User login
Study finds more adverse maternal outcomes in women with disabilities
Women with physical, intellectual, and sensory disabilities had higher risk for almost all pregnancy complications, obstetric interventions, and adverse outcomes, including severe maternal morbidity (SMM) and mortality compared to women without disabilities, according to an analysis of a large, retrospective cohort.
The findings, published in JAMA Network Open (2021;4[12]:e2138414 doi: 10.1001/jamanetworkopen.2021.38414), “may be a direct reflection of the challenges women with all types of disabilities face when accessing and receiving care, which is likely compounded by poorer preconception health,” suggested lead author Jessica L. Gleason, PhD, MPH, and co-authors, all from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Md.
“Women with disabilities have long been ignored in obstetric research and clinical practice,” added Hilary K. Brown, PhD, from the University of Toronto, in an accompanying editorial. “Inclusion of disability indicators needs to be the norm – not the exception – in health administrative data so that these disparities can be regularly tracked and addressed.”
The investigators used data from the Consortium on Safe Labor (CSL), a retrospective cohort of deliveries from 12 U.S. clinical centers between Jan. 2002 and Jan. 2008, to analyze obstetric interventions and adverse maternal outcomes in women with and without disabilities.
The analysis included a total of 223,385 women, mean age 27.6 years, of whom 2,074 (0.9%) had a disability, and 221,311 did not. Among those with disabilities, 1,733 (83.5%) were physical, 91 (4.4%) were intellectual, and 250 (12.1%) were sensory. While almost half (49.4%) of the women were White, 22.5% were Black, 17.5% were Hispanic, and 4.1% were Asian or Pacific Islander.
Outcomes were analyzed with three composite measures:
- Pregnancy-related complications (pregnancy-related hypertensive diseases, gestational diabetes, placental abruption, placenta previa, premature rupture of membranes, preterm PROM);
- All labor, delivery, and postpartum complications (chorioamnionitis, hemorrhage, blood transfusion, thromboembolism, postpartum fever, infection, cardiovascular events, cardiomyopathy, and maternal death);
- SMM only, including severe pre-eclampsia/eclampsia, hemorrhage, thromboembolism, fever, infection, cardiomyopathy, and cardiovascular events during labor and delivery.
After adjustment for covariates, women with disabilities had higher risk of pregnancy-related complications. This included a 48% higher risk of mild pre-eclampsia and double the risk of severe pre-eclampsia/eclampsia. The composite risk of any pregnancy complication was 27% higher for women with physical disabilities, 49% higher for women with intellectual disabilities, and 53% higher for women with sensory disabilities.
The findings were similar for labor, delivery, and postpartum complications, showing women with disabilities had higher risk for a range of obstetrical interventions, including cesarean delivery – both planned and intrapartum (aRR, 1.34). Additionally, women with disabilities were less likely to have a cesarean delivery that was “solely clinically indicated” (aRR, 0.79), and more likely to have a cesarean delivery for “softer” mixed indication (aRR, 1.16), “supporting a possible overuse of cesarean delivery among women with disability,” they suggested.
Women with disabilities also had a higher risk of postpartum hemorrhage (aRR, 1.27), blood transfusion (aRR, 1.64), and maternal mortality (aRR, 11.19), as well as individual markers of severe maternal morbidity, such as cardiovascular events (aRR, 4.02), infection (aRR, 2.69), and venous thromboembolism (aRR, 6.08).
The authors speculate that the increased risks for women with disabilities “may be the result of a combination of independent risk factors, including the higher rate of obstetric intervention via cesarean delivery, under-recognition of women with disabilities as a population with higher-risk pregnancies, and lack of health care practitioner knowledge or comfort in managing pregnancies among women with disabilities.”
Dr. Brown noted in her commentary that there is a need for better education of health care professionals in this area. “Given that 12% of reproductive-aged women have a disability, that pregnancy rates are similar among women with and without disabilities, and that women with disabilities are at elevated risk of a range of adverse maternal outcomes, including severe maternal morbidity and maternal mortality, disability modules should be a mandatory component of education for obstetricians and midwives as well as other obstetrical health care professionals.”
Calling the study “a serious wake-up call,” Monika Mitra, PhD, told this publication that the findings highlight the need for “urgent attention” on improving obstetric care for people with disabilities “with a focus on accessibility and inclusion, changing clinical practice to better serve disabled people, integrating disability-related training for health care practitioners, and developing evidence-based interventions to support people with disabilities during this time.” The associate professor and director of the Lurie Institute for Disability Policy, in Brandeis University, Waltham, Mass. said the risk factors for poor outcomes are present early in pregnancy or even preconception. “We know that disabled women report barriers in accessing health care and receive lower-quality care compared to nondisabled women and are more likely to experience poverty, housing and food insecurity, educational and employment barriers, abuse, chronic health conditions, and mental illness than women without disabilities.”
She noted that the study’s sample of people with disabilities was small, and the measure of disability used was based on ICD-9 codes, which captures only severe disabilities. “As noted in the commentary by [Dr.] Brown, our standard sources of health administrative data do not give us the full picture on disability, and we need other, more equitable ways of identifying disability based, for example, on self-reports of activity or participation limitations if we are to be able to understand the effects on obstetric outcomes of health and health care disparities and of social determinants of health. Moreover, researchers have generally not yet begun to incorporate knowledge of the experiences of transgender people during pregnancy, which will impact our measures and study of obstetric outcomes among people with disabilities as well as the language we use.”
The study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The study authors and Dr. Brown reported no conflicts of interest. Dr. Mitra receives funding from the NICHD and the National Institute on Disability, Independent Living for research on pregnancy outcomes among people with disabilities.
Women with physical, intellectual, and sensory disabilities had higher risk for almost all pregnancy complications, obstetric interventions, and adverse outcomes, including severe maternal morbidity (SMM) and mortality compared to women without disabilities, according to an analysis of a large, retrospective cohort.
The findings, published in JAMA Network Open (2021;4[12]:e2138414 doi: 10.1001/jamanetworkopen.2021.38414), “may be a direct reflection of the challenges women with all types of disabilities face when accessing and receiving care, which is likely compounded by poorer preconception health,” suggested lead author Jessica L. Gleason, PhD, MPH, and co-authors, all from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Md.
“Women with disabilities have long been ignored in obstetric research and clinical practice,” added Hilary K. Brown, PhD, from the University of Toronto, in an accompanying editorial. “Inclusion of disability indicators needs to be the norm – not the exception – in health administrative data so that these disparities can be regularly tracked and addressed.”
The investigators used data from the Consortium on Safe Labor (CSL), a retrospective cohort of deliveries from 12 U.S. clinical centers between Jan. 2002 and Jan. 2008, to analyze obstetric interventions and adverse maternal outcomes in women with and without disabilities.
The analysis included a total of 223,385 women, mean age 27.6 years, of whom 2,074 (0.9%) had a disability, and 221,311 did not. Among those with disabilities, 1,733 (83.5%) were physical, 91 (4.4%) were intellectual, and 250 (12.1%) were sensory. While almost half (49.4%) of the women were White, 22.5% were Black, 17.5% were Hispanic, and 4.1% were Asian or Pacific Islander.
Outcomes were analyzed with three composite measures:
- Pregnancy-related complications (pregnancy-related hypertensive diseases, gestational diabetes, placental abruption, placenta previa, premature rupture of membranes, preterm PROM);
- All labor, delivery, and postpartum complications (chorioamnionitis, hemorrhage, blood transfusion, thromboembolism, postpartum fever, infection, cardiovascular events, cardiomyopathy, and maternal death);
- SMM only, including severe pre-eclampsia/eclampsia, hemorrhage, thromboembolism, fever, infection, cardiomyopathy, and cardiovascular events during labor and delivery.
After adjustment for covariates, women with disabilities had higher risk of pregnancy-related complications. This included a 48% higher risk of mild pre-eclampsia and double the risk of severe pre-eclampsia/eclampsia. The composite risk of any pregnancy complication was 27% higher for women with physical disabilities, 49% higher for women with intellectual disabilities, and 53% higher for women with sensory disabilities.
The findings were similar for labor, delivery, and postpartum complications, showing women with disabilities had higher risk for a range of obstetrical interventions, including cesarean delivery – both planned and intrapartum (aRR, 1.34). Additionally, women with disabilities were less likely to have a cesarean delivery that was “solely clinically indicated” (aRR, 0.79), and more likely to have a cesarean delivery for “softer” mixed indication (aRR, 1.16), “supporting a possible overuse of cesarean delivery among women with disability,” they suggested.
Women with disabilities also had a higher risk of postpartum hemorrhage (aRR, 1.27), blood transfusion (aRR, 1.64), and maternal mortality (aRR, 11.19), as well as individual markers of severe maternal morbidity, such as cardiovascular events (aRR, 4.02), infection (aRR, 2.69), and venous thromboembolism (aRR, 6.08).
The authors speculate that the increased risks for women with disabilities “may be the result of a combination of independent risk factors, including the higher rate of obstetric intervention via cesarean delivery, under-recognition of women with disabilities as a population with higher-risk pregnancies, and lack of health care practitioner knowledge or comfort in managing pregnancies among women with disabilities.”
Dr. Brown noted in her commentary that there is a need for better education of health care professionals in this area. “Given that 12% of reproductive-aged women have a disability, that pregnancy rates are similar among women with and without disabilities, and that women with disabilities are at elevated risk of a range of adverse maternal outcomes, including severe maternal morbidity and maternal mortality, disability modules should be a mandatory component of education for obstetricians and midwives as well as other obstetrical health care professionals.”
Calling the study “a serious wake-up call,” Monika Mitra, PhD, told this publication that the findings highlight the need for “urgent attention” on improving obstetric care for people with disabilities “with a focus on accessibility and inclusion, changing clinical practice to better serve disabled people, integrating disability-related training for health care practitioners, and developing evidence-based interventions to support people with disabilities during this time.” The associate professor and director of the Lurie Institute for Disability Policy, in Brandeis University, Waltham, Mass. said the risk factors for poor outcomes are present early in pregnancy or even preconception. “We know that disabled women report barriers in accessing health care and receive lower-quality care compared to nondisabled women and are more likely to experience poverty, housing and food insecurity, educational and employment barriers, abuse, chronic health conditions, and mental illness than women without disabilities.”
She noted that the study’s sample of people with disabilities was small, and the measure of disability used was based on ICD-9 codes, which captures only severe disabilities. “As noted in the commentary by [Dr.] Brown, our standard sources of health administrative data do not give us the full picture on disability, and we need other, more equitable ways of identifying disability based, for example, on self-reports of activity or participation limitations if we are to be able to understand the effects on obstetric outcomes of health and health care disparities and of social determinants of health. Moreover, researchers have generally not yet begun to incorporate knowledge of the experiences of transgender people during pregnancy, which will impact our measures and study of obstetric outcomes among people with disabilities as well as the language we use.”
The study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The study authors and Dr. Brown reported no conflicts of interest. Dr. Mitra receives funding from the NICHD and the National Institute on Disability, Independent Living for research on pregnancy outcomes among people with disabilities.
Women with physical, intellectual, and sensory disabilities had higher risk for almost all pregnancy complications, obstetric interventions, and adverse outcomes, including severe maternal morbidity (SMM) and mortality compared to women without disabilities, according to an analysis of a large, retrospective cohort.
The findings, published in JAMA Network Open (2021;4[12]:e2138414 doi: 10.1001/jamanetworkopen.2021.38414), “may be a direct reflection of the challenges women with all types of disabilities face when accessing and receiving care, which is likely compounded by poorer preconception health,” suggested lead author Jessica L. Gleason, PhD, MPH, and co-authors, all from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Md.
“Women with disabilities have long been ignored in obstetric research and clinical practice,” added Hilary K. Brown, PhD, from the University of Toronto, in an accompanying editorial. “Inclusion of disability indicators needs to be the norm – not the exception – in health administrative data so that these disparities can be regularly tracked and addressed.”
The investigators used data from the Consortium on Safe Labor (CSL), a retrospective cohort of deliveries from 12 U.S. clinical centers between Jan. 2002 and Jan. 2008, to analyze obstetric interventions and adverse maternal outcomes in women with and without disabilities.
The analysis included a total of 223,385 women, mean age 27.6 years, of whom 2,074 (0.9%) had a disability, and 221,311 did not. Among those with disabilities, 1,733 (83.5%) were physical, 91 (4.4%) were intellectual, and 250 (12.1%) were sensory. While almost half (49.4%) of the women were White, 22.5% were Black, 17.5% were Hispanic, and 4.1% were Asian or Pacific Islander.
Outcomes were analyzed with three composite measures:
- Pregnancy-related complications (pregnancy-related hypertensive diseases, gestational diabetes, placental abruption, placenta previa, premature rupture of membranes, preterm PROM);
- All labor, delivery, and postpartum complications (chorioamnionitis, hemorrhage, blood transfusion, thromboembolism, postpartum fever, infection, cardiovascular events, cardiomyopathy, and maternal death);
- SMM only, including severe pre-eclampsia/eclampsia, hemorrhage, thromboembolism, fever, infection, cardiomyopathy, and cardiovascular events during labor and delivery.
After adjustment for covariates, women with disabilities had higher risk of pregnancy-related complications. This included a 48% higher risk of mild pre-eclampsia and double the risk of severe pre-eclampsia/eclampsia. The composite risk of any pregnancy complication was 27% higher for women with physical disabilities, 49% higher for women with intellectual disabilities, and 53% higher for women with sensory disabilities.
The findings were similar for labor, delivery, and postpartum complications, showing women with disabilities had higher risk for a range of obstetrical interventions, including cesarean delivery – both planned and intrapartum (aRR, 1.34). Additionally, women with disabilities were less likely to have a cesarean delivery that was “solely clinically indicated” (aRR, 0.79), and more likely to have a cesarean delivery for “softer” mixed indication (aRR, 1.16), “supporting a possible overuse of cesarean delivery among women with disability,” they suggested.
Women with disabilities also had a higher risk of postpartum hemorrhage (aRR, 1.27), blood transfusion (aRR, 1.64), and maternal mortality (aRR, 11.19), as well as individual markers of severe maternal morbidity, such as cardiovascular events (aRR, 4.02), infection (aRR, 2.69), and venous thromboembolism (aRR, 6.08).
The authors speculate that the increased risks for women with disabilities “may be the result of a combination of independent risk factors, including the higher rate of obstetric intervention via cesarean delivery, under-recognition of women with disabilities as a population with higher-risk pregnancies, and lack of health care practitioner knowledge or comfort in managing pregnancies among women with disabilities.”
Dr. Brown noted in her commentary that there is a need for better education of health care professionals in this area. “Given that 12% of reproductive-aged women have a disability, that pregnancy rates are similar among women with and without disabilities, and that women with disabilities are at elevated risk of a range of adverse maternal outcomes, including severe maternal morbidity and maternal mortality, disability modules should be a mandatory component of education for obstetricians and midwives as well as other obstetrical health care professionals.”
Calling the study “a serious wake-up call,” Monika Mitra, PhD, told this publication that the findings highlight the need for “urgent attention” on improving obstetric care for people with disabilities “with a focus on accessibility and inclusion, changing clinical practice to better serve disabled people, integrating disability-related training for health care practitioners, and developing evidence-based interventions to support people with disabilities during this time.” The associate professor and director of the Lurie Institute for Disability Policy, in Brandeis University, Waltham, Mass. said the risk factors for poor outcomes are present early in pregnancy or even preconception. “We know that disabled women report barriers in accessing health care and receive lower-quality care compared to nondisabled women and are more likely to experience poverty, housing and food insecurity, educational and employment barriers, abuse, chronic health conditions, and mental illness than women without disabilities.”
She noted that the study’s sample of people with disabilities was small, and the measure of disability used was based on ICD-9 codes, which captures only severe disabilities. “As noted in the commentary by [Dr.] Brown, our standard sources of health administrative data do not give us the full picture on disability, and we need other, more equitable ways of identifying disability based, for example, on self-reports of activity or participation limitations if we are to be able to understand the effects on obstetric outcomes of health and health care disparities and of social determinants of health. Moreover, researchers have generally not yet begun to incorporate knowledge of the experiences of transgender people during pregnancy, which will impact our measures and study of obstetric outcomes among people with disabilities as well as the language we use.”
The study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The study authors and Dr. Brown reported no conflicts of interest. Dr. Mitra receives funding from the NICHD and the National Institute on Disability, Independent Living for research on pregnancy outcomes among people with disabilities.
Fixing the maternal health problem in the U.S.: Signs of hope?
In the United States, nearly 4 million women a year prepare to give birth, looking forward to the joy to come. But for some, the dream turns tragic. And another 60,000 have pregnancy-related or childbirth-related health issues.
Causes of death vary greatly, including hemorrhage during pregnancy or during delivery, heart conditions, and mental health issues such as substance abuse and suicide after the birth.
In 2019, the U.S. maternal death rate was 20.1 per 100,000 women, according to the CDC, significantly higher than the 17.4 per 100,000 recorded in 2018. For Black women, the maternal death rate was more than double the overall – 44 per 100,000 in 2019.
“We have to address our horrendous maternal health care system and also need to address the inequities,” says Laurie Zephyrin, MD, vice president for advancing health equity for the Commonwealth Fund, a foundation supporting independent research on health care issues. “This is an issue that has needed national attention for a long time.”
“If we look overall, our maternal death rate is more than twice that of more than 10 other high-income countries,” she said.
As sobering as the problem is, recent developments have sparked hope that reversing the course is possible. Among them:
U.S. News & World Report, long known for its rankings of hospitals, issued its first ever “Best Hospitals for Maternity” rankings Dec. 7, highlighting facilities that perform well on key quality indicators. It plans to update the report annually.
At the first-ever White House Maternal Health Day of Action on Dec. 7, Vice President Kamala Harris urged a call to action to reduce maternal deaths and pregnancy-related health problems, with extension of postpartum coverage through Medicaid programs, among other actions.
A new hospital designation called ‘’Birthing Friendly” will be established by the Centers for Medicare & Medicaid Services. The label will be given to facilities that take part in a program aimed at improving maternal outcomes and that use patient safety practices.
President Joe Biden’s proposed Build Back Better plan includes maternal health provisions, including $3 billion in new maternal health funding. The money will aim to grow and diversify the workforce caring for pregnant women, coordinate care better, and step up research on maternal health, among other projects.
Ongoing efforts in Congress are aimed at fixing the wide disparities in maternal health affecting Black women. Regardless of income level or education, Black women are at a higher risk of maternal death and other health issues than are White women. A Black woman with a college education is at 60% higher risk of maternal death than a White or Hispanic woman who didn’t graduate high school, according to the Commonwealth Fund.
Best hospitals for maternity
For its rankings, U.S. News and World Report reached out to the 2,700 U.S. hospitals that offer maternity services, said Ben Harder, chief of health analysis and managing editor at U.S. News & World Report.
To be recognized, a hospital had to submit data from 2019 and meet the publication’s maternity care standards. The publication received responses from just 571 hospitals, representing about two of every five births in the country.
Of those, 237 were identified as best for maternity.
As to why the response rate was not higher, Mr. Harder cited the reporting burden and says it is understandable. Some hospitals likely did not have the staff available, especially during the pandemic, to gather the data needed to be evaluated by U.S. News & World Report.
On their other evaluations, the rankings are based on Medicare data, “so hospitals don’t have to lift a finger.” He expects more hospitals will respond for their future evaluations of maternity care.
The evaluators focused on five quality measures, making a score based on the cesarean section delivery rate among first-time mothers, early elective delivery rates, unexpected newborn complication rates, breastfeeding rates, and option for vaginal birth after C-section.
A call to action: Expand coverage
Speaking at the White House Maternal Health Day of Action, Mrs. Harris told participants: “The challenge is urgent, and it is important, and it will take all of us.”
Being pregnant and giving birth, she said, should not carry such great risks. She zeroed in on systemic inequities in the way women are treated and the dramatic impact maternal death and health issues have on the economy.
“A healthy economy requires healthy mothers and healthy babies,” Mrs. Harris said.
“Before, during, and after childbirth, women in our nation are dying at a higher rate than any other developed nation in our world,” she said, noting that research shows that Black women, Native Americans, and women in rural America more likely to suffer.
A major strategy in the call to action, according to Mrs. Harris, is encouraging states to expand postpartum coverage to pregnant women enrolled in Medicaid or the Children’s Health Insurance Program from the existing 60 days to a full year. Together, these two programs cover over 42% of births in the country, so expanding the coverage is expected to have a great impact.
