User login
Serum interleukin-36 alpha: A potential biomarker to differentiate PsA from Behçet’s syndrome
Key clinical point: Patients with psoriatic arthritis (PsA) and those with Behçet’s syndrome (BS) had significantly elevated levels of serum interleukin-36 alpha (IL-36α), although the extent was lesser in BS, highlighting the potential role of the serum IL-36α level in differential diagnosis between PsA and BS.
Major finding: The median serum IL-36α level in patients with BS (201.7 pg/mL) was significantly higher than that in control individuals (16.9 pg/mL; P < .001) but lower than that in patients with PsA (544 pg/mL; P < .001). An empirical cut-off level of 420.6 pg/mL for IL-36α showed a specificity of 0.93 and sensitivity of 0.70 to distinguish patients with PsA from those with BS.
Study details: The data come from a cross-sectional study including patients with PsA (n = 80) and BS (n = 90) and control individuals without immune-mediated inflammatory disease (n = 80) who were assessed for serum IL-36α levels.
Disclosures: This study did not receive any external funding. The authors declared no conflicts of interest.
Source: Bettiol A et al. Serum interleukin-36 α as a candidate biomarker to distinguish Behçet’s syndrome and psoriatic arthritis. Int J Mol Sci. 2023;24:8817 (May 16). doi: 10.3390/ijms24108817
Key clinical point: Patients with psoriatic arthritis (PsA) and those with Behçet’s syndrome (BS) had significantly elevated levels of serum interleukin-36 alpha (IL-36α), although the extent was lesser in BS, highlighting the potential role of the serum IL-36α level in differential diagnosis between PsA and BS.
Major finding: The median serum IL-36α level in patients with BS (201.7 pg/mL) was significantly higher than that in control individuals (16.9 pg/mL; P < .001) but lower than that in patients with PsA (544 pg/mL; P < .001). An empirical cut-off level of 420.6 pg/mL for IL-36α showed a specificity of 0.93 and sensitivity of 0.70 to distinguish patients with PsA from those with BS.
Study details: The data come from a cross-sectional study including patients with PsA (n = 80) and BS (n = 90) and control individuals without immune-mediated inflammatory disease (n = 80) who were assessed for serum IL-36α levels.
Disclosures: This study did not receive any external funding. The authors declared no conflicts of interest.
Source: Bettiol A et al. Serum interleukin-36 α as a candidate biomarker to distinguish Behçet’s syndrome and psoriatic arthritis. Int J Mol Sci. 2023;24:8817 (May 16). doi: 10.3390/ijms24108817
Key clinical point: Patients with psoriatic arthritis (PsA) and those with Behçet’s syndrome (BS) had significantly elevated levels of serum interleukin-36 alpha (IL-36α), although the extent was lesser in BS, highlighting the potential role of the serum IL-36α level in differential diagnosis between PsA and BS.
Major finding: The median serum IL-36α level in patients with BS (201.7 pg/mL) was significantly higher than that in control individuals (16.9 pg/mL; P < .001) but lower than that in patients with PsA (544 pg/mL; P < .001). An empirical cut-off level of 420.6 pg/mL for IL-36α showed a specificity of 0.93 and sensitivity of 0.70 to distinguish patients with PsA from those with BS.
Study details: The data come from a cross-sectional study including patients with PsA (n = 80) and BS (n = 90) and control individuals without immune-mediated inflammatory disease (n = 80) who were assessed for serum IL-36α levels.
Disclosures: This study did not receive any external funding. The authors declared no conflicts of interest.
Source: Bettiol A et al. Serum interleukin-36 α as a candidate biomarker to distinguish Behçet’s syndrome and psoriatic arthritis. Int J Mol Sci. 2023;24:8817 (May 16). doi: 10.3390/ijms24108817
No clinically meaningful difference in response to ustekinumab in younger vs older patients with PsA
Key clinical point: No clinically meaningful treatment-related differences were observed in the efficacy, safety, and treatment persistence of ustekinumab over 3 years in younger (<60 years) and older (≥60 years) patients with psoriatic arthritis (PsA).
Major finding: At 6 months, 51.7% and 43.8% of patients aged <60 and ≥60 years achieved clinical Disease Activity Index for Psoriatic Arthritis low disease activity, respectively, with the efficacy being maintained through 36 months. The proportions of patients reporting at least one (32.7% vs 40.9%) and serious (5.3% vs 9.6%) adverse events and treatment persistence were not significantly different among patients age < 60 vs ≥ 60 years.
Study details: This post hoc analysis of the PsABio trial included patients with PsA who received ustekinumab and were subgrouped into those age < 60 years (n = 336) and ≥ 60 years (n = 103).
Disclosures: This study was sponsored by Janssen. Six authors declared being current or former employees of Janssen or shareholders of Johnson & Johnson. Three authors reported ties with various sources, including Janssen.
Source: Gossec L et al. Response to treatment in psoriatic arthritis, the effect of age: analysis of patients receiving ustekinumab in the PsABio real-world study. Arthritis Res Ther. 2023;25:100 (Jun 9). doi: 10.1186/s13075-023-03078-8
Key clinical point: No clinically meaningful treatment-related differences were observed in the efficacy, safety, and treatment persistence of ustekinumab over 3 years in younger (<60 years) and older (≥60 years) patients with psoriatic arthritis (PsA).
Major finding: At 6 months, 51.7% and 43.8% of patients aged <60 and ≥60 years achieved clinical Disease Activity Index for Psoriatic Arthritis low disease activity, respectively, with the efficacy being maintained through 36 months. The proportions of patients reporting at least one (32.7% vs 40.9%) and serious (5.3% vs 9.6%) adverse events and treatment persistence were not significantly different among patients age < 60 vs ≥ 60 years.
Study details: This post hoc analysis of the PsABio trial included patients with PsA who received ustekinumab and were subgrouped into those age < 60 years (n = 336) and ≥ 60 years (n = 103).
Disclosures: This study was sponsored by Janssen. Six authors declared being current or former employees of Janssen or shareholders of Johnson & Johnson. Three authors reported ties with various sources, including Janssen.
Source: Gossec L et al. Response to treatment in psoriatic arthritis, the effect of age: analysis of patients receiving ustekinumab in the PsABio real-world study. Arthritis Res Ther. 2023;25:100 (Jun 9). doi: 10.1186/s13075-023-03078-8
Key clinical point: No clinically meaningful treatment-related differences were observed in the efficacy, safety, and treatment persistence of ustekinumab over 3 years in younger (<60 years) and older (≥60 years) patients with psoriatic arthritis (PsA).
Major finding: At 6 months, 51.7% and 43.8% of patients aged <60 and ≥60 years achieved clinical Disease Activity Index for Psoriatic Arthritis low disease activity, respectively, with the efficacy being maintained through 36 months. The proportions of patients reporting at least one (32.7% vs 40.9%) and serious (5.3% vs 9.6%) adverse events and treatment persistence were not significantly different among patients age < 60 vs ≥ 60 years.
Study details: This post hoc analysis of the PsABio trial included patients with PsA who received ustekinumab and were subgrouped into those age < 60 years (n = 336) and ≥ 60 years (n = 103).
Disclosures: This study was sponsored by Janssen. Six authors declared being current or former employees of Janssen or shareholders of Johnson & Johnson. Three authors reported ties with various sources, including Janssen.
Source: Gossec L et al. Response to treatment in psoriatic arthritis, the effect of age: analysis of patients receiving ustekinumab in the PsABio real-world study. Arthritis Res Ther. 2023;25:100 (Jun 9). doi: 10.1186/s13075-023-03078-8
Apremilast significantly improves dactylitis and enthesitis in PsA
Key clinical point: Apremilast led to a significant improvement in enthesitis and dactylitis activity among patients with psoriatic arthritis (PsA) presenting with enthesitis and dactylitis phenotypes, with more than one-third of patients achieving remission after 1 year of treatment.
Major finding: After 6 and 12 months of apremilast treatment, remission was achieved by 25% and 34% of patients with enthesitis and 47% and 44% of patients with dactylitis, respectively, with significant improvements in the Leeds Enthesitis and Dactylitis Indexes (P < .001).
Study details: Findings are from a retrospective study including patients with PsA who presented with either enthesitis (n = 118) or dactylitis (n = 96) phenotype and received apremilast.
Disclosures: This study received no external funding. The authors declared no conflicts of interest.
Source: Lo Gullo A et al. Therapeutic effects of apremilast on enthesitis and dactylitis in real clinical setting: An Italian multicenter study. J Clin Med. 2023;12:3892 (Jun 7). doi: 10.3390/jcm12123892
Key clinical point: Apremilast led to a significant improvement in enthesitis and dactylitis activity among patients with psoriatic arthritis (PsA) presenting with enthesitis and dactylitis phenotypes, with more than one-third of patients achieving remission after 1 year of treatment.
Major finding: After 6 and 12 months of apremilast treatment, remission was achieved by 25% and 34% of patients with enthesitis and 47% and 44% of patients with dactylitis, respectively, with significant improvements in the Leeds Enthesitis and Dactylitis Indexes (P < .001).
Study details: Findings are from a retrospective study including patients with PsA who presented with either enthesitis (n = 118) or dactylitis (n = 96) phenotype and received apremilast.
Disclosures: This study received no external funding. The authors declared no conflicts of interest.
Source: Lo Gullo A et al. Therapeutic effects of apremilast on enthesitis and dactylitis in real clinical setting: An Italian multicenter study. J Clin Med. 2023;12:3892 (Jun 7). doi: 10.3390/jcm12123892
Key clinical point: Apremilast led to a significant improvement in enthesitis and dactylitis activity among patients with psoriatic arthritis (PsA) presenting with enthesitis and dactylitis phenotypes, with more than one-third of patients achieving remission after 1 year of treatment.
