User login
Ondansetron not linked to any adverse fetal outcomes
Fetal exposure to ondansetron showed no association with adverse pregnancy outcomes in a nationwide Danish cohort study that included more than 608,000 pregnancies, according to a report published online Feb. 28 in the New England Journal of Medicine.
Ondansetron, an antiemetic often prescribed for nausea and vomiting during pregnancy, was not associated with an increased rate of spontaneous abortion, stillbirth, any major birth defect, preterm delivery, low-birth-weight (LBW) infants, or small-for-gestational-age (SGA) infants, reported Björn Pasternak, M.D., Ph.D., of the department of epidemiology research at Statens Serum Institut, Copenhagen, and his associates.
"Although these results cannot definitively rule out the possibility of adverse effects in association with ondansetron, the results do provide reassurance regarding the use of this agent for nausea and vomiting in pregnancy," the investigators noted.
To date, only two controlled studies have assessed the fetal safety of the drug, which nevertheless is the most frequently prescribed antiemetic in the United States.
Dr. Pasternak and his colleagues used data from Danish national registries to construct a nationwide historical cohort of all pregnancies that resulted in a singleton birth, stillbirth, or any abortive outcome between 2004 and March 31, 2011. (Ondansetron was rarely used during pregnancy before 2004 in Denmark.)
The investigators assessed outcomes in 608,385 pregnancies. Mothers took ondansetron in 1,970 of these pregnancies. The median number of doses dispensed was 30 per pregnancy.
In an initial unadjusted analysis, exposure to ondansetron did not increase the risk of stillbirth, major birth defects, LBW infants, or SGA infants.
The researchers then conducted several propensity-matched analyses for six possible adverse outcomes.
For the 1,233 pregnancies in this analysis in which the mother took ondansetron during the first trimester, 36 infants (2.9%) had a major birth defect. In comparison, 141 of 4,932 infants (also 2.9%) not exposed to the drug had a major birth defect. This study was not powered to assess the risks of individual birth defects.
The rate of preterm birth was 6.2% among women who took ondansetron and 5.2% among women who did not, a difference that was not significant. Similarly, the rates of stillbirth were 0.3% and 0.4%, respectively, also a nonsignificant difference.
The rates of LBW infants were 4.1% with exposure to ondansetron and 3.7% without exposure, also a nonsignificant difference. And the rates of SGA infants were 10.4% and 9.2%, another nonsignificant difference, Dr. Pasternak and his associates reported (New Engl. J. Med. 2013;368:814-23).
These results remained robust in several sensitivity analyses, including one that compared rates of adverse fetal outcomes between women who filled only one prescription for ondansetron and women who filled two or more such prescriptions.
Previously, a case-control study found an increase in the risk of cleft palate with in utero exposure to ondansetron. In this cohort, there were no cases of cleft palate, Dr. Pasternak and his associates said.
This study was funded by the Danish Medical Research Council. No financial conflicts of interest were reported.
Fetal exposure to ondansetron showed no association with adverse pregnancy outcomes in a nationwide Danish cohort study that included more than 608,000 pregnancies, according to a report published online Feb. 28 in the New England Journal of Medicine.
Ondansetron, an antiemetic often prescribed for nausea and vomiting during pregnancy, was not associated with an increased rate of spontaneous abortion, stillbirth, any major birth defect, preterm delivery, low-birth-weight (LBW) infants, or small-for-gestational-age (SGA) infants, reported Björn Pasternak, M.D., Ph.D., of the department of epidemiology research at Statens Serum Institut, Copenhagen, and his associates.
"Although these results cannot definitively rule out the possibility of adverse effects in association with ondansetron, the results do provide reassurance regarding the use of this agent for nausea and vomiting in pregnancy," the investigators noted.
To date, only two controlled studies have assessed the fetal safety of the drug, which nevertheless is the most frequently prescribed antiemetic in the United States.
Dr. Pasternak and his colleagues used data from Danish national registries to construct a nationwide historical cohort of all pregnancies that resulted in a singleton birth, stillbirth, or any abortive outcome between 2004 and March 31, 2011. (Ondansetron was rarely used during pregnancy before 2004 in Denmark.)
The investigators assessed outcomes in 608,385 pregnancies. Mothers took ondansetron in 1,970 of these pregnancies. The median number of doses dispensed was 30 per pregnancy.
In an initial unadjusted analysis, exposure to ondansetron did not increase the risk of stillbirth, major birth defects, LBW infants, or SGA infants.
The researchers then conducted several propensity-matched analyses for six possible adverse outcomes.
For the 1,233 pregnancies in this analysis in which the mother took ondansetron during the first trimester, 36 infants (2.9%) had a major birth defect. In comparison, 141 of 4,932 infants (also 2.9%) not exposed to the drug had a major birth defect. This study was not powered to assess the risks of individual birth defects.
The rate of preterm birth was 6.2% among women who took ondansetron and 5.2% among women who did not, a difference that was not significant. Similarly, the rates of stillbirth were 0.3% and 0.4%, respectively, also a nonsignificant difference.
The rates of LBW infants were 4.1% with exposure to ondansetron and 3.7% without exposure, also a nonsignificant difference. And the rates of SGA infants were 10.4% and 9.2%, another nonsignificant difference, Dr. Pasternak and his associates reported (New Engl. J. Med. 2013;368:814-23).
These results remained robust in several sensitivity analyses, including one that compared rates of adverse fetal outcomes between women who filled only one prescription for ondansetron and women who filled two or more such prescriptions.
Previously, a case-control study found an increase in the risk of cleft palate with in utero exposure to ondansetron. In this cohort, there were no cases of cleft palate, Dr. Pasternak and his associates said.
This study was funded by the Danish Medical Research Council. No financial conflicts of interest were reported.
Fetal exposure to ondansetron showed no association with adverse pregnancy outcomes in a nationwide Danish cohort study that included more than 608,000 pregnancies, according to a report published online Feb. 28 in the New England Journal of Medicine.
Ondansetron, an antiemetic often prescribed for nausea and vomiting during pregnancy, was not associated with an increased rate of spontaneous abortion, stillbirth, any major birth defect, preterm delivery, low-birth-weight (LBW) infants, or small-for-gestational-age (SGA) infants, reported Björn Pasternak, M.D., Ph.D., of the department of epidemiology research at Statens Serum Institut, Copenhagen, and his associates.
"Although these results cannot definitively rule out the possibility of adverse effects in association with ondansetron, the results do provide reassurance regarding the use of this agent for nausea and vomiting in pregnancy," the investigators noted.
To date, only two controlled studies have assessed the fetal safety of the drug, which nevertheless is the most frequently prescribed antiemetic in the United States.
Dr. Pasternak and his colleagues used data from Danish national registries to construct a nationwide historical cohort of all pregnancies that resulted in a singleton birth, stillbirth, or any abortive outcome between 2004 and March 31, 2011. (Ondansetron was rarely used during pregnancy before 2004 in Denmark.)
The investigators assessed outcomes in 608,385 pregnancies. Mothers took ondansetron in 1,970 of these pregnancies. The median number of doses dispensed was 30 per pregnancy.
In an initial unadjusted analysis, exposure to ondansetron did not increase the risk of stillbirth, major birth defects, LBW infants, or SGA infants.
The researchers then conducted several propensity-matched analyses for six possible adverse outcomes.
For the 1,233 pregnancies in this analysis in which the mother took ondansetron during the first trimester, 36 infants (2.9%) had a major birth defect. In comparison, 141 of 4,932 infants (also 2.9%) not exposed to the drug had a major birth defect. This study was not powered to assess the risks of individual birth defects.
The rate of preterm birth was 6.2% among women who took ondansetron and 5.2% among women who did not, a difference that was not significant. Similarly, the rates of stillbirth were 0.3% and 0.4%, respectively, also a nonsignificant difference.
The rates of LBW infants were 4.1% with exposure to ondansetron and 3.7% without exposure, also a nonsignificant difference. And the rates of SGA infants were 10.4% and 9.2%, another nonsignificant difference, Dr. Pasternak and his associates reported (New Engl. J. Med. 2013;368:814-23).
These results remained robust in several sensitivity analyses, including one that compared rates of adverse fetal outcomes between women who filled only one prescription for ondansetron and women who filled two or more such prescriptions.
Previously, a case-control study found an increase in the risk of cleft palate with in utero exposure to ondansetron. In this cohort, there were no cases of cleft palate, Dr. Pasternak and his associates said.
This study was funded by the Danish Medical Research Council. No financial conflicts of interest were reported.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Major finding: For pregnancies exposed to ondansetron, the rate of major birth defects was 2.9% (vs. 2.9% without exposure), that of stillbirth was 0.3% (vs. 0.4%), that of preterm birth was 6.2% (vs. 5.2%), that of LBW infants was 4.1% (vs. 3.7%), and that of SGA infants was 10.4% (vs. 9.2%).
Data source: An historical cohort study of adverse fetal outcomes in 608,385 pregnancies in Denmark during 2004-2011.
Disclosures: This study was funded by the Danish Medical Research Council. No financial conflicts of interest were reported.
Mobile technology in pregnancy catching on
With all the mobile technology available today, many patients pull out their phones, smartphones, or tablets while in waiting rooms or exam rooms, until the physician arrives.
You might be surprised to learn how many people are using these tools in ways related to pregnancy. Two new reports provide snapshots.
On average, 47% of subscribers to mobile data plans who use at least one health-related application (app) on their smartphones or tablets use an app related to pregnancy. That’s more than the 39% of app-using subscribers who used fitness-specific apps, a 2013 report from Citrix ByteMobile found.
The data are based on real-world use. Citrix ByteMobile works with 130 mobile operators in 60 countries to help them understand what people are doing on their mobile phones and devices, in order to try and optimize the data and video services on the operators’ 3G and 4G networks, according to MobiHealthNews.
Sounds impressive, but how many people is that, really? Let’s do a quick calculation. There were more than 239 million U.S. adults in 2012, according to the U.S. Census Bureau. Approximately 9% of all U.S. adults have at least one health or medical app on a smartphone, according to the "Mobile Health 2012" report by the Pew Research Center. That’s more than 21 million people, so far.
The different kinds of apps get used in different ways, the Citrix ByteMobile analysis found. Even though a greater proportion of app-using mobile subscribers use pregnancy-related apps, the fitness app users generated a lot more data, accounting for 50% of all mobile health-related data traffic on wireless networks, compared with 9% of health-related data traffic from the pregnancy app users.
The apps get used at different times of day, too. The busiest time of day for apps that monitor women’s health (such as pregnancy or menstrual tracking apps) is 9 a.m. For fitness apps, 6 p.m. is the busy hour.
Other popular kinds of personal-health apps include calorie counters, apps that provide medical information, sleep cycle trackers, and relaxation tools, the report noted.
And it’s not just smartphones and iPads catching pregnant women’s attention. Fully 85% of U.S. adults own a cell phone, according to the Pew report, and many are using them in ways related to health.
My colleague Naseem Miller reported that the public-private partnership textforbaby had 260,000 pregnant women and new moms receiving free educational texts and health-related reminders on their phones in 2011, up from 150,000 in less than a year. A small preliminary study suggests that this tool is making a difference in promoting timely immunizations, prompting conversations between women and their doctors, and more.
So, the next time you walk into an exam room and find your female patient on her phone or tablet, don’t assume she’s just playing Angry Birds to pass the time. She might actually be doing something for her or her baby’s health.
–By Sherry Boschert
On Twitter @sherryboschert
With all the mobile technology available today, many patients pull out their phones, smartphones, or tablets while in waiting rooms or exam rooms, until the physician arrives.
You might be surprised to learn how many people are using these tools in ways related to pregnancy. Two new reports provide snapshots.
On average, 47% of subscribers to mobile data plans who use at least one health-related application (app) on their smartphones or tablets use an app related to pregnancy. That’s more than the 39% of app-using subscribers who used fitness-specific apps, a 2013 report from Citrix ByteMobile found.
The data are based on real-world use. Citrix ByteMobile works with 130 mobile operators in 60 countries to help them understand what people are doing on their mobile phones and devices, in order to try and optimize the data and video services on the operators’ 3G and 4G networks, according to MobiHealthNews.
Sounds impressive, but how many people is that, really? Let’s do a quick calculation. There were more than 239 million U.S. adults in 2012, according to the U.S. Census Bureau. Approximately 9% of all U.S. adults have at least one health or medical app on a smartphone, according to the "Mobile Health 2012" report by the Pew Research Center. That’s more than 21 million people, so far.
The different kinds of apps get used in different ways, the Citrix ByteMobile analysis found. Even though a greater proportion of app-using mobile subscribers use pregnancy-related apps, the fitness app users generated a lot more data, accounting for 50% of all mobile health-related data traffic on wireless networks, compared with 9% of health-related data traffic from the pregnancy app users.
The apps get used at different times of day, too. The busiest time of day for apps that monitor women’s health (such as pregnancy or menstrual tracking apps) is 9 a.m. For fitness apps, 6 p.m. is the busy hour.
Other popular kinds of personal-health apps include calorie counters, apps that provide medical information, sleep cycle trackers, and relaxation tools, the report noted.
And it’s not just smartphones and iPads catching pregnant women’s attention. Fully 85% of U.S. adults own a cell phone, according to the Pew report, and many are using them in ways related to health.
My colleague Naseem Miller reported that the public-private partnership textforbaby had 260,000 pregnant women and new moms receiving free educational texts and health-related reminders on their phones in 2011, up from 150,000 in less than a year. A small preliminary study suggests that this tool is making a difference in promoting timely immunizations, prompting conversations between women and their doctors, and more.
So, the next time you walk into an exam room and find your female patient on her phone or tablet, don’t assume she’s just playing Angry Birds to pass the time. She might actually be doing something for her or her baby’s health.
–By Sherry Boschert
On Twitter @sherryboschert
With all the mobile technology available today, many patients pull out their phones, smartphones, or tablets while in waiting rooms or exam rooms, until the physician arrives.
You might be surprised to learn how many people are using these tools in ways related to pregnancy. Two new reports provide snapshots.
On average, 47% of subscribers to mobile data plans who use at least one health-related application (app) on their smartphones or tablets use an app related to pregnancy. That’s more than the 39% of app-using subscribers who used fitness-specific apps, a 2013 report from Citrix ByteMobile found.
The data are based on real-world use. Citrix ByteMobile works with 130 mobile operators in 60 countries to help them understand what people are doing on their mobile phones and devices, in order to try and optimize the data and video services on the operators’ 3G and 4G networks, according to MobiHealthNews.
Sounds impressive, but how many people is that, really? Let’s do a quick calculation. There were more than 239 million U.S. adults in 2012, according to the U.S. Census Bureau. Approximately 9% of all U.S. adults have at least one health or medical app on a smartphone, according to the "Mobile Health 2012" report by the Pew Research Center. That’s more than 21 million people, so far.
The different kinds of apps get used in different ways, the Citrix ByteMobile analysis found. Even though a greater proportion of app-using mobile subscribers use pregnancy-related apps, the fitness app users generated a lot more data, accounting for 50% of all mobile health-related data traffic on wireless networks, compared with 9% of health-related data traffic from the pregnancy app users.
The apps get used at different times of day, too. The busiest time of day for apps that monitor women’s health (such as pregnancy or menstrual tracking apps) is 9 a.m. For fitness apps, 6 p.m. is the busy hour.
Other popular kinds of personal-health apps include calorie counters, apps that provide medical information, sleep cycle trackers, and relaxation tools, the report noted.
And it’s not just smartphones and iPads catching pregnant women’s attention. Fully 85% of U.S. adults own a cell phone, according to the Pew report, and many are using them in ways related to health.
My colleague Naseem Miller reported that the public-private partnership textforbaby had 260,000 pregnant women and new moms receiving free educational texts and health-related reminders on their phones in 2011, up from 150,000 in less than a year. A small preliminary study suggests that this tool is making a difference in promoting timely immunizations, prompting conversations between women and their doctors, and more.
So, the next time you walk into an exam room and find your female patient on her phone or tablet, don’t assume she’s just playing Angry Birds to pass the time. She might actually be doing something for her or her baby’s health.
–By Sherry Boschert
On Twitter @sherryboschert
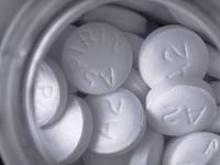
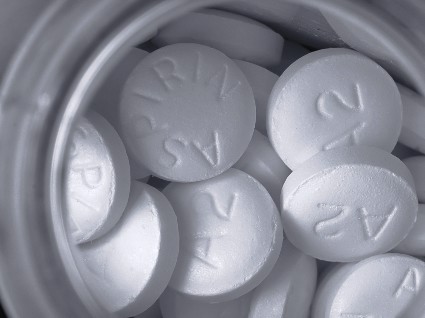
Aspirin improves chance of live birth after recent early pregnancy loss
Some women who have experienced a pregnancy loss can increase their odds of a live birth in the next pregnancy simply by taking aspirin, investigators reported at the Pregnancy Meeting, the annual meeting of the Society for Maternal-Fetal Medicine.
A team led by Enrique F. Schisterman, Ph.D., conducted a randomized trial of 1,228 healthy young women who had had up to two prior pregnancy losses, but did not have infertility and were attempting to conceive again.
The women were assigned evenly to take low-dose aspirin (81 mg) or placebo daily, along with folic acid, for up to six menstrual cycles or, if they conceived, up to the 36th week of pregnancy.
Results showed that low-dose aspirin was associated with an absolute 9.2% increase in the rate of live birth among the subset of women who met restricted criteria for pregnancy loss, namely a single pregnancy loss before 20 weeks’ gestation in the past year, reported Dr. Schisterman, who is a senior investigator and chief of the epidemiology branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Rockville, Md.
The benefit was mainly due to early effects. "There was an effect on becoming pregnant and early pregnancy [maintenance], but there were no differences after that," he elaborated. "The implications of that are not only that aspirin will help women become pregnant, but if you start too late, then the effects of aspirin are not there any more."
Analyses in the group with restricted criteria suggested that only about 11 women would need to be treated with low-dose aspirin to achieve one additional live birth.
In contrast, there was no significant benefit of low-dose aspirin among the subset of women who met general criteria for pregnancy loss that required one or two pregnancy losses at any time in the past, but excluded those meeting restricted criteria.
Low-dose aspirin was associated with somewhat higher rates of minor vaginal bleeding and minor gastrointestinal upset, but the drug was not associated with pregnancy loss or with an increased risk of major fetal, neonatal, or maternal complications.
An attendee wondered about the difference between the two subsets of women having differing histories of pregnancy loss, saying, "You would expect more or less the same effect."
Dr. Schisterman maintained that the two groups were not all that similar. "I am not sure I would expect the same result, although when we did some analyses in which we compared those who had a single loss in the restricted stratum to those who had a single loss in the general stratum, we found attenuated but in a similar direction results in the general stratum," he commented. "So it seems that the number of losses is the driving force. But we are still analyzing that data."
Another attendee raised the issue of the timing of the previous pregnancy loss in the subset meeting restricted criteria. "Were you able to identify any influence of the gestational age of the previous loss on the effectiveness of aspirin in the next pregnancy, the randomized pregnancy?" he asked.
"Not yet," Dr. Schisterman replied, noting that all of the losses were fairly early. However, here too, analyses are still ongoing.
Giving some background to the trial, he noted, "We know that inflammation and abnormal blood flow, especially in the uterus, endometrium, ovaries, and placenta, ... are unifying features of outcomes like infertility, pregnancy loss, preeclampsia, preterm delivery, and small for gestational age. So clearly, an ideal therapy that would reduce inflammation and improve blood flow will be the one that we are looking for. Low-dose aspirin could be such a therapy."
The drug has seldom been studied when given in the preconceptional period, but there is a strong rationale for such use, he maintained.
"It impacts endometrial vascularization and placentation. It has very well documented anti-inflammatory effects. It has very few maternal and fetal side effects. It’s safe, widely available, and more importantly, it’s cheap – it costs $2 for the whole pregnancy to treat a woman," he elaborated.
Women enrolled in the trial, known as EAGeR (The Effects of Aspirin in Gestation and Reproduction), were aged 18-39 years. They were roughly evenly split between meeting the restricted criteria and the general criteria for previous pregnancy loss.
On average, the women were 29 years old and had a body mass index of about 27 kg/m2. Most were married and white.
Overall, there was only a trend toward a higher rate of live births in the low-dose aspirin group compared with the placebo group (57.8% vs. 52.7%, P = .09), reported Dr. Schisterman.
In stratified analyses, there was a significant benefit of low-dose aspirin in the subset meeting the restricted pregnancy loss criteria (62.4% vs. 53.2%, P = .04) but not in the subset meeting the general pregnancy loss criteria (53.9% vs. 52.2%).
When the investigators more closely assessed the reason for benefit in the women meeting restricted criteria, they found a higher rate of achieving a positive pregnancy test with low-dose aspirin (70.5% vs. 61.7%, P = .03). Rates of progression thereafter to confirmed pregnancy by ultrasound at 6 weeks and ultimately to live birth were similar for the two treatment groups.
Dr. Schisterman disclosed no relevant conflicts of interest.
Some women who have experienced a pregnancy loss can increase their odds of a live birth in the next pregnancy simply by taking aspirin, investigators reported at the Pregnancy Meeting, the annual meeting of the Society for Maternal-Fetal Medicine.
A team led by Enrique F. Schisterman, Ph.D., conducted a randomized trial of 1,228 healthy young women who had had up to two prior pregnancy losses, but did not have infertility and were attempting to conceive again.
The women were assigned evenly to take low-dose aspirin (81 mg) or placebo daily, along with folic acid, for up to six menstrual cycles or, if they conceived, up to the 36th week of pregnancy.
Results showed that low-dose aspirin was associated with an absolute 9.2% increase in the rate of live birth among the subset of women who met restricted criteria for pregnancy loss, namely a single pregnancy loss before 20 weeks’ gestation in the past year, reported Dr. Schisterman, who is a senior investigator and chief of the epidemiology branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Rockville, Md.
The benefit was mainly due to early effects. "There was an effect on becoming pregnant and early pregnancy [maintenance], but there were no differences after that," he elaborated. "The implications of that are not only that aspirin will help women become pregnant, but if you start too late, then the effects of aspirin are not there any more."
Analyses in the group with restricted criteria suggested that only about 11 women would need to be treated with low-dose aspirin to achieve one additional live birth.
In contrast, there was no significant benefit of low-dose aspirin among the subset of women who met general criteria for pregnancy loss that required one or two pregnancy losses at any time in the past, but excluded those meeting restricted criteria.
Low-dose aspirin was associated with somewhat higher rates of minor vaginal bleeding and minor gastrointestinal upset, but the drug was not associated with pregnancy loss or with an increased risk of major fetal, neonatal, or maternal complications.
An attendee wondered about the difference between the two subsets of women having differing histories of pregnancy loss, saying, "You would expect more or less the same effect."
Dr. Schisterman maintained that the two groups were not all that similar. "I am not sure I would expect the same result, although when we did some analyses in which we compared those who had a single loss in the restricted stratum to those who had a single loss in the general stratum, we found attenuated but in a similar direction results in the general stratum," he commented. "So it seems that the number of losses is the driving force. But we are still analyzing that data."
