User login
Obesity in healthy women linked to poor pregnancy and neonatal outcomes
Obese women without chronic disease are at greater risk of pregnancy complications and poor neonatal outcomes than are normal weight women, according to a study published in Obstetrics & Gynecology.
Sung Soo Kim, Ph.D., of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and her colleagues, conducted a retrospective cohort study of medical records from the Consortium on Safe Labor, collected from 2002-2008. Singleton pregnancies among U.S. women without prepregnancy diseases were examined for obstetric and neonatal complications based on the prepregnancy body mass index (BMI) of the mother, categorized as either normal weight (18.5-24.9 kg/m2), overweight (25-29.9), obese class I (30-34.9), obese class II (35-39.9), or obese class III (40 or greater). The investigators assessed 112,309 deliveries among 106,552 women (Obstet Gynecol. 2016;128:104-12. doi: 10.1097/AOG.0000000000001465).
Women with higher prepregnancy BMIs were at greater risk for several types of maternal and neonatal outcomes, according to the findings. For example, the relative risk for gestational diabetes mellitus, compared with women with normal BMIs, was 1.99 for overweight women, 2.94 for obese class I women, 3.97 for obese class II women, and 5.47 for obese class III women.
Similar findings were noted for other maternal outcomes, including higher risks for gestational hypertensive disorders, cesarean delivery, prelabor cesarean delivery, and acute cardiovascular events among women with higher BMIs.
Higher prepregnancy maternal BMI was also associated with increased neonatal risks, including preterm birth at less than 32 weeks of gestation, large for gestational age (LGA) neonates, transient tachypnea, sepsis, and neonatal intensive care unit admission.
The relative risk for LGA neonates, compared with women with normal BMIs, was 1.52 for overweight women, 1.74 for obese class I women, 1.93 for obese class II women, and 2.32 for obese class III women.
In an analysis of a composite variable that included all obstetric and neonatal complications with the exception of interventions, the researchers detected an 18%-47% increased risk of any pregnancy complication among overweight or obese women.
Even obese women who were otherwise healthy at the start of their pregnancy, did not develop pregnancy complications, and gained weight within recommended limits, still had an elevated risk for obstetric and neonatal complications, according to the researchers. “We found increased risks of relatively rare outcomes that other studies could not observe, including maternal acute cardiovascular events and neonatal transient tachypnea, necrotizing enterocolitis, peri- and intraventricular hemorrhage, and retinopathy of prematurity among deliveries to overweight or obese women,” they wrote.
The researchers received support for the work from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The authors reported having no potential conflicts of interest.
Obese women without chronic disease are at greater risk of pregnancy complications and poor neonatal outcomes than are normal weight women, according to a study published in Obstetrics & Gynecology.
Sung Soo Kim, Ph.D., of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and her colleagues, conducted a retrospective cohort study of medical records from the Consortium on Safe Labor, collected from 2002-2008. Singleton pregnancies among U.S. women without prepregnancy diseases were examined for obstetric and neonatal complications based on the prepregnancy body mass index (BMI) of the mother, categorized as either normal weight (18.5-24.9 kg/m2), overweight (25-29.9), obese class I (30-34.9), obese class II (35-39.9), or obese class III (40 or greater). The investigators assessed 112,309 deliveries among 106,552 women (Obstet Gynecol. 2016;128:104-12. doi: 10.1097/AOG.0000000000001465).
Women with higher prepregnancy BMIs were at greater risk for several types of maternal and neonatal outcomes, according to the findings. For example, the relative risk for gestational diabetes mellitus, compared with women with normal BMIs, was 1.99 for overweight women, 2.94 for obese class I women, 3.97 for obese class II women, and 5.47 for obese class III women.
Similar findings were noted for other maternal outcomes, including higher risks for gestational hypertensive disorders, cesarean delivery, prelabor cesarean delivery, and acute cardiovascular events among women with higher BMIs.
Higher prepregnancy maternal BMI was also associated with increased neonatal risks, including preterm birth at less than 32 weeks of gestation, large for gestational age (LGA) neonates, transient tachypnea, sepsis, and neonatal intensive care unit admission.
The relative risk for LGA neonates, compared with women with normal BMIs, was 1.52 for overweight women, 1.74 for obese class I women, 1.93 for obese class II women, and 2.32 for obese class III women.
In an analysis of a composite variable that included all obstetric and neonatal complications with the exception of interventions, the researchers detected an 18%-47% increased risk of any pregnancy complication among overweight or obese women.
Even obese women who were otherwise healthy at the start of their pregnancy, did not develop pregnancy complications, and gained weight within recommended limits, still had an elevated risk for obstetric and neonatal complications, according to the researchers. “We found increased risks of relatively rare outcomes that other studies could not observe, including maternal acute cardiovascular events and neonatal transient tachypnea, necrotizing enterocolitis, peri- and intraventricular hemorrhage, and retinopathy of prematurity among deliveries to overweight or obese women,” they wrote.
The researchers received support for the work from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The authors reported having no potential conflicts of interest.
Obese women without chronic disease are at greater risk of pregnancy complications and poor neonatal outcomes than are normal weight women, according to a study published in Obstetrics & Gynecology.
Sung Soo Kim, Ph.D., of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and her colleagues, conducted a retrospective cohort study of medical records from the Consortium on Safe Labor, collected from 2002-2008. Singleton pregnancies among U.S. women without prepregnancy diseases were examined for obstetric and neonatal complications based on the prepregnancy body mass index (BMI) of the mother, categorized as either normal weight (18.5-24.9 kg/m2), overweight (25-29.9), obese class I (30-34.9), obese class II (35-39.9), or obese class III (40 or greater). The investigators assessed 112,309 deliveries among 106,552 women (Obstet Gynecol. 2016;128:104-12. doi: 10.1097/AOG.0000000000001465).
Women with higher prepregnancy BMIs were at greater risk for several types of maternal and neonatal outcomes, according to the findings. For example, the relative risk for gestational diabetes mellitus, compared with women with normal BMIs, was 1.99 for overweight women, 2.94 for obese class I women, 3.97 for obese class II women, and 5.47 for obese class III women.
Similar findings were noted for other maternal outcomes, including higher risks for gestational hypertensive disorders, cesarean delivery, prelabor cesarean delivery, and acute cardiovascular events among women with higher BMIs.
Higher prepregnancy maternal BMI was also associated with increased neonatal risks, including preterm birth at less than 32 weeks of gestation, large for gestational age (LGA) neonates, transient tachypnea, sepsis, and neonatal intensive care unit admission.
The relative risk for LGA neonates, compared with women with normal BMIs, was 1.52 for overweight women, 1.74 for obese class I women, 1.93 for obese class II women, and 2.32 for obese class III women.
In an analysis of a composite variable that included all obstetric and neonatal complications with the exception of interventions, the researchers detected an 18%-47% increased risk of any pregnancy complication among overweight or obese women.
Even obese women who were otherwise healthy at the start of their pregnancy, did not develop pregnancy complications, and gained weight within recommended limits, still had an elevated risk for obstetric and neonatal complications, according to the researchers. “We found increased risks of relatively rare outcomes that other studies could not observe, including maternal acute cardiovascular events and neonatal transient tachypnea, necrotizing enterocolitis, peri- and intraventricular hemorrhage, and retinopathy of prematurity among deliveries to overweight or obese women,” they wrote.
The researchers received support for the work from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The authors reported having no potential conflicts of interest.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: For women without chronic diseases, prepregnancy obesity increases risks for adverse pregnancy and neonatal outcomes.
Major finding: The relative risk for gestational diabetes mellitus, compared with women with normal BMIs, was 1.99 for overweight women, 2.94 for obese class I women, 3.97 for obese class II women, and 5.47 for obese class III women.
Data sources: Maternal and neonatal medical records from 112,309 singleton deliveries from 2002-2008.
Disclosures: The researchers received support for the work from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The authors reported having no potential conflicts of interest.
Postpartum glycemic screening rates are low for women with GDM history
Most women with a history of gestational diabetes mellitus are not receiving recommended glycemic screenings in their first postpartum year. And screening rates vary based on geography, race, and use of antiglycemic medication in pregnancy, according to the results of a study published in Obstetrics & Gynecology.
Currently, the American College of Obstetricians and Gynecologists recommends screening all women with gestational diabetes mellitus (GDM) at 6-12 weeks postpartum with either fasting plasma glucose (FPG) or a 75-g 2-hour oral glucose tolerance test (OGTT). A hemoglobin A1c (HBA1c) is not recommended in the early postpartum period.
Dr. Emma Morton Eggleston of Harvard Pilgrim Health Care Institute in Boston and her colleagues performed a retrospective analysis of medical records from a large U.S. health plan database to determine the rates of glycemic screenings – 75-g OGTT, HBA1c only, FPG only, or HbA1c plus FPG – in women with a history of GDM who were enrolled in the health plan from 2000-2012.
Rates were also measured for specific geographic regions, races/ethnicities, and patient clinical characteristics, including comorbidity in or before pregnancy, a visit to a nutritionist or diabetes educator during pregnancy, a visit to an endocrinologist during pregnancy, and the use of any antiglycemic agent during pregnancy (Obstet Gynecol. 2016;128:159-67. doi: 10.1097/AOG.0000000000001467).
Of all 447,556 women continuously enrolled in the health plan for 1 year before and after delivery, 32,253 (7.2%) had a history of GDM. The majority of women (76.1%) did not receive any of the glycemic screening tests in their first postpartum year.
The rates of these recommended tests were found to be low in general, although improvements in rates were noted between 2001 and 2011 for all but FPG alone, which declined from 7% within 12 weeks postpartum to 2% (adjusted odds ratio, 0.2). Conversely, the rate of receiving a 75-g OGTT within 12 weeks postpartum increased from 3% to 8% (adjusted OR, 3.2).
Geography was a predictor of postpartum screening. Women who lived in the West were the most likely to receive any screening within 12 weeks (18%) and at 1 year (31%). Among those who were screened, women in the West were most likely to receive a 75-g OGTT within 12 weeks (36%), compared with women in the Northeast (19%) and South (18%).
Race also played a role. Black women were the least likely to receive a 75-g OGTT and the most likely to receive HbA1c alone, even though this group has the highest rates of conversion to type 2 diabetes.
The strongest predictor of screening was the use of antiglycemic medication during pregnancy, according to the study. Women on antiglycemic medication in pregnancy (21%) were twice as likely to receive any type of screening, compared with women who were not on medication. Women who saw a nutritionist or diabetes educator during pregnancy, as well as those who saw an endocrinologist, were also more likely to receive any type of glycemic screening.
“Whether at the level of health system or population, quality improvement efforts must identify effective means of postpartum screening that are feasible for both women and health care providers and based on risk factors rather than geography or disparities in care,” the researchers wrote.
The researchers received grant support from the National Institutes of Health and the Centers for Disease Control and Prevention. No potential conflicts of interest were reported.
Most women with a history of gestational diabetes mellitus are not receiving recommended glycemic screenings in their first postpartum year. And screening rates vary based on geography, race, and use of antiglycemic medication in pregnancy, according to the results of a study published in Obstetrics & Gynecology.
Currently, the American College of Obstetricians and Gynecologists recommends screening all women with gestational diabetes mellitus (GDM) at 6-12 weeks postpartum with either fasting plasma glucose (FPG) or a 75-g 2-hour oral glucose tolerance test (OGTT). A hemoglobin A1c (HBA1c) is not recommended in the early postpartum period.
Dr. Emma Morton Eggleston of Harvard Pilgrim Health Care Institute in Boston and her colleagues performed a retrospective analysis of medical records from a large U.S. health plan database to determine the rates of glycemic screenings – 75-g OGTT, HBA1c only, FPG only, or HbA1c plus FPG – in women with a history of GDM who were enrolled in the health plan from 2000-2012.
Rates were also measured for specific geographic regions, races/ethnicities, and patient clinical characteristics, including comorbidity in or before pregnancy, a visit to a nutritionist or diabetes educator during pregnancy, a visit to an endocrinologist during pregnancy, and the use of any antiglycemic agent during pregnancy (Obstet Gynecol. 2016;128:159-67. doi: 10.1097/AOG.0000000000001467).
Of all 447,556 women continuously enrolled in the health plan for 1 year before and after delivery, 32,253 (7.2%) had a history of GDM. The majority of women (76.1%) did not receive any of the glycemic screening tests in their first postpartum year.
The rates of these recommended tests were found to be low in general, although improvements in rates were noted between 2001 and 2011 for all but FPG alone, which declined from 7% within 12 weeks postpartum to 2% (adjusted odds ratio, 0.2). Conversely, the rate of receiving a 75-g OGTT within 12 weeks postpartum increased from 3% to 8% (adjusted OR, 3.2).
Geography was a predictor of postpartum screening. Women who lived in the West were the most likely to receive any screening within 12 weeks (18%) and at 1 year (31%). Among those who were screened, women in the West were most likely to receive a 75-g OGTT within 12 weeks (36%), compared with women in the Northeast (19%) and South (18%).
Race also played a role. Black women were the least likely to receive a 75-g OGTT and the most likely to receive HbA1c alone, even though this group has the highest rates of conversion to type 2 diabetes.
The strongest predictor of screening was the use of antiglycemic medication during pregnancy, according to the study. Women on antiglycemic medication in pregnancy (21%) were twice as likely to receive any type of screening, compared with women who were not on medication. Women who saw a nutritionist or diabetes educator during pregnancy, as well as those who saw an endocrinologist, were also more likely to receive any type of glycemic screening.
“Whether at the level of health system or population, quality improvement efforts must identify effective means of postpartum screening that are feasible for both women and health care providers and based on risk factors rather than geography or disparities in care,” the researchers wrote.
The researchers received grant support from the National Institutes of Health and the Centers for Disease Control and Prevention. No potential conflicts of interest were reported.
Most women with a history of gestational diabetes mellitus are not receiving recommended glycemic screenings in their first postpartum year. And screening rates vary based on geography, race, and use of antiglycemic medication in pregnancy, according to the results of a study published in Obstetrics & Gynecology.
Currently, the American College of Obstetricians and Gynecologists recommends screening all women with gestational diabetes mellitus (GDM) at 6-12 weeks postpartum with either fasting plasma glucose (FPG) or a 75-g 2-hour oral glucose tolerance test (OGTT). A hemoglobin A1c (HBA1c) is not recommended in the early postpartum period.
Dr. Emma Morton Eggleston of Harvard Pilgrim Health Care Institute in Boston and her colleagues performed a retrospective analysis of medical records from a large U.S. health plan database to determine the rates of glycemic screenings – 75-g OGTT, HBA1c only, FPG only, or HbA1c plus FPG – in women with a history of GDM who were enrolled in the health plan from 2000-2012.
Rates were also measured for specific geographic regions, races/ethnicities, and patient clinical characteristics, including comorbidity in or before pregnancy, a visit to a nutritionist or diabetes educator during pregnancy, a visit to an endocrinologist during pregnancy, and the use of any antiglycemic agent during pregnancy (Obstet Gynecol. 2016;128:159-67. doi: 10.1097/AOG.0000000000001467).
Of all 447,556 women continuously enrolled in the health plan for 1 year before and after delivery, 32,253 (7.2%) had a history of GDM. The majority of women (76.1%) did not receive any of the glycemic screening tests in their first postpartum year.
The rates of these recommended tests were found to be low in general, although improvements in rates were noted between 2001 and 2011 for all but FPG alone, which declined from 7% within 12 weeks postpartum to 2% (adjusted odds ratio, 0.2). Conversely, the rate of receiving a 75-g OGTT within 12 weeks postpartum increased from 3% to 8% (adjusted OR, 3.2).
Geography was a predictor of postpartum screening. Women who lived in the West were the most likely to receive any screening within 12 weeks (18%) and at 1 year (31%). Among those who were screened, women in the West were most likely to receive a 75-g OGTT within 12 weeks (36%), compared with women in the Northeast (19%) and South (18%).
Race also played a role. Black women were the least likely to receive a 75-g OGTT and the most likely to receive HbA1c alone, even though this group has the highest rates of conversion to type 2 diabetes.
The strongest predictor of screening was the use of antiglycemic medication during pregnancy, according to the study. Women on antiglycemic medication in pregnancy (21%) were twice as likely to receive any type of screening, compared with women who were not on medication. Women who saw a nutritionist or diabetes educator during pregnancy, as well as those who saw an endocrinologist, were also more likely to receive any type of glycemic screening.
“Whether at the level of health system or population, quality improvement efforts must identify effective means of postpartum screening that are feasible for both women and health care providers and based on risk factors rather than geography or disparities in care,” the researchers wrote.
The researchers received grant support from the National Institutes of Health and the Centers for Disease Control and Prevention. No potential conflicts of interest were reported.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Glycemic screening rates for women who had gestational diabetes mellitus are generally low and vary based on race, geography, and medication treatment in pregnancy.
Major finding: More than three-quarters of women with a history of gestational diabetes mellitus did not receive a glycemic screen in their first year postpartum.
Data sources: A retrospective analysis of medical records from women enrolled in a large, U.S. commercial health plan from 2000-2012.
Disclosures: The researchers received grant support from the National Institutes of Health and the Centers for Disease Control and Prevention. No potential conflicts of interest were reported.
2016 Update on infectious disease
Chlorhexidine-alcohol is superior to iodine-alcohol for reducing SSIs after cesarean deliveryTuuli Mg, Liu J, Stout Mj, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647-655.