Major finding: After 6 and 12 months of apremilast treatment, remission was achieved by 25% and 34% of patients with enthesitis and 47% and 44% of patients with dactylitis, respectively, with significant improvements in the Leeds Enthesitis and Dactylitis Indexes (P < .001).
Study details: Findings are from a retrospective study including patients with PsA who presented with either enthesitis (n = 118) or dactylitis (n = 96) phenotype and received apremilast.
Disclosures: This study received no external funding. The authors declared no conflicts of interest.
Source: Lo Gullo A et al. Therapeutic effects of apremilast on enthesitis and dactylitis in real clinical setting: An Italian multicenter study. J Clin Med. 2023;12:3892 (Jun 7). doi: 10.3390/jcm12123892
Axial spondyloarthritis and PsA with axial involvement are distinct entities
Key clinical point: Axial spondyloarthritis (axSpA) with or without concomitant psoriasis and axial psoriatic arthritis (PsA) appear distinct entities based on marked demographic, clinical, and genetic differences.
Major finding: Patients with axial PsA vs axSpA with or without psoriasis were older at symptom onset (48.6 vs 44.7 or 41.4 years, respectively; P < .001), had a higher prevalence of dactylitis (43.2% vs 18.3% or 8.4%, respectively; P < .001) and peripheral arthritis (86.7% vs 58.1% or 44.3%, respectively; P < .001), and were less frequently HLA-B27 positive (22.3% vs 55.4% or 65.5%, respectively; P < .001).
Study details: This study included 5208 patients with axSpA (with or without psoriasis) and 2771 with PsA (axial or peripheral arthritis) from the Swiss Clinical Quality Management (SCQM) registry.
Disclosures: This study was funded by Eli Lilly. Two authors declared being employees of SCQM with salary partly financed by Eli Lilly. Several authors declared receiving honoraria, speaking or consulting fees, research grants, or other financial support from various sources, including Lilly and other SCQM supporters. Two authors declared no conflicts of interest.
Source: Ciurea A et al. Characterisation of patients with axial psoriatic arthritis and patients with axial spondyloarthritis and concomitant psoriasis in the SCQM registry. RMD Open. 2023;9:e002956 (Jun 5). doi: 10.1136/rmdopen-2022-002956
Key clinical point: Axial spondyloarthritis (axSpA) with or without concomitant psoriasis and axial psoriatic arthritis (PsA) appear distinct entities based on marked demographic, clinical, and genetic differences.
Major finding: Patients with axial PsA vs axSpA with or without psoriasis were older at symptom onset (48.6 vs 44.7 or 41.4 years, respectively; P < .001), had a higher prevalence of dactylitis (43.2% vs 18.3% or 8.4%, respectively; P < .001) and peripheral arthritis (86.7% vs 58.1% or 44.3%, respectively; P < .001), and were less frequently HLA-B27 positive (22.3% vs 55.4% or 65.5%, respectively; P < .001).
Study details: This study included 5208 patients with axSpA (with or without psoriasis) and 2771 with PsA (axial or peripheral arthritis) from the Swiss Clinical Quality Management (SCQM) registry.
Disclosures: This study was funded by Eli Lilly. Two authors declared being employees of SCQM with salary partly financed by Eli Lilly. Several authors declared receiving honoraria, speaking or consulting fees, research grants, or other financial support from various sources, including Lilly and other SCQM supporters. Two authors declared no conflicts of interest.
Source: Ciurea A et al. Characterisation of patients with axial psoriatic arthritis and patients with axial spondyloarthritis and concomitant psoriasis in the SCQM registry. RMD Open. 2023;9:e002956 (Jun 5). doi: 10.1136/rmdopen-2022-002956
Key clinical point: Axial spondyloarthritis (axSpA) with or without concomitant psoriasis and axial psoriatic arthritis (PsA) appear distinct entities based on marked demographic, clinical, and genetic differences.
Major finding: Patients with axial PsA vs axSpA with or without psoriasis were older at symptom onset (48.6 vs 44.7 or 41.4 years, respectively; P < .001), had a higher prevalence of dactylitis (43.2% vs 18.3% or 8.4%, respectively; P < .001) and peripheral arthritis (86.7% vs 58.1% or 44.3%, respectively; P < .001), and were less frequently HLA-B27 positive (22.3% vs 55.4% or 65.5%, respectively; P < .001).
Study details: This study included 5208 patients with axSpA (with or without psoriasis) and 2771 with PsA (axial or peripheral arthritis) from the Swiss Clinical Quality Management (SCQM) registry.
Disclosures: This study was funded by Eli Lilly. Two authors declared being employees of SCQM with salary partly financed by Eli Lilly. Several authors declared receiving honoraria, speaking or consulting fees, research grants, or other financial support from various sources, including Lilly and other SCQM supporters. Two authors declared no conflicts of interest.
Source: Ciurea A et al. Characterisation of patients with axial psoriatic arthritis and patients with axial spondyloarthritis and concomitant psoriasis in the SCQM registry. RMD Open. 2023;9:e002956 (Jun 5). doi: 10.1136/rmdopen-2022-002956
Etanercept safe and effective in juvenile psoriatic arthritis
Key clinical point: Etanercept was safe and effective with low rates of adverse events and led to better clinical outcomes in children with juvenile psoriatic arthritis (JPsA).
Major finding: The overall incidence of adverse events of special interest and serious adverse events were low and included 3 cases of uveitis (incidence rate [IR]/100 person-years 0.55; 95% CI 0.18-1.69), 1 of neuropathy (IR/100 person-years 0.18; 95% CI 0.03-1.29), and 1 of malignancy (IR/100 person-years 0.13; 95% CI 0.02-0.90). The American College of Rheumatology provisional criteria for inactive disease were achieved by 51.9% and 43.8% of patients at 6- and 12-month follow-ups.
Study details: This study included 226 patients with JPsA (aged ≥2 to <18 years) who received etanercept.
Disclosures: This study was sponsored by Immunex, a wholly owned subsidiary of Amgen Inc. S Stryker and D Collier declared being employees of and owning stocks in Amgen. SJ Balevic and T Beukelman declared receiving grants or research support, honoraria, or consulting fees or participating in data safety monitoring boards for various sources. The other authors declared no conflicts of interest.
Source: Correll CK et al. Occurrence of adverse events and change in disease activity after initiation of etanercept in paediatric patients with juvenile psoriatic arthritis in the CARRA Registry. RMD Open. 2023;9:e002943 (May 25). doi: 10.1136/rmdopen-2022-002943
Key clinical point: Etanercept was safe and effective with low rates of adverse events and led to better clinical outcomes in children with juvenile psoriatic arthritis (JPsA).
Major finding: The overall incidence of adverse events of special interest and serious adverse events were low and included 3 cases of uveitis (incidence rate [IR]/100 person-years 0.55; 95% CI 0.18-1.69), 1 of neuropathy (IR/100 person-years 0.18; 95% CI 0.03-1.29), and 1 of malignancy (IR/100 person-years 0.13; 95% CI 0.02-0.90). The American College of Rheumatology provisional criteria for inactive disease were achieved by 51.9% and 43.8% of patients at 6- and 12-month follow-ups.
Study details: This study included 226 patients with JPsA (aged ≥2 to <18 years) who received etanercept.
Disclosures: This study was sponsored by Immunex, a wholly owned subsidiary of Amgen Inc. S Stryker and D Collier declared being employees of and owning stocks in Amgen. SJ Balevic and T Beukelman declared receiving grants or research support, honoraria, or consulting fees or participating in data safety monitoring boards for various sources. The other authors declared no conflicts of interest.
Source: Correll CK et al. Occurrence of adverse events and change in disease activity after initiation of etanercept in paediatric patients with juvenile psoriatic arthritis in the CARRA Registry. RMD Open. 2023;9:e002943 (May 25). doi: 10.1136/rmdopen-2022-002943
Key clinical point: Etanercept was safe and effective with low rates of adverse events and led to better clinical outcomes in children with juvenile psoriatic arthritis (JPsA).
Major finding: The overall incidence of adverse events of special interest and serious adverse events were low and included 3 cases of uveitis (incidence rate [IR]/100 person-years 0.55; 95% CI 0.18-1.69), 1 of neuropathy (IR/100 person-years 0.18; 95% CI 0.03-1.29), and 1 of malignancy (IR/100 person-years 0.13; 95% CI 0.02-0.90). The American College of Rheumatology provisional criteria for inactive disease were achieved by 51.9% and 43.8% of patients at 6- and 12-month follow-ups.
Study details: This study included 226 patients with JPsA (aged ≥2 to <18 years) who received etanercept.
Disclosures: This study was sponsored by Immunex, a wholly owned subsidiary of Amgen Inc. S Stryker and D Collier declared being employees of and owning stocks in Amgen. SJ Balevic and T Beukelman declared receiving grants or research support, honoraria, or consulting fees or participating in data safety monitoring boards for various sources. The other authors declared no conflicts of interest.
Source: Correll CK et al. Occurrence of adverse events and change in disease activity after initiation of etanercept in paediatric patients with juvenile psoriatic arthritis in the CARRA Registry. RMD Open. 2023;9:e002943 (May 25). doi: 10.1136/rmdopen-2022-002943
Study supports position of methotrexate in treatment algorithm for PsA
Key clinical point: Patients with newly diagnosed psoriatic arthritis (PsA) and rheumatoid arthritis (RA) who initiated methotrexate showed similar rates of methotrexate retention; however, the addition of any other disease-modifying antirheumatic drugs (DMARD) to the treatment regimen was more rapid in RA vs PsA.
Major finding: Overall, 71% of patients with PsA and 76% of patients with RA remained on methotrexate at 2 years after initiating methotrexate. The risk for adding any other DMARD to the treatment regimen was greater in the RA vs PsA group (adjusted hazard ratio 0.86; 95% CI 0.77-0.96), with methotrexate monotherapy improving disease activity in both the groups.
