User login
Obstetric perineal trauma prevented with SAFE PASSAGES
LAS VEGAS – Severe obstetric perineal trauma can often be avoided, even in operative deliveries, with the use of a suite of evidence-based interventions, according to findings from two prospective studies.
Collectively, these interventions resulted in significant reductions in third- and fourth-degree perineal lacerations in both military and civilian settings, according to research presented at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
Developed by the Military Health System, the SAFE PASSAGES protocol brings together interventions that help achieve a controlled delivery over a relaxed perineum, minimizing risk for maternal obstetric trauma.
The entire SAFE PASSAGES curriculum is available free online.
Military results

In a prospective cohort design, 272,161 deliveries conducted before the 2011 implementation of the SAFE PASSAGES training program were compared with 451,446 postimplementation deliveries. Primary outcome measures were the incidence of third- and fourth-degree perineal lacerations during vaginal deliveries with and without instrumentation.
For vaginal deliveries with instrumentation within one service branch of the military medical system (Service X), implementation of SAFE PASSAGES training was associated with a 63.6% reduction in third- and fourth-degree perineal lacerations, compared with preintervention rates (P less than .001).
The other two services – Service Y and Service Z – received just administrative encouragement and saw a 15.5% reduction and a 12.6% increase in significant obstetric trauma when instrumentation-assisted vaginal deliveries were performed (P = .04 and .30, respectively), according to Merlin Fausett, MD, an ob.gyn. currently in private practice in Missoula, Mont., who led the SAFE PASSAGES efforts before retiring from the Air Force.
For vaginal deliveries performed without instrumentation, the rates also fell for Service X, which saw a 41.8% reduction in third- and fourth-degree perineal lacerations (P less than .002). The other services saw a 16% increase and a 12% decrease with administrative encouragement alone (P = .48 and .08, respectively).
Though the military training program had initially been conducted in person, Dr. Fausett said that the program was switched to web-based simulations because of budget constraints. Efficacy remained high, he said.
Civilian results
When the team-based simulation that formed the core of the military SAFE PASSAGES training was rolled out in a large civilian health care system, similar improvements were seen.
Over an 18-month period, 675 nurses, midwives, and physicians received simulation-based training in the SAFE PASSAGES techniques. Overall, severe perineal laceration rates in the civilian facilities were down by 38.53% after adoption of SAFE PASSAGES.
“We have really achieved a culture shift,” said Emily Marko, MD, an ob.gyn. and clerkship director for the Virginia Commonwealth University School of Medicine Inova Campus in Falls Church, Va. “This really requires the whole delivery team to get involved: the patient, the patient’s support person, the nurses, any midwives or doulas that are there,” she said. “To tell you the truth, this whole program is about paying attention to the perineum, and not rushing delivery.”
Posttraining surveys showed that 95% of providers had changed their practice patterns after training in the delivery strategies.
Interventions
The emphasis in SAFE PASSAGES is to achieve a slow, controlled delivery and to minimize strain on the perineum by means of conditioning, relaxation, and positioning.
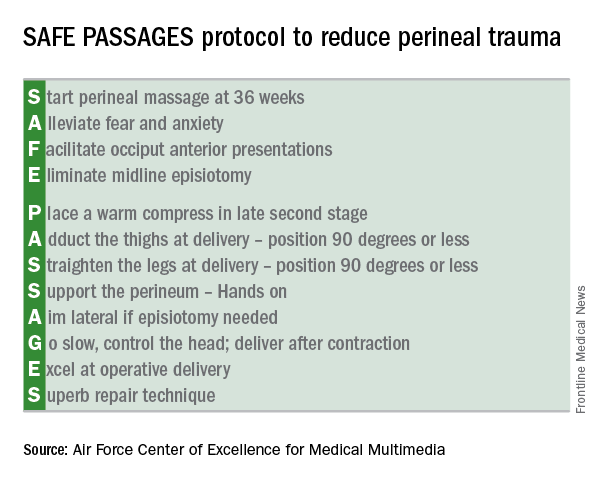
The first intervention is to have pregnant women “start” perineal massage at 36 weeks. Next, providers are urged to “alleviate” maternal fears. Providers are also encouraged to recognize posterior presentations, and to “facilitate” an anterior presentation through rotation. The “E” in SAFE stands for “eliminating” midline episiotomies – one of the more difficult practices to shift, according to both Dr. Fausett and Dr. Marko.
Despite a wealth of evidence showing fewer anal sphincter disruptions and better overall outcomes, it’s been difficult to convince U.S. physicians to adopt the mediolateral episiotomy technique that’s widely adopted in Europe, they said.
The protocol calls for “placing” a warm compress over the perineum during labor to encourage relaxation and stretching. Though prenatal perineal massage is encouraged, Dr. Marko said that intrapartum massage is not, as it’s thought to contribute to edema when performed during labor.
During delivery, leg positioning to reduce stretching of the perineum is also important: The thighs should be “adducted” to 90 degrees or less, and “straightened” to 90 degrees or less as well. Though this can make “a bit of a tight space” for the delivering physician, Dr. Marko said, it really “helps engage the pelvic muscles to support the perineum,” and a few technique adjustments make the position workable.
The perineum should be “supported” during delivery by one hand of the delivering practitioner forming a U shape with the thumb and forefinger, with the first webspace overlying the posterior fourchette. Reinforcing the importance of avoiding a midline episiotomy, the “A” of passages stands for “aiming” lateral when an episiotomy is needed.
During the delivery, the physician should “go” slow, controlling the head and delivering after, rather than during, a contraction.
It’s important to be comfortable with forceps deliveries and vacuum extractions in order to minimize both maternal and fetal trauma; thus, physicians should “excel” at operative delivery, according to the protocol.
The SAFE PASSAGES website includes comprehensive explanations, with graphics and demonstrations using a model, of both forceps and vacuum delivery techniques.
Finally, should a laceration occur, the SAFE PASSAGES website provides detailed explanations of repair techniques, with an emphasis on understanding perineal, vaginal, and anal anatomy so “superb” approximation and repair can be achieved.
Though obstetric trauma may not be life threatening, it’s still associated with significant and persistent morbidity. When perineal and pelvic floor trauma disrupts the anal sphincter, anal incontinence can occur, even after a meticulous attempt at repair. Perineal and pelvic floor trauma can result in a host of urinary and sexual problems as well.
After the intensive training period, Dr. Fausett said, “laceration rates can creep back up if people forget about it and stop paying attention to it. But where it becomes a culture, the rates can stay low. Standardized training can reduce perineal trauma rates without increasing cesarean or neonatal trauma rates,” Dr. Fausett said.
Dr. Marko and Dr. Fausett reported having no conflicts of interest.
[email protected]
On Twitter @karioakes
LAS VEGAS – Severe obstetric perineal trauma can often be avoided, even in operative deliveries, with the use of a suite of evidence-based interventions, according to findings from two prospective studies.
Collectively, these interventions resulted in significant reductions in third- and fourth-degree perineal lacerations in both military and civilian settings, according to research presented at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
Developed by the Military Health System, the SAFE PASSAGES protocol brings together interventions that help achieve a controlled delivery over a relaxed perineum, minimizing risk for maternal obstetric trauma.
The entire SAFE PASSAGES curriculum is available free online.

Military results

In a prospective cohort design, 272,161 deliveries conducted before the 2011 implementation of the SAFE PASSAGES training program were compared with 451,446 postimplementation deliveries. Primary outcome measures were the incidence of third- and fourth-degree perineal lacerations during vaginal deliveries with and without instrumentation.
For vaginal deliveries with instrumentation within one service branch of the military medical system (Service X), implementation of SAFE PASSAGES training was associated with a 63.6% reduction in third- and fourth-degree perineal lacerations, compared with preintervention rates (P less than .001).
The other two services – Service Y and Service Z – received just administrative encouragement and saw a 15.5% reduction and a 12.6% increase in significant obstetric trauma when instrumentation-assisted vaginal deliveries were performed (P = .04 and .30, respectively), according to Merlin Fausett, MD, an ob.gyn. currently in private practice in Missoula, Mont., who led the SAFE PASSAGES efforts before retiring from the Air Force.
For vaginal deliveries performed without instrumentation, the rates also fell for Service X, which saw a 41.8% reduction in third- and fourth-degree perineal lacerations (P less than .002). The other services saw a 16% increase and a 12% decrease with administrative encouragement alone (P = .48 and .08, respectively).
Though the military training program had initially been conducted in person, Dr. Fausett said that the program was switched to web-based simulations because of budget constraints. Efficacy remained high, he said.
Civilian results
When the team-based simulation that formed the core of the military SAFE PASSAGES training was rolled out in a large civilian health care system, similar improvements were seen.
Over an 18-month period, 675 nurses, midwives, and physicians received simulation-based training in the SAFE PASSAGES techniques. Overall, severe perineal laceration rates in the civilian facilities were down by 38.53% after adoption of SAFE PASSAGES.
“We have really achieved a culture shift,” said Emily Marko, MD, an ob.gyn. and clerkship director for the Virginia Commonwealth University School of Medicine Inova Campus in Falls Church, Va. “This really requires the whole delivery team to get involved: the patient, the patient’s support person, the nurses, any midwives or doulas that are there,” she said. “To tell you the truth, this whole program is about paying attention to the perineum, and not rushing delivery.”
Posttraining surveys showed that 95% of providers had changed their practice patterns after training in the delivery strategies.
Interventions
The emphasis in SAFE PASSAGES is to achieve a slow, controlled delivery and to minimize strain on the perineum by means of conditioning, relaxation, and positioning.
The first intervention is to have pregnant women “start” perineal massage at 36 weeks. Next, providers are urged to “alleviate” maternal fears. Providers are also encouraged to recognize posterior presentations, and to “facilitate” an anterior presentation through rotation. The “E” in SAFE stands for “eliminating” midline episiotomies – one of the more difficult practices to shift, according to both Dr. Fausett and Dr. Marko.
Despite a wealth of evidence showing fewer anal sphincter disruptions and better overall outcomes, it’s been difficult to convince U.S. physicians to adopt the mediolateral episiotomy technique that’s widely adopted in Europe, they said.
The protocol calls for “placing” a warm compress over the perineum during labor to encourage relaxation and stretching. Though prenatal perineal massage is encouraged, Dr. Marko said that intrapartum massage is not, as it’s thought to contribute to edema when performed during labor.
During delivery, leg positioning to reduce stretching of the perineum is also important: The thighs should be “adducted” to 90 degrees or less, and “straightened” to 90 degrees or less as well. Though this can make “a bit of a tight space” for the delivering physician, Dr. Marko said, it really “helps engage the pelvic muscles to support the perineum,” and a few technique adjustments make the position workable.
The perineum should be “supported” during delivery by one hand of the delivering practitioner forming a U shape with the thumb and forefinger, with the first webspace overlying the posterior fourchette. Reinforcing the importance of avoiding a midline episiotomy, the “A” of passages stands for “aiming” lateral when an episiotomy is needed.
During the delivery, the physician should “go” slow, controlling the head and delivering after, rather than during, a contraction.
It’s important to be comfortable with forceps deliveries and vacuum extractions in order to minimize both maternal and fetal trauma; thus, physicians should “excel” at operative delivery, according to the protocol.
The SAFE PASSAGES website includes comprehensive explanations, with graphics and demonstrations using a model, of both forceps and vacuum delivery techniques.
Finally, should a laceration occur, the SAFE PASSAGES website provides detailed explanations of repair techniques, with an emphasis on understanding perineal, vaginal, and anal anatomy so “superb” approximation and repair can be achieved.
Though obstetric trauma may not be life threatening, it’s still associated with significant and persistent morbidity. When perineal and pelvic floor trauma disrupts the anal sphincter, anal incontinence can occur, even after a meticulous attempt at repair. Perineal and pelvic floor trauma can result in a host of urinary and sexual problems as well.
After the intensive training period, Dr. Fausett said, “laceration rates can creep back up if people forget about it and stop paying attention to it. But where it becomes a culture, the rates can stay low. Standardized training can reduce perineal trauma rates without increasing cesarean or neonatal trauma rates,” Dr. Fausett said.
Dr. Marko and Dr. Fausett reported having no conflicts of interest.
[email protected]
On Twitter @karioakes
LAS VEGAS – Severe obstetric perineal trauma can often be avoided, even in operative deliveries, with the use of a suite of evidence-based interventions, according to findings from two prospective studies.
Collectively, these interventions resulted in significant reductions in third- and fourth-degree perineal lacerations in both military and civilian settings, according to research presented at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
Developed by the Military Health System, the SAFE PASSAGES protocol brings together interventions that help achieve a controlled delivery over a relaxed perineum, minimizing risk for maternal obstetric trauma.
The entire SAFE PASSAGES curriculum is available free online.

Military results

In a prospective cohort design, 272,161 deliveries conducted before the 2011 implementation of the SAFE PASSAGES training program were compared with 451,446 postimplementation deliveries. Primary outcome measures were the incidence of third- and fourth-degree perineal lacerations during vaginal deliveries with and without instrumentation.
For vaginal deliveries with instrumentation within one service branch of the military medical system (Service X), implementation of SAFE PASSAGES training was associated with a 63.6% reduction in third- and fourth-degree perineal lacerations, compared with preintervention rates (P less than .001).
The other two services – Service Y and Service Z – received just administrative encouragement and saw a 15.5% reduction and a 12.6% increase in significant obstetric trauma when instrumentation-assisted vaginal deliveries were performed (P = .04 and .30, respectively), according to Merlin Fausett, MD, an ob.gyn. currently in private practice in Missoula, Mont., who led the SAFE PASSAGES efforts before retiring from the Air Force.
For vaginal deliveries performed without instrumentation, the rates also fell for Service X, which saw a 41.8% reduction in third- and fourth-degree perineal lacerations (P less than .002). The other services saw a 16% increase and a 12% decrease with administrative encouragement alone (P = .48 and .08, respectively).
Though the military training program had initially been conducted in person, Dr. Fausett said that the program was switched to web-based simulations because of budget constraints. Efficacy remained high, he said.
Civilian results
When the team-based simulation that formed the core of the military SAFE PASSAGES training was rolled out in a large civilian health care system, similar improvements were seen.
Over an 18-month period, 675 nurses, midwives, and physicians received simulation-based training in the SAFE PASSAGES techniques. Overall, severe perineal laceration rates in the civilian facilities were down by 38.53% after adoption of SAFE PASSAGES.
“We have really achieved a culture shift,” said Emily Marko, MD, an ob.gyn. and clerkship director for the Virginia Commonwealth University School of Medicine Inova Campus in Falls Church, Va. “This really requires the whole delivery team to get involved: the patient, the patient’s support person, the nurses, any midwives or doulas that are there,” she said. “To tell you the truth, this whole program is about paying attention to the perineum, and not rushing delivery.”
Posttraining surveys showed that 95% of providers had changed their practice patterns after training in the delivery strategies.
Interventions
The emphasis in SAFE PASSAGES is to achieve a slow, controlled delivery and to minimize strain on the perineum by means of conditioning, relaxation, and positioning.
The first intervention is to have pregnant women “start” perineal massage at 36 weeks. Next, providers are urged to “alleviate” maternal fears. Providers are also encouraged to recognize posterior presentations, and to “facilitate” an anterior presentation through rotation. The “E” in SAFE stands for “eliminating” midline episiotomies – one of the more difficult practices to shift, according to both Dr. Fausett and Dr. Marko.
Despite a wealth of evidence showing fewer anal sphincter disruptions and better overall outcomes, it’s been difficult to convince U.S. physicians to adopt the mediolateral episiotomy technique that’s widely adopted in Europe, they said.
The protocol calls for “placing” a warm compress over the perineum during labor to encourage relaxation and stretching. Though prenatal perineal massage is encouraged, Dr. Marko said that intrapartum massage is not, as it’s thought to contribute to edema when performed during labor.
During delivery, leg positioning to reduce stretching of the perineum is also important: The thighs should be “adducted” to 90 degrees or less, and “straightened” to 90 degrees or less as well. Though this can make “a bit of a tight space” for the delivering physician, Dr. Marko said, it really “helps engage the pelvic muscles to support the perineum,” and a few technique adjustments make the position workable.
The perineum should be “supported” during delivery by one hand of the delivering practitioner forming a U shape with the thumb and forefinger, with the first webspace overlying the posterior fourchette. Reinforcing the importance of avoiding a midline episiotomy, the “A” of passages stands for “aiming” lateral when an episiotomy is needed.
During the delivery, the physician should “go” slow, controlling the head and delivering after, rather than during, a contraction.
It’s important to be comfortable with forceps deliveries and vacuum extractions in order to minimize both maternal and fetal trauma; thus, physicians should “excel” at operative delivery, according to the protocol.
The SAFE PASSAGES website includes comprehensive explanations, with graphics and demonstrations using a model, of both forceps and vacuum delivery techniques.
Finally, should a laceration occur, the SAFE PASSAGES website provides detailed explanations of repair techniques, with an emphasis on understanding perineal, vaginal, and anal anatomy so “superb” approximation and repair can be achieved.
Though obstetric trauma may not be life threatening, it’s still associated with significant and persistent morbidity. When perineal and pelvic floor trauma disrupts the anal sphincter, anal incontinence can occur, even after a meticulous attempt at repair. Perineal and pelvic floor trauma can result in a host of urinary and sexual problems as well.
After the intensive training period, Dr. Fausett said, “laceration rates can creep back up if people forget about it and stop paying attention to it. But where it becomes a culture, the rates can stay low. Standardized training can reduce perineal trauma rates without increasing cesarean or neonatal trauma rates,” Dr. Fausett said.
Dr. Marko and Dr. Fausett reported having no conflicts of interest.
[email protected]
On Twitter @karioakes
AT THE PREGNANCY MEETING
Key clinical point:
Major finding: Third- and fourth-degree perineal lacerations dropped by as much as 64% after SAFE PASSAGES training.
Data source: Prospective cohort study of 723,607 military deliveries; prospective study of 675 providers involved in labor and delivery at four civilian hospitals in a large health care system.
Disclosures: Dr. Marko and Dr. Fausett reported having no conflicts of interest.
Fewer infant deaths during ‘39-week rule’ era
LAS VEGAS – Closer adherence by U.S. physicians to the “39-week rule” for elective deliveries appears to have cut net neonatal mortality in an analysis of more than 14 million deliveries during 2008-2012.
This net drop in mortality occurred despite a concurrent rise in stillbirths, Rachel A. Pilliod, MD, said at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine. The increase in stillbirths was more than counterbalanced by a larger drop in infant deaths during the same period.
“It’s not a one-to-one trade, where each stillbirth corresponds to an infant death that is subsequently avoided. It’s hard to make this trade-off when counseling parents,” she said. “We think that there has been some effect from increasing gestational age on reducing overall mortality, but we need to do even better on identifying high risk [deliveries].”
What is “unacceptable,” Dr. Pilliod said, is if a woman needs an earlier delivery but it gets pushed back because of a poorly informed application of the 39-week rule.
Her study used data collected by the National Center for Health Statistics on U.S. deliveries each year, focusing on pregnancies that were singletons and nonanomalous.
She compared the 7,388,782 deliveries during 2008 and 2009 and 6,980,962 births during 2011 and 2012, selecting the 2-year time periods on either side of the Joint Commission’s 2010 adoption of a quality measure aimed at decreasing elective deliveries prior to 39 weeks gestation.
The Joint Commission’s action had its desired effect. Deliveries at 39 weeks jumped from 36% of all elective births in 2008 and 2009 to 43% in 2011 and 2012, while deliveries at 38 weeks show the biggest drop, from 22% to 20%, Dr. Pilliod reported (Am J Obstet Gynecol. 2017 Jan. doi: 10.1016/j.ajog.2016.11.959).
Concurrent with the rise in 39-week births and a drop in neonates with shorter gestation times, the incidence of stillbirths rose from 9.32 per 10,000 births in 2008 and 2009 to 10.15, an increase of 0.83 per 10,000 births.
But during the same periods the incidence of infant deaths fell, from 20.63 per 10,000 births in 2008 and 2009 to 19.0 in 2011 and 2012, a reduction of 1.63 per 10,000. Overall the stillbirth and infant death data combined for a net mortality reduction of 0.8 per 10,000 births.
[email protected]
On Twitter @mitchelzoler
LAS VEGAS – Closer adherence by U.S. physicians to the “39-week rule” for elective deliveries appears to have cut net neonatal mortality in an analysis of more than 14 million deliveries during 2008-2012.
This net drop in mortality occurred despite a concurrent rise in stillbirths, Rachel A. Pilliod, MD, said at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine. The increase in stillbirths was more than counterbalanced by a larger drop in infant deaths during the same period.
“It’s not a one-to-one trade, where each stillbirth corresponds to an infant death that is subsequently avoided. It’s hard to make this trade-off when counseling parents,” she said. “We think that there has been some effect from increasing gestational age on reducing overall mortality, but we need to do even better on identifying high risk [deliveries].”
What is “unacceptable,” Dr. Pilliod said, is if a woman needs an earlier delivery but it gets pushed back because of a poorly informed application of the 39-week rule.
Her study used data collected by the National Center for Health Statistics on U.S. deliveries each year, focusing on pregnancies that were singletons and nonanomalous.
She compared the 7,388,782 deliveries during 2008 and 2009 and 6,980,962 births during 2011 and 2012, selecting the 2-year time periods on either side of the Joint Commission’s 2010 adoption of a quality measure aimed at decreasing elective deliveries prior to 39 weeks gestation.
The Joint Commission’s action had its desired effect. Deliveries at 39 weeks jumped from 36% of all elective births in 2008 and 2009 to 43% in 2011 and 2012, while deliveries at 38 weeks show the biggest drop, from 22% to 20%, Dr. Pilliod reported (Am J Obstet Gynecol. 2017 Jan. doi: 10.1016/j.ajog.2016.11.959).
Concurrent with the rise in 39-week births and a drop in neonates with shorter gestation times, the incidence of stillbirths rose from 9.32 per 10,000 births in 2008 and 2009 to 10.15, an increase of 0.83 per 10,000 births.
But during the same periods the incidence of infant deaths fell, from 20.63 per 10,000 births in 2008 and 2009 to 19.0 in 2011 and 2012, a reduction of 1.63 per 10,000. Overall the stillbirth and infant death data combined for a net mortality reduction of 0.8 per 10,000 births.
[email protected]
On Twitter @mitchelzoler
LAS VEGAS – Closer adherence by U.S. physicians to the “39-week rule” for elective deliveries appears to have cut net neonatal mortality in an analysis of more than 14 million deliveries during 2008-2012.
This net drop in mortality occurred despite a concurrent rise in stillbirths, Rachel A. Pilliod, MD, said at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine. The increase in stillbirths was more than counterbalanced by a larger drop in infant deaths during the same period.
“It’s not a one-to-one trade, where each stillbirth corresponds to an infant death that is subsequently avoided. It’s hard to make this trade-off when counseling parents,” she said. “We think that there has been some effect from increasing gestational age on reducing overall mortality, but we need to do even better on identifying high risk [deliveries].”
What is “unacceptable,” Dr. Pilliod said, is if a woman needs an earlier delivery but it gets pushed back because of a poorly informed application of the 39-week rule.
Her study used data collected by the National Center for Health Statistics on U.S. deliveries each year, focusing on pregnancies that were singletons and nonanomalous.
She compared the 7,388,782 deliveries during 2008 and 2009 and 6,980,962 births during 2011 and 2012, selecting the 2-year time periods on either side of the Joint Commission’s 2010 adoption of a quality measure aimed at decreasing elective deliveries prior to 39 weeks gestation.
The Joint Commission’s action had its desired effect. Deliveries at 39 weeks jumped from 36% of all elective births in 2008 and 2009 to 43% in 2011 and 2012, while deliveries at 38 weeks show the biggest drop, from 22% to 20%, Dr. Pilliod reported (Am J Obstet Gynecol. 2017 Jan. doi: 10.1016/j.ajog.2016.11.959).
Concurrent with the rise in 39-week births and a drop in neonates with shorter gestation times, the incidence of stillbirths rose from 9.32 per 10,000 births in 2008 and 2009 to 10.15, an increase of 0.83 per 10,000 births.
But during the same periods the incidence of infant deaths fell, from 20.63 per 10,000 births in 2008 and 2009 to 19.0 in 2011 and 2012, a reduction of 1.63 per 10,000. Overall the stillbirth and infant death data combined for a net mortality reduction of 0.8 per 10,000 births.
[email protected]
On Twitter @mitchelzoler
AT THE PREGNANCY MEETING
Key clinical point:
Major finding: Net mortality dropped by 0.8 per 10,000 births from 2008 and 2009 to 2011 and 2012.
Data source: Review of U.S. birth records from the National Center for Health Statistics during 2008-2012.
Disclosures: Dr. Pilliod reported having no financial disclosures.