In the United States, cesarean delivery is the most commonly performed major surgical procedure, with 32.7% of births--or 1.3 million--occurring in this fashion in 2013.1,2 In general, for all surgical procedures, the SSI rate is 2% to 5%, with the rate rising to 5% to 12% for cesarean delivery, especially in obese patients.3-6 Not only do SSIs increase morbidity for the patient but they also contribute to high medical costs, with an estimated additional expense of $3,529 per cesarean-associated infection.7
Skin pathogens are a major source of SSIs. Choosing the proper antiseptic has the potential to decrease infection risk. While current guidelines recommend use of an antiseptic containing alcohol, it is unclear which disinfectant is the most effective agent to combine with the alcohol.3
Most trials evaluating preoperative antiseptic skin preparation have studied patients undergoing general surgery procedures. A well-designed trial by Darouiche and coauthors demonstrated that chlorhexidine was superior to iodine when used as an antiseptic for skin preparation.8 Interestingly, however, this trial, like most others, compared chlorhexidine-alcohol to iodine without alcohol. It is therefore unclear whether the chlorhexidine or the alcohol is responsible for the enhanced antiseptic effect.
Details of the studyIn the single-center randomized trial conducted by Tuuli and colleagues, patients were assigned to preoperative skin antisepsis with either chlorhexidine-alcohol (2% chlorhexidine gluconate with 70% isopropyl alcohol) or iodine-alcohol (8.3% povidone-iodine with 72.5% isopropyl alcohol). Antiseptic was applied according to the manufacturer's instructions, with a standard wait time of 3 minutes between application and skin incision. However, wait time was eliminated for patients undergoing emergency cesarean delivery. Additionally, patients received standard, weight-based preoperative antibiotic prophylaxis (agent not specified).
The authors estimated the necessary sample size for the trial by assuming an 8% baseline SSI rate and an anticipated 50% reduction of infection in the chlorhexidine-alcohol group. Exclusion criteria included a known allergy to chlorhexidine, alcohol, iodine, or shellfish or a preexisting skin infection adjacent to the operative site.
In addition to assessing the primary outcome of SSI with the 2 preparations, the authors conducted 4 prespecified subgroup analyses. These subgroups were based on: type of cesarean delivery (scheduled vs unscheduled), body weight (obese vs nonobese), type of skin closure (subcuticular suture vs staples), and presence or absence of chronic medical conditions (diabetes, hypertension, renal disease). Additionally, a post hoc analysis was performed, comparing women with diabetes (gestational and pregestational) to those without diabetes.
A total of 1,636 pregnant women were screened for eligibility. Of these, 489 women were excluded because they did not meet inclusion criteria or declined to participate or because informed consent could not be obtained. Baseline characteristics were similar across both groups.
Patients were followed for 30 days after surgery. A total of 1,082 women (94.3% of sample size) completed the follow-up. Among these patients, the rate of SSI was significantly lower in the chlorhexidine-alcohol group (4.3%) compared with the iodine-alcohol group (7.7%, P = .02).
In the subgroup analyses, the frequency of SSI remained lower for the chlorhexidine-alcohol group than for the iodine-alcohol group. These reductions were not affected by whether the cesarean was scheduled or unscheduled, the presence or absence of obesity, the type of skin closure, the presence of chronic disease, or diabetes status.
Several secondary outcomes also were examined in this study. There were no significant differences between the 2 antiseptic groups with respect to rates of endometritis, hospital readmission for infection-related complications, length of hospital stay, use of other health care services (such as emergency department visits, additional wound surgery, and home health services), and rates of other wound complications (seroma, hematoma, and cellulitis). Patients in the chlorhexidine-alcohol group were significantly less likely than those in the iodine-alcohol group to have physician office visits for concerns about possible wound complications (P = .009).
The authors concluded that the use of chlorhexidine-alcohol was superior to iodine-alcohol in preventing SSI after cesarean delivery.
Study strengths and limitations The authors acknowledged that their study had some minor limitations. First, the trial was conducted at a single site, which may limit the generalizability of the findings. However, the study population was racially and economically diverse. Second, the lack of blinding among providers and participants may have introduced bias, although, as the authors explain, we would expect this bias to be largely nondirectional.
A major strength of this study is its randomized design. Another strength is that the authors included emergency cesarean deliveries in their analysis. Emergency procedures represent a substantial proportion of cesarean deliveries, and they place the patient at increased risk for SSIs because of limited time available to prepare the skin before surgery begins. Thus, it is of great interest that chlorhexidine-alcohol was so effective even in the highest-risk patients.
Several properties may make chlorhexidine superior to iodine as an antiseptic: high binding affinity for the skin, high antibacterial activity against both gram-positive and gram-negative bacteria, and longer residual effects than iodine. Additionally, iodine is inactivated by organic matter, such as body fluids, whereas chlorhexidine is not.
A recent study by Ngai and colleagues9 compared chlorhexidine-alcohol with iodine-alcohol for skin preparation before cesarean delivery. These authors found no difference in SSI when comparing the 2 solutions used separately or sequentially, except in morbidly obese women. In these women, sequential application of both solutions reduced the infection rate. However, this study specifically excluded emergency cesarean deliveries, making the generalizability of the results questionable.9
What this evidence means for practiceThis large, randomized study found chlorhexidine-alcohol to be superior to iodine-alchol in reducing the risk of SSIs after cesarean delivery. These results confirm those of previous studies from both the obstetric and general surgery literature. Although chlorhexidine-alcohol is more expensive than iodine-alcohol, we strongly recommend its use in patients having cesarean delivery.
Five effective oral and intramuscular antibiotic regimens for treating postpartum endometritisMeaney-Delman D, Bartlett LA, Gravett MG, Jamieson DJ. Oral and intramuscular treatment options for early postpartum endometritis in low-resource settings: a systematic review. Obstet Gynecol. 2015;125(4):789-800.
The authors of this excellent systematic review on antibiotic treatments for early postpartum endometritis conducted their study in 3 phases. Initially, Meaney-Delman and colleagues searched the literature for reports of prospective studies that evaluated the use of oral and intramuscular (IM) antibiotics for treatment of patients who developed endometritis following either cesarean or vaginal delivery. When they discovered that these initial trials were few in number and of relatively poor quality, they reviewed more rigorous trials of intravenous (IV) antibiotics. Finally, they evaluated clinical trials that specifically identified microorganisms isolated from the uterus in patients with endometritis and used this information to help inform their recommendations for treatment options.
Details of the studyIn evaluating the trials of oral and IM antibiotics, the authors set as a standard for effectiveness a cure rate of 85%, a figure comparable to that generally achieved with IV antibiotics. They identified 2 oral antibiotic regimens that met this standard of effectiveness: amoxicillin-clavulanate (100% cure in 36 patients; 95% confidence interval [CI], 90-100) and ampicillin plus metronidazole (97% cure in 37 patients; 95% CI, 86-100).
Two studies demonstrated acceptable levels of cure with single-agent IM antibiotics: aztreonam (100% cure in 16 patients; 95% CI, 81-100) and imipenem (91% cure in 23 patients; 95% CI, 73-98). One additional trial demonstrated an acceptable clinical response rate when IV clindamycin was combined with IM gentamicin (100% cure in 54 patients; 95% CI, 94-100). By contrast, the authors noted, many different IV regimens--either as a single agent or as a drug combination--provided cure rates that equaled or exceeded 85%.
In the study's final phase, the authors provided an excellent overview of the polymicrobial nature of puerperal endometritis. As documented in multiple prior reports, the most common pathogens are the gram-negative anaerobic bacilli, such as Bacteroides and Prevotella species; the anaerobic gram-positive organisms, including Peptococcus and Peptostreptococcus species; aerobic gram-negative bacilli, such as Escherichia coli, Klebsiella pneumoniae, and Proteus species; and aerobic gram-positive cocci, such as group B streptococci, enterococci, and staphylococci.
Recommended regimens. Based on their review of clinical and microbiological studies, the authors proposed 5 oral or combined oral-IM treatment regimens that could be used in low-resource settings:
- oral clindamycin (600 mg every 6 hours)
- plus IM gentamicin (4.5 g every 24 hours)
- oral amoxicillin-clavulanic acid (875 mg every 12 hours)
- IM cefotetan (2 g every 8 hours)
- IM meropenem or imipenem-cilastatin (500 mg every 8 hours)
- oral amoxicillin (500 mg every 8 hours) plus oral metronidazole (500 mg every 8 hours).
Typical endometritis treamentEndometritis is the single most common complication following cesarean delivery. The frequency of its occurrence depends on several factors, including: the socioeconomic characteristics of the patient population, length of labor, length of ruptured membranes, number of internal vaginal examinations, presence of preexisting lower genital tract infection, type of anesthesia, surgical technique, and use of prophylactic antibiotics. Endometritis is much less common after vaginal delivery but still may occur in 3% to 5% of patients.10
Endometritis is clearly a polymicrobial infection that includes multiple aerobic and anaerobic organisms. Accordingly, antibiotic therapy must target all the major groups of pathogens. The usual standard of care for treatment of early-onset endometritis is IV antibiotics, and patients typically are treated until they have been afebrile and asymptomatic for a minimum of 24 hours. Several different IV regimens provide acceptable treatment10:
- clindamycin plus gentamicin
- metronidazole plus ampicillin plus gentamicin
- extended-spectrum cephalosporins, such as cefepime, cefotetan, and cefoxitin
- extended-spectrum penicillins, such as ampicillin-sulbactam, piperacillin- tazobactam, and ticarcillin-clavulanic acid
- carbapenems, such as imipenem-cilastatin and meropenem.
What this evidence means for practiceClearly, IV antibiotics, even generic drugs, are more expensive than oral agents. They also are more difficult to administer than oral or IM drugs. The systematic review by Meaney-Delman and co-workers is therefore a very important contribution to the literature and should reassure clinicians practicing in low-resource settings that oral and oral-IM regimens can provide safe and effective treatment for endometritis. Until more rigorous comparative trials are conducted, however, we agree with the authors' caveat that, for now, such treatment should be limited to individuals whose infection occurred after vaginal delivery or who have evidence of only mild postcesarean endometritis.
Treatment options for chlamydia infection: How does azithromycin compare with doxycycline?Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373(26):2512-2521.
The Centers for Disease Control and Prevention recommendations for treatment of chlamydia genital tract infection are either oral doxycycline, 100 mg twice daily for 7 days, or azithromycin, 1,000 mg in a single dose.11 Recent reports have raised questions about the relative effectiveness of single-dose azithromycin compared with the multiple-day doxycycline regimen. Accordingly, Geisler and colleagues conducted an interesting randomized controlled trial to determine if azithromycin is noninferior to doxycycline.
Details of the studyThe study took place in a unique institutional setting--the Los Angeles County youth correctional facilities. Participants were young men and women, aged 12 to 21 years, who tested positive for chlamydia infection by a nucleic acid amplification test on entry to the correctional facility. Participants then were randomly assigned to receive either doxycycline or azithromycin in the doses described above. The primary outcome was the percent of individuals who still tested positive for chlamydia 28 days after treatment.
Of note, all patients took their medication under direct observation of corrections officers and, with rare exceptions, did not engage in sexual activity during the period of observation. Because this was a noninferiority trial, Geisler and colleagues analyzed the outcomes only of the individuals who actually took their medication in accordance with the assigned protocol. A priori, the authors established a 95% CI of <5% difference in effectiveness as indicative of noninferiority.
Overall, 155 patients in each treatment group completed the trial according to the assigned protocol. No treatment failures occurred in the doxycycline group (0%; 95% CI, 0.0-2.4). Five treatment failures occurred in the azithromycin group (3.2%; 95% CI, 0.4-7.4), in 1 female and 4 male participants. Because the 95% CI for the difference in treatment outcome exceeded 5%, the authors were unable to conclude that azithromycin was noninferior to doxycycline.
Consider real-world treatment adherence in these resultsFor several reasons, we do not conclude from this article that ObGyns should now stop using azithromycin to treat patients with chlamydia infection. First, the actual per protocol sample size was still relatively small. If there had been just 2 fewer failures in the azithromycin group, the 95% CI for the difference in outcomes would have been less than 5%, and the authors would have concluded that the 2 drug regimens were noninferior. Second, 4 of the 5 treatment failures in the azithromycin group were in male rather than female participants. Third, the unique study design resulted in almost perfect adherence with the 7-day doxycycline treatment regimen. Such adherence is very unlikely in other practice settings, and patients who do not complete their treatment regimen are significantly more likely to fail therapy. Finally, azithromycin is definitely preferred in pregnancy because we try to avoid maternal/fetal exposure to drugs such as tetracycline and doxycycline.
What this evidence means for practiceIn this study, both doxycycline and azithromycin were highly effective (100% and 97%, respectively) for treating chlamydia genital tract infection, and they are comparable in cost. In our opinion, the improved adherence that is possible with single-dose azithromycin, the greater safety in pregnancy, and the excellent tolerability of this drug outweigh its slightly deceased rate of microbiologic cure.
Vaccine effective against hepatitis E for 4+ yearsZhang J, Zhang XF, Huang SJ, et al. Long-term efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372(10):914-922.
This study conducted by Zhang and colleagues in Dongtai, China, is an extended follow-up study of the hepatitis E virus (HEV) vaccine (Hecolin; Xiamen Innovax Biotech). A recombinant vaccine directed against HEV genotype 1, Hecolin has been used in China since 2012.
In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine (vaccine group, 56,302 participants) or the hepatitis B vaccine (control group, 56,302 participants). Vaccine administration occurred at 0, 1, and 6 months, and participants were followed for a total of 19 months.
Details of the studyThe follow-up study was designed to assess the efficacy, immunogenicity, and safety of the HEV vaccine up to 4.5 years postvaccination. All health care centers (205 village and private clinics) in the study area were enrolled in the program. The treatment assignments of all patients remained double blinded. Unblinding occurred only after the data on safety, efficacy, and immunogenicity had been locked.
A diagnosis of HEV infection was made if at least 2 of the following markers were present: a positive test for immunoglobulin M antibodies against HEV, a positive test for HEV RNA, or a serum concentration of immunoglobulin G (IgG) antibodies against HEV that was at least 4 times higher than previously measured at any time during the same illness. Vaccine immunogenicity was assessed by testing serum samples for IgG antibodies against HEV at regular intervals after the vaccination was given.
Over the 4.5-year study period, 7 cases of hepatitis E occurred in the vaccine group, and 53 in the control group. Vaccine efficacy was 86.8% (P<.001) in the modified intention-to-treat analysis. Among patients who received 3 doses of HEV vaccine and who were seronegative at the start of the study, 87% maintained antibodies against HEV for 4.5 years. Within the control group, HEV titers developed in 9% of participants. The vaccine and control groups had similar rates of adverse events.
The authors concluded that the HEV vaccine induced antibodies against hepatitis E that lasted up to 4.5 years. Additionally, 2 doses of vaccine induced slightly lower levels of antibody than those produced by 3 doses of the vaccine. Finally, all participants in the vaccine group who developed HEV had antibodies with high or moderate avidity, indicating an anamnestic response from previous immunity. Most participants in the control group who developed HEV, however, had antibodies with low avidity, indicating no previous immunity.
The burden of HEVHepatitis E is a serious infection and is the most common waterborne illness in the world. It occurs mainly in developing countries with limited resources. HEV infection is caused by genotypes 1, 2, 3, or 4, although all 4 genotypes belong to the same serotype. Genotypes 1 and 2 are typically waterborne, and genotypes 3 and 4 are typically transmitted from animals and humans. In general, the case fatality rate associated with HEV infection is 1% to 3%.12 In pregnancy, this rate increases to 5% to 25%.13,14 In Bangladesh, for example, hepatitis E is responsible for more than 1,000 deaths per year among pregnant women.15
Clinical presentation of HEV infection is a spectrum, with most symptomatic patients presenting with acute, self-limited hepatitis. Severe cases may be associated with pancreatitis, arthritis, aplastic anemia, and neurologic complications, such as seizures. Populations at risk for more severe cases include pregnant women, elderly men, and patients with pre‑ existing, chronic liver disease.
What this evidence means for practiceStandard sanitary precautions, such as clean drinking water, traditionally have been considered the mainstay of hepatitis E prevention. However, as the study authors indicate, recent severe outbreaks of HEV infection in Sudan and Uganda have occurred despite these measures. Thus, an effective vaccine that produces long-standing immunity has great potential for reducing morbidity and mortality in these countries. The present vaccine appears to be highly effective and safe. The principal unanswered question is the duration of immunity.
My patients are asking, "What is the best insect repellent to try to avoid Zika virus?"
With summer upon us we have received questions from colleagues about the best over-the-counter insect repellents to advise their pregnant patients to use.
The preferred insect repellent for skin coverage is DEET (N,N-diethyl-meta-toluamide) (TABLE). Oil of lemon/eucalyptus/para-menthane-diol and IR3535 are also acceptable repellents to use on the skin that are safe for use in pregnancy. In addition, instruct patients to spray permethrin on their clothing or to buy clothing (boots, pants, socks) that has been pretreated with permethrin.1,2
Anushka Chelliah, MD, and Patrick Duff, MD.
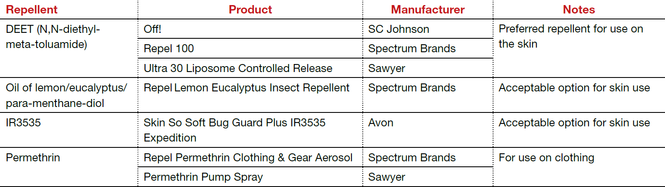
Abbreviation: OTC, over the counter.
