User login
Smokers with periodontitis are at soaring risk for psoriasis
Key clinical point: Periodontitis serves as an independent risk factor for psoriasis and in combination with smoking synergistically contributes to psoriasis development.
Major finding: The risk for psoriasis was higher in patients with vs. without periodontitis (adjusted hazard ratio [aHR], 1.116; 95% confidence interval [CI], 1.101-1.13). Compared with nonsmokers without periodontitis, the risk for psoriasis in nonsmokers with periodontitis and smokers with periodontitis increased by 11% (aHR, 1.11; 95% CI, 1.094-1.127) and 26.5% (aHR, 1.265; 95% CI, 1.234-1.296), respectively.
Study details: The data come from a 9-year follow-up, nationwide, population-based cohort study that included 1,063,004 and 8,655,587 patients with and without periodontitis, respectively, and not pre-diagnosed with psoriasis.
Disclosures: The study was supported by a National Research Foundation of Korea grant funded by the Korean government. The authors declared no potential conflict of interests.
Source: Han JH et al. Dermatology. 2021 Sep 15. doi: 10.1159/000518296.
Key clinical point: Periodontitis serves as an independent risk factor for psoriasis and in combination with smoking synergistically contributes to psoriasis development.
Major finding: The risk for psoriasis was higher in patients with vs. without periodontitis (adjusted hazard ratio [aHR], 1.116; 95% confidence interval [CI], 1.101-1.13). Compared with nonsmokers without periodontitis, the risk for psoriasis in nonsmokers with periodontitis and smokers with periodontitis increased by 11% (aHR, 1.11; 95% CI, 1.094-1.127) and 26.5% (aHR, 1.265; 95% CI, 1.234-1.296), respectively.
Study details: The data come from a 9-year follow-up, nationwide, population-based cohort study that included 1,063,004 and 8,655,587 patients with and without periodontitis, respectively, and not pre-diagnosed with psoriasis.
Disclosures: The study was supported by a National Research Foundation of Korea grant funded by the Korean government. The authors declared no potential conflict of interests.
Source: Han JH et al. Dermatology. 2021 Sep 15. doi: 10.1159/000518296.
Key clinical point: Periodontitis serves as an independent risk factor for psoriasis and in combination with smoking synergistically contributes to psoriasis development.
Major finding: The risk for psoriasis was higher in patients with vs. without periodontitis (adjusted hazard ratio [aHR], 1.116; 95% confidence interval [CI], 1.101-1.13). Compared with nonsmokers without periodontitis, the risk for psoriasis in nonsmokers with periodontitis and smokers with periodontitis increased by 11% (aHR, 1.11; 95% CI, 1.094-1.127) and 26.5% (aHR, 1.265; 95% CI, 1.234-1.296), respectively.
Study details: The data come from a 9-year follow-up, nationwide, population-based cohort study that included 1,063,004 and 8,655,587 patients with and without periodontitis, respectively, and not pre-diagnosed with psoriasis.
Disclosures: The study was supported by a National Research Foundation of Korea grant funded by the Korean government. The authors declared no potential conflict of interests.
Source: Han JH et al. Dermatology. 2021 Sep 15. doi: 10.1159/000518296.
Cal/BD foam gains ground among patients with plaque psoriasis
Key clinical point: Proactive management with calcipotriene 50 μg/g and betamethasone dipropionate 0.5 mg/g (Cal/BD) foam vs. reactive management with vehicle foam decreased the severity of patient-reported symptoms in patients with plaque psoriasis.
Major finding: Proactive vs. reactive management during 52-week maintenance showed greater improvement in Psoriasis Symptom Inventory (difference, −0.75; P = .0128) and Dermatology Life Quality Index (difference −0.45; P = .007) and nonsignificantly higher EuroQol-5D for psoriasis (0.89 vs 0.88; P = .0842) scores.
Study details: Findings are from a post hoc analysis of phase 3 PSO-LONG trial including 521 patients with plaque psoriasis randomly assigned to proactive management (Cal/BD foam twice weekly) or reactive management (vehicle foam twice weekly) arms.
Disclosures: The study was sponsored by Leo Pharma. The authors declared serving as consultants, advisory board members, and clinical trial investigators or receiving grants/speaker honoraria from various sources, including LEO Pharma.
Source: Jalili A et al. J Eur Acad Dermatol Venereol. 2021 Sep 20. doi: 10.1111/jdv.17673.
Key clinical point: Proactive management with calcipotriene 50 μg/g and betamethasone dipropionate 0.5 mg/g (Cal/BD) foam vs. reactive management with vehicle foam decreased the severity of patient-reported symptoms in patients with plaque psoriasis.
Major finding: Proactive vs. reactive management during 52-week maintenance showed greater improvement in Psoriasis Symptom Inventory (difference, −0.75; P = .0128) and Dermatology Life Quality Index (difference −0.45; P = .007) and nonsignificantly higher EuroQol-5D for psoriasis (0.89 vs 0.88; P = .0842) scores.
Study details: Findings are from a post hoc analysis of phase 3 PSO-LONG trial including 521 patients with plaque psoriasis randomly assigned to proactive management (Cal/BD foam twice weekly) or reactive management (vehicle foam twice weekly) arms.
Disclosures: The study was sponsored by Leo Pharma. The authors declared serving as consultants, advisory board members, and clinical trial investigators or receiving grants/speaker honoraria from various sources, including LEO Pharma.
Source: Jalili A et al. J Eur Acad Dermatol Venereol. 2021 Sep 20. doi: 10.1111/jdv.17673.
Key clinical point: Proactive management with calcipotriene 50 μg/g and betamethasone dipropionate 0.5 mg/g (Cal/BD) foam vs. reactive management with vehicle foam decreased the severity of patient-reported symptoms in patients with plaque psoriasis.
Major finding: Proactive vs. reactive management during 52-week maintenance showed greater improvement in Psoriasis Symptom Inventory (difference, −0.75; P = .0128) and Dermatology Life Quality Index (difference −0.45; P = .007) and nonsignificantly higher EuroQol-5D for psoriasis (0.89 vs 0.88; P = .0842) scores.
Study details: Findings are from a post hoc analysis of phase 3 PSO-LONG trial including 521 patients with plaque psoriasis randomly assigned to proactive management (Cal/BD foam twice weekly) or reactive management (vehicle foam twice weekly) arms.
Disclosures: The study was sponsored by Leo Pharma. The authors declared serving as consultants, advisory board members, and clinical trial investigators or receiving grants/speaker honoraria from various sources, including LEO Pharma.
Source: Jalili A et al. J Eur Acad Dermatol Venereol. 2021 Sep 20. doi: 10.1111/jdv.17673.
Patient-reported outcome measures complement clinician-reported outcomes in psoriasis
Key clinical point: The disease severity assessment, guiding initiation of systemic therapy, differed when either patient- or clinician-reported outcomes were considered alone, highlighting the complementary value of both for appropriate treatment.
Major finding: Overall, 72.4% (95% confidence interval [CI], 67.3%-77.0%) of patients who qualified for systemic therapy initiation on account of Psoriasis Area Severity Index (PASI) scores or body surface area (BSA) of 10 or higher had a Dermatology Life Quality Index (DLQI) score of less than 10. Conversely, 10.4% (95% CI, 8.8%-12.1%) of patients with a DLQI score higher than 10 did not qualify for systemic therapy based on PASI scores or BSA.
Study details: Findings are from a cross-sectional, observational study including 1,733 patients with mild, moderate, or severe psoriasis who met the criteria for initiation of systemic therapy based on DLQI score, PASI score, or BSA.
Disclosures: No specific funding for the study was disclosed. Dr. Gelfand declared receiving consultation fees and research grants from various sources.
Source: Barbieri JS et al. JAMA Dermatol. 2021 Sep 8. doi: 10.1001/jamadermatol.2021.3341.
Key clinical point: The disease severity assessment, guiding initiation of systemic therapy, differed when either patient- or clinician-reported outcomes were considered alone, highlighting the complementary value of both for appropriate treatment.
Major finding: Overall, 72.4% (95% confidence interval [CI], 67.3%-77.0%) of patients who qualified for systemic therapy initiation on account of Psoriasis Area Severity Index (PASI) scores or body surface area (BSA) of 10 or higher had a Dermatology Life Quality Index (DLQI) score of less than 10. Conversely, 10.4% (95% CI, 8.8%-12.1%) of patients with a DLQI score higher than 10 did not qualify for systemic therapy based on PASI scores or BSA.
Study details: Findings are from a cross-sectional, observational study including 1,733 patients with mild, moderate, or severe psoriasis who met the criteria for initiation of systemic therapy based on DLQI score, PASI score, or BSA.
Disclosures: No specific funding for the study was disclosed. Dr. Gelfand declared receiving consultation fees and research grants from various sources.
Source: Barbieri JS et al. JAMA Dermatol. 2021 Sep 8. doi: 10.1001/jamadermatol.2021.3341.
Key clinical point: The disease severity assessment, guiding initiation of systemic therapy, differed when either patient- or clinician-reported outcomes were considered alone, highlighting the complementary value of both for appropriate treatment.
Major finding: Overall, 72.4% (95% confidence interval [CI], 67.3%-77.0%) of patients who qualified for systemic therapy initiation on account of Psoriasis Area Severity Index (PASI) scores or body surface area (BSA) of 10 or higher had a Dermatology Life Quality Index (DLQI) score of less than 10. Conversely, 10.4% (95% CI, 8.8%-12.1%) of patients with a DLQI score higher than 10 did not qualify for systemic therapy based on PASI scores or BSA.
Study details: Findings are from a cross-sectional, observational study including 1,733 patients with mild, moderate, or severe psoriasis who met the criteria for initiation of systemic therapy based on DLQI score, PASI score, or BSA.
Disclosures: No specific funding for the study was disclosed. Dr. Gelfand declared receiving consultation fees and research grants from various sources.
Source: Barbieri JS et al. JAMA Dermatol. 2021 Sep 8. doi: 10.1001/jamadermatol.2021.3341.
Risankizumab shows excellent long-term PASI response in psoriasis under real-world conditions
Key clinical point: Risankizumab displayed superior long-term clinical efficacy against moderate-to-severe psoriasis, with no new safety signals in the 52-week update of a multicenter real-life study.
Major finding: Psoriasis Area and Severity Index (PASI) 75, PASI 90, and PASI 100 response rates at week 36 were 92.7%, 83.6%, and 65.5%, which were maintained through week 52 at 96.4%, 85.5%, and 60.0%, respectively. Unlike in phase 3 trials, more biologic-experienced patients reached PASI 100 at weeks 36 and 52 (71.8% and 69.2%, respectively) compared with biologic-naïve patients (50.0% and 37.5%, respectively).
Study details: Findings are from a real-life multicenter study including 57 adult patients with moderate-to-severe psoriasis who were receiving risankizumab therapy.
Disclosures: The study did not receive any funding. Some of the authors including the lead author declared receiving consultation/personal fees or research grants from various sources.
Source: Hansel K et al. J Eur Acad Dermatol Venereol. 2021 Sep 12. doi: 10.1111/jdv.17656.
Key clinical point: Risankizumab displayed superior long-term clinical efficacy against moderate-to-severe psoriasis, with no new safety signals in the 52-week update of a multicenter real-life study.
Major finding: Psoriasis Area and Severity Index (PASI) 75, PASI 90, and PASI 100 response rates at week 36 were 92.7%, 83.6%, and 65.5%, which were maintained through week 52 at 96.4%, 85.5%, and 60.0%, respectively. Unlike in phase 3 trials, more biologic-experienced patients reached PASI 100 at weeks 36 and 52 (71.8% and 69.2%, respectively) compared with biologic-naïve patients (50.0% and 37.5%, respectively).
Study details: Findings are from a real-life multicenter study including 57 adult patients with moderate-to-severe psoriasis who were receiving risankizumab therapy.
Disclosures: The study did not receive any funding. Some of the authors including the lead author declared receiving consultation/personal fees or research grants from various sources.
Source: Hansel K et al. J Eur Acad Dermatol Venereol. 2021 Sep 12. doi: 10.1111/jdv.17656.
Key clinical point: Risankizumab displayed superior long-term clinical efficacy against moderate-to-severe psoriasis, with no new safety signals in the 52-week update of a multicenter real-life study.
Major finding: Psoriasis Area and Severity Index (PASI) 75, PASI 90, and PASI 100 response rates at week 36 were 92.7%, 83.6%, and 65.5%, which were maintained through week 52 at 96.4%, 85.5%, and 60.0%, respectively. Unlike in phase 3 trials, more biologic-experienced patients reached PASI 100 at weeks 36 and 52 (71.8% and 69.2%, respectively) compared with biologic-naïve patients (50.0% and 37.5%, respectively).
Study details: Findings are from a real-life multicenter study including 57 adult patients with moderate-to-severe psoriasis who were receiving risankizumab therapy.
Disclosures: The study did not receive any funding. Some of the authors including the lead author declared receiving consultation/personal fees or research grants from various sources.
Source: Hansel K et al. J Eur Acad Dermatol Venereol. 2021 Sep 12. doi: 10.1111/jdv.17656.
Secukinumab hits mark against pediatric plaque psoriasis
Key clinical point: Secukinumab demonstrated a high skin-clearing effect and favorable safety in pediatric patients with moderate-to-severe plaque psoriasis through week 24.
Major finding: At week 12, low dose (LD) and high dose (HD) groups showed a Psoriasis Area Severity Index (PASI) 75 response rate (RR) of 92.9%, whereas their Investigator's Global Assessment (IGA) 0/1 RRs were 78.5% and 83.4%, respectively. At week 24, the PASI 75 RR for LD and HD groups increased to 95.2% and IGA 0/1 RRs increased to 88.1% and 92.9%, respectively. Both groups displayed similar treatment-emergent adverse events.
Study details: These are 24-week findings of a 224-week, open-label, phase 3 study including 84 pediatric patients with moderate-to-severe plaque psoriasis randomly assigned to LD or HD secukinumab (75/75/150 or 75/150/300 mg for body weight (kg) less than 25/25 to less than 50/50 or more, respectively).
Disclosures: The study was sponsored by Novartis Pharma AG, Switzerland. R Mazur, P Forrer, and M Patekar declared being employees of Novartis Pharma AG. Some of the authors declared serving on advisory board, speaker, consultant, or principal/subinvestigator for various organizations including Novartis.
Source: Magnolo N et al. J Am Acad Dermatol. 2021 Sep 20. doi: 10.1016/j.jaad.2021.08.066.
Key clinical point: Secukinumab demonstrated a high skin-clearing effect and favorable safety in pediatric patients with moderate-to-severe plaque psoriasis through week 24.
Major finding: At week 12, low dose (LD) and high dose (HD) groups showed a Psoriasis Area Severity Index (PASI) 75 response rate (RR) of 92.9%, whereas their Investigator's Global Assessment (IGA) 0/1 RRs were 78.5% and 83.4%, respectively. At week 24, the PASI 75 RR for LD and HD groups increased to 95.2% and IGA 0/1 RRs increased to 88.1% and 92.9%, respectively. Both groups displayed similar treatment-emergent adverse events.
Study details: These are 24-week findings of a 224-week, open-label, phase 3 study including 84 pediatric patients with moderate-to-severe plaque psoriasis randomly assigned to LD or HD secukinumab (75/75/150 or 75/150/300 mg for body weight (kg) less than 25/25 to less than 50/50 or more, respectively).
Disclosures: The study was sponsored by Novartis Pharma AG, Switzerland. R Mazur, P Forrer, and M Patekar declared being employees of Novartis Pharma AG. Some of the authors declared serving on advisory board, speaker, consultant, or principal/subinvestigator for various organizations including Novartis.
Source: Magnolo N et al. J Am Acad Dermatol. 2021 Sep 20. doi: 10.1016/j.jaad.2021.08.066.
Key clinical point: Secukinumab demonstrated a high skin-clearing effect and favorable safety in pediatric patients with moderate-to-severe plaque psoriasis through week 24.
Major finding: At week 12, low dose (LD) and high dose (HD) groups showed a Psoriasis Area Severity Index (PASI) 75 response rate (RR) of 92.9%, whereas their Investigator's Global Assessment (IGA) 0/1 RRs were 78.5% and 83.4%, respectively. At week 24, the PASI 75 RR for LD and HD groups increased to 95.2% and IGA 0/1 RRs increased to 88.1% and 92.9%, respectively. Both groups displayed similar treatment-emergent adverse events.
Study details: These are 24-week findings of a 224-week, open-label, phase 3 study including 84 pediatric patients with moderate-to-severe plaque psoriasis randomly assigned to LD or HD secukinumab (75/75/150 or 75/150/300 mg for body weight (kg) less than 25/25 to less than 50/50 or more, respectively).
Disclosures: The study was sponsored by Novartis Pharma AG, Switzerland. R Mazur, P Forrer, and M Patekar declared being employees of Novartis Pharma AG. Some of the authors declared serving on advisory board, speaker, consultant, or principal/subinvestigator for various organizations including Novartis.
Source: Magnolo N et al. J Am Acad Dermatol. 2021 Sep 20. doi: 10.1016/j.jaad.2021.08.066.
Study of biologics’ impact on psoriasis-to-PsA transition contradicts previous findings
Data source likely contributes biases
A new study has found that patients with psoriasis who were treated with biologics were more likely to develop psoriatic arthritis (PsA) than those treated with phototherapy, oral therapy, or no therapy at all, although the authors cautioned readers to consider potential biases when reviewing their findings.
“We do not suggest that these results should be interpreted causally; in other words, biologics likely do not cause PsA,” Elana Meer of the University of Pennsylvania, Philadelphia, and coauthors wrote. The study was published in Annals of the Rheumatic Diseases.
Three studies in dermatology clinic-based populations published this past summer – one from Italy, one from Argentina, and one from Israel – suggested that biologics can decrease a psoriasis patient’s risk of developing PsA. To further assess the impact of treatment with biologics, Ms. Meer and associates retrospectively examined the health records of thousands of patients with psoriasis between the ages of 16 and 90 who were initiating therapy. All told, data from 193,709 patients with psoriasis and without PsA who were treated between 2006 and 2017 were gathered from the OptumInsights Electronic Health Record Database.
A total of 14,569 patients from that cohort initiated biologic therapy while 20,321 patients initiated either oral therapy or phototherapy. The mean age in the biologics group was 45.9 years, compared with 49.8 years in the oral and phototherapy group.
The incidence of PsA across all patients was 9.75 cases per 1,000 person-years, compared with 77.26 among the biologic group, 61.99 among the oral therapy group, 26.11 among the phototherapy group, and 5.85 among those who did not receive therapy. After a multivariable adjustment in which biologics were a time-varying exposure, receiving biologics was associated with a higher incidence of PsA (hazard ratio, 4.48; 95% confidence interval, 4.23-4.75). In a model where time starts at the first use of biologics, the incidence was lower – but still notable – after multivariable adjustment (HR, 2.14; 95% CI, 2.00-2.28) and propensity score matching (HR, 2.17; 95% CI, 2.03-2.33).
Bias likely plays a large role in retrospective PsA study
“We’ve been struggling for the last several years to find a database that allows us to really address this question retrospectively,” study coauthor Christopher T. Ritchlin, MD, of the University of Rochester (N.Y.), said in an interview. “It looks like the model you use for a retrospective analysis heavily influences what you come out with.”
He described the potential biases they identified, including the possibility of protopathic bias indicating that patients being treated with biologics who then report joint pain have developed PsA – and are coded accordingly after visiting a rheumatologist.
“This has convinced us that you have to do a prospective study,” he said. “We’ve known that there were flaws with previous studies in this area. We tried to overcome them with our methodology, but there’s no way you can overcome a coding issue when you’re looking at such a large database.”
He noted another likely bias: The patients who are more likely to develop PsA are the ones with severe psoriasis, and they are also the patients most likely to be prescribed biologics.
