User login
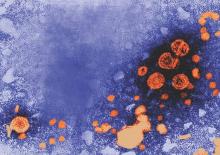
NYC launches surveillance system to detect local Zika virus transmission
The New York City Department of Health and Mental Hygiene (DOHMH) will implement a sentinel surveillance system to detect cases of local mosquito-borne transmission of Zika virus for the upcoming peak mosquito-biting season.
With the large number of people who travel frequently from New York City to active Zika virus transmission areas, DOHMH has chosen 21 primary care clinics and emergency departments as the sentinel sites across all five New York City boroughs. Once any suspected cases are reported to DOHMH, urine will be obtained for reverse transcription–polymerase chain reaction (RT-PCR) testing.
During Jan. 1.–June 17, 2016, DOHMH coordinated diagnostic laboratory testing of 3,605 patients with travel-associated exposure, including 182 (5.0%) who had the Zika virus infection. Out of the 182 patients, 20 (11.0%) were pregnant at the time of diagnosis and two cases of Zika virus–associated Guillain-Barré syndrome were diagnosed.
“Because of the known potential for Aedes mosquitoes to transmit Zika virus among humans, the anticipated large number of imported human cases into NYC, and the temporal lag between viremia and disease diagnosis in an infected patient, DOHMH is augmenting its mosquito control program, specifically source control, as well as larviciding and adult mosquito control,” Dr. Christopher T. Lee and his associates wrote in the Morbidity and Mortality Weekly Report.
Additionally, the National Institutes of Health (NIH) is partnering with the Oswaldo Cruz Foundation (known as Fiocruz) to begin a multi-country study to investigate the magnitude of health risks that the Zika virus poses to pregnant women and their developing fetuses and infants. The study will begin in Puerto Rico and expand to other locations in Brazil, Colombia, and other areas impacted by active local transmission of the virus.
“This study, in partnership with NIH, is essential to elucidating the scientific complexity of the Zika virus,” Fiocruz President Paulo Gadelha said in a statement. “It will be fundamental to developing prevention and treatment strategies against the disease.”
Read the full report on the New York City Zika experience in the Morbidity and Mortality Weekly Report (doi: 10.15585/mmwr.mm6524e3).
The New York City Department of Health and Mental Hygiene (DOHMH) will implement a sentinel surveillance system to detect cases of local mosquito-borne transmission of Zika virus for the upcoming peak mosquito-biting season.
With the large number of people who travel frequently from New York City to active Zika virus transmission areas, DOHMH has chosen 21 primary care clinics and emergency departments as the sentinel sites across all five New York City boroughs. Once any suspected cases are reported to DOHMH, urine will be obtained for reverse transcription–polymerase chain reaction (RT-PCR) testing.
During Jan. 1.–June 17, 2016, DOHMH coordinated diagnostic laboratory testing of 3,605 patients with travel-associated exposure, including 182 (5.0%) who had the Zika virus infection. Out of the 182 patients, 20 (11.0%) were pregnant at the time of diagnosis and two cases of Zika virus–associated Guillain-Barré syndrome were diagnosed.
“Because of the known potential for Aedes mosquitoes to transmit Zika virus among humans, the anticipated large number of imported human cases into NYC, and the temporal lag between viremia and disease diagnosis in an infected patient, DOHMH is augmenting its mosquito control program, specifically source control, as well as larviciding and adult mosquito control,” Dr. Christopher T. Lee and his associates wrote in the Morbidity and Mortality Weekly Report.
Additionally, the National Institutes of Health (NIH) is partnering with the Oswaldo Cruz Foundation (known as Fiocruz) to begin a multi-country study to investigate the magnitude of health risks that the Zika virus poses to pregnant women and their developing fetuses and infants. The study will begin in Puerto Rico and expand to other locations in Brazil, Colombia, and other areas impacted by active local transmission of the virus.
“This study, in partnership with NIH, is essential to elucidating the scientific complexity of the Zika virus,” Fiocruz President Paulo Gadelha said in a statement. “It will be fundamental to developing prevention and treatment strategies against the disease.”
Read the full report on the New York City Zika experience in the Morbidity and Mortality Weekly Report (doi: 10.15585/mmwr.mm6524e3).
The New York City Department of Health and Mental Hygiene (DOHMH) will implement a sentinel surveillance system to detect cases of local mosquito-borne transmission of Zika virus for the upcoming peak mosquito-biting season.
With the large number of people who travel frequently from New York City to active Zika virus transmission areas, DOHMH has chosen 21 primary care clinics and emergency departments as the sentinel sites across all five New York City boroughs. Once any suspected cases are reported to DOHMH, urine will be obtained for reverse transcription–polymerase chain reaction (RT-PCR) testing.
During Jan. 1.–June 17, 2016, DOHMH coordinated diagnostic laboratory testing of 3,605 patients with travel-associated exposure, including 182 (5.0%) who had the Zika virus infection. Out of the 182 patients, 20 (11.0%) were pregnant at the time of diagnosis and two cases of Zika virus–associated Guillain-Barré syndrome were diagnosed.
“Because of the known potential for Aedes mosquitoes to transmit Zika virus among humans, the anticipated large number of imported human cases into NYC, and the temporal lag between viremia and disease diagnosis in an infected patient, DOHMH is augmenting its mosquito control program, specifically source control, as well as larviciding and adult mosquito control,” Dr. Christopher T. Lee and his associates wrote in the Morbidity and Mortality Weekly Report.
Additionally, the National Institutes of Health (NIH) is partnering with the Oswaldo Cruz Foundation (known as Fiocruz) to begin a multi-country study to investigate the magnitude of health risks that the Zika virus poses to pregnant women and their developing fetuses and infants. The study will begin in Puerto Rico and expand to other locations in Brazil, Colombia, and other areas impacted by active local transmission of the virus.
“This study, in partnership with NIH, is essential to elucidating the scientific complexity of the Zika virus,” Fiocruz President Paulo Gadelha said in a statement. “It will be fundamental to developing prevention and treatment strategies against the disease.”
Read the full report on the New York City Zika experience in the Morbidity and Mortality Weekly Report (doi: 10.15585/mmwr.mm6524e3).
FROM MMWR
Zika virus: The path to fetal infection
The question of how viruses can enter the intrauterine compartment and infect the fetus has long been a focus of research. It is of particular urgency today as the Zika virus spreads and causes perinatal infection that threatens the developing fetus with serious adverse outcomes such microcephaly and other brain anomalies, placental insufficiency, and fetal growth restriction.
We know that viruses can take a variety of routes to the fetal compartment, but we have also learned that the placenta has a robust level of inherent resistance to viruses. This resistance likely explains why we don’t see more viral infections in pregnancy.
Recent studies performed at our institution suggest that placental trophoblasts – the placenta’s primary line of defense – have inherent resistance to viruses such as Zika. It appears, therefore, that the Zika virus invades the intrauterine cavity by crossing the trophoblasts, perhaps earlier in pregnancy and prior to the development of full trophoblast resistance, by entering through breaks in this outer layer, or by utilizing alternative pathways to access the fetal compartment.
Further study of the placenta and its various cell types and mechanisms of viral defense will be critical for designing therapeutic strategies for preventing perinatal infections.
Various routes and affinities
Viruses have long been known to affect mothers and their unborn children. The rubella virus, for instance, posed a significant threat to the fetus until a vaccine program was introduced almost 50 years ago. Cytomegalovirus (CMV), on the other hand, continues be passed from mothers to their unborn children. While not as threatening as rubella once was, it can in some cases cause severe defects.
One might expect viruses to infect the placenta and then secondarily infect the fetus. While this may indeed occur, direct placental infection is not the only route by which viruses may enter the intrauterine compartment. Some viruses may be carried by macrophages or other immune cells through the placenta and into the fetal compartment, while others colonize the uterine cavity prior to conception, ready to proliferate during pregnancy.
In still other cases, viruses may be inadvertently introduced during medical procedures such as amniocentesis or transmitted through transvaginal ascending infection, most likely after rupture of the membranes. Viruses may also be transported through infected sperm (this appears to be one of the Zika virus’s modes of transportation), and as is the case with HIV and herpes simplex viruses, transmission sometimes occurs during delivery.
When we investigate whether or not the fetus is protected against particular viruses, we must therefore think about the multifaceted mechanisms by which viruses may be transmitted. With respect to the placenta specifically, we seek to understand how viruses enter the placenta, and how the placenta resists the propagation of some viruses while allowing other viruses to gain entry to the intrauterine compartment.
An additional consideration – one that is of utmost importance in the case of Zika – is whether viruses have any special affinity for particular fetal tissues. Some viruses, like CMV, infect multiple types of fetal tissue. The Zika virus, on the other hand, appears to target neuronal tissue in the fetus. In May, investigators of two studies reported that a strain of the Zika virus efficiently infected human cortical neural progenitor cells (Cell Stem Cell. 2016 May 5;18[5]:587-90), and that Zika infection of mice early in pregnancy resulted in infection of the placenta and of the fetal brain (Cell. 2016 May 19;165[5]:1081-91).
Interestingly, other flaviviruses such as the dengue and chikungunya viruses have not been associated with microcephaly or other congenital disorders. This suggests that the Zika virus employs unique mechanisms to infect or bypass the placental barrier and, in turn, to cause neuronal-focused damage.
Placental passage
The villous trophoblasts, cells that are bathed in maternal blood, form the placenta’s first line of defense. Viruses, including the Zika virus, must cross or somehow bypass this initial barrier before crossing the placental basement membrane and endothelial cells, if they are to potentially invade the intrauterine cavity and infect the fetal brain and other tissues.
Research has demonstrated that cells of various types of tissue may express certain proteins, such as AXL, MER and TYRO3. While not yet proven, these proteins may mediate the entry of viruses such as Zika, enabling them to cross the placental trophoblast layer. These proteins are indeed expressed in trophoblasts, especially in early pregnancy, but we do not yet know if the proteins actually aid Zika’s passage through the placenta.
Another mechanism that has been postulated in the case of Zika infection is antibody-dependent enhancement, a process by which a current infection is enhanced by prior infection with another virus from the same family. Some experts believe that pre-existing immunity to the dengue virus – another member of the flavivirus family that has been endemic in Brazil – may be enhancing the spread of Zika infection as antibodies against dengue cross-react with the Zika virus.
While antibody-dependent enhancement has been shown to occur and to advance infection in various body systems, it has not been proven to affect the placenta. Until we learn more, we must simply appreciate that the presence of antibodies from another member of a family of viruses does not necessarily confer resistance. Instead, it may enable new infections to advance.
One might view pregnancy as a time of immune compromise, but we have shown in our laboratories that trophoblasts in fact have inherent resistance to a number of viruses. In a recent study, we found that trophoblasts are refractory to direct infection with the Zika virus. We isolated trophoblast cells from healthy full-term human placentas, cultured these cells for several days, and infected them with the Zika virus. We then measured viral replication and compared the infectivity of these cells with the infectivity of human brain microvascular endothelial cells – nontrophoblast cells that served as a control.
Our findings were extremely interesting to us: The trophoblast cells appeared to be significantly more resistant to the Zika virus than the nontrophoblast cells.
We learned, moreover, that this resistance was mediated by a particular interferon released by the trophoblast cells – type III interferon IFN1 – and that this type III interferon appeared to protect not only the trophoblasts but the nontrophoblast cells as well. It acted in both an autocrine and a paracrine manner to protect cells from the Zika virus. When we blocked the antiviral signaling of this interferon, resistance to the virus was attenuated.
These findings suggest that while Zika appears able to cross through the placenta and infect the fetus, the mechanism does not involve direct infection of the trophoblasts, at least in the later stages of pregnancy. The virus must either evade the type III interferon antiviral signals generated by the trophoblasts or somehow bypass these cells to cross the placenta (Cell Host Microbe. 2016 May 11;19[5]:705-12).
Interestingly, the Cell study mentioned above, in which Zika infection of mice early in pregnancy infected placental cells and the brain, also showed reduced Zika presence in the mouse mononuclear trophoblasts and syncytiotrophoblasts, in areas of the placenta analogous to the human villi.
Some experts have suggested, based the study of other viruses, that the Zika virus is better able to infect the placenta when the infection occurs early in the first trimester or the second trimester. It is indeed possible – and makes intuitive sense – that first-trimester trophoblasts confer less resistance and a lower level of protection than the mature trophoblasts we studied. At this point, however, we cannot say with certainty whether or not the placenta is more or less permissive to Zika infection at different points in pregnancy.
Interestingly, investigators who prospectively followed a small cohort of pregnant women in Brazil with suspected Zika infection identified abnormalities in fetuses of women who were infected at various points of their pregnancies, even in the third trimester. Fetuses infected in the first trimester had findings suggestive of pathologic change during embryogenesis, but central nervous system abnormalities were seen in fetuses infected as late as 27 weeks of gestation, the investigators said (N Engl J Med. 2016 Mar 4. doi: 10.1056/NEJMoa1602412).
The interferon-conferred resistance demonstrated in our recent study is one of two mechanisms we’ve identified by which placental trophoblasts orchestrate resistance to viral infection. In earlier research, we found that resistance can be conferred to nontrophoblast cells by the delivery of micro-RNAs. These micro-RNAs (C19MC miRNAs) are uniquely expressed in the placenta and packaged within trophoblast-derived nanovesicles called exosomes. The nanovesicles can latch onto other cells in the vicinity of the trophoblasts, attenuating viral replication in these recipient cells.
This earlier in-vitro study involved a panel of diverse and unrelated viruses, including coxsackievirus B3, poliovirus, vesicular stomatitis virus, and human cytomegalovirus (Proc Natl Acad Sci U S A. 2013 Jul 16;110[29]:12048-53). It did not include the Zika virus, but our ongoing preliminary research suggests that the same mechanisms might be active against Zika.
Furthering research
Research at our institution and in other laboratories has shed light on various ways in which the fetus is protected from viruses, but we must learn more in order to understand how particular viruses, such as Zika, are able to reach the fetal compartment and cause particular birth defects.
We must further investigate the role and importance of antibody-dependent enhancement, and we must continue to study the placenta and its various cell types. Continuing efforts to better elucidate the placenta’s defense mechanisms and to identify cell types that are more or less resistant to the Zika virus – and understand their differences – may lead us to potential therapeutic strategies.
Dr. Sadovsky is scientific director of the Magee-Womens Research Institute, Elsie Hilliard Hillman Chair of Women’s Health Research, and professor of ob.gyn., reproductive sciences, microbiology, and molecular genetics at the University of Pittsburgh. Dr. Coyne is associate professor of microbiology and molecular genetics, and ob.gyn. and reproductive sciences, at the University of Pittsburgh.* Their research addressed in this Master Class was supported by grants from the National Institutes of Health, State of Pennsylvania Formula Research Funds, and Burroughs Wellcome Fund.
*Correction, 7/05/2016: An earlier version of this article misstated Dr. Coyne's academic title.
The question of how viruses can enter the intrauterine compartment and infect the fetus has long been a focus of research. It is of particular urgency today as the Zika virus spreads and causes perinatal infection that threatens the developing fetus with serious adverse outcomes such microcephaly and other brain anomalies, placental insufficiency, and fetal growth restriction.