Coming soon to OBG Management
Drs. Chelliah and Duff follow-up on their March 2016 examination of Zika virus infection with:
- Latest information on Zika virus-associated birth defects
- Ultrasonographic and radiologic evidence of abnormalities in the fetus and newborn exposed to Zika virus infection
- Link between Zika virus infection and serious neurologic complications in adults
- New recommendations for preventing sexual transmission of Zika virus infection
Dr. Chelliah is a Maternal Fetal Medicine-Fellow in the Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, University of Florida College of Medicine, Gainesville.
Dr. Duff is Associate Dean for Student Affairs and Professor of Obstetrics and Gynecology in the Division of Maternal-Fetal Medicine, University of Florida College of Medicine.
The author reports no financial relationships relevant to this article.
References
- Peterson EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak--United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30-33.
- Centers for Disease Control and Prevention. CDC Features: Avoid mosquito bites. http://www.cdc.gov/Features/stopmosquitoes/index.html. Updated March 18, 2016. Accessed May 10, 2016.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2007:165:1–209.
- Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65.
- Anderson DJ, Podgorny K, Berrios-Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):605–627.
- Conroy K, Koenig AF, Yu YH, Courtney A, Lee HJ, Norwitz ER. Infectious morbidity after cesarean delivery: 10 strategies to reduce risk. Rev Obstet Gynecol. 2012;5(2):69–77.
- Scifres CM, Leighton BL, Fogertey PJ, Macones GA, Stamilio DM. Supplemental oxygen for the prevention of postcesarean infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2011;205(3)267.e1–e9.
- Wloch C, Wilson J, Lamagni T, Harrington P, Charlett A, Sheridan E. Risk factors for surgical site infection following cesarean section in England: results from a multicenter cohort study. BJOG. 2012;119(11):1324–1333.
- Olsen MA, Butler AM, Willers DM, Gross GA, Hamilton BH, Fraser VJ. Attributable costs of surgical site infection and endometritis after low transverse cesarean delivery. Infect Control Hosp Epidemiol. 2010;31(3):276–282.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;126(6):1251–1257.
- Duff P. Maternal and perinatal infection—bacterial. In: Gabbe SG, Niebyl JR, Simpson JL, et al, eds. Obstetrics: normal and problem pregnancies. 6th ed. Philadelphia, PA: Elsevier/Saunders; 2012.
- Workowski KA, Bolan GH; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1−137. Erratum in: MMWR Recomm Rep. 2015;64(33):924.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13(3):145–154.
- Khuroo MS, Teli MR, Skidmore S, Sofi MA, Khuroo MI. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70(2):252–255.
- Khuroo MS, Kamili S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J Viral Hepat. 2003;10(1):61–69.
- Labrique AB, Sikder SS, Krain IJ, et al. Hepatitis E, a vaccine-preventable cause of maternal deaths. Emerg Infect Dis. 2012;18(9):1401–1404.
Chlorhexidine-alcohol is superior to iodine-alcohol for reducing SSIs after cesarean deliveryTuuli Mg, Liu J, Stout Mj, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647-655.
In the United States, cesarean delivery is the most commonly performed major surgical procedure, with 32.7% of births--or 1.3 million--occurring in this fashion in 2013.1,2 In general, for all surgical procedures, the SSI rate is 2% to 5%, with the rate rising to 5% to 12% for cesarean delivery, especially in obese patients.3-6 Not only do SSIs increase morbidity for the patient but they also contribute to high medical costs, with an estimated additional expense of $3,529 per cesarean-associated infection.7
Skin pathogens are a major source of SSIs. Choosing the proper antiseptic has the potential to decrease infection risk. While current guidelines recommend use of an antiseptic containing alcohol, it is unclear which disinfectant is the most effective agent to combine with the alcohol.3
Most trials evaluating preoperative antiseptic skin preparation have studied patients undergoing general surgery procedures. A well-designed trial by Darouiche and coauthors demonstrated that chlorhexidine was superior to iodine when used as an antiseptic for skin preparation.8 Interestingly, however, this trial, like most others, compared chlorhexidine-alcohol to iodine without alcohol. It is therefore unclear whether the chlorhexidine or the alcohol is responsible for the enhanced antiseptic effect.
Details of the studyIn the single-center randomized trial conducted by Tuuli and colleagues, patients were assigned to preoperative skin antisepsis with either chlorhexidine-alcohol (2% chlorhexidine gluconate with 70% isopropyl alcohol) or iodine-alcohol (8.3% povidone-iodine with 72.5% isopropyl alcohol). Antiseptic was applied according to the manufacturer's instructions, with a standard wait time of 3 minutes between application and skin incision. However, wait time was eliminated for patients undergoing emergency cesarean delivery. Additionally, patients received standard, weight-based preoperative antibiotic prophylaxis (agent not specified).
The authors estimated the necessary sample size for the trial by assuming an 8% baseline SSI rate and an anticipated 50% reduction of infection in the chlorhexidine-alcohol group. Exclusion criteria included a known allergy to chlorhexidine, alcohol, iodine, or shellfish or a preexisting skin infection adjacent to the operative site.
In addition to assessing the primary outcome of SSI with the 2 preparations, the authors conducted 4 prespecified subgroup analyses. These subgroups were based on: type of cesarean delivery (scheduled vs unscheduled), body weight (obese vs nonobese), type of skin closure (subcuticular suture vs staples), and presence or absence of chronic medical conditions (diabetes, hypertension, renal disease). Additionally, a post hoc analysis was performed, comparing women with diabetes (gestational and pregestational) to those without diabetes.
A total of 1,636 pregnant women were screened for eligibility. Of these, 489 women were excluded because they did not meet inclusion criteria or declined to participate or because informed consent could not be obtained. Baseline characteristics were similar across both groups.
Patients were followed for 30 days after surgery. A total of 1,082 women (94.3% of sample size) completed the follow-up. Among these patients, the rate of SSI was significantly lower in the chlorhexidine-alcohol group (4.3%) compared with the iodine-alcohol group (7.7%, P = .02).
In the subgroup analyses, the frequency of SSI remained lower for the chlorhexidine-alcohol group than for the iodine-alcohol group. These reductions were not affected by whether the cesarean was scheduled or unscheduled, the presence or absence of obesity, the type of skin closure, the presence of chronic disease, or diabetes status.
Several secondary outcomes also were examined in this study. There were no significant differences between the 2 antiseptic groups with respect to rates of endometritis, hospital readmission for infection-related complications, length of hospital stay, use of other health care services (such as emergency department visits, additional wound surgery, and home health services), and rates of other wound complications (seroma, hematoma, and cellulitis). Patients in the chlorhexidine-alcohol group were significantly less likely than those in the iodine-alcohol group to have physician office visits for concerns about possible wound complications (P = .009).
The authors concluded that the use of chlorhexidine-alcohol was superior to iodine-alcohol in preventing SSI after cesarean delivery.
Study strengths and limitations The authors acknowledged that their study had some minor limitations. First, the trial was conducted at a single site, which may limit the generalizability of the findings. However, the study population was racially and economically diverse. Second, the lack of blinding among providers and participants may have introduced bias, although, as the authors explain, we would expect this bias to be largely nondirectional.
A major strength of this study is its randomized design. Another strength is that the authors included emergency cesarean deliveries in their analysis. Emergency procedures represent a substantial proportion of cesarean deliveries, and they place the patient at increased risk for SSIs because of limited time available to prepare the skin before surgery begins. Thus, it is of great interest that chlorhexidine-alcohol was so effective even in the highest-risk patients.
Several properties may make chlorhexidine superior to iodine as an antiseptic: high binding affinity for the skin, high antibacterial activity against both gram-positive and gram-negative bacteria, and longer residual effects than iodine. Additionally, iodine is inactivated by organic matter, such as body fluids, whereas chlorhexidine is not.
A recent study by Ngai and colleagues9 compared chlorhexidine-alcohol with iodine-alcohol for skin preparation before cesarean delivery. These authors found no difference in SSI when comparing the 2 solutions used separately or sequentially, except in morbidly obese women. In these women, sequential application of both solutions reduced the infection rate. However, this study specifically excluded emergency cesarean deliveries, making the generalizability of the results questionable.9
What this evidence means for practiceThis large, randomized study found chlorhexidine-alcohol to be superior to iodine-alchol in reducing the risk of SSIs after cesarean delivery. These results confirm those of previous studies from both the obstetric and general surgery literature. Although chlorhexidine-alcohol is more expensive than iodine-alcohol, we strongly recommend its use in patients having cesarean delivery.
Five effective oral and intramuscular antibiotic regimens for treating postpartum endometritisMeaney-Delman D, Bartlett LA, Gravett MG, Jamieson DJ. Oral and intramuscular treatment options for early postpartum endometritis in low-resource settings: a systematic review. Obstet Gynecol. 2015;125(4):789-800.
The authors of this excellent systematic review on antibiotic treatments for early postpartum endometritis conducted their study in 3 phases. Initially, Meaney-Delman and colleagues searched the literature for reports of prospective studies that evaluated the use of oral and intramuscular (IM) antibiotics for treatment of patients who developed endometritis following either cesarean or vaginal delivery. When they discovered that these initial trials were few in number and of relatively poor quality, they reviewed more rigorous trials of intravenous (IV) antibiotics. Finally, they evaluated clinical trials that specifically identified microorganisms isolated from the uterus in patients with endometritis and used this information to help inform their recommendations for treatment options.
Details of the studyIn evaluating the trials of oral and IM antibiotics, the authors set as a standard for effectiveness a cure rate of 85%, a figure comparable to that generally achieved with IV antibiotics. They identified 2 oral antibiotic regimens that met this standard of effectiveness: amoxicillin-clavulanate (100% cure in 36 patients; 95% confidence interval [CI], 90-100) and ampicillin plus metronidazole (97% cure in 37 patients; 95% CI, 86-100).
Two studies demonstrated acceptable levels of cure with single-agent IM antibiotics: aztreonam (100% cure in 16 patients; 95% CI, 81-100) and imipenem (91% cure in 23 patients; 95% CI, 73-98). One additional trial demonstrated an acceptable clinical response rate when IV clindamycin was combined with IM gentamicin (100% cure in 54 patients; 95% CI, 94-100). By contrast, the authors noted, many different IV regimens--either as a single agent or as a drug combination--provided cure rates that equaled or exceeded 85%.
In the study's final phase, the authors provided an excellent overview of the polymicrobial nature of puerperal endometritis. As documented in multiple prior reports, the most common pathogens are the gram-negative anaerobic bacilli, such as Bacteroides and Prevotella species; the anaerobic gram-positive organisms, including Peptococcus and Peptostreptococcus species; aerobic gram-negative bacilli, such as Escherichia coli, Klebsiella pneumoniae, and Proteus species; and aerobic gram-positive cocci, such as group B streptococci, enterococci, and staphylococci.
Recommended regimens. Based on their review of clinical and microbiological studies, the authors proposed 5 oral or combined oral-IM treatment regimens that could be used in low-resource settings:
- oral clindamycin (600 mg every 6 hours)
- plus IM gentamicin (4.5 g every 24 hours)
- oral amoxicillin-clavulanic acid (875 mg every 12 hours)
- IM cefotetan (2 g every 8 hours)
- IM meropenem or imipenem-cilastatin (500 mg every 8 hours)
- oral amoxicillin (500 mg every 8 hours) plus oral metronidazole (500 mg every 8 hours).
Typical endometritis treamentEndometritis is the single most common complication following cesarean delivery. The frequency of its occurrence depends on several factors, including: the socioeconomic characteristics of the patient population, length of labor, length of ruptured membranes, number of internal vaginal examinations, presence of preexisting lower genital tract infection, type of anesthesia, surgical technique, and use of prophylactic antibiotics. Endometritis is much less common after vaginal delivery but still may occur in 3% to 5% of patients.10
Endometritis is clearly a polymicrobial infection that includes multiple aerobic and anaerobic organisms. Accordingly, antibiotic therapy must target all the major groups of pathogens. The usual standard of care for treatment of early-onset endometritis is IV antibiotics, and patients typically are treated until they have been afebrile and asymptomatic for a minimum of 24 hours. Several different IV regimens provide acceptable treatment10:
- clindamycin plus gentamicin
- metronidazole plus ampicillin plus gentamicin
- extended-spectrum cephalosporins, such as cefepime, cefotetan, and cefoxitin
- extended-spectrum penicillins, such as ampicillin-sulbactam, piperacillin- tazobactam, and ticarcillin-clavulanic acid
- carbapenems, such as imipenem-cilastatin and meropenem.
What this evidence means for practiceClearly, IV antibiotics, even generic drugs, are more expensive than oral agents. They also are more difficult to administer than oral or IM drugs. The systematic review by Meaney-Delman and co-workers is therefore a very important contribution to the literature and should reassure clinicians practicing in low-resource settings that oral and oral-IM regimens can provide safe and effective treatment for endometritis. Until more rigorous comparative trials are conducted, however, we agree with the authors' caveat that, for now, such treatment should be limited to individuals whose infection occurred after vaginal delivery or who have evidence of only mild postcesarean endometritis.
Treatment options for chlamydia infection: How does azithromycin compare with doxycycline?Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373(26):2512-2521.
The Centers for Disease Control and Prevention recommendations for treatment of chlamydia genital tract infection are either oral doxycycline, 100 mg twice daily for 7 days, or azithromycin, 1,000 mg in a single dose.11 Recent reports have raised questions about the relative effectiveness of single-dose azithromycin compared with the multiple-day doxycycline regimen. Accordingly, Geisler and colleagues conducted an interesting randomized controlled trial to determine if azithromycin is noninferior to doxycycline.
Details of the studyThe study took place in a unique institutional setting--the Los Angeles County youth correctional facilities. Participants were young men and women, aged 12 to 21 years, who tested positive for chlamydia infection by a nucleic acid amplification test on entry to the correctional facility. Participants then were randomly assigned to receive either doxycycline or azithromycin in the doses described above. The primary outcome was the percent of individuals who still tested positive for chlamydia 28 days after treatment.
Of note, all patients took their medication under direct observation of corrections officers and, with rare exceptions, did not engage in sexual activity during the period of observation. Because this was a noninferiority trial, Geisler and colleagues analyzed the outcomes only of the individuals who actually took their medication in accordance with the assigned protocol. A priori, the authors established a 95% CI of <5% difference in effectiveness as indicative of noninferiority.
Overall, 155 patients in each treatment group completed the trial according to the assigned protocol. No treatment failures occurred in the doxycycline group (0%; 95% CI, 0.0-2.4). Five treatment failures occurred in the azithromycin group (3.2%; 95% CI, 0.4-7.4), in 1 female and 4 male participants. Because the 95% CI for the difference in treatment outcome exceeded 5%, the authors were unable to conclude that azithromycin was noninferior to doxycycline.
Consider real-world treatment adherence in these resultsFor several reasons, we do not conclude from this article that ObGyns should now stop using azithromycin to treat patients with chlamydia infection. First, the actual per protocol sample size was still relatively small. If there had been just 2 fewer failures in the azithromycin group, the 95% CI for the difference in outcomes would have been less than 5%, and the authors would have concluded that the 2 drug regimens were noninferior. Second, 4 of the 5 treatment failures in the azithromycin group were in male rather than female participants. Third, the unique study design resulted in almost perfect adherence with the 7-day doxycycline treatment regimen. Such adherence is very unlikely in other practice settings, and patients who do not complete their treatment regimen are significantly more likely to fail therapy. Finally, azithromycin is definitely preferred in pregnancy because we try to avoid maternal/fetal exposure to drugs such as tetracycline and doxycycline.
What this evidence means for practiceIn this study, both doxycycline and azithromycin were highly effective (100% and 97%, respectively) for treating chlamydia genital tract infection, and they are comparable in cost. In our opinion, the improved adherence that is possible with single-dose azithromycin, the greater safety in pregnancy, and the excellent tolerability of this drug outweigh its slightly deceased rate of microbiologic cure.
Vaccine effective against hepatitis E for 4+ yearsZhang J, Zhang XF, Huang SJ, et al. Long-term efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372(10):914-922.
This study conducted by Zhang and colleagues in Dongtai, China, is an extended follow-up study of the hepatitis E virus (HEV) vaccine (Hecolin; Xiamen Innovax Biotech). A recombinant vaccine directed against HEV genotype 1, Hecolin has been used in China since 2012.
In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine (vaccine group, 56,302 participants) or the hepatitis B vaccine (control group, 56,302 participants). Vaccine administration occurred at 0, 1, and 6 months, and participants were followed for a total of 19 months.
Details of the studyThe follow-up study was designed to assess the efficacy, immunogenicity, and safety of the HEV vaccine up to 4.5 years postvaccination. All health care centers (205 village and private clinics) in the study area were enrolled in the program. The treatment assignments of all patients remained double blinded. Unblinding occurred only after the data on safety, efficacy, and immunogenicity had been locked.
A diagnosis of HEV infection was made if at least 2 of the following markers were present: a positive test for immunoglobulin M antibodies against HEV, a positive test for HEV RNA, or a serum concentration of immunoglobulin G (IgG) antibodies against HEV that was at least 4 times higher than previously measured at any time during the same illness. Vaccine immunogenicity was assessed by testing serum samples for IgG antibodies against HEV at regular intervals after the vaccination was given.
Over the 4.5-year study period, 7 cases of hepatitis E occurred in the vaccine group, and 53 in the control group. Vaccine efficacy was 86.8% (P<.001) in the modified intention-to-treat analysis. Among patients who received 3 doses of HEV vaccine and who were seronegative at the start of the study, 87% maintained antibodies against HEV for 4.5 years. Within the control group, HEV titers developed in 9% of participants. The vaccine and control groups had similar rates of adverse events.
