User login
Medicaid expansion curbs disparities, increases immigrant access, in postpartum care
Expanding Medicaid coverage has proved beneficial to postpartum women and may even help reduce disparities, say two new papers.
In the first study, expansion of Medicaid coverage under the Affordable Care Act was associated with higher rates of postpartum coverage and outpatient visits, according to results published in JAMA Health Forum.
Racial and ethnic disparities were also reduced in postpartum coverage, although these disparities remained between Black and White women for outpatient visits.
In the second study, published in JAMA Network Open, researchers found that when postpartum care is covered as part of Emergency Medicaid, women who have been denied access because of their citizenship status are able to use these services, which includes contraception.
Federal law currently prohibits undocumented and documented immigrants who have been in the United States for less than 5 years from receiving full-benefit Medicaid. Coverage is limited to Emergency Medicaid, which offers benefits only for life-threatening conditions, including hospital admission for childbirth. Coverage is not available for prenatal or postpartum care, including contraception.
For the first article, lead author Maria W. Steenland, SD, of Brown University, Providence, R.I., and colleagues point out that compared with other high-income countries, maternal mortality is higher in the United States and largely driven by persistent racial disparities. Compared with non-Hispanic White women, the rates of maternal death are more than twice as high among American Indian and Alaska Native women, and more than threefold greater in non-Hispanic Black women.
“To be clear, visits increased by around the same amount for Black and White individuals after Medicaid expansion, it is just that visits started off lower among Black women, and remained lower by a similar degree,” said Dr. Steenland.
One explanation is that Black women experience racial discrimination during pregnancy-related health care including childbirth hospitalizations and this may make them more reticent to seek postpartum care, she explained. “In addition, the ability to seek health care is determined by insurance as well as other social factors such as paid leave from work, childcare, and transportation, and these other factors may have remained a larger barrier for Black women after expansion.”
In this cohort study, they looked at the association of Medicaid expansion in Arkansas with continuous postpartum coverage, postpartum health care use, and change in racial disparities in the study outcomes. Using the Arkansas All-Payer Claims Database for persons with a childbirth between 2013 and 2015, the authors identified 60,990 childbirths. Of this group, 67% were White, 22% Black, and 7% Hispanic, and 72.3% were covered by Medicaid. The remaining 27.7% were paid for by a commercial payer.
Before Medicaid expansion, 50.6% of women with Medicaid had continuous coverage during the 6 months postpartum, and the share of women with Medicaid childbirth coverage who were continuously covered for 6 months postpartum increased to 69.3% in 2014 and 90.0% in 2015. Medicaid expansion was associated with a 27.8% increase in continuous coverage for 6-12 months postpartum, and 0.9 increase in visits or a relative increase of 75.0% in outpatient care compared with the visit rate of 1.2 visits within the first 6 months postpartum during the pre-expansion period.
A subgroup analysis was conducted to see if Medicaid expansion had any effect on the disparities between White and Black patients. In the 2-year period after expansion, the percentage of both Black and White women with continuous 6-month postpartum coverage increased to 87.9% and 85.9%, respectively. White individuals averaged 2 visits in the first 6 months postpartum versus 1.6 for Black individuals before expansion, and even though there was no difference in postpartum insurance coverage after expansion, racial disparities in the number of visits during the first 6 months postpartum remained after Medicaid expansion (2.5 vs. 2).
Commenting on the paper, Catherine Cansino, MD, MPH, associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis, noted that she has seen the benefits of Medicaid expansion among obstetric population in California. “I’m glad to see similar expansion in other states,” she said. “But to address persistent health care inequities, I think concierge services or patient care navigation serve a role and can hopefully put a little dent in narrowing gaps.”
Dr. Cansino noted that there are many postpartum patients who need help arranging both pediatric and postpartum care, often prioritizing the newborn appointments. “They also need childcare help so they can focus on their own care as well as transportation,” she said, adding that “it would also be interesting to review racial/ethnic differences with regard to knowledge about contraceptive need immediately postpartum and also about the stigma related to postpartum mental health disorders. If patients don’t see the value of a postpartum visit, they would tend not to attend this visit especially given the many other challenges in the postpartum period.”
Access for immigrants
In the second study, the authors note that the decision to expand Emergency Medicaid options is largely up to individual states. Led by Maria I. Rodriguez, MD, MPH, of the department of obstetrics and gynecology, Oregon Health & Science University, Portland, and colleagues, they decided to compare two states – Oregon, which expanded Emergency Medicaid to include postpartum services and South Carolina, which kept only the federal minimum services – to see how it affected postpartum care among immigrant women.
Compared with South Carolina, there was a 40.6 percentage-point increase (95% confidence interval [CI] in postpartum care visits, P < .001) and postpartum contraception within 60 days grew by 33.2 percentage points (95% CI, P < .001), in Oregon after expansion went into effect.
“When postpartum care was covered for women who would have qualified for Medicaid, except for their citizenship status, their rates of attendance at a postpartum visit and use of postpartum contraception increased to levels observed in the traditional Medicaid population,” the authors wrote.
The calculations, drawn from Medicaid claims and birth certificate data from 2010 to 2019, assumed parallel trends, meaning the researchers made the assumption that use patterns would have remained the same in Oregon if the Emergency Medicaid expansion hadn’t happened and use in South Carolina would have remained consistent as well. A differential trend analysis showed significant increases in use of the services in Oregon relative to South Carolina.
“We included Oregon and South Carolina because both states have experienced similar growth in their immigrant population and have comparable immigrant populations, in terms of size and country of origin, residing in each state,” the authors noted.
Commenting on the study, Laura Mercer MD, MBA, MPH, associate professor in obstetrics and gynecology and director of the obstetrics and gynecology clerkship at the University of Arizona in Phoenix, said she was “excited and encouraged by the results” but not surprised, as it’s logical to assume that there would be more uptake of the services when they are provided free of charge or at low cost.
“Oftentimes, the mother of the family deprioritizes her own health and well-being in favor of diverting those resources to her children and her family,” said Dr. Mercer, who specializes in prenatal and postpartum care.
She added that the significant increase in contraception is a particularly representative sign of improvement as it is easier to quantify, compared to improvements in mental health or counseling.
But comprehensive postpartum care extends to physical, psychological, and social well-being. “Its components include counseling on the importance of birth spacing and providing the contraceptive method of their choice,” the authors wrote. “An absence of postpartum care has been associated with unintended pregnancy, short interpregnancy intervals, exacerbation of chronic diseases, and preterm birth.”
Dr. Mercer noted that closely spaced pregnancies, particularly less than 6 months but at least less than 18 months carry increased risk for mother and child. And for those who would say that immigrant women should be excluded from the Emergency Medicaid postpartum services, Dr. Mercer said she would encourage them to look at the data around the improved outcomes of comprehensive maternal care.
Being able to track health markers and intervene before a woman requires emergency care will reduce costs in the long run, she pointed out. But, regardless of the cost, policymakers have to ask themselves, “What do we value as a society? If we value families and healthy families and we want to promote the best possible outcomes, then I think this question becomes very easy to answer.”
The first study was funded by the National Institute for Health Care Management. Dr. Steenland was also supported by the Agency for Healthcare Research and Quality and the National Institutes of Health. Dr. Steenland reported grants from the Agency for Healthcare Research and Quality and from the National Institute for Child Health and Human Development during the conduct of the study. The second study was supported by the National Institute on Minority Health and Health Disparities. Dr. Rodriguez reports grants from Arnold Ventures and personal fees from the American College of Obstetricians and Gynecologists, Bayer, and Merck outside the submitted work. A coauthor reports grants from Merck/Organon and the Office of Population Affairs outside the submitted work, as well as membership on the board of directors of the Society of Family Planning and the ACOG Gynecology Clinical Practice Guideline committee. Dr. Mercer reported no relevant financial relationships.
Expanding Medicaid coverage has proved beneficial to postpartum women and may even help reduce disparities, say two new papers.
In the first study, expansion of Medicaid coverage under the Affordable Care Act was associated with higher rates of postpartum coverage and outpatient visits, according to results published in JAMA Health Forum.
Racial and ethnic disparities were also reduced in postpartum coverage, although these disparities remained between Black and White women for outpatient visits.
In the second study, published in JAMA Network Open, researchers found that when postpartum care is covered as part of Emergency Medicaid, women who have been denied access because of their citizenship status are able to use these services, which includes contraception.
Federal law currently prohibits undocumented and documented immigrants who have been in the United States for less than 5 years from receiving full-benefit Medicaid. Coverage is limited to Emergency Medicaid, which offers benefits only for life-threatening conditions, including hospital admission for childbirth. Coverage is not available for prenatal or postpartum care, including contraception.
For the first article, lead author Maria W. Steenland, SD, of Brown University, Providence, R.I., and colleagues point out that compared with other high-income countries, maternal mortality is higher in the United States and largely driven by persistent racial disparities. Compared with non-Hispanic White women, the rates of maternal death are more than twice as high among American Indian and Alaska Native women, and more than threefold greater in non-Hispanic Black women.
“To be clear, visits increased by around the same amount for Black and White individuals after Medicaid expansion, it is just that visits started off lower among Black women, and remained lower by a similar degree,” said Dr. Steenland.
One explanation is that Black women experience racial discrimination during pregnancy-related health care including childbirth hospitalizations and this may make them more reticent to seek postpartum care, she explained. “In addition, the ability to seek health care is determined by insurance as well as other social factors such as paid leave from work, childcare, and transportation, and these other factors may have remained a larger barrier for Black women after expansion.”
In this cohort study, they looked at the association of Medicaid expansion in Arkansas with continuous postpartum coverage, postpartum health care use, and change in racial disparities in the study outcomes. Using the Arkansas All-Payer Claims Database for persons with a childbirth between 2013 and 2015, the authors identified 60,990 childbirths. Of this group, 67% were White, 22% Black, and 7% Hispanic, and 72.3% were covered by Medicaid. The remaining 27.7% were paid for by a commercial payer.
Before Medicaid expansion, 50.6% of women with Medicaid had continuous coverage during the 6 months postpartum, and the share of women with Medicaid childbirth coverage who were continuously covered for 6 months postpartum increased to 69.3% in 2014 and 90.0% in 2015. Medicaid expansion was associated with a 27.8% increase in continuous coverage for 6-12 months postpartum, and 0.9 increase in visits or a relative increase of 75.0% in outpatient care compared with the visit rate of 1.2 visits within the first 6 months postpartum during the pre-expansion period.
A subgroup analysis was conducted to see if Medicaid expansion had any effect on the disparities between White and Black patients. In the 2-year period after expansion, the percentage of both Black and White women with continuous 6-month postpartum coverage increased to 87.9% and 85.9%, respectively. White individuals averaged 2 visits in the first 6 months postpartum versus 1.6 for Black individuals before expansion, and even though there was no difference in postpartum insurance coverage after expansion, racial disparities in the number of visits during the first 6 months postpartum remained after Medicaid expansion (2.5 vs. 2).
Commenting on the paper, Catherine Cansino, MD, MPH, associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis, noted that she has seen the benefits of Medicaid expansion among obstetric population in California. “I’m glad to see similar expansion in other states,” she said. “But to address persistent health care inequities, I think concierge services or patient care navigation serve a role and can hopefully put a little dent in narrowing gaps.”
Dr. Cansino noted that there are many postpartum patients who need help arranging both pediatric and postpartum care, often prioritizing the newborn appointments. “They also need childcare help so they can focus on their own care as well as transportation,” she said, adding that “it would also be interesting to review racial/ethnic differences with regard to knowledge about contraceptive need immediately postpartum and also about the stigma related to postpartum mental health disorders. If patients don’t see the value of a postpartum visit, they would tend not to attend this visit especially given the many other challenges in the postpartum period.”
Access for immigrants
In the second study, the authors note that the decision to expand Emergency Medicaid options is largely up to individual states. Led by Maria I. Rodriguez, MD, MPH, of the department of obstetrics and gynecology, Oregon Health & Science University, Portland, and colleagues, they decided to compare two states – Oregon, which expanded Emergency Medicaid to include postpartum services and South Carolina, which kept only the federal minimum services – to see how it affected postpartum care among immigrant women.
Compared with South Carolina, there was a 40.6 percentage-point increase (95% confidence interval [CI] in postpartum care visits, P < .001) and postpartum contraception within 60 days grew by 33.2 percentage points (95% CI, P < .001), in Oregon after expansion went into effect.
“When postpartum care was covered for women who would have qualified for Medicaid, except for their citizenship status, their rates of attendance at a postpartum visit and use of postpartum contraception increased to levels observed in the traditional Medicaid population,” the authors wrote.
The calculations, drawn from Medicaid claims and birth certificate data from 2010 to 2019, assumed parallel trends, meaning the researchers made the assumption that use patterns would have remained the same in Oregon if the Emergency Medicaid expansion hadn’t happened and use in South Carolina would have remained consistent as well. A differential trend analysis showed significant increases in use of the services in Oregon relative to South Carolina.
“We included Oregon and South Carolina because both states have experienced similar growth in their immigrant population and have comparable immigrant populations, in terms of size and country of origin, residing in each state,” the authors noted.
Commenting on the study, Laura Mercer MD, MBA, MPH, associate professor in obstetrics and gynecology and director of the obstetrics and gynecology clerkship at the University of Arizona in Phoenix, said she was “excited and encouraged by the results” but not surprised, as it’s logical to assume that there would be more uptake of the services when they are provided free of charge or at low cost.
“Oftentimes, the mother of the family deprioritizes her own health and well-being in favor of diverting those resources to her children and her family,” said Dr. Mercer, who specializes in prenatal and postpartum care.
She added that the significant increase in contraception is a particularly representative sign of improvement as it is easier to quantify, compared to improvements in mental health or counseling.
But comprehensive postpartum care extends to physical, psychological, and social well-being. “Its components include counseling on the importance of birth spacing and providing the contraceptive method of their choice,” the authors wrote. “An absence of postpartum care has been associated with unintended pregnancy, short interpregnancy intervals, exacerbation of chronic diseases, and preterm birth.”
Dr. Mercer noted that closely spaced pregnancies, particularly less than 6 months but at least less than 18 months carry increased risk for mother and child. And for those who would say that immigrant women should be excluded from the Emergency Medicaid postpartum services, Dr. Mercer said she would encourage them to look at the data around the improved outcomes of comprehensive maternal care.
Being able to track health markers and intervene before a woman requires emergency care will reduce costs in the long run, she pointed out. But, regardless of the cost, policymakers have to ask themselves, “What do we value as a society? If we value families and healthy families and we want to promote the best possible outcomes, then I think this question becomes very easy to answer.”
The first study was funded by the National Institute for Health Care Management. Dr. Steenland was also supported by the Agency for Healthcare Research and Quality and the National Institutes of Health. Dr. Steenland reported grants from the Agency for Healthcare Research and Quality and from the National Institute for Child Health and Human Development during the conduct of the study. The second study was supported by the National Institute on Minority Health and Health Disparities. Dr. Rodriguez reports grants from Arnold Ventures and personal fees from the American College of Obstetricians and Gynecologists, Bayer, and Merck outside the submitted work. A coauthor reports grants from Merck/Organon and the Office of Population Affairs outside the submitted work, as well as membership on the board of directors of the Society of Family Planning and the ACOG Gynecology Clinical Practice Guideline committee. Dr. Mercer reported no relevant financial relationships.
Expanding Medicaid coverage has proved beneficial to postpartum women and may even help reduce disparities, say two new papers.
In the first study, expansion of Medicaid coverage under the Affordable Care Act was associated with higher rates of postpartum coverage and outpatient visits, according to results published in JAMA Health Forum.
Racial and ethnic disparities were also reduced in postpartum coverage, although these disparities remained between Black and White women for outpatient visits.
In the second study, published in JAMA Network Open, researchers found that when postpartum care is covered as part of Emergency Medicaid, women who have been denied access because of their citizenship status are able to use these services, which includes contraception.
Federal law currently prohibits undocumented and documented immigrants who have been in the United States for less than 5 years from receiving full-benefit Medicaid. Coverage is limited to Emergency Medicaid, which offers benefits only for life-threatening conditions, including hospital admission for childbirth. Coverage is not available for prenatal or postpartum care, including contraception.
For the first article, lead author Maria W. Steenland, SD, of Brown University, Providence, R.I., and colleagues point out that compared with other high-income countries, maternal mortality is higher in the United States and largely driven by persistent racial disparities. Compared with non-Hispanic White women, the rates of maternal death are more than twice as high among American Indian and Alaska Native women, and more than threefold greater in non-Hispanic Black women.
“To be clear, visits increased by around the same amount for Black and White individuals after Medicaid expansion, it is just that visits started off lower among Black women, and remained lower by a similar degree,” said Dr. Steenland.
One explanation is that Black women experience racial discrimination during pregnancy-related health care including childbirth hospitalizations and this may make them more reticent to seek postpartum care, she explained. “In addition, the ability to seek health care is determined by insurance as well as other social factors such as paid leave from work, childcare, and transportation, and these other factors may have remained a larger barrier for Black women after expansion.”
In this cohort study, they looked at the association of Medicaid expansion in Arkansas with continuous postpartum coverage, postpartum health care use, and change in racial disparities in the study outcomes. Using the Arkansas All-Payer Claims Database for persons with a childbirth between 2013 and 2015, the authors identified 60,990 childbirths. Of this group, 67% were White, 22% Black, and 7% Hispanic, and 72.3% were covered by Medicaid. The remaining 27.7% were paid for by a commercial payer.
Before Medicaid expansion, 50.6% of women with Medicaid had continuous coverage during the 6 months postpartum, and the share of women with Medicaid childbirth coverage who were continuously covered for 6 months postpartum increased to 69.3% in 2014 and 90.0% in 2015. Medicaid expansion was associated with a 27.8% increase in continuous coverage for 6-12 months postpartum, and 0.9 increase in visits or a relative increase of 75.0% in outpatient care compared with the visit rate of 1.2 visits within the first 6 months postpartum during the pre-expansion period.