“In my clinical experience, I have seen many patients develop psoriatic arthritis while on biologics for their psoriasis,” coauthor Joel M. Gelfand, MD, of the University of Pennsylvania, added in an interview. “Currently, we do not have adequate data to recommend treating psoriasis with a particular modality in order to prevent psoriatic arthritis. This question, however, is very important to patients and clinicians and ultimately is best answered with a large-scale pragmatic trial.”
Dr. Ritchlin reported that a prospective study in which “patients with psoriasis who do not have arthritis but do have certain risk factors and abnormal findings on musculoskeletal ultrasounds” will be treated with either biologic agents or placebo is about to begin, with a goal of “either attenuating or preventing the onset of PsA.”
The authors recognized their study’s additional limitations, including electronic health records being used as the primary data source and the possibility that medications were prescribed but never filled. That said, they did attempt to address the latter by using two prescriptions for a given therapy as the primary analysis, “suggesting a refill was initiated.”
The authors said that no commercial entities provided support for the study. Two of the authors acknowledged receiving funding from the National Psoriasis Foundation, and several authors declared potential conflicts of interests that included consulting and receiving honoraria from various pharmaceutical companies.
Data source likely contributes biases
Data source likely contributes biases
A new study has found that patients with psoriasis who were treated with biologics were more likely to develop psoriatic arthritis (PsA) than those treated with phototherapy, oral therapy, or no therapy at all, although the authors cautioned readers to consider potential biases when reviewing their findings.
“We do not suggest that these results should be interpreted causally; in other words, biologics likely do not cause PsA,” Elana Meer of the University of Pennsylvania, Philadelphia, and coauthors wrote. The study was published in Annals of the Rheumatic Diseases.
Three studies in dermatology clinic-based populations published this past summer – one from Italy, one from Argentina, and one from Israel – suggested that biologics can decrease a psoriasis patient’s risk of developing PsA. To further assess the impact of treatment with biologics, Ms. Meer and associates retrospectively examined the health records of thousands of patients with psoriasis between the ages of 16 and 90 who were initiating therapy. All told, data from 193,709 patients with psoriasis and without PsA who were treated between 2006 and 2017 were gathered from the OptumInsights Electronic Health Record Database.
A total of 14,569 patients from that cohort initiated biologic therapy while 20,321 patients initiated either oral therapy or phototherapy. The mean age in the biologics group was 45.9 years, compared with 49.8 years in the oral and phototherapy group.
The incidence of PsA across all patients was 9.75 cases per 1,000 person-years, compared with 77.26 among the biologic group, 61.99 among the oral therapy group, 26.11 among the phototherapy group, and 5.85 among those who did not receive therapy. After a multivariable adjustment in which biologics were a time-varying exposure, receiving biologics was associated with a higher incidence of PsA (hazard ratio, 4.48; 95% confidence interval, 4.23-4.75). In a model where time starts at the first use of biologics, the incidence was lower – but still notable – after multivariable adjustment (HR, 2.14; 95% CI, 2.00-2.28) and propensity score matching (HR, 2.17; 95% CI, 2.03-2.33).
Bias likely plays a large role in retrospective PsA study
“We’ve been struggling for the last several years to find a database that allows us to really address this question retrospectively,” study coauthor Christopher T. Ritchlin, MD, of the University of Rochester (N.Y.), said in an interview. “It looks like the model you use for a retrospective analysis heavily influences what you come out with.”
He described the potential biases they identified, including the possibility of protopathic bias indicating that patients being treated with biologics who then report joint pain have developed PsA – and are coded accordingly after visiting a rheumatologist.
“This has convinced us that you have to do a prospective study,” he said. “We’ve known that there were flaws with previous studies in this area. We tried to overcome them with our methodology, but there’s no way you can overcome a coding issue when you’re looking at such a large database.”
He noted another likely bias: The patients who are more likely to develop PsA are the ones with severe psoriasis, and they are also the patients most likely to be prescribed biologics.
“In my clinical experience, I have seen many patients develop psoriatic arthritis while on biologics for their psoriasis,” coauthor Joel M. Gelfand, MD, of the University of Pennsylvania, added in an interview. “Currently, we do not have adequate data to recommend treating psoriasis with a particular modality in order to prevent psoriatic arthritis. This question, however, is very important to patients and clinicians and ultimately is best answered with a large-scale pragmatic trial.”
Dr. Ritchlin reported that a prospective study in which “patients with psoriasis who do not have arthritis but do have certain risk factors and abnormal findings on musculoskeletal ultrasounds” will be treated with either biologic agents or placebo is about to begin, with a goal of “either attenuating or preventing the onset of PsA.”
The authors recognized their study’s additional limitations, including electronic health records being used as the primary data source and the possibility that medications were prescribed but never filled. That said, they did attempt to address the latter by using two prescriptions for a given therapy as the primary analysis, “suggesting a refill was initiated.”
The authors said that no commercial entities provided support for the study. Two of the authors acknowledged receiving funding from the National Psoriasis Foundation, and several authors declared potential conflicts of interests that included consulting and receiving honoraria from various pharmaceutical companies.
A new study has found that patients with psoriasis who were treated with biologics were more likely to develop psoriatic arthritis (PsA) than those treated with phototherapy, oral therapy, or no therapy at all, although the authors cautioned readers to consider potential biases when reviewing their findings.
“We do not suggest that these results should be interpreted causally; in other words, biologics likely do not cause PsA,” Elana Meer of the University of Pennsylvania, Philadelphia, and coauthors wrote. The study was published in Annals of the Rheumatic Diseases.
Three studies in dermatology clinic-based populations published this past summer – one from Italy, one from Argentina, and one from Israel – suggested that biologics can decrease a psoriasis patient’s risk of developing PsA. To further assess the impact of treatment with biologics, Ms. Meer and associates retrospectively examined the health records of thousands of patients with psoriasis between the ages of 16 and 90 who were initiating therapy. All told, data from 193,709 patients with psoriasis and without PsA who were treated between 2006 and 2017 were gathered from the OptumInsights Electronic Health Record Database.
A total of 14,569 patients from that cohort initiated biologic therapy while 20,321 patients initiated either oral therapy or phototherapy. The mean age in the biologics group was 45.9 years, compared with 49.8 years in the oral and phototherapy group.
The incidence of PsA across all patients was 9.75 cases per 1,000 person-years, compared with 77.26 among the biologic group, 61.99 among the oral therapy group, 26.11 among the phototherapy group, and 5.85 among those who did not receive therapy. After a multivariable adjustment in which biologics were a time-varying exposure, receiving biologics was associated with a higher incidence of PsA (hazard ratio, 4.48; 95% confidence interval, 4.23-4.75). In a model where time starts at the first use of biologics, the incidence was lower – but still notable – after multivariable adjustment (HR, 2.14; 95% CI, 2.00-2.28) and propensity score matching (HR, 2.17; 95% CI, 2.03-2.33).
Bias likely plays a large role in retrospective PsA study
“We’ve been struggling for the last several years to find a database that allows us to really address this question retrospectively,” study coauthor Christopher T. Ritchlin, MD, of the University of Rochester (N.Y.), said in an interview. “It looks like the model you use for a retrospective analysis heavily influences what you come out with.”
He described the potential biases they identified, including the possibility of protopathic bias indicating that patients being treated with biologics who then report joint pain have developed PsA – and are coded accordingly after visiting a rheumatologist.
“This has convinced us that you have to do a prospective study,” he said. “We’ve known that there were flaws with previous studies in this area. We tried to overcome them with our methodology, but there’s no way you can overcome a coding issue when you’re looking at such a large database.”
He noted another likely bias: The patients who are more likely to develop PsA are the ones with severe psoriasis, and they are also the patients most likely to be prescribed biologics.
“In my clinical experience, I have seen many patients develop psoriatic arthritis while on biologics for their psoriasis,” coauthor Joel M. Gelfand, MD, of the University of Pennsylvania, added in an interview. “Currently, we do not have adequate data to recommend treating psoriasis with a particular modality in order to prevent psoriatic arthritis. This question, however, is very important to patients and clinicians and ultimately is best answered with a large-scale pragmatic trial.”
Dr. Ritchlin reported that a prospective study in which “patients with psoriasis who do not have arthritis but do have certain risk factors and abnormal findings on musculoskeletal ultrasounds” will be treated with either biologic agents or placebo is about to begin, with a goal of “either attenuating or preventing the onset of PsA.”
The authors recognized their study’s additional limitations, including electronic health records being used as the primary data source and the possibility that medications were prescribed but never filled. That said, they did attempt to address the latter by using two prescriptions for a given therapy as the primary analysis, “suggesting a refill was initiated.”
The authors said that no commercial entities provided support for the study. Two of the authors acknowledged receiving funding from the National Psoriasis Foundation, and several authors declared potential conflicts of interests that included consulting and receiving honoraria from various pharmaceutical companies.
FROM ANNALS OF THE RHEUMATIC DISEASES
Skin of Color in Preclinical Medical Education: A Cross-Institutional Comparison and A Call to Action
A ccording to the US Census Bureau, more than half of all Americans are projected to belong to a minority group, defined as any group other than non-Hispanic White alone, by 2044. 1 Consequently, the United States rapidly is becoming a country in which the majority of citizens will have skin of color. Individuals with skin of color are of diverse ethnic backgrounds and include people of African, Latin American, Native American, Pacific Islander, and Asian descent, as well as interethnic backgrounds. 2 Throughout the country, dermatologists along with primary care practitioners may be confronted with certain cutaneous conditions that have varying disease presentations or processes in patients with skin of color. It also is important to note that racial categories are socially rather than biologically constructed, and the term skin of color includes a wide variety of diverse skin types. Nevertheless, the current literature thoroughly supports unique pathophysiologic differences in skin of color as well as variations in disease manifestation compared to White patients. 3-5 For example, the increased lability of melanosomes in skin of color patients, which increases their risk for postinflammatory hyperpigmentation, has been well documented. 5-7 There are various dermatologic conditions that also occur with higher frequency and manifest uniquely in people with darker, more pigmented skin, 7-9 and dermatologists, along with primary care physicians, should feel prepared to recognize and address them.
Extensive evidence also indicates that there are unique aspects to consider while managing certain skin diseases in patients with skin of color.8,10,11 Consequently, as noted on the Skin of Color Society (SOCS) website, “[a]n increase in the body of dermatological literature concerning skin of color as well as the advancement of both basic science and clinical investigational research is necessary to meet the needs of the expanding skin of color population.”2 In the meantime, current knowledge regarding cutaneous conditions that diversely or disproportionately affect skin of color should be actively disseminated to physicians in training. Although patients with skin of color should always have access to comprehensive care and knowledgeable practitioners, the current changes in national and regional demographics further underscore the need for a more thorough understanding of skin of color with regard to disease pathogenesis, diagnosis, and treatment.
Several studies have found that medical students in the United States are minimally exposed to dermatology in general compared to other clinical specialties,12-14 which can easily lead to the underrecognition of disorders that may uniquely or disproportionately affect individuals with pigmented skin. Recent data showed that medical schools typically required fewer than 10 hours of dermatology instruction,12 and on average, dermatologic training made up less than 1% of a medical student’s undergraduate medical education.13,15,16 Consequently, less than 40% of primary care residents felt that their medical school curriculum adequately prepared them to manage common skin conditions.14 Although not all physicians should be expected to fully grasp the complexities of skin of color and its diagnostic and therapeutic implications, both practicing and training dermatologists have acknowledged a lack of exposure to skin of color. In one study, approximately 47% of dermatologists and dermatology residents reported that their medical training (medical school and/or residency) was inadequate in training them on skin conditions in Black patients. Furthermore, many who felt their training was lacking in skin of color identified the need for greater exposure to Black patients and training materials.15 The absence of comprehensive medical education regarding skin of color ultimately can be a disadvantage for both practitioners and patients, resulting in poorer outcomes. Furthermore, underrepresentation of skin of color may persist beyond undergraduate and graduate medical education. There also is evidence to suggest that noninclusion of skin of color pervades foundational dermatologic educational resources, including commonly used textbooks as well as continuing medical education disseminated at national conferences and meetings.17 Taken together, these findings highlight the need for more diverse and representative exposure to skin of color throughout medical training, which begins with a diverse inclusive undergraduate medical education in dermatology.
The objective of this study was to determine if the preclinical dermatology curriculum at 3 US medical schools provided adequate representation of skin of color patients in their didactic presentation slides.
Methods
Participants—Three US medical schools, a blend of private and public medical schools located across different geographic boundaries, agreed to participate in the study. All 3 institutions were current members of the American Medical Association (AMA) Accelerating Change in Medical Education consortium, whose primary goal is to create the medical school of the future and transform physician training.18 All 32 member institutions of the AMA consortium were contacted to request their participation in the study. As part of the consortium, these institutions have vowed to collectively work to develop and share the best models for educational advancement to improve care for patients, populations, and communities18 and would expectedly provide a more racially and ethnically inclusive curriculum than an institution not accountable to a group dedicated to identifying the best ways to deliver care for increasingly diverse communities.
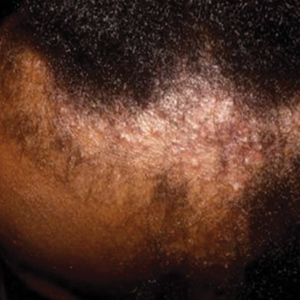
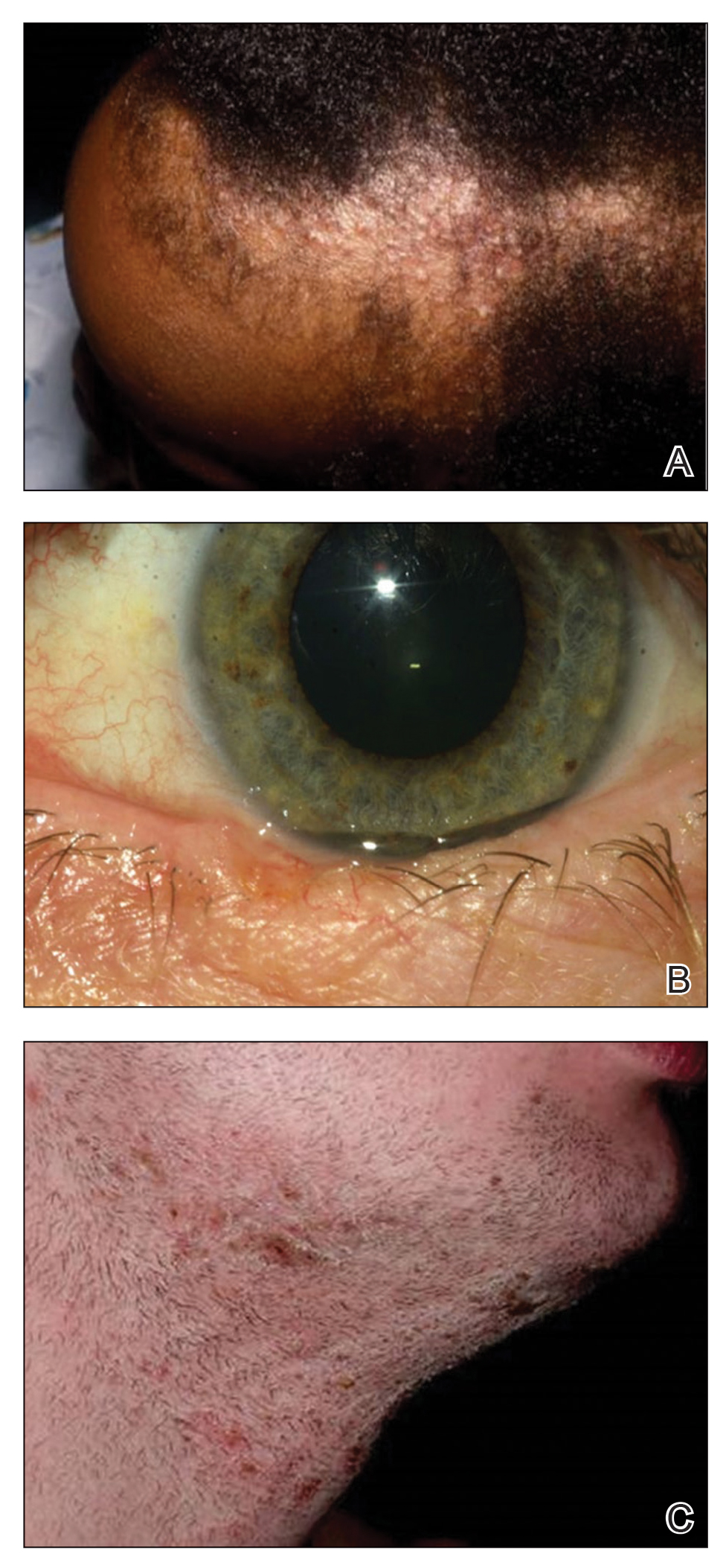
Data Collection—Lectures were included if they were presented during dermatology preclinical courses in the 2015 to 2016 academic year. An uninvolved third party removed the names and identities of instructors to preserve anonymity. Two independent coders from different institutions extracted the data—lecture title, total number of clinical and histologic images, and number of skin of color images—from each of the anonymized lectures using a standardized coding form. We documented differences in skin of color noted in lectures and the disease context for the discussed differences, such as variations in clinical presentation, disease process, epidemiology/risk, and treatment between different skin phenotypes or ethnic groups. Photographs in which the coders were unable to differentiate whether the patient had skin of color were designated as indeterminate or unclear. Photographs appearing to represent Fitzpatrick skin types IV, V, and VI19 were categorically designated as skin of color, and those appearing to represent Fitzpatrick skin types I and II were described as not skin of color; however, images appearing to represent Fitzpatrick skin type III often were classified as not skin of color or indeterminate and occasionally skin of color. The Figure shows examples of images classified as skin of color, indeterminate, and not skin of color. Photographs often were classified as indeterminate due to poor lighting, close-up view photographs, or highlighted pathology obscuring the surrounding skin. We excluded duplicate photographs and histologic images from the analyses.
We also reviewed 19 conditions previously highlighted by the SOCS as areas of importance to skin of color patients.20 The coders tracked how many of these conditions were noted in each lecture. Duplicate discussion of these conditions was not included in the analyses. Any discrepancies between coders were resolved through additional slide review and discussion. The final coded data with the agreed upon changes were used for statistical analyses. Recent national demographic data from the US Census Bureau in 2019 describe approximately 39.9% of the population as belonging to racial/ethnic groups other than non-Hispanic/Latinx White.21 Consequently, the standard for adequate representation for skin of color photographs was set at 35% for the purpose of this study.
Results
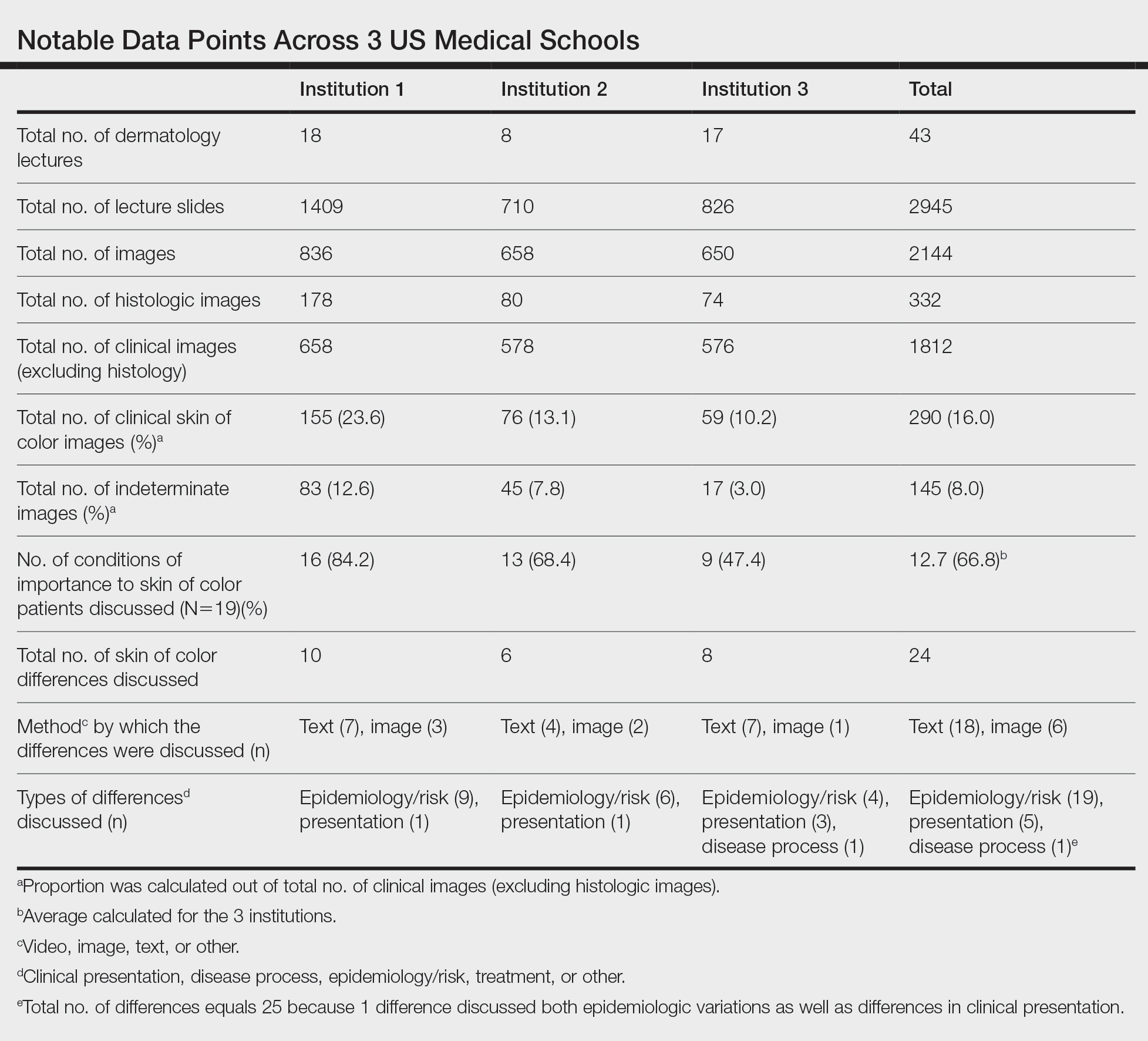
Across all 3 institutions included in the study, the proportion of the total number of clinical photographs showing skin of color was 16% (290/1812). Eight percent of the total photographs (145/1812) were noted to be indeterminate (Table). For institution 1, 23.6% of photographs (155/658) showed skin of color, and 12.6% (83/658) were indeterminate. For institution 2, 13.1% (76/578) showed skin of color and 7.8% (45/578) were indeterminate. For institution 3, 10.2% (59/576) showed skin of color and 3% (17/576) were indeterminate.
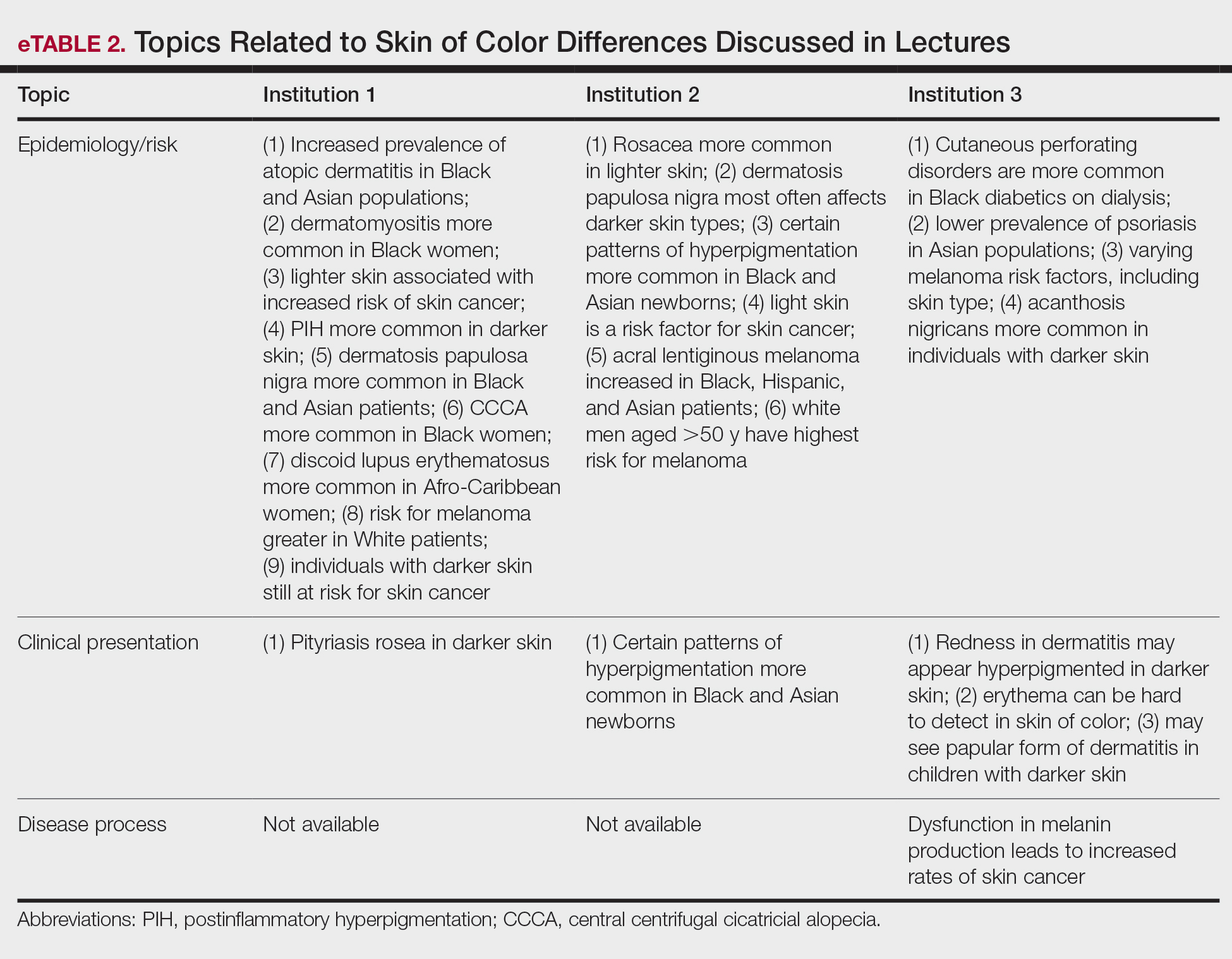
Institutions 1, 2, and 3 had 18, 8, and 17 total dermatology lectures, respectively. Of the 19 conditions designated as areas of importance to skin of color patients by the SOCS, 16 (84.2%) were discussed by institution 1, 11 (57.9%) by institution 2, and 9 (47.4%) by institution 3 (eTable 1). Institution 3 did not include photographs of skin of color patients in its acne, psoriasis, or cutaneous malignancy lectures. Institution 1 also did not include any skin of color patients in its malignancy lecture. Lectures that focused on pigmentary disorders, atopic dermatitis, infectious conditions, and benign cutaneous neoplasms were more likely to display photographs of skin of color patients; for example, lectures that discussed infectious conditions, such as superficial mycoses, herpes viruses, human papillomavirus, syphilis, and atypical mycobacterial infections, were consistently among those with higher proportions of photographs of skin of color patients.

