User login
Does the use of frankincense make sense in dermatology?
The Boswellia serrata exudate or gum (known in India as “guggulu”) that forms an aromatic resin traditionally used as incense – and known as frankincense (especially when retrieved from Boswellia species found in Eritrea and Somalia but also from the Indian variety) – has been considered for thousands of years to possess therapeutic properties. It is used in Ayurvedic medicine, as well as in traditional medicine in China and the Middle East, particularly for its anti-inflammatory effects to treat chronic conditions.1-8 In fact, such essential oils have been used since 2800 BC to treat various inflammatory conditions, including skin sores and wounds, as well as in perfumes and incense.2,9 In the West, use of frankincense dates back to thousands of years as well, more often found in the form of incense for religious and cultural ceremonies.7 Over the past 2 decades, .3 This column focuses on some of the emerging data on this ancient botanical agent.
Chemical constituents
Terpenoids and essential oils are the primary components of frankincense and are known to impart anti-inflammatory and anticancer activity. The same is true for myrrh, which has been combined with frankincense in traditional Chinese medicine as a single medication for millennia, with the two acting synergistically and considered still to be a potent combination in conferring various biological benefits.7
In 2010, in a systematic review of the anti-inflammatory and anticancer activities of Boswellia species and their chemical ingredients, Efferth and Oesch found that frankincense blocks the production of leukotrienes, cyclooxygenase (COX) 1 and 2, as well as 5-lipoxygenase; and oxidative stress. It also contributes to regulation of immune cells from the innate and acquired immune systems and exerts anticancer activity by influencing signaling transduction responsible for cell cycle arrest, as well as inhibition of proliferation, angiogenesis, invasion, and metastasis. The investigators also reported on clinical trial results that have found efficacy of frankincense and its constituents in ameliorating symptoms of psoriasis and erythematous eczema, among other disorders.3
Anti-inflammatory activity
Li et al. completed a study in 2016 to identify the active ingredients responsible for the anti-inflammatory and analgesic effects of frankincense. They found that alpha-pinene, linalool, and 1-octanol were key contributors. These constituents were noted for suppressing COX-2 overexpression in mice, as well as nociceptive stimulus-induced inflammatory infiltrates.10
Noting the increasing popularity of frankincense essential oil in skin care, despite a paucity of data, in 2017, Han et al. evaluated the biological activities of the essential oil in pre-inflamed human dermal fibroblasts using 17 key protein biomarkers. Frankincense essential oil displayed significant antiproliferative activity and suppressed collagen III, interferon gamma-induced protein 10, and intracellular adhesion molecule 1. The investigators referred to the overall encouraging potential of frankincense essential oil to exert influence over inflammation and tissue remodeling in human skin and called for additional research into its mechanisms of action and active constituents.11
Anticancer activity
The main active ingredient in frankincense, boswellic acid, has been shown to promote apoptosis, suppress matrix metalloproteinase secretion, and hinder migration in metastatic melanoma cell lines in mice.6,12
In 2019, Hakkim et al. demonstrated that frankincense essential oil yielded substantial antimelanoma activity in vitro and in vivo and ameliorated hepatotoxicity caused by acetaminophen.13
There is one case report in the literature on the use of frankincense as a treatment for skin cancer. A 56-year-old man received frankincense oil multiple times a day for 4 months to treat a nodular basal cell carcinoma on one arm (which resolved) and an infiltrative BCC on the chest (some focal residual tumor remained).6,14 Topical frankincense or boswellic acid has been given a grade D recommendation for treating skin cancer, however, because of only one level-of-evidence-5 study.6
Antimicrobial activity
In 2012, de Rapper et al. collected samples of three essential oils of frankincense (Boswellia rivae, Boswellia neglecta, and Boswellia papyrifera) and two essential oil samples of myrrh and sweet myrrh from different regions of Ethiopia to study their anti-infective properties alone and in combination. The investigators observed synergistic and additive effects, particularly between B. papyrifera and Commiphora myrrha. While noting the long history of the combined use of frankincense and myrrh essential oils since 1500 BC, the investigators highlighted their study as the first antimicrobial work to verify the effectiveness of this combination, validating the use of this combination to thwart particular pathogens.15
Just 2 years ago, Ljaljević Grbić et al. evaluated the in vitro antimicrobial potential of the liquid and vapor phases of B. carteri and C. myrrha (frankincense and myrrh, respectively) essential oils, finding that frankincense demonstrated marked capacity to act as a natural antimicrobial agent.9
Transdermal delivery
In 2017, Zhu et al. showed that frankincense and myrrh essential oils promoted the permeability of the Chinese herb Chuanxiong and may facilitate drug elimination from the epidermis via dermal capillaries by dint of improved cutaneous blood flow, thereby augmenting transdermal drug delivery.16 The same team also showed that frankincense and myrrh essential oils, by fostering permeation by enhancing drug delivery across the stratum corneum, can also alter the structure of the stratum corneum.17
Conclusion
The use of frankincense in traditional medicine has a long and impressive track record. Recent research provides reason for optimism, and further investigating the possible incorporation of this botanical agent into modern dermatologic therapies appears warranted. Clearly, however, much more research is needed.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Kimmatkar N et al. Phytomedicine. 2003 Jan;10(1):3-7.
2. Ammon HP. Wien Med Wochenschr. 2002;152(15-16):373-8.
3. Efferth T & Oesch F. Semin Cancer Biol. 2020 Feb 4;S1044-579X(20)30034-1.
4. Banno N et al. J Ethnopharmacol. 2006 Sep 19;107(2):249-53.
5. Poeckel D & Werz O. Curr Med Chem. 2006;13(28):3359-69.
6. Li JY, Kampp JT. Dermatol Surg. 2019 Jan;45(1):58-67.
7. Cao B et al. Molecules. 2019 Aug 24;24(17): 3076.
8. Mertens M et al. Flavour Fragr J. 2009;24:279-300.
9. Ljaljević Grbić M et al. J Ethnopharmacol. 2018 Jun 12;219:1-14.
10. Li XJ et al. J Ethnopharmacol. 2016 Feb 17;179:22-6.
11. Han X et al. Biochim Open. 2017 Feb 3;4:31-5.
12. Zhao W et al. Cancer Detect Prev. 2003;27:67-75.
13. Hakkim FL et al. Oncotarget. 2019 May 28;10(37):3472-90.
14. Fung K et al. OA Altern Med 2013;1:14.
15. de Rapper S et al. Lett Appl Microbiol. 2012 Apr;54(4):352-8.
16. Zhu XF et al. Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(4):680-5.
17. Guan YM et al. Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3350-5.
The Boswellia serrata exudate or gum (known in India as “guggulu”) that forms an aromatic resin traditionally used as incense – and known as frankincense (especially when retrieved from Boswellia species found in Eritrea and Somalia but also from the Indian variety) – has been considered for thousands of years to possess therapeutic properties. It is used in Ayurvedic medicine, as well as in traditional medicine in China and the Middle East, particularly for its anti-inflammatory effects to treat chronic conditions.1-8 In fact, such essential oils have been used since 2800 BC to treat various inflammatory conditions, including skin sores and wounds, as well as in perfumes and incense.2,9 In the West, use of frankincense dates back to thousands of years as well, more often found in the form of incense for religious and cultural ceremonies.7 Over the past 2 decades, .3 This column focuses on some of the emerging data on this ancient botanical agent.
Chemical constituents
Terpenoids and essential oils are the primary components of frankincense and are known to impart anti-inflammatory and anticancer activity. The same is true for myrrh, which has been combined with frankincense in traditional Chinese medicine as a single medication for millennia, with the two acting synergistically and considered still to be a potent combination in conferring various biological benefits.7
In 2010, in a systematic review of the anti-inflammatory and anticancer activities of Boswellia species and their chemical ingredients, Efferth and Oesch found that frankincense blocks the production of leukotrienes, cyclooxygenase (COX) 1 and 2, as well as 5-lipoxygenase; and oxidative stress. It also contributes to regulation of immune cells from the innate and acquired immune systems and exerts anticancer activity by influencing signaling transduction responsible for cell cycle arrest, as well as inhibition of proliferation, angiogenesis, invasion, and metastasis. The investigators also reported on clinical trial results that have found efficacy of frankincense and its constituents in ameliorating symptoms of psoriasis and erythematous eczema, among other disorders.3
Anti-inflammatory activity
Li et al. completed a study in 2016 to identify the active ingredients responsible for the anti-inflammatory and analgesic effects of frankincense. They found that alpha-pinene, linalool, and 1-octanol were key contributors. These constituents were noted for suppressing COX-2 overexpression in mice, as well as nociceptive stimulus-induced inflammatory infiltrates.10
Noting the increasing popularity of frankincense essential oil in skin care, despite a paucity of data, in 2017, Han et al. evaluated the biological activities of the essential oil in pre-inflamed human dermal fibroblasts using 17 key protein biomarkers. Frankincense essential oil displayed significant antiproliferative activity and suppressed collagen III, interferon gamma-induced protein 10, and intracellular adhesion molecule 1. The investigators referred to the overall encouraging potential of frankincense essential oil to exert influence over inflammation and tissue remodeling in human skin and called for additional research into its mechanisms of action and active constituents.11
Anticancer activity
The main active ingredient in frankincense, boswellic acid, has been shown to promote apoptosis, suppress matrix metalloproteinase secretion, and hinder migration in metastatic melanoma cell lines in mice.6,12
In 2019, Hakkim et al. demonstrated that frankincense essential oil yielded substantial antimelanoma activity in vitro and in vivo and ameliorated hepatotoxicity caused by acetaminophen.13
There is one case report in the literature on the use of frankincense as a treatment for skin cancer. A 56-year-old man received frankincense oil multiple times a day for 4 months to treat a nodular basal cell carcinoma on one arm (which resolved) and an infiltrative BCC on the chest (some focal residual tumor remained).6,14 Topical frankincense or boswellic acid has been given a grade D recommendation for treating skin cancer, however, because of only one level-of-evidence-5 study.6
Antimicrobial activity
In 2012, de Rapper et al. collected samples of three essential oils of frankincense (Boswellia rivae, Boswellia neglecta, and Boswellia papyrifera) and two essential oil samples of myrrh and sweet myrrh from different regions of Ethiopia to study their anti-infective properties alone and in combination. The investigators observed synergistic and additive effects, particularly between B. papyrifera and Commiphora myrrha. While noting the long history of the combined use of frankincense and myrrh essential oils since 1500 BC, the investigators highlighted their study as the first antimicrobial work to verify the effectiveness of this combination, validating the use of this combination to thwart particular pathogens.15
Just 2 years ago, Ljaljević Grbić et al. evaluated the in vitro antimicrobial potential of the liquid and vapor phases of B. carteri and C. myrrha (frankincense and myrrh, respectively) essential oils, finding that frankincense demonstrated marked capacity to act as a natural antimicrobial agent.9
Transdermal delivery
In 2017, Zhu et al. showed that frankincense and myrrh essential oils promoted the permeability of the Chinese herb Chuanxiong and may facilitate drug elimination from the epidermis via dermal capillaries by dint of improved cutaneous blood flow, thereby augmenting transdermal drug delivery.16 The same team also showed that frankincense and myrrh essential oils, by fostering permeation by enhancing drug delivery across the stratum corneum, can also alter the structure of the stratum corneum.17
Conclusion
The use of frankincense in traditional medicine has a long and impressive track record. Recent research provides reason for optimism, and further investigating the possible incorporation of this botanical agent into modern dermatologic therapies appears warranted. Clearly, however, much more research is needed.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Kimmatkar N et al. Phytomedicine. 2003 Jan;10(1):3-7.
2. Ammon HP. Wien Med Wochenschr. 2002;152(15-16):373-8.
3. Efferth T & Oesch F. Semin Cancer Biol. 2020 Feb 4;S1044-579X(20)30034-1.
4. Banno N et al. J Ethnopharmacol. 2006 Sep 19;107(2):249-53.
5. Poeckel D & Werz O. Curr Med Chem. 2006;13(28):3359-69.
6. Li JY, Kampp JT. Dermatol Surg. 2019 Jan;45(1):58-67.
7. Cao B et al. Molecules. 2019 Aug 24;24(17): 3076.
8. Mertens M et al. Flavour Fragr J. 2009;24:279-300.
9. Ljaljević Grbić M et al. J Ethnopharmacol. 2018 Jun 12;219:1-14.
10. Li XJ et al. J Ethnopharmacol. 2016 Feb 17;179:22-6.
11. Han X et al. Biochim Open. 2017 Feb 3;4:31-5.
12. Zhao W et al. Cancer Detect Prev. 2003;27:67-75.
13. Hakkim FL et al. Oncotarget. 2019 May 28;10(37):3472-90.
14. Fung K et al. OA Altern Med 2013;1:14.
15. de Rapper S et al. Lett Appl Microbiol. 2012 Apr;54(4):352-8.
16. Zhu XF et al. Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(4):680-5.
17. Guan YM et al. Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3350-5.
The Boswellia serrata exudate or gum (known in India as “guggulu”) that forms an aromatic resin traditionally used as incense – and known as frankincense (especially when retrieved from Boswellia species found in Eritrea and Somalia but also from the Indian variety) – has been considered for thousands of years to possess therapeutic properties. It is used in Ayurvedic medicine, as well as in traditional medicine in China and the Middle East, particularly for its anti-inflammatory effects to treat chronic conditions.1-8 In fact, such essential oils have been used since 2800 BC to treat various inflammatory conditions, including skin sores and wounds, as well as in perfumes and incense.2,9 In the West, use of frankincense dates back to thousands of years as well, more often found in the form of incense for religious and cultural ceremonies.7 Over the past 2 decades, .3 This column focuses on some of the emerging data on this ancient botanical agent.