The 60 days of coverage is not enough, as many deaths and complications happen more than 60 days after childbirth, Mrs. Harris said. The logistics for states to extend coverage were established by the American Rescue Plan and will become available by April 2022. Some states have already extended the postpartum coverage.
According to the Centers for Medicare and Medicaid Services, if every state did adopt an extension, as the Build Back Better Act proposes, the number of Americans getting coverage for a full year after childbirth would about double, extending the coverage for about 720,000 each year.
Congressional actions
Congress is working on the issue as well. The Black Maternal Health Momnibus Act of 2021, for instance, proposes several measures, including improving maternal nutrition, expanding affordable housing, and extending the maternal workforce to include more doulas and midwives.
“And for so many women, let’s note doulas are literally a lifeline,” Mrs. Harris said at the White House event.
Doulas are trained to offer women physical, emotional, and informational support before, during, and after childbirth. No reliable statistics are available on their numbers in the United States, but a March of Dimes report estimates that about 9,000 were included in a registration database in 2018.
Explaining and fixing the disparities
No one can explain for sure why Black women, in particular, are at higher risk of dying from pregnancy-related complications. Systemic inequity is one likely reason, Mrs. Harris said, noting there are differences in how people are treated based on who they are.
Inherent and unconscious bias in offering women treatment plays a role, experts say. Training could reverse or reduce that bias. Some women of color also may have less access to care, as do women in some rural areas.
According to Mrs. Harris, more than 20 companies and nonprofits have pledged to invest more than $20 million in maternal health efforts in the United States and more than $150 million globally. Among the proposed programs: remote-care monitors in rural areas, better care models for the postpartum period, and improved education programs for maternal health providers.
When statistics hit home
Many who work to improve maternal health have gone through issues themselves or had loved ones who did.
Jill Arnold, founder of the Maternal Safety Foundation in Bentonville, Ark., became a consumer advocate after giving birth to her two daughters, now teenagers. With the first birth, Ms. Arnold said she was intensely pressured at the last minute to have a C-section. She held out, resisted, and delivered a healthy baby vaginally.
For her second childbirth, she chose an accredited birth center that allowed her to have a doula and a midwife.
“The care I received was night and day,” she said. “The overwhelming pressure to consent to a C-section wasn’t there.”
She welcomes the information provided by the new U.S. News & World Report rankings as well as the upcoming “Birthing Friendly” designations.
“The onus shouldn’t be on patients, on individuals, on pregnant people to do the research,” Ms. Arnold said.
Rather, women and their partners need information at their fingertips so they can make an informed decision about how to give birth and where.
U.S. Rep. Lauren Underwood (D-Ill.), who cofounded the Black Maternal Health Caucus in April 2019, with Rep. Alma Adams (D-N.C.), wrote a touching blog in the journal Health Affairs to explain her passion in improving maternal health.
Her former classmate, Shalon Irving, who went on to become a CDC epidemiologist, died in February 2017 at age 36, just 3 weeks after giving birth, when she developed complications from high blood pressure.
In the blog, Ms. Underwood cited statistics and provides details of the Black Maternal Health Momnibus Act of 2021, then ends the blog, published in 2020, with an update on how Ms. Irving’s then 3-year-old daughter, raised by her grandmother, is doing. While Soleil is “curious, joyful, and brilliant,” the grandmother told Ms. Underwood that she has also walked into a room and found the little girl clutching a framed photograph of her mother.
The child’s question is understandable and heartbreaking: She wants to know where her mommy is.
“Soleil’s question is my motivation,” Ms. Underwood wrote. “To honor Shalon, and all the women like her who we have lost, let us take the serious and urgent action that is required to save our moms.”
A version of this article first appeared on WebMD.com.
In the United States, nearly 4 million women a year prepare to give birth, looking forward to the joy to come. But for some, the dream turns tragic. And another 60,000 have pregnancy-related or childbirth-related health issues.
Causes of death vary greatly, including hemorrhage during pregnancy or during delivery, heart conditions, and mental health issues such as substance abuse and suicide after the birth.
In 2019, the U.S. maternal death rate was 20.1 per 100,000 women, according to the CDC, significantly higher than the 17.4 per 100,000 recorded in 2018. For Black women, the maternal death rate was more than double the overall – 44 per 100,000 in 2019.
“We have to address our horrendous maternal health care system and also need to address the inequities,” says Laurie Zephyrin, MD, vice president for advancing health equity for the Commonwealth Fund, a foundation supporting independent research on health care issues. “This is an issue that has needed national attention for a long time.”
“If we look overall, our maternal death rate is more than twice that of more than 10 other high-income countries,” she said.
As sobering as the problem is, recent developments have sparked hope that reversing the course is possible. Among them:
U.S. News & World Report, long known for its rankings of hospitals, issued its first ever “Best Hospitals for Maternity” rankings Dec. 7, highlighting facilities that perform well on key quality indicators. It plans to update the report annually.
At the first-ever White House Maternal Health Day of Action on Dec. 7, Vice President Kamala Harris urged a call to action to reduce maternal deaths and pregnancy-related health problems, with extension of postpartum coverage through Medicaid programs, among other actions.
A new hospital designation called ‘’Birthing Friendly” will be established by the Centers for Medicare & Medicaid Services. The label will be given to facilities that take part in a program aimed at improving maternal outcomes and that use patient safety practices.
President Joe Biden’s proposed Build Back Better plan includes maternal health provisions, including $3 billion in new maternal health funding. The money will aim to grow and diversify the workforce caring for pregnant women, coordinate care better, and step up research on maternal health, among other projects.
Ongoing efforts in Congress are aimed at fixing the wide disparities in maternal health affecting Black women. Regardless of income level or education, Black women are at a higher risk of maternal death and other health issues than are White women. A Black woman with a college education is at 60% higher risk of maternal death than a White or Hispanic woman who didn’t graduate high school, according to the Commonwealth Fund.
Best hospitals for maternity
For its rankings, U.S. News and World Report reached out to the 2,700 U.S. hospitals that offer maternity services, said Ben Harder, chief of health analysis and managing editor at U.S. News & World Report.
To be recognized, a hospital had to submit data from 2019 and meet the publication’s maternity care standards. The publication received responses from just 571 hospitals, representing about two of every five births in the country.
Of those, 237 were identified as best for maternity.
As to why the response rate was not higher, Mr. Harder cited the reporting burden and says it is understandable. Some hospitals likely did not have the staff available, especially during the pandemic, to gather the data needed to be evaluated by U.S. News & World Report.
On their other evaluations, the rankings are based on Medicare data, “so hospitals don’t have to lift a finger.” He expects more hospitals will respond for their future evaluations of maternity care.
The evaluators focused on five quality measures, making a score based on the cesarean section delivery rate among first-time mothers, early elective delivery rates, unexpected newborn complication rates, breastfeeding rates, and option for vaginal birth after C-section.
A call to action: Expand coverage
Speaking at the White House Maternal Health Day of Action, Mrs. Harris told participants: “The challenge is urgent, and it is important, and it will take all of us.”
Being pregnant and giving birth, she said, should not carry such great risks. She zeroed in on systemic inequities in the way women are treated and the dramatic impact maternal death and health issues have on the economy.
“A healthy economy requires healthy mothers and healthy babies,” Mrs. Harris said.
“Before, during, and after childbirth, women in our nation are dying at a higher rate than any other developed nation in our world,” she said, noting that research shows that Black women, Native Americans, and women in rural America more likely to suffer.
A major strategy in the call to action, according to Mrs. Harris, is encouraging states to expand postpartum coverage to pregnant women enrolled in Medicaid or the Children’s Health Insurance Program from the existing 60 days to a full year. Together, these two programs cover over 42% of births in the country, so expanding the coverage is expected to have a great impact.
The 60 days of coverage is not enough, as many deaths and complications happen more than 60 days after childbirth, Mrs. Harris said. The logistics for states to extend coverage were established by the American Rescue Plan and will become available by April 2022. Some states have already extended the postpartum coverage.
According to the Centers for Medicare and Medicaid Services, if every state did adopt an extension, as the Build Back Better Act proposes, the number of Americans getting coverage for a full year after childbirth would about double, extending the coverage for about 720,000 each year.
Congressional actions
Congress is working on the issue as well. The Black Maternal Health Momnibus Act of 2021, for instance, proposes several measures, including improving maternal nutrition, expanding affordable housing, and extending the maternal workforce to include more doulas and midwives.
“And for so many women, let’s note doulas are literally a lifeline,” Mrs. Harris said at the White House event.
Doulas are trained to offer women physical, emotional, and informational support before, during, and after childbirth. No reliable statistics are available on their numbers in the United States, but a March of Dimes report estimates that about 9,000 were included in a registration database in 2018.
Explaining and fixing the disparities
No one can explain for sure why Black women, in particular, are at higher risk of dying from pregnancy-related complications. Systemic inequity is one likely reason, Mrs. Harris said, noting there are differences in how people are treated based on who they are.
Inherent and unconscious bias in offering women treatment plays a role, experts say. Training could reverse or reduce that bias. Some women of color also may have less access to care, as do women in some rural areas.
According to Mrs. Harris, more than 20 companies and nonprofits have pledged to invest more than $20 million in maternal health efforts in the United States and more than $150 million globally. Among the proposed programs: remote-care monitors in rural areas, better care models for the postpartum period, and improved education programs for maternal health providers.
When statistics hit home
Many who work to improve maternal health have gone through issues themselves or had loved ones who did.
Jill Arnold, founder of the Maternal Safety Foundation in Bentonville, Ark., became a consumer advocate after giving birth to her two daughters, now teenagers. With the first birth, Ms. Arnold said she was intensely pressured at the last minute to have a C-section. She held out, resisted, and delivered a healthy baby vaginally.
For her second childbirth, she chose an accredited birth center that allowed her to have a doula and a midwife.
“The care I received was night and day,” she said. “The overwhelming pressure to consent to a C-section wasn’t there.”
She welcomes the information provided by the new U.S. News & World Report rankings as well as the upcoming “Birthing Friendly” designations.
“The onus shouldn’t be on patients, on individuals, on pregnant people to do the research,” Ms. Arnold said.
Rather, women and their partners need information at their fingertips so they can make an informed decision about how to give birth and where.
U.S. Rep. Lauren Underwood (D-Ill.), who cofounded the Black Maternal Health Caucus in April 2019, with Rep. Alma Adams (D-N.C.), wrote a touching blog in the journal Health Affairs to explain her passion in improving maternal health.
Her former classmate, Shalon Irving, who went on to become a CDC epidemiologist, died in February 2017 at age 36, just 3 weeks after giving birth, when she developed complications from high blood pressure.
In the blog, Ms. Underwood cited statistics and provides details of the Black Maternal Health Momnibus Act of 2021, then ends the blog, published in 2020, with an update on how Ms. Irving’s then 3-year-old daughter, raised by her grandmother, is doing. While Soleil is “curious, joyful, and brilliant,” the grandmother told Ms. Underwood that she has also walked into a room and found the little girl clutching a framed photograph of her mother.
The child’s question is understandable and heartbreaking: She wants to know where her mommy is.
“Soleil’s question is my motivation,” Ms. Underwood wrote. “To honor Shalon, and all the women like her who we have lost, let us take the serious and urgent action that is required to save our moms.”
A version of this article first appeared on WebMD.com.
In the United States, nearly 4 million women a year prepare to give birth, looking forward to the joy to come. But for some, the dream turns tragic. And another 60,000 have pregnancy-related or childbirth-related health issues.
Causes of death vary greatly, including hemorrhage during pregnancy or during delivery, heart conditions, and mental health issues such as substance abuse and suicide after the birth.
In 2019, the U.S. maternal death rate was 20.1 per 100,000 women, according to the CDC, significantly higher than the 17.4 per 100,000 recorded in 2018. For Black women, the maternal death rate was more than double the overall – 44 per 100,000 in 2019.
“We have to address our horrendous maternal health care system and also need to address the inequities,” says Laurie Zephyrin, MD, vice president for advancing health equity for the Commonwealth Fund, a foundation supporting independent research on health care issues. “This is an issue that has needed national attention for a long time.”
“If we look overall, our maternal death rate is more than twice that of more than 10 other high-income countries,” she said.
As sobering as the problem is, recent developments have sparked hope that reversing the course is possible. Among them:
U.S. News & World Report, long known for its rankings of hospitals, issued its first ever “Best Hospitals for Maternity” rankings Dec. 7, highlighting facilities that perform well on key quality indicators. It plans to update the report annually.
At the first-ever White House Maternal Health Day of Action on Dec. 7, Vice President Kamala Harris urged a call to action to reduce maternal deaths and pregnancy-related health problems, with extension of postpartum coverage through Medicaid programs, among other actions.
A new hospital designation called ‘’Birthing Friendly” will be established by the Centers for Medicare & Medicaid Services. The label will be given to facilities that take part in a program aimed at improving maternal outcomes and that use patient safety practices.
President Joe Biden’s proposed Build Back Better plan includes maternal health provisions, including $3 billion in new maternal health funding. The money will aim to grow and diversify the workforce caring for pregnant women, coordinate care better, and step up research on maternal health, among other projects.
Ongoing efforts in Congress are aimed at fixing the wide disparities in maternal health affecting Black women. Regardless of income level or education, Black women are at a higher risk of maternal death and other health issues than are White women. A Black woman with a college education is at 60% higher risk of maternal death than a White or Hispanic woman who didn’t graduate high school, according to the Commonwealth Fund.
Best hospitals for maternity
For its rankings, U.S. News and World Report reached out to the 2,700 U.S. hospitals that offer maternity services, said Ben Harder, chief of health analysis and managing editor at U.S. News & World Report.
To be recognized, a hospital had to submit data from 2019 and meet the publication’s maternity care standards. The publication received responses from just 571 hospitals, representing about two of every five births in the country.
Of those, 237 were identified as best for maternity.
As to why the response rate was not higher, Mr. Harder cited the reporting burden and says it is understandable. Some hospitals likely did not have the staff available, especially during the pandemic, to gather the data needed to be evaluated by U.S. News & World Report.
On their other evaluations, the rankings are based on Medicare data, “so hospitals don’t have to lift a finger.” He expects more hospitals will respond for their future evaluations of maternity care.
The evaluators focused on five quality measures, making a score based on the cesarean section delivery rate among first-time mothers, early elective delivery rates, unexpected newborn complication rates, breastfeeding rates, and option for vaginal birth after C-section.
A call to action: Expand coverage
Speaking at the White House Maternal Health Day of Action, Mrs. Harris told participants: “The challenge is urgent, and it is important, and it will take all of us.”
Being pregnant and giving birth, she said, should not carry such great risks. She zeroed in on systemic inequities in the way women are treated and the dramatic impact maternal death and health issues have on the economy.
“A healthy economy requires healthy mothers and healthy babies,” Mrs. Harris said.
“Before, during, and after childbirth, women in our nation are dying at a higher rate than any other developed nation in our world,” she said, noting that research shows that Black women, Native Americans, and women in rural America more likely to suffer.
A major strategy in the call to action, according to Mrs. Harris, is encouraging states to expand postpartum coverage to pregnant women enrolled in Medicaid or the Children’s Health Insurance Program from the existing 60 days to a full year. Together, these two programs cover over 42% of births in the country, so expanding the coverage is expected to have a great impact.
The 60 days of coverage is not enough, as many deaths and complications happen more than 60 days after childbirth, Mrs. Harris said. The logistics for states to extend coverage were established by the American Rescue Plan and will become available by April 2022. Some states have already extended the postpartum coverage.
According to the Centers for Medicare and Medicaid Services, if every state did adopt an extension, as the Build Back Better Act proposes, the number of Americans getting coverage for a full year after childbirth would about double, extending the coverage for about 720,000 each year.
Congressional actions
Congress is working on the issue as well. The Black Maternal Health Momnibus Act of 2021, for instance, proposes several measures, including improving maternal nutrition, expanding affordable housing, and extending the maternal workforce to include more doulas and midwives.
“And for so many women, let’s note doulas are literally a lifeline,” Mrs. Harris said at the White House event.
Doulas are trained to offer women physical, emotional, and informational support before, during, and after childbirth. No reliable statistics are available on their numbers in the United States, but a March of Dimes report estimates that about 9,000 were included in a registration database in 2018.
Explaining and fixing the disparities
No one can explain for sure why Black women, in particular, are at higher risk of dying from pregnancy-related complications. Systemic inequity is one likely reason, Mrs. Harris said, noting there are differences in how people are treated based on who they are.
Inherent and unconscious bias in offering women treatment plays a role, experts say. Training could reverse or reduce that bias. Some women of color also may have less access to care, as do women in some rural areas.
According to Mrs. Harris, more than 20 companies and nonprofits have pledged to invest more than $20 million in maternal health efforts in the United States and more than $150 million globally. Among the proposed programs: remote-care monitors in rural areas, better care models for the postpartum period, and improved education programs for maternal health providers.
When statistics hit home
Many who work to improve maternal health have gone through issues themselves or had loved ones who did.
Jill Arnold, founder of the Maternal Safety Foundation in Bentonville, Ark., became a consumer advocate after giving birth to her two daughters, now teenagers. With the first birth, Ms. Arnold said she was intensely pressured at the last minute to have a C-section. She held out, resisted, and delivered a healthy baby vaginally.
For her second childbirth, she chose an accredited birth center that allowed her to have a doula and a midwife.
“The care I received was night and day,” she said. “The overwhelming pressure to consent to a C-section wasn’t there.”
She welcomes the information provided by the new U.S. News & World Report rankings as well as the upcoming “Birthing Friendly” designations.
“The onus shouldn’t be on patients, on individuals, on pregnant people to do the research,” Ms. Arnold said.
Rather, women and their partners need information at their fingertips so they can make an informed decision about how to give birth and where.
U.S. Rep. Lauren Underwood (D-Ill.), who cofounded the Black Maternal Health Caucus in April 2019, with Rep. Alma Adams (D-N.C.), wrote a touching blog in the journal Health Affairs to explain her passion in improving maternal health.
Her former classmate, Shalon Irving, who went on to become a CDC epidemiologist, died in February 2017 at age 36, just 3 weeks after giving birth, when she developed complications from high blood pressure.
In the blog, Ms. Underwood cited statistics and provides details of the Black Maternal Health Momnibus Act of 2021, then ends the blog, published in 2020, with an update on how Ms. Irving’s then 3-year-old daughter, raised by her grandmother, is doing. While Soleil is “curious, joyful, and brilliant,” the grandmother told Ms. Underwood that she has also walked into a room and found the little girl clutching a framed photograph of her mother.
The child’s question is understandable and heartbreaking: She wants to know where her mommy is.
“Soleil’s question is my motivation,” Ms. Underwood wrote. “To honor Shalon, and all the women like her who we have lost, let us take the serious and urgent action that is required to save our moms.”
A version of this article first appeared on WebMD.com.
Spice in breast milk could shape taste preferences later
They say you are what you eat, but scientists have long wondered whether breastfeeding babies are what their mothers eat, too. Their question: How much of a nursing mother’s diet eventually plays a role in a child’s food preferences later in life?
The aroma, taste, and makeup of breast milk change from day to day, based mostly on the mother’s diet. But previous research has already shown that the foods a mother eats do not directly translate into the same smells and tastes of that food in breast milk. Some substances from the mother’s diet enter her breast milk, some don’t, and even ones that do may have a different scent or flavor than what the mother experiences.
But a new study suggests that the active ingredient in black pepper makes its way into breast milk and may help the infant develop a tolerance to pepper later. The researchers published their findings in the journal Molecular Nutrition & Food Research.
Pinch of pepper
The study authors thought that maybe some food preferences could result from sensory programming that occurs through breast milk in the first few months of life. Though past studies have looked at which odor-producing substances transfer into breast milk, not many have explored specific substances that give food its distinctive flavor, or even what makes up the taste of breast milk. So they decided to investigate what happens when a mother consumes a meal containing three specific compounds: those that give pepper, chili, and ginger their particularly pungent flavors.
The researchers recruited 18 healthy, nonsmoking, nursing mothers who were producing more than enough milk for their baby’s needs. Their breastfeeding children ranged in age from 8 weeks to 1 year old. The women all ate a curry dish after having spent 2 days avoiding onion, garlic, and the spices in the curry. Then they provided pumped breast milk samples at 1, 2, and 3 hours after eating the curry.
Within an hour of the women eating the curry, the scientists were able to detect piperine, the compound that gives black pepper its bite, in the mothers’ breast milk. They did not find the compounds from ginger, chili, or curcumin – the main active ingredient in turmeric – in the breast milk. The piperine remained there for several hours, but there wasn’t enough for an adult to be able to taste it. It wasn’t possible to reliably tell whether the infants could consciously detect the flavor, but the researchers don’t think it’s likely they did.
