 12 months after initiation of AOM), metabolic laboratory values/vital signs (HbA1c, LDL, and BP at baseline and 12 months after initiation of AOM), diagnosis of T2DM or prediabetes, reported AEs associated with AOM therapy, and date of AOM initiation and discontinuation (if applicable). Baseline values were defined at the time of medication initiation or values documented within 6 months prior to medication initiation if true baseline data were not reported. If values were not recorded at months 6 and 12 after AOM initiation, values documented closest to those targets were used. Weights were used for baseline, 6-, and 12-month data unless they were unavailable due to use of virtual care modalities. In these cases, patient-reported weights were used. Patients were included in the 6-month data, but not the 12-month data, if they were taking AOMs for > 6 months but not for 12 months. If patients had been on multiple AOMs, baseline data were recorded at the start of the first medication that was used for 6 months or longer. Twelve-month data were recorded after subsequent medication change. Twelve-month metabolic laboratory values/vital signs were recorded for patients included in the study even if they did not complete ≥ 12 months of AOM therapy.
Statistical Analysis
Data from patients who were prescribed an AOM from January 2021 to June 2023 and who remained on the medication for ≥ 6 months were analyzed. Baseline characteristics were analyzed using descriptive statistics. The primary and secondary endpoints were evaluated using the t test. The safety endpoints were analyzed using descriptive statistics. An analysis of variance test was used for the subset analysis. Results with P < .05 were statistically significant.
Results
A total of 144 participants were included in this study, 116 in the adult group (aged < 65 years) and 28 in the older adult group (aged ≥ 65 years). Sixty-seven patients were excluded due to prespecified inclusion and exclusion criteria.
Other than the predetermined mean age differences (48 years vs 71 years), there were multiple differences in patient baseline characteristics. When comparing older adults and adults, average weight (283 lb vs 269 lb) and White race (89% vs 87%) were slightly higher in the older adult group. Also, a higher prevalence of T2DM (54% and 18%) and a lower prevalence of prediabetes (21% and 33%) was noted in the older adult group. HbA1c and BP were similar between both groups at baseline, while LDL was slightly lower in the older adult group (Table 1).

Patients in the adult group lost a mean 7.0% and 8.7% of body weight at 6 and 12 months, respectively, while the older adult group lost 5.0% and 6.6% body weight at 6 and 12 months, respectively. The difference in percent change in body weight was not statistically different at 6 (P = .08) or 12 (P = .26) months between patients in the adult group vs the older adult group or in the specific age groups (18-40 years, 41-64 years, ≥ 65 years) at 6 months (P = .24) or 12 months (P = .53) (Figure).

At 12 months, the difference between the adult group vs the older adult group was not statistically significant for HbA1c in patients with T2DM or prediabetes (P = .73), LDL (P = .95), systolic BP (P = .58), or diastolic BP (P = .51) (Table 2).

For the safety endpoint, the incidence of AEs was found to be different between groups. There were more reported AEs (61.2% vs 39.3%) and a greater increase in therapy discontinuation due to AEs (6.0% vs 0%) in the adult group compared to the older adult group (Table 3).

Discussion
Patients taking AOMs revealed no statistically significant difference in percent change in body weight at 6 or 12 months between adults aged < 65 years and older adults aged ≥ 65 years. The subset analysis also showed no statistically significant difference in change in percent body weight between more narrowly defined age groups of 18 to 40 years, 41 to 64 years, and ≥ 65 years. This suggests that AOM may have similar efficacy for weight loss in all ages of adults.
Secondary endpoint findings showed no statistically significant difference in HbA1c (in patients with T2DM or prediabetes), LDL, or BP at 12 months between the 2 groups. Although this study did not differentiate secondary outcomes based on the individual AOM, the change in HbA1c in both groups was expected, given that 70% of the patients included in this study were taking a glucagon-like peptide-1 agonist (liraglutide and semaglutide) at some point during the study. It’s also worth noting that secondary endpoints were collected for patients who discontinued the AOM between 6 and 12 months. Therefore, the patients’ HbA1c, LDL, and BP may not have accurately reflected the change that could have been expected if they had continued AOM therapy beyond the 12-month period.
Due to the different mechanisms and range in efficacy that AOMs have in regard to weight loss, changes in all outcomes, including weight, HbA1c, LDL, and BP were expected to vary as patients were included even after switching AOM (collection of data started after ≥ 6 months on a single AOM). Switching of AOM after the first 6 months of therapy was recorded in 25% of the patients in the ≥ 65 years group and 330% of the patients in the < 65 years group.
The incidence of AEs and subsequent discontinuation of AOMs in this study was higher in the adult group. This study excluded patients who did not continue taking an AOM for at least 6 months. As a result, the incidence of AEs between the 2 groups within the first 6 months of AOM therapy remains unknown. It is possible that during the first 6 months of therapy, patients aged < 65 years were more willing to tolerate or had fewer severe AEs compared with the older adult group. It’s also possible that the smaller number of patients in the older adult group was due to increased AEs that led them to discontinue early (before completion of 6 months of therapy) and/or prescriber discomfort in using AOMs in the older adult population. In addition, because the specific medication(s) taken by patients in each group were not detailed, it is unknown whether the adult group was taking AOMs associated with a greater number of AEs.
Limitations
This was a retrospective study with a relatively small sample size. A larger sample size may have shown more precise differences between age groups and may be more representative of the general population. Additionally, data were reliant on appropriate documentation, and adherence to AOM therapy was not assessed due to the retrospective nature of this study. At times, the study relied on patient reported data points, such as weight, if a clinic weight was not available. Also, this study did not account for many potential confounding factors such as other medications taken by the patient, which can affect outcomes including weight, HbA1c, LDL, blood pressure, and AEs.
Conclusions
This retrospective study of patients taking AOMs showed no statistically significant difference in weight loss at 6 or 12 months between adults aged < 65 years and older adults aged ≥ 65 years. A subset analysis found no statistically significant difference in change in body weight between specific age groups (18-40 years, 41-64 years, and ≥ 65 years). There was also no statistically significant difference in secondary outcomes, including change in HbA1c (in patients with T2DM or prediabetes), LDL or BP between age groups. The safety endpoints showed a higher incidence of medication AEs in the adult group, with more of these adults discontinuing therapy due to AEs. This study indicates that AOM may have similar outcomes for weight loss and metabolic laboratory values/vital sign changes between adults and older adults. Also, our findings suggest that patients aged < 65 years may experience more AEs than patients aged ≥ 65 years after ≥ 6 months of AOM therapy. Larger studies are needed to further evaluate these age-specific findings.
The impact of obesity in the United States is significant. Between August 2021 and August 2023, the prevalence of obesity (body mass index ≥ 30) in US adults was 40.3%.1 The prevalence of obesity in adults aged 40 to 59 years was 46.4%, higher than the prevalence in adults aged 20 to 39 years (35.5%) and those aged ≥ 60 years (38.9%).1 The excess annual medical costs associated with obesity in the US are estimated at nearly $173 billion.2
The first-line treatment for obesity is lifestyle modifications, including a healthy diet and exercise. When lifestyle modifications are not enough to achieve weight-loss goals, bariatric surgery and anti-obesity medications (AOMs) are often considered. Five medications were approved for the long-term tretament of obesity by the US Food and Drug Administration (FDA) between 2021 and 2023, when this study was conducted: semaglutide (Wegovy), liraglutide (Saxenda), phentermine and topiramate, naltrexone and bupropion, and orlistat. The clinically meaningful (and commonly accepted) weight-loss target for these medications is ≥ 5% from baseline by week 12 of the maximally tolerated dose of therapy. A 5% weight loss has been shown to be clinically significant in improving cardiometabolic risk factors.3,4 These medications are intended to be used as an adjunct to healthy diet and exercise. Of note, semaglutide and liraglutide carry brand names, which are associated with different dosing for the treatment of type 2 diabetes mellitus (T2DM).
All 5 FDA-approved AOMs were available at the Veterans Affairs Sioux Falls Health Care System (VASFHCS) for the treatment of obesity at the time of the study. To qualify for an AOM, a veteran at VASFHCS must first work with a dietitian or be enrolled in the MOVE! clinic to participate in the weight management program, which focuses on dietary, exercise, and behavioral changes. At VASFHCS, AOMs are prescribed by primary care practitioners, clinical pharmacy providers, and advanced practitioners within the MOVE! program.
Ample data exist for the efficacy of AOMs. However, no published research has reported on AOM efficacy by age group (Appendix).5-11 While most of the AOM clinical trials included older adults, the average age of participants was typically between 40 and 50 years. It is well-known that pharmacokinetic and pharmacodynamic changes occur as age increases. Renal and hepatic clearance is reduced while the volume distribution and sensitivities to some medications may increase. 12 Although this study did not focus on specific pharmacokinetic and pharmacodynamic changes with respect to AOM, it is important to recognize that this may play a role in the efficacy and safety of AOMs in older adults.

Methods
This retrospective single-center chart review was performed using the VASFHCS Computerized Patient Record System to compare the efficacy of AOMs in older adults (aged ≥ 65 years) vs adults (aged < 65 years). The primary endpoint was the percent change in body weight from baseline to 6 and 12 months after initiation of AOM therapy in the older adult vs adult population. Secondary endpoints included changes in low-density lipoprotein (LDL), hemoglobin A1c (HbA1c), and blood pressure (BP) from baseline compared to 12 months on AOM therapy. HbA1c was assessed in patients with T2DM or prediabetes at the time of AOM initiation. Two safety endpoints were also explored to determine the incidence of medication adverse events (AEs) and subsequent discontinuation of AOM. A subset analysis was performed to determine whether there was a difference in percent change in body weight between patients in 3 age groups: 18 to 40 years, 41 to 64 years, and ≥ 65 years.