A subgroup analysis was conducted to see if Medicaid expansion had any effect on the disparities between White and Black patients. In the 2-year period after expansion, the percentage of both Black and White women with continuous 6-month postpartum coverage increased to 87.9% and 85.9%, respectively. White individuals averaged 2 visits in the first 6 months postpartum versus 1.6 for Black individuals before expansion, and even though there was no difference in postpartum insurance coverage after expansion, racial disparities in the number of visits during the first 6 months postpartum remained after Medicaid expansion (2.5 vs. 2).
Commenting on the paper, Catherine Cansino, MD, MPH, associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis, noted that she has seen the benefits of Medicaid expansion among obstetric population in California. “I’m glad to see similar expansion in other states,” she said. “But to address persistent health care inequities, I think concierge services or patient care navigation serve a role and can hopefully put a little dent in narrowing gaps.”
Dr. Cansino noted that there are many postpartum patients who need help arranging both pediatric and postpartum care, often prioritizing the newborn appointments. “They also need childcare help so they can focus on their own care as well as transportation,” she said, adding that “it would also be interesting to review racial/ethnic differences with regard to knowledge about contraceptive need immediately postpartum and also about the stigma related to postpartum mental health disorders. If patients don’t see the value of a postpartum visit, they would tend not to attend this visit especially given the many other challenges in the postpartum period.”
Access for immigrants
In the second study, the authors note that the decision to expand Emergency Medicaid options is largely up to individual states. Led by Maria I. Rodriguez, MD, MPH, of the department of obstetrics and gynecology, Oregon Health & Science University, Portland, and colleagues, they decided to compare two states – Oregon, which expanded Emergency Medicaid to include postpartum services and South Carolina, which kept only the federal minimum services – to see how it affected postpartum care among immigrant women.
Compared with South Carolina, there was a 40.6 percentage-point increase (95% confidence interval [CI] in postpartum care visits, P < .001) and postpartum contraception within 60 days grew by 33.2 percentage points (95% CI, P < .001), in Oregon after expansion went into effect.
“When postpartum care was covered for women who would have qualified for Medicaid, except for their citizenship status, their rates of attendance at a postpartum visit and use of postpartum contraception increased to levels observed in the traditional Medicaid population,” the authors wrote.
The calculations, drawn from Medicaid claims and birth certificate data from 2010 to 2019, assumed parallel trends, meaning the researchers made the assumption that use patterns would have remained the same in Oregon if the Emergency Medicaid expansion hadn’t happened and use in South Carolina would have remained consistent as well. A differential trend analysis showed significant increases in use of the services in Oregon relative to South Carolina.
“We included Oregon and South Carolina because both states have experienced similar growth in their immigrant population and have comparable immigrant populations, in terms of size and country of origin, residing in each state,” the authors noted.
Commenting on the study, Laura Mercer MD, MBA, MPH, associate professor in obstetrics and gynecology and director of the obstetrics and gynecology clerkship at the University of Arizona in Phoenix, said she was “excited and encouraged by the results” but not surprised, as it’s logical to assume that there would be more uptake of the services when they are provided free of charge or at low cost.
“Oftentimes, the mother of the family deprioritizes her own health and well-being in favor of diverting those resources to her children and her family,” said Dr. Mercer, who specializes in prenatal and postpartum care.
She added that the significant increase in contraception is a particularly representative sign of improvement as it is easier to quantify, compared to improvements in mental health or counseling.
But comprehensive postpartum care extends to physical, psychological, and social well-being. “Its components include counseling on the importance of birth spacing and providing the contraceptive method of their choice,” the authors wrote. “An absence of postpartum care has been associated with unintended pregnancy, short interpregnancy intervals, exacerbation of chronic diseases, and preterm birth.”
Dr. Mercer noted that closely spaced pregnancies, particularly less than 6 months but at least less than 18 months carry increased risk for mother and child. And for those who would say that immigrant women should be excluded from the Emergency Medicaid postpartum services, Dr. Mercer said she would encourage them to look at the data around the improved outcomes of comprehensive maternal care.
Being able to track health markers and intervene before a woman requires emergency care will reduce costs in the long run, she pointed out. But, regardless of the cost, policymakers have to ask themselves, “What do we value as a society? If we value families and healthy families and we want to promote the best possible outcomes, then I think this question becomes very easy to answer.”
The first study was funded by the National Institute for Health Care Management. Dr. Steenland was also supported by the Agency for Healthcare Research and Quality and the National Institutes of Health. Dr. Steenland reported grants from the Agency for Healthcare Research and Quality and from the National Institute for Child Health and Human Development during the conduct of the study. The second study was supported by the National Institute on Minority Health and Health Disparities. Dr. Rodriguez reports grants from Arnold Ventures and personal fees from the American College of Obstetricians and Gynecologists, Bayer, and Merck outside the submitted work. A coauthor reports grants from Merck/Organon and the Office of Population Affairs outside the submitted work, as well as membership on the board of directors of the Society of Family Planning and the ACOG Gynecology Clinical Practice Guideline committee. Dr. Mercer reported no relevant financial relationships.
FROM JAMA HEALTH FORUM AND JAMA NETWORK OPEN
At-home cervical ripening linked with less time in L&D unit
Women who undergo balloon cervical ripening at home spend less time in the labor and delivery unit and have fewer cesarean deliveries than those who have the induction procedure in a hospital, researchers have found.
The findings, from a meta-analysis of eight previously conducted randomized clinical trials involving 740 women, should spur hospitals to “create and adhere to evidence-based guidelines” for outpatient balloon use, according to the researchers.
“Outpatient balloon cervical ripening is a safe alternative for low-risk patients and has the potential for significant benefits to patients and labor and delivery units,” the authors reported Jan. 6 in Obstetrics and Gynecology.
The rate of labor induction in the United States rose to 29.4% in 2019, the year following publication of the ARRIVE trial of low-risk nulliparous pregnant women, which found that induction at 39 weeks resulted in fewer cesarean deliveries with no difference in neonatal outcomes compared with expectant management, defined as continuing pregnancy until at least 40 weeks 5 days unless induction was medically indicated. Most women require preparation with a balloon-tipped catheter that slowly inflates to stretch and thin out the cervix, a process that can take many hours.
The devices have been shown to be safe, effective, and inexpensive, but the data on outpatient use are limited, according to the researchers. The new study is the “most comprehensive” examination of randomized clinical trials comparing outpatient and inpatient balloon cervical ripening, they say.
The trials included singleton gestations of at least 37 weeks of primarily low-risk patients. Body mass index was slightly lower in the outpatient group, with no differences in maternal age, gestational age at induction, or parity.
Six studies with 571 patients reported on the primary outcome, defined as time from labor unit admission to delivery. The outpatient group had a mean 16.3 hours compared with 23.8 hours for the inpatient group, a difference of 7.24 hours. However, data from three of the studies showed the inpatient group experienced 5.19 hours on average less between balloon expulsion and delivery, potentially due to more frequent adjustments and evaluation for expulsion.
The researchers observed no differences in adverse maternal or neonatal outcomes, and no stillbirths were reported among 378 patients who had the outpatient procedure. Cesarean delivery occurred less often in the outpatient group (21%) versus the inpatient group (27%) (risk ratio, 0.76; 95% confidence interval, 0.59-0.98).
Corresponding author Vincenzo Berghella, MD, director of the Division of Maternal-Fetal Medicine at Jefferson University Hospitals, Philadelphia, called the data “very assuring.” He said, “We knew induction was good in the hospital for many indications. We now know that induction can be started at home and it’s safe.”
Dr. Berghella added that the lower rate of cesarean delivery in the outpatient group likely reflected less use of fetal heart-rate monitoring, which can produce false-positive predictions of fetal compromise.
Still, too few patients have been studied to completely rule out rare adverse events with use of the balloons in the outpatient setting, the researchers acknowledge.
Aaron B. Caughey, MD, PhD, of Oregon Health and Science University, Portland, who was not involved in the study, said current data do not put to rest all safety concerns with the balloons, and it will be vital for health systems to report outcomes as outpatient use of the devices increases.
“Outcomes such as chorioamnionitis and postpartum hemorrhage will be important to have more data on, though there do not appear to be trends from these data,” Dr. Caughey told this news organization. Rarer outcomes, such as cervical injury, placenta abruption, and fetal injury, he added, “will require much larger studies to examine these potential but unlikely risks.”
The authors and Dr. Caughey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*Correction, 1/19/22: An earlier version of the headline of this article misstated a study finding. The study found that women who undergo balloon cervical ripening spend less time in the labor and delivery unit than those who have the induction procedure in a hospital.
Women who undergo balloon cervical ripening at home spend less time in the labor and delivery unit and have fewer cesarean deliveries than those who have the induction procedure in a hospital, researchers have found.
The findings, from a meta-analysis of eight previously conducted randomized clinical trials involving 740 women, should spur hospitals to “create and adhere to evidence-based guidelines” for outpatient balloon use, according to the researchers.
“Outpatient balloon cervical ripening is a safe alternative for low-risk patients and has the potential for significant benefits to patients and labor and delivery units,” the authors reported Jan. 6 in Obstetrics and Gynecology.
The rate of labor induction in the United States rose to 29.4% in 2019, the year following publication of the ARRIVE trial of low-risk nulliparous pregnant women, which found that induction at 39 weeks resulted in fewer cesarean deliveries with no difference in neonatal outcomes compared with expectant management, defined as continuing pregnancy until at least 40 weeks 5 days unless induction was medically indicated. Most women require preparation with a balloon-tipped catheter that slowly inflates to stretch and thin out the cervix, a process that can take many hours.
The devices have been shown to be safe, effective, and inexpensive, but the data on outpatient use are limited, according to the researchers. The new study is the “most comprehensive” examination of randomized clinical trials comparing outpatient and inpatient balloon cervical ripening, they say.
The trials included singleton gestations of at least 37 weeks of primarily low-risk patients. Body mass index was slightly lower in the outpatient group, with no differences in maternal age, gestational age at induction, or parity.
Six studies with 571 patients reported on the primary outcome, defined as time from labor unit admission to delivery. The outpatient group had a mean 16.3 hours compared with 23.8 hours for the inpatient group, a difference of 7.24 hours. However, data from three of the studies showed the inpatient group experienced 5.19 hours on average less between balloon expulsion and delivery, potentially due to more frequent adjustments and evaluation for expulsion.
The researchers observed no differences in adverse maternal or neonatal outcomes, and no stillbirths were reported among 378 patients who had the outpatient procedure. Cesarean delivery occurred less often in the outpatient group (21%) versus the inpatient group (27%) (risk ratio, 0.76; 95% confidence interval, 0.59-0.98).
Corresponding author Vincenzo Berghella, MD, director of the Division of Maternal-Fetal Medicine at Jefferson University Hospitals, Philadelphia, called the data “very assuring.” He said, “We knew induction was good in the hospital for many indications. We now know that induction can be started at home and it’s safe.”
Dr. Berghella added that the lower rate of cesarean delivery in the outpatient group likely reflected less use of fetal heart-rate monitoring, which can produce false-positive predictions of fetal compromise.
Still, too few patients have been studied to completely rule out rare adverse events with use of the balloons in the outpatient setting, the researchers acknowledge.
Aaron B. Caughey, MD, PhD, of Oregon Health and Science University, Portland, who was not involved in the study, said current data do not put to rest all safety concerns with the balloons, and it will be vital for health systems to report outcomes as outpatient use of the devices increases.
“Outcomes such as chorioamnionitis and postpartum hemorrhage will be important to have more data on, though there do not appear to be trends from these data,” Dr. Caughey told this news organization. Rarer outcomes, such as cervical injury, placenta abruption, and fetal injury, he added, “will require much larger studies to examine these potential but unlikely risks.”
The authors and Dr. Caughey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*Correction, 1/19/22: An earlier version of the headline of this article misstated a study finding. The study found that women who undergo balloon cervical ripening spend less time in the labor and delivery unit than those who have the induction procedure in a hospital.
Women who undergo balloon cervical ripening at home spend less time in the labor and delivery unit and have fewer cesarean deliveries than those who have the induction procedure in a hospital, researchers have found.
The findings, from a meta-analysis of eight previously conducted randomized clinical trials involving 740 women, should spur hospitals to “create and adhere to evidence-based guidelines” for outpatient balloon use, according to the researchers.
“Outpatient balloon cervical ripening is a safe alternative for low-risk patients and has the potential for significant benefits to patients and labor and delivery units,” the authors reported Jan. 6 in Obstetrics and Gynecology.
The rate of labor induction in the United States rose to 29.4% in 2019, the year following publication of the ARRIVE trial of low-risk nulliparous pregnant women, which found that induction at 39 weeks resulted in fewer cesarean deliveries with no difference in neonatal outcomes compared with expectant management, defined as continuing pregnancy until at least 40 weeks 5 days unless induction was medically indicated. Most women require preparation with a balloon-tipped catheter that slowly inflates to stretch and thin out the cervix, a process that can take many hours.
The devices have been shown to be safe, effective, and inexpensive, but the data on outpatient use are limited, according to the researchers. The new study is the “most comprehensive” examination of randomized clinical trials comparing outpatient and inpatient balloon cervical ripening, they say.
The trials included singleton gestations of at least 37 weeks of primarily low-risk patients. Body mass index was slightly lower in the outpatient group, with no differences in maternal age, gestational age at induction, or parity.
Six studies with 571 patients reported on the primary outcome, defined as time from labor unit admission to delivery. The outpatient group had a mean 16.3 hours compared with 23.8 hours for the inpatient group, a difference of 7.24 hours. However, data from three of the studies showed the inpatient group experienced 5.19 hours on average less between balloon expulsion and delivery, potentially due to more frequent adjustments and evaluation for expulsion.
The researchers observed no differences in adverse maternal or neonatal outcomes, and no stillbirths were reported among 378 patients who had the outpatient procedure. Cesarean delivery occurred less often in the outpatient group (21%) versus the inpatient group (27%) (risk ratio, 0.76; 95% confidence interval, 0.59-0.98).
Corresponding author Vincenzo Berghella, MD, director of the Division of Maternal-Fetal Medicine at Jefferson University Hospitals, Philadelphia, called the data “very assuring.” He said, “We knew induction was good in the hospital for many indications. We now know that induction can be started at home and it’s safe.”
Dr. Berghella added that the lower rate of cesarean delivery in the outpatient group likely reflected less use of fetal heart-rate monitoring, which can produce false-positive predictions of fetal compromise.
Still, too few patients have been studied to completely rule out rare adverse events with use of the balloons in the outpatient setting, the researchers acknowledge.
Aaron B. Caughey, MD, PhD, of Oregon Health and Science University, Portland, who was not involved in the study, said current data do not put to rest all safety concerns with the balloons, and it will be vital for health systems to report outcomes as outpatient use of the devices increases.
“Outcomes such as chorioamnionitis and postpartum hemorrhage will be important to have more data on, though there do not appear to be trends from these data,” Dr. Caughey told this news organization. Rarer outcomes, such as cervical injury, placenta abruption, and fetal injury, he added, “will require much larger studies to examine these potential but unlikely risks.”
The authors and Dr. Caughey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*Correction, 1/19/22: An earlier version of the headline of this article misstated a study finding. The study found that women who undergo balloon cervical ripening spend less time in the labor and delivery unit than those who have the induction procedure in a hospital.
Pregnancy diet linked to risk of obesity in child
A new study suggests that a healthy diet initiated by women before conception could lower the risk of obesity in the offspring.
Childhood obesity is a major public health concern in the United Kingdom, with nearly a quarter of children under 5 and more than a third of children starting secondary school being overweight or obese. Furthermore, childhood obesity is likely to persist in adulthood and have long-term health consequences.
Researchers at the University of Southampton (England) analyzed dietary data of 2,963 mother-child dyads identified from the U.K. Southampton Women’s Survey. Using the dietary data, each mother-child dyad was assigned combined diet quality score, based on which they were categorized into 5 groups: poor, poor-medium, medium, medium-better and best. Childhood adiposity was evaluated using dual-energy x-ray absorptiometry (DXA) and body mass index (BMI) z-scores.
The findings, published in the International Journal of Obesity, showed that mother-offspring diet quality trajectories were stable from preconception in mothers to age 8-9 years in the offspring. A poorer diet quality trajectory was linked to higher prepregnancy maternal BMI, lower maternal age at birth, lower educational levels, smoking, and multiparity.
After adjusting for confounders, a 1-category reduction in the dietary trajectory was associated with higher DXA percentage body fat (standard deviation, 0.08; 95% confidence interval, 0.01-0.15) and BMI z-score (SD, 0.08; 95% CI, 0.00-0.16) in the offspring aged 8-9 years.
Lead author Sarah Crozier, PhD, University of Southampton, said: “This research shows the importance of intervening at the earliest possible stage in a child’s life, in pregnancy or even before conception, to enable us to tackle it.” The authors believe that the preconception period serves as a crucial window to introduce favorable changes in the maternal dietary quality.
The research was funded by grants from the Medical Research Council, Project EarlyNutrition, and the European Union’s Seventh Framework and Horizon 2020 programs. The study also received support from National Institute for Health Research Southampton Biomedical Research Centre, the University of Southampton and University Hospital Southampton NHS Foundation Trust. The authors reported no competing interests.
A version of this article first appeared on Medscape UK.
A new study suggests that a healthy diet initiated by women before conception could lower the risk of obesity in the offspring.
Childhood obesity is a major public health concern in the United Kingdom, with nearly a quarter of children under 5 and more than a third of children starting secondary school being overweight or obese. Furthermore, childhood obesity is likely to persist in adulthood and have long-term health consequences.
Researchers at the University of Southampton (England) analyzed dietary data of 2,963 mother-child dyads identified from the U.K. Southampton Women’s Survey. Using the dietary data, each mother-child dyad was assigned combined diet quality score, based on which they were categorized into 5 groups: poor, poor-medium, medium, medium-better and best. Childhood adiposity was evaluated using dual-energy x-ray absorptiometry (DXA) and body mass index (BMI) z-scores.
The findings, published in the International Journal of Obesity, showed that mother-offspring diet quality trajectories were stable from preconception in mothers to age 8-9 years in the offspring. A poorer diet quality trajectory was linked to higher prepregnancy maternal BMI, lower maternal age at birth, lower educational levels, smoking, and multiparity.
