User login
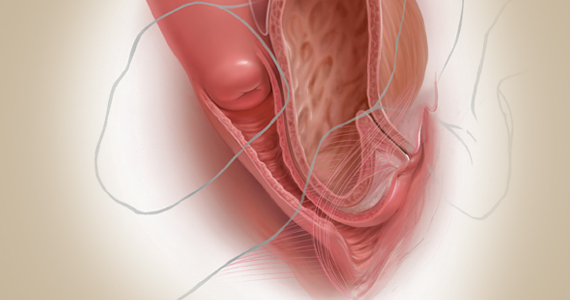
Exploring options for POP treatment: Patient selection, surgical approaches, and ways to manage risks
A number of presentations at the 2019 Pelvic Anatomy and Gynecologic Surgery (PAGS) Symposium (Las Vegas, Nevada, December 12-14, 2019) focused on pelvic organ prolapse (POP) repair, including anatomic considerations, the evolution of surgical procedures, and transvaginal repair. OBG M
Nonsurgical approaches for POP: A good option for the right patient
John B. Gebhart, MD, MS: What are the nonsurgical options for POP?
Mark D. Walters, MD: Women who have prolapse could, of course, choose to continue to live with the prolapse. If they desire treatment, however, the main nonsurgical option is a combination of pessary use, possibly with some estrogen, and possibly with pelvic muscle exercises. Women who have a well-fitting pessary can be managed satisfactorily for years. If possible, women should be taught to take the pessary in and out on a regular basis to minimize their long-term complications.
Dr. Gebhart: How can nonsurgical treatment options be maximized?
Beri M. Ridgeway, MD: It depends on patient commitment. This is important to assess at the first visit when you are making management decisions, because if someone is not going to attend physical therapy or not going to continue to do the exercises, the expectation for the outcome is not going to be great.
Also, if a patient feels very uncomfortable using a pessary and really does not want it, I am fine proceeding with surgery as a first-line treatment. If the patient is committed, the ideal is to educate her and connect her with the right people, either a pelvic floor physical therapist or someone in your office who will encourage her and manage pessary use.
Dr. Gebhart: It goes back to assessing patient goals and expectations.
Mickey M. Karram, MD: If you have a patient who is a good candidate for a pessary—say she has a well-supported distal vagina and maybe a cervical prolapse or an apical prolapse—and you can fit a small pessary that will sit in the upper vagina in a comfortable fashion, it is worthwhile to explain to the patient that she is a really good candidate for this option. By contrast, someone who has a wide genital hiatus and a large rectocele will not have good success with a pessary.
Dr. Gebhart: That is important: Choose your nonsurgical patients well, those who will respond to therapy and maybe not get frustrated with it.
Dr. Walters: A problem I see is that some people are good at fitting a pessary, but they do not teach how to use it very well. When I see the patient back, she says, “What’s my long term on the pessary?” I say, “If we teach you to take it in and out, you are less likely to have any problems with it, and then you can manage it for years that way. Otherwise, you have to keep visiting a practitioner to change it and that is not necessarily a good long-term option.” At the very first visit, I teach them what a pessary is, its purpose, and how to maintain it themselves. I think that gives patients the best chance for long-term satisfaction.
Dr. Gebhart: Surgery is always an option if pessary management is not satisfactory.
Dr. Ridgeway: I also tell patients, especially those uncertain about using a pessary, “Worst case, you spend a little time to figure this out, but if it works, you can avoid surgery. If it doesn’t—the risks are very low and you perhaps wasted some time—but at least you’ll know you tried the conservative management.”
Dr. Gebhart: Mickey made an excellent point earlier that it can be a diagnostic treatment strategy as well.
Dr. Karram: If you are concerned about the prolapse worsening or negatively impacting a functional problem related to the bladder or bowel, it is good to place a pessary for a short period of time. This can potentially give you an idea of how your surgery will impact a patient’s bladder or bowel function.
Continue to: Decisions to make before choosing a surgical approach...
Decisions to make before choosing a surgical approach
Dr. Gebhart: Would you elaborate on the surgical options for managing POP?
Dr. Walters: For women with prolapse who decide they want to have surgery, the woman and the surgeon need to make a number of decisions. Some of these include whether the uterus, if present, needs to be removed; whether the woman would like to maintain sexual function or not; whether the repair would best be done vaginally only with native tissue suturing, vaginally with some augmentation (although that is not likely in the United States at this time), or through the abdomen, usually laparoscopically or robotically with a mesh-augmented sacrocolpopexy repair.
Also, we must decide whether to do additional cystocele and rectocele repairs and whether to add slings for stress incontinence, which can coexist or could develop after the prolapse repair. A lot of different decisions need to be made when choosing a prolapse repair for different women.
Dr. Ridgeway: It is shared decision-making with the patient. You need to understand her goals, the degree of prolapse, whether she has contraindications to uterine preservation, and how much risk she is willing to take.
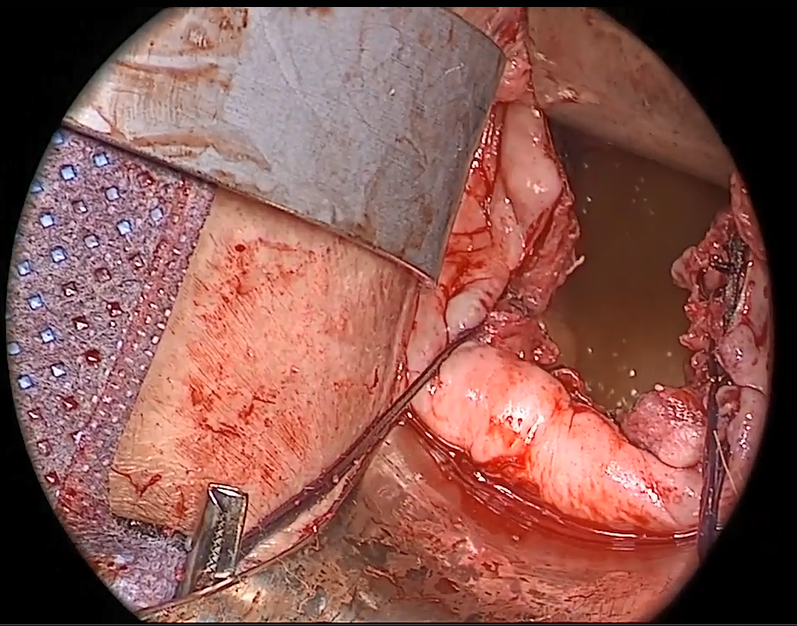
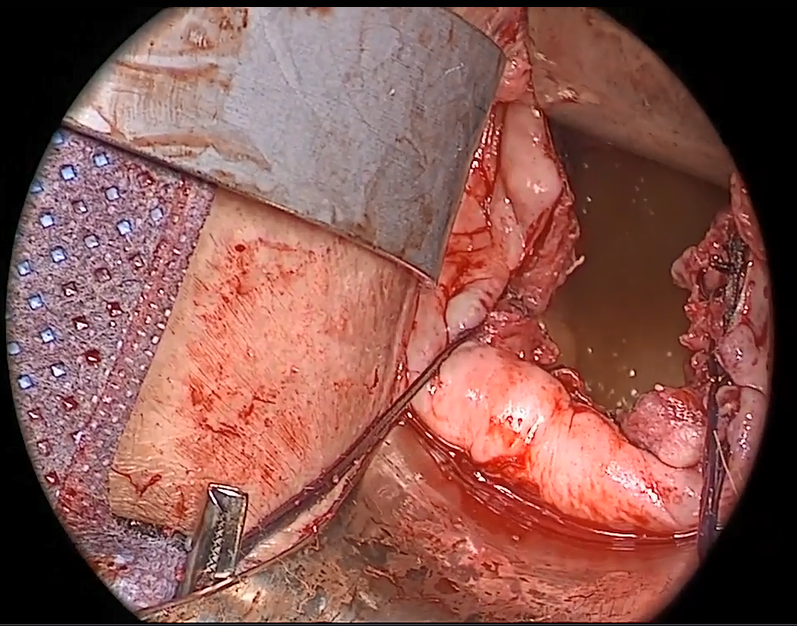
Fundamentals of the clinical evaluation
Dr. Gebhart: For a woman who wants to manage her prolapse surgically, let us consider some fundamentals of clinical diagnosis. Take me through your office evaluation of the patient reporting prolapse symptoms—her history, yes, but from a physical exam standpoint, what is important?
Dr. Karram: You want to know if this is a primary prolapse or recurrent prolapse. You want to distinguish the various segments of the pelvic floor that are prolapsing and try to quantitate that in whatever way you would like. A standardized quantification system is useful, but you should have a system within your practice that you can standardize. Then, determine if there are coexisting functional derangements and how those are being impacted by the prolapse, because that is very important.
Take a good history, and identify how badly the prolapse bothers the patient and affects her quality of life. Understand how much she is willing to do about it. Does she just want to know what it is and has no interest in a surgical intervention, versus something she definitely wants to get corrected? Then do whatever potential testing around the bladder, and bowel, based on any functional derangements and finally determine interest in maintaining sexual function. Once all this information is obtained, a detailed discussion of surgical options can be undertaken.
Dr. Gebhart: What are your clinical pearls for a patient who has prolapse and does not describe any incontinence, voiding dysfunction, or defecatory symptoms? Do we need imaging testing of any sort or is the physical exam adequate for assessing prolapse?
Dr. Walters: When you do the standardized examination of the prolapse, it is important to measure how much prolapse affects the anterior wall of the apex and/or cervix and the posterior wall. Then note that in your notes and plan your surgery accordingly.
It is useful to have the patient fully bear down and then make your measurements; then, especially if she has a full bladder, have her cough while you hold up the prolapse with a speculum or your hand to see if she has stress urinary incontinence.
Continue to: I agree that to diagnose prolapse...
Dr. Ridgeway: I agree that to diagnose prolapse, it is physical exam alone. I would not recommend any significant testing other than testing for the potential for stress incontinence.
Dr. Gebhart: Is it necessary to use the POP-Q (Pelvic Organ Prolapse Quantification system) in a nonacademic private practice setting? Or are other systems, like a Baden-Walker scoring system, adequate in the everyday practice of the experienced generalist?
Dr. Walters: The Baden-Walker system actually is adequate for use in everyday practice. However, Baden-Walker is an outdated measurement system that really is not taught anymore. I think that as older physicians finish and newer doctors come in, no one will even know what Baden-Walker is.
It is better to go ahead and start learning the POP-Q system. Everyone has electronic charts now and if you learn to use the POP-Q, you can do it very quickly and get a grading system for your chart that is reproducible for everyone.
Dr. Ridgeway: The most important thing is to assess all 3 compartments and document the amount of prolapse of each compartment. A modified POP-Q is often adequate. To do this, perform a split speculum exam and use the hymen as the reference. Zero is at the hymen, +1 is 1 cm beyond the hyman. Covering the rectum, how much does the anterior compartment prolapse in reference to the hymen? Covering the anterior compartment, get an idea of what is happening posteriorly. And the crux of any decision in my mind is what is happening at the apex or to the uterus/cervix if it is still present. It is really important to document at least those 3 compartments.
Dr. Karram: I agree. The POP-Q is the ideal, but I don’t think generalists are motivated to use it. It is very important, though, to have some anatomic landmarks, as already mentioned by Dr. Ridgeway.
Choose a surgical approach based on the clinical situation
Dr. Gebhart: How do you choose the surgical approach for someone with prolapse?
Dr. Karram: Most surgeons do what they think they do best. I have spent the majority of my career operating through the vagina, and most of that involves native tissue repairs. I almost always will do a primary prolapse through the vagina and not consider augmentation except in rare circumstances. A recurrent prolapse, a prolapsed shortened vagina, scarring, or a situation that is not straightforward has to be individualized. My basic intervention initially is almost always vaginally with native tissue.
Dr. Ridgeway: For a primary prolapse repair, I also will almost always use native tissue repair as firstline. Whether that is with hysterectomy or without, most people in the long term do very well with that. At least 70% of my repairs are done with a native tissue approach.
For a woman who has a significant prolapse posthysterectomy, especially of the anterior wall or with recurrent prolapse, I offer a laparoscopic sacrocolpopexy. The only other time I offer that as a primary approach would be for a younger woman with very significant prolapse. In that case, I will review risks and benefits with the patient and, using shared decision-making, offer either a native tissue repair or a sacrocolpopexy. For that patient, no matter what you do, given that she has many years to live, the chances are that she will likely need a second intervention.
Dr. Gebhart: Mark, how do you choose an approach for prolapse?
Dr. Walters: I do things pretty much the way Dr. Karram and Dr. Ridgeway do. For women who have a primary prolapse, I usually take a vaginal approach, and for recurrences I frequently do sacrocolpopexy with mesh or I refer to one of my partners who does more laparoscopic or robotic sacrocolpopexy.
Whether the patient needs a hysterectomy or not is evolving. Traditionally, hysterectomy is almost always done at the first prolapse repair. That is being reassessed in the United States to match what is happening in some other countries. It is possible to do nice primary prolapse repair vaginally or laparoscopically and leave the uterus in, in selected women who desire that.
Continue to: Transvaginal prolapse repair: Mesh is no longer an option...
Transvaginal prolapse repair: Mesh is no longer an option
Dr. Gebhart: What led up to the US Food and Drug Administration’s (FDA) market removal of mesh for transvaginal repair of POP?
Dr. Ridgeway: To clarify, it was not a recall—a word that many people use—it was an order to stop producing and distributing surgical mesh intended for transvaginal repair of POP.1 There is a very long history. Transvaginal mesh was introduced with the goal of improving prolapse anatomic and subjective outcomes. Over the last 13 years or so, there were adverse events that led to FDA public health notifications. Consequently, these devices were reclassified, and now require additional testing prior to approval. The newest transvaginal mesh kits were studied.
These 522 studies were completed recently and needed to show superior outcomes because, historically, the risks associated with transvaginal mesh compared to those associated with native tissue repairs are higher: higher reoperation rates, higher rates of other complications, and very minimal improvements in subjective and objective outcomes. Data were presented to the FDA, and it was deemed that these mesh kits did not improve outcomes significantly compared with native tissue repairs.
Dr. Karram: Beri, you stated that very accurately. The pro-mesh advocates were taken back by the idea that the FDA made this recommendation without allowing the outcomes to be followed longer.
Dr. Gebhart: My understanding is that the FDA had a timeline where they had to do a report and the studies had not matured to that end point; thus, they had to go with the data they had even though the studies were not completed. I think they are requesting that they be completed.
Dr. Ridgeway: Additional data will be available, some through the 522 studies, others through randomized controlled trials in which patients were already enrolled and had surgery. As far as I know, I do not think that the decision will be reversed.
Continue to: Native tissue repair and failure risk...
Native tissue repair and failure risk
Dr. Gebhart: I hear a lot that native tissue repairs fail. Mickey, as you do a lot of vaginal surgery, what are your thoughts? Should you use augmentation of some sort because native tissue fails?
Dr. Karram: There is going to be a failure rate with whatever surgery you do. I think that the failure rate with native tissue is somewhat overstated. I think a lot of that dates back to some of the things that were being promoted by mesh advocates. Initially, there was a lot of cherry-picking of native tissue data in some of those studies to promote the idea that the recurrent prolapse rates were 40% to 80%. We certainly do not see that in our patient population.
Based on our 5-year data, we have a recurrence rate of about 15% and a reoperation rate of less than 10%. That is the best I can quote based on our data. We have not followed patients longer than 5 years.
I can’t do much better than that with an augmentation; even if I get another 5% or 10% better anatomic outcome, that will be at the expense of some erosions and other complications specific to the mesh. I do think that the native tissue failure rate being promoted by a lot of individuals is a higher failure rate than what we are seeing.
Dr. Gebhart: What do you think, Mark?
Dr. Walters: Large cohort studies both at your institution, Mayo Clinic, and ours at the Cleveland Clinic mirror what Dr. Karram said, in that we have a reoperation rate somewhere between 8% and 15%. Of course, we have some failures that are stage 2 failures where patients choose not to have another operation. In general, a 10% or 12% reoperation rate at 5 to 7 years is acceptable.
Native tissue repairs probably fail at the apex a little more than mesh sacrocolpopexy. Mesh sacrocolpopexy, depending on what else you do with that operation, may have more distal vaginal failures, rates like distal rectoceles and more de novo stress urinary incontinence than we probably get with native tissue. I get some failures of the apex with native tissue repairs, but I am okay with using sacrocolpopexy as the second-line therapy in those patients.
Hysteropexy technique and pros and cons
Dr. Gebhart: Is hysteropexy a fad, or is there something to this?
Dr. Ridgeway: I do not think it is a fad. Women do feel strongly about this, and we now have data supporting this choice: randomized controlled trials of hysterectomy and prolapse repair versus hysteropexy with comparable outcomes at the short and medium term.2
The outcomes are similar, but as we said, outcomes for all prolapse repair types are not perfect. We have recurrences with sacrocolpopexy, native tissue repair, and hysteropexy. We need more data on types of hysteropexy and long-term outcomes for uterine preservation.
Dr. Walters: We have been discussing what patients think of their uterus, and some patients have very strong opinions. Some prefer to have a hysterectomy because then they don’t need to worry about cancer or do screening for cancer, and they are very happy with that. Other women with the same kind of prolapse prefer not to have a hysterectomy because philosophically they think they are better off keeping their organs. Since satisfaction is an outcome, it is useful to know what the patient wants and what she thinks about the surgical procedure.
Dr. Gebhart: For hysteropexy, do the data show that suture or a mesh augment provide an advantage one way or the other? Do we know that yet?
Dr. Walters: No, there are not enough studies with suture. There are only a few very good studies with suture hysteropexy, and they are mostly sacrospinous suture hysteropexies. Only a few studies look at mesh hysteropexy (with the Uphold device that was put on hold), or with variations of uterosacral support using strips of mesh, mostly done in other countries.
A point I want to add, if native tissue repairs fail at the apex more, why don’t you just always do sacrocolpopexy? One reason is because it might have a little higher complication rate due to the abdominal access and the fact that you are putting mesh in. If you have, for example, a 4% complication rate with the mesh but you get a better cure rate, those things balance out, and the woman may not be that much better off because of the extra complications. You have to assess the pro and con with each patient to pick what is best for her—either a more durable repair with a mesh or a little safer repair with native tissue.
Continue to: Women feel very strongly about risk...
Dr. Ridgeway: Women feel very strongly about risk. Within the same clinic I will have similar patients, and I say, “Probably in the long term this one may last a little longer but the surgery takes longer and it has a little higher complication rate.” One patient will say, “I’m not worried about the risk, I want what’s going to last the longest,” whereas a very similar patient will say, “Why would anyone pick the higher-risk operation? I want the lower risk that probably will last a long time.”
Dr. Gebhart: Beri, who should not have a hysteropexy?
Dr. Ridgeway: The biggest factor would be someone who has ever had postmenopausal bleeding. From our data, we know that if they have even had a work-up with benign results, the risk of unanticipated pathology is high. I do not recommend hysteropexy for anyone who has had postmenopausal bleeding.
For a premenopausal woman who has irregular bleeding, I also do not recommend it, because you just do not know what that future will hold. If a patient has anatomic abnormalities like large fibroids, I would not recommend it either. I would like patients to have had standard cervical cancer screening without any abnormalities for about 10 years or so.
Dr. Gebhart: What about prior cervical dysplasia?
Dr. Ridgeway: If a patient had ASCUS or low-grade dysplasia decades ago, has been normal for at least 10 years, and is currently negative for human papillomavirus, I have no problem.
Dr. Gebhart: How about women at high genetic risk for cancer?
Dr. Ridgeway: If they are at high risk for endometrial cancer, I would not recommend hysteropexy. If they are going to need an oophorectomy and/or salpingectomy for risk reduction during prolapse treatment, I usually perform a hysterectomy.
Plan surgical steps and prepare for “what if’s”
Dr. Gebhart: What tips can you provide, either regarding the evaluation or something you do surgically, that are important in a transvaginal native tissue repair?
Dr. Karram: If you have a case of posthysterectomy apical prolapse, that you think is an indication for sacrocolpopexy, in reality these are very good candidates for either sacrospinous or uterosacral suspensions. I prefer a uterosacral suspension as I feel there is less distortion of the vaginal apex compared to a sacrospinous suspension.
Dr. Ridgeway: The most critical step is setting up the OR and positioning the patient. That sets up the case for success, preventing struggles during the case. I use a high lithotomy, with careful positioning of course, but I use candy cane stirrups so that I can have an instrument stand in front of me and not struggle during the case.
Dr. Walters: My tip for everyone who is doing native tissue surgery, whether it is high McCall colpopexy or uterosacral ligament suspension or sacrocolpopexy, would be to really learn well the anatomy of each operation, including how close the ureter is, where the risk for bleeding is, and where the risk for nerve damage is.
The complications for each of these surgeries are slightly different, but there is a small risk of kinking the ureter with both uterosacral ligament suspension and the McCall, so you should do a cystoscopy as part of that operation. If you do a sacrospinous ligament suspension, use an instrument that can get a stitch into a ligament—not too close to the ischial spine and not too close to the sacrum—to avoid the risk of damage to major nerves and blood vessels and to minimize buttock and leg pain.
Continue to: Another tip is to understand...
Dr. Karram: Another tip is to understand that you are going to have potential complications intraoperatively. Think through those presurgically. You do not want to start thinking about these things and making decisions as they are happening. For example, what if I do a uterosacral suspension and I don’t see efflux of urine from the ureter? What am I going to do, and how long am I going to wait before I intervene? If I do a sacrospinous and I start to see a lot of bleeding from that area, what am I going to do? My plan would be, “I will pack the area, get extra suction, etc.” Thinking these ideas through before they occur is very helpful.
Dr. Gebhart: That is critical, to have an algorithm or a scheme in your mind. You want to think through it before it occurs because you are not always thinking as clearly when things are not going well.
I would say get good at physical examination skills in the office, then have a plan for the OR based on what you see in the office. If what is going on with the prolapse is not completely investigated and other issues are not addressed, then failure results because you did not make the diagnosis. Certainly, modify the procedure according to what you find intraoperatively, but follow through.
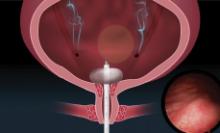
Indications and tips for sacrocolpopexy
Dr. Gebhart: What are the indications for sacrocolpopexy?
Dr. Ridgeway: Indications include recurrent apical prolapse, posthysterectomy prolapse, or severe prolapse in someone quite young. It is a fantastic operation with overall low risks, but this needs to be discussed with the patient.
Dr. Walters: There are some unusual circumstances—for example, the woman has a short prolapsed vagina, usually after a prior surgery—in which the best repair is a bridging piece of mesh, usually done laparoscopically, because those operations cannot be done very well vaginally to obtain a durable result.
Dr. Karram: I agree. I do not think that all recurrent prolapses mandate a sacrocolpopexy. You need to individualize, but in general the short prolapsed vagina and patients who are very young are at high risk for a recurrence.
Dr. Gebhart: An older patient might be a very good candidate, even if she had recurrence from another vaginal repair.
Beri, does the patient with a high body mass index need augmentation?
Dr. Ridgeway: That is a great question, and this has to be individualized because, while heavier patients can benefit from augmentation, in a very heavy patient, getting into that abdomen has its own set of challenges. Anatomically they get a better repair with a mesh-augmented repair like a sacrocolpopexy, but they do have increased risks. That is important to acknowledge and clarify with the patient.
Dr. Gebhart: Any surgical tip you might offer on sacrocolpopexy?
Dr. Ridgeway: Perform the operation in the same way you would an open procedure. Meaning, use the same materials, the same sutures, the same placement, and the same type of dissection in order to obtain results similar to those with an open operation. Using your assistants to manipulate the vagina and rectum is important, as well as exposure and typical careful surgical technique.
Dr. Gebhart: What is important about the placement of sutures on the anterior longitudinal ligament, and what do you need to be cognizant of?
Dr. Ridgeway: Be careful of that left common iliac vein that is a little more medial than you would expect and of the middle sacral artery, and try to differentiate between L5 and S1. In an ideal circumstance, place the suture at S1 or L5 but not the inner disc space, which is the area to avoid placement.
Historically, the recommendation is S1. Some people do L5 because of some pull out strength studies, but also because it is easier, and sometimes in that area of the anterior longitudinal ligament is much better. The key is to do enough dissection and use haptic feedback, especially with conventional laparoscopy or an open approach, to avoid placing sutures through the disc space, as there is some concern that it increases the risk for discitis or osteomyelitis in that area.
Continue to: We also have found...
Dr. Gebhart: We also have found that if you have a combined surgery with colorectal colleagues, like a rectal prolapse repair, there is a little higher risk of discitis.
Dr. Ridgeway: In my own practice I saw a combined case with a rectopexy in someone who had a biologic mesh erosion. When we reviewed the literature, a number of reported cases of discitis had either an early post-op or concurrent urinary tract infection or vaginal infection that likely predisposed them to an infection that traveled up the material.
Dr. Karram: My final comment is that a sacrocolpopexy is not a few stitches or a little mesh right at the apex. If the patient has an isolated enterocele, okay, but it is a wide mesh for a reason and it should connect to the endopelvic fascia anteriorly, posteriorly. It is a mistake to suture just a little bit of the cuff and grab it and think, “I’ve done a colpopexy” when the procedure has not been executed as it should be.
Dr. Gebhart: I want to thank our expert panel and OBG M
Continue to: Some procedures call for cystoscopy...
Dr. Gebhart: Is cystoscopy necessary in patients undergoing native tissue repair or abdominal approaches to prolapse, and should the experienced generalist have this skill?
Dr. Walters: If you are going to do prolapse surgery or surgery for stress urinary incontinence, you need to learn to do cystoscopy. Almost all specialists in urogynecology and urology would do a cystoscopy at the time of a native tissue prolapse repair, a mesh-augmented prolapse repair, or a sling procedure. Whether a generalist doing simple hysterectomies needs to do cystoscopy is controversial, and it is probably based on risk assessment of the kind of hysterectomy being done. Definitely, if you are doing prolapse repair, you probably should be doing cystoscopy at the same time.
Dr. Karram: I would take it further. For certain procedures, cystoscopy is standard of care. For example, if you are doing anything around the uterosacral ligaments, whether a McCall culdoplasty or uterosacral suspension, it is standard of care. It would be a difficult medical-legal defense issue if it was not done in those cases.
To Mark’s point, it is controversial whether universal cystoscopy should be performed on every hysterectomy or every anterior to posterior repair. We are not there yet, but certainly it is in your best interest to have a very low threshold, so if you think about doing cystoscopy, you should probably do it.
Dr. Gebhart: Is cystoscopy needed in sacrocolpopexy?
Dr. Ridgeway: We know from our own data that the risk of lower urinary tract injury is very low with sacrocolpopexy. Having said that, I agree with the position statement of the American Urogynecologic Society that says, “Universal cystoscopy should be performed at the time of all pelvic reconstruction surgeries, with the exception of operations solely for posterior compartment defects.”1
Dr. Gebhart: The reality is that we just want to identify if there is a problem or not at the time of the surgery. It does not mean you have to manage it. You could get your partner, your urologist, or another person with expertise to come in to help you.
Dr. Ridgeway: Absolutely, because intraoperative identification and treatment will prevent many unfavorable outcomes in the postoperative period.
Reference
1. Cohen SA, Carberry CL, Smilen SW. American Urogynecologic Society Consensus Statement: cystoscopy at the time of prolapse repair. Female Pelvic Med Reconstr Surg. 2018;24:258-259.
Dr. Gebhart: If a patient is a smoker and/or utilizes tobacco and you think she is a candidate for a sacrocolpopexy, are there any special considerations? How would you counsel that patient?
Dr. Walters: The risk of mesh erosion is high enough that I would try to not do any mesh prolapse repair in a woman who was a smoker, especially a heavy smoker. A more common situation is, would I put a polypropylene midurethral sling in that patient? I usually am willing to do that because it is still the best option compared with the no-mesh options. In a patient who would be a good candidate for sacrocolpopexy, I can usually do a no-mesh surgery and keep the risk low. I could always give the woman an option to quit smoking, but that tends not to be successful.
Dr. Gebhart: What is the risk of using mesh in a smoker?
Dr. Walters: An increased risk of erosion through the vaginal walls. I am not sure of the magnitude of risk, maybe 2 or 3 times higher. That is high enough that I probably would not take the risk except in unusual circumstances.
Dr. Ridgeway: A good amount of data show increased risk of mesh exposure for smokers. Those patients also tend to have a higher risk of prolapse recurrence because of coughing. Sacrocolpopexy is not my favorite operation to do in a smoker. I will work with the patient to quit, but often if it is the right operation, I will do it, with preoperative estrogen and appropriate conseling.
Dr. Gebhart: Is there still a role for vaginal mesh? While it is no longer being sold in the United States, could you fashion your own mesh for a prolapse procedure?
Dr. Walters: I can do pretty much everything I need to do without adding transvaginal mesh, and if I need a meshaugmented repair, then I would go with the sacrocolpopexy route. Having said that, data for hysteropexy do show that a mesh-augmented hysteropexy could have some advantages, whether you do it with a kit or some fashioned pieces of mesh. Most of the experiences with this are outside of the United States, so we need much more standardization of technique and tracking to answer that question.
Dr. Gebhart: Mickey, what are your thoughts regarding someone who thinks, “Mesh has been good for me, I want to stay with that. I’m going to cut my own mesh”? Are they assuming some liability now that companies are no longer marketing mesh for vaginal repair?
Dr. Karram: Unfortunately, I really think they are. It would be easy to be put in a legal corner and asked, the FDA felt that this should be pulled off the market, why are you still utilizing it? At the end of the day, what the FDA said was not inaccurate.
The studies have not shown a significant better outcome with mesh, and it is an extra intervention that, again, in the best of hands is going to have some issues. That is a dilemma many surgeons faced because they felt that that was their main way of treating prolapse—”they took away my way of successfully treating patients for years.” I do think it increases their medical-legal liability.
Dr. Ridgeway: I agree that it does increase medical-legal liability, and I can’t imagine a situation in which I would offer that. Dr. Gebhart: There are risks with all procedures, including slings for stress incontinence, but sling use is appropriate in appropriately counseled patients.
Dr. Ridgeway: Correct. I feel very strongly that the risk profile for the midurethral sling is very different from that for transvaginal mesh. Very large data sets in large groups of people support that the outcomes are favorable and the risk profile is low. Having said that, slings are not risk free, but living with severe incontinence is not risk free either.
- US Food and Drug Administration. FDA takes action to protect women's health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical-mesh-intended-transvaginal. April 16, 2019. Accessed January 14, 2020.
- Detollenaere RJ, den Boon J, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with suspension of the uterosacral ligaments in women with uterine prolapse stage 2 or higher: multicentre randomised non-inferiority trial. BMJ. 2015;351:h3717.
A number of presentations at the 2019 Pelvic Anatomy and Gynecologic Surgery (PAGS) Symposium (Las Vegas, Nevada, December 12-14, 2019) focused on pelvic organ prolapse (POP) repair, including anatomic considerations, the evolution of surgical procedures, and transvaginal repair. OBG M
Nonsurgical approaches for POP: A good option for the right patient
John B. Gebhart, MD, MS: What are the nonsurgical options for POP?
Mark D. Walters, MD: Women who have prolapse could, of course, choose to continue to live with the prolapse. If they desire treatment, however, the main nonsurgical option is a combination of pessary use, possibly with some estrogen, and possibly with pelvic muscle exercises. Women who have a well-fitting pessary can be managed satisfactorily for years. If possible, women should be taught to take the pessary in and out on a regular basis to minimize their long-term complications.
Dr. Gebhart: How can nonsurgical treatment options be maximized?
Beri M. Ridgeway, MD: It depends on patient commitment. This is important to assess at the first visit when you are making management decisions, because if someone is not going to attend physical therapy or not going to continue to do the exercises, the expectation for the outcome is not going to be great.
Also, if a patient feels very uncomfortable using a pessary and really does not want it, I am fine proceeding with surgery as a first-line treatment. If the patient is committed, the ideal is to educate her and connect her with the right people, either a pelvic floor physical therapist or someone in your office who will encourage her and manage pessary use.
Dr. Gebhart: It goes back to assessing patient goals and expectations.
Mickey M. Karram, MD: If you have a patient who is a good candidate for a pessary—say she has a well-supported distal vagina and maybe a cervical prolapse or an apical prolapse—and you can fit a small pessary that will sit in the upper vagina in a comfortable fashion, it is worthwhile to explain to the patient that she is a really good candidate for this option. By contrast, someone who has a wide genital hiatus and a large rectocele will not have good success with a pessary.
Dr. Gebhart: That is important: Choose your nonsurgical patients well, those who will respond to therapy and maybe not get frustrated with it.
Dr. Walters: A problem I see is that some people are good at fitting a pessary, but they do not teach how to use it very well. When I see the patient back, she says, “What’s my long term on the pessary?” I say, “If we teach you to take it in and out, you are less likely to have any problems with it, and then you can manage it for years that way. Otherwise, you have to keep visiting a practitioner to change it and that is not necessarily a good long-term option.” At the very first visit, I teach them what a pessary is, its purpose, and how to maintain it themselves. I think that gives patients the best chance for long-term satisfaction.
Dr. Gebhart: Surgery is always an option if pessary management is not satisfactory.
Dr. Ridgeway: I also tell patients, especially those uncertain about using a pessary, “Worst case, you spend a little time to figure this out, but if it works, you can avoid surgery. If it doesn’t—the risks are very low and you perhaps wasted some time—but at least you’ll know you tried the conservative management.”
Dr. Gebhart: Mickey made an excellent point earlier that it can be a diagnostic treatment strategy as well.
Dr. Karram: If you are concerned about the prolapse worsening or negatively impacting a functional problem related to the bladder or bowel, it is good to place a pessary for a short period of time. This can potentially give you an idea of how your surgery will impact a patient’s bladder or bowel function.
Continue to: Decisions to make before choosing a surgical approach...
Decisions to make before choosing a surgical approach
Dr. Gebhart: Would you elaborate on the surgical options for managing POP?
Dr. Walters: For women with prolapse who decide they want to have surgery, the woman and the surgeon need to make a number of decisions. Some of these include whether the uterus, if present, needs to be removed; whether the woman would like to maintain sexual function or not; whether the repair would best be done vaginally only with native tissue suturing, vaginally with some augmentation (although that is not likely in the United States at this time), or through the abdomen, usually laparoscopically or robotically with a mesh-augmented sacrocolpopexy repair.
Also, we must decide whether to do additional cystocele and rectocele repairs and whether to add slings for stress incontinence, which can coexist or could develop after the prolapse repair. A lot of different decisions need to be made when choosing a prolapse repair for different women.
Dr. Ridgeway: It is shared decision-making with the patient. You need to understand her goals, the degree of prolapse, whether she has contraindications to uterine preservation, and how much risk she is willing to take.