Chemical constituents
Terpenoids and essential oils are the primary components of frankincense and are known to impart anti-inflammatory and anticancer activity. The same is true for myrrh, which has been combined with frankincense in traditional Chinese medicine as a single medication for millennia, with the two acting synergistically and considered still to be a potent combination in conferring various biological benefits.7
In 2010, in a systematic review of the anti-inflammatory and anticancer activities of Boswellia species and their chemical ingredients, Efferth and Oesch found that frankincense blocks the production of leukotrienes, cyclooxygenase (COX) 1 and 2, as well as 5-lipoxygenase; and oxidative stress. It also contributes to regulation of immune cells from the innate and acquired immune systems and exerts anticancer activity by influencing signaling transduction responsible for cell cycle arrest, as well as inhibition of proliferation, angiogenesis, invasion, and metastasis. The investigators also reported on clinical trial results that have found efficacy of frankincense and its constituents in ameliorating symptoms of psoriasis and erythematous eczema, among other disorders.3
Anti-inflammatory activity
Li et al. completed a study in 2016 to identify the active ingredients responsible for the anti-inflammatory and analgesic effects of frankincense. They found that alpha-pinene, linalool, and 1-octanol were key contributors. These constituents were noted for suppressing COX-2 overexpression in mice, as well as nociceptive stimulus-induced inflammatory infiltrates.10
Noting the increasing popularity of frankincense essential oil in skin care, despite a paucity of data, in 2017, Han et al. evaluated the biological activities of the essential oil in pre-inflamed human dermal fibroblasts using 17 key protein biomarkers. Frankincense essential oil displayed significant antiproliferative activity and suppressed collagen III, interferon gamma-induced protein 10, and intracellular adhesion molecule 1. The investigators referred to the overall encouraging potential of frankincense essential oil to exert influence over inflammation and tissue remodeling in human skin and called for additional research into its mechanisms of action and active constituents.11
Anticancer activity
The main active ingredient in frankincense, boswellic acid, has been shown to promote apoptosis, suppress matrix metalloproteinase secretion, and hinder migration in metastatic melanoma cell lines in mice.6,12
In 2019, Hakkim et al. demonstrated that frankincense essential oil yielded substantial antimelanoma activity in vitro and in vivo and ameliorated hepatotoxicity caused by acetaminophen.13
There is one case report in the literature on the use of frankincense as a treatment for skin cancer. A 56-year-old man received frankincense oil multiple times a day for 4 months to treat a nodular basal cell carcinoma on one arm (which resolved) and an infiltrative BCC on the chest (some focal residual tumor remained).6,14 Topical frankincense or boswellic acid has been given a grade D recommendation for treating skin cancer, however, because of only one level-of-evidence-5 study.6
Antimicrobial activity
In 2012, de Rapper et al. collected samples of three essential oils of frankincense (Boswellia rivae, Boswellia neglecta, and Boswellia papyrifera) and two essential oil samples of myrrh and sweet myrrh from different regions of Ethiopia to study their anti-infective properties alone and in combination. The investigators observed synergistic and additive effects, particularly between B. papyrifera and Commiphora myrrha. While noting the long history of the combined use of frankincense and myrrh essential oils since 1500 BC, the investigators highlighted their study as the first antimicrobial work to verify the effectiveness of this combination, validating the use of this combination to thwart particular pathogens.15
Just 2 years ago, Ljaljević Grbić et al. evaluated the in vitro antimicrobial potential of the liquid and vapor phases of B. carteri and C. myrrha (frankincense and myrrh, respectively) essential oils, finding that frankincense demonstrated marked capacity to act as a natural antimicrobial agent.9
Transdermal delivery
In 2017, Zhu et al. showed that frankincense and myrrh essential oils promoted the permeability of the Chinese herb Chuanxiong and may facilitate drug elimination from the epidermis via dermal capillaries by dint of improved cutaneous blood flow, thereby augmenting transdermal drug delivery.16 The same team also showed that frankincense and myrrh essential oils, by fostering permeation by enhancing drug delivery across the stratum corneum, can also alter the structure of the stratum corneum.17
Conclusion
The use of frankincense in traditional medicine has a long and impressive track record. Recent research provides reason for optimism, and further investigating the possible incorporation of this botanical agent into modern dermatologic therapies appears warranted. Clearly, however, much more research is needed.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Kimmatkar N et al. Phytomedicine. 2003 Jan;10(1):3-7.
2. Ammon HP. Wien Med Wochenschr. 2002;152(15-16):373-8.
3. Efferth T & Oesch F. Semin Cancer Biol. 2020 Feb 4;S1044-579X(20)30034-1.
4. Banno N et al. J Ethnopharmacol. 2006 Sep 19;107(2):249-53.
5. Poeckel D & Werz O. Curr Med Chem. 2006;13(28):3359-69.
6. Li JY, Kampp JT. Dermatol Surg. 2019 Jan;45(1):58-67.
7. Cao B et al. Molecules. 2019 Aug 24;24(17): 3076.
8. Mertens M et al. Flavour Fragr J. 2009;24:279-300.
9. Ljaljević Grbić M et al. J Ethnopharmacol. 2018 Jun 12;219:1-14.
10. Li XJ et al. J Ethnopharmacol. 2016 Feb 17;179:22-6.
11. Han X et al. Biochim Open. 2017 Feb 3;4:31-5.
12. Zhao W et al. Cancer Detect Prev. 2003;27:67-75.
13. Hakkim FL et al. Oncotarget. 2019 May 28;10(37):3472-90.
14. Fung K et al. OA Altern Med 2013;1:14.
15. de Rapper S et al. Lett Appl Microbiol. 2012 Apr;54(4):352-8.
16. Zhu XF et al. Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(4):680-5.
17. Guan YM et al. Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3350-5.
Management of Pediatric Nail Psoriasis
Pediatric nail psoriasis is a condition that has not been extensively studied. The prevalence of nail alterations in pediatric patients with psoriasis varies among different studies, ranging from 17% to 39.2%.1 Nail pitting, onycholysis associated with subungual hyperkeratosis, paronychia, and pachyonychia are the most frequent features of psoriatic nail involvement in children.2,3 The management of nail psoriasis in children and adolescents is critical due to the quality-of-life impact, from potential functional impairment issues to the obvious cosmetic problems, which can aggravate the psychologic distress and social embarrassment of patients with psoriasis. Despite the emergence of modern potent systemic agents to treat chronic plaque psoriasis, nail psoriasis often is refractory to treatment.4 Coupled with the limited on-label options for psoriasis treatment in children, the management of nail psoriasis in this special patient group constitutes an even greater therapeutic challenge. This report aims to summarize the limited existing data on the successful management of nail psoriasis in the pediatric population.
Reviewing the Literature on Nail Psoriasis
We conducted a search of PubMed articles indexed for MEDLINE, Embase, and Scopus using the following Medical Subject Headings key terms: nail psoriasis and children, juvenile, pediatric. Additional articles were identified from the reference lists of the retrieved articles and citations. Our search included reports in the English language published from 2000 to 2019. The selection process included the following 2 steps: screening of the titles and abstracts, followed by evaluation of the selected full-text articles.
Topical Treatments for Nail Psoriasis
Because most systemic antipsoriatic treatments that can be administered in adult patients have not yet been granted an official license for administration in children, topical treatments are considered by many physicians as the preferred first-line therapy for psoriatic nail involvement in pediatric patients.5,6 However, only scarce data are available in the literature concerning the successful use of local agents in pediatric patients with psoriasis.
The main limitation of local treatments relates mostly to their impaired penetration into the affected area (nails). To optimize drug penetration, some authors suggest the use of potent keratolytic topical preparations to reduce the nail volume and facilitate drug absorption.7 A popular suggestion is trimming the onycholytic nail plate followed by 40% urea avulsion to treat subungual hyperkeratosis8 or simply the use of occlusive 40% urea in petroleum jelly.9 Other approaches include clipping the onycholytic nail over the diseased nail bed or processing the nail plate through grinding or even drilling holes with the use of mechanical burrs or ablative lasers to enhance the penetration of the topical agent.7
A frequent approach in pediatric patients is clipping the detached nails combined with daily application of calcipotriene (calcipotriol) and steroids, such as betamethasone dipropionate.5,8 Reports on the use of regimens with clobetasol propionate ointment 0.05% under occlusion, with or without the concomitant use of calcipotriol solution 0.005%, also are present in the literature but not always with satisfactory results.10,11 Another successfully administered topical steroid is mometasone furoate cream 0.1%.12 Although the use of intralesional triamcinolone acetonide also has demonstrated encouraging outcomes in isolated reports,13 associated adverse events, such as pain and hematomas, can result in tolerability issues for pediatric patients.7
Piraccini et al14 described the case of an 8-year-old patient with pustular nail psoriasis who showed improvement within 3 to 6 months of treatment with topical calcipotriol 5 μg/g as monotherapy applied to the nail and periungual tissues twice daily. Another approach, described by Diluvio et al,15 is the use of tazarotene gel 0.05% applied once daily to the affected nail plates, nail folds, and periungual skin without occlusion. In a 6-year-old patient with isolated nail psoriasis, this treatment regimen demonstrated notable improvement within 8 weeks.15
Systemic Treatments for Nail Psoriasis
Data on the successful administration of systemic agents in pediatric patients also are extremely scarce. Due to the lack of clinical trials, everyday practice is mostly based on isolated case series and case reports.
Methotrexate—Lee11 described the case of an 11-year-old girl with severe, symptomatic, 20-nail psoriatic onychodystrophy who showed a complete response to oral methotrexate 5 mg/wk after topical clobetasol propionate and calcipotriol failed. Improvement was seen as early as 4 weeks after therapy initiation, and complete resolution of the lesions was documented after 9 and 13 months of methotrexate therapy for the fingers and toes, respectively.11 The successful use of methotrexate in the improvement of psoriatic nail dystrophy in a pediatric patient also was documented by Teran et al.16 In this case, a 9-year-old girl with erythrodermic psoriasis, psoriatic arthritis, and severe onychodystrophy showed notable amelioration of all psoriatic manifestations, including the nail findings, with systemic methotrexate therapy (dose not specified).16 Notably, the authors reported that the improvement of onychodystrophy occurred with considerable delay compared to the other psoriatic lesions,16 indicating the already-known refractoriness of nail psoriasis to the various therapeutic attempts.9-15
Acitretin—Another agent that has been linked with partial improvement of acrodermatitis continua of Hallopeau (ACH)–associated onychodystrophy is acitretin. In a case series of 15 pediatric patients with pustular psoriasis, a 5-year-old boy with severe nail involvement presented with partial amelioration of nail changes with acitretin within the first 6 weeks of treatment using the following regimen: initial dosage of 0.8 mg/kg/d for 6 weeks, followed by 0.3 mg/kg/d for 4 weeks.17
Biologics—The emerging use of biologics in pediatric psoriasis also has brought important advances in the successful management of nail psoriasis in children and adolescents.18-21 Wells et al18 presented the case of an 8-year-old girl with nail psoriasis, psoriatic arthritis, and plaque psoriasis who showed complete resolution of all psoriatic manifestations, including nail involvement, within 3 months of treatment with secukinumab 150 mg subcutaneously every 4 weeks. Prior failed treatments included various systemic agents (ie, subcutaneous methotrexate 20 mg/m2, etanercept 0.8 mg/kg weekly, adalimumab 40 mg every 2 weeks) as well as topical agents (ie, urea, tazarotene, corticosteroids) and intralesional triamcinolone.18
Infliximab also has been successfully used for pediatric nail psoriasis. Watabe et al19 presented the case of an 8-year-old girl with psoriatic onychodystrophy in addition to psoriatic onycho-pachydermo-periostitis. Prior therapy with adalimumab 20 mg every other week combined with methotrexate 10 mg weekly failed. She experienced notable amelioration of the nail dystrophy within 3 months of using a combination of infliximab and methotrexate (infliximab 5 mg/kg intravenously on weeks 0, 2, and 6, and every 8 weeks thereafter; methotrexate 10 mg/wk).19
Cases in which infliximab has resulted in rapid yet only transient restoration of psoriatic onychodystrophy also are present in the literature. Pereira et al20 reported that a 3-year-old patient with severe 20-digit onychodystrophy in addition to pustular psoriasis had complete resolution of nail lesions within 2 weeks of treatment with infliximab (5 mg/kg at weeks 0, 2, and 6, and then every 7 weeks thereafter), which was sustained over the course of 1 year. The therapy had to be discontinued because of exacerbation of the cutaneous symptoms; thereafter, etanercept was initiated. Although the patient noted major improvement of all skin lesions under etanercept, only moderate amelioration of the psoriatic nail lesions was demonstrated.20
Dini et al21 described a 9-year-old girl with severe ACH-associated psoriatic onychodystrophy who showed complete clearance of all lesions within 8 weeks of treatment with adalimumab (initially 80 mg, followed by 40 mg after 1 week and then 40 mg every other week). Prior treatment with potent topical corticosteroids, cyclosporine (3 mg/kg/d for 6 months), and etanercept (0.4 mg/kg twice weekly for 3 months) was ineffective.21
Phototherapy—Other systemic agents with reported satisfactory outcomes in the treatment of psoriatic onychodystrophy include thalidomide combined with UVB phototherapy. Kiszewski et al22 described a 2-year-old patient with ACH and severe 19-digit onychodystrophy. Prior failed therapies included occluded clobetasol ointment 0.05%, occluded pimecrolimus 0.1%, and systemic methotrexate, while systemic acitretin (0.8 mg⁄kg⁄d) resulted in elevated cholesterol levels and therefore had to be interrupted. Improvement was seen 2 months after the initiation of a combined broadband UVB and thalidomide (50 mg⁄d) treatment, with no documented relapses after discontinuation of therapy.22
Narrowband UVB (311 nm) also has been used as monotherapy for ACH-associated onychodystrophy, as demonstrated by Bordignon et al.23 They reported a 9-year-old patient who showed partial improvement of isolated onychodystrophy of the fourth nail plate of the left hand after 36 sessions of narrowband UVB using a 311-nm filtering handpiece with a square spot size of 19×19 mm.23
Conclusion
Nail psoriasis constitutes a type of psoriasis that is not only refractory to most treatments but is accompanied by substantial psychological and occasionally functional burden for the affected individuals.24 Data concerning therapeutic options in the pediatric population are extremely limited, and therefore the everyday practice often involves administration of off-label medications, which can constitute a dilemma for many physicians, especially for safety.10 We suggest a simple therapeutic algorithm for the management of pediatric nail psoriasis based on the summarized data that are currently available in the literature. This algorithm is shown in the eFigure.
