User login
E-order sets for Vitamin D testing cut deficiency among elderly
Making physicians aware of their track record assessing vitamin D deficiency and adding computerized order sets to check vitamin D levels and initiate vitamin D supplementation led to "significant improvement" in assessment and treatment of vitamin D deficiency in elderly patients with hip fracture at a North Carolina hospital, according to a study published online in the Journal of Hospital Medicine.
After these interventions, the percentage of patients screened for vitamin D deficiency improved from 37.2% to 93.5% (P less than .001), and the percentage of deficient or insufficient patients discharged on the recommended vitamin D dose improved from 40.9% to 68% (P = .008), Dr. John R. Stephens of the University of North Carolina Hospitals and his colleagues reported (J. Hosp. Med. 2014 Sept. 5 [doi:10.1002/jhm.2255]).
The study authors reviewed the literature on the prevalence of vitamin D deficiency in elderly patients and used Endocrine Society guidelines "to define vitamin D deficiency, insufficiency, and recommended treatment dosing for each condition." They presented this information along with a review of data from their hospitalist group practice at a staff meeting.
They also revised the computerized physician order entry (CPOE) set for patients with hip fractures to include two new orders: an automatic order for 25-OH vitamin D level to be drawn the morning after admission and an order for initiation of 1,000 IU daily of vitamin D at admission.
They compared records of 196 patients (mean age 80), with hip fracture treated in the 28 months before these interventions and 107 similar patients treated in the 12 months following the interventions.
Three-quarters of the patients were female and at least 81% of the patients in both the intervention and preintervention groups were white in the single-center study.
"Our study demonstrates a systematic method groups may use to adopt and reliably implement practice guidelines," the authors wrote.
"With safeguards in the electronic system to flag duplicate medications, low toxicity of standard doses of vitamin D, and minimal economic harm with duplicate laboratory therapy in the context of a hospitalization for hip fracture," the researchers wrote, any possible risks "are outweighed by the benefits of screening."
They reported no conflicts of interest.
Making physicians aware of their track record assessing vitamin D deficiency and adding computerized order sets to check vitamin D levels and initiate vitamin D supplementation led to "significant improvement" in assessment and treatment of vitamin D deficiency in elderly patients with hip fracture at a North Carolina hospital, according to a study published online in the Journal of Hospital Medicine.
After these interventions, the percentage of patients screened for vitamin D deficiency improved from 37.2% to 93.5% (P less than .001), and the percentage of deficient or insufficient patients discharged on the recommended vitamin D dose improved from 40.9% to 68% (P = .008), Dr. John R. Stephens of the University of North Carolina Hospitals and his colleagues reported (J. Hosp. Med. 2014 Sept. 5 [doi:10.1002/jhm.2255]).
The study authors reviewed the literature on the prevalence of vitamin D deficiency in elderly patients and used Endocrine Society guidelines "to define vitamin D deficiency, insufficiency, and recommended treatment dosing for each condition." They presented this information along with a review of data from their hospitalist group practice at a staff meeting.
They also revised the computerized physician order entry (CPOE) set for patients with hip fractures to include two new orders: an automatic order for 25-OH vitamin D level to be drawn the morning after admission and an order for initiation of 1,000 IU daily of vitamin D at admission.
They compared records of 196 patients (mean age 80), with hip fracture treated in the 28 months before these interventions and 107 similar patients treated in the 12 months following the interventions.
Three-quarters of the patients were female and at least 81% of the patients in both the intervention and preintervention groups were white in the single-center study.
"Our study demonstrates a systematic method groups may use to adopt and reliably implement practice guidelines," the authors wrote.
"With safeguards in the electronic system to flag duplicate medications, low toxicity of standard doses of vitamin D, and minimal economic harm with duplicate laboratory therapy in the context of a hospitalization for hip fracture," the researchers wrote, any possible risks "are outweighed by the benefits of screening."
They reported no conflicts of interest.
Making physicians aware of their track record assessing vitamin D deficiency and adding computerized order sets to check vitamin D levels and initiate vitamin D supplementation led to "significant improvement" in assessment and treatment of vitamin D deficiency in elderly patients with hip fracture at a North Carolina hospital, according to a study published online in the Journal of Hospital Medicine.
After these interventions, the percentage of patients screened for vitamin D deficiency improved from 37.2% to 93.5% (P less than .001), and the percentage of deficient or insufficient patients discharged on the recommended vitamin D dose improved from 40.9% to 68% (P = .008), Dr. John R. Stephens of the University of North Carolina Hospitals and his colleagues reported (J. Hosp. Med. 2014 Sept. 5 [doi:10.1002/jhm.2255]).
The study authors reviewed the literature on the prevalence of vitamin D deficiency in elderly patients and used Endocrine Society guidelines "to define vitamin D deficiency, insufficiency, and recommended treatment dosing for each condition." They presented this information along with a review of data from their hospitalist group practice at a staff meeting.
They also revised the computerized physician order entry (CPOE) set for patients with hip fractures to include two new orders: an automatic order for 25-OH vitamin D level to be drawn the morning after admission and an order for initiation of 1,000 IU daily of vitamin D at admission.
They compared records of 196 patients (mean age 80), with hip fracture treated in the 28 months before these interventions and 107 similar patients treated in the 12 months following the interventions.
Three-quarters of the patients were female and at least 81% of the patients in both the intervention and preintervention groups were white in the single-center study.
"Our study demonstrates a systematic method groups may use to adopt and reliably implement practice guidelines," the authors wrote.
"With safeguards in the electronic system to flag duplicate medications, low toxicity of standard doses of vitamin D, and minimal economic harm with duplicate laboratory therapy in the context of a hospitalization for hip fracture," the researchers wrote, any possible risks "are outweighed by the benefits of screening."
They reported no conflicts of interest.
FROM THE JOURNAL OF HOSPITAL MEDICINE
Key clinical point: Having CPOE prompt screening can help reduce vitamin D deficiency in elderly inpatients.
Major finding: The percentage of patients screened for vitamin D deficiency improved from 37.2% to 93.5%, and the percentage of deficient or insufficient patients discharged on the recommended vitamin D dose improved from 40.9% to 68%.
Data source: An internal review of hospital records for 196 patients treated before CPOE set interventions and 107 patients treated after interventions.
Disclosures: The authors reported no conflicts of interest.
Anti-inflammatory treatment could slow bone loss in early rheumatoid arthritis
Aggressive anti-inflammatory treatment correlated with slowed bone loss in patients with early rheumatoid arthritis, according to findings from a prospective cohort study.
The results show that "modern aggressive treatment" can reduce osteoporosis in patients with RA, said Dr. Glenn Haugeberg of the rheumatology department at the Hospital of Southern Norway Trust in Kristiansand and his associates.
In all, 18.5% of patients with RA used biologic disease-modifying antirheumatic drugs during the first 2 years of the study, while 62.6% used these drugs during the next 8 years, at the same time that average bone mineral density loss slowed substantially, the researchers said.
The average yearly rate of bone loss at 2 years and 10 years slowed from –1.00% to –0.56% for the femoral neck, from –0.96% to –0.41% for total spine, and from –0.42% to 0.00% for the L1-L4 vertebrae, the researchers reported (BMC Musculoskelet. Disord. 2014 Sept. 2 [doi: 10.1186/1471-2474-15-289]).
The study included 92 patients (mean age, 50.9 years) with RA, of whom about two-thirds were women, and 80% had their bone mineral densities assessed at 10 years. Patients had experienced symptoms for a mean of 12.4 months.
Contrary to findings from some prior studies, glucocorticoid use was linked to bone loss during only the first 2 years of the study period and only for total hip measurements, the researchers said.
Almost half the patients had missing baseline data on bone mineral density. The study also lacked matched controls and did not assess the prevalence or incidence of vertebral fractures, the authors added.
Funding information for the study was not available. The authors reported having no conflicts of interest.
Aggressive anti-inflammatory treatment correlated with slowed bone loss in patients with early rheumatoid arthritis, according to findings from a prospective cohort study.
The results show that "modern aggressive treatment" can reduce osteoporosis in patients with RA, said Dr. Glenn Haugeberg of the rheumatology department at the Hospital of Southern Norway Trust in Kristiansand and his associates.
In all, 18.5% of patients with RA used biologic disease-modifying antirheumatic drugs during the first 2 years of the study, while 62.6% used these drugs during the next 8 years, at the same time that average bone mineral density loss slowed substantially, the researchers said.
The average yearly rate of bone loss at 2 years and 10 years slowed from –1.00% to –0.56% for the femoral neck, from –0.96% to –0.41% for total spine, and from –0.42% to 0.00% for the L1-L4 vertebrae, the researchers reported (BMC Musculoskelet. Disord. 2014 Sept. 2 [doi: 10.1186/1471-2474-15-289]).
The study included 92 patients (mean age, 50.9 years) with RA, of whom about two-thirds were women, and 80% had their bone mineral densities assessed at 10 years. Patients had experienced symptoms for a mean of 12.4 months.
Contrary to findings from some prior studies, glucocorticoid use was linked to bone loss during only the first 2 years of the study period and only for total hip measurements, the researchers said.
Almost half the patients had missing baseline data on bone mineral density. The study also lacked matched controls and did not assess the prevalence or incidence of vertebral fractures, the authors added.
Funding information for the study was not available. The authors reported having no conflicts of interest.
Aggressive anti-inflammatory treatment correlated with slowed bone loss in patients with early rheumatoid arthritis, according to findings from a prospective cohort study.
The results show that "modern aggressive treatment" can reduce osteoporosis in patients with RA, said Dr. Glenn Haugeberg of the rheumatology department at the Hospital of Southern Norway Trust in Kristiansand and his associates.
In all, 18.5% of patients with RA used biologic disease-modifying antirheumatic drugs during the first 2 years of the study, while 62.6% used these drugs during the next 8 years, at the same time that average bone mineral density loss slowed substantially, the researchers said.
The average yearly rate of bone loss at 2 years and 10 years slowed from –1.00% to –0.56% for the femoral neck, from –0.96% to –0.41% for total spine, and from –0.42% to 0.00% for the L1-L4 vertebrae, the researchers reported (BMC Musculoskelet. Disord. 2014 Sept. 2 [doi: 10.1186/1471-2474-15-289]).
The study included 92 patients (mean age, 50.9 years) with RA, of whom about two-thirds were women, and 80% had their bone mineral densities assessed at 10 years. Patients had experienced symptoms for a mean of 12.4 months.
Contrary to findings from some prior studies, glucocorticoid use was linked to bone loss during only the first 2 years of the study period and only for total hip measurements, the researchers said.
Almost half the patients had missing baseline data on bone mineral density. The study also lacked matched controls and did not assess the prevalence or incidence of vertebral fractures, the authors added.
Funding information for the study was not available. The authors reported having no conflicts of interest.
FROM BMC MUSCULOSKELETAL DISORDERS
Key clinical point: Aggressive anti-inflammatory treatment correlated with slowed bone loss in patients with early rheumatoid arthritis.
