User login
Motherhood and mortality: Navigating miscarriages as a physician
One clinic morning in an office visit, I stood next to the door talking, hand on the doorknob ready to exit. My elderly patient was sitting in the chair next to the door, family member in another, as I attempted my exit. Suddenly, as if looking for something, my patient locked her gaze to my abdomen and began to slowly advance herself forward, eyes squinting for a better view. She had found something. Poke, poke, poke. Three pokes in quick succession into my apparently protruding abdomen stoked an internal horror that I dared not release onto my face. How in the hell could she know? My heart sank – the signs were still there.
“There’s something in there,” she said with a seasoned certainty.
“No there’s not,” I said trying hard to hide any emotion.
“Yes, there is,” she said flatly.
“Grannie, no there isn’t,” her family member interrupted, unknowingly saving me. I thanked them again and quickly left the room.
My patient had the ongoings of slowly progressing dementia. Little did she know she was right. Maybe she had known something in another time and space. Either way, I wasn’t prepared to tell the story. She wasn’t prepared to fully understand.
I tried to forge on to see the next patient. Tears began welling in both eyes. I tilted my head back slightly to prevent the water from falling. I wanted to feel offended, but she couldn’t have known the war my body was fighting at the time. I had not yet shared the pregnancy news with this particular patient, and yet her knowing was telling in a sense. I’m learning that the old folks always know.
I was at work, actively having yet another miscarriage. This was the second of two. This most recent time, we found out at 9 weeks that our baby had stopped growing about a week or so earlier. Cue the denial. Cue the rage. Cue the devastation.
Thinking back, with each pregnancy discovery, we did not wait the customary 3 months before telling anyone. Just about everyone knew. We were immediately excited to start sharing with friends, family, coworkers, and even patients early on. We knew the risks in my 40-something age group but were quintessentially optimistic.
I am a family medicine physician with expert-level knowledge and clinical experiences in women’s health counseling, contraception, conception, and pregnancy. In my training, I’ve delivered babies, been elbow-deep searching for wayward tissue from bleeding uteri, and sutured gaping vaginal lacerations. I’ve cried with new mothers at the end of long labors. I’ve been bear-hugged by doting new fathers. I have an abundance of medical knowledge, and yet the pain and struggle of miscarriage over the past 2.5 years has twice reduced me to absolute pieces. There was no course to teach me how to navigate loss within my own body, no textbook to study so that I could test out of the experience. Life hit us dead-on, and I was broken.
I can say that the experience of a miscarriage does not get easier with each subsequent loss. At least for me, the emotions were always raw and tender. Each one was a new gash to my emotional and physical health. My sanity bled out. I was physically exhausted. The struggles of being a health care worker in the midst of a global pandemic I’m sure did not help the situation. My first miscarriage was just before the start of the pandemic. I was in New York visiting family and after dinner at Tavern on the Green, of all places, when I began showing signs. Two days later, I was at the coffee station in our clinic cafeteria adding my cream and sugar when my ob.gyn.’s office called. The hCG levels were probably too low; a miscarriage was likely. I kept my composure, walked out of the cafeteria, got my car keys, went to my car, and proceeded to scream at the top of my lungs for a few minutes. Afterward, I went back to finish up my work and canceled my clinic for the rest of the day.
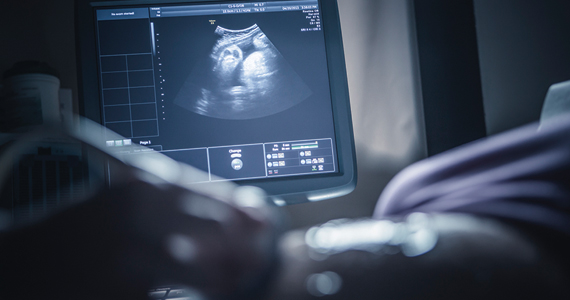
For my second miscarriage, I was laying in my doctor’s office getting an ultrasound. I had started bleeding the previous day but thought that the subchorionic hemorrhage noted on the last ultrasound might be the culprit. The bleeding was light. That’s the thing about being a pregnant physician: We know too much. The image on the screen looked abnormal, the remnants a ghost of its former self. I knew something was wrong but held out some hope. She searched and turned and pressed the transducer into my belly for a seemingly better view. She apologized for not finding the heartbeat. How is this happening again?
So how does one get through the loss of multiple pregnancies? I know my husband and I worked hard to get through each loss. We did all the right things a good therapist would recommend: Be present in the moment, go with your feelings, allow yourself to feel everything. There were no wrong emotions. Little by little we grieved and healed, grieved and healed. Having a successful pregnancy did help. Miracles are not promised but I believe we were sent one, and her name is Giavonna Barbara. Bookended by miscarriages, she has made me realize just how precious and delicate life really is. She is our absolute world and joy.
I’ve learned twice now that men mourn differently than women. Not any less, just in a different way. There is a pain in the silence that often goes unvocalized, but it is of no less value. My husband and I allowed each other to heal in our own unique ways, and that has made all of the difference. I think I knew I was doing okay when one day I found something funny and I let out the heartiest laugh my belly could muster. A different purpose was renewed. Tears were harder to come by. Hope for the future again sprung eternal. Life went on and so did we.
Looking back, I realize that having a miscarriage and working as a physician in the middle of a global pandemic pushed me to my emotional and physical limits. There is a second-guessing of sorts that occurs. Did the miscarriage happen because I was under so much stress at work? It had happened in the past, was this going to continue to happen?
I can say that I was great at compartmentalizing emotions. I’d try and box them away until I got off of work and then turn them on like a switch once I hit the driver’s seat. It’s easy as a busy physician with so many patients to see, messages to return, notes to write, students and residents to teach, and programs to run to completely tune out the thought of mourning. Temporarily anyway. Work was actually a welcome distraction at times. A purpose. The journey to healing is individualized and can’t be rushed. I like to think that I heal a little bit more every day thinking about the losses and gains that I’ve had. I’m grateful for the experience and growth.
In 2022, I’m looking forward to continuing my healing journey among the twists and turns of the pandemic. I now bring a different level of understanding and empathy to my patients who are undergoing or who have undergone a miscarriage. There will always be a piece of me that viscerally mourns with them. We have a hidden shared experience. I believe I am a better physician because of those lessons learned from my own personal tragedy. Now, I look forward to sharing big belly laughs with my family and friends and savoring the small, quiet moments with my husband and daughter.
A version of this article first appeared on Medscape.com.
One clinic morning in an office visit, I stood next to the door talking, hand on the doorknob ready to exit. My elderly patient was sitting in the chair next to the door, family member in another, as I attempted my exit. Suddenly, as if looking for something, my patient locked her gaze to my abdomen and began to slowly advance herself forward, eyes squinting for a better view. She had found something. Poke, poke, poke. Three pokes in quick succession into my apparently protruding abdomen stoked an internal horror that I dared not release onto my face. How in the hell could she know? My heart sank – the signs were still there.
“There’s something in there,” she said with a seasoned certainty.
“No there’s not,” I said trying hard to hide any emotion.
“Yes, there is,” she said flatly.
“Grannie, no there isn’t,” her family member interrupted, unknowingly saving me. I thanked them again and quickly left the room.
My patient had the ongoings of slowly progressing dementia. Little did she know she was right. Maybe she had known something in another time and space. Either way, I wasn’t prepared to tell the story. She wasn’t prepared to fully understand.
I tried to forge on to see the next patient. Tears began welling in both eyes. I tilted my head back slightly to prevent the water from falling. I wanted to feel offended, but she couldn’t have known the war my body was fighting at the time. I had not yet shared the pregnancy news with this particular patient, and yet her knowing was telling in a sense. I’m learning that the old folks always know.
I was at work, actively having yet another miscarriage. This was the second of two. This most recent time, we found out at 9 weeks that our baby had stopped growing about a week or so earlier. Cue the denial. Cue the rage. Cue the devastation.
Thinking back, with each pregnancy discovery, we did not wait the customary 3 months before telling anyone. Just about everyone knew. We were immediately excited to start sharing with friends, family, coworkers, and even patients early on. We knew the risks in my 40-something age group but were quintessentially optimistic.
I am a family medicine physician with expert-level knowledge and clinical experiences in women’s health counseling, contraception, conception, and pregnancy. In my training, I’ve delivered babies, been elbow-deep searching for wayward tissue from bleeding uteri, and sutured gaping vaginal lacerations. I’ve cried with new mothers at the end of long labors. I’ve been bear-hugged by doting new fathers. I have an abundance of medical knowledge, and yet the pain and struggle of miscarriage over the past 2.5 years has twice reduced me to absolute pieces. There was no course to teach me how to navigate loss within my own body, no textbook to study so that I could test out of the experience. Life hit us dead-on, and I was broken.
I can say that the experience of a miscarriage does not get easier with each subsequent loss. At least for me, the emotions were always raw and tender. Each one was a new gash to my emotional and physical health. My sanity bled out. I was physically exhausted. The struggles of being a health care worker in the midst of a global pandemic I’m sure did not help the situation. My first miscarriage was just before the start of the pandemic. I was in New York visiting family and after dinner at Tavern on the Green, of all places, when I began showing signs. Two days later, I was at the coffee station in our clinic cafeteria adding my cream and sugar when my ob.gyn.’s office called. The hCG levels were probably too low; a miscarriage was likely. I kept my composure, walked out of the cafeteria, got my car keys, went to my car, and proceeded to scream at the top of my lungs for a few minutes. Afterward, I went back to finish up my work and canceled my clinic for the rest of the day.
For my second miscarriage, I was laying in my doctor’s office getting an ultrasound. I had started bleeding the previous day but thought that the subchorionic hemorrhage noted on the last ultrasound might be the culprit. The bleeding was light. That’s the thing about being a pregnant physician: We know too much. The image on the screen looked abnormal, the remnants a ghost of its former self. I knew something was wrong but held out some hope. She searched and turned and pressed the transducer into my belly for a seemingly better view. She apologized for not finding the heartbeat. How is this happening again?
So how does one get through the loss of multiple pregnancies? I know my husband and I worked hard to get through each loss. We did all the right things a good therapist would recommend: Be present in the moment, go with your feelings, allow yourself to feel everything. There were no wrong emotions. Little by little we grieved and healed, grieved and healed. Having a successful pregnancy did help. Miracles are not promised but I believe we were sent one, and her name is Giavonna Barbara. Bookended by miscarriages, she has made me realize just how precious and delicate life really is. She is our absolute world and joy.
I’ve learned twice now that men mourn differently than women. Not any less, just in a different way. There is a pain in the silence that often goes unvocalized, but it is of no less value. My husband and I allowed each other to heal in our own unique ways, and that has made all of the difference. I think I knew I was doing okay when one day I found something funny and I let out the heartiest laugh my belly could muster. A different purpose was renewed. Tears were harder to come by. Hope for the future again sprung eternal. Life went on and so did we.
Looking back, I realize that having a miscarriage and working as a physician in the middle of a global pandemic pushed me to my emotional and physical limits. There is a second-guessing of sorts that occurs. Did the miscarriage happen because I was under so much stress at work? It had happened in the past, was this going to continue to happen?
I can say that I was great at compartmentalizing emotions. I’d try and box them away until I got off of work and then turn them on like a switch once I hit the driver’s seat. It’s easy as a busy physician with so many patients to see, messages to return, notes to write, students and residents to teach, and programs to run to completely tune out the thought of mourning. Temporarily anyway. Work was actually a welcome distraction at times. A purpose. The journey to healing is individualized and can’t be rushed. I like to think that I heal a little bit more every day thinking about the losses and gains that I’ve had. I’m grateful for the experience and growth.
In 2022, I’m looking forward to continuing my healing journey among the twists and turns of the pandemic. I now bring a different level of understanding and empathy to my patients who are undergoing or who have undergone a miscarriage. There will always be a piece of me that viscerally mourns with them. We have a hidden shared experience. I believe I am a better physician because of those lessons learned from my own personal tragedy. Now, I look forward to sharing big belly laughs with my family and friends and savoring the small, quiet moments with my husband and daughter.
A version of this article first appeared on Medscape.com.
One clinic morning in an office visit, I stood next to the door talking, hand on the doorknob ready to exit. My elderly patient was sitting in the chair next to the door, family member in another, as I attempted my exit. Suddenly, as if looking for something, my patient locked her gaze to my abdomen and began to slowly advance herself forward, eyes squinting for a better view. She had found something. Poke, poke, poke. Three pokes in quick succession into my apparently protruding abdomen stoked an internal horror that I dared not release onto my face. How in the hell could she know? My heart sank – the signs were still there.
“There’s something in there,” she said with a seasoned certainty.
“No there’s not,” I said trying hard to hide any emotion.
“Yes, there is,” she said flatly.
“Grannie, no there isn’t,” her family member interrupted, unknowingly saving me. I thanked them again and quickly left the room.
My patient had the ongoings of slowly progressing dementia. Little did she know she was right. Maybe she had known something in another time and space. Either way, I wasn’t prepared to tell the story. She wasn’t prepared to fully understand.
I tried to forge on to see the next patient. Tears began welling in both eyes. I tilted my head back slightly to prevent the water from falling. I wanted to feel offended, but she couldn’t have known the war my body was fighting at the time. I had not yet shared the pregnancy news with this particular patient, and yet her knowing was telling in a sense. I’m learning that the old folks always know.
I was at work, actively having yet another miscarriage. This was the second of two. This most recent time, we found out at 9 weeks that our baby had stopped growing about a week or so earlier. Cue the denial. Cue the rage. Cue the devastation.
Thinking back, with each pregnancy discovery, we did not wait the customary 3 months before telling anyone. Just about everyone knew. We were immediately excited to start sharing with friends, family, coworkers, and even patients early on. We knew the risks in my 40-something age group but were quintessentially optimistic.
I am a family medicine physician with expert-level knowledge and clinical experiences in women’s health counseling, contraception, conception, and pregnancy. In my training, I’ve delivered babies, been elbow-deep searching for wayward tissue from bleeding uteri, and sutured gaping vaginal lacerations. I’ve cried with new mothers at the end of long labors. I’ve been bear-hugged by doting new fathers. I have an abundance of medical knowledge, and yet the pain and struggle of miscarriage over the past 2.5 years has twice reduced me to absolute pieces. There was no course to teach me how to navigate loss within my own body, no textbook to study so that I could test out of the experience. Life hit us dead-on, and I was broken.
I can say that the experience of a miscarriage does not get easier with each subsequent loss. At least for me, the emotions were always raw and tender. Each one was a new gash to my emotional and physical health. My sanity bled out. I was physically exhausted. The struggles of being a health care worker in the midst of a global pandemic I’m sure did not help the situation. My first miscarriage was just before the start of the pandemic. I was in New York visiting family and after dinner at Tavern on the Green, of all places, when I began showing signs. Two days later, I was at the coffee station in our clinic cafeteria adding my cream and sugar when my ob.gyn.’s office called. The hCG levels were probably too low; a miscarriage was likely. I kept my composure, walked out of the cafeteria, got my car keys, went to my car, and proceeded to scream at the top of my lungs for a few minutes. Afterward, I went back to finish up my work and canceled my clinic for the rest of the day.
For my second miscarriage, I was laying in my doctor’s office getting an ultrasound. I had started bleeding the previous day but thought that the subchorionic hemorrhage noted on the last ultrasound might be the culprit. The bleeding was light. That’s the thing about being a pregnant physician: We know too much. The image on the screen looked abnormal, the remnants a ghost of its former self. I knew something was wrong but held out some hope. She searched and turned and pressed the transducer into my belly for a seemingly better view. She apologized for not finding the heartbeat. How is this happening again?
So how does one get through the loss of multiple pregnancies? I know my husband and I worked hard to get through each loss. We did all the right things a good therapist would recommend: Be present in the moment, go with your feelings, allow yourself to feel everything. There were no wrong emotions. Little by little we grieved and healed, grieved and healed. Having a successful pregnancy did help. Miracles are not promised but I believe we were sent one, and her name is Giavonna Barbara. Bookended by miscarriages, she has made me realize just how precious and delicate life really is. She is our absolute world and joy.
I’ve learned twice now that men mourn differently than women. Not any less, just in a different way. There is a pain in the silence that often goes unvocalized, but it is of no less value. My husband and I allowed each other to heal in our own unique ways, and that has made all of the difference. I think I knew I was doing okay when one day I found something funny and I let out the heartiest laugh my belly could muster. A different purpose was renewed. Tears were harder to come by. Hope for the future again sprung eternal. Life went on and so did we.
Looking back, I realize that having a miscarriage and working as a physician in the middle of a global pandemic pushed me to my emotional and physical limits. There is a second-guessing of sorts that occurs. Did the miscarriage happen because I was under so much stress at work? It had happened in the past, was this going to continue to happen?
I can say that I was great at compartmentalizing emotions. I’d try and box them away until I got off of work and then turn them on like a switch once I hit the driver’s seat. It’s easy as a busy physician with so many patients to see, messages to return, notes to write, students and residents to teach, and programs to run to completely tune out the thought of mourning. Temporarily anyway. Work was actually a welcome distraction at times. A purpose. The journey to healing is individualized and can’t be rushed. I like to think that I heal a little bit more every day thinking about the losses and gains that I’ve had. I’m grateful for the experience and growth.
In 2022, I’m looking forward to continuing my healing journey among the twists and turns of the pandemic. I now bring a different level of understanding and empathy to my patients who are undergoing or who have undergone a miscarriage. There will always be a piece of me that viscerally mourns with them. We have a hidden shared experience. I believe I am a better physician because of those lessons learned from my own personal tragedy. Now, I look forward to sharing big belly laughs with my family and friends and savoring the small, quiet moments with my husband and daughter.
A version of this article first appeared on Medscape.com.
Marijuana use during pregnancy raised risk of adverse neonatal outcomes
Women who used marijuana during pregnancy were at increased risk for adverse neonatal outcomes, based on data from a meta-analysis of nearly 60,000 individuals.
Marijuana misuse remains a top substance use disorder and studies of prenatal use show a prevalence as high as 22% worldwide, wrote Greg J. Marchand, MD, of the Marchand Institute for Minimally Invasive Surgery, Mesa, Ariz., and colleagues.
“The prevalence of marijuana use during pregnancy may continue to increase, given that there is a suggested association between legalized recreational marijuana and increased use in prenatal and postpartum periods,” they wrote. “Remarkably, 34%-60% of individuals who use marijuana keep using it during pregnancy,” and many women cite a belief that marijuana is safe to use while pregnant, they noted.
Cannabinoid receptors are present in the developing fetus by the start of the second trimester, and exposure to exogenous cannabinoids may be associated with changes in the prefrontal cortex, including development and function, the researchers said. However, previous studies of an association between maternal marijuana use and poor neonatal outcomes have been inconsistent, they added.
In a study published in JAMA Network Open, the researchers identified 16 interventional and observational studies including 59,138 patients; each study included pregnant women who were exposed to marijuana, compared with those not exposed to marijuana, along with neonatal outcomes. The data selection included studies published until Aug. 16, 2021, and 10 studies were published in 2015 or later.
Overall, the risk for seven adverse neonatal outcomes was significantly increased among women who were exposed to marijuana during pregnancy, compared with those not exposed. The researchers identified increased risk for birth weight less than 2,500 g (relative risk, 2.06; P = .005), small for gestational age (RR, 1.61; P < .001), preterm delivery (RR, 1.28; P < .001), and NICU admission (RR, 1.38; P < .001). In addition, they found significant differences in mean birth weight (mean difference, −112.30 g; P < .001), Apgar score at 1 minute (mean difference, −0.26; P = .002), and infant head circumference (mean difference, −0.34cm; P = .02) between women who used marijuana during pregnancy and those who did not.
The study findings were limited by several factors, including the assessment of only cohort studies, which might suffer from bias given their retrospective designs, the researchers noted. Other limitations included the reliance on self-reports, the inability to adjust for tobacco/marijuana coexposure, and the lack of differentiation between levels of use and between different types of marijuana ingestion, they added.
However, the results support an association between marijuana use and adverse neonatal outcomes, and the researchers recommended additional studies of both maternal and neonatal outcomes associated with marijuana exposure. “Given increasing marijuana legalization and use worldwide, raising awareness and educating patients about these adverse outcomes may help to improve neonatal health,” they concluded.
New research prompted new review
The motivation to conduct this analysis at this time was prompted by the publication of several new, high-quality studies on the use of marijuana in pregnancy, according to Dr. Marchand. “It’s been a few years since a full analysis of all of the available data had been done, so we decided it was time to see if the old conclusions still held,” he said in an interview.
Dr. Marchand said he was surprised to see such a clear connection to preterm deliveries and lower birth weights. “When we perform a meta-analysis, we use all of the available data, and some important studies performed as recently as the past few years provided the depth of evidence behind these connections,” he said. “We didn’t have that level of evidence the last time this topic was studied only a few years ago,” he added.
The study is the largest meta-analysis on this topic to date, so the message to clinicians is highly significant, Dr. Marchand said. That message is “that we now have a very high level of evidence to say that smoking marijuana during pregnancy is harmful, and we (physicians especially) can no longer state that we just don’t know,” he said. “This is going to mean that deciding to smoke marijuana during your pregnancy is also deciding to do something that can harm your baby,” he emphasized. “This paper also will force some difficult decisions for mothers who use marijuana to treat medical problems, and there may not be good substitute treatments for some of these conditions, especially chronic pain and anxiety,” Dr. Marchand noted. “This will set up a difficult risk-versus-benefits situation, where these mothers, ideally with the help of their physicians, will have to decide if the risks of stopping marijuana outweigh the possible harm to the unborn baby,” he said.
As for additional research, long-term studies to assess behavioral changes as exposed children grow up would be beneficial, Dr. Marchand said. Such studies “could really help us balance the risk of marijuana exposure in pregnancy, especially if it is being used to treat serious medical conditions,” he noted.
Findings are a call to action
The view among many women that prenatal cannabis use is safe and without consequence “is a false narrative perpetuated by a combination of outdated evidence and recent changes to state-level cannabis policies,” wrote Kara R. Skelton, PhD, of Towson (Md.) University, and Sara E. Benjamin-Neelon, PhD, of Johns Hopkins University, Baltimore, in an accompanying editorial.
The findings from the current study add to the growing evidence that prenatal cannabis use is associated with adverse birth outcomes, they wrote. “Clinician-directed communication about cannabis has been criticized by pregnant women, with recent findings supporting a need for increased cannabis communication by clinicians,” and not only clinicians, but all health professionals who encounter women who are pregnant or attempting pregnancy should not miss the opportunity to communicate the risks of prenatal cannabis use, they emphasized.
The authors highlighted some of the current study’s limitations, including the inability to determine a dose-response association, the reliance on self-reports, and the lack of adjustment for tobacco/marijuana coexposure. However, they noted that the inclusion of recent studies (10 published in 2015 or later) strengthens the results because of the significant increase in the potency of Δ-9-tetrahydrocannabinol (THC), the main psychoactive ingredient in cannabis, in recent decades.
