User login
The mesh mess, enmeshed in controversy
CASE Complications with mesh placement for SUI
A 47-year-old woman (G4 P3013) presents 5 months posthysterectomy with evidence of urinary tract infection (UTI). Escherichia coli is isolated, and she responds to antibiotic therapy.
Her surgical history includes a mini-sling procedure using a needleless device and mesh placement in order to correct progressive worsening of loss of urine when coughing and sneezing. She also reported slight pelvic pain, dysuria, and urgency upon urination at that time. After subsequent development of pelvic organ prolapse (POP), she underwent the vaginal hysterectomy.
Following her UTI treatment, a host of problems occur for the patient, including pelvic pain and dyspareunia. Her male partner reports “feeling something during sex,” especially at the anterior vaginal wall. A plain radiograph of the abdomen identifies a 2 cm x 2 cm stone over the vaginal mesh. In consultation with female pelvic medicine and reconstructive surgery subspecialists, lithotripsy is performed, with the stone fragmented. The patient remains symptomatic, however.
The mesh is noted to be eroding through the vaginal wall. An attempt is made to excise the mesh, initially via transuretheral resection, then through a laparoscopic approach. Due to the mesh being embedded in the tissue, however, an open approach is undertaken. Extensive excision of the mesh and stone fragments is performed. Postoperatively, the patient reports “dry vagina,” with no other genitourinary complaints.
The patient sues. She sues the mesh manufacturer. She also seeks to sue the gynecologist who placed the sling and vaginal mesh (as she says she was not informed of “all the risks” of vaginal mesh placement. She is part of a class action lawsuit, along with thousands of other women.
WHAT’S THE VERDICT?
The device manufacturer settled out of court with the class action suit. (The gynecologist was never formally a defendant because the patient/plaintiff was advised to “drop the physician from the suit.”) The attorneys representing the class action received 40% of the award plus presented costs for the representation. The class as a whole received a little more than 50% of the negotiated award. The patient in this case received $60,000.
Medical background
Stress urinary incontinence (SUI) is a prevalent condition; it affects 35% of women.1 Overall, 80% of women aged 80 or younger will undergo some form of surgery for POP during their lifetime.2 The pathophysiology of SUI includes urethral hypermobility and intrinsic sphincter deficiency.3
Surgical correction for urinary incontinence: A timeline
Use of the gracilis muscle flap to surgically correct urinary incontinence was introduced in 1907. This technique has been replaced by today’s more common Burch procedure, which was first described in 1961. Surgical mesh use dates back to the 1950s, when it was primarily used for abdominal hernia repair. Tension-free tape was introduced in 1995.4-6
Continue to: In the late 1990s the US Food and Drug Administration...
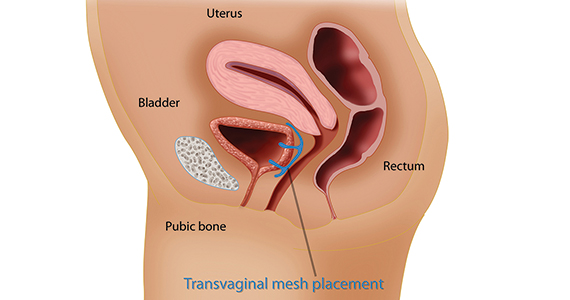
In the late 1990s the US Food and Drug Administration (FDA) permitted use of the first transvaginal meshes, which were designed to treat SUI—the midurethral sling. These mesh slings were so successful that similar meshes were developed to treat POP.7 Almost immediately there were problems with the new POP devices, and 3 years later Boston Scientific recalled its device.8 Nonetheless, the FDA cleared more than 150 devices using surgical mesh for urogynecologic indications (FIGURE).9

Mesh complications
Managing complications from intravesical mesh is a clinically challenging problem. Bladder perforation, stone formation, and penetration through the vagina can occur. Bladder-related complications can manifest as recurrent UTIs and obstructive urinary symptoms, especially in association with stone formation. From the gynecologic perspective, the more common complications with mesh utilization are pelvic pain, groin pain, dyspareunia, contracture and scarring of mesh, and narrowing of the vaginal canal.10 Mesh erosion problems will occur in an estimated 10% to 25% of transvaginal mesh POP implants.11
In 2008, a comparison of transvaginal mesh to native tissue repair (suture-based) or other (biologic) grafts was published.12 The bottom line: there is insufficient evidence to suggest that transvaginal mesh significantly improves outcomes for both posterior and apical defects.
Legal background
Mesh used for surgical purposes is a medical device, which legally is a product—a special product to be sure, but a product nonetheless. Products are subject to product liability rules. Mesh is also subject to an FDA regulatory system. We will briefly discuss products liability and the regulation of devices, both of which have played important roles in mesh-related injuries.
Products liability
As a general matter, defective products subject their manufacturer and seller to liability. There are several legal theories regarding product liability: negligence (in which the defect was caused through carelessness), breach of warranty or guarantee (in addition to express warranties, there are a number of implied warranties for products, including that it is fit for its intended purpose), and strict liability (there was a defect in the product, but it may not have been because of negligence). The product may be defective in the way it was designed, manufactured, or packaged, or it may be defective because adequate instructions and warning were not given to consumers.
Of course, not every product involved in an injury is defective—most automobile accidents, for example, are not the result of any defect in the automobile. In medicine, almost no product (device or pharmaceutical) is entirely safe. In some ways they are unavoidably unsafe and bound to cause some injuries. But when injuries are caused by a defect in the product (design or manufacturing defect or failure to warn), then there may be products liability. Most products liability cases arise under state law.
FDA’s device regulations
Both drugs and medical devices are subject to FDA review and ordinarily require some form of FDA clearance before they may be marketed. In the case of devices, the FDA has 3 classes, with an increase in risk to the user from Class I to III. Various levels of FDA review are required before marketing of the device is permitted, again with the intensity of review increasing from I to III as follows:
- Class I devices pose the least risk, have the least regulation, and are subject to general controls (ie, manufacturing and marketing practices).
- Class II devices pose slightly higher risks and are subject to special controls in addition to the criteria for Class I.
- Class III devices pose the most risk to patients and require premarket approval (scientific review and studies are required to ensure efficacy and safety).13
Continue to: There are a number of limits on manufacturer liability for defective devices...
There are a number of limits on manufacturer liability for defective devices. For Class III devices, the thorough FDA review of the safety of a device may limit the ability of an injured patient to sue based on the state product liability laws.14 For the most part, this “preemption” of state law has not played a major role in mesh litigation because they were initially classified as Class II devices which did not require or include a detailed FDA review.15
The duty to warn of the dangers and risk of medical devices means that manufacturers (or sellers) of devices are obligated to inform health care providers and other medical personnel of the risks. Unlike other manufacturers, device manufacturers do not have to directly warn consumers—because physicians deal directly with patients and prescribe the devices. Therefore, the health care providers, rather than the manufacturers, are obligated to inform the patient.16 This is known as the learned intermediary rule. Manufacturers may still be liable for failure to warn if they do not convey to health care providers proper warnings.
Manufacturers and sellers are not the only entities that may be subject to liability caused by medical devices. Hospitals or other entities that stock and care for devices are responsible for maintaining the safety and functionality of devices in their care.
Health care providers also may be responsible for injuries from medical devices. Generally, that liability is based on negligence. Negligence may relate to selecting an improper device, installing or using it incorrectly, or failing to give the patient adequate information (or informed consent) about the device and alternatives to it.17
A look at the mesh mess
There are a lot of distressing problems and professional disappointments in dissecting the “mesh mess,” including a failure of the FDA to regulate effectively, the extended sale and promotion of intrinsic sphincter deficiency mesh products, the improper use of mesh by physicians even after the risks were known, and, in some instances, the taking advantage of injured patients by attorneys and businesses.18 A lot of finger pointing also has occurred.19 We will recount some of the lowlights of this unfortunate tale.
Continue to: The FDA, in the 1990s, classified the first POP and SUI mesh...
The FDA, in the 1990s, classified the first POP and SUI mesh as Class II after deciding these products were “substantially equivalent” to older surgical meshes. This, of course, proved not to be the case.20 The FDA started receiving thousands of reports of adverse events and, in 2008, warned physicians to be vigilant for adverse events from the mesh. The FDA’s notification recommendations regarding mesh included the following13:
- Obtain specialized training for each mesh implantation technique, and be cognizant of risks.
- Be vigilant for potential adverse events from mesh, including erosion and infection.
- Be observant for complications associated with tools of transvaginal placement (ie, bowel, bladder, and vessel perforation).
- Inform patients that implantation of mesh is permanent and complications may require additional surgery for correction.
- Be aware that complications may affect quality of life—eg, pain with intercourse, scarring, and vaginal wall narrowing (POP repair).
- Provide patients with written copy of patient labeling from the surgical mesh manufacturer.
In 2011, the FDA issued a formal warning to providers that transvaginal mesh posed meaningful risks beyond nonmesh surgery. The FDA’s bulletin draws attention to how the mesh is placed more so than the material per se.19,21 Mesh was a Class II device for sacrocolpopexy or midurethral sling and, similarly, the transvaginal kit was also a Class II device. Overall, use of mesh midurethral slings has been well received as treatment for SUI. The FDA also accepted it for POP, however, but with increasingly strong warnings. The FDA’s 2011 communication stated, “This update is to inform you that serious complications associated with surgical mesh for transvaginal repair of POP are not rare….Furthermore, it is not clear that transvaginal POP repair with mesh is more effective than traditional non-mesh repair in all patients with POP and it may expose patients to greater risk.”7,13
In 2014 the FDA proposed reclassifying mesh to a Class III device, which would require that manufacturers obtain approval, based on safety and effectiveness, before selling mesh. Not until 2016 did the FDA actually reclassify the mess as Class III. Of course, during this time, mesh manufacturers were well aware of the substantial problems the products were causing.13
After serious problems with mesh became well known, and especially after FDA warnings, the use of mesh other than as indicated by the FDA was increasingly risky from a legal (as well as a health) standpoint. As long as mesh was still on the market, of course, it was available for use. But use of mesh for POP procedures without good indications in a way that was contrary to the FDA warnings might well be negligent.
Changes to informed consent
The FDA warnings also should have changed the informed consent for the use of mesh.22 Informed consent commonly consists of the following:
- informing the patient of the proposed procedure
- describing risks (and benefits) of the proposed process
- explaining reasonable alternatives
- noting the risks of taking no action.
Information that is material to a decision should be disclosed. If mesh were going to be used, after the problems of mesh were known and identified by the FDA (other than midurethral slings as treatment of SUI), the risks should have been clearly identified for patients, with alternatives outlined. The American College of Obstetricians and Gynecologists Committee on Ethics has 8 fundamental concepts with regard to informed consent that are worth keeping in mind23:
- Obtaining informed consent for medical treatment and research is an ethical requirement.
- The process expresses respect for the patient as a person.
- It protects patients against unwanted treatment and allows patients’ active involvement in medical planning and care.
- Communication is of paramount importance.
- Informed consent is a process and not a signature on a form.
- A commitment to informed consent and to provision of medical benefit to the patient are linked to provision of care.
- If obtaining informed consent is impossible, a designated surrogate should be identified representing the patient’s best interests.
- Knowledge on the part of the provider regarding state and federal requirements is necessary.
Continue to: Lawsuits line up...
Lawsuits line up
The widespread use of a product with a significant percentage of injuries and eventually with warnings about injuries from use sounds like the formula for a lot of lawsuits. This certainly has happened. A large number of suits—both class actions and individual actions—were filed as a result of mesh injuries.24 These suits were overwhelmingly against the manufacturer, although some included physicians.7 Device makers are more attractive defendants for several reasons. First, they have very deep pockets. In addition, jurors are generally much less sympathetic to large companies than to doctors. Large class actions meant that there were many different patients among the plaintiffs, and medical malpractice claims in most states have a number of trial difficulties not present in other product liability cases. Common defendants have included Johnson & Johnson, Boston Scientific, and Medtronic.
Some of the cases resulted in very large damage awards against manufacturers based on various kinds of product(s) liability. Many other cases were settled or tried with relatively small damages. There were, in addition, a number of instances in which the manufacturers were not liable. Of the 32 plaintiffs who have gone to trial thus far, 24 have obtained verdicts totaling $345 million ($14 million average). The cases that have settled have been for much less—perhaps $60,000 on average. A number of cases remain unresolved. To date, the estimate is that 100,000 women have received almost $8 billion from 7 device manufacturers to resolve claims.25
Some state attorneys general have gotten into the process as well. Attorneys general from California, Kentucky, Mississippi, and Washington have filed lawsuits against Johnson & Johnson, claiming that they deceived doctors and patients about the risks of their pelvic mesh. The states claim that marketing and instructional literature should have contained more information about the risks. Some physicians in these states have expressed concern that these lawsuit risks may do more harm than good because the suits conflate mesh used to treat incontinence with the more risky mesh for POP.26
The “ugly” of class action lawsuits
We have discussed both the sad (the injuries to patients) and the bad (the slow regulatory response and continuing injuries). (The ethics of the marketing by the manufacturers might also be raised as the bad.27) Next, let’s look briefly at the ugly.
Some of the patients affected by mesh injuries have been victimized a second time by medical “lenders” and some of their attorneys. Press reports describe patients with modest awards paying 40% in attorney fees (on the high side for personal injury settlements) plus extravagant costs—leaving modest amounts of actual recovery.25
Worse still, a process of “medical lending” has arisen in mesh cases.28 Medical lenders may contact mesh victims offering to pay up front for surgery to remove mesh, and then place a lien against the settlement for repayment at a much higher rate. They might pay the surgeon $2,500 for the surgery, but place a lien on the settlement amount for $60,000.29,30 In addition, there are allegations that lawyers may recruit the doctors to overstate the injuries or do unnecessary removal surgery because that will likely up the award.31 A quick Google search indicates dozens of offers of cash now for your mesh lawsuit (transvaginal and hernia repair).
The patient in our hypothetical case at the beginning had a fairly typical experience. She was a member of a class filing and received a modest settlement. The attorneys representing the class were allowed by the court to charge substantial attorneys’ fees and costs. The patient had the good sense to avoid medical lenders, although other members of the class did use medical lenders and are now filing complaints about the way they were treated by these lenders.
- Maintain surgical skills and be open to new technology. Medical practice requires constant updating and use of new and improved technology as it comes along. By definition, new technology often requires new skills and understanding. A significant portion of surgeons using mesh indicated that they had not read the instructions for use, or had done so only once.1 CME programs that include surgical education remain of particular value.
- Whether new technology or old, it is essential to keep up to date on all FDA bulletins pertinent to devices and pharmaceuticals that you use and prescribe. For example, in 2016 and 2018 the FDA warned that the use of a very old class of drugs (fluoroquinolones) should be limited. It advised "that the serious side effects associated with fluoroquinolones generally outweigh the benefits for patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infections who have other treatment options. For patients with these conditions, fluoroquinolones should be reserved for those who do not have alternative treatment options."2 Continued, unnecessary prescriptions for fluoroquinolones would put a physician at some legal risk whether or not the physician had paid any attention to the warning.
- Informed consent is a very important legal and medical process. Take it seriously, and make sure the patient has the information necessary to make informed decisions about treatment. Document the process and the information provided. In some cases consider directing patients to appropriate literature or websites of the manufacturers.
- As to the use of mesh, if not following FDA advice, it is important to document the reason for this and to document the informed consent especially carefully.
- Follow patients after mesh placement for a minimum of 1 year and emphasize to patients they should convey signs and symptoms of complications from initial placement.3 High-risk patients should be of particular concern and be monitored very closely.
References
- Kirkpatrick G, Faber KD, Fromer DL. Transvaginal mesh placement and the instructions for use: a survey of North American urologists. J Urol. https://doi.org/10.1016/j.urpr.2018.05.004.
- FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. July 26, 2016. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed June 19, 2019.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Maral I, Ozkardeş H, Peşkircioğlu L, et al. Prevalence of stress urinary incontinence in both sexes at or after age 15 years: a cross-sectional study. J Urol. 2001;165:408-412.
- Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
- Chang J, Lee D. Midurethral slings in the mesh litigation era. Transl Androl Urol. 2017;6(suppl 2): S68-S75.
- Mattingly R, ed. TeLinde's Operative Gynecology, 5th edition. Lippincott, William, and Wilkins: Philadelphia, PA; 1997.
- Burch J. Urethrovaginal fixation to Cooper's ligament for correction of stress incontinence, cystocele, and prolapse. Am J Obstet Gynecol. 1961;81:281-290.
- Ulmsten U, Falconer C, Johnson P, et al. A multicenter study of tension-free vaginal tape (TVT) for surgical treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:210-213.
- Kuhlmann-Capek MJ, Kilic GS, Shah AB, et al. Enmeshed in controversy: use of vaginal mesh in the current medicolegal environment. Female Pelvic Med Reconstr Surg. 2015;21:241-243.
- Powell SF. Changing our minds: reforming the FDA medical device reclassification process. Food Drug Law J. 2018;73:177-209.
- US Food and Drug Administration. Surgical Mesh for Treatment of Women with Pelvic Organ Prolapse and Stress Urinary Incontinence. September 2011. https://www.thesenatorsfirm.com/documents/OBS.pdf. Accessed June 19, 2019.
- Maher C, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;(4):CD004014.
- Ganj FA, Ibeanu OA, Bedestani A, Nolan TE, Chesson RR. Complications of transvaginal monofilament polypropylene mesh in pelvic organ prolapse repair. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:919-925.
- Sung VW, Rogers RG, Schaffer JI, et al. Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstet Gynecol. 2008;112:1131-1142.
- FDA public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. October 20, 2008. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm. Accessed February 14, 2019.
- Riegel v. Medtronic, 552 U.S. 312 (2008).
- Whitney DW. Guide to preemption of state-law claims against Class III PMA medical devices. Food Drug Law J. 2010;65:113-139.
- Alam P, Iglesia CB. Informed consent for reconstructive pelvic surgery. Obstet Gynecol Clin North Am. 2016;43:131-139.
- Nosti PA, Iglesia CB. Medicolegal issues surrounding devices and mesh for surgical treatment of prolapse and incontinence. Clin Obstet Gynecol. 2013;56:221-228.
- Shepherd CG. Transvaginal mesh litigation: a new opportunity to resolve mass medical device failure claims. Tennessee Law Rev. 2012;80:3:477-94.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Cohn JA, Timbrook Brown E, Kowalik CG, et al. The mesh controversy. F1000Research website. https://f1000research.com/articles/5-2423/v1. Accessed June 17, 2019.
- Obstetrics and Gynecology Devices Panel Meeting, February 12, 2019. US Food and Drug Administration website. https://www.fda.gov/media/122867/download. Accessed June 19, 2019.
- Mucowski SJ, Jurnalov C, Phelps JY. Use of vaginal mesh in the face of recent FDA warnings and litigation. Am J Obstet Gynecol. 2010;203:103.e1-e4.
- American College of Obstetricians and Gynecologists Committee on Ethics. ACOG Committee Opinion No. 439: informed consent. Obstet Gynecol. 2009;114(2 pt 1):401-408.
- Souders CP, Eilber KS, McClelland L, et al. The truth behind transvaginal mesh litigation: devices, timelines, and provider characteristics. Female Pelvic Med Reconstr Surg. 2018;24:21-25.
- Goldstein M. As pelvic mesh settlements near $8 billion, women question lawyers' fees. New York Times. February 1, 2019. https://www.nytimes.com/2019/02/01/business/pelvic-mesh-settlements-lawyers.html. Accessed June 19, 2019.
- Johnson G. Surgeons fear pelvic mesh lawsuits will spook patients. Associated Press News. January 10, 2019. https://www.apnews.com/25777c3c33e3489283b1dc2ebdde6b55. Accessed June 19, 2019.
- Clarke RN. Medical device marketing and the ethics of vaginal mesh kit marketing. In The Innovation and Evolution of Medical Devices. New York, NY: Springer; 2019:103-123.
- Top 5 drug and medical device developments of 2018. Law 360. January 1, 2019. Accessed through LexisNexis.
- Frankel A, Dye J. The Lien Machine. New breed of investor profits by financing surgeries for desperate women patients. Reuters. August 18, 2015. https://www.reuters.com/investigates/special-report/usa-litigation-mesh/. Accessed June 19, 2019.
- Sullivan T. New report looks at intersection of "medical lending" and pelvic mesh lawsuits. Policy & Medicine. May 5, 2018. https://www.policymed.com/2015/08/medical-lending-and-pelvic-mesh-litigation.html. Accessed June 19, 2019.
- Goldstein M, Sliver-Greensberg J. How profiteers lure women into often-unneeded surgery. New York Times. April 14, 2018. https://www.nytimes.com/2018/04/14/business/vaginal-mesh-surgery-lawsuits-financing.html. Accessed June 19, 2019.
CASE Complications with mesh placement for SUI
A 47-year-old woman (G4 P3013) presents 5 months posthysterectomy with evidence of urinary tract infection (UTI). Escherichia coli is isolated, and she responds to antibiotic therapy.
Her surgical history includes a mini-sling procedure using a needleless device and mesh placement in order to correct progressive worsening of loss of urine when coughing and sneezing. She also reported slight pelvic pain, dysuria, and urgency upon urination at that time. After subsequent development of pelvic organ prolapse (POP), she underwent the vaginal hysterectomy.
Following her UTI treatment, a host of problems occur for the patient, including pelvic pain and dyspareunia. Her male partner reports “feeling something during sex,” especially at the anterior vaginal wall. A plain radiograph of the abdomen identifies a 2 cm x 2 cm stone over the vaginal mesh. In consultation with female pelvic medicine and reconstructive surgery subspecialists, lithotripsy is performed, with the stone fragmented. The patient remains symptomatic, however.
The mesh is noted to be eroding through the vaginal wall. An attempt is made to excise the mesh, initially via transuretheral resection, then through a laparoscopic approach. Due to the mesh being embedded in the tissue, however, an open approach is undertaken. Extensive excision of the mesh and stone fragments is performed. Postoperatively, the patient reports “dry vagina,” with no other genitourinary complaints.
The patient sues. She sues the mesh manufacturer. She also seeks to sue the gynecologist who placed the sling and vaginal mesh (as she says she was not informed of “all the risks” of vaginal mesh placement. She is part of a class action lawsuit, along with thousands of other women.
WHAT’S THE VERDICT?
The device manufacturer settled out of court with the class action suit. (The gynecologist was never formally a defendant because the patient/plaintiff was advised to “drop the physician from the suit.”) The attorneys representing the class action received 40% of the award plus presented costs for the representation. The class as a whole received a little more than 50% of the negotiated award. The patient in this case received $60,000.
Medical background
Stress urinary incontinence (SUI) is a prevalent condition; it affects 35% of women.1 Overall, 80% of women aged 80 or younger will undergo some form of surgery for POP during their lifetime.2 The pathophysiology of SUI includes urethral hypermobility and intrinsic sphincter deficiency.3
Surgical correction for urinary incontinence: A timeline
Use of the gracilis muscle flap to surgically correct urinary incontinence was introduced in 1907. This technique has been replaced by today’s more common Burch procedure, which was first described in 1961. Surgical mesh use dates back to the 1950s, when it was primarily used for abdominal hernia repair. Tension-free tape was introduced in 1995.4-6
Continue to: In the late 1990s the US Food and Drug Administration...
In the late 1990s the US Food and Drug Administration (FDA) permitted use of the first transvaginal meshes, which were designed to treat SUI—the midurethral sling. These mesh slings were so successful that similar meshes were developed to treat POP.7 Almost immediately there were problems with the new POP devices, and 3 years later Boston Scientific recalled its device.8 Nonetheless, the FDA cleared more than 150 devices using surgical mesh for urogynecologic indications (FIGURE).9

Mesh complications
Managing complications from intravesical mesh is a clinically challenging problem. Bladder perforation, stone formation, and penetration through the vagina can occur. Bladder-related complications can manifest as recurrent UTIs and obstructive urinary symptoms, especially in association with stone formation. From the gynecologic perspective, the more common complications with mesh utilization are pelvic pain, groin pain, dyspareunia, contracture and scarring of mesh, and narrowing of the vaginal canal.10 Mesh erosion problems will occur in an estimated 10% to 25% of transvaginal mesh POP implants.11
In 2008, a comparison of transvaginal mesh to native tissue repair (suture-based) or other (biologic) grafts was published.12 The bottom line: there is insufficient evidence to suggest that transvaginal mesh significantly improves outcomes for both posterior and apical defects.