Another attendee raised the issue of the timing of the previous pregnancy loss in the subset meeting restricted criteria. "Were you able to identify any influence of the gestational age of the previous loss on the effectiveness of aspirin in the next pregnancy, the randomized pregnancy?" he asked.
"Not yet," Dr. Schisterman replied, noting that all of the losses were fairly early. However, here too, analyses are still ongoing.
Giving some background to the trial, he noted, "We know that inflammation and abnormal blood flow, especially in the uterus, endometrium, ovaries, and placenta, ... are unifying features of outcomes like infertility, pregnancy loss, preeclampsia, preterm delivery, and small for gestational age. So clearly, an ideal therapy that would reduce inflammation and improve blood flow will be the one that we are looking for. Low-dose aspirin could be such a therapy."
The drug has seldom been studied when given in the preconceptional period, but there is a strong rationale for such use, he maintained.
"It impacts endometrial vascularization and placentation. It has very well documented anti-inflammatory effects. It has very few maternal and fetal side effects. It’s safe, widely available, and more importantly, it’s cheap – it costs $2 for the whole pregnancy to treat a woman," he elaborated.
Women enrolled in the trial, known as EAGeR (The Effects of Aspirin in Gestation and Reproduction), were aged 18-39 years. They were roughly evenly split between meeting the restricted criteria and the general criteria for previous pregnancy loss.
On average, the women were 29 years old and had a body mass index of about 27 kg/m2. Most were married and white.
Overall, there was only a trend toward a higher rate of live births in the low-dose aspirin group compared with the placebo group (57.8% vs. 52.7%, P = .09), reported Dr. Schisterman.
In stratified analyses, there was a significant benefit of low-dose aspirin in the subset meeting the restricted pregnancy loss criteria (62.4% vs. 53.2%, P = .04) but not in the subset meeting the general pregnancy loss criteria (53.9% vs. 52.2%).
When the investigators more closely assessed the reason for benefit in the women meeting restricted criteria, they found a higher rate of achieving a positive pregnancy test with low-dose aspirin (70.5% vs. 61.7%, P = .03). Rates of progression thereafter to confirmed pregnancy by ultrasound at 6 weeks and ultimately to live birth were similar for the two treatment groups.
Dr. Schisterman disclosed no relevant conflicts of interest.
Some women who have experienced a pregnancy loss can increase their odds of a live birth in the next pregnancy simply by taking aspirin, investigators reported at the Pregnancy Meeting, the annual meeting of the Society for Maternal-Fetal Medicine.
A team led by Enrique F. Schisterman, Ph.D., conducted a randomized trial of 1,228 healthy young women who had had up to two prior pregnancy losses, but did not have infertility and were attempting to conceive again.
The women were assigned evenly to take low-dose aspirin (81 mg) or placebo daily, along with folic acid, for up to six menstrual cycles or, if they conceived, up to the 36th week of pregnancy.
Results showed that low-dose aspirin was associated with an absolute 9.2% increase in the rate of live birth among the subset of women who met restricted criteria for pregnancy loss, namely a single pregnancy loss before 20 weeks’ gestation in the past year, reported Dr. Schisterman, who is a senior investigator and chief of the epidemiology branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Rockville, Md.
The benefit was mainly due to early effects. "There was an effect on becoming pregnant and early pregnancy [maintenance], but there were no differences after that," he elaborated. "The implications of that are not only that aspirin will help women become pregnant, but if you start too late, then the effects of aspirin are not there any more."
Analyses in the group with restricted criteria suggested that only about 11 women would need to be treated with low-dose aspirin to achieve one additional live birth.
In contrast, there was no significant benefit of low-dose aspirin among the subset of women who met general criteria for pregnancy loss that required one or two pregnancy losses at any time in the past, but excluded those meeting restricted criteria.
Low-dose aspirin was associated with somewhat higher rates of minor vaginal bleeding and minor gastrointestinal upset, but the drug was not associated with pregnancy loss or with an increased risk of major fetal, neonatal, or maternal complications.
An attendee wondered about the difference between the two subsets of women having differing histories of pregnancy loss, saying, "You would expect more or less the same effect."
Dr. Schisterman maintained that the two groups were not all that similar. "I am not sure I would expect the same result, although when we did some analyses in which we compared those who had a single loss in the restricted stratum to those who had a single loss in the general stratum, we found attenuated but in a similar direction results in the general stratum," he commented. "So it seems that the number of losses is the driving force. But we are still analyzing that data."
Another attendee raised the issue of the timing of the previous pregnancy loss in the subset meeting restricted criteria. "Were you able to identify any influence of the gestational age of the previous loss on the effectiveness of aspirin in the next pregnancy, the randomized pregnancy?" he asked.
"Not yet," Dr. Schisterman replied, noting that all of the losses were fairly early. However, here too, analyses are still ongoing.
Giving some background to the trial, he noted, "We know that inflammation and abnormal blood flow, especially in the uterus, endometrium, ovaries, and placenta, ... are unifying features of outcomes like infertility, pregnancy loss, preeclampsia, preterm delivery, and small for gestational age. So clearly, an ideal therapy that would reduce inflammation and improve blood flow will be the one that we are looking for. Low-dose aspirin could be such a therapy."
The drug has seldom been studied when given in the preconceptional period, but there is a strong rationale for such use, he maintained.
"It impacts endometrial vascularization and placentation. It has very well documented anti-inflammatory effects. It has very few maternal and fetal side effects. It’s safe, widely available, and more importantly, it’s cheap – it costs $2 for the whole pregnancy to treat a woman," he elaborated.
Women enrolled in the trial, known as EAGeR (The Effects of Aspirin in Gestation and Reproduction), were aged 18-39 years. They were roughly evenly split between meeting the restricted criteria and the general criteria for previous pregnancy loss.
On average, the women were 29 years old and had a body mass index of about 27 kg/m2. Most were married and white.
Overall, there was only a trend toward a higher rate of live births in the low-dose aspirin group compared with the placebo group (57.8% vs. 52.7%, P = .09), reported Dr. Schisterman.
In stratified analyses, there was a significant benefit of low-dose aspirin in the subset meeting the restricted pregnancy loss criteria (62.4% vs. 53.2%, P = .04) but not in the subset meeting the general pregnancy loss criteria (53.9% vs. 52.2%).
When the investigators more closely assessed the reason for benefit in the women meeting restricted criteria, they found a higher rate of achieving a positive pregnancy test with low-dose aspirin (70.5% vs. 61.7%, P = .03). Rates of progression thereafter to confirmed pregnancy by ultrasound at 6 weeks and ultimately to live birth were similar for the two treatment groups.
Dr. Schisterman disclosed no relevant conflicts of interest.
AT THE PREGNANCY MEETING 2013
Major finding: Among women who had experienced a single pregnancy loss before 20 weeks’ gestation in the past year, those assigned to low-dose aspirin were more likely than those assigned to placebo to have a live birth (62.4% vs. 53.2%, P = .04).
Data source: A randomized double-blind trial among 1,228 healthy young women with up to two prior pregnancy losses who did not have infertility and were trying to conceive (EAGeR trial).
Disclosures: Dr. Schisterman disclosed no relevant conflicts of interest.
Drugs, Pregnancy, and Lactation: New Weight Loss Drugs
The need for effective weight management medications as an adjunct to diet and exercise has escalated in the United States as obesity has reached epidemic proportions.
However, in recent years, several Food and Drug Administration–approved medications for weight loss have been plagued with safety concerns and many have been removed from the market, leaving clinicians with limited choices for treatment of overweight or obese patients.
In 2012, two new weight loss medications were approved by the FDA – the first new medications approved for this indication in over a decade (N. Engl. J. Med. 2012;367:1577-9).
As of February 2013, one of the two products, a combination product containing the anorexant phentermine and the anticonvulsant topiramate in an extended-release form, is currently available by prescription in the United States. Marketed as Qysmia, the product is intended to be used together with a reduced-calorie diet and increased physical activity for chronic weight management in adults with an initial body mass index of 30 kg/m2 or greater (obese).
The medication is also indicated for adults with a BMI of 27 or greater (overweight) who also have at least one weight-related medical condition such as high blood pressure, type 2 diabetes, or high cholesterol. The recommended starting daily dose contains 3.75 mg of phentermine and 23 mg of topiramate; the maximum dose contains 15 mg of phentermine and 92 mg of topiramate.
In part, due to concerns about the teratogenicity of topiramate, Qysmia has been designated a category X drug, and specific pregnancy prevention measures in the form of a Risk Evaluation and Mitigation Strategy (REMS) have been put in place. The medication can be obtained only by prescription obtained directly from a health care provider, and providers receive training on the risks of birth defects. A prescription for Qysmia can only be filled by specially certified mail order pharmacies in the United States.
Educational materials indicate that the drug should not be prescribed to women who are pregnant or who are planning on becoming pregnant. Women who are not planning pregnancy but have the potential to become pregnant should have a negative pregnancy test before starting the drug and again every month while taking the drug, and they should use an effective method or combination of methods of contraception. The manufacturer has also initiated a pregnancy surveillance system.
Given the likelihood that many women of reproductive age will use this medication, even with a REMS in place, the potential for unintentional exposure in pregnancy exists. In the inevitable event of an exposed pregnancy, what are the specific risks and their magnitude? The concern about birth defects with this medication stems from previously published data suggesting that topiramate used in monotherapy for other indications, most commonly epilepsy, is associated with an increased risk for oral clefts (cleft lip with or without cleft palate). Although numbers are still small, a few studies have suggested the risk for oral clefts, with the most recent a large pooled case-control analysis from two data sources in the United States (Am. J. Obstet. Gynecol. 2012;207:405e1-7). The pooled estimate of the risk of oral clefts was 5.36 with very wide confidence intervals (1.49-20.07), based on seven exposed children with cleft lip with or without cleft palate. To the extent that this estimate is correct, this translates to an absolute risk of about 5 in 1,000 first-trimester topiramate-exposed pregnancies, compared with a baseline risk of about 1 in 1,000 in unexposed pregnancies.
Published studies of topiramate and oral clefts have not involved sufficient numbers of exposed and affected children to allow examination of a dose threshold; however, the range of recommended doses for seizure prevention in adults treated with topiramate monotherapy (50-400 mg/day) overlaps with the dosing range of topiramate contained in Qysmia. It is important to note that based on the published reports suggesting an increased risk for oral clefts, the pregnancy category for topiramate alone was recently changed from a C to a D, while the pregnancy category for Qysmia is an X. The rationale behind the category D is likely that the benefits of topiramate might outweigh the risks in a pregnant woman with a seizure disorder for whom topiramate is the only effective medication. However, topiramate use for weight loss would typically never be indicated in pregnancy.
The second drug, lorcaserin (Belviq), is a single-ingredient serotonergic medication – a selective agonist of the 5-HT2C receptor. Lorcaserin was approved by the FDA in 2012, but as of February 2013, it is not yet available in the United States. This medication also received a pregnancy category X designation; however, in this situation, it was presumably for the sole reason that intentional weight loss in pregnancy is not recommended. Preclinical data for lorcaserin did not suggest teratogenicity, but maternal exposure in rats late in gestation resulted in lower pup body weight that persisted into adulthood.
To the extent that these new medications are effective in reducing and maintaining BMI within a healthier range in women who are currently overweight or obese, they may lead to improvement in subsequent pregnancy outcomes. However, avoiding exposure to these medications during early pregnancy will be a challenge, even with pregnancy prevention guidance and restricted distribution programs. Postmarketing surveillance for outcomes of inadvertently exposed pregnancies will be essential.
Dr. Chambers is associate professor of pediatrics and family and preventive medicine at the University of California, San Diego. She is director of the California Teratogen Information Service and Clinical Research Program. Dr. Chambers is a past president of the Organization of Teratology Information Specialists and past president of the Teratology Society. She said she had no relevant financial disclosures. To comment, e-mail her at [email protected].
The need for effective weight management medications as an adjunct to diet and exercise has escalated in the United States as obesity has reached epidemic proportions.
However, in recent years, several Food and Drug Administration–approved medications for weight loss have been plagued with safety concerns and many have been removed from the market, leaving clinicians with limited choices for treatment of overweight or obese patients.
In 2012, two new weight loss medications were approved by the FDA – the first new medications approved for this indication in over a decade (N. Engl. J. Med. 2012;367:1577-9).
As of February 2013, one of the two products, a combination product containing the anorexant phentermine and the anticonvulsant topiramate in an extended-release form, is currently available by prescription in the United States. Marketed as Qysmia, the product is intended to be used together with a reduced-calorie diet and increased physical activity for chronic weight management in adults with an initial body mass index of 30 kg/m2 or greater (obese).
The medication is also indicated for adults with a BMI of 27 or greater (overweight) who also have at least one weight-related medical condition such as high blood pressure, type 2 diabetes, or high cholesterol. The recommended starting daily dose contains 3.75 mg of phentermine and 23 mg of topiramate; the maximum dose contains 15 mg of phentermine and 92 mg of topiramate.
In part, due to concerns about the teratogenicity of topiramate, Qysmia has been designated a category X drug, and specific pregnancy prevention measures in the form of a Risk Evaluation and Mitigation Strategy (REMS) have been put in place. The medication can be obtained only by prescription obtained directly from a health care provider, and providers receive training on the risks of birth defects. A prescription for Qysmia can only be filled by specially certified mail order pharmacies in the United States.
Educational materials indicate that the drug should not be prescribed to women who are pregnant or who are planning on becoming pregnant. Women who are not planning pregnancy but have the potential to become pregnant should have a negative pregnancy test before starting the drug and again every month while taking the drug, and they should use an effective method or combination of methods of contraception. The manufacturer has also initiated a pregnancy surveillance system.
Given the likelihood that many women of reproductive age will use this medication, even with a REMS in place, the potential for unintentional exposure in pregnancy exists. In the inevitable event of an exposed pregnancy, what are the specific risks and their magnitude? The concern about birth defects with this medication stems from previously published data suggesting that topiramate used in monotherapy for other indications, most commonly epilepsy, is associated with an increased risk for oral clefts (cleft lip with or without cleft palate). Although numbers are still small, a few studies have suggested the risk for oral clefts, with the most recent a large pooled case-control analysis from two data sources in the United States (Am. J. Obstet. Gynecol. 2012;207:405e1-7). The pooled estimate of the risk of oral clefts was 5.36 with very wide confidence intervals (1.49-20.07), based on seven exposed children with cleft lip with or without cleft palate. To the extent that this estimate is correct, this translates to an absolute risk of about 5 in 1,000 first-trimester topiramate-exposed pregnancies, compared with a baseline risk of about 1 in 1,000 in unexposed pregnancies.
Published studies of topiramate and oral clefts have not involved sufficient numbers of exposed and affected children to allow examination of a dose threshold; however, the range of recommended doses for seizure prevention in adults treated with topiramate monotherapy (50-400 mg/day) overlaps with the dosing range of topiramate contained in Qysmia. It is important to note that based on the published reports suggesting an increased risk for oral clefts, the pregnancy category for topiramate alone was recently changed from a C to a D, while the pregnancy category for Qysmia is an X. The rationale behind the category D is likely that the benefits of topiramate might outweigh the risks in a pregnant woman with a seizure disorder for whom topiramate is the only effective medication. However, topiramate use for weight loss would typically never be indicated in pregnancy.
The second drug, lorcaserin (Belviq), is a single-ingredient serotonergic medication – a selective agonist of the 5-HT2C receptor. Lorcaserin was approved by the FDA in 2012, but as of February 2013, it is not yet available in the United States. This medication also received a pregnancy category X designation; however, in this situation, it was presumably for the sole reason that intentional weight loss in pregnancy is not recommended. Preclinical data for lorcaserin did not suggest teratogenicity, but maternal exposure in rats late in gestation resulted in lower pup body weight that persisted into adulthood.
To the extent that these new medications are effective in reducing and maintaining BMI within a healthier range in women who are currently overweight or obese, they may lead to improvement in subsequent pregnancy outcomes. However, avoiding exposure to these medications during early pregnancy will be a challenge, even with pregnancy prevention guidance and restricted distribution programs. Postmarketing surveillance for outcomes of inadvertently exposed pregnancies will be essential.
Dr. Chambers is associate professor of pediatrics and family and preventive medicine at the University of California, San Diego. She is director of the California Teratogen Information Service and Clinical Research Program. Dr. Chambers is a past president of the Organization of Teratology Information Specialists and past president of the Teratology Society. She said she had no relevant financial disclosures. To comment, e-mail her at [email protected].
The need for effective weight management medications as an adjunct to diet and exercise has escalated in the United States as obesity has reached epidemic proportions.
However, in recent years, several Food and Drug Administration–approved medications for weight loss have been plagued with safety concerns and many have been removed from the market, leaving clinicians with limited choices for treatment of overweight or obese patients.
In 2012, two new weight loss medications were approved by the FDA – the first new medications approved for this indication in over a decade (N. Engl. J. Med. 2012;367:1577-9).
As of February 2013, one of the two products, a combination product containing the anorexant phentermine and the anticonvulsant topiramate in an extended-release form, is currently available by prescription in the United States. Marketed as Qysmia, the product is intended to be used together with a reduced-calorie diet and increased physical activity for chronic weight management in adults with an initial body mass index of 30 kg/m2 or greater (obese).
The medication is also indicated for adults with a BMI of 27 or greater (overweight) who also have at least one weight-related medical condition such as high blood pressure, type 2 diabetes, or high cholesterol. The recommended starting daily dose contains 3.75 mg of phentermine and 23 mg of topiramate; the maximum dose contains 15 mg of phentermine and 92 mg of topiramate.
In part, due to concerns about the teratogenicity of topiramate, Qysmia has been designated a category X drug, and specific pregnancy prevention measures in the form of a Risk Evaluation and Mitigation Strategy (REMS) have been put in place. The medication can be obtained only by prescription obtained directly from a health care provider, and providers receive training on the risks of birth defects. A prescription for Qysmia can only be filled by specially certified mail order pharmacies in the United States.
Educational materials indicate that the drug should not be prescribed to women who are pregnant or who are planning on becoming pregnant. Women who are not planning pregnancy but have the potential to become pregnant should have a negative pregnancy test before starting the drug and again every month while taking the drug, and they should use an effective method or combination of methods of contraception. The manufacturer has also initiated a pregnancy surveillance system.
Given the likelihood that many women of reproductive age will use this medication, even with a REMS in place, the potential for unintentional exposure in pregnancy exists. In the inevitable event of an exposed pregnancy, what are the specific risks and their magnitude? The concern about birth defects with this medication stems from previously published data suggesting that topiramate used in monotherapy for other indications, most commonly epilepsy, is associated with an increased risk for oral clefts (cleft lip with or without cleft palate). Although numbers are still small, a few studies have suggested the risk for oral clefts, with the most recent a large pooled case-control analysis from two data sources in the United States (Am. J. Obstet. Gynecol. 2012;207:405e1-7). The pooled estimate of the risk of oral clefts was 5.36 with very wide confidence intervals (1.49-20.07), based on seven exposed children with cleft lip with or without cleft palate. To the extent that this estimate is correct, this translates to an absolute risk of about 5 in 1,000 first-trimester topiramate-exposed pregnancies, compared with a baseline risk of about 1 in 1,000 in unexposed pregnancies.
Published studies of topiramate and oral clefts have not involved sufficient numbers of exposed and affected children to allow examination of a dose threshold; however, the range of recommended doses for seizure prevention in adults treated with topiramate monotherapy (50-400 mg/day) overlaps with the dosing range of topiramate contained in Qysmia. It is important to note that based on the published reports suggesting an increased risk for oral clefts, the pregnancy category for topiramate alone was recently changed from a C to a D, while the pregnancy category for Qysmia is an X. The rationale behind the category D is likely that the benefits of topiramate might outweigh the risks in a pregnant woman with a seizure disorder for whom topiramate is the only effective medication. However, topiramate use for weight loss would typically never be indicated in pregnancy.
The second drug, lorcaserin (Belviq), is a single-ingredient serotonergic medication – a selective agonist of the 5-HT2C receptor. Lorcaserin was approved by the FDA in 2012, but as of February 2013, it is not yet available in the United States. This medication also received a pregnancy category X designation; however, in this situation, it was presumably for the sole reason that intentional weight loss in pregnancy is not recommended. Preclinical data for lorcaserin did not suggest teratogenicity, but maternal exposure in rats late in gestation resulted in lower pup body weight that persisted into adulthood.
To the extent that these new medications are effective in reducing and maintaining BMI within a healthier range in women who are currently overweight or obese, they may lead to improvement in subsequent pregnancy outcomes. However, avoiding exposure to these medications during early pregnancy will be a challenge, even with pregnancy prevention guidance and restricted distribution programs. Postmarketing surveillance for outcomes of inadvertently exposed pregnancies will be essential.
Dr. Chambers is associate professor of pediatrics and family and preventive medicine at the University of California, San Diego. She is director of the California Teratogen Information Service and Clinical Research Program. Dr. Chambers is a past president of the Organization of Teratology Information Specialists and past president of the Teratology Society. She said she had no relevant financial disclosures. To comment, e-mail her at [email protected].
Watch for postpartum exacerbation of psoriasis
The postpartum period is often a time when women with moderate to severe psoriasis experience a significant disease flare – and if they’re breastfeeding, treatment options are limited, according to Dr. Alan Menter.
This postpartum major flare of psoriasis is an underappreciated phenomenon that catches many dermatologists and most ob.gyns. off guard, he said.
"Fifty to 60% of psoriasis patients have genital involvement. A woman with genital psoriasis in the postpartum period or during delivery really needs help, and I think we in dermatology should be addressing these issues because most of the obstetricians are not sure how to treat these patients," said Dr. Menter, chief of the division of dermatology at Baylor University Medical Center, Dallas, and chair of the American Academy of Dermatology psoriasis guidelines committee.
Psoriasis is equally common in men and women, and two-thirds of affected individuals present before age 40 – for women, the childbearing years. Thorny psoriasis management issues in pregnancy and postpartum are common.
Psoriasis slowly improves during pregnancy in roughly two-thirds of patients, as is true for other immune-mediated diseases. But for that other third, many of the mainstay therapies for tough psoriasis are off limits during pregnancy and/or post partum. UVB is a good, safe option, albeit inconvenient. Retinoids and cyclosporine are out because of teratogenicity.
Cyclosporine probably should be considered the go-to drug for significant disease during pregnancy. Its strengths are its fast onset of action and the safety reassurance provided by vast patient registries started back in the 1980s when the drug was first used in transplant recipients.
"We’re all comfortable using cyclosporine," Dr. Menter said. "Our AAD guidelines state it is appropriate for 1 year of continuous use. The European guidelines say, ‘2 years of continuous use.’ But I think most of us use it as an interventional therapy for 3-6 months. I actually think we should be using it a little more frequently as an interventional therapy."
Cyclosporine must be stopped in month 8 of pregnancy to allow the drug to clear from the patient’s system before delivery, since it is secreted in breast milk.
For the breastfeeding woman experiencing a major disease flare, the options are basically potent topical steroids, which physicians should feel comfortable in prescribing according to the standard dosing schedule used in nonpregnant patients, or – when topical therapy won’t get the job done – the biologic agents, listed by the Food and Drug Administration as category B.