We know that viruses can take a variety of routes to the fetal compartment, but we have also learned that the placenta has a robust level of inherent resistance to viruses. This resistance likely explains why we don’t see more viral infections in pregnancy.
Recent studies performed at our institution suggest that placental trophoblasts – the placenta’s primary line of defense – have inherent resistance to viruses such as Zika. It appears, therefore, that the Zika virus invades the intrauterine cavity by crossing the trophoblasts, perhaps earlier in pregnancy and prior to the development of full trophoblast resistance, by entering through breaks in this outer layer, or by utilizing alternative pathways to access the fetal compartment.
Further study of the placenta and its various cell types and mechanisms of viral defense will be critical for designing therapeutic strategies for preventing perinatal infections.
Various routes and affinities
Viruses have long been known to affect mothers and their unborn children. The rubella virus, for instance, posed a significant threat to the fetus until a vaccine program was introduced almost 50 years ago. Cytomegalovirus (CMV), on the other hand, continues be passed from mothers to their unborn children. While not as threatening as rubella once was, it can in some cases cause severe defects.
One might expect viruses to infect the placenta and then secondarily infect the fetus. While this may indeed occur, direct placental infection is not the only route by which viruses may enter the intrauterine compartment. Some viruses may be carried by macrophages or other immune cells through the placenta and into the fetal compartment, while others colonize the uterine cavity prior to conception, ready to proliferate during pregnancy.
In still other cases, viruses may be inadvertently introduced during medical procedures such as amniocentesis or transmitted through transvaginal ascending infection, most likely after rupture of the membranes. Viruses may also be transported through infected sperm (this appears to be one of the Zika virus’s modes of transportation), and as is the case with HIV and herpes simplex viruses, transmission sometimes occurs during delivery.
When we investigate whether or not the fetus is protected against particular viruses, we must therefore think about the multifaceted mechanisms by which viruses may be transmitted. With respect to the placenta specifically, we seek to understand how viruses enter the placenta, and how the placenta resists the propagation of some viruses while allowing other viruses to gain entry to the intrauterine compartment.
An additional consideration – one that is of utmost importance in the case of Zika – is whether viruses have any special affinity for particular fetal tissues. Some viruses, like CMV, infect multiple types of fetal tissue. The Zika virus, on the other hand, appears to target neuronal tissue in the fetus. In May, investigators of two studies reported that a strain of the Zika virus efficiently infected human cortical neural progenitor cells (Cell Stem Cell. 2016 May 5;18[5]:587-90), and that Zika infection of mice early in pregnancy resulted in infection of the placenta and of the fetal brain (Cell. 2016 May 19;165[5]:1081-91).
Interestingly, other flaviviruses such as the dengue and chikungunya viruses have not been associated with microcephaly or other congenital disorders. This suggests that the Zika virus employs unique mechanisms to infect or bypass the placental barrier and, in turn, to cause neuronal-focused damage.
Placental passage
The villous trophoblasts, cells that are bathed in maternal blood, form the placenta’s first line of defense. Viruses, including the Zika virus, must cross or somehow bypass this initial barrier before crossing the placental basement membrane and endothelial cells, if they are to potentially invade the intrauterine cavity and infect the fetal brain and other tissues.
Research has demonstrated that cells of various types of tissue may express certain proteins, such as AXL, MER and TYRO3. While not yet proven, these proteins may mediate the entry of viruses such as Zika, enabling them to cross the placental trophoblast layer. These proteins are indeed expressed in trophoblasts, especially in early pregnancy, but we do not yet know if the proteins actually aid Zika’s passage through the placenta.
Another mechanism that has been postulated in the case of Zika infection is antibody-dependent enhancement, a process by which a current infection is enhanced by prior infection with another virus from the same family. Some experts believe that pre-existing immunity to the dengue virus – another member of the flavivirus family that has been endemic in Brazil – may be enhancing the spread of Zika infection as antibodies against dengue cross-react with the Zika virus.
While antibody-dependent enhancement has been shown to occur and to advance infection in various body systems, it has not been proven to affect the placenta. Until we learn more, we must simply appreciate that the presence of antibodies from another member of a family of viruses does not necessarily confer resistance. Instead, it may enable new infections to advance.
One might view pregnancy as a time of immune compromise, but we have shown in our laboratories that trophoblasts in fact have inherent resistance to a number of viruses. In a recent study, we found that trophoblasts are refractory to direct infection with the Zika virus. We isolated trophoblast cells from healthy full-term human placentas, cultured these cells for several days, and infected them with the Zika virus. We then measured viral replication and compared the infectivity of these cells with the infectivity of human brain microvascular endothelial cells – nontrophoblast cells that served as a control.
Our findings were extremely interesting to us: The trophoblast cells appeared to be significantly more resistant to the Zika virus than the nontrophoblast cells.
We learned, moreover, that this resistance was mediated by a particular interferon released by the trophoblast cells – type III interferon IFN1 – and that this type III interferon appeared to protect not only the trophoblasts but the nontrophoblast cells as well. It acted in both an autocrine and a paracrine manner to protect cells from the Zika virus. When we blocked the antiviral signaling of this interferon, resistance to the virus was attenuated.
These findings suggest that while Zika appears able to cross through the placenta and infect the fetus, the mechanism does not involve direct infection of the trophoblasts, at least in the later stages of pregnancy. The virus must either evade the type III interferon antiviral signals generated by the trophoblasts or somehow bypass these cells to cross the placenta (Cell Host Microbe. 2016 May 11;19[5]:705-12).
Interestingly, the Cell study mentioned above, in which Zika infection of mice early in pregnancy infected placental cells and the brain, also showed reduced Zika presence in the mouse mononuclear trophoblasts and syncytiotrophoblasts, in areas of the placenta analogous to the human villi.
Some experts have suggested, based the study of other viruses, that the Zika virus is better able to infect the placenta when the infection occurs early in the first trimester or the second trimester. It is indeed possible – and makes intuitive sense – that first-trimester trophoblasts confer less resistance and a lower level of protection than the mature trophoblasts we studied. At this point, however, we cannot say with certainty whether or not the placenta is more or less permissive to Zika infection at different points in pregnancy.
Interestingly, investigators who prospectively followed a small cohort of pregnant women in Brazil with suspected Zika infection identified abnormalities in fetuses of women who were infected at various points of their pregnancies, even in the third trimester. Fetuses infected in the first trimester had findings suggestive of pathologic change during embryogenesis, but central nervous system abnormalities were seen in fetuses infected as late as 27 weeks of gestation, the investigators said (N Engl J Med. 2016 Mar 4. doi: 10.1056/NEJMoa1602412).
The interferon-conferred resistance demonstrated in our recent study is one of two mechanisms we’ve identified by which placental trophoblasts orchestrate resistance to viral infection. In earlier research, we found that resistance can be conferred to nontrophoblast cells by the delivery of micro-RNAs. These micro-RNAs (C19MC miRNAs) are uniquely expressed in the placenta and packaged within trophoblast-derived nanovesicles called exosomes. The nanovesicles can latch onto other cells in the vicinity of the trophoblasts, attenuating viral replication in these recipient cells.
This earlier in-vitro study involved a panel of diverse and unrelated viruses, including coxsackievirus B3, poliovirus, vesicular stomatitis virus, and human cytomegalovirus (Proc Natl Acad Sci U S A. 2013 Jul 16;110[29]:12048-53). It did not include the Zika virus, but our ongoing preliminary research suggests that the same mechanisms might be active against Zika.
Furthering research
Research at our institution and in other laboratories has shed light on various ways in which the fetus is protected from viruses, but we must learn more in order to understand how particular viruses, such as Zika, are able to reach the fetal compartment and cause particular birth defects.
We must further investigate the role and importance of antibody-dependent enhancement, and we must continue to study the placenta and its various cell types. Continuing efforts to better elucidate the placenta’s defense mechanisms and to identify cell types that are more or less resistant to the Zika virus – and understand their differences – may lead us to potential therapeutic strategies.
Dr. Sadovsky is scientific director of the Magee-Womens Research Institute, Elsie Hilliard Hillman Chair of Women’s Health Research, and professor of ob.gyn., reproductive sciences, microbiology, and molecular genetics at the University of Pittsburgh. Dr. Coyne is associate professor of microbiology and molecular genetics, and ob.gyn. and reproductive sciences, at the University of Pittsburgh.* Their research addressed in this Master Class was supported by grants from the National Institutes of Health, State of Pennsylvania Formula Research Funds, and Burroughs Wellcome Fund.
*Correction, 7/05/2016: An earlier version of this article misstated Dr. Coyne's academic title.
The question of how viruses can enter the intrauterine compartment and infect the fetus has long been a focus of research. It is of particular urgency today as the Zika virus spreads and causes perinatal infection that threatens the developing fetus with serious adverse outcomes such microcephaly and other brain anomalies, placental insufficiency, and fetal growth restriction.
We know that viruses can take a variety of routes to the fetal compartment, but we have also learned that the placenta has a robust level of inherent resistance to viruses. This resistance likely explains why we don’t see more viral infections in pregnancy.
Recent studies performed at our institution suggest that placental trophoblasts – the placenta’s primary line of defense – have inherent resistance to viruses such as Zika. It appears, therefore, that the Zika virus invades the intrauterine cavity by crossing the trophoblasts, perhaps earlier in pregnancy and prior to the development of full trophoblast resistance, by entering through breaks in this outer layer, or by utilizing alternative pathways to access the fetal compartment.
Further study of the placenta and its various cell types and mechanisms of viral defense will be critical for designing therapeutic strategies for preventing perinatal infections.
Various routes and affinities
Viruses have long been known to affect mothers and their unborn children. The rubella virus, for instance, posed a significant threat to the fetus until a vaccine program was introduced almost 50 years ago. Cytomegalovirus (CMV), on the other hand, continues be passed from mothers to their unborn children. While not as threatening as rubella once was, it can in some cases cause severe defects.
One might expect viruses to infect the placenta and then secondarily infect the fetus. While this may indeed occur, direct placental infection is not the only route by which viruses may enter the intrauterine compartment. Some viruses may be carried by macrophages or other immune cells through the placenta and into the fetal compartment, while others colonize the uterine cavity prior to conception, ready to proliferate during pregnancy.
In still other cases, viruses may be inadvertently introduced during medical procedures such as amniocentesis or transmitted through transvaginal ascending infection, most likely after rupture of the membranes. Viruses may also be transported through infected sperm (this appears to be one of the Zika virus’s modes of transportation), and as is the case with HIV and herpes simplex viruses, transmission sometimes occurs during delivery.
When we investigate whether or not the fetus is protected against particular viruses, we must therefore think about the multifaceted mechanisms by which viruses may be transmitted. With respect to the placenta specifically, we seek to understand how viruses enter the placenta, and how the placenta resists the propagation of some viruses while allowing other viruses to gain entry to the intrauterine compartment.
An additional consideration – one that is of utmost importance in the case of Zika – is whether viruses have any special affinity for particular fetal tissues. Some viruses, like CMV, infect multiple types of fetal tissue. The Zika virus, on the other hand, appears to target neuronal tissue in the fetus. In May, investigators of two studies reported that a strain of the Zika virus efficiently infected human cortical neural progenitor cells (Cell Stem Cell. 2016 May 5;18[5]:587-90), and that Zika infection of mice early in pregnancy resulted in infection of the placenta and of the fetal brain (Cell. 2016 May 19;165[5]:1081-91).
Interestingly, other flaviviruses such as the dengue and chikungunya viruses have not been associated with microcephaly or other congenital disorders. This suggests that the Zika virus employs unique mechanisms to infect or bypass the placental barrier and, in turn, to cause neuronal-focused damage.
Placental passage
The villous trophoblasts, cells that are bathed in maternal blood, form the placenta’s first line of defense. Viruses, including the Zika virus, must cross or somehow bypass this initial barrier before crossing the placental basement membrane and endothelial cells, if they are to potentially invade the intrauterine cavity and infect the fetal brain and other tissues.
Research has demonstrated that cells of various types of tissue may express certain proteins, such as AXL, MER and TYRO3. While not yet proven, these proteins may mediate the entry of viruses such as Zika, enabling them to cross the placental trophoblast layer. These proteins are indeed expressed in trophoblasts, especially in early pregnancy, but we do not yet know if the proteins actually aid Zika’s passage through the placenta.
Another mechanism that has been postulated in the case of Zika infection is antibody-dependent enhancement, a process by which a current infection is enhanced by prior infection with another virus from the same family. Some experts believe that pre-existing immunity to the dengue virus – another member of the flavivirus family that has been endemic in Brazil – may be enhancing the spread of Zika infection as antibodies against dengue cross-react with the Zika virus.
While antibody-dependent enhancement has been shown to occur and to advance infection in various body systems, it has not been proven to affect the placenta. Until we learn more, we must simply appreciate that the presence of antibodies from another member of a family of viruses does not necessarily confer resistance. Instead, it may enable new infections to advance.
One might view pregnancy as a time of immune compromise, but we have shown in our laboratories that trophoblasts in fact have inherent resistance to a number of viruses. In a recent study, we found that trophoblasts are refractory to direct infection with the Zika virus. We isolated trophoblast cells from healthy full-term human placentas, cultured these cells for several days, and infected them with the Zika virus. We then measured viral replication and compared the infectivity of these cells with the infectivity of human brain microvascular endothelial cells – nontrophoblast cells that served as a control.
Our findings were extremely interesting to us: The trophoblast cells appeared to be significantly more resistant to the Zika virus than the nontrophoblast cells.
We learned, moreover, that this resistance was mediated by a particular interferon released by the trophoblast cells – type III interferon IFN1 – and that this type III interferon appeared to protect not only the trophoblasts but the nontrophoblast cells as well. It acted in both an autocrine and a paracrine manner to protect cells from the Zika virus. When we blocked the antiviral signaling of this interferon, resistance to the virus was attenuated.
These findings suggest that while Zika appears able to cross through the placenta and infect the fetus, the mechanism does not involve direct infection of the trophoblasts, at least in the later stages of pregnancy. The virus must either evade the type III interferon antiviral signals generated by the trophoblasts or somehow bypass these cells to cross the placenta (Cell Host Microbe. 2016 May 11;19[5]:705-12).
Interestingly, the Cell study mentioned above, in which Zika infection of mice early in pregnancy infected placental cells and the brain, also showed reduced Zika presence in the mouse mononuclear trophoblasts and syncytiotrophoblasts, in areas of the placenta analogous to the human villi.
Some experts have suggested, based the study of other viruses, that the Zika virus is better able to infect the placenta when the infection occurs early in the first trimester or the second trimester. It is indeed possible – and makes intuitive sense – that first-trimester trophoblasts confer less resistance and a lower level of protection than the mature trophoblasts we studied. At this point, however, we cannot say with certainty whether or not the placenta is more or less permissive to Zika infection at different points in pregnancy.