Legal background
Mesh used for surgical purposes is a medical device, which legally is a product—a special product to be sure, but a product nonetheless. Products are subject to product liability rules. Mesh is also subject to an FDA regulatory system. We will briefly discuss products liability and the regulation of devices, both of which have played important roles in mesh-related injuries.
Products liability
As a general matter, defective products subject their manufacturer and seller to liability. There are several legal theories regarding product liability: negligence (in which the defect was caused through carelessness), breach of warranty or guarantee (in addition to express warranties, there are a number of implied warranties for products, including that it is fit for its intended purpose), and strict liability (there was a defect in the product, but it may not have been because of negligence). The product may be defective in the way it was designed, manufactured, or packaged, or it may be defective because adequate instructions and warning were not given to consumers.
Of course, not every product involved in an injury is defective—most automobile accidents, for example, are not the result of any defect in the automobile. In medicine, almost no product (device or pharmaceutical) is entirely safe. In some ways they are unavoidably unsafe and bound to cause some injuries. But when injuries are caused by a defect in the product (design or manufacturing defect or failure to warn), then there may be products liability. Most products liability cases arise under state law.
FDA’s device regulations
Both drugs and medical devices are subject to FDA review and ordinarily require some form of FDA clearance before they may be marketed. In the case of devices, the FDA has 3 classes, with an increase in risk to the user from Class I to III. Various levels of FDA review are required before marketing of the device is permitted, again with the intensity of review increasing from I to III as follows:
- Class I devices pose the least risk, have the least regulation, and are subject to general controls (ie, manufacturing and marketing practices).
- Class II devices pose slightly higher risks and are subject to special controls in addition to the criteria for Class I.
- Class III devices pose the most risk to patients and require premarket approval (scientific review and studies are required to ensure efficacy and safety).13
Continue to: There are a number of limits on manufacturer liability for defective devices...
There are a number of limits on manufacturer liability for defective devices. For Class III devices, the thorough FDA review of the safety of a device may limit the ability of an injured patient to sue based on the state product liability laws.14 For the most part, this “preemption” of state law has not played a major role in mesh litigation because they were initially classified as Class II devices which did not require or include a detailed FDA review.15
The duty to warn of the dangers and risk of medical devices means that manufacturers (or sellers) of devices are obligated to inform health care providers and other medical personnel of the risks. Unlike other manufacturers, device manufacturers do not have to directly warn consumers—because physicians deal directly with patients and prescribe the devices. Therefore, the health care providers, rather than the manufacturers, are obligated to inform the patient.16 This is known as the learned intermediary rule. Manufacturers may still be liable for failure to warn if they do not convey to health care providers proper warnings.
Manufacturers and sellers are not the only entities that may be subject to liability caused by medical devices. Hospitals or other entities that stock and care for devices are responsible for maintaining the safety and functionality of devices in their care.
Health care providers also may be responsible for injuries from medical devices. Generally, that liability is based on negligence. Negligence may relate to selecting an improper device, installing or using it incorrectly, or failing to give the patient adequate information (or informed consent) about the device and alternatives to it.17
A look at the mesh mess
There are a lot of distressing problems and professional disappointments in dissecting the “mesh mess,” including a failure of the FDA to regulate effectively, the extended sale and promotion of intrinsic sphincter deficiency mesh products, the improper use of mesh by physicians even after the risks were known, and, in some instances, the taking advantage of injured patients by attorneys and businesses.18 A lot of finger pointing also has occurred.19 We will recount some of the lowlights of this unfortunate tale.
Continue to: The FDA, in the 1990s, classified the first POP and SUI mesh...
The FDA, in the 1990s, classified the first POP and SUI mesh as Class II after deciding these products were “substantially equivalent” to older surgical meshes. This, of course, proved not to be the case.20 The FDA started receiving thousands of reports of adverse events and, in 2008, warned physicians to be vigilant for adverse events from the mesh. The FDA’s notification recommendations regarding mesh included the following13:
- Obtain specialized training for each mesh implantation technique, and be cognizant of risks.
- Be vigilant for potential adverse events from mesh, including erosion and infection.
- Be observant for complications associated with tools of transvaginal placement (ie, bowel, bladder, and vessel perforation).
- Inform patients that implantation of mesh is permanent and complications may require additional surgery for correction.
- Be aware that complications may affect quality of life—eg, pain with intercourse, scarring, and vaginal wall narrowing (POP repair).
- Provide patients with written copy of patient labeling from the surgical mesh manufacturer.
In 2011, the FDA issued a formal warning to providers that transvaginal mesh posed meaningful risks beyond nonmesh surgery. The FDA’s bulletin draws attention to how the mesh is placed more so than the material per se.19,21 Mesh was a Class II device for sacrocolpopexy or midurethral sling and, similarly, the transvaginal kit was also a Class II device. Overall, use of mesh midurethral slings has been well received as treatment for SUI. The FDA also accepted it for POP, however, but with increasingly strong warnings. The FDA’s 2011 communication stated, “This update is to inform you that serious complications associated with surgical mesh for transvaginal repair of POP are not rare….Furthermore, it is not clear that transvaginal POP repair with mesh is more effective than traditional non-mesh repair in all patients with POP and it may expose patients to greater risk.”7,13
In 2014 the FDA proposed reclassifying mesh to a Class III device, which would require that manufacturers obtain approval, based on safety and effectiveness, before selling mesh. Not until 2016 did the FDA actually reclassify the mess as Class III. Of course, during this time, mesh manufacturers were well aware of the substantial problems the products were causing.13
After serious problems with mesh became well known, and especially after FDA warnings, the use of mesh other than as indicated by the FDA was increasingly risky from a legal (as well as a health) standpoint. As long as mesh was still on the market, of course, it was available for use. But use of mesh for POP procedures without good indications in a way that was contrary to the FDA warnings might well be negligent.
Changes to informed consent
The FDA warnings also should have changed the informed consent for the use of mesh.22 Informed consent commonly consists of the following:
- informing the patient of the proposed procedure
- describing risks (and benefits) of the proposed process
- explaining reasonable alternatives
- noting the risks of taking no action.
Information that is material to a decision should be disclosed. If mesh were going to be used, after the problems of mesh were known and identified by the FDA (other than midurethral slings as treatment of SUI), the risks should have been clearly identified for patients, with alternatives outlined. The American College of Obstetricians and Gynecologists Committee on Ethics has 8 fundamental concepts with regard to informed consent that are worth keeping in mind23:
- Obtaining informed consent for medical treatment and research is an ethical requirement.
- The process expresses respect for the patient as a person.
- It protects patients against unwanted treatment and allows patients’ active involvement in medical planning and care.
- Communication is of paramount importance.
- Informed consent is a process and not a signature on a form.
- A commitment to informed consent and to provision of medical benefit to the patient are linked to provision of care.
- If obtaining informed consent is impossible, a designated surrogate should be identified representing the patient’s best interests.
- Knowledge on the part of the provider regarding state and federal requirements is necessary.
Continue to: Lawsuits line up...
Lawsuits line up
The widespread use of a product with a significant percentage of injuries and eventually with warnings about injuries from use sounds like the formula for a lot of lawsuits. This certainly has happened. A large number of suits—both class actions and individual actions—were filed as a result of mesh injuries.24 These suits were overwhelmingly against the manufacturer, although some included physicians.7 Device makers are more attractive defendants for several reasons. First, they have very deep pockets. In addition, jurors are generally much less sympathetic to large companies than to doctors. Large class actions meant that there were many different patients among the plaintiffs, and medical malpractice claims in most states have a number of trial difficulties not present in other product liability cases. Common defendants have included Johnson & Johnson, Boston Scientific, and Medtronic.
Some of the cases resulted in very large damage awards against manufacturers based on various kinds of product(s) liability. Many other cases were settled or tried with relatively small damages. There were, in addition, a number of instances in which the manufacturers were not liable. Of the 32 plaintiffs who have gone to trial thus far, 24 have obtained verdicts totaling $345 million ($14 million average). The cases that have settled have been for much less—perhaps $60,000 on average. A number of cases remain unresolved. To date, the estimate is that 100,000 women have received almost $8 billion from 7 device manufacturers to resolve claims.25
Some state attorneys general have gotten into the process as well. Attorneys general from California, Kentucky, Mississippi, and Washington have filed lawsuits against Johnson & Johnson, claiming that they deceived doctors and patients about the risks of their pelvic mesh. The states claim that marketing and instructional literature should have contained more information about the risks. Some physicians in these states have expressed concern that these lawsuit risks may do more harm than good because the suits conflate mesh used to treat incontinence with the more risky mesh for POP.26
The “ugly” of class action lawsuits
We have discussed both the sad (the injuries to patients) and the bad (the slow regulatory response and continuing injuries). (The ethics of the marketing by the manufacturers might also be raised as the bad.27) Next, let’s look briefly at the ugly.
Some of the patients affected by mesh injuries have been victimized a second time by medical “lenders” and some of their attorneys. Press reports describe patients with modest awards paying 40% in attorney fees (on the high side for personal injury settlements) plus extravagant costs—leaving modest amounts of actual recovery.25
Worse still, a process of “medical lending” has arisen in mesh cases.28 Medical lenders may contact mesh victims offering to pay up front for surgery to remove mesh, and then place a lien against the settlement for repayment at a much higher rate. They might pay the surgeon $2,500 for the surgery, but place a lien on the settlement amount for $60,000.29,30 In addition, there are allegations that lawyers may recruit the doctors to overstate the injuries or do unnecessary removal surgery because that will likely up the award.31 A quick Google search indicates dozens of offers of cash now for your mesh lawsuit (transvaginal and hernia repair).
The patient in our hypothetical case at the beginning had a fairly typical experience. She was a member of a class filing and received a modest settlement. The attorneys representing the class were allowed by the court to charge substantial attorneys’ fees and costs. The patient had the good sense to avoid medical lenders, although other members of the class did use medical lenders and are now filing complaints about the way they were treated by these lenders.
- Maintain surgical skills and be open to new technology. Medical practice requires constant updating and use of new and improved technology as it comes along. By definition, new technology often requires new skills and understanding. A significant portion of surgeons using mesh indicated that they had not read the instructions for use, or had done so only once.1 CME programs that include surgical education remain of particular value.
- Whether new technology or old, it is essential to keep up to date on all FDA bulletins pertinent to devices and pharmaceuticals that you use and prescribe. For example, in 2016 and 2018 the FDA warned that the use of a very old class of drugs (fluoroquinolones) should be limited. It advised "that the serious side effects associated with fluoroquinolones generally outweigh the benefits for patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infections who have other treatment options. For patients with these conditions, fluoroquinolones should be reserved for those who do not have alternative treatment options."2 Continued, unnecessary prescriptions for fluoroquinolones would put a physician at some legal risk whether or not the physician had paid any attention to the warning.
- Informed consent is a very important legal and medical process. Take it seriously, and make sure the patient has the information necessary to make informed decisions about treatment. Document the process and the information provided. In some cases consider directing patients to appropriate literature or websites of the manufacturers.
- As to the use of mesh, if not following FDA advice, it is important to document the reason for this and to document the informed consent especially carefully.
- Follow patients after mesh placement for a minimum of 1 year and emphasize to patients they should convey signs and symptoms of complications from initial placement.3 High-risk patients should be of particular concern and be monitored very closely.
References
- Kirkpatrick G, Faber KD, Fromer DL. Transvaginal mesh placement and the instructions for use: a survey of North American urologists. J Urol. https://doi.org/10.1016/j.urpr.2018.05.004.
- FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. July 26, 2016. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed June 19, 2019.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
CASE Complications with mesh placement for SUI
A 47-year-old woman (G4 P3013) presents 5 months posthysterectomy with evidence of urinary tract infection (UTI). Escherichia coli is isolated, and she responds to antibiotic therapy.
Her surgical history includes a mini-sling procedure using a needleless device and mesh placement in order to correct progressive worsening of loss of urine when coughing and sneezing. She also reported slight pelvic pain, dysuria, and urgency upon urination at that time. After subsequent development of pelvic organ prolapse (POP), she underwent the vaginal hysterectomy.
Following her UTI treatment, a host of problems occur for the patient, including pelvic pain and dyspareunia. Her male partner reports “feeling something during sex,” especially at the anterior vaginal wall. A plain radiograph of the abdomen identifies a 2 cm x 2 cm stone over the vaginal mesh. In consultation with female pelvic medicine and reconstructive surgery subspecialists, lithotripsy is performed, with the stone fragmented. The patient remains symptomatic, however.
The mesh is noted to be eroding through the vaginal wall. An attempt is made to excise the mesh, initially via transuretheral resection, then through a laparoscopic approach. Due to the mesh being embedded in the tissue, however, an open approach is undertaken. Extensive excision of the mesh and stone fragments is performed. Postoperatively, the patient reports “dry vagina,” with no other genitourinary complaints.
The patient sues. She sues the mesh manufacturer. She also seeks to sue the gynecologist who placed the sling and vaginal mesh (as she says she was not informed of “all the risks” of vaginal mesh placement. She is part of a class action lawsuit, along with thousands of other women.
WHAT’S THE VERDICT?
The device manufacturer settled out of court with the class action suit. (The gynecologist was never formally a defendant because the patient/plaintiff was advised to “drop the physician from the suit.”) The attorneys representing the class action received 40% of the award plus presented costs for the representation. The class as a whole received a little more than 50% of the negotiated award. The patient in this case received $60,000.
Medical background
Stress urinary incontinence (SUI) is a prevalent condition; it affects 35% of women.1 Overall, 80% of women aged 80 or younger will undergo some form of surgery for POP during their lifetime.2 The pathophysiology of SUI includes urethral hypermobility and intrinsic sphincter deficiency.3
Surgical correction for urinary incontinence: A timeline
Use of the gracilis muscle flap to surgically correct urinary incontinence was introduced in 1907. This technique has been replaced by today’s more common Burch procedure, which was first described in 1961. Surgical mesh use dates back to the 1950s, when it was primarily used for abdominal hernia repair. Tension-free tape was introduced in 1995.4-6
Continue to: In the late 1990s the US Food and Drug Administration...
In the late 1990s the US Food and Drug Administration (FDA) permitted use of the first transvaginal meshes, which were designed to treat SUI—the midurethral sling. These mesh slings were so successful that similar meshes were developed to treat POP.7 Almost immediately there were problems with the new POP devices, and 3 years later Boston Scientific recalled its device.8 Nonetheless, the FDA cleared more than 150 devices using surgical mesh for urogynecologic indications (FIGURE).9

Mesh complications
Managing complications from intravesical mesh is a clinically challenging problem. Bladder perforation, stone formation, and penetration through the vagina can occur. Bladder-related complications can manifest as recurrent UTIs and obstructive urinary symptoms, especially in association with stone formation. From the gynecologic perspective, the more common complications with mesh utilization are pelvic pain, groin pain, dyspareunia, contracture and scarring of mesh, and narrowing of the vaginal canal.10 Mesh erosion problems will occur in an estimated 10% to 25% of transvaginal mesh POP implants.11
In 2008, a comparison of transvaginal mesh to native tissue repair (suture-based) or other (biologic) grafts was published.12 The bottom line: there is insufficient evidence to suggest that transvaginal mesh significantly improves outcomes for both posterior and apical defects.
Legal background
Mesh used for surgical purposes is a medical device, which legally is a product—a special product to be sure, but a product nonetheless. Products are subject to product liability rules. Mesh is also subject to an FDA regulatory system. We will briefly discuss products liability and the regulation of devices, both of which have played important roles in mesh-related injuries.
Products liability
As a general matter, defective products subject their manufacturer and seller to liability. There are several legal theories regarding product liability: negligence (in which the defect was caused through carelessness), breach of warranty or guarantee (in addition to express warranties, there are a number of implied warranties for products, including that it is fit for its intended purpose), and strict liability (there was a defect in the product, but it may not have been because of negligence). The product may be defective in the way it was designed, manufactured, or packaged, or it may be defective because adequate instructions and warning were not given to consumers.
Of course, not every product involved in an injury is defective—most automobile accidents, for example, are not the result of any defect in the automobile. In medicine, almost no product (device or pharmaceutical) is entirely safe. In some ways they are unavoidably unsafe and bound to cause some injuries. But when injuries are caused by a defect in the product (design or manufacturing defect or failure to warn), then there may be products liability. Most products liability cases arise under state law.
FDA’s device regulations
Both drugs and medical devices are subject to FDA review and ordinarily require some form of FDA clearance before they may be marketed. In the case of devices, the FDA has 3 classes, with an increase in risk to the user from Class I to III. Various levels of FDA review are required before marketing of the device is permitted, again with the intensity of review increasing from I to III as follows:
- Class I devices pose the least risk, have the least regulation, and are subject to general controls (ie, manufacturing and marketing practices).
- Class II devices pose slightly higher risks and are subject to special controls in addition to the criteria for Class I.
- Class III devices pose the most risk to patients and require premarket approval (scientific review and studies are required to ensure efficacy and safety).13
Continue to: There are a number of limits on manufacturer liability for defective devices...
There are a number of limits on manufacturer liability for defective devices. For Class III devices, the thorough FDA review of the safety of a device may limit the ability of an injured patient to sue based on the state product liability laws.14 For the most part, this “preemption” of state law has not played a major role in mesh litigation because they were initially classified as Class II devices which did not require or include a detailed FDA review.15
The duty to warn of the dangers and risk of medical devices means that manufacturers (or sellers) of devices are obligated to inform health care providers and other medical personnel of the risks. Unlike other manufacturers, device manufacturers do not have to directly warn consumers—because physicians deal directly with patients and prescribe the devices. Therefore, the health care providers, rather than the manufacturers, are obligated to inform the patient.16 This is known as the learned intermediary rule. Manufacturers may still be liable for failure to warn if they do not convey to health care providers proper warnings.
Manufacturers and sellers are not the only entities that may be subject to liability caused by medical devices. Hospitals or other entities that stock and care for devices are responsible for maintaining the safety and functionality of devices in their care.
Health care providers also may be responsible for injuries from medical devices. Generally, that liability is based on negligence. Negligence may relate to selecting an improper device, installing or using it incorrectly, or failing to give the patient adequate information (or informed consent) about the device and alternatives to it.17
A look at the mesh mess
There are a lot of distressing problems and professional disappointments in dissecting the “mesh mess,” including a failure of the FDA to regulate effectively, the extended sale and promotion of intrinsic sphincter deficiency mesh products, the improper use of mesh by physicians even after the risks were known, and, in some instances, the taking advantage of injured patients by attorneys and businesses.18 A lot of finger pointing also has occurred.19 We will recount some of the lowlights of this unfortunate tale.
Continue to: The FDA, in the 1990s, classified the first POP and SUI mesh...
The FDA, in the 1990s, classified the first POP and SUI mesh as Class II after deciding these products were “substantially equivalent” to older surgical meshes. This, of course, proved not to be the case.20 The FDA started receiving thousands of reports of adverse events and, in 2008, warned physicians to be vigilant for adverse events from the mesh. The FDA’s notification recommendations regarding mesh included the following13:
- Obtain specialized training for each mesh implantation technique, and be cognizant of risks.
- Be vigilant for potential adverse events from mesh, including erosion and infection.
- Be observant for complications associated with tools of transvaginal placement (ie, bowel, bladder, and vessel perforation).
- Inform patients that implantation of mesh is permanent and complications may require additional surgery for correction.
- Be aware that complications may affect quality of life—eg, pain with intercourse, scarring, and vaginal wall narrowing (POP repair).
- Provide patients with written copy of patient labeling from the surgical mesh manufacturer.
In 2011, the FDA issued a formal warning to providers that transvaginal mesh posed meaningful risks beyond nonmesh surgery. The FDA’s bulletin draws attention to how the mesh is placed more so than the material per se.19,21 Mesh was a Class II device for sacrocolpopexy or midurethral sling and, similarly, the transvaginal kit was also a Class II device. Overall, use of mesh midurethral slings has been well received as treatment for SUI. The FDA also accepted it for POP, however, but with increasingly strong warnings. The FDA’s 2011 communication stated, “This update is to inform you that serious complications associated with surgical mesh for transvaginal repair of POP are not rare….Furthermore, it is not clear that transvaginal POP repair with mesh is more effective than traditional non-mesh repair in all patients with POP and it may expose patients to greater risk.”7,13
In 2014 the FDA proposed reclassifying mesh to a Class III device, which would require that manufacturers obtain approval, based on safety and effectiveness, before selling mesh. Not until 2016 did the FDA actually reclassify the mess as Class III. Of course, during this time, mesh manufacturers were well aware of the substantial problems the products were causing.13
After serious problems with mesh became well known, and especially after FDA warnings, the use of mesh other than as indicated by the FDA was increasingly risky from a legal (as well as a health) standpoint. As long as mesh was still on the market, of course, it was available for use. But use of mesh for POP procedures without good indications in a way that was contrary to the FDA warnings might well be negligent.
Changes to informed consent
The FDA warnings also should have changed the informed consent for the use of mesh.22 Informed consent commonly consists of the following:
- informing the patient of the proposed procedure
- describing risks (and benefits) of the proposed process
- explaining reasonable alternatives
- noting the risks of taking no action.
Information that is material to a decision should be disclosed. If mesh were going to be used, after the problems of mesh were known and identified by the FDA (other than midurethral slings as treatment of SUI), the risks should have been clearly identified for patients, with alternatives outlined. The American College of Obstetricians and Gynecologists Committee on Ethics has 8 fundamental concepts with regard to informed consent that are worth keeping in mind23:
- Obtaining informed consent for medical treatment and research is an ethical requirement.
- The process expresses respect for the patient as a person.
- It protects patients against unwanted treatment and allows patients’ active involvement in medical planning and care.
- Communication is of paramount importance.
- Informed consent is a process and not a signature on a form.
- A commitment to informed consent and to provision of medical benefit to the patient are linked to provision of care.
- If obtaining informed consent is impossible, a designated surrogate should be identified representing the patient’s best interests.
- Knowledge on the part of the provider regarding state and federal requirements is necessary.
Continue to: Lawsuits line up...
Lawsuits line up
The widespread use of a product with a significant percentage of injuries and eventually with warnings about injuries from use sounds like the formula for a lot of lawsuits. This certainly has happened. A large number of suits—both class actions and individual actions—were filed as a result of mesh injuries.24 These suits were overwhelmingly against the manufacturer, although some included physicians.7 Device makers are more attractive defendants for several reasons. First, they have very deep pockets. In addition, jurors are generally much less sympathetic to large companies than to doctors. Large class actions meant that there were many different patients among the plaintiffs, and medical malpractice claims in most states have a number of trial difficulties not present in other product liability cases. Common defendants have included Johnson & Johnson, Boston Scientific, and Medtronic.
Some of the cases resulted in very large damage awards against manufacturers based on various kinds of product(s) liability. Many other cases were settled or tried with relatively small damages. There were, in addition, a number of instances in which the manufacturers were not liable. Of the 32 plaintiffs who have gone to trial thus far, 24 have obtained verdicts totaling $345 million ($14 million average). The cases that have settled have been for much less—perhaps $60,000 on average. A number of cases remain unresolved. To date, the estimate is that 100,000 women have received almost $8 billion from 7 device manufacturers to resolve claims.25
Some state attorneys general have gotten into the process as well. Attorneys general from California, Kentucky, Mississippi, and Washington have filed lawsuits against Johnson & Johnson, claiming that they deceived doctors and patients about the risks of their pelvic mesh. The states claim that marketing and instructional literature should have contained more information about the risks. Some physicians in these states have expressed concern that these lawsuit risks may do more harm than good because the suits conflate mesh used to treat incontinence with the more risky mesh for POP.26
The “ugly” of class action lawsuits
We have discussed both the sad (the injuries to patients) and the bad (the slow regulatory response and continuing injuries). (The ethics of the marketing by the manufacturers might also be raised as the bad.27) Next, let’s look briefly at the ugly.