The most forward-thinking approach to take with young women who require systemic therapy for psoriasis is to discuss pregnancy-related issues before pregnancy occurs. In particular, a prospective case-control study from the Organization of Teratology Information Specialists Autoimmune Diseases in Pregnancy Project concluded that women with psoriasis were significantly more likely to smoke, carry a diagnosis of depression, and be overweight or obese before pregnancy – factors that increase their risk for adverse pregnancy outcomes (Br. J. Dermatol. 2010;163:334-9).
Moreover, other studies have shown that psoriasis patients, men as well as women, have an increased prevalence of the metabolic syndrome, which increases their long-term risk of cardiovascular disease. Women with an adverse cardiovascular risk profile who are considering pregnancy and parenthood may be in a teachable moment where they are more amenable to lifestyle changes that reduce the risks both to their baby and themselves, Dr. Menter said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
Of course, half of pregnancies in the United States are unplanned, so the potential for unintended first-trimester fetal exposure to a teratogenic drug is substantial. While methotrexate is rated by the FDA as category X in pregnancy, dermatologists can derive some comfort from a well-executed review of 101 methotrexate-exposed pregnancies in rheumatology patients (Clin. Exp. Rheumatol. 2009;27:678-84). The 23% miscarriage rate wasn’t significantly different from that seen in pregnant psoriasis patients not on systemic agents. The live birth rate was 66%, with a 5% rate of neonatal malformations, all minor.
"The outcomes were actually better than any of us would have anticipated," Dr. Menter commented.
Psoriasis appears to have an inherent adverse impact upon pregnancy, he continued, pointing to an Israeli study of 68 deliveries in 35 women with moderate to severe psoriasis and 237 deliveries in 236 controls without psoriasis matched for age, parity, and gestational age.
"I think this is something we have to very gently discuss with our female patients who are considering pregnancy. We should tell them to be cautious in pregnancy because of this link between psoriasis and a slightly increased risk of spontaneous abortions. And I also discuss it with our ob.gyn. colleagues, who really are not aware of this link," the dermatologist said.
Dr. Menter reported receiving research support and/or consultant or lecture fees from roughly 20 pharmaceutical companies. SDEF and this news organization are owned by the same parent company.
*This story was updated March 1, 2013.
The postpartum period is often a time when women with moderate to severe psoriasis experience a significant disease flare – and if they’re breastfeeding, treatment options are limited, according to Dr. Alan Menter.
This postpartum major flare of psoriasis is an underappreciated phenomenon that catches many dermatologists and most ob.gyns. off guard, he said.
"Fifty to 60% of psoriasis patients have genital involvement. A woman with genital psoriasis in the postpartum period or during delivery really needs help, and I think we in dermatology should be addressing these issues because most of the obstetricians are not sure how to treat these patients," said Dr. Menter, chief of the division of dermatology at Baylor University Medical Center, Dallas, and chair of the American Academy of Dermatology psoriasis guidelines committee.
Psoriasis is equally common in men and women, and two-thirds of affected individuals present before age 40 – for women, the childbearing years. Thorny psoriasis management issues in pregnancy and postpartum are common.
Psoriasis slowly improves during pregnancy in roughly two-thirds of patients, as is true for other immune-mediated diseases. But for that other third, many of the mainstay therapies for tough psoriasis are off limits during pregnancy and/or post partum. UVB is a good, safe option, albeit inconvenient. Retinoids and cyclosporine are out because of teratogenicity.
Cyclosporine probably should be considered the go-to drug for significant disease during pregnancy. Its strengths are its fast onset of action and the safety reassurance provided by vast patient registries started back in the 1980s when the drug was first used in transplant recipients.
"We’re all comfortable using cyclosporine," Dr. Menter said. "Our AAD guidelines state it is appropriate for 1 year of continuous use. The European guidelines say, ‘2 years of continuous use.’ But I think most of us use it as an interventional therapy for 3-6 months. I actually think we should be using it a little more frequently as an interventional therapy."
Cyclosporine must be stopped in month 8 of pregnancy to allow the drug to clear from the patient’s system before delivery, since it is secreted in breast milk.
For the breastfeeding woman experiencing a major disease flare, the options are basically potent topical steroids, which physicians should feel comfortable in prescribing according to the standard dosing schedule used in nonpregnant patients, or – when topical therapy won’t get the job done – the biologic agents, listed by the Food and Drug Administration as category B.
The most forward-thinking approach to take with young women who require systemic therapy for psoriasis is to discuss pregnancy-related issues before pregnancy occurs. In particular, a prospective case-control study from the Organization of Teratology Information Specialists Autoimmune Diseases in Pregnancy Project concluded that women with psoriasis were significantly more likely to smoke, carry a diagnosis of depression, and be overweight or obese before pregnancy – factors that increase their risk for adverse pregnancy outcomes (Br. J. Dermatol. 2010;163:334-9).
Moreover, other studies have shown that psoriasis patients, men as well as women, have an increased prevalence of the metabolic syndrome, which increases their long-term risk of cardiovascular disease. Women with an adverse cardiovascular risk profile who are considering pregnancy and parenthood may be in a teachable moment where they are more amenable to lifestyle changes that reduce the risks both to their baby and themselves, Dr. Menter said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
Of course, half of pregnancies in the United States are unplanned, so the potential for unintended first-trimester fetal exposure to a teratogenic drug is substantial. While methotrexate is rated by the FDA as category X in pregnancy, dermatologists can derive some comfort from a well-executed review of 101 methotrexate-exposed pregnancies in rheumatology patients (Clin. Exp. Rheumatol. 2009;27:678-84). The 23% miscarriage rate wasn’t significantly different from that seen in pregnant psoriasis patients not on systemic agents. The live birth rate was 66%, with a 5% rate of neonatal malformations, all minor.
"The outcomes were actually better than any of us would have anticipated," Dr. Menter commented.
Psoriasis appears to have an inherent adverse impact upon pregnancy, he continued, pointing to an Israeli study of 68 deliveries in 35 women with moderate to severe psoriasis and 237 deliveries in 236 controls without psoriasis matched for age, parity, and gestational age.
"I think this is something we have to very gently discuss with our female patients who are considering pregnancy. We should tell them to be cautious in pregnancy because of this link between psoriasis and a slightly increased risk of spontaneous abortions. And I also discuss it with our ob.gyn. colleagues, who really are not aware of this link," the dermatologist said.
Dr. Menter reported receiving research support and/or consultant or lecture fees from roughly 20 pharmaceutical companies. SDEF and this news organization are owned by the same parent company.
*This story was updated March 1, 2013.
The postpartum period is often a time when women with moderate to severe psoriasis experience a significant disease flare – and if they’re breastfeeding, treatment options are limited, according to Dr. Alan Menter.
This postpartum major flare of psoriasis is an underappreciated phenomenon that catches many dermatologists and most ob.gyns. off guard, he said.
"Fifty to 60% of psoriasis patients have genital involvement. A woman with genital psoriasis in the postpartum period or during delivery really needs help, and I think we in dermatology should be addressing these issues because most of the obstetricians are not sure how to treat these patients," said Dr. Menter, chief of the division of dermatology at Baylor University Medical Center, Dallas, and chair of the American Academy of Dermatology psoriasis guidelines committee.
Psoriasis is equally common in men and women, and two-thirds of affected individuals present before age 40 – for women, the childbearing years. Thorny psoriasis management issues in pregnancy and postpartum are common.
Psoriasis slowly improves during pregnancy in roughly two-thirds of patients, as is true for other immune-mediated diseases. But for that other third, many of the mainstay therapies for tough psoriasis are off limits during pregnancy and/or post partum. UVB is a good, safe option, albeit inconvenient. Retinoids and cyclosporine are out because of teratogenicity.
Cyclosporine probably should be considered the go-to drug for significant disease during pregnancy. Its strengths are its fast onset of action and the safety reassurance provided by vast patient registries started back in the 1980s when the drug was first used in transplant recipients.
"We’re all comfortable using cyclosporine," Dr. Menter said. "Our AAD guidelines state it is appropriate for 1 year of continuous use. The European guidelines say, ‘2 years of continuous use.’ But I think most of us use it as an interventional therapy for 3-6 months. I actually think we should be using it a little more frequently as an interventional therapy."
Cyclosporine must be stopped in month 8 of pregnancy to allow the drug to clear from the patient’s system before delivery, since it is secreted in breast milk.
For the breastfeeding woman experiencing a major disease flare, the options are basically potent topical steroids, which physicians should feel comfortable in prescribing according to the standard dosing schedule used in nonpregnant patients, or – when topical therapy won’t get the job done – the biologic agents, listed by the Food and Drug Administration as category B.
The most forward-thinking approach to take with young women who require systemic therapy for psoriasis is to discuss pregnancy-related issues before pregnancy occurs. In particular, a prospective case-control study from the Organization of Teratology Information Specialists Autoimmune Diseases in Pregnancy Project concluded that women with psoriasis were significantly more likely to smoke, carry a diagnosis of depression, and be overweight or obese before pregnancy – factors that increase their risk for adverse pregnancy outcomes (Br. J. Dermatol. 2010;163:334-9).
Moreover, other studies have shown that psoriasis patients, men as well as women, have an increased prevalence of the metabolic syndrome, which increases their long-term risk of cardiovascular disease. Women with an adverse cardiovascular risk profile who are considering pregnancy and parenthood may be in a teachable moment where they are more amenable to lifestyle changes that reduce the risks both to their baby and themselves, Dr. Menter said at the Hawaii Dermatology Seminar sponsored by Global Academy for Medical Education/Skin Disease Education Foundation.
Of course, half of pregnancies in the United States are unplanned, so the potential for unintended first-trimester fetal exposure to a teratogenic drug is substantial. While methotrexate is rated by the FDA as category X in pregnancy, dermatologists can derive some comfort from a well-executed review of 101 methotrexate-exposed pregnancies in rheumatology patients (Clin. Exp. Rheumatol. 2009;27:678-84). The 23% miscarriage rate wasn’t significantly different from that seen in pregnant psoriasis patients not on systemic agents. The live birth rate was 66%, with a 5% rate of neonatal malformations, all minor.
"The outcomes were actually better than any of us would have anticipated," Dr. Menter commented.
Psoriasis appears to have an inherent adverse impact upon pregnancy, he continued, pointing to an Israeli study of 68 deliveries in 35 women with moderate to severe psoriasis and 237 deliveries in 236 controls without psoriasis matched for age, parity, and gestational age.
"I think this is something we have to very gently discuss with our female patients who are considering pregnancy. We should tell them to be cautious in pregnancy because of this link between psoriasis and a slightly increased risk of spontaneous abortions. And I also discuss it with our ob.gyn. colleagues, who really are not aware of this link," the dermatologist said.
Dr. Menter reported receiving research support and/or consultant or lecture fees from roughly 20 pharmaceutical companies. SDEF and this news organization are owned by the same parent company.
*This story was updated March 1, 2013.
EXPERT ANALYSIS FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
Folic acid supplements linked to lower autism risk
Maternal use of folic acid supplements around the time of conception was associated with a lower risk of autistic disorder, the most severe form of autism spectrum disorders, in the children, according to a Norwegian study reported in the Feb. 13 issue of JAMA.
"This finding does not establish a causal relation between folic acid use and autistic disorder, but provides a rationale for replicating the analyses in other study samples and further investigating genetic factors and other biologic mechanisms that may explain the inverse association," said Dr. Pål Surén of the Norwegian Institute of Public Health, Oslo, and his associates.
Folic acid supplements during pregnancy reduce the risk of neural tube defects in the offspring, and there is some evidence that they may also reduce the risk of other neurodevelopmental disorders. A recent analysis of data from the Norwegian Mother and Child Cohort Study found that maternal use of folic acid supplements was associated with a lower risk of severe language delay in their children at age 3 years.
Dr. Surén and his colleagues used data from the same cohort study to examine a possible association between the supplements and risk of autism spectrum disorders. The Norwegian Mother and Child Cohort Study is a national registry of 109,020 children born between 1999 and 2009.
For this analysis, the researchers assessed data concerning 85,176 children in the registry. At final follow-up, the subjects ranged in age from 3.3 years to 10.2 years (mean age, 6.4 years).
A total of 270 of these children (0.32%) were diagnosed as having autism spectrum disorders: 0.13% had autistic disorder, 0.07% had Asperger’s syndrome, and 0.12% had pervasive developmental disorder not otherwise specified.
Approximately 33% of the mothers took folic acid supplements during the interval from 4 weeks before conception to 8 weeks afterward. This period was chosen for the analysis because folic acid’s effects on the developing central nervous system of the fetus are most prominent during this time. "The interval covers or precedes events of critical importance to the fetal brain, such as the closure of the neural tube 28 days after conception and the embryonic period that includes development of the basic brain structures 15-56 days after conception," the investigators noted.
They found an inverse association between the mother’s use of folic acid supplements periconceptually and the risk that the child would develop autistic disorder. Of the children whose mothers took the supplements, 0.10% developed autistic disorder, compared with 0.21% in children whose mothers did not.
The adjusted odds ratio of autistic disorder was 0.61 in children of folic acid users. Further adjusting the data to account for comorbid maternal illness and concomitant medication use did not change this OR, Dr. Surén and his associates reported (JAMA 2013;309:570-7).
Women who took folic acid supplements were more likely to have a college-level education, to have planned the pregnancy, to be nonsmokers, and to have a prepregnancy body mass index less than 25 kg/m2, which are all factors that could confound the association with autism. To address this issue, the investigators assessed the use of fish oil supplements in the study sample.
Use of fish oil supplements correlated with the same parental characteristics as did use of folic acid supplements, but it did not correlate with the risk of autistic disorder, they noted.
Similarly, the inverse association for folic acid use in the periconceptual period was not evident in mothers who took the supplements only later in pregnancy.
"Our findings indicate that the inverse association may be largely driven by the children with autistic disorder and severe language delay at 35 months, who were presumably the more severely affected children," the investigators added.
This study was funded in part by the Research Council of Norway. The investigators did not report having any financial conflicts of interest.
Maternal use of folic acid supplements around the time of conception was associated with a lower risk of autistic disorder, the most severe form of autism spectrum disorders, in the children, according to a Norwegian study reported in the Feb. 13 issue of JAMA.
"This finding does not establish a causal relation between folic acid use and autistic disorder, but provides a rationale for replicating the analyses in other study samples and further investigating genetic factors and other biologic mechanisms that may explain the inverse association," said Dr. Pål Surén of the Norwegian Institute of Public Health, Oslo, and his associates.
Folic acid supplements during pregnancy reduce the risk of neural tube defects in the offspring, and there is some evidence that they may also reduce the risk of other neurodevelopmental disorders. A recent analysis of data from the Norwegian Mother and Child Cohort Study found that maternal use of folic acid supplements was associated with a lower risk of severe language delay in their children at age 3 years.
Dr. Surén and his colleagues used data from the same cohort study to examine a possible association between the supplements and risk of autism spectrum disorders. The Norwegian Mother and Child Cohort Study is a national registry of 109,020 children born between 1999 and 2009.
For this analysis, the researchers assessed data concerning 85,176 children in the registry. At final follow-up, the subjects ranged in age from 3.3 years to 10.2 years (mean age, 6.4 years).
A total of 270 of these children (0.32%) were diagnosed as having autism spectrum disorders: 0.13% had autistic disorder, 0.07% had Asperger’s syndrome, and 0.12% had pervasive developmental disorder not otherwise specified.
Approximately 33% of the mothers took folic acid supplements during the interval from 4 weeks before conception to 8 weeks afterward. This period was chosen for the analysis because folic acid’s effects on the developing central nervous system of the fetus are most prominent during this time. "The interval covers or precedes events of critical importance to the fetal brain, such as the closure of the neural tube 28 days after conception and the embryonic period that includes development of the basic brain structures 15-56 days after conception," the investigators noted.
They found an inverse association between the mother’s use of folic acid supplements periconceptually and the risk that the child would develop autistic disorder. Of the children whose mothers took the supplements, 0.10% developed autistic disorder, compared with 0.21% in children whose mothers did not.
The adjusted odds ratio of autistic disorder was 0.61 in children of folic acid users. Further adjusting the data to account for comorbid maternal illness and concomitant medication use did not change this OR, Dr. Surén and his associates reported (JAMA 2013;309:570-7).
Women who took folic acid supplements were more likely to have a college-level education, to have planned the pregnancy, to be nonsmokers, and to have a prepregnancy body mass index less than 25 kg/m2, which are all factors that could confound the association with autism. To address this issue, the investigators assessed the use of fish oil supplements in the study sample.
Use of fish oil supplements correlated with the same parental characteristics as did use of folic acid supplements, but it did not correlate with the risk of autistic disorder, they noted.
Similarly, the inverse association for folic acid use in the periconceptual period was not evident in mothers who took the supplements only later in pregnancy.
"Our findings indicate that the inverse association may be largely driven by the children with autistic disorder and severe language delay at 35 months, who were presumably the more severely affected children," the investigators added.
This study was funded in part by the Research Council of Norway. The investigators did not report having any financial conflicts of interest.
Maternal use of folic acid supplements around the time of conception was associated with a lower risk of autistic disorder, the most severe form of autism spectrum disorders, in the children, according to a Norwegian study reported in the Feb. 13 issue of JAMA.
"This finding does not establish a causal relation between folic acid use and autistic disorder, but provides a rationale for replicating the analyses in other study samples and further investigating genetic factors and other biologic mechanisms that may explain the inverse association," said Dr. Pål Surén of the Norwegian Institute of Public Health, Oslo, and his associates.
Folic acid supplements during pregnancy reduce the risk of neural tube defects in the offspring, and there is some evidence that they may also reduce the risk of other neurodevelopmental disorders. A recent analysis of data from the Norwegian Mother and Child Cohort Study found that maternal use of folic acid supplements was associated with a lower risk of severe language delay in their children at age 3 years.
Dr. Surén and his colleagues used data from the same cohort study to examine a possible association between the supplements and risk of autism spectrum disorders. The Norwegian Mother and Child Cohort Study is a national registry of 109,020 children born between 1999 and 2009.
For this analysis, the researchers assessed data concerning 85,176 children in the registry. At final follow-up, the subjects ranged in age from 3.3 years to 10.2 years (mean age, 6.4 years).
A total of 270 of these children (0.32%) were diagnosed as having autism spectrum disorders: 0.13% had autistic disorder, 0.07% had Asperger’s syndrome, and 0.12% had pervasive developmental disorder not otherwise specified.
Approximately 33% of the mothers took folic acid supplements during the interval from 4 weeks before conception to 8 weeks afterward. This period was chosen for the analysis because folic acid’s effects on the developing central nervous system of the fetus are most prominent during this time. "The interval covers or precedes events of critical importance to the fetal brain, such as the closure of the neural tube 28 days after conception and the embryonic period that includes development of the basic brain structures 15-56 days after conception," the investigators noted.
They found an inverse association between the mother’s use of folic acid supplements periconceptually and the risk that the child would develop autistic disorder. Of the children whose mothers took the supplements, 0.10% developed autistic disorder, compared with 0.21% in children whose mothers did not.
The adjusted odds ratio of autistic disorder was 0.61 in children of folic acid users. Further adjusting the data to account for comorbid maternal illness and concomitant medication use did not change this OR, Dr. Surén and his associates reported (JAMA 2013;309:570-7).
Women who took folic acid supplements were more likely to have a college-level education, to have planned the pregnancy, to be nonsmokers, and to have a prepregnancy body mass index less than 25 kg/m2, which are all factors that could confound the association with autism. To address this issue, the investigators assessed the use of fish oil supplements in the study sample.
Use of fish oil supplements correlated with the same parental characteristics as did use of folic acid supplements, but it did not correlate with the risk of autistic disorder, they noted.
Similarly, the inverse association for folic acid use in the periconceptual period was not evident in mothers who took the supplements only later in pregnancy.
"Our findings indicate that the inverse association may be largely driven by the children with autistic disorder and severe language delay at 35 months, who were presumably the more severely affected children," the investigators added.
This study was funded in part by the Research Council of Norway. The investigators did not report having any financial conflicts of interest.
FROM JAMA
Major Finding: Autistic disorder was diagnosed in 0.10% of children whose mothers took folic acid supplements periconceptually, compared with 0.21% in children whose mothers did not.
Data Source: An analysis of autism spectrum disorders in 85,176 children participating in the Norwegian Mother and Child Cohort Study.
Disclosures: This study was funded in part by the Research Council of Norway. The investigators did not report having any financial conflicts of interest.
VIDEO: Treating women for psoriasis before and after delivery
At the SDEF Hawaii Dermatology Seminar, Dr. Alan Menter of Baylor University discussed the particular challenges women with psoriasis face in the peripartum period. Many patients – and many ob.gyns.– are not well informed about postpartum psoriasis flare or what to do if genital psoriasis is present at delivery. The SDEF Hawaii Dermatology Seminar is presented by the Skin Disease Education Foundation/Global Academy for Medical Education and is owned by the same parent company as this news organization.
At the SDEF Hawaii Dermatology Seminar, Dr. Alan Menter of Baylor University discussed the particular challenges women with psoriasis face in the peripartum period. Many patients – and many ob.gyns.– are not well informed about postpartum psoriasis flare or what to do if genital psoriasis is present at delivery. The SDEF Hawaii Dermatology Seminar is presented by the Skin Disease Education Foundation/Global Academy for Medical Education and is owned by the same parent company as this news organization.
At the SDEF Hawaii Dermatology Seminar, Dr. Alan Menter of Baylor University discussed the particular challenges women with psoriasis face in the peripartum period. Many patients – and many ob.gyns.– are not well informed about postpartum psoriasis flare or what to do if genital psoriasis is present at delivery. The SDEF Hawaii Dermatology Seminar is presented by the Skin Disease Education Foundation/Global Academy for Medical Education and is owned by the same parent company as this news organization.
Pregnancy and Marfan: New insight into risks
SNOWMASS, COLO–Pregnancy increases the long-term risk of aortic complications in women with Marfan syndrome, according to a recent prospective study causing a stir among adult congenital heart disease specialists.
"This is the first study that says, ‘Even if the aortic root size is okay before pregnancy, the aorta is going to get bigger during pregnancy and it’s not going to go back to baseline. And if your aorta is bigger at the outset, there is a risk for long-term adverse outcomes,’ " Dr. Carole A. Warnes explained at the annual cardiovascular conference at Snowmass sponsored by the American College of Cardiology (ACC).
This study on pregnancy’s impact on aortic growth rate and complications in patients with Marfan syndrome sheds much needed light on an area where there has been a paucity of data. The deficiency of data is reflected in discordant recommendations in the current U.S., European, and Canadian guidelines, said Dr. Warnes, professor of medicine at the Mayo Clinic, Rochester, Minn.
The U.S. guidelines put forth jointly by the ACC, American Heart Association, American Association for Thoracic Surgery, and other groups advocate that Marfan syndrome patients avoid pregnancy if their aortic root diameter exceeds 40 mm and recommend prophylactic aortic replacement in those interested in pregnancy (J. Am. Coll. Cardiol. 2010;55:e27-129).