Fundamentals of the clinical evaluation
Dr. Gebhart: For a woman who wants to manage her prolapse surgically, let us consider some fundamentals of clinical diagnosis. Take me through your office evaluation of the patient reporting prolapse symptoms—her history, yes, but from a physical exam standpoint, what is important?
Dr. Karram: You want to know if this is a primary prolapse or recurrent prolapse. You want to distinguish the various segments of the pelvic floor that are prolapsing and try to quantitate that in whatever way you would like. A standardized quantification system is useful, but you should have a system within your practice that you can standardize. Then, determine if there are coexisting functional derangements and how those are being impacted by the prolapse, because that is very important.
Take a good history, and identify how badly the prolapse bothers the patient and affects her quality of life. Understand how much she is willing to do about it. Does she just want to know what it is and has no interest in a surgical intervention, versus something she definitely wants to get corrected? Then do whatever potential testing around the bladder, and bowel, based on any functional derangements and finally determine interest in maintaining sexual function. Once all this information is obtained, a detailed discussion of surgical options can be undertaken.
Dr. Gebhart: What are your clinical pearls for a patient who has prolapse and does not describe any incontinence, voiding dysfunction, or defecatory symptoms? Do we need imaging testing of any sort or is the physical exam adequate for assessing prolapse?
Dr. Walters: When you do the standardized examination of the prolapse, it is important to measure how much prolapse affects the anterior wall of the apex and/or cervix and the posterior wall. Then note that in your notes and plan your surgery accordingly.
It is useful to have the patient fully bear down and then make your measurements; then, especially if she has a full bladder, have her cough while you hold up the prolapse with a speculum or your hand to see if she has stress urinary incontinence.
Continue to: I agree that to diagnose prolapse...
Dr. Ridgeway: I agree that to diagnose prolapse, it is physical exam alone. I would not recommend any significant testing other than testing for the potential for stress incontinence.
Dr. Gebhart: Is it necessary to use the POP-Q (Pelvic Organ Prolapse Quantification system) in a nonacademic private practice setting? Or are other systems, like a Baden-Walker scoring system, adequate in the everyday practice of the experienced generalist?
Dr. Walters: The Baden-Walker system actually is adequate for use in everyday practice. However, Baden-Walker is an outdated measurement system that really is not taught anymore. I think that as older physicians finish and newer doctors come in, no one will even know what Baden-Walker is.
It is better to go ahead and start learning the POP-Q system. Everyone has electronic charts now and if you learn to use the POP-Q, you can do it very quickly and get a grading system for your chart that is reproducible for everyone.
Dr. Ridgeway: The most important thing is to assess all 3 compartments and document the amount of prolapse of each compartment. A modified POP-Q is often adequate. To do this, perform a split speculum exam and use the hymen as the reference. Zero is at the hymen, +1 is 1 cm beyond the hyman. Covering the rectum, how much does the anterior compartment prolapse in reference to the hymen? Covering the anterior compartment, get an idea of what is happening posteriorly. And the crux of any decision in my mind is what is happening at the apex or to the uterus/cervix if it is still present. It is really important to document at least those 3 compartments.
Dr. Karram: I agree. The POP-Q is the ideal, but I don’t think generalists are motivated to use it. It is very important, though, to have some anatomic landmarks, as already mentioned by Dr. Ridgeway.
Choose a surgical approach based on the clinical situation
Dr. Gebhart: How do you choose the surgical approach for someone with prolapse?
Dr. Karram: Most surgeons do what they think they do best. I have spent the majority of my career operating through the vagina, and most of that involves native tissue repairs. I almost always will do a primary prolapse through the vagina and not consider augmentation except in rare circumstances. A recurrent prolapse, a prolapsed shortened vagina, scarring, or a situation that is not straightforward has to be individualized. My basic intervention initially is almost always vaginally with native tissue.
Dr. Ridgeway: For a primary prolapse repair, I also will almost always use native tissue repair as firstline. Whether that is with hysterectomy or without, most people in the long term do very well with that. At least 70% of my repairs are done with a native tissue approach.
For a woman who has a significant prolapse posthysterectomy, especially of the anterior wall or with recurrent prolapse, I offer a laparoscopic sacrocolpopexy. The only other time I offer that as a primary approach would be for a younger woman with very significant prolapse. In that case, I will review risks and benefits with the patient and, using shared decision-making, offer either a native tissue repair or a sacrocolpopexy. For that patient, no matter what you do, given that she has many years to live, the chances are that she will likely need a second intervention.
Dr. Gebhart: Mark, how do you choose an approach for prolapse?
Dr. Walters: I do things pretty much the way Dr. Karram and Dr. Ridgeway do. For women who have a primary prolapse, I usually take a vaginal approach, and for recurrences I frequently do sacrocolpopexy with mesh or I refer to one of my partners who does more laparoscopic or robotic sacrocolpopexy.
Whether the patient needs a hysterectomy or not is evolving. Traditionally, hysterectomy is almost always done at the first prolapse repair. That is being reassessed in the United States to match what is happening in some other countries. It is possible to do nice primary prolapse repair vaginally or laparoscopically and leave the uterus in, in selected women who desire that.
Continue to: Transvaginal prolapse repair: Mesh is no longer an option...
Transvaginal prolapse repair: Mesh is no longer an option
Dr. Gebhart: What led up to the US Food and Drug Administration’s (FDA) market removal of mesh for transvaginal repair of POP?
Dr. Ridgeway: To clarify, it was not a recall—a word that many people use—it was an order to stop producing and distributing surgical mesh intended for transvaginal repair of POP.1 There is a very long history. Transvaginal mesh was introduced with the goal of improving prolapse anatomic and subjective outcomes. Over the last 13 years or so, there were adverse events that led to FDA public health notifications. Consequently, these devices were reclassified, and now require additional testing prior to approval. The newest transvaginal mesh kits were studied.
These 522 studies were completed recently and needed to show superior outcomes because, historically, the risks associated with transvaginal mesh compared to those associated with native tissue repairs are higher: higher reoperation rates, higher rates of other complications, and very minimal improvements in subjective and objective outcomes. Data were presented to the FDA, and it was deemed that these mesh kits did not improve outcomes significantly compared with native tissue repairs.
Dr. Karram: Beri, you stated that very accurately. The pro-mesh advocates were taken back by the idea that the FDA made this recommendation without allowing the outcomes to be followed longer.
Dr. Gebhart: My understanding is that the FDA had a timeline where they had to do a report and the studies had not matured to that end point; thus, they had to go with the data they had even though the studies were not completed. I think they are requesting that they be completed.
Dr. Ridgeway: Additional data will be available, some through the 522 studies, others through randomized controlled trials in which patients were already enrolled and had surgery. As far as I know, I do not think that the decision will be reversed.
Continue to: Native tissue repair and failure risk...
Native tissue repair and failure risk
Dr. Gebhart: I hear a lot that native tissue repairs fail. Mickey, as you do a lot of vaginal surgery, what are your thoughts? Should you use augmentation of some sort because native tissue fails?
Dr. Karram: There is going to be a failure rate with whatever surgery you do. I think that the failure rate with native tissue is somewhat overstated. I think a lot of that dates back to some of the things that were being promoted by mesh advocates. Initially, there was a lot of cherry-picking of native tissue data in some of those studies to promote the idea that the recurrent prolapse rates were 40% to 80%. We certainly do not see that in our patient population.
Based on our 5-year data, we have a recurrence rate of about 15% and a reoperation rate of less than 10%. That is the best I can quote based on our data. We have not followed patients longer than 5 years.
I can’t do much better than that with an augmentation; even if I get another 5% or 10% better anatomic outcome, that will be at the expense of some erosions and other complications specific to the mesh. I do think that the native tissue failure rate being promoted by a lot of individuals is a higher failure rate than what we are seeing.
Dr. Gebhart: What do you think, Mark?
Dr. Walters: Large cohort studies both at your institution, Mayo Clinic, and ours at the Cleveland Clinic mirror what Dr. Karram said, in that we have a reoperation rate somewhere between 8% and 15%. Of course, we have some failures that are stage 2 failures where patients choose not to have another operation. In general, a 10% or 12% reoperation rate at 5 to 7 years is acceptable.
Native tissue repairs probably fail at the apex a little more than mesh sacrocolpopexy. Mesh sacrocolpopexy, depending on what else you do with that operation, may have more distal vaginal failures, rates like distal rectoceles and more de novo stress urinary incontinence than we probably get with native tissue. I get some failures of the apex with native tissue repairs, but I am okay with using sacrocolpopexy as the second-line therapy in those patients.
Hysteropexy technique and pros and cons
Dr. Gebhart: Is hysteropexy a fad, or is there something to this?
Dr. Ridgeway: I do not think it is a fad. Women do feel strongly about this, and we now have data supporting this choice: randomized controlled trials of hysterectomy and prolapse repair versus hysteropexy with comparable outcomes at the short and medium term.2
The outcomes are similar, but as we said, outcomes for all prolapse repair types are not perfect. We have recurrences with sacrocolpopexy, native tissue repair, and hysteropexy. We need more data on types of hysteropexy and long-term outcomes for uterine preservation.
Dr. Walters: We have been discussing what patients think of their uterus, and some patients have very strong opinions. Some prefer to have a hysterectomy because then they don’t need to worry about cancer or do screening for cancer, and they are very happy with that. Other women with the same kind of prolapse prefer not to have a hysterectomy because philosophically they think they are better off keeping their organs. Since satisfaction is an outcome, it is useful to know what the patient wants and what she thinks about the surgical procedure.
Dr. Gebhart: For hysteropexy, do the data show that suture or a mesh augment provide an advantage one way or the other? Do we know that yet?
Dr. Walters: No, there are not enough studies with suture. There are only a few very good studies with suture hysteropexy, and they are mostly sacrospinous suture hysteropexies. Only a few studies look at mesh hysteropexy (with the Uphold device that was put on hold), or with variations of uterosacral support using strips of mesh, mostly done in other countries.
A point I want to add, if native tissue repairs fail at the apex more, why don’t you just always do sacrocolpopexy? One reason is because it might have a little higher complication rate due to the abdominal access and the fact that you are putting mesh in. If you have, for example, a 4% complication rate with the mesh but you get a better cure rate, those things balance out, and the woman may not be that much better off because of the extra complications. You have to assess the pro and con with each patient to pick what is best for her—either a more durable repair with a mesh or a little safer repair with native tissue.
Continue to: Women feel very strongly about risk...
Dr. Ridgeway: Women feel very strongly about risk. Within the same clinic I will have similar patients, and I say, “Probably in the long term this one may last a little longer but the surgery takes longer and it has a little higher complication rate.” One patient will say, “I’m not worried about the risk, I want what’s going to last the longest,” whereas a very similar patient will say, “Why would anyone pick the higher-risk operation? I want the lower risk that probably will last a long time.”
Dr. Gebhart: Beri, who should not have a hysteropexy?
Dr. Ridgeway: The biggest factor would be someone who has ever had postmenopausal bleeding. From our data, we know that if they have even had a work-up with benign results, the risk of unanticipated pathology is high. I do not recommend hysteropexy for anyone who has had postmenopausal bleeding.
For a premenopausal woman who has irregular bleeding, I also do not recommend it, because you just do not know what that future will hold. If a patient has anatomic abnormalities like large fibroids, I would not recommend it either. I would like patients to have had standard cervical cancer screening without any abnormalities for about 10 years or so.
Dr. Gebhart: What about prior cervical dysplasia?
Dr. Ridgeway: If a patient had ASCUS or low-grade dysplasia decades ago, has been normal for at least 10 years, and is currently negative for human papillomavirus, I have no problem.
Dr. Gebhart: How about women at high genetic risk for cancer?
Dr. Ridgeway: If they are at high risk for endometrial cancer, I would not recommend hysteropexy. If they are going to need an oophorectomy and/or salpingectomy for risk reduction during prolapse treatment, I usually perform a hysterectomy.
Plan surgical steps and prepare for “what if’s”
Dr. Gebhart: What tips can you provide, either regarding the evaluation or something you do surgically, that are important in a transvaginal native tissue repair?
Dr. Karram: If you have a case of posthysterectomy apical prolapse, that you think is an indication for sacrocolpopexy, in reality these are very good candidates for either sacrospinous or uterosacral suspensions. I prefer a uterosacral suspension as I feel there is less distortion of the vaginal apex compared to a sacrospinous suspension.
Dr. Ridgeway: The most critical step is setting up the OR and positioning the patient. That sets up the case for success, preventing struggles during the case. I use a high lithotomy, with careful positioning of course, but I use candy cane stirrups so that I can have an instrument stand in front of me and not struggle during the case.
Dr. Walters: My tip for everyone who is doing native tissue surgery, whether it is high McCall colpopexy or uterosacral ligament suspension or sacrocolpopexy, would be to really learn well the anatomy of each operation, including how close the ureter is, where the risk for bleeding is, and where the risk for nerve damage is.
The complications for each of these surgeries are slightly different, but there is a small risk of kinking the ureter with both uterosacral ligament suspension and the McCall, so you should do a cystoscopy as part of that operation. If you do a sacrospinous ligament suspension, use an instrument that can get a stitch into a ligament—not too close to the ischial spine and not too close to the sacrum—to avoid the risk of damage to major nerves and blood vessels and to minimize buttock and leg pain.
Continue to: Another tip is to understand...
Dr. Karram: Another tip is to understand that you are going to have potential complications intraoperatively. Think through those presurgically. You do not want to start thinking about these things and making decisions as they are happening. For example, what if I do a uterosacral suspension and I don’t see efflux of urine from the ureter? What am I going to do, and how long am I going to wait before I intervene? If I do a sacrospinous and I start to see a lot of bleeding from that area, what am I going to do? My plan would be, “I will pack the area, get extra suction, etc.” Thinking these ideas through before they occur is very helpful.
Dr. Gebhart: That is critical, to have an algorithm or a scheme in your mind. You want to think through it before it occurs because you are not always thinking as clearly when things are not going well.
I would say get good at physical examination skills in the office, then have a plan for the OR based on what you see in the office. If what is going on with the prolapse is not completely investigated and other issues are not addressed, then failure results because you did not make the diagnosis. Certainly, modify the procedure according to what you find intraoperatively, but follow through.
Indications and tips for sacrocolpopexy
Dr. Gebhart: What are the indications for sacrocolpopexy?
Dr. Ridgeway: Indications include recurrent apical prolapse, posthysterectomy prolapse, or severe prolapse in someone quite young. It is a fantastic operation with overall low risks, but this needs to be discussed with the patient.
Dr. Walters: There are some unusual circumstances—for example, the woman has a short prolapsed vagina, usually after a prior surgery—in which the best repair is a bridging piece of mesh, usually done laparoscopically, because those operations cannot be done very well vaginally to obtain a durable result.
Dr. Karram: I agree. I do not think that all recurrent prolapses mandate a sacrocolpopexy. You need to individualize, but in general the short prolapsed vagina and patients who are very young are at high risk for a recurrence.
Dr. Gebhart: An older patient might be a very good candidate, even if she had recurrence from another vaginal repair.
Beri, does the patient with a high body mass index need augmentation?
Dr. Ridgeway: That is a great question, and this has to be individualized because, while heavier patients can benefit from augmentation, in a very heavy patient, getting into that abdomen has its own set of challenges. Anatomically they get a better repair with a mesh-augmented repair like a sacrocolpopexy, but they do have increased risks. That is important to acknowledge and clarify with the patient.
Dr. Gebhart: Any surgical tip you might offer on sacrocolpopexy?
Dr. Ridgeway: Perform the operation in the same way you would an open procedure. Meaning, use the same materials, the same sutures, the same placement, and the same type of dissection in order to obtain results similar to those with an open operation. Using your assistants to manipulate the vagina and rectum is important, as well as exposure and typical careful surgical technique.
Dr. Gebhart: What is important about the placement of sutures on the anterior longitudinal ligament, and what do you need to be cognizant of?
Dr. Ridgeway: Be careful of that left common iliac vein that is a little more medial than you would expect and of the middle sacral artery, and try to differentiate between L5 and S1. In an ideal circumstance, place the suture at S1 or L5 but not the inner disc space, which is the area to avoid placement.
Historically, the recommendation is S1. Some people do L5 because of some pull out strength studies, but also because it is easier, and sometimes in that area of the anterior longitudinal ligament is much better. The key is to do enough dissection and use haptic feedback, especially with conventional laparoscopy or an open approach, to avoid placing sutures through the disc space, as there is some concern that it increases the risk for discitis or osteomyelitis in that area.
Continue to: We also have found...
Dr. Gebhart: We also have found that if you have a combined surgery with colorectal colleagues, like a rectal prolapse repair, there is a little higher risk of discitis.
Dr. Ridgeway: In my own practice I saw a combined case with a rectopexy in someone who had a biologic mesh erosion. When we reviewed the literature, a number of reported cases of discitis had either an early post-op or concurrent urinary tract infection or vaginal infection that likely predisposed them to an infection that traveled up the material.
Dr. Karram: My final comment is that a sacrocolpopexy is not a few stitches or a little mesh right at the apex. If the patient has an isolated enterocele, okay, but it is a wide mesh for a reason and it should connect to the endopelvic fascia anteriorly, posteriorly. It is a mistake to suture just a little bit of the cuff and grab it and think, “I’ve done a colpopexy” when the procedure has not been executed as it should be.
Dr. Gebhart: I want to thank our expert panel and OBG M
Continue to: Some procedures call for cystoscopy...
Dr. Gebhart: Is cystoscopy necessary in patients undergoing native tissue repair or abdominal approaches to prolapse, and should the experienced generalist have this skill?
Dr. Walters: If you are going to do prolapse surgery or surgery for stress urinary incontinence, you need to learn to do cystoscopy. Almost all specialists in urogynecology and urology would do a cystoscopy at the time of a native tissue prolapse repair, a mesh-augmented prolapse repair, or a sling procedure. Whether a generalist doing simple hysterectomies needs to do cystoscopy is controversial, and it is probably based on risk assessment of the kind of hysterectomy being done. Definitely, if you are doing prolapse repair, you probably should be doing cystoscopy at the same time.
Dr. Karram: I would take it further. For certain procedures, cystoscopy is standard of care. For example, if you are doing anything around the uterosacral ligaments, whether a McCall culdoplasty or uterosacral suspension, it is standard of care. It would be a difficult medical-legal defense issue if it was not done in those cases.
To Mark’s point, it is controversial whether universal cystoscopy should be performed on every hysterectomy or every anterior to posterior repair. We are not there yet, but certainly it is in your best interest to have a very low threshold, so if you think about doing cystoscopy, you should probably do it.
Dr. Gebhart: Is cystoscopy needed in sacrocolpopexy?
Dr. Ridgeway: We know from our own data that the risk of lower urinary tract injury is very low with sacrocolpopexy. Having said that, I agree with the position statement of the American Urogynecologic Society that says, “Universal cystoscopy should be performed at the time of all pelvic reconstruction surgeries, with the exception of operations solely for posterior compartment defects.”1
Dr. Gebhart: The reality is that we just want to identify if there is a problem or not at the time of the surgery. It does not mean you have to manage it. You could get your partner, your urologist, or another person with expertise to come in to help you.
Dr. Ridgeway: Absolutely, because intraoperative identification and treatment will prevent many unfavorable outcomes in the postoperative period.
Reference
1. Cohen SA, Carberry CL, Smilen SW. American Urogynecologic Society Consensus Statement: cystoscopy at the time of prolapse repair. Female Pelvic Med Reconstr Surg. 2018;24:258-259.
Dr. Gebhart: If a patient is a smoker and/or utilizes tobacco and you think she is a candidate for a sacrocolpopexy, are there any special considerations? How would you counsel that patient?
Dr. Walters: The risk of mesh erosion is high enough that I would try to not do any mesh prolapse repair in a woman who was a smoker, especially a heavy smoker. A more common situation is, would I put a polypropylene midurethral sling in that patient? I usually am willing to do that because it is still the best option compared with the no-mesh options. In a patient who would be a good candidate for sacrocolpopexy, I can usually do a no-mesh surgery and keep the risk low. I could always give the woman an option to quit smoking, but that tends not to be successful.
Dr. Gebhart: What is the risk of using mesh in a smoker?
Dr. Walters: An increased risk of erosion through the vaginal walls. I am not sure of the magnitude of risk, maybe 2 or 3 times higher. That is high enough that I probably would not take the risk except in unusual circumstances.
Dr. Ridgeway: A good amount of data show increased risk of mesh exposure for smokers. Those patients also tend to have a higher risk of prolapse recurrence because of coughing. Sacrocolpopexy is not my favorite operation to do in a smoker. I will work with the patient to quit, but often if it is the right operation, I will do it, with preoperative estrogen and appropriate conseling.
Dr. Gebhart: Is there still a role for vaginal mesh? While it is no longer being sold in the United States, could you fashion your own mesh for a prolapse procedure?
Dr. Walters: I can do pretty much everything I need to do without adding transvaginal mesh, and if I need a meshaugmented repair, then I would go with the sacrocolpopexy route. Having said that, data for hysteropexy do show that a mesh-augmented hysteropexy could have some advantages, whether you do it with a kit or some fashioned pieces of mesh. Most of the experiences with this are outside of the United States, so we need much more standardization of technique and tracking to answer that question.
Dr. Gebhart: Mickey, what are your thoughts regarding someone who thinks, “Mesh has been good for me, I want to stay with that. I’m going to cut my own mesh”? Are they assuming some liability now that companies are no longer marketing mesh for vaginal repair?
Dr. Karram: Unfortunately, I really think they are. It would be easy to be put in a legal corner and asked, the FDA felt that this should be pulled off the market, why are you still utilizing it? At the end of the day, what the FDA said was not inaccurate.
The studies have not shown a significant better outcome with mesh, and it is an extra intervention that, again, in the best of hands is going to have some issues. That is a dilemma many surgeons faced because they felt that that was their main way of treating prolapse—”they took away my way of successfully treating patients for years.” I do think it increases their medical-legal liability.
Dr. Ridgeway: I agree that it does increase medical-legal liability, and I can’t imagine a situation in which I would offer that. Dr. Gebhart: There are risks with all procedures, including slings for stress incontinence, but sling use is appropriate in appropriately counseled patients.
Dr. Ridgeway: Correct. I feel very strongly that the risk profile for the midurethral sling is very different from that for transvaginal mesh. Very large data sets in large groups of people support that the outcomes are favorable and the risk profile is low. Having said that, slings are not risk free, but living with severe incontinence is not risk free either.
A number of presentations at the 2019 Pelvic Anatomy and Gynecologic Surgery (PAGS) Symposium (Las Vegas, Nevada, December 12-14, 2019) focused on pelvic organ prolapse (POP) repair, including anatomic considerations, the evolution of surgical procedures, and transvaginal repair. OBG M
Nonsurgical approaches for POP: A good option for the right patient
John B. Gebhart, MD, MS: What are the nonsurgical options for POP?
Mark D. Walters, MD: Women who have prolapse could, of course, choose to continue to live with the prolapse. If they desire treatment, however, the main nonsurgical option is a combination of pessary use, possibly with some estrogen, and possibly with pelvic muscle exercises. Women who have a well-fitting pessary can be managed satisfactorily for years. If possible, women should be taught to take the pessary in and out on a regular basis to minimize their long-term complications.
Dr. Gebhart: How can nonsurgical treatment options be maximized?
Beri M. Ridgeway, MD: It depends on patient commitment. This is important to assess at the first visit when you are making management decisions, because if someone is not going to attend physical therapy or not going to continue to do the exercises, the expectation for the outcome is not going to be great.
Also, if a patient feels very uncomfortable using a pessary and really does not want it, I am fine proceeding with surgery as a first-line treatment. If the patient is committed, the ideal is to educate her and connect her with the right people, either a pelvic floor physical therapist or someone in your office who will encourage her and manage pessary use.
Dr. Gebhart: It goes back to assessing patient goals and expectations.
Mickey M. Karram, MD: If you have a patient who is a good candidate for a pessary—say she has a well-supported distal vagina and maybe a cervical prolapse or an apical prolapse—and you can fit a small pessary that will sit in the upper vagina in a comfortable fashion, it is worthwhile to explain to the patient that she is a really good candidate for this option. By contrast, someone who has a wide genital hiatus and a large rectocele will not have good success with a pessary.
Dr. Gebhart: That is important: Choose your nonsurgical patients well, those who will respond to therapy and maybe not get frustrated with it.
Dr. Walters: A problem I see is that some people are good at fitting a pessary, but they do not teach how to use it very well. When I see the patient back, she says, “What’s my long term on the pessary?” I say, “If we teach you to take it in and out, you are less likely to have any problems with it, and then you can manage it for years that way. Otherwise, you have to keep visiting a practitioner to change it and that is not necessarily a good long-term option.” At the very first visit, I teach them what a pessary is, its purpose, and how to maintain it themselves. I think that gives patients the best chance for long-term satisfaction.
Dr. Gebhart: Surgery is always an option if pessary management is not satisfactory.
Dr. Ridgeway: I also tell patients, especially those uncertain about using a pessary, “Worst case, you spend a little time to figure this out, but if it works, you can avoid surgery. If it doesn’t—the risks are very low and you perhaps wasted some time—but at least you’ll know you tried the conservative management.”
Dr. Gebhart: Mickey made an excellent point earlier that it can be a diagnostic treatment strategy as well.
Dr. Karram: If you are concerned about the prolapse worsening or negatively impacting a functional problem related to the bladder or bowel, it is good to place a pessary for a short period of time. This can potentially give you an idea of how your surgery will impact a patient’s bladder or bowel function.
Continue to: Decisions to make before choosing a surgical approach...
Decisions to make before choosing a surgical approach
Dr. Gebhart: Would you elaborate on the surgical options for managing POP?
Dr. Walters: For women with prolapse who decide they want to have surgery, the woman and the surgeon need to make a number of decisions. Some of these include whether the uterus, if present, needs to be removed; whether the woman would like to maintain sexual function or not; whether the repair would best be done vaginally only with native tissue suturing, vaginally with some augmentation (although that is not likely in the United States at this time), or through the abdomen, usually laparoscopically or robotically with a mesh-augmented sacrocolpopexy repair.
Also, we must decide whether to do additional cystocele and rectocele repairs and whether to add slings for stress incontinence, which can coexist or could develop after the prolapse repair. A lot of different decisions need to be made when choosing a prolapse repair for different women.
Dr. Ridgeway: It is shared decision-making with the patient. You need to understand her goals, the degree of prolapse, whether she has contraindications to uterine preservation, and how much risk she is willing to take.

