User login
Satisfaction high among psoriasis patients on apremilast
MADRID – within the next 12 months than were patients on their first tumor necrosis factor (TNF) inhibitor, in a large retrospective national propensity score-matched study.
“This was surprising to us,” David L. Kaplan, MD, admitted in presenting the study findings at the annual congress of the European Academy of Dermatology and Venereology.
The surprise came because apremilast, a phosphodiesterase 4 (PDE4)-inhibitor, is less potent than the injectable biologics at driving down Psoriasis Area and Severity Index (PASI) scores.
“This is real-world data. And this is what patients are saying at 1 year: that they’re actually happier [with apremilast] and they’re not interested in changing,” said Dr. Kaplan, a dermatologist at the University of Kansas and in private practice in Overland Park, Kan.
He and his coinvestigators tapped the IBM Watson MarketScan health insurance claims database for 2015-2016 and identified 1,645 biologic-naive adults with psoriasis who started on apremilast therapy and an equal number of biologic-naive psoriasis patients who initiated treatment with a biologic, of whom 1,207 started on an TNF inhibitor and 438 began on an interleukin inhibitor, which was ustekinumab in 81% of cases. The TNF inhibitor cohort was split 80/20 between adalimumab and etanercept. The three groups – new users of apremilast, a TNF inhibitor, or an interleukin inhibitor – were propensity-matched based upon age, prior usage of systemic psoriasis therapies, Charlson Comorbidity Index scores, and other potential confounders.
The primary endpoint was the switch rate to a different psoriasis treatment within 12 months. The switch rate was significantly lower in patients who had started on apremilast than in those on a TNF inhibitor by a margin of 14% to 25%, while the 11% switch rate among patients on an interleukin inhibitor was not significantly different from the rate in the apremilast group.
“I think this data kind of gives us pause,” the dermatologist said. “As a clinician myself, when patients come back in the first question I always ask is, ‘How’re you doing? Are you happy?’ And at the end of the day, the data in terms of switch rates shows where patients are at. And that doesn’t really follow what we see with PASI scores.”
A secondary endpoint was the switch rate through 24 months. The same pattern held true: 24.9% in the apremilast starters, which was similar to the 22.9% in patients initiated on an interleukin inhibitor, and significantly less than the 39.1% rate in the TNF inhibitor group.
Among patients who switched medications within the first 12 months, the mean number of days to the switch was similar across all three groups.
The study had several limitations. Propensity score–matching is not a cure-all that can eradicate all potential biases. And the claims database didn’t include information on why patients switched, nor what their PASI scores were. “This is real-world data, and clinicians don’t do PASI scores in the real world,” he noted.
Audience member Andrew Blauvelt, MD, a dermatologist and president of the Oregon Medical Research Center, Portland, rose to challenge Dr. Kaplan’s conclusion that patients on apremilast were happier with their care.
“How can you rule out that it’s just practices that don’t use biologics, and they’re keeping patients on apremilast regardless of whether they’re better or happy because they’re not using biologics?” inquired Dr. Blauvelt.
Dr. Kaplan conceded that might well be a partial explanation for the results.
“Reluctance to use biologics is out there,” he agreed.
Dr. Kaplan reported serving as a consultant and paid speaker for Celgene, the study sponsor, as well as several other pharmaceutical companies.
SOURCE: Kaplan DL. EADV Abstract FC04.04.
MADRID – within the next 12 months than were patients on their first tumor necrosis factor (TNF) inhibitor, in a large retrospective national propensity score-matched study.
“This was surprising to us,” David L. Kaplan, MD, admitted in presenting the study findings at the annual congress of the European Academy of Dermatology and Venereology.
The surprise came because apremilast, a phosphodiesterase 4 (PDE4)-inhibitor, is less potent than the injectable biologics at driving down Psoriasis Area and Severity Index (PASI) scores.
“This is real-world data. And this is what patients are saying at 1 year: that they’re actually happier [with apremilast] and they’re not interested in changing,” said Dr. Kaplan, a dermatologist at the University of Kansas and in private practice in Overland Park, Kan.
He and his coinvestigators tapped the IBM Watson MarketScan health insurance claims database for 2015-2016 and identified 1,645 biologic-naive adults with psoriasis who started on apremilast therapy and an equal number of biologic-naive psoriasis patients who initiated treatment with a biologic, of whom 1,207 started on an TNF inhibitor and 438 began on an interleukin inhibitor, which was ustekinumab in 81% of cases. The TNF inhibitor cohort was split 80/20 between adalimumab and etanercept. The three groups – new users of apremilast, a TNF inhibitor, or an interleukin inhibitor – were propensity-matched based upon age, prior usage of systemic psoriasis therapies, Charlson Comorbidity Index scores, and other potential confounders.
The primary endpoint was the switch rate to a different psoriasis treatment within 12 months. The switch rate was significantly lower in patients who had started on apremilast than in those on a TNF inhibitor by a margin of 14% to 25%, while the 11% switch rate among patients on an interleukin inhibitor was not significantly different from the rate in the apremilast group.
“I think this data kind of gives us pause,” the dermatologist said. “As a clinician myself, when patients come back in the first question I always ask is, ‘How’re you doing? Are you happy?’ And at the end of the day, the data in terms of switch rates shows where patients are at. And that doesn’t really follow what we see with PASI scores.”
A secondary endpoint was the switch rate through 24 months. The same pattern held true: 24.9% in the apremilast starters, which was similar to the 22.9% in patients initiated on an interleukin inhibitor, and significantly less than the 39.1% rate in the TNF inhibitor group.
Among patients who switched medications within the first 12 months, the mean number of days to the switch was similar across all three groups.
The study had several limitations. Propensity score–matching is not a cure-all that can eradicate all potential biases. And the claims database didn’t include information on why patients switched, nor what their PASI scores were. “This is real-world data, and clinicians don’t do PASI scores in the real world,” he noted.
Audience member Andrew Blauvelt, MD, a dermatologist and president of the Oregon Medical Research Center, Portland, rose to challenge Dr. Kaplan’s conclusion that patients on apremilast were happier with their care.
“How can you rule out that it’s just practices that don’t use biologics, and they’re keeping patients on apremilast regardless of whether they’re better or happy because they’re not using biologics?” inquired Dr. Blauvelt.
Dr. Kaplan conceded that might well be a partial explanation for the results.
“Reluctance to use biologics is out there,” he agreed.
Dr. Kaplan reported serving as a consultant and paid speaker for Celgene, the study sponsor, as well as several other pharmaceutical companies.
SOURCE: Kaplan DL. EADV Abstract FC04.04.
MADRID – within the next 12 months than were patients on their first tumor necrosis factor (TNF) inhibitor, in a large retrospective national propensity score-matched study.
“This was surprising to us,” David L. Kaplan, MD, admitted in presenting the study findings at the annual congress of the European Academy of Dermatology and Venereology.
The surprise came because apremilast, a phosphodiesterase 4 (PDE4)-inhibitor, is less potent than the injectable biologics at driving down Psoriasis Area and Severity Index (PASI) scores.
“This is real-world data. And this is what patients are saying at 1 year: that they’re actually happier [with apremilast] and they’re not interested in changing,” said Dr. Kaplan, a dermatologist at the University of Kansas and in private practice in Overland Park, Kan.
He and his coinvestigators tapped the IBM Watson MarketScan health insurance claims database for 2015-2016 and identified 1,645 biologic-naive adults with psoriasis who started on apremilast therapy and an equal number of biologic-naive psoriasis patients who initiated treatment with a biologic, of whom 1,207 started on an TNF inhibitor and 438 began on an interleukin inhibitor, which was ustekinumab in 81% of cases. The TNF inhibitor cohort was split 80/20 between adalimumab and etanercept. The three groups – new users of apremilast, a TNF inhibitor, or an interleukin inhibitor – were propensity-matched based upon age, prior usage of systemic psoriasis therapies, Charlson Comorbidity Index scores, and other potential confounders.
The primary endpoint was the switch rate to a different psoriasis treatment within 12 months. The switch rate was significantly lower in patients who had started on apremilast than in those on a TNF inhibitor by a margin of 14% to 25%, while the 11% switch rate among patients on an interleukin inhibitor was not significantly different from the rate in the apremilast group.
“I think this data kind of gives us pause,” the dermatologist said. “As a clinician myself, when patients come back in the first question I always ask is, ‘How’re you doing? Are you happy?’ And at the end of the day, the data in terms of switch rates shows where patients are at. And that doesn’t really follow what we see with PASI scores.”
A secondary endpoint was the switch rate through 24 months. The same pattern held true: 24.9% in the apremilast starters, which was similar to the 22.9% in patients initiated on an interleukin inhibitor, and significantly less than the 39.1% rate in the TNF inhibitor group.
Among patients who switched medications within the first 12 months, the mean number of days to the switch was similar across all three groups.
The study had several limitations. Propensity score–matching is not a cure-all that can eradicate all potential biases. And the claims database didn’t include information on why patients switched, nor what their PASI scores were. “This is real-world data, and clinicians don’t do PASI scores in the real world,” he noted.
Audience member Andrew Blauvelt, MD, a dermatologist and president of the Oregon Medical Research Center, Portland, rose to challenge Dr. Kaplan’s conclusion that patients on apremilast were happier with their care.
“How can you rule out that it’s just practices that don’t use biologics, and they’re keeping patients on apremilast regardless of whether they’re better or happy because they’re not using biologics?” inquired Dr. Blauvelt.
Dr. Kaplan conceded that might well be a partial explanation for the results.
“Reluctance to use biologics is out there,” he agreed.
Dr. Kaplan reported serving as a consultant and paid speaker for Celgene, the study sponsor, as well as several other pharmaceutical companies.
SOURCE: Kaplan DL. EADV Abstract FC04.04.
REPORTING FROM EADV 2019
Ixekizumab effective over long term for psoriasis
The according to a study published in the Journal of the European Academy of Dermatology and Venereology.
The UNCOVER-3 trial was a double-blind, multicenter, phase 3 study in 1,346 individuals with moderate to severe psoriasis who were randomized to placebo, 80 mg of ixekizumab every 2 or 4 weeks, or 50 mg of etanercept twice weekly. At week 12, all patients were transferred to 80 mg of ixekizumab every 4 weeks for the long-term extension period, and after 60 weeks, some were dose adjusted to 80 mg every 2 weeks.
At week 204, 48.3% of the 385 patients who were receiving treatment either every 2 or 4 weeks achieved Psoriasis Area and Severity Index (PASI) 100 according to the modified nonresponder imputation method to summarize efficacy – in which patients who drop out of the study are counted as nonresponders – 66.4% achieved PASI 90 and 82.8% achieved PASI 75.
Using the as-observed method for assessing efficacy, 67.1% of patients achieved PASI 100, 87.8% achieved PASI 90, and 98.2% achieved PASI 75. Using the multiple-imputation method, those same figures were 52.7%, 73.3%, and 94.8% respectively.
They also saw consistently high response rates according to the static Physician’s Global Assessment (sPGA) score, which goes from 0 (clear) to 5 (severe disease). Using the as-observed, multiple imputation and modified nonresponder imputation methods, 68.9%, 54.6%, and 49.7% of patients respectively achieved a score of 0.
The study also saw complete resolution in 95.8% of patients with baseline palmoplantar involvement, 75.9% of those with baseline nail involvement, and 87.1% of those with scalp involvement, using the as-observed method.
“These results corroborate the results that were reported previously in patients with moderate to severe psoriasis,” wrote Mark G. Lebwohl, MD, professor and chair of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and coauthors. “Sustained high response was observed with all the efficacy parameters such as PASI 75, 90, 100, and sPGA (0) or (0, 1), regardless of the statistical analyses ... performed.”
The majority of adverse events were mild or moderate, but serious adverse events were seen in 18.1% of patients in the long-term extension and 9.4% of patients stopped taking the study drug because of adverse events.
The most frequently reported adverse events of special interest were infections, such as nasopharyngitis (28.5% of patients), upper respiratory tract infections (10.8%), and Candida infection (5.2%). However, clinically significant neutropenia only occurred in 0.7% of patients, and malignancies occurred in 2.2% of patients.
“These safety findings support the consistency in the safety profile of [ixekizumab] treatment with no new signals occurring even after the longer exposure,” the authors wrote.
The study was funded by Eli Lilly, which manufactures ixekizumab. Two authors were employees of Eli Lilly and own company stocks. The remaining three authors reported receiving research funding and consultancies from different pharmaceutical companies, including Eli Lilly.
SOURCE: Lebwohl MG et al. J Eur Acad Dermatol Venereol. 2019 Sep 3. doi: 10.1111/jdv.15921.
The according to a study published in the Journal of the European Academy of Dermatology and Venereology.
The UNCOVER-3 trial was a double-blind, multicenter, phase 3 study in 1,346 individuals with moderate to severe psoriasis who were randomized to placebo, 80 mg of ixekizumab every 2 or 4 weeks, or 50 mg of etanercept twice weekly. At week 12, all patients were transferred to 80 mg of ixekizumab every 4 weeks for the long-term extension period, and after 60 weeks, some were dose adjusted to 80 mg every 2 weeks.
At week 204, 48.3% of the 385 patients who were receiving treatment either every 2 or 4 weeks achieved Psoriasis Area and Severity Index (PASI) 100 according to the modified nonresponder imputation method to summarize efficacy – in which patients who drop out of the study are counted as nonresponders – 66.4% achieved PASI 90 and 82.8% achieved PASI 75.
Using the as-observed method for assessing efficacy, 67.1% of patients achieved PASI 100, 87.8% achieved PASI 90, and 98.2% achieved PASI 75. Using the multiple-imputation method, those same figures were 52.7%, 73.3%, and 94.8% respectively.
They also saw consistently high response rates according to the static Physician’s Global Assessment (sPGA) score, which goes from 0 (clear) to 5 (severe disease). Using the as-observed, multiple imputation and modified nonresponder imputation methods, 68.9%, 54.6%, and 49.7% of patients respectively achieved a score of 0.