In contrast, the European guidelines (Eur. Heart J. 2010;31:2915-57) consider an aortic root diameter of 45 mm or less to be generally safe, while strongly discouraging pregnancy in Marfan syndrome patients with a measurement above that threshold because of the associated increased dissection risk. The Canadian guidelines take a similar stance, albeit with a safety threshold of 44 mm rather than 45 mm (Can. J. Cardiol. 2010;26:e80-e97).
The Europeans qualify their position by noting that patients with a prepregnancy aortic root diameter of 40-45 mm who have a rapid aortic root growth rate or a family history of dissection ought to be considered high risk for pregnancy. The European and Canadian guidelines characterize dissection as a rare problem in patients with an aortic root diameter of less than 40 mm.
The recent Utah study included 98 women with Marfan syndrome, 69 of whom collectively had 199 pregnancies, with 170 live births, 26 spontaneous abortions, and 2 ectopic pregnancies.
Serial echocardiograms demonstrated that the aortic growth rate was significantly greater during pregnancy than beforehand, and after pregnancy it didn’t return to baseline. Obstetric complications occurred in 10% of pregnancies. Adverse fetal outcomes occurred in 13%.
Reassuringly, there were no catastrophic peripartum complications. No one required cardiac surgery or experienced aortic dissection during pregnancy. However, women with a prior pregnancy had a greater prevalence of both aortic dissection and elective aortic surgery during long-term follow-up, compared with matched childless women with Marfan syndrome. Thus, it’s important during prepregnancy counseling of women with Marfan syndrome to let them know they’ll need to have elective aortic root surgery at a younger age than if they remain childless, Dr. Warnes noted.
A larger initial root diameter and a faster increase in diameter were independent predictors of long-term adverse cardiovascular events in the Utah study.
Besides the recent Utah study, only two other prospective studies of pregnancy’s impact on aortic growth and complications have been done. Both were much smaller. In an editorial accompanying the Utah study, Dutch physicians combined the three studies to get a fuller picture. No type A dissections occurred during 145 pregnancies in 78 nonoperated women with Marfan syndrome. Of 25 women with an aortic root diameter of 40-51 mm during 29 pregnancies, one experienced a type B dissection, two had carotid artery dissections, and one developed accelerated aortic regurgitation, which went from mild to severe during pregnancy.
Five women underwent aortic root replacement (three electively), prior to six pregnancies. Two of them developed a type B dissection during pregnancy. Both women who underwent a valve-sparing elective aortic root replacement prior to pregnancy had pregnancies complicated by a worsening of aortic regurgitation, which went from trivial to moderate. These findings raise a red flag for Dr. Warnes.
"Even if they’ve had a successful root replacement, it doesn’t mean they’re out of the woods in terms of pregnancy. I think we have to question the role of prophylactic root replacement [as recommended in the U.S. guidelines] because these women will still have type B dissections, and trying to look for a type B dissection during pregnancy is a real difficult issue," the cardiologist observed.
The authors of the editorial concluded that Marfan syndrome patients without previous cardiac complications and who have a baseline aortic root diameter not in excess of 45 mm seem to tolerate pregnancy well as long as they receive good clinical care before, during, and after pregnancy. In contrast, pregnancy should be discouraged in patients with a history of aortic dissection because they are at elevated risk for aortic complications (J. Am. Coll. Cardiol. 2012;60:230-1).
Marfan syndrome is a genetic connective tissue disorder with an incidence of roughly 1 in 5,000 and autosomal dominant inheritance, so the fetus of an affected mom has a 50% chance of having the disorder. Dr. Warnes said that because the diagnostic criteria were overhauled in 2010, patients believed to have Marfan syndrome really ought to be referred to a specialized center in order to confirm or refute the diagnosis according to the contemporary Ghent criteria.
Dr. Warnes reported having no relevant financial interests.
SNOWMASS, COLO–Pregnancy increases the long-term risk of aortic complications in women with Marfan syndrome, according to a recent prospective study causing a stir among adult congenital heart disease specialists.
"This is the first study that says, ‘Even if the aortic root size is okay before pregnancy, the aorta is going to get bigger during pregnancy and it’s not going to go back to baseline. And if your aorta is bigger at the outset, there is a risk for long-term adverse outcomes,’ " Dr. Carole A. Warnes explained at the annual cardiovascular conference at Snowmass sponsored by the American College of Cardiology (ACC).
This study on pregnancy’s impact on aortic growth rate and complications in patients with Marfan syndrome sheds much needed light on an area where there has been a paucity of data. The deficiency of data is reflected in discordant recommendations in the current U.S., European, and Canadian guidelines, said Dr. Warnes, professor of medicine at the Mayo Clinic, Rochester, Minn.
The U.S. guidelines put forth jointly by the ACC, American Heart Association, American Association for Thoracic Surgery, and other groups advocate that Marfan syndrome patients avoid pregnancy if their aortic root diameter exceeds 40 mm and recommend prophylactic aortic replacement in those interested in pregnancy (J. Am. Coll. Cardiol. 2010;55:e27-129).
In contrast, the European guidelines (Eur. Heart J. 2010;31:2915-57) consider an aortic root diameter of 45 mm or less to be generally safe, while strongly discouraging pregnancy in Marfan syndrome patients with a measurement above that threshold because of the associated increased dissection risk. The Canadian guidelines take a similar stance, albeit with a safety threshold of 44 mm rather than 45 mm (Can. J. Cardiol. 2010;26:e80-e97).
The Europeans qualify their position by noting that patients with a prepregnancy aortic root diameter of 40-45 mm who have a rapid aortic root growth rate or a family history of dissection ought to be considered high risk for pregnancy. The European and Canadian guidelines characterize dissection as a rare problem in patients with an aortic root diameter of less than 40 mm.
The recent Utah study included 98 women with Marfan syndrome, 69 of whom collectively had 199 pregnancies, with 170 live births, 26 spontaneous abortions, and 2 ectopic pregnancies.
Serial echocardiograms demonstrated that the aortic growth rate was significantly greater during pregnancy than beforehand, and after pregnancy it didn’t return to baseline. Obstetric complications occurred in 10% of pregnancies. Adverse fetal outcomes occurred in 13%.
Reassuringly, there were no catastrophic peripartum complications. No one required cardiac surgery or experienced aortic dissection during pregnancy. However, women with a prior pregnancy had a greater prevalence of both aortic dissection and elective aortic surgery during long-term follow-up, compared with matched childless women with Marfan syndrome. Thus, it’s important during prepregnancy counseling of women with Marfan syndrome to let them know they’ll need to have elective aortic root surgery at a younger age than if they remain childless, Dr. Warnes noted.
A larger initial root diameter and a faster increase in diameter were independent predictors of long-term adverse cardiovascular events in the Utah study.
Besides the recent Utah study, only two other prospective studies of pregnancy’s impact on aortic growth and complications have been done. Both were much smaller. In an editorial accompanying the Utah study, Dutch physicians combined the three studies to get a fuller picture. No type A dissections occurred during 145 pregnancies in 78 nonoperated women with Marfan syndrome. Of 25 women with an aortic root diameter of 40-51 mm during 29 pregnancies, one experienced a type B dissection, two had carotid artery dissections, and one developed accelerated aortic regurgitation, which went from mild to severe during pregnancy.
Five women underwent aortic root replacement (three electively), prior to six pregnancies. Two of them developed a type B dissection during pregnancy. Both women who underwent a valve-sparing elective aortic root replacement prior to pregnancy had pregnancies complicated by a worsening of aortic regurgitation, which went from trivial to moderate. These findings raise a red flag for Dr. Warnes.
"Even if they’ve had a successful root replacement, it doesn’t mean they’re out of the woods in terms of pregnancy. I think we have to question the role of prophylactic root replacement [as recommended in the U.S. guidelines] because these women will still have type B dissections, and trying to look for a type B dissection during pregnancy is a real difficult issue," the cardiologist observed.
The authors of the editorial concluded that Marfan syndrome patients without previous cardiac complications and who have a baseline aortic root diameter not in excess of 45 mm seem to tolerate pregnancy well as long as they receive good clinical care before, during, and after pregnancy. In contrast, pregnancy should be discouraged in patients with a history of aortic dissection because they are at elevated risk for aortic complications (J. Am. Coll. Cardiol. 2012;60:230-1).
Marfan syndrome is a genetic connective tissue disorder with an incidence of roughly 1 in 5,000 and autosomal dominant inheritance, so the fetus of an affected mom has a 50% chance of having the disorder. Dr. Warnes said that because the diagnostic criteria were overhauled in 2010, patients believed to have Marfan syndrome really ought to be referred to a specialized center in order to confirm or refute the diagnosis according to the contemporary Ghent criteria.
Dr. Warnes reported having no relevant financial interests.
SNOWMASS, COLO–Pregnancy increases the long-term risk of aortic complications in women with Marfan syndrome, according to a recent prospective study causing a stir among adult congenital heart disease specialists.
"This is the first study that says, ‘Even if the aortic root size is okay before pregnancy, the aorta is going to get bigger during pregnancy and it’s not going to go back to baseline. And if your aorta is bigger at the outset, there is a risk for long-term adverse outcomes,’ " Dr. Carole A. Warnes explained at the annual cardiovascular conference at Snowmass sponsored by the American College of Cardiology (ACC).
This study on pregnancy’s impact on aortic growth rate and complications in patients with Marfan syndrome sheds much needed light on an area where there has been a paucity of data. The deficiency of data is reflected in discordant recommendations in the current U.S., European, and Canadian guidelines, said Dr. Warnes, professor of medicine at the Mayo Clinic, Rochester, Minn.
The U.S. guidelines put forth jointly by the ACC, American Heart Association, American Association for Thoracic Surgery, and other groups advocate that Marfan syndrome patients avoid pregnancy if their aortic root diameter exceeds 40 mm and recommend prophylactic aortic replacement in those interested in pregnancy (J. Am. Coll. Cardiol. 2010;55:e27-129).
In contrast, the European guidelines (Eur. Heart J. 2010;31:2915-57) consider an aortic root diameter of 45 mm or less to be generally safe, while strongly discouraging pregnancy in Marfan syndrome patients with a measurement above that threshold because of the associated increased dissection risk. The Canadian guidelines take a similar stance, albeit with a safety threshold of 44 mm rather than 45 mm (Can. J. Cardiol. 2010;26:e80-e97).
The Europeans qualify their position by noting that patients with a prepregnancy aortic root diameter of 40-45 mm who have a rapid aortic root growth rate or a family history of dissection ought to be considered high risk for pregnancy. The European and Canadian guidelines characterize dissection as a rare problem in patients with an aortic root diameter of less than 40 mm.
The recent Utah study included 98 women with Marfan syndrome, 69 of whom collectively had 199 pregnancies, with 170 live births, 26 spontaneous abortions, and 2 ectopic pregnancies.
Serial echocardiograms demonstrated that the aortic growth rate was significantly greater during pregnancy than beforehand, and after pregnancy it didn’t return to baseline. Obstetric complications occurred in 10% of pregnancies. Adverse fetal outcomes occurred in 13%.
Reassuringly, there were no catastrophic peripartum complications. No one required cardiac surgery or experienced aortic dissection during pregnancy. However, women with a prior pregnancy had a greater prevalence of both aortic dissection and elective aortic surgery during long-term follow-up, compared with matched childless women with Marfan syndrome. Thus, it’s important during prepregnancy counseling of women with Marfan syndrome to let them know they’ll need to have elective aortic root surgery at a younger age than if they remain childless, Dr. Warnes noted.
A larger initial root diameter and a faster increase in diameter were independent predictors of long-term adverse cardiovascular events in the Utah study.
Besides the recent Utah study, only two other prospective studies of pregnancy’s impact on aortic growth and complications have been done. Both were much smaller. In an editorial accompanying the Utah study, Dutch physicians combined the three studies to get a fuller picture. No type A dissections occurred during 145 pregnancies in 78 nonoperated women with Marfan syndrome. Of 25 women with an aortic root diameter of 40-51 mm during 29 pregnancies, one experienced a type B dissection, two had carotid artery dissections, and one developed accelerated aortic regurgitation, which went from mild to severe during pregnancy.
Five women underwent aortic root replacement (three electively), prior to six pregnancies. Two of them developed a type B dissection during pregnancy. Both women who underwent a valve-sparing elective aortic root replacement prior to pregnancy had pregnancies complicated by a worsening of aortic regurgitation, which went from trivial to moderate. These findings raise a red flag for Dr. Warnes.
"Even if they’ve had a successful root replacement, it doesn’t mean they’re out of the woods in terms of pregnancy. I think we have to question the role of prophylactic root replacement [as recommended in the U.S. guidelines] because these women will still have type B dissections, and trying to look for a type B dissection during pregnancy is a real difficult issue," the cardiologist observed.
The authors of the editorial concluded that Marfan syndrome patients without previous cardiac complications and who have a baseline aortic root diameter not in excess of 45 mm seem to tolerate pregnancy well as long as they receive good clinical care before, during, and after pregnancy. In contrast, pregnancy should be discouraged in patients with a history of aortic dissection because they are at elevated risk for aortic complications (J. Am. Coll. Cardiol. 2012;60:230-1).
Marfan syndrome is a genetic connective tissue disorder with an incidence of roughly 1 in 5,000 and autosomal dominant inheritance, so the fetus of an affected mom has a 50% chance of having the disorder. Dr. Warnes said that because the diagnostic criteria were overhauled in 2010, patients believed to have Marfan syndrome really ought to be referred to a specialized center in order to confirm or refute the diagnosis according to the contemporary Ghent criteria.
Dr. Warnes reported having no relevant financial interests.
EXPERT ANALYSIS FROM THE ANNUAL CARDIOVASCULAR CONFERENCE AT SNOWMASS
Pregnant Woman, 39, With Hypertension and New-Onset Proteinuria
A 39-year-old black woman, gravida 1, para 0, with an intrauterine pregnancy of 34 weeks and three days (according to last menstrual period and nine-week ultrasound) presented to her Ob-Gyn office for a routine prenatal visit. She was found to have an elevated blood pressure with new onset of 2+ proteinuria. The patient was sent to the labor and delivery unit at the adjoining hospital for serial blood pressure readings, laboratory work, and fetal monitoring.
The patient’s previous medical history was limited to sinusitis. She was taking no prescription medications, and her only listed allergy was to pineapple. Initial lab studies revealed elevations in liver enzymes, lactate dehydrogenase (LDH), uric acid, and serum creatinine, as well as thrombocytopenia (see Table 11-5). She also had a critically low blood glucose level, which conflicted with a normal follow-up reading.
At this point, the patient was thought to have HELLP syndrome6 (ie, hemolysis, elevated liver enzymes, low platelet count), or possibly acute fatty liver of pregnancy (AFLP).2,4,7-11 Additional labs were drawn immediately to confirm or rule out AFLP. These included repeat serum glucose (following a second reading with normal results), a serum ammonia level, prothrombin time (PT), and partial thromboplastin time (PTT). The most reliable values to distinguish AFLP from HELLP are profound hypoglycemia (found in 94% of women with AFLP12) and an elevated serum ammonia level.4
Given the serious nature of either diagnosis, immediate delivery of the infant was deemed necessary. Because the patient’s cervix was not found favorable for induction, she underwent low-transverse cesarean delivery without complications. She was noted to have essentially normal anatomy with the exception of a small subserosal fibroid posteriorly. Meconium-stained amniotic fluid was present. A male infant was delivered, weighing 5 lb with 1-minute and 5-minute Apgar scores of 8 and 9, respectively.
Postoperatively, the patient remained in the recovery area, where she received intensive monitoring. She experienced fluctuations in blood glucose, ranging from 33 to 144 mg/dL; she was started on 5% dextrose in lactated Ringer’s solution and treated with IV dextrose 50 g. While the patient was in surgical recovery, results from the second set of labs, drawn before surgery, were returned; findings included an elevated ammonia level and an abnormal coagulation panel, including PT of 25.3 sec, PTT of 48.4 sec, and a fibrinogen level of 116 mg/dL, confirming the suspected diagnosis of AFLP.
Magnesium sulfate, which had been started immediately postop, was discontinued on confirmation of the diagnosis of AFLP. The patient was initially somnolent as a result of general anesthesia but gradually returned to a fully normal sensorium by early morning on postop day 1. Postoperatively, the patient’s hemoglobin was found to be low (8.6 g/dL; reference range, 13.5 to 18.5 g/dL), so she was transfused with two units of packed red blood cells (PRBCs) and given fresh frozen plasma (FFP) to correct this coagulopathy. The patient’s platelets were also low at 82,000/mm3 (reference range, 140,000 to 340,000/mm3).
On postop day 1, the patient’s serum creatinine rose to 4.2 mg/dL and her total bilirubin increased to 14.4 mg/dL (reference ranges, 0.6 to 1.2 mg/dL and < 1.0 mg/dL, respectively). Given the multiple systems affected by AFLP and the need for intensive supportive care, the patient was transferred to the ICU.
On her arrival at the ICU, the patient’s vital signs were initially stable, and she was alert and oriented. However, within the next few hours, she became hypotensive and encephalopathic. She required aggressive fluid resuscitation and multiple transfusions of PRBCs and FFP due to persistent anemia and coagulopathy. Her vital signs were stabilized, but she continued to need blood transfusions.
Postop day 2, the patient became less responsive and was soon unable to follow commands or speak clearly. Her breathing remained stable with just 3 L of oxygen by nasal cannula, but in order to prevent aspiration and in consideration of a postoperative ileus, it was necessary to place a nasogastric tube with low intermittent suction. This produced a bloody return, but no intervention other than close monitoring and transfusion was performed at that time.
Abdominal ultrasound showed ascites and mild left-sided hydronephrosis with no gallstones. The common bile duct measured 3 mm in diameter.
Although liver biopsy is considered the gold standard for a confirmed diagnosis of AFLP,13,14 this procedure was contraindicated by the patient’s coagulopathy. Concern was also expressed by one consultant that the patient might have thrombotic thrombocytopenic purpura (TTP) in addition to AFLP. TTP can manifest with similar findings, such as anemia, thrombocytopenia, neurologic symptoms, and renal abnormalities, but usually fever is involved, and the patient was afebrile. A catheter was placed for hemodialysis and therapeutic plasma exchange (TPE). Given that TTP-associated mortality is significantly decreased by use of TPE,15 this intervention was deemed prudent. The patient underwent TPE on three consecutive days, postop days 2 through 4.
The patient’s mental status began to improve, and by postop day 6, she was able to follow commands and engage in brief conversations. By postop day 9, she had returned almost completely to her baseline mental status.
The patient’s liver function test results and total bilirubin, ammonia, and creatinine levels all improved over the first few postoperative days but began to rise again by day 6. In response to worsening renal and hepatic functioning, the decision was made on postop day 9 to transfer the patient to a hospital with liver transplantation capabilities, should this procedure become necessary.
Discussion
AFLP is a rare condition specific to pregnancy, affecting 1/7,000 to 1/20,000 pregnancies. Due to the low incidence of this disease, randomized controlled trials to study it are not possible. Instead, clinicians must learn either from individual case studies or from retrospective syntheses of cases reported over time.1,2,7 Fortunately, the wealth of information gleaned over the past 30 years has significantly reduced AFLP-associated maternal and fetal mortality and morbidity rates. In the 1980s, maternal and fetal mortality rates as high as 85% were reported.3 Worldwide, maternal mortality associated with AFLP has decreased significantly to 7% to 18%, whereas the fetal mortality rate has fallen to between 9% and 23%.1,16,17
Common trends among women who have developed AFLP include nulliparity, multiparity, and advanced maternal age. One retrospective study of 57 women who had developed AFLP revealed that 35 cases (61%) involved first-time pregnancies. It also showed that 10 (18%) of the women had twins, and 14 (25%) were older than 35.2 In another study of 35 cases of AFLP, 40% of the women were nulliparous, and 11.4% were multiparous, including one triplet gestation.12 In a third, smaller study, 80% of women affected by AFLP were multiparous.10 Currently, there is no known evidence linking any maternal behavior to development of AFLP.