Some of the patients affected by mesh injuries have been victimized a second time by medical “lenders” and some of their attorneys. Press reports describe patients with modest awards paying 40% in attorney fees (on the high side for personal injury settlements) plus extravagant costs—leaving modest amounts of actual recovery.25
Worse still, a process of “medical lending” has arisen in mesh cases.28 Medical lenders may contact mesh victims offering to pay up front for surgery to remove mesh, and then place a lien against the settlement for repayment at a much higher rate. They might pay the surgeon $2,500 for the surgery, but place a lien on the settlement amount for $60,000.29,30 In addition, there are allegations that lawyers may recruit the doctors to overstate the injuries or do unnecessary removal surgery because that will likely up the award.31 A quick Google search indicates dozens of offers of cash now for your mesh lawsuit (transvaginal and hernia repair).
The patient in our hypothetical case at the beginning had a fairly typical experience. She was a member of a class filing and received a modest settlement. The attorneys representing the class were allowed by the court to charge substantial attorneys’ fees and costs. The patient had the good sense to avoid medical lenders, although other members of the class did use medical lenders and are now filing complaints about the way they were treated by these lenders.
- Maintain surgical skills and be open to new technology. Medical practice requires constant updating and use of new and improved technology as it comes along. By definition, new technology often requires new skills and understanding. A significant portion of surgeons using mesh indicated that they had not read the instructions for use, or had done so only once.1 CME programs that include surgical education remain of particular value.
- Whether new technology or old, it is essential to keep up to date on all FDA bulletins pertinent to devices and pharmaceuticals that you use and prescribe. For example, in 2016 and 2018 the FDA warned that the use of a very old class of drugs (fluoroquinolones) should be limited. It advised "that the serious side effects associated with fluoroquinolones generally outweigh the benefits for patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infections who have other treatment options. For patients with these conditions, fluoroquinolones should be reserved for those who do not have alternative treatment options."2 Continued, unnecessary prescriptions for fluoroquinolones would put a physician at some legal risk whether or not the physician had paid any attention to the warning.
- Informed consent is a very important legal and medical process. Take it seriously, and make sure the patient has the information necessary to make informed decisions about treatment. Document the process and the information provided. In some cases consider directing patients to appropriate literature or websites of the manufacturers.
- As to the use of mesh, if not following FDA advice, it is important to document the reason for this and to document the informed consent especially carefully.
- Follow patients after mesh placement for a minimum of 1 year and emphasize to patients they should convey signs and symptoms of complications from initial placement.3 High-risk patients should be of particular concern and be monitored very closely.
References
- Kirkpatrick G, Faber KD, Fromer DL. Transvaginal mesh placement and the instructions for use: a survey of North American urologists. J Urol. https://doi.org/10.1016/j.urpr.2018.05.004.
- FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. July 26, 2016. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed June 19, 2019.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Maral I, Ozkardeş H, Peşkircioğlu L, et al. Prevalence of stress urinary incontinence in both sexes at or after age 15 years: a cross-sectional study. J Urol. 2001;165:408-412.
- Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
- Chang J, Lee D. Midurethral slings in the mesh litigation era. Transl Androl Urol. 2017;6(suppl 2): S68-S75.
- Mattingly R, ed. TeLinde's Operative Gynecology, 5th edition. Lippincott, William, and Wilkins: Philadelphia, PA; 1997.
- Burch J. Urethrovaginal fixation to Cooper's ligament for correction of stress incontinence, cystocele, and prolapse. Am J Obstet Gynecol. 1961;81:281-290.
- Ulmsten U, Falconer C, Johnson P, et al. A multicenter study of tension-free vaginal tape (TVT) for surgical treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:210-213.
- Kuhlmann-Capek MJ, Kilic GS, Shah AB, et al. Enmeshed in controversy: use of vaginal mesh in the current medicolegal environment. Female Pelvic Med Reconstr Surg. 2015;21:241-243.
- Powell SF. Changing our minds: reforming the FDA medical device reclassification process. Food Drug Law J. 2018;73:177-209.
- US Food and Drug Administration. Surgical Mesh for Treatment of Women with Pelvic Organ Prolapse and Stress Urinary Incontinence. September 2011. https://www.thesenatorsfirm.com/documents/OBS.pdf. Accessed June 19, 2019.
- Maher C, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;(4):CD004014.
- Ganj FA, Ibeanu OA, Bedestani A, Nolan TE, Chesson RR. Complications of transvaginal monofilament polypropylene mesh in pelvic organ prolapse repair. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:919-925.
- Sung VW, Rogers RG, Schaffer JI, et al. Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstet Gynecol. 2008;112:1131-1142.
- FDA public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. October 20, 2008. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm. Accessed February 14, 2019.
- Riegel v. Medtronic, 552 U.S. 312 (2008).
- Whitney DW. Guide to preemption of state-law claims against Class III PMA medical devices. Food Drug Law J. 2010;65:113-139.
- Alam P, Iglesia CB. Informed consent for reconstructive pelvic surgery. Obstet Gynecol Clin North Am. 2016;43:131-139.
- Nosti PA, Iglesia CB. Medicolegal issues surrounding devices and mesh for surgical treatment of prolapse and incontinence. Clin Obstet Gynecol. 2013;56:221-228.
- Shepherd CG. Transvaginal mesh litigation: a new opportunity to resolve mass medical device failure claims. Tennessee Law Rev. 2012;80:3:477-94.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Cohn JA, Timbrook Brown E, Kowalik CG, et al. The mesh controversy. F1000Research website. https://f1000research.com/articles/5-2423/v1. Accessed June 17, 2019.
- Obstetrics and Gynecology Devices Panel Meeting, February 12, 2019. US Food and Drug Administration website. https://www.fda.gov/media/122867/download. Accessed June 19, 2019.
- Mucowski SJ, Jurnalov C, Phelps JY. Use of vaginal mesh in the face of recent FDA warnings and litigation. Am J Obstet Gynecol. 2010;203:103.e1-e4.
- American College of Obstetricians and Gynecologists Committee on Ethics. ACOG Committee Opinion No. 439: informed consent. Obstet Gynecol. 2009;114(2 pt 1):401-408.
- Souders CP, Eilber KS, McClelland L, et al. The truth behind transvaginal mesh litigation: devices, timelines, and provider characteristics. Female Pelvic Med Reconstr Surg. 2018;24:21-25.
- Goldstein M. As pelvic mesh settlements near $8 billion, women question lawyers' fees. New York Times. February 1, 2019. https://www.nytimes.com/2019/02/01/business/pelvic-mesh-settlements-lawyers.html. Accessed June 19, 2019.
- Johnson G. Surgeons fear pelvic mesh lawsuits will spook patients. Associated Press News. January 10, 2019. https://www.apnews.com/25777c3c33e3489283b1dc2ebdde6b55. Accessed June 19, 2019.
- Clarke RN. Medical device marketing and the ethics of vaginal mesh kit marketing. In The Innovation and Evolution of Medical Devices. New York, NY: Springer; 2019:103-123.
- Top 5 drug and medical device developments of 2018. Law 360. January 1, 2019. Accessed through LexisNexis.
- Frankel A, Dye J. The Lien Machine. New breed of investor profits by financing surgeries for desperate women patients. Reuters. August 18, 2015. https://www.reuters.com/investigates/special-report/usa-litigation-mesh/. Accessed June 19, 2019.
- Sullivan T. New report looks at intersection of "medical lending" and pelvic mesh lawsuits. Policy & Medicine. May 5, 2018. https://www.policymed.com/2015/08/medical-lending-and-pelvic-mesh-litigation.html. Accessed June 19, 2019.
- Goldstein M, Sliver-Greensberg J. How profiteers lure women into often-unneeded surgery. New York Times. April 14, 2018. https://www.nytimes.com/2018/04/14/business/vaginal-mesh-surgery-lawsuits-financing.html. Accessed June 19, 2019.
- Maral I, Ozkardeş H, Peşkircioğlu L, et al. Prevalence of stress urinary incontinence in both sexes at or after age 15 years: a cross-sectional study. J Urol. 2001;165:408-412.
- Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
- Chang J, Lee D. Midurethral slings in the mesh litigation era. Transl Androl Urol. 2017;6(suppl 2): S68-S75.
- Mattingly R, ed. TeLinde's Operative Gynecology, 5th edition. Lippincott, William, and Wilkins: Philadelphia, PA; 1997.
- Burch J. Urethrovaginal fixation to Cooper's ligament for correction of stress incontinence, cystocele, and prolapse. Am J Obstet Gynecol. 1961;81:281-290.
- Ulmsten U, Falconer C, Johnson P, et al. A multicenter study of tension-free vaginal tape (TVT) for surgical treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:210-213.
- Kuhlmann-Capek MJ, Kilic GS, Shah AB, et al. Enmeshed in controversy: use of vaginal mesh in the current medicolegal environment. Female Pelvic Med Reconstr Surg. 2015;21:241-243.
- Powell SF. Changing our minds: reforming the FDA medical device reclassification process. Food Drug Law J. 2018;73:177-209.
- US Food and Drug Administration. Surgical Mesh for Treatment of Women with Pelvic Organ Prolapse and Stress Urinary Incontinence. September 2011. https://www.thesenatorsfirm.com/documents/OBS.pdf. Accessed June 19, 2019.
- Maher C, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;(4):CD004014.
- Ganj FA, Ibeanu OA, Bedestani A, Nolan TE, Chesson RR. Complications of transvaginal monofilament polypropylene mesh in pelvic organ prolapse repair. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:919-925.
- Sung VW, Rogers RG, Schaffer JI, et al. Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstet Gynecol. 2008;112:1131-1142.
- FDA public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. October 20, 2008. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm. Accessed February 14, 2019.
- Riegel v. Medtronic, 552 U.S. 312 (2008).
- Whitney DW. Guide to preemption of state-law claims against Class III PMA medical devices. Food Drug Law J. 2010;65:113-139.
- Alam P, Iglesia CB. Informed consent for reconstructive pelvic surgery. Obstet Gynecol Clin North Am. 2016;43:131-139.
- Nosti PA, Iglesia CB. Medicolegal issues surrounding devices and mesh for surgical treatment of prolapse and incontinence. Clin Obstet Gynecol. 2013;56:221-228.
- Shepherd CG. Transvaginal mesh litigation: a new opportunity to resolve mass medical device failure claims. Tennessee Law Rev. 2012;80:3:477-94.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Cohn JA, Timbrook Brown E, Kowalik CG, et al. The mesh controversy. F1000Research website. https://f1000research.com/articles/5-2423/v1. Accessed June 17, 2019.
- Obstetrics and Gynecology Devices Panel Meeting, February 12, 2019. US Food and Drug Administration website. https://www.fda.gov/media/122867/download. Accessed June 19, 2019.
- Mucowski SJ, Jurnalov C, Phelps JY. Use of vaginal mesh in the face of recent FDA warnings and litigation. Am J Obstet Gynecol. 2010;203:103.e1-e4.
- American College of Obstetricians and Gynecologists Committee on Ethics. ACOG Committee Opinion No. 439: informed consent. Obstet Gynecol. 2009;114(2 pt 1):401-408.
- Souders CP, Eilber KS, McClelland L, et al. The truth behind transvaginal mesh litigation: devices, timelines, and provider characteristics. Female Pelvic Med Reconstr Surg. 2018;24:21-25.
- Goldstein M. As pelvic mesh settlements near $8 billion, women question lawyers' fees. New York Times. February 1, 2019. https://www.nytimes.com/2019/02/01/business/pelvic-mesh-settlements-lawyers.html. Accessed June 19, 2019.
- Johnson G. Surgeons fear pelvic mesh lawsuits will spook patients. Associated Press News. January 10, 2019. https://www.apnews.com/25777c3c33e3489283b1dc2ebdde6b55. Accessed June 19, 2019.
- Clarke RN. Medical device marketing and the ethics of vaginal mesh kit marketing. In The Innovation and Evolution of Medical Devices. New York, NY: Springer; 2019:103-123.
- Top 5 drug and medical device developments of 2018. Law 360. January 1, 2019. Accessed through LexisNexis.
- Frankel A, Dye J. The Lien Machine. New breed of investor profits by financing surgeries for desperate women patients. Reuters. August 18, 2015. https://www.reuters.com/investigates/special-report/usa-litigation-mesh/. Accessed June 19, 2019.
- Sullivan T. New report looks at intersection of "medical lending" and pelvic mesh lawsuits. Policy & Medicine. May 5, 2018. https://www.policymed.com/2015/08/medical-lending-and-pelvic-mesh-litigation.html. Accessed June 19, 2019.
- Goldstein M, Sliver-Greensberg J. How profiteers lure women into often-unneeded surgery. New York Times. April 14, 2018. https://www.nytimes.com/2018/04/14/business/vaginal-mesh-surgery-lawsuits-financing.html. Accessed June 19, 2019.
SUI cure definition may need updating
TUCSON, ARIZ. – The definition of a surgical cure for stress urinary incontinence (SUI) varies significantly from one clinical trial to another, but the best choice might be an International Consultation on Incontinence Questionnaire (ICIQ) score of 5 or less, according to a study that correlated a patient’s definition of success with various measures of success or failure.
Adoption of a standard definition could make clinical trial results easier to interpret, as well as improve consistency in clinical practice.
The study was a planned secondary analysis of a randomized, controlled trial that compared midurethral sling to Burch colpopexy in women undergoing abdominal sacrocolpopexy. The original study found no difference in outcomes between the two approaches with respect to stress-specific incontinence rates at 6 months, although the midurethral sling was associated with better secondary, patient-reported outcomes.
That incongruity between objective and subjective outcomes raised questions. “I would frequently have the nurse tell me that a patient didn’t do well [on the stress incontinence test], but you would talk to the patient, and she was happy as could be. She wasn’t using pads, she was perfectly dry. So I thought there was a little bit of a disconnect between the definitions we were using, and what the patients wanted from the procedure,” Emanuel Trabuco, MD, said in an interview.
Dr. Trabuco is a consultant and the chair of the division of urogynecology at Mayo Clinic in Rochester, Minn. He presented the study at the annual scientific meeting of the Society of Gynecologic Surgeons.
because as things currently stand, different clinical trials use a range of different outcomes, and as the nurse’s experience shows, an objective outcome might not match patient perception. In fact, objective urinary incontinence tests may not be so objective at all.
“Urodynamics is inherently [challenging]. You can have women that come in with stress incontinence symptoms asking for treatment, and we do urodynamics and they don’t leak. It’s a false negative. Conversely, other women presenting with other issues like overactive bladder – you do urodynamics, and they leak. So that’s a false positive. We have this desire for objectivity, but the tests we have are neither sensitive nor specific,” said Dr. Trabuco.
The researchers examined 13 different methods of determining SUI cure, and then linked them to answers to two questions from 104 trial participants. The first question: “In your opinion, how successful has treatment for your urinary leakage been?” Responses ranged from 0 (not at all) to 10 (very successful). The second question: “Compared to how you were before your recent surgery, how are your urinary leakage symptoms now?” Responses ranged from 0 (much worse) to 10 (much better).
At 6 months, the largest Cohen’s d value for patient perception of symptom improvement was associated with ICIQ score greater than or equal to 5 (–13.5, mean ratings of 9.7 versus 4.6), which was better than definitions based on a negative cough stress test (–6.5) and the strict composite definition, which included a negative cough stress test, ICIQ = 0, and no retreatment (–6.4).
The researchers examined the correlation between each definition of SUI cure and the answers to the above questions, and found that the highest Cohen’s d values for agreement with patient’s perception of symptom improvement were: ICIQ score greater than or equal to 5 (Cohen’s d at 6 months, 12 months, and 24 months; –13.5; –13.0; –12.6, respectively); ICIQ score less than or equal to 5 with no (“not-at-all” or “somewhat”) SUI symptoms on Urinary Distress Inventory, Short Form (UDI-6) (–7.2; –7.2; and –8.1); and ICIQ score less than or equal to 5 with no SUI symptoms (never or rarely) on Medical, Epidemiologic, and Social aspects of Aging (MESA) urinary incontinence questionnaire (–7.0, –7.0, –6.4).
The results argue against the use of cough stress test, said Dr. Trabuco. “If you think about the time commitment that our patients give us to participate in a trial, we should make that participation as least onerous as we can. If the cough stress test doesn’t really add anything to patient perception of surgical success and improvement, why put the poor patient through a catheterization and a cough test and a prolonged visit? For all of those reasons, I hope this is something that others will look at and try to standardize,” said Dr. Trabuco.
Mayo Medical School, Rochester, Minn., funded the study. Dr. Trabuco has no relevant financial disclosures.
SOURCE: Trabuco E et al. SGS 2019, oral poster 14.
TUCSON, ARIZ. – The definition of a surgical cure for stress urinary incontinence (SUI) varies significantly from one clinical trial to another, but the best choice might be an International Consultation on Incontinence Questionnaire (ICIQ) score of 5 or less, according to a study that correlated a patient’s definition of success with various measures of success or failure.
Adoption of a standard definition could make clinical trial results easier to interpret, as well as improve consistency in clinical practice.
The study was a planned secondary analysis of a randomized, controlled trial that compared midurethral sling to Burch colpopexy in women undergoing abdominal sacrocolpopexy. The original study found no difference in outcomes between the two approaches with respect to stress-specific incontinence rates at 6 months, although the midurethral sling was associated with better secondary, patient-reported outcomes.
That incongruity between objective and subjective outcomes raised questions. “I would frequently have the nurse tell me that a patient didn’t do well [on the stress incontinence test], but you would talk to the patient, and she was happy as could be. She wasn’t using pads, she was perfectly dry. So I thought there was a little bit of a disconnect between the definitions we were using, and what the patients wanted from the procedure,” Emanuel Trabuco, MD, said in an interview.
Dr. Trabuco is a consultant and the chair of the division of urogynecology at Mayo Clinic in Rochester, Minn. He presented the study at the annual scientific meeting of the Society of Gynecologic Surgeons.
because as things currently stand, different clinical trials use a range of different outcomes, and as the nurse’s experience shows, an objective outcome might not match patient perception. In fact, objective urinary incontinence tests may not be so objective at all.
“Urodynamics is inherently [challenging]. You can have women that come in with stress incontinence symptoms asking for treatment, and we do urodynamics and they don’t leak. It’s a false negative. Conversely, other women presenting with other issues like overactive bladder – you do urodynamics, and they leak. So that’s a false positive. We have this desire for objectivity, but the tests we have are neither sensitive nor specific,” said Dr. Trabuco.
The researchers examined 13 different methods of determining SUI cure, and then linked them to answers to two questions from 104 trial participants. The first question: “In your opinion, how successful has treatment for your urinary leakage been?” Responses ranged from 0 (not at all) to 10 (very successful). The second question: “Compared to how you were before your recent surgery, how are your urinary leakage symptoms now?” Responses ranged from 0 (much worse) to 10 (much better).
At 6 months, the largest Cohen’s d value for patient perception of symptom improvement was associated with ICIQ score greater than or equal to 5 (–13.5, mean ratings of 9.7 versus 4.6), which was better than definitions based on a negative cough stress test (–6.5) and the strict composite definition, which included a negative cough stress test, ICIQ = 0, and no retreatment (–6.4).
The researchers examined the correlation between each definition of SUI cure and the answers to the above questions, and found that the highest Cohen’s d values for agreement with patient’s perception of symptom improvement were: ICIQ score greater than or equal to 5 (Cohen’s d at 6 months, 12 months, and 24 months; –13.5; –13.0; –12.6, respectively); ICIQ score less than or equal to 5 with no (“not-at-all” or “somewhat”) SUI symptoms on Urinary Distress Inventory, Short Form (UDI-6) (–7.2; –7.2; and –8.1); and ICIQ score less than or equal to 5 with no SUI symptoms (never or rarely) on Medical, Epidemiologic, and Social aspects of Aging (MESA) urinary incontinence questionnaire (–7.0, –7.0, –6.4).
The results argue against the use of cough stress test, said Dr. Trabuco. “If you think about the time commitment that our patients give us to participate in a trial, we should make that participation as least onerous as we can. If the cough stress test doesn’t really add anything to patient perception of surgical success and improvement, why put the poor patient through a catheterization and a cough test and a prolonged visit? For all of those reasons, I hope this is something that others will look at and try to standardize,” said Dr. Trabuco.
Mayo Medical School, Rochester, Minn., funded the study. Dr. Trabuco has no relevant financial disclosures.
SOURCE: Trabuco E et al. SGS 2019, oral poster 14.
TUCSON, ARIZ. – The definition of a surgical cure for stress urinary incontinence (SUI) varies significantly from one clinical trial to another, but the best choice might be an International Consultation on Incontinence Questionnaire (ICIQ) score of 5 or less, according to a study that correlated a patient’s definition of success with various measures of success or failure.
Adoption of a standard definition could make clinical trial results easier to interpret, as well as improve consistency in clinical practice.
The study was a planned secondary analysis of a randomized, controlled trial that compared midurethral sling to Burch colpopexy in women undergoing abdominal sacrocolpopexy. The original study found no difference in outcomes between the two approaches with respect to stress-specific incontinence rates at 6 months, although the midurethral sling was associated with better secondary, patient-reported outcomes.
That incongruity between objective and subjective outcomes raised questions. “I would frequently have the nurse tell me that a patient didn’t do well [on the stress incontinence test], but you would talk to the patient, and she was happy as could be. She wasn’t using pads, she was perfectly dry. So I thought there was a little bit of a disconnect between the definitions we were using, and what the patients wanted from the procedure,” Emanuel Trabuco, MD, said in an interview.
Dr. Trabuco is a consultant and the chair of the division of urogynecology at Mayo Clinic in Rochester, Minn. He presented the study at the annual scientific meeting of the Society of Gynecologic Surgeons.
because as things currently stand, different clinical trials use a range of different outcomes, and as the nurse’s experience shows, an objective outcome might not match patient perception. In fact, objective urinary incontinence tests may not be so objective at all.
“Urodynamics is inherently [challenging]. You can have women that come in with stress incontinence symptoms asking for treatment, and we do urodynamics and they don’t leak. It’s a false negative. Conversely, other women presenting with other issues like overactive bladder – you do urodynamics, and they leak. So that’s a false positive. We have this desire for objectivity, but the tests we have are neither sensitive nor specific,” said Dr. Trabuco.
The researchers examined 13 different methods of determining SUI cure, and then linked them to answers to two questions from 104 trial participants. The first question: “In your opinion, how successful has treatment for your urinary leakage been?” Responses ranged from 0 (not at all) to 10 (very successful). The second question: “Compared to how you were before your recent surgery, how are your urinary leakage symptoms now?” Responses ranged from 0 (much worse) to 10 (much better).
At 6 months, the largest Cohen’s d value for patient perception of symptom improvement was associated with ICIQ score greater than or equal to 5 (–13.5, mean ratings of 9.7 versus 4.6), which was better than definitions based on a negative cough stress test (–6.5) and the strict composite definition, which included a negative cough stress test, ICIQ = 0, and no retreatment (–6.4).
The researchers examined the correlation between each definition of SUI cure and the answers to the above questions, and found that the highest Cohen’s d values for agreement with patient’s perception of symptom improvement were: ICIQ score greater than or equal to 5 (Cohen’s d at 6 months, 12 months, and 24 months; –13.5; –13.0; –12.6, respectively); ICIQ score less than or equal to 5 with no (“not-at-all” or “somewhat”) SUI symptoms on Urinary Distress Inventory, Short Form (UDI-6) (–7.2; –7.2; and –8.1); and ICIQ score less than or equal to 5 with no SUI symptoms (never or rarely) on Medical, Epidemiologic, and Social aspects of Aging (MESA) urinary incontinence questionnaire (–7.0, –7.0, –6.4).
