User login
GLP-1 agonists linked to higher risk for rare but serious GI complications
Patients taking either of these glucagonlike peptide-1 (GLP-1) receptor agonists had a 9-fold elevation in risk for pancreatitis. They were also 4 times more likely to develop bowel obstruction and over 3.5 times more likely to experience gastroparesis.
The research letter was published online in JAMA.
Investigators say their findings are not about scaring people off the weight-loss drugs, but instead about increasing awareness that these potential adverse outcomes can happen.
“Given the wide use of these drugs, these adverse events, although rare, must be considered by patients thinking about using them for weight loss,” said lead author Mohit Sodhi, MSc, in a news release about the study. Mr. Sodhi is a graduate of the experimental medicine program at the University of British Columbia in Vancouver, and also a 4th-year medical student at UBC.
People taking a GLP-1 agonist to treat diabetes might be more willing to accept the risks, given their potential advantages, especially that of lowering the risk for heart problems, said Mahyar Etminan, PharmD, MSc, the study’s senior author and an expert in drug safety and pharmacoepidemiology at UBC. “But those who are otherwise healthy and just taking them for weight loss might want to be more careful in weighing the risk–benefit equation.”
People taking these drugs for weight loss have an approximately 1%-2% chance of experiencing these events, including a 1% risk for gastroparesis, Dr. Etminan said.
Key findings
The study included 4,144 people taking liraglutide, 613 taking semaglutide, and 654 taking naltrexone/bupropion based on medical records between 2006 and 2020.
They included patients with a recent history of obesity but excluded those with diabetes or who had been prescribed another diabetes medication.
The use of GLP-1 agonists, compared with naltrexone/bupropion, was associated with an increased risk for pancreatitis (adjusted hazard ratio, 9.09; 95% confidence interval, 1.25-66.00), bowel obstruction (HR, 4.22; 95% CI, 1.02-17.40), and gastroparesis (HR, 3.67; 95% CI, 1.15-11.90).
The study also found a higher incidence of biliary disease, but the difference was not statistically significant (HR, 1.50; 95% CI, 0.89-2.53). The incidence of biliary disease (per 1,000 person-years) was 11.7 for semaglutide, 18.6 for liraglutide, and 12.6 for naltrexone/bupropion.
Not the first report of GI issues
“This important paper confirms the safety signals hinted at in previous randomized controlled trials,” said Carel Le Roux, MBChB, PhD, professor of metabolic medicine, Ulster University, Coleraine, Ireland, and professor of experimental pathology at University College Dublin.
“The limitations of the paper are acknowledged but do not detract from the value of the robust data,” Dr. Le Roux said. “Patients should be informed of the low risk of serious complications, such as pancreatitis, gastroparesis, and bowel obstruction, before they start semaglutide or liraglutide.”
This is not the first report of GI issues associated with GLP-1 agonists, but it’s one of the largest. Most reports have been anecdotal. The U.S. Food and Drug Administration announced on Sept. 28 that it would require manufacturers to include a warning about gastrointestinal ileus on the Ozempic (semaglutide) label.
“The results from this study highlight how important it is that patients access these drugs only through trusted medical professionals, and only with ongoing support and monitoring,” noted Simon Cork, PhD, senior lecturer in physiology, Anglia Ruskin University in Cambridge, England.
Dr. Cork added that “it’s important to look at this in the proper context.” Obesity significantly increases the risk for developing cardiovascular disease, type 2 diabetes, cancer, gallbladder disease, and stroke, risks that fall dramatically with clinically meaningful and sustained weight loss, he said.
“For the overwhelming majority of patients for whom these drugs are targeted (those with the most severe forms of obesity), the benefits of weight loss far outweigh the risks,” Dr. Cork said.
The study was independently supported. Mr. Sodhi, Dr. Etminan, and Dr. Cork report no relevant financial relationships. Dr. Le Roux is a consultant and has received research funding and reimbursement of travel expenses from Novo Nordisk.
A version of this article first appeared on Medscape.com.
Patients taking either of these glucagonlike peptide-1 (GLP-1) receptor agonists had a 9-fold elevation in risk for pancreatitis. They were also 4 times more likely to develop bowel obstruction and over 3.5 times more likely to experience gastroparesis.
The research letter was published online in JAMA.
Investigators say their findings are not about scaring people off the weight-loss drugs, but instead about increasing awareness that these potential adverse outcomes can happen.
“Given the wide use of these drugs, these adverse events, although rare, must be considered by patients thinking about using them for weight loss,” said lead author Mohit Sodhi, MSc, in a news release about the study. Mr. Sodhi is a graduate of the experimental medicine program at the University of British Columbia in Vancouver, and also a 4th-year medical student at UBC.
People taking a GLP-1 agonist to treat diabetes might be more willing to accept the risks, given their potential advantages, especially that of lowering the risk for heart problems, said Mahyar Etminan, PharmD, MSc, the study’s senior author and an expert in drug safety and pharmacoepidemiology at UBC. “But those who are otherwise healthy and just taking them for weight loss might want to be more careful in weighing the risk–benefit equation.”
People taking these drugs for weight loss have an approximately 1%-2% chance of experiencing these events, including a 1% risk for gastroparesis, Dr. Etminan said.
Key findings
The study included 4,144 people taking liraglutide, 613 taking semaglutide, and 654 taking naltrexone/bupropion based on medical records between 2006 and 2020.
They included patients with a recent history of obesity but excluded those with diabetes or who had been prescribed another diabetes medication.
The use of GLP-1 agonists, compared with naltrexone/bupropion, was associated with an increased risk for pancreatitis (adjusted hazard ratio, 9.09; 95% confidence interval, 1.25-66.00), bowel obstruction (HR, 4.22; 95% CI, 1.02-17.40), and gastroparesis (HR, 3.67; 95% CI, 1.15-11.90).
The study also found a higher incidence of biliary disease, but the difference was not statistically significant (HR, 1.50; 95% CI, 0.89-2.53). The incidence of biliary disease (per 1,000 person-years) was 11.7 for semaglutide, 18.6 for liraglutide, and 12.6 for naltrexone/bupropion.
Not the first report of GI issues
“This important paper confirms the safety signals hinted at in previous randomized controlled trials,” said Carel Le Roux, MBChB, PhD, professor of metabolic medicine, Ulster University, Coleraine, Ireland, and professor of experimental pathology at University College Dublin.
“The limitations of the paper are acknowledged but do not detract from the value of the robust data,” Dr. Le Roux said. “Patients should be informed of the low risk of serious complications, such as pancreatitis, gastroparesis, and bowel obstruction, before they start semaglutide or liraglutide.”
This is not the first report of GI issues associated with GLP-1 agonists, but it’s one of the largest. Most reports have been anecdotal. The U.S. Food and Drug Administration announced on Sept. 28 that it would require manufacturers to include a warning about gastrointestinal ileus on the Ozempic (semaglutide) label.
“The results from this study highlight how important it is that patients access these drugs only through trusted medical professionals, and only with ongoing support and monitoring,” noted Simon Cork, PhD, senior lecturer in physiology, Anglia Ruskin University in Cambridge, England.
Dr. Cork added that “it’s important to look at this in the proper context.” Obesity significantly increases the risk for developing cardiovascular disease, type 2 diabetes, cancer, gallbladder disease, and stroke, risks that fall dramatically with clinically meaningful and sustained weight loss, he said.
“For the overwhelming majority of patients for whom these drugs are targeted (those with the most severe forms of obesity), the benefits of weight loss far outweigh the risks,” Dr. Cork said.
The study was independently supported. Mr. Sodhi, Dr. Etminan, and Dr. Cork report no relevant financial relationships. Dr. Le Roux is a consultant and has received research funding and reimbursement of travel expenses from Novo Nordisk.
A version of this article first appeared on Medscape.com.
Patients taking either of these glucagonlike peptide-1 (GLP-1) receptor agonists had a 9-fold elevation in risk for pancreatitis. They were also 4 times more likely to develop bowel obstruction and over 3.5 times more likely to experience gastroparesis.
The research letter was published online in JAMA.
Investigators say their findings are not about scaring people off the weight-loss drugs, but instead about increasing awareness that these potential adverse outcomes can happen.
“Given the wide use of these drugs, these adverse events, although rare, must be considered by patients thinking about using them for weight loss,” said lead author Mohit Sodhi, MSc, in a news release about the study. Mr. Sodhi is a graduate of the experimental medicine program at the University of British Columbia in Vancouver, and also a 4th-year medical student at UBC.
People taking a GLP-1 agonist to treat diabetes might be more willing to accept the risks, given their potential advantages, especially that of lowering the risk for heart problems, said Mahyar Etminan, PharmD, MSc, the study’s senior author and an expert in drug safety and pharmacoepidemiology at UBC. “But those who are otherwise healthy and just taking them for weight loss might want to be more careful in weighing the risk–benefit equation.”
People taking these drugs for weight loss have an approximately 1%-2% chance of experiencing these events, including a 1% risk for gastroparesis, Dr. Etminan said.
Key findings
The study included 4,144 people taking liraglutide, 613 taking semaglutide, and 654 taking naltrexone/bupropion based on medical records between 2006 and 2020.
They included patients with a recent history of obesity but excluded those with diabetes or who had been prescribed another diabetes medication.
The use of GLP-1 agonists, compared with naltrexone/bupropion, was associated with an increased risk for pancreatitis (adjusted hazard ratio, 9.09; 95% confidence interval, 1.25-66.00), bowel obstruction (HR, 4.22; 95% CI, 1.02-17.40), and gastroparesis (HR, 3.67; 95% CI, 1.15-11.90).
The study also found a higher incidence of biliary disease, but the difference was not statistically significant (HR, 1.50; 95% CI, 0.89-2.53). The incidence of biliary disease (per 1,000 person-years) was 11.7 for semaglutide, 18.6 for liraglutide, and 12.6 for naltrexone/bupropion.
Not the first report of GI issues
“This important paper confirms the safety signals hinted at in previous randomized controlled trials,” said Carel Le Roux, MBChB, PhD, professor of metabolic medicine, Ulster University, Coleraine, Ireland, and professor of experimental pathology at University College Dublin.
“The limitations of the paper are acknowledged but do not detract from the value of the robust data,” Dr. Le Roux said. “Patients should be informed of the low risk of serious complications, such as pancreatitis, gastroparesis, and bowel obstruction, before they start semaglutide or liraglutide.”
This is not the first report of GI issues associated with GLP-1 agonists, but it’s one of the largest. Most reports have been anecdotal. The U.S. Food and Drug Administration announced on Sept. 28 that it would require manufacturers to include a warning about gastrointestinal ileus on the Ozempic (semaglutide) label.
“The results from this study highlight how important it is that patients access these drugs only through trusted medical professionals, and only with ongoing support and monitoring,” noted Simon Cork, PhD, senior lecturer in physiology, Anglia Ruskin University in Cambridge, England.
Dr. Cork added that “it’s important to look at this in the proper context.” Obesity significantly increases the risk for developing cardiovascular disease, type 2 diabetes, cancer, gallbladder disease, and stroke, risks that fall dramatically with clinically meaningful and sustained weight loss, he said.
“For the overwhelming majority of patients for whom these drugs are targeted (those with the most severe forms of obesity), the benefits of weight loss far outweigh the risks,” Dr. Cork said.
The study was independently supported. Mr. Sodhi, Dr. Etminan, and Dr. Cork report no relevant financial relationships. Dr. Le Roux is a consultant and has received research funding and reimbursement of travel expenses from Novo Nordisk.
A version of this article first appeared on Medscape.com.
FROM JAMA
Obesity linked to multiple ills in MS study
MILAN – Swedish researchers reported at the 9th Joint ECTRIMS-ACTRIMS meeting.
In a group of 3,249 subjects tracked for up to 5 years (74% female; mean age, 37.8 years), patients who were obese at diagnosis were 1.41 times more likely than normal-weight patients to reach an Expanded Disability Status Scale (EDSS) score of 3. About 35% of 355 obese subjects (body mass index > 30 kg/m2) reached that level versus 29% of 713 overweight patients (BMI, 25-30) and 28% of 1,475 normal-weight patients (BMI, 18.5-24.99).
Among subjects whose BMI category didn’t change over follow-up, those who were obese at diagnosis were more likely to develop cognitive worsening than those who weren’t obese (hazard ratio, 1.47, 95% confidence interval, 1.08-2.01).
Lars Alfredsson, PhD, a professor at the Karolinska Institutet, Stockholm, who presented the study findings, said in an interview that they fill a gap in knowledge about obesity and MS. “It is known that obesity around the age of 20 or in adolescence is a risk factor for developing MS. But much less is known in regard to progression, and the studies have been very inconclusive.”
The researchers tracked patients via the Swedish MS registry: 1,475 of normal weight, 713 overweight, and 355 obese. Before adjustment for factors such as age, gender, and baseline EDSS, obese subjects were 1.51 times more likely to reach EDSS score 3 than normal-weight subjects.
Obese subjects whose BMI level didn’t change over time were 1.70 times more likely than the nonobese to develop physical worsening as measured by an increased Multiple Sclerosis Impact Scale physical score of 7.5 points or more, and they were 1.36 times more likely to have psychological worsening as measured by increased MSIS-28 psychological score of 7.5 points or more.
Also, among subjects whose BMI didn’t change over time, the likelihood of cognitive disability worsening was 1.47 times higher among obese participants versus nonobese participants. Worsening was defined as an increased Symbol Digit Modalities Test score of 8 points or more.
The level of excess cognitive decline “will affect people significantly,” Dr. Alfredsson said.
While obesity can counterintuitively provide a protective effect in some diseases, he said there’s no sign of such an effect in the subjects.
As for limitations, Dr. Alfredsson noted in his presentation that BMI data is self-reported, and it’s possible that the researchers didn’t adjust their statistics to reflect important confounders.
A 2023 German study of outcomes in MS patients with obesity came to similar conclusions. It tracked 1,066 subjects for up to 6 years and found that “median time to reach EDSS 3 was 0.99 years for patients with BMI of 30 or higher and 1.46 years for nonobese patients. Risk to reach EDSS 3 over 6 years was significantly increased in patients with BMI of at least 30, compared with patients with BMI less than 30 after adjustment for sex, age, smoking (HR, 1.87; 95% CI, 1.3-2.6; P < .001), and independent of disease-modifying therapies.”
However, the German researchers found no link between obesity and higher levels of relapse, contrast-enhancing MRI lesions, or MRI T2 lesion burden.
Interpretation and commentary
Could obesity be causing worse outcomes? The new study doesn’t provide insight into cause and effect. However, obesity may speed up progression via low-grade inflammation, Dr. Alfredsson said.
What can clinicians do with the information from the study? If patients are obese, it can be a good idea to more carefully monitor them and use reliable tools to improve their progression, Dr. Alfredsson said.
In an interview, Michael D. Kornberg, MD, PhD, an assistant professor of neurology at Johns Hopkins University, Baltimore, who was not involved with the study, agreed with Dr. Alfredsson that other research has linked obesity early in life to higher rates of MS. He added that “a number of studies have shown that comorbidities in general are usually associated with a higher rate of disability.”
Dr. Kornberg said the new research is important, and he noted that it has a “robust” cohort because of its larger size.
Could patients with MS reverse the risk of progression and other poor outcomes by losing weight? “It’s hard to say,” Dr. Kornberg said. “We have to be cautious when we assume causation. There’s a plausible rationale that obesity might worsen progression in MS, but it could just be a marker of some other factor that reflects a different phenotype of MS.”
He doesn’t think it’s likely that weight loss would “dramatically reverse the biology of MS,” but he said reversing the obesity epidemic would still be a good thing. An interventional study could examine the effects of weight-loss intervention on disability measures, he said, “and that’s the next step.”
Also contacted for commentary, Adil Harroud, MD, a neurologist at McGill University who studies obesity in MS, said research suggests that “obesity seems to exacerbate MS disability. While some studies show no effect, the majority indicate a detrimental impact.”
However, “the effect of obesity on MS progression remains unclear. Animal studies suggest that shifts in immune cell subsets and functions may play a role, but the relevance to humans is yet to be determined,” he said.
Dr. Harroud, who did not take part in the new study, said it’s “one of the largest examining the impact of obesity on MS disability.” He added that “the cohort was relatively early in their disease course, suggesting that obesity impacts even the early stages of MS. This underscores the importance of obesity as a modifiable risk factor for disability accumulation.”
As for why obesity affects MS, he said one theory is that obesity plays a role through its impact on vitamin D levels. “However, using a genetic approach, we have demonstrated that, at least for MS risk, the effect of obesity is independent of vitamin D. This is also likely true for MS progression, as recent trials of vitamin D supplementation have not shown a meaningful impact on MS outcomes.”
According to Dr. Harroud, “other theories suggest that obesity leads to a pro-inflammatory immune shift. Additionally, it has been proposed that obesity may influence the response to disease-modifying therapy by reducing drug bioavailability, potentially necessitating weight-based dosing for some therapies.”
Dr. Alfredsson reported receiving grants from the Swedish Research Council, the Swedish Research Council for Health Working Life and Welfare, and the Swedish Brain Foundation and personal fees from Teva and Biogene Idec. Some of the other study authors reported various disclosures. Dr. Kornberg and Dr. Harroud reported no relevant disclosures.
This article was updated 10/20/23.
MILAN – Swedish researchers reported at the 9th Joint ECTRIMS-ACTRIMS meeting.
In a group of 3,249 subjects tracked for up to 5 years (74% female; mean age, 37.8 years), patients who were obese at diagnosis were 1.41 times more likely than normal-weight patients to reach an Expanded Disability Status Scale (EDSS) score of 3. About 35% of 355 obese subjects (body mass index > 30 kg/m2) reached that level versus 29% of 713 overweight patients (BMI, 25-30) and 28% of 1,475 normal-weight patients (BMI, 18.5-24.99).
Among subjects whose BMI category didn’t change over follow-up, those who were obese at diagnosis were more likely to develop cognitive worsening than those who weren’t obese (hazard ratio, 1.47, 95% confidence interval, 1.08-2.01).
Lars Alfredsson, PhD, a professor at the Karolinska Institutet, Stockholm, who presented the study findings, said in an interview that they fill a gap in knowledge about obesity and MS. “It is known that obesity around the age of 20 or in adolescence is a risk factor for developing MS. But much less is known in regard to progression, and the studies have been very inconclusive.”
The researchers tracked patients via the Swedish MS registry: 1,475 of normal weight, 713 overweight, and 355 obese. Before adjustment for factors such as age, gender, and baseline EDSS, obese subjects were 1.51 times more likely to reach EDSS score 3 than normal-weight subjects.
Obese subjects whose BMI level didn’t change over time were 1.70 times more likely than the nonobese to develop physical worsening as measured by an increased Multiple Sclerosis Impact Scale physical score of 7.5 points or more, and they were 1.36 times more likely to have psychological worsening as measured by increased MSIS-28 psychological score of 7.5 points or more.
Also, among subjects whose BMI didn’t change over time, the likelihood of cognitive disability worsening was 1.47 times higher among obese participants versus nonobese participants. Worsening was defined as an increased Symbol Digit Modalities Test score of 8 points or more.
The level of excess cognitive decline “will affect people significantly,” Dr. Alfredsson said.
While obesity can counterintuitively provide a protective effect in some diseases, he said there’s no sign of such an effect in the subjects.
As for limitations, Dr. Alfredsson noted in his presentation that BMI data is self-reported, and it’s possible that the researchers didn’t adjust their statistics to reflect important confounders.
A 2023 German study of outcomes in MS patients with obesity came to similar conclusions. It tracked 1,066 subjects for up to 6 years and found that “median time to reach EDSS 3 was 0.99 years for patients with BMI of 30 or higher and 1.46 years for nonobese patients. Risk to reach EDSS 3 over 6 years was significantly increased in patients with BMI of at least 30, compared with patients with BMI less than 30 after adjustment for sex, age, smoking (HR, 1.87; 95% CI, 1.3-2.6; P < .001), and independent of disease-modifying therapies.”
However, the German researchers found no link between obesity and higher levels of relapse, contrast-enhancing MRI lesions, or MRI T2 lesion burden.
Interpretation and commentary
Could obesity be causing worse outcomes? The new study doesn’t provide insight into cause and effect. However, obesity may speed up progression via low-grade inflammation, Dr. Alfredsson said.
What can clinicians do with the information from the study? If patients are obese, it can be a good idea to more carefully monitor them and use reliable tools to improve their progression, Dr. Alfredsson said.
In an interview, Michael D. Kornberg, MD, PhD, an assistant professor of neurology at Johns Hopkins University, Baltimore, who was not involved with the study, agreed with Dr. Alfredsson that other research has linked obesity early in life to higher rates of MS. He added that “a number of studies have shown that comorbidities in general are usually associated with a higher rate of disability.”
Dr. Kornberg said the new research is important, and he noted that it has a “robust” cohort because of its larger size.
Could patients with MS reverse the risk of progression and other poor outcomes by losing weight? “It’s hard to say,” Dr. Kornberg said. “We have to be cautious when we assume causation. There’s a plausible rationale that obesity might worsen progression in MS, but it could just be a marker of some other factor that reflects a different phenotype of MS.”
He doesn’t think it’s likely that weight loss would “dramatically reverse the biology of MS,” but he said reversing the obesity epidemic would still be a good thing. An interventional study could examine the effects of weight-loss intervention on disability measures, he said, “and that’s the next step.”
Also contacted for commentary, Adil Harroud, MD, a neurologist at McGill University who studies obesity in MS, said research suggests that “obesity seems to exacerbate MS disability. While some studies show no effect, the majority indicate a detrimental impact.”
However, “the effect of obesity on MS progression remains unclear. Animal studies suggest that shifts in immune cell subsets and functions may play a role, but the relevance to humans is yet to be determined,” he said.
Dr. Harroud, who did not take part in the new study, said it’s “one of the largest examining the impact of obesity on MS disability.” He added that “the cohort was relatively early in their disease course, suggesting that obesity impacts even the early stages of MS. This underscores the importance of obesity as a modifiable risk factor for disability accumulation.”
As for why obesity affects MS, he said one theory is that obesity plays a role through its impact on vitamin D levels. “However, using a genetic approach, we have demonstrated that, at least for MS risk, the effect of obesity is independent of vitamin D. This is also likely true for MS progression, as recent trials of vitamin D supplementation have not shown a meaningful impact on MS outcomes.”
According to Dr. Harroud, “other theories suggest that obesity leads to a pro-inflammatory immune shift. Additionally, it has been proposed that obesity may influence the response to disease-modifying therapy by reducing drug bioavailability, potentially necessitating weight-based dosing for some therapies.”
Dr. Alfredsson reported receiving grants from the Swedish Research Council, the Swedish Research Council for Health Working Life and Welfare, and the Swedish Brain Foundation and personal fees from Teva and Biogene Idec. Some of the other study authors reported various disclosures. Dr. Kornberg and Dr. Harroud reported no relevant disclosures.
This article was updated 10/20/23.
MILAN – Swedish researchers reported at the 9th Joint ECTRIMS-ACTRIMS meeting.
In a group of 3,249 subjects tracked for up to 5 years (74% female; mean age, 37.8 years), patients who were obese at diagnosis were 1.41 times more likely than normal-weight patients to reach an Expanded Disability Status Scale (EDSS) score of 3. About 35% of 355 obese subjects (body mass index > 30 kg/m2) reached that level versus 29% of 713 overweight patients (BMI, 25-30) and 28% of 1,475 normal-weight patients (BMI, 18.5-24.99).
Among subjects whose BMI category didn’t change over follow-up, those who were obese at diagnosis were more likely to develop cognitive worsening than those who weren’t obese (hazard ratio, 1.47, 95% confidence interval, 1.08-2.01).
Lars Alfredsson, PhD, a professor at the Karolinska Institutet, Stockholm, who presented the study findings, said in an interview that they fill a gap in knowledge about obesity and MS. “It is known that obesity around the age of 20 or in adolescence is a risk factor for developing MS. But much less is known in regard to progression, and the studies have been very inconclusive.”
The researchers tracked patients via the Swedish MS registry: 1,475 of normal weight, 713 overweight, and 355 obese. Before adjustment for factors such as age, gender, and baseline EDSS, obese subjects were 1.51 times more likely to reach EDSS score 3 than normal-weight subjects.
Obese subjects whose BMI level didn’t change over time were 1.70 times more likely than the nonobese to develop physical worsening as measured by an increased Multiple Sclerosis Impact Scale physical score of 7.5 points or more, and they were 1.36 times more likely to have psychological worsening as measured by increased MSIS-28 psychological score of 7.5 points or more.
Also, among subjects whose BMI didn’t change over time, the likelihood of cognitive disability worsening was 1.47 times higher among obese participants versus nonobese participants. Worsening was defined as an increased Symbol Digit Modalities Test score of 8 points or more.
The level of excess cognitive decline “will affect people significantly,” Dr. Alfredsson said.
While obesity can counterintuitively provide a protective effect in some diseases, he said there’s no sign of such an effect in the subjects.
As for limitations, Dr. Alfredsson noted in his presentation that BMI data is self-reported, and it’s possible that the researchers didn’t adjust their statistics to reflect important confounders.