Presentation
Women who present with AFLP often experience vague, nonspecific symptoms, leading to misdiagnosis or delayed diagnosis. Objective measurements, including physical exam findings, laboratory studies, and other diagnostic tests, will help with a diagnosis. The most frequent initial symptoms are nausea and vomiting (in 70% of patients) and abdominal pain (50% to 80%), epigastric or right upper-quadrant.3 Other common symptoms include fatigue, malaise, anorexia, weight gain, polyuria, and polydipsia.2,3,9,18,19
Because the presenting symptoms in AFLP can be vague, clinicians should complete a thorough physical exam to differentiate accurately among conditions associated with pregnancy. Physical signs present in women with AFLP can include jaundice, ascites, edema, confusion, abdominal tenderness, and fever. More severe cases can present with multisystem involvement, including acute renal failure, gastrointestinal bleeding, pancreatitis, coagulopathy, and hepatic encephalopathy.3,4,9,18
Diagnostic Tests
Relevant laboratory tests include a complete blood count (CBC), liver studies, chemistry, coagulation studies, and urinalysis (see Table 1). Viral causes should be ruled out by way of a hepatitis panel.3 In AFLP, the CBC may show elevated white blood cells, decreased hemoglobin and hematocrit, and decreased platelets. Liver studies show elevated hepatic aminotransferase, bilirubin, LDH, and ammonia levels. Chemistry results show elevated blood urea nitrogen and creatinine, and decreased blood glucose. Coagulation factors are affected, and prolonged PTT, decreased fibrinogen, and proteinurea may also be found.9
Though invasive and not often necessary4,13 (and not possible for the case patient), the definitive diagnostic test for AFLP is liver biopsy.13,14 Biopsy reveals a microvesicular fatty infiltration of the hepatocytes as minute fat droplets surrounding a centrally located nucleus. These fatty infiltrates stain with oil red O, specific for fat. Inflammation is present in 50% of cases. There may also be a picture similar to cholestasis with bile thrombi or deposits within the hepatocytes.20
Due to the risk for hemorrhage and the critical status of women with AFLP, biopsy is often not possible. Ultrasonography may show increased echogenicity; CT may show decreased or diffuse attenuation in the liver. These imaging studies, though possibly helpful in severe cases, often yield false-negative results.3,20
In the absence of another explanation for the patient’s symptoms, the Swansea criteria are used for diagnosis of AFLP.1 Six or more of the following criteria must be present to confirm this diagnosis: vomiting, abdominal pain, polydipsia or polyuria, encephalopathy, leukocytosis, elevated bilirubin, elevated liver enzymes, elevated ammonia, hypoglycemia, renal dysfunction, coagulopathy, elevated uric acid, ascites on ultrasound, and microvesicular steatosis on liver biopsy.1,2,5
Pathophysiology
Normal functions of the liver include metabolism, protein synthesis, and manufacturing of blood coagulation proteins. These functions are disturbed in the presence of AFLP. Thus, women with this disease experience signs and symptoms related directly to the dysfunction of these processes.20-22
Disturbances in the hepatocytes due to excess fatty acids impair the liver’s ability to convert unconjugated bilirubin into conjugated bilirubin, causing plasma levels of unconjugated bilirubin to rise. This increase in bilirubin explains the jaundiced appearance of women with AFLP. AFLP is often thought to occur in conjunction with preeclampsia in many, but not all, patients. Thrombocytopenia in these patients is felt to be secondary to peripheral vascular consumption. Conjugated bilirubin levels may also be increased due to decreased flow of conjugated bilirubin into the common bile duct.21
Another liver function that is disrupted is that of glycogen storage and conversion to glucose, and the liver’s ability to convert nutrients into glycogen is also impaired. Decreased storage of glycogen, along with the liver’s inability to break down previously stored glycogen, causes a decrease in serum glucose levels. Women with AFLP often require treatment with IV dextrose in response to marked hypoglycemia.16,21,23
The liver dysfunction associated with AFLP reduces adequate production of clotting factors and coagulation proteins. Thrombocytopenia, elevated clotting times, and bleeding are all problems seen in AFLP. Mild to moderate elevations in serum aminotransferases and elevated LDH also occur in patients with AFLP.23,24
Genetic Factor
There is little known about the etiology of AFLP, although recent data point to a genetic component that was found in as many as 62% of mothers in one study and in 25% of infants in another study.20-22 Fatty acid oxidation (FAO) is one of the processes of hepatic mitochondria, a process that relies on several enzymes. When FAO is interrupted, fatty acids are deposited in the liver cells, as seen in histologic studies of AFLP.25,26 The common thread in women with this disease is a mutation in one of the enzymes needed for FAO. This enzyme is the long-chain 3-hydroxyacyl-CoA dehydrogenase. Deficiencies in this enzyme are common in mothers with AFLP and their infants.3,16,20,23,27
Differential Diagnosis
Several complications of pregnancy that involve the liver may, on presentation, mimic AFLP.16,20,23,24,28 The most common are hyperemesis gravidarum and intrahepatic cholestasis of pregnancy23 (see Table 216,20,23,24,28); others are preeclampsia/eclampsia and HELLP syndrome. It is important to distinguish between the signs and symptoms associated with each of these disorders in order to provide the most effective treatment. Hepatitis serologies are important in the differential diagnosis when liver enzyme levels are exceptionally high.4,16,22,28
Treatment
The most effective treatment for AFLP is delivery of the infant; often, this alone causes the signs and symptoms of AFLP to resolve.8,21,27,29 In two of three cases in a small study by Aso et al,8 early delivery of the fetus led to complete resolution of symptoms and return to normal liver function. One of these patients was sent home four days after delivery; the other, 14 days later. Other patients may require more invasive treatment and support.8
Management in the ICU is often required to provide appropriate supportive care to the mother after delivery. Acute respiratory distress syndrome, pancreatitis, hemorrhage, encephalopathy, renal failure, and continual liver failure are among the severe complications associated with AFLP.4,8,10 Many women require intubation, dialysis, fluid resuscitation, blood product transfusion, and vasopressor therapy.3,8,11 Prophylactic antibiotics, IV steroids, and glucose may all be required in the supportive care and recovery of a mother with AFLP.3,8,11
TPE has also been useful in instances of severe complications.1,3,6 In one retrospective study, Martin et al1 recommended administration of TPE in patients with AFLP under the following circumstances:
(1) Deteriorating central nervous system abnormalities, such as sensorium changes or coma;
(2) Persistent coagulopathy requiring continued and aggressive blood product support with plasma, red cells, and/or cryoprecipitate;
(3) Advanced renal dysfunction that compromised fluid management;
(4) Progressive cardiopulmonary compromise; and/or
(5) Ongoing fluid management concerns, including significant ascites, edema, anuria/oliguria, and/or fluid overload.1
In rare cases, liver transplantation is needed in patients with AFLP. Westbrook et al18 reviewed 54 cases of liver disease in pregnancy in one UK hospital between 1997 and 2008. Of these, six patients with encephalopathy or elevated lactate were listed for liver transplant, including just one with a diagnosis of AFLP. This woman never actually underwent transplant but recovered in response to medical management alone.18 According to data reported in June 2011 by the Organ Procurement and Transplantation Network,30 liver transplantation was needed in only three US patients with AFLP between 2000 and 2011. Further retrospective studies on outcomes from transplant versus medical management should be considered to guide future decision making involving this invasive therapy.
The Case Patient
This 39-year-old patient presented during a routine prenatal visit with proteinuria and hypertension, possibly indicative of preeclampsia. Because of the serious nature of this potential diagnosis in pregnancy, she was admitted for monitoring and further testing. Although the diagnosis of AFLP was not confirmed until later, the patient’s preliminary lab studies showed elevated liver enzymes and low platelet counts, signifying the need for prompt intervention and delivery of the infant. At this point, the patient met criteria for HELLP syndrome, but AFLP was suspected after the initial finding of profound hypoglycemia led to further testing.
As an older mother experiencing pregnancy for the first time, this patient fit the profile for AFLP. She initially responded well after delivery of her infant but continued to experience complications. On the days that the patient was treated with TPE, her total bilirubin and liver enzymes were at their lowest. Perhaps this treatment should be considered in more cases of AFLP.
The patient was transferred to a hospital with liver transplantation capabilities, but she ultimately recovered without undergoing transplant.
Conclusion
For the primary obstetric care provider, being aware of the possible complications associated with pregnancy is important. Though uncommon, AFLP is a serious complication that should be ruled out in women who present with vague symptoms such as nausea, vomiting, and abdominal pain in the third trimester of pregnancy. The reduction in AFLP-associated morbidity and mortality during the past 20 years is a direct result of increased early recognition and therapeutic delivery.
Referral to a maternal fetal medicine specialist, gastroenterologist, hematologist, and/or nephrologist may be necessary and appropriate in the management of a woman with AFLP. Further study is indicated for use of TPE in more severe cases of AFLP, particularly in women affected by persistent thrombocytopenia and anemia.
The author would like to thank C. Leanne Browning, MD, obstetrics/gynecology, for her invaluable guidance and advice on this project.
References
1. Martin JN Jr, Briery CM, Rose CH, et al. Postpartum plasma exchange as adjunctive therapy for severe acute fatty liver of pregnancy. J Clin Apher. 2009;23(4):138-143.
2. Knight M, Nelson-Piercy C, Kurinczuk JJ; UK Obstetric Surveillance System. A prospective national study of acute fatty liver of pregnancy in the UK. Gut. 2008;57(7):951-956.
3. Barsoom MJ, Tierney BJ. Acute fatty liver of pregnancy (2011). http://emedicine.medscape.com/article/1562425-overview. Accessed January 21, 2013.
4. Ko HH, Yoshida E. Acute fatty liver of pregnancy. Can J Gastroenterol. 2006;20(1):25-30.
5. Rathi U, Bapat M, Rathi P, Abraham P. Effect of liver disease on maternal and fetal outcome: a prospective study. Indian J Gastroenterol. 2007;26(2):59-63.
6. Myers L. Postpartum plasma exchange in a woman with suspected thrombotic thrombocytopenic purpura (TTP) vs hemolysis, elevated liver enzymes, and low platelet syndrome (HELLP): a case study. Nephrol Nurs J. 2010;37(4):399-402.
7. Vigil-de Gracia P. Acute fatty liver and HELLP syndrome: two distinct pregnancy disorders. Int J Gynaecol Obstet. 2001;73(3):215-220.
8. Aso K, Hojo S, Yumoto Y, et al. Three cases of acute fatty liver of pregnancy: postpartum clinical course depends on interval between onset of symptoms and termination of pregnancy. J Matern Fetal Neonatal Med. 2010;23(9):1047-1049.
9. Wei Q, Zhang L, Liu X. Clinical diagnosis and treatment of acute fatty liver of pregnancy: a literature review and 11 new cases. J Obstet Gynaecol Res. 2010;36(4):751-756.
10. Barber MA, Eguiluz I, Martin A, et al. Acute fatty liver of pregnancy: analysis of five consecutive cases from a tertiary centre. J Obstet Gynaecol. 2010;30(3):241-243.
11. Ajayi AO, Alao MO. Case report: acute fatty liver of pregnancy in a 30-year-old Nigerian primigravida. Niger J Clin Pract. 2008;11(4):389-391.
12. Vigíl-de Gracia P, Montufar-Rueda C. Acute fatty liver of pregnancy: diagnosis, treatment, and outcome based on 35 consecutive cases. J Matern Fetal Neonatal Med. 2011;24(9):1143-1146.
13. Dey M, Reema K. Acute fatty liver of pregnancy. N Am J Med Sci. 2012;4(11):611-612.
14. Castro MA, Goodwin TM, Shaw KJ, et al. Disseminated intravascular coagulation and antithrombin III depression in acute fatty liver of pregnancy. Am J Obstet Gynecol. 1996;174(1 pt 1):211-216.
15. Altuntas F, Aydogdu I, Kabukcu S, et al. Therapeutic plasma exchange for the treatment of thrombotic thrombocytopenic purpura: a retrospective multicenter study. Transfus Apher Sci. 2007;36(1):57-67.
16. Hay JE. Liver disease in pregnancy. Hepatology. 2008;47(3):1067-1076.
17. Wand S, Waeschle RM, Von Ahsen N, et al. Acute fatty liver failure due to acute fatty liver of pregnancy. Minerva Anesthesiol. 2012;78(4):503-506.
18. Westbrook RH, Yeoman AD, Joshi D, et al. Outcomes of severe pregnancy-related liver disease: refining the role of transplantation. Am J Transplant. 2010;10(11):2520-2526.
19. Fesenmeier MF, Coppage KH, Lambers DS, et al. Acute fatty liver of pregnancy in 3 tertiary care centers. Am J Obstet Gynecol. 2005;192(5):1416-1419.
20. Bacq Y. Liver diseases unique to pregnancy: a 2010 update. Clin Res Hepatol Gastroenterol. 2011;35(3):182-193.
21. Huether SE. Alterations of digestive function. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. St. Louis, MO: Mosby; 2009:1452-1515.
22. Huether SE. Structure and function of the digestive system. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. St. Louis, MO: Mosby; 2009:1420-1451.
23. Schutt VA, Minuk GY. Liver diseases unique to pregnancy. Best Pract Res Clin Gastroenterol. 2007;21(5):771-792.
24. Pan C, Perumalswami PV. Pregnancy-related liver diseases. Clin Liver Dis. 2011;15(1):199-208.
25. Ibdah JA. Acute fatty liver of pregnancy: an update on pathogenesis and clinical implications. World J Gastroenterol. 2006;12(46):7397-7404.
26. Browning MF, Levy HL, Wilkins-Haug LE, et al. Fetal fatty acid oxidation defects and maternal liver disease in pregnancy. Obstet Gynecol. 2006;107(1):115-120.
27. Dekker RR, Schutte JM, Stekelenburg J, et al. Maternal mortality and severe maternal morbidity from acute fatty liver of pregnancy in the Netherlands. Eur J Obstet Gynecol Reprod Biol. 2011;157(1):27-31.
28. Lee NM, Brady CW. Liver disease in pregnancy. World J Gastroenterol. 2009;15(8):897-906.
29. Vora KS, Shah VR, Parikh GP. Acute fatty liver of pregnancy: a case report of an uncommon disease. Indian J Crit Care Med. 2009;13(1):34-36.
30. Organ Procurement and Transplantation Network, Scientific Registry of Transplant Recipients. OPTN/SRTR 2011 Annual Data Report: Liver. http://srtr.transplant.hrsa.gov/annual_reports/2011/pdf/03_%20liver_12.pdf. Accessed January 18, 2013.
A 39-year-old black woman, gravida 1, para 0, with an intrauterine pregnancy of 34 weeks and three days (according to last menstrual period and nine-week ultrasound) presented to her Ob-Gyn office for a routine prenatal visit. She was found to have an elevated blood pressure with new onset of 2+ proteinuria. The patient was sent to the labor and delivery unit at the adjoining hospital for serial blood pressure readings, laboratory work, and fetal monitoring.
The patient’s previous medical history was limited to sinusitis. She was taking no prescription medications, and her only listed allergy was to pineapple. Initial lab studies revealed elevations in liver enzymes, lactate dehydrogenase (LDH), uric acid, and serum creatinine, as well as thrombocytopenia (see Table 11-5). She also had a critically low blood glucose level, which conflicted with a normal follow-up reading.
At this point, the patient was thought to have HELLP syndrome6 (ie, hemolysis, elevated liver enzymes, low platelet count), or possibly acute fatty liver of pregnancy (AFLP).2,4,7-11 Additional labs were drawn immediately to confirm or rule out AFLP. These included repeat serum glucose (following a second reading with normal results), a serum ammonia level, prothrombin time (PT), and partial thromboplastin time (PTT). The most reliable values to distinguish AFLP from HELLP are profound hypoglycemia (found in 94% of women with AFLP12) and an elevated serum ammonia level.4
Given the serious nature of either diagnosis, immediate delivery of the infant was deemed necessary. Because the patient’s cervix was not found favorable for induction, she underwent low-transverse cesarean delivery without complications. She was noted to have essentially normal anatomy with the exception of a small subserosal fibroid posteriorly. Meconium-stained amniotic fluid was present. A male infant was delivered, weighing 5 lb with 1-minute and 5-minute Apgar scores of 8 and 9, respectively.
Postoperatively, the patient remained in the recovery area, where she received intensive monitoring. She experienced fluctuations in blood glucose, ranging from 33 to 144 mg/dL; she was started on 5% dextrose in lactated Ringer’s solution and treated with IV dextrose 50 g. While the patient was in surgical recovery, results from the second set of labs, drawn before surgery, were returned; findings included an elevated ammonia level and an abnormal coagulation panel, including PT of 25.3 sec, PTT of 48.4 sec, and a fibrinogen level of 116 mg/dL, confirming the suspected diagnosis of AFLP.
Magnesium sulfate, which had been started immediately postop, was discontinued on confirmation of the diagnosis of AFLP. The patient was initially somnolent as a result of general anesthesia but gradually returned to a fully normal sensorium by early morning on postop day 1. Postoperatively, the patient’s hemoglobin was found to be low (8.6 g/dL; reference range, 13.5 to 18.5 g/dL), so she was transfused with two units of packed red blood cells (PRBCs) and given fresh frozen plasma (FFP) to correct this coagulopathy. The patient’s platelets were also low at 82,000/mm3 (reference range, 140,000 to 340,000/mm3).
On postop day 1, the patient’s serum creatinine rose to 4.2 mg/dL and her total bilirubin increased to 14.4 mg/dL (reference ranges, 0.6 to 1.2 mg/dL and < 1.0 mg/dL, respectively). Given the multiple systems affected by AFLP and the need for intensive supportive care, the patient was transferred to the ICU.
On her arrival at the ICU, the patient’s vital signs were initially stable, and she was alert and oriented. However, within the next few hours, she became hypotensive and encephalopathic. She required aggressive fluid resuscitation and multiple transfusions of PRBCs and FFP due to persistent anemia and coagulopathy. Her vital signs were stabilized, but she continued to need blood transfusions.
Postop day 2, the patient became less responsive and was soon unable to follow commands or speak clearly. Her breathing remained stable with just 3 L of oxygen by nasal cannula, but in order to prevent aspiration and in consideration of a postoperative ileus, it was necessary to place a nasogastric tube with low intermittent suction. This produced a bloody return, but no intervention other than close monitoring and transfusion was performed at that time.
Abdominal ultrasound showed ascites and mild left-sided hydronephrosis with no gallstones. The common bile duct measured 3 mm in diameter.
Although liver biopsy is considered the gold standard for a confirmed diagnosis of AFLP,13,14 this procedure was contraindicated by the patient’s coagulopathy. Concern was also expressed by one consultant that the patient might have thrombotic thrombocytopenic purpura (TTP) in addition to AFLP. TTP can manifest with similar findings, such as anemia, thrombocytopenia, neurologic symptoms, and renal abnormalities, but usually fever is involved, and the patient was afebrile. A catheter was placed for hemodialysis and therapeutic plasma exchange (TPE). Given that TTP-associated mortality is significantly decreased by use of TPE,15 this intervention was deemed prudent. The patient underwent TPE on three consecutive days, postop days 2 through 4.
The patient’s mental status began to improve, and by postop day 6, she was able to follow commands and engage in brief conversations. By postop day 9, she had returned almost completely to her baseline mental status.
The patient’s liver function test results and total bilirubin, ammonia, and creatinine levels all improved over the first few postoperative days but began to rise again by day 6. In response to worsening renal and hepatic functioning, the decision was made on postop day 9 to transfer the patient to a hospital with liver transplantation capabilities, should this procedure become necessary.
Discussion
AFLP is a rare condition specific to pregnancy, affecting 1/7,000 to 1/20,000 pregnancies. Due to the low incidence of this disease, randomized controlled trials to study it are not possible. Instead, clinicians must learn either from individual case studies or from retrospective syntheses of cases reported over time.1,2,7 Fortunately, the wealth of information gleaned over the past 30 years has significantly reduced AFLP-associated maternal and fetal mortality and morbidity rates. In the 1980s, maternal and fetal mortality rates as high as 85% were reported.3 Worldwide, maternal mortality associated with AFLP has decreased significantly to 7% to 18%, whereas the fetal mortality rate has fallen to between 9% and 23%.1,16,17
Common trends among women who have developed AFLP include nulliparity, multiparity, and advanced maternal age. One retrospective study of 57 women who had developed AFLP revealed that 35 cases (61%) involved first-time pregnancies. It also showed that 10 (18%) of the women had twins, and 14 (25%) were older than 35.2 In another study of 35 cases of AFLP, 40% of the women were nulliparous, and 11.4% were multiparous, including one triplet gestation.12 In a third, smaller study, 80% of women affected by AFLP were multiparous.10 Currently, there is no known evidence linking any maternal behavior to development of AFLP.
Presentation
Women who present with AFLP often experience vague, nonspecific symptoms, leading to misdiagnosis or delayed diagnosis. Objective measurements, including physical exam findings, laboratory studies, and other diagnostic tests, will help with a diagnosis. The most frequent initial symptoms are nausea and vomiting (in 70% of patients) and abdominal pain (50% to 80%), epigastric or right upper-quadrant.3 Other common symptoms include fatigue, malaise, anorexia, weight gain, polyuria, and polydipsia.2,3,9,18,19
Because the presenting symptoms in AFLP can be vague, clinicians should complete a thorough physical exam to differentiate accurately among conditions associated with pregnancy. Physical signs present in women with AFLP can include jaundice, ascites, edema, confusion, abdominal tenderness, and fever. More severe cases can present with multisystem involvement, including acute renal failure, gastrointestinal bleeding, pancreatitis, coagulopathy, and hepatic encephalopathy.3,4,9,18
Diagnostic Tests
Relevant laboratory tests include a complete blood count (CBC), liver studies, chemistry, coagulation studies, and urinalysis (see Table 1). Viral causes should be ruled out by way of a hepatitis panel.3 In AFLP, the CBC may show elevated white blood cells, decreased hemoglobin and hematocrit, and decreased platelets. Liver studies show elevated hepatic aminotransferase, bilirubin, LDH, and ammonia levels. Chemistry results show elevated blood urea nitrogen and creatinine, and decreased blood glucose. Coagulation factors are affected, and prolonged PTT, decreased fibrinogen, and proteinurea may also be found.9
Though invasive and not often necessary4,13 (and not possible for the case patient), the definitive diagnostic test for AFLP is liver biopsy.13,14 Biopsy reveals a microvesicular fatty infiltration of the hepatocytes as minute fat droplets surrounding a centrally located nucleus. These fatty infiltrates stain with oil red O, specific for fat. Inflammation is present in 50% of cases. There may also be a picture similar to cholestasis with bile thrombi or deposits within the hepatocytes.20
Due to the risk for hemorrhage and the critical status of women with AFLP, biopsy is often not possible. Ultrasonography may show increased echogenicity; CT may show decreased or diffuse attenuation in the liver. These imaging studies, though possibly helpful in severe cases, often yield false-negative results.3,20
In the absence of another explanation for the patient’s symptoms, the Swansea criteria are used for diagnosis of AFLP.1 Six or more of the following criteria must be present to confirm this diagnosis: vomiting, abdominal pain, polydipsia or polyuria, encephalopathy, leukocytosis, elevated bilirubin, elevated liver enzymes, elevated ammonia, hypoglycemia, renal dysfunction, coagulopathy, elevated uric acid, ascites on ultrasound, and microvesicular steatosis on liver biopsy.1,2,5
Pathophysiology
Normal functions of the liver include metabolism, protein synthesis, and manufacturing of blood coagulation proteins. These functions are disturbed in the presence of AFLP. Thus, women with this disease experience signs and symptoms related directly to the dysfunction of these processes.20-22
Disturbances in the hepatocytes due to excess fatty acids impair the liver’s ability to convert unconjugated bilirubin into conjugated bilirubin, causing plasma levels of unconjugated bilirubin to rise. This increase in bilirubin explains the jaundiced appearance of women with AFLP. AFLP is often thought to occur in conjunction with preeclampsia in many, but not all, patients. Thrombocytopenia in these patients is felt to be secondary to peripheral vascular consumption. Conjugated bilirubin levels may also be increased due to decreased flow of conjugated bilirubin into the common bile duct.21
Another liver function that is disrupted is that of glycogen storage and conversion to glucose, and the liver’s ability to convert nutrients into glycogen is also impaired. Decreased storage of glycogen, along with the liver’s inability to break down previously stored glycogen, causes a decrease in serum glucose levels. Women with AFLP often require treatment with IV dextrose in response to marked hypoglycemia.16,21,23
The liver dysfunction associated with AFLP reduces adequate production of clotting factors and coagulation proteins. Thrombocytopenia, elevated clotting times, and bleeding are all problems seen in AFLP. Mild to moderate elevations in serum aminotransferases and elevated LDH also occur in patients with AFLP.23,24
Genetic Factor
There is little known about the etiology of AFLP, although recent data point to a genetic component that was found in as many as 62% of mothers in one study and in 25% of infants in another study.20-22 Fatty acid oxidation (FAO) is one of the processes of hepatic mitochondria, a process that relies on several enzymes. When FAO is interrupted, fatty acids are deposited in the liver cells, as seen in histologic studies of AFLP.25,26 The common thread in women with this disease is a mutation in one of the enzymes needed for FAO. This enzyme is the long-chain 3-hydroxyacyl-CoA dehydrogenase. Deficiencies in this enzyme are common in mothers with AFLP and their infants.3,16,20,23,27
Differential Diagnosis
Several complications of pregnancy that involve the liver may, on presentation, mimic AFLP.16,20,23,24,28 The most common are hyperemesis gravidarum and intrahepatic cholestasis of pregnancy23 (see Table 216,20,23,24,28); others are preeclampsia/eclampsia and HELLP syndrome. It is important to distinguish between the signs and symptoms associated with each of these disorders in order to provide the most effective treatment. Hepatitis serologies are important in the differential diagnosis when liver enzyme levels are exceptionally high.4,16,22,28
Treatment
The most effective treatment for AFLP is delivery of the infant; often, this alone causes the signs and symptoms of AFLP to resolve.8,21,27,29 In two of three cases in a small study by Aso et al,8 early delivery of the fetus led to complete resolution of symptoms and return to normal liver function. One of these patients was sent home four days after delivery; the other, 14 days later. Other patients may require more invasive treatment and support.8
Management in the ICU is often required to provide appropriate supportive care to the mother after delivery. Acute respiratory distress syndrome, pancreatitis, hemorrhage, encephalopathy, renal failure, and continual liver failure are among the severe complications associated with AFLP.4,8,10 Many women require intubation, dialysis, fluid resuscitation, blood product transfusion, and vasopressor therapy.3,8,11 Prophylactic antibiotics, IV steroids, and glucose may all be required in the supportive care and recovery of a mother with AFLP.3,8,11
TPE has also been useful in instances of severe complications.1,3,6 In one retrospective study, Martin et al1 recommended administration of TPE in patients with AFLP under the following circumstances:
(1) Deteriorating central nervous system abnormalities, such as sensorium changes or coma;
(2) Persistent coagulopathy requiring continued and aggressive blood product support with plasma, red cells, and/or cryoprecipitate;
(3) Advanced renal dysfunction that compromised fluid management;
(4) Progressive cardiopulmonary compromise; and/or
(5) Ongoing fluid management concerns, including significant ascites, edema, anuria/oliguria, and/or fluid overload.1
In rare cases, liver transplantation is needed in patients with AFLP. Westbrook et al18 reviewed 54 cases of liver disease in pregnancy in one UK hospital between 1997 and 2008. Of these, six patients with encephalopathy or elevated lactate were listed for liver transplant, including just one with a diagnosis of AFLP. This woman never actually underwent transplant but recovered in response to medical management alone.18 According to data reported in June 2011 by the Organ Procurement and Transplantation Network,30 liver transplantation was needed in only three US patients with AFLP between 2000 and 2011. Further retrospective studies on outcomes from transplant versus medical management should be considered to guide future decision making involving this invasive therapy.