The results argue against the use of cough stress test, said Dr. Trabuco. “If you think about the time commitment that our patients give us to participate in a trial, we should make that participation as least onerous as we can. If the cough stress test doesn’t really add anything to patient perception of surgical success and improvement, why put the poor patient through a catheterization and a cough test and a prolonged visit? For all of those reasons, I hope this is something that others will look at and try to standardize,” said Dr. Trabuco.
Mayo Medical School, Rochester, Minn., funded the study. Dr. Trabuco has no relevant financial disclosures.
SOURCE: Trabuco E et al. SGS 2019, oral poster 14.
REPORTING FROM SGS 2019
In endometrial cancer and SUI, concomitant surgery improves outcomes
TUCSON, ARIZ. – according to a study examining the effects of an SUI screen among endometrial cancer patients.
An estimated 40%-80% of women with endometrial cancer experience SUI. The malignancy often is caught early enough to be treated with curative intent, and that is leading physicians and patients to think more about quality of life outcomes.
And yet, few patients receive concomitant surgery. Twenty percent of the women in the current study opted for concomitant surgeries, yet large database studies show the frequency of concomitant surgeries is about 2.5%. “There’s huge room for improvement in this area. The take-home message is that this is prevalent, this is doable, and this is something that could truly benefit this population,” Evelyn Hall, MD, said in an interview. Dr. Hall is a fellow in female pelvic medicine and reconstructive medicine at Brown University, Providence, R.I. She presented the study at the annual scientific meeting of the Society of Gynecologic Surgeons.
It’s not entirely surprising that SUI tends to be overlooked in patients with endometrial cancer. After all, they are going through a life-changing medical diagnosis, and oncologists are laser focused on achieving a cure when possible. But a bigger picture view, especially in light of the high cure rate for endometrial cancer when detected early, should encourage physicians to think differently about patient management.
The biggest trick may be incorporating concomitant surgeries into the surgical work flow. “It can be challenging logistically. It requires surgical planning and coordination between the two surgeons,” said Dr. Hall. But she said the experience at Brown University showed that it was possible with some patience. “It took a while to get the balls rolling, but once we figured out [it] worked for our institution, we’ve seen a continued uptake,” she said.
An important remaining question is the safety of the concomitant surgeries. Dr. Hall did not report any between-group differences in her presentation, but analysis is ongoing. They found a statistically significant increase in the number of readmissions among the concomitant surgery group, but most were deemed unlikely to be related to concomitant surgery.
In the study, 1,322 endometrial surgical candidates were screened for SUI, and 53% tested positive. Of these, 556 patients were offered concomitant surgical or nonsurgical SUI treatment: 21% chose concomitant surgery, 19% chose nonsurgical SUI treatment, and 60% of patients opted for no SUI treatment.
At 6 months after surgery, the concomitant surgery group was more likely to have a Urinary Distress Inventory (UDI)–Stress score of 0 than were those who were treated nonsurgically (odds ratio, 2.8; P = .0001) and those in the no-treatment group (OR, 3.7; P less than .0001). The concomitant group also was more likely to have a surgical site infection (SSI) score of 0 than was the nonsurgical group (OR, 2.9; P = .0008) and the no-treatment group (OR, 2.7; P less than .0001). Severe/very severe SSI scores occurred in 57% of the concomitant group at baseline, and this frequency dropped to 14% at 6 weeks (P less than .0001).
The study was funded by the Patient-Centered Outcomes Research Institute. Dr. Hall has no relevant financial disclosures.
SOURCE: Hall E et al. SGS 2019, oral presentation 12.
TUCSON, ARIZ. – according to a study examining the effects of an SUI screen among endometrial cancer patients.
An estimated 40%-80% of women with endometrial cancer experience SUI. The malignancy often is caught early enough to be treated with curative intent, and that is leading physicians and patients to think more about quality of life outcomes.
And yet, few patients receive concomitant surgery. Twenty percent of the women in the current study opted for concomitant surgeries, yet large database studies show the frequency of concomitant surgeries is about 2.5%. “There’s huge room for improvement in this area. The take-home message is that this is prevalent, this is doable, and this is something that could truly benefit this population,” Evelyn Hall, MD, said in an interview. Dr. Hall is a fellow in female pelvic medicine and reconstructive medicine at Brown University, Providence, R.I. She presented the study at the annual scientific meeting of the Society of Gynecologic Surgeons.
It’s not entirely surprising that SUI tends to be overlooked in patients with endometrial cancer. After all, they are going through a life-changing medical diagnosis, and oncologists are laser focused on achieving a cure when possible. But a bigger picture view, especially in light of the high cure rate for endometrial cancer when detected early, should encourage physicians to think differently about patient management.
The biggest trick may be incorporating concomitant surgeries into the surgical work flow. “It can be challenging logistically. It requires surgical planning and coordination between the two surgeons,” said Dr. Hall. But she said the experience at Brown University showed that it was possible with some patience. “It took a while to get the balls rolling, but once we figured out [it] worked for our institution, we’ve seen a continued uptake,” she said.
An important remaining question is the safety of the concomitant surgeries. Dr. Hall did not report any between-group differences in her presentation, but analysis is ongoing. They found a statistically significant increase in the number of readmissions among the concomitant surgery group, but most were deemed unlikely to be related to concomitant surgery.
In the study, 1,322 endometrial surgical candidates were screened for SUI, and 53% tested positive. Of these, 556 patients were offered concomitant surgical or nonsurgical SUI treatment: 21% chose concomitant surgery, 19% chose nonsurgical SUI treatment, and 60% of patients opted for no SUI treatment.
At 6 months after surgery, the concomitant surgery group was more likely to have a Urinary Distress Inventory (UDI)–Stress score of 0 than were those who were treated nonsurgically (odds ratio, 2.8; P = .0001) and those in the no-treatment group (OR, 3.7; P less than .0001). The concomitant group also was more likely to have a surgical site infection (SSI) score of 0 than was the nonsurgical group (OR, 2.9; P = .0008) and the no-treatment group (OR, 2.7; P less than .0001). Severe/very severe SSI scores occurred in 57% of the concomitant group at baseline, and this frequency dropped to 14% at 6 weeks (P less than .0001).
The study was funded by the Patient-Centered Outcomes Research Institute. Dr. Hall has no relevant financial disclosures.
SOURCE: Hall E et al. SGS 2019, oral presentation 12.
TUCSON, ARIZ. – according to a study examining the effects of an SUI screen among endometrial cancer patients.
An estimated 40%-80% of women with endometrial cancer experience SUI. The malignancy often is caught early enough to be treated with curative intent, and that is leading physicians and patients to think more about quality of life outcomes.
And yet, few patients receive concomitant surgery. Twenty percent of the women in the current study opted for concomitant surgeries, yet large database studies show the frequency of concomitant surgeries is about 2.5%. “There’s huge room for improvement in this area. The take-home message is that this is prevalent, this is doable, and this is something that could truly benefit this population,” Evelyn Hall, MD, said in an interview. Dr. Hall is a fellow in female pelvic medicine and reconstructive medicine at Brown University, Providence, R.I. She presented the study at the annual scientific meeting of the Society of Gynecologic Surgeons.
It’s not entirely surprising that SUI tends to be overlooked in patients with endometrial cancer. After all, they are going through a life-changing medical diagnosis, and oncologists are laser focused on achieving a cure when possible. But a bigger picture view, especially in light of the high cure rate for endometrial cancer when detected early, should encourage physicians to think differently about patient management.
The biggest trick may be incorporating concomitant surgeries into the surgical work flow. “It can be challenging logistically. It requires surgical planning and coordination between the two surgeons,” said Dr. Hall. But she said the experience at Brown University showed that it was possible with some patience. “It took a while to get the balls rolling, but once we figured out [it] worked for our institution, we’ve seen a continued uptake,” she said.
An important remaining question is the safety of the concomitant surgeries. Dr. Hall did not report any between-group differences in her presentation, but analysis is ongoing. They found a statistically significant increase in the number of readmissions among the concomitant surgery group, but most were deemed unlikely to be related to concomitant surgery.
In the study, 1,322 endometrial surgical candidates were screened for SUI, and 53% tested positive. Of these, 556 patients were offered concomitant surgical or nonsurgical SUI treatment: 21% chose concomitant surgery, 19% chose nonsurgical SUI treatment, and 60% of patients opted for no SUI treatment.
At 6 months after surgery, the concomitant surgery group was more likely to have a Urinary Distress Inventory (UDI)–Stress score of 0 than were those who were treated nonsurgically (odds ratio, 2.8; P = .0001) and those in the no-treatment group (OR, 3.7; P less than .0001). The concomitant group also was more likely to have a surgical site infection (SSI) score of 0 than was the nonsurgical group (OR, 2.9; P = .0008) and the no-treatment group (OR, 2.7; P less than .0001). Severe/very severe SSI scores occurred in 57% of the concomitant group at baseline, and this frequency dropped to 14% at 6 weeks (P less than .0001).
The study was funded by the Patient-Centered Outcomes Research Institute. Dr. Hall has no relevant financial disclosures.
SOURCE: Hall E et al. SGS 2019, oral presentation 12.
REPORTING FROM SGS 2019
Stress incontinence surgery improves sexual dysfunction
TUCSON, ARIZ. –
The finding comes from a secondary analysis of two randomized, controlled trials comparing Burch colposuspension, autologous fascial slings, retropubic midurethral polypropylene slings, and transobturator midurethral polypropylene slings. The analysis looked at outcomes at 24 months after surgery. Stephanie Glass Clark, MD, a resident at Virginia Commonwealth University, Richmond, presented the results at the annual scientific meeting of the Society of Gynecologic Surgeons.
In the secondary analysis of the Stress Incontinence Surgical Treatment Efficacy Trial (SISTEr) and the Trial of Midurethral Slings (TOMUS) trials, Dr. Clark and her fellow researchers looked at the effect of surgical failure on sexual dysfunction outcomes. Subjective failure was defined as self-reported SUI symptoms or self-reported leakage by 3-day voiding diary beyond 3 months after the surgery. Objective failure was defined as any treatment for SUI after the surgery or a positive stress test or pad test beyond 3 months after the surgery.
Participants were excluded from the two studies if they were sexually inactive in the previous 6 months at baseline, at 12 months post baseline, or at 24 months. The studies employed the short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12), which had 12 questions with scores ranging from 0 to 4. The secondary analysis sample included 488 women from SISTEr and 436 women from TOMUS.
There were some baseline differences among groups between the two trials, including vaginal deliveries, race/ethnicity, stage of prolapse, and concomitant surgeries performed at time of the anti-incontinence procedure.
All four surgeries were associated with improvements in sexual function, with no statistically significant between-group differences. Mean PISQ-12 scores improved from a range of 31-33 to a range of 36-38 at 24 months. Although there is no published minimum important difference for PISQ-12 scores, an improvement of at least one-half of a standard deviation is generally accepted as clinically meaningful. “In this case, the standard deviation at baseline was just under 3 and so the improvement of each treatment group by more than 1.5 is a clinically meaningful improvement in their sexual function,” Dr. Clark said.
“Sexual dysfunction is a much more common problem than we previously thought, so we’ve been trying to figure out if patients with pelvic floor disorders like stress incontinence are going to have any improvement in sexual dysfunction by surgically treating their stress incontinence. Previously published data had been pretty conflicting,” Dr. Clark added in an interview.
That previous research was mostly retrospective and could have been impacted by patient selection bias. By analyzing clinical trials, the researchers hoped to test their idea that the pelvic floor symptoms themselves may be key to sexual dysfunction and that treating it surgically would improve matters.
The positive result is encouraging, but it still leaves unanswered questions about the mechanism behind the relationship. Dr. Clark wondered whether leaking urine leakage during sex might be the culprit, or whether it is fear or shame associated with the condition.
The answer may come from further analysis of women who were sexually inactive at baseline, but became sexually active over the course of the studies. “I think looking at that patient population in particular is going to be an interesting area of research. Is it that it was completely related to their pelvic floor disorder, and then we fixed it [so] they could have a more fulfilling sexual life?” speculated Dr. Clark.
The study received some funding from the National Institutes of Health. Dr. Clark reported no relevant financial disclosures.
SOURCE: Clark SG et al. SGS 2019, Oral Presentation 11.
TUCSON, ARIZ. –
The finding comes from a secondary analysis of two randomized, controlled trials comparing Burch colposuspension, autologous fascial slings, retropubic midurethral polypropylene slings, and transobturator midurethral polypropylene slings. The analysis looked at outcomes at 24 months after surgery. Stephanie Glass Clark, MD, a resident at Virginia Commonwealth University, Richmond, presented the results at the annual scientific meeting of the Society of Gynecologic Surgeons.
In the secondary analysis of the Stress Incontinence Surgical Treatment Efficacy Trial (SISTEr) and the Trial of Midurethral Slings (TOMUS) trials, Dr. Clark and her fellow researchers looked at the effect of surgical failure on sexual dysfunction outcomes. Subjective failure was defined as self-reported SUI symptoms or self-reported leakage by 3-day voiding diary beyond 3 months after the surgery. Objective failure was defined as any treatment for SUI after the surgery or a positive stress test or pad test beyond 3 months after the surgery.
Participants were excluded from the two studies if they were sexually inactive in the previous 6 months at baseline, at 12 months post baseline, or at 24 months. The studies employed the short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12), which had 12 questions with scores ranging from 0 to 4. The secondary analysis sample included 488 women from SISTEr and 436 women from TOMUS.
There were some baseline differences among groups between the two trials, including vaginal deliveries, race/ethnicity, stage of prolapse, and concomitant surgeries performed at time of the anti-incontinence procedure.
All four surgeries were associated with improvements in sexual function, with no statistically significant between-group differences. Mean PISQ-12 scores improved from a range of 31-33 to a range of 36-38 at 24 months. Although there is no published minimum important difference for PISQ-12 scores, an improvement of at least one-half of a standard deviation is generally accepted as clinically meaningful. “In this case, the standard deviation at baseline was just under 3 and so the improvement of each treatment group by more than 1.5 is a clinically meaningful improvement in their sexual function,” Dr. Clark said.
“Sexual dysfunction is a much more common problem than we previously thought, so we’ve been trying to figure out if patients with pelvic floor disorders like stress incontinence are going to have any improvement in sexual dysfunction by surgically treating their stress incontinence. Previously published data had been pretty conflicting,” Dr. Clark added in an interview.
That previous research was mostly retrospective and could have been impacted by patient selection bias. By analyzing clinical trials, the researchers hoped to test their idea that the pelvic floor symptoms themselves may be key to sexual dysfunction and that treating it surgically would improve matters.
The positive result is encouraging, but it still leaves unanswered questions about the mechanism behind the relationship. Dr. Clark wondered whether leaking urine leakage during sex might be the culprit, or whether it is fear or shame associated with the condition.
The answer may come from further analysis of women who were sexually inactive at baseline, but became sexually active over the course of the studies. “I think looking at that patient population in particular is going to be an interesting area of research. Is it that it was completely related to their pelvic floor disorder, and then we fixed it [so] they could have a more fulfilling sexual life?” speculated Dr. Clark.
The study received some funding from the National Institutes of Health. Dr. Clark reported no relevant financial disclosures.
SOURCE: Clark SG et al. SGS 2019, Oral Presentation 11.
TUCSON, ARIZ. –
The finding comes from a secondary analysis of two randomized, controlled trials comparing Burch colposuspension, autologous fascial slings, retropubic midurethral polypropylene slings, and transobturator midurethral polypropylene slings. The analysis looked at outcomes at 24 months after surgery. Stephanie Glass Clark, MD, a resident at Virginia Commonwealth University, Richmond, presented the results at the annual scientific meeting of the Society of Gynecologic Surgeons.
In the secondary analysis of the Stress Incontinence Surgical Treatment Efficacy Trial (SISTEr) and the Trial of Midurethral Slings (TOMUS) trials, Dr. Clark and her fellow researchers looked at the effect of surgical failure on sexual dysfunction outcomes. Subjective failure was defined as self-reported SUI symptoms or self-reported leakage by 3-day voiding diary beyond 3 months after the surgery. Objective failure was defined as any treatment for SUI after the surgery or a positive stress test or pad test beyond 3 months after the surgery.
Participants were excluded from the two studies if they were sexually inactive in the previous 6 months at baseline, at 12 months post baseline, or at 24 months. The studies employed the short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12), which had 12 questions with scores ranging from 0 to 4. The secondary analysis sample included 488 women from SISTEr and 436 women from TOMUS.
There were some baseline differences among groups between the two trials, including vaginal deliveries, race/ethnicity, stage of prolapse, and concomitant surgeries performed at time of the anti-incontinence procedure.
All four surgeries were associated with improvements in sexual function, with no statistically significant between-group differences. Mean PISQ-12 scores improved from a range of 31-33 to a range of 36-38 at 24 months. Although there is no published minimum important difference for PISQ-12 scores, an improvement of at least one-half of a standard deviation is generally accepted as clinically meaningful. “In this case, the standard deviation at baseline was just under 3 and so the improvement of each treatment group by more than 1.5 is a clinically meaningful improvement in their sexual function,” Dr. Clark said.
“Sexual dysfunction is a much more common problem than we previously thought, so we’ve been trying to figure out if patients with pelvic floor disorders like stress incontinence are going to have any improvement in sexual dysfunction by surgically treating their stress incontinence. Previously published data had been pretty conflicting,” Dr. Clark added in an interview.
That previous research was mostly retrospective and could have been impacted by patient selection bias. By analyzing clinical trials, the researchers hoped to test their idea that the pelvic floor symptoms themselves may be key to sexual dysfunction and that treating it surgically would improve matters.
The positive result is encouraging, but it still leaves unanswered questions about the mechanism behind the relationship. Dr. Clark wondered whether leaking urine leakage during sex might be the culprit, or whether it is fear or shame associated with the condition.
The answer may come from further analysis of women who were sexually inactive at baseline, but became sexually active over the course of the studies. “I think looking at that patient population in particular is going to be an interesting area of research. Is it that it was completely related to their pelvic floor disorder, and then we fixed it [so] they could have a more fulfilling sexual life?” speculated Dr. Clark.
The study received some funding from the National Institutes of Health. Dr. Clark reported no relevant financial disclosures.
SOURCE: Clark SG et al. SGS 2019, Oral Presentation 11.
REPORTING FROM SGS 2019
Older women have good functional recovery after POP surgery
TUCSON, ARIZ. – according to a new study.
Functional status was defined as the ability to perform activities essential to self-care, independent living, and recreation.
Patients with the highest level of function at baseline actually had a slight decrease in functionality scores, although this difference was not clinically meaningful.
“We can tell patients: You’re high functioning, your prolapse is bothering you in other ways, but we’re going to keep you as high functioning as you are now. I think that’s really important for older women who are retired and want to stay active. They want to make sure they don’t have a surgery that’s going to make them less so,” Daniel Lee, MD, of the University of Pennsylvania, Philadelphia, said in an interview. Dr. Lee presented the study at the annual scientific meeting of the Society of Gynecologic Surgeons.
The study used data from multiple centers across the United States and a range of patient ethnicities. “That helped strengthen the conclusions,” Dr. Lee said. Most previous studies used generalized questionnaires rather than functional outcome questionnaires to determine patient outcomes.
One confounder is the potential presence of cognitive dysfunction, which can occur sometimes in older women following surgery. The study relied on surveys and excluded patients who weren’t able to understand them. Two patients were much worse after the surgery, and Dr. Lee speculated that cognitive dysfunction could have been the cause, although the team could not confirm that. “There’s a possibility that preexisting dementia or cognitive dysfunction could be unmasked by the surgery,” he said. The team is working to incorporate accelerometers to more objectively measure outcomes and will soon publish a feasibility study of their use in older women with cognitive dysfunction.
One limitation of the study was that it excluded women who were considered poor candidates for surgery. But that also suggests that patient selection is working as intended. “I think it just shows that surgeons do a very good job of figuring out who is a good surgical candidate, that they turn out to be better off [functionally] or that their prolapse gets improved,” Dr. Lee said.
The researchers analyzed questionnaire data from 176 women. The mean age was 72 years, and mean body mass index was 27 kg/m2; 87% of the women were Caucasian and 10% were black. Using the Activities Assessment Scale (AAS), which covers sedentary, ambulatory, and work/exercise domains, as well as the Patient Health Questionnaire to measure depression, the researchers found that, by 3 months, 59% of patients had an improved functional status, 35% had returned to within one standard deviation of baseline, and 6% had worsened, compared with their baseline scores.
Patients in the improved group started at a mean baseline total AAS score of 85 and improved to 100 at 3 months. Those who returned to baseline started at 100 on average and returned to 100 at 3 months, while those in the worsened group had a mean baseline score of 100 and a mean score of 93 at 3 months (P less than .001 for all comparisons).
The study was funded by the Fellows Pelvic Research Network. The investigators reported no relevant financial disclosures.
SOURCE: Lee D et al. SGS 2019, Abstract 09.
TUCSON, ARIZ. – according to a new study.
Functional status was defined as the ability to perform activities essential to self-care, independent living, and recreation.
Patients with the highest level of function at baseline actually had a slight decrease in functionality scores, although this difference was not clinically meaningful.
“We can tell patients: You’re high functioning, your prolapse is bothering you in other ways, but we’re going to keep you as high functioning as you are now. I think that’s really important for older women who are retired and want to stay active. They want to make sure they don’t have a surgery that’s going to make them less so,” Daniel Lee, MD, of the University of Pennsylvania, Philadelphia, said in an interview. Dr. Lee presented the study at the annual scientific meeting of the Society of Gynecologic Surgeons.
The study used data from multiple centers across the United States and a range of patient ethnicities. “That helped strengthen the conclusions,” Dr. Lee said. Most previous studies used generalized questionnaires rather than functional outcome questionnaires to determine patient outcomes.
One confounder is the potential presence of cognitive dysfunction, which can occur sometimes in older women following surgery. The study relied on surveys and excluded patients who weren’t able to understand them. Two patients were much worse after the surgery, and Dr. Lee speculated that cognitive dysfunction could have been the cause, although the team could not confirm that. “There’s a possibility that preexisting dementia or cognitive dysfunction could be unmasked by the surgery,” he said. The team is working to incorporate accelerometers to more objectively measure outcomes and will soon publish a feasibility study of their use in older women with cognitive dysfunction.
One limitation of the study was that it excluded women who were considered poor candidates for surgery. But that also suggests that patient selection is working as intended. “I think it just shows that surgeons do a very good job of figuring out who is a good surgical candidate, that they turn out to be better off [functionally] or that their prolapse gets improved,” Dr. Lee said.
The researchers analyzed questionnaire data from 176 women. The mean age was 72 years, and mean body mass index was 27 kg/m2; 87% of the women were Caucasian and 10% were black. Using the Activities Assessment Scale (AAS), which covers sedentary, ambulatory, and work/exercise domains, as well as the Patient Health Questionnaire to measure depression, the researchers found that, by 3 months, 59% of patients had an improved functional status, 35% had returned to within one standard deviation of baseline, and 6% had worsened, compared with their baseline scores.
Patients in the improved group started at a mean baseline total AAS score of 85 and improved to 100 at 3 months. Those who returned to baseline started at 100 on average and returned to 100 at 3 months, while those in the worsened group had a mean baseline score of 100 and a mean score of 93 at 3 months (P less than .001 for all comparisons).
The study was funded by the Fellows Pelvic Research Network. The investigators reported no relevant financial disclosures.
SOURCE: Lee D et al. SGS 2019, Abstract 09.
TUCSON, ARIZ. – according to a new study.
Functional status was defined as the ability to perform activities essential to self-care, independent living, and recreation.
Patients with the highest level of function at baseline actually had a slight decrease in functionality scores, although this difference was not clinically meaningful.
“We can tell patients: You’re high functioning, your prolapse is bothering you in other ways, but we’re going to keep you as high functioning as you are now. I think that’s really important for older women who are retired and want to stay active. They want to make sure they don’t have a surgery that’s going to make them less so,” Daniel Lee, MD, of the University of Pennsylvania, Philadelphia, said in an interview. Dr. Lee presented the study at the annual scientific meeting of the Society of Gynecologic Surgeons.