More risk factors boost mortality in home births
LAS VEGAS – Analysis of nearly 13 million U.S. deliveries during 2009-2013 identified two new, significant dangers posed to neonates delivered by planned home births: nulliparous pregnancies and deliveries at 41 weeks gestational age or older.
Both conditions linked with a substantially increased risk for neonatal mortality, compared with babies delivered at a hospital, either by a nurse midwife or a physician, said Amos Grünebaum, MD, at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
The critical difference between a home birth–like setting at a hospital and home birth in the field is distance from a hospital when emergency care is needed, he said.
“Women want less intervention during delivery and should get less intervention,” but a midwife run, home birth–like clinic should operate adjacent to a hospital able to handle obstetrical and neonatal emergencies, Dr. Grünebaum said in an interview. “Women need to understand the risks of home births.”
He and his associates used data collected by the Centers for Disease Control and Prevention on 12,953,671 U.S. deliveries during 2009-2013 for singleton, nonanomalous neonates with at least 37 weeks gestation at birth and weighing at least 2,500 grams. The total included 91% hospital deliveries by a physician, 8% hospital deliveries by a nurse-midwife, and 96,815 home births or 0.75% of U.S. deliveries during this period. Despite that low percentage, the number of U.S. home births nearly tripled from 2007 to 2015, he noted.
The rate of neonatal deaths for each 10,000 live births was 3 among infants delivered by nurse midwives at hospitals, 5 for infants delivered by physicians at hospitals, and 12 for infants delivered by home births. The standard mortality ratio was 66% higher for physicians at hospitals, compared with nurse-midwives at hospitals, because physicians handle higher-risk deliveries, and more than fourfold higher for home births, compared with hospital deliveries by nurse-midwives, Dr. Grünebaum reported.
Further analysis showed that the death rate per 10,000 neonates for pregnancies that continued to a gestational age of 41 weeks or more was 17.2, and for deliveries among nulliparous women, neonatal mortality was 22.5 deaths per 10,000 births. These rates were in the same ballpark as three conditions cited by an ACOG committee in a 2016 report as contraindications for home birth: prior cesarean delivery, which had home birth mortality of 18.9 per 10,000 neonates in the current study, multiple gestations, and breach presentation, with home birth mortality in the current study of 127.5 per 10,000.Maternal age of 35 years or greater at the time of delivery linked with a death rate of 13.6 per 10,000 births, a rate that Dr. Grünebaum did not consider high enough to specifically label it a contraindication to home birth. But Dr. Grünebaum took a dim view of home births in general. For any type of pregnancy, a birth center not adjacent to a hospital is “unprofessional,” he declared.
A journal article with this report also appeared online (Am J Ob Gyn. 2017 Jan 29. doi: 10.1016/j.ajog.2017.01.012).
[email protected]
On Twitter @mitchelzoler
LAS VEGAS – Analysis of nearly 13 million U.S. deliveries during 2009-2013 identified two new, significant dangers posed to neonates delivered by planned home births: nulliparous pregnancies and deliveries at 41 weeks gestational age or older.
Both conditions linked with a substantially increased risk for neonatal mortality, compared with babies delivered at a hospital, either by a nurse midwife or a physician, said Amos Grünebaum, MD, at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
The critical difference between a home birth–like setting at a hospital and home birth in the field is distance from a hospital when emergency care is needed, he said.
“Women want less intervention during delivery and should get less intervention,” but a midwife run, home birth–like clinic should operate adjacent to a hospital able to handle obstetrical and neonatal emergencies, Dr. Grünebaum said in an interview. “Women need to understand the risks of home births.”
He and his associates used data collected by the Centers for Disease Control and Prevention on 12,953,671 U.S. deliveries during 2009-2013 for singleton, nonanomalous neonates with at least 37 weeks gestation at birth and weighing at least 2,500 grams. The total included 91% hospital deliveries by a physician, 8% hospital deliveries by a nurse-midwife, and 96,815 home births or 0.75% of U.S. deliveries during this period. Despite that low percentage, the number of U.S. home births nearly tripled from 2007 to 2015, he noted.
The rate of neonatal deaths for each 10,000 live births was 3 among infants delivered by nurse midwives at hospitals, 5 for infants delivered by physicians at hospitals, and 12 for infants delivered by home births. The standard mortality ratio was 66% higher for physicians at hospitals, compared with nurse-midwives at hospitals, because physicians handle higher-risk deliveries, and more than fourfold higher for home births, compared with hospital deliveries by nurse-midwives, Dr. Grünebaum reported.
Further analysis showed that the death rate per 10,000 neonates for pregnancies that continued to a gestational age of 41 weeks or more was 17.2, and for deliveries among nulliparous women, neonatal mortality was 22.5 deaths per 10,000 births. These rates were in the same ballpark as three conditions cited by an ACOG committee in a 2016 report as contraindications for home birth: prior cesarean delivery, which had home birth mortality of 18.9 per 10,000 neonates in the current study, multiple gestations, and breach presentation, with home birth mortality in the current study of 127.5 per 10,000.Maternal age of 35 years or greater at the time of delivery linked with a death rate of 13.6 per 10,000 births, a rate that Dr. Grünebaum did not consider high enough to specifically label it a contraindication to home birth. But Dr. Grünebaum took a dim view of home births in general. For any type of pregnancy, a birth center not adjacent to a hospital is “unprofessional,” he declared.
A journal article with this report also appeared online (Am J Ob Gyn. 2017 Jan 29. doi: 10.1016/j.ajog.2017.01.012).
[email protected]
On Twitter @mitchelzoler
LAS VEGAS – Analysis of nearly 13 million U.S. deliveries during 2009-2013 identified two new, significant dangers posed to neonates delivered by planned home births: nulliparous pregnancies and deliveries at 41 weeks gestational age or older.
Both conditions linked with a substantially increased risk for neonatal mortality, compared with babies delivered at a hospital, either by a nurse midwife or a physician, said Amos Grünebaum, MD, at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
The critical difference between a home birth–like setting at a hospital and home birth in the field is distance from a hospital when emergency care is needed, he said.
“Women want less intervention during delivery and should get less intervention,” but a midwife run, home birth–like clinic should operate adjacent to a hospital able to handle obstetrical and neonatal emergencies, Dr. Grünebaum said in an interview. “Women need to understand the risks of home births.”
He and his associates used data collected by the Centers for Disease Control and Prevention on 12,953,671 U.S. deliveries during 2009-2013 for singleton, nonanomalous neonates with at least 37 weeks gestation at birth and weighing at least 2,500 grams. The total included 91% hospital deliveries by a physician, 8% hospital deliveries by a nurse-midwife, and 96,815 home births or 0.75% of U.S. deliveries during this period. Despite that low percentage, the number of U.S. home births nearly tripled from 2007 to 2015, he noted.
The rate of neonatal deaths for each 10,000 live births was 3 among infants delivered by nurse midwives at hospitals, 5 for infants delivered by physicians at hospitals, and 12 for infants delivered by home births. The standard mortality ratio was 66% higher for physicians at hospitals, compared with nurse-midwives at hospitals, because physicians handle higher-risk deliveries, and more than fourfold higher for home births, compared with hospital deliveries by nurse-midwives, Dr. Grünebaum reported.
Further analysis showed that the death rate per 10,000 neonates for pregnancies that continued to a gestational age of 41 weeks or more was 17.2, and for deliveries among nulliparous women, neonatal mortality was 22.5 deaths per 10,000 births. These rates were in the same ballpark as three conditions cited by an ACOG committee in a 2016 report as contraindications for home birth: prior cesarean delivery, which had home birth mortality of 18.9 per 10,000 neonates in the current study, multiple gestations, and breach presentation, with home birth mortality in the current study of 127.5 per 10,000.Maternal age of 35 years or greater at the time of delivery linked with a death rate of 13.6 per 10,000 births, a rate that Dr. Grünebaum did not consider high enough to specifically label it a contraindication to home birth. But Dr. Grünebaum took a dim view of home births in general. For any type of pregnancy, a birth center not adjacent to a hospital is “unprofessional,” he declared.
A journal article with this report also appeared online (Am J Ob Gyn. 2017 Jan 29. doi: 10.1016/j.ajog.2017.01.012).
[email protected]
On Twitter @mitchelzoler
AT THE PREGNANCY MEETING
Key clinical point:
Major finding: Home birth neonatal mortality per 10,000 births was 22.5 from nulliparous pregnancies and 17.2 with 41 weeks gestational age or greater.
Data source: Analysis of data from 12,953,671 selected full-term U.S. deliveries during 2009-2013, collected by the Centers for Disease Control and Prevention.
Disclosures: Dr. Grünebaum had no disclosures.
New Zika-infected pregnancies down for most of United States
New cases of Zika infection in pregnant women were down again for the 50 states over the 2-week reporting period ending Jan. 24, but U.S. territories saw a big increase, according to the Centers for Disease Control and Prevention.
It is important to note, however, that Puerto Rico, where most U.S. Zika cases are occurring, has been retroactively reporting cases for months, which can result in larger-than-normal increases.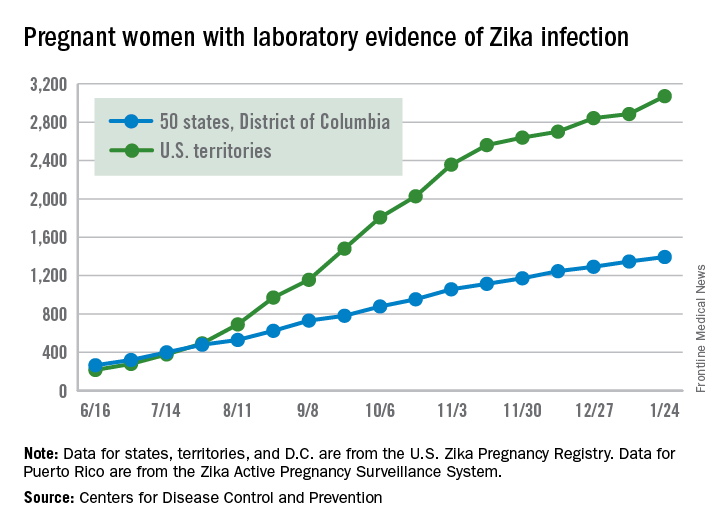
The territories had 186 new cases of pregnant women with laboratory evidence of Zika virus infection for the 2 weeks ending Jan. 24, compared with 43 for the previous 2-week period. The corresponding numbers for the 50 states and the District of Columbia were 47 (Jan. 24) and 55 (Jan. 10), the CDC data show.
So far for 2016-2017, there have been 4,465 pregnant women reported to have Zika virus infection in the United States: 3,071 in the territories and 1,394 in the 50 states and D.C., according to the U.S. Zika Pregnancy Registry (states, territories, and the District of Columbia) and the U.S. Zika Active Pregnancy Surveillance System (Puerto Rico).
Of the nearly 1,400 state/D.C. Zika-infected pregnancies, 999 have been completed, with 38 infants born with birth defects and 5 defect-related pregnancy losses, the CDC said. There have been no Zika-related pregnancy losses reported in the states/D.C. since late June. The CDC is no longer reporting adverse pregnancy outcomes for the territories because Puerto Rico is not using the same inclusion criteria.
Among all Americans in 2015-2017, there have been 41,387 cases of Zika infection as of Feb. 1. Of that total, 35,334 cases, which is more than 85%, have occurred in Puerto Rico, according to data from the CDC’s Arboviral Disease Branch.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
New cases of Zika infection in pregnant women were down again for the 50 states over the 2-week reporting period ending Jan. 24, but U.S. territories saw a big increase, according to the Centers for Disease Control and Prevention.
It is important to note, however, that Puerto Rico, where most U.S. Zika cases are occurring, has been retroactively reporting cases for months, which can result in larger-than-normal increases.
The territories had 186 new cases of pregnant women with laboratory evidence of Zika virus infection for the 2 weeks ending Jan. 24, compared with 43 for the previous 2-week period. The corresponding numbers for the 50 states and the District of Columbia were 47 (Jan. 24) and 55 (Jan. 10), the CDC data show.
So far for 2016-2017, there have been 4,465 pregnant women reported to have Zika virus infection in the United States: 3,071 in the territories and 1,394 in the 50 states and D.C., according to the U.S. Zika Pregnancy Registry (states, territories, and the District of Columbia) and the U.S. Zika Active Pregnancy Surveillance System (Puerto Rico).
Of the nearly 1,400 state/D.C. Zika-infected pregnancies, 999 have been completed, with 38 infants born with birth defects and 5 defect-related pregnancy losses, the CDC said. There have been no Zika-related pregnancy losses reported in the states/D.C. since late June. The CDC is no longer reporting adverse pregnancy outcomes for the territories because Puerto Rico is not using the same inclusion criteria.
Among all Americans in 2015-2017, there have been 41,387 cases of Zika infection as of Feb. 1. Of that total, 35,334 cases, which is more than 85%, have occurred in Puerto Rico, according to data from the CDC’s Arboviral Disease Branch.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
New cases of Zika infection in pregnant women were down again for the 50 states over the 2-week reporting period ending Jan. 24, but U.S. territories saw a big increase, according to the Centers for Disease Control and Prevention.
It is important to note, however, that Puerto Rico, where most U.S. Zika cases are occurring, has been retroactively reporting cases for months, which can result in larger-than-normal increases.
The territories had 186 new cases of pregnant women with laboratory evidence of Zika virus infection for the 2 weeks ending Jan. 24, compared with 43 for the previous 2-week period. The corresponding numbers for the 50 states and the District of Columbia were 47 (Jan. 24) and 55 (Jan. 10), the CDC data show.
So far for 2016-2017, there have been 4,465 pregnant women reported to have Zika virus infection in the United States: 3,071 in the territories and 1,394 in the 50 states and D.C., according to the U.S. Zika Pregnancy Registry (states, territories, and the District of Columbia) and the U.S. Zika Active Pregnancy Surveillance System (Puerto Rico).
Of the nearly 1,400 state/D.C. Zika-infected pregnancies, 999 have been completed, with 38 infants born with birth defects and 5 defect-related pregnancy losses, the CDC said. There have been no Zika-related pregnancy losses reported in the states/D.C. since late June. The CDC is no longer reporting adverse pregnancy outcomes for the territories because Puerto Rico is not using the same inclusion criteria.
Among all Americans in 2015-2017, there have been 41,387 cases of Zika infection as of Feb. 1. Of that total, 35,334 cases, which is more than 85%, have occurred in Puerto Rico, according to data from the CDC’s Arboviral Disease Branch.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
Detecting and managing monochorionic twin complications
Approximately 20% of all twin pregnancies are monochorionic, with the fetuses sharing a single placenta. Although the majority of these pregnancies are uncomplicated, monochorionic twins are significantly more likely than dichorionic twins to incur complications that can threaten the life and health of one or both fetuses.
The death of one monochorionic twin leaves the other twin with a 15% risk of demise. Survival after the loss of a co-twin is also associated with a 25% incidence of neurologic injury, compared with a 2% incidence in dichorionic pregnancies. Additionally, monochorionic pregnancies carry the risk of unique complications such as twin-to-twin transfusion syndrome, selective fetal growth restriction, twin anemia polycythemia sequence, and twin reversed arterial perfusion.
Increased ultrasonographic surveillance recommended for monochorionic twin pregnancies has been outlined in a recent consensus statement from the North American Fetal Therapy Network (Obstet Gynecol. 2015 Jan;125[1]:118-23). Beginning at 16 weeks’ gestation, monochorionic twins should be assessed every 2 weeks using amniotic fluid balance, presence/absence of fluid within the fetal bladder, and with fetal Doppler (umbilical artery, middle cerebral artery, and ductus venosus) studies. Fetal growth should also be assessed at least every 4 weeks.
Since monochorionic twins are at increased risk for congenital heart disease, echocardiography is also performed between 18 and 22 weeks, with surveillance intervals of 2 weeks or shorter if potential complications are identified. Early detection of these and other complications allows for earlier intervention, earlier referral if necessary, and potentially better outcomes.
Twin-to-twin transfusion syndrome
Twin-to-twin transfusion syndrome (TTTS) is one of the most common and most serious complications, affecting approximately 10% of monochorionic pregnancies. Significant imbalances in blood-flow exchange lead to progressive cardiovascular decompensation that causes one twin to become a “donor” of blood volume, and the other twin to become a “recipient.” Without proper treatment between 16 and 26 weeks’ gestation, the perinatal mortality rate has been estimated to be 70% or higher.
Disease severity is classified according to the Quintero staging system. Stage I is characterized by amniotic fluid discordance. In stage II, the bladder of the donor twin is no longer visible sonographically. Stage III is marked by critically abnormal Doppler waveforms in either twin (absent/reverse end-diastolic velocity in the umbilical artery, reverse flow in the ductus venosus, or pulsatile flow in the umbilical vein). In stage IV, one of the twins has developed hydrops, and stage V is characterized by the death of one or both of the twins.
Amnioreduction to decrease intra-amniotic pressure had been the treatment of choice until a randomized controlled trial, published in 2004, demonstrated that fetoscopic laser coagulation of anastomoses was superior as a first-line treatment for severe TTTS that is diagnosed before 26 weeks. Perinatal mortality and morbidity were significantly lower after the laser treatment (N Engl J Med. 2004 Jul 8;351[2]:136-44).
Outcomes were further improved over the next decade as the laser surgery technique was modified to cover the entire vascular equator rather than selective components of the vasculature. In an open-label randomized controlled trial comparing the two approaches for severe TTTS, fetoscopic laser coagulation of the vascular equator (known as the Solomon technique) reduced the risk of twin anemia polycythemia sequence and recurrence of TTTS – the two main postoperative complications associated with residual anastomoses after selective coagulation (Lancet. 2014 Jun 21;383[9935]:2144-51).
The procedure has many challenges and can be impacted by one’s inability to see the entire vascular equator because of poor access, by the patient’s history of other interventions, and by the stage of TTTS.
Laser coagulation is regarded as the standard treatment for Quintero stage II-IV disease, and it is offered in some cases of stage I disease, such as those involving severe polyhydramnios and shortened cervix. Research currently underway is examining the outcomes of treatment for stage I disease, but data thus far suggest that intervening at stage I is generally better than expectant management.
With laser coagulation treatment, the survival rate in pregnancies complicated by TTTS is about 85%-90% for one fetus, and about 70% for both. TTTS sometimes causes one twin, particularly the recipient, to develop pulmonary valve stenosis, but this is generally a functional problem that resolves when the syndrome is treated.
After treatment, it is important to monitor for the development of twin anemia-polycythemia sequence, which may still occur if full visualization of the vascular equator was not possible or if a fine vessel was missed. Such monitoring involves weekly ultrasound surveillance with middle cerebral artery peak systolic velocity measurements.
Patients should also be monitored for abnormal neurologic development, ventriculomegaly, and other signs of abnormal brain development. Even “perfect” laser treatment with seemingly complete placental separation has been associated with abnormal neurologic development in about 10%-15% of cases.
Maternal complications with TTTS include placental abruption and preterm membrane rupture, the latter of which occurs about 15%-20% of the time.
Currently under much discussion is fetoscopic laser coagulation of TTTS placentas that have “proximate cord insertions.” The surgery in these cases – where the cords are less than 4 cm apart – is much more challenging because of technical difficulties in visualizing the vascular equator, and outcomes are being studied. Some centers will not perform laser surgery on placentas with proximate cord insertions, which fortunately are uncommon. However, the surgery is possible; I have completed three cases thus far, each with dual survival.
Selective fetal growth restriction
Selective fetal growth restriction (sFGR) stems from unequal placental sharing and affects approximately 15%-20% of all monochorionic pregnancies, making it a bit more common than TTTS. Diagnostic criteria vary, but the North American Fetal Therapy Network recommends using either an estimated fetal weight below the 10th percentile, with or without significant growth discordance (greater than 25%), or just growth discordance greater than 25%. Either provides an acceptable definition of sFGR.
With sFGR, in general, the normally growing twin has normal fluid and the growth-restricted twin has less fluid. This makes it different from TTTS, in which the twins may have different sizes but fluid discordance is always present. Also in TTTS, there is a finding of polyhydramnios in the recipient.
There are three types of sFGR, based on umbilical artery Doppler findings. In type I there is no cardiovascular imbalance, and management typically involves weekly monitoring with Doppler ultrasound. If Doppler findings remain normal for some time, monitoring every 2-4 weeks will suffice. Elective delivery is generally set for 35 or 36 weeks.
Type II sFGR involves cardiovascular compromise early in pregnancy, with umbilical artery Doppler showing persistent reversed or absent end-diastolic flow. Treatment options include monitoring closely and, in general, delivering by 32 weeks. In these cases, prematurity may jeopardize the life or health of the normally growing twin while saving the life of the growth-restricted twin.
When type II sFGR is diagnosed early, selective termination of the growth-restricted fetus may be another option. This is a relatively safe procedure overall but it carries risks such as ruptured membrane and damage to the normal twin (10%-35% risk).
Type III sFGR is uniquely unpredictable, with intermittently absent or reversed flow stemming from a large artery-artery anastomosis. The direction of blood flow may suddenly change; in fact, the diagnosis is made by placing the Doppler caliper close to the placenta cord insertion and watching the end-diastolic flow. Present, absent, and reverse flow within a minute of observation demonstrates the presence of a large artery-artery anastomosis.
The risk of unexpected fetal death with severe sFGR is estimated to be 15% or higher, and the spontaneous death of the poorly growing twin threatens both the survival and the neurologic health of the co-twin. The risk of a parenchymal lesion for the co-twin is about 20%-40%.
Management decisions can be extremely difficult. As with type II, one could manage expectantly and generally deliver by 32 weeks. Fetoscopic laser coagulation to achieve complete dichorionization, as done with TTTS, could also be discussed; this approach could save the life of one twin in the event that the co-twin dies. Finally, selective termination may again be an option. None is a perfect treatment, and parents must be thoroughly counseled and supported in understanding the options and risks.
Twin anemia polycythemia sequence
Unlike TTTS, twin anemia polycythemia sequence (TAPS) does not involve a fluid shift. Rather, red blood cells shift from one fetus to the other through extremely small-caliber vessels, leading to severe anemia of one fetus and polycythemia of the other. The chronic and unbalanced transfusion occurs in about 5% of monochorionic twins, generally after 26 weeks’ gestation.
TAPS also occurs after laser treatment for TTTS in about 10%-15% of cases (generally within 4 weeks of treatment), though this incidence is significantly reduced when complete dichorionization is achieved using the Solomon technique for fetoscopic laser coagulation. Diagnosis is made when the middle cerebral artery peak systolic velocity of the red blood cell donor is greater than 1.5 MoM and the peak systolic velocity of the recipient is less than 1.0 MoM, without amniotic fluid discordance.
There are no established preferred treatments, but fetoscopic laser coagulation is an option for some patients. Visibility can be extremely poor when TAPS occurs after a laser treatment and vessels can be difficult to identify, but in selected cases it is possible with an experienced team. When performed, treatment can be followed by delivery or by intrauterine transfusion of the anemic fetus. Intrauterine transfusion has been studied as a primary treatment, but it generally is problematic because the small vessels at the root of TAPS continue to exist.
Twin reversed arterial perfusion
In about 1% of monochorionic pregnancies, an arterial incident prevents one of the twins from developing a heart and upper body. Some research has suggested that the condition is associated with trisomies in about 10% of the cases.
The viable, structurally normal co-twin therefore acts like a pump, continually perfusing the nonviable twin through an abnormal vascular circuit that allows arterial blood to flow in a reverse direction. In the process, the normal twin, or “pump twin,” can develop heart failure and hydrops. Mortality appears to be about 55%.
Diagnosis is straightforward, but it has been challenging to determine which pregnancies will require intervention. Some research has suggested that the risk of hydrops and mortality increases significantly – and favors intervention – when the weight difference is greater than 70%. On the other hand, if the difference is less than 50%, survival of the pump twin approaches 80% and continuing surveillance may be most appropriate.
Radiofrequency ablation of the cord of the nonviable twin is one of the treatment methods and has about an 80% success rate. Another option is coagulation of the blood supply in the abnormal twin using a laser fiber via a fine needle during the first trimester. An ongoing European trial of the procedure is showing success rates of approximately 70%.
Dr. Turan is director of fetal therapy and complex obstetric surgery, and an associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. He reported having no relevant financial disclosures.
Approximately 20% of all twin pregnancies are monochorionic, with the fetuses sharing a single placenta. Although the majority of these pregnancies are uncomplicated, monochorionic twins are significantly more likely than dichorionic twins to incur complications that can threaten the life and health of one or both fetuses.
The death of one monochorionic twin leaves the other twin with a 15% risk of demise. Survival after the loss of a co-twin is also associated with a 25% incidence of neurologic injury, compared with a 2% incidence in dichorionic pregnancies. Additionally, monochorionic pregnancies carry the risk of unique complications such as twin-to-twin transfusion syndrome, selective fetal growth restriction, twin anemia polycythemia sequence, and twin reversed arterial perfusion.