The study population included patients who were prescribed an AOM between January 1, 2021, and June 30, 2023. Patients were excluded if they did not continue AOM therapy for ≥ 6 months after initiation or if they underwent gastric bypass surgery while undergoing AOM therapy. Patients taking semaglutide (Ozempic) or liraglutide (Victoza) for both T2DM and weight loss who were eventually switched to the weight loss formulations (Wegovy or Saxenda) were included. Patients who switched between semaglutide and liraglutide for weight loss were also included. Those taking semaglutide or liraglutide solely for T2DM treatment were excluded because they are dosed differently.
Collected data included age, gender, race, weight (baseline, 6 and 12 months after initiation of AOM), metabolic laboratory values/vital signs (HbA1c, LDL, and BP at baseline and 12 months after initiation of AOM), diagnosis of T2DM or prediabetes, reported AEs associated with AOM therapy, and date of AOM initiation and discontinuation (if applicable). Baseline values were defined at the time of medication initiation or values documented within 6 months prior to medication initiation if true baseline data were not reported. If values were not recorded at months 6 and 12 after AOM initiation, values documented closest to those targets were used. Weights were used for baseline, 6-, and 12-month data unless they were unavailable due to use of virtual care modalities. In these cases, patient-reported weights were used. Patients were included in the 6-month data, but not the 12-month data, if they were taking AOMs for > 6 months but not for 12 months. If patients had been on multiple AOMs, baseline data were recorded at the start of the first medication that was used for 6 months or longer. Twelve-month data were recorded after subsequent medication change. Twelve-month metabolic laboratory values/vital signs were recorded for patients included in the study even if they did not complete ≥ 12 months of AOM therapy.
Statistical Analysis
Data from patients who were prescribed an AOM from January 2021 to June 2023 and who remained on the medication for ≥ 6 months were analyzed. Baseline characteristics were analyzed using descriptive statistics. The primary and secondary endpoints were evaluated using the t test. The safety endpoints were analyzed using descriptive statistics. An analysis of variance test was used for the subset analysis. Results with P < .05 were statistically significant.
Results
A total of 144 participants were included in this study, 116 in the adult group (aged < 65 years) and 28 in the older adult group (aged ≥ 65 years). Sixty-seven patients were excluded due to prespecified inclusion and exclusion criteria.
Other than the predetermined mean age differences (48 years vs 71 years), there were multiple differences in patient baseline characteristics. When comparing older adults and adults, average weight (283 lb vs 269 lb) and White race (89% vs 87%) were slightly higher in the older adult group. Also, a higher prevalence of T2DM (54% and 18%) and a lower prevalence of prediabetes (21% and 33%) was noted in the older adult group. HbA1c and BP were similar between both groups at baseline, while LDL was slightly lower in the older adult group (Table 1).

Patients in the adult group lost a mean 7.0% and 8.7% of body weight at 6 and 12 months, respectively, while the older adult group lost 5.0% and 6.6% body weight at 6 and 12 months, respectively. The difference in percent change in body weight was not statistically different at 6 (P = .08) or 12 (P = .26) months between patients in the adult group vs the older adult group or in the specific age groups (18-40 years, 41-64 years, ≥ 65 years) at 6 months (P = .24) or 12 months (P = .53) (Figure).

At 12 months, the difference between the adult group vs the older adult group was not statistically significant for HbA1c in patients with T2DM or prediabetes (P = .73), LDL (P = .95), systolic BP (P = .58), or diastolic BP (P = .51) (Table 2).

For the safety endpoint, the incidence of AEs was found to be different between groups. There were more reported AEs (61.2% vs 39.3%) and a greater increase in therapy discontinuation due to AEs (6.0% vs 0%) in the adult group compared to the older adult group (Table 3).

Discussion
Patients taking AOMs revealed no statistically significant difference in percent change in body weight at 6 or 12 months between adults aged < 65 years and older adults aged ≥ 65 years. The subset analysis also showed no statistically significant difference in change in percent body weight between more narrowly defined age groups of 18 to 40 years, 41 to 64 years, and ≥ 65 years. This suggests that AOM may have similar efficacy for weight loss in all ages of adults.
Secondary endpoint findings showed no statistically significant difference in HbA1c (in patients with T2DM or prediabetes), LDL, or BP at 12 months between the 2 groups. Although this study did not differentiate secondary outcomes based on the individual AOM, the change in HbA1c in both groups was expected, given that 70% of the patients included in this study were taking a glucagon-like peptide-1 agonist (liraglutide and semaglutide) at some point during the study. It’s also worth noting that secondary endpoints were collected for patients who discontinued the AOM between 6 and 12 months. Therefore, the patients’ HbA1c, LDL, and BP may not have accurately reflected the change that could have been expected if they had continued AOM therapy beyond the 12-month period.
Due to the different mechanisms and range in efficacy that AOMs have in regard to weight loss, changes in all outcomes, including weight, HbA1c, LDL, and BP were expected to vary as patients were included even after switching AOM (collection of data started after ≥ 6 months on a single AOM). Switching of AOM after the first 6 months of therapy was recorded in 25% of the patients in the ≥ 65 years group and 330% of the patients in the < 65 years group.
The incidence of AEs and subsequent discontinuation of AOMs in this study was higher in the adult group. This study excluded patients who did not continue taking an AOM for at least 6 months. As a result, the incidence of AEs between the 2 groups within the first 6 months of AOM therapy remains unknown. It is possible that during the first 6 months of therapy, patients aged < 65 years were more willing to tolerate or had fewer severe AEs compared with the older adult group. It’s also possible that the smaller number of patients in the older adult group was due to increased AEs that led them to discontinue early (before completion of 6 months of therapy) and/or prescriber discomfort in using AOMs in the older adult population. In addition, because the specific medication(s) taken by patients in each group were not detailed, it is unknown whether the adult group was taking AOMs associated with a greater number of AEs.
Limitations
This was a retrospective study with a relatively small sample size. A larger sample size may have shown more precise differences between age groups and may be more representative of the general population. Additionally, data were reliant on appropriate documentation, and adherence to AOM therapy was not assessed due to the retrospective nature of this study. At times, the study relied on patient reported data points, such as weight, if a clinic weight was not available. Also, this study did not account for many potential confounding factors such as other medications taken by the patient, which can affect outcomes including weight, HbA1c, LDL, blood pressure, and AEs.
Conclusions
This retrospective study of patients taking AOMs showed no statistically significant difference in weight loss at 6 or 12 months between adults aged < 65 years and older adults aged ≥ 65 years. A subset analysis found no statistically significant difference in change in body weight between specific age groups (18-40 years, 41-64 years, and ≥ 65 years). There was also no statistically significant difference in secondary outcomes, including change in HbA1c (in patients with T2DM or prediabetes), LDL or BP between age groups. The safety endpoints showed a higher incidence of medication AEs in the adult group, with more of these adults discontinuing therapy due to AEs. This study indicates that AOM may have similar outcomes for weight loss and metabolic laboratory values/vital sign changes between adults and older adults. Also, our findings suggest that patients aged < 65 years may experience more AEs than patients aged ≥ 65 years after ≥ 6 months of AOM therapy. Larger studies are needed to further evaluate these age-specific findings.
- Emmerich SD, Fryar CD, Stierman B, Ogden CL. Obesity and severe obesity prevalence in adults: United States, August 2021-August 2023. NCHS Data Brief No. 508. National Center for Health Statistics; 2024. Accessed December 11, 2024. https://www.cdc.gov/nchs/products/databriefs/db508.htm
- Ward ZJ, Bleich SN, Long MW, Gortmaker SL. Association of body mass index with health care expenditures in the United States by age and sex. PLoS One. 2021;16(3):e0247307. doi:10.1371/journal.pone.0247307
- Horn DB, Almandoz JP, Look M. What is clinically relevant weight loss for your patients and how can it be achieved? A narrative review. Postgrad Med. 2022;134(4):359-375. doi:10.1080/00325481.2022.2051366
- American Diabetes Association (ADA). Standards of care in diabetes–2023. Diabetes Care. 2023;46(suppl 1):S128- S2139. doi:10.2337/dc23-S008
- Wilding JPH, Batterham RL, Calanna S, et al. Onceweekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002. doi:10.1056/NEJMoa2032183
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11-22. doi:10.1056/NEJMoa1411892
- Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20(2):330-342. doi:10.1038/oby.2011.330
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341-1352. doi:10.1016/S0140-6736(11)60205-5
- Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297-308. doi:10.3945/ajcn.111.024927
- Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595-605. doi:10.1016/S0140-6736(10)60888-4
- Sjöström L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352(9123):167-172. doi:10.1016s0140-6736(97)11509-4
- Mangoni AA, Jackson SHD. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6-14. doi:10.1046/j.1365-2125.2003.02007.x
- Emmerich SD, Fryar CD, Stierman B, Ogden CL. Obesity and severe obesity prevalence in adults: United States, August 2021-August 2023. NCHS Data Brief No. 508. National Center for Health Statistics; 2024. Accessed December 11, 2024. https://www.cdc.gov/nchs/products/databriefs/db508.htm
- Ward ZJ, Bleich SN, Long MW, Gortmaker SL. Association of body mass index with health care expenditures in the United States by age and sex. PLoS One. 2021;16(3):e0247307. doi:10.1371/journal.pone.0247307
- Horn DB, Almandoz JP, Look M. What is clinically relevant weight loss for your patients and how can it be achieved? A narrative review. Postgrad Med. 2022;134(4):359-375. doi:10.1080/00325481.2022.2051366
- American Diabetes Association (ADA). Standards of care in diabetes–2023. Diabetes Care. 2023;46(suppl 1):S128- S2139. doi:10.2337/dc23-S008
- Wilding JPH, Batterham RL, Calanna S, et al. Onceweekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002. doi:10.1056/NEJMoa2032183
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11-22. doi:10.1056/NEJMoa1411892
- Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20(2):330-342. doi:10.1038/oby.2011.330
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341-1352. doi:10.1016/S0140-6736(11)60205-5
- Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297-308. doi:10.3945/ajcn.111.024927
- Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595-605. doi:10.1016/S0140-6736(10)60888-4
- Sjöström L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352(9123):167-172. doi:10.1016s0140-6736(97)11509-4
- Mangoni AA, Jackson SHD. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6-14. doi:10.1046/j.1365-2125.2003.02007.x
Efficacy of Anti-Obesity Medications in Adult and Older Adult Veteran Populations
Efficacy of Anti-Obesity Medications in Adult and Older Adult Veteran Populations
Quality, Not Type, of Diet Linked to Microbiome Health
new research suggested.