Major finding: Average use of biologic disease-modifying antirheumatic drugs rose from 18.5% during the first 2 years to 62.6% during the next 8 years, while average bone loss slowed substantially (from –1.00% to –0.56% for the femoral neck, from –0.96% to –0.41% for the total spine, and from –0.42% to 0.00% for the L1-L4 vertebrae).
Data source: Prospective cohort study of 92 patients with rheumatoid arthritis followed for up to 10 years.
Disclosures: Funding information for the study was not available. The authors reported having no conflicts of interest.
Hemodialysis patients forget to take metabolic bone disease medications
LAS VEGAS – The most common reason why end-stage-renal disease patients missed doses of their metabolic bone disease medications was that they forgot to take them, a multicenter study showed.
"Medication adherence is a constant struggle for patients with end-stage renal disease," Maureen McKinley, M.S.W., said in an interview after a meeting sponsored by the National Kidney Foundation, where the study was presented. "Dialysis patients are prescribed an average of 21 pills a day which would be difficult for even a healthy person to track and manage. The average dialysis patient misses one out of every two medication doses, which has a direct impact on their health, frequency of hospitalizations and, in turn, on costs to our health care system."
Ms. McKinley, a clinical social worker at Irvine, Calif.–based DaVita HealthCare Partners, and her associates interviewed 50 patients across 17 hemodialysis clinics over a period of 12 weeks to determine root causes of missed metabolic bone disease (MBD) medication doses. Of the 50 patients, 70% reported having missed doses of their MBD medication over the 12-week period. Of these, 66% missed a dose fewer than five times while 10% missed doses five times or more.
The most frequent reason for missing doses was "forgot to take" (41%), followed by "ill and not eating that many meals" (10%), "don’t understand importance" (7%), and "difficulty swallowing" (7%). Other reasons included "side effects" (6%), "forgot to refill" (4%), and financial barriers (3%).
"From my background as a social worker, I was expecting financial reasons to be the most common cause for patients not taking all their prescribed medication," Ms. McKinley said. "After years of sitting with families struggling to make ends meet and stay on top of payments, I know the burden that dialysis can place on a patient and their loved ones. Yet, 41% of respondents cited "forgot to take" as the reason for not taking medications."
With the complexity of so many medications and the reality of the disease, "it’s all about keeping it simple and as easy as possible for our patients," Ms. McKinley continued. "Trying to assist with simple reminders, setting an alarm, putting pills where you can see them or carrying your phosphate binders with you. Having a support system is such a help for patients on dialysis. As clinicians, we try to assist in these ways, but having the support of loved ones is a huge benefit for these daily struggles."
The study was supported by DaVita Rx. Ms. McKinley is an employee of DaVita HealthCare Partners.
LAS VEGAS – The most common reason why end-stage-renal disease patients missed doses of their metabolic bone disease medications was that they forgot to take them, a multicenter study showed.
"Medication adherence is a constant struggle for patients with end-stage renal disease," Maureen McKinley, M.S.W., said in an interview after a meeting sponsored by the National Kidney Foundation, where the study was presented. "Dialysis patients are prescribed an average of 21 pills a day which would be difficult for even a healthy person to track and manage. The average dialysis patient misses one out of every two medication doses, which has a direct impact on their health, frequency of hospitalizations and, in turn, on costs to our health care system."
Ms. McKinley, a clinical social worker at Irvine, Calif.–based DaVita HealthCare Partners, and her associates interviewed 50 patients across 17 hemodialysis clinics over a period of 12 weeks to determine root causes of missed metabolic bone disease (MBD) medication doses. Of the 50 patients, 70% reported having missed doses of their MBD medication over the 12-week period. Of these, 66% missed a dose fewer than five times while 10% missed doses five times or more.
The most frequent reason for missing doses was "forgot to take" (41%), followed by "ill and not eating that many meals" (10%), "don’t understand importance" (7%), and "difficulty swallowing" (7%). Other reasons included "side effects" (6%), "forgot to refill" (4%), and financial barriers (3%).
"From my background as a social worker, I was expecting financial reasons to be the most common cause for patients not taking all their prescribed medication," Ms. McKinley said. "After years of sitting with families struggling to make ends meet and stay on top of payments, I know the burden that dialysis can place on a patient and their loved ones. Yet, 41% of respondents cited "forgot to take" as the reason for not taking medications."
With the complexity of so many medications and the reality of the disease, "it’s all about keeping it simple and as easy as possible for our patients," Ms. McKinley continued. "Trying to assist with simple reminders, setting an alarm, putting pills where you can see them or carrying your phosphate binders with you. Having a support system is such a help for patients on dialysis. As clinicians, we try to assist in these ways, but having the support of loved ones is a huge benefit for these daily struggles."
The study was supported by DaVita Rx. Ms. McKinley is an employee of DaVita HealthCare Partners.
LAS VEGAS – The most common reason why end-stage-renal disease patients missed doses of their metabolic bone disease medications was that they forgot to take them, a multicenter study showed.
"Medication adherence is a constant struggle for patients with end-stage renal disease," Maureen McKinley, M.S.W., said in an interview after a meeting sponsored by the National Kidney Foundation, where the study was presented. "Dialysis patients are prescribed an average of 21 pills a day which would be difficult for even a healthy person to track and manage. The average dialysis patient misses one out of every two medication doses, which has a direct impact on their health, frequency of hospitalizations and, in turn, on costs to our health care system."
Ms. McKinley, a clinical social worker at Irvine, Calif.–based DaVita HealthCare Partners, and her associates interviewed 50 patients across 17 hemodialysis clinics over a period of 12 weeks to determine root causes of missed metabolic bone disease (MBD) medication doses. Of the 50 patients, 70% reported having missed doses of their MBD medication over the 12-week period. Of these, 66% missed a dose fewer than five times while 10% missed doses five times or more.
The most frequent reason for missing doses was "forgot to take" (41%), followed by "ill and not eating that many meals" (10%), "don’t understand importance" (7%), and "difficulty swallowing" (7%). Other reasons included "side effects" (6%), "forgot to refill" (4%), and financial barriers (3%).
"From my background as a social worker, I was expecting financial reasons to be the most common cause for patients not taking all their prescribed medication," Ms. McKinley said. "After years of sitting with families struggling to make ends meet and stay on top of payments, I know the burden that dialysis can place on a patient and their loved ones. Yet, 41% of respondents cited "forgot to take" as the reason for not taking medications."
With the complexity of so many medications and the reality of the disease, "it’s all about keeping it simple and as easy as possible for our patients," Ms. McKinley continued. "Trying to assist with simple reminders, setting an alarm, putting pills where you can see them or carrying your phosphate binders with you. Having a support system is such a help for patients on dialysis. As clinicians, we try to assist in these ways, but having the support of loved ones is a huge benefit for these daily struggles."
The study was supported by DaVita Rx. Ms. McKinley is an employee of DaVita HealthCare Partners.
AT SCM 14
Key clinical point: Discuss simple reminders, such as setting an alarm, to improve medication adherence for dialysis patients.
Major finding: Over a period of 12 weeks, 76% of patients being treated at hemodialysis clinics reported missing doses of their metabolic bone disease medications.
Data source: A survey of 50 patients at 17 hemodialysis clinics.
Disclosures: The study was supported by DaVita Rx. Ms. McKinley is an employee of DaVita HealthCare Partners.
Bisphosphonates don’t cut risk of breast cancer
Three to four years of therapy with the bisphosphonates alendronate and zoledronic acid, taken at doses used to treat osteoporosis, did not decrease the risk of incident breast cancer in postmenopausal women, according to a report published online Aug. 11 in JAMA Internal Medicine.
In a post hoc analysis of data from two large multicenter, randomized, double-blind, controlled clinical trials assessing the effectiveness of alendronate or zoledronic acid for osteoporosis, the development of incident breast cancer was not significantly different between women taking the drugs and women taking placebo, said Trisha F. Hue, Ph.D., of the department of epidemiology and biostatistics at the University of California, San Francisco, and her associates.
Numerous previous observational studies, as well as a metaanalysis pooling the data from several observational studies, had shown that bisphosphonates taken for osteoporosis significantly lowered the risk of breast cancer by 32%-39%. Their findings, however, may have been confounded by indication, because other conditions in postmenopausal women – notably, low levels of estradiol and high levels of sex hormone–binding globulin (SHBG) – are strongly associated both with low bone density, fractures, and bone loss with a low risk of ER-positive breast cancer, Dr. Hue and her associates said. Thus, they suggested, the postmenopausal women who are most likely to be given bisphosphonates for bone health already have a lower risk of breast cancer.
Such confounding can be averted by using a randomized trial design, so Dr. Hue and her colleagues assessed whether bisphosphonates reduced incident breast cancer by analyzing data from the Fracture Intervention Trial (FIT) and the Health Outcomes and Reduced Incidence With Zoledronic Acid Once Yearly-Pivotal Fracture Trial (HORIZON-PFT).
Among the 6,194 FIT participants in this study, there were 103 cases of invasive breast cancer during a mean of 3.8 years of follow-up. The incidence of breast cancer was 1.8% (57 women) among women taking alendronate and 1.5% (46 women) in those taking placebo, a nonsignificant difference in favor of placebo.
Among the 7,580 HORIZON-PFT participants in this study, there were 62 cases of invasive breast cancer during 3 years of follow-up. The incidence of breast cancer was 0.9% (33 women) among women taking zoledronic acid and 0.8% (29 women) among those taking placebo, which was, again, a nonsignificant difference in favor of placebo, the investigators said (JAMA Intern. Med. 2014 Aug. 11 [doi:10.1001/jamainternmed.2014.3634]).
The total of breast cancer cases was relatively small, so Dr. Hue and her associates pooled the data from both studies to increase the sample size. The combined incidence of breast cancer was 1.3% (90 women) taking bisphosphonates and 1.1% (75 women) taking placebo – again, a nonsignificant difference favoring placebo.
The discrepancy between these findings from randomized controlled trials and the results of previous observational studies illustrates "the hazard of drawing conclusions about treatment effects from observational studies (even those that are very well done)" and highlights the value of confirming such findings in randomized controlled trials, Dr. Hue and her associates said.
This study received no industry support. FIT was supported by Merck, and HORIZON-PFT was supported by Novartis; both companies were involved in data collection and management. Dr. Hue reported no potential financial conflicts of interest. One of her associates reported serving as a consultant for Merck Sharpe & Dohme.
Three to four years of therapy with the bisphosphonates alendronate and zoledronic acid, taken at doses used to treat osteoporosis, did not decrease the risk of incident breast cancer in postmenopausal women, according to a report published online Aug. 11 in JAMA Internal Medicine.
In a post hoc analysis of data from two large multicenter, randomized, double-blind, controlled clinical trials assessing the effectiveness of alendronate or zoledronic acid for osteoporosis, the development of incident breast cancer was not significantly different between women taking the drugs and women taking placebo, said Trisha F. Hue, Ph.D., of the department of epidemiology and biostatistics at the University of California, San Francisco, and her associates.