“We urge clinicians, public health professionals, and policy makers to carefully consider the consequences of in utero cannabis exposure identified by Marchand et al. and partner to ensure prioritization of infant and child health during this time of precipitous cannabis legalization and commercialization,” the authors emphasized. “Without necessary safeguards to protect neonatal health, prenatal cannabis use poses a substantial threat to current and future generations of children,” they wrote.
The study received no outside funding. The researchers had no financial conflicts to disclose. The editorialists had no financial conflicts to disclose.
Women who used marijuana during pregnancy were at increased risk for adverse neonatal outcomes, based on data from a meta-analysis of nearly 60,000 individuals.
Marijuana misuse remains a top substance use disorder and studies of prenatal use show a prevalence as high as 22% worldwide, wrote Greg J. Marchand, MD, of the Marchand Institute for Minimally Invasive Surgery, Mesa, Ariz., and colleagues.
“The prevalence of marijuana use during pregnancy may continue to increase, given that there is a suggested association between legalized recreational marijuana and increased use in prenatal and postpartum periods,” they wrote. “Remarkably, 34%-60% of individuals who use marijuana keep using it during pregnancy,” and many women cite a belief that marijuana is safe to use while pregnant, they noted.
Cannabinoid receptors are present in the developing fetus by the start of the second trimester, and exposure to exogenous cannabinoids may be associated with changes in the prefrontal cortex, including development and function, the researchers said. However, previous studies of an association between maternal marijuana use and poor neonatal outcomes have been inconsistent, they added.
In a study published in JAMA Network Open, the researchers identified 16 interventional and observational studies including 59,138 patients; each study included pregnant women who were exposed to marijuana, compared with those not exposed to marijuana, along with neonatal outcomes. The data selection included studies published until Aug. 16, 2021, and 10 studies were published in 2015 or later.
Overall, the risk for seven adverse neonatal outcomes was significantly increased among women who were exposed to marijuana during pregnancy, compared with those not exposed. The researchers identified increased risk for birth weight less than 2,500 g (relative risk, 2.06; P = .005), small for gestational age (RR, 1.61; P < .001), preterm delivery (RR, 1.28; P < .001), and NICU admission (RR, 1.38; P < .001). In addition, they found significant differences in mean birth weight (mean difference, −112.30 g; P < .001), Apgar score at 1 minute (mean difference, −0.26; P = .002), and infant head circumference (mean difference, −0.34cm; P = .02) between women who used marijuana during pregnancy and those who did not.
The study findings were limited by several factors, including the assessment of only cohort studies, which might suffer from bias given their retrospective designs, the researchers noted. Other limitations included the reliance on self-reports, the inability to adjust for tobacco/marijuana coexposure, and the lack of differentiation between levels of use and between different types of marijuana ingestion, they added.
However, the results support an association between marijuana use and adverse neonatal outcomes, and the researchers recommended additional studies of both maternal and neonatal outcomes associated with marijuana exposure. “Given increasing marijuana legalization and use worldwide, raising awareness and educating patients about these adverse outcomes may help to improve neonatal health,” they concluded.
New research prompted new review
The motivation to conduct this analysis at this time was prompted by the publication of several new, high-quality studies on the use of marijuana in pregnancy, according to Dr. Marchand. “It’s been a few years since a full analysis of all of the available data had been done, so we decided it was time to see if the old conclusions still held,” he said in an interview.
Dr. Marchand said he was surprised to see such a clear connection to preterm deliveries and lower birth weights. “When we perform a meta-analysis, we use all of the available data, and some important studies performed as recently as the past few years provided the depth of evidence behind these connections,” he said. “We didn’t have that level of evidence the last time this topic was studied only a few years ago,” he added.
The study is the largest meta-analysis on this topic to date, so the message to clinicians is highly significant, Dr. Marchand said. That message is “that we now have a very high level of evidence to say that smoking marijuana during pregnancy is harmful, and we (physicians especially) can no longer state that we just don’t know,” he said. “This is going to mean that deciding to smoke marijuana during your pregnancy is also deciding to do something that can harm your baby,” he emphasized. “This paper also will force some difficult decisions for mothers who use marijuana to treat medical problems, and there may not be good substitute treatments for some of these conditions, especially chronic pain and anxiety,” Dr. Marchand noted. “This will set up a difficult risk-versus-benefits situation, where these mothers, ideally with the help of their physicians, will have to decide if the risks of stopping marijuana outweigh the possible harm to the unborn baby,” he said.
As for additional research, long-term studies to assess behavioral changes as exposed children grow up would be beneficial, Dr. Marchand said. Such studies “could really help us balance the risk of marijuana exposure in pregnancy, especially if it is being used to treat serious medical conditions,” he noted.
Findings are a call to action
The view among many women that prenatal cannabis use is safe and without consequence “is a false narrative perpetuated by a combination of outdated evidence and recent changes to state-level cannabis policies,” wrote Kara R. Skelton, PhD, of Towson (Md.) University, and Sara E. Benjamin-Neelon, PhD, of Johns Hopkins University, Baltimore, in an accompanying editorial.
The findings from the current study add to the growing evidence that prenatal cannabis use is associated with adverse birth outcomes, they wrote. “Clinician-directed communication about cannabis has been criticized by pregnant women, with recent findings supporting a need for increased cannabis communication by clinicians,” and not only clinicians, but all health professionals who encounter women who are pregnant or attempting pregnancy should not miss the opportunity to communicate the risks of prenatal cannabis use, they emphasized.
The authors highlighted some of the current study’s limitations, including the inability to determine a dose-response association, the reliance on self-reports, and the lack of adjustment for tobacco/marijuana coexposure. However, they noted that the inclusion of recent studies (10 published in 2015 or later) strengthens the results because of the significant increase in the potency of Δ-9-tetrahydrocannabinol (THC), the main psychoactive ingredient in cannabis, in recent decades.
“We urge clinicians, public health professionals, and policy makers to carefully consider the consequences of in utero cannabis exposure identified by Marchand et al. and partner to ensure prioritization of infant and child health during this time of precipitous cannabis legalization and commercialization,” the authors emphasized. “Without necessary safeguards to protect neonatal health, prenatal cannabis use poses a substantial threat to current and future generations of children,” they wrote.
The study received no outside funding. The researchers had no financial conflicts to disclose. The editorialists had no financial conflicts to disclose.
Women who used marijuana during pregnancy were at increased risk for adverse neonatal outcomes, based on data from a meta-analysis of nearly 60,000 individuals.
Marijuana misuse remains a top substance use disorder and studies of prenatal use show a prevalence as high as 22% worldwide, wrote Greg J. Marchand, MD, of the Marchand Institute for Minimally Invasive Surgery, Mesa, Ariz., and colleagues.
“The prevalence of marijuana use during pregnancy may continue to increase, given that there is a suggested association between legalized recreational marijuana and increased use in prenatal and postpartum periods,” they wrote. “Remarkably, 34%-60% of individuals who use marijuana keep using it during pregnancy,” and many women cite a belief that marijuana is safe to use while pregnant, they noted.
Cannabinoid receptors are present in the developing fetus by the start of the second trimester, and exposure to exogenous cannabinoids may be associated with changes in the prefrontal cortex, including development and function, the researchers said. However, previous studies of an association between maternal marijuana use and poor neonatal outcomes have been inconsistent, they added.
In a study published in JAMA Network Open, the researchers identified 16 interventional and observational studies including 59,138 patients; each study included pregnant women who were exposed to marijuana, compared with those not exposed to marijuana, along with neonatal outcomes. The data selection included studies published until Aug. 16, 2021, and 10 studies were published in 2015 or later.
Overall, the risk for seven adverse neonatal outcomes was significantly increased among women who were exposed to marijuana during pregnancy, compared with those not exposed. The researchers identified increased risk for birth weight less than 2,500 g (relative risk, 2.06; P = .005), small for gestational age (RR, 1.61; P < .001), preterm delivery (RR, 1.28; P < .001), and NICU admission (RR, 1.38; P < .001). In addition, they found significant differences in mean birth weight (mean difference, −112.30 g; P < .001), Apgar score at 1 minute (mean difference, −0.26; P = .002), and infant head circumference (mean difference, −0.34cm; P = .02) between women who used marijuana during pregnancy and those who did not.
The study findings were limited by several factors, including the assessment of only cohort studies, which might suffer from bias given their retrospective designs, the researchers noted. Other limitations included the reliance on self-reports, the inability to adjust for tobacco/marijuana coexposure, and the lack of differentiation between levels of use and between different types of marijuana ingestion, they added.
However, the results support an association between marijuana use and adverse neonatal outcomes, and the researchers recommended additional studies of both maternal and neonatal outcomes associated with marijuana exposure. “Given increasing marijuana legalization and use worldwide, raising awareness and educating patients about these adverse outcomes may help to improve neonatal health,” they concluded.
New research prompted new review
The motivation to conduct this analysis at this time was prompted by the publication of several new, high-quality studies on the use of marijuana in pregnancy, according to Dr. Marchand. “It’s been a few years since a full analysis of all of the available data had been done, so we decided it was time to see if the old conclusions still held,” he said in an interview.
Dr. Marchand said he was surprised to see such a clear connection to preterm deliveries and lower birth weights. “When we perform a meta-analysis, we use all of the available data, and some important studies performed as recently as the past few years provided the depth of evidence behind these connections,” he said. “We didn’t have that level of evidence the last time this topic was studied only a few years ago,” he added.
The study is the largest meta-analysis on this topic to date, so the message to clinicians is highly significant, Dr. Marchand said. That message is “that we now have a very high level of evidence to say that smoking marijuana during pregnancy is harmful, and we (physicians especially) can no longer state that we just don’t know,” he said. “This is going to mean that deciding to smoke marijuana during your pregnancy is also deciding to do something that can harm your baby,” he emphasized. “This paper also will force some difficult decisions for mothers who use marijuana to treat medical problems, and there may not be good substitute treatments for some of these conditions, especially chronic pain and anxiety,” Dr. Marchand noted. “This will set up a difficult risk-versus-benefits situation, where these mothers, ideally with the help of their physicians, will have to decide if the risks of stopping marijuana outweigh the possible harm to the unborn baby,” he said.
As for additional research, long-term studies to assess behavioral changes as exposed children grow up would be beneficial, Dr. Marchand said. Such studies “could really help us balance the risk of marijuana exposure in pregnancy, especially if it is being used to treat serious medical conditions,” he noted.
Findings are a call to action
The view among many women that prenatal cannabis use is safe and without consequence “is a false narrative perpetuated by a combination of outdated evidence and recent changes to state-level cannabis policies,” wrote Kara R. Skelton, PhD, of Towson (Md.) University, and Sara E. Benjamin-Neelon, PhD, of Johns Hopkins University, Baltimore, in an accompanying editorial.
The findings from the current study add to the growing evidence that prenatal cannabis use is associated with adverse birth outcomes, they wrote. “Clinician-directed communication about cannabis has been criticized by pregnant women, with recent findings supporting a need for increased cannabis communication by clinicians,” and not only clinicians, but all health professionals who encounter women who are pregnant or attempting pregnancy should not miss the opportunity to communicate the risks of prenatal cannabis use, they emphasized.
The authors highlighted some of the current study’s limitations, including the inability to determine a dose-response association, the reliance on self-reports, and the lack of adjustment for tobacco/marijuana coexposure. However, they noted that the inclusion of recent studies (10 published in 2015 or later) strengthens the results because of the significant increase in the potency of Δ-9-tetrahydrocannabinol (THC), the main psychoactive ingredient in cannabis, in recent decades.
“We urge clinicians, public health professionals, and policy makers to carefully consider the consequences of in utero cannabis exposure identified by Marchand et al. and partner to ensure prioritization of infant and child health during this time of precipitous cannabis legalization and commercialization,” the authors emphasized. “Without necessary safeguards to protect neonatal health, prenatal cannabis use poses a substantial threat to current and future generations of children,” they wrote.
The study received no outside funding. The researchers had no financial conflicts to disclose. The editorialists had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Digital algorithm better predicts risk for postpartum hemorrhage
A digital algorithm using 24 patient characteristics identifies far more women who are likely to develop a postpartum hemorrhage than currently used tools to predict the risk for bleeding after delivery, according to a study published in the Journal of the American Medical Informatics Association.
About 1 in 10 of the roughly 700 pregnancy-related deaths in the United States are caused by postpartum hemorrhage, according to the U.S. Centers for Disease Control and Prevention. These deaths disproportionately occur among Black women, for whom studies show the risk of dying from a postpartum hemorrhage is fivefold greater than that of White women.
“Postpartum hemorrhage is a preventable medical emergency but remains the leading cause of maternal mortality globally,” the study’s senior author Li Li, MD, senior vice president of clinical informatics at Sema4, a company that uses artificial intelligence and machine learning to develop data-based clinical tools, told this news organization. “Early intervention is critical for reducing postpartum hemorrhage morbidity and mortality.”
Porous predictors
Existing tools for risk prediction are not particularly effective, Dr. Li said. For example, the American College of Obstetricians and Gynecologists’ (ACOG) Safe Motherhood Initiative offers checklists of clinical characteristics to classify women as low, medium, or high risk. However, 40% of the women classified as low risk based on this type of tool experience a hemorrhage.
ACOG also recommends quantifying blood loss during delivery or immediately after to identify women who are hemorrhaging, because imprecise estimates from clinicians may delay urgently needed care. Yet many hospitals have not implemented methods for measuring bleeding, said Dr. Li, who also is an assistant professor of genetics and genomic sciences at the Icahn School of Medicine at Mount Sinai, New York.
To develop a more precise way of identifying women at risk, Dr. Li and colleagues turned to artificial intelligence technology to create a “digital phenotype” based on approximately 72,000 births in the Mount Sinai Health System.
The digital tool retrospectively identified about 6,600 cases of postpartum hemorrhage, about 3.8 times the roughly 1,700 cases that would have been predicted based on methods that estimate blood loss. A blinded physician review of a subset of 45 patient charts – including 26 patients who experienced a hemorrhage, 11 who didn’t, and 6 with unclear outcomes – found that the digital approach was 89% percent accurate at identifying cases, whereas blood loss–based methods were accurate 67% of the time.
Several of the 24 characteristics included in the model appear in other risk predictors, including whether a woman has had a previous cesarean delivery or prior postdelivery bleeding and whether she has anemia or related blood disorders. Among the rest were risk factors that have been identified in the literature, including maternal blood pressure, time from admission to delivery, and average pulse during hospitalization. Five more features were new: red blood cell count and distribution width, mean corpuscular hemoglobin, absolute neutrophil count, and white blood cell count.
“These [new] values are easily obtainable from standard blood draws in the hospital but are not currently used in clinical practice to estimate postpartum hemorrhage risk,” Dr. Li said.
In a related retrospective study, Dr. Li and her colleagues used the new tool to classify women into high, low, or medium risk categories. They found that 28% of the women the algorithm classified as high risk experienced a postpartum hemorrhage compared with 15% to 19% of the women classified as high risk by standard predictive tools. They also identified potential “inflection points” where changes in vital signs may suggest a substantial increase in risk. For example, women whose median blood pressure during labor and delivery was above 132 mm Hg had an 11% average increase in their risk for bleeding.
By more precisely identifying women at risk, the new method “could be used to pre-emptively allocate resources that can ultimately reduce postpartum hemorrhage morbidity and mortality,” Dr. Li said. Sema4 is launching a prospective clinical trial to further assess the algorithm, she added.
Finding the continuum of risk
Holly Ende, MD, an obstetric anesthesiologist at Vanderbilt University Medical Center, Nashville, Tenn., said approaches that leverage electronic health records to identify women at risk for hemorrhage have many advantages over currently used tools.
“Machine learning models or statistical models are able to take into account many more risk factors and weigh each of those risk factors based on how much they contribute to risk,” Dr. Ende, who was not involved in the new studies, told this news organization. “We can stratify women more on a continuum.”
But digital approaches have potential downsides.
“Machine learning algorithms can be developed in such a way that perpetuates racial bias, and it’s important to be aware of potential biases in coded algorithms,” Dr. Li said. To help reduce such bias, they used a database that included a racially and ethnically diverse patient population, but she acknowledged that additional research is needed.
Dr. Ende, the coauthor of a commentary in Obstetrics & Gynecology on risk assessment for postpartum hemorrhage, said algorithm developers must be sensitive to pre-existing disparities in health care that may affect the data they use to build the software.
She pointed to uterine atony – a known risk factor for hemorrhage – as an example. In her own research, she and her colleagues identified women with atony by searching their medical records for medications used to treat the condition. But when they ran their model, Black women were less likely to develop uterine atony, which the team knew wasn’t true in the real world. They traced the problem to an existing disparity in obstetric care: Black women with uterine atony were less likely than women of other races to receive medications for the condition.
“People need to be cognizant as they are developing these types of prediction models and be extremely careful to avoid perpetuating any disparities in care,” Dr. Ende cautioned. On the other hand, if carefully developed, these tools might also help reduce disparities in health care by standardizing risk stratification and clinical practices, she said.
In addition to independent validation of data-based risk prediction tools, Dr. Ende said ensuring that clinicians are properly trained to use these tools is crucial.
“Implementation may be the biggest limitation,” she said.
Dr. Ende and Dr. Li have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A digital algorithm using 24 patient characteristics identifies far more women who are likely to develop a postpartum hemorrhage than currently used tools to predict the risk for bleeding after delivery, according to a study published in the Journal of the American Medical Informatics Association.
About 1 in 10 of the roughly 700 pregnancy-related deaths in the United States are caused by postpartum hemorrhage, according to the U.S. Centers for Disease Control and Prevention. These deaths disproportionately occur among Black women, for whom studies show the risk of dying from a postpartum hemorrhage is fivefold greater than that of White women.
“Postpartum hemorrhage is a preventable medical emergency but remains the leading cause of maternal mortality globally,” the study’s senior author Li Li, MD, senior vice president of clinical informatics at Sema4, a company that uses artificial intelligence and machine learning to develop data-based clinical tools, told this news organization. “Early intervention is critical for reducing postpartum hemorrhage morbidity and mortality.”
Porous predictors
Existing tools for risk prediction are not particularly effective, Dr. Li said. For example, the American College of Obstetricians and Gynecologists’ (ACOG) Safe Motherhood Initiative offers checklists of clinical characteristics to classify women as low, medium, or high risk. However, 40% of the women classified as low risk based on this type of tool experience a hemorrhage.
ACOG also recommends quantifying blood loss during delivery or immediately after to identify women who are hemorrhaging, because imprecise estimates from clinicians may delay urgently needed care. Yet many hospitals have not implemented methods for measuring bleeding, said Dr. Li, who also is an assistant professor of genetics and genomic sciences at the Icahn School of Medicine at Mount Sinai, New York.
To develop a more precise way of identifying women at risk, Dr. Li and colleagues turned to artificial intelligence technology to create a “digital phenotype” based on approximately 72,000 births in the Mount Sinai Health System.
The digital tool retrospectively identified about 6,600 cases of postpartum hemorrhage, about 3.8 times the roughly 1,700 cases that would have been predicted based on methods that estimate blood loss. A blinded physician review of a subset of 45 patient charts – including 26 patients who experienced a hemorrhage, 11 who didn’t, and 6 with unclear outcomes – found that the digital approach was 89% percent accurate at identifying cases, whereas blood loss–based methods were accurate 67% of the time.
Several of the 24 characteristics included in the model appear in other risk predictors, including whether a woman has had a previous cesarean delivery or prior postdelivery bleeding and whether she has anemia or related blood disorders. Among the rest were risk factors that have been identified in the literature, including maternal blood pressure, time from admission to delivery, and average pulse during hospitalization. Five more features were new: red blood cell count and distribution width, mean corpuscular hemoglobin, absolute neutrophil count, and white blood cell count.
“These [new] values are easily obtainable from standard blood draws in the hospital but are not currently used in clinical practice to estimate postpartum hemorrhage risk,” Dr. Li said.
In a related retrospective study, Dr. Li and her colleagues used the new tool to classify women into high, low, or medium risk categories. They found that 28% of the women the algorithm classified as high risk experienced a postpartum hemorrhage compared with 15% to 19% of the women classified as high risk by standard predictive tools. They also identified potential “inflection points” where changes in vital signs may suggest a substantial increase in risk. For example, women whose median blood pressure during labor and delivery was above 132 mm Hg had an 11% average increase in their risk for bleeding.
By more precisely identifying women at risk, the new method “could be used to pre-emptively allocate resources that can ultimately reduce postpartum hemorrhage morbidity and mortality,” Dr. Li said. Sema4 is launching a prospective clinical trial to further assess the algorithm, she added.
Finding the continuum of risk
Holly Ende, MD, an obstetric anesthesiologist at Vanderbilt University Medical Center, Nashville, Tenn., said approaches that leverage electronic health records to identify women at risk for hemorrhage have many advantages over currently used tools.
“Machine learning models or statistical models are able to take into account many more risk factors and weigh each of those risk factors based on how much they contribute to risk,” Dr. Ende, who was not involved in the new studies, told this news organization. “We can stratify women more on a continuum.”
But digital approaches have potential downsides.
“Machine learning algorithms can be developed in such a way that perpetuates racial bias, and it’s important to be aware of potential biases in coded algorithms,” Dr. Li said. To help reduce such bias, they used a database that included a racially and ethnically diverse patient population, but she acknowledged that additional research is needed.
Dr. Ende, the coauthor of a commentary in Obstetrics & Gynecology on risk assessment for postpartum hemorrhage, said algorithm developers must be sensitive to pre-existing disparities in health care that may affect the data they use to build the software.
She pointed to uterine atony – a known risk factor for hemorrhage – as an example. In her own research, she and her colleagues identified women with atony by searching their medical records for medications used to treat the condition. But when they ran their model, Black women were less likely to develop uterine atony, which the team knew wasn’t true in the real world. They traced the problem to an existing disparity in obstetric care: Black women with uterine atony were less likely than women of other races to receive medications for the condition.
“People need to be cognizant as they are developing these types of prediction models and be extremely careful to avoid perpetuating any disparities in care,” Dr. Ende cautioned. On the other hand, if carefully developed, these tools might also help reduce disparities in health care by standardizing risk stratification and clinical practices, she said.
In addition to independent validation of data-based risk prediction tools, Dr. Ende said ensuring that clinicians are properly trained to use these tools is crucial.
“Implementation may be the biggest limitation,” she said.
Dr. Ende and Dr. Li have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A digital algorithm using 24 patient characteristics identifies far more women who are likely to develop a postpartum hemorrhage than currently used tools to predict the risk for bleeding after delivery, according to a study published in the Journal of the American Medical Informatics Association.
About 1 in 10 of the roughly 700 pregnancy-related deaths in the United States are caused by postpartum hemorrhage, according to the U.S. Centers for Disease Control and Prevention. These deaths disproportionately occur among Black women, for whom studies show the risk of dying from a postpartum hemorrhage is fivefold greater than that of White women.