Increased ultrasonographic surveillance recommended for monochorionic twin pregnancies has been outlined in a recent consensus statement from the North American Fetal Therapy Network (Obstet Gynecol. 2015 Jan;125[1]:118-23). Beginning at 16 weeks’ gestation, monochorionic twins should be assessed every 2 weeks using amniotic fluid balance, presence/absence of fluid within the fetal bladder, and with fetal Doppler (umbilical artery, middle cerebral artery, and ductus venosus) studies. Fetal growth should also be assessed at least every 4 weeks.
Since monochorionic twins are at increased risk for congenital heart disease, echocardiography is also performed between 18 and 22 weeks, with surveillance intervals of 2 weeks or shorter if potential complications are identified. Early detection of these and other complications allows for earlier intervention, earlier referral if necessary, and potentially better outcomes.
Twin-to-twin transfusion syndrome
Twin-to-twin transfusion syndrome (TTTS) is one of the most common and most serious complications, affecting approximately 10% of monochorionic pregnancies. Significant imbalances in blood-flow exchange lead to progressive cardiovascular decompensation that causes one twin to become a “donor” of blood volume, and the other twin to become a “recipient.” Without proper treatment between 16 and 26 weeks’ gestation, the perinatal mortality rate has been estimated to be 70% or higher.
Disease severity is classified according to the Quintero staging system. Stage I is characterized by amniotic fluid discordance. In stage II, the bladder of the donor twin is no longer visible sonographically. Stage III is marked by critically abnormal Doppler waveforms in either twin (absent/reverse end-diastolic velocity in the umbilical artery, reverse flow in the ductus venosus, or pulsatile flow in the umbilical vein). In stage IV, one of the twins has developed hydrops, and stage V is characterized by the death of one or both of the twins.
Amnioreduction to decrease intra-amniotic pressure had been the treatment of choice until a randomized controlled trial, published in 2004, demonstrated that fetoscopic laser coagulation of anastomoses was superior as a first-line treatment for severe TTTS that is diagnosed before 26 weeks. Perinatal mortality and morbidity were significantly lower after the laser treatment (N Engl J Med. 2004 Jul 8;351[2]:136-44).
Outcomes were further improved over the next decade as the laser surgery technique was modified to cover the entire vascular equator rather than selective components of the vasculature. In an open-label randomized controlled trial comparing the two approaches for severe TTTS, fetoscopic laser coagulation of the vascular equator (known as the Solomon technique) reduced the risk of twin anemia polycythemia sequence and recurrence of TTTS – the two main postoperative complications associated with residual anastomoses after selective coagulation (Lancet. 2014 Jun 21;383[9935]:2144-51).
The procedure has many challenges and can be impacted by one’s inability to see the entire vascular equator because of poor access, by the patient’s history of other interventions, and by the stage of TTTS.
Laser coagulation is regarded as the standard treatment for Quintero stage II-IV disease, and it is offered in some cases of stage I disease, such as those involving severe polyhydramnios and shortened cervix. Research currently underway is examining the outcomes of treatment for stage I disease, but data thus far suggest that intervening at stage I is generally better than expectant management.
With laser coagulation treatment, the survival rate in pregnancies complicated by TTTS is about 85%-90% for one fetus, and about 70% for both. TTTS sometimes causes one twin, particularly the recipient, to develop pulmonary valve stenosis, but this is generally a functional problem that resolves when the syndrome is treated.
After treatment, it is important to monitor for the development of twin anemia-polycythemia sequence, which may still occur if full visualization of the vascular equator was not possible or if a fine vessel was missed. Such monitoring involves weekly ultrasound surveillance with middle cerebral artery peak systolic velocity measurements.
Patients should also be monitored for abnormal neurologic development, ventriculomegaly, and other signs of abnormal brain development. Even “perfect” laser treatment with seemingly complete placental separation has been associated with abnormal neurologic development in about 10%-15% of cases.
Maternal complications with TTTS include placental abruption and preterm membrane rupture, the latter of which occurs about 15%-20% of the time.
Currently under much discussion is fetoscopic laser coagulation of TTTS placentas that have “proximate cord insertions.” The surgery in these cases – where the cords are less than 4 cm apart – is much more challenging because of technical difficulties in visualizing the vascular equator, and outcomes are being studied. Some centers will not perform laser surgery on placentas with proximate cord insertions, which fortunately are uncommon. However, the surgery is possible; I have completed three cases thus far, each with dual survival.
Selective fetal growth restriction
Selective fetal growth restriction (sFGR) stems from unequal placental sharing and affects approximately 15%-20% of all monochorionic pregnancies, making it a bit more common than TTTS. Diagnostic criteria vary, but the North American Fetal Therapy Network recommends using either an estimated fetal weight below the 10th percentile, with or without significant growth discordance (greater than 25%), or just growth discordance greater than 25%. Either provides an acceptable definition of sFGR.
With sFGR, in general, the normally growing twin has normal fluid and the growth-restricted twin has less fluid. This makes it different from TTTS, in which the twins may have different sizes but fluid discordance is always present. Also in TTTS, there is a finding of polyhydramnios in the recipient.
There are three types of sFGR, based on umbilical artery Doppler findings. In type I there is no cardiovascular imbalance, and management typically involves weekly monitoring with Doppler ultrasound. If Doppler findings remain normal for some time, monitoring every 2-4 weeks will suffice. Elective delivery is generally set for 35 or 36 weeks.
Type II sFGR involves cardiovascular compromise early in pregnancy, with umbilical artery Doppler showing persistent reversed or absent end-diastolic flow. Treatment options include monitoring closely and, in general, delivering by 32 weeks. In these cases, prematurity may jeopardize the life or health of the normally growing twin while saving the life of the growth-restricted twin.
When type II sFGR is diagnosed early, selective termination of the growth-restricted fetus may be another option. This is a relatively safe procedure overall but it carries risks such as ruptured membrane and damage to the normal twin (10%-35% risk).
Type III sFGR is uniquely unpredictable, with intermittently absent or reversed flow stemming from a large artery-artery anastomosis. The direction of blood flow may suddenly change; in fact, the diagnosis is made by placing the Doppler caliper close to the placenta cord insertion and watching the end-diastolic flow. Present, absent, and reverse flow within a minute of observation demonstrates the presence of a large artery-artery anastomosis.
The risk of unexpected fetal death with severe sFGR is estimated to be 15% or higher, and the spontaneous death of the poorly growing twin threatens both the survival and the neurologic health of the co-twin. The risk of a parenchymal lesion for the co-twin is about 20%-40%.
Management decisions can be extremely difficult. As with type II, one could manage expectantly and generally deliver by 32 weeks. Fetoscopic laser coagulation to achieve complete dichorionization, as done with TTTS, could also be discussed; this approach could save the life of one twin in the event that the co-twin dies. Finally, selective termination may again be an option. None is a perfect treatment, and parents must be thoroughly counseled and supported in understanding the options and risks.
Twin anemia polycythemia sequence
Unlike TTTS, twin anemia polycythemia sequence (TAPS) does not involve a fluid shift. Rather, red blood cells shift from one fetus to the other through extremely small-caliber vessels, leading to severe anemia of one fetus and polycythemia of the other. The chronic and unbalanced transfusion occurs in about 5% of monochorionic twins, generally after 26 weeks’ gestation.
TAPS also occurs after laser treatment for TTTS in about 10%-15% of cases (generally within 4 weeks of treatment), though this incidence is significantly reduced when complete dichorionization is achieved using the Solomon technique for fetoscopic laser coagulation. Diagnosis is made when the middle cerebral artery peak systolic velocity of the red blood cell donor is greater than 1.5 MoM and the peak systolic velocity of the recipient is less than 1.0 MoM, without amniotic fluid discordance.
There are no established preferred treatments, but fetoscopic laser coagulation is an option for some patients. Visibility can be extremely poor when TAPS occurs after a laser treatment and vessels can be difficult to identify, but in selected cases it is possible with an experienced team. When performed, treatment can be followed by delivery or by intrauterine transfusion of the anemic fetus. Intrauterine transfusion has been studied as a primary treatment, but it generally is problematic because the small vessels at the root of TAPS continue to exist.
Twin reversed arterial perfusion
In about 1% of monochorionic pregnancies, an arterial incident prevents one of the twins from developing a heart and upper body. Some research has suggested that the condition is associated with trisomies in about 10% of the cases.
The viable, structurally normal co-twin therefore acts like a pump, continually perfusing the nonviable twin through an abnormal vascular circuit that allows arterial blood to flow in a reverse direction. In the process, the normal twin, or “pump twin,” can develop heart failure and hydrops. Mortality appears to be about 55%.
Diagnosis is straightforward, but it has been challenging to determine which pregnancies will require intervention. Some research has suggested that the risk of hydrops and mortality increases significantly – and favors intervention – when the weight difference is greater than 70%. On the other hand, if the difference is less than 50%, survival of the pump twin approaches 80% and continuing surveillance may be most appropriate.
Radiofrequency ablation of the cord of the nonviable twin is one of the treatment methods and has about an 80% success rate. Another option is coagulation of the blood supply in the abnormal twin using a laser fiber via a fine needle during the first trimester. An ongoing European trial of the procedure is showing success rates of approximately 70%.
Dr. Turan is director of fetal therapy and complex obstetric surgery, and an associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. He reported having no relevant financial disclosures.
Approximately 20% of all twin pregnancies are monochorionic, with the fetuses sharing a single placenta. Although the majority of these pregnancies are uncomplicated, monochorionic twins are significantly more likely than dichorionic twins to incur complications that can threaten the life and health of one or both fetuses.
The death of one monochorionic twin leaves the other twin with a 15% risk of demise. Survival after the loss of a co-twin is also associated with a 25% incidence of neurologic injury, compared with a 2% incidence in dichorionic pregnancies. Additionally, monochorionic pregnancies carry the risk of unique complications such as twin-to-twin transfusion syndrome, selective fetal growth restriction, twin anemia polycythemia sequence, and twin reversed arterial perfusion.
Increased ultrasonographic surveillance recommended for monochorionic twin pregnancies has been outlined in a recent consensus statement from the North American Fetal Therapy Network (Obstet Gynecol. 2015 Jan;125[1]:118-23). Beginning at 16 weeks’ gestation, monochorionic twins should be assessed every 2 weeks using amniotic fluid balance, presence/absence of fluid within the fetal bladder, and with fetal Doppler (umbilical artery, middle cerebral artery, and ductus venosus) studies. Fetal growth should also be assessed at least every 4 weeks.
Since monochorionic twins are at increased risk for congenital heart disease, echocardiography is also performed between 18 and 22 weeks, with surveillance intervals of 2 weeks or shorter if potential complications are identified. Early detection of these and other complications allows for earlier intervention, earlier referral if necessary, and potentially better outcomes.
Twin-to-twin transfusion syndrome
Twin-to-twin transfusion syndrome (TTTS) is one of the most common and most serious complications, affecting approximately 10% of monochorionic pregnancies. Significant imbalances in blood-flow exchange lead to progressive cardiovascular decompensation that causes one twin to become a “donor” of blood volume, and the other twin to become a “recipient.” Without proper treatment between 16 and 26 weeks’ gestation, the perinatal mortality rate has been estimated to be 70% or higher.
Disease severity is classified according to the Quintero staging system. Stage I is characterized by amniotic fluid discordance. In stage II, the bladder of the donor twin is no longer visible sonographically. Stage III is marked by critically abnormal Doppler waveforms in either twin (absent/reverse end-diastolic velocity in the umbilical artery, reverse flow in the ductus venosus, or pulsatile flow in the umbilical vein). In stage IV, one of the twins has developed hydrops, and stage V is characterized by the death of one or both of the twins.
Amnioreduction to decrease intra-amniotic pressure had been the treatment of choice until a randomized controlled trial, published in 2004, demonstrated that fetoscopic laser coagulation of anastomoses was superior as a first-line treatment for severe TTTS that is diagnosed before 26 weeks. Perinatal mortality and morbidity were significantly lower after the laser treatment (N Engl J Med. 2004 Jul 8;351[2]:136-44).
Outcomes were further improved over the next decade as the laser surgery technique was modified to cover the entire vascular equator rather than selective components of the vasculature. In an open-label randomized controlled trial comparing the two approaches for severe TTTS, fetoscopic laser coagulation of the vascular equator (known as the Solomon technique) reduced the risk of twin anemia polycythemia sequence and recurrence of TTTS – the two main postoperative complications associated with residual anastomoses after selective coagulation (Lancet. 2014 Jun 21;383[9935]:2144-51).
The procedure has many challenges and can be impacted by one’s inability to see the entire vascular equator because of poor access, by the patient’s history of other interventions, and by the stage of TTTS.
Laser coagulation is regarded as the standard treatment for Quintero stage II-IV disease, and it is offered in some cases of stage I disease, such as those involving severe polyhydramnios and shortened cervix. Research currently underway is examining the outcomes of treatment for stage I disease, but data thus far suggest that intervening at stage I is generally better than expectant management.
With laser coagulation treatment, the survival rate in pregnancies complicated by TTTS is about 85%-90% for one fetus, and about 70% for both. TTTS sometimes causes one twin, particularly the recipient, to develop pulmonary valve stenosis, but this is generally a functional problem that resolves when the syndrome is treated.
After treatment, it is important to monitor for the development of twin anemia-polycythemia sequence, which may still occur if full visualization of the vascular equator was not possible or if a fine vessel was missed. Such monitoring involves weekly ultrasound surveillance with middle cerebral artery peak systolic velocity measurements.
Patients should also be monitored for abnormal neurologic development, ventriculomegaly, and other signs of abnormal brain development. Even “perfect” laser treatment with seemingly complete placental separation has been associated with abnormal neurologic development in about 10%-15% of cases.
Maternal complications with TTTS include placental abruption and preterm membrane rupture, the latter of which occurs about 15%-20% of the time.
Currently under much discussion is fetoscopic laser coagulation of TTTS placentas that have “proximate cord insertions.” The surgery in these cases – where the cords are less than 4 cm apart – is much more challenging because of technical difficulties in visualizing the vascular equator, and outcomes are being studied. Some centers will not perform laser surgery on placentas with proximate cord insertions, which fortunately are uncommon. However, the surgery is possible; I have completed three cases thus far, each with dual survival.
Selective fetal growth restriction
Selective fetal growth restriction (sFGR) stems from unequal placental sharing and affects approximately 15%-20% of all monochorionic pregnancies, making it a bit more common than TTTS. Diagnostic criteria vary, but the North American Fetal Therapy Network recommends using either an estimated fetal weight below the 10th percentile, with or without significant growth discordance (greater than 25%), or just growth discordance greater than 25%. Either provides an acceptable definition of sFGR.
With sFGR, in general, the normally growing twin has normal fluid and the growth-restricted twin has less fluid. This makes it different from TTTS, in which the twins may have different sizes but fluid discordance is always present. Also in TTTS, there is a finding of polyhydramnios in the recipient.
There are three types of sFGR, based on umbilical artery Doppler findings. In type I there is no cardiovascular imbalance, and management typically involves weekly monitoring with Doppler ultrasound. If Doppler findings remain normal for some time, monitoring every 2-4 weeks will suffice. Elective delivery is generally set for 35 or 36 weeks.
Type II sFGR involves cardiovascular compromise early in pregnancy, with umbilical artery Doppler showing persistent reversed or absent end-diastolic flow. Treatment options include monitoring closely and, in general, delivering by 32 weeks. In these cases, prematurity may jeopardize the life or health of the normally growing twin while saving the life of the growth-restricted twin.
When type II sFGR is diagnosed early, selective termination of the growth-restricted fetus may be another option. This is a relatively safe procedure overall but it carries risks such as ruptured membrane and damage to the normal twin (10%-35% risk).
Type III sFGR is uniquely unpredictable, with intermittently absent or reversed flow stemming from a large artery-artery anastomosis. The direction of blood flow may suddenly change; in fact, the diagnosis is made by placing the Doppler caliper close to the placenta cord insertion and watching the end-diastolic flow. Present, absent, and reverse flow within a minute of observation demonstrates the presence of a large artery-artery anastomosis.
The risk of unexpected fetal death with severe sFGR is estimated to be 15% or higher, and the spontaneous death of the poorly growing twin threatens both the survival and the neurologic health of the co-twin. The risk of a parenchymal lesion for the co-twin is about 20%-40%.
Management decisions can be extremely difficult. As with type II, one could manage expectantly and generally deliver by 32 weeks. Fetoscopic laser coagulation to achieve complete dichorionization, as done with TTTS, could also be discussed; this approach could save the life of one twin in the event that the co-twin dies. Finally, selective termination may again be an option. None is a perfect treatment, and parents must be thoroughly counseled and supported in understanding the options and risks.
Twin anemia polycythemia sequence
Unlike TTTS, twin anemia polycythemia sequence (TAPS) does not involve a fluid shift. Rather, red blood cells shift from one fetus to the other through extremely small-caliber vessels, leading to severe anemia of one fetus and polycythemia of the other. The chronic and unbalanced transfusion occurs in about 5% of monochorionic twins, generally after 26 weeks’ gestation.
TAPS also occurs after laser treatment for TTTS in about 10%-15% of cases (generally within 4 weeks of treatment), though this incidence is significantly reduced when complete dichorionization is achieved using the Solomon technique for fetoscopic laser coagulation. Diagnosis is made when the middle cerebral artery peak systolic velocity of the red blood cell donor is greater than 1.5 MoM and the peak systolic velocity of the recipient is less than 1.0 MoM, without amniotic fluid discordance.
There are no established preferred treatments, but fetoscopic laser coagulation is an option for some patients. Visibility can be extremely poor when TAPS occurs after a laser treatment and vessels can be difficult to identify, but in selected cases it is possible with an experienced team. When performed, treatment can be followed by delivery or by intrauterine transfusion of the anemic fetus. Intrauterine transfusion has been studied as a primary treatment, but it generally is problematic because the small vessels at the root of TAPS continue to exist.
Twin reversed arterial perfusion
In about 1% of monochorionic pregnancies, an arterial incident prevents one of the twins from developing a heart and upper body. Some research has suggested that the condition is associated with trisomies in about 10% of the cases.
The viable, structurally normal co-twin therefore acts like a pump, continually perfusing the nonviable twin through an abnormal vascular circuit that allows arterial blood to flow in a reverse direction. In the process, the normal twin, or “pump twin,” can develop heart failure and hydrops. Mortality appears to be about 55%.
Diagnosis is straightforward, but it has been challenging to determine which pregnancies will require intervention. Some research has suggested that the risk of hydrops and mortality increases significantly – and favors intervention – when the weight difference is greater than 70%. On the other hand, if the difference is less than 50%, survival of the pump twin approaches 80% and continuing surveillance may be most appropriate.
Radiofrequency ablation of the cord of the nonviable twin is one of the treatment methods and has about an 80% success rate. Another option is coagulation of the blood supply in the abnormal twin using a laser fiber via a fine needle during the first trimester. An ongoing European trial of the procedure is showing success rates of approximately 70%.
Dr. Turan is director of fetal therapy and complex obstetric surgery, and an associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. He reported having no relevant financial disclosures.
How great is the risk from binge drinking in pregnancy?
Imagine this scenario: A couple goes on a Caribbean cruise with an all-you-can-eat buffet and an open bar. During the trip, they engage in 2 or 3 days of binge-style drinking, which is considered about four drinks in a single sitting. A few weeks after the trip, the woman finds out that she’s pregnant and calls your office wondering if there will be any harm to the fetus.
This is not a theoretical question. I have received many of these calls over the years, and in some cases the fear of adverse effects on the baby has led the couple to terminate the pregnancy.
Unfortunately, the literature has not been clear on the long-term impact of binge drinking in pregnancy. Animal studies suggest that it is the peak in the alcohol level created by binge drinking that causes damage to the fetus, rather than a sustained level of alcohol (although that obviously carries risk as well). The literature in humans has been controversial.
One of the most recent studies is a prospective cohort study of more than 1,600 women and their children sampled from the Danish National Birth Cohort. The investigators collected information on maternal alcohol use in early pregnancy and examined children at age 5 years using the Strengths and Difficulties Questionnaire (SDQ) completed by the mothers and a preschool teacher. It found no statistically significant association between binge drinking in early pregnancy and child behavior at age 5 years (BJOG. 2013 Aug;120[9]:1042-50).
In this study, the investigators corrected for parental education, maternal IQ, prenatal maternal smoking and postnatal parental smoking, the child’s age at testing, the child’s gender, maternal age, parity, marital status, family-home environment, prepregnancy maternal body mass index, and the child’s health status. After adjusting for confounders, they found no association between binge drinking and scores on the SDQ (odds ratio, 1.2; 95% confidence interval, 0.8-1.7 for behavioral scores and OR, 0.8; 95% CI, 0.6-1.2 for total difficulties scores). Additionally, the investigators analyzed low to moderate weekly alcohol consumption in early pregnancy and also could not find a significant effect (OR, 1.1; 95% CI, 0.5-2.3 for behavioral scores and OR, 1.1; 95% CI, 0.6-2.1 for the total difficulties scores).
This finding received a lot of press attention at the time, but it’s not the only study that has shown a lack of effect from binge drinking in contrast to the conventional wisdom on this subject.
A meta-analysis published in early 2014 further adds to the literature on this topic (Alcohol Clin Exp Res. 2014 Jan;38[1]:214-26).
The meta-analysis showed a small, but statistically significant effect on child’s cognition associated with binge drinking in pregnancy (Cohen’s d [a standardized mean difference score] −0.13; 95% CI, −0.21, −0.05). It did not include the 2013 Danish study.
The meta-analysis examined other levels of drinking in pregnancy, not just binging. Out of more than 1,500 papers that were examined, 34 studies met the criteria for inclusion, and just eight were included in the binge drinking analysis. The eight studies comprised more than 10,000 children who were tested from ages 6 months to 14 years. The researchers analyzed eight functional domains: academic performance, attention, behavior, cognition, memory, language and verbal development, executive function, and visual and motor development.
The researchers also separated the studies based on quality. When they analyzed the results for only high-quality studies, the cognition effect was not significant and no other associations were found with other child neuropsychological outcomes.
Several other studies have examined different endpoints, particularly hyperactivity and externalizing behaviors. While several studies show a trend toward those effects, mothers who binge drink also tend to be more externalizing in their own behavior.
An examination of the literature shows just how difficult it is to produce clear results that inform clinical practice. Adjusting for confounding factors from marital status to maternal IQ is just one hurdle. Another area that plagues researchers is that knowledge of drinking in early pregnancy is based on self-reports, and it is nearly impossible to know for sure if the reports of binging are accurate and also if there has been chronic alcohol use.
So what does all of this mean when it comes to advising women? There is no question that women should be advised not to drink when they are pregnant or planning a pregnancy. For a woman who engaged in binge drinking before she knew she was pregnant, it’s difficult to say that there is no effect. Instead, the collective evidence suggests there may be a small effect on cognition. In cases where binge drinking has occurred, children should be monitored as early as possible for any potential developmental effects.
Dr. Koren is professor of physiology/pharmacology and pediatrics at Western University in Ontario. He is the founder of the Motherisk Program. He reported having no relevant financial disclosures. Email him at [email protected].
Imagine this scenario: A couple goes on a Caribbean cruise with an all-you-can-eat buffet and an open bar. During the trip, they engage in 2 or 3 days of binge-style drinking, which is considered about four drinks in a single sitting. A few weeks after the trip, the woman finds out that she’s pregnant and calls your office wondering if there will be any harm to the fetus.
This is not a theoretical question. I have received many of these calls over the years, and in some cases the fear of adverse effects on the baby has led the couple to terminate the pregnancy.
Unfortunately, the literature has not been clear on the long-term impact of binge drinking in pregnancy. Animal studies suggest that it is the peak in the alcohol level created by binge drinking that causes damage to the fetus, rather than a sustained level of alcohol (although that obviously carries risk as well). The literature in humans has been controversial.
One of the most recent studies is a prospective cohort study of more than 1,600 women and their children sampled from the Danish National Birth Cohort. The investigators collected information on maternal alcohol use in early pregnancy and examined children at age 5 years using the Strengths and Difficulties Questionnaire (SDQ) completed by the mothers and a preschool teacher. It found no statistically significant association between binge drinking in early pregnancy and child behavior at age 5 years (BJOG. 2013 Aug;120[9]:1042-50).
In this study, the investigators corrected for parental education, maternal IQ, prenatal maternal smoking and postnatal parental smoking, the child’s age at testing, the child’s gender, maternal age, parity, marital status, family-home environment, prepregnancy maternal body mass index, and the child’s health status. After adjusting for confounders, they found no association between binge drinking and scores on the SDQ (odds ratio, 1.2; 95% confidence interval, 0.8-1.7 for behavioral scores and OR, 0.8; 95% CI, 0.6-1.2 for total difficulties scores). Additionally, the investigators analyzed low to moderate weekly alcohol consumption in early pregnancy and also could not find a significant effect (OR, 1.1; 95% CI, 0.5-2.3 for behavioral scores and OR, 1.1; 95% CI, 0.6-2.1 for the total difficulties scores).