Numerous previous observational studies, as well as a metaanalysis pooling the data from several observational studies, had shown that bisphosphonates taken for osteoporosis significantly lowered the risk of breast cancer by 32%-39%. Their findings, however, may have been confounded by indication, because other conditions in postmenopausal women – notably, low levels of estradiol and high levels of sex hormone–binding globulin (SHBG) – are strongly associated both with low bone density, fractures, and bone loss with a low risk of ER-positive breast cancer, Dr. Hue and her associates said. Thus, they suggested, the postmenopausal women who are most likely to be given bisphosphonates for bone health already have a lower risk of breast cancer.
Such confounding can be averted by using a randomized trial design, so Dr. Hue and her colleagues assessed whether bisphosphonates reduced incident breast cancer by analyzing data from the Fracture Intervention Trial (FIT) and the Health Outcomes and Reduced Incidence With Zoledronic Acid Once Yearly-Pivotal Fracture Trial (HORIZON-PFT).
Among the 6,194 FIT participants in this study, there were 103 cases of invasive breast cancer during a mean of 3.8 years of follow-up. The incidence of breast cancer was 1.8% (57 women) among women taking alendronate and 1.5% (46 women) in those taking placebo, a nonsignificant difference in favor of placebo.
Among the 7,580 HORIZON-PFT participants in this study, there were 62 cases of invasive breast cancer during 3 years of follow-up. The incidence of breast cancer was 0.9% (33 women) among women taking zoledronic acid and 0.8% (29 women) among those taking placebo, which was, again, a nonsignificant difference in favor of placebo, the investigators said (JAMA Intern. Med. 2014 Aug. 11 [doi:10.1001/jamainternmed.2014.3634]).
The total of breast cancer cases was relatively small, so Dr. Hue and her associates pooled the data from both studies to increase the sample size. The combined incidence of breast cancer was 1.3% (90 women) taking bisphosphonates and 1.1% (75 women) taking placebo – again, a nonsignificant difference favoring placebo.
The discrepancy between these findings from randomized controlled trials and the results of previous observational studies illustrates "the hazard of drawing conclusions about treatment effects from observational studies (even those that are very well done)" and highlights the value of confirming such findings in randomized controlled trials, Dr. Hue and her associates said.
This study received no industry support. FIT was supported by Merck, and HORIZON-PFT was supported by Novartis; both companies were involved in data collection and management. Dr. Hue reported no potential financial conflicts of interest. One of her associates reported serving as a consultant for Merck Sharpe & Dohme.
Three to four years of therapy with the bisphosphonates alendronate and zoledronic acid, taken at doses used to treat osteoporosis, did not decrease the risk of incident breast cancer in postmenopausal women, according to a report published online Aug. 11 in JAMA Internal Medicine.
In a post hoc analysis of data from two large multicenter, randomized, double-blind, controlled clinical trials assessing the effectiveness of alendronate or zoledronic acid for osteoporosis, the development of incident breast cancer was not significantly different between women taking the drugs and women taking placebo, said Trisha F. Hue, Ph.D., of the department of epidemiology and biostatistics at the University of California, San Francisco, and her associates.
Numerous previous observational studies, as well as a metaanalysis pooling the data from several observational studies, had shown that bisphosphonates taken for osteoporosis significantly lowered the risk of breast cancer by 32%-39%. Their findings, however, may have been confounded by indication, because other conditions in postmenopausal women – notably, low levels of estradiol and high levels of sex hormone–binding globulin (SHBG) – are strongly associated both with low bone density, fractures, and bone loss with a low risk of ER-positive breast cancer, Dr. Hue and her associates said. Thus, they suggested, the postmenopausal women who are most likely to be given bisphosphonates for bone health already have a lower risk of breast cancer.
Such confounding can be averted by using a randomized trial design, so Dr. Hue and her colleagues assessed whether bisphosphonates reduced incident breast cancer by analyzing data from the Fracture Intervention Trial (FIT) and the Health Outcomes and Reduced Incidence With Zoledronic Acid Once Yearly-Pivotal Fracture Trial (HORIZON-PFT).
Among the 6,194 FIT participants in this study, there were 103 cases of invasive breast cancer during a mean of 3.8 years of follow-up. The incidence of breast cancer was 1.8% (57 women) among women taking alendronate and 1.5% (46 women) in those taking placebo, a nonsignificant difference in favor of placebo.
Among the 7,580 HORIZON-PFT participants in this study, there were 62 cases of invasive breast cancer during 3 years of follow-up. The incidence of breast cancer was 0.9% (33 women) among women taking zoledronic acid and 0.8% (29 women) among those taking placebo, which was, again, a nonsignificant difference in favor of placebo, the investigators said (JAMA Intern. Med. 2014 Aug. 11 [doi:10.1001/jamainternmed.2014.3634]).
The total of breast cancer cases was relatively small, so Dr. Hue and her associates pooled the data from both studies to increase the sample size. The combined incidence of breast cancer was 1.3% (90 women) taking bisphosphonates and 1.1% (75 women) taking placebo – again, a nonsignificant difference favoring placebo.
The discrepancy between these findings from randomized controlled trials and the results of previous observational studies illustrates "the hazard of drawing conclusions about treatment effects from observational studies (even those that are very well done)" and highlights the value of confirming such findings in randomized controlled trials, Dr. Hue and her associates said.
This study received no industry support. FIT was supported by Merck, and HORIZON-PFT was supported by Novartis; both companies were involved in data collection and management. Dr. Hue reported no potential financial conflicts of interest. One of her associates reported serving as a consultant for Merck Sharpe & Dohme.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Alendronate and zoledronic acid do not appear to reduce the risk of breast cancer.
Major finding: The incidence of breast cancer was 1.8% in women taking alendronate and 1.5% in those taking placebo, a nonsignificant difference; the incidence of breast cancer was 0.9% in women taking zoledronic acid and 0.8% in those taking placebo, again, a nonsignificant difference.
Data source: A post hoc analysis of data from two large randomized clinical trials involving 6,194 postmenopausal women with osteoporosis who received either alendronate or placebo and 7,580 who received either zoledronic acid or placebo.
Disclosures: This study received no industry support. FIT was supported by Merck, and HORIZON-PFT was supported by Novartis; both companies were involved in data collection and management. Dr. Hue reported no potential financial conflicts of interest. One of her associates reported serving as a consultant for Merck Sharpe & Dohme.
Vitamin D deficiency linked to schizophrenia
More than half of schizophrenia patients have a vitamin D deficiency, according to a recent systematic review.
A meta-analysis of 13 of the total 19 studies in the review revealed that schizophrenia patients had an average 5.91 ng/mL lower serum 25-hydroxyvitamin D (25[OH])D) levels than control participants, Ghazaleh Valipour of Isfahan (Iran) University, and her colleagues reported online (J. Clin. Endocrinol. Metab. 2014 [doi:10.1210/jc.2014-1887]).
The 19 studies, published between 1988 and 2013, measured serum vitamin D levels in schizophrenic patients, including those with schizoaffective or other schizophrenia spectrum disorders. The articles included 8 cross-sectional studies, 10 case-control studies, and 1 nested case-control study, with a total of 2,804 participants aged 18-65. Sample sizes ranged from 17 to 848 participants.
A second meta-analysis of 8 of the 19 studies found that 65% of schizophrenia patients had a vitamin D deficiency, with prevalence ranging from 14% to 98% across studies. A third meta-analysis of eight studies found participants with a vitamin D deficiency had twice the odds of schizophrenia (odds ratio, 2.16) than those with sufficient levels of vitamin D, with studies’ odds ratios ranging from 0.6 to 13.6. Differences in findings across the studies could not be attributed to study design, the patient’s hospitalization status, study quality or study location; but use of 25-dihydroxyvitamin D3 (25[OH]D3) to measure serum levels explained some variation.
However, in all but one study, odds ratios "were calculated using the prevalence of schizophrenia in vitamin D–sufficient and –deficient persons," the researchers wrote. "Therefore, causality cannot be inferred from this finding because in most studies the exposure and the outcome coexisted."
The research was funded by the Research Council of the Food Security Research Center at Isfahan University. The authors had no disclosures.
More than half of schizophrenia patients have a vitamin D deficiency, according to a recent systematic review.
A meta-analysis of 13 of the total 19 studies in the review revealed that schizophrenia patients had an average 5.91 ng/mL lower serum 25-hydroxyvitamin D (25[OH])D) levels than control participants, Ghazaleh Valipour of Isfahan (Iran) University, and her colleagues reported online (J. Clin. Endocrinol. Metab. 2014 [doi:10.1210/jc.2014-1887]).
The 19 studies, published between 1988 and 2013, measured serum vitamin D levels in schizophrenic patients, including those with schizoaffective or other schizophrenia spectrum disorders. The articles included 8 cross-sectional studies, 10 case-control studies, and 1 nested case-control study, with a total of 2,804 participants aged 18-65. Sample sizes ranged from 17 to 848 participants.
A second meta-analysis of 8 of the 19 studies found that 65% of schizophrenia patients had a vitamin D deficiency, with prevalence ranging from 14% to 98% across studies. A third meta-analysis of eight studies found participants with a vitamin D deficiency had twice the odds of schizophrenia (odds ratio, 2.16) than those with sufficient levels of vitamin D, with studies’ odds ratios ranging from 0.6 to 13.6. Differences in findings across the studies could not be attributed to study design, the patient’s hospitalization status, study quality or study location; but use of 25-dihydroxyvitamin D3 (25[OH]D3) to measure serum levels explained some variation.
However, in all but one study, odds ratios "were calculated using the prevalence of schizophrenia in vitamin D–sufficient and –deficient persons," the researchers wrote. "Therefore, causality cannot be inferred from this finding because in most studies the exposure and the outcome coexisted."
The research was funded by the Research Council of the Food Security Research Center at Isfahan University. The authors had no disclosures.
More than half of schizophrenia patients have a vitamin D deficiency, according to a recent systematic review.
A meta-analysis of 13 of the total 19 studies in the review revealed that schizophrenia patients had an average 5.91 ng/mL lower serum 25-hydroxyvitamin D (25[OH])D) levels than control participants, Ghazaleh Valipour of Isfahan (Iran) University, and her colleagues reported online (J. Clin. Endocrinol. Metab. 2014 [doi:10.1210/jc.2014-1887]).
The 19 studies, published between 1988 and 2013, measured serum vitamin D levels in schizophrenic patients, including those with schizoaffective or other schizophrenia spectrum disorders. The articles included 8 cross-sectional studies, 10 case-control studies, and 1 nested case-control study, with a total of 2,804 participants aged 18-65. Sample sizes ranged from 17 to 848 participants.
A second meta-analysis of 8 of the 19 studies found that 65% of schizophrenia patients had a vitamin D deficiency, with prevalence ranging from 14% to 98% across studies. A third meta-analysis of eight studies found participants with a vitamin D deficiency had twice the odds of schizophrenia (odds ratio, 2.16) than those with sufficient levels of vitamin D, with studies’ odds ratios ranging from 0.6 to 13.6. Differences in findings across the studies could not be attributed to study design, the patient’s hospitalization status, study quality or study location; but use of 25-dihydroxyvitamin D3 (25[OH]D3) to measure serum levels explained some variation.
However, in all but one study, odds ratios "were calculated using the prevalence of schizophrenia in vitamin D–sufficient and –deficient persons," the researchers wrote. "Therefore, causality cannot be inferred from this finding because in most studies the exposure and the outcome coexisted."