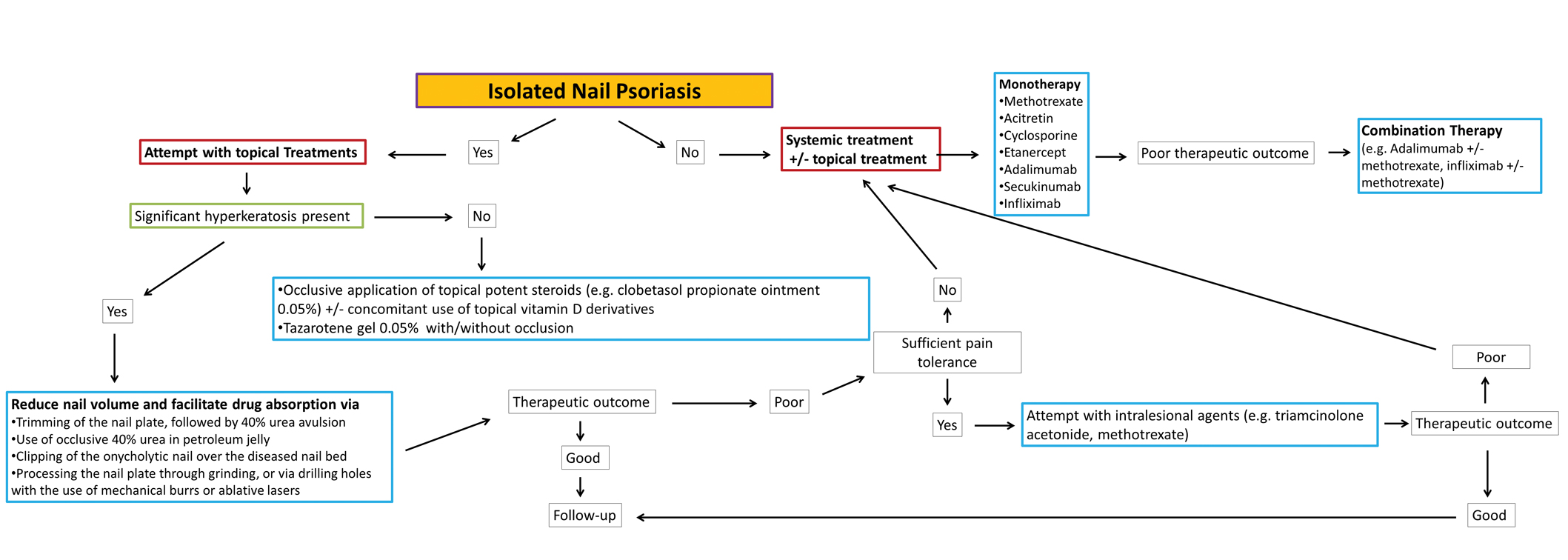
As progressively more agents—especially biologics—receive approval for use in plaque psoriasis in pediatric patients,25 it is expected that gradually more real-life data on their side efficacy for plaque psoriasis of the nails in children also will come to light. Furthermore, their on-label use in pediatric psoriasis patients will facilitate further relevant clinical trials to this target group so that the potential of these medications in the management of nail psoriasis can be fully explored.
- Uber M, Carvalho VO, Abagge KT, et al. Clinical features and nail clippings in 52 children with psoriasis. Pediatr Dermatol. 2018;35:202-207.
- Pourchot D, Bodemer C, Phan A, et al. Nail psoriasis: a systematic evaluation in 313 children with psoriasis. Pediatr Dermatol. 2017;34:58-63.
- Piraccini BM, Triantafyllopoulou I, Prevezas C, et al. Nail psoriasis in children: common or uncommon? results from a 10-year double-center study. Skin Appendage Disord. 2015;1:43-48.
- Baran R. The burden of nail psoriasis: an introduction. Dermatology. 2010;221(suppl 1):1-5.
- Richert B, André J. Nail disorders in children: diagnosis and management. Am J Clin Dermatol. 2011;12:101-112.
- Trüeb RM. Therapies for childhood psoriasis. Curr Probl Dermatol. 2009;38:137-159.
- Haneke E. Nail psoriasis: clinical features, pathogenesis, differential diagnoses, and management. Psoriasis (Auckl). 2017;7:51-63.
- Piraccini BM, Starace M. Nail disorders in infants and children. Curr Opin Pediatr. 2014;26:440-445.
- Duran-McKinster C, Ortiz-Solis D, Granados J, et al. Juvenile psoriatic arthritis with nail psoriasis in the absence of cutaneous lesions. Int J Dermatol. 2000;39:32-35.
- Holzberg M, Ruben BS, Baran R. Psoriasis restricted to the nail in a 7-year-old child. should biologics be an appropriate treatment modality when considering quality of life? J Eur Acad Dermatol Venereol. 2014;28:668-670.
- Lee JY. Severe 20-nail psoriasis successfully treated by low dose methotrexate. Dermatol Online J. 2009;15:8.
- Liao YC, Lee JY. Psoriasis in a 3-month-old infant with Kawasaki disease. Dermatol Online J. 2009;15:10.
- Khoo BP, Giam YC. A pilot study on the role of intralesional triamcinolone acetonide in the treatment of pitted nails in children. Singapore Med J. 2000;41:66-68.
- Piraccini BM, Tosti A, Iorizzo M, et al. Pustular psoriasis of the nails: treatment and long-term follow-up of 46 patients. Br J Dermatol. 2001;144:1000-1005.
- Diluvio L, Campione E, Paternò EJ, et al. Childhood nail psoriasis: a useful treatment with tazarotene 0.05%. Pediatr Dermatol. 2007;24:332-333.
- Teran CG, Teran-Escalera CN, Balderrama C. A severe case of erythrodermic psoriasis associated with advanced nail and joint manifestations: a case report. J Med Case Rep. 2010;4:179.
- Chen P, Li C, Xue R, et al. Efficacy and safety of acitretin monotherapy in children with pustular psoriasis: results from 15 cases and a literature review. J Dermatolog Treat. 2018;29:353-363.
- Wells LE, Evans T, Hilton R, et al. Use of secukinumab in a pediatric patient leads to significant improvement in nail psoriasis and psoriatic arthritis. Pediatr Dermatol. 2019;36:384-385.
- Watabe D, Endoh K, Maeda F, et al. Childhood-onset psoriatic onycho-pachydermo-periostitis treated successfully with infliximab. Eur J Dermatol. 2015;25:506-508.
- Pereira TM, Vieira AP, Fernandes JC, et al. Anti-TNF-alpha therapy in childhood pustular psoriasis. Dermatology. 2006;213:350-352.
- Dini V, Barbanera S, Romanelli M. Efficacy of adalimumab for the treatment of refractory paediatric acrodermatitis continua of Hallopeau. Acta Derm Venereol. 2013;93:588-589.
- Kiszewski AE, De Villa D, Scheibel I, et al. An infant with acrodermatitis continua of Hallopeau: successful treatment with thalidomide and UVB therapy. Pediatr Dermatol. 2009;26:105-106.
- Bordignon M, Zattra E, Albertin C, et al. Successful treatment of a 9-year-old boy affected by acrodermatitis continua of Hallopeau with targeted ultraviolet B narrow-band phototherapy. Photodermatol Photoimmunol Photomed. 2010;26:41-43.
- Fabroni C, Gori A, Troiano M, et al. Infliximab efficacy in nail psoriasis. a retrospective study in 48 patients. J Eur Acad Dermatol Venereol. 2011;25:549-553.
- Lilly’s Taltz® (ixekizumab) receives U.S. FDA approval for the treatment of pediatric patients with moderate to severe plaque psoriasis. Eli Lilly and Company. March 30, 2020. Accessed September 24, 2021. https://investor.lilly.com/news-releases/news-release-details/lillys-taltzr-ixekizumab-receives-us-fda-approval-treatment-1
Pediatric nail psoriasis is a condition that has not been extensively studied. The prevalence of nail alterations in pediatric patients with psoriasis varies among different studies, ranging from 17% to 39.2%.1 Nail pitting, onycholysis associated with subungual hyperkeratosis, paronychia, and pachyonychia are the most frequent features of psoriatic nail involvement in children.2,3 The management of nail psoriasis in children and adolescents is critical due to the quality-of-life impact, from potential functional impairment issues to the obvious cosmetic problems, which can aggravate the psychologic distress and social embarrassment of patients with psoriasis. Despite the emergence of modern potent systemic agents to treat chronic plaque psoriasis, nail psoriasis often is refractory to treatment.4 Coupled with the limited on-label options for psoriasis treatment in children, the management of nail psoriasis in this special patient group constitutes an even greater therapeutic challenge. This report aims to summarize the limited existing data on the successful management of nail psoriasis in the pediatric population.
Reviewing the Literature on Nail Psoriasis
We conducted a search of PubMed articles indexed for MEDLINE, Embase, and Scopus using the following Medical Subject Headings key terms: nail psoriasis and children, juvenile, pediatric. Additional articles were identified from the reference lists of the retrieved articles and citations. Our search included reports in the English language published from 2000 to 2019. The selection process included the following 2 steps: screening of the titles and abstracts, followed by evaluation of the selected full-text articles.
Topical Treatments for Nail Psoriasis
Because most systemic antipsoriatic treatments that can be administered in adult patients have not yet been granted an official license for administration in children, topical treatments are considered by many physicians as the preferred first-line therapy for psoriatic nail involvement in pediatric patients.5,6 However, only scarce data are available in the literature concerning the successful use of local agents in pediatric patients with psoriasis.
The main limitation of local treatments relates mostly to their impaired penetration into the affected area (nails). To optimize drug penetration, some authors suggest the use of potent keratolytic topical preparations to reduce the nail volume and facilitate drug absorption.7 A popular suggestion is trimming the onycholytic nail plate followed by 40% urea avulsion to treat subungual hyperkeratosis8 or simply the use of occlusive 40% urea in petroleum jelly.9 Other approaches include clipping the onycholytic nail over the diseased nail bed or processing the nail plate through grinding or even drilling holes with the use of mechanical burrs or ablative lasers to enhance the penetration of the topical agent.7
A frequent approach in pediatric patients is clipping the detached nails combined with daily application of calcipotriene (calcipotriol) and steroids, such as betamethasone dipropionate.5,8 Reports on the use of regimens with clobetasol propionate ointment 0.05% under occlusion, with or without the concomitant use of calcipotriol solution 0.005%, also are present in the literature but not always with satisfactory results.10,11 Another successfully administered topical steroid is mometasone furoate cream 0.1%.12 Although the use of intralesional triamcinolone acetonide also has demonstrated encouraging outcomes in isolated reports,13 associated adverse events, such as pain and hematomas, can result in tolerability issues for pediatric patients.7
Piraccini et al14 described the case of an 8-year-old patient with pustular nail psoriasis who showed improvement within 3 to 6 months of treatment with topical calcipotriol 5 μg/g as monotherapy applied to the nail and periungual tissues twice daily. Another approach, described by Diluvio et al,15 is the use of tazarotene gel 0.05% applied once daily to the affected nail plates, nail folds, and periungual skin without occlusion. In a 6-year-old patient with isolated nail psoriasis, this treatment regimen demonstrated notable improvement within 8 weeks.15
Systemic Treatments for Nail Psoriasis
Data on the successful administration of systemic agents in pediatric patients also are extremely scarce. Due to the lack of clinical trials, everyday practice is mostly based on isolated case series and case reports.
Methotrexate—Lee11 described the case of an 11-year-old girl with severe, symptomatic, 20-nail psoriatic onychodystrophy who showed a complete response to oral methotrexate 5 mg/wk after topical clobetasol propionate and calcipotriol failed. Improvement was seen as early as 4 weeks after therapy initiation, and complete resolution of the lesions was documented after 9 and 13 months of methotrexate therapy for the fingers and toes, respectively.11 The successful use of methotrexate in the improvement of psoriatic nail dystrophy in a pediatric patient also was documented by Teran et al.16 In this case, a 9-year-old girl with erythrodermic psoriasis, psoriatic arthritis, and severe onychodystrophy showed notable amelioration of all psoriatic manifestations, including the nail findings, with systemic methotrexate therapy (dose not specified).16 Notably, the authors reported that the improvement of onychodystrophy occurred with considerable delay compared to the other psoriatic lesions,16 indicating the already-known refractoriness of nail psoriasis to the various therapeutic attempts.9-15
Acitretin—Another agent that has been linked with partial improvement of acrodermatitis continua of Hallopeau (ACH)–associated onychodystrophy is acitretin. In a case series of 15 pediatric patients with pustular psoriasis, a 5-year-old boy with severe nail involvement presented with partial amelioration of nail changes with acitretin within the first 6 weeks of treatment using the following regimen: initial dosage of 0.8 mg/kg/d for 6 weeks, followed by 0.3 mg/kg/d for 4 weeks.17
Biologics—The emerging use of biologics in pediatric psoriasis also has brought important advances in the successful management of nail psoriasis in children and adolescents.18-21 Wells et al18 presented the case of an 8-year-old girl with nail psoriasis, psoriatic arthritis, and plaque psoriasis who showed complete resolution of all psoriatic manifestations, including nail involvement, within 3 months of treatment with secukinumab 150 mg subcutaneously every 4 weeks. Prior failed treatments included various systemic agents (ie, subcutaneous methotrexate 20 mg/m2, etanercept 0.8 mg/kg weekly, adalimumab 40 mg every 2 weeks) as well as topical agents (ie, urea, tazarotene, corticosteroids) and intralesional triamcinolone.18
Infliximab also has been successfully used for pediatric nail psoriasis. Watabe et al19 presented the case of an 8-year-old girl with psoriatic onychodystrophy in addition to psoriatic onycho-pachydermo-periostitis. Prior therapy with adalimumab 20 mg every other week combined with methotrexate 10 mg weekly failed. She experienced notable amelioration of the nail dystrophy within 3 months of using a combination of infliximab and methotrexate (infliximab 5 mg/kg intravenously on weeks 0, 2, and 6, and every 8 weeks thereafter; methotrexate 10 mg/wk).19
Cases in which infliximab has resulted in rapid yet only transient restoration of psoriatic onychodystrophy also are present in the literature. Pereira et al20 reported that a 3-year-old patient with severe 20-digit onychodystrophy in addition to pustular psoriasis had complete resolution of nail lesions within 2 weeks of treatment with infliximab (5 mg/kg at weeks 0, 2, and 6, and then every 7 weeks thereafter), which was sustained over the course of 1 year. The therapy had to be discontinued because of exacerbation of the cutaneous symptoms; thereafter, etanercept was initiated. Although the patient noted major improvement of all skin lesions under etanercept, only moderate amelioration of the psoriatic nail lesions was demonstrated.20
Dini et al21 described a 9-year-old girl with severe ACH-associated psoriatic onychodystrophy who showed complete clearance of all lesions within 8 weeks of treatment with adalimumab (initially 80 mg, followed by 40 mg after 1 week and then 40 mg every other week). Prior treatment with potent topical corticosteroids, cyclosporine (3 mg/kg/d for 6 months), and etanercept (0.4 mg/kg twice weekly for 3 months) was ineffective.21
Phototherapy—Other systemic agents with reported satisfactory outcomes in the treatment of psoriatic onychodystrophy include thalidomide combined with UVB phototherapy. Kiszewski et al22 described a 2-year-old patient with ACH and severe 19-digit onychodystrophy. Prior failed therapies included occluded clobetasol ointment 0.05%, occluded pimecrolimus 0.1%, and systemic methotrexate, while systemic acitretin (0.8 mg⁄kg⁄d) resulted in elevated cholesterol levels and therefore had to be interrupted. Improvement was seen 2 months after the initiation of a combined broadband UVB and thalidomide (50 mg⁄d) treatment, with no documented relapses after discontinuation of therapy.22
Narrowband UVB (311 nm) also has been used as monotherapy for ACH-associated onychodystrophy, as demonstrated by Bordignon et al.23 They reported a 9-year-old patient who showed partial improvement of isolated onychodystrophy of the fourth nail plate of the left hand after 36 sessions of narrowband UVB using a 311-nm filtering handpiece with a square spot size of 19×19 mm.23
Conclusion
Nail psoriasis constitutes a type of psoriasis that is not only refractory to most treatments but is accompanied by substantial psychological and occasionally functional burden for the affected individuals.24 Data concerning therapeutic options in the pediatric population are extremely limited, and therefore the everyday practice often involves administration of off-label medications, which can constitute a dilemma for many physicians, especially for safety.10 We suggest a simple therapeutic algorithm for the management of pediatric nail psoriasis based on the summarized data that are currently available in the literature. This algorithm is shown in the eFigure.