The authors concluded that the HEV vaccine induced antibodies against hepatitis E that lasted up to 4.5 years. Additionally, 2 doses of vaccine induced slightly lower levels of antibody than those produced by 3 doses of the vaccine. Finally, all participants in the vaccine group who developed HEV had antibodies with high or moderate avidity, indicating an anamnestic response from previous immunity. Most participants in the control group who developed HEV, however, had antibodies with low avidity, indicating no previous immunity.
The burden of HEVHepatitis E is a serious infection and is the most common waterborne illness in the world. It occurs mainly in developing countries with limited resources. HEV infection is caused by genotypes 1, 2, 3, or 4, although all 4 genotypes belong to the same serotype. Genotypes 1 and 2 are typically waterborne, and genotypes 3 and 4 are typically transmitted from animals and humans. In general, the case fatality rate associated with HEV infection is 1% to 3%.12 In pregnancy, this rate increases to 5% to 25%.13,14 In Bangladesh, for example, hepatitis E is responsible for more than 1,000 deaths per year among pregnant women.15
Clinical presentation of HEV infection is a spectrum, with most symptomatic patients presenting with acute, self-limited hepatitis. Severe cases may be associated with pancreatitis, arthritis, aplastic anemia, and neurologic complications, such as seizures. Populations at risk for more severe cases include pregnant women, elderly men, and patients with pre‑ existing, chronic liver disease.
What this evidence means for practiceStandard sanitary precautions, such as clean drinking water, traditionally have been considered the mainstay of hepatitis E prevention. However, as the study authors indicate, recent severe outbreaks of HEV infection in Sudan and Uganda have occurred despite these measures. Thus, an effective vaccine that produces long-standing immunity has great potential for reducing morbidity and mortality in these countries. The present vaccine appears to be highly effective and safe. The principal unanswered question is the duration of immunity.
My patients are asking, "What is the best insect repellent to try to avoid Zika virus?"
With summer upon us we have received questions from colleagues about the best over-the-counter insect repellents to advise their pregnant patients to use.
The preferred insect repellent for skin coverage is DEET (N,N-diethyl-meta-toluamide) (TABLE). Oil of lemon/eucalyptus/para-menthane-diol and IR3535 are also acceptable repellents to use on the skin that are safe for use in pregnancy. In addition, instruct patients to spray permethrin on their clothing or to buy clothing (boots, pants, socks) that has been pretreated with permethrin.1,2
Anushka Chelliah, MD, and Patrick Duff, MD.

Abbreviation: OTC, over the counter.
Coming soon to OBG Management
Drs. Chelliah and Duff follow-up on their March 2016 examination of Zika virus infection with:
- Latest information on Zika virus-associated birth defects
- Ultrasonographic and radiologic evidence of abnormalities in the fetus and newborn exposed to Zika virus infection
- Link between Zika virus infection and serious neurologic complications in adults
- New recommendations for preventing sexual transmission of Zika virus infection
Dr. Chelliah is a Maternal Fetal Medicine-Fellow in the Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, University of Florida College of Medicine, Gainesville.
Dr. Duff is Associate Dean for Student Affairs and Professor of Obstetrics and Gynecology in the Division of Maternal-Fetal Medicine, University of Florida College of Medicine.
The author reports no financial relationships relevant to this article.
References
- Peterson EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak--United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30-33.
- Centers for Disease Control and Prevention. CDC Features: Avoid mosquito bites. http://www.cdc.gov/Features/stopmosquitoes/index.html. Updated March 18, 2016. Accessed May 10, 2016.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Chlorhexidine-alcohol is superior to iodine-alcohol for reducing SSIs after cesarean deliveryTuuli Mg, Liu J, Stout Mj, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647-655.
In the United States, cesarean delivery is the most commonly performed major surgical procedure, with 32.7% of births--or 1.3 million--occurring in this fashion in 2013.1,2 In general, for all surgical procedures, the SSI rate is 2% to 5%, with the rate rising to 5% to 12% for cesarean delivery, especially in obese patients.3-6 Not only do SSIs increase morbidity for the patient but they also contribute to high medical costs, with an estimated additional expense of $3,529 per cesarean-associated infection.7
Skin pathogens are a major source of SSIs. Choosing the proper antiseptic has the potential to decrease infection risk. While current guidelines recommend use of an antiseptic containing alcohol, it is unclear which disinfectant is the most effective agent to combine with the alcohol.3
Most trials evaluating preoperative antiseptic skin preparation have studied patients undergoing general surgery procedures. A well-designed trial by Darouiche and coauthors demonstrated that chlorhexidine was superior to iodine when used as an antiseptic for skin preparation.8 Interestingly, however, this trial, like most others, compared chlorhexidine-alcohol to iodine without alcohol. It is therefore unclear whether the chlorhexidine or the alcohol is responsible for the enhanced antiseptic effect.
Details of the studyIn the single-center randomized trial conducted by Tuuli and colleagues, patients were assigned to preoperative skin antisepsis with either chlorhexidine-alcohol (2% chlorhexidine gluconate with 70% isopropyl alcohol) or iodine-alcohol (8.3% povidone-iodine with 72.5% isopropyl alcohol). Antiseptic was applied according to the manufacturer's instructions, with a standard wait time of 3 minutes between application and skin incision. However, wait time was eliminated for patients undergoing emergency cesarean delivery. Additionally, patients received standard, weight-based preoperative antibiotic prophylaxis (agent not specified).
The authors estimated the necessary sample size for the trial by assuming an 8% baseline SSI rate and an anticipated 50% reduction of infection in the chlorhexidine-alcohol group. Exclusion criteria included a known allergy to chlorhexidine, alcohol, iodine, or shellfish or a preexisting skin infection adjacent to the operative site.
In addition to assessing the primary outcome of SSI with the 2 preparations, the authors conducted 4 prespecified subgroup analyses. These subgroups were based on: type of cesarean delivery (scheduled vs unscheduled), body weight (obese vs nonobese), type of skin closure (subcuticular suture vs staples), and presence or absence of chronic medical conditions (diabetes, hypertension, renal disease). Additionally, a post hoc analysis was performed, comparing women with diabetes (gestational and pregestational) to those without diabetes.
A total of 1,636 pregnant women were screened for eligibility. Of these, 489 women were excluded because they did not meet inclusion criteria or declined to participate or because informed consent could not be obtained. Baseline characteristics were similar across both groups.
Patients were followed for 30 days after surgery. A total of 1,082 women (94.3% of sample size) completed the follow-up. Among these patients, the rate of SSI was significantly lower in the chlorhexidine-alcohol group (4.3%) compared with the iodine-alcohol group (7.7%, P = .02).
In the subgroup analyses, the frequency of SSI remained lower for the chlorhexidine-alcohol group than for the iodine-alcohol group. These reductions were not affected by whether the cesarean was scheduled or unscheduled, the presence or absence of obesity, the type of skin closure, the presence of chronic disease, or diabetes status.
Several secondary outcomes also were examined in this study. There were no significant differences between the 2 antiseptic groups with respect to rates of endometritis, hospital readmission for infection-related complications, length of hospital stay, use of other health care services (such as emergency department visits, additional wound surgery, and home health services), and rates of other wound complications (seroma, hematoma, and cellulitis). Patients in the chlorhexidine-alcohol group were significantly less likely than those in the iodine-alcohol group to have physician office visits for concerns about possible wound complications (P = .009).
The authors concluded that the use of chlorhexidine-alcohol was superior to iodine-alcohol in preventing SSI after cesarean delivery.
Study strengths and limitations The authors acknowledged that their study had some minor limitations. First, the trial was conducted at a single site, which may limit the generalizability of the findings. However, the study population was racially and economically diverse. Second, the lack of blinding among providers and participants may have introduced bias, although, as the authors explain, we would expect this bias to be largely nondirectional.
A major strength of this study is its randomized design. Another strength is that the authors included emergency cesarean deliveries in their analysis. Emergency procedures represent a substantial proportion of cesarean deliveries, and they place the patient at increased risk for SSIs because of limited time available to prepare the skin before surgery begins. Thus, it is of great interest that chlorhexidine-alcohol was so effective even in the highest-risk patients.
Several properties may make chlorhexidine superior to iodine as an antiseptic: high binding affinity for the skin, high antibacterial activity against both gram-positive and gram-negative bacteria, and longer residual effects than iodine. Additionally, iodine is inactivated by organic matter, such as body fluids, whereas chlorhexidine is not.
A recent study by Ngai and colleagues9 compared chlorhexidine-alcohol with iodine-alcohol for skin preparation before cesarean delivery. These authors found no difference in SSI when comparing the 2 solutions used separately or sequentially, except in morbidly obese women. In these women, sequential application of both solutions reduced the infection rate. However, this study specifically excluded emergency cesarean deliveries, making the generalizability of the results questionable.9
What this evidence means for practiceThis large, randomized study found chlorhexidine-alcohol to be superior to iodine-alchol in reducing the risk of SSIs after cesarean delivery. These results confirm those of previous studies from both the obstetric and general surgery literature. Although chlorhexidine-alcohol is more expensive than iodine-alcohol, we strongly recommend its use in patients having cesarean delivery.
Five effective oral and intramuscular antibiotic regimens for treating postpartum endometritisMeaney-Delman D, Bartlett LA, Gravett MG, Jamieson DJ. Oral and intramuscular treatment options for early postpartum endometritis in low-resource settings: a systematic review. Obstet Gynecol. 2015;125(4):789-800.
The authors of this excellent systematic review on antibiotic treatments for early postpartum endometritis conducted their study in 3 phases. Initially, Meaney-Delman and colleagues searched the literature for reports of prospective studies that evaluated the use of oral and intramuscular (IM) antibiotics for treatment of patients who developed endometritis following either cesarean or vaginal delivery. When they discovered that these initial trials were few in number and of relatively poor quality, they reviewed more rigorous trials of intravenous (IV) antibiotics. Finally, they evaluated clinical trials that specifically identified microorganisms isolated from the uterus in patients with endometritis and used this information to help inform their recommendations for treatment options.
Details of the studyIn evaluating the trials of oral and IM antibiotics, the authors set as a standard for effectiveness a cure rate of 85%, a figure comparable to that generally achieved with IV antibiotics. They identified 2 oral antibiotic regimens that met this standard of effectiveness: amoxicillin-clavulanate (100% cure in 36 patients; 95% confidence interval [CI], 90-100) and ampicillin plus metronidazole (97% cure in 37 patients; 95% CI, 86-100).
Two studies demonstrated acceptable levels of cure with single-agent IM antibiotics: aztreonam (100% cure in 16 patients; 95% CI, 81-100) and imipenem (91% cure in 23 patients; 95% CI, 73-98). One additional trial demonstrated an acceptable clinical response rate when IV clindamycin was combined with IM gentamicin (100% cure in 54 patients; 95% CI, 94-100). By contrast, the authors noted, many different IV regimens--either as a single agent or as a drug combination--provided cure rates that equaled or exceeded 85%.
In the study's final phase, the authors provided an excellent overview of the polymicrobial nature of puerperal endometritis. As documented in multiple prior reports, the most common pathogens are the gram-negative anaerobic bacilli, such as Bacteroides and Prevotella species; the anaerobic gram-positive organisms, including Peptococcus and Peptostreptococcus species; aerobic gram-negative bacilli, such as Escherichia coli, Klebsiella pneumoniae, and Proteus species; and aerobic gram-positive cocci, such as group B streptococci, enterococci, and staphylococci.
Recommended regimens. Based on their review of clinical and microbiological studies, the authors proposed 5 oral or combined oral-IM treatment regimens that could be used in low-resource settings:
- oral clindamycin (600 mg every 6 hours)
- plus IM gentamicin (4.5 g every 24 hours)
- oral amoxicillin-clavulanic acid (875 mg every 12 hours)
- IM cefotetan (2 g every 8 hours)
- IM meropenem or imipenem-cilastatin (500 mg every 8 hours)
- oral amoxicillin (500 mg every 8 hours) plus oral metronidazole (500 mg every 8 hours).
Typical endometritis treamentEndometritis is the single most common complication following cesarean delivery. The frequency of its occurrence depends on several factors, including: the socioeconomic characteristics of the patient population, length of labor, length of ruptured membranes, number of internal vaginal examinations, presence of preexisting lower genital tract infection, type of anesthesia, surgical technique, and use of prophylactic antibiotics. Endometritis is much less common after vaginal delivery but still may occur in 3% to 5% of patients.10
Endometritis is clearly a polymicrobial infection that includes multiple aerobic and anaerobic organisms. Accordingly, antibiotic therapy must target all the major groups of pathogens. The usual standard of care for treatment of early-onset endometritis is IV antibiotics, and patients typically are treated until they have been afebrile and asymptomatic for a minimum of 24 hours. Several different IV regimens provide acceptable treatment10:
- clindamycin plus gentamicin
- metronidazole plus ampicillin plus gentamicin
- extended-spectrum cephalosporins, such as cefepime, cefotetan, and cefoxitin
- extended-spectrum penicillins, such as ampicillin-sulbactam, piperacillin- tazobactam, and ticarcillin-clavulanic acid
- carbapenems, such as imipenem-cilastatin and meropenem.
What this evidence means for practiceClearly, IV antibiotics, even generic drugs, are more expensive than oral agents. They also are more difficult to administer than oral or IM drugs. The systematic review by Meaney-Delman and co-workers is therefore a very important contribution to the literature and should reassure clinicians practicing in low-resource settings that oral and oral-IM regimens can provide safe and effective treatment for endometritis. Until more rigorous comparative trials are conducted, however, we agree with the authors' caveat that, for now, such treatment should be limited to individuals whose infection occurred after vaginal delivery or who have evidence of only mild postcesarean endometritis.
Treatment options for chlamydia infection: How does azithromycin compare with doxycycline?Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373(26):2512-2521.
The Centers for Disease Control and Prevention recommendations for treatment of chlamydia genital tract infection are either oral doxycycline, 100 mg twice daily for 7 days, or azithromycin, 1,000 mg in a single dose.11 Recent reports have raised questions about the relative effectiveness of single-dose azithromycin compared with the multiple-day doxycycline regimen. Accordingly, Geisler and colleagues conducted an interesting randomized controlled trial to determine if azithromycin is noninferior to doxycycline.
Details of the studyThe study took place in a unique institutional setting--the Los Angeles County youth correctional facilities. Participants were young men and women, aged 12 to 21 years, who tested positive for chlamydia infection by a nucleic acid amplification test on entry to the correctional facility. Participants then were randomly assigned to receive either doxycycline or azithromycin in the doses described above. The primary outcome was the percent of individuals who still tested positive for chlamydia 28 days after treatment.
Of note, all patients took their medication under direct observation of corrections officers and, with rare exceptions, did not engage in sexual activity during the period of observation. Because this was a noninferiority trial, Geisler and colleagues analyzed the outcomes only of the individuals who actually took their medication in accordance with the assigned protocol. A priori, the authors established a 95% CI of <5% difference in effectiveness as indicative of noninferiority.
Overall, 155 patients in each treatment group completed the trial according to the assigned protocol. No treatment failures occurred in the doxycycline group (0%; 95% CI, 0.0-2.4). Five treatment failures occurred in the azithromycin group (3.2%; 95% CI, 0.4-7.4), in 1 female and 4 male participants. Because the 95% CI for the difference in treatment outcome exceeded 5%, the authors were unable to conclude that azithromycin was noninferior to doxycycline.
Consider real-world treatment adherence in these resultsFor several reasons, we do not conclude from this article that ObGyns should now stop using azithromycin to treat patients with chlamydia infection. First, the actual per protocol sample size was still relatively small. If there had been just 2 fewer failures in the azithromycin group, the 95% CI for the difference in outcomes would have been less than 5%, and the authors would have concluded that the 2 drug regimens were noninferior. Second, 4 of the 5 treatment failures in the azithromycin group were in male rather than female participants. Third, the unique study design resulted in almost perfect adherence with the 7-day doxycycline treatment regimen. Such adherence is very unlikely in other practice settings, and patients who do not complete their treatment regimen are significantly more likely to fail therapy. Finally, azithromycin is definitely preferred in pregnancy because we try to avoid maternal/fetal exposure to drugs such as tetracycline and doxycycline.
What this evidence means for practiceIn this study, both doxycycline and azithromycin were highly effective (100% and 97%, respectively) for treating chlamydia genital tract infection, and they are comparable in cost. In our opinion, the improved adherence that is possible with single-dose azithromycin, the greater safety in pregnancy, and the excellent tolerability of this drug outweigh its slightly deceased rate of microbiologic cure.
Vaccine effective against hepatitis E for 4+ yearsZhang J, Zhang XF, Huang SJ, et al. Long-term efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372(10):914-922.
This study conducted by Zhang and colleagues in Dongtai, China, is an extended follow-up study of the hepatitis E virus (HEV) vaccine (Hecolin; Xiamen Innovax Biotech). A recombinant vaccine directed against HEV genotype 1, Hecolin has been used in China since 2012.
In the initial efficacy study, healthy adults aged 16 to 65 years were randomly assigned to receive either the hepatitis E vaccine (vaccine group, 56,302 participants) or the hepatitis B vaccine (control group, 56,302 participants). Vaccine administration occurred at 0, 1, and 6 months, and participants were followed for a total of 19 months.
Details of the studyThe follow-up study was designed to assess the efficacy, immunogenicity, and safety of the HEV vaccine up to 4.5 years postvaccination. All health care centers (205 village and private clinics) in the study area were enrolled in the program. The treatment assignments of all patients remained double blinded. Unblinding occurred only after the data on safety, efficacy, and immunogenicity had been locked.