Fundamentals of the clinical evaluation
Dr. Gebhart: For a woman who wants to manage her prolapse surgically, let us consider some fundamentals of clinical diagnosis. Take me through your office evaluation of the patient reporting prolapse symptoms—her history, yes, but from a physical exam standpoint, what is important?
Dr. Karram: You want to know if this is a primary prolapse or recurrent prolapse. You want to distinguish the various segments of the pelvic floor that are prolapsing and try to quantitate that in whatever way you would like. A standardized quantification system is useful, but you should have a system within your practice that you can standardize. Then, determine if there are coexisting functional derangements and how those are being impacted by the prolapse, because that is very important.
Take a good history, and identify how badly the prolapse bothers the patient and affects her quality of life. Understand how much she is willing to do about it. Does she just want to know what it is and has no interest in a surgical intervention, versus something she definitely wants to get corrected? Then do whatever potential testing around the bladder, and bowel, based on any functional derangements and finally determine interest in maintaining sexual function. Once all this information is obtained, a detailed discussion of surgical options can be undertaken.
Dr. Gebhart: What are your clinical pearls for a patient who has prolapse and does not describe any incontinence, voiding dysfunction, or defecatory symptoms? Do we need imaging testing of any sort or is the physical exam adequate for assessing prolapse?
Dr. Walters: When you do the standardized examination of the prolapse, it is important to measure how much prolapse affects the anterior wall of the apex and/or cervix and the posterior wall. Then note that in your notes and plan your surgery accordingly.
It is useful to have the patient fully bear down and then make your measurements; then, especially if she has a full bladder, have her cough while you hold up the prolapse with a speculum or your hand to see if she has stress urinary incontinence.
Continue to: I agree that to diagnose prolapse...
Dr. Ridgeway: I agree that to diagnose prolapse, it is physical exam alone. I would not recommend any significant testing other than testing for the potential for stress incontinence.
Dr. Gebhart: Is it necessary to use the POP-Q (Pelvic Organ Prolapse Quantification system) in a nonacademic private practice setting? Or are other systems, like a Baden-Walker scoring system, adequate in the everyday practice of the experienced generalist?
Dr. Walters: The Baden-Walker system actually is adequate for use in everyday practice. However, Baden-Walker is an outdated measurement system that really is not taught anymore. I think that as older physicians finish and newer doctors come in, no one will even know what Baden-Walker is.
It is better to go ahead and start learning the POP-Q system. Everyone has electronic charts now and if you learn to use the POP-Q, you can do it very quickly and get a grading system for your chart that is reproducible for everyone.
Dr. Ridgeway: The most important thing is to assess all 3 compartments and document the amount of prolapse of each compartment. A modified POP-Q is often adequate. To do this, perform a split speculum exam and use the hymen as the reference. Zero is at the hymen, +1 is 1 cm beyond the hyman. Covering the rectum, how much does the anterior compartment prolapse in reference to the hymen? Covering the anterior compartment, get an idea of what is happening posteriorly. And the crux of any decision in my mind is what is happening at the apex or to the uterus/cervix if it is still present. It is really important to document at least those 3 compartments.
Dr. Karram: I agree. The POP-Q is the ideal, but I don’t think generalists are motivated to use it. It is very important, though, to have some anatomic landmarks, as already mentioned by Dr. Ridgeway.
Choose a surgical approach based on the clinical situation
Dr. Gebhart: How do you choose the surgical approach for someone with prolapse?
Dr. Karram: Most surgeons do what they think they do best. I have spent the majority of my career operating through the vagina, and most of that involves native tissue repairs. I almost always will do a primary prolapse through the vagina and not consider augmentation except in rare circumstances. A recurrent prolapse, a prolapsed shortened vagina, scarring, or a situation that is not straightforward has to be individualized. My basic intervention initially is almost always vaginally with native tissue.
Dr. Ridgeway: For a primary prolapse repair, I also will almost always use native tissue repair as firstline. Whether that is with hysterectomy or without, most people in the long term do very well with that. At least 70% of my repairs are done with a native tissue approach.
For a woman who has a significant prolapse posthysterectomy, especially of the anterior wall or with recurrent prolapse, I offer a laparoscopic sacrocolpopexy. The only other time I offer that as a primary approach would be for a younger woman with very significant prolapse. In that case, I will review risks and benefits with the patient and, using shared decision-making, offer either a native tissue repair or a sacrocolpopexy. For that patient, no matter what you do, given that she has many years to live, the chances are that she will likely need a second intervention.
Dr. Gebhart: Mark, how do you choose an approach for prolapse?
Dr. Walters: I do things pretty much the way Dr. Karram and Dr. Ridgeway do. For women who have a primary prolapse, I usually take a vaginal approach, and for recurrences I frequently do sacrocolpopexy with mesh or I refer to one of my partners who does more laparoscopic or robotic sacrocolpopexy.
Whether the patient needs a hysterectomy or not is evolving. Traditionally, hysterectomy is almost always done at the first prolapse repair. That is being reassessed in the United States to match what is happening in some other countries. It is possible to do nice primary prolapse repair vaginally or laparoscopically and leave the uterus in, in selected women who desire that.
Continue to: Transvaginal prolapse repair: Mesh is no longer an option...
Transvaginal prolapse repair: Mesh is no longer an option
Dr. Gebhart: What led up to the US Food and Drug Administration’s (FDA) market removal of mesh for transvaginal repair of POP?
Dr. Ridgeway: To clarify, it was not a recall—a word that many people use—it was an order to stop producing and distributing surgical mesh intended for transvaginal repair of POP.1 There is a very long history. Transvaginal mesh was introduced with the goal of improving prolapse anatomic and subjective outcomes. Over the last 13 years or so, there were adverse events that led to FDA public health notifications. Consequently, these devices were reclassified, and now require additional testing prior to approval. The newest transvaginal mesh kits were studied.
These 522 studies were completed recently and needed to show superior outcomes because, historically, the risks associated with transvaginal mesh compared to those associated with native tissue repairs are higher: higher reoperation rates, higher rates of other complications, and very minimal improvements in subjective and objective outcomes. Data were presented to the FDA, and it was deemed that these mesh kits did not improve outcomes significantly compared with native tissue repairs.
Dr. Karram: Beri, you stated that very accurately. The pro-mesh advocates were taken back by the idea that the FDA made this recommendation without allowing the outcomes to be followed longer.
Dr. Gebhart: My understanding is that the FDA had a timeline where they had to do a report and the studies had not matured to that end point; thus, they had to go with the data they had even though the studies were not completed. I think they are requesting that they be completed.
Dr. Ridgeway: Additional data will be available, some through the 522 studies, others through randomized controlled trials in which patients were already enrolled and had surgery. As far as I know, I do not think that the decision will be reversed.
Continue to: Native tissue repair and failure risk...
Native tissue repair and failure risk
Dr. Gebhart: I hear a lot that native tissue repairs fail. Mickey, as you do a lot of vaginal surgery, what are your thoughts? Should you use augmentation of some sort because native tissue fails?
Dr. Karram: There is going to be a failure rate with whatever surgery you do. I think that the failure rate with native tissue is somewhat overstated. I think a lot of that dates back to some of the things that were being promoted by mesh advocates. Initially, there was a lot of cherry-picking of native tissue data in some of those studies to promote the idea that the recurrent prolapse rates were 40% to 80%. We certainly do not see that in our patient population.
Based on our 5-year data, we have a recurrence rate of about 15% and a reoperation rate of less than 10%. That is the best I can quote based on our data. We have not followed patients longer than 5 years.
I can’t do much better than that with an augmentation; even if I get another 5% or 10% better anatomic outcome, that will be at the expense of some erosions and other complications specific to the mesh. I do think that the native tissue failure rate being promoted by a lot of individuals is a higher failure rate than what we are seeing.
Dr. Gebhart: What do you think, Mark?
Dr. Walters: Large cohort studies both at your institution, Mayo Clinic, and ours at the Cleveland Clinic mirror what Dr. Karram said, in that we have a reoperation rate somewhere between 8% and 15%. Of course, we have some failures that are stage 2 failures where patients choose not to have another operation. In general, a 10% or 12% reoperation rate at 5 to 7 years is acceptable.
Native tissue repairs probably fail at the apex a little more than mesh sacrocolpopexy. Mesh sacrocolpopexy, depending on what else you do with that operation, may have more distal vaginal failures, rates like distal rectoceles and more de novo stress urinary incontinence than we probably get with native tissue. I get some failures of the apex with native tissue repairs, but I am okay with using sacrocolpopexy as the second-line therapy in those patients.
Hysteropexy technique and pros and cons
Dr. Gebhart: Is hysteropexy a fad, or is there something to this?
Dr. Ridgeway: I do not think it is a fad. Women do feel strongly about this, and we now have data supporting this choice: randomized controlled trials of hysterectomy and prolapse repair versus hysteropexy with comparable outcomes at the short and medium term.2
The outcomes are similar, but as we said, outcomes for all prolapse repair types are not perfect. We have recurrences with sacrocolpopexy, native tissue repair, and hysteropexy. We need more data on types of hysteropexy and long-term outcomes for uterine preservation.
Dr. Walters: We have been discussing what patients think of their uterus, and some patients have very strong opinions. Some prefer to have a hysterectomy because then they don’t need to worry about cancer or do screening for cancer, and they are very happy with that. Other women with the same kind of prolapse prefer not to have a hysterectomy because philosophically they think they are better off keeping their organs. Since satisfaction is an outcome, it is useful to know what the patient wants and what she thinks about the surgical procedure.
Dr. Gebhart: For hysteropexy, do the data show that suture or a mesh augment provide an advantage one way or the other? Do we know that yet?
Dr. Walters: No, there are not enough studies with suture. There are only a few very good studies with suture hysteropexy, and they are mostly sacrospinous suture hysteropexies. Only a few studies look at mesh hysteropexy (with the Uphold device that was put on hold), or with variations of uterosacral support using strips of mesh, mostly done in other countries.
A point I want to add, if native tissue repairs fail at the apex more, why don’t you just always do sacrocolpopexy? One reason is because it might have a little higher complication rate due to the abdominal access and the fact that you are putting mesh in. If you have, for example, a 4% complication rate with the mesh but you get a better cure rate, those things balance out, and the woman may not be that much better off because of the extra complications. You have to assess the pro and con with each patient to pick what is best for her—either a more durable repair with a mesh or a little safer repair with native tissue.
Continue to: Women feel very strongly about risk...
Dr. Ridgeway: Women feel very strongly about risk. Within the same clinic I will have similar patients, and I say, “Probably in the long term this one may last a little longer but the surgery takes longer and it has a little higher complication rate.” One patient will say, “I’m not worried about the risk, I want what’s going to last the longest,” whereas a very similar patient will say, “Why would anyone pick the higher-risk operation? I want the lower risk that probably will last a long time.”
Dr. Gebhart: Beri, who should not have a hysteropexy?
Dr. Ridgeway: The biggest factor would be someone who has ever had postmenopausal bleeding. From our data, we know that if they have even had a work-up with benign results, the risk of unanticipated pathology is high. I do not recommend hysteropexy for anyone who has had postmenopausal bleeding.
For a premenopausal woman who has irregular bleeding, I also do not recommend it, because you just do not know what that future will hold. If a patient has anatomic abnormalities like large fibroids, I would not recommend it either. I would like patients to have had standard cervical cancer screening without any abnormalities for about 10 years or so.
Dr. Gebhart: What about prior cervical dysplasia?
Dr. Ridgeway: If a patient had ASCUS or low-grade dysplasia decades ago, has been normal for at least 10 years, and is currently negative for human papillomavirus, I have no problem.
Dr. Gebhart: How about women at high genetic risk for cancer?
Dr. Ridgeway: If they are at high risk for endometrial cancer, I would not recommend hysteropexy. If they are going to need an oophorectomy and/or salpingectomy for risk reduction during prolapse treatment, I usually perform a hysterectomy.
Plan surgical steps and prepare for “what if’s”
Dr. Gebhart: What tips can you provide, either regarding the evaluation or something you do surgically, that are important in a transvaginal native tissue repair?
Dr. Karram: If you have a case of posthysterectomy apical prolapse, that you think is an indication for sacrocolpopexy, in reality these are very good candidates for either sacrospinous or uterosacral suspensions. I prefer a uterosacral suspension as I feel there is less distortion of the vaginal apex compared to a sacrospinous suspension.
Dr. Ridgeway: The most critical step is setting up the OR and positioning the patient. That sets up the case for success, preventing struggles during the case. I use a high lithotomy, with careful positioning of course, but I use candy cane stirrups so that I can have an instrument stand in front of me and not struggle during the case.
Dr. Walters: My tip for everyone who is doing native tissue surgery, whether it is high McCall colpopexy or uterosacral ligament suspension or sacrocolpopexy, would be to really learn well the anatomy of each operation, including how close the ureter is, where the risk for bleeding is, and where the risk for nerve damage is.
The complications for each of these surgeries are slightly different, but there is a small risk of kinking the ureter with both uterosacral ligament suspension and the McCall, so you should do a cystoscopy as part of that operation. If you do a sacrospinous ligament suspension, use an instrument that can get a stitch into a ligament—not too close to the ischial spine and not too close to the sacrum—to avoid the risk of damage to major nerves and blood vessels and to minimize buttock and leg pain.
Continue to: Another tip is to understand...
Dr. Karram: Another tip is to understand that you are going to have potential complications intraoperatively. Think through those presurgically. You do not want to start thinking about these things and making decisions as they are happening. For example, what if I do a uterosacral suspension and I don’t see efflux of urine from the ureter? What am I going to do, and how long am I going to wait before I intervene? If I do a sacrospinous and I start to see a lot of bleeding from that area, what am I going to do? My plan would be, “I will pack the area, get extra suction, etc.” Thinking these ideas through before they occur is very helpful.
Dr. Gebhart: That is critical, to have an algorithm or a scheme in your mind. You want to think through it before it occurs because you are not always thinking as clearly when things are not going well.
I would say get good at physical examination skills in the office, then have a plan for the OR based on what you see in the office. If what is going on with the prolapse is not completely investigated and other issues are not addressed, then failure results because you did not make the diagnosis. Certainly, modify the procedure according to what you find intraoperatively, but follow through.
Indications and tips for sacrocolpopexy
Dr. Gebhart: What are the indications for sacrocolpopexy?
Dr. Ridgeway: Indications include recurrent apical prolapse, posthysterectomy prolapse, or severe prolapse in someone quite young. It is a fantastic operation with overall low risks, but this needs to be discussed with the patient.
Dr. Walters: There are some unusual circumstances—for example, the woman has a short prolapsed vagina, usually after a prior surgery—in which the best repair is a bridging piece of mesh, usually done laparoscopically, because those operations cannot be done very well vaginally to obtain a durable result.
Dr. Karram: I agree. I do not think that all recurrent prolapses mandate a sacrocolpopexy. You need to individualize, but in general the short prolapsed vagina and patients who are very young are at high risk for a recurrence.
Dr. Gebhart: An older patient might be a very good candidate, even if she had recurrence from another vaginal repair.
Beri, does the patient with a high body mass index need augmentation?
Dr. Ridgeway: That is a great question, and this has to be individualized because, while heavier patients can benefit from augmentation, in a very heavy patient, getting into that abdomen has its own set of challenges. Anatomically they get a better repair with a mesh-augmented repair like a sacrocolpopexy, but they do have increased risks. That is important to acknowledge and clarify with the patient.
Dr. Gebhart: Any surgical tip you might offer on sacrocolpopexy?
Dr. Ridgeway: Perform the operation in the same way you would an open procedure. Meaning, use the same materials, the same sutures, the same placement, and the same type of dissection in order to obtain results similar to those with an open operation. Using your assistants to manipulate the vagina and rectum is important, as well as exposure and typical careful surgical technique.
Dr. Gebhart: What is important about the placement of sutures on the anterior longitudinal ligament, and what do you need to be cognizant of?
Dr. Ridgeway: Be careful of that left common iliac vein that is a little more medial than you would expect and of the middle sacral artery, and try to differentiate between L5 and S1. In an ideal circumstance, place the suture at S1 or L5 but not the inner disc space, which is the area to avoid placement.
Historically, the recommendation is S1. Some people do L5 because of some pull out strength studies, but also because it is easier, and sometimes in that area of the anterior longitudinal ligament is much better. The key is to do enough dissection and use haptic feedback, especially with conventional laparoscopy or an open approach, to avoid placing sutures through the disc space, as there is some concern that it increases the risk for discitis or osteomyelitis in that area.
Continue to: We also have found...
Dr. Gebhart: We also have found that if you have a combined surgery with colorectal colleagues, like a rectal prolapse repair, there is a little higher risk of discitis.
Dr. Ridgeway: In my own practice I saw a combined case with a rectopexy in someone who had a biologic mesh erosion. When we reviewed the literature, a number of reported cases of discitis had either an early post-op or concurrent urinary tract infection or vaginal infection that likely predisposed them to an infection that traveled up the material.
Dr. Karram: My final comment is that a sacrocolpopexy is not a few stitches or a little mesh right at the apex. If the patient has an isolated enterocele, okay, but it is a wide mesh for a reason and it should connect to the endopelvic fascia anteriorly, posteriorly. It is a mistake to suture just a little bit of the cuff and grab it and think, “I’ve done a colpopexy” when the procedure has not been executed as it should be.
Dr. Gebhart: I want to thank our expert panel and OBG M
Continue to: Some procedures call for cystoscopy...
Dr. Gebhart: Is cystoscopy necessary in patients undergoing native tissue repair or abdominal approaches to prolapse, and should the experienced generalist have this skill?
Dr. Walters: If you are going to do prolapse surgery or surgery for stress urinary incontinence, you need to learn to do cystoscopy. Almost all specialists in urogynecology and urology would do a cystoscopy at the time of a native tissue prolapse repair, a mesh-augmented prolapse repair, or a sling procedure. Whether a generalist doing simple hysterectomies needs to do cystoscopy is controversial, and it is probably based on risk assessment of the kind of hysterectomy being done. Definitely, if you are doing prolapse repair, you probably should be doing cystoscopy at the same time.
Dr. Karram: I would take it further. For certain procedures, cystoscopy is standard of care. For example, if you are doing anything around the uterosacral ligaments, whether a McCall culdoplasty or uterosacral suspension, it is standard of care. It would be a difficult medical-legal defense issue if it was not done in those cases.
To Mark’s point, it is controversial whether universal cystoscopy should be performed on every hysterectomy or every anterior to posterior repair. We are not there yet, but certainly it is in your best interest to have a very low threshold, so if you think about doing cystoscopy, you should probably do it.
Dr. Gebhart: Is cystoscopy needed in sacrocolpopexy?
Dr. Ridgeway: We know from our own data that the risk of lower urinary tract injury is very low with sacrocolpopexy. Having said that, I agree with the position statement of the American Urogynecologic Society that says, “Universal cystoscopy should be performed at the time of all pelvic reconstruction surgeries, with the exception of operations solely for posterior compartment defects.”1
Dr. Gebhart: The reality is that we just want to identify if there is a problem or not at the time of the surgery. It does not mean you have to manage it. You could get your partner, your urologist, or another person with expertise to come in to help you.
Dr. Ridgeway: Absolutely, because intraoperative identification and treatment will prevent many unfavorable outcomes in the postoperative period.
Reference
1. Cohen SA, Carberry CL, Smilen SW. American Urogynecologic Society Consensus Statement: cystoscopy at the time of prolapse repair. Female Pelvic Med Reconstr Surg. 2018;24:258-259.
Dr. Gebhart: If a patient is a smoker and/or utilizes tobacco and you think she is a candidate for a sacrocolpopexy, are there any special considerations? How would you counsel that patient?
Dr. Walters: The risk of mesh erosion is high enough that I would try to not do any mesh prolapse repair in a woman who was a smoker, especially a heavy smoker. A more common situation is, would I put a polypropylene midurethral sling in that patient? I usually am willing to do that because it is still the best option compared with the no-mesh options. In a patient who would be a good candidate for sacrocolpopexy, I can usually do a no-mesh surgery and keep the risk low. I could always give the woman an option to quit smoking, but that tends not to be successful.
Dr. Gebhart: What is the risk of using mesh in a smoker?
Dr. Walters: An increased risk of erosion through the vaginal walls. I am not sure of the magnitude of risk, maybe 2 or 3 times higher. That is high enough that I probably would not take the risk except in unusual circumstances.
Dr. Ridgeway: A good amount of data show increased risk of mesh exposure for smokers. Those patients also tend to have a higher risk of prolapse recurrence because of coughing. Sacrocolpopexy is not my favorite operation to do in a smoker. I will work with the patient to quit, but often if it is the right operation, I will do it, with preoperative estrogen and appropriate conseling.
Dr. Gebhart: Is there still a role for vaginal mesh? While it is no longer being sold in the United States, could you fashion your own mesh for a prolapse procedure?
Dr. Walters: I can do pretty much everything I need to do without adding transvaginal mesh, and if I need a meshaugmented repair, then I would go with the sacrocolpopexy route. Having said that, data for hysteropexy do show that a mesh-augmented hysteropexy could have some advantages, whether you do it with a kit or some fashioned pieces of mesh. Most of the experiences with this are outside of the United States, so we need much more standardization of technique and tracking to answer that question.
Dr. Gebhart: Mickey, what are your thoughts regarding someone who thinks, “Mesh has been good for me, I want to stay with that. I’m going to cut my own mesh”? Are they assuming some liability now that companies are no longer marketing mesh for vaginal repair?
Dr. Karram: Unfortunately, I really think they are. It would be easy to be put in a legal corner and asked, the FDA felt that this should be pulled off the market, why are you still utilizing it? At the end of the day, what the FDA said was not inaccurate.
The studies have not shown a significant better outcome with mesh, and it is an extra intervention that, again, in the best of hands is going to have some issues. That is a dilemma many surgeons faced because they felt that that was their main way of treating prolapse—”they took away my way of successfully treating patients for years.” I do think it increases their medical-legal liability.
Dr. Ridgeway: I agree that it does increase medical-legal liability, and I can’t imagine a situation in which I would offer that. Dr. Gebhart: There are risks with all procedures, including slings for stress incontinence, but sling use is appropriate in appropriately counseled patients.
Dr. Ridgeway: Correct. I feel very strongly that the risk profile for the midurethral sling is very different from that for transvaginal mesh. Very large data sets in large groups of people support that the outcomes are favorable and the risk profile is low. Having said that, slings are not risk free, but living with severe incontinence is not risk free either.
- US Food and Drug Administration. FDA takes action to protect women's health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical-mesh-intended-transvaginal. April 16, 2019. Accessed January 14, 2020.
- Detollenaere RJ, den Boon J, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with suspension of the uterosacral ligaments in women with uterine prolapse stage 2 or higher: multicentre randomised non-inferiority trial. BMJ. 2015;351:h3717.
- US Food and Drug Administration. FDA takes action to protect women's health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical-mesh-intended-transvaginal. April 16, 2019. Accessed January 14, 2020.
- Detollenaere RJ, den Boon J, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with suspension of the uterosacral ligaments in women with uterine prolapse stage 2 or higher: multicentre randomised non-inferiority trial. BMJ. 2015;351:h3717.
Consider sparing the uterus in prolapse procedures
LAS VEGAS – A female pelvic medicine and reconstructive surgeon urged colleagues to consider uterus-sparing hysteropexies instead of hysterectomies in pelvic organ prolapse repairs.
said Beri M. Ridgeway, MD, of the Cleveland Clinic, at the Pelvic Anatomy and Gynecologic Surgery Symposium. Even so, “in the U.S., gynecologists rarely offer uterine preservation for women who desire repair of their uterovaginal prolapse.”
According to research compiled by Dr. Ridgeway, about 74,000 hysterectomies are performed each year in the United States to treat pelvic organ prolapse. The procedure became standard in the second half of the 20th century, in part to reduce cancer risk.
But attitudes evolved starting in the 1990s “as we have had better cancer screening and more focus on patient sexuality, patient autonomy, and quality of life,” Dr. Ridgeway said.
She offered these reasons to question hysterectomies to treat pelvic organ prolapse repairs:
- It’s not clear whether hysterectomies address the anatomic problems that produce prolapse in the first place. “Prolapse is caused by weakened or damaged tissue – connective tissue, muscles, etc.,” she said in an interview. “The problem is what is supporting the uterus, not the uterus itself.”
- Despite assumptions, women don’t necessarily prefer hysterectomy. Dr. Ridgeway pointed to a 2013 study in which researchers surveyed 213 women with prolapse symptoms about their preferred treatment, assuming that outcomes were the same. The results: 36% preferred uterine preservation, 20% preferred hysterectomy, and 44% reported no strong preference (Am J Obstet Gynecol. 2013 Nov;209[5]:470.e1-6.).
- Hysterectomies hasten menopause.
There has been a perception that uterus removal is appropriate in women who don’t wish to have any more children, Dr. Ridgeway said. “You had your babies, you’re done, you don’t need this anymore.” In fact, “that’s basically not true.”
As she explained, hysterectomy is linked to earlier menopause, and “even losing one ovary pushed patients into menopause significantly earlier.” She pointed to a 2016 Australian study, which found that “women who have a hysterectomy (with ovarian conservation) have a higher risk of hot flushes and night sweats that persist over an extended period” (Maturitas. 2016 Sep;91:1-7).
There’s no consensus on how hysterectomy affects sexual function. However, Dr. Ridgeway noted, it’s clear that pelvic floor disorders disrupt sexual function, and most women see improvement after surgical treatment.
The rate of uterine pathology is low in hysterectomy. Dr. Ridgeway highlighted a 2018 study of 24,076 women who underwent hysterectomy for benign indications. The study reported that “prevalence of occult corpus uteri, cervical, and ovarian malignancy was 1.44%, 0.60%, and 0.19%, respectively, among women undergoing hysterectomy and it varied by patient age and surgical route” (Obstet Gynecol. 2018 Apr;131[4]:642-51).
As an alternative, Dr. Ridgeway pointed to hysteropexy, which can be performed as a vaginal, laparoscopic, robot, or open procedure.
She highlighted a 2018 systematic review of pelvic organ prolapse surgeries that provided a meta-analysis and clinical practice guidelines. It found that “uterine-preserving prolapse surgeries improve operating time, blood loss, and risk of mesh exposure, compared with similar surgical routes with concomitant hysterectomy and do not significantly change short-term prolapse outcomes” (Am J Obstet Gynecol. 2018 Aug;219[2]:129-46.e2).
Dr. Ridgeway reported no relevant disclosures. This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – A female pelvic medicine and reconstructive surgeon urged colleagues to consider uterus-sparing hysteropexies instead of hysterectomies in pelvic organ prolapse repairs.
said Beri M. Ridgeway, MD, of the Cleveland Clinic, at the Pelvic Anatomy and Gynecologic Surgery Symposium. Even so, “in the U.S., gynecologists rarely offer uterine preservation for women who desire repair of their uterovaginal prolapse.”
According to research compiled by Dr. Ridgeway, about 74,000 hysterectomies are performed each year in the United States to treat pelvic organ prolapse. The procedure became standard in the second half of the 20th century, in part to reduce cancer risk.
But attitudes evolved starting in the 1990s “as we have had better cancer screening and more focus on patient sexuality, patient autonomy, and quality of life,” Dr. Ridgeway said.
She offered these reasons to question hysterectomies to treat pelvic organ prolapse repairs:
- It’s not clear whether hysterectomies address the anatomic problems that produce prolapse in the first place. “Prolapse is caused by weakened or damaged tissue – connective tissue, muscles, etc.,” she said in an interview. “The problem is what is supporting the uterus, not the uterus itself.”
- Despite assumptions, women don’t necessarily prefer hysterectomy. Dr. Ridgeway pointed to a 2013 study in which researchers surveyed 213 women with prolapse symptoms about their preferred treatment, assuming that outcomes were the same. The results: 36% preferred uterine preservation, 20% preferred hysterectomy, and 44% reported no strong preference (Am J Obstet Gynecol. 2013 Nov;209[5]:470.e1-6.).
- Hysterectomies hasten menopause.
There has been a perception that uterus removal is appropriate in women who don’t wish to have any more children, Dr. Ridgeway said. “You had your babies, you’re done, you don’t need this anymore.” In fact, “that’s basically not true.”
As she explained, hysterectomy is linked to earlier menopause, and “even losing one ovary pushed patients into menopause significantly earlier.” She pointed to a 2016 Australian study, which found that “women who have a hysterectomy (with ovarian conservation) have a higher risk of hot flushes and night sweats that persist over an extended period” (Maturitas. 2016 Sep;91:1-7).
There’s no consensus on how hysterectomy affects sexual function. However, Dr. Ridgeway noted, it’s clear that pelvic floor disorders disrupt sexual function, and most women see improvement after surgical treatment.
The rate of uterine pathology is low in hysterectomy. Dr. Ridgeway highlighted a 2018 study of 24,076 women who underwent hysterectomy for benign indications. The study reported that “prevalence of occult corpus uteri, cervical, and ovarian malignancy was 1.44%, 0.60%, and 0.19%, respectively, among women undergoing hysterectomy and it varied by patient age and surgical route” (Obstet Gynecol. 2018 Apr;131[4]:642-51).
As an alternative, Dr. Ridgeway pointed to hysteropexy, which can be performed as a vaginal, laparoscopic, robot, or open procedure.
She highlighted a 2018 systematic review of pelvic organ prolapse surgeries that provided a meta-analysis and clinical practice guidelines. It found that “uterine-preserving prolapse surgeries improve operating time, blood loss, and risk of mesh exposure, compared with similar surgical routes with concomitant hysterectomy and do not significantly change short-term prolapse outcomes” (Am J Obstet Gynecol. 2018 Aug;219[2]:129-46.e2).
Dr. Ridgeway reported no relevant disclosures. This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – A female pelvic medicine and reconstructive surgeon urged colleagues to consider uterus-sparing hysteropexies instead of hysterectomies in pelvic organ prolapse repairs.
said Beri M. Ridgeway, MD, of the Cleveland Clinic, at the Pelvic Anatomy and Gynecologic Surgery Symposium. Even so, “in the U.S., gynecologists rarely offer uterine preservation for women who desire repair of their uterovaginal prolapse.”
According to research compiled by Dr. Ridgeway, about 74,000 hysterectomies are performed each year in the United States to treat pelvic organ prolapse. The procedure became standard in the second half of the 20th century, in part to reduce cancer risk.
But attitudes evolved starting in the 1990s “as we have had better cancer screening and more focus on patient sexuality, patient autonomy, and quality of life,” Dr. Ridgeway said.
She offered these reasons to question hysterectomies to treat pelvic organ prolapse repairs:
- It’s not clear whether hysterectomies address the anatomic problems that produce prolapse in the first place. “Prolapse is caused by weakened or damaged tissue – connective tissue, muscles, etc.,” she said in an interview. “The problem is what is supporting the uterus, not the uterus itself.”
- Despite assumptions, women don’t necessarily prefer hysterectomy. Dr. Ridgeway pointed to a 2013 study in which researchers surveyed 213 women with prolapse symptoms about their preferred treatment, assuming that outcomes were the same. The results: 36% preferred uterine preservation, 20% preferred hysterectomy, and 44% reported no strong preference (Am J Obstet Gynecol. 2013 Nov;209[5]:470.e1-6.).
- Hysterectomies hasten menopause.
There has been a perception that uterus removal is appropriate in women who don’t wish to have any more children, Dr. Ridgeway said. “You had your babies, you’re done, you don’t need this anymore.” In fact, “that’s basically not true.”
As she explained, hysterectomy is linked to earlier menopause, and “even losing one ovary pushed patients into menopause significantly earlier.” She pointed to a 2016 Australian study, which found that “women who have a hysterectomy (with ovarian conservation) have a higher risk of hot flushes and night sweats that persist over an extended period” (Maturitas. 2016 Sep;91:1-7).
There’s no consensus on how hysterectomy affects sexual function. However, Dr. Ridgeway noted, it’s clear that pelvic floor disorders disrupt sexual function, and most women see improvement after surgical treatment.
The rate of uterine pathology is low in hysterectomy. Dr. Ridgeway highlighted a 2018 study of 24,076 women who underwent hysterectomy for benign indications. The study reported that “prevalence of occult corpus uteri, cervical, and ovarian malignancy was 1.44%, 0.60%, and 0.19%, respectively, among women undergoing hysterectomy and it varied by patient age and surgical route” (Obstet Gynecol. 2018 Apr;131[4]:642-51).
As an alternative, Dr. Ridgeway pointed to hysteropexy, which can be performed as a vaginal, laparoscopic, robot, or open procedure.
She highlighted a 2018 systematic review of pelvic organ prolapse surgeries that provided a meta-analysis and clinical practice guidelines. It found that “uterine-preserving prolapse surgeries improve operating time, blood loss, and risk of mesh exposure, compared with similar surgical routes with concomitant hysterectomy and do not significantly change short-term prolapse outcomes” (Am J Obstet Gynecol. 2018 Aug;219[2]:129-46.e2).
Dr. Ridgeway reported no relevant disclosures. This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAGS 2019
Product update: Neuromodulation device, cystoscopy simplified, hysteroscopy seal, next immunization frontier
NEW SACRAL NEUROMODULATION DEVICE
FOR MORE INFORMATION, VISIT: https://www.axonics.com/
CERVICAL SEAL FOR HYSTEROSCOPIC DEVICES
For more information, visit: https://gynsurgicalsolutions.com/product/omni-lok/
UNIVERSAL CYSTOSCOPY SIMPLIFIED
FOR MORE INFORMATION, VISIT: https://cystosure.com/
NEXT FRONTIER IN VACCINE IMMUNIZATION
Globally, there are 410,000 cases of GBS every year. GBS is most common in newborns; women who are carriers of the GBS bacteria may pass it on to their newborns during labor and birth. An estimated 10% to 30% of pregnant women carry the GBS bacteria. The disease can manifest as sepsis, pneumonia, and meningitis, with potentially fatal outcomes for some. A maternal vaccine may prevent 231,000 infant and maternal GBS cases, says Pfizer.
According to Pfizer, RSV causes more hospitalizations each year than influenza among young children, with an estimated 33 million cases globally each year in children less than age 5 years.
FOR MORE INFORMATION, VISIT: https://www.pfizer.com/
NEW SACRAL NEUROMODULATION DEVICE
FOR MORE INFORMATION, VISIT: https://www.axonics.com/
CERVICAL SEAL FOR HYSTEROSCOPIC DEVICES
For more information, visit: https://gynsurgicalsolutions.com/product/omni-lok/
UNIVERSAL CYSTOSCOPY SIMPLIFIED
FOR MORE INFORMATION, VISIT: https://cystosure.com/
NEXT FRONTIER IN VACCINE IMMUNIZATION
Globally, there are 410,000 cases of GBS every year. GBS is most common in newborns; women who are carriers of the GBS bacteria may pass it on to their newborns during labor and birth. An estimated 10% to 30% of pregnant women carry the GBS bacteria. The disease can manifest as sepsis, pneumonia, and meningitis, with potentially fatal outcomes for some. A maternal vaccine may prevent 231,000 infant and maternal GBS cases, says Pfizer.
According to Pfizer, RSV causes more hospitalizations each year than influenza among young children, with an estimated 33 million cases globally each year in children less than age 5 years.
FOR MORE INFORMATION, VISIT: https://www.pfizer.com/
NEW SACRAL NEUROMODULATION DEVICE
FOR MORE INFORMATION, VISIT: https://www.axonics.com/
CERVICAL SEAL FOR HYSTEROSCOPIC DEVICES
For more information, visit: https://gynsurgicalsolutions.com/product/omni-lok/
UNIVERSAL CYSTOSCOPY SIMPLIFIED
FOR MORE INFORMATION, VISIT: https://cystosure.com/
NEXT FRONTIER IN VACCINE IMMUNIZATION
Globally, there are 410,000 cases of GBS every year. GBS is most common in newborns; women who are carriers of the GBS bacteria may pass it on to their newborns during labor and birth. An estimated 10% to 30% of pregnant women carry the GBS bacteria. The disease can manifest as sepsis, pneumonia, and meningitis, with potentially fatal outcomes for some. A maternal vaccine may prevent 231,000 infant and maternal GBS cases, says Pfizer.
According to Pfizer, RSV causes more hospitalizations each year than influenza among young children, with an estimated 33 million cases globally each year in children less than age 5 years.
FOR MORE INFORMATION, VISIT: https://www.pfizer.com/
Can the office visit interval for routine pessary care be extended safely?
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2019 Dec 5. Doi: 10.1097/AOG.0000000000003580.
EXPERT COMMENTARY
Vaginal pessaries are a common and effective approach for managing pelvic organ prolapse (POP) as well as stress urinary incontinence (SUI). Vaginal mucosal erosions, however, may complicate pessary use. The risk for erosions may be associated with the frequency of pessary change, which involves removing the pessary, washing it, and replacing it in the vagina. Existing data do not address the frequency of pessary change. Recently, however, investigators conducted a randomized noninferiority trial to evaluate the effect of pessary visit intervals on the development of vaginal epithelial abnormalities.
Details of the study
At a single US hospital, Propst and colleagues randomly assigned women who used pessaries for POP, SUI, or both to routine pessary care (offices visits every 12 weeks) or to extended interval pessary care (office visits every 24 weeks). The women used ring, incontinence dish, or Gelhorn pessaries, did not change their pessaries on their own, and had no vaginal mucosal abnormalities.
A total of 130 women were randomly assigned, 64 to the routine care group and 66 to the extended interval care group. The mean age was 79 years and 90% were white, 4.6% were black, and 4% were Hispanic. Approximately 74% of the women used vaginal estrogen.
The primary outcome was the rate of vaginal epithelial abnormalities, including epithelial breaks or erosions. The predetermined noninferiority margin was set at 7.5%.
Results. At the 48-week follow-up, the rate of epithelial erosion was 7.4% in the routine care group and 1.7% in the extended interval care group, thus meeting the prespecified criteria for noninferiority of extended interval pessary care.
Women in each care group reported a similar amount of bothersome vaginal discharge. This was reported on a 5-point scale, with higher numbers indicating greater degree of bother. The mean scores were 1.39 in the routine care group and 1.34 in the extended interval care group. No other pessary-related adverse events occurred in either care group.
Study strengths and limitations
This trial provides good evidence that the timing of office pessary care can be extended to 24 weeks without compromising outcomes. However, since nearly three-quarters of the study participants used vaginal estrogen, the results may not be applicable to pessary users who do not use vaginal estrogen.
Many women change their pessary at home as often as weekly or daily. For women who rely on office visits for pessary care, however, the trial by Propst and colleagues provides good quality evidence that pessaries can be changed as infrequently as every 24 weeks without compromising outcomes. An important limitation of these data is that since most study participants used vaginal estrogen, the findings may not apply to pessary use among women who do not use vaginal estrogen.
ANDREW M. KAUNITZ, MD, NCMP
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2019 Dec 5. Doi: 10.1097/AOG.0000000000003580.
EXPERT COMMENTARY
Vaginal pessaries are a common and effective approach for managing pelvic organ prolapse (POP) as well as stress urinary incontinence (SUI). Vaginal mucosal erosions, however, may complicate pessary use. The risk for erosions may be associated with the frequency of pessary change, which involves removing the pessary, washing it, and replacing it in the vagina. Existing data do not address the frequency of pessary change. Recently, however, investigators conducted a randomized noninferiority trial to evaluate the effect of pessary visit intervals on the development of vaginal epithelial abnormalities.
Details of the study
At a single US hospital, Propst and colleagues randomly assigned women who used pessaries for POP, SUI, or both to routine pessary care (offices visits every 12 weeks) or to extended interval pessary care (office visits every 24 weeks). The women used ring, incontinence dish, or Gelhorn pessaries, did not change their pessaries on their own, and had no vaginal mucosal abnormalities.
A total of 130 women were randomly assigned, 64 to the routine care group and 66 to the extended interval care group. The mean age was 79 years and 90% were white, 4.6% were black, and 4% were Hispanic. Approximately 74% of the women used vaginal estrogen.
The primary outcome was the rate of vaginal epithelial abnormalities, including epithelial breaks or erosions. The predetermined noninferiority margin was set at 7.5%.
Results. At the 48-week follow-up, the rate of epithelial erosion was 7.4% in the routine care group and 1.7% in the extended interval care group, thus meeting the prespecified criteria for noninferiority of extended interval pessary care.
Women in each care group reported a similar amount of bothersome vaginal discharge. This was reported on a 5-point scale, with higher numbers indicating greater degree of bother. The mean scores were 1.39 in the routine care group and 1.34 in the extended interval care group. No other pessary-related adverse events occurred in either care group.
Study strengths and limitations
This trial provides good evidence that the timing of office pessary care can be extended to 24 weeks without compromising outcomes. However, since nearly three-quarters of the study participants used vaginal estrogen, the results may not be applicable to pessary users who do not use vaginal estrogen.
Many women change their pessary at home as often as weekly or daily. For women who rely on office visits for pessary care, however, the trial by Propst and colleagues provides good quality evidence that pessaries can be changed as infrequently as every 24 weeks without compromising outcomes. An important limitation of these data is that since most study participants used vaginal estrogen, the findings may not apply to pessary use among women who do not use vaginal estrogen.
ANDREW M. KAUNITZ, MD, NCMP
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2019 Dec 5. Doi: 10.1097/AOG.0000000000003580.
EXPERT COMMENTARY
Vaginal pessaries are a common and effective approach for managing pelvic organ prolapse (POP) as well as stress urinary incontinence (SUI). Vaginal mucosal erosions, however, may complicate pessary use. The risk for erosions may be associated with the frequency of pessary change, which involves removing the pessary, washing it, and replacing it in the vagina. Existing data do not address the frequency of pessary change. Recently, however, investigators conducted a randomized noninferiority trial to evaluate the effect of pessary visit intervals on the development of vaginal epithelial abnormalities.
Details of the study
At a single US hospital, Propst and colleagues randomly assigned women who used pessaries for POP, SUI, or both to routine pessary care (offices visits every 12 weeks) or to extended interval pessary care (office visits every 24 weeks). The women used ring, incontinence dish, or Gelhorn pessaries, did not change their pessaries on their own, and had no vaginal mucosal abnormalities.
A total of 130 women were randomly assigned, 64 to the routine care group and 66 to the extended interval care group. The mean age was 79 years and 90% were white, 4.6% were black, and 4% were Hispanic. Approximately 74% of the women used vaginal estrogen.
The primary outcome was the rate of vaginal epithelial abnormalities, including epithelial breaks or erosions. The predetermined noninferiority margin was set at 7.5%.
Results. At the 48-week follow-up, the rate of epithelial erosion was 7.4% in the routine care group and 1.7% in the extended interval care group, thus meeting the prespecified criteria for noninferiority of extended interval pessary care.
Women in each care group reported a similar amount of bothersome vaginal discharge. This was reported on a 5-point scale, with higher numbers indicating greater degree of bother. The mean scores were 1.39 in the routine care group and 1.34 in the extended interval care group. No other pessary-related adverse events occurred in either care group.
Study strengths and limitations
This trial provides good evidence that the timing of office pessary care can be extended to 24 weeks without compromising outcomes. However, since nearly three-quarters of the study participants used vaginal estrogen, the results may not be applicable to pessary users who do not use vaginal estrogen.
Many women change their pessary at home as often as weekly or daily. For women who rely on office visits for pessary care, however, the trial by Propst and colleagues provides good quality evidence that pessaries can be changed as infrequently as every 24 weeks without compromising outcomes. An important limitation of these data is that since most study participants used vaginal estrogen, the findings may not apply to pessary use among women who do not use vaginal estrogen.
ANDREW M. KAUNITZ, MD, NCMP
Medical management of abnormal uterine bleeding in reproductive-age women
Case 1 Multiparous woman presents with heavy regular menses
Over the past several years, a 34-year-old woman has noted increasing intensity and duration of menstrual flow, which now persists for 8 days and includes clots “the size of quarters” and soaks a pad within 1 hour. Sometimes she misses or leaves work on her heaviest days of flow. She reports that menstrual cramps prior to and during flow are increasingly bothersome and do not respond adequately to ibuprofen. She intermittently uses condoms for contraception. She does not wish to be pregnant currently; however, she recently entered into a new relationship and may wish to conceive in the future.
On bimanual examination, the uterus appears bulky. Her hemoglobin is 10.9 g/dL with low mean corpuscular volume and a serum ferritin level indicating iron depletion. Pelvic ultrasonography suggests uterine adenomyosis; no fibroids are imaged (FIGURE 1).
You advise the patient to take ferrous sulfate 325 mg every other day. After discussion with the patient regarding different treatment options, she chooses to proceed with placement of a 52-mg levonorgestrel (LNG) intrauterine device (IUD; Mirena or Liletta).
Case 2 Older adolescent presents with irregular bleeding
A 19-year-old patient reports approximately 6 bleeding episodes each year. She reports the duration of her bleeding as variable, and sometimes the bleeding is heavy with small clots passed. She has been previously diagnosed with polycystic ovary syndrome (PCOS). Combination estrogen-progestin oral contraceptives have been prescribed several times in the past, but she always has discontinued them due to nausea. The patient is in a same-sex relationship and does not anticipate being sexually active with a male. She reports having to shave her mustache and chin twice weekly for the past 1 to 2 years.
On physical examination, the patient is obese (body mass index [BMI], 32 kg/m2), facial acne and hirsutism are present, and hair extends from the mons toward the umbilicus. Bimanual examination reveals a normal size, mobile, nontender uterus without obvious adnexal pathology. Pelvic ultrasonography demonstrates a normal-appearing uterus with multiplanar endometrium (consistent with proliferative changes) (FIGURE 2). Ovarian imaging demonstrates ≥12 follicles per image (FIGURE 3).
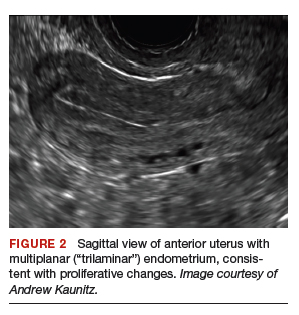

After reviewing various treatment options, you prescribe oral medroxyprogesterone acetate 20 mg (two 10-mg tablets) daily in a continuous fashion. You counsel her that she should not be surprised or concerned if frequent or even continuous bleeding occurs initially, and that she should continue this medication despite the occurrence of such.
About one-third of all women experience abnormal uterine bleeding (AUB) sometime during their lifetime and AUB can impair quality of life.1 Surgical management, including hysterectomy and endometrial ablation, plays an important role in the management of AUB in patients who do not desire future pregnancies. However, many cases of AUB occur in women who may not have completed childbearing or in women who prefer to avoid surgery.2 AUB can be managed effectively medically in most cases.1 Accordingly, in this review, we focus on nonsurgical management of AUB.
Continue to: Because previously used terms, including...
Because previously used terms, including menorrhagia and meno-metrorrhagia, were inconsistently defined and confusing, the International Federation of Gynecology and Obstetrics introduced updated terminology in 2011 to better describe and characterize AUB in nonpregnant women. Heavy menstrual bleeding (HMB) refers to ovulatory (cyclic) bleeding that is more than 8 days’ duration, or sufficiently heavy to impair a woman’s quality of life. HMB is a pattern of AUB distinct from the irregular bleeding pattern typically caused by ovulatory dysfunction (AUB-O).1
Clinical evaluation
Obtain menstrual history. In addition to a medical, surgical, and gynecologic history, a thorough menstrual history should be obtained to further characterize the patient’s bleeding pattern. In contrast to the cyclical or ovulatory bleeding seen with HMB, bleeding associated with inconsistent ovulation (AUB-O) is unpredictable or irregular, and is commonly associated with PCOS. AUB-O is also encountered in recently menarchal girls (secondary to immaturity of the hypothalamic-pituitary-gonadal axis) and in those who are perimenopausal. In addition, medications that can induce hyperprolactinemia (such as certain antipsychotics) can cause AUB-O.
Evaluate for all sources of bleeding. Be sure to evaluate for extrauterine causes of bleeding, including the cervix, vagina, vulva, or the urinary or gastrointestinal tracts for bleeding. Intermenstrual bleeding occurring between normal regular menses may be caused by an endometrial polyp, submucosal fibroid, endometritis, or an IUD. The patient report of postcoital bleeding suggests that cervical disease (cervicitis, polyp, or malignancy) may be present. Uterine leiomyoma or adenomyosis represent common causes of HMB. However, HMB also may be caused by a copper IUD, coagulation disorders (including von Willebrand disease), or use of anticoagulant medications. Hormonal contraceptives also can cause irregular bleeding.
Perform a pelvic examination and measure vital signs. The presence of fever suggests the possible presence of pelvic inflammatory disease (PID), while orthostatic hypotension raises the possibility of hypovolemia. When vaginal speculum examination is performed, a cervical cause of abnormal bleeding may be noted. The presence of fresh or old blood or finding clots in the vaginal vault or at the cervical os are all consistent with AUB. A bimanual examination that reveals an enlarged or lobular uterus suggests leiomyoma or adenomyosis. Cervical or adnexal tenderness is often noted in women with PID, which itself may be associated with endometritis. The presence of hyperandrogenic signs on physical examination (eg, acne, hirsutism, or clitoromegaly) suggests PCOS. The finding of galactorrhea suggests that hyperprolactinemia may be present.
Laboratory assessment
Test for pregnancy, cervical disease, and sexually transmitted infection when appropriate. Pregnancy testing is appropriate for women with AUB aged 55 years or younger. If patients with AUB are not up to date with normal cervical cancer screening results, cervical cytology and/or human papillomavirus testing should be performed. Testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis should be performed in patients:
- younger than 25 years
- when the history indicates new or multiple sexual partners, or
- when vaginal discharge, cervicitis, cervical motion, or adnexal tenderness is present.
Continue to: Obtain a complete blood count and serum ferritin levels...
Obtain a complete blood count and serum ferritin levels. In women presenting with HMB, iron depletion and iron deficiency anemia are common. The finding of leukocytosis raises the possibility of PID or postpartum endometritis. In women with presumptive AUB-O, checking the levels of thyroid-stimulating hormone, free T4, and prolactin should be performed.
Screen for a hemostasis disorder. Women with excessive menstrual bleeding should be clinically screened for an underlying disorder of hemostasis (TABLE 1).3 When a hemostasis disorder is suspected, initial laboratory evaluation includes a partial thromboplastin time, prothrombin time, activated partial thromboplastin time, and fibrinogen. Women who have a positive clinical screen for a possible bleeding disorder or abnormal initial laboratory test results for disorders of hemostasis should undergo further laboratory evaluation, including von Willebrand factor antigen, ristocetin cofactor assay, and factor VIII. Consultation with a hematologist should be considered in these cases.