As progressively more agents—especially biologics—receive approval for use in plaque psoriasis in pediatric patients,25 it is expected that gradually more real-life data on their side efficacy for plaque psoriasis of the nails in children also will come to light. Furthermore, their on-label use in pediatric psoriasis patients will facilitate further relevant clinical trials to this target group so that the potential of these medications in the management of nail psoriasis can be fully explored.
Pediatric nail psoriasis is a condition that has not been extensively studied. The prevalence of nail alterations in pediatric patients with psoriasis varies among different studies, ranging from 17% to 39.2%.1 Nail pitting, onycholysis associated with subungual hyperkeratosis, paronychia, and pachyonychia are the most frequent features of psoriatic nail involvement in children.2,3 The management of nail psoriasis in children and adolescents is critical due to the quality-of-life impact, from potential functional impairment issues to the obvious cosmetic problems, which can aggravate the psychologic distress and social embarrassment of patients with psoriasis. Despite the emergence of modern potent systemic agents to treat chronic plaque psoriasis, nail psoriasis often is refractory to treatment.4 Coupled with the limited on-label options for psoriasis treatment in children, the management of nail psoriasis in this special patient group constitutes an even greater therapeutic challenge. This report aims to summarize the limited existing data on the successful management of nail psoriasis in the pediatric population.
Reviewing the Literature on Nail Psoriasis
We conducted a search of PubMed articles indexed for MEDLINE, Embase, and Scopus using the following Medical Subject Headings key terms: nail psoriasis and children, juvenile, pediatric. Additional articles were identified from the reference lists of the retrieved articles and citations. Our search included reports in the English language published from 2000 to 2019. The selection process included the following 2 steps: screening of the titles and abstracts, followed by evaluation of the selected full-text articles.
Topical Treatments for Nail Psoriasis
Because most systemic antipsoriatic treatments that can be administered in adult patients have not yet been granted an official license for administration in children, topical treatments are considered by many physicians as the preferred first-line therapy for psoriatic nail involvement in pediatric patients.5,6 However, only scarce data are available in the literature concerning the successful use of local agents in pediatric patients with psoriasis.
The main limitation of local treatments relates mostly to their impaired penetration into the affected area (nails). To optimize drug penetration, some authors suggest the use of potent keratolytic topical preparations to reduce the nail volume and facilitate drug absorption.7 A popular suggestion is trimming the onycholytic nail plate followed by 40% urea avulsion to treat subungual hyperkeratosis8 or simply the use of occlusive 40% urea in petroleum jelly.9 Other approaches include clipping the onycholytic nail over the diseased nail bed or processing the nail plate through grinding or even drilling holes with the use of mechanical burrs or ablative lasers to enhance the penetration of the topical agent.7
A frequent approach in pediatric patients is clipping the detached nails combined with daily application of calcipotriene (calcipotriol) and steroids, such as betamethasone dipropionate.5,8 Reports on the use of regimens with clobetasol propionate ointment 0.05% under occlusion, with or without the concomitant use of calcipotriol solution 0.005%, also are present in the literature but not always with satisfactory results.10,11 Another successfully administered topical steroid is mometasone furoate cream 0.1%.12 Although the use of intralesional triamcinolone acetonide also has demonstrated encouraging outcomes in isolated reports,13 associated adverse events, such as pain and hematomas, can result in tolerability issues for pediatric patients.7
Piraccini et al14 described the case of an 8-year-old patient with pustular nail psoriasis who showed improvement within 3 to 6 months of treatment with topical calcipotriol 5 μg/g as monotherapy applied to the nail and periungual tissues twice daily. Another approach, described by Diluvio et al,15 is the use of tazarotene gel 0.05% applied once daily to the affected nail plates, nail folds, and periungual skin without occlusion. In a 6-year-old patient with isolated nail psoriasis, this treatment regimen demonstrated notable improvement within 8 weeks.15
Systemic Treatments for Nail Psoriasis
Data on the successful administration of systemic agents in pediatric patients also are extremely scarce. Due to the lack of clinical trials, everyday practice is mostly based on isolated case series and case reports.
Methotrexate—Lee11 described the case of an 11-year-old girl with severe, symptomatic, 20-nail psoriatic onychodystrophy who showed a complete response to oral methotrexate 5 mg/wk after topical clobetasol propionate and calcipotriol failed. Improvement was seen as early as 4 weeks after therapy initiation, and complete resolution of the lesions was documented after 9 and 13 months of methotrexate therapy for the fingers and toes, respectively.11 The successful use of methotrexate in the improvement of psoriatic nail dystrophy in a pediatric patient also was documented by Teran et al.16 In this case, a 9-year-old girl with erythrodermic psoriasis, psoriatic arthritis, and severe onychodystrophy showed notable amelioration of all psoriatic manifestations, including the nail findings, with systemic methotrexate therapy (dose not specified).16 Notably, the authors reported that the improvement of onychodystrophy occurred with considerable delay compared to the other psoriatic lesions,16 indicating the already-known refractoriness of nail psoriasis to the various therapeutic attempts.9-15
Acitretin—Another agent that has been linked with partial improvement of acrodermatitis continua of Hallopeau (ACH)–associated onychodystrophy is acitretin. In a case series of 15 pediatric patients with pustular psoriasis, a 5-year-old boy with severe nail involvement presented with partial amelioration of nail changes with acitretin within the first 6 weeks of treatment using the following regimen: initial dosage of 0.8 mg/kg/d for 6 weeks, followed by 0.3 mg/kg/d for 4 weeks.17
Biologics—The emerging use of biologics in pediatric psoriasis also has brought important advances in the successful management of nail psoriasis in children and adolescents.18-21 Wells et al18 presented the case of an 8-year-old girl with nail psoriasis, psoriatic arthritis, and plaque psoriasis who showed complete resolution of all psoriatic manifestations, including nail involvement, within 3 months of treatment with secukinumab 150 mg subcutaneously every 4 weeks. Prior failed treatments included various systemic agents (ie, subcutaneous methotrexate 20 mg/m2, etanercept 0.8 mg/kg weekly, adalimumab 40 mg every 2 weeks) as well as topical agents (ie, urea, tazarotene, corticosteroids) and intralesional triamcinolone.18
Infliximab also has been successfully used for pediatric nail psoriasis. Watabe et al19 presented the case of an 8-year-old girl with psoriatic onychodystrophy in addition to psoriatic onycho-pachydermo-periostitis. Prior therapy with adalimumab 20 mg every other week combined with methotrexate 10 mg weekly failed. She experienced notable amelioration of the nail dystrophy within 3 months of using a combination of infliximab and methotrexate (infliximab 5 mg/kg intravenously on weeks 0, 2, and 6, and every 8 weeks thereafter; methotrexate 10 mg/wk).19
Cases in which infliximab has resulted in rapid yet only transient restoration of psoriatic onychodystrophy also are present in the literature. Pereira et al20 reported that a 3-year-old patient with severe 20-digit onychodystrophy in addition to pustular psoriasis had complete resolution of nail lesions within 2 weeks of treatment with infliximab (5 mg/kg at weeks 0, 2, and 6, and then every 7 weeks thereafter), which was sustained over the course of 1 year. The therapy had to be discontinued because of exacerbation of the cutaneous symptoms; thereafter, etanercept was initiated. Although the patient noted major improvement of all skin lesions under etanercept, only moderate amelioration of the psoriatic nail lesions was demonstrated.20
Dini et al21 described a 9-year-old girl with severe ACH-associated psoriatic onychodystrophy who showed complete clearance of all lesions within 8 weeks of treatment with adalimumab (initially 80 mg, followed by 40 mg after 1 week and then 40 mg every other week). Prior treatment with potent topical corticosteroids, cyclosporine (3 mg/kg/d for 6 months), and etanercept (0.4 mg/kg twice weekly for 3 months) was ineffective.21
Phototherapy—Other systemic agents with reported satisfactory outcomes in the treatment of psoriatic onychodystrophy include thalidomide combined with UVB phototherapy. Kiszewski et al22 described a 2-year-old patient with ACH and severe 19-digit onychodystrophy. Prior failed therapies included occluded clobetasol ointment 0.05%, occluded pimecrolimus 0.1%, and systemic methotrexate, while systemic acitretin (0.8 mg⁄kg⁄d) resulted in elevated cholesterol levels and therefore had to be interrupted. Improvement was seen 2 months after the initiation of a combined broadband UVB and thalidomide (50 mg⁄d) treatment, with no documented relapses after discontinuation of therapy.22
Narrowband UVB (311 nm) also has been used as monotherapy for ACH-associated onychodystrophy, as demonstrated by Bordignon et al.23 They reported a 9-year-old patient who showed partial improvement of isolated onychodystrophy of the fourth nail plate of the left hand after 36 sessions of narrowband UVB using a 311-nm filtering handpiece with a square spot size of 19×19 mm.23
Conclusion
Nail psoriasis constitutes a type of psoriasis that is not only refractory to most treatments but is accompanied by substantial psychological and occasionally functional burden for the affected individuals.24 Data concerning therapeutic options in the pediatric population are extremely limited, and therefore the everyday practice often involves administration of off-label medications, which can constitute a dilemma for many physicians, especially for safety.10 We suggest a simple therapeutic algorithm for the management of pediatric nail psoriasis based on the summarized data that are currently available in the literature. This algorithm is shown in the eFigure.

As progressively more agents—especially biologics—receive approval for use in plaque psoriasis in pediatric patients,25 it is expected that gradually more real-life data on their side efficacy for plaque psoriasis of the nails in children also will come to light. Furthermore, their on-label use in pediatric psoriasis patients will facilitate further relevant clinical trials to this target group so that the potential of these medications in the management of nail psoriasis can be fully explored.
- Uber M, Carvalho VO, Abagge KT, et al. Clinical features and nail clippings in 52 children with psoriasis. Pediatr Dermatol. 2018;35:202-207.
- Pourchot D, Bodemer C, Phan A, et al. Nail psoriasis: a systematic evaluation in 313 children with psoriasis. Pediatr Dermatol. 2017;34:58-63.
- Piraccini BM, Triantafyllopoulou I, Prevezas C, et al. Nail psoriasis in children: common or uncommon? results from a 10-year double-center study. Skin Appendage Disord. 2015;1:43-48.
- Baran R. The burden of nail psoriasis: an introduction. Dermatology. 2010;221(suppl 1):1-5.
- Richert B, André J. Nail disorders in children: diagnosis and management. Am J Clin Dermatol. 2011;12:101-112.
- Trüeb RM. Therapies for childhood psoriasis. Curr Probl Dermatol. 2009;38:137-159.
- Haneke E. Nail psoriasis: clinical features, pathogenesis, differential diagnoses, and management. Psoriasis (Auckl). 2017;7:51-63.
- Piraccini BM, Starace M. Nail disorders in infants and children. Curr Opin Pediatr. 2014;26:440-445.
- Duran-McKinster C, Ortiz-Solis D, Granados J, et al. Juvenile psoriatic arthritis with nail psoriasis in the absence of cutaneous lesions. Int J Dermatol. 2000;39:32-35.
- Holzberg M, Ruben BS, Baran R. Psoriasis restricted to the nail in a 7-year-old child. should biologics be an appropriate treatment modality when considering quality of life? J Eur Acad Dermatol Venereol. 2014;28:668-670.
- Lee JY. Severe 20-nail psoriasis successfully treated by low dose methotrexate. Dermatol Online J. 2009;15:8.
- Liao YC, Lee JY. Psoriasis in a 3-month-old infant with Kawasaki disease. Dermatol Online J. 2009;15:10.
- Khoo BP, Giam YC. A pilot study on the role of intralesional triamcinolone acetonide in the treatment of pitted nails in children. Singapore Med J. 2000;41:66-68.
- Piraccini BM, Tosti A, Iorizzo M, et al. Pustular psoriasis of the nails: treatment and long-term follow-up of 46 patients. Br J Dermatol. 2001;144:1000-1005.
- Diluvio L, Campione E, Paternò EJ, et al. Childhood nail psoriasis: a useful treatment with tazarotene 0.05%. Pediatr Dermatol. 2007;24:332-333.
- Teran CG, Teran-Escalera CN, Balderrama C. A severe case of erythrodermic psoriasis associated with advanced nail and joint manifestations: a case report. J Med Case Rep. 2010;4:179.
- Chen P, Li C, Xue R, et al. Efficacy and safety of acitretin monotherapy in children with pustular psoriasis: results from 15 cases and a literature review. J Dermatolog Treat. 2018;29:353-363.
- Wells LE, Evans T, Hilton R, et al. Use of secukinumab in a pediatric patient leads to significant improvement in nail psoriasis and psoriatic arthritis. Pediatr Dermatol. 2019;36:384-385.
- Watabe D, Endoh K, Maeda F, et al. Childhood-onset psoriatic onycho-pachydermo-periostitis treated successfully with infliximab. Eur J Dermatol. 2015;25:506-508.
- Pereira TM, Vieira AP, Fernandes JC, et al. Anti-TNF-alpha therapy in childhood pustular psoriasis. Dermatology. 2006;213:350-352.
- Dini V, Barbanera S, Romanelli M. Efficacy of adalimumab for the treatment of refractory paediatric acrodermatitis continua of Hallopeau. Acta Derm Venereol. 2013;93:588-589.
- Kiszewski AE, De Villa D, Scheibel I, et al. An infant with acrodermatitis continua of Hallopeau: successful treatment with thalidomide and UVB therapy. Pediatr Dermatol. 2009;26:105-106.