Study details: This observational study included DMARD-naive patients with newly diagnosed PsA (n = 3642) who initiated methotrexate and matched comparator patients with RA (n = 3642).
Disclosures: This study was funded by grants from the Swedish Rheumatism Association and others. Some authors declared serving as consultants or receiving lecture fees, speakers’ bureau fees, or research support from various sources.
Source: Lindström U et al. Methotrexate treatment in early psoriatic arthritis in comparison to rheumatoid arthritis: An observational nationwide study. RMD Open. 2023;9:e002883 (May 12). doi: 10.1136/rmdopen-2022-002883
Key clinical point: Patients with newly diagnosed psoriatic arthritis (PsA) and rheumatoid arthritis (RA) who initiated methotrexate showed similar rates of methotrexate retention; however, the addition of any other disease-modifying antirheumatic drugs (DMARD) to the treatment regimen was more rapid in RA vs PsA.
Major finding: Overall, 71% of patients with PsA and 76% of patients with RA remained on methotrexate at 2 years after initiating methotrexate. The risk for adding any other DMARD to the treatment regimen was greater in the RA vs PsA group (adjusted hazard ratio 0.86; 95% CI 0.77-0.96), with methotrexate monotherapy improving disease activity in both the groups.
Study details: This observational study included DMARD-naive patients with newly diagnosed PsA (n = 3642) who initiated methotrexate and matched comparator patients with RA (n = 3642).
Disclosures: This study was funded by grants from the Swedish Rheumatism Association and others. Some authors declared serving as consultants or receiving lecture fees, speakers’ bureau fees, or research support from various sources.
Source: Lindström U et al. Methotrexate treatment in early psoriatic arthritis in comparison to rheumatoid arthritis: An observational nationwide study. RMD Open. 2023;9:e002883 (May 12). doi: 10.1136/rmdopen-2022-002883
Key clinical point: Patients with newly diagnosed psoriatic arthritis (PsA) and rheumatoid arthritis (RA) who initiated methotrexate showed similar rates of methotrexate retention; however, the addition of any other disease-modifying antirheumatic drugs (DMARD) to the treatment regimen was more rapid in RA vs PsA.
Major finding: Overall, 71% of patients with PsA and 76% of patients with RA remained on methotrexate at 2 years after initiating methotrexate. The risk for adding any other DMARD to the treatment regimen was greater in the RA vs PsA group (adjusted hazard ratio 0.86; 95% CI 0.77-0.96), with methotrexate monotherapy improving disease activity in both the groups.
Study details: This observational study included DMARD-naive patients with newly diagnosed PsA (n = 3642) who initiated methotrexate and matched comparator patients with RA (n = 3642).
Disclosures: This study was funded by grants from the Swedish Rheumatism Association and others. Some authors declared serving as consultants or receiving lecture fees, speakers’ bureau fees, or research support from various sources.
Source: Lindström U et al. Methotrexate treatment in early psoriatic arthritis in comparison to rheumatoid arthritis: An observational nationwide study. RMD Open. 2023;9:e002883 (May 12). doi: 10.1136/rmdopen-2022-002883
Brepocitinib shows promise in phase 2 trial for psoriatic arthritis
Key clinical point: Brepocitinib, the tyrosine kinase 2/Janus kinase 1 inhibitor, was superior to placebo in reducing signs and symptoms of psoriatic arthritis (PsA) and was well-tolerated throughout the 52-week study period.
Major finding: At week 16, American College of Rheumatology 20 response was achieved by a significantly higher proportion of patients receiving brepocitinib at doses of 30 mg (66.7%; P = .0197) and 60 mg (74.6%; P = .0006) compared with placebo (43.3%), with the response being maintained through week 52. Overall, 12 serious adverse events were reported in the brepocitinib arms (30 and 60 mg) by week 52. No deaths were reported.
Study details: Findings are from a phase 2b, dose-ranging, parallel treatment group trial including 218 patients with active PsA who were randomly assigned to receive either brepocitinib (60, 30, or 10 mg once daily) or placebo.
Disclosures: This study was sponsored by Pfizer Inc. Several authors declared being current or former employees and shareholders of Pfizer. The other authors reported ties with various sources, including Pfizer.
Source: Mease P et al. Efficacy and safety of tyrosine kinase 2/Janus kinase 1 Inhibitor brepocitinib for active psoriatic arthritis: A phase IIb randomized controlled trial. Arthritis Rheumatol. 2023 (May 17). doi: 10.1002/art.42519
Key clinical point: Brepocitinib, the tyrosine kinase 2/Janus kinase 1 inhibitor, was superior to placebo in reducing signs and symptoms of psoriatic arthritis (PsA) and was well-tolerated throughout the 52-week study period.
Major finding: At week 16, American College of Rheumatology 20 response was achieved by a significantly higher proportion of patients receiving brepocitinib at doses of 30 mg (66.7%; P = .0197) and 60 mg (74.6%; P = .0006) compared with placebo (43.3%), with the response being maintained through week 52. Overall, 12 serious adverse events were reported in the brepocitinib arms (30 and 60 mg) by week 52. No deaths were reported.
Study details: Findings are from a phase 2b, dose-ranging, parallel treatment group trial including 218 patients with active PsA who were randomly assigned to receive either brepocitinib (60, 30, or 10 mg once daily) or placebo.
Disclosures: This study was sponsored by Pfizer Inc. Several authors declared being current or former employees and shareholders of Pfizer. The other authors reported ties with various sources, including Pfizer.
Source: Mease P et al. Efficacy and safety of tyrosine kinase 2/Janus kinase 1 Inhibitor brepocitinib for active psoriatic arthritis: A phase IIb randomized controlled trial. Arthritis Rheumatol. 2023 (May 17). doi: 10.1002/art.42519
Key clinical point: Brepocitinib, the tyrosine kinase 2/Janus kinase 1 inhibitor, was superior to placebo in reducing signs and symptoms of psoriatic arthritis (PsA) and was well-tolerated throughout the 52-week study period.
Major finding: At week 16, American College of Rheumatology 20 response was achieved by a significantly higher proportion of patients receiving brepocitinib at doses of 30 mg (66.7%; P = .0197) and 60 mg (74.6%; P = .0006) compared with placebo (43.3%), with the response being maintained through week 52. Overall, 12 serious adverse events were reported in the brepocitinib arms (30 and 60 mg) by week 52. No deaths were reported.
Study details: Findings are from a phase 2b, dose-ranging, parallel treatment group trial including 218 patients with active PsA who were randomly assigned to receive either brepocitinib (60, 30, or 10 mg once daily) or placebo.
Disclosures: This study was sponsored by Pfizer Inc. Several authors declared being current or former employees and shareholders of Pfizer. The other authors reported ties with various sources, including Pfizer.
Source: Mease P et al. Efficacy and safety of tyrosine kinase 2/Janus kinase 1 Inhibitor brepocitinib for active psoriatic arthritis: A phase IIb randomized controlled trial. Arthritis Rheumatol. 2023 (May 17). doi: 10.1002/art.42519
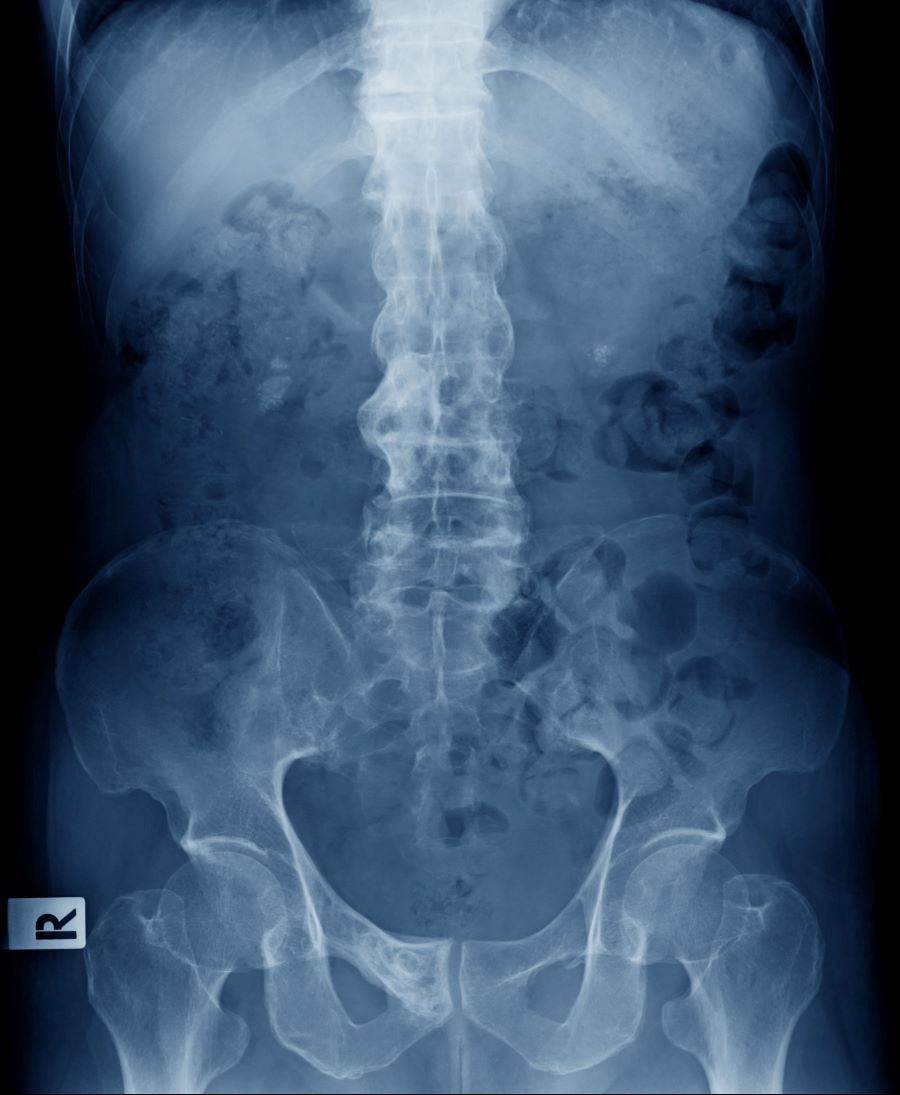
Intermittent pain and stiffness
The history and findings in this case are consistent with a diagnosis of psoriatic spondylitis.