Perform endometrial biopsy when indicated
After excluding pregnancy, endometrial biopsy (through pipelle biospy or brush sampling; FIGURE 4) should be performed in women with AUB who are at increased risk for endometrial neoplasia. The prevalence of endometrial neoplasia is substantially higher among women ≥45 years of age4 and among patients with AUB who are also obese (BMI, ≥30 kg/m2).5 In addition, AUB patients with unopposed estrogen exposure (presumed anovulation/PCOS), as well as those with persistent AUB or failed medical management, should undergo endometrial biopsy.6
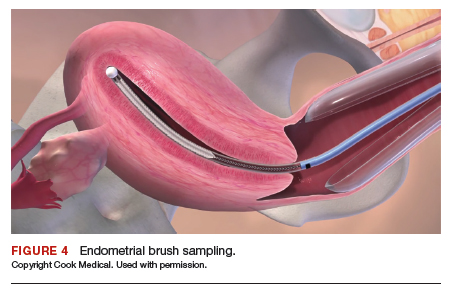
Utilize transvaginal ultrasonography
Transvaginal ultrasonography is often useful in the evaluation of patients with AUB, as it may identify uterine fibroids or adenomyosis, suggest intracavitary pathology (such as an endometrial polyp or submucosal fibroid), or raise the possibility of PCOS. In virginal patients or those in whom vaginal ultrasound is not appropriate, abdominal pelvic ultrasonography represents appropriate imaging. If unenhanced ultrasound suggests endometrial polyps or fibroids within the endometrial cavity, an office-based saline infusion sonogram (sonohysterogram) (FIGURE 5) or hysteroscopy should be performed. Targeted endometrial sampling and biopsy of intracavitary pathology can be performed at the time of hysteroscopy.
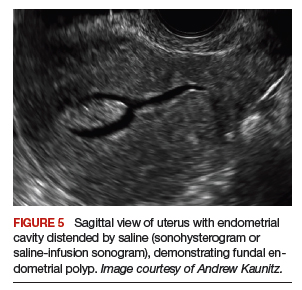
Treatment
When HMB impairs quality of life, is bothersome to the patient, or results in anemia, treatment is appropriate. Although bleeding episodes in women with AUB-O may be infrequent (as with Case 2), treatment prevents heavy or prolonged bleeding episodes as well as endometrial neoplasia that may otherwise occur in anovulatory women.
Many women with AUB can be managed medically. However, treatment choices will vary with respect to the patient’s desire for future fertility, medical comorbidities, personal preferences, and financial barriers. While many women may prefer outpatient medical management (TABLE 2),7-14 others might desire surgical therapy, including endometrial ablation or hysterectomy.

Oral contraceptives
Combination estrogen-progestin oral contraceptives represent appropriate initial therapy for many women in the reproductive-age group with AUB, whether women have HMB or AUB-O. However, contraceptive doses of estrogen are not appropriate for some women with risk factors for cardiovascular disease, including those who smoke cigarettes and are age ≥35 years or those who have hypertension (TABLE 3).15,16
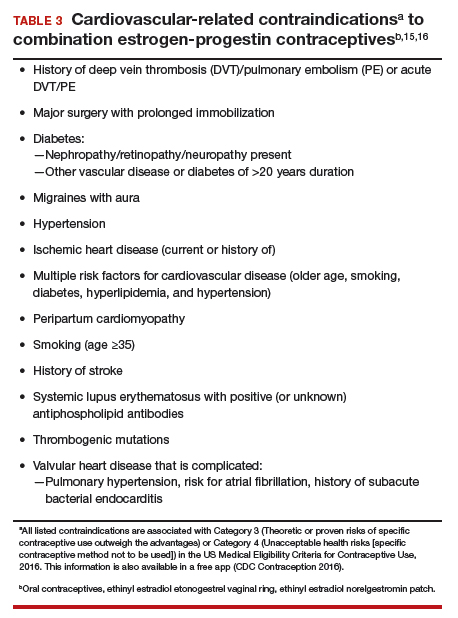
Continue to: Menopausal dosages of HT...
Menopausal dosages of HT
If use of contraceptive doses of estrogen is not appropriate, continuous off-label use of menopausal combination formulations (physiologic dosage) of hormonal therapy (HT; ie, lower doses of estrogen than contraceptives) may be effective in reducing or eliminating AUB. Options for menopausal combination formulations include generic ethinyl estradiol 5 µg/norethindrone acetate 1 mg or estradiol 1 mg/norethindrone acetate 0.5 mg.7 High-dose oral progestin therapy (norethindrone acetate 5 mg tablet once daily or medroxyprogesterone acetate 10 mg tablets 1–3 times daily) also can be used when combination contraceptives are contraindicated and may be more effective than lower-dose combination formulations.
Package labeling, as well as some guidelines, indicate that oral progestins used to treat AUB should be taken cyclically.8 However, continuous daily use is easier for many patients and may be more effective in reducing bleeding. Accordingly, we counsel patients with AUB who are using progestins and who do not wish to conceive to take these medications continuously. High-dose oral progestin therapy may cause bloating, dysphoria, and increased appetite/weight gain. Women initiating hormonal management (including the progestin IUDs detailed below) for AUB should be counseled that irregular or even continuous light bleeding/spotting is common initially, but this bleeding pattern typically decreases with continued use.
IUDs
The LNG 52 mg IUD (Mirena or Liletta) effectively treats HMB, reducing bleeding in a manner comparable to that of endometrial ablation.9,10 The Mirena IUD is approved for treatment of HMB in women desiring intrauterine contraception. In contrast to oral medications, use of progestin IUDs does not involve daily administration and may represent an attractive option for women with HMB who would like to avoid surgery or preserve fertility. With ongoing use, continuous oral or intrauterine hormonal management may result in amenorrhea in some women with AUB.
When the LNG 52 mg IUD is used to treat HMB, the menstrual suppression impact may begin to attenuate after approximately 4 years of use; in this setting, replacing the IUD often restores effective menstrual suppression.11 The LNG 52 mg IUD effectively suppresses menses in women with coagulation disorders; if menstrual suppression with the progestin IUD is not adequate in this setting, it may be appropriate to add an oral combination estrogen-progestin contraceptive or high-dose oral progestin.11,12
NSAIDs and tranexamic acid
Off-label use of nonsteroidal anti-inflammatory drugs (naproxen 500–1,000 mg daily for 5 days beginning at the onset of menstrual flow or tranexamic acid two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can suppress HMB and is useful for women who prefer to avoid or have contraindications to hormonal treatments.13,14 Unfortunately, these agents are not as effective as hormonal management in treating AUB.
Iron supplementation is often needed
Iron depletion commonly results from HMB, often resulting in iron deficiency anemia. When iron depletion (readily identified by checking a serum ferritin level) or iron deficiency anemia is identified, iron supplementation should be recommended. Every-other-day administration of iron supplements maximizes iron absorption while minimizing the adverse effects of unabsorbed iron, such as nausea. Sixty mg of elemental iron (ferrous sulfate 325 mg) administered every other day represents an inexpensive and effective treatment for iron deficiency/anemia.17 In patients who cannot tolerate oral iron supplementation or for those in whom oral therapy is not appropriate or effective, newer intravenous iron formulations are safe and effective.18
Continue to: Case 1 Follow-up...
Case 1 Follow-up
The patient noted marked improvement in her menstrual cramps following LNG-containing IUD placement. Although she also reported that she no longer experienced heavy menstrual flow or cramps, she was bothered by frequent, unpredictable light bleeding/spotting. You prescribed norethindrone acetate (NETA) 5-mg tablet orally once daily, to be used in addition to her IUD. After using the IUD with concomitant NETA for 2 months’ duration, she noted that her bleeding/spotting almost completely resolved; however, she did report feeling irritable with use of the progestin tablets. She subsequently stopped the NETA tablets and, after 6 months of additional follow-up, reported only minimal spotting and no cramps.
At this later follow-up visit, you noted that her hemoglobin level increased to 12.6 g/dL, and the ferritin level no longer indicated iron depletion. After the IUD had been in place for 4 years, she reported that she was beginning to experience frequent light bleeding again. A follow-up vaginal sonogram noted a well-positioned IUD, there was no suggestion of intracavitary pathology, and adenomyosis continued to be imaged. She underwent IUD removal and placement of a new LNG 52 mg IUD. This resulted in marked reduction in her bleeding.
Case 2 Follow-up
Two weeks after beginning continuous oral progestin therapy, the patient called reporting frequent irregular bleeding. She was reassured that this was not unexpected and encouraged to continue oral progestin therapy. During a 3-month follow-up visit, the patient noted little, if any, bleeding over the previous 2 months and was pleased with this result. She continued to note acne and hirsutism and asked about the possibility of adding spironolactone to her oral progestin regimen.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet. 2018;143:393-408.
- Kaunitz AM. Abnormal uterine bleeding in reproductive-age women. JAMA. 2019;321:2126-2127.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer. http://seer.cancer.gov/statfacts/html/corp.html. Accessed October 10, 2019.
- Wise MR, Gill P, Lensen S, et al. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215:598.e1-598.e8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- The North American Menopause Society. Menopause Practice–A Clinician’s Guide. 5th ed. NAMS: Mayfield Heights, OH; 2014.
- National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. https://www.nice.org.uk/guidance/ng88. Accessed October 10, 2019.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
- Kaunitz AM, Meredith S, Inki P, et al. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104-1116.
- Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8.
- Ylikorkala O, Pekonen F. Naproxen reduces idiopathic but not fibromyoma-induced menorrhagia. Obstet Gynecol. 1986;68:10-12.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:865-875.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1–103.
- ACOG Practice Bulletin no. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
Case 1 Multiparous woman presents with heavy regular menses
Over the past several years, a 34-year-old woman has noted increasing intensity and duration of menstrual flow, which now persists for 8 days and includes clots “the size of quarters” and soaks a pad within 1 hour. Sometimes she misses or leaves work on her heaviest days of flow. She reports that menstrual cramps prior to and during flow are increasingly bothersome and do not respond adequately to ibuprofen. She intermittently uses condoms for contraception. She does not wish to be pregnant currently; however, she recently entered into a new relationship and may wish to conceive in the future.
On bimanual examination, the uterus appears bulky. Her hemoglobin is 10.9 g/dL with low mean corpuscular volume and a serum ferritin level indicating iron depletion. Pelvic ultrasonography suggests uterine adenomyosis; no fibroids are imaged (FIGURE 1).
You advise the patient to take ferrous sulfate 325 mg every other day. After discussion with the patient regarding different treatment options, she chooses to proceed with placement of a 52-mg levonorgestrel (LNG) intrauterine device (IUD; Mirena or Liletta).
Case 2 Older adolescent presents with irregular bleeding
A 19-year-old patient reports approximately 6 bleeding episodes each year. She reports the duration of her bleeding as variable, and sometimes the bleeding is heavy with small clots passed. She has been previously diagnosed with polycystic ovary syndrome (PCOS). Combination estrogen-progestin oral contraceptives have been prescribed several times in the past, but she always has discontinued them due to nausea. The patient is in a same-sex relationship and does not anticipate being sexually active with a male. She reports having to shave her mustache and chin twice weekly for the past 1 to 2 years.
On physical examination, the patient is obese (body mass index [BMI], 32 kg/m2), facial acne and hirsutism are present, and hair extends from the mons toward the umbilicus. Bimanual examination reveals a normal size, mobile, nontender uterus without obvious adnexal pathology. Pelvic ultrasonography demonstrates a normal-appearing uterus with multiplanar endometrium (consistent with proliferative changes) (FIGURE 2). Ovarian imaging demonstrates ≥12 follicles per image (FIGURE 3).


After reviewing various treatment options, you prescribe oral medroxyprogesterone acetate 20 mg (two 10-mg tablets) daily in a continuous fashion. You counsel her that she should not be surprised or concerned if frequent or even continuous bleeding occurs initially, and that she should continue this medication despite the occurrence of such.
About one-third of all women experience abnormal uterine bleeding (AUB) sometime during their lifetime and AUB can impair quality of life.1 Surgical management, including hysterectomy and endometrial ablation, plays an important role in the management of AUB in patients who do not desire future pregnancies. However, many cases of AUB occur in women who may not have completed childbearing or in women who prefer to avoid surgery.2 AUB can be managed effectively medically in most cases.1 Accordingly, in this review, we focus on nonsurgical management of AUB.
Continue to: Because previously used terms, including...
Because previously used terms, including menorrhagia and meno-metrorrhagia, were inconsistently defined and confusing, the International Federation of Gynecology and Obstetrics introduced updated terminology in 2011 to better describe and characterize AUB in nonpregnant women. Heavy menstrual bleeding (HMB) refers to ovulatory (cyclic) bleeding that is more than 8 days’ duration, or sufficiently heavy to impair a woman’s quality of life. HMB is a pattern of AUB distinct from the irregular bleeding pattern typically caused by ovulatory dysfunction (AUB-O).1
Clinical evaluation
Obtain menstrual history. In addition to a medical, surgical, and gynecologic history, a thorough menstrual history should be obtained to further characterize the patient’s bleeding pattern. In contrast to the cyclical or ovulatory bleeding seen with HMB, bleeding associated with inconsistent ovulation (AUB-O) is unpredictable or irregular, and is commonly associated with PCOS. AUB-O is also encountered in recently menarchal girls (secondary to immaturity of the hypothalamic-pituitary-gonadal axis) and in those who are perimenopausal. In addition, medications that can induce hyperprolactinemia (such as certain antipsychotics) can cause AUB-O.
Evaluate for all sources of bleeding. Be sure to evaluate for extrauterine causes of bleeding, including the cervix, vagina, vulva, or the urinary or gastrointestinal tracts for bleeding. Intermenstrual bleeding occurring between normal regular menses may be caused by an endometrial polyp, submucosal fibroid, endometritis, or an IUD. The patient report of postcoital bleeding suggests that cervical disease (cervicitis, polyp, or malignancy) may be present. Uterine leiomyoma or adenomyosis represent common causes of HMB. However, HMB also may be caused by a copper IUD, coagulation disorders (including von Willebrand disease), or use of anticoagulant medications. Hormonal contraceptives also can cause irregular bleeding.
Perform a pelvic examination and measure vital signs. The presence of fever suggests the possible presence of pelvic inflammatory disease (PID), while orthostatic hypotension raises the possibility of hypovolemia. When vaginal speculum examination is performed, a cervical cause of abnormal bleeding may be noted. The presence of fresh or old blood or finding clots in the vaginal vault or at the cervical os are all consistent with AUB. A bimanual examination that reveals an enlarged or lobular uterus suggests leiomyoma or adenomyosis. Cervical or adnexal tenderness is often noted in women with PID, which itself may be associated with endometritis. The presence of hyperandrogenic signs on physical examination (eg, acne, hirsutism, or clitoromegaly) suggests PCOS. The finding of galactorrhea suggests that hyperprolactinemia may be present.
Laboratory assessment
Test for pregnancy, cervical disease, and sexually transmitted infection when appropriate. Pregnancy testing is appropriate for women with AUB aged 55 years or younger. If patients with AUB are not up to date with normal cervical cancer screening results, cervical cytology and/or human papillomavirus testing should be performed. Testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis should be performed in patients:
- younger than 25 years
- when the history indicates new or multiple sexual partners, or
- when vaginal discharge, cervicitis, cervical motion, or adnexal tenderness is present.
Continue to: Obtain a complete blood count and serum ferritin levels...
Obtain a complete blood count and serum ferritin levels. In women presenting with HMB, iron depletion and iron deficiency anemia are common. The finding of leukocytosis raises the possibility of PID or postpartum endometritis. In women with presumptive AUB-O, checking the levels of thyroid-stimulating hormone, free T4, and prolactin should be performed.
Screen for a hemostasis disorder. Women with excessive menstrual bleeding should be clinically screened for an underlying disorder of hemostasis (TABLE 1).3 When a hemostasis disorder is suspected, initial laboratory evaluation includes a partial thromboplastin time, prothrombin time, activated partial thromboplastin time, and fibrinogen. Women who have a positive clinical screen for a possible bleeding disorder or abnormal initial laboratory test results for disorders of hemostasis should undergo further laboratory evaluation, including von Willebrand factor antigen, ristocetin cofactor assay, and factor VIII. Consultation with a hematologist should be considered in these cases.

Perform endometrial biopsy when indicated
After excluding pregnancy, endometrial biopsy (through pipelle biospy or brush sampling; FIGURE 4) should be performed in women with AUB who are at increased risk for endometrial neoplasia. The prevalence of endometrial neoplasia is substantially higher among women ≥45 years of age4 and among patients with AUB who are also obese (BMI, ≥30 kg/m2).5 In addition, AUB patients with unopposed estrogen exposure (presumed anovulation/PCOS), as well as those with persistent AUB or failed medical management, should undergo endometrial biopsy.6

Utilize transvaginal ultrasonography
Transvaginal ultrasonography is often useful in the evaluation of patients with AUB, as it may identify uterine fibroids or adenomyosis, suggest intracavitary pathology (such as an endometrial polyp or submucosal fibroid), or raise the possibility of PCOS. In virginal patients or those in whom vaginal ultrasound is not appropriate, abdominal pelvic ultrasonography represents appropriate imaging. If unenhanced ultrasound suggests endometrial polyps or fibroids within the endometrial cavity, an office-based saline infusion sonogram (sonohysterogram) (FIGURE 5) or hysteroscopy should be performed. Targeted endometrial sampling and biopsy of intracavitary pathology can be performed at the time of hysteroscopy.

Treatment
When HMB impairs quality of life, is bothersome to the patient, or results in anemia, treatment is appropriate. Although bleeding episodes in women with AUB-O may be infrequent (as with Case 2), treatment prevents heavy or prolonged bleeding episodes as well as endometrial neoplasia that may otherwise occur in anovulatory women.
Many women with AUB can be managed medically. However, treatment choices will vary with respect to the patient’s desire for future fertility, medical comorbidities, personal preferences, and financial barriers. While many women may prefer outpatient medical management (TABLE 2),7-14 others might desire surgical therapy, including endometrial ablation or hysterectomy.

Oral contraceptives
Combination estrogen-progestin oral contraceptives represent appropriate initial therapy for many women in the reproductive-age group with AUB, whether women have HMB or AUB-O. However, contraceptive doses of estrogen are not appropriate for some women with risk factors for cardiovascular disease, including those who smoke cigarettes and are age ≥35 years or those who have hypertension (TABLE 3).15,16

Continue to: Menopausal dosages of HT...
Menopausal dosages of HT
If use of contraceptive doses of estrogen is not appropriate, continuous off-label use of menopausal combination formulations (physiologic dosage) of hormonal therapy (HT; ie, lower doses of estrogen than contraceptives) may be effective in reducing or eliminating AUB. Options for menopausal combination formulations include generic ethinyl estradiol 5 µg/norethindrone acetate 1 mg or estradiol 1 mg/norethindrone acetate 0.5 mg.7 High-dose oral progestin therapy (norethindrone acetate 5 mg tablet once daily or medroxyprogesterone acetate 10 mg tablets 1–3 times daily) also can be used when combination contraceptives are contraindicated and may be more effective than lower-dose combination formulations.
Package labeling, as well as some guidelines, indicate that oral progestins used to treat AUB should be taken cyclically.8 However, continuous daily use is easier for many patients and may be more effective in reducing bleeding. Accordingly, we counsel patients with AUB who are using progestins and who do not wish to conceive to take these medications continuously. High-dose oral progestin therapy may cause bloating, dysphoria, and increased appetite/weight gain. Women initiating hormonal management (including the progestin IUDs detailed below) for AUB should be counseled that irregular or even continuous light bleeding/spotting is common initially, but this bleeding pattern typically decreases with continued use.
IUDs
The LNG 52 mg IUD (Mirena or Liletta) effectively treats HMB, reducing bleeding in a manner comparable to that of endometrial ablation.9,10 The Mirena IUD is approved for treatment of HMB in women desiring intrauterine contraception. In contrast to oral medications, use of progestin IUDs does not involve daily administration and may represent an attractive option for women with HMB who would like to avoid surgery or preserve fertility. With ongoing use, continuous oral or intrauterine hormonal management may result in amenorrhea in some women with AUB.
When the LNG 52 mg IUD is used to treat HMB, the menstrual suppression impact may begin to attenuate after approximately 4 years of use; in this setting, replacing the IUD often restores effective menstrual suppression.11 The LNG 52 mg IUD effectively suppresses menses in women with coagulation disorders; if menstrual suppression with the progestin IUD is not adequate in this setting, it may be appropriate to add an oral combination estrogen-progestin contraceptive or high-dose oral progestin.11,12
NSAIDs and tranexamic acid
Off-label use of nonsteroidal anti-inflammatory drugs (naproxen 500–1,000 mg daily for 5 days beginning at the onset of menstrual flow or tranexamic acid two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can suppress HMB and is useful for women who prefer to avoid or have contraindications to hormonal treatments.13,14 Unfortunately, these agents are not as effective as hormonal management in treating AUB.
Iron supplementation is often needed
Iron depletion commonly results from HMB, often resulting in iron deficiency anemia. When iron depletion (readily identified by checking a serum ferritin level) or iron deficiency anemia is identified, iron supplementation should be recommended. Every-other-day administration of iron supplements maximizes iron absorption while minimizing the adverse effects of unabsorbed iron, such as nausea. Sixty mg of elemental iron (ferrous sulfate 325 mg) administered every other day represents an inexpensive and effective treatment for iron deficiency/anemia.17 In patients who cannot tolerate oral iron supplementation or for those in whom oral therapy is not appropriate or effective, newer intravenous iron formulations are safe and effective.18
Continue to: Case 1 Follow-up...
Case 1 Follow-up
The patient noted marked improvement in her menstrual cramps following LNG-containing IUD placement. Although she also reported that she no longer experienced heavy menstrual flow or cramps, she was bothered by frequent, unpredictable light bleeding/spotting. You prescribed norethindrone acetate (NETA) 5-mg tablet orally once daily, to be used in addition to her IUD. After using the IUD with concomitant NETA for 2 months’ duration, she noted that her bleeding/spotting almost completely resolved; however, she did report feeling irritable with use of the progestin tablets. She subsequently stopped the NETA tablets and, after 6 months of additional follow-up, reported only minimal spotting and no cramps.
At this later follow-up visit, you noted that her hemoglobin level increased to 12.6 g/dL, and the ferritin level no longer indicated iron depletion. After the IUD had been in place for 4 years, she reported that she was beginning to experience frequent light bleeding again. A follow-up vaginal sonogram noted a well-positioned IUD, there was no suggestion of intracavitary pathology, and adenomyosis continued to be imaged. She underwent IUD removal and placement of a new LNG 52 mg IUD. This resulted in marked reduction in her bleeding.
Case 2 Follow-up
Two weeks after beginning continuous oral progestin therapy, the patient called reporting frequent irregular bleeding. She was reassured that this was not unexpected and encouraged to continue oral progestin therapy. During a 3-month follow-up visit, the patient noted little, if any, bleeding over the previous 2 months and was pleased with this result. She continued to note acne and hirsutism and asked about the possibility of adding spironolactone to her oral progestin regimen.
Case 1 Multiparous woman presents with heavy regular menses
Over the past several years, a 34-year-old woman has noted increasing intensity and duration of menstrual flow, which now persists for 8 days and includes clots “the size of quarters” and soaks a pad within 1 hour. Sometimes she misses or leaves work on her heaviest days of flow. She reports that menstrual cramps prior to and during flow are increasingly bothersome and do not respond adequately to ibuprofen. She intermittently uses condoms for contraception. She does not wish to be pregnant currently; however, she recently entered into a new relationship and may wish to conceive in the future.
On bimanual examination, the uterus appears bulky. Her hemoglobin is 10.9 g/dL with low mean corpuscular volume and a serum ferritin level indicating iron depletion. Pelvic ultrasonography suggests uterine adenomyosis; no fibroids are imaged (FIGURE 1).
You advise the patient to take ferrous sulfate 325 mg every other day. After discussion with the patient regarding different treatment options, she chooses to proceed with placement of a 52-mg levonorgestrel (LNG) intrauterine device (IUD; Mirena or Liletta).
Case 2 Older adolescent presents with irregular bleeding
A 19-year-old patient reports approximately 6 bleeding episodes each year. She reports the duration of her bleeding as variable, and sometimes the bleeding is heavy with small clots passed. She has been previously diagnosed with polycystic ovary syndrome (PCOS). Combination estrogen-progestin oral contraceptives have been prescribed several times in the past, but she always has discontinued them due to nausea. The patient is in a same-sex relationship and does not anticipate being sexually active with a male. She reports having to shave her mustache and chin twice weekly for the past 1 to 2 years.
On physical examination, the patient is obese (body mass index [BMI], 32 kg/m2), facial acne and hirsutism are present, and hair extends from the mons toward the umbilicus. Bimanual examination reveals a normal size, mobile, nontender uterus without obvious adnexal pathology. Pelvic ultrasonography demonstrates a normal-appearing uterus with multiplanar endometrium (consistent with proliferative changes) (FIGURE 2). Ovarian imaging demonstrates ≥12 follicles per image (FIGURE 3).


After reviewing various treatment options, you prescribe oral medroxyprogesterone acetate 20 mg (two 10-mg tablets) daily in a continuous fashion. You counsel her that she should not be surprised or concerned if frequent or even continuous bleeding occurs initially, and that she should continue this medication despite the occurrence of such.
About one-third of all women experience abnormal uterine bleeding (AUB) sometime during their lifetime and AUB can impair quality of life.1 Surgical management, including hysterectomy and endometrial ablation, plays an important role in the management of AUB in patients who do not desire future pregnancies. However, many cases of AUB occur in women who may not have completed childbearing or in women who prefer to avoid surgery.2 AUB can be managed effectively medically in most cases.1 Accordingly, in this review, we focus on nonsurgical management of AUB.
Continue to: Because previously used terms, including...
Because previously used terms, including menorrhagia and meno-metrorrhagia, were inconsistently defined and confusing, the International Federation of Gynecology and Obstetrics introduced updated terminology in 2011 to better describe and characterize AUB in nonpregnant women. Heavy menstrual bleeding (HMB) refers to ovulatory (cyclic) bleeding that is more than 8 days’ duration, or sufficiently heavy to impair a woman’s quality of life. HMB is a pattern of AUB distinct from the irregular bleeding pattern typically caused by ovulatory dysfunction (AUB-O).1
Clinical evaluation
Obtain menstrual history. In addition to a medical, surgical, and gynecologic history, a thorough menstrual history should be obtained to further characterize the patient’s bleeding pattern. In contrast to the cyclical or ovulatory bleeding seen with HMB, bleeding associated with inconsistent ovulation (AUB-O) is unpredictable or irregular, and is commonly associated with PCOS. AUB-O is also encountered in recently menarchal girls (secondary to immaturity of the hypothalamic-pituitary-gonadal axis) and in those who are perimenopausal. In addition, medications that can induce hyperprolactinemia (such as certain antipsychotics) can cause AUB-O.
Evaluate for all sources of bleeding. Be sure to evaluate for extrauterine causes of bleeding, including the cervix, vagina, vulva, or the urinary or gastrointestinal tracts for bleeding. Intermenstrual bleeding occurring between normal regular menses may be caused by an endometrial polyp, submucosal fibroid, endometritis, or an IUD. The patient report of postcoital bleeding suggests that cervical disease (cervicitis, polyp, or malignancy) may be present. Uterine leiomyoma or adenomyosis represent common causes of HMB. However, HMB also may be caused by a copper IUD, coagulation disorders (including von Willebrand disease), or use of anticoagulant medications. Hormonal contraceptives also can cause irregular bleeding.
Perform a pelvic examination and measure vital signs. The presence of fever suggests the possible presence of pelvic inflammatory disease (PID), while orthostatic hypotension raises the possibility of hypovolemia. When vaginal speculum examination is performed, a cervical cause of abnormal bleeding may be noted. The presence of fresh or old blood or finding clots in the vaginal vault or at the cervical os are all consistent with AUB. A bimanual examination that reveals an enlarged or lobular uterus suggests leiomyoma or adenomyosis. Cervical or adnexal tenderness is often noted in women with PID, which itself may be associated with endometritis. The presence of hyperandrogenic signs on physical examination (eg, acne, hirsutism, or clitoromegaly) suggests PCOS. The finding of galactorrhea suggests that hyperprolactinemia may be present.
Laboratory assessment
Test for pregnancy, cervical disease, and sexually transmitted infection when appropriate. Pregnancy testing is appropriate for women with AUB aged 55 years or younger. If patients with AUB are not up to date with normal cervical cancer screening results, cervical cytology and/or human papillomavirus testing should be performed. Testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis should be performed in patients:
- younger than 25 years
- when the history indicates new or multiple sexual partners, or
- when vaginal discharge, cervicitis, cervical motion, or adnexal tenderness is present.
Continue to: Obtain a complete blood count and serum ferritin levels...
Obtain a complete blood count and serum ferritin levels. In women presenting with HMB, iron depletion and iron deficiency anemia are common. The finding of leukocytosis raises the possibility of PID or postpartum endometritis. In women with presumptive AUB-O, checking the levels of thyroid-stimulating hormone, free T4, and prolactin should be performed.
Screen for a hemostasis disorder. Women with excessive menstrual bleeding should be clinically screened for an underlying disorder of hemostasis (TABLE 1).3 When a hemostasis disorder is suspected, initial laboratory evaluation includes a partial thromboplastin time, prothrombin time, activated partial thromboplastin time, and fibrinogen. Women who have a positive clinical screen for a possible bleeding disorder or abnormal initial laboratory test results for disorders of hemostasis should undergo further laboratory evaluation, including von Willebrand factor antigen, ristocetin cofactor assay, and factor VIII. Consultation with a hematologist should be considered in these cases.

Perform endometrial biopsy when indicated
After excluding pregnancy, endometrial biopsy (through pipelle biospy or brush sampling; FIGURE 4) should be performed in women with AUB who are at increased risk for endometrial neoplasia. The prevalence of endometrial neoplasia is substantially higher among women ≥45 years of age4 and among patients with AUB who are also obese (BMI, ≥30 kg/m2).5 In addition, AUB patients with unopposed estrogen exposure (presumed anovulation/PCOS), as well as those with persistent AUB or failed medical management, should undergo endometrial biopsy.6

Utilize transvaginal ultrasonography
Transvaginal ultrasonography is often useful in the evaluation of patients with AUB, as it may identify uterine fibroids or adenomyosis, suggest intracavitary pathology (such as an endometrial polyp or submucosal fibroid), or raise the possibility of PCOS. In virginal patients or those in whom vaginal ultrasound is not appropriate, abdominal pelvic ultrasonography represents appropriate imaging. If unenhanced ultrasound suggests endometrial polyps or fibroids within the endometrial cavity, an office-based saline infusion sonogram (sonohysterogram) (FIGURE 5) or hysteroscopy should be performed. Targeted endometrial sampling and biopsy of intracavitary pathology can be performed at the time of hysteroscopy.

Treatment
When HMB impairs quality of life, is bothersome to the patient, or results in anemia, treatment is appropriate. Although bleeding episodes in women with AUB-O may be infrequent (as with Case 2), treatment prevents heavy or prolonged bleeding episodes as well as endometrial neoplasia that may otherwise occur in anovulatory women.
Many women with AUB can be managed medically. However, treatment choices will vary with respect to the patient’s desire for future fertility, medical comorbidities, personal preferences, and financial barriers. While many women may prefer outpatient medical management (TABLE 2),7-14 others might desire surgical therapy, including endometrial ablation or hysterectomy.