Interestingly, investigators who prospectively followed a small cohort of pregnant women in Brazil with suspected Zika infection identified abnormalities in fetuses of women who were infected at various points of their pregnancies, even in the third trimester. Fetuses infected in the first trimester had findings suggestive of pathologic change during embryogenesis, but central nervous system abnormalities were seen in fetuses infected as late as 27 weeks of gestation, the investigators said (N Engl J Med. 2016 Mar 4. doi: 10.1056/NEJMoa1602412).
The interferon-conferred resistance demonstrated in our recent study is one of two mechanisms we’ve identified by which placental trophoblasts orchestrate resistance to viral infection. In earlier research, we found that resistance can be conferred to nontrophoblast cells by the delivery of micro-RNAs. These micro-RNAs (C19MC miRNAs) are uniquely expressed in the placenta and packaged within trophoblast-derived nanovesicles called exosomes. The nanovesicles can latch onto other cells in the vicinity of the trophoblasts, attenuating viral replication in these recipient cells.
This earlier in-vitro study involved a panel of diverse and unrelated viruses, including coxsackievirus B3, poliovirus, vesicular stomatitis virus, and human cytomegalovirus (Proc Natl Acad Sci U S A. 2013 Jul 16;110[29]:12048-53). It did not include the Zika virus, but our ongoing preliminary research suggests that the same mechanisms might be active against Zika.
Furthering research
Research at our institution and in other laboratories has shed light on various ways in which the fetus is protected from viruses, but we must learn more in order to understand how particular viruses, such as Zika, are able to reach the fetal compartment and cause particular birth defects.
We must further investigate the role and importance of antibody-dependent enhancement, and we must continue to study the placenta and its various cell types. Continuing efforts to better elucidate the placenta’s defense mechanisms and to identify cell types that are more or less resistant to the Zika virus – and understand their differences – may lead us to potential therapeutic strategies.
Dr. Sadovsky is scientific director of the Magee-Womens Research Institute, Elsie Hilliard Hillman Chair of Women’s Health Research, and professor of ob.gyn., reproductive sciences, microbiology, and molecular genetics at the University of Pittsburgh. Dr. Coyne is associate professor of microbiology and molecular genetics, and ob.gyn. and reproductive sciences, at the University of Pittsburgh.* Their research addressed in this Master Class was supported by grants from the National Institutes of Health, State of Pennsylvania Formula Research Funds, and Burroughs Wellcome Fund.
*Correction, 7/05/2016: An earlier version of this article misstated Dr. Coyne's academic title.
Zika virus challenges ob.gyn. practice
Viral illnesses in pregnancy are not unheard of. When a patient presents with symptoms, we often think of an influenza type of infection that will be cleared within a short period of time and with few negative consequences for the developing fetus. Other infections that can occur include TORCH – Toxoplasmosis, Other (syphilis, varicella-zoster, parvovirus B19), Rubella, Cytomegalovirus (CMV), and Herpes – infections, but these are also relatively common.
Rarely do we in the United States consider a gravida’s vulnerability to tropical infectious diseases such as dengue, chikungunya, and now Zika virus. With the popularity and ease of international travel, and the potential for women’s exposure to more exotic diseases, the practice of ob.gyn. must undergo a significant transition in perspective. It is vital for us to understand these illnesses because of their potency and reported injury to both the mother and baby, for several reasons.
First, there is the public health concern. As of June 16, 2016, the Pan American Health Organization of the World Health Organization, reported 39 countries and territories in the Americas with confirmed cases of Zika virus, with 21 of those countries having confirmed cases in pregnant women.
As of June 9, 2016, the Centers for Disease Control and Prevention reported that 234 pregnant women in the United States have laboratory evidence of possible Zika infection, along with 189 pregnant women living in U.S. territories. Since the current outbreak, which began in July 2015 in Brazil, seven countries – accounting for more than 1,600 cases – have reported babies with congenital syndrome associated with Zika virus, the majority of which have been in Brazil. With the Summer Olympics in Rio starting in August 2016, the potential spread of Zika virus is dizzying.
Second, there is the counseling and management concern. Without a treatment or vaccine available, ob.gyns. must stay current on the latest research and findings to inform their patients of the risks associated with travel to an area with confirmed, or areas at risk for developing, Zika virus transmission.
Third, there is a diagnostic concern. Women who have visited areas with Zika virus, or who have had intimate contact with someone who has traveled to these areas, must be diagnosed and then counseled immediately.
We have devoted this Master Class to a discussion of Zika virus and the work being conducted in the United States to understand this disease. We have invited Dr. Yoel Sadovsky, an expert on placental development and trophoblast function, and his colleague, Carolyn Coyne, Ph.D., a leading researcher on host-virus interactions, to address this important topic.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Viral illnesses in pregnancy are not unheard of. When a patient presents with symptoms, we often think of an influenza type of infection that will be cleared within a short period of time and with few negative consequences for the developing fetus. Other infections that can occur include TORCH – Toxoplasmosis, Other (syphilis, varicella-zoster, parvovirus B19), Rubella, Cytomegalovirus (CMV), and Herpes – infections, but these are also relatively common.
Rarely do we in the United States consider a gravida’s vulnerability to tropical infectious diseases such as dengue, chikungunya, and now Zika virus. With the popularity and ease of international travel, and the potential for women’s exposure to more exotic diseases, the practice of ob.gyn. must undergo a significant transition in perspective. It is vital for us to understand these illnesses because of their potency and reported injury to both the mother and baby, for several reasons.
First, there is the public health concern. As of June 16, 2016, the Pan American Health Organization of the World Health Organization, reported 39 countries and territories in the Americas with confirmed cases of Zika virus, with 21 of those countries having confirmed cases in pregnant women.
As of June 9, 2016, the Centers for Disease Control and Prevention reported that 234 pregnant women in the United States have laboratory evidence of possible Zika infection, along with 189 pregnant women living in U.S. territories. Since the current outbreak, which began in July 2015 in Brazil, seven countries – accounting for more than 1,600 cases – have reported babies with congenital syndrome associated with Zika virus, the majority of which have been in Brazil. With the Summer Olympics in Rio starting in August 2016, the potential spread of Zika virus is dizzying.
Second, there is the counseling and management concern. Without a treatment or vaccine available, ob.gyns. must stay current on the latest research and findings to inform their patients of the risks associated with travel to an area with confirmed, or areas at risk for developing, Zika virus transmission.
Third, there is a diagnostic concern. Women who have visited areas with Zika virus, or who have had intimate contact with someone who has traveled to these areas, must be diagnosed and then counseled immediately.
We have devoted this Master Class to a discussion of Zika virus and the work being conducted in the United States to understand this disease. We have invited Dr. Yoel Sadovsky, an expert on placental development and trophoblast function, and his colleague, Carolyn Coyne, Ph.D., a leading researcher on host-virus interactions, to address this important topic.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Viral illnesses in pregnancy are not unheard of. When a patient presents with symptoms, we often think of an influenza type of infection that will be cleared within a short period of time and with few negative consequences for the developing fetus. Other infections that can occur include TORCH – Toxoplasmosis, Other (syphilis, varicella-zoster, parvovirus B19), Rubella, Cytomegalovirus (CMV), and Herpes – infections, but these are also relatively common.
Rarely do we in the United States consider a gravida’s vulnerability to tropical infectious diseases such as dengue, chikungunya, and now Zika virus. With the popularity and ease of international travel, and the potential for women’s exposure to more exotic diseases, the practice of ob.gyn. must undergo a significant transition in perspective. It is vital for us to understand these illnesses because of their potency and reported injury to both the mother and baby, for several reasons.
First, there is the public health concern. As of June 16, 2016, the Pan American Health Organization of the World Health Organization, reported 39 countries and territories in the Americas with confirmed cases of Zika virus, with 21 of those countries having confirmed cases in pregnant women.
As of June 9, 2016, the Centers for Disease Control and Prevention reported that 234 pregnant women in the United States have laboratory evidence of possible Zika infection, along with 189 pregnant women living in U.S. territories. Since the current outbreak, which began in July 2015 in Brazil, seven countries – accounting for more than 1,600 cases – have reported babies with congenital syndrome associated with Zika virus, the majority of which have been in Brazil. With the Summer Olympics in Rio starting in August 2016, the potential spread of Zika virus is dizzying.
Second, there is the counseling and management concern. Without a treatment or vaccine available, ob.gyns. must stay current on the latest research and findings to inform their patients of the risks associated with travel to an area with confirmed, or areas at risk for developing, Zika virus transmission.
Third, there is a diagnostic concern. Women who have visited areas with Zika virus, or who have had intimate contact with someone who has traveled to these areas, must be diagnosed and then counseled immediately.
We have devoted this Master Class to a discussion of Zika virus and the work being conducted in the United States to understand this disease. We have invited Dr. Yoel Sadovsky, an expert on placental development and trophoblast function, and his colleague, Carolyn Coyne, Ph.D., a leading researcher on host-virus interactions, to address this important topic.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Donor blood testing highlights increasing Zika risk in Puerto Rico
An increasing prevalence of Zika virus infection detected among blood donors in Puerto Rico using a highly sensitive, investigational nucleic acid test likely reflects an overall increase in the incidence of infection in the population at large, according to the Centers for Disease Control and Prevention.
“The implications and importance of this information are that in the coming months, it is possible that thousands of women in Puerto Rico could become infected with Zika. This could lead to dozens or hundreds of infants being born with microcephaly in the coming year,” CDC Director Dr. Tom Frieden said in a telebriefing on June 17.
“For the thousands of other infants born to women infected with Zika who don’t have microcephaly, we simply don’t know, and might not know for years, if there will be long-term consequences on brain development,” Dr. Frieden added.
The incidence of infection among blood donors in Puerto Rico the week of June 5-11 was 1.1%. This is the highest weekly incidence since testing using a newly developed nucleic acid test authorized by the Food and Drug Administration under an investigational new drug application that was implemented in Puerto Rico in April.
Between April 3 and June 11, 2016, a total of 68 presumptive viremic donors were identified from 12,777 donations tested. The incidence of positive findings has increased steadily over time (MMWR. 2016 Jun 17. doi: 10.15585/mmwr.mm6524e2).
In the wake of the finding, which was reported June 17 in an early release of the Morbidity and Mortality Weekly Report, Dr. Frieden and Dr. Matthew J. Kuehnert, director of the CDC Office of Blood, Organ, and Other Tissue Safety sought to allay concerns regarding transmission of the virus via blood transfusion.
“The test being used to test blood in Puerto Rico is extremely accurate,” Dr. Kuehnert said, noting the importance of this testing, as several mosquito-borne illnesses are know to be transmissible by blood transfusion and many infected individuals are asymptomatic.
Further, at least one case of transfusion-transmitted Zika infection has been reported in Brazil, Dr. Kuehnert said.
The blood supply in Puerto Rico is being protected by laboratory testing, and in most other areas – including the continental United States – it is being protected by deferral of people who report travel to areas with such transmission. Some centers are electing to implement blood testing, but this is not currently a requirement, he said.
All donations that test positive for Zika are removed from the blood supply, and donors who test positive are provided with information on how to prevent spreading the virus to others.
The larger concern is the increasing prevalence of infections, as the nucleic acid test results “may be the most accurate, real time, leading indicator of Zika activity in Puerto Rico,” Dr. Frieden said.
The 1% prevalence of infection suggests substantial ongoing community transmission, given that viral nucleic acid can be detected for only 7-10 days after acute infection, and it translates to a greater than 1% rate of infection each month in the community, he noted.
“Our concern here is about protecting pregnant women, and with this rate of infection the possibility that there could be thousands of pregnant women affected, leading to dozens to hundreds of affected babies, is what’s of most concern,” Dr. Frieden said, adding that efforts are underway to reduce risk.
“We’re working intensively, in addition to keeping the blood supply safe, with the Puerto Rico health department, the government, communities, and people throughout Puerto Rico, to provide services for pregnant women – DEET, long sleeves, measures in their homes to reduce mosquito exposure that might reduce their risk of getting infected, as well as to control mosquitoes,” he said.
Dr. Frieden explained that controlling the Aedes aegypti mosquito is very difficult, and that it requires the effort of the entire community working together to protect a pregnant woman.
“We can’t make the risk zero … but even if we can reduce it by 10%, 30%, or 50%, that is a significant number of tragedies that we can prevent, and we’re doing everything that we can to do that … so that we don’t look back in 3, 6, or 12 months and say we wish we had done more back in June,” he said.
An increasing prevalence of Zika virus infection detected among blood donors in Puerto Rico using a highly sensitive, investigational nucleic acid test likely reflects an overall increase in the incidence of infection in the population at large, according to the Centers for Disease Control and Prevention.
“The implications and importance of this information are that in the coming months, it is possible that thousands of women in Puerto Rico could become infected with Zika. This could lead to dozens or hundreds of infants being born with microcephaly in the coming year,” CDC Director Dr. Tom Frieden said in a telebriefing on June 17.
“For the thousands of other infants born to women infected with Zika who don’t have microcephaly, we simply don’t know, and might not know for years, if there will be long-term consequences on brain development,” Dr. Frieden added.
The incidence of infection among blood donors in Puerto Rico the week of June 5-11 was 1.1%. This is the highest weekly incidence since testing using a newly developed nucleic acid test authorized by the Food and Drug Administration under an investigational new drug application that was implemented in Puerto Rico in April.
Between April 3 and June 11, 2016, a total of 68 presumptive viremic donors were identified from 12,777 donations tested. The incidence of positive findings has increased steadily over time (MMWR. 2016 Jun 17. doi: 10.15585/mmwr.mm6524e2).
In the wake of the finding, which was reported June 17 in an early release of the Morbidity and Mortality Weekly Report, Dr. Frieden and Dr. Matthew J. Kuehnert, director of the CDC Office of Blood, Organ, and Other Tissue Safety sought to allay concerns regarding transmission of the virus via blood transfusion.
“The test being used to test blood in Puerto Rico is extremely accurate,” Dr. Kuehnert said, noting the importance of this testing, as several mosquito-borne illnesses are know to be transmissible by blood transfusion and many infected individuals are asymptomatic.
Further, at least one case of transfusion-transmitted Zika infection has been reported in Brazil, Dr. Kuehnert said.
The blood supply in Puerto Rico is being protected by laboratory testing, and in most other areas – including the continental United States – it is being protected by deferral of people who report travel to areas with such transmission. Some centers are electing to implement blood testing, but this is not currently a requirement, he said.
All donations that test positive for Zika are removed from the blood supply, and donors who test positive are provided with information on how to prevent spreading the virus to others.
The larger concern is the increasing prevalence of infections, as the nucleic acid test results “may be the most accurate, real time, leading indicator of Zika activity in Puerto Rico,” Dr. Frieden said.
The 1% prevalence of infection suggests substantial ongoing community transmission, given that viral nucleic acid can be detected for only 7-10 days after acute infection, and it translates to a greater than 1% rate of infection each month in the community, he noted.
“Our concern here is about protecting pregnant women, and with this rate of infection the possibility that there could be thousands of pregnant women affected, leading to dozens to hundreds of affected babies, is what’s of most concern,” Dr. Frieden said, adding that efforts are underway to reduce risk.