The study used data from multiple centers across the United States and a range of patient ethnicities. “That helped strengthen the conclusions,” Dr. Lee said. Most previous studies used generalized questionnaires rather than functional outcome questionnaires to determine patient outcomes.
One confounder is the potential presence of cognitive dysfunction, which can occur sometimes in older women following surgery. The study relied on surveys and excluded patients who weren’t able to understand them. Two patients were much worse after the surgery, and Dr. Lee speculated that cognitive dysfunction could have been the cause, although the team could not confirm that. “There’s a possibility that preexisting dementia or cognitive dysfunction could be unmasked by the surgery,” he said. The team is working to incorporate accelerometers to more objectively measure outcomes and will soon publish a feasibility study of their use in older women with cognitive dysfunction.
One limitation of the study was that it excluded women who were considered poor candidates for surgery. But that also suggests that patient selection is working as intended. “I think it just shows that surgeons do a very good job of figuring out who is a good surgical candidate, that they turn out to be better off [functionally] or that their prolapse gets improved,” Dr. Lee said.
The researchers analyzed questionnaire data from 176 women. The mean age was 72 years, and mean body mass index was 27 kg/m2; 87% of the women were Caucasian and 10% were black. Using the Activities Assessment Scale (AAS), which covers sedentary, ambulatory, and work/exercise domains, as well as the Patient Health Questionnaire to measure depression, the researchers found that, by 3 months, 59% of patients had an improved functional status, 35% had returned to within one standard deviation of baseline, and 6% had worsened, compared with their baseline scores.
Patients in the improved group started at a mean baseline total AAS score of 85 and improved to 100 at 3 months. Those who returned to baseline started at 100 on average and returned to 100 at 3 months, while those in the worsened group had a mean baseline score of 100 and a mean score of 93 at 3 months (P less than .001 for all comparisons).
The study was funded by the Fellows Pelvic Research Network. The investigators reported no relevant financial disclosures.
SOURCE: Lee D et al. SGS 2019, Abstract 09.
REPORTING FROM SGS 2019
FDA panel tackles mesh for anterior repair of POP
Patient-reported outcomes should be the priority consideration for determining whether the three synthetic mesh devices currently available for transvaginal repair of pelvic organ prolapse (POP) in the anterior vaginal compartment should remain on the market, according to the Food and Drug Administration Obstetrics and Gynecology Devices panel.
The panel was convened in February 2019 to advise the Food and Drug Administration on how it should evaluate the safety and effectiveness of the three currently marketed devices – each of which has ongoing postmarket surveillance studies – as well as any other similar devices that come up for premarket approval in the future.
The panel’s main messages: Subjective outcomes are what really matter – even more so than anatomic or objective outcomes – as does long-term follow-up.
“ “compared to native tissue repair,” said panel chair Keith Isaacson, MD, medical director of the Newton-Wellesley Hospital in Newton, Mass. “But we feel that, if we had to score [each category of outcome], about 75% should be subjective.”
The three devices currently marketed for transvaginal repair of POP (Boston Scientific’s Uphold LITE and Xenform, as well as Coloplast’s Restorelle DirectFix Anterior) are being scrutinized under a new regulatory paradigm and amid a charged backdrop of safety warnings and years of lawsuits regarding debilitating complications following surgeries that involved the implantation of synthetic vaginal mesh.
The two manufacturers of the currently available devices launched postmarket surveillance studies, called 522 studies, after the FDA issued postmarket surveillance study orders in 2012 to all manufacturers of surgical mesh for transvaginal repair of POP. (Most companies chose at the time to pull their products from the market.) This FDA action, along with a reclassification of the devices from class II to the high-risk class III, had been recommended at a 2011 meeting of the Obstetrics and Gynecology Devices panel.
In anticipation of a future reclassification, the 522 studies were designed at the time to support future premarket approval (PMA) applications, as advised by the FDA. Now, as a result of the 2016 reclassification of surgical mesh for transvaginal POP repair to class III – and the companies’ subsequent PMA applications – the FDA is reviewing the ongoing postmarket study results with a PMA lens to determine each device’s benefit/risk profile.
It’s a challenging assessment to make, FDA officials said.
The agency reported to the panel that a search of medical device reports from 2008 to 2018 identified 11,274 adverse events associated with mesh placed in the anterior vaginal compartment to treat POP. These included 10,391 reports of serious injury, 806 reports of device malfunctions, and 77 reports of death.
Findings from an FDA literature review covering the same period and also focusing on anterior and/or apical repair show that synthetic mesh may have some advantage over native tissue repair for objective effectiveness outcomes – but not necessarily subjective outcomes – over 1-3 years of follow-up. And the risks of using mesh are greater, particularly with respect to reoperation for recurrence and mesh complications, the latter of which continued beyond the first year of follow-up and through 5 years, the agency said.
Although the review may help the FDA frame its questions moving forward, it has limited utility beyond that, according to urogynecologic surgeons who testified on behalf of three professional societies. The review does not delineate differences between the newer materials used today and older mesh materials that were of heavier weight/higher mesh density and often placed using more invasive delivery systems. Nor does it offer any insight on the use of mesh for secondary repair.
“Much of the existing data on the use of transvaginal mesh in POP surgery comes from low to moderate quality, short-term studies of synthetic mesh that is no longer used in clinical practice,” said Cheryl Iglesia, MD, a Washington-based ob.gyn. who spoke to the advisory panel on behalf of the American College of Obstetricians and Gynecologists. “There’s a critical need for data from high-quality studies on the use of the newer, lightweight type 1 transvaginal meshes used in POP surgery.”
The FDA’s 522 orders requested that manufacturers conduct a randomized, controlled study or parallel cohort study comparing their device to native tissue repair. Requested effectiveness endpoints included anatomic success, subjective success, and retreatment for prolapse. For safety endpoints, the agency requested all device- and procedure-related adverse events, as well as the rate of individual adverse events, such as mesh erosion and de novo dyspareunia and urinary dysfunction. The FDA asked for all endpoints at 6-month intervals out to 24 months and at 36 months.
The panel advised that superiority should be the standard for the general population of women with POP – that mesh used in the anterior/apical vaginal compartment should be shown to be superior to native tissue repair at each time point. In specific patient populations for whom native tissue repair is not deemed feasible or appropriate, demonstrating equivalence is sufficient, they advised.
They called for “more diligent” presurgical assessments of sexual function and activity, as well as other symptoms that will be assessed later. And the panel agreed with the FDA that concomitant procedures (for example, hysterectomy and sling placement) and certain preexisting medical conditions and patient characteristics (such as obesity and diabetes) can affect outcomes and should be delineated and considered in the FDA’s evaluations and interpretation of study results.
Regarding surgeon characteristics, the panel’s biostatisticians and physicians (largely urogynecologists, but also one community ob.gyn.) advised the FDA to pay attention to surgeon training, experience, and volume, but they declined to offer any specific recommendations. Discussions often came back to the value of a registry that would capture both surgeon data and patient experience. And throughout the panel’s discussion, surgeons stepped away from the main questions at hand and emphasized the individualized nature of risk-benefit ratios and decision making.
Registries have been successfully used for cardiology and orthopedic implants and, within obstetrics and gynecology, for assisted reproductive technologies, Dr. Iglesia said in an interview after the meeting. “We have models … we just need to make it easy for physicians, using our EMRs. But I’m hopeful.”
The American Urogynecologic Society (AUGS) operates a quality improvement registry (AQUIRE) that is collecting information on surgical and nonsurgical treatment of POP and stress urinary incontinence – including surgical complications – from a diverse group of physicians, not just those at academic medical centers. AUGS is growing its registry this year to include device identifiers and patient-reported outcomes that are sent directly to the registry by the patient.
The panel generally agreed that postmarket follow-up of synthetic mesh for transvaginal anterior repair of POP should extend up to 5 years, Dr. Isaacson said, though “from the patients’ perspective, 10 years of experience [is meaningful].”
Geoffrey Cundiff, MD, who is AUGS president, told the committee that there are lessons to be gleaned from the CARE trial, which looked at outcomes up to 7 years after abdominal sacrocolpopexy (JAMA. 2013 May 15;309[19]:2016-24). “At 7 years, the complications [including rates of mesh erosion] had increased,” he said. “It’s a different procedure, but it’s a good example.”
Prior to its deliberations, the panel heard preliminary results of the ongoing SUPeR trial (Female Pelvic Med Reconstr Surg. 2016 Jul-Aug;22[4]:182-9), a randomized, controlled superiority trial of vaginal hysterectomy with suture apical suspension versus uterine conservation with vaginal mesh (Boston Scientific’s Uphold LITE) hysteropexy for uterovaginal prolapse. Researchers have found comparable rates of primary outcome success – no objective prolapse beyond the hymen, no retreatment, and no bulge symptoms – through 36 months and no differences in patient-reported outcomes thus far.
Hysteroplexy mesh exposure rates were approximately 8% at 36 months, and suture exposure and excessive granulation were 11%-20% in the hysterectomy group. None of these exposure cases has required reoperation. Both groups have shown improvements in sexual function and decreases in dyspareunia, said Charles W. Nager, MD, a San Diego ob.gyn. who is primary investigator of the trial.
The trial is sponsored by the Pelvic Floor Disorders Network of the National Institute of Child Health and Human Development, as was the CARE trial of abdominal sacrocolpopexy. It is following patients for 60 months and collecting data every 6 months, including data from validated functional and quality of life assessments. Patients were masked to their treatment assignment to eliminate patient reporting bias. At 36 months, approximately three-quarters of the patients in each group remained masked.
In addition to the ongoing 522 studies for anterior/apical prolapse, there is another 522 study underway of a mesh device designed for transvaginal repair of total prolapse (the Acell Matristem Pelvic Floor Repair Matrix). In addition, Coloplast is studying a mesh device designed for posterior/apical prolapse (Restorelle DirectFix) as part of its 522 study. Neither device is being marketed currently, however.
Patient-reported outcomes should be the priority consideration for determining whether the three synthetic mesh devices currently available for transvaginal repair of pelvic organ prolapse (POP) in the anterior vaginal compartment should remain on the market, according to the Food and Drug Administration Obstetrics and Gynecology Devices panel.
The panel was convened in February 2019 to advise the Food and Drug Administration on how it should evaluate the safety and effectiveness of the three currently marketed devices – each of which has ongoing postmarket surveillance studies – as well as any other similar devices that come up for premarket approval in the future.
The panel’s main messages: Subjective outcomes are what really matter – even more so than anatomic or objective outcomes – as does long-term follow-up.
“ “compared to native tissue repair,” said panel chair Keith Isaacson, MD, medical director of the Newton-Wellesley Hospital in Newton, Mass. “But we feel that, if we had to score [each category of outcome], about 75% should be subjective.”
The three devices currently marketed for transvaginal repair of POP (Boston Scientific’s Uphold LITE and Xenform, as well as Coloplast’s Restorelle DirectFix Anterior) are being scrutinized under a new regulatory paradigm and amid a charged backdrop of safety warnings and years of lawsuits regarding debilitating complications following surgeries that involved the implantation of synthetic vaginal mesh.
The two manufacturers of the currently available devices launched postmarket surveillance studies, called 522 studies, after the FDA issued postmarket surveillance study orders in 2012 to all manufacturers of surgical mesh for transvaginal repair of POP. (Most companies chose at the time to pull their products from the market.) This FDA action, along with a reclassification of the devices from class II to the high-risk class III, had been recommended at a 2011 meeting of the Obstetrics and Gynecology Devices panel.
In anticipation of a future reclassification, the 522 studies were designed at the time to support future premarket approval (PMA) applications, as advised by the FDA. Now, as a result of the 2016 reclassification of surgical mesh for transvaginal POP repair to class III – and the companies’ subsequent PMA applications – the FDA is reviewing the ongoing postmarket study results with a PMA lens to determine each device’s benefit/risk profile.
It’s a challenging assessment to make, FDA officials said.
The agency reported to the panel that a search of medical device reports from 2008 to 2018 identified 11,274 adverse events associated with mesh placed in the anterior vaginal compartment to treat POP. These included 10,391 reports of serious injury, 806 reports of device malfunctions, and 77 reports of death.
Findings from an FDA literature review covering the same period and also focusing on anterior and/or apical repair show that synthetic mesh may have some advantage over native tissue repair for objective effectiveness outcomes – but not necessarily subjective outcomes – over 1-3 years of follow-up. And the risks of using mesh are greater, particularly with respect to reoperation for recurrence and mesh complications, the latter of which continued beyond the first year of follow-up and through 5 years, the agency said.
Although the review may help the FDA frame its questions moving forward, it has limited utility beyond that, according to urogynecologic surgeons who testified on behalf of three professional societies. The review does not delineate differences between the newer materials used today and older mesh materials that were of heavier weight/higher mesh density and often placed using more invasive delivery systems. Nor does it offer any insight on the use of mesh for secondary repair.
“Much of the existing data on the use of transvaginal mesh in POP surgery comes from low to moderate quality, short-term studies of synthetic mesh that is no longer used in clinical practice,” said Cheryl Iglesia, MD, a Washington-based ob.gyn. who spoke to the advisory panel on behalf of the American College of Obstetricians and Gynecologists. “There’s a critical need for data from high-quality studies on the use of the newer, lightweight type 1 transvaginal meshes used in POP surgery.”
The FDA’s 522 orders requested that manufacturers conduct a randomized, controlled study or parallel cohort study comparing their device to native tissue repair. Requested effectiveness endpoints included anatomic success, subjective success, and retreatment for prolapse. For safety endpoints, the agency requested all device- and procedure-related adverse events, as well as the rate of individual adverse events, such as mesh erosion and de novo dyspareunia and urinary dysfunction. The FDA asked for all endpoints at 6-month intervals out to 24 months and at 36 months.
The panel advised that superiority should be the standard for the general population of women with POP – that mesh used in the anterior/apical vaginal compartment should be shown to be superior to native tissue repair at each time point. In specific patient populations for whom native tissue repair is not deemed feasible or appropriate, demonstrating equivalence is sufficient, they advised.
They called for “more diligent” presurgical assessments of sexual function and activity, as well as other symptoms that will be assessed later. And the panel agreed with the FDA that concomitant procedures (for example, hysterectomy and sling placement) and certain preexisting medical conditions and patient characteristics (such as obesity and diabetes) can affect outcomes and should be delineated and considered in the FDA’s evaluations and interpretation of study results.
Regarding surgeon characteristics, the panel’s biostatisticians and physicians (largely urogynecologists, but also one community ob.gyn.) advised the FDA to pay attention to surgeon training, experience, and volume, but they declined to offer any specific recommendations. Discussions often came back to the value of a registry that would capture both surgeon data and patient experience. And throughout the panel’s discussion, surgeons stepped away from the main questions at hand and emphasized the individualized nature of risk-benefit ratios and decision making.
Registries have been successfully used for cardiology and orthopedic implants and, within obstetrics and gynecology, for assisted reproductive technologies, Dr. Iglesia said in an interview after the meeting. “We have models … we just need to make it easy for physicians, using our EMRs. But I’m hopeful.”
The American Urogynecologic Society (AUGS) operates a quality improvement registry (AQUIRE) that is collecting information on surgical and nonsurgical treatment of POP and stress urinary incontinence – including surgical complications – from a diverse group of physicians, not just those at academic medical centers. AUGS is growing its registry this year to include device identifiers and patient-reported outcomes that are sent directly to the registry by the patient.
The panel generally agreed that postmarket follow-up of synthetic mesh for transvaginal anterior repair of POP should extend up to 5 years, Dr. Isaacson said, though “from the patients’ perspective, 10 years of experience [is meaningful].”
Geoffrey Cundiff, MD, who is AUGS president, told the committee that there are lessons to be gleaned from the CARE trial, which looked at outcomes up to 7 years after abdominal sacrocolpopexy (JAMA. 2013 May 15;309[19]:2016-24). “At 7 years, the complications [including rates of mesh erosion] had increased,” he said. “It’s a different procedure, but it’s a good example.”
Prior to its deliberations, the panel heard preliminary results of the ongoing SUPeR trial (Female Pelvic Med Reconstr Surg. 2016 Jul-Aug;22[4]:182-9), a randomized, controlled superiority trial of vaginal hysterectomy with suture apical suspension versus uterine conservation with vaginal mesh (Boston Scientific’s Uphold LITE) hysteropexy for uterovaginal prolapse. Researchers have found comparable rates of primary outcome success – no objective prolapse beyond the hymen, no retreatment, and no bulge symptoms – through 36 months and no differences in patient-reported outcomes thus far.
Hysteroplexy mesh exposure rates were approximately 8% at 36 months, and suture exposure and excessive granulation were 11%-20% in the hysterectomy group. None of these exposure cases has required reoperation. Both groups have shown improvements in sexual function and decreases in dyspareunia, said Charles W. Nager, MD, a San Diego ob.gyn. who is primary investigator of the trial.
The trial is sponsored by the Pelvic Floor Disorders Network of the National Institute of Child Health and Human Development, as was the CARE trial of abdominal sacrocolpopexy. It is following patients for 60 months and collecting data every 6 months, including data from validated functional and quality of life assessments. Patients were masked to their treatment assignment to eliminate patient reporting bias. At 36 months, approximately three-quarters of the patients in each group remained masked.
In addition to the ongoing 522 studies for anterior/apical prolapse, there is another 522 study underway of a mesh device designed for transvaginal repair of total prolapse (the Acell Matristem Pelvic Floor Repair Matrix). In addition, Coloplast is studying a mesh device designed for posterior/apical prolapse (Restorelle DirectFix) as part of its 522 study. Neither device is being marketed currently, however.
Patient-reported outcomes should be the priority consideration for determining whether the three synthetic mesh devices currently available for transvaginal repair of pelvic organ prolapse (POP) in the anterior vaginal compartment should remain on the market, according to the Food and Drug Administration Obstetrics and Gynecology Devices panel.
The panel was convened in February 2019 to advise the Food and Drug Administration on how it should evaluate the safety and effectiveness of the three currently marketed devices – each of which has ongoing postmarket surveillance studies – as well as any other similar devices that come up for premarket approval in the future.
The panel’s main messages: Subjective outcomes are what really matter – even more so than anatomic or objective outcomes – as does long-term follow-up.
“ “compared to native tissue repair,” said panel chair Keith Isaacson, MD, medical director of the Newton-Wellesley Hospital in Newton, Mass. “But we feel that, if we had to score [each category of outcome], about 75% should be subjective.”
The three devices currently marketed for transvaginal repair of POP (Boston Scientific’s Uphold LITE and Xenform, as well as Coloplast’s Restorelle DirectFix Anterior) are being scrutinized under a new regulatory paradigm and amid a charged backdrop of safety warnings and years of lawsuits regarding debilitating complications following surgeries that involved the implantation of synthetic vaginal mesh.
The two manufacturers of the currently available devices launched postmarket surveillance studies, called 522 studies, after the FDA issued postmarket surveillance study orders in 2012 to all manufacturers of surgical mesh for transvaginal repair of POP. (Most companies chose at the time to pull their products from the market.) This FDA action, along with a reclassification of the devices from class II to the high-risk class III, had been recommended at a 2011 meeting of the Obstetrics and Gynecology Devices panel.
In anticipation of a future reclassification, the 522 studies were designed at the time to support future premarket approval (PMA) applications, as advised by the FDA. Now, as a result of the 2016 reclassification of surgical mesh for transvaginal POP repair to class III – and the companies’ subsequent PMA applications – the FDA is reviewing the ongoing postmarket study results with a PMA lens to determine each device’s benefit/risk profile.
It’s a challenging assessment to make, FDA officials said.
The agency reported to the panel that a search of medical device reports from 2008 to 2018 identified 11,274 adverse events associated with mesh placed in the anterior vaginal compartment to treat POP. These included 10,391 reports of serious injury, 806 reports of device malfunctions, and 77 reports of death.
Findings from an FDA literature review covering the same period and also focusing on anterior and/or apical repair show that synthetic mesh may have some advantage over native tissue repair for objective effectiveness outcomes – but not necessarily subjective outcomes – over 1-3 years of follow-up. And the risks of using mesh are greater, particularly with respect to reoperation for recurrence and mesh complications, the latter of which continued beyond the first year of follow-up and through 5 years, the agency said.
Although the review may help the FDA frame its questions moving forward, it has limited utility beyond that, according to urogynecologic surgeons who testified on behalf of three professional societies. The review does not delineate differences between the newer materials used today and older mesh materials that were of heavier weight/higher mesh density and often placed using more invasive delivery systems. Nor does it offer any insight on the use of mesh for secondary repair.
“Much of the existing data on the use of transvaginal mesh in POP surgery comes from low to moderate quality, short-term studies of synthetic mesh that is no longer used in clinical practice,” said Cheryl Iglesia, MD, a Washington-based ob.gyn. who spoke to the advisory panel on behalf of the American College of Obstetricians and Gynecologists. “There’s a critical need for data from high-quality studies on the use of the newer, lightweight type 1 transvaginal meshes used in POP surgery.”
The FDA’s 522 orders requested that manufacturers conduct a randomized, controlled study or parallel cohort study comparing their device to native tissue repair. Requested effectiveness endpoints included anatomic success, subjective success, and retreatment for prolapse. For safety endpoints, the agency requested all device- and procedure-related adverse events, as well as the rate of individual adverse events, such as mesh erosion and de novo dyspareunia and urinary dysfunction. The FDA asked for all endpoints at 6-month intervals out to 24 months and at 36 months.
The panel advised that superiority should be the standard for the general population of women with POP – that mesh used in the anterior/apical vaginal compartment should be shown to be superior to native tissue repair at each time point. In specific patient populations for whom native tissue repair is not deemed feasible or appropriate, demonstrating equivalence is sufficient, they advised.
They called for “more diligent” presurgical assessments of sexual function and activity, as well as other symptoms that will be assessed later. And the panel agreed with the FDA that concomitant procedures (for example, hysterectomy and sling placement) and certain preexisting medical conditions and patient characteristics (such as obesity and diabetes) can affect outcomes and should be delineated and considered in the FDA’s evaluations and interpretation of study results.
Regarding surgeon characteristics, the panel’s biostatisticians and physicians (largely urogynecologists, but also one community ob.gyn.) advised the FDA to pay attention to surgeon training, experience, and volume, but they declined to offer any specific recommendations. Discussions often came back to the value of a registry that would capture both surgeon data and patient experience. And throughout the panel’s discussion, surgeons stepped away from the main questions at hand and emphasized the individualized nature of risk-benefit ratios and decision making.
Registries have been successfully used for cardiology and orthopedic implants and, within obstetrics and gynecology, for assisted reproductive technologies, Dr. Iglesia said in an interview after the meeting. “We have models … we just need to make it easy for physicians, using our EMRs. But I’m hopeful.”
The American Urogynecologic Society (AUGS) operates a quality improvement registry (AQUIRE) that is collecting information on surgical and nonsurgical treatment of POP and stress urinary incontinence – including surgical complications – from a diverse group of physicians, not just those at academic medical centers. AUGS is growing its registry this year to include device identifiers and patient-reported outcomes that are sent directly to the registry by the patient.
The panel generally agreed that postmarket follow-up of synthetic mesh for transvaginal anterior repair of POP should extend up to 5 years, Dr. Isaacson said, though “from the patients’ perspective, 10 years of experience [is meaningful].”
Geoffrey Cundiff, MD, who is AUGS president, told the committee that there are lessons to be gleaned from the CARE trial, which looked at outcomes up to 7 years after abdominal sacrocolpopexy (JAMA. 2013 May 15;309[19]:2016-24). “At 7 years, the complications [including rates of mesh erosion] had increased,” he said. “It’s a different procedure, but it’s a good example.”
Prior to its deliberations, the panel heard preliminary results of the ongoing SUPeR trial (Female Pelvic Med Reconstr Surg. 2016 Jul-Aug;22[4]:182-9), a randomized, controlled superiority trial of vaginal hysterectomy with suture apical suspension versus uterine conservation with vaginal mesh (Boston Scientific’s Uphold LITE) hysteropexy for uterovaginal prolapse. Researchers have found comparable rates of primary outcome success – no objective prolapse beyond the hymen, no retreatment, and no bulge symptoms – through 36 months and no differences in patient-reported outcomes thus far.