After adjusting for confounders, a 1-category reduction in the dietary trajectory was associated with higher DXA percentage body fat (standard deviation, 0.08; 95% confidence interval, 0.01-0.15) and BMI z-score (SD, 0.08; 95% CI, 0.00-0.16) in the offspring aged 8-9 years.
Lead author Sarah Crozier, PhD, University of Southampton, said: “This research shows the importance of intervening at the earliest possible stage in a child’s life, in pregnancy or even before conception, to enable us to tackle it.” The authors believe that the preconception period serves as a crucial window to introduce favorable changes in the maternal dietary quality.
The research was funded by grants from the Medical Research Council, Project EarlyNutrition, and the European Union’s Seventh Framework and Horizon 2020 programs. The study also received support from National Institute for Health Research Southampton Biomedical Research Centre, the University of Southampton and University Hospital Southampton NHS Foundation Trust. The authors reported no competing interests.
A version of this article first appeared on Medscape UK.
A new study suggests that a healthy diet initiated by women before conception could lower the risk of obesity in the offspring.
Childhood obesity is a major public health concern in the United Kingdom, with nearly a quarter of children under 5 and more than a third of children starting secondary school being overweight or obese. Furthermore, childhood obesity is likely to persist in adulthood and have long-term health consequences.
Researchers at the University of Southampton (England) analyzed dietary data of 2,963 mother-child dyads identified from the U.K. Southampton Women’s Survey. Using the dietary data, each mother-child dyad was assigned combined diet quality score, based on which they were categorized into 5 groups: poor, poor-medium, medium, medium-better and best. Childhood adiposity was evaluated using dual-energy x-ray absorptiometry (DXA) and body mass index (BMI) z-scores.
The findings, published in the International Journal of Obesity, showed that mother-offspring diet quality trajectories were stable from preconception in mothers to age 8-9 years in the offspring. A poorer diet quality trajectory was linked to higher prepregnancy maternal BMI, lower maternal age at birth, lower educational levels, smoking, and multiparity.
After adjusting for confounders, a 1-category reduction in the dietary trajectory was associated with higher DXA percentage body fat (standard deviation, 0.08; 95% confidence interval, 0.01-0.15) and BMI z-score (SD, 0.08; 95% CI, 0.00-0.16) in the offspring aged 8-9 years.
Lead author Sarah Crozier, PhD, University of Southampton, said: “This research shows the importance of intervening at the earliest possible stage in a child’s life, in pregnancy or even before conception, to enable us to tackle it.” The authors believe that the preconception period serves as a crucial window to introduce favorable changes in the maternal dietary quality.
The research was funded by grants from the Medical Research Council, Project EarlyNutrition, and the European Union’s Seventh Framework and Horizon 2020 programs. The study also received support from National Institute for Health Research Southampton Biomedical Research Centre, the University of Southampton and University Hospital Southampton NHS Foundation Trust. The authors reported no competing interests.
A version of this article first appeared on Medscape UK.
FROM THE INTERNATIONAL JOURNAL OF OBESITY
Are there perinatal benefits to pregnant patients after bariatric surgery?
Getahun D, Fassett MJ, Jacobsen SJ, et al. Perinatal outcomes after bariatric surgery. Am J Obstet Gynecol. 2021;S0002-9378(21)00771-7. doi: 10.1016/j.ajog.2021 .06.087.
EXPERT COMMENTARY
Prepregnancy obesity continues to rise in the United States, with a prevalence of 29% among reproductive-age women in 2019, an 11% increase from 2016.1 Pregnant patients with obesity are at increased risk for multiple adverse perinatal outcomes, including gestational diabetes and preeclampsia. Bariatric surgery is effective for weight loss and has been shown to improve comorbidities associated with obesity,2 and it may have potential benefits for pregnancy outcomes, such as reducing rates of gestational diabetes and preeclampsia.3-5 However, little was known about other outcomes as well as other potential factors before a recent study in which investigators examined perinatal outcomes after bariatric surgery.
Details of the study
Getahun and colleagues conducted a population-based, retrospective study of pregnant patients who were eligible for bariatric surgery (body mass index [BMI] ≥40 kg/m2 with no comorbidities or a BMI between 35 and 40 kg/m2 with obesity-related comorbidities, such as diabetes). They aimed to evaluate the association of bariatric surgery with adverse perinatal outcomes.
Results. In a large sample of pregnant patients eligible for bariatric surgery (N = 20,213), the authors found that patients who had bariatric surgery (n = 1,886) had a reduced risk of macrosomia (aOR, 0.24), preeclampsia (aOR, 0.53), gestational diabetes (aOR, 0.60), and cesarean delivery (aOR, 0.65) compared with those who did not have bariatric surgery (n = 18,327). They also found that patients who had bariatric surgery had an increased risk of small-for-gestational age neonates (aOR, 2.46) and postpartum hemorrhage (aOR, 1.79).
These results remained after adjusting for other potential confounders. The authors evaluated the outcomes based on the timing of surgery and the patients’ pregnancy (<1 year, 1-1.5 years, 1.5-2 years, >2 years). The outcomes were more favorable among the patients who had the bariatric surgery regardless of the time interval of surgery to pregnancy than those who did not have the surgery. In addition, the benefits of bariatric surgery did not differ between the 2 most common types of bariatric surgery (Roux-en-Y gastric bypass and vertical sleeve gastrectomy) performed in this study, and both had better outcomes than those who did not have the surgery. Finally, patients with chronic hypertension and pregestational diabetes who had bariatric surgery also had lower risks of adverse outcomes than those without bariatric surgery.
Study strengths and limitations
Given the study’s retrospective design, uncertainties and important confounders could not be addressed, such as why certain eligible patients had the surgery and others did not. However, with its large sample size and an appropriate comparison group, the study findings further support the perinatal benefits of bariatric surgery in obese patients. Of note, this study also had a large sample of Black and Hispanic patients, populations known to have higher rates of obesity1 and pregnancy complications. Subgroup analyses within each racial/ethnic group revealed that those who had the surgery had lower risks of adverse perinatal outcomes than those who did not.
Patients who had the bariatric surgery had an increased risk of postpartum hemorrhage; however, there is no physiologic basis or theory to explain this finding, so further studies are needed. Lastly, although patients who had bariatric surgery had an increased risk of small-for-gestational-age babies and the study was not powered for the risk of stillbirth, the patients who had the surgery had a reduced risk of neonates admitted to the neonatal intensive care unit. More data would have been beneficial to assess if these small-for-gestational-age babies were healthy. In general, obese patients tend to have larger and unhealthy babies; thus, healthier babies, even if small for gestational age, would not be an adverse outcome.
Benefits of bariatric surgery extend to perinatal outcomes
This study reinforces current practice that includes eligible patients being counseled about the health-related benefits of bariatric surgery, which now includes more perinatal outcomes. The finding of the increased risk of small-for-gestational-age fetuses supports the practice of a screening growth ultrasound exam in patients who had bariatric surgery. ●
An important, modifiable risk factor for adverse perinatal outcomes is the patient’s prepregnancy BMI at the time of pregnancy. Bariatric surgery is an effective procedure for weight loss. There are many perinatal benefits for eligible patients who have bariatric surgery before pregnancy. Clinicians should counsel their obese patients who are considering or planning pregnancy about the benefits of bariatric surgery.
RODNEY A. MCLAREN, JR, MD, AND VINCENZO BERGHELLA, MD
- Driscoll AK, Gregory ECW. Increases in prepregnancy obesity: United States, 2016-2019. NCHS Data Brief. 2020 Nov;(392):1-8.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724- 1737. doi: 10.1001/jama.292.14.1724.
- Maggard MA, Yermilov I, Li Z, et al. Pregnancy and fertility following bariatric surgery: a systematic review. JAMA. 2008;300:2286-2296. doi: 10.1001/jama.2008.641.
- Watanabe A, Seki Y, Haruta H, et al. Maternal impacts and perinatal outcomes after three types of bariatric surgery at a single institution. Arch Gynecol Obstet. 2019;300:145-152. doi: 10.1007/s00404-019-05195-9.
- Balestrin B, Urbanetz AA, Barbieri MM, et al. Pregnancy after bariatric surgery: a comparative study of post-bariatric pregnant women versus non-bariatric obese pregnant women. Obes Surg. 2019;29:3142-3148. doi: 10.1007/s11695- 019-03961-x.
Getahun D, Fassett MJ, Jacobsen SJ, et al. Perinatal outcomes after bariatric surgery. Am J Obstet Gynecol. 2021;S0002-9378(21)00771-7. doi: 10.1016/j.ajog.2021 .06.087.
EXPERT COMMENTARY
Prepregnancy obesity continues to rise in the United States, with a prevalence of 29% among reproductive-age women in 2019, an 11% increase from 2016.1 Pregnant patients with obesity are at increased risk for multiple adverse perinatal outcomes, including gestational diabetes and preeclampsia. Bariatric surgery is effective for weight loss and has been shown to improve comorbidities associated with obesity,2 and it may have potential benefits for pregnancy outcomes, such as reducing rates of gestational diabetes and preeclampsia.3-5 However, little was known about other outcomes as well as other potential factors before a recent study in which investigators examined perinatal outcomes after bariatric surgery.
Details of the study
Getahun and colleagues conducted a population-based, retrospective study of pregnant patients who were eligible for bariatric surgery (body mass index [BMI] ≥40 kg/m2 with no comorbidities or a BMI between 35 and 40 kg/m2 with obesity-related comorbidities, such as diabetes). They aimed to evaluate the association of bariatric surgery with adverse perinatal outcomes.
Results. In a large sample of pregnant patients eligible for bariatric surgery (N = 20,213), the authors found that patients who had bariatric surgery (n = 1,886) had a reduced risk of macrosomia (aOR, 0.24), preeclampsia (aOR, 0.53), gestational diabetes (aOR, 0.60), and cesarean delivery (aOR, 0.65) compared with those who did not have bariatric surgery (n = 18,327). They also found that patients who had bariatric surgery had an increased risk of small-for-gestational age neonates (aOR, 2.46) and postpartum hemorrhage (aOR, 1.79).
These results remained after adjusting for other potential confounders. The authors evaluated the outcomes based on the timing of surgery and the patients’ pregnancy (<1 year, 1-1.5 years, 1.5-2 years, >2 years). The outcomes were more favorable among the patients who had the bariatric surgery regardless of the time interval of surgery to pregnancy than those who did not have the surgery. In addition, the benefits of bariatric surgery did not differ between the 2 most common types of bariatric surgery (Roux-en-Y gastric bypass and vertical sleeve gastrectomy) performed in this study, and both had better outcomes than those who did not have the surgery. Finally, patients with chronic hypertension and pregestational diabetes who had bariatric surgery also had lower risks of adverse outcomes than those without bariatric surgery.
Study strengths and limitations
Given the study’s retrospective design, uncertainties and important confounders could not be addressed, such as why certain eligible patients had the surgery and others did not. However, with its large sample size and an appropriate comparison group, the study findings further support the perinatal benefits of bariatric surgery in obese patients. Of note, this study also had a large sample of Black and Hispanic patients, populations known to have higher rates of obesity1 and pregnancy complications. Subgroup analyses within each racial/ethnic group revealed that those who had the surgery had lower risks of adverse perinatal outcomes than those who did not.
Patients who had the bariatric surgery had an increased risk of postpartum hemorrhage; however, there is no physiologic basis or theory to explain this finding, so further studies are needed. Lastly, although patients who had bariatric surgery had an increased risk of small-for-gestational-age babies and the study was not powered for the risk of stillbirth, the patients who had the surgery had a reduced risk of neonates admitted to the neonatal intensive care unit. More data would have been beneficial to assess if these small-for-gestational-age babies were healthy. In general, obese patients tend to have larger and unhealthy babies; thus, healthier babies, even if small for gestational age, would not be an adverse outcome.
Benefits of bariatric surgery extend to perinatal outcomes
This study reinforces current practice that includes eligible patients being counseled about the health-related benefits of bariatric surgery, which now includes more perinatal outcomes. The finding of the increased risk of small-for-gestational-age fetuses supports the practice of a screening growth ultrasound exam in patients who had bariatric surgery. ●
An important, modifiable risk factor for adverse perinatal outcomes is the patient’s prepregnancy BMI at the time of pregnancy. Bariatric surgery is an effective procedure for weight loss. There are many perinatal benefits for eligible patients who have bariatric surgery before pregnancy. Clinicians should counsel their obese patients who are considering or planning pregnancy about the benefits of bariatric surgery.
RODNEY A. MCLAREN, JR, MD, AND VINCENZO BERGHELLA, MD
Getahun D, Fassett MJ, Jacobsen SJ, et al. Perinatal outcomes after bariatric surgery. Am J Obstet Gynecol. 2021;S0002-9378(21)00771-7. doi: 10.1016/j.ajog.2021 .06.087.
EXPERT COMMENTARY
Prepregnancy obesity continues to rise in the United States, with a prevalence of 29% among reproductive-age women in 2019, an 11% increase from 2016.1 Pregnant patients with obesity are at increased risk for multiple adverse perinatal outcomes, including gestational diabetes and preeclampsia. Bariatric surgery is effective for weight loss and has been shown to improve comorbidities associated with obesity,2 and it may have potential benefits for pregnancy outcomes, such as reducing rates of gestational diabetes and preeclampsia.3-5 However, little was known about other outcomes as well as other potential factors before a recent study in which investigators examined perinatal outcomes after bariatric surgery.
Details of the study
Getahun and colleagues conducted a population-based, retrospective study of pregnant patients who were eligible for bariatric surgery (body mass index [BMI] ≥40 kg/m2 with no comorbidities or a BMI between 35 and 40 kg/m2 with obesity-related comorbidities, such as diabetes). They aimed to evaluate the association of bariatric surgery with adverse perinatal outcomes.
Results. In a large sample of pregnant patients eligible for bariatric surgery (N = 20,213), the authors found that patients who had bariatric surgery (n = 1,886) had a reduced risk of macrosomia (aOR, 0.24), preeclampsia (aOR, 0.53), gestational diabetes (aOR, 0.60), and cesarean delivery (aOR, 0.65) compared with those who did not have bariatric surgery (n = 18,327). They also found that patients who had bariatric surgery had an increased risk of small-for-gestational age neonates (aOR, 2.46) and postpartum hemorrhage (aOR, 1.79).
These results remained after adjusting for other potential confounders. The authors evaluated the outcomes based on the timing of surgery and the patients’ pregnancy (<1 year, 1-1.5 years, 1.5-2 years, >2 years). The outcomes were more favorable among the patients who had the bariatric surgery regardless of the time interval of surgery to pregnancy than those who did not have the surgery. In addition, the benefits of bariatric surgery did not differ between the 2 most common types of bariatric surgery (Roux-en-Y gastric bypass and vertical sleeve gastrectomy) performed in this study, and both had better outcomes than those who did not have the surgery. Finally, patients with chronic hypertension and pregestational diabetes who had bariatric surgery also had lower risks of adverse outcomes than those without bariatric surgery.
Study strengths and limitations
Given the study’s retrospective design, uncertainties and important confounders could not be addressed, such as why certain eligible patients had the surgery and others did not. However, with its large sample size and an appropriate comparison group, the study findings further support the perinatal benefits of bariatric surgery in obese patients. Of note, this study also had a large sample of Black and Hispanic patients, populations known to have higher rates of obesity1 and pregnancy complications. Subgroup analyses within each racial/ethnic group revealed that those who had the surgery had lower risks of adverse perinatal outcomes than those who did not.
Patients who had the bariatric surgery had an increased risk of postpartum hemorrhage; however, there is no physiologic basis or theory to explain this finding, so further studies are needed. Lastly, although patients who had bariatric surgery had an increased risk of small-for-gestational-age babies and the study was not powered for the risk of stillbirth, the patients who had the surgery had a reduced risk of neonates admitted to the neonatal intensive care unit. More data would have been beneficial to assess if these small-for-gestational-age babies were healthy. In general, obese patients tend to have larger and unhealthy babies; thus, healthier babies, even if small for gestational age, would not be an adverse outcome.
Benefits of bariatric surgery extend to perinatal outcomes
This study reinforces current practice that includes eligible patients being counseled about the health-related benefits of bariatric surgery, which now includes more perinatal outcomes. The finding of the increased risk of small-for-gestational-age fetuses supports the practice of a screening growth ultrasound exam in patients who had bariatric surgery. ●
An important, modifiable risk factor for adverse perinatal outcomes is the patient’s prepregnancy BMI at the time of pregnancy. Bariatric surgery is an effective procedure for weight loss. There are many perinatal benefits for eligible patients who have bariatric surgery before pregnancy. Clinicians should counsel their obese patients who are considering or planning pregnancy about the benefits of bariatric surgery.
RODNEY A. MCLAREN, JR, MD, AND VINCENZO BERGHELLA, MD
- Driscoll AK, Gregory ECW. Increases in prepregnancy obesity: United States, 2016-2019. NCHS Data Brief. 2020 Nov;(392):1-8.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724- 1737. doi: 10.1001/jama.292.14.1724.
- Maggard MA, Yermilov I, Li Z, et al. Pregnancy and fertility following bariatric surgery: a systematic review. JAMA. 2008;300:2286-2296. doi: 10.1001/jama.2008.641.
- Watanabe A, Seki Y, Haruta H, et al. Maternal impacts and perinatal outcomes after three types of bariatric surgery at a single institution. Arch Gynecol Obstet. 2019;300:145-152. doi: 10.1007/s00404-019-05195-9.
- Balestrin B, Urbanetz AA, Barbieri MM, et al. Pregnancy after bariatric surgery: a comparative study of post-bariatric pregnant women versus non-bariatric obese pregnant women. Obes Surg. 2019;29:3142-3148. doi: 10.1007/s11695- 019-03961-x.
- Driscoll AK, Gregory ECW. Increases in prepregnancy obesity: United States, 2016-2019. NCHS Data Brief. 2020 Nov;(392):1-8.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724- 1737. doi: 10.1001/jama.292.14.1724.
- Maggard MA, Yermilov I, Li Z, et al. Pregnancy and fertility following bariatric surgery: a systematic review. JAMA. 2008;300:2286-2296. doi: 10.1001/jama.2008.641.