“We’re working intensively, in addition to keeping the blood supply safe, with the Puerto Rico health department, the government, communities, and people throughout Puerto Rico, to provide services for pregnant women – DEET, long sleeves, measures in their homes to reduce mosquito exposure that might reduce their risk of getting infected, as well as to control mosquitoes,” he said.
Dr. Frieden explained that controlling the Aedes aegypti mosquito is very difficult, and that it requires the effort of the entire community working together to protect a pregnant woman.
“We can’t make the risk zero … but even if we can reduce it by 10%, 30%, or 50%, that is a significant number of tragedies that we can prevent, and we’re doing everything that we can to do that … so that we don’t look back in 3, 6, or 12 months and say we wish we had done more back in June,” he said.
An increasing prevalence of Zika virus infection detected among blood donors in Puerto Rico using a highly sensitive, investigational nucleic acid test likely reflects an overall increase in the incidence of infection in the population at large, according to the Centers for Disease Control and Prevention.
“The implications and importance of this information are that in the coming months, it is possible that thousands of women in Puerto Rico could become infected with Zika. This could lead to dozens or hundreds of infants being born with microcephaly in the coming year,” CDC Director Dr. Tom Frieden said in a telebriefing on June 17.
“For the thousands of other infants born to women infected with Zika who don’t have microcephaly, we simply don’t know, and might not know for years, if there will be long-term consequences on brain development,” Dr. Frieden added.
The incidence of infection among blood donors in Puerto Rico the week of June 5-11 was 1.1%. This is the highest weekly incidence since testing using a newly developed nucleic acid test authorized by the Food and Drug Administration under an investigational new drug application that was implemented in Puerto Rico in April.
Between April 3 and June 11, 2016, a total of 68 presumptive viremic donors were identified from 12,777 donations tested. The incidence of positive findings has increased steadily over time (MMWR. 2016 Jun 17. doi: 10.15585/mmwr.mm6524e2).
In the wake of the finding, which was reported June 17 in an early release of the Morbidity and Mortality Weekly Report, Dr. Frieden and Dr. Matthew J. Kuehnert, director of the CDC Office of Blood, Organ, and Other Tissue Safety sought to allay concerns regarding transmission of the virus via blood transfusion.
“The test being used to test blood in Puerto Rico is extremely accurate,” Dr. Kuehnert said, noting the importance of this testing, as several mosquito-borne illnesses are know to be transmissible by blood transfusion and many infected individuals are asymptomatic.
Further, at least one case of transfusion-transmitted Zika infection has been reported in Brazil, Dr. Kuehnert said.
The blood supply in Puerto Rico is being protected by laboratory testing, and in most other areas – including the continental United States – it is being protected by deferral of people who report travel to areas with such transmission. Some centers are electing to implement blood testing, but this is not currently a requirement, he said.
All donations that test positive for Zika are removed from the blood supply, and donors who test positive are provided with information on how to prevent spreading the virus to others.
The larger concern is the increasing prevalence of infections, as the nucleic acid test results “may be the most accurate, real time, leading indicator of Zika activity in Puerto Rico,” Dr. Frieden said.
The 1% prevalence of infection suggests substantial ongoing community transmission, given that viral nucleic acid can be detected for only 7-10 days after acute infection, and it translates to a greater than 1% rate of infection each month in the community, he noted.
“Our concern here is about protecting pregnant women, and with this rate of infection the possibility that there could be thousands of pregnant women affected, leading to dozens to hundreds of affected babies, is what’s of most concern,” Dr. Frieden said, adding that efforts are underway to reduce risk.
“We’re working intensively, in addition to keeping the blood supply safe, with the Puerto Rico health department, the government, communities, and people throughout Puerto Rico, to provide services for pregnant women – DEET, long sleeves, measures in their homes to reduce mosquito exposure that might reduce their risk of getting infected, as well as to control mosquitoes,” he said.
Dr. Frieden explained that controlling the Aedes aegypti mosquito is very difficult, and that it requires the effort of the entire community working together to protect a pregnant woman.
“We can’t make the risk zero … but even if we can reduce it by 10%, 30%, or 50%, that is a significant number of tragedies that we can prevent, and we’re doing everything that we can to do that … so that we don’t look back in 3, 6, or 12 months and say we wish we had done more back in June,” he said.
FROM MMWR
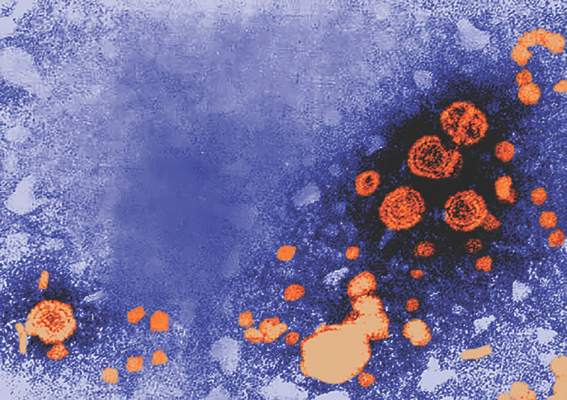
Three U.S. infants born with birth defects linked to Zika virus
There have been three infants born with birth defects and three pregnancy losses as a result of likely maternal Zika virus infection among U.S. women, according to figures released by the Centers for Disease Control and Prevention.
The figures, posted by the CDC on June 16, reflect reporting to the U.S. Zika Pregnancy Registry as of June 9. This is not real-time data and reflects only pregnancy outcomes for women with any laboratory evidence of possible Zika virus infection, though it is not known if Zika virus was the cause of the poor outcomes. The numbers also do not reflect outcomes among ongoing pregnancies.
Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The current numbers include outcomes reported in U.S. states and the District of Columbia. The CDC will begin reporting outcomes in U.S. territories in the coming weeks.
CDC officials plan to update the pregnancy outcome data every Thursday at http://www.cdc.gov/zika/geo/pregnancy-outcomes.html.
On Twitter @maryellenny
There have been three infants born with birth defects and three pregnancy losses as a result of likely maternal Zika virus infection among U.S. women, according to figures released by the Centers for Disease Control and Prevention.
The figures, posted by the CDC on June 16, reflect reporting to the U.S. Zika Pregnancy Registry as of June 9. This is not real-time data and reflects only pregnancy outcomes for women with any laboratory evidence of possible Zika virus infection, though it is not known if Zika virus was the cause of the poor outcomes. The numbers also do not reflect outcomes among ongoing pregnancies.
Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The current numbers include outcomes reported in U.S. states and the District of Columbia. The CDC will begin reporting outcomes in U.S. territories in the coming weeks.
CDC officials plan to update the pregnancy outcome data every Thursday at http://www.cdc.gov/zika/geo/pregnancy-outcomes.html.
On Twitter @maryellenny
There have been three infants born with birth defects and three pregnancy losses as a result of likely maternal Zika virus infection among U.S. women, according to figures released by the Centers for Disease Control and Prevention.
The figures, posted by the CDC on June 16, reflect reporting to the U.S. Zika Pregnancy Registry as of June 9. This is not real-time data and reflects only pregnancy outcomes for women with any laboratory evidence of possible Zika virus infection, though it is not known if Zika virus was the cause of the poor outcomes. The numbers also do not reflect outcomes among ongoing pregnancies.
Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The current numbers include outcomes reported in U.S. states and the District of Columbia. The CDC will begin reporting outcomes in U.S. territories in the coming weeks.
CDC officials plan to update the pregnancy outcome data every Thursday at http://www.cdc.gov/zika/geo/pregnancy-outcomes.html.
On Twitter @maryellenny
Tenofovir may prevent mother-to-child transmission of hepatitis B
The antiviral drug tenofovir disoproxil fumarate (TDF) can be used by expectant mothers infected with the hepatitis B virus to decrease HBV DNA levels and, therefore, significantly decrease the risk of passing the virus onto their children, according to a new study.
“A small but growing body of evidence has suggested that antiviral treatment may reduce the risk of mother-to-child transmission among mothers with an HBV DNA level of more than 6 log10 copies per milliliter, although quality studies are lacking and the existing studies have shown conflicting results,” wrote Dr. Calvin Q. Pan of New York University and coauthors in a study published in the New England Journal of Medicine (2016;374[24]:2324-34. doi: 10.1056/NEJMoa1508660).
An international research team recruited pregnant women in China – all of whom were positive for hepatitis B e antigen and had a viral load of at least 200,000 IU/mL – from March 2012 through June 2013. A total of 200 women were selected for inclusion and randomized into either a control cohort, or a cohort taking TDF; 100 mothers with 88 infants ultimately completed the study in the control group, while 97 mothers with 92 children completed the study in the TDF cohort.
Women on TDF were given a 300-mg dose orally every day, beginning 30-32 weeks into their pregnancy and continuing through 4 weeks after giving birth. Follow-up examinations were conducted at 4, 12, 24, and 28 weeks post partum, and the primary outcome was determining transmission of HBV from mother to child, with transmission defined as an infant having an HBV DNA level higher than 20 IU/mL. Patients in the control group were given “usual care without antiviral therapy.”
Mothers on TDF had lower HBV DNA levels at delivery, with 68% (66/97) dropping to under 200,000 IU/mL levels versus just 2% (2/100) in the control group having levels under that threshold (P less than .001). At 28-week follow-up examinations, intent-to-treat analysis showed that 5% (5/97) of infants born to mothers on TDF were found to have HBV DNA levels higher than 20 IU/mL, compared with 18% of infants born to mothers in the control group (P = .007). In per-protocol analysis, none of the infants whose mothers were taking TDF had contracted the disease; on the other hand, 7% (6/88) of those in the control group had (P = .01). Rates of birth defects and HBV serologic outcomes were not significantly different between the two cohorts.
“TDF [may] be useful for preventing mother-to-child transmission, which is a critical step toward the global eradication of HBV and a reduction in the incidence of liver cancer,” Dr. Pan and his colleagues said, adding that “the postpartum cessation of TDF requires close monitoring” in order to further understand the drug’s longer-term effects.
The study was funded by Gilead Sciences. Dr. Pan reported grant support from Gilead Sciences during the conduct of the study; grant support from Merck, grant support and personal fees from Gilead Sciences and Bristol-Myers Squibb; and personal fees from Johnson and Johnson and AbbVie outside the submitted work.
The antiviral drug tenofovir disoproxil fumarate (TDF) can be used by expectant mothers infected with the hepatitis B virus to decrease HBV DNA levels and, therefore, significantly decrease the risk of passing the virus onto their children, according to a new study.
“A small but growing body of evidence has suggested that antiviral treatment may reduce the risk of mother-to-child transmission among mothers with an HBV DNA level of more than 6 log10 copies per milliliter, although quality studies are lacking and the existing studies have shown conflicting results,” wrote Dr. Calvin Q. Pan of New York University and coauthors in a study published in the New England Journal of Medicine (2016;374[24]:2324-34. doi: 10.1056/NEJMoa1508660).
An international research team recruited pregnant women in China – all of whom were positive for hepatitis B e antigen and had a viral load of at least 200,000 IU/mL – from March 2012 through June 2013. A total of 200 women were selected for inclusion and randomized into either a control cohort, or a cohort taking TDF; 100 mothers with 88 infants ultimately completed the study in the control group, while 97 mothers with 92 children completed the study in the TDF cohort.
Women on TDF were given a 300-mg dose orally every day, beginning 30-32 weeks into their pregnancy and continuing through 4 weeks after giving birth. Follow-up examinations were conducted at 4, 12, 24, and 28 weeks post partum, and the primary outcome was determining transmission of HBV from mother to child, with transmission defined as an infant having an HBV DNA level higher than 20 IU/mL. Patients in the control group were given “usual care without antiviral therapy.”
Mothers on TDF had lower HBV DNA levels at delivery, with 68% (66/97) dropping to under 200,000 IU/mL levels versus just 2% (2/100) in the control group having levels under that threshold (P less than .001). At 28-week follow-up examinations, intent-to-treat analysis showed that 5% (5/97) of infants born to mothers on TDF were found to have HBV DNA levels higher than 20 IU/mL, compared with 18% of infants born to mothers in the control group (P = .007). In per-protocol analysis, none of the infants whose mothers were taking TDF had contracted the disease; on the other hand, 7% (6/88) of those in the control group had (P = .01). Rates of birth defects and HBV serologic outcomes were not significantly different between the two cohorts.
“TDF [may] be useful for preventing mother-to-child transmission, which is a critical step toward the global eradication of HBV and a reduction in the incidence of liver cancer,” Dr. Pan and his colleagues said, adding that “the postpartum cessation of TDF requires close monitoring” in order to further understand the drug’s longer-term effects.
The study was funded by Gilead Sciences. Dr. Pan reported grant support from Gilead Sciences during the conduct of the study; grant support from Merck, grant support and personal fees from Gilead Sciences and Bristol-Myers Squibb; and personal fees from Johnson and Johnson and AbbVie outside the submitted work.
The antiviral drug tenofovir disoproxil fumarate (TDF) can be used by expectant mothers infected with the hepatitis B virus to decrease HBV DNA levels and, therefore, significantly decrease the risk of passing the virus onto their children, according to a new study.
“A small but growing body of evidence has suggested that antiviral treatment may reduce the risk of mother-to-child transmission among mothers with an HBV DNA level of more than 6 log10 copies per milliliter, although quality studies are lacking and the existing studies have shown conflicting results,” wrote Dr. Calvin Q. Pan of New York University and coauthors in a study published in the New England Journal of Medicine (2016;374[24]:2324-34. doi: 10.1056/NEJMoa1508660).
An international research team recruited pregnant women in China – all of whom were positive for hepatitis B e antigen and had a viral load of at least 200,000 IU/mL – from March 2012 through June 2013. A total of 200 women were selected for inclusion and randomized into either a control cohort, or a cohort taking TDF; 100 mothers with 88 infants ultimately completed the study in the control group, while 97 mothers with 92 children completed the study in the TDF cohort.
Women on TDF were given a 300-mg dose orally every day, beginning 30-32 weeks into their pregnancy and continuing through 4 weeks after giving birth. Follow-up examinations were conducted at 4, 12, 24, and 28 weeks post partum, and the primary outcome was determining transmission of HBV from mother to child, with transmission defined as an infant having an HBV DNA level higher than 20 IU/mL. Patients in the control group were given “usual care without antiviral therapy.”
Mothers on TDF had lower HBV DNA levels at delivery, with 68% (66/97) dropping to under 200,000 IU/mL levels versus just 2% (2/100) in the control group having levels under that threshold (P less than .001). At 28-week follow-up examinations, intent-to-treat analysis showed that 5% (5/97) of infants born to mothers on TDF were found to have HBV DNA levels higher than 20 IU/mL, compared with 18% of infants born to mothers in the control group (P = .007). In per-protocol analysis, none of the infants whose mothers were taking TDF had contracted the disease; on the other hand, 7% (6/88) of those in the control group had (P = .01). Rates of birth defects and HBV serologic outcomes were not significantly different between the two cohorts.