The study also saw complete resolution in 95.8% of patients with baseline palmoplantar involvement, 75.9% of those with baseline nail involvement, and 87.1% of those with scalp involvement, using the as-observed method.
“These results corroborate the results that were reported previously in patients with moderate to severe psoriasis,” wrote Mark G. Lebwohl, MD, professor and chair of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and coauthors. “Sustained high response was observed with all the efficacy parameters such as PASI 75, 90, 100, and sPGA (0) or (0, 1), regardless of the statistical analyses ... performed.”
The majority of adverse events were mild or moderate, but serious adverse events were seen in 18.1% of patients in the long-term extension and 9.4% of patients stopped taking the study drug because of adverse events.
The most frequently reported adverse events of special interest were infections, such as nasopharyngitis (28.5% of patients), upper respiratory tract infections (10.8%), and Candida infection (5.2%). However, clinically significant neutropenia only occurred in 0.7% of patients, and malignancies occurred in 2.2% of patients.
“These safety findings support the consistency in the safety profile of [ixekizumab] treatment with no new signals occurring even after the longer exposure,” the authors wrote.
The study was funded by Eli Lilly, which manufactures ixekizumab. Two authors were employees of Eli Lilly and own company stocks. The remaining three authors reported receiving research funding and consultancies from different pharmaceutical companies, including Eli Lilly.
SOURCE: Lebwohl MG et al. J Eur Acad Dermatol Venereol. 2019 Sep 3. doi: 10.1111/jdv.15921.
The according to a study published in the Journal of the European Academy of Dermatology and Venereology.
The UNCOVER-3 trial was a double-blind, multicenter, phase 3 study in 1,346 individuals with moderate to severe psoriasis who were randomized to placebo, 80 mg of ixekizumab every 2 or 4 weeks, or 50 mg of etanercept twice weekly. At week 12, all patients were transferred to 80 mg of ixekizumab every 4 weeks for the long-term extension period, and after 60 weeks, some were dose adjusted to 80 mg every 2 weeks.
At week 204, 48.3% of the 385 patients who were receiving treatment either every 2 or 4 weeks achieved Psoriasis Area and Severity Index (PASI) 100 according to the modified nonresponder imputation method to summarize efficacy – in which patients who drop out of the study are counted as nonresponders – 66.4% achieved PASI 90 and 82.8% achieved PASI 75.
Using the as-observed method for assessing efficacy, 67.1% of patients achieved PASI 100, 87.8% achieved PASI 90, and 98.2% achieved PASI 75. Using the multiple-imputation method, those same figures were 52.7%, 73.3%, and 94.8% respectively.
They also saw consistently high response rates according to the static Physician’s Global Assessment (sPGA) score, which goes from 0 (clear) to 5 (severe disease). Using the as-observed, multiple imputation and modified nonresponder imputation methods, 68.9%, 54.6%, and 49.7% of patients respectively achieved a score of 0.
The study also saw complete resolution in 95.8% of patients with baseline palmoplantar involvement, 75.9% of those with baseline nail involvement, and 87.1% of those with scalp involvement, using the as-observed method.
“These results corroborate the results that were reported previously in patients with moderate to severe psoriasis,” wrote Mark G. Lebwohl, MD, professor and chair of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and coauthors. “Sustained high response was observed with all the efficacy parameters such as PASI 75, 90, 100, and sPGA (0) or (0, 1), regardless of the statistical analyses ... performed.”
The majority of adverse events were mild or moderate, but serious adverse events were seen in 18.1% of patients in the long-term extension and 9.4% of patients stopped taking the study drug because of adverse events.
The most frequently reported adverse events of special interest were infections, such as nasopharyngitis (28.5% of patients), upper respiratory tract infections (10.8%), and Candida infection (5.2%). However, clinically significant neutropenia only occurred in 0.7% of patients, and malignancies occurred in 2.2% of patients.
“These safety findings support the consistency in the safety profile of [ixekizumab] treatment with no new signals occurring even after the longer exposure,” the authors wrote.
The study was funded by Eli Lilly, which manufactures ixekizumab. Two authors were employees of Eli Lilly and own company stocks. The remaining three authors reported receiving research funding and consultancies from different pharmaceutical companies, including Eli Lilly.
SOURCE: Lebwohl MG et al. J Eur Acad Dermatol Venereol. 2019 Sep 3. doi: 10.1111/jdv.15921.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Key clinical point: Long-term follow-up shows ixekizumab efficacy in moderate to severe psoriasis.
Major finding: At 4 years, 66.5% of patients on ixekizumab for psoriasis achieved Psoriasis Area and Severity Index 100.
Study details: A long-term extension of the UNCOVER-3 double-blind, multicenter, phase 3 study in 1,346 individuals with psoriasis.
Disclosures: The study was funded by Eli Lilly, which manufactures ixekizumab. Two authors were employees of Eli Lilly and own company stocks. The remaining three authors reported receiving research funding and consultancies from different pharmaceutical companies, including Eli Lilly.
Source: Lebwohl MG et al. J Eur Acad Dermatol Venereol. 2019 Sep 3. doi: 10.1111/jdv.15921.
FDA announces approval of fifth adalimumab biosimilar, Abrilada
The Food and Drug Administration has cleared adalimumab-afzb (Abrilada) as the fifth approved Humira biosimilar and the 25th approved biosimilar drug overall, the agency said in a Nov. 15 announcement.
According to a press release from Pfizer, approval for Abrilada was based on review of a comprehensive data package demonstrating biosimilarity of the drug to the reference product. This included data from a clinical comparative study, which found no clinically meaningful difference between Abrilada and the reference in terms of efficacy, safety, and immunogenicity in patients with moderate to severe rheumatoid arthritis (RA). In addition to RA, Abrilada is indicated for juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, ulcerative colitis, and plaque psoriasis.
Common adverse events in adalimumab clinical trials included infection, injection-site reactions, headache, and rash.
Pfizer said that it “is working to make Abrilada available to U.S. patients as soon as feasible based on the terms of our agreement with AbbVie [the manufacturer of Humira]. Our current plans are to launch in 2023.”
The Food and Drug Administration has cleared adalimumab-afzb (Abrilada) as the fifth approved Humira biosimilar and the 25th approved biosimilar drug overall, the agency said in a Nov. 15 announcement.
According to a press release from Pfizer, approval for Abrilada was based on review of a comprehensive data package demonstrating biosimilarity of the drug to the reference product. This included data from a clinical comparative study, which found no clinically meaningful difference between Abrilada and the reference in terms of efficacy, safety, and immunogenicity in patients with moderate to severe rheumatoid arthritis (RA). In addition to RA, Abrilada is indicated for juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, ulcerative colitis, and plaque psoriasis.
Common adverse events in adalimumab clinical trials included infection, injection-site reactions, headache, and rash.
Pfizer said that it “is working to make Abrilada available to U.S. patients as soon as feasible based on the terms of our agreement with AbbVie [the manufacturer of Humira]. Our current plans are to launch in 2023.”
The Food and Drug Administration has cleared adalimumab-afzb (Abrilada) as the fifth approved Humira biosimilar and the 25th approved biosimilar drug overall, the agency said in a Nov. 15 announcement.
According to a press release from Pfizer, approval for Abrilada was based on review of a comprehensive data package demonstrating biosimilarity of the drug to the reference product. This included data from a clinical comparative study, which found no clinically meaningful difference between Abrilada and the reference in terms of efficacy, safety, and immunogenicity in patients with moderate to severe rheumatoid arthritis (RA). In addition to RA, Abrilada is indicated for juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, ulcerative colitis, and plaque psoriasis.
Common adverse events in adalimumab clinical trials included infection, injection-site reactions, headache, and rash.
Pfizer said that it “is working to make Abrilada available to U.S. patients as soon as feasible based on the terms of our agreement with AbbVie [the manufacturer of Humira]. Our current plans are to launch in 2023.”
Early use of ustekinumab levels could predict psoriasis outcomes
Early ustekinumab levels are significantly associated with a 75% reduction in Psoriasis Area and Severity Index scores from baseline, according to data from 491 adults with psoriasis.
“Evidence suggests that ustekinumab dosing is suboptimal in some patients,” because of factors including weight-based dosing and dosing intervals; therefore “individualized dose optimization and therapeutic drug monitoring (TDM) of ustekinumab may have clinical utility,” Teresa Tsakok, MRCP, of King’s College London, and colleagues wrote in JAMA Dermatology.
The researchers identified 491 adults with psoriasis who were part of the BSTOP (Biomarkers of Systemic Treatment Outcomes in Psoriasis) cohort study in the United Kingdom. Blood samples were collected during clinical reviews to assess ustekinumab levels, and primary treatment response was 75% reduction in Psoriasis Area and Severity Index (PASI 75) scores.
Ustekinumab levels measured from 1-12 weeks after the start of treatment were significantly associated with PASI 75 after 6 months (odds ratio, 1.38) after controlling for factors including baseline PASI scores, age, and ustekinumab dose. The association, however, did not hold for other PASI outcomes, including PASI 90 and PASI scores of 1.5 or less.
The participants had at least one serum sample collected at 0-56 weeks from the start of treatment and at least one PSAI score measured within the first year of treatment. The average baseline PASI score was 13.3, the average body mass index was 32 kg/m2, and 65% of the patients were male.
Antidrug antibodies were detected in 17 patients (3.5% of the study population), compared with a rate of 37.5% in patients from the same study cohort who were taking adalimumab, the researchers noted.
The study findings were limited by several factors, including a dropoff in patient numbers over the study period and the difficulty in accounting for the association between drug level and treatment response in a logistic regression model, the researchers said.
The results, however, suggest “that adequate drug exposure early in the treatment cycle may be particularly important in determining clinical outcome with ustekinumab,” and that “future work should focus on pharmacokinetic-pharmacodynamic modeling of the whole time course of response to ustekinumab” as a key step toward personalized treatment regimens, they concluded.
The study was supported by several entities, including the Medical Research Council (MRC), the National Institute for Health Research Biomedical Research Center and the Psoriasis Association. Dr. Tsakok had no financial conflicts to disclose and was supported by an MRC Clinical Research Training Fellowship.
SOURCE: Tsakok T et al. JAMA Dermatol. 2019 Sep 18. doi: 10.1001/jamadermatol.2019.1783.
Early ustekinumab levels are significantly associated with a 75% reduction in Psoriasis Area and Severity Index scores from baseline, according to data from 491 adults with psoriasis.
“Evidence suggests that ustekinumab dosing is suboptimal in some patients,” because of factors including weight-based dosing and dosing intervals; therefore “individualized dose optimization and therapeutic drug monitoring (TDM) of ustekinumab may have clinical utility,” Teresa Tsakok, MRCP, of King’s College London, and colleagues wrote in JAMA Dermatology.
The researchers identified 491 adults with psoriasis who were part of the BSTOP (Biomarkers of Systemic Treatment Outcomes in Psoriasis) cohort study in the United Kingdom. Blood samples were collected during clinical reviews to assess ustekinumab levels, and primary treatment response was 75% reduction in Psoriasis Area and Severity Index (PASI 75) scores.
Ustekinumab levels measured from 1-12 weeks after the start of treatment were significantly associated with PASI 75 after 6 months (odds ratio, 1.38) after controlling for factors including baseline PASI scores, age, and ustekinumab dose. The association, however, did not hold for other PASI outcomes, including PASI 90 and PASI scores of 1.5 or less.
The participants had at least one serum sample collected at 0-56 weeks from the start of treatment and at least one PSAI score measured within the first year of treatment. The average baseline PASI score was 13.3, the average body mass index was 32 kg/m2, and 65% of the patients were male.
Antidrug antibodies were detected in 17 patients (3.5% of the study population), compared with a rate of 37.5% in patients from the same study cohort who were taking adalimumab, the researchers noted.
The study findings were limited by several factors, including a dropoff in patient numbers over the study period and the difficulty in accounting for the association between drug level and treatment response in a logistic regression model, the researchers said.
The results, however, suggest “that adequate drug exposure early in the treatment cycle may be particularly important in determining clinical outcome with ustekinumab,” and that “future work should focus on pharmacokinetic-pharmacodynamic modeling of the whole time course of response to ustekinumab” as a key step toward personalized treatment regimens, they concluded.
The study was supported by several entities, including the Medical Research Council (MRC), the National Institute for Health Research Biomedical Research Center and the Psoriasis Association. Dr. Tsakok had no financial conflicts to disclose and was supported by an MRC Clinical Research Training Fellowship.
SOURCE: Tsakok T et al. JAMA Dermatol. 2019 Sep 18. doi: 10.1001/jamadermatol.2019.1783.
Early ustekinumab levels are significantly associated with a 75% reduction in Psoriasis Area and Severity Index scores from baseline, according to data from 491 adults with psoriasis.
“Evidence suggests that ustekinumab dosing is suboptimal in some patients,” because of factors including weight-based dosing and dosing intervals; therefore “individualized dose optimization and therapeutic drug monitoring (TDM) of ustekinumab may have clinical utility,” Teresa Tsakok, MRCP, of King’s College London, and colleagues wrote in JAMA Dermatology.
The researchers identified 491 adults with psoriasis who were part of the BSTOP (Biomarkers of Systemic Treatment Outcomes in Psoriasis) cohort study in the United Kingdom. Blood samples were collected during clinical reviews to assess ustekinumab levels, and primary treatment response was 75% reduction in Psoriasis Area and Severity Index (PASI 75) scores.
Ustekinumab levels measured from 1-12 weeks after the start of treatment were significantly associated with PASI 75 after 6 months (odds ratio, 1.38) after controlling for factors including baseline PASI scores, age, and ustekinumab dose. The association, however, did not hold for other PASI outcomes, including PASI 90 and PASI scores of 1.5 or less.