- Bordignon M, Zattra E, Albertin C, et al. Successful treatment of a 9-year-old boy affected by acrodermatitis continua of Hallopeau with targeted ultraviolet B narrow-band phototherapy. Photodermatol Photoimmunol Photomed. 2010;26:41-43.
- Fabroni C, Gori A, Troiano M, et al. Infliximab efficacy in nail psoriasis. a retrospective study in 48 patients. J Eur Acad Dermatol Venereol. 2011;25:549-553.
- Lilly’s Taltz® (ixekizumab) receives U.S. FDA approval for the treatment of pediatric patients with moderate to severe plaque psoriasis. Eli Lilly and Company. March 30, 2020. Accessed September 24, 2021. https://investor.lilly.com/news-releases/news-release-details/lillys-taltzr-ixekizumab-receives-us-fda-approval-treatment-1
- Uber M, Carvalho VO, Abagge KT, et al. Clinical features and nail clippings in 52 children with psoriasis. Pediatr Dermatol. 2018;35:202-207.
- Pourchot D, Bodemer C, Phan A, et al. Nail psoriasis: a systematic evaluation in 313 children with psoriasis. Pediatr Dermatol. 2017;34:58-63.
- Piraccini BM, Triantafyllopoulou I, Prevezas C, et al. Nail psoriasis in children: common or uncommon? results from a 10-year double-center study. Skin Appendage Disord. 2015;1:43-48.
- Baran R. The burden of nail psoriasis: an introduction. Dermatology. 2010;221(suppl 1):1-5.
- Richert B, André J. Nail disorders in children: diagnosis and management. Am J Clin Dermatol. 2011;12:101-112.
- Trüeb RM. Therapies for childhood psoriasis. Curr Probl Dermatol. 2009;38:137-159.
- Haneke E. Nail psoriasis: clinical features, pathogenesis, differential diagnoses, and management. Psoriasis (Auckl). 2017;7:51-63.
- Piraccini BM, Starace M. Nail disorders in infants and children. Curr Opin Pediatr. 2014;26:440-445.
- Duran-McKinster C, Ortiz-Solis D, Granados J, et al. Juvenile psoriatic arthritis with nail psoriasis in the absence of cutaneous lesions. Int J Dermatol. 2000;39:32-35.
- Holzberg M, Ruben BS, Baran R. Psoriasis restricted to the nail in a 7-year-old child. should biologics be an appropriate treatment modality when considering quality of life? J Eur Acad Dermatol Venereol. 2014;28:668-670.
- Lee JY. Severe 20-nail psoriasis successfully treated by low dose methotrexate. Dermatol Online J. 2009;15:8.
- Liao YC, Lee JY. Psoriasis in a 3-month-old infant with Kawasaki disease. Dermatol Online J. 2009;15:10.
- Khoo BP, Giam YC. A pilot study on the role of intralesional triamcinolone acetonide in the treatment of pitted nails in children. Singapore Med J. 2000;41:66-68.
- Piraccini BM, Tosti A, Iorizzo M, et al. Pustular psoriasis of the nails: treatment and long-term follow-up of 46 patients. Br J Dermatol. 2001;144:1000-1005.
- Diluvio L, Campione E, Paternò EJ, et al. Childhood nail psoriasis: a useful treatment with tazarotene 0.05%. Pediatr Dermatol. 2007;24:332-333.
- Teran CG, Teran-Escalera CN, Balderrama C. A severe case of erythrodermic psoriasis associated with advanced nail and joint manifestations: a case report. J Med Case Rep. 2010;4:179.
- Chen P, Li C, Xue R, et al. Efficacy and safety of acitretin monotherapy in children with pustular psoriasis: results from 15 cases and a literature review. J Dermatolog Treat. 2018;29:353-363.
- Wells LE, Evans T, Hilton R, et al. Use of secukinumab in a pediatric patient leads to significant improvement in nail psoriasis and psoriatic arthritis. Pediatr Dermatol. 2019;36:384-385.
- Watabe D, Endoh K, Maeda F, et al. Childhood-onset psoriatic onycho-pachydermo-periostitis treated successfully with infliximab. Eur J Dermatol. 2015;25:506-508.
- Pereira TM, Vieira AP, Fernandes JC, et al. Anti-TNF-alpha therapy in childhood pustular psoriasis. Dermatology. 2006;213:350-352.
- Dini V, Barbanera S, Romanelli M. Efficacy of adalimumab for the treatment of refractory paediatric acrodermatitis continua of Hallopeau. Acta Derm Venereol. 2013;93:588-589.
- Kiszewski AE, De Villa D, Scheibel I, et al. An infant with acrodermatitis continua of Hallopeau: successful treatment with thalidomide and UVB therapy. Pediatr Dermatol. 2009;26:105-106.
- Bordignon M, Zattra E, Albertin C, et al. Successful treatment of a 9-year-old boy affected by acrodermatitis continua of Hallopeau with targeted ultraviolet B narrow-band phototherapy. Photodermatol Photoimmunol Photomed. 2010;26:41-43.
- Fabroni C, Gori A, Troiano M, et al. Infliximab efficacy in nail psoriasis. a retrospective study in 48 patients. J Eur Acad Dermatol Venereol. 2011;25:549-553.
- Lilly’s Taltz® (ixekizumab) receives U.S. FDA approval for the treatment of pediatric patients with moderate to severe plaque psoriasis. Eli Lilly and Company. March 30, 2020. Accessed September 24, 2021. https://investor.lilly.com/news-releases/news-release-details/lillys-taltzr-ixekizumab-receives-us-fda-approval-treatment-1
Practice Points
- No clinical trials assessing the management of pediatric nail psoriasis currently are present in the literature. Limited information on the treatment of pediatric nail psoriasis exists, mostly in the form of small case series and case reports.
- As more agents are approved for on-label use in plaque psoriasis in pediatric patients, gradually more real-life data on their efficacy for nail psoriasis in children are expected to come to light.
Clinical Edge Journal Scan Commentary: Psoriasis November 2021
Biologic therapy is generally reserved for patients with more moderate-to-severe psoriasis, often defined as BSA ≥10% or PASI of ≥10. Notably, these criteria are all clinician-performed. The Dermatology Life Quality Index (DLQI) measures the impact of the disease on the patient with a score of 0-5, 6-10, and 11-30 indicating mild, moderate, and severe disease, respectively. In a cross-sectional, observational study, 72.4% of psoriasis patients who qualified for systemic therapy initiation based on PASI and/or BSA had a DLQI of less than 10. Conversely, 10.4% of patients with a DLQI score higher than 10 did not qualify for systemic therapy based on PASI and/or BSA. This study highlights the complementary value of considering both clinician and patient determinants of disease severity when choosing a psoriasis therapy (Barbieri JS et al.)
With so many therapeutic options, it is increasingly common for patients to switch from one biologic to another. Many factors that go into the decision to switch therapies. A retrospective study of 115 adult patients with psoriasis found that the primary factor driving switching was lack of sufficient efficacy in treating skin disease. Having concomitant psoriatic arthritis increased the likelihood of switching by 2.69-fold (Akdogan N et al.). These findings suggest that shared decision making with dermatology, rheumatology, and the patient may help in choosing a treatment plan that best addresses both skin and joints.
One consideration when counseling patients about their likelihood of response to a second or third biologic is that in several clinical trials, biologic-naïve patients tend to have higher PASI responses to biologics than do heavier and biologic-experienced patients. A recent retrospective study found that receiving one or more biologic receiving was associated with a lower likelihood of achieving a PASI75 response to guselkumab (Hung YT et al.). However, in a real-life multicenter study including 57 adult patients with moderate-to-severe psoriasis receiving risankizumab, more biologic-experienced patients reached PASI 100 at weeks 36 and 52 (71.8% and 69.2%, respectively) compared with biologic-naïve patients (50.0% and 37.5%, respectively) (Gerdes S et al.). While both studies were small and no firm conclusions or comparisons can be drawn, real-world data from large multicenter studies that evaluate multiple therapies may guide us in choosing the best therapy for challenging patients who are often underrepresented in clinical trials. Understanding how switching within vs. between classes (TNF, IL-17, and IL-23 inhibitors) impacts efficacy will also help in making choices about biologic switches.
Biologic therapy is generally reserved for patients with more moderate-to-severe psoriasis, often defined as BSA ≥10% or PASI of ≥10. Notably, these criteria are all clinician-performed. The Dermatology Life Quality Index (DLQI) measures the impact of the disease on the patient with a score of 0-5, 6-10, and 11-30 indicating mild, moderate, and severe disease, respectively. In a cross-sectional, observational study, 72.4% of psoriasis patients who qualified for systemic therapy initiation based on PASI and/or BSA had a DLQI of less than 10. Conversely, 10.4% of patients with a DLQI score higher than 10 did not qualify for systemic therapy based on PASI and/or BSA. This study highlights the complementary value of considering both clinician and patient determinants of disease severity when choosing a psoriasis therapy (Barbieri JS et al.)
With so many therapeutic options, it is increasingly common for patients to switch from one biologic to another. Many factors that go into the decision to switch therapies. A retrospective study of 115 adult patients with psoriasis found that the primary factor driving switching was lack of sufficient efficacy in treating skin disease. Having concomitant psoriatic arthritis increased the likelihood of switching by 2.69-fold (Akdogan N et al.). These findings suggest that shared decision making with dermatology, rheumatology, and the patient may help in choosing a treatment plan that best addresses both skin and joints.
One consideration when counseling patients about their likelihood of response to a second or third biologic is that in several clinical trials, biologic-naïve patients tend to have higher PASI responses to biologics than do heavier and biologic-experienced patients. A recent retrospective study found that receiving one or more biologic receiving was associated with a lower likelihood of achieving a PASI75 response to guselkumab (Hung YT et al.). However, in a real-life multicenter study including 57 adult patients with moderate-to-severe psoriasis receiving risankizumab, more biologic-experienced patients reached PASI 100 at weeks 36 and 52 (71.8% and 69.2%, respectively) compared with biologic-naïve patients (50.0% and 37.5%, respectively) (Gerdes S et al.). While both studies were small and no firm conclusions or comparisons can be drawn, real-world data from large multicenter studies that evaluate multiple therapies may guide us in choosing the best therapy for challenging patients who are often underrepresented in clinical trials. Understanding how switching within vs. between classes (TNF, IL-17, and IL-23 inhibitors) impacts efficacy will also help in making choices about biologic switches.
Biologic therapy is generally reserved for patients with more moderate-to-severe psoriasis, often defined as BSA ≥10% or PASI of ≥10. Notably, these criteria are all clinician-performed. The Dermatology Life Quality Index (DLQI) measures the impact of the disease on the patient with a score of 0-5, 6-10, and 11-30 indicating mild, moderate, and severe disease, respectively. In a cross-sectional, observational study, 72.4% of psoriasis patients who qualified for systemic therapy initiation based on PASI and/or BSA had a DLQI of less than 10. Conversely, 10.4% of patients with a DLQI score higher than 10 did not qualify for systemic therapy based on PASI and/or BSA. This study highlights the complementary value of considering both clinician and patient determinants of disease severity when choosing a psoriasis therapy (Barbieri JS et al.)
With so many therapeutic options, it is increasingly common for patients to switch from one biologic to another. Many factors that go into the decision to switch therapies. A retrospective study of 115 adult patients with psoriasis found that the primary factor driving switching was lack of sufficient efficacy in treating skin disease. Having concomitant psoriatic arthritis increased the likelihood of switching by 2.69-fold (Akdogan N et al.). These findings suggest that shared decision making with dermatology, rheumatology, and the patient may help in choosing a treatment plan that best addresses both skin and joints.
One consideration when counseling patients about their likelihood of response to a second or third biologic is that in several clinical trials, biologic-naïve patients tend to have higher PASI responses to biologics than do heavier and biologic-experienced patients. A recent retrospective study found that receiving one or more biologic receiving was associated with a lower likelihood of achieving a PASI75 response to guselkumab (Hung YT et al.). However, in a real-life multicenter study including 57 adult patients with moderate-to-severe psoriasis receiving risankizumab, more biologic-experienced patients reached PASI 100 at weeks 36 and 52 (71.8% and 69.2%, respectively) compared with biologic-naïve patients (50.0% and 37.5%, respectively) (Gerdes S et al.). While both studies were small and no firm conclusions or comparisons can be drawn, real-world data from large multicenter studies that evaluate multiple therapies may guide us in choosing the best therapy for challenging patients who are often underrepresented in clinical trials. Understanding how switching within vs. between classes (TNF, IL-17, and IL-23 inhibitors) impacts efficacy will also help in making choices about biologic switches.
Certain DMTs in MS linked to more psoriasis
, a new study finds. However, overall rates of reported disease are very low, and there’s no confirmation of a connection.
“People with MS and comorbid psoriasis – or those at a known high-risk for developing psoriasis – may benefit from a careful consideration of disease-modifying therapy (DMT), specifically when B cell-depleting therapies are considered,” study coauthor and Medical College of Wisconsin neurologist Ahmed Obeidat, MD, PhD, said in an interview. The findings were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Dr. Obeidat and colleagues launched the study after noticing cases of psoriasis that developed months to years after patients started taking ocrelizumab, a B cell-depleting therapy. “We referred to the published literature and only found very scant reports of MS, psoriasis, and B cell-depleting therapy use,” he said. “Thus we decided to pursue an investigation of a large [Food and Drug Administration] database to examine for possible out-of-proportion reports for psoriasis in patients with MS who were receiving B cell-depleting therapy.”
The researchers tracked case reports of psoriasis in patients with MS on DMTs from 2009 to 2020 via the FDA Adverse Event Reporting System. They found 517 psoriasis reports among 45,547 reports of skin/cutaneous conditions. The reports were linked to interferon beta 1a (136 reports, 26% of total), natalizumab (107, 21%), fingolimod (75, 15%), dimethyl fumarate (64, 12%), ocrelizumab (49, 10%), teriflunomide (28, 5%), interferon beta 1b (22, 4%), glatiramer acetate (12, 2%), rituximab (10, 2%), and alemtuzumab (9, 2%).
The total numbers of cases is low, but this may reflect underreporting due to the assumption that “autoimmunity begets autoimmunity” and therefore cases of psoriasis in MS are not alarming, medical student Mokshal H. Porwal, the study lead author, said in an interview.
The average age of patients – 48-51 – was similar for all of the drugs except alemtuzumab (mean age 41), which had a very small number of cases. The percentage of cases in females was 71%-77% for most of the drugs, with a few exceptions: rituximab (60%), ocrelizumab (63%), and alemtuzumab (33%).
Other drugs – cladribine, siponimod, and ozanimod – had 1, 1, and 0 reports, respectively, and were not included in the age and gender analyses.
The researchers also found that psoriasis made up about 65% of all skin/cutaneous adverse reports for rituximab, the highest number among DMTs. By comparison, that number was about 30% for ocrelizumab and under 1% for dimethyl fumarate and alemtuzumab.
Links between psoriasis and MS are murky, Dr. Obeidat said. “Some studies consider the presence of psoriasis as a possible indicator of increased future risk for MS, but there’s no clear association between the two conditions,” he said.
As for DMTs, “a few case reports of psoriasis in association with interferon-beta and rare case reports in association with ocrelizumab therapy have been published. However, the possible association between certain DMTs and psoriasis remains unclear,” he said.
Going forward, “we advise that patients with psoriasis on B cell-depleting agents are monitored more closely,” Dr. Obeidat said. “If the psoriasis worsens, it may be beneficial to think about potential alternative therapies.”
No study funding is reported. Dr. Obeidat reports various disclosures; the other authors report no disclosures.
, a new study finds. However, overall rates of reported disease are very low, and there’s no confirmation of a connection.
“People with MS and comorbid psoriasis – or those at a known high-risk for developing psoriasis – may benefit from a careful consideration of disease-modifying therapy (DMT), specifically when B cell-depleting therapies are considered,” study coauthor and Medical College of Wisconsin neurologist Ahmed Obeidat, MD, PhD, said in an interview. The findings were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Dr. Obeidat and colleagues launched the study after noticing cases of psoriasis that developed months to years after patients started taking ocrelizumab, a B cell-depleting therapy. “We referred to the published literature and only found very scant reports of MS, psoriasis, and B cell-depleting therapy use,” he said. “Thus we decided to pursue an investigation of a large [Food and Drug Administration] database to examine for possible out-of-proportion reports for psoriasis in patients with MS who were receiving B cell-depleting therapy.”
The researchers tracked case reports of psoriasis in patients with MS on DMTs from 2009 to 2020 via the FDA Adverse Event Reporting System. They found 517 psoriasis reports among 45,547 reports of skin/cutaneous conditions. The reports were linked to interferon beta 1a (136 reports, 26% of total), natalizumab (107, 21%), fingolimod (75, 15%), dimethyl fumarate (64, 12%), ocrelizumab (49, 10%), teriflunomide (28, 5%), interferon beta 1b (22, 4%), glatiramer acetate (12, 2%), rituximab (10, 2%), and alemtuzumab (9, 2%).
The total numbers of cases is low, but this may reflect underreporting due to the assumption that “autoimmunity begets autoimmunity” and therefore cases of psoriasis in MS are not alarming, medical student Mokshal H. Porwal, the study lead author, said in an interview.
The average age of patients – 48-51 – was similar for all of the drugs except alemtuzumab (mean age 41), which had a very small number of cases. The percentage of cases in females was 71%-77% for most of the drugs, with a few exceptions: rituximab (60%), ocrelizumab (63%), and alemtuzumab (33%).
Other drugs – cladribine, siponimod, and ozanimod – had 1, 1, and 0 reports, respectively, and were not included in the age and gender analyses.
The researchers also found that psoriasis made up about 65% of all skin/cutaneous adverse reports for rituximab, the highest number among DMTs. By comparison, that number was about 30% for ocrelizumab and under 1% for dimethyl fumarate and alemtuzumab.
Links between psoriasis and MS are murky, Dr. Obeidat said. “Some studies consider the presence of psoriasis as a possible indicator of increased future risk for MS, but there’s no clear association between the two conditions,” he said.
As for DMTs, “a few case reports of psoriasis in association with interferon-beta and rare case reports in association with ocrelizumab therapy have been published. However, the possible association between certain DMTs and psoriasis remains unclear,” he said.
Going forward, “we advise that patients with psoriasis on B cell-depleting agents are monitored more closely,” Dr. Obeidat said. “If the psoriasis worsens, it may be beneficial to think about potential alternative therapies.”
No study funding is reported. Dr. Obeidat reports various disclosures; the other authors report no disclosures.
, a new study finds. However, overall rates of reported disease are very low, and there’s no confirmation of a connection.
“People with MS and comorbid psoriasis – or those at a known high-risk for developing psoriasis – may benefit from a careful consideration of disease-modifying therapy (DMT), specifically when B cell-depleting therapies are considered,” study coauthor and Medical College of Wisconsin neurologist Ahmed Obeidat, MD, PhD, said in an interview. The findings were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Dr. Obeidat and colleagues launched the study after noticing cases of psoriasis that developed months to years after patients started taking ocrelizumab, a B cell-depleting therapy. “We referred to the published literature and only found very scant reports of MS, psoriasis, and B cell-depleting therapy use,” he said. “Thus we decided to pursue an investigation of a large [Food and Drug Administration] database to examine for possible out-of-proportion reports for psoriasis in patients with MS who were receiving B cell-depleting therapy.”
The researchers tracked case reports of psoriasis in patients with MS on DMTs from 2009 to 2020 via the FDA Adverse Event Reporting System. They found 517 psoriasis reports among 45,547 reports of skin/cutaneous conditions. The reports were linked to interferon beta 1a (136 reports, 26% of total), natalizumab (107, 21%), fingolimod (75, 15%), dimethyl fumarate (64, 12%), ocrelizumab (49, 10%), teriflunomide (28, 5%), interferon beta 1b (22, 4%), glatiramer acetate (12, 2%), rituximab (10, 2%), and alemtuzumab (9, 2%).
The total numbers of cases is low, but this may reflect underreporting due to the assumption that “autoimmunity begets autoimmunity” and therefore cases of psoriasis in MS are not alarming, medical student Mokshal H. Porwal, the study lead author, said in an interview.
The average age of patients – 48-51 – was similar for all of the drugs except alemtuzumab (mean age 41), which had a very small number of cases. The percentage of cases in females was 71%-77% for most of the drugs, with a few exceptions: rituximab (60%), ocrelizumab (63%), and alemtuzumab (33%).
Other drugs – cladribine, siponimod, and ozanimod – had 1, 1, and 0 reports, respectively, and were not included in the age and gender analyses.
The researchers also found that psoriasis made up about 65% of all skin/cutaneous adverse reports for rituximab, the highest number among DMTs. By comparison, that number was about 30% for ocrelizumab and under 1% for dimethyl fumarate and alemtuzumab.
Links between psoriasis and MS are murky, Dr. Obeidat said. “Some studies consider the presence of psoriasis as a possible indicator of increased future risk for MS, but there’s no clear association between the two conditions,” he said.
As for DMTs, “a few case reports of psoriasis in association with interferon-beta and rare case reports in association with ocrelizumab therapy have been published. However, the possible association between certain DMTs and psoriasis remains unclear,” he said.
Going forward, “we advise that patients with psoriasis on B cell-depleting agents are monitored more closely,” Dr. Obeidat said. “If the psoriasis worsens, it may be beneficial to think about potential alternative therapies.”
No study funding is reported. Dr. Obeidat reports various disclosures; the other authors report no disclosures.
FROM CMSC 2021
Better COVID-19 outcomes confirmed in TNF inhibitor users
Among patients with immune-mediated inflammatory diseases (IMIDs) who get COVID-19, the risk for hospitalization and death is lower if they are receiving tumor necrosis factor (TNF) inhibitor monotherapy, compared with receiving most other common drugs for these conditions, with or without TNF inhibitors, according to a study published in JAMA Network Open The only combination not associated with an increased risk for hospitalization or death was TNF inhibitor therapy with methotrexate.
“These findings support the continued use of TNF inhibitor monotherapy during the pandemic and warrant further research investigating the association of other biologic therapies with COVID-19 outcomes,” write Zara Izadi, MPharm, of the University of California, San Francisco, and her colleagues. “Treatment with TNF inhibitor combination therapy was associated with a more favorable safety profile when methotrexate rather than azathioprine/6-mercaptopurine was used, suggesting that clinicians would benefit from weighing the risks versus benefits of deescalating treatment or changing medications when a patient is receiving concomitant TNF inhibitors and azathioprine/6-mercaptopurine,” they write.
Findings mirror those seen in other settings
These findings are in line with what has been found in other settings, according to Joel M. Gelfand, MD, director of the psoriasis and phototherapy treatment center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, Philadelphia.
“In the beginning of the pandemic, there was concern about use of immune-modulating treatments, and many patients self-discontinued treatments like TNF inhibitors,” Dr. Gelfand, who was not involved in the study, told this news organization. “This has ultimately proved unnecessary and unfortunately resulted in harm to many patients due to flaring of their underlying disease.”
Dr. Gelfand emphasized the importance of vaccinating patients against COVID-19 as soon as possible and of getting a third dose for those who are already fully vaccinated with the Pfizer or Moderna shots, as recommended by the Centers for Disease Control and Prevention.
“I typically recommend this third dose be taken 6 months after the second dose,” Dr. Gelfand said. “The good news is that TNF inhibitors do not seem to meaningfully impact response to mRNA vaccines.”
Study details
The researchers analyzed data from three international registries of adults with rheumatic diseases, inflammatory bowel disease, and psoriasis who had COVID-19 between March 12, 2020, and Feb. 1, 2021. The registries included the Secure Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD) registry, the Psoriasis Patient Registry for Outcomes, Therapy and Epidemiology of COVID-19 Infection (PsoProtect), and the physician-reported registry from the Global Rheumatology Alliance (GRA).
The population included 6,077 patients from 74 countries. About half of the cohort (52.9%) were from Europe; more than half were women (58.6%). The average age was 48 years. A little over one-third of the patients (35.3%) had rheumatoid arthritis, 25.3% had Crohn’s disease, 12.5% had ulcerative colitis, 10.3% had spondyloarthritis, and 9.3% had psoriatic arthritis. Smaller percentages had psoriasis (4.9%), another type of arthritis or multiple types (1.7%), or another inflammatory bowel disease (0.6%).
One in five patients (21.3%) were hospitalized, and 3.1% died. The researchers compared outcomes for those who were receiving TNF inhibitor therapy alone to outcomes for those who were taking azathioprine/6-mercaptopurine therapy (alone or with a TNF inhibitor), methotrexate (alone or with a TNF inhibitor), and Janus kinase (JAK) inhibitors. They adjusted their analysis to account for active disease and common comorbidities, as well as geography and the period during the pandemic in which the person was admitted, because treatment regimens and hospitalization indications have varied over time.
All of the therapies except the combination of TNF inhibitors and methotrexate were associated with higher odds of hospitalization and death than TNF inhibitor monotherapy.
The researchers explored several possible explanations for the findings, including the possibility that high serum TNF concentrations may have been associated with more organ damage at the time of COVID-19 admission, owing to interaction with SARS-CoV-2–associated hyperinflammation.
“Therefore, blocking TNF could inhibit this detrimental immune response,” the authors write. “Multiple case series reporting favorable outcomes among patients receiving TNF inhibitor therapy support this assertion.”
Another possibility relates to the effects of taking non–TNF inhibitor medications for immunosuppression. The authors note that thiopurine medications are linked to a greater risk for opportunistic viral infections and that JAK inhibitors may reduce the body’s ability to clear the virus because of its suppression of innate immune response.
The authors also postulate that methotrexate may lower the likelihood of cytokine storm linked to COVID-19, even though methotrexate monotherapy was associated with poorer outcomes. “This association could mean that TNF inhibitor therapy is exerting a protective benefit or that methotrexate therapy is exerting a harmful consequence,” the authors write.
Caution needed in interpreting uncontrolled, registry-based data
The findings were not surprising to Stephen B. Hanauer, MD, medical director of the Digestive Health Center at Northwestern University, Chicago, who was not involved in the research.
“We’ve been monitoring IBD [inflammatory bowel disease] patients through the Secure registry similar to the rheumatologic and dermatologic societies and have not identified a signal of harm from any international groups,” Dr. Hanauer told this news organization. He noted that these registries also have not shown an increased risk for COVID-19 complications among patients receiving TNF inhibitors, antiadhesion therapies, or anti–IL12/23 inhibitors, compared with the general population not taking these therapies.
The study’s size and the diversity of patients strengthen its findings. However, the registries’ use of convenience sampling increases the potential for reporting bias, although the results remained similar after a sensitivity analysis. The study also lacked a control group, and the registries did not collect data uniformly.
“These are databases that rely on reporting from investigators and are not comprehensive prospective studies,” Dr. Hanauer noted as another study limitation.
Dr. Gelfand similarly advised caution in interpreting these findings, inasmuch as the study is a “collection of spontaneous reports” that should be viewed as hypothesis-generating rather than testing.
“Fortunately, more rigorous studies have been conducted, typically in large medical record systems, and have confirmed the hypothesis that TNF inhibitors are associated with a lower risk of poor COVID-19 outcomes, compared to other treatments,” Dr. Gelfand said.
Previous smaller studies similarly found better outcomes among patients taking TNF inhibitors, compared with other therapies, but their participants were predominantly from North America and Europe, noted Licio A. Velloso, MD, PhD, of the University of Campinas, in São Paulo, in an accompanying commentary.
On the basis of the findings of this study, “which included a much larger sample comprising distinct diseases and patients with a multitude of genetic backgrounds, the evidence in favor of the continued use of TNF inhibitor monotherapy for patients with IMIDs during the COVID-19 pandemic has become more substantial,” Dr. Velloso writes. “The finding that maintenance of TNF inhibitor monotherapy is associated with reductions in the risk of severe COVID-19 among patients with IMIDs offers new perspective that may guide health care professionals in the difficult decisions regarding therapeutic approaches among this specific group of patients.”
The research was funded by the American College of Rheumatology, the European Alliance of Associations for Rheumatology, the United Kingdom’s National Institute for Health Research Biomedical Research Center, and the Psoriasis Association. Many authors reported receiving grants and/or personal fees from a variety of pharmaceutical companies. Dr. Velloso has disclosed no relevant financial relationships. Dr. Hanauer has served as a consultant to companies that market TNF inhibitors. Dr. Gelfand has consulted for and received research grants from companies that market TNF inhibitors.
A version of this article first appeared on Medscape.com.
Among patients with immune-mediated inflammatory diseases (IMIDs) who get COVID-19, the risk for hospitalization and death is lower if they are receiving tumor necrosis factor (TNF) inhibitor monotherapy, compared with receiving most other common drugs for these conditions, with or without TNF inhibitors, according to a study published in JAMA Network Open The only combination not associated with an increased risk for hospitalization or death was TNF inhibitor therapy with methotrexate.
“These findings support the continued use of TNF inhibitor monotherapy during the pandemic and warrant further research investigating the association of other biologic therapies with COVID-19 outcomes,” write Zara Izadi, MPharm, of the University of California, San Francisco, and her colleagues. “Treatment with TNF inhibitor combination therapy was associated with a more favorable safety profile when methotrexate rather than azathioprine/6-mercaptopurine was used, suggesting that clinicians would benefit from weighing the risks versus benefits of deescalating treatment or changing medications when a patient is receiving concomitant TNF inhibitors and azathioprine/6-mercaptopurine,” they write.
Findings mirror those seen in other settings
These findings are in line with what has been found in other settings, according to Joel M. Gelfand, MD, director of the psoriasis and phototherapy treatment center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, Philadelphia.
“In the beginning of the pandemic, there was concern about use of immune-modulating treatments, and many patients self-discontinued treatments like TNF inhibitors,” Dr. Gelfand, who was not involved in the study, told this news organization. “This has ultimately proved unnecessary and unfortunately resulted in harm to many patients due to flaring of their underlying disease.”
Dr. Gelfand emphasized the importance of vaccinating patients against COVID-19 as soon as possible and of getting a third dose for those who are already fully vaccinated with the Pfizer or Moderna shots, as recommended by the Centers for Disease Control and Prevention.
“I typically recommend this third dose be taken 6 months after the second dose,” Dr. Gelfand said. “The good news is that TNF inhibitors do not seem to meaningfully impact response to mRNA vaccines.”
Study details
The researchers analyzed data from three international registries of adults with rheumatic diseases, inflammatory bowel disease, and psoriasis who had COVID-19 between March 12, 2020, and Feb. 1, 2021. The registries included the Secure Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD) registry, the Psoriasis Patient Registry for Outcomes, Therapy and Epidemiology of COVID-19 Infection (PsoProtect), and the physician-reported registry from the Global Rheumatology Alliance (GRA).
The population included 6,077 patients from 74 countries. About half of the cohort (52.9%) were from Europe; more than half were women (58.6%). The average age was 48 years. A little over one-third of the patients (35.3%) had rheumatoid arthritis, 25.3% had Crohn’s disease, 12.5% had ulcerative colitis, 10.3% had spondyloarthritis, and 9.3% had psoriatic arthritis. Smaller percentages had psoriasis (4.9%), another type of arthritis or multiple types (1.7%), or another inflammatory bowel disease (0.6%).
One in five patients (21.3%) were hospitalized, and 3.1% died. The researchers compared outcomes for those who were receiving TNF inhibitor therapy alone to outcomes for those who were taking azathioprine/6-mercaptopurine therapy (alone or with a TNF inhibitor), methotrexate (alone or with a TNF inhibitor), and Janus kinase (JAK) inhibitors. They adjusted their analysis to account for active disease and common comorbidities, as well as geography and the period during the pandemic in which the person was admitted, because treatment regimens and hospitalization indications have varied over time.
All of the therapies except the combination of TNF inhibitors and methotrexate were associated with higher odds of hospitalization and death than TNF inhibitor monotherapy.
The researchers explored several possible explanations for the findings, including the possibility that high serum TNF concentrations may have been associated with more organ damage at the time of COVID-19 admission, owing to interaction with SARS-CoV-2–associated hyperinflammation.
“Therefore, blocking TNF could inhibit this detrimental immune response,” the authors write. “Multiple case series reporting favorable outcomes among patients receiving TNF inhibitor therapy support this assertion.”
Another possibility relates to the effects of taking non–TNF inhibitor medications for immunosuppression. The authors note that thiopurine medications are linked to a greater risk for opportunistic viral infections and that JAK inhibitors may reduce the body’s ability to clear the virus because of its suppression of innate immune response.
The authors also postulate that methotrexate may lower the likelihood of cytokine storm linked to COVID-19, even though methotrexate monotherapy was associated with poorer outcomes. “This association could mean that TNF inhibitor therapy is exerting a protective benefit or that methotrexate therapy is exerting a harmful consequence,” the authors write.
Caution needed in interpreting uncontrolled, registry-based data
The findings were not surprising to Stephen B. Hanauer, MD, medical director of the Digestive Health Center at Northwestern University, Chicago, who was not involved in the research.
“We’ve been monitoring IBD [inflammatory bowel disease] patients through the Secure registry similar to the rheumatologic and dermatologic societies and have not identified a signal of harm from any international groups,” Dr. Hanauer told this news organization. He noted that these registries also have not shown an increased risk for COVID-19 complications among patients receiving TNF inhibitors, antiadhesion therapies, or anti–IL12/23 inhibitors, compared with the general population not taking these therapies.
The study’s size and the diversity of patients strengthen its findings. However, the registries’ use of convenience sampling increases the potential for reporting bias, although the results remained similar after a sensitivity analysis. The study also lacked a control group, and the registries did not collect data uniformly.
“These are databases that rely on reporting from investigators and are not comprehensive prospective studies,” Dr. Hanauer noted as another study limitation.
Dr. Gelfand similarly advised caution in interpreting these findings, inasmuch as the study is a “collection of spontaneous reports” that should be viewed as hypothesis-generating rather than testing.
“Fortunately, more rigorous studies have been conducted, typically in large medical record systems, and have confirmed the hypothesis that TNF inhibitors are associated with a lower risk of poor COVID-19 outcomes, compared to other treatments,” Dr. Gelfand said.
Previous smaller studies similarly found better outcomes among patients taking TNF inhibitors, compared with other therapies, but their participants were predominantly from North America and Europe, noted Licio A. Velloso, MD, PhD, of the University of Campinas, in São Paulo, in an accompanying commentary.
On the basis of the findings of this study, “which included a much larger sample comprising distinct diseases and patients with a multitude of genetic backgrounds, the evidence in favor of the continued use of TNF inhibitor monotherapy for patients with IMIDs during the COVID-19 pandemic has become more substantial,” Dr. Velloso writes. “The finding that maintenance of TNF inhibitor monotherapy is associated with reductions in the risk of severe COVID-19 among patients with IMIDs offers new perspective that may guide health care professionals in the difficult decisions regarding therapeutic approaches among this specific group of patients.”
The research was funded by the American College of Rheumatology, the European Alliance of Associations for Rheumatology, the United Kingdom’s National Institute for Health Research Biomedical Research Center, and the Psoriasis Association. Many authors reported receiving grants and/or personal fees from a variety of pharmaceutical companies. Dr. Velloso has disclosed no relevant financial relationships. Dr. Hanauer has served as a consultant to companies that market TNF inhibitors. Dr. Gelfand has consulted for and received research grants from companies that market TNF inhibitors.
A version of this article first appeared on Medscape.com.
Among patients with immune-mediated inflammatory diseases (IMIDs) who get COVID-19, the risk for hospitalization and death is lower if they are receiving tumor necrosis factor (TNF) inhibitor monotherapy, compared with receiving most other common drugs for these conditions, with or without TNF inhibitors, according to a study published in JAMA Network Open The only combination not associated with an increased risk for hospitalization or death was TNF inhibitor therapy with methotrexate.
“These findings support the continued use of TNF inhibitor monotherapy during the pandemic and warrant further research investigating the association of other biologic therapies with COVID-19 outcomes,” write Zara Izadi, MPharm, of the University of California, San Francisco, and her colleagues. “Treatment with TNF inhibitor combination therapy was associated with a more favorable safety profile when methotrexate rather than azathioprine/6-mercaptopurine was used, suggesting that clinicians would benefit from weighing the risks versus benefits of deescalating treatment or changing medications when a patient is receiving concomitant TNF inhibitors and azathioprine/6-mercaptopurine,” they write.
Findings mirror those seen in other settings
These findings are in line with what has been found in other settings, according to Joel M. Gelfand, MD, director of the psoriasis and phototherapy treatment center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, Philadelphia.
“In the beginning of the pandemic, there was concern about use of immune-modulating treatments, and many patients self-discontinued treatments like TNF inhibitors,” Dr. Gelfand, who was not involved in the study, told this news organization. “This has ultimately proved unnecessary and unfortunately resulted in harm to many patients due to flaring of their underlying disease.”
Dr. Gelfand emphasized the importance of vaccinating patients against COVID-19 as soon as possible and of getting a third dose for those who are already fully vaccinated with the Pfizer or Moderna shots, as recommended by the Centers for Disease Control and Prevention.
“I typically recommend this third dose be taken 6 months after the second dose,” Dr. Gelfand said. “The good news is that TNF inhibitors do not seem to meaningfully impact response to mRNA vaccines.”
Study details
The researchers analyzed data from three international registries of adults with rheumatic diseases, inflammatory bowel disease, and psoriasis who had COVID-19 between March 12, 2020, and Feb. 1, 2021. The registries included the Secure Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD) registry, the Psoriasis Patient Registry for Outcomes, Therapy and Epidemiology of COVID-19 Infection (PsoProtect), and the physician-reported registry from the Global Rheumatology Alliance (GRA).
The population included 6,077 patients from 74 countries. About half of the cohort (52.9%) were from Europe; more than half were women (58.6%). The average age was 48 years. A little over one-third of the patients (35.3%) had rheumatoid arthritis, 25.3% had Crohn’s disease, 12.5% had ulcerative colitis, 10.3% had spondyloarthritis, and 9.3% had psoriatic arthritis. Smaller percentages had psoriasis (4.9%), another type of arthritis or multiple types (1.7%), or another inflammatory bowel disease (0.6%).
One in five patients (21.3%) were hospitalized, and 3.1% died. The researchers compared outcomes for those who were receiving TNF inhibitor therapy alone to outcomes for those who were taking azathioprine/6-mercaptopurine therapy (alone or with a TNF inhibitor), methotrexate (alone or with a TNF inhibitor), and Janus kinase (JAK) inhibitors. They adjusted their analysis to account for active disease and common comorbidities, as well as geography and the period during the pandemic in which the person was admitted, because treatment regimens and hospitalization indications have varied over time.
All of the therapies except the combination of TNF inhibitors and methotrexate were associated with higher odds of hospitalization and death than TNF inhibitor monotherapy.
The researchers explored several possible explanations for the findings, including the possibility that high serum TNF concentrations may have been associated with more organ damage at the time of COVID-19 admission, owing to interaction with SARS-CoV-2–associated hyperinflammation.
“Therefore, blocking TNF could inhibit this detrimental immune response,” the authors write. “Multiple case series reporting favorable outcomes among patients receiving TNF inhibitor therapy support this assertion.”
Another possibility relates to the effects of taking non–TNF inhibitor medications for immunosuppression. The authors note that thiopurine medications are linked to a greater risk for opportunistic viral infections and that JAK inhibitors may reduce the body’s ability to clear the virus because of its suppression of innate immune response.
The authors also postulate that methotrexate may lower the likelihood of cytokine storm linked to COVID-19, even though methotrexate monotherapy was associated with poorer outcomes. “This association could mean that TNF inhibitor therapy is exerting a protective benefit or that methotrexate therapy is exerting a harmful consequence,” the authors write.
Caution needed in interpreting uncontrolled, registry-based data
The findings were not surprising to Stephen B. Hanauer, MD, medical director of the Digestive Health Center at Northwestern University, Chicago, who was not involved in the research.
“We’ve been monitoring IBD [inflammatory bowel disease] patients through the Secure registry similar to the rheumatologic and dermatologic societies and have not identified a signal of harm from any international groups,” Dr. Hanauer told this news organization. He noted that these registries also have not shown an increased risk for COVID-19 complications among patients receiving TNF inhibitors, antiadhesion therapies, or anti–IL12/23 inhibitors, compared with the general population not taking these therapies.
The study’s size and the diversity of patients strengthen its findings. However, the registries’ use of convenience sampling increases the potential for reporting bias, although the results remained similar after a sensitivity analysis. The study also lacked a control group, and the registries did not collect data uniformly.
“These are databases that rely on reporting from investigators and are not comprehensive prospective studies,” Dr. Hanauer noted as another study limitation.
Dr. Gelfand similarly advised caution in interpreting these findings, inasmuch as the study is a “collection of spontaneous reports” that should be viewed as hypothesis-generating rather than testing.
“Fortunately, more rigorous studies have been conducted, typically in large medical record systems, and have confirmed the hypothesis that TNF inhibitors are associated with a lower risk of poor COVID-19 outcomes, compared to other treatments,” Dr. Gelfand said.
Previous smaller studies similarly found better outcomes among patients taking TNF inhibitors, compared with other therapies, but their participants were predominantly from North America and Europe, noted Licio A. Velloso, MD, PhD, of the University of Campinas, in São Paulo, in an accompanying commentary.
On the basis of the findings of this study, “which included a much larger sample comprising distinct diseases and patients with a multitude of genetic backgrounds, the evidence in favor of the continued use of TNF inhibitor monotherapy for patients with IMIDs during the COVID-19 pandemic has become more substantial,” Dr. Velloso writes. “The finding that maintenance of TNF inhibitor monotherapy is associated with reductions in the risk of severe COVID-19 among patients with IMIDs offers new perspective that may guide health care professionals in the difficult decisions regarding therapeutic approaches among this specific group of patients.”
The research was funded by the American College of Rheumatology, the European Alliance of Associations for Rheumatology, the United Kingdom’s National Institute for Health Research Biomedical Research Center, and the Psoriasis Association. Many authors reported receiving grants and/or personal fees from a variety of pharmaceutical companies. Dr. Velloso has disclosed no relevant financial relationships. Dr. Hanauer has served as a consultant to companies that market TNF inhibitors. Dr. Gelfand has consulted for and received research grants from companies that market TNF inhibitors.
A version of this article first appeared on Medscape.com.
How is psoriasis related to coronary inflammation and atherosclerotic burden?
Key clinical point: Patients with psoriasis showed lower coronary inflammation and higher atherosclerotic burden than matched control participants.
Major finding: Compared with cardiovascular disease (CVD) risk factor-matched control participants, patients with psoriasis showed a lower perivascular fat attenuation index (−80.19 ± 7.48 vs −78.14 ± 7.81 Hounsfield unit; P less than .001), indicating lower coronary inflammation and a higher overall computed tomography-adapted Leaman score (5.86 vs 4.69; P = .030).
Study details: This was a retrospective, single-center study including 98 patients with psoriasis and 196 CVD risk factor-matched control participants.
Disclosures: No specific funding for the study was disclosed. Z Xu declared being an employee of Siemens Healthineers CT Collaboration. No other potential conflict of interests was declared.
Source: Bao W et al. Dermatology. 2021 Sep 15. doi: 10.1159/000518771.
Key clinical point: Patients with psoriasis showed lower coronary inflammation and higher atherosclerotic burden than matched control participants.
Major finding: Compared with cardiovascular disease (CVD) risk factor-matched control participants, patients with psoriasis showed a lower perivascular fat attenuation index (−80.19 ± 7.48 vs −78.14 ± 7.81 Hounsfield unit; P less than .001), indicating lower coronary inflammation and a higher overall computed tomography-adapted Leaman score (5.86 vs 4.69; P = .030).