“TDF [may] be useful for preventing mother-to-child transmission, which is a critical step toward the global eradication of HBV and a reduction in the incidence of liver cancer,” Dr. Pan and his colleagues said, adding that “the postpartum cessation of TDF requires close monitoring” in order to further understand the drug’s longer-term effects.
The study was funded by Gilead Sciences. Dr. Pan reported grant support from Gilead Sciences during the conduct of the study; grant support from Merck, grant support and personal fees from Gilead Sciences and Bristol-Myers Squibb; and personal fees from Johnson and Johnson and AbbVie outside the submitted work.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: A 300-mg daily dose of tenofovir disoproxil fumarate significantly reduced the risk of pregnant mothers with HBV infection transmitting the disease to their children.
Major finding: In an intent-to-treat analysis, only 5% of children born to mothers taking TDF during pregnancy were found to have contracted the disease, versus 18% in the control group (P = .007); similarly, in the per-protocol analysis, 0% of children born to mothers taking TDF had HBV vs. 7% in the control group (P = .01).
Data source: A multicenter, open label, randomized, parallel-group study of 197 HBV-positive pregnant women from March 2012 through June 2013.
Disclosures: The study was funded by Gilead Sciences. Dr. Pan reported grant support from Gilead Sciences during the conduct of the study; grant support from Merck, grant support and personal fees from Gilead Sciences and Bristol-Myers Squibb; and personal fees from Johnson and Johnson and AbbVie outside the submitted work.
Insomnia linked to increased risk of pregnancy loss
DENVER – Women who experience difficulty staying asleep are at increased risk of having one or more pregnancies that don’t result in a live birth, a large epidemiologic study suggests.
In contrast, other expressions of insomnia – difficulty in falling asleep, early morning awakening, or nonrestorative sleep – were not significantly associated with pregnancy loss in this analysis of a nationally representative sample comprised of 5,554 women aged 18-45 years, Sara Nowakowski, Ph.D., reported at the annual meeting of the Associated Professional Sleep Societies.
The women were participants in the National Health and Nutrition Examination Survey for 2005-2008, which collected data on reproductive history as well as sleep patterns. Roughly 20% of the women self-reported experiencing insomnia. Eighty-three percent of the 18- to 45-year-old women had been pregnant at least once, and 1,870 (40%) of them had one or more prior pregnancies that didn’t result in a live birth.
In a multivariate logistic regression analysis adjusted for age, race, education level, and frequency of sleep apnea symptoms, such as snoring or snorting/gasping, frequent difficulty in maintaining sleep was independently associated with an 85% increased risk of having experienced a pregnancy that didn’t result in a live birth, according to Dr. Nowakowski, a clinical psychologist in the department of ob.gyn. at the University of Texas, Galveston.
In an interview, she was quick to note that these are correlational, hypothesis-generating data, and that an epidemiologic study such as this can’t establish causality.
Dr. Nowakowski and her coinvestigators hope to conduct a prospective randomized trial of cognitive behavioral therapy for insomnia – a well-established treatment – in a group of women with prior spontaneous abortion, miscarriage, or other infertility issues, to determine whether insomnia is a modifiable risk factor for adverse pregnancy outcomes.
One physician at the meeting remarked that if women with fertility problems and insomnia knew of Dr. Nowakowski’s work showing an association between insomnia and unsuccessful pregnancies, they would be pounding down the doors of sleep specialists. Dr. Nowakowski agreed.
“I surf the online networks set up for infertile women. They’re very distraught over their trouble conceiving. They’re doing yoga, de-stress programs, taking all sorts of supplements – including melatonin – to try to improve their chances of fertility,” she said. “If insomnia turns out to partially account for the risk of having pregnancies that don’t result in live birth, and treating the insomnia reduces that risk, there would be a huge amount of patient interest.”
The study was supported by the National Institutes of Health. Dr. Nowakowski reported having no financial conflicts.
DENVER – Women who experience difficulty staying asleep are at increased risk of having one or more pregnancies that don’t result in a live birth, a large epidemiologic study suggests.
In contrast, other expressions of insomnia – difficulty in falling asleep, early morning awakening, or nonrestorative sleep – were not significantly associated with pregnancy loss in this analysis of a nationally representative sample comprised of 5,554 women aged 18-45 years, Sara Nowakowski, Ph.D., reported at the annual meeting of the Associated Professional Sleep Societies.
The women were participants in the National Health and Nutrition Examination Survey for 2005-2008, which collected data on reproductive history as well as sleep patterns. Roughly 20% of the women self-reported experiencing insomnia. Eighty-three percent of the 18- to 45-year-old women had been pregnant at least once, and 1,870 (40%) of them had one or more prior pregnancies that didn’t result in a live birth.
In a multivariate logistic regression analysis adjusted for age, race, education level, and frequency of sleep apnea symptoms, such as snoring or snorting/gasping, frequent difficulty in maintaining sleep was independently associated with an 85% increased risk of having experienced a pregnancy that didn’t result in a live birth, according to Dr. Nowakowski, a clinical psychologist in the department of ob.gyn. at the University of Texas, Galveston.
In an interview, she was quick to note that these are correlational, hypothesis-generating data, and that an epidemiologic study such as this can’t establish causality.
Dr. Nowakowski and her coinvestigators hope to conduct a prospective randomized trial of cognitive behavioral therapy for insomnia – a well-established treatment – in a group of women with prior spontaneous abortion, miscarriage, or other infertility issues, to determine whether insomnia is a modifiable risk factor for adverse pregnancy outcomes.
One physician at the meeting remarked that if women with fertility problems and insomnia knew of Dr. Nowakowski’s work showing an association between insomnia and unsuccessful pregnancies, they would be pounding down the doors of sleep specialists. Dr. Nowakowski agreed.
“I surf the online networks set up for infertile women. They’re very distraught over their trouble conceiving. They’re doing yoga, de-stress programs, taking all sorts of supplements – including melatonin – to try to improve their chances of fertility,” she said. “If insomnia turns out to partially account for the risk of having pregnancies that don’t result in live birth, and treating the insomnia reduces that risk, there would be a huge amount of patient interest.”
The study was supported by the National Institutes of Health. Dr. Nowakowski reported having no financial conflicts.
DENVER – Women who experience difficulty staying asleep are at increased risk of having one or more pregnancies that don’t result in a live birth, a large epidemiologic study suggests.
In contrast, other expressions of insomnia – difficulty in falling asleep, early morning awakening, or nonrestorative sleep – were not significantly associated with pregnancy loss in this analysis of a nationally representative sample comprised of 5,554 women aged 18-45 years, Sara Nowakowski, Ph.D., reported at the annual meeting of the Associated Professional Sleep Societies.
The women were participants in the National Health and Nutrition Examination Survey for 2005-2008, which collected data on reproductive history as well as sleep patterns. Roughly 20% of the women self-reported experiencing insomnia. Eighty-three percent of the 18- to 45-year-old women had been pregnant at least once, and 1,870 (40%) of them had one or more prior pregnancies that didn’t result in a live birth.
In a multivariate logistic regression analysis adjusted for age, race, education level, and frequency of sleep apnea symptoms, such as snoring or snorting/gasping, frequent difficulty in maintaining sleep was independently associated with an 85% increased risk of having experienced a pregnancy that didn’t result in a live birth, according to Dr. Nowakowski, a clinical psychologist in the department of ob.gyn. at the University of Texas, Galveston.
In an interview, she was quick to note that these are correlational, hypothesis-generating data, and that an epidemiologic study such as this can’t establish causality.
Dr. Nowakowski and her coinvestigators hope to conduct a prospective randomized trial of cognitive behavioral therapy for insomnia – a well-established treatment – in a group of women with prior spontaneous abortion, miscarriage, or other infertility issues, to determine whether insomnia is a modifiable risk factor for adverse pregnancy outcomes.
One physician at the meeting remarked that if women with fertility problems and insomnia knew of Dr. Nowakowski’s work showing an association between insomnia and unsuccessful pregnancies, they would be pounding down the doors of sleep specialists. Dr. Nowakowski agreed.
“I surf the online networks set up for infertile women. They’re very distraught over their trouble conceiving. They’re doing yoga, de-stress programs, taking all sorts of supplements – including melatonin – to try to improve their chances of fertility,” she said. “If insomnia turns out to partially account for the risk of having pregnancies that don’t result in live birth, and treating the insomnia reduces that risk, there would be a huge amount of patient interest.”
The study was supported by the National Institutes of Health. Dr. Nowakowski reported having no financial conflicts.
AT SLEEP 2016
Key clinical point: Insomnia may be a factor in some cases of poor pregnancy outcomes.
Major finding: Reproductive-age women who experienced difficulty staying asleep had an 85% greater likelihood of having a pregnancy that didn’t result in a live birth.
Data source: This epidemiologic study included 5,554 women aged 18-45 who provided details of their reproductive history and sleep status in the National Health and Nutrition Examination Survey.
Disclosures: This study was supported by the National Institutes of Health. Dr. Nowakowski reported having no financial conflicts.
A review of 2015 drug approvals: Safety in pregnancy and lactation
The Food and Drug Administration approved 45 new drugs in 2015. Currently, lesinurad (Zurampic) to treat high blood uric levels associated with gout, is not yet available. Another agent, aripiprazole (Aristada) for the treatment of schizophrenia, was initially approved in 2004 and is included in “Drugs in Pregnancy and Lactation,” 10th ed. (Philadelphia: Walters Kluwer: 2014, pp. 83-5).
There are three new multidrug combinations. Ceftazidime-avibactam (Avycaz) is indicated for complicated intra-abdominal and urinary tract infections, including pyelonephritis, when there are limited or no alternative treatment options. Lumacaftor-ivacaftor (Orkambi) is used to treat cystic fibrosis. There are no human pregnancy data for these two combination products but, based on animal data, they can be classified as low risk (no developmental toxicity in two or more animal species). There also is a four-drug combination that is a complete regimen for the treatment of HIV-1 infections: elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide (Genvoya). There is one reference citing the use of this combination drug in human pregnancy. It resulted in a healthy full-term infant. Because of its indication, this drug product should not be withheld because of pregnancy.
Asfotase (Strensiq) is a tissue nonspecific alkaline phosphatase. It is indicated for the treatment of perinatal/infantile and juvenile-onset hypophosphatasia. The animal data suggest low risk but it is doubtful that this drug will be used in pregnancy. In studies conducted by the manufacturer, the oldest patient age was 12.6 years.
Dinutuximab (Unituxin) is indicated for the treatment of neuroblastoma, an extracranial solid cancer that occurs in children. There are no studies in pregnant animals or humans.
The remaining 38 agents can be classified into the following categories: anticoagulant (2), antidiarrheal (1), antidiabetic (1), antidote (3), antiemetic (1), antifungal (1), antilipemic (2), antineoplastic (13), antipsychotic (2), antiviral (1), bile acid (2), cardiovascular (2), female sexual dysfunction (1), immunomodulator (1), lysosomal acid lipase deficiency (1), parathyroid hormone (1), pyrimidine analog (1), and respiratory (2).
Only two (cholic acid and ivabradine) of these 38 drugs have any human pregnancy data. Thus, the embryo-fetal risk has to be estimated using only animal data. However, an analysis of 1,154 drugs found that animal data raised the possibility of human developmental toxicity in 311 drugs, which was eventually confirmed in 75 drugs (Am J Obstet Gynecol. 2015 Dec;213[6]:810-5). Nevertheless, in some cases the maternal benefit will outweigh the risk based on animal data.
Anticoagulants
The two anticoagulants are cangrelor (Kengreal), indicated as an adjunct to percutaneous coronary intervention to reduce the risk of myocardial infarction, and edoxaban (Savaysa), to reduce the risk of stroke and embolism in patients with atrial fibrillation and for the treatment of deep vein thrombosis and pulmonary embolism. Although the animal data for cangrelor suggest moderate risk (developmental toxicity in one animal species), the potential benefit to the mother outweighs the risk. The animal data for edoxaban suggest low risk.
Antidiarrheal
Eluxadoline (Viberzi) is indicated for the treatment of irritable bowel syndrome with diarrhea. The animal data suggest low risk.
Antidiabetic
The animal data also suggest low risk for insulin degludec (Tresiba), a long-acting human insulin analog.
Antidote
Sugammadex (Bridion), an antidote, is indicated for the reversal of neuromuscular blockade induced by rocuronium and vecuronium in adults undergoing surgery. The animal data suggest risk (developmental toxicity in two or more animal species). Another antidote, idarucizumab (Praxbind) is a humanized monoclonal antibody fragment. It is indicated to reverse the anticoagulant effects of dabigatran. Animal reproduction studies have not been conducted. If indicated, the drug should not be withheld because of pregnancy. The third antidote is patiromer (Veltassa). It is a potassium binder used for the treatment of hyperkalemia. It should not be used for life-threatening hyperkalemia because of its delayed onset of action. It is not absorbed systemically. Because it can bind other drugs in the gut, other oral medications should be given at least 6 hours before or after patiromer.
Antiemetic
The antiemetic drug rolapitant (Varubi) is used, in combination with other antiemetics, for patients receiving antineoplastics. Animal data suggest moderate risk.
Antifungal
The triazole antifungal, isavuconazonium (Cresemba) is used for the treatment of invasive aspergillosis or invasive mucormycosis. Low doses of the drug caused perinatal death and skeletal defects in rats, similar to other azole antifungal agents.
Antilipemic agents
There are two antilipemic agents; alirocumab (Praluent) and evolocumab (Repatha). Alirocumab, a monoclonal antibody, is combined with statin therapy. Statins are contraindicated in pregnancy because interruption of cholesterol-lowering therapy during pregnancy should have no effect on the long-term treatment of hyperlipidemia and there is potential for embryo-fetal risk. Thus, alirocumab is also contraindicated during pregnancy. The same reasoning applies to evolocumab, an immunoglobulin, that is also given with statins.
Antineoplastic
There are 13 new antineoplastic agents that are often combined with other agents. They all can be classified as contraindicated in pregnancy, but the maternal benefit has to be weighed against the potential for embryo-fetal harm. Moreover, they are usually indicated when older drugs and treatments have not been effective. If the drug must be used in pregnancy, avoiding the first trimester should be a priority.
Sonidegib (Odomzo) is used for the treatment of basal cell carcinoma. The new agent for breast cancer is palbociclib (Ibrance). It is combined with letrozole, an antineoplastic that has caused spontaneous abortions and birth defects. A combination drug indicated for metastatic colorectal cancer is trifluridine/tipiracil (Lonsurf).
There are three new drugs that are indicated for non–small-cell lung cancer: alectinib (Alecensa), necitumumab (Portrazza), and osimertinib (Tagrisso). Alectinib and osimertinib are kinase inhibitors, whereas necitumumab is a epidermal growth factor receptor antagonist. The latter agent is a first-line drug that is combined with gemcitabine and cisplatin, both of which can cause birth defects.
The drug used for metastatic liposarcoma or leiomyosarcoma is trabectedin (Yondelis), an alkylating drug. There are no animal data, but the drug’s mechanism of action suggests that it can cause fetal harm. Cobimetinib (Cotellic), a kinase inhibitor, is indicated for the treatment of metastatic melanoma in combination with vemurafenib, another kinase inhibitor. The animal data and mechanism of action suggest that the drug could cause fetal harm.