Oral contraceptives
Combination estrogen-progestin oral contraceptives represent appropriate initial therapy for many women in the reproductive-age group with AUB, whether women have HMB or AUB-O. However, contraceptive doses of estrogen are not appropriate for some women with risk factors for cardiovascular disease, including those who smoke cigarettes and are age ≥35 years or those who have hypertension (TABLE 3).15,16

Continue to: Menopausal dosages of HT...
Menopausal dosages of HT
If use of contraceptive doses of estrogen is not appropriate, continuous off-label use of menopausal combination formulations (physiologic dosage) of hormonal therapy (HT; ie, lower doses of estrogen than contraceptives) may be effective in reducing or eliminating AUB. Options for menopausal combination formulations include generic ethinyl estradiol 5 µg/norethindrone acetate 1 mg or estradiol 1 mg/norethindrone acetate 0.5 mg.7 High-dose oral progestin therapy (norethindrone acetate 5 mg tablet once daily or medroxyprogesterone acetate 10 mg tablets 1–3 times daily) also can be used when combination contraceptives are contraindicated and may be more effective than lower-dose combination formulations.
Package labeling, as well as some guidelines, indicate that oral progestins used to treat AUB should be taken cyclically.8 However, continuous daily use is easier for many patients and may be more effective in reducing bleeding. Accordingly, we counsel patients with AUB who are using progestins and who do not wish to conceive to take these medications continuously. High-dose oral progestin therapy may cause bloating, dysphoria, and increased appetite/weight gain. Women initiating hormonal management (including the progestin IUDs detailed below) for AUB should be counseled that irregular or even continuous light bleeding/spotting is common initially, but this bleeding pattern typically decreases with continued use.
IUDs
The LNG 52 mg IUD (Mirena or Liletta) effectively treats HMB, reducing bleeding in a manner comparable to that of endometrial ablation.9,10 The Mirena IUD is approved for treatment of HMB in women desiring intrauterine contraception. In contrast to oral medications, use of progestin IUDs does not involve daily administration and may represent an attractive option for women with HMB who would like to avoid surgery or preserve fertility. With ongoing use, continuous oral or intrauterine hormonal management may result in amenorrhea in some women with AUB.
When the LNG 52 mg IUD is used to treat HMB, the menstrual suppression impact may begin to attenuate after approximately 4 years of use; in this setting, replacing the IUD often restores effective menstrual suppression.11 The LNG 52 mg IUD effectively suppresses menses in women with coagulation disorders; if menstrual suppression with the progestin IUD is not adequate in this setting, it may be appropriate to add an oral combination estrogen-progestin contraceptive or high-dose oral progestin.11,12
NSAIDs and tranexamic acid
Off-label use of nonsteroidal anti-inflammatory drugs (naproxen 500–1,000 mg daily for 5 days beginning at the onset of menstrual flow or tranexamic acid two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can suppress HMB and is useful for women who prefer to avoid or have contraindications to hormonal treatments.13,14 Unfortunately, these agents are not as effective as hormonal management in treating AUB.
Iron supplementation is often needed
Iron depletion commonly results from HMB, often resulting in iron deficiency anemia. When iron depletion (readily identified by checking a serum ferritin level) or iron deficiency anemia is identified, iron supplementation should be recommended. Every-other-day administration of iron supplements maximizes iron absorption while minimizing the adverse effects of unabsorbed iron, such as nausea. Sixty mg of elemental iron (ferrous sulfate 325 mg) administered every other day represents an inexpensive and effective treatment for iron deficiency/anemia.17 In patients who cannot tolerate oral iron supplementation or for those in whom oral therapy is not appropriate or effective, newer intravenous iron formulations are safe and effective.18
Continue to: Case 1 Follow-up...
Case 1 Follow-up
The patient noted marked improvement in her menstrual cramps following LNG-containing IUD placement. Although she also reported that she no longer experienced heavy menstrual flow or cramps, she was bothered by frequent, unpredictable light bleeding/spotting. You prescribed norethindrone acetate (NETA) 5-mg tablet orally once daily, to be used in addition to her IUD. After using the IUD with concomitant NETA for 2 months’ duration, she noted that her bleeding/spotting almost completely resolved; however, she did report feeling irritable with use of the progestin tablets. She subsequently stopped the NETA tablets and, after 6 months of additional follow-up, reported only minimal spotting and no cramps.
At this later follow-up visit, you noted that her hemoglobin level increased to 12.6 g/dL, and the ferritin level no longer indicated iron depletion. After the IUD had been in place for 4 years, she reported that she was beginning to experience frequent light bleeding again. A follow-up vaginal sonogram noted a well-positioned IUD, there was no suggestion of intracavitary pathology, and adenomyosis continued to be imaged. She underwent IUD removal and placement of a new LNG 52 mg IUD. This resulted in marked reduction in her bleeding.
Case 2 Follow-up
Two weeks after beginning continuous oral progestin therapy, the patient called reporting frequent irregular bleeding. She was reassured that this was not unexpected and encouraged to continue oral progestin therapy. During a 3-month follow-up visit, the patient noted little, if any, bleeding over the previous 2 months and was pleased with this result. She continued to note acne and hirsutism and asked about the possibility of adding spironolactone to her oral progestin regimen.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet. 2018;143:393-408.
- Kaunitz AM. Abnormal uterine bleeding in reproductive-age women. JAMA. 2019;321:2126-2127.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer. http://seer.cancer.gov/statfacts/html/corp.html. Accessed October 10, 2019.
- Wise MR, Gill P, Lensen S, et al. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215:598.e1-598.e8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- The North American Menopause Society. Menopause Practice–A Clinician’s Guide. 5th ed. NAMS: Mayfield Heights, OH; 2014.
- National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. https://www.nice.org.uk/guidance/ng88. Accessed October 10, 2019.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
- Kaunitz AM, Meredith S, Inki P, et al. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104-1116.
- Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8.
- Ylikorkala O, Pekonen F. Naproxen reduces idiopathic but not fibromyoma-induced menorrhagia. Obstet Gynecol. 1986;68:10-12.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:865-875.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1–103.
- ACOG Practice Bulletin no. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet. 2018;143:393-408.
- Kaunitz AM. Abnormal uterine bleeding in reproductive-age women. JAMA. 2019;321:2126-2127.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer. http://seer.cancer.gov/statfacts/html/corp.html. Accessed October 10, 2019.
- Wise MR, Gill P, Lensen S, et al. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215:598.e1-598.e8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- The North American Menopause Society. Menopause Practice–A Clinician’s Guide. 5th ed. NAMS: Mayfield Heights, OH; 2014.
- National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. https://www.nice.org.uk/guidance/ng88. Accessed October 10, 2019.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
- Kaunitz AM, Meredith S, Inki P, et al. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104-1116.
- Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8.
- Ylikorkala O, Pekonen F. Naproxen reduces idiopathic but not fibromyoma-induced menorrhagia. Obstet Gynecol. 1986;68:10-12.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:865-875.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1–103.
- ACOG Practice Bulletin no. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
Using slings for the surgical management of urinary incontinence: A safe, effective, evidence-based approach
Urinary incontinence affects approximately 50% of women, with up to 80% of these women experiencing stress urinary incontinence (SUI) at some point in their lives.1-3 While conservative measures can offer some improvement in symptoms, the mainstay of treatment for SUI is surgical intervention.4,5 The lifetime risk of undergoing surgery for SUI is 13.6%, and surgery leads to a major improvement in quality of life and productivity.1,6
Types of slings used for SUI
Sling procedures are the most commonly used surgical approach for the treatment of SUI. Two types of urethral slings are used: the midurethral sling and the autologous fascial (pubovaginal) sling. The midurethral sling, which is the most frequently used sling today, can be further characterized as the retropubic sling, the transobturator sling, and the mini sling (FIGURE 1).
Retropubic sling
A retropubic sling is a midurethral mesh sling that is placed beneath the urethra at the midpoint between the urethral meatus and the bladder neck. The arms of the sling extend behind the pubic symphysis, providing a hammock-like support that helps prevent leakage with increased abdominal pressures. The retropubic sling is the most commonly used type of sling. For women presenting with uncomplicated SUI who desire surgical correction, it often is the best choice for providing long-term treatment success.7
Transobturator sling
A transobturator sling is a midurethral mesh sling that is placed beneath the urethra as described above, but the arms of the sling extend outward through the obturator foramen and into the groin. This enables support of the midurethra, but this sling is less likely to result in such complications as bladder perforation or postoperative urinary retention. Transobturator slings also are associated with lower rates of voiding dysfunction and urinary urgency than retropubic slings.7-9 However, transobturator slings have higher rates of groin pain, and they are less effective in maintaining long-term cure of SUI.7
First introduced in 1996, the midurethral sling quickly grew in popularity for the treatment of SUI because of its high success rates and its minimally invasive approach.10 Both retropubic and transobturator slings are safe, extensively researched surgical approaches for the management of SUI.3 Midurethral slings have a very high rate of incontinence cure (80%–90%) and extremely high patient satisfaction rates (85%–90%), as even patients without complete cure report meaningful symptomatic improvement.7,8,11
Single-incision (mini) sling
A single-incision sling is a midurethral mesh sling that is designed to be shorter in length than standard midurethral slings. The placed sling lies under the midurethra and extends toward the superior edge of the obturator foramen but does not penetrate it. The sling is held in place by small pledgets on either side of the mesh hammock that anchor it in place to the obturator internus muscular fascia. Because this “mini” sling was introduced in 2006, fewer long-term data are available for this sling than for standard midurethral slings.
Continue to: Autologous (fascial) sling...
Autologous (fascial) sling
An autologous sling is a retropubic sling made from the patient’s own fascia; it is harvested from either the fascia lata of the lateral thigh or the rectus fascia of the abdomen. The sling is placed beneath the urethra in the bladder neck region, and sutures affixed to the sling edges pass behind the pubic symphysis and through the abdominal fascia to anchor it in place.
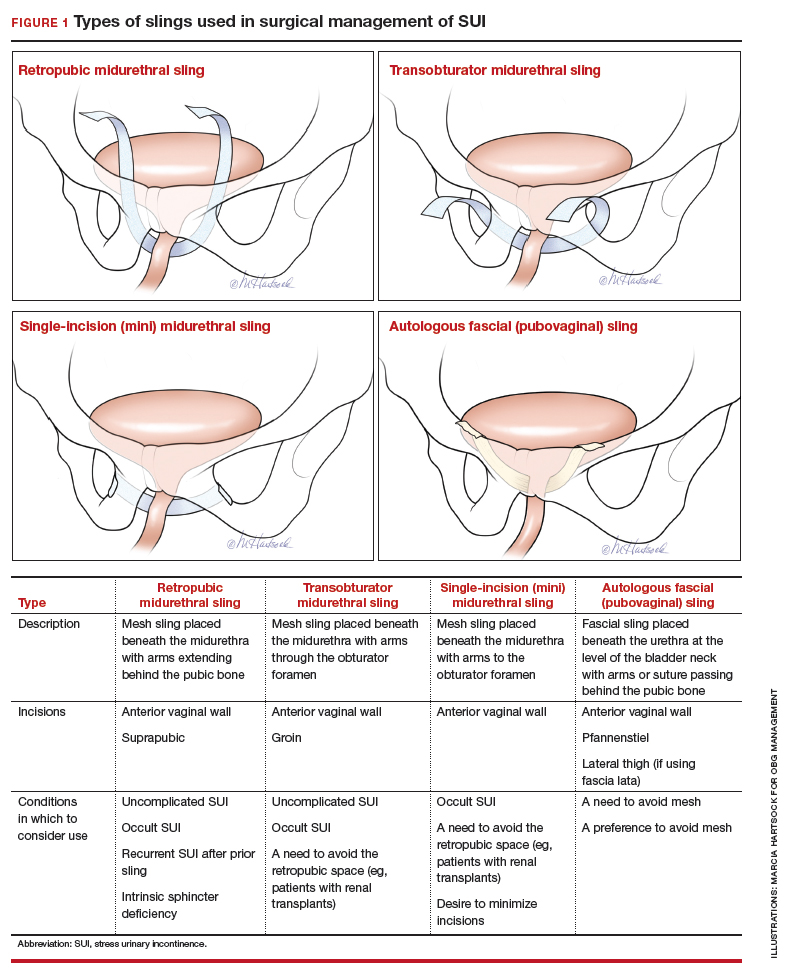
Choose a sling based on the clinical situation and patient goals
Consider the unique features of each sling when selecting the proper sling; this should be a shared decision with the patient after thorough counseling. Below, we present 4 clinical cases to exemplify scenarios in which different slings are appropriate, and we review the rationale for each selection.
CASE 1 SUI that interferes with exercise routine
Ms. P. is a 46-year-old (G3P3) active mother. She loves to exercise, but she has been working out less frequently because of embarrassing urinary leakage that occurs with activity. She has tried pelvic floor exercises and changing her fluid intake habits, but improvements have been minimal with these interventions. On evaluation, she has a positive cough stress test with a recently emptied bladder and a normal postvoid residual volume.
What type of sling would be best?
Because this patient is young, active, and has significant leakage with an empty bladder, a sling with good long-term treatment success is likely to provide her with the best results (Figure 1). We therefore offered her a retropubic midurethral sling. The retropubic approach is preferred here as it is less likely than the transobturator sling to cause groin/thigh pain, which is an important consideration in this young, active patient.
Further testing is not needed
For women with uncomplicated SUI who demonstrate leakage with stress (coughing, Valsalva stress test) and who have a normal postvoid residual volume, additional testing, such as urodynamic evaluation, is not necessary.12 These patients can be counseled on the range of conservative management options and as well as surgical inventions.
CASE 2 Return of SUI symptoms after transobturator sling placement
Ms. E. is a 70-year-old woman who had a transobturator sling placed 5 years ago. Initially, her SUI symptoms improved after surgery. Recently, however, she noticed a return of her SUI, which she finds bothersome and limiting to her quality of life.
How would you manage this patient?
While midurethral slings are highly effective, there are instances in which patients will have symptom recurrence. For women who already have a midurethral sling, consider the following important questions.
Is this truly recurrent SUI, or is it a new process?
Like any reconstructive procedure, midurethral sling success rates decline over time and recurrent SUI can develop.7 However, it also is possible for urge urinary incontinence to develop as a new process, and it is important to distinguish which type of urinary incontinence your patient has prior to counseling about treatment options.
To further evaluate patients with recurrent incontinence and a prior sling, we recommend urodynamic studies with cystoscopy (in addition to a detailed history and physical exam). This not only helps rule out other forms of incontinence, such as overactive bladder, but also evaluates for possible mesh erosion into the urethra or bladder, which can cause irritative voiding symptoms and incontinence.
Continue to: What type of sling did the patient have initially...
What type of sling did the patient have initially, and how does this impact a repeat procedure?
Regardless of the initial sling type used, repeat midurethral sling procedures have a significantly lower cure rate than primary midurethral sling procedures.13 Retropubic slings are more effective than transobturator slings for patients with recurrent SUI who have failed a prior sling. When a patient presents with recurrent SUI after a prior transobturator sling, the best option for a repeat procedure is usually a retropubic sling, as it achieves higher objective and subjective cure rates.13,14 (See FIGURE 2 for a comparison of retropubic and transobturator slings.)
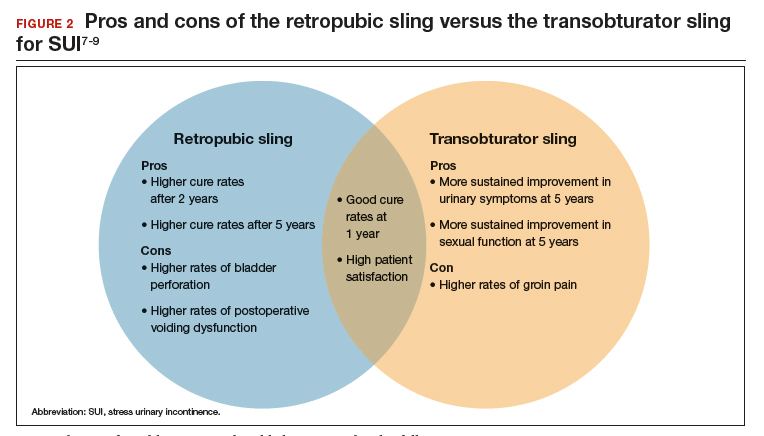
Should I remove the old sling prior to placing a new one?
While it is recommended to remove the vaginal portion of the sling if the patient has a mesh exposure or is experiencing other symptoms, such as pain or bleeding, removal of the old sling is not necessarily indicated prior to (or during) a repeat incontinence procedure.15,16 Removing the sling, removing a portion of the sling, or leaving the sling in situ are all reasonable options.
CASE 3 Treated SUI has mesh exposure
Ms. R. is a 60-year-old woman with a history of SUI that was previously managed with a retropubic midurethral sling placed at an outside hospital. She is a smoker and has developed a vaginal mesh exposure. Although she would like the mesh removed, she does not want her incontinence to come back. She tells you that she does not think she would be able to quit smoking.
What would be a reasonable next option for Ms. R.?
While complications from a midurethral sling are rare, mesh exposures occur in approximately 2% of patients, and urinary retention requiring release of the sling occurs in about 1% of patients.3,6 It often helps to clarify for patients that the US Food and Drug Administration public health advisories on the use of transvaginal mesh have been directed specifically toward the use of transvaginal mesh for the treatment of pelvic organ prolapse (POP), not the use of mesh for midurethral slings for SUI or transabdominal mesh for POP.10,17
When considering use of a mesh sling, a thorough discussion of the potential risks, as well as the benefits and alternatives, is imperative. Patients must personally balance the probability of benefit with the potential risk of complications, and while physicians can help outline the benefits and risks through shared decision-making, ultimately it is the patient who should make this decision.
Certain patient populations may be at higher risk for mesh complications18 (See "Risk factors for mesh-related complications," below). These complications are managed in various ways (FIGURE 3). Patients who have experienced mesh complications previously are typically not good candidates for a repeat mesh sling, particularly when the risk factor for complications cannot be modified.
• Smoking
• Poorly controlled diabetes
• Decreased estrogen status
• Chronic steroid use
• Prior urethral surgery (urethral diverticulum, urethroplasty)
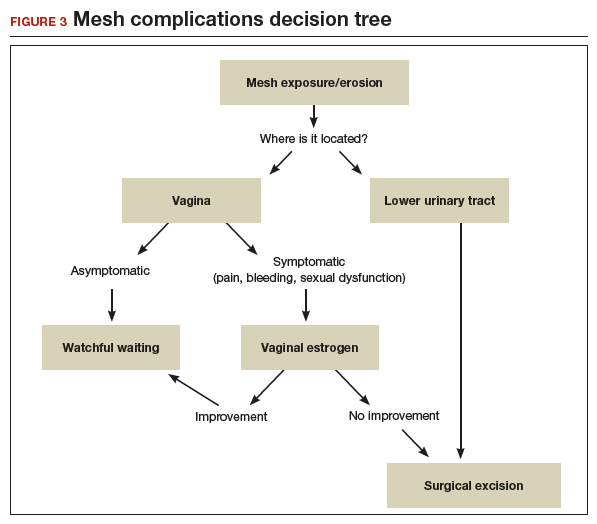
A mesh sling alternative
The most effective way to manage SUI in patients who are not good candidates for a mesh sling is to consider employing a sling that uses the patient’s own tissue.19-21 Common approaches include harvesting a graft of rectus fascia through a Pfannenstiel skin incision or using fascia lata from the patient’s iliotibial band in the lateral thigh. Autologous slings are safe and effective, and even after a mesh sling has failed, autologous slings have an almost 70% cure rate for SUI.20,21
Continue to: Timing of mesh removal and placement of an autologous fascial sling...
Timing of mesh removal and placement of an autologous fascial sling
Either concomitant or delayed placement of a pubovaginal sling is acceptable when removing mesh, though this should be a joint decision with the patient after counseling. If the risk for surgical complications is modifiable (for example, poorly controlled diabetes that could be improved with blood glucose control), it may be advisable to delay the fascial sling until the risk factors have been addressed. Similarly, if the reason for mesh removal is pain, it may be advisable to remove the mesh prior to placing a new sling to ensure that the pain resolves completely. Otherwise, if pain persists, it can be unclear whether the new sling is contributing to the pain, and this may lead to difficulties treating pain or incontinence in the future.
In this patient, who was an active smoker, we excised the exposed mesh and concomitantly placed an autologous fascial sling utilizing rectus fascia. This maintained continence without introducing mesh in a high-risk patient.
CASE 4 POP and occult SUI
Ms. B. is a 79-year-old woman with stage 3 POP planned for surgical repair. While she does not report urinary leakage, preoperative urodynamic testing revealed occult SUI with reduction of her prolapse. Her priorities are to avoid needing another surgery and to limit the chances of postoperative leakage, but she is nervous about her postoperative recovery and wants to avoid pain.
What approach would be appropriate?
Consider a mini sling for this patient
The single-incision (mini) sling is an option to consider for patients with mild incontinence or for those without evidence of intrinsic sphincter deficiency. It is also a good option for those who want to avoid the additional incisions required for full-length slings.
While currently there is not sufficient evidence to clearly state if single-incision slings are equivalent to other slings, recent studies show that single-incision slings appear to be safe and effective in the short term, with possibly fewer complications than traditional transobturator slings.22-24 As patients are often concerned about the potential for groin pain with a transobturator sling, a single-incision sling is an acceptable alternative that avoids groin incisions and also avoids the retropubic space.
Patient counseling is crucial
Regardless of the route, sling procedures are highly effective and safe for treating women with SUI.3 Understanding the characteristics of each type of sling and the distinct surgical approaches enables informed counseling for patients who are navigating the treatment options for SUI.
- Wu JM, Matthews CA, Conover MM, et al. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol. 2014;123:1201-1206.
- Jonsson Funk M, Levin PJ, Wu JM. Trends in the surgical management of stress urinary incontinence. Obstet Gynecol. 2012;119:845-851.
- Ford AA, Rogerson L, Cody JD, et al. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD006375.
- Dumoulin C, Hay-Smith J, Habee-Seguin GM, et al. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women: a short version Cochrane systematic review with meta-analysis. Neurourol Urodyn. 2015;34:300-308.
- Cox A, Herschorn S, Lee L. Surgical management of female SUI: is there a gold standard? Nat Rev Urol. 2013;10:78-89.
- Schimpf MO, Rahn DD, Wheeler TL, et al; Society of Gynecologic Surgeons Systematic Review Group. Sling surgery for stress urinary incontinence in women: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;211:71.e1-71.e27.
- Kenton K, Stoddard AM, Zyczynski H, et al. 5-year longitudinal followup after retropubic and transobturator mid urethral slings. J Urol. 2015;193:203-210.
- Richter HE, Albo ME, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066-2076.
- Albo ME, Litman HJ, Richter HE, et al; Urinary Incontinence Treatment Network. Treatment success of retropubic and transobturator midurethral slings at 24-months. J Urol. 2012;188:2281-2287.
- US Food and Drug Administration. Urogynecologic surgical mesh: update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. July 2011;1-15. https://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM262760.pdf. Accessed September 16, 2019.
- Nilsson CG, Palva K, Aarnio R, et al. Seventeen years’ follow up of the tension-free vaginal tape procedure for female stress urinary incontinence. Int Urogynecol J. 2013;24:1265-1269.
- Nager CW, Brubaker L, Litman HJ, et al; Urinary Incontinence Treatment Network. A randomized trial of urodynamic testing before stress-incontinence surgery. N Engl J Med. 2012;366:1987-1997.
- Stav K, Dwyer PL, Rosamilia A, et al. Repeat synthetic mid urethral sling procedure for women with recurrent stress urinary incontinence. J Urol. 2010;183:241-246.
- Kim A, Kim MS, Park YJ, et al. Retropubic versus transobturator mid urethral slings in patients at high risk for recurrent stress incontinence: a systematic review and meta-analysis. J Urol. 2019;202:132-142.
- Kavanagh A, Sanaee M, Carison KV, et al. Management of patients with stress urinary incontinence after failed midurethral sling. Can Urol Assoc J. 2017;11(6 suppl 2):S143-S146.
- Steele SE, Hill AJ, Unger CA. Concurrent midurethral sling excision or lysis at the time of repeat sling for treatment of recurrent or persistent stress urinary incontinence. Int Urogynecol J. 2018;29:285-290.
- US Food and Drug Administration. Urogynecologic surgical mesh implants. https://www.fda.gov/medicaldevices/productsandmedicalprocedures/implantsandprosthetics/urogynsurgicalmesh/. Content current as of July 10, 2019. Accessed September 16, 2019.
- Kokanali MK, Doganay M, Aksakal O, et al. Risk factors for mesh erosion after vaginal sling procedures for urinary incontinence. Eur J Obstet Gynecol Reprod Biol. 2014;177:146-150.
- Nikolopoulos KI, Betschart C, Doumouchtsis SK. The surgical management of recurrent stress urinary incontinence: a systematic review. Acta Obstet Gynecol Scand. 2015;94:568-576.
- Milose JC, Sharp KM, He C, et al. Success of autologous pubovaginal sling after failed synthetic mid urethral sling. J Urol. 2015;193:916-920.
- Albo ME, Richter HE, Brubaker L, et al; Urinary Incontinence Treatment Network. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med. 2007;356:2143-2155.
- Imamura M, Hudson J, Wallace SA, et al. Surgical interventions for women with stress urinary incontinence: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2019;365:I1842.
- Jiao B, Lai S, Xu X, et al. A systematic review and meta-analysis of single-incision mini-slings (MiniArc) versus transobturator mid-urethral slings in surgical management of female stress urinary incontinence. Medicine (Baltimore). 2018;97:e0283.
- Sun Z, Wang X, Lang J, et al. Comparison of outcomes between single-incision sling and transobturator sling for treating stress urinary incontinence: a 10-year prospective study. Neurourol Urodyn. 2019;38:1852-1858.
Urinary incontinence affects approximately 50% of women, with up to 80% of these women experiencing stress urinary incontinence (SUI) at some point in their lives.1-3 While conservative measures can offer some improvement in symptoms, the mainstay of treatment for SUI is surgical intervention.4,5 The lifetime risk of undergoing surgery for SUI is 13.6%, and surgery leads to a major improvement in quality of life and productivity.1,6

Types of slings used for SUI
Sling procedures are the most commonly used surgical approach for the treatment of SUI. Two types of urethral slings are used: the midurethral sling and the autologous fascial (pubovaginal) sling. The midurethral sling, which is the most frequently used sling today, can be further characterized as the retropubic sling, the transobturator sling, and the mini sling (FIGURE 1).
Retropubic sling
A retropubic sling is a midurethral mesh sling that is placed beneath the urethra at the midpoint between the urethral meatus and the bladder neck. The arms of the sling extend behind the pubic symphysis, providing a hammock-like support that helps prevent leakage with increased abdominal pressures. The retropubic sling is the most commonly used type of sling. For women presenting with uncomplicated SUI who desire surgical correction, it often is the best choice for providing long-term treatment success.7
Transobturator sling
A transobturator sling is a midurethral mesh sling that is placed beneath the urethra as described above, but the arms of the sling extend outward through the obturator foramen and into the groin. This enables support of the midurethra, but this sling is less likely to result in such complications as bladder perforation or postoperative urinary retention. Transobturator slings also are associated with lower rates of voiding dysfunction and urinary urgency than retropubic slings.7-9 However, transobturator slings have higher rates of groin pain, and they are less effective in maintaining long-term cure of SUI.7
First introduced in 1996, the midurethral sling quickly grew in popularity for the treatment of SUI because of its high success rates and its minimally invasive approach.10 Both retropubic and transobturator slings are safe, extensively researched surgical approaches for the management of SUI.3 Midurethral slings have a very high rate of incontinence cure (80%–90%) and extremely high patient satisfaction rates (85%–90%), as even patients without complete cure report meaningful symptomatic improvement.7,8,11
Single-incision (mini) sling
A single-incision sling is a midurethral mesh sling that is designed to be shorter in length than standard midurethral slings. The placed sling lies under the midurethra and extends toward the superior edge of the obturator foramen but does not penetrate it. The sling is held in place by small pledgets on either side of the mesh hammock that anchor it in place to the obturator internus muscular fascia. Because this “mini” sling was introduced in 2006, fewer long-term data are available for this sling than for standard midurethral slings.
Continue to: Autologous (fascial) sling...
Autologous (fascial) sling
An autologous sling is a retropubic sling made from the patient’s own fascia; it is harvested from either the fascia lata of the lateral thigh or the rectus fascia of the abdomen. The sling is placed beneath the urethra in the bladder neck region, and sutures affixed to the sling edges pass behind the pubic symphysis and through the abdominal fascia to anchor it in place.

Choose a sling based on the clinical situation and patient goals
Consider the unique features of each sling when selecting the proper sling; this should be a shared decision with the patient after thorough counseling. Below, we present 4 clinical cases to exemplify scenarios in which different slings are appropriate, and we review the rationale for each selection.
CASE 1 SUI that interferes with exercise routine
Ms. P. is a 46-year-old (G3P3) active mother. She loves to exercise, but she has been working out less frequently because of embarrassing urinary leakage that occurs with activity. She has tried pelvic floor exercises and changing her fluid intake habits, but improvements have been minimal with these interventions. On evaluation, she has a positive cough stress test with a recently emptied bladder and a normal postvoid residual volume.
What type of sling would be best?
Because this patient is young, active, and has significant leakage with an empty bladder, a sling with good long-term treatment success is likely to provide her with the best results (Figure 1). We therefore offered her a retropubic midurethral sling. The retropubic approach is preferred here as it is less likely than the transobturator sling to cause groin/thigh pain, which is an important consideration in this young, active patient.
Further testing is not needed
For women with uncomplicated SUI who demonstrate leakage with stress (coughing, Valsalva stress test) and who have a normal postvoid residual volume, additional testing, such as urodynamic evaluation, is not necessary.12 These patients can be counseled on the range of conservative management options and as well as surgical inventions.
CASE 2 Return of SUI symptoms after transobturator sling placement
Ms. E. is a 70-year-old woman who had a transobturator sling placed 5 years ago. Initially, her SUI symptoms improved after surgery. Recently, however, she noticed a return of her SUI, which she finds bothersome and limiting to her quality of life.
How would you manage this patient?
While midurethral slings are highly effective, there are instances in which patients will have symptom recurrence. For women who already have a midurethral sling, consider the following important questions.
Is this truly recurrent SUI, or is it a new process?
Like any reconstructive procedure, midurethral sling success rates decline over time and recurrent SUI can develop.7 However, it also is possible for urge urinary incontinence to develop as a new process, and it is important to distinguish which type of urinary incontinence your patient has prior to counseling about treatment options.
To further evaluate patients with recurrent incontinence and a prior sling, we recommend urodynamic studies with cystoscopy (in addition to a detailed history and physical exam). This not only helps rule out other forms of incontinence, such as overactive bladder, but also evaluates for possible mesh erosion into the urethra or bladder, which can cause irritative voiding symptoms and incontinence.
Continue to: What type of sling did the patient have initially...
What type of sling did the patient have initially, and how does this impact a repeat procedure?
Regardless of the initial sling type used, repeat midurethral sling procedures have a significantly lower cure rate than primary midurethral sling procedures.13 Retropubic slings are more effective than transobturator slings for patients with recurrent SUI who have failed a prior sling. When a patient presents with recurrent SUI after a prior transobturator sling, the best option for a repeat procedure is usually a retropubic sling, as it achieves higher objective and subjective cure rates.13,14 (See FIGURE 2 for a comparison of retropubic and transobturator slings.)

Should I remove the old sling prior to placing a new one?
While it is recommended to remove the vaginal portion of the sling if the patient has a mesh exposure or is experiencing other symptoms, such as pain or bleeding, removal of the old sling is not necessarily indicated prior to (or during) a repeat incontinence procedure.15,16 Removing the sling, removing a portion of the sling, or leaving the sling in situ are all reasonable options.
CASE 3 Treated SUI has mesh exposure
Ms. R. is a 60-year-old woman with a history of SUI that was previously managed with a retropubic midurethral sling placed at an outside hospital. She is a smoker and has developed a vaginal mesh exposure. Although she would like the mesh removed, she does not want her incontinence to come back. She tells you that she does not think she would be able to quit smoking.
What would be a reasonable next option for Ms. R.?
While complications from a midurethral sling are rare, mesh exposures occur in approximately 2% of patients, and urinary retention requiring release of the sling occurs in about 1% of patients.3,6 It often helps to clarify for patients that the US Food and Drug Administration public health advisories on the use of transvaginal mesh have been directed specifically toward the use of transvaginal mesh for the treatment of pelvic organ prolapse (POP), not the use of mesh for midurethral slings for SUI or transabdominal mesh for POP.10,17
When considering use of a mesh sling, a thorough discussion of the potential risks, as well as the benefits and alternatives, is imperative. Patients must personally balance the probability of benefit with the potential risk of complications, and while physicians can help outline the benefits and risks through shared decision-making, ultimately it is the patient who should make this decision.
Certain patient populations may be at higher risk for mesh complications18 (See "Risk factors for mesh-related complications," below). These complications are managed in various ways (FIGURE 3). Patients who have experienced mesh complications previously are typically not good candidates for a repeat mesh sling, particularly when the risk factor for complications cannot be modified.
• Smoking
• Poorly controlled diabetes
• Decreased estrogen status
• Chronic steroid use
• Prior urethral surgery (urethral diverticulum, urethroplasty)

A mesh sling alternative
The most effective way to manage SUI in patients who are not good candidates for a mesh sling is to consider employing a sling that uses the patient’s own tissue.19-21 Common approaches include harvesting a graft of rectus fascia through a Pfannenstiel skin incision or using fascia lata from the patient’s iliotibial band in the lateral thigh. Autologous slings are safe and effective, and even after a mesh sling has failed, autologous slings have an almost 70% cure rate for SUI.20,21
Continue to: Timing of mesh removal and placement of an autologous fascial sling...
Timing of mesh removal and placement of an autologous fascial sling
Either concomitant or delayed placement of a pubovaginal sling is acceptable when removing mesh, though this should be a joint decision with the patient after counseling. If the risk for surgical complications is modifiable (for example, poorly controlled diabetes that could be improved with blood glucose control), it may be advisable to delay the fascial sling until the risk factors have been addressed. Similarly, if the reason for mesh removal is pain, it may be advisable to remove the mesh prior to placing a new sling to ensure that the pain resolves completely. Otherwise, if pain persists, it can be unclear whether the new sling is contributing to the pain, and this may lead to difficulties treating pain or incontinence in the future.
In this patient, who was an active smoker, we excised the exposed mesh and concomitantly placed an autologous fascial sling utilizing rectus fascia. This maintained continence without introducing mesh in a high-risk patient.
CASE 4 POP and occult SUI
Ms. B. is a 79-year-old woman with stage 3 POP planned for surgical repair. While she does not report urinary leakage, preoperative urodynamic testing revealed occult SUI with reduction of her prolapse. Her priorities are to avoid needing another surgery and to limit the chances of postoperative leakage, but she is nervous about her postoperative recovery and wants to avoid pain.
What approach would be appropriate?
Consider a mini sling for this patient
The single-incision (mini) sling is an option to consider for patients with mild incontinence or for those without evidence of intrinsic sphincter deficiency. It is also a good option for those who want to avoid the additional incisions required for full-length slings.
While currently there is not sufficient evidence to clearly state if single-incision slings are equivalent to other slings, recent studies show that single-incision slings appear to be safe and effective in the short term, with possibly fewer complications than traditional transobturator slings.22-24 As patients are often concerned about the potential for groin pain with a transobturator sling, a single-incision sling is an acceptable alternative that avoids groin incisions and also avoids the retropubic space.
Patient counseling is crucial
Regardless of the route, sling procedures are highly effective and safe for treating women with SUI.3 Understanding the characteristics of each type of sling and the distinct surgical approaches enables informed counseling for patients who are navigating the treatment options for SUI.
Urinary incontinence affects approximately 50% of women, with up to 80% of these women experiencing stress urinary incontinence (SUI) at some point in their lives.1-3 While conservative measures can offer some improvement in symptoms, the mainstay of treatment for SUI is surgical intervention.4,5 The lifetime risk of undergoing surgery for SUI is 13.6%, and surgery leads to a major improvement in quality of life and productivity.1,6

Types of slings used for SUI
Sling procedures are the most commonly used surgical approach for the treatment of SUI. Two types of urethral slings are used: the midurethral sling and the autologous fascial (pubovaginal) sling. The midurethral sling, which is the most frequently used sling today, can be further characterized as the retropubic sling, the transobturator sling, and the mini sling (FIGURE 1).
Retropubic sling
A retropubic sling is a midurethral mesh sling that is placed beneath the urethra at the midpoint between the urethral meatus and the bladder neck. The arms of the sling extend behind the pubic symphysis, providing a hammock-like support that helps prevent leakage with increased abdominal pressures. The retropubic sling is the most commonly used type of sling. For women presenting with uncomplicated SUI who desire surgical correction, it often is the best choice for providing long-term treatment success.7
Transobturator sling
A transobturator sling is a midurethral mesh sling that is placed beneath the urethra as described above, but the arms of the sling extend outward through the obturator foramen and into the groin. This enables support of the midurethra, but this sling is less likely to result in such complications as bladder perforation or postoperative urinary retention. Transobturator slings also are associated with lower rates of voiding dysfunction and urinary urgency than retropubic slings.7-9 However, transobturator slings have higher rates of groin pain, and they are less effective in maintaining long-term cure of SUI.7
First introduced in 1996, the midurethral sling quickly grew in popularity for the treatment of SUI because of its high success rates and its minimally invasive approach.10 Both retropubic and transobturator slings are safe, extensively researched surgical approaches for the management of SUI.3 Midurethral slings have a very high rate of incontinence cure (80%–90%) and extremely high patient satisfaction rates (85%–90%), as even patients without complete cure report meaningful symptomatic improvement.7,8,11
Single-incision (mini) sling
A single-incision sling is a midurethral mesh sling that is designed to be shorter in length than standard midurethral slings. The placed sling lies under the midurethra and extends toward the superior edge of the obturator foramen but does not penetrate it. The sling is held in place by small pledgets on either side of the mesh hammock that anchor it in place to the obturator internus muscular fascia. Because this “mini” sling was introduced in 2006, fewer long-term data are available for this sling than for standard midurethral slings.
Continue to: Autologous (fascial) sling...
Autologous (fascial) sling
An autologous sling is a retropubic sling made from the patient’s own fascia; it is harvested from either the fascia lata of the lateral thigh or the rectus fascia of the abdomen. The sling is placed beneath the urethra in the bladder neck region, and sutures affixed to the sling edges pass behind the pubic symphysis and through the abdominal fascia to anchor it in place.