Throughout the entire preclinical dermatology course at all 3 institutions, of 2945 lecture slides, only 24 (0.8%) unique differences were noted between skin color and non–skin of color patients, with 10 total differences noted by institution 1, 6 by institution 2, and 8 by institution 3 (Table). The majority of these differences (19/24) were related to epidemiologic differences in prevalence among varying racial/ethnic groups, with only 5 instances highlighting differences in clinical presentation. There was only a single instance that elaborated on the underlying pathophysiologic mechanisms of the discussed difference. Of all 24 unique differences discussed, 8 were related to skin cancer, 3 were related to dermatitis, and 2 were related to the difference in manifestation of erythema in patients with darker skin (eTable 2).
Comment
The results of this study demonstrated that skin of color is underrepresented in the preclinical dermatology curriculum at these 3 institutions. Although only 16% of all included clinical photographs were of skin of color, individuals with skin of color will soon represent more than half of the total US population within the next 2 decades.1 To increase representation of skin of color patients, teaching faculty should consciously and deliberately include more photographs of skin of color patients for a wider variety of common conditions, including atopic dermatitis and psoriasis, in addition to those that tend to disparately affect skin of color patients, such as pseudofolliculitis barbae or melasma. Furthermore, they also can incorporate more detailed discussions about important differences seen in skin of color patients.
More Skin of Color Photographs in Psoriasis Lectures—At institution 3, there were no skin of color patients included in the psoriasis lecture, even though there is considerable data in the literature indicating notable differences in the clinical presentation, quality-of-life impact, and treatment of psoriasis in skin of color patients.11,22 There are multiple nuances in psoriasis manifestation in patients with skin of color, including less-conspicuous erythema in darker skin, higher degrees of dyspigmentation, and greater body surface area involvement. For Black patients with scalp psoriasis, the impact of hair texture, styling practices, and washing frequency are additional considerations that may impact disease severity and selection of topical therapy.11 The lack of inclusion of any skin of color patients in the psoriasis lecture at one institution further underscores the pressing need to prioritize communities of color in medical education.
More Skin of Color Photographs in Cutaneous Malignancy Lectures—Similarly, while a lecturer at institution 2 noted that acral lentiginous melanoma accounts for a considerable proportion of melanoma among skin of color patients,23 there was no mention of how melanoma generally is substantially more deadly in this population, potentially due to decreased awareness and inconsistent screening.24 Furthermore, at institutions 1 and 3, there were no photographs or discussion of skin of color patients during the cutaneous malignancy lectures. Evidence shows that more emphasis is needed for melanoma screening and awareness in skin of color populations to improve survival outcomes,24 and this begins with educating not only future dermatologists but all future physicians as well. The failure to include photographs of skin of color patients in discussions or lectures regarding cutaneous malignancies may serve to further perpetuate the harmful misperception that individuals with skin of color are unaffected by skin cancer.25,26
Analysis of Skin of Color Photographs in Infectious Disease Lectures—In addition, lectures discussing infectious etiologies were among those with the highest proportion of skin of color photographs. This relatively disproportionate representation of skin of color compared to the other lectures may contribute to the development of harmful stereotypes or the stigmatization of skin of color patients. Although skin of color should continue to be represented in similar lectures, teaching faculty should remain mindful of the potential unintended impact from lectures including relatively disproportionate amounts of skin of color, particularly when other lectures may have sparse to absent representation of skin of color.
More Photographs Available for Education—Overall, our findings may help to inform changes to preclinical dermatology medical education at other institutions to create more inclusive and representative curricula for skin of color patients. The ability of instructors to provide visual representation of various dermatologic conditions may be limited by the photographs available in certain textbooks with few examples of patients with skin of color; however, concerns regarding the lack of skin of color representation in dermatology training is not a novel discussion.17 Although it is the responsibility of all dermatologists to advocate for the inclusion of skin of color, many dermatologists of color have been leading the way in this movement for decades, publishing several textbooks to document various skin conditions in those with darker skin types and discuss unique considerations for patients with skin of color.27-29 Images from these textbooks can be utilized by programs to increase representation of skin of color in dermatology training. There also are multiple expanding online dermatologic databases, such as VisualDx, with an increasing focus on skin of color patients, some of which allow users to filter images by degree of skin pigmentation.30 Moreover, instructors also can work to diversify their curricula by highlighting more of the SOCS conditions of importance to skin of color patients, which have since been renamed and highlighted on the Patient Dermatology Education section of the SOCS website.20 These conditions, while not completely comprehensive, provide a useful starting point for medical educators to reevaluate for potential areas of improvement and inclusion.
There are several potential strategies that can be used to better represent skin of color in dermatologic preclinical medical education, including increasing awareness, especially among dermatology teaching faculty, of existing disparities in the representation of skin of color in the preclinical curricula. Additionally, all dermatology teaching materials could be reviewed at the department level prior to being disseminated to medical students to assess for instances in which skin of color could be prioritized for discussion or varying disease presentations in skin of color could be demonstrated. Finally, teaching faculty may consider photographing more clinical images of their skin of color patients to further develop a catalog of diverse images that can be used to teach students.
Study Limitations—Our study was unable to account for verbal discussion of skin of color not otherwise denoted or captured in lecture slides. Additional limitations include the utilization of Fitzpatrick skin types to describe and differentiate varying skin tones, as the Fitzpatrick scale originally was developed as a method to describe an individual’s response to UV exposure.19 The inability to further delineate the representation of darker skin types, such as those that may be classified as Fitzpatrick skin types V or VI,19 compared to those with lighter skin of color also was a limiting factor. This study was unable to assess for discussion of other common conditions affecting skin of color patients that were not listed as one of the priority conditions by SOCS. Photographs that were designated as indeterminate were difficult to elucidate as skin of color; however, it is possible that instructors may have verbally described these images as skin of color during lectures. Nonetheless, it may be beneficial for learners if teaching faculty were to clearly label instances where skin of color patients are shown or when notable differences are present.
Conclusion
Future studies would benefit from the inclusion of audio data from lectures, syllabi, and small group teaching materials from preclinical courses to more accurately assess representation of skin of color in dermatology training. Additionally, future studies also may expand to include images from lectures of overlapping clinical specialties, particularly infectious disease and rheumatology, to provide a broader assessment of skin of color exposure. Furthermore, repeat assessment may be beneficial to assess the longitudinal effectiveness of curricular changes at the institutions included in this study, comparing older lectures to more recent, updated lectures. This study also may be replicated at other medical schools to allow for wider comparison of curricula.
Acknowledgment—The authors wish to thank the institutions that offered and agreed to participate in this study with the hopes of improving medical education.
- Colby SL, Ortman JM. Projections of the size and composition of the US population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed September 14, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Learn more about SOCS. Skin of Color Society website. Accessed September 14, 2021. http://skinofcolorsociety.org/about-socs/
- Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
- Berardesca E, Maibach H. Ethnic skin: overview of structure and function. J Am Acad Dermatol. 2003;48(suppl 6):S139-S142.
- Callender VD, Surin-Lord SS, Davis EC, et al. Postinflammatory hyperpigmentation. Am J Clin Dermatol. 2011;12:87-99.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE, Stockton T. Pigmentary disorders in blacks. Dermatol Clin. 1988;6:271-281.
- Halder RM, Nootheti PK. Ethnic skin disorders overview. J Am Acad Dermatol. 2003;48(suppl 6):S143-S148.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Ramsay DL, Mayer F. National survey of undergraduate dermatologic medical education. Arch Dermatol.1985;121:1529-1530.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii.
- Knable A, Hood AF, Pearson TG. Undergraduate medical education in dermatology: report from the AAD Interdisciplinary Education Committee, Subcommittee on Undergraduate Medical Education. J Am Acad Dermatol. 1997;36:467-470.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Skin of Color Society. Patient dermatology education. Accessed September 22, 2021. https://skinofcolorsociety.org/patient-dermatology-education
- QuickFacts: United States. US Census Bureau website. Updated July 1, 2019. Accessed September 14, 2021. https://www.census.gov/quickfacts/fact/table/US#
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Pipitone M, Robinson JK, Camara C, et al. Skin cancer awareness in suburban employees: a Hispanic perspective. J Am Acad Dermatol. 2002;47:118-123.
- Imahiyerobo-Ip J, Ip I, Jamal S, et al. Skin cancer awareness in communities of color. J Am Acad Dermatol. 2011;64:198-200.
- Taylor SSC, Serrano AMA, Kelly AP, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Dadzie OE, Petit A, Alexis AF, eds. Ethnic Dermatology: Principles and Practice. Wiley-Blackwell; 2013.
- Jackson-Richards D, Pandya AG, eds. Dermatology Atlas for Skin of Color. Springer; 2014.
- VisualDx. New VisualDx feature: skin of color sort. Published October 14, 2020. Accessed September 22, 2021. https://www.visualdx.com/blog/new-visualdx-feature-skin-of-color-sort/
A ccording to the US Census Bureau, more than half of all Americans are projected to belong to a minority group, defined as any group other than non-Hispanic White alone, by 2044. 1 Consequently, the United States rapidly is becoming a country in which the majority of citizens will have skin of color. Individuals with skin of color are of diverse ethnic backgrounds and include people of African, Latin American, Native American, Pacific Islander, and Asian descent, as well as interethnic backgrounds. 2 Throughout the country, dermatologists along with primary care practitioners may be confronted with certain cutaneous conditions that have varying disease presentations or processes in patients with skin of color. It also is important to note that racial categories are socially rather than biologically constructed, and the term skin of color includes a wide variety of diverse skin types. Nevertheless, the current literature thoroughly supports unique pathophysiologic differences in skin of color as well as variations in disease manifestation compared to White patients. 3-5 For example, the increased lability of melanosomes in skin of color patients, which increases their risk for postinflammatory hyperpigmentation, has been well documented. 5-7 There are various dermatologic conditions that also occur with higher frequency and manifest uniquely in people with darker, more pigmented skin, 7-9 and dermatologists, along with primary care physicians, should feel prepared to recognize and address them.
Extensive evidence also indicates that there are unique aspects to consider while managing certain skin diseases in patients with skin of color.8,10,11 Consequently, as noted on the Skin of Color Society (SOCS) website, “[a]n increase in the body of dermatological literature concerning skin of color as well as the advancement of both basic science and clinical investigational research is necessary to meet the needs of the expanding skin of color population.”2 In the meantime, current knowledge regarding cutaneous conditions that diversely or disproportionately affect skin of color should be actively disseminated to physicians in training. Although patients with skin of color should always have access to comprehensive care and knowledgeable practitioners, the current changes in national and regional demographics further underscore the need for a more thorough understanding of skin of color with regard to disease pathogenesis, diagnosis, and treatment.
Several studies have found that medical students in the United States are minimally exposed to dermatology in general compared to other clinical specialties,12-14 which can easily lead to the underrecognition of disorders that may uniquely or disproportionately affect individuals with pigmented skin. Recent data showed that medical schools typically required fewer than 10 hours of dermatology instruction,12 and on average, dermatologic training made up less than 1% of a medical student’s undergraduate medical education.13,15,16 Consequently, less than 40% of primary care residents felt that their medical school curriculum adequately prepared them to manage common skin conditions.14 Although not all physicians should be expected to fully grasp the complexities of skin of color and its diagnostic and therapeutic implications, both practicing and training dermatologists have acknowledged a lack of exposure to skin of color. In one study, approximately 47% of dermatologists and dermatology residents reported that their medical training (medical school and/or residency) was inadequate in training them on skin conditions in Black patients. Furthermore, many who felt their training was lacking in skin of color identified the need for greater exposure to Black patients and training materials.15 The absence of comprehensive medical education regarding skin of color ultimately can be a disadvantage for both practitioners and patients, resulting in poorer outcomes. Furthermore, underrepresentation of skin of color may persist beyond undergraduate and graduate medical education. There also is evidence to suggest that noninclusion of skin of color pervades foundational dermatologic educational resources, including commonly used textbooks as well as continuing medical education disseminated at national conferences and meetings.17 Taken together, these findings highlight the need for more diverse and representative exposure to skin of color throughout medical training, which begins with a diverse inclusive undergraduate medical education in dermatology.
The objective of this study was to determine if the preclinical dermatology curriculum at 3 US medical schools provided adequate representation of skin of color patients in their didactic presentation slides.
Methods
Participants—Three US medical schools, a blend of private and public medical schools located across different geographic boundaries, agreed to participate in the study. All 3 institutions were current members of the American Medical Association (AMA) Accelerating Change in Medical Education consortium, whose primary goal is to create the medical school of the future and transform physician training.18 All 32 member institutions of the AMA consortium were contacted to request their participation in the study. As part of the consortium, these institutions have vowed to collectively work to develop and share the best models for educational advancement to improve care for patients, populations, and communities18 and would expectedly provide a more racially and ethnically inclusive curriculum than an institution not accountable to a group dedicated to identifying the best ways to deliver care for increasingly diverse communities.
Data Collection—Lectures were included if they were presented during dermatology preclinical courses in the 2015 to 2016 academic year. An uninvolved third party removed the names and identities of instructors to preserve anonymity. Two independent coders from different institutions extracted the data—lecture title, total number of clinical and histologic images, and number of skin of color images—from each of the anonymized lectures using a standardized coding form. We documented differences in skin of color noted in lectures and the disease context for the discussed differences, such as variations in clinical presentation, disease process, epidemiology/risk, and treatment between different skin phenotypes or ethnic groups. Photographs in which the coders were unable to differentiate whether the patient had skin of color were designated as indeterminate or unclear. Photographs appearing to represent Fitzpatrick skin types IV, V, and VI19 were categorically designated as skin of color, and those appearing to represent Fitzpatrick skin types I and II were described as not skin of color; however, images appearing to represent Fitzpatrick skin type III often were classified as not skin of color or indeterminate and occasionally skin of color. The Figure shows examples of images classified as skin of color, indeterminate, and not skin of color. Photographs often were classified as indeterminate due to poor lighting, close-up view photographs, or highlighted pathology obscuring the surrounding skin. We excluded duplicate photographs and histologic images from the analyses.

