User login
Polydactyly of the Hand
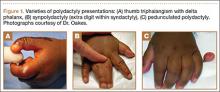
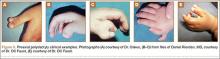
Polydactyly is the presence of extra digits. Its incidence is likely underestimated because many practitioners treat simple “nubbins” without referring them to orthopedic specialists.1-3 Polydactyly can be detected by ultrasound as early as 14 weeks’ gestational age, with partial autoamputation seen in most isolated polydactylies.4 The thumb, responsible for 40% of hand function, must be able to oppose the other digits with a stable pinch.5 Polydactyly encumbers this motion when the duplicated digits deviate from normal alignment. Ezaki6 noted that the anatomy is better described as “split” than “duplicated.” There are many dichotomous ways to classify polydactyly: preaxial (radial) versus postaxial (ulnar), thumb versus triphalangeal, simple versus complex (Figure 1). Mixed polydactyly is defined as the presence of preaxial and postaxial polydactyly.7 Surgical management seeks to allow normal hand function and to restore cosmesis.
Epidemiology
Sun and colleagues8 reported the overall polydactyly incidence as 2 per 1000 live births in China from 1998 to 2009, with a slight male predominance; polydactyly was also 3 times more common than syndactyly in this population. Ivy,9 in a 5-year audit of Pennsylvania Department of Health records, found polydactyly to be the fourth most common congenital anomaly after clubfoot, cleft lip/palate, and spina bifida. Thumb duplication occurs in 0.08 to 1.4 per 1000 live births and is more common in American Indians and Asians than in other races.5,10 It occurs in a male-to-female ratio of 2.5 to 1 and is most often unilateral.5 Postaxial polydactyly is predominant in black infants; it is most often inherited in an autosomal dominant fashion, if isolated, or in an autosomal recessive pattern, if syndromic.1 A prospective San Diego study of 11,161 newborns found postaxial type B polydactyly in 1 per 531 live births (1 per 143 black infants, 1 per 1339 white infants); 76% of cases were bilateral, and 86% had a positive family history.3 In patients of non-African descent, it is associated with anomalies in other organs. Central duplication is rare and often autosomal dominant.5,10
Genetics and Development
As early as 1896, the heritability of polydactyly was noted.11 As of 2010, polydactyly has been associated with 310 diseases.12 Ninety-nine genes, most involved in regulation of anterior-posterior formation of the limb bud, have been implicated.12,13
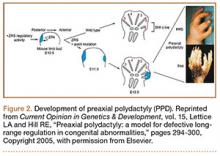
The upper limb begins to form at day 26 in utero.14 Apoptosis in the interdigital necrotic zones results in the formation of individual digits. It is presumed that, in polydactyly, the involved tissue is hypoplastic because of an abnormal interaction between mesoderm and ectoderm.5 Presence of an apical ectodermal ridge determines the formation of a limb bud, and on it the zone of polarizing activity (ZPA) dictates preaxial and postaxial alignment.14,15 The ZPA is located on the posterior zone of the developing limb bud. The levels of GLI3, a zinc finger-containing DNA-binding protein, are highest in the anterior area, and HAND2, a basic helix-loop-helix DNA-binding protein, is found in the ZPA. This polarity promotes sonic hedgehog (Shh) gene expression in the posterior region, which in turn prevents GLI3 cleavage into its repressed form. GLI3R (repressed) and GLI3A (active) concentrations are highest, therefore, in the anterior and posterior portions of the bud, respectively. The GLI3A:GLI3R ratio is responsible for the identity and number of digits in the hand (ie, the thumb develops in regions of high GLI3R). GLI and Shh mutations lead to polydactylous hands with absent thumbs (Figure 2).16
Ciliopathies have also been shown to cause postaxial polydactyly, possibly because of the role that nonmotile cilia play in hedgehog signaling.17 Mutations in Shh genomic regulators cause preaxial polydactyly.18 HoxD activates Shh in the ZPA; HoxD13 mutations are associated with synpolydactyly.16,19 In each of these mutations, Shh production is altered, and some form of polydactyly results.
Associations
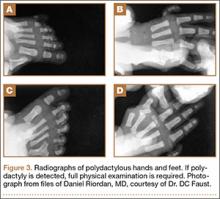
Many syndromes have been associated with polydactyly. Not all polydactyly is associated with other disorders, but the more complex the polydactyly, the more likely that other anomalies are present. Every patient who presents with polydactyly should have a full history taken and a physical examination performed (Figure 3). Any patient with syndromic findings or atypical presentations (eg, triphalangism, postaxial polydactyly in a patient of non-African descent, central and index polydactyly) should be referred to a geneticist.
Classifications
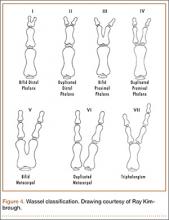
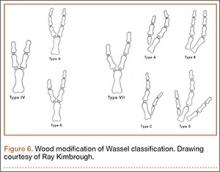
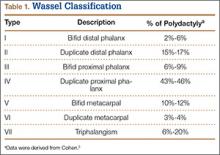
The Wassel20 classification describes the anatomical presentation of thumb duplication on the basis of 70 cases in Iowa (Figures 4, 5; Table 1). Because some duplications fall outside the Wassel classification, many researchers have proposed modifications (Figure 6).21-25
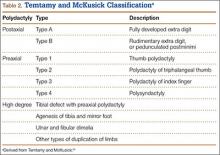
The Temtamy and McKusick10 classification, which is the product of geneticists, classifies duplications by grouping genetically related presentations (Table 2). It provides the most commonly used postaxial classification, with type A being a fully developed digit and type B a rudimentary and pedunculated digit, informally referred to as a nubbin. Type B is more common than type A. Given inheritance patterns, it is assumed that type A is likely multifactorial and type B autosomal dominant.10 Thumb polydactyly inheritance is still unclear. The other types of preaxial polydactyly and high degrees of polydactyly are rare but seem to be passed on in an autosomal dominant fashion on pedigree analysis.10
The Stelling and Turek classification presents the duplication from a tissue perspective: Type I duplication is a rudimentary mass devoid of other tissue elements; type II is a subtotal duplication with some normal structures; and type III is a duplication of the entire “osteoarticular column,” including the metacarpal.1 It is interesting to note that histology of type I duplications shows neuroma-like tissue.26-28 Again, normal is a relative term because, in polydactyly, the duplications are hypoplastic and deviated, with anomalous anatomy.
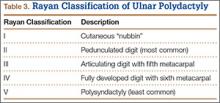
The Rayan classification describes ulnar polydactyly and was derived from a case study series of 148 patients in Oklahoma (Table 3).29
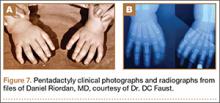
There are also some complex polydactylies that are not easily classified: ulnar dimelia, cleft hand, pentadactyly, and hyperphalangism. Ulnar dimelia, also known as “mirror hand,” is typically 7 digits with no thumb, but other variations are seen. The radius is often absent, and the elbow is abnormal. There is some debate about whether it is a fusion of 2 hands. Pentadactyly, or the 5-fingered hand, appears as 5 triphalangeal digits with no thumb (Figure 7).
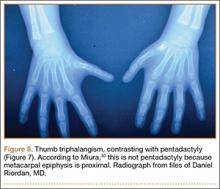
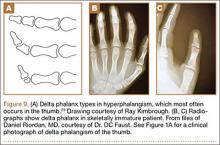
Isolated thumb triphalangism might appear similar to pentadactyly. Miura30,31 pointed out that the radial digit in the pentadactylous hand may be opposable (thumb-like) or nonopposable; in his studies, the patients with the opposable thumb had a metacarpal with a proximal epiphysis (Figure 8). Consequently, the triphalangeal thumb metacarpal with a distal epiphysis is true pentadactyly, whereas that with a proximal epiphysis is hyperphalangism (Figure 9). Treatment of these complex polydactylies involves the same underlying principles as for preaxial and postaxial polydactyly, albeit with additional proximal upper extremity considerations.
When to Operate (Timing)
Ezaki6 recommended surgical intervention at age 6 to 9 months, before fine motor skills have developed with the abnormal anatomy. Cortical learning occurs as the child begins prehensile activities before 6 months, but the risks of anesthesia outweigh functional benefits until the child is older. Waiting until 1 year of age is not uncommon, though surgery at an earlier age may be beneficial if the polydactyly affects hand function.32 It is not uncommon to wait with the more balanced thumb polydactylies to assess thumb function. Hypoplasia might also delay surgical intervention until there is enough tissue inventory for reconstruction. Wassel20 noted that surgical intervention ideally occurs before the supernumerary elements displace the normal elements, as tends to happen with growth. Suture ligation is an option in the neonatal unit for some pedunculated digits.33 Studies have shown satisfactory results in adults treated for polydactyly, if the patient presents later than expected.34
Surgical Considerations
Knavel recommended simple excision, stating that “ablation requires no ingenuity and creates no problems.”5 This belief, though true for some duplications, will not lead to the best outcome for more complex polydactylies. The goal of surgery is a stable and well-aligned thumb for pinch and prehensile activity, as well as a cosmetically pleasing hand. Incisions should not be made linearly along the axis of the digit, as the scar will cause deviation with growth.24
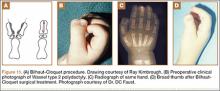
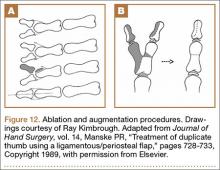
Wassel type I polydactyly might appear incidentally as a broad thumb, in which case it requires no intervention (Figure 10). However, in Wassel types I and II polydactyly with deformity, the Bilhaut-Cloquet procedure is useful for both bifid and duplicated phalanges (Figure 11).5,6,30,32,35 Collateral ligaments may need to be released in type II because of difficulty in opposing the tissue. Cosmetic results with Bilhaut-Cloquet are unpredictable. The original technique required symmetrically sized digits; results today have been improved with microtechniques and preservation of an entire nail.36 Another option is ablation of the more hypoplastic osseous element and soft-tissue augmentation of the residual digit. The theme of ablation and augmentation is seen throughout the literature for the surgical treatment of polydactyly (Figure 12).1
For type III polydactyly, the bifid proximal phalanx is narrowed by resection and realigned with osteotomy of the remaining diaphysis. Type IV polydactyly, the most common thumb duplication, often requires advancement of the abductor pollicis brevis to the base of the proximal phalanx to aid in metacarpophalangeal (MCP) stabilization, abduction, and opposition. The metacarpal head, if broad and with 2 facets, can be shaped to form a single articulating surface. The metacarpal, occasionally with the proximal phalanx, often requires realignment by closing wedge osteotomy. Last, tendons on the resected bony elements should be rebalanced on the remaining digit, and anomalous slips must be released. For instance, given a radial insertion of the long flexor tendon on the distal phalanx, the tendon should be moved centrally. A strong flexor or extensor tendon on the amputated digit should be transferred to the remaining digit.24
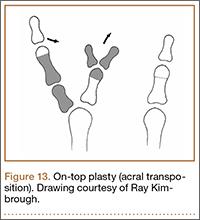
Types V and VI are treated similarly to type IV, with the addition of a first web space Z-plasty or web widening if there is thenar eminence contracture. Acral transposition has also been described, with transposition of the tip of the ablated digit in place of the tip of the kept digit; this technique is ideal if one digit has a more normal proximal part while the other has a more normal distal part (Figure 13).35
Type VII thumb polydactyly, the type most likely inherited and associated with other disorders, should be treated like type VI. The nail should be preserved; amputation of the distal phalanx is not advised. Resection of the delta phalanx or 1 interphalangeal (IP) joint is an option. Articular surfaces will remodel if done before the age of 1 year. If the thenar eminence is hypoplastic, then Huber transfer of the abductor digiti minimi should be considered.37 Resection of the triphalangeal thumb is also advised, even if the biphalangeal thumb is more hypoplastic, with transfer of the ligaments and tendons, as described earlier.5,6,24,30,32,35
Thumb triphalangism, if isolated, and hyperphalangism in the other digits, can be treated with resection of the delta phalanx or one of the IP joints if it is affecting function or cosmesis.1,6 Wood and Flatt23 recommended early resection of a thumb delta phalanx because of the likelihood of deviation that impedes thumb function. For children, they recommended delta phalanx resection and Kirschner wire fixation for 6 weeks; for adults, they recommended resection or fusion of the joint, with osteotomy as needed for deviation.23,24 For thumb triphalangism, multiple surgeries are the norm, as Wood24 reported in his study of 21 patients who underwent 78 operations in total.
Index polydactyly may present as a simple pedunculated skin tag, which can be simply excised, or as a more complex musculoskeletal duplication. More complex presentations can be treated with procedures similar to those used for the thumb. Typically, the additional digit is radially deviated and angulated, eventually leading to impingement of thumb pinch and the first web space. Ray amputation is also an option if no reconstructive surgery will produce the stable, sensate radial pinch that is essential to hand function.32
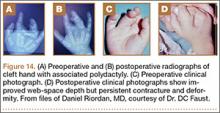
Ring-finger polydactyly and long-finger polydactyly are often complicated by some element of syndactyly, resulting in a relative paucity of skin (Figure 14). There is failure of both formation (hypoplasia) and differentiation (syndactyly). The hypoplasia particularly affects the function of these digits by tethering them; multiple surgeries to restore proper hand function are the norm.1 Reconstructive surgery for these digits requires preoperative tissue inventory followed by resection and augmentation; as in syndactyly, skin for coverage is at a premium. Creation of a 3-fingered hand is an option.23
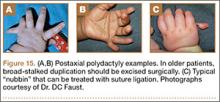
Temtamy and McKusick10 type A little-finger polydactyly is treated similarly to the thumb, with the caveat that hypothenar and intrinsic muscles that insert on the resected little finger are transferred to the remaining digit. In contrast to thumb polydactyly, the extrinsic musculature tends to be in good position. Suture ligation of type B polydactyly, as described by Flatt, is likely more common than orthopedists appreciate, as pediatricians and neonatal unit practitioners commonly perform this procedure in the nursery.1-3 It has been described with 2-0 Vicryl3 (Ethicon, Somerville, New Jersey) and 4-0 silk sutures,32 with the goal of necrosis and autoamputation. Parents should be told the finger generally falls off about 10 days (range, 4-21 days) after ligation.3 Multiple authors have cited a report of exsanguination from suture ligation, but we could not locate the primary source. It is advisable to wait until a patient is 6 months of age if planning to resect the nubbin in the operating room, given the anesthesia risk and the lack of functional impairment. Katz and Linder33 indicated they remove type B polydactyly in the nursery suite used for circumcisions; they use anesthetizing cream on the skin, and sharp excision with a scalpel, followed by direct pressure and Steri-Strip (3M, St. Paul, Minnesota) application. Suture ligation is recommended only if there is a narrow, thin (<2 mm) soft-tissue stalk; any broad or bony stalk necessitates surgical removal to avoid neuroma formation and failure of autonecrosis (Figure 15).27 Other options are a single swipe of a scalpel and elliptical excision; sharp transaction of the digital nerve with subsequent retraction is advised to avoid neuroma formation.2
Barton described ulnar dimelia operations as “spare parts surgery.”1 Extra digits are ablated and a thumb created (Figure 16). The hand might have a digit in relatively good rotational position for thumbplasty, or the principles of pollicization may need to be used. If the patient is already using the hand, the surgeon should note which finger the patient uses as a thumb.24 Any accompanying wrist flexion contracture must be corrected with careful attention to musculotendinous balancing. Because the forearm and elbow, and occasionally even the more proximal limb, will be abnormal in this disorder, multiple surgeries are again the norm.1
Pentadactyly is treated like thumb hypoplasia, with first web space creation.1
Complications
In polydactyly, a reoperation rate of up to 25% has been reported, with most reoperations performed because of residual or subsequent deformity.5,30,31,38 Risk factors for reoperation are type IV thumb duplication, preoperative “zigzag” deformity, and radially deviated thumb elements at presentation.5 The delta phalanx may not show on radiographs until the patient is 18 months old, but functional deformity will worsen as long as it is present. Zigzag deformity may be due to the delta phalanx or to musculotendinous imbalance, such as a radially inserted flexor pollicis longus (FPL) or lack of stable MCP abduction. Miura31 found that careful reconstruction of the joint capsule and thenar muscles from the ablated digit to the remnant digit is the key to a successful initial surgery. Lee and colleagues39 defined zigzag deformity as more than 20° MCP and IP angulation; for cases present before surgery, they recommended FPL relocation by the pullout technique in addition to osteotomies to prevent further interphalangeal deviation (Figures 17, 18).
Abnormal physeal growth, joint instability, and stiffness can all occur. Stiffness is particularly difficult to treat but seldom presents a functional problem. Joint enlargement, which is not uncommon, results from either broad articular surfaces or retained cartilage from the perichondral ring after resection that later ossifies.5,38 Nubbin-type duplications may not fall off after suture ligation, necessitating further excision, and a cosmetic bump is seen after 40% of suture ligations.3 Patillo and Rayan28 and Rayan and Frey29 warned against suture ligation unless the nubbin has a small stalk because of the possibility of infection and gangrene. The excised nubbin tissue is histologically nervous, and there have been reports of painful neuromas in the remaining scar of a ligated nubbin that respond well to excision.26,27,40 It is thought that these painful lesions form because the ligature prevents the digital nerves to the vestigial digit from retracting.27 Nail deformity and IP joint stiffness are seen with the Bilhaut-Cloquet procedure, though often finger function remains satisfactory.
Conclusion
Polydactyly is a common congenital hand abnormality. Its true incidence is unknown because of inconsistent documentation. Surgeons must strive for a functional, cosmetic hand, given a diverse set of possible anomalies. Hypoplasia is the rule; tissue should be ablated and augmented as necessary. Musculotendinous insertions may need to be centralized. Patients’ family members should always be counseled that more surgery may be needed in the future, as further deformity can occur with growth. Surgically corrected thumb duplications will be stiffer, shorter, and thinner than their normal counterparts. Nail ridges are common. However, it should be noted that 88% of these patients are satisfied with their results.41 Some amount of contracture and abnormal function should be expected with index-, long-, and ring-finger duplications. The only remnant of type B postaxial duplications may be a slight discoloration or bump, though stiffness and deformity can happen with a type A deformity. A “duplicated” digit that requires surgical correction will never be completely normal, but acceptable function is routinely achievable.
1. Graham TJ, Ress AM. Finger polydactyly. Hand Clin. 1998;14(1):49-64.
2. Abzug JM, Kozin SH. Treatment of postaxial polydactyly type B. J Hand Surg Am. 2013;38(6):1223-1225.
3. Watson BT, Hennrikus WL. Postaxial type-B polydactyly—prevalence and treatment. J Bone Joint Surg Am. 1997;79(1):65-68.
4. Zimmer EZ, Bronshtein M. Fetal polydactyly diagnosis during early pregnancy: clinical applications. Am J Obstet Gynecol. 2000;183(3):755-758.
5. Cohen MS. Thumb duplication. Hand Clin. 1998;14(1):17-27.
6. Ezaki M. Radial polydactyly. Hand Clin. 1990;6(4):577-588.
7. Nathan PA, Keniston RC. Crossed polydactyly: case report and review of the literature. J Bone Joint Surg Am. 1975;57(6):847-849.
8. Sun G, Xu ZM, Liang JF, Li L, Tang DX. Twelve-year prevalence of common neonatal congenital malformations in Zhejiang Province, China. World J Pediatr. 2011;7(4):331-336.
9. Ivy RH. Congenital anomalies as recorded on birth certificates in the Division of Vital Statistics of the Pennsylvania Department of Health, for the period of 1951–1955, inclusive. Plast Reconstr Surg. 1957;20(5):400-411.
10. Temtamy SA, McKusick VA. Polydactyly as a part of syndromes. In: Bergsma D, ed. Mudge JR, Paul NW, Conde Greene S, associate eds. The Genetics of Hand Malformations. New York, NY: Liss. Birth Defects Original Article Series. 1978;14(3):364-439.
11. Gould W, Pyle L. Anomalies and Curiosities of Medicine. New York, NY: Bell; 1896.
12. Biesecker LG. Polydactyly: how many disorders and how many genes: 2010 update. Dev Dyn. 2011;250(5):931-942.
13. Grzeschik K. Human limb malformations; an approach to the molecular basis of development. Int J Dev Biol. 2001;46(7):983-991.
14. Zaleske DJ. Development of the upper limb. Hand Clin. 1985;1(3):383-390.
15. Beatty E. Upper limb tissue differentiation in the human embryo. Hand Clin. 1985;1(3):391-404.
16. Anderson E, Peluso S, Lettice LA, Hill RE. Human limb abnormalities caused by disruption of hedgehog signaling. Trends Genet. 2012;28(8):364-373.
17. Ware SM, Aygun MG, Heldebrandt F. Spectrum of clinical diseases caused by disorders of primary cilia. Proc Am Thorac Soc. 2011;8(5):444-450.
18. Lettice LA, Hill RE. Preaxial polydactyly: a model for defective long-range regulation in congenital abnormalities. Curr Opin Genet Dev. 2005;15(3):294-300.
19. Al-Qattan MA. Type II familial synpolydactyly: report on two families with an emphasis on variations of expression. Eur J Hum Genet. 2011;19(1):112-114.
20. Wassel HD. The results of surgery for polydactyly of the thumb. Clin Orthop. 1969;(64):175-193.
21. Blauth W, Olason AT. Classification of polydactyly of the hands and feet. Arch Orthop Trauma Surg. 1988;107(6):334-344.
22. Wood VE. Super digit. Hand Clin. 1990;6(4):673-684.
23. Wood VE, Flatt AE. Congenital triangular bones in the hand. J Hand Surg Am. 1977;2(3):179-193.
24. Wood VE. Polydactyly and the triphalangeal thumb. J Hand Surg Am. 1978;3(5):436-444.
25. Zuidam JM, Selles RW, Ananta M, Runia J, Hovius SER. A classification system of radial polydactyly: inclusion of triphalangeal thumb and triplication. J Hand Surg Am. 2008;33(3):373-377.
26. Leber GE, Gosain AK. Surgical excision of pedunculated supernumerary digits prevents traumatic amputation neuromas. Pediatr Dermatol. 2003;20(2):108-112.
27. Mullick S, Borschel GH. A selective approach to treatment of ulnar polydactyly: preventing painful neuroma and incomplete excision. Pediatr Dermatol. 2001;27(1):39-42.
28. Patillo D, Rayan GM. Complications of suture ligation ablation for ulnar polydactyly: a report of two cases. Hand (N Y). 2011;6(1):102-105.
29. Rayan GM, Frey B. Ulnar polydactyly. Plastic Reconstr Surg. 2001;107(6):1449-1454.
30. Miura T. Triphalangeal thumb. Plastic Reconstr Surg. 1976;58(5):587-594.
31. Miura T. Duplicated thumb. Plastic Reconstr Surg. 1982;69(3):470-481.
32. Simmons BP. Polydactyly. Hand Clin. 1985;1(3):545-566.
33. Katz K, Linder N. Postaxial type B polydactyly treated by excision in the neonatal nursery. J Pediatr Orthop. 2011;31(4):448-449.
34. Manohar A, Beard AJ. Outcome of reconstruction for duplication of the thumb in adults aged over 40. Hand Surg. 2011;16(2):207-210.
35. Watt AJ, Chung KC. Duplication. Hand Clin. 2009;25(2):215-228.
36. Tonkin MA. Thumb duplication: concepts and techniques. Clin Orthop Surg. 2012;4(1):1-17.
37. Huber E. Relief operation in the case of paralysis of the median nerve. J Hand Surg Eur. 2004;29(1):35-37.
38. Mih AD. Complications of duplicate thumb reconstruction. Hand Clin. 1998;14(1):143-149.
39. Lee CC, Park HY, Yoon JO, Lee KW. Correction of Wassel type IV thumb duplication with zigzag deformity: results of a new method of flexor pollicis longus tendon relocation. J Hand Surg Eur. 2013;38(3):272-280.
40. Hare PJ. Rudimentary polydactyly. Br J Dermatol. 1954;66(11):402-408.
41. Yen CH, Chan WL, Leung HB, Mak KH. Thumb polydactyly: clinical outcome after reconstruction. J Orthop Surg (Hong Kong). 2006;14(3):295-302.
42. Edmunds JO. A tribute to Daniel C. Riordan, MD (1917–2012). Tulane University School of Medicine, Department of Orthopaedics website. http://tulane.edu/som/departments/orthopaedics/news-and-events/danriordantribute.cfm. Accessed March 31, 2015.
43. Faust DC, Herms R. Daniel C. Riordan, MD, 1917–2012. J Hand Surg Am. 2013;38(1):202-205.
Polydactyly is the presence of extra digits. Its incidence is likely underestimated because many practitioners treat simple “nubbins” without referring them to orthopedic specialists.1-3 Polydactyly can be detected by ultrasound as early as 14 weeks’ gestational age, with partial autoamputation seen in most isolated polydactylies.4 The thumb, responsible for 40% of hand function, must be able to oppose the other digits with a stable pinch.5 Polydactyly encumbers this motion when the duplicated digits deviate from normal alignment. Ezaki6 noted that the anatomy is better described as “split” than “duplicated.” There are many dichotomous ways to classify polydactyly: preaxial (radial) versus postaxial (ulnar), thumb versus triphalangeal, simple versus complex (Figure 1). Mixed polydactyly is defined as the presence of preaxial and postaxial polydactyly.7 Surgical management seeks to allow normal hand function and to restore cosmesis.
Epidemiology
Sun and colleagues8 reported the overall polydactyly incidence as 2 per 1000 live births in China from 1998 to 2009, with a slight male predominance; polydactyly was also 3 times more common than syndactyly in this population. Ivy,9 in a 5-year audit of Pennsylvania Department of Health records, found polydactyly to be the fourth most common congenital anomaly after clubfoot, cleft lip/palate, and spina bifida. Thumb duplication occurs in 0.08 to 1.4 per 1000 live births and is more common in American Indians and Asians than in other races.5,10 It occurs in a male-to-female ratio of 2.5 to 1 and is most often unilateral.5 Postaxial polydactyly is predominant in black infants; it is most often inherited in an autosomal dominant fashion, if isolated, or in an autosomal recessive pattern, if syndromic.1 A prospective San Diego study of 11,161 newborns found postaxial type B polydactyly in 1 per 531 live births (1 per 143 black infants, 1 per 1339 white infants); 76% of cases were bilateral, and 86% had a positive family history.3 In patients of non-African descent, it is associated with anomalies in other organs. Central duplication is rare and often autosomal dominant.5,10
Genetics and Development
As early as 1896, the heritability of polydactyly was noted.11 As of 2010, polydactyly has been associated with 310 diseases.12 Ninety-nine genes, most involved in regulation of anterior-posterior formation of the limb bud, have been implicated.12,13
The upper limb begins to form at day 26 in utero.14 Apoptosis in the interdigital necrotic zones results in the formation of individual digits. It is presumed that, in polydactyly, the involved tissue is hypoplastic because of an abnormal interaction between mesoderm and ectoderm.5 Presence of an apical ectodermal ridge determines the formation of a limb bud, and on it the zone of polarizing activity (ZPA) dictates preaxial and postaxial alignment.14,15 The ZPA is located on the posterior zone of the developing limb bud. The levels of GLI3, a zinc finger-containing DNA-binding protein, are highest in the anterior area, and HAND2, a basic helix-loop-helix DNA-binding protein, is found in the ZPA. This polarity promotes sonic hedgehog (Shh) gene expression in the posterior region, which in turn prevents GLI3 cleavage into its repressed form. GLI3R (repressed) and GLI3A (active) concentrations are highest, therefore, in the anterior and posterior portions of the bud, respectively. The GLI3A:GLI3R ratio is responsible for the identity and number of digits in the hand (ie, the thumb develops in regions of high GLI3R). GLI and Shh mutations lead to polydactylous hands with absent thumbs (Figure 2).16
Ciliopathies have also been shown to cause postaxial polydactyly, possibly because of the role that nonmotile cilia play in hedgehog signaling.17 Mutations in Shh genomic regulators cause preaxial polydactyly.18 HoxD activates Shh in the ZPA; HoxD13 mutations are associated with synpolydactyly.16,19 In each of these mutations, Shh production is altered, and some form of polydactyly results.
Associations
Many syndromes have been associated with polydactyly. Not all polydactyly is associated with other disorders, but the more complex the polydactyly, the more likely that other anomalies are present. Every patient who presents with polydactyly should have a full history taken and a physical examination performed (Figure 3). Any patient with syndromic findings or atypical presentations (eg, triphalangism, postaxial polydactyly in a patient of non-African descent, central and index polydactyly) should be referred to a geneticist.
Classifications
The Wassel20 classification describes the anatomical presentation of thumb duplication on the basis of 70 cases in Iowa (Figures 4, 5; Table 1). Because some duplications fall outside the Wassel classification, many researchers have proposed modifications (Figure 6).21-25
The Temtamy and McKusick10 classification, which is the product of geneticists, classifies duplications by grouping genetically related presentations (Table 2). It provides the most commonly used postaxial classification, with type A being a fully developed digit and type B a rudimentary and pedunculated digit, informally referred to as a nubbin. Type B is more common than type A. Given inheritance patterns, it is assumed that type A is likely multifactorial and type B autosomal dominant.10 Thumb polydactyly inheritance is still unclear. The other types of preaxial polydactyly and high degrees of polydactyly are rare but seem to be passed on in an autosomal dominant fashion on pedigree analysis.10
The Stelling and Turek classification presents the duplication from a tissue perspective: Type I duplication is a rudimentary mass devoid of other tissue elements; type II is a subtotal duplication with some normal structures; and type III is a duplication of the entire “osteoarticular column,” including the metacarpal.1 It is interesting to note that histology of type I duplications shows neuroma-like tissue.26-28 Again, normal is a relative term because, in polydactyly, the duplications are hypoplastic and deviated, with anomalous anatomy.
The Rayan classification describes ulnar polydactyly and was derived from a case study series of 148 patients in Oklahoma (Table 3).29
There are also some complex polydactylies that are not easily classified: ulnar dimelia, cleft hand, pentadactyly, and hyperphalangism. Ulnar dimelia, also known as “mirror hand,” is typically 7 digits with no thumb, but other variations are seen. The radius is often absent, and the elbow is abnormal. There is some debate about whether it is a fusion of 2 hands. Pentadactyly, or the 5-fingered hand, appears as 5 triphalangeal digits with no thumb (Figure 7).
Isolated thumb triphalangism might appear similar to pentadactyly. Miura30,31 pointed out that the radial digit in the pentadactylous hand may be opposable (thumb-like) or nonopposable; in his studies, the patients with the opposable thumb had a metacarpal with a proximal epiphysis (Figure 8). Consequently, the triphalangeal thumb metacarpal with a distal epiphysis is true pentadactyly, whereas that with a proximal epiphysis is hyperphalangism (Figure 9). Treatment of these complex polydactylies involves the same underlying principles as for preaxial and postaxial polydactyly, albeit with additional proximal upper extremity considerations.
When to Operate (Timing)
Ezaki6 recommended surgical intervention at age 6 to 9 months, before fine motor skills have developed with the abnormal anatomy. Cortical learning occurs as the child begins prehensile activities before 6 months, but the risks of anesthesia outweigh functional benefits until the child is older. Waiting until 1 year of age is not uncommon, though surgery at an earlier age may be beneficial if the polydactyly affects hand function.32 It is not uncommon to wait with the more balanced thumb polydactylies to assess thumb function. Hypoplasia might also delay surgical intervention until there is enough tissue inventory for reconstruction. Wassel20 noted that surgical intervention ideally occurs before the supernumerary elements displace the normal elements, as tends to happen with growth. Suture ligation is an option in the neonatal unit for some pedunculated digits.33 Studies have shown satisfactory results in adults treated for polydactyly, if the patient presents later than expected.34
Surgical Considerations
Knavel recommended simple excision, stating that “ablation requires no ingenuity and creates no problems.”5 This belief, though true for some duplications, will not lead to the best outcome for more complex polydactylies. The goal of surgery is a stable and well-aligned thumb for pinch and prehensile activity, as well as a cosmetically pleasing hand. Incisions should not be made linearly along the axis of the digit, as the scar will cause deviation with growth.24
Wassel type I polydactyly might appear incidentally as a broad thumb, in which case it requires no intervention (Figure 10). However, in Wassel types I and II polydactyly with deformity, the Bilhaut-Cloquet procedure is useful for both bifid and duplicated phalanges (Figure 11).5,6,30,32,35 Collateral ligaments may need to be released in type II because of difficulty in opposing the tissue. Cosmetic results with Bilhaut-Cloquet are unpredictable. The original technique required symmetrically sized digits; results today have been improved with microtechniques and preservation of an entire nail.36 Another option is ablation of the more hypoplastic osseous element and soft-tissue augmentation of the residual digit. The theme of ablation and augmentation is seen throughout the literature for the surgical treatment of polydactyly (Figure 12).1
For type III polydactyly, the bifid proximal phalanx is narrowed by resection and realigned with osteotomy of the remaining diaphysis. Type IV polydactyly, the most common thumb duplication, often requires advancement of the abductor pollicis brevis to the base of the proximal phalanx to aid in metacarpophalangeal (MCP) stabilization, abduction, and opposition. The metacarpal head, if broad and with 2 facets, can be shaped to form a single articulating surface. The metacarpal, occasionally with the proximal phalanx, often requires realignment by closing wedge osteotomy. Last, tendons on the resected bony elements should be rebalanced on the remaining digit, and anomalous slips must be released. For instance, given a radial insertion of the long flexor tendon on the distal phalanx, the tendon should be moved centrally. A strong flexor or extensor tendon on the amputated digit should be transferred to the remaining digit.24
Types V and VI are treated similarly to type IV, with the addition of a first web space Z-plasty or web widening if there is thenar eminence contracture. Acral transposition has also been described, with transposition of the tip of the ablated digit in place of the tip of the kept digit; this technique is ideal if one digit has a more normal proximal part while the other has a more normal distal part (Figure 13).35
Type VII thumb polydactyly, the type most likely inherited and associated with other disorders, should be treated like type VI. The nail should be preserved; amputation of the distal phalanx is not advised. Resection of the delta phalanx or 1 interphalangeal (IP) joint is an option. Articular surfaces will remodel if done before the age of 1 year. If the thenar eminence is hypoplastic, then Huber transfer of the abductor digiti minimi should be considered.37 Resection of the triphalangeal thumb is also advised, even if the biphalangeal thumb is more hypoplastic, with transfer of the ligaments and tendons, as described earlier.5,6,24,30,32,35
Thumb triphalangism, if isolated, and hyperphalangism in the other digits, can be treated with resection of the delta phalanx or one of the IP joints if it is affecting function or cosmesis.1,6 Wood and Flatt23 recommended early resection of a thumb delta phalanx because of the likelihood of deviation that impedes thumb function. For children, they recommended delta phalanx resection and Kirschner wire fixation for 6 weeks; for adults, they recommended resection or fusion of the joint, with osteotomy as needed for deviation.23,24 For thumb triphalangism, multiple surgeries are the norm, as Wood24 reported in his study of 21 patients who underwent 78 operations in total.