Psoriatic spondylitis is a form of psoriatic arthritis (PsA) that affects the spine and the joints in the pelvis (axial involvement). PsA is a chronic, heterogeneous condition that affects approximately 25%-30% of patients with psoriasis, particularly those with severe psoriasis or nail or scalp involvement. It is characterized by musculoskeletal inflammation (arthritis, enthesitis, spondylitis, and dactylitis). PsA is a spondyloarthritis that can be found either in the peripheral or axial skeleton. If not treated, it may result in permanent joint damage and loss of function.
Patients with PsA may present with nail and skin changes, peripheral arthritis, enthesitis, dactylitis, and axial spondyloarthritis (SpA), either alone or in combination. Common symptoms of axial involvement in PsA include morning back/neck stiffness that lasts longer than 30 minutes, neck or back pain that improves with activity and worsens after prolonged inactivity, and diminished mobility. PsA affects men and women equally, and typically develops when patients are between 30 and 50 years of age. As with psoriasis, PsA is associated with numerous comorbidities, such as cardiovascular disease, metabolic syndrome, obesity, diabetes, depression, uveitis, and anxiety.
The diagnosis of psoriatic spondylitis is confirmed by physical examination and imaging. Axial PsA characteristics, including sacroiliitis and spondylitis, are distinguished by the development of syndesmophytes (ie, ossification of the annulus fibrosus). Useful imaging tools for evaluating patients with PsA include plain radiography, CT, ultrasound, and MRI. Although MRI and ultrasound may be more sensitive than plain radiography for detecting early joint inflammation and damage and axial changes, including sacroiliitis, they are not mandatory for a diagnosis of PsA to be made.
International guidelines have been developed by the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), the European Alliance of Associations for Rheumatology (EULAR), and the Assessment of Spondyloarthritis International Society to guide the treatment of axial PsA. The goals of treatment include minimizing pain, stiffness, and fatigue; improving and preserving spinal flexibility and posture; improving functional capacity; and maintaining the ability to work, with a target of remission or minimal/low disease activity.
Treatment options for symptomatic relief include nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and sacroiliac joint injections with glucocorticoids for mild disease; long-term treatment with systemic glucocorticoids is not recommended. If patients remain symptomatic or have erosive disease or other indications of high disease activity, guidelines recommend initiation of a tumor necrosis factor (TNF) inhibitor (eg, adalimumab, etanercept, infliximab, golimumab, certolizumab pegol). Disease-modifying antirheumatic drugs (eg, methotrexate) are not routinely prescribed for patients with axial disease because they have not been shown to be effective. In patients with significant skin involvement, treatment with interleukin-17A inhibitors may be preferred to TNF inhibitors.
If patients have an inadequate response to a first trial of a TNF inhibitor, guidelines recommend trying a second TNF inhibitor before switching to a different class of biologic. For patients who do not respond to TNF inhibitors, a Janus kinase inhibitor (tofacitinib) may be considered. Additionally, nonpharmacologic therapies (eg, exercise, physical therapy, massage therapy, occupational therapy, acupuncture) are recommended for all patients with active PsA.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are consistent with a diagnosis of psoriatic spondylitis.
Psoriatic spondylitis is a form of psoriatic arthritis (PsA) that affects the spine and the joints in the pelvis (axial involvement). PsA is a chronic, heterogeneous condition that affects approximately 25%-30% of patients with psoriasis, particularly those with severe psoriasis or nail or scalp involvement. It is characterized by musculoskeletal inflammation (arthritis, enthesitis, spondylitis, and dactylitis). PsA is a spondyloarthritis that can be found either in the peripheral or axial skeleton. If not treated, it may result in permanent joint damage and loss of function.
Patients with PsA may present with nail and skin changes, peripheral arthritis, enthesitis, dactylitis, and axial spondyloarthritis (SpA), either alone or in combination. Common symptoms of axial involvement in PsA include morning back/neck stiffness that lasts longer than 30 minutes, neck or back pain that improves with activity and worsens after prolonged inactivity, and diminished mobility. PsA affects men and women equally, and typically develops when patients are between 30 and 50 years of age. As with psoriasis, PsA is associated with numerous comorbidities, such as cardiovascular disease, metabolic syndrome, obesity, diabetes, depression, uveitis, and anxiety.
The diagnosis of psoriatic spondylitis is confirmed by physical examination and imaging. Axial PsA characteristics, including sacroiliitis and spondylitis, are distinguished by the development of syndesmophytes (ie, ossification of the annulus fibrosus). Useful imaging tools for evaluating patients with PsA include plain radiography, CT, ultrasound, and MRI. Although MRI and ultrasound may be more sensitive than plain radiography for detecting early joint inflammation and damage and axial changes, including sacroiliitis, they are not mandatory for a diagnosis of PsA to be made.
International guidelines have been developed by the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), the European Alliance of Associations for Rheumatology (EULAR), and the Assessment of Spondyloarthritis International Society to guide the treatment of axial PsA. The goals of treatment include minimizing pain, stiffness, and fatigue; improving and preserving spinal flexibility and posture; improving functional capacity; and maintaining the ability to work, with a target of remission or minimal/low disease activity.
Treatment options for symptomatic relief include nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and sacroiliac joint injections with glucocorticoids for mild disease; long-term treatment with systemic glucocorticoids is not recommended. If patients remain symptomatic or have erosive disease or other indications of high disease activity, guidelines recommend initiation of a tumor necrosis factor (TNF) inhibitor (eg, adalimumab, etanercept, infliximab, golimumab, certolizumab pegol). Disease-modifying antirheumatic drugs (eg, methotrexate) are not routinely prescribed for patients with axial disease because they have not been shown to be effective. In patients with significant skin involvement, treatment with interleukin-17A inhibitors may be preferred to TNF inhibitors.
If patients have an inadequate response to a first trial of a TNF inhibitor, guidelines recommend trying a second TNF inhibitor before switching to a different class of biologic. For patients who do not respond to TNF inhibitors, a Janus kinase inhibitor (tofacitinib) may be considered. Additionally, nonpharmacologic therapies (eg, exercise, physical therapy, massage therapy, occupational therapy, acupuncture) are recommended for all patients with active PsA.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are consistent with a diagnosis of psoriatic spondylitis.
Psoriatic spondylitis is a form of psoriatic arthritis (PsA) that affects the spine and the joints in the pelvis (axial involvement). PsA is a chronic, heterogeneous condition that affects approximately 25%-30% of patients with psoriasis, particularly those with severe psoriasis or nail or scalp involvement. It is characterized by musculoskeletal inflammation (arthritis, enthesitis, spondylitis, and dactylitis). PsA is a spondyloarthritis that can be found either in the peripheral or axial skeleton. If not treated, it may result in permanent joint damage and loss of function.
Patients with PsA may present with nail and skin changes, peripheral arthritis, enthesitis, dactylitis, and axial spondyloarthritis (SpA), either alone or in combination. Common symptoms of axial involvement in PsA include morning back/neck stiffness that lasts longer than 30 minutes, neck or back pain that improves with activity and worsens after prolonged inactivity, and diminished mobility. PsA affects men and women equally, and typically develops when patients are between 30 and 50 years of age. As with psoriasis, PsA is associated with numerous comorbidities, such as cardiovascular disease, metabolic syndrome, obesity, diabetes, depression, uveitis, and anxiety.
The diagnosis of psoriatic spondylitis is confirmed by physical examination and imaging. Axial PsA characteristics, including sacroiliitis and spondylitis, are distinguished by the development of syndesmophytes (ie, ossification of the annulus fibrosus). Useful imaging tools for evaluating patients with PsA include plain radiography, CT, ultrasound, and MRI. Although MRI and ultrasound may be more sensitive than plain radiography for detecting early joint inflammation and damage and axial changes, including sacroiliitis, they are not mandatory for a diagnosis of PsA to be made.
International guidelines have been developed by the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), the European Alliance of Associations for Rheumatology (EULAR), and the Assessment of Spondyloarthritis International Society to guide the treatment of axial PsA. The goals of treatment include minimizing pain, stiffness, and fatigue; improving and preserving spinal flexibility and posture; improving functional capacity; and maintaining the ability to work, with a target of remission or minimal/low disease activity.
Treatment options for symptomatic relief include nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and sacroiliac joint injections with glucocorticoids for mild disease; long-term treatment with systemic glucocorticoids is not recommended. If patients remain symptomatic or have erosive disease or other indications of high disease activity, guidelines recommend initiation of a tumor necrosis factor (TNF) inhibitor (eg, adalimumab, etanercept, infliximab, golimumab, certolizumab pegol). Disease-modifying antirheumatic drugs (eg, methotrexate) are not routinely prescribed for patients with axial disease because they have not been shown to be effective. In patients with significant skin involvement, treatment with interleukin-17A inhibitors may be preferred to TNF inhibitors.