We also reviewed 19 conditions previously highlighted by the SOCS as areas of importance to skin of color patients.20 The coders tracked how many of these conditions were noted in each lecture. Duplicate discussion of these conditions was not included in the analyses. Any discrepancies between coders were resolved through additional slide review and discussion. The final coded data with the agreed upon changes were used for statistical analyses. Recent national demographic data from the US Census Bureau in 2019 describe approximately 39.9% of the population as belonging to racial/ethnic groups other than non-Hispanic/Latinx White.21 Consequently, the standard for adequate representation for skin of color photographs was set at 35% for the purpose of this study.
Results
Across all 3 institutions included in the study, the proportion of the total number of clinical photographs showing skin of color was 16% (290/1812). Eight percent of the total photographs (145/1812) were noted to be indeterminate (Table). For institution 1, 23.6% of photographs (155/658) showed skin of color, and 12.6% (83/658) were indeterminate. For institution 2, 13.1% (76/578) showed skin of color and 7.8% (45/578) were indeterminate. For institution 3, 10.2% (59/576) showed skin of color and 3% (17/576) were indeterminate.

Institutions 1, 2, and 3 had 18, 8, and 17 total dermatology lectures, respectively. Of the 19 conditions designated as areas of importance to skin of color patients by the SOCS, 16 (84.2%) were discussed by institution 1, 11 (57.9%) by institution 2, and 9 (47.4%) by institution 3 (eTable 1). Institution 3 did not include photographs of skin of color patients in its acne, psoriasis, or cutaneous malignancy lectures. Institution 1 also did not include any skin of color patients in its malignancy lecture. Lectures that focused on pigmentary disorders, atopic dermatitis, infectious conditions, and benign cutaneous neoplasms were more likely to display photographs of skin of color patients; for example, lectures that discussed infectious conditions, such as superficial mycoses, herpes viruses, human papillomavirus, syphilis, and atypical mycobacterial infections, were consistently among those with higher proportions of photographs of skin of color patients.

Throughout the entire preclinical dermatology course at all 3 institutions, of 2945 lecture slides, only 24 (0.8%) unique differences were noted between skin color and non–skin of color patients, with 10 total differences noted by institution 1, 6 by institution 2, and 8 by institution 3 (Table). The majority of these differences (19/24) were related to epidemiologic differences in prevalence among varying racial/ethnic groups, with only 5 instances highlighting differences in clinical presentation. There was only a single instance that elaborated on the underlying pathophysiologic mechanisms of the discussed difference. Of all 24 unique differences discussed, 8 were related to skin cancer, 3 were related to dermatitis, and 2 were related to the difference in manifestation of erythema in patients with darker skin (eTable 2).

Comment
The results of this study demonstrated that skin of color is underrepresented in the preclinical dermatology curriculum at these 3 institutions. Although only 16% of all included clinical photographs were of skin of color, individuals with skin of color will soon represent more than half of the total US population within the next 2 decades.1 To increase representation of skin of color patients, teaching faculty should consciously and deliberately include more photographs of skin of color patients for a wider variety of common conditions, including atopic dermatitis and psoriasis, in addition to those that tend to disparately affect skin of color patients, such as pseudofolliculitis barbae or melasma. Furthermore, they also can incorporate more detailed discussions about important differences seen in skin of color patients.
More Skin of Color Photographs in Psoriasis Lectures—At institution 3, there were no skin of color patients included in the psoriasis lecture, even though there is considerable data in the literature indicating notable differences in the clinical presentation, quality-of-life impact, and treatment of psoriasis in skin of color patients.11,22 There are multiple nuances in psoriasis manifestation in patients with skin of color, including less-conspicuous erythema in darker skin, higher degrees of dyspigmentation, and greater body surface area involvement. For Black patients with scalp psoriasis, the impact of hair texture, styling practices, and washing frequency are additional considerations that may impact disease severity and selection of topical therapy.11 The lack of inclusion of any skin of color patients in the psoriasis lecture at one institution further underscores the pressing need to prioritize communities of color in medical education.
More Skin of Color Photographs in Cutaneous Malignancy Lectures—Similarly, while a lecturer at institution 2 noted that acral lentiginous melanoma accounts for a considerable proportion of melanoma among skin of color patients,23 there was no mention of how melanoma generally is substantially more deadly in this population, potentially due to decreased awareness and inconsistent screening.24 Furthermore, at institutions 1 and 3, there were no photographs or discussion of skin of color patients during the cutaneous malignancy lectures. Evidence shows that more emphasis is needed for melanoma screening and awareness in skin of color populations to improve survival outcomes,24 and this begins with educating not only future dermatologists but all future physicians as well. The failure to include photographs of skin of color patients in discussions or lectures regarding cutaneous malignancies may serve to further perpetuate the harmful misperception that individuals with skin of color are unaffected by skin cancer.25,26
Analysis of Skin of Color Photographs in Infectious Disease Lectures—In addition, lectures discussing infectious etiologies were among those with the highest proportion of skin of color photographs. This relatively disproportionate representation of skin of color compared to the other lectures may contribute to the development of harmful stereotypes or the stigmatization of skin of color patients. Although skin of color should continue to be represented in similar lectures, teaching faculty should remain mindful of the potential unintended impact from lectures including relatively disproportionate amounts of skin of color, particularly when other lectures may have sparse to absent representation of skin of color.
More Photographs Available for Education—Overall, our findings may help to inform changes to preclinical dermatology medical education at other institutions to create more inclusive and representative curricula for skin of color patients. The ability of instructors to provide visual representation of various dermatologic conditions may be limited by the photographs available in certain textbooks with few examples of patients with skin of color; however, concerns regarding the lack of skin of color representation in dermatology training is not a novel discussion.17 Although it is the responsibility of all dermatologists to advocate for the inclusion of skin of color, many dermatologists of color have been leading the way in this movement for decades, publishing several textbooks to document various skin conditions in those with darker skin types and discuss unique considerations for patients with skin of color.27-29 Images from these textbooks can be utilized by programs to increase representation of skin of color in dermatology training. There also are multiple expanding online dermatologic databases, such as VisualDx, with an increasing focus on skin of color patients, some of which allow users to filter images by degree of skin pigmentation.30 Moreover, instructors also can work to diversify their curricula by highlighting more of the SOCS conditions of importance to skin of color patients, which have since been renamed and highlighted on the Patient Dermatology Education section of the SOCS website.20 These conditions, while not completely comprehensive, provide a useful starting point for medical educators to reevaluate for potential areas of improvement and inclusion.
There are several potential strategies that can be used to better represent skin of color in dermatologic preclinical medical education, including increasing awareness, especially among dermatology teaching faculty, of existing disparities in the representation of skin of color in the preclinical curricula. Additionally, all dermatology teaching materials could be reviewed at the department level prior to being disseminated to medical students to assess for instances in which skin of color could be prioritized for discussion or varying disease presentations in skin of color could be demonstrated. Finally, teaching faculty may consider photographing more clinical images of their skin of color patients to further develop a catalog of diverse images that can be used to teach students.
Study Limitations—Our study was unable to account for verbal discussion of skin of color not otherwise denoted or captured in lecture slides. Additional limitations include the utilization of Fitzpatrick skin types to describe and differentiate varying skin tones, as the Fitzpatrick scale originally was developed as a method to describe an individual’s response to UV exposure.19 The inability to further delineate the representation of darker skin types, such as those that may be classified as Fitzpatrick skin types V or VI,19 compared to those with lighter skin of color also was a limiting factor. This study was unable to assess for discussion of other common conditions affecting skin of color patients that were not listed as one of the priority conditions by SOCS. Photographs that were designated as indeterminate were difficult to elucidate as skin of color; however, it is possible that instructors may have verbally described these images as skin of color during lectures. Nonetheless, it may be beneficial for learners if teaching faculty were to clearly label instances where skin of color patients are shown or when notable differences are present.
Conclusion
Future studies would benefit from the inclusion of audio data from lectures, syllabi, and small group teaching materials from preclinical courses to more accurately assess representation of skin of color in dermatology training. Additionally, future studies also may expand to include images from lectures of overlapping clinical specialties, particularly infectious disease and rheumatology, to provide a broader assessment of skin of color exposure. Furthermore, repeat assessment may be beneficial to assess the longitudinal effectiveness of curricular changes at the institutions included in this study, comparing older lectures to more recent, updated lectures. This study also may be replicated at other medical schools to allow for wider comparison of curricula.
Acknowledgment—The authors wish to thank the institutions that offered and agreed to participate in this study with the hopes of improving medical education.
A ccording to the US Census Bureau, more than half of all Americans are projected to belong to a minority group, defined as any group other than non-Hispanic White alone, by 2044. 1 Consequently, the United States rapidly is becoming a country in which the majority of citizens will have skin of color. Individuals with skin of color are of diverse ethnic backgrounds and include people of African, Latin American, Native American, Pacific Islander, and Asian descent, as well as interethnic backgrounds. 2 Throughout the country, dermatologists along with primary care practitioners may be confronted with certain cutaneous conditions that have varying disease presentations or processes in patients with skin of color. It also is important to note that racial categories are socially rather than biologically constructed, and the term skin of color includes a wide variety of diverse skin types. Nevertheless, the current literature thoroughly supports unique pathophysiologic differences in skin of color as well as variations in disease manifestation compared to White patients. 3-5 For example, the increased lability of melanosomes in skin of color patients, which increases their risk for postinflammatory hyperpigmentation, has been well documented. 5-7 There are various dermatologic conditions that also occur with higher frequency and manifest uniquely in people with darker, more pigmented skin, 7-9 and dermatologists, along with primary care physicians, should feel prepared to recognize and address them.
Extensive evidence also indicates that there are unique aspects to consider while managing certain skin diseases in patients with skin of color.8,10,11 Consequently, as noted on the Skin of Color Society (SOCS) website, “[a]n increase in the body of dermatological literature concerning skin of color as well as the advancement of both basic science and clinical investigational research is necessary to meet the needs of the expanding skin of color population.”2 In the meantime, current knowledge regarding cutaneous conditions that diversely or disproportionately affect skin of color should be actively disseminated to physicians in training. Although patients with skin of color should always have access to comprehensive care and knowledgeable practitioners, the current changes in national and regional demographics further underscore the need for a more thorough understanding of skin of color with regard to disease pathogenesis, diagnosis, and treatment.
Several studies have found that medical students in the United States are minimally exposed to dermatology in general compared to other clinical specialties,12-14 which can easily lead to the underrecognition of disorders that may uniquely or disproportionately affect individuals with pigmented skin. Recent data showed that medical schools typically required fewer than 10 hours of dermatology instruction,12 and on average, dermatologic training made up less than 1% of a medical student’s undergraduate medical education.13,15,16 Consequently, less than 40% of primary care residents felt that their medical school curriculum adequately prepared them to manage common skin conditions.14 Although not all physicians should be expected to fully grasp the complexities of skin of color and its diagnostic and therapeutic implications, both practicing and training dermatologists have acknowledged a lack of exposure to skin of color. In one study, approximately 47% of dermatologists and dermatology residents reported that their medical training (medical school and/or residency) was inadequate in training them on skin conditions in Black patients. Furthermore, many who felt their training was lacking in skin of color identified the need for greater exposure to Black patients and training materials.15 The absence of comprehensive medical education regarding skin of color ultimately can be a disadvantage for both practitioners and patients, resulting in poorer outcomes. Furthermore, underrepresentation of skin of color may persist beyond undergraduate and graduate medical education. There also is evidence to suggest that noninclusion of skin of color pervades foundational dermatologic educational resources, including commonly used textbooks as well as continuing medical education disseminated at national conferences and meetings.17 Taken together, these findings highlight the need for more diverse and representative exposure to skin of color throughout medical training, which begins with a diverse inclusive undergraduate medical education in dermatology.
The objective of this study was to determine if the preclinical dermatology curriculum at 3 US medical schools provided adequate representation of skin of color patients in their didactic presentation slides.
Methods
Participants—Three US medical schools, a blend of private and public medical schools located across different geographic boundaries, agreed to participate in the study. All 3 institutions were current members of the American Medical Association (AMA) Accelerating Change in Medical Education consortium, whose primary goal is to create the medical school of the future and transform physician training.18 All 32 member institutions of the AMA consortium were contacted to request their participation in the study. As part of the consortium, these institutions have vowed to collectively work to develop and share the best models for educational advancement to improve care for patients, populations, and communities18 and would expectedly provide a more racially and ethnically inclusive curriculum than an institution not accountable to a group dedicated to identifying the best ways to deliver care for increasingly diverse communities.
Data Collection—Lectures were included if they were presented during dermatology preclinical courses in the 2015 to 2016 academic year. An uninvolved third party removed the names and identities of instructors to preserve anonymity. Two independent coders from different institutions extracted the data—lecture title, total number of clinical and histologic images, and number of skin of color images—from each of the anonymized lectures using a standardized coding form. We documented differences in skin of color noted in lectures and the disease context for the discussed differences, such as variations in clinical presentation, disease process, epidemiology/risk, and treatment between different skin phenotypes or ethnic groups. Photographs in which the coders were unable to differentiate whether the patient had skin of color were designated as indeterminate or unclear. Photographs appearing to represent Fitzpatrick skin types IV, V, and VI19 were categorically designated as skin of color, and those appearing to represent Fitzpatrick skin types I and II were described as not skin of color; however, images appearing to represent Fitzpatrick skin type III often were classified as not skin of color or indeterminate and occasionally skin of color. The Figure shows examples of images classified as skin of color, indeterminate, and not skin of color. Photographs often were classified as indeterminate due to poor lighting, close-up view photographs, or highlighted pathology obscuring the surrounding skin. We excluded duplicate photographs and histologic images from the analyses.

We also reviewed 19 conditions previously highlighted by the SOCS as areas of importance to skin of color patients.20 The coders tracked how many of these conditions were noted in each lecture. Duplicate discussion of these conditions was not included in the analyses. Any discrepancies between coders were resolved through additional slide review and discussion. The final coded data with the agreed upon changes were used for statistical analyses. Recent national demographic data from the US Census Bureau in 2019 describe approximately 39.9% of the population as belonging to racial/ethnic groups other than non-Hispanic/Latinx White.21 Consequently, the standard for adequate representation for skin of color photographs was set at 35% for the purpose of this study.
Results
Across all 3 institutions included in the study, the proportion of the total number of clinical photographs showing skin of color was 16% (290/1812). Eight percent of the total photographs (145/1812) were noted to be indeterminate (Table). For institution 1, 23.6% of photographs (155/658) showed skin of color, and 12.6% (83/658) were indeterminate. For institution 2, 13.1% (76/578) showed skin of color and 7.8% (45/578) were indeterminate. For institution 3, 10.2% (59/576) showed skin of color and 3% (17/576) were indeterminate.

Institutions 1, 2, and 3 had 18, 8, and 17 total dermatology lectures, respectively. Of the 19 conditions designated as areas of importance to skin of color patients by the SOCS, 16 (84.2%) were discussed by institution 1, 11 (57.9%) by institution 2, and 9 (47.4%) by institution 3 (eTable 1). Institution 3 did not include photographs of skin of color patients in its acne, psoriasis, or cutaneous malignancy lectures. Institution 1 also did not include any skin of color patients in its malignancy lecture. Lectures that focused on pigmentary disorders, atopic dermatitis, infectious conditions, and benign cutaneous neoplasms were more likely to display photographs of skin of color patients; for example, lectures that discussed infectious conditions, such as superficial mycoses, herpes viruses, human papillomavirus, syphilis, and atypical mycobacterial infections, were consistently among those with higher proportions of photographs of skin of color patients.

Throughout the entire preclinical dermatology course at all 3 institutions, of 2945 lecture slides, only 24 (0.8%) unique differences were noted between skin color and non–skin of color patients, with 10 total differences noted by institution 1, 6 by institution 2, and 8 by institution 3 (Table). The majority of these differences (19/24) were related to epidemiologic differences in prevalence among varying racial/ethnic groups, with only 5 instances highlighting differences in clinical presentation. There was only a single instance that elaborated on the underlying pathophysiologic mechanisms of the discussed difference. Of all 24 unique differences discussed, 8 were related to skin cancer, 3 were related to dermatitis, and 2 were related to the difference in manifestation of erythema in patients with darker skin (eTable 2).