Index polydactyly may present as a simple pedunculated skin tag, which can be simply excised, or as a more complex musculoskeletal duplication. More complex presentations can be treated with procedures similar to those used for the thumb. Typically, the additional digit is radially deviated and angulated, eventually leading to impingement of thumb pinch and the first web space. Ray amputation is also an option if no reconstructive surgery will produce the stable, sensate radial pinch that is essential to hand function.32
Ring-finger polydactyly and long-finger polydactyly are often complicated by some element of syndactyly, resulting in a relative paucity of skin (Figure 14). There is failure of both formation (hypoplasia) and differentiation (syndactyly). The hypoplasia particularly affects the function of these digits by tethering them; multiple surgeries to restore proper hand function are the norm.1 Reconstructive surgery for these digits requires preoperative tissue inventory followed by resection and augmentation; as in syndactyly, skin for coverage is at a premium. Creation of a 3-fingered hand is an option.23
Temtamy and McKusick10 type A little-finger polydactyly is treated similarly to the thumb, with the caveat that hypothenar and intrinsic muscles that insert on the resected little finger are transferred to the remaining digit. In contrast to thumb polydactyly, the extrinsic musculature tends to be in good position. Suture ligation of type B polydactyly, as described by Flatt, is likely more common than orthopedists appreciate, as pediatricians and neonatal unit practitioners commonly perform this procedure in the nursery.1-3 It has been described with 2-0 Vicryl3 (Ethicon, Somerville, New Jersey) and 4-0 silk sutures,32 with the goal of necrosis and autoamputation. Parents should be told the finger generally falls off about 10 days (range, 4-21 days) after ligation.3 Multiple authors have cited a report of exsanguination from suture ligation, but we could not locate the primary source. It is advisable to wait until a patient is 6 months of age if planning to resect the nubbin in the operating room, given the anesthesia risk and the lack of functional impairment. Katz and Linder33 indicated they remove type B polydactyly in the nursery suite used for circumcisions; they use anesthetizing cream on the skin, and sharp excision with a scalpel, followed by direct pressure and Steri-Strip (3M, St. Paul, Minnesota) application. Suture ligation is recommended only if there is a narrow, thin (<2 mm) soft-tissue stalk; any broad or bony stalk necessitates surgical removal to avoid neuroma formation and failure of autonecrosis (Figure 15).27 Other options are a single swipe of a scalpel and elliptical excision; sharp transaction of the digital nerve with subsequent retraction is advised to avoid neuroma formation.2
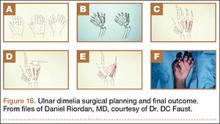
Barton described ulnar dimelia operations as “spare parts surgery.”1 Extra digits are ablated and a thumb created (Figure 16). The hand might have a digit in relatively good rotational position for thumbplasty, or the principles of pollicization may need to be used. If the patient is already using the hand, the surgeon should note which finger the patient uses as a thumb.24 Any accompanying wrist flexion contracture must be corrected with careful attention to musculotendinous balancing. Because the forearm and elbow, and occasionally even the more proximal limb, will be abnormal in this disorder, multiple surgeries are again the norm.1
Pentadactyly is treated like thumb hypoplasia, with first web space creation.1
Complications
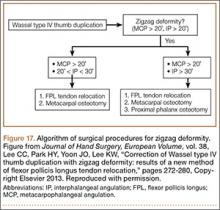
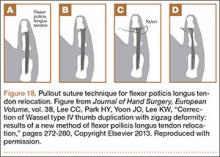
In polydactyly, a reoperation rate of up to 25% has been reported, with most reoperations performed because of residual or subsequent deformity.5,30,31,38 Risk factors for reoperation are type IV thumb duplication, preoperative “zigzag” deformity, and radially deviated thumb elements at presentation.5 The delta phalanx may not show on radiographs until the patient is 18 months old, but functional deformity will worsen as long as it is present. Zigzag deformity may be due to the delta phalanx or to musculotendinous imbalance, such as a radially inserted flexor pollicis longus (FPL) or lack of stable MCP abduction. Miura31 found that careful reconstruction of the joint capsule and thenar muscles from the ablated digit to the remnant digit is the key to a successful initial surgery. Lee and colleagues39 defined zigzag deformity as more than 20° MCP and IP angulation; for cases present before surgery, they recommended FPL relocation by the pullout technique in addition to osteotomies to prevent further interphalangeal deviation (Figures 17, 18).
Abnormal physeal growth, joint instability, and stiffness can all occur. Stiffness is particularly difficult to treat but seldom presents a functional problem. Joint enlargement, which is not uncommon, results from either broad articular surfaces or retained cartilage from the perichondral ring after resection that later ossifies.5,38 Nubbin-type duplications may not fall off after suture ligation, necessitating further excision, and a cosmetic bump is seen after 40% of suture ligations.3 Patillo and Rayan28 and Rayan and Frey29 warned against suture ligation unless the nubbin has a small stalk because of the possibility of infection and gangrene. The excised nubbin tissue is histologically nervous, and there have been reports of painful neuromas in the remaining scar of a ligated nubbin that respond well to excision.26,27,40 It is thought that these painful lesions form because the ligature prevents the digital nerves to the vestigial digit from retracting.27 Nail deformity and IP joint stiffness are seen with the Bilhaut-Cloquet procedure, though often finger function remains satisfactory.
Conclusion
Polydactyly is a common congenital hand abnormality. Its true incidence is unknown because of inconsistent documentation. Surgeons must strive for a functional, cosmetic hand, given a diverse set of possible anomalies. Hypoplasia is the rule; tissue should be ablated and augmented as necessary. Musculotendinous insertions may need to be centralized. Patients’ family members should always be counseled that more surgery may be needed in the future, as further deformity can occur with growth. Surgically corrected thumb duplications will be stiffer, shorter, and thinner than their normal counterparts. Nail ridges are common. However, it should be noted that 88% of these patients are satisfied with their results.41 Some amount of contracture and abnormal function should be expected with index-, long-, and ring-finger duplications. The only remnant of type B postaxial duplications may be a slight discoloration or bump, though stiffness and deformity can happen with a type A deformity. A “duplicated” digit that requires surgical correction will never be completely normal, but acceptable function is routinely achievable.
Polydactyly is the presence of extra digits. Its incidence is likely underestimated because many practitioners treat simple “nubbins” without referring them to orthopedic specialists.1-3 Polydactyly can be detected by ultrasound as early as 14 weeks’ gestational age, with partial autoamputation seen in most isolated polydactylies.4 The thumb, responsible for 40% of hand function, must be able to oppose the other digits with a stable pinch.5 Polydactyly encumbers this motion when the duplicated digits deviate from normal alignment. Ezaki6 noted that the anatomy is better described as “split” than “duplicated.” There are many dichotomous ways to classify polydactyly: preaxial (radial) versus postaxial (ulnar), thumb versus triphalangeal, simple versus complex (Figure 1). Mixed polydactyly is defined as the presence of preaxial and postaxial polydactyly.7 Surgical management seeks to allow normal hand function and to restore cosmesis.
Epidemiology
Sun and colleagues8 reported the overall polydactyly incidence as 2 per 1000 live births in China from 1998 to 2009, with a slight male predominance; polydactyly was also 3 times more common than syndactyly in this population. Ivy,9 in a 5-year audit of Pennsylvania Department of Health records, found polydactyly to be the fourth most common congenital anomaly after clubfoot, cleft lip/palate, and spina bifida. Thumb duplication occurs in 0.08 to 1.4 per 1000 live births and is more common in American Indians and Asians than in other races.5,10 It occurs in a male-to-female ratio of 2.5 to 1 and is most often unilateral.5 Postaxial polydactyly is predominant in black infants; it is most often inherited in an autosomal dominant fashion, if isolated, or in an autosomal recessive pattern, if syndromic.1 A prospective San Diego study of 11,161 newborns found postaxial type B polydactyly in 1 per 531 live births (1 per 143 black infants, 1 per 1339 white infants); 76% of cases were bilateral, and 86% had a positive family history.3 In patients of non-African descent, it is associated with anomalies in other organs. Central duplication is rare and often autosomal dominant.5,10
Genetics and Development
As early as 1896, the heritability of polydactyly was noted.11 As of 2010, polydactyly has been associated with 310 diseases.12 Ninety-nine genes, most involved in regulation of anterior-posterior formation of the limb bud, have been implicated.12,13
The upper limb begins to form at day 26 in utero.14 Apoptosis in the interdigital necrotic zones results in the formation of individual digits. It is presumed that, in polydactyly, the involved tissue is hypoplastic because of an abnormal interaction between mesoderm and ectoderm.5 Presence of an apical ectodermal ridge determines the formation of a limb bud, and on it the zone of polarizing activity (ZPA) dictates preaxial and postaxial alignment.14,15 The ZPA is located on the posterior zone of the developing limb bud. The levels of GLI3, a zinc finger-containing DNA-binding protein, are highest in the anterior area, and HAND2, a basic helix-loop-helix DNA-binding protein, is found in the ZPA. This polarity promotes sonic hedgehog (Shh) gene expression in the posterior region, which in turn prevents GLI3 cleavage into its repressed form. GLI3R (repressed) and GLI3A (active) concentrations are highest, therefore, in the anterior and posterior portions of the bud, respectively. The GLI3A:GLI3R ratio is responsible for the identity and number of digits in the hand (ie, the thumb develops in regions of high GLI3R). GLI and Shh mutations lead to polydactylous hands with absent thumbs (Figure 2).16
Ciliopathies have also been shown to cause postaxial polydactyly, possibly because of the role that nonmotile cilia play in hedgehog signaling.17 Mutations in Shh genomic regulators cause preaxial polydactyly.18 HoxD activates Shh in the ZPA; HoxD13 mutations are associated with synpolydactyly.16,19 In each of these mutations, Shh production is altered, and some form of polydactyly results.
Associations
Many syndromes have been associated with polydactyly. Not all polydactyly is associated with other disorders, but the more complex the polydactyly, the more likely that other anomalies are present. Every patient who presents with polydactyly should have a full history taken and a physical examination performed (Figure 3). Any patient with syndromic findings or atypical presentations (eg, triphalangism, postaxial polydactyly in a patient of non-African descent, central and index polydactyly) should be referred to a geneticist.
Classifications
The Wassel20 classification describes the anatomical presentation of thumb duplication on the basis of 70 cases in Iowa (Figures 4, 5; Table 1). Because some duplications fall outside the Wassel classification, many researchers have proposed modifications (Figure 6).21-25
The Temtamy and McKusick10 classification, which is the product of geneticists, classifies duplications by grouping genetically related presentations (Table 2). It provides the most commonly used postaxial classification, with type A being a fully developed digit and type B a rudimentary and pedunculated digit, informally referred to as a nubbin. Type B is more common than type A. Given inheritance patterns, it is assumed that type A is likely multifactorial and type B autosomal dominant.10 Thumb polydactyly inheritance is still unclear. The other types of preaxial polydactyly and high degrees of polydactyly are rare but seem to be passed on in an autosomal dominant fashion on pedigree analysis.10
The Stelling and Turek classification presents the duplication from a tissue perspective: Type I duplication is a rudimentary mass devoid of other tissue elements; type II is a subtotal duplication with some normal structures; and type III is a duplication of the entire “osteoarticular column,” including the metacarpal.1 It is interesting to note that histology of type I duplications shows neuroma-like tissue.26-28 Again, normal is a relative term because, in polydactyly, the duplications are hypoplastic and deviated, with anomalous anatomy.
The Rayan classification describes ulnar polydactyly and was derived from a case study series of 148 patients in Oklahoma (Table 3).29
There are also some complex polydactylies that are not easily classified: ulnar dimelia, cleft hand, pentadactyly, and hyperphalangism. Ulnar dimelia, also known as “mirror hand,” is typically 7 digits with no thumb, but other variations are seen. The radius is often absent, and the elbow is abnormal. There is some debate about whether it is a fusion of 2 hands. Pentadactyly, or the 5-fingered hand, appears as 5 triphalangeal digits with no thumb (Figure 7).
Isolated thumb triphalangism might appear similar to pentadactyly. Miura30,31 pointed out that the radial digit in the pentadactylous hand may be opposable (thumb-like) or nonopposable; in his studies, the patients with the opposable thumb had a metacarpal with a proximal epiphysis (Figure 8). Consequently, the triphalangeal thumb metacarpal with a distal epiphysis is true pentadactyly, whereas that with a proximal epiphysis is hyperphalangism (Figure 9). Treatment of these complex polydactylies involves the same underlying principles as for preaxial and postaxial polydactyly, albeit with additional proximal upper extremity considerations.
When to Operate (Timing)
Ezaki6 recommended surgical intervention at age 6 to 9 months, before fine motor skills have developed with the abnormal anatomy. Cortical learning occurs as the child begins prehensile activities before 6 months, but the risks of anesthesia outweigh functional benefits until the child is older. Waiting until 1 year of age is not uncommon, though surgery at an earlier age may be beneficial if the polydactyly affects hand function.32 It is not uncommon to wait with the more balanced thumb polydactylies to assess thumb function. Hypoplasia might also delay surgical intervention until there is enough tissue inventory for reconstruction. Wassel20 noted that surgical intervention ideally occurs before the supernumerary elements displace the normal elements, as tends to happen with growth. Suture ligation is an option in the neonatal unit for some pedunculated digits.33 Studies have shown satisfactory results in adults treated for polydactyly, if the patient presents later than expected.34
Surgical Considerations
Knavel recommended simple excision, stating that “ablation requires no ingenuity and creates no problems.”5 This belief, though true for some duplications, will not lead to the best outcome for more complex polydactylies. The goal of surgery is a stable and well-aligned thumb for pinch and prehensile activity, as well as a cosmetically pleasing hand. Incisions should not be made linearly along the axis of the digit, as the scar will cause deviation with growth.24
Wassel type I polydactyly might appear incidentally as a broad thumb, in which case it requires no intervention (Figure 10). However, in Wassel types I and II polydactyly with deformity, the Bilhaut-Cloquet procedure is useful for both bifid and duplicated phalanges (Figure 11).5,6,30,32,35 Collateral ligaments may need to be released in type II because of difficulty in opposing the tissue. Cosmetic results with Bilhaut-Cloquet are unpredictable. The original technique required symmetrically sized digits; results today have been improved with microtechniques and preservation of an entire nail.36 Another option is ablation of the more hypoplastic osseous element and soft-tissue augmentation of the residual digit. The theme of ablation and augmentation is seen throughout the literature for the surgical treatment of polydactyly (Figure 12).1
For type III polydactyly, the bifid proximal phalanx is narrowed by resection and realigned with osteotomy of the remaining diaphysis. Type IV polydactyly, the most common thumb duplication, often requires advancement of the abductor pollicis brevis to the base of the proximal phalanx to aid in metacarpophalangeal (MCP) stabilization, abduction, and opposition. The metacarpal head, if broad and with 2 facets, can be shaped to form a single articulating surface. The metacarpal, occasionally with the proximal phalanx, often requires realignment by closing wedge osteotomy. Last, tendons on the resected bony elements should be rebalanced on the remaining digit, and anomalous slips must be released. For instance, given a radial insertion of the long flexor tendon on the distal phalanx, the tendon should be moved centrally. A strong flexor or extensor tendon on the amputated digit should be transferred to the remaining digit.24
Types V and VI are treated similarly to type IV, with the addition of a first web space Z-plasty or web widening if there is thenar eminence contracture. Acral transposition has also been described, with transposition of the tip of the ablated digit in place of the tip of the kept digit; this technique is ideal if one digit has a more normal proximal part while the other has a more normal distal part (Figure 13).35
Type VII thumb polydactyly, the type most likely inherited and associated with other disorders, should be treated like type VI. The nail should be preserved; amputation of the distal phalanx is not advised. Resection of the delta phalanx or 1 interphalangeal (IP) joint is an option. Articular surfaces will remodel if done before the age of 1 year. If the thenar eminence is hypoplastic, then Huber transfer of the abductor digiti minimi should be considered.37 Resection of the triphalangeal thumb is also advised, even if the biphalangeal thumb is more hypoplastic, with transfer of the ligaments and tendons, as described earlier.5,6,24,30,32,35
Thumb triphalangism, if isolated, and hyperphalangism in the other digits, can be treated with resection of the delta phalanx or one of the IP joints if it is affecting function or cosmesis.1,6 Wood and Flatt23 recommended early resection of a thumb delta phalanx because of the likelihood of deviation that impedes thumb function. For children, they recommended delta phalanx resection and Kirschner wire fixation for 6 weeks; for adults, they recommended resection or fusion of the joint, with osteotomy as needed for deviation.23,24 For thumb triphalangism, multiple surgeries are the norm, as Wood24 reported in his study of 21 patients who underwent 78 operations in total.
Index polydactyly may present as a simple pedunculated skin tag, which can be simply excised, or as a more complex musculoskeletal duplication. More complex presentations can be treated with procedures similar to those used for the thumb. Typically, the additional digit is radially deviated and angulated, eventually leading to impingement of thumb pinch and the first web space. Ray amputation is also an option if no reconstructive surgery will produce the stable, sensate radial pinch that is essential to hand function.32
Ring-finger polydactyly and long-finger polydactyly are often complicated by some element of syndactyly, resulting in a relative paucity of skin (Figure 14). There is failure of both formation (hypoplasia) and differentiation (syndactyly). The hypoplasia particularly affects the function of these digits by tethering them; multiple surgeries to restore proper hand function are the norm.1 Reconstructive surgery for these digits requires preoperative tissue inventory followed by resection and augmentation; as in syndactyly, skin for coverage is at a premium. Creation of a 3-fingered hand is an option.23
Temtamy and McKusick10 type A little-finger polydactyly is treated similarly to the thumb, with the caveat that hypothenar and intrinsic muscles that insert on the resected little finger are transferred to the remaining digit. In contrast to thumb polydactyly, the extrinsic musculature tends to be in good position. Suture ligation of type B polydactyly, as described by Flatt, is likely more common than orthopedists appreciate, as pediatricians and neonatal unit practitioners commonly perform this procedure in the nursery.1-3 It has been described with 2-0 Vicryl3 (Ethicon, Somerville, New Jersey) and 4-0 silk sutures,32 with the goal of necrosis and autoamputation. Parents should be told the finger generally falls off about 10 days (range, 4-21 days) after ligation.3 Multiple authors have cited a report of exsanguination from suture ligation, but we could not locate the primary source. It is advisable to wait until a patient is 6 months of age if planning to resect the nubbin in the operating room, given the anesthesia risk and the lack of functional impairment. Katz and Linder33 indicated they remove type B polydactyly in the nursery suite used for circumcisions; they use anesthetizing cream on the skin, and sharp excision with a scalpel, followed by direct pressure and Steri-Strip (3M, St. Paul, Minnesota) application. Suture ligation is recommended only if there is a narrow, thin (<2 mm) soft-tissue stalk; any broad or bony stalk necessitates surgical removal to avoid neuroma formation and failure of autonecrosis (Figure 15).27 Other options are a single swipe of a scalpel and elliptical excision; sharp transaction of the digital nerve with subsequent retraction is advised to avoid neuroma formation.2
Barton described ulnar dimelia operations as “spare parts surgery.”1 Extra digits are ablated and a thumb created (Figure 16). The hand might have a digit in relatively good rotational position for thumbplasty, or the principles of pollicization may need to be used. If the patient is already using the hand, the surgeon should note which finger the patient uses as a thumb.24 Any accompanying wrist flexion contracture must be corrected with careful attention to musculotendinous balancing. Because the forearm and elbow, and occasionally even the more proximal limb, will be abnormal in this disorder, multiple surgeries are again the norm.1
Pentadactyly is treated like thumb hypoplasia, with first web space creation.1
Complications
In polydactyly, a reoperation rate of up to 25% has been reported, with most reoperations performed because of residual or subsequent deformity.5,30,31,38 Risk factors for reoperation are type IV thumb duplication, preoperative “zigzag” deformity, and radially deviated thumb elements at presentation.5 The delta phalanx may not show on radiographs until the patient is 18 months old, but functional deformity will worsen as long as it is present. Zigzag deformity may be due to the delta phalanx or to musculotendinous imbalance, such as a radially inserted flexor pollicis longus (FPL) or lack of stable MCP abduction. Miura31 found that careful reconstruction of the joint capsule and thenar muscles from the ablated digit to the remnant digit is the key to a successful initial surgery. Lee and colleagues39 defined zigzag deformity as more than 20° MCP and IP angulation; for cases present before surgery, they recommended FPL relocation by the pullout technique in addition to osteotomies to prevent further interphalangeal deviation (Figures 17, 18).
Abnormal physeal growth, joint instability, and stiffness can all occur. Stiffness is particularly difficult to treat but seldom presents a functional problem. Joint enlargement, which is not uncommon, results from either broad articular surfaces or retained cartilage from the perichondral ring after resection that later ossifies.5,38 Nubbin-type duplications may not fall off after suture ligation, necessitating further excision, and a cosmetic bump is seen after 40% of suture ligations.3 Patillo and Rayan28 and Rayan and Frey29 warned against suture ligation unless the nubbin has a small stalk because of the possibility of infection and gangrene. The excised nubbin tissue is histologically nervous, and there have been reports of painful neuromas in the remaining scar of a ligated nubbin that respond well to excision.26,27,40 It is thought that these painful lesions form because the ligature prevents the digital nerves to the vestigial digit from retracting.27 Nail deformity and IP joint stiffness are seen with the Bilhaut-Cloquet procedure, though often finger function remains satisfactory.
Conclusion
Polydactyly is a common congenital hand abnormality. Its true incidence is unknown because of inconsistent documentation. Surgeons must strive for a functional, cosmetic hand, given a diverse set of possible anomalies. Hypoplasia is the rule; tissue should be ablated and augmented as necessary. Musculotendinous insertions may need to be centralized. Patients’ family members should always be counseled that more surgery may be needed in the future, as further deformity can occur with growth. Surgically corrected thumb duplications will be stiffer, shorter, and thinner than their normal counterparts. Nail ridges are common. However, it should be noted that 88% of these patients are satisfied with their results.41 Some amount of contracture and abnormal function should be expected with index-, long-, and ring-finger duplications. The only remnant of type B postaxial duplications may be a slight discoloration or bump, though stiffness and deformity can happen with a type A deformity. A “duplicated” digit that requires surgical correction will never be completely normal, but acceptable function is routinely achievable.
1. Graham TJ, Ress AM. Finger polydactyly. Hand Clin. 1998;14(1):49-64.
2. Abzug JM, Kozin SH. Treatment of postaxial polydactyly type B. J Hand Surg Am. 2013;38(6):1223-1225.
3. Watson BT, Hennrikus WL. Postaxial type-B polydactyly—prevalence and treatment. J Bone Joint Surg Am. 1997;79(1):65-68.
4. Zimmer EZ, Bronshtein M. Fetal polydactyly diagnosis during early pregnancy: clinical applications. Am J Obstet Gynecol. 2000;183(3):755-758.
5. Cohen MS. Thumb duplication. Hand Clin. 1998;14(1):17-27.
6. Ezaki M. Radial polydactyly. Hand Clin. 1990;6(4):577-588.
7. Nathan PA, Keniston RC. Crossed polydactyly: case report and review of the literature. J Bone Joint Surg Am. 1975;57(6):847-849.
8. Sun G, Xu ZM, Liang JF, Li L, Tang DX. Twelve-year prevalence of common neonatal congenital malformations in Zhejiang Province, China. World J Pediatr. 2011;7(4):331-336.
9. Ivy RH. Congenital anomalies as recorded on birth certificates in the Division of Vital Statistics of the Pennsylvania Department of Health, for the period of 1951–1955, inclusive. Plast Reconstr Surg. 1957;20(5):400-411.
10. Temtamy SA, McKusick VA. Polydactyly as a part of syndromes. In: Bergsma D, ed. Mudge JR, Paul NW, Conde Greene S, associate eds. The Genetics of Hand Malformations. New York, NY: Liss. Birth Defects Original Article Series. 1978;14(3):364-439.
11. Gould W, Pyle L. Anomalies and Curiosities of Medicine. New York, NY: Bell; 1896.
12. Biesecker LG. Polydactyly: how many disorders and how many genes: 2010 update. Dev Dyn. 2011;250(5):931-942.
13. Grzeschik K. Human limb malformations; an approach to the molecular basis of development. Int J Dev Biol. 2001;46(7):983-991.
14. Zaleske DJ. Development of the upper limb. Hand Clin. 1985;1(3):383-390.
15. Beatty E. Upper limb tissue differentiation in the human embryo. Hand Clin. 1985;1(3):391-404.
16. Anderson E, Peluso S, Lettice LA, Hill RE. Human limb abnormalities caused by disruption of hedgehog signaling. Trends Genet. 2012;28(8):364-373.
17. Ware SM, Aygun MG, Heldebrandt F. Spectrum of clinical diseases caused by disorders of primary cilia. Proc Am Thorac Soc. 2011;8(5):444-450.
18. Lettice LA, Hill RE. Preaxial polydactyly: a model for defective long-range regulation in congenital abnormalities. Curr Opin Genet Dev. 2005;15(3):294-300.
19. Al-Qattan MA. Type II familial synpolydactyly: report on two families with an emphasis on variations of expression. Eur J Hum Genet. 2011;19(1):112-114.
20. Wassel HD. The results of surgery for polydactyly of the thumb. Clin Orthop. 1969;(64):175-193.
21. Blauth W, Olason AT. Classification of polydactyly of the hands and feet. Arch Orthop Trauma Surg. 1988;107(6):334-344.
22. Wood VE. Super digit. Hand Clin. 1990;6(4):673-684.
23. Wood VE, Flatt AE. Congenital triangular bones in the hand. J Hand Surg Am. 1977;2(3):179-193.
24. Wood VE. Polydactyly and the triphalangeal thumb. J Hand Surg Am. 1978;3(5):436-444.
25. Zuidam JM, Selles RW, Ananta M, Runia J, Hovius SER. A classification system of radial polydactyly: inclusion of triphalangeal thumb and triplication. J Hand Surg Am. 2008;33(3):373-377.
26. Leber GE, Gosain AK. Surgical excision of pedunculated supernumerary digits prevents traumatic amputation neuromas. Pediatr Dermatol. 2003;20(2):108-112.
27. Mullick S, Borschel GH. A selective approach to treatment of ulnar polydactyly: preventing painful neuroma and incomplete excision. Pediatr Dermatol. 2001;27(1):39-42.
28. Patillo D, Rayan GM. Complications of suture ligation ablation for ulnar polydactyly: a report of two cases. Hand (N Y). 2011;6(1):102-105.
29. Rayan GM, Frey B. Ulnar polydactyly. Plastic Reconstr Surg. 2001;107(6):1449-1454.
30. Miura T. Triphalangeal thumb. Plastic Reconstr Surg. 1976;58(5):587-594.
31. Miura T. Duplicated thumb. Plastic Reconstr Surg. 1982;69(3):470-481.
32. Simmons BP. Polydactyly. Hand Clin. 1985;1(3):545-566.
33. Katz K, Linder N. Postaxial type B polydactyly treated by excision in the neonatal nursery. J Pediatr Orthop. 2011;31(4):448-449.
34. Manohar A, Beard AJ. Outcome of reconstruction for duplication of the thumb in adults aged over 40. Hand Surg. 2011;16(2):207-210.
35. Watt AJ, Chung KC. Duplication. Hand Clin. 2009;25(2):215-228.
36. Tonkin MA. Thumb duplication: concepts and techniques. Clin Orthop Surg. 2012;4(1):1-17.
37. Huber E. Relief operation in the case of paralysis of the median nerve. J Hand Surg Eur. 2004;29(1):35-37.
38. Mih AD. Complications of duplicate thumb reconstruction. Hand Clin. 1998;14(1):143-149.
39. Lee CC, Park HY, Yoon JO, Lee KW. Correction of Wassel type IV thumb duplication with zigzag deformity: results of a new method of flexor pollicis longus tendon relocation. J Hand Surg Eur. 2013;38(3):272-280.
40. Hare PJ. Rudimentary polydactyly. Br J Dermatol. 1954;66(11):402-408.
41. Yen CH, Chan WL, Leung HB, Mak KH. Thumb polydactyly: clinical outcome after reconstruction. J Orthop Surg (Hong Kong). 2006;14(3):295-302.
42. Edmunds JO. A tribute to Daniel C. Riordan, MD (1917–2012). Tulane University School of Medicine, Department of Orthopaedics website. http://tulane.edu/som/departments/orthopaedics/news-and-events/danriordantribute.cfm. Accessed March 31, 2015.
43. Faust DC, Herms R. Daniel C. Riordan, MD, 1917–2012. J Hand Surg Am. 2013;38(1):202-205.
1. Graham TJ, Ress AM. Finger polydactyly. Hand Clin. 1998;14(1):49-64.
2. Abzug JM, Kozin SH. Treatment of postaxial polydactyly type B. J Hand Surg Am. 2013;38(6):1223-1225.
3. Watson BT, Hennrikus WL. Postaxial type-B polydactyly—prevalence and treatment. J Bone Joint Surg Am. 1997;79(1):65-68.
4. Zimmer EZ, Bronshtein M. Fetal polydactyly diagnosis during early pregnancy: clinical applications. Am J Obstet Gynecol. 2000;183(3):755-758.
5. Cohen MS. Thumb duplication. Hand Clin. 1998;14(1):17-27.
6. Ezaki M. Radial polydactyly. Hand Clin. 1990;6(4):577-588.
7. Nathan PA, Keniston RC. Crossed polydactyly: case report and review of the literature. J Bone Joint Surg Am. 1975;57(6):847-849.
8. Sun G, Xu ZM, Liang JF, Li L, Tang DX. Twelve-year prevalence of common neonatal congenital malformations in Zhejiang Province, China. World J Pediatr. 2011;7(4):331-336.
9. Ivy RH. Congenital anomalies as recorded on birth certificates in the Division of Vital Statistics of the Pennsylvania Department of Health, for the period of 1951–1955, inclusive. Plast Reconstr Surg. 1957;20(5):400-411.
10. Temtamy SA, McKusick VA. Polydactyly as a part of syndromes. In: Bergsma D, ed. Mudge JR, Paul NW, Conde Greene S, associate eds. The Genetics of Hand Malformations. New York, NY: Liss. Birth Defects Original Article Series. 1978;14(3):364-439.
11. Gould W, Pyle L. Anomalies and Curiosities of Medicine. New York, NY: Bell; 1896.
12. Biesecker LG. Polydactyly: how many disorders and how many genes: 2010 update. Dev Dyn. 2011;250(5):931-942.
13. Grzeschik K. Human limb malformations; an approach to the molecular basis of development. Int J Dev Biol. 2001;46(7):983-991.
14. Zaleske DJ. Development of the upper limb. Hand Clin. 1985;1(3):383-390.
15. Beatty E. Upper limb tissue differentiation in the human embryo. Hand Clin. 1985;1(3):391-404.
16. Anderson E, Peluso S, Lettice LA, Hill RE. Human limb abnormalities caused by disruption of hedgehog signaling. Trends Genet. 2012;28(8):364-373.
17. Ware SM, Aygun MG, Heldebrandt F. Spectrum of clinical diseases caused by disorders of primary cilia. Proc Am Thorac Soc. 2011;8(5):444-450.
18. Lettice LA, Hill RE. Preaxial polydactyly: a model for defective long-range regulation in congenital abnormalities. Curr Opin Genet Dev. 2005;15(3):294-300.
19. Al-Qattan MA. Type II familial synpolydactyly: report on two families with an emphasis on variations of expression. Eur J Hum Genet. 2011;19(1):112-114.
20. Wassel HD. The results of surgery for polydactyly of the thumb. Clin Orthop. 1969;(64):175-193.
21. Blauth W, Olason AT. Classification of polydactyly of the hands and feet. Arch Orthop Trauma Surg. 1988;107(6):334-344.
22. Wood VE. Super digit. Hand Clin. 1990;6(4):673-684.
23. Wood VE, Flatt AE. Congenital triangular bones in the hand. J Hand Surg Am. 1977;2(3):179-193.
24. Wood VE. Polydactyly and the triphalangeal thumb. J Hand Surg Am. 1978;3(5):436-444.
25. Zuidam JM, Selles RW, Ananta M, Runia J, Hovius SER. A classification system of radial polydactyly: inclusion of triphalangeal thumb and triplication. J Hand Surg Am. 2008;33(3):373-377.