But the scientists do suggest it’s possible that the piperine in breast milk could regularly activate a protein that detects pungent or potentially harmful substances. This is the same protein that produces the sensation of heat when eating a spicy food. If the piperine frequently activates that protein in a nursing baby at levels too low for the baby to notice, it may increase the baby’s tolerance for similar spicy substances later in life.
Ultimately, the findings suggest that .
A version of this story first appeared on WebMD.com.
They say you are what you eat, but scientists have long wondered whether breastfeeding babies are what their mothers eat, too. Their question: How much of a nursing mother’s diet eventually plays a role in a child’s food preferences later in life?
The aroma, taste, and makeup of breast milk change from day to day, based mostly on the mother’s diet. But previous research has already shown that the foods a mother eats do not directly translate into the same smells and tastes of that food in breast milk. Some substances from the mother’s diet enter her breast milk, some don’t, and even ones that do may have a different scent or flavor than what the mother experiences.
But a new study suggests that the active ingredient in black pepper makes its way into breast milk and may help the infant develop a tolerance to pepper later. The researchers published their findings in the journal Molecular Nutrition & Food Research.
Pinch of pepper
The study authors thought that maybe some food preferences could result from sensory programming that occurs through breast milk in the first few months of life. Though past studies have looked at which odor-producing substances transfer into breast milk, not many have explored specific substances that give food its distinctive flavor, or even what makes up the taste of breast milk. So they decided to investigate what happens when a mother consumes a meal containing three specific compounds: those that give pepper, chili, and ginger their particularly pungent flavors.
The researchers recruited 18 healthy, nonsmoking, nursing mothers who were producing more than enough milk for their baby’s needs. Their breastfeeding children ranged in age from 8 weeks to 1 year old. The women all ate a curry dish after having spent 2 days avoiding onion, garlic, and the spices in the curry. Then they provided pumped breast milk samples at 1, 2, and 3 hours after eating the curry.
Within an hour of the women eating the curry, the scientists were able to detect piperine, the compound that gives black pepper its bite, in the mothers’ breast milk. They did not find the compounds from ginger, chili, or curcumin – the main active ingredient in turmeric – in the breast milk. The piperine remained there for several hours, but there wasn’t enough for an adult to be able to taste it. It wasn’t possible to reliably tell whether the infants could consciously detect the flavor, but the researchers don’t think it’s likely they did.
But the scientists do suggest it’s possible that the piperine in breast milk could regularly activate a protein that detects pungent or potentially harmful substances. This is the same protein that produces the sensation of heat when eating a spicy food. If the piperine frequently activates that protein in a nursing baby at levels too low for the baby to notice, it may increase the baby’s tolerance for similar spicy substances later in life.
Ultimately, the findings suggest that .
A version of this story first appeared on WebMD.com.
They say you are what you eat, but scientists have long wondered whether breastfeeding babies are what their mothers eat, too. Their question: How much of a nursing mother’s diet eventually plays a role in a child’s food preferences later in life?
The aroma, taste, and makeup of breast milk change from day to day, based mostly on the mother’s diet. But previous research has already shown that the foods a mother eats do not directly translate into the same smells and tastes of that food in breast milk. Some substances from the mother’s diet enter her breast milk, some don’t, and even ones that do may have a different scent or flavor than what the mother experiences.
But a new study suggests that the active ingredient in black pepper makes its way into breast milk and may help the infant develop a tolerance to pepper later. The researchers published their findings in the journal Molecular Nutrition & Food Research.
Pinch of pepper
The study authors thought that maybe some food preferences could result from sensory programming that occurs through breast milk in the first few months of life. Though past studies have looked at which odor-producing substances transfer into breast milk, not many have explored specific substances that give food its distinctive flavor, or even what makes up the taste of breast milk. So they decided to investigate what happens when a mother consumes a meal containing three specific compounds: those that give pepper, chili, and ginger their particularly pungent flavors.
The researchers recruited 18 healthy, nonsmoking, nursing mothers who were producing more than enough milk for their baby’s needs. Their breastfeeding children ranged in age from 8 weeks to 1 year old. The women all ate a curry dish after having spent 2 days avoiding onion, garlic, and the spices in the curry. Then they provided pumped breast milk samples at 1, 2, and 3 hours after eating the curry.
Within an hour of the women eating the curry, the scientists were able to detect piperine, the compound that gives black pepper its bite, in the mothers’ breast milk. They did not find the compounds from ginger, chili, or curcumin – the main active ingredient in turmeric – in the breast milk. The piperine remained there for several hours, but there wasn’t enough for an adult to be able to taste it. It wasn’t possible to reliably tell whether the infants could consciously detect the flavor, but the researchers don’t think it’s likely they did.
But the scientists do suggest it’s possible that the piperine in breast milk could regularly activate a protein that detects pungent or potentially harmful substances. This is the same protein that produces the sensation of heat when eating a spicy food. If the piperine frequently activates that protein in a nursing baby at levels too low for the baby to notice, it may increase the baby’s tolerance for similar spicy substances later in life.
Ultimately, the findings suggest that .
A version of this story first appeared on WebMD.com.
FROM MOLECULAR NUTRITION & FOOD RESEARCH
Booster recommendations for pregnant women, teens, and other groups explained
These recommendations have been widened because of the continued emergence of new variants of the virus and the wane of protection over time for both vaccinations and previous disease.
The new recommendations take away some of the questions surrounding eligibility for booster vaccinations while potentially leaving some additional questions. All in all, they provide flexibility for individuals to help protect themselves against the COVID-19 virus, as many are considering celebrating the holidays with friends and family.
The first item that has become clear is that all individuals over 18 are now not only eligible for a booster vaccination a certain time after they have completed their series, but have a recommendation for one.1
But what about a fourth dose? There is a possibility that some patients should be receiving one. For those who require a three-dose series due to a condition that makes them immunocompromised, they should receive their booster vaccination six months after completion of the three-dose series. This distinction may cause confusion for some, but is important for those immunocompromised.
Boosters in women who are pregnant
The recommendations also include specific comments about individuals who are pregnant. Although initial studies did not include pregnant individuals, there has been increasing real world data that vaccination against COVID, including booster vaccinations, is safe and recommended. As pregnancy increases the risk of severe disease if infected by COVID-19, both the CDC and the American College of Obstetricians and Gynecologists,2 along with other specialty organizations, such as the Royal College of Obstetricians and Gynaecologists, recommend vaccinations for pregnant individuals.
The CDC goes on to describe that there is no evidence of vaccination increasing the risk of infertility. The vaccine protects the pregnant individual and also provides protection to the baby once born. The same is true of breastfeeding individuals.3
I hope that this information allows physicians to feel comfortable recommending vaccinations and boosters to those who are pregnant and breast feeding.
Expanded recommendations for those aged 16-17 years
Recently, the CDC also expanded booster recommendations to include those aged 16-17 years, 6 months after completing their vaccine series.
Those under 18 are currently only able to receive the Pfizer-BioNtech vaccine. This new guidance has left some parents wondering if there will also be approval for booster vaccinations soon for those aged 12-16 who are approaching or have reached six months past the initial vaccine.1
Booster brand for those over 18 years?
Although the recommendation has been simplified for all over age 18 years, there is still a decision to be made about which vaccine to use as the booster.
The recommendations allow individuals to decide which brand of vaccine they would like to have as a booster. They may choose to be vaccinated with the same vaccine they originally received or with a different vaccine. This vaccine flexibility may cause confusion, but ultimately is a good thing as it allows individuals to receive whatever vaccine is available and most convenient. This also allows individuals who have been vaccinated outside of the United States by a different brand of vaccine to also receive a booster vaccination with one of the options available here.
Take home message
Overall, the expansion of booster recommendations will help everyone avoid severe disease from COVID-19 infections. Physicians now have more clarity on who should be receiving these vaccines. Along with testing, masking, and appropriate distancing, these recommendations should help prevent severe disease and death from COVID-19.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program, also in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. 2021 Dec 9.
2. COVID-19 Vaccines and Pregnancy: Conversation Guide. American College of Obstetricians and Gynecologists. 2021 November.
3. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. 2021 Dec 6.
These recommendations have been widened because of the continued emergence of new variants of the virus and the wane of protection over time for both vaccinations and previous disease.
The new recommendations take away some of the questions surrounding eligibility for booster vaccinations while potentially leaving some additional questions. All in all, they provide flexibility for individuals to help protect themselves against the COVID-19 virus, as many are considering celebrating the holidays with friends and family.
The first item that has become clear is that all individuals over 18 are now not only eligible for a booster vaccination a certain time after they have completed their series, but have a recommendation for one.1
But what about a fourth dose? There is a possibility that some patients should be receiving one. For those who require a three-dose series due to a condition that makes them immunocompromised, they should receive their booster vaccination six months after completion of the three-dose series. This distinction may cause confusion for some, but is important for those immunocompromised.
Boosters in women who are pregnant
The recommendations also include specific comments about individuals who are pregnant. Although initial studies did not include pregnant individuals, there has been increasing real world data that vaccination against COVID, including booster vaccinations, is safe and recommended. As pregnancy increases the risk of severe disease if infected by COVID-19, both the CDC and the American College of Obstetricians and Gynecologists,2 along with other specialty organizations, such as the Royal College of Obstetricians and Gynaecologists, recommend vaccinations for pregnant individuals.
The CDC goes on to describe that there is no evidence of vaccination increasing the risk of infertility. The vaccine protects the pregnant individual and also provides protection to the baby once born. The same is true of breastfeeding individuals.3
I hope that this information allows physicians to feel comfortable recommending vaccinations and boosters to those who are pregnant and breast feeding.
Expanded recommendations for those aged 16-17 years
Recently, the CDC also expanded booster recommendations to include those aged 16-17 years, 6 months after completing their vaccine series.
Those under 18 are currently only able to receive the Pfizer-BioNtech vaccine. This new guidance has left some parents wondering if there will also be approval for booster vaccinations soon for those aged 12-16 who are approaching or have reached six months past the initial vaccine.1
Booster brand for those over 18 years?
Although the recommendation has been simplified for all over age 18 years, there is still a decision to be made about which vaccine to use as the booster.
The recommendations allow individuals to decide which brand of vaccine they would like to have as a booster. They may choose to be vaccinated with the same vaccine they originally received or with a different vaccine. This vaccine flexibility may cause confusion, but ultimately is a good thing as it allows individuals to receive whatever vaccine is available and most convenient. This also allows individuals who have been vaccinated outside of the United States by a different brand of vaccine to also receive a booster vaccination with one of the options available here.
Take home message
Overall, the expansion of booster recommendations will help everyone avoid severe disease from COVID-19 infections. Physicians now have more clarity on who should be receiving these vaccines. Along with testing, masking, and appropriate distancing, these recommendations should help prevent severe disease and death from COVID-19.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program, also in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. 2021 Dec 9.
2. COVID-19 Vaccines and Pregnancy: Conversation Guide. American College of Obstetricians and Gynecologists. 2021 November.
3. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. 2021 Dec 6.
These recommendations have been widened because of the continued emergence of new variants of the virus and the wane of protection over time for both vaccinations and previous disease.
The new recommendations take away some of the questions surrounding eligibility for booster vaccinations while potentially leaving some additional questions. All in all, they provide flexibility for individuals to help protect themselves against the COVID-19 virus, as many are considering celebrating the holidays with friends and family.
The first item that has become clear is that all individuals over 18 are now not only eligible for a booster vaccination a certain time after they have completed their series, but have a recommendation for one.1
But what about a fourth dose? There is a possibility that some patients should be receiving one. For those who require a three-dose series due to a condition that makes them immunocompromised, they should receive their booster vaccination six months after completion of the three-dose series. This distinction may cause confusion for some, but is important for those immunocompromised.
Boosters in women who are pregnant
The recommendations also include specific comments about individuals who are pregnant. Although initial studies did not include pregnant individuals, there has been increasing real world data that vaccination against COVID, including booster vaccinations, is safe and recommended. As pregnancy increases the risk of severe disease if infected by COVID-19, both the CDC and the American College of Obstetricians and Gynecologists,2 along with other specialty organizations, such as the Royal College of Obstetricians and Gynaecologists, recommend vaccinations for pregnant individuals.
The CDC goes on to describe that there is no evidence of vaccination increasing the risk of infertility. The vaccine protects the pregnant individual and also provides protection to the baby once born. The same is true of breastfeeding individuals.3
I hope that this information allows physicians to feel comfortable recommending vaccinations and boosters to those who are pregnant and breast feeding.
Expanded recommendations for those aged 16-17 years
Recently, the CDC also expanded booster recommendations to include those aged 16-17 years, 6 months after completing their vaccine series.
Those under 18 are currently only able to receive the Pfizer-BioNtech vaccine. This new guidance has left some parents wondering if there will also be approval for booster vaccinations soon for those aged 12-16 who are approaching or have reached six months past the initial vaccine.1
Booster brand for those over 18 years?
Although the recommendation has been simplified for all over age 18 years, there is still a decision to be made about which vaccine to use as the booster.
The recommendations allow individuals to decide which brand of vaccine they would like to have as a booster. They may choose to be vaccinated with the same vaccine they originally received or with a different vaccine. This vaccine flexibility may cause confusion, but ultimately is a good thing as it allows individuals to receive whatever vaccine is available and most convenient. This also allows individuals who have been vaccinated outside of the United States by a different brand of vaccine to also receive a booster vaccination with one of the options available here.
Take home message
Overall, the expansion of booster recommendations will help everyone avoid severe disease from COVID-19 infections. Physicians now have more clarity on who should be receiving these vaccines. Along with testing, masking, and appropriate distancing, these recommendations should help prevent severe disease and death from COVID-19.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program, also in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. 2021 Dec 9.
2. COVID-19 Vaccines and Pregnancy: Conversation Guide. American College of Obstetricians and Gynecologists. 2021 November.
3. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. 2021 Dec 6.
Supreme Court leaves Texas abortion law in place
In a highly anticipated decision, the U.S. Supreme Court ruled Dec. 10 that the controversial Texas abortion law that restricts the procedure to women pregnant for 6 weeks or less may continue to be enforced, but allowed for state and federal courts to hear challenges to whether it violates the Constitution.
As anti-abortion organizations celebrate and abortion rights groups confer on what the decision could mean for women not only in Texas but across the United States, there is another, bigger implication as well.
The Texas law generated a lot of controversy, in part, because it took an unusual approach. In authorizing essentially anyone across the nation to file a lawsuit against a woman in the lone star state who seeks the procedure outside the law, or anyone who assists her -- including healthcare professionals, it opens up the potential for similar legal challenges to other Supreme Court rulings on marriage, guns, and other rights.
The vote was 5-4, with Chief Justice John Roberts joining the liberal members of the court in dissenting.
The ruling allows abortion rights supporters to sue in state court, where a Texas judge on Dec. 9 ruled the law unconstitutional. He stopped short, however, of issuing an injunction against. Abortion rights opponents have vowed to appeal District Judge David Peeples’ ruling.
A timeline on the case
The law took effect on Sept. 1, 2021. The day before, the Supreme Court did not act to put a hold on the law as requested by abortion rights organizations. As a result, many Texas women seeking the procedure after 6 weeks traveled to nearby states. On Oct. 25, the Court agreed to hear a challenge to the law by the Biden Administration.
The Dec. 10 Supreme Court decision to uphold the Texas law contrasts with a general consensus among many legal observers that the justices were receptive to blocking the law, based on questions and issues the judges raised during oral arguments on Nov. 1, 2021.
A separate legal challenge to abortion rights involves a Mississippi law banning the procedure starting at 15 weeks of pregnancy. The Supreme Court justices scheduled oral arguments in that case for Dec. 1, and are expected to issue a ruling in that case in June 2022.
A version of this article first appeared on WebMD.com .
In a highly anticipated decision, the U.S. Supreme Court ruled Dec. 10 that the controversial Texas abortion law that restricts the procedure to women pregnant for 6 weeks or less may continue to be enforced, but allowed for state and federal courts to hear challenges to whether it violates the Constitution.
As anti-abortion organizations celebrate and abortion rights groups confer on what the decision could mean for women not only in Texas but across the United States, there is another, bigger implication as well.
The Texas law generated a lot of controversy, in part, because it took an unusual approach. In authorizing essentially anyone across the nation to file a lawsuit against a woman in the lone star state who seeks the procedure outside the law, or anyone who assists her -- including healthcare professionals, it opens up the potential for similar legal challenges to other Supreme Court rulings on marriage, guns, and other rights.
The vote was 5-4, with Chief Justice John Roberts joining the liberal members of the court in dissenting.
The ruling allows abortion rights supporters to sue in state court, where a Texas judge on Dec. 9 ruled the law unconstitutional. He stopped short, however, of issuing an injunction against. Abortion rights opponents have vowed to appeal District Judge David Peeples’ ruling.
A timeline on the case
The law took effect on Sept. 1, 2021. The day before, the Supreme Court did not act to put a hold on the law as requested by abortion rights organizations. As a result, many Texas women seeking the procedure after 6 weeks traveled to nearby states. On Oct. 25, the Court agreed to hear a challenge to the law by the Biden Administration.
The Dec. 10 Supreme Court decision to uphold the Texas law contrasts with a general consensus among many legal observers that the justices were receptive to blocking the law, based on questions and issues the judges raised during oral arguments on Nov. 1, 2021.
A separate legal challenge to abortion rights involves a Mississippi law banning the procedure starting at 15 weeks of pregnancy. The Supreme Court justices scheduled oral arguments in that case for Dec. 1, and are expected to issue a ruling in that case in June 2022.
A version of this article first appeared on WebMD.com .
In a highly anticipated decision, the U.S. Supreme Court ruled Dec. 10 that the controversial Texas abortion law that restricts the procedure to women pregnant for 6 weeks or less may continue to be enforced, but allowed for state and federal courts to hear challenges to whether it violates the Constitution.
As anti-abortion organizations celebrate and abortion rights groups confer on what the decision could mean for women not only in Texas but across the United States, there is another, bigger implication as well.
The Texas law generated a lot of controversy, in part, because it took an unusual approach. In authorizing essentially anyone across the nation to file a lawsuit against a woman in the lone star state who seeks the procedure outside the law, or anyone who assists her -- including healthcare professionals, it opens up the potential for similar legal challenges to other Supreme Court rulings on marriage, guns, and other rights.
The vote was 5-4, with Chief Justice John Roberts joining the liberal members of the court in dissenting.
The ruling allows abortion rights supporters to sue in state court, where a Texas judge on Dec. 9 ruled the law unconstitutional. He stopped short, however, of issuing an injunction against. Abortion rights opponents have vowed to appeal District Judge David Peeples’ ruling.
A timeline on the case
The law took effect on Sept. 1, 2021. The day before, the Supreme Court did not act to put a hold on the law as requested by abortion rights organizations. As a result, many Texas women seeking the procedure after 6 weeks traveled to nearby states. On Oct. 25, the Court agreed to hear a challenge to the law by the Biden Administration.
The Dec. 10 Supreme Court decision to uphold the Texas law contrasts with a general consensus among many legal observers that the justices were receptive to blocking the law, based on questions and issues the judges raised during oral arguments on Nov. 1, 2021.
A separate legal challenge to abortion rights involves a Mississippi law banning the procedure starting at 15 weeks of pregnancy. The Supreme Court justices scheduled oral arguments in that case for Dec. 1, and are expected to issue a ruling in that case in June 2022.
A version of this article first appeared on WebMD.com .
Should ‘advanced maternal age’ be redefined? Study suggests benefits.
Pregnant women who were at or above the advanced maternal age (AMA) cutoff of 35 years on their due date received significantly more prenatal care, resulting in a slight decline in perinatal mortality, compared with women who were just a few months younger, according to a new study published in JAMA Health Forum. The findings “suggest that clinicians use the cutoff as a heuristic in their clinical recommendations and service provision,” noted lead author Caroline K. Geiger, PhD, who was a PhD student at Harvard University in Cambridge, Mass., during the course of the study, and now works as an associate health economist at Genentech in San Francisco. She and her coauthors suggest a slightly younger AMA cutoff might be beneficial. “Our results suggest that 3.9 perinatal deaths per 1,000 deliveries in this age range could be averted if patients just a few months younger than the AMA cutoff received similar care to those older than the cutoff,” they wrote. “Although the risk of adverse outcomes increases with maternal age, individuals 4 months older or younger than 35 years should not have different underlying risks.”