There are four new agents for the treatment of multiple myeloma: panobinostat (Farydak), daratumumab (Darzalex), elotuzumab (Empliciti), and ixazomib (Ninlaro). Panobinostat, a histone deacetylase inhibitor, is combined with bortezomib (a proteasome inhibitor) and dexamethasone. The animal data suggest risk. The manufacturer recommends effective contraception while taking the drug and for at least 1 month after the last dose. Daratumumab is a monoclonal antibody. There are no animal data, but based on its mechanism of action, the drug may cause fetal myeloid or lymphoid-cell depletion and decreased bone density. The immunostimulatory antibody elotuzumab is given in combination with lenalidomide, an analog of thalidomide, and dexamethasone. There are no pregnancy data in animals or humans with lenalidomide, but this agent is contraindicated in pregnancy because of its relationship to thalidomide. Ixazomib, a proteasome inhibitor, is also given in combination with lenalidomide and dexamethasone, so the concerns are the same.
A new drug for thyroid cancer is lenvatinib (Lenvima), a tyrosine kinase inhibitor. The animal data suggest risk.
Antipsychotics
The two atypical antipsychotics are brexpiprazole (Rexulti) and cariprazine (Vraylar). Both drugs are included in National Pregnancy Registry for Atypical Antipsychotics. Information concerning the registry can be obtained by calling 1-866-961-2388 or at http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/.
Brexpiprazole is indicated for use as an adjunctive therapy to antidepressants for the treatment of major depressive disorder and for the treatment of schizophrenia. The animal data suggest low risk. Cariprazine is indicated for the treatment of schizophrenia and for the treatment of manic or mixed episodes associated with bipolar I disorder. The animal data suggest moderate risk.
Antiviral
Daclatasvir (Daklinza) is an antiviral agent indicated for use with sofosbuvir, with or without ribavirin, for the treatment of chronic hepatitis C virus genotype 1 or 3 infection. The animal data suggest low risk but, if ribavirin is used, the combination is contraindicated.
Bile acid
There are two new bile acid products. Cholic acid (Cholbam), is indicated for the treatment of bile acid synthesis disorders due to single enzyme defects and for peroxisomal disorders, including Zellweger spectrum disorders. Although studies in pregnant women or animals have not been conducted, the manufacturer is aware of some case reports that resulted in healthy infants.
Deoxycholic acid (Kybella) is a cytolytic agent that is indicated for improvement in the appearance of moderate to severe convexity or fullness associated with submental fat in adults. The drug caused no fetal harm in rats but did in rabbits, which may have been due to maternal toxicity.
Cardiovascular agents
There are two new cardiovascular agents. Ivabradine (Corlanor) is a hyperpolarization-activated cyclic nucleotide-gated channel blocker that is indicated to reduce the risk of hospitalization for worsening heart failure. The animal data suggest risk. A 2001 case report described the use of the drug in a pregnant woman. She suffered a cardiopulmonary arrest in her 10th week of pregnancy and was successfully treated with several drugs, including ivabradine. Ivabradine was stopped 1 month later. She gave birth at term to a normal healthy male infant.
Entresto is a combination of sacubitril, a neprilysin inhibitor, and valsartan, an angiotensin II receptor blocker. The combination is indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure and reduced ejection fraction. There is ample evidence that exposure to valsartan in the second half of pregnancy can cause severe fetal/neonatal toxicity including death.
Sexual dysfunction
Flibanserin (Addyi) is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder. The animal data suggest moderate risk. The simultaneous use of alcohol increases the risk of severe hypotension and syncope. The drug is available only through a restricted program called the Addyi REMS Program.
Immunomodulator
The new immunomodulator is secukinumab (Cosentyx). It is a human interleukin-17A antagonist indicated for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy of phototherapy, adults with psoriatic arthritis, and adults with active ankylosing spondylitis. The animal data suggest low risk.
Lysosomal acid lipase deficiency treatment
Sebelipase (Kanuma) is a lysosomal cholesteryl ester and triacylglycerol-specific enzyme that is indicated for the treatment of lysosomal acid lipase deficiency. The animal data suggest low risk.
Parathyroid hormone
Parathyroid hormone (Natpara) is used as an adjunct to calcium and vitamin D to control hypocalcemia in patients with hypoparathryoidism. The animal data in one species suggest moderate risk. Moreover, because of the risk of osteosarcoma, the drug should be used only in patients who cannot be well controlled on calcium and active forms of vitamin D alone.
Pyrimidine analog
Uridine (Xuriden) is a pyrimidine analog for uridine replacement. It is indicated for the treatment of hereditary orotic aciduria. The animal data suggest low risk.
Respiratory agents
The two respiratory agents are mepolizumab (Nucala) and selexipag (Uptravi). Mepolizumab is an interleukin-5 monoclonal antibody. It is indicated for the add-on maintenance treatment of patients with severe asthma aged 12 years and older and with an eosinophilic phenotype. The animal data suggest low risk. Selexipag is a prostacyclin receptor agonist that is used for the treatment of pulmonary arterial hypertension to delay disease progression and reduce the risk of hospitalization. The animal data suggest low risk.
Lactation
Use of the above drugs during human lactation has not been reported. It is likely that many of these agents will be excreted into breast milk. If a new agent is being taken by a woman who is or will be breastfeeding, the drug’s product information should be checked for the most common adverse reactions noted in nonpregnant patients and the nursing infant should be monitored for these effects.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He has no relevant financial disclosures.
The Food and Drug Administration approved 45 new drugs in 2015. Currently, lesinurad (Zurampic) to treat high blood uric levels associated with gout, is not yet available. Another agent, aripiprazole (Aristada) for the treatment of schizophrenia, was initially approved in 2004 and is included in “Drugs in Pregnancy and Lactation,” 10th ed. (Philadelphia: Walters Kluwer: 2014, pp. 83-5).
There are three new multidrug combinations. Ceftazidime-avibactam (Avycaz) is indicated for complicated intra-abdominal and urinary tract infections, including pyelonephritis, when there are limited or no alternative treatment options. Lumacaftor-ivacaftor (Orkambi) is used to treat cystic fibrosis. There are no human pregnancy data for these two combination products but, based on animal data, they can be classified as low risk (no developmental toxicity in two or more animal species). There also is a four-drug combination that is a complete regimen for the treatment of HIV-1 infections: elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide (Genvoya). There is one reference citing the use of this combination drug in human pregnancy. It resulted in a healthy full-term infant. Because of its indication, this drug product should not be withheld because of pregnancy.
Asfotase (Strensiq) is a tissue nonspecific alkaline phosphatase. It is indicated for the treatment of perinatal/infantile and juvenile-onset hypophosphatasia. The animal data suggest low risk but it is doubtful that this drug will be used in pregnancy. In studies conducted by the manufacturer, the oldest patient age was 12.6 years.
Dinutuximab (Unituxin) is indicated for the treatment of neuroblastoma, an extracranial solid cancer that occurs in children. There are no studies in pregnant animals or humans.
The remaining 38 agents can be classified into the following categories: anticoagulant (2), antidiarrheal (1), antidiabetic (1), antidote (3), antiemetic (1), antifungal (1), antilipemic (2), antineoplastic (13), antipsychotic (2), antiviral (1), bile acid (2), cardiovascular (2), female sexual dysfunction (1), immunomodulator (1), lysosomal acid lipase deficiency (1), parathyroid hormone (1), pyrimidine analog (1), and respiratory (2).
Only two (cholic acid and ivabradine) of these 38 drugs have any human pregnancy data. Thus, the embryo-fetal risk has to be estimated using only animal data. However, an analysis of 1,154 drugs found that animal data raised the possibility of human developmental toxicity in 311 drugs, which was eventually confirmed in 75 drugs (Am J Obstet Gynecol. 2015 Dec;213[6]:810-5). Nevertheless, in some cases the maternal benefit will outweigh the risk based on animal data.
Anticoagulants
The two anticoagulants are cangrelor (Kengreal), indicated as an adjunct to percutaneous coronary intervention to reduce the risk of myocardial infarction, and edoxaban (Savaysa), to reduce the risk of stroke and embolism in patients with atrial fibrillation and for the treatment of deep vein thrombosis and pulmonary embolism. Although the animal data for cangrelor suggest moderate risk (developmental toxicity in one animal species), the potential benefit to the mother outweighs the risk. The animal data for edoxaban suggest low risk.
Antidiarrheal
Eluxadoline (Viberzi) is indicated for the treatment of irritable bowel syndrome with diarrhea. The animal data suggest low risk.
Antidiabetic
The animal data also suggest low risk for insulin degludec (Tresiba), a long-acting human insulin analog.
Antidote
Sugammadex (Bridion), an antidote, is indicated for the reversal of neuromuscular blockade induced by rocuronium and vecuronium in adults undergoing surgery. The animal data suggest risk (developmental toxicity in two or more animal species). Another antidote, idarucizumab (Praxbind) is a humanized monoclonal antibody fragment. It is indicated to reverse the anticoagulant effects of dabigatran. Animal reproduction studies have not been conducted. If indicated, the drug should not be withheld because of pregnancy. The third antidote is patiromer (Veltassa). It is a potassium binder used for the treatment of hyperkalemia. It should not be used for life-threatening hyperkalemia because of its delayed onset of action. It is not absorbed systemically. Because it can bind other drugs in the gut, other oral medications should be given at least 6 hours before or after patiromer.
Antiemetic
The antiemetic drug rolapitant (Varubi) is used, in combination with other antiemetics, for patients receiving antineoplastics. Animal data suggest moderate risk.
Antifungal
The triazole antifungal, isavuconazonium (Cresemba) is used for the treatment of invasive aspergillosis or invasive mucormycosis. Low doses of the drug caused perinatal death and skeletal defects in rats, similar to other azole antifungal agents.
Antilipemic agents
There are two antilipemic agents; alirocumab (Praluent) and evolocumab (Repatha). Alirocumab, a monoclonal antibody, is combined with statin therapy. Statins are contraindicated in pregnancy because interruption of cholesterol-lowering therapy during pregnancy should have no effect on the long-term treatment of hyperlipidemia and there is potential for embryo-fetal risk. Thus, alirocumab is also contraindicated during pregnancy. The same reasoning applies to evolocumab, an immunoglobulin, that is also given with statins.
Antineoplastic
There are 13 new antineoplastic agents that are often combined with other agents. They all can be classified as contraindicated in pregnancy, but the maternal benefit has to be weighed against the potential for embryo-fetal harm. Moreover, they are usually indicated when older drugs and treatments have not been effective. If the drug must be used in pregnancy, avoiding the first trimester should be a priority.
Sonidegib (Odomzo) is used for the treatment of basal cell carcinoma. The new agent for breast cancer is palbociclib (Ibrance). It is combined with letrozole, an antineoplastic that has caused spontaneous abortions and birth defects. A combination drug indicated for metastatic colorectal cancer is trifluridine/tipiracil (Lonsurf).
There are three new drugs that are indicated for non–small-cell lung cancer: alectinib (Alecensa), necitumumab (Portrazza), and osimertinib (Tagrisso). Alectinib and osimertinib are kinase inhibitors, whereas necitumumab is a epidermal growth factor receptor antagonist. The latter agent is a first-line drug that is combined with gemcitabine and cisplatin, both of which can cause birth defects.
The drug used for metastatic liposarcoma or leiomyosarcoma is trabectedin (Yondelis), an alkylating drug. There are no animal data, but the drug’s mechanism of action suggests that it can cause fetal harm. Cobimetinib (Cotellic), a kinase inhibitor, is indicated for the treatment of metastatic melanoma in combination with vemurafenib, another kinase inhibitor. The animal data and mechanism of action suggest that the drug could cause fetal harm.
There are four new agents for the treatment of multiple myeloma: panobinostat (Farydak), daratumumab (Darzalex), elotuzumab (Empliciti), and ixazomib (Ninlaro). Panobinostat, a histone deacetylase inhibitor, is combined with bortezomib (a proteasome inhibitor) and dexamethasone. The animal data suggest risk. The manufacturer recommends effective contraception while taking the drug and for at least 1 month after the last dose. Daratumumab is a monoclonal antibody. There are no animal data, but based on its mechanism of action, the drug may cause fetal myeloid or lymphoid-cell depletion and decreased bone density. The immunostimulatory antibody elotuzumab is given in combination with lenalidomide, an analog of thalidomide, and dexamethasone. There are no pregnancy data in animals or humans with lenalidomide, but this agent is contraindicated in pregnancy because of its relationship to thalidomide. Ixazomib, a proteasome inhibitor, is also given in combination with lenalidomide and dexamethasone, so the concerns are the same.
A new drug for thyroid cancer is lenvatinib (Lenvima), a tyrosine kinase inhibitor. The animal data suggest risk.
Antipsychotics
The two atypical antipsychotics are brexpiprazole (Rexulti) and cariprazine (Vraylar). Both drugs are included in National Pregnancy Registry for Atypical Antipsychotics. Information concerning the registry can be obtained by calling 1-866-961-2388 or at http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/.
Brexpiprazole is indicated for use as an adjunctive therapy to antidepressants for the treatment of major depressive disorder and for the treatment of schizophrenia. The animal data suggest low risk. Cariprazine is indicated for the treatment of schizophrenia and for the treatment of manic or mixed episodes associated with bipolar I disorder. The animal data suggest moderate risk.
Antiviral
Daclatasvir (Daklinza) is an antiviral agent indicated for use with sofosbuvir, with or without ribavirin, for the treatment of chronic hepatitis C virus genotype 1 or 3 infection. The animal data suggest low risk but, if ribavirin is used, the combination is contraindicated.
Bile acid
There are two new bile acid products. Cholic acid (Cholbam), is indicated for the treatment of bile acid synthesis disorders due to single enzyme defects and for peroxisomal disorders, including Zellweger spectrum disorders. Although studies in pregnant women or animals have not been conducted, the manufacturer is aware of some case reports that resulted in healthy infants.
Deoxycholic acid (Kybella) is a cytolytic agent that is indicated for improvement in the appearance of moderate to severe convexity or fullness associated with submental fat in adults. The drug caused no fetal harm in rats but did in rabbits, which may have been due to maternal toxicity.
Cardiovascular agents
There are two new cardiovascular agents. Ivabradine (Corlanor) is a hyperpolarization-activated cyclic nucleotide-gated channel blocker that is indicated to reduce the risk of hospitalization for worsening heart failure. The animal data suggest risk. A 2001 case report described the use of the drug in a pregnant woman. She suffered a cardiopulmonary arrest in her 10th week of pregnancy and was successfully treated with several drugs, including ivabradine. Ivabradine was stopped 1 month later. She gave birth at term to a normal healthy male infant.
Entresto is a combination of sacubitril, a neprilysin inhibitor, and valsartan, an angiotensin II receptor blocker. The combination is indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure and reduced ejection fraction. There is ample evidence that exposure to valsartan in the second half of pregnancy can cause severe fetal/neonatal toxicity including death.