For example, red meat was a strong driver of omnivore microbiomes, with corresponding signature microbes that are negatively correlated with host cardiometabolic health.
In contrast, the signature microbes found in vegans’ gut microbiomes were correlated with favorable cardiometabolic markers and were enriched in omnivores who ate more plant-based foods.
“From the viewpoint of the impact of diet on the gut microbiome, what seems to be more important is the diversity of healthy plant-based foods that are consumed,” principal author Nicola Segata, PhD, University of Trento in Italy, said in an interview. “Whether this comes within a vegan or an omnivore diet is less crucial, as long as there is no specific overconsumption of unhealthy food categories, such as red meat.”
Excluding broad categories of foods also can have consequences, he added. “For example, we saw that the exclusion of dairy fermented foods is associated with decreased presence of potentially probiotic microbes that are constitutive of such foods. Avoiding meat or dairy products does not necessarily have a positive effect if it does not come with a variety of quality plant-based products.”
The study was published online in Nature Microbiology.
Diet Tied to Microbial Signature
The researchers analyzed biological samples from 21,561 individuals across five multi-national cohorts to map how differences in diet patterns (omnivore, vegetarian, and vegan) are reflected in gut microbiomes.
They found that the three diet patterns are highly distinguishable by their microbial profiles and that each diet has corresponding unique signature microbes, including those tied to digestion of specific types of food and sometimes those derived from food itself.
The microbiomes of omnivores had an increased presence of bacteria associated with meat digestion, such as Alistipes putredinis, which is involved in protein fermentation. Omnivores also had more bacteria associated with both inflammatory bowel disease and increased colon cancer risk, such as Ruminococcus torques and Bilophila wadsworthia.
The microbiomes of vegans had an abundance of bacteria involved in fiber fermentation, such as several species of Bacteroides and Firmicutes phyla, which help produce short-chain fatty acids. These compounds have beneficial effects on gut health by reducing inflammation and helping to maintain a better homeostatic balance between an individual’s metabolism and immune system.
The main difference between vegetarians and vegans was the presence in vegetarians’ microbiomes of Streptococcus thermophilus, a bacterium found mainly in dairy products and used in the production of yogurt.
Dietary factors within each diet pattern, such as the amount of plant-based food, shape the microbiome more than the type of diet and are important for gut health, according to the authors. For example, by eating more plant-based foods, people with an omnivorous diet can bring the proportion of beneficial signature microbes in their microbiomes more in line with the levels in people who are vegan or vegetarian.
“Since our data showed that omnivores on average ingest significantly fewer healthy plant-based foods than vegetarians or vegans, optimizing the quality of omnivore diets by increasing dietary plant diversity could lead to better gut health,” they wrote.
The ultimate goal, Segata said, is “a precision nutrition approach that recommends foods based on the configuration of the microbiome of patients and of the aspects of the microbiome one wants to enhance. We are not there yet, but it is nonetheless important to know which foods are usually boosting which types of members of the gut microbiome.”
His team is currently analyzing changes in the gut microbiome induced by diet changes among thousands of participants in various cohorts.
“This is one of the next steps toward unraveling causality along the diet-microbiome-health axis, together with the cultivation of specific microbiome members of interest for potential prebiotic and probiotic strategies,” he said.
Conventional Dietary Advice for Now
The findings are consistent with those of previous studies, Jack Gilbert, MD, director of the Microbiome and Metagenomics Center at the University of California, San Diego, and president of Applied Microbiology International, Cambridge, England, said in an interview.
“Future research needs to focus on whether the gut microbial signature can predict those that develop cardiovascular disease in each cohort — ie, the n-of-1 studies, whereby a vegan develops cardiovascular disease, or a carnivore does not,” said Gilbert, who was not involved in the study.
With more data, he said, “we can also start examining these trends over time to understand what might be going on with these ‘oddballs.’ ”
“There is not much you can do with the ‘eat a healthy balanced diet’ routine,” he noted. “If I got a microbiome signature, I could potentially tell you what to eat to optimize your blood glucose trends and your lipid panels but not to handle long-term disease risk, yet. So sticking with the guideline-recommended dietary advice seems best, until we can provide more nuanced advice for the patient.
“Importantly, I would also like to see time-resolved data,” he added. “Signatures can fluctuate over time, even over days, and so collecting a few weeks of stool samples would help us to better align the microbiome signatures to clinical endpoints.”
Segata is a consultant to and receives options from ZOE. Gilbert is a member of the scientific advisory boards of Holobiome, BiomeSense, EcoBiomics Canadian Research Program, MASTER EU, Sun Genomics, and Oath; the editorial advisory board for The Scientist; and the external advisory board for the Binational Early Asthma & Microbiome Study. He is also an adviser for Bened Life.
A version of this article appeared on Medscape.com.
new research suggested.
For example, red meat was a strong driver of omnivore microbiomes, with corresponding signature microbes that are negatively correlated with host cardiometabolic health.
In contrast, the signature microbes found in vegans’ gut microbiomes were correlated with favorable cardiometabolic markers and were enriched in omnivores who ate more plant-based foods.
“From the viewpoint of the impact of diet on the gut microbiome, what seems to be more important is the diversity of healthy plant-based foods that are consumed,” principal author Nicola Segata, PhD, University of Trento in Italy, said in an interview. “Whether this comes within a vegan or an omnivore diet is less crucial, as long as there is no specific overconsumption of unhealthy food categories, such as red meat.”
Excluding broad categories of foods also can have consequences, he added. “For example, we saw that the exclusion of dairy fermented foods is associated with decreased presence of potentially probiotic microbes that are constitutive of such foods. Avoiding meat or dairy products does not necessarily have a positive effect if it does not come with a variety of quality plant-based products.”
The study was published online in Nature Microbiology.
Diet Tied to Microbial Signature
The researchers analyzed biological samples from 21,561 individuals across five multi-national cohorts to map how differences in diet patterns (omnivore, vegetarian, and vegan) are reflected in gut microbiomes.
They found that the three diet patterns are highly distinguishable by their microbial profiles and that each diet has corresponding unique signature microbes, including those tied to digestion of specific types of food and sometimes those derived from food itself.
The microbiomes of omnivores had an increased presence of bacteria associated with meat digestion, such as Alistipes putredinis, which is involved in protein fermentation. Omnivores also had more bacteria associated with both inflammatory bowel disease and increased colon cancer risk, such as Ruminococcus torques and Bilophila wadsworthia.
The microbiomes of vegans had an abundance of bacteria involved in fiber fermentation, such as several species of Bacteroides and Firmicutes phyla, which help produce short-chain fatty acids. These compounds have beneficial effects on gut health by reducing inflammation and helping to maintain a better homeostatic balance between an individual’s metabolism and immune system.
The main difference between vegetarians and vegans was the presence in vegetarians’ microbiomes of Streptococcus thermophilus, a bacterium found mainly in dairy products and used in the production of yogurt.
Dietary factors within each diet pattern, such as the amount of plant-based food, shape the microbiome more than the type of diet and are important for gut health, according to the authors. For example, by eating more plant-based foods, people with an omnivorous diet can bring the proportion of beneficial signature microbes in their microbiomes more in line with the levels in people who are vegan or vegetarian.
“Since our data showed that omnivores on average ingest significantly fewer healthy plant-based foods than vegetarians or vegans, optimizing the quality of omnivore diets by increasing dietary plant diversity could lead to better gut health,” they wrote.
The ultimate goal, Segata said, is “a precision nutrition approach that recommends foods based on the configuration of the microbiome of patients and of the aspects of the microbiome one wants to enhance. We are not there yet, but it is nonetheless important to know which foods are usually boosting which types of members of the gut microbiome.”
His team is currently analyzing changes in the gut microbiome induced by diet changes among thousands of participants in various cohorts.
“This is one of the next steps toward unraveling causality along the diet-microbiome-health axis, together with the cultivation of specific microbiome members of interest for potential prebiotic and probiotic strategies,” he said.
Conventional Dietary Advice for Now
The findings are consistent with those of previous studies, Jack Gilbert, MD, director of the Microbiome and Metagenomics Center at the University of California, San Diego, and president of Applied Microbiology International, Cambridge, England, said in an interview.
“Future research needs to focus on whether the gut microbial signature can predict those that develop cardiovascular disease in each cohort — ie, the n-of-1 studies, whereby a vegan develops cardiovascular disease, or a carnivore does not,” said Gilbert, who was not involved in the study.