The participants had at least one serum sample collected at 0-56 weeks from the start of treatment and at least one PSAI score measured within the first year of treatment. The average baseline PASI score was 13.3, the average body mass index was 32 kg/m2, and 65% of the patients were male.
Antidrug antibodies were detected in 17 patients (3.5% of the study population), compared with a rate of 37.5% in patients from the same study cohort who were taking adalimumab, the researchers noted.
The study findings were limited by several factors, including a dropoff in patient numbers over the study period and the difficulty in accounting for the association between drug level and treatment response in a logistic regression model, the researchers said.
The results, however, suggest “that adequate drug exposure early in the treatment cycle may be particularly important in determining clinical outcome with ustekinumab,” and that “future work should focus on pharmacokinetic-pharmacodynamic modeling of the whole time course of response to ustekinumab” as a key step toward personalized treatment regimens, they concluded.
The study was supported by several entities, including the Medical Research Council (MRC), the National Institute for Health Research Biomedical Research Center and the Psoriasis Association. Dr. Tsakok had no financial conflicts to disclose and was supported by an MRC Clinical Research Training Fellowship.
SOURCE: Tsakok T et al. JAMA Dermatol. 2019 Sep 18. doi: 10.1001/jamadermatol.2019.1783.
FROM JAMA DERMATOLOGY
Key clinical point: Measurement of ustekinumab levels early in treatment could help direct strategies and ensure correct dosage in psoriasis patients.
Major finding: Early serum ustekinumab levels 1-12 weeks after starting treatment were associated with a 75% reduction in PASI scores from baseline at 6 months.
Study details: The prospective, observational study involved 491 adults with psoriasis.
Disclosures: The study was supported by several entities, including the Medical Research Council (MRC), the National Institute for Health Research Biomedical Research Center, and the Psoriasis Association. Dr. Tsakok disclosed financial conflicts and was supported by an MRC Clinical Research Training Fellowship.
Source: Tsakok T et al. JAMA Dermatol. 2019 Sep 18. doi: 10.1001/jamadermatol.2019.1783.
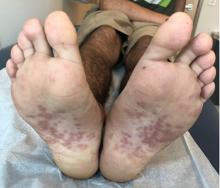
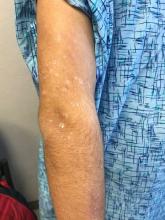
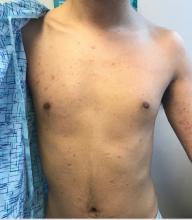
Pink scaly rash on torso and extremities
Tumor necrosis factor–alpha (TNF-alpha) inhibitors are therapeutic agents used to treat a variety of inflammatory conditions such as rheumatoid arthritis and inflammatory bowel disease, as well as psoriasis of the skin (PSO) and psoriatic arthritis. In a 2017 systematic review, there were 216 reported cases of new-onset TNF-alpha inhibitor–induced psoriasis, with an estimated rate of 1 per 1,000. The cases thus far have had a wide range of presentations, the most common being plaque psoriasis, scalp psoriasis, as well as palmoplantar pustular psoriasis.1
A retrospective chart review study at Mayo clinic published in 2017 evaluated children younger than 19 years seen in 2003-2015 who developed new-onset or recurrent PSO with a history of inflammatory bowel disease being treated with anti-TNF-alpha therapy. The review showed variable latency in the development of PSO in these patients, although it typically occurred during inflammatory bowel disease remission.2 It is unclear whether there is an association between a personal or family history of psoriasis and development of these lesions.
TNF-alpha, interleukin (IL)–17) and interferon-alpha (IFN-alpha) are main cytokines that contribute to the development of psoriasis. The mechanism of action for paradoxical PSO/psoriasis in patients treated with anti-TNF is not clearly understood; however, many hypotheses are based on an imbalance between TNF-alpha and interferon-alpha – more specifically, an increased production of interferon-alpha. TNF-alpha inhibits the activity of plasmacytoid dendritic cells which are key producers of IFN-alpha. Because of this blockade, there is unopposed IFN-alpha production. Interferon-alpha allows for the expression of chemokines such as CXCR3, which favor T cells homing to the skin. IFN-alpha also stimulates and activates T cells to produce TNF-alpha and IL-17, which in turn sustains inflammatory mechanisms and allows for the development of psoriatic lesions.3
There are no universal management guidelines. Most of these patients’ treatment plans mirror standard psoriasis therapies while the main question remains the decision to continue the same anti-TNF therapy, change anti-TNF agents, or entirely switch classes of biologic or other systemic therapy. This decision in management requires several considerations: treatability of TNF-alpha inhibitor-induced psoriasis, the severity of background disease (i.e., rheumatoid arthritis, inflammatory bowel disease, other systemic condition), and whether the underlying disease is well controlled on current therapy, as well as the consideration of possible loss in efficacy if a drug is discontinued and then restarted at a later date.4
A reasonable initial approach in patients with well-controlled underlying disease and mild skin eruption is to continue anti-TNF therapy and manage skin topically with topical corticosteroids and/or phototherapy. In patients that either do not have well-controlled underlying disease or moderate skin involvement, changing to an alternative anti-TNF or other agent may be reasonable, and requires coordinated care with involved specialists. In the 2017 pediatric review mentioned previously, nearly half of the patients required a change in their initial anti-TNF-alpha agent despite conventional skin-directed therapies, and one-third of patients discontinued all anti-TNF-alpha therapy because of PSO.2
The psoriasiform papulosquamous features of this case along with the history suggests the diagnosis. Pityriasis rosea would be highly atypical on the feet and with the duration of findings. Lichen planus and atopic dermatitis morphology are inconsistent with this eruption, and coxsackie viral infection would have a shorter course.
Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital–San Diego and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Email them at [email protected].
References
1. J Am Acad Dermatol. 2017 Feb;76(2):334-41.
2. Pediatr Dermatol. 2017 May;34(3):253-60.
3. RMD Open. 2016 Jul 15;2(2):e000239.
4. J Psoriasis Psoriatic Arthritis. 2019 Apr;4(2):70-80.
Tumor necrosis factor–alpha (TNF-alpha) inhibitors are therapeutic agents used to treat a variety of inflammatory conditions such as rheumatoid arthritis and inflammatory bowel disease, as well as psoriasis of the skin (PSO) and psoriatic arthritis. In a 2017 systematic review, there were 216 reported cases of new-onset TNF-alpha inhibitor–induced psoriasis, with an estimated rate of 1 per 1,000. The cases thus far have had a wide range of presentations, the most common being plaque psoriasis, scalp psoriasis, as well as palmoplantar pustular psoriasis.1
A retrospective chart review study at Mayo clinic published in 2017 evaluated children younger than 19 years seen in 2003-2015 who developed new-onset or recurrent PSO with a history of inflammatory bowel disease being treated with anti-TNF-alpha therapy. The review showed variable latency in the development of PSO in these patients, although it typically occurred during inflammatory bowel disease remission.2 It is unclear whether there is an association between a personal or family history of psoriasis and development of these lesions.
TNF-alpha, interleukin (IL)–17) and interferon-alpha (IFN-alpha) are main cytokines that contribute to the development of psoriasis. The mechanism of action for paradoxical PSO/psoriasis in patients treated with anti-TNF is not clearly understood; however, many hypotheses are based on an imbalance between TNF-alpha and interferon-alpha – more specifically, an increased production of interferon-alpha. TNF-alpha inhibits the activity of plasmacytoid dendritic cells which are key producers of IFN-alpha. Because of this blockade, there is unopposed IFN-alpha production. Interferon-alpha allows for the expression of chemokines such as CXCR3, which favor T cells homing to the skin. IFN-alpha also stimulates and activates T cells to produce TNF-alpha and IL-17, which in turn sustains inflammatory mechanisms and allows for the development of psoriatic lesions.3
There are no universal management guidelines. Most of these patients’ treatment plans mirror standard psoriasis therapies while the main question remains the decision to continue the same anti-TNF therapy, change anti-TNF agents, or entirely switch classes of biologic or other systemic therapy. This decision in management requires several considerations: treatability of TNF-alpha inhibitor-induced psoriasis, the severity of background disease (i.e., rheumatoid arthritis, inflammatory bowel disease, other systemic condition), and whether the underlying disease is well controlled on current therapy, as well as the consideration of possible loss in efficacy if a drug is discontinued and then restarted at a later date.4
A reasonable initial approach in patients with well-controlled underlying disease and mild skin eruption is to continue anti-TNF therapy and manage skin topically with topical corticosteroids and/or phototherapy. In patients that either do not have well-controlled underlying disease or moderate skin involvement, changing to an alternative anti-TNF or other agent may be reasonable, and requires coordinated care with involved specialists. In the 2017 pediatric review mentioned previously, nearly half of the patients required a change in their initial anti-TNF-alpha agent despite conventional skin-directed therapies, and one-third of patients discontinued all anti-TNF-alpha therapy because of PSO.2
The psoriasiform papulosquamous features of this case along with the history suggests the diagnosis. Pityriasis rosea would be highly atypical on the feet and with the duration of findings. Lichen planus and atopic dermatitis morphology are inconsistent with this eruption, and coxsackie viral infection would have a shorter course.
Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital–San Diego and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Email them at [email protected].
References
1. J Am Acad Dermatol. 2017 Feb;76(2):334-41.
2. Pediatr Dermatol. 2017 May;34(3):253-60.
3. RMD Open. 2016 Jul 15;2(2):e000239.
4. J Psoriasis Psoriatic Arthritis. 2019 Apr;4(2):70-80.
Tumor necrosis factor–alpha (TNF-alpha) inhibitors are therapeutic agents used to treat a variety of inflammatory conditions such as rheumatoid arthritis and inflammatory bowel disease, as well as psoriasis of the skin (PSO) and psoriatic arthritis. In a 2017 systematic review, there were 216 reported cases of new-onset TNF-alpha inhibitor–induced psoriasis, with an estimated rate of 1 per 1,000. The cases thus far have had a wide range of presentations, the most common being plaque psoriasis, scalp psoriasis, as well as palmoplantar pustular psoriasis.1
A retrospective chart review study at Mayo clinic published in 2017 evaluated children younger than 19 years seen in 2003-2015 who developed new-onset or recurrent PSO with a history of inflammatory bowel disease being treated with anti-TNF-alpha therapy. The review showed variable latency in the development of PSO in these patients, although it typically occurred during inflammatory bowel disease remission.2 It is unclear whether there is an association between a personal or family history of psoriasis and development of these lesions.
TNF-alpha, interleukin (IL)–17) and interferon-alpha (IFN-alpha) are main cytokines that contribute to the development of psoriasis. The mechanism of action for paradoxical PSO/psoriasis in patients treated with anti-TNF is not clearly understood; however, many hypotheses are based on an imbalance between TNF-alpha and interferon-alpha – more specifically, an increased production of interferon-alpha. TNF-alpha inhibits the activity of plasmacytoid dendritic cells which are key producers of IFN-alpha. Because of this blockade, there is unopposed IFN-alpha production. Interferon-alpha allows for the expression of chemokines such as CXCR3, which favor T cells homing to the skin. IFN-alpha also stimulates and activates T cells to produce TNF-alpha and IL-17, which in turn sustains inflammatory mechanisms and allows for the development of psoriatic lesions.3
There are no universal management guidelines. Most of these patients’ treatment plans mirror standard psoriasis therapies while the main question remains the decision to continue the same anti-TNF therapy, change anti-TNF agents, or entirely switch classes of biologic or other systemic therapy. This decision in management requires several considerations: treatability of TNF-alpha inhibitor-induced psoriasis, the severity of background disease (i.e., rheumatoid arthritis, inflammatory bowel disease, other systemic condition), and whether the underlying disease is well controlled on current therapy, as well as the consideration of possible loss in efficacy if a drug is discontinued and then restarted at a later date.4
A reasonable initial approach in patients with well-controlled underlying disease and mild skin eruption is to continue anti-TNF therapy and manage skin topically with topical corticosteroids and/or phototherapy. In patients that either do not have well-controlled underlying disease or moderate skin involvement, changing to an alternative anti-TNF or other agent may be reasonable, and requires coordinated care with involved specialists. In the 2017 pediatric review mentioned previously, nearly half of the patients required a change in their initial anti-TNF-alpha agent despite conventional skin-directed therapies, and one-third of patients discontinued all anti-TNF-alpha therapy because of PSO.2
The psoriasiform papulosquamous features of this case along with the history suggests the diagnosis. Pityriasis rosea would be highly atypical on the feet and with the duration of findings. Lichen planus and atopic dermatitis morphology are inconsistent with this eruption, and coxsackie viral infection would have a shorter course.
Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital–San Diego and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Email them at [email protected].
References
1. J Am Acad Dermatol. 2017 Feb;76(2):334-41.
2. Pediatr Dermatol. 2017 May;34(3):253-60.
3. RMD Open. 2016 Jul 15;2(2):e000239.
4. J Psoriasis Psoriatic Arthritis. 2019 Apr;4(2):70-80.
A 17-year-old male with a history of Crohn's disease, well controlled on infliximab, is seen for evaluation of a pink scaly rash on the torso, upper extremities, and lower extremities. The rash began 5 months previously and has been mostly asymptomatic. The patient denies pruritus or pain at the affected areas. There is no fever or drainage from any of the sites. The patient has not undergone any treatments. He does not have a personal or family history of chronic skin conditions.
On physical exam, he is noted to have numerous pink papules and plaques with overlying scale on his trunk, as well as the dorsal aspects of bilateral hands and the plantar surfaces of bilateral feet. His skin is otherwise unremarkable.
Expert shares tips for TNF-alpha inhibitor use in special populations
LAS VEGAS – Francisco A. Kerdel, BSc, MBBS, said at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
Dr. Kerdel, professor and vice chair of the department of dermatology at Florida International University, Miami, noted that, while tumor necrosis factor (TNF)–alpha inhibitors are category B drugs, inadequate data exist regarding lactation and exposure throughout pregnancy. “Rates of malformations and spontaneous abortions with therapy are similar to those in the general population, higher concentrations of infliximab and adalimumab have been found in infant and cord blood, compared with certolizumab pegol,” an anti-TNF biologic, he said.