A diagnosis of HEV infection was made if at least 2 of the following markers were present: a positive test for immunoglobulin M antibodies against HEV, a positive test for HEV RNA, or a serum concentration of immunoglobulin G (IgG) antibodies against HEV that was at least 4 times higher than previously measured at any time during the same illness. Vaccine immunogenicity was assessed by testing serum samples for IgG antibodies against HEV at regular intervals after the vaccination was given.
Over the 4.5-year study period, 7 cases of hepatitis E occurred in the vaccine group, and 53 in the control group. Vaccine efficacy was 86.8% (P<.001) in the modified intention-to-treat analysis. Among patients who received 3 doses of HEV vaccine and who were seronegative at the start of the study, 87% maintained antibodies against HEV for 4.5 years. Within the control group, HEV titers developed in 9% of participants. The vaccine and control groups had similar rates of adverse events.
The authors concluded that the HEV vaccine induced antibodies against hepatitis E that lasted up to 4.5 years. Additionally, 2 doses of vaccine induced slightly lower levels of antibody than those produced by 3 doses of the vaccine. Finally, all participants in the vaccine group who developed HEV had antibodies with high or moderate avidity, indicating an anamnestic response from previous immunity. Most participants in the control group who developed HEV, however, had antibodies with low avidity, indicating no previous immunity.
The burden of HEVHepatitis E is a serious infection and is the most common waterborne illness in the world. It occurs mainly in developing countries with limited resources. HEV infection is caused by genotypes 1, 2, 3, or 4, although all 4 genotypes belong to the same serotype. Genotypes 1 and 2 are typically waterborne, and genotypes 3 and 4 are typically transmitted from animals and humans. In general, the case fatality rate associated with HEV infection is 1% to 3%.12 In pregnancy, this rate increases to 5% to 25%.13,14 In Bangladesh, for example, hepatitis E is responsible for more than 1,000 deaths per year among pregnant women.15
Clinical presentation of HEV infection is a spectrum, with most symptomatic patients presenting with acute, self-limited hepatitis. Severe cases may be associated with pancreatitis, arthritis, aplastic anemia, and neurologic complications, such as seizures. Populations at risk for more severe cases include pregnant women, elderly men, and patients with pre‑ existing, chronic liver disease.
What this evidence means for practiceStandard sanitary precautions, such as clean drinking water, traditionally have been considered the mainstay of hepatitis E prevention. However, as the study authors indicate, recent severe outbreaks of HEV infection in Sudan and Uganda have occurred despite these measures. Thus, an effective vaccine that produces long-standing immunity has great potential for reducing morbidity and mortality in these countries. The present vaccine appears to be highly effective and safe. The principal unanswered question is the duration of immunity.
My patients are asking, "What is the best insect repellent to try to avoid Zika virus?"
With summer upon us we have received questions from colleagues about the best over-the-counter insect repellents to advise their pregnant patients to use.
The preferred insect repellent for skin coverage is DEET (N,N-diethyl-meta-toluamide) (TABLE). Oil of lemon/eucalyptus/para-menthane-diol and IR3535 are also acceptable repellents to use on the skin that are safe for use in pregnancy. In addition, instruct patients to spray permethrin on their clothing or to buy clothing (boots, pants, socks) that has been pretreated with permethrin.1,2
Anushka Chelliah, MD, and Patrick Duff, MD.

Abbreviation: OTC, over the counter.
Coming soon to OBG Management
Drs. Chelliah and Duff follow-up on their March 2016 examination of Zika virus infection with:
- Latest information on Zika virus-associated birth defects
- Ultrasonographic and radiologic evidence of abnormalities in the fetus and newborn exposed to Zika virus infection
- Link between Zika virus infection and serious neurologic complications in adults
- New recommendations for preventing sexual transmission of Zika virus infection
Dr. Chelliah is a Maternal Fetal Medicine-Fellow in the Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, University of Florida College of Medicine, Gainesville.
Dr. Duff is Associate Dean for Student Affairs and Professor of Obstetrics and Gynecology in the Division of Maternal-Fetal Medicine, University of Florida College of Medicine.
The author reports no financial relationships relevant to this article.
References
- Peterson EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak--United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(2):30-33.
- Centers for Disease Control and Prevention. CDC Features: Avoid mosquito bites. http://www.cdc.gov/Features/stopmosquitoes/index.html. Updated March 18, 2016. Accessed May 10, 2016.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2007:165:1–209.
- Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65.
- Anderson DJ, Podgorny K, Berrios-Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):605–627.
- Conroy K, Koenig AF, Yu YH, Courtney A, Lee HJ, Norwitz ER. Infectious morbidity after cesarean delivery: 10 strategies to reduce risk. Rev Obstet Gynecol. 2012;5(2):69–77.
- Scifres CM, Leighton BL, Fogertey PJ, Macones GA, Stamilio DM. Supplemental oxygen for the prevention of postcesarean infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2011;205(3)267.e1–e9.
- Wloch C, Wilson J, Lamagni T, Harrington P, Charlett A, Sheridan E. Risk factors for surgical site infection following cesarean section in England: results from a multicenter cohort study. BJOG. 2012;119(11):1324–1333.
- Olsen MA, Butler AM, Willers DM, Gross GA, Hamilton BH, Fraser VJ. Attributable costs of surgical site infection and endometritis after low transverse cesarean delivery. Infect Control Hosp Epidemiol. 2010;31(3):276–282.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;126(6):1251–1257.
- Duff P. Maternal and perinatal infection—bacterial. In: Gabbe SG, Niebyl JR, Simpson JL, et al, eds. Obstetrics: normal and problem pregnancies. 6th ed. Philadelphia, PA: Elsevier/Saunders; 2012.
- Workowski KA, Bolan GH; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1−137. Erratum in: MMWR Recomm Rep. 2015;64(33):924.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13(3):145–154.
- Khuroo MS, Teli MR, Skidmore S, Sofi MA, Khuroo MI. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70(2):252–255.
- Khuroo MS, Kamili S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J Viral Hepat. 2003;10(1):61–69.
- Labrique AB, Sikder SS, Krain IJ, et al. Hepatitis E, a vaccine-preventable cause of maternal deaths. Emerg Infect Dis. 2012;18(9):1401–1404.
- DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2007:165:1–209.
- Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65.
- Anderson DJ, Podgorny K, Berrios-Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):605–627.
- Conroy K, Koenig AF, Yu YH, Courtney A, Lee HJ, Norwitz ER. Infectious morbidity after cesarean delivery: 10 strategies to reduce risk. Rev Obstet Gynecol. 2012;5(2):69–77.
- Scifres CM, Leighton BL, Fogertey PJ, Macones GA, Stamilio DM. Supplemental oxygen for the prevention of postcesarean infectious morbidity: a randomized controlled trial. Am J Obstet Gynecol. 2011;205(3)267.e1–e9.
- Wloch C, Wilson J, Lamagni T, Harrington P, Charlett A, Sheridan E. Risk factors for surgical site infection following cesarean section in England: results from a multicenter cohort study. BJOG. 2012;119(11):1324–1333.
- Olsen MA, Butler AM, Willers DM, Gross GA, Hamilton BH, Fraser VJ. Attributable costs of surgical site infection and endometritis after low transverse cesarean delivery. Infect Control Hosp Epidemiol. 2010;31(3):276–282.
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26.
- Ngai IM, Van Arsdale A, Govindappagari S, et al. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;126(6):1251–1257.
- Duff P. Maternal and perinatal infection—bacterial. In: Gabbe SG, Niebyl JR, Simpson JL, et al, eds. Obstetrics: normal and problem pregnancies. 6th ed. Philadelphia, PA: Elsevier/Saunders; 2012.
- Workowski KA, Bolan GH; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1−137. Erratum in: MMWR Recomm Rep. 2015;64(33):924.
- Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13(3):145–154.
- Khuroo MS, Teli MR, Skidmore S, Sofi MA, Khuroo MI. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70(2):252–255.
- Khuroo MS, Kamili S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J Viral Hepat. 2003;10(1):61–69.
- Labrique AB, Sikder SS, Krain IJ, et al. Hepatitis E, a vaccine-preventable cause of maternal deaths. Emerg Infect Dis. 2012;18(9):1401–1404.
In this article
• Azithromycin vs doxycycline for chlamydia
• Insect repellents to prevent Zika virus
Study helps set upper boundary for leukocytosis after prenatal corticosteroids
WASHINGTON – Maternal leukocytosis after prenatal corticosteroid administration peaks at up to 24 hours after therapy, with the highest second standard deviation from the mean being 18.3 x 109/L, according to a systematic review and meta-analysis.
There has been limited data available on the magnitude and timing of leukocytosis after corticosteroid administration, making it difficult to interpret the significance of elevated white blood cell counts, Dr. Samuel Bauer, of Beaumont Health, Royal Oak, Mich., said at the annual meeting of the American College of Obstetricians and Gynecologists.
“We know corticosteroids cause leukocytosis, but we haven’t really known what the upper boundary is,” he said.
Driven by concerns about maternal sepsis and the ability to recognize early signs, Dr. Bauer and his colleagues identified six studies that reported white blood cell counts prior to corticosteroid administration, and between 24 and 96 hours afterward in healthy women with singleton gestations. The studies also met the inclusion criterion of having “excluded infected parturients between 23 and 34 weeks of gestation,” he said.
Mean maternal white blood cell count values prior to corticosteroid administration and up to 24 hours, 48 hours, 72 hours, and 96 hours after corticosteroid administration were 10.2, 13.7, 12.8, 11.5, and 11.1 x 109/L, respectively.
The highest second standard deviation from the mean of 18.3 x 109/L did not occur after 24 hours, he emphasized, and by 72 hours, mean values had returned to 11.5 x 109/L.
The findings need to be applied “cautiously” in practice, Dr. Bauer said, since the analysis did not include women with signs of infection and because some women who develop serious infections “have a very low white blood cell count.”
Still, the findings “establish a temporal trend and give us an upper boundary” for the level of leukocytosis that can be expected with prenatal corticosteroids. This can be helpful – along with other considerations – in determining whether an infectious workup is needed when white blood cell counts are high, he said in an interview.
It is not uncommon in clinical practice for maternal leukocytosis at 5-6 days or a week after corticosteroid administration to be attributed to the corticosteroids, he said. But the parameters drawn by this analysis of healthy, non-infected patients show this is a faulty assumption, he added.
The analysis covered 524 patients and 1,406 observations. Dr. Bauer and his coinvestigators did not report any financial disclosures.
WASHINGTON – Maternal leukocytosis after prenatal corticosteroid administration peaks at up to 24 hours after therapy, with the highest second standard deviation from the mean being 18.3 x 109/L, according to a systematic review and meta-analysis.
There has been limited data available on the magnitude and timing of leukocytosis after corticosteroid administration, making it difficult to interpret the significance of elevated white blood cell counts, Dr. Samuel Bauer, of Beaumont Health, Royal Oak, Mich., said at the annual meeting of the American College of Obstetricians and Gynecologists.
“We know corticosteroids cause leukocytosis, but we haven’t really known what the upper boundary is,” he said.
Driven by concerns about maternal sepsis and the ability to recognize early signs, Dr. Bauer and his colleagues identified six studies that reported white blood cell counts prior to corticosteroid administration, and between 24 and 96 hours afterward in healthy women with singleton gestations. The studies also met the inclusion criterion of having “excluded infected parturients between 23 and 34 weeks of gestation,” he said.
Mean maternal white blood cell count values prior to corticosteroid administration and up to 24 hours, 48 hours, 72 hours, and 96 hours after corticosteroid administration were 10.2, 13.7, 12.8, 11.5, and 11.1 x 109/L, respectively.
The highest second standard deviation from the mean of 18.3 x 109/L did not occur after 24 hours, he emphasized, and by 72 hours, mean values had returned to 11.5 x 109/L.
The findings need to be applied “cautiously” in practice, Dr. Bauer said, since the analysis did not include women with signs of infection and because some women who develop serious infections “have a very low white blood cell count.”
Still, the findings “establish a temporal trend and give us an upper boundary” for the level of leukocytosis that can be expected with prenatal corticosteroids. This can be helpful – along with other considerations – in determining whether an infectious workup is needed when white blood cell counts are high, he said in an interview.
It is not uncommon in clinical practice for maternal leukocytosis at 5-6 days or a week after corticosteroid administration to be attributed to the corticosteroids, he said. But the parameters drawn by this analysis of healthy, non-infected patients show this is a faulty assumption, he added.
The analysis covered 524 patients and 1,406 observations. Dr. Bauer and his coinvestigators did not report any financial disclosures.
WASHINGTON – Maternal leukocytosis after prenatal corticosteroid administration peaks at up to 24 hours after therapy, with the highest second standard deviation from the mean being 18.3 x 109/L, according to a systematic review and meta-analysis.
There has been limited data available on the magnitude and timing of leukocytosis after corticosteroid administration, making it difficult to interpret the significance of elevated white blood cell counts, Dr. Samuel Bauer, of Beaumont Health, Royal Oak, Mich., said at the annual meeting of the American College of Obstetricians and Gynecologists.
“We know corticosteroids cause leukocytosis, but we haven’t really known what the upper boundary is,” he said.
Driven by concerns about maternal sepsis and the ability to recognize early signs, Dr. Bauer and his colleagues identified six studies that reported white blood cell counts prior to corticosteroid administration, and between 24 and 96 hours afterward in healthy women with singleton gestations. The studies also met the inclusion criterion of having “excluded infected parturients between 23 and 34 weeks of gestation,” he said.
Mean maternal white blood cell count values prior to corticosteroid administration and up to 24 hours, 48 hours, 72 hours, and 96 hours after corticosteroid administration were 10.2, 13.7, 12.8, 11.5, and 11.1 x 109/L, respectively.
The highest second standard deviation from the mean of 18.3 x 109/L did not occur after 24 hours, he emphasized, and by 72 hours, mean values had returned to 11.5 x 109/L.
The findings need to be applied “cautiously” in practice, Dr. Bauer said, since the analysis did not include women with signs of infection and because some women who develop serious infections “have a very low white blood cell count.”
Still, the findings “establish a temporal trend and give us an upper boundary” for the level of leukocytosis that can be expected with prenatal corticosteroids. This can be helpful – along with other considerations – in determining whether an infectious workup is needed when white blood cell counts are high, he said in an interview.
It is not uncommon in clinical practice for maternal leukocytosis at 5-6 days or a week after corticosteroid administration to be attributed to the corticosteroids, he said. But the parameters drawn by this analysis of healthy, non-infected patients show this is a faulty assumption, he added.
The analysis covered 524 patients and 1,406 observations. Dr. Bauer and his coinvestigators did not report any financial disclosures.
AT ACOG 2016
Key clinical point: Leukocytosis attributable to prenatal corticosteroids, rather than infection, has a definable upper boundary.
Major finding: Maternal leukocytosis peaks at up to 24 hours after administration of antenatal corticosteroids. The highest second standard deviation from the mean was 18.3 x 109/L.
Data source: A systematic review and meta-analysis.
Disclosures: Dr. Bauer reported that he and his coinvestigators had no financial disclosures.
When could use of antenatal corticosteroids in the late preterm birth period be beneficial?
The use of antenatal corticosteroids for preterm deliveries between 24 and 34 weeks has been standard of care in obstetric practice. But approximately 70% of preterm deliveries in the United States occur after 34 weeks, in the so-called late preterm period (34 weeks 0 days to 36 weeks 6 days). Recently, Gyamfi-Bannerman and colleagues at the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network completed a trial that examined the use of antenatal betamethasone in women at risk for delivery in the late preterm period.
Details of the study
The Antenatal Late Preterm Steroids (ALPS) trial was a randomized, double-blind, placebo-controlled study that included women with a singleton gestation between 34 weeks 0 days and 36 weeks 5 days who had a high probability risk of delivery in the late preterm period. The authors defined “high probability of delivery” as spontaneous labor with cervical change (at least 3-cm dilation or 75% effacement), preterm premature rupture of the membranes, or a planned delivery scheduled in the late preterm period for specific obstetric indications, such as oligohydramnios, preeclampsia, gestational hypertension, and intrauterine growth restriction.
Women were excluded from the study if they had previously received a course of steroids or had multiple gestations, pregestational diabetes, chorioamnionitis, or were expected to deliver in less than 12 hours due to advanced labor, vaginal bleeding, or nonreassuring fetal status.
Study participants were randomly assigned to receive 2 doses (12 mg intramuscularly) of betamethasone 24 hours apart (1,429 participants) or identical-appearing placebo (1,402 participants). Tocolysis was not allowed in the protocol.
Positive outcomes for neonates
The use of corticosteroids was associated with a significant reduction in the primary outcome of need for respiratory support in the first 72 hours of life (14.4% in the placebo group vs 11.6% in the betamethasone group; relative risk [RR], 0.80; 95% confidence interval [CI], 0.66–0.97; P = .02). Steroid use also decreased the incidence of severe respiratory complications, the need for resuscitation at birth, the need for surfactant therapy, the incidence of transient tachypnea of the newborn, and the incidence of bronchopulmonary dysplasia. Neonatal hypoglycemia was more frequent among infants exposed to betamethasone (24% vs 15%; RR, 1.6; 95% CI, 1.37–1.87; P<.001).
New guidelines issued
The ALPS study is the largest randomized trial to evaluate the benefit of antenatal steroids during the late preterm period. The study’s findings certainly will change clinical practice. Based on the study’s large sample size, rigorous design and protocol, and a cohort generalizable to the US population, SMFM has issued new recommendations for practitioners on using antenatal steroids in the late preterm period in women at risk for preterm delivery.