A 2023 German study of outcomes in MS patients with obesity came to similar conclusions. It tracked 1,066 subjects for up to 6 years and found that “median time to reach EDSS 3 was 0.99 years for patients with BMI of 30 or higher and 1.46 years for nonobese patients. Risk to reach EDSS 3 over 6 years was significantly increased in patients with BMI of at least 30, compared with patients with BMI less than 30 after adjustment for sex, age, smoking (HR, 1.87; 95% CI, 1.3-2.6; P < .001), and independent of disease-modifying therapies.”
However, the German researchers found no link between obesity and higher levels of relapse, contrast-enhancing MRI lesions, or MRI T2 lesion burden.
Interpretation and commentary
Could obesity be causing worse outcomes? The new study doesn’t provide insight into cause and effect. However, obesity may speed up progression via low-grade inflammation, Dr. Alfredsson said.
What can clinicians do with the information from the study? If patients are obese, it can be a good idea to more carefully monitor them and use reliable tools to improve their progression, Dr. Alfredsson said.
In an interview, Michael D. Kornberg, MD, PhD, an assistant professor of neurology at Johns Hopkins University, Baltimore, who was not involved with the study, agreed with Dr. Alfredsson that other research has linked obesity early in life to higher rates of MS. He added that “a number of studies have shown that comorbidities in general are usually associated with a higher rate of disability.”
Dr. Kornberg said the new research is important, and he noted that it has a “robust” cohort because of its larger size.
Could patients with MS reverse the risk of progression and other poor outcomes by losing weight? “It’s hard to say,” Dr. Kornberg said. “We have to be cautious when we assume causation. There’s a plausible rationale that obesity might worsen progression in MS, but it could just be a marker of some other factor that reflects a different phenotype of MS.”
He doesn’t think it’s likely that weight loss would “dramatically reverse the biology of MS,” but he said reversing the obesity epidemic would still be a good thing. An interventional study could examine the effects of weight-loss intervention on disability measures, he said, “and that’s the next step.”
Also contacted for commentary, Adil Harroud, MD, a neurologist at McGill University who studies obesity in MS, said research suggests that “obesity seems to exacerbate MS disability. While some studies show no effect, the majority indicate a detrimental impact.”
However, “the effect of obesity on MS progression remains unclear. Animal studies suggest that shifts in immune cell subsets and functions may play a role, but the relevance to humans is yet to be determined,” he said.
Dr. Harroud, who did not take part in the new study, said it’s “one of the largest examining the impact of obesity on MS disability.” He added that “the cohort was relatively early in their disease course, suggesting that obesity impacts even the early stages of MS. This underscores the importance of obesity as a modifiable risk factor for disability accumulation.”
As for why obesity affects MS, he said one theory is that obesity plays a role through its impact on vitamin D levels. “However, using a genetic approach, we have demonstrated that, at least for MS risk, the effect of obesity is independent of vitamin D. This is also likely true for MS progression, as recent trials of vitamin D supplementation have not shown a meaningful impact on MS outcomes.”
According to Dr. Harroud, “other theories suggest that obesity leads to a pro-inflammatory immune shift. Additionally, it has been proposed that obesity may influence the response to disease-modifying therapy by reducing drug bioavailability, potentially necessitating weight-based dosing for some therapies.”
Dr. Alfredsson reported receiving grants from the Swedish Research Council, the Swedish Research Council for Health Working Life and Welfare, and the Swedish Brain Foundation and personal fees from Teva and Biogene Idec. Some of the other study authors reported various disclosures. Dr. Kornberg and Dr. Harroud reported no relevant disclosures.
This article was updated 10/20/23.
AT ECTRIMS 2023
Are they ‘antiobesity medications’ or ‘weight-loss drugs’?
A simple Google search for the terms “weight-loss pens,” “weight-loss drugs,” and “weight-loss medications” displays seven times more results than a search for terms like “antiobesity medications,” “antiobesity drugs,” or “drugs (or medications) to treat obesity.” The same search applied to academic databases yields the opposite results: fewer than 500 results for “weight-loss drugs/agents/medications” and 19,000 results for “antiobesity agents,” for example.
To highlight the importance of the language used to talk about obesity treatment, researchers affiliated with the Brazilian Society of Endocrinology and Metabolism (SBEM) and the Brazilian Association for the Study of Obesity and Metabolic Syndrome (ABESO) released a statement on the subject at the Brazilian Congress of Update in Endocrinology and Metabolism 2023. On the basis of the study by the ABESO and the SBEM, the statement proposes abandoning the use of the term “weight-loss medications” in scientific publications and, most importantly, in the media.
“Put together, we believe that the common use of the term ‘weight-loss medications’ by media and the public, as well as by doctors and the scientific community, contributes to stigma, and certainly that language matters,” study author Paulo Augusto Carvalho Miranda, MD, PhD, chair of SBEM, said in an interview.
“When we refer to these medications as ‘weight-loss drugs,’ we are using derogatory terms to refer to medications that were extensively studied before their launch onto the market and approved by a regulatory authority to treat a disease called obesity,” said study author Márcio Mancini, MD, PhD, deputy chair of the SBEM’s Obesity Department.
Beyond semantics
Another article published by this news organization presents the initiative of a global task force comprising 60 leaders in the clinical management of obesity, who proposed a new name for the disease. According to the leader of the project, Francesco Rubino, MD, “The word is so stigmatized, with so much misunderstanding and misperception, that some might say the only solution is to change the name.” Following this same logic, the authors of the Brazilian study believe that changing how we refer to medications may improve perceptions of health care professionals and patients toward prevention and treatment strategies for obesity.
According to Dr. Miranda, the first step is “remembering that how we refer to people, diseases, and treatments makes all the difference, especially in situations like obesity, a stigmatized disease loaded with misconceptions. It is not merely an issue of semantics, but also an issue of reducing the stigma surrounding the subject.”
According to Dr. Miranda, the primary purpose of the statement is to highlight the uniqueness of the situation and the importance of encouraging the use of the expressions “antiobesity medications” and “medications to treat obesity” to help reduce the stigma and improve adherence and persistence in obesity treatment.
Impact in practice
The statement also emphasizes that obesity pharmacotherapy is widely underused in patients with obesity and that, in the United States, it is prescribed only for approximately 3% of adults with the disease. Weight management programs for this patient population stress implementing lifestyle changes, and only 1.1% of participants are prescribed medications.
According to the statement, the term “weight-loss medications” contributes to the concept that their use has an aesthetic goal and can be consumed by anyone who desires to lose weight.
In addition to ensuring the correct use of language, Dr. Mancini adds that it is essential for doctors to seek and present pharmacologic treatment for obesity as something that will improve patient health. This means stressing that obesity can be controlled with a 10% loss in body weight, just as other chronic diseases, such as diabetes, can be controlled. Moreover, it is important to point out that medications also have a crucial role in optimizing weight maintenance in the long term.
Another issue Dr. Mancini raised is the prejudice that many doctors have against people with obesity. Health professionals should recognize they are also subject to weight bias and that the way they communicate with patients could have a profound effect on health-related outcomes.
“The stigma surrounding obesity can lead to bullying, even in the patient’s home by their relatives; this is very common. Weight stigma is so strong that it hinders patient health and decreases the likelihood of the patient seeking specialized care,” Dr. Mancini warned.
According to the authors, it is of utmost importance to understand that an individual should not be defined by his or disease (as by the use of the terms “obese” or “diabetic”) but rather understood to live with this disease (“individual with obesity” or “with diabetes”). Dr. Mancini suggests the following strategies that health care professionals can adopt while caring for patients with obesity:
- Speak to patients with empathy and respect, avoiding the use of judgmental words.
- Ask if they would like to discuss the “weight issue,” “BMI issue,” terms that are better received by the public, instead of saying “excess fat” or “excess weight.”
- If the patient agrees to talk about the subject, reinforce that this is a chronic health problem that requires longterm treatment and give him or her short, medium, and longterm options.
Lastly, the authors highlighted the importance of differentiating between regulatory agency–approved medications and over-the-counter drugs and supplements that are often sold as “weight-loss agents” and are responsible for an unacceptably high rate of emergency visits.
This article was translated from the Medscape Portuguese Edition. A version appeared on Medscape.com.
A simple Google search for the terms “weight-loss pens,” “weight-loss drugs,” and “weight-loss medications” displays seven times more results than a search for terms like “antiobesity medications,” “antiobesity drugs,” or “drugs (or medications) to treat obesity.” The same search applied to academic databases yields the opposite results: fewer than 500 results for “weight-loss drugs/agents/medications” and 19,000 results for “antiobesity agents,” for example.
To highlight the importance of the language used to talk about obesity treatment, researchers affiliated with the Brazilian Society of Endocrinology and Metabolism (SBEM) and the Brazilian Association for the Study of Obesity and Metabolic Syndrome (ABESO) released a statement on the subject at the Brazilian Congress of Update in Endocrinology and Metabolism 2023. On the basis of the study by the ABESO and the SBEM, the statement proposes abandoning the use of the term “weight-loss medications” in scientific publications and, most importantly, in the media.
“Put together, we believe that the common use of the term ‘weight-loss medications’ by media and the public, as well as by doctors and the scientific community, contributes to stigma, and certainly that language matters,” study author Paulo Augusto Carvalho Miranda, MD, PhD, chair of SBEM, said in an interview.
“When we refer to these medications as ‘weight-loss drugs,’ we are using derogatory terms to refer to medications that were extensively studied before their launch onto the market and approved by a regulatory authority to treat a disease called obesity,” said study author Márcio Mancini, MD, PhD, deputy chair of the SBEM’s Obesity Department.
Beyond semantics
Another article published by this news organization presents the initiative of a global task force comprising 60 leaders in the clinical management of obesity, who proposed a new name for the disease. According to the leader of the project, Francesco Rubino, MD, “The word is so stigmatized, with so much misunderstanding and misperception, that some might say the only solution is to change the name.” Following this same logic, the authors of the Brazilian study believe that changing how we refer to medications may improve perceptions of health care professionals and patients toward prevention and treatment strategies for obesity.
According to Dr. Miranda, the first step is “remembering that how we refer to people, diseases, and treatments makes all the difference, especially in situations like obesity, a stigmatized disease loaded with misconceptions. It is not merely an issue of semantics, but also an issue of reducing the stigma surrounding the subject.”
According to Dr. Miranda, the primary purpose of the statement is to highlight the uniqueness of the situation and the importance of encouraging the use of the expressions “antiobesity medications” and “medications to treat obesity” to help reduce the stigma and improve adherence and persistence in obesity treatment.
Impact in practice
The statement also emphasizes that obesity pharmacotherapy is widely underused in patients with obesity and that, in the United States, it is prescribed only for approximately 3% of adults with the disease. Weight management programs for this patient population stress implementing lifestyle changes, and only 1.1% of participants are prescribed medications.
According to the statement, the term “weight-loss medications” contributes to the concept that their use has an aesthetic goal and can be consumed by anyone who desires to lose weight.
In addition to ensuring the correct use of language, Dr. Mancini adds that it is essential for doctors to seek and present pharmacologic treatment for obesity as something that will improve patient health. This means stressing that obesity can be controlled with a 10% loss in body weight, just as other chronic diseases, such as diabetes, can be controlled. Moreover, it is important to point out that medications also have a crucial role in optimizing weight maintenance in the long term.
Another issue Dr. Mancini raised is the prejudice that many doctors have against people with obesity. Health professionals should recognize they are also subject to weight bias and that the way they communicate with patients could have a profound effect on health-related outcomes.
“The stigma surrounding obesity can lead to bullying, even in the patient’s home by their relatives; this is very common. Weight stigma is so strong that it hinders patient health and decreases the likelihood of the patient seeking specialized care,” Dr. Mancini warned.
According to the authors, it is of utmost importance to understand that an individual should not be defined by his or disease (as by the use of the terms “obese” or “diabetic”) but rather understood to live with this disease (“individual with obesity” or “with diabetes”). Dr. Mancini suggests the following strategies that health care professionals can adopt while caring for patients with obesity:
- Speak to patients with empathy and respect, avoiding the use of judgmental words.
- Ask if they would like to discuss the “weight issue,” “BMI issue,” terms that are better received by the public, instead of saying “excess fat” or “excess weight.”
- If the patient agrees to talk about the subject, reinforce that this is a chronic health problem that requires longterm treatment and give him or her short, medium, and longterm options.
Lastly, the authors highlighted the importance of differentiating between regulatory agency–approved medications and over-the-counter drugs and supplements that are often sold as “weight-loss agents” and are responsible for an unacceptably high rate of emergency visits.
This article was translated from the Medscape Portuguese Edition. A version appeared on Medscape.com.
A simple Google search for the terms “weight-loss pens,” “weight-loss drugs,” and “weight-loss medications” displays seven times more results than a search for terms like “antiobesity medications,” “antiobesity drugs,” or “drugs (or medications) to treat obesity.” The same search applied to academic databases yields the opposite results: fewer than 500 results for “weight-loss drugs/agents/medications” and 19,000 results for “antiobesity agents,” for example.
To highlight the importance of the language used to talk about obesity treatment, researchers affiliated with the Brazilian Society of Endocrinology and Metabolism (SBEM) and the Brazilian Association for the Study of Obesity and Metabolic Syndrome (ABESO) released a statement on the subject at the Brazilian Congress of Update in Endocrinology and Metabolism 2023. On the basis of the study by the ABESO and the SBEM, the statement proposes abandoning the use of the term “weight-loss medications” in scientific publications and, most importantly, in the media.
“Put together, we believe that the common use of the term ‘weight-loss medications’ by media and the public, as well as by doctors and the scientific community, contributes to stigma, and certainly that language matters,” study author Paulo Augusto Carvalho Miranda, MD, PhD, chair of SBEM, said in an interview.
“When we refer to these medications as ‘weight-loss drugs,’ we are using derogatory terms to refer to medications that were extensively studied before their launch onto the market and approved by a regulatory authority to treat a disease called obesity,” said study author Márcio Mancini, MD, PhD, deputy chair of the SBEM’s Obesity Department.
Beyond semantics
Another article published by this news organization presents the initiative of a global task force comprising 60 leaders in the clinical management of obesity, who proposed a new name for the disease. According to the leader of the project, Francesco Rubino, MD, “The word is so stigmatized, with so much misunderstanding and misperception, that some might say the only solution is to change the name.” Following this same logic, the authors of the Brazilian study believe that changing how we refer to medications may improve perceptions of health care professionals and patients toward prevention and treatment strategies for obesity.
According to Dr. Miranda, the first step is “remembering that how we refer to people, diseases, and treatments makes all the difference, especially in situations like obesity, a stigmatized disease loaded with misconceptions. It is not merely an issue of semantics, but also an issue of reducing the stigma surrounding the subject.”
According to Dr. Miranda, the primary purpose of the statement is to highlight the uniqueness of the situation and the importance of encouraging the use of the expressions “antiobesity medications” and “medications to treat obesity” to help reduce the stigma and improve adherence and persistence in obesity treatment.
Impact in practice
The statement also emphasizes that obesity pharmacotherapy is widely underused in patients with obesity and that, in the United States, it is prescribed only for approximately 3% of adults with the disease. Weight management programs for this patient population stress implementing lifestyle changes, and only 1.1% of participants are prescribed medications.
According to the statement, the term “weight-loss medications” contributes to the concept that their use has an aesthetic goal and can be consumed by anyone who desires to lose weight.
In addition to ensuring the correct use of language, Dr. Mancini adds that it is essential for doctors to seek and present pharmacologic treatment for obesity as something that will improve patient health. This means stressing that obesity can be controlled with a 10% loss in body weight, just as other chronic diseases, such as diabetes, can be controlled. Moreover, it is important to point out that medications also have a crucial role in optimizing weight maintenance in the long term.
Another issue Dr. Mancini raised is the prejudice that many doctors have against people with obesity. Health professionals should recognize they are also subject to weight bias and that the way they communicate with patients could have a profound effect on health-related outcomes.
“The stigma surrounding obesity can lead to bullying, even in the patient’s home by their relatives; this is very common. Weight stigma is so strong that it hinders patient health and decreases the likelihood of the patient seeking specialized care,” Dr. Mancini warned.
According to the authors, it is of utmost importance to understand that an individual should not be defined by his or disease (as by the use of the terms “obese” or “diabetic”) but rather understood to live with this disease (“individual with obesity” or “with diabetes”). Dr. Mancini suggests the following strategies that health care professionals can adopt while caring for patients with obesity:
- Speak to patients with empathy and respect, avoiding the use of judgmental words.
- Ask if they would like to discuss the “weight issue,” “BMI issue,” terms that are better received by the public, instead of saying “excess fat” or “excess weight.”
- If the patient agrees to talk about the subject, reinforce that this is a chronic health problem that requires longterm treatment and give him or her short, medium, and longterm options.
Lastly, the authors highlighted the importance of differentiating between regulatory agency–approved medications and over-the-counter drugs and supplements that are often sold as “weight-loss agents” and are responsible for an unacceptably high rate of emergency visits.
This article was translated from the Medscape Portuguese Edition. A version appeared on Medscape.com.
Inadequate sleep & obesity: Breaking the vicious cycle
Sleep is fundamental to overall health and longevity, with the average person spending about one-third of their life sleeping.1 Adequate sleep is critical for optimal cognition, memory consolidation, mood regulation, metabolism, appetite regulation, and immune and hormone functioning. According to the American Academy of Sleep Medicine and the Sleep Research Society, adults should sleep at least 7 hours per night on a regular basis “to promote optimal health.”2 Yet, between 2013 and 2020, only about 65% of adults in the United States were meeting this amount.3 Insufficient sleep is associated with an increased risk for chronic health conditions, including obesity, diabetes, cardiovascular diseases, and even premature death.4

In a population-based longitudinal study of sleep disorders, short sleep duration was associated with increased body mass index (BMI), low blood levels of leptin, and high ghrelin levels.5 In addition to physical impairments, poor sleep can impair cognitive performance and lead to vehicular accidents and increased accidents at work.4 The potential economic impact that this may have is significant, and includes increased costs and loss of productivity in the workplace.6
Many factors may contribute to short sleep duration: environment, mental and physical condition, and social influences such as occupation, family responsibilities, travel, group activities, and personal care. Furthermore, the rapidly evolving and developing media, communication, and entertainment industries are already strongly implicated in poor sleep quality and quantity, both contributing to excessive daytime sleepiness.7 Poor sleep quality is most notable in modern societies, and it correlates with the increasing prevalence of obesity, likely due to sleep’s effect on food consumption and physical activity.8 Optimizing a person’s sleep will improve overall health and longevity by inhibiting the development of chronic disease.
How insufficient sleep raises the risk for obesity
Not only is sleep beneficial for brain health, memory, learning, and growth, its effect on food consumption and physical activity likely correlates with the increased prevalence of obesity in modern society. Yet the optimal amount of sleep is controversial, and current recommendations of 7 or more hours of sleep per night for adults are derived from expert panels only.2 The recommended sleep duration for children is longer, and it varies by age.9 The quality of sleep and its impact on neuroendocrine hormones, not just the quantity of sleep, needs to be factored into these recommendations.
Sleep restriction activates the orexigenic system via the hormones leptin and ghrelin. These hormones control the food reward system, essentially increasing hunger and food intake. Leptin, created by white adipose tissue, is responsible for satiety and decreased food consumption.10 Ghrelin, made by oxyntic glands in the stomach, is responsible for the sensation of hunger.
In a 2004 study by Spiegel et al,11 leptin and ghrelin levels were measured during 2 days of sleep restriction (4 hours in bed) and sleep extension (10 hours in bed). Sleep restriction was associated with a decrease in leptin levels and an increase in ghrelin levels. The researchers reported that participants experienced an increase in hunger and appetite—especially for calorie-dense foods with high carbohydrate content.
Although research design has limitations with predominantly self-reported sleep data, studies have shown that short sleep time leads to increased food intake by increasing hunger signals and craving of unhealthy foods, and by providing more opportunities to eat while awake. It also may lead to decreased physical activity, creating a sedentary lifestyle that further encourages obesity.8 Reduced sleep is even correlated to decreased efficacy of weight-loss treatments.12
Continue to: Other sleep characteristics weakly correlated with obesity
Other sleep characteristics weakly correlated with obesity are sleep variability, timing, efficiency, quality, and daytime napping.8 Sleep variability causes dysregulation of eating patterns, leading to increased food intake. A shift to later sleep and waking times often results in higher consumption of calories after 8
Poor sleep efficiency and quality decreases N3-stage (deep non-REM) sleep, affects the autonomic nervous system, and has been associated with increased abdominal obesity. Daytime napping, which can cause irregular circadian rhythms and sleep schedules, is associated with increased obesity.15 Thus, each component of sleep needs to be assessed to promote optimal regulation of the orexigenic system.
Another study showed that inadequate sleep not only promotes unhealthy lifestyle habits that can lead to obesity but also decreases the ability to lose weight.16 This small study with 10 overweight patients provided its subjects with a controlled caloric intake over 2 weeks. Patients spent two 14-day periods 3 months apart in the laboratory, divided into 2 time-in-bed arms of 8.5 and 5.5 hours per night. Neuroendocrine changes caused by decreased sleep were associated with a significant lean body mass loss while conserving energy-dense fat.16 This study highlights the importance of sleep hygiene counseling when developing a weight-management plan with patients.
Sleep, and its many components, play an integral role in the prevention and treatment of obesity.17 Poor sleep will increase the risk for obesity and hinder its treatment. Therefore, sleep quality and duration are vital components of obesity management.
The sleep–obesity link in children and the elderly
Childhood obesity is linked to several chronic diseases in adulthood, including type 2 diabetes, cardiovascular disease, nonalcoholic fatty liver disease, asthma, and obstructive sleep apnea (OSA).18 According to 2017-2018 NHANES (National Health and Nutrition Examination Surveys) data, obesity (BMI ≥ 95th percentile) prevalence among children and adolescents was reported at 19.3% and severe obesity (BMI ≥ 120% of the 95th percentile) at 6.1%. Pediatric overweight prevalence (≥ 85th percentile and < 95th percentile) was 16.1%.19
Continue to: Although poor sleep is associated...
Although poor sleep is associated with increased risk for obesity, there is no proven cause-effect relationship.20 Nutrition and physical activity have been identified as 2 critical factors in childhood obesity, but sleep health also needs to be investigated. Shorter sleep duration is strongly associated with the development of obesity. Furthermore, children with obesity are more likely to have shorter sleep duration.21 A short sleep duration alters plasma levels of insulin, low-density lipoprotein, and high-sensitivity C-reactive protein. It is associated with lower diet quality, an increased intake of nutrient-poor foods, and a lower intake of vegetables and fruits.22 Recent studies have shown that interventions to promote earlier bedtimes can improve sleep duration in children.