This finding received a lot of press attention at the time, but it’s not the only study that has shown a lack of effect from binge drinking in contrast to the conventional wisdom on this subject.
A meta-analysis published in early 2014 further adds to the literature on this topic (Alcohol Clin Exp Res. 2014 Jan;38[1]:214-26).
The meta-analysis showed a small, but statistically significant effect on child’s cognition associated with binge drinking in pregnancy (Cohen’s d [a standardized mean difference score] −0.13; 95% CI, −0.21, −0.05). It did not include the 2013 Danish study.
The meta-analysis examined other levels of drinking in pregnancy, not just binging. Out of more than 1,500 papers that were examined, 34 studies met the criteria for inclusion, and just eight were included in the binge drinking analysis. The eight studies comprised more than 10,000 children who were tested from ages 6 months to 14 years. The researchers analyzed eight functional domains: academic performance, attention, behavior, cognition, memory, language and verbal development, executive function, and visual and motor development.
The researchers also separated the studies based on quality. When they analyzed the results for only high-quality studies, the cognition effect was not significant and no other associations were found with other child neuropsychological outcomes.
Several other studies have examined different endpoints, particularly hyperactivity and externalizing behaviors. While several studies show a trend toward those effects, mothers who binge drink also tend to be more externalizing in their own behavior.
An examination of the literature shows just how difficult it is to produce clear results that inform clinical practice. Adjusting for confounding factors from marital status to maternal IQ is just one hurdle. Another area that plagues researchers is that knowledge of drinking in early pregnancy is based on self-reports, and it is nearly impossible to know for sure if the reports of binging are accurate and also if there has been chronic alcohol use.
So what does all of this mean when it comes to advising women? There is no question that women should be advised not to drink when they are pregnant or planning a pregnancy. For a woman who engaged in binge drinking before she knew she was pregnant, it’s difficult to say that there is no effect. Instead, the collective evidence suggests there may be a small effect on cognition. In cases where binge drinking has occurred, children should be monitored as early as possible for any potential developmental effects.
Dr. Koren is professor of physiology/pharmacology and pediatrics at Western University in Ontario. He is the founder of the Motherisk Program. He reported having no relevant financial disclosures. Email him at [email protected].
Imagine this scenario: A couple goes on a Caribbean cruise with an all-you-can-eat buffet and an open bar. During the trip, they engage in 2 or 3 days of binge-style drinking, which is considered about four drinks in a single sitting. A few weeks after the trip, the woman finds out that she’s pregnant and calls your office wondering if there will be any harm to the fetus.
This is not a theoretical question. I have received many of these calls over the years, and in some cases the fear of adverse effects on the baby has led the couple to terminate the pregnancy.
Unfortunately, the literature has not been clear on the long-term impact of binge drinking in pregnancy. Animal studies suggest that it is the peak in the alcohol level created by binge drinking that causes damage to the fetus, rather than a sustained level of alcohol (although that obviously carries risk as well). The literature in humans has been controversial.
One of the most recent studies is a prospective cohort study of more than 1,600 women and their children sampled from the Danish National Birth Cohort. The investigators collected information on maternal alcohol use in early pregnancy and examined children at age 5 years using the Strengths and Difficulties Questionnaire (SDQ) completed by the mothers and a preschool teacher. It found no statistically significant association between binge drinking in early pregnancy and child behavior at age 5 years (BJOG. 2013 Aug;120[9]:1042-50).
In this study, the investigators corrected for parental education, maternal IQ, prenatal maternal smoking and postnatal parental smoking, the child’s age at testing, the child’s gender, maternal age, parity, marital status, family-home environment, prepregnancy maternal body mass index, and the child’s health status. After adjusting for confounders, they found no association between binge drinking and scores on the SDQ (odds ratio, 1.2; 95% confidence interval, 0.8-1.7 for behavioral scores and OR, 0.8; 95% CI, 0.6-1.2 for total difficulties scores). Additionally, the investigators analyzed low to moderate weekly alcohol consumption in early pregnancy and also could not find a significant effect (OR, 1.1; 95% CI, 0.5-2.3 for behavioral scores and OR, 1.1; 95% CI, 0.6-2.1 for the total difficulties scores).
This finding received a lot of press attention at the time, but it’s not the only study that has shown a lack of effect from binge drinking in contrast to the conventional wisdom on this subject.
A meta-analysis published in early 2014 further adds to the literature on this topic (Alcohol Clin Exp Res. 2014 Jan;38[1]:214-26).
The meta-analysis showed a small, but statistically significant effect on child’s cognition associated with binge drinking in pregnancy (Cohen’s d [a standardized mean difference score] −0.13; 95% CI, −0.21, −0.05). It did not include the 2013 Danish study.
The meta-analysis examined other levels of drinking in pregnancy, not just binging. Out of more than 1,500 papers that were examined, 34 studies met the criteria for inclusion, and just eight were included in the binge drinking analysis. The eight studies comprised more than 10,000 children who were tested from ages 6 months to 14 years. The researchers analyzed eight functional domains: academic performance, attention, behavior, cognition, memory, language and verbal development, executive function, and visual and motor development.
The researchers also separated the studies based on quality. When they analyzed the results for only high-quality studies, the cognition effect was not significant and no other associations were found with other child neuropsychological outcomes.
Several other studies have examined different endpoints, particularly hyperactivity and externalizing behaviors. While several studies show a trend toward those effects, mothers who binge drink also tend to be more externalizing in their own behavior.
An examination of the literature shows just how difficult it is to produce clear results that inform clinical practice. Adjusting for confounding factors from marital status to maternal IQ is just one hurdle. Another area that plagues researchers is that knowledge of drinking in early pregnancy is based on self-reports, and it is nearly impossible to know for sure if the reports of binging are accurate and also if there has been chronic alcohol use.
So what does all of this mean when it comes to advising women? There is no question that women should be advised not to drink when they are pregnant or planning a pregnancy. For a woman who engaged in binge drinking before she knew she was pregnant, it’s difficult to say that there is no effect. Instead, the collective evidence suggests there may be a small effect on cognition. In cases where binge drinking has occurred, children should be monitored as early as possible for any potential developmental effects.
Dr. Koren is professor of physiology/pharmacology and pediatrics at Western University in Ontario. He is the founder of the Motherisk Program. He reported having no relevant financial disclosures. Email him at [email protected].
Unpublished study on Bendectin prompts questions on hidden data
Doubt is being cast on the efficacy of Diclegis – the only prescription drug approved in the United States for treating nausea and vomiting in pregnancy – after researchers exposed flaws in previously unpublished data that served as the basis for the drug’s approval.
But the larger point, according to the researcher who brought the unpublished study to light, is the danger of relying too heavily on hidden data.
“It’s not like there’s some special concern over the safety of Diclegis. It’s that there is this commonly prescribed medication that hasn’t been proven to be effective,” Navindra Persaud, MD, a family physician and researcher at St. Michael’s Hospital in Toronto, said in an interview.
The 8-Way Bendectin Study was a double-blind, multicentered, randomized, placebo-controlled study of 2,359 women with morning sickness in the first trimester, conducted in the United States across multiple sites in 1976 by the now-defunct Wm. S. Merrell Co. The aim was to find a replacement formulation of a three-agent formula (Bendectin) for morning sickness, after one of the ingredients – dicyclomine hydrochloride – was determined ineffective for pregnancy-related nausea and vomiting.
Participants in the study, which had seven treatment arms and one control group, were asked to keep diaries for a week, detailing their bouts of nausea and vomiting. Clinicians then evaluated and rated the diary entries. In all, data for 1,599 of the women were analyzed, with all seven treatment arms besting placebo. Doxylamine-pyridoxine was rated “moderate or excellent” with a 21% absolute difference, compared with placebo (95% confidence interval, 11-30). The most commonly reported side effect across the study was drowsiness.
Dr. Persaud said he thinks the study was never published because of multiple flaws. For instance, there was not a clear baseline for symptoms, or clear parameters for how the clinicians rated those symptoms; outcome data for more than a third of controls were missing, as were completed reports about potential adverse outcomes; and P values were one sided and not adjusted to account for all eight study arms, he said.
“While the analyzed data indicate differences from placebo for several combinations, the questionable data integrity, high dropout rate, and other methodological concerns mean that the prescribing of this medication should not be based on this trial,” wrote Dr. Persaud and Dr. Zhang in their analysis.
The newly published analysis brings back the rocky history of morning sickness treatments in the United States, notably the withdrawal of Bendectin in 1983 following a barrage of teratogenicity claims against the drug maker that made it unprofitable to continue marketing.
This is all beside the point, according to Dr. Persaud. “For every medication, you’re going to find some of these associations. They might be real; they might be not. So you have to weigh potential harm against the benefit. The real problem here is that there is no demonstrated benefit even though the claim seems to be that there is,” he said.
Duchesnay defended the efficacy of the drug.
“The conclusions expressed in the report published in PLOS ONE are highly inconsistent with the large and comprehensive body of evidence regarding this combination drug,” Michael Gallo, Duchesnay vice president for regulatory and medical affairs, said in a statement posted on the company’s website. In its response to Dr. Persaud and Dr. Zhang’s analysis, the company also said that doxylamine succinate and pyridoxine hydrochloride – the two agents in the treatment – are “ the most studied drug combination used in pregnancy. The safety and efficacy of [Diclegis] have been proven in 16 cohort studies, two meta-analyses, an ecological study, a neurological development study, and numerous others.”
“It’s unclear if the [unpublished] study was carefully reassessed in the lead-up to the recent approval of Diclegis,” he said. “The available FDA review documents for the recent approval of Diclectin [pyridoxine/doxylamine] do not mention the problems with the study.”
One factor in the treatment’s place in standard of care might be anecdotal influences from some of the more than 35 million women around the world thought to have used the treatment, according to Dr. Persaud. “Lots of women have taken this medication and felt better shortly after, so they feel strongly that the medication is effective,” he said, but because nausea and vomiting in pregnancy is common in more than three-quarters of women, and typically does not last more than several weeks, most likely the patients would have gotten better over time anyway.
“Some women suffer greatly and do seem to get relief from medication,” Dr. Chambers said, but noted that Diclegis is not the only option available for women.
When Bendectin was pulled from the U.S. market, for example, Dr. Chambers said women turned to combinations of vitamin B6 and over-the-counter medications that contain the antihistamine doxylamine.
Dr. Persaud said his interest in the review started after a patient expressed her concerns over the medication. “She was reluctant to take it, and asked me if I was sure about it. I reassured her, but then after she left, I did wonder if I was correct,” he recalled. He said he checked all the guidelines, but could not find anything to justify its use other than the manufacturer’s monograph.
He said he suspects this is not the only prescription medication that would not withstand such scrutiny, but that uncovering the necessary data would be very difficult. “I was shocked it was very difficult to get access to this information as a clinician,” he said, adding that it also is impractical to expect physicians to spend 5 years to track the information down.
In their analysis of the study, Dr. Persaud and Dr. Zhang stated that their objective is to contribute to a movement across all of medicine to end the risks of data secrecy, and instead “restore invisible and abandoned trials” (RIAT). The U.S. Department of Health & Human Services has been pushing to make more clinical trials data public through ClinicalTrials.gov, including issuing federal regulations requiring information to be made public for certain trials involving drugs and devices regulated by the FDA.
As for how his own practice has been impacted by this research, Dr. Persaud said he no longer prescribes Diclegis.
Dr. Persaud, Dr. Zhang, and Dr. Chambers had no relevant financial disclosures.
[email protected]
On Twitter @whitneymcknight
Between 1956 and 1983, the primary treatment for nausea/vomiting of pregnancy (NVP) was Bendectin, a combination of doxylamine and pyridoxine. In 1983, it was removed from the market by the manufacturer because of litigation expense. Following this, there was a marked increase in the incidence of hyperemesis gravidarum, the most severe form of NVP, which was probably due to ineffective treatment of the condition.
Several organizations have stated that the combination of doxylamine/pyridoxine is safe and effective for use in pregnancy. In 2002, the Society of Obstetricians and Gynaecologists of Canada concluded that the doxylamine/pyridoxine combination should be the standard of care because it had the greatest evidence to support its efficacy and safety. In 2004, the American College of Obstetricians and Gynecologists stated that the combination was safe and effective and was the first-line treatment for NVP.
If NVP is not controlled with 2 tablets at bedtime, the Diclegis dose can be increased up to 4 tablets per day – 1 in the morning, 1 in midafternoon, and 2 at bedtime.
Gerald G. Briggs, BPharm, FCCP, is a clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He reported having no relevant financial disclosures.
Between 1956 and 1983, the primary treatment for nausea/vomiting of pregnancy (NVP) was Bendectin, a combination of doxylamine and pyridoxine. In 1983, it was removed from the market by the manufacturer because of litigation expense. Following this, there was a marked increase in the incidence of hyperemesis gravidarum, the most severe form of NVP, which was probably due to ineffective treatment of the condition.
Several organizations have stated that the combination of doxylamine/pyridoxine is safe and effective for use in pregnancy. In 2002, the Society of Obstetricians and Gynaecologists of Canada concluded that the doxylamine/pyridoxine combination should be the standard of care because it had the greatest evidence to support its efficacy and safety. In 2004, the American College of Obstetricians and Gynecologists stated that the combination was safe and effective and was the first-line treatment for NVP.
If NVP is not controlled with 2 tablets at bedtime, the Diclegis dose can be increased up to 4 tablets per day – 1 in the morning, 1 in midafternoon, and 2 at bedtime.
Gerald G. Briggs, BPharm, FCCP, is a clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He reported having no relevant financial disclosures.
Between 1956 and 1983, the primary treatment for nausea/vomiting of pregnancy (NVP) was Bendectin, a combination of doxylamine and pyridoxine. In 1983, it was removed from the market by the manufacturer because of litigation expense. Following this, there was a marked increase in the incidence of hyperemesis gravidarum, the most severe form of NVP, which was probably due to ineffective treatment of the condition.
Several organizations have stated that the combination of doxylamine/pyridoxine is safe and effective for use in pregnancy. In 2002, the Society of Obstetricians and Gynaecologists of Canada concluded that the doxylamine/pyridoxine combination should be the standard of care because it had the greatest evidence to support its efficacy and safety. In 2004, the American College of Obstetricians and Gynecologists stated that the combination was safe and effective and was the first-line treatment for NVP.
If NVP is not controlled with 2 tablets at bedtime, the Diclegis dose can be increased up to 4 tablets per day – 1 in the morning, 1 in midafternoon, and 2 at bedtime.
Gerald G. Briggs, BPharm, FCCP, is a clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He reported having no relevant financial disclosures.
Doubt is being cast on the efficacy of Diclegis – the only prescription drug approved in the United States for treating nausea and vomiting in pregnancy – after researchers exposed flaws in previously unpublished data that served as the basis for the drug’s approval.
But the larger point, according to the researcher who brought the unpublished study to light, is the danger of relying too heavily on hidden data.
“It’s not like there’s some special concern over the safety of Diclegis. It’s that there is this commonly prescribed medication that hasn’t been proven to be effective,” Navindra Persaud, MD, a family physician and researcher at St. Michael’s Hospital in Toronto, said in an interview.
The 8-Way Bendectin Study was a double-blind, multicentered, randomized, placebo-controlled study of 2,359 women with morning sickness in the first trimester, conducted in the United States across multiple sites in 1976 by the now-defunct Wm. S. Merrell Co. The aim was to find a replacement formulation of a three-agent formula (Bendectin) for morning sickness, after one of the ingredients – dicyclomine hydrochloride – was determined ineffective for pregnancy-related nausea and vomiting.
Participants in the study, which had seven treatment arms and one control group, were asked to keep diaries for a week, detailing their bouts of nausea and vomiting. Clinicians then evaluated and rated the diary entries. In all, data for 1,599 of the women were analyzed, with all seven treatment arms besting placebo. Doxylamine-pyridoxine was rated “moderate or excellent” with a 21% absolute difference, compared with placebo (95% confidence interval, 11-30). The most commonly reported side effect across the study was drowsiness.
Dr. Persaud said he thinks the study was never published because of multiple flaws. For instance, there was not a clear baseline for symptoms, or clear parameters for how the clinicians rated those symptoms; outcome data for more than a third of controls were missing, as were completed reports about potential adverse outcomes; and P values were one sided and not adjusted to account for all eight study arms, he said.
“While the analyzed data indicate differences from placebo for several combinations, the questionable data integrity, high dropout rate, and other methodological concerns mean that the prescribing of this medication should not be based on this trial,” wrote Dr. Persaud and Dr. Zhang in their analysis.
The newly published analysis brings back the rocky history of morning sickness treatments in the United States, notably the withdrawal of Bendectin in 1983 following a barrage of teratogenicity claims against the drug maker that made it unprofitable to continue marketing.
This is all beside the point, according to Dr. Persaud. “For every medication, you’re going to find some of these associations. They might be real; they might be not. So you have to weigh potential harm against the benefit. The real problem here is that there is no demonstrated benefit even though the claim seems to be that there is,” he said.
Duchesnay defended the efficacy of the drug.
“The conclusions expressed in the report published in PLOS ONE are highly inconsistent with the large and comprehensive body of evidence regarding this combination drug,” Michael Gallo, Duchesnay vice president for regulatory and medical affairs, said in a statement posted on the company’s website. In its response to Dr. Persaud and Dr. Zhang’s analysis, the company also said that doxylamine succinate and pyridoxine hydrochloride – the two agents in the treatment – are “ the most studied drug combination used in pregnancy. The safety and efficacy of [Diclegis] have been proven in 16 cohort studies, two meta-analyses, an ecological study, a neurological development study, and numerous others.”
“It’s unclear if the [unpublished] study was carefully reassessed in the lead-up to the recent approval of Diclegis,” he said. “The available FDA review documents for the recent approval of Diclectin [pyridoxine/doxylamine] do not mention the problems with the study.”
One factor in the treatment’s place in standard of care might be anecdotal influences from some of the more than 35 million women around the world thought to have used the treatment, according to Dr. Persaud. “Lots of women have taken this medication and felt better shortly after, so they feel strongly that the medication is effective,” he said, but because nausea and vomiting in pregnancy is common in more than three-quarters of women, and typically does not last more than several weeks, most likely the patients would have gotten better over time anyway.
“Some women suffer greatly and do seem to get relief from medication,” Dr. Chambers said, but noted that Diclegis is not the only option available for women.
When Bendectin was pulled from the U.S. market, for example, Dr. Chambers said women turned to combinations of vitamin B6 and over-the-counter medications that contain the antihistamine doxylamine.
Dr. Persaud said his interest in the review started after a patient expressed her concerns over the medication. “She was reluctant to take it, and asked me if I was sure about it. I reassured her, but then after she left, I did wonder if I was correct,” he recalled. He said he checked all the guidelines, but could not find anything to justify its use other than the manufacturer’s monograph.
He said he suspects this is not the only prescription medication that would not withstand such scrutiny, but that uncovering the necessary data would be very difficult. “I was shocked it was very difficult to get access to this information as a clinician,” he said, adding that it also is impractical to expect physicians to spend 5 years to track the information down.
In their analysis of the study, Dr. Persaud and Dr. Zhang stated that their objective is to contribute to a movement across all of medicine to end the risks of data secrecy, and instead “restore invisible and abandoned trials” (RIAT). The U.S. Department of Health & Human Services has been pushing to make more clinical trials data public through ClinicalTrials.gov, including issuing federal regulations requiring information to be made public for certain trials involving drugs and devices regulated by the FDA.
As for how his own practice has been impacted by this research, Dr. Persaud said he no longer prescribes Diclegis.
Dr. Persaud, Dr. Zhang, and Dr. Chambers had no relevant financial disclosures.
[email protected]
On Twitter @whitneymcknight
Doubt is being cast on the efficacy of Diclegis – the only prescription drug approved in the United States for treating nausea and vomiting in pregnancy – after researchers exposed flaws in previously unpublished data that served as the basis for the drug’s approval.
But the larger point, according to the researcher who brought the unpublished study to light, is the danger of relying too heavily on hidden data.
“It’s not like there’s some special concern over the safety of Diclegis. It’s that there is this commonly prescribed medication that hasn’t been proven to be effective,” Navindra Persaud, MD, a family physician and researcher at St. Michael’s Hospital in Toronto, said in an interview.
The 8-Way Bendectin Study was a double-blind, multicentered, randomized, placebo-controlled study of 2,359 women with morning sickness in the first trimester, conducted in the United States across multiple sites in 1976 by the now-defunct Wm. S. Merrell Co. The aim was to find a replacement formulation of a three-agent formula (Bendectin) for morning sickness, after one of the ingredients – dicyclomine hydrochloride – was determined ineffective for pregnancy-related nausea and vomiting.
Participants in the study, which had seven treatment arms and one control group, were asked to keep diaries for a week, detailing their bouts of nausea and vomiting. Clinicians then evaluated and rated the diary entries. In all, data for 1,599 of the women were analyzed, with all seven treatment arms besting placebo. Doxylamine-pyridoxine was rated “moderate or excellent” with a 21% absolute difference, compared with placebo (95% confidence interval, 11-30). The most commonly reported side effect across the study was drowsiness.
Dr. Persaud said he thinks the study was never published because of multiple flaws. For instance, there was not a clear baseline for symptoms, or clear parameters for how the clinicians rated those symptoms; outcome data for more than a third of controls were missing, as were completed reports about potential adverse outcomes; and P values were one sided and not adjusted to account for all eight study arms, he said.
“While the analyzed data indicate differences from placebo for several combinations, the questionable data integrity, high dropout rate, and other methodological concerns mean that the prescribing of this medication should not be based on this trial,” wrote Dr. Persaud and Dr. Zhang in their analysis.
The newly published analysis brings back the rocky history of morning sickness treatments in the United States, notably the withdrawal of Bendectin in 1983 following a barrage of teratogenicity claims against the drug maker that made it unprofitable to continue marketing.
This is all beside the point, according to Dr. Persaud. “For every medication, you’re going to find some of these associations. They might be real; they might be not. So you have to weigh potential harm against the benefit. The real problem here is that there is no demonstrated benefit even though the claim seems to be that there is,” he said.
Duchesnay defended the efficacy of the drug.
“The conclusions expressed in the report published in PLOS ONE are highly inconsistent with the large and comprehensive body of evidence regarding this combination drug,” Michael Gallo, Duchesnay vice president for regulatory and medical affairs, said in a statement posted on the company’s website. In its response to Dr. Persaud and Dr. Zhang’s analysis, the company also said that doxylamine succinate and pyridoxine hydrochloride – the two agents in the treatment – are “ the most studied drug combination used in pregnancy. The safety and efficacy of [Diclegis] have been proven in 16 cohort studies, two meta-analyses, an ecological study, a neurological development study, and numerous others.”
“It’s unclear if the [unpublished] study was carefully reassessed in the lead-up to the recent approval of Diclegis,” he said. “The available FDA review documents for the recent approval of Diclectin [pyridoxine/doxylamine] do not mention the problems with the study.”
One factor in the treatment’s place in standard of care might be anecdotal influences from some of the more than 35 million women around the world thought to have used the treatment, according to Dr. Persaud. “Lots of women have taken this medication and felt better shortly after, so they feel strongly that the medication is effective,” he said, but because nausea and vomiting in pregnancy is common in more than three-quarters of women, and typically does not last more than several weeks, most likely the patients would have gotten better over time anyway.
“Some women suffer greatly and do seem to get relief from medication,” Dr. Chambers said, but noted that Diclegis is not the only option available for women.
When Bendectin was pulled from the U.S. market, for example, Dr. Chambers said women turned to combinations of vitamin B6 and over-the-counter medications that contain the antihistamine doxylamine.
Dr. Persaud said his interest in the review started after a patient expressed her concerns over the medication. “She was reluctant to take it, and asked me if I was sure about it. I reassured her, but then after she left, I did wonder if I was correct,” he recalled. He said he checked all the guidelines, but could not find anything to justify its use other than the manufacturer’s monograph.
He said he suspects this is not the only prescription medication that would not withstand such scrutiny, but that uncovering the necessary data would be very difficult. “I was shocked it was very difficult to get access to this information as a clinician,” he said, adding that it also is impractical to expect physicians to spend 5 years to track the information down.
In their analysis of the study, Dr. Persaud and Dr. Zhang stated that their objective is to contribute to a movement across all of medicine to end the risks of data secrecy, and instead “restore invisible and abandoned trials” (RIAT). The U.S. Department of Health & Human Services has been pushing to make more clinical trials data public through ClinicalTrials.gov, including issuing federal regulations requiring information to be made public for certain trials involving drugs and devices regulated by the FDA.
As for how his own practice has been impacted by this research, Dr. Persaud said he no longer prescribes Diclegis.