In a prospective, postmarketing, multicenter pharmacokinetic study, researchers found a lack of placental transfer of certolizumab pegol during pregnancy (Ann Rheum Dis. 2018;77:228-33). Specifically, certolizumab levels were below the lower limit of quantification (less than 0.032 mcg/mL) in 13 of 14 infant samples at birth and in all infant samples at weeks 4 and 8. Only one infant had a minimal certolizumab level at birth (infant/mother ratio of 0.0009). No antibodies were detected at any time point during the study. Safety data in mothers were in line with the known safety profile of certolizumab and pregnancy profile of these underlying diseases. Adverse events experienced by the infants did not show any patterns or clusters of events suggesting a specific safety signal in children.
In a separate postmarketing pharmacokinetic study, investigators evaluated the transfer of certolizumab into breast milk (Ann Rheum Dis. 2017;76:1890-6). They found that the average daily infant dose of certolizumab was minimal. Specifically, the highest concentration of certolizumab in breast milk (0.0758 mcg/mL) was less than 1% of the expected mean plasma trough concentration of a therapeutic dose.
How do TNF-alpha inhibitors fare in the pediatric population? In a retrospective study of 390 children with psoriasis treated at 20 centers in the United States, Canada, and Europe, researchers evaluated the safety of systemic agents (JAMA Dermatol. 2017;153[11]:1147-57). Most (69%) were prescribed methotrexate, followed by biologics, acitretin, cyclosporine, and fumaric acid. Drug discontinuation (because of adverse events), which is sometimes used as an efficacy parameter, occurred in 12% of those who were on methotrexate, compared with 3% of those on biologics, 67% of those on acitretin, and 68% of those on fumaric acid.
At the other end of the age spectrum, biologic therapy is generally effective and well tolerated in elderly patients. “Sometimes, they may be more effective than other traditional drugs,” Dr. Kerdel said. “We’re a little bit concerned about immunosenescence, which can increase the risk for severe infections and malignancies. And, 90% of elderly patients with psoriasis may have comorbidities that need to be taken into account when treating psoriasis.”
Other factors come into play when choosing the right anti-TNF agent, including weight. While clinical trials show efficacy across weight groups, infliximab has weight-based dosing, “which may make it a better choice,” Dr. Kerdel said. “Patients taking etanercept may need a biweekly dose.”
Treatment flexibility also comes into play. For example, stopping therapy because of an infection or surgery may be problematic in drugs with a long half-life. Then there’s the issue of patient preference. “Some people don’t want to be injected frequently,” he said. “Some people don’t want to be injected at all and may require a simpler dosing regimen.”
Optimizing anti-TNF-alpha treatment starts with recognizing that there is a loss of response over time, Dr. Kerdel said, “or there may not be a response at all.” Contributing factors may include immunogenicity, suboptimal dosing, and poor patient adherence. In order to optimize treatment, clinicians can try switching agents or combination therapy, and explore continuous versus intermittent dosing.
“We really don’t have good data on the best protocol for switching treatment after failure of an anti-TNF-alpha agent,” he added. In cases of primary and secondary treatment failure, there is no consensus or guidelines on which second-line agent to use, nor good data on which measures to use.
No evidence-based guidelines are available for screening and monitoring patients receiving biologic therapy for psoriasis, either. “Evidence is strongest [grade B] for tuberculosis screening in patients treated with biologic agents,” Dr. Kerdel said. “Among known hepatitis B virus carriers, consider monitoring liver function tests and viral load [grade C]. High-grade evidence is lacking to support other routine testing. Physicians should use clinical judgment when screening and monitoring patients.”
He concluded his presentation by noting that there are a number of biosimilar agents available or in the pipeline for infliximab, adalimumab, and etanercept. This raises a number of questions for current and future consideration. For one, “will biosimilars show the same long-term efficacy and safety as the innovator products?” he asked. “Real-world, postmarketing, and registry data are needed. Will biosimilar agents offer significant cost benefits? Will biosimilar labeling be adequately transparent? Will we find biomarkers to help us target biologic agents to specific patients and subtypes of psoriasis?”
Dr. Kerdel reported that he is a member of the speaker’s bureau for AbbVie, Amgen, Celgene, Janssen, Novartis, Lilly, Leo, Ortho, and Novartis. He has also received grant/research support from AbbVie, Amgen, AstraZeneca, Celgene, Janssen, Leo, Lilly, Menlo Therapeutics, Novartis, Pfizer, and XBiotech.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – Francisco A. Kerdel, BSc, MBBS, said at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
Dr. Kerdel, professor and vice chair of the department of dermatology at Florida International University, Miami, noted that, while tumor necrosis factor (TNF)–alpha inhibitors are category B drugs, inadequate data exist regarding lactation and exposure throughout pregnancy. “Rates of malformations and spontaneous abortions with therapy are similar to those in the general population, higher concentrations of infliximab and adalimumab have been found in infant and cord blood, compared with certolizumab pegol,” an anti-TNF biologic, he said.
In a prospective, postmarketing, multicenter pharmacokinetic study, researchers found a lack of placental transfer of certolizumab pegol during pregnancy (Ann Rheum Dis. 2018;77:228-33). Specifically, certolizumab levels were below the lower limit of quantification (less than 0.032 mcg/mL) in 13 of 14 infant samples at birth and in all infant samples at weeks 4 and 8. Only one infant had a minimal certolizumab level at birth (infant/mother ratio of 0.0009). No antibodies were detected at any time point during the study. Safety data in mothers were in line with the known safety profile of certolizumab and pregnancy profile of these underlying diseases. Adverse events experienced by the infants did not show any patterns or clusters of events suggesting a specific safety signal in children.
In a separate postmarketing pharmacokinetic study, investigators evaluated the transfer of certolizumab into breast milk (Ann Rheum Dis. 2017;76:1890-6). They found that the average daily infant dose of certolizumab was minimal. Specifically, the highest concentration of certolizumab in breast milk (0.0758 mcg/mL) was less than 1% of the expected mean plasma trough concentration of a therapeutic dose.
How do TNF-alpha inhibitors fare in the pediatric population? In a retrospective study of 390 children with psoriasis treated at 20 centers in the United States, Canada, and Europe, researchers evaluated the safety of systemic agents (JAMA Dermatol. 2017;153[11]:1147-57). Most (69%) were prescribed methotrexate, followed by biologics, acitretin, cyclosporine, and fumaric acid. Drug discontinuation (because of adverse events), which is sometimes used as an efficacy parameter, occurred in 12% of those who were on methotrexate, compared with 3% of those on biologics, 67% of those on acitretin, and 68% of those on fumaric acid.
At the other end of the age spectrum, biologic therapy is generally effective and well tolerated in elderly patients. “Sometimes, they may be more effective than other traditional drugs,” Dr. Kerdel said. “We’re a little bit concerned about immunosenescence, which can increase the risk for severe infections and malignancies. And, 90% of elderly patients with psoriasis may have comorbidities that need to be taken into account when treating psoriasis.”
Other factors come into play when choosing the right anti-TNF agent, including weight. While clinical trials show efficacy across weight groups, infliximab has weight-based dosing, “which may make it a better choice,” Dr. Kerdel said. “Patients taking etanercept may need a biweekly dose.”
Treatment flexibility also comes into play. For example, stopping therapy because of an infection or surgery may be problematic in drugs with a long half-life. Then there’s the issue of patient preference. “Some people don’t want to be injected frequently,” he said. “Some people don’t want to be injected at all and may require a simpler dosing regimen.”
Optimizing anti-TNF-alpha treatment starts with recognizing that there is a loss of response over time, Dr. Kerdel said, “or there may not be a response at all.” Contributing factors may include immunogenicity, suboptimal dosing, and poor patient adherence. In order to optimize treatment, clinicians can try switching agents or combination therapy, and explore continuous versus intermittent dosing.
“We really don’t have good data on the best protocol for switching treatment after failure of an anti-TNF-alpha agent,” he added. In cases of primary and secondary treatment failure, there is no consensus or guidelines on which second-line agent to use, nor good data on which measures to use.
No evidence-based guidelines are available for screening and monitoring patients receiving biologic therapy for psoriasis, either. “Evidence is strongest [grade B] for tuberculosis screening in patients treated with biologic agents,” Dr. Kerdel said. “Among known hepatitis B virus carriers, consider monitoring liver function tests and viral load [grade C]. High-grade evidence is lacking to support other routine testing. Physicians should use clinical judgment when screening and monitoring patients.”
He concluded his presentation by noting that there are a number of biosimilar agents available or in the pipeline for infliximab, adalimumab, and etanercept. This raises a number of questions for current and future consideration. For one, “will biosimilars show the same long-term efficacy and safety as the innovator products?” he asked. “Real-world, postmarketing, and registry data are needed. Will biosimilar agents offer significant cost benefits? Will biosimilar labeling be adequately transparent? Will we find biomarkers to help us target biologic agents to specific patients and subtypes of psoriasis?”
Dr. Kerdel reported that he is a member of the speaker’s bureau for AbbVie, Amgen, Celgene, Janssen, Novartis, Lilly, Leo, Ortho, and Novartis. He has also received grant/research support from AbbVie, Amgen, AstraZeneca, Celgene, Janssen, Leo, Lilly, Menlo Therapeutics, Novartis, Pfizer, and XBiotech.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – Francisco A. Kerdel, BSc, MBBS, said at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
Dr. Kerdel, professor and vice chair of the department of dermatology at Florida International University, Miami, noted that, while tumor necrosis factor (TNF)–alpha inhibitors are category B drugs, inadequate data exist regarding lactation and exposure throughout pregnancy. “Rates of malformations and spontaneous abortions with therapy are similar to those in the general population, higher concentrations of infliximab and adalimumab have been found in infant and cord blood, compared with certolizumab pegol,” an anti-TNF biologic, he said.
In a prospective, postmarketing, multicenter pharmacokinetic study, researchers found a lack of placental transfer of certolizumab pegol during pregnancy (Ann Rheum Dis. 2018;77:228-33). Specifically, certolizumab levels were below the lower limit of quantification (less than 0.032 mcg/mL) in 13 of 14 infant samples at birth and in all infant samples at weeks 4 and 8. Only one infant had a minimal certolizumab level at birth (infant/mother ratio of 0.0009). No antibodies were detected at any time point during the study. Safety data in mothers were in line with the known safety profile of certolizumab and pregnancy profile of these underlying diseases. Adverse events experienced by the infants did not show any patterns or clusters of events suggesting a specific safety signal in children.
In a separate postmarketing pharmacokinetic study, investigators evaluated the transfer of certolizumab into breast milk (Ann Rheum Dis. 2017;76:1890-6). They found that the average daily infant dose of certolizumab was minimal. Specifically, the highest concentration of certolizumab in breast milk (0.0758 mcg/mL) was less than 1% of the expected mean plasma trough concentration of a therapeutic dose.
How do TNF-alpha inhibitors fare in the pediatric population? In a retrospective study of 390 children with psoriasis treated at 20 centers in the United States, Canada, and Europe, researchers evaluated the safety of systemic agents (JAMA Dermatol. 2017;153[11]:1147-57). Most (69%) were prescribed methotrexate, followed by biologics, acitretin, cyclosporine, and fumaric acid. Drug discontinuation (because of adverse events), which is sometimes used as an efficacy parameter, occurred in 12% of those who were on methotrexate, compared with 3% of those on biologics, 67% of those on acitretin, and 68% of those on fumaric acid.
At the other end of the age spectrum, biologic therapy is generally effective and well tolerated in elderly patients. “Sometimes, they may be more effective than other traditional drugs,” Dr. Kerdel said. “We’re a little bit concerned about immunosenescence, which can increase the risk for severe infections and malignancies. And, 90% of elderly patients with psoriasis may have comorbidities that need to be taken into account when treating psoriasis.”
Other factors come into play when choosing the right anti-TNF agent, including weight. While clinical trials show efficacy across weight groups, infliximab has weight-based dosing, “which may make it a better choice,” Dr. Kerdel said. “Patients taking etanercept may need a biweekly dose.”
Treatment flexibility also comes into play. For example, stopping therapy because of an infection or surgery may be problematic in drugs with a long half-life. Then there’s the issue of patient preference. “Some people don’t want to be injected frequently,” he said. “Some people don’t want to be injected at all and may require a simpler dosing regimen.”
Optimizing anti-TNF-alpha treatment starts with recognizing that there is a loss of response over time, Dr. Kerdel said, “or there may not be a response at all.” Contributing factors may include immunogenicity, suboptimal dosing, and poor patient adherence. In order to optimize treatment, clinicians can try switching agents or combination therapy, and explore continuous versus intermittent dosing.
“We really don’t have good data on the best protocol for switching treatment after failure of an anti-TNF-alpha agent,” he added. In cases of primary and secondary treatment failure, there is no consensus or guidelines on which second-line agent to use, nor good data on which measures to use.
No evidence-based guidelines are available for screening and monitoring patients receiving biologic therapy for psoriasis, either. “Evidence is strongest [grade B] for tuberculosis screening in patients treated with biologic agents,” Dr. Kerdel said. “Among known hepatitis B virus carriers, consider monitoring liver function tests and viral load [grade C]. High-grade evidence is lacking to support other routine testing. Physicians should use clinical judgment when screening and monitoring patients.”
He concluded his presentation by noting that there are a number of biosimilar agents available or in the pipeline for infliximab, adalimumab, and etanercept. This raises a number of questions for current and future consideration. For one, “will biosimilars show the same long-term efficacy and safety as the innovator products?” he asked. “Real-world, postmarketing, and registry data are needed. Will biosimilar agents offer significant cost benefits? Will biosimilar labeling be adequately transparent? Will we find biomarkers to help us target biologic agents to specific patients and subtypes of psoriasis?”