26. Leber GE, Gosain AK. Surgical excision of pedunculated supernumerary digits prevents traumatic amputation neuromas. Pediatr Dermatol. 2003;20(2):108-112.
27. Mullick S, Borschel GH. A selective approach to treatment of ulnar polydactyly: preventing painful neuroma and incomplete excision. Pediatr Dermatol. 2001;27(1):39-42.
28. Patillo D, Rayan GM. Complications of suture ligation ablation for ulnar polydactyly: a report of two cases. Hand (N Y). 2011;6(1):102-105.
29. Rayan GM, Frey B. Ulnar polydactyly. Plastic Reconstr Surg. 2001;107(6):1449-1454.
30. Miura T. Triphalangeal thumb. Plastic Reconstr Surg. 1976;58(5):587-594.
31. Miura T. Duplicated thumb. Plastic Reconstr Surg. 1982;69(3):470-481.
32. Simmons BP. Polydactyly. Hand Clin. 1985;1(3):545-566.
33. Katz K, Linder N. Postaxial type B polydactyly treated by excision in the neonatal nursery. J Pediatr Orthop. 2011;31(4):448-449.
34. Manohar A, Beard AJ. Outcome of reconstruction for duplication of the thumb in adults aged over 40. Hand Surg. 2011;16(2):207-210.
35. Watt AJ, Chung KC. Duplication. Hand Clin. 2009;25(2):215-228.
36. Tonkin MA. Thumb duplication: concepts and techniques. Clin Orthop Surg. 2012;4(1):1-17.
37. Huber E. Relief operation in the case of paralysis of the median nerve. J Hand Surg Eur. 2004;29(1):35-37.
38. Mih AD. Complications of duplicate thumb reconstruction. Hand Clin. 1998;14(1):143-149.
39. Lee CC, Park HY, Yoon JO, Lee KW. Correction of Wassel type IV thumb duplication with zigzag deformity: results of a new method of flexor pollicis longus tendon relocation. J Hand Surg Eur. 2013;38(3):272-280.
40. Hare PJ. Rudimentary polydactyly. Br J Dermatol. 1954;66(11):402-408.
41. Yen CH, Chan WL, Leung HB, Mak KH. Thumb polydactyly: clinical outcome after reconstruction. J Orthop Surg (Hong Kong). 2006;14(3):295-302.
42. Edmunds JO. A tribute to Daniel C. Riordan, MD (1917–2012). Tulane University School of Medicine, Department of Orthopaedics website. http://tulane.edu/som/departments/orthopaedics/news-and-events/danriordantribute.cfm. Accessed March 31, 2015.
43. Faust DC, Herms R. Daniel C. Riordan, MD, 1917–2012. J Hand Surg Am. 2013;38(1):202-205.
Nontraumatic Knee Pain: A Diagnostic & Treatment Guide
Jane, age 42, presents with right knee pain that she’s had for about six months. She denies any trauma. Jane describes the pain as “vague and poorly localized” but worse with activity. She says she started a walking/running program nine months ago, when she was told she was overweight (BMI, 29). She has lost 10 pounds since then and hopes to lose more by continuing to exercise. Further review reveals that Jane has experienced increasing pain while ascending and descending stairs and that the pain is also exacerbated when she stands after prolonged sitting.
If Jane were your patient, what would you include in a physical examination, and how would you diagnose and treat her?
Knee pain is a common presentation in primary care. While traumatic knee pain is frequently addressed in the medical literature, little has been written about chronic nontraumatic nonarthritic knee pain such as Jane’s. Thus, while physical exam tests often lead to the correct diagnosis for traumatic knee pain, there is limited information on the use of such tests to determine the etiology of chronic knee pain.
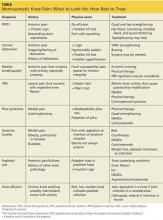
This review was developed to fill that gap. The pages that follow contain general guidance on the diagnosis and treatment of chronic nontraumatic knee pain. The conditions are presented anatomically—anterior, lateral, medial, or posterior—with common etiologies, history and physical exam findings, and diagnosis and treatment options for each (see Table, page 28).1-31
ANTERIOR KNEE PAIN
Patellofemoral pain syndrome (PFPS)
The most common cause of anterior knee pain, PFPS is a complex entity with an etiology that has not been well described.2 The quadriceps tendon, medial and lateral retinacula, iliotibial band (ITB), vastus medialis and lateralis, and the insertion of the patellar tendon on the anterior tibial tubercle all play a role in proper tracking of the patellofemoral joint; an imbalance in any of these forces leads to abnormal patellar tracking over the femoral condyles, and pain ensues. PFPS can also be secondary to joint overload, in which excessive physical activity (eg, running, lunges, or squats) overloads the patellofemoral joint and causes pain.
Risk factors for PFPS include strength imbalances in the quadriceps, hamstring, and hip muscle groups, and increased training, such as running longer distances.4,32 A recent review showed no relationship between an increased quadriceps (Q)-angle and PFPS, so that is no longer considered a major risk factor.5
Diagnosis. PFPS is a diagnosis of exclusion and is primarily based on history and physical exam. Anterior knee pain that is exacerbated when seated for long periods of time (the “theater sign”) or by descending stairs is a classic indication of PFPS.1 Patients may complain of knee stiffness or “giving out” secondary to sharp knee pain and a sensation of popping or crepitus in the joint. Swelling is not a common finding.2
A recent meta-analysis revealed limited evidence for the use of any specific physical exam tests to diagnose PFPS. But pain during squatting and pain with a patellar tilt test were most consistent with a diagnosis of PFPS. (The patellar tilt test involves lifting the lateral edge of the patella superiorly while the patient lies supine with knee extended; pain with < 20° of lift suggests a tight lateral retinaculum). Conversely, the absence of pain during squatting or the absence of lateral retinacular pain helps rule it out.2 A physical exam of the cruciate and collateral ligaments should be performed in a patient with a history of instability. Radiography is not needed for a diagnosis but may be considered if examination reveals an effusion, the patient is 50 or older, or no improvement occurs after eight to 12 weeks of treatment.33
Treatment. The most effective and strongly supported treatment for PFPS is a six-week physiotherapy program focusing on strengthening the quadriceps and hip muscles and stretching the quadriceps, ITB, hamstrings, and hip flexors.4,5 There is limited information about the use of NSAIDs, but they can be considered for short-term management.2
Patellar taping and bracing have shown some promise as adjunct therapies for PFPS, although the data for both are nonconclusive. There is a paucity of prospective randomized trials of patellar bracing, and a 2012 Cochrane review found limited evidence of its efficacy.34 But a 2014 meta-analysis revealed moderate evidence in support of patellar taping early on to help decrease pain,6 and a recent review suggests that it can be helpful in both the short and long term.7
Taping or bracing may be useful when combined with a tailored physical therapy program. Evidence for treatments such as biofeedback, chiropractic manipulation, and orthotics is limited, and they should be used only as adjunctive therapy.4
When you examine Jane, you find no swelling of the affected knee. You perform the tilt test, which elicits pain. Squatting causes some pain, as well. You diagnose PFPS and provide a referral for six weeks of physiotherapy.
Patellofemoral instability (PFI)
PFI occurs when the patella disengages completely from the trochlear groove.11 PFI’s etiology also relates to the complexity of the patellofemoral joint. Here, too, stability of the joint is achieved with a combination of soft-tissue and bony restraints. At full extension and early flexion of the knee, however, the mechanisms of stability are limited, resulting in increased instability. Other associated factors include Q-angle, lateral pull from a tight ITB, and opposing forces from the vastus lateralis and vastus medialis obliquus (VMO).8-10
Risk factors for PFI. The most common predisposing factors for PFI are trochlear dysplasia, patella alta, and lateralization of the tibial tuberosity or patella.10,11 Older patients, predominately women, have an increased risk for PFI.9 Patients usually have a history of patellar subluxation or dislocation in their youth, with approximately 17% of those who had a first dislocation experiencing a recurrence.9 A family history of PFI is common, as well.10
Diagnosis. Patients with PFI often present with nonspecific anterior knee pain secondary to recurrent dislocation.13 Notable exam findings include
• A positive J sign (noted if the patella suddenly shifts medially during early knee flexion or laterally during full extension)
• Decreased quadriceps (specifically VMO) and hamstring strength and flexibility
• Patellar hypermobility, which should be no more than a quarter to a half of the patellar diameter bilaterally
• Pain during a patellar tilt test
• A positive patellar apprehension test.10 (With the patient lying with the knee flexed to 20°, place thumbs on the medial patella and push laterally; the patient will straighten leg with pain or “apprehension” prior to patellar dislocation.)
Plain radiography should be ordered in all cases to assess for osseous trauma/deformity and to help guide surgical consideration. MRI can provide additional information when significant soft-tissue damage is suspected or the patient does not improve with conservative therapy.8,11
Treatment. A recent Cochrane review showed that conservative treatment (VMO strengthening, bracing, and proprioceptive therapy) prevented future dislocations more effectively than surgical intervention.11 However, surgery is indicated when obvious predisposing anatomic conditions (osteochondral fracture, intra-articular deformity, or a major tear of a medial soft-tissue stabilizer) are clearly shown on imaging.8,11
Patellar tendinopathy
An overuse injury often called “jumper’s knee” because it is associated with high-intensity jumping sports (eg, volleyball and basketball), patellar tendinopathy is an insertional tendinopathy with pain most commonly at the proximal patellar tendon.10 The pathology of the injury, though poorly understood, is believed to result from an impaired healing response to microtears.12,14
Diagnosis. Patients with patellar tendinopathy typically present with anterior suprapatellar pain aggravated by activity. Classically, the pain can occur in any of four phases12
1. Pain isolated after activity
2. Pain that occurs during activity but does not impede activity
3. Pain that occurs both during and after the activity and interferes with competition
4. A complete tendon disruption.
Examination should include an assessment of the patellar tendon for localized thickening, nodularity, crepitus, and focal suprapatellar tenderness. The muscle tendon function should be evaluated by assessing knee mobility and strength of the quads via straight-leg raise, decline squat, or single-leg squats.12 The Victorian Institute of Sport Assessment (VISA) questionnaire can be used to quantify the symptoms and to help track the patient’s progress throughout therapy.31 There are no proven special tests or radiologic studies to aid in the diagnosis of patellar tendinopathy,14 but MRI can be used for further evaluation when findings are equivocal.35
Treatment. A wide range of options, from eccentric training (eg, three sets of 15 repetitions performed twice a day for 12 weeks) and physical therapy to platelet-rich plasma (PRP) injections, sclerosing injections, and surgery, are available for the treatment of patellar tendinopathy.13-15 While no specific data have proven the superiority of any one therapy, expert consensus recommends eccentric exercise as initial therapy, performed for 12 weeks.14,15
It’s also interesting to note that a recently published study showed that three weekly PRP injections helped 75% of patients—all of whom failed to respond to four months of eccentric therapy—return to their presymptom activity level within 90 days.16 Corticosteroid injections should not be used to treat patellar tendinopathy due to the risk for tendon rupture.15 Orthopedic referral for surgical intervention should be considered for patients who fail to respond after three to six months of conservative therapy.14
Next: Lateral knee pain >>
LATERAL KNEE PAIN
Iliotibial band syndrome (ITBS)
A common source of lateral knee pain, ITBS is found particularly in runners, cyclists, and endurance athletes.17-19,36,37 The exact pathophysiology behind this diagnosis is debatable, but the most accepted etiology is inflammation generated from microtrauma to the soft tissues with inadequate healing time, resulting in persistent inflammation. ITBS is often associated with excessive overall running mileage, a sudden increase in mileage, or an abrupt change in training.18,37
Diagnosis. Patients often complain of persistent nontraumatic lateral knee pain that worsens with repetitive knee flexion (eg, running or cycling).17-19,37 A physical exam will often reveal pain over the lateral femoral condyle and a positive Noble’s test (see Figure 1, page 30). A positive Ober’s test (see Figure 2, page 32) is suggestive of ITBS, as well. The sensitivity and specificity of these tests are not well established, but in patients performing repetitive knee flexion activities with subjective lateral knee pain, pain over the lateral femoral condyle and a positive Ober’s and/or Noble’s test suggest an ITBS diagnosis.18 Imaging is not indicated initially, but MRI should be used in refractory cases to rule out other etiologies.17,19
Treatment. First-line therapy for ITBS is conservative,17-19,36,37 often involving a combination of techniques such as refraining from the activity that triggers the pain, NSAIDs, activity modification to reduce the strain over the ITB, myofascial release via foam rollers, and physical therapy focused on stretching the ITB, tensor fasciae latae, and gluteus medius while strengthening the gluteus medius and core muscles.17 No single program has been shown to be better than another.
Corticosteroid injections are second-line therapy and have been shown to improve pain compared with placebo up to two weeks postinjection.17,19 When symptoms persist for more than six months despite conservative treatment, surgical intervention may be indicated.18,19 Patients who experience temporary pain relief with corticosteroid injections often respond best to surgery.36
MEDIAL KNEE PAIN
Medial plica syndrome
Because of its anatomic location, the medial plica—which can be palpated in up to 84% of the population20—is susceptible to impingement by the medial femoral condyle or the patellofemoral joint. Trauma with repetitive knee movement leads to inflammation and thickening of the plica, resulting in medial plica syndrome.20,38 Initial inflammation may be triggered by blunt trauma, a sudden increase in activity, or transient synovitis.22
Diagnosis. Medial plica syndrome is a challenging diagnosis. Patients generally have nonspecific complaints of aching medial knee pain, locking, and catching similar to complaints of a medial meniscal injury.20
Evaluation should include the mediopatellar plica test, which is performed with the patient lying supine with the knee fully extended. Pressure is placed over the inferomedial patellofemoral joint, creating an impingement of the medial plica between the finger and the medial femoral condyle. Elimination or marked diminishing of pain with knee flexion to 90° is considered a positive test.21
A recent systematic review found this test to be more diagnostically accurate than an MRI (sensitivity of the test is 90% and specificity is 89%, vs 77% and 58%, respectively, for MRI) for detection of medial plica syndrome. Ultrasound is almost as accurate, with a sensitivity of 90% and specificity of 83%.39
Treatment of medial plica syndrome centers on physiotherapy and quadriceps strengthening,20 augmented with NSAIDs. Intra-articular corticosteroid injections are considered second-line treatment.20,22 An orthopedics referral is indicated to consider arthroscopic plica removal for refractory cases.20,22
Pes anserine bursitis
The anserine bursal complex, located approximately 5 cm distal to the medial joint line, is formed by the combined insertion of the sartorius, gracilis, and semitendinosus tendons,39 but the exact mechanism of pain is not well understood. Whether the pathophysiology is from an insertional tendonitis or overt bursitis is unknown, and no studies have focused on prevalence or risk factors. What is known is that overweight individuals and women with a wide pelvis seem to have a greater predilection and those with pes planus, diabetes, or knee osteoarthritis are at increased risk.23
Diagnosis. Medial knee pain reproduced on palpation of the anatomic site of insertion of the pes anserine tendon complex supports a diagnosis of pes anserine bursitis, with or without edema. Radiologic studies are not needed but may be helpful if significant bony pathology is suspected. Ultrasound, CT, and MRI are not recommended.23
Treatment. Resting the affected knee, cryotherapy, NSAIDs, and use of a pillow at night to relieve direct bursal pressure are recommended.33 Weight loss in obese patients, treatment of pes planus, and control of diabetes may be helpful, as well. Although the literature is limited and dated, corticosteroid injection has been found to reduce the pain and may be considered as second-line treatment.24-26
POSTERIOR KNEE PAIN
Popliteal (Baker’s) cyst
The popliteal fossa contains six of the numerous bursa of the knee; the bursa beneath the medial head of the gastrocnemius muscle and the semimembranosus tendon is most commonly involved in the formation of a popliteal cyst.40 It is postulated that increased intra-articular pressure forces fluid into the bursa, leading to expansion and pain. This can be idiopathic or secondary to internal derangement or trauma to the knee.41 Older age, a remote history of knee trauma, or a coexisting joint disease such as osteoarthritis, meniscal pathology, or rheumatoid arthritis are significant risk factors for popliteal cysts.27
Diagnosis. Most popliteal cysts are asymptomatic in adults and discovered incidentally after routine imaging to evaluate other knee pathology. However, symptomatic popliteal cysts present as a palpable mass in the popliteal fossa, resulting in pain and limited range of motion.
During the physical exam with the patient lying supine, a medial popliteal mass that is most prominent with the knee fully extended is common. A positive Foucher’s sign (the painful mass is palpated posteriorly in the popliteal fossa with the knee fully extended; pain is relieved and/or the mass reduced in size with knee flexion to 45°) suggests a diagnosis of popliteal cyst.27,28
Radiologic studies are generally not needed to diagnose a popliteal cyst. However, if diagnostic uncertainty remains after the history and physical exam, plain knee radiographs and ultrasound should be obtained. This combination provides complementary information and helps rule out a fracture, arthritis, and thrombosis as the cause of the pain.27 MRI is helpful if the diagnosis is still in doubt or if patients are suspected of having significant internal derangement leading to cyst formation. Arthrography or CT is generally not needed.27,41
Treatment. As popliteal cysts are often associated with other knee pathology, management of the underlying condition often leads to cyst regression. Keeping the knee in flexion can decrease the available space and assist in pain control in the acute phase.27 Cold packs and NSAIDs can also be used initially. Cyst aspiration and intra-articular corticosteroid injection have been shown to be effective for cysts that do not respond to this conservative approach.27 However, addressing and managing the underlying knee pathology (eg, osteoarthritis, meniscal pathology, or rheumatoid arthritis) will prevent popliteal cysts from recurring.
Continue for when the problem is painful knee effusion >>
WHEN THE PROBLEM IS PAINFUL KNEE EFFUSION
Nontraumatic knee effusion can be the primary source of knee pain or the result of underlying pathology. It is mentioned here because clinical suspicion is paramount to diagnosis of a septic joint—a serious cause of painful knee effusion that warrants prompt treatment.
As in other causes of knee pain, a detailed history of the character of the pain is essential. Septic arthritis and crystalline disease (gout, pseudogout) should be suspected in patients without a history of trauma who cannot bear weight. Systemic complaints point to an infection and, with the exception of a possible low-grade fever, are not typically seen in crystalline disease. Notable findings include an erythematous, hot, swollen knee and pain with both active and passive movement.
Plain radiographs of the knee should be ordered to rule out significant trauma or arthritis as the etiology. It is important to perform joint aspiration with synovial fluid analysis. Fluid analysis should include a white blood cell (WBC) count with differential, Gram stain and cultures, and polarized light microscopy (not readily available in an outpatient setting).29
Synovial fluid analysis characteristics suggestive of a septic joint include turbid quality, WBC > 50,000/mL, an elevated protein content, and a low glucose concentration.30 Gram stain and culture will help identify the infectious agent. Orthopedic referral should not be delayed in patients with a suspected infectious joint. Corticosteroids should not be injected during aspiration if infection is being ruled out.
When Jane returns for a follow-up visit eight weeks later, she states that the knee pain has resolved and that she has returned to running. She has lost an additional eight pounds and continues to diet. And, at the advice of her physical therapist, she is continuing her physiotherapy regimen at home to prevent a recurrence of PFPS.
REFERENCES
1. Earl JE, Vetter CS. Patellofemoral pain. Phys Med Rehabil Clin N Am. 2007;18:439-458,viii.
2. McGowan HJ, Beutler A. Patellofemoral syndrome. Essential Evidence Plus. www.essentialevidenceplus.com. Accessed April 14, 2015.
3. Nunes GS, Stapait EL, Kirsten MH, et al. Clinical test for diagnosis of patellofemoral pain syndrome: systematic review with meta-analysis. Phys Ther Sport. 2013;14:54-59.
4. Rixe JA, Glick JE, Brady J, et al. A review of the management of patellofemoral pain syndrome. Phys Sportsmed. 2013;41:19-28.
5. Bolgla LA, Boling MC. An update for the conservative management of patellofemoral pain syndrome: a systematic review of the literature from 2000 to 2010. Int J Sports Phys Ther. 2011;6:112-125.
6. Barton C, Balachandar V, Lack S, et al. Patellar taping for patellofemoral pain: a systematic review and meta-analysis to evaluate clinical outcomes and biomechanical mechanisms. Br J Sports Med. 2014;48:417-424.
7. Dutton RA, Khadavi MJ, Fredericson M. Update on rehabilitation of patellofemoral pain. Curr Sports Med Rep. 2014;13:172-178.
8. Kapur S, Wissman RD, Robertson M, et al. Acute knee dislocation: review of an elusive entity. Curr Probl Diagn Radiol. 2009;38:237-250.
9. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90:2751-2762.
10. Tscholl PM, Koch PP, Fucentese SF. Treatment options for patellofemoral instability in sports traumatology. Orthop Rev (Pavia). 2013;5:e23.
11. Earhart C, Patel DB, White EA, et al. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20:11-23.
12. Tan SC, Chan O. Achilles and patellar tendinopathy: current understanding of pathophysiology and management. Disabil Rehabil. 2008;30:1608-1615.
13. Gaida JE, Cook J. Treatment options for patellar tendinopathy: critical review. Curr Sports Med Rep. 2011;10:255-270.
14. Rodriguez-Merchan EC. The treatment of patellar tendinopathy. J Orthop Traumatol. 2013;14:77-81.
15. Childress MA, Beutler A. Management of chronic tendon injuries. Am Fam Physician. 2013;87:486-490.
16. Charousset C, Zaoui A, Bellaiche L, et al. Are multiple platelet-rich plasma injections useful for treatment of chronic patellar tendinopathy in athletes? A prospective study. Am J Sports Med. 2014;42:906-911.
17. Strauss EJ, Kim S, Calcei JG, et al. Iliotibial band syndrome: evaluation and management. J Am Acad Orthop Surg. 2011;19:728-736.
18. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
19. Hong JH, Kim JS. Diagnosis of iliotibial band friction syndrome and ultrasound guided steroid injection. Korean J Pain. 2013;26:387-391.
20. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
21. Kim SJ, Jeong JH, Cheon YM, et al. MPP test in the diagnosis of medial patellar plica syndrome. Arthroscopy. 2004;20:1101-1103.
22. Schindler OS. ‘The Sneaky Plica’ revisited: morphology, pathophysiology and treatment of synovial plicae of the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22:247-262.
23. Helfenstein M Jr, Kuromoto J. Anserine syndrome. Rev Bras Rheumatol. 2010;50:313-327.
24. Abeles M. Osteoarthritis of the knee: anserine bursitis as an extra-articular cause of pain. Clin Res. 1983;31:4471-4476.
25. Kang I, Han SW. Anserine bursitis in patients with osteoarthritis of the knee. South Med J. 2000;93:207-209.
26. Yoon HS, Kim SE, Suh YR, et al. Correlation between ultrasonographic findings and the response to corticosteroid injection in pes anserinus tendinobursitis syndrome in knee osteoarthritis patients. J Korean Med Sci. 2005;20:109-112.
27. Stein D, Cantlon M, MacKay B, et al. Cysts about the knee: evaluation and management. J Am Acad Orthop Surg. 2013;21:469-479.
28. Canoso JJ, Goldsmith MR, Gerzof SG, et al. Foucher’s sign of the Baker’s cyst. Ann Rheum Dis. 1987;46:228-232.
29. Palmer T. Knee pain. Essential Evidence Plus. www.essentialevidenceplus.com. Accessed April 14, 2015.
30. Franks AG Jr. Rheumatologic aspects of knee disorders. In: Scott WN, ed. The Knee. St. Louis: Mosby; 1994:315-329.
31. Visentini PJ, Khan KM, Cook JL, et al. The VISA score: an index of severity of symptoms in patients with jumper’s knee (patellar tendinosis). Victorian Institute of Sport Tendon Study Group. J Sci Med Sport. 1998;1:22-28.
32. Halabchi F, Mazaheri R, Seif-Barghi T. Patellofemoral pain syndrome and modifiable intrinsic risk factors; how to assess and address? Asian J Sports Med. 2013;4:85-100.
33. Dixit S, DiFiori JP, Burton M, et al. Management of patellofemoral pain syndrome. Am Fam Physician. 2007;75:194-202.
34. Callaghan MJ, Selfe J. Patellar taping for patellofemoral pain syndrome in adults. Cochrane Database Syst Rev. 2012;4:CD006717.
35. Atanda AJ Jr, Ruiz D, Dodson CC, et al. Approach to the active patient with chronic anterior knee pain. Phys Sportsmed. 2012;40:41-50.
36. Ellis R, Hing W, Reid D. Iliotibial band friction syndrome—a systematic review. Man Ther. 2007;12:200-208.
37. Kirk KL, Kuklo T, Klemme W. Iliotibial band friction syndrome. Orthopedics. 2000;23:1209-1217.
38. Stubbings N, Smith T. Diagnostic test accuracy of clinical and radiological assessments for medial patella plica syndrome: a systematic review and meta-analysis. Knee. 2014;21:486-490.
39. Alvarez-Nemegyei J, Canoso JJ. Evidence-based soft tissue rheumatology IV: anserine bursitis. J Clin Rheumatol. 2004;10:205-206.
40. Fritschy D, Fasel J, Imbert JC, et al. The popliteal cyst. Knee Surg Sports Traumatol Arthrosc. 2006;14:623-628.
41. Handy JR. Popliteal cysts in adults: a review. Semin Arthritis Rheum. 2001;31:108-118.
Jane, age 42, presents with right knee pain that she’s had for about six months. She denies any trauma. Jane describes the pain as “vague and poorly localized” but worse with activity. She says she started a walking/running program nine months ago, when she was told she was overweight (BMI, 29). She has lost 10 pounds since then and hopes to lose more by continuing to exercise. Further review reveals that Jane has experienced increasing pain while ascending and descending stairs and that the pain is also exacerbated when she stands after prolonged sitting.
If Jane were your patient, what would you include in a physical examination, and how would you diagnose and treat her?
Knee pain is a common presentation in primary care. While traumatic knee pain is frequently addressed in the medical literature, little has been written about chronic nontraumatic nonarthritic knee pain such as Jane’s. Thus, while physical exam tests often lead to the correct diagnosis for traumatic knee pain, there is limited information on the use of such tests to determine the etiology of chronic knee pain.
This review was developed to fill that gap. The pages that follow contain general guidance on the diagnosis and treatment of chronic nontraumatic knee pain. The conditions are presented anatomically—anterior, lateral, medial, or posterior—with common etiologies, history and physical exam findings, and diagnosis and treatment options for each (see Table, page 28).1-31
ANTERIOR KNEE PAIN
Patellofemoral pain syndrome (PFPS)
The most common cause of anterior knee pain, PFPS is a complex entity with an etiology that has not been well described.2 The quadriceps tendon, medial and lateral retinacula, iliotibial band (ITB), vastus medialis and lateralis, and the insertion of the patellar tendon on the anterior tibial tubercle all play a role in proper tracking of the patellofemoral joint; an imbalance in any of these forces leads to abnormal patellar tracking over the femoral condyles, and pain ensues. PFPS can also be secondary to joint overload, in which excessive physical activity (eg, running, lunges, or squats) overloads the patellofemoral joint and causes pain.
Risk factors for PFPS include strength imbalances in the quadriceps, hamstring, and hip muscle groups, and increased training, such as running longer distances.4,32 A recent review showed no relationship between an increased quadriceps (Q)-angle and PFPS, so that is no longer considered a major risk factor.5
Diagnosis. PFPS is a diagnosis of exclusion and is primarily based on history and physical exam. Anterior knee pain that is exacerbated when seated for long periods of time (the “theater sign”) or by descending stairs is a classic indication of PFPS.1 Patients may complain of knee stiffness or “giving out” secondary to sharp knee pain and a sensation of popping or crepitus in the joint. Swelling is not a common finding.2
A recent meta-analysis revealed limited evidence for the use of any specific physical exam tests to diagnose PFPS. But pain during squatting and pain with a patellar tilt test were most consistent with a diagnosis of PFPS. (The patellar tilt test involves lifting the lateral edge of the patella superiorly while the patient lies supine with knee extended; pain with < 20° of lift suggests a tight lateral retinaculum). Conversely, the absence of pain during squatting or the absence of lateral retinacular pain helps rule it out.2 A physical exam of the cruciate and collateral ligaments should be performed in a patient with a history of instability. Radiography is not needed for a diagnosis but may be considered if examination reveals an effusion, the patient is 50 or older, or no improvement occurs after eight to 12 weeks of treatment.33
Treatment. The most effective and strongly supported treatment for PFPS is a six-week physiotherapy program focusing on strengthening the quadriceps and hip muscles and stretching the quadriceps, ITB, hamstrings, and hip flexors.4,5 There is limited information about the use of NSAIDs, but they can be considered for short-term management.2
Patellar taping and bracing have shown some promise as adjunct therapies for PFPS, although the data for both are nonconclusive. There is a paucity of prospective randomized trials of patellar bracing, and a 2012 Cochrane review found limited evidence of its efficacy.34 But a 2014 meta-analysis revealed moderate evidence in support of patellar taping early on to help decrease pain,6 and a recent review suggests that it can be helpful in both the short and long term.7
Taping or bracing may be useful when combined with a tailored physical therapy program. Evidence for treatments such as biofeedback, chiropractic manipulation, and orthotics is limited, and they should be used only as adjunctive therapy.4
When you examine Jane, you find no swelling of the affected knee. You perform the tilt test, which elicits pain. Squatting causes some pain, as well. You diagnose PFPS and provide a referral for six weeks of physiotherapy.
Patellofemoral instability (PFI)
PFI occurs when the patella disengages completely from the trochlear groove.11 PFI’s etiology also relates to the complexity of the patellofemoral joint. Here, too, stability of the joint is achieved with a combination of soft-tissue and bony restraints. At full extension and early flexion of the knee, however, the mechanisms of stability are limited, resulting in increased instability. Other associated factors include Q-angle, lateral pull from a tight ITB, and opposing forces from the vastus lateralis and vastus medialis obliquus (VMO).8-10
Risk factors for PFI. The most common predisposing factors for PFI are trochlear dysplasia, patella alta, and lateralization of the tibial tuberosity or patella.10,11 Older patients, predominately women, have an increased risk for PFI.9 Patients usually have a history of patellar subluxation or dislocation in their youth, with approximately 17% of those who had a first dislocation experiencing a recurrence.9 A family history of PFI is common, as well.10
Diagnosis. Patients with PFI often present with nonspecific anterior knee pain secondary to recurrent dislocation.13 Notable exam findings include
• A positive J sign (noted if the patella suddenly shifts medially during early knee flexion or laterally during full extension)
• Decreased quadriceps (specifically VMO) and hamstring strength and flexibility
• Patellar hypermobility, which should be no more than a quarter to a half of the patellar diameter bilaterally
• Pain during a patellar tilt test
• A positive patellar apprehension test.10 (With the patient lying with the knee flexed to 20°, place thumbs on the medial patella and push laterally; the patient will straighten leg with pain or “apprehension” prior to patellar dislocation.)
Plain radiography should be ordered in all cases to assess for osseous trauma/deformity and to help guide surgical consideration. MRI can provide additional information when significant soft-tissue damage is suspected or the patient does not improve with conservative therapy.8,11
Treatment. A recent Cochrane review showed that conservative treatment (VMO strengthening, bracing, and proprioceptive therapy) prevented future dislocations more effectively than surgical intervention.11 However, surgery is indicated when obvious predisposing anatomic conditions (osteochondral fracture, intra-articular deformity, or a major tear of a medial soft-tissue stabilizer) are clearly shown on imaging.8,11
Patellar tendinopathy
An overuse injury often called “jumper’s knee” because it is associated with high-intensity jumping sports (eg, volleyball and basketball), patellar tendinopathy is an insertional tendinopathy with pain most commonly at the proximal patellar tendon.10 The pathology of the injury, though poorly understood, is believed to result from an impaired healing response to microtears.12,14
Diagnosis. Patients with patellar tendinopathy typically present with anterior suprapatellar pain aggravated by activity. Classically, the pain can occur in any of four phases12
1. Pain isolated after activity
2. Pain that occurs during activity but does not impede activity
3. Pain that occurs both during and after the activity and interferes with competition
4. A complete tendon disruption.
Examination should include an assessment of the patellar tendon for localized thickening, nodularity, crepitus, and focal suprapatellar tenderness. The muscle tendon function should be evaluated by assessing knee mobility and strength of the quads via straight-leg raise, decline squat, or single-leg squats.12 The Victorian Institute of Sport Assessment (VISA) questionnaire can be used to quantify the symptoms and to help track the patient’s progress throughout therapy.31 There are no proven special tests or radiologic studies to aid in the diagnosis of patellar tendinopathy,14 but MRI can be used for further evaluation when findings are equivocal.35
Treatment. A wide range of options, from eccentric training (eg, three sets of 15 repetitions performed twice a day for 12 weeks) and physical therapy to platelet-rich plasma (PRP) injections, sclerosing injections, and surgery, are available for the treatment of patellar tendinopathy.13-15 While no specific data have proven the superiority of any one therapy, expert consensus recommends eccentric exercise as initial therapy, performed for 12 weeks.14,15
It’s also interesting to note that a recently published study showed that three weekly PRP injections helped 75% of patients—all of whom failed to respond to four months of eccentric therapy—return to their presymptom activity level within 90 days.16 Corticosteroid injections should not be used to treat patellar tendinopathy due to the risk for tendon rupture.15 Orthopedic referral for surgical intervention should be considered for patients who fail to respond after three to six months of conservative therapy.14
Next: Lateral knee pain >>
LATERAL KNEE PAIN
Iliotibial band syndrome (ITBS)
A common source of lateral knee pain, ITBS is found particularly in runners, cyclists, and endurance athletes.17-19,36,37 The exact pathophysiology behind this diagnosis is debatable, but the most accepted etiology is inflammation generated from microtrauma to the soft tissues with inadequate healing time, resulting in persistent inflammation. ITBS is often associated with excessive overall running mileage, a sudden increase in mileage, or an abrupt change in training.18,37
Diagnosis. Patients often complain of persistent nontraumatic lateral knee pain that worsens with repetitive knee flexion (eg, running or cycling).17-19,37 A physical exam will often reveal pain over the lateral femoral condyle and a positive Noble’s test (see Figure 1, page 30). A positive Ober’s test (see Figure 2, page 32) is suggestive of ITBS, as well. The sensitivity and specificity of these tests are not well established, but in patients performing repetitive knee flexion activities with subjective lateral knee pain, pain over the lateral femoral condyle and a positive Ober’s and/or Noble’s test suggest an ITBS diagnosis.18 Imaging is not indicated initially, but MRI should be used in refractory cases to rule out other etiologies.17,19
Treatment. First-line therapy for ITBS is conservative,17-19,36,37 often involving a combination of techniques such as refraining from the activity that triggers the pain, NSAIDs, activity modification to reduce the strain over the ITB, myofascial release via foam rollers, and physical therapy focused on stretching the ITB, tensor fasciae latae, and gluteus medius while strengthening the gluteus medius and core muscles.17 No single program has been shown to be better than another.