Dr. Persaud, Dr. Zhang, and Dr. Chambers had no relevant financial disclosures.
[email protected]
On Twitter @whitneymcknight
2017 Update on fertility
Zika virus is a serious problem. Education and infection prevention are critical to effective management, and why we chose to include Zika virus as a topic for this year’s Update. We also discuss obesity’s effects on reproduction—a very relevant concern for all ObGyns and patients alike as about half of reproductive-age women are obese. Finally, subclinical hypothyroidism can present unique management challenges, such as determining when it is present and when treatment is indicated.
Read about counseling patients about Zika virus
Managing attempted pregnancy in the era of Zika virus
Oduyebo T, Igbinosa I, Petersen EE, et al. Update: interim guidance for health care providers caring for pregnant women with possible Zika virus exposure--United States, July 2016. MMWR Morb Mortal Wkly Rep. 2016;65(29):739-744.
Petersen EE, Meaney-Delman D, Neblett-Fanfair R, et al. Update: interim guidance for preconception counseling and prevention of sexual transmission of Zika virus for persons with possible Zika virus exposure--United States, September 2016. MMWR Morb Mortal Wkly Rep. 2016;65(39):1077-1081.
US Food and Drug Administration. Donor Screening Recommendations to Reduce the Risk of Transmission of Zika Virus by Human Cells, Tissues, and Cellular and Tissue-Based Products. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/UCM488582.pdf. Published March 2016. Accessed January 12, 2017.
National Institutes of Health. Zika: Overview. https://www.nichd.nih.gov/health/topics/zika/Pages/default.aspx. Accessed January 12, 2017.
World Health Organization. Prevention of sexual transmission of Zika virus interim guidance. WHO reference number: WHO/ZIKV/MOC/16. 1 Rev. 3, September 6, 2016.
Zika Virus Guidance Task Force of the American Society for Reproductive Medicine. Rev. 13, September 2016.
Zika virus presents unique challenges to physicians managing the care of patients attempting pregnancy, with or without fertility treatment. Neonatal Zika virus infection sequelae only recently have been appreciated; microcephaly was associated with Zika virus in October 2015, followed by other neurologic conditions including brain abnormalities, neural tube defects, and eye abnormalities. Results of recent studies involving the US Zika Pregnancy Registry show that 6% of women with Zika at any time in pregnancy had affected babies, but 11% of those who contracted the disease in the first trimester were affected.
Diagnosis is difficult because symptoms are generally mild, with 80% of affected patients asymptomatic. Possible Zika virus exposure is defined as travel to or residence in an area of active Zika virus transmission, or sex without a condom with a partner who traveled to or lived in an area of active transmission. Much is unknown about the interval from exposure to symptoms. Testing availability is limited and variable, and much is unknown about sensitivity and specificity of direct viral RNA testing, appearance and disappearance of detectable immunoglobulin (Ig) M and IgG antibodies that affect false positive and false negative test results, duration of infectious phase, risk of transmission, and numerous other factors.
Positive serum viral testing likely indicates virus in semen or other bodily fluids, but a negative serum viral test cannot definitively preclude virus in other bodily fluids. Zika virus likely can be passed from any combination of semen and vaginal and cervical fluids, but validating tests for these fluids are not yet available. It is not known if sperm preparation and assisted reproductive technology (ART) procedures that minimize risk of HIV transmission are effective against Zika virus or whether or not cryopreservation can destroy the virus.
Pregnancy timing
The Centers for Disease Control and Prevention now recommends that all men with possible Zika virus exposure who are considering attempting pregnancy with their partner wait to get pregnant until at least 6 months after symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic). Women with possible Zika virus exposure are recommended to wait to get pregnant until at least 8 weeks after symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic).
Women and men with possible exposure to Zika virus but without clinical symptoms of illness should consider testing for Zika viral RNA within 2 weeks of suspected exposure and wait at least 8 weeks after the last date of exposure before being re-tested. If direct viral testing (using rRT-PCR) results initially are negative, ideally, antibody testing would be obtained, if available, at 8 weeks. However, no testing paradigm will absolutely guarantee lack of Zika virus infectivity.
Virus management problems are dramatically compounded in areas endemic for Zika. Women and men who have had Zika virus disease should wait at least 6 months after illness onset to attempt reproduction. The temporal relationship between the presence of viral RNA and infectivity is not known definitively, and so the absolute duration of time to wait before attempting pregnancy is unknown. Male and female partners who become infected should avoid all forms of intimate sexual conduct or use condoms for the same 6 months. There is no evidence Zika will cause congenital infection in pregnancies initiated after resolution of maternal Zika viremia. However, any testing performed at a time other than the time of treatment might not reflect true viral status, particularly in areas of active Zika virus transmission.
Prevention
Women and men, especially those residing in areas of active Zika virus transmission, should talk with their physicians regarding pregnancy plans and avoid mosquito bites using the usual precautions: avoid mosquito areas, drain standing water, use mosquito repellent containing DEET, and use mosquito netting. Some people have gone so far as to relocate to nonendemic areas.
Those contemplating pregnancy should be advised to consider what they would do if they become exposed to or have suspected or confirmed Zika virus during pregnancy. Additional considerations are gamete or embryo cryopreservation and quarantine until a subsequent rRT-PCR test result is negative in both the male and female and at least 8 weeks have passed from gamete collection.

Patient counseling essentials
Counsel patients considering reproduction about:
- Zika virus as a new reproductive hazard
- the significance of the hazard to the fetus if infected
- the areas of active transmission, and that they are constantly changing
- avoidance of Zika areas if possible
- methods of transmission through mosquito bites or sex
- avoidance of mosquito bites
- symptoms of Zika infection
- safe sex practices
- testing limitations and knowledge deficiency about Zika.
Not uncommonly, clinical situations require complex individualized management decisions regarding trade-offs of risks, especially in older patients with decreased ovarian reserve. Consultation with infectious disease and reproductive specialists should be obtained when complicated and consequential decisions have to be made.
All practitioners should inform their patients, especially those undergoing fertility treatments, about Zika, and develop language in their informed consent that conveys the gap in knowledge to these patients.
Read how obesity specifically affects reproduction in an adverse way
Obesity adversely affects reproduction, but how specifically?
Practice Committee of the American Society for Reproductive Medicine. Obesity and Reproduction: A committee opinion. Fertil Steril. 2015;104(5):1116-1126.
The prevalence of obesity has increased substantially over the past 2 decades. Almost two-thirds of women and three-fourths of men in the United States are overweight or obese (defined as a body mass index [BMI] ≥25 kg/m2 and BMI ≥30 kg/m2, respectively; TABLE). Nearly 50% of reproductive-age women are obese.
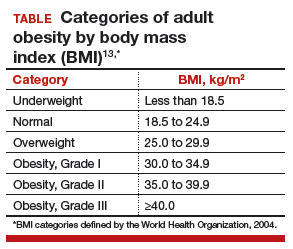
A disease of excess body fat and insulin resistance, obesity increases the risks of hypertension, diabetes, dyslipidemia, cardiovascular disease, sleep apnea, respiratory problems, and cancer as well as other serious health problems. While not all individuals with obesity will have infertility, obesity is associated with impaired reproduction in both women and men, adverse obstetric outcomes, and health problems in offspring. The American Society for Reproductive Medicine (ASRM) reviewed this important issue in a recent practice committee opinion.
Menstrual cycle and ovulatory dysfunction
Menstrual cycle abnormalities are more common in women with obesity. Elevated levels of insulin in obese women suppress sex hormone−binding globulin (SHBG) which in turn reduces gonadotropin secretion due to increased production of estrogen from conversion of androgens by adipose aromatase.1 Adipose tissue produces adipokines, which directly can suppress ovarian function.2
Ovulatory dysfunction is common among obese women; the relative risk of such dysfunction is 3.1 (95% confidence interval [CI], 2.2−4.4) among women with BMI levels >27 kg/m2 versus BMI levels 20.0 to 24.9 kg/m2.3,4 Obesity decreases fecundity even in women with normal menstrual cycles.5 This may in part be due to altered ovulatory dynamics with reduced early follicular luteinizing hormone pulse amplitude accompanied by prolonged folliculogenesis and reduced luteal progesterone levels.6
Compared with normal-weight women, obese women have a lower chance of conception within 1 year of stopping contraception; about 66% of obese women conceive within 1 year of stopping contraception, compared with about 81% of women with normal weight.7 Results of a Dutch study of 3,029 women with regular ovulation, at least one patent tube, and a partner with a normal semen analysis indicated a direct correlation between obesity and delayed conception, with a 4% lower spontaneous pregnancy rate per kg/m2 increase in women with a BMI >29 kg/m2 versus a BMI of 21 to 29 kg/m2 (hazard ratio, 0.96; 95% CI, 0.91−0.99).8
Assisted reproduction
Assisted reproduction in women with obesity is associated with lower success rates than in women with normal weight. A systematic review of 27 in vitro fertilization (IVF) studies (23 of which were retrospective) reveals 10% lower live-birth rate in overweight (BMI >25 kg/m2) versus normal-weight women (BMI <25 kg/m2) undergoing IVF (odds ratio [OR], 0.90; 95% CI, 0.82−1.0).9 Data from a meta-analysis of 33 IVF studies, including 47,967 cycles, show that, compared with women with a BMI <25 kg/m2, overweight or obese women have significantly reduced rates of clinical pregnancy (relative risk [RR], 0.90; P<.0001) and live birth (RR, 0.84; P = .0002).10
Results of a retrospective study of 4,609 women undergoing first IVF or IVF/intracytoplasmic sperm injection cycles revealed impaired embryo implantation (controlling for embryo quality and transfer day), reducing the age-adjusted odds of live birth in a BMI-dependent manner by 37% (BMI, 30.0−34.9 kg/m2), 61% (BMI, 35.0−39.9 kg/m2), and 68% (BMI, >40 kg/m2) compared with women with a BMI of 18.5 to 24.9 kg/m2.11 In a study of 12,566 Danish couples undergoing assisted reproduction, overweight and obese ovulatory women had a 12% (95% CI, 0.79−0.99) and 25% (95% CI, 0.63−0.90) reduction in IVF-related live birth rate, respectively (referent BMI, 18.5−24.9 kg/m2), with a 2% (95% CI, 0.97−0.99) decrease in live-birth rate for every one-unit increase in BMI.12 Putative mechanisms for these findings include altered oocyte morphology and reduced fertilization in eggs from obese women,13 and impaired embryo quality in women less than age 35.14 Oocytes from women with a BMI >25 kg/m2 are smaller and less likely to complete development postfertilization, with embryos arrested prior to blastulation containing more triglyceride than those forming blastocysts.15
Blastocysts developed from oocytes of high-BMI women are smaller, contain fewer cells and have a higher content of triglycerides, lower glucose consumption, and altered amino acid metabolism compared with embryos of normal-weight women (BMI <24.9 kg/m2).15 Obesity may alter endometrial receptivity during IVF given the finding that third-party surrogate women with a BMI >35 kg/m2 have a lower live-birth rate (25%) compared with women with a BMI <35 kg/m2 (49%; P<.05).16
Pregnancy outcomes
Obesity is linked to an increased risk of miscarriage. Results of a meta-analysis of 33 IVF studies including 47,967 cycles indicated that overweight or obese women have a higher rate of miscarriage (RR, 1.31; P<.0001) than normal-weight women (BMI <25 kg/m2).17 Maternal and perinatal morbid obesity are strongly associated with obstetric and perinatal complications, including gestational diabetes, hypertension, preeclampsia, preterm delivery, shoulder dystocia, fetal distress, early neonatal death, and small- as well as large-for-gestational age infants.
Obese women who conceive by IVF are at increased risk for preeclampsia, gestational diabetes, preterm delivery, and cesarean delivery.13 Authors of a meta-analysis of 18 observational studies concluded that obese mothers were at increased odds of pregnancies affected by such birth defects as neural tube defects, cardiovascular anomalies, and cleft lip and palate, among others.18
In addition to being the cause of these fetal abnormalities, maternal metabolic dysfunction is linked to promoting obesity in offspring, thereby perpetuating a cycle of obesity and adverse health outcomes that include an increased risk of premature death in adult offspring in subsequent generations.13
Treatment for obesity
Lifestyle modification is the first-line treatment for obesity.
Pre-fertility therapy and pregnancy goals. Targets for pregnancy should include:
- preconception weight loss to a BMI of 35 kg/m2
- prevention of excess weight gain in pregnancy
- long-term reduction in weight.
For all obese individuals, lifestyle modifications should include a weight loss of 7% of body weight and increased physical activity to at least 150 minutes of moderate activity, such as walking, per week. Calorie restriction should be emphasized. A 500 to 1,000 kcal/day decrease from usual dietary intake is expected to result in a 1- to 2-lb weight loss per week. A low-calorie diet of 1,000 to 1,200 kcal/day can lead to an average 10% decrease in total body weight over 6 months.
Adjunct supervised medical therapy or bariatric surgery can play an important role in successful weight loss prepregnancy but are not appropriate for women actively attempting conception. Importantly, pregnancy should be deferred for a minimum of 1 year after bariatric surgery. The decision to postpone pregnancy to achieve weight loss must be balanced against the risk of declining fertility with advancing age of the woman.
Read about when to treat subclinical hypothyroidism
Optimal management of subclinical hypothyroidism in women with infertility
Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545-553.
Thyroid disorders long have been associated with the potential for adverse reproductive outcomes. While overt hypothyroidism has been linked to infertility, increased miscarriage risk, and poor maternal and fetal outcomes, controversy has existed regarding the association between subclinical hypothyroidism (SCH) and reproductive problems. The ASRM recently published a guideline on the role of SCH in the infertile female population.
How is subclinical hypothyroidism defined?
SCH is classically defined as a thyrotropin (TSH) level above the upper limit of normal range (4.5−5.0 mIU/L) with normal free thyroxine (FT4) levels. The National Health and Nutrition Examination Survey (NHANES III) population has been used to establish normative data for TSH for a disease-free population. These include a median serum level for TSH of 1.5 mIU/L, with the corresponding 2.5 and 97.5 percentiles of 0.41 and 6.10, respectively.19 Data from the National Academy of Clinical Biochemistry, however, reveal that 95% of individuals without evidence of thyroid disease have a TSH level <2.5 mIU/L, and that the normal reference range is skewed to the right.20 Adjusting the upper limit of the normal range to 2.5 mIU/L would result in an additional 11.8% to 14.2% of the United States population (22 to 28 million individuals) being diagnosed with hypothyroidism.
This information raises several important questions.
1. Should nonpregnant women be treated for SCH?
No. There is no benefit from the standpoint of lipid profile or alteration of cardiovascular risk in the treatment of TSH levels between 5 and 10 mIU/L and, therefore, treatment of individuals with TSH <5 mIU/L is questionable. Furthermore, the risk of overtreatment resulting in bone loss is a concern. The Endocrine Society does not recommend changing the current normal TSH range for nonpregnant women.
2. What are normal TSH levels in pregnant women?
Because human chorionic gonadotropin (hCG) can bind to and affect the TSH receptor, thereby influencing TSH values, the normal range for TSH is modified in pregnancy. The Endocrine Society recommends the following pregnancy trimester guidelines for TSH levels: 2.5 mIU/L is the recommended upper limit of normal in the first trimester, 3.0 mIU/L in the second trimester, and 3.5 mIU/L in the third trimester.
3. Is untreated SCH associated with miscarriage?
There is fair evidence that SCH, defined as a TSH level >4 mIU/L during pregnancy, is associated with miscarriage, but there is insufficient evidence that TSH levels between 2.5 and 4 mIU/L are associated with miscarriage.
4. Is untreated SCH associated with infertility?
Limited data are available to assess the effect of SCH on infertility. While a few studies show an association between SCH on unexplained infertility and ovulatory disorders, SCH does not appear to be increased in other causes of infertility.
5. Is SCH associated with adverse obstetric outcomes?
Available data reveal that SCH with TSH levels outside the normal pregnancy range are associated with an increased risk of such obstetric complications as placental abruption, preterm birth, fetal death, and preterm premature rupture of membranes (PPROM). However, it is unclear if prepregnancy TSH levels between 2.5 and 4 mIU/L are associated with adverse obstetric outcomes.
6. Does untreated SCH affect developmental outcomes in children?
The fetus is solely dependent on maternal thyroid hormone in early pregnancy because the fetal thyroid does not produce thyroid hormone before 10 to 13 weeks of gestation. Significant evidence has associated untreated maternal hypothyroidism with delayed fetal neurologic development, impaired school performance, and lower intelligence quotient (IQ) among offspring.21 There is fair evidence that SCH diagnosed in pregnancy is associated with adverse neurologic development. There is no evidence that SCH prior to pregnancy is associated with adverse neurodevelopmental outcomes. It should be noted that only one study has examined whether treatment of SCH improves developmental outcomes (measured by IQ scored at age 3 years) and no significant differences were observed in women with SCH who were treated with levothyroxine versus those who were not.22
7. Does treatment of SCH improve miscarriage rates, live-birth rates, and/or clinical pregnancy rates?
Small randomized controlled studies of women undergoing infertility treatment and a few observational studies in the general population yield good evidence that levothyroxine treatment in women with SCH defined as TSH >4.0 mIU/L is associated with improvement in pregnancy, live birth, and miscarriage rates. There are no randomized trials assessing whether levothyroxine treatment in women with TSH levels between 2.5 and 4 mIU/L would yield similar benefits to those observed in women with TSH levels above 4 mIU/L.
8. Are thyroid antibodies associated with infertility or adverse reproductive outcomes?
There is good evidence that the thyroid autoimmunity, or the presence of TPO-Ab, is associated with miscarriage and fair evidence that it is associated with infertility. Treatment with levothyroxine may improve pregnancy outcomes especially if the TSH level is above 2.5 mIU/L.
9. Should there be universal screening for hypothyroidism in the first trimester of pregnancy?
Current evidence does not reveal a benefit of universal screening at this time. The American College of Obstetricians and Gynecologists does not recommend routine screening for hypothyroidism in pregnancy unless women have risk factors for thyroid disease, including a personal or family history of thyroid disease, physical findings or symptoms of goiter or hypothyroidism, type 1 diabetes mellitus, infertility, history of miscarriage or preterm delivery, and/or personal or family history of autoimmune disease.
The bottom line
SCH, defined as a TSH level greater than the upper limit of normal range (4.5−5.0 mIU/L)with normal FT4 levels, is associated with adverse reproductive outcomes including miscarriage, pregnancy complications, and delayed fetal neurodevelopment. Thyroid supplementation is beneficial; however, treatment has not been shown to improve long-term neurologic developmental outcomes in offspring. Data are limited on whether TSH values between 2.5 mIU/L and the upper range of normal are associated with adverse pregnancy outcomes and therefore treatment in this group remains controversial. Although available evidence is weak, there may be a benefit in some subgroups, and because risk is minimal, it may be reasonable to treat or to monitor levels and treat above nonpregnant and pregnancy ranges. There is fair evidence that thyroid autoimmunity (positive thyroid antibody) is associated with miscarriage and infertility. Levothyroxine therapy may improve pregnancy outcomes especially if the TSH level is above 2.5 mIU/L. While universal screening of thyroid function in pregnancy is not recommended, women at high risk for thyroid disease should be screened.23
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. Obesity and reproductive disorders in women. Hum Reprod Update. 2003;9(4):359-372.
- Greisen S, Ledet T, Møller N, et al. Effects of leptin on basal and FSH stimulated steroidogenesis in human granulosa luteal cells. Acta Obstet Gynecol Scand. 2000;79(11):931-935.
- Rich-Edwards JW, Goldman MB, Willett WC, et al. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171(1):171-177.
- Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994;5(2):247-250.
- Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22(2):414-420.
- Jain A, Polotsky AJ, Rochester D, et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92(7):2468-2473.
- Lake JK, Power C, Cole TJ. Women's reproductive health: the role of body mass index in early and adult life. Int J Obes Relat Metab Disord. 1997;21(6):432-438.
- van der Steeg JW, Steures P, Eijkemans MJ, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod. 2008;23(2):324-328.
- Koning AM, Mutsaerts MA, Kuchenbecker WK, et al. Complications and outcome of assisted reproduction technologies in overweight and obese women [Published correction appears in Hum Reprod. 2012;27(8):2570.] Hum Reprod. 2012;27(2):457-467.
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421-439.
- Moragianni VA, Jones SM, Ryley DA. The effect of body mass index on the outcomes of first assisted reproductive technology cycles. Fertil Steril. 2012;98(1):102-108.
- Petersen GL, Schmidt L, Pinborg A, Kamper-Jørgensen M. The influence of female and male body mass index on live births after assisted reproductive technology treatment: a nationwide register-based cohort study. Fertil Steril. 2013;99(6):1654-1662.
- Practice Committee of the American Society for Reproductive Medicine. Obesity and Reproduction: A committee opinion. Fertil Steril. 2015;104(5):1116-1126.
- Metwally M, Cutting R, Tipton A, Skull J, Ledger WL, Li TC. Effect of increased body mass index on oocyte and embryo quality in IVF patients. Reprod Biomed Online. 2007;15(5):532-538.
- Leary C, Leese HJ, Sturmey RG. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum Reprod. 2015;30(1):122-132.
- Deugarte D, Deugarte C, Sahakian V. Surrogate obesity negatively impacts pregnancy rates in third-party reproduction. Fertil Steril. 2010;93(3):1008-1010.
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421-439.
- Stothard KJ, Tennant PWG, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301(6):636-650.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499.
- Baloch Z, Carayon P, Conte-Devolx B, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13(1):3-126.
- Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf). 1999;50(2):149-155.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366(17):493-501.
- Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545-553.
Zika virus is a serious problem. Education and infection prevention are critical to effective management, and why we chose to include Zika virus as a topic for this year’s Update. We also discuss obesity’s effects on reproduction—a very relevant concern for all ObGyns and patients alike as about half of reproductive-age women are obese. Finally, subclinical hypothyroidism can present unique management challenges, such as determining when it is present and when treatment is indicated.
Read about counseling patients about Zika virus
Managing attempted pregnancy in the era of Zika virus
Oduyebo T, Igbinosa I, Petersen EE, et al. Update: interim guidance for health care providers caring for pregnant women with possible Zika virus exposure--United States, July 2016. MMWR Morb Mortal Wkly Rep. 2016;65(29):739-744.
Petersen EE, Meaney-Delman D, Neblett-Fanfair R, et al. Update: interim guidance for preconception counseling and prevention of sexual transmission of Zika virus for persons with possible Zika virus exposure--United States, September 2016. MMWR Morb Mortal Wkly Rep. 2016;65(39):1077-1081.
US Food and Drug Administration. Donor Screening Recommendations to Reduce the Risk of Transmission of Zika Virus by Human Cells, Tissues, and Cellular and Tissue-Based Products. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/UCM488582.pdf. Published March 2016. Accessed January 12, 2017.
National Institutes of Health. Zika: Overview. https://www.nichd.nih.gov/health/topics/zika/Pages/default.aspx. Accessed January 12, 2017.
World Health Organization. Prevention of sexual transmission of Zika virus interim guidance. WHO reference number: WHO/ZIKV/MOC/16. 1 Rev. 3, September 6, 2016.
Zika Virus Guidance Task Force of the American Society for Reproductive Medicine. Rev. 13, September 2016.
Zika virus presents unique challenges to physicians managing the care of patients attempting pregnancy, with or without fertility treatment. Neonatal Zika virus infection sequelae only recently have been appreciated; microcephaly was associated with Zika virus in October 2015, followed by other neurologic conditions including brain abnormalities, neural tube defects, and eye abnormalities. Results of recent studies involving the US Zika Pregnancy Registry show that 6% of women with Zika at any time in pregnancy had affected babies, but 11% of those who contracted the disease in the first trimester were affected.
Diagnosis is difficult because symptoms are generally mild, with 80% of affected patients asymptomatic. Possible Zika virus exposure is defined as travel to or residence in an area of active Zika virus transmission, or sex without a condom with a partner who traveled to or lived in an area of active transmission. Much is unknown about the interval from exposure to symptoms. Testing availability is limited and variable, and much is unknown about sensitivity and specificity of direct viral RNA testing, appearance and disappearance of detectable immunoglobulin (Ig) M and IgG antibodies that affect false positive and false negative test results, duration of infectious phase, risk of transmission, and numerous other factors.
Positive serum viral testing likely indicates virus in semen or other bodily fluids, but a negative serum viral test cannot definitively preclude virus in other bodily fluids. Zika virus likely can be passed from any combination of semen and vaginal and cervical fluids, but validating tests for these fluids are not yet available. It is not known if sperm preparation and assisted reproductive technology (ART) procedures that minimize risk of HIV transmission are effective against Zika virus or whether or not cryopreservation can destroy the virus.
Pregnancy timing
The Centers for Disease Control and Prevention now recommends that all men with possible Zika virus exposure who are considering attempting pregnancy with their partner wait to get pregnant until at least 6 months after symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic). Women with possible Zika virus exposure are recommended to wait to get pregnant until at least 8 weeks after symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic).