“Postpartum hemorrhage is a preventable medical emergency but remains the leading cause of maternal mortality globally,” the study’s senior author Li Li, MD, senior vice president of clinical informatics at Sema4, a company that uses artificial intelligence and machine learning to develop data-based clinical tools, told this news organization. “Early intervention is critical for reducing postpartum hemorrhage morbidity and mortality.”
Porous predictors
Existing tools for risk prediction are not particularly effective, Dr. Li said. For example, the American College of Obstetricians and Gynecologists’ (ACOG) Safe Motherhood Initiative offers checklists of clinical characteristics to classify women as low, medium, or high risk. However, 40% of the women classified as low risk based on this type of tool experience a hemorrhage.
ACOG also recommends quantifying blood loss during delivery or immediately after to identify women who are hemorrhaging, because imprecise estimates from clinicians may delay urgently needed care. Yet many hospitals have not implemented methods for measuring bleeding, said Dr. Li, who also is an assistant professor of genetics and genomic sciences at the Icahn School of Medicine at Mount Sinai, New York.
To develop a more precise way of identifying women at risk, Dr. Li and colleagues turned to artificial intelligence technology to create a “digital phenotype” based on approximately 72,000 births in the Mount Sinai Health System.
The digital tool retrospectively identified about 6,600 cases of postpartum hemorrhage, about 3.8 times the roughly 1,700 cases that would have been predicted based on methods that estimate blood loss. A blinded physician review of a subset of 45 patient charts – including 26 patients who experienced a hemorrhage, 11 who didn’t, and 6 with unclear outcomes – found that the digital approach was 89% percent accurate at identifying cases, whereas blood loss–based methods were accurate 67% of the time.
Several of the 24 characteristics included in the model appear in other risk predictors, including whether a woman has had a previous cesarean delivery or prior postdelivery bleeding and whether she has anemia or related blood disorders. Among the rest were risk factors that have been identified in the literature, including maternal blood pressure, time from admission to delivery, and average pulse during hospitalization. Five more features were new: red blood cell count and distribution width, mean corpuscular hemoglobin, absolute neutrophil count, and white blood cell count.
“These [new] values are easily obtainable from standard blood draws in the hospital but are not currently used in clinical practice to estimate postpartum hemorrhage risk,” Dr. Li said.
In a related retrospective study, Dr. Li and her colleagues used the new tool to classify women into high, low, or medium risk categories. They found that 28% of the women the algorithm classified as high risk experienced a postpartum hemorrhage compared with 15% to 19% of the women classified as high risk by standard predictive tools. They also identified potential “inflection points” where changes in vital signs may suggest a substantial increase in risk. For example, women whose median blood pressure during labor and delivery was above 132 mm Hg had an 11% average increase in their risk for bleeding.
By more precisely identifying women at risk, the new method “could be used to pre-emptively allocate resources that can ultimately reduce postpartum hemorrhage morbidity and mortality,” Dr. Li said. Sema4 is launching a prospective clinical trial to further assess the algorithm, she added.
Finding the continuum of risk
Holly Ende, MD, an obstetric anesthesiologist at Vanderbilt University Medical Center, Nashville, Tenn., said approaches that leverage electronic health records to identify women at risk for hemorrhage have many advantages over currently used tools.
“Machine learning models or statistical models are able to take into account many more risk factors and weigh each of those risk factors based on how much they contribute to risk,” Dr. Ende, who was not involved in the new studies, told this news organization. “We can stratify women more on a continuum.”
But digital approaches have potential downsides.
“Machine learning algorithms can be developed in such a way that perpetuates racial bias, and it’s important to be aware of potential biases in coded algorithms,” Dr. Li said. To help reduce such bias, they used a database that included a racially and ethnically diverse patient population, but she acknowledged that additional research is needed.
Dr. Ende, the coauthor of a commentary in Obstetrics & Gynecology on risk assessment for postpartum hemorrhage, said algorithm developers must be sensitive to pre-existing disparities in health care that may affect the data they use to build the software.
She pointed to uterine atony – a known risk factor for hemorrhage – as an example. In her own research, she and her colleagues identified women with atony by searching their medical records for medications used to treat the condition. But when they ran their model, Black women were less likely to develop uterine atony, which the team knew wasn’t true in the real world. They traced the problem to an existing disparity in obstetric care: Black women with uterine atony were less likely than women of other races to receive medications for the condition.
“People need to be cognizant as they are developing these types of prediction models and be extremely careful to avoid perpetuating any disparities in care,” Dr. Ende cautioned. On the other hand, if carefully developed, these tools might also help reduce disparities in health care by standardizing risk stratification and clinical practices, she said.
In addition to independent validation of data-based risk prediction tools, Dr. Ende said ensuring that clinicians are properly trained to use these tools is crucial.
“Implementation may be the biggest limitation,” she said.
Dr. Ende and Dr. Li have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Alternative birthing practices tied to neonatal infection risk
Increasingly popular alternative peripartum practices such as water immersion and nonseverance of the umbilical cord may increase the risk of infections in newborns, a new clinical report from the American Academy of Pediatrics found.
Another perinatal measure potentially raising infection risk was placentophagy, according to a review led by Dawn Nolt, MD, MPH, a professor of pediatric infectious diseases at Oregon Health & Science University, Portland.
“Awareness of emerging alternative peripartum and neonatal practices helps pediatricians provide counseling to families before birth and to appropriately evaluate and treat neonates who have been exposed to these practices,” Dr. Nolt and colleagues wrote online in Pediatrics.
Amid growing inquiries made from women seeking a positive and meaningful birth experience through alternative approaches as well as reports of possibly related illness in newborns, Dr. Nolt’s group reviewed observational studies, case series, and medical society guidance on the risks associated with seven alternative birthing practices.
Based on their summation, it was not possible to quantify the actual risk associated with any one practice. “But of the seven we reviewed, as an infectious disease pediatrician I would say the most discernible immediate risk is likely attached to nonseverance of the cord,” Dr. Nolt said in an interview. “Left attached, the tissue can potentially necrote and transfer bacteria directly to the child.”
The authors made the following recommendations:
- Water immersion for labor and delivery. While this can increase the comfort of the mother in the first stages of labor, the water can become contaminated and increase the infant’s exposure to water-borne pathogens such as Legionella and Pseudomonas. It is not recommended after the second stage of labor and if offered, requires rigorous prophylactic and infection-control measures. This practice has also been linked to aspiration, drowning, hyponatremia, cord rupture, and death.
- Vaginal seeding. The skin, noses, and mouths of infants born by cesarean section are inoculated with swabs of vaginal fluid in order to expose them to vaginal bacteria that positively influence the infant’s microbiome. Of no known benefit, this measure can expose newborns to microbes such as group B Streptococcus and herpes simplex virus. Infants born by C-section receiving vaginal seeding should be evaluated the same way as those delivered vaginally.
- Umbilical cord nonseverance. Colloquially known as lotus birth, this is another practice with no evidence of advantage but with the potential to raise the risk of neonatal sepsis owing to the presence of necrotic umbilical or placental tissue. Some parents may view the placenta as a spiritual entity and fail to recognize it may be contaminated with harmful pathogens. Any placenta and umbilical cord attached to a febrile or ill-seeming neonate should be immediately removed.
- Placentophagy. Proponents believe placental consumption has antidepressive, analgesic, galactogogic, and nutritional properties. But eating raw, cooked, or dehydrated afterbirth tissue – viewed by some as a spiritual event – can expose a neonate to flora from the mother’s genitourinary tract and other sources encountered during preparation. Placentophagy has been associated with a case of recurrent late-onset group B streptococcal sepsis in a newborn. Strict food-handling practices at the level for raw meat should be maintained.
- HBV vaccine deferral. Viewed as “a critical safety net in preventing HBV infection,” the birth dose of the hepatitis B virus vaccine should not be postponed except for medical reasons. An estimated 1,000 new perinatally acquired HBV cases occurred annually in the United States from 2000 to 2009.
- Deferral of ocular prophylaxis. While ocular prophylaxis with topical erythromycin protects against gonococcal ophthalmia neonatorum, particularly in infants of high-risk mothers, it is not effective against other common pathogens. Parents and health care providers have recently questioned the need for its routine application, with concerns including its limited range of effectiveness as well as antibiotic resistance and shortages. With adequate prenatal testing, the risk of this neonatal conjunctivitis is significantly reduced, and deferral of prophylaxis may be considered in low-risk situations although it may be mandated by state legislation.
- Delayed bathing. The practice of delaying the infant’s first bath until several hours after birth may have several benefits. These include the initiation and exclusivity of breastfeeding, decreased mother/child separation time and risk of hypothermia, and protection of the neonatal skin microbiome. It should be discouraged, however, in neonates exposed to active herpes simplex virus lesions or whose mothers have a known history of HIV infection.
When women inquire about alternative practices, physicians need to strike a diplomatic balance between respecting women’s wishes and the benefits they hope to gain and at the same time informing them of potential risks, Dr. Nolt said. “The conversation we want to have with them should show compassion and sympathy but also tell them what the medical literature shows.” Patient and doctor should engage in shared decision-making about the safety of various alternative approaches.
“Over the last decade information on a variety of birth practices have become more widely available through social media and other Internet forums, which certainly has increased the variety of questions to health professionals, Amy C. Hermesch, MD, PhD, director of obstetric services at OHSC, said in an interview.
“We counsel about rare but serious risk, as noted in Dr. Nolt’s article,” said Dr. Hermesch, who was not involved in the AAP report. Most important is a discussion about appropriate pregnancy risk stratification. “For example, persons considering water immersion birth, probably the most common one I get inquiries about, should have an otherwise uncomplicated pregnancy with good mobility to get in and out of tub in the event of an emergency.”
While adverse events can happen during any birth, she sees these more often in mothers who underestimate the risk level of their situation or pregnancy when declining provider-recommended interventions. “I encourage pregnant persons to find a health care professional they trust who is knowledgeable about the benefits and the risk of all birth environments and interventions.”
Dr. Hermesch added that most alternative practices have little data to guide decisions, so she offers professional society recommendations, evidence review, and her own professional experiences. “The patient must weight the risk and benefits in the context of their value system and sometimes this means not following my advice or recommendations. My medical recommendation with the best of intentions does not remove patient autonomy.”
This report had no external funding. The authors had no potential conflicts of interest to disclose. Dr. Hermesch had no competing interests to declare.
Increasingly popular alternative peripartum practices such as water immersion and nonseverance of the umbilical cord may increase the risk of infections in newborns, a new clinical report from the American Academy of Pediatrics found.
Another perinatal measure potentially raising infection risk was placentophagy, according to a review led by Dawn Nolt, MD, MPH, a professor of pediatric infectious diseases at Oregon Health & Science University, Portland.
“Awareness of emerging alternative peripartum and neonatal practices helps pediatricians provide counseling to families before birth and to appropriately evaluate and treat neonates who have been exposed to these practices,” Dr. Nolt and colleagues wrote online in Pediatrics.
Amid growing inquiries made from women seeking a positive and meaningful birth experience through alternative approaches as well as reports of possibly related illness in newborns, Dr. Nolt’s group reviewed observational studies, case series, and medical society guidance on the risks associated with seven alternative birthing practices.
Based on their summation, it was not possible to quantify the actual risk associated with any one practice. “But of the seven we reviewed, as an infectious disease pediatrician I would say the most discernible immediate risk is likely attached to nonseverance of the cord,” Dr. Nolt said in an interview. “Left attached, the tissue can potentially necrote and transfer bacteria directly to the child.”
The authors made the following recommendations:
- Water immersion for labor and delivery. While this can increase the comfort of the mother in the first stages of labor, the water can become contaminated and increase the infant’s exposure to water-borne pathogens such as Legionella and Pseudomonas. It is not recommended after the second stage of labor and if offered, requires rigorous prophylactic and infection-control measures. This practice has also been linked to aspiration, drowning, hyponatremia, cord rupture, and death.
- Vaginal seeding. The skin, noses, and mouths of infants born by cesarean section are inoculated with swabs of vaginal fluid in order to expose them to vaginal bacteria that positively influence the infant’s microbiome. Of no known benefit, this measure can expose newborns to microbes such as group B Streptococcus and herpes simplex virus. Infants born by C-section receiving vaginal seeding should be evaluated the same way as those delivered vaginally.
- Umbilical cord nonseverance. Colloquially known as lotus birth, this is another practice with no evidence of advantage but with the potential to raise the risk of neonatal sepsis owing to the presence of necrotic umbilical or placental tissue. Some parents may view the placenta as a spiritual entity and fail to recognize it may be contaminated with harmful pathogens. Any placenta and umbilical cord attached to a febrile or ill-seeming neonate should be immediately removed.
- Placentophagy. Proponents believe placental consumption has antidepressive, analgesic, galactogogic, and nutritional properties. But eating raw, cooked, or dehydrated afterbirth tissue – viewed by some as a spiritual event – can expose a neonate to flora from the mother’s genitourinary tract and other sources encountered during preparation. Placentophagy has been associated with a case of recurrent late-onset group B streptococcal sepsis in a newborn. Strict food-handling practices at the level for raw meat should be maintained.
- HBV vaccine deferral. Viewed as “a critical safety net in preventing HBV infection,” the birth dose of the hepatitis B virus vaccine should not be postponed except for medical reasons. An estimated 1,000 new perinatally acquired HBV cases occurred annually in the United States from 2000 to 2009.
- Deferral of ocular prophylaxis. While ocular prophylaxis with topical erythromycin protects against gonococcal ophthalmia neonatorum, particularly in infants of high-risk mothers, it is not effective against other common pathogens. Parents and health care providers have recently questioned the need for its routine application, with concerns including its limited range of effectiveness as well as antibiotic resistance and shortages. With adequate prenatal testing, the risk of this neonatal conjunctivitis is significantly reduced, and deferral of prophylaxis may be considered in low-risk situations although it may be mandated by state legislation.
- Delayed bathing. The practice of delaying the infant’s first bath until several hours after birth may have several benefits. These include the initiation and exclusivity of breastfeeding, decreased mother/child separation time and risk of hypothermia, and protection of the neonatal skin microbiome. It should be discouraged, however, in neonates exposed to active herpes simplex virus lesions or whose mothers have a known history of HIV infection.
When women inquire about alternative practices, physicians need to strike a diplomatic balance between respecting women’s wishes and the benefits they hope to gain and at the same time informing them of potential risks, Dr. Nolt said. “The conversation we want to have with them should show compassion and sympathy but also tell them what the medical literature shows.” Patient and doctor should engage in shared decision-making about the safety of various alternative approaches.
“Over the last decade information on a variety of birth practices have become more widely available through social media and other Internet forums, which certainly has increased the variety of questions to health professionals, Amy C. Hermesch, MD, PhD, director of obstetric services at OHSC, said in an interview.
“We counsel about rare but serious risk, as noted in Dr. Nolt’s article,” said Dr. Hermesch, who was not involved in the AAP report. Most important is a discussion about appropriate pregnancy risk stratification. “For example, persons considering water immersion birth, probably the most common one I get inquiries about, should have an otherwise uncomplicated pregnancy with good mobility to get in and out of tub in the event of an emergency.”
While adverse events can happen during any birth, she sees these more often in mothers who underestimate the risk level of their situation or pregnancy when declining provider-recommended interventions. “I encourage pregnant persons to find a health care professional they trust who is knowledgeable about the benefits and the risk of all birth environments and interventions.”
Dr. Hermesch added that most alternative practices have little data to guide decisions, so she offers professional society recommendations, evidence review, and her own professional experiences. “The patient must weight the risk and benefits in the context of their value system and sometimes this means not following my advice or recommendations. My medical recommendation with the best of intentions does not remove patient autonomy.”
This report had no external funding. The authors had no potential conflicts of interest to disclose. Dr. Hermesch had no competing interests to declare.
Increasingly popular alternative peripartum practices such as water immersion and nonseverance of the umbilical cord may increase the risk of infections in newborns, a new clinical report from the American Academy of Pediatrics found.
Another perinatal measure potentially raising infection risk was placentophagy, according to a review led by Dawn Nolt, MD, MPH, a professor of pediatric infectious diseases at Oregon Health & Science University, Portland.
“Awareness of emerging alternative peripartum and neonatal practices helps pediatricians provide counseling to families before birth and to appropriately evaluate and treat neonates who have been exposed to these practices,” Dr. Nolt and colleagues wrote online in Pediatrics.
Amid growing inquiries made from women seeking a positive and meaningful birth experience through alternative approaches as well as reports of possibly related illness in newborns, Dr. Nolt’s group reviewed observational studies, case series, and medical society guidance on the risks associated with seven alternative birthing practices.
Based on their summation, it was not possible to quantify the actual risk associated with any one practice. “But of the seven we reviewed, as an infectious disease pediatrician I would say the most discernible immediate risk is likely attached to nonseverance of the cord,” Dr. Nolt said in an interview. “Left attached, the tissue can potentially necrote and transfer bacteria directly to the child.”
The authors made the following recommendations:
- Water immersion for labor and delivery. While this can increase the comfort of the mother in the first stages of labor, the water can become contaminated and increase the infant’s exposure to water-borne pathogens such as Legionella and Pseudomonas. It is not recommended after the second stage of labor and if offered, requires rigorous prophylactic and infection-control measures. This practice has also been linked to aspiration, drowning, hyponatremia, cord rupture, and death.
- Vaginal seeding. The skin, noses, and mouths of infants born by cesarean section are inoculated with swabs of vaginal fluid in order to expose them to vaginal bacteria that positively influence the infant’s microbiome. Of no known benefit, this measure can expose newborns to microbes such as group B Streptococcus and herpes simplex virus. Infants born by C-section receiving vaginal seeding should be evaluated the same way as those delivered vaginally.
- Umbilical cord nonseverance. Colloquially known as lotus birth, this is another practice with no evidence of advantage but with the potential to raise the risk of neonatal sepsis owing to the presence of necrotic umbilical or placental tissue. Some parents may view the placenta as a spiritual entity and fail to recognize it may be contaminated with harmful pathogens. Any placenta and umbilical cord attached to a febrile or ill-seeming neonate should be immediately removed.
- Placentophagy. Proponents believe placental consumption has antidepressive, analgesic, galactogogic, and nutritional properties. But eating raw, cooked, or dehydrated afterbirth tissue – viewed by some as a spiritual event – can expose a neonate to flora from the mother’s genitourinary tract and other sources encountered during preparation. Placentophagy has been associated with a case of recurrent late-onset group B streptococcal sepsis in a newborn. Strict food-handling practices at the level for raw meat should be maintained.
- HBV vaccine deferral. Viewed as “a critical safety net in preventing HBV infection,” the birth dose of the hepatitis B virus vaccine should not be postponed except for medical reasons. An estimated 1,000 new perinatally acquired HBV cases occurred annually in the United States from 2000 to 2009.
- Deferral of ocular prophylaxis. While ocular prophylaxis with topical erythromycin protects against gonococcal ophthalmia neonatorum, particularly in infants of high-risk mothers, it is not effective against other common pathogens. Parents and health care providers have recently questioned the need for its routine application, with concerns including its limited range of effectiveness as well as antibiotic resistance and shortages. With adequate prenatal testing, the risk of this neonatal conjunctivitis is significantly reduced, and deferral of prophylaxis may be considered in low-risk situations although it may be mandated by state legislation.
- Delayed bathing. The practice of delaying the infant’s first bath until several hours after birth may have several benefits. These include the initiation and exclusivity of breastfeeding, decreased mother/child separation time and risk of hypothermia, and protection of the neonatal skin microbiome. It should be discouraged, however, in neonates exposed to active herpes simplex virus lesions or whose mothers have a known history of HIV infection.
When women inquire about alternative practices, physicians need to strike a diplomatic balance between respecting women’s wishes and the benefits they hope to gain and at the same time informing them of potential risks, Dr. Nolt said. “The conversation we want to have with them should show compassion and sympathy but also tell them what the medical literature shows.” Patient and doctor should engage in shared decision-making about the safety of various alternative approaches.
“Over the last decade information on a variety of birth practices have become more widely available through social media and other Internet forums, which certainly has increased the variety of questions to health professionals, Amy C. Hermesch, MD, PhD, director of obstetric services at OHSC, said in an interview.
“We counsel about rare but serious risk, as noted in Dr. Nolt’s article,” said Dr. Hermesch, who was not involved in the AAP report. Most important is a discussion about appropriate pregnancy risk stratification. “For example, persons considering water immersion birth, probably the most common one I get inquiries about, should have an otherwise uncomplicated pregnancy with good mobility to get in and out of tub in the event of an emergency.”
While adverse events can happen during any birth, she sees these more often in mothers who underestimate the risk level of their situation or pregnancy when declining provider-recommended interventions. “I encourage pregnant persons to find a health care professional they trust who is knowledgeable about the benefits and the risk of all birth environments and interventions.”
Dr. Hermesch added that most alternative practices have little data to guide decisions, so she offers professional society recommendations, evidence review, and her own professional experiences. “The patient must weight the risk and benefits in the context of their value system and sometimes this means not following my advice or recommendations. My medical recommendation with the best of intentions does not remove patient autonomy.”
This report had no external funding. The authors had no potential conflicts of interest to disclose. Dr. Hermesch had no competing interests to declare.
FROM PEDIATRICS
Things reproductive psychiatrists might ‘always’ or ‘never’ do in 2022
The experience of practicing reproductive psychiatry in the context of the pandemic has highlighted unique situations I’ve written about in previous columns that have affected pregnant and postpartum women during the pandemic, such as the management of anxiety and insomnia.
The pandemic has also seen a shift to telemedicine and an opportunity to use virtual platforms to engage with colleagues in our subspecialty across the country. These forums of engagement, which we realize virtually with so many of our colleagues, has prompted me to refine and galvanize what I consider to be some principles that guide frequently encountered clinical scenarios in reproductive psychiatry.
To open 2022, I wanted to revisit the practices I nearly “always” (or conversely, “never”) follow as a reproductive psychiatrist across the numerous clinical situations and variations on the associated clinical themes encountered as we see patients during pregnancy and the postpartum period.
Things we ‘always’ do
1. I continue to make maternal euthymia the North Star of treatment before, during, and after pregnancy.
Before pregnancy, maternal euthymia may be realized through optimization of pharmacologic and nonpharmacologic treatments and waiting to conceive until patients are emotionally well. Sustaining euthymia during pregnancy is a critical issue because of the extent to which euthymia during pregnancy predicts postpartum course. According to many studies, postpartum euthymia is the strongest predictor of long-term neurobehavioral outcome and risk for later child psychopathology. At the end of the day, there are few things I would not do with respect to treatment of maternal psychiatric disorder if the upside afforded maternal euthymia.
2. I almost always treat with consistency of medication across the peripartum period.
Although there have been discussions about the wisdom of changing medications, such as antidepressants, benzodiazepines, and mood stabilizers, that have afforded euthymia during pregnancy as patients approach their delivery date, the evidence base supporting switching medications at that time is exceedingly sparse. The time to adjust or to modify is typically not just prior to delivery unless it is to prevent postpartum psychiatric disorder (see below).