Older adults have many sleeping issues, including insomnia, circadian rhythm sleep-wake disorders, sleep-related movement disorders, and sleep-breathing disorders. Additionally, the older population has increased sleep latency, decreased sleep efficiency and total sleep time, decreased REM sleep, more frequent nighttime awakenings, and more daytime napping.23 The increased sleep disturbance with age is mainly related to higher risk factors for sleep disorders than the aging process itself. Sleeping 5 or fewer hours is associated with an increased risk for obesity and central abdominal fat compared with those who sleep 7 to 8 hours per night.24 Similar to children and youth, older adults also show a strong correlation between inadequate sleep and obesity.24
The consequence: A vicious cycle
Obesity in turn leads to shorter sleep duration and more disruptions. This negatively affects the orexigenic system, and the resulting hormonal derangement promotes worsening obesity. It is a cycle of poor sleep causing obesity and obesity causing poor sleep. Insomnia, in combination with shorter (and longer) sleep times, also has been linked with obesity.25 These patients experience more daytime sleepiness, fatigue, and nighttime sleep disturbances, all correlated with decreased quality of life and higher prevalence of medical comorbidities.8,26 Additional comorbidities secondary to obesity, including gastroesophageal reflux, depression, and asthma, also have been linked to sleep disturbances.8
OSA is a common sleep complication associated with obesity. With the increasing prevalence of obesity, the prevalence of OSA is rising.8,27 Factors that heighten the risk for OSA are male sex, age 40 to 70 years, postmenopausal status, elevated BMI, and craniofacial and upper airway abnormality.28 However, the US Preventive Services Task Force found insufficient evidence to screen for or treat OSA in asymptomatic adults.28 Signs and symptoms of OSA include nighttime awakenings with choking, loud snoring, and feeling unrefreshed after sleep.29
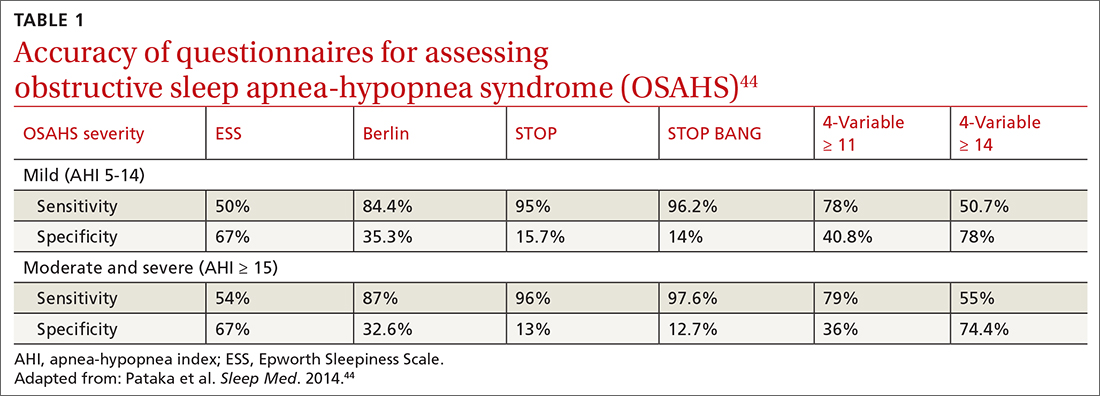
OSA is caused by the intermittent narrowing and obstruction of the pharyngeal airway due to anatomical and structural irregularities or neuromuscular impairments. Untreated OSA is associated with cardiovascular disease and cardiac arrhythmias such as atrial fibrillation. Even with this correlation between obesity and sleep, it is estimated that 80% of OSA remains undiagnosed.30 Approximately half of primary care clinicians do not screen at-risk patients for OSA, and 90% do not use validated OSA screening tools.31 Screening tools that have been validated are the STOP, STOP-BANG, Epworth Sleepiness Scale, and 4-Variable Screening Tool. However, the US Department of Veterans Affairs and the US Department of Defense have a more recent guideline recommending STOP as an easier-to-administer screen for OSA.32 A positive result with a screening tool should be confirmed with polysomnography.32
Continue to: Intervention for OSA
Intervention for OSA. The longest randomized controlled study to date, Sleep AHEAD, evaluated over a period of 10 years the effect of weight loss on OSA severity achieved with either an intensive lifestyle intervention (ILI) or with diabetes support and education (DSE).33 OSA severity is rated on an Apnea-Hypopnea Index (AHI), with scores reflecting the number of sleep apnea events per hour. This study demonstrated that weight loss was associated with decreased OSA severity. At 4-year follow-up, the greater the weight loss with ILI intervention, the lower the patients’ OSA severity scores. The study found an average decrease in AHI of 0.68 events per hour for every kilogram of weight loss in the ILI group (P < .0001).33,34 Over the follow-up visits, the ILI participants had 7.4 events per hour, a more significantly reduced AHI than the DSE participants (P < .0001).33,34
Additionally, a small cohort of study participants achieved OSA remission (ILI, 34.4%; DSE, 22.2%), indicated by a low AHI score (< 5 events per hour). At the conclusion of the study, OSA severity decreased to a greater degree with ILI intervention.33,34
Alcohol and drug use can negatively influence sleep patterns and obesity. Higher alcohol consumption is associated with poorer sleep quality and higher chances of developing short sleep duration and snoring.35 Alcohol, a muscle relaxant, causes upper airway narrowing and reduced tongue muscle tone, thereby increasing snoring and OSA as demonstrated by increased AHI on polysomnography after alcohol intake. Alcohol also changes sleep architecture by increasing slow-wave sleep, decreasing REM sleep duration, and increasing sleep arousal in the second half of the night.36 Disrupted circadian rhythm after alcohol consumption was correlated with increased adenosine neurotransmitters derived from ethanol metabolism.37 Alcohol dependence may be related to other psychiatric symptoms, and chronic alcohol use eventually alters sleep mechanisms leading to persistent insomnia, further perpetuating adverse outcomes such as suicidal ideation.36 There are positive associations between beer drinking and measures of abdominal adiposity in men, and “the combination of short sleep duration [and] disinhibited eating … is associated with greater alcohol intake and excess weight.”38
Therefore, counsel patients to avoid alcohol since it is a modifiable risk factor with pervasive adverse health effects.
Many drugs have a profound effect on sleep patterns. Illicit drug use in particular can affect the brain’s neurotransmitter serotonin system. For example, ecstasy users have an increased risk for OSA.39 People with cocaine and heroin use disorder tend to have more sleep-maintenance insomnia.40
Continue to: In contrast, those with alcohol...
In contrast, those with alcohol or cannabis use disorder tend to have more sleep-onset insomnia.40 Not only do illicit drugs interrupt sleep, but daily tobacco use also has been correlated with increased insomnia and shorter sleep duration since nicotine is a stimulant.41
Insomnia is commonly treated with sedative antidepressants and hypnotics—eg, mirtazapine and olanzapine—that contribute to weight gain.42 In addition, other common pharmaceuticals used for sleep disorders, such as diphenhydramine, have sedative properties and tend to lead to weight gain.43 Because so many medications affect sleep and weight, carefully review patients’ medication lists and switch offending agents to weight-neutral drugs if possible.
Treatment and tools to improve sleep in patients with obesity
Given the strong correlation between obesity and sleep disorders, validated screening tools should be used to assess sleep quality, including onset and potential symptoms associated with poor sleep (TABLE 144). For weight management to succeed in patients with obesity, it is crucial to address sleep in addition to nutrition and physical activity.17,45
Physical activity has many benefits to overall health, especially for chronic diseases such as type 2 diabetes and hypertension. The Centers for Disease Control and Prevention recommends at least 150 minutes of moderate-intensity aerobic activity or 75 minutes of vigorous-intensity aerobic exercise per week in addition to muscle-strengthening activities 2 or more days per week.46 However, approximately 300 minutes of moderate-
Physical activity and diet in combination are vital, but diet restriction has a more substantial effect on weight loss than physical activity alone.48 Still, physical activity is essential in helping maintain and prevent weight regain.
Continue to: Nonpharmacologic interventions
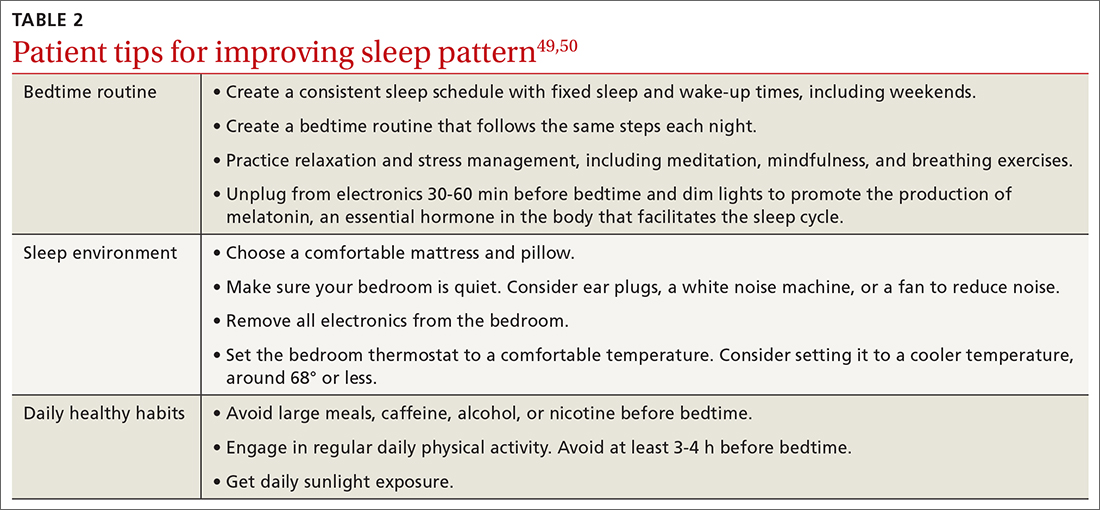
Nonpharmacologic interventions include promoting greater sleep quality and quantity by emphasizing good sleep hygiene practices. Developing a practical and effective bedtime routine, creating a quiet sleep environment, and practicing healthy daily habits are essential components to sleep hygiene (TABLE 249,50). Relaxation techniques and cognitive behavioral therapy (CBT) also can help. CBT for insomnia (CBT-I) is the first-line intervention for chronic insomnia.51 Sleep restriction is a type of CBT used to treat insomnia, encouraging short-term sleep loss in the hopes of improving insomnia. A trial by Logue et al showed that patients with overweight and obesity randomized to undergo CBT with better sleep hygiene (nonpharmacologic) interventions had a greater mean weight loss percentage (5% vs 2%; P = .04) than did those who received CBT alone.52
Eastern medicine including herbal interventions lack evidence of efficacy and safety. Further studies need to be done on the effects that chamomile, kava, valerian root (Valeriana officinalis), tryptophan, and Wu Ling (from mycelia Xylaria nigripes) might have on sleep.53
Proceed cautiously with medication. The American College of Physicians recommends a shared decision-making approach when considering pharmacologic therapy for chronic insomnia and the American Academy of Sleep Medicine (AASM) offers guidance on options.51,54 However, the evidence behind AASM sleep pharmacologic recommendations is weak, implying a lesser degree of confidence in the outcome and, therefore, in its appropriateness. Thus, it falls upon the clinician and patient to weigh the benefits and burdens of the pharmacologic treatments of insomnia. If indicated, medications suggested to treat sleep onset and sleep maintenance insomnia are eszopiclone, zolpidem, and temazepam. Zaleplon, triazolam, and ramelteon may improve sleep initiation. Suvorexant and doxepin are used for sleep-maintenance insomnia.54 Exploring patient preferences, cost of treatment, health care options, and available resources should all be considered.
CORRESPONDENCE
Ecler Ercole Jaqua, MD, MBA, FAAFP, AGSF, FACLM, DipABOM, Loma Linda University Health, 25455 Barton Road, Suite 206A, Loma Linda, CA 92354; [email protected]
1. Aminoff MJ, Boller F, Swaab DF. We spend about one-third of our life either sleeping or attempting to do so. Handb Clin Neurol. 2011;98:vii. doi: 10.1016/B978-0-444-52006-7.00047-2
2. Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
3. CDC. Sleep and sleep disorders, adults. Accessed September 21, 2023. www.cdc.gov/sleep/data-and-statistics/adults.html
4. Chattu VK, Manzar MD, Kumary S. The global problem of insufficient sleep and its serious public health implications. Healthcare (Basel). 2019;7:1. doi: 10.3390/healthcare7010001
5. Taheri S, Lin L, Austin D, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062
6. Hafner M, Stepanek M, Taylor J, et al. Why sleep matters—the economic costs of insufficient sleep. Rand Health Q. 2017;6:11.
7. Hisler G, Twenge JM, Krizan Z. Associations between screen time and short sleep duration among adolescents varies by media type: evidence from a cohort study. Sleep Med. 2020;66:92-102. doi: 10.1016/j.sleep.2019.08.007
8. Ogilvie RP, Patel SR. The epidemiology of sleep and obesity. Sleep Health. 2017;3:383-388. doi: 10.1016/j.sleh.2017.07.013
9. CDC. Sleep and sleep disorders: How much sleep do I need? Accessed September 21, 2023. www.cdc.gov/sleep/about_sleep/how_much_sleep.html
10. van Egmond LT, Meth EMS, Engström J, et al. Effects of acute sleep loss on leptin, ghrelin, and adiponectin in adults with healthy weight and obesity: a laboratory study. Obesity (Silver Spring). 2023;31:635-641. doi: 10.1002/oby.23616
11. Spiegel K, Tasali E, Penev P, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846-850. doi: 10.7326/0003-4819-141-11-200412070-00008
12. Antza C, Kostopoulos G, Mostafa S, et al. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol. 2021;252:125-141. doi: 10.1530/JOE-21-0155
13. Baron KG, Reid KJ, Kern AS, et al. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring). 2011;19:1374-1381. doi: 10.1038/oby.2011.100
14. Liu XY, Zheng CL, Xu C, et al. Nighttime snacking is associated with risk of obesity and hyperglycemia in adults: a cross-sectional survey from Chinese adult teachers J Biomed Res. 2017;31:541-547. doi: 10.7555/JBR.31.20160083
15. Cai Z, Yang Y, Zhang J, et al. The relationship between daytime napping and obesity: a systematic review and meta-analysis. Sci Rep. 2023.13:12124. doi: 10.1038/s41598-023-37883-7
16. Nedeltcheva AV, Kilkus JM, Imperial J, et al. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435-441. doi: 10.7326/0003-4819-153-7-201010050-00006
17. Chaput JP, Tremblay A. Adequate sleep to improve the treatment of obesity. CMAJ. 2012;184:1975-1976. doi: 10.1503/cmaj.120876
18. Kelsey MM, Zaepfel A, Bjornstad P, et al. Age-related consequences of childhood obesity. Gerontology. 2014;60:222-228. doi: 10.1159/000356023
19. Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2-19 years: United States, 1963-1965 through 2017-2018. National Center for Health Statistics Health E-Stats. Updated January 29, 2021. Accessed September 21, 2021. www.cdc.gov/nchs/data/hestat/obesity-child-17-18/overweight-obesity-child-H.pdf
20. Fatima Y, Doi SAR, Mamun AA. Sleep quality and obesity in young subjects: a meta-analysis. Obes Rev. 2016;17:1154-1166. doi: 10.1111/obr.12444
21. Gohil A, Hannon TS. Poor sleep and obesity: concurrent epidemics in adolescent youth. Front Endocrinol. 2018;9:364. doi: 10.3389/fendo.2018.00364
22. Golley RK, Maher CA, Matricciani L, et al. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes (Lond). 2013;37:546-551. doi: 10.1038/ijo.2012.212
23. Alessi CA. Sleep issues. In: Harper GM, Lyons WL, Potter JF, eds. Geriatrics Review Syllabus (GRS 10). Updated January 2021. Accessed August 29, 2023. http://geriatricscareonline.org
24. Patel SR, Blackwell T, Redline S, et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond). 2008;32:1825-1834. doi: 10.1038/ijo.2008.198
25. Cai GH, Theorell-Haglöw J, Janson C, et al. Insomnia symptoms and sleep duration and their combined effects in relation to associations with obesity and central obesity. Sleep Med. 2018;46:81-87. doi: 10.1016/j.sleep.2018.03.009
26. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14:402-412. doi: 10.1097/MCO.0b013 e3283479109
27. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population–a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311-1322. doi: 10.3978/j.issn.2072-1439.2015.06.11
28. USPSTF. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for obstructive sleep apnea in adults: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:407-414. doi: 10.1001/jama.2016.20325
29. Goyal M, Johnson J. Obstructive sleep apnea diagnosis and management. Mo Med. 2017;114:120-124.
30. American Academy of Sleep Medicine. Hidden health crisis costing America billions: underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. 2016. Accessed September 25, 2023. https://aasm.org/wp-content/uploads/2017/10/sleep-apnea-economic-crisis.pdf
31. Devaraj, NK. Knowledge, attitude, and practice regarding obstructive sleep apnea among primary care physicians. Sleep Breath. 2020;24:1581-1590. doi: 10.1007/s11325-020-02040-1
32. Mysliwiec V, Martin JL, Ulmer CS, et al. The management of chronic insomnia disorder and obstructive sleep apnea: synopsis of the 2019 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guidelines. Ann Intern Med. 2020;172:325-336. doi: 10.7326/M19-3575
33. Kuna ST, Reboussin DM, Strotmeyer ES, et al. Effects of weight loss on obstructive sleep apnea severity. Ten-year results of the Sleep AHEAD study. Am J Respir Crit Care Med. 2021;203:221-229. doi: 10.1164/rccm.201912-2511OC
34. St-Onge MP, Tasali E. Weight loss is integral to obstructive sleep apnea management. Ten-year follow-up in Sleep AHEAD. Am J Respir Crit Care Med. 2021;203:161-162. doi: 10.1164/rccm.202007-2906ED
35. Zheng D, Yuan X, Ma C, et al. Alcohol consumption and sleep quality: a community-based study. Public Health Nutr. 2021;24:4851-4858. doi: 10.1017/S1368980020004553
36. Chakravorty S, Chaudhary NS, Brower KJ. Alcohol dependence and its relationship with insomnia and other sleep disorders. Alcohol Clin Exp Res. 2016;40:2271-2282. doi: 10.1111/acer.13217
37. Elmenhorst EM, Elmenhorst D, Benderoth S, et al. Cognitive impairments by alcohol and sleep deprivation indicate trait characteristics and a potential role for adenosine A1 receptors. Proc Natl Acad Sci U S A. 2018;115:8009-8014. doi: 10.1073/pnas.1803770115
38. Traversy G, Chaput JP. Alcohol consumption and obesity: an update. Curr Obes Rep. 2015;4:122-130. doi: 10.1007/s13679-014-0129-4
39. McCann UD, Sgambati FP, Schwartz AR, et al. Sleep apnea in young abstinent recreational MDMA (“ecstasy”) consumers. Neurology. 2009;73:2011-2017. doi: 10.1212/WNL.0b013e3181c51a62
40. Grau-López L, Grau-López L, Daigre C, et al. Insomnia symptoms in patients with substance use disorders during detoxification and associated clinical features. Front Psychiatry. 2020;11:540022. doi: 10.3389/fpsyt.2020.540022
41. Boehm MA, Lei QM, Lloyd RM, et al. Depression, anxiety, and tobacco use: overlapping impediments to sleep in a national sample of college students. J Am Coll Health. 2016;64:565-574. doi: 10.1080/07448481.2016.1205073
42. Gracious BL, Meyer AE. Psychotropic-induced weight gain and potential pharmacologic treatment strategies. Psychiatry (Edgmont). 2005;2:36-42.
43. Ratliff JC, Barber JA, Palmese LB, et al. Association of prescription H1 antihistamine use with obesity: results from the National Health and Nutrition Examination Survey. Obesity (Silver Spring). 2010;18:2398-2400. doi: 10.1038/oby.2010.176
44. Pataka A, Daskalopoulou E, Kalamaras G, et al. Evaluation of five different questionnaires for assessing sleep apnea syndrome in a sleep clinic. Sleep Med. 2014;15:776-781. doi: 10.1016/j.sleep.2014.03.012
45. Kline CE, Chasens ER, Bizhanova Z, et al. The association between sleep health and weight change during a 12-month behavioral weight loss intervention. Int J Obes (Lond). 2021;45:639-649. doi: 10.1038/s41366-020-00728-8
46. CDC. How much physical activity do adults need? Accessed August 23, 2023. www.cdc.gov/physicalactivity/basics/adults/index.htm
47. Flack KD, Hays HM, Moreland J, et al. Exercise for weight loss: further evaluating energy compensation with exercise. Med Sci Sports Exerc. 2020;52:2466-2475. doi: 10.1249/MSS.0000000000002376
48. Swift DL, Johannsen NM, Lavie CJ, et al. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56:441-447. doi: 10.1016/j.pcad.2013.09.012
49. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36. doi: 10.1016/j.smrv.2014.10.001
50. CDC. Tips for better sleep. 2022. Accessed August 4, 2023. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html
51. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133. doi: 10.7326/M15-2175
52. Logue EE, Bourguet CC, Palmieri PA, et al. The better weight-better sleep study: a pilot intervention in primary care. Am J Health Behav. 2012;36:319-334. doi: 10.5993/AJHB.36.3.4
53. Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1-12. doi: 10.1016/j.smrv.2014.12.003
54. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
Sleep is fundamental to overall health and longevity, with the average person spending about one-third of their life sleeping.1 Adequate sleep is critical for optimal cognition, memory consolidation, mood regulation, metabolism, appetite regulation, and immune and hormone functioning. According to the American Academy of Sleep Medicine and the Sleep Research Society, adults should sleep at least 7 hours per night on a regular basis “to promote optimal health.”2 Yet, between 2013 and 2020, only about 65% of adults in the United States were meeting this amount.3 Insufficient sleep is associated with an increased risk for chronic health conditions, including obesity, diabetes, cardiovascular diseases, and even premature death.4

In a population-based longitudinal study of sleep disorders, short sleep duration was associated with increased body mass index (BMI), low blood levels of leptin, and high ghrelin levels.5 In addition to physical impairments, poor sleep can impair cognitive performance and lead to vehicular accidents and increased accidents at work.4 The potential economic impact that this may have is significant, and includes increased costs and loss of productivity in the workplace.6
Many factors may contribute to short sleep duration: environment, mental and physical condition, and social influences such as occupation, family responsibilities, travel, group activities, and personal care. Furthermore, the rapidly evolving and developing media, communication, and entertainment industries are already strongly implicated in poor sleep quality and quantity, both contributing to excessive daytime sleepiness.7 Poor sleep quality is most notable in modern societies, and it correlates with the increasing prevalence of obesity, likely due to sleep’s effect on food consumption and physical activity.8 Optimizing a person’s sleep will improve overall health and longevity by inhibiting the development of chronic disease.
How insufficient sleep raises the risk for obesity
Not only is sleep beneficial for brain health, memory, learning, and growth, its effect on food consumption and physical activity likely correlates with the increased prevalence of obesity in modern society. Yet the optimal amount of sleep is controversial, and current recommendations of 7 or more hours of sleep per night for adults are derived from expert panels only.2 The recommended sleep duration for children is longer, and it varies by age.9 The quality of sleep and its impact on neuroendocrine hormones, not just the quantity of sleep, needs to be factored into these recommendations.
Sleep restriction activates the orexigenic system via the hormones leptin and ghrelin. These hormones control the food reward system, essentially increasing hunger and food intake. Leptin, created by white adipose tissue, is responsible for satiety and decreased food consumption.10 Ghrelin, made by oxyntic glands in the stomach, is responsible for the sensation of hunger.
In a 2004 study by Spiegel et al,11 leptin and ghrelin levels were measured during 2 days of sleep restriction (4 hours in bed) and sleep extension (10 hours in bed). Sleep restriction was associated with a decrease in leptin levels and an increase in ghrelin levels. The researchers reported that participants experienced an increase in hunger and appetite—especially for calorie-dense foods with high carbohydrate content.
Although research design has limitations with predominantly self-reported sleep data, studies have shown that short sleep time leads to increased food intake by increasing hunger signals and craving of unhealthy foods, and by providing more opportunities to eat while awake. It also may lead to decreased physical activity, creating a sedentary lifestyle that further encourages obesity.8 Reduced sleep is even correlated to decreased efficacy of weight-loss treatments.12
Continue to: Other sleep characteristics weakly correlated with obesity
Other sleep characteristics weakly correlated with obesity are sleep variability, timing, efficiency, quality, and daytime napping.8 Sleep variability causes dysregulation of eating patterns, leading to increased food intake. A shift to later sleep and waking times often results in higher consumption of calories after 8
Poor sleep efficiency and quality decreases N3-stage (deep non-REM) sleep, affects the autonomic nervous system, and has been associated with increased abdominal obesity. Daytime napping, which can cause irregular circadian rhythms and sleep schedules, is associated with increased obesity.15 Thus, each component of sleep needs to be assessed to promote optimal regulation of the orexigenic system.
Another study showed that inadequate sleep not only promotes unhealthy lifestyle habits that can lead to obesity but also decreases the ability to lose weight.16 This small study with 10 overweight patients provided its subjects with a controlled caloric intake over 2 weeks. Patients spent two 14-day periods 3 months apart in the laboratory, divided into 2 time-in-bed arms of 8.5 and 5.5 hours per night. Neuroendocrine changes caused by decreased sleep were associated with a significant lean body mass loss while conserving energy-dense fat.16 This study highlights the importance of sleep hygiene counseling when developing a weight-management plan with patients.
Sleep, and its many components, play an integral role in the prevention and treatment of obesity.17 Poor sleep will increase the risk for obesity and hinder its treatment. Therefore, sleep quality and duration are vital components of obesity management.
The sleep–obesity link in children and the elderly
Childhood obesity is linked to several chronic diseases in adulthood, including type 2 diabetes, cardiovascular disease, nonalcoholic fatty liver disease, asthma, and obstructive sleep apnea (OSA).18 According to 2017-2018 NHANES (National Health and Nutrition Examination Surveys) data, obesity (BMI ≥ 95th percentile) prevalence among children and adolescents was reported at 19.3% and severe obesity (BMI ≥ 120% of the 95th percentile) at 6.1%. Pediatric overweight prevalence (≥ 85th percentile and < 95th percentile) was 16.1%.19
Continue to: Although poor sleep is associated...
Although poor sleep is associated with increased risk for obesity, there is no proven cause-effect relationship.20 Nutrition and physical activity have been identified as 2 critical factors in childhood obesity, but sleep health also needs to be investigated. Shorter sleep duration is strongly associated with the development of obesity. Furthermore, children with obesity are more likely to have shorter sleep duration.21 A short sleep duration alters plasma levels of insulin, low-density lipoprotein, and high-sensitivity C-reactive protein. It is associated with lower diet quality, an increased intake of nutrient-poor foods, and a lower intake of vegetables and fruits.22 Recent studies have shown that interventions to promote earlier bedtimes can improve sleep duration in children.