The Case Patient
This 39-year-old patient presented during a routine prenatal visit with proteinuria and hypertension, possibly indicative of preeclampsia. Because of the serious nature of this potential diagnosis in pregnancy, she was admitted for monitoring and further testing. Although the diagnosis of AFLP was not confirmed until later, the patient’s preliminary lab studies showed elevated liver enzymes and low platelet counts, signifying the need for prompt intervention and delivery of the infant. At this point, the patient met criteria for HELLP syndrome, but AFLP was suspected after the initial finding of profound hypoglycemia led to further testing.
As an older mother experiencing pregnancy for the first time, this patient fit the profile for AFLP. She initially responded well after delivery of her infant but continued to experience complications. On the days that the patient was treated with TPE, her total bilirubin and liver enzymes were at their lowest. Perhaps this treatment should be considered in more cases of AFLP.
The patient was transferred to a hospital with liver transplantation capabilities, but she ultimately recovered without undergoing transplant.
Conclusion
For the primary obstetric care provider, being aware of the possible complications associated with pregnancy is important. Though uncommon, AFLP is a serious complication that should be ruled out in women who present with vague symptoms such as nausea, vomiting, and abdominal pain in the third trimester of pregnancy. The reduction in AFLP-associated morbidity and mortality during the past 20 years is a direct result of increased early recognition and therapeutic delivery.
Referral to a maternal fetal medicine specialist, gastroenterologist, hematologist, and/or nephrologist may be necessary and appropriate in the management of a woman with AFLP. Further study is indicated for use of TPE in more severe cases of AFLP, particularly in women affected by persistent thrombocytopenia and anemia.
The author would like to thank C. Leanne Browning, MD, obstetrics/gynecology, for her invaluable guidance and advice on this project.
References
1. Martin JN Jr, Briery CM, Rose CH, et al. Postpartum plasma exchange as adjunctive therapy for severe acute fatty liver of pregnancy. J Clin Apher. 2009;23(4):138-143.
2. Knight M, Nelson-Piercy C, Kurinczuk JJ; UK Obstetric Surveillance System. A prospective national study of acute fatty liver of pregnancy in the UK. Gut. 2008;57(7):951-956.
3. Barsoom MJ, Tierney BJ. Acute fatty liver of pregnancy (2011). http://emedicine.medscape.com/article/1562425-overview. Accessed January 21, 2013.
4. Ko HH, Yoshida E. Acute fatty liver of pregnancy. Can J Gastroenterol. 2006;20(1):25-30.
5. Rathi U, Bapat M, Rathi P, Abraham P. Effect of liver disease on maternal and fetal outcome: a prospective study. Indian J Gastroenterol. 2007;26(2):59-63.
6. Myers L. Postpartum plasma exchange in a woman with suspected thrombotic thrombocytopenic purpura (TTP) vs hemolysis, elevated liver enzymes, and low platelet syndrome (HELLP): a case study. Nephrol Nurs J. 2010;37(4):399-402.
7. Vigil-de Gracia P. Acute fatty liver and HELLP syndrome: two distinct pregnancy disorders. Int J Gynaecol Obstet. 2001;73(3):215-220.
8. Aso K, Hojo S, Yumoto Y, et al. Three cases of acute fatty liver of pregnancy: postpartum clinical course depends on interval between onset of symptoms and termination of pregnancy. J Matern Fetal Neonatal Med. 2010;23(9):1047-1049.
9. Wei Q, Zhang L, Liu X. Clinical diagnosis and treatment of acute fatty liver of pregnancy: a literature review and 11 new cases. J Obstet Gynaecol Res. 2010;36(4):751-756.
10. Barber MA, Eguiluz I, Martin A, et al. Acute fatty liver of pregnancy: analysis of five consecutive cases from a tertiary centre. J Obstet Gynaecol. 2010;30(3):241-243.
11. Ajayi AO, Alao MO. Case report: acute fatty liver of pregnancy in a 30-year-old Nigerian primigravida. Niger J Clin Pract. 2008;11(4):389-391.
12. Vigíl-de Gracia P, Montufar-Rueda C. Acute fatty liver of pregnancy: diagnosis, treatment, and outcome based on 35 consecutive cases. J Matern Fetal Neonatal Med. 2011;24(9):1143-1146.
13. Dey M, Reema K. Acute fatty liver of pregnancy. N Am J Med Sci. 2012;4(11):611-612.
14. Castro MA, Goodwin TM, Shaw KJ, et al. Disseminated intravascular coagulation and antithrombin III depression in acute fatty liver of pregnancy. Am J Obstet Gynecol. 1996;174(1 pt 1):211-216.
15. Altuntas F, Aydogdu I, Kabukcu S, et al. Therapeutic plasma exchange for the treatment of thrombotic thrombocytopenic purpura: a retrospective multicenter study. Transfus Apher Sci. 2007;36(1):57-67.
16. Hay JE. Liver disease in pregnancy. Hepatology. 2008;47(3):1067-1076.
17. Wand S, Waeschle RM, Von Ahsen N, et al. Acute fatty liver failure due to acute fatty liver of pregnancy. Minerva Anesthesiol. 2012;78(4):503-506.
18. Westbrook RH, Yeoman AD, Joshi D, et al. Outcomes of severe pregnancy-related liver disease: refining the role of transplantation. Am J Transplant. 2010;10(11):2520-2526.
19. Fesenmeier MF, Coppage KH, Lambers DS, et al. Acute fatty liver of pregnancy in 3 tertiary care centers. Am J Obstet Gynecol. 2005;192(5):1416-1419.
20. Bacq Y. Liver diseases unique to pregnancy: a 2010 update. Clin Res Hepatol Gastroenterol. 2011;35(3):182-193.
21. Huether SE. Alterations of digestive function. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. St. Louis, MO: Mosby; 2009:1452-1515.
22. Huether SE. Structure and function of the digestive system. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. St. Louis, MO: Mosby; 2009:1420-1451.
23. Schutt VA, Minuk GY. Liver diseases unique to pregnancy. Best Pract Res Clin Gastroenterol. 2007;21(5):771-792.
24. Pan C, Perumalswami PV. Pregnancy-related liver diseases. Clin Liver Dis. 2011;15(1):199-208.
25. Ibdah JA. Acute fatty liver of pregnancy: an update on pathogenesis and clinical implications. World J Gastroenterol. 2006;12(46):7397-7404.
26. Browning MF, Levy HL, Wilkins-Haug LE, et al. Fetal fatty acid oxidation defects and maternal liver disease in pregnancy. Obstet Gynecol. 2006;107(1):115-120.
27. Dekker RR, Schutte JM, Stekelenburg J, et al. Maternal mortality and severe maternal morbidity from acute fatty liver of pregnancy in the Netherlands. Eur J Obstet Gynecol Reprod Biol. 2011;157(1):27-31.
28. Lee NM, Brady CW. Liver disease in pregnancy. World J Gastroenterol. 2009;15(8):897-906.
29. Vora KS, Shah VR, Parikh GP. Acute fatty liver of pregnancy: a case report of an uncommon disease. Indian J Crit Care Med. 2009;13(1):34-36.
30. Organ Procurement and Transplantation Network, Scientific Registry of Transplant Recipients. OPTN/SRTR 2011 Annual Data Report: Liver. http://srtr.transplant.hrsa.gov/annual_reports/2011/pdf/03_%20liver_12.pdf. Accessed January 18, 2013.
A 39-year-old black woman, gravida 1, para 0, with an intrauterine pregnancy of 34 weeks and three days (according to last menstrual period and nine-week ultrasound) presented to her Ob-Gyn office for a routine prenatal visit. She was found to have an elevated blood pressure with new onset of 2+ proteinuria. The patient was sent to the labor and delivery unit at the adjoining hospital for serial blood pressure readings, laboratory work, and fetal monitoring.
The patient’s previous medical history was limited to sinusitis. She was taking no prescription medications, and her only listed allergy was to pineapple. Initial lab studies revealed elevations in liver enzymes, lactate dehydrogenase (LDH), uric acid, and serum creatinine, as well as thrombocytopenia (see Table 11-5). She also had a critically low blood glucose level, which conflicted with a normal follow-up reading.
At this point, the patient was thought to have HELLP syndrome6 (ie, hemolysis, elevated liver enzymes, low platelet count), or possibly acute fatty liver of pregnancy (AFLP).2,4,7-11 Additional labs were drawn immediately to confirm or rule out AFLP. These included repeat serum glucose (following a second reading with normal results), a serum ammonia level, prothrombin time (PT), and partial thromboplastin time (PTT). The most reliable values to distinguish AFLP from HELLP are profound hypoglycemia (found in 94% of women with AFLP12) and an elevated serum ammonia level.4
Given the serious nature of either diagnosis, immediate delivery of the infant was deemed necessary. Because the patient’s cervix was not found favorable for induction, she underwent low-transverse cesarean delivery without complications. She was noted to have essentially normal anatomy with the exception of a small subserosal fibroid posteriorly. Meconium-stained amniotic fluid was present. A male infant was delivered, weighing 5 lb with 1-minute and 5-minute Apgar scores of 8 and 9, respectively.
Postoperatively, the patient remained in the recovery area, where she received intensive monitoring. She experienced fluctuations in blood glucose, ranging from 33 to 144 mg/dL; she was started on 5% dextrose in lactated Ringer’s solution and treated with IV dextrose 50 g. While the patient was in surgical recovery, results from the second set of labs, drawn before surgery, were returned; findings included an elevated ammonia level and an abnormal coagulation panel, including PT of 25.3 sec, PTT of 48.4 sec, and a fibrinogen level of 116 mg/dL, confirming the suspected diagnosis of AFLP.
Magnesium sulfate, which had been started immediately postop, was discontinued on confirmation of the diagnosis of AFLP. The patient was initially somnolent as a result of general anesthesia but gradually returned to a fully normal sensorium by early morning on postop day 1. Postoperatively, the patient’s hemoglobin was found to be low (8.6 g/dL; reference range, 13.5 to 18.5 g/dL), so she was transfused with two units of packed red blood cells (PRBCs) and given fresh frozen plasma (FFP) to correct this coagulopathy. The patient’s platelets were also low at 82,000/mm3 (reference range, 140,000 to 340,000/mm3).
On postop day 1, the patient’s serum creatinine rose to 4.2 mg/dL and her total bilirubin increased to 14.4 mg/dL (reference ranges, 0.6 to 1.2 mg/dL and < 1.0 mg/dL, respectively). Given the multiple systems affected by AFLP and the need for intensive supportive care, the patient was transferred to the ICU.
On her arrival at the ICU, the patient’s vital signs were initially stable, and she was alert and oriented. However, within the next few hours, she became hypotensive and encephalopathic. She required aggressive fluid resuscitation and multiple transfusions of PRBCs and FFP due to persistent anemia and coagulopathy. Her vital signs were stabilized, but she continued to need blood transfusions.
Postop day 2, the patient became less responsive and was soon unable to follow commands or speak clearly. Her breathing remained stable with just 3 L of oxygen by nasal cannula, but in order to prevent aspiration and in consideration of a postoperative ileus, it was necessary to place a nasogastric tube with low intermittent suction. This produced a bloody return, but no intervention other than close monitoring and transfusion was performed at that time.
Abdominal ultrasound showed ascites and mild left-sided hydronephrosis with no gallstones. The common bile duct measured 3 mm in diameter.
Although liver biopsy is considered the gold standard for a confirmed diagnosis of AFLP,13,14 this procedure was contraindicated by the patient’s coagulopathy. Concern was also expressed by one consultant that the patient might have thrombotic thrombocytopenic purpura (TTP) in addition to AFLP. TTP can manifest with similar findings, such as anemia, thrombocytopenia, neurologic symptoms, and renal abnormalities, but usually fever is involved, and the patient was afebrile. A catheter was placed for hemodialysis and therapeutic plasma exchange (TPE). Given that TTP-associated mortality is significantly decreased by use of TPE,15 this intervention was deemed prudent. The patient underwent TPE on three consecutive days, postop days 2 through 4.
The patient’s mental status began to improve, and by postop day 6, she was able to follow commands and engage in brief conversations. By postop day 9, she had returned almost completely to her baseline mental status.
The patient’s liver function test results and total bilirubin, ammonia, and creatinine levels all improved over the first few postoperative days but began to rise again by day 6. In response to worsening renal and hepatic functioning, the decision was made on postop day 9 to transfer the patient to a hospital with liver transplantation capabilities, should this procedure become necessary.
Discussion
AFLP is a rare condition specific to pregnancy, affecting 1/7,000 to 1/20,000 pregnancies. Due to the low incidence of this disease, randomized controlled trials to study it are not possible. Instead, clinicians must learn either from individual case studies or from retrospective syntheses of cases reported over time.1,2,7 Fortunately, the wealth of information gleaned over the past 30 years has significantly reduced AFLP-associated maternal and fetal mortality and morbidity rates. In the 1980s, maternal and fetal mortality rates as high as 85% were reported.3 Worldwide, maternal mortality associated with AFLP has decreased significantly to 7% to 18%, whereas the fetal mortality rate has fallen to between 9% and 23%.1,16,17
Common trends among women who have developed AFLP include nulliparity, multiparity, and advanced maternal age. One retrospective study of 57 women who had developed AFLP revealed that 35 cases (61%) involved first-time pregnancies. It also showed that 10 (18%) of the women had twins, and 14 (25%) were older than 35.2 In another study of 35 cases of AFLP, 40% of the women were nulliparous, and 11.4% were multiparous, including one triplet gestation.12 In a third, smaller study, 80% of women affected by AFLP were multiparous.10 Currently, there is no known evidence linking any maternal behavior to development of AFLP.
Presentation
Women who present with AFLP often experience vague, nonspecific symptoms, leading to misdiagnosis or delayed diagnosis. Objective measurements, including physical exam findings, laboratory studies, and other diagnostic tests, will help with a diagnosis. The most frequent initial symptoms are nausea and vomiting (in 70% of patients) and abdominal pain (50% to 80%), epigastric or right upper-quadrant.3 Other common symptoms include fatigue, malaise, anorexia, weight gain, polyuria, and polydipsia.2,3,9,18,19
Because the presenting symptoms in AFLP can be vague, clinicians should complete a thorough physical exam to differentiate accurately among conditions associated with pregnancy. Physical signs present in women with AFLP can include jaundice, ascites, edema, confusion, abdominal tenderness, and fever. More severe cases can present with multisystem involvement, including acute renal failure, gastrointestinal bleeding, pancreatitis, coagulopathy, and hepatic encephalopathy.3,4,9,18
Diagnostic Tests
Relevant laboratory tests include a complete blood count (CBC), liver studies, chemistry, coagulation studies, and urinalysis (see Table 1). Viral causes should be ruled out by way of a hepatitis panel.3 In AFLP, the CBC may show elevated white blood cells, decreased hemoglobin and hematocrit, and decreased platelets. Liver studies show elevated hepatic aminotransferase, bilirubin, LDH, and ammonia levels. Chemistry results show elevated blood urea nitrogen and creatinine, and decreased blood glucose. Coagulation factors are affected, and prolonged PTT, decreased fibrinogen, and proteinurea may also be found.9
Though invasive and not often necessary4,13 (and not possible for the case patient), the definitive diagnostic test for AFLP is liver biopsy.13,14 Biopsy reveals a microvesicular fatty infiltration of the hepatocytes as minute fat droplets surrounding a centrally located nucleus. These fatty infiltrates stain with oil red O, specific for fat. Inflammation is present in 50% of cases. There may also be a picture similar to cholestasis with bile thrombi or deposits within the hepatocytes.20
Due to the risk for hemorrhage and the critical status of women with AFLP, biopsy is often not possible. Ultrasonography may show increased echogenicity; CT may show decreased or diffuse attenuation in the liver. These imaging studies, though possibly helpful in severe cases, often yield false-negative results.3,20
In the absence of another explanation for the patient’s symptoms, the Swansea criteria are used for diagnosis of AFLP.1 Six or more of the following criteria must be present to confirm this diagnosis: vomiting, abdominal pain, polydipsia or polyuria, encephalopathy, leukocytosis, elevated bilirubin, elevated liver enzymes, elevated ammonia, hypoglycemia, renal dysfunction, coagulopathy, elevated uric acid, ascites on ultrasound, and microvesicular steatosis on liver biopsy.1,2,5
Pathophysiology
Normal functions of the liver include metabolism, protein synthesis, and manufacturing of blood coagulation proteins. These functions are disturbed in the presence of AFLP. Thus, women with this disease experience signs and symptoms related directly to the dysfunction of these processes.20-22
Disturbances in the hepatocytes due to excess fatty acids impair the liver’s ability to convert unconjugated bilirubin into conjugated bilirubin, causing plasma levels of unconjugated bilirubin to rise. This increase in bilirubin explains the jaundiced appearance of women with AFLP. AFLP is often thought to occur in conjunction with preeclampsia in many, but not all, patients. Thrombocytopenia in these patients is felt to be secondary to peripheral vascular consumption. Conjugated bilirubin levels may also be increased due to decreased flow of conjugated bilirubin into the common bile duct.21
Another liver function that is disrupted is that of glycogen storage and conversion to glucose, and the liver’s ability to convert nutrients into glycogen is also impaired. Decreased storage of glycogen, along with the liver’s inability to break down previously stored glycogen, causes a decrease in serum glucose levels. Women with AFLP often require treatment with IV dextrose in response to marked hypoglycemia.16,21,23
The liver dysfunction associated with AFLP reduces adequate production of clotting factors and coagulation proteins. Thrombocytopenia, elevated clotting times, and bleeding are all problems seen in AFLP. Mild to moderate elevations in serum aminotransferases and elevated LDH also occur in patients with AFLP.23,24
Genetic Factor
There is little known about the etiology of AFLP, although recent data point to a genetic component that was found in as many as 62% of mothers in one study and in 25% of infants in another study.20-22 Fatty acid oxidation (FAO) is one of the processes of hepatic mitochondria, a process that relies on several enzymes. When FAO is interrupted, fatty acids are deposited in the liver cells, as seen in histologic studies of AFLP.25,26 The common thread in women with this disease is a mutation in one of the enzymes needed for FAO. This enzyme is the long-chain 3-hydroxyacyl-CoA dehydrogenase. Deficiencies in this enzyme are common in mothers with AFLP and their infants.3,16,20,23,27
Differential Diagnosis
Several complications of pregnancy that involve the liver may, on presentation, mimic AFLP.16,20,23,24,28 The most common are hyperemesis gravidarum and intrahepatic cholestasis of pregnancy23 (see Table 216,20,23,24,28); others are preeclampsia/eclampsia and HELLP syndrome. It is important to distinguish between the signs and symptoms associated with each of these disorders in order to provide the most effective treatment. Hepatitis serologies are important in the differential diagnosis when liver enzyme levels are exceptionally high.4,16,22,28
Treatment
The most effective treatment for AFLP is delivery of the infant; often, this alone causes the signs and symptoms of AFLP to resolve.8,21,27,29 In two of three cases in a small study by Aso et al,8 early delivery of the fetus led to complete resolution of symptoms and return to normal liver function. One of these patients was sent home four days after delivery; the other, 14 days later. Other patients may require more invasive treatment and support.8
Management in the ICU is often required to provide appropriate supportive care to the mother after delivery. Acute respiratory distress syndrome, pancreatitis, hemorrhage, encephalopathy, renal failure, and continual liver failure are among the severe complications associated with AFLP.4,8,10 Many women require intubation, dialysis, fluid resuscitation, blood product transfusion, and vasopressor therapy.3,8,11 Prophylactic antibiotics, IV steroids, and glucose may all be required in the supportive care and recovery of a mother with AFLP.3,8,11
TPE has also been useful in instances of severe complications.1,3,6 In one retrospective study, Martin et al1 recommended administration of TPE in patients with AFLP under the following circumstances:
(1) Deteriorating central nervous system abnormalities, such as sensorium changes or coma;
(2) Persistent coagulopathy requiring continued and aggressive blood product support with plasma, red cells, and/or cryoprecipitate;
(3) Advanced renal dysfunction that compromised fluid management;
(4) Progressive cardiopulmonary compromise; and/or
(5) Ongoing fluid management concerns, including significant ascites, edema, anuria/oliguria, and/or fluid overload.1
In rare cases, liver transplantation is needed in patients with AFLP. Westbrook et al18 reviewed 54 cases of liver disease in pregnancy in one UK hospital between 1997 and 2008. Of these, six patients with encephalopathy or elevated lactate were listed for liver transplant, including just one with a diagnosis of AFLP. This woman never actually underwent transplant but recovered in response to medical management alone.18 According to data reported in June 2011 by the Organ Procurement and Transplantation Network,30 liver transplantation was needed in only three US patients with AFLP between 2000 and 2011. Further retrospective studies on outcomes from transplant versus medical management should be considered to guide future decision making involving this invasive therapy.
The Case Patient
This 39-year-old patient presented during a routine prenatal visit with proteinuria and hypertension, possibly indicative of preeclampsia. Because of the serious nature of this potential diagnosis in pregnancy, she was admitted for monitoring and further testing. Although the diagnosis of AFLP was not confirmed until later, the patient’s preliminary lab studies showed elevated liver enzymes and low platelet counts, signifying the need for prompt intervention and delivery of the infant. At this point, the patient met criteria for HELLP syndrome, but AFLP was suspected after the initial finding of profound hypoglycemia led to further testing.
As an older mother experiencing pregnancy for the first time, this patient fit the profile for AFLP. She initially responded well after delivery of her infant but continued to experience complications. On the days that the patient was treated with TPE, her total bilirubin and liver enzymes were at their lowest. Perhaps this treatment should be considered in more cases of AFLP.
The patient was transferred to a hospital with liver transplantation capabilities, but she ultimately recovered without undergoing transplant.