The cross-sectional study used a national sample of 51,290 commercially insured individuals who were pregnant between 2008 and 2019 and had delivery dates within 120 days of their 35th birthday. Just over half (50.9%) of the individuals were aged 34.7-34.9 years on their expected delivery date – just below the AMA cutoff – while 49.1% were just over the cutoff at age 35.0-35.3 years. A total of 4.7% had multiple gestation, 4.8% had pregestational diabetes, 4.4% had chronic hypertension, and 9.7% had obesity. There was also a subgroup analysis among individuals with low-risk pregnancy (defined as singleton, with no pregestational diabetes, chronic hypertension, or obesity) because they were less likely to have indications for additional prenatal care.
Although there was a slight, nonstatistically significant increase in the overall number of ob.gyn. visits at the AMA cutoff, compared with below it, the percentage of individuals with any maternal-fetal medicine visit increased by 4.27 percentage points (P < .001) at the cutoff. Additionally, while there was a “modest” increase in total ultrasounds (P = .006), there was a significant increase in detailed ultrasounds (P < .001) at the cutoff, and a “substantial” increase in antepartum surveillance (P < .001), the authors reported.
The AMA designation was associated with a 0.39 percentage-point decline in perinatal mortality (P = .04), “however, there were no significant changes in the proportion of individuals with severe maternal morbidity or with preterm birth or low birth weight at age 35 years,” they wrote.
In the subgroup analysis of low-risk pregnancies, “prenatal care services increased substantially at the 35-year cutoff, and in all cases, the increases at age 35 years for this group were larger than for the full sample,” they noted, adding that there was also a “substantially larger” decline in perinatal mortality at the AMA cutoff (P = .002), compared with the full sample.
The authors noted the need for more rigorous evidence on the value and effect of prenatal care guidelines on pregnancy outcomes. “Although pregnancy-related risks increase with maternal age, there is no known abrupt biological increase in underlying risk precisely at age 35 years,” they wrote, adding that “much of the content of prenatal care guidelines has persisted for decades without strong causal evidence to demonstrate its value.”
Their words echo those of Alex F. Peahl, MD, an ob.gyn. and assistant professor at the Institute for Healthcare Policy and Innovation, at the University of Michigan, in Ann Arbor, MI. In a recent review, Dr. Peahl and her colleague Joel D. Howell, MD, PhD, from the same university (Am J Obstet Gynecol. 2021 Apr;224[4]:339-47), note that the COVID-19 pandemic forced a much-needed rethink of prenatal care and its delivery. A look through the history of prenatal care shows “we have treated visit frequency and modality as fixed boxes, into which we must fit an ever-changing set of care recommendations,” they wrote. “We do not have data to support a specific prenatal visit schedule, recommended number of telemedicine visits, or specifications of additional services, and we never have. However, one thing is clear: we are long overdue for new prenatal care delivery guidelines in the United States.”
But when reached for comment on the new study Dr. Peahl cautioned that its conclusions are “limited and warrant future investigation. … While increased prenatal services may explain the improvement in outcomes, several other explanations should be considered,” she told this publication. “Perhaps, maternity care professional behavior differs for patients who are over the age of 35, resulting in increased caution in interpreting test results and symptoms; perhaps patients are more routinely induced at 39 weeks, limiting stillbirth rate; or perhaps patients are more hypervigilant when given the diagnosis of AMA.”
Priya Rajan, MD, agreed that while the paper showed an association between intensified antenatal interventions and decreased perinatal mortality, it did not show a causal relationship. “The study did not include information on other important factors that are also associated with perinatal risk,” noted Dr. Rajan, who is an associate professor in the department of ob.gyn. at Northwestern University in Chicago. Yet, she acknowledged that the findings “support what many clinicians know, which is that age 35 isn’t some tipping point; rather, obstetric risk is influenced by a range of factors, of which age may be one. This study, particularly when considered in the context of other studies and articles we have seen recently, confirms the need for us to rethink how we care for people during pregnancy and post partum. This includes delving further into understanding what aspects of the prenatal care that we provide have the biggest impact for both maternal and perinatal adverse outcomes.”
The study was supported by grant DGE1745303 from the National Science Foundation Graduate Research Fellowship Program. Dr. Geiger reported being a PhD student during the conduction of the study, but had no other disclosures. Dr. Peahl will soon be a consultant for Maven Clinic. Dr. Rajan had no relevant disclosures.
Pregnant women who were at or above the advanced maternal age (AMA) cutoff of 35 years on their due date received significantly more prenatal care, resulting in a slight decline in perinatal mortality, compared with women who were just a few months younger, according to a new study published in JAMA Health Forum. The findings “suggest that clinicians use the cutoff as a heuristic in their clinical recommendations and service provision,” noted lead author Caroline K. Geiger, PhD, who was a PhD student at Harvard University in Cambridge, Mass., during the course of the study, and now works as an associate health economist at Genentech in San Francisco. She and her coauthors suggest a slightly younger AMA cutoff might be beneficial. “Our results suggest that 3.9 perinatal deaths per 1,000 deliveries in this age range could be averted if patients just a few months younger than the AMA cutoff received similar care to those older than the cutoff,” they wrote. “Although the risk of adverse outcomes increases with maternal age, individuals 4 months older or younger than 35 years should not have different underlying risks.”
The cross-sectional study used a national sample of 51,290 commercially insured individuals who were pregnant between 2008 and 2019 and had delivery dates within 120 days of their 35th birthday. Just over half (50.9%) of the individuals were aged 34.7-34.9 years on their expected delivery date – just below the AMA cutoff – while 49.1% were just over the cutoff at age 35.0-35.3 years. A total of 4.7% had multiple gestation, 4.8% had pregestational diabetes, 4.4% had chronic hypertension, and 9.7% had obesity. There was also a subgroup analysis among individuals with low-risk pregnancy (defined as singleton, with no pregestational diabetes, chronic hypertension, or obesity) because they were less likely to have indications for additional prenatal care.
Although there was a slight, nonstatistically significant increase in the overall number of ob.gyn. visits at the AMA cutoff, compared with below it, the percentage of individuals with any maternal-fetal medicine visit increased by 4.27 percentage points (P < .001) at the cutoff. Additionally, while there was a “modest” increase in total ultrasounds (P = .006), there was a significant increase in detailed ultrasounds (P < .001) at the cutoff, and a “substantial” increase in antepartum surveillance (P < .001), the authors reported.
The AMA designation was associated with a 0.39 percentage-point decline in perinatal mortality (P = .04), “however, there were no significant changes in the proportion of individuals with severe maternal morbidity or with preterm birth or low birth weight at age 35 years,” they wrote.
In the subgroup analysis of low-risk pregnancies, “prenatal care services increased substantially at the 35-year cutoff, and in all cases, the increases at age 35 years for this group were larger than for the full sample,” they noted, adding that there was also a “substantially larger” decline in perinatal mortality at the AMA cutoff (P = .002), compared with the full sample.
The authors noted the need for more rigorous evidence on the value and effect of prenatal care guidelines on pregnancy outcomes. “Although pregnancy-related risks increase with maternal age, there is no known abrupt biological increase in underlying risk precisely at age 35 years,” they wrote, adding that “much of the content of prenatal care guidelines has persisted for decades without strong causal evidence to demonstrate its value.”
Their words echo those of Alex F. Peahl, MD, an ob.gyn. and assistant professor at the Institute for Healthcare Policy and Innovation, at the University of Michigan, in Ann Arbor, MI. In a recent review, Dr. Peahl and her colleague Joel D. Howell, MD, PhD, from the same university (Am J Obstet Gynecol. 2021 Apr;224[4]:339-47), note that the COVID-19 pandemic forced a much-needed rethink of prenatal care and its delivery. A look through the history of prenatal care shows “we have treated visit frequency and modality as fixed boxes, into which we must fit an ever-changing set of care recommendations,” they wrote. “We do not have data to support a specific prenatal visit schedule, recommended number of telemedicine visits, or specifications of additional services, and we never have. However, one thing is clear: we are long overdue for new prenatal care delivery guidelines in the United States.”
But when reached for comment on the new study Dr. Peahl cautioned that its conclusions are “limited and warrant future investigation. … While increased prenatal services may explain the improvement in outcomes, several other explanations should be considered,” she told this publication. “Perhaps, maternity care professional behavior differs for patients who are over the age of 35, resulting in increased caution in interpreting test results and symptoms; perhaps patients are more routinely induced at 39 weeks, limiting stillbirth rate; or perhaps patients are more hypervigilant when given the diagnosis of AMA.”
Priya Rajan, MD, agreed that while the paper showed an association between intensified antenatal interventions and decreased perinatal mortality, it did not show a causal relationship. “The study did not include information on other important factors that are also associated with perinatal risk,” noted Dr. Rajan, who is an associate professor in the department of ob.gyn. at Northwestern University in Chicago. Yet, she acknowledged that the findings “support what many clinicians know, which is that age 35 isn’t some tipping point; rather, obstetric risk is influenced by a range of factors, of which age may be one. This study, particularly when considered in the context of other studies and articles we have seen recently, confirms the need for us to rethink how we care for people during pregnancy and post partum. This includes delving further into understanding what aspects of the prenatal care that we provide have the biggest impact for both maternal and perinatal adverse outcomes.”
The study was supported by grant DGE1745303 from the National Science Foundation Graduate Research Fellowship Program. Dr. Geiger reported being a PhD student during the conduction of the study, but had no other disclosures. Dr. Peahl will soon be a consultant for Maven Clinic. Dr. Rajan had no relevant disclosures.
Pregnant women who were at or above the advanced maternal age (AMA) cutoff of 35 years on their due date received significantly more prenatal care, resulting in a slight decline in perinatal mortality, compared with women who were just a few months younger, according to a new study published in JAMA Health Forum. The findings “suggest that clinicians use the cutoff as a heuristic in their clinical recommendations and service provision,” noted lead author Caroline K. Geiger, PhD, who was a PhD student at Harvard University in Cambridge, Mass., during the course of the study, and now works as an associate health economist at Genentech in San Francisco. She and her coauthors suggest a slightly younger AMA cutoff might be beneficial. “Our results suggest that 3.9 perinatal deaths per 1,000 deliveries in this age range could be averted if patients just a few months younger than the AMA cutoff received similar care to those older than the cutoff,” they wrote. “Although the risk of adverse outcomes increases with maternal age, individuals 4 months older or younger than 35 years should not have different underlying risks.”
The cross-sectional study used a national sample of 51,290 commercially insured individuals who were pregnant between 2008 and 2019 and had delivery dates within 120 days of their 35th birthday. Just over half (50.9%) of the individuals were aged 34.7-34.9 years on their expected delivery date – just below the AMA cutoff – while 49.1% were just over the cutoff at age 35.0-35.3 years. A total of 4.7% had multiple gestation, 4.8% had pregestational diabetes, 4.4% had chronic hypertension, and 9.7% had obesity. There was also a subgroup analysis among individuals with low-risk pregnancy (defined as singleton, with no pregestational diabetes, chronic hypertension, or obesity) because they were less likely to have indications for additional prenatal care.
Although there was a slight, nonstatistically significant increase in the overall number of ob.gyn. visits at the AMA cutoff, compared with below it, the percentage of individuals with any maternal-fetal medicine visit increased by 4.27 percentage points (P < .001) at the cutoff. Additionally, while there was a “modest” increase in total ultrasounds (P = .006), there was a significant increase in detailed ultrasounds (P < .001) at the cutoff, and a “substantial” increase in antepartum surveillance (P < .001), the authors reported.
The AMA designation was associated with a 0.39 percentage-point decline in perinatal mortality (P = .04), “however, there were no significant changes in the proportion of individuals with severe maternal morbidity or with preterm birth or low birth weight at age 35 years,” they wrote.
In the subgroup analysis of low-risk pregnancies, “prenatal care services increased substantially at the 35-year cutoff, and in all cases, the increases at age 35 years for this group were larger than for the full sample,” they noted, adding that there was also a “substantially larger” decline in perinatal mortality at the AMA cutoff (P = .002), compared with the full sample.
The authors noted the need for more rigorous evidence on the value and effect of prenatal care guidelines on pregnancy outcomes. “Although pregnancy-related risks increase with maternal age, there is no known abrupt biological increase in underlying risk precisely at age 35 years,” they wrote, adding that “much of the content of prenatal care guidelines has persisted for decades without strong causal evidence to demonstrate its value.”
Their words echo those of Alex F. Peahl, MD, an ob.gyn. and assistant professor at the Institute for Healthcare Policy and Innovation, at the University of Michigan, in Ann Arbor, MI. In a recent review, Dr. Peahl and her colleague Joel D. Howell, MD, PhD, from the same university (Am J Obstet Gynecol. 2021 Apr;224[4]:339-47), note that the COVID-19 pandemic forced a much-needed rethink of prenatal care and its delivery. A look through the history of prenatal care shows “we have treated visit frequency and modality as fixed boxes, into which we must fit an ever-changing set of care recommendations,” they wrote. “We do not have data to support a specific prenatal visit schedule, recommended number of telemedicine visits, or specifications of additional services, and we never have. However, one thing is clear: we are long overdue for new prenatal care delivery guidelines in the United States.”
But when reached for comment on the new study Dr. Peahl cautioned that its conclusions are “limited and warrant future investigation. … While increased prenatal services may explain the improvement in outcomes, several other explanations should be considered,” she told this publication. “Perhaps, maternity care professional behavior differs for patients who are over the age of 35, resulting in increased caution in interpreting test results and symptoms; perhaps patients are more routinely induced at 39 weeks, limiting stillbirth rate; or perhaps patients are more hypervigilant when given the diagnosis of AMA.”
Priya Rajan, MD, agreed that while the paper showed an association between intensified antenatal interventions and decreased perinatal mortality, it did not show a causal relationship. “The study did not include information on other important factors that are also associated with perinatal risk,” noted Dr. Rajan, who is an associate professor in the department of ob.gyn. at Northwestern University in Chicago. Yet, she acknowledged that the findings “support what many clinicians know, which is that age 35 isn’t some tipping point; rather, obstetric risk is influenced by a range of factors, of which age may be one. This study, particularly when considered in the context of other studies and articles we have seen recently, confirms the need for us to rethink how we care for people during pregnancy and post partum. This includes delving further into understanding what aspects of the prenatal care that we provide have the biggest impact for both maternal and perinatal adverse outcomes.”
The study was supported by grant DGE1745303 from the National Science Foundation Graduate Research Fellowship Program. Dr. Geiger reported being a PhD student during the conduction of the study, but had no other disclosures. Dr. Peahl will soon be a consultant for Maven Clinic. Dr. Rajan had no relevant disclosures.
JAMA HEALTH FORUM
‘Alarming’ rate of abuse in pregnant women with epilepsy
, new research shows.
Study investigator Naveed Chaudhry, MD, a recent epilepsy fellow and assistant professor of neurology, University of Colorado School of Medicine, described the finding as “alarming” and called for more support for this patient population.
Investigators found that women with epilepsy are also more likely to report other stressors, including divorce, illness, lost pay, and partner discord, while expecting.
“As epilepsy physicians, it’s important that we ask the right questions and dive a little bit deeper with these patients, even if it’s uncomfortable and not something we’re used to,” said Dr. Chaudhry.
The findings were presented at the annual meeting of the American Epilepsy Society.
Cause for concern
Women with epilepsy may be under stress for a variety of social and economic reasons. In some women, stress can trigger seizures, and during pregnancy, this can lead to complications such as preterm labor and low birth weight.
For the study, researchers tapped into the Center for Disease Control and Prevention Pregnancy Risk Assessment and Monitoring System (PRAMS). This database includes information from surveys asking women across the U.S. about their pregnancy and postpartum period.
Thirteen states collected data on stresses in women with and without epilepsy. Respondents were asked about 14 economic and other worries in the year prior to their baby’s birth, including the pregnancy period.
The analysis included 64,951 women, 1,140 of whom had epilepsy, who were included in surveys from 2012-2020. There were no significant demographic differences between those with and those without the disorder.
After adjusting for maternal age, race, ethnicity, marital status, education, and socioeconomic status, the study found that women with epilepsy experienced an average of 2.41 of the stressors compared with 1.72 for women without epilepsy.
Women with epilepsy were more likely to have experienced family illness, divorce, homelessness, partner job loss, reduced work or pay, increased arguments, having a partner in jail, drug use, and the death of someone close to them.
The results showed that unmarried and younger women as well as those with lower incomes were particularly prone to experience stress during pregnancy.
It’s not clear why women with epilepsy report more stressors. “Looking at the literature, no one has really looked at the exact reason for this, but we postulate it could be a lack of supports and support systems,” said Dr. Chaudhry.
Women were asked about physical, sexual, and emotional abuse. Results showed that substantially more women with epilepsy than those without the disorder reported such abuse during pregnancy – 10.6% versus 4.1%. The adjusted odds ratio for women with epilepsy reporting abuse was 2.78 (95% CI, 2.07-3.74).
“That raises our concern and needs to be looked at in more detail,” said Dr. Chaudhry.
It is unclear whether some women might have had psychogenic non-epileptic seizures (PNES), which are linked to a higher rate of abuse, said Dr. Chaudhry. “But the prevalence of PNES in the general population is quite low, so we don’t think it’s contributing to a large extent to this finding.”
The findings highlight the importance of addressing stress in women with epilepsy during pregnancy, he said. “We need to have good support services and we need to counsel women to optimize good outcomes.”
This applies to all women of childbearing age. “We suspect abuse and stressors are going to be going on throughout that period,” said Dr. Chaudhry. “It’s important to ask about it and have appropriate support staff and social work and people available to help when an issue is identified.”
Stress a common seizure trigger
Commenting on the research, Kimford Meador, MD, professor, Department of Neurology and Neurological Sciences, Stanford University School of Medicine, noted the study was well conducted and had a large sample size.
The findings are important, as stress is a common trigger for seizures in people with epilepsy and is associated with mood and anxiety, which can affect quality of life, said Dr. Meador.
Results of his analysis from the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study, also presented at this year’s AES meeting, showed that women with epilepsy had more depressive symptoms during the postpartum period and more anxiety symptoms during pregnancy and postpartum in comparison with those without epilepsy.
Dr. Meador’s group also recently conducted a study that was published in JAMA Neurology, showing that in women with epilepsy during the postpartum period, anxiety is associated with lower cognitive ability in their children at age 2 years.
“All these findings highlight the importance of assessing and managing stress, anxiety, and mood in women with epilepsy,” said Dr. Meador. “Interventions could impact seizures and quality of life in pregnant women with epilepsy and long-term outcomes in their children.”
A version of this article first appeared on Medscape.com.
, new research shows.
Study investigator Naveed Chaudhry, MD, a recent epilepsy fellow and assistant professor of neurology, University of Colorado School of Medicine, described the finding as “alarming” and called for more support for this patient population.
Investigators found that women with epilepsy are also more likely to report other stressors, including divorce, illness, lost pay, and partner discord, while expecting.
“As epilepsy physicians, it’s important that we ask the right questions and dive a little bit deeper with these patients, even if it’s uncomfortable and not something we’re used to,” said Dr. Chaudhry.
The findings were presented at the annual meeting of the American Epilepsy Society.
Cause for concern
Women with epilepsy may be under stress for a variety of social and economic reasons. In some women, stress can trigger seizures, and during pregnancy, this can lead to complications such as preterm labor and low birth weight.
For the study, researchers tapped into the Center for Disease Control and Prevention Pregnancy Risk Assessment and Monitoring System (PRAMS). This database includes information from surveys asking women across the U.S. about their pregnancy and postpartum period.
Thirteen states collected data on stresses in women with and without epilepsy. Respondents were asked about 14 economic and other worries in the year prior to their baby’s birth, including the pregnancy period.
The analysis included 64,951 women, 1,140 of whom had epilepsy, who were included in surveys from 2012-2020. There were no significant demographic differences between those with and those without the disorder.
After adjusting for maternal age, race, ethnicity, marital status, education, and socioeconomic status, the study found that women with epilepsy experienced an average of 2.41 of the stressors compared with 1.72 for women without epilepsy.
Women with epilepsy were more likely to have experienced family illness, divorce, homelessness, partner job loss, reduced work or pay, increased arguments, having a partner in jail, drug use, and the death of someone close to them.
The results showed that unmarried and younger women as well as those with lower incomes were particularly prone to experience stress during pregnancy.
It’s not clear why women with epilepsy report more stressors. “Looking at the literature, no one has really looked at the exact reason for this, but we postulate it could be a lack of supports and support systems,” said Dr. Chaudhry.