Comment
The results of this study demonstrated that skin of color is underrepresented in the preclinical dermatology curriculum at these 3 institutions. Although only 16% of all included clinical photographs were of skin of color, individuals with skin of color will soon represent more than half of the total US population within the next 2 decades.1 To increase representation of skin of color patients, teaching faculty should consciously and deliberately include more photographs of skin of color patients for a wider variety of common conditions, including atopic dermatitis and psoriasis, in addition to those that tend to disparately affect skin of color patients, such as pseudofolliculitis barbae or melasma. Furthermore, they also can incorporate more detailed discussions about important differences seen in skin of color patients.
More Skin of Color Photographs in Psoriasis Lectures—At institution 3, there were no skin of color patients included in the psoriasis lecture, even though there is considerable data in the literature indicating notable differences in the clinical presentation, quality-of-life impact, and treatment of psoriasis in skin of color patients.11,22 There are multiple nuances in psoriasis manifestation in patients with skin of color, including less-conspicuous erythema in darker skin, higher degrees of dyspigmentation, and greater body surface area involvement. For Black patients with scalp psoriasis, the impact of hair texture, styling practices, and washing frequency are additional considerations that may impact disease severity and selection of topical therapy.11 The lack of inclusion of any skin of color patients in the psoriasis lecture at one institution further underscores the pressing need to prioritize communities of color in medical education.
More Skin of Color Photographs in Cutaneous Malignancy Lectures—Similarly, while a lecturer at institution 2 noted that acral lentiginous melanoma accounts for a considerable proportion of melanoma among skin of color patients,23 there was no mention of how melanoma generally is substantially more deadly in this population, potentially due to decreased awareness and inconsistent screening.24 Furthermore, at institutions 1 and 3, there were no photographs or discussion of skin of color patients during the cutaneous malignancy lectures. Evidence shows that more emphasis is needed for melanoma screening and awareness in skin of color populations to improve survival outcomes,24 and this begins with educating not only future dermatologists but all future physicians as well. The failure to include photographs of skin of color patients in discussions or lectures regarding cutaneous malignancies may serve to further perpetuate the harmful misperception that individuals with skin of color are unaffected by skin cancer.25,26
Analysis of Skin of Color Photographs in Infectious Disease Lectures—In addition, lectures discussing infectious etiologies were among those with the highest proportion of skin of color photographs. This relatively disproportionate representation of skin of color compared to the other lectures may contribute to the development of harmful stereotypes or the stigmatization of skin of color patients. Although skin of color should continue to be represented in similar lectures, teaching faculty should remain mindful of the potential unintended impact from lectures including relatively disproportionate amounts of skin of color, particularly when other lectures may have sparse to absent representation of skin of color.
More Photographs Available for Education—Overall, our findings may help to inform changes to preclinical dermatology medical education at other institutions to create more inclusive and representative curricula for skin of color patients. The ability of instructors to provide visual representation of various dermatologic conditions may be limited by the photographs available in certain textbooks with few examples of patients with skin of color; however, concerns regarding the lack of skin of color representation in dermatology training is not a novel discussion.17 Although it is the responsibility of all dermatologists to advocate for the inclusion of skin of color, many dermatologists of color have been leading the way in this movement for decades, publishing several textbooks to document various skin conditions in those with darker skin types and discuss unique considerations for patients with skin of color.27-29 Images from these textbooks can be utilized by programs to increase representation of skin of color in dermatology training. There also are multiple expanding online dermatologic databases, such as VisualDx, with an increasing focus on skin of color patients, some of which allow users to filter images by degree of skin pigmentation.30 Moreover, instructors also can work to diversify their curricula by highlighting more of the SOCS conditions of importance to skin of color patients, which have since been renamed and highlighted on the Patient Dermatology Education section of the SOCS website.20 These conditions, while not completely comprehensive, provide a useful starting point for medical educators to reevaluate for potential areas of improvement and inclusion.
There are several potential strategies that can be used to better represent skin of color in dermatologic preclinical medical education, including increasing awareness, especially among dermatology teaching faculty, of existing disparities in the representation of skin of color in the preclinical curricula. Additionally, all dermatology teaching materials could be reviewed at the department level prior to being disseminated to medical students to assess for instances in which skin of color could be prioritized for discussion or varying disease presentations in skin of color could be demonstrated. Finally, teaching faculty may consider photographing more clinical images of their skin of color patients to further develop a catalog of diverse images that can be used to teach students.
Study Limitations—Our study was unable to account for verbal discussion of skin of color not otherwise denoted or captured in lecture slides. Additional limitations include the utilization of Fitzpatrick skin types to describe and differentiate varying skin tones, as the Fitzpatrick scale originally was developed as a method to describe an individual’s response to UV exposure.19 The inability to further delineate the representation of darker skin types, such as those that may be classified as Fitzpatrick skin types V or VI,19 compared to those with lighter skin of color also was a limiting factor. This study was unable to assess for discussion of other common conditions affecting skin of color patients that were not listed as one of the priority conditions by SOCS. Photographs that were designated as indeterminate were difficult to elucidate as skin of color; however, it is possible that instructors may have verbally described these images as skin of color during lectures. Nonetheless, it may be beneficial for learners if teaching faculty were to clearly label instances where skin of color patients are shown or when notable differences are present.
Conclusion
Future studies would benefit from the inclusion of audio data from lectures, syllabi, and small group teaching materials from preclinical courses to more accurately assess representation of skin of color in dermatology training. Additionally, future studies also may expand to include images from lectures of overlapping clinical specialties, particularly infectious disease and rheumatology, to provide a broader assessment of skin of color exposure. Furthermore, repeat assessment may be beneficial to assess the longitudinal effectiveness of curricular changes at the institutions included in this study, comparing older lectures to more recent, updated lectures. This study also may be replicated at other medical schools to allow for wider comparison of curricula.
Acknowledgment—The authors wish to thank the institutions that offered and agreed to participate in this study with the hopes of improving medical education.
- Colby SL, Ortman JM. Projections of the size and composition of the US population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed September 14, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Learn more about SOCS. Skin of Color Society website. Accessed September 14, 2021. http://skinofcolorsociety.org/about-socs/
- Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
- Berardesca E, Maibach H. Ethnic skin: overview of structure and function. J Am Acad Dermatol. 2003;48(suppl 6):S139-S142.
- Callender VD, Surin-Lord SS, Davis EC, et al. Postinflammatory hyperpigmentation. Am J Clin Dermatol. 2011;12:87-99.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE, Stockton T. Pigmentary disorders in blacks. Dermatol Clin. 1988;6:271-281.
- Halder RM, Nootheti PK. Ethnic skin disorders overview. J Am Acad Dermatol. 2003;48(suppl 6):S143-S148.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Ramsay DL, Mayer F. National survey of undergraduate dermatologic medical education. Arch Dermatol.1985;121:1529-1530.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii.
- Knable A, Hood AF, Pearson TG. Undergraduate medical education in dermatology: report from the AAD Interdisciplinary Education Committee, Subcommittee on Undergraduate Medical Education. J Am Acad Dermatol. 1997;36:467-470.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Skin of Color Society. Patient dermatology education. Accessed September 22, 2021. https://skinofcolorsociety.org/patient-dermatology-education
- QuickFacts: United States. US Census Bureau website. Updated July 1, 2019. Accessed September 14, 2021. https://www.census.gov/quickfacts/fact/table/US#
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Pipitone M, Robinson JK, Camara C, et al. Skin cancer awareness in suburban employees: a Hispanic perspective. J Am Acad Dermatol. 2002;47:118-123.
- Imahiyerobo-Ip J, Ip I, Jamal S, et al. Skin cancer awareness in communities of color. J Am Acad Dermatol. 2011;64:198-200.
- Taylor SSC, Serrano AMA, Kelly AP, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Dadzie OE, Petit A, Alexis AF, eds. Ethnic Dermatology: Principles and Practice. Wiley-Blackwell; 2013.
- Jackson-Richards D, Pandya AG, eds. Dermatology Atlas for Skin of Color. Springer; 2014.
- VisualDx. New VisualDx feature: skin of color sort. Published October 14, 2020. Accessed September 22, 2021. https://www.visualdx.com/blog/new-visualdx-feature-skin-of-color-sort/
- Colby SL, Ortman JM. Projections of the size and composition of the US population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed September 14, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Learn more about SOCS. Skin of Color Society website. Accessed September 14, 2021. http://skinofcolorsociety.org/about-socs/
- Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
- Berardesca E, Maibach H. Ethnic skin: overview of structure and function. J Am Acad Dermatol. 2003;48(suppl 6):S139-S142.
- Callender VD, Surin-Lord SS, Davis EC, et al. Postinflammatory hyperpigmentation. Am J Clin Dermatol. 2011;12:87-99.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE, Stockton T. Pigmentary disorders in blacks. Dermatol Clin. 1988;6:271-281.
- Halder RM, Nootheti PK. Ethnic skin disorders overview. J Am Acad Dermatol. 2003;48(suppl 6):S143-S148.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Ramsay DL, Mayer F. National survey of undergraduate dermatologic medical education. Arch Dermatol.1985;121:1529-1530.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii.
- Knable A, Hood AF, Pearson TG. Undergraduate medical education in dermatology: report from the AAD Interdisciplinary Education Committee, Subcommittee on Undergraduate Medical Education. J Am Acad Dermatol. 1997;36:467-470.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Skin of Color Society. Patient dermatology education. Accessed September 22, 2021. https://skinofcolorsociety.org/patient-dermatology-education
- QuickFacts: United States. US Census Bureau website. Updated July 1, 2019. Accessed September 14, 2021. https://www.census.gov/quickfacts/fact/table/US#
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Pipitone M, Robinson JK, Camara C, et al. Skin cancer awareness in suburban employees: a Hispanic perspective. J Am Acad Dermatol. 2002;47:118-123.
- Imahiyerobo-Ip J, Ip I, Jamal S, et al. Skin cancer awareness in communities of color. J Am Acad Dermatol. 2011;64:198-200.
- Taylor SSC, Serrano AMA, Kelly AP, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Dadzie OE, Petit A, Alexis AF, eds. Ethnic Dermatology: Principles and Practice. Wiley-Blackwell; 2013.
- Jackson-Richards D, Pandya AG, eds. Dermatology Atlas for Skin of Color. Springer; 2014.
- VisualDx. New VisualDx feature: skin of color sort. Published October 14, 2020. Accessed September 22, 2021. https://www.visualdx.com/blog/new-visualdx-feature-skin-of-color-sort/
Practice Points
- The United States rapidly is becoming a country in which the majority of citizens will have skin of color.
- Our study results strongly suggest that skin of color may be seriously underrepresented in medical education and can guide modifications to preclinical dermatology medical education to develop a more comprehensive and inclusive curriculum.
- Efforts should be made to increase images and discussion of skin of color in preclinical didactics.
The Role of Inpatient Dermatology Consultations
Dermatology is an often-underutilized resource in the hospital setting. As the health care landscape has evolved, so has the role of the inpatient dermatologist.1-3 Structural changes in the health system and advances in therapies have shifted dermatology from an admitting service to an almost exclusively outpatient practice. Improved treatment modalities led to decreases in the number of patients requiring admission for chronic dermatoses, and outpatient clinics began offering therapies once limited to hospitals.1,4 Inpatient dermatology consultations emerged and continue to have profound effects on hospitalized patients regardless of their reason for admission.1-11
Inpatient dermatologists supply knowledge in areas primary medical teams lack, and there is evidence that dermatology consultations improve the quality of care while decreasing cost.2,5-7 Establishing correct diagnoses, preventing exposure to unnecessary medications, and reducing hospitalization duration and readmission rates are a few ways dermatology consultations positively impact hospitalized patients.2,5-7,9,10 This study highlights the role of the dermatologist in the care of hospitalized patients at a large academic medical center in an urban setting and reveals how consultation supports the efficiency and efficacy of other services.
Materials and Methods
Study Design—This single-institution, cross-sectional retrospective study included all hospitalized patients at the Thomas Jefferson University Hospital (Philadelphia, Pennsylvania), who received an inpatient dermatology consultation completed by physicians of Jefferson Dermatology Associates between January 1, 2019, and December 31, 2019. The institutional review board at Thomas Jefferson University approved this study.
Data Collection—A list of all inpatient dermatology consultations in 2019 was provided by Jefferson Dermatology Associates. Through a retrospective chart review, data regarding the consultations were collected from the electronic medical record (Epic Systems) and recorded into the Research Electronic Data Capture system. Data on patient demographics, the primary medical team, the dermatology evaluation, and the hospital course of the patient were collected.
Results
Patient Characteristics—Dermatology received 253 inpatient consultation requests during this time period; 53% of patients were female and 47% were male, with a mean age of 55 years. Most patients were White (57%), while 34% were Black. Five percent and 4% of patients were Asian and Hispanic or Latino, respectively (Table 1). The mean duration of hospitalization for all patients was 15 days, and the average number of days to discharge following the first encounter with dermatology was 10 days.
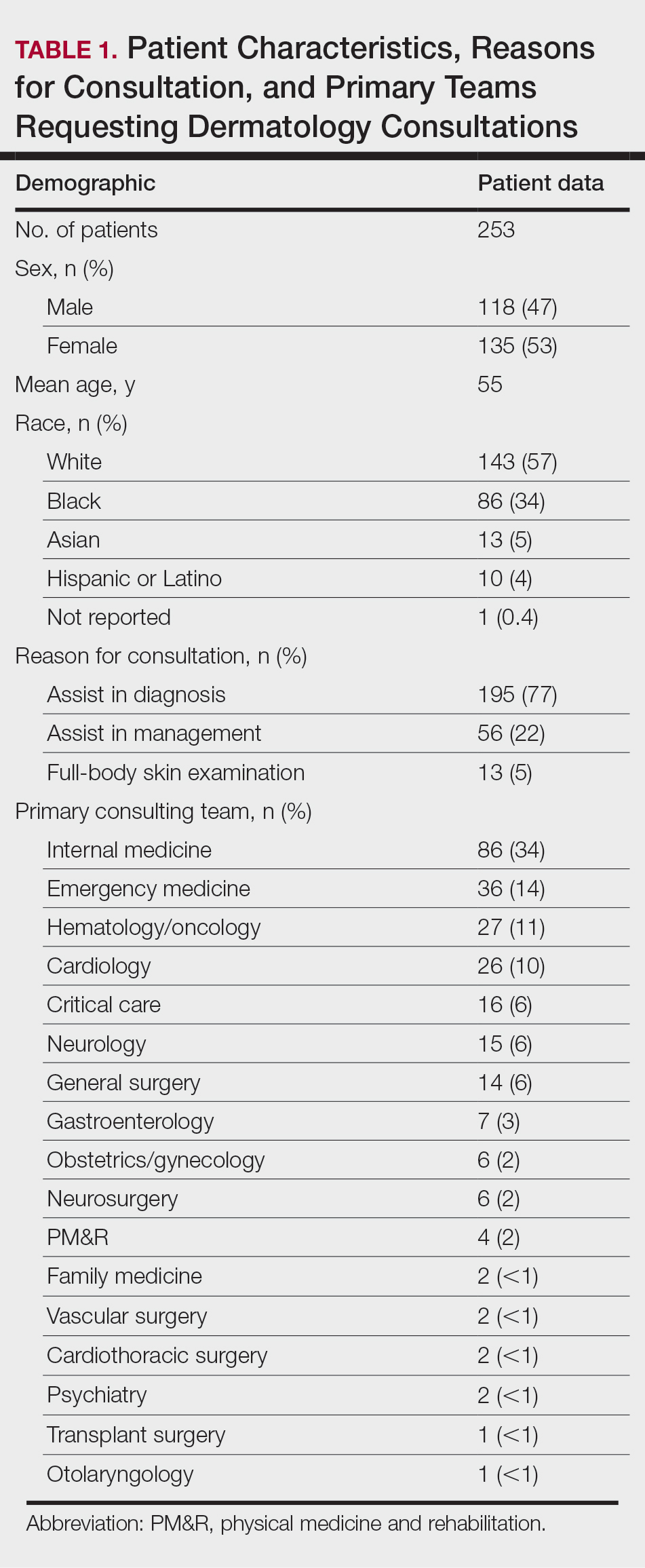
Requesting Team and Reason for Consultation—Internal medicine consulted dermatology most frequently (34% of all consultations), followed by emergency medicine (14%) and a variety of other services (Table 1). Most dermatology consultations were placed to assist in achieving a diagnosis of a cutaneous condition (77%), while a minority were to assist in the management of a previously diagnosed disease (22%). A small fraction of consultations (5%) were to complete full-body skin examinations (FBSEs) to rule out infection or malignancy in candidates for organ transplantation, left ventricular assist devices, or certain chemotherapies. One FBSE was conducted to search for a primary tumor in a patient diagnosed with metastatic melanoma.
Most Common Final Diagnoses and Consultation Impact—Table 2 lists the most common final diagnosis categories, as well as the effects of the consultation on diagnosis, management, biopsies, hospitalization, and clinical improvement as documented by the primary medical provider. The most common final diagnoses were inflammatory and autoimmune (39%), such as contact dermatitis and seborrheic dermatitis; infectious (23%), such as varicella (primary or zoster) and bacterial furunculosis; drug reactions (20%), such as morbilliform drug eruptions; vascular (8%), such as vasculitis and calciphylaxis; neoplastic (7%), such as keratinocyte carcinomas and leukemia cutis; and other (15%), such as xerosis, keratosis pilaris, and miliaria rubra.
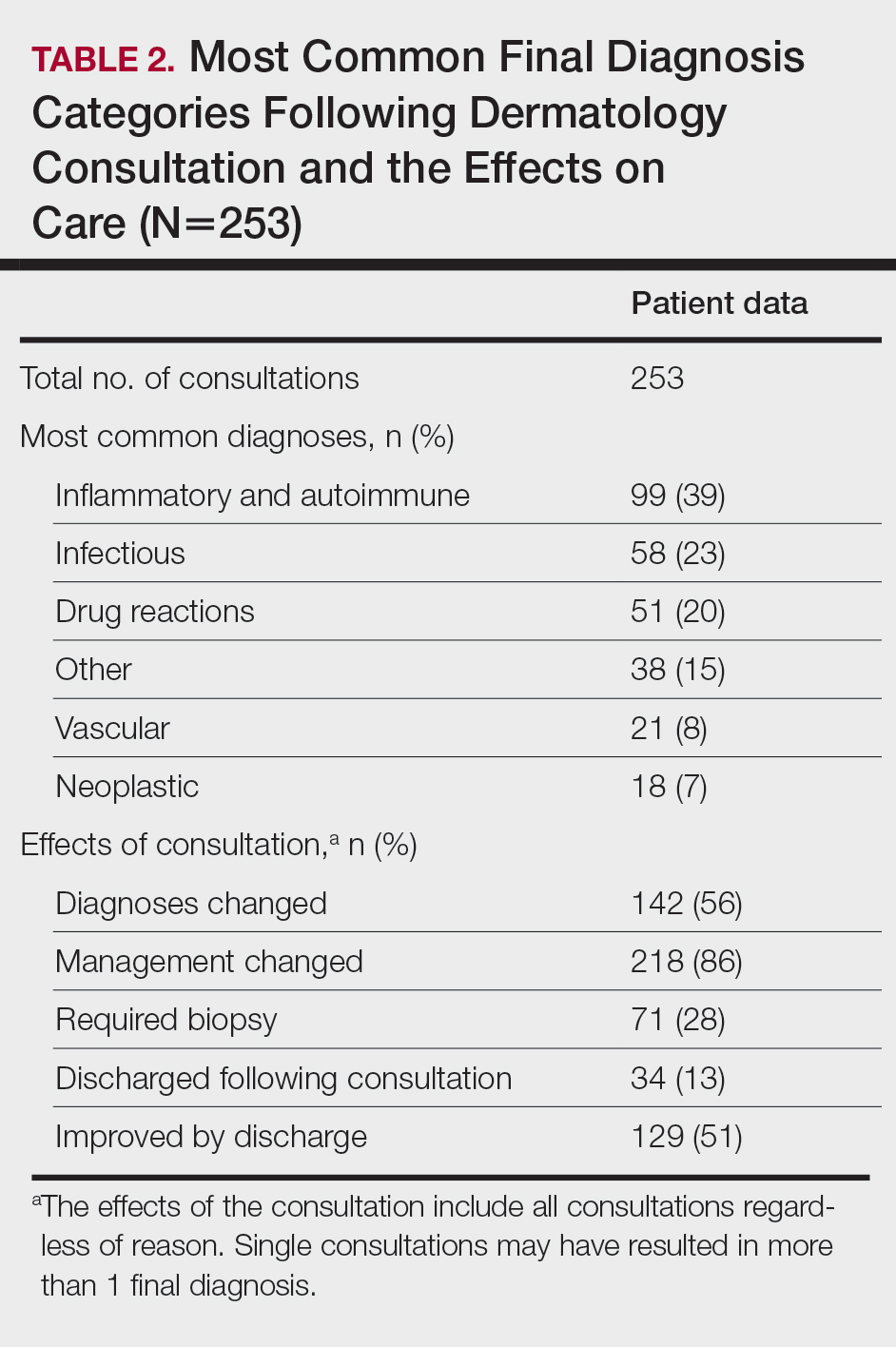
Impact on Diagnosis—Fifty-six percent of all consultations resulted in a change in diagnosis. When dermatology was consulted specifically to assist in the diagnosis of a patient (195 consultations), the working diagnosis of the primary team was changed 69% of the time. Thirty-five of these consultation requests had no preliminary diagnosis, and the primary team listed the working diagnosis as either rash or a morphologic description of the lesion(s). Sixty-three percent of suspected drug eruptions ended with a diagnosis of a form of drug eruption, while 20% of consultations for suspected cellulitis or bacterial infections were confirmed to be cellulitis or soft tissue infections.
Impact on Management—Regardless of the reason for the consultation, most consultations (86%) resulted in a change in management. The remaining 14% consisted of FBSEs with benign findings; cases of cutaneous metastases and leukemia cutis managed by oncology; as well as select cases of purpura fulminans, postfebrile desquamation, and postinflammatory hyperpigmentation.
Changes in management included alterations in medications, requests for additional laboratory work or imaging, additional consultation requests, biopsies, or specific wound care instructions. Seventy-five percent of all consultations were given specific medication recommendations by dermatology. Most (61%) were recommended to be given a topical steroid, antibiotic, or both. However, 45% of all consultations were recommended to initiate a systemic medication, most commonly antihistamines, antibiotics, steroids, antivirals, or immunomodulators. Dermatology recommended discontinuing specific medications in 16% of all consultations, with antibiotics being the most frequent culprit (17 antibiotics discontinued), owing to drug eruptions or misdiagnosed infections. Vancomycin, piperacillin-tazobactam, and trimethoprim-sulfamethoxazole were the most frequently discontinued antibiotics.
Dermatology was consulted for assistance in management of previously diagnosed cutaneous conditions 56 times (22% of all consultations), often regarding complicated cases of hidradenitis suppurativa (9 cases), pyoderma gangrenosum (5 cases), bullous pemphigoid (4 cases), or erythroderma (4 cases). Most of these cases required a single dermatology encounter to provide recommendations (71%), and 21% required 1 additional follow-up. Sixty-three percent of patients consulted for management assistance were noted to have improvement in their cutaneous condition by time of discharge, as documented by the primary provider in the medical record.
Twenty-eight percent of all consultations required at least 1 biopsy. Seventy-two percent of all biopsies were consistent with the dermatologist’s working diagnosis or highest-ranked differential diagnosis, and 16% of biopsy results were consistent with the second- or third-ranked diagnosis. The primary teams requested a biopsy 38 times to assist in diagnosis, as documented in the progress note or consultation request. Only 21 of these consultations (55% of requests) received at least 1 biopsy, as the remaining consultations did not require a biopsy to establish a diagnosis. The most common final diagnoses of consultations receiving biopsies included drug eruptions (5), leukemia cutis (4), vasculopathies (4), vasculitis (4), and calciphylaxis (3).
Impact on Hospitalization and Efficacy—Dermatology performed 217 consultations regarding patients already admitted to the hospital, and 92% remained hospitalized either due to comorbidities or complicated cutaneous conditions following the consultation. The remaining 8% were cleared for discharge. Dermatology received 36 consultation requests from emergency medicine physicians. Fifty-three percent of these patients were admitted, while the remaining 47% were discharged from the emergency department or its observation unit following evaluation.
Fifty-one percent of all consultations were noted to have improvement in their cutaneous condition by the time of discharge, as noted in the physical examination, progress note, or discharge summary of the primary team. Thirty percent of cases remained stable, where improvement was not noted in in the medical record. Most of these cases involved keratinocyte carcinomas scheduled for outpatient excision, benign melanocytic nevi found on FBSE, and benign etiologies that led to immediate discharge following consultation. Three percent of all consultations were noted to have worsened following consultation, including cases of calciphylaxis, vasculopathies, and purpura fulminans, as well as patients who elected for palliative care and hospice. The cutaneous condition by the time of discharge could not be determined from the medical record in 16% of all consultations.
Eighty-five percent of all consultations required a single encounter with dermatology. An additional 10% required a single follow-up with dermatology, while only 5% of patients required 3 or more encounters. Notably, these cases included patients with 1 or more severe cutaneous diseases, such as Sweet syndrome, calciphylaxis, Stevens-Johnson syndrome/toxic epidermal necrolysis, and hidradenitis suppurativa.
Comment
Although dermatology often is viewed as an outpatient specialty, this study provides a glimpse into the ways inpatient dermatology consultations optimize the care of hospitalized patients. Most consultations involved assistance in diagnosing an unknown condition, but several regarded pre-existing skin disorders requiring management aid. As a variety of medical specialties requested consultations, dermatology was able to provide care to a diverse group of patients with conditions varying in complexity and severity. Several specialties benefited from niche dermatologic expertise: hematology and oncology frequently requested dermatology to assist in diagnosis and management of the toxic effects of chemotherapy, cutaneous metastasis, or suspected cutaneous infections in immunocompromised patients. Cardiology patients were frequently evaluated for potential malignancy or infection prior to heart transplantation and initiation of antirejection immunosuppressants. Dermatology was consulted to differentiate cutaneous manifestations of critical illness from underlying systemic disease in the intensive care unit, and patients presenting to the emergency department often were examined to determine if hospital admission was necessary, with 47% of these consultations resulting in a discharge following evaluation by a dermatologist.
Our results were consistent with prior studies1,5,6 that have reported frequent changes in final diagnosis following dermatology consultation, with 69% of working diagnoses changed in this study when consultation was requested for diagnostic assistance. When dermatology was consulted for diagnostic assistance, several of these cases lacked a preliminary differential diagnosis. Although the absence of a documented differential diagnosis may not necessarily reflect a lack of suspicion for a particular etiology, 86% of all consultations included a ranked differential or working diagnosis either in the consultation request or progress note prior to consultation. The final diagnoses of consultations without a preliminary diagnosis varied from the mild and localized to systemic and severe, further suggesting these cases reflected knowledge gaps of the primary medical team.
Integration of dermatology into the care of hospitalized patients could provide an opportunity for education of primary medical teams. With frequent consultation, primary medical teams may become more comfortable diagnosing and managing common cutaneous conditions specific to their specialty or extended hospitalizations.
Several consultations were requested to aid in management of cases of hidradenitis suppurativa, pyoderma gangrenosum, or bullous pemphigoid that either failed outpatient therapy or were complicated by superinfections. Despite the ranges in complexity, the majority of all consultations required a single encounter and led to improvement by the time of discharge, demonstrating the efficacy and efficiency of inpatient dermatologists.
Dermatology consultations often led to changes in management involving medications and additional workup. Changes in management also extended to specific wound care instructions provided by dermatology, as expected for cases of Stevens-Johnson syndrome/toxic epidermal necrolysis, Sweet syndrome, hidradenitis suppurativa, and pyoderma gangrenosum. However, patients with the sequelae of extended hospitalizations, such as chronic wounds, pressure ulcers, and edema bullae, also benefited from this expertise.
When patients required a biopsy, the final diagnoses were consistent with the dermatologist’s number one differential diagnosis or top 3 differential diagnoses 72% and 88% of the time, respectively. Only 55% of cases where the primary team requested a biopsy ultimately required a biopsy, as many involved clinical diagnoses such as urticaria. Not only was dermatology accurate in their preliminary diagnoses, but they decreased cost and morbidity by avoiding unnecessary procedures.
This study provided additional evidence to support the integration of dermatology into the hospital setting for the benefit of patients, primary medical teams, and hospital systems. Dermatology offers high-value care through the efficient diagnosis and management of hospitalized patients, which contributes to decreased cost and improved outcomes.2,5-7,9,10 This study highlighted lesser-known areas of impact, such as the various specialty-specific services dermatology provides as well as the high rates of reported improvement following consultation. Future studies should continue to explore the field’s unique impact on hospitalized medicine as well as other avenues of care delivery, such as telemedicine, that may encourage dermatologists to participate in consultations and increase the volume of patients who may benefit from their care.
- Madigan LM, Fox LP. Where are we now with inpatient consultative dermatology?: assessing the value and evolution of this subspecialty over the past decade. J Am Acad Dermatol. 2019;80:1804-1808. doi:10.1016/j.jaad.2019.01.031
- Noe MH, Rosenbach M. Inpatient dermatologists—crucial for the management of skin diseases in hospitalized patients [editorial]. JAMA Dermatol. 2018;154:524-525. doi:10.1001/jamadermatol.2017.6195
- Strowd LC. Inpatient dermatology: a paradigm shift in the management of skin disease in the hospital. Br J Dermatol. 2019;180:966-967. doi:10.1111/bjd.17778
- Kirsner RS, Yang DG, Kerdel FA. The changing status of inpatient dermatology at American academic dermatology programs. J Am Acad Dermatol. 1999;40:755-757. doi:10.1016/s0190-9622(99)70158-1
- Kroshinsky D, Cotliar J, Hughey LC, et al. Association of dermatology consultation with accuracy of cutaneous disorder diagnoses in hospitalized patients: a multicenter analysis. JAMA Dermatol. 2016;152:477-480. doi:10.1001/jamadermatol.2015.5098
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis. JAMA Dermatol. 2018;154:529-533. doi:10.1001/jamadermatol.2017.6196
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528. doi:10.1001/jamadermatol.2016.6130
- Imadojemu S, Rosenbach M. Dermatologists must take an active role in the diagnosis of cellulitis. JAMA Dermatol. 2017;153:134-135. doi:10.1001/jamadermatol.2016.4230
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062. doi:10.1001/jamadermatol.2014.1164
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558. doi:10.1111/ijd.13939
Dermatology is an often-underutilized resource in the hospital setting. As the health care landscape has evolved, so has the role of the inpatient dermatologist.1-3 Structural changes in the health system and advances in therapies have shifted dermatology from an admitting service to an almost exclusively outpatient practice. Improved treatment modalities led to decreases in the number of patients requiring admission for chronic dermatoses, and outpatient clinics began offering therapies once limited to hospitals.1,4 Inpatient dermatology consultations emerged and continue to have profound effects on hospitalized patients regardless of their reason for admission.1-11
Inpatient dermatologists supply knowledge in areas primary medical teams lack, and there is evidence that dermatology consultations improve the quality of care while decreasing cost.2,5-7 Establishing correct diagnoses, preventing exposure to unnecessary medications, and reducing hospitalization duration and readmission rates are a few ways dermatology consultations positively impact hospitalized patients.2,5-7,9,10 This study highlights the role of the dermatologist in the care of hospitalized patients at a large academic medical center in an urban setting and reveals how consultation supports the efficiency and efficacy of other services.
Materials and Methods
Study Design—This single-institution, cross-sectional retrospective study included all hospitalized patients at the Thomas Jefferson University Hospital (Philadelphia, Pennsylvania), who received an inpatient dermatology consultation completed by physicians of Jefferson Dermatology Associates between January 1, 2019, and December 31, 2019. The institutional review board at Thomas Jefferson University approved this study.
Data Collection—A list of all inpatient dermatology consultations in 2019 was provided by Jefferson Dermatology Associates. Through a retrospective chart review, data regarding the consultations were collected from the electronic medical record (Epic Systems) and recorded into the Research Electronic Data Capture system. Data on patient demographics, the primary medical team, the dermatology evaluation, and the hospital course of the patient were collected.
Results
Patient Characteristics—Dermatology received 253 inpatient consultation requests during this time period; 53% of patients were female and 47% were male, with a mean age of 55 years. Most patients were White (57%), while 34% were Black. Five percent and 4% of patients were Asian and Hispanic or Latino, respectively (Table 1). The mean duration of hospitalization for all patients was 15 days, and the average number of days to discharge following the first encounter with dermatology was 10 days.