Corticosteroid injections are second-line therapy and have been shown to improve pain compared with placebo up to two weeks postinjection.17,19 When symptoms persist for more than six months despite conservative treatment, surgical intervention may be indicated.18,19 Patients who experience temporary pain relief with corticosteroid injections often respond best to surgery.36
MEDIAL KNEE PAIN
Medial plica syndrome
Because of its anatomic location, the medial plica—which can be palpated in up to 84% of the population20—is susceptible to impingement by the medial femoral condyle or the patellofemoral joint. Trauma with repetitive knee movement leads to inflammation and thickening of the plica, resulting in medial plica syndrome.20,38 Initial inflammation may be triggered by blunt trauma, a sudden increase in activity, or transient synovitis.22
Diagnosis. Medial plica syndrome is a challenging diagnosis. Patients generally have nonspecific complaints of aching medial knee pain, locking, and catching similar to complaints of a medial meniscal injury.20
Evaluation should include the mediopatellar plica test, which is performed with the patient lying supine with the knee fully extended. Pressure is placed over the inferomedial patellofemoral joint, creating an impingement of the medial plica between the finger and the medial femoral condyle. Elimination or marked diminishing of pain with knee flexion to 90° is considered a positive test.21
A recent systematic review found this test to be more diagnostically accurate than an MRI (sensitivity of the test is 90% and specificity is 89%, vs 77% and 58%, respectively, for MRI) for detection of medial plica syndrome. Ultrasound is almost as accurate, with a sensitivity of 90% and specificity of 83%.39
Treatment of medial plica syndrome centers on physiotherapy and quadriceps strengthening,20 augmented with NSAIDs. Intra-articular corticosteroid injections are considered second-line treatment.20,22 An orthopedics referral is indicated to consider arthroscopic plica removal for refractory cases.20,22
Pes anserine bursitis
The anserine bursal complex, located approximately 5 cm distal to the medial joint line, is formed by the combined insertion of the sartorius, gracilis, and semitendinosus tendons,39 but the exact mechanism of pain is not well understood. Whether the pathophysiology is from an insertional tendonitis or overt bursitis is unknown, and no studies have focused on prevalence or risk factors. What is known is that overweight individuals and women with a wide pelvis seem to have a greater predilection and those with pes planus, diabetes, or knee osteoarthritis are at increased risk.23
Diagnosis. Medial knee pain reproduced on palpation of the anatomic site of insertion of the pes anserine tendon complex supports a diagnosis of pes anserine bursitis, with or without edema. Radiologic studies are not needed but may be helpful if significant bony pathology is suspected. Ultrasound, CT, and MRI are not recommended.23
Treatment. Resting the affected knee, cryotherapy, NSAIDs, and use of a pillow at night to relieve direct bursal pressure are recommended.33 Weight loss in obese patients, treatment of pes planus, and control of diabetes may be helpful, as well. Although the literature is limited and dated, corticosteroid injection has been found to reduce the pain and may be considered as second-line treatment.24-26
POSTERIOR KNEE PAIN
Popliteal (Baker’s) cyst
The popliteal fossa contains six of the numerous bursa of the knee; the bursa beneath the medial head of the gastrocnemius muscle and the semimembranosus tendon is most commonly involved in the formation of a popliteal cyst.40 It is postulated that increased intra-articular pressure forces fluid into the bursa, leading to expansion and pain. This can be idiopathic or secondary to internal derangement or trauma to the knee.41 Older age, a remote history of knee trauma, or a coexisting joint disease such as osteoarthritis, meniscal pathology, or rheumatoid arthritis are significant risk factors for popliteal cysts.27
Diagnosis. Most popliteal cysts are asymptomatic in adults and discovered incidentally after routine imaging to evaluate other knee pathology. However, symptomatic popliteal cysts present as a palpable mass in the popliteal fossa, resulting in pain and limited range of motion.
During the physical exam with the patient lying supine, a medial popliteal mass that is most prominent with the knee fully extended is common. A positive Foucher’s sign (the painful mass is palpated posteriorly in the popliteal fossa with the knee fully extended; pain is relieved and/or the mass reduced in size with knee flexion to 45°) suggests a diagnosis of popliteal cyst.27,28
Radiologic studies are generally not needed to diagnose a popliteal cyst. However, if diagnostic uncertainty remains after the history and physical exam, plain knee radiographs and ultrasound should be obtained. This combination provides complementary information and helps rule out a fracture, arthritis, and thrombosis as the cause of the pain.27 MRI is helpful if the diagnosis is still in doubt or if patients are suspected of having significant internal derangement leading to cyst formation. Arthrography or CT is generally not needed.27,41
Treatment. As popliteal cysts are often associated with other knee pathology, management of the underlying condition often leads to cyst regression. Keeping the knee in flexion can decrease the available space and assist in pain control in the acute phase.27 Cold packs and NSAIDs can also be used initially. Cyst aspiration and intra-articular corticosteroid injection have been shown to be effective for cysts that do not respond to this conservative approach.27 However, addressing and managing the underlying knee pathology (eg, osteoarthritis, meniscal pathology, or rheumatoid arthritis) will prevent popliteal cysts from recurring.
Continue for when the problem is painful knee effusion >>
WHEN THE PROBLEM IS PAINFUL KNEE EFFUSION
Nontraumatic knee effusion can be the primary source of knee pain or the result of underlying pathology. It is mentioned here because clinical suspicion is paramount to diagnosis of a septic joint—a serious cause of painful knee effusion that warrants prompt treatment.
As in other causes of knee pain, a detailed history of the character of the pain is essential. Septic arthritis and crystalline disease (gout, pseudogout) should be suspected in patients without a history of trauma who cannot bear weight. Systemic complaints point to an infection and, with the exception of a possible low-grade fever, are not typically seen in crystalline disease. Notable findings include an erythematous, hot, swollen knee and pain with both active and passive movement.
Plain radiographs of the knee should be ordered to rule out significant trauma or arthritis as the etiology. It is important to perform joint aspiration with synovial fluid analysis. Fluid analysis should include a white blood cell (WBC) count with differential, Gram stain and cultures, and polarized light microscopy (not readily available in an outpatient setting).29
Synovial fluid analysis characteristics suggestive of a septic joint include turbid quality, WBC > 50,000/mL, an elevated protein content, and a low glucose concentration.30 Gram stain and culture will help identify the infectious agent. Orthopedic referral should not be delayed in patients with a suspected infectious joint. Corticosteroids should not be injected during aspiration if infection is being ruled out.
When Jane returns for a follow-up visit eight weeks later, she states that the knee pain has resolved and that she has returned to running. She has lost an additional eight pounds and continues to diet. And, at the advice of her physical therapist, she is continuing her physiotherapy regimen at home to prevent a recurrence of PFPS.
REFERENCES
1. Earl JE, Vetter CS. Patellofemoral pain. Phys Med Rehabil Clin N Am. 2007;18:439-458,viii.
2. McGowan HJ, Beutler A. Patellofemoral syndrome. Essential Evidence Plus. www.essentialevidenceplus.com. Accessed April 14, 2015.
3. Nunes GS, Stapait EL, Kirsten MH, et al. Clinical test for diagnosis of patellofemoral pain syndrome: systematic review with meta-analysis. Phys Ther Sport. 2013;14:54-59.
4. Rixe JA, Glick JE, Brady J, et al. A review of the management of patellofemoral pain syndrome. Phys Sportsmed. 2013;41:19-28.
5. Bolgla LA, Boling MC. An update for the conservative management of patellofemoral pain syndrome: a systematic review of the literature from 2000 to 2010. Int J Sports Phys Ther. 2011;6:112-125.
6. Barton C, Balachandar V, Lack S, et al. Patellar taping for patellofemoral pain: a systematic review and meta-analysis to evaluate clinical outcomes and biomechanical mechanisms. Br J Sports Med. 2014;48:417-424.
7. Dutton RA, Khadavi MJ, Fredericson M. Update on rehabilitation of patellofemoral pain. Curr Sports Med Rep. 2014;13:172-178.
8. Kapur S, Wissman RD, Robertson M, et al. Acute knee dislocation: review of an elusive entity. Curr Probl Diagn Radiol. 2009;38:237-250.
9. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90:2751-2762.
10. Tscholl PM, Koch PP, Fucentese SF. Treatment options for patellofemoral instability in sports traumatology. Orthop Rev (Pavia). 2013;5:e23.
11. Earhart C, Patel DB, White EA, et al. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20:11-23.
12. Tan SC, Chan O. Achilles and patellar tendinopathy: current understanding of pathophysiology and management. Disabil Rehabil. 2008;30:1608-1615.
13. Gaida JE, Cook J. Treatment options for patellar tendinopathy: critical review. Curr Sports Med Rep. 2011;10:255-270.
14. Rodriguez-Merchan EC. The treatment of patellar tendinopathy. J Orthop Traumatol. 2013;14:77-81.
15. Childress MA, Beutler A. Management of chronic tendon injuries. Am Fam Physician. 2013;87:486-490.
16. Charousset C, Zaoui A, Bellaiche L, et al. Are multiple platelet-rich plasma injections useful for treatment of chronic patellar tendinopathy in athletes? A prospective study. Am J Sports Med. 2014;42:906-911.
17. Strauss EJ, Kim S, Calcei JG, et al. Iliotibial band syndrome: evaluation and management. J Am Acad Orthop Surg. 2011;19:728-736.
18. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
19. Hong JH, Kim JS. Diagnosis of iliotibial band friction syndrome and ultrasound guided steroid injection. Korean J Pain. 2013;26:387-391.
20. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
21. Kim SJ, Jeong JH, Cheon YM, et al. MPP test in the diagnosis of medial patellar plica syndrome. Arthroscopy. 2004;20:1101-1103.
22. Schindler OS. ‘The Sneaky Plica’ revisited: morphology, pathophysiology and treatment of synovial plicae of the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22:247-262.
23. Helfenstein M Jr, Kuromoto J. Anserine syndrome. Rev Bras Rheumatol. 2010;50:313-327.
24. Abeles M. Osteoarthritis of the knee: anserine bursitis as an extra-articular cause of pain. Clin Res. 1983;31:4471-4476.
25. Kang I, Han SW. Anserine bursitis in patients with osteoarthritis of the knee. South Med J. 2000;93:207-209.
26. Yoon HS, Kim SE, Suh YR, et al. Correlation between ultrasonographic findings and the response to corticosteroid injection in pes anserinus tendinobursitis syndrome in knee osteoarthritis patients. J Korean Med Sci. 2005;20:109-112.
27. Stein D, Cantlon M, MacKay B, et al. Cysts about the knee: evaluation and management. J Am Acad Orthop Surg. 2013;21:469-479.
28. Canoso JJ, Goldsmith MR, Gerzof SG, et al. Foucher’s sign of the Baker’s cyst. Ann Rheum Dis. 1987;46:228-232.
29. Palmer T. Knee pain. Essential Evidence Plus. www.essentialevidenceplus.com. Accessed April 14, 2015.
30. Franks AG Jr. Rheumatologic aspects of knee disorders. In: Scott WN, ed. The Knee. St. Louis: Mosby; 1994:315-329.
31. Visentini PJ, Khan KM, Cook JL, et al. The VISA score: an index of severity of symptoms in patients with jumper’s knee (patellar tendinosis). Victorian Institute of Sport Tendon Study Group. J Sci Med Sport. 1998;1:22-28.
32. Halabchi F, Mazaheri R, Seif-Barghi T. Patellofemoral pain syndrome and modifiable intrinsic risk factors; how to assess and address? Asian J Sports Med. 2013;4:85-100.
33. Dixit S, DiFiori JP, Burton M, et al. Management of patellofemoral pain syndrome. Am Fam Physician. 2007;75:194-202.
34. Callaghan MJ, Selfe J. Patellar taping for patellofemoral pain syndrome in adults. Cochrane Database Syst Rev. 2012;4:CD006717.
35. Atanda AJ Jr, Ruiz D, Dodson CC, et al. Approach to the active patient with chronic anterior knee pain. Phys Sportsmed. 2012;40:41-50.
36. Ellis R, Hing W, Reid D. Iliotibial band friction syndrome—a systematic review. Man Ther. 2007;12:200-208.
37. Kirk KL, Kuklo T, Klemme W. Iliotibial band friction syndrome. Orthopedics. 2000;23:1209-1217.
38. Stubbings N, Smith T. Diagnostic test accuracy of clinical and radiological assessments for medial patella plica syndrome: a systematic review and meta-analysis. Knee. 2014;21:486-490.
39. Alvarez-Nemegyei J, Canoso JJ. Evidence-based soft tissue rheumatology IV: anserine bursitis. J Clin Rheumatol. 2004;10:205-206.
40. Fritschy D, Fasel J, Imbert JC, et al. The popliteal cyst. Knee Surg Sports Traumatol Arthrosc. 2006;14:623-628.
41. Handy JR. Popliteal cysts in adults: a review. Semin Arthritis Rheum. 2001;31:108-118.
Jane, age 42, presents with right knee pain that she’s had for about six months. She denies any trauma. Jane describes the pain as “vague and poorly localized” but worse with activity. She says she started a walking/running program nine months ago, when she was told she was overweight (BMI, 29). She has lost 10 pounds since then and hopes to lose more by continuing to exercise. Further review reveals that Jane has experienced increasing pain while ascending and descending stairs and that the pain is also exacerbated when she stands after prolonged sitting.
If Jane were your patient, what would you include in a physical examination, and how would you diagnose and treat her?
Knee pain is a common presentation in primary care. While traumatic knee pain is frequently addressed in the medical literature, little has been written about chronic nontraumatic nonarthritic knee pain such as Jane’s. Thus, while physical exam tests often lead to the correct diagnosis for traumatic knee pain, there is limited information on the use of such tests to determine the etiology of chronic knee pain.
This review was developed to fill that gap. The pages that follow contain general guidance on the diagnosis and treatment of chronic nontraumatic knee pain. The conditions are presented anatomically—anterior, lateral, medial, or posterior—with common etiologies, history and physical exam findings, and diagnosis and treatment options for each (see Table, page 28).1-31
ANTERIOR KNEE PAIN
Patellofemoral pain syndrome (PFPS)
The most common cause of anterior knee pain, PFPS is a complex entity with an etiology that has not been well described.2 The quadriceps tendon, medial and lateral retinacula, iliotibial band (ITB), vastus medialis and lateralis, and the insertion of the patellar tendon on the anterior tibial tubercle all play a role in proper tracking of the patellofemoral joint; an imbalance in any of these forces leads to abnormal patellar tracking over the femoral condyles, and pain ensues. PFPS can also be secondary to joint overload, in which excessive physical activity (eg, running, lunges, or squats) overloads the patellofemoral joint and causes pain.
Risk factors for PFPS include strength imbalances in the quadriceps, hamstring, and hip muscle groups, and increased training, such as running longer distances.4,32 A recent review showed no relationship between an increased quadriceps (Q)-angle and PFPS, so that is no longer considered a major risk factor.5
Diagnosis. PFPS is a diagnosis of exclusion and is primarily based on history and physical exam. Anterior knee pain that is exacerbated when seated for long periods of time (the “theater sign”) or by descending stairs is a classic indication of PFPS.1 Patients may complain of knee stiffness or “giving out” secondary to sharp knee pain and a sensation of popping or crepitus in the joint. Swelling is not a common finding.2
A recent meta-analysis revealed limited evidence for the use of any specific physical exam tests to diagnose PFPS. But pain during squatting and pain with a patellar tilt test were most consistent with a diagnosis of PFPS. (The patellar tilt test involves lifting the lateral edge of the patella superiorly while the patient lies supine with knee extended; pain with < 20° of lift suggests a tight lateral retinaculum). Conversely, the absence of pain during squatting or the absence of lateral retinacular pain helps rule it out.2 A physical exam of the cruciate and collateral ligaments should be performed in a patient with a history of instability. Radiography is not needed for a diagnosis but may be considered if examination reveals an effusion, the patient is 50 or older, or no improvement occurs after eight to 12 weeks of treatment.33
Treatment. The most effective and strongly supported treatment for PFPS is a six-week physiotherapy program focusing on strengthening the quadriceps and hip muscles and stretching the quadriceps, ITB, hamstrings, and hip flexors.4,5 There is limited information about the use of NSAIDs, but they can be considered for short-term management.2
Patellar taping and bracing have shown some promise as adjunct therapies for PFPS, although the data for both are nonconclusive. There is a paucity of prospective randomized trials of patellar bracing, and a 2012 Cochrane review found limited evidence of its efficacy.34 But a 2014 meta-analysis revealed moderate evidence in support of patellar taping early on to help decrease pain,6 and a recent review suggests that it can be helpful in both the short and long term.7
Taping or bracing may be useful when combined with a tailored physical therapy program. Evidence for treatments such as biofeedback, chiropractic manipulation, and orthotics is limited, and they should be used only as adjunctive therapy.4
When you examine Jane, you find no swelling of the affected knee. You perform the tilt test, which elicits pain. Squatting causes some pain, as well. You diagnose PFPS and provide a referral for six weeks of physiotherapy.
Patellofemoral instability (PFI)
PFI occurs when the patella disengages completely from the trochlear groove.11 PFI’s etiology also relates to the complexity of the patellofemoral joint. Here, too, stability of the joint is achieved with a combination of soft-tissue and bony restraints. At full extension and early flexion of the knee, however, the mechanisms of stability are limited, resulting in increased instability. Other associated factors include Q-angle, lateral pull from a tight ITB, and opposing forces from the vastus lateralis and vastus medialis obliquus (VMO).8-10
Risk factors for PFI. The most common predisposing factors for PFI are trochlear dysplasia, patella alta, and lateralization of the tibial tuberosity or patella.10,11 Older patients, predominately women, have an increased risk for PFI.9 Patients usually have a history of patellar subluxation or dislocation in their youth, with approximately 17% of those who had a first dislocation experiencing a recurrence.9 A family history of PFI is common, as well.10
Diagnosis. Patients with PFI often present with nonspecific anterior knee pain secondary to recurrent dislocation.13 Notable exam findings include
• A positive J sign (noted if the patella suddenly shifts medially during early knee flexion or laterally during full extension)
• Decreased quadriceps (specifically VMO) and hamstring strength and flexibility
• Patellar hypermobility, which should be no more than a quarter to a half of the patellar diameter bilaterally
• Pain during a patellar tilt test
• A positive patellar apprehension test.10 (With the patient lying with the knee flexed to 20°, place thumbs on the medial patella and push laterally; the patient will straighten leg with pain or “apprehension” prior to patellar dislocation.)
Plain radiography should be ordered in all cases to assess for osseous trauma/deformity and to help guide surgical consideration. MRI can provide additional information when significant soft-tissue damage is suspected or the patient does not improve with conservative therapy.8,11
Treatment. A recent Cochrane review showed that conservative treatment (VMO strengthening, bracing, and proprioceptive therapy) prevented future dislocations more effectively than surgical intervention.11 However, surgery is indicated when obvious predisposing anatomic conditions (osteochondral fracture, intra-articular deformity, or a major tear of a medial soft-tissue stabilizer) are clearly shown on imaging.8,11
Patellar tendinopathy
An overuse injury often called “jumper’s knee” because it is associated with high-intensity jumping sports (eg, volleyball and basketball), patellar tendinopathy is an insertional tendinopathy with pain most commonly at the proximal patellar tendon.10 The pathology of the injury, though poorly understood, is believed to result from an impaired healing response to microtears.12,14
Diagnosis. Patients with patellar tendinopathy typically present with anterior suprapatellar pain aggravated by activity. Classically, the pain can occur in any of four phases12
1. Pain isolated after activity
2. Pain that occurs during activity but does not impede activity
3. Pain that occurs both during and after the activity and interferes with competition
4. A complete tendon disruption.
Examination should include an assessment of the patellar tendon for localized thickening, nodularity, crepitus, and focal suprapatellar tenderness. The muscle tendon function should be evaluated by assessing knee mobility and strength of the quads via straight-leg raise, decline squat, or single-leg squats.12 The Victorian Institute of Sport Assessment (VISA) questionnaire can be used to quantify the symptoms and to help track the patient’s progress throughout therapy.31 There are no proven special tests or radiologic studies to aid in the diagnosis of patellar tendinopathy,14 but MRI can be used for further evaluation when findings are equivocal.35
Treatment. A wide range of options, from eccentric training (eg, three sets of 15 repetitions performed twice a day for 12 weeks) and physical therapy to platelet-rich plasma (PRP) injections, sclerosing injections, and surgery, are available for the treatment of patellar tendinopathy.13-15 While no specific data have proven the superiority of any one therapy, expert consensus recommends eccentric exercise as initial therapy, performed for 12 weeks.14,15
It’s also interesting to note that a recently published study showed that three weekly PRP injections helped 75% of patients—all of whom failed to respond to four months of eccentric therapy—return to their presymptom activity level within 90 days.16 Corticosteroid injections should not be used to treat patellar tendinopathy due to the risk for tendon rupture.15 Orthopedic referral for surgical intervention should be considered for patients who fail to respond after three to six months of conservative therapy.14
Next: Lateral knee pain >>
LATERAL KNEE PAIN
Iliotibial band syndrome (ITBS)
A common source of lateral knee pain, ITBS is found particularly in runners, cyclists, and endurance athletes.17-19,36,37 The exact pathophysiology behind this diagnosis is debatable, but the most accepted etiology is inflammation generated from microtrauma to the soft tissues with inadequate healing time, resulting in persistent inflammation. ITBS is often associated with excessive overall running mileage, a sudden increase in mileage, or an abrupt change in training.18,37
Diagnosis. Patients often complain of persistent nontraumatic lateral knee pain that worsens with repetitive knee flexion (eg, running or cycling).17-19,37 A physical exam will often reveal pain over the lateral femoral condyle and a positive Noble’s test (see Figure 1, page 30). A positive Ober’s test (see Figure 2, page 32) is suggestive of ITBS, as well. The sensitivity and specificity of these tests are not well established, but in patients performing repetitive knee flexion activities with subjective lateral knee pain, pain over the lateral femoral condyle and a positive Ober’s and/or Noble’s test suggest an ITBS diagnosis.18 Imaging is not indicated initially, but MRI should be used in refractory cases to rule out other etiologies.17,19
Treatment. First-line therapy for ITBS is conservative,17-19,36,37 often involving a combination of techniques such as refraining from the activity that triggers the pain, NSAIDs, activity modification to reduce the strain over the ITB, myofascial release via foam rollers, and physical therapy focused on stretching the ITB, tensor fasciae latae, and gluteus medius while strengthening the gluteus medius and core muscles.17 No single program has been shown to be better than another.
Corticosteroid injections are second-line therapy and have been shown to improve pain compared with placebo up to two weeks postinjection.17,19 When symptoms persist for more than six months despite conservative treatment, surgical intervention may be indicated.18,19 Patients who experience temporary pain relief with corticosteroid injections often respond best to surgery.36
MEDIAL KNEE PAIN
Medial plica syndrome
Because of its anatomic location, the medial plica—which can be palpated in up to 84% of the population20—is susceptible to impingement by the medial femoral condyle or the patellofemoral joint. Trauma with repetitive knee movement leads to inflammation and thickening of the plica, resulting in medial plica syndrome.20,38 Initial inflammation may be triggered by blunt trauma, a sudden increase in activity, or transient synovitis.22
Diagnosis. Medial plica syndrome is a challenging diagnosis. Patients generally have nonspecific complaints of aching medial knee pain, locking, and catching similar to complaints of a medial meniscal injury.20
Evaluation should include the mediopatellar plica test, which is performed with the patient lying supine with the knee fully extended. Pressure is placed over the inferomedial patellofemoral joint, creating an impingement of the medial plica between the finger and the medial femoral condyle. Elimination or marked diminishing of pain with knee flexion to 90° is considered a positive test.21
A recent systematic review found this test to be more diagnostically accurate than an MRI (sensitivity of the test is 90% and specificity is 89%, vs 77% and 58%, respectively, for MRI) for detection of medial plica syndrome. Ultrasound is almost as accurate, with a sensitivity of 90% and specificity of 83%.39
Treatment of medial plica syndrome centers on physiotherapy and quadriceps strengthening,20 augmented with NSAIDs. Intra-articular corticosteroid injections are considered second-line treatment.20,22 An orthopedics referral is indicated to consider arthroscopic plica removal for refractory cases.20,22
Pes anserine bursitis
The anserine bursal complex, located approximately 5 cm distal to the medial joint line, is formed by the combined insertion of the sartorius, gracilis, and semitendinosus tendons,39 but the exact mechanism of pain is not well understood. Whether the pathophysiology is from an insertional tendonitis or overt bursitis is unknown, and no studies have focused on prevalence or risk factors. What is known is that overweight individuals and women with a wide pelvis seem to have a greater predilection and those with pes planus, diabetes, or knee osteoarthritis are at increased risk.23
Diagnosis. Medial knee pain reproduced on palpation of the anatomic site of insertion of the pes anserine tendon complex supports a diagnosis of pes anserine bursitis, with or without edema. Radiologic studies are not needed but may be helpful if significant bony pathology is suspected. Ultrasound, CT, and MRI are not recommended.23
Treatment. Resting the affected knee, cryotherapy, NSAIDs, and use of a pillow at night to relieve direct bursal pressure are recommended.33 Weight loss in obese patients, treatment of pes planus, and control of diabetes may be helpful, as well. Although the literature is limited and dated, corticosteroid injection has been found to reduce the pain and may be considered as second-line treatment.24-26
POSTERIOR KNEE PAIN
Popliteal (Baker’s) cyst
The popliteal fossa contains six of the numerous bursa of the knee; the bursa beneath the medial head of the gastrocnemius muscle and the semimembranosus tendon is most commonly involved in the formation of a popliteal cyst.40 It is postulated that increased intra-articular pressure forces fluid into the bursa, leading to expansion and pain. This can be idiopathic or secondary to internal derangement or trauma to the knee.41 Older age, a remote history of knee trauma, or a coexisting joint disease such as osteoarthritis, meniscal pathology, or rheumatoid arthritis are significant risk factors for popliteal cysts.27
Diagnosis. Most popliteal cysts are asymptomatic in adults and discovered incidentally after routine imaging to evaluate other knee pathology. However, symptomatic popliteal cysts present as a palpable mass in the popliteal fossa, resulting in pain and limited range of motion.
During the physical exam with the patient lying supine, a medial popliteal mass that is most prominent with the knee fully extended is common. A positive Foucher’s sign (the painful mass is palpated posteriorly in the popliteal fossa with the knee fully extended; pain is relieved and/or the mass reduced in size with knee flexion to 45°) suggests a diagnosis of popliteal cyst.27,28
Radiologic studies are generally not needed to diagnose a popliteal cyst. However, if diagnostic uncertainty remains after the history and physical exam, plain knee radiographs and ultrasound should be obtained. This combination provides complementary information and helps rule out a fracture, arthritis, and thrombosis as the cause of the pain.27 MRI is helpful if the diagnosis is still in doubt or if patients are suspected of having significant internal derangement leading to cyst formation. Arthrography or CT is generally not needed.27,41
Treatment. As popliteal cysts are often associated with other knee pathology, management of the underlying condition often leads to cyst regression. Keeping the knee in flexion can decrease the available space and assist in pain control in the acute phase.27 Cold packs and NSAIDs can also be used initially. Cyst aspiration and intra-articular corticosteroid injection have been shown to be effective for cysts that do not respond to this conservative approach.27 However, addressing and managing the underlying knee pathology (eg, osteoarthritis, meniscal pathology, or rheumatoid arthritis) will prevent popliteal cysts from recurring.
Continue for when the problem is painful knee effusion >>
WHEN THE PROBLEM IS PAINFUL KNEE EFFUSION
Nontraumatic knee effusion can be the primary source of knee pain or the result of underlying pathology. It is mentioned here because clinical suspicion is paramount to diagnosis of a septic joint—a serious cause of painful knee effusion that warrants prompt treatment.
As in other causes of knee pain, a detailed history of the character of the pain is essential. Septic arthritis and crystalline disease (gout, pseudogout) should be suspected in patients without a history of trauma who cannot bear weight. Systemic complaints point to an infection and, with the exception of a possible low-grade fever, are not typically seen in crystalline disease. Notable findings include an erythematous, hot, swollen knee and pain with both active and passive movement.
Plain radiographs of the knee should be ordered to rule out significant trauma or arthritis as the etiology. It is important to perform joint aspiration with synovial fluid analysis. Fluid analysis should include a white blood cell (WBC) count with differential, Gram stain and cultures, and polarized light microscopy (not readily available in an outpatient setting).29
Synovial fluid analysis characteristics suggestive of a septic joint include turbid quality, WBC > 50,000/mL, an elevated protein content, and a low glucose concentration.30 Gram stain and culture will help identify the infectious agent. Orthopedic referral should not be delayed in patients with a suspected infectious joint. Corticosteroids should not be injected during aspiration if infection is being ruled out.
When Jane returns for a follow-up visit eight weeks later, she states that the knee pain has resolved and that she has returned to running. She has lost an additional eight pounds and continues to diet. And, at the advice of her physical therapist, she is continuing her physiotherapy regimen at home to prevent a recurrence of PFPS.
REFERENCES
1. Earl JE, Vetter CS. Patellofemoral pain. Phys Med Rehabil Clin N Am. 2007;18:439-458,viii.
2. McGowan HJ, Beutler A. Patellofemoral syndrome. Essential Evidence Plus. www.essentialevidenceplus.com. Accessed April 14, 2015.
3. Nunes GS, Stapait EL, Kirsten MH, et al. Clinical test for diagnosis of patellofemoral pain syndrome: systematic review with meta-analysis. Phys Ther Sport. 2013;14:54-59.
4. Rixe JA, Glick JE, Brady J, et al. A review of the management of patellofemoral pain syndrome. Phys Sportsmed. 2013;41:19-28.
5. Bolgla LA, Boling MC. An update for the conservative management of patellofemoral pain syndrome: a systematic review of the literature from 2000 to 2010. Int J Sports Phys Ther. 2011;6:112-125.
6. Barton C, Balachandar V, Lack S, et al. Patellar taping for patellofemoral pain: a systematic review and meta-analysis to evaluate clinical outcomes and biomechanical mechanisms. Br J Sports Med. 2014;48:417-424.
7. Dutton RA, Khadavi MJ, Fredericson M. Update on rehabilitation of patellofemoral pain. Curr Sports Med Rep. 2014;13:172-178.
8. Kapur S, Wissman RD, Robertson M, et al. Acute knee dislocation: review of an elusive entity. Curr Probl Diagn Radiol. 2009;38:237-250.
9. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90:2751-2762.
10. Tscholl PM, Koch PP, Fucentese SF. Treatment options for patellofemoral instability in sports traumatology. Orthop Rev (Pavia). 2013;5:e23.
11. Earhart C, Patel DB, White EA, et al. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20:11-23.
12. Tan SC, Chan O. Achilles and patellar tendinopathy: current understanding of pathophysiology and management. Disabil Rehabil. 2008;30:1608-1615.
13. Gaida JE, Cook J. Treatment options for patellar tendinopathy: critical review. Curr Sports Med Rep. 2011;10:255-270.
14. Rodriguez-Merchan EC. The treatment of patellar tendinopathy. J Orthop Traumatol. 2013;14:77-81.
15. Childress MA, Beutler A. Management of chronic tendon injuries. Am Fam Physician. 2013;87:486-490.
16. Charousset C, Zaoui A, Bellaiche L, et al. Are multiple platelet-rich plasma injections useful for treatment of chronic patellar tendinopathy in athletes? A prospective study. Am J Sports Med. 2014;42:906-911.
17. Strauss EJ, Kim S, Calcei JG, et al. Iliotibial band syndrome: evaluation and management. J Am Acad Orthop Surg. 2011;19:728-736.
18. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
19. Hong JH, Kim JS. Diagnosis of iliotibial band friction syndrome and ultrasound guided steroid injection. Korean J Pain. 2013;26:387-391.
20. Bellary SS, Lynch G, Housman B, et al. Medial plica syndrome: a review of the literature. Clin Anat. 2012;25:423-428.
21. Kim SJ, Jeong JH, Cheon YM, et al. MPP test in the diagnosis of medial patellar plica syndrome. Arthroscopy. 2004;20:1101-1103.
22. Schindler OS. ‘The Sneaky Plica’ revisited: morphology, pathophysiology and treatment of synovial plicae of the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22:247-262.
23. Helfenstein M Jr, Kuromoto J. Anserine syndrome. Rev Bras Rheumatol. 2010;50:313-327.
24. Abeles M. Osteoarthritis of the knee: anserine bursitis as an extra-articular cause of pain. Clin Res. 1983;31:4471-4476.
25. Kang I, Han SW. Anserine bursitis in patients with osteoarthritis of the knee. South Med J. 2000;93:207-209.
26. Yoon HS, Kim SE, Suh YR, et al. Correlation between ultrasonographic findings and the response to corticosteroid injection in pes anserinus tendinobursitis syndrome in knee osteoarthritis patients. J Korean Med Sci. 2005;20:109-112.
27. Stein D, Cantlon M, MacKay B, et al. Cysts about the knee: evaluation and management. J Am Acad Orthop Surg. 2013;21:469-479.
28. Canoso JJ, Goldsmith MR, Gerzof SG, et al. Foucher’s sign of the Baker’s cyst. Ann Rheum Dis. 1987;46:228-232.
29. Palmer T. Knee pain. Essential Evidence Plus. www.essentialevidenceplus.com. Accessed April 14, 2015.
30. Franks AG Jr. Rheumatologic aspects of knee disorders. In: Scott WN, ed. The Knee. St. Louis: Mosby; 1994:315-329.
31. Visentini PJ, Khan KM, Cook JL, et al. The VISA score: an index of severity of symptoms in patients with jumper’s knee (patellar tendinosis). Victorian Institute of Sport Tendon Study Group. J Sci Med Sport. 1998;1:22-28.
32. Halabchi F, Mazaheri R, Seif-Barghi T. Patellofemoral pain syndrome and modifiable intrinsic risk factors; how to assess and address? Asian J Sports Med. 2013;4:85-100.
33. Dixit S, DiFiori JP, Burton M, et al. Management of patellofemoral pain syndrome. Am Fam Physician. 2007;75:194-202.
34. Callaghan MJ, Selfe J. Patellar taping for patellofemoral pain syndrome in adults. Cochrane Database Syst Rev. 2012;4:CD006717.
35. Atanda AJ Jr, Ruiz D, Dodson CC, et al. Approach to the active patient with chronic anterior knee pain. Phys Sportsmed. 2012;40:41-50.
36. Ellis R, Hing W, Reid D. Iliotibial band friction syndrome—a systematic review. Man Ther. 2007;12:200-208.
37. Kirk KL, Kuklo T, Klemme W. Iliotibial band friction syndrome. Orthopedics. 2000;23:1209-1217.
38. Stubbings N, Smith T. Diagnostic test accuracy of clinical and radiological assessments for medial patella plica syndrome: a systematic review and meta-analysis. Knee. 2014;21:486-490.
39. Alvarez-Nemegyei J, Canoso JJ. Evidence-based soft tissue rheumatology IV: anserine bursitis. J Clin Rheumatol. 2004;10:205-206.
40. Fritschy D, Fasel J, Imbert JC, et al. The popliteal cyst. Knee Surg Sports Traumatol Arthrosc. 2006;14:623-628.
41. Handy JR. Popliteal cysts in adults: a review. Semin Arthritis Rheum. 2001;31:108-118.
Seizure Prompts Man to Fall
ANSWER
The radiograph shows a fracture dislocation of the ankle. The distal tibia is dislocated medially relative to the talus, as evidenced by the widened joint space. There is also an oblique fracture of the distal fibula.
Since the patient was experiencing neurovascular compromise, the dislocation was promptly reduced in the emergency department. Subsequently, he was taken to the operating room for open reduction and internal fixation of his fibula fracture.
ANSWER
The radiograph shows a fracture dislocation of the ankle. The distal tibia is dislocated medially relative to the talus, as evidenced by the widened joint space. There is also an oblique fracture of the distal fibula.
Since the patient was experiencing neurovascular compromise, the dislocation was promptly reduced in the emergency department. Subsequently, he was taken to the operating room for open reduction and internal fixation of his fibula fracture.
ANSWER
The radiograph shows a fracture dislocation of the ankle. The distal tibia is dislocated medially relative to the talus, as evidenced by the widened joint space. There is also an oblique fracture of the distal fibula.
Since the patient was experiencing neurovascular compromise, the dislocation was promptly reduced in the emergency department. Subsequently, he was taken to the operating room for open reduction and internal fixation of his fibula fracture.
A 70-year-old man is brought to your facility by EMS following a new-onset, witnessed seizure. He reportedly fell down some steps. On arrival, he has returned to baseline but is complaining of left-sided weakness and right ankle pain. Medical history is significant for mild hypertension. Vital signs are stable. The patient exhibits slight confusion. He reports some mild weakness on his left side, especially in his lower extremity. There also appears to be moderate soft-tissue swelling of his right ankle, with a slight deformity noted. Dorsalis pedal pulse appears to be slightly diminished in that foot as well. You send the patient for noncontrast CT of the head, as well as a radiograph of the right ankle (the latter of which is shown). What is your impression?
A Blood Test for Osteoarthritis?
The first blood test to detect rheumatoid arthritis and osteoarthritis may soon be developed, according to a study published March 19 in Scientific Reports. The research findings could potentially lead to patients being tested for rheumatoid arthritis and osteoarthritis several years before the onset of physical symptoms.
Lead researcher Dr. Naila Rabbani, Reader of Experimental Systems Biology at the University of Warwick in Coventry United Kingdom, and colleagues have identified a biomarker that is linked to both rheumatoid arthritis and osteoarthritis. While there are established tests for rheumatoid arthritis, the newly identified biomarker could lead to one that can diagnose rheumatoid arthritis and osteoarthritis.
Initially, the research's focus was on citrullinated proteins, a biomarker suspected to be present in the blood of patients with early stage rheumatoid arthritis. It had previously been established that patients with rheumatoid arthritis have citrullinated protein antibodies, but it was not believed that the same held true for people with osteoarthritis. However, investigators found that there was an increase in citrullinated protein levels in both early-stage osteoarthritis and rheumatoid arthritis.
Study authors then produced an algorithm of 3 biomarkers, plasma/serum citrullinated protein, 4-hydroxyproline, and anti-cyclic citrullinated peptide. Based on this algorithm, the researchers found that with a single test they could potentially detect and discriminate between the major types of arthritis at the early stages, before joint damage has occurred.
“Detection of early stage osteoarthritis made the study very promising and we would have been satisfied with this only, but beyond this we also found we could detect and discriminate early-stage rheumatoid arthritis and other inflammatory joint diseases at the same,” said Dr. Rabbani.
“This discovery raises the potential of a blood test that can help diagnose both rheumatoid arthritis and osteoarthritis several years before the onset of physical symptoms,” Dr. Rabbani stated.
Suggested Reading
Ahmed U, Anwar A, Savage RS, et al. Biomarkers of early stage osteoarthritis, rheumatoid arthritis and musculoskeletal health. Sci Rep. 2015 Mar 19;5:9259.
The first blood test to detect rheumatoid arthritis and osteoarthritis may soon be developed, according to a study published March 19 in Scientific Reports. The research findings could potentially lead to patients being tested for rheumatoid arthritis and osteoarthritis several years before the onset of physical symptoms.
Lead researcher Dr. Naila Rabbani, Reader of Experimental Systems Biology at the University of Warwick in Coventry United Kingdom, and colleagues have identified a biomarker that is linked to both rheumatoid arthritis and osteoarthritis. While there are established tests for rheumatoid arthritis, the newly identified biomarker could lead to one that can diagnose rheumatoid arthritis and osteoarthritis.
Initially, the research's focus was on citrullinated proteins, a biomarker suspected to be present in the blood of patients with early stage rheumatoid arthritis. It had previously been established that patients with rheumatoid arthritis have citrullinated protein antibodies, but it was not believed that the same held true for people with osteoarthritis. However, investigators found that there was an increase in citrullinated protein levels in both early-stage osteoarthritis and rheumatoid arthritis.
Study authors then produced an algorithm of 3 biomarkers, plasma/serum citrullinated protein, 4-hydroxyproline, and anti-cyclic citrullinated peptide. Based on this algorithm, the researchers found that with a single test they could potentially detect and discriminate between the major types of arthritis at the early stages, before joint damage has occurred.
“Detection of early stage osteoarthritis made the study very promising and we would have been satisfied with this only, but beyond this we also found we could detect and discriminate early-stage rheumatoid arthritis and other inflammatory joint diseases at the same,” said Dr. Rabbani.
“This discovery raises the potential of a blood test that can help diagnose both rheumatoid arthritis and osteoarthritis several years before the onset of physical symptoms,” Dr. Rabbani stated.
The first blood test to detect rheumatoid arthritis and osteoarthritis may soon be developed, according to a study published March 19 in Scientific Reports. The research findings could potentially lead to patients being tested for rheumatoid arthritis and osteoarthritis several years before the onset of physical symptoms.
Lead researcher Dr. Naila Rabbani, Reader of Experimental Systems Biology at the University of Warwick in Coventry United Kingdom, and colleagues have identified a biomarker that is linked to both rheumatoid arthritis and osteoarthritis. While there are established tests for rheumatoid arthritis, the newly identified biomarker could lead to one that can diagnose rheumatoid arthritis and osteoarthritis.
Initially, the research's focus was on citrullinated proteins, a biomarker suspected to be present in the blood of patients with early stage rheumatoid arthritis. It had previously been established that patients with rheumatoid arthritis have citrullinated protein antibodies, but it was not believed that the same held true for people with osteoarthritis. However, investigators found that there was an increase in citrullinated protein levels in both early-stage osteoarthritis and rheumatoid arthritis.
Study authors then produced an algorithm of 3 biomarkers, plasma/serum citrullinated protein, 4-hydroxyproline, and anti-cyclic citrullinated peptide. Based on this algorithm, the researchers found that with a single test they could potentially detect and discriminate between the major types of arthritis at the early stages, before joint damage has occurred.
“Detection of early stage osteoarthritis made the study very promising and we would have been satisfied with this only, but beyond this we also found we could detect and discriminate early-stage rheumatoid arthritis and other inflammatory joint diseases at the same,” said Dr. Rabbani.
“This discovery raises the potential of a blood test that can help diagnose both rheumatoid arthritis and osteoarthritis several years before the onset of physical symptoms,” Dr. Rabbani stated.
Suggested Reading
Ahmed U, Anwar A, Savage RS, et al. Biomarkers of early stage osteoarthritis, rheumatoid arthritis and musculoskeletal health. Sci Rep. 2015 Mar 19;5:9259.
Suggested Reading
Ahmed U, Anwar A, Savage RS, et al. Biomarkers of early stage osteoarthritis, rheumatoid arthritis and musculoskeletal health. Sci Rep. 2015 Mar 19;5:9259.
Twin Study Offers New Insights Into the Link Between Back Pain and Depression
Genetic factors help to explain the common association between low back pain and depression, according to a large study of twins published in the March issue of Pain.
Marina B. Pinheiro, MSc, and her research colleagues at the University of Sydney in Australia, analyzed data from the Murcia Twin Registry of nearly 2,150 Spanish twins. Questionnaire responses were assessed to determine whether participants with symptoms of depression had a higher prevalence of back pain. A series of statistical analyses were then performed to clarify genetic factors and to determine how an environment that is shared early on can contribute to the linkage between depression and back pain.
The results showed a significant association between symptoms of depression and low back pain. On the initial analysis, which considered the participants as individuals, the odds of having back pain were about 1.6 higher for those with symptoms of depression and anxiety.
For the analysis of twin pairs, which controlled for genetic and familial factors that could influence the relationship between depression and back pain, there was a 1.7 increase in odds. The association was even stronger—more than a 2.3 increase in odds of low back pain associated with depression and anxiety—on the analysis of dizygotic twins.
Upon further analysis of monozygotic twins, the association between symptoms of depression and low back pain disappeared. This suggested that the strong association found in non-identical twins resulted from the confounding effects of common genetic factors influencing both conditions.
Overall, the finding that the association between symptoms of depression and low back pain disappears after fully adjusting for genetics and familial confounders in identical twins suggests that genetics is the main confounder of the relationship between depression and back pain.
Suggested Reading
Pinheiro MB, Ferreira ML, Refshauge K, et al. Genetics and the environment affect the relationship between depression and low back pain: a co-twin control study of Spanish twins. Pain. 2015;156(3):496-503.
Genetic factors help to explain the common association between low back pain and depression, according to a large study of twins published in the March issue of Pain.
Marina B. Pinheiro, MSc, and her research colleagues at the University of Sydney in Australia, analyzed data from the Murcia Twin Registry of nearly 2,150 Spanish twins. Questionnaire responses were assessed to determine whether participants with symptoms of depression had a higher prevalence of back pain. A series of statistical analyses were then performed to clarify genetic factors and to determine how an environment that is shared early on can contribute to the linkage between depression and back pain.
The results showed a significant association between symptoms of depression and low back pain. On the initial analysis, which considered the participants as individuals, the odds of having back pain were about 1.6 higher for those with symptoms of depression and anxiety.
For the analysis of twin pairs, which controlled for genetic and familial factors that could influence the relationship between depression and back pain, there was a 1.7 increase in odds. The association was even stronger—more than a 2.3 increase in odds of low back pain associated with depression and anxiety—on the analysis of dizygotic twins.
Upon further analysis of monozygotic twins, the association between symptoms of depression and low back pain disappeared. This suggested that the strong association found in non-identical twins resulted from the confounding effects of common genetic factors influencing both conditions.
Overall, the finding that the association between symptoms of depression and low back pain disappears after fully adjusting for genetics and familial confounders in identical twins suggests that genetics is the main confounder of the relationship between depression and back pain.
Genetic factors help to explain the common association between low back pain and depression, according to a large study of twins published in the March issue of Pain.
Marina B. Pinheiro, MSc, and her research colleagues at the University of Sydney in Australia, analyzed data from the Murcia Twin Registry of nearly 2,150 Spanish twins. Questionnaire responses were assessed to determine whether participants with symptoms of depression had a higher prevalence of back pain. A series of statistical analyses were then performed to clarify genetic factors and to determine how an environment that is shared early on can contribute to the linkage between depression and back pain.
The results showed a significant association between symptoms of depression and low back pain. On the initial analysis, which considered the participants as individuals, the odds of having back pain were about 1.6 higher for those with symptoms of depression and anxiety.
For the analysis of twin pairs, which controlled for genetic and familial factors that could influence the relationship between depression and back pain, there was a 1.7 increase in odds. The association was even stronger—more than a 2.3 increase in odds of low back pain associated with depression and anxiety—on the analysis of dizygotic twins.
Upon further analysis of monozygotic twins, the association between symptoms of depression and low back pain disappeared. This suggested that the strong association found in non-identical twins resulted from the confounding effects of common genetic factors influencing both conditions.
Overall, the finding that the association between symptoms of depression and low back pain disappears after fully adjusting for genetics and familial confounders in identical twins suggests that genetics is the main confounder of the relationship between depression and back pain.
Suggested Reading
Pinheiro MB, Ferreira ML, Refshauge K, et al. Genetics and the environment affect the relationship between depression and low back pain: a co-twin control study of Spanish twins. Pain. 2015;156(3):496-503.
Suggested Reading
Pinheiro MB, Ferreira ML, Refshauge K, et al. Genetics and the environment affect the relationship between depression and low back pain: a co-twin control study of Spanish twins. Pain. 2015;156(3):496-503.
Common OTC Analgesic Proven Inefficacious for Treating Low Back Pain
Paracetamol (acetaminophen) is ineffective for the treatment of spinal pain and provides negligible benefits for low back pain or osteoarthritis of the hip or knee, its usage also may affect the liver, according to a study published March 31 in BMJ.
Lead study author Gustavo Machado, a PhD student from The George Institute for Global Health at the University of Sydney in Australia, and his research colleagues conducted a systematic review and meta-analysis to examine the efficacy and safety of paracetamol for lower back pain and osteoarthritis of the hip or knee. The reduction of pain intensity, improvement of disability, quality of life, safety, and patient adherence were analyzed in this trial.
The study included 13 randomized controlled trials that examined the effects of paracetamol use compared with placebo. Ten trials included 3,541 patients and evaluated the use of paracetamol for osteoarthritis of the hip or knee, and 3 trials included 1,825 patients that were evaluated for the use of paracetamol for lower back pain.
Among the study’s findings:
• For lower back pain, paracetamol had no effect and did not reduce disability or improve quality of life compared with placebo.
• Paracetamol use for osteoarthritis was shown to increase the likelihood of receiving abnormal results on liver function tests by almost 4 times compared with placebo.
• For osteoarthritis, the researchers found small, but not clinically important benefits in the reduction of pain and disability compared with placebo.
“This latest research, the most comprehensive systematic review of its kind, reaffirms this with an even larger, global patient base, and has for the first time also established that the effects of paracetamol for knee and hip osteoarthritis are too small to be of clinical importance,” Mr. Machado stated.
The study also found that adverse side effects varied across all of the trials. But no differences were found in the number of patients using paracetamol reporting these effects or being withdrawn from studies because of adverse events compared with those using a placebo. The adherence to treatment schedule rates was similar among patients taking paracetamol compared with those taking placebo.
“Use of paracetamol for low back pain or osteoarthritis was also shown to be associated with higher risk of liver toxicity in patients," Mr. Machado said. “Patients were nearly 4 times more likely to have abnormal results on liver function tests compared to those taking placebo pills.”
“World-wide, paracetamol is the most widely used over-the counter medicine for musculoskeletal conditions, so it is important to reconsider treatment recommendations given this new evidence,” stated Mr. Machado.
Suggested Reading
Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015 Mar 31;350:h1225.
Paracetamol (acetaminophen) is ineffective for the treatment of spinal pain and provides negligible benefits for low back pain or osteoarthritis of the hip or knee, its usage also may affect the liver, according to a study published March 31 in BMJ.
Lead study author Gustavo Machado, a PhD student from The George Institute for Global Health at the University of Sydney in Australia, and his research colleagues conducted a systematic review and meta-analysis to examine the efficacy and safety of paracetamol for lower back pain and osteoarthritis of the hip or knee. The reduction of pain intensity, improvement of disability, quality of life, safety, and patient adherence were analyzed in this trial.
The study included 13 randomized controlled trials that examined the effects of paracetamol use compared with placebo. Ten trials included 3,541 patients and evaluated the use of paracetamol for osteoarthritis of the hip or knee, and 3 trials included 1,825 patients that were evaluated for the use of paracetamol for lower back pain.
Among the study’s findings:
• For lower back pain, paracetamol had no effect and did not reduce disability or improve quality of life compared with placebo.
• Paracetamol use for osteoarthritis was shown to increase the likelihood of receiving abnormal results on liver function tests by almost 4 times compared with placebo.
• For osteoarthritis, the researchers found small, but not clinically important benefits in the reduction of pain and disability compared with placebo.
“This latest research, the most comprehensive systematic review of its kind, reaffirms this with an even larger, global patient base, and has for the first time also established that the effects of paracetamol for knee and hip osteoarthritis are too small to be of clinical importance,” Mr. Machado stated.
The study also found that adverse side effects varied across all of the trials. But no differences were found in the number of patients using paracetamol reporting these effects or being withdrawn from studies because of adverse events compared with those using a placebo. The adherence to treatment schedule rates was similar among patients taking paracetamol compared with those taking placebo.
“Use of paracetamol for low back pain or osteoarthritis was also shown to be associated with higher risk of liver toxicity in patients," Mr. Machado said. “Patients were nearly 4 times more likely to have abnormal results on liver function tests compared to those taking placebo pills.”
“World-wide, paracetamol is the most widely used over-the counter medicine for musculoskeletal conditions, so it is important to reconsider treatment recommendations given this new evidence,” stated Mr. Machado.
Paracetamol (acetaminophen) is ineffective for the treatment of spinal pain and provides negligible benefits for low back pain or osteoarthritis of the hip or knee, its usage also may affect the liver, according to a study published March 31 in BMJ.
Lead study author Gustavo Machado, a PhD student from The George Institute for Global Health at the University of Sydney in Australia, and his research colleagues conducted a systematic review and meta-analysis to examine the efficacy and safety of paracetamol for lower back pain and osteoarthritis of the hip or knee. The reduction of pain intensity, improvement of disability, quality of life, safety, and patient adherence were analyzed in this trial.
The study included 13 randomized controlled trials that examined the effects of paracetamol use compared with placebo. Ten trials included 3,541 patients and evaluated the use of paracetamol for osteoarthritis of the hip or knee, and 3 trials included 1,825 patients that were evaluated for the use of paracetamol for lower back pain.
Among the study’s findings:
• For lower back pain, paracetamol had no effect and did not reduce disability or improve quality of life compared with placebo.
• Paracetamol use for osteoarthritis was shown to increase the likelihood of receiving abnormal results on liver function tests by almost 4 times compared with placebo.
• For osteoarthritis, the researchers found small, but not clinically important benefits in the reduction of pain and disability compared with placebo.
“This latest research, the most comprehensive systematic review of its kind, reaffirms this with an even larger, global patient base, and has for the first time also established that the effects of paracetamol for knee and hip osteoarthritis are too small to be of clinical importance,” Mr. Machado stated.
The study also found that adverse side effects varied across all of the trials. But no differences were found in the number of patients using paracetamol reporting these effects or being withdrawn from studies because of adverse events compared with those using a placebo. The adherence to treatment schedule rates was similar among patients taking paracetamol compared with those taking placebo.
“Use of paracetamol for low back pain or osteoarthritis was also shown to be associated with higher risk of liver toxicity in patients," Mr. Machado said. “Patients were nearly 4 times more likely to have abnormal results on liver function tests compared to those taking placebo pills.”
“World-wide, paracetamol is the most widely used over-the counter medicine for musculoskeletal conditions, so it is important to reconsider treatment recommendations given this new evidence,” stated Mr. Machado.
Suggested Reading
Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015 Mar 31;350:h1225.
Suggested Reading
Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015 Mar 31;350:h1225.
Phone Counseling Bolsters Recovery and Reduces Pain Following Spinal Surgery
Participating in a short series of phone conversations with trained counselors can substantially boost recovery and reduce pain in patients after spinal surgery, according to a study published online ahead of print March 28 in Archives of Physical Medicine and Rehabilitation.
The phone calls were designed to enhance standard pre- and post-operative care by reinforcing the value of continuing with physical therapy and back-strengthening exercise regimens.
“Phone counseling appears to be an easy, low-cost strategy that yields meaningful results by improving patient engagement in physical therapy and at-home exercise programs that are so vital for their recovery,” said lead study author Richard Skolasky Jr., ScD, Associate Professor of Orthopedic Surgery at the Johns Hopkins University School of Medicine in Baltimore.
The study included 122 patients ages 46 to 72, who underwent surgery at Johns Hopkins University between 2009 and 2012 to correct spinal stenosis. Each patient was assigned either home exercise programs or physical therapy to help accelerate their recovery time. About half of the patients also received a series of phone counseling sessions from a trained spinal surgery counselor to discuss the importance of exercise in their recovery. The first and most detailed phone session took place a few weeks before the patients had their surgeries. Two follow-up sessions occurred at 6 weeks and at 3 months after the operation was performed.
The study found that patients who received phone calls participated in physical therapy and home exercise at higher rates, and had less pain and less disability 6 months after their surgery, compared with the standard-approach group. Six months after surgery, 74% of patients who received phone counseling experienced significant improvements on standard measures of physical functioning and self-reported measures of pain, compared with 41% of people who did not receive phone calls.
“Modern orthopedic science has made great strides in surgical techniques to correct spinal deformities and achieved significant progress in developing physical therapies that boost the benefits of surgery, but we have not been all that good at motivating and engaging patients to partake in such post-surgical recovery programs,” said co-investigator Stephen Wegener, PhD, Associate Professor of Physical Medicine and Rehabilitation at Johns Hopkins University.
“The findings of our research suggest we may have found a way to add that missing ingredient that draws patients to be more active participants in their physical rehabilitation and recovery,” stated Dr. Wegener.
Suggested Reading
Skolasky RL, Maggard AM, Li D, et al. Health behavior change counseling in surgery for degenerative lumbar spinal stenosis. part I: improvement in rehabilitation engagement and functional outcomes. Arch Phys Med Rehabil. 2015 Mar 28 [Epub ahead of print].
Participating in a short series of phone conversations with trained counselors can substantially boost recovery and reduce pain in patients after spinal surgery, according to a study published online ahead of print March 28 in Archives of Physical Medicine and Rehabilitation.
The phone calls were designed to enhance standard pre- and post-operative care by reinforcing the value of continuing with physical therapy and back-strengthening exercise regimens.
“Phone counseling appears to be an easy, low-cost strategy that yields meaningful results by improving patient engagement in physical therapy and at-home exercise programs that are so vital for their recovery,” said lead study author Richard Skolasky Jr., ScD, Associate Professor of Orthopedic Surgery at the Johns Hopkins University School of Medicine in Baltimore.
The study included 122 patients ages 46 to 72, who underwent surgery at Johns Hopkins University between 2009 and 2012 to correct spinal stenosis. Each patient was assigned either home exercise programs or physical therapy to help accelerate their recovery time. About half of the patients also received a series of phone counseling sessions from a trained spinal surgery counselor to discuss the importance of exercise in their recovery. The first and most detailed phone session took place a few weeks before the patients had their surgeries. Two follow-up sessions occurred at 6 weeks and at 3 months after the operation was performed.
The study found that patients who received phone calls participated in physical therapy and home exercise at higher rates, and had less pain and less disability 6 months after their surgery, compared with the standard-approach group. Six months after surgery, 74% of patients who received phone counseling experienced significant improvements on standard measures of physical functioning and self-reported measures of pain, compared with 41% of people who did not receive phone calls.
“Modern orthopedic science has made great strides in surgical techniques to correct spinal deformities and achieved significant progress in developing physical therapies that boost the benefits of surgery, but we have not been all that good at motivating and engaging patients to partake in such post-surgical recovery programs,” said co-investigator Stephen Wegener, PhD, Associate Professor of Physical Medicine and Rehabilitation at Johns Hopkins University.
“The findings of our research suggest we may have found a way to add that missing ingredient that draws patients to be more active participants in their physical rehabilitation and recovery,” stated Dr. Wegener.
Participating in a short series of phone conversations with trained counselors can substantially boost recovery and reduce pain in patients after spinal surgery, according to a study published online ahead of print March 28 in Archives of Physical Medicine and Rehabilitation.
The phone calls were designed to enhance standard pre- and post-operative care by reinforcing the value of continuing with physical therapy and back-strengthening exercise regimens.
“Phone counseling appears to be an easy, low-cost strategy that yields meaningful results by improving patient engagement in physical therapy and at-home exercise programs that are so vital for their recovery,” said lead study author Richard Skolasky Jr., ScD, Associate Professor of Orthopedic Surgery at the Johns Hopkins University School of Medicine in Baltimore.
The study included 122 patients ages 46 to 72, who underwent surgery at Johns Hopkins University between 2009 and 2012 to correct spinal stenosis. Each patient was assigned either home exercise programs or physical therapy to help accelerate their recovery time. About half of the patients also received a series of phone counseling sessions from a trained spinal surgery counselor to discuss the importance of exercise in their recovery. The first and most detailed phone session took place a few weeks before the patients had their surgeries. Two follow-up sessions occurred at 6 weeks and at 3 months after the operation was performed.
The study found that patients who received phone calls participated in physical therapy and home exercise at higher rates, and had less pain and less disability 6 months after their surgery, compared with the standard-approach group. Six months after surgery, 74% of patients who received phone counseling experienced significant improvements on standard measures of physical functioning and self-reported measures of pain, compared with 41% of people who did not receive phone calls.
“Modern orthopedic science has made great strides in surgical techniques to correct spinal deformities and achieved significant progress in developing physical therapies that boost the benefits of surgery, but we have not been all that good at motivating and engaging patients to partake in such post-surgical recovery programs,” said co-investigator Stephen Wegener, PhD, Associate Professor of Physical Medicine and Rehabilitation at Johns Hopkins University.
“The findings of our research suggest we may have found a way to add that missing ingredient that draws patients to be more active participants in their physical rehabilitation and recovery,” stated Dr. Wegener.
Suggested Reading
Skolasky RL, Maggard AM, Li D, et al. Health behavior change counseling in surgery for degenerative lumbar spinal stenosis. part I: improvement in rehabilitation engagement and functional outcomes. Arch Phys Med Rehabil. 2015 Mar 28 [Epub ahead of print].
Suggested Reading
Skolasky RL, Maggard AM, Li D, et al. Health behavior change counseling in surgery for degenerative lumbar spinal stenosis. part I: improvement in rehabilitation engagement and functional outcomes. Arch Phys Med Rehabil. 2015 Mar 28 [Epub ahead of print].
21st-Century Patient Collections: Implement a Point-of-Service Collections Program Now
An 8-surgeon group in the Southeast had a history of high patient receivables, the result of a long-held culture of “We’ll submit to your insurance and bill you after insurance pays.”
The billing and collections staff worked in the basement—far away and out of sight of the patients who showed up for their postoperative visits owing big bucks.
In a flash of wisdom, the administrator agreed to move the patient-balance collector into a converted closet near the check-out area, and provided the information, tools, and training that enabled her to speak with patients about their balances when they came in for an appointment. In her first month in this role and location, this employee collected more than her annual salary from patients.
It Takes a Program
This is one of our favorite client success stories, and it illustrates a key point: point-of-service (POS) collections do not have to be complicated. But the process does have to be deliberate and coordinated. Practices cannot simply update the financial policy and hope the staff members magically begin collecting. If this is your strategy, we promise that it will fail.
Successful POS collecting requires a program approach. And this approach starts at the front-end of the billing cycle, not “after insurance pays.”
POS collections have never been more important. Health insurance exchanges and payers are increasing deductibles and coinsurances. Physicians are opting out of network. Given these realities, POS collections are vital to your cash flow and effective receivables management.
If you are starting practice, you have a perfect opportunity to open with POS collecting in place. A solo surgeon whom we set up in practice did so, and has collected up-front for office services, scans, and surgeries from his first day in practice. Today, the practice’s only outstanding patient receivables are those of patients on payment plans—and these are less than 1% of total accounts receivable.
We also converted the “after insurance pays” philosophy of a surgeon in the South, implementing both POS collections and surgical deposits. In the first month, his patient payments increased by 40%. Another solo orthopedist reported an increased take-home salary of $90,000 in the first year after we helped his staff collect surgery deposits.
Six POS Program Elements
In 30 years of implementing or training staff to implement POS collections, we have come to recognize the following 6 key elements to include in your program approach: Policies + Procedures + Technology + Training + Monitoring + Coaching.
At a high level, here are the actions your practice will need to take:
1. Update the financial policy with 1 written standard for all physicians.
2. Develop granular procedures driven by the policy; these are the “how-tos” that enable the staff to collect successfully.
3. Implement new technologies, such as cost estimators, recurring payments, and online bill pay.
4. Schedule formal training to ensure that staff members know how to ask for money. (Do not assume they are, can, or will without training.)
5. Measure and monitor the outcome of patient collections and staff performance.
6. Provide ongoing coaching and oversight to maintain motivation and skills.
A blueprint for addressing each one of these actions follows.
1. Update the financial policy
The policy is the set of expectations on which to build all procedures and training. Dust off this document, and review it as a group with the practice administrator. First, strike old language that says the patient will be balance-billed, or will only be asked in the office for his visit copay. Next, strive for clarity. “You will be asked to pay your financial responsibility at the time of service,” really says nothing. Instead, the policy should be direct:
If you are recommended for surgery, our staff will calculate your coinsurance and unmet deductible amounts: 50% of this amount will be collected as a surgery deposit, and the remaining 50% is due on or before the day of surgery. Payment plans are available.
For office visits and services, break down the policy by coverage type. We find that a table such as the one shown makes expectations clear.
Finally, strive for 1 standard policy for all providers. If every provider is allowed to create his or her own set of collection policies, the practice is setting staff up for complexity overload, and collections will suffer.
2. Develop granular procedures
Few practices take the time to translate the financial policy into written procedures that can be followed by staff. The policy establishes the rules, but the procedures tell staff what to do to implement those rules. For instance:
Create a “POS Playbook” that contains information such as procedures, cost-quotation worksheets, US Poverty guidelines1, and financing brochures. As old-school as it sounds, a 3-ring binder is great for this information, and makes information access and updates easy.
3. Implement collection technologies
Modern practices use inexpensive (and often free) tools that increase patient convenience and staff efficiency. Implement at least 2 of these useful technologies and watch your POS collections increase:
Reports from your practice management system (PMS). Use the technology you already have. There are 2 standard reports in your PMS or clearinghouse that give front-desk staff the data to ask patients for money. Eligibility status and past-due balance reports indicate amounts owed, unmet deductibles, and the ineligible patients they can collect from when they come in for their appointment.
Online cost estimators. These free, online tools are offered by payers and provide staff with real-time data about a patient’s unmet deductible and coinsurance. When staff members enter Current Procedural Terminology (CPT) codes and the patient’s benefit information into the online cost estimator, they can access valuable information. Many insurance plans offer cost estimators on their web sites. Others deliver the data through statewide or regional portals, such as Availity (www.availity.com). The accuracy of cost-estimator data can vary by region and depends on the data links with payers. Ask your team to evaluate which estimators are best for you based on your payer mix.
Online bill pay. Everyone appreciates the convenience of paying bills online. Most patient portals offer this feature. If yours does not or you do not have a portal, you can offer PayPal (www.paypal.com) on your practice website, or use a system such as Intuit Health (www.intuithealth.com).
Recurring billing. Recurring billing is how you pay for services, such as Netflix, Pandora, or your gym membership: it is automatically billed to a credit card each month. Offer this option to patients as a payment plan method, and staff will no longer need to send costly statements, post monthly check payments, or follow up when a patient is delinquent. Plus, it guarantees payment every month; patients can no longer say, “I forgot.”
TransFirst (www.transfirstassociation.com) and a-claim (www.a-claim.com) offer recurring billing through a “virtual terminal” that staff logs in to at checkout, or during the preprocedure patient counseling process. Both vendors also offer the option of automatically charging a patient’s credit card after their insurance pays, speeding patient account pay-off and negating the need for statements.
Real-time collections scripts based on payer rules. Patient Access, offered by Availity, combines real-time payer data with financial policies that are entered during set-up to create instant, patient-specific scripts that staff members read to the patient in front of them.
4. Schedule formal training
Just because someone can collect a copay does not mean he or she is comfortable with or capable of asking patients for past-due balances, surgical deposits, or large coinsurances. It is the rare staff person who is a “natural” at asking patients for money in a polished and professional manner.
That’s why training staff how to ask patients for money is vital. A front-office supervisor or manager should conduct several training sessions to cover policies and procedures. Training materials should include talking points and scenarios for collecting for office services and past-due balances, and calculating what patients owe, using technology tools. Use role-playing to ensure staff can explain payment plan options and how to apply for patient financing or financial assistance.
Few practices can skip this part of the POS program and still be successful. If your manager or supervisor is not capable of training, it is worth the investment to hire an outside expert. Without thorough training, staff efforts will be suboptimal or, at worst, fail because the staff members will not know how or what to collect.
5. Measure and monitor the outcome
The Hawthorne effect is a psychological phenomenon that says people perform better and make more positive changes as a result of increased attention.2 In other words, staff members will perform better, and collect more, if they know someone is paying attention. Trust us on this one.
Employees respect what management inspects. So even if the implementation of POS collections has been a big success, do not take your eyes off the ball.
Stop by the front desk or surgery coordinator’s office a few times a month and ask how much has been collected. Randomly review daily over-the-counter collections logs. And always put POS collections performance on the monthly partner meeting agenda; review a graph that shows monthly collections at checkout and surgery deposits. Keeping tabs on performance enables the practice to take action quickly when collections drop, and before that decline becomes acute.
6. Provide ongoing coaching and oversight
Most practices train once, then wonder why staff motivation (and collections too) fall off after a while. Like that new couch you bought: it was all you could talk about the week after it was delivered. Now, it is only a comfy place to sit. It is the same with collections efforts. When the newness wears off, staff motivation does too, and training principles can be forgotten. That’s human nature. Conduct role-playing in staff meetings each quarter and discuss best practices for handling patient objections. Encourage peer-to-peer observation and coaching to address knowledge gaps and missed collection opportunities. Ongoing training and coaching will tease out training needs and boost your team’s collection confidence and success.
1. 2015 Poverty Guidelines. US Department of Health and Human Services website. http://aspe.hhs.gov/poverty/15poverty.cfm. Accessed March 25, 2015.
2. The Hawthorne effect. The Economist website. http://www.economist.com/node/12510632. Published November 3, 2008. Accessed March 25, 2015.
An 8-surgeon group in the Southeast had a history of high patient receivables, the result of a long-held culture of “We’ll submit to your insurance and bill you after insurance pays.”
The billing and collections staff worked in the basement—far away and out of sight of the patients who showed up for their postoperative visits owing big bucks.
In a flash of wisdom, the administrator agreed to move the patient-balance collector into a converted closet near the check-out area, and provided the information, tools, and training that enabled her to speak with patients about their balances when they came in for an appointment. In her first month in this role and location, this employee collected more than her annual salary from patients.
It Takes a Program
This is one of our favorite client success stories, and it illustrates a key point: point-of-service (POS) collections do not have to be complicated. But the process does have to be deliberate and coordinated. Practices cannot simply update the financial policy and hope the staff members magically begin collecting. If this is your strategy, we promise that it will fail.
Successful POS collecting requires a program approach. And this approach starts at the front-end of the billing cycle, not “after insurance pays.”
POS collections have never been more important. Health insurance exchanges and payers are increasing deductibles and coinsurances. Physicians are opting out of network. Given these realities, POS collections are vital to your cash flow and effective receivables management.
If you are starting practice, you have a perfect opportunity to open with POS collecting in place. A solo surgeon whom we set up in practice did so, and has collected up-front for office services, scans, and surgeries from his first day in practice. Today, the practice’s only outstanding patient receivables are those of patients on payment plans—and these are less than 1% of total accounts receivable.
We also converted the “after insurance pays” philosophy of a surgeon in the South, implementing both POS collections and surgical deposits. In the first month, his patient payments increased by 40%. Another solo orthopedist reported an increased take-home salary of $90,000 in the first year after we helped his staff collect surgery deposits.
Six POS Program Elements
In 30 years of implementing or training staff to implement POS collections, we have come to recognize the following 6 key elements to include in your program approach: Policies + Procedures + Technology + Training + Monitoring + Coaching.
At a high level, here are the actions your practice will need to take:
1. Update the financial policy with 1 written standard for all physicians.
2. Develop granular procedures driven by the policy; these are the “how-tos” that enable the staff to collect successfully.
3. Implement new technologies, such as cost estimators, recurring payments, and online bill pay.
4. Schedule formal training to ensure that staff members know how to ask for money. (Do not assume they are, can, or will without training.)
5. Measure and monitor the outcome of patient collections and staff performance.
6. Provide ongoing coaching and oversight to maintain motivation and skills.
A blueprint for addressing each one of these actions follows.
1. Update the financial policy
The policy is the set of expectations on which to build all procedures and training. Dust off this document, and review it as a group with the practice administrator. First, strike old language that says the patient will be balance-billed, or will only be asked in the office for his visit copay. Next, strive for clarity. “You will be asked to pay your financial responsibility at the time of service,” really says nothing. Instead, the policy should be direct:
If you are recommended for surgery, our staff will calculate your coinsurance and unmet deductible amounts: 50% of this amount will be collected as a surgery deposit, and the remaining 50% is due on or before the day of surgery. Payment plans are available.
For office visits and services, break down the policy by coverage type. We find that a table such as the one shown makes expectations clear.
Finally, strive for 1 standard policy for all providers. If every provider is allowed to create his or her own set of collection policies, the practice is setting staff up for complexity overload, and collections will suffer.
2. Develop granular procedures
Few practices take the time to translate the financial policy into written procedures that can be followed by staff. The policy establishes the rules, but the procedures tell staff what to do to implement those rules. For instance:
Create a “POS Playbook” that contains information such as procedures, cost-quotation worksheets, US Poverty guidelines1, and financing brochures. As old-school as it sounds, a 3-ring binder is great for this information, and makes information access and updates easy.
3. Implement collection technologies
Modern practices use inexpensive (and often free) tools that increase patient convenience and staff efficiency. Implement at least 2 of these useful technologies and watch your POS collections increase:
Reports from your practice management system (PMS). Use the technology you already have. There are 2 standard reports in your PMS or clearinghouse that give front-desk staff the data to ask patients for money. Eligibility status and past-due balance reports indicate amounts owed, unmet deductibles, and the ineligible patients they can collect from when they come in for their appointment.
Online cost estimators. These free, online tools are offered by payers and provide staff with real-time data about a patient’s unmet deductible and coinsurance. When staff members enter Current Procedural Terminology (CPT) codes and the patient’s benefit information into the online cost estimator, they can access valuable information. Many insurance plans offer cost estimators on their web sites. Others deliver the data through statewide or regional portals, such as Availity (www.availity.com). The accuracy of cost-estimator data can vary by region and depends on the data links with payers. Ask your team to evaluate which estimators are best for you based on your payer mix.
Online bill pay. Everyone appreciates the convenience of paying bills online. Most patient portals offer this feature. If yours does not or you do not have a portal, you can offer PayPal (www.paypal.com) on your practice website, or use a system such as Intuit Health (www.intuithealth.com).
Recurring billing. Recurring billing is how you pay for services, such as Netflix, Pandora, or your gym membership: it is automatically billed to a credit card each month. Offer this option to patients as a payment plan method, and staff will no longer need to send costly statements, post monthly check payments, or follow up when a patient is delinquent. Plus, it guarantees payment every month; patients can no longer say, “I forgot.”
TransFirst (www.transfirstassociation.com) and a-claim (www.a-claim.com) offer recurring billing through a “virtual terminal” that staff logs in to at checkout, or during the preprocedure patient counseling process. Both vendors also offer the option of automatically charging a patient’s credit card after their insurance pays, speeding patient account pay-off and negating the need for statements.
Real-time collections scripts based on payer rules. Patient Access, offered by Availity, combines real-time payer data with financial policies that are entered during set-up to create instant, patient-specific scripts that staff members read to the patient in front of them.
4. Schedule formal training
Just because someone can collect a copay does not mean he or she is comfortable with or capable of asking patients for past-due balances, surgical deposits, or large coinsurances. It is the rare staff person who is a “natural” at asking patients for money in a polished and professional manner.
That’s why training staff how to ask patients for money is vital. A front-office supervisor or manager should conduct several training sessions to cover policies and procedures. Training materials should include talking points and scenarios for collecting for office services and past-due balances, and calculating what patients owe, using technology tools. Use role-playing to ensure staff can explain payment plan options and how to apply for patient financing or financial assistance.
Few practices can skip this part of the POS program and still be successful. If your manager or supervisor is not capable of training, it is worth the investment to hire an outside expert. Without thorough training, staff efforts will be suboptimal or, at worst, fail because the staff members will not know how or what to collect.
5. Measure and monitor the outcome
The Hawthorne effect is a psychological phenomenon that says people perform better and make more positive changes as a result of increased attention.2 In other words, staff members will perform better, and collect more, if they know someone is paying attention. Trust us on this one.
Employees respect what management inspects. So even if the implementation of POS collections has been a big success, do not take your eyes off the ball.
Stop by the front desk or surgery coordinator’s office a few times a month and ask how much has been collected. Randomly review daily over-the-counter collections logs. And always put POS collections performance on the monthly partner meeting agenda; review a graph that shows monthly collections at checkout and surgery deposits. Keeping tabs on performance enables the practice to take action quickly when collections drop, and before that decline becomes acute.
6. Provide ongoing coaching and oversight
Most practices train once, then wonder why staff motivation (and collections too) fall off after a while. Like that new couch you bought: it was all you could talk about the week after it was delivered. Now, it is only a comfy place to sit. It is the same with collections efforts. When the newness wears off, staff motivation does too, and training principles can be forgotten. That’s human nature. Conduct role-playing in staff meetings each quarter and discuss best practices for handling patient objections. Encourage peer-to-peer observation and coaching to address knowledge gaps and missed collection opportunities. Ongoing training and coaching will tease out training needs and boost your team’s collection confidence and success.
An 8-surgeon group in the Southeast had a history of high patient receivables, the result of a long-held culture of “We’ll submit to your insurance and bill you after insurance pays.”
The billing and collections staff worked in the basement—far away and out of sight of the patients who showed up for their postoperative visits owing big bucks.
In a flash of wisdom, the administrator agreed to move the patient-balance collector into a converted closet near the check-out area, and provided the information, tools, and training that enabled her to speak with patients about their balances when they came in for an appointment. In her first month in this role and location, this employee collected more than her annual salary from patients.
It Takes a Program
This is one of our favorite client success stories, and it illustrates a key point: point-of-service (POS) collections do not have to be complicated. But the process does have to be deliberate and coordinated. Practices cannot simply update the financial policy and hope the staff members magically begin collecting. If this is your strategy, we promise that it will fail.
Successful POS collecting requires a program approach. And this approach starts at the front-end of the billing cycle, not “after insurance pays.”
POS collections have never been more important. Health insurance exchanges and payers are increasing deductibles and coinsurances. Physicians are opting out of network. Given these realities, POS collections are vital to your cash flow and effective receivables management.
If you are starting practice, you have a perfect opportunity to open with POS collecting in place. A solo surgeon whom we set up in practice did so, and has collected up-front for office services, scans, and surgeries from his first day in practice. Today, the practice’s only outstanding patient receivables are those of patients on payment plans—and these are less than 1% of total accounts receivable.
We also converted the “after insurance pays” philosophy of a surgeon in the South, implementing both POS collections and surgical deposits. In the first month, his patient payments increased by 40%. Another solo orthopedist reported an increased take-home salary of $90,000 in the first year after we helped his staff collect surgery deposits.
Six POS Program Elements
In 30 years of implementing or training staff to implement POS collections, we have come to recognize the following 6 key elements to include in your program approach: Policies + Procedures + Technology + Training + Monitoring + Coaching.
At a high level, here are the actions your practice will need to take:
1. Update the financial policy with 1 written standard for all physicians.
2. Develop granular procedures driven by the policy; these are the “how-tos” that enable the staff to collect successfully.
3. Implement new technologies, such as cost estimators, recurring payments, and online bill pay.
4. Schedule formal training to ensure that staff members know how to ask for money. (Do not assume they are, can, or will without training.)
5. Measure and monitor the outcome of patient collections and staff performance.
6. Provide ongoing coaching and oversight to maintain motivation and skills.
A blueprint for addressing each one of these actions follows.
1. Update the financial policy
The policy is the set of expectations on which to build all procedures and training. Dust off this document, and review it as a group with the practice administrator. First, strike old language that says the patient will be balance-billed, or will only be asked in the office for his visit copay. Next, strive for clarity. “You will be asked to pay your financial responsibility at the time of service,” really says nothing. Instead, the policy should be direct:
If you are recommended for surgery, our staff will calculate your coinsurance and unmet deductible amounts: 50% of this amount will be collected as a surgery deposit, and the remaining 50% is due on or before the day of surgery. Payment plans are available.
For office visits and services, break down the policy by coverage type. We find that a table such as the one shown makes expectations clear.
Finally, strive for 1 standard policy for all providers. If every provider is allowed to create his or her own set of collection policies, the practice is setting staff up for complexity overload, and collections will suffer.
2. Develop granular procedures
Few practices take the time to translate the financial policy into written procedures that can be followed by staff. The policy establishes the rules, but the procedures tell staff what to do to implement those rules. For instance:
Create a “POS Playbook” that contains information such as procedures, cost-quotation worksheets, US Poverty guidelines1, and financing brochures. As old-school as it sounds, a 3-ring binder is great for this information, and makes information access and updates easy.
3. Implement collection technologies
Modern practices use inexpensive (and often free) tools that increase patient convenience and staff efficiency. Implement at least 2 of these useful technologies and watch your POS collections increase:
Reports from your practice management system (PMS). Use the technology you already have. There are 2 standard reports in your PMS or clearinghouse that give front-desk staff the data to ask patients for money. Eligibility status and past-due balance reports indicate amounts owed, unmet deductibles, and the ineligible patients they can collect from when they come in for their appointment.
Online cost estimators. These free, online tools are offered by payers and provide staff with real-time data about a patient’s unmet deductible and coinsurance. When staff members enter Current Procedural Terminology (CPT) codes and the patient’s benefit information into the online cost estimator, they can access valuable information. Many insurance plans offer cost estimators on their web sites. Others deliver the data through statewide or regional portals, such as Availity (www.availity.com). The accuracy of cost-estimator data can vary by region and depends on the data links with payers. Ask your team to evaluate which estimators are best for you based on your payer mix.
Online bill pay. Everyone appreciates the convenience of paying bills online. Most patient portals offer this feature. If yours does not or you do not have a portal, you can offer PayPal (www.paypal.com) on your practice website, or use a system such as Intuit Health (www.intuithealth.com).
Recurring billing. Recurring billing is how you pay for services, such as Netflix, Pandora, or your gym membership: it is automatically billed to a credit card each month. Offer this option to patients as a payment plan method, and staff will no longer need to send costly statements, post monthly check payments, or follow up when a patient is delinquent. Plus, it guarantees payment every month; patients can no longer say, “I forgot.”
TransFirst (www.transfirstassociation.com) and a-claim (www.a-claim.com) offer recurring billing through a “virtual terminal” that staff logs in to at checkout, or during the preprocedure patient counseling process. Both vendors also offer the option of automatically charging a patient’s credit card after their insurance pays, speeding patient account pay-off and negating the need for statements.
Real-time collections scripts based on payer rules. Patient Access, offered by Availity, combines real-time payer data with financial policies that are entered during set-up to create instant, patient-specific scripts that staff members read to the patient in front of them.
4. Schedule formal training
Just because someone can collect a copay does not mean he or she is comfortable with or capable of asking patients for past-due balances, surgical deposits, or large coinsurances. It is the rare staff person who is a “natural” at asking patients for money in a polished and professional manner.
That’s why training staff how to ask patients for money is vital. A front-office supervisor or manager should conduct several training sessions to cover policies and procedures. Training materials should include talking points and scenarios for collecting for office services and past-due balances, and calculating what patients owe, using technology tools. Use role-playing to ensure staff can explain payment plan options and how to apply for patient financing or financial assistance.
Few practices can skip this part of the POS program and still be successful. If your manager or supervisor is not capable of training, it is worth the investment to hire an outside expert. Without thorough training, staff efforts will be suboptimal or, at worst, fail because the staff members will not know how or what to collect.
5. Measure and monitor the outcome
The Hawthorne effect is a psychological phenomenon that says people perform better and make more positive changes as a result of increased attention.2 In other words, staff members will perform better, and collect more, if they know someone is paying attention. Trust us on this one.
Employees respect what management inspects. So even if the implementation of POS collections has been a big success, do not take your eyes off the ball.
Stop by the front desk or surgery coordinator’s office a few times a month and ask how much has been collected. Randomly review daily over-the-counter collections logs. And always put POS collections performance on the monthly partner meeting agenda; review a graph that shows monthly collections at checkout and surgery deposits. Keeping tabs on performance enables the practice to take action quickly when collections drop, and before that decline becomes acute.
6. Provide ongoing coaching and oversight
Most practices train once, then wonder why staff motivation (and collections too) fall off after a while. Like that new couch you bought: it was all you could talk about the week after it was delivered. Now, it is only a comfy place to sit. It is the same with collections efforts. When the newness wears off, staff motivation does too, and training principles can be forgotten. That’s human nature. Conduct role-playing in staff meetings each quarter and discuss best practices for handling patient objections. Encourage peer-to-peer observation and coaching to address knowledge gaps and missed collection opportunities. Ongoing training and coaching will tease out training needs and boost your team’s collection confidence and success.
1. 2015 Poverty Guidelines. US Department of Health and Human Services website. http://aspe.hhs.gov/poverty/15poverty.cfm. Accessed March 25, 2015.
2. The Hawthorne effect. The Economist website. http://www.economist.com/node/12510632. Published November 3, 2008. Accessed March 25, 2015.
1. 2015 Poverty Guidelines. US Department of Health and Human Services website. http://aspe.hhs.gov/poverty/15poverty.cfm. Accessed March 25, 2015.
2. The Hawthorne effect. The Economist website. http://www.economist.com/node/12510632. Published November 3, 2008. Accessed March 25, 2015.
Total Hip Arthroplasty After Contralateral Hip Disarticulation: A Challenging “Simple Primary”
Patients with lower limb amputation have a high incidence of hip and knee osteoarthritis (OA) in the residual limb as well as the contralateral limb. A radical surgery, hip disarticulation is generally performed in younger patients after malignancy or trauma. Compliance is poor with existing prostheses, resulting in increased dependency on and use of the remaining sound limb.
In this case report, a crutch-walking 51-year-old woman presented with severe left hip arthritis 25 years after a right hip disarticulation. She underwent total hip arthroplasty (THA), a challenging procedure in a person without a contralateral hip joint. The many complex technical considerations associated with her THA included precise perioperative planning, the selection of appropriate prostheses and bearing surfaces, and the preoperative and intraoperative assessment of limb length and offset. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman presented to our service with a 3-year history of debilitating left hip pain. Twenty-five years earlier, she had been diagnosed with synovial sarcoma of the right knee and underwent limb-sparing surgery, followed by a true hip disarticulation performed for local recurrence. After her surgery, she declined the use of a prosthesis and mobilized with the use of 2 crutches. She has remained otherwise healthy and active, and runs her own business, which involves some lifting and carrying of objects. During the 3 years prior to presentation, she developed progressively debilitating left hip and groin pain, which radiated to the medial aspect of her left knee. Her mobilization distance had reduced to a few hundred meters, and she experienced significant night pain, and start-up pain. Activity modification, weight loss, and nonsteroidal anti-inflammatory medication afforded no relief. She denied any back pain or radicular symptoms.
Clinical examination showed a well-healed scar and pristine stump under her right hemipelvis. Passive range of movement of her left hip was painful for all movements, reduced at flexion (90º) and internal (10º) and external rotation (5º). Examination of her left knee was normal, with a full range of movement and no joint-line tenderness. A high body mass index (>30) was noted. Radiographic imaging confirmed significant OA of the hip joint (Figure 1). Informed consent was obtained for THA. The implants were selected—an uncemented collared Corail Stem (DePuy, Warsaw, Indiana) with a stainless steel dual mobility (DM) Novae SunFit acetabular cup (Serf, Decines, France), with bearing components of ceramic on polyethylene. A preoperative computed tomography (CT) scan of the left hip was performed (Figure 2) to aid templating, which was accomplished using plain films and CT images, with reference to the proximal femur for deciding level of neck cut, planning stem size, and optimizing length and offset, while determining cup size, depth, inclination, and height for the acetabular component.
Prior to surgery, the patient was positioned in the lateral decubitus position, using folded pillows under the medial aspect of her left proximal and distal thigh in lieu of her amputated limb. Pillows were secured to the table with elastic bandage tape. Standard pubic symphysis, lumbosacral, and midthoracic padded bolsters stabilized the pelvis in the normal fashion, with additional elastic bandage tape to further secure the pelvis brim to the table and reduce intraoperative motion. A posterior approach was used. A capsulotomy was performed with the hip in extension and slight abduction, with meticulous preservation of the capsule as the guide for the patient’s native length and offset. Reaming of the acetabulum was line to line, with insertion of an uncemented DM metal-back press-fit hydroxyapatite-coated shell placed in a standard fashion parallel with the transverse acetabular ligament, as described by Archbold and colleagues.1 The femur was sequentially reamed with broaches until press fit was achieved, and a calcar reamer was used to optimize interface with the collared implant. The surgeon’s standard 4 clinical tests were performed with trial implants after reduction to gauge hip tension, length, and offset. These tests are positive shuck test with hip and knee extension, lack of shuck in hip extension with knee flexion, lack of kick sign in hip extension and knee flexion, and palpation of gluteus medius belly to determine tension. Finally, with the hip returned to the extended and slightly abducted position, the capsule was tested for length and tension. The definitive stem implant was inserted, final testing with trial heads was repeated prior to definitive neck length and head selection, and final reduction was performed. A layered closure was performed, after generous washout. Pillows were taped together and positioned from the bed railing across the midline of the bed to prevent abduction, in the fashion of an abduction pillow.
The patient was mobilized the day after surgery and permitted full weight-bearing. Recovery was uneventful, and the patient returned to work within 6 weeks of surgery after her scheduled appointment and radiographic examination (Figure 3). Ongoing regular clinical and radiologic surveillance are planned.
Discussion
Hip and knee OA in the residual limb is more common for amputees than for the general population.2,3 THA for OA in amputees has been reported after below-knee amputation in both the ipsilateral and the contralateral hip.4 A true hip disarticulation is a rarely performed radical surgical procedure, involving the removal of the entire femur, and is most often related to surgical oncologic treatment or combat-related injuries, both being more common in younger people. Like many patients who have had a hip disarticulation,5 our patient declined a prosthesis, finding the design cosmetically unappealing and uncomfortable, in favor of crutch-walking. This accelerated wear of the remaining hip, and is a sobering reminder of the high demand on the bearing surfaces of the implants after her procedure.
The implants chosen for this procedure are critical. We use implants which are proven and reliable. Our institution uses the Corail Stem, an uncemented collared stem with an Orthopaedic Data Evaluation Panel (ODEP) 10A rating,6 widely used for THA.7 For the acetabulum, we chose the Novae SunFit, a modern version based on Bousquet’s 1976 DM design. The DM cup is a tripolar cup with a fixed porous-coated or cemented metal cup, which articulates with a large mobile polyethylene liner. A standard head in either metal or ceramic is inserted into this liner. The articulation between the head and the liner is constrained, while the articulation between the liner and the metal cup is unconstrained. This interposition of a mobile insert increases the effective head diameter, and the favorable head-neck ratio allows increased range of motion while avoiding early femoral neck impingement with a fixed liner or metal cup. A growing body of evidence indicates that DM cups reduce dislocation rates in primary and revision total knee arthroplasty and, when used with prudence, in selected tumor cases.8 A study of 1905 hips, using second-generation DM cups, reported cumulative survival rate of 98.6% at 12.2 years,9 with favorable outcomes compared with standard prostheses in the medium term for younger patients,10 and in the longer term,11 without increasing polyethylene wear.12
We use DM cups for 2 patient cohorts: first, for all patients older than 75 years because, in this age group, the risk of dislocation is higher than the risk of revision for wear-induced lysis; and second, in younger patients with any neuromuscular, cognitive, or mechanical risk factors that would excessively increase the risk of dislocation. This reflects the balance of risks in arthroplasty, with the ever-present trade-off between polyethylene-induced osteolysis and stability. Dislocation of the remaining sound limb for this young, active, agile patient would be a catastrophic complication. Given our patient’s risk factors for dislocation—female, an amputee with a high risk of falling, high body mass index, and lack of a contralateral limb to restrict adduction—the balance of risks favored hip stability over wear. We chose, therefore, a DM cup, using a ceramic-head-on-polyethylene-insert surface-bearing combination.
CT scanning is routinely performed in our institution to optimize preoperative templating. The preoperative CT images enable accurate planning, notably for the extramedullary reconstruction,13 and are used in addition to acetates and standard radiographs. This encourages preservation of acetabular bone stock by selecting the smallest suitable cup, reduces the risk of femoral fracture by giving an accurate prediction of the stem size, and ensures accuracy of restoring the patient’s offset and length. Although limb-length discrepancy was not an issue for this patient with a single sound limb, the sequalae of excessively increasing offset or length (eg, gluteus medius tendinopathy and trochanteric bursitis) would arguably be more debilitating than for someone who could offload weight to the “good hip.” For these reasons, marrying the preoperative templating with on-table testing with trial prostheses and restoring the native capsular tension is vital.
The importance of on-table positioning for proximal amputees undergoing hip arthroplasty has been highlighted.14 Lacking the normal bony constraints increases the risk of intraoperative on-table movement, which, in turn, risks reducing the accuracy of implant positioning. Crude limb-length checking using the contralateral knee is not possible. In addition, the lack of a contralateral hip joint causes a degree of compensatory pelvic tilt, which raises the option of increasing the coverage to compensate for obligate adduction during single-leg, crutch-walking gait. Lacking established guidelines to accommodate these variables, we inserted the cup in a standard fashion, at 45º, referencing acetabular version using the transverse acetabular ligament,1 and used the smallest stable cup after line-to-line reaming.
This case of THA in a young, crutch-walking patient with a contralateral true hip disarticulation highlights the importance of meticulous preoperative planning, implant selection appropriate for the patient in question, perioperative positioning, and the technical and operative challenges of restoring the patient’s normal hip architecture.
1. Archbold HA, Mockford B, Molloy D, McConway J, Ogonda L, Beverland D. The transverse acetabular ligament: an aid to orientation of the acetabular component during primary total hip replacement: a preliminary study of 1000 cases investigating postoperative stability. J Bone Joint Surg Br. 2006;88(7):883-886.
2. Kulkarni J, Adams J, Thomas E, Silman A. Association between amputation, arthritis and osteopenia in British male war veterans with major lower limb amputations. Clin Rehabil. 1998;12(4):348-353.
3. Struyf PA, van Heugten CM, Hitters MW, Smeets RJ. The prevalence of osteoarthritis of the intact hip and knee among traumatic leg amputees. Arch Phys Med Rehabil. 2009;90(3):440-446.
4. Nejat EJ, Meyer A, Sánchez PM, Schaefer SH, Westrich GH. Total hip arthroplasty and rehabilitation in ambulatory lower extremity amputees--a case series. Iowa Orthop J. 2005;25:38-41.
5. Zaffer SM, Braddom RL, Conti A, Goff J, Bokma D. Total hip disarticulation prosthesis with suction socket: report of two cases. Am J Phys Med Rehabil. 1999;78(2):160-162.
6. Lewis P. ODEP [Orthopaedic Data Evaluation Panel]. NHS Supply Chain website. http://www.supplychain.nhs.uk/odep. Accessed April 2, 2015.
7. National Joint Registry for England and Wales. 8th Annual Report, 2011. National Joint Registry website. www.njrcentre.org.uk/NjrCentre/Portals/0/Documents/NJR%208th%20Annual%20Report%202011.pdf. Accessed April 2, 2015.
8. Grazioli A, Ek ET, Rüdiger HA. Biomechanical concept and clinical outcome of dual mobility cups. Int Orthop. 2012;36(12):2411-2418.
9. Massin P, Orain V, Philippot R, Farizon F, Fessy MH. Fixation failures of dual mobility cups: a mid-term study of 2601 hip replacements. Clin Orthop. 2012;470(7):1932-1940.
10. Epinette JA, Béracassat R, Tracol P, Pagazani G, Vandenbussche E. Are modern dual mobility cups a valuable option in reducing instability after primary hip arthroplasty, even in younger patients? J Arthroplasty. 2014;29(6):1323-1328.
11. Philippot R, Meucci JF, Boyer B, Farizon F. Modern dual-mobility cup implanted with an uncemented stem: about 100 cases with 12-year follow-up. Surg Technol Int. 2013;23:208-212.
12. Prudhon JL, Ferreira A, Verdier R. Dual mobility cup: dislocation rate and survivorship at ten years of follow-up. Int Orthop. 2013;37(12):2345-2350.
13. Sariali E, Mouttet A, Pasquier G, Durante E, Catone Y. Accuracy of reconstruction of the hip using computerised three-dimensional pre-operative planning and a cementless modular neck. J Bone Joint Surg Br. 2009;91(13):333-340.
14. Bong MR, Kaplan KM, Jaffe WL. Total hip arthroplasty in a patient with contralateral hemipelvectomy. J Arthroplasty. 2006;21(5):762-764.
Patients with lower limb amputation have a high incidence of hip and knee osteoarthritis (OA) in the residual limb as well as the contralateral limb. A radical surgery, hip disarticulation is generally performed in younger patients after malignancy or trauma. Compliance is poor with existing prostheses, resulting in increased dependency on and use of the remaining sound limb.
In this case report, a crutch-walking 51-year-old woman presented with severe left hip arthritis 25 years after a right hip disarticulation. She underwent total hip arthroplasty (THA), a challenging procedure in a person without a contralateral hip joint. The many complex technical considerations associated with her THA included precise perioperative planning, the selection of appropriate prostheses and bearing surfaces, and the preoperative and intraoperative assessment of limb length and offset. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman presented to our service with a 3-year history of debilitating left hip pain. Twenty-five years earlier, she had been diagnosed with synovial sarcoma of the right knee and underwent limb-sparing surgery, followed by a true hip disarticulation performed for local recurrence. After her surgery, she declined the use of a prosthesis and mobilized with the use of 2 crutches. She has remained otherwise healthy and active, and runs her own business, which involves some lifting and carrying of objects. During the 3 years prior to presentation, she developed progressively debilitating left hip and groin pain, which radiated to the medial aspect of her left knee. Her mobilization distance had reduced to a few hundred meters, and she experienced significant night pain, and start-up pain. Activity modification, weight loss, and nonsteroidal anti-inflammatory medication afforded no relief. She denied any back pain or radicular symptoms.
Clinical examination showed a well-healed scar and pristine stump under her right hemipelvis. Passive range of movement of her left hip was painful for all movements, reduced at flexion (90º) and internal (10º) and external rotation (5º). Examination of her left knee was normal, with a full range of movement and no joint-line tenderness. A high body mass index (>30) was noted. Radiographic imaging confirmed significant OA of the hip joint (Figure 1). Informed consent was obtained for THA. The implants were selected—an uncemented collared Corail Stem (DePuy, Warsaw, Indiana) with a stainless steel dual mobility (DM) Novae SunFit acetabular cup (Serf, Decines, France), with bearing components of ceramic on polyethylene. A preoperative computed tomography (CT) scan of the left hip was performed (Figure 2) to aid templating, which was accomplished using plain films and CT images, with reference to the proximal femur for deciding level of neck cut, planning stem size, and optimizing length and offset, while determining cup size, depth, inclination, and height for the acetabular component.
Prior to surgery, the patient was positioned in the lateral decubitus position, using folded pillows under the medial aspect of her left proximal and distal thigh in lieu of her amputated limb. Pillows were secured to the table with elastic bandage tape. Standard pubic symphysis, lumbosacral, and midthoracic padded bolsters stabilized the pelvis in the normal fashion, with additional elastic bandage tape to further secure the pelvis brim to the table and reduce intraoperative motion. A posterior approach was used. A capsulotomy was performed with the hip in extension and slight abduction, with meticulous preservation of the capsule as the guide for the patient’s native length and offset. Reaming of the acetabulum was line to line, with insertion of an uncemented DM metal-back press-fit hydroxyapatite-coated shell placed in a standard fashion parallel with the transverse acetabular ligament, as described by Archbold and colleagues.1 The femur was sequentially reamed with broaches until press fit was achieved, and a calcar reamer was used to optimize interface with the collared implant. The surgeon’s standard 4 clinical tests were performed with trial implants after reduction to gauge hip tension, length, and offset. These tests are positive shuck test with hip and knee extension, lack of shuck in hip extension with knee flexion, lack of kick sign in hip extension and knee flexion, and palpation of gluteus medius belly to determine tension. Finally, with the hip returned to the extended and slightly abducted position, the capsule was tested for length and tension. The definitive stem implant was inserted, final testing with trial heads was repeated prior to definitive neck length and head selection, and final reduction was performed. A layered closure was performed, after generous washout. Pillows were taped together and positioned from the bed railing across the midline of the bed to prevent abduction, in the fashion of an abduction pillow.
The patient was mobilized the day after surgery and permitted full weight-bearing. Recovery was uneventful, and the patient returned to work within 6 weeks of surgery after her scheduled appointment and radiographic examination (Figure 3). Ongoing regular clinical and radiologic surveillance are planned.
Discussion
Hip and knee OA in the residual limb is more common for amputees than for the general population.2,3 THA for OA in amputees has been reported after below-knee amputation in both the ipsilateral and the contralateral hip.4 A true hip disarticulation is a rarely performed radical surgical procedure, involving the removal of the entire femur, and is most often related to surgical oncologic treatment or combat-related injuries, both being more common in younger people. Like many patients who have had a hip disarticulation,5 our patient declined a prosthesis, finding the design cosmetically unappealing and uncomfortable, in favor of crutch-walking. This accelerated wear of the remaining hip, and is a sobering reminder of the high demand on the bearing surfaces of the implants after her procedure.
The implants chosen for this procedure are critical. We use implants which are proven and reliable. Our institution uses the Corail Stem, an uncemented collared stem with an Orthopaedic Data Evaluation Panel (ODEP) 10A rating,6 widely used for THA.7 For the acetabulum, we chose the Novae SunFit, a modern version based on Bousquet’s 1976 DM design. The DM cup is a tripolar cup with a fixed porous-coated or cemented metal cup, which articulates with a large mobile polyethylene liner. A standard head in either metal or ceramic is inserted into this liner. The articulation between the head and the liner is constrained, while the articulation between the liner and the metal cup is unconstrained. This interposition of a mobile insert increases the effective head diameter, and the favorable head-neck ratio allows increased range of motion while avoiding early femoral neck impingement with a fixed liner or metal cup. A growing body of evidence indicates that DM cups reduce dislocation rates in primary and revision total knee arthroplasty and, when used with prudence, in selected tumor cases.8 A study of 1905 hips, using second-generation DM cups, reported cumulative survival rate of 98.6% at 12.2 years,9 with favorable outcomes compared with standard prostheses in the medium term for younger patients,10 and in the longer term,11 without increasing polyethylene wear.12
We use DM cups for 2 patient cohorts: first, for all patients older than 75 years because, in this age group, the risk of dislocation is higher than the risk of revision for wear-induced lysis; and second, in younger patients with any neuromuscular, cognitive, or mechanical risk factors that would excessively increase the risk of dislocation. This reflects the balance of risks in arthroplasty, with the ever-present trade-off between polyethylene-induced osteolysis and stability. Dislocation of the remaining sound limb for this young, active, agile patient would be a catastrophic complication. Given our patient’s risk factors for dislocation—female, an amputee with a high risk of falling, high body mass index, and lack of a contralateral limb to restrict adduction—the balance of risks favored hip stability over wear. We chose, therefore, a DM cup, using a ceramic-head-on-polyethylene-insert surface-bearing combination.
CT scanning is routinely performed in our institution to optimize preoperative templating. The preoperative CT images enable accurate planning, notably for the extramedullary reconstruction,13 and are used in addition to acetates and standard radiographs. This encourages preservation of acetabular bone stock by selecting the smallest suitable cup, reduces the risk of femoral fracture by giving an accurate prediction of the stem size, and ensures accuracy of restoring the patient’s offset and length. Although limb-length discrepancy was not an issue for this patient with a single sound limb, the sequalae of excessively increasing offset or length (eg, gluteus medius tendinopathy and trochanteric bursitis) would arguably be more debilitating than for someone who could offload weight to the “good hip.” For these reasons, marrying the preoperative templating with on-table testing with trial prostheses and restoring the native capsular tension is vital.
The importance of on-table positioning for proximal amputees undergoing hip arthroplasty has been highlighted.14 Lacking the normal bony constraints increases the risk of intraoperative on-table movement, which, in turn, risks reducing the accuracy of implant positioning. Crude limb-length checking using the contralateral knee is not possible. In addition, the lack of a contralateral hip joint causes a degree of compensatory pelvic tilt, which raises the option of increasing the coverage to compensate for obligate adduction during single-leg, crutch-walking gait. Lacking established guidelines to accommodate these variables, we inserted the cup in a standard fashion, at 45º, referencing acetabular version using the transverse acetabular ligament,1 and used the smallest stable cup after line-to-line reaming.
This case of THA in a young, crutch-walking patient with a contralateral true hip disarticulation highlights the importance of meticulous preoperative planning, implant selection appropriate for the patient in question, perioperative positioning, and the technical and operative challenges of restoring the patient’s normal hip architecture.
Patients with lower limb amputation have a high incidence of hip and knee osteoarthritis (OA) in the residual limb as well as the contralateral limb. A radical surgery, hip disarticulation is generally performed in younger patients after malignancy or trauma. Compliance is poor with existing prostheses, resulting in increased dependency on and use of the remaining sound limb.
In this case report, a crutch-walking 51-year-old woman presented with severe left hip arthritis 25 years after a right hip disarticulation. She underwent total hip arthroplasty (THA), a challenging procedure in a person without a contralateral hip joint. The many complex technical considerations associated with her THA included precise perioperative planning, the selection of appropriate prostheses and bearing surfaces, and the preoperative and intraoperative assessment of limb length and offset. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman presented to our service with a 3-year history of debilitating left hip pain. Twenty-five years earlier, she had been diagnosed with synovial sarcoma of the right knee and underwent limb-sparing surgery, followed by a true hip disarticulation performed for local recurrence. After her surgery, she declined the use of a prosthesis and mobilized with the use of 2 crutches. She has remained otherwise healthy and active, and runs her own business, which involves some lifting and carrying of objects. During the 3 years prior to presentation, she developed progressively debilitating left hip and groin pain, which radiated to the medial aspect of her left knee. Her mobilization distance had reduced to a few hundred meters, and she experienced significant night pain, and start-up pain. Activity modification, weight loss, and nonsteroidal anti-inflammatory medication afforded no relief. She denied any back pain or radicular symptoms.
Clinical examination showed a well-healed scar and pristine stump under her right hemipelvis. Passive range of movement of her left hip was painful for all movements, reduced at flexion (90º) and internal (10º) and external rotation (5º). Examination of her left knee was normal, with a full range of movement and no joint-line tenderness. A high body mass index (>30) was noted. Radiographic imaging confirmed significant OA of the hip joint (Figure 1). Informed consent was obtained for THA. The implants were selected—an uncemented collared Corail Stem (DePuy, Warsaw, Indiana) with a stainless steel dual mobility (DM) Novae SunFit acetabular cup (Serf, Decines, France), with bearing components of ceramic on polyethylene. A preoperative computed tomography (CT) scan of the left hip was performed (Figure 2) to aid templating, which was accomplished using plain films and CT images, with reference to the proximal femur for deciding level of neck cut, planning stem size, and optimizing length and offset, while determining cup size, depth, inclination, and height for the acetabular component.
Prior to surgery, the patient was positioned in the lateral decubitus position, using folded pillows under the medial aspect of her left proximal and distal thigh in lieu of her amputated limb. Pillows were secured to the table with elastic bandage tape. Standard pubic symphysis, lumbosacral, and midthoracic padded bolsters stabilized the pelvis in the normal fashion, with additional elastic bandage tape to further secure the pelvis brim to the table and reduce intraoperative motion. A posterior approach was used. A capsulotomy was performed with the hip in extension and slight abduction, with meticulous preservation of the capsule as the guide for the patient’s native length and offset. Reaming of the acetabulum was line to line, with insertion of an uncemented DM metal-back press-fit hydroxyapatite-coated shell placed in a standard fashion parallel with the transverse acetabular ligament, as described by Archbold and colleagues.1 The femur was sequentially reamed with broaches until press fit was achieved, and a calcar reamer was used to optimize interface with the collared implant. The surgeon’s standard 4 clinical tests were performed with trial implants after reduction to gauge hip tension, length, and offset. These tests are positive shuck test with hip and knee extension, lack of shuck in hip extension with knee flexion, lack of kick sign in hip extension and knee flexion, and palpation of gluteus medius belly to determine tension. Finally, with the hip returned to the extended and slightly abducted position, the capsule was tested for length and tension. The definitive stem implant was inserted, final testing with trial heads was repeated prior to definitive neck length and head selection, and final reduction was performed. A layered closure was performed, after generous washout. Pillows were taped together and positioned from the bed railing across the midline of the bed to prevent abduction, in the fashion of an abduction pillow.
The patient was mobilized the day after surgery and permitted full weight-bearing. Recovery was uneventful, and the patient returned to work within 6 weeks of surgery after her scheduled appointment and radiographic examination (Figure 3). Ongoing regular clinical and radiologic surveillance are planned.
Discussion
Hip and knee OA in the residual limb is more common for amputees than for the general population.2,3 THA for OA in amputees has been reported after below-knee amputation in both the ipsilateral and the contralateral hip.4 A true hip disarticulation is a rarely performed radical surgical procedure, involving the removal of the entire femur, and is most often related to surgical oncologic treatment or combat-related injuries, both being more common in younger people. Like many patients who have had a hip disarticulation,5 our patient declined a prosthesis, finding the design cosmetically unappealing and uncomfortable, in favor of crutch-walking. This accelerated wear of the remaining hip, and is a sobering reminder of the high demand on the bearing surfaces of the implants after her procedure.
The implants chosen for this procedure are critical. We use implants which are proven and reliable. Our institution uses the Corail Stem, an uncemented collared stem with an Orthopaedic Data Evaluation Panel (ODEP) 10A rating,6 widely used for THA.7 For the acetabulum, we chose the Novae SunFit, a modern version based on Bousquet’s 1976 DM design. The DM cup is a tripolar cup with a fixed porous-coated or cemented metal cup, which articulates with a large mobile polyethylene liner. A standard head in either metal or ceramic is inserted into this liner. The articulation between the head and the liner is constrained, while the articulation between the liner and the metal cup is unconstrained. This interposition of a mobile insert increases the effective head diameter, and the favorable head-neck ratio allows increased range of motion while avoiding early femoral neck impingement with a fixed liner or metal cup. A growing body of evidence indicates that DM cups reduce dislocation rates in primary and revision total knee arthroplasty and, when used with prudence, in selected tumor cases.8 A study of 1905 hips, using second-generation DM cups, reported cumulative survival rate of 98.6% at 12.2 years,9 with favorable outcomes compared with standard prostheses in the medium term for younger patients,10 and in the longer term,11 without increasing polyethylene wear.12
We use DM cups for 2 patient cohorts: first, for all patients older than 75 years because, in this age group, the risk of dislocation is higher than the risk of revision for wear-induced lysis; and second, in younger patients with any neuromuscular, cognitive, or mechanical risk factors that would excessively increase the risk of dislocation. This reflects the balance of risks in arthroplasty, with the ever-present trade-off between polyethylene-induced osteolysis and stability. Dislocation of the remaining sound limb for this young, active, agile patient would be a catastrophic complication. Given our patient’s risk factors for dislocation—female, an amputee with a high risk of falling, high body mass index, and lack of a contralateral limb to restrict adduction—the balance of risks favored hip stability over wear. We chose, therefore, a DM cup, using a ceramic-head-on-polyethylene-insert surface-bearing combination.
CT scanning is routinely performed in our institution to optimize preoperative templating. The preoperative CT images enable accurate planning, notably for the extramedullary reconstruction,13 and are used in addition to acetates and standard radiographs. This encourages preservation of acetabular bone stock by selecting the smallest suitable cup, reduces the risk of femoral fracture by giving an accurate prediction of the stem size, and ensures accuracy of restoring the patient’s offset and length. Although limb-length discrepancy was not an issue for this patient with a single sound limb, the sequalae of excessively increasing offset or length (eg, gluteus medius tendinopathy and trochanteric bursitis) would arguably be more debilitating than for someone who could offload weight to the “good hip.” For these reasons, marrying the preoperative templating with on-table testing with trial prostheses and restoring the native capsular tension is vital.
The importance of on-table positioning for proximal amputees undergoing hip arthroplasty has been highlighted.14 Lacking the normal bony constraints increases the risk of intraoperative on-table movement, which, in turn, risks reducing the accuracy of implant positioning. Crude limb-length checking using the contralateral knee is not possible. In addition, the lack of a contralateral hip joint causes a degree of compensatory pelvic tilt, which raises the option of increasing the coverage to compensate for obligate adduction during single-leg, crutch-walking gait. Lacking established guidelines to accommodate these variables, we inserted the cup in a standard fashion, at 45º, referencing acetabular version using the transverse acetabular ligament,1 and used the smallest stable cup after line-to-line reaming.
This case of THA in a young, crutch-walking patient with a contralateral true hip disarticulation highlights the importance of meticulous preoperative planning, implant selection appropriate for the patient in question, perioperative positioning, and the technical and operative challenges of restoring the patient’s normal hip architecture.
1. Archbold HA, Mockford B, Molloy D, McConway J, Ogonda L, Beverland D. The transverse acetabular ligament: an aid to orientation of the acetabular component during primary total hip replacement: a preliminary study of 1000 cases investigating postoperative stability. J Bone Joint Surg Br. 2006;88(7):883-886.
2. Kulkarni J, Adams J, Thomas E, Silman A. Association between amputation, arthritis and osteopenia in British male war veterans with major lower limb amputations. Clin Rehabil. 1998;12(4):348-353.
3. Struyf PA, van Heugten CM, Hitters MW, Smeets RJ. The prevalence of osteoarthritis of the intact hip and knee among traumatic leg amputees. Arch Phys Med Rehabil. 2009;90(3):440-446.
4. Nejat EJ, Meyer A, Sánchez PM, Schaefer SH, Westrich GH. Total hip arthroplasty and rehabilitation in ambulatory lower extremity amputees--a case series. Iowa Orthop J. 2005;25:38-41.
5. Zaffer SM, Braddom RL, Conti A, Goff J, Bokma D. Total hip disarticulation prosthesis with suction socket: report of two cases. Am J Phys Med Rehabil. 1999;78(2):160-162.
6. Lewis P. ODEP [Orthopaedic Data Evaluation Panel]. NHS Supply Chain website. http://www.supplychain.nhs.uk/odep. Accessed April 2, 2015.
7. National Joint Registry for England and Wales. 8th Annual Report, 2011. National Joint Registry website. www.njrcentre.org.uk/NjrCentre/Portals/0/Documents/NJR%208th%20Annual%20Report%202011.pdf. Accessed April 2, 2015.
8. Grazioli A, Ek ET, Rüdiger HA. Biomechanical concept and clinical outcome of dual mobility cups. Int Orthop. 2012;36(12):2411-2418.
9. Massin P, Orain V, Philippot R, Farizon F, Fessy MH. Fixation failures of dual mobility cups: a mid-term study of 2601 hip replacements. Clin Orthop. 2012;470(7):1932-1940.
10. Epinette JA, Béracassat R, Tracol P, Pagazani G, Vandenbussche E. Are modern dual mobility cups a valuable option in reducing instability after primary hip arthroplasty, even in younger patients? J Arthroplasty. 2014;29(6):1323-1328.
11. Philippot R, Meucci JF, Boyer B, Farizon F. Modern dual-mobility cup implanted with an uncemented stem: about 100 cases with 12-year follow-up. Surg Technol Int. 2013;23:208-212.
12. Prudhon JL, Ferreira A, Verdier R. Dual mobility cup: dislocation rate and survivorship at ten years of follow-up. Int Orthop. 2013;37(12):2345-2350.
13. Sariali E, Mouttet A, Pasquier G, Durante E, Catone Y. Accuracy of reconstruction of the hip using computerised three-dimensional pre-operative planning and a cementless modular neck. J Bone Joint Surg Br. 2009;91(13):333-340.
14. Bong MR, Kaplan KM, Jaffe WL. Total hip arthroplasty in a patient with contralateral hemipelvectomy. J Arthroplasty. 2006;21(5):762-764.
1. Archbold HA, Mockford B, Molloy D, McConway J, Ogonda L, Beverland D. The transverse acetabular ligament: an aid to orientation of the acetabular component during primary total hip replacement: a preliminary study of 1000 cases investigating postoperative stability. J Bone Joint Surg Br. 2006;88(7):883-886.
2. Kulkarni J, Adams J, Thomas E, Silman A. Association between amputation, arthritis and osteopenia in British male war veterans with major lower limb amputations. Clin Rehabil. 1998;12(4):348-353.
3. Struyf PA, van Heugten CM, Hitters MW, Smeets RJ. The prevalence of osteoarthritis of the intact hip and knee among traumatic leg amputees. Arch Phys Med Rehabil. 2009;90(3):440-446.
4. Nejat EJ, Meyer A, Sánchez PM, Schaefer SH, Westrich GH. Total hip arthroplasty and rehabilitation in ambulatory lower extremity amputees--a case series. Iowa Orthop J. 2005;25:38-41.
5. Zaffer SM, Braddom RL, Conti A, Goff J, Bokma D. Total hip disarticulation prosthesis with suction socket: report of two cases. Am J Phys Med Rehabil. 1999;78(2):160-162.
6. Lewis P. ODEP [Orthopaedic Data Evaluation Panel]. NHS Supply Chain website. http://www.supplychain.nhs.uk/odep. Accessed April 2, 2015.
7. National Joint Registry for England and Wales. 8th Annual Report, 2011. National Joint Registry website. www.njrcentre.org.uk/NjrCentre/Portals/0/Documents/NJR%208th%20Annual%20Report%202011.pdf. Accessed April 2, 2015.
8. Grazioli A, Ek ET, Rüdiger HA. Biomechanical concept and clinical outcome of dual mobility cups. Int Orthop. 2012;36(12):2411-2418.
9. Massin P, Orain V, Philippot R, Farizon F, Fessy MH. Fixation failures of dual mobility cups: a mid-term study of 2601 hip replacements. Clin Orthop. 2012;470(7):1932-1940.
10. Epinette JA, Béracassat R, Tracol P, Pagazani G, Vandenbussche E. Are modern dual mobility cups a valuable option in reducing instability after primary hip arthroplasty, even in younger patients? J Arthroplasty. 2014;29(6):1323-1328.
11. Philippot R, Meucci JF, Boyer B, Farizon F. Modern dual-mobility cup implanted with an uncemented stem: about 100 cases with 12-year follow-up. Surg Technol Int. 2013;23:208-212.
12. Prudhon JL, Ferreira A, Verdier R. Dual mobility cup: dislocation rate and survivorship at ten years of follow-up. Int Orthop. 2013;37(12):2345-2350.
13. Sariali E, Mouttet A, Pasquier G, Durante E, Catone Y. Accuracy of reconstruction of the hip using computerised three-dimensional pre-operative planning and a cementless modular neck. J Bone Joint Surg Br. 2009;91(13):333-340.
14. Bong MR, Kaplan KM, Jaffe WL. Total hip arthroplasty in a patient with contralateral hemipelvectomy. J Arthroplasty. 2006;21(5):762-764.
Operative Intervention for Geriatric Hip Fracture: Does Type of Surgery Affect Hospital Length of Stay?
Hip fractures, the most severe and costly fall-related fractures, account for 350,000 hospital admissions per year.1 The majority of hip fractures result from low-impact falls, typically in patients over age 60 years. In fact, the increase in hip fracture with age is nearly exponential.2,3 With the predicted aging of our population, hip fractures will continue to increase in volume. Between 2000 and 2050, the elderly US population will increase by 135%,4 proportionately increasing the number of projected hip fractures. Considering that hip fractures account for 72% of total costs in terms of orthopedic fracture care in the elderly, the dramatic rise in hip fractures is of great concern for future costs of health care delivery in this field.5-7
In an effort to move toward a value-based system in which costs are reduced while quality of care is maintained, Medicare recently unveiled a new bundled payment system of reimbursement. Through this system, hospitals will be reimbursed for treatment provided to Medicare beneficiaries based on the expected costs of care, instead of through the traditional fee-for-service model. Given this development, orthopedic surgeons will need to develop interventions that reduce costs while maintaining quality of care after hip fracture surgery.
One of the most significant ramifications of a value-based system is that reimbursement for hip fractures may be standardized based on a single diagnosis regardless of the actual costs associated with treatment.8 In hip fracture cases, however, a wide range of factors, including degree of communition of the bone, presence of medical comorbidities,9 and amount of soft-tissue injury, can dramatically increase recovery time. In fact, one of the most important determinants of treatment costs related to hospital length of stay (LOS) is whether the fracture is a femoral neck or intertrochanteric fracture.10,11 Type of fracture is a significant determinant of surgical options, and these can dramatically change patient outcomes and costs of surgical care.12-16 In addition, hospital recovery time or LOS can vary widely based on type of surgery. As hospitalization costs account for 44% of the direct medical costs for hip fractures,17 differences in LOS can have major financial implications in a value-based system of reimbursement in which all forms of hip fracture are reimbursed a standard amount.
We conducted a study to analyze differences in hospital LOS for different forms of hip fracture repair to determine the potential financial repercussions of a bundled payment model of reimbursement. By performing a retrospective chart review at a large, level I trauma center, we were able to compare LOS and associated costs for total hip arthroplasty (THA), hemiarthroplasty (HA), cephalomedullary nailing (CMN), open reduction and internal fixation (ORIF), and closed reduction and percutaneous pinning (CRPP).
Materials and Methods
After receiving institutional review board approval for this study, we retrospectively reviewed all hip fracture cases treated at a level I trauma center between January 2000 and December 2009. Current Procedural Terminology (CPT) codes were searched for cases of low-energy falls that caused hip fractures that were resolved with THA, HA, CMN, ORIF, or CRPP. Patients who underwent HA or THA were grouped for analysis. Patients who were over age 60 years and had acetabular, proximal femoral, trochanteric, or femoral neck fractures were included in our search. Patients who had incomplete medical records or did not meet the age criterion were excluded from analysis.
We reviewed patient charts in our institutional electronic medical records database to collect these data: date of birth, age, sex, date of admission, date of discharge, American Society of Anesthesiologists (ASA) Physical Status score, complications, height, weight, start and stop times of procedure, whether or not the procedure was an emergent procedure, days from admission to surgery, 90-day readmissions, days from surgery to discharge, and general category of operation. We also recorded individual comorbidities, including prior myocardial infarction, dysrhythmia, atrial fibrillation, congestive heart failure, heart block, cerebrovascular disease, chronic obstructive pulmonary disease, emphysema, current smoking status, smoking history, renal disease, dialysis, cancer, and diabetes. Duration of surgery was calculated from recorded start and stop times. Body mass index was calculated using height and weight recorded during initial stay. LOS was recorded as the difference between the admission and discharge dates.
Mean total cost to the hospital ($4530/d patient was hospitalized) was obtained from the institution’s financial services. All fractional LOS values were rounded to the nearest whole number and multiplied by the per diem cost. Student t test was used to compare mean LOS and costs of HA/THA with those of all the other procedures. Additional tests were run to analyze differences in LOS and type of surgeries performed throughout the 9-year period. A multivariate regression model controlling for ASA score, body mass index, age, sex, and comorbidities was developed to analyze differences in LOS and costs for patients who underwent HA/THA versus CMN, ORIF, and CRPP. Significance was set at P = .05.
Results
Our search identified 720 patients who were over age 60 years and underwent operative fixation for hip fracture at our level I trauma center between 2000 and 2009. Of these 720 patients, 105 who had incomplete charts or did not meet the age criteria were excluded, leaving 615 patients (with complete records of isolated low-energy hip fractures) for analysis.
Table 1 lists the demographics of our patient population. The majority of patients had undergone ORIF (30.24%) or HA/THA (45.69%). CRPP was the least common procedure (9.92%) after CMN (14.15%). Mean age was 78.4 years; the majority of patients were between 75 and 89 years of age. Mean hospital LOS was 6.91 days. The majority of patients (n = 414; 67.32%) were female. ASA scores had a narrow distribution, with most patients assigned a score of 3. The readmission rate was significantly higher for HA/THA (39.1%) than for ORIF (28.5%; P = .02) and CRPP (24.6%; P = .04).
Table 2 lists mean LOS and associated costs for each procedure compared with HA/THA. Mean LOS for all patients was 6.91 days, with associated hospitalization costs of $30,011.25. Patients who underwent HA/THA had the longest mean LOS (7.43 days) and highest mean hospitalization costs ($33,657.90). In comparison, patients who underwent ORIF had a mean LOS of 6.59 days with $29,852.70 in costs (P = .04). CRPP also had a significantly (P < .003) shorter LOS (5.59 days) and lower costs ($25,322.70). Although CMN had a mean LOS of 6.89 days and $31,211.70 in costs, the difference in LOS was not significantly different from that of HA/THA. The proportion of surgeries that were HA/THA, CMN, ORIF, and CRPP did not change significantly through the 9-year period (P = .19). Similarly, mean LOS did not change significantly for any of the types of surgery through this period (Table 3).
Figure 1 provides the distribution of LOS for all 4 procedures. The interquartile range (IQR) for patients who underwent HA/THA was 4 to 9 days (median, 6 days). Patients who underwent CMN also had a median LOS of 6 days and an IQR of 4 to 8 days. Both ORIF (IQR, 4-8 days) and CRPP (IQR, 3-6 days) were associated with a median LOS of 5 days.
Figure 2 shows mean hospitalization costs based on type of procedure. HA/THA had the highest mean cost, $33,657.90, or $8335.20 more than CRPP ($25,322.70). Patients who underwent CMN had a mean cost of $31,211.70, versus $29,852.70 for patients who underwent ORIF.
Table 4 summarizes the multivariate analysis results. After ASA score, sex, age, and comorbidities were controlled for, there was an overall significant relationship involving surgical treatment, LOS, and associated hospitalization costs for HA/THA, ORIF, and CRPP. Compared with HA/THA, ORIF had $3805.20 less in costs (P = .042) and 0.84 fewer hospital days. Patients who underwent CRPP were hospitalized for significantly fewer days (1.63) and associated costs ($7383.90) (P = .0076). There was no significant difference in LOS and costs between HA/THA and CMN. Of the controlled variables, only ASA score (P < .001) and male sex (P = .001) were significantly associated with changes in LOS and costs. There was no significant association with comorbidities, LOS, or costs.
Discussion
In this study of surgical intervention in patients with hip fractures, we determined that HA/THA was associated with significantly increased hospital LOS and costs than ORIF and CRPP. Although arthroplasty had an increased mean LOS compared with CMN, the difference was not statistically significant. In addition to type of procedure, both male sex (P = .001) and preoperative ASA score (P < .001) were significant predictors of LOS and costs. These findings are supported by other studies in which preoperative functioning was found to be a strong predictor of increased LOS and costs among hip fracture patients,18 most likely because of increased risk for complications.19
Although our study was the first to directly compare LOS and costs for HA/THA and CMN, other investigators have analyzed the effect of surgical complications on LOS for patients treated with THA, HA, and CMN. In a study on the effects of surgical complications on LOS after hip fracture surgery, Foss and colleagues17 reported that the proportion of CMN patients (31%) with complications was larger than that of HA patients (19%) and THA patients (0%). They also reported that surgical complications were associated with significantly increased LOS during primary admission. Similarly, Edwards and colleagues20 found that the infection risk was higher with CMN (3.1%) than with THA (0%) and HA (0%-2.3%) and that infections were associated with increased LOS (P > .001). However, further statistical analysis revealed that the odds of developing an infection were not significantly higher with CMN than with other studies.20 Similarly, other studies have reported low rates of complications, including nonunion, with CMN.21,22 In our study, we found no significant difference in LOS and costs for CMN and HA/THA after controlling for ASA score, which is known to be associated with a higher risk for complications.18,19
The largest difference in LOS and costs after controlling for potential confounding variables was between HA/THA and CRPP ($7383.90). To our knowledge, only one study has performed a comparative analysis of LOS for CRPP and other surgical treatments for hip fractures. For femoral neck fractures treated between 1990 and 1994, Fekete and colleagues23 found that LOS was 14.9 days for ORIF cases and 12.1 days for CRPP cases—a difference of 2.8 days. In comparison, we found a 1-day difference in mean LOS between ORIF cases (6.59 days) and CRPP cases (5.59 days).
Other studies of LOS and associated costs over a 2-year period have found that ORIF is overall more costly than HA/THA. For example, Keating and colleagues13 compared total costs of care, including LOS, for healthy older patients with displaced intracapsular hip fractures treated with ORIF, bipolar HA, or THA. Although ORIF was initially less costly than HA/THA, overall ORIF costs over 2 years were significantly higher because of readmissions, which increased overall LOS. Similarly, in cases of displaced femoral fractures, Iorio and colleagues15 found that LOS was 6.4 days for ORIF, 4.9 days for unipolar HA, 6.2 days for bipolar HA, and 5.5 days for cemented and hybrid THA. However, when overall projected costs were estimated, including the costs of rehabilitation and of (probable) revision arthroplasty, ORIF was estimated to cost more over a 2-year period because of the need for additional care and in-patient stays. In contrast, we found that hospitalization costs were $3805.20 lower for ORIF than for HA/THA, even after adjusting for comorbidities, and that ORIF had a lower overall readmission rate. Early discharge of patients who are at risk for subsequent complications may have played a significant role in increasing readmission rates for arthroplasty patients. These findings indicate the complexities involved in a bundled payment system of reimbursement, in which a single payment for both initial stay and related readmissions will force orthopedists to consider long-term hospitalization costs when deciding on length of postoperative care and the most cost-effective surgical treatment.
One of the limitations of this study is its retrospective design. Although selection of our sample from a single level I trauma center reduced differences in cost and patient care protocols between institutions, it also reduced the generalizability of our actual costs. In addition, for some patients, LOS may have increased because of delays in surgery or discharge, lack of operating room availability, or need for further medical clearance for additional procedures. Day of admission could also have significantly affected LOS. However, the effects of these confounding factors were reduced because of the large sample analyzed. As stated earlier, overall LOS depends on both initial in-patient stays and readmissions. Therefore, long-term prospective studies that compare LOS and associated costs for patients with hip fractures treated with ORIF, CRPP, HA/THA, and CMN are needed.
Conclusion
It has been recently suggested that hip fracture repair be included in the National Pilot Program on Payment Bundling, which will potentially reimburse orthopedic surgeons a standardized amount for hip fracture surgery regardless of actual treatment costs.8 In this model, it will be essential to understand how type of fracture and surgical procedure can influence LOS and therefore hip fracture treatment costs. We found that, based on these factors, mean LOS ranged from 5.59 to 7.43 days, which translates to a cost range of $25,322.70 to $33,657.90. Before a standardized bundled payment system is implemented, further studies are needed to identify other factors that can significantly affect the cost of hip fracture repair.
1. American Academy of Orthopaedic Surgeons. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
2. Samelson EJ, Zhang Y, Kiel DP, Hannan MT, Felson DT. Effect of birth cohort on risk of hip fracture: age-specific incidence rates in the Framingham study. Am J Public Health. 2002;92(5):858-862.
3. Scott JC. Osteoporosis and hip fractures. Rheum Dis Clin North Am. 1990;16(3):717-740.
4. Wiener JM, Tilly J. Population ageing in the United States of America: implications for public programmes. Int J Epidemiol. 2002;31(4):776-781.
5. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465-475.
6. Burge RT, King AB, Balda E, Worley D. Methodology for estimating current and future burden of osteoporosis in state populations: application to Florida in 2000 through 2025. Value Health. 2003;6(5):574-583.
7. Tosteson AN, Burge RT, Marshall DA, Lindsay R. Therapies for treatment of osteoporosis in US women: cost-effectiveness and budget impact considerations. Am J Manag Care. 2008;14(9):605-615.
8. Sood N, Huckfeldt PJ, Escarce JJ, Grabowski DC, Newhouse JP. Medicare’s bundled payment pilot for acute and postacute care: analysis and recommendations on where to begin. Health Aff. 2011;30(9):1708-1717.
9. Shah A, Eissler J, Radomisli T. Algorithms for the treatment of femoral neck fractures. Clin Orthop. 2002;(399):28-34.
10. Sund R, Riihimäki J, Mäkelä M, et al. Modeling the length of the care episode after hip fracture: does the type of fracture matter? Scand J Surg. 2009;98(3):169-174.
11. Fox KM, Magaziner J, Hebel JR, Kenzora JE, Kashner TM. Intertrochanteric versus femoral neck hip fractures: differential characteristics, treatment, and sequelae. J Gerontol A Biol Sci Med Sci. 1999;54(12):M635-M640.
12. Carroll C, Stevenson M, Scope A, Evans P, Buckley S. Hemiarthroplasty and total hip arthroplasty for treating primary intracapsular fracture of the hip: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2011;15(36):1-74.
13. Keating JF, Grant A, Masson M, Scott NW, Forbes JF. Randomized comparison of reduction and fixation, bipolar hemiarthroplasty, and total hip arthroplasty. Treatment of displaced intracapsular hip fractures in healthy older patients. J Bone Joint Surg Am. 2006;88(2):249-260.
14. Rogmark C, Carlsson A, Johnell O, Sembo I. Costs of internal fixation and arthroplasty for displaced femoral neck fractures: a randomized study of 68 patients. Acta Orthop Scand. 2003;74(3):293-298.
15. Iorio R, Healy WL, Lemos DW, Appleby D, Lucchesi CA, Saleh KJ. Displaced femoral neck fractures in the elderly: outcomes and cost effectiveness. Clin Orthop. 2001;(383):229-242.
16. Slover J, Hoffman MV, Malchau H, Tosteson AN, Koval KJ. A cost-effectiveness analysis of the arthroplasty options for displaced femoral neck fractures in the active, healthy, elderly population. J Arthroplasty. 2009;24(6):854-860.