Older adults have many sleeping issues, including insomnia, circadian rhythm sleep-wake disorders, sleep-related movement disorders, and sleep-breathing disorders. Additionally, the older population has increased sleep latency, decreased sleep efficiency and total sleep time, decreased REM sleep, more frequent nighttime awakenings, and more daytime napping.23 The increased sleep disturbance with age is mainly related to higher risk factors for sleep disorders than the aging process itself. Sleeping 5 or fewer hours is associated with an increased risk for obesity and central abdominal fat compared with those who sleep 7 to 8 hours per night.24 Similar to children and youth, older adults also show a strong correlation between inadequate sleep and obesity.24
The consequence: A vicious cycle
Obesity in turn leads to shorter sleep duration and more disruptions. This negatively affects the orexigenic system, and the resulting hormonal derangement promotes worsening obesity. It is a cycle of poor sleep causing obesity and obesity causing poor sleep. Insomnia, in combination with shorter (and longer) sleep times, also has been linked with obesity.25 These patients experience more daytime sleepiness, fatigue, and nighttime sleep disturbances, all correlated with decreased quality of life and higher prevalence of medical comorbidities.8,26 Additional comorbidities secondary to obesity, including gastroesophageal reflux, depression, and asthma, also have been linked to sleep disturbances.8
OSA is a common sleep complication associated with obesity. With the increasing prevalence of obesity, the prevalence of OSA is rising.8,27 Factors that heighten the risk for OSA are male sex, age 40 to 70 years, postmenopausal status, elevated BMI, and craniofacial and upper airway abnormality.28 However, the US Preventive Services Task Force found insufficient evidence to screen for or treat OSA in asymptomatic adults.28 Signs and symptoms of OSA include nighttime awakenings with choking, loud snoring, and feeling unrefreshed after sleep.29
OSA is caused by the intermittent narrowing and obstruction of the pharyngeal airway due to anatomical and structural irregularities or neuromuscular impairments. Untreated OSA is associated with cardiovascular disease and cardiac arrhythmias such as atrial fibrillation. Even with this correlation between obesity and sleep, it is estimated that 80% of OSA remains undiagnosed.30 Approximately half of primary care clinicians do not screen at-risk patients for OSA, and 90% do not use validated OSA screening tools.31 Screening tools that have been validated are the STOP, STOP-BANG, Epworth Sleepiness Scale, and 4-Variable Screening Tool. However, the US Department of Veterans Affairs and the US Department of Defense have a more recent guideline recommending STOP as an easier-to-administer screen for OSA.32 A positive result with a screening tool should be confirmed with polysomnography.32
Continue to: Intervention for OSA
Intervention for OSA. The longest randomized controlled study to date, Sleep AHEAD, evaluated over a period of 10 years the effect of weight loss on OSA severity achieved with either an intensive lifestyle intervention (ILI) or with diabetes support and education (DSE).33 OSA severity is rated on an Apnea-Hypopnea Index (AHI), with scores reflecting the number of sleep apnea events per hour. This study demonstrated that weight loss was associated with decreased OSA severity. At 4-year follow-up, the greater the weight loss with ILI intervention, the lower the patients’ OSA severity scores. The study found an average decrease in AHI of 0.68 events per hour for every kilogram of weight loss in the ILI group (P < .0001).33,34 Over the follow-up visits, the ILI participants had 7.4 events per hour, a more significantly reduced AHI than the DSE participants (P < .0001).33,34
Additionally, a small cohort of study participants achieved OSA remission (ILI, 34.4%; DSE, 22.2%), indicated by a low AHI score (< 5 events per hour). At the conclusion of the study, OSA severity decreased to a greater degree with ILI intervention.33,34
Alcohol and drug use can negatively influence sleep patterns and obesity. Higher alcohol consumption is associated with poorer sleep quality and higher chances of developing short sleep duration and snoring.35 Alcohol, a muscle relaxant, causes upper airway narrowing and reduced tongue muscle tone, thereby increasing snoring and OSA as demonstrated by increased AHI on polysomnography after alcohol intake. Alcohol also changes sleep architecture by increasing slow-wave sleep, decreasing REM sleep duration, and increasing sleep arousal in the second half of the night.36 Disrupted circadian rhythm after alcohol consumption was correlated with increased adenosine neurotransmitters derived from ethanol metabolism.37 Alcohol dependence may be related to other psychiatric symptoms, and chronic alcohol use eventually alters sleep mechanisms leading to persistent insomnia, further perpetuating adverse outcomes such as suicidal ideation.36 There are positive associations between beer drinking and measures of abdominal adiposity in men, and “the combination of short sleep duration [and] disinhibited eating … is associated with greater alcohol intake and excess weight.”38
Therefore, counsel patients to avoid alcohol since it is a modifiable risk factor with pervasive adverse health effects.
Many drugs have a profound effect on sleep patterns. Illicit drug use in particular can affect the brain’s neurotransmitter serotonin system. For example, ecstasy users have an increased risk for OSA.39 People with cocaine and heroin use disorder tend to have more sleep-maintenance insomnia.40
Continue to: In contrast, those with alcohol...
In contrast, those with alcohol or cannabis use disorder tend to have more sleep-onset insomnia.40 Not only do illicit drugs interrupt sleep, but daily tobacco use also has been correlated with increased insomnia and shorter sleep duration since nicotine is a stimulant.41
Insomnia is commonly treated with sedative antidepressants and hypnotics—eg, mirtazapine and olanzapine—that contribute to weight gain.42 In addition, other common pharmaceuticals used for sleep disorders, such as diphenhydramine, have sedative properties and tend to lead to weight gain.43 Because so many medications affect sleep and weight, carefully review patients’ medication lists and switch offending agents to weight-neutral drugs if possible.
Treatment and tools to improve sleep in patients with obesity
Given the strong correlation between obesity and sleep disorders, validated screening tools should be used to assess sleep quality, including onset and potential symptoms associated with poor sleep (TABLE 144). For weight management to succeed in patients with obesity, it is crucial to address sleep in addition to nutrition and physical activity.17,45

Physical activity has many benefits to overall health, especially for chronic diseases such as type 2 diabetes and hypertension. The Centers for Disease Control and Prevention recommends at least 150 minutes of moderate-intensity aerobic activity or 75 minutes of vigorous-intensity aerobic exercise per week in addition to muscle-strengthening activities 2 or more days per week.46 However, approximately 300 minutes of moderate-
Physical activity and diet in combination are vital, but diet restriction has a more substantial effect on weight loss than physical activity alone.48 Still, physical activity is essential in helping maintain and prevent weight regain.
Continue to: Nonpharmacologic interventions
Nonpharmacologic interventions include promoting greater sleep quality and quantity by emphasizing good sleep hygiene practices. Developing a practical and effective bedtime routine, creating a quiet sleep environment, and practicing healthy daily habits are essential components to sleep hygiene (TABLE 249,50). Relaxation techniques and cognitive behavioral therapy (CBT) also can help. CBT for insomnia (CBT-I) is the first-line intervention for chronic insomnia.51 Sleep restriction is a type of CBT used to treat insomnia, encouraging short-term sleep loss in the hopes of improving insomnia. A trial by Logue et al showed that patients with overweight and obesity randomized to undergo CBT with better sleep hygiene (nonpharmacologic) interventions had a greater mean weight loss percentage (5% vs 2%; P = .04) than did those who received CBT alone.52

Eastern medicine including herbal interventions lack evidence of efficacy and safety. Further studies need to be done on the effects that chamomile, kava, valerian root (Valeriana officinalis), tryptophan, and Wu Ling (from mycelia Xylaria nigripes) might have on sleep.53
Proceed cautiously with medication. The American College of Physicians recommends a shared decision-making approach when considering pharmacologic therapy for chronic insomnia and the American Academy of Sleep Medicine (AASM) offers guidance on options.51,54 However, the evidence behind AASM sleep pharmacologic recommendations is weak, implying a lesser degree of confidence in the outcome and, therefore, in its appropriateness. Thus, it falls upon the clinician and patient to weigh the benefits and burdens of the pharmacologic treatments of insomnia. If indicated, medications suggested to treat sleep onset and sleep maintenance insomnia are eszopiclone, zolpidem, and temazepam. Zaleplon, triazolam, and ramelteon may improve sleep initiation. Suvorexant and doxepin are used for sleep-maintenance insomnia.54 Exploring patient preferences, cost of treatment, health care options, and available resources should all be considered.
CORRESPONDENCE
Ecler Ercole Jaqua, MD, MBA, FAAFP, AGSF, FACLM, DipABOM, Loma Linda University Health, 25455 Barton Road, Suite 206A, Loma Linda, CA 92354; [email protected]
Sleep is fundamental to overall health and longevity, with the average person spending about one-third of their life sleeping.1 Adequate sleep is critical for optimal cognition, memory consolidation, mood regulation, metabolism, appetite regulation, and immune and hormone functioning. According to the American Academy of Sleep Medicine and the Sleep Research Society, adults should sleep at least 7 hours per night on a regular basis “to promote optimal health.”2 Yet, between 2013 and 2020, only about 65% of adults in the United States were meeting this amount.3 Insufficient sleep is associated with an increased risk for chronic health conditions, including obesity, diabetes, cardiovascular diseases, and even premature death.4

In a population-based longitudinal study of sleep disorders, short sleep duration was associated with increased body mass index (BMI), low blood levels of leptin, and high ghrelin levels.5 In addition to physical impairments, poor sleep can impair cognitive performance and lead to vehicular accidents and increased accidents at work.4 The potential economic impact that this may have is significant, and includes increased costs and loss of productivity in the workplace.6
Many factors may contribute to short sleep duration: environment, mental and physical condition, and social influences such as occupation, family responsibilities, travel, group activities, and personal care. Furthermore, the rapidly evolving and developing media, communication, and entertainment industries are already strongly implicated in poor sleep quality and quantity, both contributing to excessive daytime sleepiness.7 Poor sleep quality is most notable in modern societies, and it correlates with the increasing prevalence of obesity, likely due to sleep’s effect on food consumption and physical activity.8 Optimizing a person’s sleep will improve overall health and longevity by inhibiting the development of chronic disease.
How insufficient sleep raises the risk for obesity
Not only is sleep beneficial for brain health, memory, learning, and growth, its effect on food consumption and physical activity likely correlates with the increased prevalence of obesity in modern society. Yet the optimal amount of sleep is controversial, and current recommendations of 7 or more hours of sleep per night for adults are derived from expert panels only.2 The recommended sleep duration for children is longer, and it varies by age.9 The quality of sleep and its impact on neuroendocrine hormones, not just the quantity of sleep, needs to be factored into these recommendations.
Sleep restriction activates the orexigenic system via the hormones leptin and ghrelin. These hormones control the food reward system, essentially increasing hunger and food intake. Leptin, created by white adipose tissue, is responsible for satiety and decreased food consumption.10 Ghrelin, made by oxyntic glands in the stomach, is responsible for the sensation of hunger.
In a 2004 study by Spiegel et al,11 leptin and ghrelin levels were measured during 2 days of sleep restriction (4 hours in bed) and sleep extension (10 hours in bed). Sleep restriction was associated with a decrease in leptin levels and an increase in ghrelin levels. The researchers reported that participants experienced an increase in hunger and appetite—especially for calorie-dense foods with high carbohydrate content.
Although research design has limitations with predominantly self-reported sleep data, studies have shown that short sleep time leads to increased food intake by increasing hunger signals and craving of unhealthy foods, and by providing more opportunities to eat while awake. It also may lead to decreased physical activity, creating a sedentary lifestyle that further encourages obesity.8 Reduced sleep is even correlated to decreased efficacy of weight-loss treatments.12
Continue to: Other sleep characteristics weakly correlated with obesity
Other sleep characteristics weakly correlated with obesity are sleep variability, timing, efficiency, quality, and daytime napping.8 Sleep variability causes dysregulation of eating patterns, leading to increased food intake. A shift to later sleep and waking times often results in higher consumption of calories after 8
Poor sleep efficiency and quality decreases N3-stage (deep non-REM) sleep, affects the autonomic nervous system, and has been associated with increased abdominal obesity. Daytime napping, which can cause irregular circadian rhythms and sleep schedules, is associated with increased obesity.15 Thus, each component of sleep needs to be assessed to promote optimal regulation of the orexigenic system.
Another study showed that inadequate sleep not only promotes unhealthy lifestyle habits that can lead to obesity but also decreases the ability to lose weight.16 This small study with 10 overweight patients provided its subjects with a controlled caloric intake over 2 weeks. Patients spent two 14-day periods 3 months apart in the laboratory, divided into 2 time-in-bed arms of 8.5 and 5.5 hours per night. Neuroendocrine changes caused by decreased sleep were associated with a significant lean body mass loss while conserving energy-dense fat.16 This study highlights the importance of sleep hygiene counseling when developing a weight-management plan with patients.
Sleep, and its many components, play an integral role in the prevention and treatment of obesity.17 Poor sleep will increase the risk for obesity and hinder its treatment. Therefore, sleep quality and duration are vital components of obesity management.
The sleep–obesity link in children and the elderly
Childhood obesity is linked to several chronic diseases in adulthood, including type 2 diabetes, cardiovascular disease, nonalcoholic fatty liver disease, asthma, and obstructive sleep apnea (OSA).18 According to 2017-2018 NHANES (National Health and Nutrition Examination Surveys) data, obesity (BMI ≥ 95th percentile) prevalence among children and adolescents was reported at 19.3% and severe obesity (BMI ≥ 120% of the 95th percentile) at 6.1%. Pediatric overweight prevalence (≥ 85th percentile and < 95th percentile) was 16.1%.19
Continue to: Although poor sleep is associated...
Although poor sleep is associated with increased risk for obesity, there is no proven cause-effect relationship.20 Nutrition and physical activity have been identified as 2 critical factors in childhood obesity, but sleep health also needs to be investigated. Shorter sleep duration is strongly associated with the development of obesity. Furthermore, children with obesity are more likely to have shorter sleep duration.21 A short sleep duration alters plasma levels of insulin, low-density lipoprotein, and high-sensitivity C-reactive protein. It is associated with lower diet quality, an increased intake of nutrient-poor foods, and a lower intake of vegetables and fruits.22 Recent studies have shown that interventions to promote earlier bedtimes can improve sleep duration in children.
Older adults have many sleeping issues, including insomnia, circadian rhythm sleep-wake disorders, sleep-related movement disorders, and sleep-breathing disorders. Additionally, the older population has increased sleep latency, decreased sleep efficiency and total sleep time, decreased REM sleep, more frequent nighttime awakenings, and more daytime napping.23 The increased sleep disturbance with age is mainly related to higher risk factors for sleep disorders than the aging process itself. Sleeping 5 or fewer hours is associated with an increased risk for obesity and central abdominal fat compared with those who sleep 7 to 8 hours per night.24 Similar to children and youth, older adults also show a strong correlation between inadequate sleep and obesity.24
The consequence: A vicious cycle
Obesity in turn leads to shorter sleep duration and more disruptions. This negatively affects the orexigenic system, and the resulting hormonal derangement promotes worsening obesity. It is a cycle of poor sleep causing obesity and obesity causing poor sleep. Insomnia, in combination with shorter (and longer) sleep times, also has been linked with obesity.25 These patients experience more daytime sleepiness, fatigue, and nighttime sleep disturbances, all correlated with decreased quality of life and higher prevalence of medical comorbidities.8,26 Additional comorbidities secondary to obesity, including gastroesophageal reflux, depression, and asthma, also have been linked to sleep disturbances.8
OSA is a common sleep complication associated with obesity. With the increasing prevalence of obesity, the prevalence of OSA is rising.8,27 Factors that heighten the risk for OSA are male sex, age 40 to 70 years, postmenopausal status, elevated BMI, and craniofacial and upper airway abnormality.28 However, the US Preventive Services Task Force found insufficient evidence to screen for or treat OSA in asymptomatic adults.28 Signs and symptoms of OSA include nighttime awakenings with choking, loud snoring, and feeling unrefreshed after sleep.29
OSA is caused by the intermittent narrowing and obstruction of the pharyngeal airway due to anatomical and structural irregularities or neuromuscular impairments. Untreated OSA is associated with cardiovascular disease and cardiac arrhythmias such as atrial fibrillation. Even with this correlation between obesity and sleep, it is estimated that 80% of OSA remains undiagnosed.30 Approximately half of primary care clinicians do not screen at-risk patients for OSA, and 90% do not use validated OSA screening tools.31 Screening tools that have been validated are the STOP, STOP-BANG, Epworth Sleepiness Scale, and 4-Variable Screening Tool. However, the US Department of Veterans Affairs and the US Department of Defense have a more recent guideline recommending STOP as an easier-to-administer screen for OSA.32 A positive result with a screening tool should be confirmed with polysomnography.32
Continue to: Intervention for OSA
Intervention for OSA. The longest randomized controlled study to date, Sleep AHEAD, evaluated over a period of 10 years the effect of weight loss on OSA severity achieved with either an intensive lifestyle intervention (ILI) or with diabetes support and education (DSE).33 OSA severity is rated on an Apnea-Hypopnea Index (AHI), with scores reflecting the number of sleep apnea events per hour. This study demonstrated that weight loss was associated with decreased OSA severity. At 4-year follow-up, the greater the weight loss with ILI intervention, the lower the patients’ OSA severity scores. The study found an average decrease in AHI of 0.68 events per hour for every kilogram of weight loss in the ILI group (P < .0001).33,34 Over the follow-up visits, the ILI participants had 7.4 events per hour, a more significantly reduced AHI than the DSE participants (P < .0001).33,34
Additionally, a small cohort of study participants achieved OSA remission (ILI, 34.4%; DSE, 22.2%), indicated by a low AHI score (< 5 events per hour). At the conclusion of the study, OSA severity decreased to a greater degree with ILI intervention.33,34
Alcohol and drug use can negatively influence sleep patterns and obesity. Higher alcohol consumption is associated with poorer sleep quality and higher chances of developing short sleep duration and snoring.35 Alcohol, a muscle relaxant, causes upper airway narrowing and reduced tongue muscle tone, thereby increasing snoring and OSA as demonstrated by increased AHI on polysomnography after alcohol intake. Alcohol also changes sleep architecture by increasing slow-wave sleep, decreasing REM sleep duration, and increasing sleep arousal in the second half of the night.36 Disrupted circadian rhythm after alcohol consumption was correlated with increased adenosine neurotransmitters derived from ethanol metabolism.37 Alcohol dependence may be related to other psychiatric symptoms, and chronic alcohol use eventually alters sleep mechanisms leading to persistent insomnia, further perpetuating adverse outcomes such as suicidal ideation.36 There are positive associations between beer drinking and measures of abdominal adiposity in men, and “the combination of short sleep duration [and] disinhibited eating … is associated with greater alcohol intake and excess weight.”38
Therefore, counsel patients to avoid alcohol since it is a modifiable risk factor with pervasive adverse health effects.
Many drugs have a profound effect on sleep patterns. Illicit drug use in particular can affect the brain’s neurotransmitter serotonin system. For example, ecstasy users have an increased risk for OSA.39 People with cocaine and heroin use disorder tend to have more sleep-maintenance insomnia.40
Continue to: In contrast, those with alcohol...
In contrast, those with alcohol or cannabis use disorder tend to have more sleep-onset insomnia.40 Not only do illicit drugs interrupt sleep, but daily tobacco use also has been correlated with increased insomnia and shorter sleep duration since nicotine is a stimulant.41
Insomnia is commonly treated with sedative antidepressants and hypnotics—eg, mirtazapine and olanzapine—that contribute to weight gain.42 In addition, other common pharmaceuticals used for sleep disorders, such as diphenhydramine, have sedative properties and tend to lead to weight gain.43 Because so many medications affect sleep and weight, carefully review patients’ medication lists and switch offending agents to weight-neutral drugs if possible.
Treatment and tools to improve sleep in patients with obesity
Given the strong correlation between obesity and sleep disorders, validated screening tools should be used to assess sleep quality, including onset and potential symptoms associated with poor sleep (TABLE 144). For weight management to succeed in patients with obesity, it is crucial to address sleep in addition to nutrition and physical activity.17,45

Physical activity has many benefits to overall health, especially for chronic diseases such as type 2 diabetes and hypertension. The Centers for Disease Control and Prevention recommends at least 150 minutes of moderate-intensity aerobic activity or 75 minutes of vigorous-intensity aerobic exercise per week in addition to muscle-strengthening activities 2 or more days per week.46 However, approximately 300 minutes of moderate-
Physical activity and diet in combination are vital, but diet restriction has a more substantial effect on weight loss than physical activity alone.48 Still, physical activity is essential in helping maintain and prevent weight regain.
Continue to: Nonpharmacologic interventions
Nonpharmacologic interventions include promoting greater sleep quality and quantity by emphasizing good sleep hygiene practices. Developing a practical and effective bedtime routine, creating a quiet sleep environment, and practicing healthy daily habits are essential components to sleep hygiene (TABLE 249,50). Relaxation techniques and cognitive behavioral therapy (CBT) also can help. CBT for insomnia (CBT-I) is the first-line intervention for chronic insomnia.51 Sleep restriction is a type of CBT used to treat insomnia, encouraging short-term sleep loss in the hopes of improving insomnia. A trial by Logue et al showed that patients with overweight and obesity randomized to undergo CBT with better sleep hygiene (nonpharmacologic) interventions had a greater mean weight loss percentage (5% vs 2%; P = .04) than did those who received CBT alone.52

Eastern medicine including herbal interventions lack evidence of efficacy and safety. Further studies need to be done on the effects that chamomile, kava, valerian root (Valeriana officinalis), tryptophan, and Wu Ling (from mycelia Xylaria nigripes) might have on sleep.53
Proceed cautiously with medication. The American College of Physicians recommends a shared decision-making approach when considering pharmacologic therapy for chronic insomnia and the American Academy of Sleep Medicine (AASM) offers guidance on options.51,54 However, the evidence behind AASM sleep pharmacologic recommendations is weak, implying a lesser degree of confidence in the outcome and, therefore, in its appropriateness. Thus, it falls upon the clinician and patient to weigh the benefits and burdens of the pharmacologic treatments of insomnia. If indicated, medications suggested to treat sleep onset and sleep maintenance insomnia are eszopiclone, zolpidem, and temazepam. Zaleplon, triazolam, and ramelteon may improve sleep initiation. Suvorexant and doxepin are used for sleep-maintenance insomnia.54 Exploring patient preferences, cost of treatment, health care options, and available resources should all be considered.
CORRESPONDENCE
Ecler Ercole Jaqua, MD, MBA, FAAFP, AGSF, FACLM, DipABOM, Loma Linda University Health, 25455 Barton Road, Suite 206A, Loma Linda, CA 92354; [email protected]
1. Aminoff MJ, Boller F, Swaab DF. We spend about one-third of our life either sleeping or attempting to do so. Handb Clin Neurol. 2011;98:vii. doi: 10.1016/B978-0-444-52006-7.00047-2
2. Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
3. CDC. Sleep and sleep disorders, adults. Accessed September 21, 2023. www.cdc.gov/sleep/data-and-statistics/adults.html
4. Chattu VK, Manzar MD, Kumary S. The global problem of insufficient sleep and its serious public health implications. Healthcare (Basel). 2019;7:1. doi: 10.3390/healthcare7010001
5. Taheri S, Lin L, Austin D, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062
6. Hafner M, Stepanek M, Taylor J, et al. Why sleep matters—the economic costs of insufficient sleep. Rand Health Q. 2017;6:11.