Choose a sling based on the clinical situation and patient goals
Consider the unique features of each sling when selecting the proper sling; this should be a shared decision with the patient after thorough counseling. Below, we present 4 clinical cases to exemplify scenarios in which different slings are appropriate, and we review the rationale for each selection.
CASE 1 SUI that interferes with exercise routine
Ms. P. is a 46-year-old (G3P3) active mother. She loves to exercise, but she has been working out less frequently because of embarrassing urinary leakage that occurs with activity. She has tried pelvic floor exercises and changing her fluid intake habits, but improvements have been minimal with these interventions. On evaluation, she has a positive cough stress test with a recently emptied bladder and a normal postvoid residual volume.
What type of sling would be best?
Because this patient is young, active, and has significant leakage with an empty bladder, a sling with good long-term treatment success is likely to provide her with the best results (Figure 1). We therefore offered her a retropubic midurethral sling. The retropubic approach is preferred here as it is less likely than the transobturator sling to cause groin/thigh pain, which is an important consideration in this young, active patient.
Further testing is not needed
For women with uncomplicated SUI who demonstrate leakage with stress (coughing, Valsalva stress test) and who have a normal postvoid residual volume, additional testing, such as urodynamic evaluation, is not necessary.12 These patients can be counseled on the range of conservative management options and as well as surgical inventions.
CASE 2 Return of SUI symptoms after transobturator sling placement
Ms. E. is a 70-year-old woman who had a transobturator sling placed 5 years ago. Initially, her SUI symptoms improved after surgery. Recently, however, she noticed a return of her SUI, which she finds bothersome and limiting to her quality of life.
How would you manage this patient?
While midurethral slings are highly effective, there are instances in which patients will have symptom recurrence. For women who already have a midurethral sling, consider the following important questions.
Is this truly recurrent SUI, or is it a new process?
Like any reconstructive procedure, midurethral sling success rates decline over time and recurrent SUI can develop.7 However, it also is possible for urge urinary incontinence to develop as a new process, and it is important to distinguish which type of urinary incontinence your patient has prior to counseling about treatment options.
To further evaluate patients with recurrent incontinence and a prior sling, we recommend urodynamic studies with cystoscopy (in addition to a detailed history and physical exam). This not only helps rule out other forms of incontinence, such as overactive bladder, but also evaluates for possible mesh erosion into the urethra or bladder, which can cause irritative voiding symptoms and incontinence.
Continue to: What type of sling did the patient have initially...
What type of sling did the patient have initially, and how does this impact a repeat procedure?
Regardless of the initial sling type used, repeat midurethral sling procedures have a significantly lower cure rate than primary midurethral sling procedures.13 Retropubic slings are more effective than transobturator slings for patients with recurrent SUI who have failed a prior sling. When a patient presents with recurrent SUI after a prior transobturator sling, the best option for a repeat procedure is usually a retropubic sling, as it achieves higher objective and subjective cure rates.13,14 (See FIGURE 2 for a comparison of retropubic and transobturator slings.)

Should I remove the old sling prior to placing a new one?
While it is recommended to remove the vaginal portion of the sling if the patient has a mesh exposure or is experiencing other symptoms, such as pain or bleeding, removal of the old sling is not necessarily indicated prior to (or during) a repeat incontinence procedure.15,16 Removing the sling, removing a portion of the sling, or leaving the sling in situ are all reasonable options.
CASE 3 Treated SUI has mesh exposure
Ms. R. is a 60-year-old woman with a history of SUI that was previously managed with a retropubic midurethral sling placed at an outside hospital. She is a smoker and has developed a vaginal mesh exposure. Although she would like the mesh removed, she does not want her incontinence to come back. She tells you that she does not think she would be able to quit smoking.
What would be a reasonable next option for Ms. R.?
While complications from a midurethral sling are rare, mesh exposures occur in approximately 2% of patients, and urinary retention requiring release of the sling occurs in about 1% of patients.3,6 It often helps to clarify for patients that the US Food and Drug Administration public health advisories on the use of transvaginal mesh have been directed specifically toward the use of transvaginal mesh for the treatment of pelvic organ prolapse (POP), not the use of mesh for midurethral slings for SUI or transabdominal mesh for POP.10,17
When considering use of a mesh sling, a thorough discussion of the potential risks, as well as the benefits and alternatives, is imperative. Patients must personally balance the probability of benefit with the potential risk of complications, and while physicians can help outline the benefits and risks through shared decision-making, ultimately it is the patient who should make this decision.
Certain patient populations may be at higher risk for mesh complications18 (See "Risk factors for mesh-related complications," below). These complications are managed in various ways (FIGURE 3). Patients who have experienced mesh complications previously are typically not good candidates for a repeat mesh sling, particularly when the risk factor for complications cannot be modified.
• Smoking
• Poorly controlled diabetes
• Decreased estrogen status
• Chronic steroid use
• Prior urethral surgery (urethral diverticulum, urethroplasty)

A mesh sling alternative
The most effective way to manage SUI in patients who are not good candidates for a mesh sling is to consider employing a sling that uses the patient’s own tissue.19-21 Common approaches include harvesting a graft of rectus fascia through a Pfannenstiel skin incision or using fascia lata from the patient’s iliotibial band in the lateral thigh. Autologous slings are safe and effective, and even after a mesh sling has failed, autologous slings have an almost 70% cure rate for SUI.20,21
Continue to: Timing of mesh removal and placement of an autologous fascial sling...
Timing of mesh removal and placement of an autologous fascial sling
Either concomitant or delayed placement of a pubovaginal sling is acceptable when removing mesh, though this should be a joint decision with the patient after counseling. If the risk for surgical complications is modifiable (for example, poorly controlled diabetes that could be improved with blood glucose control), it may be advisable to delay the fascial sling until the risk factors have been addressed. Similarly, if the reason for mesh removal is pain, it may be advisable to remove the mesh prior to placing a new sling to ensure that the pain resolves completely. Otherwise, if pain persists, it can be unclear whether the new sling is contributing to the pain, and this may lead to difficulties treating pain or incontinence in the future.
In this patient, who was an active smoker, we excised the exposed mesh and concomitantly placed an autologous fascial sling utilizing rectus fascia. This maintained continence without introducing mesh in a high-risk patient.
CASE 4 POP and occult SUI
Ms. B. is a 79-year-old woman with stage 3 POP planned for surgical repair. While she does not report urinary leakage, preoperative urodynamic testing revealed occult SUI with reduction of her prolapse. Her priorities are to avoid needing another surgery and to limit the chances of postoperative leakage, but she is nervous about her postoperative recovery and wants to avoid pain.
What approach would be appropriate?
Consider a mini sling for this patient
The single-incision (mini) sling is an option to consider for patients with mild incontinence or for those without evidence of intrinsic sphincter deficiency. It is also a good option for those who want to avoid the additional incisions required for full-length slings.
While currently there is not sufficient evidence to clearly state if single-incision slings are equivalent to other slings, recent studies show that single-incision slings appear to be safe and effective in the short term, with possibly fewer complications than traditional transobturator slings.22-24 As patients are often concerned about the potential for groin pain with a transobturator sling, a single-incision sling is an acceptable alternative that avoids groin incisions and also avoids the retropubic space.
Patient counseling is crucial
Regardless of the route, sling procedures are highly effective and safe for treating women with SUI.3 Understanding the characteristics of each type of sling and the distinct surgical approaches enables informed counseling for patients who are navigating the treatment options for SUI.
- Wu JM, Matthews CA, Conover MM, et al. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol. 2014;123:1201-1206.
- Jonsson Funk M, Levin PJ, Wu JM. Trends in the surgical management of stress urinary incontinence. Obstet Gynecol. 2012;119:845-851.
- Ford AA, Rogerson L, Cody JD, et al. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD006375.
- Dumoulin C, Hay-Smith J, Habee-Seguin GM, et al. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women: a short version Cochrane systematic review with meta-analysis. Neurourol Urodyn. 2015;34:300-308.
- Cox A, Herschorn S, Lee L. Surgical management of female SUI: is there a gold standard? Nat Rev Urol. 2013;10:78-89.
- Schimpf MO, Rahn DD, Wheeler TL, et al; Society of Gynecologic Surgeons Systematic Review Group. Sling surgery for stress urinary incontinence in women: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;211:71.e1-71.e27.
- Kenton K, Stoddard AM, Zyczynski H, et al. 5-year longitudinal followup after retropubic and transobturator mid urethral slings. J Urol. 2015;193:203-210.
- Richter HE, Albo ME, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066-2076.
- Albo ME, Litman HJ, Richter HE, et al; Urinary Incontinence Treatment Network. Treatment success of retropubic and transobturator midurethral slings at 24-months. J Urol. 2012;188:2281-2287.
- US Food and Drug Administration. Urogynecologic surgical mesh: update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. July 2011;1-15. https://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM262760.pdf. Accessed September 16, 2019.
- Nilsson CG, Palva K, Aarnio R, et al. Seventeen years’ follow up of the tension-free vaginal tape procedure for female stress urinary incontinence. Int Urogynecol J. 2013;24:1265-1269.
- Nager CW, Brubaker L, Litman HJ, et al; Urinary Incontinence Treatment Network. A randomized trial of urodynamic testing before stress-incontinence surgery. N Engl J Med. 2012;366:1987-1997.
- Stav K, Dwyer PL, Rosamilia A, et al. Repeat synthetic mid urethral sling procedure for women with recurrent stress urinary incontinence. J Urol. 2010;183:241-246.
- Kim A, Kim MS, Park YJ, et al. Retropubic versus transobturator mid urethral slings in patients at high risk for recurrent stress incontinence: a systematic review and meta-analysis. J Urol. 2019;202:132-142.
- Kavanagh A, Sanaee M, Carison KV, et al. Management of patients with stress urinary incontinence after failed midurethral sling. Can Urol Assoc J. 2017;11(6 suppl 2):S143-S146.
- Steele SE, Hill AJ, Unger CA. Concurrent midurethral sling excision or lysis at the time of repeat sling for treatment of recurrent or persistent stress urinary incontinence. Int Urogynecol J. 2018;29:285-290.
- US Food and Drug Administration. Urogynecologic surgical mesh implants. https://www.fda.gov/medicaldevices/productsandmedicalprocedures/implantsandprosthetics/urogynsurgicalmesh/. Content current as of July 10, 2019. Accessed September 16, 2019.
- Kokanali MK, Doganay M, Aksakal O, et al. Risk factors for mesh erosion after vaginal sling procedures for urinary incontinence. Eur J Obstet Gynecol Reprod Biol. 2014;177:146-150.
- Nikolopoulos KI, Betschart C, Doumouchtsis SK. The surgical management of recurrent stress urinary incontinence: a systematic review. Acta Obstet Gynecol Scand. 2015;94:568-576.
- Milose JC, Sharp KM, He C, et al. Success of autologous pubovaginal sling after failed synthetic mid urethral sling. J Urol. 2015;193:916-920.
- Albo ME, Richter HE, Brubaker L, et al; Urinary Incontinence Treatment Network. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med. 2007;356:2143-2155.
- Imamura M, Hudson J, Wallace SA, et al. Surgical interventions for women with stress urinary incontinence: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2019;365:I1842.
- Jiao B, Lai S, Xu X, et al. A systematic review and meta-analysis of single-incision mini-slings (MiniArc) versus transobturator mid-urethral slings in surgical management of female stress urinary incontinence. Medicine (Baltimore). 2018;97:e0283.
- Sun Z, Wang X, Lang J, et al. Comparison of outcomes between single-incision sling and transobturator sling for treating stress urinary incontinence: a 10-year prospective study. Neurourol Urodyn. 2019;38:1852-1858.
- Wu JM, Matthews CA, Conover MM, et al. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol. 2014;123:1201-1206.
- Jonsson Funk M, Levin PJ, Wu JM. Trends in the surgical management of stress urinary incontinence. Obstet Gynecol. 2012;119:845-851.
- Ford AA, Rogerson L, Cody JD, et al. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD006375.
- Dumoulin C, Hay-Smith J, Habee-Seguin GM, et al. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women: a short version Cochrane systematic review with meta-analysis. Neurourol Urodyn. 2015;34:300-308.
- Cox A, Herschorn S, Lee L. Surgical management of female SUI: is there a gold standard? Nat Rev Urol. 2013;10:78-89.
- Schimpf MO, Rahn DD, Wheeler TL, et al; Society of Gynecologic Surgeons Systematic Review Group. Sling surgery for stress urinary incontinence in women: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;211:71.e1-71.e27.
- Kenton K, Stoddard AM, Zyczynski H, et al. 5-year longitudinal followup after retropubic and transobturator mid urethral slings. J Urol. 2015;193:203-210.
- Richter HE, Albo ME, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066-2076.
- Albo ME, Litman HJ, Richter HE, et al; Urinary Incontinence Treatment Network. Treatment success of retropubic and transobturator midurethral slings at 24-months. J Urol. 2012;188:2281-2287.
- US Food and Drug Administration. Urogynecologic surgical mesh: update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. July 2011;1-15. https://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM262760.pdf. Accessed September 16, 2019.
- Nilsson CG, Palva K, Aarnio R, et al. Seventeen years’ follow up of the tension-free vaginal tape procedure for female stress urinary incontinence. Int Urogynecol J. 2013;24:1265-1269.
- Nager CW, Brubaker L, Litman HJ, et al; Urinary Incontinence Treatment Network. A randomized trial of urodynamic testing before stress-incontinence surgery. N Engl J Med. 2012;366:1987-1997.
- Stav K, Dwyer PL, Rosamilia A, et al. Repeat synthetic mid urethral sling procedure for women with recurrent stress urinary incontinence. J Urol. 2010;183:241-246.
- Kim A, Kim MS, Park YJ, et al. Retropubic versus transobturator mid urethral slings in patients at high risk for recurrent stress incontinence: a systematic review and meta-analysis. J Urol. 2019;202:132-142.
- Kavanagh A, Sanaee M, Carison KV, et al. Management of patients with stress urinary incontinence after failed midurethral sling. Can Urol Assoc J. 2017;11(6 suppl 2):S143-S146.
- Steele SE, Hill AJ, Unger CA. Concurrent midurethral sling excision or lysis at the time of repeat sling for treatment of recurrent or persistent stress urinary incontinence. Int Urogynecol J. 2018;29:285-290.
- US Food and Drug Administration. Urogynecologic surgical mesh implants. https://www.fda.gov/medicaldevices/productsandmedicalprocedures/implantsandprosthetics/urogynsurgicalmesh/. Content current as of July 10, 2019. Accessed September 16, 2019.
- Kokanali MK, Doganay M, Aksakal O, et al. Risk factors for mesh erosion after vaginal sling procedures for urinary incontinence. Eur J Obstet Gynecol Reprod Biol. 2014;177:146-150.
- Nikolopoulos KI, Betschart C, Doumouchtsis SK. The surgical management of recurrent stress urinary incontinence: a systematic review. Acta Obstet Gynecol Scand. 2015;94:568-576.
- Milose JC, Sharp KM, He C, et al. Success of autologous pubovaginal sling after failed synthetic mid urethral sling. J Urol. 2015;193:916-920.
- Albo ME, Richter HE, Brubaker L, et al; Urinary Incontinence Treatment Network. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med. 2007;356:2143-2155.
- Imamura M, Hudson J, Wallace SA, et al. Surgical interventions for women with stress urinary incontinence: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2019;365:I1842.
- Jiao B, Lai S, Xu X, et al. A systematic review and meta-analysis of single-incision mini-slings (MiniArc) versus transobturator mid-urethral slings in surgical management of female stress urinary incontinence. Medicine (Baltimore). 2018;97:e0283.
- Sun Z, Wang X, Lang J, et al. Comparison of outcomes between single-incision sling and transobturator sling for treating stress urinary incontinence: a 10-year prospective study. Neurourol Urodyn. 2019;38:1852-1858.
A novel approach to complete transobturator sling mesh removal

Additional videos from SGS are available here, including these recent offerings:
- Embryologic development of the external genitalia as it relates to vaginoplasty for the transgender woman
- A stepwise approach to the difficult bladder flap to prevent urinary tract injury during laparoscopic hysterectomy
- Novel method to demarcate bladder dissection during posthysterectomy sacrocolpopexy

Additional videos from SGS are available here, including these recent offerings:
- Embryologic development of the external genitalia as it relates to vaginoplasty for the transgender woman
- A stepwise approach to the difficult bladder flap to prevent urinary tract injury during laparoscopic hysterectomy
- Novel method to demarcate bladder dissection during posthysterectomy sacrocolpopexy

Additional videos from SGS are available here, including these recent offerings:
- Embryologic development of the external genitalia as it relates to vaginoplasty for the transgender woman
- A stepwise approach to the difficult bladder flap to prevent urinary tract injury during laparoscopic hysterectomy
- Novel method to demarcate bladder dissection during posthysterectomy sacrocolpopexy
Native tissue repair of POP: Apical suspension, anterior repair, and posterior repair



Videos courtesy of Mayo Clinic
Read the related article: Native tissue repair of POP: Surgical techniques to improve outcomes



Videos courtesy of Mayo Clinic
Read the related article: Native tissue repair of POP: Surgical techniques to improve outcomes



Videos courtesy of Mayo Clinic
Read the related article: Native tissue repair of POP: Surgical techniques to improve outcomes
2019 Update on pelvic floor dysfunction
Fecal incontinence (FI), also known as accidental bowel leakage, is the involuntary loss of feces, which includes both liquid and solid stool as defined by the International Continence Society (ICS) and the International Urogynecological Association (IUGA).1,2 Fecal incontinence is common, occurring in 7% to 25% of community-dwelling women, and it increases with age.2-6 The condition is rarely addressed, with only 30% of women seeking care.6-8 This is due to patient embarrassment and the lack of a reliable screening tool. However, FI affects quality of life and mental health, and the associated economic burden likely will rise given the increased prevalence of FI among older women.2,4,7,9
Fecal incontinence occurs due to poor stool consistency, anal and pelvic muscle weakness, reduced rectal compliance, reduced or increased rectal sensation, or bowel inflammation or dysfunction. Many conditions can cause FI (TABLE 1).5,10,11 It is therefore important to elicit a full medical history with a focus on specific bowel symptoms, such as stool consistency type (TABLE 2),12 FI frequency, and duration of symptoms, as well as to perform a complete examination to identify any readily reversible or malignant causes. A colonoscopy is recommended for individuals who meet screening criteria or present with a change in bowel symptoms, such as diarrhea, bleeding, or obstruction.13,14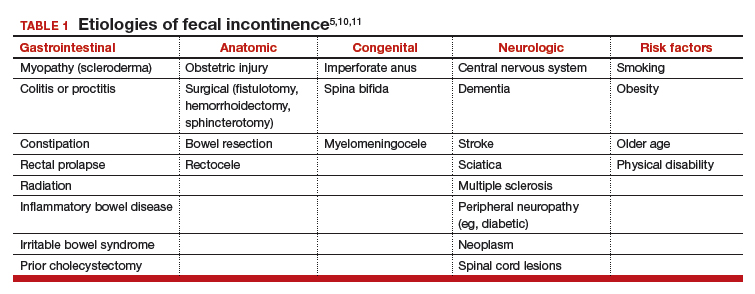
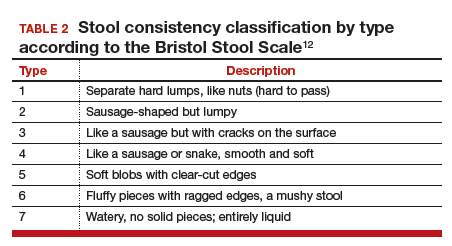
Fecal incontinence treatments include a range of approaches categorized from conservative, or first-line therapy, to fourth-line surgical managements (FIGURE 1).1,10,13,14 In this Update, we review the results of 3 well-designed trials that enrolled women with frequent nonneurogenic FI.
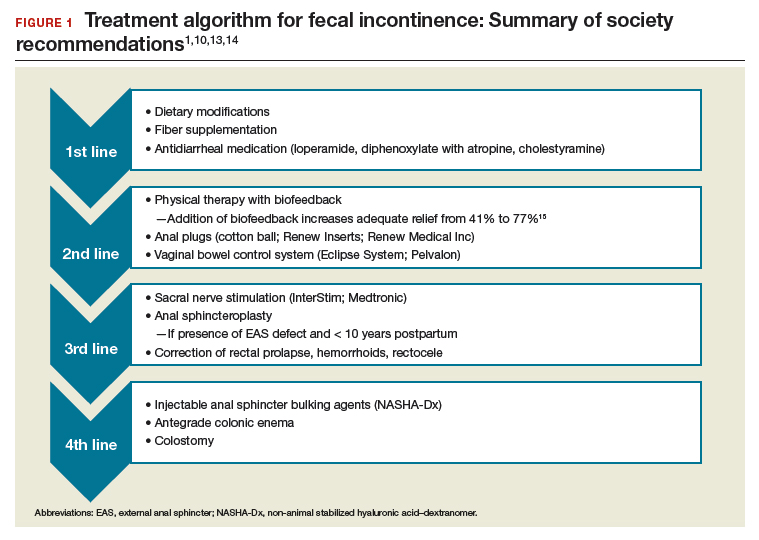
Common first- and second-line treatments produce equivalent improvements in FI symptoms at
6 months
Jelovsek JE, Markland AD, Whitehead WE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Controlling faecal incontinence in women by performing anal exercises with biofeedback or loperamide: a randomized clinical trial. Lancet Gastroenterol Hepatol. 2019;4:698-710.
In a multicenter, randomized trial of first- and second-line treatments for FI, Jelovsek and colleagues evaluated the efficacy of oral placebo, loperamide, pelvic floor physical therapy (PFPT) with biofeedback using anorectal manometry, or combination therapy over a 24-week period.
Continue to: Four treatments compared...
Four treatments compared
Three hundred women with FI occurring monthly for 3 months were included in the trial. Women were excluded if they had a stool classification of type 1 or type 7 on the Bristol Stool Scale, inflammatory bowel disease (IBD), history of rectovaginal fistula or cloacal defect, rectal prolapse, prior bowel diversion, fecal impaction, neurologic disorder leading to incontinence, use of loperamide or diphenoxylate within the last 30 days, childbirth within the last 3 months, need for antiretroviral drugs, hepatic impairment, or chronic abdominal pain without diarrhea.
Baseline characteristics and symptoms severity were similar among participants. The average age of the women was 63 years, with 79% white and 85% postmenopausal. Participants had a mean (SD) of 1.6 (1.8) leaks per day.
Participants were randomly assigned in a 0.5:1:1:1 fashion to receive oral placebo, loperamide, oral placebo with PFPT/biofeedback, or loperamide with PFPT/biofeedback. All participants received a standardized educational pamphlet that outlined dietary and behavioral recommendations.
Women assigned to PFPT/biofeedback received 6 sessions every other week. Loperamide was started at a dosage of 2 mg per day with the possibility of dose maintenance, escalation, reduction, or discontinuation.
Study outcomes. The primary outcome was a change from baseline to 24 weeks in the Vaizey FI symptom severity score, which assesses fecal frequency, urgency, and use of pads and medications. Secondary outcomes included assessment of a 7-day bowel diary and other quality-of-life measures. Data at 24 weeks were available for 89% of the women.
All treatment groups experienced improved FI symptoms
Based on changes in Vaizey scores after 24 weeks of treatment, women in all treatment groups had similar improvement in symptoms severity. However, those who received loperamide and PFPT/biofeedback had decreased pad changes per week and more accident-free days compared with women treated with placebo and biofeedback. Quality of life at 24 weeks was not statistically different between treatment groups as improvement was seen in all groups, including those who received oral placebo and patient education.
Adverse events. The proportion of gastrointestinal adverse effects was similar between treatment groups, ranging from 45% to 63%. Constipation was the most common adverse event overall and was more common in those taking loperamide, occurring in 51% of the loperamide plus PFPT/biofeedback group, 38% of those who received loperamide alone, 23% of the biofeedback with placebo group, and 12% of the placebo-alone group.
Strengths and limitations. Strengths of this study include its multisite, large sample size, low dropout rate, and sufficiently powered design to compare various combinations of first- and second-line therapies in women with a mean baseline FI of 1.6 leaks per day. Another strength is the robustness of the PFPT/biofeedback sessions that used anorectal manometry. This may, however, limit the study's external validity given that clinical use of this device is likely rare. Additionally, the population was comprised largely of postmenopausal and white women, which may make the findings less generalizable to other populations.
Women who suffer from frequent FI may require both loperamide and PFPT/biofeedback if they want to increase the likelihood of accident-free days and use of fewer pads. Should they note increased constipation or are not amenable to scheduled PFPT sessions, formalized education about dietary modifications, according to this study, will provide improvement in symptom severity.
Continue to: Novel vaginal bowel control system...
Novel vaginal bowel control system is effective, durable over 12 months for FI treatment
Richter HE, Dunivan G, Brown HW, et al. A 12-month clinical durability of effectiveness and safety evaluation of a vaginal bowel control system for the nonsurgical treatment of fecal incontinence. Female Pelvic Med Reconstr Surg. 2019;25:113-119.
Richter and colleagues characterized clinical success, effect on quality of life, and durability over 12 months of a novel vaginal bowel control device (Eclipse System; Pelvalon) for FI in a prospective cohort study. The device is a silicone-coated vaginal insert with a detachable pump and balloon that deflects the rectovaginal septum posteriorly, thus impeding the passage of stool in the rectum (FIGURE 2).
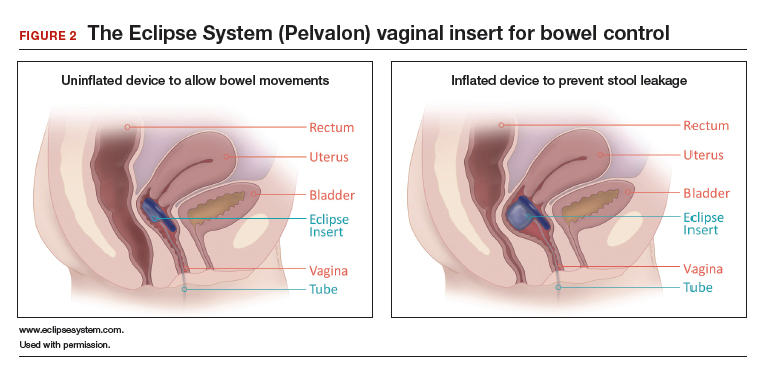
Study eligibility criteria and treatment protocol
Women were eligible for the study if they had 4 or more episodes of fecal soiling on a 2-week bowel diary and had FI for at least 6 months. Participants were excluded if they had prolapse outside the hymen, rectovaginal fistula, IBD, congenital anorectal malformation, urinary or colorectal infection, chronic pelvic or anorectal pain, pregnancy or planning pregnancy in the next 5 months, unmanaged chronic watery diarrhea, presence of an open wound or tear in the vagina, significant urogenital atrophy, or any psychiatric or neurologic disorder that would hinder the ability to participate.
Participants successfully fitted with the device (3 attempts were allowed) were entered into the study's run-in phase. Those who were successfully fitted and had a 50% or greater reduction in FI continued into the treatment phase with 12 months of follow-up.
Of the 137 women eligible for device fitting, 62% were successfully fitted. The 73 (86%) women who had a 50% or greater reduction in FI during the run-in period comprised the intent-to-treat study population. On average, these women were 61.3 years of age, with 70% white and 82% postmenopausal. At baseline, they had a mean of 14.1 episodes of FI over 2 weeks. (Prior to enrollment, 97.3% of women attempted self-management strategies, 17.8% to 23% failed conservative therapy, and 7.8% to 13.7% failed surgical therapy.) The follow-up rate at 12 months was 74%.
Study outcomes. The primary outcome was treatment success, defined as proportion of subjects with a 50% or greater reduction in FI episodes at 3 months; this outcome also was evaluated at 6 and 12 months. Secondary outcomes were the number of FI episodes and quality-of-life measures at 3, 6, and 12 months.
Treatment success, patient satisfaction high
In the treatment phase, women had sustained improvements in symptom severity and quality-of-life measures over 12 months. Treatment success was 73% at 3 months, 71% at 6 months, and 70% at 12 months. Complete continence was achieved in 46% of participants at 12 months, and major FI episodes (requiring immediate change of undergarments) decreased from 5.0 at baseline to 0.5 at 12 months. Quality-of-life measures were improved at 3 months, and improvement was sustained over 12 months. Satisfaction was 94% at 12 months.
Adverse events. No serious device-related adverse events occurred. Mild device-related adverse events were experienced by 45% of women during the fitting process and by 38% during treatment period. These included vaginal wall injury such as hyperemia and erosion; vaginal or pelvic discomfort; vaginal infection; constipation; and lower urinary tract issues such as urinary tract infection, urinary incontinence, and voiding dysfunction. No adverse events led to treatment discontinuation.
Strengths and limitations. Strengths of this study include that it was conducted at multiple clinical sites, had a large sample size, and had a 1-year follow-up period in a population with daily FI. A limitation was that only women who had a 50% or greater reduction in FI episodes during the run-in period were followed for 12 months; however, this was 86% of the original cohort. The use of a comparative group using other devices, such as anal plugs, would have strengthened this study.
The Eclipse intravaginal bowel control device (approved by the US Food and Drug Administration in 2015) provided a sustained 50% or greater reduction in FI episodes in more than 70% of women wearing the device for 1 year, with high patient satisfaction. Thus, for women who fail conservative treatment methods for FI, clinicians should consider referring them to a urogynecologist or specialist who is knowledgeable in fitting this vaginal bowel control device.
Continue to: Sacroneuromodulation for FI…
Sacral neuromodulation for FI is effective long-term
Hull T, Giese C, Wexner SD, et al; for the SNS Study Group. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56:234-245.
In this multicenter, prospective cohort study, Hull and colleagues evaluated the 5-year efficacy of sacral neuromodulation (SNM), also known as sacral nerve stimulation, for treatment of FI. This study followed an earlier investigation by Wexner and colleagues, which reported that 83% of 120 patients treated with SNM had a 50% or greater improvement in FI episodes at 12 months.16
Details of the study
The investigators enrolled 133 participants (92% female) who had more than 2 episodes of FI per week for longer than 6 months (12 months after vaginal delivery). Participants were excluded if they had congenital anorectal malformations, prior rectal surgery within the past 12 months (or 24 months if due to cancer), defects greater than 120° of the external anal sphincter (EAS), IBD, unmanaged chronic watery diarrhea, stool consistency type 6 or type 7 on the Bristol Stool Scale, sequela of pelvic radiation, active anal abscess or fistula, pregnancy, or planned pregnancy.
Eligible participants underwent a 2-stage procedure with the InterStim bowel control device (Medtronic). If participants experienced a 50% or greater reduction in incontinence episodes with a wearable external SNM device in the test stimulation (stage 1), they received the chronic SNM implant device (stage 2).
Participants who underwent device implantation were followed at 1, 3, and 6 months and annually for 5 years or until they exited the study. Bowel diaries and quality of life assessments were completed at baseline and at follow-up.
The primary outcome was therapeutic success, defined as 50% or greater improvement in FI episodes per week.
A total of 120 participants (90%) underwent implantation of the chronic lead and neuromodulator, and 76 (63%) were followed for 5 years. Baseline characteristics available in the initial study of 133 participants showed that the mean age was 60.5 years; 25% had undergone a prior anal sphincteroplasty; and 16.5% and 10.5% had EAS or internal anal sphincter (IAS) defects, respectively, on endoanal ultrasonography.16
Therapeutic success was high at 5 years
At the 5-year follow-up, 89% (64/72) of participants met therapeutic success, with a reduction in weekly FI episodes from 9.1 at baseline to 1.7 at 5 years. The number of incontinence pads required decreased, and more participants wore no pads at 5 years. In the intention-to-treat analysis, carrying forward the baseline FI rate in participants who lacked follow-up data, the therapeutic success rate was 69%. Quality-of-life measures improved at 5 years, both statistically and by minimal clinical difference.
Adverse events. Sixty-eight percent of participants experienced device-related adverse events, including implant site pain, change in sensation of stimulation, change in efficacy, implant site infection, or neurostimulator battery depletion (neurostimulator use commonly expires after 3 to 5 years). Of these events, 80% were successfully treated with medications, reprogramming, or no intervention. The 5-year probability of device revision or replacement was 24.4%, and the 5-year probability of device explant was 19.0%.
Strengths and limitations. Overall, this study was a well-designed, multicenter trial with long-term follow-up that showed significant improvement in FI with the use of SNM. Its strengths include the enrollment of postmenopausal women who had current defects in EAS and/or IAS on endoanal ultrasonography and 25% who had a prior sphincteroplasty. The findings therefore are relevant to the gynecologic population in whom anal sphincteroplasty would not be recommended. The study also accounted for dropouts and reported the adjusted success rate of 69% at 5 years in that group.
The lack of a control arm to rule out the placebo effect is a limitation of this study, although randomized trials comparing the effect of SNM "on" versus "off" showed greater improvement with the device "on."17
Sacral neuromodulation is an excellent therapy for women with daily FI who have failed noninvasive options and desire to proceed to a more durable, long-lasting device therapy. Although adverse events may occur, they are mild and most often resolve with device reprogramming.
- Sultan AH, Monga A, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female anorectal dysfunction. Neurourol Urodyn. 2017;36:10-34.
- Bharucha AE, Dunivan G, Goode PS, et al. Epidemiology, pathophysiology, and classification of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. Am J Gastroenterol. 2015;110:127-136.
- Bharucha AE, Zinsmeister AR, Locke GR, et al. Symptoms and quality of life in community women with fecal incontinence. Clin Gastroenterol Hepatol. 2006;4:1004-1008.
- Perry S, Shaw C, McGrother C, et al; Leicestershire MRC Incontinence Study Team. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut. 2002;50:480-484.
- Ditah I, Devaki P, Luma HN, et al. Prevalence, trends, and risk factors for fecal incontinence in United States adults, 2005-2010. Clin Gastroenterol Hepatol. 2014;12:636-643.e1-2.
- Brown HW, Wexner SD, Lukacz ES. Factors associated with care seeking among women with accidental bowel leakage. Female Pelvic Med Reconstr Surg. 2013;19:66-71.
- Norton NJ. The perspective of the patient. Gastroenterology. 2004;126(1 suppl 1):S175-S179.
- Guan W, Schmuhl NB, Brown HW. Response re: If we don't ask, they won't tell: screening for urinary and fecal incontinence by primary care providers. J Am Board Fam Med. 2019;32:119.3-120.
- Whitehead WE, Borrud L, Goode PS, et al; Pelvic Floor Disorders Network. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512-517.
- Wald A, Bharucha AE, Cosman BC, et al. ACG clinical guideline: management of benign anorectal disorders. Am J Gastroenterol. 2014;109:1141-1157.
- Bharucha AE, Zinsmeister AR, Schleck CD, et al. Bowel disturbances are the most important risk factors for late onset fecal incontinence: a population-based case-control study in women. Gastroenterology. 2010;139:1559-1566.
- Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920-924.
- Paquette IM, Varma MG, Kaiser AM, et al. The American Society of Colon and Rectal Surgeons' clinical practice guideline for the treatment of fecal incontinence. Dis Colon Rectum. 2015;58:623-636.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 210: Fecal incontinence. Obstet Gynecol. 2019;133:e260-e273.
- Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum. 2009;52:1730-1737.
- Wexner SD, Coller JA, Devroede G, et al. Sacral nerve stimulation for fecal incontinence: results of a 120-patient prospective multicenter study. Ann Surg. 2010;251:441-449.
- Leroi AM, Parc Y, Lehur PA, et al. Efficacy of sacral nerve stimulation for fecal incontinence: results of a multicenter double-blind crossover study. Ann Surg. 2005;242:662-669.
Fecal incontinence (FI), also known as accidental bowel leakage, is the involuntary loss of feces, which includes both liquid and solid stool as defined by the International Continence Society (ICS) and the International Urogynecological Association (IUGA).1,2 Fecal incontinence is common, occurring in 7% to 25% of community-dwelling women, and it increases with age.2-6 The condition is rarely addressed, with only 30% of women seeking care.6-8 This is due to patient embarrassment and the lack of a reliable screening tool. However, FI affects quality of life and mental health, and the associated economic burden likely will rise given the increased prevalence of FI among older women.2,4,7,9
Fecal incontinence occurs due to poor stool consistency, anal and pelvic muscle weakness, reduced rectal compliance, reduced or increased rectal sensation, or bowel inflammation or dysfunction. Many conditions can cause FI (TABLE 1).5,10,11 It is therefore important to elicit a full medical history with a focus on specific bowel symptoms, such as stool consistency type (TABLE 2),12 FI frequency, and duration of symptoms, as well as to perform a complete examination to identify any readily reversible or malignant causes. A colonoscopy is recommended for individuals who meet screening criteria or present with a change in bowel symptoms, such as diarrhea, bleeding, or obstruction.13,14

Fecal incontinence treatments include a range of approaches categorized from conservative, or first-line therapy, to fourth-line surgical managements (FIGURE 1).1,10,13,14 In this Update, we review the results of 3 well-designed trials that enrolled women with frequent nonneurogenic FI.

Common first- and second-line treatments produce equivalent improvements in FI symptoms at
6 months
Jelovsek JE, Markland AD, Whitehead WE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Controlling faecal incontinence in women by performing anal exercises with biofeedback or loperamide: a randomized clinical trial. Lancet Gastroenterol Hepatol. 2019;4:698-710.
In a multicenter, randomized trial of first- and second-line treatments for FI, Jelovsek and colleagues evaluated the efficacy of oral placebo, loperamide, pelvic floor physical therapy (PFPT) with biofeedback using anorectal manometry, or combination therapy over a 24-week period.
Continue to: Four treatments compared...
Four treatments compared
Three hundred women with FI occurring monthly for 3 months were included in the trial. Women were excluded if they had a stool classification of type 1 or type 7 on the Bristol Stool Scale, inflammatory bowel disease (IBD), history of rectovaginal fistula or cloacal defect, rectal prolapse, prior bowel diversion, fecal impaction, neurologic disorder leading to incontinence, use of loperamide or diphenoxylate within the last 30 days, childbirth within the last 3 months, need for antiretroviral drugs, hepatic impairment, or chronic abdominal pain without diarrhea.
Baseline characteristics and symptoms severity were similar among participants. The average age of the women was 63 years, with 79% white and 85% postmenopausal. Participants had a mean (SD) of 1.6 (1.8) leaks per day.
Participants were randomly assigned in a 0.5:1:1:1 fashion to receive oral placebo, loperamide, oral placebo with PFPT/biofeedback, or loperamide with PFPT/biofeedback. All participants received a standardized educational pamphlet that outlined dietary and behavioral recommendations.
Women assigned to PFPT/biofeedback received 6 sessions every other week. Loperamide was started at a dosage of 2 mg per day with the possibility of dose maintenance, escalation, reduction, or discontinuation.
Study outcomes. The primary outcome was a change from baseline to 24 weeks in the Vaizey FI symptom severity score, which assesses fecal frequency, urgency, and use of pads and medications. Secondary outcomes included assessment of a 7-day bowel diary and other quality-of-life measures. Data at 24 weeks were available for 89% of the women.
All treatment groups experienced improved FI symptoms
Based on changes in Vaizey scores after 24 weeks of treatment, women in all treatment groups had similar improvement in symptoms severity. However, those who received loperamide and PFPT/biofeedback had decreased pad changes per week and more accident-free days compared with women treated with placebo and biofeedback. Quality of life at 24 weeks was not statistically different between treatment groups as improvement was seen in all groups, including those who received oral placebo and patient education.
Adverse events. The proportion of gastrointestinal adverse effects was similar between treatment groups, ranging from 45% to 63%. Constipation was the most common adverse event overall and was more common in those taking loperamide, occurring in 51% of the loperamide plus PFPT/biofeedback group, 38% of those who received loperamide alone, 23% of the biofeedback with placebo group, and 12% of the placebo-alone group.
Strengths and limitations. Strengths of this study include its multisite, large sample size, low dropout rate, and sufficiently powered design to compare various combinations of first- and second-line therapies in women with a mean baseline FI of 1.6 leaks per day. Another strength is the robustness of the PFPT/biofeedback sessions that used anorectal manometry. This may, however, limit the study's external validity given that clinical use of this device is likely rare. Additionally, the population was comprised largely of postmenopausal and white women, which may make the findings less generalizable to other populations.
Women who suffer from frequent FI may require both loperamide and PFPT/biofeedback if they want to increase the likelihood of accident-free days and use of fewer pads. Should they note increased constipation or are not amenable to scheduled PFPT sessions, formalized education about dietary modifications, according to this study, will provide improvement in symptom severity.
Continue to: Novel vaginal bowel control system...
Novel vaginal bowel control system is effective, durable over 12 months for FI treatment
Richter HE, Dunivan G, Brown HW, et al. A 12-month clinical durability of effectiveness and safety evaluation of a vaginal bowel control system for the nonsurgical treatment of fecal incontinence. Female Pelvic Med Reconstr Surg. 2019;25:113-119.
Richter and colleagues characterized clinical success, effect on quality of life, and durability over 12 months of a novel vaginal bowel control device (Eclipse System; Pelvalon) for FI in a prospective cohort study. The device is a silicone-coated vaginal insert with a detachable pump and balloon that deflects the rectovaginal septum posteriorly, thus impeding the passage of stool in the rectum (FIGURE 2).

Study eligibility criteria and treatment protocol
Women were eligible for the study if they had 4 or more episodes of fecal soiling on a 2-week bowel diary and had FI for at least 6 months. Participants were excluded if they had prolapse outside the hymen, rectovaginal fistula, IBD, congenital anorectal malformation, urinary or colorectal infection, chronic pelvic or anorectal pain, pregnancy or planning pregnancy in the next 5 months, unmanaged chronic watery diarrhea, presence of an open wound or tear in the vagina, significant urogenital atrophy, or any psychiatric or neurologic disorder that would hinder the ability to participate.
Participants successfully fitted with the device (3 attempts were allowed) were entered into the study's run-in phase. Those who were successfully fitted and had a 50% or greater reduction in FI continued into the treatment phase with 12 months of follow-up.
Of the 137 women eligible for device fitting, 62% were successfully fitted. The 73 (86%) women who had a 50% or greater reduction in FI during the run-in period comprised the intent-to-treat study population. On average, these women were 61.3 years of age, with 70% white and 82% postmenopausal. At baseline, they had a mean of 14.1 episodes of FI over 2 weeks. (Prior to enrollment, 97.3% of women attempted self-management strategies, 17.8% to 23% failed conservative therapy, and 7.8% to 13.7% failed surgical therapy.) The follow-up rate at 12 months was 74%.
Study outcomes. The primary outcome was treatment success, defined as proportion of subjects with a 50% or greater reduction in FI episodes at 3 months; this outcome also was evaluated at 6 and 12 months. Secondary outcomes were the number of FI episodes and quality-of-life measures at 3, 6, and 12 months.
Treatment success, patient satisfaction high
In the treatment phase, women had sustained improvements in symptom severity and quality-of-life measures over 12 months. Treatment success was 73% at 3 months, 71% at 6 months, and 70% at 12 months. Complete continence was achieved in 46% of participants at 12 months, and major FI episodes (requiring immediate change of undergarments) decreased from 5.0 at baseline to 0.5 at 12 months. Quality-of-life measures were improved at 3 months, and improvement was sustained over 12 months. Satisfaction was 94% at 12 months.
Adverse events. No serious device-related adverse events occurred. Mild device-related adverse events were experienced by 45% of women during the fitting process and by 38% during treatment period. These included vaginal wall injury such as hyperemia and erosion; vaginal or pelvic discomfort; vaginal infection; constipation; and lower urinary tract issues such as urinary tract infection, urinary incontinence, and voiding dysfunction. No adverse events led to treatment discontinuation.
Strengths and limitations. Strengths of this study include that it was conducted at multiple clinical sites, had a large sample size, and had a 1-year follow-up period in a population with daily FI. A limitation was that only women who had a 50% or greater reduction in FI episodes during the run-in period were followed for 12 months; however, this was 86% of the original cohort. The use of a comparative group using other devices, such as anal plugs, would have strengthened this study.
The Eclipse intravaginal bowel control device (approved by the US Food and Drug Administration in 2015) provided a sustained 50% or greater reduction in FI episodes in more than 70% of women wearing the device for 1 year, with high patient satisfaction. Thus, for women who fail conservative treatment methods for FI, clinicians should consider referring them to a urogynecologist or specialist who is knowledgeable in fitting this vaginal bowel control device.
Continue to: Sacroneuromodulation for FI…
Sacral neuromodulation for FI is effective long-term
Hull T, Giese C, Wexner SD, et al; for the SNS Study Group. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56:234-245.
In this multicenter, prospective cohort study, Hull and colleagues evaluated the 5-year efficacy of sacral neuromodulation (SNM), also known as sacral nerve stimulation, for treatment of FI. This study followed an earlier investigation by Wexner and colleagues, which reported that 83% of 120 patients treated with SNM had a 50% or greater improvement in FI episodes at 12 months.16
Details of the study
The investigators enrolled 133 participants (92% female) who had more than 2 episodes of FI per week for longer than 6 months (12 months after vaginal delivery). Participants were excluded if they had congenital anorectal malformations, prior rectal surgery within the past 12 months (or 24 months if due to cancer), defects greater than 120° of the external anal sphincter (EAS), IBD, unmanaged chronic watery diarrhea, stool consistency type 6 or type 7 on the Bristol Stool Scale, sequela of pelvic radiation, active anal abscess or fistula, pregnancy, or planned pregnancy.
Eligible participants underwent a 2-stage procedure with the InterStim bowel control device (Medtronic). If participants experienced a 50% or greater reduction in incontinence episodes with a wearable external SNM device in the test stimulation (stage 1), they received the chronic SNM implant device (stage 2).
Participants who underwent device implantation were followed at 1, 3, and 6 months and annually for 5 years or until they exited the study. Bowel diaries and quality of life assessments were completed at baseline and at follow-up.
The primary outcome was therapeutic success, defined as 50% or greater improvement in FI episodes per week.
A total of 120 participants (90%) underwent implantation of the chronic lead and neuromodulator, and 76 (63%) were followed for 5 years. Baseline characteristics available in the initial study of 133 participants showed that the mean age was 60.5 years; 25% had undergone a prior anal sphincteroplasty; and 16.5% and 10.5% had EAS or internal anal sphincter (IAS) defects, respectively, on endoanal ultrasonography.16
Therapeutic success was high at 5 years
At the 5-year follow-up, 89% (64/72) of participants met therapeutic success, with a reduction in weekly FI episodes from 9.1 at baseline to 1.7 at 5 years. The number of incontinence pads required decreased, and more participants wore no pads at 5 years. In the intention-to-treat analysis, carrying forward the baseline FI rate in participants who lacked follow-up data, the therapeutic success rate was 69%. Quality-of-life measures improved at 5 years, both statistically and by minimal clinical difference.
Adverse events. Sixty-eight percent of participants experienced device-related adverse events, including implant site pain, change in sensation of stimulation, change in efficacy, implant site infection, or neurostimulator battery depletion (neurostimulator use commonly expires after 3 to 5 years). Of these events, 80% were successfully treated with medications, reprogramming, or no intervention. The 5-year probability of device revision or replacement was 24.4%, and the 5-year probability of device explant was 19.0%.
Strengths and limitations. Overall, this study was a well-designed, multicenter trial with long-term follow-up that showed significant improvement in FI with the use of SNM. Its strengths include the enrollment of postmenopausal women who had current defects in EAS and/or IAS on endoanal ultrasonography and 25% who had a prior sphincteroplasty. The findings therefore are relevant to the gynecologic population in whom anal sphincteroplasty would not be recommended. The study also accounted for dropouts and reported the adjusted success rate of 69% at 5 years in that group.
The lack of a control arm to rule out the placebo effect is a limitation of this study, although randomized trials comparing the effect of SNM "on" versus "off" showed greater improvement with the device "on."17
Sacral neuromodulation is an excellent therapy for women with daily FI who have failed noninvasive options and desire to proceed to a more durable, long-lasting device therapy. Although adverse events may occur, they are mild and most often resolve with device reprogramming.
Fecal incontinence (FI), also known as accidental bowel leakage, is the involuntary loss of feces, which includes both liquid and solid stool as defined by the International Continence Society (ICS) and the International Urogynecological Association (IUGA).1,2 Fecal incontinence is common, occurring in 7% to 25% of community-dwelling women, and it increases with age.2-6 The condition is rarely addressed, with only 30% of women seeking care.6-8 This is due to patient embarrassment and the lack of a reliable screening tool. However, FI affects quality of life and mental health, and the associated economic burden likely will rise given the increased prevalence of FI among older women.2,4,7,9
Fecal incontinence occurs due to poor stool consistency, anal and pelvic muscle weakness, reduced rectal compliance, reduced or increased rectal sensation, or bowel inflammation or dysfunction. Many conditions can cause FI (TABLE 1).5,10,11 It is therefore important to elicit a full medical history with a focus on specific bowel symptoms, such as stool consistency type (TABLE 2),12 FI frequency, and duration of symptoms, as well as to perform a complete examination to identify any readily reversible or malignant causes. A colonoscopy is recommended for individuals who meet screening criteria or present with a change in bowel symptoms, such as diarrhea, bleeding, or obstruction.13,14

Fecal incontinence treatments include a range of approaches categorized from conservative, or first-line therapy, to fourth-line surgical managements (FIGURE 1).1,10,13,14 In this Update, we review the results of 3 well-designed trials that enrolled women with frequent nonneurogenic FI.

Common first- and second-line treatments produce equivalent improvements in FI symptoms at
6 months
Jelovsek JE, Markland AD, Whitehead WE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Controlling faecal incontinence in women by performing anal exercises with biofeedback or loperamide: a randomized clinical trial. Lancet Gastroenterol Hepatol. 2019;4:698-710.
In a multicenter, randomized trial of first- and second-line treatments for FI, Jelovsek and colleagues evaluated the efficacy of oral placebo, loperamide, pelvic floor physical therapy (PFPT) with biofeedback using anorectal manometry, or combination therapy over a 24-week period.
Continue to: Four treatments compared...
Four treatments compared
Three hundred women with FI occurring monthly for 3 months were included in the trial. Women were excluded if they had a stool classification of type 1 or type 7 on the Bristol Stool Scale, inflammatory bowel disease (IBD), history of rectovaginal fistula or cloacal defect, rectal prolapse, prior bowel diversion, fecal impaction, neurologic disorder leading to incontinence, use of loperamide or diphenoxylate within the last 30 days, childbirth within the last 3 months, need for antiretroviral drugs, hepatic impairment, or chronic abdominal pain without diarrhea.
Baseline characteristics and symptoms severity were similar among participants. The average age of the women was 63 years, with 79% white and 85% postmenopausal. Participants had a mean (SD) of 1.6 (1.8) leaks per day.
Participants were randomly assigned in a 0.5:1:1:1 fashion to receive oral placebo, loperamide, oral placebo with PFPT/biofeedback, or loperamide with PFPT/biofeedback. All participants received a standardized educational pamphlet that outlined dietary and behavioral recommendations.
Women assigned to PFPT/biofeedback received 6 sessions every other week. Loperamide was started at a dosage of 2 mg per day with the possibility of dose maintenance, escalation, reduction, or discontinuation.
Study outcomes. The primary outcome was a change from baseline to 24 weeks in the Vaizey FI symptom severity score, which assesses fecal frequency, urgency, and use of pads and medications. Secondary outcomes included assessment of a 7-day bowel diary and other quality-of-life measures. Data at 24 weeks were available for 89% of the women.
All treatment groups experienced improved FI symptoms
Based on changes in Vaizey scores after 24 weeks of treatment, women in all treatment groups had similar improvement in symptoms severity. However, those who received loperamide and PFPT/biofeedback had decreased pad changes per week and more accident-free days compared with women treated with placebo and biofeedback. Quality of life at 24 weeks was not statistically different between treatment groups as improvement was seen in all groups, including those who received oral placebo and patient education.
Adverse events. The proportion of gastrointestinal adverse effects was similar between treatment groups, ranging from 45% to 63%. Constipation was the most common adverse event overall and was more common in those taking loperamide, occurring in 51% of the loperamide plus PFPT/biofeedback group, 38% of those who received loperamide alone, 23% of the biofeedback with placebo group, and 12% of the placebo-alone group.
Strengths and limitations. Strengths of this study include its multisite, large sample size, low dropout rate, and sufficiently powered design to compare various combinations of first- and second-line therapies in women with a mean baseline FI of 1.6 leaks per day. Another strength is the robustness of the PFPT/biofeedback sessions that used anorectal manometry. This may, however, limit the study's external validity given that clinical use of this device is likely rare. Additionally, the population was comprised largely of postmenopausal and white women, which may make the findings less generalizable to other populations.
Women who suffer from frequent FI may require both loperamide and PFPT/biofeedback if they want to increase the likelihood of accident-free days and use of fewer pads. Should they note increased constipation or are not amenable to scheduled PFPT sessions, formalized education about dietary modifications, according to this study, will provide improvement in symptom severity.
Continue to: Novel vaginal bowel control system...
Novel vaginal bowel control system is effective, durable over 12 months for FI treatment
Richter HE, Dunivan G, Brown HW, et al. A 12-month clinical durability of effectiveness and safety evaluation of a vaginal bowel control system for the nonsurgical treatment of fecal incontinence. Female Pelvic Med Reconstr Surg. 2019;25:113-119.
Richter and colleagues characterized clinical success, effect on quality of life, and durability over 12 months of a novel vaginal bowel control device (Eclipse System; Pelvalon) for FI in a prospective cohort study. The device is a silicone-coated vaginal insert with a detachable pump and balloon that deflects the rectovaginal septum posteriorly, thus impeding the passage of stool in the rectum (FIGURE 2).

Study eligibility criteria and treatment protocol
Women were eligible for the study if they had 4 or more episodes of fecal soiling on a 2-week bowel diary and had FI for at least 6 months. Participants were excluded if they had prolapse outside the hymen, rectovaginal fistula, IBD, congenital anorectal malformation, urinary or colorectal infection, chronic pelvic or anorectal pain, pregnancy or planning pregnancy in the next 5 months, unmanaged chronic watery diarrhea, presence of an open wound or tear in the vagina, significant urogenital atrophy, or any psychiatric or neurologic disorder that would hinder the ability to participate.
Participants successfully fitted with the device (3 attempts were allowed) were entered into the study's run-in phase. Those who were successfully fitted and had a 50% or greater reduction in FI continued into the treatment phase with 12 months of follow-up.
Of the 137 women eligible for device fitting, 62% were successfully fitted. The 73 (86%) women who had a 50% or greater reduction in FI during the run-in period comprised the intent-to-treat study population. On average, these women were 61.3 years of age, with 70% white and 82% postmenopausal. At baseline, they had a mean of 14.1 episodes of FI over 2 weeks. (Prior to enrollment, 97.3% of women attempted self-management strategies, 17.8% to 23% failed conservative therapy, and 7.8% to 13.7% failed surgical therapy.) The follow-up rate at 12 months was 74%.
Study outcomes. The primary outcome was treatment success, defined as proportion of subjects with a 50% or greater reduction in FI episodes at 3 months; this outcome also was evaluated at 6 and 12 months. Secondary outcomes were the number of FI episodes and quality-of-life measures at 3, 6, and 12 months.
Treatment success, patient satisfaction high
In the treatment phase, women had sustained improvements in symptom severity and quality-of-life measures over 12 months. Treatment success was 73% at 3 months, 71% at 6 months, and 70% at 12 months. Complete continence was achieved in 46% of participants at 12 months, and major FI episodes (requiring immediate change of undergarments) decreased from 5.0 at baseline to 0.5 at 12 months. Quality-of-life measures were improved at 3 months, and improvement was sustained over 12 months. Satisfaction was 94% at 12 months.
Adverse events. No serious device-related adverse events occurred. Mild device-related adverse events were experienced by 45% of women during the fitting process and by 38% during treatment period. These included vaginal wall injury such as hyperemia and erosion; vaginal or pelvic discomfort; vaginal infection; constipation; and lower urinary tract issues such as urinary tract infection, urinary incontinence, and voiding dysfunction. No adverse events led to treatment discontinuation.
Strengths and limitations. Strengths of this study include that it was conducted at multiple clinical sites, had a large sample size, and had a 1-year follow-up period in a population with daily FI. A limitation was that only women who had a 50% or greater reduction in FI episodes during the run-in period were followed for 12 months; however, this was 86% of the original cohort. The use of a comparative group using other devices, such as anal plugs, would have strengthened this study.
The Eclipse intravaginal bowel control device (approved by the US Food and Drug Administration in 2015) provided a sustained 50% or greater reduction in FI episodes in more than 70% of women wearing the device for 1 year, with high patient satisfaction. Thus, for women who fail conservative treatment methods for FI, clinicians should consider referring them to a urogynecologist or specialist who is knowledgeable in fitting this vaginal bowel control device.
Continue to: Sacroneuromodulation for FI…
Sacral neuromodulation for FI is effective long-term
Hull T, Giese C, Wexner SD, et al; for the SNS Study Group. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56:234-245.
In this multicenter, prospective cohort study, Hull and colleagues evaluated the 5-year efficacy of sacral neuromodulation (SNM), also known as sacral nerve stimulation, for treatment of FI. This study followed an earlier investigation by Wexner and colleagues, which reported that 83% of 120 patients treated with SNM had a 50% or greater improvement in FI episodes at 12 months.16
Details of the study
The investigators enrolled 133 participants (92% female) who had more than 2 episodes of FI per week for longer than 6 months (12 months after vaginal delivery). Participants were excluded if they had congenital anorectal malformations, prior rectal surgery within the past 12 months (or 24 months if due to cancer), defects greater than 120° of the external anal sphincter (EAS), IBD, unmanaged chronic watery diarrhea, stool consistency type 6 or type 7 on the Bristol Stool Scale, sequela of pelvic radiation, active anal abscess or fistula, pregnancy, or planned pregnancy.
Eligible participants underwent a 2-stage procedure with the InterStim bowel control device (Medtronic). If participants experienced a 50% or greater reduction in incontinence episodes with a wearable external SNM device in the test stimulation (stage 1), they received the chronic SNM implant device (stage 2).
Participants who underwent device implantation were followed at 1, 3, and 6 months and annually for 5 years or until they exited the study. Bowel diaries and quality of life assessments were completed at baseline and at follow-up.
The primary outcome was therapeutic success, defined as 50% or greater improvement in FI episodes per week.
A total of 120 participants (90%) underwent implantation of the chronic lead and neuromodulator, and 76 (63%) were followed for 5 years. Baseline characteristics available in the initial study of 133 participants showed that the mean age was 60.5 years; 25% had undergone a prior anal sphincteroplasty; and 16.5% and 10.5% had EAS or internal anal sphincter (IAS) defects, respectively, on endoanal ultrasonography.16
Therapeutic success was high at 5 years
At the 5-year follow-up, 89% (64/72) of participants met therapeutic success, with a reduction in weekly FI episodes from 9.1 at baseline to 1.7 at 5 years. The number of incontinence pads required decreased, and more participants wore no pads at 5 years. In the intention-to-treat analysis, carrying forward the baseline FI rate in participants who lacked follow-up data, the therapeutic success rate was 69%. Quality-of-life measures improved at 5 years, both statistically and by minimal clinical difference.
Adverse events. Sixty-eight percent of participants experienced device-related adverse events, including implant site pain, change in sensation of stimulation, change in efficacy, implant site infection, or neurostimulator battery depletion (neurostimulator use commonly expires after 3 to 5 years). Of these events, 80% were successfully treated with medications, reprogramming, or no intervention. The 5-year probability of device revision or replacement was 24.4%, and the 5-year probability of device explant was 19.0%.
Strengths and limitations. Overall, this study was a well-designed, multicenter trial with long-term follow-up that showed significant improvement in FI with the use of SNM. Its strengths include the enrollment of postmenopausal women who had current defects in EAS and/or IAS on endoanal ultrasonography and 25% who had a prior sphincteroplasty. The findings therefore are relevant to the gynecologic population in whom anal sphincteroplasty would not be recommended. The study also accounted for dropouts and reported the adjusted success rate of 69% at 5 years in that group.
The lack of a control arm to rule out the placebo effect is a limitation of this study, although randomized trials comparing the effect of SNM "on" versus "off" showed greater improvement with the device "on."17
Sacral neuromodulation is an excellent therapy for women with daily FI who have failed noninvasive options and desire to proceed to a more durable, long-lasting device therapy. Although adverse events may occur, they are mild and most often resolve with device reprogramming.
- Sultan AH, Monga A, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female anorectal dysfunction. Neurourol Urodyn. 2017;36:10-34.
- Bharucha AE, Dunivan G, Goode PS, et al. Epidemiology, pathophysiology, and classification of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. Am J Gastroenterol. 2015;110:127-136.
- Bharucha AE, Zinsmeister AR, Locke GR, et al. Symptoms and quality of life in community women with fecal incontinence. Clin Gastroenterol Hepatol. 2006;4:1004-1008.
- Perry S, Shaw C, McGrother C, et al; Leicestershire MRC Incontinence Study Team. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut. 2002;50:480-484.
- Ditah I, Devaki P, Luma HN, et al. Prevalence, trends, and risk factors for fecal incontinence in United States adults, 2005-2010. Clin Gastroenterol Hepatol. 2014;12:636-643.e1-2.
- Brown HW, Wexner SD, Lukacz ES. Factors associated with care seeking among women with accidental bowel leakage. Female Pelvic Med Reconstr Surg. 2013;19:66-71.
- Norton NJ. The perspective of the patient. Gastroenterology. 2004;126(1 suppl 1):S175-S179.
- Guan W, Schmuhl NB, Brown HW. Response re: If we don't ask, they won't tell: screening for urinary and fecal incontinence by primary care providers. J Am Board Fam Med. 2019;32:119.3-120.
- Whitehead WE, Borrud L, Goode PS, et al; Pelvic Floor Disorders Network. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512-517.
- Wald A, Bharucha AE, Cosman BC, et al. ACG clinical guideline: management of benign anorectal disorders. Am J Gastroenterol. 2014;109:1141-1157.
- Bharucha AE, Zinsmeister AR, Schleck CD, et al. Bowel disturbances are the most important risk factors for late onset fecal incontinence: a population-based case-control study in women. Gastroenterology. 2010;139:1559-1566.
- Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920-924.
- Paquette IM, Varma MG, Kaiser AM, et al. The American Society of Colon and Rectal Surgeons' clinical practice guideline for the treatment of fecal incontinence. Dis Colon Rectum. 2015;58:623-636.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 210: Fecal incontinence. Obstet Gynecol. 2019;133:e260-e273.
- Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum. 2009;52:1730-1737.
- Wexner SD, Coller JA, Devroede G, et al. Sacral nerve stimulation for fecal incontinence: results of a 120-patient prospective multicenter study. Ann Surg. 2010;251:441-449.
- Leroi AM, Parc Y, Lehur PA, et al. Efficacy of sacral nerve stimulation for fecal incontinence: results of a multicenter double-blind crossover study. Ann Surg. 2005;242:662-669.
- Sultan AH, Monga A, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female anorectal dysfunction. Neurourol Urodyn. 2017;36:10-34.
- Bharucha AE, Dunivan G, Goode PS, et al. Epidemiology, pathophysiology, and classification of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. Am J Gastroenterol. 2015;110:127-136.
- Bharucha AE, Zinsmeister AR, Locke GR, et al. Symptoms and quality of life in community women with fecal incontinence. Clin Gastroenterol Hepatol. 2006;4:1004-1008.
- Perry S, Shaw C, McGrother C, et al; Leicestershire MRC Incontinence Study Team. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut. 2002;50:480-484.
- Ditah I, Devaki P, Luma HN, et al. Prevalence, trends, and risk factors for fecal incontinence in United States adults, 2005-2010. Clin Gastroenterol Hepatol. 2014;12:636-643.e1-2.
- Brown HW, Wexner SD, Lukacz ES. Factors associated with care seeking among women with accidental bowel leakage. Female Pelvic Med Reconstr Surg. 2013;19:66-71.
- Norton NJ. The perspective of the patient. Gastroenterology. 2004;126(1 suppl 1):S175-S179.
- Guan W, Schmuhl NB, Brown HW. Response re: If we don't ask, they won't tell: screening for urinary and fecal incontinence by primary care providers. J Am Board Fam Med. 2019;32:119.3-120.
- Whitehead WE, Borrud L, Goode PS, et al; Pelvic Floor Disorders Network. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512-517.
- Wald A, Bharucha AE, Cosman BC, et al. ACG clinical guideline: management of benign anorectal disorders. Am J Gastroenterol. 2014;109:1141-1157.
- Bharucha AE, Zinsmeister AR, Schleck CD, et al. Bowel disturbances are the most important risk factors for late onset fecal incontinence: a population-based case-control study in women. Gastroenterology. 2010;139:1559-1566.
- Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920-924.
- Paquette IM, Varma MG, Kaiser AM, et al. The American Society of Colon and Rectal Surgeons' clinical practice guideline for the treatment of fecal incontinence. Dis Colon Rectum. 2015;58:623-636.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 210: Fecal incontinence. Obstet Gynecol. 2019;133:e260-e273.
- Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum. 2009;52:1730-1737.
- Wexner SD, Coller JA, Devroede G, et al. Sacral nerve stimulation for fecal incontinence: results of a 120-patient prospective multicenter study. Ann Surg. 2010;251:441-449.
- Leroi AM, Parc Y, Lehur PA, et al. Efficacy of sacral nerve stimulation for fecal incontinence: results of a multicenter double-blind crossover study. Ann Surg. 2005;242:662-669.
Native tissue repair of POP: Surgical techniques to improve outcomes
“Take pride in your surgical work. Do it in such a way that you would be willing to sign your name to it…the operation was performed by me.”
—Raymond A. Lee, MD
The US Food and Drug Administration (FDA) recently ordered companies to cease selling transvaginal mesh intended for pelvic organ prolapse (POP) repair (but not for the treatment of stress urinary incontinence [SUI] or for abdominal sacrocolpopexy).1,2 The FDA is also requiring companies preparing premarket approval applications for mesh products for the treatment of transvaginal POP to continue safety and efficacy follow-up in existing section 522 postmarket surveillance studies.3
It is, therefore, incumbent upon gynecologic surgeons to understand the surgical options that remain and perfect their surgical approach to POP to optimize patient outcomes. POP may be performed transvaginally or transabdominally, with each approach offering its own set of risks and benefits. The ability to perform both effectively allows the surgeon to tailor the approach to the condition and circumstances encountered. It is also important to realize that “cures” are elusive in POP surgery. While we can frequently alleviate patient symptoms and improve quality of life, a lifelong “cure” is an unrealistic goal for most prolapse procedures.
This article focuses on transvaginal native tissue repair,4 specifically the Mayo approach.
Watch video here
Vaginal surgery fundamentals
Before we explore the details of the Mayo technique, let’s review some basic principles of vaginal surgery. First, it is important to make a good clinical diagnosis so that you know which compartments (apex, anterior, or posterior) are involved. Although single compartment defects exist, multicompartment defects are far more common. Failing to recognize all compartment defects often results in incomplete repair, which can mean recurrent prolapse and additional interventions.
Second, exposure is critical when performing surgery by any route. You must be able to see your surgical field completely in order to properly execute your surgical approach. Table height, lighting, and retraction are all important to surgical success.
Lastly, it is important to know how to effectively execute your intended procedure. Native tissue repair is often criticized for having a high failure rate. It makes sense that mesh augmentation offers greater durability of a repair, but an effective native tissue repair will also effectively treat the majority of patients. An ineffective repair does not benefit the patient and contributes to high failure rates.
- Mesh slings for urinary incontinence and mesh use in sacrocolpopexy have not been banned by the FDA.
- Apical support is helpful to all other compartment support.
- Fixing the fascial defect between the base of the bladder and the apex will improve your anterior compartment outcomes.
- Monitor vaginal caliber throughout your posterior compartment repair.
Vaginal apex repairs
Data from the OPTIMAL trial suggest that uterosacral ligament suspension and sacrospinous ligament fixation are equally effective in treating apical prolapse.5 Our preference is a McCall culdoplasty (uterosacral ligament plication). It allows direct visualization (internally or externally) to place apical support stitches and plicates the ligaments in the midline of the vaginal cuff to help prevent enterocele protrusion. DeLancey has described the levels of support in the female pelvis and places importance on apical support.6 Keep in mind that anterior and posterior compartment prolapse is often accompanied by apical prolapse. Therefore, treating the apex is critical for overall success.
External vs internal McCall sutures: My technique. Envision the open vaginal cuff after completing a vaginal hysterectomy or after opening the vaginal cuff for a posthysterectomy vaginal vault prolapse (FIGURE 1). External (suture placed through the vaginal cuff epithelium into the peritoneal cavity, incorporating the uterosacral ligaments and intervening peritoneum, and ultimately brought back out through the posterior cuff and tied) or internal (suture placed in the intraperitoneal space, incorporating the uterosacral ligaments and intervening peritoneum, and tied internally) McCall sutures can be utilized (FIGURE 2). I prefer a combination of both. I use 0-polyglactin for external sutures, as the sutures will ultimately dissolve and not remain in the vaginal cavity. I usually place at least 2 external sutures with the lowest suture on the vaginal cuff being the deepest uterosacral stitch. Each subsequent suture is placed closer to the vaginal cuff and closer to the ends of the ligamentous stumps, starting deepest and working back toward the cuff with each stitch. I place 1 or 2 internal sutures (delayed absorbable or permanent) between my 2 external sutures. Because these sutures will be tied internally and located in the intraperitoneal space, permanent sutures may be used.
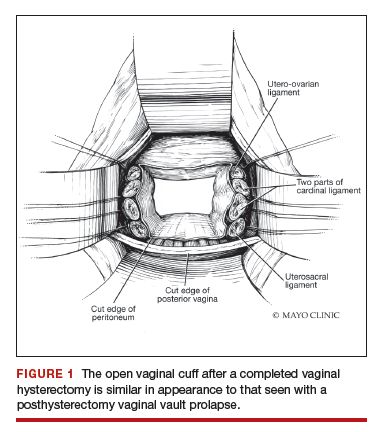
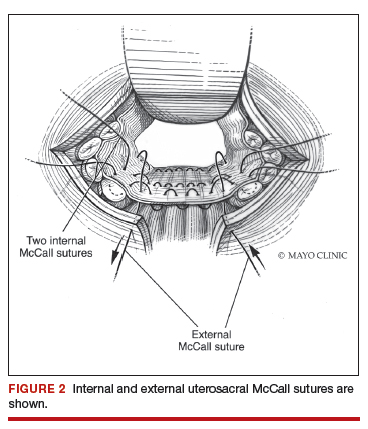
Avoiding ureteral injury: Tips for cystoscopy. A known risk of performing uterosacral ligament stitches is kinking or injury to the ureter. Therefore, cystoscopy is mandatory when performing this procedure. I tie one suture at a time starting with the internal sutures. I then perform cystoscopy after each suture tying. If I do not get ureteral spill after tying the suture, I remove and replace the suture and repeat cystoscopy until normal bilateral ureteral spill is achieved.
Key points for uterosacral ligament suspension. Achieving apical support at this point gives me the ability to build my anterior and posterior repair procedures off of this support. It is critical when performing uterosacral ligament suspension that you define the space between the ureter and rectum on each side. (Elevation of the cardinal pedicle and medial retraction of the rectum facilitate this.) The ligament runs down toward the sacrum when the patient is supine. You must follow that trajectory to be successful and avoid injury. One must also be careful not to be too deep on the ligament, as plication at that level may cause defecatory dysfunction.
Continue to: Anterior compartment repairs...
Anterior compartment repairs
The anterior compartment seems the most susceptible to forces within the pelvis and is a common site of prolapse. Many theories exist as to what causes a cystocele—distension, displacement, detachment, etc. While paravaginal defects exist, I believe that most cystoceles arise horizontally at the base of the bladder as the anterior endopelvic fascia detaches from the apex or cervix. The tissue then attenuates as the hernia progresses.
For surgical success: Make certain your repair addresses re-establishing continuity of the anterior endopelvic fascia with the fascia and ligaments at the vaginal apex; it will increase your success in treating anterior compartment prolapse.
We prefer to mobilize the epithelium in the midline from the vaginal apex to the mid‑urethra (if performing a midurethral sling, we stop short of the bladder neck and perform a separate suburethral incision). When incising the epithelium in the midline, the underlying fascia is also split in the midline, creating a midline defect. Once the epithelium is split and mobilized laterally off the underlying fascia, we can begin reconstruction.
The midline fascial defect that was just created is closed with a running 2-0 polyglactin from just beneath the bladder neck down to and including the fascia and uterosacral ligaments at the apex. This is accomplished in an upside down ‘T’ orientation (FIGURE 3). It is critical that the fascia is reunited at the base or you will leave the patient with a hernia.
For surgical success: To check intraoperatively that the fascia is reunited at the base, try to place an index finger between the base of the cystocele repair and the apex. If you can insert your finger, that is where the hernia still exists. If you meet resistance with your finger, you are palpating reunification of the anterior and apical fascia.
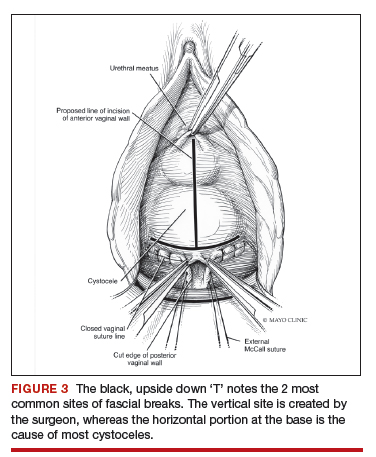
Technique for Kelly-Kennedy bladder neck plication. If the patient has mild incontinence that does not require a sling procedure, we now complete the second portion of the anterior repair starting with a Kelly-Kennedy bladder neck plication. Utilizing interrupted 1-0 polyglactin suture, vertical bites are taken periurethrally, starting at the midurethra and then the bladder neck. This nicely supports the urethra and proximal bladder neck and is very helpful for mild incontinence or for prophylactic benefit. Then starting beneath the bladder neck, the fascia is plicated again in the midline, reinforcing the suture line of the inverse ‘T’ with 2-0 polyglactin. The redundant epithelium is trimmed and reapproximated with interrupted 2-0 polyglactin (FIGURE 4). We tend to be more aggressive by adding the Kelly-Kennedy plication, which can lead to temporary voiding delay. We offer placement of a suprapubic catheter at the time of surgery or self-intermittent catherization.
Lastly, given that we have just dissected and then plicated the tissues beneath the bladder, I like to perform cystoscopy to be certain the bladder has not been violated. It is also important not to over-plicate the anterior fascia so that the sutures shear through the fascia and weaken the support or narrow the vaginal lumen.
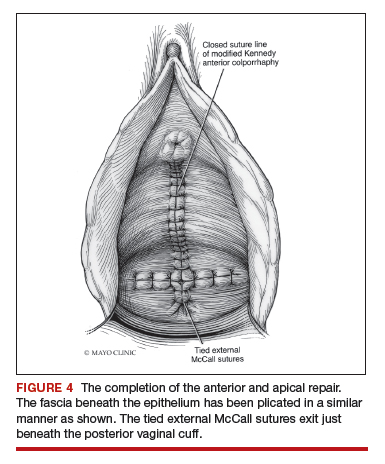
Continue to: Posterior compartment repairs...
Posterior compartment repairs
Like with the anterior compartment, opinions differ as to the site of posterior compartment prolapse. Midline, lateral, distal, and site-specific defects and surgical approaches have been described. Research suggests that there is no benefit to the use of mesh in the posterior compartment.7 It is very important to recognize that over-plication of the posterior compartment can lead to narrowing/stricture and dyspareunia. Therefore, monitor vaginal caliber throughout repair of the posterior compartment.
Although we believe that a midline defect in the endopelvic fascia is primarily responsible for rectoceles, we also appreciate that the fascia must be reconstructed all the way to the perineal body and that narrowing the genital hiatus is very important and often underappreciated (FIGURE 5). Thus, perineal reconstruction is universally performed. I will emphasize again that reconstruction must be performed while also monitoring vaginal caliber. If it is too tight with the patient under anesthesia, it will be too tight when the patient recovers. Avoidance is the best option. If the patient does not desire a functional vagina (eg, an elderly patient), then narrowing is a desired goal.