Sexual dysfunction
Flibanserin (Addyi) is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder. The animal data suggest moderate risk. The simultaneous use of alcohol increases the risk of severe hypotension and syncope. The drug is available only through a restricted program called the Addyi REMS Program.
Immunomodulator
The new immunomodulator is secukinumab (Cosentyx). It is a human interleukin-17A antagonist indicated for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy of phototherapy, adults with psoriatic arthritis, and adults with active ankylosing spondylitis. The animal data suggest low risk.
Lysosomal acid lipase deficiency treatment
Sebelipase (Kanuma) is a lysosomal cholesteryl ester and triacylglycerol-specific enzyme that is indicated for the treatment of lysosomal acid lipase deficiency. The animal data suggest low risk.
Parathyroid hormone
Parathyroid hormone (Natpara) is used as an adjunct to calcium and vitamin D to control hypocalcemia in patients with hypoparathryoidism. The animal data in one species suggest moderate risk. Moreover, because of the risk of osteosarcoma, the drug should be used only in patients who cannot be well controlled on calcium and active forms of vitamin D alone.
Pyrimidine analog
Uridine (Xuriden) is a pyrimidine analog for uridine replacement. It is indicated for the treatment of hereditary orotic aciduria. The animal data suggest low risk.
Respiratory agents
The two respiratory agents are mepolizumab (Nucala) and selexipag (Uptravi). Mepolizumab is an interleukin-5 monoclonal antibody. It is indicated for the add-on maintenance treatment of patients with severe asthma aged 12 years and older and with an eosinophilic phenotype. The animal data suggest low risk. Selexipag is a prostacyclin receptor agonist that is used for the treatment of pulmonary arterial hypertension to delay disease progression and reduce the risk of hospitalization. The animal data suggest low risk.
Lactation
Use of the above drugs during human lactation has not been reported. It is likely that many of these agents will be excreted into breast milk. If a new agent is being taken by a woman who is or will be breastfeeding, the drug’s product information should be checked for the most common adverse reactions noted in nonpregnant patients and the nursing infant should be monitored for these effects.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He has no relevant financial disclosures.
The Food and Drug Administration approved 45 new drugs in 2015. Currently, lesinurad (Zurampic) to treat high blood uric levels associated with gout, is not yet available. Another agent, aripiprazole (Aristada) for the treatment of schizophrenia, was initially approved in 2004 and is included in “Drugs in Pregnancy and Lactation,” 10th ed. (Philadelphia: Walters Kluwer: 2014, pp. 83-5).
There are three new multidrug combinations. Ceftazidime-avibactam (Avycaz) is indicated for complicated intra-abdominal and urinary tract infections, including pyelonephritis, when there are limited or no alternative treatment options. Lumacaftor-ivacaftor (Orkambi) is used to treat cystic fibrosis. There are no human pregnancy data for these two combination products but, based on animal data, they can be classified as low risk (no developmental toxicity in two or more animal species). There also is a four-drug combination that is a complete regimen for the treatment of HIV-1 infections: elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide (Genvoya). There is one reference citing the use of this combination drug in human pregnancy. It resulted in a healthy full-term infant. Because of its indication, this drug product should not be withheld because of pregnancy.
Asfotase (Strensiq) is a tissue nonspecific alkaline phosphatase. It is indicated for the treatment of perinatal/infantile and juvenile-onset hypophosphatasia. The animal data suggest low risk but it is doubtful that this drug will be used in pregnancy. In studies conducted by the manufacturer, the oldest patient age was 12.6 years.
Dinutuximab (Unituxin) is indicated for the treatment of neuroblastoma, an extracranial solid cancer that occurs in children. There are no studies in pregnant animals or humans.
The remaining 38 agents can be classified into the following categories: anticoagulant (2), antidiarrheal (1), antidiabetic (1), antidote (3), antiemetic (1), antifungal (1), antilipemic (2), antineoplastic (13), antipsychotic (2), antiviral (1), bile acid (2), cardiovascular (2), female sexual dysfunction (1), immunomodulator (1), lysosomal acid lipase deficiency (1), parathyroid hormone (1), pyrimidine analog (1), and respiratory (2).
Only two (cholic acid and ivabradine) of these 38 drugs have any human pregnancy data. Thus, the embryo-fetal risk has to be estimated using only animal data. However, an analysis of 1,154 drugs found that animal data raised the possibility of human developmental toxicity in 311 drugs, which was eventually confirmed in 75 drugs (Am J Obstet Gynecol. 2015 Dec;213[6]:810-5). Nevertheless, in some cases the maternal benefit will outweigh the risk based on animal data.
Anticoagulants
The two anticoagulants are cangrelor (Kengreal), indicated as an adjunct to percutaneous coronary intervention to reduce the risk of myocardial infarction, and edoxaban (Savaysa), to reduce the risk of stroke and embolism in patients with atrial fibrillation and for the treatment of deep vein thrombosis and pulmonary embolism. Although the animal data for cangrelor suggest moderate risk (developmental toxicity in one animal species), the potential benefit to the mother outweighs the risk. The animal data for edoxaban suggest low risk.
Antidiarrheal
Eluxadoline (Viberzi) is indicated for the treatment of irritable bowel syndrome with diarrhea. The animal data suggest low risk.
Antidiabetic
The animal data also suggest low risk for insulin degludec (Tresiba), a long-acting human insulin analog.
Antidote
Sugammadex (Bridion), an antidote, is indicated for the reversal of neuromuscular blockade induced by rocuronium and vecuronium in adults undergoing surgery. The animal data suggest risk (developmental toxicity in two or more animal species). Another antidote, idarucizumab (Praxbind) is a humanized monoclonal antibody fragment. It is indicated to reverse the anticoagulant effects of dabigatran. Animal reproduction studies have not been conducted. If indicated, the drug should not be withheld because of pregnancy. The third antidote is patiromer (Veltassa). It is a potassium binder used for the treatment of hyperkalemia. It should not be used for life-threatening hyperkalemia because of its delayed onset of action. It is not absorbed systemically. Because it can bind other drugs in the gut, other oral medications should be given at least 6 hours before or after patiromer.
Antiemetic
The antiemetic drug rolapitant (Varubi) is used, in combination with other antiemetics, for patients receiving antineoplastics. Animal data suggest moderate risk.
Antifungal
The triazole antifungal, isavuconazonium (Cresemba) is used for the treatment of invasive aspergillosis or invasive mucormycosis. Low doses of the drug caused perinatal death and skeletal defects in rats, similar to other azole antifungal agents.
Antilipemic agents
There are two antilipemic agents; alirocumab (Praluent) and evolocumab (Repatha). Alirocumab, a monoclonal antibody, is combined with statin therapy. Statins are contraindicated in pregnancy because interruption of cholesterol-lowering therapy during pregnancy should have no effect on the long-term treatment of hyperlipidemia and there is potential for embryo-fetal risk. Thus, alirocumab is also contraindicated during pregnancy. The same reasoning applies to evolocumab, an immunoglobulin, that is also given with statins.
Antineoplastic
There are 13 new antineoplastic agents that are often combined with other agents. They all can be classified as contraindicated in pregnancy, but the maternal benefit has to be weighed against the potential for embryo-fetal harm. Moreover, they are usually indicated when older drugs and treatments have not been effective. If the drug must be used in pregnancy, avoiding the first trimester should be a priority.
Sonidegib (Odomzo) is used for the treatment of basal cell carcinoma. The new agent for breast cancer is palbociclib (Ibrance). It is combined with letrozole, an antineoplastic that has caused spontaneous abortions and birth defects. A combination drug indicated for metastatic colorectal cancer is trifluridine/tipiracil (Lonsurf).
There are three new drugs that are indicated for non–small-cell lung cancer: alectinib (Alecensa), necitumumab (Portrazza), and osimertinib (Tagrisso). Alectinib and osimertinib are kinase inhibitors, whereas necitumumab is a epidermal growth factor receptor antagonist. The latter agent is a first-line drug that is combined with gemcitabine and cisplatin, both of which can cause birth defects.
The drug used for metastatic liposarcoma or leiomyosarcoma is trabectedin (Yondelis), an alkylating drug. There are no animal data, but the drug’s mechanism of action suggests that it can cause fetal harm. Cobimetinib (Cotellic), a kinase inhibitor, is indicated for the treatment of metastatic melanoma in combination with vemurafenib, another kinase inhibitor. The animal data and mechanism of action suggest that the drug could cause fetal harm.
There are four new agents for the treatment of multiple myeloma: panobinostat (Farydak), daratumumab (Darzalex), elotuzumab (Empliciti), and ixazomib (Ninlaro). Panobinostat, a histone deacetylase inhibitor, is combined with bortezomib (a proteasome inhibitor) and dexamethasone. The animal data suggest risk. The manufacturer recommends effective contraception while taking the drug and for at least 1 month after the last dose. Daratumumab is a monoclonal antibody. There are no animal data, but based on its mechanism of action, the drug may cause fetal myeloid or lymphoid-cell depletion and decreased bone density. The immunostimulatory antibody elotuzumab is given in combination with lenalidomide, an analog of thalidomide, and dexamethasone. There are no pregnancy data in animals or humans with lenalidomide, but this agent is contraindicated in pregnancy because of its relationship to thalidomide. Ixazomib, a proteasome inhibitor, is also given in combination with lenalidomide and dexamethasone, so the concerns are the same.
A new drug for thyroid cancer is lenvatinib (Lenvima), a tyrosine kinase inhibitor. The animal data suggest risk.
Antipsychotics
The two atypical antipsychotics are brexpiprazole (Rexulti) and cariprazine (Vraylar). Both drugs are included in National Pregnancy Registry for Atypical Antipsychotics. Information concerning the registry can be obtained by calling 1-866-961-2388 or at http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/.
Brexpiprazole is indicated for use as an adjunctive therapy to antidepressants for the treatment of major depressive disorder and for the treatment of schizophrenia. The animal data suggest low risk. Cariprazine is indicated for the treatment of schizophrenia and for the treatment of manic or mixed episodes associated with bipolar I disorder. The animal data suggest moderate risk.
Antiviral
Daclatasvir (Daklinza) is an antiviral agent indicated for use with sofosbuvir, with or without ribavirin, for the treatment of chronic hepatitis C virus genotype 1 or 3 infection. The animal data suggest low risk but, if ribavirin is used, the combination is contraindicated.
Bile acid
There are two new bile acid products. Cholic acid (Cholbam), is indicated for the treatment of bile acid synthesis disorders due to single enzyme defects and for peroxisomal disorders, including Zellweger spectrum disorders. Although studies in pregnant women or animals have not been conducted, the manufacturer is aware of some case reports that resulted in healthy infants.
Deoxycholic acid (Kybella) is a cytolytic agent that is indicated for improvement in the appearance of moderate to severe convexity or fullness associated with submental fat in adults. The drug caused no fetal harm in rats but did in rabbits, which may have been due to maternal toxicity.
Cardiovascular agents
There are two new cardiovascular agents. Ivabradine (Corlanor) is a hyperpolarization-activated cyclic nucleotide-gated channel blocker that is indicated to reduce the risk of hospitalization for worsening heart failure. The animal data suggest risk. A 2001 case report described the use of the drug in a pregnant woman. She suffered a cardiopulmonary arrest in her 10th week of pregnancy and was successfully treated with several drugs, including ivabradine. Ivabradine was stopped 1 month later. She gave birth at term to a normal healthy male infant.
Entresto is a combination of sacubitril, a neprilysin inhibitor, and valsartan, an angiotensin II receptor blocker. The combination is indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure and reduced ejection fraction. There is ample evidence that exposure to valsartan in the second half of pregnancy can cause severe fetal/neonatal toxicity including death.
Sexual dysfunction
Flibanserin (Addyi) is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder. The animal data suggest moderate risk. The simultaneous use of alcohol increases the risk of severe hypotension and syncope. The drug is available only through a restricted program called the Addyi REMS Program.
Immunomodulator
The new immunomodulator is secukinumab (Cosentyx). It is a human interleukin-17A antagonist indicated for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy of phototherapy, adults with psoriatic arthritis, and adults with active ankylosing spondylitis. The animal data suggest low risk.
Lysosomal acid lipase deficiency treatment
Sebelipase (Kanuma) is a lysosomal cholesteryl ester and triacylglycerol-specific enzyme that is indicated for the treatment of lysosomal acid lipase deficiency. The animal data suggest low risk.
Parathyroid hormone
Parathyroid hormone (Natpara) is used as an adjunct to calcium and vitamin D to control hypocalcemia in patients with hypoparathryoidism. The animal data in one species suggest moderate risk. Moreover, because of the risk of osteosarcoma, the drug should be used only in patients who cannot be well controlled on calcium and active forms of vitamin D alone.
Pyrimidine analog
Uridine (Xuriden) is a pyrimidine analog for uridine replacement. It is indicated for the treatment of hereditary orotic aciduria. The animal data suggest low risk.
Respiratory agents
The two respiratory agents are mepolizumab (Nucala) and selexipag (Uptravi). Mepolizumab is an interleukin-5 monoclonal antibody. It is indicated for the add-on maintenance treatment of patients with severe asthma aged 12 years and older and with an eosinophilic phenotype. The animal data suggest low risk. Selexipag is a prostacyclin receptor agonist that is used for the treatment of pulmonary arterial hypertension to delay disease progression and reduce the risk of hospitalization. The animal data suggest low risk.
Lactation
Use of the above drugs during human lactation has not been reported. It is likely that many of these agents will be excreted into breast milk. If a new agent is being taken by a woman who is or will be breastfeeding, the drug’s product information should be checked for the most common adverse reactions noted in nonpregnant patients and the nursing infant should be monitored for these effects.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He has no relevant financial disclosures.
Cesarean Delivery Rates Among Nulliparous Women Are Lower After Guidance
For at least one medical center, the adoption of March 2014 consensus guidelines on cesarean delivery was associated with a significant reduction in the primary C-section rate among nulliparous patients attempting vaginal delivery.
“This investigation confirms that adoption of consensus guidelines was associated with a reduction in the primary cesarean delivery rate among nulliparous patients at our institution. The observed decrease is both statistically and clinically significant,” Dr. Jonas G. Wilson-Leedy of the department of obstetrics and gynecology at the Penn State Milton S. Hershey Medical Center and colleagues wrote (Obstet Gynecol. 2016;128:145-52. doi: 10.1097/AOG.0000000000001488).
The researchers conducted a before-after retrospective analysis of medical records at their hospital, assessing the rates of the induced or augmented cesarean delivery rate, the overall cesarean delivery rate, and the performance of cesarean for arrest disorder at less than 6 cm dilatation in women delivering before (Sept. 13, 2013 to Feb. 28, 2014) and after (May 1, 2014 to Sept. 28, 2014) guideline implementation.
The guidelines – issued in March 2014 by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine – encouraged changes in labor management, including defining 6 cm dilatation as the threshold for active labor and urging longer durations of expectant management before cesarean delivery for labor arrest.
The researchers analyzed records of 275 and 292 women before guideline adoption and after guideline adoption, respectively.