Hysteroplexy mesh exposure rates were approximately 8% at 36 months, and suture exposure and excessive granulation were 11%-20% in the hysterectomy group. None of these exposure cases has required reoperation. Both groups have shown improvements in sexual function and decreases in dyspareunia, said Charles W. Nager, MD, a San Diego ob.gyn. who is primary investigator of the trial.
The trial is sponsored by the Pelvic Floor Disorders Network of the National Institute of Child Health and Human Development, as was the CARE trial of abdominal sacrocolpopexy. It is following patients for 60 months and collecting data every 6 months, including data from validated functional and quality of life assessments. Patients were masked to their treatment assignment to eliminate patient reporting bias. At 36 months, approximately three-quarters of the patients in each group remained masked.
In addition to the ongoing 522 studies for anterior/apical prolapse, there is another 522 study underway of a mesh device designed for transvaginal repair of total prolapse (the Acell Matristem Pelvic Floor Repair Matrix). In addition, Coloplast is studying a mesh device designed for posterior/apical prolapse (Restorelle DirectFix) as part of its 522 study. Neither device is being marketed currently, however.
Surgeon: Sacral colpopexy can be smart strategy in POP repairs
LAS VEGAS – While research suggests that vaginal mesh grafts are inappropriate for many prolapse repairs, an obstetrician-gynecologist told colleagues that they’re still a valid tool in the repair procedure known as sacral colpopexy, in which mesh is attached via an abdominal route.
Beri M. Ridgeway, MD, of Cleveland Clinic, spoke about the role of mesh grafts and prolapse repairs at the Pelvic Anatomy and Gynecologic Surgery Symposium.
As Dr. Ridgeway noted, vaginal mesh grafts are controversial because of concerns about their safety. Although many women had favorable outcomes, an unacceptable proportion have experienced complications.
In 2011, the Food and Drug Administration warned that urogynecologic surgical mesh had been linked to 2,874 reports of injuries, deaths, and malfunctions, mostly in pelvic organ prolapse (POP) repairs, over 3 years. The other injuries were in stress urinary incontinence repairs. The report focuses on transvaginal mesh for prolapse and not sacral colpopexy or synthetic midurethral slings, which are considered to have a more favorable risk profile.
The FDA declared that “serious adverse events are NOT rare ... and transvaginally placed mesh in POP repair does NOT conclusively improve clinical outcomes over traditional non-mesh repair.” Subsequently, most companies stopped marketing mesh for transvaginal repair of POP.
Since 2011, research has offered new perspective on the use of mesh in specific POP situations.
“We know that mesh does have some slight improvement in medium-term outcome for subjective and objective symptoms,” Dr. Ridgeway said at the meeting, which was jointly provided by Global Academy for Medical Education and the University of Cincinnati. “This all comes at a price. There’s more blood loss, and you can actually have prolapse in other compartments and de novo SUI.”
She pointed out that these outcomes were noted in a 2013 Cochrane Review. It found improvements in subjective and objective results after treatment with polypropylene mesh vs. native tissue for anterior compartment POP repairs. But the review found multiple disadvantages for mesh vs. native tissue in operating time, blood loss, and reoperations (Cochrane Database Syst Rev. 2013 Apr 30;[4]:CD004014).
In 2016, an updated Cochrane Review declared that “current evidence does not support the use of mesh repair compared with native tissue repair for anterior compartment prolapse owing to increased morbidity.” The review also cautioned that while new light-weight transvaginal meshes are available, they haven’t been fully studied. “Clinicians and women should be cautious when utilizing these products, as their safety and efficacy have not been established,” according to the review (Cochrane Database of Syst Rev. 2016[11];CD004014).
In a follow-up interview, Dr. Ridgeway said “the data are scarce, so it is hard to have an opinion on this.”
She focused much of her presentation on sacral colpopexy. .
“Compared to native tissue prolapse repair using a vaginal approach, sacral colpopexy does have an increased risk profile but likely is associated with better durability,” she said in the interview. “The long-term outcomes following sacral colpopexy are favorable and the risk profile is acceptably low.”
She prefers the approach for recurrent prolapse and post-hysterectomy prolapse, especially in patients with a shorter vagina. She also offers this procedure for younger patients with significant prolapse and those women who are very active or perform repetitive heavy lifting.
In the interview, she offered these tips about the procedure:
- “Identify pertinent anatomy and set yourself up for success. Restore anatomy, retract the colon if necessary, use angled laparoscopes to optimize visualization, and don’t place the vagina on significant tension.”
- “In cases with unusual anatomy, one must recheck anatomic landmarks because it is critical to avoid the middle sacral artery and left common iliac vein, which is often located close to the midline.”
- “The vagina should be well supported but not on tension. One must communicate with assistants to elevate the vagina but not push it too much. I often demonstrate to the assistant how I like it to be.”
- “In regard to closing the peritoneum over the mesh, I like to make sure this dissection is sufficient at the beginning of the case so this part is not a struggle.”
Dr. Ridgeway discloses consulting for Coloplast and serving as an independent contractor (Legal) for Ethicon.
Global Academy and this news organization are owned by the same company.
LAS VEGAS – While research suggests that vaginal mesh grafts are inappropriate for many prolapse repairs, an obstetrician-gynecologist told colleagues that they’re still a valid tool in the repair procedure known as sacral colpopexy, in which mesh is attached via an abdominal route.
Beri M. Ridgeway, MD, of Cleveland Clinic, spoke about the role of mesh grafts and prolapse repairs at the Pelvic Anatomy and Gynecologic Surgery Symposium.
As Dr. Ridgeway noted, vaginal mesh grafts are controversial because of concerns about their safety. Although many women had favorable outcomes, an unacceptable proportion have experienced complications.
In 2011, the Food and Drug Administration warned that urogynecologic surgical mesh had been linked to 2,874 reports of injuries, deaths, and malfunctions, mostly in pelvic organ prolapse (POP) repairs, over 3 years. The other injuries were in stress urinary incontinence repairs. The report focuses on transvaginal mesh for prolapse and not sacral colpopexy or synthetic midurethral slings, which are considered to have a more favorable risk profile.
The FDA declared that “serious adverse events are NOT rare ... and transvaginally placed mesh in POP repair does NOT conclusively improve clinical outcomes over traditional non-mesh repair.” Subsequently, most companies stopped marketing mesh for transvaginal repair of POP.
Since 2011, research has offered new perspective on the use of mesh in specific POP situations.
“We know that mesh does have some slight improvement in medium-term outcome for subjective and objective symptoms,” Dr. Ridgeway said at the meeting, which was jointly provided by Global Academy for Medical Education and the University of Cincinnati. “This all comes at a price. There’s more blood loss, and you can actually have prolapse in other compartments and de novo SUI.”
She pointed out that these outcomes were noted in a 2013 Cochrane Review. It found improvements in subjective and objective results after treatment with polypropylene mesh vs. native tissue for anterior compartment POP repairs. But the review found multiple disadvantages for mesh vs. native tissue in operating time, blood loss, and reoperations (Cochrane Database Syst Rev. 2013 Apr 30;[4]:CD004014).
In 2016, an updated Cochrane Review declared that “current evidence does not support the use of mesh repair compared with native tissue repair for anterior compartment prolapse owing to increased morbidity.” The review also cautioned that while new light-weight transvaginal meshes are available, they haven’t been fully studied. “Clinicians and women should be cautious when utilizing these products, as their safety and efficacy have not been established,” according to the review (Cochrane Database of Syst Rev. 2016[11];CD004014).
In a follow-up interview, Dr. Ridgeway said “the data are scarce, so it is hard to have an opinion on this.”
She focused much of her presentation on sacral colpopexy. .
“Compared to native tissue prolapse repair using a vaginal approach, sacral colpopexy does have an increased risk profile but likely is associated with better durability,” she said in the interview. “The long-term outcomes following sacral colpopexy are favorable and the risk profile is acceptably low.”
She prefers the approach for recurrent prolapse and post-hysterectomy prolapse, especially in patients with a shorter vagina. She also offers this procedure for younger patients with significant prolapse and those women who are very active or perform repetitive heavy lifting.
In the interview, she offered these tips about the procedure:
- “Identify pertinent anatomy and set yourself up for success. Restore anatomy, retract the colon if necessary, use angled laparoscopes to optimize visualization, and don’t place the vagina on significant tension.”
- “In cases with unusual anatomy, one must recheck anatomic landmarks because it is critical to avoid the middle sacral artery and left common iliac vein, which is often located close to the midline.”
- “The vagina should be well supported but not on tension. One must communicate with assistants to elevate the vagina but not push it too much. I often demonstrate to the assistant how I like it to be.”
- “In regard to closing the peritoneum over the mesh, I like to make sure this dissection is sufficient at the beginning of the case so this part is not a struggle.”
Dr. Ridgeway discloses consulting for Coloplast and serving as an independent contractor (Legal) for Ethicon.
Global Academy and this news organization are owned by the same company.
LAS VEGAS – While research suggests that vaginal mesh grafts are inappropriate for many prolapse repairs, an obstetrician-gynecologist told colleagues that they’re still a valid tool in the repair procedure known as sacral colpopexy, in which mesh is attached via an abdominal route.
Beri M. Ridgeway, MD, of Cleveland Clinic, spoke about the role of mesh grafts and prolapse repairs at the Pelvic Anatomy and Gynecologic Surgery Symposium.
As Dr. Ridgeway noted, vaginal mesh grafts are controversial because of concerns about their safety. Although many women had favorable outcomes, an unacceptable proportion have experienced complications.
In 2011, the Food and Drug Administration warned that urogynecologic surgical mesh had been linked to 2,874 reports of injuries, deaths, and malfunctions, mostly in pelvic organ prolapse (POP) repairs, over 3 years. The other injuries were in stress urinary incontinence repairs. The report focuses on transvaginal mesh for prolapse and not sacral colpopexy or synthetic midurethral slings, which are considered to have a more favorable risk profile.
The FDA declared that “serious adverse events are NOT rare ... and transvaginally placed mesh in POP repair does NOT conclusively improve clinical outcomes over traditional non-mesh repair.” Subsequently, most companies stopped marketing mesh for transvaginal repair of POP.
Since 2011, research has offered new perspective on the use of mesh in specific POP situations.
“We know that mesh does have some slight improvement in medium-term outcome for subjective and objective symptoms,” Dr. Ridgeway said at the meeting, which was jointly provided by Global Academy for Medical Education and the University of Cincinnati. “This all comes at a price. There’s more blood loss, and you can actually have prolapse in other compartments and de novo SUI.”
She pointed out that these outcomes were noted in a 2013 Cochrane Review. It found improvements in subjective and objective results after treatment with polypropylene mesh vs. native tissue for anterior compartment POP repairs. But the review found multiple disadvantages for mesh vs. native tissue in operating time, blood loss, and reoperations (Cochrane Database Syst Rev. 2013 Apr 30;[4]:CD004014).
In 2016, an updated Cochrane Review declared that “current evidence does not support the use of mesh repair compared with native tissue repair for anterior compartment prolapse owing to increased morbidity.” The review also cautioned that while new light-weight transvaginal meshes are available, they haven’t been fully studied. “Clinicians and women should be cautious when utilizing these products, as their safety and efficacy have not been established,” according to the review (Cochrane Database of Syst Rev. 2016[11];CD004014).
In a follow-up interview, Dr. Ridgeway said “the data are scarce, so it is hard to have an opinion on this.”
She focused much of her presentation on sacral colpopexy. .
“Compared to native tissue prolapse repair using a vaginal approach, sacral colpopexy does have an increased risk profile but likely is associated with better durability,” she said in the interview. “The long-term outcomes following sacral colpopexy are favorable and the risk profile is acceptably low.”
She prefers the approach for recurrent prolapse and post-hysterectomy prolapse, especially in patients with a shorter vagina. She also offers this procedure for younger patients with significant prolapse and those women who are very active or perform repetitive heavy lifting.
In the interview, she offered these tips about the procedure:
- “Identify pertinent anatomy and set yourself up for success. Restore anatomy, retract the colon if necessary, use angled laparoscopes to optimize visualization, and don’t place the vagina on significant tension.”
- “In cases with unusual anatomy, one must recheck anatomic landmarks because it is critical to avoid the middle sacral artery and left common iliac vein, which is often located close to the midline.”
- “The vagina should be well supported but not on tension. One must communicate with assistants to elevate the vagina but not push it too much. I often demonstrate to the assistant how I like it to be.”
- “In regard to closing the peritoneum over the mesh, I like to make sure this dissection is sufficient at the beginning of the case so this part is not a struggle.”
Dr. Ridgeway discloses consulting for Coloplast and serving as an independent contractor (Legal) for Ethicon.
Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAGS
Despite concerns, synthetic slings are still ‘standard of care’ in SUI
LAS VEGAS – A few weeks before she was scheduled to speak at the annual Pelvic Anatomy and Gynecologic Surgery Symposium, Beri M. Ridgeway, MD, received an anonymous note about her upcoming presentation. “Someone wanted me to think very carefully about what I’d be talking about during my presentation on synthetics,” she recalled.
The note reflects the deep controversy over the use of transvaginal synthetic mesh products, which have been linked to a long list of serious adverse effects. “There are women who have been harmed, and I take care of a lot of those,” said Dr. Ridgeway, who’s based at Cleveland Clinic. One key distinction is that there is a very different risk profile between transvaginal synthetic mesh prolapse kits and polypropylene midurethral slings. While it’s important to be thoughtful about the use of mesh in synthetic midurethral (MU) slings, she said, they remain well supported as an effective treatment for stress urinary incontinence (SUI).
Even so, she said, the news about the risks of mesh “weighs on our patients’ minds” and spawns fear among physicians. Meanwhile, she said, “there is quite a bit of flux” in the marketplace as companies withdraw products because of their perception of risk.
Even amid the controversy, she said, it’s important to remember how crucial it is to treat women in need. “SUI is a very common problem, and women suffer significantly. With our aging population, the prevalence will increase even more,” she said. “It is critical that we screen patients for SUI and have the ability to offer treatment. Having different treatment options benefit women significantly.”
Dr. Ridgeway offered these pearls about the use of synthetic MU slings and alternative approaches to treating SUI.
It’s helpful to find a single strategy and embrace it.
“For ob.gyn. specialists who treat primary, uncomplicated SUI, I recommend surgeons become comfortable with an approach and focus on becoming high-volume surgeons in that approach,” Dr. Ridgeway said. “It is also good to partner with a female pelvic medicine & reconstructive surgery specialist who can back one up for more complicated cases, complications, or recurrent SUI. These specialists should be able to offer a full array of procedures to treat SUI and tailor the treatment to the individual patient, especially in more complex cases.”
Synthetic MU slings are the “definitive standard of care.”
More than 17 years of research suggest the efficacy of the slings is durable, she said, especially when the goal is to resolve symptoms in patients with pure SUI symptoms. she said, pointing to more than 500 articles and more than 40 randomized controlled trials.
According to her, synthetic slings have similar efficacy to traditional slings but require less time in the operating room and produce less voiding dysfunction and de novo urgency. “The revision rate of synthetic MU slings is very low,” she added. “In large studies, the revision rate at 10 years is 3%-4%.”
It’s important to keep patient consent in mind, she said. “Patients should know and understand the specific risks of any procedure, including MU slings, so that they can share in decision making.”
Transobdurator (TOT) slings offer benefits.
There’s less risk of bladder and vascular injury from the TOT procedure, which is easy to learn and teach, Dr. Ridgeway said. Research suggests the tension-free vaginal tape (TVT) approach is more likely to cause voiding dysfunction, she added.
But TOT is probably less effective in patients with SUI linked to intrinsic sphincter deficiency and in longer-term follow-up, she said. And there are cases of male sex partners injuring their penises during contact with TOT slings during intercourse.
Single-incision slings are up-and-coming options.
These slings offer promising results in short-term studies, but long-term results aren’t available yet. They may be a good option for cases of mild and occult SUI, she said.
Alternative treatments for SUI have limitations.
These include urethral bulking agents, which mainly lead to improvement rather than cure. Autologous fascial pubovaginal slings are another option, especially if patients don’t want a mesh-based treatment or have recurrent SUI following a synthetic mesh complication. However, she noted that research points to morbidity and de novo urinary urgency, she said.
The Pelvic Anatomy & Gynecologic Surgery Symposium was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
Dr. Ridgeway disclosed consulting for Coloplast and having served as an independent contractor (legal) for Ethicon.
LAS VEGAS – A few weeks before she was scheduled to speak at the annual Pelvic Anatomy and Gynecologic Surgery Symposium, Beri M. Ridgeway, MD, received an anonymous note about her upcoming presentation. “Someone wanted me to think very carefully about what I’d be talking about during my presentation on synthetics,” she recalled.
The note reflects the deep controversy over the use of transvaginal synthetic mesh products, which have been linked to a long list of serious adverse effects. “There are women who have been harmed, and I take care of a lot of those,” said Dr. Ridgeway, who’s based at Cleveland Clinic. One key distinction is that there is a very different risk profile between transvaginal synthetic mesh prolapse kits and polypropylene midurethral slings. While it’s important to be thoughtful about the use of mesh in synthetic midurethral (MU) slings, she said, they remain well supported as an effective treatment for stress urinary incontinence (SUI).
Even so, she said, the news about the risks of mesh “weighs on our patients’ minds” and spawns fear among physicians. Meanwhile, she said, “there is quite a bit of flux” in the marketplace as companies withdraw products because of their perception of risk.
Even amid the controversy, she said, it’s important to remember how crucial it is to treat women in need. “SUI is a very common problem, and women suffer significantly. With our aging population, the prevalence will increase even more,” she said. “It is critical that we screen patients for SUI and have the ability to offer treatment. Having different treatment options benefit women significantly.”
Dr. Ridgeway offered these pearls about the use of synthetic MU slings and alternative approaches to treating SUI.
It’s helpful to find a single strategy and embrace it.
“For ob.gyn. specialists who treat primary, uncomplicated SUI, I recommend surgeons become comfortable with an approach and focus on becoming high-volume surgeons in that approach,” Dr. Ridgeway said. “It is also good to partner with a female pelvic medicine & reconstructive surgery specialist who can back one up for more complicated cases, complications, or recurrent SUI. These specialists should be able to offer a full array of procedures to treat SUI and tailor the treatment to the individual patient, especially in more complex cases.”
Synthetic MU slings are the “definitive standard of care.”
More than 17 years of research suggest the efficacy of the slings is durable, she said, especially when the goal is to resolve symptoms in patients with pure SUI symptoms. she said, pointing to more than 500 articles and more than 40 randomized controlled trials.
According to her, synthetic slings have similar efficacy to traditional slings but require less time in the operating room and produce less voiding dysfunction and de novo urgency. “The revision rate of synthetic MU slings is very low,” she added. “In large studies, the revision rate at 10 years is 3%-4%.”
It’s important to keep patient consent in mind, she said. “Patients should know and understand the specific risks of any procedure, including MU slings, so that they can share in decision making.”
Transobdurator (TOT) slings offer benefits.
There’s less risk of bladder and vascular injury from the TOT procedure, which is easy to learn and teach, Dr. Ridgeway said. Research suggests the tension-free vaginal tape (TVT) approach is more likely to cause voiding dysfunction, she added.
But TOT is probably less effective in patients with SUI linked to intrinsic sphincter deficiency and in longer-term follow-up, she said. And there are cases of male sex partners injuring their penises during contact with TOT slings during intercourse.
Single-incision slings are up-and-coming options.
These slings offer promising results in short-term studies, but long-term results aren’t available yet. They may be a good option for cases of mild and occult SUI, she said.
Alternative treatments for SUI have limitations.
These include urethral bulking agents, which mainly lead to improvement rather than cure. Autologous fascial pubovaginal slings are another option, especially if patients don’t want a mesh-based treatment or have recurrent SUI following a synthetic mesh complication. However, she noted that research points to morbidity and de novo urinary urgency, she said.
The Pelvic Anatomy & Gynecologic Surgery Symposium was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
Dr. Ridgeway disclosed consulting for Coloplast and having served as an independent contractor (legal) for Ethicon.
LAS VEGAS – A few weeks before she was scheduled to speak at the annual Pelvic Anatomy and Gynecologic Surgery Symposium, Beri M. Ridgeway, MD, received an anonymous note about her upcoming presentation. “Someone wanted me to think very carefully about what I’d be talking about during my presentation on synthetics,” she recalled.
The note reflects the deep controversy over the use of transvaginal synthetic mesh products, which have been linked to a long list of serious adverse effects. “There are women who have been harmed, and I take care of a lot of those,” said Dr. Ridgeway, who’s based at Cleveland Clinic. One key distinction is that there is a very different risk profile between transvaginal synthetic mesh prolapse kits and polypropylene midurethral slings. While it’s important to be thoughtful about the use of mesh in synthetic midurethral (MU) slings, she said, they remain well supported as an effective treatment for stress urinary incontinence (SUI).
Even so, she said, the news about the risks of mesh “weighs on our patients’ minds” and spawns fear among physicians. Meanwhile, she said, “there is quite a bit of flux” in the marketplace as companies withdraw products because of their perception of risk.
Even amid the controversy, she said, it’s important to remember how crucial it is to treat women in need. “SUI is a very common problem, and women suffer significantly. With our aging population, the prevalence will increase even more,” she said. “It is critical that we screen patients for SUI and have the ability to offer treatment. Having different treatment options benefit women significantly.”
Dr. Ridgeway offered these pearls about the use of synthetic MU slings and alternative approaches to treating SUI.
It’s helpful to find a single strategy and embrace it.
“For ob.gyn. specialists who treat primary, uncomplicated SUI, I recommend surgeons become comfortable with an approach and focus on becoming high-volume surgeons in that approach,” Dr. Ridgeway said. “It is also good to partner with a female pelvic medicine & reconstructive surgery specialist who can back one up for more complicated cases, complications, or recurrent SUI. These specialists should be able to offer a full array of procedures to treat SUI and tailor the treatment to the individual patient, especially in more complex cases.”
Synthetic MU slings are the “definitive standard of care.”
More than 17 years of research suggest the efficacy of the slings is durable, she said, especially when the goal is to resolve symptoms in patients with pure SUI symptoms. she said, pointing to more than 500 articles and more than 40 randomized controlled trials.
According to her, synthetic slings have similar efficacy to traditional slings but require less time in the operating room and produce less voiding dysfunction and de novo urgency. “The revision rate of synthetic MU slings is very low,” she added. “In large studies, the revision rate at 10 years is 3%-4%.”
It’s important to keep patient consent in mind, she said. “Patients should know and understand the specific risks of any procedure, including MU slings, so that they can share in decision making.”
Transobdurator (TOT) slings offer benefits.
There’s less risk of bladder and vascular injury from the TOT procedure, which is easy to learn and teach, Dr. Ridgeway said. Research suggests the tension-free vaginal tape (TVT) approach is more likely to cause voiding dysfunction, she added.
But TOT is probably less effective in patients with SUI linked to intrinsic sphincter deficiency and in longer-term follow-up, she said. And there are cases of male sex partners injuring their penises during contact with TOT slings during intercourse.
Single-incision slings are up-and-coming options.
These slings offer promising results in short-term studies, but long-term results aren’t available yet. They may be a good option for cases of mild and occult SUI, she said.
Alternative treatments for SUI have limitations.
These include urethral bulking agents, which mainly lead to improvement rather than cure. Autologous fascial pubovaginal slings are another option, especially if patients don’t want a mesh-based treatment or have recurrent SUI following a synthetic mesh complication. However, she noted that research points to morbidity and de novo urinary urgency, she said.
The Pelvic Anatomy & Gynecologic Surgery Symposium was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
Dr. Ridgeway disclosed consulting for Coloplast and having served as an independent contractor (legal) for Ethicon.
EXPERT ANALYSIS FROM PAGS
Gap in care: Female patients with incontinence
LAS VEGAS – A pelvic surgeon brought a bold message to a gathering of gynecologists: There’s a great gap in American care for pelvic floor disorders such as urinary incontinence, and they’re the right physicians to make a difference by treating these common conditions.