- Watanabe A, Seki Y, Haruta H, et al. Maternal impacts and perinatal outcomes after three types of bariatric surgery at a single institution. Arch Gynecol Obstet. 2019;300:145-152. doi: 10.1007/s00404-019-05195-9.
- Balestrin B, Urbanetz AA, Barbieri MM, et al. Pregnancy after bariatric surgery: a comparative study of post-bariatric pregnant women versus non-bariatric obese pregnant women. Obes Surg. 2019;29:3142-3148. doi: 10.1007/s11695- 019-03961-x.
Heavy snoring in early pregnancy linked to increased insulin resistance
Severe maternal sleep-disordered breathing (SDB) is a known risk factor for gestational diabetes, which is commonly diagnosed in the second or third trimester of pregnancy.
Now, a new study suggests that increases in insulin resistance, a precursor for gestational diabetes, may take place as early as the first trimester of pregnancy in women with risk factors for obstructive sleep apnea (OSA), such as overweight and habitual snoring.
This finding could potentially provide physicians with a window of opportunity to improve outcomes by screening at-risk women early in pregnancy or even prior to conception, Laura Sanapo, MD, assistant professor of medicine (research) at Brown University, Providence, R.I., and colleagues wrote in Sleep.
“Further studies are needed to investigate the association and its impact on the development of gestational diabetes, and to establish whether early-gestation or pregestational treatment of SDB would improve glucose metabolic outcomes in pregnancy,” they wrote.
”What this paper demonstrates is that the changes that predate gestational diabetes are seen much earlier in pregnancy,” senior study author Ghada Bourjeily, MD, professor of medicine at Brown University, said in an interview. Women should be screened for SDB rather than insulin resistance in early pregnancy since continuous positive airway pressure therapy (CPAP) is a highly effective intervention.
Waiting until midpregnancy to screen for OSA “is too late to make significant changes in the care of these women,” said Dr. Bourjeily, who is also director of research and training at the Women’s Medicine Collaborative at The Miriam Hospital in Providence, R.I. “By the time you diagnose gestational diabetes, the cat is out of the bag.”
For the study, women with early singleton pregnancies and risk factors for OSA such as habitual snoring and a median body mass index (BMI) of at least 27 kg/m2 were recruited from two prospective clinical trial studies enriched for OSA positivity. Women with a history of pregestational diabetes and those using CPAP or receiving chronic steroid therapy were excluded from the current study.
A total of 192 study participants underwent in-home sleep study (HSAT) and homeostatic model assessment (HOMA) between 11 and 15 gestational weeks, respectively. The association between continuous measures of SDB as a respiratory-event index as well as oxygen-desaturation index and glucose metabolism parameters such as insulin resistance (HOMA-IR) were analyzed after adjusting for gestational age, maternal age, BMI, ethnicity, race, and parity.
In all, 61 women (32%) were diagnosed with OSA based on respiratory event index values greater than or equal to five events per hour. These participants were more likely to be older, to have a high BMI, and to be multipara, compared with women who didn’t have a diagnosis of OSA. Women with a diagnosis of OSA exhibited higher glucose and C-peptide values and a higher degree of insulin resistance, compared with women without OSA, the researchers found. An increase of 0.3 in HOMA-IR related to maternal SDB in early pregnancy may significantly affect glucose metabolism.
Although the findings of the current study cannot be extrapolated to women who don’t have overweight or obesity, some women with normal-range BMI (18.5-24.9) are also at increased risk of glucose metabolism changes, Dr. Bourjeily pointed out. This includes those of Southeast Asian descent. “We found that the association of SDB parameters with insulin resistance was actually happening independently of BMI and other factors.”
Ideally, screening for SDB would begin prior to pregnancy, Dr. Bourjeily said. A BMI greater than 25 should be taken into account and patients asked if they snore and if so, whether it’s loud enough to wake their partner. They should also be asked about experiencing daytime sleepiness.
“Based on these answers, especially in women screened prior to pregnancy, there will be time to make the diagnosis of sleep apnea and get the patient on CPAP,” Dr. Bourjeily said.
“This is an interesting study and one of the rare ones looking at early pregnancy and some of the mechanisms that could possibly be contributing to gestational diabetes,” commented Grenye O’Malley, MD, assistant professor in the division of endocrinology, diabetes, and bone disease at the Icahn School of Medicine at Mount Sinai, New York. Dr. O’Malley was not involved in the study.
“It confirms our suspicions that there’s probably a lot of things happening earlier in pregnancy before a diagnosis of gestational diabetes. It also confirms that some of the mechanisms are probably very similar to those involved in the association between disordered sleep and the development of type 2 diabetes.”
However, it’s too early to determine whether screening for SDB and the use of CPAP will prevent glycemic changes, Dr. O’Malley said in an interview. “Whenever we screen, we ask whether we have an intervention that changes outcomes and we don’t know that yet.”
Some of the symptoms of SDB are also common in early pregnancy, such as a BMI greater than 25 and daytime sleepiness, Dr. O’Malley pointed out. It was unclear whether the study participants had a propensity to develop type 2 diabetes or whether they were at risk of gestational diabetes.
This study was funded by the National Heart, Lung, and Blood Institute; the National Institute for Child Health; and the National Institute of General Medical Sciences. Dr. Bourjeily and colleagues, as well as Dr. O’Malley, reported having no potential financial conflicts of interest.
Severe maternal sleep-disordered breathing (SDB) is a known risk factor for gestational diabetes, which is commonly diagnosed in the second or third trimester of pregnancy.
Now, a new study suggests that increases in insulin resistance, a precursor for gestational diabetes, may take place as early as the first trimester of pregnancy in women with risk factors for obstructive sleep apnea (OSA), such as overweight and habitual snoring.
This finding could potentially provide physicians with a window of opportunity to improve outcomes by screening at-risk women early in pregnancy or even prior to conception, Laura Sanapo, MD, assistant professor of medicine (research) at Brown University, Providence, R.I., and colleagues wrote in Sleep.
“Further studies are needed to investigate the association and its impact on the development of gestational diabetes, and to establish whether early-gestation or pregestational treatment of SDB would improve glucose metabolic outcomes in pregnancy,” they wrote.
”What this paper demonstrates is that the changes that predate gestational diabetes are seen much earlier in pregnancy,” senior study author Ghada Bourjeily, MD, professor of medicine at Brown University, said in an interview. Women should be screened for SDB rather than insulin resistance in early pregnancy since continuous positive airway pressure therapy (CPAP) is a highly effective intervention.
Waiting until midpregnancy to screen for OSA “is too late to make significant changes in the care of these women,” said Dr. Bourjeily, who is also director of research and training at the Women’s Medicine Collaborative at The Miriam Hospital in Providence, R.I. “By the time you diagnose gestational diabetes, the cat is out of the bag.”
For the study, women with early singleton pregnancies and risk factors for OSA such as habitual snoring and a median body mass index (BMI) of at least 27 kg/m2 were recruited from two prospective clinical trial studies enriched for OSA positivity. Women with a history of pregestational diabetes and those using CPAP or receiving chronic steroid therapy were excluded from the current study.
A total of 192 study participants underwent in-home sleep study (HSAT) and homeostatic model assessment (HOMA) between 11 and 15 gestational weeks, respectively. The association between continuous measures of SDB as a respiratory-event index as well as oxygen-desaturation index and glucose metabolism parameters such as insulin resistance (HOMA-IR) were analyzed after adjusting for gestational age, maternal age, BMI, ethnicity, race, and parity.
In all, 61 women (32%) were diagnosed with OSA based on respiratory event index values greater than or equal to five events per hour. These participants were more likely to be older, to have a high BMI, and to be multipara, compared with women who didn’t have a diagnosis of OSA. Women with a diagnosis of OSA exhibited higher glucose and C-peptide values and a higher degree of insulin resistance, compared with women without OSA, the researchers found. An increase of 0.3 in HOMA-IR related to maternal SDB in early pregnancy may significantly affect glucose metabolism.
Although the findings of the current study cannot be extrapolated to women who don’t have overweight or obesity, some women with normal-range BMI (18.5-24.9) are also at increased risk of glucose metabolism changes, Dr. Bourjeily pointed out. This includes those of Southeast Asian descent. “We found that the association of SDB parameters with insulin resistance was actually happening independently of BMI and other factors.”
Ideally, screening for SDB would begin prior to pregnancy, Dr. Bourjeily said. A BMI greater than 25 should be taken into account and patients asked if they snore and if so, whether it’s loud enough to wake their partner. They should also be asked about experiencing daytime sleepiness.
“Based on these answers, especially in women screened prior to pregnancy, there will be time to make the diagnosis of sleep apnea and get the patient on CPAP,” Dr. Bourjeily said.
“This is an interesting study and one of the rare ones looking at early pregnancy and some of the mechanisms that could possibly be contributing to gestational diabetes,” commented Grenye O’Malley, MD, assistant professor in the division of endocrinology, diabetes, and bone disease at the Icahn School of Medicine at Mount Sinai, New York. Dr. O’Malley was not involved in the study.
“It confirms our suspicions that there’s probably a lot of things happening earlier in pregnancy before a diagnosis of gestational diabetes. It also confirms that some of the mechanisms are probably very similar to those involved in the association between disordered sleep and the development of type 2 diabetes.”
However, it’s too early to determine whether screening for SDB and the use of CPAP will prevent glycemic changes, Dr. O’Malley said in an interview. “Whenever we screen, we ask whether we have an intervention that changes outcomes and we don’t know that yet.”
Some of the symptoms of SDB are also common in early pregnancy, such as a BMI greater than 25 and daytime sleepiness, Dr. O’Malley pointed out. It was unclear whether the study participants had a propensity to develop type 2 diabetes or whether they were at risk of gestational diabetes.
This study was funded by the National Heart, Lung, and Blood Institute; the National Institute for Child Health; and the National Institute of General Medical Sciences. Dr. Bourjeily and colleagues, as well as Dr. O’Malley, reported having no potential financial conflicts of interest.
Severe maternal sleep-disordered breathing (SDB) is a known risk factor for gestational diabetes, which is commonly diagnosed in the second or third trimester of pregnancy.
Now, a new study suggests that increases in insulin resistance, a precursor for gestational diabetes, may take place as early as the first trimester of pregnancy in women with risk factors for obstructive sleep apnea (OSA), such as overweight and habitual snoring.
This finding could potentially provide physicians with a window of opportunity to improve outcomes by screening at-risk women early in pregnancy or even prior to conception, Laura Sanapo, MD, assistant professor of medicine (research) at Brown University, Providence, R.I., and colleagues wrote in Sleep.
“Further studies are needed to investigate the association and its impact on the development of gestational diabetes, and to establish whether early-gestation or pregestational treatment of SDB would improve glucose metabolic outcomes in pregnancy,” they wrote.
”What this paper demonstrates is that the changes that predate gestational diabetes are seen much earlier in pregnancy,” senior study author Ghada Bourjeily, MD, professor of medicine at Brown University, said in an interview. Women should be screened for SDB rather than insulin resistance in early pregnancy since continuous positive airway pressure therapy (CPAP) is a highly effective intervention.
Waiting until midpregnancy to screen for OSA “is too late to make significant changes in the care of these women,” said Dr. Bourjeily, who is also director of research and training at the Women’s Medicine Collaborative at The Miriam Hospital in Providence, R.I. “By the time you diagnose gestational diabetes, the cat is out of the bag.”
For the study, women with early singleton pregnancies and risk factors for OSA such as habitual snoring and a median body mass index (BMI) of at least 27 kg/m2 were recruited from two prospective clinical trial studies enriched for OSA positivity. Women with a history of pregestational diabetes and those using CPAP or receiving chronic steroid therapy were excluded from the current study.
A total of 192 study participants underwent in-home sleep study (HSAT) and homeostatic model assessment (HOMA) between 11 and 15 gestational weeks, respectively. The association between continuous measures of SDB as a respiratory-event index as well as oxygen-desaturation index and glucose metabolism parameters such as insulin resistance (HOMA-IR) were analyzed after adjusting for gestational age, maternal age, BMI, ethnicity, race, and parity.
In all, 61 women (32%) were diagnosed with OSA based on respiratory event index values greater than or equal to five events per hour. These participants were more likely to be older, to have a high BMI, and to be multipara, compared with women who didn’t have a diagnosis of OSA. Women with a diagnosis of OSA exhibited higher glucose and C-peptide values and a higher degree of insulin resistance, compared with women without OSA, the researchers found. An increase of 0.3 in HOMA-IR related to maternal SDB in early pregnancy may significantly affect glucose metabolism.
Although the findings of the current study cannot be extrapolated to women who don’t have overweight or obesity, some women with normal-range BMI (18.5-24.9) are also at increased risk of glucose metabolism changes, Dr. Bourjeily pointed out. This includes those of Southeast Asian descent. “We found that the association of SDB parameters with insulin resistance was actually happening independently of BMI and other factors.”
Ideally, screening for SDB would begin prior to pregnancy, Dr. Bourjeily said. A BMI greater than 25 should be taken into account and patients asked if they snore and if so, whether it’s loud enough to wake their partner. They should also be asked about experiencing daytime sleepiness.
“Based on these answers, especially in women screened prior to pregnancy, there will be time to make the diagnosis of sleep apnea and get the patient on CPAP,” Dr. Bourjeily said.
“This is an interesting study and one of the rare ones looking at early pregnancy and some of the mechanisms that could possibly be contributing to gestational diabetes,” commented Grenye O’Malley, MD, assistant professor in the division of endocrinology, diabetes, and bone disease at the Icahn School of Medicine at Mount Sinai, New York. Dr. O’Malley was not involved in the study.
“It confirms our suspicions that there’s probably a lot of things happening earlier in pregnancy before a diagnosis of gestational diabetes. It also confirms that some of the mechanisms are probably very similar to those involved in the association between disordered sleep and the development of type 2 diabetes.”
However, it’s too early to determine whether screening for SDB and the use of CPAP will prevent glycemic changes, Dr. O’Malley said in an interview. “Whenever we screen, we ask whether we have an intervention that changes outcomes and we don’t know that yet.”
Some of the symptoms of SDB are also common in early pregnancy, such as a BMI greater than 25 and daytime sleepiness, Dr. O’Malley pointed out. It was unclear whether the study participants had a propensity to develop type 2 diabetes or whether they were at risk of gestational diabetes.
This study was funded by the National Heart, Lung, and Blood Institute; the National Institute for Child Health; and the National Institute of General Medical Sciences. Dr. Bourjeily and colleagues, as well as Dr. O’Malley, reported having no potential financial conflicts of interest.
FROM SLEEP
Individualize the duration of postpartum magnesium treatment for patients with preeclampsia to best balance the benefits and harms of treatment
Preeclampsia complicates 3% to 8% of pregnancies.1-3 The incidence of preeclampsia is influenced by the clinical characteristics of the pregnant population, including the prevalence of overweight, obesity, chronic hypertension, diabetes, nulliparity, advanced maternal age, multiple gestations, kidney disease, and a history of preeclampsia in a prior pregnancy.4
Magnesium treatment reduces the rate of eclampsia among patients with preeclampsia
For patients with preeclampsia, magnesium treatment reduces the risk of seizure. In the Magpie trial, 9,992 pregnant patients were treated for 24 hours with magnesium or placebo.5 The magnesium treatment regimen was either a 4-g IV bolus over 10 to 15 minutes followed by a continuous infusion of 1 g/hr or an intramuscular regimen (10-g intramuscular loading dose followed by 5 g IM every 4 hours). Eclamptic seizures occurred in 0.8% and 1.9% of patients treated with magnesium or placebo, respectively (relative risk [RR], 0.42; 95% confidence interval [CI], 0.29 to 0.60). Among patients with a multiple gestation, the rate of eclampsia was 2% and 6% in the patients treated with magnesium or placebo, respectively. The number of patients who needed to be treated to prevent one eclamptic event was 63 and 109 for patients with preeclampsia with and without severe features, respectively. Intrapartum treatment with magnesium also reduced the risk of placental abruption from 3.2% for the patients receiving placebo to 2.0% among the patients treated with magnesium (RR, 0.67; 99% CI, 0.45- 0.89). Maternal death was reduced with magnesium treatment compared with placebo (0.2% vs 0.4%), but the difference was not statistically significant.
In the Magpie trial, side effects were reported by 24% and 5% of patients treated with magnesium and placebo, respectively. The most common side effects were flushing, nausea, vomiting, and muscle weakness. Of note, magnesium treatment is contraindicated in patients with myasthenia gravis because it can cause muscle weakness and hypoventilation.6 For patients with preeclampsia and myasthenia gravis, levetiracetam may be utilized to reduce the risk of seizure.6
Duration of postpartum magnesium treatment
There are no studies with a sufficient number of participants to definitively determine the optimal duration of postpartum magnesium therapy. A properly powered study would likely require more than 16,000 to 20,000 participants to identify clinically meaningful differences in the rate of postpartum eclampsia among patients treated with magnesium for 12 or 24 hours.7,8 It is unlikely that such a study will be completed. Hence, the duration of postpartum magnesium must be based on clinical judgment, balancing the risks and benefits of treatment.
The American College of Obstetricians and Gynecologists (ACOG) recommends continuing magnesium treatment for 24 hours postpartum. They advise, “For patients requiring cesarean delivery (before the onset of labor), the infusion should ideally begin before surgery and continue during surgery, as well as 24 hours afterwards. For patients who deliver vaginally, the infusion should continue for 24 hours after delivery.”9
Multiple randomized trials have reported on the outcomes associated with 12 hours versus 24 hours of postpartum magnesium therapy (TABLE). Because the rate of postpartum eclamptic seizure is very low, none of the studies were sufficiently powered to provide a definitive answer to the benefits and harms of the shorter versus longer time frame of magnesium therapy.10-15
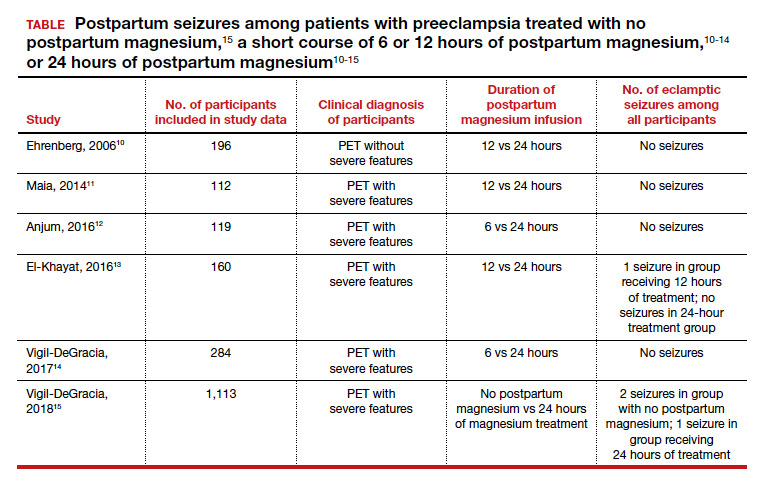
Continue to: The harms of prolonged postpartum magnesium infusion...