With more data, he said, “we can also start examining these trends over time to understand what might be going on with these ‘oddballs.’ ”
“There is not much you can do with the ‘eat a healthy balanced diet’ routine,” he noted. “If I got a microbiome signature, I could potentially tell you what to eat to optimize your blood glucose trends and your lipid panels but not to handle long-term disease risk, yet. So sticking with the guideline-recommended dietary advice seems best, until we can provide more nuanced advice for the patient.
“Importantly, I would also like to see time-resolved data,” he added. “Signatures can fluctuate over time, even over days, and so collecting a few weeks of stool samples would help us to better align the microbiome signatures to clinical endpoints.”
Segata is a consultant to and receives options from ZOE. Gilbert is a member of the scientific advisory boards of Holobiome, BiomeSense, EcoBiomics Canadian Research Program, MASTER EU, Sun Genomics, and Oath; the editorial advisory board for The Scientist; and the external advisory board for the Binational Early Asthma & Microbiome Study. He is also an adviser for Bened Life.
A version of this article appeared on Medscape.com.
new research suggested.
For example, red meat was a strong driver of omnivore microbiomes, with corresponding signature microbes that are negatively correlated with host cardiometabolic health.
In contrast, the signature microbes found in vegans’ gut microbiomes were correlated with favorable cardiometabolic markers and were enriched in omnivores who ate more plant-based foods.
“From the viewpoint of the impact of diet on the gut microbiome, what seems to be more important is the diversity of healthy plant-based foods that are consumed,” principal author Nicola Segata, PhD, University of Trento in Italy, said in an interview. “Whether this comes within a vegan or an omnivore diet is less crucial, as long as there is no specific overconsumption of unhealthy food categories, such as red meat.”
Excluding broad categories of foods also can have consequences, he added. “For example, we saw that the exclusion of dairy fermented foods is associated with decreased presence of potentially probiotic microbes that are constitutive of such foods. Avoiding meat or dairy products does not necessarily have a positive effect if it does not come with a variety of quality plant-based products.”
The study was published online in Nature Microbiology.
Diet Tied to Microbial Signature
The researchers analyzed biological samples from 21,561 individuals across five multi-national cohorts to map how differences in diet patterns (omnivore, vegetarian, and vegan) are reflected in gut microbiomes.
They found that the three diet patterns are highly distinguishable by their microbial profiles and that each diet has corresponding unique signature microbes, including those tied to digestion of specific types of food and sometimes those derived from food itself.
The microbiomes of omnivores had an increased presence of bacteria associated with meat digestion, such as Alistipes putredinis, which is involved in protein fermentation. Omnivores also had more bacteria associated with both inflammatory bowel disease and increased colon cancer risk, such as Ruminococcus torques and Bilophila wadsworthia.
The microbiomes of vegans had an abundance of bacteria involved in fiber fermentation, such as several species of Bacteroides and Firmicutes phyla, which help produce short-chain fatty acids. These compounds have beneficial effects on gut health by reducing inflammation and helping to maintain a better homeostatic balance between an individual’s metabolism and immune system.
The main difference between vegetarians and vegans was the presence in vegetarians’ microbiomes of Streptococcus thermophilus, a bacterium found mainly in dairy products and used in the production of yogurt.
Dietary factors within each diet pattern, such as the amount of plant-based food, shape the microbiome more than the type of diet and are important for gut health, according to the authors. For example, by eating more plant-based foods, people with an omnivorous diet can bring the proportion of beneficial signature microbes in their microbiomes more in line with the levels in people who are vegan or vegetarian.
“Since our data showed that omnivores on average ingest significantly fewer healthy plant-based foods than vegetarians or vegans, optimizing the quality of omnivore diets by increasing dietary plant diversity could lead to better gut health,” they wrote.
The ultimate goal, Segata said, is “a precision nutrition approach that recommends foods based on the configuration of the microbiome of patients and of the aspects of the microbiome one wants to enhance. We are not there yet, but it is nonetheless important to know which foods are usually boosting which types of members of the gut microbiome.”
His team is currently analyzing changes in the gut microbiome induced by diet changes among thousands of participants in various cohorts.
“This is one of the next steps toward unraveling causality along the diet-microbiome-health axis, together with the cultivation of specific microbiome members of interest for potential prebiotic and probiotic strategies,” he said.
Conventional Dietary Advice for Now
The findings are consistent with those of previous studies, Jack Gilbert, MD, director of the Microbiome and Metagenomics Center at the University of California, San Diego, and president of Applied Microbiology International, Cambridge, England, said in an interview.
“Future research needs to focus on whether the gut microbial signature can predict those that develop cardiovascular disease in each cohort — ie, the n-of-1 studies, whereby a vegan develops cardiovascular disease, or a carnivore does not,” said Gilbert, who was not involved in the study.
With more data, he said, “we can also start examining these trends over time to understand what might be going on with these ‘oddballs.’ ”
“There is not much you can do with the ‘eat a healthy balanced diet’ routine,” he noted. “If I got a microbiome signature, I could potentially tell you what to eat to optimize your blood glucose trends and your lipid panels but not to handle long-term disease risk, yet. So sticking with the guideline-recommended dietary advice seems best, until we can provide more nuanced advice for the patient.
“Importantly, I would also like to see time-resolved data,” he added. “Signatures can fluctuate over time, even over days, and so collecting a few weeks of stool samples would help us to better align the microbiome signatures to clinical endpoints.”
Segata is a consultant to and receives options from ZOE. Gilbert is a member of the scientific advisory boards of Holobiome, BiomeSense, EcoBiomics Canadian Research Program, MASTER EU, Sun Genomics, and Oath; the editorial advisory board for The Scientist; and the external advisory board for the Binational Early Asthma & Microbiome Study. He is also an adviser for Bened Life.
A version of this article appeared on Medscape.com.
FROM NATURE MICROBIOLOGY
Obesity Linked with Malignant Progression of Barrett’s Esophagus
A dose-response relationship exists between body mass index (BMI) and esophageal adenocarcinoma (EAC) or high-grade dysplasia (HGD), the authors found.
“Obesity has been implicated in the pathogenesis of many reflux-related esophageal disorders such as gastroesophageal reflux disease (GERD), BE, and EAC,” said senior author Leo Alexandre, MRCP, PhD, a clinical associate professor and member of the Norwich Epidemiology Centre at the University of East Anglia and gastroenterologist with the Norfolk & Norwich University Hospital NHS Foundation Trust, both in Norwich, England.
“Guidelines advocate obesity as a criterion for targeted screening for BE in patients with chronic reflux symptoms,” he said. “While obesity is a recognized risk factor for both BE and EAC, it’s been unclear whether obesity is a risk factor for malignant progression.”
The study was published in Clinical Gastroenterology and Hepatology.
Analyzing Risk
BE, which is the only recognized precursor lesion to EAC, is associated with a 30-fold increase in the incidence of the aggressive cancer. Typically, malignant progression occurs when nondysplastic BE epithelium progresses to low-grade dysplasia (LGD) and then HGD, followed by invasive adenocarcinoma.
Current guidelines suggest that patients with BE undergo endoscopic surveillance for early detection of adenocarcinoma. However, clinical risk factors could help with risk stratification and a personalized approach to long-term BE management, the authors wrote.
Alexandre and colleagues reviewed case-control or cohort studies that reported on the effect of BMI on the progression of nondysplastic BE or LGD to EAC, HGD, or esophageal cancer (EC). Then they estimated the dose-response relationship with a two-stage dose-response meta-analysis.
Overall, 20 observational studies reported data on 38,565 adult patients, including 1684 patients who were diagnosed with EAC, HGD, or EC. The studies enrolled patients between 1976 and 2019 and were published between 2005 and 2022. Most were based in Europe or the United States, and 74.4% of participants were men.
Among 12 cohort studies with 19,223 patients who had baseline nondysplastic BE or LGD, 816 progressed to EAC, HGD, or EC. The pooled annual rate of progression was .03%.
Among eight cohort studies with 6647 male patients who had baseline nondysplastic BE or LGD, 555 progressed to EAC, HGD, or EC. The pooled annual rate of progression was .02%.
In addition, among 1992 female patients with baseline nondysplastic BE or LGD, 110 progressed to EAC, HGD, or EC. The pooled annual rate of progression was .01%, which wasn’t a significant difference compared with the progression rate among male patients.
Based on meta-analyses, obesity was associated with a 4% increase in the risk for malignant progression among patients with BE (unadjusted odds ratio, 1.04; 95% CI, 1.00-1.07; P < .001).
Notably, each 5 unit increase in BMI was associated with a 6% increase in the risk of developing HGD or EAC (adjusted odds ratio, 1.06; 95% CI, 1.02-1.10; P < .001).
“Although the exact mechanisms by which obesity promotes esophageal carcinogenesis is not fully understood, several possible mechanisms may explain it,” Alexandre said. “The most obvious pathologic link is via GERD, with the mechanical effect of visceral obesity promoting the GERD directly, and the sequence of Barrett’s dysplasia to cancer indirectly. In addition, it has been demonstrated in experimental studies that gastric acid and bile acid drive malignant changes in esophageal epithelium through stimulation of proliferation, inhibition of apoptosis, and generation of free radicals.”
Considering Risk
This study highlights the importance of recognizing the association between obesity and cancer risks, said Prateek Sharma, MD, professor of medicine and director of gastrointestinal training at the University of Kansas School of Medicine, Kansas City, Kansas.
Sharma, who wasn’t involved with this study, coauthored an American Gastroenterological Association technical review on the management of BE.
“Obesity is a known risk factor for esophageal adenocarcinoma and may be a modifiable risk factor,” he said. “Showing that BMI is related to neoplastic progression in Barrett’s esophagus may impact surveillance intervals.”