“There are never going to be enough specialists to deal with these problems. This is a natural progression for many of you,” said urogynecologist and pelvic surgeon Mickey M. Karram, MD, in a joint presentation at the Pelvic Anatomy and Gynecologic Surgery Symposium. In fact, he said, “there’s so much disease out there to fix that you may become more overwhelmed.”
Dr. Karram, who has offices in Cincinnati, Beverly Hills, and Orange County, Calif., spoke about female urinary incontinence with obstetrician-gynecologist Beri M. Ridgeway, MD, of Cleveland Clinic. They offered these tips:
Test for stress incontinence
Dr. Karram recommends using a “quick and easy” cystometrogram (CMG) test to “corroborate or refute what the patient thinks is going on” in regard to urinary function. “With this simple test, you’ll get a clear understanding of sensation [to urinate] and of what their fullness and capacity numbers are,” he said. And if you have the patient cough or strain during the test, “you should be able to duplicate a sign of stress incontinence 90% of the time.”
If patients don’t leak when they take this test, there may be another problem such as overactive bladder, a condition that can’t be duplicated via the test, he said.
Ask the right questions
When it comes to identifying when they have urinary difficulties, some patients “say yes to every question we ask,” said Dr. Ridgeway, and they may not be able to distinguish between urgency and leakage.
A better approach is to ask women to provide specific examples of when they have continence issues, she said. It’s also useful to ask patients about what bothers them the most if they have multiple symptoms: Is it urgency (“Gotta go; gotta go”)? Leakage during certain situations like coughing and laughing? “That helps me decide how to go about treating them first and foremost,” she said. “It doesn’t mean you won’t treat both [problems], but it really gives you a reference point of where to start.”
Research suggests that women tend to be more bothered by urge incontinence than stress incontinence, she said, because they can regulate their activities or avoid the stress form.
Beware of acute incontinence cases
“If a woman walks in and says ‘Everything was great until a week or two ago, but now I’m living in pads,’ it could be a fecal impaction or a pelvic mass,” Dr. Karram said at the meeting jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
Discuss the many treatment options
In some cases of incontinence, Dr. Ridgeway said she’ll mention “the array of treatment options, such as pelvic floor physical therapy, bladder retraining, vaginal estrogen, medications, and Botox.”
She added: “I explain that we’ll work together, and sometimes it will take a couple tries, or we’ll try a couple things at once.”
Dr. Ridgeway disclosed consulting for Coloplast and serving as an independent contractor (legal) for Ethicon. Dr. Karram disclosed speaking for Allergan, Astellas Pharma, Coloplast, and Cynosure/Hologic; consulting for Coloplast and Cynosure/Hologic; and receiving royalties from BihlerMed.
LAS VEGAS – A pelvic surgeon brought a bold message to a gathering of gynecologists: There’s a great gap in American care for pelvic floor disorders such as urinary incontinence, and they’re the right physicians to make a difference by treating these common conditions.
“There are never going to be enough specialists to deal with these problems. This is a natural progression for many of you,” said urogynecologist and pelvic surgeon Mickey M. Karram, MD, in a joint presentation at the Pelvic Anatomy and Gynecologic Surgery Symposium. In fact, he said, “there’s so much disease out there to fix that you may become more overwhelmed.”
Dr. Karram, who has offices in Cincinnati, Beverly Hills, and Orange County, Calif., spoke about female urinary incontinence with obstetrician-gynecologist Beri M. Ridgeway, MD, of Cleveland Clinic. They offered these tips:
Test for stress incontinence
Dr. Karram recommends using a “quick and easy” cystometrogram (CMG) test to “corroborate or refute what the patient thinks is going on” in regard to urinary function. “With this simple test, you’ll get a clear understanding of sensation [to urinate] and of what their fullness and capacity numbers are,” he said. And if you have the patient cough or strain during the test, “you should be able to duplicate a sign of stress incontinence 90% of the time.”
If patients don’t leak when they take this test, there may be another problem such as overactive bladder, a condition that can’t be duplicated via the test, he said.
Ask the right questions
When it comes to identifying when they have urinary difficulties, some patients “say yes to every question we ask,” said Dr. Ridgeway, and they may not be able to distinguish between urgency and leakage.
A better approach is to ask women to provide specific examples of when they have continence issues, she said. It’s also useful to ask patients about what bothers them the most if they have multiple symptoms: Is it urgency (“Gotta go; gotta go”)? Leakage during certain situations like coughing and laughing? “That helps me decide how to go about treating them first and foremost,” she said. “It doesn’t mean you won’t treat both [problems], but it really gives you a reference point of where to start.”
Research suggests that women tend to be more bothered by urge incontinence than stress incontinence, she said, because they can regulate their activities or avoid the stress form.
Beware of acute incontinence cases
“If a woman walks in and says ‘Everything was great until a week or two ago, but now I’m living in pads,’ it could be a fecal impaction or a pelvic mass,” Dr. Karram said at the meeting jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
Discuss the many treatment options
In some cases of incontinence, Dr. Ridgeway said she’ll mention “the array of treatment options, such as pelvic floor physical therapy, bladder retraining, vaginal estrogen, medications, and Botox.”
She added: “I explain that we’ll work together, and sometimes it will take a couple tries, or we’ll try a couple things at once.”
Dr. Ridgeway disclosed consulting for Coloplast and serving as an independent contractor (legal) for Ethicon. Dr. Karram disclosed speaking for Allergan, Astellas Pharma, Coloplast, and Cynosure/Hologic; consulting for Coloplast and Cynosure/Hologic; and receiving royalties from BihlerMed.
LAS VEGAS – A pelvic surgeon brought a bold message to a gathering of gynecologists: There’s a great gap in American care for pelvic floor disorders such as urinary incontinence, and they’re the right physicians to make a difference by treating these common conditions.
“There are never going to be enough specialists to deal with these problems. This is a natural progression for many of you,” said urogynecologist and pelvic surgeon Mickey M. Karram, MD, in a joint presentation at the Pelvic Anatomy and Gynecologic Surgery Symposium. In fact, he said, “there’s so much disease out there to fix that you may become more overwhelmed.”
Dr. Karram, who has offices in Cincinnati, Beverly Hills, and Orange County, Calif., spoke about female urinary incontinence with obstetrician-gynecologist Beri M. Ridgeway, MD, of Cleveland Clinic. They offered these tips:
Test for stress incontinence
Dr. Karram recommends using a “quick and easy” cystometrogram (CMG) test to “corroborate or refute what the patient thinks is going on” in regard to urinary function. “With this simple test, you’ll get a clear understanding of sensation [to urinate] and of what their fullness and capacity numbers are,” he said. And if you have the patient cough or strain during the test, “you should be able to duplicate a sign of stress incontinence 90% of the time.”
If patients don’t leak when they take this test, there may be another problem such as overactive bladder, a condition that can’t be duplicated via the test, he said.
Ask the right questions
When it comes to identifying when they have urinary difficulties, some patients “say yes to every question we ask,” said Dr. Ridgeway, and they may not be able to distinguish between urgency and leakage.
A better approach is to ask women to provide specific examples of when they have continence issues, she said. It’s also useful to ask patients about what bothers them the most if they have multiple symptoms: Is it urgency (“Gotta go; gotta go”)? Leakage during certain situations like coughing and laughing? “That helps me decide how to go about treating them first and foremost,” she said. “It doesn’t mean you won’t treat both [problems], but it really gives you a reference point of where to start.”
Research suggests that women tend to be more bothered by urge incontinence than stress incontinence, she said, because they can regulate their activities or avoid the stress form.
Beware of acute incontinence cases
“If a woman walks in and says ‘Everything was great until a week or two ago, but now I’m living in pads,’ it could be a fecal impaction or a pelvic mass,” Dr. Karram said at the meeting jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
Discuss the many treatment options
In some cases of incontinence, Dr. Ridgeway said she’ll mention “the array of treatment options, such as pelvic floor physical therapy, bladder retraining, vaginal estrogen, medications, and Botox.”
She added: “I explain that we’ll work together, and sometimes it will take a couple tries, or we’ll try a couple things at once.”
Dr. Ridgeway disclosed consulting for Coloplast and serving as an independent contractor (legal) for Ethicon. Dr. Karram disclosed speaking for Allergan, Astellas Pharma, Coloplast, and Cynosure/Hologic; consulting for Coloplast and Cynosure/Hologic; and receiving royalties from BihlerMed.
EXPERT ANALYSIS FROM PAGS
Meaningful endometriosis treatment requires a holistic approach and an understanding of chronic pain
Although it has been more than 100 years since endometriosis was first described in the literature, deciphering the mechanisms that cause pain in women with this enigmatic disease is an ongoing pursuit.
Pain is the most debilitating symptom of endometriosis.1,2 In many cases, it has a profoundly negative impact on a patient’s quality of life, and contributes significantly to disease burden, as well as to personal and societal costs from lost productivity.3,4 Women with endometriosis often experience chronic pelvic pain, deep dyspareunia, dysmenorrhea, and subfertility.5 The majority of women with the disease also have one or more comorbidities, including adenomyosis, adhesive disease, and other pelvic pain conditions such as interstitial cystitis, irritable bowel disease, inflammatory bowel disease, and pelvic floor myalgia.6-8
Recent studies have yielded new insights into the development of endometriosis-associated pelvic pain. The role of peritoneal inflammation, de novo innervation of endometriosis implants, and changes in the central nervous system are becoming increasingly clear.5,9,10 These discoveries have important treatment implications.
In this article, Andrea J. Rapkin, MD, Professor of Obstetrics and Gynecology at the University of California, Los Angeles, and Founder and Director of the UCLA Pelvic Pain Center, offers her expert opinion on the findings of key studies and their clinical implications, including the importance of a multidisciplinary treatment approach that focuses on the whole patient.
Q What mechanisms underlie the chronic pain that many women with endometriosis feel?
Although pain is the primary symptom experienced by women with endometriosis, the disease burden and symptom severity do not often correlate.11,12 “This was the first conundrum presented to clinicians,” noted Dr. Rapkin. “In fact, we do not know the true prevalence of endometriosis because women with endometriosis only come to diagnosis either based on pain or infertility. When infertility is the problem, very often we are surprised by how much disease is present in an individual with either no pain or minimal pain. Conversely, in other individuals with very severe pain, upon laparoscopic surgery, have minimal or mild endometriosis.”
Efforts to solve this clinical puzzle began decades ago. “Dr. Michael Vernon discovered that the small, red, endometriosis implants that looked like petechial hemorrhages produced more prostaglandin E2 (PGE2) in vitro than the older black-brown lesions. PGE2 is a pain-producing (algesic) chemical produced after cytokines stimulation,” said Dr. Rapkin. “This was the first evidence that, yes, there is a reason for pain in many individuals with lower-stage disease.”
“Prostaglandins are known to be a major cause of dysmenorrhea. Prostaglandins induce uterine cramping, sensitize nerve endings, and promote other inflammatory factors responsible for attracting monocytes that become macrophages, further contributing to inflammation,” Dr. Rapkin continued. “PGE2 also stimulates the enzyme aromatase, which allows androgens to be converted to estrogen, which promotes growth of endometriotic lesions. This is a self-feeding aspect of endometriosis.”
Continue to: These discoveries were followed by the realization that deeply infiltrating endometriosis...
These discoveries were followed by the realization that deeply infiltrating endometriosis (defined by disease infiltration of more than 5 mm, often in the uterosacral ligaments) was more likely to be painful than superficial disease, said Dr. Rapkin. “In some women with endometriosis, the disease we see laparoscopically is really the tip of the iceberg.”
In 2005, landmark studies performed by Karen J. Berkley, PhD, were summarized in a paper coauthored by Dr. Berkley, Dr. Rapkin, and Raymond E. Papka, PhD.13 “In a rodent model where endometriosis was developed by suturing pieces of endometrium in the mesentery, the endometriosis implants developed a vascular supply and a nerve supply. These nerves were not just functioning to govern the dilation and contraction of the blood vessels (in other words the sympathetic type nerves), but these nerves stained for neurotransmitters associated with pain (algesic agents, such as substance P and CGRP),” said Dr. Rapkin. “At UCLA, we acquired tissue from women with endometriosis and analyzed in Dr. Papka’s lab. Those tissues also showed nerves staining for pain-producing chemicals.” Other studies performed worldwide also demonstrated nerve endings with neurotrophic and algesic chemicals in endometriotic tissues. In addition to prostaglandins and cytokines, increased expression of various neuropeptides, neurotrophins, and alterations in ion channels contribute to hypersensitivity and pain.
Q What other chronic pain conditions might women with endometriosis experience?
Overlapping chronic pain conditions are common in women with endometriosis. “There is a very high co-occurrence of interstitial cystitis/painful bladder syndrome,” said Dr. Rapkin. “Irritable bowel syndrome is more common in women with endometriosis, as is vulvodynia. Fibromyalgia, migraine headache, temporo-mandibular joint pain (TMJ), anxiety, and depression also commonly co-occur in women with endometriosis.”
“Two concepts may be relevant to why these overlapping pain conditions develop,” Dr. Rapkin continued. “First, visceral sensitization: If one organ or tissue is inflamed and becomes hyperalgesic then other organs in the adjacent region with shared thoracolumbar and sacral innervation can become sensitized through shared cell bodies in the spinal cord, cross-sensitization in the cord, or at higher regions of the CNS. In addition, visceral somatic conversion occurs, whereby somatic tissues such as muscles and subcutaneous tissues with the same nerve supply as the affected organs become sensitized. This process may explain why abdominal wall and pelvic floor muscles become painful. The involvement of surrounding musculature is an important contributor to the pain in many women with endometriosis.”
“Finally, genetic studies of alterations in genes that encode for chemicals affecting the sensitivity and perception of pain are shedding light on the development of chronic pain. Ultimately these studies will advance our understanding of pain related to endometriosis.”
Continue to: Q How have new understandings about the pain mechanisms...
Q How have new understandings about the pain mechanisms involved with endometriosis-caused pelvic pain improved treatment?
According to Dr. Rapkin, the increased understanding of the mechanisms involved in endometriosis-associated pain gained from these key studies led to a paradigm shift, with endometriosis being viewed not just as a condition with mechanical hypersensitivity due to altered anatomy and inflammation but also as a neurologic condition, or a nerve pain condition with peripheral and central sensitization. “This means there is upregulation or hyperactivity both in the periphery (in the pelvis) and centrally (in the spinal cord and brain),” said Dr. Rapkin.
“In the periphery, the endometriotic lesions develop an afferent sensory innervation and communicate with the brain. Stimulation of these nerves by the inflammatory milieu contributes to pain.” Dr. Rapkin noted research by Maria Adele Giamberardino, which demonstrated that women with endometriosis and pain have a lower threshold for feeling pain in the tissues overlying the pelvis (the abdominal wall and back).14 This also has been shown by Dr. Berkley in rodents given endometriosis.
“The muscles develop trigger points and tender hyperalgesic points as part of the sensitization process. In addition, distant sensitization develops—women with pelvic pain and endometriosis have a lower threshold for sensing experimental pain in areas outside the pelvis, for example the back, leg, or shoulder. These discoveries clearly reflect up regulation for pain processing in the central nervous system.”
Dr. Rapkin also pointed to research published in 2016 by Sawson As-Sanie, MD, MPH, that showed an association between endometriosis-associated pelvic pain and altered brain chemistry and function.16 “Dr. As-Sanie demonstrated a decrease in gray matter volume in key neural pain processing areas in the brain in women with pain with endometriosis. This was not found in women with endometriosis who did not have pain,” she said. “Altered connectivity in brain areas related to perception and inhibition of pain is important in maintaining pain. Dr. As-Sanie’s studies also found that these changes are correlated with anxiety, depression, and pain intensity in patients with endometriosis and chronic pain.”
Continue to: Q What are some newer treatment approaches to chronic pain with endometriosis?
Q What are some newer treatment approaches to chronic pain with endometriosis?
“Multidisciplinary approaches to endometriosis-related pain are important,” said Dr. Rapkin. “Although it is important to excise or cauterize endometriosis lesions, or debulk as much as can safely be removed during laparoscopic surgery, it is now standard of care that medical therapy, not surgery, is the first approach to treatment. Endometriosis is a chronic condition. Inflammatory factors will continue to proliferate in patients who menstruate and produce high levels of estrogen with ovulation. The goal of medical therapy is to decrease the levels of estrogen that contribute to maintenance and proliferation of the implants. We want to suppress estrogen in a way that is compatible with long-term quality of life for our patients. Wiping out estrogen and placing patients into a chemical or surgical menopause for most of their reproductive years is not desirable.”
Approaches to hormonally modulate endometriosis include combined hormonal contraceptives and progestin-only medications, such as the levonogestrol-containing IUD, progestin-containing contraceptive implants, injections, or tablets. Second-line medical therapy consists of gonadotropin-releasing hormone agonists and antagonists that can be used for 6 months to 2 years and allows for further lowering of estrogen levels. These may not provide sufficient pain relief for some patients. “There is some evidence from Dr. Giamberadino’s studies that after women with dysmenorrhea were treated with oral contraceptives, the abdominal wall hyperalgesia decreased,” said Dr. Rapkin. “The question is, why don’t we see this in all patients? We come to the realization that endometriosis has to be treated as a neurologically mediated disorder. We have to treat the peripheral and central sensitization in a multidisciplinary way.”
A holistic approach to endometriosis is a new and exciting area for the field, said Dr. Rapkin. “We have to treat ‘bottom-up’, and ‘top-down.’ Bottom-up means we are addressing the peripheral factors that contribute to pain: endometriotic lesions, other pelvic organ pain, myofascial pain, trigger points, the tender points, and the muscle dysfunction in the abdominal wall, the back, and the pelvic floor. Pelvic floor physical therapists help women with pain and endometriosis. Often, women with endometriosis have myofascial pain and pain related to the other comorbid pain conditions they may have developed. Peripheral nerve blocks and medications used for neuropathic pain that alter nerve firing can be helpful in many situations. Pain can be augmented by cognitions and beliefs about pain, and by anxiety and depression. So the top-down approach addresses the cognitions, depression, and anxiety. We do not consider endometriosis a psychosomatic condition, but we know that if you do not address the central upregulation, including anxiety and depression, we may not get anywhere.”
“Interestingly, neurotransmitters and brain regions governing mood contribute to nerve pain. Medications such as tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors, anticonvulsants, and calcium channel blocking agents may prove fruitful. Cognitive behavioral therapy is another approach—to stimulate the prefrontal cortex, the area that is involved in pain inhibition, and other areas of the brain that may produce endogenous opioids to help with inhibiting pain. Bringing in complementary approaches is very important—for example, mindfulness-based meditation or yoga. There is growing evidence for acupuncture as well. Physical therapists, pain psychologists, anesthesiologists, or gynecologists who are facile with nerve blocks, to help tone down hyperalgesic tissues, in addition to medical and surgical therapy, have the possibility of really improving the lives of women with endometriosis.”
Q What key pearls would you like to share with readers?
“It is important to evaluate the entire individual,” she said. “Do not just viscerally focus on the uterus, the ovaries, fallopian tubes, and the peritoneum; investigate the adjacent organs and somatic tissues. Think about the abdominal wall, think about the pelvic floor. Learn how to evaluate these structures. There are simple evaluation techniques that gynecologists can learn and should include with every patient with pelvic pain, whether or not they are suspected of having endometriosis. You also want to get a complete history to determine if there are other co-occurring pain conditions. If there are, it is already a sign that there may be central sensitization.”
“Very often, it is necessary to bring in a pain psychologist—not because the disease is psychosomatic but because therapy can help the patient to learn how to use their brain to erase pain memory, and of course to address the concomitant anxiety, depression, and social isolation that happens with pain.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Olive DL, Lindheim SR, Pritts EA. New medical treatments for endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):319-328.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447): 1789-1799.
- Nnoaham KE, Hummelshoj L, Webster P, et al; World Endometriosis Research Foundation Global Study of Women’s Health consortium. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366-373.e8.
- Simoens S, Dunselman G, Dirksen C, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27(5):1292–1299.
- Bruner-Tran KL, Mokshagundam S, Herington JL. Rodent models of experimental endometriosis: identifying mechanisms of disease and therapeutic targets. Curr Womens Health Rev. 2018;14(2):173-188.
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715-2724.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23(2):164-185.
- Tirlapur SA, Kuhrt K, Chaliha C. The ‘evil twin syndrome’ in chronic pelvic pain: a systematic review of prevalence studies of bladder pain syndrome and endometriosis. Int J Surg. 2013;11(3):233-237.
- Coxon L, Horne AW, Vincent K. Pathophysiology of endometriosis-associated pain: a review of pelvic and central nervous system mechanisms. Best Pract Res Clin Obstet Gynaecol. 2018 Feb 15. pii: S1521-6934(18)30032-4. doi: 10.1016/j.bpobgyn.2018.01.014. [Epub ahead of print]
- Yan D, Liu X, Guo SW. Nerve fibers and endometriotic lesions: partners in crime in inflicting pains in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017;209:14-24.
- Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266-271.
- Fedele L, Parazzini F, Bianchi S. Stage and localization of pelvic endometriosis and pain. Fertil Steril. 1990;53(1):155-158.
- Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308(5728):1587-1589.
- Giamberardino MA, Tana C, Costantini R. Pain thresholds in women with chronic pelvic pain. Curr Opin Obstet Gynecol. 2014;26(4):253-259.
- Giamberardino MA, Berkley KJ, Affaitati G. Influence of endometriosis on pain behaviors and muscle hyperalgesia induced by a ureteral calculosis in female rats. Pain. 2002;95(3):247-257.
- As-Sanie S, Kim J, Schmidt-Wilcke T. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17(1):1-13.
Although it has been more than 100 years since endometriosis was first described in the literature, deciphering the mechanisms that cause pain in women with this enigmatic disease is an ongoing pursuit.
Pain is the most debilitating symptom of endometriosis.1,2 In many cases, it has a profoundly negative impact on a patient’s quality of life, and contributes significantly to disease burden, as well as to personal and societal costs from lost productivity.3,4 Women with endometriosis often experience chronic pelvic pain, deep dyspareunia, dysmenorrhea, and subfertility.5 The majority of women with the disease also have one or more comorbidities, including adenomyosis, adhesive disease, and other pelvic pain conditions such as interstitial cystitis, irritable bowel disease, inflammatory bowel disease, and pelvic floor myalgia.6-8
Recent studies have yielded new insights into the development of endometriosis-associated pelvic pain. The role of peritoneal inflammation, de novo innervation of endometriosis implants, and changes in the central nervous system are becoming increasingly clear.5,9,10 These discoveries have important treatment implications.
In this article, Andrea J. Rapkin, MD, Professor of Obstetrics and Gynecology at the University of California, Los Angeles, and Founder and Director of the UCLA Pelvic Pain Center, offers her expert opinion on the findings of key studies and their clinical implications, including the importance of a multidisciplinary treatment approach that focuses on the whole patient.
Q What mechanisms underlie the chronic pain that many women with endometriosis feel?
Although pain is the primary symptom experienced by women with endometriosis, the disease burden and symptom severity do not often correlate.11,12 “This was the first conundrum presented to clinicians,” noted Dr. Rapkin. “In fact, we do not know the true prevalence of endometriosis because women with endometriosis only come to diagnosis either based on pain or infertility. When infertility is the problem, very often we are surprised by how much disease is present in an individual with either no pain or minimal pain. Conversely, in other individuals with very severe pain, upon laparoscopic surgery, have minimal or mild endometriosis.”
Efforts to solve this clinical puzzle began decades ago. “Dr. Michael Vernon discovered that the small, red, endometriosis implants that looked like petechial hemorrhages produced more prostaglandin E2 (PGE2) in vitro than the older black-brown lesions. PGE2 is a pain-producing (algesic) chemical produced after cytokines stimulation,” said Dr. Rapkin. “This was the first evidence that, yes, there is a reason for pain in many individuals with lower-stage disease.”