The harms of prolonged postpartum magnesium infusion
The harms of prolonging treatment with postpartum magnesium infusion are generally not emphasized in the medical literature. However, side effects that can occur are flushing, nausea, vomiting, and muscle weakness, delayed early ambulation, delayed return to full diet, delayed discontinuation of a bladder catheter, and delayed initiation of breastfeeding.5,15 In one large clinical trial, 1,113 patients with preeclampsia with severe features who received intrapartum magnesium for ≥8 hours were randomized after birth to immediate discontinuation of magnesium or continuation of magnesium for 24 hours.15 There was 1 seizure in the group of 555 patients who received 24 hours of postpartum magnesium and 2 seizures in the group of 558 patients who received no magnesium after birth. In this trial, continuation of magnesium postpartum resulted in delayed initiation of breastfeeding and delayed ambulation.15
Balancing the benefits and harms of postpartum magnesium infusion
An important clinical point is that magnesium treatment will not prevent all seizures associated with preeclampsia; in the Magpie trial, among the 5,055 patients with preeclampsia treated with magnesium there were 40 (0.8%) seizures.5 Magnesium treatment will reduce but not eliminate the risk of seizure. Clinicians should have a plan to treat seizures that occur while a woman is being treated with magnesium.
In the absence of high-quality data to guide the duration of postpartum magnesium therapy it is best to use clinical parameters to balance the benefits and harms of postpartum magnesium treatment.16-18 Patients may want to participate in the decision about the duration of postpartum magnesium treatment after receiving counseling about the benefits and harms.
For patients with preeclampsia without severe features, many clinicians are no longer ordering intrapartum magnesium for prevention of seizures because they believe the risk of seizure in patients without severe disease is very low. Hence, these patients will not receive postpartum magnesium treatment unless they evolve to preeclampsia with severe features or develop a “red flag” warning postpartum (see below).
For patients with preeclampsia without severe features who received intrapartum magnesium, after birth, the magnesium infusion could be stopped immediately or within 12 hours of birth. For patients with preeclampsia without severe features, early termination of the magnesium infusion best balances the benefit of seizure reduction with the harms of delayed early ambulation, return to full diet, discontinuation of the bladder catheter, and initiation of breastfeeding.
For patients with preeclampsia with severe features, 24 hours of magnesium may best balance the benefits and harms of treatment. However, if the patient continues to have “red flag” findings, continued magnesium treatment beyond 24 hours may be warranted.
Red flag findings include: an eclamptic seizure before or after birth, ongoing or recurring severe headaches, visual scotomata, nausea, vomiting, epigastric pain, severe hypertension, oliguria, rising creatinine, or liver transaminases and declining platelet count.
The hypertensive diseases of pregnancy, including preeclampsia often appear suddenly and may evolve rapidly, threatening the health of both mother and fetus. A high level of suspicion that a hypertensive disease might be the cause of vague symptoms such as epigastric discomfort or headache may accelerate early diagnosis. Rapid treatment of severe hypertension with intravenous labetalol and hydralazine, and intrapartum plus postpartum administration of magnesium to prevent placental abruption and eclampsia will optimize patient outcomes. No patient, patient’s family members, or clinician, wants to experience the grief of a preventable maternal, fetal, or newborn death due to hypertension.19 Obstetricians, midwives, labor nurses, obstetrical anesthesiologists and doulas play key roles in preventing maternal, fetal, and newborn morbidity and death from hypertensive diseases of pregnancy. As a team we are the last line of defense protecting the health of our patients. ●
- World Health Organization. WHO International Collaborative Study of Hypertensive Disorders of Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. Am J Obstet Gynecol. 1988;158:80-83.
- Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209:544.e1-e12. doi: 10.1016 /j.ajog.2013.08.019.
- Mayrink K, Souza RT, Feitosa FE, et al. Incidence and risk factors for preeclampsia in a cohort of healthy nulliparous patients: a nested casecontrol study. Sci Rep. 2019;9:9517. doi: 10.1038 /s41598-019-46011-3.
- Bartsch E, Medcalf KE, Park AL, et al. High risk of pre-eclampsia identification group. BMJ. 2016;353:i1753. doi: 10.1136/bmj.i1753.
- Altman D, Carroli G, Duley L; The Magpie Trial Collaborative Group. Do patients with preeclampsia, and their babies, benefit from magnesium sulfate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877- 1890. doi: 10.1016/s0140-6736(02)08778-0.
- Lake AJ, Al Hkabbaz A, Keeney R. Severe preeclampsia in the setting of myasthenia gravis. Case Rep Obstet Gynecol. 2017;9204930. doi: 10.1155/2017/9204930.
- Hurd WW, Ventolini G, Stolfi A. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;102: 196-197. doi: 10.1016/s0029-7844(03)00471-x.
- Scott JR. Safety of eliminating postpartum magnesium sulphate: intriguing but not yet proven. BJOG. 2018;125:1312. doi: 10.1111/1471 -0528.15317.
- Gestational hypertension and preeclampsia. ACOG Practice Bulletin No. 222. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2020;135:e237-e260. doi: 10.1097/AOG .0000000000003891.
- Ehrenberg H, Mercer BM. Abbreviated postpartum magnesium sulfate therapy for patients with mild preeclampsia: a randomized controlled trial. Obstet Gynecol. 2006;108:833-888. doi: 10.1097 /01.AOG.0000236493.35347.d8.
- Maia SB, Katz L, Neto CN, et al. Abbreviated (12- hour) versus traditional (24-hour) postpartum magnesium sulfate therapy in severe pre-eclampsia. Int J Gynaecol Obstet. 2014;126:260-264. doi: 10.1016/j.ijgo.2014.03.024.
- Anjum S, Rajaram GP, Bano I. Short-course (6-h) magnesium sulfate therapy in severe preeclampsia. Arch Gynecol Obstet. 2016;293:983-986. doi: 10.1007/s00404-015-3903-y.
- El-Khayat W, Atef A, Abdelatty S, et al. A novel protocol for postpartum magnesium sulphate in severe pre-eclampsia: a randomized controlled pilot trial. J Matern Fetal Neonatal Med. 2016;29: 154-158. doi: 10.3109/14767058.2014.991915.
- Vigil-De Gracia P, Ramirez R, Duran Y, et al. Magnesium sulfate for 6 vs 24 hours post-delivery in patients who received magnesium sulfate for less than 8 hours before birth: a randomized clinical trial. BMC Pregnancy Childbirth. 2017;17:241. doi: 10.1186/s12884-017-1424-3.
- Vigil-DeGracia P, Ludmir J, Ng J, et al. Is there benefit to continue magnesium sulphate postpartum in patients receiving magnesium sulphate before delivery? A randomized controlled study. BJOG. 2018;125:1304-1311. doi: 10.1111/1471 -0528.15320.
- Ascarelli MH, Johnson V, May WL, et al. Individually determined postpartum magnesium sulfate therapy with clinical parameters to safety and cost-effectively shorten treatment for preeclampsia. Am J Obstet Gynecol. 1998;179:952-956. doi: 10.1016/s0002-9378(98)70195-4.
- Isler CM, Barrilleaux PS, Rinehart BK, et al. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;101:66-69. doi: 10.1016/s0029 -7844(02)02317-7.
- Fontenot MT, Lewis DF, Frederick JB, et al. A prospective randomized trial of magnesium sulfate in severe preeclampsia: use of diuresis as a clinical parameter to determine the duration of postpartum therapy. Am J Obstet Gynecol. 2005;192:1788- 1793. doi: 10.1016/j.ajog.2004.12.056.
- Tsigas EZ. The Preeclampsia Foundation: the voice and views of the patient and family. Am J Obstet Gynecol. Epub August 23, 2021. doi: 10.1016/j.ajog.2020.10.053.
Preeclampsia complicates 3% to 8% of pregnancies.1-3 The incidence of preeclampsia is influenced by the clinical characteristics of the pregnant population, including the prevalence of overweight, obesity, chronic hypertension, diabetes, nulliparity, advanced maternal age, multiple gestations, kidney disease, and a history of preeclampsia in a prior pregnancy.4
Magnesium treatment reduces the rate of eclampsia among patients with preeclampsia
For patients with preeclampsia, magnesium treatment reduces the risk of seizure. In the Magpie trial, 9,992 pregnant patients were treated for 24 hours with magnesium or placebo.5 The magnesium treatment regimen was either a 4-g IV bolus over 10 to 15 minutes followed by a continuous infusion of 1 g/hr or an intramuscular regimen (10-g intramuscular loading dose followed by 5 g IM every 4 hours). Eclamptic seizures occurred in 0.8% and 1.9% of patients treated with magnesium or placebo, respectively (relative risk [RR], 0.42; 95% confidence interval [CI], 0.29 to 0.60). Among patients with a multiple gestation, the rate of eclampsia was 2% and 6% in the patients treated with magnesium or placebo, respectively. The number of patients who needed to be treated to prevent one eclamptic event was 63 and 109 for patients with preeclampsia with and without severe features, respectively. Intrapartum treatment with magnesium also reduced the risk of placental abruption from 3.2% for the patients receiving placebo to 2.0% among the patients treated with magnesium (RR, 0.67; 99% CI, 0.45- 0.89). Maternal death was reduced with magnesium treatment compared with placebo (0.2% vs 0.4%), but the difference was not statistically significant.
In the Magpie trial, side effects were reported by 24% and 5% of patients treated with magnesium and placebo, respectively. The most common side effects were flushing, nausea, vomiting, and muscle weakness. Of note, magnesium treatment is contraindicated in patients with myasthenia gravis because it can cause muscle weakness and hypoventilation.6 For patients with preeclampsia and myasthenia gravis, levetiracetam may be utilized to reduce the risk of seizure.6
Duration of postpartum magnesium treatment
There are no studies with a sufficient number of participants to definitively determine the optimal duration of postpartum magnesium therapy. A properly powered study would likely require more than 16,000 to 20,000 participants to identify clinically meaningful differences in the rate of postpartum eclampsia among patients treated with magnesium for 12 or 24 hours.7,8 It is unlikely that such a study will be completed. Hence, the duration of postpartum magnesium must be based on clinical judgment, balancing the risks and benefits of treatment.
The American College of Obstetricians and Gynecologists (ACOG) recommends continuing magnesium treatment for 24 hours postpartum. They advise, “For patients requiring cesarean delivery (before the onset of labor), the infusion should ideally begin before surgery and continue during surgery, as well as 24 hours afterwards. For patients who deliver vaginally, the infusion should continue for 24 hours after delivery.”9
Multiple randomized trials have reported on the outcomes associated with 12 hours versus 24 hours of postpartum magnesium therapy (TABLE). Because the rate of postpartum eclamptic seizure is very low, none of the studies were sufficiently powered to provide a definitive answer to the benefits and harms of the shorter versus longer time frame of magnesium therapy.10-15

Continue to: The harms of prolonged postpartum magnesium infusion...
The harms of prolonged postpartum magnesium infusion
The harms of prolonging treatment with postpartum magnesium infusion are generally not emphasized in the medical literature. However, side effects that can occur are flushing, nausea, vomiting, and muscle weakness, delayed early ambulation, delayed return to full diet, delayed discontinuation of a bladder catheter, and delayed initiation of breastfeeding.5,15 In one large clinical trial, 1,113 patients with preeclampsia with severe features who received intrapartum magnesium for ≥8 hours were randomized after birth to immediate discontinuation of magnesium or continuation of magnesium for 24 hours.15 There was 1 seizure in the group of 555 patients who received 24 hours of postpartum magnesium and 2 seizures in the group of 558 patients who received no magnesium after birth. In this trial, continuation of magnesium postpartum resulted in delayed initiation of breastfeeding and delayed ambulation.15
Balancing the benefits and harms of postpartum magnesium infusion
An important clinical point is that magnesium treatment will not prevent all seizures associated with preeclampsia; in the Magpie trial, among the 5,055 patients with preeclampsia treated with magnesium there were 40 (0.8%) seizures.5 Magnesium treatment will reduce but not eliminate the risk of seizure. Clinicians should have a plan to treat seizures that occur while a woman is being treated with magnesium.
In the absence of high-quality data to guide the duration of postpartum magnesium therapy it is best to use clinical parameters to balance the benefits and harms of postpartum magnesium treatment.16-18 Patients may want to participate in the decision about the duration of postpartum magnesium treatment after receiving counseling about the benefits and harms.
For patients with preeclampsia without severe features, many clinicians are no longer ordering intrapartum magnesium for prevention of seizures because they believe the risk of seizure in patients without severe disease is very low. Hence, these patients will not receive postpartum magnesium treatment unless they evolve to preeclampsia with severe features or develop a “red flag” warning postpartum (see below).
For patients with preeclampsia without severe features who received intrapartum magnesium, after birth, the magnesium infusion could be stopped immediately or within 12 hours of birth. For patients with preeclampsia without severe features, early termination of the magnesium infusion best balances the benefit of seizure reduction with the harms of delayed early ambulation, return to full diet, discontinuation of the bladder catheter, and initiation of breastfeeding.
For patients with preeclampsia with severe features, 24 hours of magnesium may best balance the benefits and harms of treatment. However, if the patient continues to have “red flag” findings, continued magnesium treatment beyond 24 hours may be warranted.
Red flag findings include: an eclamptic seizure before or after birth, ongoing or recurring severe headaches, visual scotomata, nausea, vomiting, epigastric pain, severe hypertension, oliguria, rising creatinine, or liver transaminases and declining platelet count.
The hypertensive diseases of pregnancy, including preeclampsia often appear suddenly and may evolve rapidly, threatening the health of both mother and fetus. A high level of suspicion that a hypertensive disease might be the cause of vague symptoms such as epigastric discomfort or headache may accelerate early diagnosis. Rapid treatment of severe hypertension with intravenous labetalol and hydralazine, and intrapartum plus postpartum administration of magnesium to prevent placental abruption and eclampsia will optimize patient outcomes. No patient, patient’s family members, or clinician, wants to experience the grief of a preventable maternal, fetal, or newborn death due to hypertension.19 Obstetricians, midwives, labor nurses, obstetrical anesthesiologists and doulas play key roles in preventing maternal, fetal, and newborn morbidity and death from hypertensive diseases of pregnancy. As a team we are the last line of defense protecting the health of our patients. ●
Preeclampsia complicates 3% to 8% of pregnancies.1-3 The incidence of preeclampsia is influenced by the clinical characteristics of the pregnant population, including the prevalence of overweight, obesity, chronic hypertension, diabetes, nulliparity, advanced maternal age, multiple gestations, kidney disease, and a history of preeclampsia in a prior pregnancy.4
Magnesium treatment reduces the rate of eclampsia among patients with preeclampsia
For patients with preeclampsia, magnesium treatment reduces the risk of seizure. In the Magpie trial, 9,992 pregnant patients were treated for 24 hours with magnesium or placebo.5 The magnesium treatment regimen was either a 4-g IV bolus over 10 to 15 minutes followed by a continuous infusion of 1 g/hr or an intramuscular regimen (10-g intramuscular loading dose followed by 5 g IM every 4 hours). Eclamptic seizures occurred in 0.8% and 1.9% of patients treated with magnesium or placebo, respectively (relative risk [RR], 0.42; 95% confidence interval [CI], 0.29 to 0.60). Among patients with a multiple gestation, the rate of eclampsia was 2% and 6% in the patients treated with magnesium or placebo, respectively. The number of patients who needed to be treated to prevent one eclamptic event was 63 and 109 for patients with preeclampsia with and without severe features, respectively. Intrapartum treatment with magnesium also reduced the risk of placental abruption from 3.2% for the patients receiving placebo to 2.0% among the patients treated with magnesium (RR, 0.67; 99% CI, 0.45- 0.89). Maternal death was reduced with magnesium treatment compared with placebo (0.2% vs 0.4%), but the difference was not statistically significant.
In the Magpie trial, side effects were reported by 24% and 5% of patients treated with magnesium and placebo, respectively. The most common side effects were flushing, nausea, vomiting, and muscle weakness. Of note, magnesium treatment is contraindicated in patients with myasthenia gravis because it can cause muscle weakness and hypoventilation.6 For patients with preeclampsia and myasthenia gravis, levetiracetam may be utilized to reduce the risk of seizure.6
Duration of postpartum magnesium treatment
There are no studies with a sufficient number of participants to definitively determine the optimal duration of postpartum magnesium therapy. A properly powered study would likely require more than 16,000 to 20,000 participants to identify clinically meaningful differences in the rate of postpartum eclampsia among patients treated with magnesium for 12 or 24 hours.7,8 It is unlikely that such a study will be completed. Hence, the duration of postpartum magnesium must be based on clinical judgment, balancing the risks and benefits of treatment.
The American College of Obstetricians and Gynecologists (ACOG) recommends continuing magnesium treatment for 24 hours postpartum. They advise, “For patients requiring cesarean delivery (before the onset of labor), the infusion should ideally begin before surgery and continue during surgery, as well as 24 hours afterwards. For patients who deliver vaginally, the infusion should continue for 24 hours after delivery.”9
Multiple randomized trials have reported on the outcomes associated with 12 hours versus 24 hours of postpartum magnesium therapy (TABLE). Because the rate of postpartum eclamptic seizure is very low, none of the studies were sufficiently powered to provide a definitive answer to the benefits and harms of the shorter versus longer time frame of magnesium therapy.10-15

Continue to: The harms of prolonged postpartum magnesium infusion...