After guideline adoption, the cesarean delivery rate was significantly reduced in the subpopulation of patients induced or augmented, dropping from 71 of 200 patients (35.5%) to 49 of 200 patients (24.5%), for an odds ratio of 0.59. The drop in the cesarean delivery rate remained significant even when spontaneously laboring patients were included in the analysis, the researchers reported.
Using a multivariable logistic regression model, the overall cesarean delivery rate was also found to be significantly reduced in the post–guideline adoption period, from 26.9% to 18.8%.
The rate of cesarean delivery for arrest of dilation at less than 6 cm declined from 7.1% to 1.1% after guideline adoption (P = .006). Rates of postpartum hemorrhage fell from 38.2% to 29.5% (OR, 0.68) in the postguidline period. A similar drop was seen in a composite measure of maternal morbidity, which declined from 45.1% to 36.3% (OR, 0.69) after adoption of the guidelines.
“Our data suggest that a significant decrease in cesarean deliveries for labor arrest at less than 6 cm dilatation contributed to the reduction in cesarean delivery rate,” the researchers wrote. “Consistent with recommendations discouraging diagnosis of arrest of dilation in latent labor, a sixfold reduction in this indication was identified postguideline.”
Although the researchers did not identify significant increases in either maternal or neonatal morbidities, the study was not sufficiently powered to assess those outcomes.
The researchers reported having no potential conflicts of interest.
For at least one medical center, the adoption of March 2014 consensus guidelines on cesarean delivery was associated with a significant reduction in the primary C-section rate among nulliparous patients attempting vaginal delivery.
“This investigation confirms that adoption of consensus guidelines was associated with a reduction in the primary cesarean delivery rate among nulliparous patients at our institution. The observed decrease is both statistically and clinically significant,” Dr. Jonas G. Wilson-Leedy of the department of obstetrics and gynecology at the Penn State Milton S. Hershey Medical Center and colleagues wrote (Obstet Gynecol. 2016;128:145-52. doi: 10.1097/AOG.0000000000001488).
The researchers conducted a before-after retrospective analysis of medical records at their hospital, assessing the rates of the induced or augmented cesarean delivery rate, the overall cesarean delivery rate, and the performance of cesarean for arrest disorder at less than 6 cm dilatation in women delivering before (Sept. 13, 2013 to Feb. 28, 2014) and after (May 1, 2014 to Sept. 28, 2014) guideline implementation.
The guidelines – issued in March 2014 by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine – encouraged changes in labor management, including defining 6 cm dilatation as the threshold for active labor and urging longer durations of expectant management before cesarean delivery for labor arrest.
The researchers analyzed records of 275 and 292 women before guideline adoption and after guideline adoption, respectively.
After guideline adoption, the cesarean delivery rate was significantly reduced in the subpopulation of patients induced or augmented, dropping from 71 of 200 patients (35.5%) to 49 of 200 patients (24.5%), for an odds ratio of 0.59. The drop in the cesarean delivery rate remained significant even when spontaneously laboring patients were included in the analysis, the researchers reported.
Using a multivariable logistic regression model, the overall cesarean delivery rate was also found to be significantly reduced in the post–guideline adoption period, from 26.9% to 18.8%.
The rate of cesarean delivery for arrest of dilation at less than 6 cm declined from 7.1% to 1.1% after guideline adoption (P = .006). Rates of postpartum hemorrhage fell from 38.2% to 29.5% (OR, 0.68) in the postguidline period. A similar drop was seen in a composite measure of maternal morbidity, which declined from 45.1% to 36.3% (OR, 0.69) after adoption of the guidelines.
“Our data suggest that a significant decrease in cesarean deliveries for labor arrest at less than 6 cm dilatation contributed to the reduction in cesarean delivery rate,” the researchers wrote. “Consistent with recommendations discouraging diagnosis of arrest of dilation in latent labor, a sixfold reduction in this indication was identified postguideline.”
Although the researchers did not identify significant increases in either maternal or neonatal morbidities, the study was not sufficiently powered to assess those outcomes.
The researchers reported having no potential conflicts of interest.
For at least one medical center, the adoption of March 2014 consensus guidelines on cesarean delivery was associated with a significant reduction in the primary C-section rate among nulliparous patients attempting vaginal delivery.
“This investigation confirms that adoption of consensus guidelines was associated with a reduction in the primary cesarean delivery rate among nulliparous patients at our institution. The observed decrease is both statistically and clinically significant,” Dr. Jonas G. Wilson-Leedy of the department of obstetrics and gynecology at the Penn State Milton S. Hershey Medical Center and colleagues wrote (Obstet Gynecol. 2016;128:145-52. doi: 10.1097/AOG.0000000000001488).
The researchers conducted a before-after retrospective analysis of medical records at their hospital, assessing the rates of the induced or augmented cesarean delivery rate, the overall cesarean delivery rate, and the performance of cesarean for arrest disorder at less than 6 cm dilatation in women delivering before (Sept. 13, 2013 to Feb. 28, 2014) and after (May 1, 2014 to Sept. 28, 2014) guideline implementation.
The guidelines – issued in March 2014 by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine – encouraged changes in labor management, including defining 6 cm dilatation as the threshold for active labor and urging longer durations of expectant management before cesarean delivery for labor arrest.
The researchers analyzed records of 275 and 292 women before guideline adoption and after guideline adoption, respectively.
After guideline adoption, the cesarean delivery rate was significantly reduced in the subpopulation of patients induced or augmented, dropping from 71 of 200 patients (35.5%) to 49 of 200 patients (24.5%), for an odds ratio of 0.59. The drop in the cesarean delivery rate remained significant even when spontaneously laboring patients were included in the analysis, the researchers reported.
Using a multivariable logistic regression model, the overall cesarean delivery rate was also found to be significantly reduced in the post–guideline adoption period, from 26.9% to 18.8%.
The rate of cesarean delivery for arrest of dilation at less than 6 cm declined from 7.1% to 1.1% after guideline adoption (P = .006). Rates of postpartum hemorrhage fell from 38.2% to 29.5% (OR, 0.68) in the postguidline period. A similar drop was seen in a composite measure of maternal morbidity, which declined from 45.1% to 36.3% (OR, 0.69) after adoption of the guidelines.
“Our data suggest that a significant decrease in cesarean deliveries for labor arrest at less than 6 cm dilatation contributed to the reduction in cesarean delivery rate,” the researchers wrote. “Consistent with recommendations discouraging diagnosis of arrest of dilation in latent labor, a sixfold reduction in this indication was identified postguideline.”
Although the researchers did not identify significant increases in either maternal or neonatal morbidities, the study was not sufficiently powered to assess those outcomes.
The researchers reported having no potential conflicts of interest.
FROM OBSTETRICS & GYNECOLOGY
Cesarean delivery rates among nulliparous women are lower after guidance
For at least one medical center, the adoption of March 2014 consensus guidelines on cesarean delivery was associated with a significant reduction in the primary C-section rate among nulliparous patients attempting vaginal delivery.
“This investigation confirms that adoption of consensus guidelines was associated with a reduction in the primary cesarean delivery rate among nulliparous patients at our institution. The observed decrease is both statistically and clinically significant,” Dr. Jonas G. Wilson-Leedy of the department of obstetrics and gynecology at the Penn State Milton S. Hershey Medical Center and colleagues wrote (Obstet Gynecol. 2016;128:145-52. doi: 10.1097/AOG.0000000000001488).
The researchers conducted a before-after retrospective analysis of medical records at their hospital, assessing the rates of the induced or augmented cesarean delivery rate, the overall cesarean delivery rate, and the performance of cesarean for arrest disorder at less than 6 cm dilatation in women delivering before (Sept. 13, 2013 to Feb. 28, 2014) and after (May 1, 2014 to Sept. 28, 2014) guideline implementation.
The guidelines – issued in March 2014 by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine – encouraged changes in labor management, including defining 6 cm dilatation as the threshold for active labor and urging longer durations of expectant management before cesarean delivery for labor arrest.
The researchers analyzed records of 275 and 292 women before guideline adoption and after guideline adoption, respectively.
After guideline adoption, the cesarean delivery rate was significantly reduced in the subpopulation of patients induced or augmented, dropping from 71 of 200 patients (35.5%) to 49 of 200 patients (24.5%), for an odds ratio of 0.59. The drop in the cesarean delivery rate remained significant even when spontaneously laboring patients were included in the analysis, the researchers reported.
Using a multivariable logistic regression model, the overall cesarean delivery rate was also found to be significantly reduced in the post–guideline adoption period, from 26.9% to 18.8%.
The rate of cesarean delivery for arrest of dilation at less than 6 cm declined from 7.1% to 1.1% after guideline adoption (P = .006). Rates of postpartum hemorrhage fell from 38.2% to 29.5% (OR, 0.68) in the postguidline period. A similar drop was seen in a composite measure of maternal morbidity, which declined from 45.1% to 36.3% (OR, 0.69) after adoption of the guidelines.
“Our data suggest that a significant decrease in cesarean deliveries for labor arrest at less than 6 cm dilatation contributed to the reduction in cesarean delivery rate,” the researchers wrote. “Consistent with recommendations discouraging diagnosis of arrest of dilation in latent labor, a sixfold reduction in this indication was identified postguideline.”
Although the researchers did not identify significant increases in either maternal or neonatal morbidities, the study was not sufficiently powered to assess those outcomes.
The researchers reported having no potential conflicts of interest.
For at least one medical center, the adoption of March 2014 consensus guidelines on cesarean delivery was associated with a significant reduction in the primary C-section rate among nulliparous patients attempting vaginal delivery.
“This investigation confirms that adoption of consensus guidelines was associated with a reduction in the primary cesarean delivery rate among nulliparous patients at our institution. The observed decrease is both statistically and clinically significant,” Dr. Jonas G. Wilson-Leedy of the department of obstetrics and gynecology at the Penn State Milton S. Hershey Medical Center and colleagues wrote (Obstet Gynecol. 2016;128:145-52. doi: 10.1097/AOG.0000000000001488).
The researchers conducted a before-after retrospective analysis of medical records at their hospital, assessing the rates of the induced or augmented cesarean delivery rate, the overall cesarean delivery rate, and the performance of cesarean for arrest disorder at less than 6 cm dilatation in women delivering before (Sept. 13, 2013 to Feb. 28, 2014) and after (May 1, 2014 to Sept. 28, 2014) guideline implementation.
The guidelines – issued in March 2014 by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine – encouraged changes in labor management, including defining 6 cm dilatation as the threshold for active labor and urging longer durations of expectant management before cesarean delivery for labor arrest.
The researchers analyzed records of 275 and 292 women before guideline adoption and after guideline adoption, respectively.
After guideline adoption, the cesarean delivery rate was significantly reduced in the subpopulation of patients induced or augmented, dropping from 71 of 200 patients (35.5%) to 49 of 200 patients (24.5%), for an odds ratio of 0.59. The drop in the cesarean delivery rate remained significant even when spontaneously laboring patients were included in the analysis, the researchers reported.
Using a multivariable logistic regression model, the overall cesarean delivery rate was also found to be significantly reduced in the post–guideline adoption period, from 26.9% to 18.8%.
The rate of cesarean delivery for arrest of dilation at less than 6 cm declined from 7.1% to 1.1% after guideline adoption (P = .006). Rates of postpartum hemorrhage fell from 38.2% to 29.5% (OR, 0.68) in the postguidline period. A similar drop was seen in a composite measure of maternal morbidity, which declined from 45.1% to 36.3% (OR, 0.69) after adoption of the guidelines.
“Our data suggest that a significant decrease in cesarean deliveries for labor arrest at less than 6 cm dilatation contributed to the reduction in cesarean delivery rate,” the researchers wrote. “Consistent with recommendations discouraging diagnosis of arrest of dilation in latent labor, a sixfold reduction in this indication was identified postguideline.”
Although the researchers did not identify significant increases in either maternal or neonatal morbidities, the study was not sufficiently powered to assess those outcomes.
The researchers reported having no potential conflicts of interest.
For at least one medical center, the adoption of March 2014 consensus guidelines on cesarean delivery was associated with a significant reduction in the primary C-section rate among nulliparous patients attempting vaginal delivery.
“This investigation confirms that adoption of consensus guidelines was associated with a reduction in the primary cesarean delivery rate among nulliparous patients at our institution. The observed decrease is both statistically and clinically significant,” Dr. Jonas G. Wilson-Leedy of the department of obstetrics and gynecology at the Penn State Milton S. Hershey Medical Center and colleagues wrote (Obstet Gynecol. 2016;128:145-52. doi: 10.1097/AOG.0000000000001488).
The researchers conducted a before-after retrospective analysis of medical records at their hospital, assessing the rates of the induced or augmented cesarean delivery rate, the overall cesarean delivery rate, and the performance of cesarean for arrest disorder at less than 6 cm dilatation in women delivering before (Sept. 13, 2013 to Feb. 28, 2014) and after (May 1, 2014 to Sept. 28, 2014) guideline implementation.
The guidelines – issued in March 2014 by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine – encouraged changes in labor management, including defining 6 cm dilatation as the threshold for active labor and urging longer durations of expectant management before cesarean delivery for labor arrest.
The researchers analyzed records of 275 and 292 women before guideline adoption and after guideline adoption, respectively.
After guideline adoption, the cesarean delivery rate was significantly reduced in the subpopulation of patients induced or augmented, dropping from 71 of 200 patients (35.5%) to 49 of 200 patients (24.5%), for an odds ratio of 0.59. The drop in the cesarean delivery rate remained significant even when spontaneously laboring patients were included in the analysis, the researchers reported.
Using a multivariable logistic regression model, the overall cesarean delivery rate was also found to be significantly reduced in the post–guideline adoption period, from 26.9% to 18.8%.
The rate of cesarean delivery for arrest of dilation at less than 6 cm declined from 7.1% to 1.1% after guideline adoption (P = .006). Rates of postpartum hemorrhage fell from 38.2% to 29.5% (OR, 0.68) in the postguidline period. A similar drop was seen in a composite measure of maternal morbidity, which declined from 45.1% to 36.3% (OR, 0.69) after adoption of the guidelines.
“Our data suggest that a significant decrease in cesarean deliveries for labor arrest at less than 6 cm dilatation contributed to the reduction in cesarean delivery rate,” the researchers wrote. “Consistent with recommendations discouraging diagnosis of arrest of dilation in latent labor, a sixfold reduction in this indication was identified postguideline.”
Although the researchers did not identify significant increases in either maternal or neonatal morbidities, the study was not sufficiently powered to assess those outcomes.
The researchers reported having no potential conflicts of interest.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Adoption of the 2014 consensus guidelines for the prevention of primary cesarean delivery significantly decreased the C-section rate at one medical center.
Major finding: After guideline adoption, the cesarean delivery among patients who were induced or augmented fell from 35.5% to 24.5%.
Data sources: A before-after retrospective cohort study of 567 patients at a single academic center between Sept. 13, 2013 and Sept. 28, 2014.
Disclosures: No funding source was reported and the researchers reported having no potential conflicts of interest.