Women and men with possible exposure to Zika virus but without clinical symptoms of illness should consider testing for Zika viral RNA within 2 weeks of suspected exposure and wait at least 8 weeks after the last date of exposure before being re-tested. If direct viral testing (using rRT-PCR) results initially are negative, ideally, antibody testing would be obtained, if available, at 8 weeks. However, no testing paradigm will absolutely guarantee lack of Zika virus infectivity.
Virus management problems are dramatically compounded in areas endemic for Zika. Women and men who have had Zika virus disease should wait at least 6 months after illness onset to attempt reproduction. The temporal relationship between the presence of viral RNA and infectivity is not known definitively, and so the absolute duration of time to wait before attempting pregnancy is unknown. Male and female partners who become infected should avoid all forms of intimate sexual conduct or use condoms for the same 6 months. There is no evidence Zika will cause congenital infection in pregnancies initiated after resolution of maternal Zika viremia. However, any testing performed at a time other than the time of treatment might not reflect true viral status, particularly in areas of active Zika virus transmission.
Prevention
Women and men, especially those residing in areas of active Zika virus transmission, should talk with their physicians regarding pregnancy plans and avoid mosquito bites using the usual precautions: avoid mosquito areas, drain standing water, use mosquito repellent containing DEET, and use mosquito netting. Some people have gone so far as to relocate to nonendemic areas.
Those contemplating pregnancy should be advised to consider what they would do if they become exposed to or have suspected or confirmed Zika virus during pregnancy. Additional considerations are gamete or embryo cryopreservation and quarantine until a subsequent rRT-PCR test result is negative in both the male and female and at least 8 weeks have passed from gamete collection.

Patient counseling essentials
Counsel patients considering reproduction about:
- Zika virus as a new reproductive hazard
- the significance of the hazard to the fetus if infected
- the areas of active transmission, and that they are constantly changing
- avoidance of Zika areas if possible
- methods of transmission through mosquito bites or sex
- avoidance of mosquito bites
- symptoms of Zika infection
- safe sex practices
- testing limitations and knowledge deficiency about Zika.
Not uncommonly, clinical situations require complex individualized management decisions regarding trade-offs of risks, especially in older patients with decreased ovarian reserve. Consultation with infectious disease and reproductive specialists should be obtained when complicated and consequential decisions have to be made.
All practitioners should inform their patients, especially those undergoing fertility treatments, about Zika, and develop language in their informed consent that conveys the gap in knowledge to these patients.
Read how obesity specifically affects reproduction in an adverse way
Obesity adversely affects reproduction, but how specifically?
Practice Committee of the American Society for Reproductive Medicine. Obesity and Reproduction: A committee opinion. Fertil Steril. 2015;104(5):1116-1126.
The prevalence of obesity has increased substantially over the past 2 decades. Almost two-thirds of women and three-fourths of men in the United States are overweight or obese (defined as a body mass index [BMI] ≥25 kg/m2 and BMI ≥30 kg/m2, respectively; TABLE). Nearly 50% of reproductive-age women are obese.

A disease of excess body fat and insulin resistance, obesity increases the risks of hypertension, diabetes, dyslipidemia, cardiovascular disease, sleep apnea, respiratory problems, and cancer as well as other serious health problems. While not all individuals with obesity will have infertility, obesity is associated with impaired reproduction in both women and men, adverse obstetric outcomes, and health problems in offspring. The American Society for Reproductive Medicine (ASRM) reviewed this important issue in a recent practice committee opinion.
Menstrual cycle and ovulatory dysfunction
Menstrual cycle abnormalities are more common in women with obesity. Elevated levels of insulin in obese women suppress sex hormone−binding globulin (SHBG) which in turn reduces gonadotropin secretion due to increased production of estrogen from conversion of androgens by adipose aromatase.1 Adipose tissue produces adipokines, which directly can suppress ovarian function.2
Ovulatory dysfunction is common among obese women; the relative risk of such dysfunction is 3.1 (95% confidence interval [CI], 2.2−4.4) among women with BMI levels >27 kg/m2 versus BMI levels 20.0 to 24.9 kg/m2.3,4 Obesity decreases fecundity even in women with normal menstrual cycles.5 This may in part be due to altered ovulatory dynamics with reduced early follicular luteinizing hormone pulse amplitude accompanied by prolonged folliculogenesis and reduced luteal progesterone levels.6
Compared with normal-weight women, obese women have a lower chance of conception within 1 year of stopping contraception; about 66% of obese women conceive within 1 year of stopping contraception, compared with about 81% of women with normal weight.7 Results of a Dutch study of 3,029 women with regular ovulation, at least one patent tube, and a partner with a normal semen analysis indicated a direct correlation between obesity and delayed conception, with a 4% lower spontaneous pregnancy rate per kg/m2 increase in women with a BMI >29 kg/m2 versus a BMI of 21 to 29 kg/m2 (hazard ratio, 0.96; 95% CI, 0.91−0.99).8
Assisted reproduction
Assisted reproduction in women with obesity is associated with lower success rates than in women with normal weight. A systematic review of 27 in vitro fertilization (IVF) studies (23 of which were retrospective) reveals 10% lower live-birth rate in overweight (BMI >25 kg/m2) versus normal-weight women (BMI <25 kg/m2) undergoing IVF (odds ratio [OR], 0.90; 95% CI, 0.82−1.0).9 Data from a meta-analysis of 33 IVF studies, including 47,967 cycles, show that, compared with women with a BMI <25 kg/m2, overweight or obese women have significantly reduced rates of clinical pregnancy (relative risk [RR], 0.90; P<.0001) and live birth (RR, 0.84; P = .0002).10
Results of a retrospective study of 4,609 women undergoing first IVF or IVF/intracytoplasmic sperm injection cycles revealed impaired embryo implantation (controlling for embryo quality and transfer day), reducing the age-adjusted odds of live birth in a BMI-dependent manner by 37% (BMI, 30.0−34.9 kg/m2), 61% (BMI, 35.0−39.9 kg/m2), and 68% (BMI, >40 kg/m2) compared with women with a BMI of 18.5 to 24.9 kg/m2.11 In a study of 12,566 Danish couples undergoing assisted reproduction, overweight and obese ovulatory women had a 12% (95% CI, 0.79−0.99) and 25% (95% CI, 0.63−0.90) reduction in IVF-related live birth rate, respectively (referent BMI, 18.5−24.9 kg/m2), with a 2% (95% CI, 0.97−0.99) decrease in live-birth rate for every one-unit increase in BMI.12 Putative mechanisms for these findings include altered oocyte morphology and reduced fertilization in eggs from obese women,13 and impaired embryo quality in women less than age 35.14 Oocytes from women with a BMI >25 kg/m2 are smaller and less likely to complete development postfertilization, with embryos arrested prior to blastulation containing more triglyceride than those forming blastocysts.15
Blastocysts developed from oocytes of high-BMI women are smaller, contain fewer cells and have a higher content of triglycerides, lower glucose consumption, and altered amino acid metabolism compared with embryos of normal-weight women (BMI <24.9 kg/m2).15 Obesity may alter endometrial receptivity during IVF given the finding that third-party surrogate women with a BMI >35 kg/m2 have a lower live-birth rate (25%) compared with women with a BMI <35 kg/m2 (49%; P<.05).16
Pregnancy outcomes
Obesity is linked to an increased risk of miscarriage. Results of a meta-analysis of 33 IVF studies including 47,967 cycles indicated that overweight or obese women have a higher rate of miscarriage (RR, 1.31; P<.0001) than normal-weight women (BMI <25 kg/m2).17 Maternal and perinatal morbid obesity are strongly associated with obstetric and perinatal complications, including gestational diabetes, hypertension, preeclampsia, preterm delivery, shoulder dystocia, fetal distress, early neonatal death, and small- as well as large-for-gestational age infants.
Obese women who conceive by IVF are at increased risk for preeclampsia, gestational diabetes, preterm delivery, and cesarean delivery.13 Authors of a meta-analysis of 18 observational studies concluded that obese mothers were at increased odds of pregnancies affected by such birth defects as neural tube defects, cardiovascular anomalies, and cleft lip and palate, among others.18
In addition to being the cause of these fetal abnormalities, maternal metabolic dysfunction is linked to promoting obesity in offspring, thereby perpetuating a cycle of obesity and adverse health outcomes that include an increased risk of premature death in adult offspring in subsequent generations.13
Treatment for obesity
Lifestyle modification is the first-line treatment for obesity.
Pre-fertility therapy and pregnancy goals. Targets for pregnancy should include:
- preconception weight loss to a BMI of 35 kg/m2
- prevention of excess weight gain in pregnancy
- long-term reduction in weight.
For all obese individuals, lifestyle modifications should include a weight loss of 7% of body weight and increased physical activity to at least 150 minutes of moderate activity, such as walking, per week. Calorie restriction should be emphasized. A 500 to 1,000 kcal/day decrease from usual dietary intake is expected to result in a 1- to 2-lb weight loss per week. A low-calorie diet of 1,000 to 1,200 kcal/day can lead to an average 10% decrease in total body weight over 6 months.
Adjunct supervised medical therapy or bariatric surgery can play an important role in successful weight loss prepregnancy but are not appropriate for women actively attempting conception. Importantly, pregnancy should be deferred for a minimum of 1 year after bariatric surgery. The decision to postpone pregnancy to achieve weight loss must be balanced against the risk of declining fertility with advancing age of the woman.
Read about when to treat subclinical hypothyroidism
Optimal management of subclinical hypothyroidism in women with infertility
Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545-553.
Thyroid disorders long have been associated with the potential for adverse reproductive outcomes. While overt hypothyroidism has been linked to infertility, increased miscarriage risk, and poor maternal and fetal outcomes, controversy has existed regarding the association between subclinical hypothyroidism (SCH) and reproductive problems. The ASRM recently published a guideline on the role of SCH in the infertile female population.
How is subclinical hypothyroidism defined?
SCH is classically defined as a thyrotropin (TSH) level above the upper limit of normal range (4.5−5.0 mIU/L) with normal free thyroxine (FT4) levels. The National Health and Nutrition Examination Survey (NHANES III) population has been used to establish normative data for TSH for a disease-free population. These include a median serum level for TSH of 1.5 mIU/L, with the corresponding 2.5 and 97.5 percentiles of 0.41 and 6.10, respectively.19 Data from the National Academy of Clinical Biochemistry, however, reveal that 95% of individuals without evidence of thyroid disease have a TSH level <2.5 mIU/L, and that the normal reference range is skewed to the right.20 Adjusting the upper limit of the normal range to 2.5 mIU/L would result in an additional 11.8% to 14.2% of the United States population (22 to 28 million individuals) being diagnosed with hypothyroidism.
This information raises several important questions.
1. Should nonpregnant women be treated for SCH?
No. There is no benefit from the standpoint of lipid profile or alteration of cardiovascular risk in the treatment of TSH levels between 5 and 10 mIU/L and, therefore, treatment of individuals with TSH <5 mIU/L is questionable. Furthermore, the risk of overtreatment resulting in bone loss is a concern. The Endocrine Society does not recommend changing the current normal TSH range for nonpregnant women.
2. What are normal TSH levels in pregnant women?
Because human chorionic gonadotropin (hCG) can bind to and affect the TSH receptor, thereby influencing TSH values, the normal range for TSH is modified in pregnancy. The Endocrine Society recommends the following pregnancy trimester guidelines for TSH levels: 2.5 mIU/L is the recommended upper limit of normal in the first trimester, 3.0 mIU/L in the second trimester, and 3.5 mIU/L in the third trimester.
3. Is untreated SCH associated with miscarriage?
There is fair evidence that SCH, defined as a TSH level >4 mIU/L during pregnancy, is associated with miscarriage, but there is insufficient evidence that TSH levels between 2.5 and 4 mIU/L are associated with miscarriage.
4. Is untreated SCH associated with infertility?
Limited data are available to assess the effect of SCH on infertility. While a few studies show an association between SCH on unexplained infertility and ovulatory disorders, SCH does not appear to be increased in other causes of infertility.
5. Is SCH associated with adverse obstetric outcomes?
Available data reveal that SCH with TSH levels outside the normal pregnancy range are associated with an increased risk of such obstetric complications as placental abruption, preterm birth, fetal death, and preterm premature rupture of membranes (PPROM). However, it is unclear if prepregnancy TSH levels between 2.5 and 4 mIU/L are associated with adverse obstetric outcomes.
6. Does untreated SCH affect developmental outcomes in children?
The fetus is solely dependent on maternal thyroid hormone in early pregnancy because the fetal thyroid does not produce thyroid hormone before 10 to 13 weeks of gestation. Significant evidence has associated untreated maternal hypothyroidism with delayed fetal neurologic development, impaired school performance, and lower intelligence quotient (IQ) among offspring.21 There is fair evidence that SCH diagnosed in pregnancy is associated with adverse neurologic development. There is no evidence that SCH prior to pregnancy is associated with adverse neurodevelopmental outcomes. It should be noted that only one study has examined whether treatment of SCH improves developmental outcomes (measured by IQ scored at age 3 years) and no significant differences were observed in women with SCH who were treated with levothyroxine versus those who were not.22
7. Does treatment of SCH improve miscarriage rates, live-birth rates, and/or clinical pregnancy rates?
Small randomized controlled studies of women undergoing infertility treatment and a few observational studies in the general population yield good evidence that levothyroxine treatment in women with SCH defined as TSH >4.0 mIU/L is associated with improvement in pregnancy, live birth, and miscarriage rates. There are no randomized trials assessing whether levothyroxine treatment in women with TSH levels between 2.5 and 4 mIU/L would yield similar benefits to those observed in women with TSH levels above 4 mIU/L.
8. Are thyroid antibodies associated with infertility or adverse reproductive outcomes?
There is good evidence that the thyroid autoimmunity, or the presence of TPO-Ab, is associated with miscarriage and fair evidence that it is associated with infertility. Treatment with levothyroxine may improve pregnancy outcomes especially if the TSH level is above 2.5 mIU/L.
9. Should there be universal screening for hypothyroidism in the first trimester of pregnancy?
Current evidence does not reveal a benefit of universal screening at this time. The American College of Obstetricians and Gynecologists does not recommend routine screening for hypothyroidism in pregnancy unless women have risk factors for thyroid disease, including a personal or family history of thyroid disease, physical findings or symptoms of goiter or hypothyroidism, type 1 diabetes mellitus, infertility, history of miscarriage or preterm delivery, and/or personal or family history of autoimmune disease.
The bottom line
SCH, defined as a TSH level greater than the upper limit of normal range (4.5−5.0 mIU/L)with normal FT4 levels, is associated with adverse reproductive outcomes including miscarriage, pregnancy complications, and delayed fetal neurodevelopment. Thyroid supplementation is beneficial; however, treatment has not been shown to improve long-term neurologic developmental outcomes in offspring. Data are limited on whether TSH values between 2.5 mIU/L and the upper range of normal are associated with adverse pregnancy outcomes and therefore treatment in this group remains controversial. Although available evidence is weak, there may be a benefit in some subgroups, and because risk is minimal, it may be reasonable to treat or to monitor levels and treat above nonpregnant and pregnancy ranges. There is fair evidence that thyroid autoimmunity (positive thyroid antibody) is associated with miscarriage and infertility. Levothyroxine therapy may improve pregnancy outcomes especially if the TSH level is above 2.5 mIU/L. While universal screening of thyroid function in pregnancy is not recommended, women at high risk for thyroid disease should be screened.23
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Zika virus is a serious problem. Education and infection prevention are critical to effective management, and why we chose to include Zika virus as a topic for this year’s Update. We also discuss obesity’s effects on reproduction—a very relevant concern for all ObGyns and patients alike as about half of reproductive-age women are obese. Finally, subclinical hypothyroidism can present unique management challenges, such as determining when it is present and when treatment is indicated.
Read about counseling patients about Zika virus
Managing attempted pregnancy in the era of Zika virus
Oduyebo T, Igbinosa I, Petersen EE, et al. Update: interim guidance for health care providers caring for pregnant women with possible Zika virus exposure--United States, July 2016. MMWR Morb Mortal Wkly Rep. 2016;65(29):739-744.
Petersen EE, Meaney-Delman D, Neblett-Fanfair R, et al. Update: interim guidance for preconception counseling and prevention of sexual transmission of Zika virus for persons with possible Zika virus exposure--United States, September 2016. MMWR Morb Mortal Wkly Rep. 2016;65(39):1077-1081.
US Food and Drug Administration. Donor Screening Recommendations to Reduce the Risk of Transmission of Zika Virus by Human Cells, Tissues, and Cellular and Tissue-Based Products. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/UCM488582.pdf. Published March 2016. Accessed January 12, 2017.
National Institutes of Health. Zika: Overview. https://www.nichd.nih.gov/health/topics/zika/Pages/default.aspx. Accessed January 12, 2017.
World Health Organization. Prevention of sexual transmission of Zika virus interim guidance. WHO reference number: WHO/ZIKV/MOC/16. 1 Rev. 3, September 6, 2016.
Zika Virus Guidance Task Force of the American Society for Reproductive Medicine. Rev. 13, September 2016.
Zika virus presents unique challenges to physicians managing the care of patients attempting pregnancy, with or without fertility treatment. Neonatal Zika virus infection sequelae only recently have been appreciated; microcephaly was associated with Zika virus in October 2015, followed by other neurologic conditions including brain abnormalities, neural tube defects, and eye abnormalities. Results of recent studies involving the US Zika Pregnancy Registry show that 6% of women with Zika at any time in pregnancy had affected babies, but 11% of those who contracted the disease in the first trimester were affected.
Diagnosis is difficult because symptoms are generally mild, with 80% of affected patients asymptomatic. Possible Zika virus exposure is defined as travel to or residence in an area of active Zika virus transmission, or sex without a condom with a partner who traveled to or lived in an area of active transmission. Much is unknown about the interval from exposure to symptoms. Testing availability is limited and variable, and much is unknown about sensitivity and specificity of direct viral RNA testing, appearance and disappearance of detectable immunoglobulin (Ig) M and IgG antibodies that affect false positive and false negative test results, duration of infectious phase, risk of transmission, and numerous other factors.
Positive serum viral testing likely indicates virus in semen or other bodily fluids, but a negative serum viral test cannot definitively preclude virus in other bodily fluids. Zika virus likely can be passed from any combination of semen and vaginal and cervical fluids, but validating tests for these fluids are not yet available. It is not known if sperm preparation and assisted reproductive technology (ART) procedures that minimize risk of HIV transmission are effective against Zika virus or whether or not cryopreservation can destroy the virus.
Pregnancy timing
The Centers for Disease Control and Prevention now recommends that all men with possible Zika virus exposure who are considering attempting pregnancy with their partner wait to get pregnant until at least 6 months after symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic). Women with possible Zika virus exposure are recommended to wait to get pregnant until at least 8 weeks after symptom onset (if symptomatic) or last possible Zika virus exposure (if asymptomatic).
Women and men with possible exposure to Zika virus but without clinical symptoms of illness should consider testing for Zika viral RNA within 2 weeks of suspected exposure and wait at least 8 weeks after the last date of exposure before being re-tested. If direct viral testing (using rRT-PCR) results initially are negative, ideally, antibody testing would be obtained, if available, at 8 weeks. However, no testing paradigm will absolutely guarantee lack of Zika virus infectivity.
Virus management problems are dramatically compounded in areas endemic for Zika. Women and men who have had Zika virus disease should wait at least 6 months after illness onset to attempt reproduction. The temporal relationship between the presence of viral RNA and infectivity is not known definitively, and so the absolute duration of time to wait before attempting pregnancy is unknown. Male and female partners who become infected should avoid all forms of intimate sexual conduct or use condoms for the same 6 months. There is no evidence Zika will cause congenital infection in pregnancies initiated after resolution of maternal Zika viremia. However, any testing performed at a time other than the time of treatment might not reflect true viral status, particularly in areas of active Zika virus transmission.
Prevention
Women and men, especially those residing in areas of active Zika virus transmission, should talk with their physicians regarding pregnancy plans and avoid mosquito bites using the usual precautions: avoid mosquito areas, drain standing water, use mosquito repellent containing DEET, and use mosquito netting. Some people have gone so far as to relocate to nonendemic areas.
Those contemplating pregnancy should be advised to consider what they would do if they become exposed to or have suspected or confirmed Zika virus during pregnancy. Additional considerations are gamete or embryo cryopreservation and quarantine until a subsequent rRT-PCR test result is negative in both the male and female and at least 8 weeks have passed from gamete collection.

Patient counseling essentials
Counsel patients considering reproduction about:
- Zika virus as a new reproductive hazard
- the significance of the hazard to the fetus if infected
- the areas of active transmission, and that they are constantly changing
- avoidance of Zika areas if possible
- methods of transmission through mosquito bites or sex
- avoidance of mosquito bites
- symptoms of Zika infection
- safe sex practices
- testing limitations and knowledge deficiency about Zika.
Not uncommonly, clinical situations require complex individualized management decisions regarding trade-offs of risks, especially in older patients with decreased ovarian reserve. Consultation with infectious disease and reproductive specialists should be obtained when complicated and consequential decisions have to be made.
All practitioners should inform their patients, especially those undergoing fertility treatments, about Zika, and develop language in their informed consent that conveys the gap in knowledge to these patients.
Read how obesity specifically affects reproduction in an adverse way
Obesity adversely affects reproduction, but how specifically?
Practice Committee of the American Society for Reproductive Medicine. Obesity and Reproduction: A committee opinion. Fertil Steril. 2015;104(5):1116-1126.
The prevalence of obesity has increased substantially over the past 2 decades. Almost two-thirds of women and three-fourths of men in the United States are overweight or obese (defined as a body mass index [BMI] ≥25 kg/m2 and BMI ≥30 kg/m2, respectively; TABLE). Nearly 50% of reproductive-age women are obese.

A disease of excess body fat and insulin resistance, obesity increases the risks of hypertension, diabetes, dyslipidemia, cardiovascular disease, sleep apnea, respiratory problems, and cancer as well as other serious health problems. While not all individuals with obesity will have infertility, obesity is associated with impaired reproduction in both women and men, adverse obstetric outcomes, and health problems in offspring. The American Society for Reproductive Medicine (ASRM) reviewed this important issue in a recent practice committee opinion.
Menstrual cycle and ovulatory dysfunction
Menstrual cycle abnormalities are more common in women with obesity. Elevated levels of insulin in obese women suppress sex hormone−binding globulin (SHBG) which in turn reduces gonadotropin secretion due to increased production of estrogen from conversion of androgens by adipose aromatase.1 Adipose tissue produces adipokines, which directly can suppress ovarian function.2
Ovulatory dysfunction is common among obese women; the relative risk of such dysfunction is 3.1 (95% confidence interval [CI], 2.2−4.4) among women with BMI levels >27 kg/m2 versus BMI levels 20.0 to 24.9 kg/m2.3,4 Obesity decreases fecundity even in women with normal menstrual cycles.5 This may in part be due to altered ovulatory dynamics with reduced early follicular luteinizing hormone pulse amplitude accompanied by prolonged folliculogenesis and reduced luteal progesterone levels.6
Compared with normal-weight women, obese women have a lower chance of conception within 1 year of stopping contraception; about 66% of obese women conceive within 1 year of stopping contraception, compared with about 81% of women with normal weight.7 Results of a Dutch study of 3,029 women with regular ovulation, at least one patent tube, and a partner with a normal semen analysis indicated a direct correlation between obesity and delayed conception, with a 4% lower spontaneous pregnancy rate per kg/m2 increase in women with a BMI >29 kg/m2 versus a BMI of 21 to 29 kg/m2 (hazard ratio, 0.96; 95% CI, 0.91−0.99).8
Assisted reproduction
Assisted reproduction in women with obesity is associated with lower success rates than in women with normal weight. A systematic review of 27 in vitro fertilization (IVF) studies (23 of which were retrospective) reveals 10% lower live-birth rate in overweight (BMI >25 kg/m2) versus normal-weight women (BMI <25 kg/m2) undergoing IVF (odds ratio [OR], 0.90; 95% CI, 0.82−1.0).9 Data from a meta-analysis of 33 IVF studies, including 47,967 cycles, show that, compared with women with a BMI <25 kg/m2, overweight or obese women have significantly reduced rates of clinical pregnancy (relative risk [RR], 0.90; P<.0001) and live birth (RR, 0.84; P = .0002).10
Results of a retrospective study of 4,609 women undergoing first IVF or IVF/intracytoplasmic sperm injection cycles revealed impaired embryo implantation (controlling for embryo quality and transfer day), reducing the age-adjusted odds of live birth in a BMI-dependent manner by 37% (BMI, 30.0−34.9 kg/m2), 61% (BMI, 35.0−39.9 kg/m2), and 68% (BMI, >40 kg/m2) compared with women with a BMI of 18.5 to 24.9 kg/m2.11 In a study of 12,566 Danish couples undergoing assisted reproduction, overweight and obese ovulatory women had a 12% (95% CI, 0.79−0.99) and 25% (95% CI, 0.63−0.90) reduction in IVF-related live birth rate, respectively (referent BMI, 18.5−24.9 kg/m2), with a 2% (95% CI, 0.97−0.99) decrease in live-birth rate for every one-unit increase in BMI.12 Putative mechanisms for these findings include altered oocyte morphology and reduced fertilization in eggs from obese women,13 and impaired embryo quality in women less than age 35.14 Oocytes from women with a BMI >25 kg/m2 are smaller and less likely to complete development postfertilization, with embryos arrested prior to blastulation containing more triglyceride than those forming blastocysts.15
Blastocysts developed from oocytes of high-BMI women are smaller, contain fewer cells and have a higher content of triglycerides, lower glucose consumption, and altered amino acid metabolism compared with embryos of normal-weight women (BMI <24.9 kg/m2).15 Obesity may alter endometrial receptivity during IVF given the finding that third-party surrogate women with a BMI >35 kg/m2 have a lower live-birth rate (25%) compared with women with a BMI <35 kg/m2 (49%; P<.05).16
Pregnancy outcomes
Obesity is linked to an increased risk of miscarriage. Results of a meta-analysis of 33 IVF studies including 47,967 cycles indicated that overweight or obese women have a higher rate of miscarriage (RR, 1.31; P<.0001) than normal-weight women (BMI <25 kg/m2).17 Maternal and perinatal morbid obesity are strongly associated with obstetric and perinatal complications, including gestational diabetes, hypertension, preeclampsia, preterm delivery, shoulder dystocia, fetal distress, early neonatal death, and small- as well as large-for-gestational age infants.