Requesting Team and Reason for Consultation—Internal medicine consulted dermatology most frequently (34% of all consultations), followed by emergency medicine (14%) and a variety of other services (Table 1). Most dermatology consultations were placed to assist in achieving a diagnosis of a cutaneous condition (77%), while a minority were to assist in the management of a previously diagnosed disease (22%). A small fraction of consultations (5%) were to complete full-body skin examinations (FBSEs) to rule out infection or malignancy in candidates for organ transplantation, left ventricular assist devices, or certain chemotherapies. One FBSE was conducted to search for a primary tumor in a patient diagnosed with metastatic melanoma.
Most Common Final Diagnoses and Consultation Impact—Table 2 lists the most common final diagnosis categories, as well as the effects of the consultation on diagnosis, management, biopsies, hospitalization, and clinical improvement as documented by the primary medical provider. The most common final diagnoses were inflammatory and autoimmune (39%), such as contact dermatitis and seborrheic dermatitis; infectious (23%), such as varicella (primary or zoster) and bacterial furunculosis; drug reactions (20%), such as morbilliform drug eruptions; vascular (8%), such as vasculitis and calciphylaxis; neoplastic (7%), such as keratinocyte carcinomas and leukemia cutis; and other (15%), such as xerosis, keratosis pilaris, and miliaria rubra.

Impact on Diagnosis—Fifty-six percent of all consultations resulted in a change in diagnosis. When dermatology was consulted specifically to assist in the diagnosis of a patient (195 consultations), the working diagnosis of the primary team was changed 69% of the time. Thirty-five of these consultation requests had no preliminary diagnosis, and the primary team listed the working diagnosis as either rash or a morphologic description of the lesion(s). Sixty-three percent of suspected drug eruptions ended with a diagnosis of a form of drug eruption, while 20% of consultations for suspected cellulitis or bacterial infections were confirmed to be cellulitis or soft tissue infections.
Impact on Management—Regardless of the reason for the consultation, most consultations (86%) resulted in a change in management. The remaining 14% consisted of FBSEs with benign findings; cases of cutaneous metastases and leukemia cutis managed by oncology; as well as select cases of purpura fulminans, postfebrile desquamation, and postinflammatory hyperpigmentation.
Changes in management included alterations in medications, requests for additional laboratory work or imaging, additional consultation requests, biopsies, or specific wound care instructions. Seventy-five percent of all consultations were given specific medication recommendations by dermatology. Most (61%) were recommended to be given a topical steroid, antibiotic, or both. However, 45% of all consultations were recommended to initiate a systemic medication, most commonly antihistamines, antibiotics, steroids, antivirals, or immunomodulators. Dermatology recommended discontinuing specific medications in 16% of all consultations, with antibiotics being the most frequent culprit (17 antibiotics discontinued), owing to drug eruptions or misdiagnosed infections. Vancomycin, piperacillin-tazobactam, and trimethoprim-sulfamethoxazole were the most frequently discontinued antibiotics.
Dermatology was consulted for assistance in management of previously diagnosed cutaneous conditions 56 times (22% of all consultations), often regarding complicated cases of hidradenitis suppurativa (9 cases), pyoderma gangrenosum (5 cases), bullous pemphigoid (4 cases), or erythroderma (4 cases). Most of these cases required a single dermatology encounter to provide recommendations (71%), and 21% required 1 additional follow-up. Sixty-three percent of patients consulted for management assistance were noted to have improvement in their cutaneous condition by time of discharge, as documented by the primary provider in the medical record.
Twenty-eight percent of all consultations required at least 1 biopsy. Seventy-two percent of all biopsies were consistent with the dermatologist’s working diagnosis or highest-ranked differential diagnosis, and 16% of biopsy results were consistent with the second- or third-ranked diagnosis. The primary teams requested a biopsy 38 times to assist in diagnosis, as documented in the progress note or consultation request. Only 21 of these consultations (55% of requests) received at least 1 biopsy, as the remaining consultations did not require a biopsy to establish a diagnosis. The most common final diagnoses of consultations receiving biopsies included drug eruptions (5), leukemia cutis (4), vasculopathies (4), vasculitis (4), and calciphylaxis (3).
Impact on Hospitalization and Efficacy—Dermatology performed 217 consultations regarding patients already admitted to the hospital, and 92% remained hospitalized either due to comorbidities or complicated cutaneous conditions following the consultation. The remaining 8% were cleared for discharge. Dermatology received 36 consultation requests from emergency medicine physicians. Fifty-three percent of these patients were admitted, while the remaining 47% were discharged from the emergency department or its observation unit following evaluation.
Fifty-one percent of all consultations were noted to have improvement in their cutaneous condition by the time of discharge, as noted in the physical examination, progress note, or discharge summary of the primary team. Thirty percent of cases remained stable, where improvement was not noted in in the medical record. Most of these cases involved keratinocyte carcinomas scheduled for outpatient excision, benign melanocytic nevi found on FBSE, and benign etiologies that led to immediate discharge following consultation. Three percent of all consultations were noted to have worsened following consultation, including cases of calciphylaxis, vasculopathies, and purpura fulminans, as well as patients who elected for palliative care and hospice. The cutaneous condition by the time of discharge could not be determined from the medical record in 16% of all consultations.
Eighty-five percent of all consultations required a single encounter with dermatology. An additional 10% required a single follow-up with dermatology, while only 5% of patients required 3 or more encounters. Notably, these cases included patients with 1 or more severe cutaneous diseases, such as Sweet syndrome, calciphylaxis, Stevens-Johnson syndrome/toxic epidermal necrolysis, and hidradenitis suppurativa.
Comment
Although dermatology often is viewed as an outpatient specialty, this study provides a glimpse into the ways inpatient dermatology consultations optimize the care of hospitalized patients. Most consultations involved assistance in diagnosing an unknown condition, but several regarded pre-existing skin disorders requiring management aid. As a variety of medical specialties requested consultations, dermatology was able to provide care to a diverse group of patients with conditions varying in complexity and severity. Several specialties benefited from niche dermatologic expertise: hematology and oncology frequently requested dermatology to assist in diagnosis and management of the toxic effects of chemotherapy, cutaneous metastasis, or suspected cutaneous infections in immunocompromised patients. Cardiology patients were frequently evaluated for potential malignancy or infection prior to heart transplantation and initiation of antirejection immunosuppressants. Dermatology was consulted to differentiate cutaneous manifestations of critical illness from underlying systemic disease in the intensive care unit, and patients presenting to the emergency department often were examined to determine if hospital admission was necessary, with 47% of these consultations resulting in a discharge following evaluation by a dermatologist.
Our results were consistent with prior studies1,5,6 that have reported frequent changes in final diagnosis following dermatology consultation, with 69% of working diagnoses changed in this study when consultation was requested for diagnostic assistance. When dermatology was consulted for diagnostic assistance, several of these cases lacked a preliminary differential diagnosis. Although the absence of a documented differential diagnosis may not necessarily reflect a lack of suspicion for a particular etiology, 86% of all consultations included a ranked differential or working diagnosis either in the consultation request or progress note prior to consultation. The final diagnoses of consultations without a preliminary diagnosis varied from the mild and localized to systemic and severe, further suggesting these cases reflected knowledge gaps of the primary medical team.
Integration of dermatology into the care of hospitalized patients could provide an opportunity for education of primary medical teams. With frequent consultation, primary medical teams may become more comfortable diagnosing and managing common cutaneous conditions specific to their specialty or extended hospitalizations.
Several consultations were requested to aid in management of cases of hidradenitis suppurativa, pyoderma gangrenosum, or bullous pemphigoid that either failed outpatient therapy or were complicated by superinfections. Despite the ranges in complexity, the majority of all consultations required a single encounter and led to improvement by the time of discharge, demonstrating the efficacy and efficiency of inpatient dermatologists.
Dermatology consultations often led to changes in management involving medications and additional workup. Changes in management also extended to specific wound care instructions provided by dermatology, as expected for cases of Stevens-Johnson syndrome/toxic epidermal necrolysis, Sweet syndrome, hidradenitis suppurativa, and pyoderma gangrenosum. However, patients with the sequelae of extended hospitalizations, such as chronic wounds, pressure ulcers, and edema bullae, also benefited from this expertise.
When patients required a biopsy, the final diagnoses were consistent with the dermatologist’s number one differential diagnosis or top 3 differential diagnoses 72% and 88% of the time, respectively. Only 55% of cases where the primary team requested a biopsy ultimately required a biopsy, as many involved clinical diagnoses such as urticaria. Not only was dermatology accurate in their preliminary diagnoses, but they decreased cost and morbidity by avoiding unnecessary procedures.
This study provided additional evidence to support the integration of dermatology into the hospital setting for the benefit of patients, primary medical teams, and hospital systems. Dermatology offers high-value care through the efficient diagnosis and management of hospitalized patients, which contributes to decreased cost and improved outcomes.2,5-7,9,10 This study highlighted lesser-known areas of impact, such as the various specialty-specific services dermatology provides as well as the high rates of reported improvement following consultation. Future studies should continue to explore the field’s unique impact on hospitalized medicine as well as other avenues of care delivery, such as telemedicine, that may encourage dermatologists to participate in consultations and increase the volume of patients who may benefit from their care.
Dermatology is an often-underutilized resource in the hospital setting. As the health care landscape has evolved, so has the role of the inpatient dermatologist.1-3 Structural changes in the health system and advances in therapies have shifted dermatology from an admitting service to an almost exclusively outpatient practice. Improved treatment modalities led to decreases in the number of patients requiring admission for chronic dermatoses, and outpatient clinics began offering therapies once limited to hospitals.1,4 Inpatient dermatology consultations emerged and continue to have profound effects on hospitalized patients regardless of their reason for admission.1-11
Inpatient dermatologists supply knowledge in areas primary medical teams lack, and there is evidence that dermatology consultations improve the quality of care while decreasing cost.2,5-7 Establishing correct diagnoses, preventing exposure to unnecessary medications, and reducing hospitalization duration and readmission rates are a few ways dermatology consultations positively impact hospitalized patients.2,5-7,9,10 This study highlights the role of the dermatologist in the care of hospitalized patients at a large academic medical center in an urban setting and reveals how consultation supports the efficiency and efficacy of other services.
Materials and Methods
Study Design—This single-institution, cross-sectional retrospective study included all hospitalized patients at the Thomas Jefferson University Hospital (Philadelphia, Pennsylvania), who received an inpatient dermatology consultation completed by physicians of Jefferson Dermatology Associates between January 1, 2019, and December 31, 2019. The institutional review board at Thomas Jefferson University approved this study.
Data Collection—A list of all inpatient dermatology consultations in 2019 was provided by Jefferson Dermatology Associates. Through a retrospective chart review, data regarding the consultations were collected from the electronic medical record (Epic Systems) and recorded into the Research Electronic Data Capture system. Data on patient demographics, the primary medical team, the dermatology evaluation, and the hospital course of the patient were collected.
Results
Patient Characteristics—Dermatology received 253 inpatient consultation requests during this time period; 53% of patients were female and 47% were male, with a mean age of 55 years. Most patients were White (57%), while 34% were Black. Five percent and 4% of patients were Asian and Hispanic or Latino, respectively (Table 1). The mean duration of hospitalization for all patients was 15 days, and the average number of days to discharge following the first encounter with dermatology was 10 days.

Requesting Team and Reason for Consultation—Internal medicine consulted dermatology most frequently (34% of all consultations), followed by emergency medicine (14%) and a variety of other services (Table 1). Most dermatology consultations were placed to assist in achieving a diagnosis of a cutaneous condition (77%), while a minority were to assist in the management of a previously diagnosed disease (22%). A small fraction of consultations (5%) were to complete full-body skin examinations (FBSEs) to rule out infection or malignancy in candidates for organ transplantation, left ventricular assist devices, or certain chemotherapies. One FBSE was conducted to search for a primary tumor in a patient diagnosed with metastatic melanoma.
Most Common Final Diagnoses and Consultation Impact—Table 2 lists the most common final diagnosis categories, as well as the effects of the consultation on diagnosis, management, biopsies, hospitalization, and clinical improvement as documented by the primary medical provider. The most common final diagnoses were inflammatory and autoimmune (39%), such as contact dermatitis and seborrheic dermatitis; infectious (23%), such as varicella (primary or zoster) and bacterial furunculosis; drug reactions (20%), such as morbilliform drug eruptions; vascular (8%), such as vasculitis and calciphylaxis; neoplastic (7%), such as keratinocyte carcinomas and leukemia cutis; and other (15%), such as xerosis, keratosis pilaris, and miliaria rubra.

Impact on Diagnosis—Fifty-six percent of all consultations resulted in a change in diagnosis. When dermatology was consulted specifically to assist in the diagnosis of a patient (195 consultations), the working diagnosis of the primary team was changed 69% of the time. Thirty-five of these consultation requests had no preliminary diagnosis, and the primary team listed the working diagnosis as either rash or a morphologic description of the lesion(s). Sixty-three percent of suspected drug eruptions ended with a diagnosis of a form of drug eruption, while 20% of consultations for suspected cellulitis or bacterial infections were confirmed to be cellulitis or soft tissue infections.
Impact on Management—Regardless of the reason for the consultation, most consultations (86%) resulted in a change in management. The remaining 14% consisted of FBSEs with benign findings; cases of cutaneous metastases and leukemia cutis managed by oncology; as well as select cases of purpura fulminans, postfebrile desquamation, and postinflammatory hyperpigmentation.
Changes in management included alterations in medications, requests for additional laboratory work or imaging, additional consultation requests, biopsies, or specific wound care instructions. Seventy-five percent of all consultations were given specific medication recommendations by dermatology. Most (61%) were recommended to be given a topical steroid, antibiotic, or both. However, 45% of all consultations were recommended to initiate a systemic medication, most commonly antihistamines, antibiotics, steroids, antivirals, or immunomodulators. Dermatology recommended discontinuing specific medications in 16% of all consultations, with antibiotics being the most frequent culprit (17 antibiotics discontinued), owing to drug eruptions or misdiagnosed infections. Vancomycin, piperacillin-tazobactam, and trimethoprim-sulfamethoxazole were the most frequently discontinued antibiotics.
Dermatology was consulted for assistance in management of previously diagnosed cutaneous conditions 56 times (22% of all consultations), often regarding complicated cases of hidradenitis suppurativa (9 cases), pyoderma gangrenosum (5 cases), bullous pemphigoid (4 cases), or erythroderma (4 cases). Most of these cases required a single dermatology encounter to provide recommendations (71%), and 21% required 1 additional follow-up. Sixty-three percent of patients consulted for management assistance were noted to have improvement in their cutaneous condition by time of discharge, as documented by the primary provider in the medical record.
Twenty-eight percent of all consultations required at least 1 biopsy. Seventy-two percent of all biopsies were consistent with the dermatologist’s working diagnosis or highest-ranked differential diagnosis, and 16% of biopsy results were consistent with the second- or third-ranked diagnosis. The primary teams requested a biopsy 38 times to assist in diagnosis, as documented in the progress note or consultation request. Only 21 of these consultations (55% of requests) received at least 1 biopsy, as the remaining consultations did not require a biopsy to establish a diagnosis. The most common final diagnoses of consultations receiving biopsies included drug eruptions (5), leukemia cutis (4), vasculopathies (4), vasculitis (4), and calciphylaxis (3).
Impact on Hospitalization and Efficacy—Dermatology performed 217 consultations regarding patients already admitted to the hospital, and 92% remained hospitalized either due to comorbidities or complicated cutaneous conditions following the consultation. The remaining 8% were cleared for discharge. Dermatology received 36 consultation requests from emergency medicine physicians. Fifty-three percent of these patients were admitted, while the remaining 47% were discharged from the emergency department or its observation unit following evaluation.
Fifty-one percent of all consultations were noted to have improvement in their cutaneous condition by the time of discharge, as noted in the physical examination, progress note, or discharge summary of the primary team. Thirty percent of cases remained stable, where improvement was not noted in in the medical record. Most of these cases involved keratinocyte carcinomas scheduled for outpatient excision, benign melanocytic nevi found on FBSE, and benign etiologies that led to immediate discharge following consultation. Three percent of all consultations were noted to have worsened following consultation, including cases of calciphylaxis, vasculopathies, and purpura fulminans, as well as patients who elected for palliative care and hospice. The cutaneous condition by the time of discharge could not be determined from the medical record in 16% of all consultations.
Eighty-five percent of all consultations required a single encounter with dermatology. An additional 10% required a single follow-up with dermatology, while only 5% of patients required 3 or more encounters. Notably, these cases included patients with 1 or more severe cutaneous diseases, such as Sweet syndrome, calciphylaxis, Stevens-Johnson syndrome/toxic epidermal necrolysis, and hidradenitis suppurativa.
Comment
Although dermatology often is viewed as an outpatient specialty, this study provides a glimpse into the ways inpatient dermatology consultations optimize the care of hospitalized patients. Most consultations involved assistance in diagnosing an unknown condition, but several regarded pre-existing skin disorders requiring management aid. As a variety of medical specialties requested consultations, dermatology was able to provide care to a diverse group of patients with conditions varying in complexity and severity. Several specialties benefited from niche dermatologic expertise: hematology and oncology frequently requested dermatology to assist in diagnosis and management of the toxic effects of chemotherapy, cutaneous metastasis, or suspected cutaneous infections in immunocompromised patients. Cardiology patients were frequently evaluated for potential malignancy or infection prior to heart transplantation and initiation of antirejection immunosuppressants. Dermatology was consulted to differentiate cutaneous manifestations of critical illness from underlying systemic disease in the intensive care unit, and patients presenting to the emergency department often were examined to determine if hospital admission was necessary, with 47% of these consultations resulting in a discharge following evaluation by a dermatologist.
Our results were consistent with prior studies1,5,6 that have reported frequent changes in final diagnosis following dermatology consultation, with 69% of working diagnoses changed in this study when consultation was requested for diagnostic assistance. When dermatology was consulted for diagnostic assistance, several of these cases lacked a preliminary differential diagnosis. Although the absence of a documented differential diagnosis may not necessarily reflect a lack of suspicion for a particular etiology, 86% of all consultations included a ranked differential or working diagnosis either in the consultation request or progress note prior to consultation. The final diagnoses of consultations without a preliminary diagnosis varied from the mild and localized to systemic and severe, further suggesting these cases reflected knowledge gaps of the primary medical team.
Integration of dermatology into the care of hospitalized patients could provide an opportunity for education of primary medical teams. With frequent consultation, primary medical teams may become more comfortable diagnosing and managing common cutaneous conditions specific to their specialty or extended hospitalizations.
Several consultations were requested to aid in management of cases of hidradenitis suppurativa, pyoderma gangrenosum, or bullous pemphigoid that either failed outpatient therapy or were complicated by superinfections. Despite the ranges in complexity, the majority of all consultations required a single encounter and led to improvement by the time of discharge, demonstrating the efficacy and efficiency of inpatient dermatologists.
Dermatology consultations often led to changes in management involving medications and additional workup. Changes in management also extended to specific wound care instructions provided by dermatology, as expected for cases of Stevens-Johnson syndrome/toxic epidermal necrolysis, Sweet syndrome, hidradenitis suppurativa, and pyoderma gangrenosum. However, patients with the sequelae of extended hospitalizations, such as chronic wounds, pressure ulcers, and edema bullae, also benefited from this expertise.
When patients required a biopsy, the final diagnoses were consistent with the dermatologist’s number one differential diagnosis or top 3 differential diagnoses 72% and 88% of the time, respectively. Only 55% of cases where the primary team requested a biopsy ultimately required a biopsy, as many involved clinical diagnoses such as urticaria. Not only was dermatology accurate in their preliminary diagnoses, but they decreased cost and morbidity by avoiding unnecessary procedures.
This study provided additional evidence to support the integration of dermatology into the hospital setting for the benefit of patients, primary medical teams, and hospital systems. Dermatology offers high-value care through the efficient diagnosis and management of hospitalized patients, which contributes to decreased cost and improved outcomes.2,5-7,9,10 This study highlighted lesser-known areas of impact, such as the various specialty-specific services dermatology provides as well as the high rates of reported improvement following consultation. Future studies should continue to explore the field’s unique impact on hospitalized medicine as well as other avenues of care delivery, such as telemedicine, that may encourage dermatologists to participate in consultations and increase the volume of patients who may benefit from their care.
- Madigan LM, Fox LP. Where are we now with inpatient consultative dermatology?: assessing the value and evolution of this subspecialty over the past decade. J Am Acad Dermatol. 2019;80:1804-1808. doi:10.1016/j.jaad.2019.01.031
- Noe MH, Rosenbach M. Inpatient dermatologists—crucial for the management of skin diseases in hospitalized patients [editorial]. JAMA Dermatol. 2018;154:524-525. doi:10.1001/jamadermatol.2017.6195
- Strowd LC. Inpatient dermatology: a paradigm shift in the management of skin disease in the hospital. Br J Dermatol. 2019;180:966-967. doi:10.1111/bjd.17778
- Kirsner RS, Yang DG, Kerdel FA. The changing status of inpatient dermatology at American academic dermatology programs. J Am Acad Dermatol. 1999;40:755-757. doi:10.1016/s0190-9622(99)70158-1
- Kroshinsky D, Cotliar J, Hughey LC, et al. Association of dermatology consultation with accuracy of cutaneous disorder diagnoses in hospitalized patients: a multicenter analysis. JAMA Dermatol. 2016;152:477-480. doi:10.1001/jamadermatol.2015.5098
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis. JAMA Dermatol. 2018;154:529-533. doi:10.1001/jamadermatol.2017.6196
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528. doi:10.1001/jamadermatol.2016.6130
- Imadojemu S, Rosenbach M. Dermatologists must take an active role in the diagnosis of cellulitis. JAMA Dermatol. 2017;153:134-135. doi:10.1001/jamadermatol.2016.4230
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062. doi:10.1001/jamadermatol.2014.1164
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558. doi:10.1111/ijd.13939
- Madigan LM, Fox LP. Where are we now with inpatient consultative dermatology?: assessing the value and evolution of this subspecialty over the past decade. J Am Acad Dermatol. 2019;80:1804-1808. doi:10.1016/j.jaad.2019.01.031
- Noe MH, Rosenbach M. Inpatient dermatologists—crucial for the management of skin diseases in hospitalized patients [editorial]. JAMA Dermatol. 2018;154:524-525. doi:10.1001/jamadermatol.2017.6195
- Strowd LC. Inpatient dermatology: a paradigm shift in the management of skin disease in the hospital. Br J Dermatol. 2019;180:966-967. doi:10.1111/bjd.17778
- Kirsner RS, Yang DG, Kerdel FA. The changing status of inpatient dermatology at American academic dermatology programs. J Am Acad Dermatol. 1999;40:755-757. doi:10.1016/s0190-9622(99)70158-1
- Kroshinsky D, Cotliar J, Hughey LC, et al. Association of dermatology consultation with accuracy of cutaneous disorder diagnoses in hospitalized patients: a multicenter analysis. JAMA Dermatol. 2016;152:477-480. doi:10.1001/jamadermatol.2015.5098
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis. JAMA Dermatol. 2018;154:529-533. doi:10.1001/jamadermatol.2017.6196
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528. doi:10.1001/jamadermatol.2016.6130
- Imadojemu S, Rosenbach M. Dermatologists must take an active role in the diagnosis of cellulitis. JAMA Dermatol. 2017;153:134-135. doi:10.1001/jamadermatol.2016.4230
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062. doi:10.1001/jamadermatol.2014.1164
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558. doi:10.1111/ijd.13939
Practice Points
- Inpatient dermatologists fill knowledge gaps that often alter the diagnosis, management, and hospital course of hospitalized patients.
- Several medical specialties benefit from niche expertise of inpatient dermatologists specific to their patient population.
- Integration of inpatient dermatology consultations can prevent unnecessary hospital admissions and medication administration.
Treatment Stacking: Optimizing Therapeutic Regimens for Hidradenitis Suppurativa
Hidradenitis suppurativa (HS) is a debilitating chronic condition that often is recalcitrant to first-line treatments, and mechanisms underlying its pathology remain unclear. Existing data suggest a multifactorial etiology with different pathophysiologic contributors, including genetic, hormonal, and immune dysregulation factors. At this time, only one medication (adalimumab) is US Food and Drug Administration approved for HS, but multiple medical and procedural therapies are available.1 Herein, we discuss the concept of treatment stacking, or the combination of unique therapeutic modalities—an approach we believe is key to optimizing management of HS patients.
Stacking Treatments for HS
Unlike psoriasis, in which a single biologic agent may provide 100% clearance (psoriasis area and severity index 100 [PASI 100]) without adjuvant treatment,2,3 the field of HS currently lacks medications that are efficacious to that degree of success as monotherapy. In HS, the benchmark for a positive treatment outcome is Hidradenitis Suppurativa Clinical Response 50 (HiSCR50),4 a 50% reduction in inflammatory lesion count—a far less stringent marker for disease improvement. Thus, providers should design HS treatment regimens with a model of combining therapies and shift away from monotherapy. Targeting different pathophysiologic pathways by stacking multiple treatments may provide synergistic benefits for HS patients. Treatment stacking is a familiar concept in acne; for instance, patients who benefit tremendously from isotretinoin may still require a hormone-modulating treatment (eg, spironolactone) to attain optimal results.
Adherence to a rigid treatment algorithm based on disease severity limits the potential to create comprehensive regimens that account for unique patient characteristics and clinical manifestations. When evaluating an HS patient, providers should systematically consider each pathophysiologic factor and target the ones that appear to be most involved in that particular patient. The North American HS guidelines illustrate this point by supporting use of several treatments across different Hurley stages, such as recommending hormonal treatment in patients with Hurley stages 1, 2, or 3.1 Of note, treatment stacking also includes procedural therapies. Surgeons typically prefer a patient’s disease management to be optimized prior to surgery, including reduced drainage and inflammation. In addition, even after surgery, patients often still require medical management to prevent continued disease worsening.
Treatment Pathways for HS
A multimodal approach with treatment stacking (Figure) can be useful to all HS patients, from those with the mildest to the most severe disease. Modifiable pathophysiologic factors and examples of their targeted treatments include (1) follicular occlusion (eg, oral retinoids), (2) metabolic dysfunction (eg, metformin), (3) hormones (eg, oral contraceptive pills, spironolactone, finasteride), (4) dysbiosis (eg, antibiotics such as clindamycin and rifampin combination therapy), (5) immune dysregulation (eg, biologic agents), and (6) friction/irritation (eg, weight loss, clothing recommendations).
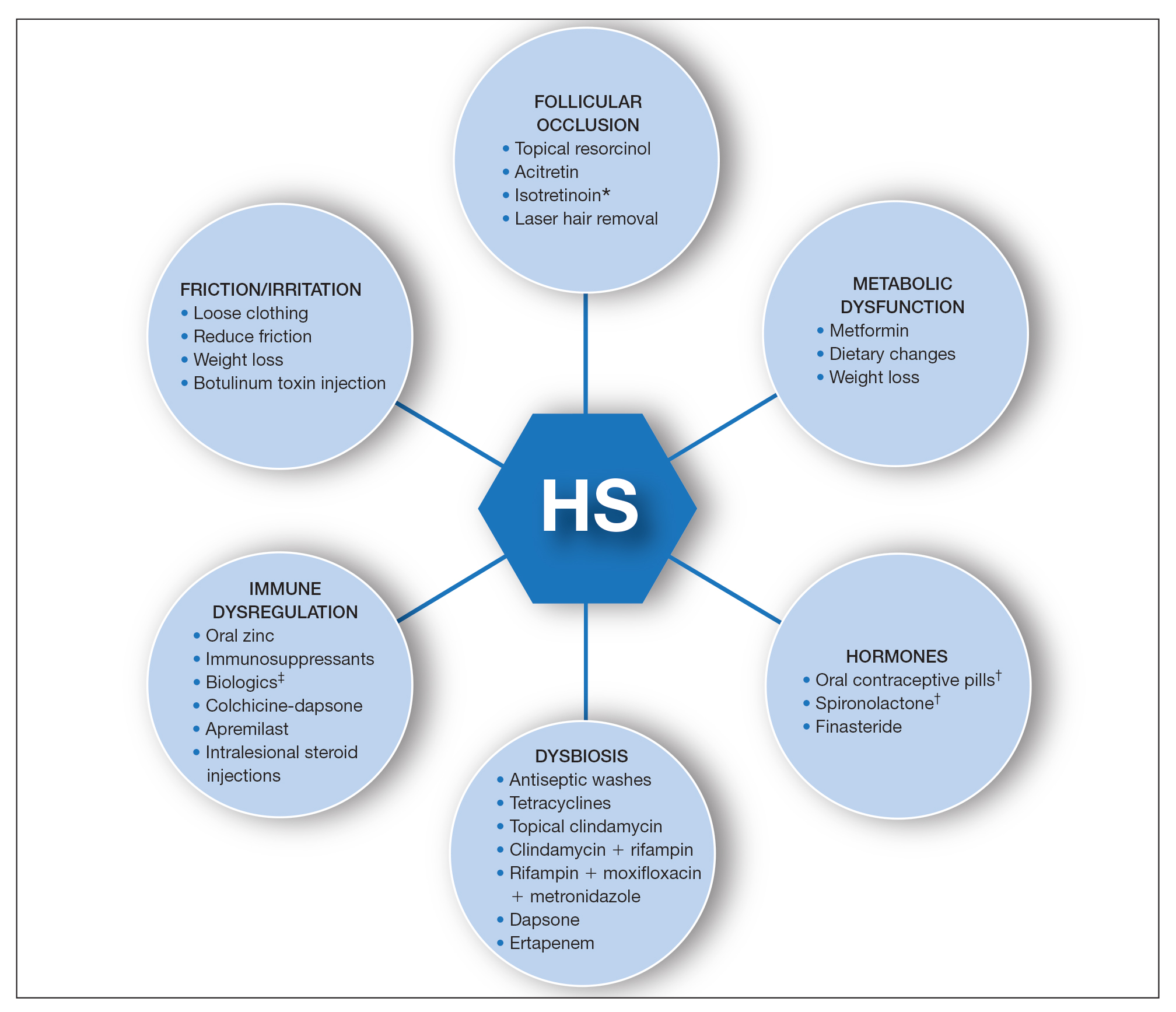
Combining treatments from different pathways enables potentiation of individual treatment efficacies. A female patient with only a few HS nodules that flare with menses may be well controlled with spironolactone as her only systemic agent; however, she still may benefit from use of an antiseptic wash, topical clindamycin, and lifestyle changes such as weight loss and reduction of mechanical irritation. A patient with severe recalcitrant HS could notably benefit from concomitant biologic, systemic antibiotic, and hormonal/metabolic treatments. If disease control is still inadequate, agents within the same class can be switched (eg, choosing a different biologic) or other disease-modifying agents such as colchicine also can be added. The goal is to create an effective treatment toolbox with therapies targeting different pathophysiologic arms of HS and working together in synergy. Each tool can be refined by modifying dosing frequency and duration of use to strive for optimal response. At this time, the literature on HS combination therapy is sparse. A retrospective study of 31 patients reported promising combinations, including isotretinoin with spironolactone for mild disease, isotretinoin or doxycycline with adalimumab for moderate disease, and cyclosporine with adalimumab for severe disease.5 Larger prospective studies on clinical response to different combination regimens are warranted.
Optimizing Therapy for HS and Its Comorbidities
Additional considerations may further optimize treatment plans. Some therapies benefit all patients; for example, providers should counsel all HS patients on healthy weight management, optimized clothing choices,6 and friction reduction in the intertriginous folds. Providers also may consider adding therapies with faster onset of efficacy as a bridge to long-term, slower-onset therapies. For instance, female HS patients with menstrual flares who are prescribed spironolactone also may benefit from a course of systemic antibiotics, which typically provides more prompt relief. Treatment regimens also can concomitantly treat HS and its comorbidities.7 For example, metformin serves a dual purpose in HS patients with diabetes mellitus, and adalimumab in patients with both HS and inflammatory bowel disease.
Final Thoughts
The last decade has seen tremendous growth in HS research8 coupled with a remarkable expansion in the therapeutic pipeline.9 However, currently no single therapy for HS can guarantee satisfactory disease remission or durability of remission. The contrast between clinical trials and real-world practice should be acknowledged; the former often is restrictive in design with monotherapy and allowance of very limited concomitant treatments, such as topical or oral antibiotics. This limits our ability to draw conclusions regarding the additive synergistic potential of different therapeutics in combination. In clinical practice, we are not restricted by monotherapy trial protocols. As we await new tools, treatment stacking allows for creating a framework to best utilize the tools that are available to us.
Although HS has continued to affect the lives of many patients, improved understanding of underlying pathophysiology and a well-placed sense of urgency from all stakeholders (ie, patients, clinicians, researchers, industry partners) has pushed this field forward. Until our therapeutic armamentarium has expanded to include highly efficacious monotherapy options, providers should consider treatment stacking for every HS patient.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101. doi:10.1016/j.jaad.2019.02.068
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152. doi:10.1056/NEJMoa2102383
- Imafuku S, Nakagawa H, Igarashi A, et al. Long-term efficacy and safety of tildrakizumab in Japanese patients with moderate to severe plaque psoriasis: results from a 5-year extension of a phase 3 study (reSURFACE 1). J Dermatol. 2021;48:844-852. doi:10.1111/1346-8138.15763
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- McPhie ML, Bridgman AC, Kirchhof MG. Combination therapies for hidradenitis suppurativa: a retrospective chart review of 31 patients. J Cutan Med Surg. 2019;23:270-276. doi:10.1177/1203475418823529
- Loh TY, Hendricks AJ, Hsiao JL, et al. Undergarment and fabric selection in the management of hidradenitis suppurativa. Dermatol Basel Switz. 2021;237:119-124. doi:10.1159/000501611
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.01.059
- Savage KT, Brant EG, Flood KS, et al. Publication trends in hidradenitis suppurativa from 2008 to 2018. J Eur Acad Dermatol Venereol. 2020;34:1885-1889. doi:10.1111/jdv.16213
- van Straalen KR, Schneider-Burrus S, Prens EP. Current and future treatment of hidradenitis suppurativa. Br J Dermatol. 2020;183:E178-E187. doi:10.1111/bjd.16768
Hidradenitis suppurativa (HS) is a debilitating chronic condition that often is recalcitrant to first-line treatments, and mechanisms underlying its pathology remain unclear. Existing data suggest a multifactorial etiology with different pathophysiologic contributors, including genetic, hormonal, and immune dysregulation factors. At this time, only one medication (adalimumab) is US Food and Drug Administration approved for HS, but multiple medical and procedural therapies are available.1 Herein, we discuss the concept of treatment stacking, or the combination of unique therapeutic modalities—an approach we believe is key to optimizing management of HS patients.
Stacking Treatments for HS
Unlike psoriasis, in which a single biologic agent may provide 100% clearance (psoriasis area and severity index 100 [PASI 100]) without adjuvant treatment,2,3 the field of HS currently lacks medications that are efficacious to that degree of success as monotherapy. In HS, the benchmark for a positive treatment outcome is Hidradenitis Suppurativa Clinical Response 50 (HiSCR50),4 a 50% reduction in inflammatory lesion count—a far less stringent marker for disease improvement. Thus, providers should design HS treatment regimens with a model of combining therapies and shift away from monotherapy. Targeting different pathophysiologic pathways by stacking multiple treatments may provide synergistic benefits for HS patients. Treatment stacking is a familiar concept in acne; for instance, patients who benefit tremendously from isotretinoin may still require a hormone-modulating treatment (eg, spironolactone) to attain optimal results.
Adherence to a rigid treatment algorithm based on disease severity limits the potential to create comprehensive regimens that account for unique patient characteristics and clinical manifestations. When evaluating an HS patient, providers should systematically consider each pathophysiologic factor and target the ones that appear to be most involved in that particular patient. The North American HS guidelines illustrate this point by supporting use of several treatments across different Hurley stages, such as recommending hormonal treatment in patients with Hurley stages 1, 2, or 3.1 Of note, treatment stacking also includes procedural therapies. Surgeons typically prefer a patient’s disease management to be optimized prior to surgery, including reduced drainage and inflammation. In addition, even after surgery, patients often still require medical management to prevent continued disease worsening.
Treatment Pathways for HS
A multimodal approach with treatment stacking (Figure) can be useful to all HS patients, from those with the mildest to the most severe disease. Modifiable pathophysiologic factors and examples of their targeted treatments include (1) follicular occlusion (eg, oral retinoids), (2) metabolic dysfunction (eg, metformin), (3) hormones (eg, oral contraceptive pills, spironolactone, finasteride), (4) dysbiosis (eg, antibiotics such as clindamycin and rifampin combination therapy), (5) immune dysregulation (eg, biologic agents), and (6) friction/irritation (eg, weight loss, clothing recommendations).