Dr. Kerdel reported that he is a member of the speaker’s bureau for AbbVie, Amgen, Celgene, Janssen, Novartis, Lilly, Leo, Ortho, and Novartis. He has also received grant/research support from AbbVie, Amgen, AstraZeneca, Celgene, Janssen, Leo, Lilly, Menlo Therapeutics, Novartis, Pfizer, and XBiotech.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM THE SDEF LAS VEGAS DERMATOLOGY SEMINAR
Narrowband UVB phototherapy an effective treatment for psoriasis
LAS VEGAS – professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee.
At the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar, Dr. Gordon said that he uses phototherapy most in patients “who can’t get to all of the spots without it,” and in those whose disease is not having a huge impact on day-to-day life. “In my experience it’s best in people with thinner plaques, though I have some question over whether we can figure out if morphology really does correlate to respond to phototherapy,” he added. “We’re in the process of conducting a study to try and figure that out.”
From an immunologic standpoint, UVB decreases responsiveness of the interleukin-17 pathway and increases both cutaneous and circulating FoxP3+ cells, he said. Activation of p38 mitogen–activated protein kinase during phototherapy inhibits proinflammatory T-cell activation. In Dr. Gordon’s opinion, phototherapy is absolutely contraindicated in patients who have a photosensitivity reaction related to the UVB spectrum, while it is relatively contraindicated in those at high risk for skin cancer, including patients with multiple squamous cell carcinomas and those who have undergone organ transplantation. “I would argue that [the use of] photosensitizing drugs are not a contraindication,” he said. “Would I prefer that someone who’s had 54 [squamous cell carcinomas] not use phototherapy? Of course. But if it’s the only weapon I have, I’ll use it.”
Although clinicians have used phototherapy for about 100 years, early standards for clinical trials were different, compared with the rigor required of those conducted in the current era. Outcomes were not standardized, he said, and they were generally smaller trials that did not incorporate proper monitoring of adverse events. “Even with these weaknesses, we can get a general impression of how well UV works,” he said. In a 6-week, split-body trial of 11 patients from 20 years ago, researchers were able to induce clinical clearing in 82% of patients after narrowband UVB (the type most commonly used today), but in only 9% of patients after broadband UVB treatment (P less than .01) (J Am Acad Dermatol. 1999 Jun;40[6 Pt 1]:893-900).
Most clinicians commence narrowband UVB phototherapy treatment with three sessions per week and safely increase the dose to reach an effective level. Erythema can develop with overdosing. “If a patient can only come in twice weekly [for phototherapy treatment], that is okay,” Dr. Gordon said. “It takes a longer time getting to treatment goals. In my opinion, going down to once a week during the escalating phase of using phototherapy doesn’t work. Three times a week is better than twice a week during the escalating phase, but one time a week isn’t enough.”
Even for maintenance therapy, it remains a question whether limiting phototherapy to once every 2 or 4 weeks is sufficient for disease control. “I let the patient choose, but I like to go down to no less than once a week and hold people steady at that dose,” he said.
Purported risks of narrowband UVB phototherapy include photoaging, sunburn, herpes simplex virus reactivation, loss of the patient’s productive time, and development of skin cancer. “I don’t think we have good data about the effect of phototherapy on photoaging,” Dr. Gordon said. “Loss of productive time is my single biggest [complaint]. People are having trouble getting to work and having bosses who are not pleased with them [leaving work for treatment].”
As for the association between phototherapy and skin cancer, he noted that published studies that show statistical differences all incorporate psoralen-UVA photochemotherapy into their analyses. “There’s really no study that has demonstrated a significant impact on nonmelanoma skin cancers with the use of phototherapy [alone],” Dr. Gordon said. A British study of 3,867 psoriasis patients found no significant association between narrowband UVB treatment and basal cell cancer, squamous cell cancer, or melanoma (Br J Dermatol. 2008 Sep;159[4]:931-5). However, among those who also were treated with psoralen-UVA photochemotherapy, there was a small increase in basal cell cancer.
In a more recent Swedish study of 162 psoriasis patients who were treated with UVB phototherapy, researchers found that, after controlling for age, the risk of skin cancer was associated with the number of treatments, but not with the type of UVB lamp used (Acta Derm Venereol. 2014 Jul;94[4]:425-30). They reported that overall, the risk of malignancy in the UVB-treated patients did not exceed that in the general population.
Dr. Gordon concluded his presentation by noting that home-based UVB phototherapy is gaining in popularity and is comparable with in-office UVB in terms of accuracy and efficacy (BMJ. 2009;338:b1542). Because of convenience and decreased time away from work, he said, “it’s easier for patients ... and it’s probably cheaper in the long run.” The National Psoriasis Foundation web site includes a list of home UVB equipment.
Dr. Gordon disclosed that he is a consultant with AbbVie, Almirall, Amgen, Bristol-Meyers Squibb, Celgene, Dermavant, Eli Lilly, Janssen, Leo, Novartis, Ortho Dermatologics, Pfizer, Sun Pharma, and UCB. He has also received grant/research support from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, and Novartis.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee.
At the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar, Dr. Gordon said that he uses phototherapy most in patients “who can’t get to all of the spots without it,” and in those whose disease is not having a huge impact on day-to-day life. “In my experience it’s best in people with thinner plaques, though I have some question over whether we can figure out if morphology really does correlate to respond to phototherapy,” he added. “We’re in the process of conducting a study to try and figure that out.”
From an immunologic standpoint, UVB decreases responsiveness of the interleukin-17 pathway and increases both cutaneous and circulating FoxP3+ cells, he said. Activation of p38 mitogen–activated protein kinase during phototherapy inhibits proinflammatory T-cell activation. In Dr. Gordon’s opinion, phototherapy is absolutely contraindicated in patients who have a photosensitivity reaction related to the UVB spectrum, while it is relatively contraindicated in those at high risk for skin cancer, including patients with multiple squamous cell carcinomas and those who have undergone organ transplantation. “I would argue that [the use of] photosensitizing drugs are not a contraindication,” he said. “Would I prefer that someone who’s had 54 [squamous cell carcinomas] not use phototherapy? Of course. But if it’s the only weapon I have, I’ll use it.”
Although clinicians have used phototherapy for about 100 years, early standards for clinical trials were different, compared with the rigor required of those conducted in the current era. Outcomes were not standardized, he said, and they were generally smaller trials that did not incorporate proper monitoring of adverse events. “Even with these weaknesses, we can get a general impression of how well UV works,” he said. In a 6-week, split-body trial of 11 patients from 20 years ago, researchers were able to induce clinical clearing in 82% of patients after narrowband UVB (the type most commonly used today), but in only 9% of patients after broadband UVB treatment (P less than .01) (J Am Acad Dermatol. 1999 Jun;40[6 Pt 1]:893-900).
Most clinicians commence narrowband UVB phototherapy treatment with three sessions per week and safely increase the dose to reach an effective level. Erythema can develop with overdosing. “If a patient can only come in twice weekly [for phototherapy treatment], that is okay,” Dr. Gordon said. “It takes a longer time getting to treatment goals. In my opinion, going down to once a week during the escalating phase of using phototherapy doesn’t work. Three times a week is better than twice a week during the escalating phase, but one time a week isn’t enough.”
Even for maintenance therapy, it remains a question whether limiting phototherapy to once every 2 or 4 weeks is sufficient for disease control. “I let the patient choose, but I like to go down to no less than once a week and hold people steady at that dose,” he said.
Purported risks of narrowband UVB phototherapy include photoaging, sunburn, herpes simplex virus reactivation, loss of the patient’s productive time, and development of skin cancer. “I don’t think we have good data about the effect of phototherapy on photoaging,” Dr. Gordon said. “Loss of productive time is my single biggest [complaint]. People are having trouble getting to work and having bosses who are not pleased with them [leaving work for treatment].”
As for the association between phototherapy and skin cancer, he noted that published studies that show statistical differences all incorporate psoralen-UVA photochemotherapy into their analyses. “There’s really no study that has demonstrated a significant impact on nonmelanoma skin cancers with the use of phototherapy [alone],” Dr. Gordon said. A British study of 3,867 psoriasis patients found no significant association between narrowband UVB treatment and basal cell cancer, squamous cell cancer, or melanoma (Br J Dermatol. 2008 Sep;159[4]:931-5). However, among those who also were treated with psoralen-UVA photochemotherapy, there was a small increase in basal cell cancer.
In a more recent Swedish study of 162 psoriasis patients who were treated with UVB phototherapy, researchers found that, after controlling for age, the risk of skin cancer was associated with the number of treatments, but not with the type of UVB lamp used (Acta Derm Venereol. 2014 Jul;94[4]:425-30). They reported that overall, the risk of malignancy in the UVB-treated patients did not exceed that in the general population.
Dr. Gordon concluded his presentation by noting that home-based UVB phototherapy is gaining in popularity and is comparable with in-office UVB in terms of accuracy and efficacy (BMJ. 2009;338:b1542). Because of convenience and decreased time away from work, he said, “it’s easier for patients ... and it’s probably cheaper in the long run.” The National Psoriasis Foundation web site includes a list of home UVB equipment.
Dr. Gordon disclosed that he is a consultant with AbbVie, Almirall, Amgen, Bristol-Meyers Squibb, Celgene, Dermavant, Eli Lilly, Janssen, Leo, Novartis, Ortho Dermatologics, Pfizer, Sun Pharma, and UCB. He has also received grant/research support from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, and Novartis.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee.
At the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar, Dr. Gordon said that he uses phototherapy most in patients “who can’t get to all of the spots without it,” and in those whose disease is not having a huge impact on day-to-day life. “In my experience it’s best in people with thinner plaques, though I have some question over whether we can figure out if morphology really does correlate to respond to phototherapy,” he added. “We’re in the process of conducting a study to try and figure that out.”
From an immunologic standpoint, UVB decreases responsiveness of the interleukin-17 pathway and increases both cutaneous and circulating FoxP3+ cells, he said. Activation of p38 mitogen–activated protein kinase during phototherapy inhibits proinflammatory T-cell activation. In Dr. Gordon’s opinion, phototherapy is absolutely contraindicated in patients who have a photosensitivity reaction related to the UVB spectrum, while it is relatively contraindicated in those at high risk for skin cancer, including patients with multiple squamous cell carcinomas and those who have undergone organ transplantation. “I would argue that [the use of] photosensitizing drugs are not a contraindication,” he said. “Would I prefer that someone who’s had 54 [squamous cell carcinomas] not use phototherapy? Of course. But if it’s the only weapon I have, I’ll use it.”
Although clinicians have used phototherapy for about 100 years, early standards for clinical trials were different, compared with the rigor required of those conducted in the current era. Outcomes were not standardized, he said, and they were generally smaller trials that did not incorporate proper monitoring of adverse events. “Even with these weaknesses, we can get a general impression of how well UV works,” he said. In a 6-week, split-body trial of 11 patients from 20 years ago, researchers were able to induce clinical clearing in 82% of patients after narrowband UVB (the type most commonly used today), but in only 9% of patients after broadband UVB treatment (P less than .01) (J Am Acad Dermatol. 1999 Jun;40[6 Pt 1]:893-900).
Most clinicians commence narrowband UVB phototherapy treatment with three sessions per week and safely increase the dose to reach an effective level. Erythema can develop with overdosing. “If a patient can only come in twice weekly [for phototherapy treatment], that is okay,” Dr. Gordon said. “It takes a longer time getting to treatment goals. In my opinion, going down to once a week during the escalating phase of using phototherapy doesn’t work. Three times a week is better than twice a week during the escalating phase, but one time a week isn’t enough.”
Even for maintenance therapy, it remains a question whether limiting phototherapy to once every 2 or 4 weeks is sufficient for disease control. “I let the patient choose, but I like to go down to no less than once a week and hold people steady at that dose,” he said.
Purported risks of narrowband UVB phototherapy include photoaging, sunburn, herpes simplex virus reactivation, loss of the patient’s productive time, and development of skin cancer. “I don’t think we have good data about the effect of phototherapy on photoaging,” Dr. Gordon said. “Loss of productive time is my single biggest [complaint]. People are having trouble getting to work and having bosses who are not pleased with them [leaving work for treatment].”
As for the association between phototherapy and skin cancer, he noted that published studies that show statistical differences all incorporate psoralen-UVA photochemotherapy into their analyses. “There’s really no study that has demonstrated a significant impact on nonmelanoma skin cancers with the use of phototherapy [alone],” Dr. Gordon said. A British study of 3,867 psoriasis patients found no significant association between narrowband UVB treatment and basal cell cancer, squamous cell cancer, or melanoma (Br J Dermatol. 2008 Sep;159[4]:931-5). However, among those who also were treated with psoralen-UVA photochemotherapy, there was a small increase in basal cell cancer.
In a more recent Swedish study of 162 psoriasis patients who were treated with UVB phototherapy, researchers found that, after controlling for age, the risk of skin cancer was associated with the number of treatments, but not with the type of UVB lamp used (Acta Derm Venereol. 2014 Jul;94[4]:425-30). They reported that overall, the risk of malignancy in the UVB-treated patients did not exceed that in the general population.
Dr. Gordon concluded his presentation by noting that home-based UVB phototherapy is gaining in popularity and is comparable with in-office UVB in terms of accuracy and efficacy (BMJ. 2009;338:b1542). Because of convenience and decreased time away from work, he said, “it’s easier for patients ... and it’s probably cheaper in the long run.” The National Psoriasis Foundation web site includes a list of home UVB equipment.
Dr. Gordon disclosed that he is a consultant with AbbVie, Almirall, Amgen, Bristol-Meyers Squibb, Celgene, Dermavant, Eli Lilly, Janssen, Leo, Novartis, Ortho Dermatologics, Pfizer, Sun Pharma, and UCB. He has also received grant/research support from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, and Novartis.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM THE SDEF LAS VEGAS DERMATOLOGY SEMINAR
Guselkumab improves psoriatic arthritis regardless of prior TNFi use
ATLANTA – Guselkumab improved outcomes in psoriatic arthritis patients regardless of past treatment with tumor necrosis factor inhibitors in the phase 3 DISCOVER-1 trial.