Future research should look at additional obesity-related factors, such as visceral obesity and malignant progression of BE, as well as whether diet, lifestyle, and bariatric interventions can reduce the risk for progression.
“The next steps also include plugging BMI into risk scores and risk stratification models to enable targeted surveillance among high-risk groups,” Sharma said.
One of the study coauthors received funding as a National Institute for Health Research Academic clinical fellow. No other funding sources were declared. Alexandre and Sharma reported no relevant disclosures.
A version of this article appeared on Medscape.com.
A dose-response relationship exists between body mass index (BMI) and esophageal adenocarcinoma (EAC) or high-grade dysplasia (HGD), the authors found.
“Obesity has been implicated in the pathogenesis of many reflux-related esophageal disorders such as gastroesophageal reflux disease (GERD), BE, and EAC,” said senior author Leo Alexandre, MRCP, PhD, a clinical associate professor and member of the Norwich Epidemiology Centre at the University of East Anglia and gastroenterologist with the Norfolk & Norwich University Hospital NHS Foundation Trust, both in Norwich, England.
“Guidelines advocate obesity as a criterion for targeted screening for BE in patients with chronic reflux symptoms,” he said. “While obesity is a recognized risk factor for both BE and EAC, it’s been unclear whether obesity is a risk factor for malignant progression.”
The study was published in Clinical Gastroenterology and Hepatology.
Analyzing Risk
BE, which is the only recognized precursor lesion to EAC, is associated with a 30-fold increase in the incidence of the aggressive cancer. Typically, malignant progression occurs when nondysplastic BE epithelium progresses to low-grade dysplasia (LGD) and then HGD, followed by invasive adenocarcinoma.
Current guidelines suggest that patients with BE undergo endoscopic surveillance for early detection of adenocarcinoma. However, clinical risk factors could help with risk stratification and a personalized approach to long-term BE management, the authors wrote.
Alexandre and colleagues reviewed case-control or cohort studies that reported on the effect of BMI on the progression of nondysplastic BE or LGD to EAC, HGD, or esophageal cancer (EC). Then they estimated the dose-response relationship with a two-stage dose-response meta-analysis.
Overall, 20 observational studies reported data on 38,565 adult patients, including 1684 patients who were diagnosed with EAC, HGD, or EC. The studies enrolled patients between 1976 and 2019 and were published between 2005 and 2022. Most were based in Europe or the United States, and 74.4% of participants were men.
Among 12 cohort studies with 19,223 patients who had baseline nondysplastic BE or LGD, 816 progressed to EAC, HGD, or EC. The pooled annual rate of progression was .03%.
Among eight cohort studies with 6647 male patients who had baseline nondysplastic BE or LGD, 555 progressed to EAC, HGD, or EC. The pooled annual rate of progression was .02%.
In addition, among 1992 female patients with baseline nondysplastic BE or LGD, 110 progressed to EAC, HGD, or EC. The pooled annual rate of progression was .01%, which wasn’t a significant difference compared with the progression rate among male patients.
Based on meta-analyses, obesity was associated with a 4% increase in the risk for malignant progression among patients with BE (unadjusted odds ratio, 1.04; 95% CI, 1.00-1.07; P < .001).
Notably, each 5 unit increase in BMI was associated with a 6% increase in the risk of developing HGD or EAC (adjusted odds ratio, 1.06; 95% CI, 1.02-1.10; P < .001).
“Although the exact mechanisms by which obesity promotes esophageal carcinogenesis is not fully understood, several possible mechanisms may explain it,” Alexandre said. “The most obvious pathologic link is via GERD, with the mechanical effect of visceral obesity promoting the GERD directly, and the sequence of Barrett’s dysplasia to cancer indirectly. In addition, it has been demonstrated in experimental studies that gastric acid and bile acid drive malignant changes in esophageal epithelium through stimulation of proliferation, inhibition of apoptosis, and generation of free radicals.”
Considering Risk
This study highlights the importance of recognizing the association between obesity and cancer risks, said Prateek Sharma, MD, professor of medicine and director of gastrointestinal training at the University of Kansas School of Medicine, Kansas City, Kansas.
Sharma, who wasn’t involved with this study, coauthored an American Gastroenterological Association technical review on the management of BE.
“Obesity is a known risk factor for esophageal adenocarcinoma and may be a modifiable risk factor,” he said. “Showing that BMI is related to neoplastic progression in Barrett’s esophagus may impact surveillance intervals.”
Future research should look at additional obesity-related factors, such as visceral obesity and malignant progression of BE, as well as whether diet, lifestyle, and bariatric interventions can reduce the risk for progression.
“The next steps also include plugging BMI into risk scores and risk stratification models to enable targeted surveillance among high-risk groups,” Sharma said.
One of the study coauthors received funding as a National Institute for Health Research Academic clinical fellow. No other funding sources were declared. Alexandre and Sharma reported no relevant disclosures.
A version of this article appeared on Medscape.com.
A dose-response relationship exists between body mass index (BMI) and esophageal adenocarcinoma (EAC) or high-grade dysplasia (HGD), the authors found.
“Obesity has been implicated in the pathogenesis of many reflux-related esophageal disorders such as gastroesophageal reflux disease (GERD), BE, and EAC,” said senior author Leo Alexandre, MRCP, PhD, a clinical associate professor and member of the Norwich Epidemiology Centre at the University of East Anglia and gastroenterologist with the Norfolk & Norwich University Hospital NHS Foundation Trust, both in Norwich, England.
“Guidelines advocate obesity as a criterion for targeted screening for BE in patients with chronic reflux symptoms,” he said. “While obesity is a recognized risk factor for both BE and EAC, it’s been unclear whether obesity is a risk factor for malignant progression.”
The study was published in Clinical Gastroenterology and Hepatology.
Analyzing Risk
BE, which is the only recognized precursor lesion to EAC, is associated with a 30-fold increase in the incidence of the aggressive cancer. Typically, malignant progression occurs when nondysplastic BE epithelium progresses to low-grade dysplasia (LGD) and then HGD, followed by invasive adenocarcinoma.
Current guidelines suggest that patients with BE undergo endoscopic surveillance for early detection of adenocarcinoma. However, clinical risk factors could help with risk stratification and a personalized approach to long-term BE management, the authors wrote.
Alexandre and colleagues reviewed case-control or cohort studies that reported on the effect of BMI on the progression of nondysplastic BE or LGD to EAC, HGD, or esophageal cancer (EC). Then they estimated the dose-response relationship with a two-stage dose-response meta-analysis.
Overall, 20 observational studies reported data on 38,565 adult patients, including 1684 patients who were diagnosed with EAC, HGD, or EC. The studies enrolled patients between 1976 and 2019 and were published between 2005 and 2022. Most were based in Europe or the United States, and 74.4% of participants were men.
Among 12 cohort studies with 19,223 patients who had baseline nondysplastic BE or LGD, 816 progressed to EAC, HGD, or EC. The pooled annual rate of progression was .03%.
Among eight cohort studies with 6647 male patients who had baseline nondysplastic BE or LGD, 555 progressed to EAC, HGD, or EC. The pooled annual rate of progression was .02%.
In addition, among 1992 female patients with baseline nondysplastic BE or LGD, 110 progressed to EAC, HGD, or EC. The pooled annual rate of progression was .01%, which wasn’t a significant difference compared with the progression rate among male patients.
Based on meta-analyses, obesity was associated with a 4% increase in the risk for malignant progression among patients with BE (unadjusted odds ratio, 1.04; 95% CI, 1.00-1.07; P < .001).
Notably, each 5 unit increase in BMI was associated with a 6% increase in the risk of developing HGD or EAC (adjusted odds ratio, 1.06; 95% CI, 1.02-1.10; P < .001).
“Although the exact mechanisms by which obesity promotes esophageal carcinogenesis is not fully understood, several possible mechanisms may explain it,” Alexandre said. “The most obvious pathologic link is via GERD, with the mechanical effect of visceral obesity promoting the GERD directly, and the sequence of Barrett’s dysplasia to cancer indirectly. In addition, it has been demonstrated in experimental studies that gastric acid and bile acid drive malignant changes in esophageal epithelium through stimulation of proliferation, inhibition of apoptosis, and generation of free radicals.”
Considering Risk
This study highlights the importance of recognizing the association between obesity and cancer risks, said Prateek Sharma, MD, professor of medicine and director of gastrointestinal training at the University of Kansas School of Medicine, Kansas City, Kansas.
Sharma, who wasn’t involved with this study, coauthored an American Gastroenterological Association technical review on the management of BE.
“Obesity is a known risk factor for esophageal adenocarcinoma and may be a modifiable risk factor,” he said. “Showing that BMI is related to neoplastic progression in Barrett’s esophagus may impact surveillance intervals.”
Future research should look at additional obesity-related factors, such as visceral obesity and malignant progression of BE, as well as whether diet, lifestyle, and bariatric interventions can reduce the risk for progression.
“The next steps also include plugging BMI into risk scores and risk stratification models to enable targeted surveillance among high-risk groups,” Sharma said.
One of the study coauthors received funding as a National Institute for Health Research Academic clinical fellow. No other funding sources were declared. Alexandre and Sharma reported no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Commission Issues ‘Radical Overhaul’ of Obesity Diagnosis
“We propose a radical overhaul of the actual diagnosis of obesity to improve global healthcare and practices and policies. The specific aims were to facilitate individualized assessment and care of people living with obesity while preserving resources by reducing overdiagnosis and unnecessary or inadequate interventions,” Professor Louise Baur, chair of Child & Adolescent Health at the University of Sydney, Australia, said during a UK Science Media Centre (SMC) news briefing.