3. I simplify regimens before pregnancy if it’s unclear which medications have afforded patients euthymia.
We have a growing appreciation that polypharmacy is the rule in treatment of affective disorder for both unipolar and bipolar illness. Consultation before pregnancy is the ideal time to take a particularly careful history and think about simplifying regimens where adding medicines hasn’t clearly provided enhanced clinical benefit to the patient.
4. When making a treatment plan for psychiatric disorder during pregnancy, I consider the impact of untreated psychiatric disorder (even if not absolutely quantifiable) on fetal, neonatal, and maternal well-being.
Perhaps now more than even 5-10 years ago, we have better data describing the adverse effects of untreated psychiatric illness on fetal, neonatal, and maternal well-being.
We always try to deliberately consider the effect of a specific treatment on fetal well-being. Less attention (and science) has focused on the effect on pregnancy of deferring treatment; historically, this has not been adequately quantified in the risk-benefit decision. Yet, there is growing evidence of the increased adverse effects of activating the stress axis on everything from intrauterine fetal programming in the brain to effects on obstetrical outcomes such as preterm labor and delivery.
5. I appreciate the value of postpartum prophylaxis for pregnant women with bipolar disorder to mitigate risk of relapse.
We have spoken over the last 20 months of the pandemic, particularly in reproductive psychiatry circles, about the importance of keeping reproductive-age women with bipolar disorder emotionally well as they plan to conceive, during pregnancy, and in the postpartum period. The management of bipolar disorder during this time can be a humbling experience. Clinical roughening can be quick and severe, and so we do everything that we can for these women.
The area in which we have the strongest evidence base for mitigating risk with bipolar women is the value of postpartum prophylaxis during the peripartum period, regardless of what patients have done with their mood-stabilizing medications during pregnancy. Given the risk for postpartum disease, even though there are varying amounts of evidence on prophylactic benefit of specific mood stabilizers (i.e., lithium vs. atypical antipsychotics), the value of prophylaxis against worsening of bipolar disorder postpartum is widely accepted.
The importance of this has been particularly underscored during the pandemic where postpartum support, although available, has been more tenuous given the fluctuations in COVID-19 status around the country. The availability of friends and loved ones as support during the postpartum period has become less reliable in certain circumstances during the pandemic. In some cases, COVID-19 surges have wreaked havoc on travel plans and support persons have contracted the virus, rendering on-site support nonviable given safety concerns. Last-minute shifts of support plans have been responsible for disruption of care plans for new moms and by extension, have affected the ability to protect the sleep of bipolar women, which is critical. Keeping bipolar women well during the postpartum period with plans and backup plans for management remains critical.
Things we ‘never’ do
1. I never taper antidepressants (just prior to delivery), I never check plasma levels of selective serotonin reuptake inhibitors (across pregnancy, or just prior to labor and delivery), and I never use sodium valproate (during pregnancy).
Although there has been some discussion about the potential to mitigate risk for maternal or neonatal toxicity with lowering of agents such as lithium or lamotrigine during pregnancy, I do not routinely check plasma levels or arbitrarily change the dose of antidepressants, lithium, or lamotrigine during pregnancy in the absence of clinical symptoms.
We know full well that plasma levels of medications decline during pregnancy because of hemodilution with lithium and antidepressants and, in the case of lamotrigine, the effects of rising estrogen concentration during pregnancy on the metabolism of lamotrigine. While several studies have shown the decrease of SSRI concentration during pregnancy absent a change in dose of medication, these data have not correlated changes in plasma concentration of SSRI with a frank change in clinical status across pregnancy. Unlike what we see in conditions like epilepsy, where doses are increased to maintain therapeutic plasma levels to mitigate risk for seizure, those therapeutic plasma levels do not clearly exist for the psychiatric medications most widely used to treat psychiatric disorders.
We also almost never use sodium valproate in reproductive-age women despite its efficacy in both the acute and maintenance treatment of bipolar disorder given the risk of both major malformations associated with first-trimester fetal exposure to valproate and the data suggesting longer-term adverse neurobehavioral effects associated with its use during pregnancy.
2. We never suggest patients defer pregnancy based on their underlying psychiatric disorder.
Our role is to provide the best information regarding reproductive safety of psychiatric medications and risks of untreated psychiatric disorder to patients as they and relevant parties weigh the risks of pursuing one treatment or another. Those are private choices, and women and their partners make private decisions applying their own calculus with respect to moving forward with plans to conceive.
3. We never switch antidepressants once a woman has become pregnant.
Although we continue to see patients switched to older SSRIs such as sertraline with documentation of pregnancy, a patient’s road to getting well is sometimes very lengthy. In the absence of indicting reproductive safety data for any particular antidepressant, for patients who have gotten well on an antidepressant, even one for which we have less information, we stay the course and do not switch arbitrarily to an older SSRI for which we may have more reproductive safety data.
If we have the luxury prior to pregnancy to switch a patient to an untried and better studied antidepressant with more data supporting safety, we do so. But this is rarely the case. More often, we see women presenting with a newly documented pregnancy (frequently unplanned, with half of pregnancies across the country still being unplanned across sociodemographic lines) on an antidepressant with varying amounts of reproductive safety information available for the medicine being taken, and frequently after failed previous trials of other antidepressants. In this scenario, we rarely see the time of a newly documented pregnancy as an opportunity to pursue a new trial of an antidepressant without known efficacy for that patient; we stay the course and hope for sustained euthymia on the drug which has afforded euthymia to date.
Final thoughts
Dos and don’ts are relative in reproductive psychiatry. We tend to apply available data and clinical experience as we guide patients on a case-by-case basis, considering the most currently available rigorous reproductive safety data, as well as the individual patient’s clinical status and her personal wishes.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
The experience of practicing reproductive psychiatry in the context of the pandemic has highlighted unique situations I’ve written about in previous columns that have affected pregnant and postpartum women during the pandemic, such as the management of anxiety and insomnia.
The pandemic has also seen a shift to telemedicine and an opportunity to use virtual platforms to engage with colleagues in our subspecialty across the country. These forums of engagement, which we realize virtually with so many of our colleagues, has prompted me to refine and galvanize what I consider to be some principles that guide frequently encountered clinical scenarios in reproductive psychiatry.
To open 2022, I wanted to revisit the practices I nearly “always” (or conversely, “never”) follow as a reproductive psychiatrist across the numerous clinical situations and variations on the associated clinical themes encountered as we see patients during pregnancy and the postpartum period.
Things we ‘always’ do
1. I continue to make maternal euthymia the North Star of treatment before, during, and after pregnancy.
Before pregnancy, maternal euthymia may be realized through optimization of pharmacologic and nonpharmacologic treatments and waiting to conceive until patients are emotionally well. Sustaining euthymia during pregnancy is a critical issue because of the extent to which euthymia during pregnancy predicts postpartum course. According to many studies, postpartum euthymia is the strongest predictor of long-term neurobehavioral outcome and risk for later child psychopathology. At the end of the day, there are few things I would not do with respect to treatment of maternal psychiatric disorder if the upside afforded maternal euthymia.
2. I almost always treat with consistency of medication across the peripartum period.
Although there have been discussions about the wisdom of changing medications, such as antidepressants, benzodiazepines, and mood stabilizers, that have afforded euthymia during pregnancy as patients approach their delivery date, the evidence base supporting switching medications at that time is exceedingly sparse. The time to adjust or to modify is typically not just prior to delivery unless it is to prevent postpartum psychiatric disorder (see below).
3. I simplify regimens before pregnancy if it’s unclear which medications have afforded patients euthymia.
We have a growing appreciation that polypharmacy is the rule in treatment of affective disorder for both unipolar and bipolar illness. Consultation before pregnancy is the ideal time to take a particularly careful history and think about simplifying regimens where adding medicines hasn’t clearly provided enhanced clinical benefit to the patient.
4. When making a treatment plan for psychiatric disorder during pregnancy, I consider the impact of untreated psychiatric disorder (even if not absolutely quantifiable) on fetal, neonatal, and maternal well-being.
Perhaps now more than even 5-10 years ago, we have better data describing the adverse effects of untreated psychiatric illness on fetal, neonatal, and maternal well-being.
We always try to deliberately consider the effect of a specific treatment on fetal well-being. Less attention (and science) has focused on the effect on pregnancy of deferring treatment; historically, this has not been adequately quantified in the risk-benefit decision. Yet, there is growing evidence of the increased adverse effects of activating the stress axis on everything from intrauterine fetal programming in the brain to effects on obstetrical outcomes such as preterm labor and delivery.
5. I appreciate the value of postpartum prophylaxis for pregnant women with bipolar disorder to mitigate risk of relapse.
We have spoken over the last 20 months of the pandemic, particularly in reproductive psychiatry circles, about the importance of keeping reproductive-age women with bipolar disorder emotionally well as they plan to conceive, during pregnancy, and in the postpartum period. The management of bipolar disorder during this time can be a humbling experience. Clinical roughening can be quick and severe, and so we do everything that we can for these women.
The area in which we have the strongest evidence base for mitigating risk with bipolar women is the value of postpartum prophylaxis during the peripartum period, regardless of what patients have done with their mood-stabilizing medications during pregnancy. Given the risk for postpartum disease, even though there are varying amounts of evidence on prophylactic benefit of specific mood stabilizers (i.e., lithium vs. atypical antipsychotics), the value of prophylaxis against worsening of bipolar disorder postpartum is widely accepted.
The importance of this has been particularly underscored during the pandemic where postpartum support, although available, has been more tenuous given the fluctuations in COVID-19 status around the country. The availability of friends and loved ones as support during the postpartum period has become less reliable in certain circumstances during the pandemic. In some cases, COVID-19 surges have wreaked havoc on travel plans and support persons have contracted the virus, rendering on-site support nonviable given safety concerns. Last-minute shifts of support plans have been responsible for disruption of care plans for new moms and by extension, have affected the ability to protect the sleep of bipolar women, which is critical. Keeping bipolar women well during the postpartum period with plans and backup plans for management remains critical.
Things we ‘never’ do
1. I never taper antidepressants (just prior to delivery), I never check plasma levels of selective serotonin reuptake inhibitors (across pregnancy, or just prior to labor and delivery), and I never use sodium valproate (during pregnancy).
Although there has been some discussion about the potential to mitigate risk for maternal or neonatal toxicity with lowering of agents such as lithium or lamotrigine during pregnancy, I do not routinely check plasma levels or arbitrarily change the dose of antidepressants, lithium, or lamotrigine during pregnancy in the absence of clinical symptoms.
We know full well that plasma levels of medications decline during pregnancy because of hemodilution with lithium and antidepressants and, in the case of lamotrigine, the effects of rising estrogen concentration during pregnancy on the metabolism of lamotrigine. While several studies have shown the decrease of SSRI concentration during pregnancy absent a change in dose of medication, these data have not correlated changes in plasma concentration of SSRI with a frank change in clinical status across pregnancy. Unlike what we see in conditions like epilepsy, where doses are increased to maintain therapeutic plasma levels to mitigate risk for seizure, those therapeutic plasma levels do not clearly exist for the psychiatric medications most widely used to treat psychiatric disorders.
We also almost never use sodium valproate in reproductive-age women despite its efficacy in both the acute and maintenance treatment of bipolar disorder given the risk of both major malformations associated with first-trimester fetal exposure to valproate and the data suggesting longer-term adverse neurobehavioral effects associated with its use during pregnancy.
2. We never suggest patients defer pregnancy based on their underlying psychiatric disorder.
Our role is to provide the best information regarding reproductive safety of psychiatric medications and risks of untreated psychiatric disorder to patients as they and relevant parties weigh the risks of pursuing one treatment or another. Those are private choices, and women and their partners make private decisions applying their own calculus with respect to moving forward with plans to conceive.
3. We never switch antidepressants once a woman has become pregnant.
Although we continue to see patients switched to older SSRIs such as sertraline with documentation of pregnancy, a patient’s road to getting well is sometimes very lengthy. In the absence of indicting reproductive safety data for any particular antidepressant, for patients who have gotten well on an antidepressant, even one for which we have less information, we stay the course and do not switch arbitrarily to an older SSRI for which we may have more reproductive safety data.
If we have the luxury prior to pregnancy to switch a patient to an untried and better studied antidepressant with more data supporting safety, we do so. But this is rarely the case. More often, we see women presenting with a newly documented pregnancy (frequently unplanned, with half of pregnancies across the country still being unplanned across sociodemographic lines) on an antidepressant with varying amounts of reproductive safety information available for the medicine being taken, and frequently after failed previous trials of other antidepressants. In this scenario, we rarely see the time of a newly documented pregnancy as an opportunity to pursue a new trial of an antidepressant without known efficacy for that patient; we stay the course and hope for sustained euthymia on the drug which has afforded euthymia to date.
Final thoughts
Dos and don’ts are relative in reproductive psychiatry. We tend to apply available data and clinical experience as we guide patients on a case-by-case basis, considering the most currently available rigorous reproductive safety data, as well as the individual patient’s clinical status and her personal wishes.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
The experience of practicing reproductive psychiatry in the context of the pandemic has highlighted unique situations I’ve written about in previous columns that have affected pregnant and postpartum women during the pandemic, such as the management of anxiety and insomnia.
The pandemic has also seen a shift to telemedicine and an opportunity to use virtual platforms to engage with colleagues in our subspecialty across the country. These forums of engagement, which we realize virtually with so many of our colleagues, has prompted me to refine and galvanize what I consider to be some principles that guide frequently encountered clinical scenarios in reproductive psychiatry.
To open 2022, I wanted to revisit the practices I nearly “always” (or conversely, “never”) follow as a reproductive psychiatrist across the numerous clinical situations and variations on the associated clinical themes encountered as we see patients during pregnancy and the postpartum period.
Things we ‘always’ do
1. I continue to make maternal euthymia the North Star of treatment before, during, and after pregnancy.
Before pregnancy, maternal euthymia may be realized through optimization of pharmacologic and nonpharmacologic treatments and waiting to conceive until patients are emotionally well. Sustaining euthymia during pregnancy is a critical issue because of the extent to which euthymia during pregnancy predicts postpartum course. According to many studies, postpartum euthymia is the strongest predictor of long-term neurobehavioral outcome and risk for later child psychopathology. At the end of the day, there are few things I would not do with respect to treatment of maternal psychiatric disorder if the upside afforded maternal euthymia.
2. I almost always treat with consistency of medication across the peripartum period.
Although there have been discussions about the wisdom of changing medications, such as antidepressants, benzodiazepines, and mood stabilizers, that have afforded euthymia during pregnancy as patients approach their delivery date, the evidence base supporting switching medications at that time is exceedingly sparse. The time to adjust or to modify is typically not just prior to delivery unless it is to prevent postpartum psychiatric disorder (see below).
3. I simplify regimens before pregnancy if it’s unclear which medications have afforded patients euthymia.
We have a growing appreciation that polypharmacy is the rule in treatment of affective disorder for both unipolar and bipolar illness. Consultation before pregnancy is the ideal time to take a particularly careful history and think about simplifying regimens where adding medicines hasn’t clearly provided enhanced clinical benefit to the patient.
4. When making a treatment plan for psychiatric disorder during pregnancy, I consider the impact of untreated psychiatric disorder (even if not absolutely quantifiable) on fetal, neonatal, and maternal well-being.
Perhaps now more than even 5-10 years ago, we have better data describing the adverse effects of untreated psychiatric illness on fetal, neonatal, and maternal well-being.
We always try to deliberately consider the effect of a specific treatment on fetal well-being. Less attention (and science) has focused on the effect on pregnancy of deferring treatment; historically, this has not been adequately quantified in the risk-benefit decision. Yet, there is growing evidence of the increased adverse effects of activating the stress axis on everything from intrauterine fetal programming in the brain to effects on obstetrical outcomes such as preterm labor and delivery.
5. I appreciate the value of postpartum prophylaxis for pregnant women with bipolar disorder to mitigate risk of relapse.
We have spoken over the last 20 months of the pandemic, particularly in reproductive psychiatry circles, about the importance of keeping reproductive-age women with bipolar disorder emotionally well as they plan to conceive, during pregnancy, and in the postpartum period. The management of bipolar disorder during this time can be a humbling experience. Clinical roughening can be quick and severe, and so we do everything that we can for these women.
The area in which we have the strongest evidence base for mitigating risk with bipolar women is the value of postpartum prophylaxis during the peripartum period, regardless of what patients have done with their mood-stabilizing medications during pregnancy. Given the risk for postpartum disease, even though there are varying amounts of evidence on prophylactic benefit of specific mood stabilizers (i.e., lithium vs. atypical antipsychotics), the value of prophylaxis against worsening of bipolar disorder postpartum is widely accepted.
The importance of this has been particularly underscored during the pandemic where postpartum support, although available, has been more tenuous given the fluctuations in COVID-19 status around the country. The availability of friends and loved ones as support during the postpartum period has become less reliable in certain circumstances during the pandemic. In some cases, COVID-19 surges have wreaked havoc on travel plans and support persons have contracted the virus, rendering on-site support nonviable given safety concerns. Last-minute shifts of support plans have been responsible for disruption of care plans for new moms and by extension, have affected the ability to protect the sleep of bipolar women, which is critical. Keeping bipolar women well during the postpartum period with plans and backup plans for management remains critical.
Things we ‘never’ do
1. I never taper antidepressants (just prior to delivery), I never check plasma levels of selective serotonin reuptake inhibitors (across pregnancy, or just prior to labor and delivery), and I never use sodium valproate (during pregnancy).
Although there has been some discussion about the potential to mitigate risk for maternal or neonatal toxicity with lowering of agents such as lithium or lamotrigine during pregnancy, I do not routinely check plasma levels or arbitrarily change the dose of antidepressants, lithium, or lamotrigine during pregnancy in the absence of clinical symptoms.
We know full well that plasma levels of medications decline during pregnancy because of hemodilution with lithium and antidepressants and, in the case of lamotrigine, the effects of rising estrogen concentration during pregnancy on the metabolism of lamotrigine. While several studies have shown the decrease of SSRI concentration during pregnancy absent a change in dose of medication, these data have not correlated changes in plasma concentration of SSRI with a frank change in clinical status across pregnancy. Unlike what we see in conditions like epilepsy, where doses are increased to maintain therapeutic plasma levels to mitigate risk for seizure, those therapeutic plasma levels do not clearly exist for the psychiatric medications most widely used to treat psychiatric disorders.
We also almost never use sodium valproate in reproductive-age women despite its efficacy in both the acute and maintenance treatment of bipolar disorder given the risk of both major malformations associated with first-trimester fetal exposure to valproate and the data suggesting longer-term adverse neurobehavioral effects associated with its use during pregnancy.
2. We never suggest patients defer pregnancy based on their underlying psychiatric disorder.
Our role is to provide the best information regarding reproductive safety of psychiatric medications and risks of untreated psychiatric disorder to patients as they and relevant parties weigh the risks of pursuing one treatment or another. Those are private choices, and women and their partners make private decisions applying their own calculus with respect to moving forward with plans to conceive.
3. We never switch antidepressants once a woman has become pregnant.
Although we continue to see patients switched to older SSRIs such as sertraline with documentation of pregnancy, a patient’s road to getting well is sometimes very lengthy. In the absence of indicting reproductive safety data for any particular antidepressant, for patients who have gotten well on an antidepressant, even one for which we have less information, we stay the course and do not switch arbitrarily to an older SSRI for which we may have more reproductive safety data.
If we have the luxury prior to pregnancy to switch a patient to an untried and better studied antidepressant with more data supporting safety, we do so. But this is rarely the case. More often, we see women presenting with a newly documented pregnancy (frequently unplanned, with half of pregnancies across the country still being unplanned across sociodemographic lines) on an antidepressant with varying amounts of reproductive safety information available for the medicine being taken, and frequently after failed previous trials of other antidepressants. In this scenario, we rarely see the time of a newly documented pregnancy as an opportunity to pursue a new trial of an antidepressant without known efficacy for that patient; we stay the course and hope for sustained euthymia on the drug which has afforded euthymia to date.
Final thoughts
Dos and don’ts are relative in reproductive psychiatry. We tend to apply available data and clinical experience as we guide patients on a case-by-case basis, considering the most currently available rigorous reproductive safety data, as well as the individual patient’s clinical status and her personal wishes.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Survey shows more women drinking during pregnancy
More pregnant Americans indulged – and overindulged – in alcohol from 2018 to 2020 than in previous years, but researchers found no sharp increase associated with the first wave of COVID-19 lockdowns, according to a new report from the Centers for Disease Control and Prevention.
The pandemic notwithstanding, health officials worry about a rising tide of pregnant women using alcohol and binge drinking since the CDC survey began in 2011. In the period ending in 2013, 1 in 10 women reported having had a drink in the previous 30 days; by 2017, that figure was 1 in 9; and in the latest survey, the number had risen to 1 in 7.
That mark is “the highest to date,” said Lucas Gosdin, PhD, MPH, an epidemic intelligence officer at the CDC’s National Center on Birth Defects and Developmental Disabilities, Atlanta, and first author of the report, which was published in Morbidity and Mortality Weekly Report.
“We’re concerned that this number has been slowly increasing,” Amanda Cohn, MD, director of the CDC’s Division of Birth Defects and Infant Disorders, told this news organization. “We need to be doing more outreach, both to pregnant persons as well as health care providers who are caring for them.”
Exposure to alcohol in the womb has been linked to a wide range of neurologic and physical problems in children, ranging from fetal alcohol syndrome to stunted learning abilities. Even if these problems are unlikely, experts insist there’s no known “safe” amount of alcohol a pregnant woman can have.
Dr. Cohn likened alcohol use to that of tobacco. “Lots of people smoke who don’t get lung cancer. Still, everyone is at an increased risk,” she said. “The safest way to not get lung cancer is to not smoke at all. It’s a behavior that can prevent additional harm.”
The report summarizes the results of a phone survey of 6,327 pregnant Americans between the ages of 18 and 49. The survey asked whether women had consumed an alcoholic beverage or had at least four drinks on one occasion – a common definition of binge drinking – in the past 30 days.
According to the report, 13.5% of women reported using alcohol, and 5.2% said they had binged on alcohol. Women who experienced frequent mental distress – describing their mental health as “not good” for 14 or more days in the past month – were twice as likely to drink and three times likelier to binge drink, the researchers found.
The increase within the 3-year period was roughly the same as in previous surveys.
“There was no evidence of increased alcohol consumption by pregnant adults in 2020 relative to 2019, despite possible increased alcohol sales and consumption among the general population during the first months of the COVID-19 pandemic,” the report states.
“That is one finding that was unexpected but that we were pleased to see,” Dr. Gosdin said.
Experts stressed that the survey covered only the first 9 months of the COVID-19 pandemic. “We’re still in the depths of it,” Samuel T. Bauer, MD, associate professor of obstetrics and gynecology at Duke University Medical Center, Durham, N.C., told this news organization. “People with alcohol use disorders certainly have been challenged during COVID. I think this is a preliminary answer.”
Dr. Gosdin said the effects of the pandemic on drinking habits bear watching. “We are concerned about the impacts of COVID-19,” he said. “We know it’s affected how people access regular care.”