Perineal reconstruction technique and tips for success
A retractor at 12 o’clock to support the apex and anterior wall can be helpful for visualization in the posterior compartment. We start with a v-shaped incision on the perineum. The width is determined by how much you want to build up the perineum and narrow the vagina (the wider the incision, the more building up of the perineal body and vaginal narrowing). A strip of epithelium is then mobilized in the midline (be careful not to excise too much). This dissection is carried all the way up the midline to just short of the tied apical suspension sutures at the posterior vaginal apex. The posterior dissection tends to be the most vascular in my experience.
Utilize cautery to obtain hemostasis along your dissection margins while protecting the underlying rectum. We have not found it necessary to dissect the posterior epithelium off the underlying fascia (that is an option at this point, however, if you feel more comfortable doing this). With an index finger in the vagina, compressing the rectum posteriorly, interrupted 1-0 polyglactin suture is placed through the epithelium and underlying fascia (avoiding the rectum) on one side, then the other, and then tied. The next sutures are placed utilizing the same technique, and the caliber of the vagina is noted with the placement of each suture (if it is too tight, then remove and replace the suture and recheck). It is important to realize you want to plicate the fascia in the midline and not perform an aggressive levatorplasty that could lead to muscle pain. Additionally, each suture should get the same purchase of tissue on each side, and the spacing of each suture should be uniform, like rungs on a ladder. Ultimately, the repair is carried down to the hymenal ring. At this point, the perineal reconstruction is performed, plicating the perineal body in the midline with deeper horizontal sutures and then closing the perineal skin with interrupted or subcuticular sutures (FIGURE 6). Completion of these repairs should orient the vagina toward the hollow of the sacrum (FIGURE 7), allowing downward forces to compress the vaginal supports posteriorly onto the pelvic floor instead of forcing it out the vaginal lumen (FIGURE 8).
Our patients generally stay in the hospital overnight, and we place a vaginal pack to provide topical pressure throughout the vagina overnight. We tell patients no lifting more than 15 lb and no intercourse for 6 weeks. While we do not tend to use hydrodissection in our repairs, it is a perfectly acceptable option.
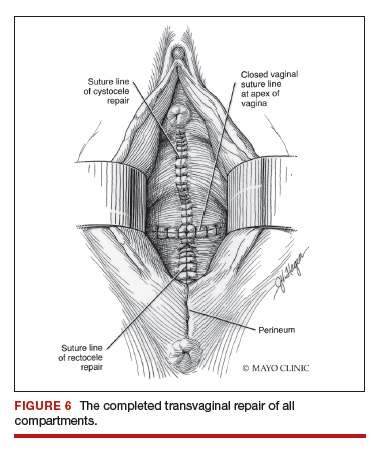
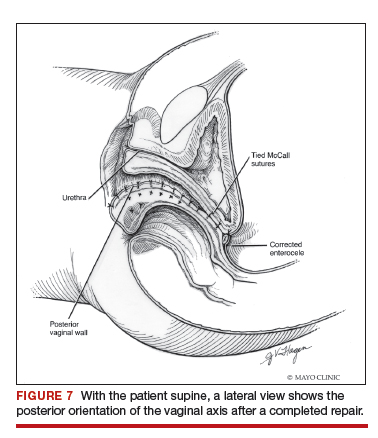
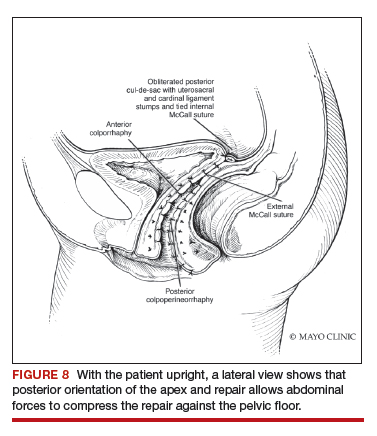
Continue to: Commit to knowledge of native tissue techniques...
Commit to knowledge of native tissue techniques
Given the recent FDA ban on the sale of transvaginal mesh for POP and the public’s negative perception of mesh (based often on misleading information in the media), it is incumbent upon gynecologic surgeons to invest in learning or relearning effective native tissue techniques for the transvaginal treatment of POP. While not perfect, they offer an effective nonmesh treatment option for many of our patients.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. . Published April 16, 2019. Accessed August 6, 2019.
- US Food and Drug Administration. Urogynecological surgical mesh implants. . Published July 10, 2019. Accessed August 5, 2019.
- US Food and Drug Administration. Effective date of requirement for premarket approval for surgical mesh for transvaginal pelvic organ prolapse repair. https://www.federalregister.gov/documents/2016/01/05/2015-33163/effective-date-of-requirement-for-premarket-approval-for-surgical-mesh-for-transvaginal-pelvic-organ. Published January 5, 2016. Accessed August 5, 2019.
- Lee RA. Atlas of Gynecologic Surgery. W.B. Saunders: Philadelphia, PA; 1992.
- Jelovsek JE, Barber MD, Brubaker L, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018;319:1554-1565.
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 part 1):1717-1728.
- Paraiso MF, Barber MD, Muir TW, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762- 1771.
“Take pride in your surgical work. Do it in such a way that you would be willing to sign your name to it…the operation was performed by me.”
—Raymond A. Lee, MD
The US Food and Drug Administration (FDA) recently ordered companies to cease selling transvaginal mesh intended for pelvic organ prolapse (POP) repair (but not for the treatment of stress urinary incontinence [SUI] or for abdominal sacrocolpopexy).1,2 The FDA is also requiring companies preparing premarket approval applications for mesh products for the treatment of transvaginal POP to continue safety and efficacy follow-up in existing section 522 postmarket surveillance studies.3
It is, therefore, incumbent upon gynecologic surgeons to understand the surgical options that remain and perfect their surgical approach to POP to optimize patient outcomes. POP may be performed transvaginally or transabdominally, with each approach offering its own set of risks and benefits. The ability to perform both effectively allows the surgeon to tailor the approach to the condition and circumstances encountered. It is also important to realize that “cures” are elusive in POP surgery. While we can frequently alleviate patient symptoms and improve quality of life, a lifelong “cure” is an unrealistic goal for most prolapse procedures.
This article focuses on transvaginal native tissue repair,4 specifically the Mayo approach.
Watch video here
Vaginal surgery fundamentals
Before we explore the details of the Mayo technique, let’s review some basic principles of vaginal surgery. First, it is important to make a good clinical diagnosis so that you know which compartments (apex, anterior, or posterior) are involved. Although single compartment defects exist, multicompartment defects are far more common. Failing to recognize all compartment defects often results in incomplete repair, which can mean recurrent prolapse and additional interventions.
Second, exposure is critical when performing surgery by any route. You must be able to see your surgical field completely in order to properly execute your surgical approach. Table height, lighting, and retraction are all important to surgical success.
Lastly, it is important to know how to effectively execute your intended procedure. Native tissue repair is often criticized for having a high failure rate. It makes sense that mesh augmentation offers greater durability of a repair, but an effective native tissue repair will also effectively treat the majority of patients. An ineffective repair does not benefit the patient and contributes to high failure rates.
- Mesh slings for urinary incontinence and mesh use in sacrocolpopexy have not been banned by the FDA.
- Apical support is helpful to all other compartment support.
- Fixing the fascial defect between the base of the bladder and the apex will improve your anterior compartment outcomes.
- Monitor vaginal caliber throughout your posterior compartment repair.
Vaginal apex repairs
Data from the OPTIMAL trial suggest that uterosacral ligament suspension and sacrospinous ligament fixation are equally effective in treating apical prolapse.5 Our preference is a McCall culdoplasty (uterosacral ligament plication). It allows direct visualization (internally or externally) to place apical support stitches and plicates the ligaments in the midline of the vaginal cuff to help prevent enterocele protrusion. DeLancey has described the levels of support in the female pelvis and places importance on apical support.6 Keep in mind that anterior and posterior compartment prolapse is often accompanied by apical prolapse. Therefore, treating the apex is critical for overall success.
External vs internal McCall sutures: My technique. Envision the open vaginal cuff after completing a vaginal hysterectomy or after opening the vaginal cuff for a posthysterectomy vaginal vault prolapse (FIGURE 1). External (suture placed through the vaginal cuff epithelium into the peritoneal cavity, incorporating the uterosacral ligaments and intervening peritoneum, and ultimately brought back out through the posterior cuff and tied) or internal (suture placed in the intraperitoneal space, incorporating the uterosacral ligaments and intervening peritoneum, and tied internally) McCall sutures can be utilized (FIGURE 2). I prefer a combination of both. I use 0-polyglactin for external sutures, as the sutures will ultimately dissolve and not remain in the vaginal cavity. I usually place at least 2 external sutures with the lowest suture on the vaginal cuff being the deepest uterosacral stitch. Each subsequent suture is placed closer to the vaginal cuff and closer to the ends of the ligamentous stumps, starting deepest and working back toward the cuff with each stitch. I place 1 or 2 internal sutures (delayed absorbable or permanent) between my 2 external sutures. Because these sutures will be tied internally and located in the intraperitoneal space, permanent sutures may be used.


Avoiding ureteral injury: Tips for cystoscopy. A known risk of performing uterosacral ligament stitches is kinking or injury to the ureter. Therefore, cystoscopy is mandatory when performing this procedure. I tie one suture at a time starting with the internal sutures. I then perform cystoscopy after each suture tying. If I do not get ureteral spill after tying the suture, I remove and replace the suture and repeat cystoscopy until normal bilateral ureteral spill is achieved.
Key points for uterosacral ligament suspension. Achieving apical support at this point gives me the ability to build my anterior and posterior repair procedures off of this support. It is critical when performing uterosacral ligament suspension that you define the space between the ureter and rectum on each side. (Elevation of the cardinal pedicle and medial retraction of the rectum facilitate this.) The ligament runs down toward the sacrum when the patient is supine. You must follow that trajectory to be successful and avoid injury. One must also be careful not to be too deep on the ligament, as plication at that level may cause defecatory dysfunction.
Continue to: Anterior compartment repairs...
Anterior compartment repairs
The anterior compartment seems the most susceptible to forces within the pelvis and is a common site of prolapse. Many theories exist as to what causes a cystocele—distension, displacement, detachment, etc. While paravaginal defects exist, I believe that most cystoceles arise horizontally at the base of the bladder as the anterior endopelvic fascia detaches from the apex or cervix. The tissue then attenuates as the hernia progresses.
For surgical success: Make certain your repair addresses re-establishing continuity of the anterior endopelvic fascia with the fascia and ligaments at the vaginal apex; it will increase your success in treating anterior compartment prolapse.
We prefer to mobilize the epithelium in the midline from the vaginal apex to the mid‑urethra (if performing a midurethral sling, we stop short of the bladder neck and perform a separate suburethral incision). When incising the epithelium in the midline, the underlying fascia is also split in the midline, creating a midline defect. Once the epithelium is split and mobilized laterally off the underlying fascia, we can begin reconstruction.
The midline fascial defect that was just created is closed with a running 2-0 polyglactin from just beneath the bladder neck down to and including the fascia and uterosacral ligaments at the apex. This is accomplished in an upside down ‘T’ orientation (FIGURE 3). It is critical that the fascia is reunited at the base or you will leave the patient with a hernia.
For surgical success: To check intraoperatively that the fascia is reunited at the base, try to place an index finger between the base of the cystocele repair and the apex. If you can insert your finger, that is where the hernia still exists. If you meet resistance with your finger, you are palpating reunification of the anterior and apical fascia.

Technique for Kelly-Kennedy bladder neck plication. If the patient has mild incontinence that does not require a sling procedure, we now complete the second portion of the anterior repair starting with a Kelly-Kennedy bladder neck plication. Utilizing interrupted 1-0 polyglactin suture, vertical bites are taken periurethrally, starting at the midurethra and then the bladder neck. This nicely supports the urethra and proximal bladder neck and is very helpful for mild incontinence or for prophylactic benefit. Then starting beneath the bladder neck, the fascia is plicated again in the midline, reinforcing the suture line of the inverse ‘T’ with 2-0 polyglactin. The redundant epithelium is trimmed and reapproximated with interrupted 2-0 polyglactin (FIGURE 4). We tend to be more aggressive by adding the Kelly-Kennedy plication, which can lead to temporary voiding delay. We offer placement of a suprapubic catheter at the time of surgery or self-intermittent catherization.
Lastly, given that we have just dissected and then plicated the tissues beneath the bladder, I like to perform cystoscopy to be certain the bladder has not been violated. It is also important not to over-plicate the anterior fascia so that the sutures shear through the fascia and weaken the support or narrow the vaginal lumen.

Continue to: Posterior compartment repairs...
Posterior compartment repairs
Like with the anterior compartment, opinions differ as to the site of posterior compartment prolapse. Midline, lateral, distal, and site-specific defects and surgical approaches have been described. Research suggests that there is no benefit to the use of mesh in the posterior compartment.7 It is very important to recognize that over-plication of the posterior compartment can lead to narrowing/stricture and dyspareunia. Therefore, monitor vaginal caliber throughout repair of the posterior compartment.
Although we believe that a midline defect in the endopelvic fascia is primarily responsible for rectoceles, we also appreciate that the fascia must be reconstructed all the way to the perineal body and that narrowing the genital hiatus is very important and often underappreciated (FIGURE 5). Thus, perineal reconstruction is universally performed. I will emphasize again that reconstruction must be performed while also monitoring vaginal caliber. If it is too tight with the patient under anesthesia, it will be too tight when the patient recovers. Avoidance is the best option. If the patient does not desire a functional vagina (eg, an elderly patient), then narrowing is a desired goal.

Perineal reconstruction technique and tips for success
A retractor at 12 o’clock to support the apex and anterior wall can be helpful for visualization in the posterior compartment. We start with a v-shaped incision on the perineum. The width is determined by how much you want to build up the perineum and narrow the vagina (the wider the incision, the more building up of the perineal body and vaginal narrowing). A strip of epithelium is then mobilized in the midline (be careful not to excise too much). This dissection is carried all the way up the midline to just short of the tied apical suspension sutures at the posterior vaginal apex. The posterior dissection tends to be the most vascular in my experience.
Utilize cautery to obtain hemostasis along your dissection margins while protecting the underlying rectum. We have not found it necessary to dissect the posterior epithelium off the underlying fascia (that is an option at this point, however, if you feel more comfortable doing this). With an index finger in the vagina, compressing the rectum posteriorly, interrupted 1-0 polyglactin suture is placed through the epithelium and underlying fascia (avoiding the rectum) on one side, then the other, and then tied. The next sutures are placed utilizing the same technique, and the caliber of the vagina is noted with the placement of each suture (if it is too tight, then remove and replace the suture and recheck). It is important to realize you want to plicate the fascia in the midline and not perform an aggressive levatorplasty that could lead to muscle pain. Additionally, each suture should get the same purchase of tissue on each side, and the spacing of each suture should be uniform, like rungs on a ladder. Ultimately, the repair is carried down to the hymenal ring. At this point, the perineal reconstruction is performed, plicating the perineal body in the midline with deeper horizontal sutures and then closing the perineal skin with interrupted or subcuticular sutures (FIGURE 6). Completion of these repairs should orient the vagina toward the hollow of the sacrum (FIGURE 7), allowing downward forces to compress the vaginal supports posteriorly onto the pelvic floor instead of forcing it out the vaginal lumen (FIGURE 8).
Our patients generally stay in the hospital overnight, and we place a vaginal pack to provide topical pressure throughout the vagina overnight. We tell patients no lifting more than 15 lb and no intercourse for 6 weeks. While we do not tend to use hydrodissection in our repairs, it is a perfectly acceptable option.



Continue to: Commit to knowledge of native tissue techniques...
Commit to knowledge of native tissue techniques
Given the recent FDA ban on the sale of transvaginal mesh for POP and the public’s negative perception of mesh (based often on misleading information in the media), it is incumbent upon gynecologic surgeons to invest in learning or relearning effective native tissue techniques for the transvaginal treatment of POP. While not perfect, they offer an effective nonmesh treatment option for many of our patients.
“Take pride in your surgical work. Do it in such a way that you would be willing to sign your name to it…the operation was performed by me.”
—Raymond A. Lee, MD
The US Food and Drug Administration (FDA) recently ordered companies to cease selling transvaginal mesh intended for pelvic organ prolapse (POP) repair (but not for the treatment of stress urinary incontinence [SUI] or for abdominal sacrocolpopexy).1,2 The FDA is also requiring companies preparing premarket approval applications for mesh products for the treatment of transvaginal POP to continue safety and efficacy follow-up in existing section 522 postmarket surveillance studies.3
It is, therefore, incumbent upon gynecologic surgeons to understand the surgical options that remain and perfect their surgical approach to POP to optimize patient outcomes. POP may be performed transvaginally or transabdominally, with each approach offering its own set of risks and benefits. The ability to perform both effectively allows the surgeon to tailor the approach to the condition and circumstances encountered. It is also important to realize that “cures” are elusive in POP surgery. While we can frequently alleviate patient symptoms and improve quality of life, a lifelong “cure” is an unrealistic goal for most prolapse procedures.
This article focuses on transvaginal native tissue repair,4 specifically the Mayo approach.
Watch video here
Vaginal surgery fundamentals
Before we explore the details of the Mayo technique, let’s review some basic principles of vaginal surgery. First, it is important to make a good clinical diagnosis so that you know which compartments (apex, anterior, or posterior) are involved. Although single compartment defects exist, multicompartment defects are far more common. Failing to recognize all compartment defects often results in incomplete repair, which can mean recurrent prolapse and additional interventions.
Second, exposure is critical when performing surgery by any route. You must be able to see your surgical field completely in order to properly execute your surgical approach. Table height, lighting, and retraction are all important to surgical success.
Lastly, it is important to know how to effectively execute your intended procedure. Native tissue repair is often criticized for having a high failure rate. It makes sense that mesh augmentation offers greater durability of a repair, but an effective native tissue repair will also effectively treat the majority of patients. An ineffective repair does not benefit the patient and contributes to high failure rates.
- Mesh slings for urinary incontinence and mesh use in sacrocolpopexy have not been banned by the FDA.
- Apical support is helpful to all other compartment support.
- Fixing the fascial defect between the base of the bladder and the apex will improve your anterior compartment outcomes.
- Monitor vaginal caliber throughout your posterior compartment repair.
Vaginal apex repairs
Data from the OPTIMAL trial suggest that uterosacral ligament suspension and sacrospinous ligament fixation are equally effective in treating apical prolapse.5 Our preference is a McCall culdoplasty (uterosacral ligament plication). It allows direct visualization (internally or externally) to place apical support stitches and plicates the ligaments in the midline of the vaginal cuff to help prevent enterocele protrusion. DeLancey has described the levels of support in the female pelvis and places importance on apical support.6 Keep in mind that anterior and posterior compartment prolapse is often accompanied by apical prolapse. Therefore, treating the apex is critical for overall success.
External vs internal McCall sutures: My technique. Envision the open vaginal cuff after completing a vaginal hysterectomy or after opening the vaginal cuff for a posthysterectomy vaginal vault prolapse (FIGURE 1). External (suture placed through the vaginal cuff epithelium into the peritoneal cavity, incorporating the uterosacral ligaments and intervening peritoneum, and ultimately brought back out through the posterior cuff and tied) or internal (suture placed in the intraperitoneal space, incorporating the uterosacral ligaments and intervening peritoneum, and tied internally) McCall sutures can be utilized (FIGURE 2). I prefer a combination of both. I use 0-polyglactin for external sutures, as the sutures will ultimately dissolve and not remain in the vaginal cavity. I usually place at least 2 external sutures with the lowest suture on the vaginal cuff being the deepest uterosacral stitch. Each subsequent suture is placed closer to the vaginal cuff and closer to the ends of the ligamentous stumps, starting deepest and working back toward the cuff with each stitch. I place 1 or 2 internal sutures (delayed absorbable or permanent) between my 2 external sutures. Because these sutures will be tied internally and located in the intraperitoneal space, permanent sutures may be used.


Avoiding ureteral injury: Tips for cystoscopy. A known risk of performing uterosacral ligament stitches is kinking or injury to the ureter. Therefore, cystoscopy is mandatory when performing this procedure. I tie one suture at a time starting with the internal sutures. I then perform cystoscopy after each suture tying. If I do not get ureteral spill after tying the suture, I remove and replace the suture and repeat cystoscopy until normal bilateral ureteral spill is achieved.
Key points for uterosacral ligament suspension. Achieving apical support at this point gives me the ability to build my anterior and posterior repair procedures off of this support. It is critical when performing uterosacral ligament suspension that you define the space between the ureter and rectum on each side. (Elevation of the cardinal pedicle and medial retraction of the rectum facilitate this.) The ligament runs down toward the sacrum when the patient is supine. You must follow that trajectory to be successful and avoid injury. One must also be careful not to be too deep on the ligament, as plication at that level may cause defecatory dysfunction.
Continue to: Anterior compartment repairs...
Anterior compartment repairs
The anterior compartment seems the most susceptible to forces within the pelvis and is a common site of prolapse. Many theories exist as to what causes a cystocele—distension, displacement, detachment, etc. While paravaginal defects exist, I believe that most cystoceles arise horizontally at the base of the bladder as the anterior endopelvic fascia detaches from the apex or cervix. The tissue then attenuates as the hernia progresses.
For surgical success: Make certain your repair addresses re-establishing continuity of the anterior endopelvic fascia with the fascia and ligaments at the vaginal apex; it will increase your success in treating anterior compartment prolapse.
We prefer to mobilize the epithelium in the midline from the vaginal apex to the mid‑urethra (if performing a midurethral sling, we stop short of the bladder neck and perform a separate suburethral incision). When incising the epithelium in the midline, the underlying fascia is also split in the midline, creating a midline defect. Once the epithelium is split and mobilized laterally off the underlying fascia, we can begin reconstruction.
The midline fascial defect that was just created is closed with a running 2-0 polyglactin from just beneath the bladder neck down to and including the fascia and uterosacral ligaments at the apex. This is accomplished in an upside down ‘T’ orientation (FIGURE 3). It is critical that the fascia is reunited at the base or you will leave the patient with a hernia.
For surgical success: To check intraoperatively that the fascia is reunited at the base, try to place an index finger between the base of the cystocele repair and the apex. If you can insert your finger, that is where the hernia still exists. If you meet resistance with your finger, you are palpating reunification of the anterior and apical fascia.

Technique for Kelly-Kennedy bladder neck plication. If the patient has mild incontinence that does not require a sling procedure, we now complete the second portion of the anterior repair starting with a Kelly-Kennedy bladder neck plication. Utilizing interrupted 1-0 polyglactin suture, vertical bites are taken periurethrally, starting at the midurethra and then the bladder neck. This nicely supports the urethra and proximal bladder neck and is very helpful for mild incontinence or for prophylactic benefit. Then starting beneath the bladder neck, the fascia is plicated again in the midline, reinforcing the suture line of the inverse ‘T’ with 2-0 polyglactin. The redundant epithelium is trimmed and reapproximated with interrupted 2-0 polyglactin (FIGURE 4). We tend to be more aggressive by adding the Kelly-Kennedy plication, which can lead to temporary voiding delay. We offer placement of a suprapubic catheter at the time of surgery or self-intermittent catherization.
Lastly, given that we have just dissected and then plicated the tissues beneath the bladder, I like to perform cystoscopy to be certain the bladder has not been violated. It is also important not to over-plicate the anterior fascia so that the sutures shear through the fascia and weaken the support or narrow the vaginal lumen.

Continue to: Posterior compartment repairs...
Posterior compartment repairs
Like with the anterior compartment, opinions differ as to the site of posterior compartment prolapse. Midline, lateral, distal, and site-specific defects and surgical approaches have been described. Research suggests that there is no benefit to the use of mesh in the posterior compartment.7 It is very important to recognize that over-plication of the posterior compartment can lead to narrowing/stricture and dyspareunia. Therefore, monitor vaginal caliber throughout repair of the posterior compartment.
Although we believe that a midline defect in the endopelvic fascia is primarily responsible for rectoceles, we also appreciate that the fascia must be reconstructed all the way to the perineal body and that narrowing the genital hiatus is very important and often underappreciated (FIGURE 5). Thus, perineal reconstruction is universally performed. I will emphasize again that reconstruction must be performed while also monitoring vaginal caliber. If it is too tight with the patient under anesthesia, it will be too tight when the patient recovers. Avoidance is the best option. If the patient does not desire a functional vagina (eg, an elderly patient), then narrowing is a desired goal.

Perineal reconstruction technique and tips for success
A retractor at 12 o’clock to support the apex and anterior wall can be helpful for visualization in the posterior compartment. We start with a v-shaped incision on the perineum. The width is determined by how much you want to build up the perineum and narrow the vagina (the wider the incision, the more building up of the perineal body and vaginal narrowing). A strip of epithelium is then mobilized in the midline (be careful not to excise too much). This dissection is carried all the way up the midline to just short of the tied apical suspension sutures at the posterior vaginal apex. The posterior dissection tends to be the most vascular in my experience.
Utilize cautery to obtain hemostasis along your dissection margins while protecting the underlying rectum. We have not found it necessary to dissect the posterior epithelium off the underlying fascia (that is an option at this point, however, if you feel more comfortable doing this). With an index finger in the vagina, compressing the rectum posteriorly, interrupted 1-0 polyglactin suture is placed through the epithelium and underlying fascia (avoiding the rectum) on one side, then the other, and then tied. The next sutures are placed utilizing the same technique, and the caliber of the vagina is noted with the placement of each suture (if it is too tight, then remove and replace the suture and recheck). It is important to realize you want to plicate the fascia in the midline and not perform an aggressive levatorplasty that could lead to muscle pain. Additionally, each suture should get the same purchase of tissue on each side, and the spacing of each suture should be uniform, like rungs on a ladder. Ultimately, the repair is carried down to the hymenal ring. At this point, the perineal reconstruction is performed, plicating the perineal body in the midline with deeper horizontal sutures and then closing the perineal skin with interrupted or subcuticular sutures (FIGURE 6). Completion of these repairs should orient the vagina toward the hollow of the sacrum (FIGURE 7), allowing downward forces to compress the vaginal supports posteriorly onto the pelvic floor instead of forcing it out the vaginal lumen (FIGURE 8).
Our patients generally stay in the hospital overnight, and we place a vaginal pack to provide topical pressure throughout the vagina overnight. We tell patients no lifting more than 15 lb and no intercourse for 6 weeks. While we do not tend to use hydrodissection in our repairs, it is a perfectly acceptable option.



Continue to: Commit to knowledge of native tissue techniques...
Commit to knowledge of native tissue techniques
Given the recent FDA ban on the sale of transvaginal mesh for POP and the public’s negative perception of mesh (based often on misleading information in the media), it is incumbent upon gynecologic surgeons to invest in learning or relearning effective native tissue techniques for the transvaginal treatment of POP. While not perfect, they offer an effective nonmesh treatment option for many of our patients.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. . Published April 16, 2019. Accessed August 6, 2019.
- US Food and Drug Administration. Urogynecological surgical mesh implants. . Published July 10, 2019. Accessed August 5, 2019.
- US Food and Drug Administration. Effective date of requirement for premarket approval for surgical mesh for transvaginal pelvic organ prolapse repair. https://www.federalregister.gov/documents/2016/01/05/2015-33163/effective-date-of-requirement-for-premarket-approval-for-surgical-mesh-for-transvaginal-pelvic-organ. Published January 5, 2016. Accessed August 5, 2019.
- Lee RA. Atlas of Gynecologic Surgery. W.B. Saunders: Philadelphia, PA; 1992.
- Jelovsek JE, Barber MD, Brubaker L, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018;319:1554-1565.
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 part 1):1717-1728.
- Paraiso MF, Barber MD, Muir TW, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762- 1771.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. . Published April 16, 2019. Accessed August 6, 2019.
- US Food and Drug Administration. Urogynecological surgical mesh implants. . Published July 10, 2019. Accessed August 5, 2019.
- US Food and Drug Administration. Effective date of requirement for premarket approval for surgical mesh for transvaginal pelvic organ prolapse repair. https://www.federalregister.gov/documents/2016/01/05/2015-33163/effective-date-of-requirement-for-premarket-approval-for-surgical-mesh-for-transvaginal-pelvic-organ. Published January 5, 2016. Accessed August 5, 2019.
- Lee RA. Atlas of Gynecologic Surgery. W.B. Saunders: Philadelphia, PA; 1992.
- Jelovsek JE, Barber MD, Brubaker L, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018;319:1554-1565.
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 part 1):1717-1728.
- Paraiso MF, Barber MD, Muir TW, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762- 1771.