Study details: This was a retrospective, single-center study including 98 patients with psoriasis and 196 CVD risk factor-matched control participants.
Disclosures: No specific funding for the study was disclosed. Z Xu declared being an employee of Siemens Healthineers CT Collaboration. No other potential conflict of interests was declared.
Source: Bao W et al. Dermatology. 2021 Sep 15. doi: 10.1159/000518771.
Key clinical point: Patients with psoriasis showed lower coronary inflammation and higher atherosclerotic burden than matched control participants.
Major finding: Compared with cardiovascular disease (CVD) risk factor-matched control participants, patients with psoriasis showed a lower perivascular fat attenuation index (−80.19 ± 7.48 vs −78.14 ± 7.81 Hounsfield unit; P less than .001), indicating lower coronary inflammation and a higher overall computed tomography-adapted Leaman score (5.86 vs 4.69; P = .030).
Study details: This was a retrospective, single-center study including 98 patients with psoriasis and 196 CVD risk factor-matched control participants.
Disclosures: No specific funding for the study was disclosed. Z Xu declared being an employee of Siemens Healthineers CT Collaboration. No other potential conflict of interests was declared.
Source: Bao W et al. Dermatology. 2021 Sep 15. doi: 10.1159/000518771.
Delineating factors behind frequent biologic switching in psoriasis
Key clinical point: Switching biologics in patients with psoriasis was mostly impelled by secondary lack of efficacy for skin symptoms with young age and the presence of psoriatic arthritis (PsA) linked to a higher frequency of switching in the long-term.
Major finding: Switching of first- and second-line biologics was likely attributed to a secondary lack of efficacy for skin disease. Each unit increase in age decreased the likelihood of switching twice or more by 4% (odds ratio [OR], 0.964; P = .038), whereas the existence of PsA increased the likelihood by 2.69-fold (OR, 2.69; P = .026).
Study details: This was a retrospective study including 115 adult patients with psoriasis who had been receiving biologics for 12 consecutive months or more and underwent at least a single biologic switch.
Disclosures: No specific funding for the study was disclosed. The authors declared no potential conflict of interests.
Source: Akdogan N et al. Expert Rev Clin Pharmacol. 2021 Sep 23. doi: 10.1080/17512433.2021.1979394.
Key clinical point: Switching biologics in patients with psoriasis was mostly impelled by secondary lack of efficacy for skin symptoms with young age and the presence of psoriatic arthritis (PsA) linked to a higher frequency of switching in the long-term.
Major finding: Switching of first- and second-line biologics was likely attributed to a secondary lack of efficacy for skin disease. Each unit increase in age decreased the likelihood of switching twice or more by 4% (odds ratio [OR], 0.964; P = .038), whereas the existence of PsA increased the likelihood by 2.69-fold (OR, 2.69; P = .026).
Study details: This was a retrospective study including 115 adult patients with psoriasis who had been receiving biologics for 12 consecutive months or more and underwent at least a single biologic switch.
Disclosures: No specific funding for the study was disclosed. The authors declared no potential conflict of interests.
Source: Akdogan N et al. Expert Rev Clin Pharmacol. 2021 Sep 23. doi: 10.1080/17512433.2021.1979394.
Key clinical point: Switching biologics in patients with psoriasis was mostly impelled by secondary lack of efficacy for skin symptoms with young age and the presence of psoriatic arthritis (PsA) linked to a higher frequency of switching in the long-term.
Major finding: Switching of first- and second-line biologics was likely attributed to a secondary lack of efficacy for skin disease. Each unit increase in age decreased the likelihood of switching twice or more by 4% (odds ratio [OR], 0.964; P = .038), whereas the existence of PsA increased the likelihood by 2.69-fold (OR, 2.69; P = .026).
Study details: This was a retrospective study including 115 adult patients with psoriasis who had been receiving biologics for 12 consecutive months or more and underwent at least a single biologic switch.
Disclosures: No specific funding for the study was disclosed. The authors declared no potential conflict of interests.
Source: Akdogan N et al. Expert Rev Clin Pharmacol. 2021 Sep 23. doi: 10.1080/17512433.2021.1979394.
High body weight and previous biologic use counter real-life guselkumab efficacy against plaque psoriasis
Key clinical point: This real-life study showed reduced efficacy of guselkumab for over 36 weeks in patients with moderate-to-severe chronic plaque psoriasis than previously reported, with body weight and exposure to biologics being the major predictors of response.
Major finding: At week 36, 67% of patients achieved Psoriasis Area Severity Index (PASI) 75 with heavier vs. low‐weight patients showing a decreased likelihood of achieving PASI 75 until week 4 (odds ratio [OR], 0.94; 95% confidence interval [CI], 0.88-0.99). Even at week 36, PASI 75 response rates were lower for patients exposed to 1 (OR, 0.07; 95% CI, 0.00-0.68) or more than 1 (OR, 0.00; 95% CI, 0.00-0.044) biologics than for biologic-naïve patients.
Study details: Findings are from a multicenter retrospective cohort study including 135 adult patients with moderate-to-severe chronic plaque psoriasis.
Disclosures: The study was supported by the Chang Gung Memorial Hospital and National Taiwan University Hospital, Hsin-Chu branch. Some of the authors declared serving as clinical trial participant or receiving speaker/consultancy honoraria from various sources.
Source: Hung YT et al. Ther Adv Chronic Dis. 2021 Sep 29. doi: 10.1177/20406223211046685.
Key clinical point: This real-life study showed reduced efficacy of guselkumab for over 36 weeks in patients with moderate-to-severe chronic plaque psoriasis than previously reported, with body weight and exposure to biologics being the major predictors of response.
Major finding: At week 36, 67% of patients achieved Psoriasis Area Severity Index (PASI) 75 with heavier vs. low‐weight patients showing a decreased likelihood of achieving PASI 75 until week 4 (odds ratio [OR], 0.94; 95% confidence interval [CI], 0.88-0.99). Even at week 36, PASI 75 response rates were lower for patients exposed to 1 (OR, 0.07; 95% CI, 0.00-0.68) or more than 1 (OR, 0.00; 95% CI, 0.00-0.044) biologics than for biologic-naïve patients.
Study details: Findings are from a multicenter retrospective cohort study including 135 adult patients with moderate-to-severe chronic plaque psoriasis.
Disclosures: The study was supported by the Chang Gung Memorial Hospital and National Taiwan University Hospital, Hsin-Chu branch. Some of the authors declared serving as clinical trial participant or receiving speaker/consultancy honoraria from various sources.
Source: Hung YT et al. Ther Adv Chronic Dis. 2021 Sep 29. doi: 10.1177/20406223211046685.
Key clinical point: This real-life study showed reduced efficacy of guselkumab for over 36 weeks in patients with moderate-to-severe chronic plaque psoriasis than previously reported, with body weight and exposure to biologics being the major predictors of response.
Major finding: At week 36, 67% of patients achieved Psoriasis Area Severity Index (PASI) 75 with heavier vs. low‐weight patients showing a decreased likelihood of achieving PASI 75 until week 4 (odds ratio [OR], 0.94; 95% confidence interval [CI], 0.88-0.99). Even at week 36, PASI 75 response rates were lower for patients exposed to 1 (OR, 0.07; 95% CI, 0.00-0.68) or more than 1 (OR, 0.00; 95% CI, 0.00-0.044) biologics than for biologic-naïve patients.
Study details: Findings are from a multicenter retrospective cohort study including 135 adult patients with moderate-to-severe chronic plaque psoriasis.
Disclosures: The study was supported by the Chang Gung Memorial Hospital and National Taiwan University Hospital, Hsin-Chu branch. Some of the authors declared serving as clinical trial participant or receiving speaker/consultancy honoraria from various sources.
Source: Hung YT et al. Ther Adv Chronic Dis. 2021 Sep 29. doi: 10.1177/20406223211046685.
Occult blood in feces tied to increased risk for psoriasis
Key clinical point: Patients with positive fecal immunochemistry test (FIT) results showed a significantly higher risk for psoriasis than those who were FIT-negative.
Major finding: During a 6.68-year median follow-up, the incidence rate of psoriasis per 1000 person-years was higher for the FIT-positive vs the FIT-negative group (4.14 vs 3.76). After multivariable adjustment, the adjusted hazard ratios for psoriasis were 1.029 (95% confidence interval [CI], 0.997-1.061), 1.118 (95% CI, 1.04-1.201), and 1.342 (95% CI, 1.157-1.557) for 1, 2, and 3 positive FIT results, respectively, vs negative FIT results.
Study details: Findings are from a retrospective nationwide population-based study of 1,395,147 participants aged 50 years or above who underwent screening for colorectal cancer.
Disclosures: The study was supported by the New Faculty Startup Fund from Seoul National University. The authors declared no potential conflict of interests.
Source: Lee HJ et al. Dermatology. 2021 Sep 16. doi: 10.1159/000518625.
Key clinical point: Patients with positive fecal immunochemistry test (FIT) results showed a significantly higher risk for psoriasis than those who were FIT-negative.
Major finding: During a 6.68-year median follow-up, the incidence rate of psoriasis per 1000 person-years was higher for the FIT-positive vs the FIT-negative group (4.14 vs 3.76). After multivariable adjustment, the adjusted hazard ratios for psoriasis were 1.029 (95% confidence interval [CI], 0.997-1.061), 1.118 (95% CI, 1.04-1.201), and 1.342 (95% CI, 1.157-1.557) for 1, 2, and 3 positive FIT results, respectively, vs negative FIT results.
Study details: Findings are from a retrospective nationwide population-based study of 1,395,147 participants aged 50 years or above who underwent screening for colorectal cancer.
Disclosures: The study was supported by the New Faculty Startup Fund from Seoul National University. The authors declared no potential conflict of interests.
Source: Lee HJ et al. Dermatology. 2021 Sep 16. doi: 10.1159/000518625.
Key clinical point: Patients with positive fecal immunochemistry test (FIT) results showed a significantly higher risk for psoriasis than those who were FIT-negative.
Major finding: During a 6.68-year median follow-up, the incidence rate of psoriasis per 1000 person-years was higher for the FIT-positive vs the FIT-negative group (4.14 vs 3.76). After multivariable adjustment, the adjusted hazard ratios for psoriasis were 1.029 (95% confidence interval [CI], 0.997-1.061), 1.118 (95% CI, 1.04-1.201), and 1.342 (95% CI, 1.157-1.557) for 1, 2, and 3 positive FIT results, respectively, vs negative FIT results.
Study details: Findings are from a retrospective nationwide population-based study of 1,395,147 participants aged 50 years or above who underwent screening for colorectal cancer.
Disclosures: The study was supported by the New Faculty Startup Fund from Seoul National University. The authors declared no potential conflict of interests.
Source: Lee HJ et al. Dermatology. 2021 Sep 16. doi: 10.1159/000518625.
Guselkumab: A promising treatment option for moderate-to-severe plaque psoriasis
Key clinical point: Guselkumab positively affected both clinician- and patient-reported outcomes in patients with moderate-to-severe plaque psoriasis, irrespective of their history of psoriasis therapies.
Major finding: After 28 weeks, 56.8% of patients attained a Dermatology Life Quality Index (DLQI) score of 1 or less, with the mean DLQI score decreasing from 13.7 at baseline to 2.8 (95% confidence interval [CI], 2.3-3.2), and mean Psoriasis Area Severity Index (PASI) decreasing from 16.4 at baseline to 3.0 (95% CI, 2.3-3.6), with a 55.3% PASI 90 response rate. Most adverse events were mild or moderate.
Study details: The data come from PERSIST, an ongoing, prospective, real-life study that enrolled 303 patients aged 18 years or above with moderate-to-severe plaque psoriasis for 2 years or more who were prescribed guselkumab.
Disclosures: This study was supported by Janssen-Cilag GmbH (Germany). S Wegner, Y Personke, and M Gomez reported being employees of Janssen-Cilag, whereas the other authors declared serving as an advisor, speaker, or clinical trial participant or receiving grants from various companies including Janssen-Cilag.
Source: Gerdes S et al. J Dermatol. 2021 Sep 12. doi: 10.1111/1346-8138.16128.
Key clinical point: Guselkumab positively affected both clinician- and patient-reported outcomes in patients with moderate-to-severe plaque psoriasis, irrespective of their history of psoriasis therapies.
Major finding: After 28 weeks, 56.8% of patients attained a Dermatology Life Quality Index (DLQI) score of 1 or less, with the mean DLQI score decreasing from 13.7 at baseline to 2.8 (95% confidence interval [CI], 2.3-3.2), and mean Psoriasis Area Severity Index (PASI) decreasing from 16.4 at baseline to 3.0 (95% CI, 2.3-3.6), with a 55.3% PASI 90 response rate. Most adverse events were mild or moderate.
Study details: The data come from PERSIST, an ongoing, prospective, real-life study that enrolled 303 patients aged 18 years or above with moderate-to-severe plaque psoriasis for 2 years or more who were prescribed guselkumab.
Disclosures: This study was supported by Janssen-Cilag GmbH (Germany). S Wegner, Y Personke, and M Gomez reported being employees of Janssen-Cilag, whereas the other authors declared serving as an advisor, speaker, or clinical trial participant or receiving grants from various companies including Janssen-Cilag.
Source: Gerdes S et al. J Dermatol. 2021 Sep 12. doi: 10.1111/1346-8138.16128.
Key clinical point: Guselkumab positively affected both clinician- and patient-reported outcomes in patients with moderate-to-severe plaque psoriasis, irrespective of their history of psoriasis therapies.
Major finding: After 28 weeks, 56.8% of patients attained a Dermatology Life Quality Index (DLQI) score of 1 or less, with the mean DLQI score decreasing from 13.7 at baseline to 2.8 (95% confidence interval [CI], 2.3-3.2), and mean Psoriasis Area Severity Index (PASI) decreasing from 16.4 at baseline to 3.0 (95% CI, 2.3-3.6), with a 55.3% PASI 90 response rate. Most adverse events were mild or moderate.
Study details: The data come from PERSIST, an ongoing, prospective, real-life study that enrolled 303 patients aged 18 years or above with moderate-to-severe plaque psoriasis for 2 years or more who were prescribed guselkumab.
Disclosures: This study was supported by Janssen-Cilag GmbH (Germany). S Wegner, Y Personke, and M Gomez reported being employees of Janssen-Cilag, whereas the other authors declared serving as an advisor, speaker, or clinical trial participant or receiving grants from various companies including Janssen-Cilag.
Source: Gerdes S et al. J Dermatol. 2021 Sep 12. doi: 10.1111/1346-8138.16128.