The research was funded by the Research Council of the Food Security Research Center at Isfahan University. The authors had no disclosures.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Key clinical point: One literature review suggests an association between vitamin D deficiency and schizophrenia.
Major finding: Persons with a vitamin D deficiency were 2.16 times more likely to have schizophrenia, and roughly 65% of schizophrenic patients had a vitamin D deficiency.
Data source: A systematic review of 19 observational studies, published between 1988 and 2013, which measured serum vitamin D levels in 2,804 schizophrenic and control participants.
Disclosures: The research was funded by the Research Council of the Food Security Research Center at Isfahan University of Medical Sciences in Iran. The authors had no disclosures.
New agent builds bone bigger, faster
CHICAGO – Abaloparatide, a synthetic analog of human parathyroid hormone–related peptide, displayed jaw-dropping superiority to teriparatide in boosting bone mineral density at multiple anatomic sites in a head-to-head, placebo-controlled phase II study.
"Given the consistency of these increases in BMD [bone mineral density] seen in the phase II studies, abaloparatide may emerge as an important therapeutic agent in the treatment of postmenopausal osteoporosis," Dr. Alan G. Harris observed in presenting the results of two separate phase II abaloparatide studies at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"There is an unmet need for anabolic agents that preferentially increase bone formation as opposed to decreasing bone resorption. There is also a challenge we’re faced with in clinical practice: that is, the lack of early hip BMD increase with teriparatide," added Dr. Harris, chief medical officer at Radius Health of Cambridge, Mass., which is developing the agent.
The phase II data suggest abaloparatide at 80 mcg by once-daily subcutaneous injection meets both needs, he added.
Indeed, based upon the highly positive phase II work, a phase III, placebo- and teriparatide-controlled clinical trial with fracture endpoints is well underway. The 18-month trial involving more than 2,400 patients is due to be completed later this year.
Separately, Gary Hattersley, Ph.D., presented encouraging results from a 231-patient, 24-week, phase-II, dose-ranging study of abaloparatide delivered by transdermal patch.
"We look at this as being a strong proof-of-concept study demonstrating that this simple transdermal patch with only a 5-minute wear time is able to deliver meaningful amounts of abaloparatide through the skin without the need for a subcutaneous injection in order to achieve meaningful increases in BMD," said Dr. Hattersley, chief scientific officer at Radius Health.
"We recognize that there’s really a significant opportunity for an alternative to daily subcutaneous injection. This has the potential to improve both patient convenience as well as patient compliance," he added.
The increases in BMD with the patch – a 2.95% increase from baseline at the spine with the 150-mcg patch and a 1.49% rise in total hip BMD – were not as robust as in controls assigned to once-daily abaloparatide at 80 mcg, which is the optimal injectable dose also being used in the ongoing phase III trial. But Dr. Hattersley said he believes that higher-dose patches now under study will achieve substantially bigger increases in BMD.
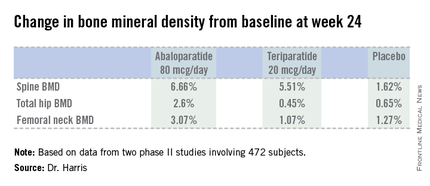
Dr. Harris presented data from two phase II studies on a total of 472 postmenopausal women with osteoporosis. In one, subjects were randomized to subcutaneous abaloparatide, teriparatide (Forteo) at its approved dose of 20 mcg by daily subcutaneous injection, or placebo. Although the primary endpoints in this study were assessed at 24 weeks, in an extension out to 48 weeks the increase in lumbar spine BMD over baseline was 12.9% with abaloparatide 80 mcg, 8.6% with teriparatide, and 0.7% with placebo.
In the other study, patients were randomized to abaloparatide or placebo. In this trial, patients on abaloparatide at 80 mcg showed a 5.8% increase over baseline in spine BMD at 24 weeks, along with a 2.74% increase in total hip BMD and a 2.76% increase in femoral neck BMD, as compared with a 0.44% increase in spine BMD with placebo and net BMD losses of less than 1% at each of the other two sites.
In both studies, side effects of abaloparatide were similar in type and incidence to placebo. Of note, the incidence of mild, transient hypercalcemia in abaloparatide-treated patients was half that of the teriparatide group.
Asked why the subcutaneous abaloparatide at 80 mcg is so much more effective at increasing BMD than teriparatide is at its approved dose, Dr. Harris replied, "They’re different peptides." In monkey studies, abaloparatide showed less increase in cortical bone porosity than in studies done using teriparatide. And in the head-to-head phase II study, the increase in bone turnover markers related to resorption was substantially greater with teriparatide. So abaloparatide’s greater BMD-building efficacy is because of greater selectivity for increased bone formation and less bone resorption relative to teriparatide, he suggested.
The abaloparatide patch utilizes proprietary technology developed by 3M. The dime-size patch contains 316 spearlike microprojections, each 500 mcm (micrometers) long. The tip of each microprojection is coated with abaloparatide. When the patch is applied to periumbilical skin, the microprojections penetrate the skin to a depth of about 250 mcm, putting the tip into the upper dermis. Patch application was painless and without side effects in the phase II study, according to Dr. Harris.
These phase II studies were funded by Radius Health.
CHICAGO – Abaloparatide, a synthetic analog of human parathyroid hormone–related peptide, displayed jaw-dropping superiority to teriparatide in boosting bone mineral density at multiple anatomic sites in a head-to-head, placebo-controlled phase II study.
"Given the consistency of these increases in BMD [bone mineral density] seen in the phase II studies, abaloparatide may emerge as an important therapeutic agent in the treatment of postmenopausal osteoporosis," Dr. Alan G. Harris observed in presenting the results of two separate phase II abaloparatide studies at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.

"There is an unmet need for anabolic agents that preferentially increase bone formation as opposed to decreasing bone resorption. There is also a challenge we’re faced with in clinical practice: that is, the lack of early hip BMD increase with teriparatide," added Dr. Harris, chief medical officer at Radius Health of Cambridge, Mass., which is developing the agent.
The phase II data suggest abaloparatide at 80 mcg by once-daily subcutaneous injection meets both needs, he added.
Indeed, based upon the highly positive phase II work, a phase III, placebo- and teriparatide-controlled clinical trial with fracture endpoints is well underway. The 18-month trial involving more than 2,400 patients is due to be completed later this year.
Separately, Gary Hattersley, Ph.D., presented encouraging results from a 231-patient, 24-week, phase-II, dose-ranging study of abaloparatide delivered by transdermal patch.
"We look at this as being a strong proof-of-concept study demonstrating that this simple transdermal patch with only a 5-minute wear time is able to deliver meaningful amounts of abaloparatide through the skin without the need for a subcutaneous injection in order to achieve meaningful increases in BMD," said Dr. Hattersley, chief scientific officer at Radius Health.
"We recognize that there’s really a significant opportunity for an alternative to daily subcutaneous injection. This has the potential to improve both patient convenience as well as patient compliance," he added.
The increases in BMD with the patch – a 2.95% increase from baseline at the spine with the 150-mcg patch and a 1.49% rise in total hip BMD – were not as robust as in controls assigned to once-daily abaloparatide at 80 mcg, which is the optimal injectable dose also being used in the ongoing phase III trial. But Dr. Hattersley said he believes that higher-dose patches now under study will achieve substantially bigger increases in BMD.
Dr. Harris presented data from two phase II studies on a total of 472 postmenopausal women with osteoporosis. In one, subjects were randomized to subcutaneous abaloparatide, teriparatide (Forteo) at its approved dose of 20 mcg by daily subcutaneous injection, or placebo. Although the primary endpoints in this study were assessed at 24 weeks, in an extension out to 48 weeks the increase in lumbar spine BMD over baseline was 12.9% with abaloparatide 80 mcg, 8.6% with teriparatide, and 0.7% with placebo.
In the other study, patients were randomized to abaloparatide or placebo. In this trial, patients on abaloparatide at 80 mcg showed a 5.8% increase over baseline in spine BMD at 24 weeks, along with a 2.74% increase in total hip BMD and a 2.76% increase in femoral neck BMD, as compared with a 0.44% increase in spine BMD with placebo and net BMD losses of less than 1% at each of the other two sites.
In both studies, side effects of abaloparatide were similar in type and incidence to placebo. Of note, the incidence of mild, transient hypercalcemia in abaloparatide-treated patients was half that of the teriparatide group.
Asked why the subcutaneous abaloparatide at 80 mcg is so much more effective at increasing BMD than teriparatide is at its approved dose, Dr. Harris replied, "They’re different peptides." In monkey studies, abaloparatide showed less increase in cortical bone porosity than in studies done using teriparatide. And in the head-to-head phase II study, the increase in bone turnover markers related to resorption was substantially greater with teriparatide. So abaloparatide’s greater BMD-building efficacy is because of greater selectivity for increased bone formation and less bone resorption relative to teriparatide, he suggested.
The abaloparatide patch utilizes proprietary technology developed by 3M. The dime-size patch contains 316 spearlike microprojections, each 500 mcm (micrometers) long. The tip of each microprojection is coated with abaloparatide. When the patch is applied to periumbilical skin, the microprojections penetrate the skin to a depth of about 250 mcm, putting the tip into the upper dermis. Patch application was painless and without side effects in the phase II study, according to Dr. Harris.
These phase II studies were funded by Radius Health.
CHICAGO – Abaloparatide, a synthetic analog of human parathyroid hormone–related peptide, displayed jaw-dropping superiority to teriparatide in boosting bone mineral density at multiple anatomic sites in a head-to-head, placebo-controlled phase II study.
"Given the consistency of these increases in BMD [bone mineral density] seen in the phase II studies, abaloparatide may emerge as an important therapeutic agent in the treatment of postmenopausal osteoporosis," Dr. Alan G. Harris observed in presenting the results of two separate phase II abaloparatide studies at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.

"There is an unmet need for anabolic agents that preferentially increase bone formation as opposed to decreasing bone resorption. There is also a challenge we’re faced with in clinical practice: that is, the lack of early hip BMD increase with teriparatide," added Dr. Harris, chief medical officer at Radius Health of Cambridge, Mass., which is developing the agent.
The phase II data suggest abaloparatide at 80 mcg by once-daily subcutaneous injection meets both needs, he added.
Indeed, based upon the highly positive phase II work, a phase III, placebo- and teriparatide-controlled clinical trial with fracture endpoints is well underway. The 18-month trial involving more than 2,400 patients is due to be completed later this year.
Separately, Gary Hattersley, Ph.D., presented encouraging results from a 231-patient, 24-week, phase-II, dose-ranging study of abaloparatide delivered by transdermal patch.
"We look at this as being a strong proof-of-concept study demonstrating that this simple transdermal patch with only a 5-minute wear time is able to deliver meaningful amounts of abaloparatide through the skin without the need for a subcutaneous injection in order to achieve meaningful increases in BMD," said Dr. Hattersley, chief scientific officer at Radius Health.
"We recognize that there’s really a significant opportunity for an alternative to daily subcutaneous injection. This has the potential to improve both patient convenience as well as patient compliance," he added.
The increases in BMD with the patch – a 2.95% increase from baseline at the spine with the 150-mcg patch and a 1.49% rise in total hip BMD – were not as robust as in controls assigned to once-daily abaloparatide at 80 mcg, which is the optimal injectable dose also being used in the ongoing phase III trial. But Dr. Hattersley said he believes that higher-dose patches now under study will achieve substantially bigger increases in BMD.
Dr. Harris presented data from two phase II studies on a total of 472 postmenopausal women with osteoporosis. In one, subjects were randomized to subcutaneous abaloparatide, teriparatide (Forteo) at its approved dose of 20 mcg by daily subcutaneous injection, or placebo. Although the primary endpoints in this study were assessed at 24 weeks, in an extension out to 48 weeks the increase in lumbar spine BMD over baseline was 12.9% with abaloparatide 80 mcg, 8.6% with teriparatide, and 0.7% with placebo.
In the other study, patients were randomized to abaloparatide or placebo. In this trial, patients on abaloparatide at 80 mcg showed a 5.8% increase over baseline in spine BMD at 24 weeks, along with a 2.74% increase in total hip BMD and a 2.76% increase in femoral neck BMD, as compared with a 0.44% increase in spine BMD with placebo and net BMD losses of less than 1% at each of the other two sites.
In both studies, side effects of abaloparatide were similar in type and incidence to placebo. Of note, the incidence of mild, transient hypercalcemia in abaloparatide-treated patients was half that of the teriparatide group.
Asked why the subcutaneous abaloparatide at 80 mcg is so much more effective at increasing BMD than teriparatide is at its approved dose, Dr. Harris replied, "They’re different peptides." In monkey studies, abaloparatide showed less increase in cortical bone porosity than in studies done using teriparatide. And in the head-to-head phase II study, the increase in bone turnover markers related to resorption was substantially greater with teriparatide. So abaloparatide’s greater BMD-building efficacy is because of greater selectivity for increased bone formation and less bone resorption relative to teriparatide, he suggested.
The abaloparatide patch utilizes proprietary technology developed by 3M. The dime-size patch contains 316 spearlike microprojections, each 500 mcm (micrometers) long. The tip of each microprojection is coated with abaloparatide. When the patch is applied to periumbilical skin, the microprojections penetrate the skin to a depth of about 250 mcm, putting the tip into the upper dermis. Patch application was painless and without side effects in the phase II study, according to Dr. Harris.
These phase II studies were funded by Radius Health.
AT ICE/ENDO 2014
Key clinical point: A novel anabolic agent being developed for the treatment of postmenopausal osteoporosis increased BMD faster and to a greater extent than did teriparatide.
Major finding: Total hip bone mineral density increased by 2.6% over baseline after 24 weeks of abaloparatide at 80 mcg daily, compared with 0.45% with teriparatide at 20 mcg daily and 0.65% with placebo.
Data source: This phase II randomized trial included 222 postmenopausal women with osteoporosis.
Disclosures: The study was sponsored by Radius Health. The presenter is the company’s chief medical officer.
Fractures increased with two diabetes drugs
SAN FRANCISCO – Adults who started taking sulfonylurea drugs for diabetes were 9% more likely to develop fractures within 5 years, and patients starting thiazolidinediones were 9% more likely to have fractures, compared with patients on other medications in a retrospective study of 99,892 adults.
Those significantly increased risks emerged after adjusting for the effects of multiple factors including age, gender, region, medical conditions, and concomitant medications, Sandhya Mehta, Ph.D. and her associates reported at the annual scientific sessions of the American Diabetes Association. Patients had no prior history of fracture.
The longitudinal retrospective analysis of a large administrative claims database found a 10% incidence of fracture on sulfonylureas and 11% on thiazolidinediones, compared with 7% on biguanides (metformin), 8% on dipeptidyl peptidase-4 (DPP-4) inhibitors, 11% on meglitinide analogues, and 6% on incretin mimetic agents. After adjusting for the confounders, the hazard ratios for each of the other three types of drugs hovered around 1 and were not statistically significantly different but increased to 1.09 for sulfonylureas and 1.4 for thiazolidinediones, reported Dr. Mehta of Inovalon Inc. in Bowie, Md., a health care data analytics company.
In the cohort as a whole, 7% of patients developed fractures. Roughly 15% of patients started sulfonylureas, 3% took thiazolidinediones, 78% were on metformin, 3% took DPP-4 inhibitors, 1% were on incretin mimetics, and 1% took meglitinides.
Previous reports have shown an increased risk of fracture in patients on thiazolidinediones, compared with those on metformin, and the current results support the hypothesis that thiazolidinediones decrease bone mineral density, stimulate adipocyte and osteoclast differentiation, and inhibit osteoblast differentiation to make fracture more likely, Dr. Mehta said.
The association between sulfonylureas and increased fracture risk appears to be new, however, and deserves further study, she added.
The study did not look at the effects of combination drug therapy.
Data came from the Medical Outcomes Research for Effectiveness and Economics (MORE) Registry, which is owned by Inovalon, drawing from the years 2008-2012.
Dr. Mehta reported having no financial disclosures.
On Twitter @sherryboschert
This study confirms that we observe more fracture risks with thiazolidinediones. To my knowledge, this is the first time that this has been shown for sulfonylureas.

|
|
One concern about the study is so-called residual confounding. The confounders were not entirely clear in the presentation. One possible confounder is what might have preceded the fracture. If, in fact, patients on sulfonylureas were having more low blood glucose values and consequently fell, then that would be interesting to know. Unfortunately, the investigators couldn’t tell us that.
With thiazolidinediones, I’ve been told that a cell that hasn’t decided yet whether it’s going to be a bone cell or a fat cell might, in the presence of a thiazolidinedione, go on to be a fat cell instead of a bone cell. In which case, you would possibly have a problem with fewer bone cells. This study set out to look at bone metabolism but, to be fair, it could not show anything about that. That was rather ambitious.
I think there is no clinical message from this as yet. The investigators might want to look back at trials that randomized people to sulfonylureas or something else and meta-analyze that to see if there were differences in fractures.
The study suggests that the other second-line agents are okay, but again, we can’t really know what they’re doing to bones without knowing if there’s something else going on. If you took a drug that made you more likely to topple over, your bones could be equally strong as those who didn’t fall over, yet you end up with a fracture.
I’m not going to change my practice on the basis of this study.
Dr. Amanda Adler is consultant physician at Cambridge University’s Addenbrooke’s Hospital and chair of the technology appraisals committee for the National Institute for Health and Clinical Excellence in England. She gave these comments in an interview at the meeting.
This study confirms that we observe more fracture risks with thiazolidinediones. To my knowledge, this is the first time that this has been shown for sulfonylureas.

|
|
One concern about the study is so-called residual confounding. The confounders were not entirely clear in the presentation. One possible confounder is what might have preceded the fracture. If, in fact, patients on sulfonylureas were having more low blood glucose values and consequently fell, then that would be interesting to know. Unfortunately, the investigators couldn’t tell us that.
With thiazolidinediones, I’ve been told that a cell that hasn’t decided yet whether it’s going to be a bone cell or a fat cell might, in the presence of a thiazolidinedione, go on to be a fat cell instead of a bone cell. In which case, you would possibly have a problem with fewer bone cells. This study set out to look at bone metabolism but, to be fair, it could not show anything about that. That was rather ambitious.
I think there is no clinical message from this as yet. The investigators might want to look back at trials that randomized people to sulfonylureas or something else and meta-analyze that to see if there were differences in fractures.
The study suggests that the other second-line agents are okay, but again, we can’t really know what they’re doing to bones without knowing if there’s something else going on. If you took a drug that made you more likely to topple over, your bones could be equally strong as those who didn’t fall over, yet you end up with a fracture.
I’m not going to change my practice on the basis of this study.
Dr. Amanda Adler is consultant physician at Cambridge University’s Addenbrooke’s Hospital and chair of the technology appraisals committee for the National Institute for Health and Clinical Excellence in England. She gave these comments in an interview at the meeting.
This study confirms that we observe more fracture risks with thiazolidinediones. To my knowledge, this is the first time that this has been shown for sulfonylureas.

|
|
One concern about the study is so-called residual confounding. The confounders were not entirely clear in the presentation. One possible confounder is what might have preceded the fracture. If, in fact, patients on sulfonylureas were having more low blood glucose values and consequently fell, then that would be interesting to know. Unfortunately, the investigators couldn’t tell us that.
With thiazolidinediones, I’ve been told that a cell that hasn’t decided yet whether it’s going to be a bone cell or a fat cell might, in the presence of a thiazolidinedione, go on to be a fat cell instead of a bone cell. In which case, you would possibly have a problem with fewer bone cells. This study set out to look at bone metabolism but, to be fair, it could not show anything about that. That was rather ambitious.
I think there is no clinical message from this as yet. The investigators might want to look back at trials that randomized people to sulfonylureas or something else and meta-analyze that to see if there were differences in fractures.
The study suggests that the other second-line agents are okay, but again, we can’t really know what they’re doing to bones without knowing if there’s something else going on. If you took a drug that made you more likely to topple over, your bones could be equally strong as those who didn’t fall over, yet you end up with a fracture.
I’m not going to change my practice on the basis of this study.
Dr. Amanda Adler is consultant physician at Cambridge University’s Addenbrooke’s Hospital and chair of the technology appraisals committee for the National Institute for Health and Clinical Excellence in England. She gave these comments in an interview at the meeting.
SAN FRANCISCO – Adults who started taking sulfonylurea drugs for diabetes were 9% more likely to develop fractures within 5 years, and patients starting thiazolidinediones were 9% more likely to have fractures, compared with patients on other medications in a retrospective study of 99,892 adults.
Those significantly increased risks emerged after adjusting for the effects of multiple factors including age, gender, region, medical conditions, and concomitant medications, Sandhya Mehta, Ph.D. and her associates reported at the annual scientific sessions of the American Diabetes Association. Patients had no prior history of fracture.
The longitudinal retrospective analysis of a large administrative claims database found a 10% incidence of fracture on sulfonylureas and 11% on thiazolidinediones, compared with 7% on biguanides (metformin), 8% on dipeptidyl peptidase-4 (DPP-4) inhibitors, 11% on meglitinide analogues, and 6% on incretin mimetic agents. After adjusting for the confounders, the hazard ratios for each of the other three types of drugs hovered around 1 and were not statistically significantly different but increased to 1.09 for sulfonylureas and 1.4 for thiazolidinediones, reported Dr. Mehta of Inovalon Inc. in Bowie, Md., a health care data analytics company.
In the cohort as a whole, 7% of patients developed fractures. Roughly 15% of patients started sulfonylureas, 3% took thiazolidinediones, 78% were on metformin, 3% took DPP-4 inhibitors, 1% were on incretin mimetics, and 1% took meglitinides.
Previous reports have shown an increased risk of fracture in patients on thiazolidinediones, compared with those on metformin, and the current results support the hypothesis that thiazolidinediones decrease bone mineral density, stimulate adipocyte and osteoclast differentiation, and inhibit osteoblast differentiation to make fracture more likely, Dr. Mehta said.
The association between sulfonylureas and increased fracture risk appears to be new, however, and deserves further study, she added.
The study did not look at the effects of combination drug therapy.
Data came from the Medical Outcomes Research for Effectiveness and Economics (MORE) Registry, which is owned by Inovalon, drawing from the years 2008-2012.
Dr. Mehta reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Adults who started taking sulfonylurea drugs for diabetes were 9% more likely to develop fractures within 5 years, and patients starting thiazolidinediones were 9% more likely to have fractures, compared with patients on other medications in a retrospective study of 99,892 adults.
Those significantly increased risks emerged after adjusting for the effects of multiple factors including age, gender, region, medical conditions, and concomitant medications, Sandhya Mehta, Ph.D. and her associates reported at the annual scientific sessions of the American Diabetes Association. Patients had no prior history of fracture.
The longitudinal retrospective analysis of a large administrative claims database found a 10% incidence of fracture on sulfonylureas and 11% on thiazolidinediones, compared with 7% on biguanides (metformin), 8% on dipeptidyl peptidase-4 (DPP-4) inhibitors, 11% on meglitinide analogues, and 6% on incretin mimetic agents. After adjusting for the confounders, the hazard ratios for each of the other three types of drugs hovered around 1 and were not statistically significantly different but increased to 1.09 for sulfonylureas and 1.4 for thiazolidinediones, reported Dr. Mehta of Inovalon Inc. in Bowie, Md., a health care data analytics company.
In the cohort as a whole, 7% of patients developed fractures. Roughly 15% of patients started sulfonylureas, 3% took thiazolidinediones, 78% were on metformin, 3% took DPP-4 inhibitors, 1% were on incretin mimetics, and 1% took meglitinides.
Previous reports have shown an increased risk of fracture in patients on thiazolidinediones, compared with those on metformin, and the current results support the hypothesis that thiazolidinediones decrease bone mineral density, stimulate adipocyte and osteoclast differentiation, and inhibit osteoblast differentiation to make fracture more likely, Dr. Mehta said.
The association between sulfonylureas and increased fracture risk appears to be new, however, and deserves further study, she added.
The study did not look at the effects of combination drug therapy.
Data came from the Medical Outcomes Research for Effectiveness and Economics (MORE) Registry, which is owned by Inovalon, drawing from the years 2008-2012.
Dr. Mehta reported having no financial disclosures.
On Twitter @sherryboschert
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Consider possible increased fracture risks with thiazolidinediones and sulfonylureas when choosing diabetes drug therapy.
Major finding: The 5-year fracture risk was 9% higher with sulfonylureas and 40% higher with thiazolidinediones, compared with other diabetes medications.
Data source: Retrospective analysis of data on 98,892 adults with no history of fracture who started a diabetes medication.
Disclosures: Dr. Mehta reported having no financial disclosures.
Meta-analysis finds limited bone loss with low-dose glucocorticoids
PARIS – Bone loss at 1 year is limited at the low doses of glucocorticoids typically used to treat adults with chronic inflammatory disorders like rheumatoid arthritis, based on a meta-analysis of prospective studies of 1,565 patients with chronic inflammatory diseases (44 studies) and 635 transplant patients (16 studies).
Glucocorticoid treatment at the high doses used in transplantation patients leads to considerable bone loss, especially in the lumbar spine. In contrast, bone loss is limited during glucocorticoid treatment at the lower doses used in chronic inflammatory disease, Dr. Maarten Boers, professor of clinical epidemiology at VU University Medical Center, Amsterdam, reported at the annual European Congress of Rheumatology.
All patients in the meta-analysis had at least two bone mineral density (BMD) measurements over at least 8 months. None received bisphosphonates or antiresorptive therapies, only vitamin D3 and calcium were allowed.
Glucocorticoid doses ranged from 1 to 16 mg/day (mean 9 mg/day) in the patients with chronic inflammatory diseases and from 6 to 53 mg/day (mean 20 mg/day) in the transplant patients. In those with chronic inflammatory diseases, bone loss at the lumbar spine at 1 year averaged –1.7%. For the patients who had measures of femoral neck bone loss, the average loss was –1.3%. In the transplantation group, average bone loss was much higher at –3.6% in the lumbar spine and –3.1% in the femoral neck.
Among the 44 studies that reported BMD in patients with chronic inflammatory diseases, BMD declined from baseline in the lumbar spine by as much as 6% in 1 study and increased by as much as 2% in another. The 39 studies that also reported changes in femoral neck BMD described a decline as high as 7% and an increase as great as 4%.
"We sought to quantify the ‘pure’ effect of GC [glucocorticoid(s)], because so little high-quality information is available," Dr. Boers explained.
The data analysis in these studies was limited to 1 year, but about two-thirds of the patients with chronic inflammatory disease were on chronic glucocorticoid therapy and almost all of the transplant patients were just starting glucocorticoids, Dr. Boers said.
"On average, the yearly loss in a wide range of doses is limited, but starters have more bone loss. The heterogeneity of studies suggests that factors other than GC dose are the main drivers in determining bone loss. Although the data were not available to study these factors, it appears likely that disease activity is very important and acts as a confounder. In other words, disease activity leads to bone loss, and high GC doses lead to bone loss. Effective treatment of high disease activity requires high GC doses, but the interaction of these factors may lead to bone loss comparable to low disease activity being treated with low doses," he explained in an interview.
Many rheumatoid arthritis patients are initially treated with "bridge" glucocorticoid therapy for a few months, until the effect of methotrexate is established, he said. "We prefer ‘COBRA [combination therapy for rheumatoid arthritis] light,’ where patients are initially treated with a higher dose of 30 mg, rapidly tapered to 7.5 mg/day, and maintained for at least 6 months. Several groups are advising to treat for longer periods, and observational data suggest many patients are kept on chronic therapy for periods longer than 1 year," he said.
Local guidelines differ, but all guidelines agree on the necessity of prophylaxis in high-risk situations and on screening for intermediate-risk patients. Unfortunately, many patients requiring antiresorptive treatment according to the guidelines are still not receiving it, with surveys showing about 70% uptake in patients treated by rheumatologists and only about 30% in patients receiving care from other specialists, he said.
"Given the effects of starting GC therapy in our review, we strongly suggest all patients requiring GC therapy for longer than 3 months at any dose should at least be assessed by DXA scan; postmenopausal women, males age 70 or older, and patients with other risk factors should be treated with antiresorptive agents from the start," Dr. Boers advised.
Dr. Boers his coinvestigators reported having no financial conflicts.
PARIS – Bone loss at 1 year is limited at the low doses of glucocorticoids typically used to treat adults with chronic inflammatory disorders like rheumatoid arthritis, based on a meta-analysis of prospective studies of 1,565 patients with chronic inflammatory diseases (44 studies) and 635 transplant patients (16 studies).
Glucocorticoid treatment at the high doses used in transplantation patients leads to considerable bone loss, especially in the lumbar spine. In contrast, bone loss is limited during glucocorticoid treatment at the lower doses used in chronic inflammatory disease, Dr. Maarten Boers, professor of clinical epidemiology at VU University Medical Center, Amsterdam, reported at the annual European Congress of Rheumatology.
All patients in the meta-analysis had at least two bone mineral density (BMD) measurements over at least 8 months. None received bisphosphonates or antiresorptive therapies, only vitamin D3 and calcium were allowed.
Glucocorticoid doses ranged from 1 to 16 mg/day (mean 9 mg/day) in the patients with chronic inflammatory diseases and from 6 to 53 mg/day (mean 20 mg/day) in the transplant patients. In those with chronic inflammatory diseases, bone loss at the lumbar spine at 1 year averaged –1.7%. For the patients who had measures of femoral neck bone loss, the average loss was –1.3%. In the transplantation group, average bone loss was much higher at –3.6% in the lumbar spine and –3.1% in the femoral neck.
Among the 44 studies that reported BMD in patients with chronic inflammatory diseases, BMD declined from baseline in the lumbar spine by as much as 6% in 1 study and increased by as much as 2% in another. The 39 studies that also reported changes in femoral neck BMD described a decline as high as 7% and an increase as great as 4%.
"We sought to quantify the ‘pure’ effect of GC [glucocorticoid(s)], because so little high-quality information is available," Dr. Boers explained.
The data analysis in these studies was limited to 1 year, but about two-thirds of the patients with chronic inflammatory disease were on chronic glucocorticoid therapy and almost all of the transplant patients were just starting glucocorticoids, Dr. Boers said.
"On average, the yearly loss in a wide range of doses is limited, but starters have more bone loss. The heterogeneity of studies suggests that factors other than GC dose are the main drivers in determining bone loss. Although the data were not available to study these factors, it appears likely that disease activity is very important and acts as a confounder. In other words, disease activity leads to bone loss, and high GC doses lead to bone loss. Effective treatment of high disease activity requires high GC doses, but the interaction of these factors may lead to bone loss comparable to low disease activity being treated with low doses," he explained in an interview.
Many rheumatoid arthritis patients are initially treated with "bridge" glucocorticoid therapy for a few months, until the effect of methotrexate is established, he said. "We prefer ‘COBRA [combination therapy for rheumatoid arthritis] light,’ where patients are initially treated with a higher dose of 30 mg, rapidly tapered to 7.5 mg/day, and maintained for at least 6 months. Several groups are advising to treat for longer periods, and observational data suggest many patients are kept on chronic therapy for periods longer than 1 year," he said.
Local guidelines differ, but all guidelines agree on the necessity of prophylaxis in high-risk situations and on screening for intermediate-risk patients. Unfortunately, many patients requiring antiresorptive treatment according to the guidelines are still not receiving it, with surveys showing about 70% uptake in patients treated by rheumatologists and only about 30% in patients receiving care from other specialists, he said.
"Given the effects of starting GC therapy in our review, we strongly suggest all patients requiring GC therapy for longer than 3 months at any dose should at least be assessed by DXA scan; postmenopausal women, males age 70 or older, and patients with other risk factors should be treated with antiresorptive agents from the start," Dr. Boers advised.
Dr. Boers his coinvestigators reported having no financial conflicts.
PARIS – Bone loss at 1 year is limited at the low doses of glucocorticoids typically used to treat adults with chronic inflammatory disorders like rheumatoid arthritis, based on a meta-analysis of prospective studies of 1,565 patients with chronic inflammatory diseases (44 studies) and 635 transplant patients (16 studies).
Glucocorticoid treatment at the high doses used in transplantation patients leads to considerable bone loss, especially in the lumbar spine. In contrast, bone loss is limited during glucocorticoid treatment at the lower doses used in chronic inflammatory disease, Dr. Maarten Boers, professor of clinical epidemiology at VU University Medical Center, Amsterdam, reported at the annual European Congress of Rheumatology.
All patients in the meta-analysis had at least two bone mineral density (BMD) measurements over at least 8 months. None received bisphosphonates or antiresorptive therapies, only vitamin D3 and calcium were allowed.
Glucocorticoid doses ranged from 1 to 16 mg/day (mean 9 mg/day) in the patients with chronic inflammatory diseases and from 6 to 53 mg/day (mean 20 mg/day) in the transplant patients. In those with chronic inflammatory diseases, bone loss at the lumbar spine at 1 year averaged –1.7%. For the patients who had measures of femoral neck bone loss, the average loss was –1.3%. In the transplantation group, average bone loss was much higher at –3.6% in the lumbar spine and –3.1% in the femoral neck.
Among the 44 studies that reported BMD in patients with chronic inflammatory diseases, BMD declined from baseline in the lumbar spine by as much as 6% in 1 study and increased by as much as 2% in another. The 39 studies that also reported changes in femoral neck BMD described a decline as high as 7% and an increase as great as 4%.
"We sought to quantify the ‘pure’ effect of GC [glucocorticoid(s)], because so little high-quality information is available," Dr. Boers explained.
The data analysis in these studies was limited to 1 year, but about two-thirds of the patients with chronic inflammatory disease were on chronic glucocorticoid therapy and almost all of the transplant patients were just starting glucocorticoids, Dr. Boers said.
"On average, the yearly loss in a wide range of doses is limited, but starters have more bone loss. The heterogeneity of studies suggests that factors other than GC dose are the main drivers in determining bone loss. Although the data were not available to study these factors, it appears likely that disease activity is very important and acts as a confounder. In other words, disease activity leads to bone loss, and high GC doses lead to bone loss. Effective treatment of high disease activity requires high GC doses, but the interaction of these factors may lead to bone loss comparable to low disease activity being treated with low doses," he explained in an interview.
Many rheumatoid arthritis patients are initially treated with "bridge" glucocorticoid therapy for a few months, until the effect of methotrexate is established, he said. "We prefer ‘COBRA [combination therapy for rheumatoid arthritis] light,’ where patients are initially treated with a higher dose of 30 mg, rapidly tapered to 7.5 mg/day, and maintained for at least 6 months. Several groups are advising to treat for longer periods, and observational data suggest many patients are kept on chronic therapy for periods longer than 1 year," he said.
Local guidelines differ, but all guidelines agree on the necessity of prophylaxis in high-risk situations and on screening for intermediate-risk patients. Unfortunately, many patients requiring antiresorptive treatment according to the guidelines are still not receiving it, with surveys showing about 70% uptake in patients treated by rheumatologists and only about 30% in patients receiving care from other specialists, he said.
"Given the effects of starting GC therapy in our review, we strongly suggest all patients requiring GC therapy for longer than 3 months at any dose should at least be assessed by DXA scan; postmenopausal women, males age 70 or older, and patients with other risk factors should be treated with antiresorptive agents from the start," Dr. Boers advised.
Dr. Boers his coinvestigators reported having no financial conflicts.
AT THE EULAR CONGRESS 2014
Key clinical point: One-year bone loss is limited during glucocorticoid treatment at the lower doses used in chronic inflammatory disease.
Major finding: In those with chronic inflammatory diseases, bone loss at the lumbar spine at 1 year averaged –1.7%. For the patients who had measures of femoral neck bone loss, the average loss was –1.3%.
Data source: A meta-analysis of prospective studies of 1,565 patients with chronic inflammatory diseases (44 studies) and 635 transplant patients (16 studies).
Disclosures: Dr. Boers his coinvestigators reported having no financial conflicts.
Hyponatremia linked to osteoporosis, fragility fractures
CHICAGO – Hyponatremia quadruples the risk of osteoporosis and fragility fractures, according to a retrospective database study presented at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
A team from Georgetown University in Washington matched 30,517 patients diagnosed with osteoporosis to 30,517 controls for age, race, sex, and how long they had been in the database of the MedStar Health System, which serves Baltimore and Washington.
The investigators found that patients with chronic hyponatremia – at least two sodium values below 135 mmol/L at least 1 year apart – were far more likely to be later diagnosed with osteoporosis (adjusted odds ratio, 3.99). Recent hyponatremia – at least one value below 135 mmol/L in the previous 30 days – also increased the risk (aOR, 3.08). In contrast, glucocorticoid use, a known osteoporosis risk factor, was associated with a far lower risk (aOR, 1.4), the team reported in a poster session at the meeting.
The researchers had similar results when they matched 46,256 patients with fragility fractures to 46,256 without: The fracture risk was substantially increased in patients with chronic hyponatremia (aOR, 4.71) and recent hyponatremia (aOR, 3.08). A previous diagnosis of osteoporosis – again, a known risk factor – increased the risk only moderately (aOR, 1.8).
The severity of hyponatremia played a role, too; patients with at least one value below 125 mmol/L had the highest risk for osteoporosis and fragility fractures.
The findings were all statistically significant.
"The results of this study support the hypothesis that hyponatremia is a significant and clinically important risk factor for both osteoporosis and bone fractures in inpatients and outpatients," the team concluded.
"We were surprised by the odds ratios and how strong a factor this was. Right now, hyponatremia is not an indication for bone mineral density [testing] because it’s never been considered to be a risk factor. It ought to be added as an indication. Patients with hyponatremia beyond an isolated single event should be evaluated for their bone density and fracture risk" no matter their age or sex, said senior investigator Dr. Joseph Verbalis, chief of the division on endocrinology and metabolism at Georgetown.
Based on the findings, "we [speculate] that early treatment of hyponatremia will prevent progression of bone disease and decrease fracture risk," and perhaps even obviate the need for bisphosphonates. "It’s an implication that needs to be followed up with definitive studies," he said.
Chronic hyponatremia increases osteoclast proliferation and activity, while recent hyponatremia reduces reaction time and makes it less likely people will catch themselves if they stumble. Elderly people are most at risk, either from overzealous salt restriction, sodium-depleting drugs like thiazide diuretics, or the syndrome of inappropriate antidiuretic hormone secretion (Indian J. Endocrinol. Metab. 2011;15(Suppl3):S208-S215).
The mean age of subjects in the osteoporosis analysis was about 75 years, and almost 90% were women. In the fragility fracture analysis, the mean age was about 60 years, and just over half the subjects were women.
Among the roughly 3 million patients the team initially sampled at the start of their work, there was a more than twofold increase in the prevalence of osteoporosis in hyponatremic (4.6%) vs. nonhyponatremic (1.8%) subjects, and a similar increase in vertebral or long-bone fractures (9.5% vs. 3.7%).
Dr. Verbalis is a consultant, investigator, speaker, and adviser for Otsuka, the maker of the hyponatremia drug tolvaptan. He is also a consultant for Cornerstone Therapeutics and Ferring Pharmaceuticals. The study had no outside funding.
CHICAGO – Hyponatremia quadruples the risk of osteoporosis and fragility fractures, according to a retrospective database study presented at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
A team from Georgetown University in Washington matched 30,517 patients diagnosed with osteoporosis to 30,517 controls for age, race, sex, and how long they had been in the database of the MedStar Health System, which serves Baltimore and Washington.
The investigators found that patients with chronic hyponatremia – at least two sodium values below 135 mmol/L at least 1 year apart – were far more likely to be later diagnosed with osteoporosis (adjusted odds ratio, 3.99). Recent hyponatremia – at least one value below 135 mmol/L in the previous 30 days – also increased the risk (aOR, 3.08). In contrast, glucocorticoid use, a known osteoporosis risk factor, was associated with a far lower risk (aOR, 1.4), the team reported in a poster session at the meeting.
The researchers had similar results when they matched 46,256 patients with fragility fractures to 46,256 without: The fracture risk was substantially increased in patients with chronic hyponatremia (aOR, 4.71) and recent hyponatremia (aOR, 3.08). A previous diagnosis of osteoporosis – again, a known risk factor – increased the risk only moderately (aOR, 1.8).
The severity of hyponatremia played a role, too; patients with at least one value below 125 mmol/L had the highest risk for osteoporosis and fragility fractures.
The findings were all statistically significant.
"The results of this study support the hypothesis that hyponatremia is a significant and clinically important risk factor for both osteoporosis and bone fractures in inpatients and outpatients," the team concluded.
"We were surprised by the odds ratios and how strong a factor this was. Right now, hyponatremia is not an indication for bone mineral density [testing] because it’s never been considered to be a risk factor. It ought to be added as an indication. Patients with hyponatremia beyond an isolated single event should be evaluated for their bone density and fracture risk" no matter their age or sex, said senior investigator Dr. Joseph Verbalis, chief of the division on endocrinology and metabolism at Georgetown.
Based on the findings, "we [speculate] that early treatment of hyponatremia will prevent progression of bone disease and decrease fracture risk," and perhaps even obviate the need for bisphosphonates. "It’s an implication that needs to be followed up with definitive studies," he said.
Chronic hyponatremia increases osteoclast proliferation and activity, while recent hyponatremia reduces reaction time and makes it less likely people will catch themselves if they stumble. Elderly people are most at risk, either from overzealous salt restriction, sodium-depleting drugs like thiazide diuretics, or the syndrome of inappropriate antidiuretic hormone secretion (Indian J. Endocrinol. Metab. 2011;15(Suppl3):S208-S215).
The mean age of subjects in the osteoporosis analysis was about 75 years, and almost 90% were women. In the fragility fracture analysis, the mean age was about 60 years, and just over half the subjects were women.
Among the roughly 3 million patients the team initially sampled at the start of their work, there was a more than twofold increase in the prevalence of osteoporosis in hyponatremic (4.6%) vs. nonhyponatremic (1.8%) subjects, and a similar increase in vertebral or long-bone fractures (9.5% vs. 3.7%).
Dr. Verbalis is a consultant, investigator, speaker, and adviser for Otsuka, the maker of the hyponatremia drug tolvaptan. He is also a consultant for Cornerstone Therapeutics and Ferring Pharmaceuticals. The study had no outside funding.
CHICAGO – Hyponatremia quadruples the risk of osteoporosis and fragility fractures, according to a retrospective database study presented at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
A team from Georgetown University in Washington matched 30,517 patients diagnosed with osteoporosis to 30,517 controls for age, race, sex, and how long they had been in the database of the MedStar Health System, which serves Baltimore and Washington.
The investigators found that patients with chronic hyponatremia – at least two sodium values below 135 mmol/L at least 1 year apart – were far more likely to be later diagnosed with osteoporosis (adjusted odds ratio, 3.99). Recent hyponatremia – at least one value below 135 mmol/L in the previous 30 days – also increased the risk (aOR, 3.08). In contrast, glucocorticoid use, a known osteoporosis risk factor, was associated with a far lower risk (aOR, 1.4), the team reported in a poster session at the meeting.
The researchers had similar results when they matched 46,256 patients with fragility fractures to 46,256 without: The fracture risk was substantially increased in patients with chronic hyponatremia (aOR, 4.71) and recent hyponatremia (aOR, 3.08). A previous diagnosis of osteoporosis – again, a known risk factor – increased the risk only moderately (aOR, 1.8).
The severity of hyponatremia played a role, too; patients with at least one value below 125 mmol/L had the highest risk for osteoporosis and fragility fractures.
The findings were all statistically significant.
"The results of this study support the hypothesis that hyponatremia is a significant and clinically important risk factor for both osteoporosis and bone fractures in inpatients and outpatients," the team concluded.
"We were surprised by the odds ratios and how strong a factor this was. Right now, hyponatremia is not an indication for bone mineral density [testing] because it’s never been considered to be a risk factor. It ought to be added as an indication. Patients with hyponatremia beyond an isolated single event should be evaluated for their bone density and fracture risk" no matter their age or sex, said senior investigator Dr. Joseph Verbalis, chief of the division on endocrinology and metabolism at Georgetown.
Based on the findings, "we [speculate] that early treatment of hyponatremia will prevent progression of bone disease and decrease fracture risk," and perhaps even obviate the need for bisphosphonates. "It’s an implication that needs to be followed up with definitive studies," he said.
Chronic hyponatremia increases osteoclast proliferation and activity, while recent hyponatremia reduces reaction time and makes it less likely people will catch themselves if they stumble. Elderly people are most at risk, either from overzealous salt restriction, sodium-depleting drugs like thiazide diuretics, or the syndrome of inappropriate antidiuretic hormone secretion (Indian J. Endocrinol. Metab. 2011;15(Suppl3):S208-S215).
The mean age of subjects in the osteoporosis analysis was about 75 years, and almost 90% were women. In the fragility fracture analysis, the mean age was about 60 years, and just over half the subjects were women.
Among the roughly 3 million patients the team initially sampled at the start of their work, there was a more than twofold increase in the prevalence of osteoporosis in hyponatremic (4.6%) vs. nonhyponatremic (1.8%) subjects, and a similar increase in vertebral or long-bone fractures (9.5% vs. 3.7%).
Dr. Verbalis is a consultant, investigator, speaker, and adviser for Otsuka, the maker of the hyponatremia drug tolvaptan. He is also a consultant for Cornerstone Therapeutics and Ferring Pharmaceuticals. The study had no outside funding.
AT ICE/ENDO 2014
Key clinical point: Early treatment of hyponatremia may one day prove to slow the progression of osteoporosis and reduce the need for bisphosphonates.
Major finding: In patients with chronic hyponatremia, the adjusted odds ratio for developing osteoporosis was 3.99, compared with those without.
Data Source: Retrospective case-control study involving more than 150,000 subjects.
Disclosures: Dr. Verbalis is a consultant, investigator, speaker, and adviser for Otsuka, the maker of the hyponatremia drug tolvaptan. The study had no outside funding.
Monitor elderly for bone loss after gastric bypass
CHICAGO – Older adults who undergo Roux-en-Y gastric bypass are at risk for lessened bone mineral density for at least 2 years after their surgery and should be monitored appropriately for osteoporosis or fragility fractures, according to investigators from Massachusetts General Hospital in Boston.
Two years postoperatively, vertebral bone mineral density (BMD) in 30 patients was about 7% lower on quantitative computed tomography (QCT) and 6% lower on dual-energy x-ray absorptiometry (DXA) – both methods were used to ensure accuracy – when compared with 20 well-matched, morbidly obese controls. Total hip BMD was 6% lower on QCT and 10% lower on DXA. Femoral neck BMD was about 6% lower by both measures.
Biomarkers of bone turnover remained markedly elevated in surgery patients, as well, but unchanged in controls (C-telopeptide 0.65 ng/mL vs. 0.3 ng/mL; amino-terminal propeptide of type I collagen 65 ng/mL vs. 40 ng/mL). The findings were all statistically significant.
The groups started to separate early on BMD, and there’s concern that bone loss will continue for more than 2 years after surgery. Preoperatively, most Roux-en-Y gastric bypass patients have a higher than normal BMD, "so even a loss of 10% over 2 years is not going to put most of them in the osteopenic or osteoporotic range. The caveat now is that we are [offering surgery] to older patients and adolescents. Elderly patients are starting with lower bone mass, so there are concerns about [post-op] skeletal fragility. The oldest patient in our study was 72. She became osteoporotic after surgery because her bone density was low to begin with," said lead investigator Dr. Elaine W. Yu, an endocrinologist at Massachusetts General.
"In adolescents, there are implications for achieving peak bone mass. Even if you have a normal bone density 2 years after surgery, what’s going to happen in 10 years, 20 years?" she asked.
In short, "people should pay attention to bone density and bone loss and discuss this as one of the potential negative effects of bariatric surgery. For patients at risk, you should definitely consider serial bone density monitoring and osteoporosis therapy if needed," she said at the joint meeting of the International Society of Endocrinology and the Endocrine Society.
At baseline, both subjects and controls were about 47 years old and 270 pounds, with a mean a mean body mass index of 45 kg/m2. About 85% were women. The study excluded patients with histories of bone disorders or use of bone-affecting medications.
Surgery patients lost a mean of about 85 pounds in the first 6 months; their weight loss then stabilized, but they continued to lose bone. Controls stayed about the same weight.
The findings can’t be explained by post-op calcium or vitamin D depletion. "These subjects were aggressively supplemented with both," and both groups maintained normal levels throughout the study. Also, there were no statistical differences in parathyroid hormone levels between the groups.
"Our theory is that there are changes in gut hormones after gastric bypass that have direct effects on bone, like ghrelin," Dr. Yu said.
The National Institutes of Health funded the work. Dr. Yu is a consultant for Amgen.
CHICAGO – Older adults who undergo Roux-en-Y gastric bypass are at risk for lessened bone mineral density for at least 2 years after their surgery and should be monitored appropriately for osteoporosis or fragility fractures, according to investigators from Massachusetts General Hospital in Boston.
Two years postoperatively, vertebral bone mineral density (BMD) in 30 patients was about 7% lower on quantitative computed tomography (QCT) and 6% lower on dual-energy x-ray absorptiometry (DXA) – both methods were used to ensure accuracy – when compared with 20 well-matched, morbidly obese controls. Total hip BMD was 6% lower on QCT and 10% lower on DXA. Femoral neck BMD was about 6% lower by both measures.
Biomarkers of bone turnover remained markedly elevated in surgery patients, as well, but unchanged in controls (C-telopeptide 0.65 ng/mL vs. 0.3 ng/mL; amino-terminal propeptide of type I collagen 65 ng/mL vs. 40 ng/mL). The findings were all statistically significant.
The groups started to separate early on BMD, and there’s concern that bone loss will continue for more than 2 years after surgery. Preoperatively, most Roux-en-Y gastric bypass patients have a higher than normal BMD, "so even a loss of 10% over 2 years is not going to put most of them in the osteopenic or osteoporotic range. The caveat now is that we are [offering surgery] to older patients and adolescents. Elderly patients are starting with lower bone mass, so there are concerns about [post-op] skeletal fragility. The oldest patient in our study was 72. She became osteoporotic after surgery because her bone density was low to begin with," said lead investigator Dr. Elaine W. Yu, an endocrinologist at Massachusetts General.
"In adolescents, there are implications for achieving peak bone mass. Even if you have a normal bone density 2 years after surgery, what’s going to happen in 10 years, 20 years?" she asked.
In short, "people should pay attention to bone density and bone loss and discuss this as one of the potential negative effects of bariatric surgery. For patients at risk, you should definitely consider serial bone density monitoring and osteoporosis therapy if needed," she said at the joint meeting of the International Society of Endocrinology and the Endocrine Society.
At baseline, both subjects and controls were about 47 years old and 270 pounds, with a mean a mean body mass index of 45 kg/m2. About 85% were women. The study excluded patients with histories of bone disorders or use of bone-affecting medications.
Surgery patients lost a mean of about 85 pounds in the first 6 months; their weight loss then stabilized, but they continued to lose bone. Controls stayed about the same weight.
The findings can’t be explained by post-op calcium or vitamin D depletion. "These subjects were aggressively supplemented with both," and both groups maintained normal levels throughout the study. Also, there were no statistical differences in parathyroid hormone levels between the groups.
"Our theory is that there are changes in gut hormones after gastric bypass that have direct effects on bone, like ghrelin," Dr. Yu said.
The National Institutes of Health funded the work. Dr. Yu is a consultant for Amgen.
CHICAGO – Older adults who undergo Roux-en-Y gastric bypass are at risk for lessened bone mineral density for at least 2 years after their surgery and should be monitored appropriately for osteoporosis or fragility fractures, according to investigators from Massachusetts General Hospital in Boston.
Two years postoperatively, vertebral bone mineral density (BMD) in 30 patients was about 7% lower on quantitative computed tomography (QCT) and 6% lower on dual-energy x-ray absorptiometry (DXA) – both methods were used to ensure accuracy – when compared with 20 well-matched, morbidly obese controls. Total hip BMD was 6% lower on QCT and 10% lower on DXA. Femoral neck BMD was about 6% lower by both measures.
Biomarkers of bone turnover remained markedly elevated in surgery patients, as well, but unchanged in controls (C-telopeptide 0.65 ng/mL vs. 0.3 ng/mL; amino-terminal propeptide of type I collagen 65 ng/mL vs. 40 ng/mL). The findings were all statistically significant.
The groups started to separate early on BMD, and there’s concern that bone loss will continue for more than 2 years after surgery. Preoperatively, most Roux-en-Y gastric bypass patients have a higher than normal BMD, "so even a loss of 10% over 2 years is not going to put most of them in the osteopenic or osteoporotic range. The caveat now is that we are [offering surgery] to older patients and adolescents. Elderly patients are starting with lower bone mass, so there are concerns about [post-op] skeletal fragility. The oldest patient in our study was 72. She became osteoporotic after surgery because her bone density was low to begin with," said lead investigator Dr. Elaine W. Yu, an endocrinologist at Massachusetts General.
"In adolescents, there are implications for achieving peak bone mass. Even if you have a normal bone density 2 years after surgery, what’s going to happen in 10 years, 20 years?" she asked.
In short, "people should pay attention to bone density and bone loss and discuss this as one of the potential negative effects of bariatric surgery. For patients at risk, you should definitely consider serial bone density monitoring and osteoporosis therapy if needed," she said at the joint meeting of the International Society of Endocrinology and the Endocrine Society.
At baseline, both subjects and controls were about 47 years old and 270 pounds, with a mean a mean body mass index of 45 kg/m2. About 85% were women. The study excluded patients with histories of bone disorders or use of bone-affecting medications.
Surgery patients lost a mean of about 85 pounds in the first 6 months; their weight loss then stabilized, but they continued to lose bone. Controls stayed about the same weight.
The findings can’t be explained by post-op calcium or vitamin D depletion. "These subjects were aggressively supplemented with both," and both groups maintained normal levels throughout the study. Also, there were no statistical differences in parathyroid hormone levels between the groups.
"Our theory is that there are changes in gut hormones after gastric bypass that have direct effects on bone, like ghrelin," Dr. Yu said.
The National Institutes of Health funded the work. Dr. Yu is a consultant for Amgen.
AT ICE/ENDO 2014
Key clinical point: Gastric bypass puts some older patients at risk for osteoporosis.
Major finding: Two years after surgery, spine BMD in 30 patients was about 7% lower on quantitative CT and 6% lower on DXA when compared with 20 well-matched, morbidly obese controls.
Data Source: Retrospective case-control study with 2 years follow-up
Disclosures: The work was funded the National Institutes of Health. Dr. Yu is a consultant for Amgen.