Obese women who conceive by IVF are at increased risk for preeclampsia, gestational diabetes, preterm delivery, and cesarean delivery.13 Authors of a meta-analysis of 18 observational studies concluded that obese mothers were at increased odds of pregnancies affected by such birth defects as neural tube defects, cardiovascular anomalies, and cleft lip and palate, among others.18
In addition to being the cause of these fetal abnormalities, maternal metabolic dysfunction is linked to promoting obesity in offspring, thereby perpetuating a cycle of obesity and adverse health outcomes that include an increased risk of premature death in adult offspring in subsequent generations.13
Treatment for obesity
Lifestyle modification is the first-line treatment for obesity.
Pre-fertility therapy and pregnancy goals. Targets for pregnancy should include:
- preconception weight loss to a BMI of 35 kg/m2
- prevention of excess weight gain in pregnancy
- long-term reduction in weight.
For all obese individuals, lifestyle modifications should include a weight loss of 7% of body weight and increased physical activity to at least 150 minutes of moderate activity, such as walking, per week. Calorie restriction should be emphasized. A 500 to 1,000 kcal/day decrease from usual dietary intake is expected to result in a 1- to 2-lb weight loss per week. A low-calorie diet of 1,000 to 1,200 kcal/day can lead to an average 10% decrease in total body weight over 6 months.
Adjunct supervised medical therapy or bariatric surgery can play an important role in successful weight loss prepregnancy but are not appropriate for women actively attempting conception. Importantly, pregnancy should be deferred for a minimum of 1 year after bariatric surgery. The decision to postpone pregnancy to achieve weight loss must be balanced against the risk of declining fertility with advancing age of the woman.
Read about when to treat subclinical hypothyroidism
Optimal management of subclinical hypothyroidism in women with infertility
Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545-553.
Thyroid disorders long have been associated with the potential for adverse reproductive outcomes. While overt hypothyroidism has been linked to infertility, increased miscarriage risk, and poor maternal and fetal outcomes, controversy has existed regarding the association between subclinical hypothyroidism (SCH) and reproductive problems. The ASRM recently published a guideline on the role of SCH in the infertile female population.
How is subclinical hypothyroidism defined?
SCH is classically defined as a thyrotropin (TSH) level above the upper limit of normal range (4.5−5.0 mIU/L) with normal free thyroxine (FT4) levels. The National Health and Nutrition Examination Survey (NHANES III) population has been used to establish normative data for TSH for a disease-free population. These include a median serum level for TSH of 1.5 mIU/L, with the corresponding 2.5 and 97.5 percentiles of 0.41 and 6.10, respectively.19 Data from the National Academy of Clinical Biochemistry, however, reveal that 95% of individuals without evidence of thyroid disease have a TSH level <2.5 mIU/L, and that the normal reference range is skewed to the right.20 Adjusting the upper limit of the normal range to 2.5 mIU/L would result in an additional 11.8% to 14.2% of the United States population (22 to 28 million individuals) being diagnosed with hypothyroidism.
This information raises several important questions.
1. Should nonpregnant women be treated for SCH?
No. There is no benefit from the standpoint of lipid profile or alteration of cardiovascular risk in the treatment of TSH levels between 5 and 10 mIU/L and, therefore, treatment of individuals with TSH <5 mIU/L is questionable. Furthermore, the risk of overtreatment resulting in bone loss is a concern. The Endocrine Society does not recommend changing the current normal TSH range for nonpregnant women.
2. What are normal TSH levels in pregnant women?
Because human chorionic gonadotropin (hCG) can bind to and affect the TSH receptor, thereby influencing TSH values, the normal range for TSH is modified in pregnancy. The Endocrine Society recommends the following pregnancy trimester guidelines for TSH levels: 2.5 mIU/L is the recommended upper limit of normal in the first trimester, 3.0 mIU/L in the second trimester, and 3.5 mIU/L in the third trimester.
3. Is untreated SCH associated with miscarriage?
There is fair evidence that SCH, defined as a TSH level >4 mIU/L during pregnancy, is associated with miscarriage, but there is insufficient evidence that TSH levels between 2.5 and 4 mIU/L are associated with miscarriage.
4. Is untreated SCH associated with infertility?
Limited data are available to assess the effect of SCH on infertility. While a few studies show an association between SCH on unexplained infertility and ovulatory disorders, SCH does not appear to be increased in other causes of infertility.
5. Is SCH associated with adverse obstetric outcomes?
Available data reveal that SCH with TSH levels outside the normal pregnancy range are associated with an increased risk of such obstetric complications as placental abruption, preterm birth, fetal death, and preterm premature rupture of membranes (PPROM). However, it is unclear if prepregnancy TSH levels between 2.5 and 4 mIU/L are associated with adverse obstetric outcomes.
6. Does untreated SCH affect developmental outcomes in children?
The fetus is solely dependent on maternal thyroid hormone in early pregnancy because the fetal thyroid does not produce thyroid hormone before 10 to 13 weeks of gestation. Significant evidence has associated untreated maternal hypothyroidism with delayed fetal neurologic development, impaired school performance, and lower intelligence quotient (IQ) among offspring.21 There is fair evidence that SCH diagnosed in pregnancy is associated with adverse neurologic development. There is no evidence that SCH prior to pregnancy is associated with adverse neurodevelopmental outcomes. It should be noted that only one study has examined whether treatment of SCH improves developmental outcomes (measured by IQ scored at age 3 years) and no significant differences were observed in women with SCH who were treated with levothyroxine versus those who were not.22
7. Does treatment of SCH improve miscarriage rates, live-birth rates, and/or clinical pregnancy rates?
Small randomized controlled studies of women undergoing infertility treatment and a few observational studies in the general population yield good evidence that levothyroxine treatment in women with SCH defined as TSH >4.0 mIU/L is associated with improvement in pregnancy, live birth, and miscarriage rates. There are no randomized trials assessing whether levothyroxine treatment in women with TSH levels between 2.5 and 4 mIU/L would yield similar benefits to those observed in women with TSH levels above 4 mIU/L.
8. Are thyroid antibodies associated with infertility or adverse reproductive outcomes?
There is good evidence that the thyroid autoimmunity, or the presence of TPO-Ab, is associated with miscarriage and fair evidence that it is associated with infertility. Treatment with levothyroxine may improve pregnancy outcomes especially if the TSH level is above 2.5 mIU/L.
9. Should there be universal screening for hypothyroidism in the first trimester of pregnancy?
Current evidence does not reveal a benefit of universal screening at this time. The American College of Obstetricians and Gynecologists does not recommend routine screening for hypothyroidism in pregnancy unless women have risk factors for thyroid disease, including a personal or family history of thyroid disease, physical findings or symptoms of goiter or hypothyroidism, type 1 diabetes mellitus, infertility, history of miscarriage or preterm delivery, and/or personal or family history of autoimmune disease.
The bottom line
SCH, defined as a TSH level greater than the upper limit of normal range (4.5−5.0 mIU/L)with normal FT4 levels, is associated with adverse reproductive outcomes including miscarriage, pregnancy complications, and delayed fetal neurodevelopment. Thyroid supplementation is beneficial; however, treatment has not been shown to improve long-term neurologic developmental outcomes in offspring. Data are limited on whether TSH values between 2.5 mIU/L and the upper range of normal are associated with adverse pregnancy outcomes and therefore treatment in this group remains controversial. Although available evidence is weak, there may be a benefit in some subgroups, and because risk is minimal, it may be reasonable to treat or to monitor levels and treat above nonpregnant and pregnancy ranges. There is fair evidence that thyroid autoimmunity (positive thyroid antibody) is associated with miscarriage and infertility. Levothyroxine therapy may improve pregnancy outcomes especially if the TSH level is above 2.5 mIU/L. While universal screening of thyroid function in pregnancy is not recommended, women at high risk for thyroid disease should be screened.23
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. Obesity and reproductive disorders in women. Hum Reprod Update. 2003;9(4):359-372.
- Greisen S, Ledet T, Møller N, et al. Effects of leptin on basal and FSH stimulated steroidogenesis in human granulosa luteal cells. Acta Obstet Gynecol Scand. 2000;79(11):931-935.
- Rich-Edwards JW, Goldman MB, Willett WC, et al. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171(1):171-177.
- Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994;5(2):247-250.
- Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22(2):414-420.
- Jain A, Polotsky AJ, Rochester D, et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92(7):2468-2473.
- Lake JK, Power C, Cole TJ. Women's reproductive health: the role of body mass index in early and adult life. Int J Obes Relat Metab Disord. 1997;21(6):432-438.
- van der Steeg JW, Steures P, Eijkemans MJ, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod. 2008;23(2):324-328.
- Koning AM, Mutsaerts MA, Kuchenbecker WK, et al. Complications and outcome of assisted reproduction technologies in overweight and obese women [Published correction appears in Hum Reprod. 2012;27(8):2570.] Hum Reprod. 2012;27(2):457-467.
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421-439.
- Moragianni VA, Jones SM, Ryley DA. The effect of body mass index on the outcomes of first assisted reproductive technology cycles. Fertil Steril. 2012;98(1):102-108.
- Petersen GL, Schmidt L, Pinborg A, Kamper-Jørgensen M. The influence of female and male body mass index on live births after assisted reproductive technology treatment: a nationwide register-based cohort study. Fertil Steril. 2013;99(6):1654-1662.
- Practice Committee of the American Society for Reproductive Medicine. Obesity and Reproduction: A committee opinion. Fertil Steril. 2015;104(5):1116-1126.
- Metwally M, Cutting R, Tipton A, Skull J, Ledger WL, Li TC. Effect of increased body mass index on oocyte and embryo quality in IVF patients. Reprod Biomed Online. 2007;15(5):532-538.
- Leary C, Leese HJ, Sturmey RG. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum Reprod. 2015;30(1):122-132.
- Deugarte D, Deugarte C, Sahakian V. Surrogate obesity negatively impacts pregnancy rates in third-party reproduction. Fertil Steril. 2010;93(3):1008-1010.
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421-439.
- Stothard KJ, Tennant PWG, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301(6):636-650.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499.
- Baloch Z, Carayon P, Conte-Devolx B, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13(1):3-126.
- Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf). 1999;50(2):149-155.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366(17):493-501.
- Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545-553.
- Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. Obesity and reproductive disorders in women. Hum Reprod Update. 2003;9(4):359-372.
- Greisen S, Ledet T, Møller N, et al. Effects of leptin on basal and FSH stimulated steroidogenesis in human granulosa luteal cells. Acta Obstet Gynecol Scand. 2000;79(11):931-935.
- Rich-Edwards JW, Goldman MB, Willett WC, et al. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171(1):171-177.
- Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994;5(2):247-250.
- Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22(2):414-420.
- Jain A, Polotsky AJ, Rochester D, et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92(7):2468-2473.
- Lake JK, Power C, Cole TJ. Women's reproductive health: the role of body mass index in early and adult life. Int J Obes Relat Metab Disord. 1997;21(6):432-438.
- van der Steeg JW, Steures P, Eijkemans MJ, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod. 2008;23(2):324-328.
- Koning AM, Mutsaerts MA, Kuchenbecker WK, et al. Complications and outcome of assisted reproduction technologies in overweight and obese women [Published correction appears in Hum Reprod. 2012;27(8):2570.] Hum Reprod. 2012;27(2):457-467.
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421-439.
- Moragianni VA, Jones SM, Ryley DA. The effect of body mass index on the outcomes of first assisted reproductive technology cycles. Fertil Steril. 2012;98(1):102-108.
- Petersen GL, Schmidt L, Pinborg A, Kamper-Jørgensen M. The influence of female and male body mass index on live births after assisted reproductive technology treatment: a nationwide register-based cohort study. Fertil Steril. 2013;99(6):1654-1662.
- Practice Committee of the American Society for Reproductive Medicine. Obesity and Reproduction: A committee opinion. Fertil Steril. 2015;104(5):1116-1126.
- Metwally M, Cutting R, Tipton A, Skull J, Ledger WL, Li TC. Effect of increased body mass index on oocyte and embryo quality in IVF patients. Reprod Biomed Online. 2007;15(5):532-538.
- Leary C, Leese HJ, Sturmey RG. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum Reprod. 2015;30(1):122-132.
- Deugarte D, Deugarte C, Sahakian V. Surrogate obesity negatively impacts pregnancy rates in third-party reproduction. Fertil Steril. 2010;93(3):1008-1010.
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23(4):421-439.
- Stothard KJ, Tennant PWG, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301(6):636-650.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499.
- Baloch Z, Carayon P, Conte-Devolx B, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13(1):3-126.
- Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf). 1999;50(2):149-155.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366(17):493-501.
- Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104(3):545-553.
Women’s Preventive Services Initiative Guidelines provide consensus for practicing ObGyns
The Patient Protection and Affordable Care Act of 2010 (ACA) intended that women have access to critical preventive health services without a copay or deductible. The Institute of Medicine (IOM) was asked to help identify those critical preventive women’s health services. In 2011, the IOM Committee on Preventive Services for Women recommended that all women have access to 9 preventive services, among them1:
- screening for gestational diabetes mellitus (GDM)
- human papilloma virus testing
- contraceptive methods and counseling
- well-woman visits.
The Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services agreed to update the recommended preventive services every 5 years.
In March 2016, HRSA entered into a 5-year cooperative agreement with the American College of Obstetricians and Gynecologists (ACOG) to update the guidelines and to develop additional recommendations to enhance women’s health.2 ACOG launched the Women’s Preventive Services Initiative (WPSI) to develop the 2016 update.
The 5-year grant with HRSA will address many more preventive health services for women across their lifespan as well as implementation strategies so that women receive consistent and appropriate care, regardless of the health care provider’s specialty. The WPSI recognizes that the selection of a provider for well-woman care will be determined as much by a woman’s needs and preferences as by her access to health care services and health plan availability.
The WPSI draft recommendations were released for public comment in September 2016,2 and HRSA approved the recommendations in December 2016.3 In this editorial, I provide a look at which organizations comprise the WPSI and a summary of the 9 recommended preventive health services.
Who makes up the Women’s Preventive Services Initiative?
The WPSI is a collaboration between professional societies and consumer organizations. The goal of the WPSI is “to promote health over the course of a woman’s lifetime through disease prevention and preventive healthcare.” The WPSI advisory panel provides oversight to the effort and the multidisciplinary steering committee develops the recommendations. The WPSI advisory panel includes leaders and experts from 4 major professional organizations, whose members provide the majority of women’s health care in the United States:
- ACOG
- American College of Physicians (ACP)
- American Academy of Family Physicians (AAFP)
- National Association of Nurse Practitioners in Women’s Health (NPWH).
The multidisciplinary steering committee includes the members of the advisory panel, representatives from 17 professional and consumer organizations, a patient representative, and representatives from 6 federal agencies. The WPSI is currently chaired by Jeanne Conry, MD, PhD, past president of ACOG. The steering committee used evidence-based best practices to develop the guidelines and relied heavily on the foundation provided by the 2011 IOM report.1

The 9 WPSI recommendations
Much of the text below is directly quoted from the final recommendations. When a recommendation is paraphrased it is not placed in quotations.
Recommendation 1: Breast cancer screening for average-risk women
“Average-risk women should initiate mammography screening for breast cancer no earlier than age 40 and no later than age 50 years. Screeningmammography should occur at least biennially and as frequently as annually. Screening should continue through at least age 74 years and age alone should not be the basis to stop screening.”
Decisions about when to initiate screening for women between 40 and 50 years of age, how often to screen, and when to stop screening should be based on shared decision making involving the woman and her clinician.
Recommendation 2: Breastfeeding services and supplies
Women should be provided “comprehensive lactation support services including counseling, education and breast feeding equipment and supplies during the antenatal, perinatal, and postpartum periods.” These services will support the successful initiation and maintenance of breastfeeding. Women should have access to double electric breast pumps.
Recommendation 3: Screening for cervical cancer
Average-risk women should initiate cervical cancer screening with cervical cytology at age 21 years and have cervical cytology testing every 3 years from 21 to 29 years of age. “Cotesting with cytology and human papillomavirus (HPV) testing is not recommended for women younger than 30 years. Women aged 30 to 65 years should be screened with cytology and HPV testing every 5 years or cytology alone every 3 years.” Women who have received the HPV vaccine should be screened using these guidelines. Cervical cancer screening is not recommended for women younger than 21 years or older than 65 years who have had adequate prior screening and are not at high risk for cervical cancer. Cervical cancer screening is also not recommended for women who have had a hysterectomy with removal of the cervix and no personal history of cervical intraepithelial neoplasia grade 2 or 3 within the past 20 years.
Recommendation 4: Contraception
Adolescent and adult women should have access to the full range of US Food and Drug Administration–approved female-controlled contraceptives to prevent unintended pregnancy and improve birth outcomes. Multiple visits with a clinician may be needed to select an optimal contraceptive.
Recommendation 5: Screening for gestational diabetes mellitus
Pregnant women should be screened for GDM between 24 and 28 weeks’ gestation to prevent adverse birth outcomes. Screening should be performed with a “50 gm oral glucose challenge test followed by a 3-hour 100 gm oral glucose tolerance test” if the results on the initial oral glucose tolerance test are abnormal. This testing sequence has high sensitivity and specificity. Women with risk factors for diabetes mellitus should be screened for diabetes at the first prenatal visit using current best clinical practice.
Recommendation 6: Screening for human immunodeficiency virus (HIV) infection
Adolescents and women should receive education and risk assessment for HIV annually and should be tested for HIV at least once during their lifetime. Based on assessed risk, screening annually may be appropriate. “Screening for HIV is recommended for all pregnant women upon initiation of prenatal care with retesting during pregnancy based on risk factors. Rapid HIV testing is recommended for pregnant women who present in active labor with an undocumented HIV status.” Risk-based screening does not identify approximately 20% of HIV-infected people. Hence screening annually may be reasonable.
Recommendation 7: Screening for interpersonal and domestic violence
All adolescents and women should be screened annually for both interpersonal violence (IPV) and domestic violence (DV). Intervention services should be available to all adolescents and women. IPV and DV are prevalent problems, and they are often undetected by clinicians. Hence annual screening is recommended.
Recommendation 8: Counseling for sexually transmitted infections
Adolescents and women should be assessed for sexually transmittedinfection (STI) risk. Risk factors include:
- “age younger than 25 years,
- a recent STI,
- a new sex partner,
- multiple partners,
- a partner with concurrent partners,
- a partner with an STI, and
- a lack of or inconsistent condom use.”
Women at increased risk for an STI should receive behavioral counseling.
Recommendation 9: Well-woman preventive visits
Women should “receive at least one preventive care visit per year beginning in adolescence and continuing across the lifespan to ensure that the recommended preventive services including preconception and many services necessary for prenatal and interconception care are obtained. The primary purpose of these visits is the delivery and coordination of recommended preventive services as determined by age and risk factors.”
- Abridged guidelines for the Women's Preventive Services Initiative can be found here: http://www.womenspreventivehealth.org/wp-content/uploads/2017/01/WPSI_2016AbridgedReport.pdf.
- Evidence-based summaries and appendices are available at this link: http://www.womenspreventivehealth.org/wp-content/uploads/2016/12/Evidence-Summaries-and-Appendices.pdf.
I plan on using these recommendations to guide my practice
Historically, many high-profile expert professional groups have developed their own women’s health services guidelines. The proliferation of conflicting guidelines confused both patients and clinicians. Dueling guidelines likely undermine public health because they result in confusion among patients and inconsistent care across the many disciplines that provide medical services to women.
The proliferation of conflicting guidelines for mammography screening for breast cancer is a good example of how dueling guidelines can undermine public health (TABLE).4−7 The WPSI has done a great service to women and clinicians by creating a shared framework for consistently providing critical services across a woman’s entire life. I plan on using these recommendations to guide my practice. Patients and clinicians will greatly benefit from the exceptionally thoughtful women’s preventive services guidelines provided by the WPSI.
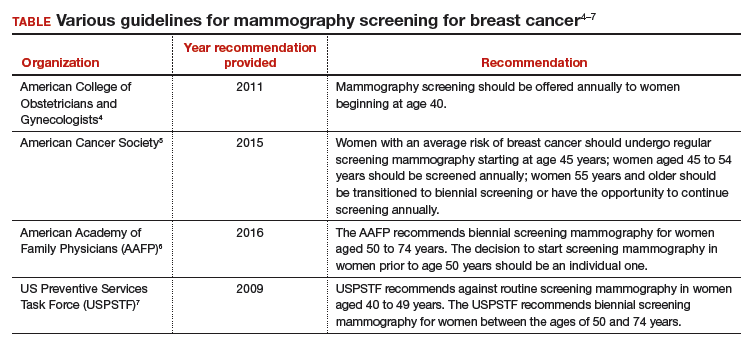
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Institute of Medicine. Clinical preventive services for women: closing the gaps. Washington DC: The National Academies Press; 2011. http://nap.edu/13181. Accessed January 16, 2017.
- American Congress of Obstetricians and Gynecologists (ACOG). Women's Preventive Services Initiative (WPSI). http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Womens-Preventive-Services-Initiative. Accessed January 16, 2017.
- Health Resources and Services Administration website. Women's preventive services guidelines. https://www.hrsa.gov/womensguidelines/. Accessed January 16, 2017.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 122: breast cancer screening. Obstet Gynecol. 2011;118(2 pt 1):372-382.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599-1614.
- American Academy of Family Physicians website. Clinical preventive service recommendation: breast cancer. http://www.aafp.org/patient-care/clinical-recommendations/all/breast-cancer.html. Accessed January 16, 2017.
- US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716-726.
The Patient Protection and Affordable Care Act of 2010 (ACA) intended that women have access to critical preventive health services without a copay or deductible. The Institute of Medicine (IOM) was asked to help identify those critical preventive women’s health services. In 2011, the IOM Committee on Preventive Services for Women recommended that all women have access to 9 preventive services, among them1:
- screening for gestational diabetes mellitus (GDM)
- human papilloma virus testing
- contraceptive methods and counseling
- well-woman visits.
The Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services agreed to update the recommended preventive services every 5 years.
In March 2016, HRSA entered into a 5-year cooperative agreement with the American College of Obstetricians and Gynecologists (ACOG) to update the guidelines and to develop additional recommendations to enhance women’s health.2 ACOG launched the Women’s Preventive Services Initiative (WPSI) to develop the 2016 update.
The 5-year grant with HRSA will address many more preventive health services for women across their lifespan as well as implementation strategies so that women receive consistent and appropriate care, regardless of the health care provider’s specialty. The WPSI recognizes that the selection of a provider for well-woman care will be determined as much by a woman’s needs and preferences as by her access to health care services and health plan availability.
The WPSI draft recommendations were released for public comment in September 2016,2 and HRSA approved the recommendations in December 2016.3 In this editorial, I provide a look at which organizations comprise the WPSI and a summary of the 9 recommended preventive health services.
Who makes up the Women’s Preventive Services Initiative?
The WPSI is a collaboration between professional societies and consumer organizations. The goal of the WPSI is “to promote health over the course of a woman’s lifetime through disease prevention and preventive healthcare.” The WPSI advisory panel provides oversight to the effort and the multidisciplinary steering committee develops the recommendations. The WPSI advisory panel includes leaders and experts from 4 major professional organizations, whose members provide the majority of women’s health care in the United States:
- ACOG
- American College of Physicians (ACP)
- American Academy of Family Physicians (AAFP)
- National Association of Nurse Practitioners in Women’s Health (NPWH).