The harms of prolonged postpartum magnesium infusion
The harms of prolonging treatment with postpartum magnesium infusion are generally not emphasized in the medical literature. However, side effects that can occur are flushing, nausea, vomiting, and muscle weakness, delayed early ambulation, delayed return to full diet, delayed discontinuation of a bladder catheter, and delayed initiation of breastfeeding.5,15 In one large clinical trial, 1,113 patients with preeclampsia with severe features who received intrapartum magnesium for ≥8 hours were randomized after birth to immediate discontinuation of magnesium or continuation of magnesium for 24 hours.15 There was 1 seizure in the group of 555 patients who received 24 hours of postpartum magnesium and 2 seizures in the group of 558 patients who received no magnesium after birth. In this trial, continuation of magnesium postpartum resulted in delayed initiation of breastfeeding and delayed ambulation.15
Balancing the benefits and harms of postpartum magnesium infusion
An important clinical point is that magnesium treatment will not prevent all seizures associated with preeclampsia; in the Magpie trial, among the 5,055 patients with preeclampsia treated with magnesium there were 40 (0.8%) seizures.5 Magnesium treatment will reduce but not eliminate the risk of seizure. Clinicians should have a plan to treat seizures that occur while a woman is being treated with magnesium.
In the absence of high-quality data to guide the duration of postpartum magnesium therapy it is best to use clinical parameters to balance the benefits and harms of postpartum magnesium treatment.16-18 Patients may want to participate in the decision about the duration of postpartum magnesium treatment after receiving counseling about the benefits and harms.
For patients with preeclampsia without severe features, many clinicians are no longer ordering intrapartum magnesium for prevention of seizures because they believe the risk of seizure in patients without severe disease is very low. Hence, these patients will not receive postpartum magnesium treatment unless they evolve to preeclampsia with severe features or develop a “red flag” warning postpartum (see below).
For patients with preeclampsia without severe features who received intrapartum magnesium, after birth, the magnesium infusion could be stopped immediately or within 12 hours of birth. For patients with preeclampsia without severe features, early termination of the magnesium infusion best balances the benefit of seizure reduction with the harms of delayed early ambulation, return to full diet, discontinuation of the bladder catheter, and initiation of breastfeeding.
For patients with preeclampsia with severe features, 24 hours of magnesium may best balance the benefits and harms of treatment. However, if the patient continues to have “red flag” findings, continued magnesium treatment beyond 24 hours may be warranted.
Red flag findings include: an eclamptic seizure before or after birth, ongoing or recurring severe headaches, visual scotomata, nausea, vomiting, epigastric pain, severe hypertension, oliguria, rising creatinine, or liver transaminases and declining platelet count.
The hypertensive diseases of pregnancy, including preeclampsia often appear suddenly and may evolve rapidly, threatening the health of both mother and fetus. A high level of suspicion that a hypertensive disease might be the cause of vague symptoms such as epigastric discomfort or headache may accelerate early diagnosis. Rapid treatment of severe hypertension with intravenous labetalol and hydralazine, and intrapartum plus postpartum administration of magnesium to prevent placental abruption and eclampsia will optimize patient outcomes. No patient, patient’s family members, or clinician, wants to experience the grief of a preventable maternal, fetal, or newborn death due to hypertension.19 Obstetricians, midwives, labor nurses, obstetrical anesthesiologists and doulas play key roles in preventing maternal, fetal, and newborn morbidity and death from hypertensive diseases of pregnancy. As a team we are the last line of defense protecting the health of our patients. ●
- World Health Organization. WHO International Collaborative Study of Hypertensive Disorders of Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. Am J Obstet Gynecol. 1988;158:80-83.
- Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209:544.e1-e12. doi: 10.1016 /j.ajog.2013.08.019.
- Mayrink K, Souza RT, Feitosa FE, et al. Incidence and risk factors for preeclampsia in a cohort of healthy nulliparous patients: a nested casecontrol study. Sci Rep. 2019;9:9517. doi: 10.1038 /s41598-019-46011-3.
- Bartsch E, Medcalf KE, Park AL, et al. High risk of pre-eclampsia identification group. BMJ. 2016;353:i1753. doi: 10.1136/bmj.i1753.
- Altman D, Carroli G, Duley L; The Magpie Trial Collaborative Group. Do patients with preeclampsia, and their babies, benefit from magnesium sulfate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877- 1890. doi: 10.1016/s0140-6736(02)08778-0.
- Lake AJ, Al Hkabbaz A, Keeney R. Severe preeclampsia in the setting of myasthenia gravis. Case Rep Obstet Gynecol. 2017;9204930. doi: 10.1155/2017/9204930.
- Hurd WW, Ventolini G, Stolfi A. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;102: 196-197. doi: 10.1016/s0029-7844(03)00471-x.
- Scott JR. Safety of eliminating postpartum magnesium sulphate: intriguing but not yet proven. BJOG. 2018;125:1312. doi: 10.1111/1471 -0528.15317.
- Gestational hypertension and preeclampsia. ACOG Practice Bulletin No. 222. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2020;135:e237-e260. doi: 10.1097/AOG .0000000000003891.
- Ehrenberg H, Mercer BM. Abbreviated postpartum magnesium sulfate therapy for patients with mild preeclampsia: a randomized controlled trial. Obstet Gynecol. 2006;108:833-888. doi: 10.1097 /01.AOG.0000236493.35347.d8.
- Maia SB, Katz L, Neto CN, et al. Abbreviated (12- hour) versus traditional (24-hour) postpartum magnesium sulfate therapy in severe pre-eclampsia. Int J Gynaecol Obstet. 2014;126:260-264. doi: 10.1016/j.ijgo.2014.03.024.
- Anjum S, Rajaram GP, Bano I. Short-course (6-h) magnesium sulfate therapy in severe preeclampsia. Arch Gynecol Obstet. 2016;293:983-986. doi: 10.1007/s00404-015-3903-y.
- El-Khayat W, Atef A, Abdelatty S, et al. A novel protocol for postpartum magnesium sulphate in severe pre-eclampsia: a randomized controlled pilot trial. J Matern Fetal Neonatal Med. 2016;29: 154-158. doi: 10.3109/14767058.2014.991915.
- Vigil-De Gracia P, Ramirez R, Duran Y, et al. Magnesium sulfate for 6 vs 24 hours post-delivery in patients who received magnesium sulfate for less than 8 hours before birth: a randomized clinical trial. BMC Pregnancy Childbirth. 2017;17:241. doi: 10.1186/s12884-017-1424-3.
- Vigil-DeGracia P, Ludmir J, Ng J, et al. Is there benefit to continue magnesium sulphate postpartum in patients receiving magnesium sulphate before delivery? A randomized controlled study. BJOG. 2018;125:1304-1311. doi: 10.1111/1471 -0528.15320.
- Ascarelli MH, Johnson V, May WL, et al. Individually determined postpartum magnesium sulfate therapy with clinical parameters to safety and cost-effectively shorten treatment for preeclampsia. Am J Obstet Gynecol. 1998;179:952-956. doi: 10.1016/s0002-9378(98)70195-4.
- Isler CM, Barrilleaux PS, Rinehart BK, et al. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;101:66-69. doi: 10.1016/s0029 -7844(02)02317-7.
- Fontenot MT, Lewis DF, Frederick JB, et al. A prospective randomized trial of magnesium sulfate in severe preeclampsia: use of diuresis as a clinical parameter to determine the duration of postpartum therapy. Am J Obstet Gynecol. 2005;192:1788- 1793. doi: 10.1016/j.ajog.2004.12.056.
- Tsigas EZ. The Preeclampsia Foundation: the voice and views of the patient and family. Am J Obstet Gynecol. Epub August 23, 2021. doi: 10.1016/j.ajog.2020.10.053.
- World Health Organization. WHO International Collaborative Study of Hypertensive Disorders of Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. Am J Obstet Gynecol. 1988;158:80-83.
- Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209:544.e1-e12. doi: 10.1016 /j.ajog.2013.08.019.
- Mayrink K, Souza RT, Feitosa FE, et al. Incidence and risk factors for preeclampsia in a cohort of healthy nulliparous patients: a nested casecontrol study. Sci Rep. 2019;9:9517. doi: 10.1038 /s41598-019-46011-3.
- Bartsch E, Medcalf KE, Park AL, et al. High risk of pre-eclampsia identification group. BMJ. 2016;353:i1753. doi: 10.1136/bmj.i1753.
- Altman D, Carroli G, Duley L; The Magpie Trial Collaborative Group. Do patients with preeclampsia, and their babies, benefit from magnesium sulfate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877- 1890. doi: 10.1016/s0140-6736(02)08778-0.
- Lake AJ, Al Hkabbaz A, Keeney R. Severe preeclampsia in the setting of myasthenia gravis. Case Rep Obstet Gynecol. 2017;9204930. doi: 10.1155/2017/9204930.
- Hurd WW, Ventolini G, Stolfi A. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;102: 196-197. doi: 10.1016/s0029-7844(03)00471-x.
- Scott JR. Safety of eliminating postpartum magnesium sulphate: intriguing but not yet proven. BJOG. 2018;125:1312. doi: 10.1111/1471 -0528.15317.
- Gestational hypertension and preeclampsia. ACOG Practice Bulletin No. 222. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2020;135:e237-e260. doi: 10.1097/AOG .0000000000003891.
- Ehrenberg H, Mercer BM. Abbreviated postpartum magnesium sulfate therapy for patients with mild preeclampsia: a randomized controlled trial. Obstet Gynecol. 2006;108:833-888. doi: 10.1097 /01.AOG.0000236493.35347.d8.
- Maia SB, Katz L, Neto CN, et al. Abbreviated (12- hour) versus traditional (24-hour) postpartum magnesium sulfate therapy in severe pre-eclampsia. Int J Gynaecol Obstet. 2014;126:260-264. doi: 10.1016/j.ijgo.2014.03.024.
- Anjum S, Rajaram GP, Bano I. Short-course (6-h) magnesium sulfate therapy in severe preeclampsia. Arch Gynecol Obstet. 2016;293:983-986. doi: 10.1007/s00404-015-3903-y.
- El-Khayat W, Atef A, Abdelatty S, et al. A novel protocol for postpartum magnesium sulphate in severe pre-eclampsia: a randomized controlled pilot trial. J Matern Fetal Neonatal Med. 2016;29: 154-158. doi: 10.3109/14767058.2014.991915.
- Vigil-De Gracia P, Ramirez R, Duran Y, et al. Magnesium sulfate for 6 vs 24 hours post-delivery in patients who received magnesium sulfate for less than 8 hours before birth: a randomized clinical trial. BMC Pregnancy Childbirth. 2017;17:241. doi: 10.1186/s12884-017-1424-3.
- Vigil-DeGracia P, Ludmir J, Ng J, et al. Is there benefit to continue magnesium sulphate postpartum in patients receiving magnesium sulphate before delivery? A randomized controlled study. BJOG. 2018;125:1304-1311. doi: 10.1111/1471 -0528.15320.
- Ascarelli MH, Johnson V, May WL, et al. Individually determined postpartum magnesium sulfate therapy with clinical parameters to safety and cost-effectively shorten treatment for preeclampsia. Am J Obstet Gynecol. 1998;179:952-956. doi: 10.1016/s0002-9378(98)70195-4.
- Isler CM, Barrilleaux PS, Rinehart BK, et al. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;101:66-69. doi: 10.1016/s0029 -7844(02)02317-7.
- Fontenot MT, Lewis DF, Frederick JB, et al. A prospective randomized trial of magnesium sulfate in severe preeclampsia: use of diuresis as a clinical parameter to determine the duration of postpartum therapy. Am J Obstet Gynecol. 2005;192:1788- 1793. doi: 10.1016/j.ajog.2004.12.056.
- Tsigas EZ. The Preeclampsia Foundation: the voice and views of the patient and family. Am J Obstet Gynecol. Epub August 23, 2021. doi: 10.1016/j.ajog.2020.10.053.
New blood test could identify pregnant women who are at risk of preeclampsia
Pregnant women who are at risk of preeclampsia can now be identified early before symptoms develop, finds new research from Kings College London and Guy’s and St Thomas’ NHS Foundation Trust, published in Nature.
The study, supported by the National Institute for Health Research and in partnership with the Mirvie RNA platform, analyzed the genetic material from over 2,500 blood samples of pregnant women from eight independent cohorts with multiple demographics, including socioeconomic background, geographic location, ethnicity, and nationality, collected 14.5 weeks before delivery.
“Because the study drew upon samples for a diverse group of women, including participants recruited across King’s Health Partners, the molecular signature is very reliable and has potential to outperform currently available tests,” said Rachel Tribe, PhD, department of women and children’s health, King’s College London.
Researchers used plasma cell-free RNA (cfRNA) transcripts to examine the standard molecular mechanism between the fetus, maternal, and placental tissues in order to determine fetal development and healthy pregnancy progression. Deviation from the standard cfRNA expression was also observed to establish the molecular pathway for those at risk of preeclampsia before clinical presentation.
A cfRNA signal from a single blood sample showed a 32.3% positive-predictive value and 75% sensitivity, which exceeds current positive-predictive values from recent clinical state-of-the-art models.
In addition, 73% of participants with a positive-predictive value were identified “as destined to have a medically indicated preterm birth over 3 months in advance of the preeclampsia symptoms,” said the authors.
With up to 1 in 12 pregnancies affected by preeclampsia, and the diagnosis most often only being made in the third trimester, these results provide a promising outlook for pregnant women “so that they can be more closely monitored and treated by the clinicians involved,” commented Dr. Tribe.
“We are now focused on ongoing clinical research to further validate these results and improve the understanding of other pregnancy complications,” she said. “As a scientist, it was also extremely interesting to see that the molecular signature tells us something about mechanisms associated with health in pregnancy and complications including preeclampsia; such knowledge will aid development of treatment strategies in the future.”
A version of this article first appeared on Medscape.com.
Pregnant women who are at risk of preeclampsia can now be identified early before symptoms develop, finds new research from Kings College London and Guy’s and St Thomas’ NHS Foundation Trust, published in Nature.
The study, supported by the National Institute for Health Research and in partnership with the Mirvie RNA platform, analyzed the genetic material from over 2,500 blood samples of pregnant women from eight independent cohorts with multiple demographics, including socioeconomic background, geographic location, ethnicity, and nationality, collected 14.5 weeks before delivery.
“Because the study drew upon samples for a diverse group of women, including participants recruited across King’s Health Partners, the molecular signature is very reliable and has potential to outperform currently available tests,” said Rachel Tribe, PhD, department of women and children’s health, King’s College London.
Researchers used plasma cell-free RNA (cfRNA) transcripts to examine the standard molecular mechanism between the fetus, maternal, and placental tissues in order to determine fetal development and healthy pregnancy progression. Deviation from the standard cfRNA expression was also observed to establish the molecular pathway for those at risk of preeclampsia before clinical presentation.
A cfRNA signal from a single blood sample showed a 32.3% positive-predictive value and 75% sensitivity, which exceeds current positive-predictive values from recent clinical state-of-the-art models.
In addition, 73% of participants with a positive-predictive value were identified “as destined to have a medically indicated preterm birth over 3 months in advance of the preeclampsia symptoms,” said the authors.
With up to 1 in 12 pregnancies affected by preeclampsia, and the diagnosis most often only being made in the third trimester, these results provide a promising outlook for pregnant women “so that they can be more closely monitored and treated by the clinicians involved,” commented Dr. Tribe.
“We are now focused on ongoing clinical research to further validate these results and improve the understanding of other pregnancy complications,” she said. “As a scientist, it was also extremely interesting to see that the molecular signature tells us something about mechanisms associated with health in pregnancy and complications including preeclampsia; such knowledge will aid development of treatment strategies in the future.”
A version of this article first appeared on Medscape.com.
Pregnant women who are at risk of preeclampsia can now be identified early before symptoms develop, finds new research from Kings College London and Guy’s and St Thomas’ NHS Foundation Trust, published in Nature.
The study, supported by the National Institute for Health Research and in partnership with the Mirvie RNA platform, analyzed the genetic material from over 2,500 blood samples of pregnant women from eight independent cohorts with multiple demographics, including socioeconomic background, geographic location, ethnicity, and nationality, collected 14.5 weeks before delivery.
“Because the study drew upon samples for a diverse group of women, including participants recruited across King’s Health Partners, the molecular signature is very reliable and has potential to outperform currently available tests,” said Rachel Tribe, PhD, department of women and children’s health, King’s College London.
Researchers used plasma cell-free RNA (cfRNA) transcripts to examine the standard molecular mechanism between the fetus, maternal, and placental tissues in order to determine fetal development and healthy pregnancy progression. Deviation from the standard cfRNA expression was also observed to establish the molecular pathway for those at risk of preeclampsia before clinical presentation.
A cfRNA signal from a single blood sample showed a 32.3% positive-predictive value and 75% sensitivity, which exceeds current positive-predictive values from recent clinical state-of-the-art models.
In addition, 73% of participants with a positive-predictive value were identified “as destined to have a medically indicated preterm birth over 3 months in advance of the preeclampsia symptoms,” said the authors.
With up to 1 in 12 pregnancies affected by preeclampsia, and the diagnosis most often only being made in the third trimester, these results provide a promising outlook for pregnant women “so that they can be more closely monitored and treated by the clinicians involved,” commented Dr. Tribe.
“We are now focused on ongoing clinical research to further validate these results and improve the understanding of other pregnancy complications,” she said. “As a scientist, it was also extremely interesting to see that the molecular signature tells us something about mechanisms associated with health in pregnancy and complications including preeclampsia; such knowledge will aid development of treatment strategies in the future.”
A version of this article first appeared on Medscape.com.
FROM NATURE
Statin therapy seems safe in pregnancy
Statins may be safe when used during pregnancy, with no increase in risk for fetal anomalies, although there may be a higher risk for low birth weight and preterm labor, results of a large study from Taiwan suggest.