7. Hisler G, Twenge JM, Krizan Z. Associations between screen time and short sleep duration among adolescents varies by media type: evidence from a cohort study. Sleep Med. 2020;66:92-102. doi: 10.1016/j.sleep.2019.08.007
8. Ogilvie RP, Patel SR. The epidemiology of sleep and obesity. Sleep Health. 2017;3:383-388. doi: 10.1016/j.sleh.2017.07.013
9. CDC. Sleep and sleep disorders: How much sleep do I need? Accessed September 21, 2023. www.cdc.gov/sleep/about_sleep/how_much_sleep.html
10. van Egmond LT, Meth EMS, Engström J, et al. Effects of acute sleep loss on leptin, ghrelin, and adiponectin in adults with healthy weight and obesity: a laboratory study. Obesity (Silver Spring). 2023;31:635-641. doi: 10.1002/oby.23616
11. Spiegel K, Tasali E, Penev P, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846-850. doi: 10.7326/0003-4819-141-11-200412070-00008
12. Antza C, Kostopoulos G, Mostafa S, et al. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol. 2021;252:125-141. doi: 10.1530/JOE-21-0155
13. Baron KG, Reid KJ, Kern AS, et al. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring). 2011;19:1374-1381. doi: 10.1038/oby.2011.100
14. Liu XY, Zheng CL, Xu C, et al. Nighttime snacking is associated with risk of obesity and hyperglycemia in adults: a cross-sectional survey from Chinese adult teachers J Biomed Res. 2017;31:541-547. doi: 10.7555/JBR.31.20160083
15. Cai Z, Yang Y, Zhang J, et al. The relationship between daytime napping and obesity: a systematic review and meta-analysis. Sci Rep. 2023.13:12124. doi: 10.1038/s41598-023-37883-7
16. Nedeltcheva AV, Kilkus JM, Imperial J, et al. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435-441. doi: 10.7326/0003-4819-153-7-201010050-00006
17. Chaput JP, Tremblay A. Adequate sleep to improve the treatment of obesity. CMAJ. 2012;184:1975-1976. doi: 10.1503/cmaj.120876
18. Kelsey MM, Zaepfel A, Bjornstad P, et al. Age-related consequences of childhood obesity. Gerontology. 2014;60:222-228. doi: 10.1159/000356023
19. Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2-19 years: United States, 1963-1965 through 2017-2018. National Center for Health Statistics Health E-Stats. Updated January 29, 2021. Accessed September 21, 2021. www.cdc.gov/nchs/data/hestat/obesity-child-17-18/overweight-obesity-child-H.pdf
20. Fatima Y, Doi SAR, Mamun AA. Sleep quality and obesity in young subjects: a meta-analysis. Obes Rev. 2016;17:1154-1166. doi: 10.1111/obr.12444
21. Gohil A, Hannon TS. Poor sleep and obesity: concurrent epidemics in adolescent youth. Front Endocrinol. 2018;9:364. doi: 10.3389/fendo.2018.00364
22. Golley RK, Maher CA, Matricciani L, et al. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes (Lond). 2013;37:546-551. doi: 10.1038/ijo.2012.212
23. Alessi CA. Sleep issues. In: Harper GM, Lyons WL, Potter JF, eds. Geriatrics Review Syllabus (GRS 10). Updated January 2021. Accessed August 29, 2023. http://geriatricscareonline.org
24. Patel SR, Blackwell T, Redline S, et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond). 2008;32:1825-1834. doi: 10.1038/ijo.2008.198
25. Cai GH, Theorell-Haglöw J, Janson C, et al. Insomnia symptoms and sleep duration and their combined effects in relation to associations with obesity and central obesity. Sleep Med. 2018;46:81-87. doi: 10.1016/j.sleep.2018.03.009
26. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14:402-412. doi: 10.1097/MCO.0b013 e3283479109
27. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population–a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311-1322. doi: 10.3978/j.issn.2072-1439.2015.06.11
28. USPSTF. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for obstructive sleep apnea in adults: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:407-414. doi: 10.1001/jama.2016.20325
29. Goyal M, Johnson J. Obstructive sleep apnea diagnosis and management. Mo Med. 2017;114:120-124.
30. American Academy of Sleep Medicine. Hidden health crisis costing America billions: underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. 2016. Accessed September 25, 2023. https://aasm.org/wp-content/uploads/2017/10/sleep-apnea-economic-crisis.pdf
31. Devaraj, NK. Knowledge, attitude, and practice regarding obstructive sleep apnea among primary care physicians. Sleep Breath. 2020;24:1581-1590. doi: 10.1007/s11325-020-02040-1
32. Mysliwiec V, Martin JL, Ulmer CS, et al. The management of chronic insomnia disorder and obstructive sleep apnea: synopsis of the 2019 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guidelines. Ann Intern Med. 2020;172:325-336. doi: 10.7326/M19-3575
33. Kuna ST, Reboussin DM, Strotmeyer ES, et al. Effects of weight loss on obstructive sleep apnea severity. Ten-year results of the Sleep AHEAD study. Am J Respir Crit Care Med. 2021;203:221-229. doi: 10.1164/rccm.201912-2511OC
34. St-Onge MP, Tasali E. Weight loss is integral to obstructive sleep apnea management. Ten-year follow-up in Sleep AHEAD. Am J Respir Crit Care Med. 2021;203:161-162. doi: 10.1164/rccm.202007-2906ED
35. Zheng D, Yuan X, Ma C, et al. Alcohol consumption and sleep quality: a community-based study. Public Health Nutr. 2021;24:4851-4858. doi: 10.1017/S1368980020004553
36. Chakravorty S, Chaudhary NS, Brower KJ. Alcohol dependence and its relationship with insomnia and other sleep disorders. Alcohol Clin Exp Res. 2016;40:2271-2282. doi: 10.1111/acer.13217
37. Elmenhorst EM, Elmenhorst D, Benderoth S, et al. Cognitive impairments by alcohol and sleep deprivation indicate trait characteristics and a potential role for adenosine A1 receptors. Proc Natl Acad Sci U S A. 2018;115:8009-8014. doi: 10.1073/pnas.1803770115
38. Traversy G, Chaput JP. Alcohol consumption and obesity: an update. Curr Obes Rep. 2015;4:122-130. doi: 10.1007/s13679-014-0129-4
39. McCann UD, Sgambati FP, Schwartz AR, et al. Sleep apnea in young abstinent recreational MDMA (“ecstasy”) consumers. Neurology. 2009;73:2011-2017. doi: 10.1212/WNL.0b013e3181c51a62
40. Grau-López L, Grau-López L, Daigre C, et al. Insomnia symptoms in patients with substance use disorders during detoxification and associated clinical features. Front Psychiatry. 2020;11:540022. doi: 10.3389/fpsyt.2020.540022
41. Boehm MA, Lei QM, Lloyd RM, et al. Depression, anxiety, and tobacco use: overlapping impediments to sleep in a national sample of college students. J Am Coll Health. 2016;64:565-574. doi: 10.1080/07448481.2016.1205073
42. Gracious BL, Meyer AE. Psychotropic-induced weight gain and potential pharmacologic treatment strategies. Psychiatry (Edgmont). 2005;2:36-42.
43. Ratliff JC, Barber JA, Palmese LB, et al. Association of prescription H1 antihistamine use with obesity: results from the National Health and Nutrition Examination Survey. Obesity (Silver Spring). 2010;18:2398-2400. doi: 10.1038/oby.2010.176
44. Pataka A, Daskalopoulou E, Kalamaras G, et al. Evaluation of five different questionnaires for assessing sleep apnea syndrome in a sleep clinic. Sleep Med. 2014;15:776-781. doi: 10.1016/j.sleep.2014.03.012
45. Kline CE, Chasens ER, Bizhanova Z, et al. The association between sleep health and weight change during a 12-month behavioral weight loss intervention. Int J Obes (Lond). 2021;45:639-649. doi: 10.1038/s41366-020-00728-8
46. CDC. How much physical activity do adults need? Accessed August 23, 2023. www.cdc.gov/physicalactivity/basics/adults/index.htm
47. Flack KD, Hays HM, Moreland J, et al. Exercise for weight loss: further evaluating energy compensation with exercise. Med Sci Sports Exerc. 2020;52:2466-2475. doi: 10.1249/MSS.0000000000002376
48. Swift DL, Johannsen NM, Lavie CJ, et al. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56:441-447. doi: 10.1016/j.pcad.2013.09.012
49. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36. doi: 10.1016/j.smrv.2014.10.001
50. CDC. Tips for better sleep. 2022. Accessed August 4, 2023. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html
51. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133. doi: 10.7326/M15-2175
52. Logue EE, Bourguet CC, Palmieri PA, et al. The better weight-better sleep study: a pilot intervention in primary care. Am J Health Behav. 2012;36:319-334. doi: 10.5993/AJHB.36.3.4
53. Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1-12. doi: 10.1016/j.smrv.2014.12.003
54. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
1. Aminoff MJ, Boller F, Swaab DF. We spend about one-third of our life either sleeping or attempting to do so. Handb Clin Neurol. 2011;98:vii. doi: 10.1016/B978-0-444-52006-7.00047-2
2. Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
3. CDC. Sleep and sleep disorders, adults. Accessed September 21, 2023. www.cdc.gov/sleep/data-and-statistics/adults.html
4. Chattu VK, Manzar MD, Kumary S. The global problem of insufficient sleep and its serious public health implications. Healthcare (Basel). 2019;7:1. doi: 10.3390/healthcare7010001
5. Taheri S, Lin L, Austin D, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062
6. Hafner M, Stepanek M, Taylor J, et al. Why sleep matters—the economic costs of insufficient sleep. Rand Health Q. 2017;6:11.
7. Hisler G, Twenge JM, Krizan Z. Associations between screen time and short sleep duration among adolescents varies by media type: evidence from a cohort study. Sleep Med. 2020;66:92-102. doi: 10.1016/j.sleep.2019.08.007
8. Ogilvie RP, Patel SR. The epidemiology of sleep and obesity. Sleep Health. 2017;3:383-388. doi: 10.1016/j.sleh.2017.07.013
9. CDC. Sleep and sleep disorders: How much sleep do I need? Accessed September 21, 2023. www.cdc.gov/sleep/about_sleep/how_much_sleep.html
10. van Egmond LT, Meth EMS, Engström J, et al. Effects of acute sleep loss on leptin, ghrelin, and adiponectin in adults with healthy weight and obesity: a laboratory study. Obesity (Silver Spring). 2023;31:635-641. doi: 10.1002/oby.23616
11. Spiegel K, Tasali E, Penev P, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846-850. doi: 10.7326/0003-4819-141-11-200412070-00008
12. Antza C, Kostopoulos G, Mostafa S, et al. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol. 2021;252:125-141. doi: 10.1530/JOE-21-0155
13. Baron KG, Reid KJ, Kern AS, et al. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring). 2011;19:1374-1381. doi: 10.1038/oby.2011.100
14. Liu XY, Zheng CL, Xu C, et al. Nighttime snacking is associated with risk of obesity and hyperglycemia in adults: a cross-sectional survey from Chinese adult teachers J Biomed Res. 2017;31:541-547. doi: 10.7555/JBR.31.20160083
15. Cai Z, Yang Y, Zhang J, et al. The relationship between daytime napping and obesity: a systematic review and meta-analysis. Sci Rep. 2023.13:12124. doi: 10.1038/s41598-023-37883-7
16. Nedeltcheva AV, Kilkus JM, Imperial J, et al. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435-441. doi: 10.7326/0003-4819-153-7-201010050-00006
17. Chaput JP, Tremblay A. Adequate sleep to improve the treatment of obesity. CMAJ. 2012;184:1975-1976. doi: 10.1503/cmaj.120876
18. Kelsey MM, Zaepfel A, Bjornstad P, et al. Age-related consequences of childhood obesity. Gerontology. 2014;60:222-228. doi: 10.1159/000356023
19. Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2-19 years: United States, 1963-1965 through 2017-2018. National Center for Health Statistics Health E-Stats. Updated January 29, 2021. Accessed September 21, 2021. www.cdc.gov/nchs/data/hestat/obesity-child-17-18/overweight-obesity-child-H.pdf
20. Fatima Y, Doi SAR, Mamun AA. Sleep quality and obesity in young subjects: a meta-analysis. Obes Rev. 2016;17:1154-1166. doi: 10.1111/obr.12444
21. Gohil A, Hannon TS. Poor sleep and obesity: concurrent epidemics in adolescent youth. Front Endocrinol. 2018;9:364. doi: 10.3389/fendo.2018.00364
22. Golley RK, Maher CA, Matricciani L, et al. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes (Lond). 2013;37:546-551. doi: 10.1038/ijo.2012.212
23. Alessi CA. Sleep issues. In: Harper GM, Lyons WL, Potter JF, eds. Geriatrics Review Syllabus (GRS 10). Updated January 2021. Accessed August 29, 2023. http://geriatricscareonline.org
24. Patel SR, Blackwell T, Redline S, et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond). 2008;32:1825-1834. doi: 10.1038/ijo.2008.198
25. Cai GH, Theorell-Haglöw J, Janson C, et al. Insomnia symptoms and sleep duration and their combined effects in relation to associations with obesity and central obesity. Sleep Med. 2018;46:81-87. doi: 10.1016/j.sleep.2018.03.009
26. Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14:402-412. doi: 10.1097/MCO.0b013 e3283479109
27. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population–a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311-1322. doi: 10.3978/j.issn.2072-1439.2015.06.11
28. USPSTF. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for obstructive sleep apnea in adults: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:407-414. doi: 10.1001/jama.2016.20325
29. Goyal M, Johnson J. Obstructive sleep apnea diagnosis and management. Mo Med. 2017;114:120-124.
30. American Academy of Sleep Medicine. Hidden health crisis costing America billions: underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. 2016. Accessed September 25, 2023. https://aasm.org/wp-content/uploads/2017/10/sleep-apnea-economic-crisis.pdf
31. Devaraj, NK. Knowledge, attitude, and practice regarding obstructive sleep apnea among primary care physicians. Sleep Breath. 2020;24:1581-1590. doi: 10.1007/s11325-020-02040-1
32. Mysliwiec V, Martin JL, Ulmer CS, et al. The management of chronic insomnia disorder and obstructive sleep apnea: synopsis of the 2019 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guidelines. Ann Intern Med. 2020;172:325-336. doi: 10.7326/M19-3575
33. Kuna ST, Reboussin DM, Strotmeyer ES, et al. Effects of weight loss on obstructive sleep apnea severity. Ten-year results of the Sleep AHEAD study. Am J Respir Crit Care Med. 2021;203:221-229. doi: 10.1164/rccm.201912-2511OC
34. St-Onge MP, Tasali E. Weight loss is integral to obstructive sleep apnea management. Ten-year follow-up in Sleep AHEAD. Am J Respir Crit Care Med. 2021;203:161-162. doi: 10.1164/rccm.202007-2906ED
35. Zheng D, Yuan X, Ma C, et al. Alcohol consumption and sleep quality: a community-based study. Public Health Nutr. 2021;24:4851-4858. doi: 10.1017/S1368980020004553
36. Chakravorty S, Chaudhary NS, Brower KJ. Alcohol dependence and its relationship with insomnia and other sleep disorders. Alcohol Clin Exp Res. 2016;40:2271-2282. doi: 10.1111/acer.13217
37. Elmenhorst EM, Elmenhorst D, Benderoth S, et al. Cognitive impairments by alcohol and sleep deprivation indicate trait characteristics and a potential role for adenosine A1 receptors. Proc Natl Acad Sci U S A. 2018;115:8009-8014. doi: 10.1073/pnas.1803770115
38. Traversy G, Chaput JP. Alcohol consumption and obesity: an update. Curr Obes Rep. 2015;4:122-130. doi: 10.1007/s13679-014-0129-4
39. McCann UD, Sgambati FP, Schwartz AR, et al. Sleep apnea in young abstinent recreational MDMA (“ecstasy”) consumers. Neurology. 2009;73:2011-2017. doi: 10.1212/WNL.0b013e3181c51a62
40. Grau-López L, Grau-López L, Daigre C, et al. Insomnia symptoms in patients with substance use disorders during detoxification and associated clinical features. Front Psychiatry. 2020;11:540022. doi: 10.3389/fpsyt.2020.540022
41. Boehm MA, Lei QM, Lloyd RM, et al. Depression, anxiety, and tobacco use: overlapping impediments to sleep in a national sample of college students. J Am Coll Health. 2016;64:565-574. doi: 10.1080/07448481.2016.1205073
42. Gracious BL, Meyer AE. Psychotropic-induced weight gain and potential pharmacologic treatment strategies. Psychiatry (Edgmont). 2005;2:36-42.
43. Ratliff JC, Barber JA, Palmese LB, et al. Association of prescription H1 antihistamine use with obesity: results from the National Health and Nutrition Examination Survey. Obesity (Silver Spring). 2010;18:2398-2400. doi: 10.1038/oby.2010.176
44. Pataka A, Daskalopoulou E, Kalamaras G, et al. Evaluation of five different questionnaires for assessing sleep apnea syndrome in a sleep clinic. Sleep Med. 2014;15:776-781. doi: 10.1016/j.sleep.2014.03.012
45. Kline CE, Chasens ER, Bizhanova Z, et al. The association between sleep health and weight change during a 12-month behavioral weight loss intervention. Int J Obes (Lond). 2021;45:639-649. doi: 10.1038/s41366-020-00728-8
46. CDC. How much physical activity do adults need? Accessed August 23, 2023. www.cdc.gov/physicalactivity/basics/adults/index.htm
47. Flack KD, Hays HM, Moreland J, et al. Exercise for weight loss: further evaluating energy compensation with exercise. Med Sci Sports Exerc. 2020;52:2466-2475. doi: 10.1249/MSS.0000000000002376
48. Swift DL, Johannsen NM, Lavie CJ, et al. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56:441-447. doi: 10.1016/j.pcad.2013.09.012
49. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36. doi: 10.1016/j.smrv.2014.10.001
50. CDC. Tips for better sleep. 2022. Accessed August 4, 2023. www.cdc.gov/sleep/about_sleep/sleep_hygiene.html
51. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133. doi: 10.7326/M15-2175
52. Logue EE, Bourguet CC, Palmieri PA, et al. The better weight-better sleep study: a pilot intervention in primary care. Am J Health Behav. 2012;36:319-334. doi: 10.5993/AJHB.36.3.4
53. Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1-12. doi: 10.1016/j.smrv.2014.12.003
54. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
PRACTICE RECOMMENDATIONS
› Consider cognitive behaviorial therapy for insomnia (CBT-I) first-line treatment for insomnia. A
› Carefully review patients’ medication lists, as many pharmaceuticals can affect weight and sleep. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Semaglutide win in HFpEF with obesity regardless of ejection fraction: STEP-HFpEF
CLEVELAND – independently of baseline left-ventricular ejection fraction (LVEF).
The finding comes from a prespecified secondary analysis of the STEP-HFpEF trial of more than 500 nondiabetic patients with obesity and HF with an initial LVEF of 45% or greater.
They suggest that for patients with the obesity phenotype of HFpEF, semaglutide (Wegovy) could potentially join SGLT2 inhibitors on the short list of meds with consistent treatment effects whether LVEF is mildly reduced, preserved, or in the normal range.
That would distinguish the drug, a glucagon-like peptide-1 (GLP-1) receptor agonist, from mineralocorticoid receptor antagonists (MRA), sacubitril-valsartan (Entresto), and other renin-angiotensin-system inhibitors (RASi), whose benefits tend to taper off with rising LVEF.
The patients assigned to semaglutide showed significant improvement in both primary endpoints – change in Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) and change in body weight at 52 weeks – whether their baseline LVEF was 45%-49%, 50%-59%, or 60% or greater.
Results were similar for improvements in 6-minute walk distance (6MWD) and levels of NT-terminal pro–brain natriuretic peptide (NT-proBNP) and C-reactive protein, observed Javed Butler, MD, when presenting the analysis at the annual meeting of the Heart Failure Society of America, Cleveland.
Dr. Butler, of Baylor Scott and White Research Institute, Dallas, and the University of Mississippi, Jackson, is also lead author of the study, which was published on the same day in the Journal of the American College of Cardiology.
In his presentation, Dr. Butler singled out the NT-proBNP finding as “very meaningful” with respect to understanding potential mechanisms of the drug effects observed in the trial.
For example, people with obesity tend to have lower than average natriuretic peptide levels that “actually go up a bit” when they lose weight, he observed. But in the trial, “we saw a reduction in NT-proBNP in spite of the weight loss,” regardless of LVEF category.
John McMurray, MD, University of Glasgow, the invited discussant for Dr. Butler’s presentation, agreed that it raises the question whether weight loss was the sole semaglutide effect responsible for the improvement in heart failure status and biomarkers. The accompanying NT-proBNP reductions – when the opposite might otherwise have been expected – may point to a possible mechanism of action that is “something more than just weight loss,” he said. “If that were the case, it becomes very important, because it means that this treatment might do good things in non-obese patients or might do good things in patients with other types of heart failure.”
‘Vital reassurance’
More definitive trials are needed “to clarify safety and efficacy of obesity-targeted therapeutics in HF across the ejection fraction spectrum,” according to an accompanying editorial).
Still, the STEP-HFpEF analysis “strengthens the role of GLP-1 [receptor agonists] to ameliorate health status” for patients with obesity and HF with mildly reduced or preserved ejection fraction, write Muthiah Vaduganathan, MD, MPH, and John W. Ostrominski, MD, Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
Its findings “provide vital reassurance” on semaglutide safety and efficacy in HF with below-normal LVEF and “tentatively support the existence of a more general, LVEF-independent, obesity-related HF phenotype capable of favorable modification with incretin-based therapies.”
The lack of heterogeneity in treatment effects across LVEF subgroups “is not surprising,” but “the findings reinforce that the benefits of this therapy in those meeting trial criteria do not vary by left ventricular ejection fraction,” Gregg C. Fonarow, MD, University of California, Los Angeles, Medical Center, said in an interview.
It remains unknown, however, “whether the improvement in health status, functional status, and reduced inflammation” will translate to reduced risk of cardiovascular death or HF hospitalization, said Dr. Fonarow, who isn’t connected to STEP-HFpEF.
It’s a question for future studies, he agreed, whether semaglutide would confer similar benefits for patients with obesity and HF with LVEF less than 45% or in non-obese HF patients.
Dr. McMurray proposed that future GLP-1 receptor agonist heart-failure trials should include non-obese patients to determine whether the effects seen in STEP-HFpEF were due to something more than weight loss. Trials in patients with obesity and HF with reduced LVEF would also be important.
“If it turns out just to be about weight loss, then we need to think about the alternatives,” including diet, exercise, and bariatric surgery but also, potentially, weight-loss drugs other than semaglutide, he said.
No heterogeneity by LVEF
STEP-HFpEF randomly assigned 529 patients free of diabetes with an LVEF greater than or equal to 45%, a body mass index (BMI) of at least 30 kg/m2, and NYHA functional status of 2-4 to either a placebo injection or 2.4-mg semaglutide subcutaneously once a week (the dose used for weight reduction) atop standard care.
As previously reported, those assigned to semaglutide showed significant improvements at 1 year in symptoms and in physical limitation, per changes in KCCQ-CSS, and weight loss, compared with the control group. Their exercise capacity, as measured by 6MWD, also improved.
The more weight patients lost while taking semaglutide, the better their KCCQ-CSS and 6MWD outcomes, a prior secondary analysis suggested. But the STEP-HFpEF researchers said weight loss did not appear to explain all of their gains, compared with usual care.
For the current analysis, the 263 patients assigned to receive semaglutide and 266 control patients were divided into three groups by baseline LVEF and compared for the same outcomes.
The semaglutide group, compared with control patients, also showed a significantly increased hierarchical composite win ratio, 1.72 (95% CI, 1.37-2.15; P < .001), that was consistent across LVEF categories and that accounted for all-cause mortality, HF events, KCCQ-CSS and 6MWD changes, and change in CRP.
Limitations make it hard to generalize the results, the authors caution. Well over 90% of the participants were White patients, for example, and the overall trial was not powered to show subgroup differences.
Given the many patients with HFpEF who have a cardiometabolic phenotype and are with overweight or obesity, write Dr. Butler and colleagues, their treatment approach “may ultimately include combination therapy with SGLT2 inhibitors and GLP-1 receptor agonists, given their non-overlapping and complementary mechanisms of action.”
Dr. Fonarow noted that both MRAs and sacubitril-valsartan offer clinical benefits for patients with HF and LVEF “in the 41%-60% range” that are evident “across BMI categories.”
So it’s likely, he said, that those medications as well as SGLT2 inhibitors will be used along with GLP-1 receptor agonists for patients with HFpEF and obesity.
STEP-HFpEF was funded by Novo Nordisk. Dr. Butler and the other authors disclose consulting for many companies, a list of which can be found in the report. Dr. Fonarow reports consulting for multiple companies. Dr. McMurray discloses consulting for AstraZeneca. Dr. Ostrominski reports no relevant disclosures. Dr. Vaduganathan discloses receiving grant support, serving on advisory boards, or speaking for multiple companies and serving on committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics.
A version of this article appeared on Medscape.com.
CLEVELAND – independently of baseline left-ventricular ejection fraction (LVEF).
The finding comes from a prespecified secondary analysis of the STEP-HFpEF trial of more than 500 nondiabetic patients with obesity and HF with an initial LVEF of 45% or greater.
They suggest that for patients with the obesity phenotype of HFpEF, semaglutide (Wegovy) could potentially join SGLT2 inhibitors on the short list of meds with consistent treatment effects whether LVEF is mildly reduced, preserved, or in the normal range.
That would distinguish the drug, a glucagon-like peptide-1 (GLP-1) receptor agonist, from mineralocorticoid receptor antagonists (MRA), sacubitril-valsartan (Entresto), and other renin-angiotensin-system inhibitors (RASi), whose benefits tend to taper off with rising LVEF.
The patients assigned to semaglutide showed significant improvement in both primary endpoints – change in Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) and change in body weight at 52 weeks – whether their baseline LVEF was 45%-49%, 50%-59%, or 60% or greater.
Results were similar for improvements in 6-minute walk distance (6MWD) and levels of NT-terminal pro–brain natriuretic peptide (NT-proBNP) and C-reactive protein, observed Javed Butler, MD, when presenting the analysis at the annual meeting of the Heart Failure Society of America, Cleveland.
Dr. Butler, of Baylor Scott and White Research Institute, Dallas, and the University of Mississippi, Jackson, is also lead author of the study, which was published on the same day in the Journal of the American College of Cardiology.
In his presentation, Dr. Butler singled out the NT-proBNP finding as “very meaningful” with respect to understanding potential mechanisms of the drug effects observed in the trial.
For example, people with obesity tend to have lower than average natriuretic peptide levels that “actually go up a bit” when they lose weight, he observed. But in the trial, “we saw a reduction in NT-proBNP in spite of the weight loss,” regardless of LVEF category.
John McMurray, MD, University of Glasgow, the invited discussant for Dr. Butler’s presentation, agreed that it raises the question whether weight loss was the sole semaglutide effect responsible for the improvement in heart failure status and biomarkers. The accompanying NT-proBNP reductions – when the opposite might otherwise have been expected – may point to a possible mechanism of action that is “something more than just weight loss,” he said. “If that were the case, it becomes very important, because it means that this treatment might do good things in non-obese patients or might do good things in patients with other types of heart failure.”
‘Vital reassurance’
More definitive trials are needed “to clarify safety and efficacy of obesity-targeted therapeutics in HF across the ejection fraction spectrum,” according to an accompanying editorial).
Still, the STEP-HFpEF analysis “strengthens the role of GLP-1 [receptor agonists] to ameliorate health status” for patients with obesity and HF with mildly reduced or preserved ejection fraction, write Muthiah Vaduganathan, MD, MPH, and John W. Ostrominski, MD, Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
Its findings “provide vital reassurance” on semaglutide safety and efficacy in HF with below-normal LVEF and “tentatively support the existence of a more general, LVEF-independent, obesity-related HF phenotype capable of favorable modification with incretin-based therapies.”
The lack of heterogeneity in treatment effects across LVEF subgroups “is not surprising,” but “the findings reinforce that the benefits of this therapy in those meeting trial criteria do not vary by left ventricular ejection fraction,” Gregg C. Fonarow, MD, University of California, Los Angeles, Medical Center, said in an interview.
It remains unknown, however, “whether the improvement in health status, functional status, and reduced inflammation” will translate to reduced risk of cardiovascular death or HF hospitalization, said Dr. Fonarow, who isn’t connected to STEP-HFpEF.
It’s a question for future studies, he agreed, whether semaglutide would confer similar benefits for patients with obesity and HF with LVEF less than 45% or in non-obese HF patients.
Dr. McMurray proposed that future GLP-1 receptor agonist heart-failure trials should include non-obese patients to determine whether the effects seen in STEP-HFpEF were due to something more than weight loss. Trials in patients with obesity and HF with reduced LVEF would also be important.
“If it turns out just to be about weight loss, then we need to think about the alternatives,” including diet, exercise, and bariatric surgery but also, potentially, weight-loss drugs other than semaglutide, he said.
No heterogeneity by LVEF
STEP-HFpEF randomly assigned 529 patients free of diabetes with an LVEF greater than or equal to 45%, a body mass index (BMI) of at least 30 kg/m2, and NYHA functional status of 2-4 to either a placebo injection or 2.4-mg semaglutide subcutaneously once a week (the dose used for weight reduction) atop standard care.
As previously reported, those assigned to semaglutide showed significant improvements at 1 year in symptoms and in physical limitation, per changes in KCCQ-CSS, and weight loss, compared with the control group. Their exercise capacity, as measured by 6MWD, also improved.
The more weight patients lost while taking semaglutide, the better their KCCQ-CSS and 6MWD outcomes, a prior secondary analysis suggested. But the STEP-HFpEF researchers said weight loss did not appear to explain all of their gains, compared with usual care.
For the current analysis, the 263 patients assigned to receive semaglutide and 266 control patients were divided into three groups by baseline LVEF and compared for the same outcomes.
The semaglutide group, compared with control patients, also showed a significantly increased hierarchical composite win ratio, 1.72 (95% CI, 1.37-2.15; P < .001), that was consistent across LVEF categories and that accounted for all-cause mortality, HF events, KCCQ-CSS and 6MWD changes, and change in CRP.
Limitations make it hard to generalize the results, the authors caution. Well over 90% of the participants were White patients, for example, and the overall trial was not powered to show subgroup differences.
Given the many patients with HFpEF who have a cardiometabolic phenotype and are with overweight or obesity, write Dr. Butler and colleagues, their treatment approach “may ultimately include combination therapy with SGLT2 inhibitors and GLP-1 receptor agonists, given their non-overlapping and complementary mechanisms of action.”
Dr. Fonarow noted that both MRAs and sacubitril-valsartan offer clinical benefits for patients with HF and LVEF “in the 41%-60% range” that are evident “across BMI categories.”
So it’s likely, he said, that those medications as well as SGLT2 inhibitors will be used along with GLP-1 receptor agonists for patients with HFpEF and obesity.
STEP-HFpEF was funded by Novo Nordisk. Dr. Butler and the other authors disclose consulting for many companies, a list of which can be found in the report. Dr. Fonarow reports consulting for multiple companies. Dr. McMurray discloses consulting for AstraZeneca. Dr. Ostrominski reports no relevant disclosures. Dr. Vaduganathan discloses receiving grant support, serving on advisory boards, or speaking for multiple companies and serving on committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics.
A version of this article appeared on Medscape.com.
CLEVELAND – independently of baseline left-ventricular ejection fraction (LVEF).
The finding comes from a prespecified secondary analysis of the STEP-HFpEF trial of more than 500 nondiabetic patients with obesity and HF with an initial LVEF of 45% or greater.
They suggest that for patients with the obesity phenotype of HFpEF, semaglutide (Wegovy) could potentially join SGLT2 inhibitors on the short list of meds with consistent treatment effects whether LVEF is mildly reduced, preserved, or in the normal range.
That would distinguish the drug, a glucagon-like peptide-1 (GLP-1) receptor agonist, from mineralocorticoid receptor antagonists (MRA), sacubitril-valsartan (Entresto), and other renin-angiotensin-system inhibitors (RASi), whose benefits tend to taper off with rising LVEF.
The patients assigned to semaglutide showed significant improvement in both primary endpoints – change in Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) and change in body weight at 52 weeks – whether their baseline LVEF was 45%-49%, 50%-59%, or 60% or greater.
Results were similar for improvements in 6-minute walk distance (6MWD) and levels of NT-terminal pro–brain natriuretic peptide (NT-proBNP) and C-reactive protein, observed Javed Butler, MD, when presenting the analysis at the annual meeting of the Heart Failure Society of America, Cleveland.
Dr. Butler, of Baylor Scott and White Research Institute, Dallas, and the University of Mississippi, Jackson, is also lead author of the study, which was published on the same day in the Journal of the American College of Cardiology.
In his presentation, Dr. Butler singled out the NT-proBNP finding as “very meaningful” with respect to understanding potential mechanisms of the drug effects observed in the trial.
For example, people with obesity tend to have lower than average natriuretic peptide levels that “actually go up a bit” when they lose weight, he observed. But in the trial, “we saw a reduction in NT-proBNP in spite of the weight loss,” regardless of LVEF category.
John McMurray, MD, University of Glasgow, the invited discussant for Dr. Butler’s presentation, agreed that it raises the question whether weight loss was the sole semaglutide effect responsible for the improvement in heart failure status and biomarkers. The accompanying NT-proBNP reductions – when the opposite might otherwise have been expected – may point to a possible mechanism of action that is “something more than just weight loss,” he said. “If that were the case, it becomes very important, because it means that this treatment might do good things in non-obese patients or might do good things in patients with other types of heart failure.”
‘Vital reassurance’
More definitive trials are needed “to clarify safety and efficacy of obesity-targeted therapeutics in HF across the ejection fraction spectrum,” according to an accompanying editorial).
Still, the STEP-HFpEF analysis “strengthens the role of GLP-1 [receptor agonists] to ameliorate health status” for patients with obesity and HF with mildly reduced or preserved ejection fraction, write Muthiah Vaduganathan, MD, MPH, and John W. Ostrominski, MD, Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
Its findings “provide vital reassurance” on semaglutide safety and efficacy in HF with below-normal LVEF and “tentatively support the existence of a more general, LVEF-independent, obesity-related HF phenotype capable of favorable modification with incretin-based therapies.”
The lack of heterogeneity in treatment effects across LVEF subgroups “is not surprising,” but “the findings reinforce that the benefits of this therapy in those meeting trial criteria do not vary by left ventricular ejection fraction,” Gregg C. Fonarow, MD, University of California, Los Angeles, Medical Center, said in an interview.
It remains unknown, however, “whether the improvement in health status, functional status, and reduced inflammation” will translate to reduced risk of cardiovascular death or HF hospitalization, said Dr. Fonarow, who isn’t connected to STEP-HFpEF.
It’s a question for future studies, he agreed, whether semaglutide would confer similar benefits for patients with obesity and HF with LVEF less than 45% or in non-obese HF patients.
Dr. McMurray proposed that future GLP-1 receptor agonist heart-failure trials should include non-obese patients to determine whether the effects seen in STEP-HFpEF were due to something more than weight loss. Trials in patients with obesity and HF with reduced LVEF would also be important.
“If it turns out just to be about weight loss, then we need to think about the alternatives,” including diet, exercise, and bariatric surgery but also, potentially, weight-loss drugs other than semaglutide, he said.
No heterogeneity by LVEF
STEP-HFpEF randomly assigned 529 patients free of diabetes with an LVEF greater than or equal to 45%, a body mass index (BMI) of at least 30 kg/m2, and NYHA functional status of 2-4 to either a placebo injection or 2.4-mg semaglutide subcutaneously once a week (the dose used for weight reduction) atop standard care.
As previously reported, those assigned to semaglutide showed significant improvements at 1 year in symptoms and in physical limitation, per changes in KCCQ-CSS, and weight loss, compared with the control group. Their exercise capacity, as measured by 6MWD, also improved.
The more weight patients lost while taking semaglutide, the better their KCCQ-CSS and 6MWD outcomes, a prior secondary analysis suggested. But the STEP-HFpEF researchers said weight loss did not appear to explain all of their gains, compared with usual care.
For the current analysis, the 263 patients assigned to receive semaglutide and 266 control patients were divided into three groups by baseline LVEF and compared for the same outcomes.
The semaglutide group, compared with control patients, also showed a significantly increased hierarchical composite win ratio, 1.72 (95% CI, 1.37-2.15; P < .001), that was consistent across LVEF categories and that accounted for all-cause mortality, HF events, KCCQ-CSS and 6MWD changes, and change in CRP.
Limitations make it hard to generalize the results, the authors caution. Well over 90% of the participants were White patients, for example, and the overall trial was not powered to show subgroup differences.
Given the many patients with HFpEF who have a cardiometabolic phenotype and are with overweight or obesity, write Dr. Butler and colleagues, their treatment approach “may ultimately include combination therapy with SGLT2 inhibitors and GLP-1 receptor agonists, given their non-overlapping and complementary mechanisms of action.”
Dr. Fonarow noted that both MRAs and sacubitril-valsartan offer clinical benefits for patients with HF and LVEF “in the 41%-60% range” that are evident “across BMI categories.”
So it’s likely, he said, that those medications as well as SGLT2 inhibitors will be used along with GLP-1 receptor agonists for patients with HFpEF and obesity.
STEP-HFpEF was funded by Novo Nordisk. Dr. Butler and the other authors disclose consulting for many companies, a list of which can be found in the report. Dr. Fonarow reports consulting for multiple companies. Dr. McMurray discloses consulting for AstraZeneca. Dr. Ostrominski reports no relevant disclosures. Dr. Vaduganathan discloses receiving grant support, serving on advisory boards, or speaking for multiple companies and serving on committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics.
A version of this article appeared on Medscape.com.
AT HFSA 2023
The new word in liver disease: The story behind NAFLD’s rebranding as MASLD
A noteworthy shift recently occurred in the field of hepatology, but it didn’t stem from a clinical trial or medical finding. Instead, the change arose from a matter of semantics.
In a special article published online in the journal Hepatology, a diverse international consensus group introduced new terminology for one of the world’s most rapidly growing diseases.
The term nonalcoholic fatty liver disease (NAFLD) was to be officially retired, replaced with a more precise and descriptive term – metabolic dysfunction–associated steatotic liver disease (MASLD).
In addition, steatotic liver disease (SLD) would be used as an umbrella term encompassing both MASLD and a new subcategory, MetALD, for individuals with MASLD whose alcohol consumption ranges from 140 to 350 g/wk for women and from 210 to 420 g/wk for men. Nonalcoholic steatohepatitis (NASH) would be known as metabolic dysfunction-associated steatohepatitis (MASH).
said the NAFLD nomenclature consensus group’s co-lead, Mary E. Rinella, MD, professor of medicine at University of Chicago and director of the metabolic and fatty liver program at University of Chicago Hospitals.
“The only really new thing we did is identify a group of people who meet criteria for MASLD and also drink more than the allowable limit,” she said. “There are tons of these patients who were not being considered before. Now they’re in a category by themselves, where they are going to be able to be studied and better understood.”
Why make a change?
The unveiling of the new nomenclature marked the culmination of 3 years of dedicated work that was built upon decades of growing understanding about the pathophysiologic underpinnings of these disease states.
The terms NAFLD and NASH emerged in 1980 to describe patients with chronic liver disease who denied excessive alcohol consumption. However, in the past 2 decades, it became increasingly evident that the existing terminology was inadequate, the consensus group’s co-lead, Philip Newsome, PhD, said in an interview.
“There was a strong desire for a name that describes what the condition is, rather than what it isn’t; avoiding use of stigmatizing terms, such as fatty and alcoholic; and finally, a nomenclature that could recognize the coexistence of conditions,” said Dr. Newsome, former secretary general of the European Association for the Study of the Liver (EASL), and director of the Centre for Liver and Gastrointestinal Research at the University of Birmingham, England.
These forces, combined with the recognition that NAFLD and alcohol-related liver disease shared biological processes, created momentum for change.
The idea gained traction with a 2020 article that proposed “MAFLD” as a more suitable term because it would link the disease with its known cardiometabolic risks, Dr. Rinella explained.
“We thought that paper was going to be the beginning of a conversation, but what happened instead is it became a full-court press,” Dr. Rinella said.
Dr. Rinella and Dr. Newsome then spearheaded a study to determine whether content experts and patients supported change. The process was led by three prominent international liver societies: EASL, the American Association for the Study of Liver Diseases (AASLD), and the Asociación Latinoamericana para el Estudio del Hígado. The organizations received input from 236 panelists from 56 countries, reflecting the diverse voices essential for addressing a disease with an expanding global prevalence rate.
In this globalized world, you cannot make a decision from on high and then expect everybody to just adopt it, Dr. Rinella noted.
The panel utilized a modified Delphi consensus approach, necessitating a supermajority of respondents (67%) to vote in favor of the changes. Seventy-four percent felt that the current nomenclature was sufficiently flawed to consider a name change, and 89% preferred terminology that describes the underlying cause of the disease. A supermajority felt that having “metabolic disease or dysfunction” in the name would help patients better understand their disease (72%) and help health care professionals better explain or understand the disease (80%).
The participants settled on the new terminology, and the study resulted in a conclusion: “The new nomenclature and diagnostic criteria are widely supported, nonstigmatizing, and can improve awareness and patient identification.”
It was by no means a simple or straightforward task, according to Dr. Rinella. “Anytime you have a contentious issue and you engage a broad range of stakeholders, many of which you know are in disagreement, you’re going to have a difficult time reaching consensus,” she said.
Reassuring reluctant adopters
The backing of international liver societies will be crucial to ensuring the smooth and relatively swift adoption of the new nomenclature. The AASLD announced in July that it would begin this process by holding conversations with key stakeholders, including the Food and Drug Administration, patient organizations, and pharmaceutical industry representatives.
“By engaging external groups, we have gained valuable insights into potential roadblocks or barriers that may impede the full implementation of the new MASLD nomenclature,” AASLD President Norah Terrault, MD, MPH, FAASLD, told this news organization. “Knowing the types of issues they face will allow us to build an implementation plan that will help guide the field through adoption.”
Even with buy-in from key stakeholders, implementing the changes will be no small feat. It’s a “vast undertaking” that may result in short-term frustrations for some groups, Dr. Terrault said.
“For instance, researchers whose work commenced under the old nomenclature may not be able to alter their research papers and will need to publish under the old nomenclature, which may impact which journals their research could be published in,” she said. “Some patient advocacy groups may have the old nomenclature in their names, resulting in a need to rebrand and revise their educational resources. Patient materials need to be updated. Primary care professionals need to be educated. The list goes on.”
These changes demand both patience and time, Dr. Terrault said. This applies to those tasked with persuading colleagues and patients, as well as clinicians, many of whom have already expressed some resistance to the updated terminology.
The panel anticipated pushback from clinicians who still advocate for NAFLD. However, Dr. Rinella countered that a diagnosis of MASLD requires only one cardiometabolic risk factor and has 99% overlap in most populations. In contrast, the MAFLD diagnostic criteria put forward in 2020 proposed even more restrictive cardiometabolic criteria and greater tolerance for alcohol consumption and would alter the disease natural history, she said.
Concerns have also been raised that replacing NAFLD with MASLD might complicate the value of prior research efforts. However, this should not be a cause for concern, as extensive examination across multiple populations has demonstrated near complete overlap between the two definitions, Dr. Rinella said. Biomarker development, natural history studies, and drug development research will remain unaffected, she said.
Some detractors argue that the term “fatty” is sufficiently descriptive and not stigmatizing. However, Dr. Newsome contends that the panel’s research unequivocally disproves this notion.
“Our Delphi process demonstrated very clearly that over 50% felt it was stigmatizing, and in particular, there were clear supportive views for this change from many patient groups,” he noted. “The new nomenclature empowers patients to explain what the condition means without the use of emotional language.”
An opportunity to improve care
One compelling way to persuade reluctant adopters of the new nomenclature’s value is to highlight the opportunities it presents.
The updated terminology opens avenues for research and clinical improvements for patients who meet MASLD criteria and consume alcohol at higher levels (MetALD), Dr. Newsome said.
“There are questions about the relative contribution of these two factors to liver injury, and I see this as an opportunity to explore this area further,” he said.
Hepatologists should embrace this change as a means of increasing awareness regarding the metabolic origins of the disease, Dr. Rinella said. This, in turn, will help identify more patients who require treatment but who are currently overlooked by the existing system, she noted.
“Right now, only around 1% of people with advanced disease are being identified by primary care physicians,” she said. “Hopefully, by elevating the role of metabolic disease, primary care physicians, endocrinologists, and gastroenterologists will be able to identify more patients and bring them to care before they develop cirrhosis.”
Such an outcome would signify much more than a mere semantic shift; it would represent a major advancement in the diagnosis and management of the disease.
A version of this article appeared on Medscape.com.
A noteworthy shift recently occurred in the field of hepatology, but it didn’t stem from a clinical trial or medical finding. Instead, the change arose from a matter of semantics.
In a special article published online in the journal Hepatology, a diverse international consensus group introduced new terminology for one of the world’s most rapidly growing diseases.
The term nonalcoholic fatty liver disease (NAFLD) was to be officially retired, replaced with a more precise and descriptive term – metabolic dysfunction–associated steatotic liver disease (MASLD).
In addition, steatotic liver disease (SLD) would be used as an umbrella term encompassing both MASLD and a new subcategory, MetALD, for individuals with MASLD whose alcohol consumption ranges from 140 to 350 g/wk for women and from 210 to 420 g/wk for men. Nonalcoholic steatohepatitis (NASH) would be known as metabolic dysfunction-associated steatohepatitis (MASH).
said the NAFLD nomenclature consensus group’s co-lead, Mary E. Rinella, MD, professor of medicine at University of Chicago and director of the metabolic and fatty liver program at University of Chicago Hospitals.
“The only really new thing we did is identify a group of people who meet criteria for MASLD and also drink more than the allowable limit,” she said. “There are tons of these patients who were not being considered before. Now they’re in a category by themselves, where they are going to be able to be studied and better understood.”
Why make a change?
The unveiling of the new nomenclature marked the culmination of 3 years of dedicated work that was built upon decades of growing understanding about the pathophysiologic underpinnings of these disease states.
The terms NAFLD and NASH emerged in 1980 to describe patients with chronic liver disease who denied excessive alcohol consumption. However, in the past 2 decades, it became increasingly evident that the existing terminology was inadequate, the consensus group’s co-lead, Philip Newsome, PhD, said in an interview.
“There was a strong desire for a name that describes what the condition is, rather than what it isn’t; avoiding use of stigmatizing terms, such as fatty and alcoholic; and finally, a nomenclature that could recognize the coexistence of conditions,” said Dr. Newsome, former secretary general of the European Association for the Study of the Liver (EASL), and director of the Centre for Liver and Gastrointestinal Research at the University of Birmingham, England.
These forces, combined with the recognition that NAFLD and alcohol-related liver disease shared biological processes, created momentum for change.
The idea gained traction with a 2020 article that proposed “MAFLD” as a more suitable term because it would link the disease with its known cardiometabolic risks, Dr. Rinella explained.
“We thought that paper was going to be the beginning of a conversation, but what happened instead is it became a full-court press,” Dr. Rinella said.
Dr. Rinella and Dr. Newsome then spearheaded a study to determine whether content experts and patients supported change. The process was led by three prominent international liver societies: EASL, the American Association for the Study of Liver Diseases (AASLD), and the Asociación Latinoamericana para el Estudio del Hígado. The organizations received input from 236 panelists from 56 countries, reflecting the diverse voices essential for addressing a disease with an expanding global prevalence rate.
In this globalized world, you cannot make a decision from on high and then expect everybody to just adopt it, Dr. Rinella noted.
The panel utilized a modified Delphi consensus approach, necessitating a supermajority of respondents (67%) to vote in favor of the changes. Seventy-four percent felt that the current nomenclature was sufficiently flawed to consider a name change, and 89% preferred terminology that describes the underlying cause of the disease. A supermajority felt that having “metabolic disease or dysfunction” in the name would help patients better understand their disease (72%) and help health care professionals better explain or understand the disease (80%).
The participants settled on the new terminology, and the study resulted in a conclusion: “The new nomenclature and diagnostic criteria are widely supported, nonstigmatizing, and can improve awareness and patient identification.”
It was by no means a simple or straightforward task, according to Dr. Rinella. “Anytime you have a contentious issue and you engage a broad range of stakeholders, many of which you know are in disagreement, you’re going to have a difficult time reaching consensus,” she said.
Reassuring reluctant adopters
The backing of international liver societies will be crucial to ensuring the smooth and relatively swift adoption of the new nomenclature. The AASLD announced in July that it would begin this process by holding conversations with key stakeholders, including the Food and Drug Administration, patient organizations, and pharmaceutical industry representatives.
“By engaging external groups, we have gained valuable insights into potential roadblocks or barriers that may impede the full implementation of the new MASLD nomenclature,” AASLD President Norah Terrault, MD, MPH, FAASLD, told this news organization. “Knowing the types of issues they face will allow us to build an implementation plan that will help guide the field through adoption.”
Even with buy-in from key stakeholders, implementing the changes will be no small feat. It’s a “vast undertaking” that may result in short-term frustrations for some groups, Dr. Terrault said.
“For instance, researchers whose work commenced under the old nomenclature may not be able to alter their research papers and will need to publish under the old nomenclature, which may impact which journals their research could be published in,” she said. “Some patient advocacy groups may have the old nomenclature in their names, resulting in a need to rebrand and revise their educational resources. Patient materials need to be updated. Primary care professionals need to be educated. The list goes on.”
These changes demand both patience and time, Dr. Terrault said. This applies to those tasked with persuading colleagues and patients, as well as clinicians, many of whom have already expressed some resistance to the updated terminology.
The panel anticipated pushback from clinicians who still advocate for NAFLD. However, Dr. Rinella countered that a diagnosis of MASLD requires only one cardiometabolic risk factor and has 99% overlap in most populations. In contrast, the MAFLD diagnostic criteria put forward in 2020 proposed even more restrictive cardiometabolic criteria and greater tolerance for alcohol consumption and would alter the disease natural history, she said.
Concerns have also been raised that replacing NAFLD with MASLD might complicate the value of prior research efforts. However, this should not be a cause for concern, as extensive examination across multiple populations has demonstrated near complete overlap between the two definitions, Dr. Rinella said. Biomarker development, natural history studies, and drug development research will remain unaffected, she said.
Some detractors argue that the term “fatty” is sufficiently descriptive and not stigmatizing. However, Dr. Newsome contends that the panel’s research unequivocally disproves this notion.
“Our Delphi process demonstrated very clearly that over 50% felt it was stigmatizing, and in particular, there were clear supportive views for this change from many patient groups,” he noted. “The new nomenclature empowers patients to explain what the condition means without the use of emotional language.”
An opportunity to improve care
One compelling way to persuade reluctant adopters of the new nomenclature’s value is to highlight the opportunities it presents.
The updated terminology opens avenues for research and clinical improvements for patients who meet MASLD criteria and consume alcohol at higher levels (MetALD), Dr. Newsome said.
“There are questions about the relative contribution of these two factors to liver injury, and I see this as an opportunity to explore this area further,” he said.
Hepatologists should embrace this change as a means of increasing awareness regarding the metabolic origins of the disease, Dr. Rinella said. This, in turn, will help identify more patients who require treatment but who are currently overlooked by the existing system, she noted.
“Right now, only around 1% of people with advanced disease are being identified by primary care physicians,” she said. “Hopefully, by elevating the role of metabolic disease, primary care physicians, endocrinologists, and gastroenterologists will be able to identify more patients and bring them to care before they develop cirrhosis.”
Such an outcome would signify much more than a mere semantic shift; it would represent a major advancement in the diagnosis and management of the disease.
A version of this article appeared on Medscape.com.
A noteworthy shift recently occurred in the field of hepatology, but it didn’t stem from a clinical trial or medical finding. Instead, the change arose from a matter of semantics.
In a special article published online in the journal Hepatology, a diverse international consensus group introduced new terminology for one of the world’s most rapidly growing diseases.
The term nonalcoholic fatty liver disease (NAFLD) was to be officially retired, replaced with a more precise and descriptive term – metabolic dysfunction–associated steatotic liver disease (MASLD).
In addition, steatotic liver disease (SLD) would be used as an umbrella term encompassing both MASLD and a new subcategory, MetALD, for individuals with MASLD whose alcohol consumption ranges from 140 to 350 g/wk for women and from 210 to 420 g/wk for men. Nonalcoholic steatohepatitis (NASH) would be known as metabolic dysfunction-associated steatohepatitis (MASH).
said the NAFLD nomenclature consensus group’s co-lead, Mary E. Rinella, MD, professor of medicine at University of Chicago and director of the metabolic and fatty liver program at University of Chicago Hospitals.