The report calls first for a diagnosis of obesity via confirmation of excess adiposity using measures such as waist circumference or waist-to-hip ratio in addition to BMI. Next, a clinical assessment of signs and symptoms of organ dysfunction due to obesity and/or functional limitations determines whether the individual has the disease “clinical obesity,” or “preclinical obesity,” a condition of health risk but not an illness itself.
Published on January 14, 2025, in The Lancet Diabetes & Endocrinology, the document also provides broad guidance on management for the two obesity conditions, emphasizing a personalized and stigma-free approach. The Lancet Commission on Obesity comprised 56 experts in relevant fields including endocrinology, surgery, nutrition, and public health, along with people living with obesity.
The report has been endorsed by more than 75 medical organizations, including the Association of British Clinical Diabetologists, the American Association of Clinical Endocrinologists, the American Diabetes Association, the American Heart Association, the Obesity Society, the World Obesity Federation, and obesity and endocrinology societies from countries in Europe, Latin America, Asia, and South Africa.
In recent years, many in the field have found fault with the current BMI-based definition of obesity (> 30 for people of European descent or other cutoffs for specific ethnic groups), primarily because BMI alone doesn’t reflect a person’s fat vs lean mass, fat distribution, or overall health. The new definition aims to overcome these limitations, as well as settle the debate about whether obesity is a “disease.”
“We now have a clinical diagnosis for obesity, which has been lacking. ... The traditional classification based on BMI ... reflects simply whether or not there is excess adiposity, and sometimes not even precise in that regard, either…It has never been a classification that was meant to diagnose a specific illness with its own clinical characteristics in the same way we diagnose any other illness,” Commission Chair Francesco Rubino, MD, professor and chair of Metabolic and Bariatric Surgery at King’s College London, England, said in an interview.
He added, “The fact that now we have a clinical diagnosis allows recognition of the nuance that obesity is generally a risk and for some can be an illness. There are some who have risk but don’t have the illness here and now. And it’s crucially important for clinical decision-making, but also for policies to have a distinction between those two things because the treatment strategy for one and the other are substantially different.”
Asked to comment, obesity specialist Michael A. Weintraub, MD, clinical assistant professor of endocrinology at New York University Langone Health, said in an interview, “I wholeheartedly agree with modifying the definition of obesity in this more accurate way. ... There has already been a lot of talk about the fallibility of BMI and that BMI over 30 does not equal obesity. ... So a major Commission article like this I think can really move those discussions even more into the forefront and start changing practice.”
However, Weintraub added, “I think there needs to be another step here of more practical guidance on how to actually implement this ... including how to measure waist circumference and to put it into a patient flow.”
Asked to comment, obesity expert Luca Busetto, MD, associate professor of internal medicine at the Department of Medicine of the University of Padova, Italy, said in an interview that he agrees with the general concept of moving beyond BMI in defining obesity. That view was expressed in a proposed “framework” from the European Association for the Study of Obesity (EASO), for which Busetto was the lead author.
Busetto also agrees with the emphasis on the need for a complete clinical evaluation of patients to define their health status. “The precise definition of the symptoms defining clinical obesity in adults and children is extremely important, emphasizing the fact that obesity is a severe and disabling disease by itself, even without or before the occurrence of obesity-related complications,” he said.
However, he takes issue with the Commission’s designation that “preclinical” obesity is not a disease. “The critical point of disagreement for me is the message that obesity is a disease only if it is clinical or only if it presents functional impairment or clinical symptoms. This remains, in my opinion, an oversimplification, not taking into account the fact that the pathophysiological mechanisms that lead to fat accumulation and ‘adipose tissue-based chronic disease’ usually start well before the occurrence of symptoms.”
Busetto pointed to examples such as type 2 diabetes and chronic kidney disease, both of which can be asymptomatic in their early phases yet are still considered diseases at those points. “I have no problem in accepting a distinction between preclinical and clinical stages of the disease, and I like the definition of clinical obesity, but why should this imply the fact that obesity is NOT a disease since its beginning?”
The Commission does state that preclinical obesity should be addressed, mostly with preventive approaches but in some cases with more intensive management. “This is highly relevant, but the risk of an undertreatment of obesity in its asymptomatic state remains in place. This could delay appropriate management for a progressive disease that certainly should not be treated only when presenting symptoms. It would be too late,” Busetto said.
And EASO framework coauthor Gijs Goossens, PhD, professor of cardiometabolic physiology of obesity at Maastricht University Medical Centre, the Netherlands, added a concern that those with excess adiposity but lower BMI might be missed entirely, noting “Since abdominal fat accumulation better predicts chronic cardiometabolic diseases and can also be accompanied by clinical manifestations in individuals with overweight as a consequence of compromised adipose tissue function, the proposed model may lead to underdiagnosis or undertreatment in individuals with BMI 25-30 who have excess abdominal fat.”
Diagnosis and Management Beyond BMI
The Commission advises the use of BMI solely as a marker to screen for potential obesity. Those with a BMI > 40 can be assumed to have excess body fat. For others with a BMI at or near the threshold for obesity in a specific country or ethnic group or for whom there is the clinical judgment of the potential for clinical obesity, confirmation of excess or abnormal adiposity is needed by one of the following:
- At least one measurement of body size and BMI
- At least two measures of body size, regardless of BMI
- Direct body fat measurement, such as a dual-energy x-ray absorptiometry (DEXA) scan
Measurement of body size can be assessed in three ways:
- Waist circumference ≥ 102 cm for men and ≥ 88 cm for women
- Waist-to-hip ratio > 0.90 for men and > 0.50 for women
- Waist-to-height ratio > 0.50 for all.
Weintraub noted, “Telemedicine is a useful tool used by many patients and providers but may also make it challenging to accurately assess someone’s body size. Having technology like an iPhone app to measure body size would circumvent this challenge but this type of tool has not yet been validated.”
If the person does not have excess adiposity, they don’t have obesity. Those with excess adiposity do have obesity. Further assessment is then needed to establish whether the person has an illness, that is, clinical obesity, indicated by signs/symptoms of organ dysfunction, and/or limitations of daily activities. If not, they have “preclinical” obesity.
The document provides a list of 18 obesity-related organ, tissue, and body system criteria for diagnosing “clinical” obesity in adults, including upper airways (eg, apneas/hypopneas), respiratory (breathlessness), cardiovascular (hypertension, heart failure), liver (fatty liver disease with hepatic fibrosis), reproductive (polycystic ovary syndrome, hypogonadism), and metabolism (hyperglycemia, hypertriglyceridemia, low high-density lipoprotein cholesterol). A list of 13 such criteria is also provided for children. “Limitations of day-to-day activities” are included on both lists.
Management Differs by Designation
For preclinical obesity, management should focus on risk reduction and prevention of progression to clinical obesity or other obesity-related diseases. Such approaches include health counseling for weight loss or prevention of weight gain, monitoring over time, and active weight loss interventions in people at higher risk of developing clinical obesity and other obesity-related diseases.
Management for clinical obesity focuses on improvements or reversal of the organ dysfunction. The type of evidence-based treatment and management should be informed by individual risk-benefit assessments and decided via “active discussion” with the patient. Success is determined by improvement in the signs and symptoms rather than measures of weight loss.
In response to a reporter’s question at the SMC briefing about the implications for the use of weight-loss medications, Rubino noted that this wasn’t the focus of the report, but nonetheless said that this new obesity definition could help with their targeted use. “The strategy and intent by which you use the drugs is different in clinical and preclinical obesity. ... Pharmacological interventions could be used for patients with high-risk preclinical obesity, with the intent of reducing risk, but we ... would use the same medication at a different intensity, dose, and maybe in combination therapies.”
As for clinical obesity, “It could be more or less severe and could affect more than one organ, and so clinical obesity might require drugs, might require surgery, or may require, in some cases, a combination of both of them, to achieve the best possible outcomes. ... We want to make sure that the person is restoring health ... with whatever it takes.”
Rubino believes this new definition will convince the remaining clinicians who haven’t yet accepted the concept of obesity as a disease. “When they see clinical obesity, I think it will be much harder to say that a biological process that is capable of causing a dysfunction in the heart or the lungs is less of a disease than another biological process that causes similar dysfunction in the heart of the lungs. ... It’s going to be objective. Obesity is a spectrum of different situations. ... When it’s an illness, clinical obesity, it’s not a matter of if or when. It’s a matter of fact.”
There were no industrial grants or other funding for this initiative. King’s Health Partners hosted the initiative and provided logistical and personnel support to facilitate administrative work and the Delphi-like consensus-development process. Rubino declared receiving research grants from Ethicon (Johnson & Johnson), Novo Nordisk, and Medtronic; consulting fees from Morphic Medical; speaking honoraria from Medtronic, Ethicon, Novo Nordisk, Eli Lilly, and Amgen. Rubino has served (unpaid) as a member of the scientific advisory board for Keyron and a member of the data safety and monitoring board for GI Metabolic Solutions; is president of the Metabolic Health Institute (non-profit); and is the sole director of Metabolic Health International and London Metabolic and Bariatric Surgery (private practice). Baur declared serving on the scientific advisory board for Novo Nordisk (for the ACTION Teens study) and Eli Lilly and receiving speaker fees (paid to the institution) from Novo Nordisk.
A version of this article first appeared on Medscape.com.
“We propose a radical overhaul of the actual diagnosis of obesity to improve global healthcare and practices and policies. The specific aims were to facilitate individualized assessment and care of people living with obesity while preserving resources by reducing overdiagnosis and unnecessary or inadequate interventions,” Professor Louise Baur, chair of Child & Adolescent Health at the University of Sydney, Australia, said during a UK Science Media Centre (SMC) news briefing.