Women were asked about physical, sexual, and emotional abuse. Results showed that substantially more women with epilepsy than those without the disorder reported such abuse during pregnancy – 10.6% versus 4.1%. The adjusted odds ratio for women with epilepsy reporting abuse was 2.78 (95% CI, 2.07-3.74).
“That raises our concern and needs to be looked at in more detail,” said Dr. Chaudhry.
It is unclear whether some women might have had psychogenic non-epileptic seizures (PNES), which are linked to a higher rate of abuse, said Dr. Chaudhry. “But the prevalence of PNES in the general population is quite low, so we don’t think it’s contributing to a large extent to this finding.”
The findings highlight the importance of addressing stress in women with epilepsy during pregnancy, he said. “We need to have good support services and we need to counsel women to optimize good outcomes.”
This applies to all women of childbearing age. “We suspect abuse and stressors are going to be going on throughout that period,” said Dr. Chaudhry. “It’s important to ask about it and have appropriate support staff and social work and people available to help when an issue is identified.”
Stress a common seizure trigger
Commenting on the research, Kimford Meador, MD, professor, Department of Neurology and Neurological Sciences, Stanford University School of Medicine, noted the study was well conducted and had a large sample size.
The findings are important, as stress is a common trigger for seizures in people with epilepsy and is associated with mood and anxiety, which can affect quality of life, said Dr. Meador.
Results of his analysis from the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study, also presented at this year’s AES meeting, showed that women with epilepsy had more depressive symptoms during the postpartum period and more anxiety symptoms during pregnancy and postpartum in comparison with those without epilepsy.
Dr. Meador’s group also recently conducted a study that was published in JAMA Neurology, showing that in women with epilepsy during the postpartum period, anxiety is associated with lower cognitive ability in their children at age 2 years.
“All these findings highlight the importance of assessing and managing stress, anxiety, and mood in women with epilepsy,” said Dr. Meador. “Interventions could impact seizures and quality of life in pregnant women with epilepsy and long-term outcomes in their children.”
A version of this article first appeared on Medscape.com.
, new research shows.
Study investigator Naveed Chaudhry, MD, a recent epilepsy fellow and assistant professor of neurology, University of Colorado School of Medicine, described the finding as “alarming” and called for more support for this patient population.
Investigators found that women with epilepsy are also more likely to report other stressors, including divorce, illness, lost pay, and partner discord, while expecting.
“As epilepsy physicians, it’s important that we ask the right questions and dive a little bit deeper with these patients, even if it’s uncomfortable and not something we’re used to,” said Dr. Chaudhry.
The findings were presented at the annual meeting of the American Epilepsy Society.
Cause for concern
Women with epilepsy may be under stress for a variety of social and economic reasons. In some women, stress can trigger seizures, and during pregnancy, this can lead to complications such as preterm labor and low birth weight.
For the study, researchers tapped into the Center for Disease Control and Prevention Pregnancy Risk Assessment and Monitoring System (PRAMS). This database includes information from surveys asking women across the U.S. about their pregnancy and postpartum period.
Thirteen states collected data on stresses in women with and without epilepsy. Respondents were asked about 14 economic and other worries in the year prior to their baby’s birth, including the pregnancy period.
The analysis included 64,951 women, 1,140 of whom had epilepsy, who were included in surveys from 2012-2020. There were no significant demographic differences between those with and those without the disorder.
After adjusting for maternal age, race, ethnicity, marital status, education, and socioeconomic status, the study found that women with epilepsy experienced an average of 2.41 of the stressors compared with 1.72 for women without epilepsy.
Women with epilepsy were more likely to have experienced family illness, divorce, homelessness, partner job loss, reduced work or pay, increased arguments, having a partner in jail, drug use, and the death of someone close to them.
The results showed that unmarried and younger women as well as those with lower incomes were particularly prone to experience stress during pregnancy.
It’s not clear why women with epilepsy report more stressors. “Looking at the literature, no one has really looked at the exact reason for this, but we postulate it could be a lack of supports and support systems,” said Dr. Chaudhry.
Women were asked about physical, sexual, and emotional abuse. Results showed that substantially more women with epilepsy than those without the disorder reported such abuse during pregnancy – 10.6% versus 4.1%. The adjusted odds ratio for women with epilepsy reporting abuse was 2.78 (95% CI, 2.07-3.74).
“That raises our concern and needs to be looked at in more detail,” said Dr. Chaudhry.
It is unclear whether some women might have had psychogenic non-epileptic seizures (PNES), which are linked to a higher rate of abuse, said Dr. Chaudhry. “But the prevalence of PNES in the general population is quite low, so we don’t think it’s contributing to a large extent to this finding.”
The findings highlight the importance of addressing stress in women with epilepsy during pregnancy, he said. “We need to have good support services and we need to counsel women to optimize good outcomes.”
This applies to all women of childbearing age. “We suspect abuse and stressors are going to be going on throughout that period,” said Dr. Chaudhry. “It’s important to ask about it and have appropriate support staff and social work and people available to help when an issue is identified.”
Stress a common seizure trigger
Commenting on the research, Kimford Meador, MD, professor, Department of Neurology and Neurological Sciences, Stanford University School of Medicine, noted the study was well conducted and had a large sample size.
The findings are important, as stress is a common trigger for seizures in people with epilepsy and is associated with mood and anxiety, which can affect quality of life, said Dr. Meador.
Results of his analysis from the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study, also presented at this year’s AES meeting, showed that women with epilepsy had more depressive symptoms during the postpartum period and more anxiety symptoms during pregnancy and postpartum in comparison with those without epilepsy.
Dr. Meador’s group also recently conducted a study that was published in JAMA Neurology, showing that in women with epilepsy during the postpartum period, anxiety is associated with lower cognitive ability in their children at age 2 years.
“All these findings highlight the importance of assessing and managing stress, anxiety, and mood in women with epilepsy,” said Dr. Meador. “Interventions could impact seizures and quality of life in pregnant women with epilepsy and long-term outcomes in their children.”
A version of this article first appeared on Medscape.com.
From AES 2021
Letter counters study that focuses on low-risk home births
A research letter published recently in the American Journal of Obstetrics and Gynecology argues that the methodology of a recent paper on the safety of planned home births presented a biased analysis.
The paper that Amos Grünebaum, MD, and colleagues with the department of obstetrics and gynecology at Lenox Hill Hospital in Hempstead, N.J., referred to is a study in Obstetrics & Gynecology which concluded that planned home births in Washington state are low risk.
In that paper, Elizabeth Nethery, RM, MS, MSM, a midwife and PhD candidate at the University of British Columbia, Vancouver, calculated the outcomes from 2015 to 2020 for “all births attended by members of a statewide midwifery professional association that were within professional association guidelines and met eligibility criteria for planned birth center birth.”
Ms. Nethery’s team concluded: “Rates of adverse outcomes for this cohort in a U.S. state with well-established and integrated community midwifery were low overall. Birth outcomes were similar for births planned at home or at a state-licensed, freestanding birth center.”
This news organization was among the publications that reported the results of that study.
But it’s the exclusion criteria in that study, primarily, that Dr. Grünebaum and colleagues take issue with.
Births excluded from the main analysis of the study by Ms. Nethery and coauthors involved “multifetal pregnancy, prior cesarean delivery, onset of labor at more than 42 0/7 weeks of gestation or preterm (less than 37 weeks), preexisting hypertension or diabetes, known amniotic fluid abnormality, gestational hypertension or preeclampsia, or malpresentation.”
Those are conditions that fall outside guidelines for planned home births. But both Ms. Nethery and Dr. Grünebaum said that sometimes these high-risk conditions are present in home births.
Different conclusion for home birth safety
Dr. Grünebaum and colleagues’ analysis of the risk profiles and outcomes for U.S. planned home births for the years 2016-2020 came to a different conclusion about the safety of home births.
They used a retrospective population-based cohort study that used the Centers for Disease Control and Prevention WONDER natality online database. They included planned home births and compared the outcomes with and without certain risk factors, including some high-risk factors such as twin deliveries, breech births, and previous cesarean.
Dr. Grünebaum’s analysis concluded that “it is an immutable truth that planned home births in the United States result in avoidable risks of increased adverse neonatal outcomes.”
Ms. Nethery said though the high-risk conditions were excluded from their main analysis, they are mentioned in the paper and detailed in the supplement.
She acknowledged in the paper that some midwives practice outside the guidelines and that was the case in 7% of births or for 800 people in the Washington state study. But she told this publication it’s a small number and high-risk births should be handled in a hospital so the team focused its research on low-risk births.
“People plan home births who are outside the guidelines everywhere in the world. There are a lot of reasons why people do it,” she said. Among them are not feeling safe in the hospital, being rejected by an obstetrician for a desired procedure, or, in some cases, because they are misinformed.
She said midwives are sometimes faced with a difficult choice, when a patient wants, for instance, a vaginal birth after cesarean (VBAC), one of the conditions not recommended for home births.
The midwife is left with the choice of saying she will not do a VBAC in the home, or she can explain to the patient why it is not recommended and explain all the reasons it is not recommended, such as an elevated risk of rupture, but honor the patient’s choice.
“Do you tell the person: ‘Sorry, go have the cesarean anyway or do you do your best to support this person?’ Birthing people have the right to autonomy of choice,” Ms. Nethery said.
Dr. Grünebaum and colleagues said: “The recent study by Nethery et al. concluded that planned home births in the state of Washington have good neonatal outcomes by focusing on results of low-risk patients.”
Dr. Grünebaum said in an interview: “It’s like reporting on smoking and lung cancer and saying I’m only going to report on patients who have smoked for less than 5 years. You need to take the whole picture into consideration.”
Ms. Nethery gave this explanation for excluding the high-risk patients: “If you are studying a drug, you exclude people from your study who got the drug even though they had risk factors that were ‘contraindications’ to that drug. Likely there was a reason they got the drug – in consultation with their doctor, the patient and the doctor decided that the potential benefit outweighed the risk – but they are not relevant to understanding how that drug impacts people who were ‘eligible’ for the drug in the first place.”
“That is part of the reason we excluded ‘high-risk’ people from our study,” she said. “The other reason is that that is what is commonly done in most research on this topic – we focus on ‘low-risk’ people who are within standards and eligibility criteria.”
She gave examples such as a 2019 meta-analysis and a 2011 Birthplace in England national prospective cohort study, both of which excluded high-risk home births.
“Third, we wanted to compare apples to apples (for our analysis of home vs. hospital) – and licensed birth centers in Washington state have restrictions based on risk,” Ms. Nethery said.
Dr. Grünebaum said his team supports the right of all women to give birth where they wish. “But you cannot choose unless you are given the right information.”
Dr. Grünebaum also said planned home births in the United States cannot be compared with home births delivered by midwives in other countries. Different from the United States, he said, in countries such as Canada, Germany, and England, midwives are well integrated in the medical system and they are typically affiliated with hospitals and they belong to organizations which support very strong guidelines.
He added that, while Washington state has its own set of guidelines, there are no national guidelines for home births and practice varies greatly by state.
The authors concluded: “It is the professional responsibility of all health care providers, obstetricians, and midwives to present unbiased information. Focusing the reporting of outcomes on low-risk deliveries underreports true adverse outcomes in U.S. home births and provides biased information to patients considering planned home births. It is an immutable truth that planned home births in the United States result in avoidable risks of increased adverse neonatal outcomes.”
Angela Martin, MD, assistant professor of maternal-fetal medicine and medical director of the labor and delivery department at University of Kansas Medical Center, Kansas City, who was not part of either study, said she did not believe it was a problem that Ms. Nethery’s study excluded the high-risk conditions in the main analysis because it was disclosed.
“The authors were clear that they excluded high-risk conditions,” she said. “Therefore, the study should not be extrapolated to women with these conditions.”
“I believe her results do make that case for low-risk women in Washington state,” Dr. Martin said. “Again, it is important that findings are not extrapolated to women outside of those included in the study.”
She said there are several things that make Washington unusual in midwifery care. Consequently, the results should not be seen as representative of the United States.
“It is one of the most integrated states for midwife care in the country,” Dr. Martin said. “Washington has licensure available for midwives, which is not true of all states. It also has a robust state professional association that publishes guidelines for midwives to follow. And midwives in Washington have a wide formulary. For example, they can administer antibiotics, carry and administer hemorrhage medications, they can carry oxygen, and they are allowed to suture.”
Iris Krishna, MD, MPH, director of perinatal quality, Emory Perinatal Center and assistant professor in the division of maternal-fetal medicine at Emory University, Atlanta, said in an interview that the arguments by Ms. Nethery and Dr. Grünebaum illustrate the controversy over home births.
Dr. Krishna, who was not part of either study, said physicians and midwives should counsel patients contemplating a planned community birth that available data is not generalizable to all birth settings or all patients.
“Women should be counseled that delivery in a hospital setting or accredited birth center is safer than home birth,” she said. “Ultimately, each woman has the right to make a medically informed decision about delivery after adequate counseling on the risks and benefits of community birth.”
Dr. Grünebaum and colleagues reported no relevant financial relationships. Ms. Nethery, Dr. Martin, and Dr. Krishna also reported no relevant financial relationships.
A research letter published recently in the American Journal of Obstetrics and Gynecology argues that the methodology of a recent paper on the safety of planned home births presented a biased analysis.
The paper that Amos Grünebaum, MD, and colleagues with the department of obstetrics and gynecology at Lenox Hill Hospital in Hempstead, N.J., referred to is a study in Obstetrics & Gynecology which concluded that planned home births in Washington state are low risk.
In that paper, Elizabeth Nethery, RM, MS, MSM, a midwife and PhD candidate at the University of British Columbia, Vancouver, calculated the outcomes from 2015 to 2020 for “all births attended by members of a statewide midwifery professional association that were within professional association guidelines and met eligibility criteria for planned birth center birth.”
Ms. Nethery’s team concluded: “Rates of adverse outcomes for this cohort in a U.S. state with well-established and integrated community midwifery were low overall. Birth outcomes were similar for births planned at home or at a state-licensed, freestanding birth center.”
This news organization was among the publications that reported the results of that study.
But it’s the exclusion criteria in that study, primarily, that Dr. Grünebaum and colleagues take issue with.
Births excluded from the main analysis of the study by Ms. Nethery and coauthors involved “multifetal pregnancy, prior cesarean delivery, onset of labor at more than 42 0/7 weeks of gestation or preterm (less than 37 weeks), preexisting hypertension or diabetes, known amniotic fluid abnormality, gestational hypertension or preeclampsia, or malpresentation.”
Those are conditions that fall outside guidelines for planned home births. But both Ms. Nethery and Dr. Grünebaum said that sometimes these high-risk conditions are present in home births.
Different conclusion for home birth safety
Dr. Grünebaum and colleagues’ analysis of the risk profiles and outcomes for U.S. planned home births for the years 2016-2020 came to a different conclusion about the safety of home births.
They used a retrospective population-based cohort study that used the Centers for Disease Control and Prevention WONDER natality online database. They included planned home births and compared the outcomes with and without certain risk factors, including some high-risk factors such as twin deliveries, breech births, and previous cesarean.
Dr. Grünebaum’s analysis concluded that “it is an immutable truth that planned home births in the United States result in avoidable risks of increased adverse neonatal outcomes.”
Ms. Nethery said though the high-risk conditions were excluded from their main analysis, they are mentioned in the paper and detailed in the supplement.
She acknowledged in the paper that some midwives practice outside the guidelines and that was the case in 7% of births or for 800 people in the Washington state study. But she told this publication it’s a small number and high-risk births should be handled in a hospital so the team focused its research on low-risk births.
“People plan home births who are outside the guidelines everywhere in the world. There are a lot of reasons why people do it,” she said. Among them are not feeling safe in the hospital, being rejected by an obstetrician for a desired procedure, or, in some cases, because they are misinformed.
She said midwives are sometimes faced with a difficult choice, when a patient wants, for instance, a vaginal birth after cesarean (VBAC), one of the conditions not recommended for home births.
The midwife is left with the choice of saying she will not do a VBAC in the home, or she can explain to the patient why it is not recommended and explain all the reasons it is not recommended, such as an elevated risk of rupture, but honor the patient’s choice.
“Do you tell the person: ‘Sorry, go have the cesarean anyway or do you do your best to support this person?’ Birthing people have the right to autonomy of choice,” Ms. Nethery said.
Dr. Grünebaum and colleagues said: “The recent study by Nethery et al. concluded that planned home births in the state of Washington have good neonatal outcomes by focusing on results of low-risk patients.”
Dr. Grünebaum said in an interview: “It’s like reporting on smoking and lung cancer and saying I’m only going to report on patients who have smoked for less than 5 years. You need to take the whole picture into consideration.”
Ms. Nethery gave this explanation for excluding the high-risk patients: “If you are studying a drug, you exclude people from your study who got the drug even though they had risk factors that were ‘contraindications’ to that drug. Likely there was a reason they got the drug – in consultation with their doctor, the patient and the doctor decided that the potential benefit outweighed the risk – but they are not relevant to understanding how that drug impacts people who were ‘eligible’ for the drug in the first place.”
“That is part of the reason we excluded ‘high-risk’ people from our study,” she said. “The other reason is that that is what is commonly done in most research on this topic – we focus on ‘low-risk’ people who are within standards and eligibility criteria.”
She gave examples such as a 2019 meta-analysis and a 2011 Birthplace in England national prospective cohort study, both of which excluded high-risk home births.
“Third, we wanted to compare apples to apples (for our analysis of home vs. hospital) – and licensed birth centers in Washington state have restrictions based on risk,” Ms. Nethery said.
Dr. Grünebaum said his team supports the right of all women to give birth where they wish. “But you cannot choose unless you are given the right information.”
Dr. Grünebaum also said planned home births in the United States cannot be compared with home births delivered by midwives in other countries. Different from the United States, he said, in countries such as Canada, Germany, and England, midwives are well integrated in the medical system and they are typically affiliated with hospitals and they belong to organizations which support very strong guidelines.
He added that, while Washington state has its own set of guidelines, there are no national guidelines for home births and practice varies greatly by state.
The authors concluded: “It is the professional responsibility of all health care providers, obstetricians, and midwives to present unbiased information. Focusing the reporting of outcomes on low-risk deliveries underreports true adverse outcomes in U.S. home births and provides biased information to patients considering planned home births. It is an immutable truth that planned home births in the United States result in avoidable risks of increased adverse neonatal outcomes.”
Angela Martin, MD, assistant professor of maternal-fetal medicine and medical director of the labor and delivery department at University of Kansas Medical Center, Kansas City, who was not part of either study, said she did not believe it was a problem that Ms. Nethery’s study excluded the high-risk conditions in the main analysis because it was disclosed.
“The authors were clear that they excluded high-risk conditions,” she said. “Therefore, the study should not be extrapolated to women with these conditions.”
“I believe her results do make that case for low-risk women in Washington state,” Dr. Martin said. “Again, it is important that findings are not extrapolated to women outside of those included in the study.”
She said there are several things that make Washington unusual in midwifery care. Consequently, the results should not be seen as representative of the United States.
“It is one of the most integrated states for midwife care in the country,” Dr. Martin said. “Washington has licensure available for midwives, which is not true of all states. It also has a robust state professional association that publishes guidelines for midwives to follow. And midwives in Washington have a wide formulary. For example, they can administer antibiotics, carry and administer hemorrhage medications, they can carry oxygen, and they are allowed to suture.”
Iris Krishna, MD, MPH, director of perinatal quality, Emory Perinatal Center and assistant professor in the division of maternal-fetal medicine at Emory University, Atlanta, said in an interview that the arguments by Ms. Nethery and Dr. Grünebaum illustrate the controversy over home births.
Dr. Krishna, who was not part of either study, said physicians and midwives should counsel patients contemplating a planned community birth that available data is not generalizable to all birth settings or all patients.
“Women should be counseled that delivery in a hospital setting or accredited birth center is safer than home birth,” she said. “Ultimately, each woman has the right to make a medically informed decision about delivery after adequate counseling on the risks and benefits of community birth.”
Dr. Grünebaum and colleagues reported no relevant financial relationships. Ms. Nethery, Dr. Martin, and Dr. Krishna also reported no relevant financial relationships.
A research letter published recently in the American Journal of Obstetrics and Gynecology argues that the methodology of a recent paper on the safety of planned home births presented a biased analysis.
The paper that Amos Grünebaum, MD, and colleagues with the department of obstetrics and gynecology at Lenox Hill Hospital in Hempstead, N.J., referred to is a study in Obstetrics & Gynecology which concluded that planned home births in Washington state are low risk.