17. Foss NB, Palm H, Krasheninnikoff M, Kehlet H, Gebuhr P. Impact of surgical complications on length of stay after hip fracture surgery. Injury. 2007;38(7):780-784.
18. Garcia AE, Bonnaig JV, Yoneda ZT. Patient variables which may predict length of stay and hospital costs in elderly patients with hip fracture. J Orthop Trauma. 2012;26(11):620-623.
19. Donegan DJ, Gay AN, Baldwin K, Morales EE, Esterhai JL Jr, Mehta S. Use of medical comorbidities to predict complications after hip fracture surgery in the elderly. J Bone Joint Surg Am. 2010;92(4):807-813.
20. Edwards C, Counsell A, Boulton C, Moran CG. Early infection after hip fracture surgery: risk factors, costs and outcome. J Bone Joint Surg Br. 2008;90(6):770-777.
21. Jain P, Maini L, Mishra P, Upadhyay A, Agarwal A. Cephalomedullary interlocked nail for ipsilateral hip and femoral shaft fractures. Injury. 2004;35(10):1031-1038.
22. Matre K, Havelin LI, Gjertsen JE, Espehaug B, Fevang JM. Intramedullary nails result in more reoperations than sliding hip screws in two-part intertrochanteric fractures. Clin Orthop. 2013;471(4):1379-1386.
23. Fekete K, Manninger J, Kazár G, Cserháti P, Bosch U. Percutaneous internal fixation of femoral neck fractures with cannulated screws and a small tension band plate. Orthop Traumatol. 2000;8(4):250-263.
Hip fractures, the most severe and costly fall-related fractures, account for 350,000 hospital admissions per year.1 The majority of hip fractures result from low-impact falls, typically in patients over age 60 years. In fact, the increase in hip fracture with age is nearly exponential.2,3 With the predicted aging of our population, hip fractures will continue to increase in volume. Between 2000 and 2050, the elderly US population will increase by 135%,4 proportionately increasing the number of projected hip fractures. Considering that hip fractures account for 72% of total costs in terms of orthopedic fracture care in the elderly, the dramatic rise in hip fractures is of great concern for future costs of health care delivery in this field.5-7
In an effort to move toward a value-based system in which costs are reduced while quality of care is maintained, Medicare recently unveiled a new bundled payment system of reimbursement. Through this system, hospitals will be reimbursed for treatment provided to Medicare beneficiaries based on the expected costs of care, instead of through the traditional fee-for-service model. Given this development, orthopedic surgeons will need to develop interventions that reduce costs while maintaining quality of care after hip fracture surgery.
One of the most significant ramifications of a value-based system is that reimbursement for hip fractures may be standardized based on a single diagnosis regardless of the actual costs associated with treatment.8 In hip fracture cases, however, a wide range of factors, including degree of communition of the bone, presence of medical comorbidities,9 and amount of soft-tissue injury, can dramatically increase recovery time. In fact, one of the most important determinants of treatment costs related to hospital length of stay (LOS) is whether the fracture is a femoral neck or intertrochanteric fracture.10,11 Type of fracture is a significant determinant of surgical options, and these can dramatically change patient outcomes and costs of surgical care.12-16 In addition, hospital recovery time or LOS can vary widely based on type of surgery. As hospitalization costs account for 44% of the direct medical costs for hip fractures,17 differences in LOS can have major financial implications in a value-based system of reimbursement in which all forms of hip fracture are reimbursed a standard amount.
We conducted a study to analyze differences in hospital LOS for different forms of hip fracture repair to determine the potential financial repercussions of a bundled payment model of reimbursement. By performing a retrospective chart review at a large, level I trauma center, we were able to compare LOS and associated costs for total hip arthroplasty (THA), hemiarthroplasty (HA), cephalomedullary nailing (CMN), open reduction and internal fixation (ORIF), and closed reduction and percutaneous pinning (CRPP).
Materials and Methods
After receiving institutional review board approval for this study, we retrospectively reviewed all hip fracture cases treated at a level I trauma center between January 2000 and December 2009. Current Procedural Terminology (CPT) codes were searched for cases of low-energy falls that caused hip fractures that were resolved with THA, HA, CMN, ORIF, or CRPP. Patients who underwent HA or THA were grouped for analysis. Patients who were over age 60 years and had acetabular, proximal femoral, trochanteric, or femoral neck fractures were included in our search. Patients who had incomplete medical records or did not meet the age criterion were excluded from analysis.
We reviewed patient charts in our institutional electronic medical records database to collect these data: date of birth, age, sex, date of admission, date of discharge, American Society of Anesthesiologists (ASA) Physical Status score, complications, height, weight, start and stop times of procedure, whether or not the procedure was an emergent procedure, days from admission to surgery, 90-day readmissions, days from surgery to discharge, and general category of operation. We also recorded individual comorbidities, including prior myocardial infarction, dysrhythmia, atrial fibrillation, congestive heart failure, heart block, cerebrovascular disease, chronic obstructive pulmonary disease, emphysema, current smoking status, smoking history, renal disease, dialysis, cancer, and diabetes. Duration of surgery was calculated from recorded start and stop times. Body mass index was calculated using height and weight recorded during initial stay. LOS was recorded as the difference between the admission and discharge dates.
Mean total cost to the hospital ($4530/d patient was hospitalized) was obtained from the institution’s financial services. All fractional LOS values were rounded to the nearest whole number and multiplied by the per diem cost. Student t test was used to compare mean LOS and costs of HA/THA with those of all the other procedures. Additional tests were run to analyze differences in LOS and type of surgeries performed throughout the 9-year period. A multivariate regression model controlling for ASA score, body mass index, age, sex, and comorbidities was developed to analyze differences in LOS and costs for patients who underwent HA/THA versus CMN, ORIF, and CRPP. Significance was set at P = .05.
Results
Our search identified 720 patients who were over age 60 years and underwent operative fixation for hip fracture at our level I trauma center between 2000 and 2009. Of these 720 patients, 105 who had incomplete charts or did not meet the age criteria were excluded, leaving 615 patients (with complete records of isolated low-energy hip fractures) for analysis.
Table 1 lists the demographics of our patient population. The majority of patients had undergone ORIF (30.24%) or HA/THA (45.69%). CRPP was the least common procedure (9.92%) after CMN (14.15%). Mean age was 78.4 years; the majority of patients were between 75 and 89 years of age. Mean hospital LOS was 6.91 days. The majority of patients (n = 414; 67.32%) were female. ASA scores had a narrow distribution, with most patients assigned a score of 3. The readmission rate was significantly higher for HA/THA (39.1%) than for ORIF (28.5%; P = .02) and CRPP (24.6%; P = .04).
Table 2 lists mean LOS and associated costs for each procedure compared with HA/THA. Mean LOS for all patients was 6.91 days, with associated hospitalization costs of $30,011.25. Patients who underwent HA/THA had the longest mean LOS (7.43 days) and highest mean hospitalization costs ($33,657.90). In comparison, patients who underwent ORIF had a mean LOS of 6.59 days with $29,852.70 in costs (P = .04). CRPP also had a significantly (P < .003) shorter LOS (5.59 days) and lower costs ($25,322.70). Although CMN had a mean LOS of 6.89 days and $31,211.70 in costs, the difference in LOS was not significantly different from that of HA/THA. The proportion of surgeries that were HA/THA, CMN, ORIF, and CRPP did not change significantly through the 9-year period (P = .19). Similarly, mean LOS did not change significantly for any of the types of surgery through this period (Table 3).
Figure 1 provides the distribution of LOS for all 4 procedures. The interquartile range (IQR) for patients who underwent HA/THA was 4 to 9 days (median, 6 days). Patients who underwent CMN also had a median LOS of 6 days and an IQR of 4 to 8 days. Both ORIF (IQR, 4-8 days) and CRPP (IQR, 3-6 days) were associated with a median LOS of 5 days.
Figure 2 shows mean hospitalization costs based on type of procedure. HA/THA had the highest mean cost, $33,657.90, or $8335.20 more than CRPP ($25,322.70). Patients who underwent CMN had a mean cost of $31,211.70, versus $29,852.70 for patients who underwent ORIF.
Table 4 summarizes the multivariate analysis results. After ASA score, sex, age, and comorbidities were controlled for, there was an overall significant relationship involving surgical treatment, LOS, and associated hospitalization costs for HA/THA, ORIF, and CRPP. Compared with HA/THA, ORIF had $3805.20 less in costs (P = .042) and 0.84 fewer hospital days. Patients who underwent CRPP were hospitalized for significantly fewer days (1.63) and associated costs ($7383.90) (P = .0076). There was no significant difference in LOS and costs between HA/THA and CMN. Of the controlled variables, only ASA score (P < .001) and male sex (P = .001) were significantly associated with changes in LOS and costs. There was no significant association with comorbidities, LOS, or costs.
Discussion
In this study of surgical intervention in patients with hip fractures, we determined that HA/THA was associated with significantly increased hospital LOS and costs than ORIF and CRPP. Although arthroplasty had an increased mean LOS compared with CMN, the difference was not statistically significant. In addition to type of procedure, both male sex (P = .001) and preoperative ASA score (P < .001) were significant predictors of LOS and costs. These findings are supported by other studies in which preoperative functioning was found to be a strong predictor of increased LOS and costs among hip fracture patients,18 most likely because of increased risk for complications.19
Although our study was the first to directly compare LOS and costs for HA/THA and CMN, other investigators have analyzed the effect of surgical complications on LOS for patients treated with THA, HA, and CMN. In a study on the effects of surgical complications on LOS after hip fracture surgery, Foss and colleagues17 reported that the proportion of CMN patients (31%) with complications was larger than that of HA patients (19%) and THA patients (0%). They also reported that surgical complications were associated with significantly increased LOS during primary admission. Similarly, Edwards and colleagues20 found that the infection risk was higher with CMN (3.1%) than with THA (0%) and HA (0%-2.3%) and that infections were associated with increased LOS (P > .001). However, further statistical analysis revealed that the odds of developing an infection were not significantly higher with CMN than with other studies.20 Similarly, other studies have reported low rates of complications, including nonunion, with CMN.21,22 In our study, we found no significant difference in LOS and costs for CMN and HA/THA after controlling for ASA score, which is known to be associated with a higher risk for complications.18,19
The largest difference in LOS and costs after controlling for potential confounding variables was between HA/THA and CRPP ($7383.90). To our knowledge, only one study has performed a comparative analysis of LOS for CRPP and other surgical treatments for hip fractures. For femoral neck fractures treated between 1990 and 1994, Fekete and colleagues23 found that LOS was 14.9 days for ORIF cases and 12.1 days for CRPP cases—a difference of 2.8 days. In comparison, we found a 1-day difference in mean LOS between ORIF cases (6.59 days) and CRPP cases (5.59 days).
Other studies of LOS and associated costs over a 2-year period have found that ORIF is overall more costly than HA/THA. For example, Keating and colleagues13 compared total costs of care, including LOS, for healthy older patients with displaced intracapsular hip fractures treated with ORIF, bipolar HA, or THA. Although ORIF was initially less costly than HA/THA, overall ORIF costs over 2 years were significantly higher because of readmissions, which increased overall LOS. Similarly, in cases of displaced femoral fractures, Iorio and colleagues15 found that LOS was 6.4 days for ORIF, 4.9 days for unipolar HA, 6.2 days for bipolar HA, and 5.5 days for cemented and hybrid THA. However, when overall projected costs were estimated, including the costs of rehabilitation and of (probable) revision arthroplasty, ORIF was estimated to cost more over a 2-year period because of the need for additional care and in-patient stays. In contrast, we found that hospitalization costs were $3805.20 lower for ORIF than for HA/THA, even after adjusting for comorbidities, and that ORIF had a lower overall readmission rate. Early discharge of patients who are at risk for subsequent complications may have played a significant role in increasing readmission rates for arthroplasty patients. These findings indicate the complexities involved in a bundled payment system of reimbursement, in which a single payment for both initial stay and related readmissions will force orthopedists to consider long-term hospitalization costs when deciding on length of postoperative care and the most cost-effective surgical treatment.
One of the limitations of this study is its retrospective design. Although selection of our sample from a single level I trauma center reduced differences in cost and patient care protocols between institutions, it also reduced the generalizability of our actual costs. In addition, for some patients, LOS may have increased because of delays in surgery or discharge, lack of operating room availability, or need for further medical clearance for additional procedures. Day of admission could also have significantly affected LOS. However, the effects of these confounding factors were reduced because of the large sample analyzed. As stated earlier, overall LOS depends on both initial in-patient stays and readmissions. Therefore, long-term prospective studies that compare LOS and associated costs for patients with hip fractures treated with ORIF, CRPP, HA/THA, and CMN are needed.
Conclusion
It has been recently suggested that hip fracture repair be included in the National Pilot Program on Payment Bundling, which will potentially reimburse orthopedic surgeons a standardized amount for hip fracture surgery regardless of actual treatment costs.8 In this model, it will be essential to understand how type of fracture and surgical procedure can influence LOS and therefore hip fracture treatment costs. We found that, based on these factors, mean LOS ranged from 5.59 to 7.43 days, which translates to a cost range of $25,322.70 to $33,657.90. Before a standardized bundled payment system is implemented, further studies are needed to identify other factors that can significantly affect the cost of hip fracture repair.
Hip fractures, the most severe and costly fall-related fractures, account for 350,000 hospital admissions per year.1 The majority of hip fractures result from low-impact falls, typically in patients over age 60 years. In fact, the increase in hip fracture with age is nearly exponential.2,3 With the predicted aging of our population, hip fractures will continue to increase in volume. Between 2000 and 2050, the elderly US population will increase by 135%,4 proportionately increasing the number of projected hip fractures. Considering that hip fractures account for 72% of total costs in terms of orthopedic fracture care in the elderly, the dramatic rise in hip fractures is of great concern for future costs of health care delivery in this field.5-7
In an effort to move toward a value-based system in which costs are reduced while quality of care is maintained, Medicare recently unveiled a new bundled payment system of reimbursement. Through this system, hospitals will be reimbursed for treatment provided to Medicare beneficiaries based on the expected costs of care, instead of through the traditional fee-for-service model. Given this development, orthopedic surgeons will need to develop interventions that reduce costs while maintaining quality of care after hip fracture surgery.
One of the most significant ramifications of a value-based system is that reimbursement for hip fractures may be standardized based on a single diagnosis regardless of the actual costs associated with treatment.8 In hip fracture cases, however, a wide range of factors, including degree of communition of the bone, presence of medical comorbidities,9 and amount of soft-tissue injury, can dramatically increase recovery time. In fact, one of the most important determinants of treatment costs related to hospital length of stay (LOS) is whether the fracture is a femoral neck or intertrochanteric fracture.10,11 Type of fracture is a significant determinant of surgical options, and these can dramatically change patient outcomes and costs of surgical care.12-16 In addition, hospital recovery time or LOS can vary widely based on type of surgery. As hospitalization costs account for 44% of the direct medical costs for hip fractures,17 differences in LOS can have major financial implications in a value-based system of reimbursement in which all forms of hip fracture are reimbursed a standard amount.
We conducted a study to analyze differences in hospital LOS for different forms of hip fracture repair to determine the potential financial repercussions of a bundled payment model of reimbursement. By performing a retrospective chart review at a large, level I trauma center, we were able to compare LOS and associated costs for total hip arthroplasty (THA), hemiarthroplasty (HA), cephalomedullary nailing (CMN), open reduction and internal fixation (ORIF), and closed reduction and percutaneous pinning (CRPP).
Materials and Methods
After receiving institutional review board approval for this study, we retrospectively reviewed all hip fracture cases treated at a level I trauma center between January 2000 and December 2009. Current Procedural Terminology (CPT) codes were searched for cases of low-energy falls that caused hip fractures that were resolved with THA, HA, CMN, ORIF, or CRPP. Patients who underwent HA or THA were grouped for analysis. Patients who were over age 60 years and had acetabular, proximal femoral, trochanteric, or femoral neck fractures were included in our search. Patients who had incomplete medical records or did not meet the age criterion were excluded from analysis.
We reviewed patient charts in our institutional electronic medical records database to collect these data: date of birth, age, sex, date of admission, date of discharge, American Society of Anesthesiologists (ASA) Physical Status score, complications, height, weight, start and stop times of procedure, whether or not the procedure was an emergent procedure, days from admission to surgery, 90-day readmissions, days from surgery to discharge, and general category of operation. We also recorded individual comorbidities, including prior myocardial infarction, dysrhythmia, atrial fibrillation, congestive heart failure, heart block, cerebrovascular disease, chronic obstructive pulmonary disease, emphysema, current smoking status, smoking history, renal disease, dialysis, cancer, and diabetes. Duration of surgery was calculated from recorded start and stop times. Body mass index was calculated using height and weight recorded during initial stay. LOS was recorded as the difference between the admission and discharge dates.
Mean total cost to the hospital ($4530/d patient was hospitalized) was obtained from the institution’s financial services. All fractional LOS values were rounded to the nearest whole number and multiplied by the per diem cost. Student t test was used to compare mean LOS and costs of HA/THA with those of all the other procedures. Additional tests were run to analyze differences in LOS and type of surgeries performed throughout the 9-year period. A multivariate regression model controlling for ASA score, body mass index, age, sex, and comorbidities was developed to analyze differences in LOS and costs for patients who underwent HA/THA versus CMN, ORIF, and CRPP. Significance was set at P = .05.
Results
Our search identified 720 patients who were over age 60 years and underwent operative fixation for hip fracture at our level I trauma center between 2000 and 2009. Of these 720 patients, 105 who had incomplete charts or did not meet the age criteria were excluded, leaving 615 patients (with complete records of isolated low-energy hip fractures) for analysis.
Table 1 lists the demographics of our patient population. The majority of patients had undergone ORIF (30.24%) or HA/THA (45.69%). CRPP was the least common procedure (9.92%) after CMN (14.15%). Mean age was 78.4 years; the majority of patients were between 75 and 89 years of age. Mean hospital LOS was 6.91 days. The majority of patients (n = 414; 67.32%) were female. ASA scores had a narrow distribution, with most patients assigned a score of 3. The readmission rate was significantly higher for HA/THA (39.1%) than for ORIF (28.5%; P = .02) and CRPP (24.6%; P = .04).
Table 2 lists mean LOS and associated costs for each procedure compared with HA/THA. Mean LOS for all patients was 6.91 days, with associated hospitalization costs of $30,011.25. Patients who underwent HA/THA had the longest mean LOS (7.43 days) and highest mean hospitalization costs ($33,657.90). In comparison, patients who underwent ORIF had a mean LOS of 6.59 days with $29,852.70 in costs (P = .04). CRPP also had a significantly (P < .003) shorter LOS (5.59 days) and lower costs ($25,322.70). Although CMN had a mean LOS of 6.89 days and $31,211.70 in costs, the difference in LOS was not significantly different from that of HA/THA. The proportion of surgeries that were HA/THA, CMN, ORIF, and CRPP did not change significantly through the 9-year period (P = .19). Similarly, mean LOS did not change significantly for any of the types of surgery through this period (Table 3).
Figure 1 provides the distribution of LOS for all 4 procedures. The interquartile range (IQR) for patients who underwent HA/THA was 4 to 9 days (median, 6 days). Patients who underwent CMN also had a median LOS of 6 days and an IQR of 4 to 8 days. Both ORIF (IQR, 4-8 days) and CRPP (IQR, 3-6 days) were associated with a median LOS of 5 days.
Figure 2 shows mean hospitalization costs based on type of procedure. HA/THA had the highest mean cost, $33,657.90, or $8335.20 more than CRPP ($25,322.70). Patients who underwent CMN had a mean cost of $31,211.70, versus $29,852.70 for patients who underwent ORIF.
Table 4 summarizes the multivariate analysis results. After ASA score, sex, age, and comorbidities were controlled for, there was an overall significant relationship involving surgical treatment, LOS, and associated hospitalization costs for HA/THA, ORIF, and CRPP. Compared with HA/THA, ORIF had $3805.20 less in costs (P = .042) and 0.84 fewer hospital days. Patients who underwent CRPP were hospitalized for significantly fewer days (1.63) and associated costs ($7383.90) (P = .0076). There was no significant difference in LOS and costs between HA/THA and CMN. Of the controlled variables, only ASA score (P < .001) and male sex (P = .001) were significantly associated with changes in LOS and costs. There was no significant association with comorbidities, LOS, or costs.
Discussion
In this study of surgical intervention in patients with hip fractures, we determined that HA/THA was associated with significantly increased hospital LOS and costs than ORIF and CRPP. Although arthroplasty had an increased mean LOS compared with CMN, the difference was not statistically significant. In addition to type of procedure, both male sex (P = .001) and preoperative ASA score (P < .001) were significant predictors of LOS and costs. These findings are supported by other studies in which preoperative functioning was found to be a strong predictor of increased LOS and costs among hip fracture patients,18 most likely because of increased risk for complications.19
Although our study was the first to directly compare LOS and costs for HA/THA and CMN, other investigators have analyzed the effect of surgical complications on LOS for patients treated with THA, HA, and CMN. In a study on the effects of surgical complications on LOS after hip fracture surgery, Foss and colleagues17 reported that the proportion of CMN patients (31%) with complications was larger than that of HA patients (19%) and THA patients (0%). They also reported that surgical complications were associated with significantly increased LOS during primary admission. Similarly, Edwards and colleagues20 found that the infection risk was higher with CMN (3.1%) than with THA (0%) and HA (0%-2.3%) and that infections were associated with increased LOS (P > .001). However, further statistical analysis revealed that the odds of developing an infection were not significantly higher with CMN than with other studies.20 Similarly, other studies have reported low rates of complications, including nonunion, with CMN.21,22 In our study, we found no significant difference in LOS and costs for CMN and HA/THA after controlling for ASA score, which is known to be associated with a higher risk for complications.18,19
The largest difference in LOS and costs after controlling for potential confounding variables was between HA/THA and CRPP ($7383.90). To our knowledge, only one study has performed a comparative analysis of LOS for CRPP and other surgical treatments for hip fractures. For femoral neck fractures treated between 1990 and 1994, Fekete and colleagues23 found that LOS was 14.9 days for ORIF cases and 12.1 days for CRPP cases—a difference of 2.8 days. In comparison, we found a 1-day difference in mean LOS between ORIF cases (6.59 days) and CRPP cases (5.59 days).
Other studies of LOS and associated costs over a 2-year period have found that ORIF is overall more costly than HA/THA. For example, Keating and colleagues13 compared total costs of care, including LOS, for healthy older patients with displaced intracapsular hip fractures treated with ORIF, bipolar HA, or THA. Although ORIF was initially less costly than HA/THA, overall ORIF costs over 2 years were significantly higher because of readmissions, which increased overall LOS. Similarly, in cases of displaced femoral fractures, Iorio and colleagues15 found that LOS was 6.4 days for ORIF, 4.9 days for unipolar HA, 6.2 days for bipolar HA, and 5.5 days for cemented and hybrid THA. However, when overall projected costs were estimated, including the costs of rehabilitation and of (probable) revision arthroplasty, ORIF was estimated to cost more over a 2-year period because of the need for additional care and in-patient stays. In contrast, we found that hospitalization costs were $3805.20 lower for ORIF than for HA/THA, even after adjusting for comorbidities, and that ORIF had a lower overall readmission rate. Early discharge of patients who are at risk for subsequent complications may have played a significant role in increasing readmission rates for arthroplasty patients. These findings indicate the complexities involved in a bundled payment system of reimbursement, in which a single payment for both initial stay and related readmissions will force orthopedists to consider long-term hospitalization costs when deciding on length of postoperative care and the most cost-effective surgical treatment.
One of the limitations of this study is its retrospective design. Although selection of our sample from a single level I trauma center reduced differences in cost and patient care protocols between institutions, it also reduced the generalizability of our actual costs. In addition, for some patients, LOS may have increased because of delays in surgery or discharge, lack of operating room availability, or need for further medical clearance for additional procedures. Day of admission could also have significantly affected LOS. However, the effects of these confounding factors were reduced because of the large sample analyzed. As stated earlier, overall LOS depends on both initial in-patient stays and readmissions. Therefore, long-term prospective studies that compare LOS and associated costs for patients with hip fractures treated with ORIF, CRPP, HA/THA, and CMN are needed.
Conclusion
It has been recently suggested that hip fracture repair be included in the National Pilot Program on Payment Bundling, which will potentially reimburse orthopedic surgeons a standardized amount for hip fracture surgery regardless of actual treatment costs.8 In this model, it will be essential to understand how type of fracture and surgical procedure can influence LOS and therefore hip fracture treatment costs. We found that, based on these factors, mean LOS ranged from 5.59 to 7.43 days, which translates to a cost range of $25,322.70 to $33,657.90. Before a standardized bundled payment system is implemented, further studies are needed to identify other factors that can significantly affect the cost of hip fracture repair.
1. American Academy of Orthopaedic Surgeons. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
2. Samelson EJ, Zhang Y, Kiel DP, Hannan MT, Felson DT. Effect of birth cohort on risk of hip fracture: age-specific incidence rates in the Framingham study. Am J Public Health. 2002;92(5):858-862.
3. Scott JC. Osteoporosis and hip fractures. Rheum Dis Clin North Am. 1990;16(3):717-740.
4. Wiener JM, Tilly J. Population ageing in the United States of America: implications for public programmes. Int J Epidemiol. 2002;31(4):776-781.
5. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465-475.
6. Burge RT, King AB, Balda E, Worley D. Methodology for estimating current and future burden of osteoporosis in state populations: application to Florida in 2000 through 2025. Value Health. 2003;6(5):574-583.
7. Tosteson AN, Burge RT, Marshall DA, Lindsay R. Therapies for treatment of osteoporosis in US women: cost-effectiveness and budget impact considerations. Am J Manag Care. 2008;14(9):605-615.
8. Sood N, Huckfeldt PJ, Escarce JJ, Grabowski DC, Newhouse JP. Medicare’s bundled payment pilot for acute and postacute care: analysis and recommendations on where to begin. Health Aff. 2011;30(9):1708-1717.
9. Shah A, Eissler J, Radomisli T. Algorithms for the treatment of femoral neck fractures. Clin Orthop. 2002;(399):28-34.
10. Sund R, Riihimäki J, Mäkelä M, et al. Modeling the length of the care episode after hip fracture: does the type of fracture matter? Scand J Surg. 2009;98(3):169-174.
11. Fox KM, Magaziner J, Hebel JR, Kenzora JE, Kashner TM. Intertrochanteric versus femoral neck hip fractures: differential characteristics, treatment, and sequelae. J Gerontol A Biol Sci Med Sci. 1999;54(12):M635-M640.
12. Carroll C, Stevenson M, Scope A, Evans P, Buckley S. Hemiarthroplasty and total hip arthroplasty for treating primary intracapsular fracture of the hip: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2011;15(36):1-74.
13. Keating JF, Grant A, Masson M, Scott NW, Forbes JF. Randomized comparison of reduction and fixation, bipolar hemiarthroplasty, and total hip arthroplasty. Treatment of displaced intracapsular hip fractures in healthy older patients. J Bone Joint Surg Am. 2006;88(2):249-260.
14. Rogmark C, Carlsson A, Johnell O, Sembo I. Costs of internal fixation and arthroplasty for displaced femoral neck fractures: a randomized study of 68 patients. Acta Orthop Scand. 2003;74(3):293-298.
15. Iorio R, Healy WL, Lemos DW, Appleby D, Lucchesi CA, Saleh KJ. Displaced femoral neck fractures in the elderly: outcomes and cost effectiveness. Clin Orthop. 2001;(383):229-242.
16. Slover J, Hoffman MV, Malchau H, Tosteson AN, Koval KJ. A cost-effectiveness analysis of the arthroplasty options for displaced femoral neck fractures in the active, healthy, elderly population. J Arthroplasty. 2009;24(6):854-860.
17. Foss NB, Palm H, Krasheninnikoff M, Kehlet H, Gebuhr P. Impact of surgical complications on length of stay after hip fracture surgery. Injury. 2007;38(7):780-784.
18. Garcia AE, Bonnaig JV, Yoneda ZT. Patient variables which may predict length of stay and hospital costs in elderly patients with hip fracture. J Orthop Trauma. 2012;26(11):620-623.
19. Donegan DJ, Gay AN, Baldwin K, Morales EE, Esterhai JL Jr, Mehta S. Use of medical comorbidities to predict complications after hip fracture surgery in the elderly. J Bone Joint Surg Am. 2010;92(4):807-813.
20. Edwards C, Counsell A, Boulton C, Moran CG. Early infection after hip fracture surgery: risk factors, costs and outcome. J Bone Joint Surg Br. 2008;90(6):770-777.
21. Jain P, Maini L, Mishra P, Upadhyay A, Agarwal A. Cephalomedullary interlocked nail for ipsilateral hip and femoral shaft fractures. Injury. 2004;35(10):1031-1038.
22. Matre K, Havelin LI, Gjertsen JE, Espehaug B, Fevang JM. Intramedullary nails result in more reoperations than sliding hip screws in two-part intertrochanteric fractures. Clin Orthop. 2013;471(4):1379-1386.
23. Fekete K, Manninger J, Kazár G, Cserháti P, Bosch U. Percutaneous internal fixation of femoral neck fractures with cannulated screws and a small tension band plate. Orthop Traumatol. 2000;8(4):250-263.
1. American Academy of Orthopaedic Surgeons. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
2. Samelson EJ, Zhang Y, Kiel DP, Hannan MT, Felson DT. Effect of birth cohort on risk of hip fracture: age-specific incidence rates in the Framingham study. Am J Public Health. 2002;92(5):858-862.
3. Scott JC. Osteoporosis and hip fractures. Rheum Dis Clin North Am. 1990;16(3):717-740.
4. Wiener JM, Tilly J. Population ageing in the United States of America: implications for public programmes. Int J Epidemiol. 2002;31(4):776-781.
5. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465-475.
6. Burge RT, King AB, Balda E, Worley D. Methodology for estimating current and future burden of osteoporosis in state populations: application to Florida in 2000 through 2025. Value Health. 2003;6(5):574-583.
7. Tosteson AN, Burge RT, Marshall DA, Lindsay R. Therapies for treatment of osteoporosis in US women: cost-effectiveness and budget impact considerations. Am J Manag Care. 2008;14(9):605-615.
8. Sood N, Huckfeldt PJ, Escarce JJ, Grabowski DC, Newhouse JP. Medicare’s bundled payment pilot for acute and postacute care: analysis and recommendations on where to begin. Health Aff. 2011;30(9):1708-1717.
9. Shah A, Eissler J, Radomisli T. Algorithms for the treatment of femoral neck fractures. Clin Orthop. 2002;(399):28-34.
10. Sund R, Riihimäki J, Mäkelä M, et al. Modeling the length of the care episode after hip fracture: does the type of fracture matter? Scand J Surg. 2009;98(3):169-174.
11. Fox KM, Magaziner J, Hebel JR, Kenzora JE, Kashner TM. Intertrochanteric versus femoral neck hip fractures: differential characteristics, treatment, and sequelae. J Gerontol A Biol Sci Med Sci. 1999;54(12):M635-M640.
12. Carroll C, Stevenson M, Scope A, Evans P, Buckley S. Hemiarthroplasty and total hip arthroplasty for treating primary intracapsular fracture of the hip: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2011;15(36):1-74.
13. Keating JF, Grant A, Masson M, Scott NW, Forbes JF. Randomized comparison of reduction and fixation, bipolar hemiarthroplasty, and total hip arthroplasty. Treatment of displaced intracapsular hip fractures in healthy older patients. J Bone Joint Surg Am. 2006;88(2):249-260.
14. Rogmark C, Carlsson A, Johnell O, Sembo I. Costs of internal fixation and arthroplasty for displaced femoral neck fractures: a randomized study of 68 patients. Acta Orthop Scand. 2003;74(3):293-298.
15. Iorio R, Healy WL, Lemos DW, Appleby D, Lucchesi CA, Saleh KJ. Displaced femoral neck fractures in the elderly: outcomes and cost effectiveness. Clin Orthop. 2001;(383):229-242.
16. Slover J, Hoffman MV, Malchau H, Tosteson AN, Koval KJ. A cost-effectiveness analysis of the arthroplasty options for displaced femoral neck fractures in the active, healthy, elderly population. J Arthroplasty. 2009;24(6):854-860.
17. Foss NB, Palm H, Krasheninnikoff M, Kehlet H, Gebuhr P. Impact of surgical complications on length of stay after hip fracture surgery. Injury. 2007;38(7):780-784.
18. Garcia AE, Bonnaig JV, Yoneda ZT. Patient variables which may predict length of stay and hospital costs in elderly patients with hip fracture. J Orthop Trauma. 2012;26(11):620-623.
19. Donegan DJ, Gay AN, Baldwin K, Morales EE, Esterhai JL Jr, Mehta S. Use of medical comorbidities to predict complications after hip fracture surgery in the elderly. J Bone Joint Surg Am. 2010;92(4):807-813.
20. Edwards C, Counsell A, Boulton C, Moran CG. Early infection after hip fracture surgery: risk factors, costs and outcome. J Bone Joint Surg Br. 2008;90(6):770-777.
21. Jain P, Maini L, Mishra P, Upadhyay A, Agarwal A. Cephalomedullary interlocked nail for ipsilateral hip and femoral shaft fractures. Injury. 2004;35(10):1031-1038.
22. Matre K, Havelin LI, Gjertsen JE, Espehaug B, Fevang JM. Intramedullary nails result in more reoperations than sliding hip screws in two-part intertrochanteric fractures. Clin Orthop. 2013;471(4):1379-1386.
23. Fekete K, Manninger J, Kazár G, Cserháti P, Bosch U. Percutaneous internal fixation of femoral neck fractures with cannulated screws and a small tension band plate. Orthop Traumatol. 2000;8(4):250-263.