If patients have an inadequate response to a first trial of a TNF inhibitor, guidelines recommend trying a second TNF inhibitor before switching to a different class of biologic. For patients who do not respond to TNF inhibitors, a Janus kinase inhibitor (tofacitinib) may be considered. Additionally, nonpharmacologic therapies (eg, exercise, physical therapy, massage therapy, occupational therapy, acupuncture) are recommended for all patients with active PsA.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 41-year-old man with a 5-year history of moderate to severe scalp psoriasis presents with complaints of intermittent pain and stiffness in his left hip and lower back of approximately 6 months' duration. The patient states that his back pain has been severe enough to wake him up on several occasions. Treatment with over-the-counter ibuprofen is moderately effective at relieving his pain. He also reports morning back stiffness that improves with motion, usually within an hour of awakening. The patient reports no fever, pain, swelling, or worsening of his scalp psoriasis. He is not aware of any injury or other triggering factor for his back pain. He takes an over-the-counter multivitamin daily and treats his scalp psoriasis with fluocinolone acetonide 0.01% oil. The patient is 5 ft 9 in and weighs 176 lb (BMI 26).
Physical examination reveals tenderness in the lumbar spine and associated decreased range of motion, as well as psoriatic plaques on the scalp. Vital signs are within normal ranges. Pertinent laboratory findings include erythrocyte sedimentation rate of 19 mm/h and C-reactive protein of 10 mg/L. Rheumatoid factor, antinuclear antibody, and anti-cyclic citrullinated peptide antibody were negative. Radiographic findings include sacroiliitis and bulky nonmarginal syndesmophytes.
High-intensity interval training has sustainable effects in patients with inflammatory arthritis
MILAN – High-intensity interval training (HIIT) has been shown to enhance cardiorespiratory fitness (CRF) and mitigate cardiovascular disease (CVD) risk factors in patients with inflammatory joint diseases (IJD) in a randomized trial. Notably, the positive response in CRF did not coincide with changes in pain or fatigue.
Kristine Norden, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases, Norwegian National Advisory Unit on Rehabilitation in Rheumatology, Diakonhjemmet Hospital, Oslo, presented the late-breaking results of the ExeHeart trial at the annual European Congress of Rheumatology. The trial aimed to evaluate the short- and long-term effects of 12 weeks of supervised HIIT in patients with IJD.
Ms. Norden said in an interview that “HIIT is a feasible physiotherapeutic intervention with sustainable effects in patients with IJD. It does not exacerbate symptoms of IJD and can be implemented in primary care settings.”
The trial
The ExeHeart trial is a randomized controlled trial designed to assess the effects of HIIT on CRF, CVD risk, and disease activity in patients with IJD. The trial is a collaborative effort with patient research partners and aligns with patients’ requests for effective nonpharmacologic treatments. The outcomes being evaluated include CRF (primary outcome), CVD risk factors, anthropometric measures, disease activity, and patient-reported outcomes related to pain, fatigue, disease, physical activity, and exercise.
A total of 60 patients with IJD were recruited from the Preventive Cardio-Rheuma clinic at Diakonhjemmet. They were randomly assigned to receive either standard care (including relevant lifestyle advice and cardiopreventive medication) or standard care along with a 12-week HIIT intervention supervised by physiotherapists. Assessments were conducted at baseline, at 3 months (primary endpoint), and at 6 months post baseline. There was no supervised intervention between the 3- and 6-month time points.
The median age of the participants was 59 years, with 34 participants (57%) being women. The types of IJD among the participants included rheumatoid arthritis in 45%, spondyloarthritis in 32%, and psoriatic arthritis in 23%. Furthermore, 49 patients (82%) had a high risk for CVD.
The participants were divided into two groups: a control group (n = 30) and a HIIT group (n = 30). The HIIT group underwent a 12-week intervention consisting of twice-a-week supervised 4x4-minute HIIT sessions at 90%-95% of peak heart rate, alternated with moderate activity at 70%. The control group engaged in unsupervised moderate-intensity exercise sessions. The primary outcome measured was the change in CRF, assessed through peak oxygen uptake (VO2 max) using a cardiopulmonary exercise test. Secondary outcomes – pain and fatigue – were evaluated using a questionnaire (Numeric Rating Scale 0-10, where 0 represents no pain or fatigue).
Following HIIT, a statistically significant difference was observed in VO2 max (2.5 mL/kg per min; P < .01) in favor of the exercise group at 3 months, while no significant differences were found in pain and fatigue. This discrepancy in VO2 max between the groups was maintained at 6 months (2.6 mL/kg per min; P < .01), with no notable disparities in pain and fatigue. A per-protocol analysis at 3 months demonstrated a difference in VO2 max between the groups (3.2 mL/kg per min; P < .01).
Ms. Norden concluded that the clinical implications of these findings are significant, as increased CRF achieved through HIIT reflects an improvement in the body’s ability to deliver oxygen to working muscles. Consequently, this enhancement in CRF can lead to overall health improvements and a reduced risk for CVD.
Long-lasting effects
Christopher Edwards, MBBS, MD, honorary consultant rheumatologist at University Hospital Southampton (England) NHS Foundation Trust Medicine, University of Southampton, was concerned about future maintenance of increased CRF. “I really wish we had data on these patients at 12 months as well, so we could see if the effects last even longer. Regarding intensity, there are clear indications that engaging in moderate and high-intensity workouts is more beneficial,” Dr. Norden said. “So, I would certainly recommend at least one high-intensity exercise session per week for those patients, while also incorporating lower and moderate-intensity exercises if desired. However, for individuals aiming to maximize their oxygen uptake, high-intensity exercise is considered the most effective approach.”
There is compelling evidence supporting the benefits of physical activity in improving disease activity among patients with IJD, making it a critical component of nonpharmacologic treatment. However, individuals with rheumatic and musculoskeletal conditions generally exhibit lower levels of physical activity, compared with their healthy counterparts. Recognizing the importance of CVD prevention in patients with IJD, EULAR recommends routine CVD screening for individuals diagnosed with IJD.
Ms. Norden and coauthors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MILAN – High-intensity interval training (HIIT) has been shown to enhance cardiorespiratory fitness (CRF) and mitigate cardiovascular disease (CVD) risk factors in patients with inflammatory joint diseases (IJD) in a randomized trial. Notably, the positive response in CRF did not coincide with changes in pain or fatigue.
Kristine Norden, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases, Norwegian National Advisory Unit on Rehabilitation in Rheumatology, Diakonhjemmet Hospital, Oslo, presented the late-breaking results of the ExeHeart trial at the annual European Congress of Rheumatology. The trial aimed to evaluate the short- and long-term effects of 12 weeks of supervised HIIT in patients with IJD.
Ms. Norden said in an interview that “HIIT is a feasible physiotherapeutic intervention with sustainable effects in patients with IJD. It does not exacerbate symptoms of IJD and can be implemented in primary care settings.”
The trial
The ExeHeart trial is a randomized controlled trial designed to assess the effects of HIIT on CRF, CVD risk, and disease activity in patients with IJD. The trial is a collaborative effort with patient research partners and aligns with patients’ requests for effective nonpharmacologic treatments. The outcomes being evaluated include CRF (primary outcome), CVD risk factors, anthropometric measures, disease activity, and patient-reported outcomes related to pain, fatigue, disease, physical activity, and exercise.
A total of 60 patients with IJD were recruited from the Preventive Cardio-Rheuma clinic at Diakonhjemmet. They were randomly assigned to receive either standard care (including relevant lifestyle advice and cardiopreventive medication) or standard care along with a 12-week HIIT intervention supervised by physiotherapists. Assessments were conducted at baseline, at 3 months (primary endpoint), and at 6 months post baseline. There was no supervised intervention between the 3- and 6-month time points.
The median age of the participants was 59 years, with 34 participants (57%) being women. The types of IJD among the participants included rheumatoid arthritis in 45%, spondyloarthritis in 32%, and psoriatic arthritis in 23%. Furthermore, 49 patients (82%) had a high risk for CVD.
The participants were divided into two groups: a control group (n = 30) and a HIIT group (n = 30). The HIIT group underwent a 12-week intervention consisting of twice-a-week supervised 4x4-minute HIIT sessions at 90%-95% of peak heart rate, alternated with moderate activity at 70%. The control group engaged in unsupervised moderate-intensity exercise sessions. The primary outcome measured was the change in CRF, assessed through peak oxygen uptake (VO2 max) using a cardiopulmonary exercise test. Secondary outcomes – pain and fatigue – were evaluated using a questionnaire (Numeric Rating Scale 0-10, where 0 represents no pain or fatigue).
Following HIIT, a statistically significant difference was observed in VO2 max (2.5 mL/kg per min; P < .01) in favor of the exercise group at 3 months, while no significant differences were found in pain and fatigue. This discrepancy in VO2 max between the groups was maintained at 6 months (2.6 mL/kg per min; P < .01), with no notable disparities in pain and fatigue. A per-protocol analysis at 3 months demonstrated a difference in VO2 max between the groups (3.2 mL/kg per min; P < .01).
Ms. Norden concluded that the clinical implications of these findings are significant, as increased CRF achieved through HIIT reflects an improvement in the body’s ability to deliver oxygen to working muscles. Consequently, this enhancement in CRF can lead to overall health improvements and a reduced risk for CVD.
Long-lasting effects
Christopher Edwards, MBBS, MD, honorary consultant rheumatologist at University Hospital Southampton (England) NHS Foundation Trust Medicine, University of Southampton, was concerned about future maintenance of increased CRF. “I really wish we had data on these patients at 12 months as well, so we could see if the effects last even longer. Regarding intensity, there are clear indications that engaging in moderate and high-intensity workouts is more beneficial,” Dr. Norden said. “So, I would certainly recommend at least one high-intensity exercise session per week for those patients, while also incorporating lower and moderate-intensity exercises if desired. However, for individuals aiming to maximize their oxygen uptake, high-intensity exercise is considered the most effective approach.”
There is compelling evidence supporting the benefits of physical activity in improving disease activity among patients with IJD, making it a critical component of nonpharmacologic treatment. However, individuals with rheumatic and musculoskeletal conditions generally exhibit lower levels of physical activity, compared with their healthy counterparts. Recognizing the importance of CVD prevention in patients with IJD, EULAR recommends routine CVD screening for individuals diagnosed with IJD.
Ms. Norden and coauthors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MILAN – High-intensity interval training (HIIT) has been shown to enhance cardiorespiratory fitness (CRF) and mitigate cardiovascular disease (CVD) risk factors in patients with inflammatory joint diseases (IJD) in a randomized trial. Notably, the positive response in CRF did not coincide with changes in pain or fatigue.
Kristine Norden, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases, Norwegian National Advisory Unit on Rehabilitation in Rheumatology, Diakonhjemmet Hospital, Oslo, presented the late-breaking results of the ExeHeart trial at the annual European Congress of Rheumatology. The trial aimed to evaluate the short- and long-term effects of 12 weeks of supervised HIIT in patients with IJD.
Ms. Norden said in an interview that “HIIT is a feasible physiotherapeutic intervention with sustainable effects in patients with IJD. It does not exacerbate symptoms of IJD and can be implemented in primary care settings.”
The trial
The ExeHeart trial is a randomized controlled trial designed to assess the effects of HIIT on CRF, CVD risk, and disease activity in patients with IJD. The trial is a collaborative effort with patient research partners and aligns with patients’ requests for effective nonpharmacologic treatments. The outcomes being evaluated include CRF (primary outcome), CVD risk factors, anthropometric measures, disease activity, and patient-reported outcomes related to pain, fatigue, disease, physical activity, and exercise.
A total of 60 patients with IJD were recruited from the Preventive Cardio-Rheuma clinic at Diakonhjemmet. They were randomly assigned to receive either standard care (including relevant lifestyle advice and cardiopreventive medication) or standard care along with a 12-week HIIT intervention supervised by physiotherapists. Assessments were conducted at baseline, at 3 months (primary endpoint), and at 6 months post baseline. There was no supervised intervention between the 3- and 6-month time points.
The median age of the participants was 59 years, with 34 participants (57%) being women. The types of IJD among the participants included rheumatoid arthritis in 45%, spondyloarthritis in 32%, and psoriatic arthritis in 23%. Furthermore, 49 patients (82%) had a high risk for CVD.
The participants were divided into two groups: a control group (n = 30) and a HIIT group (n = 30). The HIIT group underwent a 12-week intervention consisting of twice-a-week supervised 4x4-minute HIIT sessions at 90%-95% of peak heart rate, alternated with moderate activity at 70%. The control group engaged in unsupervised moderate-intensity exercise sessions. The primary outcome measured was the change in CRF, assessed through peak oxygen uptake (VO2 max) using a cardiopulmonary exercise test. Secondary outcomes – pain and fatigue – were evaluated using a questionnaire (Numeric Rating Scale 0-10, where 0 represents no pain or fatigue).
Following HIIT, a statistically significant difference was observed in VO2 max (2.5 mL/kg per min; P < .01) in favor of the exercise group at 3 months, while no significant differences were found in pain and fatigue. This discrepancy in VO2 max between the groups was maintained at 6 months (2.6 mL/kg per min; P < .01), with no notable disparities in pain and fatigue. A per-protocol analysis at 3 months demonstrated a difference in VO2 max between the groups (3.2 mL/kg per min; P < .01).
Ms. Norden concluded that the clinical implications of these findings are significant, as increased CRF achieved through HIIT reflects an improvement in the body’s ability to deliver oxygen to working muscles. Consequently, this enhancement in CRF can lead to overall health improvements and a reduced risk for CVD.
Long-lasting effects
Christopher Edwards, MBBS, MD, honorary consultant rheumatologist at University Hospital Southampton (England) NHS Foundation Trust Medicine, University of Southampton, was concerned about future maintenance of increased CRF. “I really wish we had data on these patients at 12 months as well, so we could see if the effects last even longer. Regarding intensity, there are clear indications that engaging in moderate and high-intensity workouts is more beneficial,” Dr. Norden said. “So, I would certainly recommend at least one high-intensity exercise session per week for those patients, while also incorporating lower and moderate-intensity exercises if desired. However, for individuals aiming to maximize their oxygen uptake, high-intensity exercise is considered the most effective approach.”
There is compelling evidence supporting the benefits of physical activity in improving disease activity among patients with IJD, making it a critical component of nonpharmacologic treatment. However, individuals with rheumatic and musculoskeletal conditions generally exhibit lower levels of physical activity, compared with their healthy counterparts. Recognizing the importance of CVD prevention in patients with IJD, EULAR recommends routine CVD screening for individuals diagnosed with IJD.
Ms. Norden and coauthors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EULAR 2023
Why not both? Dual biologics for treatment-resistant RA and PsA
The introduction of tumor necrosis factor (TNF) inhibitors in the late 1990s revolutionized treatment of rheumatic diseases, such as rheumatoid arthritis (RA) and psoriatic arthritis (PsA), providing patients with another treatment option when conventional therapies were ineffective. However, when these diseases don’t respond to anti-TNF therapy, it is still difficult to determine the next best course of action.
“One of the big challenges we have in treatment of psoriatic arthritis, and I would say rheumatoid arthritis was well, is how to handle patients who have failed their first biologic therapy,” Christopher T. Ritchlin, MD, MPH, professor of allergy, immunology, and rheumatology at the University of Rochester (N.Y.), told this news organization. “In the case of both RA and PsA, that’s quite frequently an anti-TNF agent.”
For an estimated 30% to 40% of patients, TNF inhibitor therapy is discontinued because of nonresponse or intolerance. Clinicians can switch to another biologic or targeted synthetic disease-modifying antirheumatic drug (DMARD) or add another conventional DMARD, such as methotrexate. Now, several case studies as well as promising findings from phase 2 clinical trials suggest that combining two biologics could be an alternative strategy to improve patient response to treatment. However, concerns about safety and higher costs remain.
Targeting multiple mechanisms of action
Rheumatic conditions affect multiple areas of the body and involve different signaling pathways, said Dr. Ritchlin, who heads the Clinical Immunology Research Unit at the University of Rochester. PsA, for example, affects the skin, peripheral joints, the axial skeleton, and the entheses.
“The question is, Are these various manifestations – of which multiple [ones] are often seen in one patient – likely to respond to one therapy that targets one single pathway?” he said.
Combination therapies have been effective in treating leukemia and lymphoma as well as infection with HIV, Melek Yalçin Mutlu, MD, and colleagues from Friedrich Alexander University Erlangen-Nuremberg and the University Clinic Erlangen (Germany), wrote in a review about combining biologic DMARDs in the treatment of RA and PsA. The review was published in Joint Bone Spine.
“Cumulative evidence on the success of combination therapies in various diseases supports an akin approach in rheumatology, and simultaneous or sequential blockade of multiple mechanisms that generate or propagate arthritis could theoretically enhance efficacy,” the authors wrote. “On the other hand, intervening on multiple targets in the immune system brings about a risk of adverse events, among which infection is a major concern.”
Failed clinical trials
Clinical trials of combination biologic therapies for rheumatic disease have been tried before, but these combinations did not show superior efficacy, and they increased patients’ risk for infection. One study published in 2004 compared monotherapy with the TNF inhibitor etanercept (Enbrel) to the combination of etanercept and anakinra (Kineret), an interleukin-1 (IL-1) antagonist, in 244 patients with active RA despite methotrexate therapy. Researchers found no statistically significant difference in achieving 20% improvement in modified American College of Rheumatology response criteria (ACR20), ACR50, or ACR70 between the groups that received etanercept and anakinra and those that received etanercept alone. There were nine serious infections among patients given etanercept and anakinra, including one death due to pneumonia. There were no serious infections in the etanercept monotherapy group.
In another RA trial, 121 patients were given etanercept 25 mg twice weekly and were randomly assigned to also receive a placebo or low-dose abatacept (Orencia), a T-cell co-stimulation inhibitor. There was no significant difference in disease improvement between the two groups, although the rate of serious adverse events was nearly six times higher in the etanercept-abatacept group (16.5% vs. 2.8%).
These studies had a “chilling effect on the whole field for some years,” Brian G. Feagan, MD, the senior scientific director of the gastrointestinal contract research firm Alimentiv in London, Ontario, told this news organization. People were reluctant to try new biologic combinations, owing to the fear that these safety issues would plague subsequent trials.
Promising combinations
But a recent phase 2 trial, led by Dr. Feagan, suggests that certain combinations can be effective. In the Janssen-sponsored VEGA trial, researchers found that a combination of guselkumab (Tremfya), an IL-23 inhibitor, and golimumab (Simponi), an anti-TNF agent, was more effective than either drug used as monotherapy for initial induction treatment for moderate to severe ulcerative colitis. Importantly, there was no difference in adverse events between any of the groups. This same combination therapy is now being tried for patients with active PsA in Janssen’s AFFINITY trial, for which Dr. Ritchlin is one of the lead investigators.
Other trials have also delivered promising results. One study enrolled 51 adults with active RA who were all receiving stable doses of both a TNF inhibitor – either etanercept or adalimumab (Humira) – and methotrexate. Patients were randomly assigned to receive one course of rituximab (Rituxan) or placebo. The researchers found that the safety profile of this TNF inhibitor/methotrexate/rituximab combination was “consistent” with the safety profiles of previous studies of methotrexate/rituximab dual combinations with no TNF inhibitor; there were no new safety signals. At 24 weeks, 30% of the group that received rituximab reached ACR20, compared with 17% of the group that was given placebo. Twelve percent of the rituximab group achieved ACR50, compared with 6% of the group that received placebo.
“B-cell depletion is fundamentally different from cytokine inhibition and even from co-stimulation blockade, making an additive effect more likely,” Dr. Mutlu and colleagues wrote in their review. Reports have also suggested possible benefits of combining a TNF inhibitor and an IL-17 inhibitor in the treatment of RA and PsA, as well as the combination of a TNF inhibitor and an IL-23 antagonist for PsA.
While these combinations require controlled clinical trials, “there’s some smoke signals out there that this might be an effective strategy for some patients,” Dr. Ritchlin said.
In addition to the AFFINITY trial, two clinical trials are underway in France. The first, CRI-RA, is evaluating the combination of baricitinib (Olumiant), a Janus kinase (JAK) inhibitor, and adalimumab. Although baricitinib is not a biologic, as a targeted synthetic DMARD, the therapy is more potent than conventional DMARDs, and the same potential safety concerns apply. However, use of a combination of tofacitinib (Xeljanz) and different biologics for RA patients has been reported; no serious side effects were reported over 11 months of therapy. The randomized, placebo-controlled trial began in July 2021 and will enroll 178 patients. The estimated study completion date is July 2025.
“Of note, baricitinib does not directly block signaling downstream of TNF, even if an indirect effect on TNF production is likely to occur,” the CRI-RA entry on clinicaltrials.gov reads. “Targeting multiple inflammatory cytokines in combination may lead to more effective treatment and enhanced clinical responses in patients with RA compared to the current second-line strategies.”
The second trial, SEQUENS-RA, is evaluating the use of TNF inhibitors followed by abatacept for patients with RA who test positive for anticitrullinated protein autoantibodies (ACPAs). In the past, the combination of a TNF inhibitor and abatacept did not lead to promising results, but in this trial, the drugs will be administered sequentially.
“Although abatacept has shown a very good tolerance profile that might be superior to other bDMARDs [biologic DMARDs], rheumatologists might be reluctant to use it as a first line bDMARD as there is a belief of a slower efficacy compared to other bDMARDs or JAK inhibitors,” according to the clinical trial’s description. “Investigators have hypothesized that first rapidly controlling the inflammation phase, using TNF inhibitors, followed by abatacept to induce an immunological remission, would optimize response and tolerance of ACPA-positive patients with RA.”
The randomized trial of 220 participants began in November 2022. The estimated completion date for the study is November 2025.
Finding the right patients
Though these studies have had some promising results, the difference in efficacy between biologic monotherapy and dual therapy has been mostly moderate, Dr. Mutlu and coauthors wrote. Identifying disease subtypes for which there might be a higher likelihood of response to dual biologic treatment, especially multidrug-resistant types, could improve efficacies in future trials, they argued. “The good effects of bDMARD combinations in resistant patients in fact point into this direction, though they were observed in uncontrolled studies,” the authors noted.
Insurance coverage remains a “huge challenge” for these dual therapies because of the higher expense, noted Dr. Ritchlin. Better targeting therapies could help convince these companies to pay for these therapies.
“I would say that if we were able to demonstrate a phenotype of a patient that would respond to biologics and not monotherapies, [then] many companies would be amenable to this kind of approach,” he said.
Dr. Ritchlin reports financial relationships with AbbVie, Bristol-Myers Squibb, Janssen, Pfizer, Eli Lilly, Novartis, and UCB. Dr. Feagan reports financial relationships with AbbVie, Amgen, Janssen, Pfizer, Takeda, and several other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
The introduction of tumor necrosis factor (TNF) inhibitors in the late 1990s revolutionized treatment of rheumatic diseases, such as rheumatoid arthritis (RA) and psoriatic arthritis (PsA), providing patients with another treatment option when conventional therapies were ineffective. However, when these diseases don’t respond to anti-TNF therapy, it is still difficult to determine the next best course of action.
“One of the big challenges we have in treatment of psoriatic arthritis, and I would say rheumatoid arthritis was well, is how to handle patients who have failed their first biologic therapy,” Christopher T. Ritchlin, MD, MPH, professor of allergy, immunology, and rheumatology at the University of Rochester (N.Y.), told this news organization. “In the case of both RA and PsA, that’s quite frequently an anti-TNF agent.”
For an estimated 30% to 40% of patients, TNF inhibitor therapy is discontinued because of nonresponse or intolerance. Clinicians can switch to another biologic or targeted synthetic disease-modifying antirheumatic drug (DMARD) or add another conventional DMARD, such as methotrexate. Now, several case studies as well as promising findings from phase 2 clinical trials suggest that combining two biologics could be an alternative strategy to improve patient response to treatment. However, concerns about safety and higher costs remain.
Targeting multiple mechanisms of action
Rheumatic conditions affect multiple areas of the body and involve different signaling pathways, said Dr. Ritchlin, who heads the Clinical Immunology Research Unit at the University of Rochester. PsA, for example, affects the skin, peripheral joints, the axial skeleton, and the entheses.
“The question is, Are these various manifestations – of which multiple [ones] are often seen in one patient – likely to respond to one therapy that targets one single pathway?” he said.
Combination therapies have been effective in treating leukemia and lymphoma as well as infection with HIV, Melek Yalçin Mutlu, MD, and colleagues from Friedrich Alexander University Erlangen-Nuremberg and the University Clinic Erlangen (Germany), wrote in a review about combining biologic DMARDs in the treatment of RA and PsA. The review was published in Joint Bone Spine.
“Cumulative evidence on the success of combination therapies in various diseases supports an akin approach in rheumatology, and simultaneous or sequential blockade of multiple mechanisms that generate or propagate arthritis could theoretically enhance efficacy,” the authors wrote. “On the other hand, intervening on multiple targets in the immune system brings about a risk of adverse events, among which infection is a major concern.”
Failed clinical trials
Clinical trials of combination biologic therapies for rheumatic disease have been tried before, but these combinations did not show superior efficacy, and they increased patients’ risk for infection. One study published in 2004 compared monotherapy with the TNF inhibitor etanercept (Enbrel) to the combination of etanercept and anakinra (Kineret), an interleukin-1 (IL-1) antagonist, in 244 patients with active RA despite methotrexate therapy. Researchers found no statistically significant difference in achieving 20% improvement in modified American College of Rheumatology response criteria (ACR20), ACR50, or ACR70 between the groups that received etanercept and anakinra and those that received etanercept alone. There were nine serious infections among patients given etanercept and anakinra, including one death due to pneumonia. There were no serious infections in the etanercept monotherapy group.
In another RA trial, 121 patients were given etanercept 25 mg twice weekly and were randomly assigned to also receive a placebo or low-dose abatacept (Orencia), a T-cell co-stimulation inhibitor. There was no significant difference in disease improvement between the two groups, although the rate of serious adverse events was nearly six times higher in the etanercept-abatacept group (16.5% vs. 2.8%).
These studies had a “chilling effect on the whole field for some years,” Brian G. Feagan, MD, the senior scientific director of the gastrointestinal contract research firm Alimentiv in London, Ontario, told this news organization. People were reluctant to try new biologic combinations, owing to the fear that these safety issues would plague subsequent trials.
Promising combinations
But a recent phase 2 trial, led by Dr. Feagan, suggests that certain combinations can be effective. In the Janssen-sponsored VEGA trial, researchers found that a combination of guselkumab (Tremfya), an IL-23 inhibitor, and golimumab (Simponi), an anti-TNF agent, was more effective than either drug used as monotherapy for initial induction treatment for moderate to severe ulcerative colitis. Importantly, there was no difference in adverse events between any of the groups. This same combination therapy is now being tried for patients with active PsA in Janssen’s AFFINITY trial, for which Dr. Ritchlin is one of the lead investigators.
Other trials have also delivered promising results. One study enrolled 51 adults with active RA who were all receiving stable doses of both a TNF inhibitor – either etanercept or adalimumab (Humira) – and methotrexate. Patients were randomly assigned to receive one course of rituximab (Rituxan) or placebo. The researchers found that the safety profile of this TNF inhibitor/methotrexate/rituximab combination was “consistent” with the safety profiles of previous studies of methotrexate/rituximab dual combinations with no TNF inhibitor; there were no new safety signals. At 24 weeks, 30% of the group that received rituximab reached ACR20, compared with 17% of the group that was given placebo. Twelve percent of the rituximab group achieved ACR50, compared with 6% of the group that received placebo.
“B-cell depletion is fundamentally different from cytokine inhibition and even from co-stimulation blockade, making an additive effect more likely,” Dr. Mutlu and colleagues wrote in their review. Reports have also suggested possible benefits of combining a TNF inhibitor and an IL-17 inhibitor in the treatment of RA and PsA, as well as the combination of a TNF inhibitor and an IL-23 antagonist for PsA.
While these combinations require controlled clinical trials, “there’s some smoke signals out there that this might be an effective strategy for some patients,” Dr. Ritchlin said.
In addition to the AFFINITY trial, two clinical trials are underway in France. The first, CRI-RA, is evaluating the combination of baricitinib (Olumiant), a Janus kinase (JAK) inhibitor, and adalimumab. Although baricitinib is not a biologic, as a targeted synthetic DMARD, the therapy is more potent than conventional DMARDs, and the same potential safety concerns apply. However, use of a combination of tofacitinib (Xeljanz) and different biologics for RA patients has been reported; no serious side effects were reported over 11 months of therapy. The randomized, placebo-controlled trial began in July 2021 and will enroll 178 patients. The estimated study completion date is July 2025.
“Of note, baricitinib does not directly block signaling downstream of TNF, even if an indirect effect on TNF production is likely to occur,” the CRI-RA entry on clinicaltrials.gov reads. “Targeting multiple inflammatory cytokines in combination may lead to more effective treatment and enhanced clinical responses in patients with RA compared to the current second-line strategies.”
The second trial, SEQUENS-RA, is evaluating the use of TNF inhibitors followed by abatacept for patients with RA who test positive for anticitrullinated protein autoantibodies (ACPAs). In the past, the combination of a TNF inhibitor and abatacept did not lead to promising results, but in this trial, the drugs will be administered sequentially.
“Although abatacept has shown a very good tolerance profile that might be superior to other bDMARDs [biologic DMARDs], rheumatologists might be reluctant to use it as a first line bDMARD as there is a belief of a slower efficacy compared to other bDMARDs or JAK inhibitors,” according to the clinical trial’s description. “Investigators have hypothesized that first rapidly controlling the inflammation phase, using TNF inhibitors, followed by abatacept to induce an immunological remission, would optimize response and tolerance of ACPA-positive patients with RA.”
The randomized trial of 220 participants began in November 2022. The estimated completion date for the study is November 2025.
Finding the right patients
Though these studies have had some promising results, the difference in efficacy between biologic monotherapy and dual therapy has been mostly moderate, Dr. Mutlu and coauthors wrote. Identifying disease subtypes for which there might be a higher likelihood of response to dual biologic treatment, especially multidrug-resistant types, could improve efficacies in future trials, they argued. “The good effects of bDMARD combinations in resistant patients in fact point into this direction, though they were observed in uncontrolled studies,” the authors noted.
Insurance coverage remains a “huge challenge” for these dual therapies because of the higher expense, noted Dr. Ritchlin. Better targeting therapies could help convince these companies to pay for these therapies.
“I would say that if we were able to demonstrate a phenotype of a patient that would respond to biologics and not monotherapies, [then] many companies would be amenable to this kind of approach,” he said.
Dr. Ritchlin reports financial relationships with AbbVie, Bristol-Myers Squibb, Janssen, Pfizer, Eli Lilly, Novartis, and UCB. Dr. Feagan reports financial relationships with AbbVie, Amgen, Janssen, Pfizer, Takeda, and several other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
The introduction of tumor necrosis factor (TNF) inhibitors in the late 1990s revolutionized treatment of rheumatic diseases, such as rheumatoid arthritis (RA) and psoriatic arthritis (PsA), providing patients with another treatment option when conventional therapies were ineffective. However, when these diseases don’t respond to anti-TNF therapy, it is still difficult to determine the next best course of action.
“One of the big challenges we have in treatment of psoriatic arthritis, and I would say rheumatoid arthritis was well, is how to handle patients who have failed their first biologic therapy,” Christopher T. Ritchlin, MD, MPH, professor of allergy, immunology, and rheumatology at the University of Rochester (N.Y.), told this news organization. “In the case of both RA and PsA, that’s quite frequently an anti-TNF agent.”
For an estimated 30% to 40% of patients, TNF inhibitor therapy is discontinued because of nonresponse or intolerance. Clinicians can switch to another biologic or targeted synthetic disease-modifying antirheumatic drug (DMARD) or add another conventional DMARD, such as methotrexate. Now, several case studies as well as promising findings from phase 2 clinical trials suggest that combining two biologics could be an alternative strategy to improve patient response to treatment. However, concerns about safety and higher costs remain.
Targeting multiple mechanisms of action
Rheumatic conditions affect multiple areas of the body and involve different signaling pathways, said Dr. Ritchlin, who heads the Clinical Immunology Research Unit at the University of Rochester. PsA, for example, affects the skin, peripheral joints, the axial skeleton, and the entheses.
“The question is, Are these various manifestations – of which multiple [ones] are often seen in one patient – likely to respond to one therapy that targets one single pathway?” he said.
Combination therapies have been effective in treating leukemia and lymphoma as well as infection with HIV, Melek Yalçin Mutlu, MD, and colleagues from Friedrich Alexander University Erlangen-Nuremberg and the University Clinic Erlangen (Germany), wrote in a review about combining biologic DMARDs in the treatment of RA and PsA. The review was published in Joint Bone Spine.
“Cumulative evidence on the success of combination therapies in various diseases supports an akin approach in rheumatology, and simultaneous or sequential blockade of multiple mechanisms that generate or propagate arthritis could theoretically enhance efficacy,” the authors wrote. “On the other hand, intervening on multiple targets in the immune system brings about a risk of adverse events, among which infection is a major concern.”
Failed clinical trials
Clinical trials of combination biologic therapies for rheumatic disease have been tried before, but these combinations did not show superior efficacy, and they increased patients’ risk for infection. One study published in 2004 compared monotherapy with the TNF inhibitor etanercept (Enbrel) to the combination of etanercept and anakinra (Kineret), an interleukin-1 (IL-1) antagonist, in 244 patients with active RA despite methotrexate therapy. Researchers found no statistically significant difference in achieving 20% improvement in modified American College of Rheumatology response criteria (ACR20), ACR50, or ACR70 between the groups that received etanercept and anakinra and those that received etanercept alone. There were nine serious infections among patients given etanercept and anakinra, including one death due to pneumonia. There were no serious infections in the etanercept monotherapy group.
In another RA trial, 121 patients were given etanercept 25 mg twice weekly and were randomly assigned to also receive a placebo or low-dose abatacept (Orencia), a T-cell co-stimulation inhibitor. There was no significant difference in disease improvement between the two groups, although the rate of serious adverse events was nearly six times higher in the etanercept-abatacept group (16.5% vs. 2.8%).
These studies had a “chilling effect on the whole field for some years,” Brian G. Feagan, MD, the senior scientific director of the gastrointestinal contract research firm Alimentiv in London, Ontario, told this news organization. People were reluctant to try new biologic combinations, owing to the fear that these safety issues would plague subsequent trials.
Promising combinations
But a recent phase 2 trial, led by Dr. Feagan, suggests that certain combinations can be effective. In the Janssen-sponsored VEGA trial, researchers found that a combination of guselkumab (Tremfya), an IL-23 inhibitor, and golimumab (Simponi), an anti-TNF agent, was more effective than either drug used as monotherapy for initial induction treatment for moderate to severe ulcerative colitis. Importantly, there was no difference in adverse events between any of the groups. This same combination therapy is now being tried for patients with active PsA in Janssen’s AFFINITY trial, for which Dr. Ritchlin is one of the lead investigators.
Other trials have also delivered promising results. One study enrolled 51 adults with active RA who were all receiving stable doses of both a TNF inhibitor – either etanercept or adalimumab (Humira) – and methotrexate. Patients were randomly assigned to receive one course of rituximab (Rituxan) or placebo. The researchers found that the safety profile of this TNF inhibitor/methotrexate/rituximab combination was “consistent” with the safety profiles of previous studies of methotrexate/rituximab dual combinations with no TNF inhibitor; there were no new safety signals. At 24 weeks, 30% of the group that received rituximab reached ACR20, compared with 17% of the group that was given placebo. Twelve percent of the rituximab group achieved ACR50, compared with 6% of the group that received placebo.
“B-cell depletion is fundamentally different from cytokine inhibition and even from co-stimulation blockade, making an additive effect more likely,” Dr. Mutlu and colleagues wrote in their review. Reports have also suggested possible benefits of combining a TNF inhibitor and an IL-17 inhibitor in the treatment of RA and PsA, as well as the combination of a TNF inhibitor and an IL-23 antagonist for PsA.
While these combinations require controlled clinical trials, “there’s some smoke signals out there that this might be an effective strategy for some patients,” Dr. Ritchlin said.
In addition to the AFFINITY trial, two clinical trials are underway in France. The first, CRI-RA, is evaluating the combination of baricitinib (Olumiant), a Janus kinase (JAK) inhibitor, and adalimumab. Although baricitinib is not a biologic, as a targeted synthetic DMARD, the therapy is more potent than conventional DMARDs, and the same potential safety concerns apply. However, use of a combination of tofacitinib (Xeljanz) and different biologics for RA patients has been reported; no serious side effects were reported over 11 months of therapy. The randomized, placebo-controlled trial began in July 2021 and will enroll 178 patients. The estimated study completion date is July 2025.
“Of note, baricitinib does not directly block signaling downstream of TNF, even if an indirect effect on TNF production is likely to occur,” the CRI-RA entry on clinicaltrials.gov reads. “Targeting multiple inflammatory cytokines in combination may lead to more effective treatment and enhanced clinical responses in patients with RA compared to the current second-line strategies.”
The second trial, SEQUENS-RA, is evaluating the use of TNF inhibitors followed by abatacept for patients with RA who test positive for anticitrullinated protein autoantibodies (ACPAs). In the past, the combination of a TNF inhibitor and abatacept did not lead to promising results, but in this trial, the drugs will be administered sequentially.
“Although abatacept has shown a very good tolerance profile that might be superior to other bDMARDs [biologic DMARDs], rheumatologists might be reluctant to use it as a first line bDMARD as there is a belief of a slower efficacy compared to other bDMARDs or JAK inhibitors,” according to the clinical trial’s description. “Investigators have hypothesized that first rapidly controlling the inflammation phase, using TNF inhibitors, followed by abatacept to induce an immunological remission, would optimize response and tolerance of ACPA-positive patients with RA.”
The randomized trial of 220 participants began in November 2022. The estimated completion date for the study is November 2025.
Finding the right patients
Though these studies have had some promising results, the difference in efficacy between biologic monotherapy and dual therapy has been mostly moderate, Dr. Mutlu and coauthors wrote. Identifying disease subtypes for which there might be a higher likelihood of response to dual biologic treatment, especially multidrug-resistant types, could improve efficacies in future trials, they argued. “The good effects of bDMARD combinations in resistant patients in fact point into this direction, though they were observed in uncontrolled studies,” the authors noted.
Insurance coverage remains a “huge challenge” for these dual therapies because of the higher expense, noted Dr. Ritchlin. Better targeting therapies could help convince these companies to pay for these therapies.
“I would say that if we were able to demonstrate a phenotype of a patient that would respond to biologics and not monotherapies, [then] many companies would be amenable to this kind of approach,” he said.
Dr. Ritchlin reports financial relationships with AbbVie, Bristol-Myers Squibb, Janssen, Pfizer, Eli Lilly, Novartis, and UCB. Dr. Feagan reports financial relationships with AbbVie, Amgen, Janssen, Pfizer, Takeda, and several other pharmaceutical companies.
A version of this article first appeared on Medscape.com.