Conclusion
For the primary obstetric care provider, being aware of the possible complications associated with pregnancy is important. Though uncommon, AFLP is a serious complication that should be ruled out in women who present with vague symptoms such as nausea, vomiting, and abdominal pain in the third trimester of pregnancy. The reduction in AFLP-associated morbidity and mortality during the past 20 years is a direct result of increased early recognition and therapeutic delivery.
Referral to a maternal fetal medicine specialist, gastroenterologist, hematologist, and/or nephrologist may be necessary and appropriate in the management of a woman with AFLP. Further study is indicated for use of TPE in more severe cases of AFLP, particularly in women affected by persistent thrombocytopenia and anemia.
The author would like to thank C. Leanne Browning, MD, obstetrics/gynecology, for her invaluable guidance and advice on this project.
References
1. Martin JN Jr, Briery CM, Rose CH, et al. Postpartum plasma exchange as adjunctive therapy for severe acute fatty liver of pregnancy. J Clin Apher. 2009;23(4):138-143.
2. Knight M, Nelson-Piercy C, Kurinczuk JJ; UK Obstetric Surveillance System. A prospective national study of acute fatty liver of pregnancy in the UK. Gut. 2008;57(7):951-956.
3. Barsoom MJ, Tierney BJ. Acute fatty liver of pregnancy (2011). http://emedicine.medscape.com/article/1562425-overview. Accessed January 21, 2013.
4. Ko HH, Yoshida E. Acute fatty liver of pregnancy. Can J Gastroenterol. 2006;20(1):25-30.
5. Rathi U, Bapat M, Rathi P, Abraham P. Effect of liver disease on maternal and fetal outcome: a prospective study. Indian J Gastroenterol. 2007;26(2):59-63.
6. Myers L. Postpartum plasma exchange in a woman with suspected thrombotic thrombocytopenic purpura (TTP) vs hemolysis, elevated liver enzymes, and low platelet syndrome (HELLP): a case study. Nephrol Nurs J. 2010;37(4):399-402.
7. Vigil-de Gracia P. Acute fatty liver and HELLP syndrome: two distinct pregnancy disorders. Int J Gynaecol Obstet. 2001;73(3):215-220.
8. Aso K, Hojo S, Yumoto Y, et al. Three cases of acute fatty liver of pregnancy: postpartum clinical course depends on interval between onset of symptoms and termination of pregnancy. J Matern Fetal Neonatal Med. 2010;23(9):1047-1049.
9. Wei Q, Zhang L, Liu X. Clinical diagnosis and treatment of acute fatty liver of pregnancy: a literature review and 11 new cases. J Obstet Gynaecol Res. 2010;36(4):751-756.
10. Barber MA, Eguiluz I, Martin A, et al. Acute fatty liver of pregnancy: analysis of five consecutive cases from a tertiary centre. J Obstet Gynaecol. 2010;30(3):241-243.
11. Ajayi AO, Alao MO. Case report: acute fatty liver of pregnancy in a 30-year-old Nigerian primigravida. Niger J Clin Pract. 2008;11(4):389-391.
12. Vigíl-de Gracia P, Montufar-Rueda C. Acute fatty liver of pregnancy: diagnosis, treatment, and outcome based on 35 consecutive cases. J Matern Fetal Neonatal Med. 2011;24(9):1143-1146.
13. Dey M, Reema K. Acute fatty liver of pregnancy. N Am J Med Sci. 2012;4(11):611-612.
14. Castro MA, Goodwin TM, Shaw KJ, et al. Disseminated intravascular coagulation and antithrombin III depression in acute fatty liver of pregnancy. Am J Obstet Gynecol. 1996;174(1 pt 1):211-216.
15. Altuntas F, Aydogdu I, Kabukcu S, et al. Therapeutic plasma exchange for the treatment of thrombotic thrombocytopenic purpura: a retrospective multicenter study. Transfus Apher Sci. 2007;36(1):57-67.
16. Hay JE. Liver disease in pregnancy. Hepatology. 2008;47(3):1067-1076.
17. Wand S, Waeschle RM, Von Ahsen N, et al. Acute fatty liver failure due to acute fatty liver of pregnancy. Minerva Anesthesiol. 2012;78(4):503-506.
18. Westbrook RH, Yeoman AD, Joshi D, et al. Outcomes of severe pregnancy-related liver disease: refining the role of transplantation. Am J Transplant. 2010;10(11):2520-2526.
19. Fesenmeier MF, Coppage KH, Lambers DS, et al. Acute fatty liver of pregnancy in 3 tertiary care centers. Am J Obstet Gynecol. 2005;192(5):1416-1419.
20. Bacq Y. Liver diseases unique to pregnancy: a 2010 update. Clin Res Hepatol Gastroenterol. 2011;35(3):182-193.
21. Huether SE. Alterations of digestive function. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. St. Louis, MO: Mosby; 2009:1452-1515.
22. Huether SE. Structure and function of the digestive system. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. St. Louis, MO: Mosby; 2009:1420-1451.
23. Schutt VA, Minuk GY. Liver diseases unique to pregnancy. Best Pract Res Clin Gastroenterol. 2007;21(5):771-792.
24. Pan C, Perumalswami PV. Pregnancy-related liver diseases. Clin Liver Dis. 2011;15(1):199-208.
25. Ibdah JA. Acute fatty liver of pregnancy: an update on pathogenesis and clinical implications. World J Gastroenterol. 2006;12(46):7397-7404.
26. Browning MF, Levy HL, Wilkins-Haug LE, et al. Fetal fatty acid oxidation defects and maternal liver disease in pregnancy. Obstet Gynecol. 2006;107(1):115-120.
27. Dekker RR, Schutte JM, Stekelenburg J, et al. Maternal mortality and severe maternal morbidity from acute fatty liver of pregnancy in the Netherlands. Eur J Obstet Gynecol Reprod Biol. 2011;157(1):27-31.
28. Lee NM, Brady CW. Liver disease in pregnancy. World J Gastroenterol. 2009;15(8):897-906.
29. Vora KS, Shah VR, Parikh GP. Acute fatty liver of pregnancy: a case report of an uncommon disease. Indian J Crit Care Med. 2009;13(1):34-36.
30. Organ Procurement and Transplantation Network, Scientific Registry of Transplant Recipients. OPTN/SRTR 2011 Annual Data Report: Liver. http://srtr.transplant.hrsa.gov/annual_reports/2011/pdf/03_%20liver_12.pdf. Accessed January 18, 2013.
The natural history of obstetric brachial plexus injury
Delivery of the shoulders often gets overlooked in discussions
of episiotomy
(Comment & Controversy, November 2012)
Difficult fetal extraction at cesarean delivery:
What should you do?
Robert L. Barbieri, MD (Editorial, January 2012)
What is the significance of the head-to-body delivery interval
in shoulder dystocia?
William A. Grobman, MD, MBA (Examining the Evidence, December 2011)
Does the use of multiple maneuvers in the management of shoulder dystocia increase the risk of neonatal injury?
Robert B. Gherman, MD (Examining the Evidence, August 2011)
CASE: Shoulder dystocia resulting in persistent injury to C5 and C6
A 30-year-old, G2P1 woman presented in labor at 39 weeks and reported a strong desire to have a natural childbirth. She was taking insulin for gestational diabetes mellitus diagnosed in the second trimester. Her body mass index was 43 kg/m2, and her height was 4 ft 11 in. The estimated fetal weight was 9 lb. She had a prior vaginal delivery. During her antepartum care the patient was extensively counseled about the risk of shoulder dystocia and obstetric brachial plexus (OBP) injury.
The patient progressed normally through labor without anesthesia. At birth, the baby delivered occiput posterior and restituted to right occiput transverse. There was a turtle sign, and the obstetrician diagnosed a shoulder dystocia, called for help, and told the mother to stop pushing. An attempt to deliver the fetal head with gentle downward guidance was unsuccessful. The McRoberts maneuver and suprapubic pressure combined with gentle downward guidance on the fetal head did not result in delivery. A mediolateral episiotomy was made and the Rubin and Wood maneuvers were attempted without success. The obstetrician then successfully delivered the posterior arm and the body of the baby was easily delivered.
The shoulder dystocia lasted 2 minutes before successful delivery. The Apgar scores were 3 and 6 at one and five minutes, respectively. The umbilical cord artery pH was 7.18. The birthweight was 9 lb 2 oz. A diagnosis of OBP injury involving C5 and C6 was made. At discharge the OBP injury persisted.
What is OBP injury?
Shoulder dystocia, which affected this mother and fetus, is a problem involving the impaction of the fetal shoulder behind the maternal symphysis pubis. Shoulder dystocia is a “bony problem.” Although shoulder dystocia cannot be predicted reliably, risk factors include:
- fetal macrosomia
- maternal diabetes
- maternal weight gain
- prepregnancy obesity
- multiparity
- operative vaginal delivery.1-3
The injury can be serious and permanent. The feared consequence of shoulder dystocia is permanent obstetric brachial plexus injury and/or fetal neurologic damage caused by reduced cord blood flow and fetal asphyxia. Occasionally, shoulder dystocia results in fetal death.
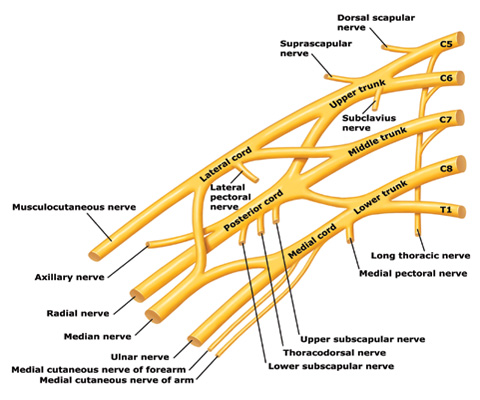
Trunks and cords of the brachial plexus
C5 and C6 roots merge to form the upper trunk, C7 root forms the middle trunk, and C8 and T1 roots merge to form the lower trunk.
How can an OBP injury occur?
Most cases are unilateral and arise when one or more of the brachial plexus nerves ( Figure ) are compromised. The forces of labor, fetal position, maternal pushing, and force applied to the fetal head and neck by a clinician all may contribute to an OBP injury.4 It is important to note that up to one-third of all OBP injuries occur in births not associated with a recognized shoulder dystocia.5
Erb’s palsy. Injuries to the C5 and C6 nerves account for about 50% of cases. The muscle groups impacted by this injury include the deltoid and infraspinatus muscles (mainly C5) and the biceps muscle (mainly C6). This pattern results in an adducted and internally rotated upper arm, an extended forearm, with preservation of hand and wrist movement.
Erb’s palsy plus. Injury to C5, C6, and C7 nerves accounts for about 35% of cases and manifests as adduction and internal rotation of the arm, extension and pronation of the forearm, plus flexion of the wrist and fingers—the so-called waiter’s tip posture.
Klumpke’s palsy. Isolated injury to the C8 nerve and T1 root occurs infrequently and manifests as isolated hand paralysis and Horner’s syndrome.
Approximately one in five OBP injuries persist for years following birth. The incidence of OBP injury ranges from 1.0 to 3.0 cases per 1,000 births.6-8 In a review of studies with at least 3 years of follow-up and less than 10% loss to follow-up, the authors reported that the three best studies observed a risk of persistent OBP of 10%, 19%, and 27%.9
Long-term management of OBP injury
The optimal approach to management of OBP injury is unknown because few high-quality studies are available to guide therapy. Typically, 3 to 9 months of observation and physical therapy are recommended following birth, with the hope that the majority of newborns will recover some or almost all function. Physical therapy is focused on reducing the occurrence of joint contractures and maintaining range of motion in the shoulder, elbow, wrist, and fingers.
Surgical exploration of the plexus is often advised when biceps muscle function is absent at 3 months of age. Some surgeons recommend waiting until 9 months before pursuing surgical exploration.10 No randomized trials have been performed demonstrating the value of surgical intervention, but case series report that surgical intervention with nerve grafting or nerve transfer is superior to management without neurosurgery.
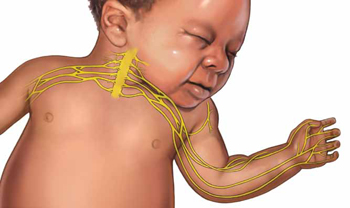
The consequences of permanent OBP injury
A lifetime of corrective therapy for the child. OBP injury may result in a permanent brachial plexus injury—permanent arm weakness requiring decades of physical therapy and multiple surgeries to try to reduce functional disabilities. Problems that frequently occur in children with OBP injury include:
- shoulder contractures
- limb length differences
- winged scapula
- behavioral and developmental problems.
A patient’s care may be coordinated at a brachial plexus injury specialty center, where coordinated access to pediatric neurologists, neurosurgeons, orthopedic surgeons, and neurophysiologists is available and physical and occupational therapy are provided.
Years of emotional response for the parents. Following a birth complicated by shoulder dystocia, the parents may experience grief and anger. During the decades following the birth, the parents of the child will wonder if the injury could have been avoided. They may be asked by friends and relatives, “Would a cesarean delivery have prevented the injury?” Physical therapists, pediatric neurologists, orthopedic and neurosurgeons may reinforce the idea that the injury was caused when the obstetrician applied excessive lateral force to the head and neck, resulting in an injury to the brachial plexus.
Decades-long exposure to these ideas, unresolved anger, financial stress, and lingering doubts may result in the parents and child pursuing civil litigation against the delivering clinician.
Can we reduce the incidence of OBP injury and our risk of litigation?
There are no interventions proven to reduce the incidence of OBP injury. However, many experts advise that a multifaceted approach to this obstetric emergency may reduce the incidence of severe OBP injury at the same time that we reduce our risk of litigation.
When counseling patients about the risk for OBP injury, use teach-back. Patients tend to misunderstand or forget much of what their clinicians teach them.11 Teach-back is an iterative communication process in which the clinician teaches the patient a concept or technique, then asks the patient to repeat the concept in their own words or demonstrate the technique. The clinician then amplifies the concept or technique and corrects misunderstandings. The patient is then asked to repeat the concept or demonstrate the technique. When using teach-back, the clinician never asks, “Do you understand?” The clinician encourages the patient to teach the concept to the clinician using the patient’s own words.12
In the case above, the patient has multiple risk factors for shoulder dystocia, including obesity, short stature, insulin-treated gestational diabetes, and a large fetus. Given the high risk for shoulder dystocia, teach-back might help the patient better understand the situation. After explaining the concepts to the patient, the teach-back questions to the patient might include:
- “Can you tell me what is a shoulder dystocia?”
- “Can you list the health conditions that you have that increase your baby’s risk of shoulder dystocia?”
- “When a shoulder dystocia occurs, what actions will we take at birth to try to fix the shoulder dystocia?”
- “When a baby has experienced a shoulder dystocia, what can happen to the baby’s arm?”
Regularly perform multidisciplinary shoulder dystocia drills. The Joint Commission recommends that clinical drills be performed to help staff prepare for high-risk labor and delivery events, including shoulder dystocia, emergency cesarean delivery, and maternal hemorrhage.13
When shoulder dystocia occurs, extensively chart the event and interventions used. The American College of Obstetricians and Gynecologists has developed a patient safety checklist focused on key clinical elements in the antepartum, intrapartum, and postpartum periods and overall timing of the delivery to document when a shoulder dystocia occurs.14
Stop using the term “traction” in the medical record and obstetric literature. Words are meaningful and open to multiple interpretations. Often, words have unintended consequences. Plaintiff attorneys often highlight the obstetrician’s use of “traction” or “excessive traction” as the cause of an OBP injury. Orthopedic surgeons and pediatricians often state in their records that the OBP injury was a “traction injury,” further supporting the plaintiff attorney’s contention that excessive traction applied by the obstetrician caused the traction injury. Obstetricians do not use traction to deliver a baby. Motorized tractors generate traction, not obstetricians. We use gentle downward guidance to deliver the fetal shoulders and body.
In a high-risk situation, proceed quickly to delivery of the posterior arm. When you recognize a shoulder dystocia in a high-risk situation (maternal diabetes and large fetus), it may be wise to move quickly to delivery of the posterior arm.15,16 In high-risk situations, delivery of the posterior arm is the maneuver with the greatest likelihood of resolving a severe shoulder dystocia, with the least force applied to the brachial plexus that is trapped under the mother’s symphysis pubis.
SHOULDER DYSTOCIA?
Most of our monthly Medical Verdicts columns include cases about shoulder dystocia, brachial plexus injury, or Erb’s palsy. CLICK HERE to read those from 2012 and 2013.
Practice, and then practice some more
A difficult-to-resolve shoulder dystocia is one of the most dramatic and frightening obstetric events. We know that we will all experience such cases. If we prepare well and frequently practice shoulder dystocia maneuvers, the dread of being responsible for resolving a shoulder dystocia will diminish. In most cases we will be able to report, “Mother and newborn safely birthed.”
INSTANT POLL: In your experience, what can clinicians do to reduce their exposure to brachial plexus injury litigation? Click here to respond.
1. Acker DB, Sachs BP, Friedman EA. Risk factors for shoulder dystocia. Obstet Gynecol. 1985;66(6):762-768.
2. Benedetti TJ, Gabbe SG. Shoulder dystocia: a complication of fetal macrosomia and prolonged second stage of labor with midpelvic delivery. Obstet Gynecol. 1978;52(5):526-529.
3. Gilbert WM, Nesbitt TS, Danielsen B. Associated factors in 1611 cases of brachial plexus injury. Obstet Gynecol. 1999;93(4):536-540.
4. Gonik B, Walker A, Grimm M. Mathematic modeling of forces associated with shoulder dystocia: a comparison of endogenous and exogenous sources. Am J Obstet Gynecol. 2000;182(3):689-691.
5. Rouse DJ, Owen J, Goldenberg RL, Cliver SP. The effectiveness and costs of elective cesarean delivery for fetal macrosomia diagnosed by ultrasound. JAMA. 1996;276(18):1480-1486.
6. Ecker JL, Greenberg JA, Norwitz ER, Nadel AS, Repke JT. Birth weight as a predictor of brachial plexus injury. Obstet Gynecol 1997;89(5 pt 1):643-647.
7. Bager B. Perinatally acquired brachial plexus palsy—a persisting challenge. Acta Paediatr. 1997;86(11):1216-1219.
8. Dawodu A, Sankaran-Kutty M, Rajan TV. Risk factors and prognosis for brachial plexus injury and clavicular fracture in neonates: a prospective analysis from the United Arab Emirates. Ann Trop Paediatr. 1997;17(3):195-200.
9. Pondaag W, Malessy MJA, van Dijk JG, Thomeer RT. Natural history of obstetric brachial plexus palsy: a systematic review. Dev Med Child Neurol. 2004;46(2):138-144.
10. Grossman JA. Early operative intervention for birth injuries to the brachial plexus. Semin Pediatr Neurol. 2000;7(1):36-43.
11. Institute of Medicine. Nielsen-Bohlman L Panzer AM, Kindig DA, eds. Health Literacy: A Prescription to End Confusion. Washington, DC: National Academies Press; 2004. http://www.nap.edu/openbook.php?isbn=0309091179. Accessed January 15, 2013.
12. Wu H, Nishimi RY, Page-Lopez CM, Kizer KW. Improving patient safety through informed consent for patients with limited health literacy. Washington DC; National Quality Forum; 2005. http://www.qualityforum.org/Publications/2005/09/Improving_Patient_Safety_Through_Informed_Consent_for_Patients_with_Limited_Health_Literacy.aspx. Accessed January 15, 2013.
13. The Joint Commission. Sentinel Event Alert Issue 30: Preventing infant death and injury during delivery. http://www.jointcommission.org/assets/1/18/SEA_30.PDF. Published July 21, 2004. Accessed January 15, 2013.
14. Patient safety checklist No. 6: documenting shoulder dystocia. Obstet Gynecol. 2012;120(2 pt 1):430-431.http://www.acog.org/Resources_And_Publications/Patient_Safety_Checklists_List. Accessed January 15 2013.
15. Poggi SH, Spong CY, Allen RH. Prioritizing posterior arm delivery during severe shoulder dystocia. Obstet Gynecol. 2003;101(5 pt 2):1068-1072.
16. Hoffman MK, Bailit JL, Branch DW, et al. Consortium on Safe Labor. A comparison of obstetric maneuvers for the acute management of shoulder dystocia. Obstet Gynecol. 2011;117(6):1272-1278.
Delivery of the shoulders often gets overlooked in discussions
of episiotomy
(Comment & Controversy, November 2012)
Difficult fetal extraction at cesarean delivery:
What should you do?
Robert L. Barbieri, MD (Editorial, January 2012)
What is the significance of the head-to-body delivery interval
in shoulder dystocia?
William A. Grobman, MD, MBA (Examining the Evidence, December 2011)
Does the use of multiple maneuvers in the management of shoulder dystocia increase the risk of neonatal injury?
Robert B. Gherman, MD (Examining the Evidence, August 2011)
CASE: Shoulder dystocia resulting in persistent injury to C5 and C6
A 30-year-old, G2P1 woman presented in labor at 39 weeks and reported a strong desire to have a natural childbirth. She was taking insulin for gestational diabetes mellitus diagnosed in the second trimester. Her body mass index was 43 kg/m2, and her height was 4 ft 11 in. The estimated fetal weight was 9 lb. She had a prior vaginal delivery. During her antepartum care the patient was extensively counseled about the risk of shoulder dystocia and obstetric brachial plexus (OBP) injury.
The patient progressed normally through labor without anesthesia. At birth, the baby delivered occiput posterior and restituted to right occiput transverse. There was a turtle sign, and the obstetrician diagnosed a shoulder dystocia, called for help, and told the mother to stop pushing. An attempt to deliver the fetal head with gentle downward guidance was unsuccessful. The McRoberts maneuver and suprapubic pressure combined with gentle downward guidance on the fetal head did not result in delivery. A mediolateral episiotomy was made and the Rubin and Wood maneuvers were attempted without success. The obstetrician then successfully delivered the posterior arm and the body of the baby was easily delivered.
The shoulder dystocia lasted 2 minutes before successful delivery. The Apgar scores were 3 and 6 at one and five minutes, respectively. The umbilical cord artery pH was 7.18. The birthweight was 9 lb 2 oz. A diagnosis of OBP injury involving C5 and C6 was made. At discharge the OBP injury persisted.
What is OBP injury?
Shoulder dystocia, which affected this mother and fetus, is a problem involving the impaction of the fetal shoulder behind the maternal symphysis pubis. Shoulder dystocia is a “bony problem.” Although shoulder dystocia cannot be predicted reliably, risk factors include:
- fetal macrosomia
- maternal diabetes
- maternal weight gain
- prepregnancy obesity
- multiparity
- operative vaginal delivery.1-3
The injury can be serious and permanent. The feared consequence of shoulder dystocia is permanent obstetric brachial plexus injury and/or fetal neurologic damage caused by reduced cord blood flow and fetal asphyxia. Occasionally, shoulder dystocia results in fetal death.

Trunks and cords of the brachial plexus
C5 and C6 roots merge to form the upper trunk, C7 root forms the middle trunk, and C8 and T1 roots merge to form the lower trunk.
How can an OBP injury occur?