The Food and Drug Administration relaxed its warning on statins in July 2021, removing the drug’s blanket contraindication in all pregnant women.
Removal of the broadly worded contraindication should “enable health care professionals and patients to make individual decisions about benefit and risk, especially for those at very high risk of heart attack or stroke,” the FDA said in their announcement.
“Our findings suggested that statins may be used during pregnancy with no increase in the rate of congenital anomalies,” wrote Jui-Chun Chang, MD, from Taichung Veterans General Hospital, Taiwan, and colleagues in the new study, published online Dec. 30, 2021, in JAMA Network Open.
“For pregnant women at low risk, statins should be used carefully after assessing the risks of low birth weight and preterm birth,” they said. “For women with dyslipidemia or high-risk cardiovascular disease, as well as those who use statins before conception, statins may be continuously used with no increased risks of neonatal adverse effects.”
The study included more than 1.4 million pregnant women aged 18 years and older who gave birth to their first child between 2004 and 2014.
A total of 469 women (mean age, 32.6 years; mean gestational age, 38.4 weeks) who used statins during pregnancy were compared with 4,690 matched controls who had no statin exposure during pregnancy.
After controlling for maternal comorbidities and age, women who used statins during pregnancy were more apt to have low-birth-weight babies weighing less than 2,500 g (risk ratio, 1.51; 95% confidence interval, 1.05-2.16) and to deliver preterm (RR, 1.99; 95% CI, 1.46-2.71).
The statin-exposed babies were also more likely to have a lower 1-minute Apgar score (RR, 1.83; 95% CI, 1.04-3.20). Importantly, however, there was no increase in risk for fetal anomalies in the statin-exposed infants, the researchers said.
In addition, for women who used statins for more than 3 months prior to pregnancy, maintaining statin use during pregnancy did not increase the risk for adverse neonatal outcomes, including congenital anomalies, low birth weight, preterm birth, very low birth weight, low Apgar scores, and fetal distress.
The researchers called for further studies to confirm their observations.
Funding for the study was provided by Taichung Veterans General Hospital. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Statins may be safe when used during pregnancy, with no increase in risk for fetal anomalies, although there may be a higher risk for low birth weight and preterm labor, results of a large study from Taiwan suggest.
The Food and Drug Administration relaxed its warning on statins in July 2021, removing the drug’s blanket contraindication in all pregnant women.
Removal of the broadly worded contraindication should “enable health care professionals and patients to make individual decisions about benefit and risk, especially for those at very high risk of heart attack or stroke,” the FDA said in their announcement.
“Our findings suggested that statins may be used during pregnancy with no increase in the rate of congenital anomalies,” wrote Jui-Chun Chang, MD, from Taichung Veterans General Hospital, Taiwan, and colleagues in the new study, published online Dec. 30, 2021, in JAMA Network Open.
“For pregnant women at low risk, statins should be used carefully after assessing the risks of low birth weight and preterm birth,” they said. “For women with dyslipidemia or high-risk cardiovascular disease, as well as those who use statins before conception, statins may be continuously used with no increased risks of neonatal adverse effects.”
The study included more than 1.4 million pregnant women aged 18 years and older who gave birth to their first child between 2004 and 2014.
A total of 469 women (mean age, 32.6 years; mean gestational age, 38.4 weeks) who used statins during pregnancy were compared with 4,690 matched controls who had no statin exposure during pregnancy.
After controlling for maternal comorbidities and age, women who used statins during pregnancy were more apt to have low-birth-weight babies weighing less than 2,500 g (risk ratio, 1.51; 95% confidence interval, 1.05-2.16) and to deliver preterm (RR, 1.99; 95% CI, 1.46-2.71).
The statin-exposed babies were also more likely to have a lower 1-minute Apgar score (RR, 1.83; 95% CI, 1.04-3.20). Importantly, however, there was no increase in risk for fetal anomalies in the statin-exposed infants, the researchers said.
In addition, for women who used statins for more than 3 months prior to pregnancy, maintaining statin use during pregnancy did not increase the risk for adverse neonatal outcomes, including congenital anomalies, low birth weight, preterm birth, very low birth weight, low Apgar scores, and fetal distress.
The researchers called for further studies to confirm their observations.
Funding for the study was provided by Taichung Veterans General Hospital. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Statins may be safe when used during pregnancy, with no increase in risk for fetal anomalies, although there may be a higher risk for low birth weight and preterm labor, results of a large study from Taiwan suggest.
The Food and Drug Administration relaxed its warning on statins in July 2021, removing the drug’s blanket contraindication in all pregnant women.
Removal of the broadly worded contraindication should “enable health care professionals and patients to make individual decisions about benefit and risk, especially for those at very high risk of heart attack or stroke,” the FDA said in their announcement.
“Our findings suggested that statins may be used during pregnancy with no increase in the rate of congenital anomalies,” wrote Jui-Chun Chang, MD, from Taichung Veterans General Hospital, Taiwan, and colleagues in the new study, published online Dec. 30, 2021, in JAMA Network Open.
“For pregnant women at low risk, statins should be used carefully after assessing the risks of low birth weight and preterm birth,” they said. “For women with dyslipidemia or high-risk cardiovascular disease, as well as those who use statins before conception, statins may be continuously used with no increased risks of neonatal adverse effects.”
The study included more than 1.4 million pregnant women aged 18 years and older who gave birth to their first child between 2004 and 2014.
A total of 469 women (mean age, 32.6 years; mean gestational age, 38.4 weeks) who used statins during pregnancy were compared with 4,690 matched controls who had no statin exposure during pregnancy.
After controlling for maternal comorbidities and age, women who used statins during pregnancy were more apt to have low-birth-weight babies weighing less than 2,500 g (risk ratio, 1.51; 95% confidence interval, 1.05-2.16) and to deliver preterm (RR, 1.99; 95% CI, 1.46-2.71).
The statin-exposed babies were also more likely to have a lower 1-minute Apgar score (RR, 1.83; 95% CI, 1.04-3.20). Importantly, however, there was no increase in risk for fetal anomalies in the statin-exposed infants, the researchers said.
In addition, for women who used statins for more than 3 months prior to pregnancy, maintaining statin use during pregnancy did not increase the risk for adverse neonatal outcomes, including congenital anomalies, low birth weight, preterm birth, very low birth weight, low Apgar scores, and fetal distress.
The researchers called for further studies to confirm their observations.
Funding for the study was provided by Taichung Veterans General Hospital. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Even healthy Black and Hispanic women have more cesareans than White women
New research offers more insight into potentially dangerous racial disparities in cesarean deliveries: In first-time live births, healthy African-American and Hispanic mothers were 21% and 26% more likely than White mothers, respectively, to deliver by cesarean section despite being low risk. The higher number of cesareans appeared to boost their risk of morbidity.
“A 20% increased odds of cesarean among otherwise healthy, low-risk, nulliparous individuals at term – with limited medical or obstetric explanation – is a significant concern, especially when considering that cesarean is the most common surgical procedure in the U.S.,” said study author Michelle P. Debbink, MD, PhD, an assistant professor with the department of obstetrics and gynecology at the University of Utah, in an interview.
Dr. Debbink and colleagues launched the study, published in the Jan. 2022 issue of Obstetrics & Gynecology, to better understand the racial gap in cesarean sections, which are considered riskier than vaginal deliveries. “Several studies have shown that Black women undergo cesarean more frequently than non-Hispanic White women. Numerous studies also show that Hispanic/Latina women undergo cesarean more frequently than White women, although these data are a bit more mixed,” she said. “What we don’t know, however, is why these differences occur and whether there are clues in the data which can point us toward interventions to close the gap.”
One theory, she said, is that Black and Hispanic women have more comorbidities and therefore more cesareans. To test that idea, the researchers found a cohort of healthy women in a randomized trial that studied the induction of labor.
For the study, they focused on 5,759 women (24.3% Black, 30% Hispanic, 46.6% White). Major differences between the groups included maternal age (average = 21 for Black, 22 for Hispanic, and 26 for White, P < .001), private insurance (17% for Black and Hispanic, 75% for White; P < .001), and full or part-time employment (37% for Black, 31% for Hispanic, and 71% for White; P < .001).
A total of 1,158 of the women (20.1%) underwent cesarean deliveries, accounting for 23% of deliveries by Black women, 22.8% of those by Hispanic women, and 17.6% of those by White women (P < .001). Black women were 21% more likely than White women to give birth via cesarean (adjusted relative risk = 1.21, 95% CI: 1.03-1.42) and Hispanic women were 26% more likely (aRR = 1.26, 95% CI: 1.08-1.46).
The study doesn’t explore why Black and Hispanic women have more cesarean deliveries. However, Dr. Debbink said, “we hypothesize that the difference likely stems more from differing treatment of Black or Hispanic individuals during labor.” It’s unlikely, she said, that these women are more likely to prefer cesarean sections. For one thing, she said, other research hasn’t shown a difference in preferences by race.
The researchers also analyzed maternal morbidity, defined as “transfusion of 4 or more units of red blood cells, any transfusion of other products, postpartum infection, intensive care unit admission, hysterectomy, venous thromboembolism, or maternal death.”
The study found that while few women (2.3%) suffered from morbidity, Black (aRR = 2.05, 95% CI: 1.21-3.47) and Hispanic (aRR = 1.92, 95% CI: 1.17-3.14) women were more likely to suffer from it than White women.
The researchers report that “cesarean birth accounted for an estimated 15.8% (95% CI: 2.1%-48.7%) and 16.5% (95% CI: 4.0%-44.0%) of excess maternal morbidity among non-Hispanic Black and Hispanic people, respectively.”
“Both endometritis and wound complications are much more common among individuals with cesarean, and blood clots, hysterectomy, and ICU admission are also more common after cesarean compared with vaginal delivery,” Dr. Debbink said.
The message of the study, she said, is that the health care system “perpetuates gaps in cesarean delivery for Black and Hispanic individuals compared to White individuals” even in low-risk, first-time live births. “We do not yet know exactly what the right levers are to address this gap, but it is important that we ob-gyns examine our practice patterns and our hospitals’ practice patterns to ensure equity for all our patients.”
Rebecca Delafield, PhD, an assistant professor of Native Hawaiian Health at the University of Hawaii, praised the study as “well-conducted” in an interview. “I agree with the assessment that while the cesarean delivery accounts for a modest proportion of excess morbidity in this study, the impact at the population level is significant,” said Dr. Delafield, who studies health disparities and didn’t take part in the study. “Delivery is complex and the causes of disparities observed are likely multifactorial, therefore research such as this is necessary and compelling.”
She added: “It is becoming increasingly evident that studies investigating racial/ethnic disparities in cesarean delivery and other maternal health outcomes must look beyond maternal behavioral or medical risk factors – e.g., obesity or hypertension – and consider the contribution of a broader set of factors, including societal prejudices.”
The study is funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Center for Advancing Translational Sciences. One study author reports funding from GlaxoSmithKline, Pfizer, Moderna, and UpToDate (contributor) and from the Centers for Disease Control and Prevention (via her institution). Dr. Debbink, the other authors, and Dr. Delafield report no disclosures.
New research offers more insight into potentially dangerous racial disparities in cesarean deliveries: In first-time live births, healthy African-American and Hispanic mothers were 21% and 26% more likely than White mothers, respectively, to deliver by cesarean section despite being low risk. The higher number of cesareans appeared to boost their risk of morbidity.
“A 20% increased odds of cesarean among otherwise healthy, low-risk, nulliparous individuals at term – with limited medical or obstetric explanation – is a significant concern, especially when considering that cesarean is the most common surgical procedure in the U.S.,” said study author Michelle P. Debbink, MD, PhD, an assistant professor with the department of obstetrics and gynecology at the University of Utah, in an interview.
Dr. Debbink and colleagues launched the study, published in the Jan. 2022 issue of Obstetrics & Gynecology, to better understand the racial gap in cesarean sections, which are considered riskier than vaginal deliveries. “Several studies have shown that Black women undergo cesarean more frequently than non-Hispanic White women. Numerous studies also show that Hispanic/Latina women undergo cesarean more frequently than White women, although these data are a bit more mixed,” she said. “What we don’t know, however, is why these differences occur and whether there are clues in the data which can point us toward interventions to close the gap.”
One theory, she said, is that Black and Hispanic women have more comorbidities and therefore more cesareans. To test that idea, the researchers found a cohort of healthy women in a randomized trial that studied the induction of labor.
For the study, they focused on 5,759 women (24.3% Black, 30% Hispanic, 46.6% White). Major differences between the groups included maternal age (average = 21 for Black, 22 for Hispanic, and 26 for White, P < .001), private insurance (17% for Black and Hispanic, 75% for White; P < .001), and full or part-time employment (37% for Black, 31% for Hispanic, and 71% for White; P < .001).
A total of 1,158 of the women (20.1%) underwent cesarean deliveries, accounting for 23% of deliveries by Black women, 22.8% of those by Hispanic women, and 17.6% of those by White women (P < .001). Black women were 21% more likely than White women to give birth via cesarean (adjusted relative risk = 1.21, 95% CI: 1.03-1.42) and Hispanic women were 26% more likely (aRR = 1.26, 95% CI: 1.08-1.46).
The study doesn’t explore why Black and Hispanic women have more cesarean deliveries. However, Dr. Debbink said, “we hypothesize that the difference likely stems more from differing treatment of Black or Hispanic individuals during labor.” It’s unlikely, she said, that these women are more likely to prefer cesarean sections. For one thing, she said, other research hasn’t shown a difference in preferences by race.
The researchers also analyzed maternal morbidity, defined as “transfusion of 4 or more units of red blood cells, any transfusion of other products, postpartum infection, intensive care unit admission, hysterectomy, venous thromboembolism, or maternal death.”
The study found that while few women (2.3%) suffered from morbidity, Black (aRR = 2.05, 95% CI: 1.21-3.47) and Hispanic (aRR = 1.92, 95% CI: 1.17-3.14) women were more likely to suffer from it than White women.
The researchers report that “cesarean birth accounted for an estimated 15.8% (95% CI: 2.1%-48.7%) and 16.5% (95% CI: 4.0%-44.0%) of excess maternal morbidity among non-Hispanic Black and Hispanic people, respectively.”
“Both endometritis and wound complications are much more common among individuals with cesarean, and blood clots, hysterectomy, and ICU admission are also more common after cesarean compared with vaginal delivery,” Dr. Debbink said.
The message of the study, she said, is that the health care system “perpetuates gaps in cesarean delivery for Black and Hispanic individuals compared to White individuals” even in low-risk, first-time live births. “We do not yet know exactly what the right levers are to address this gap, but it is important that we ob-gyns examine our practice patterns and our hospitals’ practice patterns to ensure equity for all our patients.”
Rebecca Delafield, PhD, an assistant professor of Native Hawaiian Health at the University of Hawaii, praised the study as “well-conducted” in an interview. “I agree with the assessment that while the cesarean delivery accounts for a modest proportion of excess morbidity in this study, the impact at the population level is significant,” said Dr. Delafield, who studies health disparities and didn’t take part in the study. “Delivery is complex and the causes of disparities observed are likely multifactorial, therefore research such as this is necessary and compelling.”
She added: “It is becoming increasingly evident that studies investigating racial/ethnic disparities in cesarean delivery and other maternal health outcomes must look beyond maternal behavioral or medical risk factors – e.g., obesity or hypertension – and consider the contribution of a broader set of factors, including societal prejudices.”
The study is funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Center for Advancing Translational Sciences. One study author reports funding from GlaxoSmithKline, Pfizer, Moderna, and UpToDate (contributor) and from the Centers for Disease Control and Prevention (via her institution). Dr. Debbink, the other authors, and Dr. Delafield report no disclosures.
New research offers more insight into potentially dangerous racial disparities in cesarean deliveries: In first-time live births, healthy African-American and Hispanic mothers were 21% and 26% more likely than White mothers, respectively, to deliver by cesarean section despite being low risk. The higher number of cesareans appeared to boost their risk of morbidity.
“A 20% increased odds of cesarean among otherwise healthy, low-risk, nulliparous individuals at term – with limited medical or obstetric explanation – is a significant concern, especially when considering that cesarean is the most common surgical procedure in the U.S.,” said study author Michelle P. Debbink, MD, PhD, an assistant professor with the department of obstetrics and gynecology at the University of Utah, in an interview.
Dr. Debbink and colleagues launched the study, published in the Jan. 2022 issue of Obstetrics & Gynecology, to better understand the racial gap in cesarean sections, which are considered riskier than vaginal deliveries. “Several studies have shown that Black women undergo cesarean more frequently than non-Hispanic White women. Numerous studies also show that Hispanic/Latina women undergo cesarean more frequently than White women, although these data are a bit more mixed,” she said. “What we don’t know, however, is why these differences occur and whether there are clues in the data which can point us toward interventions to close the gap.”
One theory, she said, is that Black and Hispanic women have more comorbidities and therefore more cesareans. To test that idea, the researchers found a cohort of healthy women in a randomized trial that studied the induction of labor.
For the study, they focused on 5,759 women (24.3% Black, 30% Hispanic, 46.6% White). Major differences between the groups included maternal age (average = 21 for Black, 22 for Hispanic, and 26 for White, P < .001), private insurance (17% for Black and Hispanic, 75% for White; P < .001), and full or part-time employment (37% for Black, 31% for Hispanic, and 71% for White; P < .001).
A total of 1,158 of the women (20.1%) underwent cesarean deliveries, accounting for 23% of deliveries by Black women, 22.8% of those by Hispanic women, and 17.6% of those by White women (P < .001). Black women were 21% more likely than White women to give birth via cesarean (adjusted relative risk = 1.21, 95% CI: 1.03-1.42) and Hispanic women were 26% more likely (aRR = 1.26, 95% CI: 1.08-1.46).
The study doesn’t explore why Black and Hispanic women have more cesarean deliveries. However, Dr. Debbink said, “we hypothesize that the difference likely stems more from differing treatment of Black or Hispanic individuals during labor.” It’s unlikely, she said, that these women are more likely to prefer cesarean sections. For one thing, she said, other research hasn’t shown a difference in preferences by race.
The researchers also analyzed maternal morbidity, defined as “transfusion of 4 or more units of red blood cells, any transfusion of other products, postpartum infection, intensive care unit admission, hysterectomy, venous thromboembolism, or maternal death.”