Although virtual care has “exploded during COVID,” Dr. Bauer said, insurers have “turned off reimbursing” for doctor-patient visits via telephone, but not for visits by Internet-based video platforms like Zoom.
That split creates equity issues in many parts of the country, including his home state of North Carolina, where broadband is scarce, and patients may live 100 miles or more away from caregivers.
The “full-blown birth defect” of fetal alcohol syndrome is just the most visible hazard of drinking. Other medical and developmental issues include speech delays, slower learning and reading skills, attention-deficit and hyperactivity disorders, and problems with the heart and kidneys.
So, when Dr. Bauer encounters patients who believe that a few drinks will not harm their baby, he says he tells them: “’Why is this going to be where you put your flag?’ That leads to a different form of conversation.”
A version of this article first appeared on Medscape.com.
More pregnant Americans indulged – and overindulged – in alcohol from 2018 to 2020 than in previous years, but researchers found no sharp increase associated with the first wave of COVID-19 lockdowns, according to a new report from the Centers for Disease Control and Prevention.
The pandemic notwithstanding, health officials worry about a rising tide of pregnant women using alcohol and binge drinking since the CDC survey began in 2011. In the period ending in 2013, 1 in 10 women reported having had a drink in the previous 30 days; by 2017, that figure was 1 in 9; and in the latest survey, the number had risen to 1 in 7.
That mark is “the highest to date,” said Lucas Gosdin, PhD, MPH, an epidemic intelligence officer at the CDC’s National Center on Birth Defects and Developmental Disabilities, Atlanta, and first author of the report, which was published in Morbidity and Mortality Weekly Report.
“We’re concerned that this number has been slowly increasing,” Amanda Cohn, MD, director of the CDC’s Division of Birth Defects and Infant Disorders, told this news organization. “We need to be doing more outreach, both to pregnant persons as well as health care providers who are caring for them.”
Exposure to alcohol in the womb has been linked to a wide range of neurologic and physical problems in children, ranging from fetal alcohol syndrome to stunted learning abilities. Even if these problems are unlikely, experts insist there’s no known “safe” amount of alcohol a pregnant woman can have.
Dr. Cohn likened alcohol use to that of tobacco. “Lots of people smoke who don’t get lung cancer. Still, everyone is at an increased risk,” she said. “The safest way to not get lung cancer is to not smoke at all. It’s a behavior that can prevent additional harm.”
The report summarizes the results of a phone survey of 6,327 pregnant Americans between the ages of 18 and 49. The survey asked whether women had consumed an alcoholic beverage or had at least four drinks on one occasion – a common definition of binge drinking – in the past 30 days.
According to the report, 13.5% of women reported using alcohol, and 5.2% said they had binged on alcohol. Women who experienced frequent mental distress – describing their mental health as “not good” for 14 or more days in the past month – were twice as likely to drink and three times likelier to binge drink, the researchers found.
The increase within the 3-year period was roughly the same as in previous surveys.
“There was no evidence of increased alcohol consumption by pregnant adults in 2020 relative to 2019, despite possible increased alcohol sales and consumption among the general population during the first months of the COVID-19 pandemic,” the report states.
“That is one finding that was unexpected but that we were pleased to see,” Dr. Gosdin said.
Experts stressed that the survey covered only the first 9 months of the COVID-19 pandemic. “We’re still in the depths of it,” Samuel T. Bauer, MD, associate professor of obstetrics and gynecology at Duke University Medical Center, Durham, N.C., told this news organization. “People with alcohol use disorders certainly have been challenged during COVID. I think this is a preliminary answer.”
Dr. Gosdin said the effects of the pandemic on drinking habits bear watching. “We are concerned about the impacts of COVID-19,” he said. “We know it’s affected how people access regular care.”
Although virtual care has “exploded during COVID,” Dr. Bauer said, insurers have “turned off reimbursing” for doctor-patient visits via telephone, but not for visits by Internet-based video platforms like Zoom.
That split creates equity issues in many parts of the country, including his home state of North Carolina, where broadband is scarce, and patients may live 100 miles or more away from caregivers.
The “full-blown birth defect” of fetal alcohol syndrome is just the most visible hazard of drinking. Other medical and developmental issues include speech delays, slower learning and reading skills, attention-deficit and hyperactivity disorders, and problems with the heart and kidneys.
So, when Dr. Bauer encounters patients who believe that a few drinks will not harm their baby, he says he tells them: “’Why is this going to be where you put your flag?’ That leads to a different form of conversation.”
A version of this article first appeared on Medscape.com.
More pregnant Americans indulged – and overindulged – in alcohol from 2018 to 2020 than in previous years, but researchers found no sharp increase associated with the first wave of COVID-19 lockdowns, according to a new report from the Centers for Disease Control and Prevention.
The pandemic notwithstanding, health officials worry about a rising tide of pregnant women using alcohol and binge drinking since the CDC survey began in 2011. In the period ending in 2013, 1 in 10 women reported having had a drink in the previous 30 days; by 2017, that figure was 1 in 9; and in the latest survey, the number had risen to 1 in 7.
That mark is “the highest to date,” said Lucas Gosdin, PhD, MPH, an epidemic intelligence officer at the CDC’s National Center on Birth Defects and Developmental Disabilities, Atlanta, and first author of the report, which was published in Morbidity and Mortality Weekly Report.
“We’re concerned that this number has been slowly increasing,” Amanda Cohn, MD, director of the CDC’s Division of Birth Defects and Infant Disorders, told this news organization. “We need to be doing more outreach, both to pregnant persons as well as health care providers who are caring for them.”
Exposure to alcohol in the womb has been linked to a wide range of neurologic and physical problems in children, ranging from fetal alcohol syndrome to stunted learning abilities. Even if these problems are unlikely, experts insist there’s no known “safe” amount of alcohol a pregnant woman can have.
Dr. Cohn likened alcohol use to that of tobacco. “Lots of people smoke who don’t get lung cancer. Still, everyone is at an increased risk,” she said. “The safest way to not get lung cancer is to not smoke at all. It’s a behavior that can prevent additional harm.”
The report summarizes the results of a phone survey of 6,327 pregnant Americans between the ages of 18 and 49. The survey asked whether women had consumed an alcoholic beverage or had at least four drinks on one occasion – a common definition of binge drinking – in the past 30 days.
According to the report, 13.5% of women reported using alcohol, and 5.2% said they had binged on alcohol. Women who experienced frequent mental distress – describing their mental health as “not good” for 14 or more days in the past month – were twice as likely to drink and three times likelier to binge drink, the researchers found.
The increase within the 3-year period was roughly the same as in previous surveys.
“There was no evidence of increased alcohol consumption by pregnant adults in 2020 relative to 2019, despite possible increased alcohol sales and consumption among the general population during the first months of the COVID-19 pandemic,” the report states.
“That is one finding that was unexpected but that we were pleased to see,” Dr. Gosdin said.
Experts stressed that the survey covered only the first 9 months of the COVID-19 pandemic. “We’re still in the depths of it,” Samuel T. Bauer, MD, associate professor of obstetrics and gynecology at Duke University Medical Center, Durham, N.C., told this news organization. “People with alcohol use disorders certainly have been challenged during COVID. I think this is a preliminary answer.”
Dr. Gosdin said the effects of the pandemic on drinking habits bear watching. “We are concerned about the impacts of COVID-19,” he said. “We know it’s affected how people access regular care.”
Although virtual care has “exploded during COVID,” Dr. Bauer said, insurers have “turned off reimbursing” for doctor-patient visits via telephone, but not for visits by Internet-based video platforms like Zoom.
That split creates equity issues in many parts of the country, including his home state of North Carolina, where broadband is scarce, and patients may live 100 miles or more away from caregivers.
The “full-blown birth defect” of fetal alcohol syndrome is just the most visible hazard of drinking. Other medical and developmental issues include speech delays, slower learning and reading skills, attention-deficit and hyperactivity disorders, and problems with the heart and kidneys.
So, when Dr. Bauer encounters patients who believe that a few drinks will not harm their baby, he says he tells them: “’Why is this going to be where you put your flag?’ That leads to a different form of conversation.”
A version of this article first appeared on Medscape.com.
FROM MMWR
Two studies detail the dangers of COVID in pregnancy
Two new studies show how COVID-19 threatens the health of pregnant people and their newborn infants.
A study conducted in Scotland showed that unvaccinated pregnant people who got COVID were much more likely to have a stillborn infant or one that dies in the first 28 days. The study also found that pregnant women infected with COVID died or needed hospitalization at a much higher rate than vaccinated women who got pregnant.
The University of Edinburgh and Public Health Scotland studied national data in 88,000 pregnancies between Dec. 2020 and Oct. 2021, according to the study published in Nature Medicine.
Overall, 77.4% of infections, 90.9% of COVID-related hospitalizations, and 98% of critical care cases occurred in the unvaccinated people, as did all newborn deaths.
The study said 2,364 babies were born to women infected with COVID, with 2,353 live births. Eleven babies were stillborn and eight live-born babies died within 28 days. Of the live births, 241 were premature.
The problems were more likely if the infection occurred 28 days or less before the delivery date, the researchers said.
The authors said the low vaccination rate among pregnant people was a problem. Only 32% of people giving birth in Oct. 2021 were fully vaccinated, while 77% of the Scottish female population aged 18-44 was fully vaccinated.
“Vaccine hesitancy in pregnancy thus requires addressing, especially in light of new recommendations for booster vaccination administration 3 months after the initial vaccination course to help protect against new variants such as Omicron,” the authors wrote. “Addressing low vaccine uptake rates in pregnant women is imperative to protect the health of women and babies in the ongoing pandemic.”
Vaccinated women who were pregnant had complication rates that were about the same for all pregnant women, the study shows.
The second study, published in The Lancet, found that women who got COVID while pregnant in five Western U.S. states were more likely to have premature births, low birth weights, and stillbirths, even when the COVID cases are mild.
The Institute for Systems Biology researchers in Seattle studied data for women who gave birth in Alaska, California, Montana, Oregon, or Washington from March 5, 2020, to July 4, 2021. About 18,000 of them were tested for COVID, with 882 testing positive. Of the positive tests, 85 came in the first trimester, 226 in the second trimester, and 571 in the third semester. None of the pregnant women had been vaccinated at the time they were infected.
Most of the birth problems occurred with first and second trimester infections, the study noted, and problems occurred even if the pregnant person didn’t have respiratory complications, a major COVID symptom.
“Pregnant people are at an increased risk of adverse outcomes following SARS-CoV-2 infection, even when maternal COVID-19 is less severe, and they may benefit from increased monitoring following infection,” Jennifer Hadlock, MD, an author of the paper, said in a news release.
The study also pointed out continuing inequities in health care, with most of the positive cases occurring among young, non-White people with Medicaid and high body mass index.
A version of this article first appeared on WebMD.com.
Two new studies show how COVID-19 threatens the health of pregnant people and their newborn infants.
A study conducted in Scotland showed that unvaccinated pregnant people who got COVID were much more likely to have a stillborn infant or one that dies in the first 28 days. The study also found that pregnant women infected with COVID died or needed hospitalization at a much higher rate than vaccinated women who got pregnant.
The University of Edinburgh and Public Health Scotland studied national data in 88,000 pregnancies between Dec. 2020 and Oct. 2021, according to the study published in Nature Medicine.
Overall, 77.4% of infections, 90.9% of COVID-related hospitalizations, and 98% of critical care cases occurred in the unvaccinated people, as did all newborn deaths.
The study said 2,364 babies were born to women infected with COVID, with 2,353 live births. Eleven babies were stillborn and eight live-born babies died within 28 days. Of the live births, 241 were premature.
The problems were more likely if the infection occurred 28 days or less before the delivery date, the researchers said.
The authors said the low vaccination rate among pregnant people was a problem. Only 32% of people giving birth in Oct. 2021 were fully vaccinated, while 77% of the Scottish female population aged 18-44 was fully vaccinated.
“Vaccine hesitancy in pregnancy thus requires addressing, especially in light of new recommendations for booster vaccination administration 3 months after the initial vaccination course to help protect against new variants such as Omicron,” the authors wrote. “Addressing low vaccine uptake rates in pregnant women is imperative to protect the health of women and babies in the ongoing pandemic.”
Vaccinated women who were pregnant had complication rates that were about the same for all pregnant women, the study shows.
The second study, published in The Lancet, found that women who got COVID while pregnant in five Western U.S. states were more likely to have premature births, low birth weights, and stillbirths, even when the COVID cases are mild.
The Institute for Systems Biology researchers in Seattle studied data for women who gave birth in Alaska, California, Montana, Oregon, or Washington from March 5, 2020, to July 4, 2021. About 18,000 of them were tested for COVID, with 882 testing positive. Of the positive tests, 85 came in the first trimester, 226 in the second trimester, and 571 in the third semester. None of the pregnant women had been vaccinated at the time they were infected.
Most of the birth problems occurred with first and second trimester infections, the study noted, and problems occurred even if the pregnant person didn’t have respiratory complications, a major COVID symptom.
“Pregnant people are at an increased risk of adverse outcomes following SARS-CoV-2 infection, even when maternal COVID-19 is less severe, and they may benefit from increased monitoring following infection,” Jennifer Hadlock, MD, an author of the paper, said in a news release.
The study also pointed out continuing inequities in health care, with most of the positive cases occurring among young, non-White people with Medicaid and high body mass index.
A version of this article first appeared on WebMD.com.
Two new studies show how COVID-19 threatens the health of pregnant people and their newborn infants.
A study conducted in Scotland showed that unvaccinated pregnant people who got COVID were much more likely to have a stillborn infant or one that dies in the first 28 days. The study also found that pregnant women infected with COVID died or needed hospitalization at a much higher rate than vaccinated women who got pregnant.
The University of Edinburgh and Public Health Scotland studied national data in 88,000 pregnancies between Dec. 2020 and Oct. 2021, according to the study published in Nature Medicine.
Overall, 77.4% of infections, 90.9% of COVID-related hospitalizations, and 98% of critical care cases occurred in the unvaccinated people, as did all newborn deaths.
The study said 2,364 babies were born to women infected with COVID, with 2,353 live births. Eleven babies were stillborn and eight live-born babies died within 28 days. Of the live births, 241 were premature.
The problems were more likely if the infection occurred 28 days or less before the delivery date, the researchers said.
The authors said the low vaccination rate among pregnant people was a problem. Only 32% of people giving birth in Oct. 2021 were fully vaccinated, while 77% of the Scottish female population aged 18-44 was fully vaccinated.
“Vaccine hesitancy in pregnancy thus requires addressing, especially in light of new recommendations for booster vaccination administration 3 months after the initial vaccination course to help protect against new variants such as Omicron,” the authors wrote. “Addressing low vaccine uptake rates in pregnant women is imperative to protect the health of women and babies in the ongoing pandemic.”
Vaccinated women who were pregnant had complication rates that were about the same for all pregnant women, the study shows.
The second study, published in The Lancet, found that women who got COVID while pregnant in five Western U.S. states were more likely to have premature births, low birth weights, and stillbirths, even when the COVID cases are mild.
The Institute for Systems Biology researchers in Seattle studied data for women who gave birth in Alaska, California, Montana, Oregon, or Washington from March 5, 2020, to July 4, 2021. About 18,000 of them were tested for COVID, with 882 testing positive. Of the positive tests, 85 came in the first trimester, 226 in the second trimester, and 571 in the third semester. None of the pregnant women had been vaccinated at the time they were infected.
Most of the birth problems occurred with first and second trimester infections, the study noted, and problems occurred even if the pregnant person didn’t have respiratory complications, a major COVID symptom.
“Pregnant people are at an increased risk of adverse outcomes following SARS-CoV-2 infection, even when maternal COVID-19 is less severe, and they may benefit from increased monitoring following infection,” Jennifer Hadlock, MD, an author of the paper, said in a news release.
The study also pointed out continuing inequities in health care, with most of the positive cases occurring among young, non-White people with Medicaid and high body mass index.
A version of this article first appeared on WebMD.com.
Trauma rates with operative vaginal delivery unexpectedly high, study finds
A new investigation has found that rates of physical trauma following operative vaginal delivery (OVD) in Canada are higher than previously reported.
The cohort study of more than 1.3 million deliveries in the country found trauma rates were highest with forceps delivery, with more than 1 in 4 pregnancies resulting in maternal trauma and 1 in 105 infants experiencing neonatal trauma. Maternal and neonatal trauma following vacuum deliveries was less common, occurring in 1 in 8 pregnancies and 1 in 104 infants, according to the researchers, who reported their findings in the Canadian Medical Association Journal .
“The rates of trauma following OVD in Canada are higher than previously reported, irrespective of region, level of obstetric care, and volume of instrument use among hospitals,” lead author Giulia Muraca, PhD, MPH, assistant professor of obstetrics and gynecology at McMaster University, Hamilton, Ont., said in an interview. “While OVDs may be associated with low rates of morbidity in carefully selected circumstances, the uniformly high rates of trauma among forceps and vacuum deliveries documented across regions, levels of obstetric care, and hospitals show that such conditions do not apply to routine obstetric practice in Canada.”
The American College of Obstetricians and Gynecologists considers OVD a way to reduce the rate of cesarean deliveries. However, the group has also pointed to a decline in familiarity with the procedures among clinicians new to the field.
Current reports also show that while OVD accounts for up to 15% of deliveries in Canada, Australia, and the United Kingdom, the risks associated with the approach are heavily dependent on the expertise of the provider. Declining use of OVD in favor of cesarean delivery has reduced opportunities for clinicians to acquire proficiency in performing these deliveries, according to the researchers.
Given these various factors, the investigators said the consensus on the safety of OVD is under scrutiny.
“An examination of maternal and neonatal trauma among OVD in contemporary practice is necessary to ensure that health care providers, policy makers, and pregnant individuals are informed regarding the risks of OVD typically experienced in routine obstetric practice, rather than those encountered under ideal conditions,” Dr. Muraca said.
Over 1 million deliveries studied
Dr. Muraca and colleagues looked at 1,326,191 deliveries occurring across Canada (except Quebec) between April 2013 and March 2019. The researchers included all singleton, term (≥37 weeks), in-hospital deliveries to women who had not undergone a previous cesarean delivery.
The study’s primary outcome measures were composite maternal trauma and composite neonatal trauma. Maternal trauma included obstetric anal sphincter injury (OASI); cervical or high vaginal laceration; pelvic hematoma; obstetric injury to the pelvic organs, pelvic joints, or ligaments; injury to the bladder or urethra; and other pelvic trauma. Neonatal trauma comprised intracranial hemorrhage and laceration, skull fracture, severe injury to the central or peripheral nervous system, fracture of the long bones, injury to the liver or spleen, seizures, and neonatal death.
The analysis found that 38,500 (2.9%) of the cases involved attempted forceps deliveries while 110,987 (8.4%) were attempted vacuum deliveries. Of the attempted forceps deliveries, 1,606 (4.2%) failed, while 8,791 (7.9%) of attempted vacuum deliveries failed.
Maternal trauma was observed in 25.3% of all forceps deliveries (n = 9,728) and 13.2% of all vacuum deliveries (n = 14,614), the researchers reported. The most common form of maternal trauma was OASI, which was observed in 21.52% of women undergoing forceps delivery and 11.67% of those undergoing vacuum delivery. The rates of all other forms of maternal trauma were higher among patients undergoing attempted forceps delivery than among their counterparts undergoing attempted vacuum delivery.
After adjusting for possible confounders, rates of maternal trauma remained higher with forceps than with vacuum deliveries (adjusted rate ratio, 1.70).
The rate of neonatal trauma was comparable for forceps (9.56/1,000 live births) and vacuum deliveries (9.58/1,000 live births). In these cases, damage to the peripheral nervous system was the most common form of neonatal trauma, occurring in 4.85/1,000 live births with forceps delivery and 3.41/1,000 live births for vacuum delivery, the researchers found.
Consider morbidity following OVD against potential alternatives, authors say
According to Dr. Muraca, the rates of maternal trauma in her study – along with accumulating evidence of the severe long-term consequences of these injuries – demonstrates the importance of reporting timely, empirically derived risk measures that accurately reflect those that pregnant individuals may encounter in typical obstetric practice.
“Although there is merit in understanding the estimates of risk that can be achieved when conditions are optimal, the interpretation of these estimates can be misleading, especially given secular shifts in patterns of practice,” she said. “The failure to do so compromises women’s autonomy in making evidence-informed decisions regarding childbirth interventions, such as evaluating the short- and long-term risks of OVD and cesarean delivery.
Her group recommended that morbidity following OVD be weighed against potential alternatives to such procedures, which carry their own risks. “This includes an extended second stage of labor and a spontaneous vaginal delivery, or a second-stage cesarean delivery, both of which are associated with significant morbidity,” Dr. Muraca said. “However, a comprehensive consideration of high population rates of OVD morbidity also prompts questions about choice of instrument, obstetrician training in OVD use, and for recognizing cases that would benefit from a cesarean delivery earlier in labor.”
Alan Peaceman, MD, professor of obstetrics and gynecology at Northwestern University, Chicago, said he was not surprised by the rates of sphincter injury, but that the rate of severe neonatal injury rate was higher than he expected. However, he added, “I don’t think clinicians should change their approach based on a single study. They should continue with the approach that they are most skilled at and is appropriate for the clinical circumstances.”
The study was funded by a grant from the Canadian Institutes of Health Research. Dr. Muraca and Dr. Peaceman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new investigation has found that rates of physical trauma following operative vaginal delivery (OVD) in Canada are higher than previously reported.
The cohort study of more than 1.3 million deliveries in the country found trauma rates were highest with forceps delivery, with more than 1 in 4 pregnancies resulting in maternal trauma and 1 in 105 infants experiencing neonatal trauma. Maternal and neonatal trauma following vacuum deliveries was less common, occurring in 1 in 8 pregnancies and 1 in 104 infants, according to the researchers, who reported their findings in the Canadian Medical Association Journal .
“The rates of trauma following OVD in Canada are higher than previously reported, irrespective of region, level of obstetric care, and volume of instrument use among hospitals,” lead author Giulia Muraca, PhD, MPH, assistant professor of obstetrics and gynecology at McMaster University, Hamilton, Ont., said in an interview. “While OVDs may be associated with low rates of morbidity in carefully selected circumstances, the uniformly high rates of trauma among forceps and vacuum deliveries documented across regions, levels of obstetric care, and hospitals show that such conditions do not apply to routine obstetric practice in Canada.”
The American College of Obstetricians and Gynecologists considers OVD a way to reduce the rate of cesarean deliveries. However, the group has also pointed to a decline in familiarity with the procedures among clinicians new to the field.
Current reports also show that while OVD accounts for up to 15% of deliveries in Canada, Australia, and the United Kingdom, the risks associated with the approach are heavily dependent on the expertise of the provider. Declining use of OVD in favor of cesarean delivery has reduced opportunities for clinicians to acquire proficiency in performing these deliveries, according to the researchers.
Given these various factors, the investigators said the consensus on the safety of OVD is under scrutiny.
“An examination of maternal and neonatal trauma among OVD in contemporary practice is necessary to ensure that health care providers, policy makers, and pregnant individuals are informed regarding the risks of OVD typically experienced in routine obstetric practice, rather than those encountered under ideal conditions,” Dr. Muraca said.
Over 1 million deliveries studied
Dr. Muraca and colleagues looked at 1,326,191 deliveries occurring across Canada (except Quebec) between April 2013 and March 2019. The researchers included all singleton, term (≥37 weeks), in-hospital deliveries to women who had not undergone a previous cesarean delivery.
The study’s primary outcome measures were composite maternal trauma and composite neonatal trauma. Maternal trauma included obstetric anal sphincter injury (OASI); cervical or high vaginal laceration; pelvic hematoma; obstetric injury to the pelvic organs, pelvic joints, or ligaments; injury to the bladder or urethra; and other pelvic trauma. Neonatal trauma comprised intracranial hemorrhage and laceration, skull fracture, severe injury to the central or peripheral nervous system, fracture of the long bones, injury to the liver or spleen, seizures, and neonatal death.
The analysis found that 38,500 (2.9%) of the cases involved attempted forceps deliveries while 110,987 (8.4%) were attempted vacuum deliveries. Of the attempted forceps deliveries, 1,606 (4.2%) failed, while 8,791 (7.9%) of attempted vacuum deliveries failed.
Maternal trauma was observed in 25.3% of all forceps deliveries (n = 9,728) and 13.2% of all vacuum deliveries (n = 14,614), the researchers reported. The most common form of maternal trauma was OASI, which was observed in 21.52% of women undergoing forceps delivery and 11.67% of those undergoing vacuum delivery. The rates of all other forms of maternal trauma were higher among patients undergoing attempted forceps delivery than among their counterparts undergoing attempted vacuum delivery.
After adjusting for possible confounders, rates of maternal trauma remained higher with forceps than with vacuum deliveries (adjusted rate ratio, 1.70).
The rate of neonatal trauma was comparable for forceps (9.56/1,000 live births) and vacuum deliveries (9.58/1,000 live births). In these cases, damage to the peripheral nervous system was the most common form of neonatal trauma, occurring in 4.85/1,000 live births with forceps delivery and 3.41/1,000 live births for vacuum delivery, the researchers found.
Consider morbidity following OVD against potential alternatives, authors say
According to Dr. Muraca, the rates of maternal trauma in her study – along with accumulating evidence of the severe long-term consequences of these injuries – demonstrates the importance of reporting timely, empirically derived risk measures that accurately reflect those that pregnant individuals may encounter in typical obstetric practice.
“Although there is merit in understanding the estimates of risk that can be achieved when conditions are optimal, the interpretation of these estimates can be misleading, especially given secular shifts in patterns of practice,” she said. “The failure to do so compromises women’s autonomy in making evidence-informed decisions regarding childbirth interventions, such as evaluating the short- and long-term risks of OVD and cesarean delivery.
Her group recommended that morbidity following OVD be weighed against potential alternatives to such procedures, which carry their own risks. “This includes an extended second stage of labor and a spontaneous vaginal delivery, or a second-stage cesarean delivery, both of which are associated with significant morbidity,” Dr. Muraca said. “However, a comprehensive consideration of high population rates of OVD morbidity also prompts questions about choice of instrument, obstetrician training in OVD use, and for recognizing cases that would benefit from a cesarean delivery earlier in labor.”
Alan Peaceman, MD, professor of obstetrics and gynecology at Northwestern University, Chicago, said he was not surprised by the rates of sphincter injury, but that the rate of severe neonatal injury rate was higher than he expected. However, he added, “I don’t think clinicians should change their approach based on a single study. They should continue with the approach that they are most skilled at and is appropriate for the clinical circumstances.”
The study was funded by a grant from the Canadian Institutes of Health Research. Dr. Muraca and Dr. Peaceman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new investigation has found that rates of physical trauma following operative vaginal delivery (OVD) in Canada are higher than previously reported.
The cohort study of more than 1.3 million deliveries in the country found trauma rates were highest with forceps delivery, with more than 1 in 4 pregnancies resulting in maternal trauma and 1 in 105 infants experiencing neonatal trauma. Maternal and neonatal trauma following vacuum deliveries was less common, occurring in 1 in 8 pregnancies and 1 in 104 infants, according to the researchers, who reported their findings in the Canadian Medical Association Journal .
“The rates of trauma following OVD in Canada are higher than previously reported, irrespective of region, level of obstetric care, and volume of instrument use among hospitals,” lead author Giulia Muraca, PhD, MPH, assistant professor of obstetrics and gynecology at McMaster University, Hamilton, Ont., said in an interview. “While OVDs may be associated with low rates of morbidity in carefully selected circumstances, the uniformly high rates of trauma among forceps and vacuum deliveries documented across regions, levels of obstetric care, and hospitals show that such conditions do not apply to routine obstetric practice in Canada.”
The American College of Obstetricians and Gynecologists considers OVD a way to reduce the rate of cesarean deliveries. However, the group has also pointed to a decline in familiarity with the procedures among clinicians new to the field.
Current reports also show that while OVD accounts for up to 15% of deliveries in Canada, Australia, and the United Kingdom, the risks associated with the approach are heavily dependent on the expertise of the provider. Declining use of OVD in favor of cesarean delivery has reduced opportunities for clinicians to acquire proficiency in performing these deliveries, according to the researchers.
Given these various factors, the investigators said the consensus on the safety of OVD is under scrutiny.
“An examination of maternal and neonatal trauma among OVD in contemporary practice is necessary to ensure that health care providers, policy makers, and pregnant individuals are informed regarding the risks of OVD typically experienced in routine obstetric practice, rather than those encountered under ideal conditions,” Dr. Muraca said.
Over 1 million deliveries studied
Dr. Muraca and colleagues looked at 1,326,191 deliveries occurring across Canada (except Quebec) between April 2013 and March 2019. The researchers included all singleton, term (≥37 weeks), in-hospital deliveries to women who had not undergone a previous cesarean delivery.
The study’s primary outcome measures were composite maternal trauma and composite neonatal trauma. Maternal trauma included obstetric anal sphincter injury (OASI); cervical or high vaginal laceration; pelvic hematoma; obstetric injury to the pelvic organs, pelvic joints, or ligaments; injury to the bladder or urethra; and other pelvic trauma. Neonatal trauma comprised intracranial hemorrhage and laceration, skull fracture, severe injury to the central or peripheral nervous system, fracture of the long bones, injury to the liver or spleen, seizures, and neonatal death.
The analysis found that 38,500 (2.9%) of the cases involved attempted forceps deliveries while 110,987 (8.4%) were attempted vacuum deliveries. Of the attempted forceps deliveries, 1,606 (4.2%) failed, while 8,791 (7.9%) of attempted vacuum deliveries failed.
Maternal trauma was observed in 25.3% of all forceps deliveries (n = 9,728) and 13.2% of all vacuum deliveries (n = 14,614), the researchers reported. The most common form of maternal trauma was OASI, which was observed in 21.52% of women undergoing forceps delivery and 11.67% of those undergoing vacuum delivery. The rates of all other forms of maternal trauma were higher among patients undergoing attempted forceps delivery than among their counterparts undergoing attempted vacuum delivery.
After adjusting for possible confounders, rates of maternal trauma remained higher with forceps than with vacuum deliveries (adjusted rate ratio, 1.70).
The rate of neonatal trauma was comparable for forceps (9.56/1,000 live births) and vacuum deliveries (9.58/1,000 live births). In these cases, damage to the peripheral nervous system was the most common form of neonatal trauma, occurring in 4.85/1,000 live births with forceps delivery and 3.41/1,000 live births for vacuum delivery, the researchers found.
Consider morbidity following OVD against potential alternatives, authors say
According to Dr. Muraca, the rates of maternal trauma in her study – along with accumulating evidence of the severe long-term consequences of these injuries – demonstrates the importance of reporting timely, empirically derived risk measures that accurately reflect those that pregnant individuals may encounter in typical obstetric practice.
“Although there is merit in understanding the estimates of risk that can be achieved when conditions are optimal, the interpretation of these estimates can be misleading, especially given secular shifts in patterns of practice,” she said. “The failure to do so compromises women’s autonomy in making evidence-informed decisions regarding childbirth interventions, such as evaluating the short- and long-term risks of OVD and cesarean delivery.
Her group recommended that morbidity following OVD be weighed against potential alternatives to such procedures, which carry their own risks. “This includes an extended second stage of labor and a spontaneous vaginal delivery, or a second-stage cesarean delivery, both of which are associated with significant morbidity,” Dr. Muraca said. “However, a comprehensive consideration of high population rates of OVD morbidity also prompts questions about choice of instrument, obstetrician training in OVD use, and for recognizing cases that would benefit from a cesarean delivery earlier in labor.”
Alan Peaceman, MD, professor of obstetrics and gynecology at Northwestern University, Chicago, said he was not surprised by the rates of sphincter injury, but that the rate of severe neonatal injury rate was higher than he expected. However, he added, “I don’t think clinicians should change their approach based on a single study. They should continue with the approach that they are most skilled at and is appropriate for the clinical circumstances.”
The study was funded by a grant from the Canadian Institutes of Health Research. Dr. Muraca and Dr. Peaceman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
2022 Update on obstetrics
Obstetrical practice saw updates in 2021 to 3 major areas of pregnancy management: preterm birth prevention, antepartum fetal surveillance, and the use of antenatal corticosteroids.
Updated guidance on predicting and preventing spontaneous PTB
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
Preterm birth (PTB) continues to pose a challenge in clinical obstetrics, with the most recently reported rate of 10.2% in the United States.1 This accounts for almost 75% of perinatal mortality and more than half of neonatal morbidity, in which effects last well past the neonatal period. PTB is classified as spontaneous (following preterm labor, preterm prelabor rupture of membranes, or cervical insufficiency) or iatrogenic (indicated due to maternal and/or fetal complications).
Assessing risk for PTB
The single strongest predictor of subsequent PTB is a history of spontaneous PTB. Recurrence risk is further increased by the number of prior PTBs and the gestational age at prior PTB. Identification of and intervention for a short cervix has been shown to prolong gestation. Transvaginal ultrasonography of the cervix is the most accurate method for evaluating cervical length (CL). Specific examination criteria exist to ensure that CL measurements are reproducible and reliable.2 A short CL is generally defined as a measurement of less than 25 mm between 16 and 24 weeks’ gestation.
Screening strategies
The American College of Obstetricians and Gynecologists (ACOG), with an endorsement from the Society for Maternal-Fetal Medicine (SMFM), recommends cervical evaluation during the anatomy ultrasound exam between 18 0/7 and 22 6/7 weeks’ gestation in all pregnant patients regardless of prior PTB.3 If transabdominal imaging is concerning for a shortened cervix, transvaginal ultrasonography should be performed to assess the CL.
Serial transvaginal CL measurements are recommended between 16 0/7 and 24 0/7 weeks’ gestation for patients with a current singleton pregnancy and history of a spontaneous PTB, but not for patients with a history of iatrogenic or indicated PTB.
Interventions: Mind your p’s and c’s
Interventions to reduce the risk of spontaneous PTB depend on whether the current pregnancy is a singleton, twins, or higher-order multiples; CL measurement; and history of spontaneous PTB. Preconception optimization of underlying medical conditions also is important to reduce the risk of recurrent indicated PTB.
Continue to: Progesterone...
Progesterone
Vaginal administration. Several trials have shown that vaginal progesterone can be used to reduce the risk of spontaneous PTB in asymptomatic patients with a singleton pregnancy, incidental finding of a short cervix (<25 mm), and no history of spontaneous PTB. This is a change from the prior recommendation of CL of less than 20 mm. In the setting of a twin pregnancy, regardless of CL, data do not definitively support the use of vaginal progesterone.
Intramuscular administration.4,5 The popularity of intramuscular progesterone has waxed and waned. At present, ACOG recommends that all patients with a singleton pregnancy and history of spontaneous PTB be offered progesterone beginning at 16 0/7 weeks’ gestation following a shared decision-making process that includes the limited data of efficacy noted in existing studies.
In a twin pregnancy with no history of spontaneous PTB, the use of intramuscular progesterone has been shown to potentially increase the risk of PTB and admission to the neonatal intensive care unit. As such, intramuscular progesterone in the setting of a twin gestation without a history of spontaneous PTB is not recommended. When a prior spontaneous PTB has occurred, there may be some benefit to intramuscular progesterone in twin gestations.
Cerclage
Ultrasound indicated. In a singleton pregnancy with an incidental finding of short cervix (<25 mm) and no history of PTB, the use of cerclage is of uncertain benefit. Effectiveness may be seen if the cervix is less than 10 mm. Ultrasound-indicated cerclage should be considered in a singleton pregnancy with a CL less than 25 mm and a history of spontaneous PTB.
Possibly one of the most controversial topics is ultrasound-indicated cerclage placement in twin gestation. As with many situations in obstetrics, data regarding ultrasound-indicated cerclage in twin gestation is based on small retrospective studies fraught with bias. Results from these studies range from no benefit, to potential benefit, to even possible increased risk of PTB. Since data are limited, as we await more evidence, it is recommended that the clinician and patient use shared decision making to decide on cerclage placement in a twin gestation.
Exam indicated. In a singleton pregnancy with a dilated cervix on digital or speculum exam between 16 0/7 to 23 6/7 weeks’ gestation, a physical exam–indicated cerclage should be offered. Exam-indicated cerclage also may reduce the incidence of PTB in twin gestations with cervical dilation between 16 0/7 and 23 6/7 weeks’ gestation. Indomethacin tocolysis and perioperative antibiotics should be considered when an exam-indicated cerclage is placed.
As the limits of viability are continually pushed earlier, more in-depth conversation is needed with patients who are considering an exam-indicated cerclage. The nuances of periviability and the likelihood that an exam-indicated cerclage will commit a pregnancy to a periviable or extremely preterm birth should be discussed in detail using a shared decision making model.
Regardless of whether the cerclage is ultrasound or exam indicated, once it is placed there is no utility in additional CL ultrasound monitoring.
Pessary
Vaginal pessaries for prevention of PTB have not gained popularity in the United States as they have in other countries. Trials are being conducted to determine the utility of vaginal pessary, but current data have not proven its effectiveness in preventing PTB in the setting of singleton pregnancy, short cervix, and no history of spontaneous PTB. So for now, pessary is not recommended. The same can be said for use in the twin gestation.
- All patients should have cervical evaluation during pregnancy. Serial imaging is reserved for those with a history of spontaneous PTB.
- Progesterone supplementation should be offered to patients with a singleton pregnancy and a history of spontaneous PTB or to patients with a singleton pregnancy and no history of spontaneous PTB who have cervical shortening at less than 24 weeks.
- Cerclage may be offered between 16 and 24 weeks for a cervical length less than 25 mm in a patient with a singleton gestation who has a history of spontaneous PTB (<10 mm if no history of spontaneous PTB) or for a dilated cervix on exam regardless of history.
- Women who have a twin gestation with cervical dilation may be offered physical exam–indicated cerclage.
Which patients may benefit from antepartum fetal surveillance and when to initiate it
American College of Obstetricians and Gynecologists’ Committee on Obstetrics Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal surveillance: ACOG committee opinion, number 828. Obstet Gynecol. 2021;137:e177-e197.
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Antepartum fetal surveillance: ACOG practice bulletin, number 229. Obstet Gynecol. 2021;137:e116-e127.
The ultimate purpose of antenatal fetal surveillance is to prevent stillbirth. However, stillbirth has multiple etiologies, not all of which are preventable with testing. In June 2021, ACOG released a new Committee Opinion containing guidelines for fetal surveillance, including suggested gestational age at initiation and frequency of testing, for the most common high-risk conditions. ACOG also released an update to the Practice Bulletin on antepartum fetal surveillance; additions include randomized controlled trial level data on the utility of fetal kick counts (FKCs) and recommendations that align with the new Committee Opinion.
Data for the efficacy of antepartum fetal surveillance are lacking, mainly due to the difficulty of performing prospective studies in stillbirth. The existing evidence is subject to intervention bias, as deliveries increase in tested patients, and recommendations rely heavily on expert consensus and nonrandomized studies. Antenatal testing is also time, cost, and labor intensive, with the risk of intervention for a false-positive result. Despite these limitations, obstetrical practices routinely perform antenatal fetal surveillance.
The new guidelines: The why, when, and how often
Why. Antepartum fetal surveillance is suggested for conditions that have a risk of stillbirth greater than 0.8 per 1,000 (that is, the false-negative rate of a biophysical profile or a modified biophysical profile) and the relative risk or odds ratio is greater than 2.0 for stillbirth compared with unaffected pregnancies.
When. For most conditions, ACOG recommends initiation of testing at 32 weeks or later, with notable earlier exceptions for some of the highest-risk patients. For certain conditions, such as fetal growth restriction and hypertensive disorders of pregnancy, the recommendation is to start “at diagnosis,” with the corollary “or at a gestational age when delivery would be considered because of abnormal results.” Shared decision making with the patient about pregnancy goals therefore is required, particularly in cases of fetal anomalies, genetic conditions, or at very early gestational ages.
How often. The recommended frequency of testing is at least weekly. Testing frequency should be increased to twice-weekly outpatient or daily inpatient for the most complicated pregnancies (for example, fetal growth restriction with abnormal umbilical artery Doppler studies, preeclampsia with severe features).
Once or twice weekly is an option for many conditions, which gives the clinician the opportunity to assess clinical stability as well as the patient’s input in terms of logistics and anxiety.
Patients with multiple conditions may fall into the “individualized” category, as do patients with suboptimal control of conditions (for example, diabetes, hypertension) that may affect the fetus as the pregnancy progresses.
New diagnoses included for surveillance
Several diagnoses not previously included now qualify for antepartum fetal surveillance under the new guidelines, most notably:
- history of obstetrical complications in the immediate preceding pregnancy
—history of prior fetal growth restriction requiring preterm delivery
—history of prior preeclampsia requiring delivery
- alcohol use of 5 or more drinks per day
- in vitro fertilization
- abnormal serum markers
—pregnancy-associated plasma protein A (PAPP-A) in the fifth or lower percentile or 0.4 multiples of the median (MoM)
—second trimester inhibin A of 2 or greater MoM
- prepregnancy body mass index (BMI)
—this is divided into 2 categories for timing of initiation of testing:
- 37 weeks for BMI of 35 to 39.9 kg/m2
- 34 weeks for BMI of or greater than 40 kg/m2.
Fetal kick counts
The major change to the updated Practice Bulletin on antenatal surveillance is the inclusion of data on FKCs, a simple modality of fetal surveillance that does not require a clinical visit. For FKCs, a meta-analysis of more than 450,000 patients did not demonstrate a difference in perinatal death between the FKC intervention group (0.54%) and the control group (0.59%). Of note, there were small but statistically significant increases in the rates of induction of labor, cesarean delivery, and preterm delivery in the FKC intervention group. Therefore, this update does not recommend a formal program of FKCs for all patients.
- The antenatal fetal surveillance guidelines are just that—guidelines, not mandates. Their use will need to be adapted for specific patient populations and practice management patterns.
- Many conditions qualify for “individualized” surveillance, which offers the opportunity for detailed discussions on the patient’s care. This includes shared decision making with patients to meet their goals for the pregnancy.
- Although patient-perceived decreased fetal movement always warrants clinical evaluation, a regular program of fetal kick count monitoring is not recommended for all patients due to lack of data supporting its benefit in reducing perinatal death.
- As with any change, new guidelines potentially are a source of frustration, so a concerted effort by obstetrical clinicians to agree on adoption of the guidelines is needed. Additional clinical resources and both clinician and patient education may be required depending on current practice style, as the new strategy may increase the number of appointments and ultrasound exams required.
Continue to: Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation...
Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation
American College of Obstetricians and Gynecologists. Use of antenatal corticosteroids at 22 weeks of gestation: ACOG practice advisory. September 2021. https://www .acog.org/clinical/clinical-guidance/practice-advisory /articles/2021/09/use-of-antenatal-corticosteroids-at -22-weeks-of-gestation. Accessed December 11, 2021.
In September 2021, ACOG and SMFM released a Practice Advisory updating the current recommendations for the administration of antenatal corticosteroids in the periviable period (22 to 25 6/7 weeks’ gestation). Whereas the prior lower limit of gestational age for consideration of steroids was 23 weeks, the new recommendation now extends this consideration down to 22 weeks.
The cited data include a meta-analysis of more than 2,200 patients in which the survival rate of infants born between 22 and 22 6/7 weeks who were exposed to antenatal steroids was 39% compared with 19.5% in the unexposed group. Another study of more than 1,000 patients demonstrated a statistically significant increase in overall survival in patients treated with antenatal steroids plus life support compared with life support only (38.5% vs 17.7%). Survival without major morbidity in this study, although increased from 1% to 4.4%, was still low.
Recommendation carries caveats
Given this information, the Practice Advisory offers a 2C level recommendation (weak recommendation, low quality of evidence) for antenatal steroids at 22 to 22 6/7 weeks’ gestation if neonatal resuscitation is planned, acknowledging the limitations and potential bias of the available data.
The Practice Advisory emphasizes the importance of counseling and patient involvement in the decision making. This requires a multidisciplinary collaboration among the neonatology and obstetrical teams, flexibility in the plan after birth depending on the infant’s condition, and redirection of care if appropriate. Estimated fetal weight, the presence of multiple gestations, fetal biologic sex, and any anomalies are also important in helping families make an informed decision for their particular pregnancy. As described in the Obstetric Care Consensus on periviable birth,6 it is important to remember that considerations and recommendations are not the same as requirements, and redirection of care to comfort and family memory making is not the same as withholding care.
The rest of the recommendations for the administration of antenatal steroids remain the same: Antenatal steroids are not recommended at less than 22 weeks due to lack of evidence of benefit, and they continue to be recommended at 24 weeks and beyond. ●
- Antenatal corticosteroids may be considered at 22 to 22 6/7 weeks’ gestation if, after thorough patient counseling, neonatal resuscitation is desired and planned by the family.
- The overall likelihood of survival and survival without major morbidities continues to be very low in the periviable period, especially at 22 weeks. Gestational age is only one of the many factors that must be considered in the shared decision making for this very difficult decision.
- Palliative care is a valid and appropriate option for patients facing a periviable delivery after appropriate counseling or after evaluation of the infant has occurred after birth.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2019. NCHS Data Brief, no 387. Hyattsville, MD: National Center for Health Statistics. October 2020. www.cdc.gov/nchs /data/databriefs/db387-H.pdf. Accessed December 20, 2021.
- To MS, Skentou C, Chan C, et al. Cervical assessment at the routine 23-week scan: standardizing techniques. Ultrasound Obstet Gynecol. 2001;17:217-219.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. Am J Obstet Gynecol. 2020;223:B16-B18.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37:127-136.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 6: Periviable birth. Obstet Gynecol. 2017;130:e187-e199.
Obstetrical practice saw updates in 2021 to 3 major areas of pregnancy management: preterm birth prevention, antepartum fetal surveillance, and the use of antenatal corticosteroids.
Updated guidance on predicting and preventing spontaneous PTB
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
Preterm birth (PTB) continues to pose a challenge in clinical obstetrics, with the most recently reported rate of 10.2% in the United States.1 This accounts for almost 75% of perinatal mortality and more than half of neonatal morbidity, in which effects last well past the neonatal period. PTB is classified as spontaneous (following preterm labor, preterm prelabor rupture of membranes, or cervical insufficiency) or iatrogenic (indicated due to maternal and/or fetal complications).
Assessing risk for PTB
The single strongest predictor of subsequent PTB is a history of spontaneous PTB. Recurrence risk is further increased by the number of prior PTBs and the gestational age at prior PTB. Identification of and intervention for a short cervix has been shown to prolong gestation. Transvaginal ultrasonography of the cervix is the most accurate method for evaluating cervical length (CL). Specific examination criteria exist to ensure that CL measurements are reproducible and reliable.2 A short CL is generally defined as a measurement of less than 25 mm between 16 and 24 weeks’ gestation.
Screening strategies
The American College of Obstetricians and Gynecologists (ACOG), with an endorsement from the Society for Maternal-Fetal Medicine (SMFM), recommends cervical evaluation during the anatomy ultrasound exam between 18 0/7 and 22 6/7 weeks’ gestation in all pregnant patients regardless of prior PTB.3 If transabdominal imaging is concerning for a shortened cervix, transvaginal ultrasonography should be performed to assess the CL.
Serial transvaginal CL measurements are recommended between 16 0/7 and 24 0/7 weeks’ gestation for patients with a current singleton pregnancy and history of a spontaneous PTB, but not for patients with a history of iatrogenic or indicated PTB.
Interventions: Mind your p’s and c’s
Interventions to reduce the risk of spontaneous PTB depend on whether the current pregnancy is a singleton, twins, or higher-order multiples; CL measurement; and history of spontaneous PTB. Preconception optimization of underlying medical conditions also is important to reduce the risk of recurrent indicated PTB.
Continue to: Progesterone...
Progesterone
Vaginal administration. Several trials have shown that vaginal progesterone can be used to reduce the risk of spontaneous PTB in asymptomatic patients with a singleton pregnancy, incidental finding of a short cervix (<25 mm), and no history of spontaneous PTB. This is a change from the prior recommendation of CL of less than 20 mm. In the setting of a twin pregnancy, regardless of CL, data do not definitively support the use of vaginal progesterone.
Intramuscular administration.4,5 The popularity of intramuscular progesterone has waxed and waned. At present, ACOG recommends that all patients with a singleton pregnancy and history of spontaneous PTB be offered progesterone beginning at 16 0/7 weeks’ gestation following a shared decision-making process that includes the limited data of efficacy noted in existing studies.
In a twin pregnancy with no history of spontaneous PTB, the use of intramuscular progesterone has been shown to potentially increase the risk of PTB and admission to the neonatal intensive care unit. As such, intramuscular progesterone in the setting of a twin gestation without a history of spontaneous PTB is not recommended. When a prior spontaneous PTB has occurred, there may be some benefit to intramuscular progesterone in twin gestations.
Cerclage
Ultrasound indicated. In a singleton pregnancy with an incidental finding of short cervix (<25 mm) and no history of PTB, the use of cerclage is of uncertain benefit. Effectiveness may be seen if the cervix is less than 10 mm. Ultrasound-indicated cerclage should be considered in a singleton pregnancy with a CL less than 25 mm and a history of spontaneous PTB.
Possibly one of the most controversial topics is ultrasound-indicated cerclage placement in twin gestation. As with many situations in obstetrics, data regarding ultrasound-indicated cerclage in twin gestation is based on small retrospective studies fraught with bias. Results from these studies range from no benefit, to potential benefit, to even possible increased risk of PTB. Since data are limited, as we await more evidence, it is recommended that the clinician and patient use shared decision making to decide on cerclage placement in a twin gestation.
Exam indicated. In a singleton pregnancy with a dilated cervix on digital or speculum exam between 16 0/7 to 23 6/7 weeks’ gestation, a physical exam–indicated cerclage should be offered. Exam-indicated cerclage also may reduce the incidence of PTB in twin gestations with cervical dilation between 16 0/7 and 23 6/7 weeks’ gestation. Indomethacin tocolysis and perioperative antibiotics should be considered when an exam-indicated cerclage is placed.
As the limits of viability are continually pushed earlier, more in-depth conversation is needed with patients who are considering an exam-indicated cerclage. The nuances of periviability and the likelihood that an exam-indicated cerclage will commit a pregnancy to a periviable or extremely preterm birth should be discussed in detail using a shared decision making model.
Regardless of whether the cerclage is ultrasound or exam indicated, once it is placed there is no utility in additional CL ultrasound monitoring.
Pessary
Vaginal pessaries for prevention of PTB have not gained popularity in the United States as they have in other countries. Trials are being conducted to determine the utility of vaginal pessary, but current data have not proven its effectiveness in preventing PTB in the setting of singleton pregnancy, short cervix, and no history of spontaneous PTB. So for now, pessary is not recommended. The same can be said for use in the twin gestation.
- All patients should have cervical evaluation during pregnancy. Serial imaging is reserved for those with a history of spontaneous PTB.
- Progesterone supplementation should be offered to patients with a singleton pregnancy and a history of spontaneous PTB or to patients with a singleton pregnancy and no history of spontaneous PTB who have cervical shortening at less than 24 weeks.
- Cerclage may be offered between 16 and 24 weeks for a cervical length less than 25 mm in a patient with a singleton gestation who has a history of spontaneous PTB (<10 mm if no history of spontaneous PTB) or for a dilated cervix on exam regardless of history.
- Women who have a twin gestation with cervical dilation may be offered physical exam–indicated cerclage.
Which patients may benefit from antepartum fetal surveillance and when to initiate it
American College of Obstetricians and Gynecologists’ Committee on Obstetrics Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal surveillance: ACOG committee opinion, number 828. Obstet Gynecol. 2021;137:e177-e197.
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Antepartum fetal surveillance: ACOG practice bulletin, number 229. Obstet Gynecol. 2021;137:e116-e127.
The ultimate purpose of antenatal fetal surveillance is to prevent stillbirth. However, stillbirth has multiple etiologies, not all of which are preventable with testing. In June 2021, ACOG released a new Committee Opinion containing guidelines for fetal surveillance, including suggested gestational age at initiation and frequency of testing, for the most common high-risk conditions. ACOG also released an update to the Practice Bulletin on antepartum fetal surveillance; additions include randomized controlled trial level data on the utility of fetal kick counts (FKCs) and recommendations that align with the new Committee Opinion.
Data for the efficacy of antepartum fetal surveillance are lacking, mainly due to the difficulty of performing prospective studies in stillbirth. The existing evidence is subject to intervention bias, as deliveries increase in tested patients, and recommendations rely heavily on expert consensus and nonrandomized studies. Antenatal testing is also time, cost, and labor intensive, with the risk of intervention for a false-positive result. Despite these limitations, obstetrical practices routinely perform antenatal fetal surveillance.
The new guidelines: The why, when, and how often
Why. Antepartum fetal surveillance is suggested for conditions that have a risk of stillbirth greater than 0.8 per 1,000 (that is, the false-negative rate of a biophysical profile or a modified biophysical profile) and the relative risk or odds ratio is greater than 2.0 for stillbirth compared with unaffected pregnancies.
When. For most conditions, ACOG recommends initiation of testing at 32 weeks or later, with notable earlier exceptions for some of the highest-risk patients. For certain conditions, such as fetal growth restriction and hypertensive disorders of pregnancy, the recommendation is to start “at diagnosis,” with the corollary “or at a gestational age when delivery would be considered because of abnormal results.” Shared decision making with the patient about pregnancy goals therefore is required, particularly in cases of fetal anomalies, genetic conditions, or at very early gestational ages.
How often. The recommended frequency of testing is at least weekly. Testing frequency should be increased to twice-weekly outpatient or daily inpatient for the most complicated pregnancies (for example, fetal growth restriction with abnormal umbilical artery Doppler studies, preeclampsia with severe features).
Once or twice weekly is an option for many conditions, which gives the clinician the opportunity to assess clinical stability as well as the patient’s input in terms of logistics and anxiety.
Patients with multiple conditions may fall into the “individualized” category, as do patients with suboptimal control of conditions (for example, diabetes, hypertension) that may affect the fetus as the pregnancy progresses.
New diagnoses included for surveillance
Several diagnoses not previously included now qualify for antepartum fetal surveillance under the new guidelines, most notably:
- history of obstetrical complications in the immediate preceding pregnancy
—history of prior fetal growth restriction requiring preterm delivery
—history of prior preeclampsia requiring delivery
- alcohol use of 5 or more drinks per day
- in vitro fertilization
- abnormal serum markers
—pregnancy-associated plasma protein A (PAPP-A) in the fifth or lower percentile or 0.4 multiples of the median (MoM)
—second trimester inhibin A of 2 or greater MoM
- prepregnancy body mass index (BMI)
—this is divided into 2 categories for timing of initiation of testing:
- 37 weeks for BMI of 35 to 39.9 kg/m2
- 34 weeks for BMI of or greater than 40 kg/m2.
Fetal kick counts
The major change to the updated Practice Bulletin on antenatal surveillance is the inclusion of data on FKCs, a simple modality of fetal surveillance that does not require a clinical visit. For FKCs, a meta-analysis of more than 450,000 patients did not demonstrate a difference in perinatal death between the FKC intervention group (0.54%) and the control group (0.59%). Of note, there were small but statistically significant increases in the rates of induction of labor, cesarean delivery, and preterm delivery in the FKC intervention group. Therefore, this update does not recommend a formal program of FKCs for all patients.
- The antenatal fetal surveillance guidelines are just that—guidelines, not mandates. Their use will need to be adapted for specific patient populations and practice management patterns.
- Many conditions qualify for “individualized” surveillance, which offers the opportunity for detailed discussions on the patient’s care. This includes shared decision making with patients to meet their goals for the pregnancy.
- Although patient-perceived decreased fetal movement always warrants clinical evaluation, a regular program of fetal kick count monitoring is not recommended for all patients due to lack of data supporting its benefit in reducing perinatal death.
- As with any change, new guidelines potentially are a source of frustration, so a concerted effort by obstetrical clinicians to agree on adoption of the guidelines is needed. Additional clinical resources and both clinician and patient education may be required depending on current practice style, as the new strategy may increase the number of appointments and ultrasound exams required.
Continue to: Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation...
Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation
American College of Obstetricians and Gynecologists. Use of antenatal corticosteroids at 22 weeks of gestation: ACOG practice advisory. September 2021. https://www .acog.org/clinical/clinical-guidance/practice-advisory /articles/2021/09/use-of-antenatal-corticosteroids-at -22-weeks-of-gestation. Accessed December 11, 2021.
In September 2021, ACOG and SMFM released a Practice Advisory updating the current recommendations for the administration of antenatal corticosteroids in the periviable period (22 to 25 6/7 weeks’ gestation). Whereas the prior lower limit of gestational age for consideration of steroids was 23 weeks, the new recommendation now extends this consideration down to 22 weeks.
The cited data include a meta-analysis of more than 2,200 patients in which the survival rate of infants born between 22 and 22 6/7 weeks who were exposed to antenatal steroids was 39% compared with 19.5% in the unexposed group. Another study of more than 1,000 patients demonstrated a statistically significant increase in overall survival in patients treated with antenatal steroids plus life support compared with life support only (38.5% vs 17.7%). Survival without major morbidity in this study, although increased from 1% to 4.4%, was still low.
Recommendation carries caveats
Given this information, the Practice Advisory offers a 2C level recommendation (weak recommendation, low quality of evidence) for antenatal steroids at 22 to 22 6/7 weeks’ gestation if neonatal resuscitation is planned, acknowledging the limitations and potential bias of the available data.
The Practice Advisory emphasizes the importance of counseling and patient involvement in the decision making. This requires a multidisciplinary collaboration among the neonatology and obstetrical teams, flexibility in the plan after birth depending on the infant’s condition, and redirection of care if appropriate. Estimated fetal weight, the presence of multiple gestations, fetal biologic sex, and any anomalies are also important in helping families make an informed decision for their particular pregnancy. As described in the Obstetric Care Consensus on periviable birth,6 it is important to remember that considerations and recommendations are not the same as requirements, and redirection of care to comfort and family memory making is not the same as withholding care.
The rest of the recommendations for the administration of antenatal steroids remain the same: Antenatal steroids are not recommended at less than 22 weeks due to lack of evidence of benefit, and they continue to be recommended at 24 weeks and beyond. ●
- Antenatal corticosteroids may be considered at 22 to 22 6/7 weeks’ gestation if, after thorough patient counseling, neonatal resuscitation is desired and planned by the family.
- The overall likelihood of survival and survival without major morbidities continues to be very low in the periviable period, especially at 22 weeks. Gestational age is only one of the many factors that must be considered in the shared decision making for this very difficult decision.
- Palliative care is a valid and appropriate option for patients facing a periviable delivery after appropriate counseling or after evaluation of the infant has occurred after birth.
Obstetrical practice saw updates in 2021 to 3 major areas of pregnancy management: preterm birth prevention, antepartum fetal surveillance, and the use of antenatal corticosteroids.
Updated guidance on predicting and preventing spontaneous PTB
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
Preterm birth (PTB) continues to pose a challenge in clinical obstetrics, with the most recently reported rate of 10.2% in the United States.1 This accounts for almost 75% of perinatal mortality and more than half of neonatal morbidity, in which effects last well past the neonatal period. PTB is classified as spontaneous (following preterm labor, preterm prelabor rupture of membranes, or cervical insufficiency) or iatrogenic (indicated due to maternal and/or fetal complications).
Assessing risk for PTB
The single strongest predictor of subsequent PTB is a history of spontaneous PTB. Recurrence risk is further increased by the number of prior PTBs and the gestational age at prior PTB. Identification of and intervention for a short cervix has been shown to prolong gestation. Transvaginal ultrasonography of the cervix is the most accurate method for evaluating cervical length (CL). Specific examination criteria exist to ensure that CL measurements are reproducible and reliable.2 A short CL is generally defined as a measurement of less than 25 mm between 16 and 24 weeks’ gestation.
Screening strategies
The American College of Obstetricians and Gynecologists (ACOG), with an endorsement from the Society for Maternal-Fetal Medicine (SMFM), recommends cervical evaluation during the anatomy ultrasound exam between 18 0/7 and 22 6/7 weeks’ gestation in all pregnant patients regardless of prior PTB.3 If transabdominal imaging is concerning for a shortened cervix, transvaginal ultrasonography should be performed to assess the CL.
Serial transvaginal CL measurements are recommended between 16 0/7 and 24 0/7 weeks’ gestation for patients with a current singleton pregnancy and history of a spontaneous PTB, but not for patients with a history of iatrogenic or indicated PTB.
Interventions: Mind your p’s and c’s
Interventions to reduce the risk of spontaneous PTB depend on whether the current pregnancy is a singleton, twins, or higher-order multiples; CL measurement; and history of spontaneous PTB. Preconception optimization of underlying medical conditions also is important to reduce the risk of recurrent indicated PTB.
Continue to: Progesterone...
Progesterone
Vaginal administration. Several trials have shown that vaginal progesterone can be used to reduce the risk of spontaneous PTB in asymptomatic patients with a singleton pregnancy, incidental finding of a short cervix (<25 mm), and no history of spontaneous PTB. This is a change from the prior recommendation of CL of less than 20 mm. In the setting of a twin pregnancy, regardless of CL, data do not definitively support the use of vaginal progesterone.
Intramuscular administration.4,5 The popularity of intramuscular progesterone has waxed and waned. At present, ACOG recommends that all patients with a singleton pregnancy and history of spontaneous PTB be offered progesterone beginning at 16 0/7 weeks’ gestation following a shared decision-making process that includes the limited data of efficacy noted in existing studies.
In a twin pregnancy with no history of spontaneous PTB, the use of intramuscular progesterone has been shown to potentially increase the risk of PTB and admission to the neonatal intensive care unit. As such, intramuscular progesterone in the setting of a twin gestation without a history of spontaneous PTB is not recommended. When a prior spontaneous PTB has occurred, there may be some benefit to intramuscular progesterone in twin gestations.
Cerclage
Ultrasound indicated. In a singleton pregnancy with an incidental finding of short cervix (<25 mm) and no history of PTB, the use of cerclage is of uncertain benefit. Effectiveness may be seen if the cervix is less than 10 mm. Ultrasound-indicated cerclage should be considered in a singleton pregnancy with a CL less than 25 mm and a history of spontaneous PTB.
Possibly one of the most controversial topics is ultrasound-indicated cerclage placement in twin gestation. As with many situations in obstetrics, data regarding ultrasound-indicated cerclage in twin gestation is based on small retrospective studies fraught with bias. Results from these studies range from no benefit, to potential benefit, to even possible increased risk of PTB. Since data are limited, as we await more evidence, it is recommended that the clinician and patient use shared decision making to decide on cerclage placement in a twin gestation.
Exam indicated. In a singleton pregnancy with a dilated cervix on digital or speculum exam between 16 0/7 to 23 6/7 weeks’ gestation, a physical exam–indicated cerclage should be offered. Exam-indicated cerclage also may reduce the incidence of PTB in twin gestations with cervical dilation between 16 0/7 and 23 6/7 weeks’ gestation. Indomethacin tocolysis and perioperative antibiotics should be considered when an exam-indicated cerclage is placed.
As the limits of viability are continually pushed earlier, more in-depth conversation is needed with patients who are considering an exam-indicated cerclage. The nuances of periviability and the likelihood that an exam-indicated cerclage will commit a pregnancy to a periviable or extremely preterm birth should be discussed in detail using a shared decision making model.
Regardless of whether the cerclage is ultrasound or exam indicated, once it is placed there is no utility in additional CL ultrasound monitoring.
Pessary
Vaginal pessaries for prevention of PTB have not gained popularity in the United States as they have in other countries. Trials are being conducted to determine the utility of vaginal pessary, but current data have not proven its effectiveness in preventing PTB in the setting of singleton pregnancy, short cervix, and no history of spontaneous PTB. So for now, pessary is not recommended. The same can be said for use in the twin gestation.
- All patients should have cervical evaluation during pregnancy. Serial imaging is reserved for those with a history of spontaneous PTB.
- Progesterone supplementation should be offered to patients with a singleton pregnancy and a history of spontaneous PTB or to patients with a singleton pregnancy and no history of spontaneous PTB who have cervical shortening at less than 24 weeks.
- Cerclage may be offered between 16 and 24 weeks for a cervical length less than 25 mm in a patient with a singleton gestation who has a history of spontaneous PTB (<10 mm if no history of spontaneous PTB) or for a dilated cervix on exam regardless of history.
- Women who have a twin gestation with cervical dilation may be offered physical exam–indicated cerclage.
Which patients may benefit from antepartum fetal surveillance and when to initiate it
American College of Obstetricians and Gynecologists’ Committee on Obstetrics Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal surveillance: ACOG committee opinion, number 828. Obstet Gynecol. 2021;137:e177-e197.
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Antepartum fetal surveillance: ACOG practice bulletin, number 229. Obstet Gynecol. 2021;137:e116-e127.
The ultimate purpose of antenatal fetal surveillance is to prevent stillbirth. However, stillbirth has multiple etiologies, not all of which are preventable with testing. In June 2021, ACOG released a new Committee Opinion containing guidelines for fetal surveillance, including suggested gestational age at initiation and frequency of testing, for the most common high-risk conditions. ACOG also released an update to the Practice Bulletin on antepartum fetal surveillance; additions include randomized controlled trial level data on the utility of fetal kick counts (FKCs) and recommendations that align with the new Committee Opinion.
Data for the efficacy of antepartum fetal surveillance are lacking, mainly due to the difficulty of performing prospective studies in stillbirth. The existing evidence is subject to intervention bias, as deliveries increase in tested patients, and recommendations rely heavily on expert consensus and nonrandomized studies. Antenatal testing is also time, cost, and labor intensive, with the risk of intervention for a false-positive result. Despite these limitations, obstetrical practices routinely perform antenatal fetal surveillance.
The new guidelines: The why, when, and how often
Why. Antepartum fetal surveillance is suggested for conditions that have a risk of stillbirth greater than 0.8 per 1,000 (that is, the false-negative rate of a biophysical profile or a modified biophysical profile) and the relative risk or odds ratio is greater than 2.0 for stillbirth compared with unaffected pregnancies.
When. For most conditions, ACOG recommends initiation of testing at 32 weeks or later, with notable earlier exceptions for some of the highest-risk patients. For certain conditions, such as fetal growth restriction and hypertensive disorders of pregnancy, the recommendation is to start “at diagnosis,” with the corollary “or at a gestational age when delivery would be considered because of abnormal results.” Shared decision making with the patient about pregnancy goals therefore is required, particularly in cases of fetal anomalies, genetic conditions, or at very early gestational ages.
How often. The recommended frequency of testing is at least weekly. Testing frequency should be increased to twice-weekly outpatient or daily inpatient for the most complicated pregnancies (for example, fetal growth restriction with abnormal umbilical artery Doppler studies, preeclampsia with severe features).
Once or twice weekly is an option for many conditions, which gives the clinician the opportunity to assess clinical stability as well as the patient’s input in terms of logistics and anxiety.
Patients with multiple conditions may fall into the “individualized” category, as do patients with suboptimal control of conditions (for example, diabetes, hypertension) that may affect the fetus as the pregnancy progresses.
New diagnoses included for surveillance
Several diagnoses not previously included now qualify for antepartum fetal surveillance under the new guidelines, most notably:
- history of obstetrical complications in the immediate preceding pregnancy
—history of prior fetal growth restriction requiring preterm delivery
—history of prior preeclampsia requiring delivery
- alcohol use of 5 or more drinks per day
- in vitro fertilization
- abnormal serum markers
—pregnancy-associated plasma protein A (PAPP-A) in the fifth or lower percentile or 0.4 multiples of the median (MoM)
—second trimester inhibin A of 2 or greater MoM
- prepregnancy body mass index (BMI)
—this is divided into 2 categories for timing of initiation of testing:
- 37 weeks for BMI of 35 to 39.9 kg/m2
- 34 weeks for BMI of or greater than 40 kg/m2.
Fetal kick counts
The major change to the updated Practice Bulletin on antenatal surveillance is the inclusion of data on FKCs, a simple modality of fetal surveillance that does not require a clinical visit. For FKCs, a meta-analysis of more than 450,000 patients did not demonstrate a difference in perinatal death between the FKC intervention group (0.54%) and the control group (0.59%). Of note, there were small but statistically significant increases in the rates of induction of labor, cesarean delivery, and preterm delivery in the FKC intervention group. Therefore, this update does not recommend a formal program of FKCs for all patients.
- The antenatal fetal surveillance guidelines are just that—guidelines, not mandates. Their use will need to be adapted for specific patient populations and practice management patterns.
- Many conditions qualify for “individualized” surveillance, which offers the opportunity for detailed discussions on the patient’s care. This includes shared decision making with patients to meet their goals for the pregnancy.
- Although patient-perceived decreased fetal movement always warrants clinical evaluation, a regular program of fetal kick count monitoring is not recommended for all patients due to lack of data supporting its benefit in reducing perinatal death.
- As with any change, new guidelines potentially are a source of frustration, so a concerted effort by obstetrical clinicians to agree on adoption of the guidelines is needed. Additional clinical resources and both clinician and patient education may be required depending on current practice style, as the new strategy may increase the number of appointments and ultrasound exams required.
Continue to: Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation...
Use of antenatal corticosteroids now may be considered at 22 weeks’ gestation
American College of Obstetricians and Gynecologists. Use of antenatal corticosteroids at 22 weeks of gestation: ACOG practice advisory. September 2021. https://www .acog.org/clinical/clinical-guidance/practice-advisory /articles/2021/09/use-of-antenatal-corticosteroids-at -22-weeks-of-gestation. Accessed December 11, 2021.
In September 2021, ACOG and SMFM released a Practice Advisory updating the current recommendations for the administration of antenatal corticosteroids in the periviable period (22 to 25 6/7 weeks’ gestation). Whereas the prior lower limit of gestational age for consideration of steroids was 23 weeks, the new recommendation now extends this consideration down to 22 weeks.
The cited data include a meta-analysis of more than 2,200 patients in which the survival rate of infants born between 22 and 22 6/7 weeks who were exposed to antenatal steroids was 39% compared with 19.5% in the unexposed group. Another study of more than 1,000 patients demonstrated a statistically significant increase in overall survival in patients treated with antenatal steroids plus life support compared with life support only (38.5% vs 17.7%). Survival without major morbidity in this study, although increased from 1% to 4.4%, was still low.
Recommendation carries caveats
Given this information, the Practice Advisory offers a 2C level recommendation (weak recommendation, low quality of evidence) for antenatal steroids at 22 to 22 6/7 weeks’ gestation if neonatal resuscitation is planned, acknowledging the limitations and potential bias of the available data.
The Practice Advisory emphasizes the importance of counseling and patient involvement in the decision making. This requires a multidisciplinary collaboration among the neonatology and obstetrical teams, flexibility in the plan after birth depending on the infant’s condition, and redirection of care if appropriate. Estimated fetal weight, the presence of multiple gestations, fetal biologic sex, and any anomalies are also important in helping families make an informed decision for their particular pregnancy. As described in the Obstetric Care Consensus on periviable birth,6 it is important to remember that considerations and recommendations are not the same as requirements, and redirection of care to comfort and family memory making is not the same as withholding care.
The rest of the recommendations for the administration of antenatal steroids remain the same: Antenatal steroids are not recommended at less than 22 weeks due to lack of evidence of benefit, and they continue to be recommended at 24 weeks and beyond. ●
- Antenatal corticosteroids may be considered at 22 to 22 6/7 weeks’ gestation if, after thorough patient counseling, neonatal resuscitation is desired and planned by the family.
- The overall likelihood of survival and survival without major morbidities continues to be very low in the periviable period, especially at 22 weeks. Gestational age is only one of the many factors that must be considered in the shared decision making for this very difficult decision.
- Palliative care is a valid and appropriate option for patients facing a periviable delivery after appropriate counseling or after evaluation of the infant has occurred after birth.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2019. NCHS Data Brief, no 387. Hyattsville, MD: National Center for Health Statistics. October 2020. www.cdc.gov/nchs /data/databriefs/db387-H.pdf. Accessed December 20, 2021.
- To MS, Skentou C, Chan C, et al. Cervical assessment at the routine 23-week scan: standardizing techniques. Ultrasound Obstet Gynecol. 2001;17:217-219.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. Am J Obstet Gynecol. 2020;223:B16-B18.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37:127-136.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 6: Periviable birth. Obstet Gynecol. 2017;130:e187-e199.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2019. NCHS Data Brief, no 387. Hyattsville, MD: National Center for Health Statistics. October 2020. www.cdc.gov/nchs /data/databriefs/db387-H.pdf. Accessed December 20, 2021.
- To MS, Skentou C, Chan C, et al. Cervical assessment at the routine 23-week scan: standardizing techniques. Ultrasound Obstet Gynecol. 2001;17:217-219.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG practice bulletin, number 234. Obstet Gynecol. 2021;138:e65-e90.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. Am J Obstet Gynecol. 2020;223:B16-B18.
- Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37:127-136.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 6: Periviable birth. Obstet Gynecol. 2017;130:e187-e199.
Preschool boys’ behaviors traced back to moms’ thyroid hormones
Pregnant women’s thyroid hormone trajectories (levels in the first, second, and third trimester) may predict whether their male offspring are aggressive or withdrawn at age 4.
Certain maternal thyroid hormone trajectories were associated with problem behaviors in preschool boys in a study of close to 2,000 mother-child pairs in China.
The researchers identified low, moderate, and high thyroid-stimulating hormone (TSH) and free thyroxine (FT4) trajectories.
Most women had a low TSH trajectory and moderate FT4 trajectory, which the researchers deemed to be reference (normal) trajectories.
The children’s primary caregiver (parent or grandparent) completed an extensive questionnaire about their child’s behavior at age 4.
The 4-year-old boys whose mothers had a high TSH trajectory during pregnancy were more likely to be withdrawn and to externalize problems (odds ratio, 2.01 and 2.69, respectively).
Boys whose mothers had a high FT4 trajectory during pregnancy were more likely to be anxious/depressed (OR, 2.22).
And boys whose mothers had a moderate TSH trajectory or low FT4 trajectory were more likely to show aggressive behavior (OR, 3.76 and 4.17, respectively), compared with boys whose mothers had normal TSH and FT4 trajectories, after adjusting for potential confounders.
However, there was no association between abnormal maternal thyroid hormone trajectories and behavior problems in 4-year-old girls.
The study by Peixuan Li, BM, and colleagues was published online Jan. 6 in the Journal of Clinical Endocrinology & Metabolism.
‘Study supports monitoring thyroid function in pregnancy’
“Our findings highlight the significance of close monitoring and management of maternal thyroid function during pregnancy,” senior author Kun Huang, PhD, said in a press release from the Endocrine Society.
“This research presents a new perspective in early intervention of children’s emotional and behavioral problems,” added Dr. Huang, from Anhui Medical University, Hefei, China.
The results add to a growing body of literature about a controversial link between maternal thyroid hormones in pregnancy, when the fetal brain is developing, and subsequent behavior in preschool children, Caroline T. Nguyen, MD, who was not involved with this research, commented in an email.
“Some studies show an association between thyroid levels and behavioral outcomes, others not,” added Dr. Nguyen, assistant professor of clinical medicine, Keck School of Medicine, University of Southern California, Los Angeles. And “some studies have found sex-specific associations with maternal thyroid levels and neurocognitive/behavioral outcomes, others have not.”
Women considering pregnancy should be evaluated for possible thyroid disease, she continued. Currently, no universal screening mandates exist for thyroid disease in pregnancy, but the 2017 American Thyroid Association guidelines do recommend screening women at risk for thyroid dysfunction.
“I think screening for thyroid peroxidase antibody (TPOAb) positivity is helpful in women desiring pregnancy,” Dr. Nguyen continued, “because we know that patients with TPOAb positivity are at increased risk for miscarriage and have a blunted response to the increased demands of pregnancy for thyroid hormone production.”
TPOAb positivity is also associated with the increased risk of postpartum and long-term thyroid dysfunction.
This current study, Dr. Nguyen summarized, “adds to a growing body of research of the relationship of thyroid hormone levels and neurocognitive outcomes [in offspring] and supports the monitoring of thyroid disease in pregnancy.”
“However, we do not have sufficient data to demonstrate the benefits of intervention with levothyroxine treatment,” she noted.
Nevertheless, the lack of positive data does not suggest there is no theoretical benefit of intervention, she said, as such studies are very challenging to do.
“Physicians can help reduce stress and anxiety in patients desiring pregnancy by [recommending] preconception counseling, screening patients at risk for thyroid disease, and optimizing thyroid hormone levels before and during pregnancy,” according to Dr. Nguyen.
Maternal TSH and FT4 trajectories and preschoolers’ behaviors
Previous studies have reported that during pregnancy, maternal subclinical hypothyroidism (elevated TSH with normal FT4) as well as isolated hypothyroxinemia (decreased FT4 with normal TSH) are associated with adverse maternal and child outcomes, including preterm delivery and low birth weight.
However, most studies have not determined maternal thyroid hormone levels in different trimesters.
Researchers recruited pregnant women going for their first antenatal checkup at the Ma’anshan Maternal and Child Health Hospital in China from May 2013 to September 2014 and identified 1,860 mother-child pairs.
They determined maternal thyroid hormone levels from blood samples taken during the first, second, and third trimester: on average, gestational week 10, 25, and 34, respectively.
The researchers found that TSH levels increased somewhat from trimester 1 to trimester 2 and then decreased slightly in trimester 3. Most women (68%) had a low TSH trajectory, 28% had a moderate TSH trajectory, and 4% had a high TSH trajectory.
FT4 levels dropped sharply from trimester 1 to trimester 2 and then increased somewhat in trimester 3. About half of the women (52%) had a moderate FT4 trajectory, 33% had a low FT4 trajectory, and 15% had a high FT4 trajectory.
Most women (96.5%) had a low and stable TPOAb level, and the rest (3.5%) had high and decreasing TPOAb levels.
When the children in the study were 4 years old, their main caregiver (parent or grandparent) completed the 100-question Achenbach Child Behavior checklist to identify whether the child often, sometimes, or never displayed three internalizing problem behaviors (emotionally reactive, anxious/depressed, or withdrawn) and/or two externalizing problem behaviors (attention problems or aggressive behavior).
Study limitations, more research needed
It is not clear why the associations between maternal hormones and offspring behavior were only seen in boys. Perhaps male fetuses are more sensitive than female fetuses to changing maternal thyroid hormone levels in pregnancy, the researchers speculate.
They acknowledge that study limitations include there were few children with aggressive behavior, so the confidence interval for the association of the moderate TSH trajectory with aggressive behavior was very wide.
In addition, evaluation of children’s behavior by caregivers was subjective. Also, the researchers did not have information about iodine levels, and low iodine levels can impair child brain development.
And there may have been residual confounders that researchers did not account for, such as differences in family upbringing, parental marital status, and the mother’s exposure to endocrine disruptors.
Therefore, further research is needed.
The study was supported by grants from the National Natural Science Foundation of China, the University Synergy Innovation Program of Anhui Province, the Sci-Tech Basic Resources Research Program of China, the National Key Research and Development Program, the Chinese Academy of Medical Sciences, and the Research Fund of Anhui Institute of Translational Medicine. The researchers and Dr. Nguyen have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women’s thyroid hormone trajectories (levels in the first, second, and third trimester) may predict whether their male offspring are aggressive or withdrawn at age 4.
Certain maternal thyroid hormone trajectories were associated with problem behaviors in preschool boys in a study of close to 2,000 mother-child pairs in China.
The researchers identified low, moderate, and high thyroid-stimulating hormone (TSH) and free thyroxine (FT4) trajectories.
Most women had a low TSH trajectory and moderate FT4 trajectory, which the researchers deemed to be reference (normal) trajectories.
The children’s primary caregiver (parent or grandparent) completed an extensive questionnaire about their child’s behavior at age 4.
The 4-year-old boys whose mothers had a high TSH trajectory during pregnancy were more likely to be withdrawn and to externalize problems (odds ratio, 2.01 and 2.69, respectively).
Boys whose mothers had a high FT4 trajectory during pregnancy were more likely to be anxious/depressed (OR, 2.22).
And boys whose mothers had a moderate TSH trajectory or low FT4 trajectory were more likely to show aggressive behavior (OR, 3.76 and 4.17, respectively), compared with boys whose mothers had normal TSH and FT4 trajectories, after adjusting for potential confounders.
However, there was no association between abnormal maternal thyroid hormone trajectories and behavior problems in 4-year-old girls.
The study by Peixuan Li, BM, and colleagues was published online Jan. 6 in the Journal of Clinical Endocrinology & Metabolism.
‘Study supports monitoring thyroid function in pregnancy’
“Our findings highlight the significance of close monitoring and management of maternal thyroid function during pregnancy,” senior author Kun Huang, PhD, said in a press release from the Endocrine Society.
“This research presents a new perspective in early intervention of children’s emotional and behavioral problems,” added Dr. Huang, from Anhui Medical University, Hefei, China.
The results add to a growing body of literature about a controversial link between maternal thyroid hormones in pregnancy, when the fetal brain is developing, and subsequent behavior in preschool children, Caroline T. Nguyen, MD, who was not involved with this research, commented in an email.
“Some studies show an association between thyroid levels and behavioral outcomes, others not,” added Dr. Nguyen, assistant professor of clinical medicine, Keck School of Medicine, University of Southern California, Los Angeles. And “some studies have found sex-specific associations with maternal thyroid levels and neurocognitive/behavioral outcomes, others have not.”
Women considering pregnancy should be evaluated for possible thyroid disease, she continued. Currently, no universal screening mandates exist for thyroid disease in pregnancy, but the 2017 American Thyroid Association guidelines do recommend screening women at risk for thyroid dysfunction.
“I think screening for thyroid peroxidase antibody (TPOAb) positivity is helpful in women desiring pregnancy,” Dr. Nguyen continued, “because we know that patients with TPOAb positivity are at increased risk for miscarriage and have a blunted response to the increased demands of pregnancy for thyroid hormone production.”
TPOAb positivity is also associated with the increased risk of postpartum and long-term thyroid dysfunction.
This current study, Dr. Nguyen summarized, “adds to a growing body of research of the relationship of thyroid hormone levels and neurocognitive outcomes [in offspring] and supports the monitoring of thyroid disease in pregnancy.”
“However, we do not have sufficient data to demonstrate the benefits of intervention with levothyroxine treatment,” she noted.
Nevertheless, the lack of positive data does not suggest there is no theoretical benefit of intervention, she said, as such studies are very challenging to do.
“Physicians can help reduce stress and anxiety in patients desiring pregnancy by [recommending] preconception counseling, screening patients at risk for thyroid disease, and optimizing thyroid hormone levels before and during pregnancy,” according to Dr. Nguyen.
Maternal TSH and FT4 trajectories and preschoolers’ behaviors
Previous studies have reported that during pregnancy, maternal subclinical hypothyroidism (elevated TSH with normal FT4) as well as isolated hypothyroxinemia (decreased FT4 with normal TSH) are associated with adverse maternal and child outcomes, including preterm delivery and low birth weight.
However, most studies have not determined maternal thyroid hormone levels in different trimesters.
Researchers recruited pregnant women going for their first antenatal checkup at the Ma’anshan Maternal and Child Health Hospital in China from May 2013 to September 2014 and identified 1,860 mother-child pairs.
They determined maternal thyroid hormone levels from blood samples taken during the first, second, and third trimester: on average, gestational week 10, 25, and 34, respectively.
The researchers found that TSH levels increased somewhat from trimester 1 to trimester 2 and then decreased slightly in trimester 3. Most women (68%) had a low TSH trajectory, 28% had a moderate TSH trajectory, and 4% had a high TSH trajectory.
FT4 levels dropped sharply from trimester 1 to trimester 2 and then increased somewhat in trimester 3. About half of the women (52%) had a moderate FT4 trajectory, 33% had a low FT4 trajectory, and 15% had a high FT4 trajectory.
Most women (96.5%) had a low and stable TPOAb level, and the rest (3.5%) had high and decreasing TPOAb levels.
When the children in the study were 4 years old, their main caregiver (parent or grandparent) completed the 100-question Achenbach Child Behavior checklist to identify whether the child often, sometimes, or never displayed three internalizing problem behaviors (emotionally reactive, anxious/depressed, or withdrawn) and/or two externalizing problem behaviors (attention problems or aggressive behavior).
Study limitations, more research needed
It is not clear why the associations between maternal hormones and offspring behavior were only seen in boys. Perhaps male fetuses are more sensitive than female fetuses to changing maternal thyroid hormone levels in pregnancy, the researchers speculate.
They acknowledge that study limitations include there were few children with aggressive behavior, so the confidence interval for the association of the moderate TSH trajectory with aggressive behavior was very wide.
In addition, evaluation of children’s behavior by caregivers was subjective. Also, the researchers did not have information about iodine levels, and low iodine levels can impair child brain development.
And there may have been residual confounders that researchers did not account for, such as differences in family upbringing, parental marital status, and the mother’s exposure to endocrine disruptors.
Therefore, further research is needed.
The study was supported by grants from the National Natural Science Foundation of China, the University Synergy Innovation Program of Anhui Province, the Sci-Tech Basic Resources Research Program of China, the National Key Research and Development Program, the Chinese Academy of Medical Sciences, and the Research Fund of Anhui Institute of Translational Medicine. The researchers and Dr. Nguyen have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women’s thyroid hormone trajectories (levels in the first, second, and third trimester) may predict whether their male offspring are aggressive or withdrawn at age 4.
Certain maternal thyroid hormone trajectories were associated with problem behaviors in preschool boys in a study of close to 2,000 mother-child pairs in China.
The researchers identified low, moderate, and high thyroid-stimulating hormone (TSH) and free thyroxine (FT4) trajectories.
Most women had a low TSH trajectory and moderate FT4 trajectory, which the researchers deemed to be reference (normal) trajectories.
The children’s primary caregiver (parent or grandparent) completed an extensive questionnaire about their child’s behavior at age 4.
The 4-year-old boys whose mothers had a high TSH trajectory during pregnancy were more likely to be withdrawn and to externalize problems (odds ratio, 2.01 and 2.69, respectively).
Boys whose mothers had a high FT4 trajectory during pregnancy were more likely to be anxious/depressed (OR, 2.22).
And boys whose mothers had a moderate TSH trajectory or low FT4 trajectory were more likely to show aggressive behavior (OR, 3.76 and 4.17, respectively), compared with boys whose mothers had normal TSH and FT4 trajectories, after adjusting for potential confounders.
However, there was no association between abnormal maternal thyroid hormone trajectories and behavior problems in 4-year-old girls.
The study by Peixuan Li, BM, and colleagues was published online Jan. 6 in the Journal of Clinical Endocrinology & Metabolism.
‘Study supports monitoring thyroid function in pregnancy’
“Our findings highlight the significance of close monitoring and management of maternal thyroid function during pregnancy,” senior author Kun Huang, PhD, said in a press release from the Endocrine Society.
“This research presents a new perspective in early intervention of children’s emotional and behavioral problems,” added Dr. Huang, from Anhui Medical University, Hefei, China.
The results add to a growing body of literature about a controversial link between maternal thyroid hormones in pregnancy, when the fetal brain is developing, and subsequent behavior in preschool children, Caroline T. Nguyen, MD, who was not involved with this research, commented in an email.
“Some studies show an association between thyroid levels and behavioral outcomes, others not,” added Dr. Nguyen, assistant professor of clinical medicine, Keck School of Medicine, University of Southern California, Los Angeles. And “some studies have found sex-specific associations with maternal thyroid levels and neurocognitive/behavioral outcomes, others have not.”
Women considering pregnancy should be evaluated for possible thyroid disease, she continued. Currently, no universal screening mandates exist for thyroid disease in pregnancy, but the 2017 American Thyroid Association guidelines do recommend screening women at risk for thyroid dysfunction.
“I think screening for thyroid peroxidase antibody (TPOAb) positivity is helpful in women desiring pregnancy,” Dr. Nguyen continued, “because we know that patients with TPOAb positivity are at increased risk for miscarriage and have a blunted response to the increased demands of pregnancy for thyroid hormone production.”
TPOAb positivity is also associated with the increased risk of postpartum and long-term thyroid dysfunction.
This current study, Dr. Nguyen summarized, “adds to a growing body of research of the relationship of thyroid hormone levels and neurocognitive outcomes [in offspring] and supports the monitoring of thyroid disease in pregnancy.”
“However, we do not have sufficient data to demonstrate the benefits of intervention with levothyroxine treatment,” she noted.
Nevertheless, the lack of positive data does not suggest there is no theoretical benefit of intervention, she said, as such studies are very challenging to do.
“Physicians can help reduce stress and anxiety in patients desiring pregnancy by [recommending] preconception counseling, screening patients at risk for thyroid disease, and optimizing thyroid hormone levels before and during pregnancy,” according to Dr. Nguyen.
Maternal TSH and FT4 trajectories and preschoolers’ behaviors
Previous studies have reported that during pregnancy, maternal subclinical hypothyroidism (elevated TSH with normal FT4) as well as isolated hypothyroxinemia (decreased FT4 with normal TSH) are associated with adverse maternal and child outcomes, including preterm delivery and low birth weight.
However, most studies have not determined maternal thyroid hormone levels in different trimesters.
Researchers recruited pregnant women going for their first antenatal checkup at the Ma’anshan Maternal and Child Health Hospital in China from May 2013 to September 2014 and identified 1,860 mother-child pairs.
They determined maternal thyroid hormone levels from blood samples taken during the first, second, and third trimester: on average, gestational week 10, 25, and 34, respectively.
The researchers found that TSH levels increased somewhat from trimester 1 to trimester 2 and then decreased slightly in trimester 3. Most women (68%) had a low TSH trajectory, 28% had a moderate TSH trajectory, and 4% had a high TSH trajectory.
FT4 levels dropped sharply from trimester 1 to trimester 2 and then increased somewhat in trimester 3. About half of the women (52%) had a moderate FT4 trajectory, 33% had a low FT4 trajectory, and 15% had a high FT4 trajectory.
Most women (96.5%) had a low and stable TPOAb level, and the rest (3.5%) had high and decreasing TPOAb levels.
When the children in the study were 4 years old, their main caregiver (parent or grandparent) completed the 100-question Achenbach Child Behavior checklist to identify whether the child often, sometimes, or never displayed three internalizing problem behaviors (emotionally reactive, anxious/depressed, or withdrawn) and/or two externalizing problem behaviors (attention problems or aggressive behavior).
Study limitations, more research needed
It is not clear why the associations between maternal hormones and offspring behavior were only seen in boys. Perhaps male fetuses are more sensitive than female fetuses to changing maternal thyroid hormone levels in pregnancy, the researchers speculate.
They acknowledge that study limitations include there were few children with aggressive behavior, so the confidence interval for the association of the moderate TSH trajectory with aggressive behavior was very wide.
In addition, evaluation of children’s behavior by caregivers was subjective. Also, the researchers did not have information about iodine levels, and low iodine levels can impair child brain development.
And there may have been residual confounders that researchers did not account for, such as differences in family upbringing, parental marital status, and the mother’s exposure to endocrine disruptors.
Therefore, further research is needed.
The study was supported by grants from the National Natural Science Foundation of China, the University Synergy Innovation Program of Anhui Province, the Sci-Tech Basic Resources Research Program of China, the National Key Research and Development Program, the Chinese Academy of Medical Sciences, and the Research Fund of Anhui Institute of Translational Medicine. The researchers and Dr. Nguyen have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM