User login
Opioid-based therapies reduce TKA needs for OA patients, but not costs
Treatment with opioids is not cost effective in osteoarthritis patients without comorbidities, according to Savannah R. Smith and her associates.
When a 10% reduction in total knee arthroplasty (TKA) effectiveness from opioid-based therapies was assumed, tramadol therapy delayed TKA by 7 years and tramadol plus oxycodone therapy delayed TKA by 9 years. Opioid-based therapy reduced primary TKA utilization by 4% for tramadol and by 10% for tramadol plus oxycodone, and reduced revision TKA use by 23% and 39%, respectively.
While both opioid-based therapies reduced dependence on TKA, treatment was more expensive and it reduced quality of life, compared with an opioid-sparing therapy. For a 60-year-old OA patient for whom TKA was not an option, the incremental cost-effectiveness ratio for tramadol was $39,600 per quality-adjusted life-year, compared with a therapy without opioids, and the incremental cost-effectiveness ratio for tramadol plus oxycodone was $116,800 per quality-adjusted life-year.
“Given the risk of diversion and its associated cost for potent opioids, policy makers may consider limiting the use of potent opioids in knee OA patients. From a cost-effectiveness standpoint, both opioid-based strategies led to higher costs without providing additional benefits, unless patients were unwilling or unable to undergo TKA later,” the investigators noted.
Find the full study in Arthritis Care and Research (doi: 10.1002/acr.22916).
Treatment with opioids is not cost effective in osteoarthritis patients without comorbidities, according to Savannah R. Smith and her associates.
When a 10% reduction in total knee arthroplasty (TKA) effectiveness from opioid-based therapies was assumed, tramadol therapy delayed TKA by 7 years and tramadol plus oxycodone therapy delayed TKA by 9 years. Opioid-based therapy reduced primary TKA utilization by 4% for tramadol and by 10% for tramadol plus oxycodone, and reduced revision TKA use by 23% and 39%, respectively.
While both opioid-based therapies reduced dependence on TKA, treatment was more expensive and it reduced quality of life, compared with an opioid-sparing therapy. For a 60-year-old OA patient for whom TKA was not an option, the incremental cost-effectiveness ratio for tramadol was $39,600 per quality-adjusted life-year, compared with a therapy without opioids, and the incremental cost-effectiveness ratio for tramadol plus oxycodone was $116,800 per quality-adjusted life-year.
“Given the risk of diversion and its associated cost for potent opioids, policy makers may consider limiting the use of potent opioids in knee OA patients. From a cost-effectiveness standpoint, both opioid-based strategies led to higher costs without providing additional benefits, unless patients were unwilling or unable to undergo TKA later,” the investigators noted.
Find the full study in Arthritis Care and Research (doi: 10.1002/acr.22916).
Treatment with opioids is not cost effective in osteoarthritis patients without comorbidities, according to Savannah R. Smith and her associates.
When a 10% reduction in total knee arthroplasty (TKA) effectiveness from opioid-based therapies was assumed, tramadol therapy delayed TKA by 7 years and tramadol plus oxycodone therapy delayed TKA by 9 years. Opioid-based therapy reduced primary TKA utilization by 4% for tramadol and by 10% for tramadol plus oxycodone, and reduced revision TKA use by 23% and 39%, respectively.
While both opioid-based therapies reduced dependence on TKA, treatment was more expensive and it reduced quality of life, compared with an opioid-sparing therapy. For a 60-year-old OA patient for whom TKA was not an option, the incremental cost-effectiveness ratio for tramadol was $39,600 per quality-adjusted life-year, compared with a therapy without opioids, and the incremental cost-effectiveness ratio for tramadol plus oxycodone was $116,800 per quality-adjusted life-year.
“Given the risk of diversion and its associated cost for potent opioids, policy makers may consider limiting the use of potent opioids in knee OA patients. From a cost-effectiveness standpoint, both opioid-based strategies led to higher costs without providing additional benefits, unless patients were unwilling or unable to undergo TKA later,” the investigators noted.
Find the full study in Arthritis Care and Research (doi: 10.1002/acr.22916).
FROM ARTHRITIS CARE AND RESEARCH
Single-Bundle, Double-Bundle Techniques Offer Similar Outcomes in ACL Reconstruction
ORLANDO, FL—Patients who undergo anterior cruciate ligament (ACL) reconstruction with a single-bundle or a double-bundle technique demonstrate similar performance during recovery, according to research presented at the American Orthopedic Society for Sports Medicine’s Specialty Day.
Researchers studied 105 patients with ACL ranging in age from 18 to 52. A total of 87 patients were available for the 5-year follow-up and were included in the study. All patients underwent post-operative rehabilitation under the same guidelines and supervision of physical therapists. Follow-up exams included multiple subjective and objective evaluation tests, including range of motion, one-leg-hop test, square-hop test, and knee injury osteoarthritis outcome score.
Patients treated with single-bundle or double-bundle ACL reconstruction showed no significant difference in major performance tests. In addition, 89% of the single-bundle and 84% of the double-bundle groups had a negative pivot-shift test, which suggests both groups had similar knee stability and health. The study also noted that the presence of osteoarthritis in patients was similar during follow-up evaluations, regardless of the technique used during ACL surgery.
ORLANDO, FL—Patients who undergo anterior cruciate ligament (ACL) reconstruction with a single-bundle or a double-bundle technique demonstrate similar performance during recovery, according to research presented at the American Orthopedic Society for Sports Medicine’s Specialty Day.
Researchers studied 105 patients with ACL ranging in age from 18 to 52. A total of 87 patients were available for the 5-year follow-up and were included in the study. All patients underwent post-operative rehabilitation under the same guidelines and supervision of physical therapists. Follow-up exams included multiple subjective and objective evaluation tests, including range of motion, one-leg-hop test, square-hop test, and knee injury osteoarthritis outcome score.
Patients treated with single-bundle or double-bundle ACL reconstruction showed no significant difference in major performance tests. In addition, 89% of the single-bundle and 84% of the double-bundle groups had a negative pivot-shift test, which suggests both groups had similar knee stability and health. The study also noted that the presence of osteoarthritis in patients was similar during follow-up evaluations, regardless of the technique used during ACL surgery.
ORLANDO, FL—Patients who undergo anterior cruciate ligament (ACL) reconstruction with a single-bundle or a double-bundle technique demonstrate similar performance during recovery, according to research presented at the American Orthopedic Society for Sports Medicine’s Specialty Day.
Researchers studied 105 patients with ACL ranging in age from 18 to 52. A total of 87 patients were available for the 5-year follow-up and were included in the study. All patients underwent post-operative rehabilitation under the same guidelines and supervision of physical therapists. Follow-up exams included multiple subjective and objective evaluation tests, including range of motion, one-leg-hop test, square-hop test, and knee injury osteoarthritis outcome score.
Patients treated with single-bundle or double-bundle ACL reconstruction showed no significant difference in major performance tests. In addition, 89% of the single-bundle and 84% of the double-bundle groups had a negative pivot-shift test, which suggests both groups had similar knee stability and health. The study also noted that the presence of osteoarthritis in patients was similar during follow-up evaluations, regardless of the technique used during ACL surgery.
Low Back Pain: Evidence-based Diagnosis and Treatment
CE/CME No: CR-1605
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify "red flag" items in the history and physical exam that make low back pain (LBP) "complicated."
• Stratify patients into three categories: simple back pain, complicated back pain, and back pain with sciatica.
• Discuss when appropriate additional testing/imaging is needed based on LBP categories.
• Discuss patient perceptions and costs associated with imaging and LBP.
• Describe basic treatment options for noncomplicated acute LBP.
FACULTY
Mike Roscoe is the PA Program Director at the University of Evansville, Indiana. Alyssa Nishihira is in her final year of the PA program at Butler University, Indianapolis; after graduation, she will be practicing at Advanced Neurosurgery in Reno, Nevada.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of May 2016.
Article begins on next page >>
Low back pain (LBP) is one of the most common reasons for an office visit, but most cases—at least 95%—have a benign underlying cause. Evaluation of LBP patients in the primary care setting, therefore, must focus on identifying “red flags” in the history and physical exam that suggest a significant underlying process requiring further work-up, including imaging. This evidence-based approach helps control costs and prevents the detrimental effects of unnecessary testing.
Low back pain (LBP) plagues many Americans and is a common reason for office visits in the United States. In 2010, back symptoms were the principal reason for 1.3% of office visits in the US.1 Recent data suggest that 75% to 85% of all Americans will experience an episode of LBP at least once in their lifetime.2 It is the leading cause of years lived with disability in the US3 and is a common reason for work disability. From a health care system standpoint, LBP imposes a considerable burden, accounting for more than $85 billion annually in direct costs.2
The etiology of LBP can be related to several anatomic and physiologic changes. Potential origins of LBP include, but are not limited to, pathology of the vertebrospinal ligaments, musculature, facet joints, fascia, vertebra and vertebral disks, and the extensive neurovascular components of the lumbar region. Although the potential causes of LBP are many, the majority of patients presenting with acute LBP usually improve with minimal clinical intervention within the first month. This is true even for patients who report limitations in daily activities and those with severe, acute cases of LBP.
A single standard of care for patients presenting with LBP has not been established. The wide array of choices for diagnosis and treatment of LBP is one factor that hinders the development of a standard diagnostic protocol. The challenge to clinicians when diagnosing LBP is to differentiate the patients with benign, self-limiting LBP (simple), who comprise the vast majority of LBP patients, from the 1% to 5% with a serious underlying pathology (complicated).4
Continue for stratification of low back pain >>
STRATIFICATION OF LOW BACK PAIN
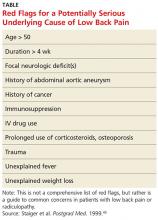
Koes and colleagues analyzed 13 different national guidelines and two international guidelines for the management of LBP.5 They found that the guidelines consistently recommend focusing the history and physical exam (HPE) on identifying features suggestive of underlying serious pathology, or “red flags,” and excluding specific diseases.5 They also found that none of the guidelines recommends the routine use of imaging in patients without suspected serious pathology.5 The American College of Radiology simplified this approach to patients with LBP by creating a list of red flags to look for during the HPE.3 The presence of red flags indicates a case of complicated LBP, and patients who present with them should undergo additional diagnostic studies to screen for serious underlying conditions (see the Table).
The HPE should ultimately separate patients into three categories to determine the need for imaging (and course of treatment): (1) simple acute back pain, (2) complicated back pain with red flag (ie, a potential underlying systemic disease), and (3) LBP with neurologic deficits potentially requiring surgery.5
Simple acute low back pain
Up to 85% of patients presenting with LBP may never receive a definitive diagnosis due to lack of specific symptoms and ambiguous imaging results.6 Clinicians can assume that LBP in these patients is due to a mechanical cause, by far the most common cause of LBP.7 It is therefore more useful to rule out serious or potentially fatal causes of LBP (complicated LBP) rather than rule in a cause for patients presenting with LBP.
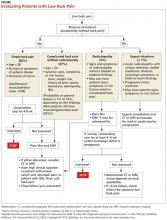
It is generally accepted among practitioners that a thorough HPE alone is sufficient for evaluating most patients presenting with acute LBP lasting less than four weeks.5 Patients presenting without red flags should be assured that improvement of acute LBP is typical, and that no diagnostic intervention is needed unless they do not improve as expected per patient or provider (eg, in terms of activities of daily living or work restrictions). The Figure depicts an appropriate approach to diagnosis and treatment in patients presenting with LBP.8 Clinicians should also offer patient education for self-care and discuss noninvasive treatment options, including pharmacologic and nonpharmacologic therapy.9
Low back pain with red flags (complicated)
Patient history is more useful than the physical exam in screening for spinal malignancies. In one particular combination (age > 50, history of cancer, unexplained weight loss, and failure to improve with conservative therapy), red flag symptoms are 100% sensitive for detecting malignancy.10 However, malignant neoplasms of the spine make up less than 1% of the diagnoses of patients presenting with LBP in primary care.4 Additionally, Deyo and Diehl reviewed five studies of a large series of consecutive spine films with large sample sizes and found the incidence of tumors, infections, and inflammatory spondyloarthropathies together were present in less than 2%.11 This low prevalence underscores the challenge of diagnosing serious pathology of the spine in the primary care setting.
Patients with complicated back pain presenting with red flags should always be examined for an underlying systemic disease. There is one red flag that, seen in isolation, meaningfully increases the likelihood of cancer: a previous history of cancer.4 Otherwise, inflammatory markers (eg, erythrocyte sedimentation rate) can be used to determine the need for advanced imaging (see the Figure).10
Low back pain with neurologic findings (sciatica)
Screening (HPE) for neurologic damage is difficult because traditional findings of neurologic injury (paresis or muscle weakness, impaired reflexes, sensory deficits, and decreased range of motion) all have low sensitivity with higher specificity.12 For this reason, these tests are of limited value as screening tools during the HPE. Specific exams, such as the straight leg raise and crossed straight leg tests, are also of limited value, especially in the primary care setting, because of inconsistent sensitivity and specificity.
This is the primary reason that the HPE in patients with LBP who have neurologic findings must include evaluation for urgent findings (see the Figure). If any red flags are present, advanced imaging is immediately warranted. Otherwise, inflammatory markers and plain radiography may be obtained, and advanced imaging may be considered if the plain radiography and/or inflammatory markers are abnormal.
There is also an approach that advocates the use of advanced imaging in patients with significant functional disability due to their LBP. Two questionnaires, the Oswestry Low Back Pain Disability Index and the Roland-Morris Disability Questionnaire, evaluate subjective data to determine a patient’s functional disability due to LBP.The validity of both tests has been confirmed.13
Continue for diagnostic imaging >>
DIAGNOSTIC IMAGING
The majority of patients presenting with LBP without concerning symptoms can be assumed to have nonspecific mechanical back pain. These patients do not need radiography unless the pain has not improved after four to six weeks of conservative care, because plain radiographs often detect findings (degenerative joint disease, bone spurs, spondylosis) that are unrelated to symptoms.9 Advanced imaging is generally recommended only for LBP patients with red flags due to the potentially critical nature of these cases.5 Patients with LBP presenting with any of these factors require further testing, even if the duration of their pain is less than four weeks.
If a patient’s LBP persists beyond four weeks, the clinician must decide which diagnostic test to order. General medical knowledge suggests that MRI is superior to plain radiography because it shows soft tissue and can detect more concerning abnormalities, such as infections, cancer, and metastatic tumors. CT is better for showing bony abnormalities, but these rarely correlate with a patient’s LBP, and CT subjects patients to levels of radiation that can increase cancer risks.14 Plain radiography in this cohort (LBP > 4 wk) is not generally recommended as it cannot show intervertebral discs or evaluate the degree of spinal stenosis as accurately as MRI. Additionally, these lumbar radiographs expose patients to more than 35 times the radiation delivered in a single chest radiograph.15
COSTS AND PATIENT OUTCOMES
The estimated cost of unnecessary imaging for LBP is $300 million per year.16 There is evidence of a strong association between advanced lumbar spine imaging and increased rates of surgery and significantly higher total medical expenditures.17,18 One study examined patients with nonspecific LBP who either received MRI within 30 days post-onset (defined as “early MRI”) or did not receive MRI. Early-MRI patients had significantly higher total medical expenses ($12,948, P < .0001) than the no-MRI group.17 The early-MRI group also had significantly longer periods of disability and were less likely to go off disability than the no-MRI group (P < .0001).
Cost-effectiveness studies of plain radiographs, dating back to 1982, have yielded similar findings. Liang et al suggested that if radiography was done routinely at the initial visit in patients with acute LBP but no red flags, the cost would be more than $2,000 (in 1982 dollars) to avert one day of pain.19 A more recent study examined patients with acute LBP who received MRI, with one group blinded (both patients and physicians) to their MRI results for six months while the other group received their results within 48 hours.20 All patients underwent a physical exam by a study coordinator, and treatment was assigned prior to imaging. At six weeks and one year, there was no significant difference in treatment assignments or self-reported surveys between groups, indicating that the MRI results had no significant influence on patient outcomes.
Despite the large increase in the use of advanced diagnostic imaging aimed at improving patient care and outcomes, there is a lack of data showing any correlative or causative connection between the two. Given this lack of evidence, and the potentially detrimental radiation exposure and increased costs to patients, clinicians should follow evidence-based guidelines when considering diagnostic imaging in patients presenting with LBP.
Continue for patient perception >>
PATIENT PERCEPTION
Patient satisfaction plays a very important role in health care and may correlate with compliance and other outcomes. One study showed that while radiography in patients with LBP was not associated with improved clinical outcomes, it did increase patients’ satisfaction with the care they received.21 A study that grouped patients requiring imaging for LBP into rapid MRI and plain film radiography cohorts found that patients who received rapid MRI were more assured by their results than were patients in the radiography group (74% vs 58%, P = .002).22 Both groups showed significant clinical improvement in the first three months, but there was no difference between groups at either the three- or 12-month mark. In both groups, reassurance was positively correlated with patient satisfaction (Pearson correlation coefficients, 0.55-0.59, P < .001).
Patients may be reassured by imaging, even when it is unnecessary. Effectively explaining symptomatology during the HPE to patients with LBP should be of high priority to clinicians. A study found that when patients with mechanical LBP did not receive an adequate explanation of the problem, they were less satisfied with their visit and wanted more diagnostic tests.11 Another study found that when low-risk patients were randomly assigned to a control group and received an educational intervention only, they reported equal satisfaction with their care and had clinical outcomes equal to those of the treatment group that received a plain radiograph.11
Given the costs, radiation risks, and other negative aspects of unnecessary imaging, additional diagnostic tests may not be in a patient’s best interest. A careful physical exam should be performed, with the clinician providing ongoing commentary to reassure patients that the clinician is neither dismissing the patient’s symptoms nor inappropriately avoiding further tests.
Often, medical providers order imaging with the intention to reassure patients with the results and thus ultimately increase the patient’s sense of well-being. However, the opposite effect may occur, with patients actually developing a decreased sense of wellness with no alteration of outcomes. A study evaluated general health (GH) scores (based on results from several screening questionnaires that assessed the patient’s current physical and mental health state) in patients receiving MRI results.20 The patients were divided into those who received results (within 48 hours), and those who did not unless it was critical to patient management (blinded group). At six weeks, the blinded group’s GH score was significantly higher than the early-informed group’s GH score. This suggests that receiving MRI results may negatively influence patients’ perception of their general health.20
The same meta-analysis that reviewed patient outcomes also evaluated mental health and quality-of-life scores of LBP patients who received either MRI, CT, or radiography.23 There was no short-term (< 3 mo) or long-term (6-12 mo) difference between patients who received radiography versus advanced imaging. This indicates that using imaging of any kind in patients with LBP but without indications of serious underlying conditions does not improve clinical outcomes and is negatively correlated with quality-of-life measures at short- and long-term intervals.23
Continue for treatment >>
TREATMENT
The prognosis of simple acute mechanical LBP is excellent. Although back pain is a leading reason for visiting health care providers, many affected individuals never seek medical care and apparently improve on their own. In a random telephone survey of North Carolina residents, only 39% of persons with LBP sought medical care.24 Therefore, patients who do seek treatment should be given reassurance, and therapies should be tailored to the individual in the least invasive and most cost-effective manner. Many treatment options are available for LBP, but often strong evidence of benefit is lacking.
Pharmacologic therapy
Anti-inflammatories. It can be assumed that when a patient comes to the practitioner for evaluation of LBP, there is an expectation that some type of medication will be recommended or prescribed for pain relief. Unless there is a contraindication, NSAIDs are often first-line therapy, and they are effective for short-term symptom relief when compared with placebo.25 A mild pain medication, such as acetaminophen, is also a common treatment. The 2007 joint practice guideline from the American Pain Society (APS) and the American College of Physicians (ACP) recommends acetaminophen or NSAIDs as first-line therapy for acute LBP.3 Neither agent—NSAIDs or acetaminophen—has shown superiority, and combining the two has shown no additional benefits.26 Caution must be used, however, as NSAIDs have a risk for gastrointestinal toxicity and nephrotoxicity, and acetaminophen has a dose- and patient-dependent risk for hepatotoxicity.
Muscle relaxants. Muscle relaxants are another pharmacologic treatment option for LBP. Most pain reduction from this class of medication occurs in the first one to two weeks of therapy, although benefit may continue for up to four weeks.27 There is also evidence that a combination of an NSAID and a muscle relaxer has added benefits.27 These medications are centrally acting, so sedation and dizziness are common; all medications in this class have these adverse effects to some degree. Carisoprodol has as its first metabolite meprobamate, which is a tranquilizer used to treat anxiety disorders; it has a potential for abuse and should be used with caution in certain populations.
Opioids. Opioids are commonly prescribed to patients with LBP, though there are limited data regarding efficacy. One trial compared an NSAID alone versus an NSAID plus oxycodone/acetaminophen and found no significant difference in pain or disability after seven days.28 In addition, the adverse effects of opioids, which include sedation, constipation, nausea, and confusion, may be amplified in the elderly population; therefore, opioids should be prescribed with caution in these patients. If prescribed to treat acute LBP, opioids should be used in short, scheduled dosing regimens since NSAIDs or acetaminophen suffice for most patients.
Corticosteroids. Oral glucocorticoids are sometimes given to patients with acute LBP, and they likely are used more frequently in patients with radicular symptoms. However, the APS/ACP 2007 joint guidelines recommend against use of systemic glucocorticoids for acute LBP due to lack of proven benefit.3 Epidural steroid injections are not generally beneficial for isolated acute LBP, but there is evidence that they are helpful with persistent radicular pain.29 Zarghooni and colleagues found significant reductions in pain and use of pain medication after single-shot epidural injections.29
Other pharmacologic therapies, acupuncture, sclerotherapy, and other methods are used to treat back pain, but these are typically reserved for chronic, not acute, LBP.
Nonpharmacologic therapy
Physical therapy. Physical therapy is a commonly prescribed treatment for LBP. Systematic literature reviews indicate that for patients with acute LBP (< 6 wk), there is no difference in the effectiveness of exercise therapy compared to no treatment and care provided by a general practitioner or to manipulations.30 For patients with subacute (6-12 wk) and chronic (≥ 12 wk) LBP, exercise therapy is effective compared to no treatment.30 There is debate, however, over which exercise activities should be used. Research supports strength/resistance and coordination/stabilization exercises.
Most therapists recommend the McKenzie method or spine stabilization exercises.31 The McKenzie method is used for LBP with sciatica; the patient moves through exercises within the prone position and focuses on extension of the spine. Spine stabilization is an active form of exercise based on a “neutral spine” position and helps strengthen muscles to maintain this position (core stabilization). The McKenzie method, when added to first-line care for LBP, does not produce significant improvements in pain or other clinical outcomes, although it may reduce health care utilization.32 Spine stabilization exercises have been shown to decrease pain, disability, and risk for recurrence after a first episode of back pain.33 The apparent success of physical therapy is attributed to compliance with directed home exercise programs, which have been shown to reduce the rate of recurrence, decrease episodes of acute LBP, and decrease the need for health services.34
Spinal traction. Traction or nonsurgical spinal decompression has emerged as a treatment for LBP. Unfortunately, there are little data to support its use as a treatment for acute LBP. Only a few randomized trials showed benefit, and these were small studies with a high risk for bias. A Cochrane review published in 2013 looked at 32 studies involving 2,762 patients with acute, subacute, and chronic LBP.35 The review did not find any evidence that traction alone or in combination with other therapy was any better than placebo treatment.35
Spinal manipulation. Spinal manipulation may be more effective than placebo treatment in reducing pain when the pain has been present for less than six weeks, but it is not more effective in reducing disability.36 There is little or no high-level evidence about spinal manipulation for acute LBP. However, there is some evidence of cost-effectiveness when using spinal manipulation in subacute to chronic pain.37 Chiropractic techniques are considered safe (when performed by a trained provider), but a systematic review found that these techniques provide no clinically relevant improvement in pain or disability when compared to other treatments.38
Bed rest. Bed rest has not been shown to improve outcomes, and in fact patients who had bed rest had less favorable outcomes than those who stayed active.39 Bed rest is less effective at reducing pain and improving function when compared to staying active.39
Continue for recommended management >>
Recommended management
A patient who presents with nonspecific acute LBP should have a thorough HPE to evaluate for the presence of red flags. If no concerning findings are present, the initial visit should focus on patient education based on the following items: (1) good prognosis with little intervention, (2) staying active and avoiding bed rest as much as possible, and (3) avoiding pain-causing movements when possible. The second step is to initiate a trial of an NSAID or acetaminophen and consider a muscle relaxant based on pain severity. Avoid opioid therapy if possible, but use conservative dosing if required for severe pain. Patients should be advised to return in two to four weeks if they do not experience significant improvement. At this time, the clinician may consider referring the patient for physical therapy, changing NSAIDs, ordering inflammatory markers, and/or referring to a specialist.
CONCLUSION
Although no single diagnostic protocol for LBP exists, the clinician must be able to distinguish simple from complex types. A thorough HPE is useful for categorizing the patient’s pain, with diagnostic imaging reserved for those patients with severe or progressive neurologic deficits, suspicion of serious underlying conditions, or LBP lasting more than four weeks without improvement. MRI, if available, is generally preferred over CT because it does not use ionizing radiation and provides better visualization of soft tissue, vertebral marrow, and the spinal cord. Symptomatology should be explained to patients with LBP during the HPE, with ongoing commentary to increase patient satisfaction and compliance. About two-thirds of patients with LBP do not seek evaluation from a health care provider; therefore, those who do seek treatment should be reassured, and therapies tailored to the individual in the least invasive and most cost-effective manner possible.
1. CDC. National Ambulatory Medical Care Survey: 2010 Summary Tables. Table 9. www.cdc.gov/nchs/data/ahcd/namcs_summary/2010_namcs_web_tables.pdf. Accessed March 29, 2016.
2. Davies C, Nitz AJ, Mattacola CG, et al. Practice patterns when treating patients with low back pain: a survey of physical therapists. Physiother Theor Pract. 2014;30(6):399-408.
3. American College of Radiology. ACR Appropriateness Criteria. Low back pain. 2015. www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/LowBackPain.pdf. Accessed March 10, 2016.
4. Henschke N, Maher CG, Ostelo RW, et al. Red flags to screen for malignancy in patients with low back pain. Cochrane Database Syst Rev. 2013;2:CD008686.
5. Koes BW, Tulder M, Lin CW, et al. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010;19(12):2075-2094.
6. Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA. 1992;268(6):760-765.
7. Jarvik JG. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med. 2002;137:586-597.
8. Diagnostic testing for low back pain. In: Post TW (ed), UpToDate, Waltham, MA. www.uptodate.com. Accessed March 16, 2016.
9. Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478-491.
10. Deyo RA, Diehl AK. Cancer as a cause of back pain: frequency, clinical presentation, and diagnostic strategies. J Gen Intern Med. 1988;3(3):230-238.
11. Deyo RA, Diehl AK. Patient satisfaction with medical care for low-back pain. Spine. 1986;11(1):28-30.
12. Pradeep S, Rainville J, Katz JN, et al. The accuracy of the physical examination for the diagnosis of midlumbar and low lumbar nerve root impingement. Spine. 2011;36(1):63-73.
13. Leclaire R, Blier F, Fortin L, Proulx R. A cross-sectional study comparing the Oswestry and Roland-Morris Functional Disability Scales in two populations of patients with low back pain of different levels of severity. Spine. 1997;22(1):68-71
14. FDA. Radiation emitting products. www.fda.gov/Radiation-EmittingProducts/RadiationEmittingProductsandProcedures/MedicalImaging/MedicalX-Rays/ucm115317.htm. Accessed March 29, 2016.
15. Simpson AK, Whang PG, Jonisch A, et al. The radiation exposure associated with cervical and lumbar spine radiographs. J Spinal Disord Tech. 2008;21(6):409-412.
16. Srinivas S, Deyo R, Berger Z. Application of “less is more” to lower back pain. Arch Intern Med. 2012;172(13):1016-1020.
17. Webster BS, Bauer AZ, Choi Y, et al. Iatrogenic consequences of early magnetic resonance imaging in acute, work-related, disabling back pain. Spine. 2013;38(22):1939-1946.
18. Webster BS, Bauer AZ, Choi Y, et al. The cascade of medical services and associated longitudinal costs due to nonadherent magnetic resonance imaging for low back pain. Spine. 2014;39(17):1433-1440.
19. Liang M, Komaroff AL. Roentgenograms in primary care patients with acute low back pain: a cost-effectiveness analysis. Arch Intern Med. 1982;142(6):1108-1112.
20. Ash LM, Modic MT, Obuchowski NA, et al. Effects of diagnostic information, per se, on patient outcomes in acute radiculopathy and low back pain. AJNR Am J Neuroradiol. 2008;29(6):1098-1103.
21. Kendrick D, Fielding K, Bentley E, et al. Radiography of the lumbar spine in primary care patients with low back pain: randomized controlled trial. BMJ. 2001;322(7283):400-405.
22. Jarvik JG, Hollingworth W, Martin B, et al. Rapid magnetic resonance imaging vs radiographs for patients with low back pain. JAMA. 2003;289(21):2810-2818.
23. Chou R, Fu R, Carrino JA, Deyo RA. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet. 2009;373(9662):463-472.
24. Carey TS, Evans AT, Hadler NM, et al. Acute severe low back pain: a population-based study of prevalence and care-seeking. Spine. 1996;21(3):339-344.
25. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain. Spine. 2008;33(16):1766-1774.
26. Hancock MJ, Maher CG, Latimer J, et al. Assessment of diclofenac or spinal manipulative therapy, or both, in addition to recommended first-line treatment for acute low back pain: a randomized controlled trial. Lancet. 2007;370(10):1638-1643.
27. Van Tulder MW, Touray T, Furlan AD, et al. Muscle relaxants for non-specific low-back pain. Cochrane Database Syst Rev. 2003;(4):CD004252.
28. Friedman BW, Dym AA, Davitt M, et al. Naproxen with cyclobenzaprine, oxycodone/acetaminophen, or placebo for treating acute low back pain: a randomized clinical trial. JAMA. 2015;314(15):1572-1580.
29. Zarghooni K, Rashidi A, Siewe, J, et al. Single-shot epidural injections in the management of radicular pain. Orthop Rev (Pavia). 2015;7(4):5985.
30. Smidt N, deVet HC, Bouter LM, et al. Effectiveness of exercise therapy: A best-evidence summary of systematic reviews. Aust J Physiother. 2005;51(2):71-85.
31. Casazza BA. Diagnosis and treatment of acute low back pain. Am Fam Physician. 2012;85(4):343-350.
32. Machado LA, Maher CG, Herbert RD, et al. The effectiveness of the McKenzie method in addition to first-line care for acute low back pain: a randomized controlled trial. BMC Med. 2010;8(10):1-10.
33. Cho I, Jeon C, Lee S, et al. Effects of lumbar stabilization exercise on functional disability and lumbar lordosis angle in patients with chronic low back pain. J Phys Ther Sci. 2015;27(6):1983-1985.
34. Choi BK, Verbeek JH, Tam WW, Jiang JY. Exercises for prevention of recurrences of low-back pain (review). Cochrane Database Syst Rev. 2010;(1):CD006555.
35. Wegner I, Widyahening IS, van Tulder MW, et al. Traction for low-back pain with or without sciatica (review). Cochrane Database Syst Rev. 2013;(8):CD003010.
36. Hoiriis KT, Pfleger B, McDuffie FC, et al. A randomized clinical trial comparing chiropractic adjustments to muscle relaxants for subacute low back pain. J Manipulative Physiol Ther. 2004;27(6):388-398.
37. Lin CC, Haas M, Maher CG, et al. Cost-effectiveness of guideline-endorsed treatments for low back pain: a systematic review. Eur Spine J. 2011;20:1024-1038.
38. Walker BF, French SD, Grant W, Green S. A Cochrane Review of combined chiropractic interventions for low-back pain. Spine. 2011;36(3): 230-242.
39. Dahm KT, Brurberg KG, Jamtvedt G, Hagen KB. Advice to rest in bed versus advice to stay active for acute low-back pain and sciatica. Cochrane Database Syst Rev. 2010;(6):CD007612.
40. Staiger T, Paauw D, Deyo A, Jarvik JG. Imaging studies for acute low back pain. When and when not to order them. Postgrad Med. 1999;105(4):161-162,165-166,171-172.
CE/CME No: CR-1605
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify "red flag" items in the history and physical exam that make low back pain (LBP) "complicated."
• Stratify patients into three categories: simple back pain, complicated back pain, and back pain with sciatica.
• Discuss when appropriate additional testing/imaging is needed based on LBP categories.
• Discuss patient perceptions and costs associated with imaging and LBP.
• Describe basic treatment options for noncomplicated acute LBP.
FACULTY
Mike Roscoe is the PA Program Director at the University of Evansville, Indiana. Alyssa Nishihira is in her final year of the PA program at Butler University, Indianapolis; after graduation, she will be practicing at Advanced Neurosurgery in Reno, Nevada.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of May 2016.
Article begins on next page >>
Low back pain (LBP) is one of the most common reasons for an office visit, but most cases—at least 95%—have a benign underlying cause. Evaluation of LBP patients in the primary care setting, therefore, must focus on identifying “red flags” in the history and physical exam that suggest a significant underlying process requiring further work-up, including imaging. This evidence-based approach helps control costs and prevents the detrimental effects of unnecessary testing.
Low back pain (LBP) plagues many Americans and is a common reason for office visits in the United States. In 2010, back symptoms were the principal reason for 1.3% of office visits in the US.1 Recent data suggest that 75% to 85% of all Americans will experience an episode of LBP at least once in their lifetime.2 It is the leading cause of years lived with disability in the US3 and is a common reason for work disability. From a health care system standpoint, LBP imposes a considerable burden, accounting for more than $85 billion annually in direct costs.2
The etiology of LBP can be related to several anatomic and physiologic changes. Potential origins of LBP include, but are not limited to, pathology of the vertebrospinal ligaments, musculature, facet joints, fascia, vertebra and vertebral disks, and the extensive neurovascular components of the lumbar region. Although the potential causes of LBP are many, the majority of patients presenting with acute LBP usually improve with minimal clinical intervention within the first month. This is true even for patients who report limitations in daily activities and those with severe, acute cases of LBP.
A single standard of care for patients presenting with LBP has not been established. The wide array of choices for diagnosis and treatment of LBP is one factor that hinders the development of a standard diagnostic protocol. The challenge to clinicians when diagnosing LBP is to differentiate the patients with benign, self-limiting LBP (simple), who comprise the vast majority of LBP patients, from the 1% to 5% with a serious underlying pathology (complicated).4
Continue for stratification of low back pain >>
STRATIFICATION OF LOW BACK PAIN
Koes and colleagues analyzed 13 different national guidelines and two international guidelines for the management of LBP.5 They found that the guidelines consistently recommend focusing the history and physical exam (HPE) on identifying features suggestive of underlying serious pathology, or “red flags,” and excluding specific diseases.5 They also found that none of the guidelines recommends the routine use of imaging in patients without suspected serious pathology.5 The American College of Radiology simplified this approach to patients with LBP by creating a list of red flags to look for during the HPE.3 The presence of red flags indicates a case of complicated LBP, and patients who present with them should undergo additional diagnostic studies to screen for serious underlying conditions (see the Table).
The HPE should ultimately separate patients into three categories to determine the need for imaging (and course of treatment): (1) simple acute back pain, (2) complicated back pain with red flag (ie, a potential underlying systemic disease), and (3) LBP with neurologic deficits potentially requiring surgery.5
Simple acute low back pain
Up to 85% of patients presenting with LBP may never receive a definitive diagnosis due to lack of specific symptoms and ambiguous imaging results.6 Clinicians can assume that LBP in these patients is due to a mechanical cause, by far the most common cause of LBP.7 It is therefore more useful to rule out serious or potentially fatal causes of LBP (complicated LBP) rather than rule in a cause for patients presenting with LBP.
It is generally accepted among practitioners that a thorough HPE alone is sufficient for evaluating most patients presenting with acute LBP lasting less than four weeks.5 Patients presenting without red flags should be assured that improvement of acute LBP is typical, and that no diagnostic intervention is needed unless they do not improve as expected per patient or provider (eg, in terms of activities of daily living or work restrictions). The Figure depicts an appropriate approach to diagnosis and treatment in patients presenting with LBP.8 Clinicians should also offer patient education for self-care and discuss noninvasive treatment options, including pharmacologic and nonpharmacologic therapy.9
Low back pain with red flags (complicated)
Patient history is more useful than the physical exam in screening for spinal malignancies. In one particular combination (age > 50, history of cancer, unexplained weight loss, and failure to improve with conservative therapy), red flag symptoms are 100% sensitive for detecting malignancy.10 However, malignant neoplasms of the spine make up less than 1% of the diagnoses of patients presenting with LBP in primary care.4 Additionally, Deyo and Diehl reviewed five studies of a large series of consecutive spine films with large sample sizes and found the incidence of tumors, infections, and inflammatory spondyloarthropathies together were present in less than 2%.11 This low prevalence underscores the challenge of diagnosing serious pathology of the spine in the primary care setting.
Patients with complicated back pain presenting with red flags should always be examined for an underlying systemic disease. There is one red flag that, seen in isolation, meaningfully increases the likelihood of cancer: a previous history of cancer.4 Otherwise, inflammatory markers (eg, erythrocyte sedimentation rate) can be used to determine the need for advanced imaging (see the Figure).10
Low back pain with neurologic findings (sciatica)
Screening (HPE) for neurologic damage is difficult because traditional findings of neurologic injury (paresis or muscle weakness, impaired reflexes, sensory deficits, and decreased range of motion) all have low sensitivity with higher specificity.12 For this reason, these tests are of limited value as screening tools during the HPE. Specific exams, such as the straight leg raise and crossed straight leg tests, are also of limited value, especially in the primary care setting, because of inconsistent sensitivity and specificity.
This is the primary reason that the HPE in patients with LBP who have neurologic findings must include evaluation for urgent findings (see the Figure). If any red flags are present, advanced imaging is immediately warranted. Otherwise, inflammatory markers and plain radiography may be obtained, and advanced imaging may be considered if the plain radiography and/or inflammatory markers are abnormal.
There is also an approach that advocates the use of advanced imaging in patients with significant functional disability due to their LBP. Two questionnaires, the Oswestry Low Back Pain Disability Index and the Roland-Morris Disability Questionnaire, evaluate subjective data to determine a patient’s functional disability due to LBP.The validity of both tests has been confirmed.13
Continue for diagnostic imaging >>
DIAGNOSTIC IMAGING
The majority of patients presenting with LBP without concerning symptoms can be assumed to have nonspecific mechanical back pain. These patients do not need radiography unless the pain has not improved after four to six weeks of conservative care, because plain radiographs often detect findings (degenerative joint disease, bone spurs, spondylosis) that are unrelated to symptoms.9 Advanced imaging is generally recommended only for LBP patients with red flags due to the potentially critical nature of these cases.5 Patients with LBP presenting with any of these factors require further testing, even if the duration of their pain is less than four weeks.
If a patient’s LBP persists beyond four weeks, the clinician must decide which diagnostic test to order. General medical knowledge suggests that MRI is superior to plain radiography because it shows soft tissue and can detect more concerning abnormalities, such as infections, cancer, and metastatic tumors. CT is better for showing bony abnormalities, but these rarely correlate with a patient’s LBP, and CT subjects patients to levels of radiation that can increase cancer risks.14 Plain radiography in this cohort (LBP > 4 wk) is not generally recommended as it cannot show intervertebral discs or evaluate the degree of spinal stenosis as accurately as MRI. Additionally, these lumbar radiographs expose patients to more than 35 times the radiation delivered in a single chest radiograph.15
COSTS AND PATIENT OUTCOMES
The estimated cost of unnecessary imaging for LBP is $300 million per year.16 There is evidence of a strong association between advanced lumbar spine imaging and increased rates of surgery and significantly higher total medical expenditures.17,18 One study examined patients with nonspecific LBP who either received MRI within 30 days post-onset (defined as “early MRI”) or did not receive MRI. Early-MRI patients had significantly higher total medical expenses ($12,948, P < .0001) than the no-MRI group.17 The early-MRI group also had significantly longer periods of disability and were less likely to go off disability than the no-MRI group (P < .0001).
Cost-effectiveness studies of plain radiographs, dating back to 1982, have yielded similar findings. Liang et al suggested that if radiography was done routinely at the initial visit in patients with acute LBP but no red flags, the cost would be more than $2,000 (in 1982 dollars) to avert one day of pain.19 A more recent study examined patients with acute LBP who received MRI, with one group blinded (both patients and physicians) to their MRI results for six months while the other group received their results within 48 hours.20 All patients underwent a physical exam by a study coordinator, and treatment was assigned prior to imaging. At six weeks and one year, there was no significant difference in treatment assignments or self-reported surveys between groups, indicating that the MRI results had no significant influence on patient outcomes.
Despite the large increase in the use of advanced diagnostic imaging aimed at improving patient care and outcomes, there is a lack of data showing any correlative or causative connection between the two. Given this lack of evidence, and the potentially detrimental radiation exposure and increased costs to patients, clinicians should follow evidence-based guidelines when considering diagnostic imaging in patients presenting with LBP.
Continue for patient perception >>
PATIENT PERCEPTION
Patient satisfaction plays a very important role in health care and may correlate with compliance and other outcomes. One study showed that while radiography in patients with LBP was not associated with improved clinical outcomes, it did increase patients’ satisfaction with the care they received.21 A study that grouped patients requiring imaging for LBP into rapid MRI and plain film radiography cohorts found that patients who received rapid MRI were more assured by their results than were patients in the radiography group (74% vs 58%, P = .002).22 Both groups showed significant clinical improvement in the first three months, but there was no difference between groups at either the three- or 12-month mark. In both groups, reassurance was positively correlated with patient satisfaction (Pearson correlation coefficients, 0.55-0.59, P < .001).
Patients may be reassured by imaging, even when it is unnecessary. Effectively explaining symptomatology during the HPE to patients with LBP should be of high priority to clinicians. A study found that when patients with mechanical LBP did not receive an adequate explanation of the problem, they were less satisfied with their visit and wanted more diagnostic tests.11 Another study found that when low-risk patients were randomly assigned to a control group and received an educational intervention only, they reported equal satisfaction with their care and had clinical outcomes equal to those of the treatment group that received a plain radiograph.11
Given the costs, radiation risks, and other negative aspects of unnecessary imaging, additional diagnostic tests may not be in a patient’s best interest. A careful physical exam should be performed, with the clinician providing ongoing commentary to reassure patients that the clinician is neither dismissing the patient’s symptoms nor inappropriately avoiding further tests.
Often, medical providers order imaging with the intention to reassure patients with the results and thus ultimately increase the patient’s sense of well-being. However, the opposite effect may occur, with patients actually developing a decreased sense of wellness with no alteration of outcomes. A study evaluated general health (GH) scores (based on results from several screening questionnaires that assessed the patient’s current physical and mental health state) in patients receiving MRI results.20 The patients were divided into those who received results (within 48 hours), and those who did not unless it was critical to patient management (blinded group). At six weeks, the blinded group’s GH score was significantly higher than the early-informed group’s GH score. This suggests that receiving MRI results may negatively influence patients’ perception of their general health.20
The same meta-analysis that reviewed patient outcomes also evaluated mental health and quality-of-life scores of LBP patients who received either MRI, CT, or radiography.23 There was no short-term (< 3 mo) or long-term (6-12 mo) difference between patients who received radiography versus advanced imaging. This indicates that using imaging of any kind in patients with LBP but without indications of serious underlying conditions does not improve clinical outcomes and is negatively correlated with quality-of-life measures at short- and long-term intervals.23
Continue for treatment >>
TREATMENT
The prognosis of simple acute mechanical LBP is excellent. Although back pain is a leading reason for visiting health care providers, many affected individuals never seek medical care and apparently improve on their own. In a random telephone survey of North Carolina residents, only 39% of persons with LBP sought medical care.24 Therefore, patients who do seek treatment should be given reassurance, and therapies should be tailored to the individual in the least invasive and most cost-effective manner. Many treatment options are available for LBP, but often strong evidence of benefit is lacking.
Pharmacologic therapy
Anti-inflammatories. It can be assumed that when a patient comes to the practitioner for evaluation of LBP, there is an expectation that some type of medication will be recommended or prescribed for pain relief. Unless there is a contraindication, NSAIDs are often first-line therapy, and they are effective for short-term symptom relief when compared with placebo.25 A mild pain medication, such as acetaminophen, is also a common treatment. The 2007 joint practice guideline from the American Pain Society (APS) and the American College of Physicians (ACP) recommends acetaminophen or NSAIDs as first-line therapy for acute LBP.3 Neither agent—NSAIDs or acetaminophen—has shown superiority, and combining the two has shown no additional benefits.26 Caution must be used, however, as NSAIDs have a risk for gastrointestinal toxicity and nephrotoxicity, and acetaminophen has a dose- and patient-dependent risk for hepatotoxicity.
Muscle relaxants. Muscle relaxants are another pharmacologic treatment option for LBP. Most pain reduction from this class of medication occurs in the first one to two weeks of therapy, although benefit may continue for up to four weeks.27 There is also evidence that a combination of an NSAID and a muscle relaxer has added benefits.27 These medications are centrally acting, so sedation and dizziness are common; all medications in this class have these adverse effects to some degree. Carisoprodol has as its first metabolite meprobamate, which is a tranquilizer used to treat anxiety disorders; it has a potential for abuse and should be used with caution in certain populations.
Opioids. Opioids are commonly prescribed to patients with LBP, though there are limited data regarding efficacy. One trial compared an NSAID alone versus an NSAID plus oxycodone/acetaminophen and found no significant difference in pain or disability after seven days.28 In addition, the adverse effects of opioids, which include sedation, constipation, nausea, and confusion, may be amplified in the elderly population; therefore, opioids should be prescribed with caution in these patients. If prescribed to treat acute LBP, opioids should be used in short, scheduled dosing regimens since NSAIDs or acetaminophen suffice for most patients.
Corticosteroids. Oral glucocorticoids are sometimes given to patients with acute LBP, and they likely are used more frequently in patients with radicular symptoms. However, the APS/ACP 2007 joint guidelines recommend against use of systemic glucocorticoids for acute LBP due to lack of proven benefit.3 Epidural steroid injections are not generally beneficial for isolated acute LBP, but there is evidence that they are helpful with persistent radicular pain.29 Zarghooni and colleagues found significant reductions in pain and use of pain medication after single-shot epidural injections.29
Other pharmacologic therapies, acupuncture, sclerotherapy, and other methods are used to treat back pain, but these are typically reserved for chronic, not acute, LBP.
Nonpharmacologic therapy
Physical therapy. Physical therapy is a commonly prescribed treatment for LBP. Systematic literature reviews indicate that for patients with acute LBP (< 6 wk), there is no difference in the effectiveness of exercise therapy compared to no treatment and care provided by a general practitioner or to manipulations.30 For patients with subacute (6-12 wk) and chronic (≥ 12 wk) LBP, exercise therapy is effective compared to no treatment.30 There is debate, however, over which exercise activities should be used. Research supports strength/resistance and coordination/stabilization exercises.
Most therapists recommend the McKenzie method or spine stabilization exercises.31 The McKenzie method is used for LBP with sciatica; the patient moves through exercises within the prone position and focuses on extension of the spine. Spine stabilization is an active form of exercise based on a “neutral spine” position and helps strengthen muscles to maintain this position (core stabilization). The McKenzie method, when added to first-line care for LBP, does not produce significant improvements in pain or other clinical outcomes, although it may reduce health care utilization.32 Spine stabilization exercises have been shown to decrease pain, disability, and risk for recurrence after a first episode of back pain.33 The apparent success of physical therapy is attributed to compliance with directed home exercise programs, which have been shown to reduce the rate of recurrence, decrease episodes of acute LBP, and decrease the need for health services.34
Spinal traction. Traction or nonsurgical spinal decompression has emerged as a treatment for LBP. Unfortunately, there are little data to support its use as a treatment for acute LBP. Only a few randomized trials showed benefit, and these were small studies with a high risk for bias. A Cochrane review published in 2013 looked at 32 studies involving 2,762 patients with acute, subacute, and chronic LBP.35 The review did not find any evidence that traction alone or in combination with other therapy was any better than placebo treatment.35
Spinal manipulation. Spinal manipulation may be more effective than placebo treatment in reducing pain when the pain has been present for less than six weeks, but it is not more effective in reducing disability.36 There is little or no high-level evidence about spinal manipulation for acute LBP. However, there is some evidence of cost-effectiveness when using spinal manipulation in subacute to chronic pain.37 Chiropractic techniques are considered safe (when performed by a trained provider), but a systematic review found that these techniques provide no clinically relevant improvement in pain or disability when compared to other treatments.38
Bed rest. Bed rest has not been shown to improve outcomes, and in fact patients who had bed rest had less favorable outcomes than those who stayed active.39 Bed rest is less effective at reducing pain and improving function when compared to staying active.39
Continue for recommended management >>
Recommended management
A patient who presents with nonspecific acute LBP should have a thorough HPE to evaluate for the presence of red flags. If no concerning findings are present, the initial visit should focus on patient education based on the following items: (1) good prognosis with little intervention, (2) staying active and avoiding bed rest as much as possible, and (3) avoiding pain-causing movements when possible. The second step is to initiate a trial of an NSAID or acetaminophen and consider a muscle relaxant based on pain severity. Avoid opioid therapy if possible, but use conservative dosing if required for severe pain. Patients should be advised to return in two to four weeks if they do not experience significant improvement. At this time, the clinician may consider referring the patient for physical therapy, changing NSAIDs, ordering inflammatory markers, and/or referring to a specialist.
CONCLUSION
Although no single diagnostic protocol for LBP exists, the clinician must be able to distinguish simple from complex types. A thorough HPE is useful for categorizing the patient’s pain, with diagnostic imaging reserved for those patients with severe or progressive neurologic deficits, suspicion of serious underlying conditions, or LBP lasting more than four weeks without improvement. MRI, if available, is generally preferred over CT because it does not use ionizing radiation and provides better visualization of soft tissue, vertebral marrow, and the spinal cord. Symptomatology should be explained to patients with LBP during the HPE, with ongoing commentary to increase patient satisfaction and compliance. About two-thirds of patients with LBP do not seek evaluation from a health care provider; therefore, those who do seek treatment should be reassured, and therapies tailored to the individual in the least invasive and most cost-effective manner possible.
CE/CME No: CR-1605
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify "red flag" items in the history and physical exam that make low back pain (LBP) "complicated."
• Stratify patients into three categories: simple back pain, complicated back pain, and back pain with sciatica.
• Discuss when appropriate additional testing/imaging is needed based on LBP categories.
• Discuss patient perceptions and costs associated with imaging and LBP.
• Describe basic treatment options for noncomplicated acute LBP.
FACULTY
Mike Roscoe is the PA Program Director at the University of Evansville, Indiana. Alyssa Nishihira is in her final year of the PA program at Butler University, Indianapolis; after graduation, she will be practicing at Advanced Neurosurgery in Reno, Nevada.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of May 2016.
Article begins on next page >>
Low back pain (LBP) is one of the most common reasons for an office visit, but most cases—at least 95%—have a benign underlying cause. Evaluation of LBP patients in the primary care setting, therefore, must focus on identifying “red flags” in the history and physical exam that suggest a significant underlying process requiring further work-up, including imaging. This evidence-based approach helps control costs and prevents the detrimental effects of unnecessary testing.
Low back pain (LBP) plagues many Americans and is a common reason for office visits in the United States. In 2010, back symptoms were the principal reason for 1.3% of office visits in the US.1 Recent data suggest that 75% to 85% of all Americans will experience an episode of LBP at least once in their lifetime.2 It is the leading cause of years lived with disability in the US3 and is a common reason for work disability. From a health care system standpoint, LBP imposes a considerable burden, accounting for more than $85 billion annually in direct costs.2
The etiology of LBP can be related to several anatomic and physiologic changes. Potential origins of LBP include, but are not limited to, pathology of the vertebrospinal ligaments, musculature, facet joints, fascia, vertebra and vertebral disks, and the extensive neurovascular components of the lumbar region. Although the potential causes of LBP are many, the majority of patients presenting with acute LBP usually improve with minimal clinical intervention within the first month. This is true even for patients who report limitations in daily activities and those with severe, acute cases of LBP.
A single standard of care for patients presenting with LBP has not been established. The wide array of choices for diagnosis and treatment of LBP is one factor that hinders the development of a standard diagnostic protocol. The challenge to clinicians when diagnosing LBP is to differentiate the patients with benign, self-limiting LBP (simple), who comprise the vast majority of LBP patients, from the 1% to 5% with a serious underlying pathology (complicated).4
Continue for stratification of low back pain >>
STRATIFICATION OF LOW BACK PAIN
Koes and colleagues analyzed 13 different national guidelines and two international guidelines for the management of LBP.5 They found that the guidelines consistently recommend focusing the history and physical exam (HPE) on identifying features suggestive of underlying serious pathology, or “red flags,” and excluding specific diseases.5 They also found that none of the guidelines recommends the routine use of imaging in patients without suspected serious pathology.5 The American College of Radiology simplified this approach to patients with LBP by creating a list of red flags to look for during the HPE.3 The presence of red flags indicates a case of complicated LBP, and patients who present with them should undergo additional diagnostic studies to screen for serious underlying conditions (see the Table).
The HPE should ultimately separate patients into three categories to determine the need for imaging (and course of treatment): (1) simple acute back pain, (2) complicated back pain with red flag (ie, a potential underlying systemic disease), and (3) LBP with neurologic deficits potentially requiring surgery.5
Simple acute low back pain
Up to 85% of patients presenting with LBP may never receive a definitive diagnosis due to lack of specific symptoms and ambiguous imaging results.6 Clinicians can assume that LBP in these patients is due to a mechanical cause, by far the most common cause of LBP.7 It is therefore more useful to rule out serious or potentially fatal causes of LBP (complicated LBP) rather than rule in a cause for patients presenting with LBP.
It is generally accepted among practitioners that a thorough HPE alone is sufficient for evaluating most patients presenting with acute LBP lasting less than four weeks.5 Patients presenting without red flags should be assured that improvement of acute LBP is typical, and that no diagnostic intervention is needed unless they do not improve as expected per patient or provider (eg, in terms of activities of daily living or work restrictions). The Figure depicts an appropriate approach to diagnosis and treatment in patients presenting with LBP.8 Clinicians should also offer patient education for self-care and discuss noninvasive treatment options, including pharmacologic and nonpharmacologic therapy.9
Low back pain with red flags (complicated)
Patient history is more useful than the physical exam in screening for spinal malignancies. In one particular combination (age > 50, history of cancer, unexplained weight loss, and failure to improve with conservative therapy), red flag symptoms are 100% sensitive for detecting malignancy.10 However, malignant neoplasms of the spine make up less than 1% of the diagnoses of patients presenting with LBP in primary care.4 Additionally, Deyo and Diehl reviewed five studies of a large series of consecutive spine films with large sample sizes and found the incidence of tumors, infections, and inflammatory spondyloarthropathies together were present in less than 2%.11 This low prevalence underscores the challenge of diagnosing serious pathology of the spine in the primary care setting.
Patients with complicated back pain presenting with red flags should always be examined for an underlying systemic disease. There is one red flag that, seen in isolation, meaningfully increases the likelihood of cancer: a previous history of cancer.4 Otherwise, inflammatory markers (eg, erythrocyte sedimentation rate) can be used to determine the need for advanced imaging (see the Figure).10
Low back pain with neurologic findings (sciatica)
Screening (HPE) for neurologic damage is difficult because traditional findings of neurologic injury (paresis or muscle weakness, impaired reflexes, sensory deficits, and decreased range of motion) all have low sensitivity with higher specificity.12 For this reason, these tests are of limited value as screening tools during the HPE. Specific exams, such as the straight leg raise and crossed straight leg tests, are also of limited value, especially in the primary care setting, because of inconsistent sensitivity and specificity.
This is the primary reason that the HPE in patients with LBP who have neurologic findings must include evaluation for urgent findings (see the Figure). If any red flags are present, advanced imaging is immediately warranted. Otherwise, inflammatory markers and plain radiography may be obtained, and advanced imaging may be considered if the plain radiography and/or inflammatory markers are abnormal.
There is also an approach that advocates the use of advanced imaging in patients with significant functional disability due to their LBP. Two questionnaires, the Oswestry Low Back Pain Disability Index and the Roland-Morris Disability Questionnaire, evaluate subjective data to determine a patient’s functional disability due to LBP.The validity of both tests has been confirmed.13
Continue for diagnostic imaging >>
DIAGNOSTIC IMAGING
The majority of patients presenting with LBP without concerning symptoms can be assumed to have nonspecific mechanical back pain. These patients do not need radiography unless the pain has not improved after four to six weeks of conservative care, because plain radiographs often detect findings (degenerative joint disease, bone spurs, spondylosis) that are unrelated to symptoms.9 Advanced imaging is generally recommended only for LBP patients with red flags due to the potentially critical nature of these cases.5 Patients with LBP presenting with any of these factors require further testing, even if the duration of their pain is less than four weeks.
If a patient’s LBP persists beyond four weeks, the clinician must decide which diagnostic test to order. General medical knowledge suggests that MRI is superior to plain radiography because it shows soft tissue and can detect more concerning abnormalities, such as infections, cancer, and metastatic tumors. CT is better for showing bony abnormalities, but these rarely correlate with a patient’s LBP, and CT subjects patients to levels of radiation that can increase cancer risks.14 Plain radiography in this cohort (LBP > 4 wk) is not generally recommended as it cannot show intervertebral discs or evaluate the degree of spinal stenosis as accurately as MRI. Additionally, these lumbar radiographs expose patients to more than 35 times the radiation delivered in a single chest radiograph.15
COSTS AND PATIENT OUTCOMES
The estimated cost of unnecessary imaging for LBP is $300 million per year.16 There is evidence of a strong association between advanced lumbar spine imaging and increased rates of surgery and significantly higher total medical expenditures.17,18 One study examined patients with nonspecific LBP who either received MRI within 30 days post-onset (defined as “early MRI”) or did not receive MRI. Early-MRI patients had significantly higher total medical expenses ($12,948, P < .0001) than the no-MRI group.17 The early-MRI group also had significantly longer periods of disability and were less likely to go off disability than the no-MRI group (P < .0001).
Cost-effectiveness studies of plain radiographs, dating back to 1982, have yielded similar findings. Liang et al suggested that if radiography was done routinely at the initial visit in patients with acute LBP but no red flags, the cost would be more than $2,000 (in 1982 dollars) to avert one day of pain.19 A more recent study examined patients with acute LBP who received MRI, with one group blinded (both patients and physicians) to their MRI results for six months while the other group received their results within 48 hours.20 All patients underwent a physical exam by a study coordinator, and treatment was assigned prior to imaging. At six weeks and one year, there was no significant difference in treatment assignments or self-reported surveys between groups, indicating that the MRI results had no significant influence on patient outcomes.
Despite the large increase in the use of advanced diagnostic imaging aimed at improving patient care and outcomes, there is a lack of data showing any correlative or causative connection between the two. Given this lack of evidence, and the potentially detrimental radiation exposure and increased costs to patients, clinicians should follow evidence-based guidelines when considering diagnostic imaging in patients presenting with LBP.
Continue for patient perception >>
PATIENT PERCEPTION
Patient satisfaction plays a very important role in health care and may correlate with compliance and other outcomes. One study showed that while radiography in patients with LBP was not associated with improved clinical outcomes, it did increase patients’ satisfaction with the care they received.21 A study that grouped patients requiring imaging for LBP into rapid MRI and plain film radiography cohorts found that patients who received rapid MRI were more assured by their results than were patients in the radiography group (74% vs 58%, P = .002).22 Both groups showed significant clinical improvement in the first three months, but there was no difference between groups at either the three- or 12-month mark. In both groups, reassurance was positively correlated with patient satisfaction (Pearson correlation coefficients, 0.55-0.59, P < .001).
Patients may be reassured by imaging, even when it is unnecessary. Effectively explaining symptomatology during the HPE to patients with LBP should be of high priority to clinicians. A study found that when patients with mechanical LBP did not receive an adequate explanation of the problem, they were less satisfied with their visit and wanted more diagnostic tests.11 Another study found that when low-risk patients were randomly assigned to a control group and received an educational intervention only, they reported equal satisfaction with their care and had clinical outcomes equal to those of the treatment group that received a plain radiograph.11
Given the costs, radiation risks, and other negative aspects of unnecessary imaging, additional diagnostic tests may not be in a patient’s best interest. A careful physical exam should be performed, with the clinician providing ongoing commentary to reassure patients that the clinician is neither dismissing the patient’s symptoms nor inappropriately avoiding further tests.
Often, medical providers order imaging with the intention to reassure patients with the results and thus ultimately increase the patient’s sense of well-being. However, the opposite effect may occur, with patients actually developing a decreased sense of wellness with no alteration of outcomes. A study evaluated general health (GH) scores (based on results from several screening questionnaires that assessed the patient’s current physical and mental health state) in patients receiving MRI results.20 The patients were divided into those who received results (within 48 hours), and those who did not unless it was critical to patient management (blinded group). At six weeks, the blinded group’s GH score was significantly higher than the early-informed group’s GH score. This suggests that receiving MRI results may negatively influence patients’ perception of their general health.20
The same meta-analysis that reviewed patient outcomes also evaluated mental health and quality-of-life scores of LBP patients who received either MRI, CT, or radiography.23 There was no short-term (< 3 mo) or long-term (6-12 mo) difference between patients who received radiography versus advanced imaging. This indicates that using imaging of any kind in patients with LBP but without indications of serious underlying conditions does not improve clinical outcomes and is negatively correlated with quality-of-life measures at short- and long-term intervals.23
Continue for treatment >>
TREATMENT
The prognosis of simple acute mechanical LBP is excellent. Although back pain is a leading reason for visiting health care providers, many affected individuals never seek medical care and apparently improve on their own. In a random telephone survey of North Carolina residents, only 39% of persons with LBP sought medical care.24 Therefore, patients who do seek treatment should be given reassurance, and therapies should be tailored to the individual in the least invasive and most cost-effective manner. Many treatment options are available for LBP, but often strong evidence of benefit is lacking.
Pharmacologic therapy
Anti-inflammatories. It can be assumed that when a patient comes to the practitioner for evaluation of LBP, there is an expectation that some type of medication will be recommended or prescribed for pain relief. Unless there is a contraindication, NSAIDs are often first-line therapy, and they are effective for short-term symptom relief when compared with placebo.25 A mild pain medication, such as acetaminophen, is also a common treatment. The 2007 joint practice guideline from the American Pain Society (APS) and the American College of Physicians (ACP) recommends acetaminophen or NSAIDs as first-line therapy for acute LBP.3 Neither agent—NSAIDs or acetaminophen—has shown superiority, and combining the two has shown no additional benefits.26 Caution must be used, however, as NSAIDs have a risk for gastrointestinal toxicity and nephrotoxicity, and acetaminophen has a dose- and patient-dependent risk for hepatotoxicity.
Muscle relaxants. Muscle relaxants are another pharmacologic treatment option for LBP. Most pain reduction from this class of medication occurs in the first one to two weeks of therapy, although benefit may continue for up to four weeks.27 There is also evidence that a combination of an NSAID and a muscle relaxer has added benefits.27 These medications are centrally acting, so sedation and dizziness are common; all medications in this class have these adverse effects to some degree. Carisoprodol has as its first metabolite meprobamate, which is a tranquilizer used to treat anxiety disorders; it has a potential for abuse and should be used with caution in certain populations.
Opioids. Opioids are commonly prescribed to patients with LBP, though there are limited data regarding efficacy. One trial compared an NSAID alone versus an NSAID plus oxycodone/acetaminophen and found no significant difference in pain or disability after seven days.28 In addition, the adverse effects of opioids, which include sedation, constipation, nausea, and confusion, may be amplified in the elderly population; therefore, opioids should be prescribed with caution in these patients. If prescribed to treat acute LBP, opioids should be used in short, scheduled dosing regimens since NSAIDs or acetaminophen suffice for most patients.
Corticosteroids. Oral glucocorticoids are sometimes given to patients with acute LBP, and they likely are used more frequently in patients with radicular symptoms. However, the APS/ACP 2007 joint guidelines recommend against use of systemic glucocorticoids for acute LBP due to lack of proven benefit.3 Epidural steroid injections are not generally beneficial for isolated acute LBP, but there is evidence that they are helpful with persistent radicular pain.29 Zarghooni and colleagues found significant reductions in pain and use of pain medication after single-shot epidural injections.29
Other pharmacologic therapies, acupuncture, sclerotherapy, and other methods are used to treat back pain, but these are typically reserved for chronic, not acute, LBP.
Nonpharmacologic therapy
Physical therapy. Physical therapy is a commonly prescribed treatment for LBP. Systematic literature reviews indicate that for patients with acute LBP (< 6 wk), there is no difference in the effectiveness of exercise therapy compared to no treatment and care provided by a general practitioner or to manipulations.30 For patients with subacute (6-12 wk) and chronic (≥ 12 wk) LBP, exercise therapy is effective compared to no treatment.30 There is debate, however, over which exercise activities should be used. Research supports strength/resistance and coordination/stabilization exercises.
Most therapists recommend the McKenzie method or spine stabilization exercises.31 The McKenzie method is used for LBP with sciatica; the patient moves through exercises within the prone position and focuses on extension of the spine. Spine stabilization is an active form of exercise based on a “neutral spine” position and helps strengthen muscles to maintain this position (core stabilization). The McKenzie method, when added to first-line care for LBP, does not produce significant improvements in pain or other clinical outcomes, although it may reduce health care utilization.32 Spine stabilization exercises have been shown to decrease pain, disability, and risk for recurrence after a first episode of back pain.33 The apparent success of physical therapy is attributed to compliance with directed home exercise programs, which have been shown to reduce the rate of recurrence, decrease episodes of acute LBP, and decrease the need for health services.34
Spinal traction. Traction or nonsurgical spinal decompression has emerged as a treatment for LBP. Unfortunately, there are little data to support its use as a treatment for acute LBP. Only a few randomized trials showed benefit, and these were small studies with a high risk for bias. A Cochrane review published in 2013 looked at 32 studies involving 2,762 patients with acute, subacute, and chronic LBP.35 The review did not find any evidence that traction alone or in combination with other therapy was any better than placebo treatment.35
Spinal manipulation. Spinal manipulation may be more effective than placebo treatment in reducing pain when the pain has been present for less than six weeks, but it is not more effective in reducing disability.36 There is little or no high-level evidence about spinal manipulation for acute LBP. However, there is some evidence of cost-effectiveness when using spinal manipulation in subacute to chronic pain.37 Chiropractic techniques are considered safe (when performed by a trained provider), but a systematic review found that these techniques provide no clinically relevant improvement in pain or disability when compared to other treatments.38
Bed rest. Bed rest has not been shown to improve outcomes, and in fact patients who had bed rest had less favorable outcomes than those who stayed active.39 Bed rest is less effective at reducing pain and improving function when compared to staying active.39
Continue for recommended management >>
Recommended management
A patient who presents with nonspecific acute LBP should have a thorough HPE to evaluate for the presence of red flags. If no concerning findings are present, the initial visit should focus on patient education based on the following items: (1) good prognosis with little intervention, (2) staying active and avoiding bed rest as much as possible, and (3) avoiding pain-causing movements when possible. The second step is to initiate a trial of an NSAID or acetaminophen and consider a muscle relaxant based on pain severity. Avoid opioid therapy if possible, but use conservative dosing if required for severe pain. Patients should be advised to return in two to four weeks if they do not experience significant improvement. At this time, the clinician may consider referring the patient for physical therapy, changing NSAIDs, ordering inflammatory markers, and/or referring to a specialist.
CONCLUSION
Although no single diagnostic protocol for LBP exists, the clinician must be able to distinguish simple from complex types. A thorough HPE is useful for categorizing the patient’s pain, with diagnostic imaging reserved for those patients with severe or progressive neurologic deficits, suspicion of serious underlying conditions, or LBP lasting more than four weeks without improvement. MRI, if available, is generally preferred over CT because it does not use ionizing radiation and provides better visualization of soft tissue, vertebral marrow, and the spinal cord. Symptomatology should be explained to patients with LBP during the HPE, with ongoing commentary to increase patient satisfaction and compliance. About two-thirds of patients with LBP do not seek evaluation from a health care provider; therefore, those who do seek treatment should be reassured, and therapies tailored to the individual in the least invasive and most cost-effective manner possible.
1. CDC. National Ambulatory Medical Care Survey: 2010 Summary Tables. Table 9. www.cdc.gov/nchs/data/ahcd/namcs_summary/2010_namcs_web_tables.pdf. Accessed March 29, 2016.
2. Davies C, Nitz AJ, Mattacola CG, et al. Practice patterns when treating patients with low back pain: a survey of physical therapists. Physiother Theor Pract. 2014;30(6):399-408.
3. American College of Radiology. ACR Appropriateness Criteria. Low back pain. 2015. www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/LowBackPain.pdf. Accessed March 10, 2016.
4. Henschke N, Maher CG, Ostelo RW, et al. Red flags to screen for malignancy in patients with low back pain. Cochrane Database Syst Rev. 2013;2:CD008686.
5. Koes BW, Tulder M, Lin CW, et al. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010;19(12):2075-2094.
6. Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA. 1992;268(6):760-765.
7. Jarvik JG. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med. 2002;137:586-597.
8. Diagnostic testing for low back pain. In: Post TW (ed), UpToDate, Waltham, MA. www.uptodate.com. Accessed March 16, 2016.
9. Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478-491.
10. Deyo RA, Diehl AK. Cancer as a cause of back pain: frequency, clinical presentation, and diagnostic strategies. J Gen Intern Med. 1988;3(3):230-238.
11. Deyo RA, Diehl AK. Patient satisfaction with medical care for low-back pain. Spine. 1986;11(1):28-30.
12. Pradeep S, Rainville J, Katz JN, et al. The accuracy of the physical examination for the diagnosis of midlumbar and low lumbar nerve root impingement. Spine. 2011;36(1):63-73.
13. Leclaire R, Blier F, Fortin L, Proulx R. A cross-sectional study comparing the Oswestry and Roland-Morris Functional Disability Scales in two populations of patients with low back pain of different levels of severity. Spine. 1997;22(1):68-71
14. FDA. Radiation emitting products. www.fda.gov/Radiation-EmittingProducts/RadiationEmittingProductsandProcedures/MedicalImaging/MedicalX-Rays/ucm115317.htm. Accessed March 29, 2016.
15. Simpson AK, Whang PG, Jonisch A, et al. The radiation exposure associated with cervical and lumbar spine radiographs. J Spinal Disord Tech. 2008;21(6):409-412.
16. Srinivas S, Deyo R, Berger Z. Application of “less is more” to lower back pain. Arch Intern Med. 2012;172(13):1016-1020.
17. Webster BS, Bauer AZ, Choi Y, et al. Iatrogenic consequences of early magnetic resonance imaging in acute, work-related, disabling back pain. Spine. 2013;38(22):1939-1946.
18. Webster BS, Bauer AZ, Choi Y, et al. The cascade of medical services and associated longitudinal costs due to nonadherent magnetic resonance imaging for low back pain. Spine. 2014;39(17):1433-1440.
19. Liang M, Komaroff AL. Roentgenograms in primary care patients with acute low back pain: a cost-effectiveness analysis. Arch Intern Med. 1982;142(6):1108-1112.
20. Ash LM, Modic MT, Obuchowski NA, et al. Effects of diagnostic information, per se, on patient outcomes in acute radiculopathy and low back pain. AJNR Am J Neuroradiol. 2008;29(6):1098-1103.
21. Kendrick D, Fielding K, Bentley E, et al. Radiography of the lumbar spine in primary care patients with low back pain: randomized controlled trial. BMJ. 2001;322(7283):400-405.
22. Jarvik JG, Hollingworth W, Martin B, et al. Rapid magnetic resonance imaging vs radiographs for patients with low back pain. JAMA. 2003;289(21):2810-2818.
23. Chou R, Fu R, Carrino JA, Deyo RA. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet. 2009;373(9662):463-472.
24. Carey TS, Evans AT, Hadler NM, et al. Acute severe low back pain: a population-based study of prevalence and care-seeking. Spine. 1996;21(3):339-344.
25. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain. Spine. 2008;33(16):1766-1774.
26. Hancock MJ, Maher CG, Latimer J, et al. Assessment of diclofenac or spinal manipulative therapy, or both, in addition to recommended first-line treatment for acute low back pain: a randomized controlled trial. Lancet. 2007;370(10):1638-1643.
27. Van Tulder MW, Touray T, Furlan AD, et al. Muscle relaxants for non-specific low-back pain. Cochrane Database Syst Rev. 2003;(4):CD004252.
28. Friedman BW, Dym AA, Davitt M, et al. Naproxen with cyclobenzaprine, oxycodone/acetaminophen, or placebo for treating acute low back pain: a randomized clinical trial. JAMA. 2015;314(15):1572-1580.
29. Zarghooni K, Rashidi A, Siewe, J, et al. Single-shot epidural injections in the management of radicular pain. Orthop Rev (Pavia). 2015;7(4):5985.
30. Smidt N, deVet HC, Bouter LM, et al. Effectiveness of exercise therapy: A best-evidence summary of systematic reviews. Aust J Physiother. 2005;51(2):71-85.
31. Casazza BA. Diagnosis and treatment of acute low back pain. Am Fam Physician. 2012;85(4):343-350.
32. Machado LA, Maher CG, Herbert RD, et al. The effectiveness of the McKenzie method in addition to first-line care for acute low back pain: a randomized controlled trial. BMC Med. 2010;8(10):1-10.
33. Cho I, Jeon C, Lee S, et al. Effects of lumbar stabilization exercise on functional disability and lumbar lordosis angle in patients with chronic low back pain. J Phys Ther Sci. 2015;27(6):1983-1985.
34. Choi BK, Verbeek JH, Tam WW, Jiang JY. Exercises for prevention of recurrences of low-back pain (review). Cochrane Database Syst Rev. 2010;(1):CD006555.
35. Wegner I, Widyahening IS, van Tulder MW, et al. Traction for low-back pain with or without sciatica (review). Cochrane Database Syst Rev. 2013;(8):CD003010.
36. Hoiriis KT, Pfleger B, McDuffie FC, et al. A randomized clinical trial comparing chiropractic adjustments to muscle relaxants for subacute low back pain. J Manipulative Physiol Ther. 2004;27(6):388-398.
37. Lin CC, Haas M, Maher CG, et al. Cost-effectiveness of guideline-endorsed treatments for low back pain: a systematic review. Eur Spine J. 2011;20:1024-1038.
38. Walker BF, French SD, Grant W, Green S. A Cochrane Review of combined chiropractic interventions for low-back pain. Spine. 2011;36(3): 230-242.
39. Dahm KT, Brurberg KG, Jamtvedt G, Hagen KB. Advice to rest in bed versus advice to stay active for acute low-back pain and sciatica. Cochrane Database Syst Rev. 2010;(6):CD007612.
40. Staiger T, Paauw D, Deyo A, Jarvik JG. Imaging studies for acute low back pain. When and when not to order them. Postgrad Med. 1999;105(4):161-162,165-166,171-172.
1. CDC. National Ambulatory Medical Care Survey: 2010 Summary Tables. Table 9. www.cdc.gov/nchs/data/ahcd/namcs_summary/2010_namcs_web_tables.pdf. Accessed March 29, 2016.
2. Davies C, Nitz AJ, Mattacola CG, et al. Practice patterns when treating patients with low back pain: a survey of physical therapists. Physiother Theor Pract. 2014;30(6):399-408.
3. American College of Radiology. ACR Appropriateness Criteria. Low back pain. 2015. www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/LowBackPain.pdf. Accessed March 10, 2016.
4. Henschke N, Maher CG, Ostelo RW, et al. Red flags to screen for malignancy in patients with low back pain. Cochrane Database Syst Rev. 2013;2:CD008686.
5. Koes BW, Tulder M, Lin CW, et al. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010;19(12):2075-2094.
6. Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA. 1992;268(6):760-765.
7. Jarvik JG. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med. 2002;137:586-597.
8. Diagnostic testing for low back pain. In: Post TW (ed), UpToDate, Waltham, MA. www.uptodate.com. Accessed March 16, 2016.
9. Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478-491.
10. Deyo RA, Diehl AK. Cancer as a cause of back pain: frequency, clinical presentation, and diagnostic strategies. J Gen Intern Med. 1988;3(3):230-238.
11. Deyo RA, Diehl AK. Patient satisfaction with medical care for low-back pain. Spine. 1986;11(1):28-30.
12. Pradeep S, Rainville J, Katz JN, et al. The accuracy of the physical examination for the diagnosis of midlumbar and low lumbar nerve root impingement. Spine. 2011;36(1):63-73.
13. Leclaire R, Blier F, Fortin L, Proulx R. A cross-sectional study comparing the Oswestry and Roland-Morris Functional Disability Scales in two populations of patients with low back pain of different levels of severity. Spine. 1997;22(1):68-71
14. FDA. Radiation emitting products. www.fda.gov/Radiation-EmittingProducts/RadiationEmittingProductsandProcedures/MedicalImaging/MedicalX-Rays/ucm115317.htm. Accessed March 29, 2016.
15. Simpson AK, Whang PG, Jonisch A, et al. The radiation exposure associated with cervical and lumbar spine radiographs. J Spinal Disord Tech. 2008;21(6):409-412.
16. Srinivas S, Deyo R, Berger Z. Application of “less is more” to lower back pain. Arch Intern Med. 2012;172(13):1016-1020.
17. Webster BS, Bauer AZ, Choi Y, et al. Iatrogenic consequences of early magnetic resonance imaging in acute, work-related, disabling back pain. Spine. 2013;38(22):1939-1946.
18. Webster BS, Bauer AZ, Choi Y, et al. The cascade of medical services and associated longitudinal costs due to nonadherent magnetic resonance imaging for low back pain. Spine. 2014;39(17):1433-1440.
19. Liang M, Komaroff AL. Roentgenograms in primary care patients with acute low back pain: a cost-effectiveness analysis. Arch Intern Med. 1982;142(6):1108-1112.
20. Ash LM, Modic MT, Obuchowski NA, et al. Effects of diagnostic information, per se, on patient outcomes in acute radiculopathy and low back pain. AJNR Am J Neuroradiol. 2008;29(6):1098-1103.
21. Kendrick D, Fielding K, Bentley E, et al. Radiography of the lumbar spine in primary care patients with low back pain: randomized controlled trial. BMJ. 2001;322(7283):400-405.
22. Jarvik JG, Hollingworth W, Martin B, et al. Rapid magnetic resonance imaging vs radiographs for patients with low back pain. JAMA. 2003;289(21):2810-2818.
23. Chou R, Fu R, Carrino JA, Deyo RA. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet. 2009;373(9662):463-472.
24. Carey TS, Evans AT, Hadler NM, et al. Acute severe low back pain: a population-based study of prevalence and care-seeking. Spine. 1996;21(3):339-344.
25. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain. Spine. 2008;33(16):1766-1774.
26. Hancock MJ, Maher CG, Latimer J, et al. Assessment of diclofenac or spinal manipulative therapy, or both, in addition to recommended first-line treatment for acute low back pain: a randomized controlled trial. Lancet. 2007;370(10):1638-1643.
27. Van Tulder MW, Touray T, Furlan AD, et al. Muscle relaxants for non-specific low-back pain. Cochrane Database Syst Rev. 2003;(4):CD004252.
28. Friedman BW, Dym AA, Davitt M, et al. Naproxen with cyclobenzaprine, oxycodone/acetaminophen, or placebo for treating acute low back pain: a randomized clinical trial. JAMA. 2015;314(15):1572-1580.
29. Zarghooni K, Rashidi A, Siewe, J, et al. Single-shot epidural injections in the management of radicular pain. Orthop Rev (Pavia). 2015;7(4):5985.
30. Smidt N, deVet HC, Bouter LM, et al. Effectiveness of exercise therapy: A best-evidence summary of systematic reviews. Aust J Physiother. 2005;51(2):71-85.
31. Casazza BA. Diagnosis and treatment of acute low back pain. Am Fam Physician. 2012;85(4):343-350.
32. Machado LA, Maher CG, Herbert RD, et al. The effectiveness of the McKenzie method in addition to first-line care for acute low back pain: a randomized controlled trial. BMC Med. 2010;8(10):1-10.
33. Cho I, Jeon C, Lee S, et al. Effects of lumbar stabilization exercise on functional disability and lumbar lordosis angle in patients with chronic low back pain. J Phys Ther Sci. 2015;27(6):1983-1985.
34. Choi BK, Verbeek JH, Tam WW, Jiang JY. Exercises for prevention of recurrences of low-back pain (review). Cochrane Database Syst Rev. 2010;(1):CD006555.
35. Wegner I, Widyahening IS, van Tulder MW, et al. Traction for low-back pain with or without sciatica (review). Cochrane Database Syst Rev. 2013;(8):CD003010.
36. Hoiriis KT, Pfleger B, McDuffie FC, et al. A randomized clinical trial comparing chiropractic adjustments to muscle relaxants for subacute low back pain. J Manipulative Physiol Ther. 2004;27(6):388-398.
37. Lin CC, Haas M, Maher CG, et al. Cost-effectiveness of guideline-endorsed treatments for low back pain: a systematic review. Eur Spine J. 2011;20:1024-1038.
38. Walker BF, French SD, Grant W, Green S. A Cochrane Review of combined chiropractic interventions for low-back pain. Spine. 2011;36(3): 230-242.
39. Dahm KT, Brurberg KG, Jamtvedt G, Hagen KB. Advice to rest in bed versus advice to stay active for acute low-back pain and sciatica. Cochrane Database Syst Rev. 2010;(6):CD007612.
40. Staiger T, Paauw D, Deyo A, Jarvik JG. Imaging studies for acute low back pain. When and when not to order them. Postgrad Med. 1999;105(4):161-162,165-166,171-172.
Editorial Board Biographies
Thomas M. DeBerardino, MD
Associate Editor for Sports Medicine
Dr. DeBerardino is an orthopedic surgeon specializing in sports medicine and arthroscopic surgery of the knee, hip, and shoulder; and team physician for the University of Connecticut athletic teams, including football and the men’s and women’s basketball programs. He attended the United States Military Academy, West Point as an undergraduate, completed medical school at the New York Medical College, and completed his residency at Tripler Army Medical Center. He also completed a John A. Feagin, Jr, Sports Medicine Fellowship at Keller Army Hospital, West Point. Previously he was head team physician for the collegiate athletic teams at the United States Military Academy, and Director of the Sports Medicine Fellowship. He is a member of many professional societies, including the American Orthopaedic Society for Sports Medicine (AOSSM), the International ACL Study Group, and the Herodicus Society.
Patrick J. Denard, MD
Associate Editor for Shoulder
Dr. Denard is a shoulder specialist at Southern Oregon Orthopedics in Medford, OR and a clinical instructor at Oregon Health & Science University. He attended college at the University of Puget Sound. He obtained his medical degree at Dartmouth College in New Hampshire. He completed his orthopedic residency training at Oregon Health & Science University, where he received the research award as a chief resident. After residency, he completed specialized fellowship training in shoulder arthroscopy in San Antonio, TX under Dr. Stephen Burkhart. Dr. Denard completed a fellowship in shoulder replacement surgery in Lyon, France under Dr. Gilles Walch, one of the developers of the current prosthesis used in reverse shoulder replacement. He has co-authored a textbook on the shoulder, and contributed to 15 book chapters and over 60 research papers. He was recently elected to the American Shoulder and Elbow Surgeons (ASES).
Anand M. Murthi, MD
Associate Editor for Shoulder
Dr. Murthi is attending orthopedic surgeon; chief, shoulder and elbow service; and fellowship director at MedStar Union Memorial Hospital in Baltimore, MD. He received his undergraduate degree in Chemistry and Psychology and his doctorate degree from Case Western Reserve University. He completed an internship in general surgery, residency in orthopedic surgery, and was a chief resident in orthopedic surgery at George Washington University Medical Center. He also completed a fellowship in shoulder and elbow surgery at Columbia-Presbyterian Medical Center. He is a member of numerous societies, including the American Academy of Orthopaedic Surgeons (AAOS), American Medical Association (AMA), and American Shoulder and Elbow Surgeons (ASES).
Jose B. Toro, MD
Associate Editor for Trauma
Dr. Toro is director for orthopaedic trauma at Peconic Bay Medical Center. He obtained his medical degree from Pontifical Xavier University in Bogota, Colombia, where he also trained in the specialty of orthopedic surgery. Dr. Toro started his fellowship training at the Hospital for Special Surgery, where he completed a fellowship in orthopedic traumatology under the tutelage of Dr. David L. Helfet and a fellowship in metabolic bone disease under the supervision of Dr. Joseph Lane. Dr. Toro started his professional career as an attending orthopaedic surgeon at the Veterans Affairs administration of the James J. Peters Bronx VA Medical Center. He also has served as director of orthopaedic trauma and assistant professor in orthopaedic surgery at the Albert Einstein College of Medicine, Jacobi Medical Center, Bronx, New York.
Thomas M. DeBerardino, MD
Associate Editor for Sports Medicine
Dr. DeBerardino is an orthopedic surgeon specializing in sports medicine and arthroscopic surgery of the knee, hip, and shoulder; and team physician for the University of Connecticut athletic teams, including football and the men’s and women’s basketball programs. He attended the United States Military Academy, West Point as an undergraduate, completed medical school at the New York Medical College, and completed his residency at Tripler Army Medical Center. He also completed a John A. Feagin, Jr, Sports Medicine Fellowship at Keller Army Hospital, West Point. Previously he was head team physician for the collegiate athletic teams at the United States Military Academy, and Director of the Sports Medicine Fellowship. He is a member of many professional societies, including the American Orthopaedic Society for Sports Medicine (AOSSM), the International ACL Study Group, and the Herodicus Society.
Patrick J. Denard, MD
Associate Editor for Shoulder
Dr. Denard is a shoulder specialist at Southern Oregon Orthopedics in Medford, OR and a clinical instructor at Oregon Health & Science University. He attended college at the University of Puget Sound. He obtained his medical degree at Dartmouth College in New Hampshire. He completed his orthopedic residency training at Oregon Health & Science University, where he received the research award as a chief resident. After residency, he completed specialized fellowship training in shoulder arthroscopy in San Antonio, TX under Dr. Stephen Burkhart. Dr. Denard completed a fellowship in shoulder replacement surgery in Lyon, France under Dr. Gilles Walch, one of the developers of the current prosthesis used in reverse shoulder replacement. He has co-authored a textbook on the shoulder, and contributed to 15 book chapters and over 60 research papers. He was recently elected to the American Shoulder and Elbow Surgeons (ASES).
Anand M. Murthi, MD
Associate Editor for Shoulder
Dr. Murthi is attending orthopedic surgeon; chief, shoulder and elbow service; and fellowship director at MedStar Union Memorial Hospital in Baltimore, MD. He received his undergraduate degree in Chemistry and Psychology and his doctorate degree from Case Western Reserve University. He completed an internship in general surgery, residency in orthopedic surgery, and was a chief resident in orthopedic surgery at George Washington University Medical Center. He also completed a fellowship in shoulder and elbow surgery at Columbia-Presbyterian Medical Center. He is a member of numerous societies, including the American Academy of Orthopaedic Surgeons (AAOS), American Medical Association (AMA), and American Shoulder and Elbow Surgeons (ASES).
Jose B. Toro, MD
Associate Editor for Trauma
Dr. Toro is director for orthopaedic trauma at Peconic Bay Medical Center. He obtained his medical degree from Pontifical Xavier University in Bogota, Colombia, where he also trained in the specialty of orthopedic surgery. Dr. Toro started his fellowship training at the Hospital for Special Surgery, where he completed a fellowship in orthopedic traumatology under the tutelage of Dr. David L. Helfet and a fellowship in metabolic bone disease under the supervision of Dr. Joseph Lane. Dr. Toro started his professional career as an attending orthopaedic surgeon at the Veterans Affairs administration of the James J. Peters Bronx VA Medical Center. He also has served as director of orthopaedic trauma and assistant professor in orthopaedic surgery at the Albert Einstein College of Medicine, Jacobi Medical Center, Bronx, New York.
Thomas M. DeBerardino, MD
Associate Editor for Sports Medicine
Dr. DeBerardino is an orthopedic surgeon specializing in sports medicine and arthroscopic surgery of the knee, hip, and shoulder; and team physician for the University of Connecticut athletic teams, including football and the men’s and women’s basketball programs. He attended the United States Military Academy, West Point as an undergraduate, completed medical school at the New York Medical College, and completed his residency at Tripler Army Medical Center. He also completed a John A. Feagin, Jr, Sports Medicine Fellowship at Keller Army Hospital, West Point. Previously he was head team physician for the collegiate athletic teams at the United States Military Academy, and Director of the Sports Medicine Fellowship. He is a member of many professional societies, including the American Orthopaedic Society for Sports Medicine (AOSSM), the International ACL Study Group, and the Herodicus Society.
Patrick J. Denard, MD
Associate Editor for Shoulder
Dr. Denard is a shoulder specialist at Southern Oregon Orthopedics in Medford, OR and a clinical instructor at Oregon Health & Science University. He attended college at the University of Puget Sound. He obtained his medical degree at Dartmouth College in New Hampshire. He completed his orthopedic residency training at Oregon Health & Science University, where he received the research award as a chief resident. After residency, he completed specialized fellowship training in shoulder arthroscopy in San Antonio, TX under Dr. Stephen Burkhart. Dr. Denard completed a fellowship in shoulder replacement surgery in Lyon, France under Dr. Gilles Walch, one of the developers of the current prosthesis used in reverse shoulder replacement. He has co-authored a textbook on the shoulder, and contributed to 15 book chapters and over 60 research papers. He was recently elected to the American Shoulder and Elbow Surgeons (ASES).
Anand M. Murthi, MD
Associate Editor for Shoulder
Dr. Murthi is attending orthopedic surgeon; chief, shoulder and elbow service; and fellowship director at MedStar Union Memorial Hospital in Baltimore, MD. He received his undergraduate degree in Chemistry and Psychology and his doctorate degree from Case Western Reserve University. He completed an internship in general surgery, residency in orthopedic surgery, and was a chief resident in orthopedic surgery at George Washington University Medical Center. He also completed a fellowship in shoulder and elbow surgery at Columbia-Presbyterian Medical Center. He is a member of numerous societies, including the American Academy of Orthopaedic Surgeons (AAOS), American Medical Association (AMA), and American Shoulder and Elbow Surgeons (ASES).
Jose B. Toro, MD
Associate Editor for Trauma
Dr. Toro is director for orthopaedic trauma at Peconic Bay Medical Center. He obtained his medical degree from Pontifical Xavier University in Bogota, Colombia, where he also trained in the specialty of orthopedic surgery. Dr. Toro started his fellowship training at the Hospital for Special Surgery, where he completed a fellowship in orthopedic traumatology under the tutelage of Dr. David L. Helfet and a fellowship in metabolic bone disease under the supervision of Dr. Joseph Lane. Dr. Toro started his professional career as an attending orthopaedic surgeon at the Veterans Affairs administration of the James J. Peters Bronx VA Medical Center. He also has served as director of orthopaedic trauma and assistant professor in orthopaedic surgery at the Albert Einstein College of Medicine, Jacobi Medical Center, Bronx, New York.
Suture Anchor
DePuy Synthes Mitek Sports Medicine
(https://www.depuysynthes.com/hcp/mitek-sports-medicine)
Gryphon® Suture Anchor with Proknot™ Technology
Paul Favorito, MD, Wellington Orthopaedic and Sports Medicine, Cincinnati, OH
The Gryphon® suture anchor with Proknot™ technology is a doubled No. 1 Permacord® high-strength orthopedic suture with a proprietary pre-tied sliding knot. The suture construct is loaded onto a 3.0-mm Gryphonsuture anchor (Peek or Biocryl Rapide® biocomposite material) and has clinical indications for labral repair of the shoulder and hip. In a laboratory setting, Proknot technology has been tested against other high-tensile sutures and commonly tied arthroscopic knots.1 Proknot technology demonstrated higher ultimate strength, significantly less knot volume, and better reproducibility among surgeons.
Surgical pearl: I use the Gryphon Proknot suture anchor for all shoulder Bankart and superior labral anterior to posterior (SLAP) repairs. I have colleagues who also use this anchor for hip arthroscopy.
Once opened on the back table, the surgical assistant may ink the free limb of suture for easy arthroscopic identification. The anchor is placed and, in the case of hard bone frequently encountered in younger patients, a 2.5-mm drill bit may be substituted for the usual 2.4-mm. One important goal of any labral repair is to position knots away from the articular surface. The free suture limb is passed through the labrum, retrieved, and delivered through the open, pre-tied knot on the suture card.
Once the knot is released and dressed, the knot pusher is placed over the suture and the knot is advanced and preliminarily tensioned medial to the articular surface. The suture limbs are separated and one limb of the suture is removed from the knot pusher. As few as 1, or up to 3, half hitches may be placed to secure the knot, taking care to direct it away from the joint surface. The result is a strong but well-positioned knot with minimal mass securing the soft tissue.
1. Rodes SA, Favorito PJ, Piccirillo JM, Spivey JT. Performance comparison of a prettied suture knot with three conventional arthroscopic knots. Arthroscopy. 2015;31(11):2183-2190.
DePuy Synthes Mitek Sports Medicine
(https://www.depuysynthes.com/hcp/mitek-sports-medicine)
Gryphon® Suture Anchor with Proknot™ Technology
Paul Favorito, MD, Wellington Orthopaedic and Sports Medicine, Cincinnati, OH
The Gryphon® suture anchor with Proknot™ technology is a doubled No. 1 Permacord® high-strength orthopedic suture with a proprietary pre-tied sliding knot. The suture construct is loaded onto a 3.0-mm Gryphonsuture anchor (Peek or Biocryl Rapide® biocomposite material) and has clinical indications for labral repair of the shoulder and hip. In a laboratory setting, Proknot technology has been tested against other high-tensile sutures and commonly tied arthroscopic knots.1 Proknot technology demonstrated higher ultimate strength, significantly less knot volume, and better reproducibility among surgeons.
Surgical pearl: I use the Gryphon Proknot suture anchor for all shoulder Bankart and superior labral anterior to posterior (SLAP) repairs. I have colleagues who also use this anchor for hip arthroscopy.
Once opened on the back table, the surgical assistant may ink the free limb of suture for easy arthroscopic identification. The anchor is placed and, in the case of hard bone frequently encountered in younger patients, a 2.5-mm drill bit may be substituted for the usual 2.4-mm. One important goal of any labral repair is to position knots away from the articular surface. The free suture limb is passed through the labrum, retrieved, and delivered through the open, pre-tied knot on the suture card.
Once the knot is released and dressed, the knot pusher is placed over the suture and the knot is advanced and preliminarily tensioned medial to the articular surface. The suture limbs are separated and one limb of the suture is removed from the knot pusher. As few as 1, or up to 3, half hitches may be placed to secure the knot, taking care to direct it away from the joint surface. The result is a strong but well-positioned knot with minimal mass securing the soft tissue.
DePuy Synthes Mitek Sports Medicine
(https://www.depuysynthes.com/hcp/mitek-sports-medicine)
Gryphon® Suture Anchor with Proknot™ Technology
Paul Favorito, MD, Wellington Orthopaedic and Sports Medicine, Cincinnati, OH
The Gryphon® suture anchor with Proknot™ technology is a doubled No. 1 Permacord® high-strength orthopedic suture with a proprietary pre-tied sliding knot. The suture construct is loaded onto a 3.0-mm Gryphonsuture anchor (Peek or Biocryl Rapide® biocomposite material) and has clinical indications for labral repair of the shoulder and hip. In a laboratory setting, Proknot technology has been tested against other high-tensile sutures and commonly tied arthroscopic knots.1 Proknot technology demonstrated higher ultimate strength, significantly less knot volume, and better reproducibility among surgeons.
Surgical pearl: I use the Gryphon Proknot suture anchor for all shoulder Bankart and superior labral anterior to posterior (SLAP) repairs. I have colleagues who also use this anchor for hip arthroscopy.
Once opened on the back table, the surgical assistant may ink the free limb of suture for easy arthroscopic identification. The anchor is placed and, in the case of hard bone frequently encountered in younger patients, a 2.5-mm drill bit may be substituted for the usual 2.4-mm. One important goal of any labral repair is to position knots away from the articular surface. The free suture limb is passed through the labrum, retrieved, and delivered through the open, pre-tied knot on the suture card.
Once the knot is released and dressed, the knot pusher is placed over the suture and the knot is advanced and preliminarily tensioned medial to the articular surface. The suture limbs are separated and one limb of the suture is removed from the knot pusher. As few as 1, or up to 3, half hitches may be placed to secure the knot, taking care to direct it away from the joint surface. The result is a strong but well-positioned knot with minimal mass securing the soft tissue.
1. Rodes SA, Favorito PJ, Piccirillo JM, Spivey JT. Performance comparison of a prettied suture knot with three conventional arthroscopic knots. Arthroscopy. 2015;31(11):2183-2190.
1. Rodes SA, Favorito PJ, Piccirillo JM, Spivey JT. Performance comparison of a prettied suture knot with three conventional arthroscopic knots. Arthroscopy. 2015;31(11):2183-2190.
ASCR Restores Stability in Patients with Large Rotator Cuff Tears
ORLANDO, FL—Using arthroscopic superior capsule reconstruction (ASCR) to treat patients with massive rotator cuff tears can improve shoulder strength and function, according to research presented at the American Orthopedic Society for Sports Medicine’s Specialty Day.
Researchers used ASCR to treat 100 patients (average age: 66) who had irreparable rotator cuff tears that failed during previous treatment. Physical exams, x-rays, and magnetic resonance imaging were performed before surgery, at 3, 6 and 12 months following surgery, and on a yearly basis thereafter. Rates of return to work or sport were analyzed in 34 patients who were employed and 26 patients who were recreational athletes before the rotator cuff tear.
Overall, 92% of patients significantly improved their strength and shoulder function. In all, 32 patients returned fully to their previous work and 2 patients returned with reduced hours and workloads. All 26 patients who played sports prior to injury fully returned to their activities.
ORLANDO, FL—Using arthroscopic superior capsule reconstruction (ASCR) to treat patients with massive rotator cuff tears can improve shoulder strength and function, according to research presented at the American Orthopedic Society for Sports Medicine’s Specialty Day.
Researchers used ASCR to treat 100 patients (average age: 66) who had irreparable rotator cuff tears that failed during previous treatment. Physical exams, x-rays, and magnetic resonance imaging were performed before surgery, at 3, 6 and 12 months following surgery, and on a yearly basis thereafter. Rates of return to work or sport were analyzed in 34 patients who were employed and 26 patients who were recreational athletes before the rotator cuff tear.
Overall, 92% of patients significantly improved their strength and shoulder function. In all, 32 patients returned fully to their previous work and 2 patients returned with reduced hours and workloads. All 26 patients who played sports prior to injury fully returned to their activities.
ORLANDO, FL—Using arthroscopic superior capsule reconstruction (ASCR) to treat patients with massive rotator cuff tears can improve shoulder strength and function, according to research presented at the American Orthopedic Society for Sports Medicine’s Specialty Day.
Researchers used ASCR to treat 100 patients (average age: 66) who had irreparable rotator cuff tears that failed during previous treatment. Physical exams, x-rays, and magnetic resonance imaging were performed before surgery, at 3, 6 and 12 months following surgery, and on a yearly basis thereafter. Rates of return to work or sport were analyzed in 34 patients who were employed and 26 patients who were recreational athletes before the rotator cuff tear.
Overall, 92% of patients significantly improved their strength and shoulder function. In all, 32 patients returned fully to their previous work and 2 patients returned with reduced hours and workloads. All 26 patients who played sports prior to injury fully returned to their activities.
A Guide to Ultrasound of the Shoulder, Part 2: The Diagnostic Evaluation
The musculoskeletal (MSK) ultrasound evaluation of the shoulder provides a cost- and time-efficient imaging modality with similar diagnostic power as magnetic resonance imaging (MRI).1,2 Its portable point-of-care applications can be used in the office, in the operating room, and in sideline athletic event coverage, as we discussed in Part 1 of this series.3
MSK ultrasound may seem difficult and daunting, and many articles have quoted steep learning curves.4,5 However, in our experience in teaching many ultrasound courses, this modality can be learned quite quickly with the proper instruction. Physicians are already familiar with anatomy and usually have had some exposure to MRI.4 Taking courses in MSK ultrasound or simply learning the basic concepts of ultrasound and then learning the machine controls is usually a good start.5-8 Practice scanning normal individuals, comparing the images from an MRI to learn how to reproduce the same planes and images. This will allow the user to become familiar with normal anatomy and how to see the images on the ultrasound screen.5-8 Vollman and colleagues9 showed that in trainees, combining MRI images with sonograms enhances the ability to correctly identify MSK ultrasound anatomy from 40.9% to 72.5%, when compared with learning from ultrasound images alone.
There are currently no certifications necessary to perform ultrasound scans or bill for them; however, some insurance carriers may require demonstrating relevant, documented training for reimbursement.3 Various organizations are trying to develop certifications and regulations for ultrasound to standardize the use of this modality. In the United States, the American Institute of Ultrasound in Medicine (AIUM) and the American Registry for Diagnostic Medical Sonography (ARDMS) provide guidelines and particular MSK ultrasound certifications.10,11
Basic Ultrasound Principles
The ultrasound machine creates electrical impulses that are turned into sound waves by piezoelectric crystals at the probe’s footprint. These sound waves bounce off tissues and return to the probe, where they are converted electronically to an image on the monitor. Depending on the echogenicity of the scanned tissue, the ultrasound beam will either reflect or be absorbed at different rates. This variance is transmitted on the monitor as a grayscale image. When ultrasound waves are highly reflective, like in bone or fat, they are characterized as hyperechoic. The opposite occurs when ultrasound waves are absorbed like in the fluid of a cystic cavity or joint effusion, and the image appears black. This is described as anechoic.12 Intermediate tissues such as tendons that are less reflective are seen as hypoechoic and appear gray. When a tissue has a similar echogenicity to its surrounding tissues, it is called isoechoic.12
The transducer is the scanning component of the ultrasound machine. Transducers come in 2 shapes: linear and curvilinear. The linear probe creates a straight image that is equal to the size of the transducer footprint. The curvilinear probe creates a wider, wedge-shaped panoramic image.
Linear probes are of higher frequency and generate higher resolution images of shallower structures, while curvilinear probes have greater depth penetration but generate lower resolution images. A high frequency of 10 to 15 MHz is preferred for anatomy between 2 cm to 4 cm depth.13 Midrange frequency of 5 to 10 MHz is preferred at 5 cm to 6 cm depth, and low-frequency 2 to 5 MHz probes are preferred for anatomical structures >6 cm depth.13
Anisotropy is the property of being directionally dependent, as opposed to isotropy, which implies identical properties in all directions. This anisotropic effect is dependent on the angle of the insonating beam. The maximum return echo occurs when the ultrasound beam is perpendicular to the tendon. Decreasing the insonating angle on a normal tendon will cause it to change from brightly hyperechoic (the actual echo from tightly bound tendon fibers) to darkly hypoechoic. If the angle is then increased, the tendon will again appear hyperechoic. If the artifact causes a normal tendon to appear hypoechoic, it may falsely lead to a diagnosis of tendinosis or tear.
Posterior acoustic shadowing is present when a hyperechoic structure reflects the ultrasound beam so much that it creates a dark shadow underneath it.12,14 This phenomenon is possible since the ultrasound beam cannot penetrate the hyperechoic structure and reflects off its inferior tissues. Reverberation is when the beam is repeated back and forth between 2 parallel highly reflective surfaces. The initial reflection will be displayed correctly, while the subsequent ultrasound waves will be delayed and appear at a farther distance from the transducer.12,14
The point where the beam is at its narrowest point generates the section of the image that is best visualized.15 This is called the focal zone, and it can be adjusted to highlight the desired area of evaluation. Gain controls adjust the amount of black, gray, and white on the monitor and can be adjusted to focus the desired image.13 Depth settings are fundamental in finding the desired targets. It is recommended to start with a higher depth setting to get an overview and progressively decrease the depth to key in on the desired anatomy.13 Color Doppler can be used to view movement within structures and to identify vessels, synovitis, and neovascularization in tendinopathy.13
Ultrasound of the Shoulder
Patients should be seated, if possible, on a rotating seat. The examiner’s shoulder should be higher than the patient’s shoulder.16 The user holds the ultrasound probe between the thumb and index fingers while resting the hypothenar eminence on the patient to serve as a fulcrum and steadying force. The examination should take 5 to 15 minutes, depending on the examiner’s expertise and the amount of anatomy being scanned.
Examining the body requires knowledge of anatomy. The examination and accuracy are determined by the technician using the probe. The probe can be angled any direction and be placed obliquely on the subject. The advantage here is that anatomy in the human body is not always planar. Muscles and tissues can run obliquely or even perpendicular to each other. When evaluating anatomy, the examiner should keep in mind what structure he or she is looking for; where it should be found; what landmarks can be used to easily locate it; what orientation it has; and what the normal anatomy should look like.
Muscle appears as a lattice with larger areas of hypoechoic muscle tissue and hyperechoic fascial perimysium layers traversing through it.17 The actual muscle tissue appears hypoechoic from the fluid or blood found within. Scarring, fibrosis, calcification, or chronic injury will change the tissue to appear denser or hyperechoic.17 Acute injury will appear hypoechoic from the inflammatory response and influx of blood. Tendon appears dense and hyperechoic with striations within the tissue, sometimes referred to as a horse’s tail.17 When torn, there will be a disassociation of the tissue with a hypoechoic region between the 2 ends. The attachment to the bone and muscle tissue should appear uniform. Hyperechoic areas within the tendon may be from calcification. Ligament appears similar to tendon but is more isoechoic and connects bone to bone. Evaluation of the entire length and the attachments to the bone are critical to evaluate for disease.
Bone appears bright hyperechoic, smooth, and flat, while hyaline cartilage is hypoechoic, smooth, and runs superiorly in a parallel pattern to its respective inferior cortical bone.17
Fibrocartilage is hyperechoic and typically triangularly shaped, such as in the glenohumeral labrum. Nerves appear fascicular and hypoechoic surrounded by hyperechoic epineurium.14
The epidermis and dermis are the most superficial structure on top of the screen, and are also hyperechoic.17
The Diagnostic Shoulder Examination
The proximal long head of the biceps tendon (LHBT) is the easiest structure in the shoulder to identify because of the anatomic structure, the bicipital groove. By keeping the arm relaxed, perpendicular to the ground, and in neutral rotation, the probe can be placed perpendicular to the arm over the proximal shoulder (Figure 1A).16-20 By finding the groove, the biceps tendon will usually be found resting within the groove (Figure 1B). This is the short axis view and is equivalent to an MRI in the axial plane.
The long axis view of the proximal biceps tendon is found by keeping the tendon in the center of the screen/probe. The probe is then rotated 90° on its center axis, keeping the tendon centered on the probe. The user should be sure to visualize the entire tendon on the screen. If only part of the tendon is seen along only part of the screen, then the probe is oblique to the tendon. In this case, the probe area showing the tendon must be stabilized as the center or set point. The other part of the probe will then pivot until all of the tendon is seen on the screen. The MRI equivalent to the long axis of the proximal biceps tendon is the sagittal view.
Ultrasound is a dynamic evaluation. Moving the probe or moving the patient will change what and how something is imaged. The proximal biceps tendon is a good example of this concept. The bicipital groove is very deep proximally and flattens out as it travels distally to the mid-humerus. The examiner should continually adjust his or her hand/probe/patient position as well as depth/gain and other console functions to adapt to the dynamics of the scan. While keeping the bicep tendon in a short axis view, the tendon can be dynamically evaluated for subluxation by internally and externally rotating the arm.
To find the subscapularis, the arm remains in a neutral position with the hand supinated and the probe is held parallel with the ground. After finding the bicipital groove, the subscapularis tendon insertion is just medial to the groove (Figure 1B). By externally rotating the arm, the subscapularis tendon/muscle will come into a long axis view.16-20 The MRI equivalent to the long axis view of the subscapularis is the axial view. Dynamic testing can be done by internally and externally rotating the arm to evaluate for impingement of the subscapularis tendon as it slides underneath the coracoid process. To view the subscapularis tendon in short axis, the tendon is kept in the center of the screen/probe, and the probe is then rotated 90° on its center axis, keeping the tendon centered on the probe. The MRI equivalent is the sagittal view.
Some have recommended using the modified Crass or Middleton position to evaluate the supraspinatus, where the hand is in the “back pocket”.19 However, many patients with shoulder pain have trouble with this position. By resting the ipsilateral hand on the ipsilateral hip and then dropping the elbow, the supraspinatus insertion can still be brought out from under the acromion. This does bring the insertion anterior out of the scapular plane, so an adjustment is required in probe positioning to properly see the supraspinatus short and long axis. To find the long axis, the probe is placed parallel to a plane that spans the contralateral shoulder and ipsilateral hip (Figure 2A). The fibers of the supraspinatus should be inserting directly lateral to the humeral head without any intervening space (Figure 2B). If any space exists, a partial articular supraspinatus tendon avulsion (PASTA) lesion is present, and its thickness can be directly measured. Moving more posterior will show the flattening of the tuberosity and the fibers of the infraspinatus moving away from the humeral head—the bare spot. The MRI equivalent is the coronal view.
To view the supraspinatus tendon in short axis, maintain the arm in the same position, keeping the tendon in the center of the screen/probe. The probe is then rotated 90° on its center axis, keeping the tendon centered on the probe. The probe should now be in a parallel plane between the ipsilateral shoulder and the contralateral hip. The biceps tendon in cross-section will be found anteriorly, and the articular cartilage will appear as a black layer over the bone. Dynamic testing includes placing the probe in a coronal plane between the acromion and greater tuberosity. When the patient abducts the arm while in internal rotation, the supraspinatus tendon will slide underneath the coracoacromial arch showing potential external impingement.15 The MRI equivalent is the sagittal plane.
The glenohumeral joint is best viewed posteriorly, limiting how much of the intra-articular portion of the joint can be imaged. The arm remains in a neutral position; palpate for the posterior acromion and place the probe just inferior to it, wedging up against it (Figure 3A). The glenohumeral joint will be seen by keeping the probe parallel to the ground (Figure 3B). The MRI equivalent is the axial plane. If a joint effusion exists, it can be seen in the posterior recess.15 A hyperechoic triangular region in between the humeral head and the glenoid will represent the glenoid labrum (Figure 3B). By internally and externally rotating the arm, the joint and labrum complex can be dynamically examined. From the labrum, scanning superior and medial can sometimes show the spinoglenoid notch where a paralabral cyst might be seen.15
Using the glenohumeral joint as a reference, the infraspinatus muscle is easily visualized. Maintaining the arm in neutral position with the probe over the glenohumeral joint, the infraspinatus will become apparent as it lays in long axis view superficially between the posterior deltoid and glenohumeral joint (Figure 3B).16-20 The teres minor lies just inferiorly. The MRI equivalent is the axial plane. To view the infraspinatus and teres minor in short axis, the probe is then rotated 90° on its center axis. The infraspinatus (superiorly) and teres minor (inferiorly) muscles will be visible in short axis within the infraspinatus fossa.15 The MRI equivalent is the sagittal view.
The acromioclavicular joint is superficial and easy to image. The arm remains in a neutral position, and we can palpate the joint for easy localization. The probe is placed anteriorly in a coronal plane over the acromion and clavicle. By scanning anteriorly and posteriorly, a joint effusion referred to as a Geyser sign might be seen. The MRI equivalent is the coronal view.
Available Certifications
The AIUM certification is a voluntary peer reviewed process that acknowledges that a practice is meeting national standards and aids in improving their respective MSK ultrasound protocols. They also provide guidelines on demonstrating training and competence on performing and/or interpreting diagnostic MSK examinations (Table).10 The ARDMS certification provides an actual individual certification referred to as “Registered” in MSK ultrasound.11 The physician must perform 150 diagnostic MSK ultrasound evaluations within 36 months of applying and pass a 200-question examination that is offered twice per year.11 None of these certifications are mandated by the American Medical Association (AMA) or American Osteopathic Association (AOA).
Maintenance and Continuing Medical Education (CME)
The AIUM recommends that a minimum of 50 diagnostic MSK ultrasound evaluations be performed per year for skill maintenance.10 Furthermore, 10 hours of AMA PRA Category 1 Credits™ or American Osteopathic Association Category 1-A Credits specific to MSK ultrasound must be completed by physicians performing and/or interpreting these examinations every 3 years.10 ARDMS recommends a minimum of 30 MSK ultrasound-specific CMEs in preparation for their “Registered” MSK evaluation.1
Conclusion
MSK ultrasound is a dynamic, real-time imaging modality that can improve cost efficiency and patient care. Its portability allows for its use anywhere. Learning the skill may seem daunting, but with the proper courses and education, the technology can be easily learned. By correlating a known modality like MRI, the user will easily begin to read ultrasound images. No current certification is needed to use or bill for ultrasound, but various institutions are developing criteria and testing. Two organizations, AIUM and ARDMS, provide guidelines and certifications to demonstrate competency, which may become necessary in the very near future.
1. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
2. Roy J-S, Braën C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterization of rotator cuff disorders: a meta-analysis [published online ahead of print February 11, 2015]. Br J Sports Med. doi:10.1136/bjsports-2014-094148.
3. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 1: coding and reimbursement. Am J Orthop. 2016;45(3):176-182.
4. Hama M, Takase K, Ihata A, et al. Challenges to expanding the clinical application of musculoskeletal ultrasonography (MSUS) among rheumatologists: from a second survey in Japan. Mod Rheumatol. 2012;2:202-208.
5. Smith MJ, Rogers A, Amso N, Kennedy J, Hall A, Mullaney P. A training, assessment and feedback package for the trainee shoulder sonographer. Ultrasound. 2015;23(1):29-41.
6. Delzell PB, Boyle A, Schneider E. Dedicated training program for shoulder sonography: the results of a quality program reverberate with everyone. J Ultrasound Med. 2015;34(6):1037-1042.
7. Finnoff JT, Berkoff D, Brennan F, et al. American Medical Society for Sports Medicine (AMSSM) recommended sports ultrasound curriculum for sports medicine fellowships. PM R. 2015;7(2)e1-e11.
8. Adelman S, Fishman P. Use of portable ultrasound machine for outpatient orthopedic diagnosis: an implementation study. Perm J. 2013;17(3):18-22.
9. Vollman A, Hulen R, Dulchavsky S, et al. Educational benefits of fusing magnetic resonance imaging with sonograms. J Clin Ultrasound. 2014;42(5) 257-263.
10. Training guidelines for physicians and chiropractors who evaluate and interpret diagnostic musculoskeletal ultrasound examinations. Laurel, MD: American Institute of Ultrasound in Medicine; 2014. http://www.aium.org/resources/viewStatement.aspx?id=51. Accessed February 26, 2016.
11. Registered in musculoskeletal (RMSK) sonography. American Registry for Diagnostic Medical Sonography Web site. http://www.ardms.org/get-certified/RMSK/Pages/RMSK.aspx. Accessed February 26, 2016.
12. Silkowski C. Ultrasound nomenclature, image orientation, and basic instrumentation. In: Abraham D, Silkowski C, Odwin C, eds. Emergency Medicine Sonography Pocket Guide to Sonographic Anatomy and Pathology. Sudbury, MA: Jones and Bartlett; 2010:1-24.
13. Ihnatsenka B, Boezaart AP. Ultrasound: basic understanding and learning the language. Int J Shoulder Surg. 2010;4(3):55-62.
14. Taljanovic MS, Melville DM, Scalcione LR, Gimber LH, Lorenz EJ, Witte RS. Artifacts in musculoskeletal ultrasonography. Semin Musculoskelet Radiol. 2014;18(1):3-11.
15. Ng A, Swanevelder J. Resolution in ultrasound imaging. Continuing Educ Anaesth Crit Care Pain. 2011;11(5):186-192. http://ceaccp.oxfordjournals.org/content/11/5/186.full. Accessed March 3, 2016.
16. Nazarian L, Bohm-Velez M, Kan JH, et al. AIUM practice parameters for the performance of a musculoskeletal ultrasound examination. Laurel, MD: American Institute of Ultrasound in Medicine; 2012. http://www.aium.org/resources/guidelines/musculoskeletal.pdf. Accessed February 26, 2016.
17. Jacobson J. Fundamentals of Musculoskeletal Ultrasound. 2nd edition. Philadelphia, PA: Elsevier Saunders; 2013.
18. The Ultrasound Subcommittee of the European Society of Musculoskeletal Radiology. Musculoskeletal ultrasound: technique guidelines. Insights Imaging. 2010;1:99-141.
19. Corazza A, Orlandi D, Fabbro E, et al. Dynamic high-resolution ultrasound of the shoulder: how we do it. Eur J Radiol. 2015;84(2):266-277.
20. Allen GM. Shoulder ultrasound imaging-integrating anatomy, biomechanics and disease processes. Eur J Radiol. 2008;68(1):137-146
The musculoskeletal (MSK) ultrasound evaluation of the shoulder provides a cost- and time-efficient imaging modality with similar diagnostic power as magnetic resonance imaging (MRI).1,2 Its portable point-of-care applications can be used in the office, in the operating room, and in sideline athletic event coverage, as we discussed in Part 1 of this series.3
MSK ultrasound may seem difficult and daunting, and many articles have quoted steep learning curves.4,5 However, in our experience in teaching many ultrasound courses, this modality can be learned quite quickly with the proper instruction. Physicians are already familiar with anatomy and usually have had some exposure to MRI.4 Taking courses in MSK ultrasound or simply learning the basic concepts of ultrasound and then learning the machine controls is usually a good start.5-8 Practice scanning normal individuals, comparing the images from an MRI to learn how to reproduce the same planes and images. This will allow the user to become familiar with normal anatomy and how to see the images on the ultrasound screen.5-8 Vollman and colleagues9 showed that in trainees, combining MRI images with sonograms enhances the ability to correctly identify MSK ultrasound anatomy from 40.9% to 72.5%, when compared with learning from ultrasound images alone.
There are currently no certifications necessary to perform ultrasound scans or bill for them; however, some insurance carriers may require demonstrating relevant, documented training for reimbursement.3 Various organizations are trying to develop certifications and regulations for ultrasound to standardize the use of this modality. In the United States, the American Institute of Ultrasound in Medicine (AIUM) and the American Registry for Diagnostic Medical Sonography (ARDMS) provide guidelines and particular MSK ultrasound certifications.10,11
Basic Ultrasound Principles
The ultrasound machine creates electrical impulses that are turned into sound waves by piezoelectric crystals at the probe’s footprint. These sound waves bounce off tissues and return to the probe, where they are converted electronically to an image on the monitor. Depending on the echogenicity of the scanned tissue, the ultrasound beam will either reflect or be absorbed at different rates. This variance is transmitted on the monitor as a grayscale image. When ultrasound waves are highly reflective, like in bone or fat, they are characterized as hyperechoic. The opposite occurs when ultrasound waves are absorbed like in the fluid of a cystic cavity or joint effusion, and the image appears black. This is described as anechoic.12 Intermediate tissues such as tendons that are less reflective are seen as hypoechoic and appear gray. When a tissue has a similar echogenicity to its surrounding tissues, it is called isoechoic.12
The transducer is the scanning component of the ultrasound machine. Transducers come in 2 shapes: linear and curvilinear. The linear probe creates a straight image that is equal to the size of the transducer footprint. The curvilinear probe creates a wider, wedge-shaped panoramic image.
Linear probes are of higher frequency and generate higher resolution images of shallower structures, while curvilinear probes have greater depth penetration but generate lower resolution images. A high frequency of 10 to 15 MHz is preferred for anatomy between 2 cm to 4 cm depth.13 Midrange frequency of 5 to 10 MHz is preferred at 5 cm to 6 cm depth, and low-frequency 2 to 5 MHz probes are preferred for anatomical structures >6 cm depth.13
Anisotropy is the property of being directionally dependent, as opposed to isotropy, which implies identical properties in all directions. This anisotropic effect is dependent on the angle of the insonating beam. The maximum return echo occurs when the ultrasound beam is perpendicular to the tendon. Decreasing the insonating angle on a normal tendon will cause it to change from brightly hyperechoic (the actual echo from tightly bound tendon fibers) to darkly hypoechoic. If the angle is then increased, the tendon will again appear hyperechoic. If the artifact causes a normal tendon to appear hypoechoic, it may falsely lead to a diagnosis of tendinosis or tear.
Posterior acoustic shadowing is present when a hyperechoic structure reflects the ultrasound beam so much that it creates a dark shadow underneath it.12,14 This phenomenon is possible since the ultrasound beam cannot penetrate the hyperechoic structure and reflects off its inferior tissues. Reverberation is when the beam is repeated back and forth between 2 parallel highly reflective surfaces. The initial reflection will be displayed correctly, while the subsequent ultrasound waves will be delayed and appear at a farther distance from the transducer.12,14
The point where the beam is at its narrowest point generates the section of the image that is best visualized.15 This is called the focal zone, and it can be adjusted to highlight the desired area of evaluation. Gain controls adjust the amount of black, gray, and white on the monitor and can be adjusted to focus the desired image.13 Depth settings are fundamental in finding the desired targets. It is recommended to start with a higher depth setting to get an overview and progressively decrease the depth to key in on the desired anatomy.13 Color Doppler can be used to view movement within structures and to identify vessels, synovitis, and neovascularization in tendinopathy.13
Ultrasound of the Shoulder
Patients should be seated, if possible, on a rotating seat. The examiner’s shoulder should be higher than the patient’s shoulder.16 The user holds the ultrasound probe between the thumb and index fingers while resting the hypothenar eminence on the patient to serve as a fulcrum and steadying force. The examination should take 5 to 15 minutes, depending on the examiner’s expertise and the amount of anatomy being scanned.
Examining the body requires knowledge of anatomy. The examination and accuracy are determined by the technician using the probe. The probe can be angled any direction and be placed obliquely on the subject. The advantage here is that anatomy in the human body is not always planar. Muscles and tissues can run obliquely or even perpendicular to each other. When evaluating anatomy, the examiner should keep in mind what structure he or she is looking for; where it should be found; what landmarks can be used to easily locate it; what orientation it has; and what the normal anatomy should look like.
Muscle appears as a lattice with larger areas of hypoechoic muscle tissue and hyperechoic fascial perimysium layers traversing through it.17 The actual muscle tissue appears hypoechoic from the fluid or blood found within. Scarring, fibrosis, calcification, or chronic injury will change the tissue to appear denser or hyperechoic.17 Acute injury will appear hypoechoic from the inflammatory response and influx of blood. Tendon appears dense and hyperechoic with striations within the tissue, sometimes referred to as a horse’s tail.17 When torn, there will be a disassociation of the tissue with a hypoechoic region between the 2 ends. The attachment to the bone and muscle tissue should appear uniform. Hyperechoic areas within the tendon may be from calcification. Ligament appears similar to tendon but is more isoechoic and connects bone to bone. Evaluation of the entire length and the attachments to the bone are critical to evaluate for disease.
Bone appears bright hyperechoic, smooth, and flat, while hyaline cartilage is hypoechoic, smooth, and runs superiorly in a parallel pattern to its respective inferior cortical bone.17
Fibrocartilage is hyperechoic and typically triangularly shaped, such as in the glenohumeral labrum. Nerves appear fascicular and hypoechoic surrounded by hyperechoic epineurium.14
The epidermis and dermis are the most superficial structure on top of the screen, and are also hyperechoic.17
The Diagnostic Shoulder Examination
The proximal long head of the biceps tendon (LHBT) is the easiest structure in the shoulder to identify because of the anatomic structure, the bicipital groove. By keeping the arm relaxed, perpendicular to the ground, and in neutral rotation, the probe can be placed perpendicular to the arm over the proximal shoulder (Figure 1A).16-20 By finding the groove, the biceps tendon will usually be found resting within the groove (Figure 1B). This is the short axis view and is equivalent to an MRI in the axial plane.
The long axis view of the proximal biceps tendon is found by keeping the tendon in the center of the screen/probe. The probe is then rotated 90° on its center axis, keeping the tendon centered on the probe. The user should be sure to visualize the entire tendon on the screen. If only part of the tendon is seen along only part of the screen, then the probe is oblique to the tendon. In this case, the probe area showing the tendon must be stabilized as the center or set point. The other part of the probe will then pivot until all of the tendon is seen on the screen. The MRI equivalent to the long axis of the proximal biceps tendon is the sagittal view.
Ultrasound is a dynamic evaluation. Moving the probe or moving the patient will change what and how something is imaged. The proximal biceps tendon is a good example of this concept. The bicipital groove is very deep proximally and flattens out as it travels distally to the mid-humerus. The examiner should continually adjust his or her hand/probe/patient position as well as depth/gain and other console functions to adapt to the dynamics of the scan. While keeping the bicep tendon in a short axis view, the tendon can be dynamically evaluated for subluxation by internally and externally rotating the arm.
To find the subscapularis, the arm remains in a neutral position with the hand supinated and the probe is held parallel with the ground. After finding the bicipital groove, the subscapularis tendon insertion is just medial to the groove (Figure 1B). By externally rotating the arm, the subscapularis tendon/muscle will come into a long axis view.16-20 The MRI equivalent to the long axis view of the subscapularis is the axial view. Dynamic testing can be done by internally and externally rotating the arm to evaluate for impingement of the subscapularis tendon as it slides underneath the coracoid process. To view the subscapularis tendon in short axis, the tendon is kept in the center of the screen/probe, and the probe is then rotated 90° on its center axis, keeping the tendon centered on the probe. The MRI equivalent is the sagittal view.
Some have recommended using the modified Crass or Middleton position to evaluate the supraspinatus, where the hand is in the “back pocket”.19 However, many patients with shoulder pain have trouble with this position. By resting the ipsilateral hand on the ipsilateral hip and then dropping the elbow, the supraspinatus insertion can still be brought out from under the acromion. This does bring the insertion anterior out of the scapular plane, so an adjustment is required in probe positioning to properly see the supraspinatus short and long axis. To find the long axis, the probe is placed parallel to a plane that spans the contralateral shoulder and ipsilateral hip (Figure 2A). The fibers of the supraspinatus should be inserting directly lateral to the humeral head without any intervening space (Figure 2B). If any space exists, a partial articular supraspinatus tendon avulsion (PASTA) lesion is present, and its thickness can be directly measured. Moving more posterior will show the flattening of the tuberosity and the fibers of the infraspinatus moving away from the humeral head—the bare spot. The MRI equivalent is the coronal view.
To view the supraspinatus tendon in short axis, maintain the arm in the same position, keeping the tendon in the center of the screen/probe. The probe is then rotated 90° on its center axis, keeping the tendon centered on the probe. The probe should now be in a parallel plane between the ipsilateral shoulder and the contralateral hip. The biceps tendon in cross-section will be found anteriorly, and the articular cartilage will appear as a black layer over the bone. Dynamic testing includes placing the probe in a coronal plane between the acromion and greater tuberosity. When the patient abducts the arm while in internal rotation, the supraspinatus tendon will slide underneath the coracoacromial arch showing potential external impingement.15 The MRI equivalent is the sagittal plane.
The glenohumeral joint is best viewed posteriorly, limiting how much of the intra-articular portion of the joint can be imaged. The arm remains in a neutral position; palpate for the posterior acromion and place the probe just inferior to it, wedging up against it (Figure 3A). The glenohumeral joint will be seen by keeping the probe parallel to the ground (Figure 3B). The MRI equivalent is the axial plane. If a joint effusion exists, it can be seen in the posterior recess.15 A hyperechoic triangular region in between the humeral head and the glenoid will represent the glenoid labrum (Figure 3B). By internally and externally rotating the arm, the joint and labrum complex can be dynamically examined. From the labrum, scanning superior and medial can sometimes show the spinoglenoid notch where a paralabral cyst might be seen.15
Using the glenohumeral joint as a reference, the infraspinatus muscle is easily visualized. Maintaining the arm in neutral position with the probe over the glenohumeral joint, the infraspinatus will become apparent as it lays in long axis view superficially between the posterior deltoid and glenohumeral joint (Figure 3B).16-20 The teres minor lies just inferiorly. The MRI equivalent is the axial plane. To view the infraspinatus and teres minor in short axis, the probe is then rotated 90° on its center axis. The infraspinatus (superiorly) and teres minor (inferiorly) muscles will be visible in short axis within the infraspinatus fossa.15 The MRI equivalent is the sagittal view.
The acromioclavicular joint is superficial and easy to image. The arm remains in a neutral position, and we can palpate the joint for easy localization. The probe is placed anteriorly in a coronal plane over the acromion and clavicle. By scanning anteriorly and posteriorly, a joint effusion referred to as a Geyser sign might be seen. The MRI equivalent is the coronal view.
Available Certifications
The AIUM certification is a voluntary peer reviewed process that acknowledges that a practice is meeting national standards and aids in improving their respective MSK ultrasound protocols. They also provide guidelines on demonstrating training and competence on performing and/or interpreting diagnostic MSK examinations (Table).10 The ARDMS certification provides an actual individual certification referred to as “Registered” in MSK ultrasound.11 The physician must perform 150 diagnostic MSK ultrasound evaluations within 36 months of applying and pass a 200-question examination that is offered twice per year.11 None of these certifications are mandated by the American Medical Association (AMA) or American Osteopathic Association (AOA).
Maintenance and Continuing Medical Education (CME)
The AIUM recommends that a minimum of 50 diagnostic MSK ultrasound evaluations be performed per year for skill maintenance.10 Furthermore, 10 hours of AMA PRA Category 1 Credits™ or American Osteopathic Association Category 1-A Credits specific to MSK ultrasound must be completed by physicians performing and/or interpreting these examinations every 3 years.10 ARDMS recommends a minimum of 30 MSK ultrasound-specific CMEs in preparation for their “Registered” MSK evaluation.1
Conclusion
MSK ultrasound is a dynamic, real-time imaging modality that can improve cost efficiency and patient care. Its portability allows for its use anywhere. Learning the skill may seem daunting, but with the proper courses and education, the technology can be easily learned. By correlating a known modality like MRI, the user will easily begin to read ultrasound images. No current certification is needed to use or bill for ultrasound, but various institutions are developing criteria and testing. Two organizations, AIUM and ARDMS, provide guidelines and certifications to demonstrate competency, which may become necessary in the very near future.
The musculoskeletal (MSK) ultrasound evaluation of the shoulder provides a cost- and time-efficient imaging modality with similar diagnostic power as magnetic resonance imaging (MRI).1,2 Its portable point-of-care applications can be used in the office, in the operating room, and in sideline athletic event coverage, as we discussed in Part 1 of this series.3
MSK ultrasound may seem difficult and daunting, and many articles have quoted steep learning curves.4,5 However, in our experience in teaching many ultrasound courses, this modality can be learned quite quickly with the proper instruction. Physicians are already familiar with anatomy and usually have had some exposure to MRI.4 Taking courses in MSK ultrasound or simply learning the basic concepts of ultrasound and then learning the machine controls is usually a good start.5-8 Practice scanning normal individuals, comparing the images from an MRI to learn how to reproduce the same planes and images. This will allow the user to become familiar with normal anatomy and how to see the images on the ultrasound screen.5-8 Vollman and colleagues9 showed that in trainees, combining MRI images with sonograms enhances the ability to correctly identify MSK ultrasound anatomy from 40.9% to 72.5%, when compared with learning from ultrasound images alone.
There are currently no certifications necessary to perform ultrasound scans or bill for them; however, some insurance carriers may require demonstrating relevant, documented training for reimbursement.3 Various organizations are trying to develop certifications and regulations for ultrasound to standardize the use of this modality. In the United States, the American Institute of Ultrasound in Medicine (AIUM) and the American Registry for Diagnostic Medical Sonography (ARDMS) provide guidelines and particular MSK ultrasound certifications.10,11
Basic Ultrasound Principles
The ultrasound machine creates electrical impulses that are turned into sound waves by piezoelectric crystals at the probe’s footprint. These sound waves bounce off tissues and return to the probe, where they are converted electronically to an image on the monitor. Depending on the echogenicity of the scanned tissue, the ultrasound beam will either reflect or be absorbed at different rates. This variance is transmitted on the monitor as a grayscale image. When ultrasound waves are highly reflective, like in bone or fat, they are characterized as hyperechoic. The opposite occurs when ultrasound waves are absorbed like in the fluid of a cystic cavity or joint effusion, and the image appears black. This is described as anechoic.12 Intermediate tissues such as tendons that are less reflective are seen as hypoechoic and appear gray. When a tissue has a similar echogenicity to its surrounding tissues, it is called isoechoic.12
The transducer is the scanning component of the ultrasound machine. Transducers come in 2 shapes: linear and curvilinear. The linear probe creates a straight image that is equal to the size of the transducer footprint. The curvilinear probe creates a wider, wedge-shaped panoramic image.
Linear probes are of higher frequency and generate higher resolution images of shallower structures, while curvilinear probes have greater depth penetration but generate lower resolution images. A high frequency of 10 to 15 MHz is preferred for anatomy between 2 cm to 4 cm depth.13 Midrange frequency of 5 to 10 MHz is preferred at 5 cm to 6 cm depth, and low-frequency 2 to 5 MHz probes are preferred for anatomical structures >6 cm depth.13
Anisotropy is the property of being directionally dependent, as opposed to isotropy, which implies identical properties in all directions. This anisotropic effect is dependent on the angle of the insonating beam. The maximum return echo occurs when the ultrasound beam is perpendicular to the tendon. Decreasing the insonating angle on a normal tendon will cause it to change from brightly hyperechoic (the actual echo from tightly bound tendon fibers) to darkly hypoechoic. If the angle is then increased, the tendon will again appear hyperechoic. If the artifact causes a normal tendon to appear hypoechoic, it may falsely lead to a diagnosis of tendinosis or tear.
Posterior acoustic shadowing is present when a hyperechoic structure reflects the ultrasound beam so much that it creates a dark shadow underneath it.12,14 This phenomenon is possible since the ultrasound beam cannot penetrate the hyperechoic structure and reflects off its inferior tissues. Reverberation is when the beam is repeated back and forth between 2 parallel highly reflective surfaces. The initial reflection will be displayed correctly, while the subsequent ultrasound waves will be delayed and appear at a farther distance from the transducer.12,14
The point where the beam is at its narrowest point generates the section of the image that is best visualized.15 This is called the focal zone, and it can be adjusted to highlight the desired area of evaluation. Gain controls adjust the amount of black, gray, and white on the monitor and can be adjusted to focus the desired image.13 Depth settings are fundamental in finding the desired targets. It is recommended to start with a higher depth setting to get an overview and progressively decrease the depth to key in on the desired anatomy.13 Color Doppler can be used to view movement within structures and to identify vessels, synovitis, and neovascularization in tendinopathy.13
Ultrasound of the Shoulder
Patients should be seated, if possible, on a rotating seat. The examiner’s shoulder should be higher than the patient’s shoulder.16 The user holds the ultrasound probe between the thumb and index fingers while resting the hypothenar eminence on the patient to serve as a fulcrum and steadying force. The examination should take 5 to 15 minutes, depending on the examiner’s expertise and the amount of anatomy being scanned.
Examining the body requires knowledge of anatomy. The examination and accuracy are determined by the technician using the probe. The probe can be angled any direction and be placed obliquely on the subject. The advantage here is that anatomy in the human body is not always planar. Muscles and tissues can run obliquely or even perpendicular to each other. When evaluating anatomy, the examiner should keep in mind what structure he or she is looking for; where it should be found; what landmarks can be used to easily locate it; what orientation it has; and what the normal anatomy should look like.
Muscle appears as a lattice with larger areas of hypoechoic muscle tissue and hyperechoic fascial perimysium layers traversing through it.17 The actual muscle tissue appears hypoechoic from the fluid or blood found within. Scarring, fibrosis, calcification, or chronic injury will change the tissue to appear denser or hyperechoic.17 Acute injury will appear hypoechoic from the inflammatory response and influx of blood. Tendon appears dense and hyperechoic with striations within the tissue, sometimes referred to as a horse’s tail.17 When torn, there will be a disassociation of the tissue with a hypoechoic region between the 2 ends. The attachment to the bone and muscle tissue should appear uniform. Hyperechoic areas within the tendon may be from calcification. Ligament appears similar to tendon but is more isoechoic and connects bone to bone. Evaluation of the entire length and the attachments to the bone are critical to evaluate for disease.
Bone appears bright hyperechoic, smooth, and flat, while hyaline cartilage is hypoechoic, smooth, and runs superiorly in a parallel pattern to its respective inferior cortical bone.17
Fibrocartilage is hyperechoic and typically triangularly shaped, such as in the glenohumeral labrum. Nerves appear fascicular and hypoechoic surrounded by hyperechoic epineurium.14
The epidermis and dermis are the most superficial structure on top of the screen, and are also hyperechoic.17
The Diagnostic Shoulder Examination
The proximal long head of the biceps tendon (LHBT) is the easiest structure in the shoulder to identify because of the anatomic structure, the bicipital groove. By keeping the arm relaxed, perpendicular to the ground, and in neutral rotation, the probe can be placed perpendicular to the arm over the proximal shoulder (Figure 1A).16-20 By finding the groove, the biceps tendon will usually be found resting within the groove (Figure 1B). This is the short axis view and is equivalent to an MRI in the axial plane.
The long axis view of the proximal biceps tendon is found by keeping the tendon in the center of the screen/probe. The probe is then rotated 90° on its center axis, keeping the tendon centered on the probe. The user should be sure to visualize the entire tendon on the screen. If only part of the tendon is seen along only part of the screen, then the probe is oblique to the tendon. In this case, the probe area showing the tendon must be stabilized as the center or set point. The other part of the probe will then pivot until all of the tendon is seen on the screen. The MRI equivalent to the long axis of the proximal biceps tendon is the sagittal view.
Ultrasound is a dynamic evaluation. Moving the probe or moving the patient will change what and how something is imaged. The proximal biceps tendon is a good example of this concept. The bicipital groove is very deep proximally and flattens out as it travels distally to the mid-humerus. The examiner should continually adjust his or her hand/probe/patient position as well as depth/gain and other console functions to adapt to the dynamics of the scan. While keeping the bicep tendon in a short axis view, the tendon can be dynamically evaluated for subluxation by internally and externally rotating the arm.
To find the subscapularis, the arm remains in a neutral position with the hand supinated and the probe is held parallel with the ground. After finding the bicipital groove, the subscapularis tendon insertion is just medial to the groove (Figure 1B). By externally rotating the arm, the subscapularis tendon/muscle will come into a long axis view.16-20 The MRI equivalent to the long axis view of the subscapularis is the axial view. Dynamic testing can be done by internally and externally rotating the arm to evaluate for impingement of the subscapularis tendon as it slides underneath the coracoid process. To view the subscapularis tendon in short axis, the tendon is kept in the center of the screen/probe, and the probe is then rotated 90° on its center axis, keeping the tendon centered on the probe. The MRI equivalent is the sagittal view.
Some have recommended using the modified Crass or Middleton position to evaluate the supraspinatus, where the hand is in the “back pocket”.19 However, many patients with shoulder pain have trouble with this position. By resting the ipsilateral hand on the ipsilateral hip and then dropping the elbow, the supraspinatus insertion can still be brought out from under the acromion. This does bring the insertion anterior out of the scapular plane, so an adjustment is required in probe positioning to properly see the supraspinatus short and long axis. To find the long axis, the probe is placed parallel to a plane that spans the contralateral shoulder and ipsilateral hip (Figure 2A). The fibers of the supraspinatus should be inserting directly lateral to the humeral head without any intervening space (Figure 2B). If any space exists, a partial articular supraspinatus tendon avulsion (PASTA) lesion is present, and its thickness can be directly measured. Moving more posterior will show the flattening of the tuberosity and the fibers of the infraspinatus moving away from the humeral head—the bare spot. The MRI equivalent is the coronal view.
To view the supraspinatus tendon in short axis, maintain the arm in the same position, keeping the tendon in the center of the screen/probe. The probe is then rotated 90° on its center axis, keeping the tendon centered on the probe. The probe should now be in a parallel plane between the ipsilateral shoulder and the contralateral hip. The biceps tendon in cross-section will be found anteriorly, and the articular cartilage will appear as a black layer over the bone. Dynamic testing includes placing the probe in a coronal plane between the acromion and greater tuberosity. When the patient abducts the arm while in internal rotation, the supraspinatus tendon will slide underneath the coracoacromial arch showing potential external impingement.15 The MRI equivalent is the sagittal plane.
The glenohumeral joint is best viewed posteriorly, limiting how much of the intra-articular portion of the joint can be imaged. The arm remains in a neutral position; palpate for the posterior acromion and place the probe just inferior to it, wedging up against it (Figure 3A). The glenohumeral joint will be seen by keeping the probe parallel to the ground (Figure 3B). The MRI equivalent is the axial plane. If a joint effusion exists, it can be seen in the posterior recess.15 A hyperechoic triangular region in between the humeral head and the glenoid will represent the glenoid labrum (Figure 3B). By internally and externally rotating the arm, the joint and labrum complex can be dynamically examined. From the labrum, scanning superior and medial can sometimes show the spinoglenoid notch where a paralabral cyst might be seen.15
Using the glenohumeral joint as a reference, the infraspinatus muscle is easily visualized. Maintaining the arm in neutral position with the probe over the glenohumeral joint, the infraspinatus will become apparent as it lays in long axis view superficially between the posterior deltoid and glenohumeral joint (Figure 3B).16-20 The teres minor lies just inferiorly. The MRI equivalent is the axial plane. To view the infraspinatus and teres minor in short axis, the probe is then rotated 90° on its center axis. The infraspinatus (superiorly) and teres minor (inferiorly) muscles will be visible in short axis within the infraspinatus fossa.15 The MRI equivalent is the sagittal view.
The acromioclavicular joint is superficial and easy to image. The arm remains in a neutral position, and we can palpate the joint for easy localization. The probe is placed anteriorly in a coronal plane over the acromion and clavicle. By scanning anteriorly and posteriorly, a joint effusion referred to as a Geyser sign might be seen. The MRI equivalent is the coronal view.
Available Certifications
The AIUM certification is a voluntary peer reviewed process that acknowledges that a practice is meeting national standards and aids in improving their respective MSK ultrasound protocols. They also provide guidelines on demonstrating training and competence on performing and/or interpreting diagnostic MSK examinations (Table).10 The ARDMS certification provides an actual individual certification referred to as “Registered” in MSK ultrasound.11 The physician must perform 150 diagnostic MSK ultrasound evaluations within 36 months of applying and pass a 200-question examination that is offered twice per year.11 None of these certifications are mandated by the American Medical Association (AMA) or American Osteopathic Association (AOA).
Maintenance and Continuing Medical Education (CME)
The AIUM recommends that a minimum of 50 diagnostic MSK ultrasound evaluations be performed per year for skill maintenance.10 Furthermore, 10 hours of AMA PRA Category 1 Credits™ or American Osteopathic Association Category 1-A Credits specific to MSK ultrasound must be completed by physicians performing and/or interpreting these examinations every 3 years.10 ARDMS recommends a minimum of 30 MSK ultrasound-specific CMEs in preparation for their “Registered” MSK evaluation.1
Conclusion
MSK ultrasound is a dynamic, real-time imaging modality that can improve cost efficiency and patient care. Its portability allows for its use anywhere. Learning the skill may seem daunting, but with the proper courses and education, the technology can be easily learned. By correlating a known modality like MRI, the user will easily begin to read ultrasound images. No current certification is needed to use or bill for ultrasound, but various institutions are developing criteria and testing. Two organizations, AIUM and ARDMS, provide guidelines and certifications to demonstrate competency, which may become necessary in the very near future.
1. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
2. Roy J-S, Braën C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterization of rotator cuff disorders: a meta-analysis [published online ahead of print February 11, 2015]. Br J Sports Med. doi:10.1136/bjsports-2014-094148.
3. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 1: coding and reimbursement. Am J Orthop. 2016;45(3):176-182.
4. Hama M, Takase K, Ihata A, et al. Challenges to expanding the clinical application of musculoskeletal ultrasonography (MSUS) among rheumatologists: from a second survey in Japan. Mod Rheumatol. 2012;2:202-208.
5. Smith MJ, Rogers A, Amso N, Kennedy J, Hall A, Mullaney P. A training, assessment and feedback package for the trainee shoulder sonographer. Ultrasound. 2015;23(1):29-41.
6. Delzell PB, Boyle A, Schneider E. Dedicated training program for shoulder sonography: the results of a quality program reverberate with everyone. J Ultrasound Med. 2015;34(6):1037-1042.
7. Finnoff JT, Berkoff D, Brennan F, et al. American Medical Society for Sports Medicine (AMSSM) recommended sports ultrasound curriculum for sports medicine fellowships. PM R. 2015;7(2)e1-e11.
8. Adelman S, Fishman P. Use of portable ultrasound machine for outpatient orthopedic diagnosis: an implementation study. Perm J. 2013;17(3):18-22.
9. Vollman A, Hulen R, Dulchavsky S, et al. Educational benefits of fusing magnetic resonance imaging with sonograms. J Clin Ultrasound. 2014;42(5) 257-263.
10. Training guidelines for physicians and chiropractors who evaluate and interpret diagnostic musculoskeletal ultrasound examinations. Laurel, MD: American Institute of Ultrasound in Medicine; 2014. http://www.aium.org/resources/viewStatement.aspx?id=51. Accessed February 26, 2016.
11. Registered in musculoskeletal (RMSK) sonography. American Registry for Diagnostic Medical Sonography Web site. http://www.ardms.org/get-certified/RMSK/Pages/RMSK.aspx. Accessed February 26, 2016.
12. Silkowski C. Ultrasound nomenclature, image orientation, and basic instrumentation. In: Abraham D, Silkowski C, Odwin C, eds. Emergency Medicine Sonography Pocket Guide to Sonographic Anatomy and Pathology. Sudbury, MA: Jones and Bartlett; 2010:1-24.
13. Ihnatsenka B, Boezaart AP. Ultrasound: basic understanding and learning the language. Int J Shoulder Surg. 2010;4(3):55-62.
14. Taljanovic MS, Melville DM, Scalcione LR, Gimber LH, Lorenz EJ, Witte RS. Artifacts in musculoskeletal ultrasonography. Semin Musculoskelet Radiol. 2014;18(1):3-11.
15. Ng A, Swanevelder J. Resolution in ultrasound imaging. Continuing Educ Anaesth Crit Care Pain. 2011;11(5):186-192. http://ceaccp.oxfordjournals.org/content/11/5/186.full. Accessed March 3, 2016.
16. Nazarian L, Bohm-Velez M, Kan JH, et al. AIUM practice parameters for the performance of a musculoskeletal ultrasound examination. Laurel, MD: American Institute of Ultrasound in Medicine; 2012. http://www.aium.org/resources/guidelines/musculoskeletal.pdf. Accessed February 26, 2016.
17. Jacobson J. Fundamentals of Musculoskeletal Ultrasound. 2nd edition. Philadelphia, PA: Elsevier Saunders; 2013.
18. The Ultrasound Subcommittee of the European Society of Musculoskeletal Radiology. Musculoskeletal ultrasound: technique guidelines. Insights Imaging. 2010;1:99-141.
19. Corazza A, Orlandi D, Fabbro E, et al. Dynamic high-resolution ultrasound of the shoulder: how we do it. Eur J Radiol. 2015;84(2):266-277.
20. Allen GM. Shoulder ultrasound imaging-integrating anatomy, biomechanics and disease processes. Eur J Radiol. 2008;68(1):137-146
1. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
2. Roy J-S, Braën C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterization of rotator cuff disorders: a meta-analysis [published online ahead of print February 11, 2015]. Br J Sports Med. doi:10.1136/bjsports-2014-094148.
3. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 1: coding and reimbursement. Am J Orthop. 2016;45(3):176-182.
4. Hama M, Takase K, Ihata A, et al. Challenges to expanding the clinical application of musculoskeletal ultrasonography (MSUS) among rheumatologists: from a second survey in Japan. Mod Rheumatol. 2012;2:202-208.
5. Smith MJ, Rogers A, Amso N, Kennedy J, Hall A, Mullaney P. A training, assessment and feedback package for the trainee shoulder sonographer. Ultrasound. 2015;23(1):29-41.
6. Delzell PB, Boyle A, Schneider E. Dedicated training program for shoulder sonography: the results of a quality program reverberate with everyone. J Ultrasound Med. 2015;34(6):1037-1042.
7. Finnoff JT, Berkoff D, Brennan F, et al. American Medical Society for Sports Medicine (AMSSM) recommended sports ultrasound curriculum for sports medicine fellowships. PM R. 2015;7(2)e1-e11.
8. Adelman S, Fishman P. Use of portable ultrasound machine for outpatient orthopedic diagnosis: an implementation study. Perm J. 2013;17(3):18-22.
9. Vollman A, Hulen R, Dulchavsky S, et al. Educational benefits of fusing magnetic resonance imaging with sonograms. J Clin Ultrasound. 2014;42(5) 257-263.
10. Training guidelines for physicians and chiropractors who evaluate and interpret diagnostic musculoskeletal ultrasound examinations. Laurel, MD: American Institute of Ultrasound in Medicine; 2014. http://www.aium.org/resources/viewStatement.aspx?id=51. Accessed February 26, 2016.
11. Registered in musculoskeletal (RMSK) sonography. American Registry for Diagnostic Medical Sonography Web site. http://www.ardms.org/get-certified/RMSK/Pages/RMSK.aspx. Accessed February 26, 2016.
12. Silkowski C. Ultrasound nomenclature, image orientation, and basic instrumentation. In: Abraham D, Silkowski C, Odwin C, eds. Emergency Medicine Sonography Pocket Guide to Sonographic Anatomy and Pathology. Sudbury, MA: Jones and Bartlett; 2010:1-24.
13. Ihnatsenka B, Boezaart AP. Ultrasound: basic understanding and learning the language. Int J Shoulder Surg. 2010;4(3):55-62.
14. Taljanovic MS, Melville DM, Scalcione LR, Gimber LH, Lorenz EJ, Witte RS. Artifacts in musculoskeletal ultrasonography. Semin Musculoskelet Radiol. 2014;18(1):3-11.
15. Ng A, Swanevelder J. Resolution in ultrasound imaging. Continuing Educ Anaesth Crit Care Pain. 2011;11(5):186-192. http://ceaccp.oxfordjournals.org/content/11/5/186.full. Accessed March 3, 2016.
16. Nazarian L, Bohm-Velez M, Kan JH, et al. AIUM practice parameters for the performance of a musculoskeletal ultrasound examination. Laurel, MD: American Institute of Ultrasound in Medicine; 2012. http://www.aium.org/resources/guidelines/musculoskeletal.pdf. Accessed February 26, 2016.
17. Jacobson J. Fundamentals of Musculoskeletal Ultrasound. 2nd edition. Philadelphia, PA: Elsevier Saunders; 2013.
18. The Ultrasound Subcommittee of the European Society of Musculoskeletal Radiology. Musculoskeletal ultrasound: technique guidelines. Insights Imaging. 2010;1:99-141.
19. Corazza A, Orlandi D, Fabbro E, et al. Dynamic high-resolution ultrasound of the shoulder: how we do it. Eur J Radiol. 2015;84(2):266-277.
20. Allen GM. Shoulder ultrasound imaging-integrating anatomy, biomechanics and disease processes. Eur J Radiol. 2008;68(1):137-146
Are Preseason Arm Injury Prevention Programs Beneficial for Young Baseball Players?
ORLANDO, FL—Preseason prevention programs can improve deficits in young baseball pitchers, according to research presented at the American Orthopedic Society for Sports Medicine’s Specialty Day.
Researchers evaluated 143 pitchers, of which 88 participated in additional preseason training and 76 continued with normal training. The median age of the pitchers was 15.7 years.
The prevention program, which was supervised by an athletic trainer and required a commitment of 15 minutes 4 times a week, included resistance training with dumbbell weights, elastic tubing, and a focused flexibility program. Pitchers who participated in the prevention program had reduced internal rotation and horizontal adduction deficits. Pitchers who had previous injuries and participated in the preseason training program were 4 times less likely to suffer an injury than those in the general arm care program.
ORLANDO, FL—Preseason prevention programs can improve deficits in young baseball pitchers, according to research presented at the American Orthopedic Society for Sports Medicine’s Specialty Day.
Researchers evaluated 143 pitchers, of which 88 participated in additional preseason training and 76 continued with normal training. The median age of the pitchers was 15.7 years.
The prevention program, which was supervised by an athletic trainer and required a commitment of 15 minutes 4 times a week, included resistance training with dumbbell weights, elastic tubing, and a focused flexibility program. Pitchers who participated in the prevention program had reduced internal rotation and horizontal adduction deficits. Pitchers who had previous injuries and participated in the preseason training program were 4 times less likely to suffer an injury than those in the general arm care program.
ORLANDO, FL—Preseason prevention programs can improve deficits in young baseball pitchers, according to research presented at the American Orthopedic Society for Sports Medicine’s Specialty Day.
Researchers evaluated 143 pitchers, of which 88 participated in additional preseason training and 76 continued with normal training. The median age of the pitchers was 15.7 years.
The prevention program, which was supervised by an athletic trainer and required a commitment of 15 minutes 4 times a week, included resistance training with dumbbell weights, elastic tubing, and a focused flexibility program. Pitchers who participated in the prevention program had reduced internal rotation and horizontal adduction deficits. Pitchers who had previous injuries and participated in the preseason training program were 4 times less likely to suffer an injury than those in the general arm care program.
Graft Choice in ACL Reconstruction May Affect Revision Rates
ORLANDO, FL—Using soft tissue allografts for anterior cruciate ligament (ACL) reconstructions may increase the risks for a revision reconstruction postoperatively, according to research presented at the American Orthopedic Society for Sports Medicine’s Specialty Day.
Researchers analyzed data from the Kaiser Permanente ACLR Registry. Of the cases analyzed, 4,557 involved bone-patellar tendon-bone (BPTB) autografts, 3,751 soft tissue allograft, and 5,707 hamstring allograft.
After a 3-year follow-up, the overall revision rates were 2.5% for BPTB autographs, 3.5% for hamstring autografts, and 3.7% for soft tissue allografts. Non-processed soft tissue allografts were not found to have a statistically significantly different risk of revision compared to BPTB autografts. However, compared to BPTB autografts, allografts processed with more than 1.8Mrads irradiation had a more than 2 times higher risk of revision, and grafts processed with more than 1.8Mrads or high pressure chemical processing had a more than 4 to 6 times higher risk of revision. This was true even after adjustments for age, gender, and race.
ORLANDO, FL—Using soft tissue allografts for anterior cruciate ligament (ACL) reconstructions may increase the risks for a revision reconstruction postoperatively, according to research presented at the American Orthopedic Society for Sports Medicine’s Specialty Day.
Researchers analyzed data from the Kaiser Permanente ACLR Registry. Of the cases analyzed, 4,557 involved bone-patellar tendon-bone (BPTB) autografts, 3,751 soft tissue allograft, and 5,707 hamstring allograft.
After a 3-year follow-up, the overall revision rates were 2.5% for BPTB autographs, 3.5% for hamstring autografts, and 3.7% for soft tissue allografts. Non-processed soft tissue allografts were not found to have a statistically significantly different risk of revision compared to BPTB autografts. However, compared to BPTB autografts, allografts processed with more than 1.8Mrads irradiation had a more than 2 times higher risk of revision, and grafts processed with more than 1.8Mrads or high pressure chemical processing had a more than 4 to 6 times higher risk of revision. This was true even after adjustments for age, gender, and race.
ORLANDO, FL—Using soft tissue allografts for anterior cruciate ligament (ACL) reconstructions may increase the risks for a revision reconstruction postoperatively, according to research presented at the American Orthopedic Society for Sports Medicine’s Specialty Day.
Researchers analyzed data from the Kaiser Permanente ACLR Registry. Of the cases analyzed, 4,557 involved bone-patellar tendon-bone (BPTB) autografts, 3,751 soft tissue allograft, and 5,707 hamstring allograft.
After a 3-year follow-up, the overall revision rates were 2.5% for BPTB autographs, 3.5% for hamstring autografts, and 3.7% for soft tissue allografts. Non-processed soft tissue allografts were not found to have a statistically significantly different risk of revision compared to BPTB autografts. However, compared to BPTB autografts, allografts processed with more than 1.8Mrads irradiation had a more than 2 times higher risk of revision, and grafts processed with more than 1.8Mrads or high pressure chemical processing had a more than 4 to 6 times higher risk of revision. This was true even after adjustments for age, gender, and race.
Management of the Biconcave (B2) Glenoid in Shoulder Arthroplasty: Technical Considerations
Total shoulder arthroplasty (TSA) has demonstrated excellent long-term clinical outcomes for the treatment of advanced glenohumeral osteoarthritis (OA).1-5 Glenohumeral OA is characterized by a broad spectrum of glenoid pathology. Both the morphology of the glenoid and humeral head subluxation are important preoperative factors to evaluate, as these have been shown to adversely impact shoulder arthroplasty outcomes.6,7
Walch and colleagues8 have previously classified glenoid morphology in cases of advanced glenohumeral arthritis based on the preoperative computed tomography (CT) scans of individuals undergoing shoulder arthroplasty (Figures 1A-1E). The biconcave (B2) glenoid is characterized by asymmetric posterior bone loss and a posterior translated humeral head that is seated in a biconcave glenoid. The degree and extent of bone loss in the B2 glenoid can be highly variable, ranging from the classic interpretation, in which 50% of the native glenoid fossa is preserved, to the more extreme case with little remaining native anterior glenoid. Scalise and colleagues9 have reported that determining the premorbid native glenoid version with a 3-dimensional (3D) glenoid vault model can aid in differentiating a pathologic B2 glenoid from a nonpathologic type C glenoid.
The B2 glenoid in particular has been associated with poor shoulder arthroplasty outcomes and component survivorship.6,10-12 There are many factors that are thought to contribute to this problem, such as glenoid component malposition, or undercorrection of the pathologic retroversion.6,13,14 Walch and colleagues10 reported that if the neoglenoid retroversion was greater than 27°, there was a 44% incidence of loosening and/or instability and 60% of the dislocations were observed when the humeral head subluxation was greater than 80%. Cases with severe posterior glenoid bone deficiency present a unique challenge to the surgeon, and the ability to accurately and securely place an implant in the correct anatomic position can be compromised. Standard TSA has proven excellent outcomes in the setting of typical glenohumeral OA, but in the B2 glenoid with significant posterior bone erosion, additional attention must be given to ensure adequate correction of the bony deformity, soft tissue balancing, and implant stability.
Several strategies that have been proposed to address extreme bone loss in the B2 glenoid will be discussed in this review. These include hemiarthroplasty, TSA with asymmetric reaming of the high side, TSA with bone grafting of the posterior glenoid bone loss, TSA with an augmented glenoid component, and reverse shoulder arthroplasty (RSA). Importantly, while these techniques have been proposed for managing the B2 glenoid, currently there is no gold standard consensus for the treatment of this condition. The purpose of this review is to highlight important characteristics of the B2 glenoid morphology on clinical outcomes and discuss the current surgical management options for this condition.
Preoperative Planning
Being able to accurately determine the amount of retroversion is critical for preoperative planning. Friedman and colleagues15 initially described a method to measure glenoid retroversion; however, this is less accurate in B2 glenoids (Figures 2A, 2B). More recently, Rouleau and colleagues16 have validated and published methods to measure glenoid retroversion and subluxation in the B2 glenoid using 3 reference lines: the paleoglenoid (native glenoid surface), intermediate glenoid (line from anterior and posterior edge), and neoglenoid (eroded posterior surface) (Figure 2).
Preoperative evaluation starts with plain radiographs; however, additional imaging is needed, as the axillary view has shown to overestimate retroversion in 86% of patients (Figures 3A-3E).17 For a detailed evaluation of the glenoid retroversion and bone deficiency, CT scans with 3D reconstructions are useful.18,19 The surgical plan should be guided by the location and extent of glenoid bone loss. One tool that has been developed to help in predicting premorbid glenoid version, inclination, and position of the joint line is the 3D virtual glenoid vault model.9,20,21 This helps determine accurate premorbid glenoid anatomy and has been shown to assist in the selection of the optimal implant in an attempt to restore native glenoid anatomy, and avoid peg perforation.21 Patient-specific instrumentation (PSI) for shoulder arthroplasty is being used more frequently and has shown promise for more accurate glenoid component placement, particularly in the complex glenoid with severe bone deficiency. PSI involves creating a custom-fitted guide that is referenced to surface anatomy derived from the preoperative CT scan, which can then direct the surgeon toward optimal implant position with regard to glenoid component location, version and inclination (Figures 4A, 4B). Early reports show that PSI has resulted in a significant reduction in the frequency of malpositioned glenoid implants, with the greatest benefit observed in patients with retroversion in excess of 16°.22
Surgical Management
Hemiarthroplasty
Shoulder hemiarthroplasty has been traditionally described as an option for younger, more active patients in whom longevity of the glenoid component is a concern, or in patients with inadequate glenoid bone stock to tolerate a glenoid component. While there are no reports of hemiarthroplasty specifically for patients with B2 glenoids, one study has examined the effect of glenoid morphology on the outcomes of hemiarthroplasty for shoulder osteoarthritis. Levine and colleagues7 reported inferior clinical outcomes after shoulder hemiarthroplasty in patients with eccentric posterior glenoid wear. Several authors have advocated a “ream-and-run” technique to create a concentric glenoid and re-center the humeral head while still maintaining the native glenoid.23,24 However, in a recent series of 162 ream-and-run procedures, Gilmer and colleagues25 reported that only 23% of patients with B2 glenoid geometry achieved a minimal clinically important change in patient-reported outcome scores and 14% required revision. Furthermore, Lynch and colleagues26 found that progressive medial erosion and recurrent posterior glenoid erosion occur in a significant percentage of patients at early follow-up. Given these recent findings, the use of hemiarthroplasty alone or a ream-and-run procedure for patients with B2 glenoid morphology should be approached with caution.
Total Shoulder Arthroplasty
As with any TSA, the primary goals in treating patients with B2 glenoid defects are to provide the patient with a pain-free, stable, and functional shoulder (Figures 5A-5D). There are, however, a few challenges that are unique to TSA in the setting of B2 glenoid defects. Because the humeral head is often subluxated posteriorly into the defect, the anterior capsule and rotator cuff can tighten while the posterior aspect of the joint becomes lax. These soft tissues must be balanced during TSA in order to stabilize the shoulder and restore the appropriate length-tension relationship of the rotator cuff. The other primary concern is restoration of appropriate glenoid version and lateralization. To accomplish this, the most common techniques utilized are asymmetric reaming, bone graft augmentation, and glenoid component augmentation.27,28
Asymmetric Reaming. One of the more readily utilized techniques for addressing the B2 glenoid during TSA is eccentric or asymmetric reaming. During this process, the anterior glenoid is preferentially reamed while little to no bone is removed posteriorly. This technique is generally felt to be sufficient to treat posterior defects up to 5 mm to 8 mm or retroversion up to 15°.28 These upper limits have been confirmed in a number of cadaveric and simulated models.29-31
The success of this technique hinges on excellent glenoid exposure. With appropriate retractors in place, the anterior capsulolabral complex, including the biceps insertion, is resected to improve visualization. The inferior capsule must be resected carefully to ensure exposure and better motion postoperatively. On the other hand, it is imperative to protect the posterior capsulolabral attachments because of the increased risk of posterior instability in patients with B2 glenoids.
Detailed imaging such as CT scans with 3D reconstructions have improved our understanding of the degree of the deformities in all directions, which can better guide the reaming. PSI and planning software developed to improve the surgeon’s ability to place the glenoid component centrally in the best possible position after version correction can be even more helpful. We find that using a burr to provisionally lower the high side (anterior) provides a more en face view, which subsequently makes the eccentric reaming easier. As a guide, we will not ream more than 1 cm of anterior bone or attempt to correct more than ~20° of retroversion. The goal should be to create a glenoid surface that is more neutral and congruent to the posterior surface of the glenoid component while not overmedializing the component.
Although eccentric reaming may be one of the more straightforward methods for addressing posterior glenoid erosion, it is not without a number of potential downsides. When attempting to correct defects >10 mm or retroversion beyond 15°, excessive medialization of the implant can occur. Although increasing the thickness of the glenoid component can compensate for small amounts of medialization, excessive medialization can lead to a number of issues.27,28,32 As reaming progresses medially, the risk of keel penetration increases as the glenoid vault narrows.30,32 Further medialization decreases posterior cortical support for the implant, which increases the risk of component loosening and subsidence.33-35 The more medial the implant is placed, the smaller the surface of available bone for implant fixation. This often requires utilization of a smaller sized glenoid component that may result in component mismatch with the humeral implant. Finally, excessive medialization has the potential to under tension the rotator cuff, leading to decreased shoulder stability, strength, and function.
Bone Graft Augmentation. When posterior erosion becomes too excessive to address with eccentric reaming alone, defect augmentation is another option to consider (Figures 6A-6E). While technically more demanding, bone graft also provides the advantage of better re-creating the natural joint line and center of rotation of the glenohumeral joint.
For most defects, the resected humeral head provides the ideal source of graft. After initial reaming of the anterior glenoid, the defect must be sized and measured. We then recommend using a guided, cannulated system to place a central pin, lying perpendicular to the glenoid axis in neutral position. The anterior glenoid is then reamed enough to create a flat surface on which to attach the bone graft. The posterior surface is then gently burred to create a bleeding surface to enhance graft incorporation. The graft is then contoured to the defect and placed flush with the anterior glenoid. Cannulated screws are placed over guidewires to fix the graft. Using an arthroscopic cannula inserted posteriorly allows for easier placement of the guidewires and easier implantation of the screws. Although a reamer or burr can be used to contour the graft once it is fixed in place, this should be minimized to prevent loss of fixation. When the graft is fixed, we then cement the glenoid component into place.
Although good clinical results have been obtained with this technique, there is concern of incomplete graft healing and component loosening in the long term. Even in clinically asymptomatic and well functioning patients, some degree of radiographic lucency may be present in over 50% of cases.31,36,37 Glenoid Component Augmentation. To address the issues related to lucency and nonunion of bone graft augmentation, several augmented glenoid components have been developed. Augmented glenoid components have the benefit of filling posterior defects and stabilizing the shoulder without requiring excessive medialization (as often occurs with eccentric reaming) or union of a bone-to-bone interface (as is required in bone graft augmentation).38 Although many of the metal back designs experienced undesirably high failure rates and have since been recalled,39 more modern all-polyethylene components hold promise. The 2 most commonly utilized designs are the posterior step augment (DePuy) and the posterior wedge (Exactech). Although biomechanical analyses of both designs have demonstrated increased stability during loading in cadaveric and simulation models, the step augment (DePuy) has demonstrated increased stability and resistance to loosening.40,41 Although midterm results are not yet available for this newest generation of augmented components, short-term results with 2 to 3 years of follow-up have demonstrated excellent clinical outcomes.28
Reverse Total Shoulder Arthroplasty
While most commonly indicated for patients with rotator cuff tear arthropathy, RSA has recently been advocated for older patients with osteoarthritis and B2 glenoids in the setting of an intact rotator cuff. The semi-constrained design of the RSA is a potential solution to the static posterior humeral head subluxation seen in patients with B2 glenoid geometry (Figure 6E).
Technically, RSA is often an easier solution than a TSA with bone grafting because there is usually enough glenoid bone stock for fixation. That said, we always get a CT scan with 3D reconstructions to better appreciate the anatomy. Note that in B2 glenoids, the bone loss is typically posterior and inferior. RSA in the setting of a B2 glenoid is one of the ideal indications to use PSI to ensure ideal placement of the central pin, which is the key to glenoid baseplate positioning. Even when using a RSA, eccentric reaming and/or bone grafting allow for more ideal component placement. Using the same eccentric reaming techniques described above, one should try to ream to place the baseplate at 10° of retroversion. In cases where retroversion cannot be corrected to 10°, graft can be taken from the humeral head, iliac crest, or allograft. A benefit to using bone graft with RSA as opposed to TSA is that the graft can be fashioned to the baseplate, impacted/compressed into the B2 glenoid, and then secured with a central compression screw and peripheral locking screws.
Mizuno and colleagues41 reported a retrospective series of 27 RSAs performed for primary glenohumeral osteoarthritis and biconcave glenoid. At a mean follow-up of nearly 5 years, the authors noted significant improvement in Constant scores and shoulder motion with minimal complications. There was no recurrence of posterior instability observed by the time of final follow-up.41
RSA is a promising treatment for primary glenohumeral arthritis with posterior glenoid bone loss and static posterior subluxation in elderly or less active patients, but the longevity of these implants has yet to be established for younger, more active patients and requires further study.
Conclusion
Reconstruction of the B2 glenoid presents a challenging clinical problem that has been associated with poor clinical outcomes and implant survivorship. The high failure rate from glenoid component loosening and subsequent premature implant failure can be substantially decreased with accurate glenoid component positioning and appropriate correction of the pathologic glenoid retroversion. Careful preoperative planning is essential for accurate preparation and execution of the optimal surgical plan. There are many surgical strategies to address the B2 glenoid, but no consensus on the optimal method exists, as the technique should be uniquely customized to the individual’s pathology and surgeon preference (Table). Cases with mild deformity may be corrected with eccentric reaming and TSA, while the more severe deformities may require posterior glenoid bone grafting and/or augmented implants to restore native version. Finally, the RSA is a reliable option to restore stability and address bone deficiency for the severe B2 glenoid in an older, lower demand patient.
1. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
2. Bryant D, Litchfield R, Sandow M, Gartsman GM, Guyatt G, Kirkley A. A comparison of pain, strength, range of motion, and functional outcomes after hemiarthroplasty and total shoulder arthroplasty in patients with osteoarthritis of the shoulder. A systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87(9):1947-1956.
3. Matsen FA 3rd. Early effectiveness of shoulder arthroplasty for patients who have primary glenohumeral degenerative joint disease. J Bone Joint Surg Am. 1996;78(2):260-264.
4. Fenlin JM Jr, Frieman BG. Indications, technique, and results of total shoulder arthroplasty in osteoarthritis. Orthop Clin North Am. 1998;29(3):423-434.
5. Singh JA, Sperling JW, Cofield RH. Revision surgery following total shoulder arthroplasty: Analysis of 2588 shoulders over three decades (1976 to 2008). J Bone Joint Surg Br. 2011;93(11):1513-1517.
6. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 2003;85-A(2):251-258.
7. Levine WN, Djurasovic M, Glasson JM, Pollock RG, Flatow EL, Bigliani LU. Hemiarthroplasty for glenohumeral osteoarthritis: Results correlated to degree of glenoid wear. J Shoulder Elbow Surg. 1997;6(5):449-454.
8. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
9. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491.
10. Walch G, Moraga C, Young A, Castellanos-Rosas J. Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Shoulder Elbow Surg. 2012;21(11):1526-1533.
11. Kany J, Katz D. How to deal with glenoid type B2 or C? How to prevent mistakes in implantation of glenoid component? Eur J Orthop Surg Traumatol. 2013;23(4):379-385.
12. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598.
13. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55.
14. Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013;95(12):e82.
15. Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am. 1992;74(7):1032-1037.
16. Rouleau DM, Kidder JF, Pons-Villanueva J, Dynamidis S, Defranco M, Walch G. Glenoid version: How to measure it? Validity of different methods in two-dimensional computed tomography scans. J Shoulder Elbow Surg. 2010;19(8):1230-1237.
17. Nyffeler RW, Jost B, Pfirrmann CW, Gerber C. Measurement of glenoid version: Conventional radiographs versus computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496.
18. Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577-583.
19. Bokor DJ, O’Sullivan MD, Hazan GJ. Variability of measurement of glenoid version on computed tomography scan. J Shoulder Elbow Surg. 1999;8(6):595-598.
20. Ganapathi A, McCarron JA, Chen X, Iannotti JP. Predicting normal glenoid version from the pathologic scapula: A comparison of 4 methods in 2- and 3-dimensional models. J Shoulder Elbow Surg. 2011;20(2):234-244.
21. Ricchetti ET, Hendel MD, Collins DN, Iannotti JP. Is premorbid glenoid anatomy altered in patients with glenohumeral osteoarthritis? Clin Orthop Relat Res. 2013;471(9):2932-2939.
22. Hendel MD, Bryan JA, Barsoum WK, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: A randomized prospective clinical trial. J Bone Joint Surg Am. 2012;94(23):2167-2175.
23. Matsen FA 3rd, Warme WJ, Jackins SE. Can the ream and run procedure improve glenohumeral relationships and function for shoulders with the arthritic triad? Clin Orthop Relat Res. 2015;473(6):2088-2096.
24. Saltzman MD, Chamberlain AM, Mercer DM, Warme WJ, Bertelsen AL, Matsen FA 3rd. Shoulder hemiarthroplasty with concentric glenoid reaming in patients 55 years old or less. J Shoulder Elbow Surg. 2011;20(4):609-615.
25. Gilmer BB, Comstock BA, Jette JL, Warme WJ, Jackins SE, Matsen FA. The prognosis for improvement in comfort and function after the ream-and-run arthroplasty for glenohumeral arthritis: An analysis of 176 consecutive cases. J Bone Joint Surg Am. 2012;94(14):e102.
26. Lynch JR, Franta AK, Montgomery WH Jr, Lenters TR, Mounce D, Matsen FA 3rd. Self-assessed outcome at two to four years after shoulder hemiarthroplasty with concentric glenoid reaming. J Bone Joint Surg Am. 2007;89(6):1284-1292.
27. Donohue KW, Ricchetti ET, Iannotti JP. Surgical management of the biconcave (B2) glenoid. Curr Rev Musculoskelet Med. 2016;9(1):30-39.
28. Clavert P, Millett PJ, Warner JJ. Glenoid resurfacing: What are the limits to asymmetric reaming for posterior erosion? J Shoulder Elbow Surg. 2007;16(6):843-848.
29. Gillespie R, Lyons R, Lazarus M. Eccentric reaming in total shoulder arthroplasty: A cadaveric study. Orthopedics. 2009;32(1):21.
30. Neer CS 2nd, Morrison DS. Glenoid bone-grafting in total shoulder arthroplasty. J Bone Joint Surg Am. 1988;70(8):1154-1162.
31. Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: The amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680-688.
32. Strauss EJ, Roche C, Flurin PH, Wright T, Zuckerman JD. The glenoid in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(5):819-833.
33. Walch G, Young AA, Boileau P, Loew M, Gazielly D, Mole D. Patterns of loosening of polyethylene keeled glenoid components after shoulder arthroplasty for primary osteoarthritis: Results of a multicenter study with more than five years of follow-up. J Bone Joint Surg Am. 2012;94(2):145-150.
34. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: Multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
35. Klika BJ, Wooten CW, Sperling JW, et al. Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):1066-1072.
36. Sabesan V, Callanan M, Sharma V, Iannotti JP. Correction of acquired glenoid bone loss in osteoarthritis with a standard versus an augmented glenoid component. J Shoulder Elbow Surg. 2014;23(7):964-973.
37. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9(5):361-367.
38. Cil A, Sperling JW, Cofield RH. Nonstandard glenoid components for bone deficiencies in shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):e149-e157.
39. Iannotti JP, Lappin KE, Klotz CL, Reber EW, Swope SW. Liftoff resistance of augmented glenoid components during cyclic fatigue loading in the posterior-superior direction. J Shoulder Elbow Surg. 2013;22(11):1530-1536.
40. Knowles NK, Ferreira LM, Athwal GS. Augmented glenoid component designs for type B2 erosions: A computational comparison by volume of bone removal and quality of remaining bone. J Shoulder Elbow Surg. 2015;24(8):1218-1226.
41. Mizuno N, Denard PJ, Raiss P, Walch G. Reverse total shoulder arthroplasty for primary glenohumeral osteoarthritis in patients with a biconcave glenoid. J Bone Joint Surg Am. 2013;95(14):1297-1304.
Total shoulder arthroplasty (TSA) has demonstrated excellent long-term clinical outcomes for the treatment of advanced glenohumeral osteoarthritis (OA).1-5 Glenohumeral OA is characterized by a broad spectrum of glenoid pathology. Both the morphology of the glenoid and humeral head subluxation are important preoperative factors to evaluate, as these have been shown to adversely impact shoulder arthroplasty outcomes.6,7
Walch and colleagues8 have previously classified glenoid morphology in cases of advanced glenohumeral arthritis based on the preoperative computed tomography (CT) scans of individuals undergoing shoulder arthroplasty (Figures 1A-1E). The biconcave (B2) glenoid is characterized by asymmetric posterior bone loss and a posterior translated humeral head that is seated in a biconcave glenoid. The degree and extent of bone loss in the B2 glenoid can be highly variable, ranging from the classic interpretation, in which 50% of the native glenoid fossa is preserved, to the more extreme case with little remaining native anterior glenoid. Scalise and colleagues9 have reported that determining the premorbid native glenoid version with a 3-dimensional (3D) glenoid vault model can aid in differentiating a pathologic B2 glenoid from a nonpathologic type C glenoid.
The B2 glenoid in particular has been associated with poor shoulder arthroplasty outcomes and component survivorship.6,10-12 There are many factors that are thought to contribute to this problem, such as glenoid component malposition, or undercorrection of the pathologic retroversion.6,13,14 Walch and colleagues10 reported that if the neoglenoid retroversion was greater than 27°, there was a 44% incidence of loosening and/or instability and 60% of the dislocations were observed when the humeral head subluxation was greater than 80%. Cases with severe posterior glenoid bone deficiency present a unique challenge to the surgeon, and the ability to accurately and securely place an implant in the correct anatomic position can be compromised. Standard TSA has proven excellent outcomes in the setting of typical glenohumeral OA, but in the B2 glenoid with significant posterior bone erosion, additional attention must be given to ensure adequate correction of the bony deformity, soft tissue balancing, and implant stability.
Several strategies that have been proposed to address extreme bone loss in the B2 glenoid will be discussed in this review. These include hemiarthroplasty, TSA with asymmetric reaming of the high side, TSA with bone grafting of the posterior glenoid bone loss, TSA with an augmented glenoid component, and reverse shoulder arthroplasty (RSA). Importantly, while these techniques have been proposed for managing the B2 glenoid, currently there is no gold standard consensus for the treatment of this condition. The purpose of this review is to highlight important characteristics of the B2 glenoid morphology on clinical outcomes and discuss the current surgical management options for this condition.
Preoperative Planning
Being able to accurately determine the amount of retroversion is critical for preoperative planning. Friedman and colleagues15 initially described a method to measure glenoid retroversion; however, this is less accurate in B2 glenoids (Figures 2A, 2B). More recently, Rouleau and colleagues16 have validated and published methods to measure glenoid retroversion and subluxation in the B2 glenoid using 3 reference lines: the paleoglenoid (native glenoid surface), intermediate glenoid (line from anterior and posterior edge), and neoglenoid (eroded posterior surface) (Figure 2).
Preoperative evaluation starts with plain radiographs; however, additional imaging is needed, as the axillary view has shown to overestimate retroversion in 86% of patients (Figures 3A-3E).17 For a detailed evaluation of the glenoid retroversion and bone deficiency, CT scans with 3D reconstructions are useful.18,19 The surgical plan should be guided by the location and extent of glenoid bone loss. One tool that has been developed to help in predicting premorbid glenoid version, inclination, and position of the joint line is the 3D virtual glenoid vault model.9,20,21 This helps determine accurate premorbid glenoid anatomy and has been shown to assist in the selection of the optimal implant in an attempt to restore native glenoid anatomy, and avoid peg perforation.21 Patient-specific instrumentation (PSI) for shoulder arthroplasty is being used more frequently and has shown promise for more accurate glenoid component placement, particularly in the complex glenoid with severe bone deficiency. PSI involves creating a custom-fitted guide that is referenced to surface anatomy derived from the preoperative CT scan, which can then direct the surgeon toward optimal implant position with regard to glenoid component location, version and inclination (Figures 4A, 4B). Early reports show that PSI has resulted in a significant reduction in the frequency of malpositioned glenoid implants, with the greatest benefit observed in patients with retroversion in excess of 16°.22
Surgical Management
Hemiarthroplasty
Shoulder hemiarthroplasty has been traditionally described as an option for younger, more active patients in whom longevity of the glenoid component is a concern, or in patients with inadequate glenoid bone stock to tolerate a glenoid component. While there are no reports of hemiarthroplasty specifically for patients with B2 glenoids, one study has examined the effect of glenoid morphology on the outcomes of hemiarthroplasty for shoulder osteoarthritis. Levine and colleagues7 reported inferior clinical outcomes after shoulder hemiarthroplasty in patients with eccentric posterior glenoid wear. Several authors have advocated a “ream-and-run” technique to create a concentric glenoid and re-center the humeral head while still maintaining the native glenoid.23,24 However, in a recent series of 162 ream-and-run procedures, Gilmer and colleagues25 reported that only 23% of patients with B2 glenoid geometry achieved a minimal clinically important change in patient-reported outcome scores and 14% required revision. Furthermore, Lynch and colleagues26 found that progressive medial erosion and recurrent posterior glenoid erosion occur in a significant percentage of patients at early follow-up. Given these recent findings, the use of hemiarthroplasty alone or a ream-and-run procedure for patients with B2 glenoid morphology should be approached with caution.
Total Shoulder Arthroplasty
As with any TSA, the primary goals in treating patients with B2 glenoid defects are to provide the patient with a pain-free, stable, and functional shoulder (Figures 5A-5D). There are, however, a few challenges that are unique to TSA in the setting of B2 glenoid defects. Because the humeral head is often subluxated posteriorly into the defect, the anterior capsule and rotator cuff can tighten while the posterior aspect of the joint becomes lax. These soft tissues must be balanced during TSA in order to stabilize the shoulder and restore the appropriate length-tension relationship of the rotator cuff. The other primary concern is restoration of appropriate glenoid version and lateralization. To accomplish this, the most common techniques utilized are asymmetric reaming, bone graft augmentation, and glenoid component augmentation.27,28
Asymmetric Reaming. One of the more readily utilized techniques for addressing the B2 glenoid during TSA is eccentric or asymmetric reaming. During this process, the anterior glenoid is preferentially reamed while little to no bone is removed posteriorly. This technique is generally felt to be sufficient to treat posterior defects up to 5 mm to 8 mm or retroversion up to 15°.28 These upper limits have been confirmed in a number of cadaveric and simulated models.29-31
The success of this technique hinges on excellent glenoid exposure. With appropriate retractors in place, the anterior capsulolabral complex, including the biceps insertion, is resected to improve visualization. The inferior capsule must be resected carefully to ensure exposure and better motion postoperatively. On the other hand, it is imperative to protect the posterior capsulolabral attachments because of the increased risk of posterior instability in patients with B2 glenoids.
Detailed imaging such as CT scans with 3D reconstructions have improved our understanding of the degree of the deformities in all directions, which can better guide the reaming. PSI and planning software developed to improve the surgeon’s ability to place the glenoid component centrally in the best possible position after version correction can be even more helpful. We find that using a burr to provisionally lower the high side (anterior) provides a more en face view, which subsequently makes the eccentric reaming easier. As a guide, we will not ream more than 1 cm of anterior bone or attempt to correct more than ~20° of retroversion. The goal should be to create a glenoid surface that is more neutral and congruent to the posterior surface of the glenoid component while not overmedializing the component.
Although eccentric reaming may be one of the more straightforward methods for addressing posterior glenoid erosion, it is not without a number of potential downsides. When attempting to correct defects >10 mm or retroversion beyond 15°, excessive medialization of the implant can occur. Although increasing the thickness of the glenoid component can compensate for small amounts of medialization, excessive medialization can lead to a number of issues.27,28,32 As reaming progresses medially, the risk of keel penetration increases as the glenoid vault narrows.30,32 Further medialization decreases posterior cortical support for the implant, which increases the risk of component loosening and subsidence.33-35 The more medial the implant is placed, the smaller the surface of available bone for implant fixation. This often requires utilization of a smaller sized glenoid component that may result in component mismatch with the humeral implant. Finally, excessive medialization has the potential to under tension the rotator cuff, leading to decreased shoulder stability, strength, and function.
Bone Graft Augmentation. When posterior erosion becomes too excessive to address with eccentric reaming alone, defect augmentation is another option to consider (Figures 6A-6E). While technically more demanding, bone graft also provides the advantage of better re-creating the natural joint line and center of rotation of the glenohumeral joint.
For most defects, the resected humeral head provides the ideal source of graft. After initial reaming of the anterior glenoid, the defect must be sized and measured. We then recommend using a guided, cannulated system to place a central pin, lying perpendicular to the glenoid axis in neutral position. The anterior glenoid is then reamed enough to create a flat surface on which to attach the bone graft. The posterior surface is then gently burred to create a bleeding surface to enhance graft incorporation. The graft is then contoured to the defect and placed flush with the anterior glenoid. Cannulated screws are placed over guidewires to fix the graft. Using an arthroscopic cannula inserted posteriorly allows for easier placement of the guidewires and easier implantation of the screws. Although a reamer or burr can be used to contour the graft once it is fixed in place, this should be minimized to prevent loss of fixation. When the graft is fixed, we then cement the glenoid component into place.
Although good clinical results have been obtained with this technique, there is concern of incomplete graft healing and component loosening in the long term. Even in clinically asymptomatic and well functioning patients, some degree of radiographic lucency may be present in over 50% of cases.31,36,37 Glenoid Component Augmentation. To address the issues related to lucency and nonunion of bone graft augmentation, several augmented glenoid components have been developed. Augmented glenoid components have the benefit of filling posterior defects and stabilizing the shoulder without requiring excessive medialization (as often occurs with eccentric reaming) or union of a bone-to-bone interface (as is required in bone graft augmentation).38 Although many of the metal back designs experienced undesirably high failure rates and have since been recalled,39 more modern all-polyethylene components hold promise. The 2 most commonly utilized designs are the posterior step augment (DePuy) and the posterior wedge (Exactech). Although biomechanical analyses of both designs have demonstrated increased stability during loading in cadaveric and simulation models, the step augment (DePuy) has demonstrated increased stability and resistance to loosening.40,41 Although midterm results are not yet available for this newest generation of augmented components, short-term results with 2 to 3 years of follow-up have demonstrated excellent clinical outcomes.28
Reverse Total Shoulder Arthroplasty
While most commonly indicated for patients with rotator cuff tear arthropathy, RSA has recently been advocated for older patients with osteoarthritis and B2 glenoids in the setting of an intact rotator cuff. The semi-constrained design of the RSA is a potential solution to the static posterior humeral head subluxation seen in patients with B2 glenoid geometry (Figure 6E).
Technically, RSA is often an easier solution than a TSA with bone grafting because there is usually enough glenoid bone stock for fixation. That said, we always get a CT scan with 3D reconstructions to better appreciate the anatomy. Note that in B2 glenoids, the bone loss is typically posterior and inferior. RSA in the setting of a B2 glenoid is one of the ideal indications to use PSI to ensure ideal placement of the central pin, which is the key to glenoid baseplate positioning. Even when using a RSA, eccentric reaming and/or bone grafting allow for more ideal component placement. Using the same eccentric reaming techniques described above, one should try to ream to place the baseplate at 10° of retroversion. In cases where retroversion cannot be corrected to 10°, graft can be taken from the humeral head, iliac crest, or allograft. A benefit to using bone graft with RSA as opposed to TSA is that the graft can be fashioned to the baseplate, impacted/compressed into the B2 glenoid, and then secured with a central compression screw and peripheral locking screws.
Mizuno and colleagues41 reported a retrospective series of 27 RSAs performed for primary glenohumeral osteoarthritis and biconcave glenoid. At a mean follow-up of nearly 5 years, the authors noted significant improvement in Constant scores and shoulder motion with minimal complications. There was no recurrence of posterior instability observed by the time of final follow-up.41
RSA is a promising treatment for primary glenohumeral arthritis with posterior glenoid bone loss and static posterior subluxation in elderly or less active patients, but the longevity of these implants has yet to be established for younger, more active patients and requires further study.
Conclusion
Reconstruction of the B2 glenoid presents a challenging clinical problem that has been associated with poor clinical outcomes and implant survivorship. The high failure rate from glenoid component loosening and subsequent premature implant failure can be substantially decreased with accurate glenoid component positioning and appropriate correction of the pathologic glenoid retroversion. Careful preoperative planning is essential for accurate preparation and execution of the optimal surgical plan. There are many surgical strategies to address the B2 glenoid, but no consensus on the optimal method exists, as the technique should be uniquely customized to the individual’s pathology and surgeon preference (Table). Cases with mild deformity may be corrected with eccentric reaming and TSA, while the more severe deformities may require posterior glenoid bone grafting and/or augmented implants to restore native version. Finally, the RSA is a reliable option to restore stability and address bone deficiency for the severe B2 glenoid in an older, lower demand patient.
Total shoulder arthroplasty (TSA) has demonstrated excellent long-term clinical outcomes for the treatment of advanced glenohumeral osteoarthritis (OA).1-5 Glenohumeral OA is characterized by a broad spectrum of glenoid pathology. Both the morphology of the glenoid and humeral head subluxation are important preoperative factors to evaluate, as these have been shown to adversely impact shoulder arthroplasty outcomes.6,7
Walch and colleagues8 have previously classified glenoid morphology in cases of advanced glenohumeral arthritis based on the preoperative computed tomography (CT) scans of individuals undergoing shoulder arthroplasty (Figures 1A-1E). The biconcave (B2) glenoid is characterized by asymmetric posterior bone loss and a posterior translated humeral head that is seated in a biconcave glenoid. The degree and extent of bone loss in the B2 glenoid can be highly variable, ranging from the classic interpretation, in which 50% of the native glenoid fossa is preserved, to the more extreme case with little remaining native anterior glenoid. Scalise and colleagues9 have reported that determining the premorbid native glenoid version with a 3-dimensional (3D) glenoid vault model can aid in differentiating a pathologic B2 glenoid from a nonpathologic type C glenoid.
The B2 glenoid in particular has been associated with poor shoulder arthroplasty outcomes and component survivorship.6,10-12 There are many factors that are thought to contribute to this problem, such as glenoid component malposition, or undercorrection of the pathologic retroversion.6,13,14 Walch and colleagues10 reported that if the neoglenoid retroversion was greater than 27°, there was a 44% incidence of loosening and/or instability and 60% of the dislocations were observed when the humeral head subluxation was greater than 80%. Cases with severe posterior glenoid bone deficiency present a unique challenge to the surgeon, and the ability to accurately and securely place an implant in the correct anatomic position can be compromised. Standard TSA has proven excellent outcomes in the setting of typical glenohumeral OA, but in the B2 glenoid with significant posterior bone erosion, additional attention must be given to ensure adequate correction of the bony deformity, soft tissue balancing, and implant stability.
Several strategies that have been proposed to address extreme bone loss in the B2 glenoid will be discussed in this review. These include hemiarthroplasty, TSA with asymmetric reaming of the high side, TSA with bone grafting of the posterior glenoid bone loss, TSA with an augmented glenoid component, and reverse shoulder arthroplasty (RSA). Importantly, while these techniques have been proposed for managing the B2 glenoid, currently there is no gold standard consensus for the treatment of this condition. The purpose of this review is to highlight important characteristics of the B2 glenoid morphology on clinical outcomes and discuss the current surgical management options for this condition.
Preoperative Planning
Being able to accurately determine the amount of retroversion is critical for preoperative planning. Friedman and colleagues15 initially described a method to measure glenoid retroversion; however, this is less accurate in B2 glenoids (Figures 2A, 2B). More recently, Rouleau and colleagues16 have validated and published methods to measure glenoid retroversion and subluxation in the B2 glenoid using 3 reference lines: the paleoglenoid (native glenoid surface), intermediate glenoid (line from anterior and posterior edge), and neoglenoid (eroded posterior surface) (Figure 2).
Preoperative evaluation starts with plain radiographs; however, additional imaging is needed, as the axillary view has shown to overestimate retroversion in 86% of patients (Figures 3A-3E).17 For a detailed evaluation of the glenoid retroversion and bone deficiency, CT scans with 3D reconstructions are useful.18,19 The surgical plan should be guided by the location and extent of glenoid bone loss. One tool that has been developed to help in predicting premorbid glenoid version, inclination, and position of the joint line is the 3D virtual glenoid vault model.9,20,21 This helps determine accurate premorbid glenoid anatomy and has been shown to assist in the selection of the optimal implant in an attempt to restore native glenoid anatomy, and avoid peg perforation.21 Patient-specific instrumentation (PSI) for shoulder arthroplasty is being used more frequently and has shown promise for more accurate glenoid component placement, particularly in the complex glenoid with severe bone deficiency. PSI involves creating a custom-fitted guide that is referenced to surface anatomy derived from the preoperative CT scan, which can then direct the surgeon toward optimal implant position with regard to glenoid component location, version and inclination (Figures 4A, 4B). Early reports show that PSI has resulted in a significant reduction in the frequency of malpositioned glenoid implants, with the greatest benefit observed in patients with retroversion in excess of 16°.22
Surgical Management
Hemiarthroplasty
Shoulder hemiarthroplasty has been traditionally described as an option for younger, more active patients in whom longevity of the glenoid component is a concern, or in patients with inadequate glenoid bone stock to tolerate a glenoid component. While there are no reports of hemiarthroplasty specifically for patients with B2 glenoids, one study has examined the effect of glenoid morphology on the outcomes of hemiarthroplasty for shoulder osteoarthritis. Levine and colleagues7 reported inferior clinical outcomes after shoulder hemiarthroplasty in patients with eccentric posterior glenoid wear. Several authors have advocated a “ream-and-run” technique to create a concentric glenoid and re-center the humeral head while still maintaining the native glenoid.23,24 However, in a recent series of 162 ream-and-run procedures, Gilmer and colleagues25 reported that only 23% of patients with B2 glenoid geometry achieved a minimal clinically important change in patient-reported outcome scores and 14% required revision. Furthermore, Lynch and colleagues26 found that progressive medial erosion and recurrent posterior glenoid erosion occur in a significant percentage of patients at early follow-up. Given these recent findings, the use of hemiarthroplasty alone or a ream-and-run procedure for patients with B2 glenoid morphology should be approached with caution.
Total Shoulder Arthroplasty
As with any TSA, the primary goals in treating patients with B2 glenoid defects are to provide the patient with a pain-free, stable, and functional shoulder (Figures 5A-5D). There are, however, a few challenges that are unique to TSA in the setting of B2 glenoid defects. Because the humeral head is often subluxated posteriorly into the defect, the anterior capsule and rotator cuff can tighten while the posterior aspect of the joint becomes lax. These soft tissues must be balanced during TSA in order to stabilize the shoulder and restore the appropriate length-tension relationship of the rotator cuff. The other primary concern is restoration of appropriate glenoid version and lateralization. To accomplish this, the most common techniques utilized are asymmetric reaming, bone graft augmentation, and glenoid component augmentation.27,28
Asymmetric Reaming. One of the more readily utilized techniques for addressing the B2 glenoid during TSA is eccentric or asymmetric reaming. During this process, the anterior glenoid is preferentially reamed while little to no bone is removed posteriorly. This technique is generally felt to be sufficient to treat posterior defects up to 5 mm to 8 mm or retroversion up to 15°.28 These upper limits have been confirmed in a number of cadaveric and simulated models.29-31
The success of this technique hinges on excellent glenoid exposure. With appropriate retractors in place, the anterior capsulolabral complex, including the biceps insertion, is resected to improve visualization. The inferior capsule must be resected carefully to ensure exposure and better motion postoperatively. On the other hand, it is imperative to protect the posterior capsulolabral attachments because of the increased risk of posterior instability in patients with B2 glenoids.
Detailed imaging such as CT scans with 3D reconstructions have improved our understanding of the degree of the deformities in all directions, which can better guide the reaming. PSI and planning software developed to improve the surgeon’s ability to place the glenoid component centrally in the best possible position after version correction can be even more helpful. We find that using a burr to provisionally lower the high side (anterior) provides a more en face view, which subsequently makes the eccentric reaming easier. As a guide, we will not ream more than 1 cm of anterior bone or attempt to correct more than ~20° of retroversion. The goal should be to create a glenoid surface that is more neutral and congruent to the posterior surface of the glenoid component while not overmedializing the component.
Although eccentric reaming may be one of the more straightforward methods for addressing posterior glenoid erosion, it is not without a number of potential downsides. When attempting to correct defects >10 mm or retroversion beyond 15°, excessive medialization of the implant can occur. Although increasing the thickness of the glenoid component can compensate for small amounts of medialization, excessive medialization can lead to a number of issues.27,28,32 As reaming progresses medially, the risk of keel penetration increases as the glenoid vault narrows.30,32 Further medialization decreases posterior cortical support for the implant, which increases the risk of component loosening and subsidence.33-35 The more medial the implant is placed, the smaller the surface of available bone for implant fixation. This often requires utilization of a smaller sized glenoid component that may result in component mismatch with the humeral implant. Finally, excessive medialization has the potential to under tension the rotator cuff, leading to decreased shoulder stability, strength, and function.
Bone Graft Augmentation. When posterior erosion becomes too excessive to address with eccentric reaming alone, defect augmentation is another option to consider (Figures 6A-6E). While technically more demanding, bone graft also provides the advantage of better re-creating the natural joint line and center of rotation of the glenohumeral joint.
For most defects, the resected humeral head provides the ideal source of graft. After initial reaming of the anterior glenoid, the defect must be sized and measured. We then recommend using a guided, cannulated system to place a central pin, lying perpendicular to the glenoid axis in neutral position. The anterior glenoid is then reamed enough to create a flat surface on which to attach the bone graft. The posterior surface is then gently burred to create a bleeding surface to enhance graft incorporation. The graft is then contoured to the defect and placed flush with the anterior glenoid. Cannulated screws are placed over guidewires to fix the graft. Using an arthroscopic cannula inserted posteriorly allows for easier placement of the guidewires and easier implantation of the screws. Although a reamer or burr can be used to contour the graft once it is fixed in place, this should be minimized to prevent loss of fixation. When the graft is fixed, we then cement the glenoid component into place.
Although good clinical results have been obtained with this technique, there is concern of incomplete graft healing and component loosening in the long term. Even in clinically asymptomatic and well functioning patients, some degree of radiographic lucency may be present in over 50% of cases.31,36,37 Glenoid Component Augmentation. To address the issues related to lucency and nonunion of bone graft augmentation, several augmented glenoid components have been developed. Augmented glenoid components have the benefit of filling posterior defects and stabilizing the shoulder without requiring excessive medialization (as often occurs with eccentric reaming) or union of a bone-to-bone interface (as is required in bone graft augmentation).38 Although many of the metal back designs experienced undesirably high failure rates and have since been recalled,39 more modern all-polyethylene components hold promise. The 2 most commonly utilized designs are the posterior step augment (DePuy) and the posterior wedge (Exactech). Although biomechanical analyses of both designs have demonstrated increased stability during loading in cadaveric and simulation models, the step augment (DePuy) has demonstrated increased stability and resistance to loosening.40,41 Although midterm results are not yet available for this newest generation of augmented components, short-term results with 2 to 3 years of follow-up have demonstrated excellent clinical outcomes.28
Reverse Total Shoulder Arthroplasty
While most commonly indicated for patients with rotator cuff tear arthropathy, RSA has recently been advocated for older patients with osteoarthritis and B2 glenoids in the setting of an intact rotator cuff. The semi-constrained design of the RSA is a potential solution to the static posterior humeral head subluxation seen in patients with B2 glenoid geometry (Figure 6E).
Technically, RSA is often an easier solution than a TSA with bone grafting because there is usually enough glenoid bone stock for fixation. That said, we always get a CT scan with 3D reconstructions to better appreciate the anatomy. Note that in B2 glenoids, the bone loss is typically posterior and inferior. RSA in the setting of a B2 glenoid is one of the ideal indications to use PSI to ensure ideal placement of the central pin, which is the key to glenoid baseplate positioning. Even when using a RSA, eccentric reaming and/or bone grafting allow for more ideal component placement. Using the same eccentric reaming techniques described above, one should try to ream to place the baseplate at 10° of retroversion. In cases where retroversion cannot be corrected to 10°, graft can be taken from the humeral head, iliac crest, or allograft. A benefit to using bone graft with RSA as opposed to TSA is that the graft can be fashioned to the baseplate, impacted/compressed into the B2 glenoid, and then secured with a central compression screw and peripheral locking screws.
Mizuno and colleagues41 reported a retrospective series of 27 RSAs performed for primary glenohumeral osteoarthritis and biconcave glenoid. At a mean follow-up of nearly 5 years, the authors noted significant improvement in Constant scores and shoulder motion with minimal complications. There was no recurrence of posterior instability observed by the time of final follow-up.41
RSA is a promising treatment for primary glenohumeral arthritis with posterior glenoid bone loss and static posterior subluxation in elderly or less active patients, but the longevity of these implants has yet to be established for younger, more active patients and requires further study.
Conclusion
Reconstruction of the B2 glenoid presents a challenging clinical problem that has been associated with poor clinical outcomes and implant survivorship. The high failure rate from glenoid component loosening and subsequent premature implant failure can be substantially decreased with accurate glenoid component positioning and appropriate correction of the pathologic glenoid retroversion. Careful preoperative planning is essential for accurate preparation and execution of the optimal surgical plan. There are many surgical strategies to address the B2 glenoid, but no consensus on the optimal method exists, as the technique should be uniquely customized to the individual’s pathology and surgeon preference (Table). Cases with mild deformity may be corrected with eccentric reaming and TSA, while the more severe deformities may require posterior glenoid bone grafting and/or augmented implants to restore native version. Finally, the RSA is a reliable option to restore stability and address bone deficiency for the severe B2 glenoid in an older, lower demand patient.
1. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
2. Bryant D, Litchfield R, Sandow M, Gartsman GM, Guyatt G, Kirkley A. A comparison of pain, strength, range of motion, and functional outcomes after hemiarthroplasty and total shoulder arthroplasty in patients with osteoarthritis of the shoulder. A systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87(9):1947-1956.
3. Matsen FA 3rd. Early effectiveness of shoulder arthroplasty for patients who have primary glenohumeral degenerative joint disease. J Bone Joint Surg Am. 1996;78(2):260-264.
4. Fenlin JM Jr, Frieman BG. Indications, technique, and results of total shoulder arthroplasty in osteoarthritis. Orthop Clin North Am. 1998;29(3):423-434.
5. Singh JA, Sperling JW, Cofield RH. Revision surgery following total shoulder arthroplasty: Analysis of 2588 shoulders over three decades (1976 to 2008). J Bone Joint Surg Br. 2011;93(11):1513-1517.
6. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 2003;85-A(2):251-258.
7. Levine WN, Djurasovic M, Glasson JM, Pollock RG, Flatow EL, Bigliani LU. Hemiarthroplasty for glenohumeral osteoarthritis: Results correlated to degree of glenoid wear. J Shoulder Elbow Surg. 1997;6(5):449-454.
8. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
9. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491.
10. Walch G, Moraga C, Young A, Castellanos-Rosas J. Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Shoulder Elbow Surg. 2012;21(11):1526-1533.
11. Kany J, Katz D. How to deal with glenoid type B2 or C? How to prevent mistakes in implantation of glenoid component? Eur J Orthop Surg Traumatol. 2013;23(4):379-385.
12. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598.
13. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55.
14. Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013;95(12):e82.
15. Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am. 1992;74(7):1032-1037.
16. Rouleau DM, Kidder JF, Pons-Villanueva J, Dynamidis S, Defranco M, Walch G. Glenoid version: How to measure it? Validity of different methods in two-dimensional computed tomography scans. J Shoulder Elbow Surg. 2010;19(8):1230-1237.
17. Nyffeler RW, Jost B, Pfirrmann CW, Gerber C. Measurement of glenoid version: Conventional radiographs versus computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496.
18. Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577-583.
19. Bokor DJ, O’Sullivan MD, Hazan GJ. Variability of measurement of glenoid version on computed tomography scan. J Shoulder Elbow Surg. 1999;8(6):595-598.
20. Ganapathi A, McCarron JA, Chen X, Iannotti JP. Predicting normal glenoid version from the pathologic scapula: A comparison of 4 methods in 2- and 3-dimensional models. J Shoulder Elbow Surg. 2011;20(2):234-244.
21. Ricchetti ET, Hendel MD, Collins DN, Iannotti JP. Is premorbid glenoid anatomy altered in patients with glenohumeral osteoarthritis? Clin Orthop Relat Res. 2013;471(9):2932-2939.
22. Hendel MD, Bryan JA, Barsoum WK, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: A randomized prospective clinical trial. J Bone Joint Surg Am. 2012;94(23):2167-2175.
23. Matsen FA 3rd, Warme WJ, Jackins SE. Can the ream and run procedure improve glenohumeral relationships and function for shoulders with the arthritic triad? Clin Orthop Relat Res. 2015;473(6):2088-2096.
24. Saltzman MD, Chamberlain AM, Mercer DM, Warme WJ, Bertelsen AL, Matsen FA 3rd. Shoulder hemiarthroplasty with concentric glenoid reaming in patients 55 years old or less. J Shoulder Elbow Surg. 2011;20(4):609-615.
25. Gilmer BB, Comstock BA, Jette JL, Warme WJ, Jackins SE, Matsen FA. The prognosis for improvement in comfort and function after the ream-and-run arthroplasty for glenohumeral arthritis: An analysis of 176 consecutive cases. J Bone Joint Surg Am. 2012;94(14):e102.
26. Lynch JR, Franta AK, Montgomery WH Jr, Lenters TR, Mounce D, Matsen FA 3rd. Self-assessed outcome at two to four years after shoulder hemiarthroplasty with concentric glenoid reaming. J Bone Joint Surg Am. 2007;89(6):1284-1292.
27. Donohue KW, Ricchetti ET, Iannotti JP. Surgical management of the biconcave (B2) glenoid. Curr Rev Musculoskelet Med. 2016;9(1):30-39.
28. Clavert P, Millett PJ, Warner JJ. Glenoid resurfacing: What are the limits to asymmetric reaming for posterior erosion? J Shoulder Elbow Surg. 2007;16(6):843-848.
29. Gillespie R, Lyons R, Lazarus M. Eccentric reaming in total shoulder arthroplasty: A cadaveric study. Orthopedics. 2009;32(1):21.
30. Neer CS 2nd, Morrison DS. Glenoid bone-grafting in total shoulder arthroplasty. J Bone Joint Surg Am. 1988;70(8):1154-1162.
31. Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: The amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680-688.
32. Strauss EJ, Roche C, Flurin PH, Wright T, Zuckerman JD. The glenoid in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(5):819-833.
33. Walch G, Young AA, Boileau P, Loew M, Gazielly D, Mole D. Patterns of loosening of polyethylene keeled glenoid components after shoulder arthroplasty for primary osteoarthritis: Results of a multicenter study with more than five years of follow-up. J Bone Joint Surg Am. 2012;94(2):145-150.
34. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: Multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
35. Klika BJ, Wooten CW, Sperling JW, et al. Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):1066-1072.
36. Sabesan V, Callanan M, Sharma V, Iannotti JP. Correction of acquired glenoid bone loss in osteoarthritis with a standard versus an augmented glenoid component. J Shoulder Elbow Surg. 2014;23(7):964-973.
37. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9(5):361-367.
38. Cil A, Sperling JW, Cofield RH. Nonstandard glenoid components for bone deficiencies in shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):e149-e157.
39. Iannotti JP, Lappin KE, Klotz CL, Reber EW, Swope SW. Liftoff resistance of augmented glenoid components during cyclic fatigue loading in the posterior-superior direction. J Shoulder Elbow Surg. 2013;22(11):1530-1536.
40. Knowles NK, Ferreira LM, Athwal GS. Augmented glenoid component designs for type B2 erosions: A computational comparison by volume of bone removal and quality of remaining bone. J Shoulder Elbow Surg. 2015;24(8):1218-1226.
41. Mizuno N, Denard PJ, Raiss P, Walch G. Reverse total shoulder arthroplasty for primary glenohumeral osteoarthritis in patients with a biconcave glenoid. J Bone Joint Surg Am. 2013;95(14):1297-1304.
1. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
2. Bryant D, Litchfield R, Sandow M, Gartsman GM, Guyatt G, Kirkley A. A comparison of pain, strength, range of motion, and functional outcomes after hemiarthroplasty and total shoulder arthroplasty in patients with osteoarthritis of the shoulder. A systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87(9):1947-1956.
3. Matsen FA 3rd. Early effectiveness of shoulder arthroplasty for patients who have primary glenohumeral degenerative joint disease. J Bone Joint Surg Am. 1996;78(2):260-264.
4. Fenlin JM Jr, Frieman BG. Indications, technique, and results of total shoulder arthroplasty in osteoarthritis. Orthop Clin North Am. 1998;29(3):423-434.
5. Singh JA, Sperling JW, Cofield RH. Revision surgery following total shoulder arthroplasty: Analysis of 2588 shoulders over three decades (1976 to 2008). J Bone Joint Surg Br. 2011;93(11):1513-1517.
6. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 2003;85-A(2):251-258.
7. Levine WN, Djurasovic M, Glasson JM, Pollock RG, Flatow EL, Bigliani LU. Hemiarthroplasty for glenohumeral osteoarthritis: Results correlated to degree of glenoid wear. J Shoulder Elbow Surg. 1997;6(5):449-454.
8. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
9. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491.
10. Walch G, Moraga C, Young A, Castellanos-Rosas J. Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Shoulder Elbow Surg. 2012;21(11):1526-1533.
11. Kany J, Katz D. How to deal with glenoid type B2 or C? How to prevent mistakes in implantation of glenoid component? Eur J Orthop Surg Traumatol. 2013;23(4):379-385.
12. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598.
13. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55.
14. Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013;95(12):e82.
15. Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am. 1992;74(7):1032-1037.
16. Rouleau DM, Kidder JF, Pons-Villanueva J, Dynamidis S, Defranco M, Walch G. Glenoid version: How to measure it? Validity of different methods in two-dimensional computed tomography scans. J Shoulder Elbow Surg. 2010;19(8):1230-1237.
17. Nyffeler RW, Jost B, Pfirrmann CW, Gerber C. Measurement of glenoid version: Conventional radiographs versus computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496.
18. Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577-583.
19. Bokor DJ, O’Sullivan MD, Hazan GJ. Variability of measurement of glenoid version on computed tomography scan. J Shoulder Elbow Surg. 1999;8(6):595-598.
20. Ganapathi A, McCarron JA, Chen X, Iannotti JP. Predicting normal glenoid version from the pathologic scapula: A comparison of 4 methods in 2- and 3-dimensional models. J Shoulder Elbow Surg. 2011;20(2):234-244.
21. Ricchetti ET, Hendel MD, Collins DN, Iannotti JP. Is premorbid glenoid anatomy altered in patients with glenohumeral osteoarthritis? Clin Orthop Relat Res. 2013;471(9):2932-2939.
22. Hendel MD, Bryan JA, Barsoum WK, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: A randomized prospective clinical trial. J Bone Joint Surg Am. 2012;94(23):2167-2175.
23. Matsen FA 3rd, Warme WJ, Jackins SE. Can the ream and run procedure improve glenohumeral relationships and function for shoulders with the arthritic triad? Clin Orthop Relat Res. 2015;473(6):2088-2096.
24. Saltzman MD, Chamberlain AM, Mercer DM, Warme WJ, Bertelsen AL, Matsen FA 3rd. Shoulder hemiarthroplasty with concentric glenoid reaming in patients 55 years old or less. J Shoulder Elbow Surg. 2011;20(4):609-615.
25. Gilmer BB, Comstock BA, Jette JL, Warme WJ, Jackins SE, Matsen FA. The prognosis for improvement in comfort and function after the ream-and-run arthroplasty for glenohumeral arthritis: An analysis of 176 consecutive cases. J Bone Joint Surg Am. 2012;94(14):e102.
26. Lynch JR, Franta AK, Montgomery WH Jr, Lenters TR, Mounce D, Matsen FA 3rd. Self-assessed outcome at two to four years after shoulder hemiarthroplasty with concentric glenoid reaming. J Bone Joint Surg Am. 2007;89(6):1284-1292.
27. Donohue KW, Ricchetti ET, Iannotti JP. Surgical management of the biconcave (B2) glenoid. Curr Rev Musculoskelet Med. 2016;9(1):30-39.
28. Clavert P, Millett PJ, Warner JJ. Glenoid resurfacing: What are the limits to asymmetric reaming for posterior erosion? J Shoulder Elbow Surg. 2007;16(6):843-848.
29. Gillespie R, Lyons R, Lazarus M. Eccentric reaming in total shoulder arthroplasty: A cadaveric study. Orthopedics. 2009;32(1):21.
30. Neer CS 2nd, Morrison DS. Glenoid bone-grafting in total shoulder arthroplasty. J Bone Joint Surg Am. 1988;70(8):1154-1162.
31. Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: The amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680-688.
32. Strauss EJ, Roche C, Flurin PH, Wright T, Zuckerman JD. The glenoid in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(5):819-833.
33. Walch G, Young AA, Boileau P, Loew M, Gazielly D, Mole D. Patterns of loosening of polyethylene keeled glenoid components after shoulder arthroplasty for primary osteoarthritis: Results of a multicenter study with more than five years of follow-up. J Bone Joint Surg Am. 2012;94(2):145-150.
34. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: Multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
35. Klika BJ, Wooten CW, Sperling JW, et al. Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):1066-1072.
36. Sabesan V, Callanan M, Sharma V, Iannotti JP. Correction of acquired glenoid bone loss in osteoarthritis with a standard versus an augmented glenoid component. J Shoulder Elbow Surg. 2014;23(7):964-973.
37. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9(5):361-367.
38. Cil A, Sperling JW, Cofield RH. Nonstandard glenoid components for bone deficiencies in shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):e149-e157.
39. Iannotti JP, Lappin KE, Klotz CL, Reber EW, Swope SW. Liftoff resistance of augmented glenoid components during cyclic fatigue loading in the posterior-superior direction. J Shoulder Elbow Surg. 2013;22(11):1530-1536.
40. Knowles NK, Ferreira LM, Athwal GS. Augmented glenoid component designs for type B2 erosions: A computational comparison by volume of bone removal and quality of remaining bone. J Shoulder Elbow Surg. 2015;24(8):1218-1226.
41. Mizuno N, Denard PJ, Raiss P, Walch G. Reverse total shoulder arthroplasty for primary glenohumeral osteoarthritis in patients with a biconcave glenoid. J Bone Joint Surg Am. 2013;95(14):1297-1304.