The multidisciplinary steering committee includes the members of the advisory panel, representatives from 17 professional and consumer organizations, a patient representative, and representatives from 6 federal agencies. The WPSI is currently chaired by Jeanne Conry, MD, PhD, past president of ACOG. The steering committee used evidence-based best practices to develop the guidelines and relied heavily on the foundation provided by the 2011 IOM report.1

The 9 WPSI recommendations
Much of the text below is directly quoted from the final recommendations. When a recommendation is paraphrased it is not placed in quotations.
Recommendation 1: Breast cancer screening for average-risk women
“Average-risk women should initiate mammography screening for breast cancer no earlier than age 40 and no later than age 50 years. Screeningmammography should occur at least biennially and as frequently as annually. Screening should continue through at least age 74 years and age alone should not be the basis to stop screening.”
Decisions about when to initiate screening for women between 40 and 50 years of age, how often to screen, and when to stop screening should be based on shared decision making involving the woman and her clinician.
Recommendation 2: Breastfeeding services and supplies
Women should be provided “comprehensive lactation support services including counseling, education and breast feeding equipment and supplies during the antenatal, perinatal, and postpartum periods.” These services will support the successful initiation and maintenance of breastfeeding. Women should have access to double electric breast pumps.
Recommendation 3: Screening for cervical cancer
Average-risk women should initiate cervical cancer screening with cervical cytology at age 21 years and have cervical cytology testing every 3 years from 21 to 29 years of age. “Cotesting with cytology and human papillomavirus (HPV) testing is not recommended for women younger than 30 years. Women aged 30 to 65 years should be screened with cytology and HPV testing every 5 years or cytology alone every 3 years.” Women who have received the HPV vaccine should be screened using these guidelines. Cervical cancer screening is not recommended for women younger than 21 years or older than 65 years who have had adequate prior screening and are not at high risk for cervical cancer. Cervical cancer screening is also not recommended for women who have had a hysterectomy with removal of the cervix and no personal history of cervical intraepithelial neoplasia grade 2 or 3 within the past 20 years.
Recommendation 4: Contraception
Adolescent and adult women should have access to the full range of US Food and Drug Administration–approved female-controlled contraceptives to prevent unintended pregnancy and improve birth outcomes. Multiple visits with a clinician may be needed to select an optimal contraceptive.
Recommendation 5: Screening for gestational diabetes mellitus
Pregnant women should be screened for GDM between 24 and 28 weeks’ gestation to prevent adverse birth outcomes. Screening should be performed with a “50 gm oral glucose challenge test followed by a 3-hour 100 gm oral glucose tolerance test” if the results on the initial oral glucose tolerance test are abnormal. This testing sequence has high sensitivity and specificity. Women with risk factors for diabetes mellitus should be screened for diabetes at the first prenatal visit using current best clinical practice.
Recommendation 6: Screening for human immunodeficiency virus (HIV) infection
Adolescents and women should receive education and risk assessment for HIV annually and should be tested for HIV at least once during their lifetime. Based on assessed risk, screening annually may be appropriate. “Screening for HIV is recommended for all pregnant women upon initiation of prenatal care with retesting during pregnancy based on risk factors. Rapid HIV testing is recommended for pregnant women who present in active labor with an undocumented HIV status.” Risk-based screening does not identify approximately 20% of HIV-infected people. Hence screening annually may be reasonable.
Recommendation 7: Screening for interpersonal and domestic violence
All adolescents and women should be screened annually for both interpersonal violence (IPV) and domestic violence (DV). Intervention services should be available to all adolescents and women. IPV and DV are prevalent problems, and they are often undetected by clinicians. Hence annual screening is recommended.
Recommendation 8: Counseling for sexually transmitted infections
Adolescents and women should be assessed for sexually transmittedinfection (STI) risk. Risk factors include:
- “age younger than 25 years,
- a recent STI,
- a new sex partner,
- multiple partners,
- a partner with concurrent partners,
- a partner with an STI, and
- a lack of or inconsistent condom use.”
Women at increased risk for an STI should receive behavioral counseling.
Recommendation 9: Well-woman preventive visits
Women should “receive at least one preventive care visit per year beginning in adolescence and continuing across the lifespan to ensure that the recommended preventive services including preconception and many services necessary for prenatal and interconception care are obtained. The primary purpose of these visits is the delivery and coordination of recommended preventive services as determined by age and risk factors.”
- Abridged guidelines for the Women's Preventive Services Initiative can be found here: http://www.womenspreventivehealth.org/wp-content/uploads/2017/01/WPSI_2016AbridgedReport.pdf.
- Evidence-based summaries and appendices are available at this link: http://www.womenspreventivehealth.org/wp-content/uploads/2016/12/Evidence-Summaries-and-Appendices.pdf.
I plan on using these recommendations to guide my practice
Historically, many high-profile expert professional groups have developed their own women’s health services guidelines. The proliferation of conflicting guidelines confused both patients and clinicians. Dueling guidelines likely undermine public health because they result in confusion among patients and inconsistent care across the many disciplines that provide medical services to women.
The proliferation of conflicting guidelines for mammography screening for breast cancer is a good example of how dueling guidelines can undermine public health (TABLE).4−7 The WPSI has done a great service to women and clinicians by creating a shared framework for consistently providing critical services across a woman’s entire life. I plan on using these recommendations to guide my practice. Patients and clinicians will greatly benefit from the exceptionally thoughtful women’s preventive services guidelines provided by the WPSI.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The Patient Protection and Affordable Care Act of 2010 (ACA) intended that women have access to critical preventive health services without a copay or deductible. The Institute of Medicine (IOM) was asked to help identify those critical preventive women’s health services. In 2011, the IOM Committee on Preventive Services for Women recommended that all women have access to 9 preventive services, among them1:
- screening for gestational diabetes mellitus (GDM)
- human papilloma virus testing
- contraceptive methods and counseling
- well-woman visits.
The Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services agreed to update the recommended preventive services every 5 years.
In March 2016, HRSA entered into a 5-year cooperative agreement with the American College of Obstetricians and Gynecologists (ACOG) to update the guidelines and to develop additional recommendations to enhance women’s health.2 ACOG launched the Women’s Preventive Services Initiative (WPSI) to develop the 2016 update.
The 5-year grant with HRSA will address many more preventive health services for women across their lifespan as well as implementation strategies so that women receive consistent and appropriate care, regardless of the health care provider’s specialty. The WPSI recognizes that the selection of a provider for well-woman care will be determined as much by a woman’s needs and preferences as by her access to health care services and health plan availability.
The WPSI draft recommendations were released for public comment in September 2016,2 and HRSA approved the recommendations in December 2016.3 In this editorial, I provide a look at which organizations comprise the WPSI and a summary of the 9 recommended preventive health services.
Who makes up the Women’s Preventive Services Initiative?
The WPSI is a collaboration between professional societies and consumer organizations. The goal of the WPSI is “to promote health over the course of a woman’s lifetime through disease prevention and preventive healthcare.” The WPSI advisory panel provides oversight to the effort and the multidisciplinary steering committee develops the recommendations. The WPSI advisory panel includes leaders and experts from 4 major professional organizations, whose members provide the majority of women’s health care in the United States:
- ACOG
- American College of Physicians (ACP)
- American Academy of Family Physicians (AAFP)
- National Association of Nurse Practitioners in Women’s Health (NPWH).
The multidisciplinary steering committee includes the members of the advisory panel, representatives from 17 professional and consumer organizations, a patient representative, and representatives from 6 federal agencies. The WPSI is currently chaired by Jeanne Conry, MD, PhD, past president of ACOG. The steering committee used evidence-based best practices to develop the guidelines and relied heavily on the foundation provided by the 2011 IOM report.1

The 9 WPSI recommendations
Much of the text below is directly quoted from the final recommendations. When a recommendation is paraphrased it is not placed in quotations.
Recommendation 1: Breast cancer screening for average-risk women
“Average-risk women should initiate mammography screening for breast cancer no earlier than age 40 and no later than age 50 years. Screeningmammography should occur at least biennially and as frequently as annually. Screening should continue through at least age 74 years and age alone should not be the basis to stop screening.”
Decisions about when to initiate screening for women between 40 and 50 years of age, how often to screen, and when to stop screening should be based on shared decision making involving the woman and her clinician.
Recommendation 2: Breastfeeding services and supplies
Women should be provided “comprehensive lactation support services including counseling, education and breast feeding equipment and supplies during the antenatal, perinatal, and postpartum periods.” These services will support the successful initiation and maintenance of breastfeeding. Women should have access to double electric breast pumps.
Recommendation 3: Screening for cervical cancer
Average-risk women should initiate cervical cancer screening with cervical cytology at age 21 years and have cervical cytology testing every 3 years from 21 to 29 years of age. “Cotesting with cytology and human papillomavirus (HPV) testing is not recommended for women younger than 30 years. Women aged 30 to 65 years should be screened with cytology and HPV testing every 5 years or cytology alone every 3 years.” Women who have received the HPV vaccine should be screened using these guidelines. Cervical cancer screening is not recommended for women younger than 21 years or older than 65 years who have had adequate prior screening and are not at high risk for cervical cancer. Cervical cancer screening is also not recommended for women who have had a hysterectomy with removal of the cervix and no personal history of cervical intraepithelial neoplasia grade 2 or 3 within the past 20 years.
Recommendation 4: Contraception
Adolescent and adult women should have access to the full range of US Food and Drug Administration–approved female-controlled contraceptives to prevent unintended pregnancy and improve birth outcomes. Multiple visits with a clinician may be needed to select an optimal contraceptive.
Recommendation 5: Screening for gestational diabetes mellitus
Pregnant women should be screened for GDM between 24 and 28 weeks’ gestation to prevent adverse birth outcomes. Screening should be performed with a “50 gm oral glucose challenge test followed by a 3-hour 100 gm oral glucose tolerance test” if the results on the initial oral glucose tolerance test are abnormal. This testing sequence has high sensitivity and specificity. Women with risk factors for diabetes mellitus should be screened for diabetes at the first prenatal visit using current best clinical practice.
Recommendation 6: Screening for human immunodeficiency virus (HIV) infection
Adolescents and women should receive education and risk assessment for HIV annually and should be tested for HIV at least once during their lifetime. Based on assessed risk, screening annually may be appropriate. “Screening for HIV is recommended for all pregnant women upon initiation of prenatal care with retesting during pregnancy based on risk factors. Rapid HIV testing is recommended for pregnant women who present in active labor with an undocumented HIV status.” Risk-based screening does not identify approximately 20% of HIV-infected people. Hence screening annually may be reasonable.
Recommendation 7: Screening for interpersonal and domestic violence
All adolescents and women should be screened annually for both interpersonal violence (IPV) and domestic violence (DV). Intervention services should be available to all adolescents and women. IPV and DV are prevalent problems, and they are often undetected by clinicians. Hence annual screening is recommended.
Recommendation 8: Counseling for sexually transmitted infections
Adolescents and women should be assessed for sexually transmittedinfection (STI) risk. Risk factors include:
- “age younger than 25 years,
- a recent STI,
- a new sex partner,
- multiple partners,
- a partner with concurrent partners,
- a partner with an STI, and
- a lack of or inconsistent condom use.”
Women at increased risk for an STI should receive behavioral counseling.
Recommendation 9: Well-woman preventive visits
Women should “receive at least one preventive care visit per year beginning in adolescence and continuing across the lifespan to ensure that the recommended preventive services including preconception and many services necessary for prenatal and interconception care are obtained. The primary purpose of these visits is the delivery and coordination of recommended preventive services as determined by age and risk factors.”
- Abridged guidelines for the Women's Preventive Services Initiative can be found here: http://www.womenspreventivehealth.org/wp-content/uploads/2017/01/WPSI_2016AbridgedReport.pdf.
- Evidence-based summaries and appendices are available at this link: http://www.womenspreventivehealth.org/wp-content/uploads/2016/12/Evidence-Summaries-and-Appendices.pdf.
I plan on using these recommendations to guide my practice
Historically, many high-profile expert professional groups have developed their own women’s health services guidelines. The proliferation of conflicting guidelines confused both patients and clinicians. Dueling guidelines likely undermine public health because they result in confusion among patients and inconsistent care across the many disciplines that provide medical services to women.
The proliferation of conflicting guidelines for mammography screening for breast cancer is a good example of how dueling guidelines can undermine public health (TABLE).4−7 The WPSI has done a great service to women and clinicians by creating a shared framework for consistently providing critical services across a woman’s entire life. I plan on using these recommendations to guide my practice. Patients and clinicians will greatly benefit from the exceptionally thoughtful women’s preventive services guidelines provided by the WPSI.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Institute of Medicine. Clinical preventive services for women: closing the gaps. Washington DC: The National Academies Press; 2011. http://nap.edu/13181. Accessed January 16, 2017.
- American Congress of Obstetricians and Gynecologists (ACOG). Women's Preventive Services Initiative (WPSI). http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Womens-Preventive-Services-Initiative. Accessed January 16, 2017.
- Health Resources and Services Administration website. Women's preventive services guidelines. https://www.hrsa.gov/womensguidelines/. Accessed January 16, 2017.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 122: breast cancer screening. Obstet Gynecol. 2011;118(2 pt 1):372-382.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599-1614.
- American Academy of Family Physicians website. Clinical preventive service recommendation: breast cancer. http://www.aafp.org/patient-care/clinical-recommendations/all/breast-cancer.html. Accessed January 16, 2017.
- US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716-726.
- Institute of Medicine. Clinical preventive services for women: closing the gaps. Washington DC: The National Academies Press; 2011. http://nap.edu/13181. Accessed January 16, 2017.
- American Congress of Obstetricians and Gynecologists (ACOG). Women's Preventive Services Initiative (WPSI). http://www.acog.org/About-ACOG/ACOG-Departments/Annual-Womens-Health-Care/Womens-Preventive-Services-Initiative. Accessed January 16, 2017.
- Health Resources and Services Administration website. Women's preventive services guidelines. https://www.hrsa.gov/womensguidelines/. Accessed January 16, 2017.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 122: breast cancer screening. Obstet Gynecol. 2011;118(2 pt 1):372-382.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599-1614.
- American Academy of Family Physicians website. Clinical preventive service recommendation: breast cancer. http://www.aafp.org/patient-care/clinical-recommendations/all/breast-cancer.html. Accessed January 16, 2017.
- US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716-726.
Letters to the Editor: Should we change instruments and gloves after closing the uterus?
“PREVENTING INFECTION AFTER CESAREAN DELIVERY: 5 MORE EVIDENCE-BASED MEASURES TO CONSIDER”
KATHRYN E. PATRICK, MD; SARA L. DEATSMAN, MD; AND PATRICK DUFF, MD (DECEMEBER 2016)
Should we change instruments and gloves after closing the uterus?
In reference to the recent article series on preventing infection after cesarean delivery by Drs. Patrick, Deatsman, and Duff, what are the thoughts on using clean instruments and changing gloves after closing the uterus?
Gerrit J. Schipper, MD
Frederick, Maryland
❯❯ Drs. Patrick, Deatsman, and Duff respond:
We appreciate Dr. Schipper’s thoughtful question concerning our recent articles. At present, we are not aware of any rigorous studies that have evaluated the possible protective effect of changing to a different set of surgical instruments after closure of the uterus.
The second part of the question concerning the effect of changing gloves at a certain point in the operation is more intriguing. In an earlier report from our institution, we showed that the dominant hand of the operator becomes heavily contaminated with bacteria during the process of extracting the fetal head from the lower uterine segment.1 The contamination is particularly heavy when the patient has had an extended duration of labor in the presence of ruptured membranes. In a subsequent investigation, we showed that avoidance of manual extraction of the placenta, a process in which the now-contaminated glove of the operator is placed between the placenta and the uterine wall, significantly reduced the frequency of postcesarean endometritis even in patients who already were receiving systemic antibiotic prophylaxis.2 Whether changing gloves after delivery of the baby will further decrease the frequency of postcesarean endometritis, beyond that which can be achieved with systemic antibiotic prophylaxis combined with delivery of the placenta by traction on the cord, has not been studied in a systematic manner.
Given the low frequency of infection that can be achieved with these 2 methods, it would require a very large sample size to show that glove change offered an additional protective effect. Nevertheless, on a practical basis, we think it is very reasonable to change the glove on the dominant hand following a difficult extraction of the presenting part in a patient who has had an extended duration of labor and ruptured membranes. The glove change is particularly important if manual extraction of the placenta is contemplated.
Of note, we would like to acknowledge that the US Food and Drug Administration finalized a ban on the use of powdered surgical gloves effective January 18, 2017.3 The aerosolized glove powder on latex gloves contains proteins that can provoke severe respiratory allergic reactions in patients who are sensitive to latex. Even powdered synthetic gloves can cause airway inflammation, wound inflammation, and postoperative adhesions.
“DOES ONE PARTICULAR CESAREAN TECHNIQUE CONFER BETTER MATERNAL AND NEONATAL OUTCOMES?”
JOHN M. THORP JR, MD (EXAMINING THE EVIDENCE; NOVEMBER 2016)
Choosing a cesarean technique based on “evidence”
I appreciate the commentary by Dr. Thorp concerning cesarean delivery techniques. I have always thought that there was no difference in the outcomes of the various techniques. However, we will continue to waver to the peer pressure of this evidence-based stuff—until we find out later, like now—until things change again. “The more things change, the more they remain the same.”
Dr. Smart Ebinne
Port Harcourt, Nigeria
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Yancey MK, Clark P, Duff P. The frequency of glove contamination during cesarean delivery. Obstet Gynecol. 1994;83(4):538–542.
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176(6):1250–1254.
- US Food and Drug Administration. Banned devices; powdered surgeon’s gloves, powdered patient examination gloves, and absorbable powder for lubricating a surgeon’s glove. Final rule. Fed Regist. 2016;81(243):91722–91731.
“PREVENTING INFECTION AFTER CESAREAN DELIVERY: 5 MORE EVIDENCE-BASED MEASURES TO CONSIDER”
KATHRYN E. PATRICK, MD; SARA L. DEATSMAN, MD; AND PATRICK DUFF, MD (DECEMEBER 2016)
Should we change instruments and gloves after closing the uterus?
In reference to the recent article series on preventing infection after cesarean delivery by Drs. Patrick, Deatsman, and Duff, what are the thoughts on using clean instruments and changing gloves after closing the uterus?
Gerrit J. Schipper, MD
Frederick, Maryland
❯❯ Drs. Patrick, Deatsman, and Duff respond:
We appreciate Dr. Schipper’s thoughtful question concerning our recent articles. At present, we are not aware of any rigorous studies that have evaluated the possible protective effect of changing to a different set of surgical instruments after closure of the uterus.
The second part of the question concerning the effect of changing gloves at a certain point in the operation is more intriguing. In an earlier report from our institution, we showed that the dominant hand of the operator becomes heavily contaminated with bacteria during the process of extracting the fetal head from the lower uterine segment.1 The contamination is particularly heavy when the patient has had an extended duration of labor in the presence of ruptured membranes. In a subsequent investigation, we showed that avoidance of manual extraction of the placenta, a process in which the now-contaminated glove of the operator is placed between the placenta and the uterine wall, significantly reduced the frequency of postcesarean endometritis even in patients who already were receiving systemic antibiotic prophylaxis.2 Whether changing gloves after delivery of the baby will further decrease the frequency of postcesarean endometritis, beyond that which can be achieved with systemic antibiotic prophylaxis combined with delivery of the placenta by traction on the cord, has not been studied in a systematic manner.
Given the low frequency of infection that can be achieved with these 2 methods, it would require a very large sample size to show that glove change offered an additional protective effect. Nevertheless, on a practical basis, we think it is very reasonable to change the glove on the dominant hand following a difficult extraction of the presenting part in a patient who has had an extended duration of labor and ruptured membranes. The glove change is particularly important if manual extraction of the placenta is contemplated.
Of note, we would like to acknowledge that the US Food and Drug Administration finalized a ban on the use of powdered surgical gloves effective January 18, 2017.3 The aerosolized glove powder on latex gloves contains proteins that can provoke severe respiratory allergic reactions in patients who are sensitive to latex. Even powdered synthetic gloves can cause airway inflammation, wound inflammation, and postoperative adhesions.
“DOES ONE PARTICULAR CESAREAN TECHNIQUE CONFER BETTER MATERNAL AND NEONATAL OUTCOMES?”
JOHN M. THORP JR, MD (EXAMINING THE EVIDENCE; NOVEMBER 2016)
Choosing a cesarean technique based on “evidence”
I appreciate the commentary by Dr. Thorp concerning cesarean delivery techniques. I have always thought that there was no difference in the outcomes of the various techniques. However, we will continue to waver to the peer pressure of this evidence-based stuff—until we find out later, like now—until things change again. “The more things change, the more they remain the same.”
Dr. Smart Ebinne
Port Harcourt, Nigeria
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“PREVENTING INFECTION AFTER CESAREAN DELIVERY: 5 MORE EVIDENCE-BASED MEASURES TO CONSIDER”
KATHRYN E. PATRICK, MD; SARA L. DEATSMAN, MD; AND PATRICK DUFF, MD (DECEMEBER 2016)
Should we change instruments and gloves after closing the uterus?
In reference to the recent article series on preventing infection after cesarean delivery by Drs. Patrick, Deatsman, and Duff, what are the thoughts on using clean instruments and changing gloves after closing the uterus?
Gerrit J. Schipper, MD
Frederick, Maryland
❯❯ Drs. Patrick, Deatsman, and Duff respond:
We appreciate Dr. Schipper’s thoughtful question concerning our recent articles. At present, we are not aware of any rigorous studies that have evaluated the possible protective effect of changing to a different set of surgical instruments after closure of the uterus.
The second part of the question concerning the effect of changing gloves at a certain point in the operation is more intriguing. In an earlier report from our institution, we showed that the dominant hand of the operator becomes heavily contaminated with bacteria during the process of extracting the fetal head from the lower uterine segment.1 The contamination is particularly heavy when the patient has had an extended duration of labor in the presence of ruptured membranes. In a subsequent investigation, we showed that avoidance of manual extraction of the placenta, a process in which the now-contaminated glove of the operator is placed between the placenta and the uterine wall, significantly reduced the frequency of postcesarean endometritis even in patients who already were receiving systemic antibiotic prophylaxis.2 Whether changing gloves after delivery of the baby will further decrease the frequency of postcesarean endometritis, beyond that which can be achieved with systemic antibiotic prophylaxis combined with delivery of the placenta by traction on the cord, has not been studied in a systematic manner.
Given the low frequency of infection that can be achieved with these 2 methods, it would require a very large sample size to show that glove change offered an additional protective effect. Nevertheless, on a practical basis, we think it is very reasonable to change the glove on the dominant hand following a difficult extraction of the presenting part in a patient who has had an extended duration of labor and ruptured membranes. The glove change is particularly important if manual extraction of the placenta is contemplated.
Of note, we would like to acknowledge that the US Food and Drug Administration finalized a ban on the use of powdered surgical gloves effective January 18, 2017.3 The aerosolized glove powder on latex gloves contains proteins that can provoke severe respiratory allergic reactions in patients who are sensitive to latex. Even powdered synthetic gloves can cause airway inflammation, wound inflammation, and postoperative adhesions.
“DOES ONE PARTICULAR CESAREAN TECHNIQUE CONFER BETTER MATERNAL AND NEONATAL OUTCOMES?”
JOHN M. THORP JR, MD (EXAMINING THE EVIDENCE; NOVEMBER 2016)
Choosing a cesarean technique based on “evidence”
I appreciate the commentary by Dr. Thorp concerning cesarean delivery techniques. I have always thought that there was no difference in the outcomes of the various techniques. However, we will continue to waver to the peer pressure of this evidence-based stuff—until we find out later, like now—until things change again. “The more things change, the more they remain the same.”
Dr. Smart Ebinne
Port Harcourt, Nigeria
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Yancey MK, Clark P, Duff P. The frequency of glove contamination during cesarean delivery. Obstet Gynecol. 1994;83(4):538–542.
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176(6):1250–1254.
- US Food and Drug Administration. Banned devices; powdered surgeon’s gloves, powdered patient examination gloves, and absorbable powder for lubricating a surgeon’s glove. Final rule. Fed Regist. 2016;81(243):91722–91731.
- Yancey MK, Clark P, Duff P. The frequency of glove contamination during cesarean delivery. Obstet Gynecol. 1994;83(4):538–542.
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176(6):1250–1254.
- US Food and Drug Administration. Banned devices; powdered surgeon’s gloves, powdered patient examination gloves, and absorbable powder for lubricating a surgeon’s glove. Final rule. Fed Regist. 2016;81(243):91722–91731.