In that paper, Elizabeth Nethery, RM, MS, MSM, a midwife and PhD candidate at the University of British Columbia, Vancouver, calculated the outcomes from 2015 to 2020 for “all births attended by members of a statewide midwifery professional association that were within professional association guidelines and met eligibility criteria for planned birth center birth.”
Ms. Nethery’s team concluded: “Rates of adverse outcomes for this cohort in a U.S. state with well-established and integrated community midwifery were low overall. Birth outcomes were similar for births planned at home or at a state-licensed, freestanding birth center.”
This news organization was among the publications that reported the results of that study.
But it’s the exclusion criteria in that study, primarily, that Dr. Grünebaum and colleagues take issue with.
Births excluded from the main analysis of the study by Ms. Nethery and coauthors involved “multifetal pregnancy, prior cesarean delivery, onset of labor at more than 42 0/7 weeks of gestation or preterm (less than 37 weeks), preexisting hypertension or diabetes, known amniotic fluid abnormality, gestational hypertension or preeclampsia, or malpresentation.”
Those are conditions that fall outside guidelines for planned home births. But both Ms. Nethery and Dr. Grünebaum said that sometimes these high-risk conditions are present in home births.
Different conclusion for home birth safety
Dr. Grünebaum and colleagues’ analysis of the risk profiles and outcomes for U.S. planned home births for the years 2016-2020 came to a different conclusion about the safety of home births.
They used a retrospective population-based cohort study that used the Centers for Disease Control and Prevention WONDER natality online database. They included planned home births and compared the outcomes with and without certain risk factors, including some high-risk factors such as twin deliveries, breech births, and previous cesarean.
Dr. Grünebaum’s analysis concluded that “it is an immutable truth that planned home births in the United States result in avoidable risks of increased adverse neonatal outcomes.”
Ms. Nethery said though the high-risk conditions were excluded from their main analysis, they are mentioned in the paper and detailed in the supplement.
She acknowledged in the paper that some midwives practice outside the guidelines and that was the case in 7% of births or for 800 people in the Washington state study. But she told this publication it’s a small number and high-risk births should be handled in a hospital so the team focused its research on low-risk births.
“People plan home births who are outside the guidelines everywhere in the world. There are a lot of reasons why people do it,” she said. Among them are not feeling safe in the hospital, being rejected by an obstetrician for a desired procedure, or, in some cases, because they are misinformed.
She said midwives are sometimes faced with a difficult choice, when a patient wants, for instance, a vaginal birth after cesarean (VBAC), one of the conditions not recommended for home births.
The midwife is left with the choice of saying she will not do a VBAC in the home, or she can explain to the patient why it is not recommended and explain all the reasons it is not recommended, such as an elevated risk of rupture, but honor the patient’s choice.
“Do you tell the person: ‘Sorry, go have the cesarean anyway or do you do your best to support this person?’ Birthing people have the right to autonomy of choice,” Ms. Nethery said.
Dr. Grünebaum and colleagues said: “The recent study by Nethery et al. concluded that planned home births in the state of Washington have good neonatal outcomes by focusing on results of low-risk patients.”
Dr. Grünebaum said in an interview: “It’s like reporting on smoking and lung cancer and saying I’m only going to report on patients who have smoked for less than 5 years. You need to take the whole picture into consideration.”
Ms. Nethery gave this explanation for excluding the high-risk patients: “If you are studying a drug, you exclude people from your study who got the drug even though they had risk factors that were ‘contraindications’ to that drug. Likely there was a reason they got the drug – in consultation with their doctor, the patient and the doctor decided that the potential benefit outweighed the risk – but they are not relevant to understanding how that drug impacts people who were ‘eligible’ for the drug in the first place.”
“That is part of the reason we excluded ‘high-risk’ people from our study,” she said. “The other reason is that that is what is commonly done in most research on this topic – we focus on ‘low-risk’ people who are within standards and eligibility criteria.”
She gave examples such as a 2019 meta-analysis and a 2011 Birthplace in England national prospective cohort study, both of which excluded high-risk home births.
“Third, we wanted to compare apples to apples (for our analysis of home vs. hospital) – and licensed birth centers in Washington state have restrictions based on risk,” Ms. Nethery said.
Dr. Grünebaum said his team supports the right of all women to give birth where they wish. “But you cannot choose unless you are given the right information.”
Dr. Grünebaum also said planned home births in the United States cannot be compared with home births delivered by midwives in other countries. Different from the United States, he said, in countries such as Canada, Germany, and England, midwives are well integrated in the medical system and they are typically affiliated with hospitals and they belong to organizations which support very strong guidelines.
He added that, while Washington state has its own set of guidelines, there are no national guidelines for home births and practice varies greatly by state.
The authors concluded: “It is the professional responsibility of all health care providers, obstetricians, and midwives to present unbiased information. Focusing the reporting of outcomes on low-risk deliveries underreports true adverse outcomes in U.S. home births and provides biased information to patients considering planned home births. It is an immutable truth that planned home births in the United States result in avoidable risks of increased adverse neonatal outcomes.”
Angela Martin, MD, assistant professor of maternal-fetal medicine and medical director of the labor and delivery department at University of Kansas Medical Center, Kansas City, who was not part of either study, said she did not believe it was a problem that Ms. Nethery’s study excluded the high-risk conditions in the main analysis because it was disclosed.
“The authors were clear that they excluded high-risk conditions,” she said. “Therefore, the study should not be extrapolated to women with these conditions.”
“I believe her results do make that case for low-risk women in Washington state,” Dr. Martin said. “Again, it is important that findings are not extrapolated to women outside of those included in the study.”
She said there are several things that make Washington unusual in midwifery care. Consequently, the results should not be seen as representative of the United States.
“It is one of the most integrated states for midwife care in the country,” Dr. Martin said. “Washington has licensure available for midwives, which is not true of all states. It also has a robust state professional association that publishes guidelines for midwives to follow. And midwives in Washington have a wide formulary. For example, they can administer antibiotics, carry and administer hemorrhage medications, they can carry oxygen, and they are allowed to suture.”
Iris Krishna, MD, MPH, director of perinatal quality, Emory Perinatal Center and assistant professor in the division of maternal-fetal medicine at Emory University, Atlanta, said in an interview that the arguments by Ms. Nethery and Dr. Grünebaum illustrate the controversy over home births.
Dr. Krishna, who was not part of either study, said physicians and midwives should counsel patients contemplating a planned community birth that available data is not generalizable to all birth settings or all patients.
“Women should be counseled that delivery in a hospital setting or accredited birth center is safer than home birth,” she said. “Ultimately, each woman has the right to make a medically informed decision about delivery after adequate counseling on the risks and benefits of community birth.”
Dr. Grünebaum and colleagues reported no relevant financial relationships. Ms. Nethery, Dr. Martin, and Dr. Krishna also reported no relevant financial relationships.
FROM THE AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
Fever following cesarean delivery: What are your steps for management?
CASE Woman who has undergone recent cesarean delivery
A 23-year-old woman had a primary cesarean delivery 72 hours ago due to an arrest of dilation at 6 cm. She was in labor for 22 hours, and her membranes were ruptured for 18 hours. She had 10 internal vaginal examinations, and the duration of internal fetal monitoring was 12 hours; 24 hours after delivery, she developed a fever of 39°C, in association with lower abdominal pain and tenderness. She was presumptively treated for endometritis with cefepime; 48 hours after the initiation of antibiotics, she remains febrile and symptomatic.
- What are the most likely causes of her persistent fever?
- What should be the next steps in her evaluation?
Cesarean delivery background
Cesarean delivery is now the most common major operation performed in US hospitals. Cesarean delivery rates hover between 25% and 30% in most medical centers in the United States.1 The most common postoperative complication of cesarean delivery is infection. Infection typically takes 1 of 3 forms: endometritis (organ space infection), wound infection (surgical site infection), and urinary tract infection (UTI).1 This article will review the initial differential diagnosis, evaluation, and management of the patient with a postoperative fever and also will describe the appropriate assessment and treatment of the patient who has a persistent postoperative fever despite therapy. The article will also highlight key interventions that help to prevent postoperative infections.
Initial evaluation of the febrile patient
In the first 24 to 48 hours after cesarean delivery, the most common cause of fever is endometritis (organ space infection). This condition is a polymicrobial, mixed aerobic-anaerobic infection (FIGURE). The principal pathogens include anaerobic gram-positive cocci (
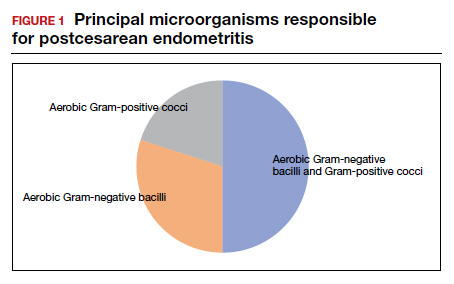
The major risk factors for postcesarean endometritis are extended duration of labor and ruptured membranes, multiple internal vaginal examinations, invasive fetal monitoring, and pre-existing colonization with group B Streptococcus and/or the organisms that cause bacterial vaginosis. Affected patients typically have a fever in the range of 38 to 39°C, tachycardia, mild tachypnea, lower abdominal pain and tenderness, and purulent lochia in some individuals.1
Differential for postoperative fever
The initial differential diagnosis of postoperative fever is relatively limited (TABLE 1). In addition to endometritis, it includes extensive atelectasis, perhaps resulting from general anesthesia; lower respiratory tract infection, either viral influenza or bacterial pneumonia; and acute pyelonephritis. A simple infection of the bladder (cystitis or asymptomatic bacteriuria) should not cause a substantial temperature elevation and systemic symptoms.1
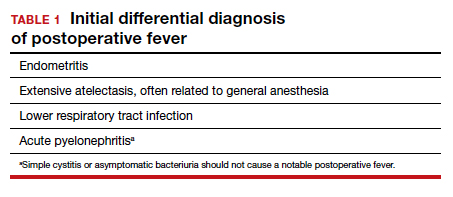
Differentiation between these entities usually is possible based on physical examination and a few laboratory tests. The peripheral white blood cell count usually is elevated, and a left shift may be evident. If a respiratory tract infection is suspected, chest radiography is indicated. A urine culture should be obtained if acute pyelonephritis strongly is considered. Lower genital tract cultures are rarely of value, and uncontaminated upper tract cultures are difficult to obtain. I do not believe that blood cultures should be performed as a matter of routine. They are expensive, and the results are often not available until after the patient has cleared her infection and left the hospital. However, I would obtain blood cultures in patients who meet one of these criteria1,2:
- They are immunocompromised (eg, HIV infection).
- They have a cardiac or vascular prosthesis and, thus, are at increased risk of complications related to bacteremia.
- They seem critically ill at the onset of evaluation.
- They fail to respond appropriately to initial therapy.
The cornerstone of therapy is broad spectrum antibiotics that target the multiple organisms responsible for endometritis.3 There are several single agents and several combination antibiotic regimens that provide excellent coverage against the usual pelvic pathogens (TABLE 2). I personally favor the generic combination regimen (clindamycin plus gentamicin) because it is relatively inexpensive and has been very well validated in multiple studies. In patients who have underlying renal dysfunction, aztreonam can be substituted for gentamicin.
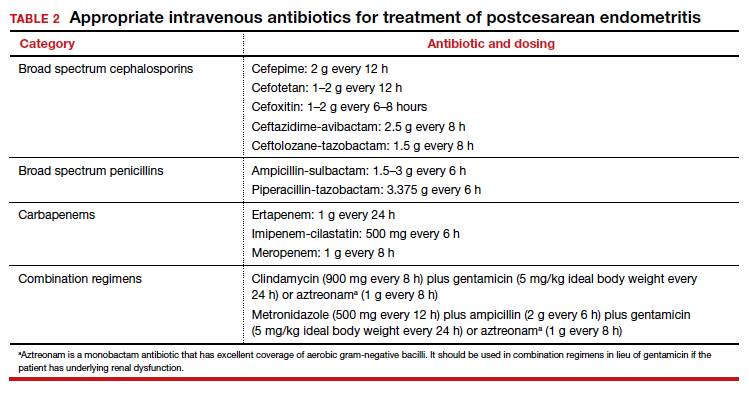
Approximately 90% of patients will show clear evidence of clinical improvement (ie, decrease in temperature and resolution of abdominopelvic pain) within 48 hours of starting antibiotics. Patients should then continue therapy until they have been afebrile and asymptomatic for approximately 24 hours. At that point, antibiotics should be discontinued, and the patient can be discharged. With rare exceptions, there is no indication for administration of oral antibiotics on an outpatient basis.1,4
Continue to: Persistent postoperative fever...
Persistent postoperative fever
Resistant microorganism
The most common cause of a persistent fever after initiating antibiotic therapy is a resistant microorganism. There are potential gaps in coverage for the antibiotic regimens commonly used to treat postcesarean endometritis (TABLE 3).1,4 Assuming there is no other obvious cause for treatment failure, I recommend that therapy be changed to the triple combination of metronidazole plus ampicillin plus gentamicin (or aztreonam). The first drug provides superb coverage against anaerobes; the second covers enterococci. Gentamicin or aztreonam cover virtually all aerobic Gram-negative bacilli likely to cause postcesarean infection. I prefer metronidazole rather than clindamycin in this regimen because, unlike clindamycin, it is less likely to trigger diarrhea when used in combination with ampicillin. The 3-drug regimen should be continued until the patient has been afebrile and asymptomatic for approximately 24 hours.1,3,4
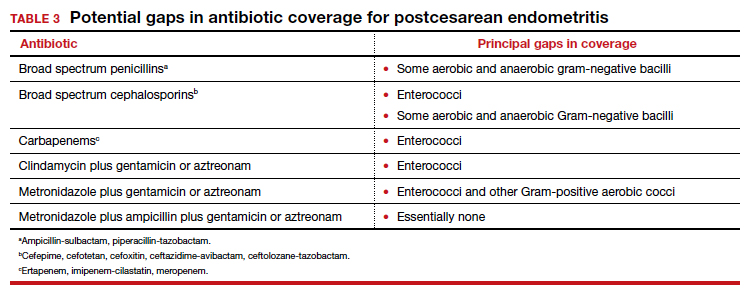
Wound infection
The second most common reason for a poor response to initial antibiotic therapy is a wound (surgical site) infection. Wound infections are caused by many of the same pelvic pathogens responsible for endometritis combined with skin flora, notably Streptococcus and Staphylococcus species, including methicillin-resistant Staphylococcus aureus (MRSA).1,4
Wound infections typically take one of two forms. The first is an actual incisional abscess. The patient is febrile; the margins of the wound are warm, indurated, erythematous, and tender; and purulent material drains from the incision. In this situation, the wound should be opened widely to drain the purulent collection. The fascia should then be probed to be certain that dehiscence has not occurred. In addition, intravenous vancomycin (1 g every 12 h) should be included in the antibiotic regimen to ensure adequate coverage of hospital-acquired MRSA.1,4
The second common presentation of a wound infection is cellulitis. The patient is febrile, and there is a spreading area of erythema, warmth, and exquisite tenderness extending from the edges of the incision; however, no purulent drainage is apparent. In this second scenario, the wound should not be opened, but intravenous vancomycin should be added to the treatment regimen.1,3,4
A third and very rare form of wound infection is necrotizing fasciitis. In affected patients, the margins of the wound are darkened and necrotic rather than erythematous and indurated. Two other key physical findings are crepitance and loss of sensation along the margins of the wound. Necrotizing fasciitis is truly a life-threatening emergency and requires immediate and extensive debridement of the devitalized tissue, combined with broad spectrum therapy with antibiotics that provide excellent coverage against anaerobes, aerobic streptococci (particularly group A streptococci), and staphylococci. The requirement for debridement may be so extensive that a skin graft subsequently is necessary to close the defect.1,4
Continue to: Unusual causes of persistent postoperative fever...
Unusual causes of persistent postoperative fever
If a resistant microorganism and wound infection can be excluded, the clinician then must begin a diligent search for “zebras” (ie, uncommon but potentially serious causes of persistent fever).1,4 One possible cause is a pelvic abscess. These purulent collections typically form in the retrovesicle space as a result of infection of a hematoma that formed between the posterior bladder wall and the lower uterine segment, in the leaves of the broad ligament, or in the posterior cul-de-sac. The abscess may or may not be palpable. The patient’s peripheral white blood cell count usually is elevated, with a preponderance of neutrophils. The best imaging test for an abscess is a computed tomography (CT) scan. Abscesses require drainage, which usually can be accomplished by insertion of a percutaneous drain under ultrasonographic or CT guidance.
A second unusual cause of persistent fever is septic pelvic vein thrombophlebitis. The infected venous emboli usually are present in the ovarian veins, with the right side predominant. The patient’s peripheral white blood cell count usually is elevated, and the infected clots are best imaged by CT scan with contrast or magnetic resonance angiography. The appropriate treatment is continuation of broad-spectrum antibiotics and administration of therapeutic doses of parenteral anticoagulants such as enoxaparin or unfractionated heparin.
A third explanation for persistent fever is retained products of conception. This diagnosis is best made by ultrasonography. The placental fragments should be removed by sharp curettage.
A fourth consideration when evaluating the patient with persistent fever is an allergic drug reaction. In most instances, the increase in the patient’s temperature will correspond with administration of the offending antibiotic(s). Affected patients typically have an increased number of eosinophils in their peripheral white blood cell count. The appropriate management of drug fever is discontinuation of antibiotics.
A final and distinctly unusual consideration is recrudescence of a connective tissue disorder such as systemic lupus erythematosus. The best test to confirm this diagnosis is the serum complement assay, which will demonstrate a decreased serum concentration of complement, reflecting consumption of this serum protein during the inflammatory process. The correct management for this condition is administration of a short course of systemic glucocorticoids. TABLE 4 summarizes a simple, systematic plan for evaluation of the patient with a persistent postoperative fever.
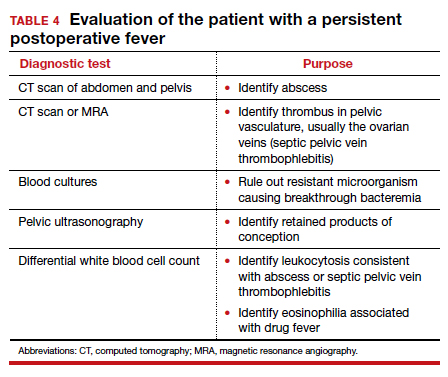
Preventive measures
We all remember the simple but profound statement by Benjamin Franklin, “An ounce of prevention is worth a pound of cure.” That folksy adage rings true with respect to postoperative infection because this complication extends hospital stay, increases hospital expense, and causes considerable discomfort and inconvenience for the patient. Therefore, we would do well to prevent as many instances of postoperative infection as possible.
Endometritis
On the basis of well-designed, prospective, randomized trials (Level 1 evidence), 3 interventions have proven effective in reducing the frequency of postcesarean endometritis. The first is irrigation of the vaginal canal preoperatively with an iodophor solution.5,6 The second is preoperative administration of systemic antibiotics.7-9 The combination of cefazolin (2 g IV within 30 minutes of incision) plus azithromycin (500 mg IV over 1 hour prior to incision) is superior to cefazolin alone.10,11 The third important preventive measure is removing the placenta by traction on the umbilical cord rather than by manual extraction.12,13
Wound infection
Several interventions are of proven effectiveness in reducing the frequency of postcesarean wound (surgical site) infection. The first is removal of hair at the incision site by clipping rather than by shaving (Level 2 evidence).14 The second is cleansing of the skin with chlorhexidine rather than iodophor (Level 1 evidence).15 The third is closing of the deep subcutaneous layer of the incision if it exceeds 2 cm in depth (Level 1 evidence).16,17 The fourth is closure of the skin with subcutaneous sutures rather than staples (Level 1 evidence).18 The monofilament suture poliglecaprone 25 is superior to the multifilament suture polyglactin 910 for this purpose (Level 1 evidence).19 Finally, in obese patients (body mass index >30 kg/m2), application of a negative pressure wound vacuum dressing may offer additional protection against infection (Level 1 evidence).20 Such dressings are too expensive, however, to be used routinely in all patients.