The report calls first for a diagnosis of obesity via confirmation of excess adiposity using measures such as waist circumference or waist-to-hip ratio in addition to BMI. Next, a clinical assessment of signs and symptoms of organ dysfunction due to obesity and/or functional limitations determines whether the individual has the disease “clinical obesity,” or “preclinical obesity,” a condition of health risk but not an illness itself.
Published on January 14, 2025, in The Lancet Diabetes & Endocrinology, the document also provides broad guidance on management for the two obesity conditions, emphasizing a personalized and stigma-free approach. The Lancet Commission on Obesity comprised 56 experts in relevant fields including endocrinology, surgery, nutrition, and public health, along with people living with obesity.
The report has been endorsed by more than 75 medical organizations, including the Association of British Clinical Diabetologists, the American Association of Clinical Endocrinologists, the American Diabetes Association, the American Heart Association, the Obesity Society, the World Obesity Federation, and obesity and endocrinology societies from countries in Europe, Latin America, Asia, and South Africa.
In recent years, many in the field have found fault with the current BMI-based definition of obesity (> 30 for people of European descent or other cutoffs for specific ethnic groups), primarily because BMI alone doesn’t reflect a person’s fat vs lean mass, fat distribution, or overall health. The new definition aims to overcome these limitations, as well as settle the debate about whether obesity is a “disease.”
“We now have a clinical diagnosis for obesity, which has been lacking. ... The traditional classification based on BMI ... reflects simply whether or not there is excess adiposity, and sometimes not even precise in that regard, either…It has never been a classification that was meant to diagnose a specific illness with its own clinical characteristics in the same way we diagnose any other illness,” Commission Chair Francesco Rubino, MD, professor and chair of Metabolic and Bariatric Surgery at King’s College London, England, said in an interview.
He added, “The fact that now we have a clinical diagnosis allows recognition of the nuance that obesity is generally a risk and for some can be an illness. There are some who have risk but don’t have the illness here and now. And it’s crucially important for clinical decision-making, but also for policies to have a distinction between those two things because the treatment strategy for one and the other are substantially different.”
Asked to comment, obesity specialist Michael A. Weintraub, MD, clinical assistant professor of endocrinology at New York University Langone Health, said in an interview, “I wholeheartedly agree with modifying the definition of obesity in this more accurate way. ... There has already been a lot of talk about the fallibility of BMI and that BMI over 30 does not equal obesity. ... So a major Commission article like this I think can really move those discussions even more into the forefront and start changing practice.”
However, Weintraub added, “I think there needs to be another step here of more practical guidance on how to actually implement this ... including how to measure waist circumference and to put it into a patient flow.”
Asked to comment, obesity expert Luca Busetto, MD, associate professor of internal medicine at the Department of Medicine of the University of Padova, Italy, said in an interview that he agrees with the general concept of moving beyond BMI in defining obesity. That view was expressed in a proposed “framework” from the European Association for the Study of Obesity (EASO), for which Busetto was the lead author.
Busetto also agrees with the emphasis on the need for a complete clinical evaluation of patients to define their health status. “The precise definition of the symptoms defining clinical obesity in adults and children is extremely important, emphasizing the fact that obesity is a severe and disabling disease by itself, even without or before the occurrence of obesity-related complications,” he said.
However, he takes issue with the Commission’s designation that “preclinical” obesity is not a disease. “The critical point of disagreement for me is the message that obesity is a disease only if it is clinical or only if it presents functional impairment or clinical symptoms. This remains, in my opinion, an oversimplification, not taking into account the fact that the pathophysiological mechanisms that lead to fat accumulation and ‘adipose tissue-based chronic disease’ usually start well before the occurrence of symptoms.”
Busetto pointed to examples such as type 2 diabetes and chronic kidney disease, both of which can be asymptomatic in their early phases yet are still considered diseases at those points. “I have no problem in accepting a distinction between preclinical and clinical stages of the disease, and I like the definition of clinical obesity, but why should this imply the fact that obesity is NOT a disease since its beginning?”
The Commission does state that preclinical obesity should be addressed, mostly with preventive approaches but in some cases with more intensive management. “This is highly relevant, but the risk of an undertreatment of obesity in its asymptomatic state remains in place. This could delay appropriate management for a progressive disease that certainly should not be treated only when presenting symptoms. It would be too late,” Busetto said.
And EASO framework coauthor Gijs Goossens, PhD, professor of cardiometabolic physiology of obesity at Maastricht University Medical Centre, the Netherlands, added a concern that those with excess adiposity but lower BMI might be missed entirely, noting “Since abdominal fat accumulation better predicts chronic cardiometabolic diseases and can also be accompanied by clinical manifestations in individuals with overweight as a consequence of compromised adipose tissue function, the proposed model may lead to underdiagnosis or undertreatment in individuals with BMI 25-30 who have excess abdominal fat.”
Diagnosis and Management Beyond BMI
The Commission advises the use of BMI solely as a marker to screen for potential obesity. Those with a BMI > 40 can be assumed to have excess body fat. For others with a BMI at or near the threshold for obesity in a specific country or ethnic group or for whom there is the clinical judgment of the potential for clinical obesity, confirmation of excess or abnormal adiposity is needed by one of the following:
- At least one measurement of body size and BMI
- At least two measures of body size, regardless of BMI
- Direct body fat measurement, such as a dual-energy x-ray absorptiometry (DEXA) scan
Measurement of body size can be assessed in three ways:
- Waist circumference ≥ 102 cm for men and ≥ 88 cm for women
- Waist-to-hip ratio > 0.90 for men and > 0.50 for women
- Waist-to-height ratio > 0.50 for all.
Weintraub noted, “Telemedicine is a useful tool used by many patients and providers but may also make it challenging to accurately assess someone’s body size. Having technology like an iPhone app to measure body size would circumvent this challenge but this type of tool has not yet been validated.”
If the person does not have excess adiposity, they don’t have obesity. Those with excess adiposity do have obesity. Further assessment is then needed to establish whether the person has an illness, that is, clinical obesity, indicated by signs/symptoms of organ dysfunction, and/or limitations of daily activities. If not, they have “preclinical” obesity.
The document provides a list of 18 obesity-related organ, tissue, and body system criteria for diagnosing “clinical” obesity in adults, including upper airways (eg, apneas/hypopneas), respiratory (breathlessness), cardiovascular (hypertension, heart failure), liver (fatty liver disease with hepatic fibrosis), reproductive (polycystic ovary syndrome, hypogonadism), and metabolism (hyperglycemia, hypertriglyceridemia, low high-density lipoprotein cholesterol). A list of 13 such criteria is also provided for children. “Limitations of day-to-day activities” are included on both lists.
Management Differs by Designation
For preclinical obesity, management should focus on risk reduction and prevention of progression to clinical obesity or other obesity-related diseases. Such approaches include health counseling for weight loss or prevention of weight gain, monitoring over time, and active weight loss interventions in people at higher risk of developing clinical obesity and other obesity-related diseases.
Management for clinical obesity focuses on improvements or reversal of the organ dysfunction. The type of evidence-based treatment and management should be informed by individual risk-benefit assessments and decided via “active discussion” with the patient. Success is determined by improvement in the signs and symptoms rather than measures of weight loss.
In response to a reporter’s question at the SMC briefing about the implications for the use of weight-loss medications, Rubino noted that this wasn’t the focus of the report, but nonetheless said that this new obesity definition could help with their targeted use. “The strategy and intent by which you use the drugs is different in clinical and preclinical obesity. ... Pharmacological interventions could be used for patients with high-risk preclinical obesity, with the intent of reducing risk, but we ... would use the same medication at a different intensity, dose, and maybe in combination therapies.”
As for clinical obesity, “It could be more or less severe and could affect more than one organ, and so clinical obesity might require drugs, might require surgery, or may require, in some cases, a combination of both of them, to achieve the best possible outcomes. ... We want to make sure that the person is restoring health ... with whatever it takes.”
Rubino believes this new definition will convince the remaining clinicians who haven’t yet accepted the concept of obesity as a disease. “When they see clinical obesity, I think it will be much harder to say that a biological process that is capable of causing a dysfunction in the heart or the lungs is less of a disease than another biological process that causes similar dysfunction in the heart of the lungs. ... It’s going to be objective. Obesity is a spectrum of different situations. ... When it’s an illness, clinical obesity, it’s not a matter of if or when. It’s a matter of fact.”
There were no industrial grants or other funding for this initiative. King’s Health Partners hosted the initiative and provided logistical and personnel support to facilitate administrative work and the Delphi-like consensus-development process. Rubino declared receiving research grants from Ethicon (Johnson & Johnson), Novo Nordisk, and Medtronic; consulting fees from Morphic Medical; speaking honoraria from Medtronic, Ethicon, Novo Nordisk, Eli Lilly, and Amgen. Rubino has served (unpaid) as a member of the scientific advisory board for Keyron and a member of the data safety and monitoring board for GI Metabolic Solutions; is president of the Metabolic Health Institute (non-profit); and is the sole director of Metabolic Health International and London Metabolic and Bariatric Surgery (private practice). Baur declared serving on the scientific advisory board for Novo Nordisk (for the ACTION Teens study) and Eli Lilly and receiving speaker fees (paid to the institution) from Novo Nordisk.
A version of this article first appeared on Medscape.com.