The anti-interleukin-23p19 monoclonal antibody is approved in the United States for the treatment of moderate to severe plaque psoriasis (PsO).
Benefits in psoriatic arthritis (PsA) were seen in both biologic-naive and tumor necrosis factor inhibitor (TNFi)–treated patients and occurred with both 4- and 8-week dosing regimens, Atul Deodhar, MD, reported during a plenary session at the annual meeting of the American College of Rheumatology.
For example, the primary endpoint of ACR 20 response at 24 weeks was achieved in 58.6% and 52.8% of patients randomized to receive 100 mg of guselkumab delivered subcutaneously either at baseline and every 4 weeks or at baseline, week 4, and then every 8 weeks, respectively, compared with 22.2% of those randomized to receive placebo, said Dr. Deodhar, professor of medicine at Oregon Health & Science University, Portland.
Greater proportions of patients in the guselkumab groups achieved ACR 20 response at week 16; ACR 50 response at weeks 16 and 24; ACR 70 response at week 24; Psoriasis Area and Severity Index 75, 90, and 100 responses at week 24; and minimal disease activity response at week 24, he said, adding that improvements were also seen with guselkumab versus placebo for the controlled major secondary endpoints of change from baseline in Health Assessment Questionnaire–Disability Index score, Short Form 36 Health Survey score, and investigator global assessment (IGA) of PsO response.
The response rates with guselkumab versus placebo were seen regardless of prior TNFi use, he said.
The study included 381 patients with active PsA, defined as three or more swollen joints, three or more tender joints, and C-reactive protein of 0.3 mg/dL or greater despite standard therapies. About 30% were exposed to up to two TNFi therapies and 10% were nonresponders or inadequate responders to those therapies.
Concomitant use of select nonbiologic disease-modifying antirheumatic drugs, oral corticosteroids, and NSAIDs was allowed, and patients with less than 5% improvement in tender plus swollen joints at week 16 could initiate or increase the dose of the permitted medications while continuing study treatment, Dr. Deodhar said.
The mean body surface area with PsO involvement was 13.4%; 42.5% of patients had an IGA of 3-4 for skin involvement. Mean swollen and tender joint counts were 9.8 and 19.3, respectively, indicating a population with moderate to severe disease, he added.
Serious adverse events, serious infections, and death occurred in 2.4%, 0.5%, and 0.3% of patients, respectively.
“Both guselkumab regimens were safe and well tolerated through week 24,” Dr. Deodhar said, noting that the safety profile was consistent with that established in the treatment of PsO and described in the label.
DISCOVER-1 was funded by Janssen Research & Development. Dr. Deodhar reported relationships (advisory board activity, consulting, and/or research grant funding) with several pharmaceutical companies including Janssen. Several coauthors are employees of Janssen.
SOURCE: Deodhar A et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 807.
ATLANTA – Guselkumab improved outcomes in psoriatic arthritis patients regardless of past treatment with tumor necrosis factor inhibitors in the phase 3 DISCOVER-1 trial.
The anti-interleukin-23p19 monoclonal antibody is approved in the United States for the treatment of moderate to severe plaque psoriasis (PsO).
Benefits in psoriatic arthritis (PsA) were seen in both biologic-naive and tumor necrosis factor inhibitor (TNFi)–treated patients and occurred with both 4- and 8-week dosing regimens, Atul Deodhar, MD, reported during a plenary session at the annual meeting of the American College of Rheumatology.
For example, the primary endpoint of ACR 20 response at 24 weeks was achieved in 58.6% and 52.8% of patients randomized to receive 100 mg of guselkumab delivered subcutaneously either at baseline and every 4 weeks or at baseline, week 4, and then every 8 weeks, respectively, compared with 22.2% of those randomized to receive placebo, said Dr. Deodhar, professor of medicine at Oregon Health & Science University, Portland.
Greater proportions of patients in the guselkumab groups achieved ACR 20 response at week 16; ACR 50 response at weeks 16 and 24; ACR 70 response at week 24; Psoriasis Area and Severity Index 75, 90, and 100 responses at week 24; and minimal disease activity response at week 24, he said, adding that improvements were also seen with guselkumab versus placebo for the controlled major secondary endpoints of change from baseline in Health Assessment Questionnaire–Disability Index score, Short Form 36 Health Survey score, and investigator global assessment (IGA) of PsO response.
The response rates with guselkumab versus placebo were seen regardless of prior TNFi use, he said.
The study included 381 patients with active PsA, defined as three or more swollen joints, three or more tender joints, and C-reactive protein of 0.3 mg/dL or greater despite standard therapies. About 30% were exposed to up to two TNFi therapies and 10% were nonresponders or inadequate responders to those therapies.
Concomitant use of select nonbiologic disease-modifying antirheumatic drugs, oral corticosteroids, and NSAIDs was allowed, and patients with less than 5% improvement in tender plus swollen joints at week 16 could initiate or increase the dose of the permitted medications while continuing study treatment, Dr. Deodhar said.
The mean body surface area with PsO involvement was 13.4%; 42.5% of patients had an IGA of 3-4 for skin involvement. Mean swollen and tender joint counts were 9.8 and 19.3, respectively, indicating a population with moderate to severe disease, he added.
Serious adverse events, serious infections, and death occurred in 2.4%, 0.5%, and 0.3% of patients, respectively.
“Both guselkumab regimens were safe and well tolerated through week 24,” Dr. Deodhar said, noting that the safety profile was consistent with that established in the treatment of PsO and described in the label.
DISCOVER-1 was funded by Janssen Research & Development. Dr. Deodhar reported relationships (advisory board activity, consulting, and/or research grant funding) with several pharmaceutical companies including Janssen. Several coauthors are employees of Janssen.
SOURCE: Deodhar A et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 807.
ATLANTA – Guselkumab improved outcomes in psoriatic arthritis patients regardless of past treatment with tumor necrosis factor inhibitors in the phase 3 DISCOVER-1 trial.
The anti-interleukin-23p19 monoclonal antibody is approved in the United States for the treatment of moderate to severe plaque psoriasis (PsO).
Benefits in psoriatic arthritis (PsA) were seen in both biologic-naive and tumor necrosis factor inhibitor (TNFi)–treated patients and occurred with both 4- and 8-week dosing regimens, Atul Deodhar, MD, reported during a plenary session at the annual meeting of the American College of Rheumatology.
For example, the primary endpoint of ACR 20 response at 24 weeks was achieved in 58.6% and 52.8% of patients randomized to receive 100 mg of guselkumab delivered subcutaneously either at baseline and every 4 weeks or at baseline, week 4, and then every 8 weeks, respectively, compared with 22.2% of those randomized to receive placebo, said Dr. Deodhar, professor of medicine at Oregon Health & Science University, Portland.
Greater proportions of patients in the guselkumab groups achieved ACR 20 response at week 16; ACR 50 response at weeks 16 and 24; ACR 70 response at week 24; Psoriasis Area and Severity Index 75, 90, and 100 responses at week 24; and minimal disease activity response at week 24, he said, adding that improvements were also seen with guselkumab versus placebo for the controlled major secondary endpoints of change from baseline in Health Assessment Questionnaire–Disability Index score, Short Form 36 Health Survey score, and investigator global assessment (IGA) of PsO response.
The response rates with guselkumab versus placebo were seen regardless of prior TNFi use, he said.
The study included 381 patients with active PsA, defined as three or more swollen joints, three or more tender joints, and C-reactive protein of 0.3 mg/dL or greater despite standard therapies. About 30% were exposed to up to two TNFi therapies and 10% were nonresponders or inadequate responders to those therapies.
Concomitant use of select nonbiologic disease-modifying antirheumatic drugs, oral corticosteroids, and NSAIDs was allowed, and patients with less than 5% improvement in tender plus swollen joints at week 16 could initiate or increase the dose of the permitted medications while continuing study treatment, Dr. Deodhar said.
The mean body surface area with PsO involvement was 13.4%; 42.5% of patients had an IGA of 3-4 for skin involvement. Mean swollen and tender joint counts were 9.8 and 19.3, respectively, indicating a population with moderate to severe disease, he added.
Serious adverse events, serious infections, and death occurred in 2.4%, 0.5%, and 0.3% of patients, respectively.
“Both guselkumab regimens were safe and well tolerated through week 24,” Dr. Deodhar said, noting that the safety profile was consistent with that established in the treatment of PsO and described in the label.
DISCOVER-1 was funded by Janssen Research & Development. Dr. Deodhar reported relationships (advisory board activity, consulting, and/or research grant funding) with several pharmaceutical companies including Janssen. Several coauthors are employees of Janssen.
SOURCE: Deodhar A et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 807.
AAD-NPF Pediatric psoriasis guideline advises on physical and mental care
Psoriasis management in children involves attention not only to treatment of the physical condition but also psychosocial wellness and quality of life, according to
Psoriasis affects approximately 1% of children, either alone or associated with comorbid conditions such as psoriatic arthritis (PsA), wrote Alan Menter, MD, of Baylor University Medical Center, Dallas, and coauthors of the guideline.
In the guideline, published in the Journal of the American Academy of Dermatology, the multidisciplinary work group identified screening tools to measure disease severity, strategies for management of comorbidities, and the safety and effectiveness of topical, systemic, and phototherapy treatments.
To assess disease severity, the work group recommended not only the use of body surface area (BSA), similar to measurement of severity in adults, but also the use of the Children’s Dermatology Life Quality Index, a 10-question quality of life survey, as BSA alone does not account for the potential negative impact of the disease on quality of life in terms of physical, emotional, social, and psychological function.
“For example, a child with psoriasis limited to the face or the entire scalp does not have severe disease based on BSA definitions, but if this involvement causes shame, social withdrawal, or bullying, it satisfies criteria for severe disease based on impact beyond the skin,” they said.
The work group stated that a variety of conditions may trigger or exacerbate psoriasis in children, including infections, cutaneous trauma, or physiological, emotional, and environmental stressors.
The majority of children with PsA develop joint inflammation before skin disease, the work group wrote. In addition, children with psoriasis are at increased risk for rheumatoid arthritis, so clinicians may need to distinguish between a combination of psoriasis and musculoskeletal issues and cases of either psoriatic or rheumatoid arthritis in young patients.
The cardiovascular risk factors associated with metabolic syndrome are greater in children with psoriasis, compared with children without psoriasis, the work group noted. In addition, pediatric psoriasis patients have a higher prevalence of obesity than children without psoriasis, and they recommended that children with psoriasis be monitored for the development of obesity, and that obese children with psoriasis should be referred for weight management.
The work group noted that data are insufficient in children to support the link between psoriasis and cardiovascular disease that has been documented in adults with psoriasis. However, “patients with pediatric psoriasis should have American Academy of Pediatrics (AAP)–recommended age-related cardiovascular screening regardless of the presence of signs or symptoms,” they said.
The guideline also recommends screening for dyslipidemia and hypertension according to AAP guidelines and educating pediatric psoriasis patients about the risk of diabetes and regularly screening for diabetes and insulin resistance in those who are obese. Overweight children with psoriasis may be screened at the provider’s discretion, they wrote. Patients with signs of inflammatory bowel disease, which also is associated with psoriasis in adults, should be considered for referral to a gastroenterologist, they noted.
Children with psoriasis should be screened regularly for mental health conditions regardless of age, and they should be asked about substance abuse, according to the guideline, and those with concerns should be referred for additional assessment and management.
The guideline divides treatment of psoriasis in children into three categories: topical, phototherapy and photochemotherapy, and systemic treatments (nonbiologic or biologic).
For topicals, the guideline recommendations include corticosteroids as an off-label therapy, as well as ultra-high-potency topical corticosteroids as monotherapy. Overall, “selection of a therapeutic routine (potency, delivery vehicle, frequency of application) should take into account sites of involvement, type and thickness of psoriasis, age of the patient, total BSA of application, anticipated occlusion, and disease acuity, among other patient-, disease-, and drug-related factors,” the authors wrote. Other topical options included in the recommendations: calcineurin inhibitors, topical vitamin D analogues, tazarotene (off label), anthralin, and coal tar.
Phototherapy has a history of use in psoriasis treatment and remains part of the current recommendations, although data in children are limited, and data on the use of phototherapy for pustular psoriasis in children are insufficient to make specific treatment and dosing recommendations, the work group noted. The researchers also noted that in-office phototherapy may not be feasible for many patients, but that in-home ultraviolet light equipment or natural sunlight in moderation could be recommended as an alternative.
The use of systemic, nonbiologic treatments for pediatric psoriasis should be “based on baseline severity of disease, subtype of psoriasis, speed of disease progression, lack of response to more conservative therapies such as topical agents and phototherapy (when appropriate), impaired physical or psychological functioning or [quality of life] due to disease extent, and the presence of comorbidities such as PsA,” the workgroup said.
Options for systemic treatment include methotrexate, cyclosporine (notably for pustular as well as plaque and erythrodermic psoriasis), and systemic retinoids. In addition, fumaric acid esters may be an option for children with moderate to severe psoriasis, with recommended clinical and laboratory monitoring.
The increasing safety and efficacy data on biologics in pediatric psoriasis patients support their consideration among first-line systemic treatments, the work group suggested. “Etanercept and ustekinumab are now [Food and Drug Administration] approved for patients with psoriasis 4 years and older and 12 years and older, respectively,” they said, and infliximab and adalimumab have been used off label in children.
The work group concluded that research and knowledge gaps about pediatric psoriasis persist and include mechanism of disease onset, development of comorbidities, and identification of ideal dosing for various treatments.
Finally, the work group emphasized the importance of collaboration between dermatologists and primary care providers for managing psoriasis in children, as well as the importance of patient education.