Combining treatments from different pathways enables potentiation of individual treatment efficacies. A female patient with only a few HS nodules that flare with menses may be well controlled with spironolactone as her only systemic agent; however, she still may benefit from use of an antiseptic wash, topical clindamycin, and lifestyle changes such as weight loss and reduction of mechanical irritation. A patient with severe recalcitrant HS could notably benefit from concomitant biologic, systemic antibiotic, and hormonal/metabolic treatments. If disease control is still inadequate, agents within the same class can be switched (eg, choosing a different biologic) or other disease-modifying agents such as colchicine also can be added. The goal is to create an effective treatment toolbox with therapies targeting different pathophysiologic arms of HS and working together in synergy. Each tool can be refined by modifying dosing frequency and duration of use to strive for optimal response. At this time, the literature on HS combination therapy is sparse. A retrospective study of 31 patients reported promising combinations, including isotretinoin with spironolactone for mild disease, isotretinoin or doxycycline with adalimumab for moderate disease, and cyclosporine with adalimumab for severe disease.5 Larger prospective studies on clinical response to different combination regimens are warranted.
Optimizing Therapy for HS and Its Comorbidities
Additional considerations may further optimize treatment plans. Some therapies benefit all patients; for example, providers should counsel all HS patients on healthy weight management, optimized clothing choices,6 and friction reduction in the intertriginous folds. Providers also may consider adding therapies with faster onset of efficacy as a bridge to long-term, slower-onset therapies. For instance, female HS patients with menstrual flares who are prescribed spironolactone also may benefit from a course of systemic antibiotics, which typically provides more prompt relief. Treatment regimens also can concomitantly treat HS and its comorbidities.7 For example, metformin serves a dual purpose in HS patients with diabetes mellitus, and adalimumab in patients with both HS and inflammatory bowel disease.
Final Thoughts
The last decade has seen tremendous growth in HS research8 coupled with a remarkable expansion in the therapeutic pipeline.9 However, currently no single therapy for HS can guarantee satisfactory disease remission or durability of remission. The contrast between clinical trials and real-world practice should be acknowledged; the former often is restrictive in design with monotherapy and allowance of very limited concomitant treatments, such as topical or oral antibiotics. This limits our ability to draw conclusions regarding the additive synergistic potential of different therapeutics in combination. In clinical practice, we are not restricted by monotherapy trial protocols. As we await new tools, treatment stacking allows for creating a framework to best utilize the tools that are available to us.
Although HS has continued to affect the lives of many patients, improved understanding of underlying pathophysiology and a well-placed sense of urgency from all stakeholders (ie, patients, clinicians, researchers, industry partners) has pushed this field forward. Until our therapeutic armamentarium has expanded to include highly efficacious monotherapy options, providers should consider treatment stacking for every HS patient.
Hidradenitis suppurativa (HS) is a debilitating chronic condition that often is recalcitrant to first-line treatments, and mechanisms underlying its pathology remain unclear. Existing data suggest a multifactorial etiology with different pathophysiologic contributors, including genetic, hormonal, and immune dysregulation factors. At this time, only one medication (adalimumab) is US Food and Drug Administration approved for HS, but multiple medical and procedural therapies are available.1 Herein, we discuss the concept of treatment stacking, or the combination of unique therapeutic modalities—an approach we believe is key to optimizing management of HS patients.
Stacking Treatments for HS
Unlike psoriasis, in which a single biologic agent may provide 100% clearance (psoriasis area and severity index 100 [PASI 100]) without adjuvant treatment,2,3 the field of HS currently lacks medications that are efficacious to that degree of success as monotherapy. In HS, the benchmark for a positive treatment outcome is Hidradenitis Suppurativa Clinical Response 50 (HiSCR50),4 a 50% reduction in inflammatory lesion count—a far less stringent marker for disease improvement. Thus, providers should design HS treatment regimens with a model of combining therapies and shift away from monotherapy. Targeting different pathophysiologic pathways by stacking multiple treatments may provide synergistic benefits for HS patients. Treatment stacking is a familiar concept in acne; for instance, patients who benefit tremendously from isotretinoin may still require a hormone-modulating treatment (eg, spironolactone) to attain optimal results.
Adherence to a rigid treatment algorithm based on disease severity limits the potential to create comprehensive regimens that account for unique patient characteristics and clinical manifestations. When evaluating an HS patient, providers should systematically consider each pathophysiologic factor and target the ones that appear to be most involved in that particular patient. The North American HS guidelines illustrate this point by supporting use of several treatments across different Hurley stages, such as recommending hormonal treatment in patients with Hurley stages 1, 2, or 3.1 Of note, treatment stacking also includes procedural therapies. Surgeons typically prefer a patient’s disease management to be optimized prior to surgery, including reduced drainage and inflammation. In addition, even after surgery, patients often still require medical management to prevent continued disease worsening.
Treatment Pathways for HS
A multimodal approach with treatment stacking (Figure) can be useful to all HS patients, from those with the mildest to the most severe disease. Modifiable pathophysiologic factors and examples of their targeted treatments include (1) follicular occlusion (eg, oral retinoids), (2) metabolic dysfunction (eg, metformin), (3) hormones (eg, oral contraceptive pills, spironolactone, finasteride), (4) dysbiosis (eg, antibiotics such as clindamycin and rifampin combination therapy), (5) immune dysregulation (eg, biologic agents), and (6) friction/irritation (eg, weight loss, clothing recommendations).

Combining treatments from different pathways enables potentiation of individual treatment efficacies. A female patient with only a few HS nodules that flare with menses may be well controlled with spironolactone as her only systemic agent; however, she still may benefit from use of an antiseptic wash, topical clindamycin, and lifestyle changes such as weight loss and reduction of mechanical irritation. A patient with severe recalcitrant HS could notably benefit from concomitant biologic, systemic antibiotic, and hormonal/metabolic treatments. If disease control is still inadequate, agents within the same class can be switched (eg, choosing a different biologic) or other disease-modifying agents such as colchicine also can be added. The goal is to create an effective treatment toolbox with therapies targeting different pathophysiologic arms of HS and working together in synergy. Each tool can be refined by modifying dosing frequency and duration of use to strive for optimal response. At this time, the literature on HS combination therapy is sparse. A retrospective study of 31 patients reported promising combinations, including isotretinoin with spironolactone for mild disease, isotretinoin or doxycycline with adalimumab for moderate disease, and cyclosporine with adalimumab for severe disease.5 Larger prospective studies on clinical response to different combination regimens are warranted.
Optimizing Therapy for HS and Its Comorbidities
Additional considerations may further optimize treatment plans. Some therapies benefit all patients; for example, providers should counsel all HS patients on healthy weight management, optimized clothing choices,6 and friction reduction in the intertriginous folds. Providers also may consider adding therapies with faster onset of efficacy as a bridge to long-term, slower-onset therapies. For instance, female HS patients with menstrual flares who are prescribed spironolactone also may benefit from a course of systemic antibiotics, which typically provides more prompt relief. Treatment regimens also can concomitantly treat HS and its comorbidities.7 For example, metformin serves a dual purpose in HS patients with diabetes mellitus, and adalimumab in patients with both HS and inflammatory bowel disease.
Final Thoughts
The last decade has seen tremendous growth in HS research8 coupled with a remarkable expansion in the therapeutic pipeline.9 However, currently no single therapy for HS can guarantee satisfactory disease remission or durability of remission. The contrast between clinical trials and real-world practice should be acknowledged; the former often is restrictive in design with monotherapy and allowance of very limited concomitant treatments, such as topical or oral antibiotics. This limits our ability to draw conclusions regarding the additive synergistic potential of different therapeutics in combination. In clinical practice, we are not restricted by monotherapy trial protocols. As we await new tools, treatment stacking allows for creating a framework to best utilize the tools that are available to us.
Although HS has continued to affect the lives of many patients, improved understanding of underlying pathophysiology and a well-placed sense of urgency from all stakeholders (ie, patients, clinicians, researchers, industry partners) has pushed this field forward. Until our therapeutic armamentarium has expanded to include highly efficacious monotherapy options, providers should consider treatment stacking for every HS patient.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101. doi:10.1016/j.jaad.2019.02.068
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152. doi:10.1056/NEJMoa2102383
- Imafuku S, Nakagawa H, Igarashi A, et al. Long-term efficacy and safety of tildrakizumab in Japanese patients with moderate to severe plaque psoriasis: results from a 5-year extension of a phase 3 study (reSURFACE 1). J Dermatol. 2021;48:844-852. doi:10.1111/1346-8138.15763
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- McPhie ML, Bridgman AC, Kirchhof MG. Combination therapies for hidradenitis suppurativa: a retrospective chart review of 31 patients. J Cutan Med Surg. 2019;23:270-276. doi:10.1177/1203475418823529
- Loh TY, Hendricks AJ, Hsiao JL, et al. Undergarment and fabric selection in the management of hidradenitis suppurativa. Dermatol Basel Switz. 2021;237:119-124. doi:10.1159/000501611
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.01.059
- Savage KT, Brant EG, Flood KS, et al. Publication trends in hidradenitis suppurativa from 2008 to 2018. J Eur Acad Dermatol Venereol. 2020;34:1885-1889. doi:10.1111/jdv.16213
- van Straalen KR, Schneider-Burrus S, Prens EP. Current and future treatment of hidradenitis suppurativa. Br J Dermatol. 2020;183:E178-E187. doi:10.1111/bjd.16768
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101. doi:10.1016/j.jaad.2019.02.068
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152. doi:10.1056/NEJMoa2102383
- Imafuku S, Nakagawa H, Igarashi A, et al. Long-term efficacy and safety of tildrakizumab in Japanese patients with moderate to severe plaque psoriasis: results from a 5-year extension of a phase 3 study (reSURFACE 1). J Dermatol. 2021;48:844-852. doi:10.1111/1346-8138.15763
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- McPhie ML, Bridgman AC, Kirchhof MG. Combination therapies for hidradenitis suppurativa: a retrospective chart review of 31 patients. J Cutan Med Surg. 2019;23:270-276. doi:10.1177/1203475418823529
- Loh TY, Hendricks AJ, Hsiao JL, et al. Undergarment and fabric selection in the management of hidradenitis suppurativa. Dermatol Basel Switz. 2021;237:119-124. doi:10.1159/000501611
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.01.059
- Savage KT, Brant EG, Flood KS, et al. Publication trends in hidradenitis suppurativa from 2008 to 2018. J Eur Acad Dermatol Venereol. 2020;34:1885-1889. doi:10.1111/jdv.16213
- van Straalen KR, Schneider-Burrus S, Prens EP. Current and future treatment of hidradenitis suppurativa. Br J Dermatol. 2020;183:E178-E187. doi:10.1111/bjd.16768
Psoriasis and Psoriatic Arthritis: A Supplement to Dermatology News
Psoriasis and Psoriatic Arthritis: A Supplement to Dermatology News 2021
- Psoriasis severity redefined by expert group
- Mitigating PSA risk with biologic therapy
- Lack of diversity seen in psoriasis trials
- PSA Comorbidities effect on treatment responses
With Commentaries by Joel M. Gelfand, MD, MSCE and Alan Menter, MD
And more…
Psoriasis and Psoriatic Arthritis: A Supplement to Dermatology News 2021
- Psoriasis severity redefined by expert group
- Mitigating PSA risk with biologic therapy
- Lack of diversity seen in psoriasis trials
- PSA Comorbidities effect on treatment responses
With Commentaries by Joel M. Gelfand, MD, MSCE and Alan Menter, MD
And more…
Psoriasis and Psoriatic Arthritis: A Supplement to Dermatology News 2021
- Psoriasis severity redefined by expert group
- Mitigating PSA risk with biologic therapy
- Lack of diversity seen in psoriasis trials
- PSA Comorbidities effect on treatment responses
With Commentaries by Joel M. Gelfand, MD, MSCE and Alan Menter, MD
And more…