“We propose a radical overhaul of the actual diagnosis of obesity to improve global healthcare and practices and policies. The specific aims were to facilitate individualized assessment and care of people living with obesity while preserving resources by reducing overdiagnosis and unnecessary or inadequate interventions,” Professor Louise Baur, chair of Child & Adolescent Health at the University of Sydney, Australia, said during a UK Science Media Centre (SMC) news briefing.
The report calls first for a diagnosis of obesity via confirmation of excess adiposity using measures such as waist circumference or waist-to-hip ratio in addition to BMI. Next, a clinical assessment of signs and symptoms of organ dysfunction due to obesity and/or functional limitations determines whether the individual has the disease “clinical obesity,” or “preclinical obesity,” a condition of health risk but not an illness itself.
Published on January 14, 2025, in The Lancet Diabetes & Endocrinology, the document also provides broad guidance on management for the two obesity conditions, emphasizing a personalized and stigma-free approach. The Lancet Commission on Obesity comprised 56 experts in relevant fields including endocrinology, surgery, nutrition, and public health, along with people living with obesity.
The report has been endorsed by more than 75 medical organizations, including the Association of British Clinical Diabetologists, the American Association of Clinical Endocrinologists, the American Diabetes Association, the American Heart Association, the Obesity Society, the World Obesity Federation, and obesity and endocrinology societies from countries in Europe, Latin America, Asia, and South Africa.
In recent years, many in the field have found fault with the current BMI-based definition of obesity (> 30 for people of European descent or other cutoffs for specific ethnic groups), primarily because BMI alone doesn’t reflect a person’s fat vs lean mass, fat distribution, or overall health. The new definition aims to overcome these limitations, as well as settle the debate about whether obesity is a “disease.”
“We now have a clinical diagnosis for obesity, which has been lacking. ... The traditional classification based on BMI ... reflects simply whether or not there is excess adiposity, and sometimes not even precise in that regard, either…It has never been a classification that was meant to diagnose a specific illness with its own clinical characteristics in the same way we diagnose any other illness,” Commission Chair Francesco Rubino, MD, professor and chair of Metabolic and Bariatric Surgery at King’s College London, England, said in an interview.
He added, “The fact that now we have a clinical diagnosis allows recognition of the nuance that obesity is generally a risk and for some can be an illness. There are some who have risk but don’t have the illness here and now. And it’s crucially important for clinical decision-making, but also for policies to have a distinction between those two things because the treatment strategy for one and the other are substantially different.”
Asked to comment, obesity specialist Michael A. Weintraub, MD, clinical assistant professor of endocrinology at New York University Langone Health, said in an interview, “I wholeheartedly agree with modifying the definition of obesity in this more accurate way. ... There has already been a lot of talk about the fallibility of BMI and that BMI over 30 does not equal obesity. ... So a major Commission article like this I think can really move those discussions even more into the forefront and start changing practice.”
However, Weintraub added, “I think there needs to be another step here of more practical guidance on how to actually implement this ... including how to measure waist circumference and to put it into a patient flow.”
Asked to comment, obesity expert Luca Busetto, MD, associate professor of internal medicine at the Department of Medicine of the University of Padova, Italy, said in an interview that he agrees with the general concept of moving beyond BMI in defining obesity. That view was expressed in a proposed “framework” from the European Association for the Study of Obesity (EASO), for which Busetto was the lead author.
Busetto also agrees with the emphasis on the need for a complete clinical evaluation of patients to define their health status. “The precise definition of the symptoms defining clinical obesity in adults and children is extremely important, emphasizing the fact that obesity is a severe and disabling disease by itself, even without or before the occurrence of obesity-related complications,” he said.
However, he takes issue with the Commission’s designation that “preclinical” obesity is not a disease. “The critical point of disagreement for me is the message that obesity is a disease only if it is clinical or only if it presents functional impairment or clinical symptoms. This remains, in my opinion, an oversimplification, not taking into account the fact that the pathophysiological mechanisms that lead to fat accumulation and ‘adipose tissue-based chronic disease’ usually start well before the occurrence of symptoms.”
Busetto pointed to examples such as type 2 diabetes and chronic kidney disease, both of which can be asymptomatic in their early phases yet are still considered diseases at those points. “I have no problem in accepting a distinction between preclinical and clinical stages of the disease, and I like the definition of clinical obesity, but why should this imply the fact that obesity is NOT a disease since its beginning?”
The Commission does state that preclinical obesity should be addressed, mostly with preventive approaches but in some cases with more intensive management. “This is highly relevant, but the risk of an undertreatment of obesity in its asymptomatic state remains in place. This could delay appropriate management for a progressive disease that certainly should not be treated only when presenting symptoms. It would be too late,” Busetto said.
And EASO framework coauthor Gijs Goossens, PhD, professor of cardiometabolic physiology of obesity at Maastricht University Medical Centre, the Netherlands, added a concern that those with excess adiposity but lower BMI might be missed entirely, noting “Since abdominal fat accumulation better predicts chronic cardiometabolic diseases and can also be accompanied by clinical manifestations in individuals with overweight as a consequence of compromised adipose tissue function, the proposed model may lead to underdiagnosis or undertreatment in individuals with BMI 25-30 who have excess abdominal fat.”
Diagnosis and Management Beyond BMI
The Commission advises the use of BMI solely as a marker to screen for potential obesity. Those with a BMI > 40 can be assumed to have excess body fat. For others with a BMI at or near the threshold for obesity in a specific country or ethnic group or for whom there is the clinical judgment of the potential for clinical obesity, confirmation of excess or abnormal adiposity is needed by one of the following:
- At least one measurement of body size and BMI
- At least two measures of body size, regardless of BMI
- Direct body fat measurement, such as a dual-energy x-ray absorptiometry (DEXA) scan
Measurement of body size can be assessed in three ways:
- Waist circumference ≥ 102 cm for men and ≥ 88 cm for women
- Waist-to-hip ratio > 0.90 for men and > 0.50 for women
- Waist-to-height ratio > 0.50 for all.
Weintraub noted, “Telemedicine is a useful tool used by many patients and providers but may also make it challenging to accurately assess someone’s body size. Having technology like an iPhone app to measure body size would circumvent this challenge but this type of tool has not yet been validated.”
If the person does not have excess adiposity, they don’t have obesity. Those with excess adiposity do have obesity. Further assessment is then needed to establish whether the person has an illness, that is, clinical obesity, indicated by signs/symptoms of organ dysfunction, and/or limitations of daily activities. If not, they have “preclinical” obesity.
The document provides a list of 18 obesity-related organ, tissue, and body system criteria for diagnosing “clinical” obesity in adults, including upper airways (eg, apneas/hypopneas), respiratory (breathlessness), cardiovascular (hypertension, heart failure), liver (fatty liver disease with hepatic fibrosis), reproductive (polycystic ovary syndrome, hypogonadism), and metabolism (hyperglycemia, hypertriglyceridemia, low high-density lipoprotein cholesterol). A list of 13 such criteria is also provided for children. “Limitations of day-to-day activities” are included on both lists.
Management Differs by Designation
For preclinical obesity, management should focus on risk reduction and prevention of progression to clinical obesity or other obesity-related diseases. Such approaches include health counseling for weight loss or prevention of weight gain, monitoring over time, and active weight loss interventions in people at higher risk of developing clinical obesity and other obesity-related diseases.
Management for clinical obesity focuses on improvements or reversal of the organ dysfunction. The type of evidence-based treatment and management should be informed by individual risk-benefit assessments and decided via “active discussion” with the patient. Success is determined by improvement in the signs and symptoms rather than measures of weight loss.
In response to a reporter’s question at the SMC briefing about the implications for the use of weight-loss medications, Rubino noted that this wasn’t the focus of the report, but nonetheless said that this new obesity definition could help with their targeted use. “The strategy and intent by which you use the drugs is different in clinical and preclinical obesity. ... Pharmacological interventions could be used for patients with high-risk preclinical obesity, with the intent of reducing risk, but we ... would use the same medication at a different intensity, dose, and maybe in combination therapies.”
As for clinical obesity, “It could be more or less severe and could affect more than one organ, and so clinical obesity might require drugs, might require surgery, or may require, in some cases, a combination of both of them, to achieve the best possible outcomes. ... We want to make sure that the person is restoring health ... with whatever it takes.”
Rubino believes this new definition will convince the remaining clinicians who haven’t yet accepted the concept of obesity as a disease. “When they see clinical obesity, I think it will be much harder to say that a biological process that is capable of causing a dysfunction in the heart or the lungs is less of a disease than another biological process that causes similar dysfunction in the heart of the lungs. ... It’s going to be objective. Obesity is a spectrum of different situations. ... When it’s an illness, clinical obesity, it’s not a matter of if or when. It’s a matter of fact.”
There were no industrial grants or other funding for this initiative. King’s Health Partners hosted the initiative and provided logistical and personnel support to facilitate administrative work and the Delphi-like consensus-development process. Rubino declared receiving research grants from Ethicon (Johnson & Johnson), Novo Nordisk, and Medtronic; consulting fees from Morphic Medical; speaking honoraria from Medtronic, Ethicon, Novo Nordisk, Eli Lilly, and Amgen. Rubino has served (unpaid) as a member of the scientific advisory board for Keyron and a member of the data safety and monitoring board for GI Metabolic Solutions; is president of the Metabolic Health Institute (non-profit); and is the sole director of Metabolic Health International and London Metabolic and Bariatric Surgery (private practice). Baur declared serving on the scientific advisory board for Novo Nordisk (for the ACTION Teens study) and Eli Lilly and receiving speaker fees (paid to the institution) from Novo Nordisk.
A version of this article first appeared on Medscape.com.
FROM THE LANCET DIABETES & ENDOCRINOLOGY