“Dermatologists should be mindful of the unique aspects of the emotional development of children and the social dynamics of having a visible difference,” they wrote. “Shared decision making with the patient (if age appropriate) and the caregivers is a useful approach, particularly as related to the use of off-label medications to treat severe disease,” they said.
“This is the first time that pediatric psoriasis has been discussed as an independent topic within the guideline,” said one of the guideline authors, Dawn M.R. Davis, MD, of the Mayo Clinic, Rochester, Minn., in an interview. “Children have unique physiology and psychosocial aspects to their care relative to adults. In addition, psoriasis has some clinical manifestations that are oftentimes distinctly seen in children,” she commented. “Creation of a guideline specific to children allows us to summarize the similarities and differences of disease presentation and management. It also allows an opportunity to clarify what research data (especially therapeutics) have been studied in children and their uses, safety profiles, and dosing,” she noted.
Psoriasis can be a psychosocially debilitating disease, she emphasized. “In children, for example, isolated or prominent facial involvement is common, which can be embarrassing and impact relationships.”
The take-home message for clinicians, Dr. Davis said, is to keep in mind the multisystemic nature of psoriasis. “It is not limited to the skin,” she said. “Treating a patient with psoriasis necessitates practicing whole-person care” and considering the multiple comorbidities that impact quality of life and overall health in children, as well as adults with psoriasis, she commented. “Dermatologists can empower patients and their caregivers by educating them on the multifocal, complex nature of the disease.” She added, “We have much to learn regarding psoriasis in the pediatric population. More research into therapeutics, topical and systemic, is necessary to optimize patient care.”
The guideline was based on studies published in the PubMed and MEDLINE databases from January 2011 through December 31, 2017.
Dr. Menter and Craig A. Elmets, MD, professor of dermatology, at the University of Alabama, Birmingham, were cochairs of the work group. The pediatric guideline is the latest in a multipart series of AAD-NPF guidelines on psoriasis being published this year in the Journal of the American Academy of Dermatology.
Many of the guideline authors, including lead author Dr. Menter, disclosed relationships with multiple companies; however, a minimum 51% of workgroup members had no relevant conflicts of interest in accordance with AAD policy. There was no funding source. Dr. Davis disclosed serving as an investigator for Regeneron, with no compensation.
SOURCE: Menter et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2019.08.049.
Psoriasis management in children involves attention not only to treatment of the physical condition but also psychosocial wellness and quality of life, according to
Psoriasis affects approximately 1% of children, either alone or associated with comorbid conditions such as psoriatic arthritis (PsA), wrote Alan Menter, MD, of Baylor University Medical Center, Dallas, and coauthors of the guideline.
In the guideline, published in the Journal of the American Academy of Dermatology, the multidisciplinary work group identified screening tools to measure disease severity, strategies for management of comorbidities, and the safety and effectiveness of topical, systemic, and phototherapy treatments.
To assess disease severity, the work group recommended not only the use of body surface area (BSA), similar to measurement of severity in adults, but also the use of the Children’s Dermatology Life Quality Index, a 10-question quality of life survey, as BSA alone does not account for the potential negative impact of the disease on quality of life in terms of physical, emotional, social, and psychological function.
“For example, a child with psoriasis limited to the face or the entire scalp does not have severe disease based on BSA definitions, but if this involvement causes shame, social withdrawal, or bullying, it satisfies criteria for severe disease based on impact beyond the skin,” they said.
The work group stated that a variety of conditions may trigger or exacerbate psoriasis in children, including infections, cutaneous trauma, or physiological, emotional, and environmental stressors.
The majority of children with PsA develop joint inflammation before skin disease, the work group wrote. In addition, children with psoriasis are at increased risk for rheumatoid arthritis, so clinicians may need to distinguish between a combination of psoriasis and musculoskeletal issues and cases of either psoriatic or rheumatoid arthritis in young patients.
The cardiovascular risk factors associated with metabolic syndrome are greater in children with psoriasis, compared with children without psoriasis, the work group noted. In addition, pediatric psoriasis patients have a higher prevalence of obesity than children without psoriasis, and they recommended that children with psoriasis be monitored for the development of obesity, and that obese children with psoriasis should be referred for weight management.
The work group noted that data are insufficient in children to support the link between psoriasis and cardiovascular disease that has been documented in adults with psoriasis. However, “patients with pediatric psoriasis should have American Academy of Pediatrics (AAP)–recommended age-related cardiovascular screening regardless of the presence of signs or symptoms,” they said.
The guideline also recommends screening for dyslipidemia and hypertension according to AAP guidelines and educating pediatric psoriasis patients about the risk of diabetes and regularly screening for diabetes and insulin resistance in those who are obese. Overweight children with psoriasis may be screened at the provider’s discretion, they wrote. Patients with signs of inflammatory bowel disease, which also is associated with psoriasis in adults, should be considered for referral to a gastroenterologist, they noted.
Children with psoriasis should be screened regularly for mental health conditions regardless of age, and they should be asked about substance abuse, according to the guideline, and those with concerns should be referred for additional assessment and management.
The guideline divides treatment of psoriasis in children into three categories: topical, phototherapy and photochemotherapy, and systemic treatments (nonbiologic or biologic).
For topicals, the guideline recommendations include corticosteroids as an off-label therapy, as well as ultra-high-potency topical corticosteroids as monotherapy. Overall, “selection of a therapeutic routine (potency, delivery vehicle, frequency of application) should take into account sites of involvement, type and thickness of psoriasis, age of the patient, total BSA of application, anticipated occlusion, and disease acuity, among other patient-, disease-, and drug-related factors,” the authors wrote. Other topical options included in the recommendations: calcineurin inhibitors, topical vitamin D analogues, tazarotene (off label), anthralin, and coal tar.
Phototherapy has a history of use in psoriasis treatment and remains part of the current recommendations, although data in children are limited, and data on the use of phototherapy for pustular psoriasis in children are insufficient to make specific treatment and dosing recommendations, the work group noted. The researchers also noted that in-office phototherapy may not be feasible for many patients, but that in-home ultraviolet light equipment or natural sunlight in moderation could be recommended as an alternative.
The use of systemic, nonbiologic treatments for pediatric psoriasis should be “based on baseline severity of disease, subtype of psoriasis, speed of disease progression, lack of response to more conservative therapies such as topical agents and phototherapy (when appropriate), impaired physical or psychological functioning or [quality of life] due to disease extent, and the presence of comorbidities such as PsA,” the workgroup said.
Options for systemic treatment include methotrexate, cyclosporine (notably for pustular as well as plaque and erythrodermic psoriasis), and systemic retinoids. In addition, fumaric acid esters may be an option for children with moderate to severe psoriasis, with recommended clinical and laboratory monitoring.
The increasing safety and efficacy data on biologics in pediatric psoriasis patients support their consideration among first-line systemic treatments, the work group suggested. “Etanercept and ustekinumab are now [Food and Drug Administration] approved for patients with psoriasis 4 years and older and 12 years and older, respectively,” they said, and infliximab and adalimumab have been used off label in children.
The work group concluded that research and knowledge gaps about pediatric psoriasis persist and include mechanism of disease onset, development of comorbidities, and identification of ideal dosing for various treatments.
Finally, the work group emphasized the importance of collaboration between dermatologists and primary care providers for managing psoriasis in children, as well as the importance of patient education.
“Dermatologists should be mindful of the unique aspects of the emotional development of children and the social dynamics of having a visible difference,” they wrote. “Shared decision making with the patient (if age appropriate) and the caregivers is a useful approach, particularly as related to the use of off-label medications to treat severe disease,” they said.
“This is the first time that pediatric psoriasis has been discussed as an independent topic within the guideline,” said one of the guideline authors, Dawn M.R. Davis, MD, of the Mayo Clinic, Rochester, Minn., in an interview. “Children have unique physiology and psychosocial aspects to their care relative to adults. In addition, psoriasis has some clinical manifestations that are oftentimes distinctly seen in children,” she commented. “Creation of a guideline specific to children allows us to summarize the similarities and differences of disease presentation and management. It also allows an opportunity to clarify what research data (especially therapeutics) have been studied in children and their uses, safety profiles, and dosing,” she noted.
Psoriasis can be a psychosocially debilitating disease, she emphasized. “In children, for example, isolated or prominent facial involvement is common, which can be embarrassing and impact relationships.”
The take-home message for clinicians, Dr. Davis said, is to keep in mind the multisystemic nature of psoriasis. “It is not limited to the skin,” she said. “Treating a patient with psoriasis necessitates practicing whole-person care” and considering the multiple comorbidities that impact quality of life and overall health in children, as well as adults with psoriasis, she commented. “Dermatologists can empower patients and their caregivers by educating them on the multifocal, complex nature of the disease.” She added, “We have much to learn regarding psoriasis in the pediatric population. More research into therapeutics, topical and systemic, is necessary to optimize patient care.”
The guideline was based on studies published in the PubMed and MEDLINE databases from January 2011 through December 31, 2017.
Dr. Menter and Craig A. Elmets, MD, professor of dermatology, at the University of Alabama, Birmingham, were cochairs of the work group. The pediatric guideline is the latest in a multipart series of AAD-NPF guidelines on psoriasis being published this year in the Journal of the American Academy of Dermatology.
Many of the guideline authors, including lead author Dr. Menter, disclosed relationships with multiple companies; however, a minimum 51% of workgroup members had no relevant conflicts of interest in accordance with AAD policy. There was no funding source. Dr. Davis disclosed serving as an investigator for Regeneron, with no compensation.
SOURCE: Menter et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2019.08.049.
Psoriasis management in children involves attention not only to treatment of the physical condition but also psychosocial wellness and quality of life, according to
Psoriasis affects approximately 1% of children, either alone or associated with comorbid conditions such as psoriatic arthritis (PsA), wrote Alan Menter, MD, of Baylor University Medical Center, Dallas, and coauthors of the guideline.
In the guideline, published in the Journal of the American Academy of Dermatology, the multidisciplinary work group identified screening tools to measure disease severity, strategies for management of comorbidities, and the safety and effectiveness of topical, systemic, and phototherapy treatments.
To assess disease severity, the work group recommended not only the use of body surface area (BSA), similar to measurement of severity in adults, but also the use of the Children’s Dermatology Life Quality Index, a 10-question quality of life survey, as BSA alone does not account for the potential negative impact of the disease on quality of life in terms of physical, emotional, social, and psychological function.
“For example, a child with psoriasis limited to the face or the entire scalp does not have severe disease based on BSA definitions, but if this involvement causes shame, social withdrawal, or bullying, it satisfies criteria for severe disease based on impact beyond the skin,” they said.
The work group stated that a variety of conditions may trigger or exacerbate psoriasis in children, including infections, cutaneous trauma, or physiological, emotional, and environmental stressors.
The majority of children with PsA develop joint inflammation before skin disease, the work group wrote. In addition, children with psoriasis are at increased risk for rheumatoid arthritis, so clinicians may need to distinguish between a combination of psoriasis and musculoskeletal issues and cases of either psoriatic or rheumatoid arthritis in young patients.
The cardiovascular risk factors associated with metabolic syndrome are greater in children with psoriasis, compared with children without psoriasis, the work group noted. In addition, pediatric psoriasis patients have a higher prevalence of obesity than children without psoriasis, and they recommended that children with psoriasis be monitored for the development of obesity, and that obese children with psoriasis should be referred for weight management.
The work group noted that data are insufficient in children to support the link between psoriasis and cardiovascular disease that has been documented in adults with psoriasis. However, “patients with pediatric psoriasis should have American Academy of Pediatrics (AAP)–recommended age-related cardiovascular screening regardless of the presence of signs or symptoms,” they said.
The guideline also recommends screening for dyslipidemia and hypertension according to AAP guidelines and educating pediatric psoriasis patients about the risk of diabetes and regularly screening for diabetes and insulin resistance in those who are obese. Overweight children with psoriasis may be screened at the provider’s discretion, they wrote. Patients with signs of inflammatory bowel disease, which also is associated with psoriasis in adults, should be considered for referral to a gastroenterologist, they noted.
Children with psoriasis should be screened regularly for mental health conditions regardless of age, and they should be asked about substance abuse, according to the guideline, and those with concerns should be referred for additional assessment and management.
The guideline divides treatment of psoriasis in children into three categories: topical, phototherapy and photochemotherapy, and systemic treatments (nonbiologic or biologic).
For topicals, the guideline recommendations include corticosteroids as an off-label therapy, as well as ultra-high-potency topical corticosteroids as monotherapy. Overall, “selection of a therapeutic routine (potency, delivery vehicle, frequency of application) should take into account sites of involvement, type and thickness of psoriasis, age of the patient, total BSA of application, anticipated occlusion, and disease acuity, among other patient-, disease-, and drug-related factors,” the authors wrote. Other topical options included in the recommendations: calcineurin inhibitors, topical vitamin D analogues, tazarotene (off label), anthralin, and coal tar.
Phototherapy has a history of use in psoriasis treatment and remains part of the current recommendations, although data in children are limited, and data on the use of phototherapy for pustular psoriasis in children are insufficient to make specific treatment and dosing recommendations, the work group noted. The researchers also noted that in-office phototherapy may not be feasible for many patients, but that in-home ultraviolet light equipment or natural sunlight in moderation could be recommended as an alternative.
The use of systemic, nonbiologic treatments for pediatric psoriasis should be “based on baseline severity of disease, subtype of psoriasis, speed of disease progression, lack of response to more conservative therapies such as topical agents and phototherapy (when appropriate), impaired physical or psychological functioning or [quality of life] due to disease extent, and the presence of comorbidities such as PsA,” the workgroup said.
Options for systemic treatment include methotrexate, cyclosporine (notably for pustular as well as plaque and erythrodermic psoriasis), and systemic retinoids. In addition, fumaric acid esters may be an option for children with moderate to severe psoriasis, with recommended clinical and laboratory monitoring.