“The only really new thing we did is identify a group of people who meet criteria for MASLD and also drink more than the allowable limit,” she said. “There are tons of these patients who were not being considered before. Now they’re in a category by themselves, where they are going to be able to be studied and better understood.”
Why make a change?
The unveiling of the new nomenclature marked the culmination of 3 years of dedicated work that was built upon decades of growing understanding about the pathophysiologic underpinnings of these disease states.
The terms NAFLD and NASH emerged in 1980 to describe patients with chronic liver disease who denied excessive alcohol consumption. However, in the past 2 decades, it became increasingly evident that the existing terminology was inadequate, the consensus group’s co-lead, Philip Newsome, PhD, said in an interview.
“There was a strong desire for a name that describes what the condition is, rather than what it isn’t; avoiding use of stigmatizing terms, such as fatty and alcoholic; and finally, a nomenclature that could recognize the coexistence of conditions,” said Dr. Newsome, former secretary general of the European Association for the Study of the Liver (EASL), and director of the Centre for Liver and Gastrointestinal Research at the University of Birmingham, England.
These forces, combined with the recognition that NAFLD and alcohol-related liver disease shared biological processes, created momentum for change.
The idea gained traction with a 2020 article that proposed “MAFLD” as a more suitable term because it would link the disease with its known cardiometabolic risks, Dr. Rinella explained.
“We thought that paper was going to be the beginning of a conversation, but what happened instead is it became a full-court press,” Dr. Rinella said.
Dr. Rinella and Dr. Newsome then spearheaded a study to determine whether content experts and patients supported change. The process was led by three prominent international liver societies: EASL, the American Association for the Study of Liver Diseases (AASLD), and the Asociación Latinoamericana para el Estudio del Hígado. The organizations received input from 236 panelists from 56 countries, reflecting the diverse voices essential for addressing a disease with an expanding global prevalence rate.
In this globalized world, you cannot make a decision from on high and then expect everybody to just adopt it, Dr. Rinella noted.
The panel utilized a modified Delphi consensus approach, necessitating a supermajority of respondents (67%) to vote in favor of the changes. Seventy-four percent felt that the current nomenclature was sufficiently flawed to consider a name change, and 89% preferred terminology that describes the underlying cause of the disease. A supermajority felt that having “metabolic disease or dysfunction” in the name would help patients better understand their disease (72%) and help health care professionals better explain or understand the disease (80%).
The participants settled on the new terminology, and the study resulted in a conclusion: “The new nomenclature and diagnostic criteria are widely supported, nonstigmatizing, and can improve awareness and patient identification.”
It was by no means a simple or straightforward task, according to Dr. Rinella. “Anytime you have a contentious issue and you engage a broad range of stakeholders, many of which you know are in disagreement, you’re going to have a difficult time reaching consensus,” she said.
Reassuring reluctant adopters
The backing of international liver societies will be crucial to ensuring the smooth and relatively swift adoption of the new nomenclature. The AASLD announced in July that it would begin this process by holding conversations with key stakeholders, including the Food and Drug Administration, patient organizations, and pharmaceutical industry representatives.
“By engaging external groups, we have gained valuable insights into potential roadblocks or barriers that may impede the full implementation of the new MASLD nomenclature,” AASLD President Norah Terrault, MD, MPH, FAASLD, told this news organization. “Knowing the types of issues they face will allow us to build an implementation plan that will help guide the field through adoption.”
Even with buy-in from key stakeholders, implementing the changes will be no small feat. It’s a “vast undertaking” that may result in short-term frustrations for some groups, Dr. Terrault said.
“For instance, researchers whose work commenced under the old nomenclature may not be able to alter their research papers and will need to publish under the old nomenclature, which may impact which journals their research could be published in,” she said. “Some patient advocacy groups may have the old nomenclature in their names, resulting in a need to rebrand and revise their educational resources. Patient materials need to be updated. Primary care professionals need to be educated. The list goes on.”
These changes demand both patience and time, Dr. Terrault said. This applies to those tasked with persuading colleagues and patients, as well as clinicians, many of whom have already expressed some resistance to the updated terminology.
The panel anticipated pushback from clinicians who still advocate for NAFLD. However, Dr. Rinella countered that a diagnosis of MASLD requires only one cardiometabolic risk factor and has 99% overlap in most populations. In contrast, the MAFLD diagnostic criteria put forward in 2020 proposed even more restrictive cardiometabolic criteria and greater tolerance for alcohol consumption and would alter the disease natural history, she said.
Concerns have also been raised that replacing NAFLD with MASLD might complicate the value of prior research efforts. However, this should not be a cause for concern, as extensive examination across multiple populations has demonstrated near complete overlap between the two definitions, Dr. Rinella said. Biomarker development, natural history studies, and drug development research will remain unaffected, she said.
Some detractors argue that the term “fatty” is sufficiently descriptive and not stigmatizing. However, Dr. Newsome contends that the panel’s research unequivocally disproves this notion.
“Our Delphi process demonstrated very clearly that over 50% felt it was stigmatizing, and in particular, there were clear supportive views for this change from many patient groups,” he noted. “The new nomenclature empowers patients to explain what the condition means without the use of emotional language.”
An opportunity to improve care
One compelling way to persuade reluctant adopters of the new nomenclature’s value is to highlight the opportunities it presents.
The updated terminology opens avenues for research and clinical improvements for patients who meet MASLD criteria and consume alcohol at higher levels (MetALD), Dr. Newsome said.
“There are questions about the relative contribution of these two factors to liver injury, and I see this as an opportunity to explore this area further,” he said.
Hepatologists should embrace this change as a means of increasing awareness regarding the metabolic origins of the disease, Dr. Rinella said. This, in turn, will help identify more patients who require treatment but who are currently overlooked by the existing system, she noted.
“Right now, only around 1% of people with advanced disease are being identified by primary care physicians,” she said. “Hopefully, by elevating the role of metabolic disease, primary care physicians, endocrinologists, and gastroenterologists will be able to identify more patients and bring them to care before they develop cirrhosis.”
Such an outcome would signify much more than a mere semantic shift; it would represent a major advancement in the diagnosis and management of the disease.
A version of this article appeared on Medscape.com.
Weight loss with semaglutide maintained for up to 3 years
Once weekly glucagon-like peptide 1 receptor agonist (GLP-1 RA) semaglutide (Ozempic, Novo Nordisk) significantly improved hemoglobin A1c level and body weight for up to 3 years in a large cohort of adults with type 2 diabetes, show real-world data from Israel.
and, in particular, in those patients with higher adherence to the therapy.
Avraham Karasik, MD, from the Institute of Research and Innovation at Maccabi Health Services, Tel Aviv, led the study and presented the work as a poster at this year’s annual meeting of the European Association for the Study of Diabetes.
“We found a clinically relevant improvement in blood sugar control and weight loss after 6 months of treatment, comparable with that seen in randomized trials,” said Dr. Karasik during an interview. “Importantly, these effects were sustained for up to 3 years, supporting the use of once weekly semaglutide for the long-term management of type 2 diabetes.”
Esther Walden, RN, deputy head of care at Diabetes UK, appreciated that the real-world findings reflected those seen in the randomized controlled trials. “This study suggests that improvements in blood sugars and weight loss can potentially be sustained in the longer term for adults with type 2 diabetes taking semaglutide as prescribed.”
Large scale, long term, and real world
Dr. Karasik explained that in Israel, there are many early adopters of once weekly semaglutide, and as such, it made for a large sample size, with a significant use duration for the retrospective study. “It’s a popular drug and there are lots of questions about durability of effect,” he pointed out.
Though evidence from randomized controlled trials support the effectiveness of once weekly semaglutide to treat type 2 diabetes, these studies are mostly of relatively short follow-up, explained Dr. Karasik, pointing out that long-term, large-scale, real-world data are needed. “In real life, people are acting differently to the trial setting and some adhere while others don’t, so it was interesting to see the durability as well as what happens when people discontinue treatment or adhere less.”
“Unsurprisingly, people who had a higher proportion of days covered ([PDC]; the total days of semaglutide use as a proportion of the total number of days followed up) had a higher effect,” explained Dr. Karasik, adding that, “if you don’t take it, it doesn’t work.”
A total of 23,442 patients were included in the study, with 6,049 followed up for 2 years or more. Mean baseline A1c was 7.6%-7.9%; body mass index (BMI) was 33.7-33.8 kg/m2; metformin was taken by 84%-88% of participants; insulin was taken by 30%; and 31% were treated with another GLP-1 RA prior to receiving semaglutide.
For study inclusion, participants were required to have had redeemed at least one prescription for subcutaneous semaglutide (0.25, 0.5, or 1 mg), and had at least one A1c measurement 12 months before and around 6 months after the start of semaglutide.
The primary outcome was change in A1c from baseline to the end of the follow-up at 6, 12, 18, 24, 30, and 36 months. Key secondary outcomes included change in body weight from baseline to the end of the follow-up (36 months); change in A1c and body weight in subgroups of patients who were persistently on therapy (at 12, 24, 36 months); and change in A1c and body weight in subgroups stratified by baseline characteristics. There was also an exploratory outcome, which was change in A1c and weight after treatment discontinuation. Dr. Karasik presented some of these results in his poster.
Median follow-up was 17.6 months in the total population and was 29.9 months in those who persisted with therapy for 2 years or more. “We have over 23,000 participants so it’s a large group, and these are not selected patients so the generalizability is better.”
Three-year sustained effect
Results from the total population showed that A1c lowered by a mean of 0.77% (from 7.6% to 6.8%) and body weight reduced by 4.7 kg (from 94.1 kg to 89.7 kg) after 6 months of treatment. These reductions were maintained during 3 years of follow-up in around 1,000 patients.
A significant 75% of participants adhered to once weekly semaglutide (PDC of more than 60%) within the first 6 months. In patients who used semaglutide for at least 2 years, those with high adherence (PDC of at least 80%) showed an A1c reduction of 0.76% after 24 months and of 0.43% after 36 months. Body weight was reduced by 6.0 kg after 24 months and 5.8 kg after 36 months.
Reductions in both A1c and weight were lower in patients with PDC of below 60%, compared with those with PDC of 60%-79% or 80% or over (statistically significant difference of P < .05 for between-groups differences for both outcomes across maximum follow-up time).
As expected, among patients who were GLP-1 RA–naive, reductions in A1c level and body weight were more pronounced, compared with GLP-1 RA–experienced patients (A1c reduction, –0.87% vs. –0.54%; weight loss, –5.5 kg vs. –3.0 kg, respectively; P < .001 for between-groups difference for both outcomes).
Dr. Karasik reported that some patients who stopped taking semaglutide did not regain weight immediately and that this potential residual effect after treatment discontinuation merits additional investigation. “This is not like in the randomized controlled trials. I don’t know how to interpret it, but that’s the observation. A1c did increase a little when they stopped therapy, compared to those with PDC [of 60%-79% or 80% or over] (P < .05 for between-groups difference for both outcomes in most follow-up time).”
He also highlighted that in regard to the long-term outcomes, “unlike many drugs where the effect fades out with time, here we don’t see that happening. This is another encouraging point.”
Dr. Karasik declares speaker fees and grants from Novo Nordisk, Boehringer Ingelheim, and AstraZeneca. The study was supported by Novo Nordisk.
A version of this article appeared on Medscape.com.
Once weekly glucagon-like peptide 1 receptor agonist (GLP-1 RA) semaglutide (Ozempic, Novo Nordisk) significantly improved hemoglobin A1c level and body weight for up to 3 years in a large cohort of adults with type 2 diabetes, show real-world data from Israel.
and, in particular, in those patients with higher adherence to the therapy.
Avraham Karasik, MD, from the Institute of Research and Innovation at Maccabi Health Services, Tel Aviv, led the study and presented the work as a poster at this year’s annual meeting of the European Association for the Study of Diabetes.
“We found a clinically relevant improvement in blood sugar control and weight loss after 6 months of treatment, comparable with that seen in randomized trials,” said Dr. Karasik during an interview. “Importantly, these effects were sustained for up to 3 years, supporting the use of once weekly semaglutide for the long-term management of type 2 diabetes.”
Esther Walden, RN, deputy head of care at Diabetes UK, appreciated that the real-world findings reflected those seen in the randomized controlled trials. “This study suggests that improvements in blood sugars and weight loss can potentially be sustained in the longer term for adults with type 2 diabetes taking semaglutide as prescribed.”
Large scale, long term, and real world
Dr. Karasik explained that in Israel, there are many early adopters of once weekly semaglutide, and as such, it made for a large sample size, with a significant use duration for the retrospective study. “It’s a popular drug and there are lots of questions about durability of effect,” he pointed out.
Though evidence from randomized controlled trials support the effectiveness of once weekly semaglutide to treat type 2 diabetes, these studies are mostly of relatively short follow-up, explained Dr. Karasik, pointing out that long-term, large-scale, real-world data are needed. “In real life, people are acting differently to the trial setting and some adhere while others don’t, so it was interesting to see the durability as well as what happens when people discontinue treatment or adhere less.”
“Unsurprisingly, people who had a higher proportion of days covered ([PDC]; the total days of semaglutide use as a proportion of the total number of days followed up) had a higher effect,” explained Dr. Karasik, adding that, “if you don’t take it, it doesn’t work.”
A total of 23,442 patients were included in the study, with 6,049 followed up for 2 years or more. Mean baseline A1c was 7.6%-7.9%; body mass index (BMI) was 33.7-33.8 kg/m2; metformin was taken by 84%-88% of participants; insulin was taken by 30%; and 31% were treated with another GLP-1 RA prior to receiving semaglutide.
For study inclusion, participants were required to have had redeemed at least one prescription for subcutaneous semaglutide (0.25, 0.5, or 1 mg), and had at least one A1c measurement 12 months before and around 6 months after the start of semaglutide.
The primary outcome was change in A1c from baseline to the end of the follow-up at 6, 12, 18, 24, 30, and 36 months. Key secondary outcomes included change in body weight from baseline to the end of the follow-up (36 months); change in A1c and body weight in subgroups of patients who were persistently on therapy (at 12, 24, 36 months); and change in A1c and body weight in subgroups stratified by baseline characteristics. There was also an exploratory outcome, which was change in A1c and weight after treatment discontinuation. Dr. Karasik presented some of these results in his poster.
Median follow-up was 17.6 months in the total population and was 29.9 months in those who persisted with therapy for 2 years or more. “We have over 23,000 participants so it’s a large group, and these are not selected patients so the generalizability is better.”
Three-year sustained effect
Results from the total population showed that A1c lowered by a mean of 0.77% (from 7.6% to 6.8%) and body weight reduced by 4.7 kg (from 94.1 kg to 89.7 kg) after 6 months of treatment. These reductions were maintained during 3 years of follow-up in around 1,000 patients.
A significant 75% of participants adhered to once weekly semaglutide (PDC of more than 60%) within the first 6 months. In patients who used semaglutide for at least 2 years, those with high adherence (PDC of at least 80%) showed an A1c reduction of 0.76% after 24 months and of 0.43% after 36 months. Body weight was reduced by 6.0 kg after 24 months and 5.8 kg after 36 months.
Reductions in both A1c and weight were lower in patients with PDC of below 60%, compared with those with PDC of 60%-79% or 80% or over (statistically significant difference of P < .05 for between-groups differences for both outcomes across maximum follow-up time).
As expected, among patients who were GLP-1 RA–naive, reductions in A1c level and body weight were more pronounced, compared with GLP-1 RA–experienced patients (A1c reduction, –0.87% vs. –0.54%; weight loss, –5.5 kg vs. –3.0 kg, respectively; P < .001 for between-groups difference for both outcomes).
Dr. Karasik reported that some patients who stopped taking semaglutide did not regain weight immediately and that this potential residual effect after treatment discontinuation merits additional investigation. “This is not like in the randomized controlled trials. I don’t know how to interpret it, but that’s the observation. A1c did increase a little when they stopped therapy, compared to those with PDC [of 60%-79% or 80% or over] (P < .05 for between-groups difference for both outcomes in most follow-up time).”
He also highlighted that in regard to the long-term outcomes, “unlike many drugs where the effect fades out with time, here we don’t see that happening. This is another encouraging point.”
Dr. Karasik declares speaker fees and grants from Novo Nordisk, Boehringer Ingelheim, and AstraZeneca. The study was supported by Novo Nordisk.
A version of this article appeared on Medscape.com.
Once weekly glucagon-like peptide 1 receptor agonist (GLP-1 RA) semaglutide (Ozempic, Novo Nordisk) significantly improved hemoglobin A1c level and body weight for up to 3 years in a large cohort of adults with type 2 diabetes, show real-world data from Israel.
and, in particular, in those patients with higher adherence to the therapy.
Avraham Karasik, MD, from the Institute of Research and Innovation at Maccabi Health Services, Tel Aviv, led the study and presented the work as a poster at this year’s annual meeting of the European Association for the Study of Diabetes.
“We found a clinically relevant improvement in blood sugar control and weight loss after 6 months of treatment, comparable with that seen in randomized trials,” said Dr. Karasik during an interview. “Importantly, these effects were sustained for up to 3 years, supporting the use of once weekly semaglutide for the long-term management of type 2 diabetes.”
Esther Walden, RN, deputy head of care at Diabetes UK, appreciated that the real-world findings reflected those seen in the randomized controlled trials. “This study suggests that improvements in blood sugars and weight loss can potentially be sustained in the longer term for adults with type 2 diabetes taking semaglutide as prescribed.”
Large scale, long term, and real world
Dr. Karasik explained that in Israel, there are many early adopters of once weekly semaglutide, and as such, it made for a large sample size, with a significant use duration for the retrospective study. “It’s a popular drug and there are lots of questions about durability of effect,” he pointed out.
Though evidence from randomized controlled trials support the effectiveness of once weekly semaglutide to treat type 2 diabetes, these studies are mostly of relatively short follow-up, explained Dr. Karasik, pointing out that long-term, large-scale, real-world data are needed. “In real life, people are acting differently to the trial setting and some adhere while others don’t, so it was interesting to see the durability as well as what happens when people discontinue treatment or adhere less.”
“Unsurprisingly, people who had a higher proportion of days covered ([PDC]; the total days of semaglutide use as a proportion of the total number of days followed up) had a higher effect,” explained Dr. Karasik, adding that, “if you don’t take it, it doesn’t work.”
A total of 23,442 patients were included in the study, with 6,049 followed up for 2 years or more. Mean baseline A1c was 7.6%-7.9%; body mass index (BMI) was 33.7-33.8 kg/m2; metformin was taken by 84%-88% of participants; insulin was taken by 30%; and 31% were treated with another GLP-1 RA prior to receiving semaglutide.
For study inclusion, participants were required to have had redeemed at least one prescription for subcutaneous semaglutide (0.25, 0.5, or 1 mg), and had at least one A1c measurement 12 months before and around 6 months after the start of semaglutide.
The primary outcome was change in A1c from baseline to the end of the follow-up at 6, 12, 18, 24, 30, and 36 months. Key secondary outcomes included change in body weight from baseline to the end of the follow-up (36 months); change in A1c and body weight in subgroups of patients who were persistently on therapy (at 12, 24, 36 months); and change in A1c and body weight in subgroups stratified by baseline characteristics. There was also an exploratory outcome, which was change in A1c and weight after treatment discontinuation. Dr. Karasik presented some of these results in his poster.
Median follow-up was 17.6 months in the total population and was 29.9 months in those who persisted with therapy for 2 years or more. “We have over 23,000 participants so it’s a large group, and these are not selected patients so the generalizability is better.”
Three-year sustained effect
Results from the total population showed that A1c lowered by a mean of 0.77% (from 7.6% to 6.8%) and body weight reduced by 4.7 kg (from 94.1 kg to 89.7 kg) after 6 months of treatment. These reductions were maintained during 3 years of follow-up in around 1,000 patients.
A significant 75% of participants adhered to once weekly semaglutide (PDC of more than 60%) within the first 6 months. In patients who used semaglutide for at least 2 years, those with high adherence (PDC of at least 80%) showed an A1c reduction of 0.76% after 24 months and of 0.43% after 36 months. Body weight was reduced by 6.0 kg after 24 months and 5.8 kg after 36 months.
Reductions in both A1c and weight were lower in patients with PDC of below 60%, compared with those with PDC of 60%-79% or 80% or over (statistically significant difference of P < .05 for between-groups differences for both outcomes across maximum follow-up time).
As expected, among patients who were GLP-1 RA–naive, reductions in A1c level and body weight were more pronounced, compared with GLP-1 RA–experienced patients (A1c reduction, –0.87% vs. –0.54%; weight loss, –5.5 kg vs. –3.0 kg, respectively; P < .001 for between-groups difference for both outcomes).
Dr. Karasik reported that some patients who stopped taking semaglutide did not regain weight immediately and that this potential residual effect after treatment discontinuation merits additional investigation. “This is not like in the randomized controlled trials. I don’t know how to interpret it, but that’s the observation. A1c did increase a little when they stopped therapy, compared to those with PDC [of 60%-79% or 80% or over] (P < .05 for between-groups difference for both outcomes in most follow-up time).”
He also highlighted that in regard to the long-term outcomes, “unlike many drugs where the effect fades out with time, here we don’t see that happening. This is another encouraging point.”
Dr. Karasik declares speaker fees and grants from Novo Nordisk, Boehringer Ingelheim, and AstraZeneca. The study was supported by Novo Nordisk.
A version of this article appeared on Medscape.com.
FROM EASD 2023
When to prescribe semaglutide?
A 36-year-old woman presents to your office for assistance with weight loss. She usually weighs around 150 lb, but she had two pregnancies in the past 4 years and has gained 70 lb. Her current weight is 220 lb with a body mass index (BMI) of 36.6 kg/m2, and she has been unable to lose any weight despite diet and exercise. She reports back pain and generalized fatigue but is primarily worried about developing type 2 diabetes, which runs in her family. Her insurance covers weight loss medications, but
More and more people are turning to “medical weight management” to drop pounds and improve their health. This is a strategy that adds pharmacotherapy to lifestyle modifications to treat the chronic disease of obesity, and it is analogous to the treatment of high blood pressure or high cholesterol with medications.
This patient meets the criteria set forth by the American Heart Association, American College of Cardiology, and The Obesity Society for the management of obesity with antiobesity medications:
- BMI ≥ 30 or BMI ≥ 27 with weight-related comorbidities and
- Has been unable to achieve ≥ 5% weight loss with lifestyle changes alone.
Several U.S. Food and Drug Administration–approved antiobesity medications have been proven to cause clinically significant weight loss:
- orlistat (Alli or Xenical).
- phentermine/topiramate (Qsymia).
- naltrexone/bupropion (Contrave).
- liraglutide 3.0 mg subcutaneously daily (Saxenda).
- semaglutide 2.4 mg subcutaneously weekly (Wegovy).
When considering an antiobesity medication for a patient, it’s important to discuss efficacy, side-effect profile, contraindications, cost and coverage, and long-term use.
In this commentary, we’ll specifically focus on semaglutide (Wegovy) as it is currently the most effective FDA-approved medication for weight loss.
Efficacy
In a phase 3 clinical trial, patients on semaglutide 2.4 mg weekly lost an average of 15% of their body weight at 68 weeks, or approximately 33 lb. It is important to note that there is variability in treatment response to semaglutide 2.4 mg, just like with any other medication. About 1 in 3 individuals lost ≥ 20% of their weight, but about 1 in every 10 patients did not lose any weight.
In this patient, who has a family history of type 2 diabetes, weight loss with semaglutide 2.4 mg will probably reduce her risk of developing diabetes. With just 5%-10% weight loss, she will see improvements in her blood glucose, blood pressure, and cholesterol. Even greater weight loss (≥ 10%) has been associated with resolution of fatty liver and sleep apnea.
Side effects
Before starting semaglutide, patients should be counseled about potential gastrointestinal side effects, including nausea, upset stomach, diarrhea, constipation, and reflux.
Side effects can be managed with dietary modifications, over-the-counter treatments, and slow dose escalation. Some common tips include:
- Eat slowly.
- Eat a bland diet.
- Avoid fatty or fried foods.
- Avoid lying down immediately after eating.
- Prioritize water and fiber intake to mitigate constipation.
- Use over-the-counter treatments as needed (for example, laxative for constipation).
Most of these side effects are present only during dose escalation and resolve once the patient is on a stable dose.
Patients should be counseled about the less than 1% risk for gallbladder issues or pancreatitis. They should be instructed to go to an urgent care or emergency room if they develop severe abdominal pain, recurrent vomiting, or the inability to eat or drink.
Contraindications
We don’t prescribe GLP-1 receptor agonists, including semaglutide 2.4 mg, in patients with a personal or family history of medullary thyroid cancer. GLP-1 agonists are contraindicated in patients with a history of pancreatitis or gastroparesis. All FDA-approved antiobesity medications are contraindicated in women who are breastfeeding or trying for pregnancy. If this patient would like to pursue pregnancy again, semaglutide 2.4 mg should be stopped 2 months prior to conception.
Access
In this case, the patient’s insurance covered semaglutide 2.4 mg with a copay of $25 per month. Without insurance, semaglutide 2.4 mg (Wegovy) costs about $1,400 per month, and semaglutide 2.0 mg (Ozempic), the formulation approved for type 2 diabetes, costs up to $1,000 per month. These price ranges are often cost-prohibitive and unsustainable, especially because these medications are intended for long-term use.
Currently, Medicare does not cover antiobesity medications nor do most state Medicaid plans. Therefore, these medications are usually not considered by patients who have Medicare or Medicaid insurance.
Because insurance coverage varies and out-of-pocket costs can be prohibitive, many individuals seek other ways of acquiring semaglutide. The off-label use of semaglutide 2.0 mg (Ozempic) for obesity is scientifically supported and safe, whereas the use of compounded semaglutide is risky due to lack of regulation.
Compounded semaglutide should be avoided, given that these products are not controlled by the FDA, and adverse events have been reported in connection with compounded semaglutide.
In our clinical practice, patients have reported advertisements for “generic semaglutide” compounded with vitamins like vitamin B12 or B6. This is a significant area of concern because some vitamins (for instance, vitamin B6) are toxic at high doses.
We discussed the dangers of compounded semaglutide with our patient and told her that this isn’t something we recommend prescribing. If the patient didn’t want to wait for semaglutide 2.4 mg to be available at her pharmacy, we discussed alternative medications used for the management of obesity, such as other FDA-approved GLP-1 agonists (that is, liraglutide 3.0 mg) and off-label medications. In this case, the patient opted to wait for semaglutide 2.4 mg because she preferred a weekly injectable medication, given her busy lifestyle as a new mom.
Dr. Schmitz, of Weill Cornell Medicine, New York, disclosed no relevant financial relationships. Dr. Tchang, of Weill Cornell Medicine and the Iris Cantor Women's Health Center, both in New York, serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for Gelesis and Novo Nordisk, and has received income from Gelesis.
A version of this article first appeared on Medscape.com.
A 36-year-old woman presents to your office for assistance with weight loss. She usually weighs around 150 lb, but she had two pregnancies in the past 4 years and has gained 70 lb. Her current weight is 220 lb with a body mass index (BMI) of 36.6 kg/m2, and she has been unable to lose any weight despite diet and exercise. She reports back pain and generalized fatigue but is primarily worried about developing type 2 diabetes, which runs in her family. Her insurance covers weight loss medications, but
More and more people are turning to “medical weight management” to drop pounds and improve their health. This is a strategy that adds pharmacotherapy to lifestyle modifications to treat the chronic disease of obesity, and it is analogous to the treatment of high blood pressure or high cholesterol with medications.
This patient meets the criteria set forth by the American Heart Association, American College of Cardiology, and The Obesity Society for the management of obesity with antiobesity medications:
- BMI ≥ 30 or BMI ≥ 27 with weight-related comorbidities and
- Has been unable to achieve ≥ 5% weight loss with lifestyle changes alone.
Several U.S. Food and Drug Administration–approved antiobesity medications have been proven to cause clinically significant weight loss:
- orlistat (Alli or Xenical).
- phentermine/topiramate (Qsymia).
- naltrexone/bupropion (Contrave).
- liraglutide 3.0 mg subcutaneously daily (Saxenda).
- semaglutide 2.4 mg subcutaneously weekly (Wegovy).
When considering an antiobesity medication for a patient, it’s important to discuss efficacy, side-effect profile, contraindications, cost and coverage, and long-term use.
In this commentary, we’ll specifically focus on semaglutide (Wegovy) as it is currently the most effective FDA-approved medication for weight loss.
Efficacy
In a phase 3 clinical trial, patients on semaglutide 2.4 mg weekly lost an average of 15% of their body weight at 68 weeks, or approximately 33 lb. It is important to note that there is variability in treatment response to semaglutide 2.4 mg, just like with any other medication. About 1 in 3 individuals lost ≥ 20% of their weight, but about 1 in every 10 patients did not lose any weight.
In this patient, who has a family history of type 2 diabetes, weight loss with semaglutide 2.4 mg will probably reduce her risk of developing diabetes. With just 5%-10% weight loss, she will see improvements in her blood glucose, blood pressure, and cholesterol. Even greater weight loss (≥ 10%) has been associated with resolution of fatty liver and sleep apnea.
Side effects
Before starting semaglutide, patients should be counseled about potential gastrointestinal side effects, including nausea, upset stomach, diarrhea, constipation, and reflux.
Side effects can be managed with dietary modifications, over-the-counter treatments, and slow dose escalation. Some common tips include:
- Eat slowly.
- Eat a bland diet.
- Avoid fatty or fried foods.
- Avoid lying down immediately after eating.
- Prioritize water and fiber intake to mitigate constipation.
- Use over-the-counter treatments as needed (for example, laxative for constipation).
Most of these side effects are present only during dose escalation and resolve once the patient is on a stable dose.
Patients should be counseled about the less than 1% risk for gallbladder issues or pancreatitis. They should be instructed to go to an urgent care or emergency room if they develop severe abdominal pain, recurrent vomiting, or the inability to eat or drink.
Contraindications
We don’t prescribe GLP-1 receptor agonists, including semaglutide 2.4 mg, in patients with a personal or family history of medullary thyroid cancer. GLP-1 agonists are contraindicated in patients with a history of pancreatitis or gastroparesis. All FDA-approved antiobesity medications are contraindicated in women who are breastfeeding or trying for pregnancy. If this patient would like to pursue pregnancy again, semaglutide 2.4 mg should be stopped 2 months prior to conception.
Access
In this case, the patient’s insurance covered semaglutide 2.4 mg with a copay of $25 per month. Without insurance, semaglutide 2.4 mg (Wegovy) costs about $1,400 per month, and semaglutide 2.0 mg (Ozempic), the formulation approved for type 2 diabetes, costs up to $1,000 per month. These price ranges are often cost-prohibitive and unsustainable, especially because these medications are intended for long-term use.
Currently, Medicare does not cover antiobesity medications nor do most state Medicaid plans. Therefore, these medications are usually not considered by patients who have Medicare or Medicaid insurance.
Because insurance coverage varies and out-of-pocket costs can be prohibitive, many individuals seek other ways of acquiring semaglutide. The off-label use of semaglutide 2.0 mg (Ozempic) for obesity is scientifically supported and safe, whereas the use of compounded semaglutide is risky due to lack of regulation.
Compounded semaglutide should be avoided, given that these products are not controlled by the FDA, and adverse events have been reported in connection with compounded semaglutide.
In our clinical practice, patients have reported advertisements for “generic semaglutide” compounded with vitamins like vitamin B12 or B6. This is a significant area of concern because some vitamins (for instance, vitamin B6) are toxic at high doses.
We discussed the dangers of compounded semaglutide with our patient and told her that this isn’t something we recommend prescribing. If the patient didn’t want to wait for semaglutide 2.4 mg to be available at her pharmacy, we discussed alternative medications used for the management of obesity, such as other FDA-approved GLP-1 agonists (that is, liraglutide 3.0 mg) and off-label medications. In this case, the patient opted to wait for semaglutide 2.4 mg because she preferred a weekly injectable medication, given her busy lifestyle as a new mom.
Dr. Schmitz, of Weill Cornell Medicine, New York, disclosed no relevant financial relationships. Dr. Tchang, of Weill Cornell Medicine and the Iris Cantor Women's Health Center, both in New York, serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for Gelesis and Novo Nordisk, and has received income from Gelesis.
A version of this article first appeared on Medscape.com.
A 36-year-old woman presents to your office for assistance with weight loss. She usually weighs around 150 lb, but she had two pregnancies in the past 4 years and has gained 70 lb. Her current weight is 220 lb with a body mass index (BMI) of 36.6 kg/m2, and she has been unable to lose any weight despite diet and exercise. She reports back pain and generalized fatigue but is primarily worried about developing type 2 diabetes, which runs in her family. Her insurance covers weight loss medications, but
More and more people are turning to “medical weight management” to drop pounds and improve their health. This is a strategy that adds pharmacotherapy to lifestyle modifications to treat the chronic disease of obesity, and it is analogous to the treatment of high blood pressure or high cholesterol with medications.
This patient meets the criteria set forth by the American Heart Association, American College of Cardiology, and The Obesity Society for the management of obesity with antiobesity medications:
- BMI ≥ 30 or BMI ≥ 27 with weight-related comorbidities and
- Has been unable to achieve ≥ 5% weight loss with lifestyle changes alone.
Several U.S. Food and Drug Administration–approved antiobesity medications have been proven to cause clinically significant weight loss:
- orlistat (Alli or Xenical).
- phentermine/topiramate (Qsymia).
- naltrexone/bupropion (Contrave).
- liraglutide 3.0 mg subcutaneously daily (Saxenda).
- semaglutide 2.4 mg subcutaneously weekly (Wegovy).
When considering an antiobesity medication for a patient, it’s important to discuss efficacy, side-effect profile, contraindications, cost and coverage, and long-term use.
In this commentary, we’ll specifically focus on semaglutide (Wegovy) as it is currently the most effective FDA-approved medication for weight loss.
Efficacy
In a phase 3 clinical trial, patients on semaglutide 2.4 mg weekly lost an average of 15% of their body weight at 68 weeks, or approximately 33 lb. It is important to note that there is variability in treatment response to semaglutide 2.4 mg, just like with any other medication. About 1 in 3 individuals lost ≥ 20% of their weight, but about 1 in every 10 patients did not lose any weight.
In this patient, who has a family history of type 2 diabetes, weight loss with semaglutide 2.4 mg will probably reduce her risk of developing diabetes. With just 5%-10% weight loss, she will see improvements in her blood glucose, blood pressure, and cholesterol. Even greater weight loss (≥ 10%) has been associated with resolution of fatty liver and sleep apnea.
Side effects
Before starting semaglutide, patients should be counseled about potential gastrointestinal side effects, including nausea, upset stomach, diarrhea, constipation, and reflux.
Side effects can be managed with dietary modifications, over-the-counter treatments, and slow dose escalation. Some common tips include:
- Eat slowly.
- Eat a bland diet.
- Avoid fatty or fried foods.
- Avoid lying down immediately after eating.
- Prioritize water and fiber intake to mitigate constipation.
- Use over-the-counter treatments as needed (for example, laxative for constipation).
Most of these side effects are present only during dose escalation and resolve once the patient is on a stable dose.
Patients should be counseled about the less than 1% risk for gallbladder issues or pancreatitis. They should be instructed to go to an urgent care or emergency room if they develop severe abdominal pain, recurrent vomiting, or the inability to eat or drink.
Contraindications
We don’t prescribe GLP-1 receptor agonists, including semaglutide 2.4 mg, in patients with a personal or family history of medullary thyroid cancer. GLP-1 agonists are contraindicated in patients with a history of pancreatitis or gastroparesis. All FDA-approved antiobesity medications are contraindicated in women who are breastfeeding or trying for pregnancy. If this patient would like to pursue pregnancy again, semaglutide 2.4 mg should be stopped 2 months prior to conception.
Access
In this case, the patient’s insurance covered semaglutide 2.4 mg with a copay of $25 per month. Without insurance, semaglutide 2.4 mg (Wegovy) costs about $1,400 per month, and semaglutide 2.0 mg (Ozempic), the formulation approved for type 2 diabetes, costs up to $1,000 per month. These price ranges are often cost-prohibitive and unsustainable, especially because these medications are intended for long-term use.
Currently, Medicare does not cover antiobesity medications nor do most state Medicaid plans. Therefore, these medications are usually not considered by patients who have Medicare or Medicaid insurance.
Because insurance coverage varies and out-of-pocket costs can be prohibitive, many individuals seek other ways of acquiring semaglutide. The off-label use of semaglutide 2.0 mg (Ozempic) for obesity is scientifically supported and safe, whereas the use of compounded semaglutide is risky due to lack of regulation.
Compounded semaglutide should be avoided, given that these products are not controlled by the FDA, and adverse events have been reported in connection with compounded semaglutide.
In our clinical practice, patients have reported advertisements for “generic semaglutide” compounded with vitamins like vitamin B12 or B6. This is a significant area of concern because some vitamins (for instance, vitamin B6) are toxic at high doses.
We discussed the dangers of compounded semaglutide with our patient and told her that this isn’t something we recommend prescribing. If the patient didn’t want to wait for semaglutide 2.4 mg to be available at her pharmacy, we discussed alternative medications used for the management of obesity, such as other FDA-approved GLP-1 agonists (that is, liraglutide 3.0 mg) and off-label medications. In this case, the patient opted to wait for semaglutide 2.4 mg because she preferred a weekly injectable medication, given her busy lifestyle as a new mom.
Dr. Schmitz, of Weill Cornell Medicine, New York, disclosed no relevant financial relationships. Dr. Tchang, of Weill Cornell Medicine and the Iris Cantor Women's Health Center, both in New York, serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for Gelesis and Novo Nordisk, and has received income from Gelesis.
A version of this article first appeared on Medscape.com.
Obesity in GI care
While AGA’s advocacy efforts related to access to colorectal cancer screening are frequently highlighted, this is one aspect of a larger advocacy agenda.
This month, I wish to highlight AGA’s extensive advocacy efforts focused on expanding access to obesity treatment. More than 2 in 5 adults in the U.S. have obesity, and weight management has been shown to be beneficial in patients with comorbid gastrointestinal diseases, such as metabolic dysfunction–associated steatotic liver disease, gastroesophageal reflux disease, gallbladder disease, pancreatitis, and GI malignancy.
In 2022, Inside Scope, a podcast by AGA, featured a 6-part seriescalled “Obesity in GI.” In July, Drs. Octavia Pickett-Blakely and Naresh Gunaratnam moderated a Gastro Bites lunch-and-learn session on “Obesity in GI Care – Embracing and Putting It into Practice” in which they discussed models of care delivery supporting obesity management in GI practice.
In November 2022, AGA released “AGA Clinical Practice Guideline on Pharmacological Interventions for Adults With Obesity,” (https://shorturl.at/bDNOV) to aid clinicians in appropriately prescribing obesity pharmacotherapy on the front lines of care.
On the policy front, in June, AGA held a Capitol Hill briefing in support of H.R.1577 - Treat and Reduce Obesity Act of 2021 (TROA), a bipartisan bill that would improve access to obesity treatment and care by expanding coverage under Medicare Part D for FDA-approved obesity pharmacotherapy, as well as related services such as behavioral, nutrition, and other counseling. Please check out our new obesity advocacy toolkit for more information.
This month we update you on important multi-society guidance regarding peri-endoscopic management of GLP-1 receptor agonists. We highlight new AGA Clinical Practice Updates on ostomy management and use of gastric POEM for treatment of gastroparesis, as well as a randomized controlled trial from Gastroenterology showing the effectiveness of hemostatic powder in the management of malignant GI bleeding as compared with standard care.
In our Member Spotlight, we feature gastroenterologist Sameer Berry, MD, MBA, who discusses his role as a physician-entrepreneur seeking to transform GI care delivery through his AGA GI Opportunity Fund–supported company, Oshi Health.
This issue includes our annual supplement, “Gastroenterology Data Trends.” It features a collection of contributions on GI and climate change, long COVID and the GI tract, and the evolution of targeted therapies for C. difficile, among others.
We hope you enjoy this, and all the exciting content included in our October issue.
Megan A. Adams, MD, JD, MSc
While AGA’s advocacy efforts related to access to colorectal cancer screening are frequently highlighted, this is one aspect of a larger advocacy agenda.
This month, I wish to highlight AGA’s extensive advocacy efforts focused on expanding access to obesity treatment. More than 2 in 5 adults in the U.S. have obesity, and weight management has been shown to be beneficial in patients with comorbid gastrointestinal diseases, such as metabolic dysfunction–associated steatotic liver disease, gastroesophageal reflux disease, gallbladder disease, pancreatitis, and GI malignancy.
In 2022, Inside Scope, a podcast by AGA, featured a 6-part seriescalled “Obesity in GI.” In July, Drs. Octavia Pickett-Blakely and Naresh Gunaratnam moderated a Gastro Bites lunch-and-learn session on “Obesity in GI Care – Embracing and Putting It into Practice” in which they discussed models of care delivery supporting obesity management in GI practice.
In November 2022, AGA released “AGA Clinical Practice Guideline on Pharmacological Interventions for Adults With Obesity,” (https://shorturl.at/bDNOV) to aid clinicians in appropriately prescribing obesity pharmacotherapy on the front lines of care.
On the policy front, in June, AGA held a Capitol Hill briefing in support of H.R.1577 - Treat and Reduce Obesity Act of 2021 (TROA), a bipartisan bill that would improve access to obesity treatment and care by expanding coverage under Medicare Part D for FDA-approved obesity pharmacotherapy, as well as related services such as behavioral, nutrition, and other counseling. Please check out our new obesity advocacy toolkit for more information.
This month we update you on important multi-society guidance regarding peri-endoscopic management of GLP-1 receptor agonists. We highlight new AGA Clinical Practice Updates on ostomy management and use of gastric POEM for treatment of gastroparesis, as well as a randomized controlled trial from Gastroenterology showing the effectiveness of hemostatic powder in the management of malignant GI bleeding as compared with standard care.
In our Member Spotlight, we feature gastroenterologist Sameer Berry, MD, MBA, who discusses his role as a physician-entrepreneur seeking to transform GI care delivery through his AGA GI Opportunity Fund–supported company, Oshi Health.
This issue includes our annual supplement, “Gastroenterology Data Trends.” It features a collection of contributions on GI and climate change, long COVID and the GI tract, and the evolution of targeted therapies for C. difficile, among others.
We hope you enjoy this, and all the exciting content included in our October issue.
Megan A. Adams, MD, JD, MSc
While AGA’s advocacy efforts related to access to colorectal cancer screening are frequently highlighted, this is one aspect of a larger advocacy agenda.
This month, I wish to highlight AGA’s extensive advocacy efforts focused on expanding access to obesity treatment. More than 2 in 5 adults in the U.S. have obesity, and weight management has been shown to be beneficial in patients with comorbid gastrointestinal diseases, such as metabolic dysfunction–associated steatotic liver disease, gastroesophageal reflux disease, gallbladder disease, pancreatitis, and GI malignancy.
In 2022, Inside Scope, a podcast by AGA, featured a 6-part seriescalled “Obesity in GI.” In July, Drs. Octavia Pickett-Blakely and Naresh Gunaratnam moderated a Gastro Bites lunch-and-learn session on “Obesity in GI Care – Embracing and Putting It into Practice” in which they discussed models of care delivery supporting obesity management in GI practice.
In November 2022, AGA released “AGA Clinical Practice Guideline on Pharmacological Interventions for Adults With Obesity,” (https://shorturl.at/bDNOV) to aid clinicians in appropriately prescribing obesity pharmacotherapy on the front lines of care.
On the policy front, in June, AGA held a Capitol Hill briefing in support of H.R.1577 - Treat and Reduce Obesity Act of 2021 (TROA), a bipartisan bill that would improve access to obesity treatment and care by expanding coverage under Medicare Part D for FDA-approved obesity pharmacotherapy, as well as related services such as behavioral, nutrition, and other counseling. Please check out our new obesity advocacy toolkit for more information.
This month we update you on important multi-society guidance regarding peri-endoscopic management of GLP-1 receptor agonists. We highlight new AGA Clinical Practice Updates on ostomy management and use of gastric POEM for treatment of gastroparesis, as well as a randomized controlled trial from Gastroenterology showing the effectiveness of hemostatic powder in the management of malignant GI bleeding as compared with standard care.
In our Member Spotlight, we feature gastroenterologist Sameer Berry, MD, MBA, who discusses his role as a physician-entrepreneur seeking to transform GI care delivery through his AGA GI Opportunity Fund–supported company, Oshi Health.
This issue includes our annual supplement, “Gastroenterology Data Trends.” It features a collection of contributions on GI and climate change, long COVID and the GI tract, and the evolution of targeted therapies for C. difficile, among others.
We hope you enjoy this, and all the exciting content included in our October issue.
Megan A. Adams, MD, JD, MSc
FDA gives semaglutide two drug safety–related label changes
The FDA added a warning to the drug-interaction section of the Ozempiclabel that reiterates a warning that is already in place in other label sections, reinforcing the message that the glucagon-like peptide-1 (GLP-1) receptor agonist Ozempiccan potentially interact with the action of certain other agents to increase a person’s risk for hypoglycemia.
The added text says: “Ozempic stimulates insulin release in the presence of elevated blood glucose concentrations. Patients receiving Ozempic in combination with an insulin secretagogue (for instance, sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia.”
This text was already included in both the “Warning and Precautions” and the “Adverse Reactions” sections of the label. The warning also advises, “The risk of hypoglycemia may be lowered by a reduction in the dose of sulfonylurea (or other concomitantly administered insulin secretagogue) or insulin. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia.”
Reports of ileus episodes after approval
The second addition concerns a new adverse reaction that was identified during the postmarketing experience.
The FDA has received more than 8,500 reports of gastrointestinal issues among patients prescribed glucagon-like peptide-1 (GLP-1) receptor agonists. Ileus is mentioned in 33 cases, including two deaths, associated with semaglutide. The FDA stopped short of saying there is a direct link between the drug and intestinal blockages.
“Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure,” the FDA stated in its approval of the label update.
The same warning for the risk of intestinal blockages is already listed on the labels for tirzepatide (Mounjaro, Lilly) and semaglutide injection 2.4 mg (Wegovy, Novo Nordisk).
The label change comes after a Louisiana woman filed a lawsuit in August that claims she was “severely injured” after using Mounjaro and Ozempic. She claimed the drug makers failed to disclose risks of vomiting and diarrhea due to inflammation of the stomach lining, as well as the risk of gastroparesis.
*Correction, 10/3/23: An earlier version of this article misstated the semaglutide formulation that received the updates.
A version of this article first appeared on Medscape.com.
The FDA added a warning to the drug-interaction section of the Ozempiclabel that reiterates a warning that is already in place in other label sections, reinforcing the message that the glucagon-like peptide-1 (GLP-1) receptor agonist Ozempiccan potentially interact with the action of certain other agents to increase a person’s risk for hypoglycemia.
The added text says: “Ozempic stimulates insulin release in the presence of elevated blood glucose concentrations. Patients receiving Ozempic in combination with an insulin secretagogue (for instance, sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia.”
This text was already included in both the “Warning and Precautions” and the “Adverse Reactions” sections of the label. The warning also advises, “The risk of hypoglycemia may be lowered by a reduction in the dose of sulfonylurea (or other concomitantly administered insulin secretagogue) or insulin. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia.”
Reports of ileus episodes after approval
The second addition concerns a new adverse reaction that was identified during the postmarketing experience.
The FDA has received more than 8,500 reports of gastrointestinal issues among patients prescribed glucagon-like peptide-1 (GLP-1) receptor agonists. Ileus is mentioned in 33 cases, including two deaths, associated with semaglutide. The FDA stopped short of saying there is a direct link between the drug and intestinal blockages.
“Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure,” the FDA stated in its approval of the label update.
The same warning for the risk of intestinal blockages is already listed on the labels for tirzepatide (Mounjaro, Lilly) and semaglutide injection 2.4 mg (Wegovy, Novo Nordisk).
The label change comes after a Louisiana woman filed a lawsuit in August that claims she was “severely injured” after using Mounjaro and Ozempic. She claimed the drug makers failed to disclose risks of vomiting and diarrhea due to inflammation of the stomach lining, as well as the risk of gastroparesis.
*Correction, 10/3/23: An earlier version of this article misstated the semaglutide formulation that received the updates.
A version of this article first appeared on Medscape.com.
The FDA added a warning to the drug-interaction section of the Ozempiclabel that reiterates a warning that is already in place in other label sections, reinforcing the message that the glucagon-like peptide-1 (GLP-1) receptor agonist Ozempiccan potentially interact with the action of certain other agents to increase a person’s risk for hypoglycemia.
The added text says: “Ozempic stimulates insulin release in the presence of elevated blood glucose concentrations. Patients receiving Ozempic in combination with an insulin secretagogue (for instance, sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia.”
This text was already included in both the “Warning and Precautions” and the “Adverse Reactions” sections of the label. The warning also advises, “The risk of hypoglycemia may be lowered by a reduction in the dose of sulfonylurea (or other concomitantly administered insulin secretagogue) or insulin. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia.”
Reports of ileus episodes after approval
The second addition concerns a new adverse reaction that was identified during the postmarketing experience.
The FDA has received more than 8,500 reports of gastrointestinal issues among patients prescribed glucagon-like peptide-1 (GLP-1) receptor agonists. Ileus is mentioned in 33 cases, including two deaths, associated with semaglutide. The FDA stopped short of saying there is a direct link between the drug and intestinal blockages.
“Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure,” the FDA stated in its approval of the label update.
The same warning for the risk of intestinal blockages is already listed on the labels for tirzepatide (Mounjaro, Lilly) and semaglutide injection 2.4 mg (Wegovy, Novo Nordisk).
The label change comes after a Louisiana woman filed a lawsuit in August that claims she was “severely injured” after using Mounjaro and Ozempic. She claimed the drug makers failed to disclose risks of vomiting and diarrhea due to inflammation of the stomach lining, as well as the risk of gastroparesis.
*Correction, 10/3/23: An earlier version of this article misstated the semaglutide formulation that received the updates.
A version of this article first appeared on Medscape.com.