Urinary tract infection
The most important measures for preventing postoperative UTIs are identifying and clearing asymptomatic bacteriuria prior to delivery, inserting the urinary catheter prior to surgery using strict sterile technique, and removing the catheter as soon as possible after surgery, ideally within 12 hours.1,4
CASE Resolved
The 2 most likely causes for this patient’s poor response to initial therapy are resistant microorganism and wound infection. If a wound infection can be excluded by physical examination, the patient’s antibiotic regimen should be changed to metronidazole plus ampicillin plus gentamicin (or aztreonam). If an incisional abscess is identified, the incision should be opened and drained, and vancomycin should be added to the treatment regimen. If a wound cellulitis is evident, the incision should not be opened, but vancomycin should be added to the treatment regimen to enhance coverage against aerobic Streptococcus and Staphylococcus species. ●
- Duff WP. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2020:1124-1146.
- Locksmith GJ, Duff P. Assessment of the value of routine blood cultures in the evaluation and treatment of patients with chorioamnionitis. Infect Dis Obstet Gynecol. 1994;2:111-114.
- Duff P. Antibiotic selection in obstetric patients. Infect Dis Clin N Am. 1997;11:1-12.
- Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams, JD, et al, eds. Creasy & Resnik’s Maternal Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Syst Rev. 2014;12:CD007892.
- Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery. a systematic review and meta-analysis. Obstet Gynecol. 2017;130:527-538.
- Sullivan SA, Smith T, Change E, et al. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity; a randomized controlled trial. Am J Obstet Gynecol. 2007;196:455.e1-455.e5.
- Tita ATN, Hauth JC, Grimes A, et al. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111:51-56.
- Tita ATN, Owen J, Stamm AM, et al. Impact of extended-spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199: 303.e1-303.e3.
- Tita ATN, Szchowski JM, Boggess K, et al. Two antibiotics before cesarean delivery reduce infection rates further than one agent. N Engl J Med. 2016;375:1231-1241.
- Harper LM, Kilgore M, Szychowski JM, et al. Economic evaluation of adjunctive azithromycin prophylaxis for cesarean delivery. Obstet Gynecol. 2017;130:328-334.
- Lasley DS, Eblen A, Yancey MK, et al. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176:1250-1254.
- Anorlu RI, Maholwana B, Hofmeyr GJ. Methods of delivering the placenta at cesarean section. Cochrane Database Syst Rev. 2008;3:CD004737.
- Cruse PJ, Foord R. A five-year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107:206-209.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374:657-665.
- Del Valle GO, Combs P, Qualls C, et al. Does closure of camper fascia reduce the incidence of post-cesarean superficial wound disruption? Obstet Gynecol. 1992;80:1013-1016.
- Chelmow D. Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: a meta-analysis. Obstet Gynecol. 2004;103:974-980.
- Tuuli MG, Rampersod RM, Carbone JF, et al. Staples compared with subcuticular suture for skin closure after cesarean delivery. a systematic review and meta-analysis. Obstet Gynecol. 2011;117:682-690.
- Buresch AM, Arsdale AV, Ferzli M, et al. Comparison of subcuticular suture type for skin closure after cesarean delivery. a randomized controlled trial. Obstet Gynecol. 2017;130:521-526.
- Yu L, Kronen RJ, Simon LE, et al. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218:200-210.
CASE Woman who has undergone recent cesarean delivery
A 23-year-old woman had a primary cesarean delivery 72 hours ago due to an arrest of dilation at 6 cm. She was in labor for 22 hours, and her membranes were ruptured for 18 hours. She had 10 internal vaginal examinations, and the duration of internal fetal monitoring was 12 hours; 24 hours after delivery, she developed a fever of 39°C, in association with lower abdominal pain and tenderness. She was presumptively treated for endometritis with cefepime; 48 hours after the initiation of antibiotics, she remains febrile and symptomatic.
- What are the most likely causes of her persistent fever?
- What should be the next steps in her evaluation?
Cesarean delivery background
Cesarean delivery is now the most common major operation performed in US hospitals. Cesarean delivery rates hover between 25% and 30% in most medical centers in the United States.1 The most common postoperative complication of cesarean delivery is infection. Infection typically takes 1 of 3 forms: endometritis (organ space infection), wound infection (surgical site infection), and urinary tract infection (UTI).1 This article will review the initial differential diagnosis, evaluation, and management of the patient with a postoperative fever and also will describe the appropriate assessment and treatment of the patient who has a persistent postoperative fever despite therapy. The article will also highlight key interventions that help to prevent postoperative infections.
Initial evaluation of the febrile patient
In the first 24 to 48 hours after cesarean delivery, the most common cause of fever is endometritis (organ space infection). This condition is a polymicrobial, mixed aerobic-anaerobic infection (FIGURE). The principal pathogens include anaerobic gram-positive cocci (

The major risk factors for postcesarean endometritis are extended duration of labor and ruptured membranes, multiple internal vaginal examinations, invasive fetal monitoring, and pre-existing colonization with group B Streptococcus and/or the organisms that cause bacterial vaginosis. Affected patients typically have a fever in the range of 38 to 39°C, tachycardia, mild tachypnea, lower abdominal pain and tenderness, and purulent lochia in some individuals.1
Differential for postoperative fever
The initial differential diagnosis of postoperative fever is relatively limited (TABLE 1). In addition to endometritis, it includes extensive atelectasis, perhaps resulting from general anesthesia; lower respiratory tract infection, either viral influenza or bacterial pneumonia; and acute pyelonephritis. A simple infection of the bladder (cystitis or asymptomatic bacteriuria) should not cause a substantial temperature elevation and systemic symptoms.1

Differentiation between these entities usually is possible based on physical examination and a few laboratory tests. The peripheral white blood cell count usually is elevated, and a left shift may be evident. If a respiratory tract infection is suspected, chest radiography is indicated. A urine culture should be obtained if acute pyelonephritis strongly is considered. Lower genital tract cultures are rarely of value, and uncontaminated upper tract cultures are difficult to obtain. I do not believe that blood cultures should be performed as a matter of routine. They are expensive, and the results are often not available until after the patient has cleared her infection and left the hospital. However, I would obtain blood cultures in patients who meet one of these criteria1,2:
- They are immunocompromised (eg, HIV infection).
- They have a cardiac or vascular prosthesis and, thus, are at increased risk of complications related to bacteremia.
- They seem critically ill at the onset of evaluation.
- They fail to respond appropriately to initial therapy.
The cornerstone of therapy is broad spectrum antibiotics that target the multiple organisms responsible for endometritis.3 There are several single agents and several combination antibiotic regimens that provide excellent coverage against the usual pelvic pathogens (TABLE 2). I personally favor the generic combination regimen (clindamycin plus gentamicin) because it is relatively inexpensive and has been very well validated in multiple studies. In patients who have underlying renal dysfunction, aztreonam can be substituted for gentamicin.

Approximately 90% of patients will show clear evidence of clinical improvement (ie, decrease in temperature and resolution of abdominopelvic pain) within 48 hours of starting antibiotics. Patients should then continue therapy until they have been afebrile and asymptomatic for approximately 24 hours. At that point, antibiotics should be discontinued, and the patient can be discharged. With rare exceptions, there is no indication for administration of oral antibiotics on an outpatient basis.1,4
Continue to: Persistent postoperative fever...
Persistent postoperative fever
Resistant microorganism
The most common cause of a persistent fever after initiating antibiotic therapy is a resistant microorganism. There are potential gaps in coverage for the antibiotic regimens commonly used to treat postcesarean endometritis (TABLE 3).1,4 Assuming there is no other obvious cause for treatment failure, I recommend that therapy be changed to the triple combination of metronidazole plus ampicillin plus gentamicin (or aztreonam). The first drug provides superb coverage against anaerobes; the second covers enterococci. Gentamicin or aztreonam cover virtually all aerobic Gram-negative bacilli likely to cause postcesarean infection. I prefer metronidazole rather than clindamycin in this regimen because, unlike clindamycin, it is less likely to trigger diarrhea when used in combination with ampicillin. The 3-drug regimen should be continued until the patient has been afebrile and asymptomatic for approximately 24 hours.1,3,4

Wound infection
The second most common reason for a poor response to initial antibiotic therapy is a wound (surgical site) infection. Wound infections are caused by many of the same pelvic pathogens responsible for endometritis combined with skin flora, notably Streptococcus and Staphylococcus species, including methicillin-resistant Staphylococcus aureus (MRSA).1,4
Wound infections typically take one of two forms. The first is an actual incisional abscess. The patient is febrile; the margins of the wound are warm, indurated, erythematous, and tender; and purulent material drains from the incision. In this situation, the wound should be opened widely to drain the purulent collection. The fascia should then be probed to be certain that dehiscence has not occurred. In addition, intravenous vancomycin (1 g every 12 h) should be included in the antibiotic regimen to ensure adequate coverage of hospital-acquired MRSA.1,4
The second common presentation of a wound infection is cellulitis. The patient is febrile, and there is a spreading area of erythema, warmth, and exquisite tenderness extending from the edges of the incision; however, no purulent drainage is apparent. In this second scenario, the wound should not be opened, but intravenous vancomycin should be added to the treatment regimen.1,3,4
A third and very rare form of wound infection is necrotizing fasciitis. In affected patients, the margins of the wound are darkened and necrotic rather than erythematous and indurated. Two other key physical findings are crepitance and loss of sensation along the margins of the wound. Necrotizing fasciitis is truly a life-threatening emergency and requires immediate and extensive debridement of the devitalized tissue, combined with broad spectrum therapy with antibiotics that provide excellent coverage against anaerobes, aerobic streptococci (particularly group A streptococci), and staphylococci. The requirement for debridement may be so extensive that a skin graft subsequently is necessary to close the defect.1,4
Continue to: Unusual causes of persistent postoperative fever...
Unusual causes of persistent postoperative fever
If a resistant microorganism and wound infection can be excluded, the clinician then must begin a diligent search for “zebras” (ie, uncommon but potentially serious causes of persistent fever).1,4 One possible cause is a pelvic abscess. These purulent collections typically form in the retrovesicle space as a result of infection of a hematoma that formed between the posterior bladder wall and the lower uterine segment, in the leaves of the broad ligament, or in the posterior cul-de-sac. The abscess may or may not be palpable. The patient’s peripheral white blood cell count usually is elevated, with a preponderance of neutrophils. The best imaging test for an abscess is a computed tomography (CT) scan. Abscesses require drainage, which usually can be accomplished by insertion of a percutaneous drain under ultrasonographic or CT guidance.
A second unusual cause of persistent fever is septic pelvic vein thrombophlebitis. The infected venous emboli usually are present in the ovarian veins, with the right side predominant. The patient’s peripheral white blood cell count usually is elevated, and the infected clots are best imaged by CT scan with contrast or magnetic resonance angiography. The appropriate treatment is continuation of broad-spectrum antibiotics and administration of therapeutic doses of parenteral anticoagulants such as enoxaparin or unfractionated heparin.
A third explanation for persistent fever is retained products of conception. This diagnosis is best made by ultrasonography. The placental fragments should be removed by sharp curettage.
A fourth consideration when evaluating the patient with persistent fever is an allergic drug reaction. In most instances, the increase in the patient’s temperature will correspond with administration of the offending antibiotic(s). Affected patients typically have an increased number of eosinophils in their peripheral white blood cell count. The appropriate management of drug fever is discontinuation of antibiotics.
A final and distinctly unusual consideration is recrudescence of a connective tissue disorder such as systemic lupus erythematosus. The best test to confirm this diagnosis is the serum complement assay, which will demonstrate a decreased serum concentration of complement, reflecting consumption of this serum protein during the inflammatory process. The correct management for this condition is administration of a short course of systemic glucocorticoids. TABLE 4 summarizes a simple, systematic plan for evaluation of the patient with a persistent postoperative fever.

Preventive measures
We all remember the simple but profound statement by Benjamin Franklin, “An ounce of prevention is worth a pound of cure.” That folksy adage rings true with respect to postoperative infection because this complication extends hospital stay, increases hospital expense, and causes considerable discomfort and inconvenience for the patient. Therefore, we would do well to prevent as many instances of postoperative infection as possible.
Endometritis
On the basis of well-designed, prospective, randomized trials (Level 1 evidence), 3 interventions have proven effective in reducing the frequency of postcesarean endometritis. The first is irrigation of the vaginal canal preoperatively with an iodophor solution.5,6 The second is preoperative administration of systemic antibiotics.7-9 The combination of cefazolin (2 g IV within 30 minutes of incision) plus azithromycin (500 mg IV over 1 hour prior to incision) is superior to cefazolin alone.10,11 The third important preventive measure is removing the placenta by traction on the umbilical cord rather than by manual extraction.12,13
Wound infection
Several interventions are of proven effectiveness in reducing the frequency of postcesarean wound (surgical site) infection. The first is removal of hair at the incision site by clipping rather than by shaving (Level 2 evidence).14 The second is cleansing of the skin with chlorhexidine rather than iodophor (Level 1 evidence).15 The third is closing of the deep subcutaneous layer of the incision if it exceeds 2 cm in depth (Level 1 evidence).16,17 The fourth is closure of the skin with subcutaneous sutures rather than staples (Level 1 evidence).18 The monofilament suture poliglecaprone 25 is superior to the multifilament suture polyglactin 910 for this purpose (Level 1 evidence).19 Finally, in obese patients (body mass index >30 kg/m2), application of a negative pressure wound vacuum dressing may offer additional protection against infection (Level 1 evidence).20 Such dressings are too expensive, however, to be used routinely in all patients.
Urinary tract infection
The most important measures for preventing postoperative UTIs are identifying and clearing asymptomatic bacteriuria prior to delivery, inserting the urinary catheter prior to surgery using strict sterile technique, and removing the catheter as soon as possible after surgery, ideally within 12 hours.1,4
CASE Resolved
The 2 most likely causes for this patient’s poor response to initial therapy are resistant microorganism and wound infection. If a wound infection can be excluded by physical examination, the patient’s antibiotic regimen should be changed to metronidazole plus ampicillin plus gentamicin (or aztreonam). If an incisional abscess is identified, the incision should be opened and drained, and vancomycin should be added to the treatment regimen. If a wound cellulitis is evident, the incision should not be opened, but vancomycin should be added to the treatment regimen to enhance coverage against aerobic Streptococcus and Staphylococcus species. ●
CASE Woman who has undergone recent cesarean delivery
A 23-year-old woman had a primary cesarean delivery 72 hours ago due to an arrest of dilation at 6 cm. She was in labor for 22 hours, and her membranes were ruptured for 18 hours. She had 10 internal vaginal examinations, and the duration of internal fetal monitoring was 12 hours; 24 hours after delivery, she developed a fever of 39°C, in association with lower abdominal pain and tenderness. She was presumptively treated for endometritis with cefepime; 48 hours after the initiation of antibiotics, she remains febrile and symptomatic.
- What are the most likely causes of her persistent fever?
- What should be the next steps in her evaluation?
Cesarean delivery background
Cesarean delivery is now the most common major operation performed in US hospitals. Cesarean delivery rates hover between 25% and 30% in most medical centers in the United States.1 The most common postoperative complication of cesarean delivery is infection. Infection typically takes 1 of 3 forms: endometritis (organ space infection), wound infection (surgical site infection), and urinary tract infection (UTI).1 This article will review the initial differential diagnosis, evaluation, and management of the patient with a postoperative fever and also will describe the appropriate assessment and treatment of the patient who has a persistent postoperative fever despite therapy. The article will also highlight key interventions that help to prevent postoperative infections.
Initial evaluation of the febrile patient
In the first 24 to 48 hours after cesarean delivery, the most common cause of fever is endometritis (organ space infection). This condition is a polymicrobial, mixed aerobic-anaerobic infection (FIGURE). The principal pathogens include anaerobic gram-positive cocci (

The major risk factors for postcesarean endometritis are extended duration of labor and ruptured membranes, multiple internal vaginal examinations, invasive fetal monitoring, and pre-existing colonization with group B Streptococcus and/or the organisms that cause bacterial vaginosis. Affected patients typically have a fever in the range of 38 to 39°C, tachycardia, mild tachypnea, lower abdominal pain and tenderness, and purulent lochia in some individuals.1
Differential for postoperative fever
The initial differential diagnosis of postoperative fever is relatively limited (TABLE 1). In addition to endometritis, it includes extensive atelectasis, perhaps resulting from general anesthesia; lower respiratory tract infection, either viral influenza or bacterial pneumonia; and acute pyelonephritis. A simple infection of the bladder (cystitis or asymptomatic bacteriuria) should not cause a substantial temperature elevation and systemic symptoms.1

Differentiation between these entities usually is possible based on physical examination and a few laboratory tests. The peripheral white blood cell count usually is elevated, and a left shift may be evident. If a respiratory tract infection is suspected, chest radiography is indicated. A urine culture should be obtained if acute pyelonephritis strongly is considered. Lower genital tract cultures are rarely of value, and uncontaminated upper tract cultures are difficult to obtain. I do not believe that blood cultures should be performed as a matter of routine. They are expensive, and the results are often not available until after the patient has cleared her infection and left the hospital. However, I would obtain blood cultures in patients who meet one of these criteria1,2:
- They are immunocompromised (eg, HIV infection).
- They have a cardiac or vascular prosthesis and, thus, are at increased risk of complications related to bacteremia.
- They seem critically ill at the onset of evaluation.
- They fail to respond appropriately to initial therapy.
The cornerstone of therapy is broad spectrum antibiotics that target the multiple organisms responsible for endometritis.3 There are several single agents and several combination antibiotic regimens that provide excellent coverage against the usual pelvic pathogens (TABLE 2). I personally favor the generic combination regimen (clindamycin plus gentamicin) because it is relatively inexpensive and has been very well validated in multiple studies. In patients who have underlying renal dysfunction, aztreonam can be substituted for gentamicin.

Approximately 90% of patients will show clear evidence of clinical improvement (ie, decrease in temperature and resolution of abdominopelvic pain) within 48 hours of starting antibiotics. Patients should then continue therapy until they have been afebrile and asymptomatic for approximately 24 hours. At that point, antibiotics should be discontinued, and the patient can be discharged. With rare exceptions, there is no indication for administration of oral antibiotics on an outpatient basis.1,4
Continue to: Persistent postoperative fever...
Persistent postoperative fever
Resistant microorganism
The most common cause of a persistent fever after initiating antibiotic therapy is a resistant microorganism. There are potential gaps in coverage for the antibiotic regimens commonly used to treat postcesarean endometritis (TABLE 3).1,4 Assuming there is no other obvious cause for treatment failure, I recommend that therapy be changed to the triple combination of metronidazole plus ampicillin plus gentamicin (or aztreonam). The first drug provides superb coverage against anaerobes; the second covers enterococci. Gentamicin or aztreonam cover virtually all aerobic Gram-negative bacilli likely to cause postcesarean infection. I prefer metronidazole rather than clindamycin in this regimen because, unlike clindamycin, it is less likely to trigger diarrhea when used in combination with ampicillin. The 3-drug regimen should be continued until the patient has been afebrile and asymptomatic for approximately 24 hours.1,3,4

Wound infection
The second most common reason for a poor response to initial antibiotic therapy is a wound (surgical site) infection. Wound infections are caused by many of the same pelvic pathogens responsible for endometritis combined with skin flora, notably Streptococcus and Staphylococcus species, including methicillin-resistant Staphylococcus aureus (MRSA).1,4
Wound infections typically take one of two forms. The first is an actual incisional abscess. The patient is febrile; the margins of the wound are warm, indurated, erythematous, and tender; and purulent material drains from the incision. In this situation, the wound should be opened widely to drain the purulent collection. The fascia should then be probed to be certain that dehiscence has not occurred. In addition, intravenous vancomycin (1 g every 12 h) should be included in the antibiotic regimen to ensure adequate coverage of hospital-acquired MRSA.1,4
The second common presentation of a wound infection is cellulitis. The patient is febrile, and there is a spreading area of erythema, warmth, and exquisite tenderness extending from the edges of the incision; however, no purulent drainage is apparent. In this second scenario, the wound should not be opened, but intravenous vancomycin should be added to the treatment regimen.1,3,4
A third and very rare form of wound infection is necrotizing fasciitis. In affected patients, the margins of the wound are darkened and necrotic rather than erythematous and indurated. Two other key physical findings are crepitance and loss of sensation along the margins of the wound. Necrotizing fasciitis is truly a life-threatening emergency and requires immediate and extensive debridement of the devitalized tissue, combined with broad spectrum therapy with antibiotics that provide excellent coverage against anaerobes, aerobic streptococci (particularly group A streptococci), and staphylococci. The requirement for debridement may be so extensive that a skin graft subsequently is necessary to close the defect.1,4
Continue to: Unusual causes of persistent postoperative fever...