The increasing safety and efficacy data on biologics in pediatric psoriasis patients support their consideration among first-line systemic treatments, the work group suggested. “Etanercept and ustekinumab are now [Food and Drug Administration] approved for patients with psoriasis 4 years and older and 12 years and older, respectively,” they said, and infliximab and adalimumab have been used off label in children.
The work group concluded that research and knowledge gaps about pediatric psoriasis persist and include mechanism of disease onset, development of comorbidities, and identification of ideal dosing for various treatments.
Finally, the work group emphasized the importance of collaboration between dermatologists and primary care providers for managing psoriasis in children, as well as the importance of patient education.
“Dermatologists should be mindful of the unique aspects of the emotional development of children and the social dynamics of having a visible difference,” they wrote. “Shared decision making with the patient (if age appropriate) and the caregivers is a useful approach, particularly as related to the use of off-label medications to treat severe disease,” they said.
“This is the first time that pediatric psoriasis has been discussed as an independent topic within the guideline,” said one of the guideline authors, Dawn M.R. Davis, MD, of the Mayo Clinic, Rochester, Minn., in an interview. “Children have unique physiology and psychosocial aspects to their care relative to adults. In addition, psoriasis has some clinical manifestations that are oftentimes distinctly seen in children,” she commented. “Creation of a guideline specific to children allows us to summarize the similarities and differences of disease presentation and management. It also allows an opportunity to clarify what research data (especially therapeutics) have been studied in children and their uses, safety profiles, and dosing,” she noted.
Psoriasis can be a psychosocially debilitating disease, she emphasized. “In children, for example, isolated or prominent facial involvement is common, which can be embarrassing and impact relationships.”
The take-home message for clinicians, Dr. Davis said, is to keep in mind the multisystemic nature of psoriasis. “It is not limited to the skin,” she said. “Treating a patient with psoriasis necessitates practicing whole-person care” and considering the multiple comorbidities that impact quality of life and overall health in children, as well as adults with psoriasis, she commented. “Dermatologists can empower patients and their caregivers by educating them on the multifocal, complex nature of the disease.” She added, “We have much to learn regarding psoriasis in the pediatric population. More research into therapeutics, topical and systemic, is necessary to optimize patient care.”
The guideline was based on studies published in the PubMed and MEDLINE databases from January 2011 through December 31, 2017.
Dr. Menter and Craig A. Elmets, MD, professor of dermatology, at the University of Alabama, Birmingham, were cochairs of the work group. The pediatric guideline is the latest in a multipart series of AAD-NPF guidelines on psoriasis being published this year in the Journal of the American Academy of Dermatology.
Many of the guideline authors, including lead author Dr. Menter, disclosed relationships with multiple companies; however, a minimum 51% of workgroup members had no relevant conflicts of interest in accordance with AAD policy. There was no funding source. Dr. Davis disclosed serving as an investigator for Regeneron, with no compensation.
SOURCE: Menter et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2019.08.049.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Observational secukinumab data reflect clinical trial results in patients with moderate to severe psoriasis
A in a report published in the Journal of the European Academy of Dermatology and Venereology.
“The safety profile of secukinumab was similar to that reported in previous clinical trials, and no new or unexpected safety signals were observed,” according to Diamant Thaci, MD, of the Comprehensive Centre of Inflammation Medicine, University of Lübeck (Germany) and coauthors. Moreover, effectiveness in those who started treatment with secukinumab at baseline, they added, “was comparable to that observed in Phase 3 trials. High levels of effectiveness were observed also in subjects who had received previous biologic therapies, although the response rates were numerically lower, as might be expected in a difficult to treat population. In addition, lower baseline PASI [Psoriasis Area and Severity Index] in patients with prior biologic treatment could also reduce the relative decrease in PASI observed over the course of the study.”
They reported on an interim analysis of the first 1,988 patients enrolled in the PROSPECT study, an observational 24-week study conducted in Germany; 1,323 patients completed the 24 week study; total cumulative exposure to secukinumab was 746.3 patient-years. Their mean baseline PASI was 17.7, slightly lower than those in typical clinical trials, and most (91%) had received systemic therapies before.
Almost half the patients (46%) experienced an adverse event during treatment, and about 4% experienced a serious adverse event; only 1% of serious adverse events were considered related to the study drug. About 7% discontinued treatment with secukinumab because of an adverse event. The most common reasons for discontinuation were lack of benefit in 2.4%, psoriasis in 2.3%, and upper respiratory tract viral infection in 0.5%.
The most common adverse events were nasopharyngitis (8.7%), pruritus (2.9%), and headache (2.4%). Rates of neoplastic disorders and major cerebrovascular events were similar to published data, with 5 patients (0.3%) experiencing a major adverse cardiovascular event and 10 (0.5%) experiencing a malignancy. Four patients (0.2%) developed inflammatory bowel disease, 42 (2.1%) developed Candida infection, 2 (0.1%) developed hepatotoxicity, and 11 (0.6%) an injection-site reaction. There were three deaths, determined not to be related to secukinumab, the authors wrote.
Efficacy was also similar to that observed in earlier studies, they noted, with positive results regardless of concomitant medication. Overall, 44% of the cohort used concomitant medications.
Of the 829 patients using concomitant topical treatments, 73% had started before baseline. In all, 110 patients were also using conventional systemic medications and phototherapy; 77 started treatment before baseline. The most commonly employed concomitant therapies were topical steroids and phototherapy.
Overall, most patients (86%) achieved a PASI 75 by week 24, with 68.5% achieving a PASI 90, and 40% achieving a PASI 100 at that time point.
Secukinumab was most effective among the 83 patients who were naive to systemic therapies; in these patients, results at week 24 were as follows: PASI 75, 93%; PASI 90, 84%; and PASI 100, 66%. Among patients who had previously received a biologic, scores were slightly lower: PASI 75, 78%; PASI 90, 55%; and PASI 100, 29%.
“These interim data from PROSPECT confirm the effectiveness and safety of secukinumab in the routine clinical setting, in a large cohort of psoriasis patients with high disease severity,” the investigators concluded.
Initially approved in the United States in 2015, secukinumab, an interleukin-17A antagonist, is indicated for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy, adults with psoriatic arthritis, and adults with active ankylosing spondylitis.
The study was funded by Novartis, Germany; four authors are employees of the company. Dr. Thaci has served as an investigator and/or consultant for multiple pharmaceutical companies, including Novartis, AbbVie, Amgen, Arena, Biogen Idec, Boehringer Ingelheim, and Celgene. Other authors also disclosed serving as investigators, consultants, and/or speakers for Novartis and other companies.
SOURCE: J Eur Acad Dermatol Venereol. 2019 Sep 21. doi: 10.1111/jdv.15962.
A in a report published in the Journal of the European Academy of Dermatology and Venereology.
“The safety profile of secukinumab was similar to that reported in previous clinical trials, and no new or unexpected safety signals were observed,” according to Diamant Thaci, MD, of the Comprehensive Centre of Inflammation Medicine, University of Lübeck (Germany) and coauthors. Moreover, effectiveness in those who started treatment with secukinumab at baseline, they added, “was comparable to that observed in Phase 3 trials. High levels of effectiveness were observed also in subjects who had received previous biologic therapies, although the response rates were numerically lower, as might be expected in a difficult to treat population. In addition, lower baseline PASI [Psoriasis Area and Severity Index] in patients with prior biologic treatment could also reduce the relative decrease in PASI observed over the course of the study.”
They reported on an interim analysis of the first 1,988 patients enrolled in the PROSPECT study, an observational 24-week study conducted in Germany; 1,323 patients completed the 24 week study; total cumulative exposure to secukinumab was 746.3 patient-years. Their mean baseline PASI was 17.7, slightly lower than those in typical clinical trials, and most (91%) had received systemic therapies before.
Almost half the patients (46%) experienced an adverse event during treatment, and about 4% experienced a serious adverse event; only 1% of serious adverse events were considered related to the study drug. About 7% discontinued treatment with secukinumab because of an adverse event. The most common reasons for discontinuation were lack of benefit in 2.4%, psoriasis in 2.3%, and upper respiratory tract viral infection in 0.5%.
The most common adverse events were nasopharyngitis (8.7%), pruritus (2.9%), and headache (2.4%). Rates of neoplastic disorders and major cerebrovascular events were similar to published data, with 5 patients (0.3%) experiencing a major adverse cardiovascular event and 10 (0.5%) experiencing a malignancy. Four patients (0.2%) developed inflammatory bowel disease, 42 (2.1%) developed Candida infection, 2 (0.1%) developed hepatotoxicity, and 11 (0.6%) an injection-site reaction. There were three deaths, determined not to be related to secukinumab, the authors wrote.
Efficacy was also similar to that observed in earlier studies, they noted, with positive results regardless of concomitant medication. Overall, 44% of the cohort used concomitant medications.
Of the 829 patients using concomitant topical treatments, 73% had started before baseline. In all, 110 patients were also using conventional systemic medications and phototherapy; 77 started treatment before baseline. The most commonly employed concomitant therapies were topical steroids and phototherapy.
Overall, most patients (86%) achieved a PASI 75 by week 24, with 68.5% achieving a PASI 90, and 40% achieving a PASI 100 at that time point.
Secukinumab was most effective among the 83 patients who were naive to systemic therapies; in these patients, results at week 24 were as follows: PASI 75, 93%; PASI 90, 84%; and PASI 100, 66%. Among patients who had previously received a biologic, scores were slightly lower: PASI 75, 78%; PASI 90, 55%; and PASI 100, 29%.
“These interim data from PROSPECT confirm the effectiveness and safety of secukinumab in the routine clinical setting, in a large cohort of psoriasis patients with high disease severity,” the investigators concluded.
Initially approved in the United States in 2015, secukinumab, an interleukin-17A antagonist, is indicated for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy, adults with psoriatic arthritis, and adults with active ankylosing spondylitis.
The study was funded by Novartis, Germany; four authors are employees of the company. Dr. Thaci has served as an investigator and/or consultant for multiple pharmaceutical companies, including Novartis, AbbVie, Amgen, Arena, Biogen Idec, Boehringer Ingelheim, and Celgene. Other authors also disclosed serving as investigators, consultants, and/or speakers for Novartis and other companies.
SOURCE: J Eur Acad Dermatol Venereol. 2019 Sep 21. doi: 10.1111/jdv.15962.
A in a report published in the Journal of the European Academy of Dermatology and Venereology.
“The safety profile of secukinumab was similar to that reported in previous clinical trials, and no new or unexpected safety signals were observed,” according to Diamant Thaci, MD, of the Comprehensive Centre of Inflammation Medicine, University of Lübeck (Germany) and coauthors. Moreover, effectiveness in those who started treatment with secukinumab at baseline, they added, “was comparable to that observed in Phase 3 trials. High levels of effectiveness were observed also in subjects who had received previous biologic therapies, although the response rates were numerically lower, as might be expected in a difficult to treat population. In addition, lower baseline PASI [Psoriasis Area and Severity Index] in patients with prior biologic treatment could also reduce the relative decrease in PASI observed over the course of the study.”
They reported on an interim analysis of the first 1,988 patients enrolled in the PROSPECT study, an observational 24-week study conducted in Germany; 1,323 patients completed the 24 week study; total cumulative exposure to secukinumab was 746.3 patient-years. Their mean baseline PASI was 17.7, slightly lower than those in typical clinical trials, and most (91%) had received systemic therapies before.
Almost half the patients (46%) experienced an adverse event during treatment, and about 4% experienced a serious adverse event; only 1% of serious adverse events were considered related to the study drug. About 7% discontinued treatment with secukinumab because of an adverse event. The most common reasons for discontinuation were lack of benefit in 2.4%, psoriasis in 2.3%, and upper respiratory tract viral infection in 0.5%.
The most common adverse events were nasopharyngitis (8.7%), pruritus (2.9%), and headache (2.4%). Rates of neoplastic disorders and major cerebrovascular events were similar to published data, with 5 patients (0.3%) experiencing a major adverse cardiovascular event and 10 (0.5%) experiencing a malignancy. Four patients (0.2%) developed inflammatory bowel disease, 42 (2.1%) developed Candida infection, 2 (0.1%) developed hepatotoxicity, and 11 (0.6%) an injection-site reaction. There were three deaths, determined not to be related to secukinumab, the authors wrote.
Efficacy was also similar to that observed in earlier studies, they noted, with positive results regardless of concomitant medication. Overall, 44% of the cohort used concomitant medications.
Of the 829 patients using concomitant topical treatments, 73% had started before baseline. In all, 110 patients were also using conventional systemic medications and phototherapy; 77 started treatment before baseline. The most commonly employed concomitant therapies were topical steroids and phototherapy.
Overall, most patients (86%) achieved a PASI 75 by week 24, with 68.5% achieving a PASI 90, and 40% achieving a PASI 100 at that time point.
Secukinumab was most effective among the 83 patients who were naive to systemic therapies; in these patients, results at week 24 were as follows: PASI 75, 93%; PASI 90, 84%; and PASI 100, 66%. Among patients who had previously received a biologic, scores were slightly lower: PASI 75, 78%; PASI 90, 55%; and PASI 100, 29%.
“These interim data from PROSPECT confirm the effectiveness and safety of secukinumab in the routine clinical setting, in a large cohort of psoriasis patients with high disease severity,” the investigators concluded.
Initially approved in the United States in 2015, secukinumab, an interleukin-17A antagonist, is indicated for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy, adults with psoriatic arthritis, and adults with active ankylosing spondylitis.
The study was funded by Novartis, Germany; four authors are employees of the company. Dr. Thaci has served as an investigator and/or consultant for multiple pharmaceutical companies, including Novartis, AbbVie, Amgen, Arena, Biogen Idec, Boehringer Ingelheim, and Celgene. Other authors also disclosed serving as investigators, consultants, and/or speakers for Novartis and other companies.
SOURCE: J Eur Acad Dermatol Venereol. 2019 Sep 21. doi: 10.1111/jdv.15962.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY