User login
Caring for women with pelvic floor disorders during pregnancy and postpartum: Expert guidance
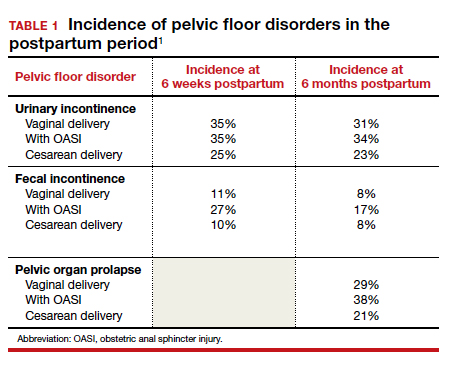
Pelvic floor disorders (PFDs) affect many pregnant and newly postpartum women. These conditions, including urinary incontinence, anal incontinence, and pelvic organ prolapse (POP), can be overshadowed by common pregnancy and postpartum concerns (TABLE 1).1 With the use of a few quick screening questions, however, PFDs easily can be identified in this at-risk population. Active management need not be delayed until after delivery for women experiencing bother, as options exist for women with PFDs during pregnancy as well as postpartum.
In this article, we discuss the common PFDs that obstetric clinicians face in the context of case scenarios and review how you can be better equipped to care for affected individuals.
CASE 1 Screening
A 30-year-old woman (G1P1) presents for her routine postpartum visit after an operative vaginal delivery with a second-degree laceration.
How would you screen this patient for PFDs?
Why screening for PFDs matters
While there are no validated PFD screening tools for this patient population, clinicians can ask a series of brief open-ended questions as part of the review of systems to efficiently evaluate for the common PFDs in peripartum patients (see “Screening questions to evaluate patients for peripartum pelvic floor disorders” below).
Pelvic floor disorders in the peripartum period can have a significant negative impact. In pregnancy, nearly half of women report psychological strain due to the presence of bowel, bladder, prolapse, or sexual dysfunction symptoms.2 Postpartum, PFDs have negative effects on overall health, well-being, and self-esteem, with significantly increased rates of postpartum depression in women who experience urinary incontinence.3,4 Proactively inquiring about PFD symptoms, providing anticipatory guidance, and recommending treatment options can positively impact a patient in multiple domains.
Sometimes during pregnancy or after having a baby, a woman experiences pelvic floor symptoms. Do you have any of the following?
- leakage with coughing, laughing, sneezing, or physical activity
- urgency to urinate or leakage due to urgency
- bulging or pressure within the vagina
- pain with intercourse
- accidental bowel leakage of stool or flatus
CASE 2 Stress urinary incontinence
A 27-year-old woman (G1P1) presents 2 months following spontaneous vaginal delivery with symptoms of urine leakage with laughing and running. Her urinary incontinence has been improving since delivery, but it continues to be bothersome.
What would you recommend for this patient?
Conservative SUI management strategies in pregnancy
Urinary tract symptoms are common in pregnancy, with up to 41.8% of women reporting urinary symptom distress in the third trimester.5 During pregnancy, estrogen and progesterone decrease urethral pressure that, together with increased intra-abdominal pressure from the gravid uterus, can cause or worsen stress urinary incontinence (SUI).6
During pregnancy, women should be offered conservative therapies for SUI. For women who can perform a pelvic floor contraction (a Kegel exercise), self-guided pelvic floor muscle exercises (PFMEs) may be helpful (see “Pelvic floor muscle exercises” below). We recommend that women start with 1 to 2 sets of 10 Kegel exercises per day and that they hold the squeeze for 2 to 3 seconds, working up to holding for 10 seconds. The goal is to strengthen and improve muscle control so that the Kegel squeeze can be paired with activities that cause SUI.
For women who are unable to perform a Kegel exercise or are not improving with a home PFME regimen, referral to pelvic floor physical therapy (PFPT) can be considered. While data support the efficacy of PFPT for SUI treatment in nonpregnant women,7 data are lacking on PFME in pregnancy.
In women without urinary incontinence, PFME in early pregnancy can prevent the onset of incontinence in late pregnancy and the postpartum period.8 By contrast, the same 2020 Cochrane Review found no evidence that antenatal pelvic floor muscle therapy in incontinent women decreases incontinence in mid- or late-pregnancy or in the postpartum period.8 As the quality of this evidence is very low and there is no evidence of harm with PFME, we continue to recommend it for women with bothersome SUI.
Incontinence pessaries or vaginal inserts (such as Poise Impressa bladder supports) can be helpful for SUI treatment. An incontinence pessary can be fitted in the office, and fitting kits are available for both. Pessaries can safely be used in pregnancy, but there are no data on the efficacy of pessaries for treating SUI in pregnancy. In nonpregnant women, evidence demonstrates 63% satisfaction 3 months post–pessary placement for SUI.7
We do not recommend invasive procedures for the treatment of SUI during pregnancy or in the first 6 months following delivery. There is no evidence that elective cesarean delivery prevents persistent SUI postpartum.9
To identify and engage the proper pelvic floor muscles:
- Insert a finger in the vagina and squeeze the vaginal muscles around your finger.
- Imagine you are sitting on a marble and have to pick it up with the vaginal muscles.
- Squeeze the muscles you would use to stop the flow of urine or hold back flatulence.
Perform sets of 10, 2 to 3 times per day as follows:
- Squeeze: Engage the pelvic floor muscles as described above; avoid performing Kegels while voiding.
- Hold: For 2 to 10 seconds; increase the duration to 10 seconds as able.
- Relax: Completely relax muscles before initating the next squeeze.
Reference
1. UpToDate. Patient education: pelvic muscle (Kegel) exercises (the basics). 2018. https://uptodatefree.ir/topic.htm?path=pelvic-muscle-kegel-exercises-the-basics. Accessed February 24, 2021.
Continue to: Managing SUI in the postpartum period...
Managing SUI in the postpartum period
After the first 6 months postpartum and exhaustion of conservative measures, we offer surgical interventions for women with persistent, bothersome incontinence. Surgery for SUI typically is not recommended until childbearing is complete, but it can be considered if the patient’s bother is significant.
For women with bothersome SUI who still desire future pregnancy, management options include periurethral bulking, a retropubic urethropexy (Burch procedure), or a midurethral sling procedure. Women who undergo an anti-incontinence procedure have an increased risk for urinary retention during a subsequent pregnancy.10 Most women with a midurethral sling will continue to be continent following an obstetric delivery.
Anticipatory guidance
At 3 months postpartum, the incidence of urinary incontinence is 6% to 40%, depending on parity and delivery type. Postpartum urinary incontinence is most common after instrumented vaginal delivery (32%) followed by spontaneous vaginal delivery (28%) and cesarean delivery (15%). The mean prevalence of any type of urinary incontinence is 33% at 3 months postpartum, and only small changes in the rate of urinary incontinence occur over the first postpartum year.11 While urinary incontinence is common postpartum, it should not be considered normal. We counsel that symptoms may improve spontaneously, but treatment can be initiated if the patient experiences significant bother.
A longitudinal cohort study that followed women from 3 months to 12 years postpartum found that, of women with urinary incontinence at 3 months postpartum, 76% continued to report incontinence at 12 years postpartum.12 We recommend that women be counseled that, even when symptoms resolve, they remain at increased risk for urinary incontinence in the future. Invasive therapies should be used to treat bothersome urinary incontinence, not to prevent future incontinence.
CASE 3 Fecal incontinence
A 24-year-old woman (G1P1) presents 3 weeks postpartum following a forceps-assisted vaginal delivery complicated by a 3c laceration. She reports fecal urgency, inability to control flatus, and once-daily fecal incontinence.
How would you evaluate these symptoms?
Steps in evaluation
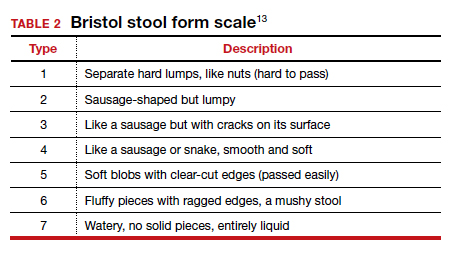
The initial evaluation should include an inquiry regarding the patient’s stool consistency and bowel regimen. The Bristol stool form scale can be used to help patients describe their typical bowel movements (TABLE 2).13 During healing, the goal is to achieve a Bristol type 4 stool, both to avoid straining and to improve continence, as loose stool is the most difficult to control.
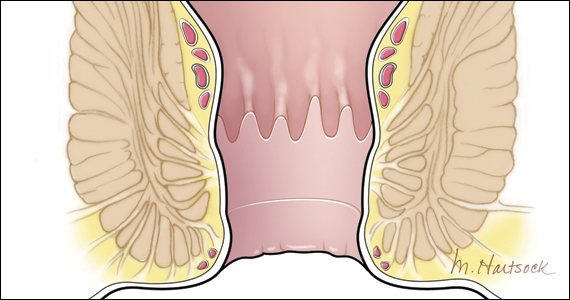
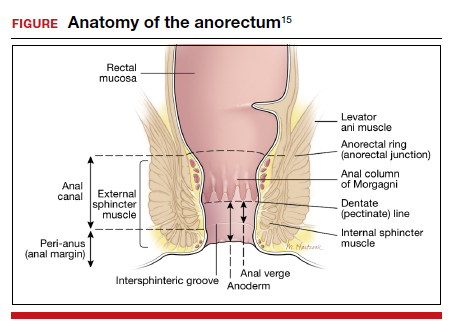
A physical examination can evaluate healing and sphincter integrity; it should include inspection of the distal vagina and perineal body and a digital rectal exam. Anal canal resting tone and squeeze strength should be evaluated, and the digital rectal examination scoring system (DRESS) can be useful for quantification (TABLE 3).14 Lack of tone at rest in the anterolateral portion of the sphincter complex can indicate an internal anal sphincter defect, as 80% of the resting tone comes from this muscle (FIGURE).15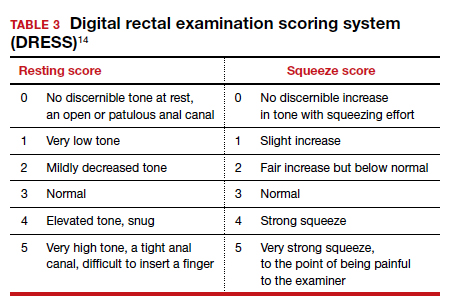
The rectovaginal septum should be assessed given the increased risk of rectovaginal fistula in women with obstetric anal sphincter injury (OASI). The patient should be instructed to contract the anal sphincter, allowing evaluation of muscular contraction. Lack of contraction anteriolaterally may indicate external anal sphincter separation.
Continue to: Conservative options for improving fecal incontinence symptoms...
Conservative options for improving fecal incontinence symptoms
The patient can be counseled regarding stool bulking, first with insoluble fiber supplementation and cessation of stool softeners if she is incontinent of liquid stool. If these measures are not effective, use of a constipating agent, such as loperamide, can improve stool consistency and thereby decrease incontinence episodes. PFPT with biofeedback can be offered as well. While typically we do not recommend initiating PFPT before 6 weeks postpartum, so the initial phases of healing can occur, early referral enables the patient to avoid a delay in access to care.
The patient also can be counseled about a referral to a pelvic floor specialist for further evaluation. A variety of peripartum pelvic floor disorder clinics are being established by Female Pelvic Medicine and Reconstructive Surgery (FPMRS) physicians. These clinics provide the benefit of comprehensive care for pelvic floor disorders in this unique population.
When conservative measures fail. If a patient has persistent bowel control issues despite conservative measures, a referral to an FPMRS physician should be initiated.
Delivery route in future pregnancies
The risk of a subsequent OASI is low. While this means that many women can safely pursue a future vaginal delivery, a scheduled cesarean delivery is indicated for women with persistent bowel control issues, wound healing complications, and those who experienced psychological trauma from their delivery.16 We recommend a shared-decision making approach, reviewing modifiable and nonmodifiable risk factors to help determine whether or not a future vaginal birth is appropriate. It is important to highlight that a cesarean delivery does not protect against fecal incontinence in women with a history of OASI; however, there is benefit in preventing worsening of anal incontinence, if present.17
CASE 4 Uterovaginal prolapse
A 36-year-old woman (G3P3) presents for her routine postpartum visit at 6 weeks after a spontaneous vaginal delivery without lacerations. She reports a persistent feeling of vaginal pressure and fullness. She thinks she felt a bulge with wiping after a bowel movement.
What options are available for this patient?
Prolapse in the peripartum population
Previous studies have revealed an increased prevalence of POP in pregnant women on examination compared with their nulligravid counterparts (47.6% vs 0%).18 With the changes in the hormonal milieu in pregnancy, as well as the weight of the gravid uterus on the pelvic floor, it is not surprising that pregnancy may be the inciting event to expose even transient defects in pelvic organ support.19
It is well established that increasing parity and, to a lesser extent, larger babies are associated with increased risk for future POP and surgery for prolapse. In the first year postpartum, nearly one-third of women have stage 2 or greater prolapse on exam, with studies demonstrating an increased prevalence of postpartum POP in women who delivered vaginally compared with those who delivered by cesarean.20,21
Initial evaluation
Diagnosis can be made during a routine pelvic exam by having the patient perform a Valsalva maneuver while in the lithotomy position. Using half of a speculum permits evaluation of the anterior and posterior vaginal walls separately, and Valsalva during a bimanual exam can aid in evaluating descensus of the uterus and cervix.
Excellent free patient education resources available online through the American Urogynecologic Society and the International Urogynecological Association can be used to direct counseling.
Continue to: Treatments you can offer for POP...
Treatments you can offer for POP
For pregnant or postpartum patients with bothersome prolapse, initial management options include pessary fitting and/or PFPT referral. In pregnancy, women often can be successfully fitted with a pessary for POP; however, as expulsion is a common issue, selection of a stiffer or space-occupying device may be more efficacious.
Often, early onset POP in pregnancy resolves as the gravid uterus lifts out of the pelvis in the second trimester, at which time the pessary can be discontinued. In the postpartum period, a pessary fitting can be undertaken similarly to that in nonpregnant patients. While data are lacking in the peripartum population, evidence supports the positive impact of PFPT on improving POP symptom bother.22 Additionally, for postpartum women who experience OASI, PFPT can produce significant improvement in subjective POP and associated bother.23
Impact of future childbearing wishes on treatment
The desire for future childbearing does not preclude treatment of patients experiencing bother from POP after conservative management options have failed. Both vaginal native tissue and mesh-augmented uterine-sparing repairs are performed by many FPMRS specialists and are associated with good outcomes. As with SUI, we do not recommend invasive treatment for POP during pregnancy or before 6 months postpartum.
In conclusion
Obstetric specialists play an essential role in caring for women with PFDs in the peripartum period. Basic evaluation, counseling, and management can be initiated using many of the resources already available in an obstetric ambulatory practice. Important adjunctive resources include those available for both providers and patients through the American Urogynecologic Society and the International Urogynecological Association. In addition, clinicians can partner with pelvic floor specialists through the growing number of FPMRS-run peripartum pelvic floor disorder clinics across the country and pelvic floor physical therapists.
If these specialty clinics and therapists are not available in your area, FPMRS specialists, urologists, gastroenterologists, and/or colorectal surgeons can aid in patient diagnosis and management to reach the ultimate goal of improving PFDs in this at-risk population. ●
- Madsen AM, Hickman LC, Propst K. Recognition and management of pelvic floor disorders in pregnancy and the postpartum period. Obstet Gynecol Clin North Am. Forthcoming 2021.
- Bodner-Adler B, Kimberger O, Laml T, et al. Prevalence and risk factors for pelvic floor disorders during early and late pregnancy in a cohort of Austrian women. Arch Gynecol Obstet. 2019;300:1325-1330.
- Swenson CW, DePorre JA, Haefner JK, et al. Postpartum depression screening and pelvic floor symptoms among women referred to a specialty postpartum perineal clinic. Am J Obstet Gynecol. 2018;218:335.e1-335.e6.
- Skinner EM, Dietz HP. Psychological and somatic sequelae of traumatic vaginal delivery: a literature review. Aust N Z J Obstet Gynaecol. 2015;55:309-314.
- Yohay D, Weintraub AY, Mauer-Perry N, et al. Prevalence and trends of pelvic floor disorders in late pregnancy and after delivery in a cohort of Israeli women using the PFDI-20. Eur J Obstet Gynecol Reprod Biol. 2016;200:35-39.
- Gregory WT, Sibai BM. Obstetrics and pelvic floor disorders. In: Walters M, Karram M, eds. Urogynecology and Reconstructive Pelvic Surgery. 4th ed. Philadelphia, PA: Saunders; 2015:224-237.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Woodley SJ, Lawrenson P, Boyle R, et al. Pelvic floor muscle training for preventing and treating urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev. 2020;6:CD007471.
- Foldspang A, Hvidman L, Mommsen S, et al. Risk of postpartum urinary incontinence associated with pregnancy and mode of delivery. Acta Obstet Gynecol Scand. 2004;83:923-927.
- Wieslander CK, Weinstein MM, Handa V, et al. Pregnancy in women with prior treatments for pelvic floor disorders. Female Pelvic Med Reconstr Surg. 2020;26:299-305.
- Thom DH, Rortveit G. Prevalence of postpartum urinary incontinence: a systematic review. Acta Obstet Gynecol Scand. 2010;89:1511-1522.
- MacArthur C, Wilson D, Herbison P, et al; Prolong Study Group. Urinary incontinence persisting after childbirth: extent, delivery history, and effects in a 12-year longitudinal cohort study. BJOG. 2016;123:1022-1029.
- Blake MR, Raker JM, Whelan K. Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44:693-703
- Orkin BA, Sinykin SB, Lloyd PC. The digital rectal examination scoring system (DRESS). Dis Colon Rectum. 2010;53:1656-1660.
- UpToDate. Repair of episiotomy and perineal lacerations associated with childbirth. 2020. https://www-uptodate-com .ccmain.ohionet.org/contents/repair-of-perineal-and-other -lacerations-associated-with-childbirth?search=repair%20 episiotomy&source=search_result&selectedTitle=1~150&usa ge_type=default&display_rank=1. Accessed February 28, 2021.
- Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 198: prevention and management of obstetric lacerations at vaginal delivery. Obstet Gynecol. 2018;132:e87-e102.
- Jangö H, Langhoff-Roos J, Rosthøj S, et al. Long-term anal incontinence after obstetric anal sphincter injury—does grade of tear matter? Am J Obstet Gynecol. 2018;218:232.e1-232.e10.
- O’Boyle AL, Woodman PJ, O’Boyle JD, et al. Pelvic organ support in nulliparous pregnant and nonpregnant women: a case control study. Am J Obstet Gynecol. 2002;187:99-102.
- Handa VL, Blomquist JL, McDermott KC, et al. Pelvic floor disorders after vaginal birth. Obstet Gynecol. 2012;119 (2, pt 1):233-239.
- Handa VL, Nygaard I, Kenton K, et al; Pelvic Floor Disorders Network. Pelvic organ support among primiparous women in the first year after childbirth. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:1407-1411.
- O’Boyle AL, O’Boyle JD, Calhoun B, et al. Pelvic organ support in pregnancy and postpartum. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:69-72.
- Hagen S, Stark D, Glazener C, et al; POPPY Trial Collaborators. Individualised pelvic floor muscle training in women with pelvic organ prolapse (POPPY): a multicentre randomised controlled trial. Lancet. 2014;383:796-806.
- Von Bargen E, Haviland MJ, Chang OH, et al. Evaluation of postpartum pelvic floor physical therapy on obstetrical anal sphincter injury: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2020. doi: 10.1097/SPV.0000000000000849.
Pelvic floor disorders (PFDs) affect many pregnant and newly postpartum women. These conditions, including urinary incontinence, anal incontinence, and pelvic organ prolapse (POP), can be overshadowed by common pregnancy and postpartum concerns (TABLE 1).1 With the use of a few quick screening questions, however, PFDs easily can be identified in this at-risk population. Active management need not be delayed until after delivery for women experiencing bother, as options exist for women with PFDs during pregnancy as well as postpartum.

In this article, we discuss the common PFDs that obstetric clinicians face in the context of case scenarios and review how you can be better equipped to care for affected individuals.
CASE 1 Screening
A 30-year-old woman (G1P1) presents for her routine postpartum visit after an operative vaginal delivery with a second-degree laceration.
How would you screen this patient for PFDs?
Why screening for PFDs matters
While there are no validated PFD screening tools for this patient population, clinicians can ask a series of brief open-ended questions as part of the review of systems to efficiently evaluate for the common PFDs in peripartum patients (see “Screening questions to evaluate patients for peripartum pelvic floor disorders” below).
Pelvic floor disorders in the peripartum period can have a significant negative impact. In pregnancy, nearly half of women report psychological strain due to the presence of bowel, bladder, prolapse, or sexual dysfunction symptoms.2 Postpartum, PFDs have negative effects on overall health, well-being, and self-esteem, with significantly increased rates of postpartum depression in women who experience urinary incontinence.3,4 Proactively inquiring about PFD symptoms, providing anticipatory guidance, and recommending treatment options can positively impact a patient in multiple domains.
Sometimes during pregnancy or after having a baby, a woman experiences pelvic floor symptoms. Do you have any of the following?
- leakage with coughing, laughing, sneezing, or physical activity
- urgency to urinate or leakage due to urgency
- bulging or pressure within the vagina
- pain with intercourse
- accidental bowel leakage of stool or flatus
CASE 2 Stress urinary incontinence
A 27-year-old woman (G1P1) presents 2 months following spontaneous vaginal delivery with symptoms of urine leakage with laughing and running. Her urinary incontinence has been improving since delivery, but it continues to be bothersome.
What would you recommend for this patient?
Conservative SUI management strategies in pregnancy
Urinary tract symptoms are common in pregnancy, with up to 41.8% of women reporting urinary symptom distress in the third trimester.5 During pregnancy, estrogen and progesterone decrease urethral pressure that, together with increased intra-abdominal pressure from the gravid uterus, can cause or worsen stress urinary incontinence (SUI).6
During pregnancy, women should be offered conservative therapies for SUI. For women who can perform a pelvic floor contraction (a Kegel exercise), self-guided pelvic floor muscle exercises (PFMEs) may be helpful (see “Pelvic floor muscle exercises” below). We recommend that women start with 1 to 2 sets of 10 Kegel exercises per day and that they hold the squeeze for 2 to 3 seconds, working up to holding for 10 seconds. The goal is to strengthen and improve muscle control so that the Kegel squeeze can be paired with activities that cause SUI.
For women who are unable to perform a Kegel exercise or are not improving with a home PFME regimen, referral to pelvic floor physical therapy (PFPT) can be considered. While data support the efficacy of PFPT for SUI treatment in nonpregnant women,7 data are lacking on PFME in pregnancy.
In women without urinary incontinence, PFME in early pregnancy can prevent the onset of incontinence in late pregnancy and the postpartum period.8 By contrast, the same 2020 Cochrane Review found no evidence that antenatal pelvic floor muscle therapy in incontinent women decreases incontinence in mid- or late-pregnancy or in the postpartum period.8 As the quality of this evidence is very low and there is no evidence of harm with PFME, we continue to recommend it for women with bothersome SUI.
Incontinence pessaries or vaginal inserts (such as Poise Impressa bladder supports) can be helpful for SUI treatment. An incontinence pessary can be fitted in the office, and fitting kits are available for both. Pessaries can safely be used in pregnancy, but there are no data on the efficacy of pessaries for treating SUI in pregnancy. In nonpregnant women, evidence demonstrates 63% satisfaction 3 months post–pessary placement for SUI.7
We do not recommend invasive procedures for the treatment of SUI during pregnancy or in the first 6 months following delivery. There is no evidence that elective cesarean delivery prevents persistent SUI postpartum.9
To identify and engage the proper pelvic floor muscles:
- Insert a finger in the vagina and squeeze the vaginal muscles around your finger.
- Imagine you are sitting on a marble and have to pick it up with the vaginal muscles.
- Squeeze the muscles you would use to stop the flow of urine or hold back flatulence.
Perform sets of 10, 2 to 3 times per day as follows:
- Squeeze: Engage the pelvic floor muscles as described above; avoid performing Kegels while voiding.
- Hold: For 2 to 10 seconds; increase the duration to 10 seconds as able.
- Relax: Completely relax muscles before initating the next squeeze.
Reference
1. UpToDate. Patient education: pelvic muscle (Kegel) exercises (the basics). 2018. https://uptodatefree.ir/topic.htm?path=pelvic-muscle-kegel-exercises-the-basics. Accessed February 24, 2021.
Continue to: Managing SUI in the postpartum period...
Managing SUI in the postpartum period
After the first 6 months postpartum and exhaustion of conservative measures, we offer surgical interventions for women with persistent, bothersome incontinence. Surgery for SUI typically is not recommended until childbearing is complete, but it can be considered if the patient’s bother is significant.
For women with bothersome SUI who still desire future pregnancy, management options include periurethral bulking, a retropubic urethropexy (Burch procedure), or a midurethral sling procedure. Women who undergo an anti-incontinence procedure have an increased risk for urinary retention during a subsequent pregnancy.10 Most women with a midurethral sling will continue to be continent following an obstetric delivery.
Anticipatory guidance
At 3 months postpartum, the incidence of urinary incontinence is 6% to 40%, depending on parity and delivery type. Postpartum urinary incontinence is most common after instrumented vaginal delivery (32%) followed by spontaneous vaginal delivery (28%) and cesarean delivery (15%). The mean prevalence of any type of urinary incontinence is 33% at 3 months postpartum, and only small changes in the rate of urinary incontinence occur over the first postpartum year.11 While urinary incontinence is common postpartum, it should not be considered normal. We counsel that symptoms may improve spontaneously, but treatment can be initiated if the patient experiences significant bother.
A longitudinal cohort study that followed women from 3 months to 12 years postpartum found that, of women with urinary incontinence at 3 months postpartum, 76% continued to report incontinence at 12 years postpartum.12 We recommend that women be counseled that, even when symptoms resolve, they remain at increased risk for urinary incontinence in the future. Invasive therapies should be used to treat bothersome urinary incontinence, not to prevent future incontinence.
CASE 3 Fecal incontinence
A 24-year-old woman (G1P1) presents 3 weeks postpartum following a forceps-assisted vaginal delivery complicated by a 3c laceration. She reports fecal urgency, inability to control flatus, and once-daily fecal incontinence.
How would you evaluate these symptoms?
Steps in evaluation
The initial evaluation should include an inquiry regarding the patient’s stool consistency and bowel regimen. The Bristol stool form scale can be used to help patients describe their typical bowel movements (TABLE 2).13 During healing, the goal is to achieve a Bristol type 4 stool, both to avoid straining and to improve continence, as loose stool is the most difficult to control.

A physical examination can evaluate healing and sphincter integrity; it should include inspection of the distal vagina and perineal body and a digital rectal exam. Anal canal resting tone and squeeze strength should be evaluated, and the digital rectal examination scoring system (DRESS) can be useful for quantification (TABLE 3).14 Lack of tone at rest in the anterolateral portion of the sphincter complex can indicate an internal anal sphincter defect, as 80% of the resting tone comes from this muscle (FIGURE).15

The rectovaginal septum should be assessed given the increased risk of rectovaginal fistula in women with obstetric anal sphincter injury (OASI). The patient should be instructed to contract the anal sphincter, allowing evaluation of muscular contraction. Lack of contraction anteriolaterally may indicate external anal sphincter separation.
Continue to: Conservative options for improving fecal incontinence symptoms...
Conservative options for improving fecal incontinence symptoms
The patient can be counseled regarding stool bulking, first with insoluble fiber supplementation and cessation of stool softeners if she is incontinent of liquid stool. If these measures are not effective, use of a constipating agent, such as loperamide, can improve stool consistency and thereby decrease incontinence episodes. PFPT with biofeedback can be offered as well. While typically we do not recommend initiating PFPT before 6 weeks postpartum, so the initial phases of healing can occur, early referral enables the patient to avoid a delay in access to care.
The patient also can be counseled about a referral to a pelvic floor specialist for further evaluation. A variety of peripartum pelvic floor disorder clinics are being established by Female Pelvic Medicine and Reconstructive Surgery (FPMRS) physicians. These clinics provide the benefit of comprehensive care for pelvic floor disorders in this unique population.
When conservative measures fail. If a patient has persistent bowel control issues despite conservative measures, a referral to an FPMRS physician should be initiated.
Delivery route in future pregnancies
The risk of a subsequent OASI is low. While this means that many women can safely pursue a future vaginal delivery, a scheduled cesarean delivery is indicated for women with persistent bowel control issues, wound healing complications, and those who experienced psychological trauma from their delivery.16 We recommend a shared-decision making approach, reviewing modifiable and nonmodifiable risk factors to help determine whether or not a future vaginal birth is appropriate. It is important to highlight that a cesarean delivery does not protect against fecal incontinence in women with a history of OASI; however, there is benefit in preventing worsening of anal incontinence, if present.17
CASE 4 Uterovaginal prolapse
A 36-year-old woman (G3P3) presents for her routine postpartum visit at 6 weeks after a spontaneous vaginal delivery without lacerations. She reports a persistent feeling of vaginal pressure and fullness. She thinks she felt a bulge with wiping after a bowel movement.
What options are available for this patient?
Prolapse in the peripartum population
Previous studies have revealed an increased prevalence of POP in pregnant women on examination compared with their nulligravid counterparts (47.6% vs 0%).18 With the changes in the hormonal milieu in pregnancy, as well as the weight of the gravid uterus on the pelvic floor, it is not surprising that pregnancy may be the inciting event to expose even transient defects in pelvic organ support.19
It is well established that increasing parity and, to a lesser extent, larger babies are associated with increased risk for future POP and surgery for prolapse. In the first year postpartum, nearly one-third of women have stage 2 or greater prolapse on exam, with studies demonstrating an increased prevalence of postpartum POP in women who delivered vaginally compared with those who delivered by cesarean.20,21
Initial evaluation
Diagnosis can be made during a routine pelvic exam by having the patient perform a Valsalva maneuver while in the lithotomy position. Using half of a speculum permits evaluation of the anterior and posterior vaginal walls separately, and Valsalva during a bimanual exam can aid in evaluating descensus of the uterus and cervix.
Excellent free patient education resources available online through the American Urogynecologic Society and the International Urogynecological Association can be used to direct counseling.
Continue to: Treatments you can offer for POP...
Treatments you can offer for POP
For pregnant or postpartum patients with bothersome prolapse, initial management options include pessary fitting and/or PFPT referral. In pregnancy, women often can be successfully fitted with a pessary for POP; however, as expulsion is a common issue, selection of a stiffer or space-occupying device may be more efficacious.
Often, early onset POP in pregnancy resolves as the gravid uterus lifts out of the pelvis in the second trimester, at which time the pessary can be discontinued. In the postpartum period, a pessary fitting can be undertaken similarly to that in nonpregnant patients. While data are lacking in the peripartum population, evidence supports the positive impact of PFPT on improving POP symptom bother.22 Additionally, for postpartum women who experience OASI, PFPT can produce significant improvement in subjective POP and associated bother.23
Impact of future childbearing wishes on treatment
The desire for future childbearing does not preclude treatment of patients experiencing bother from POP after conservative management options have failed. Both vaginal native tissue and mesh-augmented uterine-sparing repairs are performed by many FPMRS specialists and are associated with good outcomes. As with SUI, we do not recommend invasive treatment for POP during pregnancy or before 6 months postpartum.
In conclusion
Obstetric specialists play an essential role in caring for women with PFDs in the peripartum period. Basic evaluation, counseling, and management can be initiated using many of the resources already available in an obstetric ambulatory practice. Important adjunctive resources include those available for both providers and patients through the American Urogynecologic Society and the International Urogynecological Association. In addition, clinicians can partner with pelvic floor specialists through the growing number of FPMRS-run peripartum pelvic floor disorder clinics across the country and pelvic floor physical therapists.
If these specialty clinics and therapists are not available in your area, FPMRS specialists, urologists, gastroenterologists, and/or colorectal surgeons can aid in patient diagnosis and management to reach the ultimate goal of improving PFDs in this at-risk population. ●
Pelvic floor disorders (PFDs) affect many pregnant and newly postpartum women. These conditions, including urinary incontinence, anal incontinence, and pelvic organ prolapse (POP), can be overshadowed by common pregnancy and postpartum concerns (TABLE 1).1 With the use of a few quick screening questions, however, PFDs easily can be identified in this at-risk population. Active management need not be delayed until after delivery for women experiencing bother, as options exist for women with PFDs during pregnancy as well as postpartum.

In this article, we discuss the common PFDs that obstetric clinicians face in the context of case scenarios and review how you can be better equipped to care for affected individuals.
CASE 1 Screening
A 30-year-old woman (G1P1) presents for her routine postpartum visit after an operative vaginal delivery with a second-degree laceration.
How would you screen this patient for PFDs?
Why screening for PFDs matters
While there are no validated PFD screening tools for this patient population, clinicians can ask a series of brief open-ended questions as part of the review of systems to efficiently evaluate for the common PFDs in peripartum patients (see “Screening questions to evaluate patients for peripartum pelvic floor disorders” below).
Pelvic floor disorders in the peripartum period can have a significant negative impact. In pregnancy, nearly half of women report psychological strain due to the presence of bowel, bladder, prolapse, or sexual dysfunction symptoms.2 Postpartum, PFDs have negative effects on overall health, well-being, and self-esteem, with significantly increased rates of postpartum depression in women who experience urinary incontinence.3,4 Proactively inquiring about PFD symptoms, providing anticipatory guidance, and recommending treatment options can positively impact a patient in multiple domains.
Sometimes during pregnancy or after having a baby, a woman experiences pelvic floor symptoms. Do you have any of the following?
- leakage with coughing, laughing, sneezing, or physical activity
- urgency to urinate or leakage due to urgency
- bulging or pressure within the vagina
- pain with intercourse
- accidental bowel leakage of stool or flatus
CASE 2 Stress urinary incontinence
A 27-year-old woman (G1P1) presents 2 months following spontaneous vaginal delivery with symptoms of urine leakage with laughing and running. Her urinary incontinence has been improving since delivery, but it continues to be bothersome.
What would you recommend for this patient?
Conservative SUI management strategies in pregnancy
Urinary tract symptoms are common in pregnancy, with up to 41.8% of women reporting urinary symptom distress in the third trimester.5 During pregnancy, estrogen and progesterone decrease urethral pressure that, together with increased intra-abdominal pressure from the gravid uterus, can cause or worsen stress urinary incontinence (SUI).6
During pregnancy, women should be offered conservative therapies for SUI. For women who can perform a pelvic floor contraction (a Kegel exercise), self-guided pelvic floor muscle exercises (PFMEs) may be helpful (see “Pelvic floor muscle exercises” below). We recommend that women start with 1 to 2 sets of 10 Kegel exercises per day and that they hold the squeeze for 2 to 3 seconds, working up to holding for 10 seconds. The goal is to strengthen and improve muscle control so that the Kegel squeeze can be paired with activities that cause SUI.
For women who are unable to perform a Kegel exercise or are not improving with a home PFME regimen, referral to pelvic floor physical therapy (PFPT) can be considered. While data support the efficacy of PFPT for SUI treatment in nonpregnant women,7 data are lacking on PFME in pregnancy.
In women without urinary incontinence, PFME in early pregnancy can prevent the onset of incontinence in late pregnancy and the postpartum period.8 By contrast, the same 2020 Cochrane Review found no evidence that antenatal pelvic floor muscle therapy in incontinent women decreases incontinence in mid- or late-pregnancy or in the postpartum period.8 As the quality of this evidence is very low and there is no evidence of harm with PFME, we continue to recommend it for women with bothersome SUI.
Incontinence pessaries or vaginal inserts (such as Poise Impressa bladder supports) can be helpful for SUI treatment. An incontinence pessary can be fitted in the office, and fitting kits are available for both. Pessaries can safely be used in pregnancy, but there are no data on the efficacy of pessaries for treating SUI in pregnancy. In nonpregnant women, evidence demonstrates 63% satisfaction 3 months post–pessary placement for SUI.7
We do not recommend invasive procedures for the treatment of SUI during pregnancy or in the first 6 months following delivery. There is no evidence that elective cesarean delivery prevents persistent SUI postpartum.9
To identify and engage the proper pelvic floor muscles:
- Insert a finger in the vagina and squeeze the vaginal muscles around your finger.
- Imagine you are sitting on a marble and have to pick it up with the vaginal muscles.
- Squeeze the muscles you would use to stop the flow of urine or hold back flatulence.
Perform sets of 10, 2 to 3 times per day as follows:
- Squeeze: Engage the pelvic floor muscles as described above; avoid performing Kegels while voiding.
- Hold: For 2 to 10 seconds; increase the duration to 10 seconds as able.
- Relax: Completely relax muscles before initating the next squeeze.
Reference
1. UpToDate. Patient education: pelvic muscle (Kegel) exercises (the basics). 2018. https://uptodatefree.ir/topic.htm?path=pelvic-muscle-kegel-exercises-the-basics. Accessed February 24, 2021.
Continue to: Managing SUI in the postpartum period...
Managing SUI in the postpartum period
After the first 6 months postpartum and exhaustion of conservative measures, we offer surgical interventions for women with persistent, bothersome incontinence. Surgery for SUI typically is not recommended until childbearing is complete, but it can be considered if the patient’s bother is significant.
For women with bothersome SUI who still desire future pregnancy, management options include periurethral bulking, a retropubic urethropexy (Burch procedure), or a midurethral sling procedure. Women who undergo an anti-incontinence procedure have an increased risk for urinary retention during a subsequent pregnancy.10 Most women with a midurethral sling will continue to be continent following an obstetric delivery.
Anticipatory guidance
At 3 months postpartum, the incidence of urinary incontinence is 6% to 40%, depending on parity and delivery type. Postpartum urinary incontinence is most common after instrumented vaginal delivery (32%) followed by spontaneous vaginal delivery (28%) and cesarean delivery (15%). The mean prevalence of any type of urinary incontinence is 33% at 3 months postpartum, and only small changes in the rate of urinary incontinence occur over the first postpartum year.11 While urinary incontinence is common postpartum, it should not be considered normal. We counsel that symptoms may improve spontaneously, but treatment can be initiated if the patient experiences significant bother.
A longitudinal cohort study that followed women from 3 months to 12 years postpartum found that, of women with urinary incontinence at 3 months postpartum, 76% continued to report incontinence at 12 years postpartum.12 We recommend that women be counseled that, even when symptoms resolve, they remain at increased risk for urinary incontinence in the future. Invasive therapies should be used to treat bothersome urinary incontinence, not to prevent future incontinence.
CASE 3 Fecal incontinence
A 24-year-old woman (G1P1) presents 3 weeks postpartum following a forceps-assisted vaginal delivery complicated by a 3c laceration. She reports fecal urgency, inability to control flatus, and once-daily fecal incontinence.
How would you evaluate these symptoms?
Steps in evaluation
The initial evaluation should include an inquiry regarding the patient’s stool consistency and bowel regimen. The Bristol stool form scale can be used to help patients describe their typical bowel movements (TABLE 2).13 During healing, the goal is to achieve a Bristol type 4 stool, both to avoid straining and to improve continence, as loose stool is the most difficult to control.

A physical examination can evaluate healing and sphincter integrity; it should include inspection of the distal vagina and perineal body and a digital rectal exam. Anal canal resting tone and squeeze strength should be evaluated, and the digital rectal examination scoring system (DRESS) can be useful for quantification (TABLE 3).14 Lack of tone at rest in the anterolateral portion of the sphincter complex can indicate an internal anal sphincter defect, as 80% of the resting tone comes from this muscle (FIGURE).15

The rectovaginal septum should be assessed given the increased risk of rectovaginal fistula in women with obstetric anal sphincter injury (OASI). The patient should be instructed to contract the anal sphincter, allowing evaluation of muscular contraction. Lack of contraction anteriolaterally may indicate external anal sphincter separation.
Continue to: Conservative options for improving fecal incontinence symptoms...
Conservative options for improving fecal incontinence symptoms
The patient can be counseled regarding stool bulking, first with insoluble fiber supplementation and cessation of stool softeners if she is incontinent of liquid stool. If these measures are not effective, use of a constipating agent, such as loperamide, can improve stool consistency and thereby decrease incontinence episodes. PFPT with biofeedback can be offered as well. While typically we do not recommend initiating PFPT before 6 weeks postpartum, so the initial phases of healing can occur, early referral enables the patient to avoid a delay in access to care.
The patient also can be counseled about a referral to a pelvic floor specialist for further evaluation. A variety of peripartum pelvic floor disorder clinics are being established by Female Pelvic Medicine and Reconstructive Surgery (FPMRS) physicians. These clinics provide the benefit of comprehensive care for pelvic floor disorders in this unique population.
When conservative measures fail. If a patient has persistent bowel control issues despite conservative measures, a referral to an FPMRS physician should be initiated.
Delivery route in future pregnancies
The risk of a subsequent OASI is low. While this means that many women can safely pursue a future vaginal delivery, a scheduled cesarean delivery is indicated for women with persistent bowel control issues, wound healing complications, and those who experienced psychological trauma from their delivery.16 We recommend a shared-decision making approach, reviewing modifiable and nonmodifiable risk factors to help determine whether or not a future vaginal birth is appropriate. It is important to highlight that a cesarean delivery does not protect against fecal incontinence in women with a history of OASI; however, there is benefit in preventing worsening of anal incontinence, if present.17
CASE 4 Uterovaginal prolapse
A 36-year-old woman (G3P3) presents for her routine postpartum visit at 6 weeks after a spontaneous vaginal delivery without lacerations. She reports a persistent feeling of vaginal pressure and fullness. She thinks she felt a bulge with wiping after a bowel movement.
What options are available for this patient?
Prolapse in the peripartum population
Previous studies have revealed an increased prevalence of POP in pregnant women on examination compared with their nulligravid counterparts (47.6% vs 0%).18 With the changes in the hormonal milieu in pregnancy, as well as the weight of the gravid uterus on the pelvic floor, it is not surprising that pregnancy may be the inciting event to expose even transient defects in pelvic organ support.19
It is well established that increasing parity and, to a lesser extent, larger babies are associated with increased risk for future POP and surgery for prolapse. In the first year postpartum, nearly one-third of women have stage 2 or greater prolapse on exam, with studies demonstrating an increased prevalence of postpartum POP in women who delivered vaginally compared with those who delivered by cesarean.20,21
Initial evaluation
Diagnosis can be made during a routine pelvic exam by having the patient perform a Valsalva maneuver while in the lithotomy position. Using half of a speculum permits evaluation of the anterior and posterior vaginal walls separately, and Valsalva during a bimanual exam can aid in evaluating descensus of the uterus and cervix.
Excellent free patient education resources available online through the American Urogynecologic Society and the International Urogynecological Association can be used to direct counseling.
Continue to: Treatments you can offer for POP...
Treatments you can offer for POP
For pregnant or postpartum patients with bothersome prolapse, initial management options include pessary fitting and/or PFPT referral. In pregnancy, women often can be successfully fitted with a pessary for POP; however, as expulsion is a common issue, selection of a stiffer or space-occupying device may be more efficacious.
Often, early onset POP in pregnancy resolves as the gravid uterus lifts out of the pelvis in the second trimester, at which time the pessary can be discontinued. In the postpartum period, a pessary fitting can be undertaken similarly to that in nonpregnant patients. While data are lacking in the peripartum population, evidence supports the positive impact of PFPT on improving POP symptom bother.22 Additionally, for postpartum women who experience OASI, PFPT can produce significant improvement in subjective POP and associated bother.23
Impact of future childbearing wishes on treatment
The desire for future childbearing does not preclude treatment of patients experiencing bother from POP after conservative management options have failed. Both vaginal native tissue and mesh-augmented uterine-sparing repairs are performed by many FPMRS specialists and are associated with good outcomes. As with SUI, we do not recommend invasive treatment for POP during pregnancy or before 6 months postpartum.
In conclusion
Obstetric specialists play an essential role in caring for women with PFDs in the peripartum period. Basic evaluation, counseling, and management can be initiated using many of the resources already available in an obstetric ambulatory practice. Important adjunctive resources include those available for both providers and patients through the American Urogynecologic Society and the International Urogynecological Association. In addition, clinicians can partner with pelvic floor specialists through the growing number of FPMRS-run peripartum pelvic floor disorder clinics across the country and pelvic floor physical therapists.
If these specialty clinics and therapists are not available in your area, FPMRS specialists, urologists, gastroenterologists, and/or colorectal surgeons can aid in patient diagnosis and management to reach the ultimate goal of improving PFDs in this at-risk population. ●
- Madsen AM, Hickman LC, Propst K. Recognition and management of pelvic floor disorders in pregnancy and the postpartum period. Obstet Gynecol Clin North Am. Forthcoming 2021.
- Bodner-Adler B, Kimberger O, Laml T, et al. Prevalence and risk factors for pelvic floor disorders during early and late pregnancy in a cohort of Austrian women. Arch Gynecol Obstet. 2019;300:1325-1330.
- Swenson CW, DePorre JA, Haefner JK, et al. Postpartum depression screening and pelvic floor symptoms among women referred to a specialty postpartum perineal clinic. Am J Obstet Gynecol. 2018;218:335.e1-335.e6.
- Skinner EM, Dietz HP. Psychological and somatic sequelae of traumatic vaginal delivery: a literature review. Aust N Z J Obstet Gynaecol. 2015;55:309-314.
- Yohay D, Weintraub AY, Mauer-Perry N, et al. Prevalence and trends of pelvic floor disorders in late pregnancy and after delivery in a cohort of Israeli women using the PFDI-20. Eur J Obstet Gynecol Reprod Biol. 2016;200:35-39.
- Gregory WT, Sibai BM. Obstetrics and pelvic floor disorders. In: Walters M, Karram M, eds. Urogynecology and Reconstructive Pelvic Surgery. 4th ed. Philadelphia, PA: Saunders; 2015:224-237.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Woodley SJ, Lawrenson P, Boyle R, et al. Pelvic floor muscle training for preventing and treating urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev. 2020;6:CD007471.
- Foldspang A, Hvidman L, Mommsen S, et al. Risk of postpartum urinary incontinence associated with pregnancy and mode of delivery. Acta Obstet Gynecol Scand. 2004;83:923-927.
- Wieslander CK, Weinstein MM, Handa V, et al. Pregnancy in women with prior treatments for pelvic floor disorders. Female Pelvic Med Reconstr Surg. 2020;26:299-305.
- Thom DH, Rortveit G. Prevalence of postpartum urinary incontinence: a systematic review. Acta Obstet Gynecol Scand. 2010;89:1511-1522.
- MacArthur C, Wilson D, Herbison P, et al; Prolong Study Group. Urinary incontinence persisting after childbirth: extent, delivery history, and effects in a 12-year longitudinal cohort study. BJOG. 2016;123:1022-1029.
- Blake MR, Raker JM, Whelan K. Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44:693-703
- Orkin BA, Sinykin SB, Lloyd PC. The digital rectal examination scoring system (DRESS). Dis Colon Rectum. 2010;53:1656-1660.
- UpToDate. Repair of episiotomy and perineal lacerations associated with childbirth. 2020. https://www-uptodate-com .ccmain.ohionet.org/contents/repair-of-perineal-and-other -lacerations-associated-with-childbirth?search=repair%20 episiotomy&source=search_result&selectedTitle=1~150&usa ge_type=default&display_rank=1. Accessed February 28, 2021.
- Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 198: prevention and management of obstetric lacerations at vaginal delivery. Obstet Gynecol. 2018;132:e87-e102.
- Jangö H, Langhoff-Roos J, Rosthøj S, et al. Long-term anal incontinence after obstetric anal sphincter injury—does grade of tear matter? Am J Obstet Gynecol. 2018;218:232.e1-232.e10.
- O’Boyle AL, Woodman PJ, O’Boyle JD, et al. Pelvic organ support in nulliparous pregnant and nonpregnant women: a case control study. Am J Obstet Gynecol. 2002;187:99-102.
- Handa VL, Blomquist JL, McDermott KC, et al. Pelvic floor disorders after vaginal birth. Obstet Gynecol. 2012;119 (2, pt 1):233-239.
- Handa VL, Nygaard I, Kenton K, et al; Pelvic Floor Disorders Network. Pelvic organ support among primiparous women in the first year after childbirth. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:1407-1411.
- O’Boyle AL, O’Boyle JD, Calhoun B, et al. Pelvic organ support in pregnancy and postpartum. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:69-72.
- Hagen S, Stark D, Glazener C, et al; POPPY Trial Collaborators. Individualised pelvic floor muscle training in women with pelvic organ prolapse (POPPY): a multicentre randomised controlled trial. Lancet. 2014;383:796-806.
- Von Bargen E, Haviland MJ, Chang OH, et al. Evaluation of postpartum pelvic floor physical therapy on obstetrical anal sphincter injury: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2020. doi: 10.1097/SPV.0000000000000849.
- Madsen AM, Hickman LC, Propst K. Recognition and management of pelvic floor disorders in pregnancy and the postpartum period. Obstet Gynecol Clin North Am. Forthcoming 2021.
- Bodner-Adler B, Kimberger O, Laml T, et al. Prevalence and risk factors for pelvic floor disorders during early and late pregnancy in a cohort of Austrian women. Arch Gynecol Obstet. 2019;300:1325-1330.
- Swenson CW, DePorre JA, Haefner JK, et al. Postpartum depression screening and pelvic floor symptoms among women referred to a specialty postpartum perineal clinic. Am J Obstet Gynecol. 2018;218:335.e1-335.e6.
- Skinner EM, Dietz HP. Psychological and somatic sequelae of traumatic vaginal delivery: a literature review. Aust N Z J Obstet Gynaecol. 2015;55:309-314.
- Yohay D, Weintraub AY, Mauer-Perry N, et al. Prevalence and trends of pelvic floor disorders in late pregnancy and after delivery in a cohort of Israeli women using the PFDI-20. Eur J Obstet Gynecol Reprod Biol. 2016;200:35-39.
- Gregory WT, Sibai BM. Obstetrics and pelvic floor disorders. In: Walters M, Karram M, eds. Urogynecology and Reconstructive Pelvic Surgery. 4th ed. Philadelphia, PA: Saunders; 2015:224-237.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Woodley SJ, Lawrenson P, Boyle R, et al. Pelvic floor muscle training for preventing and treating urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev. 2020;6:CD007471.
- Foldspang A, Hvidman L, Mommsen S, et al. Risk of postpartum urinary incontinence associated with pregnancy and mode of delivery. Acta Obstet Gynecol Scand. 2004;83:923-927.
- Wieslander CK, Weinstein MM, Handa V, et al. Pregnancy in women with prior treatments for pelvic floor disorders. Female Pelvic Med Reconstr Surg. 2020;26:299-305.
- Thom DH, Rortveit G. Prevalence of postpartum urinary incontinence: a systematic review. Acta Obstet Gynecol Scand. 2010;89:1511-1522.
- MacArthur C, Wilson D, Herbison P, et al; Prolong Study Group. Urinary incontinence persisting after childbirth: extent, delivery history, and effects in a 12-year longitudinal cohort study. BJOG. 2016;123:1022-1029.
- Blake MR, Raker JM, Whelan K. Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44:693-703
- Orkin BA, Sinykin SB, Lloyd PC. The digital rectal examination scoring system (DRESS). Dis Colon Rectum. 2010;53:1656-1660.
- UpToDate. Repair of episiotomy and perineal lacerations associated with childbirth. 2020. https://www-uptodate-com .ccmain.ohionet.org/contents/repair-of-perineal-and-other -lacerations-associated-with-childbirth?search=repair%20 episiotomy&source=search_result&selectedTitle=1~150&usa ge_type=default&display_rank=1. Accessed February 28, 2021.
- Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 198: prevention and management of obstetric lacerations at vaginal delivery. Obstet Gynecol. 2018;132:e87-e102.
- Jangö H, Langhoff-Roos J, Rosthøj S, et al. Long-term anal incontinence after obstetric anal sphincter injury—does grade of tear matter? Am J Obstet Gynecol. 2018;218:232.e1-232.e10.
- O’Boyle AL, Woodman PJ, O’Boyle JD, et al. Pelvic organ support in nulliparous pregnant and nonpregnant women: a case control study. Am J Obstet Gynecol. 2002;187:99-102.
- Handa VL, Blomquist JL, McDermott KC, et al. Pelvic floor disorders after vaginal birth. Obstet Gynecol. 2012;119 (2, pt 1):233-239.
- Handa VL, Nygaard I, Kenton K, et al; Pelvic Floor Disorders Network. Pelvic organ support among primiparous women in the first year after childbirth. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:1407-1411.
- O’Boyle AL, O’Boyle JD, Calhoun B, et al. Pelvic organ support in pregnancy and postpartum. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:69-72.
- Hagen S, Stark D, Glazener C, et al; POPPY Trial Collaborators. Individualised pelvic floor muscle training in women with pelvic organ prolapse (POPPY): a multicentre randomised controlled trial. Lancet. 2014;383:796-806.
- Von Bargen E, Haviland MJ, Chang OH, et al. Evaluation of postpartum pelvic floor physical therapy on obstetrical anal sphincter injury: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2020. doi: 10.1097/SPV.0000000000000849.
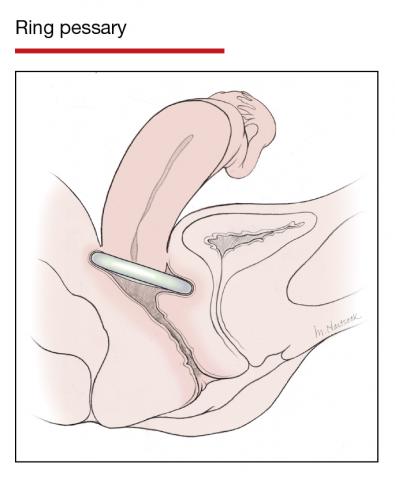
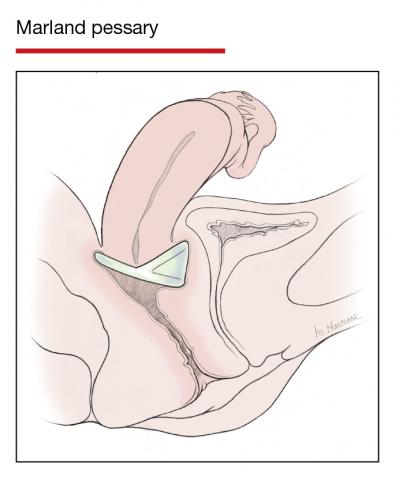
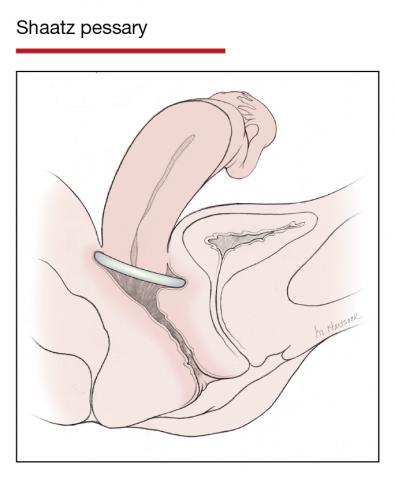
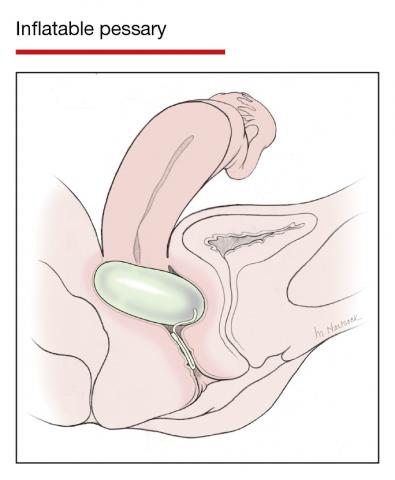
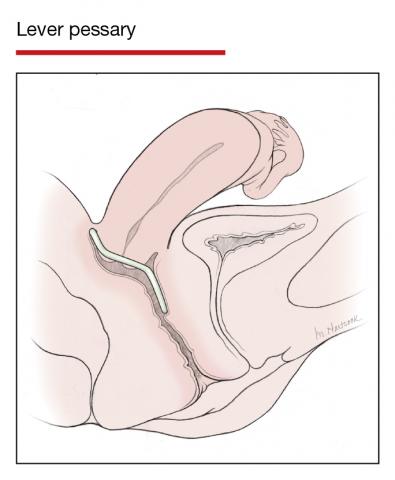
Pessaries for POP and SUI: Their fitting, care, and effectiveness in various disorders
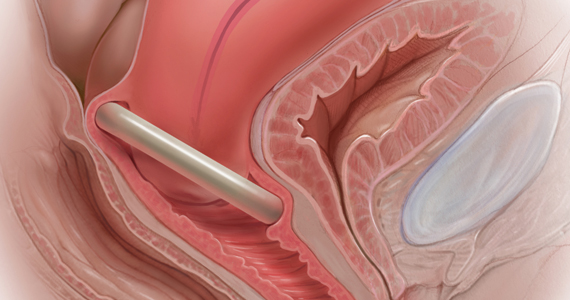
In Part 1 of this article in the December 2020 issue of OBG Management, I discussed the reasons that pessaries are an effective treatment option for many women with pelvic organ prolapse (POP) and stress urinary incontinence (SUI) and provided details on the types of pessaries available.
In this article, I highlight the steps in fitting a pessary, pessary aftercare, and potential complications associated with pessary use. In addition, I discuss the effectiveness of pessary treatment for POP and SUI as well as for preterm labor prevention and defecatory disorders.
The pessary fitting process
For a given patient, the best size pessary is the smallest one that will not fall out. The only “rule” for fitting a pessary is that a woman’s internal vaginal caliber should be wider than her introitus.
When fitting a pessary, goals include that the selected pessary:
- should be comfortable for the patient to wear
- is not easily expelled
- does not interfere with urination or defecation
- does not cause vaginal irritation.
The presence or absence of a cervix or uterus does not affect pessary choice.
Most experts agree that the process for fitting the right size pessary is one of trial and error. As with fitting a contraceptive diaphragm, the clinician should perform a manual examination to estimate the integrity and width of the perineum and the depth of the vagina to roughly approximate the pessary size that might best fit. Using a set of “fitting pessaries,” a pessary of the estimated size should be placed into the vagina and the fit evaluated as to whether the device is too big, too small, or appropriate. If the pessary is easily expelled, larger sizes should be tried until the pessary remains in place or the patient is uncomfortable. Once the pessary is in place, the clinician should be able to run his or her finger around the entire pessary; if this is not possible, the pessary is too tight. In addition, the pessary should remain more than one finger breadth above the introitus when the patient is standing or bearing down.
Since many patients who require a pessary are elderly, their perineal skin and vaginal mucosa may be atrophic and fragile. Inserting a pessary can be uncomfortable and can cause abrasions or tears. Successfully fitting a pessary may require extra care under these circumstances. The following steps may help alleviate these difficulties:
- Explain the fitting process to the patient in detail.
- Employ lubrication liberally.
- Enlarge the introitus by applying gentle digital pressure on the posterior fourchette.
- Apply 2% lidocaine ointment several minutes prior to pessary fitting to help decrease patient discomfort.
- Treat the patient for several weeks with vaginal estrogen cream before attempting to fit a pessary if severe vulvovaginal atrophy is present.
Once the type and size of the pessary are selected and a pessary is inserted, evaluate the patient with the pessary in place. Assess for the following:
Discomfort. Ask the patient if she feels discomfort with the pessary in position. A patient with a properly fitting pessary should not feel that it is in place. If she does feel discomfort initially, the discomfort will only increase with time and the issue should be addressed at that time.
Expulsion. Test to make certain that the pessary is not easily expelled from the vagina. Have the patient walk, cough, squat, and even jump if possible.
Urination. Have the patient urinate with the pessary in place. This tests for her ability to void while wearing the pessary and shows whether the contraction of pelvic muscles during voiding results in expulsion of the pessary. (Experience shows that it is best to do this with a plastic “hat” over the toilet so that if the pessary is expelled, it does not drop into the bowl.)
Re-examination. After these provocative tests, examine the patient again to ensure that the pessary has not slid out of place.
Depending on whether or not your office stocks pessaries, at this point the patient is either given the correct type and size of pessary or it is ordered for her. If the former, the patient should try placing it herself; if she is unable to, the clinician should place it for her. In either event, its position should be checked. If the pessary has to be ordered, the patient must schedule an appointment to return for pessary insertion.
Whether the pessary is supplied by the office or ordered, instruct the patient on how to insert and remove the pessary, how frequently to remove it for cleansing (see below), and signs to watch for, such as vaginal bleeding, inability to void or defecate, or pelvic pain.
It is advisable to schedule a subsequent visit for 2 to 3 weeks after initial pessary placement to assess how the patient is doing and to address any issues that have developed.
Continue to: Special circumstances...
Special circumstances
It is safe for a patient with a pessary in place to undergo magnetic resonance imaging.1 Patients should be informed, however, that full body scans, such as at airports, will detect pessaries. Patients may need to obtain a physician’s note to document that the pessary is a medical device.
Finally, several factors may prevent successful pessary fitting. These include prior pelvic surgery, obesity, short vaginal length (less than 6–7 cm), and a vaginal introitus width of greater than 4 finger breadths.
Necessary pessary aftercare
Once a pessary is in place and the patient is comfortable with it, the only maintenance necessary is the pessary’s intermittent removal for cleansing and for evaluation of the vaginal mucosa for erosion and ulcerations. How frequently this should be done varies based on the type of pessary, the amount of discharge that a woman produces, whether or not an odor develops after prolonged wearing of the pessary, and whether or not the patient’s vaginal mucosa has been abraded.
The question of timing for pessary cleaning
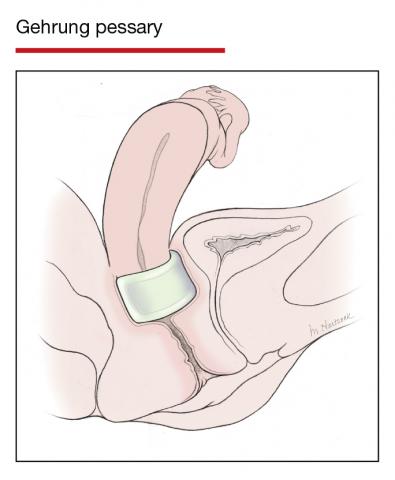
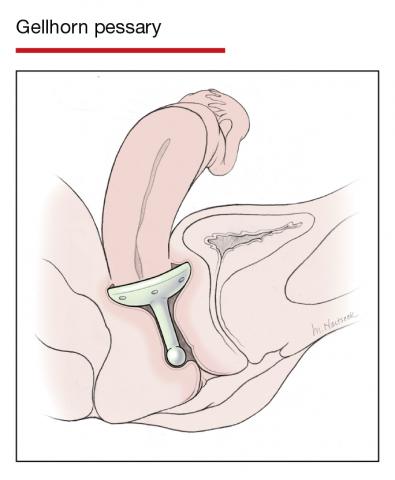
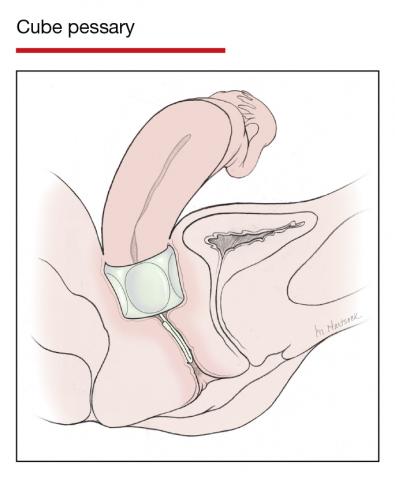
Although there are many opinions about how often pessaries should be removed and cleaned, no data in the literature support any specific interval. Pessaries that are easily removed by women themselves can be cleaned as frequently as desired, often on a weekly basis. The patient simply removes the pessary, washes it with soap and water, and reinserts it. For pessaries that are difficult to remove (such as the Gellhorn, cube, or donut) or for women who are physically unable to remove their own ring pessary, the clinician should remove and clean the pessary in the office every 3 to 6 months. It has been shown that there is no difference in complications from pessary use with either of these intervals.2
Prior to any vaginal surgical procedure, patients must be instructed to remove their pessary 10 to 14 days beforehand so that the surgeon can see the full extent of prolapse when making decisions about reconstruction and so that any vaginal mucosal erosions or abrasions have time to heal.
Office visits for follow-up care
The pessary “cleaning visit” has several goals, including to:
- see if the pessary is meeting the patient’s needs in terms of resolving symptoms of prolapse and/or restoring urinary continence
- discuss with the patient any problems she may be having, such as pelvic discomfort or pressure, difficulty voiding or defecating, excessive vaginal discharge, or vaginal odor
- check for vaginal mucosal erosion or ulceration; such vaginal lesions often can be prevented by the prophylactic use of either estrogen vaginal cream twice weekly or the continuous use of an estradiol vaginal ring in addition to the pessary
- evaluate the condition of the pessary itself and clean it with soap and water.
Continue to: Potential complications of pessary use...
Potential complications of pessary use
The most common complications experienced by pessary users are:
Odor or excessive discharge. Bacterial vaginosis (BV) occurs more frequently in women who use pessaries. The symptoms of BV can be minimized—but unfortunately not totally eliminated—by the prophylactic use of antiseptic vaginal creams or gels, such as metronidazole, clindamycin, Trimo-San (oxyquinoline sulfate and sodium lauryl sulfate), and others. Inserting the gel vaginally once a week can significantly reduce discharge and odor.3
Vaginal mucosal erosion and ulceration. These are treated by removing the pessary for 2 weeks during which time estrogen cream is applied daily or an estradiol vaginal ring is put in place. If no resolution occurs after 2 weeks, the nonhealing vaginal mucosa should be biopsied.
Pressure on the rectum or bladder. If the pessary causes significant discomfort or interferes with voiding function, then either a different size or a different type pessary should be tried
Patients may discontinue pessary use for a variety of reasons. Among these are:
- discomfort
- inadequate improvement of POP or incontinence symptoms
- expulsion of the pessary during daily activities
- the patient’s desire for surgery instead
- worsening of urine leakage
- difficulty inserting or removing the pessary
- damage to the vaginal mucosa
- pain during removal of the pessary in the office.
Pessary effectiveness for POP and SUI symptoms
As might be expected with a device that is available in so many forms and is used to treat varied types of POP and SUI, the data concerning the success rates of pessary use vary considerably. These rates depend on the definition of success, that is, complete or partial control of prolapse and/or incontinence; which devices are being evaluated; and the nature and severity of the POP and/or SUI being treated.
That being said, a review of the literature reveals that the rates of prolapse symptom relief vary from 48% to 92% (TABLE 1).4-13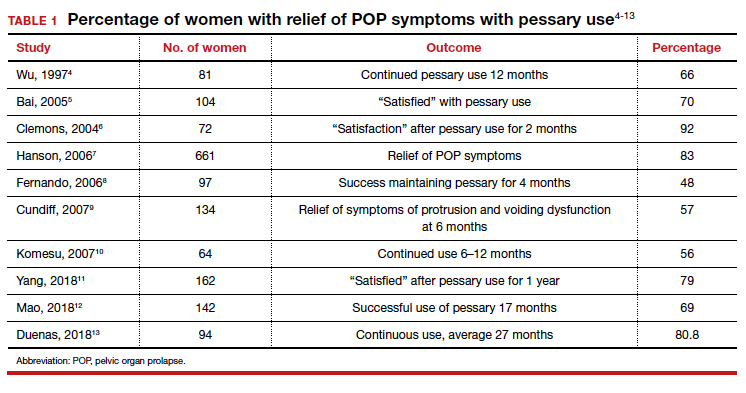
As for success in relieving symptoms of incontinence, studies show improvements in from 40% to 77% of patients (TABLE 2).6,8,14-17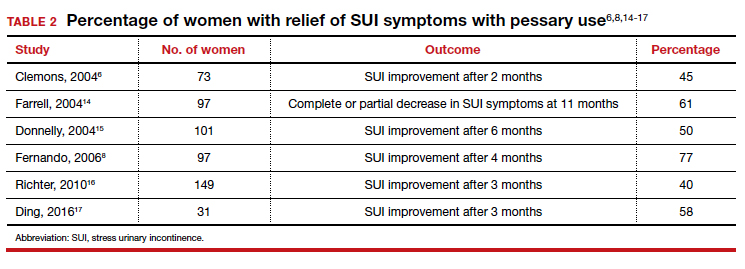
In addition, some studies show a 50% improvement in bowel symptoms (urgency, obstruction, and anal incontinence) with the use of a pessary.9,18
How pessaries compare with surgery
While surgery has the advantage of being a one-time fix with a very high rate of initial success in correcting both POP and incontinence, surgery also has potential drawbacks:
- It is an invasive procedure with the discomfort and risk of complications any surgery entails.
- There is a relatively high rate of prolapse recurrence.
- It exposes the patient to the possibility of mesh erosion if mesh is employed either for POP support or incontinence treatment.
Pessaries, on the other hand, are inexpensive, nonsurgical, removable, and allow for immediate correction of symptoms. Moreover, if the pessary is tried and is found to be unsatisfactory, surgery always can be performed subsequently.
Drawbacks of pessary treatment compared with surgery include the:
- ongoing need to wear an artificial internal device
- need for intermittent pessary removal and cleansing
- inability to have sexual intercourse with certain kinds of pessaries in place
- possible accumulation of vaginal discharge and odor.
Sexual activity and pessaries
Studies by Fernando, Meriwether, and Kuhn concur that for a substantial number of pessary users who are sexually active, both frequency and satisfaction with sexual intercourse are increased.8,19,20 Kuhn further showed that desire, orgasm, and lubrication improved with the use of pessaries.20 While some types of pessaries do require removal for intercourse, Clemons reported that issues involving sexual activity are not associated with pessary discontinuation.21
Using a pessary to predict a surgical outcome
Because a pessary elevates the pelvic organs, supports the vaginal walls, and lifts the bladder and urethra into a position that simulates the results of surgical repair, trial placement of a pessary can be used as a fairly accurate predictive tool to model what pelvic support and continence status will be after a proposed surgical procedure.22,23 This is especially important because a significant number of patients with POP will have their occult stress incontinence unmasked following a reparative procedure.24 A brief pessary trial prior to surgery, therefore, can be a useful tool for both patient and surgeon.
Continue to: Pessaries for prevention of preterm labor...
Pessaries for prevention of preterm labor
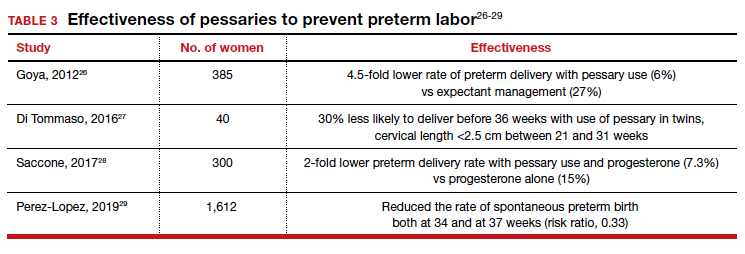
Almost 1 in 10 births in the United States occurs before 37 completed weeks of gestation.25 Obstetricians have long thought that in women at risk for preterm delivery, the use of a pessary might help reduce the pressure of the growing uterus on the cervix and thus help prevent premature cervical dilation. It also has been thought that use of a pessary would be a safer and less invasive alternative to cervical cerclage. Many studies have evaluated the use of pessaries for the prevention of preterm labor with a mixture of positive (TABLE 3)26-29 and negative results (TABLE 4).30-33
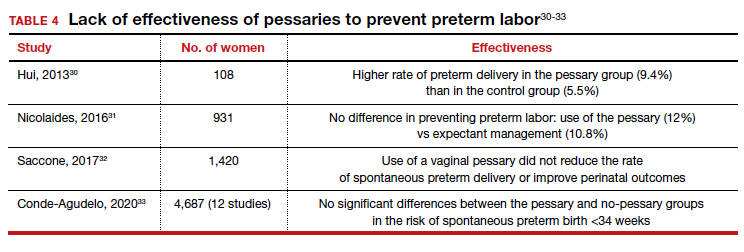
From these data, it is reasonable to conclude that:
- The final answer concerning the effectiveness or lack thereof of pessary use in preventing preterm delivery is not yet in.
- Any advantage there might be to using pessaries to prevent preterm delivery cannot be too significant if multiple studies show as many negative outcomes as positive ones.
Pessary effectiveness in defecatory disorders
Vaginal birth has the potential to create multiple anatomic injuries in the anus, lower pelvis, and perineum that can affect defecation and bowel control. Tears of the anal sphincter, whether obvious or occult, may heal incompletely or be repaired inadequately.34 Nerve innervation of the perianal and perineal areas can be interrupted or damaged by stretching, tearing, or prolonged compression. Of healthy parous adult women, 7% to 16% admit incontinence of gas or feces.35,36
In addition, when a rectocele is present, stool in the lower rectum may cause bulging of the anterior rectal wall into the vagina, preventing stool from passing out of the anus. This sometimes requires women to digitally press their posterior vaginal walls during defecation to evacuate stool successfully. The question thus arises as to whether or not pessary placement and subsequent relief of rectoceles might facilitate bowel movements and decrease or eliminate defecatory dysfunction.
As with the issue of pessary use for prevention of preterm delivery, the answer is mixed. For instance, while Brazell18 showed that there was an overall improvement in bowel symptoms in pessary users, a study by Komesu10 did not demonstrate improvement.
There is, however, a relatively new device specifically designed to control defecatory problems: the vaginal bowel control system (Eclipse; Pelvalon). The silicon device is placed intravaginally as one does a pessary. After insertion, it is inflated via a valve and syringe. It works by putting pressure on and reversibly closing the lower rectum, thus blocking the uncontrolled passage of stool and gas. It can be worn continuously or intermittently, but it does need to be deflated for normal bowel movements. One trial of this device demonstrated a 50% reduction in incontinence episodes with a patient satisfaction rate of 84% at 3 months.37 This device may well prove to be a valuable nonsurgical approach to the treatment of fecal incontinence. Unfortunately, the device is relatively expensive and usually is not covered by insurance as third-party payers do not consider it to be a pessary (which generally is covered).
Practice management particulars
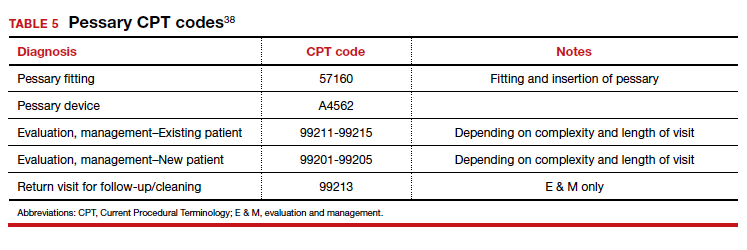
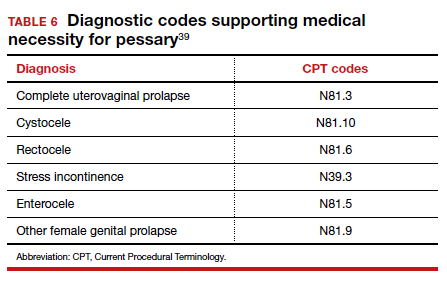
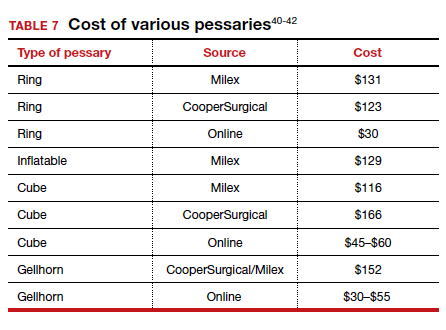
Useful information on Current Procedural Terminology codes for pessaries, diagnostic codes, and the cost of various pessaries is provided in TABLE 5,38TABLE 6,39 and TABLE 7.40-42
A contemporary device used since antiquity
Pessaries, considered “old-fashioned” by many gynecologists, are actually a very cost-effective and useful tool for the correction of POP and SUI. It behooves all who provide medical care to women to be familiar with them, to know when they might be useful, and to know how to fit and prescribe them. ●
- O’Dell K, Atnip S. Pessary care: follow up and management of complications. Urol Nurs. 2012;32:126-136, 145.
- Gorti M, Hudelist G, Simons A. Evaluation of vaginal pessary management: a UK-based survey. J Obstet Gynaecol. 2009;29:129-131.
- Meriwether KV, Rogers RG, Craig E, et al. The effect of hydroxyquinoline-based gel on pessary-associated bacterial vaginosis: a multicenter randomized controlled trial. Am J Obstet Gynecol. 2015;213:729.e1-9.
- Wu V, Farrell SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Bai SW, Yoon BS, Kwon JY, et al. Survey of the characteristics and satisfaction degree of the patients using a pessary. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:182-186.
- Clemons JL, Aguilar VC, Tillinghast TA, et al. Patient satisfaction and changes in prolapse and urinary symptoms in women who were fitted successfully with a pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2004;190:1025-1029.
- Hanson LM, Schulz JA, Flood CG, et al. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17: 155-159.
- Fernando RJ, Thakar R, Sultan AH, et al. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108:93-99.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-405e.8.
- Komesu YM Rogers RG, Rode MA, et al. Pelvic floor symptom changes in pessary users. Am J Obstet Gynecol. 2007;197: 620.e1-6.
- Yang J, Han J, Zhu F, et al. Ring and Gellhorn pessaries used inpatients with pelvic organ prolapse: a retrospective study of 8 years. Arch Gynecol Obstet. 2018;298:623-629.
- Mao M, Ai F, Zhang Y, et al. Changes in the symptoms and quality of life of women with symptomatic pelvic organ prolapse fitted with a ring with support pessary. Maturitas. 2018;117:51-56.
- Duenas JL, Miceli A. Effectiveness of a continuous-use ringshaped vaginal pessary without support for advanced pelvic organ prolapse in postmenopausal women. Int Urogynecol J. 2018;29:1629-1636.
- Farrell S, Singh B, Aldakhil L. Continence pessaries in the management of urinary incontinence in women. J Obstet Gynaecol Canada. 2004;26:113-117.
- Donnelly MJ, Powell-Morgan SP, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:302-307.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Ding J, Chen C, Song XC, et al. Changes in prolapse and urinary symptoms after successful fitting of a ring pessary with support in women with advanced pelvic organ prolapse: a prospective study. Urology. 2016;87:70-75.
- Brazell HD, Patel M, O’Sullivan DM, et al. The impact of pessary use on bowel symptoms: one-year outcomes. Female Pelvic Med Reconstr Surg. 2014;20:95-98.
- Meriwether KV, Komesu YM, Craig C, et al. Sexual function and pessary management among women using a pessary for pelvic floor disorders. J Sex Med. 2015;12:2339-2349.
- Kuhn A, Bapst D, Stadlmayr W, et al. Sexual and organ function in patients with symptomatic prolapse: are pessaries helpful? Fertil Steril. 2009;91:1914-1918.
- Clemons JL, Aguilar VC, Sokol ER, et al. Patient characteristics that are associated with continued pessary use versus surgery after 1 year. Am J Obstet Gynecol. 2004;191:159-164.
- Liang CC, Chang YL, Chang SD, et al. Pessary test to predict postoperative urinary incontinence in women undergoing hysterectomy for prolapse. Obstet Gynecol. 2004;104:795-800.
- Liapis A, Bakas P, Georgantopoulou C, et al. The use of the pessary test in preoperative assessment of women with severe genital prolapse. Eur J Obstet Gynecol Reprod Biol. 2011; 155:110-113.
- Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366:2358-2367.
- March of Dimes. Quick facts: preterm birth. https://www .marchofdimes.org/Peristats/ViewTopic.aspx?reg=99 &top=3&lev=0&slev=1&gclid=EAIaIQobChMI4r. Accessed December 10, 2020.
- Goya M, Pratcorona L, Merced C, et al; PECEP Trial Group. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomized controlled trial. Lancet. 2012;379:1800-1806.
- Di Tommaso M, Seravalli V, Arduino S, et al. Arabin cervical pessary to prevent preterm birth in twin pregnancies with short cervix. J Obstet Gynaecol. 2016;36:715-718.
- Saccone G, Maruotti GM, Giudicepietro A, et al; Italian Preterm Birth Prevention (IPP) Working Group. Effect of cervical pessary on spontaneous preterm birth in women with singleton pregnancies and short cervical length: a randomized clinical trial. JAMA. 2017;318:2317-2324.
- Perez-Lopez FR, Chedraui P, Perez-Roncero GR, et al; Health Outcomes and Systematic Analyses (HOUSSAY) Project. Effectiveness of the cervical pessary for the prevention of preterm birth in singleton pregnancies with a short cervix: a meta-analysis of randomized trials. Arch Gynecol Obstet. 2019;299:1215-1231.
- Hui SYA, Chor CM, Lau TK, et al. Cerclage pessary for preventing preterm birth in women with a singleton pregnancy and a short cervix at 20 to 24 weeks: a randomized controlled trial. Am J Perinatol. 2013;30:283-288.
- Nicolaides KH, Syngelaki A, Poon LC, et al. A randomized trial of a cervical pessary to prevent preterm singleton birth. N Engl J Med. 2016;374:1044-1052.
- Saccone G, Ciardulli A, Xodo S, et al. Cervical pessary for preventing preterm birth in singleton pregnancies with short cervical length: a systematic review and meta-analyses. J Ultrasound Med. 2017;36:1535-1543.
- Conde-Agudelo A, Romero R, Nicolaides KH. Cervical pessary to prevent preterm birth in asymptomatic high-risk women: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:42-65.e2.
- Sultan AH, Kamm MA, Hudson CN, et al. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329: 1905-1911.
- Talley NJ, O’Keefe EA, Zinsmeister AR, et al. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895-901.
- Denis P, Bercoff E, Bizien MF, et al. Prevalence of anal incontinence in adults [in French]. Gastroenterol Clin Biol. 1992;16:344-350.
- Richter HE, Matthew CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125:540-547.
- 2019 Current Procedural Coding Expert. Optum360; 2018.
- ICD-10-CM Expert for Physicians. Optum360; 2019.
- MDS Medical Department Store website. http://www .medicaldepartmentstore.com/Pessary-Vaginal -Pessaries-/3788.htm?gclid=CjwKCAiAlNf-BRB _EiwA2osbxdqln8fQg-AxOUEMphM9aYlTIft Skwy0xXLT0PrcpIZnb5gBhiLc1RoCsbMQAvD_BwE. Accessed December 15, 2020.
- Monarch Medical Products website. https://www .monarchmedicalproducts.com/index.php?route=product /category&path=99_67. Accessed December 15, 2020.
- CooperSurgical Medical Devices website. https://www .coopersurgical.com/our-brands/milex/. Accessed December 15, 2020.

In Part 1 of this article in the December 2020 issue of OBG Management, I discussed the reasons that pessaries are an effective treatment option for many women with pelvic organ prolapse (POP) and stress urinary incontinence (SUI) and provided details on the types of pessaries available.
In this article, I highlight the steps in fitting a pessary, pessary aftercare, and potential complications associated with pessary use. In addition, I discuss the effectiveness of pessary treatment for POP and SUI as well as for preterm labor prevention and defecatory disorders.
The pessary fitting process
For a given patient, the best size pessary is the smallest one that will not fall out. The only “rule” for fitting a pessary is that a woman’s internal vaginal caliber should be wider than her introitus.
When fitting a pessary, goals include that the selected pessary:
- should be comfortable for the patient to wear
- is not easily expelled
- does not interfere with urination or defecation
- does not cause vaginal irritation.
The presence or absence of a cervix or uterus does not affect pessary choice.
Most experts agree that the process for fitting the right size pessary is one of trial and error. As with fitting a contraceptive diaphragm, the clinician should perform a manual examination to estimate the integrity and width of the perineum and the depth of the vagina to roughly approximate the pessary size that might best fit. Using a set of “fitting pessaries,” a pessary of the estimated size should be placed into the vagina and the fit evaluated as to whether the device is too big, too small, or appropriate. If the pessary is easily expelled, larger sizes should be tried until the pessary remains in place or the patient is uncomfortable. Once the pessary is in place, the clinician should be able to run his or her finger around the entire pessary; if this is not possible, the pessary is too tight. In addition, the pessary should remain more than one finger breadth above the introitus when the patient is standing or bearing down.
Since many patients who require a pessary are elderly, their perineal skin and vaginal mucosa may be atrophic and fragile. Inserting a pessary can be uncomfortable and can cause abrasions or tears. Successfully fitting a pessary may require extra care under these circumstances. The following steps may help alleviate these difficulties:
- Explain the fitting process to the patient in detail.
- Employ lubrication liberally.
- Enlarge the introitus by applying gentle digital pressure on the posterior fourchette.
- Apply 2% lidocaine ointment several minutes prior to pessary fitting to help decrease patient discomfort.
- Treat the patient for several weeks with vaginal estrogen cream before attempting to fit a pessary if severe vulvovaginal atrophy is present.
Once the type and size of the pessary are selected and a pessary is inserted, evaluate the patient with the pessary in place. Assess for the following:
Discomfort. Ask the patient if she feels discomfort with the pessary in position. A patient with a properly fitting pessary should not feel that it is in place. If she does feel discomfort initially, the discomfort will only increase with time and the issue should be addressed at that time.
Expulsion. Test to make certain that the pessary is not easily expelled from the vagina. Have the patient walk, cough, squat, and even jump if possible.
Urination. Have the patient urinate with the pessary in place. This tests for her ability to void while wearing the pessary and shows whether the contraction of pelvic muscles during voiding results in expulsion of the pessary. (Experience shows that it is best to do this with a plastic “hat” over the toilet so that if the pessary is expelled, it does not drop into the bowl.)
Re-examination. After these provocative tests, examine the patient again to ensure that the pessary has not slid out of place.
Depending on whether or not your office stocks pessaries, at this point the patient is either given the correct type and size of pessary or it is ordered for her. If the former, the patient should try placing it herself; if she is unable to, the clinician should place it for her. In either event, its position should be checked. If the pessary has to be ordered, the patient must schedule an appointment to return for pessary insertion.
Whether the pessary is supplied by the office or ordered, instruct the patient on how to insert and remove the pessary, how frequently to remove it for cleansing (see below), and signs to watch for, such as vaginal bleeding, inability to void or defecate, or pelvic pain.
It is advisable to schedule a subsequent visit for 2 to 3 weeks after initial pessary placement to assess how the patient is doing and to address any issues that have developed.
Continue to: Special circumstances...
Special circumstances
It is safe for a patient with a pessary in place to undergo magnetic resonance imaging.1 Patients should be informed, however, that full body scans, such as at airports, will detect pessaries. Patients may need to obtain a physician’s note to document that the pessary is a medical device.
Finally, several factors may prevent successful pessary fitting. These include prior pelvic surgery, obesity, short vaginal length (less than 6–7 cm), and a vaginal introitus width of greater than 4 finger breadths.
Necessary pessary aftercare
Once a pessary is in place and the patient is comfortable with it, the only maintenance necessary is the pessary’s intermittent removal for cleansing and for evaluation of the vaginal mucosa for erosion and ulcerations. How frequently this should be done varies based on the type of pessary, the amount of discharge that a woman produces, whether or not an odor develops after prolonged wearing of the pessary, and whether or not the patient’s vaginal mucosa has been abraded.
The question of timing for pessary cleaning
Although there are many opinions about how often pessaries should be removed and cleaned, no data in the literature support any specific interval. Pessaries that are easily removed by women themselves can be cleaned as frequently as desired, often on a weekly basis. The patient simply removes the pessary, washes it with soap and water, and reinserts it. For pessaries that are difficult to remove (such as the Gellhorn, cube, or donut) or for women who are physically unable to remove their own ring pessary, the clinician should remove and clean the pessary in the office every 3 to 6 months. It has been shown that there is no difference in complications from pessary use with either of these intervals.2
Prior to any vaginal surgical procedure, patients must be instructed to remove their pessary 10 to 14 days beforehand so that the surgeon can see the full extent of prolapse when making decisions about reconstruction and so that any vaginal mucosal erosions or abrasions have time to heal.
Office visits for follow-up care
The pessary “cleaning visit” has several goals, including to:
- see if the pessary is meeting the patient’s needs in terms of resolving symptoms of prolapse and/or restoring urinary continence
- discuss with the patient any problems she may be having, such as pelvic discomfort or pressure, difficulty voiding or defecating, excessive vaginal discharge, or vaginal odor
- check for vaginal mucosal erosion or ulceration; such vaginal lesions often can be prevented by the prophylactic use of either estrogen vaginal cream twice weekly or the continuous use of an estradiol vaginal ring in addition to the pessary
- evaluate the condition of the pessary itself and clean it with soap and water.
Continue to: Potential complications of pessary use...
Potential complications of pessary use
The most common complications experienced by pessary users are:
Odor or excessive discharge. Bacterial vaginosis (BV) occurs more frequently in women who use pessaries. The symptoms of BV can be minimized—but unfortunately not totally eliminated—by the prophylactic use of antiseptic vaginal creams or gels, such as metronidazole, clindamycin, Trimo-San (oxyquinoline sulfate and sodium lauryl sulfate), and others. Inserting the gel vaginally once a week can significantly reduce discharge and odor.3
Vaginal mucosal erosion and ulceration. These are treated by removing the pessary for 2 weeks during which time estrogen cream is applied daily or an estradiol vaginal ring is put in place. If no resolution occurs after 2 weeks, the nonhealing vaginal mucosa should be biopsied.
Pressure on the rectum or bladder. If the pessary causes significant discomfort or interferes with voiding function, then either a different size or a different type pessary should be tried
Patients may discontinue pessary use for a variety of reasons. Among these are:
- discomfort
- inadequate improvement of POP or incontinence symptoms
- expulsion of the pessary during daily activities
- the patient’s desire for surgery instead
- worsening of urine leakage
- difficulty inserting or removing the pessary
- damage to the vaginal mucosa
- pain during removal of the pessary in the office.
Pessary effectiveness for POP and SUI symptoms
As might be expected with a device that is available in so many forms and is used to treat varied types of POP and SUI, the data concerning the success rates of pessary use vary considerably. These rates depend on the definition of success, that is, complete or partial control of prolapse and/or incontinence; which devices are being evaluated; and the nature and severity of the POP and/or SUI being treated.
That being said, a review of the literature reveals that the rates of prolapse symptom relief vary from 48% to 92% (TABLE 1).4-13
As for success in relieving symptoms of incontinence, studies show improvements in from 40% to 77% of patients (TABLE 2).6,8,14-17
In addition, some studies show a 50% improvement in bowel symptoms (urgency, obstruction, and anal incontinence) with the use of a pessary.9,18
How pessaries compare with surgery
While surgery has the advantage of being a one-time fix with a very high rate of initial success in correcting both POP and incontinence, surgery also has potential drawbacks:
- It is an invasive procedure with the discomfort and risk of complications any surgery entails.
- There is a relatively high rate of prolapse recurrence.
- It exposes the patient to the possibility of mesh erosion if mesh is employed either for POP support or incontinence treatment.
Pessaries, on the other hand, are inexpensive, nonsurgical, removable, and allow for immediate correction of symptoms. Moreover, if the pessary is tried and is found to be unsatisfactory, surgery always can be performed subsequently.
Drawbacks of pessary treatment compared with surgery include the:
- ongoing need to wear an artificial internal device
- need for intermittent pessary removal and cleansing
- inability to have sexual intercourse with certain kinds of pessaries in place
- possible accumulation of vaginal discharge and odor.
Sexual activity and pessaries
Studies by Fernando, Meriwether, and Kuhn concur that for a substantial number of pessary users who are sexually active, both frequency and satisfaction with sexual intercourse are increased.8,19,20 Kuhn further showed that desire, orgasm, and lubrication improved with the use of pessaries.20 While some types of pessaries do require removal for intercourse, Clemons reported that issues involving sexual activity are not associated with pessary discontinuation.21
Using a pessary to predict a surgical outcome
Because a pessary elevates the pelvic organs, supports the vaginal walls, and lifts the bladder and urethra into a position that simulates the results of surgical repair, trial placement of a pessary can be used as a fairly accurate predictive tool to model what pelvic support and continence status will be after a proposed surgical procedure.22,23 This is especially important because a significant number of patients with POP will have their occult stress incontinence unmasked following a reparative procedure.24 A brief pessary trial prior to surgery, therefore, can be a useful tool for both patient and surgeon.
Continue to: Pessaries for prevention of preterm labor...
Pessaries for prevention of preterm labor
Almost 1 in 10 births in the United States occurs before 37 completed weeks of gestation.25 Obstetricians have long thought that in women at risk for preterm delivery, the use of a pessary might help reduce the pressure of the growing uterus on the cervix and thus help prevent premature cervical dilation. It also has been thought that use of a pessary would be a safer and less invasive alternative to cervical cerclage. Many studies have evaluated the use of pessaries for the prevention of preterm labor with a mixture of positive (TABLE 3)26-29 and negative results (TABLE 4).30-33


From these data, it is reasonable to conclude that:
- The final answer concerning the effectiveness or lack thereof of pessary use in preventing preterm delivery is not yet in.
- Any advantage there might be to using pessaries to prevent preterm delivery cannot be too significant if multiple studies show as many negative outcomes as positive ones.
Pessary effectiveness in defecatory disorders
Vaginal birth has the potential to create multiple anatomic injuries in the anus, lower pelvis, and perineum that can affect defecation and bowel control. Tears of the anal sphincter, whether obvious or occult, may heal incompletely or be repaired inadequately.34 Nerve innervation of the perianal and perineal areas can be interrupted or damaged by stretching, tearing, or prolonged compression. Of healthy parous adult women, 7% to 16% admit incontinence of gas or feces.35,36
In addition, when a rectocele is present, stool in the lower rectum may cause bulging of the anterior rectal wall into the vagina, preventing stool from passing out of the anus. This sometimes requires women to digitally press their posterior vaginal walls during defecation to evacuate stool successfully. The question thus arises as to whether or not pessary placement and subsequent relief of rectoceles might facilitate bowel movements and decrease or eliminate defecatory dysfunction.
As with the issue of pessary use for prevention of preterm delivery, the answer is mixed. For instance, while Brazell18 showed that there was an overall improvement in bowel symptoms in pessary users, a study by Komesu10 did not demonstrate improvement.
There is, however, a relatively new device specifically designed to control defecatory problems: the vaginal bowel control system (Eclipse; Pelvalon). The silicon device is placed intravaginally as one does a pessary. After insertion, it is inflated via a valve and syringe. It works by putting pressure on and reversibly closing the lower rectum, thus blocking the uncontrolled passage of stool and gas. It can be worn continuously or intermittently, but it does need to be deflated for normal bowel movements. One trial of this device demonstrated a 50% reduction in incontinence episodes with a patient satisfaction rate of 84% at 3 months.37 This device may well prove to be a valuable nonsurgical approach to the treatment of fecal incontinence. Unfortunately, the device is relatively expensive and usually is not covered by insurance as third-party payers do not consider it to be a pessary (which generally is covered).
Practice management particulars
Useful information on Current Procedural Terminology codes for pessaries, diagnostic codes, and the cost of various pessaries is provided in TABLE 5,38TABLE 6,39 and TABLE 7.40-42



A contemporary device used since antiquity
Pessaries, considered “old-fashioned” by many gynecologists, are actually a very cost-effective and useful tool for the correction of POP and SUI. It behooves all who provide medical care to women to be familiar with them, to know when they might be useful, and to know how to fit and prescribe them. ●

In Part 1 of this article in the December 2020 issue of OBG Management, I discussed the reasons that pessaries are an effective treatment option for many women with pelvic organ prolapse (POP) and stress urinary incontinence (SUI) and provided details on the types of pessaries available.
In this article, I highlight the steps in fitting a pessary, pessary aftercare, and potential complications associated with pessary use. In addition, I discuss the effectiveness of pessary treatment for POP and SUI as well as for preterm labor prevention and defecatory disorders.
The pessary fitting process
For a given patient, the best size pessary is the smallest one that will not fall out. The only “rule” for fitting a pessary is that a woman’s internal vaginal caliber should be wider than her introitus.
When fitting a pessary, goals include that the selected pessary:
- should be comfortable for the patient to wear
- is not easily expelled
- does not interfere with urination or defecation
- does not cause vaginal irritation.
The presence or absence of a cervix or uterus does not affect pessary choice.
Most experts agree that the process for fitting the right size pessary is one of trial and error. As with fitting a contraceptive diaphragm, the clinician should perform a manual examination to estimate the integrity and width of the perineum and the depth of the vagina to roughly approximate the pessary size that might best fit. Using a set of “fitting pessaries,” a pessary of the estimated size should be placed into the vagina and the fit evaluated as to whether the device is too big, too small, or appropriate. If the pessary is easily expelled, larger sizes should be tried until the pessary remains in place or the patient is uncomfortable. Once the pessary is in place, the clinician should be able to run his or her finger around the entire pessary; if this is not possible, the pessary is too tight. In addition, the pessary should remain more than one finger breadth above the introitus when the patient is standing or bearing down.
Since many patients who require a pessary are elderly, their perineal skin and vaginal mucosa may be atrophic and fragile. Inserting a pessary can be uncomfortable and can cause abrasions or tears. Successfully fitting a pessary may require extra care under these circumstances. The following steps may help alleviate these difficulties:
- Explain the fitting process to the patient in detail.
- Employ lubrication liberally.
- Enlarge the introitus by applying gentle digital pressure on the posterior fourchette.
- Apply 2% lidocaine ointment several minutes prior to pessary fitting to help decrease patient discomfort.
- Treat the patient for several weeks with vaginal estrogen cream before attempting to fit a pessary if severe vulvovaginal atrophy is present.
Once the type and size of the pessary are selected and a pessary is inserted, evaluate the patient with the pessary in place. Assess for the following:
Discomfort. Ask the patient if she feels discomfort with the pessary in position. A patient with a properly fitting pessary should not feel that it is in place. If she does feel discomfort initially, the discomfort will only increase with time and the issue should be addressed at that time.
Expulsion. Test to make certain that the pessary is not easily expelled from the vagina. Have the patient walk, cough, squat, and even jump if possible.
Urination. Have the patient urinate with the pessary in place. This tests for her ability to void while wearing the pessary and shows whether the contraction of pelvic muscles during voiding results in expulsion of the pessary. (Experience shows that it is best to do this with a plastic “hat” over the toilet so that if the pessary is expelled, it does not drop into the bowl.)
Re-examination. After these provocative tests, examine the patient again to ensure that the pessary has not slid out of place.
Depending on whether or not your office stocks pessaries, at this point the patient is either given the correct type and size of pessary or it is ordered for her. If the former, the patient should try placing it herself; if she is unable to, the clinician should place it for her. In either event, its position should be checked. If the pessary has to be ordered, the patient must schedule an appointment to return for pessary insertion.
Whether the pessary is supplied by the office or ordered, instruct the patient on how to insert and remove the pessary, how frequently to remove it for cleansing (see below), and signs to watch for, such as vaginal bleeding, inability to void or defecate, or pelvic pain.
It is advisable to schedule a subsequent visit for 2 to 3 weeks after initial pessary placement to assess how the patient is doing and to address any issues that have developed.
Continue to: Special circumstances...
Special circumstances
It is safe for a patient with a pessary in place to undergo magnetic resonance imaging.1 Patients should be informed, however, that full body scans, such as at airports, will detect pessaries. Patients may need to obtain a physician’s note to document that the pessary is a medical device.
Finally, several factors may prevent successful pessary fitting. These include prior pelvic surgery, obesity, short vaginal length (less than 6–7 cm), and a vaginal introitus width of greater than 4 finger breadths.
Necessary pessary aftercare
Once a pessary is in place and the patient is comfortable with it, the only maintenance necessary is the pessary’s intermittent removal for cleansing and for evaluation of the vaginal mucosa for erosion and ulcerations. How frequently this should be done varies based on the type of pessary, the amount of discharge that a woman produces, whether or not an odor develops after prolonged wearing of the pessary, and whether or not the patient’s vaginal mucosa has been abraded.
The question of timing for pessary cleaning
Although there are many opinions about how often pessaries should be removed and cleaned, no data in the literature support any specific interval. Pessaries that are easily removed by women themselves can be cleaned as frequently as desired, often on a weekly basis. The patient simply removes the pessary, washes it with soap and water, and reinserts it. For pessaries that are difficult to remove (such as the Gellhorn, cube, or donut) or for women who are physically unable to remove their own ring pessary, the clinician should remove and clean the pessary in the office every 3 to 6 months. It has been shown that there is no difference in complications from pessary use with either of these intervals.2
Prior to any vaginal surgical procedure, patients must be instructed to remove their pessary 10 to 14 days beforehand so that the surgeon can see the full extent of prolapse when making decisions about reconstruction and so that any vaginal mucosal erosions or abrasions have time to heal.
Office visits for follow-up care
The pessary “cleaning visit” has several goals, including to:
- see if the pessary is meeting the patient’s needs in terms of resolving symptoms of prolapse and/or restoring urinary continence
- discuss with the patient any problems she may be having, such as pelvic discomfort or pressure, difficulty voiding or defecating, excessive vaginal discharge, or vaginal odor
- check for vaginal mucosal erosion or ulceration; such vaginal lesions often can be prevented by the prophylactic use of either estrogen vaginal cream twice weekly or the continuous use of an estradiol vaginal ring in addition to the pessary
- evaluate the condition of the pessary itself and clean it with soap and water.
Continue to: Potential complications of pessary use...
Potential complications of pessary use
The most common complications experienced by pessary users are:
Odor or excessive discharge. Bacterial vaginosis (BV) occurs more frequently in women who use pessaries. The symptoms of BV can be minimized—but unfortunately not totally eliminated—by the prophylactic use of antiseptic vaginal creams or gels, such as metronidazole, clindamycin, Trimo-San (oxyquinoline sulfate and sodium lauryl sulfate), and others. Inserting the gel vaginally once a week can significantly reduce discharge and odor.3
Vaginal mucosal erosion and ulceration. These are treated by removing the pessary for 2 weeks during which time estrogen cream is applied daily or an estradiol vaginal ring is put in place. If no resolution occurs after 2 weeks, the nonhealing vaginal mucosa should be biopsied.
Pressure on the rectum or bladder. If the pessary causes significant discomfort or interferes with voiding function, then either a different size or a different type pessary should be tried
Patients may discontinue pessary use for a variety of reasons. Among these are:
- discomfort
- inadequate improvement of POP or incontinence symptoms
- expulsion of the pessary during daily activities
- the patient’s desire for surgery instead
- worsening of urine leakage
- difficulty inserting or removing the pessary
- damage to the vaginal mucosa
- pain during removal of the pessary in the office.
Pessary effectiveness for POP and SUI symptoms
As might be expected with a device that is available in so many forms and is used to treat varied types of POP and SUI, the data concerning the success rates of pessary use vary considerably. These rates depend on the definition of success, that is, complete or partial control of prolapse and/or incontinence; which devices are being evaluated; and the nature and severity of the POP and/or SUI being treated.
That being said, a review of the literature reveals that the rates of prolapse symptom relief vary from 48% to 92% (TABLE 1).4-13
As for success in relieving symptoms of incontinence, studies show improvements in from 40% to 77% of patients (TABLE 2).6,8,14-17
In addition, some studies show a 50% improvement in bowel symptoms (urgency, obstruction, and anal incontinence) with the use of a pessary.9,18
How pessaries compare with surgery
While surgery has the advantage of being a one-time fix with a very high rate of initial success in correcting both POP and incontinence, surgery also has potential drawbacks:
- It is an invasive procedure with the discomfort and risk of complications any surgery entails.
- There is a relatively high rate of prolapse recurrence.
- It exposes the patient to the possibility of mesh erosion if mesh is employed either for POP support or incontinence treatment.
Pessaries, on the other hand, are inexpensive, nonsurgical, removable, and allow for immediate correction of symptoms. Moreover, if the pessary is tried and is found to be unsatisfactory, surgery always can be performed subsequently.
Drawbacks of pessary treatment compared with surgery include the:
- ongoing need to wear an artificial internal device
- need for intermittent pessary removal and cleansing
- inability to have sexual intercourse with certain kinds of pessaries in place
- possible accumulation of vaginal discharge and odor.
Sexual activity and pessaries
Studies by Fernando, Meriwether, and Kuhn concur that for a substantial number of pessary users who are sexually active, both frequency and satisfaction with sexual intercourse are increased.8,19,20 Kuhn further showed that desire, orgasm, and lubrication improved with the use of pessaries.20 While some types of pessaries do require removal for intercourse, Clemons reported that issues involving sexual activity are not associated with pessary discontinuation.21
Using a pessary to predict a surgical outcome
Because a pessary elevates the pelvic organs, supports the vaginal walls, and lifts the bladder and urethra into a position that simulates the results of surgical repair, trial placement of a pessary can be used as a fairly accurate predictive tool to model what pelvic support and continence status will be after a proposed surgical procedure.22,23 This is especially important because a significant number of patients with POP will have their occult stress incontinence unmasked following a reparative procedure.24 A brief pessary trial prior to surgery, therefore, can be a useful tool for both patient and surgeon.
Continue to: Pessaries for prevention of preterm labor...
Pessaries for prevention of preterm labor
Almost 1 in 10 births in the United States occurs before 37 completed weeks of gestation.25 Obstetricians have long thought that in women at risk for preterm delivery, the use of a pessary might help reduce the pressure of the growing uterus on the cervix and thus help prevent premature cervical dilation. It also has been thought that use of a pessary would be a safer and less invasive alternative to cervical cerclage. Many studies have evaluated the use of pessaries for the prevention of preterm labor with a mixture of positive (TABLE 3)26-29 and negative results (TABLE 4).30-33


From these data, it is reasonable to conclude that:
- The final answer concerning the effectiveness or lack thereof of pessary use in preventing preterm delivery is not yet in.
- Any advantage there might be to using pessaries to prevent preterm delivery cannot be too significant if multiple studies show as many negative outcomes as positive ones.
Pessary effectiveness in defecatory disorders
Vaginal birth has the potential to create multiple anatomic injuries in the anus, lower pelvis, and perineum that can affect defecation and bowel control. Tears of the anal sphincter, whether obvious or occult, may heal incompletely or be repaired inadequately.34 Nerve innervation of the perianal and perineal areas can be interrupted or damaged by stretching, tearing, or prolonged compression. Of healthy parous adult women, 7% to 16% admit incontinence of gas or feces.35,36
In addition, when a rectocele is present, stool in the lower rectum may cause bulging of the anterior rectal wall into the vagina, preventing stool from passing out of the anus. This sometimes requires women to digitally press their posterior vaginal walls during defecation to evacuate stool successfully. The question thus arises as to whether or not pessary placement and subsequent relief of rectoceles might facilitate bowel movements and decrease or eliminate defecatory dysfunction.
As with the issue of pessary use for prevention of preterm delivery, the answer is mixed. For instance, while Brazell18 showed that there was an overall improvement in bowel symptoms in pessary users, a study by Komesu10 did not demonstrate improvement.
There is, however, a relatively new device specifically designed to control defecatory problems: the vaginal bowel control system (Eclipse; Pelvalon). The silicon device is placed intravaginally as one does a pessary. After insertion, it is inflated via a valve and syringe. It works by putting pressure on and reversibly closing the lower rectum, thus blocking the uncontrolled passage of stool and gas. It can be worn continuously or intermittently, but it does need to be deflated for normal bowel movements. One trial of this device demonstrated a 50% reduction in incontinence episodes with a patient satisfaction rate of 84% at 3 months.37 This device may well prove to be a valuable nonsurgical approach to the treatment of fecal incontinence. Unfortunately, the device is relatively expensive and usually is not covered by insurance as third-party payers do not consider it to be a pessary (which generally is covered).
Practice management particulars
Useful information on Current Procedural Terminology codes for pessaries, diagnostic codes, and the cost of various pessaries is provided in TABLE 5,38TABLE 6,39 and TABLE 7.40-42



A contemporary device used since antiquity
Pessaries, considered “old-fashioned” by many gynecologists, are actually a very cost-effective and useful tool for the correction of POP and SUI. It behooves all who provide medical care to women to be familiar with them, to know when they might be useful, and to know how to fit and prescribe them. ●
- O’Dell K, Atnip S. Pessary care: follow up and management of complications. Urol Nurs. 2012;32:126-136, 145.
- Gorti M, Hudelist G, Simons A. Evaluation of vaginal pessary management: a UK-based survey. J Obstet Gynaecol. 2009;29:129-131.
- Meriwether KV, Rogers RG, Craig E, et al. The effect of hydroxyquinoline-based gel on pessary-associated bacterial vaginosis: a multicenter randomized controlled trial. Am J Obstet Gynecol. 2015;213:729.e1-9.
- Wu V, Farrell SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Bai SW, Yoon BS, Kwon JY, et al. Survey of the characteristics and satisfaction degree of the patients using a pessary. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:182-186.
- Clemons JL, Aguilar VC, Tillinghast TA, et al. Patient satisfaction and changes in prolapse and urinary symptoms in women who were fitted successfully with a pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2004;190:1025-1029.
- Hanson LM, Schulz JA, Flood CG, et al. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17: 155-159.
- Fernando RJ, Thakar R, Sultan AH, et al. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108:93-99.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-405e.8.
- Komesu YM Rogers RG, Rode MA, et al. Pelvic floor symptom changes in pessary users. Am J Obstet Gynecol. 2007;197: 620.e1-6.
- Yang J, Han J, Zhu F, et al. Ring and Gellhorn pessaries used inpatients with pelvic organ prolapse: a retrospective study of 8 years. Arch Gynecol Obstet. 2018;298:623-629.
- Mao M, Ai F, Zhang Y, et al. Changes in the symptoms and quality of life of women with symptomatic pelvic organ prolapse fitted with a ring with support pessary. Maturitas. 2018;117:51-56.
- Duenas JL, Miceli A. Effectiveness of a continuous-use ringshaped vaginal pessary without support for advanced pelvic organ prolapse in postmenopausal women. Int Urogynecol J. 2018;29:1629-1636.
- Farrell S, Singh B, Aldakhil L. Continence pessaries in the management of urinary incontinence in women. J Obstet Gynaecol Canada. 2004;26:113-117.
- Donnelly MJ, Powell-Morgan SP, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:302-307.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Ding J, Chen C, Song XC, et al. Changes in prolapse and urinary symptoms after successful fitting of a ring pessary with support in women with advanced pelvic organ prolapse: a prospective study. Urology. 2016;87:70-75.
- Brazell HD, Patel M, O’Sullivan DM, et al. The impact of pessary use on bowel symptoms: one-year outcomes. Female Pelvic Med Reconstr Surg. 2014;20:95-98.
- Meriwether KV, Komesu YM, Craig C, et al. Sexual function and pessary management among women using a pessary for pelvic floor disorders. J Sex Med. 2015;12:2339-2349.
- Kuhn A, Bapst D, Stadlmayr W, et al. Sexual and organ function in patients with symptomatic prolapse: are pessaries helpful? Fertil Steril. 2009;91:1914-1918.
- Clemons JL, Aguilar VC, Sokol ER, et al. Patient characteristics that are associated with continued pessary use versus surgery after 1 year. Am J Obstet Gynecol. 2004;191:159-164.
- Liang CC, Chang YL, Chang SD, et al. Pessary test to predict postoperative urinary incontinence in women undergoing hysterectomy for prolapse. Obstet Gynecol. 2004;104:795-800.
- Liapis A, Bakas P, Georgantopoulou C, et al. The use of the pessary test in preoperative assessment of women with severe genital prolapse. Eur J Obstet Gynecol Reprod Biol. 2011; 155:110-113.
- Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366:2358-2367.
- March of Dimes. Quick facts: preterm birth. https://www .marchofdimes.org/Peristats/ViewTopic.aspx?reg=99 &top=3&lev=0&slev=1&gclid=EAIaIQobChMI4r. Accessed December 10, 2020.
- Goya M, Pratcorona L, Merced C, et al; PECEP Trial Group. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomized controlled trial. Lancet. 2012;379:1800-1806.
- Di Tommaso M, Seravalli V, Arduino S, et al. Arabin cervical pessary to prevent preterm birth in twin pregnancies with short cervix. J Obstet Gynaecol. 2016;36:715-718.
- Saccone G, Maruotti GM, Giudicepietro A, et al; Italian Preterm Birth Prevention (IPP) Working Group. Effect of cervical pessary on spontaneous preterm birth in women with singleton pregnancies and short cervical length: a randomized clinical trial. JAMA. 2017;318:2317-2324.
- Perez-Lopez FR, Chedraui P, Perez-Roncero GR, et al; Health Outcomes and Systematic Analyses (HOUSSAY) Project. Effectiveness of the cervical pessary for the prevention of preterm birth in singleton pregnancies with a short cervix: a meta-analysis of randomized trials. Arch Gynecol Obstet. 2019;299:1215-1231.
- Hui SYA, Chor CM, Lau TK, et al. Cerclage pessary for preventing preterm birth in women with a singleton pregnancy and a short cervix at 20 to 24 weeks: a randomized controlled trial. Am J Perinatol. 2013;30:283-288.
- Nicolaides KH, Syngelaki A, Poon LC, et al. A randomized trial of a cervical pessary to prevent preterm singleton birth. N Engl J Med. 2016;374:1044-1052.
- Saccone G, Ciardulli A, Xodo S, et al. Cervical pessary for preventing preterm birth in singleton pregnancies with short cervical length: a systematic review and meta-analyses. J Ultrasound Med. 2017;36:1535-1543.
- Conde-Agudelo A, Romero R, Nicolaides KH. Cervical pessary to prevent preterm birth in asymptomatic high-risk women: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:42-65.e2.
- Sultan AH, Kamm MA, Hudson CN, et al. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329: 1905-1911.
- Talley NJ, O’Keefe EA, Zinsmeister AR, et al. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895-901.
- Denis P, Bercoff E, Bizien MF, et al. Prevalence of anal incontinence in adults [in French]. Gastroenterol Clin Biol. 1992;16:344-350.
- Richter HE, Matthew CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125:540-547.
- 2019 Current Procedural Coding Expert. Optum360; 2018.
- ICD-10-CM Expert for Physicians. Optum360; 2019.
- MDS Medical Department Store website. http://www .medicaldepartmentstore.com/Pessary-Vaginal -Pessaries-/3788.htm?gclid=CjwKCAiAlNf-BRB _EiwA2osbxdqln8fQg-AxOUEMphM9aYlTIft Skwy0xXLT0PrcpIZnb5gBhiLc1RoCsbMQAvD_BwE. Accessed December 15, 2020.
- Monarch Medical Products website. https://www .monarchmedicalproducts.com/index.php?route=product /category&path=99_67. Accessed December 15, 2020.
- CooperSurgical Medical Devices website. https://www .coopersurgical.com/our-brands/milex/. Accessed December 15, 2020.
- O’Dell K, Atnip S. Pessary care: follow up and management of complications. Urol Nurs. 2012;32:126-136, 145.
- Gorti M, Hudelist G, Simons A. Evaluation of vaginal pessary management: a UK-based survey. J Obstet Gynaecol. 2009;29:129-131.
- Meriwether KV, Rogers RG, Craig E, et al. The effect of hydroxyquinoline-based gel on pessary-associated bacterial vaginosis: a multicenter randomized controlled trial. Am J Obstet Gynecol. 2015;213:729.e1-9.
- Wu V, Farrell SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Bai SW, Yoon BS, Kwon JY, et al. Survey of the characteristics and satisfaction degree of the patients using a pessary. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:182-186.
- Clemons JL, Aguilar VC, Tillinghast TA, et al. Patient satisfaction and changes in prolapse and urinary symptoms in women who were fitted successfully with a pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2004;190:1025-1029.
- Hanson LM, Schulz JA, Flood CG, et al. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17: 155-159.
- Fernando RJ, Thakar R, Sultan AH, et al. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108:93-99.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-405e.8.
- Komesu YM Rogers RG, Rode MA, et al. Pelvic floor symptom changes in pessary users. Am J Obstet Gynecol. 2007;197: 620.e1-6.
- Yang J, Han J, Zhu F, et al. Ring and Gellhorn pessaries used inpatients with pelvic organ prolapse: a retrospective study of 8 years. Arch Gynecol Obstet. 2018;298:623-629.
- Mao M, Ai F, Zhang Y, et al. Changes in the symptoms and quality of life of women with symptomatic pelvic organ prolapse fitted with a ring with support pessary. Maturitas. 2018;117:51-56.
- Duenas JL, Miceli A. Effectiveness of a continuous-use ringshaped vaginal pessary without support for advanced pelvic organ prolapse in postmenopausal women. Int Urogynecol J. 2018;29:1629-1636.
- Farrell S, Singh B, Aldakhil L. Continence pessaries in the management of urinary incontinence in women. J Obstet Gynaecol Canada. 2004;26:113-117.
- Donnelly MJ, Powell-Morgan SP, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:302-307.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Ding J, Chen C, Song XC, et al. Changes in prolapse and urinary symptoms after successful fitting of a ring pessary with support in women with advanced pelvic organ prolapse: a prospective study. Urology. 2016;87:70-75.
- Brazell HD, Patel M, O’Sullivan DM, et al. The impact of pessary use on bowel symptoms: one-year outcomes. Female Pelvic Med Reconstr Surg. 2014;20:95-98.
- Meriwether KV, Komesu YM, Craig C, et al. Sexual function and pessary management among women using a pessary for pelvic floor disorders. J Sex Med. 2015;12:2339-2349.
- Kuhn A, Bapst D, Stadlmayr W, et al. Sexual and organ function in patients with symptomatic prolapse: are pessaries helpful? Fertil Steril. 2009;91:1914-1918.
- Clemons JL, Aguilar VC, Sokol ER, et al. Patient characteristics that are associated with continued pessary use versus surgery after 1 year. Am J Obstet Gynecol. 2004;191:159-164.
- Liang CC, Chang YL, Chang SD, et al. Pessary test to predict postoperative urinary incontinence in women undergoing hysterectomy for prolapse. Obstet Gynecol. 2004;104:795-800.
- Liapis A, Bakas P, Georgantopoulou C, et al. The use of the pessary test in preoperative assessment of women with severe genital prolapse. Eur J Obstet Gynecol Reprod Biol. 2011; 155:110-113.
- Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366:2358-2367.
- March of Dimes. Quick facts: preterm birth. https://www .marchofdimes.org/Peristats/ViewTopic.aspx?reg=99 &top=3&lev=0&slev=1&gclid=EAIaIQobChMI4r. Accessed December 10, 2020.
- Goya M, Pratcorona L, Merced C, et al; PECEP Trial Group. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomized controlled trial. Lancet. 2012;379:1800-1806.
- Di Tommaso M, Seravalli V, Arduino S, et al. Arabin cervical pessary to prevent preterm birth in twin pregnancies with short cervix. J Obstet Gynaecol. 2016;36:715-718.
- Saccone G, Maruotti GM, Giudicepietro A, et al; Italian Preterm Birth Prevention (IPP) Working Group. Effect of cervical pessary on spontaneous preterm birth in women with singleton pregnancies and short cervical length: a randomized clinical trial. JAMA. 2017;318:2317-2324.
- Perez-Lopez FR, Chedraui P, Perez-Roncero GR, et al; Health Outcomes and Systematic Analyses (HOUSSAY) Project. Effectiveness of the cervical pessary for the prevention of preterm birth in singleton pregnancies with a short cervix: a meta-analysis of randomized trials. Arch Gynecol Obstet. 2019;299:1215-1231.
- Hui SYA, Chor CM, Lau TK, et al. Cerclage pessary for preventing preterm birth in women with a singleton pregnancy and a short cervix at 20 to 24 weeks: a randomized controlled trial. Am J Perinatol. 2013;30:283-288.
- Nicolaides KH, Syngelaki A, Poon LC, et al. A randomized trial of a cervical pessary to prevent preterm singleton birth. N Engl J Med. 2016;374:1044-1052.
- Saccone G, Ciardulli A, Xodo S, et al. Cervical pessary for preventing preterm birth in singleton pregnancies with short cervical length: a systematic review and meta-analyses. J Ultrasound Med. 2017;36:1535-1543.
- Conde-Agudelo A, Romero R, Nicolaides KH. Cervical pessary to prevent preterm birth in asymptomatic high-risk women: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:42-65.e2.
- Sultan AH, Kamm MA, Hudson CN, et al. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329: 1905-1911.
- Talley NJ, O’Keefe EA, Zinsmeister AR, et al. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895-901.
- Denis P, Bercoff E, Bizien MF, et al. Prevalence of anal incontinence in adults [in French]. Gastroenterol Clin Biol. 1992;16:344-350.
- Richter HE, Matthew CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125:540-547.
- 2019 Current Procedural Coding Expert. Optum360; 2018.
- ICD-10-CM Expert for Physicians. Optum360; 2019.
- MDS Medical Department Store website. http://www .medicaldepartmentstore.com/Pessary-Vaginal -Pessaries-/3788.htm?gclid=CjwKCAiAlNf-BRB _EiwA2osbxdqln8fQg-AxOUEMphM9aYlTIft Skwy0xXLT0PrcpIZnb5gBhiLc1RoCsbMQAvD_BwE. Accessed December 15, 2020.
- Monarch Medical Products website. https://www .monarchmedicalproducts.com/index.php?route=product /category&path=99_67. Accessed December 15, 2020.
- CooperSurgical Medical Devices website. https://www .coopersurgical.com/our-brands/milex/. Accessed December 15, 2020.
Two at-home apps for patients with pelvic floor disorders
In the “You asked, Dr. Jen Gunter answered” series in The New York Times, Dr. Gunter writes that “pelvic floor exercises (also known as Kegel exercises) can be very helpful for urinary incontinence, pelvic organ prolapse, and fecal incontinence.” She continues to say that “pelvic floor exercises can be hard to master correctly, so it is important to make sure [one has] the correct technique. Many women can learn to do them after reading instructions like the ones found at the National Association for Continence, but some women may need their technique checked by their doctor, or help from a specialized pelvic floor physical therapist.”1
Similarly, Sudol and colleagues write that “guidelines from multiple medical societies emphasize the importance of patient education, behavioral therapy, and/or exercise regimens in the initial treatment and management of women with pelvic floor disorders. However, even with well-established recommendations, engaging patients and maintaining adherence to treatment plans and unmonitored programs at home are often difficult.”2 To help patients, those authors identified and evaluated patient-centered apps on topics in female pelvic medicine and reconstructive surgery.2
Two apps that assist patients in Kegel exercises are presented here. The Squeezy app includes guided pelvic floor muscle exercises with reminders, and the Kegel Nation app has a biofeedback feature.
The TABLE details the features of the 2 apps based on a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature used, and important special features).3
I hope clinicians find these apps helpful to their patients with pelvic floor disorders.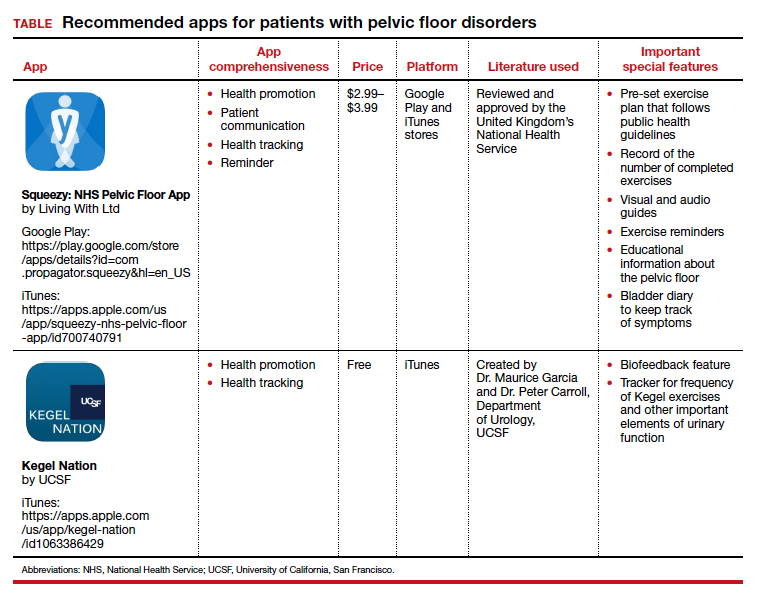
- Gunter J. You asked, Dr. Jen Gunter answered. New York Times. https://www.nytimes.com/ask/answers/kegels-pelvic-floor-exercises-yoni-eggs. Accessed December 22, 2020.
- Sudol NT, Adams-Piper E, Perry R, et al. In search of mobile applications for patients with pelvic floor disorders. Female Pelvic Med Reconstr Surg. 2019;25:252-256.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
In the “You asked, Dr. Jen Gunter answered” series in The New York Times, Dr. Gunter writes that “pelvic floor exercises (also known as Kegel exercises) can be very helpful for urinary incontinence, pelvic organ prolapse, and fecal incontinence.” She continues to say that “pelvic floor exercises can be hard to master correctly, so it is important to make sure [one has] the correct technique. Many women can learn to do them after reading instructions like the ones found at the National Association for Continence, but some women may need their technique checked by their doctor, or help from a specialized pelvic floor physical therapist.”1
Similarly, Sudol and colleagues write that “guidelines from multiple medical societies emphasize the importance of patient education, behavioral therapy, and/or exercise regimens in the initial treatment and management of women with pelvic floor disorders. However, even with well-established recommendations, engaging patients and maintaining adherence to treatment plans and unmonitored programs at home are often difficult.”2 To help patients, those authors identified and evaluated patient-centered apps on topics in female pelvic medicine and reconstructive surgery.2
Two apps that assist patients in Kegel exercises are presented here. The Squeezy app includes guided pelvic floor muscle exercises with reminders, and the Kegel Nation app has a biofeedback feature.
The TABLE details the features of the 2 apps based on a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature used, and important special features).3
I hope clinicians find these apps helpful to their patients with pelvic floor disorders.
In the “You asked, Dr. Jen Gunter answered” series in The New York Times, Dr. Gunter writes that “pelvic floor exercises (also known as Kegel exercises) can be very helpful for urinary incontinence, pelvic organ prolapse, and fecal incontinence.” She continues to say that “pelvic floor exercises can be hard to master correctly, so it is important to make sure [one has] the correct technique. Many women can learn to do them after reading instructions like the ones found at the National Association for Continence, but some women may need their technique checked by their doctor, or help from a specialized pelvic floor physical therapist.”1
Similarly, Sudol and colleagues write that “guidelines from multiple medical societies emphasize the importance of patient education, behavioral therapy, and/or exercise regimens in the initial treatment and management of women with pelvic floor disorders. However, even with well-established recommendations, engaging patients and maintaining adherence to treatment plans and unmonitored programs at home are often difficult.”2 To help patients, those authors identified and evaluated patient-centered apps on topics in female pelvic medicine and reconstructive surgery.2
Two apps that assist patients in Kegel exercises are presented here. The Squeezy app includes guided pelvic floor muscle exercises with reminders, and the Kegel Nation app has a biofeedback feature.
The TABLE details the features of the 2 apps based on a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature used, and important special features).3
I hope clinicians find these apps helpful to their patients with pelvic floor disorders.
- Gunter J. You asked, Dr. Jen Gunter answered. New York Times. https://www.nytimes.com/ask/answers/kegels-pelvic-floor-exercises-yoni-eggs. Accessed December 22, 2020.
- Sudol NT, Adams-Piper E, Perry R, et al. In search of mobile applications for patients with pelvic floor disorders. Female Pelvic Med Reconstr Surg. 2019;25:252-256.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
- Gunter J. You asked, Dr. Jen Gunter answered. New York Times. https://www.nytimes.com/ask/answers/kegels-pelvic-floor-exercises-yoni-eggs. Accessed December 22, 2020.
- Sudol NT, Adams-Piper E, Perry R, et al. In search of mobile applications for patients with pelvic floor disorders. Female Pelvic Med Reconstr Surg. 2019;25:252-256.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
Pessaries for POP and SUI: Your options and guidance on use
Over the last 30 years, surgical correction of the common condition pelvic organ prolapse (POP) and stress urinary incontinence (SUI) has become so routine and straightforward that many gynecologists and urogynecologists choose surgery as their first choice for treating these conditions, withholding it only from the riskiest patients or from those who, for a variety of reasons, do not choose surgery. Moreover, as generalist gynecologists increasingly refer patients with POP or incontinence to their urogynecologist colleagues, they increasingly lack the skills, or have not been trained, to use conservative treatment strategies for these disorders. Thus, pessaries—devices constructed of inert plastic, silicone, or latex and placed inside the vagina to support prolapsed pelvic structures—frequently are not part of the general gynecologist’s armamentarium.
When properly selected, however, pessaries used for indicated purposes and correctly fitted are an excellent, inexpensive, low-risk, and noninvasive tool that can provide immediate relief not only of POP but also of SUI and defecatory difficulties. As an alternative to surgery, pessaries are especially valuable, because the other major nonsurgical modality for treatment of POP and incontinence—pelvic floor muscle training—often is not covered by insurance (making it expensive for patients), takes many weekly sessions to complete (which can make access challenging), and frequently is not readily available.1
POP is very common. An estimated 15% to 30% of women in North America have some degree of prolapse, and more than 500,000 surgeries for this condition are performed in the United States each year.2 Risk factors for POP include:
- vaginal childbirth, especially higher parity
- advancing age
- high body mass index (BMI)
- prior hysterectomy
- raised intra-abdominal pressure, such as from obesity, chronic cough, or heavy lifting.
In addition to the discomfort caused by the herniation of pelvic and vaginal structures, POP also is associated with urinary incontinence (73%), urinary urgency and frequency (86%), and fecal incontinence (31%).3
Moreover, according to the US Census Bureau, the number of American women aged 65 or older will double to more than 40 million by 2030.4 This will greatly increase the population of women at risk for POP who may be candidates for pessary use. It therefore behooves gynecologists to become familiar with the correct usage, fitting, and maintenance of this effective, nonsurgical mode of treatment for POP.
In this article, I discuss why pessaries are a good option for many patients with POP, review the types of pessaries available, and offer guidance on how to choose the right pessary for an individual patient’s needs. In addition, the box at the end of this article provides an interesting timeline of pessary history dating back to antiquity.
Next month in Part 2 of this article, I cover how to fit a pessary; device aftercare; potential complications of use; and effectiveness of pessaries for POP, SUI, preterm labor prevention, and defecatory disorders.
Continue to: Potential candidates for pessary use...
Potential candidates for pessary use
Almost all women with POP—and in many cases accompanying SUI—are potential candidates for a pessary. In fact, many urogynecologists believe that a trial of pessary usage should be the first treatment modality offered for POP.5 Women who cannot use a pessary include those with an extremely short vagina (<6 cm) and those who have severely eroded vaginal mucosa. In the latter situation, the mucosa can be treated with estrogen cream for several weeks and, once the tissue has healed, a pessary can be fitted.
Given that surgical repair is generally a straightforward, one-time procedure that obviates the need for long-term use of an artificial device worn internally, why might a patient or her physician opt for a pessary instead?
Some of the many reasons include:
- Many patients prefer to avoid surgery.
- Many patients are not appropriate candidates for surgery because they have significant comorbid risk factors or high BMI.
- Patients may have recurrent prolapse or incontinence and wish to avoid repeat surgery.
- Patients with SUI may have heard of the occurrence of mesh erosion and wish to avoid that possibility.
- Women who live in low-resource environments or countries where elective surgical care is relatively unavailable may not have the option of surgery.
A clinician might also recommend pessary use:
- as a diagnostic tool to attempt to assess the potential results of vaginal repair surgery
- to estimate the potential effectiveness of a midurethral sling procedure; several investigators have found this to be approximately as accurate as urodynamic testing6,7
- as prophylaxis for pregnant women with either a history of preterm cervical dilation or a short cervix detected on ultrasonography
- for pregnant women with POP that is worsening and becoming increasingly uncomfortable
- for women with POP who wish to have more children
- for short-term use while a patient is delaying or awaiting POP surgery or to allow time for other medical issues to resolve
- for patients who wish only intermittent, temporary support while exercising or engaging in sports.
Patient acceptance may be contingent on counseling
Numerous studies show that women who choose pessaries to treat POP are generally older than women who elect surgery. Still, patient acceptance of a trial of pessary use depends much on the counseling and information she receives. Properly informed, many patients with POP will opt for a trial of pessary placement. One study showed that, of women with untreated POP, 36% preferred pessary placement to surgery.8 Other investigators reported that when women with symptomatic POP had the benefits of a pessary versus surgery explained to them, nearly two-thirds opted for a pessary as their mode of treatment.9
Exceptions to pessary use
Fortunately, there are relatively few contraindications to pessary use. These are vaginal or pelvic infection and an exposed foreign body in the vagina, such as eroded vaginal mesh. In addition, patients at risk for nonadherence with follow-up care are poor candidates, as it could lead to missing such problems as mucosal erosion, ulceration, or even (extremely rarely) fistula formation. Pessaries may be inappropriate for sexually active women who on their own are unable to remove and reinsert pessary types that do not allow for intercourse while in place (see below).
Continue to: Types of pessaries...
Types of pessaries
The numerous kinds of pessaries available fall into 3 general categories: support, space filling, and lever, and devices within each group have modifications and variations. As with most areas of prescribing and treatment in medicine, it is best to become very familiar with just a few kinds of pessaries, know their indications, and use them when appropriate.
Most pessaries are constructed of inert silicone which, unlike earlier rubber pessaries, does not absorb odor or discharge. They are easy to clean, long lasting, and are autoclavable and hypoallergenic.
Support pessaries
Support pessaries look like contraceptive diaphragms. They are easy to place and remove, are comfortable, and do an excellent job correcting moderate POP. They also can control or eliminate symptoms of SUI by the pressure they exert on the urethra and their alteration of the urethrovesicular angle.
Ring pessaries. The most commonly used type of pessary, the ring pessary,10 comes in 4 variations:
- a simple open ring
- a ring with a web of material, called a “support shield,” that fills the ring
- an open ring with a firm 2-cm “incontinence knob” attached that is positioned over the urethra
- a ring with support shield and incontinence knob.
When in position, the deepest edge of a ring pessary fits behind the cervix (or in the vaginal apex for women who have had a hysterectomy) while the front of the ring slips into place behind the pubic symphysis, just like a diaphragm. When a ring with an incontinence knob is used, the ring is rotated until the knob is directly over the urethra.
Sexual intercourse is possible with any of the ring pessaries in place. Of the various types of pessaries, the ring pessary is the easiest to insert and remove. Some women tie a piece of dental floss to the edge of the ring to make its removal even easier.
The ring pessary is available in sizes 0 (44.5 mm) to 13 (127 mm). For most women a size 3, 4, or 5 ring pessary fits well.
The Marland pessary is similar to the ring pessary with the addition of a wedge-shaped piece of material approximately 3 cm in height that arises from half of the ring. It rarely is used in the United States because most American gynecologists are unfamiliar with it, and there is little evidence that it is more effective than the ring pessary.11
The Shaatz pessary is a rigid round pessary, smaller in diameter than the standard ring pessary, and similar to the Gellhorn pessary (discussed below) but without a stem. It is placed the same way one places a ring pessary but with its concave surface up against the cervix or, if there is no cervix, against the upper anterior vaginal wall. Its main benefit is that it provides firmer support than the ring pessary. This pessary is not widely used in the United States.
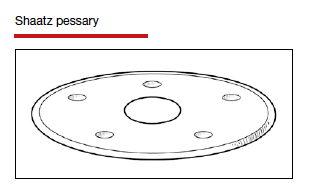
The Gehrung pessary looks like a flat strip of material that has been bent into the shape of a “U.” It is designed to correct severe cystoceles and rectoceles. For insertion, the edges at the open end of the pessary are squeezed together and the pessary is inserted with the closed part of the “U” facing the anterior vaginal wall. The upper edge is advanced until it rests in the anterior fornix of the vagina (or in the vaginal apex in women who have had a hysterectomy). Although it is more efficacious than some other pessaries for control of vaginal wall prolapse, its unfamiliarity to clinicians and its unusual shape result in it being used rarely.
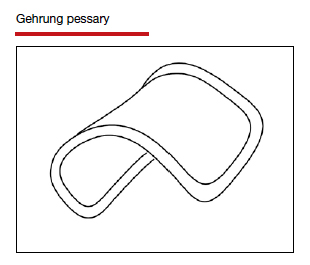
Continue to: Space-filling pessaries...
Space-filling pessaries
Space-filling pessaries are used when more severe degrees of prolapse are present than can be managed by the ring or other support pessaries. This is especially the case when the vagina is so capacious or the introitus so lax that a standard ring pessary cannot be kept in place, resulting in frequent expulsions.
Space-filling pessaries are 3 dimensional and work by filling the vagina with a relatively large object that prevents the cervix/vaginal apex from dropping down and the vaginal walls from prolapsing. They have a special role for women who:
- are posthysterectomy and have an enterocele and/or vaginal apex prolapse
- have significant rectoceles for which support pessaries are not effective
- have a wide vaginal hiatus and thus are prone to expel support pessaries.
Space-filling pessaries do have some drawbacks compared with support pessaries. For example, they do not help in controlling SUI, and they are difficult for patients to remove on their own for cleaning. In addition, sexual intercourse is impossible with a space-filling pessary in place.
The Gellhorn pessary is the most common of the space-filling pessaries, and it is the one gynecologists and urogynecologists most often use for severe prolapse. It has a concave disc that fits up against the cervix or vaginal apex and a solid stem that points down the vagina. The stem itself is supported by the perineal body. It offers excellent support for severe uterine and vaginal wall prolapse, as long as the perineal body is intact. The stem stabilizes the disc portion of the pessary and prevents pessary expulsion. Gellhorn pessaries are available with long or short stems.
The Gellhorn is inserted into the vagina by folding the stem 90 degrees until it is in the same plane as the disc. With lubricated fingers, the patient’s perineal body is depressed and the disc of the pessary is folded and slid in. The disc is then placed up against the cervix or vaginal apex with the stem pointing down the vagina and tucked just inside the posterior edge of the introitus.
Removing the Gellhorn pessary can be problematic and is difficult for patients to do on their own. Clinicians often must use a ring forceps to grasp the stem of the pessary in order to bring it into the lower vagina, where the stem is folded up against the disc and the entire pessary removed. As with all space-filling pessaries, the Gellhorn must be taken out prior to intercourse.
The Gellhorn pessary is available in sizes that range from a disc diameter of 1.5 to 3.75 inches. Those measuring 2.5, 2.75, or 3 inches are used most commonly.
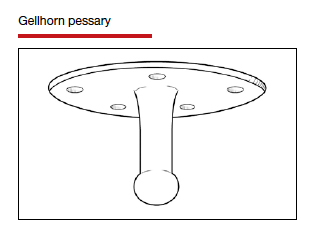
The cube pessary is a soft, dice-shaped piece of silicone with an indentation in each of its 6 sides. It is used for severe prolapse.
Squeezing the cube allows for easier insertion into the vagina; once it is at the top of the vagina, the cube expands back to its normal shape. The indentations on each side of the cube attach to the vaginal walls with moderate suction, which helps to keep the pessary in place. Because of the suction, the cube pessary can be used in cases of severe prolapse when other pessaries will not stay in place; a drawback is that the suction created by the indented sides can cause vaginal mucosal erosion.10 Ideally, the cube pessary should be removed every night for cleansing as discharge and accompanying odor can accumulate. The string attached to the cube pessary aids in its removal.
The cube pessary is available in sizes 0 to 7, with edge lengths that range from 1 to 2.25 inches.
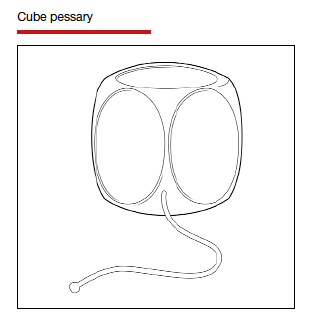
The donut pessary, as its name suggests, has the form of a large donut. It can be compressed slightly to help with insertion. Because it occupies a large space within the vagina, it is used (like the cube pessary) for treatment of severe prolapse. The size and shape of the donut pessary, however, can make it difficult for patients to insert and take out on their own.
The donut pessary is available in sizes 0 (51 mm) to 8 (95 mm).
The inflatable pessary has the same basic shape as the donut pessary and serves the same purpose: It acts as a large semisoft object that fills the vagina to support the vaginal walls and cervix (or vaginal apex) in cases of severe prolapse. The inflatable pessary differs in that it has a valve on a stem through which air can be inserted and removed. This allows the uninflated pessary to be placed relatively easily into the vagina and then pumped full of air to the dimensions necessary to prevent vaginal, cervical, uterine, or apex prolapse. Air likewise can be removed to facilitate pessary removal.
One drawback of the inflatable pessary is that it is made of latex and thus cannot be used by anyone with a latex allergy. Also, as latex retains discharge and odors, this pessary should be removed and washed daily.
The inflatable pessary is available in sizes that range from 2 to 2.75 inches in 0.25-inch increments.
Continue to: Space-filling pessaries...
Lever pessaries
In addition to the more commonly used support and space-filling pessaries, there is a third kind that is rarely used in current practice: the lever pessaries. These pessaries—the Hodge, the Smith, and the Risser—are rectangles made of inert plastic that are folded into 3 planes to facilitate positioning in the vagina. The narrower of the 2 shorter ends of the folded rectangle is placed behind the cervix or at the vaginal apex while the other short end is placed behind the symphysis pubis.
Although sometimes used to correct POP in nonpregnant women, the lever pessary’s main purpose is to antivert a retroflexed uterus and to support the cervix and uterus in cases of prolapse during pregnancy or impending cervical incompetence.
The 3 lever pessaries differ in terms of whether the narrow ends of the pessary are straight or curved and wider or narrower.
How to choose the right pessary for your patient
If a patient’s POP or urinary incontinence symptoms would best be treated with a pessary, the next step is to select the pessary type and size best suited for that patient’s needs and the size that should be prescribed. While there is controversy among experts as to whether or not certain pessaries are better than others for different indications,12 most gynecologists and urogynecologists who use pessaries on a regular basis agree on the following:
1. Support pessaries will meet the needs of most women with moderate POP and/or SUI. These include the ring pessary with or without the support shield and with or without an incontinence knob. A support pessary is the go-to pessary in most cases. Most women find it comfortable to wear, it is easy to put in and take out, and sexual intercourse is possible with the pessary in place.
2. The specific degree of a patient’s prolapse and/or incontinence dictates whether or not to prescribe the support shield feature or the incontinence knob with a ring pessary. The shield helps support a prolapsed cervix and uterus when they are present.5,13 The knob is a useful feature if incontinence is a prominent symptom.
3. The Gellhorn pessary is usually the first choice for more severe prolapse. As long as there is some degree of posterior perineal support, this pessary does an excellent job of correcting even severe prolapse whether of a cervix and uterus or of vaginal walls and apex. It does require the patient to have some practice and dexterity for inserting and removing it on her own; individuals not comfortable or physically able to do so will need to have the pessary removed and cleaned by a clinician on a regular basis in the office. (Part 2 of this article will discuss pessary cleansing intervals).
4. Space-filling pessaries (such as the cube and donut) are useful when there is a severe degree of prolapse and insufficient perineal support to maintain a Gellhorn pessary. In practice, they are generally used less frequently—which is unfortunate, as they are a potentially useful solution for older women with severe prolapse who might not be candidates for surgical repair. As mentioned, both the cube and donut pessaries require more frequent removal for cleaning.
5. In unusual cases, the use of 2 pessaries simultaneously may resolve a difficult problem, such as when a pessary is the only option for treatment, the prolapse is severe, or it is impossible to find a pessary that resists being expelled from the vagina.14 A space-filling pessary in the most cephalad aspect of the vagina used in conjunction with a ring pessary with support shield below it can sometimes resolve even the worst cases of prolapse.
Continue to: Stay tuned...
Stay tuned
Part 2 of this article next month will provide more information on pessaries, including fitting, aftercare, potential complications, and effectiveness in various disorders. ●
Pessaries have been used in one form or another to help resolve pelvic organ prolapse (POP) in women for at least 2,500 years. They have come in many shapes and have been made of many materials. Here is a brief sketch of the history of the pessary.
Antiquity
Kahun papyrus (ancient Egypt, c. 2000 BCE)
Women with POP were made to stand over a fire in which different ingredients were burned. It was thought that the disagreeable odors emitted would cause the uterus to “rebel” and thus revert back into place.1
Hippocrates (c. 460–375 BCE)
Used several techniques to resolve uterine prolapse:
- Tipping the woman upside down and shaking her, using gravity as an aid to return the prolapsed organs into the pelvis2
- Cupping of the buttocks and the lower abdomen in hopes of “sucking” the prolapsed uterus back into place3
The Greek physician Polybus (c. 400 BCE)
Placed half a pomegranate in the vagina to hold prolapsed structures in place2
Cleopatra (c. 70–30 BCE)
Treated prolapse with the vaginal application of an astringent liquid2
Celsus (c. 25 BCE–50 CE)
Used cone-shaped pessaries made of bronze with a perforated circular plate on the lower edge through which bands were attached. The bands were then tied around the body to keep the device in place4
The Greek physician Soranus (c. 98–138)
Utilized linen tampons soaked with vinegar—along with a piece of beef—to treat prolapse. These were then held in place by bands passed around the loins2
Galen (c. 130–210)
Used fumigation to “encourage” the uterus to return to the pelvis2
Middle Ages
Paulus of Aegina (c. 625–690) and Abbas (c. 949–982)
Both wrote about the use of pessaries made of wax3
Myrepsus (late 13th century)
Described the preparation of 45 types of pessaries consisting of different solid materials treated with perfumes, wax, honey, and herbs5
16th century
Caspar Stromayr (Practica Copiosa, 1559)
Used as pessaries tightly rolled sponges bound with string, dipped in wax, and covered with oil or butter6
Ambroise Paré (c. 1510–1590)
Developed the first ring-type pessary in the late 16th century. He used hammered brass and waxed cork in the shape of an oval to treat uterine prolapse. He also made ring-shaped devices of gold, silver, or brass which were kept in place by a belt around the waist.7
17th century de Castro (1546–1627)
Urged “attacking” uterine prolapse with application of a red-hot iron thus “frightening it” into receding back into the vagina8
Hendrik van Roonhuyse (1625–1672)
In his gynecology textbook, discussed the etiology and treatment of prolapse. He utilized a cork with a hole in it (to allow for passage of discharge) as prolapse treatment. He also wrote of removing an obstructed wax pessary that had blocked discharge of a patient’s vaginal secretions for many years4
18th century Thomas Simson (1696-1764)
Invented a metal spring device that kept a pessary made of cork in place9
John Leake (1729-1792)
Recommended the use of sponges as pessaries to avoid vaginal prolapse10
Juville (1783)
Was the first to use rubber pessaries, resembling today’s contraceptive cup, to avoid injuring the vaginal mucosa. The center of the cup was perforated with a gold tip which allowed for the discharge of vaginal secretions10
19th century
Scanzoni (1821-1891)
Recommended massage and the application of leeches to reduce local congestion and swelling of prolapsed pelvic organs before manual replacement11
Hugh Lenox Hodge (1796-1873)
In his 1860 textbook Diseases Peculiar to Women, Hodge discussed at length the use of pessaries for uterine displacement. He suggested that metals, alloys, glass, and porcelain be used for pessaries rather than cork, wax, and sponges12
20th century
1950s—
Pessaries made of rubber, which absorb discharge and odor, are replaced by polystyrene pessaries. Currently, pessaries are made of silicone, plastic, and latex.
References
- Stevens JM. Gynecology from ancient Egypt: the papyrus Kahun, a translation of the oldest treatise on gynecology that has survived from the ancient world. Med J Austr. 1975;2:949-952.
- Emge LA, Durfee RB. Pelvic organ prolapse: four thousand years of treatment. Clin Obstet Gynecol. 1966;9:997-1032.
- Van Dongen L. The anatomy of genital prolapse. South Afr Med J. 1981;60:357-359.
- Cianfrani T. Short History of Obstetrics and Gynecology. Springfield, IL: Charles C Thomas; 1960.
- Leonardo RA. History of Gynecology. New York, NY: Froben Press; 1944.
- Tizzano AP, Muffly TM. Historical milestones in female pelvic surgery, gynecology, and female urology. In: Walters M, Karram M. Urogynecology and Reconstructive Pelvic Surgery, 4th ed. Philadelphia, PA: Elsevier Saunders; 2015
- Farrell SA. Pessaries in Clinical Practice. Switzerland: Springer-Verlag; 2006.
- Tam T, Davies MF, eds. Vaginal Pessaries. Boca Raton, FL: CRC Press; 2019.
- Ricci JV. Genealogy of Gynaecology. Philadelphia, PA: Blakiston; 1950.
- Miller DS. Contemporary use of the pessary. In Sciarra JJ, ed. Gynecology and Obstetrics. Philadelphia, PA: JB Lippincott Company; 1995.
- Thomas TG. A Practical Treatise on the Disorders of Women. Philadelphia, PA: Lea Brothers and Co; 1891.
- Hodge HL. Diseases Peculiar to Women, Including Displacements of the Uterus. Philadelphia, PA: Blanchard and Lea; 1860.
- Zoorob D, Higgins M, Swan K, et al. Barriers to pelvic floor physical therapy regarding treatment of high-tone pelvic floor dysfunction. Female Pelvic Med Reconstr Surg. 2017;23:444-448.
- Kirby AC, Luber KM, Menefee SA. An update on the current and future demand for care of pelvic floor disorders in the United States. Am J Obstet Gynecol. 2013;209:584.e1-584.e5.
- Ellerkmann RM, Cundiff GW, Melick CF, et al. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185:1332-1337.
- US Census Bureau. United States population projections: 2000 to 2050. https://www.census.gov/library/workingpapers/2009/demo/us-pop-proj-2000-2050.html. Accessed November 13, 2020.
- Pott-Grinstein E, Newcomer JR. Gynecologists’ patterns of prescribing pessaries. J Reprod Med. 2001;46:205-208.
- Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
- Reena C, Kekre AN, Kekre N. Occult stress incontinence in women with pelvic organ prolapse. Int J Gynaecol Obstet. 2007;97:31-34.
- Thys SD, Roovers JP, Geomini PM, et al. Do patients prefer a pessary or surgery as primary treatment for pelvic organ prolapse. Gynecol Obstet Invest. 2012;74:6-12.
- Kapoor DS, Thakar R, Sultan AH, et al. Conservative versus surgical management of prolapse: what dictates patient choice? Int Urogynecol J Pelvic Floor Dysfunct. 2009;20: 1157-1161.
- Wu V, Farrel SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Culligan PJ. Nonsurgical management of pelvic organ prolapse. Obstet Gynecol. 2012;119:852-860.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-404.e8.
- Cundiff GW, Weidner AC, Visco AG, et al. A survey of pessary use by members of the American Urogynecologic Society. Obstet Gynecol. 2000;95(6 pt 1):931-935.
- Singh K, Reid W. Nonsurgical treatment of uterovaginal prolapse using double vaginal rings. Br J Obstet Gynecol. 2001;108:112-113.

Over the last 30 years, surgical correction of the common condition pelvic organ prolapse (POP) and stress urinary incontinence (SUI) has become so routine and straightforward that many gynecologists and urogynecologists choose surgery as their first choice for treating these conditions, withholding it only from the riskiest patients or from those who, for a variety of reasons, do not choose surgery. Moreover, as generalist gynecologists increasingly refer patients with POP or incontinence to their urogynecologist colleagues, they increasingly lack the skills, or have not been trained, to use conservative treatment strategies for these disorders. Thus, pessaries—devices constructed of inert plastic, silicone, or latex and placed inside the vagina to support prolapsed pelvic structures—frequently are not part of the general gynecologist’s armamentarium.
When properly selected, however, pessaries used for indicated purposes and correctly fitted are an excellent, inexpensive, low-risk, and noninvasive tool that can provide immediate relief not only of POP but also of SUI and defecatory difficulties. As an alternative to surgery, pessaries are especially valuable, because the other major nonsurgical modality for treatment of POP and incontinence—pelvic floor muscle training—often is not covered by insurance (making it expensive for patients), takes many weekly sessions to complete (which can make access challenging), and frequently is not readily available.1
POP is very common. An estimated 15% to 30% of women in North America have some degree of prolapse, and more than 500,000 surgeries for this condition are performed in the United States each year.2 Risk factors for POP include:
- vaginal childbirth, especially higher parity
- advancing age
- high body mass index (BMI)
- prior hysterectomy
- raised intra-abdominal pressure, such as from obesity, chronic cough, or heavy lifting.
In addition to the discomfort caused by the herniation of pelvic and vaginal structures, POP also is associated with urinary incontinence (73%), urinary urgency and frequency (86%), and fecal incontinence (31%).3
Moreover, according to the US Census Bureau, the number of American women aged 65 or older will double to more than 40 million by 2030.4 This will greatly increase the population of women at risk for POP who may be candidates for pessary use. It therefore behooves gynecologists to become familiar with the correct usage, fitting, and maintenance of this effective, nonsurgical mode of treatment for POP.
In this article, I discuss why pessaries are a good option for many patients with POP, review the types of pessaries available, and offer guidance on how to choose the right pessary for an individual patient’s needs. In addition, the box at the end of this article provides an interesting timeline of pessary history dating back to antiquity.
Next month in Part 2 of this article, I cover how to fit a pessary; device aftercare; potential complications of use; and effectiveness of pessaries for POP, SUI, preterm labor prevention, and defecatory disorders.
Continue to: Potential candidates for pessary use...
Potential candidates for pessary use
Almost all women with POP—and in many cases accompanying SUI—are potential candidates for a pessary. In fact, many urogynecologists believe that a trial of pessary usage should be the first treatment modality offered for POP.5 Women who cannot use a pessary include those with an extremely short vagina (<6 cm) and those who have severely eroded vaginal mucosa. In the latter situation, the mucosa can be treated with estrogen cream for several weeks and, once the tissue has healed, a pessary can be fitted.
Given that surgical repair is generally a straightforward, one-time procedure that obviates the need for long-term use of an artificial device worn internally, why might a patient or her physician opt for a pessary instead?
Some of the many reasons include:
- Many patients prefer to avoid surgery.
- Many patients are not appropriate candidates for surgery because they have significant comorbid risk factors or high BMI.
- Patients may have recurrent prolapse or incontinence and wish to avoid repeat surgery.
- Patients with SUI may have heard of the occurrence of mesh erosion and wish to avoid that possibility.
- Women who live in low-resource environments or countries where elective surgical care is relatively unavailable may not have the option of surgery.
A clinician might also recommend pessary use:
- as a diagnostic tool to attempt to assess the potential results of vaginal repair surgery
- to estimate the potential effectiveness of a midurethral sling procedure; several investigators have found this to be approximately as accurate as urodynamic testing6,7
- as prophylaxis for pregnant women with either a history of preterm cervical dilation or a short cervix detected on ultrasonography
- for pregnant women with POP that is worsening and becoming increasingly uncomfortable
- for women with POP who wish to have more children
- for short-term use while a patient is delaying or awaiting POP surgery or to allow time for other medical issues to resolve
- for patients who wish only intermittent, temporary support while exercising or engaging in sports.
Patient acceptance may be contingent on counseling
Numerous studies show that women who choose pessaries to treat POP are generally older than women who elect surgery. Still, patient acceptance of a trial of pessary use depends much on the counseling and information she receives. Properly informed, many patients with POP will opt for a trial of pessary placement. One study showed that, of women with untreated POP, 36% preferred pessary placement to surgery.8 Other investigators reported that when women with symptomatic POP had the benefits of a pessary versus surgery explained to them, nearly two-thirds opted for a pessary as their mode of treatment.9
Exceptions to pessary use
Fortunately, there are relatively few contraindications to pessary use. These are vaginal or pelvic infection and an exposed foreign body in the vagina, such as eroded vaginal mesh. In addition, patients at risk for nonadherence with follow-up care are poor candidates, as it could lead to missing such problems as mucosal erosion, ulceration, or even (extremely rarely) fistula formation. Pessaries may be inappropriate for sexually active women who on their own are unable to remove and reinsert pessary types that do not allow for intercourse while in place (see below).
Continue to: Types of pessaries...
Types of pessaries
The numerous kinds of pessaries available fall into 3 general categories: support, space filling, and lever, and devices within each group have modifications and variations. As with most areas of prescribing and treatment in medicine, it is best to become very familiar with just a few kinds of pessaries, know their indications, and use them when appropriate.
Most pessaries are constructed of inert silicone which, unlike earlier rubber pessaries, does not absorb odor or discharge. They are easy to clean, long lasting, and are autoclavable and hypoallergenic.
Support pessaries
Support pessaries look like contraceptive diaphragms. They are easy to place and remove, are comfortable, and do an excellent job correcting moderate POP. They also can control or eliminate symptoms of SUI by the pressure they exert on the urethra and their alteration of the urethrovesicular angle.
Ring pessaries. The most commonly used type of pessary, the ring pessary,10 comes in 4 variations:
- a simple open ring
- a ring with a web of material, called a “support shield,” that fills the ring
- an open ring with a firm 2-cm “incontinence knob” attached that is positioned over the urethra
- a ring with support shield and incontinence knob.
When in position, the deepest edge of a ring pessary fits behind the cervix (or in the vaginal apex for women who have had a hysterectomy) while the front of the ring slips into place behind the pubic symphysis, just like a diaphragm. When a ring with an incontinence knob is used, the ring is rotated until the knob is directly over the urethra.
Sexual intercourse is possible with any of the ring pessaries in place. Of the various types of pessaries, the ring pessary is the easiest to insert and remove. Some women tie a piece of dental floss to the edge of the ring to make its removal even easier.
The ring pessary is available in sizes 0 (44.5 mm) to 13 (127 mm). For most women a size 3, 4, or 5 ring pessary fits well.

The Marland pessary is similar to the ring pessary with the addition of a wedge-shaped piece of material approximately 3 cm in height that arises from half of the ring. It rarely is used in the United States because most American gynecologists are unfamiliar with it, and there is little evidence that it is more effective than the ring pessary.11

The Shaatz pessary is a rigid round pessary, smaller in diameter than the standard ring pessary, and similar to the Gellhorn pessary (discussed below) but without a stem. It is placed the same way one places a ring pessary but with its concave surface up against the cervix or, if there is no cervix, against the upper anterior vaginal wall. Its main benefit is that it provides firmer support than the ring pessary. This pessary is not widely used in the United States.

The Gehrung pessary looks like a flat strip of material that has been bent into the shape of a “U.” It is designed to correct severe cystoceles and rectoceles. For insertion, the edges at the open end of the pessary are squeezed together and the pessary is inserted with the closed part of the “U” facing the anterior vaginal wall. The upper edge is advanced until it rests in the anterior fornix of the vagina (or in the vaginal apex in women who have had a hysterectomy). Although it is more efficacious than some other pessaries for control of vaginal wall prolapse, its unfamiliarity to clinicians and its unusual shape result in it being used rarely.

Continue to: Space-filling pessaries...
Space-filling pessaries
Space-filling pessaries are used when more severe degrees of prolapse are present than can be managed by the ring or other support pessaries. This is especially the case when the vagina is so capacious or the introitus so lax that a standard ring pessary cannot be kept in place, resulting in frequent expulsions.
Space-filling pessaries are 3 dimensional and work by filling the vagina with a relatively large object that prevents the cervix/vaginal apex from dropping down and the vaginal walls from prolapsing. They have a special role for women who:
- are posthysterectomy and have an enterocele and/or vaginal apex prolapse
- have significant rectoceles for which support pessaries are not effective
- have a wide vaginal hiatus and thus are prone to expel support pessaries.
Space-filling pessaries do have some drawbacks compared with support pessaries. For example, they do not help in controlling SUI, and they are difficult for patients to remove on their own for cleaning. In addition, sexual intercourse is impossible with a space-filling pessary in place.
The Gellhorn pessary is the most common of the space-filling pessaries, and it is the one gynecologists and urogynecologists most often use for severe prolapse. It has a concave disc that fits up against the cervix or vaginal apex and a solid stem that points down the vagina. The stem itself is supported by the perineal body. It offers excellent support for severe uterine and vaginal wall prolapse, as long as the perineal body is intact. The stem stabilizes the disc portion of the pessary and prevents pessary expulsion. Gellhorn pessaries are available with long or short stems.
The Gellhorn is inserted into the vagina by folding the stem 90 degrees until it is in the same plane as the disc. With lubricated fingers, the patient’s perineal body is depressed and the disc of the pessary is folded and slid in. The disc is then placed up against the cervix or vaginal apex with the stem pointing down the vagina and tucked just inside the posterior edge of the introitus.
Removing the Gellhorn pessary can be problematic and is difficult for patients to do on their own. Clinicians often must use a ring forceps to grasp the stem of the pessary in order to bring it into the lower vagina, where the stem is folded up against the disc and the entire pessary removed. As with all space-filling pessaries, the Gellhorn must be taken out prior to intercourse.
The Gellhorn pessary is available in sizes that range from a disc diameter of 1.5 to 3.75 inches. Those measuring 2.5, 2.75, or 3 inches are used most commonly.

The cube pessary is a soft, dice-shaped piece of silicone with an indentation in each of its 6 sides. It is used for severe prolapse.
Squeezing the cube allows for easier insertion into the vagina; once it is at the top of the vagina, the cube expands back to its normal shape. The indentations on each side of the cube attach to the vaginal walls with moderate suction, which helps to keep the pessary in place. Because of the suction, the cube pessary can be used in cases of severe prolapse when other pessaries will not stay in place; a drawback is that the suction created by the indented sides can cause vaginal mucosal erosion.10 Ideally, the cube pessary should be removed every night for cleansing as discharge and accompanying odor can accumulate. The string attached to the cube pessary aids in its removal.
The cube pessary is available in sizes 0 to 7, with edge lengths that range from 1 to 2.25 inches.

The donut pessary, as its name suggests, has the form of a large donut. It can be compressed slightly to help with insertion. Because it occupies a large space within the vagina, it is used (like the cube pessary) for treatment of severe prolapse. The size and shape of the donut pessary, however, can make it difficult for patients to insert and take out on their own.
The donut pessary is available in sizes 0 (51 mm) to 8 (95 mm).

The inflatable pessary has the same basic shape as the donut pessary and serves the same purpose: It acts as a large semisoft object that fills the vagina to support the vaginal walls and cervix (or vaginal apex) in cases of severe prolapse. The inflatable pessary differs in that it has a valve on a stem through which air can be inserted and removed. This allows the uninflated pessary to be placed relatively easily into the vagina and then pumped full of air to the dimensions necessary to prevent vaginal, cervical, uterine, or apex prolapse. Air likewise can be removed to facilitate pessary removal.
One drawback of the inflatable pessary is that it is made of latex and thus cannot be used by anyone with a latex allergy. Also, as latex retains discharge and odors, this pessary should be removed and washed daily.
The inflatable pessary is available in sizes that range from 2 to 2.75 inches in 0.25-inch increments.

Continue to: Space-filling pessaries...
Lever pessaries
In addition to the more commonly used support and space-filling pessaries, there is a third kind that is rarely used in current practice: the lever pessaries. These pessaries—the Hodge, the Smith, and the Risser—are rectangles made of inert plastic that are folded into 3 planes to facilitate positioning in the vagina. The narrower of the 2 shorter ends of the folded rectangle is placed behind the cervix or at the vaginal apex while the other short end is placed behind the symphysis pubis.
Although sometimes used to correct POP in nonpregnant women, the lever pessary’s main purpose is to antivert a retroflexed uterus and to support the cervix and uterus in cases of prolapse during pregnancy or impending cervical incompetence.
The 3 lever pessaries differ in terms of whether the narrow ends of the pessary are straight or curved and wider or narrower.
How to choose the right pessary for your patient
If a patient’s POP or urinary incontinence symptoms would best be treated with a pessary, the next step is to select the pessary type and size best suited for that patient’s needs and the size that should be prescribed. While there is controversy among experts as to whether or not certain pessaries are better than others for different indications,12 most gynecologists and urogynecologists who use pessaries on a regular basis agree on the following:
1. Support pessaries will meet the needs of most women with moderate POP and/or SUI. These include the ring pessary with or without the support shield and with or without an incontinence knob. A support pessary is the go-to pessary in most cases. Most women find it comfortable to wear, it is easy to put in and take out, and sexual intercourse is possible with the pessary in place.
2. The specific degree of a patient’s prolapse and/or incontinence dictates whether or not to prescribe the support shield feature or the incontinence knob with a ring pessary. The shield helps support a prolapsed cervix and uterus when they are present.5,13 The knob is a useful feature if incontinence is a prominent symptom.
3. The Gellhorn pessary is usually the first choice for more severe prolapse. As long as there is some degree of posterior perineal support, this pessary does an excellent job of correcting even severe prolapse whether of a cervix and uterus or of vaginal walls and apex. It does require the patient to have some practice and dexterity for inserting and removing it on her own; individuals not comfortable or physically able to do so will need to have the pessary removed and cleaned by a clinician on a regular basis in the office. (Part 2 of this article will discuss pessary cleansing intervals).
4. Space-filling pessaries (such as the cube and donut) are useful when there is a severe degree of prolapse and insufficient perineal support to maintain a Gellhorn pessary. In practice, they are generally used less frequently—which is unfortunate, as they are a potentially useful solution for older women with severe prolapse who might not be candidates for surgical repair. As mentioned, both the cube and donut pessaries require more frequent removal for cleaning.
5. In unusual cases, the use of 2 pessaries simultaneously may resolve a difficult problem, such as when a pessary is the only option for treatment, the prolapse is severe, or it is impossible to find a pessary that resists being expelled from the vagina.14 A space-filling pessary in the most cephalad aspect of the vagina used in conjunction with a ring pessary with support shield below it can sometimes resolve even the worst cases of prolapse.
Continue to: Stay tuned...
Stay tuned
Part 2 of this article next month will provide more information on pessaries, including fitting, aftercare, potential complications, and effectiveness in various disorders. ●
Pessaries have been used in one form or another to help resolve pelvic organ prolapse (POP) in women for at least 2,500 years. They have come in many shapes and have been made of many materials. Here is a brief sketch of the history of the pessary.
Antiquity
Kahun papyrus (ancient Egypt, c. 2000 BCE)
Women with POP were made to stand over a fire in which different ingredients were burned. It was thought that the disagreeable odors emitted would cause the uterus to “rebel” and thus revert back into place.1
Hippocrates (c. 460–375 BCE)
Used several techniques to resolve uterine prolapse:
- Tipping the woman upside down and shaking her, using gravity as an aid to return the prolapsed organs into the pelvis2
- Cupping of the buttocks and the lower abdomen in hopes of “sucking” the prolapsed uterus back into place3
The Greek physician Polybus (c. 400 BCE)
Placed half a pomegranate in the vagina to hold prolapsed structures in place2
Cleopatra (c. 70–30 BCE)
Treated prolapse with the vaginal application of an astringent liquid2
Celsus (c. 25 BCE–50 CE)
Used cone-shaped pessaries made of bronze with a perforated circular plate on the lower edge through which bands were attached. The bands were then tied around the body to keep the device in place4
The Greek physician Soranus (c. 98–138)
Utilized linen tampons soaked with vinegar—along with a piece of beef—to treat prolapse. These were then held in place by bands passed around the loins2
Galen (c. 130–210)
Used fumigation to “encourage” the uterus to return to the pelvis2
Middle Ages
Paulus of Aegina (c. 625–690) and Abbas (c. 949–982)
Both wrote about the use of pessaries made of wax3
Myrepsus (late 13th century)
Described the preparation of 45 types of pessaries consisting of different solid materials treated with perfumes, wax, honey, and herbs5
16th century
Caspar Stromayr (Practica Copiosa, 1559)
Used as pessaries tightly rolled sponges bound with string, dipped in wax, and covered with oil or butter6
Ambroise Paré (c. 1510–1590)
Developed the first ring-type pessary in the late 16th century. He used hammered brass and waxed cork in the shape of an oval to treat uterine prolapse. He also made ring-shaped devices of gold, silver, or brass which were kept in place by a belt around the waist.7
17th century de Castro (1546–1627)
Urged “attacking” uterine prolapse with application of a red-hot iron thus “frightening it” into receding back into the vagina8
Hendrik van Roonhuyse (1625–1672)
In his gynecology textbook, discussed the etiology and treatment of prolapse. He utilized a cork with a hole in it (to allow for passage of discharge) as prolapse treatment. He also wrote of removing an obstructed wax pessary that had blocked discharge of a patient’s vaginal secretions for many years4
18th century Thomas Simson (1696-1764)
Invented a metal spring device that kept a pessary made of cork in place9
John Leake (1729-1792)
Recommended the use of sponges as pessaries to avoid vaginal prolapse10
Juville (1783)
Was the first to use rubber pessaries, resembling today’s contraceptive cup, to avoid injuring the vaginal mucosa. The center of the cup was perforated with a gold tip which allowed for the discharge of vaginal secretions10
19th century
Scanzoni (1821-1891)
Recommended massage and the application of leeches to reduce local congestion and swelling of prolapsed pelvic organs before manual replacement11
Hugh Lenox Hodge (1796-1873)
In his 1860 textbook Diseases Peculiar to Women, Hodge discussed at length the use of pessaries for uterine displacement. He suggested that metals, alloys, glass, and porcelain be used for pessaries rather than cork, wax, and sponges12
20th century
1950s—
Pessaries made of rubber, which absorb discharge and odor, are replaced by polystyrene pessaries. Currently, pessaries are made of silicone, plastic, and latex.
References
- Stevens JM. Gynecology from ancient Egypt: the papyrus Kahun, a translation of the oldest treatise on gynecology that has survived from the ancient world. Med J Austr. 1975;2:949-952.
- Emge LA, Durfee RB. Pelvic organ prolapse: four thousand years of treatment. Clin Obstet Gynecol. 1966;9:997-1032.
- Van Dongen L. The anatomy of genital prolapse. South Afr Med J. 1981;60:357-359.
- Cianfrani T. Short History of Obstetrics and Gynecology. Springfield, IL: Charles C Thomas; 1960.
- Leonardo RA. History of Gynecology. New York, NY: Froben Press; 1944.
- Tizzano AP, Muffly TM. Historical milestones in female pelvic surgery, gynecology, and female urology. In: Walters M, Karram M. Urogynecology and Reconstructive Pelvic Surgery, 4th ed. Philadelphia, PA: Elsevier Saunders; 2015
- Farrell SA. Pessaries in Clinical Practice. Switzerland: Springer-Verlag; 2006.
- Tam T, Davies MF, eds. Vaginal Pessaries. Boca Raton, FL: CRC Press; 2019.
- Ricci JV. Genealogy of Gynaecology. Philadelphia, PA: Blakiston; 1950.
- Miller DS. Contemporary use of the pessary. In Sciarra JJ, ed. Gynecology and Obstetrics. Philadelphia, PA: JB Lippincott Company; 1995.
- Thomas TG. A Practical Treatise on the Disorders of Women. Philadelphia, PA: Lea Brothers and Co; 1891.
- Hodge HL. Diseases Peculiar to Women, Including Displacements of the Uterus. Philadelphia, PA: Blanchard and Lea; 1860.

Over the last 30 years, surgical correction of the common condition pelvic organ prolapse (POP) and stress urinary incontinence (SUI) has become so routine and straightforward that many gynecologists and urogynecologists choose surgery as their first choice for treating these conditions, withholding it only from the riskiest patients or from those who, for a variety of reasons, do not choose surgery. Moreover, as generalist gynecologists increasingly refer patients with POP or incontinence to their urogynecologist colleagues, they increasingly lack the skills, or have not been trained, to use conservative treatment strategies for these disorders. Thus, pessaries—devices constructed of inert plastic, silicone, or latex and placed inside the vagina to support prolapsed pelvic structures—frequently are not part of the general gynecologist’s armamentarium.
When properly selected, however, pessaries used for indicated purposes and correctly fitted are an excellent, inexpensive, low-risk, and noninvasive tool that can provide immediate relief not only of POP but also of SUI and defecatory difficulties. As an alternative to surgery, pessaries are especially valuable, because the other major nonsurgical modality for treatment of POP and incontinence—pelvic floor muscle training—often is not covered by insurance (making it expensive for patients), takes many weekly sessions to complete (which can make access challenging), and frequently is not readily available.1
POP is very common. An estimated 15% to 30% of women in North America have some degree of prolapse, and more than 500,000 surgeries for this condition are performed in the United States each year.2 Risk factors for POP include:
- vaginal childbirth, especially higher parity
- advancing age
- high body mass index (BMI)
- prior hysterectomy
- raised intra-abdominal pressure, such as from obesity, chronic cough, or heavy lifting.
In addition to the discomfort caused by the herniation of pelvic and vaginal structures, POP also is associated with urinary incontinence (73%), urinary urgency and frequency (86%), and fecal incontinence (31%).3
Moreover, according to the US Census Bureau, the number of American women aged 65 or older will double to more than 40 million by 2030.4 This will greatly increase the population of women at risk for POP who may be candidates for pessary use. It therefore behooves gynecologists to become familiar with the correct usage, fitting, and maintenance of this effective, nonsurgical mode of treatment for POP.
In this article, I discuss why pessaries are a good option for many patients with POP, review the types of pessaries available, and offer guidance on how to choose the right pessary for an individual patient’s needs. In addition, the box at the end of this article provides an interesting timeline of pessary history dating back to antiquity.
Next month in Part 2 of this article, I cover how to fit a pessary; device aftercare; potential complications of use; and effectiveness of pessaries for POP, SUI, preterm labor prevention, and defecatory disorders.
Continue to: Potential candidates for pessary use...
Potential candidates for pessary use
Almost all women with POP—and in many cases accompanying SUI—are potential candidates for a pessary. In fact, many urogynecologists believe that a trial of pessary usage should be the first treatment modality offered for POP.5 Women who cannot use a pessary include those with an extremely short vagina (<6 cm) and those who have severely eroded vaginal mucosa. In the latter situation, the mucosa can be treated with estrogen cream for several weeks and, once the tissue has healed, a pessary can be fitted.
Given that surgical repair is generally a straightforward, one-time procedure that obviates the need for long-term use of an artificial device worn internally, why might a patient or her physician opt for a pessary instead?
Some of the many reasons include:
- Many patients prefer to avoid surgery.
- Many patients are not appropriate candidates for surgery because they have significant comorbid risk factors or high BMI.
- Patients may have recurrent prolapse or incontinence and wish to avoid repeat surgery.
- Patients with SUI may have heard of the occurrence of mesh erosion and wish to avoid that possibility.
- Women who live in low-resource environments or countries where elective surgical care is relatively unavailable may not have the option of surgery.
A clinician might also recommend pessary use:
- as a diagnostic tool to attempt to assess the potential results of vaginal repair surgery
- to estimate the potential effectiveness of a midurethral sling procedure; several investigators have found this to be approximately as accurate as urodynamic testing6,7
- as prophylaxis for pregnant women with either a history of preterm cervical dilation or a short cervix detected on ultrasonography
- for pregnant women with POP that is worsening and becoming increasingly uncomfortable
- for women with POP who wish to have more children
- for short-term use while a patient is delaying or awaiting POP surgery or to allow time for other medical issues to resolve
- for patients who wish only intermittent, temporary support while exercising or engaging in sports.
Patient acceptance may be contingent on counseling
Numerous studies show that women who choose pessaries to treat POP are generally older than women who elect surgery. Still, patient acceptance of a trial of pessary use depends much on the counseling and information she receives. Properly informed, many patients with POP will opt for a trial of pessary placement. One study showed that, of women with untreated POP, 36% preferred pessary placement to surgery.8 Other investigators reported that when women with symptomatic POP had the benefits of a pessary versus surgery explained to them, nearly two-thirds opted for a pessary as their mode of treatment.9
Exceptions to pessary use
Fortunately, there are relatively few contraindications to pessary use. These are vaginal or pelvic infection and an exposed foreign body in the vagina, such as eroded vaginal mesh. In addition, patients at risk for nonadherence with follow-up care are poor candidates, as it could lead to missing such problems as mucosal erosion, ulceration, or even (extremely rarely) fistula formation. Pessaries may be inappropriate for sexually active women who on their own are unable to remove and reinsert pessary types that do not allow for intercourse while in place (see below).
Continue to: Types of pessaries...
Types of pessaries
The numerous kinds of pessaries available fall into 3 general categories: support, space filling, and lever, and devices within each group have modifications and variations. As with most areas of prescribing and treatment in medicine, it is best to become very familiar with just a few kinds of pessaries, know their indications, and use them when appropriate.
Most pessaries are constructed of inert silicone which, unlike earlier rubber pessaries, does not absorb odor or discharge. They are easy to clean, long lasting, and are autoclavable and hypoallergenic.
Support pessaries
Support pessaries look like contraceptive diaphragms. They are easy to place and remove, are comfortable, and do an excellent job correcting moderate POP. They also can control or eliminate symptoms of SUI by the pressure they exert on the urethra and their alteration of the urethrovesicular angle.
Ring pessaries. The most commonly used type of pessary, the ring pessary,10 comes in 4 variations:
- a simple open ring
- a ring with a web of material, called a “support shield,” that fills the ring
- an open ring with a firm 2-cm “incontinence knob” attached that is positioned over the urethra
- a ring with support shield and incontinence knob.
When in position, the deepest edge of a ring pessary fits behind the cervix (or in the vaginal apex for women who have had a hysterectomy) while the front of the ring slips into place behind the pubic symphysis, just like a diaphragm. When a ring with an incontinence knob is used, the ring is rotated until the knob is directly over the urethra.
Sexual intercourse is possible with any of the ring pessaries in place. Of the various types of pessaries, the ring pessary is the easiest to insert and remove. Some women tie a piece of dental floss to the edge of the ring to make its removal even easier.
The ring pessary is available in sizes 0 (44.5 mm) to 13 (127 mm). For most women a size 3, 4, or 5 ring pessary fits well.

The Marland pessary is similar to the ring pessary with the addition of a wedge-shaped piece of material approximately 3 cm in height that arises from half of the ring. It rarely is used in the United States because most American gynecologists are unfamiliar with it, and there is little evidence that it is more effective than the ring pessary.11

The Shaatz pessary is a rigid round pessary, smaller in diameter than the standard ring pessary, and similar to the Gellhorn pessary (discussed below) but without a stem. It is placed the same way one places a ring pessary but with its concave surface up against the cervix or, if there is no cervix, against the upper anterior vaginal wall. Its main benefit is that it provides firmer support than the ring pessary. This pessary is not widely used in the United States.

The Gehrung pessary looks like a flat strip of material that has been bent into the shape of a “U.” It is designed to correct severe cystoceles and rectoceles. For insertion, the edges at the open end of the pessary are squeezed together and the pessary is inserted with the closed part of the “U” facing the anterior vaginal wall. The upper edge is advanced until it rests in the anterior fornix of the vagina (or in the vaginal apex in women who have had a hysterectomy). Although it is more efficacious than some other pessaries for control of vaginal wall prolapse, its unfamiliarity to clinicians and its unusual shape result in it being used rarely.

Continue to: Space-filling pessaries...
Space-filling pessaries
Space-filling pessaries are used when more severe degrees of prolapse are present than can be managed by the ring or other support pessaries. This is especially the case when the vagina is so capacious or the introitus so lax that a standard ring pessary cannot be kept in place, resulting in frequent expulsions.
Space-filling pessaries are 3 dimensional and work by filling the vagina with a relatively large object that prevents the cervix/vaginal apex from dropping down and the vaginal walls from prolapsing. They have a special role for women who:
- are posthysterectomy and have an enterocele and/or vaginal apex prolapse
- have significant rectoceles for which support pessaries are not effective
- have a wide vaginal hiatus and thus are prone to expel support pessaries.
Space-filling pessaries do have some drawbacks compared with support pessaries. For example, they do not help in controlling SUI, and they are difficult for patients to remove on their own for cleaning. In addition, sexual intercourse is impossible with a space-filling pessary in place.
The Gellhorn pessary is the most common of the space-filling pessaries, and it is the one gynecologists and urogynecologists most often use for severe prolapse. It has a concave disc that fits up against the cervix or vaginal apex and a solid stem that points down the vagina. The stem itself is supported by the perineal body. It offers excellent support for severe uterine and vaginal wall prolapse, as long as the perineal body is intact. The stem stabilizes the disc portion of the pessary and prevents pessary expulsion. Gellhorn pessaries are available with long or short stems.
The Gellhorn is inserted into the vagina by folding the stem 90 degrees until it is in the same plane as the disc. With lubricated fingers, the patient’s perineal body is depressed and the disc of the pessary is folded and slid in. The disc is then placed up against the cervix or vaginal apex with the stem pointing down the vagina and tucked just inside the posterior edge of the introitus.
Removing the Gellhorn pessary can be problematic and is difficult for patients to do on their own. Clinicians often must use a ring forceps to grasp the stem of the pessary in order to bring it into the lower vagina, where the stem is folded up against the disc and the entire pessary removed. As with all space-filling pessaries, the Gellhorn must be taken out prior to intercourse.
The Gellhorn pessary is available in sizes that range from a disc diameter of 1.5 to 3.75 inches. Those measuring 2.5, 2.75, or 3 inches are used most commonly.

The cube pessary is a soft, dice-shaped piece of silicone with an indentation in each of its 6 sides. It is used for severe prolapse.
Squeezing the cube allows for easier insertion into the vagina; once it is at the top of the vagina, the cube expands back to its normal shape. The indentations on each side of the cube attach to the vaginal walls with moderate suction, which helps to keep the pessary in place. Because of the suction, the cube pessary can be used in cases of severe prolapse when other pessaries will not stay in place; a drawback is that the suction created by the indented sides can cause vaginal mucosal erosion.10 Ideally, the cube pessary should be removed every night for cleansing as discharge and accompanying odor can accumulate. The string attached to the cube pessary aids in its removal.
The cube pessary is available in sizes 0 to 7, with edge lengths that range from 1 to 2.25 inches.

The donut pessary, as its name suggests, has the form of a large donut. It can be compressed slightly to help with insertion. Because it occupies a large space within the vagina, it is used (like the cube pessary) for treatment of severe prolapse. The size and shape of the donut pessary, however, can make it difficult for patients to insert and take out on their own.
The donut pessary is available in sizes 0 (51 mm) to 8 (95 mm).

The inflatable pessary has the same basic shape as the donut pessary and serves the same purpose: It acts as a large semisoft object that fills the vagina to support the vaginal walls and cervix (or vaginal apex) in cases of severe prolapse. The inflatable pessary differs in that it has a valve on a stem through which air can be inserted and removed. This allows the uninflated pessary to be placed relatively easily into the vagina and then pumped full of air to the dimensions necessary to prevent vaginal, cervical, uterine, or apex prolapse. Air likewise can be removed to facilitate pessary removal.
One drawback of the inflatable pessary is that it is made of latex and thus cannot be used by anyone with a latex allergy. Also, as latex retains discharge and odors, this pessary should be removed and washed daily.
The inflatable pessary is available in sizes that range from 2 to 2.75 inches in 0.25-inch increments.

Continue to: Space-filling pessaries...
Lever pessaries
In addition to the more commonly used support and space-filling pessaries, there is a third kind that is rarely used in current practice: the lever pessaries. These pessaries—the Hodge, the Smith, and the Risser—are rectangles made of inert plastic that are folded into 3 planes to facilitate positioning in the vagina. The narrower of the 2 shorter ends of the folded rectangle is placed behind the cervix or at the vaginal apex while the other short end is placed behind the symphysis pubis.
Although sometimes used to correct POP in nonpregnant women, the lever pessary’s main purpose is to antivert a retroflexed uterus and to support the cervix and uterus in cases of prolapse during pregnancy or impending cervical incompetence.
The 3 lever pessaries differ in terms of whether the narrow ends of the pessary are straight or curved and wider or narrower.
How to choose the right pessary for your patient
If a patient’s POP or urinary incontinence symptoms would best be treated with a pessary, the next step is to select the pessary type and size best suited for that patient’s needs and the size that should be prescribed. While there is controversy among experts as to whether or not certain pessaries are better than others for different indications,12 most gynecologists and urogynecologists who use pessaries on a regular basis agree on the following:
1. Support pessaries will meet the needs of most women with moderate POP and/or SUI. These include the ring pessary with or without the support shield and with or without an incontinence knob. A support pessary is the go-to pessary in most cases. Most women find it comfortable to wear, it is easy to put in and take out, and sexual intercourse is possible with the pessary in place.
2. The specific degree of a patient’s prolapse and/or incontinence dictates whether or not to prescribe the support shield feature or the incontinence knob with a ring pessary. The shield helps support a prolapsed cervix and uterus when they are present.5,13 The knob is a useful feature if incontinence is a prominent symptom.
3. The Gellhorn pessary is usually the first choice for more severe prolapse. As long as there is some degree of posterior perineal support, this pessary does an excellent job of correcting even severe prolapse whether of a cervix and uterus or of vaginal walls and apex. It does require the patient to have some practice and dexterity for inserting and removing it on her own; individuals not comfortable or physically able to do so will need to have the pessary removed and cleaned by a clinician on a regular basis in the office. (Part 2 of this article will discuss pessary cleansing intervals).
4. Space-filling pessaries (such as the cube and donut) are useful when there is a severe degree of prolapse and insufficient perineal support to maintain a Gellhorn pessary. In practice, they are generally used less frequently—which is unfortunate, as they are a potentially useful solution for older women with severe prolapse who might not be candidates for surgical repair. As mentioned, both the cube and donut pessaries require more frequent removal for cleaning.
5. In unusual cases, the use of 2 pessaries simultaneously may resolve a difficult problem, such as when a pessary is the only option for treatment, the prolapse is severe, or it is impossible to find a pessary that resists being expelled from the vagina.14 A space-filling pessary in the most cephalad aspect of the vagina used in conjunction with a ring pessary with support shield below it can sometimes resolve even the worst cases of prolapse.
Continue to: Stay tuned...
Stay tuned
Part 2 of this article next month will provide more information on pessaries, including fitting, aftercare, potential complications, and effectiveness in various disorders. ●
Pessaries have been used in one form or another to help resolve pelvic organ prolapse (POP) in women for at least 2,500 years. They have come in many shapes and have been made of many materials. Here is a brief sketch of the history of the pessary.
Antiquity
Kahun papyrus (ancient Egypt, c. 2000 BCE)
Women with POP were made to stand over a fire in which different ingredients were burned. It was thought that the disagreeable odors emitted would cause the uterus to “rebel” and thus revert back into place.1
Hippocrates (c. 460–375 BCE)
Used several techniques to resolve uterine prolapse:
- Tipping the woman upside down and shaking her, using gravity as an aid to return the prolapsed organs into the pelvis2
- Cupping of the buttocks and the lower abdomen in hopes of “sucking” the prolapsed uterus back into place3
The Greek physician Polybus (c. 400 BCE)
Placed half a pomegranate in the vagina to hold prolapsed structures in place2
Cleopatra (c. 70–30 BCE)
Treated prolapse with the vaginal application of an astringent liquid2
Celsus (c. 25 BCE–50 CE)
Used cone-shaped pessaries made of bronze with a perforated circular plate on the lower edge through which bands were attached. The bands were then tied around the body to keep the device in place4
The Greek physician Soranus (c. 98–138)
Utilized linen tampons soaked with vinegar—along with a piece of beef—to treat prolapse. These were then held in place by bands passed around the loins2
Galen (c. 130–210)
Used fumigation to “encourage” the uterus to return to the pelvis2
Middle Ages
Paulus of Aegina (c. 625–690) and Abbas (c. 949–982)
Both wrote about the use of pessaries made of wax3
Myrepsus (late 13th century)
Described the preparation of 45 types of pessaries consisting of different solid materials treated with perfumes, wax, honey, and herbs5
16th century
Caspar Stromayr (Practica Copiosa, 1559)
Used as pessaries tightly rolled sponges bound with string, dipped in wax, and covered with oil or butter6
Ambroise Paré (c. 1510–1590)
Developed the first ring-type pessary in the late 16th century. He used hammered brass and waxed cork in the shape of an oval to treat uterine prolapse. He also made ring-shaped devices of gold, silver, or brass which were kept in place by a belt around the waist.7
17th century de Castro (1546–1627)
Urged “attacking” uterine prolapse with application of a red-hot iron thus “frightening it” into receding back into the vagina8
Hendrik van Roonhuyse (1625–1672)
In his gynecology textbook, discussed the etiology and treatment of prolapse. He utilized a cork with a hole in it (to allow for passage of discharge) as prolapse treatment. He also wrote of removing an obstructed wax pessary that had blocked discharge of a patient’s vaginal secretions for many years4
18th century Thomas Simson (1696-1764)
Invented a metal spring device that kept a pessary made of cork in place9
John Leake (1729-1792)
Recommended the use of sponges as pessaries to avoid vaginal prolapse10
Juville (1783)
Was the first to use rubber pessaries, resembling today’s contraceptive cup, to avoid injuring the vaginal mucosa. The center of the cup was perforated with a gold tip which allowed for the discharge of vaginal secretions10
19th century
Scanzoni (1821-1891)
Recommended massage and the application of leeches to reduce local congestion and swelling of prolapsed pelvic organs before manual replacement11
Hugh Lenox Hodge (1796-1873)
In his 1860 textbook Diseases Peculiar to Women, Hodge discussed at length the use of pessaries for uterine displacement. He suggested that metals, alloys, glass, and porcelain be used for pessaries rather than cork, wax, and sponges12
20th century
1950s—
Pessaries made of rubber, which absorb discharge and odor, are replaced by polystyrene pessaries. Currently, pessaries are made of silicone, plastic, and latex.
References
- Stevens JM. Gynecology from ancient Egypt: the papyrus Kahun, a translation of the oldest treatise on gynecology that has survived from the ancient world. Med J Austr. 1975;2:949-952.
- Emge LA, Durfee RB. Pelvic organ prolapse: four thousand years of treatment. Clin Obstet Gynecol. 1966;9:997-1032.
- Van Dongen L. The anatomy of genital prolapse. South Afr Med J. 1981;60:357-359.
- Cianfrani T. Short History of Obstetrics and Gynecology. Springfield, IL: Charles C Thomas; 1960.
- Leonardo RA. History of Gynecology. New York, NY: Froben Press; 1944.
- Tizzano AP, Muffly TM. Historical milestones in female pelvic surgery, gynecology, and female urology. In: Walters M, Karram M. Urogynecology and Reconstructive Pelvic Surgery, 4th ed. Philadelphia, PA: Elsevier Saunders; 2015
- Farrell SA. Pessaries in Clinical Practice. Switzerland: Springer-Verlag; 2006.
- Tam T, Davies MF, eds. Vaginal Pessaries. Boca Raton, FL: CRC Press; 2019.
- Ricci JV. Genealogy of Gynaecology. Philadelphia, PA: Blakiston; 1950.
- Miller DS. Contemporary use of the pessary. In Sciarra JJ, ed. Gynecology and Obstetrics. Philadelphia, PA: JB Lippincott Company; 1995.
- Thomas TG. A Practical Treatise on the Disorders of Women. Philadelphia, PA: Lea Brothers and Co; 1891.
- Hodge HL. Diseases Peculiar to Women, Including Displacements of the Uterus. Philadelphia, PA: Blanchard and Lea; 1860.
- Zoorob D, Higgins M, Swan K, et al. Barriers to pelvic floor physical therapy regarding treatment of high-tone pelvic floor dysfunction. Female Pelvic Med Reconstr Surg. 2017;23:444-448.
- Kirby AC, Luber KM, Menefee SA. An update on the current and future demand for care of pelvic floor disorders in the United States. Am J Obstet Gynecol. 2013;209:584.e1-584.e5.
- Ellerkmann RM, Cundiff GW, Melick CF, et al. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185:1332-1337.
- US Census Bureau. United States population projections: 2000 to 2050. https://www.census.gov/library/workingpapers/2009/demo/us-pop-proj-2000-2050.html. Accessed November 13, 2020.
- Pott-Grinstein E, Newcomer JR. Gynecologists’ patterns of prescribing pessaries. J Reprod Med. 2001;46:205-208.
- Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
- Reena C, Kekre AN, Kekre N. Occult stress incontinence in women with pelvic organ prolapse. Int J Gynaecol Obstet. 2007;97:31-34.
- Thys SD, Roovers JP, Geomini PM, et al. Do patients prefer a pessary or surgery as primary treatment for pelvic organ prolapse. Gynecol Obstet Invest. 2012;74:6-12.
- Kapoor DS, Thakar R, Sultan AH, et al. Conservative versus surgical management of prolapse: what dictates patient choice? Int Urogynecol J Pelvic Floor Dysfunct. 2009;20: 1157-1161.
- Wu V, Farrel SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Culligan PJ. Nonsurgical management of pelvic organ prolapse. Obstet Gynecol. 2012;119:852-860.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-404.e8.
- Cundiff GW, Weidner AC, Visco AG, et al. A survey of pessary use by members of the American Urogynecologic Society. Obstet Gynecol. 2000;95(6 pt 1):931-935.
- Singh K, Reid W. Nonsurgical treatment of uterovaginal prolapse using double vaginal rings. Br J Obstet Gynecol. 2001;108:112-113.
- Zoorob D, Higgins M, Swan K, et al. Barriers to pelvic floor physical therapy regarding treatment of high-tone pelvic floor dysfunction. Female Pelvic Med Reconstr Surg. 2017;23:444-448.
- Kirby AC, Luber KM, Menefee SA. An update on the current and future demand for care of pelvic floor disorders in the United States. Am J Obstet Gynecol. 2013;209:584.e1-584.e5.
- Ellerkmann RM, Cundiff GW, Melick CF, et al. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185:1332-1337.
- US Census Bureau. United States population projections: 2000 to 2050. https://www.census.gov/library/workingpapers/2009/demo/us-pop-proj-2000-2050.html. Accessed November 13, 2020.
- Pott-Grinstein E, Newcomer JR. Gynecologists’ patterns of prescribing pessaries. J Reprod Med. 2001;46:205-208.
- Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
- Reena C, Kekre AN, Kekre N. Occult stress incontinence in women with pelvic organ prolapse. Int J Gynaecol Obstet. 2007;97:31-34.
- Thys SD, Roovers JP, Geomini PM, et al. Do patients prefer a pessary or surgery as primary treatment for pelvic organ prolapse. Gynecol Obstet Invest. 2012;74:6-12.
- Kapoor DS, Thakar R, Sultan AH, et al. Conservative versus surgical management of prolapse: what dictates patient choice? Int Urogynecol J Pelvic Floor Dysfunct. 2009;20: 1157-1161.
- Wu V, Farrel SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Culligan PJ. Nonsurgical management of pelvic organ prolapse. Obstet Gynecol. 2012;119:852-860.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-404.e8.
- Cundiff GW, Weidner AC, Visco AG, et al. A survey of pessary use by members of the American Urogynecologic Society. Obstet Gynecol. 2000;95(6 pt 1):931-935.
- Singh K, Reid W. Nonsurgical treatment of uterovaginal prolapse using double vaginal rings. Br J Obstet Gynecol. 2001;108:112-113.
Even in a virtual environment, the Society of Gynecologic Surgeons delivers without a “glitch”
Earlier this year, I was honored to serve as the Scientific Program Chair for the 46th Annual Scientific Meeting of the Society of Gynecologic Surgeons (SGS). This year’s meeting was the first ever (and hopefully last) “virtual” scientific meeting, which consisted of a hybrid of prerecorded and live presentations. Although faculty and attendees were not able to be together physically, the essence of the lively SGS meetings came through loud and clear. We still had “discussants” comment on the oral presentations and ask questions of the presenters. These questions and answers were all done live—without a glitch! Many thanks to all who made this meeting possible.
In addition to the outstanding abstract and video presentations, there were 4 superb postgraduate courses:
- Mikio Nihira, MD, chaired “Enhanced recovery after surgery: Overcoming barriers to implementation.”
- Charles Hanes, MD, headed up “It’s all about the apex: The key to successful POP surgery.”
- Cara King, DO, MS, led “Total laparoscopic hysterectomy: Pushing the envelope.”
- Vincent Lucente, MD, chaired “Transvaginal reconstructive pelvic surgery using graft augmentation post-FDA.”
Many special thanks to Dr. Lucente who transformed his course into a wonderful article for this special section of
One of our exceptional keynote speakers was Marc Beer (a serial entrepreneur and cofounder, chairman, and CEO of Renovia, Inc.), whose talk was entitled “A primer on medical device innovation—How to avoid common pitfalls while realizing your vision.” Mr. Beer has turned this topic into a unique article for this special section (see next month’s issue for Part 2).
Our TeLinde Lecture, entitled “Artificial intelligence in surgery,” was delivered by the dynamic Vicente Gracias, MD, professor of surgery at Robert Wood Johnson University Hospital, New Brunswick, New Jersey. We also held 2 live panel discussions that were very popular. The first, “Work-life balance and gynecologic surgery,” featured various perspectives from Drs. Kristie Green, Sally Huber, Catherine Matthews, and Charles Rardin. The second panel discussion, entitled “Understanding, managing, and benefiting from your e-presence,” by experts Heather Schueppert; Chief Marketing Officer at Unified Physician Management, Brad Bowman, MD; and Peter Lotze, MD. Both of these panel discussions are included in this special section as well.
I hope you enjoy the content of this special section of
Earlier this year, I was honored to serve as the Scientific Program Chair for the 46th Annual Scientific Meeting of the Society of Gynecologic Surgeons (SGS). This year’s meeting was the first ever (and hopefully last) “virtual” scientific meeting, which consisted of a hybrid of prerecorded and live presentations. Although faculty and attendees were not able to be together physically, the essence of the lively SGS meetings came through loud and clear. We still had “discussants” comment on the oral presentations and ask questions of the presenters. These questions and answers were all done live—without a glitch! Many thanks to all who made this meeting possible.
In addition to the outstanding abstract and video presentations, there were 4 superb postgraduate courses:
- Mikio Nihira, MD, chaired “Enhanced recovery after surgery: Overcoming barriers to implementation.”
- Charles Hanes, MD, headed up “It’s all about the apex: The key to successful POP surgery.”
- Cara King, DO, MS, led “Total laparoscopic hysterectomy: Pushing the envelope.”
- Vincent Lucente, MD, chaired “Transvaginal reconstructive pelvic surgery using graft augmentation post-FDA.”
Many special thanks to Dr. Lucente who transformed his course into a wonderful article for this special section of
One of our exceptional keynote speakers was Marc Beer (a serial entrepreneur and cofounder, chairman, and CEO of Renovia, Inc.), whose talk was entitled “A primer on medical device innovation—How to avoid common pitfalls while realizing your vision.” Mr. Beer has turned this topic into a unique article for this special section (see next month’s issue for Part 2).
Our TeLinde Lecture, entitled “Artificial intelligence in surgery,” was delivered by the dynamic Vicente Gracias, MD, professor of surgery at Robert Wood Johnson University Hospital, New Brunswick, New Jersey. We also held 2 live panel discussions that were very popular. The first, “Work-life balance and gynecologic surgery,” featured various perspectives from Drs. Kristie Green, Sally Huber, Catherine Matthews, and Charles Rardin. The second panel discussion, entitled “Understanding, managing, and benefiting from your e-presence,” by experts Heather Schueppert; Chief Marketing Officer at Unified Physician Management, Brad Bowman, MD; and Peter Lotze, MD. Both of these panel discussions are included in this special section as well.
I hope you enjoy the content of this special section of
Earlier this year, I was honored to serve as the Scientific Program Chair for the 46th Annual Scientific Meeting of the Society of Gynecologic Surgeons (SGS). This year’s meeting was the first ever (and hopefully last) “virtual” scientific meeting, which consisted of a hybrid of prerecorded and live presentations. Although faculty and attendees were not able to be together physically, the essence of the lively SGS meetings came through loud and clear. We still had “discussants” comment on the oral presentations and ask questions of the presenters. These questions and answers were all done live—without a glitch! Many thanks to all who made this meeting possible.
In addition to the outstanding abstract and video presentations, there were 4 superb postgraduate courses:
- Mikio Nihira, MD, chaired “Enhanced recovery after surgery: Overcoming barriers to implementation.”
- Charles Hanes, MD, headed up “It’s all about the apex: The key to successful POP surgery.”
- Cara King, DO, MS, led “Total laparoscopic hysterectomy: Pushing the envelope.”
- Vincent Lucente, MD, chaired “Transvaginal reconstructive pelvic surgery using graft augmentation post-FDA.”
Many special thanks to Dr. Lucente who transformed his course into a wonderful article for this special section of
One of our exceptional keynote speakers was Marc Beer (a serial entrepreneur and cofounder, chairman, and CEO of Renovia, Inc.), whose talk was entitled “A primer on medical device innovation—How to avoid common pitfalls while realizing your vision.” Mr. Beer has turned this topic into a unique article for this special section (see next month’s issue for Part 2).
Our TeLinde Lecture, entitled “Artificial intelligence in surgery,” was delivered by the dynamic Vicente Gracias, MD, professor of surgery at Robert Wood Johnson University Hospital, New Brunswick, New Jersey. We also held 2 live panel discussions that were very popular. The first, “Work-life balance and gynecologic surgery,” featured various perspectives from Drs. Kristie Green, Sally Huber, Catherine Matthews, and Charles Rardin. The second panel discussion, entitled “Understanding, managing, and benefiting from your e-presence,” by experts Heather Schueppert; Chief Marketing Officer at Unified Physician Management, Brad Bowman, MD; and Peter Lotze, MD. Both of these panel discussions are included in this special section as well.
I hope you enjoy the content of this special section of
Transvaginal reconstructive surgery for POP: Innovative approach to graft augmentation in the post-mesh era
Pelvic organ prolapse (POP) is a common occurrence over the course of a woman’s lifetime, especially in parous women (up to 50% of women who have given birth).1 The anterior vaginal wall is the most common site of POP and has the highest recurrence rate of up to 70%.2 The risk of developing POP increases with age, obesity, White race, family history, and prior pelvic surgery, such as hysterectomy. It affects more than 3 million women in the United States alone, often negatively impacting sexual function and overall quality of life.3,4
Because women are living longer than ever before and are more active in their senior years, a long-lasting, durable surgical repair is desirable, if not necessary. To be cost-effective and to avoid general anesthesia, the surgical approach ideally should be vaginal.
Biologic and synthetic grafts to augment transvaginal repair traditionally are used to improve on the well-recognized high failure rate of native-tissue repair that is often seen at both short-term and medium-term follow-up.5 The failure rate is commonly referenced as 30% to 40% at 2-year follow-up and 61% to 70% at 5-year follow-up, well-established by the results of the OPTIMAL randomized clinical trial.6 The more recent Descent trial likewise demonstrates a higher failure rate of native-tissue repair versus transvaginal mesh repair at a shorter term of 30 to 42 months.7 Furthermore, the use of permanent versus absorbable suture in suspension of the vaginal apex is associated with lower short-term failure rates.8
Despite this Level I evidence that demonstrates a clear advantage for obtaining a longer or more durable repair with permanent materials, native-tissue repairs with absorbable suture are still performed routinely. Since the US Food and Drug Administration (FDA) ordered that the use of transvaginal surgical mesh augmentation for pelvic reconstructive surgery be discontinued, it is more important than ever to explore evolving alternative native-tissue augmentation repair techniques that hopefully can preserve the advantages and merits of vaginal surgery and achieve longer durability.9
Biologic graft augmentation use in transvaginal reconstruction
All biologic grafts, including allografts derived from human tissue and xenografts derived from animal tissue, are acellular constructs composed of extracellular matrix (ECM) that acts as scaffolding for the host tissue. The ECM is predominantly composed of collagen (types I and III) and noncollagenous fibronectin, laminin, and glycosaminoglycans in various amounts depending on the source tissue. The 3D presentation of ECM’s complex molecules allows for rapid repopulation of host cells and revascularization with eventual regeneration.
Once a biologic graft is placed surgically, the body’s response to the scaffold ECM mimics the normal wound-healing process, beginning with fibrin-rich matrix hemostasis and the subsequent innate immune response of neutrophil and M1 macrophage infiltration. M1 macrophages are proinflammatory and clear cellular debris and begin the process of graft scaffold degradation. The host tissue then begins the process of remodeling through pro-remodeling M2 macrophages and stem cell recruitment, proliferation, and differentiation.10 As the biologic graft provides initial structure and strength for pelvic repairs, the ideal ECM scaffold would not degrade before the host is able to fully undergo regeneration and maintain its structure and strength.
Biologic grafts differ in source (allograft or xenograft), type (pericardium, dermis, or bladder), developmental stage (fetal or adult), decellularization processing, and sterilization techniques. These 5 aspects determine the distinct 3D ECM scaffold structure, strength, and longevity. If the ECM scaffold is damaged or retains noncollagenous proteins during the preparation process, an inflammatory response is triggered in which the graft is degraded, resorbed, and replaced with scar tissue. Furthermore, certain processing techniques aimed at extending the ECM’s durability—that is, cross-linking collagen—results in the foreign body response in which there is no vascular infiltration or cellular penetration of the graft and a collagen capsule is created around the empty matrix.11 To avoid resorption or encapsulation of the graft, the ECM scaffolds of biologic grafts must be optimized to induce regeneration.
Continue to: Choosing surgical POP repair...
Choosing surgical POP repair
The decision to undergo surgical treatment for prolapse is a shared decision-making process between the patient and surgeon and always should be individualized. The type of procedure and the surgical approach will depend on the patient’s goals, the degree of prolapse, clinical history, risk tolerance, the surgeon’s skill set, and whether or not there is an indication or relative contraindication for uterine removal at the time of prolapse repair.
While the FDA’s order does not apply to transabdominally placed surgical mesh, such as sacrocolpopexy, not all patients are ideal candidates for an abdominal sacrocolpopexy. Most notable are women with a history of multiple prior abdominal surgeries with higher rates of intraperitoneal adhesions. Ideally, to be cost-effective and to avoid general anesthesia, the surgical approach should be vaginal whenever possible.
Biologic versus native-tissue grafts
Currently, only low-quality evidence exists that compares the outcomes of biologic grafts with traditional native-tissue repairs in POP. Studies have been limited by poor reporting of methods, inconsistency in technique and materials used, and imprecise definitions. One Cochrane Review on the surgical management of POP concluded that biologic graft augmentation was associated with a lower failure rate (18%) within 1 to 2 years when compared with a traditional anterior colporrhaphy (28%).12
Based on consideration of all Cochrane Database Reviews and recent large systematic reviews, there clearly is a paucity of information on which to draw well-defined conclusions regarding the advantage of biomaterials in prolapse surgery.12-14 This is due in part to the variation in graft material used and the surgical technique employed.
Similarly, evidence is lacking regarding the superiority of one type of biologic graft over another. Furthermore, some of the grafts previously studied are no longer on the market.15 With the FDA’s removal of all transvaginal mesh, including xenografts, only allografts are available for pelvic floor reconstruction. Currently, only 3 commercial manufacturers market allografts for pelvic floor reconstruction. Each allograft is available in various sizes and all can be trimmed at the time of the surgical procedure to customize both the size and shape to fit the individual patient.
A novel technique using Axis Dermis and polypropylene suture
One of the commercially available allografts, Axis Dermis (Coloplast), is non–cross-linked and is derived from human cadaveric dermal tissue from the back and dorsum of the upper leg. It is sterilized by a proprietary Tutoplast️ sterilization process that uses gamma irradiation to inactivate and prevent the transmission of pathogens. This unique technique involving solvent dehydration means the graft is never freeze dried; thus, the natural tissue matrix is preserved.
Additionally, the allograft is antigen-free, which decreases the risk of tissue reaction (scarring/fibrosis) and aids in the process of host tissue remodeling; invasion by growth factors, blood cells, collagen, elastin, and neovascularization. This natural tissue remodeling facilitates the anticipated “reabsorption” of the graft by the host tissue, leaving the patient with a tissue scaffold, that is, a stronger layer of “fascia” beneath the muscularis.16 As a result of this “biocompatible” graft, the host tissue remodeling has been shown in the rat model to involve early cellular infiltration and angiogenesis (in the first week after implantation), that leads to an organized cellular architecture with greater tensile strength by week 4, and ultimately inability to distinguish host collagen from the implant by 8 to 12 weeks.17,18
Continue to: Steps in performing the technique...
Steps in performing the technique
To ensure that the graft is placed adjacent to the vaginal serosa, a full-thickness dissection is carried out to enter the true vesicovaginal space, which lies below all 4 histologic layers of the vagina (nonkeratinized stratified squamous epithelium, lamina propria, muscularis, and serosa). For the anterior dissection, a Tuohy epidural needle is used to achieve an accurate and consistent depth when injecting fluid (hydrodissection) to enter this true pelvic space (FIGURE 1). Correct entry into the vesicovaginal space can be confirmed visually by the presence of adipose tissue.
Many pelvic surgeons use the sacrospinous ligament (SSL) as a strong and reliable point of attachment for vaginal prolapse repair. It can be approached either anteriorly or posteriorly with careful dissection. Permanent suture (0-Prolene) is used to “bridge” the attachment between the SSL, the Axis Dermis graft, and the cervix (or vaginal apex). The suture is placed in the middle third and lower half of the ligament to avoid injury to nearby neurovascular structures.
While the surgeon may use any suture-capturing device, we prefer the Anchosure System (Neomedic). This device delivers a small anchor securely into the ligament through a single point of entry, minimizing the risk of postoperative pain for the patient. A 6 cm x 8 cm size Axis Dermis graft is then trimmed to meet the specifications of the patient’s anatomy.
Most commonly, we measure, mark, and trim the body of the graft to 5.5 cm in length with a width of 3 cm. The bilateral arms are approximately 1 cm in width and comprise the remaining length of the 8 cm graft (FIGURE 2). As shown in Figure 2, pre-made holes are marked and punched out using a large hollow needle. These serve as the points of attachment for the permanent suture to be “weaved” into the graft arms and delayed absorbable “tacking suture” to be attached from the pubocervical fascia at the bladder neck to the distal end of the graft. This facilitates fixation of the graft in the midline of the anterior vaginal wall, overlying any central distention-type defect.
Finally, following attachment of the SSL permanent suture to the distal graft arm, this suture is then attached to the proximal U-shaped end of the graft body (in the midline), followed by a deep and secure bite through the cervix (or vaginal vault apex) and back through the proximal graft. These SSL suspension sutures are then tied such that the distal arms of the graft advance down to the ligament. Care is taken not to tie down to the SSL itself, rather until the cervix (or apex) is reduced to its normal anatomical location.
After the graft is secured in place, the full-thickness vaginal wall is closed with delayed absorbable suture. Sterile 1-inch ribbon packing is placed in the vagina immediately to close any dead space between the vagina and the graft to decrease the risk of seroma or hematoma formation.
This newly developed technique, like many surgeries for POP, requires extensive knowledge of pelvic anatomy and skill in vaginal surgery, and we recommend referral to a subspecialist in Female Pelvic Medicine and Reconstructive Surgery.

Continue to: Upcoming plans to share outcomes data...
Upcoming plans to share outcomes data
We are in the process of performing a retrospective review of all of the cases we have performed at our institution using this technique of permanent suture bridging to the SSL within the arm of the biograft. Given the relatively recent FDA announcement, we have yet to establish any long-term outcomes data. However, the preliminary results at 6-month follow-up are promising and demonstrate a low (2.6%) failure rate, without significant safety concerns. We hope to publish these data as well as more data on longitudinal outcomes in the future.
In summary
Many women are at risk for native-tissue repair failure or are not well suited for an abdominal procedure to correct their pelvic support defect and restore their quality of life. As expert pelvic surgeons, we play an important role in the search for innovative solutions for these women. There is ample opportunity for future research and clinical trials to determine the best biologic materials and their optimal use in pelvic reconstructive surgery.
Originally, polypropylene mesh was designed for use in augmenting abdominal hernia repairs and later was adapted by manufacturers for use in POP repair. The FDA removal from the market of existing transvaginal synthetic mesh kits was a unique catalyst that challenged our community to develop transvaginal repairs using biologic grafts that are genuinely tailored to the unique needs of the female pelvic anatomy. ●
- Maher C, Feiner B, Baessler K. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013:CD004014.
- Weber AM, Walters MD, Piedmonte MR, et al. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1304.
- Walters MD, Ridgeway BM. Surgical treatment of vaginal apex prolapse. Obstet Gynecol. 2013;121(2 pt 1):354-374.
- Meister MRL, Sutcliffe S, Lowder JL. Definitions of apical vaginal support loss: a systematic review. Am J Obstet Gynecol. 2017;216:232. e1-232.e14.
- Cox A, Herschorn S. Evaluation of current biologic meshes in pelvic organ prolapse repair. Curr Urol Rep. 2012;13:247-255.
- Jelovsek JE, Barber M, Brubaker K, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018:319:1554-1565.
- Bowen ST, Moalli P, Abramowitch S, et al. Outcomes of the defining mechanisms of anterior vaginal wall descent trial [abstract 15]. Am J Obstet Gynecol. 2020;222:S770-S771.
- Chung CP, Miskimins R, Kuehl TJ, et al. Permanent suture used in uterosacral ligament suspension offers better anatomical support than delayed absorbable suture. Int Urogynecol J. 2012;23:223-227.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical -mesh-intended-transvaginal. April 16, 2019. Accessed September 1, 2020.
- Londono R, Badylak SF. Biologic scaffolds for regenerative medicine: mechanisms of in vivo remodeling. Ann Biomed Eng. 2015;43:577-592.
- Cornwell KG, Landsman A, James KS. Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg. 2009;26: 507-523.
- Maher CM, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women: the updated summary version Cochrane review. Int Urogynecol J. 2011;22:1445-1447.
- Maher C, Feiner B, Baessler K, et al. Surgery for women with anterior compartment prolapse. Cochrane Database Syst Rev. 2016;11:CD004014.
- Maher C, Feiner B, Baessler K, et al. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cochrane Database Syst Rev. 2016;2:CD012179.
- Rosenblatt P, Von Bargen E. Use of biologic grafts in pelvic organ prolapse surgery. Contemporary OB/GYN. 2017;62:14-19.
- Greenspan DC, Hernandez R, Faleris J. Histology of surgically implanted Tutoplast processed dermis. http://www.zimmerbiomet .co.il/images/lib_artHistologyDermis%2010.pdf. Accessed September 2, 2020.
- Williams D. Revisiting the definition of biocompatibility. Med Device Technol. 2003;14:10-13.
- Nosti PA, Carter CM, Sokol AI, et al. Transvaginal versus transabdominal placement of synthetic mesh at time of sacrocolpopexy. Female Pelvic Med Reconstr Surg. 2016;22:151-155.
Pelvic organ prolapse (POP) is a common occurrence over the course of a woman’s lifetime, especially in parous women (up to 50% of women who have given birth).1 The anterior vaginal wall is the most common site of POP and has the highest recurrence rate of up to 70%.2 The risk of developing POP increases with age, obesity, White race, family history, and prior pelvic surgery, such as hysterectomy. It affects more than 3 million women in the United States alone, often negatively impacting sexual function and overall quality of life.3,4
Because women are living longer than ever before and are more active in their senior years, a long-lasting, durable surgical repair is desirable, if not necessary. To be cost-effective and to avoid general anesthesia, the surgical approach ideally should be vaginal.
Biologic and synthetic grafts to augment transvaginal repair traditionally are used to improve on the well-recognized high failure rate of native-tissue repair that is often seen at both short-term and medium-term follow-up.5 The failure rate is commonly referenced as 30% to 40% at 2-year follow-up and 61% to 70% at 5-year follow-up, well-established by the results of the OPTIMAL randomized clinical trial.6 The more recent Descent trial likewise demonstrates a higher failure rate of native-tissue repair versus transvaginal mesh repair at a shorter term of 30 to 42 months.7 Furthermore, the use of permanent versus absorbable suture in suspension of the vaginal apex is associated with lower short-term failure rates.8
Despite this Level I evidence that demonstrates a clear advantage for obtaining a longer or more durable repair with permanent materials, native-tissue repairs with absorbable suture are still performed routinely. Since the US Food and Drug Administration (FDA) ordered that the use of transvaginal surgical mesh augmentation for pelvic reconstructive surgery be discontinued, it is more important than ever to explore evolving alternative native-tissue augmentation repair techniques that hopefully can preserve the advantages and merits of vaginal surgery and achieve longer durability.9
Biologic graft augmentation use in transvaginal reconstruction
All biologic grafts, including allografts derived from human tissue and xenografts derived from animal tissue, are acellular constructs composed of extracellular matrix (ECM) that acts as scaffolding for the host tissue. The ECM is predominantly composed of collagen (types I and III) and noncollagenous fibronectin, laminin, and glycosaminoglycans in various amounts depending on the source tissue. The 3D presentation of ECM’s complex molecules allows for rapid repopulation of host cells and revascularization with eventual regeneration.
Once a biologic graft is placed surgically, the body’s response to the scaffold ECM mimics the normal wound-healing process, beginning with fibrin-rich matrix hemostasis and the subsequent innate immune response of neutrophil and M1 macrophage infiltration. M1 macrophages are proinflammatory and clear cellular debris and begin the process of graft scaffold degradation. The host tissue then begins the process of remodeling through pro-remodeling M2 macrophages and stem cell recruitment, proliferation, and differentiation.10 As the biologic graft provides initial structure and strength for pelvic repairs, the ideal ECM scaffold would not degrade before the host is able to fully undergo regeneration and maintain its structure and strength.
Biologic grafts differ in source (allograft or xenograft), type (pericardium, dermis, or bladder), developmental stage (fetal or adult), decellularization processing, and sterilization techniques. These 5 aspects determine the distinct 3D ECM scaffold structure, strength, and longevity. If the ECM scaffold is damaged or retains noncollagenous proteins during the preparation process, an inflammatory response is triggered in which the graft is degraded, resorbed, and replaced with scar tissue. Furthermore, certain processing techniques aimed at extending the ECM’s durability—that is, cross-linking collagen—results in the foreign body response in which there is no vascular infiltration or cellular penetration of the graft and a collagen capsule is created around the empty matrix.11 To avoid resorption or encapsulation of the graft, the ECM scaffolds of biologic grafts must be optimized to induce regeneration.
Continue to: Choosing surgical POP repair...
Choosing surgical POP repair
The decision to undergo surgical treatment for prolapse is a shared decision-making process between the patient and surgeon and always should be individualized. The type of procedure and the surgical approach will depend on the patient’s goals, the degree of prolapse, clinical history, risk tolerance, the surgeon’s skill set, and whether or not there is an indication or relative contraindication for uterine removal at the time of prolapse repair.
While the FDA’s order does not apply to transabdominally placed surgical mesh, such as sacrocolpopexy, not all patients are ideal candidates for an abdominal sacrocolpopexy. Most notable are women with a history of multiple prior abdominal surgeries with higher rates of intraperitoneal adhesions. Ideally, to be cost-effective and to avoid general anesthesia, the surgical approach should be vaginal whenever possible.
Biologic versus native-tissue grafts
Currently, only low-quality evidence exists that compares the outcomes of biologic grafts with traditional native-tissue repairs in POP. Studies have been limited by poor reporting of methods, inconsistency in technique and materials used, and imprecise definitions. One Cochrane Review on the surgical management of POP concluded that biologic graft augmentation was associated with a lower failure rate (18%) within 1 to 2 years when compared with a traditional anterior colporrhaphy (28%).12
Based on consideration of all Cochrane Database Reviews and recent large systematic reviews, there clearly is a paucity of information on which to draw well-defined conclusions regarding the advantage of biomaterials in prolapse surgery.12-14 This is due in part to the variation in graft material used and the surgical technique employed.
Similarly, evidence is lacking regarding the superiority of one type of biologic graft over another. Furthermore, some of the grafts previously studied are no longer on the market.15 With the FDA’s removal of all transvaginal mesh, including xenografts, only allografts are available for pelvic floor reconstruction. Currently, only 3 commercial manufacturers market allografts for pelvic floor reconstruction. Each allograft is available in various sizes and all can be trimmed at the time of the surgical procedure to customize both the size and shape to fit the individual patient.
A novel technique using Axis Dermis and polypropylene suture
One of the commercially available allografts, Axis Dermis (Coloplast), is non–cross-linked and is derived from human cadaveric dermal tissue from the back and dorsum of the upper leg. It is sterilized by a proprietary Tutoplast️ sterilization process that uses gamma irradiation to inactivate and prevent the transmission of pathogens. This unique technique involving solvent dehydration means the graft is never freeze dried; thus, the natural tissue matrix is preserved.
Additionally, the allograft is antigen-free, which decreases the risk of tissue reaction (scarring/fibrosis) and aids in the process of host tissue remodeling; invasion by growth factors, blood cells, collagen, elastin, and neovascularization. This natural tissue remodeling facilitates the anticipated “reabsorption” of the graft by the host tissue, leaving the patient with a tissue scaffold, that is, a stronger layer of “fascia” beneath the muscularis.16 As a result of this “biocompatible” graft, the host tissue remodeling has been shown in the rat model to involve early cellular infiltration and angiogenesis (in the first week after implantation), that leads to an organized cellular architecture with greater tensile strength by week 4, and ultimately inability to distinguish host collagen from the implant by 8 to 12 weeks.17,18
Continue to: Steps in performing the technique...
Steps in performing the technique
To ensure that the graft is placed adjacent to the vaginal serosa, a full-thickness dissection is carried out to enter the true vesicovaginal space, which lies below all 4 histologic layers of the vagina (nonkeratinized stratified squamous epithelium, lamina propria, muscularis, and serosa). For the anterior dissection, a Tuohy epidural needle is used to achieve an accurate and consistent depth when injecting fluid (hydrodissection) to enter this true pelvic space (FIGURE 1). Correct entry into the vesicovaginal space can be confirmed visually by the presence of adipose tissue.

Many pelvic surgeons use the sacrospinous ligament (SSL) as a strong and reliable point of attachment for vaginal prolapse repair. It can be approached either anteriorly or posteriorly with careful dissection. Permanent suture (0-Prolene) is used to “bridge” the attachment between the SSL, the Axis Dermis graft, and the cervix (or vaginal apex). The suture is placed in the middle third and lower half of the ligament to avoid injury to nearby neurovascular structures.
While the surgeon may use any suture-capturing device, we prefer the Anchosure System (Neomedic). This device delivers a small anchor securely into the ligament through a single point of entry, minimizing the risk of postoperative pain for the patient. A 6 cm x 8 cm size Axis Dermis graft is then trimmed to meet the specifications of the patient’s anatomy.
Most commonly, we measure, mark, and trim the body of the graft to 5.5 cm in length with a width of 3 cm. The bilateral arms are approximately 1 cm in width and comprise the remaining length of the 8 cm graft (FIGURE 2). As shown in Figure 2, pre-made holes are marked and punched out using a large hollow needle. These serve as the points of attachment for the permanent suture to be “weaved” into the graft arms and delayed absorbable “tacking suture” to be attached from the pubocervical fascia at the bladder neck to the distal end of the graft. This facilitates fixation of the graft in the midline of the anterior vaginal wall, overlying any central distention-type defect.

Finally, following attachment of the SSL permanent suture to the distal graft arm, this suture is then attached to the proximal U-shaped end of the graft body (in the midline), followed by a deep and secure bite through the cervix (or vaginal vault apex) and back through the proximal graft. These SSL suspension sutures are then tied such that the distal arms of the graft advance down to the ligament. Care is taken not to tie down to the SSL itself, rather until the cervix (or apex) is reduced to its normal anatomical location.
After the graft is secured in place, the full-thickness vaginal wall is closed with delayed absorbable suture. Sterile 1-inch ribbon packing is placed in the vagina immediately to close any dead space between the vagina and the graft to decrease the risk of seroma or hematoma formation.
This newly developed technique, like many surgeries for POP, requires extensive knowledge of pelvic anatomy and skill in vaginal surgery, and we recommend referral to a subspecialist in Female Pelvic Medicine and Reconstructive Surgery.

Continue to: Upcoming plans to share outcomes data...
Upcoming plans to share outcomes data
We are in the process of performing a retrospective review of all of the cases we have performed at our institution using this technique of permanent suture bridging to the SSL within the arm of the biograft. Given the relatively recent FDA announcement, we have yet to establish any long-term outcomes data. However, the preliminary results at 6-month follow-up are promising and demonstrate a low (2.6%) failure rate, without significant safety concerns. We hope to publish these data as well as more data on longitudinal outcomes in the future.
In summary
Many women are at risk for native-tissue repair failure or are not well suited for an abdominal procedure to correct their pelvic support defect and restore their quality of life. As expert pelvic surgeons, we play an important role in the search for innovative solutions for these women. There is ample opportunity for future research and clinical trials to determine the best biologic materials and their optimal use in pelvic reconstructive surgery.
Originally, polypropylene mesh was designed for use in augmenting abdominal hernia repairs and later was adapted by manufacturers for use in POP repair. The FDA removal from the market of existing transvaginal synthetic mesh kits was a unique catalyst that challenged our community to develop transvaginal repairs using biologic grafts that are genuinely tailored to the unique needs of the female pelvic anatomy. ●
Pelvic organ prolapse (POP) is a common occurrence over the course of a woman’s lifetime, especially in parous women (up to 50% of women who have given birth).1 The anterior vaginal wall is the most common site of POP and has the highest recurrence rate of up to 70%.2 The risk of developing POP increases with age, obesity, White race, family history, and prior pelvic surgery, such as hysterectomy. It affects more than 3 million women in the United States alone, often negatively impacting sexual function and overall quality of life.3,4
Because women are living longer than ever before and are more active in their senior years, a long-lasting, durable surgical repair is desirable, if not necessary. To be cost-effective and to avoid general anesthesia, the surgical approach ideally should be vaginal.
Biologic and synthetic grafts to augment transvaginal repair traditionally are used to improve on the well-recognized high failure rate of native-tissue repair that is often seen at both short-term and medium-term follow-up.5 The failure rate is commonly referenced as 30% to 40% at 2-year follow-up and 61% to 70% at 5-year follow-up, well-established by the results of the OPTIMAL randomized clinical trial.6 The more recent Descent trial likewise demonstrates a higher failure rate of native-tissue repair versus transvaginal mesh repair at a shorter term of 30 to 42 months.7 Furthermore, the use of permanent versus absorbable suture in suspension of the vaginal apex is associated with lower short-term failure rates.8
Despite this Level I evidence that demonstrates a clear advantage for obtaining a longer or more durable repair with permanent materials, native-tissue repairs with absorbable suture are still performed routinely. Since the US Food and Drug Administration (FDA) ordered that the use of transvaginal surgical mesh augmentation for pelvic reconstructive surgery be discontinued, it is more important than ever to explore evolving alternative native-tissue augmentation repair techniques that hopefully can preserve the advantages and merits of vaginal surgery and achieve longer durability.9
Biologic graft augmentation use in transvaginal reconstruction
All biologic grafts, including allografts derived from human tissue and xenografts derived from animal tissue, are acellular constructs composed of extracellular matrix (ECM) that acts as scaffolding for the host tissue. The ECM is predominantly composed of collagen (types I and III) and noncollagenous fibronectin, laminin, and glycosaminoglycans in various amounts depending on the source tissue. The 3D presentation of ECM’s complex molecules allows for rapid repopulation of host cells and revascularization with eventual regeneration.
Once a biologic graft is placed surgically, the body’s response to the scaffold ECM mimics the normal wound-healing process, beginning with fibrin-rich matrix hemostasis and the subsequent innate immune response of neutrophil and M1 macrophage infiltration. M1 macrophages are proinflammatory and clear cellular debris and begin the process of graft scaffold degradation. The host tissue then begins the process of remodeling through pro-remodeling M2 macrophages and stem cell recruitment, proliferation, and differentiation.10 As the biologic graft provides initial structure and strength for pelvic repairs, the ideal ECM scaffold would not degrade before the host is able to fully undergo regeneration and maintain its structure and strength.
Biologic grafts differ in source (allograft or xenograft), type (pericardium, dermis, or bladder), developmental stage (fetal or adult), decellularization processing, and sterilization techniques. These 5 aspects determine the distinct 3D ECM scaffold structure, strength, and longevity. If the ECM scaffold is damaged or retains noncollagenous proteins during the preparation process, an inflammatory response is triggered in which the graft is degraded, resorbed, and replaced with scar tissue. Furthermore, certain processing techniques aimed at extending the ECM’s durability—that is, cross-linking collagen—results in the foreign body response in which there is no vascular infiltration or cellular penetration of the graft and a collagen capsule is created around the empty matrix.11 To avoid resorption or encapsulation of the graft, the ECM scaffolds of biologic grafts must be optimized to induce regeneration.
Continue to: Choosing surgical POP repair...
Choosing surgical POP repair
The decision to undergo surgical treatment for prolapse is a shared decision-making process between the patient and surgeon and always should be individualized. The type of procedure and the surgical approach will depend on the patient’s goals, the degree of prolapse, clinical history, risk tolerance, the surgeon’s skill set, and whether or not there is an indication or relative contraindication for uterine removal at the time of prolapse repair.
While the FDA’s order does not apply to transabdominally placed surgical mesh, such as sacrocolpopexy, not all patients are ideal candidates for an abdominal sacrocolpopexy. Most notable are women with a history of multiple prior abdominal surgeries with higher rates of intraperitoneal adhesions. Ideally, to be cost-effective and to avoid general anesthesia, the surgical approach should be vaginal whenever possible.
Biologic versus native-tissue grafts
Currently, only low-quality evidence exists that compares the outcomes of biologic grafts with traditional native-tissue repairs in POP. Studies have been limited by poor reporting of methods, inconsistency in technique and materials used, and imprecise definitions. One Cochrane Review on the surgical management of POP concluded that biologic graft augmentation was associated with a lower failure rate (18%) within 1 to 2 years when compared with a traditional anterior colporrhaphy (28%).12
Based on consideration of all Cochrane Database Reviews and recent large systematic reviews, there clearly is a paucity of information on which to draw well-defined conclusions regarding the advantage of biomaterials in prolapse surgery.12-14 This is due in part to the variation in graft material used and the surgical technique employed.
Similarly, evidence is lacking regarding the superiority of one type of biologic graft over another. Furthermore, some of the grafts previously studied are no longer on the market.15 With the FDA’s removal of all transvaginal mesh, including xenografts, only allografts are available for pelvic floor reconstruction. Currently, only 3 commercial manufacturers market allografts for pelvic floor reconstruction. Each allograft is available in various sizes and all can be trimmed at the time of the surgical procedure to customize both the size and shape to fit the individual patient.
A novel technique using Axis Dermis and polypropylene suture
One of the commercially available allografts, Axis Dermis (Coloplast), is non–cross-linked and is derived from human cadaveric dermal tissue from the back and dorsum of the upper leg. It is sterilized by a proprietary Tutoplast️ sterilization process that uses gamma irradiation to inactivate and prevent the transmission of pathogens. This unique technique involving solvent dehydration means the graft is never freeze dried; thus, the natural tissue matrix is preserved.
Additionally, the allograft is antigen-free, which decreases the risk of tissue reaction (scarring/fibrosis) and aids in the process of host tissue remodeling; invasion by growth factors, blood cells, collagen, elastin, and neovascularization. This natural tissue remodeling facilitates the anticipated “reabsorption” of the graft by the host tissue, leaving the patient with a tissue scaffold, that is, a stronger layer of “fascia” beneath the muscularis.16 As a result of this “biocompatible” graft, the host tissue remodeling has been shown in the rat model to involve early cellular infiltration and angiogenesis (in the first week after implantation), that leads to an organized cellular architecture with greater tensile strength by week 4, and ultimately inability to distinguish host collagen from the implant by 8 to 12 weeks.17,18
Continue to: Steps in performing the technique...
Steps in performing the technique
To ensure that the graft is placed adjacent to the vaginal serosa, a full-thickness dissection is carried out to enter the true vesicovaginal space, which lies below all 4 histologic layers of the vagina (nonkeratinized stratified squamous epithelium, lamina propria, muscularis, and serosa). For the anterior dissection, a Tuohy epidural needle is used to achieve an accurate and consistent depth when injecting fluid (hydrodissection) to enter this true pelvic space (FIGURE 1). Correct entry into the vesicovaginal space can be confirmed visually by the presence of adipose tissue.

Many pelvic surgeons use the sacrospinous ligament (SSL) as a strong and reliable point of attachment for vaginal prolapse repair. It can be approached either anteriorly or posteriorly with careful dissection. Permanent suture (0-Prolene) is used to “bridge” the attachment between the SSL, the Axis Dermis graft, and the cervix (or vaginal apex). The suture is placed in the middle third and lower half of the ligament to avoid injury to nearby neurovascular structures.
While the surgeon may use any suture-capturing device, we prefer the Anchosure System (Neomedic). This device delivers a small anchor securely into the ligament through a single point of entry, minimizing the risk of postoperative pain for the patient. A 6 cm x 8 cm size Axis Dermis graft is then trimmed to meet the specifications of the patient’s anatomy.
Most commonly, we measure, mark, and trim the body of the graft to 5.5 cm in length with a width of 3 cm. The bilateral arms are approximately 1 cm in width and comprise the remaining length of the 8 cm graft (FIGURE 2). As shown in Figure 2, pre-made holes are marked and punched out using a large hollow needle. These serve as the points of attachment for the permanent suture to be “weaved” into the graft arms and delayed absorbable “tacking suture” to be attached from the pubocervical fascia at the bladder neck to the distal end of the graft. This facilitates fixation of the graft in the midline of the anterior vaginal wall, overlying any central distention-type defect.

Finally, following attachment of the SSL permanent suture to the distal graft arm, this suture is then attached to the proximal U-shaped end of the graft body (in the midline), followed by a deep and secure bite through the cervix (or vaginal vault apex) and back through the proximal graft. These SSL suspension sutures are then tied such that the distal arms of the graft advance down to the ligament. Care is taken not to tie down to the SSL itself, rather until the cervix (or apex) is reduced to its normal anatomical location.
After the graft is secured in place, the full-thickness vaginal wall is closed with delayed absorbable suture. Sterile 1-inch ribbon packing is placed in the vagina immediately to close any dead space between the vagina and the graft to decrease the risk of seroma or hematoma formation.
This newly developed technique, like many surgeries for POP, requires extensive knowledge of pelvic anatomy and skill in vaginal surgery, and we recommend referral to a subspecialist in Female Pelvic Medicine and Reconstructive Surgery.

Continue to: Upcoming plans to share outcomes data...
Upcoming plans to share outcomes data
We are in the process of performing a retrospective review of all of the cases we have performed at our institution using this technique of permanent suture bridging to the SSL within the arm of the biograft. Given the relatively recent FDA announcement, we have yet to establish any long-term outcomes data. However, the preliminary results at 6-month follow-up are promising and demonstrate a low (2.6%) failure rate, without significant safety concerns. We hope to publish these data as well as more data on longitudinal outcomes in the future.
In summary
Many women are at risk for native-tissue repair failure or are not well suited for an abdominal procedure to correct their pelvic support defect and restore their quality of life. As expert pelvic surgeons, we play an important role in the search for innovative solutions for these women. There is ample opportunity for future research and clinical trials to determine the best biologic materials and their optimal use in pelvic reconstructive surgery.
Originally, polypropylene mesh was designed for use in augmenting abdominal hernia repairs and later was adapted by manufacturers for use in POP repair. The FDA removal from the market of existing transvaginal synthetic mesh kits was a unique catalyst that challenged our community to develop transvaginal repairs using biologic grafts that are genuinely tailored to the unique needs of the female pelvic anatomy. ●
- Maher C, Feiner B, Baessler K. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013:CD004014.
- Weber AM, Walters MD, Piedmonte MR, et al. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1304.
- Walters MD, Ridgeway BM. Surgical treatment of vaginal apex prolapse. Obstet Gynecol. 2013;121(2 pt 1):354-374.
- Meister MRL, Sutcliffe S, Lowder JL. Definitions of apical vaginal support loss: a systematic review. Am J Obstet Gynecol. 2017;216:232. e1-232.e14.
- Cox A, Herschorn S. Evaluation of current biologic meshes in pelvic organ prolapse repair. Curr Urol Rep. 2012;13:247-255.
- Jelovsek JE, Barber M, Brubaker K, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018:319:1554-1565.
- Bowen ST, Moalli P, Abramowitch S, et al. Outcomes of the defining mechanisms of anterior vaginal wall descent trial [abstract 15]. Am J Obstet Gynecol. 2020;222:S770-S771.
- Chung CP, Miskimins R, Kuehl TJ, et al. Permanent suture used in uterosacral ligament suspension offers better anatomical support than delayed absorbable suture. Int Urogynecol J. 2012;23:223-227.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical -mesh-intended-transvaginal. April 16, 2019. Accessed September 1, 2020.
- Londono R, Badylak SF. Biologic scaffolds for regenerative medicine: mechanisms of in vivo remodeling. Ann Biomed Eng. 2015;43:577-592.
- Cornwell KG, Landsman A, James KS. Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg. 2009;26: 507-523.
- Maher CM, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women: the updated summary version Cochrane review. Int Urogynecol J. 2011;22:1445-1447.
- Maher C, Feiner B, Baessler K, et al. Surgery for women with anterior compartment prolapse. Cochrane Database Syst Rev. 2016;11:CD004014.
- Maher C, Feiner B, Baessler K, et al. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cochrane Database Syst Rev. 2016;2:CD012179.
- Rosenblatt P, Von Bargen E. Use of biologic grafts in pelvic organ prolapse surgery. Contemporary OB/GYN. 2017;62:14-19.
- Greenspan DC, Hernandez R, Faleris J. Histology of surgically implanted Tutoplast processed dermis. http://www.zimmerbiomet .co.il/images/lib_artHistologyDermis%2010.pdf. Accessed September 2, 2020.
- Williams D. Revisiting the definition of biocompatibility. Med Device Technol. 2003;14:10-13.
- Nosti PA, Carter CM, Sokol AI, et al. Transvaginal versus transabdominal placement of synthetic mesh at time of sacrocolpopexy. Female Pelvic Med Reconstr Surg. 2016;22:151-155.
- Maher C, Feiner B, Baessler K. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013:CD004014.
- Weber AM, Walters MD, Piedmonte MR, et al. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1304.
- Walters MD, Ridgeway BM. Surgical treatment of vaginal apex prolapse. Obstet Gynecol. 2013;121(2 pt 1):354-374.
- Meister MRL, Sutcliffe S, Lowder JL. Definitions of apical vaginal support loss: a systematic review. Am J Obstet Gynecol. 2017;216:232. e1-232.e14.
- Cox A, Herschorn S. Evaluation of current biologic meshes in pelvic organ prolapse repair. Curr Urol Rep. 2012;13:247-255.
- Jelovsek JE, Barber M, Brubaker K, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018:319:1554-1565.
- Bowen ST, Moalli P, Abramowitch S, et al. Outcomes of the defining mechanisms of anterior vaginal wall descent trial [abstract 15]. Am J Obstet Gynecol. 2020;222:S770-S771.
- Chung CP, Miskimins R, Kuehl TJ, et al. Permanent suture used in uterosacral ligament suspension offers better anatomical support than delayed absorbable suture. Int Urogynecol J. 2012;23:223-227.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical -mesh-intended-transvaginal. April 16, 2019. Accessed September 1, 2020.
- Londono R, Badylak SF. Biologic scaffolds for regenerative medicine: mechanisms of in vivo remodeling. Ann Biomed Eng. 2015;43:577-592.
- Cornwell KG, Landsman A, James KS. Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg. 2009;26: 507-523.
- Maher CM, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women: the updated summary version Cochrane review. Int Urogynecol J. 2011;22:1445-1447.
- Maher C, Feiner B, Baessler K, et al. Surgery for women with anterior compartment prolapse. Cochrane Database Syst Rev. 2016;11:CD004014.
- Maher C, Feiner B, Baessler K, et al. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cochrane Database Syst Rev. 2016;2:CD012179.
- Rosenblatt P, Von Bargen E. Use of biologic grafts in pelvic organ prolapse surgery. Contemporary OB/GYN. 2017;62:14-19.
- Greenspan DC, Hernandez R, Faleris J. Histology of surgically implanted Tutoplast processed dermis. http://www.zimmerbiomet .co.il/images/lib_artHistologyDermis%2010.pdf. Accessed September 2, 2020.
- Williams D. Revisiting the definition of biocompatibility. Med Device Technol. 2003;14:10-13.
- Nosti PA, Carter CM, Sokol AI, et al. Transvaginal versus transabdominal placement of synthetic mesh at time of sacrocolpopexy. Female Pelvic Med Reconstr Surg. 2016;22:151-155.
How to build your identity as a physician online
To have a thriving business in today’s world, a functioning website is crucial to the overall business health. For a medical practice in general, and for its physicians specifically, it is one of the first steps for maintaining a practice. But to grow that practice, it is crucial to take the steps beyond just having a website. Growth requires website optimization for search engines, an expanding referral base, and the knowledge to use web tools and social media at your disposal to promote the practice and its physicians. In this roundtable, several marketing experts and web-savvy physicians discuss using available tools to best position and grow a practice.
Choosing a web upgrade
Patrick J. Culligan, MD: Peter, can you start us off by describing your relationship with Heather, and how your practice benefitted from her expertise?
Peter M. Lotze, MD: Sure. I am a urogynecologist in the competitive market of pelvic reconstructive surgery in Houston, Texas. Within that market, my main approach was to reach out to other physicians to refer patients to my practice. It generally would work, but took increasingly greater amounts of time to call these physicians up, write them letters, and maintain relationships. I felt that the large, national practice group that I am in did not have a significant web presence optimized to promote my practice, which makes it difficult for patients seeking your services to find you in their search for a doctor. It is helpful for patients to be able to understand from your website who you are, what you do, and what their experience may be like.
Glaring to me was that a web search specific for me or things that I do, would not produce our company’s results until page 2 or more on Google. This can be devastating for a practice because most people don’t go past the first page, and you can end up with fewer self-referrals, which should be a significant portion of new patients to your practice. I knew I needed guidance; I knew of Heather’s expertise given her exceptional past work building marketing strategies.
Digital go-tos for marketing
Heather Schueppert: Yes, I was pleased to work with Dr. Lotze, and at the time was a marketing consultant for practices such as his. But gone are the days of printed material—brochures, pamphlets, or even billboards—to effectively promote a business, or in this case, a practice. What still withstands the test of time, however, as the number 1 marketing referral source is word of mouth—from your trusted friend, family member, or coworker.
It is now proven that the number 2 most trusted form of advertising, the most persuasive and the most motivating, is online marketing.1 It is the “digital word of mouth”—the review. Patients are actively online, and a strong digital presence is critical to provide that direct value to retain and grow your patient base.
Continue to: Foundations of private practice reach out...
Foundations of private practice reach out
There are 3 important areas that I consider the foundation of any private practice marketing strategy (TABLE). First is an updated website that is search engine optimized (SEO). You can’t just set it and forget it, it needs to be an updated website. The algorithms for search engines are changing constantly to try to make it as fair and relevant as possible for patients or consumers to find the businesses they are searching for online.

The second area is review management, and for a physician, or even a care center, to do this on your own is a daunting task. It is a critical component, however, to making sure that your reputation out there, that online word of mouth, is as high a star rating as possible.
The third component is local search, which is basically a form of SEO that helps businesses show up in relevant local searches. We are all familiar with the search, “find a restaurant near me,” anything that pushes those search engines to find something local.
Those are what I call the effective triad: that updated website, the review management, and the local search, and all of these are tied together. I think Dr. Lotze and his practice did these effectively well, and I believe that he achieved his goals for the longer term.
Review/reputation management
Dr. Culligan: Brad, is there something that doctors may not know about Healthgrades, and are there opportunities to take full advantage of this physician-rating site?
Brad Bowman, MD: I agree with everything that Dr. Lotze and Heather have said. Start with yourself—what is it that you want to be, the one thing you want to stand for? Get your own marketing, your website right, then, the point is, once you do all that and you are number 1 in SEO, you are still only going to get about 25% of the people looking for you by name to come to your website. The other 75% are going to look at all the other different sites that are out there to provide information to consumers. So the question becomes what do you do with all these other third-party sites? Healthgrades is the most comprehensive and has the highest traffic of the third-party “find a doctor” sites. In 2020, half of all Americans who go to a doctor will use Healthgrades at some point to help select and connect with that doctor.
Physicians have their focus on the quality of the care they provide. Patients, however, focus on the quality of the entire health care experience. Did I get better? How long did I have to wait? Was the office staff helpful? Scarily enough, we still spend more time shopping for a refrigerator or mattress than we do shopping for a doctor. We still tend to think that all doctors are the same. It is the reality of how we have been trained by our insurance companies and by the health care system. That is why getting your marketing right and getting what is it that you want to be known for out there is important, so that you can get the types of patients you want.
Listings management is very important. Make sure that you are findable everywhere. There are services that will do this: Doctor.com, Reputation.com, and many others. They can help you make sure you get all your basic materials right: addresses, phone numbers, your picture. Because 75% of people are going to end up on third-party websites, if your phone number is wrong there, you could lose that patient.
Then the second piece of working with third-party sites is reputation management. Physician reviews are not a bad thing, they are the new word of mouth, as Heather pointed out. Most (80%) of the reviews are going to be positive. The others will be negative, and that is okay. It is important that you get at least 1 or 2 reviews on all the different sites. We know from Healthgrades.com that going from zero reviews to 1 review will increase your call volume by 60%. If you have the choice between 2 physicians and one practice looks like people have been there before, you will go to that one.
You can learn from reviews as well, consumers provide valid feedback. Best practice is to respond to every positive and negative review. Thank them, indicate that you have listened to them, and address any concerns as necessary.
Continue to: Dr. Lotze...
Dr. Lotze: As an example, one of the paramount things that Heather introduced me to was the third party I use to run my website. That company sends a HIPAA-compliant review out to each patient we have seen that day and gives them the opportunity to rate our services and leave comments. If a patient brings up a concern, we can respond immediately, which is important. Patients appreciate feeling that they have been heard. Typically, communicating with a patient will turn the 3-star review into a 5-star as she follows up with the practice.
Ms. Schueppert: Timeliness is important. And just to mention, there certainly is a time commitment to this (and it is a marathon versus a sprint) and there is some financial investment to get it going, but it could truly be detrimental to a practice if you decide not to do anything at all.
Dr. Bowman: Agencies can really help with the time commitment.
Handling bad reviews
Dr. Culligan: What about that person who seems to have it out for you, perhaps giving you multiple bad reviews?
Dr. Bowman: I have seen this before. At Healthgrades, we recently analyzed 8.4 million patient reviews to see what people wrote about.2 The first thing they will talk about is quality of care as they see it. Did I get better or not? You can’t “fix” every patient; there will be some that you cannot help. The next thing patients comment on is bedside manner. With negative reviews, you will see more comments about the office staff.2
A single negative review actually helps make the positive ones look more credible. But if you do believe someone is trolling you, we can flag it and will investigate to the best of our ability. (Different sites likely have different editorial policies.) For example, we look at the IP addresses of all reviews, and if multiple reviews are coming from the same location, we would only let one through, overwriting the previous review from that address.
Patients just want to be heard. We have seen people change their views, based on how their review is handled and responded to.
Dr. Lotze: Is there a response by the physician that you think tends to work better in terms of resolving the issue that can minimize a perceived caustic reaction to a patient’s criticism?
Dr. Bowman: First, just like with any stressful situation, take a deep breath and respond when you feel like you can be constructive. When you do respond, be gracious. Thank them for their feedback. Make sure you reference something about their concern: “I understand that you had to wait longer than you would have liked.” Acknowledge the problem they reference, and then just apologize: “I’m sorry we didn’t meet your expectations.” Then, if they waited too long for example, “We have a new system where no one should have to wait more than 30 minutes….” You can respond privately or publically. Generally, public responses are better as it shows other consumers that you are willing to listen and consider their point of view.
Continue to: The next phase at Healthgrades...
The next phase at Healthgrades
Dr. Culligan: Do you see changes to the way physician-rating sites are working now? Are we going to stay status quo over the next 10 years, or do you see frontiers in how your site is going to develop?
Dr. Bowman: For Healthgrades, we rely on quantitative and objective measures, not just the qualitative. We are investing heavily right now in trying to help consumers understand what are the relative volumes of different procedures or different patient types that each individual doctor sees. Orthopedics is an easy example—if you have a knee problem, you want to go to someone who specializes in knees. Our job is to help consumers easily identify, “This is a shoulder doctor, this is a knee doctor, and this is why that matters.”
In the meantime, as a physician, you can always go into our site and state your care philosophy, identifying what is the sort of patient that you like to treat. Transparency is good for everyone, and especially physicians. It helps the right patient show up for you, and it helps you do a better job providing referrals.
Social media: Avoid pitfalls, and use it to your benefit
Dr. Lotze: Branding was one of the things that I was confused about, and Heather really helped me out. As physicians, we put ourselves out there on our websites, which we try to make professional sources of information for patients. But patients often want to see what else they can find out about us, including Healthgrades and social media. I think the thing that is important to know with social media is that it is a place where people learn about you as a person. Your social media should be another avenue of promotion. Whether it is your personal or professional Facebook page, people are going to see those sites. You have an opportunity to promote yourself as a good physician and a good person with a wholesome practice that you want people to come to. If a physician is posting questionable things about themselves on any kind of social media, it could be perceived as inappropriate by the patient. That can impact how patients think of you as a person, and how they are going to grade you. If people lose sight of who you are due to a questionable social media posting, everything else (SEO, the website) can be for naught.
Dr. Culligan: What are the most important social media tools to invest your time in?
Ms. Schueppert: Before anybody jumps into social media, I firmly recommend that you make sure your local search and your Google 3-pack is set up—which is basically a method Google uses to display the top 3 results on its listings page. Then make sure you have a review management system in place. Make sure you have that updated website. Those are the foundational elements. Once you have that going, social media is the next added layer to that digital presence.
I usually recommend LinkedIn. It is huge because you are staying in contact with your colleagues, that business-to-business type of connection. It remains a way for physicians to set themselves up as experts in their level of specialty.
From there, it’s either Instagram or Facebook. If you are serving more of the younger generations, the millennials and younger, then Instagram is the way to go. If you are focusing on your 40+, 50+, they are going to be far more on Facebook.
Continue to: Dr. Lotze...
Dr. Lotze: For me, a Facebook page was a great place to start. The cost of those Google ads—the first things we see at the top of a Google search in their own separate box—is significant. If a practice has that kind of money to invest, great; it is an instant way to be first on the page during a search. But there are more cost-effective ways of doing that, especially as you are getting your name out. Facebook provides, at a smaller cost, promotion of whatever it is that you are seeking to promote. You can find people within a certain zip code, for instance, and use a Facebook ad campaign that can drive people to your Facebook page—which should have both routinely updated new posts and a link to your website. The posts should be interesting topics relevant to the patients you wish to treat (avoiding personal stories or controversial discussions). You can put a post together, or you can have a third-party service do this. People who follow your page will get reminders of you and your practice with each new post. As your page followers increase, your Facebook rank will improve, and your page will more likely be discovered by Facebook searches for your services. With an added link to your office practice website, those patients go straight to your site without getting lost in the noise of Google search results.
For Instagram, a short video or an interesting picture, along with a brief statement, are the essentials. You can add a single link. Marketing here is by direct messaging or having patients going to your website through a link. Instagram, like Facebook, offers analytics to help show you what your audience likes to read about, improving the quality of your posts and increasing number of followers.
YouTube is the number 2 search engine behind Google. A Google search for your field of medicine may be filled with pages of competitors. However, YouTube has a much lower volume of competing practices, making it easier for patients to find you. The only downside to YouTube is that it will list your video along with other competing videos, which can draw attention away from your practice.
If you want to promote your website or practice with video, using a company such as Vimeo is a better choice compared with YouTube, as YouTube gets credit for video views—which improves YouTube’s SEO and not your own website. Vimeo allows for your website to get credit each time the video is watched. Regardless of where you place your videos, make them short and to the point, with links to your website. Videos only need to be long enough to get your message across and stimulate interest in your practice.
If you can have a blog on your website, it also will help with SEO. What a search engine like Google wants to see is that a patient is on your web page and looking at something for at least 60 seconds. If so, the website is deemed to have information that is relevant, improving your SEO ranking.
Finally, Twitter also can be used for getting messaging out and for branding. The problem with it is that many people go to Twitter to follow a Hollywood celebrity, a sports star, or are looking for mass communication. There is less interest on Twitter for physician outreach.
Continue to: Measuring ROI...
Measuring ROI
Dr. Culligan: What’s the best way to track your return on investment?
Dr. Lotze: First for me was to find out what didn’t work in the office and fix that before really promoting my practice. It’s about the global experience for a patient, as Brad mentioned. As a marketing expert, Heather met with me to understand my goals. She then called my office as a patient to set up an appointment and went through that entire office experience. We identified issues needing improvement.
The next step was to develop a working relationship with my webmaster—someone who can help manage Internet image and SEO. Together, you will develop goals for what the SEO should promote specific to your practice. Once a good SEO program is in place, your website’s ranking will go up—although it can take a minimum of 6 months to see a significant increase. To help understand your website’s performance, your webmaster should provide you with reports on your site’s analytics.
As you go through this process, it is great to have a marketing expert to be the point person. You will work closely together for a while, but eventually you can back off over time. The time and expense you invest on the front end have huge rewards on the back end. Currently, I still spend a reasonable amount of money every month. I have a high self-referral base because of these efforts, however, which results in more patient surgeries and easily covers my expenses. It is money well invested. My website traffic increased by 268% over 2 years (FIGURE). I’ll propose that currently more than half of my patients are self-referrals due to online marketing.
Ms. Schueppert: The only thing I would add is training your front staff. They are checking people in, taking appointments, checking your patients out. Have them be mindful that there are campaigns going on, whether it is a social media push, or a new video that went on the website. They can ask, “How did you hear about us?” when a new patient calls.
Dr. Bowman: Unless you are a large university hospital, where the analytics get significantly more advanced in terms of measuring return on investment (ROI), I think you should just be looking at your schedule and looking at your monthly billings and seeing how they change over time. You can calculate how much a new patient is worth because you can figure out how many patients you have and how much you bill and what your profits are.
Dr. Culligan: For those of us who are hospital employees, you can try to convince the hospital that you can do a detailed ROI analysis, or you can just look at it like (say it’s $3,000 per month), how many surgeries does this project have to generate before the hospital makes that back? The answer is a fraction of 1 case.
Thank you to all of you for your expertise on this roundtable.
- Anderson A. Online reviews vs. word of mouth: Which one is more important. https://www.revlocal.com/blog/reviewandreputationmanagement/onlinereviewsvswordofmouthwhichoneismoreimportant. Accessed July 17, 2020.
- 2020 Patient sentiment report. Healthgrades; Medical Group Management Association. https://www.healthgrades.com/content /patientsentimentreport. Accessed July 17, 2020
To have a thriving business in today’s world, a functioning website is crucial to the overall business health. For a medical practice in general, and for its physicians specifically, it is one of the first steps for maintaining a practice. But to grow that practice, it is crucial to take the steps beyond just having a website. Growth requires website optimization for search engines, an expanding referral base, and the knowledge to use web tools and social media at your disposal to promote the practice and its physicians. In this roundtable, several marketing experts and web-savvy physicians discuss using available tools to best position and grow a practice.
Choosing a web upgrade
Patrick J. Culligan, MD: Peter, can you start us off by describing your relationship with Heather, and how your practice benefitted from her expertise?
Peter M. Lotze, MD: Sure. I am a urogynecologist in the competitive market of pelvic reconstructive surgery in Houston, Texas. Within that market, my main approach was to reach out to other physicians to refer patients to my practice. It generally would work, but took increasingly greater amounts of time to call these physicians up, write them letters, and maintain relationships. I felt that the large, national practice group that I am in did not have a significant web presence optimized to promote my practice, which makes it difficult for patients seeking your services to find you in their search for a doctor. It is helpful for patients to be able to understand from your website who you are, what you do, and what their experience may be like.
Glaring to me was that a web search specific for me or things that I do, would not produce our company’s results until page 2 or more on Google. This can be devastating for a practice because most people don’t go past the first page, and you can end up with fewer self-referrals, which should be a significant portion of new patients to your practice. I knew I needed guidance; I knew of Heather’s expertise given her exceptional past work building marketing strategies.
Digital go-tos for marketing
Heather Schueppert: Yes, I was pleased to work with Dr. Lotze, and at the time was a marketing consultant for practices such as his. But gone are the days of printed material—brochures, pamphlets, or even billboards—to effectively promote a business, or in this case, a practice. What still withstands the test of time, however, as the number 1 marketing referral source is word of mouth—from your trusted friend, family member, or coworker.
It is now proven that the number 2 most trusted form of advertising, the most persuasive and the most motivating, is online marketing.1 It is the “digital word of mouth”—the review. Patients are actively online, and a strong digital presence is critical to provide that direct value to retain and grow your patient base.
Continue to: Foundations of private practice reach out...
Foundations of private practice reach out
There are 3 important areas that I consider the foundation of any private practice marketing strategy (TABLE). First is an updated website that is search engine optimized (SEO). You can’t just set it and forget it, it needs to be an updated website. The algorithms for search engines are changing constantly to try to make it as fair and relevant as possible for patients or consumers to find the businesses they are searching for online.

The second area is review management, and for a physician, or even a care center, to do this on your own is a daunting task. It is a critical component, however, to making sure that your reputation out there, that online word of mouth, is as high a star rating as possible.
The third component is local search, which is basically a form of SEO that helps businesses show up in relevant local searches. We are all familiar with the search, “find a restaurant near me,” anything that pushes those search engines to find something local.
Those are what I call the effective triad: that updated website, the review management, and the local search, and all of these are tied together. I think Dr. Lotze and his practice did these effectively well, and I believe that he achieved his goals for the longer term.
Review/reputation management
Dr. Culligan: Brad, is there something that doctors may not know about Healthgrades, and are there opportunities to take full advantage of this physician-rating site?
Brad Bowman, MD: I agree with everything that Dr. Lotze and Heather have said. Start with yourself—what is it that you want to be, the one thing you want to stand for? Get your own marketing, your website right, then, the point is, once you do all that and you are number 1 in SEO, you are still only going to get about 25% of the people looking for you by name to come to your website. The other 75% are going to look at all the other different sites that are out there to provide information to consumers. So the question becomes what do you do with all these other third-party sites? Healthgrades is the most comprehensive and has the highest traffic of the third-party “find a doctor” sites. In 2020, half of all Americans who go to a doctor will use Healthgrades at some point to help select and connect with that doctor.
Physicians have their focus on the quality of the care they provide. Patients, however, focus on the quality of the entire health care experience. Did I get better? How long did I have to wait? Was the office staff helpful? Scarily enough, we still spend more time shopping for a refrigerator or mattress than we do shopping for a doctor. We still tend to think that all doctors are the same. It is the reality of how we have been trained by our insurance companies and by the health care system. That is why getting your marketing right and getting what is it that you want to be known for out there is important, so that you can get the types of patients you want.
Listings management is very important. Make sure that you are findable everywhere. There are services that will do this: Doctor.com, Reputation.com, and many others. They can help you make sure you get all your basic materials right: addresses, phone numbers, your picture. Because 75% of people are going to end up on third-party websites, if your phone number is wrong there, you could lose that patient.
Then the second piece of working with third-party sites is reputation management. Physician reviews are not a bad thing, they are the new word of mouth, as Heather pointed out. Most (80%) of the reviews are going to be positive. The others will be negative, and that is okay. It is important that you get at least 1 or 2 reviews on all the different sites. We know from Healthgrades.com that going from zero reviews to 1 review will increase your call volume by 60%. If you have the choice between 2 physicians and one practice looks like people have been there before, you will go to that one.
You can learn from reviews as well, consumers provide valid feedback. Best practice is to respond to every positive and negative review. Thank them, indicate that you have listened to them, and address any concerns as necessary.
Continue to: Dr. Lotze...
Dr. Lotze: As an example, one of the paramount things that Heather introduced me to was the third party I use to run my website. That company sends a HIPAA-compliant review out to each patient we have seen that day and gives them the opportunity to rate our services and leave comments. If a patient brings up a concern, we can respond immediately, which is important. Patients appreciate feeling that they have been heard. Typically, communicating with a patient will turn the 3-star review into a 5-star as she follows up with the practice.
Ms. Schueppert: Timeliness is important. And just to mention, there certainly is a time commitment to this (and it is a marathon versus a sprint) and there is some financial investment to get it going, but it could truly be detrimental to a practice if you decide not to do anything at all.
Dr. Bowman: Agencies can really help with the time commitment.
Handling bad reviews
Dr. Culligan: What about that person who seems to have it out for you, perhaps giving you multiple bad reviews?
Dr. Bowman: I have seen this before. At Healthgrades, we recently analyzed 8.4 million patient reviews to see what people wrote about.2 The first thing they will talk about is quality of care as they see it. Did I get better or not? You can’t “fix” every patient; there will be some that you cannot help. The next thing patients comment on is bedside manner. With negative reviews, you will see more comments about the office staff.2
A single negative review actually helps make the positive ones look more credible. But if you do believe someone is trolling you, we can flag it and will investigate to the best of our ability. (Different sites likely have different editorial policies.) For example, we look at the IP addresses of all reviews, and if multiple reviews are coming from the same location, we would only let one through, overwriting the previous review from that address.
Patients just want to be heard. We have seen people change their views, based on how their review is handled and responded to.
Dr. Lotze: Is there a response by the physician that you think tends to work better in terms of resolving the issue that can minimize a perceived caustic reaction to a patient’s criticism?
Dr. Bowman: First, just like with any stressful situation, take a deep breath and respond when you feel like you can be constructive. When you do respond, be gracious. Thank them for their feedback. Make sure you reference something about their concern: “I understand that you had to wait longer than you would have liked.” Acknowledge the problem they reference, and then just apologize: “I’m sorry we didn’t meet your expectations.” Then, if they waited too long for example, “We have a new system where no one should have to wait more than 30 minutes….” You can respond privately or publically. Generally, public responses are better as it shows other consumers that you are willing to listen and consider their point of view.
Continue to: The next phase at Healthgrades...
The next phase at Healthgrades
Dr. Culligan: Do you see changes to the way physician-rating sites are working now? Are we going to stay status quo over the next 10 years, or do you see frontiers in how your site is going to develop?
Dr. Bowman: For Healthgrades, we rely on quantitative and objective measures, not just the qualitative. We are investing heavily right now in trying to help consumers understand what are the relative volumes of different procedures or different patient types that each individual doctor sees. Orthopedics is an easy example—if you have a knee problem, you want to go to someone who specializes in knees. Our job is to help consumers easily identify, “This is a shoulder doctor, this is a knee doctor, and this is why that matters.”
In the meantime, as a physician, you can always go into our site and state your care philosophy, identifying what is the sort of patient that you like to treat. Transparency is good for everyone, and especially physicians. It helps the right patient show up for you, and it helps you do a better job providing referrals.
Social media: Avoid pitfalls, and use it to your benefit
Dr. Lotze: Branding was one of the things that I was confused about, and Heather really helped me out. As physicians, we put ourselves out there on our websites, which we try to make professional sources of information for patients. But patients often want to see what else they can find out about us, including Healthgrades and social media. I think the thing that is important to know with social media is that it is a place where people learn about you as a person. Your social media should be another avenue of promotion. Whether it is your personal or professional Facebook page, people are going to see those sites. You have an opportunity to promote yourself as a good physician and a good person with a wholesome practice that you want people to come to. If a physician is posting questionable things about themselves on any kind of social media, it could be perceived as inappropriate by the patient. That can impact how patients think of you as a person, and how they are going to grade you. If people lose sight of who you are due to a questionable social media posting, everything else (SEO, the website) can be for naught.
Dr. Culligan: What are the most important social media tools to invest your time in?
Ms. Schueppert: Before anybody jumps into social media, I firmly recommend that you make sure your local search and your Google 3-pack is set up—which is basically a method Google uses to display the top 3 results on its listings page. Then make sure you have a review management system in place. Make sure you have that updated website. Those are the foundational elements. Once you have that going, social media is the next added layer to that digital presence.
I usually recommend LinkedIn. It is huge because you are staying in contact with your colleagues, that business-to-business type of connection. It remains a way for physicians to set themselves up as experts in their level of specialty.
From there, it’s either Instagram or Facebook. If you are serving more of the younger generations, the millennials and younger, then Instagram is the way to go. If you are focusing on your 40+, 50+, they are going to be far more on Facebook.
Continue to: Dr. Lotze...
Dr. Lotze: For me, a Facebook page was a great place to start. The cost of those Google ads—the first things we see at the top of a Google search in their own separate box—is significant. If a practice has that kind of money to invest, great; it is an instant way to be first on the page during a search. But there are more cost-effective ways of doing that, especially as you are getting your name out. Facebook provides, at a smaller cost, promotion of whatever it is that you are seeking to promote. You can find people within a certain zip code, for instance, and use a Facebook ad campaign that can drive people to your Facebook page—which should have both routinely updated new posts and a link to your website. The posts should be interesting topics relevant to the patients you wish to treat (avoiding personal stories or controversial discussions). You can put a post together, or you can have a third-party service do this. People who follow your page will get reminders of you and your practice with each new post. As your page followers increase, your Facebook rank will improve, and your page will more likely be discovered by Facebook searches for your services. With an added link to your office practice website, those patients go straight to your site without getting lost in the noise of Google search results.
For Instagram, a short video or an interesting picture, along with a brief statement, are the essentials. You can add a single link. Marketing here is by direct messaging or having patients going to your website through a link. Instagram, like Facebook, offers analytics to help show you what your audience likes to read about, improving the quality of your posts and increasing number of followers.
YouTube is the number 2 search engine behind Google. A Google search for your field of medicine may be filled with pages of competitors. However, YouTube has a much lower volume of competing practices, making it easier for patients to find you. The only downside to YouTube is that it will list your video along with other competing videos, which can draw attention away from your practice.
If you want to promote your website or practice with video, using a company such as Vimeo is a better choice compared with YouTube, as YouTube gets credit for video views—which improves YouTube’s SEO and not your own website. Vimeo allows for your website to get credit each time the video is watched. Regardless of where you place your videos, make them short and to the point, with links to your website. Videos only need to be long enough to get your message across and stimulate interest in your practice.
If you can have a blog on your website, it also will help with SEO. What a search engine like Google wants to see is that a patient is on your web page and looking at something for at least 60 seconds. If so, the website is deemed to have information that is relevant, improving your SEO ranking.
Finally, Twitter also can be used for getting messaging out and for branding. The problem with it is that many people go to Twitter to follow a Hollywood celebrity, a sports star, or are looking for mass communication. There is less interest on Twitter for physician outreach.
Continue to: Measuring ROI...
Measuring ROI
Dr. Culligan: What’s the best way to track your return on investment?
Dr. Lotze: First for me was to find out what didn’t work in the office and fix that before really promoting my practice. It’s about the global experience for a patient, as Brad mentioned. As a marketing expert, Heather met with me to understand my goals. She then called my office as a patient to set up an appointment and went through that entire office experience. We identified issues needing improvement.
The next step was to develop a working relationship with my webmaster—someone who can help manage Internet image and SEO. Together, you will develop goals for what the SEO should promote specific to your practice. Once a good SEO program is in place, your website’s ranking will go up—although it can take a minimum of 6 months to see a significant increase. To help understand your website’s performance, your webmaster should provide you with reports on your site’s analytics.
As you go through this process, it is great to have a marketing expert to be the point person. You will work closely together for a while, but eventually you can back off over time. The time and expense you invest on the front end have huge rewards on the back end. Currently, I still spend a reasonable amount of money every month. I have a high self-referral base because of these efforts, however, which results in more patient surgeries and easily covers my expenses. It is money well invested. My website traffic increased by 268% over 2 years (FIGURE). I’ll propose that currently more than half of my patients are self-referrals due to online marketing.

Ms. Schueppert: The only thing I would add is training your front staff. They are checking people in, taking appointments, checking your patients out. Have them be mindful that there are campaigns going on, whether it is a social media push, or a new video that went on the website. They can ask, “How did you hear about us?” when a new patient calls.
Dr. Bowman: Unless you are a large university hospital, where the analytics get significantly more advanced in terms of measuring return on investment (ROI), I think you should just be looking at your schedule and looking at your monthly billings and seeing how they change over time. You can calculate how much a new patient is worth because you can figure out how many patients you have and how much you bill and what your profits are.
Dr. Culligan: For those of us who are hospital employees, you can try to convince the hospital that you can do a detailed ROI analysis, or you can just look at it like (say it’s $3,000 per month), how many surgeries does this project have to generate before the hospital makes that back? The answer is a fraction of 1 case.
Thank you to all of you for your expertise on this roundtable.
To have a thriving business in today’s world, a functioning website is crucial to the overall business health. For a medical practice in general, and for its physicians specifically, it is one of the first steps for maintaining a practice. But to grow that practice, it is crucial to take the steps beyond just having a website. Growth requires website optimization for search engines, an expanding referral base, and the knowledge to use web tools and social media at your disposal to promote the practice and its physicians. In this roundtable, several marketing experts and web-savvy physicians discuss using available tools to best position and grow a practice.
Choosing a web upgrade
Patrick J. Culligan, MD: Peter, can you start us off by describing your relationship with Heather, and how your practice benefitted from her expertise?
Peter M. Lotze, MD: Sure. I am a urogynecologist in the competitive market of pelvic reconstructive surgery in Houston, Texas. Within that market, my main approach was to reach out to other physicians to refer patients to my practice. It generally would work, but took increasingly greater amounts of time to call these physicians up, write them letters, and maintain relationships. I felt that the large, national practice group that I am in did not have a significant web presence optimized to promote my practice, which makes it difficult for patients seeking your services to find you in their search for a doctor. It is helpful for patients to be able to understand from your website who you are, what you do, and what their experience may be like.
Glaring to me was that a web search specific for me or things that I do, would not produce our company’s results until page 2 or more on Google. This can be devastating for a practice because most people don’t go past the first page, and you can end up with fewer self-referrals, which should be a significant portion of new patients to your practice. I knew I needed guidance; I knew of Heather’s expertise given her exceptional past work building marketing strategies.
Digital go-tos for marketing
Heather Schueppert: Yes, I was pleased to work with Dr. Lotze, and at the time was a marketing consultant for practices such as his. But gone are the days of printed material—brochures, pamphlets, or even billboards—to effectively promote a business, or in this case, a practice. What still withstands the test of time, however, as the number 1 marketing referral source is word of mouth—from your trusted friend, family member, or coworker.
It is now proven that the number 2 most trusted form of advertising, the most persuasive and the most motivating, is online marketing.1 It is the “digital word of mouth”—the review. Patients are actively online, and a strong digital presence is critical to provide that direct value to retain and grow your patient base.
Continue to: Foundations of private practice reach out...
Foundations of private practice reach out
There are 3 important areas that I consider the foundation of any private practice marketing strategy (TABLE). First is an updated website that is search engine optimized (SEO). You can’t just set it and forget it, it needs to be an updated website. The algorithms for search engines are changing constantly to try to make it as fair and relevant as possible for patients or consumers to find the businesses they are searching for online.

The second area is review management, and for a physician, or even a care center, to do this on your own is a daunting task. It is a critical component, however, to making sure that your reputation out there, that online word of mouth, is as high a star rating as possible.
The third component is local search, which is basically a form of SEO that helps businesses show up in relevant local searches. We are all familiar with the search, “find a restaurant near me,” anything that pushes those search engines to find something local.
Those are what I call the effective triad: that updated website, the review management, and the local search, and all of these are tied together. I think Dr. Lotze and his practice did these effectively well, and I believe that he achieved his goals for the longer term.
Review/reputation management
Dr. Culligan: Brad, is there something that doctors may not know about Healthgrades, and are there opportunities to take full advantage of this physician-rating site?
Brad Bowman, MD: I agree with everything that Dr. Lotze and Heather have said. Start with yourself—what is it that you want to be, the one thing you want to stand for? Get your own marketing, your website right, then, the point is, once you do all that and you are number 1 in SEO, you are still only going to get about 25% of the people looking for you by name to come to your website. The other 75% are going to look at all the other different sites that are out there to provide information to consumers. So the question becomes what do you do with all these other third-party sites? Healthgrades is the most comprehensive and has the highest traffic of the third-party “find a doctor” sites. In 2020, half of all Americans who go to a doctor will use Healthgrades at some point to help select and connect with that doctor.
Physicians have their focus on the quality of the care they provide. Patients, however, focus on the quality of the entire health care experience. Did I get better? How long did I have to wait? Was the office staff helpful? Scarily enough, we still spend more time shopping for a refrigerator or mattress than we do shopping for a doctor. We still tend to think that all doctors are the same. It is the reality of how we have been trained by our insurance companies and by the health care system. That is why getting your marketing right and getting what is it that you want to be known for out there is important, so that you can get the types of patients you want.
Listings management is very important. Make sure that you are findable everywhere. There are services that will do this: Doctor.com, Reputation.com, and many others. They can help you make sure you get all your basic materials right: addresses, phone numbers, your picture. Because 75% of people are going to end up on third-party websites, if your phone number is wrong there, you could lose that patient.
Then the second piece of working with third-party sites is reputation management. Physician reviews are not a bad thing, they are the new word of mouth, as Heather pointed out. Most (80%) of the reviews are going to be positive. The others will be negative, and that is okay. It is important that you get at least 1 or 2 reviews on all the different sites. We know from Healthgrades.com that going from zero reviews to 1 review will increase your call volume by 60%. If you have the choice between 2 physicians and one practice looks like people have been there before, you will go to that one.
You can learn from reviews as well, consumers provide valid feedback. Best practice is to respond to every positive and negative review. Thank them, indicate that you have listened to them, and address any concerns as necessary.
Continue to: Dr. Lotze...
Dr. Lotze: As an example, one of the paramount things that Heather introduced me to was the third party I use to run my website. That company sends a HIPAA-compliant review out to each patient we have seen that day and gives them the opportunity to rate our services and leave comments. If a patient brings up a concern, we can respond immediately, which is important. Patients appreciate feeling that they have been heard. Typically, communicating with a patient will turn the 3-star review into a 5-star as she follows up with the practice.
Ms. Schueppert: Timeliness is important. And just to mention, there certainly is a time commitment to this (and it is a marathon versus a sprint) and there is some financial investment to get it going, but it could truly be detrimental to a practice if you decide not to do anything at all.
Dr. Bowman: Agencies can really help with the time commitment.
Handling bad reviews
Dr. Culligan: What about that person who seems to have it out for you, perhaps giving you multiple bad reviews?
Dr. Bowman: I have seen this before. At Healthgrades, we recently analyzed 8.4 million patient reviews to see what people wrote about.2 The first thing they will talk about is quality of care as they see it. Did I get better or not? You can’t “fix” every patient; there will be some that you cannot help. The next thing patients comment on is bedside manner. With negative reviews, you will see more comments about the office staff.2
A single negative review actually helps make the positive ones look more credible. But if you do believe someone is trolling you, we can flag it and will investigate to the best of our ability. (Different sites likely have different editorial policies.) For example, we look at the IP addresses of all reviews, and if multiple reviews are coming from the same location, we would only let one through, overwriting the previous review from that address.
Patients just want to be heard. We have seen people change their views, based on how their review is handled and responded to.
Dr. Lotze: Is there a response by the physician that you think tends to work better in terms of resolving the issue that can minimize a perceived caustic reaction to a patient’s criticism?
Dr. Bowman: First, just like with any stressful situation, take a deep breath and respond when you feel like you can be constructive. When you do respond, be gracious. Thank them for their feedback. Make sure you reference something about their concern: “I understand that you had to wait longer than you would have liked.” Acknowledge the problem they reference, and then just apologize: “I’m sorry we didn’t meet your expectations.” Then, if they waited too long for example, “We have a new system where no one should have to wait more than 30 minutes….” You can respond privately or publically. Generally, public responses are better as it shows other consumers that you are willing to listen and consider their point of view.
Continue to: The next phase at Healthgrades...
The next phase at Healthgrades
Dr. Culligan: Do you see changes to the way physician-rating sites are working now? Are we going to stay status quo over the next 10 years, or do you see frontiers in how your site is going to develop?
Dr. Bowman: For Healthgrades, we rely on quantitative and objective measures, not just the qualitative. We are investing heavily right now in trying to help consumers understand what are the relative volumes of different procedures or different patient types that each individual doctor sees. Orthopedics is an easy example—if you have a knee problem, you want to go to someone who specializes in knees. Our job is to help consumers easily identify, “This is a shoulder doctor, this is a knee doctor, and this is why that matters.”
In the meantime, as a physician, you can always go into our site and state your care philosophy, identifying what is the sort of patient that you like to treat. Transparency is good for everyone, and especially physicians. It helps the right patient show up for you, and it helps you do a better job providing referrals.
Social media: Avoid pitfalls, and use it to your benefit
Dr. Lotze: Branding was one of the things that I was confused about, and Heather really helped me out. As physicians, we put ourselves out there on our websites, which we try to make professional sources of information for patients. But patients often want to see what else they can find out about us, including Healthgrades and social media. I think the thing that is important to know with social media is that it is a place where people learn about you as a person. Your social media should be another avenue of promotion. Whether it is your personal or professional Facebook page, people are going to see those sites. You have an opportunity to promote yourself as a good physician and a good person with a wholesome practice that you want people to come to. If a physician is posting questionable things about themselves on any kind of social media, it could be perceived as inappropriate by the patient. That can impact how patients think of you as a person, and how they are going to grade you. If people lose sight of who you are due to a questionable social media posting, everything else (SEO, the website) can be for naught.
Dr. Culligan: What are the most important social media tools to invest your time in?
Ms. Schueppert: Before anybody jumps into social media, I firmly recommend that you make sure your local search and your Google 3-pack is set up—which is basically a method Google uses to display the top 3 results on its listings page. Then make sure you have a review management system in place. Make sure you have that updated website. Those are the foundational elements. Once you have that going, social media is the next added layer to that digital presence.
I usually recommend LinkedIn. It is huge because you are staying in contact with your colleagues, that business-to-business type of connection. It remains a way for physicians to set themselves up as experts in their level of specialty.
From there, it’s either Instagram or Facebook. If you are serving more of the younger generations, the millennials and younger, then Instagram is the way to go. If you are focusing on your 40+, 50+, they are going to be far more on Facebook.
Continue to: Dr. Lotze...
Dr. Lotze: For me, a Facebook page was a great place to start. The cost of those Google ads—the first things we see at the top of a Google search in their own separate box—is significant. If a practice has that kind of money to invest, great; it is an instant way to be first on the page during a search. But there are more cost-effective ways of doing that, especially as you are getting your name out. Facebook provides, at a smaller cost, promotion of whatever it is that you are seeking to promote. You can find people within a certain zip code, for instance, and use a Facebook ad campaign that can drive people to your Facebook page—which should have both routinely updated new posts and a link to your website. The posts should be interesting topics relevant to the patients you wish to treat (avoiding personal stories or controversial discussions). You can put a post together, or you can have a third-party service do this. People who follow your page will get reminders of you and your practice with each new post. As your page followers increase, your Facebook rank will improve, and your page will more likely be discovered by Facebook searches for your services. With an added link to your office practice website, those patients go straight to your site without getting lost in the noise of Google search results.
For Instagram, a short video or an interesting picture, along with a brief statement, are the essentials. You can add a single link. Marketing here is by direct messaging or having patients going to your website through a link. Instagram, like Facebook, offers analytics to help show you what your audience likes to read about, improving the quality of your posts and increasing number of followers.
YouTube is the number 2 search engine behind Google. A Google search for your field of medicine may be filled with pages of competitors. However, YouTube has a much lower volume of competing practices, making it easier for patients to find you. The only downside to YouTube is that it will list your video along with other competing videos, which can draw attention away from your practice.
If you want to promote your website or practice with video, using a company such as Vimeo is a better choice compared with YouTube, as YouTube gets credit for video views—which improves YouTube’s SEO and not your own website. Vimeo allows for your website to get credit each time the video is watched. Regardless of where you place your videos, make them short and to the point, with links to your website. Videos only need to be long enough to get your message across and stimulate interest in your practice.
If you can have a blog on your website, it also will help with SEO. What a search engine like Google wants to see is that a patient is on your web page and looking at something for at least 60 seconds. If so, the website is deemed to have information that is relevant, improving your SEO ranking.
Finally, Twitter also can be used for getting messaging out and for branding. The problem with it is that many people go to Twitter to follow a Hollywood celebrity, a sports star, or are looking for mass communication. There is less interest on Twitter for physician outreach.
Continue to: Measuring ROI...
Measuring ROI
Dr. Culligan: What’s the best way to track your return on investment?
Dr. Lotze: First for me was to find out what didn’t work in the office and fix that before really promoting my practice. It’s about the global experience for a patient, as Brad mentioned. As a marketing expert, Heather met with me to understand my goals. She then called my office as a patient to set up an appointment and went through that entire office experience. We identified issues needing improvement.
The next step was to develop a working relationship with my webmaster—someone who can help manage Internet image and SEO. Together, you will develop goals for what the SEO should promote specific to your practice. Once a good SEO program is in place, your website’s ranking will go up—although it can take a minimum of 6 months to see a significant increase. To help understand your website’s performance, your webmaster should provide you with reports on your site’s analytics.
As you go through this process, it is great to have a marketing expert to be the point person. You will work closely together for a while, but eventually you can back off over time. The time and expense you invest on the front end have huge rewards on the back end. Currently, I still spend a reasonable amount of money every month. I have a high self-referral base because of these efforts, however, which results in more patient surgeries and easily covers my expenses. It is money well invested. My website traffic increased by 268% over 2 years (FIGURE). I’ll propose that currently more than half of my patients are self-referrals due to online marketing.

Ms. Schueppert: The only thing I would add is training your front staff. They are checking people in, taking appointments, checking your patients out. Have them be mindful that there are campaigns going on, whether it is a social media push, or a new video that went on the website. They can ask, “How did you hear about us?” when a new patient calls.
Dr. Bowman: Unless you are a large university hospital, where the analytics get significantly more advanced in terms of measuring return on investment (ROI), I think you should just be looking at your schedule and looking at your monthly billings and seeing how they change over time. You can calculate how much a new patient is worth because you can figure out how many patients you have and how much you bill and what your profits are.
Dr. Culligan: For those of us who are hospital employees, you can try to convince the hospital that you can do a detailed ROI analysis, or you can just look at it like (say it’s $3,000 per month), how many surgeries does this project have to generate before the hospital makes that back? The answer is a fraction of 1 case.
Thank you to all of you for your expertise on this roundtable.
- Anderson A. Online reviews vs. word of mouth: Which one is more important. https://www.revlocal.com/blog/reviewandreputationmanagement/onlinereviewsvswordofmouthwhichoneismoreimportant. Accessed July 17, 2020.
- 2020 Patient sentiment report. Healthgrades; Medical Group Management Association. https://www.healthgrades.com/content /patientsentimentreport. Accessed July 17, 2020
- Anderson A. Online reviews vs. word of mouth: Which one is more important. https://www.revlocal.com/blog/reviewandreputationmanagement/onlinereviewsvswordofmouthwhichoneismoreimportant. Accessed July 17, 2020.
- 2020 Patient sentiment report. Healthgrades; Medical Group Management Association. https://www.healthgrades.com/content /patientsentimentreport. Accessed July 17, 2020
Hysteroscopy and COVID-19: Have recommended techniques changed due to the pandemic?
The emergence of the coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) in December 2019, has resulted in a global pandemic that has challenged the medical community and will continue to represent a public health emergency for the next several months.1 It has rapidly spread globally, infecting many individuals in an unprecedented rate of infection and worldwide reach. On March 11, 2020, the World Health Organization designated COVID-19 as a pandemic. While the majority of infected individuals are asymptomatic or develop only mild symptoms, some have an unfortunate clinical course resulting in multi-organ failure and death.2
It is accepted that the virus mainly spreads during close contact and via respiratory droplets.3 The average time from infection to onset of symptoms ranges from 2 to 14 days, with an average of 5 days.4 Recommended measures to prevent the spread of the infection include social distancing (at least 6 feet from others), meticulous hand hygiene, and wearing a mask covering the mouth and nose when in public.5 Aiming to mitigate the risk of viral dissemination for patients and health care providers, and to preserve hospital resources, all nonessential medical interventions were initially suspended. Recently, the American College of Surgeons in a joint statement with 9 women’s health care societies have provided recommendations on how to resume clinical activities as we recover from the pandemic.6
As we reinitiate clinical activities, gynecologists have been alerted of the potential risk of viral dissemination during gynecologic minimally invasive surgical procedures due to the presence of the virus in blood, stool, and the potential risk of aerosolization of the virus, especially when using smoke-generating devices.7,8 This risk is not limited to intubation and extubation of the airway during anesthesia; the risk also presents itself during other aerosol-generating procedures, such as laparoscopy or robotic surgery.9,10
Hysteroscopy is considered the gold standard procedure for the diagnosis and management of intrauterine pathologies.11 It is frequently performed in an office setting without the use of anesthesia.11,12 It is usually well tolerated, with only a few patients reporting discomfort.12 It allows for immediate treatment (using the “see and treat” approach) while avoiding not only the risk of anesthesia, as stated, but also the need for intubation—which has a high risk of droplet contamination in COVID-19–infected individuals.13
Is there risk of viral dissemination during hysteroscopic procedures?
The novel and rapidly changing nature of the COVID-19 pandemic present many challenges to the gynecologist. Significant concerns have been raised regarding potential risk of viral dissemination during laparoscopic surgery due to aerosolization of viral particles and the presence of the virus in blood and the gastrointestinal tract of infected patients.7 Diagnostic, and some simple, hysteroscopic procedures are commonly performed in an outpatient setting, with the patient awake. Complex hysteroscopic interventions, however, are generally performed in the operating room, typically with the use of general anesthesia. Hysteroscopy has the theoretical risks of viral dissemination when performed in COVID-19–positive patients. Two important questions must be addressed to better understand the potential risk of COVID-19 viral dissemination during hysteroscopic procedures.
Continue to: 1. Is the virus present in the vaginal fluid of women infected with COVID-19?...
1. Is the virus present in the vaginal fluid of women infected with COVID-19?
Recent studies have confirmed the presence of viral particles in urine, feces, blood, and tears in addition to the respiratory tract in patients infected with COVID-19.3,14,15 The presence of the SARS-CoV-2 virus in the female genital system is currently unknown. Previous studies, of other epidemic viral infections, have demonstrated the presence of the virus in the female genital tract in affected patients of Zika virus and Ebola.16,17 However, 2 recent studies have failed to demonstrate the presence of the SARS-CoV-2 virus in the vaginal fluid of pregnant14 and not pregnant18 women with severe COVID-19 infection.
2. Is there risk of viral dissemination during hysteroscopy if using electrosurgery?
There are significant concerns with possible risk of COVID-19 transmission to health care providers in direct contact with infected patients during minimally invasive gynecologic procedures due to direct contamination and aerosolization of the virus.10,19 Current data on COVID-19 transmission during surgery are limited. However, it is important to recognize that viral aerosolization has been documented with other viral diseases, such as human papillomavirus and hepatitis B.20 A recent report called for awareness in the surgical community about the potential risks of COVID-19 viral dissemination during laparoscopic surgery. Among other recommendations, international experts advised minimizing the use of electrosurgery to reduce the creation of surgical plume, decreasing the pneumoperitoneum pressure to minimum levels, and using suction devices in a closed system.21 Although these preventive measures apply to laparoscopic surgery, it is important to consider that hysteroscopy is performed in a unique environment.
During hysteroscopy the uterine cavity is distended with a liquid medium (normal saline or electrolyte-free solutions); this is opposed to gynecologic laparoscopy, in which the peritoneal cavity is distended with carbon dioxide.22 The smoke produced with the use of hysteroscopic electrosurgical instruments generates bubbles that are immediately cooled down to the temperature of the distention media and subsequently dissolve into it. Therefore, there are no bubbles generated during hysteroscopic surgery that are subsequently released into the air. This results in a low risk for viral dissemination during hysteroscopic procedures. Nevertheless, the necessary precautions to minimize the risk of COVID-19 transmission during hysteroscopic intervention are extremely important.
Recommendations for hysteroscopic procedures during the COVID-19 pandemic
We provide our overall recommendations for hysteroscopy, as well as those specific to the office and hospital setting.
Recommendations: General
Limit hysteroscopic procedures to COVID-19–negative patients and to those patients in whom delaying the procedure could result in adverse clinical outcomes.23
Universally screen for potential COVID-19 infection. When possible, a phone interview to triage patients based on their symptoms and infection exposure status should take place before the patient arrives to the health care center. Patients with suspected or confirmed COVID-19 infection who require immediate evaluation should be directed to COVID-19–designated emergency areas.
Universally test for SARS-CoV-2 before procedures performed in the operating room (OR). Using nasopharyngeal swabs for the detection of viral RNA, employing molecular methods such as polymerase chain reaction (PCR), within 48 to 72 hours prior to all OR hysteroscopic procedures is strongly recommended. Adopting this testing strategy will aid to identify asymptomatic SARS-CoV-2‒infected patients, allowing to defer the procedure, if possible, among patients testing positive. If tests are limited, testing only patients scheduled for hysteroscopic procedures in which general or regional anesthesia will be required is acceptable.
Universal SARS-CoV-2 testing of patients undergoing in-office hysteroscopic diagnostic or minor operative procedures without the use of anesthesia is not required.
Limit the presence of a companion. It is understood that visitor policies may vary at the discretion of each institution’s guidelines. Children and individuals over the age of 60 years should not be granted access to the center. Companions will be subjected to the same screening criteria as patients.
Provide for social distancing and other precautionary measures. If more than one patient is scheduled to be at the facility at the same time, ensure that the facility provides adequate space to allow the appropriate social distancing recommendations between patients. Hand sanitizers and facemasks should be available for patients and companions.
Provide PPE for clinicians. All health care providers in close contact with the patient must wear personal protective equipment (PPE), which includes an apron and gown, a surgical mask, eye protection, and gloves. Health care providers should wear PPE deemed appropriate by their regulatory institutions following their local and national guidelines during clinical patient interactions.
Restrict surgical attendees to vital personnel. The participation of learners by physical presence in the office or operating room should be restricted.
Continue to: Recommendations: Office setting...
Recommendations: Office setting
Preprocedural recommendations
- Advise patients to come to the office alone. If the patient requires a companion, a maximum of one adult companion under the age of 60 should be accepted.
- Limit the number of health care team members present in the procedure room.
Intraprocedural recommendations
- Choose the appropriate device(s) that will allow for an effective and fast procedure.
- Use the recommended PPE for all clinicians.
- Limit the movement of staff members in and out of the procedure room.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same procedure room, allow enough time in between cases to grant a thorough OR decontamination.
- Allow for patients to recover from the procedure in the same room as the procedure took place in order to avoid potential contamination of multiple rooms.
- Expedite patient discharge.
- Follow up after the procedure by phone or telemedicine.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Continue to: Recommendations: Operating room setting...
Recommendations: Operating room setting
Preprocedural recommendations
- Perform adequate patient screening for potential COVID-19 infection. (Screening should be independent of symptoms and not be limited to those with clinical symptoms.)
- Limit the number of health care team members in the operating procedure room.
- To minimize unnecessary staff exposure, have surgeons and staff not needed for intubation remain outside the OR until intubation is completed and leave the OR before extubation.
Intraprocedure recommendations
- Limit personnel in the OR to a minimum.
- Staff should not enter or leave the room during the procedure.
- When possible, use conscious sedation or regional anesthesia to avoid the risk of viral dissemination at the time of intubation/extubation.
- Choose the device that will allow an effective and fast procedure.
- Favor non–smoke-generating devices, such as hysteroscopic scissors, graspers, and tissue retrieval systems.
- Connect active suction to the outflow, especially when using smoke-generating instruments, to facilitate the extraction of surgical smoke.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same room, allow enough time in between cases to grant a thorough OR decontamination.
- Expedite postprocedure recovery and patient discharge.
- After completion of the procedure, staff should remove scrubs and change into clean clothing.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Conclusions
The COVID-19 pandemic has caused a global health emergency. Our knowledge of this devastating virus is constantly evolving as we continue to fight this overwhelming disease. Theoretical risk of “viral” dissemination is considered extremely low, or negligible, during hysterosocopy. Hysteroscopic procedures in COVID-19–positive patients with life-threatening conditions or in patients in whom delaying the procedure could worsen outcomes should be performed taking appropriate measures. Patients who test negative for COVID-19 (confirmed by PCR) and require hysteroscopic procedures, should be treated using universal precautions. ●
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25:e936-e945.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. February 24, 2020. doi:10.1001/jama.2020.2648.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843-1844.
- Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71:793-798.
- Prem K, Liu Y, Russell TW, et al; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261-e270.
- American College of Surgeons, American Society of Aesthesiologists, Association of periOperative Registered Nurses, American Hospital Association. Joint Statement: Roadmap for resuming elective surgery after COVID-19 pandemic. April 16, 2020. https://www.aorn.org/guidelines/aorn-support/roadmap-for-resuming-elective-surgery-after-covid-19. Accessed August 27, 2020.
- Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386-389.
- Mowbray NG, Ansell J, Horwood J, et al. Safe management of surgical smoke in the age of COVID-19. Br J Surg. May 3, 2020. doi: 10.1002/bjs.11679.
- Cohen SL, Liu G, Abrao M, et al. Perspectives on surgery in the time of COVID-19: safety first. J Minim Invasive Gynecol. 2020;27:792-793.
- COVID-19: protecting health-care workers. Lancet. 2020;395:922.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Cicinelli E. Hysteroscopy without anesthesia: review of recent literature. J Minim Invasive Gynecol. 2010;17:703-708.
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568-576.
- Aslan MM, Yuvaci HU, Köse O, et al. SARS-CoV-2 is not present in the vaginal fluid of pregnant women with COVID-19. J Matern Fetal Neonatal Med. 2020:1-3. doi: 10.1080/14767058.2020.1793318.
- Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833-840.
- Prisant N, Bujan L, Benichou H, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1001.
- Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179 Suppl 1:S170-S176.
- Qiu L, Liu X, Xiao M, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis. 2020;71:813-817.
- Brat GA, Hersey S, Chhabra K, et al. Protecting surgical teams during the COVID-19 outbreak: a narrative review and clinical considerations. Ann Surg. April 17, 2020. doi: 10.1097/SLA.0000000000003926.
- Kwak HD, Kim SH, Seo YS, et al. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73:857-863.
- Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. 2020;272:e5-e6.
- Catena U. Surgical smoke in hysteroscopic surgery: does it really matter in COVID-19 times? Facts Views Vis Obgyn. 2020;12:67-68.
- Carugno J, Di Spiezio Sardo A, Alonso L, et al. COVID-19 pandemic. Impact on hysteroscopic procedures: a consensus statement from the Global Congress of Hysteroscopy Scientific Committee. J Minim Invasive Gynecol. 2020;27:988-992.
The emergence of the coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) in December 2019, has resulted in a global pandemic that has challenged the medical community and will continue to represent a public health emergency for the next several months.1 It has rapidly spread globally, infecting many individuals in an unprecedented rate of infection and worldwide reach. On March 11, 2020, the World Health Organization designated COVID-19 as a pandemic. While the majority of infected individuals are asymptomatic or develop only mild symptoms, some have an unfortunate clinical course resulting in multi-organ failure and death.2
It is accepted that the virus mainly spreads during close contact and via respiratory droplets.3 The average time from infection to onset of symptoms ranges from 2 to 14 days, with an average of 5 days.4 Recommended measures to prevent the spread of the infection include social distancing (at least 6 feet from others), meticulous hand hygiene, and wearing a mask covering the mouth and nose when in public.5 Aiming to mitigate the risk of viral dissemination for patients and health care providers, and to preserve hospital resources, all nonessential medical interventions were initially suspended. Recently, the American College of Surgeons in a joint statement with 9 women’s health care societies have provided recommendations on how to resume clinical activities as we recover from the pandemic.6
As we reinitiate clinical activities, gynecologists have been alerted of the potential risk of viral dissemination during gynecologic minimally invasive surgical procedures due to the presence of the virus in blood, stool, and the potential risk of aerosolization of the virus, especially when using smoke-generating devices.7,8 This risk is not limited to intubation and extubation of the airway during anesthesia; the risk also presents itself during other aerosol-generating procedures, such as laparoscopy or robotic surgery.9,10
Hysteroscopy is considered the gold standard procedure for the diagnosis and management of intrauterine pathologies.11 It is frequently performed in an office setting without the use of anesthesia.11,12 It is usually well tolerated, with only a few patients reporting discomfort.12 It allows for immediate treatment (using the “see and treat” approach) while avoiding not only the risk of anesthesia, as stated, but also the need for intubation—which has a high risk of droplet contamination in COVID-19–infected individuals.13

Is there risk of viral dissemination during hysteroscopic procedures?
The novel and rapidly changing nature of the COVID-19 pandemic present many challenges to the gynecologist. Significant concerns have been raised regarding potential risk of viral dissemination during laparoscopic surgery due to aerosolization of viral particles and the presence of the virus in blood and the gastrointestinal tract of infected patients.7 Diagnostic, and some simple, hysteroscopic procedures are commonly performed in an outpatient setting, with the patient awake. Complex hysteroscopic interventions, however, are generally performed in the operating room, typically with the use of general anesthesia. Hysteroscopy has the theoretical risks of viral dissemination when performed in COVID-19–positive patients. Two important questions must be addressed to better understand the potential risk of COVID-19 viral dissemination during hysteroscopic procedures.
Continue to: 1. Is the virus present in the vaginal fluid of women infected with COVID-19?...
1. Is the virus present in the vaginal fluid of women infected with COVID-19?
Recent studies have confirmed the presence of viral particles in urine, feces, blood, and tears in addition to the respiratory tract in patients infected with COVID-19.3,14,15 The presence of the SARS-CoV-2 virus in the female genital system is currently unknown. Previous studies, of other epidemic viral infections, have demonstrated the presence of the virus in the female genital tract in affected patients of Zika virus and Ebola.16,17 However, 2 recent studies have failed to demonstrate the presence of the SARS-CoV-2 virus in the vaginal fluid of pregnant14 and not pregnant18 women with severe COVID-19 infection.
2. Is there risk of viral dissemination during hysteroscopy if using electrosurgery?
There are significant concerns with possible risk of COVID-19 transmission to health care providers in direct contact with infected patients during minimally invasive gynecologic procedures due to direct contamination and aerosolization of the virus.10,19 Current data on COVID-19 transmission during surgery are limited. However, it is important to recognize that viral aerosolization has been documented with other viral diseases, such as human papillomavirus and hepatitis B.20 A recent report called for awareness in the surgical community about the potential risks of COVID-19 viral dissemination during laparoscopic surgery. Among other recommendations, international experts advised minimizing the use of electrosurgery to reduce the creation of surgical plume, decreasing the pneumoperitoneum pressure to minimum levels, and using suction devices in a closed system.21 Although these preventive measures apply to laparoscopic surgery, it is important to consider that hysteroscopy is performed in a unique environment.
During hysteroscopy the uterine cavity is distended with a liquid medium (normal saline or electrolyte-free solutions); this is opposed to gynecologic laparoscopy, in which the peritoneal cavity is distended with carbon dioxide.22 The smoke produced with the use of hysteroscopic electrosurgical instruments generates bubbles that are immediately cooled down to the temperature of the distention media and subsequently dissolve into it. Therefore, there are no bubbles generated during hysteroscopic surgery that are subsequently released into the air. This results in a low risk for viral dissemination during hysteroscopic procedures. Nevertheless, the necessary precautions to minimize the risk of COVID-19 transmission during hysteroscopic intervention are extremely important.
Recommendations for hysteroscopic procedures during the COVID-19 pandemic
We provide our overall recommendations for hysteroscopy, as well as those specific to the office and hospital setting.
Recommendations: General
Limit hysteroscopic procedures to COVID-19–negative patients and to those patients in whom delaying the procedure could result in adverse clinical outcomes.23
Universally screen for potential COVID-19 infection. When possible, a phone interview to triage patients based on their symptoms and infection exposure status should take place before the patient arrives to the health care center. Patients with suspected or confirmed COVID-19 infection who require immediate evaluation should be directed to COVID-19–designated emergency areas.
Universally test for SARS-CoV-2 before procedures performed in the operating room (OR). Using nasopharyngeal swabs for the detection of viral RNA, employing molecular methods such as polymerase chain reaction (PCR), within 48 to 72 hours prior to all OR hysteroscopic procedures is strongly recommended. Adopting this testing strategy will aid to identify asymptomatic SARS-CoV-2‒infected patients, allowing to defer the procedure, if possible, among patients testing positive. If tests are limited, testing only patients scheduled for hysteroscopic procedures in which general or regional anesthesia will be required is acceptable.
Universal SARS-CoV-2 testing of patients undergoing in-office hysteroscopic diagnostic or minor operative procedures without the use of anesthesia is not required.
Limit the presence of a companion. It is understood that visitor policies may vary at the discretion of each institution’s guidelines. Children and individuals over the age of 60 years should not be granted access to the center. Companions will be subjected to the same screening criteria as patients.
Provide for social distancing and other precautionary measures. If more than one patient is scheduled to be at the facility at the same time, ensure that the facility provides adequate space to allow the appropriate social distancing recommendations between patients. Hand sanitizers and facemasks should be available for patients and companions.
Provide PPE for clinicians. All health care providers in close contact with the patient must wear personal protective equipment (PPE), which includes an apron and gown, a surgical mask, eye protection, and gloves. Health care providers should wear PPE deemed appropriate by their regulatory institutions following their local and national guidelines during clinical patient interactions.
Restrict surgical attendees to vital personnel. The participation of learners by physical presence in the office or operating room should be restricted.
Continue to: Recommendations: Office setting...
Recommendations: Office setting
Preprocedural recommendations
- Advise patients to come to the office alone. If the patient requires a companion, a maximum of one adult companion under the age of 60 should be accepted.
- Limit the number of health care team members present in the procedure room.
Intraprocedural recommendations
- Choose the appropriate device(s) that will allow for an effective and fast procedure.
- Use the recommended PPE for all clinicians.
- Limit the movement of staff members in and out of the procedure room.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same procedure room, allow enough time in between cases to grant a thorough OR decontamination.
- Allow for patients to recover from the procedure in the same room as the procedure took place in order to avoid potential contamination of multiple rooms.
- Expedite patient discharge.
- Follow up after the procedure by phone or telemedicine.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Continue to: Recommendations: Operating room setting...
Recommendations: Operating room setting
Preprocedural recommendations
- Perform adequate patient screening for potential COVID-19 infection. (Screening should be independent of symptoms and not be limited to those with clinical symptoms.)
- Limit the number of health care team members in the operating procedure room.
- To minimize unnecessary staff exposure, have surgeons and staff not needed for intubation remain outside the OR until intubation is completed and leave the OR before extubation.
Intraprocedure recommendations
- Limit personnel in the OR to a minimum.
- Staff should not enter or leave the room during the procedure.
- When possible, use conscious sedation or regional anesthesia to avoid the risk of viral dissemination at the time of intubation/extubation.
- Choose the device that will allow an effective and fast procedure.
- Favor non–smoke-generating devices, such as hysteroscopic scissors, graspers, and tissue retrieval systems.
- Connect active suction to the outflow, especially when using smoke-generating instruments, to facilitate the extraction of surgical smoke.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same room, allow enough time in between cases to grant a thorough OR decontamination.
- Expedite postprocedure recovery and patient discharge.
- After completion of the procedure, staff should remove scrubs and change into clean clothing.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Conclusions
The COVID-19 pandemic has caused a global health emergency. Our knowledge of this devastating virus is constantly evolving as we continue to fight this overwhelming disease. Theoretical risk of “viral” dissemination is considered extremely low, or negligible, during hysterosocopy. Hysteroscopic procedures in COVID-19–positive patients with life-threatening conditions or in patients in whom delaying the procedure could worsen outcomes should be performed taking appropriate measures. Patients who test negative for COVID-19 (confirmed by PCR) and require hysteroscopic procedures, should be treated using universal precautions. ●
The emergence of the coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) in December 2019, has resulted in a global pandemic that has challenged the medical community and will continue to represent a public health emergency for the next several months.1 It has rapidly spread globally, infecting many individuals in an unprecedented rate of infection and worldwide reach. On March 11, 2020, the World Health Organization designated COVID-19 as a pandemic. While the majority of infected individuals are asymptomatic or develop only mild symptoms, some have an unfortunate clinical course resulting in multi-organ failure and death.2
It is accepted that the virus mainly spreads during close contact and via respiratory droplets.3 The average time from infection to onset of symptoms ranges from 2 to 14 days, with an average of 5 days.4 Recommended measures to prevent the spread of the infection include social distancing (at least 6 feet from others), meticulous hand hygiene, and wearing a mask covering the mouth and nose when in public.5 Aiming to mitigate the risk of viral dissemination for patients and health care providers, and to preserve hospital resources, all nonessential medical interventions were initially suspended. Recently, the American College of Surgeons in a joint statement with 9 women’s health care societies have provided recommendations on how to resume clinical activities as we recover from the pandemic.6
As we reinitiate clinical activities, gynecologists have been alerted of the potential risk of viral dissemination during gynecologic minimally invasive surgical procedures due to the presence of the virus in blood, stool, and the potential risk of aerosolization of the virus, especially when using smoke-generating devices.7,8 This risk is not limited to intubation and extubation of the airway during anesthesia; the risk also presents itself during other aerosol-generating procedures, such as laparoscopy or robotic surgery.9,10
Hysteroscopy is considered the gold standard procedure for the diagnosis and management of intrauterine pathologies.11 It is frequently performed in an office setting without the use of anesthesia.11,12 It is usually well tolerated, with only a few patients reporting discomfort.12 It allows for immediate treatment (using the “see and treat” approach) while avoiding not only the risk of anesthesia, as stated, but also the need for intubation—which has a high risk of droplet contamination in COVID-19–infected individuals.13

Is there risk of viral dissemination during hysteroscopic procedures?
The novel and rapidly changing nature of the COVID-19 pandemic present many challenges to the gynecologist. Significant concerns have been raised regarding potential risk of viral dissemination during laparoscopic surgery due to aerosolization of viral particles and the presence of the virus in blood and the gastrointestinal tract of infected patients.7 Diagnostic, and some simple, hysteroscopic procedures are commonly performed in an outpatient setting, with the patient awake. Complex hysteroscopic interventions, however, are generally performed in the operating room, typically with the use of general anesthesia. Hysteroscopy has the theoretical risks of viral dissemination when performed in COVID-19–positive patients. Two important questions must be addressed to better understand the potential risk of COVID-19 viral dissemination during hysteroscopic procedures.
Continue to: 1. Is the virus present in the vaginal fluid of women infected with COVID-19?...
1. Is the virus present in the vaginal fluid of women infected with COVID-19?
Recent studies have confirmed the presence of viral particles in urine, feces, blood, and tears in addition to the respiratory tract in patients infected with COVID-19.3,14,15 The presence of the SARS-CoV-2 virus in the female genital system is currently unknown. Previous studies, of other epidemic viral infections, have demonstrated the presence of the virus in the female genital tract in affected patients of Zika virus and Ebola.16,17 However, 2 recent studies have failed to demonstrate the presence of the SARS-CoV-2 virus in the vaginal fluid of pregnant14 and not pregnant18 women with severe COVID-19 infection.
2. Is there risk of viral dissemination during hysteroscopy if using electrosurgery?
There are significant concerns with possible risk of COVID-19 transmission to health care providers in direct contact with infected patients during minimally invasive gynecologic procedures due to direct contamination and aerosolization of the virus.10,19 Current data on COVID-19 transmission during surgery are limited. However, it is important to recognize that viral aerosolization has been documented with other viral diseases, such as human papillomavirus and hepatitis B.20 A recent report called for awareness in the surgical community about the potential risks of COVID-19 viral dissemination during laparoscopic surgery. Among other recommendations, international experts advised minimizing the use of electrosurgery to reduce the creation of surgical plume, decreasing the pneumoperitoneum pressure to minimum levels, and using suction devices in a closed system.21 Although these preventive measures apply to laparoscopic surgery, it is important to consider that hysteroscopy is performed in a unique environment.
During hysteroscopy the uterine cavity is distended with a liquid medium (normal saline or electrolyte-free solutions); this is opposed to gynecologic laparoscopy, in which the peritoneal cavity is distended with carbon dioxide.22 The smoke produced with the use of hysteroscopic electrosurgical instruments generates bubbles that are immediately cooled down to the temperature of the distention media and subsequently dissolve into it. Therefore, there are no bubbles generated during hysteroscopic surgery that are subsequently released into the air. This results in a low risk for viral dissemination during hysteroscopic procedures. Nevertheless, the necessary precautions to minimize the risk of COVID-19 transmission during hysteroscopic intervention are extremely important.
Recommendations for hysteroscopic procedures during the COVID-19 pandemic
We provide our overall recommendations for hysteroscopy, as well as those specific to the office and hospital setting.
Recommendations: General
Limit hysteroscopic procedures to COVID-19–negative patients and to those patients in whom delaying the procedure could result in adverse clinical outcomes.23
Universally screen for potential COVID-19 infection. When possible, a phone interview to triage patients based on their symptoms and infection exposure status should take place before the patient arrives to the health care center. Patients with suspected or confirmed COVID-19 infection who require immediate evaluation should be directed to COVID-19–designated emergency areas.
Universally test for SARS-CoV-2 before procedures performed in the operating room (OR). Using nasopharyngeal swabs for the detection of viral RNA, employing molecular methods such as polymerase chain reaction (PCR), within 48 to 72 hours prior to all OR hysteroscopic procedures is strongly recommended. Adopting this testing strategy will aid to identify asymptomatic SARS-CoV-2‒infected patients, allowing to defer the procedure, if possible, among patients testing positive. If tests are limited, testing only patients scheduled for hysteroscopic procedures in which general or regional anesthesia will be required is acceptable.
Universal SARS-CoV-2 testing of patients undergoing in-office hysteroscopic diagnostic or minor operative procedures without the use of anesthesia is not required.
Limit the presence of a companion. It is understood that visitor policies may vary at the discretion of each institution’s guidelines. Children and individuals over the age of 60 years should not be granted access to the center. Companions will be subjected to the same screening criteria as patients.
Provide for social distancing and other precautionary measures. If more than one patient is scheduled to be at the facility at the same time, ensure that the facility provides adequate space to allow the appropriate social distancing recommendations between patients. Hand sanitizers and facemasks should be available for patients and companions.
Provide PPE for clinicians. All health care providers in close contact with the patient must wear personal protective equipment (PPE), which includes an apron and gown, a surgical mask, eye protection, and gloves. Health care providers should wear PPE deemed appropriate by their regulatory institutions following their local and national guidelines during clinical patient interactions.
Restrict surgical attendees to vital personnel. The participation of learners by physical presence in the office or operating room should be restricted.
Continue to: Recommendations: Office setting...
Recommendations: Office setting
Preprocedural recommendations
- Advise patients to come to the office alone. If the patient requires a companion, a maximum of one adult companion under the age of 60 should be accepted.
- Limit the number of health care team members present in the procedure room.
Intraprocedural recommendations
- Choose the appropriate device(s) that will allow for an effective and fast procedure.
- Use the recommended PPE for all clinicians.
- Limit the movement of staff members in and out of the procedure room.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same procedure room, allow enough time in between cases to grant a thorough OR decontamination.
- Allow for patients to recover from the procedure in the same room as the procedure took place in order to avoid potential contamination of multiple rooms.
- Expedite patient discharge.
- Follow up after the procedure by phone or telemedicine.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Continue to: Recommendations: Operating room setting...
Recommendations: Operating room setting
Preprocedural recommendations
- Perform adequate patient screening for potential COVID-19 infection. (Screening should be independent of symptoms and not be limited to those with clinical symptoms.)
- Limit the number of health care team members in the operating procedure room.
- To minimize unnecessary staff exposure, have surgeons and staff not needed for intubation remain outside the OR until intubation is completed and leave the OR before extubation.
Intraprocedure recommendations
- Limit personnel in the OR to a minimum.
- Staff should not enter or leave the room during the procedure.
- When possible, use conscious sedation or regional anesthesia to avoid the risk of viral dissemination at the time of intubation/extubation.
- Choose the device that will allow an effective and fast procedure.
- Favor non–smoke-generating devices, such as hysteroscopic scissors, graspers, and tissue retrieval systems.
- Connect active suction to the outflow, especially when using smoke-generating instruments, to facilitate the extraction of surgical smoke.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same room, allow enough time in between cases to grant a thorough OR decontamination.
- Expedite postprocedure recovery and patient discharge.
- After completion of the procedure, staff should remove scrubs and change into clean clothing.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Conclusions
The COVID-19 pandemic has caused a global health emergency. Our knowledge of this devastating virus is constantly evolving as we continue to fight this overwhelming disease. Theoretical risk of “viral” dissemination is considered extremely low, or negligible, during hysterosocopy. Hysteroscopic procedures in COVID-19–positive patients with life-threatening conditions or in patients in whom delaying the procedure could worsen outcomes should be performed taking appropriate measures. Patients who test negative for COVID-19 (confirmed by PCR) and require hysteroscopic procedures, should be treated using universal precautions. ●
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25:e936-e945.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. February 24, 2020. doi:10.1001/jama.2020.2648.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843-1844.
- Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71:793-798.
- Prem K, Liu Y, Russell TW, et al; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261-e270.
- American College of Surgeons, American Society of Aesthesiologists, Association of periOperative Registered Nurses, American Hospital Association. Joint Statement: Roadmap for resuming elective surgery after COVID-19 pandemic. April 16, 2020. https://www.aorn.org/guidelines/aorn-support/roadmap-for-resuming-elective-surgery-after-covid-19. Accessed August 27, 2020.
- Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386-389.
- Mowbray NG, Ansell J, Horwood J, et al. Safe management of surgical smoke in the age of COVID-19. Br J Surg. May 3, 2020. doi: 10.1002/bjs.11679.
- Cohen SL, Liu G, Abrao M, et al. Perspectives on surgery in the time of COVID-19: safety first. J Minim Invasive Gynecol. 2020;27:792-793.
- COVID-19: protecting health-care workers. Lancet. 2020;395:922.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Cicinelli E. Hysteroscopy without anesthesia: review of recent literature. J Minim Invasive Gynecol. 2010;17:703-708.
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568-576.
- Aslan MM, Yuvaci HU, Köse O, et al. SARS-CoV-2 is not present in the vaginal fluid of pregnant women with COVID-19. J Matern Fetal Neonatal Med. 2020:1-3. doi: 10.1080/14767058.2020.1793318.
- Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833-840.
- Prisant N, Bujan L, Benichou H, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1001.
- Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179 Suppl 1:S170-S176.
- Qiu L, Liu X, Xiao M, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis. 2020;71:813-817.
- Brat GA, Hersey S, Chhabra K, et al. Protecting surgical teams during the COVID-19 outbreak: a narrative review and clinical considerations. Ann Surg. April 17, 2020. doi: 10.1097/SLA.0000000000003926.
- Kwak HD, Kim SH, Seo YS, et al. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73:857-863.
- Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. 2020;272:e5-e6.
- Catena U. Surgical smoke in hysteroscopic surgery: does it really matter in COVID-19 times? Facts Views Vis Obgyn. 2020;12:67-68.
- Carugno J, Di Spiezio Sardo A, Alonso L, et al. COVID-19 pandemic. Impact on hysteroscopic procedures: a consensus statement from the Global Congress of Hysteroscopy Scientific Committee. J Minim Invasive Gynecol. 2020;27:988-992.
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25:e936-e945.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. February 24, 2020. doi:10.1001/jama.2020.2648.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843-1844.
- Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71:793-798.
- Prem K, Liu Y, Russell TW, et al; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261-e270.
- American College of Surgeons, American Society of Aesthesiologists, Association of periOperative Registered Nurses, American Hospital Association. Joint Statement: Roadmap for resuming elective surgery after COVID-19 pandemic. April 16, 2020. https://www.aorn.org/guidelines/aorn-support/roadmap-for-resuming-elective-surgery-after-covid-19. Accessed August 27, 2020.
- Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386-389.
- Mowbray NG, Ansell J, Horwood J, et al. Safe management of surgical smoke in the age of COVID-19. Br J Surg. May 3, 2020. doi: 10.1002/bjs.11679.
- Cohen SL, Liu G, Abrao M, et al. Perspectives on surgery in the time of COVID-19: safety first. J Minim Invasive Gynecol. 2020;27:792-793.
- COVID-19: protecting health-care workers. Lancet. 2020;395:922.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Cicinelli E. Hysteroscopy without anesthesia: review of recent literature. J Minim Invasive Gynecol. 2010;17:703-708.
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568-576.
- Aslan MM, Yuvaci HU, Köse O, et al. SARS-CoV-2 is not present in the vaginal fluid of pregnant women with COVID-19. J Matern Fetal Neonatal Med. 2020:1-3. doi: 10.1080/14767058.2020.1793318.
- Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833-840.
- Prisant N, Bujan L, Benichou H, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1001.
- Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179 Suppl 1:S170-S176.
- Qiu L, Liu X, Xiao M, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis. 2020;71:813-817.
- Brat GA, Hersey S, Chhabra K, et al. Protecting surgical teams during the COVID-19 outbreak: a narrative review and clinical considerations. Ann Surg. April 17, 2020. doi: 10.1097/SLA.0000000000003926.
- Kwak HD, Kim SH, Seo YS, et al. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73:857-863.
- Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. 2020;272:e5-e6.
- Catena U. Surgical smoke in hysteroscopic surgery: does it really matter in COVID-19 times? Facts Views Vis Obgyn. 2020;12:67-68.
- Carugno J, Di Spiezio Sardo A, Alonso L, et al. COVID-19 pandemic. Impact on hysteroscopic procedures: a consensus statement from the Global Congress of Hysteroscopy Scientific Committee. J Minim Invasive Gynecol. 2020;27:988-992.
2020 Update on pelvic floor dysfunction
Postoperative voiding dysfunction refers to the acute inability to spontaneously and adequately empty the bladder after surgery. Postoperative voiding dysfunction occurs in 21% to 42% of pelvic reconstructive surgeries, as well as 7% to 21% of benign gynecologic surgeries.1-4 While much of its peril lies in patient discomfort or dissatisfaction with temporary bladder drainage, serious consequences of the disorder include bladder overdistension injury with inadequate drainage and urinary tract infection (UTI) associated with prolonged catheterization.4-6
Although transient postoperative voiding dysfunction is associated with anti-incontinence surgery, tricyclic antidepressant use, diabetes, preoperative voiding dysfunction, and postoperative narcotic use, it also may occur in patients without risk factors.4,7,8 Thus, all gynecologic surgeons should be prepared to assess and manage the patient with postoperative voiding dysfunction.
Diagnosis of postoperative voiding dysfunction can be approached in myriad ways, including spontaneous (or natural) bladder filling or bladder backfill followed by spontaneous void. When compared with spontaneous void trials, backfill-assisted void trial is associated with improved accuracy in predicting voiding dysfunction in patients who undergo urogynecologic surgery, leading to widespread adoption of the procedure following pelvic reconstructive surgeries.9,10
Criteria for “passing” a void trial may include the patient’s subjective feeling of having emptied her bladder; having a near-baseline force of stream; or commonly by objective parameters of voided volume and postvoid residual (PVR), assessed via catheterization or bladder scan.3,6,10 Completing a postoperative void trial typically requires significant nursing effort because of the technical demands of backfilling the bladder, obtaining the voided volume and PVR, or assessing subjective emptying.
Management of postoperative voiding dysfunction typically consists of continuous drainage with a transurethral catheter or clean intermittent self-catheterization (CISC). Patients discharged home with a bladder drainage method also may be prescribed various medications, such as antibiotics, anticholinergics, and bladder analgesics, which often depends on provider practice.
Given the minimal universal guidance available for gynecologic surgeons on postoperative voiding dysfunction, we review several articles that contribute new evidence on the assessment and management of this condition.
Continue to: How can we efficiently approach the postoperative void trial for pelvic floor surgery?
How can we efficiently approach the postoperative void trial for pelvic floor surgery?
Chao L, Mansuria S. Postoperative bladder filling after outpatient laparoscopic hysterectomy and time to discharge: a randomized controlled trial. Obstet Gynecol. 2019;133:879-887.
Despite efforts to implement and promote enhanced recovery after surgery pathways, waiting for spontaneous void can be a barrier to efficient same-day discharge. Chao and Mansuria conducted a randomized controlled trial (RCT) to determine whether backfilling the bladder intraoperatively, compared with spontaneous (physiologic) filling, would reduce time to discharge in patients undergoing total laparoscopic hysterectomy (TLH) or supracervical hysterectomy (SCH).
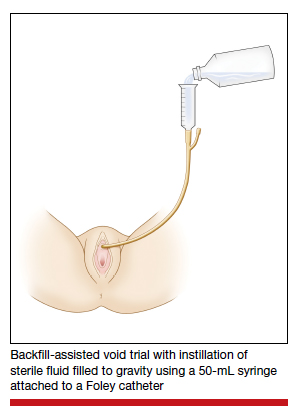
Study details

Women undergoing TLH or laparoscopic SCH for benign indications were randomly assigned to undergo either a backfill-assisted void trial in the operating room with 200 mL of sterile normal saline (n = 75) or Foley catheter removal with spontaneous fill in the postanesthesia care unit (PACU) (n = 78).
For both groups, the maximum time allowed for spontaneous void was 5 hours. A successful void trial was defined as a voided volume of at least 200 mL. If a patient was unable to void at least 200 mL, a bladder scan was performed, and the patient was considered to have failed the void trial if a PVR of 200 mL or greater was noted. If the PVR was less than 200 mL, the patient was given an additional 1 hour to spontaneously void 200 mL by 6 hours after the surgery. Patients who failed the void trial were discharged home with a transurethral catheter.
The primary outcome was time to discharge, and the sample size (153 participants included in the analysis) allowed 80% power to detect a 30-minute difference in time to discharge. Participant baseline characteristics, concomitant procedures, and indication for hysterectomy were similar for both groups.
Results. The mean time to discharge was 273.4 minutes for the backfill-assisted void trial group and 283.2 minutes for the spontaneous fill group, a difference of 9.8 minutes that was not statistically significant (P = .45).
Although it was not a primary outcome, time to spontaneous void was 24.9 minutes shorter in the backfill group (P = .04). Rates of postoperative voiding dysfunction did not differ between the 2 groups (6.7% for the backfill group and 12.8% for the spontaneous fill group; P = .2). There were no significant differences in emergency department visits, UTI rates, or readmissions.
Bladder backfill is safe, simple, and may reduce time to spontaneous void
Strengths of the study included its prospective randomized design, blinded outcome assessors, and diversity in benign gynecologic surgeries performed. Although this study found a reduced time to spontaneous void in the backfill group, it was not powered to assess this difference, limiting ability to draw conclusions from those data. Data on postoperative nausea and pain scores also were not collected, which likely influenced the overall time to discharge.
Void trial completion is one of many criteria to fulfill prior to patient discharge, and a reduced time to first void may not decrease the overall length of PACU stay if other factors, such as nausea or pain, are not controlled. Nonetheless, backfilling the bladder intraoperatively is a safe alternative that may decrease the time to first spontaneous void, and it is a relatively simple alteration in the surgical workflow that could significantly lessen PACU nursing demands.
Backfilling the bladder in the operating room prior to catheter discontinuation can reduce time to first spontaneous void, but not the overall time to discharge.
Continue to: Algorithm assesses need for PVR, although further study required...
Algorithm assesses need for PVR, although further study required
Meekins AR, Siddiqui N, Amundsen CL, et al. Improving postoperative efficiency: an algorithm for expedited void trials after urogynecologic surgery. South Med J. 2017;110:785-790.
To determine ways to further maximize postoperative efficiency, Meekins and colleagues sought to determine whether certain voided volumes during backfill-assisted void trials could obviate the need for PVR assessment.
Void trial results calculated to develop algorithm
The study was a secondary analysis of a previously conducted RCT that assessed antibiotics for the prevention of UTI after urogynecologic surgery. Void trials from the parent RCT were performed via the backfill-assisted method in which the bladder was backfilled in the PACU with 300 mL of normal saline or until the patient reported urgency to void, after which the catheter was removed and the patient was prompted to void immediately.
Postvoid residual levels were assessed via ultrasonography or catheterization. A void trial was considered to be passed when a PVR was less than 100 mL or less than 50% of the total bladder volume, with a minimum voided volume of 200 mL.
In the follow-up study, the authors analyzed the void trial results of 255 women of the original 264 in the parent RCT. A total of 69% of patients passed their void trial. The authors assessed the optimal positive predictive value (PPV) and negative predictive value (NPV) combinations, which were then used to create lower and upper voided volume thresholds that would best predict a failed or passed trial, thus obviating PVR measurement.
Results. When patients voided less than 100 mL, the NPV was 96.7% (meaning that they had a 96.7% chance of failing the void trial). When patients voided 200 mL or more, the PPV was 97% (meaning that they had a 97% chance of passing the void trial). Receiver operating characteristic analysis confirmed that voided volume alone was an excellent predictor of final void trial results, with area under the curve of 0.97. The authors estimated that applying this algorithm to their study population would have eliminated the need for assessing PVR in 85% of patients. Ultimately, they proposed the algorithm shown in TABLE 1.
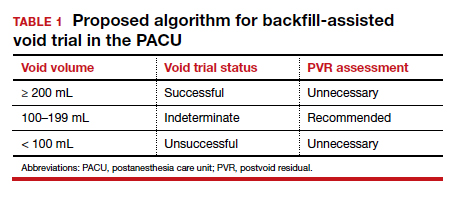
A potential alternative for assessing PVR
This study's strengths include the use of prospectively and systematically collected void trial data in a large patient population undergoing various urogynecologic procedures. By contrast, the generalizability of the results is limited regarding other void trial methods, such as spontaneous filling and void, as well as populations outside of the studied institution.
With the algorithm, the authors estimated that the majority of postoperative patients would no longer require a PVR assessment in the PACU. This could have beneficial downstream implications, including decreasing the nursing workload, reducing total time in the PACU, and minimizing patient discomfort with PVR assessment.
While further studies are needed to validate the proposed algorithm in larger populations, this study provides evidence of an efficient alternative to the traditional approach to PVR assessment in the PACU.
Application of the algorithm proposed by the study investigators has the potential to eliminate the need for a PVR assessment in most patients following a backfill-assisted void trial.
Continue to: An alternative to Foley use if a patient does not know CISC...
An alternative to Foley use if a patient does not know CISC
Boyd SS, O'Sullivan DM, Tunitsky-Bitton E. A comparison of two methods of catheter management after pelvic reconstructive surgery: a randomized controlled trial. Obstet Gynecol. 2019;134:1037-1045.
The traditional indwelling catheter as a postoperative bladder drainage method has a number of drawbacks, including an increased rate of UTI, patient discomfort, and potential limitations in mobility due to the presence of a drainage bag.5
Boyd and colleagues reported on a variation of traditional transurethral catheterization that hypothetically allows for improved mobility. With this method, the transurethral catheter is occluded with a plastic plug that is intermittently plugged and unplugged (plug-unplug method) for bladder drainage. To test whether activity levels are improved with the plug-unplug method versus the continuous drainage approach, the authors conducted an RCT in women undergoing pelvic reconstructive surgery to compare the plug-unplug method with transurethral catheterization (with a continuous drainage bag) and a reference group of freely voiding women.
Study particulars and outcomes
The trial's primary outcome was the patients' activity score as measured by the Activity Assessment Scale (AAS) at 5 to 7 days postoperatively. Because of the theoretically increased risk of a UTI with opening and closing a closed drainage system, secondary outcomes included the UTI rate, the time to pass an outpatient void trial, postoperative pain, patient satisfaction, and catheter effect. To detect an effect size of 0.33 in the primary outcome between the 3 groups, 90 participants were needed along with a difference in proportions of 0.3 between the catheterized and noncatheterized groups.
The participants were randomly assigned 1:1 preoperatively to the continuous drainage or plug-unplug method. All patients underwent a backfill-assisted void trial prior to hospital discharge; the first 30 randomly assigned patients to pass their void trial comprised the reference group. Patients in the plug-unplug arm were instructed to uncap the plastic plug to drain their bladder when they felt the urge to void or at least every 4 hours. All catheterized patients were provided with a large drainage bag for gravity-based drainage for overnight use.
Participants who were discharged home with a catheter underwent an outpatient void trial between postoperative days 5 and 7. A urinalysis was performed at that time and a urine culture was done if a patient reported UTI symptoms. All patients underwent routine follow-up until they passed the office void trial.
Results. Ninety-three women were included in the primary analysis. There were no differences in baseline characteristics between groups. No difference was detected in activity by AAS scores between all 3 groups (scores: plug-unplug, 70.3; continuous drainage, 67.7; reference arm, 79.4; P = .09). The 2 treatment arms had no overall difference in culture-positive UTI (plug-unplug, 68.8%; continuous drainage, 48.4%; P = .625). No significant difference was found in the percentage of patients who passed their initial outpatient void trial (plug-unplug, 71.9%, vs continuous drainage, 58.1%; P = .25) (TABLE 2).
Catheter impact on postoperative activity considered
Strengths of the study include the prospective randomized design, the inclusion of a noncatheterized reference arm, and use of a validated questionnaire to assess activity. The study was limited, however, by the inability to blind patients to treatment and the lack of power to assess other important outcomes, such as UTI rates.
Although the authors did not find a difference in activity scores between the 2 catheterization methods, no significant difference was found between the catheterized and noncatheterized groups, which suggests that catheters in general may not significantly impact postoperative activity. The theoretical concern that opening and closing a transurethral drainage system would increase UTI rates was not substantiated, although the study was not powered specifically for this outcome.
Ultimately, the plug-unplug method may be a safe alternative for patients who desire to avoid attachment to a drainage bag postoperatively.
Based on the results of an RCT that compared 2 methods of catheter management after pelvic reconstructive surgery, the plug-unplug catheterization method may be an acceptable alternative to traditional catheterization.
- Bladder backfill in the operating room followed by spontaneous void in the postanesthesia care unit (PACU) is a safe and efficient way to assess for postoperative voiding dysfunction.
- Voids of 200 mL or more (following a 300-mL backfill) may not require a PACU postvoid residual assessment.
- Postoperative activity does not appear to be impacted by the presence of an indwelling catheter.
Continue to: Does antibiotic prophylaxis reduce UTI for patients catheter-managed postoperatively?
Does antibiotic prophylaxis reduce UTI for patients catheter-managed postoperatively?
Lavelle ES, Alam P, Meister M, et al. Antibiotic prophylaxis during catheter-managed postoperative urinary retention after pelvic reconstructive surgery: a randomized controlled trial. Obstet Gynecol. 2019;134:727-735.
Limited high-quality evidence supports the use of prophylactic antibiotics during catheterization following prolapse or incontinence surgery, and the Infectious Disease Society of America cautions against routine antibiotic prophylaxis for those requiring catheterization.11
Lavelle and colleagues conducted a multicenter RCT to determine whether nitrofurantoin is more effective than placebo in decreasing UTIs among patients with postoperative voiding dysfunction following surgery for prolapse or incontinence.
Focus of the study
The investigators conducted a double-blind RCT at 5 academic sites that included women with postoperative voiding dysfunction who required catheter management (transurethral indwelling catheter or CISC). Voiding dysfunction was diagnosed by backfill or spontaneous fill void trial and was defined as a PVR of greater than 100 mL. Women were randomly assigned 1:1 to nitrofurantoin 100 mg or placebo taken daily during catheter use. Catheter use was discontinued once an outpatient void trial confirmed efficient voiding.
The primary outcome was symptomatic culture-confirmed UTI within 6 weeks of surgery. Secondary outcomes included frequency of urine cultures with nitrofurantoin-resistant or intermediate-sensitivity isolates and adverse symptoms possibly related to nitrofurantoin. The authors calculated that 154 participants would provide 80% power to detect a decrease in UTI incidence from 33% to 13%, allowing for 10% dropout.
A total of 151 women were randomly assigned and included in the intention-to-treat analysis. There were no differences in baseline characteristics. The median duration of catheter use was 4 days (interquartile range, 3-7).
Results. Overall, 13 women in the nitrofurantoin group and 13 in the placebo group experienced the primary outcome of UTI within 6 weeks postoperatively (17.3% nitrofurantoin vs 17.1% placebo; P = .97; relative risk [RR], 1.01; 95% confidence interval [CI], 0.50-2.04). The number needed to treat with nitrofurantoin to prevent 1 UTI was 500. A subanalysis found no difference in UTI incidence stratified by CISC versus indwelling catheter.
Urine cultures were obtained for 94.5% of all patients reporting UTI symptoms. Four isolates of the 13 cultures in the nitrofurantoin group (30.8%) and 3 in the placebo group (21.4%) showed nitrofurantoin resistance (P = .58). The rate of endorsing at least 1 adverse symptom attributable to nitrofurantoin was similar between groups (68.0% vs 60.5%, respectively; P = .34).
Study strong points and limitations
This study's randomized, placebo-controlled design and multicenter recruitment increase the generalizability of the results. An additional strength is that the authors chose a clinically relevant definition of UTI. The study was likely underpowered, however, to detect differences in secondary outcomes, such as nitrofurantoin resistance. We cannot conclude on the role of antibiotics for patients who require more prolonged catheterization.
Notably, a similar RCT by Dieter and colleagues of 159 patients undergoing daily nitrofurantoin versus placebo during CISC or transurethral catheterization failed to detect a difference in the rate of UTI treatment up to 3 weeks postoperatively with nitrofurantoin prophylaxis.12
Ultimately, the study by Lavelle and colleagues contributes to a growing body of evidence that supports the avoidance of antibiotic prophylaxis during short-term postoperative catheterization.
Nitrofurantoin prophylaxis did not reduce the incidence of postoperative UTI in patients with catheter-managed postoperative voiding dysfunction.
- Prophylactic antibiotics are not necessary for short-term catheterization in postoperative patients.
- Baessler K, Maher C. Pelvic organ prolapse surgery and bladder function. Int Urogynecol J. 2013;24:1843-1852.
- Yune JJ, Cheng JW, Wagner H, et al. Postoperative urinary retention after pelvic organ prolapse repair: vaginal versus robotic transabdominal approach. Neurourol Urodyn. 2018;37:1794-1800.
- Ghezzi F, Cromi A, Uccella S, et al. Immediate Foley removal after laparoscopic and vaginal hysterectomy: determinants of postoperative urinary retention. J Minim Invasive Gynecol. 2007;14:706-711.
- Smorgick N, DeLancey J, Patzkowsky K, et al. Risk factors for postoperative urinary retention after laparoscopic and robotic hysterectomy for benign indications. Obstet Gynecol. 2012;120:581-586.
- Dieter AA, Amundsen CL, Visco AG, et al. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18:175-178.
- Tunitsky-Bitton E, Murphy A, Barber MD, et al. Assessment of voiding after sling: a randomized trial of 2 methods of postoperative catheter management after midurethral sling surgery for stress urinary incontinence in women. Am J Obstet Gynecol. 2015;212:597.e1-e9.
- Kandadai P, Saini J, Patterson D, et al. Urinary retention after hysterectomy and postoperative analgesic use. Female Pelvic Med Reconstr Surg. 2015;21:257-262.
- Liang CC, Lee CL, Chang TC, et al. Postoperative urinary outcomes in catheterized and non-catheterized patients undergoing laparoscopic-assisted vaginal hysterectomy--a randomized controlled trial. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:295-300.
- Foster RT Sr, Borawski KM, South MM, et al. A randomized, controlled trial evaluating 2 techniques of postoperative bladder testing after transvaginal surgery. Am J Obstet Gynecol. 2007;197:627.e1-e4.
- Geller EJ, Hankins KJ, Parnell BA, et al. Diagnostic accuracy of retrograde and spontaneous voiding trials for postoperative voiding dysfunction: a randomized controlled trial. Obstet Gynecol. 2011;118:637-642.
Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Disease Society of America. Clin Infect Dis. 2010;50:625-663.
Dieter AA, Amundsen CL, Edenfield AL, et al. Oral antibiotics to prevent postoperative urinary tract infection: a randomized controlled trial. Obstet Gynecol. 2014;123:96-103.
Postoperative voiding dysfunction refers to the acute inability to spontaneously and adequately empty the bladder after surgery. Postoperative voiding dysfunction occurs in 21% to 42% of pelvic reconstructive surgeries, as well as 7% to 21% of benign gynecologic surgeries.1-4 While much of its peril lies in patient discomfort or dissatisfaction with temporary bladder drainage, serious consequences of the disorder include bladder overdistension injury with inadequate drainage and urinary tract infection (UTI) associated with prolonged catheterization.4-6
Although transient postoperative voiding dysfunction is associated with anti-incontinence surgery, tricyclic antidepressant use, diabetes, preoperative voiding dysfunction, and postoperative narcotic use, it also may occur in patients without risk factors.4,7,8 Thus, all gynecologic surgeons should be prepared to assess and manage the patient with postoperative voiding dysfunction.
Diagnosis of postoperative voiding dysfunction can be approached in myriad ways, including spontaneous (or natural) bladder filling or bladder backfill followed by spontaneous void. When compared with spontaneous void trials, backfill-assisted void trial is associated with improved accuracy in predicting voiding dysfunction in patients who undergo urogynecologic surgery, leading to widespread adoption of the procedure following pelvic reconstructive surgeries.9,10
Criteria for “passing” a void trial may include the patient’s subjective feeling of having emptied her bladder; having a near-baseline force of stream; or commonly by objective parameters of voided volume and postvoid residual (PVR), assessed via catheterization or bladder scan.3,6,10 Completing a postoperative void trial typically requires significant nursing effort because of the technical demands of backfilling the bladder, obtaining the voided volume and PVR, or assessing subjective emptying.
Management of postoperative voiding dysfunction typically consists of continuous drainage with a transurethral catheter or clean intermittent self-catheterization (CISC). Patients discharged home with a bladder drainage method also may be prescribed various medications, such as antibiotics, anticholinergics, and bladder analgesics, which often depends on provider practice.
Given the minimal universal guidance available for gynecologic surgeons on postoperative voiding dysfunction, we review several articles that contribute new evidence on the assessment and management of this condition.
Continue to: How can we efficiently approach the postoperative void trial for pelvic floor surgery?
How can we efficiently approach the postoperative void trial for pelvic floor surgery?
Chao L, Mansuria S. Postoperative bladder filling after outpatient laparoscopic hysterectomy and time to discharge: a randomized controlled trial. Obstet Gynecol. 2019;133:879-887.
Despite efforts to implement and promote enhanced recovery after surgery pathways, waiting for spontaneous void can be a barrier to efficient same-day discharge. Chao and Mansuria conducted a randomized controlled trial (RCT) to determine whether backfilling the bladder intraoperatively, compared with spontaneous (physiologic) filling, would reduce time to discharge in patients undergoing total laparoscopic hysterectomy (TLH) or supracervical hysterectomy (SCH).

Study details

Women undergoing TLH or laparoscopic SCH for benign indications were randomly assigned to undergo either a backfill-assisted void trial in the operating room with 200 mL of sterile normal saline (n = 75) or Foley catheter removal with spontaneous fill in the postanesthesia care unit (PACU) (n = 78).
For both groups, the maximum time allowed for spontaneous void was 5 hours. A successful void trial was defined as a voided volume of at least 200 mL. If a patient was unable to void at least 200 mL, a bladder scan was performed, and the patient was considered to have failed the void trial if a PVR of 200 mL or greater was noted. If the PVR was less than 200 mL, the patient was given an additional 1 hour to spontaneously void 200 mL by 6 hours after the surgery. Patients who failed the void trial were discharged home with a transurethral catheter.
The primary outcome was time to discharge, and the sample size (153 participants included in the analysis) allowed 80% power to detect a 30-minute difference in time to discharge. Participant baseline characteristics, concomitant procedures, and indication for hysterectomy were similar for both groups.
Results. The mean time to discharge was 273.4 minutes for the backfill-assisted void trial group and 283.2 minutes for the spontaneous fill group, a difference of 9.8 minutes that was not statistically significant (P = .45).
Although it was not a primary outcome, time to spontaneous void was 24.9 minutes shorter in the backfill group (P = .04). Rates of postoperative voiding dysfunction did not differ between the 2 groups (6.7% for the backfill group and 12.8% for the spontaneous fill group; P = .2). There were no significant differences in emergency department visits, UTI rates, or readmissions.
Bladder backfill is safe, simple, and may reduce time to spontaneous void
Strengths of the study included its prospective randomized design, blinded outcome assessors, and diversity in benign gynecologic surgeries performed. Although this study found a reduced time to spontaneous void in the backfill group, it was not powered to assess this difference, limiting ability to draw conclusions from those data. Data on postoperative nausea and pain scores also were not collected, which likely influenced the overall time to discharge.
Void trial completion is one of many criteria to fulfill prior to patient discharge, and a reduced time to first void may not decrease the overall length of PACU stay if other factors, such as nausea or pain, are not controlled. Nonetheless, backfilling the bladder intraoperatively is a safe alternative that may decrease the time to first spontaneous void, and it is a relatively simple alteration in the surgical workflow that could significantly lessen PACU nursing demands.
Backfilling the bladder in the operating room prior to catheter discontinuation can reduce time to first spontaneous void, but not the overall time to discharge.
Continue to: Algorithm assesses need for PVR, although further study required...
Algorithm assesses need for PVR, although further study required
Meekins AR, Siddiqui N, Amundsen CL, et al. Improving postoperative efficiency: an algorithm for expedited void trials after urogynecologic surgery. South Med J. 2017;110:785-790.
To determine ways to further maximize postoperative efficiency, Meekins and colleagues sought to determine whether certain voided volumes during backfill-assisted void trials could obviate the need for PVR assessment.
Void trial results calculated to develop algorithm
The study was a secondary analysis of a previously conducted RCT that assessed antibiotics for the prevention of UTI after urogynecologic surgery. Void trials from the parent RCT were performed via the backfill-assisted method in which the bladder was backfilled in the PACU with 300 mL of normal saline or until the patient reported urgency to void, after which the catheter was removed and the patient was prompted to void immediately.
Postvoid residual levels were assessed via ultrasonography or catheterization. A void trial was considered to be passed when a PVR was less than 100 mL or less than 50% of the total bladder volume, with a minimum voided volume of 200 mL.
In the follow-up study, the authors analyzed the void trial results of 255 women of the original 264 in the parent RCT. A total of 69% of patients passed their void trial. The authors assessed the optimal positive predictive value (PPV) and negative predictive value (NPV) combinations, which were then used to create lower and upper voided volume thresholds that would best predict a failed or passed trial, thus obviating PVR measurement.
Results. When patients voided less than 100 mL, the NPV was 96.7% (meaning that they had a 96.7% chance of failing the void trial). When patients voided 200 mL or more, the PPV was 97% (meaning that they had a 97% chance of passing the void trial). Receiver operating characteristic analysis confirmed that voided volume alone was an excellent predictor of final void trial results, with area under the curve of 0.97. The authors estimated that applying this algorithm to their study population would have eliminated the need for assessing PVR in 85% of patients. Ultimately, they proposed the algorithm shown in TABLE 1.

A potential alternative for assessing PVR
This study's strengths include the use of prospectively and systematically collected void trial data in a large patient population undergoing various urogynecologic procedures. By contrast, the generalizability of the results is limited regarding other void trial methods, such as spontaneous filling and void, as well as populations outside of the studied institution.
With the algorithm, the authors estimated that the majority of postoperative patients would no longer require a PVR assessment in the PACU. This could have beneficial downstream implications, including decreasing the nursing workload, reducing total time in the PACU, and minimizing patient discomfort with PVR assessment.
While further studies are needed to validate the proposed algorithm in larger populations, this study provides evidence of an efficient alternative to the traditional approach to PVR assessment in the PACU.
Application of the algorithm proposed by the study investigators has the potential to eliminate the need for a PVR assessment in most patients following a backfill-assisted void trial.
Continue to: An alternative to Foley use if a patient does not know CISC...
An alternative to Foley use if a patient does not know CISC
Boyd SS, O'Sullivan DM, Tunitsky-Bitton E. A comparison of two methods of catheter management after pelvic reconstructive surgery: a randomized controlled trial. Obstet Gynecol. 2019;134:1037-1045.
The traditional indwelling catheter as a postoperative bladder drainage method has a number of drawbacks, including an increased rate of UTI, patient discomfort, and potential limitations in mobility due to the presence of a drainage bag.5
Boyd and colleagues reported on a variation of traditional transurethral catheterization that hypothetically allows for improved mobility. With this method, the transurethral catheter is occluded with a plastic plug that is intermittently plugged and unplugged (plug-unplug method) for bladder drainage. To test whether activity levels are improved with the plug-unplug method versus the continuous drainage approach, the authors conducted an RCT in women undergoing pelvic reconstructive surgery to compare the plug-unplug method with transurethral catheterization (with a continuous drainage bag) and a reference group of freely voiding women.
Study particulars and outcomes
The trial's primary outcome was the patients' activity score as measured by the Activity Assessment Scale (AAS) at 5 to 7 days postoperatively. Because of the theoretically increased risk of a UTI with opening and closing a closed drainage system, secondary outcomes included the UTI rate, the time to pass an outpatient void trial, postoperative pain, patient satisfaction, and catheter effect. To detect an effect size of 0.33 in the primary outcome between the 3 groups, 90 participants were needed along with a difference in proportions of 0.3 between the catheterized and noncatheterized groups.
The participants were randomly assigned 1:1 preoperatively to the continuous drainage or plug-unplug method. All patients underwent a backfill-assisted void trial prior to hospital discharge; the first 30 randomly assigned patients to pass their void trial comprised the reference group. Patients in the plug-unplug arm were instructed to uncap the plastic plug to drain their bladder when they felt the urge to void or at least every 4 hours. All catheterized patients were provided with a large drainage bag for gravity-based drainage for overnight use.
Participants who were discharged home with a catheter underwent an outpatient void trial between postoperative days 5 and 7. A urinalysis was performed at that time and a urine culture was done if a patient reported UTI symptoms. All patients underwent routine follow-up until they passed the office void trial.
Results. Ninety-three women were included in the primary analysis. There were no differences in baseline characteristics between groups. No difference was detected in activity by AAS scores between all 3 groups (scores: plug-unplug, 70.3; continuous drainage, 67.7; reference arm, 79.4; P = .09). The 2 treatment arms had no overall difference in culture-positive UTI (plug-unplug, 68.8%; continuous drainage, 48.4%; P = .625). No significant difference was found in the percentage of patients who passed their initial outpatient void trial (plug-unplug, 71.9%, vs continuous drainage, 58.1%; P = .25) (TABLE 2).

Catheter impact on postoperative activity considered
Strengths of the study include the prospective randomized design, the inclusion of a noncatheterized reference arm, and use of a validated questionnaire to assess activity. The study was limited, however, by the inability to blind patients to treatment and the lack of power to assess other important outcomes, such as UTI rates.
Although the authors did not find a difference in activity scores between the 2 catheterization methods, no significant difference was found between the catheterized and noncatheterized groups, which suggests that catheters in general may not significantly impact postoperative activity. The theoretical concern that opening and closing a transurethral drainage system would increase UTI rates was not substantiated, although the study was not powered specifically for this outcome.
Ultimately, the plug-unplug method may be a safe alternative for patients who desire to avoid attachment to a drainage bag postoperatively.
Based on the results of an RCT that compared 2 methods of catheter management after pelvic reconstructive surgery, the plug-unplug catheterization method may be an acceptable alternative to traditional catheterization.
- Bladder backfill in the operating room followed by spontaneous void in the postanesthesia care unit (PACU) is a safe and efficient way to assess for postoperative voiding dysfunction.
- Voids of 200 mL or more (following a 300-mL backfill) may not require a PACU postvoid residual assessment.
- Postoperative activity does not appear to be impacted by the presence of an indwelling catheter.
Continue to: Does antibiotic prophylaxis reduce UTI for patients catheter-managed postoperatively?
Does antibiotic prophylaxis reduce UTI for patients catheter-managed postoperatively?
Lavelle ES, Alam P, Meister M, et al. Antibiotic prophylaxis during catheter-managed postoperative urinary retention after pelvic reconstructive surgery: a randomized controlled trial. Obstet Gynecol. 2019;134:727-735.
Limited high-quality evidence supports the use of prophylactic antibiotics during catheterization following prolapse or incontinence surgery, and the Infectious Disease Society of America cautions against routine antibiotic prophylaxis for those requiring catheterization.11
Lavelle and colleagues conducted a multicenter RCT to determine whether nitrofurantoin is more effective than placebo in decreasing UTIs among patients with postoperative voiding dysfunction following surgery for prolapse or incontinence.
Focus of the study
The investigators conducted a double-blind RCT at 5 academic sites that included women with postoperative voiding dysfunction who required catheter management (transurethral indwelling catheter or CISC). Voiding dysfunction was diagnosed by backfill or spontaneous fill void trial and was defined as a PVR of greater than 100 mL. Women were randomly assigned 1:1 to nitrofurantoin 100 mg or placebo taken daily during catheter use. Catheter use was discontinued once an outpatient void trial confirmed efficient voiding.
The primary outcome was symptomatic culture-confirmed UTI within 6 weeks of surgery. Secondary outcomes included frequency of urine cultures with nitrofurantoin-resistant or intermediate-sensitivity isolates and adverse symptoms possibly related to nitrofurantoin. The authors calculated that 154 participants would provide 80% power to detect a decrease in UTI incidence from 33% to 13%, allowing for 10% dropout.
A total of 151 women were randomly assigned and included in the intention-to-treat analysis. There were no differences in baseline characteristics. The median duration of catheter use was 4 days (interquartile range, 3-7).
Results. Overall, 13 women in the nitrofurantoin group and 13 in the placebo group experienced the primary outcome of UTI within 6 weeks postoperatively (17.3% nitrofurantoin vs 17.1% placebo; P = .97; relative risk [RR], 1.01; 95% confidence interval [CI], 0.50-2.04). The number needed to treat with nitrofurantoin to prevent 1 UTI was 500. A subanalysis found no difference in UTI incidence stratified by CISC versus indwelling catheter.
Urine cultures were obtained for 94.5% of all patients reporting UTI symptoms. Four isolates of the 13 cultures in the nitrofurantoin group (30.8%) and 3 in the placebo group (21.4%) showed nitrofurantoin resistance (P = .58). The rate of endorsing at least 1 adverse symptom attributable to nitrofurantoin was similar between groups (68.0% vs 60.5%, respectively; P = .34).
Study strong points and limitations
This study's randomized, placebo-controlled design and multicenter recruitment increase the generalizability of the results. An additional strength is that the authors chose a clinically relevant definition of UTI. The study was likely underpowered, however, to detect differences in secondary outcomes, such as nitrofurantoin resistance. We cannot conclude on the role of antibiotics for patients who require more prolonged catheterization.
Notably, a similar RCT by Dieter and colleagues of 159 patients undergoing daily nitrofurantoin versus placebo during CISC or transurethral catheterization failed to detect a difference in the rate of UTI treatment up to 3 weeks postoperatively with nitrofurantoin prophylaxis.12
Ultimately, the study by Lavelle and colleagues contributes to a growing body of evidence that supports the avoidance of antibiotic prophylaxis during short-term postoperative catheterization.
Nitrofurantoin prophylaxis did not reduce the incidence of postoperative UTI in patients with catheter-managed postoperative voiding dysfunction.
- Prophylactic antibiotics are not necessary for short-term catheterization in postoperative patients.
Postoperative voiding dysfunction refers to the acute inability to spontaneously and adequately empty the bladder after surgery. Postoperative voiding dysfunction occurs in 21% to 42% of pelvic reconstructive surgeries, as well as 7% to 21% of benign gynecologic surgeries.1-4 While much of its peril lies in patient discomfort or dissatisfaction with temporary bladder drainage, serious consequences of the disorder include bladder overdistension injury with inadequate drainage and urinary tract infection (UTI) associated with prolonged catheterization.4-6
Although transient postoperative voiding dysfunction is associated with anti-incontinence surgery, tricyclic antidepressant use, diabetes, preoperative voiding dysfunction, and postoperative narcotic use, it also may occur in patients without risk factors.4,7,8 Thus, all gynecologic surgeons should be prepared to assess and manage the patient with postoperative voiding dysfunction.
Diagnosis of postoperative voiding dysfunction can be approached in myriad ways, including spontaneous (or natural) bladder filling or bladder backfill followed by spontaneous void. When compared with spontaneous void trials, backfill-assisted void trial is associated with improved accuracy in predicting voiding dysfunction in patients who undergo urogynecologic surgery, leading to widespread adoption of the procedure following pelvic reconstructive surgeries.9,10
Criteria for “passing” a void trial may include the patient’s subjective feeling of having emptied her bladder; having a near-baseline force of stream; or commonly by objective parameters of voided volume and postvoid residual (PVR), assessed via catheterization or bladder scan.3,6,10 Completing a postoperative void trial typically requires significant nursing effort because of the technical demands of backfilling the bladder, obtaining the voided volume and PVR, or assessing subjective emptying.
Management of postoperative voiding dysfunction typically consists of continuous drainage with a transurethral catheter or clean intermittent self-catheterization (CISC). Patients discharged home with a bladder drainage method also may be prescribed various medications, such as antibiotics, anticholinergics, and bladder analgesics, which often depends on provider practice.
Given the minimal universal guidance available for gynecologic surgeons on postoperative voiding dysfunction, we review several articles that contribute new evidence on the assessment and management of this condition.
Continue to: How can we efficiently approach the postoperative void trial for pelvic floor surgery?
How can we efficiently approach the postoperative void trial for pelvic floor surgery?
Chao L, Mansuria S. Postoperative bladder filling after outpatient laparoscopic hysterectomy and time to discharge: a randomized controlled trial. Obstet Gynecol. 2019;133:879-887.
Despite efforts to implement and promote enhanced recovery after surgery pathways, waiting for spontaneous void can be a barrier to efficient same-day discharge. Chao and Mansuria conducted a randomized controlled trial (RCT) to determine whether backfilling the bladder intraoperatively, compared with spontaneous (physiologic) filling, would reduce time to discharge in patients undergoing total laparoscopic hysterectomy (TLH) or supracervical hysterectomy (SCH).

Study details

Women undergoing TLH or laparoscopic SCH for benign indications were randomly assigned to undergo either a backfill-assisted void trial in the operating room with 200 mL of sterile normal saline (n = 75) or Foley catheter removal with spontaneous fill in the postanesthesia care unit (PACU) (n = 78).
For both groups, the maximum time allowed for spontaneous void was 5 hours. A successful void trial was defined as a voided volume of at least 200 mL. If a patient was unable to void at least 200 mL, a bladder scan was performed, and the patient was considered to have failed the void trial if a PVR of 200 mL or greater was noted. If the PVR was less than 200 mL, the patient was given an additional 1 hour to spontaneously void 200 mL by 6 hours after the surgery. Patients who failed the void trial were discharged home with a transurethral catheter.
The primary outcome was time to discharge, and the sample size (153 participants included in the analysis) allowed 80% power to detect a 30-minute difference in time to discharge. Participant baseline characteristics, concomitant procedures, and indication for hysterectomy were similar for both groups.
Results. The mean time to discharge was 273.4 minutes for the backfill-assisted void trial group and 283.2 minutes for the spontaneous fill group, a difference of 9.8 minutes that was not statistically significant (P = .45).
Although it was not a primary outcome, time to spontaneous void was 24.9 minutes shorter in the backfill group (P = .04). Rates of postoperative voiding dysfunction did not differ between the 2 groups (6.7% for the backfill group and 12.8% for the spontaneous fill group; P = .2). There were no significant differences in emergency department visits, UTI rates, or readmissions.
Bladder backfill is safe, simple, and may reduce time to spontaneous void
Strengths of the study included its prospective randomized design, blinded outcome assessors, and diversity in benign gynecologic surgeries performed. Although this study found a reduced time to spontaneous void in the backfill group, it was not powered to assess this difference, limiting ability to draw conclusions from those data. Data on postoperative nausea and pain scores also were not collected, which likely influenced the overall time to discharge.
Void trial completion is one of many criteria to fulfill prior to patient discharge, and a reduced time to first void may not decrease the overall length of PACU stay if other factors, such as nausea or pain, are not controlled. Nonetheless, backfilling the bladder intraoperatively is a safe alternative that may decrease the time to first spontaneous void, and it is a relatively simple alteration in the surgical workflow that could significantly lessen PACU nursing demands.
Backfilling the bladder in the operating room prior to catheter discontinuation can reduce time to first spontaneous void, but not the overall time to discharge.
Continue to: Algorithm assesses need for PVR, although further study required...
Algorithm assesses need for PVR, although further study required
Meekins AR, Siddiqui N, Amundsen CL, et al. Improving postoperative efficiency: an algorithm for expedited void trials after urogynecologic surgery. South Med J. 2017;110:785-790.
To determine ways to further maximize postoperative efficiency, Meekins and colleagues sought to determine whether certain voided volumes during backfill-assisted void trials could obviate the need for PVR assessment.
Void trial results calculated to develop algorithm
The study was a secondary analysis of a previously conducted RCT that assessed antibiotics for the prevention of UTI after urogynecologic surgery. Void trials from the parent RCT were performed via the backfill-assisted method in which the bladder was backfilled in the PACU with 300 mL of normal saline or until the patient reported urgency to void, after which the catheter was removed and the patient was prompted to void immediately.
Postvoid residual levels were assessed via ultrasonography or catheterization. A void trial was considered to be passed when a PVR was less than 100 mL or less than 50% of the total bladder volume, with a minimum voided volume of 200 mL.
In the follow-up study, the authors analyzed the void trial results of 255 women of the original 264 in the parent RCT. A total of 69% of patients passed their void trial. The authors assessed the optimal positive predictive value (PPV) and negative predictive value (NPV) combinations, which were then used to create lower and upper voided volume thresholds that would best predict a failed or passed trial, thus obviating PVR measurement.
Results. When patients voided less than 100 mL, the NPV was 96.7% (meaning that they had a 96.7% chance of failing the void trial). When patients voided 200 mL or more, the PPV was 97% (meaning that they had a 97% chance of passing the void trial). Receiver operating characteristic analysis confirmed that voided volume alone was an excellent predictor of final void trial results, with area under the curve of 0.97. The authors estimated that applying this algorithm to their study population would have eliminated the need for assessing PVR in 85% of patients. Ultimately, they proposed the algorithm shown in TABLE 1.

A potential alternative for assessing PVR
This study's strengths include the use of prospectively and systematically collected void trial data in a large patient population undergoing various urogynecologic procedures. By contrast, the generalizability of the results is limited regarding other void trial methods, such as spontaneous filling and void, as well as populations outside of the studied institution.
With the algorithm, the authors estimated that the majority of postoperative patients would no longer require a PVR assessment in the PACU. This could have beneficial downstream implications, including decreasing the nursing workload, reducing total time in the PACU, and minimizing patient discomfort with PVR assessment.
While further studies are needed to validate the proposed algorithm in larger populations, this study provides evidence of an efficient alternative to the traditional approach to PVR assessment in the PACU.
Application of the algorithm proposed by the study investigators has the potential to eliminate the need for a PVR assessment in most patients following a backfill-assisted void trial.
Continue to: An alternative to Foley use if a patient does not know CISC...
An alternative to Foley use if a patient does not know CISC
Boyd SS, O'Sullivan DM, Tunitsky-Bitton E. A comparison of two methods of catheter management after pelvic reconstructive surgery: a randomized controlled trial. Obstet Gynecol. 2019;134:1037-1045.
The traditional indwelling catheter as a postoperative bladder drainage method has a number of drawbacks, including an increased rate of UTI, patient discomfort, and potential limitations in mobility due to the presence of a drainage bag.5
Boyd and colleagues reported on a variation of traditional transurethral catheterization that hypothetically allows for improved mobility. With this method, the transurethral catheter is occluded with a plastic plug that is intermittently plugged and unplugged (plug-unplug method) for bladder drainage. To test whether activity levels are improved with the plug-unplug method versus the continuous drainage approach, the authors conducted an RCT in women undergoing pelvic reconstructive surgery to compare the plug-unplug method with transurethral catheterization (with a continuous drainage bag) and a reference group of freely voiding women.
Study particulars and outcomes
The trial's primary outcome was the patients' activity score as measured by the Activity Assessment Scale (AAS) at 5 to 7 days postoperatively. Because of the theoretically increased risk of a UTI with opening and closing a closed drainage system, secondary outcomes included the UTI rate, the time to pass an outpatient void trial, postoperative pain, patient satisfaction, and catheter effect. To detect an effect size of 0.33 in the primary outcome between the 3 groups, 90 participants were needed along with a difference in proportions of 0.3 between the catheterized and noncatheterized groups.
The participants were randomly assigned 1:1 preoperatively to the continuous drainage or plug-unplug method. All patients underwent a backfill-assisted void trial prior to hospital discharge; the first 30 randomly assigned patients to pass their void trial comprised the reference group. Patients in the plug-unplug arm were instructed to uncap the plastic plug to drain their bladder when they felt the urge to void or at least every 4 hours. All catheterized patients were provided with a large drainage bag for gravity-based drainage for overnight use.
Participants who were discharged home with a catheter underwent an outpatient void trial between postoperative days 5 and 7. A urinalysis was performed at that time and a urine culture was done if a patient reported UTI symptoms. All patients underwent routine follow-up until they passed the office void trial.
Results. Ninety-three women were included in the primary analysis. There were no differences in baseline characteristics between groups. No difference was detected in activity by AAS scores between all 3 groups (scores: plug-unplug, 70.3; continuous drainage, 67.7; reference arm, 79.4; P = .09). The 2 treatment arms had no overall difference in culture-positive UTI (plug-unplug, 68.8%; continuous drainage, 48.4%; P = .625). No significant difference was found in the percentage of patients who passed their initial outpatient void trial (plug-unplug, 71.9%, vs continuous drainage, 58.1%; P = .25) (TABLE 2).

Catheter impact on postoperative activity considered
Strengths of the study include the prospective randomized design, the inclusion of a noncatheterized reference arm, and use of a validated questionnaire to assess activity. The study was limited, however, by the inability to blind patients to treatment and the lack of power to assess other important outcomes, such as UTI rates.
Although the authors did not find a difference in activity scores between the 2 catheterization methods, no significant difference was found between the catheterized and noncatheterized groups, which suggests that catheters in general may not significantly impact postoperative activity. The theoretical concern that opening and closing a transurethral drainage system would increase UTI rates was not substantiated, although the study was not powered specifically for this outcome.
Ultimately, the plug-unplug method may be a safe alternative for patients who desire to avoid attachment to a drainage bag postoperatively.
Based on the results of an RCT that compared 2 methods of catheter management after pelvic reconstructive surgery, the plug-unplug catheterization method may be an acceptable alternative to traditional catheterization.
- Bladder backfill in the operating room followed by spontaneous void in the postanesthesia care unit (PACU) is a safe and efficient way to assess for postoperative voiding dysfunction.
- Voids of 200 mL or more (following a 300-mL backfill) may not require a PACU postvoid residual assessment.
- Postoperative activity does not appear to be impacted by the presence of an indwelling catheter.
Continue to: Does antibiotic prophylaxis reduce UTI for patients catheter-managed postoperatively?
Does antibiotic prophylaxis reduce UTI for patients catheter-managed postoperatively?
Lavelle ES, Alam P, Meister M, et al. Antibiotic prophylaxis during catheter-managed postoperative urinary retention after pelvic reconstructive surgery: a randomized controlled trial. Obstet Gynecol. 2019;134:727-735.
Limited high-quality evidence supports the use of prophylactic antibiotics during catheterization following prolapse or incontinence surgery, and the Infectious Disease Society of America cautions against routine antibiotic prophylaxis for those requiring catheterization.11
Lavelle and colleagues conducted a multicenter RCT to determine whether nitrofurantoin is more effective than placebo in decreasing UTIs among patients with postoperative voiding dysfunction following surgery for prolapse or incontinence.
Focus of the study
The investigators conducted a double-blind RCT at 5 academic sites that included women with postoperative voiding dysfunction who required catheter management (transurethral indwelling catheter or CISC). Voiding dysfunction was diagnosed by backfill or spontaneous fill void trial and was defined as a PVR of greater than 100 mL. Women were randomly assigned 1:1 to nitrofurantoin 100 mg or placebo taken daily during catheter use. Catheter use was discontinued once an outpatient void trial confirmed efficient voiding.
The primary outcome was symptomatic culture-confirmed UTI within 6 weeks of surgery. Secondary outcomes included frequency of urine cultures with nitrofurantoin-resistant or intermediate-sensitivity isolates and adverse symptoms possibly related to nitrofurantoin. The authors calculated that 154 participants would provide 80% power to detect a decrease in UTI incidence from 33% to 13%, allowing for 10% dropout.
A total of 151 women were randomly assigned and included in the intention-to-treat analysis. There were no differences in baseline characteristics. The median duration of catheter use was 4 days (interquartile range, 3-7).
Results. Overall, 13 women in the nitrofurantoin group and 13 in the placebo group experienced the primary outcome of UTI within 6 weeks postoperatively (17.3% nitrofurantoin vs 17.1% placebo; P = .97; relative risk [RR], 1.01; 95% confidence interval [CI], 0.50-2.04). The number needed to treat with nitrofurantoin to prevent 1 UTI was 500. A subanalysis found no difference in UTI incidence stratified by CISC versus indwelling catheter.
Urine cultures were obtained for 94.5% of all patients reporting UTI symptoms. Four isolates of the 13 cultures in the nitrofurantoin group (30.8%) and 3 in the placebo group (21.4%) showed nitrofurantoin resistance (P = .58). The rate of endorsing at least 1 adverse symptom attributable to nitrofurantoin was similar between groups (68.0% vs 60.5%, respectively; P = .34).
Study strong points and limitations
This study's randomized, placebo-controlled design and multicenter recruitment increase the generalizability of the results. An additional strength is that the authors chose a clinically relevant definition of UTI. The study was likely underpowered, however, to detect differences in secondary outcomes, such as nitrofurantoin resistance. We cannot conclude on the role of antibiotics for patients who require more prolonged catheterization.
Notably, a similar RCT by Dieter and colleagues of 159 patients undergoing daily nitrofurantoin versus placebo during CISC or transurethral catheterization failed to detect a difference in the rate of UTI treatment up to 3 weeks postoperatively with nitrofurantoin prophylaxis.12
Ultimately, the study by Lavelle and colleagues contributes to a growing body of evidence that supports the avoidance of antibiotic prophylaxis during short-term postoperative catheterization.
Nitrofurantoin prophylaxis did not reduce the incidence of postoperative UTI in patients with catheter-managed postoperative voiding dysfunction.
- Prophylactic antibiotics are not necessary for short-term catheterization in postoperative patients.
- Baessler K, Maher C. Pelvic organ prolapse surgery and bladder function. Int Urogynecol J. 2013;24:1843-1852.
- Yune JJ, Cheng JW, Wagner H, et al. Postoperative urinary retention after pelvic organ prolapse repair: vaginal versus robotic transabdominal approach. Neurourol Urodyn. 2018;37:1794-1800.
- Ghezzi F, Cromi A, Uccella S, et al. Immediate Foley removal after laparoscopic and vaginal hysterectomy: determinants of postoperative urinary retention. J Minim Invasive Gynecol. 2007;14:706-711.
- Smorgick N, DeLancey J, Patzkowsky K, et al. Risk factors for postoperative urinary retention after laparoscopic and robotic hysterectomy for benign indications. Obstet Gynecol. 2012;120:581-586.
- Dieter AA, Amundsen CL, Visco AG, et al. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18:175-178.
- Tunitsky-Bitton E, Murphy A, Barber MD, et al. Assessment of voiding after sling: a randomized trial of 2 methods of postoperative catheter management after midurethral sling surgery for stress urinary incontinence in women. Am J Obstet Gynecol. 2015;212:597.e1-e9.
- Kandadai P, Saini J, Patterson D, et al. Urinary retention after hysterectomy and postoperative analgesic use. Female Pelvic Med Reconstr Surg. 2015;21:257-262.
- Liang CC, Lee CL, Chang TC, et al. Postoperative urinary outcomes in catheterized and non-catheterized patients undergoing laparoscopic-assisted vaginal hysterectomy--a randomized controlled trial. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:295-300.
- Foster RT Sr, Borawski KM, South MM, et al. A randomized, controlled trial evaluating 2 techniques of postoperative bladder testing after transvaginal surgery. Am J Obstet Gynecol. 2007;197:627.e1-e4.
- Geller EJ, Hankins KJ, Parnell BA, et al. Diagnostic accuracy of retrograde and spontaneous voiding trials for postoperative voiding dysfunction: a randomized controlled trial. Obstet Gynecol. 2011;118:637-642.
Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Disease Society of America. Clin Infect Dis. 2010;50:625-663.
Dieter AA, Amundsen CL, Edenfield AL, et al. Oral antibiotics to prevent postoperative urinary tract infection: a randomized controlled trial. Obstet Gynecol. 2014;123:96-103.
- Baessler K, Maher C. Pelvic organ prolapse surgery and bladder function. Int Urogynecol J. 2013;24:1843-1852.
- Yune JJ, Cheng JW, Wagner H, et al. Postoperative urinary retention after pelvic organ prolapse repair: vaginal versus robotic transabdominal approach. Neurourol Urodyn. 2018;37:1794-1800.
- Ghezzi F, Cromi A, Uccella S, et al. Immediate Foley removal after laparoscopic and vaginal hysterectomy: determinants of postoperative urinary retention. J Minim Invasive Gynecol. 2007;14:706-711.
- Smorgick N, DeLancey J, Patzkowsky K, et al. Risk factors for postoperative urinary retention after laparoscopic and robotic hysterectomy for benign indications. Obstet Gynecol. 2012;120:581-586.
- Dieter AA, Amundsen CL, Visco AG, et al. Treatment for urinary tract infection after midurethral sling: a retrospective study comparing patients who receive short-term postoperative catheterization and patients who pass a void trial on the day of surgery. Female Pelvic Med Reconstr Surg. 2012;18:175-178.
- Tunitsky-Bitton E, Murphy A, Barber MD, et al. Assessment of voiding after sling: a randomized trial of 2 methods of postoperative catheter management after midurethral sling surgery for stress urinary incontinence in women. Am J Obstet Gynecol. 2015;212:597.e1-e9.
- Kandadai P, Saini J, Patterson D, et al. Urinary retention after hysterectomy and postoperative analgesic use. Female Pelvic Med Reconstr Surg. 2015;21:257-262.
- Liang CC, Lee CL, Chang TC, et al. Postoperative urinary outcomes in catheterized and non-catheterized patients undergoing laparoscopic-assisted vaginal hysterectomy--a randomized controlled trial. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:295-300.
- Foster RT Sr, Borawski KM, South MM, et al. A randomized, controlled trial evaluating 2 techniques of postoperative bladder testing after transvaginal surgery. Am J Obstet Gynecol. 2007;197:627.e1-e4.
- Geller EJ, Hankins KJ, Parnell BA, et al. Diagnostic accuracy of retrograde and spontaneous voiding trials for postoperative voiding dysfunction: a randomized controlled trial. Obstet Gynecol. 2011;118:637-642.
Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Disease Society of America. Clin Infect Dis. 2010;50:625-663.
Dieter AA, Amundsen CL, Edenfield AL, et al. Oral antibiotics to prevent postoperative urinary tract infection: a randomized controlled trial. Obstet Gynecol. 2014;123:96-103.
Botox: A new option for endometriosis pain?
NATIONAL HARBOR, MD. – Botulinum toxin injection into the vagina appears to relieve pain associated with endometriosis by relaxing the pelvic floor muscles, new research suggests.
In a randomized study, women with surgically diagnosed endometriosis who had chronic pelvic pain despite optimal surgical and hormonal treatment had less pain after injection vs their counterparts who received placebo.
This result suggests pelvic floor spasm may be an important factor in endometriosis-associated pelvic pain, the investigators note.
“Botulinum toxin injection offers an alternative approach for women with pelvic pain,” lead author Barbara Illowsky Karp, MD, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Md., told Medscape Medical News.
“We focused on endometriosis, but there are reasons to think it may be effective for pelvic pain from other causes if there is spasm of the muscle,” said Karp, a neurologist who has used botulinum toxin therapeutically since it was first developed in the 1980s.
She noted that it is unknown whether the toxin will work in women who do not have actual spasm, “but the effect on spasm is not the sole effect of toxin,” as demonstrated by its use in other pain conditions.
“It seems to have a direct effect on the pain pathways in the nervous system as well,” Karp added.
The study findings were presented here at the American Academy of Pain Medicine (AAPM) 2020 Annual Meeting.
Less pain
The investigators randomly assigned 29 women between the ages of 18 and 50 years to receive injections with 100 U onabotulinumtoxinA (n = 15) or saline placebo (n = 14).
All of the women had endometriosis with chronic pelvic pain lasting at least 3 months (mean time, 6 years) and confirmed pelvic floor spasm as a main pain generator.
One month after treatment, participants were asked if they had improvement in their pain.
“Our primary outcome was just simply asking the women if they felt better or not, because we were blinded as to what treatment they received. One month is typically when the toxin reaches its maximal effect,” Karp said.
At 1 month, 11 women in the placebo group reported that they had no benefit, compared with only 4 women in the botulinum toxin group (P = .027).
The botulinum toxin group reported a greater degree of benefit compared with the placebo group (P = .030) and greater percent of improvement (P = .034).
Neither group reported substantial changes in pain rating on the visual analog scale. However, a definite pain score is often difficult to measure in women with chronic pelvic pain, coinvestigator Pamela Stratton, MD, a gynecologist in Bethesda, said.
“Some women report their pain as a 2, some as an 8. Also, women may have a lot of pain one day and not have that much pain another day, and how do you measure that? This is why we have not focused solely on the pain score but instead wanted the women to tell us if they were improved or not,” Stratton told Medscape Medical News.
Disability worsened considerably in the placebo group, but remained consistent in the botulinum toxin group. Five patients in the botulinum toxin group were able to reduce pain medication compared with one patient in the placebo group.
“Compelling” findings but early days
Commenting on the findings for Medscape Medical News, Ann E. Hansen, MD, Chronic Pain Wellness Center, Phoenix VA Health Care System, Arizona, noted that this “preliminary study” showed some benefit for a complex and challenging-to-treat syndrome.
“Injection of botulinum toxin prevents local muscle contraction, thus effectively relieving a variety of neuromuscular conditions such as torticollis; spasticity; pain syndromes such as headache and migraine; and some neurologic disorders, for instance, overactive bladder,” said Hansen, who was not involved with the research.
“Using botulinum toxin injection to target pelvic floor muscle spasm, a known pain generator in women suffering from chronic pelvic pain, makes sense. Future studies will be helpful in elucidating optimal treatment protocols for this debilitating condition,” she added.
Also commenting for Medscape Medical News, Kathryn T. Hall, PhD, MPH, Brigham & Women’s Hospital, Boston, Massachusetts, called the results “quite compelling” although, “it’s still early days.”
“It remains to be seen if the treatment effect will endure or if side effects will emerge. Hopefully all will go well,” Hall said.
The study was funded by an unrestricted grant from the National Institutes of Health. Allergan provided the botulinum toxin that was used in the study. Karp, Stratton, Hansen, and Hall have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
NATIONAL HARBOR, MD. – Botulinum toxin injection into the vagina appears to relieve pain associated with endometriosis by relaxing the pelvic floor muscles, new research suggests.
In a randomized study, women with surgically diagnosed endometriosis who had chronic pelvic pain despite optimal surgical and hormonal treatment had less pain after injection vs their counterparts who received placebo.
This result suggests pelvic floor spasm may be an important factor in endometriosis-associated pelvic pain, the investigators note.
“Botulinum toxin injection offers an alternative approach for women with pelvic pain,” lead author Barbara Illowsky Karp, MD, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Md., told Medscape Medical News.
“We focused on endometriosis, but there are reasons to think it may be effective for pelvic pain from other causes if there is spasm of the muscle,” said Karp, a neurologist who has used botulinum toxin therapeutically since it was first developed in the 1980s.
She noted that it is unknown whether the toxin will work in women who do not have actual spasm, “but the effect on spasm is not the sole effect of toxin,” as demonstrated by its use in other pain conditions.
“It seems to have a direct effect on the pain pathways in the nervous system as well,” Karp added.
The study findings were presented here at the American Academy of Pain Medicine (AAPM) 2020 Annual Meeting.
Less pain
The investigators randomly assigned 29 women between the ages of 18 and 50 years to receive injections with 100 U onabotulinumtoxinA (n = 15) or saline placebo (n = 14).
All of the women had endometriosis with chronic pelvic pain lasting at least 3 months (mean time, 6 years) and confirmed pelvic floor spasm as a main pain generator.
One month after treatment, participants were asked if they had improvement in their pain.
“Our primary outcome was just simply asking the women if they felt better or not, because we were blinded as to what treatment they received. One month is typically when the toxin reaches its maximal effect,” Karp said.
At 1 month, 11 women in the placebo group reported that they had no benefit, compared with only 4 women in the botulinum toxin group (P = .027).
The botulinum toxin group reported a greater degree of benefit compared with the placebo group (P = .030) and greater percent of improvement (P = .034).
Neither group reported substantial changes in pain rating on the visual analog scale. However, a definite pain score is often difficult to measure in women with chronic pelvic pain, coinvestigator Pamela Stratton, MD, a gynecologist in Bethesda, said.
“Some women report their pain as a 2, some as an 8. Also, women may have a lot of pain one day and not have that much pain another day, and how do you measure that? This is why we have not focused solely on the pain score but instead wanted the women to tell us if they were improved or not,” Stratton told Medscape Medical News.
Disability worsened considerably in the placebo group, but remained consistent in the botulinum toxin group. Five patients in the botulinum toxin group were able to reduce pain medication compared with one patient in the placebo group.
“Compelling” findings but early days
Commenting on the findings for Medscape Medical News, Ann E. Hansen, MD, Chronic Pain Wellness Center, Phoenix VA Health Care System, Arizona, noted that this “preliminary study” showed some benefit for a complex and challenging-to-treat syndrome.
“Injection of botulinum toxin prevents local muscle contraction, thus effectively relieving a variety of neuromuscular conditions such as torticollis; spasticity; pain syndromes such as headache and migraine; and some neurologic disorders, for instance, overactive bladder,” said Hansen, who was not involved with the research.
“Using botulinum toxin injection to target pelvic floor muscle spasm, a known pain generator in women suffering from chronic pelvic pain, makes sense. Future studies will be helpful in elucidating optimal treatment protocols for this debilitating condition,” she added.
Also commenting for Medscape Medical News, Kathryn T. Hall, PhD, MPH, Brigham & Women’s Hospital, Boston, Massachusetts, called the results “quite compelling” although, “it’s still early days.”
“It remains to be seen if the treatment effect will endure or if side effects will emerge. Hopefully all will go well,” Hall said.
The study was funded by an unrestricted grant from the National Institutes of Health. Allergan provided the botulinum toxin that was used in the study. Karp, Stratton, Hansen, and Hall have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
NATIONAL HARBOR, MD. – Botulinum toxin injection into the vagina appears to relieve pain associated with endometriosis by relaxing the pelvic floor muscles, new research suggests.
In a randomized study, women with surgically diagnosed endometriosis who had chronic pelvic pain despite optimal surgical and hormonal treatment had less pain after injection vs their counterparts who received placebo.
This result suggests pelvic floor spasm may be an important factor in endometriosis-associated pelvic pain, the investigators note.
“Botulinum toxin injection offers an alternative approach for women with pelvic pain,” lead author Barbara Illowsky Karp, MD, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Md., told Medscape Medical News.
“We focused on endometriosis, but there are reasons to think it may be effective for pelvic pain from other causes if there is spasm of the muscle,” said Karp, a neurologist who has used botulinum toxin therapeutically since it was first developed in the 1980s.
She noted that it is unknown whether the toxin will work in women who do not have actual spasm, “but the effect on spasm is not the sole effect of toxin,” as demonstrated by its use in other pain conditions.
“It seems to have a direct effect on the pain pathways in the nervous system as well,” Karp added.
The study findings were presented here at the American Academy of Pain Medicine (AAPM) 2020 Annual Meeting.
Less pain
The investigators randomly assigned 29 women between the ages of 18 and 50 years to receive injections with 100 U onabotulinumtoxinA (n = 15) or saline placebo (n = 14).
All of the women had endometriosis with chronic pelvic pain lasting at least 3 months (mean time, 6 years) and confirmed pelvic floor spasm as a main pain generator.
One month after treatment, participants were asked if they had improvement in their pain.
“Our primary outcome was just simply asking the women if they felt better or not, because we were blinded as to what treatment they received. One month is typically when the toxin reaches its maximal effect,” Karp said.
At 1 month, 11 women in the placebo group reported that they had no benefit, compared with only 4 women in the botulinum toxin group (P = .027).
The botulinum toxin group reported a greater degree of benefit compared with the placebo group (P = .030) and greater percent of improvement (P = .034).
Neither group reported substantial changes in pain rating on the visual analog scale. However, a definite pain score is often difficult to measure in women with chronic pelvic pain, coinvestigator Pamela Stratton, MD, a gynecologist in Bethesda, said.
“Some women report their pain as a 2, some as an 8. Also, women may have a lot of pain one day and not have that much pain another day, and how do you measure that? This is why we have not focused solely on the pain score but instead wanted the women to tell us if they were improved or not,” Stratton told Medscape Medical News.
Disability worsened considerably in the placebo group, but remained consistent in the botulinum toxin group. Five patients in the botulinum toxin group were able to reduce pain medication compared with one patient in the placebo group.
“Compelling” findings but early days
Commenting on the findings for Medscape Medical News, Ann E. Hansen, MD, Chronic Pain Wellness Center, Phoenix VA Health Care System, Arizona, noted that this “preliminary study” showed some benefit for a complex and challenging-to-treat syndrome.
“Injection of botulinum toxin prevents local muscle contraction, thus effectively relieving a variety of neuromuscular conditions such as torticollis; spasticity; pain syndromes such as headache and migraine; and some neurologic disorders, for instance, overactive bladder,” said Hansen, who was not involved with the research.
“Using botulinum toxin injection to target pelvic floor muscle spasm, a known pain generator in women suffering from chronic pelvic pain, makes sense. Future studies will be helpful in elucidating optimal treatment protocols for this debilitating condition,” she added.
Also commenting for Medscape Medical News, Kathryn T. Hall, PhD, MPH, Brigham & Women’s Hospital, Boston, Massachusetts, called the results “quite compelling” although, “it’s still early days.”
“It remains to be seen if the treatment effect will endure or if side effects will emerge. Hopefully all will go well,” Hall said.
The study was funded by an unrestricted grant from the National Institutes of Health. Allergan provided the botulinum toxin that was used in the study. Karp, Stratton, Hansen, and Hall have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.