What this EVIDENCE means for practice
In light of the new SMFM recommendations, in my practice, I will adhere to the inclusion criteria used in the ALPS study, and be careful not to apply the same approach used before 34 weeks, when delivery is often delayed intentionally in order to achieve steroid benefit. If considering adoption of this same practice, clinicians should not use tocolytics when administering corticosteroids in the late preterm period. When indicated, such as in women with severe preeclampsia or ruptured membranes, delivery should not be delayed. A patient with high probability of delivery in the late preterm period is eligible for treatment as long as the clinician thinks that she is not going to deliver within 12 hours. On the other hand, clinicians should not overtreat women, and should maintain a high suspicion for delivery in patients with preterm labor (a cervix that is at least 3 cm dilated or 75% effaced).
The ALPS trial did not allow the administration of more than one course of steroids. The eligibility criteria for corticosteroid use in the late preterm period should not be extended to include subpopulations that were not studied in the trial (including patients with multiple gestations, pregestational diabetes, or those who already had received a complete course of steroids).
— Luis Pacheco, MD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The use of antenatal corticosteroids for preterm deliveries between 24 and 34 weeks has been standard of care in obstetric practice. But approximately 70% of preterm deliveries in the United States occur after 34 weeks, in the so-called late preterm period (34 weeks 0 days to 36 weeks 6 days). Recently, Gyamfi-Bannerman and colleagues at the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network completed a trial that examined the use of antenatal betamethasone in women at risk for delivery in the late preterm period.
Details of the study
The Antenatal Late Preterm Steroids (ALPS) trial was a randomized, double-blind, placebo-controlled study that included women with a singleton gestation between 34 weeks 0 days and 36 weeks 5 days who had a high probability risk of delivery in the late preterm period. The authors defined “high probability of delivery” as spontaneous labor with cervical change (at least 3-cm dilation or 75% effacement), preterm premature rupture of the membranes, or a planned delivery scheduled in the late preterm period for specific obstetric indications, such as oligohydramnios, preeclampsia, gestational hypertension, and intrauterine growth restriction.
Women were excluded from the study if they had previously received a course of steroids or had multiple gestations, pregestational diabetes, chorioamnionitis, or were expected to deliver in less than 12 hours due to advanced labor, vaginal bleeding, or nonreassuring fetal status.
Study participants were randomly assigned to receive 2 doses (12 mg intramuscularly) of betamethasone 24 hours apart (1,429 participants) or identical-appearing placebo (1,402 participants). Tocolysis was not allowed in the protocol.
Positive outcomes for neonates
The use of corticosteroids was associated with a significant reduction in the primary outcome of need for respiratory support in the first 72 hours of life (14.4% in the placebo group vs 11.6% in the betamethasone group; relative risk [RR], 0.80; 95% confidence interval [CI], 0.66–0.97; P = .02). Steroid use also decreased the incidence of severe respiratory complications, the need for resuscitation at birth, the need for surfactant therapy, the incidence of transient tachypnea of the newborn, and the incidence of bronchopulmonary dysplasia. Neonatal hypoglycemia was more frequent among infants exposed to betamethasone (24% vs 15%; RR, 1.6; 95% CI, 1.37–1.87; P<.001).
New guidelines issued
The ALPS study is the largest randomized trial to evaluate the benefit of antenatal steroids during the late preterm period. The study’s findings certainly will change clinical practice. Based on the study’s large sample size, rigorous design and protocol, and a cohort generalizable to the US population, SMFM has issued new recommendations for practitioners on using antenatal steroids in the late preterm period in women at risk for preterm delivery.
What this EVIDENCE means for practice
In light of the new SMFM recommendations, in my practice, I will adhere to the inclusion criteria used in the ALPS study, and be careful not to apply the same approach used before 34 weeks, when delivery is often delayed intentionally in order to achieve steroid benefit. If considering adoption of this same practice, clinicians should not use tocolytics when administering corticosteroids in the late preterm period. When indicated, such as in women with severe preeclampsia or ruptured membranes, delivery should not be delayed. A patient with high probability of delivery in the late preterm period is eligible for treatment as long as the clinician thinks that she is not going to deliver within 12 hours. On the other hand, clinicians should not overtreat women, and should maintain a high suspicion for delivery in patients with preterm labor (a cervix that is at least 3 cm dilated or 75% effaced).
The ALPS trial did not allow the administration of more than one course of steroids. The eligibility criteria for corticosteroid use in the late preterm period should not be extended to include subpopulations that were not studied in the trial (including patients with multiple gestations, pregestational diabetes, or those who already had received a complete course of steroids).
— Luis Pacheco, MD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The use of antenatal corticosteroids for preterm deliveries between 24 and 34 weeks has been standard of care in obstetric practice. But approximately 70% of preterm deliveries in the United States occur after 34 weeks, in the so-called late preterm period (34 weeks 0 days to 36 weeks 6 days). Recently, Gyamfi-Bannerman and colleagues at the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network completed a trial that examined the use of antenatal betamethasone in women at risk for delivery in the late preterm period.
Details of the study
The Antenatal Late Preterm Steroids (ALPS) trial was a randomized, double-blind, placebo-controlled study that included women with a singleton gestation between 34 weeks 0 days and 36 weeks 5 days who had a high probability risk of delivery in the late preterm period. The authors defined “high probability of delivery” as spontaneous labor with cervical change (at least 3-cm dilation or 75% effacement), preterm premature rupture of the membranes, or a planned delivery scheduled in the late preterm period for specific obstetric indications, such as oligohydramnios, preeclampsia, gestational hypertension, and intrauterine growth restriction.
Women were excluded from the study if they had previously received a course of steroids or had multiple gestations, pregestational diabetes, chorioamnionitis, or were expected to deliver in less than 12 hours due to advanced labor, vaginal bleeding, or nonreassuring fetal status.
Study participants were randomly assigned to receive 2 doses (12 mg intramuscularly) of betamethasone 24 hours apart (1,429 participants) or identical-appearing placebo (1,402 participants). Tocolysis was not allowed in the protocol.
Positive outcomes for neonates
The use of corticosteroids was associated with a significant reduction in the primary outcome of need for respiratory support in the first 72 hours of life (14.4% in the placebo group vs 11.6% in the betamethasone group; relative risk [RR], 0.80; 95% confidence interval [CI], 0.66–0.97; P = .02). Steroid use also decreased the incidence of severe respiratory complications, the need for resuscitation at birth, the need for surfactant therapy, the incidence of transient tachypnea of the newborn, and the incidence of bronchopulmonary dysplasia. Neonatal hypoglycemia was more frequent among infants exposed to betamethasone (24% vs 15%; RR, 1.6; 95% CI, 1.37–1.87; P<.001).
New guidelines issued
The ALPS study is the largest randomized trial to evaluate the benefit of antenatal steroids during the late preterm period. The study’s findings certainly will change clinical practice. Based on the study’s large sample size, rigorous design and protocol, and a cohort generalizable to the US population, SMFM has issued new recommendations for practitioners on using antenatal steroids in the late preterm period in women at risk for preterm delivery.
What this EVIDENCE means for practice
In light of the new SMFM recommendations, in my practice, I will adhere to the inclusion criteria used in the ALPS study, and be careful not to apply the same approach used before 34 weeks, when delivery is often delayed intentionally in order to achieve steroid benefit. If considering adoption of this same practice, clinicians should not use tocolytics when administering corticosteroids in the late preterm period. When indicated, such as in women with severe preeclampsia or ruptured membranes, delivery should not be delayed. A patient with high probability of delivery in the late preterm period is eligible for treatment as long as the clinician thinks that she is not going to deliver within 12 hours. On the other hand, clinicians should not overtreat women, and should maintain a high suspicion for delivery in patients with preterm labor (a cervix that is at least 3 cm dilated or 75% effaced).
The ALPS trial did not allow the administration of more than one course of steroids. The eligibility criteria for corticosteroid use in the late preterm period should not be extended to include subpopulations that were not studied in the trial (including patients with multiple gestations, pregestational diabetes, or those who already had received a complete course of steroids).
— Luis Pacheco, MD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Remote prenatal care monitoring is a hit with patients
WASHINGTON – Is it time to reconsider the standard prenatal care model of 12-14 in-office prenatal visits?
As pregnant women increasingly use digital technology, and as the array of available health monitoring tools grows larger and smarter, the question looms.
Research findings reported at the annual meeting of the American College of Obstetricians and Gynecologists suggest that women with low-risk pregnancies have equivalent outcomes but are more satisfied with models that reduce the number of office visits and utilize remote monitoring.
At the Mayo Clinic in Rochester, Minn., 300 women deemed to have low-risk pregnancies were randomized to either 12 planned office visits with a physician or midwife, or to the clinic’s “OB Nest” model of care consisting of 8 planned clinic visits with a physician or midwife, 6 virtual visits with a nurse (by phone or email), home monitoring with an automatic blood pressure cuff and a hand-held fetal Doppler monitor, and access to an online prenatal care community.
The clinic’s OB Nest model “was born out of concern that the traditional model no longer met the needs of our patients,” said Dr. Yvonne S. Butler Tobah, a senior associate consultant to the department of obstetrics and gynecology at Mayo.
The goal, she said, is to “shift our prenatal clinic’s culture … to a wellness care model and to strengthen the autonomy, confidence, self-awareness and empowerment of our patients.”
Patients in the OB Nest group were encouraged to get blood pressure readings once a week, and weekly Doppler readings of fetal heart rate between weeks 12 and 28. They could use the cuff and monitor anytime they chose, however.
Values were recorded in a pregnancy journal – along with weight – and reported during the scheduled virtual visits. The patients could send in concerning readings or otherwise communicate with dedicated OB Nest nurses at any time they chose by phone or via an online portal. Emergencies were to be reported immediately.
The online prenatal care community is an invitation-only, Mayo-specific social platform, monitored by the OB Nest nurses, which gave patients the opportunity to share and discuss issues.
Patient satisfaction, as measured at 36 weeks with a 16-item validated satisfaction scale, was significantly higher in the OB Nest group; these patients had a mean score of 93.9 on a 1-100 point scale, compared with a mean score of 78.9 in the usual care group.
Levels of pregnancy-related stress – measured at three points in time with a 9-item prenatal maternal stress survey – were also significantly lower at 14 weeks and lower at 36 weeks in the OB Nest group compared with usual care. Stress levels were similar in both groups at 24 weeks.
Perceived quality of care was assessed at 36 weeks using a modified version of a prenatal processes-of-care scale that addressed communication and decision making, and no differences were observed.
“OB Nest significantly improved patient satisfaction with care and reduced maternal stress,” Dr. Tobah said. “And it did this while maintaining perceived quality of care and maintaining [safe] outcomes.”
The study was not sufficiently powered to detect statistically significant differences in clinical outcomes, which were the study’s secondary outcomes. However, there were no differences observed in maternal-fetal events or delivery outcomes, with the exception of a 4.5% rate of gestational diabetes in the OB Nest group, compared with none in the usual care group, Dr. Tobah explained.
Of the 150 patients randomized to each group, 19 and 20 were lost to follow-up in the OB Nest group and usual care group, respectively. Patients in the study had a mean age of 29, and the majority were white and married. “It was a highly educated, low-risk group,” she said. “Patients said [at the end] that they liked the general accessibility and consistent communication with a provider on an ongoing basis.”
While patients in the OB Nest group ultimately had 3.4 fewer in-office appointments than did usual care patients, they required more out-of-office nursing time and the length of in-office visits was significantly higher, Dr. Tobah noted.
In another study of remote prenatal care monitoring reported at the ACOG meeting, low-risk patients assigned to an alternative prenatal care schedule of 8 in-office visits supplemented with digital monitoring of blood pressure and weight similarly had higher patient satisfaction scores than did low-risk patients assigned to 14 prenatal visits.
Patient satisfaction was measured several times during pregnancy. Scores were significantly higher at 20 weeks in the 49-patient alternative care group “and evened out [with the 41-patient usual-care group] at the tail end of pregnancy,” said Dr. Nihar Ganju of George Washington University, Washington. “And there was no difference in pregnancy outcomes.”
This study used the Babyscripts mobile app connected to a wireless weight scale and a wireless blood pressure cuff. Patients were instructed to check their weight and blood pressure at least once a week.
They were “highly engaged,” Dr. Ganju said, checking their blood pressure a mean of 1.4 times weekly and their weight almost twice weekly. Providers received “four notifications of abnormal values,” he said.
Dr. Ganju and his colleagues are hopeful that the digital health tool “can really be effective in addressing the issue of excessive weight gain,” he said at the ACOG meeting. They are also beginning a study on remote monitoring for patients with chronic hypertension.
Findings on the effectiveness of remote personalized weight management are also expected to come from the soon-to-be-completed LIFE-Moms study, a national project looking at how overweight and obese women can best manage weight gain in pregnancy and improve their maternal and fetal outcomes.
Dr. Ganju reported having no disclosures. Two coauthors reported a nonfinancial advisory relationship with 1Eq Inc., the mobile app company that developed Babyscripts and helped fund the study. Another author is an employee of 1Eq. Dr. Tobah reported that she had no disclosures.
WASHINGTON – Is it time to reconsider the standard prenatal care model of 12-14 in-office prenatal visits?
As pregnant women increasingly use digital technology, and as the array of available health monitoring tools grows larger and smarter, the question looms.
Research findings reported at the annual meeting of the American College of Obstetricians and Gynecologists suggest that women with low-risk pregnancies have equivalent outcomes but are more satisfied with models that reduce the number of office visits and utilize remote monitoring.
At the Mayo Clinic in Rochester, Minn., 300 women deemed to have low-risk pregnancies were randomized to either 12 planned office visits with a physician or midwife, or to the clinic’s “OB Nest” model of care consisting of 8 planned clinic visits with a physician or midwife, 6 virtual visits with a nurse (by phone or email), home monitoring with an automatic blood pressure cuff and a hand-held fetal Doppler monitor, and access to an online prenatal care community.
The clinic’s OB Nest model “was born out of concern that the traditional model no longer met the needs of our patients,” said Dr. Yvonne S. Butler Tobah, a senior associate consultant to the department of obstetrics and gynecology at Mayo.
The goal, she said, is to “shift our prenatal clinic’s culture … to a wellness care model and to strengthen the autonomy, confidence, self-awareness and empowerment of our patients.”
Patients in the OB Nest group were encouraged to get blood pressure readings once a week, and weekly Doppler readings of fetal heart rate between weeks 12 and 28. They could use the cuff and monitor anytime they chose, however.
Values were recorded in a pregnancy journal – along with weight – and reported during the scheduled virtual visits. The patients could send in concerning readings or otherwise communicate with dedicated OB Nest nurses at any time they chose by phone or via an online portal. Emergencies were to be reported immediately.
The online prenatal care community is an invitation-only, Mayo-specific social platform, monitored by the OB Nest nurses, which gave patients the opportunity to share and discuss issues.
Patient satisfaction, as measured at 36 weeks with a 16-item validated satisfaction scale, was significantly higher in the OB Nest group; these patients had a mean score of 93.9 on a 1-100 point scale, compared with a mean score of 78.9 in the usual care group.
Levels of pregnancy-related stress – measured at three points in time with a 9-item prenatal maternal stress survey – were also significantly lower at 14 weeks and lower at 36 weeks in the OB Nest group compared with usual care. Stress levels were similar in both groups at 24 weeks.
Perceived quality of care was assessed at 36 weeks using a modified version of a prenatal processes-of-care scale that addressed communication and decision making, and no differences were observed.
“OB Nest significantly improved patient satisfaction with care and reduced maternal stress,” Dr. Tobah said. “And it did this while maintaining perceived quality of care and maintaining [safe] outcomes.”
The study was not sufficiently powered to detect statistically significant differences in clinical outcomes, which were the study’s secondary outcomes. However, there were no differences observed in maternal-fetal events or delivery outcomes, with the exception of a 4.5% rate of gestational diabetes in the OB Nest group, compared with none in the usual care group, Dr. Tobah explained.
Of the 150 patients randomized to each group, 19 and 20 were lost to follow-up in the OB Nest group and usual care group, respectively. Patients in the study had a mean age of 29, and the majority were white and married. “It was a highly educated, low-risk group,” she said. “Patients said [at the end] that they liked the general accessibility and consistent communication with a provider on an ongoing basis.”
While patients in the OB Nest group ultimately had 3.4 fewer in-office appointments than did usual care patients, they required more out-of-office nursing time and the length of in-office visits was significantly higher, Dr. Tobah noted.
In another study of remote prenatal care monitoring reported at the ACOG meeting, low-risk patients assigned to an alternative prenatal care schedule of 8 in-office visits supplemented with digital monitoring of blood pressure and weight similarly had higher patient satisfaction scores than did low-risk patients assigned to 14 prenatal visits.
Patient satisfaction was measured several times during pregnancy. Scores were significantly higher at 20 weeks in the 49-patient alternative care group “and evened out [with the 41-patient usual-care group] at the tail end of pregnancy,” said Dr. Nihar Ganju of George Washington University, Washington. “And there was no difference in pregnancy outcomes.”
This study used the Babyscripts mobile app connected to a wireless weight scale and a wireless blood pressure cuff. Patients were instructed to check their weight and blood pressure at least once a week.
They were “highly engaged,” Dr. Ganju said, checking their blood pressure a mean of 1.4 times weekly and their weight almost twice weekly. Providers received “four notifications of abnormal values,” he said.
Dr. Ganju and his colleagues are hopeful that the digital health tool “can really be effective in addressing the issue of excessive weight gain,” he said at the ACOG meeting. They are also beginning a study on remote monitoring for patients with chronic hypertension.
Findings on the effectiveness of remote personalized weight management are also expected to come from the soon-to-be-completed LIFE-Moms study, a national project looking at how overweight and obese women can best manage weight gain in pregnancy and improve their maternal and fetal outcomes.
Dr. Ganju reported having no disclosures. Two coauthors reported a nonfinancial advisory relationship with 1Eq Inc., the mobile app company that developed Babyscripts and helped fund the study. Another author is an employee of 1Eq. Dr. Tobah reported that she had no disclosures.
WASHINGTON – Is it time to reconsider the standard prenatal care model of 12-14 in-office prenatal visits?
As pregnant women increasingly use digital technology, and as the array of available health monitoring tools grows larger and smarter, the question looms.
Research findings reported at the annual meeting of the American College of Obstetricians and Gynecologists suggest that women with low-risk pregnancies have equivalent outcomes but are more satisfied with models that reduce the number of office visits and utilize remote monitoring.
At the Mayo Clinic in Rochester, Minn., 300 women deemed to have low-risk pregnancies were randomized to either 12 planned office visits with a physician or midwife, or to the clinic’s “OB Nest” model of care consisting of 8 planned clinic visits with a physician or midwife, 6 virtual visits with a nurse (by phone or email), home monitoring with an automatic blood pressure cuff and a hand-held fetal Doppler monitor, and access to an online prenatal care community.
The clinic’s OB Nest model “was born out of concern that the traditional model no longer met the needs of our patients,” said Dr. Yvonne S. Butler Tobah, a senior associate consultant to the department of obstetrics and gynecology at Mayo.
The goal, she said, is to “shift our prenatal clinic’s culture … to a wellness care model and to strengthen the autonomy, confidence, self-awareness and empowerment of our patients.”
Patients in the OB Nest group were encouraged to get blood pressure readings once a week, and weekly Doppler readings of fetal heart rate between weeks 12 and 28. They could use the cuff and monitor anytime they chose, however.
Values were recorded in a pregnancy journal – along with weight – and reported during the scheduled virtual visits. The patients could send in concerning readings or otherwise communicate with dedicated OB Nest nurses at any time they chose by phone or via an online portal. Emergencies were to be reported immediately.
The online prenatal care community is an invitation-only, Mayo-specific social platform, monitored by the OB Nest nurses, which gave patients the opportunity to share and discuss issues.
Patient satisfaction, as measured at 36 weeks with a 16-item validated satisfaction scale, was significantly higher in the OB Nest group; these patients had a mean score of 93.9 on a 1-100 point scale, compared with a mean score of 78.9 in the usual care group.
Levels of pregnancy-related stress – measured at three points in time with a 9-item prenatal maternal stress survey – were also significantly lower at 14 weeks and lower at 36 weeks in the OB Nest group compared with usual care. Stress levels were similar in both groups at 24 weeks.
Perceived quality of care was assessed at 36 weeks using a modified version of a prenatal processes-of-care scale that addressed communication and decision making, and no differences were observed.
“OB Nest significantly improved patient satisfaction with care and reduced maternal stress,” Dr. Tobah said. “And it did this while maintaining perceived quality of care and maintaining [safe] outcomes.”
The study was not sufficiently powered to detect statistically significant differences in clinical outcomes, which were the study’s secondary outcomes. However, there were no differences observed in maternal-fetal events or delivery outcomes, with the exception of a 4.5% rate of gestational diabetes in the OB Nest group, compared with none in the usual care group, Dr. Tobah explained.
Of the 150 patients randomized to each group, 19 and 20 were lost to follow-up in the OB Nest group and usual care group, respectively. Patients in the study had a mean age of 29, and the majority were white and married. “It was a highly educated, low-risk group,” she said. “Patients said [at the end] that they liked the general accessibility and consistent communication with a provider on an ongoing basis.”
While patients in the OB Nest group ultimately had 3.4 fewer in-office appointments than did usual care patients, they required more out-of-office nursing time and the length of in-office visits was significantly higher, Dr. Tobah noted.
In another study of remote prenatal care monitoring reported at the ACOG meeting, low-risk patients assigned to an alternative prenatal care schedule of 8 in-office visits supplemented with digital monitoring of blood pressure and weight similarly had higher patient satisfaction scores than did low-risk patients assigned to 14 prenatal visits.
Patient satisfaction was measured several times during pregnancy. Scores were significantly higher at 20 weeks in the 49-patient alternative care group “and evened out [with the 41-patient usual-care group] at the tail end of pregnancy,” said Dr. Nihar Ganju of George Washington University, Washington. “And there was no difference in pregnancy outcomes.”
This study used the Babyscripts mobile app connected to a wireless weight scale and a wireless blood pressure cuff. Patients were instructed to check their weight and blood pressure at least once a week.
They were “highly engaged,” Dr. Ganju said, checking their blood pressure a mean of 1.4 times weekly and their weight almost twice weekly. Providers received “four notifications of abnormal values,” he said.
Dr. Ganju and his colleagues are hopeful that the digital health tool “can really be effective in addressing the issue of excessive weight gain,” he said at the ACOG meeting. They are also beginning a study on remote monitoring for patients with chronic hypertension.
Findings on the effectiveness of remote personalized weight management are also expected to come from the soon-to-be-completed LIFE-Moms study, a national project looking at how overweight and obese women can best manage weight gain in pregnancy and improve their maternal and fetal outcomes.
Dr. Ganju reported having no disclosures. Two coauthors reported a nonfinancial advisory relationship with 1Eq Inc., the mobile app company that developed Babyscripts and helped fund the study. Another author is an employee of 1Eq. Dr. Tobah reported that she had no disclosures.
AT ACOG 2016
Key clinical point: Patient satisfaction improves with reduced clinic visits supplemented with remote monitoring.
Major finding: Patient satisfaction scores were significantly higher in alternative care groups, compared with usual care groups (12-14 in-office visits) in two studies.
Data source: A 300-patient randomized controlled trial of the OB Nest model at the Mayo Clinic, Rochester, Minn., and a 90-patient controlled study involving the Babyscripts tool at George Washington University, Washington.
Disclosures: Dr. Ganju reported having no disclosures. Two coauthors reported a nonfinancial advisory relationship with 1Eq Inc., the mobile app company that developed Babyscripts and helped fund the study. Another author is an employee of 1Eq. Dr. Tobah reported that she had no disclosures.
Maternal vaccination against pertussis can protect premature infants
Maternal immunization in the early third trimester (from 28 weeks’ gestation) may protect premature infants from pertussis, study results found.
This was the finding of an observational substudy of a larger multicenter, randomized, controlled vaccination trial of premature infants (the PUNS trial), which compared pertussis antibody concentrations before and after primary immunization in premature infants born to mothers who were or were not vaccinated with Repevax. Dr. Alison Kent of St George’s, University of London, and colleagues assessed the levels of the five vaccine antigens present in the maternal combination Repevax vaccine (pertussis toxoid, filamentous hemagglutinin, fimbriae types 2 and 3, diphtheria toxoid, tetanus toxoid, and inactivated poliovirus) in premature infants born to mothers who either received or did not receive Repevax from 28 weeks’ gestation. Antigen quantifications were conducted in these premature infants at approximately 2, 5, and 12 months of age.
Thirty-one (19%) of the 160 premature infants in the substudy were born to mothers who had been vaccinated. Two months after their premature birth, infants born to vaccinated mothers had significantly higher concentrations of all five measured antigens, compared with those born to unvaccinated mothers (all P values less than .001). Although fewer infants were sampled at 5 months of age, significantly higher concentrations of filamentous hemagglutinin and diphtheria toxoid were still found in those born to vaccinated mothers (both P = .003). Data collected at the 12-month assessment indicated that only tetanus antibody concentrations remained significantly higher in those born to vaccinated mothers (P = .015). A positive correlation between the number of days from maternal vaccination to delivery was found for all measured antigens, with the exception of fimbriae types 2 and 3.
“The emergency introduction of a maternal immunization program to control a national pertussis outbreak serendipitously provided an opportunity to assess antibody concentrations to maternal vaccine antigens in premature infants,” Dr. Kent and associates noted in Pediatrics (June 2016 doi: 10.1542/peds.2015-3854). This unexpected opportunity resulted in evidence supporting a protective effect against pertussis in the early lives of infants born prematurely to mothers immunized in their early third trimester.
Pfizer and the National Institute for Health Research Clinical Research Network funded the study. Professor Heath and Dr. Ladhani disclosed conducting studies on behalf of St George’s, University of London funded by vaccine manufacturers without receiving personal payments or travel support. The other authors reported no conflicts of interest.
Maternal immunization in the early third trimester (from 28 weeks’ gestation) may protect premature infants from pertussis, study results found.
This was the finding of an observational substudy of a larger multicenter, randomized, controlled vaccination trial of premature infants (the PUNS trial), which compared pertussis antibody concentrations before and after primary immunization in premature infants born to mothers who were or were not vaccinated with Repevax. Dr. Alison Kent of St George’s, University of London, and colleagues assessed the levels of the five vaccine antigens present in the maternal combination Repevax vaccine (pertussis toxoid, filamentous hemagglutinin, fimbriae types 2 and 3, diphtheria toxoid, tetanus toxoid, and inactivated poliovirus) in premature infants born to mothers who either received or did not receive Repevax from 28 weeks’ gestation. Antigen quantifications were conducted in these premature infants at approximately 2, 5, and 12 months of age.
Thirty-one (19%) of the 160 premature infants in the substudy were born to mothers who had been vaccinated. Two months after their premature birth, infants born to vaccinated mothers had significantly higher concentrations of all five measured antigens, compared with those born to unvaccinated mothers (all P values less than .001). Although fewer infants were sampled at 5 months of age, significantly higher concentrations of filamentous hemagglutinin and diphtheria toxoid were still found in those born to vaccinated mothers (both P = .003). Data collected at the 12-month assessment indicated that only tetanus antibody concentrations remained significantly higher in those born to vaccinated mothers (P = .015). A positive correlation between the number of days from maternal vaccination to delivery was found for all measured antigens, with the exception of fimbriae types 2 and 3.
“The emergency introduction of a maternal immunization program to control a national pertussis outbreak serendipitously provided an opportunity to assess antibody concentrations to maternal vaccine antigens in premature infants,” Dr. Kent and associates noted in Pediatrics (June 2016 doi: 10.1542/peds.2015-3854). This unexpected opportunity resulted in evidence supporting a protective effect against pertussis in the early lives of infants born prematurely to mothers immunized in their early third trimester.
Pfizer and the National Institute for Health Research Clinical Research Network funded the study. Professor Heath and Dr. Ladhani disclosed conducting studies on behalf of St George’s, University of London funded by vaccine manufacturers without receiving personal payments or travel support. The other authors reported no conflicts of interest.
Maternal immunization in the early third trimester (from 28 weeks’ gestation) may protect premature infants from pertussis, study results found.
This was the finding of an observational substudy of a larger multicenter, randomized, controlled vaccination trial of premature infants (the PUNS trial), which compared pertussis antibody concentrations before and after primary immunization in premature infants born to mothers who were or were not vaccinated with Repevax. Dr. Alison Kent of St George’s, University of London, and colleagues assessed the levels of the five vaccine antigens present in the maternal combination Repevax vaccine (pertussis toxoid, filamentous hemagglutinin, fimbriae types 2 and 3, diphtheria toxoid, tetanus toxoid, and inactivated poliovirus) in premature infants born to mothers who either received or did not receive Repevax from 28 weeks’ gestation. Antigen quantifications were conducted in these premature infants at approximately 2, 5, and 12 months of age.
Thirty-one (19%) of the 160 premature infants in the substudy were born to mothers who had been vaccinated. Two months after their premature birth, infants born to vaccinated mothers had significantly higher concentrations of all five measured antigens, compared with those born to unvaccinated mothers (all P values less than .001). Although fewer infants were sampled at 5 months of age, significantly higher concentrations of filamentous hemagglutinin and diphtheria toxoid were still found in those born to vaccinated mothers (both P = .003). Data collected at the 12-month assessment indicated that only tetanus antibody concentrations remained significantly higher in those born to vaccinated mothers (P = .015). A positive correlation between the number of days from maternal vaccination to delivery was found for all measured antigens, with the exception of fimbriae types 2 and 3.
“The emergency introduction of a maternal immunization program to control a national pertussis outbreak serendipitously provided an opportunity to assess antibody concentrations to maternal vaccine antigens in premature infants,” Dr. Kent and associates noted in Pediatrics (June 2016 doi: 10.1542/peds.2015-3854). This unexpected opportunity resulted in evidence supporting a protective effect against pertussis in the early lives of infants born prematurely to mothers immunized in their early third trimester.
Pfizer and the National Institute for Health Research Clinical Research Network funded the study. Professor Heath and Dr. Ladhani disclosed conducting studies on behalf of St George’s, University of London funded by vaccine manufacturers without receiving personal payments or travel support. The other authors reported no conflicts of interest.
FROM PEDIATRICS
Key clinical point: Vaccination of pregnant women early in their third trimester may protect premature infants against pertussis.
Major finding: At 2 months after birth, infants born to vaccinated mothers displayed higher antibody concentrations to vaccine antigens than did infants born to unvaccinated mothers.
Data source: Premature infants born to mothers that did or did not receive Repevax from 28 weeks of pregnancy.
Disclosures: Pfizer Ltd. and the National Institute for Health Research Clinical Research Network funded the study. Professor Heath and Dr. Ladhani disclosed conducting studies on behalf of St George’s, University of London funded by vaccine manufacturers without receiving personal payments or travel support. The other authors reported no conflicts of interest.
Women prescribed opioids during pregnancy have outcomes similar to those of illicit users
WASHINGTON – Women using chronic prescription opioids while pregnant have obstetrics outcomes similar to those of women chronically using illegal opioids and/or other forms of opioid substitution therapy.
Dr. Kaitlin Hanmer of Brigham & Women’s Hospital and Massachusetts General Hospital, Boston, analyzed 76 women who presented to the medical center for care between 2000 and 2015, comparing 22 women in the prescription opioid group and 54 women in the illicit opioid/opioid substitution therapy group. Among the 22 women in the prescription group, 13.6% of those pregnancies had a neonatal intensive care unit (NICU) consult, compared with 44.4% of the 54 women in the illicit opioid/opioid substitution therapy. She presented the findings at the annual meeting of the American College of Obstetricians and Gynecologists.
“The surprising finding was that women who used prescription opioids were less likely to be offered, or to have, an NICU consult during their pregnancy – prior to the birth – than were the women in the illicit opioid/opioid substitution therapy group, even though infants from both groups were significantly more likely to have longer hospital stays than their mothers,” Dr. Hanmer said in an interview.
In both groups, the hospital stays for the infants were significantly longer than for their mothers. Among infants born to mothers in the prescribed opioids group, 54.5% were hospitalized for more than 4 days longer than their mothers. This was true for 83.3% of infants born to mothers in the illicit opioid/opioid substitution therapy group.
The study also examined neonatal abstinence syndrome and found that 50% of infants of mothers using prescribed opioids were likely to be diagnosed with neonatal abstinence syndrome, compared with 81.5% of the infants of mothers in the illicit opioid/opioid substitution therapy group.
“Regardless of type of opioid use in pregnancy or preconceived notions about certain patients, we as practicing doctors need to use our points of care during the antenatal period to maximize the success of transition to the post partum period for both mom and baby,” Dr. Hanmer said. “At the end of the day, the goal is healthy mom and healthy baby.”
Dr. Hanmer reported having no financial disclosures.
WASHINGTON – Women using chronic prescription opioids while pregnant have obstetrics outcomes similar to those of women chronically using illegal opioids and/or other forms of opioid substitution therapy.
Dr. Kaitlin Hanmer of Brigham & Women’s Hospital and Massachusetts General Hospital, Boston, analyzed 76 women who presented to the medical center for care between 2000 and 2015, comparing 22 women in the prescription opioid group and 54 women in the illicit opioid/opioid substitution therapy group. Among the 22 women in the prescription group, 13.6% of those pregnancies had a neonatal intensive care unit (NICU) consult, compared with 44.4% of the 54 women in the illicit opioid/opioid substitution therapy. She presented the findings at the annual meeting of the American College of Obstetricians and Gynecologists.
“The surprising finding was that women who used prescription opioids were less likely to be offered, or to have, an NICU consult during their pregnancy – prior to the birth – than were the women in the illicit opioid/opioid substitution therapy group, even though infants from both groups were significantly more likely to have longer hospital stays than their mothers,” Dr. Hanmer said in an interview.
In both groups, the hospital stays for the infants were significantly longer than for their mothers. Among infants born to mothers in the prescribed opioids group, 54.5% were hospitalized for more than 4 days longer than their mothers. This was true for 83.3% of infants born to mothers in the illicit opioid/opioid substitution therapy group.
The study also examined neonatal abstinence syndrome and found that 50% of infants of mothers using prescribed opioids were likely to be diagnosed with neonatal abstinence syndrome, compared with 81.5% of the infants of mothers in the illicit opioid/opioid substitution therapy group.
“Regardless of type of opioid use in pregnancy or preconceived notions about certain patients, we as practicing doctors need to use our points of care during the antenatal period to maximize the success of transition to the post partum period for both mom and baby,” Dr. Hanmer said. “At the end of the day, the goal is healthy mom and healthy baby.”
Dr. Hanmer reported having no financial disclosures.
WASHINGTON – Women using chronic prescription opioids while pregnant have obstetrics outcomes similar to those of women chronically using illegal opioids and/or other forms of opioid substitution therapy.
Dr. Kaitlin Hanmer of Brigham & Women’s Hospital and Massachusetts General Hospital, Boston, analyzed 76 women who presented to the medical center for care between 2000 and 2015, comparing 22 women in the prescription opioid group and 54 women in the illicit opioid/opioid substitution therapy group. Among the 22 women in the prescription group, 13.6% of those pregnancies had a neonatal intensive care unit (NICU) consult, compared with 44.4% of the 54 women in the illicit opioid/opioid substitution therapy. She presented the findings at the annual meeting of the American College of Obstetricians and Gynecologists.
“The surprising finding was that women who used prescription opioids were less likely to be offered, or to have, an NICU consult during their pregnancy – prior to the birth – than were the women in the illicit opioid/opioid substitution therapy group, even though infants from both groups were significantly more likely to have longer hospital stays than their mothers,” Dr. Hanmer said in an interview.
In both groups, the hospital stays for the infants were significantly longer than for their mothers. Among infants born to mothers in the prescribed opioids group, 54.5% were hospitalized for more than 4 days longer than their mothers. This was true for 83.3% of infants born to mothers in the illicit opioid/opioid substitution therapy group.
The study also examined neonatal abstinence syndrome and found that 50% of infants of mothers using prescribed opioids were likely to be diagnosed with neonatal abstinence syndrome, compared with 81.5% of the infants of mothers in the illicit opioid/opioid substitution therapy group.
“Regardless of type of opioid use in pregnancy or preconceived notions about certain patients, we as practicing doctors need to use our points of care during the antenatal period to maximize the success of transition to the post partum period for both mom and baby,” Dr. Hanmer said. “At the end of the day, the goal is healthy mom and healthy baby.”
Dr. Hanmer reported having no financial disclosures.
AT ACOG 2016
Key clinical point: Use of chronic prescription opioids in pregnancy results in similar obstetrics outcomes, compared with use of illegal opioids and other forms of opioid substitution therapy.
Major finding: Half of infants whose mothers used prescribed opioids were likely to be diagnosed with neonatal abstinence syndrome, compared with 81.5% of the infants of mothers using illicit opioid and/or opioid substitution therapy.
Data source: A retrospective chart review of 76 pregnancies between 2000 and 2015.
Disclosures: Dr. Hanmer reported having no financial disclosures.
CDC updates guidelines for Zika virus testing, interpretation of results
Patients with suspected Zika virus infection who have negative real-time reverse transcription–polymerase chain reaction (rRT-PCR) test results are not automatically in the clear, officials at the Centers for Disease Control and Prevention warned in new guidelines on the interpretation of Zika virus antibody test results.
The CDC cautions that while a positive rRT-PCR test confirms a Zika virus infection, a negative test cannot exclude infection, because of Zika’s viremic similarity to other mosquito-borne flaviviruses such as dengue, West Nile, Japanese encephalitis, and yellow fever. In cases of a negative rRT-PCR results, patients should undergo immunoglobulin M (IgM) antibody testing; IgM testing should be done on any patient with a negative rRT-PCR test, regardless of the day on which the sample was originally collected. For confirmation of a positive, equivocal, or inconclusive result on the IgM test, patients will require a plaque reduction neutralization test (PRNT) as well.
“Recent evidence suggests that a 4-fold higher titer by PRNT might not discriminate between anti-Zika virus antibodies and crossreacting antibodies in all persons who have been previously infected with or vaccinated against a related flavivirus. Thus, a more conservative approach to interpreting PRNT results is now recommended to reduce the possibility of missing the diagnosis of either Zika or dengue virus infection,” CDC officials wrote. The report is in the May 31 issue of Morbidity and Mortality Weekly Report (doi: http://dx.doi.org/10.15585/mmwr.mm6521e1).
Any PRNT titer greater than 10 should be interpreted as proof of infection with the specific tested flavivirus, if the titer is less than 10 for other tested flaviviruses. If multiple flaviviruses are tested and all result in a titer of 10 or greater, than the result is proof of a recent infection with a flavivirus, not Zika specifically. And if the PRNT titer is less than 10 for a specific flavivirus, then that should be interpreted as there being no evidence of infection with that virus.
The CDC urges health care providers who have patients suspected of Zika virus infection to consult with their local and state health agencies to properly interpret any test results. Any pregnant women who have a confirmed Zika virus infection should be reported to the U.S. Zika Pregnancy Registry or the Puerto Rico Zika Active Pregnancy Surveillance System, with continued monitoring for any possible adverse effects to the pregnancy.
“If serologic testing indicates recent flavivirus infection that could be caused by either Zika or dengue virus, patients should be clinically managed for both infections because they might have been infected with either virus,” the CDC cautioned.
Patients with suspected Zika virus infection who have negative real-time reverse transcription–polymerase chain reaction (rRT-PCR) test results are not automatically in the clear, officials at the Centers for Disease Control and Prevention warned in new guidelines on the interpretation of Zika virus antibody test results.
The CDC cautions that while a positive rRT-PCR test confirms a Zika virus infection, a negative test cannot exclude infection, because of Zika’s viremic similarity to other mosquito-borne flaviviruses such as dengue, West Nile, Japanese encephalitis, and yellow fever. In cases of a negative rRT-PCR results, patients should undergo immunoglobulin M (IgM) antibody testing; IgM testing should be done on any patient with a negative rRT-PCR test, regardless of the day on which the sample was originally collected. For confirmation of a positive, equivocal, or inconclusive result on the IgM test, patients will require a plaque reduction neutralization test (PRNT) as well.
“Recent evidence suggests that a 4-fold higher titer by PRNT might not discriminate between anti-Zika virus antibodies and crossreacting antibodies in all persons who have been previously infected with or vaccinated against a related flavivirus. Thus, a more conservative approach to interpreting PRNT results is now recommended to reduce the possibility of missing the diagnosis of either Zika or dengue virus infection,” CDC officials wrote. The report is in the May 31 issue of Morbidity and Mortality Weekly Report (doi: http://dx.doi.org/10.15585/mmwr.mm6521e1).
Any PRNT titer greater than 10 should be interpreted as proof of infection with the specific tested flavivirus, if the titer is less than 10 for other tested flaviviruses. If multiple flaviviruses are tested and all result in a titer of 10 or greater, than the result is proof of a recent infection with a flavivirus, not Zika specifically. And if the PRNT titer is less than 10 for a specific flavivirus, then that should be interpreted as there being no evidence of infection with that virus.
The CDC urges health care providers who have patients suspected of Zika virus infection to consult with their local and state health agencies to properly interpret any test results. Any pregnant women who have a confirmed Zika virus infection should be reported to the U.S. Zika Pregnancy Registry or the Puerto Rico Zika Active Pregnancy Surveillance System, with continued monitoring for any possible adverse effects to the pregnancy.
“If serologic testing indicates recent flavivirus infection that could be caused by either Zika or dengue virus, patients should be clinically managed for both infections because they might have been infected with either virus,” the CDC cautioned.
Patients with suspected Zika virus infection who have negative real-time reverse transcription–polymerase chain reaction (rRT-PCR) test results are not automatically in the clear, officials at the Centers for Disease Control and Prevention warned in new guidelines on the interpretation of Zika virus antibody test results.
The CDC cautions that while a positive rRT-PCR test confirms a Zika virus infection, a negative test cannot exclude infection, because of Zika’s viremic similarity to other mosquito-borne flaviviruses such as dengue, West Nile, Japanese encephalitis, and yellow fever. In cases of a negative rRT-PCR results, patients should undergo immunoglobulin M (IgM) antibody testing; IgM testing should be done on any patient with a negative rRT-PCR test, regardless of the day on which the sample was originally collected. For confirmation of a positive, equivocal, or inconclusive result on the IgM test, patients will require a plaque reduction neutralization test (PRNT) as well.
“Recent evidence suggests that a 4-fold higher titer by PRNT might not discriminate between anti-Zika virus antibodies and crossreacting antibodies in all persons who have been previously infected with or vaccinated against a related flavivirus. Thus, a more conservative approach to interpreting PRNT results is now recommended to reduce the possibility of missing the diagnosis of either Zika or dengue virus infection,” CDC officials wrote. The report is in the May 31 issue of Morbidity and Mortality Weekly Report (doi: http://dx.doi.org/10.15585/mmwr.mm6521e1).
Any PRNT titer greater than 10 should be interpreted as proof of infection with the specific tested flavivirus, if the titer is less than 10 for other tested flaviviruses. If multiple flaviviruses are tested and all result in a titer of 10 or greater, than the result is proof of a recent infection with a flavivirus, not Zika specifically. And if the PRNT titer is less than 10 for a specific flavivirus, then that should be interpreted as there being no evidence of infection with that virus.
The CDC urges health care providers who have patients suspected of Zika virus infection to consult with their local and state health agencies to properly interpret any test results. Any pregnant women who have a confirmed Zika virus infection should be reported to the U.S. Zika Pregnancy Registry or the Puerto Rico Zika Active Pregnancy Surveillance System, with continued monitoring for any possible adverse effects to the pregnancy.
“If serologic testing indicates recent flavivirus infection that could be caused by either Zika or dengue virus, patients should be clinically managed for both infections because they might have been infected with either virus,” the CDC cautioned.
FROM MMWR
When should we stop aspirin during pregnancy?
When should we stop aspirin during pregnancy?
In his Editorial, Dr. Barbieri discusses the ideal time to start aspirin in women at high risk for preeclampsia, but does not identify when to stop this medication. At our community health center, we have been stopping oral aspirin 81 mg at 36 weeks’ gestation because of the “potential” for postpartum hemorrhage or intrapartum hemorrhage after this time. Is there any literature regarding the evidence behind this date?
Tammy R. Gruenberg, MD, MPH
Bronx, New York
Dr. Barbieri responds
I appreciate Dr. Gruenberg’s important advice for our readers. Although low-dose aspirin is not known to be a major risk factor for adverse maternal or fetal outcomes, it is wise to stop the therapy a week prior to delivery, to reduce the theoretical risk of postpartum hemorrhage. Stopping aspirin at 36 or 37 weeks’ gestation will ensure that the majority of women are not taking aspirin at delivery.
Another shoulder dystocia maneuver?
An additional technique that I use for managing shoulder dystocia is to simply track upward with the baby’s head, delivering the posterior shoulder without injury to the arm. Once the posterior shoulder clears the plane of the pubis and the anterior shoulder is mobilized, the posterior shoulder is rotated anterior in front of the pubic plane and the body unscrews itself from the pelvis. I also described this technique in a published letter to the editor in August 2013.
Dr. Barbieri’s suggestions in his April 2016 article are complicated for the less experienced ObGyn and could be dangerous for the baby (with potential fractures, nerve, and vascular injuries). Think about the described Gaskin maneuver: you flip the patient over on all fours, pull the baby’s head down, and deliver the superior shoulder (formerly the posterior shoulder).
Many obese and exhausted patients with epidurals will not be able to flip over for the Gaskin maneuver. The beauty of what I suggest is that this repositioning is not needed, and pulling on arms and axillae can endanger the baby.
Robert Graebe, MD
Long Branch, New Jersey
Dr. Barbieri responds
I thank Dr. Graebe for describing his approach to resolving an intractable shoulder dystocia. Personally, I try to avoid applying force to the fetal head once a shoulder dystocia has been diagnosed.
QUICK POLL RESULTS
Preferred approaches to resolving the difficult shoulder dystocia
In his article, "Intractable shoulder dystocia: A posterior axilla maneuver may save the day," which appeared in the April 2016 issue of OBG Management, Editor in Chief Robert L. Barbieri, MD, offered several posterior axilla maneuvers to use when initial shoulder dystocia management steps are not enough.
He indicated his preferred maneuver as the Menticoglou, and asked readers: "What is your preferred approach to resolving the difficult shoulder dystocia?"
More than 100 readers weighed in:
- 33.6% (38 readers) prefer the Menicoglou maneuver
- 21.2% (24 readers) prefer the Schramm maneuver
- 19.5% (22 readers) prefer the Holman maneuver
- 15% (17 readers) prefer the Willughby maneuver
- 10.6% (12 readers) prefer the Hofmeyr-Cluver maneuver.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
When should we stop aspirin during pregnancy?
In his Editorial, Dr. Barbieri discusses the ideal time to start aspirin in women at high risk for preeclampsia, but does not identify when to stop this medication. At our community health center, we have been stopping oral aspirin 81 mg at 36 weeks’ gestation because of the “potential” for postpartum hemorrhage or intrapartum hemorrhage after this time. Is there any literature regarding the evidence behind this date?
Tammy R. Gruenberg, MD, MPH
Bronx, New York
Dr. Barbieri responds
I appreciate Dr. Gruenberg’s important advice for our readers. Although low-dose aspirin is not known to be a major risk factor for adverse maternal or fetal outcomes, it is wise to stop the therapy a week prior to delivery, to reduce the theoretical risk of postpartum hemorrhage. Stopping aspirin at 36 or 37 weeks’ gestation will ensure that the majority of women are not taking aspirin at delivery.
Another shoulder dystocia maneuver?
An additional technique that I use for managing shoulder dystocia is to simply track upward with the baby’s head, delivering the posterior shoulder without injury to the arm. Once the posterior shoulder clears the plane of the pubis and the anterior shoulder is mobilized, the posterior shoulder is rotated anterior in front of the pubic plane and the body unscrews itself from the pelvis. I also described this technique in a published letter to the editor in August 2013.
Dr. Barbieri’s suggestions in his April 2016 article are complicated for the less experienced ObGyn and could be dangerous for the baby (with potential fractures, nerve, and vascular injuries). Think about the described Gaskin maneuver: you flip the patient over on all fours, pull the baby’s head down, and deliver the superior shoulder (formerly the posterior shoulder).
Many obese and exhausted patients with epidurals will not be able to flip over for the Gaskin maneuver. The beauty of what I suggest is that this repositioning is not needed, and pulling on arms and axillae can endanger the baby.
Robert Graebe, MD
Long Branch, New Jersey
Dr. Barbieri responds
I thank Dr. Graebe for describing his approach to resolving an intractable shoulder dystocia. Personally, I try to avoid applying force to the fetal head once a shoulder dystocia has been diagnosed.
QUICK POLL RESULTS
Preferred approaches to resolving the difficult shoulder dystocia
In his article, "Intractable shoulder dystocia: A posterior axilla maneuver may save the day," which appeared in the April 2016 issue of OBG Management, Editor in Chief Robert L. Barbieri, MD, offered several posterior axilla maneuvers to use when initial shoulder dystocia management steps are not enough.
He indicated his preferred maneuver as the Menticoglou, and asked readers: "What is your preferred approach to resolving the difficult shoulder dystocia?"
More than 100 readers weighed in:
- 33.6% (38 readers) prefer the Menicoglou maneuver
- 21.2% (24 readers) prefer the Schramm maneuver
- 19.5% (22 readers) prefer the Holman maneuver
- 15% (17 readers) prefer the Willughby maneuver
- 10.6% (12 readers) prefer the Hofmeyr-Cluver maneuver.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
When should we stop aspirin during pregnancy?
In his Editorial, Dr. Barbieri discusses the ideal time to start aspirin in women at high risk for preeclampsia, but does not identify when to stop this medication. At our community health center, we have been stopping oral aspirin 81 mg at 36 weeks’ gestation because of the “potential” for postpartum hemorrhage or intrapartum hemorrhage after this time. Is there any literature regarding the evidence behind this date?
Tammy R. Gruenberg, MD, MPH
Bronx, New York
Dr. Barbieri responds
I appreciate Dr. Gruenberg’s important advice for our readers. Although low-dose aspirin is not known to be a major risk factor for adverse maternal or fetal outcomes, it is wise to stop the therapy a week prior to delivery, to reduce the theoretical risk of postpartum hemorrhage. Stopping aspirin at 36 or 37 weeks’ gestation will ensure that the majority of women are not taking aspirin at delivery.
Another shoulder dystocia maneuver?
An additional technique that I use for managing shoulder dystocia is to simply track upward with the baby’s head, delivering the posterior shoulder without injury to the arm. Once the posterior shoulder clears the plane of the pubis and the anterior shoulder is mobilized, the posterior shoulder is rotated anterior in front of the pubic plane and the body unscrews itself from the pelvis. I also described this technique in a published letter to the editor in August 2013.
Dr. Barbieri’s suggestions in his April 2016 article are complicated for the less experienced ObGyn and could be dangerous for the baby (with potential fractures, nerve, and vascular injuries). Think about the described Gaskin maneuver: you flip the patient over on all fours, pull the baby’s head down, and deliver the superior shoulder (formerly the posterior shoulder).
Many obese and exhausted patients with epidurals will not be able to flip over for the Gaskin maneuver. The beauty of what I suggest is that this repositioning is not needed, and pulling on arms and axillae can endanger the baby.
Robert Graebe, MD
Long Branch, New Jersey
Dr. Barbieri responds
I thank Dr. Graebe for describing his approach to resolving an intractable shoulder dystocia. Personally, I try to avoid applying force to the fetal head once a shoulder dystocia has been diagnosed.
QUICK POLL RESULTS
Preferred approaches to resolving the difficult shoulder dystocia
In his article, "Intractable shoulder dystocia: A posterior axilla maneuver may save the day," which appeared in the April 2016 issue of OBG Management, Editor in Chief Robert L. Barbieri, MD, offered several posterior axilla maneuvers to use when initial shoulder dystocia management steps are not enough.
He indicated his preferred maneuver as the Menticoglou, and asked readers: "What is your preferred approach to resolving the difficult shoulder dystocia?"
More than 100 readers weighed in:
- 33.6% (38 readers) prefer the Menicoglou maneuver
- 21.2% (24 readers) prefer the Schramm maneuver
- 19.5% (22 readers) prefer the Holman maneuver
- 15% (17 readers) prefer the Willughby maneuver
- 10.6% (12 readers) prefer the Hofmeyr-Cluver maneuver.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.