The study found that while few women (2.3%) suffered from morbidity, Black (aRR = 2.05, 95% CI: 1.21-3.47) and Hispanic (aRR = 1.92, 95% CI: 1.17-3.14) women were more likely to suffer from it than White women.
The researchers report that “cesarean birth accounted for an estimated 15.8% (95% CI: 2.1%-48.7%) and 16.5% (95% CI: 4.0%-44.0%) of excess maternal morbidity among non-Hispanic Black and Hispanic people, respectively.”
“Both endometritis and wound complications are much more common among individuals with cesarean, and blood clots, hysterectomy, and ICU admission are also more common after cesarean compared with vaginal delivery,” Dr. Debbink said.
The message of the study, she said, is that the health care system “perpetuates gaps in cesarean delivery for Black and Hispanic individuals compared to White individuals” even in low-risk, first-time live births. “We do not yet know exactly what the right levers are to address this gap, but it is important that we ob-gyns examine our practice patterns and our hospitals’ practice patterns to ensure equity for all our patients.”
Rebecca Delafield, PhD, an assistant professor of Native Hawaiian Health at the University of Hawaii, praised the study as “well-conducted” in an interview. “I agree with the assessment that while the cesarean delivery accounts for a modest proportion of excess morbidity in this study, the impact at the population level is significant,” said Dr. Delafield, who studies health disparities and didn’t take part in the study. “Delivery is complex and the causes of disparities observed are likely multifactorial, therefore research such as this is necessary and compelling.”
She added: “It is becoming increasingly evident that studies investigating racial/ethnic disparities in cesarean delivery and other maternal health outcomes must look beyond maternal behavioral or medical risk factors – e.g., obesity or hypertension – and consider the contribution of a broader set of factors, including societal prejudices.”
The study is funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Center for Advancing Translational Sciences. One study author reports funding from GlaxoSmithKline, Pfizer, Moderna, and UpToDate (contributor) and from the Centers for Disease Control and Prevention (via her institution). Dr. Debbink, the other authors, and Dr. Delafield report no disclosures.
FROM OBSTETRICS & GYNECOLOGY
FDA agrees that mifepristone is safe enough to dispense by mail
The Food and Drug Administration has announced that women no longer will have to pick up the abortion pill mifepristone (Mifeprex) in person at certain certified sites and can get a prescription via an online consultation and delivery through the mail.
In April 2021, the FDA lifted the in-person dispensing requirement for mifepristone for the duration of the COVID-19 pandemic and in December the agency made that decision permanent.
As this news organization reported on April 12, 2021, acting commissioner of food and drugs, Janet Woodcock, MD, stated that the FDA would “permit the dispensing of mifepristone through the mail when done by or under the supervision of a certified prescriber; or through a mail-order pharmacy under the supervision of a certified prescriber.”
That decision came after suspension of the in-person dispensing requirement in response to COVID-19 safety concerns for patients as well as providers associated with in-person clinic visits.
Decision comes amid Supreme Court debate
The FDA decision comes as the Supreme Court nears a decision on whether to overturn its 1973 ruling on Roe v. Wade.
Additionally, the Supreme Court on returned a lawsuit over Texas’ ban on abortions after 6 weeks to a federal appeals court that has twice allowed the law to stay in effect, rather than to a district judge who wanted it blocked.
Alexis McGill Johnson, president and CEO, of the Planned Parenthood Federation of America, said in a statement, “Abortion is time sensitive, essential health care, and this decision will remove a sometimes insurmountable barrier for patients seeking an abortion. With abortion rights at risk like never before, the FDA’s decision is a long overdue step toward expanding people’s access to safe medication abortion.”
Georgeanne Usova, senior legislative counsel at the American Civil Liberties Union told CNBC News: “The FDA’s decision will come as a tremendous relief for countless abortion and miscarriage patients.”
Catherine D. Cansino, MD, MPH, associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis, and member of the editorial advisory board for ObGyn News said in an interview: “I think that this change is a long time coming and speaks to the fact that science matters and medicine prevails over politics. We need to protect health rights first!”
Others expressed doubt or outrage.
Fidelma Rigby, MD, a professor in the department of obstetrics and gynecology, division of maternal fetal medicine, Virginia Commonwealth University Medical Center, Richmond, said in an interview: “My concern is that what if there is an ectopic pregnancy? I’m not as enthusiastic as some of my partners would be about this announcement.”
“The FDA’s decision today places women at risk,” said Carol Tobias, president of the National Right to Life Committee. “These changes do not make this abortion process safer for women. What these changes do is make the process easier for the abortion industry.”
The antiabortion groups Charlotte Lozier Institute and the Susan B. Anthony List were among other organizations issuing statements against Dec. 16’s FDA ruling.
The FDA stated that mifepristone prescribers will still need to earn certification and training. Additionally, the agency said dispensing pharmacies will have to be certified.
The FDA said in updated guidance on its website that after conducting a review of the Risk Evaluation and Mitigation Strategy for mifepristone, it “determined that the data support modification of the REMS to reduce burden on patient access and the health care delivery system and to ensure the benefits of the product outweigh the risks.”
The modifications include:
- “Removing the requirement that mifepristone be dispensed only in certain health care settings, specifically clinics, medical offices, and hospitals (referred to as the ‘in-person dispensing requirement’).”
- Adding a requirement that pharmacies must be certified to dispense the drug.
The FDA said removing the in-person dispensing rule will allow delivery of mifepristone by mail via certified prescribers or pharmacies, in addition to in-person dispensing in clinics, medical offices, and hospitals.
In 2018, an expert National Academies of Science, Engineering, and Medicine panel concluded that requiring that medication abortion be provided at only certain facilities, solely by a physician or in the physical presence of certain providers, did not improve safety or quality of care.
Mifepristone is used, together with misoprostol, to end an early pregnancy. The FDA first approved Mifeprex in 2000 for use through 10 weeks’ gestation. According to the FDA, mifepristone is approved in more than 60 other countries.
Many states bar mailing of abortion pills
However, according to the Guttmacher Institute, 19 U.S. states have laws that bar telehealth consultations or mailing of abortion pills.
Reuters reported that women in those states would not be able to make use of the rule change get the drug delivered to their home but could potentially travel to other states to obtain medication abortion.
“States such as California and New York that have sought to strengthen access to abortion may make the drug available to women from other states,” Reuters reported.
Jessica Arons, senior advocacy and policy counsel for reproductive freedom at the ACLU, told CBS News, “Medication abortion is one more lens through which we see that we are witnessing a tale of two countries. Half the states are protecting access to abortion and half are trying every single way they can to eliminate access to abortion care.”
Positive results when Canada lifted restrictions
As this news organization has reported, a study found positive results when Canada lifted restrictions on access to the abortion pills and a good safety profile for mifepristone.
A study in the New England Journal of Medicine found abortion rates remained stable and adverse events were rare after mifepristone prescribing restrictions were lifted in Canada.
Senior author Wendy V. Norman, MD, professor in the department of family practice at the University of British Columbia, Vancouver, said in a statement, “Our study is a signal to other countries that restrictions are not necessary to ensure patient safety.”
Another recent study in JAMA Network Open (2021 Aug 24. doi: 10.1001/jamanetworkopen.2021.22320) found that abortion via telehealth prescriptions may be just as safe and effective as in-person care.
The study investigators said that, “of the 110 women from whom researchers collected remote abortion outcome data, 95% had a complete abortion without additional medical interventions, such as aspiration or surgery, and none experienced adverse events. Researchers said this efficacy rate is similar to in-person visits.”
The Food and Drug Administration has announced that women no longer will have to pick up the abortion pill mifepristone (Mifeprex) in person at certain certified sites and can get a prescription via an online consultation and delivery through the mail.
In April 2021, the FDA lifted the in-person dispensing requirement for mifepristone for the duration of the COVID-19 pandemic and in December the agency made that decision permanent.
As this news organization reported on April 12, 2021, acting commissioner of food and drugs, Janet Woodcock, MD, stated that the FDA would “permit the dispensing of mifepristone through the mail when done by or under the supervision of a certified prescriber; or through a mail-order pharmacy under the supervision of a certified prescriber.”
That decision came after suspension of the in-person dispensing requirement in response to COVID-19 safety concerns for patients as well as providers associated with in-person clinic visits.
Decision comes amid Supreme Court debate
The FDA decision comes as the Supreme Court nears a decision on whether to overturn its 1973 ruling on Roe v. Wade.
Additionally, the Supreme Court on returned a lawsuit over Texas’ ban on abortions after 6 weeks to a federal appeals court that has twice allowed the law to stay in effect, rather than to a district judge who wanted it blocked.
Alexis McGill Johnson, president and CEO, of the Planned Parenthood Federation of America, said in a statement, “Abortion is time sensitive, essential health care, and this decision will remove a sometimes insurmountable barrier for patients seeking an abortion. With abortion rights at risk like never before, the FDA’s decision is a long overdue step toward expanding people’s access to safe medication abortion.”
Georgeanne Usova, senior legislative counsel at the American Civil Liberties Union told CNBC News: “The FDA’s decision will come as a tremendous relief for countless abortion and miscarriage patients.”
Catherine D. Cansino, MD, MPH, associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis, and member of the editorial advisory board for ObGyn News said in an interview: “I think that this change is a long time coming and speaks to the fact that science matters and medicine prevails over politics. We need to protect health rights first!”
Others expressed doubt or outrage.
Fidelma Rigby, MD, a professor in the department of obstetrics and gynecology, division of maternal fetal medicine, Virginia Commonwealth University Medical Center, Richmond, said in an interview: “My concern is that what if there is an ectopic pregnancy? I’m not as enthusiastic as some of my partners would be about this announcement.”
“The FDA’s decision today places women at risk,” said Carol Tobias, president of the National Right to Life Committee. “These changes do not make this abortion process safer for women. What these changes do is make the process easier for the abortion industry.”
The antiabortion groups Charlotte Lozier Institute and the Susan B. Anthony List were among other organizations issuing statements against Dec. 16’s FDA ruling.
The FDA stated that mifepristone prescribers will still need to earn certification and training. Additionally, the agency said dispensing pharmacies will have to be certified.
The FDA said in updated guidance on its website that after conducting a review of the Risk Evaluation and Mitigation Strategy for mifepristone, it “determined that the data support modification of the REMS to reduce burden on patient access and the health care delivery system and to ensure the benefits of the product outweigh the risks.”
The modifications include:
- “Removing the requirement that mifepristone be dispensed only in certain health care settings, specifically clinics, medical offices, and hospitals (referred to as the ‘in-person dispensing requirement’).”
- Adding a requirement that pharmacies must be certified to dispense the drug.
The FDA said removing the in-person dispensing rule will allow delivery of mifepristone by mail via certified prescribers or pharmacies, in addition to in-person dispensing in clinics, medical offices, and hospitals.
In 2018, an expert National Academies of Science, Engineering, and Medicine panel concluded that requiring that medication abortion be provided at only certain facilities, solely by a physician or in the physical presence of certain providers, did not improve safety or quality of care.
Mifepristone is used, together with misoprostol, to end an early pregnancy. The FDA first approved Mifeprex in 2000 for use through 10 weeks’ gestation. According to the FDA, mifepristone is approved in more than 60 other countries.
Many states bar mailing of abortion pills
However, according to the Guttmacher Institute, 19 U.S. states have laws that bar telehealth consultations or mailing of abortion pills.
Reuters reported that women in those states would not be able to make use of the rule change get the drug delivered to their home but could potentially travel to other states to obtain medication abortion.
“States such as California and New York that have sought to strengthen access to abortion may make the drug available to women from other states,” Reuters reported.
Jessica Arons, senior advocacy and policy counsel for reproductive freedom at the ACLU, told CBS News, “Medication abortion is one more lens through which we see that we are witnessing a tale of two countries. Half the states are protecting access to abortion and half are trying every single way they can to eliminate access to abortion care.”
Positive results when Canada lifted restrictions
As this news organization has reported, a study found positive results when Canada lifted restrictions on access to the abortion pills and a good safety profile for mifepristone.
A study in the New England Journal of Medicine found abortion rates remained stable and adverse events were rare after mifepristone prescribing restrictions were lifted in Canada.
Senior author Wendy V. Norman, MD, professor in the department of family practice at the University of British Columbia, Vancouver, said in a statement, “Our study is a signal to other countries that restrictions are not necessary to ensure patient safety.”
Another recent study in JAMA Network Open (2021 Aug 24. doi: 10.1001/jamanetworkopen.2021.22320) found that abortion via telehealth prescriptions may be just as safe and effective as in-person care.
The study investigators said that, “of the 110 women from whom researchers collected remote abortion outcome data, 95% had a complete abortion without additional medical interventions, such as aspiration or surgery, and none experienced adverse events. Researchers said this efficacy rate is similar to in-person visits.”
The Food and Drug Administration has announced that women no longer will have to pick up the abortion pill mifepristone (Mifeprex) in person at certain certified sites and can get a prescription via an online consultation and delivery through the mail.
In April 2021, the FDA lifted the in-person dispensing requirement for mifepristone for the duration of the COVID-19 pandemic and in December the agency made that decision permanent.
As this news organization reported on April 12, 2021, acting commissioner of food and drugs, Janet Woodcock, MD, stated that the FDA would “permit the dispensing of mifepristone through the mail when done by or under the supervision of a certified prescriber; or through a mail-order pharmacy under the supervision of a certified prescriber.”
That decision came after suspension of the in-person dispensing requirement in response to COVID-19 safety concerns for patients as well as providers associated with in-person clinic visits.
Decision comes amid Supreme Court debate
The FDA decision comes as the Supreme Court nears a decision on whether to overturn its 1973 ruling on Roe v. Wade.
Additionally, the Supreme Court on returned a lawsuit over Texas’ ban on abortions after 6 weeks to a federal appeals court that has twice allowed the law to stay in effect, rather than to a district judge who wanted it blocked.
Alexis McGill Johnson, president and CEO, of the Planned Parenthood Federation of America, said in a statement, “Abortion is time sensitive, essential health care, and this decision will remove a sometimes insurmountable barrier for patients seeking an abortion. With abortion rights at risk like never before, the FDA’s decision is a long overdue step toward expanding people’s access to safe medication abortion.”
Georgeanne Usova, senior legislative counsel at the American Civil Liberties Union told CNBC News: “The FDA’s decision will come as a tremendous relief for countless abortion and miscarriage patients.”
Catherine D. Cansino, MD, MPH, associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis, and member of the editorial advisory board for ObGyn News said in an interview: “I think that this change is a long time coming and speaks to the fact that science matters and medicine prevails over politics. We need to protect health rights first!”
Others expressed doubt or outrage.
Fidelma Rigby, MD, a professor in the department of obstetrics and gynecology, division of maternal fetal medicine, Virginia Commonwealth University Medical Center, Richmond, said in an interview: “My concern is that what if there is an ectopic pregnancy? I’m not as enthusiastic as some of my partners would be about this announcement.”
“The FDA’s decision today places women at risk,” said Carol Tobias, president of the National Right to Life Committee. “These changes do not make this abortion process safer for women. What these changes do is make the process easier for the abortion industry.”
The antiabortion groups Charlotte Lozier Institute and the Susan B. Anthony List were among other organizations issuing statements against Dec. 16’s FDA ruling.
The FDA stated that mifepristone prescribers will still need to earn certification and training. Additionally, the agency said dispensing pharmacies will have to be certified.
The FDA said in updated guidance on its website that after conducting a review of the Risk Evaluation and Mitigation Strategy for mifepristone, it “determined that the data support modification of the REMS to reduce burden on patient access and the health care delivery system and to ensure the benefits of the product outweigh the risks.”
The modifications include:
- “Removing the requirement that mifepristone be dispensed only in certain health care settings, specifically clinics, medical offices, and hospitals (referred to as the ‘in-person dispensing requirement’).”
- Adding a requirement that pharmacies must be certified to dispense the drug.
The FDA said removing the in-person dispensing rule will allow delivery of mifepristone by mail via certified prescribers or pharmacies, in addition to in-person dispensing in clinics, medical offices, and hospitals.
In 2018, an expert National Academies of Science, Engineering, and Medicine panel concluded that requiring that medication abortion be provided at only certain facilities, solely by a physician or in the physical presence of certain providers, did not improve safety or quality of care.
Mifepristone is used, together with misoprostol, to end an early pregnancy. The FDA first approved Mifeprex in 2000 for use through 10 weeks’ gestation. According to the FDA, mifepristone is approved in more than 60 other countries.
Many states bar mailing of abortion pills
However, according to the Guttmacher Institute, 19 U.S. states have laws that bar telehealth consultations or mailing of abortion pills.
Reuters reported that women in those states would not be able to make use of the rule change get the drug delivered to their home but could potentially travel to other states to obtain medication abortion.
“States such as California and New York that have sought to strengthen access to abortion may make the drug available to women from other states,” Reuters reported.
Jessica Arons, senior advocacy and policy counsel for reproductive freedom at the ACLU, told CBS News, “Medication abortion is one more lens through which we see that we are witnessing a tale of two countries. Half the states are protecting access to abortion and half are trying every single way they can to eliminate access to abortion care.”
Positive results when Canada lifted restrictions
As this news organization has reported, a study found positive results when Canada lifted restrictions on access to the abortion pills and a good safety profile for mifepristone.
A study in the New England Journal of Medicine found abortion rates remained stable and adverse events were rare after mifepristone prescribing restrictions were lifted in Canada.
Senior author Wendy V. Norman, MD, professor in the department of family practice at the University of British Columbia, Vancouver, said in a statement, “Our study is a signal to other countries that restrictions are not necessary to ensure patient safety.”
Another recent study in JAMA Network Open (2021 Aug 24. doi: 10.1001/jamanetworkopen.2021.22320) found that abortion via telehealth prescriptions may be just as safe and effective as in-person care.
The study investigators said that, “of the 110 women from whom researchers collected remote abortion outcome data, 95% had a complete abortion without additional medical interventions, such as aspiration or surgery, and none experienced adverse events. Researchers said this efficacy rate is similar to in-person visits.”