Most cases are unilateral and arise when one or more of the brachial plexus nerves ( Figure ) are compromised. The forces of labor, fetal position, maternal pushing, and force applied to the fetal head and neck by a clinician all may contribute to an OBP injury.4 It is important to note that up to one-third of all OBP injuries occur in births not associated with a recognized shoulder dystocia.5
Erb’s palsy. Injuries to the C5 and C6 nerves account for about 50% of cases. The muscle groups impacted by this injury include the deltoid and infraspinatus muscles (mainly C5) and the biceps muscle (mainly C6). This pattern results in an adducted and internally rotated upper arm, an extended forearm, with preservation of hand and wrist movement.
Erb’s palsy plus. Injury to C5, C6, and C7 nerves accounts for about 35% of cases and manifests as adduction and internal rotation of the arm, extension and pronation of the forearm, plus flexion of the wrist and fingers—the so-called waiter’s tip posture.
Klumpke’s palsy. Isolated injury to the C8 nerve and T1 root occurs infrequently and manifests as isolated hand paralysis and Horner’s syndrome.
Approximately one in five OBP injuries persist for years following birth. The incidence of OBP injury ranges from 1.0 to 3.0 cases per 1,000 births.6-8 In a review of studies with at least 3 years of follow-up and less than 10% loss to follow-up, the authors reported that the three best studies observed a risk of persistent OBP of 10%, 19%, and 27%.9
Long-term management of OBP injury
The optimal approach to management of OBP injury is unknown because few high-quality studies are available to guide therapy. Typically, 3 to 9 months of observation and physical therapy are recommended following birth, with the hope that the majority of newborns will recover some or almost all function. Physical therapy is focused on reducing the occurrence of joint contractures and maintaining range of motion in the shoulder, elbow, wrist, and fingers.
Surgical exploration of the plexus is often advised when biceps muscle function is absent at 3 months of age. Some surgeons recommend waiting until 9 months before pursuing surgical exploration.10 No randomized trials have been performed demonstrating the value of surgical intervention, but case series report that surgical intervention with nerve grafting or nerve transfer is superior to management without neurosurgery.

The consequences of permanent OBP injury
A lifetime of corrective therapy for the child. OBP injury may result in a permanent brachial plexus injury—permanent arm weakness requiring decades of physical therapy and multiple surgeries to try to reduce functional disabilities. Problems that frequently occur in children with OBP injury include:
- shoulder contractures
- limb length differences
- winged scapula
- behavioral and developmental problems.
A patient’s care may be coordinated at a brachial plexus injury specialty center, where coordinated access to pediatric neurologists, neurosurgeons, orthopedic surgeons, and neurophysiologists is available and physical and occupational therapy are provided.
Years of emotional response for the parents. Following a birth complicated by shoulder dystocia, the parents may experience grief and anger. During the decades following the birth, the parents of the child will wonder if the injury could have been avoided. They may be asked by friends and relatives, “Would a cesarean delivery have prevented the injury?” Physical therapists, pediatric neurologists, orthopedic and neurosurgeons may reinforce the idea that the injury was caused when the obstetrician applied excessive lateral force to the head and neck, resulting in an injury to the brachial plexus.
Decades-long exposure to these ideas, unresolved anger, financial stress, and lingering doubts may result in the parents and child pursuing civil litigation against the delivering clinician.
Can we reduce the incidence of OBP injury and our risk of litigation?
There are no interventions proven to reduce the incidence of OBP injury. However, many experts advise that a multifaceted approach to this obstetric emergency may reduce the incidence of severe OBP injury at the same time that we reduce our risk of litigation.
When counseling patients about the risk for OBP injury, use teach-back. Patients tend to misunderstand or forget much of what their clinicians teach them.11 Teach-back is an iterative communication process in which the clinician teaches the patient a concept or technique, then asks the patient to repeat the concept in their own words or demonstrate the technique. The clinician then amplifies the concept or technique and corrects misunderstandings. The patient is then asked to repeat the concept or demonstrate the technique. When using teach-back, the clinician never asks, “Do you understand?” The clinician encourages the patient to teach the concept to the clinician using the patient’s own words.12
In the case above, the patient has multiple risk factors for shoulder dystocia, including obesity, short stature, insulin-treated gestational diabetes, and a large fetus. Given the high risk for shoulder dystocia, teach-back might help the patient better understand the situation. After explaining the concepts to the patient, the teach-back questions to the patient might include:
- “Can you tell me what is a shoulder dystocia?”
- “Can you list the health conditions that you have that increase your baby’s risk of shoulder dystocia?”
- “When a shoulder dystocia occurs, what actions will we take at birth to try to fix the shoulder dystocia?”
- “When a baby has experienced a shoulder dystocia, what can happen to the baby’s arm?”
Regularly perform multidisciplinary shoulder dystocia drills. The Joint Commission recommends that clinical drills be performed to help staff prepare for high-risk labor and delivery events, including shoulder dystocia, emergency cesarean delivery, and maternal hemorrhage.13
When shoulder dystocia occurs, extensively chart the event and interventions used. The American College of Obstetricians and Gynecologists has developed a patient safety checklist focused on key clinical elements in the antepartum, intrapartum, and postpartum periods and overall timing of the delivery to document when a shoulder dystocia occurs.14
Stop using the term “traction” in the medical record and obstetric literature. Words are meaningful and open to multiple interpretations. Often, words have unintended consequences. Plaintiff attorneys often highlight the obstetrician’s use of “traction” or “excessive traction” as the cause of an OBP injury. Orthopedic surgeons and pediatricians often state in their records that the OBP injury was a “traction injury,” further supporting the plaintiff attorney’s contention that excessive traction applied by the obstetrician caused the traction injury. Obstetricians do not use traction to deliver a baby. Motorized tractors generate traction, not obstetricians. We use gentle downward guidance to deliver the fetal shoulders and body.
In a high-risk situation, proceed quickly to delivery of the posterior arm. When you recognize a shoulder dystocia in a high-risk situation (maternal diabetes and large fetus), it may be wise to move quickly to delivery of the posterior arm.15,16 In high-risk situations, delivery of the posterior arm is the maneuver with the greatest likelihood of resolving a severe shoulder dystocia, with the least force applied to the brachial plexus that is trapped under the mother’s symphysis pubis.
SHOULDER DYSTOCIA?
Most of our monthly Medical Verdicts columns include cases about shoulder dystocia, brachial plexus injury, or Erb’s palsy. CLICK HERE to read those from 2012 and 2013.
Practice, and then practice some more
A difficult-to-resolve shoulder dystocia is one of the most dramatic and frightening obstetric events. We know that we will all experience such cases. If we prepare well and frequently practice shoulder dystocia maneuvers, the dread of being responsible for resolving a shoulder dystocia will diminish. In most cases we will be able to report, “Mother and newborn safely birthed.”
INSTANT POLL: In your experience, what can clinicians do to reduce their exposure to brachial plexus injury litigation? Click here to respond.
Delivery of the shoulders often gets overlooked in discussions
of episiotomy
(Comment & Controversy, November 2012)
Difficult fetal extraction at cesarean delivery:
What should you do?
Robert L. Barbieri, MD (Editorial, January 2012)
What is the significance of the head-to-body delivery interval
in shoulder dystocia?
William A. Grobman, MD, MBA (Examining the Evidence, December 2011)
Does the use of multiple maneuvers in the management of shoulder dystocia increase the risk of neonatal injury?
Robert B. Gherman, MD (Examining the Evidence, August 2011)
CASE: Shoulder dystocia resulting in persistent injury to C5 and C6
A 30-year-old, G2P1 woman presented in labor at 39 weeks and reported a strong desire to have a natural childbirth. She was taking insulin for gestational diabetes mellitus diagnosed in the second trimester. Her body mass index was 43 kg/m2, and her height was 4 ft 11 in. The estimated fetal weight was 9 lb. She had a prior vaginal delivery. During her antepartum care the patient was extensively counseled about the risk of shoulder dystocia and obstetric brachial plexus (OBP) injury.
The patient progressed normally through labor without anesthesia. At birth, the baby delivered occiput posterior and restituted to right occiput transverse. There was a turtle sign, and the obstetrician diagnosed a shoulder dystocia, called for help, and told the mother to stop pushing. An attempt to deliver the fetal head with gentle downward guidance was unsuccessful. The McRoberts maneuver and suprapubic pressure combined with gentle downward guidance on the fetal head did not result in delivery. A mediolateral episiotomy was made and the Rubin and Wood maneuvers were attempted without success. The obstetrician then successfully delivered the posterior arm and the body of the baby was easily delivered.
The shoulder dystocia lasted 2 minutes before successful delivery. The Apgar scores were 3 and 6 at one and five minutes, respectively. The umbilical cord artery pH was 7.18. The birthweight was 9 lb 2 oz. A diagnosis of OBP injury involving C5 and C6 was made. At discharge the OBP injury persisted.
What is OBP injury?
Shoulder dystocia, which affected this mother and fetus, is a problem involving the impaction of the fetal shoulder behind the maternal symphysis pubis. Shoulder dystocia is a “bony problem.” Although shoulder dystocia cannot be predicted reliably, risk factors include:
- fetal macrosomia
- maternal diabetes
- maternal weight gain
- prepregnancy obesity
- multiparity
- operative vaginal delivery.1-3
The injury can be serious and permanent. The feared consequence of shoulder dystocia is permanent obstetric brachial plexus injury and/or fetal neurologic damage caused by reduced cord blood flow and fetal asphyxia. Occasionally, shoulder dystocia results in fetal death.

Trunks and cords of the brachial plexus
C5 and C6 roots merge to form the upper trunk, C7 root forms the middle trunk, and C8 and T1 roots merge to form the lower trunk.
How can an OBP injury occur?
Most cases are unilateral and arise when one or more of the brachial plexus nerves ( Figure ) are compromised. The forces of labor, fetal position, maternal pushing, and force applied to the fetal head and neck by a clinician all may contribute to an OBP injury.4 It is important to note that up to one-third of all OBP injuries occur in births not associated with a recognized shoulder dystocia.5
Erb’s palsy. Injuries to the C5 and C6 nerves account for about 50% of cases. The muscle groups impacted by this injury include the deltoid and infraspinatus muscles (mainly C5) and the biceps muscle (mainly C6). This pattern results in an adducted and internally rotated upper arm, an extended forearm, with preservation of hand and wrist movement.
Erb’s palsy plus. Injury to C5, C6, and C7 nerves accounts for about 35% of cases and manifests as adduction and internal rotation of the arm, extension and pronation of the forearm, plus flexion of the wrist and fingers—the so-called waiter’s tip posture.
Klumpke’s palsy. Isolated injury to the C8 nerve and T1 root occurs infrequently and manifests as isolated hand paralysis and Horner’s syndrome.
Approximately one in five OBP injuries persist for years following birth. The incidence of OBP injury ranges from 1.0 to 3.0 cases per 1,000 births.6-8 In a review of studies with at least 3 years of follow-up and less than 10% loss to follow-up, the authors reported that the three best studies observed a risk of persistent OBP of 10%, 19%, and 27%.9
Long-term management of OBP injury
The optimal approach to management of OBP injury is unknown because few high-quality studies are available to guide therapy. Typically, 3 to 9 months of observation and physical therapy are recommended following birth, with the hope that the majority of newborns will recover some or almost all function. Physical therapy is focused on reducing the occurrence of joint contractures and maintaining range of motion in the shoulder, elbow, wrist, and fingers.
Surgical exploration of the plexus is often advised when biceps muscle function is absent at 3 months of age. Some surgeons recommend waiting until 9 months before pursuing surgical exploration.10 No randomized trials have been performed demonstrating the value of surgical intervention, but case series report that surgical intervention with nerve grafting or nerve transfer is superior to management without neurosurgery.

The consequences of permanent OBP injury
A lifetime of corrective therapy for the child. OBP injury may result in a permanent brachial plexus injury—permanent arm weakness requiring decades of physical therapy and multiple surgeries to try to reduce functional disabilities. Problems that frequently occur in children with OBP injury include:
- shoulder contractures
- limb length differences
- winged scapula
- behavioral and developmental problems.
A patient’s care may be coordinated at a brachial plexus injury specialty center, where coordinated access to pediatric neurologists, neurosurgeons, orthopedic surgeons, and neurophysiologists is available and physical and occupational therapy are provided.
Years of emotional response for the parents. Following a birth complicated by shoulder dystocia, the parents may experience grief and anger. During the decades following the birth, the parents of the child will wonder if the injury could have been avoided. They may be asked by friends and relatives, “Would a cesarean delivery have prevented the injury?” Physical therapists, pediatric neurologists, orthopedic and neurosurgeons may reinforce the idea that the injury was caused when the obstetrician applied excessive lateral force to the head and neck, resulting in an injury to the brachial plexus.
Decades-long exposure to these ideas, unresolved anger, financial stress, and lingering doubts may result in the parents and child pursuing civil litigation against the delivering clinician.
Can we reduce the incidence of OBP injury and our risk of litigation?
There are no interventions proven to reduce the incidence of OBP injury. However, many experts advise that a multifaceted approach to this obstetric emergency may reduce the incidence of severe OBP injury at the same time that we reduce our risk of litigation.
When counseling patients about the risk for OBP injury, use teach-back. Patients tend to misunderstand or forget much of what their clinicians teach them.11 Teach-back is an iterative communication process in which the clinician teaches the patient a concept or technique, then asks the patient to repeat the concept in their own words or demonstrate the technique. The clinician then amplifies the concept or technique and corrects misunderstandings. The patient is then asked to repeat the concept or demonstrate the technique. When using teach-back, the clinician never asks, “Do you understand?” The clinician encourages the patient to teach the concept to the clinician using the patient’s own words.12
In the case above, the patient has multiple risk factors for shoulder dystocia, including obesity, short stature, insulin-treated gestational diabetes, and a large fetus. Given the high risk for shoulder dystocia, teach-back might help the patient better understand the situation. After explaining the concepts to the patient, the teach-back questions to the patient might include:
- “Can you tell me what is a shoulder dystocia?”
- “Can you list the health conditions that you have that increase your baby’s risk of shoulder dystocia?”
- “When a shoulder dystocia occurs, what actions will we take at birth to try to fix the shoulder dystocia?”
- “When a baby has experienced a shoulder dystocia, what can happen to the baby’s arm?”
Regularly perform multidisciplinary shoulder dystocia drills. The Joint Commission recommends that clinical drills be performed to help staff prepare for high-risk labor and delivery events, including shoulder dystocia, emergency cesarean delivery, and maternal hemorrhage.13
When shoulder dystocia occurs, extensively chart the event and interventions used. The American College of Obstetricians and Gynecologists has developed a patient safety checklist focused on key clinical elements in the antepartum, intrapartum, and postpartum periods and overall timing of the delivery to document when a shoulder dystocia occurs.14
Stop using the term “traction” in the medical record and obstetric literature. Words are meaningful and open to multiple interpretations. Often, words have unintended consequences. Plaintiff attorneys often highlight the obstetrician’s use of “traction” or “excessive traction” as the cause of an OBP injury. Orthopedic surgeons and pediatricians often state in their records that the OBP injury was a “traction injury,” further supporting the plaintiff attorney’s contention that excessive traction applied by the obstetrician caused the traction injury. Obstetricians do not use traction to deliver a baby. Motorized tractors generate traction, not obstetricians. We use gentle downward guidance to deliver the fetal shoulders and body.
In a high-risk situation, proceed quickly to delivery of the posterior arm. When you recognize a shoulder dystocia in a high-risk situation (maternal diabetes and large fetus), it may be wise to move quickly to delivery of the posterior arm.15,16 In high-risk situations, delivery of the posterior arm is the maneuver with the greatest likelihood of resolving a severe shoulder dystocia, with the least force applied to the brachial plexus that is trapped under the mother’s symphysis pubis.
SHOULDER DYSTOCIA?
Most of our monthly Medical Verdicts columns include cases about shoulder dystocia, brachial plexus injury, or Erb’s palsy. CLICK HERE to read those from 2012 and 2013.
Practice, and then practice some more
A difficult-to-resolve shoulder dystocia is one of the most dramatic and frightening obstetric events. We know that we will all experience such cases. If we prepare well and frequently practice shoulder dystocia maneuvers, the dread of being responsible for resolving a shoulder dystocia will diminish. In most cases we will be able to report, “Mother and newborn safely birthed.”
INSTANT POLL: In your experience, what can clinicians do to reduce their exposure to brachial plexus injury litigation? Click here to respond.
1. Acker DB, Sachs BP, Friedman EA. Risk factors for shoulder dystocia. Obstet Gynecol. 1985;66(6):762-768.
2. Benedetti TJ, Gabbe SG. Shoulder dystocia: a complication of fetal macrosomia and prolonged second stage of labor with midpelvic delivery. Obstet Gynecol. 1978;52(5):526-529.
3. Gilbert WM, Nesbitt TS, Danielsen B. Associated factors in 1611 cases of brachial plexus injury. Obstet Gynecol. 1999;93(4):536-540.
4. Gonik B, Walker A, Grimm M. Mathematic modeling of forces associated with shoulder dystocia: a comparison of endogenous and exogenous sources. Am J Obstet Gynecol. 2000;182(3):689-691.
5. Rouse DJ, Owen J, Goldenberg RL, Cliver SP. The effectiveness and costs of elective cesarean delivery for fetal macrosomia diagnosed by ultrasound. JAMA. 1996;276(18):1480-1486.
6. Ecker JL, Greenberg JA, Norwitz ER, Nadel AS, Repke JT. Birth weight as a predictor of brachial plexus injury. Obstet Gynecol 1997;89(5 pt 1):643-647.
7. Bager B. Perinatally acquired brachial plexus palsy—a persisting challenge. Acta Paediatr. 1997;86(11):1216-1219.
8. Dawodu A, Sankaran-Kutty M, Rajan TV. Risk factors and prognosis for brachial plexus injury and clavicular fracture in neonates: a prospective analysis from the United Arab Emirates. Ann Trop Paediatr. 1997;17(3):195-200.
9. Pondaag W, Malessy MJA, van Dijk JG, Thomeer RT. Natural history of obstetric brachial plexus palsy: a systematic review. Dev Med Child Neurol. 2004;46(2):138-144.
10. Grossman JA. Early operative intervention for birth injuries to the brachial plexus. Semin Pediatr Neurol. 2000;7(1):36-43.
11. Institute of Medicine. Nielsen-Bohlman L Panzer AM, Kindig DA, eds. Health Literacy: A Prescription to End Confusion. Washington, DC: National Academies Press; 2004. http://www.nap.edu/openbook.php?isbn=0309091179. Accessed January 15, 2013.
12. Wu H, Nishimi RY, Page-Lopez CM, Kizer KW. Improving patient safety through informed consent for patients with limited health literacy. Washington DC; National Quality Forum; 2005. http://www.qualityforum.org/Publications/2005/09/Improving_Patient_Safety_Through_Informed_Consent_for_Patients_with_Limited_Health_Literacy.aspx. Accessed January 15, 2013.
13. The Joint Commission. Sentinel Event Alert Issue 30: Preventing infant death and injury during delivery. http://www.jointcommission.org/assets/1/18/SEA_30.PDF. Published July 21, 2004. Accessed January 15, 2013.
14. Patient safety checklist No. 6: documenting shoulder dystocia. Obstet Gynecol. 2012;120(2 pt 1):430-431.http://www.acog.org/Resources_And_Publications/Patient_Safety_Checklists_List. Accessed January 15 2013.
15. Poggi SH, Spong CY, Allen RH. Prioritizing posterior arm delivery during severe shoulder dystocia. Obstet Gynecol. 2003;101(5 pt 2):1068-1072.
16. Hoffman MK, Bailit JL, Branch DW, et al. Consortium on Safe Labor. A comparison of obstetric maneuvers for the acute management of shoulder dystocia. Obstet Gynecol. 2011;117(6):1272-1278.
1. Acker DB, Sachs BP, Friedman EA. Risk factors for shoulder dystocia. Obstet Gynecol. 1985;66(6):762-768.
2. Benedetti TJ, Gabbe SG. Shoulder dystocia: a complication of fetal macrosomia and prolonged second stage of labor with midpelvic delivery. Obstet Gynecol. 1978;52(5):526-529.
3. Gilbert WM, Nesbitt TS, Danielsen B. Associated factors in 1611 cases of brachial plexus injury. Obstet Gynecol. 1999;93(4):536-540.
4. Gonik B, Walker A, Grimm M. Mathematic modeling of forces associated with shoulder dystocia: a comparison of endogenous and exogenous sources. Am J Obstet Gynecol. 2000;182(3):689-691.
5. Rouse DJ, Owen J, Goldenberg RL, Cliver SP. The effectiveness and costs of elective cesarean delivery for fetal macrosomia diagnosed by ultrasound. JAMA. 1996;276(18):1480-1486.
6. Ecker JL, Greenberg JA, Norwitz ER, Nadel AS, Repke JT. Birth weight as a predictor of brachial plexus injury. Obstet Gynecol 1997;89(5 pt 1):643-647.
7. Bager B. Perinatally acquired brachial plexus palsy—a persisting challenge. Acta Paediatr. 1997;86(11):1216-1219.
8. Dawodu A, Sankaran-Kutty M, Rajan TV. Risk factors and prognosis for brachial plexus injury and clavicular fracture in neonates: a prospective analysis from the United Arab Emirates. Ann Trop Paediatr. 1997;17(3):195-200.
9. Pondaag W, Malessy MJA, van Dijk JG, Thomeer RT. Natural history of obstetric brachial plexus palsy: a systematic review. Dev Med Child Neurol. 2004;46(2):138-144.
10. Grossman JA. Early operative intervention for birth injuries to the brachial plexus. Semin Pediatr Neurol. 2000;7(1):36-43.
11. Institute of Medicine. Nielsen-Bohlman L Panzer AM, Kindig DA, eds. Health Literacy: A Prescription to End Confusion. Washington, DC: National Academies Press; 2004. http://www.nap.edu/openbook.php?isbn=0309091179. Accessed January 15, 2013.
12. Wu H, Nishimi RY, Page-Lopez CM, Kizer KW. Improving patient safety through informed consent for patients with limited health literacy. Washington DC; National Quality Forum; 2005. http://www.qualityforum.org/Publications/2005/09/Improving_Patient_Safety_Through_Informed_Consent_for_Patients_with_Limited_Health_Literacy.aspx. Accessed January 15, 2013.
13. The Joint Commission. Sentinel Event Alert Issue 30: Preventing infant death and injury during delivery. http://www.jointcommission.org/assets/1/18/SEA_30.PDF. Published July 21, 2004. Accessed January 15, 2013.
14. Patient safety checklist No. 6: documenting shoulder dystocia. Obstet Gynecol. 2012;120(2 pt 1):430-431.http://www.acog.org/Resources_And_Publications/Patient_Safety_Checklists_List. Accessed January 15 2013.
15. Poggi SH, Spong CY, Allen RH. Prioritizing posterior arm delivery during severe shoulder dystocia. Obstet Gynecol. 2003;101(5 pt 2):1068-1072.
16. Hoffman MK, Bailit JL, Branch DW, et al. Consortium on Safe Labor. A comparison of obstetric maneuvers for the acute management of shoulder dystocia. Obstet Gynecol. 2011;117(6):1272-1278.