Unusual causes of persistent postoperative fever
If a resistant microorganism and wound infection can be excluded, the clinician then must begin a diligent search for “zebras” (ie, uncommon but potentially serious causes of persistent fever).1,4 One possible cause is a pelvic abscess. These purulent collections typically form in the retrovesicle space as a result of infection of a hematoma that formed between the posterior bladder wall and the lower uterine segment, in the leaves of the broad ligament, or in the posterior cul-de-sac. The abscess may or may not be palpable. The patient’s peripheral white blood cell count usually is elevated, with a preponderance of neutrophils. The best imaging test for an abscess is a computed tomography (CT) scan. Abscesses require drainage, which usually can be accomplished by insertion of a percutaneous drain under ultrasonographic or CT guidance.
A second unusual cause of persistent fever is septic pelvic vein thrombophlebitis. The infected venous emboli usually are present in the ovarian veins, with the right side predominant. The patient’s peripheral white blood cell count usually is elevated, and the infected clots are best imaged by CT scan with contrast or magnetic resonance angiography. The appropriate treatment is continuation of broad-spectrum antibiotics and administration of therapeutic doses of parenteral anticoagulants such as enoxaparin or unfractionated heparin.
A third explanation for persistent fever is retained products of conception. This diagnosis is best made by ultrasonography. The placental fragments should be removed by sharp curettage.
A fourth consideration when evaluating the patient with persistent fever is an allergic drug reaction. In most instances, the increase in the patient’s temperature will correspond with administration of the offending antibiotic(s). Affected patients typically have an increased number of eosinophils in their peripheral white blood cell count. The appropriate management of drug fever is discontinuation of antibiotics.
A final and distinctly unusual consideration is recrudescence of a connective tissue disorder such as systemic lupus erythematosus. The best test to confirm this diagnosis is the serum complement assay, which will demonstrate a decreased serum concentration of complement, reflecting consumption of this serum protein during the inflammatory process. The correct management for this condition is administration of a short course of systemic glucocorticoids. TABLE 4 summarizes a simple, systematic plan for evaluation of the patient with a persistent postoperative fever.

Preventive measures
We all remember the simple but profound statement by Benjamin Franklin, “An ounce of prevention is worth a pound of cure.” That folksy adage rings true with respect to postoperative infection because this complication extends hospital stay, increases hospital expense, and causes considerable discomfort and inconvenience for the patient. Therefore, we would do well to prevent as many instances of postoperative infection as possible.
Endometritis
On the basis of well-designed, prospective, randomized trials (Level 1 evidence), 3 interventions have proven effective in reducing the frequency of postcesarean endometritis. The first is irrigation of the vaginal canal preoperatively with an iodophor solution.5,6 The second is preoperative administration of systemic antibiotics.7-9 The combination of cefazolin (2 g IV within 30 minutes of incision) plus azithromycin (500 mg IV over 1 hour prior to incision) is superior to cefazolin alone.10,11 The third important preventive measure is removing the placenta by traction on the umbilical cord rather than by manual extraction.12,13
Wound infection
Several interventions are of proven effectiveness in reducing the frequency of postcesarean wound (surgical site) infection. The first is removal of hair at the incision site by clipping rather than by shaving (Level 2 evidence).14 The second is cleansing of the skin with chlorhexidine rather than iodophor (Level 1 evidence).15 The third is closing of the deep subcutaneous layer of the incision if it exceeds 2 cm in depth (Level 1 evidence).16,17 The fourth is closure of the skin with subcutaneous sutures rather than staples (Level 1 evidence).18 The monofilament suture poliglecaprone 25 is superior to the multifilament suture polyglactin 910 for this purpose (Level 1 evidence).19 Finally, in obese patients (body mass index >30 kg/m2), application of a negative pressure wound vacuum dressing may offer additional protection against infection (Level 1 evidence).20 Such dressings are too expensive, however, to be used routinely in all patients.
Urinary tract infection
The most important measures for preventing postoperative UTIs are identifying and clearing asymptomatic bacteriuria prior to delivery, inserting the urinary catheter prior to surgery using strict sterile technique, and removing the catheter as soon as possible after surgery, ideally within 12 hours.1,4
CASE Resolved
The 2 most likely causes for this patient’s poor response to initial therapy are resistant microorganism and wound infection. If a wound infection can be excluded by physical examination, the patient’s antibiotic regimen should be changed to metronidazole plus ampicillin plus gentamicin (or aztreonam). If an incisional abscess is identified, the incision should be opened and drained, and vancomycin should be added to the treatment regimen. If a wound cellulitis is evident, the incision should not be opened, but vancomycin should be added to the treatment regimen to enhance coverage against aerobic Streptococcus and Staphylococcus species. ●
- Duff WP. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2020:1124-1146.
- Locksmith GJ, Duff P. Assessment of the value of routine blood cultures in the evaluation and treatment of patients with chorioamnionitis. Infect Dis Obstet Gynecol. 1994;2:111-114.
- Duff P. Antibiotic selection in obstetric patients. Infect Dis Clin N Am. 1997;11:1-12.
- Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams, JD, et al, eds. Creasy & Resnik’s Maternal Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Syst Rev. 2014;12:CD007892.
- Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery. a systematic review and meta-analysis. Obstet Gynecol. 2017;130:527-538.
- Sullivan SA, Smith T, Change E, et al. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity; a randomized controlled trial. Am J Obstet Gynecol. 2007;196:455.e1-455.e5.
- Tita ATN, Hauth JC, Grimes A, et al. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111:51-56.
- Tita ATN, Owen J, Stamm AM, et al. Impact of extended-spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199: 303.e1-303.e3.
- Tita ATN, Szchowski JM, Boggess K, et al. Two antibiotics before cesarean delivery reduce infection rates further than one agent. N Engl J Med. 2016;375:1231-1241.
- Harper LM, Kilgore M, Szychowski JM, et al. Economic evaluation of adjunctive azithromycin prophylaxis for cesarean delivery. Obstet Gynecol. 2017;130:328-334.
- Lasley DS, Eblen A, Yancey MK, et al. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176:1250-1254.
- Anorlu RI, Maholwana B, Hofmeyr GJ. Methods of delivering the placenta at cesarean section. Cochrane Database Syst Rev. 2008;3:CD004737.
- Cruse PJ, Foord R. A five-year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107:206-209.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374:657-665.
- Del Valle GO, Combs P, Qualls C, et al. Does closure of camper fascia reduce the incidence of post-cesarean superficial wound disruption? Obstet Gynecol. 1992;80:1013-1016.
- Chelmow D. Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: a meta-analysis. Obstet Gynecol. 2004;103:974-980.
- Tuuli MG, Rampersod RM, Carbone JF, et al. Staples compared with subcuticular suture for skin closure after cesarean delivery. a systematic review and meta-analysis. Obstet Gynecol. 2011;117:682-690.
- Buresch AM, Arsdale AV, Ferzli M, et al. Comparison of subcuticular suture type for skin closure after cesarean delivery. a randomized controlled trial. Obstet Gynecol. 2017;130:521-526.
- Yu L, Kronen RJ, Simon LE, et al. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218:200-210.
- Duff WP. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2020:1124-1146.
- Locksmith GJ, Duff P. Assessment of the value of routine blood cultures in the evaluation and treatment of patients with chorioamnionitis. Infect Dis Obstet Gynecol. 1994;2:111-114.
- Duff P. Antibiotic selection in obstetric patients. Infect Dis Clin N Am. 1997;11:1-12.
- Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams, JD, et al, eds. Creasy & Resnik’s Maternal Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Syst Rev. 2014;12:CD007892.
- Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery. a systematic review and meta-analysis. Obstet Gynecol. 2017;130:527-538.
- Sullivan SA, Smith T, Change E, et al. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity; a randomized controlled trial. Am J Obstet Gynecol. 2007;196:455.e1-455.e5.
- Tita ATN, Hauth JC, Grimes A, et al. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111:51-56.
- Tita ATN, Owen J, Stamm AM, et al. Impact of extended-spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199: 303.e1-303.e3.
- Tita ATN, Szchowski JM, Boggess K, et al. Two antibiotics before cesarean delivery reduce infection rates further than one agent. N Engl J Med. 2016;375:1231-1241.
- Harper LM, Kilgore M, Szychowski JM, et al. Economic evaluation of adjunctive azithromycin prophylaxis for cesarean delivery. Obstet Gynecol. 2017;130:328-334.
- Lasley DS, Eblen A, Yancey MK, et al. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176:1250-1254.
- Anorlu RI, Maholwana B, Hofmeyr GJ. Methods of delivering the placenta at cesarean section. Cochrane Database Syst Rev. 2008;3:CD004737.
- Cruse PJ, Foord R. A five-year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107:206-209.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374:657-665.
- Del Valle GO, Combs P, Qualls C, et al. Does closure of camper fascia reduce the incidence of post-cesarean superficial wound disruption? Obstet Gynecol. 1992;80:1013-1016.
- Chelmow D. Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: a meta-analysis. Obstet Gynecol. 2004;103:974-980.
- Tuuli MG, Rampersod RM, Carbone JF, et al. Staples compared with subcuticular suture for skin closure after cesarean delivery. a systematic review and meta-analysis. Obstet Gynecol. 2011;117:682-690.
- Buresch AM, Arsdale AV, Ferzli M, et al. Comparison of subcuticular suture type for skin closure after cesarean delivery. a randomized controlled trial. Obstet Gynecol. 2017;130:521-526.
- Yu L, Kronen RJ, Simon LE, et al. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218:200-210.
Does prophylactic manual rotation of OP and OT positions in early second stage of labor decrease operative vaginal and/or CDs?
Blanc J, Castel P, Mauviel F, et al. Prophylactic manual rotation of occiput posterior and transverse positions to decrease operative delivery: the PROPOP randomized clinical trial. Am J Obstet Gynecol. 2021;225:444.e1-444.e8. doi: 10.1016/j.ajog.2021.05.020.
EXPERT COMMENTARY
Occiput posterior or occiput transverse positions are reported at a rate of 20% in labor, with 5% persistent at the time of delivery. These lead to a higher risk of maternal complications, such as cesarean delivery (CD), prolonged second stage, severe perineal lacerations, postpartum hemorrhage, chorioamnionitis, and operative vaginal delivery.
Several options are available for rotation to occiput anterior (OA) to increase the likelihood of spontaneous delivery. These include instrument (which requires forceps or vacuum experience in rotation), maternal positioning changes, or manual rotation. Timing of manual rotation can be at full dilation (“prophylactic”) or at failure to progress (“therapeutic”), with the latter less likely to succeed.
Although the existing literature is somewhat limited, both the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine recommend consideration of manual rotation to reduce the rate of operative delivery. A recent study by Blanc and colleagues sought to add to the evidence for the effectiveness of manual rotation in reducing operative delivery.
Details of the study
The multicenter, open-label, randomized clinical trial included 257 patients at 4 French hospitals (2 academic, 2 community). The 126 patients in the intervention group underwent a trial of prophylactic manual rotation, while the 131 in the standard group had no trial of prophylactic manual rotation. The study’s primary objective was to determine the effect of prophylactic manual rotation on operative delivery (vaginal or cesarean). The hypothesis was that manual rotation would decrease the risk of operative delivery.
The inclusion criteria were patients with a singleton pregnancy at more than 37 weeks, epidural anesthesia, and OP or OT presentation (confirmed by ultrasonography) in the early second stage of labor at diagnosis of full dilation. Manual rotation was attempted using the previously described Tarnier and Chantreiul technique, and all investigators were trained in this technique at the beginning of the study using a mannequin.
The primary outcome was vaginal or cesarean operative delivery. Secondary outcomes included length of the second stage of labor as well as maternal and neonatal complications.
Results. The intervention group had a significantly lower rate of operative delivery (29.4%) compared with the standard group (41.2%). Length of the second stage was also lower in the intervention group (146.7 minutes) compared with that of the standard group (164.4 minutes). The 5-minute Apgar score was reported as significantly higher in the intervention group as well (9.8 vs 9.6). There were no other differences between the groups in either maternal or neonatal complications.
Study strengths and limitations
The strengths of this study included randomization and no loss to follow-up. The 4 different study sites with different levels of care and acuity added to the generalizability of the results. Given the potential for inaccuracy of digital exam for fetal head positioning, the use of ultrasonography for confirmation of the OP or OT position is a study strength. Additional strengths are the prestudy training in the maneuver using simulation and the high level of success in the rotations (89.7%).
The study’s main limitation is that it was not double blinded; therefore, bias in management was a possibility. Additionally, the study looked only at short-term outcomes for the delivery itself and not at the potential long-term pelvic floor outcomes. The authors reported that the study was underpowered for operative vaginal delivery and cesarean delivery separately, as well as the secondary outcomes. Other limitations were the high frequency of operative vaginal delivery, low rate of consent for the study, and lack of patient satisfaction data. ●
In this study, a trial of prophylactic manual rotation of the occiput posterior or occiput transverse presentation decreased the rate of operative delivery and reduced the length of the second stage of labor without differences in maternal or neonatal complications. Obstetrical providers should consider this strategy to resolve the OP or OT presentation prior to performing an operative vaginal delivery or cesarean delivery. Simulation training in this maneuver may be a useful adjunct for both trainees and providers unfamiliar with the procedure.
JAIMEY M. PAULI, MD
Blanc J, Castel P, Mauviel F, et al. Prophylactic manual rotation of occiput posterior and transverse positions to decrease operative delivery: the PROPOP randomized clinical trial. Am J Obstet Gynecol. 2021;225:444.e1-444.e8. doi: 10.1016/j.ajog.2021.05.020.
EXPERT COMMENTARY
Occiput posterior or occiput transverse positions are reported at a rate of 20% in labor, with 5% persistent at the time of delivery. These lead to a higher risk of maternal complications, such as cesarean delivery (CD), prolonged second stage, severe perineal lacerations, postpartum hemorrhage, chorioamnionitis, and operative vaginal delivery.
Several options are available for rotation to occiput anterior (OA) to increase the likelihood of spontaneous delivery. These include instrument (which requires forceps or vacuum experience in rotation), maternal positioning changes, or manual rotation. Timing of manual rotation can be at full dilation (“prophylactic”) or at failure to progress (“therapeutic”), with the latter less likely to succeed.
Although the existing literature is somewhat limited, both the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine recommend consideration of manual rotation to reduce the rate of operative delivery. A recent study by Blanc and colleagues sought to add to the evidence for the effectiveness of manual rotation in reducing operative delivery.
Details of the study
The multicenter, open-label, randomized clinical trial included 257 patients at 4 French hospitals (2 academic, 2 community). The 126 patients in the intervention group underwent a trial of prophylactic manual rotation, while the 131 in the standard group had no trial of prophylactic manual rotation. The study’s primary objective was to determine the effect of prophylactic manual rotation on operative delivery (vaginal or cesarean). The hypothesis was that manual rotation would decrease the risk of operative delivery.
The inclusion criteria were patients with a singleton pregnancy at more than 37 weeks, epidural anesthesia, and OP or OT presentation (confirmed by ultrasonography) in the early second stage of labor at diagnosis of full dilation. Manual rotation was attempted using the previously described Tarnier and Chantreiul technique, and all investigators were trained in this technique at the beginning of the study using a mannequin.
The primary outcome was vaginal or cesarean operative delivery. Secondary outcomes included length of the second stage of labor as well as maternal and neonatal complications.
Results. The intervention group had a significantly lower rate of operative delivery (29.4%) compared with the standard group (41.2%). Length of the second stage was also lower in the intervention group (146.7 minutes) compared with that of the standard group (164.4 minutes). The 5-minute Apgar score was reported as significantly higher in the intervention group as well (9.8 vs 9.6). There were no other differences between the groups in either maternal or neonatal complications.
Study strengths and limitations
The strengths of this study included randomization and no loss to follow-up. The 4 different study sites with different levels of care and acuity added to the generalizability of the results. Given the potential for inaccuracy of digital exam for fetal head positioning, the use of ultrasonography for confirmation of the OP or OT position is a study strength. Additional strengths are the prestudy training in the maneuver using simulation and the high level of success in the rotations (89.7%).
The study’s main limitation is that it was not double blinded; therefore, bias in management was a possibility. Additionally, the study looked only at short-term outcomes for the delivery itself and not at the potential long-term pelvic floor outcomes. The authors reported that the study was underpowered for operative vaginal delivery and cesarean delivery separately, as well as the secondary outcomes. Other limitations were the high frequency of operative vaginal delivery, low rate of consent for the study, and lack of patient satisfaction data. ●
In this study, a trial of prophylactic manual rotation of the occiput posterior or occiput transverse presentation decreased the rate of operative delivery and reduced the length of the second stage of labor without differences in maternal or neonatal complications. Obstetrical providers should consider this strategy to resolve the OP or OT presentation prior to performing an operative vaginal delivery or cesarean delivery. Simulation training in this maneuver may be a useful adjunct for both trainees and providers unfamiliar with the procedure.
JAIMEY M. PAULI, MD
Blanc J, Castel P, Mauviel F, et al. Prophylactic manual rotation of occiput posterior and transverse positions to decrease operative delivery: the PROPOP randomized clinical trial. Am J Obstet Gynecol. 2021;225:444.e1-444.e8. doi: 10.1016/j.ajog.2021.05.020.
EXPERT COMMENTARY
Occiput posterior or occiput transverse positions are reported at a rate of 20% in labor, with 5% persistent at the time of delivery. These lead to a higher risk of maternal complications, such as cesarean delivery (CD), prolonged second stage, severe perineal lacerations, postpartum hemorrhage, chorioamnionitis, and operative vaginal delivery.
Several options are available for rotation to occiput anterior (OA) to increase the likelihood of spontaneous delivery. These include instrument (which requires forceps or vacuum experience in rotation), maternal positioning changes, or manual rotation. Timing of manual rotation can be at full dilation (“prophylactic”) or at failure to progress (“therapeutic”), with the latter less likely to succeed.
Although the existing literature is somewhat limited, both the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine recommend consideration of manual rotation to reduce the rate of operative delivery. A recent study by Blanc and colleagues sought to add to the evidence for the effectiveness of manual rotation in reducing operative delivery.
Details of the study
The multicenter, open-label, randomized clinical trial included 257 patients at 4 French hospitals (2 academic, 2 community). The 126 patients in the intervention group underwent a trial of prophylactic manual rotation, while the 131 in the standard group had no trial of prophylactic manual rotation. The study’s primary objective was to determine the effect of prophylactic manual rotation on operative delivery (vaginal or cesarean). The hypothesis was that manual rotation would decrease the risk of operative delivery.
The inclusion criteria were patients with a singleton pregnancy at more than 37 weeks, epidural anesthesia, and OP or OT presentation (confirmed by ultrasonography) in the early second stage of labor at diagnosis of full dilation. Manual rotation was attempted using the previously described Tarnier and Chantreiul technique, and all investigators were trained in this technique at the beginning of the study using a mannequin.
The primary outcome was vaginal or cesarean operative delivery. Secondary outcomes included length of the second stage of labor as well as maternal and neonatal complications.
Results. The intervention group had a significantly lower rate of operative delivery (29.4%) compared with the standard group (41.2%). Length of the second stage was also lower in the intervention group (146.7 minutes) compared with that of the standard group (164.4 minutes). The 5-minute Apgar score was reported as significantly higher in the intervention group as well (9.8 vs 9.6). There were no other differences between the groups in either maternal or neonatal complications.
Study strengths and limitations
The strengths of this study included randomization and no loss to follow-up. The 4 different study sites with different levels of care and acuity added to the generalizability of the results. Given the potential for inaccuracy of digital exam for fetal head positioning, the use of ultrasonography for confirmation of the OP or OT position is a study strength. Additional strengths are the prestudy training in the maneuver using simulation and the high level of success in the rotations (89.7%).
The study’s main limitation is that it was not double blinded; therefore, bias in management was a possibility. Additionally, the study looked only at short-term outcomes for the delivery itself and not at the potential long-term pelvic floor outcomes. The authors reported that the study was underpowered for operative vaginal delivery and cesarean delivery separately, as well as the secondary outcomes. Other limitations were the high frequency of operative vaginal delivery, low rate of consent for the study, and lack of patient satisfaction data. ●
In this study, a trial of prophylactic manual rotation of the occiput posterior or occiput transverse presentation decreased the rate of operative delivery and reduced the length of the second stage of labor without differences in maternal or neonatal complications. Obstetrical providers should consider this strategy to resolve the OP or OT presentation prior to performing an operative vaginal delivery or cesarean delivery. Simulation training in this maneuver may be a useful adjunct for both trainees and providers unfamiliar with the procedure.
JAIMEY M. PAULI, MD