“Prostaglandins are known to be a major cause of dysmenorrhea. Prostaglandins induce uterine cramping, sensitize nerve endings, and promote other inflammatory factors responsible for attracting monocytes that become macrophages, further contributing to inflammation,” Dr. Rapkin continued. “PGE2 also stimulates the enzyme aromatase, which allows androgens to be converted to estrogen, which promotes growth of endometriotic lesions. This is a self-feeding aspect of endometriosis.”
Continue to: These discoveries were followed by the realization that deeply infiltrating endometriosis...
These discoveries were followed by the realization that deeply infiltrating endometriosis (defined by disease infiltration of more than 5 mm, often in the uterosacral ligaments) was more likely to be painful than superficial disease, said Dr. Rapkin. “In some women with endometriosis, the disease we see laparoscopically is really the tip of the iceberg.”
In 2005, landmark studies performed by Karen J. Berkley, PhD, were summarized in a paper coauthored by Dr. Berkley, Dr. Rapkin, and Raymond E. Papka, PhD.13 “In a rodent model where endometriosis was developed by suturing pieces of endometrium in the mesentery, the endometriosis implants developed a vascular supply and a nerve supply. These nerves were not just functioning to govern the dilation and contraction of the blood vessels (in other words the sympathetic type nerves), but these nerves stained for neurotransmitters associated with pain (algesic agents, such as substance P and CGRP),” said Dr. Rapkin. “At UCLA, we acquired tissue from women with endometriosis and analyzed in Dr. Papka’s lab. Those tissues also showed nerves staining for pain-producing chemicals.” Other studies performed worldwide also demonstrated nerve endings with neurotrophic and algesic chemicals in endometriotic tissues. In addition to prostaglandins and cytokines, increased expression of various neuropeptides, neurotrophins, and alterations in ion channels contribute to hypersensitivity and pain.
Q What other chronic pain conditions might women with endometriosis experience?
Overlapping chronic pain conditions are common in women with endometriosis. “There is a very high co-occurrence of interstitial cystitis/painful bladder syndrome,” said Dr. Rapkin. “Irritable bowel syndrome is more common in women with endometriosis, as is vulvodynia. Fibromyalgia, migraine headache, temporo-mandibular joint pain (TMJ), anxiety, and depression also commonly co-occur in women with endometriosis.”
“Two concepts may be relevant to why these overlapping pain conditions develop,” Dr. Rapkin continued. “First, visceral sensitization: If one organ or tissue is inflamed and becomes hyperalgesic then other organs in the adjacent region with shared thoracolumbar and sacral innervation can become sensitized through shared cell bodies in the spinal cord, cross-sensitization in the cord, or at higher regions of the CNS. In addition, visceral somatic conversion occurs, whereby somatic tissues such as muscles and subcutaneous tissues with the same nerve supply as the affected organs become sensitized. This process may explain why abdominal wall and pelvic floor muscles become painful. The involvement of surrounding musculature is an important contributor to the pain in many women with endometriosis.”
“Finally, genetic studies of alterations in genes that encode for chemicals affecting the sensitivity and perception of pain are shedding light on the development of chronic pain. Ultimately these studies will advance our understanding of pain related to endometriosis.”
Continue to: Q How have new understandings about the pain mechanisms...
Q How have new understandings about the pain mechanisms involved with endometriosis-caused pelvic pain improved treatment?
According to Dr. Rapkin, the increased understanding of the mechanisms involved in endometriosis-associated pain gained from these key studies led to a paradigm shift, with endometriosis being viewed not just as a condition with mechanical hypersensitivity due to altered anatomy and inflammation but also as a neurologic condition, or a nerve pain condition with peripheral and central sensitization. “This means there is upregulation or hyperactivity both in the periphery (in the pelvis) and centrally (in the spinal cord and brain),” said Dr. Rapkin.
“In the periphery, the endometriotic lesions develop an afferent sensory innervation and communicate with the brain. Stimulation of these nerves by the inflammatory milieu contributes to pain.” Dr. Rapkin noted research by Maria Adele Giamberardino, which demonstrated that women with endometriosis and pain have a lower threshold for feeling pain in the tissues overlying the pelvis (the abdominal wall and back).14 This also has been shown by Dr. Berkley in rodents given endometriosis.
“The muscles develop trigger points and tender hyperalgesic points as part of the sensitization process. In addition, distant sensitization develops—women with pelvic pain and endometriosis have a lower threshold for sensing experimental pain in areas outside the pelvis, for example the back, leg, or shoulder. These discoveries clearly reflect up regulation for pain processing in the central nervous system.”
Dr. Rapkin also pointed to research published in 2016 by Sawson As-Sanie, MD, MPH, that showed an association between endometriosis-associated pelvic pain and altered brain chemistry and function.16 “Dr. As-Sanie demonstrated a decrease in gray matter volume in key neural pain processing areas in the brain in women with pain with endometriosis. This was not found in women with endometriosis who did not have pain,” she said. “Altered connectivity in brain areas related to perception and inhibition of pain is important in maintaining pain. Dr. As-Sanie’s studies also found that these changes are correlated with anxiety, depression, and pain intensity in patients with endometriosis and chronic pain.”
Continue to: Q What are some newer treatment approaches to chronic pain with endometriosis?
Q What are some newer treatment approaches to chronic pain with endometriosis?
“Multidisciplinary approaches to endometriosis-related pain are important,” said Dr. Rapkin. “Although it is important to excise or cauterize endometriosis lesions, or debulk as much as can safely be removed during laparoscopic surgery, it is now standard of care that medical therapy, not surgery, is the first approach to treatment. Endometriosis is a chronic condition. Inflammatory factors will continue to proliferate in patients who menstruate and produce high levels of estrogen with ovulation. The goal of medical therapy is to decrease the levels of estrogen that contribute to maintenance and proliferation of the implants. We want to suppress estrogen in a way that is compatible with long-term quality of life for our patients. Wiping out estrogen and placing patients into a chemical or surgical menopause for most of their reproductive years is not desirable.”
Approaches to hormonally modulate endometriosis include combined hormonal contraceptives and progestin-only medications, such as the levonogestrol-containing IUD, progestin-containing contraceptive implants, injections, or tablets. Second-line medical therapy consists of gonadotropin-releasing hormone agonists and antagonists that can be used for 6 months to 2 years and allows for further lowering of estrogen levels. These may not provide sufficient pain relief for some patients. “There is some evidence from Dr. Giamberadino’s studies that after women with dysmenorrhea were treated with oral contraceptives, the abdominal wall hyperalgesia decreased,” said Dr. Rapkin. “The question is, why don’t we see this in all patients? We come to the realization that endometriosis has to be treated as a neurologically mediated disorder. We have to treat the peripheral and central sensitization in a multidisciplinary way.”
A holistic approach to endometriosis is a new and exciting area for the field, said Dr. Rapkin. “We have to treat ‘bottom-up’, and ‘top-down.’ Bottom-up means we are addressing the peripheral factors that contribute to pain: endometriotic lesions, other pelvic organ pain, myofascial pain, trigger points, the tender points, and the muscle dysfunction in the abdominal wall, the back, and the pelvic floor. Pelvic floor physical therapists help women with pain and endometriosis. Often, women with endometriosis have myofascial pain and pain related to the other comorbid pain conditions they may have developed. Peripheral nerve blocks and medications used for neuropathic pain that alter nerve firing can be helpful in many situations. Pain can be augmented by cognitions and beliefs about pain, and by anxiety and depression. So the top-down approach addresses the cognitions, depression, and anxiety. We do not consider endometriosis a psychosomatic condition, but we know that if you do not address the central upregulation, including anxiety and depression, we may not get anywhere.”
“Interestingly, neurotransmitters and brain regions governing mood contribute to nerve pain. Medications such as tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors, anticonvulsants, and calcium channel blocking agents may prove fruitful. Cognitive behavioral therapy is another approach—to stimulate the prefrontal cortex, the area that is involved in pain inhibition, and other areas of the brain that may produce endogenous opioids to help with inhibiting pain. Bringing in complementary approaches is very important—for example, mindfulness-based meditation or yoga. There is growing evidence for acupuncture as well. Physical therapists, pain psychologists, anesthesiologists, or gynecologists who are facile with nerve blocks, to help tone down hyperalgesic tissues, in addition to medical and surgical therapy, have the possibility of really improving the lives of women with endometriosis.”
Q What key pearls would you like to share with readers?
“It is important to evaluate the entire individual,” she said. “Do not just viscerally focus on the uterus, the ovaries, fallopian tubes, and the peritoneum; investigate the adjacent organs and somatic tissues. Think about the abdominal wall, think about the pelvic floor. Learn how to evaluate these structures. There are simple evaluation techniques that gynecologists can learn and should include with every patient with pelvic pain, whether or not they are suspected of having endometriosis. You also want to get a complete history to determine if there are other co-occurring pain conditions. If there are, it is already a sign that there may be central sensitization.”
“Very often, it is necessary to bring in a pain psychologist—not because the disease is psychosomatic but because therapy can help the patient to learn how to use their brain to erase pain memory, and of course to address the concomitant anxiety, depression, and social isolation that happens with pain.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Although it has been more than 100 years since endometriosis was first described in the literature, deciphering the mechanisms that cause pain in women with this enigmatic disease is an ongoing pursuit.
Pain is the most debilitating symptom of endometriosis.1,2 In many cases, it has a profoundly negative impact on a patient’s quality of life, and contributes significantly to disease burden, as well as to personal and societal costs from lost productivity.3,4 Women with endometriosis often experience chronic pelvic pain, deep dyspareunia, dysmenorrhea, and subfertility.5 The majority of women with the disease also have one or more comorbidities, including adenomyosis, adhesive disease, and other pelvic pain conditions such as interstitial cystitis, irritable bowel disease, inflammatory bowel disease, and pelvic floor myalgia.6-8
Recent studies have yielded new insights into the development of endometriosis-associated pelvic pain. The role of peritoneal inflammation, de novo innervation of endometriosis implants, and changes in the central nervous system are becoming increasingly clear.5,9,10 These discoveries have important treatment implications.
In this article, Andrea J. Rapkin, MD, Professor of Obstetrics and Gynecology at the University of California, Los Angeles, and Founder and Director of the UCLA Pelvic Pain Center, offers her expert opinion on the findings of key studies and their clinical implications, including the importance of a multidisciplinary treatment approach that focuses on the whole patient.
Q What mechanisms underlie the chronic pain that many women with endometriosis feel?
Although pain is the primary symptom experienced by women with endometriosis, the disease burden and symptom severity do not often correlate.11,12 “This was the first conundrum presented to clinicians,” noted Dr. Rapkin. “In fact, we do not know the true prevalence of endometriosis because women with endometriosis only come to diagnosis either based on pain or infertility. When infertility is the problem, very often we are surprised by how much disease is present in an individual with either no pain or minimal pain. Conversely, in other individuals with very severe pain, upon laparoscopic surgery, have minimal or mild endometriosis.”
Efforts to solve this clinical puzzle began decades ago. “Dr. Michael Vernon discovered that the small, red, endometriosis implants that looked like petechial hemorrhages produced more prostaglandin E2 (PGE2) in vitro than the older black-brown lesions. PGE2 is a pain-producing (algesic) chemical produced after cytokines stimulation,” said Dr. Rapkin. “This was the first evidence that, yes, there is a reason for pain in many individuals with lower-stage disease.”
“Prostaglandins are known to be a major cause of dysmenorrhea. Prostaglandins induce uterine cramping, sensitize nerve endings, and promote other inflammatory factors responsible for attracting monocytes that become macrophages, further contributing to inflammation,” Dr. Rapkin continued. “PGE2 also stimulates the enzyme aromatase, which allows androgens to be converted to estrogen, which promotes growth of endometriotic lesions. This is a self-feeding aspect of endometriosis.”
Continue to: These discoveries were followed by the realization that deeply infiltrating endometriosis...
These discoveries were followed by the realization that deeply infiltrating endometriosis (defined by disease infiltration of more than 5 mm, often in the uterosacral ligaments) was more likely to be painful than superficial disease, said Dr. Rapkin. “In some women with endometriosis, the disease we see laparoscopically is really the tip of the iceberg.”
In 2005, landmark studies performed by Karen J. Berkley, PhD, were summarized in a paper coauthored by Dr. Berkley, Dr. Rapkin, and Raymond E. Papka, PhD.13 “In a rodent model where endometriosis was developed by suturing pieces of endometrium in the mesentery, the endometriosis implants developed a vascular supply and a nerve supply. These nerves were not just functioning to govern the dilation and contraction of the blood vessels (in other words the sympathetic type nerves), but these nerves stained for neurotransmitters associated with pain (algesic agents, such as substance P and CGRP),” said Dr. Rapkin. “At UCLA, we acquired tissue from women with endometriosis and analyzed in Dr. Papka’s lab. Those tissues also showed nerves staining for pain-producing chemicals.” Other studies performed worldwide also demonstrated nerve endings with neurotrophic and algesic chemicals in endometriotic tissues. In addition to prostaglandins and cytokines, increased expression of various neuropeptides, neurotrophins, and alterations in ion channels contribute to hypersensitivity and pain.
Q What other chronic pain conditions might women with endometriosis experience?
Overlapping chronic pain conditions are common in women with endometriosis. “There is a very high co-occurrence of interstitial cystitis/painful bladder syndrome,” said Dr. Rapkin. “Irritable bowel syndrome is more common in women with endometriosis, as is vulvodynia. Fibromyalgia, migraine headache, temporo-mandibular joint pain (TMJ), anxiety, and depression also commonly co-occur in women with endometriosis.”
“Two concepts may be relevant to why these overlapping pain conditions develop,” Dr. Rapkin continued. “First, visceral sensitization: If one organ or tissue is inflamed and becomes hyperalgesic then other organs in the adjacent region with shared thoracolumbar and sacral innervation can become sensitized through shared cell bodies in the spinal cord, cross-sensitization in the cord, or at higher regions of the CNS. In addition, visceral somatic conversion occurs, whereby somatic tissues such as muscles and subcutaneous tissues with the same nerve supply as the affected organs become sensitized. This process may explain why abdominal wall and pelvic floor muscles become painful. The involvement of surrounding musculature is an important contributor to the pain in many women with endometriosis.”
“Finally, genetic studies of alterations in genes that encode for chemicals affecting the sensitivity and perception of pain are shedding light on the development of chronic pain. Ultimately these studies will advance our understanding of pain related to endometriosis.”
Continue to: Q How have new understandings about the pain mechanisms...
Q How have new understandings about the pain mechanisms involved with endometriosis-caused pelvic pain improved treatment?
According to Dr. Rapkin, the increased understanding of the mechanisms involved in endometriosis-associated pain gained from these key studies led to a paradigm shift, with endometriosis being viewed not just as a condition with mechanical hypersensitivity due to altered anatomy and inflammation but also as a neurologic condition, or a nerve pain condition with peripheral and central sensitization. “This means there is upregulation or hyperactivity both in the periphery (in the pelvis) and centrally (in the spinal cord and brain),” said Dr. Rapkin.
“In the periphery, the endometriotic lesions develop an afferent sensory innervation and communicate with the brain. Stimulation of these nerves by the inflammatory milieu contributes to pain.” Dr. Rapkin noted research by Maria Adele Giamberardino, which demonstrated that women with endometriosis and pain have a lower threshold for feeling pain in the tissues overlying the pelvis (the abdominal wall and back).14 This also has been shown by Dr. Berkley in rodents given endometriosis.
“The muscles develop trigger points and tender hyperalgesic points as part of the sensitization process. In addition, distant sensitization develops—women with pelvic pain and endometriosis have a lower threshold for sensing experimental pain in areas outside the pelvis, for example the back, leg, or shoulder. These discoveries clearly reflect up regulation for pain processing in the central nervous system.”
Dr. Rapkin also pointed to research published in 2016 by Sawson As-Sanie, MD, MPH, that showed an association between endometriosis-associated pelvic pain and altered brain chemistry and function.16 “Dr. As-Sanie demonstrated a decrease in gray matter volume in key neural pain processing areas in the brain in women with pain with endometriosis. This was not found in women with endometriosis who did not have pain,” she said. “Altered connectivity in brain areas related to perception and inhibition of pain is important in maintaining pain. Dr. As-Sanie’s studies also found that these changes are correlated with anxiety, depression, and pain intensity in patients with endometriosis and chronic pain.”
Continue to: Q What are some newer treatment approaches to chronic pain with endometriosis?
Q What are some newer treatment approaches to chronic pain with endometriosis?
“Multidisciplinary approaches to endometriosis-related pain are important,” said Dr. Rapkin. “Although it is important to excise or cauterize endometriosis lesions, or debulk as much as can safely be removed during laparoscopic surgery, it is now standard of care that medical therapy, not surgery, is the first approach to treatment. Endometriosis is a chronic condition. Inflammatory factors will continue to proliferate in patients who menstruate and produce high levels of estrogen with ovulation. The goal of medical therapy is to decrease the levels of estrogen that contribute to maintenance and proliferation of the implants. We want to suppress estrogen in a way that is compatible with long-term quality of life for our patients. Wiping out estrogen and placing patients into a chemical or surgical menopause for most of their reproductive years is not desirable.”
Approaches to hormonally modulate endometriosis include combined hormonal contraceptives and progestin-only medications, such as the levonogestrol-containing IUD, progestin-containing contraceptive implants, injections, or tablets. Second-line medical therapy consists of gonadotropin-releasing hormone agonists and antagonists that can be used for 6 months to 2 years and allows for further lowering of estrogen levels. These may not provide sufficient pain relief for some patients. “There is some evidence from Dr. Giamberadino’s studies that after women with dysmenorrhea were treated with oral contraceptives, the abdominal wall hyperalgesia decreased,” said Dr. Rapkin. “The question is, why don’t we see this in all patients? We come to the realization that endometriosis has to be treated as a neurologically mediated disorder. We have to treat the peripheral and central sensitization in a multidisciplinary way.”
A holistic approach to endometriosis is a new and exciting area for the field, said Dr. Rapkin. “We have to treat ‘bottom-up’, and ‘top-down.’ Bottom-up means we are addressing the peripheral factors that contribute to pain: endometriotic lesions, other pelvic organ pain, myofascial pain, trigger points, the tender points, and the muscle dysfunction in the abdominal wall, the back, and the pelvic floor. Pelvic floor physical therapists help women with pain and endometriosis. Often, women with endometriosis have myofascial pain and pain related to the other comorbid pain conditions they may have developed. Peripheral nerve blocks and medications used for neuropathic pain that alter nerve firing can be helpful in many situations. Pain can be augmented by cognitions and beliefs about pain, and by anxiety and depression. So the top-down approach addresses the cognitions, depression, and anxiety. We do not consider endometriosis a psychosomatic condition, but we know that if you do not address the central upregulation, including anxiety and depression, we may not get anywhere.”
“Interestingly, neurotransmitters and brain regions governing mood contribute to nerve pain. Medications such as tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors, anticonvulsants, and calcium channel blocking agents may prove fruitful. Cognitive behavioral therapy is another approach—to stimulate the prefrontal cortex, the area that is involved in pain inhibition, and other areas of the brain that may produce endogenous opioids to help with inhibiting pain. Bringing in complementary approaches is very important—for example, mindfulness-based meditation or yoga. There is growing evidence for acupuncture as well. Physical therapists, pain psychologists, anesthesiologists, or gynecologists who are facile with nerve blocks, to help tone down hyperalgesic tissues, in addition to medical and surgical therapy, have the possibility of really improving the lives of women with endometriosis.”
Q What key pearls would you like to share with readers?
“It is important to evaluate the entire individual,” she said. “Do not just viscerally focus on the uterus, the ovaries, fallopian tubes, and the peritoneum; investigate the adjacent organs and somatic tissues. Think about the abdominal wall, think about the pelvic floor. Learn how to evaluate these structures. There are simple evaluation techniques that gynecologists can learn and should include with every patient with pelvic pain, whether or not they are suspected of having endometriosis. You also want to get a complete history to determine if there are other co-occurring pain conditions. If there are, it is already a sign that there may be central sensitization.”
“Very often, it is necessary to bring in a pain psychologist—not because the disease is psychosomatic but because therapy can help the patient to learn how to use their brain to erase pain memory, and of course to address the concomitant anxiety, depression, and social isolation that happens with pain.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Olive DL, Lindheim SR, Pritts EA. New medical treatments for endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):319-328.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447): 1789-1799.
- Nnoaham KE, Hummelshoj L, Webster P, et al; World Endometriosis Research Foundation Global Study of Women’s Health consortium. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366-373.e8.
- Simoens S, Dunselman G, Dirksen C, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27(5):1292–1299.
- Bruner-Tran KL, Mokshagundam S, Herington JL. Rodent models of experimental endometriosis: identifying mechanisms of disease and therapeutic targets. Curr Womens Health Rev. 2018;14(2):173-188.
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715-2724.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23(2):164-185.
- Tirlapur SA, Kuhrt K, Chaliha C. The ‘evil twin syndrome’ in chronic pelvic pain: a systematic review of prevalence studies of bladder pain syndrome and endometriosis. Int J Surg. 2013;11(3):233-237.
- Coxon L, Horne AW, Vincent K. Pathophysiology of endometriosis-associated pain: a review of pelvic and central nervous system mechanisms. Best Pract Res Clin Obstet Gynaecol. 2018 Feb 15. pii: S1521-6934(18)30032-4. doi: 10.1016/j.bpobgyn.2018.01.014. [Epub ahead of print]
- Yan D, Liu X, Guo SW. Nerve fibers and endometriotic lesions: partners in crime in inflicting pains in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017;209:14-24.
- Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266-271.
- Fedele L, Parazzini F, Bianchi S. Stage and localization of pelvic endometriosis and pain. Fertil Steril. 1990;53(1):155-158.
- Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308(5728):1587-1589.
- Giamberardino MA, Tana C, Costantini R. Pain thresholds in women with chronic pelvic pain. Curr Opin Obstet Gynecol. 2014;26(4):253-259.
- Giamberardino MA, Berkley KJ, Affaitati G. Influence of endometriosis on pain behaviors and muscle hyperalgesia induced by a ureteral calculosis in female rats. Pain. 2002;95(3):247-257.
- As-Sanie S, Kim J, Schmidt-Wilcke T. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17(1):1-13.
- Olive DL, Lindheim SR, Pritts EA. New medical treatments for endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):319-328.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447): 1789-1799.
- Nnoaham KE, Hummelshoj L, Webster P, et al; World Endometriosis Research Foundation Global Study of Women’s Health consortium. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366-373.e8.
- Simoens S, Dunselman G, Dirksen C, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27(5):1292–1299.
- Bruner-Tran KL, Mokshagundam S, Herington JL. Rodent models of experimental endometriosis: identifying mechanisms of disease and therapeutic targets. Curr Womens Health Rev. 2018;14(2):173-188.
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715-2724.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23(2):164-185.
- Tirlapur SA, Kuhrt K, Chaliha C. The ‘evil twin syndrome’ in chronic pelvic pain: a systematic review of prevalence studies of bladder pain syndrome and endometriosis. Int J Surg. 2013;11(3):233-237.
- Coxon L, Horne AW, Vincent K. Pathophysiology of endometriosis-associated pain: a review of pelvic and central nervous system mechanisms. Best Pract Res Clin Obstet Gynaecol. 2018 Feb 15. pii: S1521-6934(18)30032-4. doi: 10.1016/j.bpobgyn.2018.01.014. [Epub ahead of print]
- Yan D, Liu X, Guo SW. Nerve fibers and endometriotic lesions: partners in crime in inflicting pains in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017;209:14-24.
- Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266-271.
- Fedele L, Parazzini F, Bianchi S. Stage and localization of pelvic endometriosis and pain. Fertil Steril. 1990;53(1):155-158.
- Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308(5728):1587-1589.
- Giamberardino MA, Tana C, Costantini R. Pain thresholds in women with chronic pelvic pain. Curr Opin Obstet Gynecol. 2014;26(4):253-259.
- Giamberardino MA, Berkley KJ, Affaitati G. Influence of endometriosis on pain behaviors and muscle hyperalgesia induced by a ureteral calculosis in female rats. Pain. 2002;95(3):247-257.
- As-Sanie S, Kim J, Schmidt-Wilcke T. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17(1):1-13.