User login
How does psoriasis affect fertility and birth outcomes?
in a U.K. cohort study.
Those are key findings from what is believed to be one of the largest studies to investigate fertility and obstetric outcomes in patients with psoriasis.
“Studies that have examined fertility and pregnancy outcomes in women with psoriasis have reported conflicting findings,” lead author Teng-Chou Chen, PhD, of the Centre for Pharmacoepidemiology and Drug Safety at the University of Manchester (England), and colleagues from the Global Psoriasis Atlas wrote in the study, published in JAMA Dermatology. Most of the studies were small, with under 100 women, “and are thus likely underpowered to detect a difference in pregnancy outcomes. The majority of those studies used disease registry data or lacked a matched comparison group and hence were unable to estimate the association of fertility and adverse pregnancy outcomes in women with psoriasis when compared with the general population.”
To determine fertility rates and birth outcomes in female patients with psoriasis, compared with age- and practice-matched patients without psoriasis, the researchers evaluated EHR data from a large U.K. primary care database, the Clinical Practice Research Datalink GOLD, from 1998 to 2019. They limited the analysis to patients aged 15-44 years and used relevant codes from clinical consultations to identify those with psoriasis. Then, for each patient with psoriasis, the researchers selected five comparators without psoriasis from the same primary care practice and matched for year of birth.
Both sets of patients were followed from the index date to age 45 years, death, transfer out of practice, last date of data collection, or end of the study period (Dec. 31, 2019), whichever came first. Pregnancy records were extracted for both sets of patients, and birth outcomes were categorized as pregnancy loss, live birth, stillbirth, and preterm birth. Adverse pregnancy outcomes were also collected. Finally, Dr. Chen and colleagues used a negative binomial model to examine the association between psoriasis and the fertility rate, and they applied logistic regression to compare the association between psoriasis and obstetric outcomes.
The analysis included 63,681 patients with psoriasis and 318,405 comparators whose median age on the index date was 30 years and who were followed for a median of 4.1 years. Among patients with psoriasis, 5.1% met criteria for moderate to severe disease in the follow-up period. The researchers observed that, compared with their age- and practice-matched counterparts, patients with psoriasis were more likely to be current smokers, alcohol drinkers, or overweight on the index date. They were also more often diagnosed with diabetes, hypertension, inflammatory bowel disease, thyroid disorders, and respiratory diseases such as asthma and chronic obstructive pulmonary disease.
Fertility, birth outcomes
When they looked at fertility outcomes, the researchers found that, compared with their matched peers without psoriasis, those with psoriasis had higher rates of fertility (risk ratio, 1.30; 95% confidence interval, 1.27-1.33; P < .001). But after the researchers stratified patients based on psoriasis severity, those with moderate to severe disease had significantly lower rates of fertility (RR, 0.75; 95% CI, 0.69-0.83; P < .001), compared those who did not have psoriasis.
As for adverse birth outcomes, compared with their matched comparators, pregnancies in patients with psoriasis were less likely to end in a live birth (odds ratio, 0.91; 95% CI, 0.88-0.93; P < .001). They also had a higher risk of pregnancy loss (OR, 1.06; 95% CI, 1.03-1.10; P < .001), most during the first trimester, at a gestation period of under 91 days.
In addition to psoriasis, patients younger than age 20 (OR, 2.04; 95% CI, 1.94-2.15; P < .011) and those aged between 20 and 24 years (OR, 1.35; 95% CI, 1.31-1.40; P < .001) had a higher risk of pregnancy loss, compared with those aged between 25 and 34 years.
However, no increases in the risks of antenatal hemorrhage, preeclampsia, or gestational diabetes were observed in patients with psoriasis, and no statistically significant differences in the odds of stillbirth and preterm birth were found between patients with psoriasis and matched comparators who did not have psoriasis.
“The mechanism to link the higher risk of pregnancy loss in patients with psoriasis is not clear, but there might be potential explanations,” the researchers wrote. “Psoriasis is characterized by the increased activity of [interleukin]-17, IL-23, and tumor necrosis factor–alpha. Those proinflammatory cytokines may negatively affect the placenta and cause impaired fetal growth.”
They recommended that further studies “evaluate the effects of better management of psoriasis and close monitoring during pregnancy on pregnancy loss.” In particular, “patients with psoriasis were more likely to have comorbidities that may be related to poor pregnancy outcomes, and hence increased emphasis of managing comorbidities as part of the routine management plan is also warranted.”
Asked to comment on the study, Alexa B. Kimball, MD, MPH, who has been involved with research on this topic, said that she and other investigators had observed some years ago that fertility rates for women with moderate to severe psoriasis might be lower than expected.
This trend was observed in some psoriasis registries, some pregnancy registries, and in clinical practice, Dr. Kimball, professor of dermatology at Harvard Medical School, Boston, said in an interview. “This study clearly demonstrates that lower fertility rates in the moderate to severe psoriasis population occurs and compels further exploration of the reason why.” The reasons could be biologic, she continued, including difficulty conceiving or an increased risk of miscarriage, sociobehavioral issues, or a combination.
“Behavioral examples could include that some women with moderate to severe psoriasis can flare during pregnancy, which might affect their choice” to become pregnant, Dr. Kimball said. “Stigma may also play a role in how women with moderate to severe psoriasis form relationships. Now that there are much better treatments for moderate to severe psoriasis and better knowledge about managing psoriasis during pregnancy, it will also be important to explore whether these trends change over time.”
The study was funded by the International League of Dermatological Societies on behalf of the Global Psoriasis Atlas. Two of the study authors reported receiving consulting fees and grant support from many pharmaceutical companies. Dr. Kimball disclosed that she serves or has served on several Organization of Teratology Information Specialists advisory board pregnancy registries, is a consultant and investigator for Abbvie, Janssen, Lilly, Bristol-Myers Squibb, Moonlake, UCB, and Amgen; has fellowship funding from Janssen; and serves on the board of Almirall.
in a U.K. cohort study.
Those are key findings from what is believed to be one of the largest studies to investigate fertility and obstetric outcomes in patients with psoriasis.
“Studies that have examined fertility and pregnancy outcomes in women with psoriasis have reported conflicting findings,” lead author Teng-Chou Chen, PhD, of the Centre for Pharmacoepidemiology and Drug Safety at the University of Manchester (England), and colleagues from the Global Psoriasis Atlas wrote in the study, published in JAMA Dermatology. Most of the studies were small, with under 100 women, “and are thus likely underpowered to detect a difference in pregnancy outcomes. The majority of those studies used disease registry data or lacked a matched comparison group and hence were unable to estimate the association of fertility and adverse pregnancy outcomes in women with psoriasis when compared with the general population.”
To determine fertility rates and birth outcomes in female patients with psoriasis, compared with age- and practice-matched patients without psoriasis, the researchers evaluated EHR data from a large U.K. primary care database, the Clinical Practice Research Datalink GOLD, from 1998 to 2019. They limited the analysis to patients aged 15-44 years and used relevant codes from clinical consultations to identify those with psoriasis. Then, for each patient with psoriasis, the researchers selected five comparators without psoriasis from the same primary care practice and matched for year of birth.
Both sets of patients were followed from the index date to age 45 years, death, transfer out of practice, last date of data collection, or end of the study period (Dec. 31, 2019), whichever came first. Pregnancy records were extracted for both sets of patients, and birth outcomes were categorized as pregnancy loss, live birth, stillbirth, and preterm birth. Adverse pregnancy outcomes were also collected. Finally, Dr. Chen and colleagues used a negative binomial model to examine the association between psoriasis and the fertility rate, and they applied logistic regression to compare the association between psoriasis and obstetric outcomes.
The analysis included 63,681 patients with psoriasis and 318,405 comparators whose median age on the index date was 30 years and who were followed for a median of 4.1 years. Among patients with psoriasis, 5.1% met criteria for moderate to severe disease in the follow-up period. The researchers observed that, compared with their age- and practice-matched counterparts, patients with psoriasis were more likely to be current smokers, alcohol drinkers, or overweight on the index date. They were also more often diagnosed with diabetes, hypertension, inflammatory bowel disease, thyroid disorders, and respiratory diseases such as asthma and chronic obstructive pulmonary disease.
Fertility, birth outcomes
When they looked at fertility outcomes, the researchers found that, compared with their matched peers without psoriasis, those with psoriasis had higher rates of fertility (risk ratio, 1.30; 95% confidence interval, 1.27-1.33; P < .001). But after the researchers stratified patients based on psoriasis severity, those with moderate to severe disease had significantly lower rates of fertility (RR, 0.75; 95% CI, 0.69-0.83; P < .001), compared those who did not have psoriasis.
As for adverse birth outcomes, compared with their matched comparators, pregnancies in patients with psoriasis were less likely to end in a live birth (odds ratio, 0.91; 95% CI, 0.88-0.93; P < .001). They also had a higher risk of pregnancy loss (OR, 1.06; 95% CI, 1.03-1.10; P < .001), most during the first trimester, at a gestation period of under 91 days.
In addition to psoriasis, patients younger than age 20 (OR, 2.04; 95% CI, 1.94-2.15; P < .011) and those aged between 20 and 24 years (OR, 1.35; 95% CI, 1.31-1.40; P < .001) had a higher risk of pregnancy loss, compared with those aged between 25 and 34 years.
However, no increases in the risks of antenatal hemorrhage, preeclampsia, or gestational diabetes were observed in patients with psoriasis, and no statistically significant differences in the odds of stillbirth and preterm birth were found between patients with psoriasis and matched comparators who did not have psoriasis.
“The mechanism to link the higher risk of pregnancy loss in patients with psoriasis is not clear, but there might be potential explanations,” the researchers wrote. “Psoriasis is characterized by the increased activity of [interleukin]-17, IL-23, and tumor necrosis factor–alpha. Those proinflammatory cytokines may negatively affect the placenta and cause impaired fetal growth.”
They recommended that further studies “evaluate the effects of better management of psoriasis and close monitoring during pregnancy on pregnancy loss.” In particular, “patients with psoriasis were more likely to have comorbidities that may be related to poor pregnancy outcomes, and hence increased emphasis of managing comorbidities as part of the routine management plan is also warranted.”
Asked to comment on the study, Alexa B. Kimball, MD, MPH, who has been involved with research on this topic, said that she and other investigators had observed some years ago that fertility rates for women with moderate to severe psoriasis might be lower than expected.
This trend was observed in some psoriasis registries, some pregnancy registries, and in clinical practice, Dr. Kimball, professor of dermatology at Harvard Medical School, Boston, said in an interview. “This study clearly demonstrates that lower fertility rates in the moderate to severe psoriasis population occurs and compels further exploration of the reason why.” The reasons could be biologic, she continued, including difficulty conceiving or an increased risk of miscarriage, sociobehavioral issues, or a combination.
“Behavioral examples could include that some women with moderate to severe psoriasis can flare during pregnancy, which might affect their choice” to become pregnant, Dr. Kimball said. “Stigma may also play a role in how women with moderate to severe psoriasis form relationships. Now that there are much better treatments for moderate to severe psoriasis and better knowledge about managing psoriasis during pregnancy, it will also be important to explore whether these trends change over time.”
The study was funded by the International League of Dermatological Societies on behalf of the Global Psoriasis Atlas. Two of the study authors reported receiving consulting fees and grant support from many pharmaceutical companies. Dr. Kimball disclosed that she serves or has served on several Organization of Teratology Information Specialists advisory board pregnancy registries, is a consultant and investigator for Abbvie, Janssen, Lilly, Bristol-Myers Squibb, Moonlake, UCB, and Amgen; has fellowship funding from Janssen; and serves on the board of Almirall.
in a U.K. cohort study.
Those are key findings from what is believed to be one of the largest studies to investigate fertility and obstetric outcomes in patients with psoriasis.
“Studies that have examined fertility and pregnancy outcomes in women with psoriasis have reported conflicting findings,” lead author Teng-Chou Chen, PhD, of the Centre for Pharmacoepidemiology and Drug Safety at the University of Manchester (England), and colleagues from the Global Psoriasis Atlas wrote in the study, published in JAMA Dermatology. Most of the studies were small, with under 100 women, “and are thus likely underpowered to detect a difference in pregnancy outcomes. The majority of those studies used disease registry data or lacked a matched comparison group and hence were unable to estimate the association of fertility and adverse pregnancy outcomes in women with psoriasis when compared with the general population.”
To determine fertility rates and birth outcomes in female patients with psoriasis, compared with age- and practice-matched patients without psoriasis, the researchers evaluated EHR data from a large U.K. primary care database, the Clinical Practice Research Datalink GOLD, from 1998 to 2019. They limited the analysis to patients aged 15-44 years and used relevant codes from clinical consultations to identify those with psoriasis. Then, for each patient with psoriasis, the researchers selected five comparators without psoriasis from the same primary care practice and matched for year of birth.
Both sets of patients were followed from the index date to age 45 years, death, transfer out of practice, last date of data collection, or end of the study period (Dec. 31, 2019), whichever came first. Pregnancy records were extracted for both sets of patients, and birth outcomes were categorized as pregnancy loss, live birth, stillbirth, and preterm birth. Adverse pregnancy outcomes were also collected. Finally, Dr. Chen and colleagues used a negative binomial model to examine the association between psoriasis and the fertility rate, and they applied logistic regression to compare the association between psoriasis and obstetric outcomes.
The analysis included 63,681 patients with psoriasis and 318,405 comparators whose median age on the index date was 30 years and who were followed for a median of 4.1 years. Among patients with psoriasis, 5.1% met criteria for moderate to severe disease in the follow-up period. The researchers observed that, compared with their age- and practice-matched counterparts, patients with psoriasis were more likely to be current smokers, alcohol drinkers, or overweight on the index date. They were also more often diagnosed with diabetes, hypertension, inflammatory bowel disease, thyroid disorders, and respiratory diseases such as asthma and chronic obstructive pulmonary disease.
Fertility, birth outcomes
When they looked at fertility outcomes, the researchers found that, compared with their matched peers without psoriasis, those with psoriasis had higher rates of fertility (risk ratio, 1.30; 95% confidence interval, 1.27-1.33; P < .001). But after the researchers stratified patients based on psoriasis severity, those with moderate to severe disease had significantly lower rates of fertility (RR, 0.75; 95% CI, 0.69-0.83; P < .001), compared those who did not have psoriasis.
As for adverse birth outcomes, compared with their matched comparators, pregnancies in patients with psoriasis were less likely to end in a live birth (odds ratio, 0.91; 95% CI, 0.88-0.93; P < .001). They also had a higher risk of pregnancy loss (OR, 1.06; 95% CI, 1.03-1.10; P < .001), most during the first trimester, at a gestation period of under 91 days.
In addition to psoriasis, patients younger than age 20 (OR, 2.04; 95% CI, 1.94-2.15; P < .011) and those aged between 20 and 24 years (OR, 1.35; 95% CI, 1.31-1.40; P < .001) had a higher risk of pregnancy loss, compared with those aged between 25 and 34 years.
However, no increases in the risks of antenatal hemorrhage, preeclampsia, or gestational diabetes were observed in patients with psoriasis, and no statistically significant differences in the odds of stillbirth and preterm birth were found between patients with psoriasis and matched comparators who did not have psoriasis.
“The mechanism to link the higher risk of pregnancy loss in patients with psoriasis is not clear, but there might be potential explanations,” the researchers wrote. “Psoriasis is characterized by the increased activity of [interleukin]-17, IL-23, and tumor necrosis factor–alpha. Those proinflammatory cytokines may negatively affect the placenta and cause impaired fetal growth.”
They recommended that further studies “evaluate the effects of better management of psoriasis and close monitoring during pregnancy on pregnancy loss.” In particular, “patients with psoriasis were more likely to have comorbidities that may be related to poor pregnancy outcomes, and hence increased emphasis of managing comorbidities as part of the routine management plan is also warranted.”
Asked to comment on the study, Alexa B. Kimball, MD, MPH, who has been involved with research on this topic, said that she and other investigators had observed some years ago that fertility rates for women with moderate to severe psoriasis might be lower than expected.
This trend was observed in some psoriasis registries, some pregnancy registries, and in clinical practice, Dr. Kimball, professor of dermatology at Harvard Medical School, Boston, said in an interview. “This study clearly demonstrates that lower fertility rates in the moderate to severe psoriasis population occurs and compels further exploration of the reason why.” The reasons could be biologic, she continued, including difficulty conceiving or an increased risk of miscarriage, sociobehavioral issues, or a combination.
“Behavioral examples could include that some women with moderate to severe psoriasis can flare during pregnancy, which might affect their choice” to become pregnant, Dr. Kimball said. “Stigma may also play a role in how women with moderate to severe psoriasis form relationships. Now that there are much better treatments for moderate to severe psoriasis and better knowledge about managing psoriasis during pregnancy, it will also be important to explore whether these trends change over time.”
The study was funded by the International League of Dermatological Societies on behalf of the Global Psoriasis Atlas. Two of the study authors reported receiving consulting fees and grant support from many pharmaceutical companies. Dr. Kimball disclosed that she serves or has served on several Organization of Teratology Information Specialists advisory board pregnancy registries, is a consultant and investigator for Abbvie, Janssen, Lilly, Bristol-Myers Squibb, Moonlake, UCB, and Amgen; has fellowship funding from Janssen; and serves on the board of Almirall.
FROM JAMA DERMATOLOGY
EULAR PsA recommendations update emphasizes safety, nonmusculoskeletal manifestations
AT EULAR 2023
MILAN – Safety considerations, particularly regarding the use of Janus kinase (JAK) inhibitors, are of utmost importance in the 2023 update to recommendations for managing psoriatic arthritis (PsA) by the European Alliance of Associations for Rheumatology (EULAR). Additionally, the selection of therapy should now take into account the complete clinical presentation, explicitly considering nonmusculoskeletal manifestations.
Safety considerations with JAK inhibitors
Expanding on the existing six overarching principles from the 2019 recommendations, the PsA EULAR recommendations now introduce a seventh principle: “The choice of treatment should consider safety considerations regarding individual modes of action to optimize the benefit-risk profile.”
This addition was prompted by recent safety data on JAK inhibitors, which revealed serious potential side effects, such as heart attacks, blood clots, cancer, and severe infections, that recently prompted the European Medicines Agency to restrict their use. As indicated by the new principle, safety considerations have been incorporated into several recommendations.
For instance, in the context of peripheral arthritis, JAK inhibitors may now be considered if there is an inadequate response to at least one conventional synthetic disease-modifying antirheumatic drug (csDMARD) such as methotrexate, sulfasalazine, or leflunomide, and at least one biologic DMARD (bDMARD).
Alternatively, JAK inhibitors may be utilized when bDMARDs are not suitable for other reasons. However, EULAR now emphasizes caution whenever JAK inhibitors are mentioned. Specifically, “careful consideration is necessary for patients aged 65 or above, current or past long-time smokers, individuals with a history of atherosclerotic cardiovascular disease or other cardiovascular risk factors, those with other malignancy risk factors, or individuals with a known risk for venous thromboembolism.”
Consider nonmusculoskeletal manifestations in treatment decisions
In another significant update, EULAR now recommends that the choice of therapy should also consider nonmusculoskeletal manifestations associated with PsA. “There is a notable shift in perspective here,” Dr. Gossec told this news organization. Clinically relevant skin involvement should prompt the use of IL-17A or IL-17A/F or IL-23 or IL-12/23 inhibitors, while uveitis should be treated with tumor necrosis factor (TNF) inhibitors.
In the case of inflammatory bowel disease, EULAR advises the use of anti-TNF agents, IL-12/23 or IL-23 inhibitors, or a JAK inhibitor. The recommended course of action within each treatment category is not ranked in order of preference, but EULAR emphasizes the importance of following EMA recommendations and considering safety.
Systemic glucocorticoids removed
Certain medications have been removed from the recommendations, reflecting the heightened focus on treatment safety. The use of systemic glucocorticoids as adjunctive therapy is no longer recommended. “We always had reservations about their use, and now we have eliminated them. We are aware that they are still utilized, with 30% of patients in Germany, for instance, receiving low doses of glucocorticoids. However, the long-term efficacy/safety balance of glucocorticoids is unfavorable in any disease, particularly in patients with psoriatic arthritis and multiple comorbidities,” Dr. Gossec explained.
NSAIDs and local glucocorticoids are now limited to specific patient populations, namely those affected by oligoarthritis without poor prognostic factors, entheseal disease, or predominant axial disease. Their use should be short-term, generally no longer than 4 weeks. Polyarthritis or oligoarthritis with poor prognostic factors should instead be treated directly with csDMARDs.
No specific biologic treatment order recommended for peripheral arthritis
Regarding patients with peripheral arthritis, recent efficacy data have led EULAR to refrain from recommending any specific order of preference for the use of bDMARDs, which encompass TNF inhibitors and drugs targeting the IL-17 and IL-12/23 pathways. “We lack the data to propose an order of preference in patients with peripheral arthritis. Different classes of molecules exhibit efficacy in joint inflammation, generally resulting in a 50% response rate and similar overall effects,” said Dr. Gossec, referencing head-to-head trials between biologics that yielded very comparable results, such as the EXCEED trial or SPIRIT-H2H trial.
The updated recommendations now consider two IL-23p19 inhibitors, guselkumab (Tremfya) and risankizumab (Skyrizi), the JAK inhibitor upadacitinib (Rinvoq), and the very recently EMA-approved bimekizumab (Bimzelx), an IL-17A/F double inhibitor.
The recommendation for patients with mono- or oligoarthritis and poor prognostic factors now aligns with the previous recommendations for polyarthritis: A csDMARD should be initiated promptly, with a preference for methotrexate if significant skin involvement is present. New data suggest that methotrexate may be beneficial for enthesitis, achieving resolution in approximately 30% of patients. When considering treatment options, JAK inhibitors may also be taken into account, with safety considerations in mind.
In cases of clinically relevant axial disease and an inadequate response to NSAIDs, therapy with an IL-17A inhibitor, a TNF inhibitor, an IL-17A/F inhibitor, or a JAK inhibitor may be considered. This approach now aligns with the most recent axial spondyloarthritis recommendation from EULAR and the Assessment of SpondyloArthritis international Society (ASAS).
Which disease manifestation to treat first?
During the discussion, chairwoman Uta Kiltz, MD, PhD, a rheumatologist at Rheumatism Center Ruhrgebiet, Herne, Germany, and clinical lecturer at Ruhr University Bochum, inquired about identifying the primary manifestation to guide the course of action.
“Psoriatic arthritis is highly heterogeneous, and determining the predominant manifestation is sometimes challenging,” Dr. Gossec said. “However, we believe that a certain order of preference is necessary when making treatment decisions. Starting with peripheral arthritis, which can lead to structural damage, allows for treatment selection based on that aspect. If peripheral arthritis is not present, attention should be directed towards axial disease, ensuring the presence of actual inflammation rather than solely axial pain, as mechanical origin axial pain can occur due to the patient’s age.”
David Liew, MBBS, PhD, consultant rheumatologist and clinical pharmacologist at Austin Health in Melbourne, commented on the update to this news organization: “We are fortunate to have a wide range of targeted therapy options for psoriatic arthritis, and these guidelines reflect this abundance of choices. They emphasize the importance of selecting therapies based on specific disease manifestations and tailoring care to each patient’s unique type of psoriatic arthritis. It’s worth noting that some changes in these guidelines were influenced by regulatory changes following ORAL Surveillance. In an era of numerous options, we can afford to be selective at times.”
Regarding safety concerns and JAK inhibitors, Dr. Liew added: “It is not surprising to see these guidelines impose certain restrictions on the use of JAK inhibitors, especially in psoriatic arthritis, where other therapies offer distinct advantages. Until high-quality evidence convincingly points away from a class effect, we can expect to see similar provisions in many more guidelines.”
Many of the recommendations’ authors report financial relationships with one or more pharmaceutical companies. These include AbbVie, Amgen, Biogen, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Celltrion, Chugai, Galapagos, Gilead, GlaxoSmithKline, Janssen, Leo, Lilly, Medac, Merck, Merck Sharp & Dohme, Novartis, Pfizer, R-Pharma, Regeneron, Roche, Sandoz, Sanofi, Takeda, UCB, and Viatris.
EULAR funded the development of the recommendations.
A version of this article originally appeared on Medscape.com.
AT EULAR 2023
MILAN – Safety considerations, particularly regarding the use of Janus kinase (JAK) inhibitors, are of utmost importance in the 2023 update to recommendations for managing psoriatic arthritis (PsA) by the European Alliance of Associations for Rheumatology (EULAR). Additionally, the selection of therapy should now take into account the complete clinical presentation, explicitly considering nonmusculoskeletal manifestations.
Safety considerations with JAK inhibitors
Expanding on the existing six overarching principles from the 2019 recommendations, the PsA EULAR recommendations now introduce a seventh principle: “The choice of treatment should consider safety considerations regarding individual modes of action to optimize the benefit-risk profile.”
This addition was prompted by recent safety data on JAK inhibitors, which revealed serious potential side effects, such as heart attacks, blood clots, cancer, and severe infections, that recently prompted the European Medicines Agency to restrict their use. As indicated by the new principle, safety considerations have been incorporated into several recommendations.
For instance, in the context of peripheral arthritis, JAK inhibitors may now be considered if there is an inadequate response to at least one conventional synthetic disease-modifying antirheumatic drug (csDMARD) such as methotrexate, sulfasalazine, or leflunomide, and at least one biologic DMARD (bDMARD).
Alternatively, JAK inhibitors may be utilized when bDMARDs are not suitable for other reasons. However, EULAR now emphasizes caution whenever JAK inhibitors are mentioned. Specifically, “careful consideration is necessary for patients aged 65 or above, current or past long-time smokers, individuals with a history of atherosclerotic cardiovascular disease or other cardiovascular risk factors, those with other malignancy risk factors, or individuals with a known risk for venous thromboembolism.”
Consider nonmusculoskeletal manifestations in treatment decisions
In another significant update, EULAR now recommends that the choice of therapy should also consider nonmusculoskeletal manifestations associated with PsA. “There is a notable shift in perspective here,” Dr. Gossec told this news organization. Clinically relevant skin involvement should prompt the use of IL-17A or IL-17A/F or IL-23 or IL-12/23 inhibitors, while uveitis should be treated with tumor necrosis factor (TNF) inhibitors.
In the case of inflammatory bowel disease, EULAR advises the use of anti-TNF agents, IL-12/23 or IL-23 inhibitors, or a JAK inhibitor. The recommended course of action within each treatment category is not ranked in order of preference, but EULAR emphasizes the importance of following EMA recommendations and considering safety.
Systemic glucocorticoids removed
Certain medications have been removed from the recommendations, reflecting the heightened focus on treatment safety. The use of systemic glucocorticoids as adjunctive therapy is no longer recommended. “We always had reservations about their use, and now we have eliminated them. We are aware that they are still utilized, with 30% of patients in Germany, for instance, receiving low doses of glucocorticoids. However, the long-term efficacy/safety balance of glucocorticoids is unfavorable in any disease, particularly in patients with psoriatic arthritis and multiple comorbidities,” Dr. Gossec explained.
NSAIDs and local glucocorticoids are now limited to specific patient populations, namely those affected by oligoarthritis without poor prognostic factors, entheseal disease, or predominant axial disease. Their use should be short-term, generally no longer than 4 weeks. Polyarthritis or oligoarthritis with poor prognostic factors should instead be treated directly with csDMARDs.
No specific biologic treatment order recommended for peripheral arthritis
Regarding patients with peripheral arthritis, recent efficacy data have led EULAR to refrain from recommending any specific order of preference for the use of bDMARDs, which encompass TNF inhibitors and drugs targeting the IL-17 and IL-12/23 pathways. “We lack the data to propose an order of preference in patients with peripheral arthritis. Different classes of molecules exhibit efficacy in joint inflammation, generally resulting in a 50% response rate and similar overall effects,” said Dr. Gossec, referencing head-to-head trials between biologics that yielded very comparable results, such as the EXCEED trial or SPIRIT-H2H trial.
The updated recommendations now consider two IL-23p19 inhibitors, guselkumab (Tremfya) and risankizumab (Skyrizi), the JAK inhibitor upadacitinib (Rinvoq), and the very recently EMA-approved bimekizumab (Bimzelx), an IL-17A/F double inhibitor.
The recommendation for patients with mono- or oligoarthritis and poor prognostic factors now aligns with the previous recommendations for polyarthritis: A csDMARD should be initiated promptly, with a preference for methotrexate if significant skin involvement is present. New data suggest that methotrexate may be beneficial for enthesitis, achieving resolution in approximately 30% of patients. When considering treatment options, JAK inhibitors may also be taken into account, with safety considerations in mind.
In cases of clinically relevant axial disease and an inadequate response to NSAIDs, therapy with an IL-17A inhibitor, a TNF inhibitor, an IL-17A/F inhibitor, or a JAK inhibitor may be considered. This approach now aligns with the most recent axial spondyloarthritis recommendation from EULAR and the Assessment of SpondyloArthritis international Society (ASAS).
Which disease manifestation to treat first?
During the discussion, chairwoman Uta Kiltz, MD, PhD, a rheumatologist at Rheumatism Center Ruhrgebiet, Herne, Germany, and clinical lecturer at Ruhr University Bochum, inquired about identifying the primary manifestation to guide the course of action.
“Psoriatic arthritis is highly heterogeneous, and determining the predominant manifestation is sometimes challenging,” Dr. Gossec said. “However, we believe that a certain order of preference is necessary when making treatment decisions. Starting with peripheral arthritis, which can lead to structural damage, allows for treatment selection based on that aspect. If peripheral arthritis is not present, attention should be directed towards axial disease, ensuring the presence of actual inflammation rather than solely axial pain, as mechanical origin axial pain can occur due to the patient’s age.”
David Liew, MBBS, PhD, consultant rheumatologist and clinical pharmacologist at Austin Health in Melbourne, commented on the update to this news organization: “We are fortunate to have a wide range of targeted therapy options for psoriatic arthritis, and these guidelines reflect this abundance of choices. They emphasize the importance of selecting therapies based on specific disease manifestations and tailoring care to each patient’s unique type of psoriatic arthritis. It’s worth noting that some changes in these guidelines were influenced by regulatory changes following ORAL Surveillance. In an era of numerous options, we can afford to be selective at times.”
Regarding safety concerns and JAK inhibitors, Dr. Liew added: “It is not surprising to see these guidelines impose certain restrictions on the use of JAK inhibitors, especially in psoriatic arthritis, where other therapies offer distinct advantages. Until high-quality evidence convincingly points away from a class effect, we can expect to see similar provisions in many more guidelines.”
Many of the recommendations’ authors report financial relationships with one or more pharmaceutical companies. These include AbbVie, Amgen, Biogen, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Celltrion, Chugai, Galapagos, Gilead, GlaxoSmithKline, Janssen, Leo, Lilly, Medac, Merck, Merck Sharp & Dohme, Novartis, Pfizer, R-Pharma, Regeneron, Roche, Sandoz, Sanofi, Takeda, UCB, and Viatris.
EULAR funded the development of the recommendations.
A version of this article originally appeared on Medscape.com.
AT EULAR 2023
MILAN – Safety considerations, particularly regarding the use of Janus kinase (JAK) inhibitors, are of utmost importance in the 2023 update to recommendations for managing psoriatic arthritis (PsA) by the European Alliance of Associations for Rheumatology (EULAR). Additionally, the selection of therapy should now take into account the complete clinical presentation, explicitly considering nonmusculoskeletal manifestations.
Safety considerations with JAK inhibitors
Expanding on the existing six overarching principles from the 2019 recommendations, the PsA EULAR recommendations now introduce a seventh principle: “The choice of treatment should consider safety considerations regarding individual modes of action to optimize the benefit-risk profile.”
This addition was prompted by recent safety data on JAK inhibitors, which revealed serious potential side effects, such as heart attacks, blood clots, cancer, and severe infections, that recently prompted the European Medicines Agency to restrict their use. As indicated by the new principle, safety considerations have been incorporated into several recommendations.
For instance, in the context of peripheral arthritis, JAK inhibitors may now be considered if there is an inadequate response to at least one conventional synthetic disease-modifying antirheumatic drug (csDMARD) such as methotrexate, sulfasalazine, or leflunomide, and at least one biologic DMARD (bDMARD).
Alternatively, JAK inhibitors may be utilized when bDMARDs are not suitable for other reasons. However, EULAR now emphasizes caution whenever JAK inhibitors are mentioned. Specifically, “careful consideration is necessary for patients aged 65 or above, current or past long-time smokers, individuals with a history of atherosclerotic cardiovascular disease or other cardiovascular risk factors, those with other malignancy risk factors, or individuals with a known risk for venous thromboembolism.”
Consider nonmusculoskeletal manifestations in treatment decisions
In another significant update, EULAR now recommends that the choice of therapy should also consider nonmusculoskeletal manifestations associated with PsA. “There is a notable shift in perspective here,” Dr. Gossec told this news organization. Clinically relevant skin involvement should prompt the use of IL-17A or IL-17A/F or IL-23 or IL-12/23 inhibitors, while uveitis should be treated with tumor necrosis factor (TNF) inhibitors.
In the case of inflammatory bowel disease, EULAR advises the use of anti-TNF agents, IL-12/23 or IL-23 inhibitors, or a JAK inhibitor. The recommended course of action within each treatment category is not ranked in order of preference, but EULAR emphasizes the importance of following EMA recommendations and considering safety.
Systemic glucocorticoids removed
Certain medications have been removed from the recommendations, reflecting the heightened focus on treatment safety. The use of systemic glucocorticoids as adjunctive therapy is no longer recommended. “We always had reservations about their use, and now we have eliminated them. We are aware that they are still utilized, with 30% of patients in Germany, for instance, receiving low doses of glucocorticoids. However, the long-term efficacy/safety balance of glucocorticoids is unfavorable in any disease, particularly in patients with psoriatic arthritis and multiple comorbidities,” Dr. Gossec explained.
NSAIDs and local glucocorticoids are now limited to specific patient populations, namely those affected by oligoarthritis without poor prognostic factors, entheseal disease, or predominant axial disease. Their use should be short-term, generally no longer than 4 weeks. Polyarthritis or oligoarthritis with poor prognostic factors should instead be treated directly with csDMARDs.
No specific biologic treatment order recommended for peripheral arthritis
Regarding patients with peripheral arthritis, recent efficacy data have led EULAR to refrain from recommending any specific order of preference for the use of bDMARDs, which encompass TNF inhibitors and drugs targeting the IL-17 and IL-12/23 pathways. “We lack the data to propose an order of preference in patients with peripheral arthritis. Different classes of molecules exhibit efficacy in joint inflammation, generally resulting in a 50% response rate and similar overall effects,” said Dr. Gossec, referencing head-to-head trials between biologics that yielded very comparable results, such as the EXCEED trial or SPIRIT-H2H trial.
The updated recommendations now consider two IL-23p19 inhibitors, guselkumab (Tremfya) and risankizumab (Skyrizi), the JAK inhibitor upadacitinib (Rinvoq), and the very recently EMA-approved bimekizumab (Bimzelx), an IL-17A/F double inhibitor.
The recommendation for patients with mono- or oligoarthritis and poor prognostic factors now aligns with the previous recommendations for polyarthritis: A csDMARD should be initiated promptly, with a preference for methotrexate if significant skin involvement is present. New data suggest that methotrexate may be beneficial for enthesitis, achieving resolution in approximately 30% of patients. When considering treatment options, JAK inhibitors may also be taken into account, with safety considerations in mind.
In cases of clinically relevant axial disease and an inadequate response to NSAIDs, therapy with an IL-17A inhibitor, a TNF inhibitor, an IL-17A/F inhibitor, or a JAK inhibitor may be considered. This approach now aligns with the most recent axial spondyloarthritis recommendation from EULAR and the Assessment of SpondyloArthritis international Society (ASAS).
Which disease manifestation to treat first?
During the discussion, chairwoman Uta Kiltz, MD, PhD, a rheumatologist at Rheumatism Center Ruhrgebiet, Herne, Germany, and clinical lecturer at Ruhr University Bochum, inquired about identifying the primary manifestation to guide the course of action.
“Psoriatic arthritis is highly heterogeneous, and determining the predominant manifestation is sometimes challenging,” Dr. Gossec said. “However, we believe that a certain order of preference is necessary when making treatment decisions. Starting with peripheral arthritis, which can lead to structural damage, allows for treatment selection based on that aspect. If peripheral arthritis is not present, attention should be directed towards axial disease, ensuring the presence of actual inflammation rather than solely axial pain, as mechanical origin axial pain can occur due to the patient’s age.”
David Liew, MBBS, PhD, consultant rheumatologist and clinical pharmacologist at Austin Health in Melbourne, commented on the update to this news organization: “We are fortunate to have a wide range of targeted therapy options for psoriatic arthritis, and these guidelines reflect this abundance of choices. They emphasize the importance of selecting therapies based on specific disease manifestations and tailoring care to each patient’s unique type of psoriatic arthritis. It’s worth noting that some changes in these guidelines were influenced by regulatory changes following ORAL Surveillance. In an era of numerous options, we can afford to be selective at times.”
Regarding safety concerns and JAK inhibitors, Dr. Liew added: “It is not surprising to see these guidelines impose certain restrictions on the use of JAK inhibitors, especially in psoriatic arthritis, where other therapies offer distinct advantages. Until high-quality evidence convincingly points away from a class effect, we can expect to see similar provisions in many more guidelines.”
Many of the recommendations’ authors report financial relationships with one or more pharmaceutical companies. These include AbbVie, Amgen, Biogen, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Celltrion, Chugai, Galapagos, Gilead, GlaxoSmithKline, Janssen, Leo, Lilly, Medac, Merck, Merck Sharp & Dohme, Novartis, Pfizer, R-Pharma, Regeneron, Roche, Sandoz, Sanofi, Takeda, UCB, and Viatris.
EULAR funded the development of the recommendations.
A version of this article originally appeared on Medscape.com.
Cell activity in psoriasis may predict disease severity and provide clues to comorbidities
The activity and clustering of certain cell types may distinguish mild and severe forms of psoriasis, with severe disease altering the cellular and metabolic composition of distal unaffected skin sites, according to a new analysis using single-cell transcriptomic technology.
On the surface, psoriasis severity is identified based on the visible lesions, Rochelle L. Castillo, MD, of the division of rheumatology and the NYU Psoriatic Arthritis Center, NYU Langone Health, New York, and colleagues wrote in their study, published in Science Immunology. Although cellular and molecular features of inflammatory skin diseases such as psoriasis have been characterized, activity at the tissue level and its systemic impact has not been explored.
“Our initial goal was to find measurable molecular signals that could tell us who is more likely to develop severe psoriasis, as well as who is at higher risk of developing related disorders that often accompany psoriasis, such as arthritis and cardiovascular disease,” study co–senior investigator Jose Scher, MD, director of the Psoriatic Arthritis Center and the Judith and Stewart Colton Center for Autoimmunity at NYU Langone Health, said in a press release accompanying the publication of the findings. “Having found signals with potential systemic consequences, we are now working to understand how skin inflammation can lead to widespread disease affecting other organs,”
In the study, the researchers used spatial transcriptomics, a technique that positions tissue sections onto genetic arrays to determine gene expression by cell type and histological location, helping to create a broad image-based map of where certain cell types are located in tissues and with what other cells they are communicating. They characterized the cell activity of skin samples from 11 men and women with mild to severe psoriasis/psoriatic arthritis, and three healthy adults who did not have psoriasis. They defined the cellular composition of 25 healthy skin biopsies and matched skin biopsies from psoriatic lesional and nonlesional skin, and identified 17 distinct clusters of cells, which they grouped into epidermal, dermis, pilosebaceous, and adipose categories.
The researchers found that cell activity associated with inflammation, as shown by clusters of fibroblasts and dermal macrophages, was more common in the upper layers of the skin in samples from patients with more severe psoriasis, compared with healthy control samples.
They also examined patterns of immune activity at the cellular level and found significant patterns around the upper follicle, around the perifollicular dermis, and within the hair follicle, where immune cells were enriched in healthy skin. Other cells enriched in these upper layer areas in healthy skin included dendritic cells, innate lymphoid cells, T helper cells, T cytotoxic cells, and myeloid cells.
Clusters of fibroblasts and macrophages, which are associated with inflammation, were clustered in psoriatic lesional skin, which also showed more inflammation at the dermal and suprabasal epidermal levels. B lymphocytes also were more prevalent in lesional skin.
The researchers then analyzed the skin samples according to disease severity; mild psoriasis was defined as a Psoriasis Area and Severity Index score less than 12; moderate to severe disease was defined as a PASI score of 12 or higher. The macrophage, fibroblast, and lymphatic endothelium–associated clusters distinguished mild and moderate to severe endotypes.
The pathology of moderate to severe psoriasis in lesional and nonlesional skin showed the extensive effects of psoriasis-related inflammation. Although nonlesional mild disease was clustered with healthy skin, in cases of moderate to severe disease, nonlesional and lesional groups were clustered together. This effect was segregated according to disease severity, independent of the presence of joint disease, and “was particularly evident in distal, nonlesional samples,” the researchers wrote.
The researchers also found evidence of increased gene activity in more than three dozen molecular pathways associated with metabolism and lipid levels in areas of lesional and nonlesional skin, Dr. Scher said.
The findings were limited by several factors including the small sample size and the limits of spatial transcriptomics technology resolution, the researchers wrote. “As this technology evolves, platforms with higher density, and by extension, resolution, of spatially barcoded beads will provide more granularity about cellular microenvironments in healthy and diseased states.”
The study was supported by the National Institutes of Health, the National Psoriasis Foundation, the NYU Colton Center for Autoimmunity, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, the Beatrice Snyder Foundation, The Riley Family Foundation, the Rheumatology Research Foundation, and the NY Stem Cell Foundation. Dr. Castillo had no financial conflicts to disclose. Dr. Scher has served as a consultant for Janssen, Abbvie, Novartis, Pfizer, Sanofi, UCB, and Bristol-Myers Squibb, and has received research funding from Janssen and Pfizer.
The activity and clustering of certain cell types may distinguish mild and severe forms of psoriasis, with severe disease altering the cellular and metabolic composition of distal unaffected skin sites, according to a new analysis using single-cell transcriptomic technology.
On the surface, psoriasis severity is identified based on the visible lesions, Rochelle L. Castillo, MD, of the division of rheumatology and the NYU Psoriatic Arthritis Center, NYU Langone Health, New York, and colleagues wrote in their study, published in Science Immunology. Although cellular and molecular features of inflammatory skin diseases such as psoriasis have been characterized, activity at the tissue level and its systemic impact has not been explored.
“Our initial goal was to find measurable molecular signals that could tell us who is more likely to develop severe psoriasis, as well as who is at higher risk of developing related disorders that often accompany psoriasis, such as arthritis and cardiovascular disease,” study co–senior investigator Jose Scher, MD, director of the Psoriatic Arthritis Center and the Judith and Stewart Colton Center for Autoimmunity at NYU Langone Health, said in a press release accompanying the publication of the findings. “Having found signals with potential systemic consequences, we are now working to understand how skin inflammation can lead to widespread disease affecting other organs,”
In the study, the researchers used spatial transcriptomics, a technique that positions tissue sections onto genetic arrays to determine gene expression by cell type and histological location, helping to create a broad image-based map of where certain cell types are located in tissues and with what other cells they are communicating. They characterized the cell activity of skin samples from 11 men and women with mild to severe psoriasis/psoriatic arthritis, and three healthy adults who did not have psoriasis. They defined the cellular composition of 25 healthy skin biopsies and matched skin biopsies from psoriatic lesional and nonlesional skin, and identified 17 distinct clusters of cells, which they grouped into epidermal, dermis, pilosebaceous, and adipose categories.
The researchers found that cell activity associated with inflammation, as shown by clusters of fibroblasts and dermal macrophages, was more common in the upper layers of the skin in samples from patients with more severe psoriasis, compared with healthy control samples.
They also examined patterns of immune activity at the cellular level and found significant patterns around the upper follicle, around the perifollicular dermis, and within the hair follicle, where immune cells were enriched in healthy skin. Other cells enriched in these upper layer areas in healthy skin included dendritic cells, innate lymphoid cells, T helper cells, T cytotoxic cells, and myeloid cells.
Clusters of fibroblasts and macrophages, which are associated with inflammation, were clustered in psoriatic lesional skin, which also showed more inflammation at the dermal and suprabasal epidermal levels. B lymphocytes also were more prevalent in lesional skin.
The researchers then analyzed the skin samples according to disease severity; mild psoriasis was defined as a Psoriasis Area and Severity Index score less than 12; moderate to severe disease was defined as a PASI score of 12 or higher. The macrophage, fibroblast, and lymphatic endothelium–associated clusters distinguished mild and moderate to severe endotypes.
The pathology of moderate to severe psoriasis in lesional and nonlesional skin showed the extensive effects of psoriasis-related inflammation. Although nonlesional mild disease was clustered with healthy skin, in cases of moderate to severe disease, nonlesional and lesional groups were clustered together. This effect was segregated according to disease severity, independent of the presence of joint disease, and “was particularly evident in distal, nonlesional samples,” the researchers wrote.
The researchers also found evidence of increased gene activity in more than three dozen molecular pathways associated with metabolism and lipid levels in areas of lesional and nonlesional skin, Dr. Scher said.
The findings were limited by several factors including the small sample size and the limits of spatial transcriptomics technology resolution, the researchers wrote. “As this technology evolves, platforms with higher density, and by extension, resolution, of spatially barcoded beads will provide more granularity about cellular microenvironments in healthy and diseased states.”
The study was supported by the National Institutes of Health, the National Psoriasis Foundation, the NYU Colton Center for Autoimmunity, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, the Beatrice Snyder Foundation, The Riley Family Foundation, the Rheumatology Research Foundation, and the NY Stem Cell Foundation. Dr. Castillo had no financial conflicts to disclose. Dr. Scher has served as a consultant for Janssen, Abbvie, Novartis, Pfizer, Sanofi, UCB, and Bristol-Myers Squibb, and has received research funding from Janssen and Pfizer.
The activity and clustering of certain cell types may distinguish mild and severe forms of psoriasis, with severe disease altering the cellular and metabolic composition of distal unaffected skin sites, according to a new analysis using single-cell transcriptomic technology.
On the surface, psoriasis severity is identified based on the visible lesions, Rochelle L. Castillo, MD, of the division of rheumatology and the NYU Psoriatic Arthritis Center, NYU Langone Health, New York, and colleagues wrote in their study, published in Science Immunology. Although cellular and molecular features of inflammatory skin diseases such as psoriasis have been characterized, activity at the tissue level and its systemic impact has not been explored.
“Our initial goal was to find measurable molecular signals that could tell us who is more likely to develop severe psoriasis, as well as who is at higher risk of developing related disorders that often accompany psoriasis, such as arthritis and cardiovascular disease,” study co–senior investigator Jose Scher, MD, director of the Psoriatic Arthritis Center and the Judith and Stewart Colton Center for Autoimmunity at NYU Langone Health, said in a press release accompanying the publication of the findings. “Having found signals with potential systemic consequences, we are now working to understand how skin inflammation can lead to widespread disease affecting other organs,”
In the study, the researchers used spatial transcriptomics, a technique that positions tissue sections onto genetic arrays to determine gene expression by cell type and histological location, helping to create a broad image-based map of where certain cell types are located in tissues and with what other cells they are communicating. They characterized the cell activity of skin samples from 11 men and women with mild to severe psoriasis/psoriatic arthritis, and three healthy adults who did not have psoriasis. They defined the cellular composition of 25 healthy skin biopsies and matched skin biopsies from psoriatic lesional and nonlesional skin, and identified 17 distinct clusters of cells, which they grouped into epidermal, dermis, pilosebaceous, and adipose categories.
The researchers found that cell activity associated with inflammation, as shown by clusters of fibroblasts and dermal macrophages, was more common in the upper layers of the skin in samples from patients with more severe psoriasis, compared with healthy control samples.
They also examined patterns of immune activity at the cellular level and found significant patterns around the upper follicle, around the perifollicular dermis, and within the hair follicle, where immune cells were enriched in healthy skin. Other cells enriched in these upper layer areas in healthy skin included dendritic cells, innate lymphoid cells, T helper cells, T cytotoxic cells, and myeloid cells.
Clusters of fibroblasts and macrophages, which are associated with inflammation, were clustered in psoriatic lesional skin, which also showed more inflammation at the dermal and suprabasal epidermal levels. B lymphocytes also were more prevalent in lesional skin.
The researchers then analyzed the skin samples according to disease severity; mild psoriasis was defined as a Psoriasis Area and Severity Index score less than 12; moderate to severe disease was defined as a PASI score of 12 or higher. The macrophage, fibroblast, and lymphatic endothelium–associated clusters distinguished mild and moderate to severe endotypes.
The pathology of moderate to severe psoriasis in lesional and nonlesional skin showed the extensive effects of psoriasis-related inflammation. Although nonlesional mild disease was clustered with healthy skin, in cases of moderate to severe disease, nonlesional and lesional groups were clustered together. This effect was segregated according to disease severity, independent of the presence of joint disease, and “was particularly evident in distal, nonlesional samples,” the researchers wrote.
The researchers also found evidence of increased gene activity in more than three dozen molecular pathways associated with metabolism and lipid levels in areas of lesional and nonlesional skin, Dr. Scher said.
The findings were limited by several factors including the small sample size and the limits of spatial transcriptomics technology resolution, the researchers wrote. “As this technology evolves, platforms with higher density, and by extension, resolution, of spatially barcoded beads will provide more granularity about cellular microenvironments in healthy and diseased states.”
The study was supported by the National Institutes of Health, the National Psoriasis Foundation, the NYU Colton Center for Autoimmunity, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, the Beatrice Snyder Foundation, The Riley Family Foundation, the Rheumatology Research Foundation, and the NY Stem Cell Foundation. Dr. Castillo had no financial conflicts to disclose. Dr. Scher has served as a consultant for Janssen, Abbvie, Novartis, Pfizer, Sanofi, UCB, and Bristol-Myers Squibb, and has received research funding from Janssen and Pfizer.
FROM SCIENCE IMMUNOLOGY
A Joint Effort to Save the Joints: What Dermatologists Need to Know About Psoriatic Arthritis
Nearly all dermatologists are aware that psoriatic arthritis (PsA) is one of the most prevalent comorbidities associated with psoriasis, yet we may lack the insight regarding how to utilize this information. After all, we specialize in the skin, not the joints, right?
When I graduated from residency in 2014, I began staffing our psoriasis clinic, where we care for the toughest, most complicated psoriasis patients, many of them struggling with both severe recalcitrant psoriasis as well as debilitating PsA. In 2016, we partnered with rheumatology to open a multidisciplinary psoriasis and PsA clinic, and I quickly began to appreciate how much PsA was being overlooked simply because patients with psoriasis were not being asked about their joints.
To start, let’s look at several facts:
- One quarter of patients with psoriasis also have PsA.1
- Skin disease most commonly develops before PsA.1
- Fifteen percent of PsA cases go undiagnosed, which dramatically increases the risk for deformed joints, erosions, osteolysis, sacroiliitis, and arthritis mutilans2 and also increases the cost of health care.3
- Everyone is crazy busy—rheumatology wait lists often are months long.
Given that dermatologists are the ones who already are seeing the majority of patients who develop PsA, we play a key role in screening for this debilitating comorbidity and starting therapy for patients with both psoriasis and PsA. We, too, are crazy busy; therefore, we need to make this process quick and efficient but also reliable. Fortunately, the Psoriasis Epidemiology Screening Tool (PEST) is effective, fast, and very easy. With only 5 questions and a sensitivity and specificity of around 70%,4 this short and simple questionnaire can be incorporated into an intake form or rooming note or can just be asked during the visit. The questions include whether the patient currently has or has had a swollen joint, nail pits, heel pain, and/or dactylitis, as well as if they have been told by a physician that they have arthritis. A score of 3 or higher is considered positive and a referral to rheumatology should be considered. At the bare minimum, I highly encourage all dermatologists to incorporate the PEST screening tool into their practice.
During the physical examination itself, be sure to look at the patient’s nails and also look for joint swelling and redness, especially in the hands. When palpating a swollen joint, the presence of inflammatory arthritis will feel spongy or boggy, while the osteophytes associated with osteoarthritis will feel hard. Radiography of the affected joint may be helpful, but keep in mind that bone changes are latter sequelae of PsA, and negative radiographs do not rule out PsA.
If you highly suspect PsA after using the PEST screening tool and palpating any swollen joints, then a rheumatology referral certainly is warranted. Medication that covers both psoriasis and PsA also can be initiated. Although methotrexate often is used for joints, higher doses (ie, >15 mg/wk) usually are needed. A 2019 Cochrane review found that low-dose methotrexate (ie, ≤15 mg/wk) may be only slightly more effective then placebo5—certainly not a ringing endorsement for its use in PsA. Additionally, quality data demonstrating methotrexate’s efficacy for enthesitis or axial spondyloarthritis is lacking, and methotrexate has not demonstrated an ability to slow the radiographic progression of joints. In contrast, the anti–tumor necrosis factor agents, including adalimumab, infliximab, etanercept, and certolizumab, as well as ustekinumab and the anti–IL-17 biologics secukinumab and ixekizumab have demonstrated efficacy in American College of Rheumatology (ACR) scores, enthesitis, dactylitis, and prevention of radiographic progression of joints.6,7 Although brodalumab, an anti–IL-17 receptor inhibitor, demonstrated improvement in ACR scores, enthesitis, and dactylitis, data on its effects on radiographic progression of joints were inconclusive given the phase III trial’s premature ending due to suicidal ideation and behavior in participants.8 Several of the anti–IL-23 agents also may help PsA, with trials demonstrating improvements in ACR scores, enthesitis, and dactylitis; however, only guselkumab 100 mg every 4 weeks decreased radiographic progression of joints.9 Additionally, with the age of the Janus kinase (JAK) inhibitor upon us, there are several JAK/TYK2 inhibitors that are approved by the US Food and Drug Administration for psoriasis (deucravacitinib) as well as for PsA (tofacitinib, upadacitinib), and there are more JAK inhibitors in the pipeline. These medications are effective; however, I do encourage caution and careful consideration in selecting the appropriate patient, as data demonstrated an increased risk for major adverse cardiovascular events and cancer in older (>50 years) rheumatoid arthritis patients who had at least 1 cardiovascular risk factor and were treated with tofacitinib.10 Although several other trials have not demonstrated this increased risk, further data are needed to determine risk for both pan-JAK inhibitors as well as selective JAK inhibitors and TYK2 inhibitors. Additionally, given psoriasis already is closely linked with many cardiovascular risk factors including heart disease, obesity, hypertension, hyperlipidemia, and diabetes mellitus,11 it will be important to have long-term safety information for JAK inhibitors in the psoriasis and PsA population.
Dermatologists are in a pivotal position to identify patients affected by PsA and start an appropriate systemic medication. We can help make an enormous impact on our patients’ lives as well as help decrease the economic impact of untreated disease. Let’s join the effort to save the joints!
- Alinaghi F, Calov M, Kristensen L, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.
- Villani A, Zouzaud M, Sevrain M, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol. 2015;73:242-248.
- Iragorri N, Hazlewood G, Manns B, et al. Model to determine the cost-effectiveness of screening psoriasis patients for psoriatic arthritis. Arth Car Res. 2021;73:266-274.
- Karreman M, Weel A, Van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology. 2017;56:597-602.
- Wilsdon TD, Whittle SL, Thynne TR, et al. Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev. 2019;1:CD012722. doi:10.1002/14651858.CD012722.pub2
- Mourad A, Gniadecki R. Treatment of dactylitis and enthesitis in psoriatic arthritis with biologic agents: a systematic review and metaanalysis. J Rheum. 2020;47:59-65.
- Wu D, Li C, Zhang S, et al. Effect of biologics on radiographic progression of peripheral joint in patients with psoriatic arthritis: meta-analysis. Rheumatology (Oxford). 2020;59:3172-3180.
- Mease P, Helliwell P, Fjellhaugen Hjuler K, et al. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis. 2021;80:185-193.
- McInnes I, Rahman P, Gottlieb A, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arth Rheum. 2022;74:475-485.
- Ytterberg S, Bhatt D, Mikuls T, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- Miller I, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. JAAD. 2013;69:1014-1024.
Nearly all dermatologists are aware that psoriatic arthritis (PsA) is one of the most prevalent comorbidities associated with psoriasis, yet we may lack the insight regarding how to utilize this information. After all, we specialize in the skin, not the joints, right?
When I graduated from residency in 2014, I began staffing our psoriasis clinic, where we care for the toughest, most complicated psoriasis patients, many of them struggling with both severe recalcitrant psoriasis as well as debilitating PsA. In 2016, we partnered with rheumatology to open a multidisciplinary psoriasis and PsA clinic, and I quickly began to appreciate how much PsA was being overlooked simply because patients with psoriasis were not being asked about their joints.
To start, let’s look at several facts:
- One quarter of patients with psoriasis also have PsA.1
- Skin disease most commonly develops before PsA.1
- Fifteen percent of PsA cases go undiagnosed, which dramatically increases the risk for deformed joints, erosions, osteolysis, sacroiliitis, and arthritis mutilans2 and also increases the cost of health care.3
- Everyone is crazy busy—rheumatology wait lists often are months long.
Given that dermatologists are the ones who already are seeing the majority of patients who develop PsA, we play a key role in screening for this debilitating comorbidity and starting therapy for patients with both psoriasis and PsA. We, too, are crazy busy; therefore, we need to make this process quick and efficient but also reliable. Fortunately, the Psoriasis Epidemiology Screening Tool (PEST) is effective, fast, and very easy. With only 5 questions and a sensitivity and specificity of around 70%,4 this short and simple questionnaire can be incorporated into an intake form or rooming note or can just be asked during the visit. The questions include whether the patient currently has or has had a swollen joint, nail pits, heel pain, and/or dactylitis, as well as if they have been told by a physician that they have arthritis. A score of 3 or higher is considered positive and a referral to rheumatology should be considered. At the bare minimum, I highly encourage all dermatologists to incorporate the PEST screening tool into their practice.
During the physical examination itself, be sure to look at the patient’s nails and also look for joint swelling and redness, especially in the hands. When palpating a swollen joint, the presence of inflammatory arthritis will feel spongy or boggy, while the osteophytes associated with osteoarthritis will feel hard. Radiography of the affected joint may be helpful, but keep in mind that bone changes are latter sequelae of PsA, and negative radiographs do not rule out PsA.
If you highly suspect PsA after using the PEST screening tool and palpating any swollen joints, then a rheumatology referral certainly is warranted. Medication that covers both psoriasis and PsA also can be initiated. Although methotrexate often is used for joints, higher doses (ie, >15 mg/wk) usually are needed. A 2019 Cochrane review found that low-dose methotrexate (ie, ≤15 mg/wk) may be only slightly more effective then placebo5—certainly not a ringing endorsement for its use in PsA. Additionally, quality data demonstrating methotrexate’s efficacy for enthesitis or axial spondyloarthritis is lacking, and methotrexate has not demonstrated an ability to slow the radiographic progression of joints. In contrast, the anti–tumor necrosis factor agents, including adalimumab, infliximab, etanercept, and certolizumab, as well as ustekinumab and the anti–IL-17 biologics secukinumab and ixekizumab have demonstrated efficacy in American College of Rheumatology (ACR) scores, enthesitis, dactylitis, and prevention of radiographic progression of joints.6,7 Although brodalumab, an anti–IL-17 receptor inhibitor, demonstrated improvement in ACR scores, enthesitis, and dactylitis, data on its effects on radiographic progression of joints were inconclusive given the phase III trial’s premature ending due to suicidal ideation and behavior in participants.8 Several of the anti–IL-23 agents also may help PsA, with trials demonstrating improvements in ACR scores, enthesitis, and dactylitis; however, only guselkumab 100 mg every 4 weeks decreased radiographic progression of joints.9 Additionally, with the age of the Janus kinase (JAK) inhibitor upon us, there are several JAK/TYK2 inhibitors that are approved by the US Food and Drug Administration for psoriasis (deucravacitinib) as well as for PsA (tofacitinib, upadacitinib), and there are more JAK inhibitors in the pipeline. These medications are effective; however, I do encourage caution and careful consideration in selecting the appropriate patient, as data demonstrated an increased risk for major adverse cardiovascular events and cancer in older (>50 years) rheumatoid arthritis patients who had at least 1 cardiovascular risk factor and were treated with tofacitinib.10 Although several other trials have not demonstrated this increased risk, further data are needed to determine risk for both pan-JAK inhibitors as well as selective JAK inhibitors and TYK2 inhibitors. Additionally, given psoriasis already is closely linked with many cardiovascular risk factors including heart disease, obesity, hypertension, hyperlipidemia, and diabetes mellitus,11 it will be important to have long-term safety information for JAK inhibitors in the psoriasis and PsA population.
Dermatologists are in a pivotal position to identify patients affected by PsA and start an appropriate systemic medication. We can help make an enormous impact on our patients’ lives as well as help decrease the economic impact of untreated disease. Let’s join the effort to save the joints!
Nearly all dermatologists are aware that psoriatic arthritis (PsA) is one of the most prevalent comorbidities associated with psoriasis, yet we may lack the insight regarding how to utilize this information. After all, we specialize in the skin, not the joints, right?
When I graduated from residency in 2014, I began staffing our psoriasis clinic, where we care for the toughest, most complicated psoriasis patients, many of them struggling with both severe recalcitrant psoriasis as well as debilitating PsA. In 2016, we partnered with rheumatology to open a multidisciplinary psoriasis and PsA clinic, and I quickly began to appreciate how much PsA was being overlooked simply because patients with psoriasis were not being asked about their joints.
To start, let’s look at several facts:
- One quarter of patients with psoriasis also have PsA.1
- Skin disease most commonly develops before PsA.1
- Fifteen percent of PsA cases go undiagnosed, which dramatically increases the risk for deformed joints, erosions, osteolysis, sacroiliitis, and arthritis mutilans2 and also increases the cost of health care.3
- Everyone is crazy busy—rheumatology wait lists often are months long.
Given that dermatologists are the ones who already are seeing the majority of patients who develop PsA, we play a key role in screening for this debilitating comorbidity and starting therapy for patients with both psoriasis and PsA. We, too, are crazy busy; therefore, we need to make this process quick and efficient but also reliable. Fortunately, the Psoriasis Epidemiology Screening Tool (PEST) is effective, fast, and very easy. With only 5 questions and a sensitivity and specificity of around 70%,4 this short and simple questionnaire can be incorporated into an intake form or rooming note or can just be asked during the visit. The questions include whether the patient currently has or has had a swollen joint, nail pits, heel pain, and/or dactylitis, as well as if they have been told by a physician that they have arthritis. A score of 3 or higher is considered positive and a referral to rheumatology should be considered. At the bare minimum, I highly encourage all dermatologists to incorporate the PEST screening tool into their practice.
During the physical examination itself, be sure to look at the patient’s nails and also look for joint swelling and redness, especially in the hands. When palpating a swollen joint, the presence of inflammatory arthritis will feel spongy or boggy, while the osteophytes associated with osteoarthritis will feel hard. Radiography of the affected joint may be helpful, but keep in mind that bone changes are latter sequelae of PsA, and negative radiographs do not rule out PsA.
If you highly suspect PsA after using the PEST screening tool and palpating any swollen joints, then a rheumatology referral certainly is warranted. Medication that covers both psoriasis and PsA also can be initiated. Although methotrexate often is used for joints, higher doses (ie, >15 mg/wk) usually are needed. A 2019 Cochrane review found that low-dose methotrexate (ie, ≤15 mg/wk) may be only slightly more effective then placebo5—certainly not a ringing endorsement for its use in PsA. Additionally, quality data demonstrating methotrexate’s efficacy for enthesitis or axial spondyloarthritis is lacking, and methotrexate has not demonstrated an ability to slow the radiographic progression of joints. In contrast, the anti–tumor necrosis factor agents, including adalimumab, infliximab, etanercept, and certolizumab, as well as ustekinumab and the anti–IL-17 biologics secukinumab and ixekizumab have demonstrated efficacy in American College of Rheumatology (ACR) scores, enthesitis, dactylitis, and prevention of radiographic progression of joints.6,7 Although brodalumab, an anti–IL-17 receptor inhibitor, demonstrated improvement in ACR scores, enthesitis, and dactylitis, data on its effects on radiographic progression of joints were inconclusive given the phase III trial’s premature ending due to suicidal ideation and behavior in participants.8 Several of the anti–IL-23 agents also may help PsA, with trials demonstrating improvements in ACR scores, enthesitis, and dactylitis; however, only guselkumab 100 mg every 4 weeks decreased radiographic progression of joints.9 Additionally, with the age of the Janus kinase (JAK) inhibitor upon us, there are several JAK/TYK2 inhibitors that are approved by the US Food and Drug Administration for psoriasis (deucravacitinib) as well as for PsA (tofacitinib, upadacitinib), and there are more JAK inhibitors in the pipeline. These medications are effective; however, I do encourage caution and careful consideration in selecting the appropriate patient, as data demonstrated an increased risk for major adverse cardiovascular events and cancer in older (>50 years) rheumatoid arthritis patients who had at least 1 cardiovascular risk factor and were treated with tofacitinib.10 Although several other trials have not demonstrated this increased risk, further data are needed to determine risk for both pan-JAK inhibitors as well as selective JAK inhibitors and TYK2 inhibitors. Additionally, given psoriasis already is closely linked with many cardiovascular risk factors including heart disease, obesity, hypertension, hyperlipidemia, and diabetes mellitus,11 it will be important to have long-term safety information for JAK inhibitors in the psoriasis and PsA population.
Dermatologists are in a pivotal position to identify patients affected by PsA and start an appropriate systemic medication. We can help make an enormous impact on our patients’ lives as well as help decrease the economic impact of untreated disease. Let’s join the effort to save the joints!
- Alinaghi F, Calov M, Kristensen L, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.
- Villani A, Zouzaud M, Sevrain M, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol. 2015;73:242-248.
- Iragorri N, Hazlewood G, Manns B, et al. Model to determine the cost-effectiveness of screening psoriasis patients for psoriatic arthritis. Arth Car Res. 2021;73:266-274.
- Karreman M, Weel A, Van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology. 2017;56:597-602.
- Wilsdon TD, Whittle SL, Thynne TR, et al. Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev. 2019;1:CD012722. doi:10.1002/14651858.CD012722.pub2
- Mourad A, Gniadecki R. Treatment of dactylitis and enthesitis in psoriatic arthritis with biologic agents: a systematic review and metaanalysis. J Rheum. 2020;47:59-65.
- Wu D, Li C, Zhang S, et al. Effect of biologics on radiographic progression of peripheral joint in patients with psoriatic arthritis: meta-analysis. Rheumatology (Oxford). 2020;59:3172-3180.
- Mease P, Helliwell P, Fjellhaugen Hjuler K, et al. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis. 2021;80:185-193.
- McInnes I, Rahman P, Gottlieb A, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arth Rheum. 2022;74:475-485.
- Ytterberg S, Bhatt D, Mikuls T, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- Miller I, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. JAAD. 2013;69:1014-1024.
- Alinaghi F, Calov M, Kristensen L, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.
- Villani A, Zouzaud M, Sevrain M, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol. 2015;73:242-248.
- Iragorri N, Hazlewood G, Manns B, et al. Model to determine the cost-effectiveness of screening psoriasis patients for psoriatic arthritis. Arth Car Res. 2021;73:266-274.
- Karreman M, Weel A, Van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology. 2017;56:597-602.
- Wilsdon TD, Whittle SL, Thynne TR, et al. Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev. 2019;1:CD012722. doi:10.1002/14651858.CD012722.pub2
- Mourad A, Gniadecki R. Treatment of dactylitis and enthesitis in psoriatic arthritis with biologic agents: a systematic review and metaanalysis. J Rheum. 2020;47:59-65.
- Wu D, Li C, Zhang S, et al. Effect of biologics on radiographic progression of peripheral joint in patients with psoriatic arthritis: meta-analysis. Rheumatology (Oxford). 2020;59:3172-3180.
- Mease P, Helliwell P, Fjellhaugen Hjuler K, et al. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis. 2021;80:185-193.
- McInnes I, Rahman P, Gottlieb A, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arth Rheum. 2022;74:475-485.
- Ytterberg S, Bhatt D, Mikuls T, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- Miller I, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. JAAD. 2013;69:1014-1024.
Glitter Effects of Nail Art on Optical Coherence Tomography
Practice Gap
Nail art can skew the results of optical coherence tomography (OCT), a noninvasive imaging technology that is used to visualize nail morphology in diseases such as psoriatic arthritis and onychomycosis, with a penetration depth of 2 mm and high-resolution images.1 Few studies have evaluated the effects of nail art on OCT. Saleah and colleagues1 found that clear, semitransparent, and red nail polishes do not interfere with visualization of the nail plate, whereas nontransparent gel polish and art stones obscure the image. They did not comment on the effect of glitter nail art in their study, though they did test 1 nail that contained glitter.1 Monpeurt et al2 compared matte and glossy nail polishes. They found that matte polish was readily identifiable from the nail plate, whereas glossy polish presented a greater number of artifacts.2
The Solution
We looked at 3 glitter nail polishes—gold, pink, and silver—that were scanned by OCT to assess the effect of the polish on the resulting image. We determined that glitter particles completely obscured the nail bed and nail plate, regardless of color (Figure 1). Glossy clear polish imparted a distinct film on the top of the nail plate that did not obscure the nail plate or the nail bed (Figure 2).
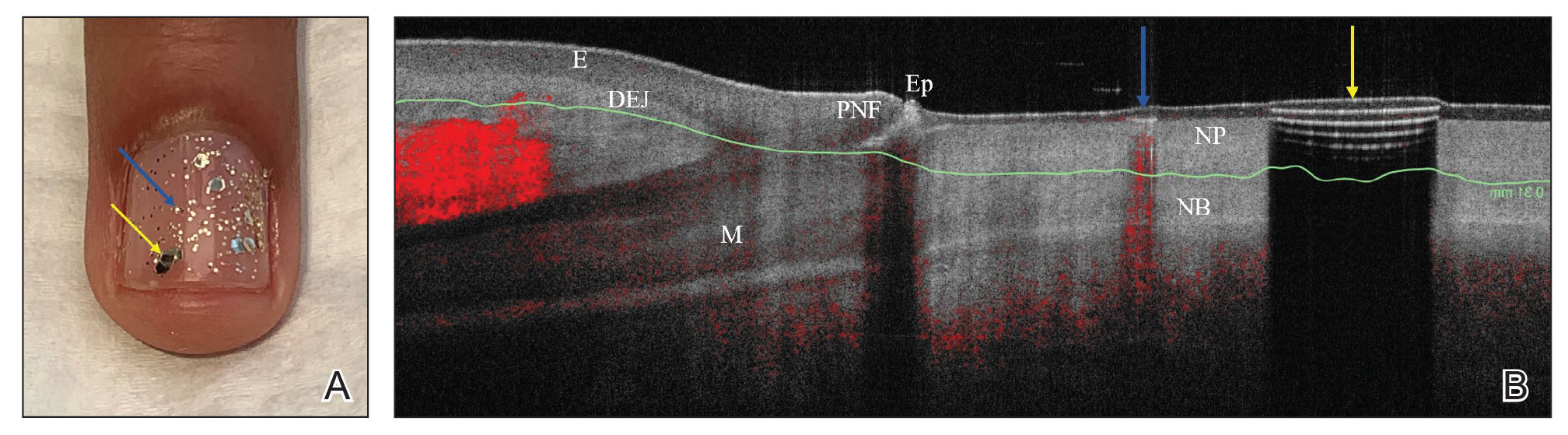
We conclude that glitter nail polish contains numerous reflective solid particles that interfere with OCT imaging of the nail plate and nail bed. As a result, we recommend removal of nail art to properly assess nail pathology. Because removal may need to be conducted by a nail technician, the treating clinician should inform the patient ahead of time to come to the appointment with bare (ie, unpolished) nails.
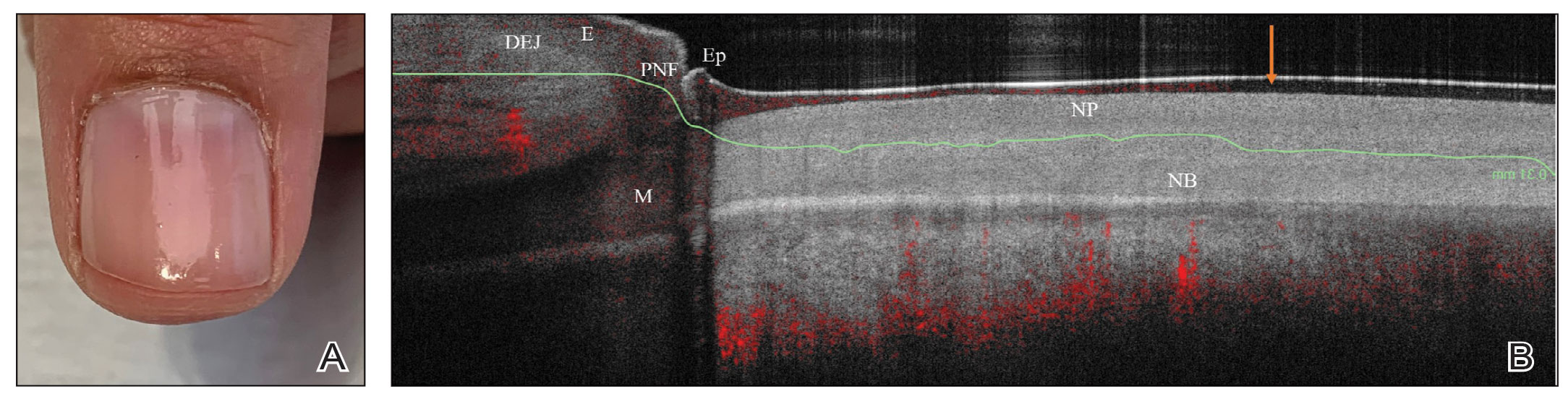
Practice Implications
Bringing awareness to the necessity of removing nail art prior to OCT imaging is crucial because many patients partake in its application, and removal may require the involvement of a professional nail technician. If a patient can be made aware that they should remove all nail art in advance, they will be better prepared for an OCT imaging session. Such a protocol increases efficiency, decreases diagnostic delay, and reduces cost associated with multiple office visits.
- Saleah S, Kim P, Seong D, et al. A preliminary study of post-progressive nail-art effects on in vivo nail plate using optical coherence tomography-based intensity profiling assessment. Sci Rep. 2021;11:666. doi:10.1038/s41598-020-79497-3
- Monpeurt C, Cinotti E, Hebert M, et al. Thickness and morphology assessment of nail polishes applied on nails by high-definition optical coherence tomography. Skin Res Technol. 2018;24:156-157. doi:10.1111/srt.12406
Practice Gap
Nail art can skew the results of optical coherence tomography (OCT), a noninvasive imaging technology that is used to visualize nail morphology in diseases such as psoriatic arthritis and onychomycosis, with a penetration depth of 2 mm and high-resolution images.1 Few studies have evaluated the effects of nail art on OCT. Saleah and colleagues1 found that clear, semitransparent, and red nail polishes do not interfere with visualization of the nail plate, whereas nontransparent gel polish and art stones obscure the image. They did not comment on the effect of glitter nail art in their study, though they did test 1 nail that contained glitter.1 Monpeurt et al2 compared matte and glossy nail polishes. They found that matte polish was readily identifiable from the nail plate, whereas glossy polish presented a greater number of artifacts.2
The Solution
We looked at 3 glitter nail polishes—gold, pink, and silver—that were scanned by OCT to assess the effect of the polish on the resulting image. We determined that glitter particles completely obscured the nail bed and nail plate, regardless of color (Figure 1). Glossy clear polish imparted a distinct film on the top of the nail plate that did not obscure the nail plate or the nail bed (Figure 2).

We conclude that glitter nail polish contains numerous reflective solid particles that interfere with OCT imaging of the nail plate and nail bed. As a result, we recommend removal of nail art to properly assess nail pathology. Because removal may need to be conducted by a nail technician, the treating clinician should inform the patient ahead of time to come to the appointment with bare (ie, unpolished) nails.

Practice Implications
Bringing awareness to the necessity of removing nail art prior to OCT imaging is crucial because many patients partake in its application, and removal may require the involvement of a professional nail technician. If a patient can be made aware that they should remove all nail art in advance, they will be better prepared for an OCT imaging session. Such a protocol increases efficiency, decreases diagnostic delay, and reduces cost associated with multiple office visits.
Practice Gap
Nail art can skew the results of optical coherence tomography (OCT), a noninvasive imaging technology that is used to visualize nail morphology in diseases such as psoriatic arthritis and onychomycosis, with a penetration depth of 2 mm and high-resolution images.1 Few studies have evaluated the effects of nail art on OCT. Saleah and colleagues1 found that clear, semitransparent, and red nail polishes do not interfere with visualization of the nail plate, whereas nontransparent gel polish and art stones obscure the image. They did not comment on the effect of glitter nail art in their study, though they did test 1 nail that contained glitter.1 Monpeurt et al2 compared matte and glossy nail polishes. They found that matte polish was readily identifiable from the nail plate, whereas glossy polish presented a greater number of artifacts.2
The Solution
We looked at 3 glitter nail polishes—gold, pink, and silver—that were scanned by OCT to assess the effect of the polish on the resulting image. We determined that glitter particles completely obscured the nail bed and nail plate, regardless of color (Figure 1). Glossy clear polish imparted a distinct film on the top of the nail plate that did not obscure the nail plate or the nail bed (Figure 2).

We conclude that glitter nail polish contains numerous reflective solid particles that interfere with OCT imaging of the nail plate and nail bed. As a result, we recommend removal of nail art to properly assess nail pathology. Because removal may need to be conducted by a nail technician, the treating clinician should inform the patient ahead of time to come to the appointment with bare (ie, unpolished) nails.

Practice Implications
Bringing awareness to the necessity of removing nail art prior to OCT imaging is crucial because many patients partake in its application, and removal may require the involvement of a professional nail technician. If a patient can be made aware that they should remove all nail art in advance, they will be better prepared for an OCT imaging session. Such a protocol increases efficiency, decreases diagnostic delay, and reduces cost associated with multiple office visits.
- Saleah S, Kim P, Seong D, et al. A preliminary study of post-progressive nail-art effects on in vivo nail plate using optical coherence tomography-based intensity profiling assessment. Sci Rep. 2021;11:666. doi:10.1038/s41598-020-79497-3
- Monpeurt C, Cinotti E, Hebert M, et al. Thickness and morphology assessment of nail polishes applied on nails by high-definition optical coherence tomography. Skin Res Technol. 2018;24:156-157. doi:10.1111/srt.12406
- Saleah S, Kim P, Seong D, et al. A preliminary study of post-progressive nail-art effects on in vivo nail plate using optical coherence tomography-based intensity profiling assessment. Sci Rep. 2021;11:666. doi:10.1038/s41598-020-79497-3
- Monpeurt C, Cinotti E, Hebert M, et al. Thickness and morphology assessment of nail polishes applied on nails by high-definition optical coherence tomography. Skin Res Technol. 2018;24:156-157. doi:10.1111/srt.12406
Enthesitis, arthritis, tenosynovitis linked to dupilumab use for atopic dermatitis
Around 5% of patients treated with dupilumab (Dupixent) for moderate-to-severe atopic dermatitis experience musculoskeletal (MSK) symptoms, according to the results of a descriptive study.
The main MSK symptom seen in the observational cohort was enthesitis, but some patients also experienced arthritis and tenosynovitis a median of 17 weeks after starting dupilumab treatment. Together these symptoms represent a new MSK syndrome, say researchers from the United Kingdom.
“The pattern of MSK symptoms and signs is characteristic of psoriatic arthritis/peripheral spondyloarthritis,” Bruce Kirkham, MD, and collaborators report in Arthritis & Rheumatology.
“We started a few years ago and have been following the patients for quite a long time,” Dr. Kirkham, a consultant rheumatologist at Guy’s and St. Thomas’ NHS Foundation Trust, London, told this news organization.
“We’re still seeing patients with the same type of syndrome presenting occasionally. It’s not a very common adverse event, but we think it continues,” he observed.
“Most of them don’t have very severe problems, and a lot of them can be treated with quite simple drugs or, alternatively, reducing the frequency of the injection,” Dr. Kirkham added.
Characterizing the MSK symptoms
Of 470 patients with atopic dermatitis who started treatment with dupilumab at Guy’s and St. Thomas’ NHS Foundation Trust between October 2018 and February 2021, 36 (7.65%) developed rheumatic symptoms and were referred to the rheumatology department. These individuals had their family history assessed and thorough MSK evaluations, which included antibody and inflammatory markers, ultrasound of the peripheral small joints, and MRI of the large joints and spine.
A total of 26 (5.5%) patients – 14 of whom were male – had inflammatory enthesitis, arthritis, and/or tenosynovitis. Of the others, seven had osteoarthritis and three had degenerative spine disease.
Enthesitis was the most common finding in those with rheumatic symptoms, occurring on its own in 11 patients, with arthritis in three patients, and tenosynovitis in two patients.
These symptoms appeared 2-48 weeks after starting dupilumab treatment and were categorized as mild in 16 (61%) cases, moderate in six cases, and severe in four cases.
No specific predictors of the MSK symptoms seen were noted. Patient age, sex, duration of their atopic dermatitis, or how their skin condition had been previously treated did not help identify those who might develop rheumatic problems.
Conservative management approach
All patients had “outstanding” responses to treatment, Dr. Kirkham noted: The mean Eczema Area and Severity Index score before dupilumab treatment was 21, falling to 4.2 with treatment, indicating a mean 80% improvement.
Co-author Joseph Nathan, MBChB, of London North West Healthcare NHS Trust, who collaborated on the research while working within Dr. Kirkham’s group, said separately: “The concern that patients have is that when they start a medication and develop a side effect is that the medication is going to be stopped.”
Clinicians treating the patients took a conservative approach, prescribing NSAIDs such as cyclooxygenase-2 inhibitors or altering the frequency with which dupilumab was given.
With this approach, MSK symptoms resolved in 15 patients who remained on treatment and in seven who had to stop dupilumab. There were four patients, however, who had unresolved symptoms even once dupilumab treatment had been stopped.
Altering the local cytokine balance
Dupilumab is a monoclonal antibody that binds to the alpha subunit of the interleukin-4 receptor. This results in blocking the function of not only IL-4 but also IL-13.
Dr. Kirkham and colleagues think this might not only alter the balance of cytokines in the skin but also in the joints and entheses with IL-17, IL-23, or even tumor necrosis factor playing a possible role. Another thought is that many circulating T-cells in the skin move to the joints and entheses to trigger symptoms.
IL-13 inhibition does seem to be important, as another British research team, from the Centre for Epidemiology Versus Arthritis at the University of Manchester (England), has found.
At the recent annual meeting of the British Society for Rheumatology, Sizheng Steven Zhao, MBChB, PhD, and colleagues reported that among people who carried a genetic variant predisposing them to having low IL-13 function, there was a higher risk for inflammatory diseases such as psoriatic arthritis and other spondyloarthropathy-related diseases.
Indeed, when the single nucleotide polymorphism rs20541 was present, the odds for having psoriatic arthritis and psoriasis were higher than when it was not.
The findings are consistent with the idea that IL-4 and IL-13 may be acting as a restraint towards MSK diseases in some patients, Dr. Zhao and co-authors suggest.
“The genetic data supports what [Dr. Kirkham and team] have said from a mechanistic point of view,” Dr. Zhao said in an interview. “What you’re observing has a genetic basis.”
Dermatology perspective
Approved by the U.S. Food and Drug Administration in 2017, dupilumab has since been hailed as a “breakthrough” in atopic dermatitis treatment. Given as a subcutaneous injection every 2 weeks, it provides a much-needed option for people who have moderate-to-severe disease and have tried other available treatments, including corticosteroids.
Dupilumab has since also been approved for asthma, chronic sinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis and is used off-label for other skin conditions such as contact dermatitis, chronic spontaneous urticaria, and alopecia areata.
“Dupilumab, like a lot of medications for atopic dermatitis, is a relatively new drug, and we are still learning about its safety,” Joel M. Gelfand, MD, MSCE, of the University of Pennsylvania Perelman School of Medicine, Philadelphia, told this news organization.
“Inflammatory arthritis has been reported in patients treated with dupilumab, and this new study provides some useful estimates,” added Dr. Gelfand, who is a professor of dermatology and epidemiology and directs the Psoriasis and Phototherapy Treatment Center, Philadelphia.
“There was no control group,” Dr. Gelfand said, so “a causal relationship cannot be well established based on these data alone. The mechanism is not known but may result from a shifting of the immune system.”
Dr. Zhao observed: “We don’t know what the natural history of these adverse events is. We don’t know if stopping the drug early will prevent long-term adverse events. So, we don’t know if people will ultimately develop permanent psoriatic arthritis if we don’t intervene quick enough when we observe an adverse event.”
Being aware of the possibility of rheumatic side effects occurring with dupilumab and similar agents is key, Dr. Gelfand and Dr. Kirkham both said independently.
“I have personally seen this entity in my practice,” Dr. Gelfand said. “It is important to clinicians prescribing dupilumab to alert patients about this potential side effect and ask about joint symptoms in follow-up.”
Dr. Kirkham said: “Prescribers need to be aware of it, because up until now it’s been just very vaguely discussed as sort of aches and pains, arthralgias, and it’s a much more specific of a kind of syndrome of enthesitis, arthritis, tenosynovitis – a little like psoriatic arthritis.”
Not everyone has come across these side effects, however, as Steven Daveluy, MD, associate professor and dermatology program director at Wayne State University, Detroit, said in an interview.
“This article and the other case series both noted the musculoskeletal symptoms occurred in about 5% of patients, which surprised me since I haven’t seen it in my practice and have enough patients being treated with dupilumab that I would expect to see a case at that rate,” Dr. Daveluy said.
“The majority of cases are mild and respond to treatment with anti-inflammatories like naproxen, which is available over the counter. It’s likely that patients with a mild case could simply treat their pain with naproxen that’s already in their medicine cabinet until it resolves, never bringing it to the doctor’s attention,” he suggested.
“Dupilumab is still a safe and effective medication that can change the lives of patients suffering from atopic dermatitis,” he said.
“Awareness of this potential side effect can help dermatologists recognize it early and work together with patients to determine the best course of action.”
All research mentioned in this article was independently supported. Dr. Kirkham, Mr. Nathan, Dr. Zhao, and Dr. Daveluy report no relevant financial relationships. Dr. Gelfand has served as a consultant for numerous pharmaceutical companies and receives research grants from Amgen, Boehringer Ingelheim, and Pfizer. He is a co-patent holder of resiquimod for treatment of cutaneous T-cell lymphoma.
A version of this article first appeared on Medscape.com.
Around 5% of patients treated with dupilumab (Dupixent) for moderate-to-severe atopic dermatitis experience musculoskeletal (MSK) symptoms, according to the results of a descriptive study.
The main MSK symptom seen in the observational cohort was enthesitis, but some patients also experienced arthritis and tenosynovitis a median of 17 weeks after starting dupilumab treatment. Together these symptoms represent a new MSK syndrome, say researchers from the United Kingdom.
“The pattern of MSK symptoms and signs is characteristic of psoriatic arthritis/peripheral spondyloarthritis,” Bruce Kirkham, MD, and collaborators report in Arthritis & Rheumatology.
“We started a few years ago and have been following the patients for quite a long time,” Dr. Kirkham, a consultant rheumatologist at Guy’s and St. Thomas’ NHS Foundation Trust, London, told this news organization.
“We’re still seeing patients with the same type of syndrome presenting occasionally. It’s not a very common adverse event, but we think it continues,” he observed.
“Most of them don’t have very severe problems, and a lot of them can be treated with quite simple drugs or, alternatively, reducing the frequency of the injection,” Dr. Kirkham added.
Characterizing the MSK symptoms
Of 470 patients with atopic dermatitis who started treatment with dupilumab at Guy’s and St. Thomas’ NHS Foundation Trust between October 2018 and February 2021, 36 (7.65%) developed rheumatic symptoms and were referred to the rheumatology department. These individuals had their family history assessed and thorough MSK evaluations, which included antibody and inflammatory markers, ultrasound of the peripheral small joints, and MRI of the large joints and spine.
A total of 26 (5.5%) patients – 14 of whom were male – had inflammatory enthesitis, arthritis, and/or tenosynovitis. Of the others, seven had osteoarthritis and three had degenerative spine disease.
Enthesitis was the most common finding in those with rheumatic symptoms, occurring on its own in 11 patients, with arthritis in three patients, and tenosynovitis in two patients.
These symptoms appeared 2-48 weeks after starting dupilumab treatment and were categorized as mild in 16 (61%) cases, moderate in six cases, and severe in four cases.
No specific predictors of the MSK symptoms seen were noted. Patient age, sex, duration of their atopic dermatitis, or how their skin condition had been previously treated did not help identify those who might develop rheumatic problems.
Conservative management approach
All patients had “outstanding” responses to treatment, Dr. Kirkham noted: The mean Eczema Area and Severity Index score before dupilumab treatment was 21, falling to 4.2 with treatment, indicating a mean 80% improvement.
Co-author Joseph Nathan, MBChB, of London North West Healthcare NHS Trust, who collaborated on the research while working within Dr. Kirkham’s group, said separately: “The concern that patients have is that when they start a medication and develop a side effect is that the medication is going to be stopped.”
Clinicians treating the patients took a conservative approach, prescribing NSAIDs such as cyclooxygenase-2 inhibitors or altering the frequency with which dupilumab was given.
With this approach, MSK symptoms resolved in 15 patients who remained on treatment and in seven who had to stop dupilumab. There were four patients, however, who had unresolved symptoms even once dupilumab treatment had been stopped.
Altering the local cytokine balance
Dupilumab is a monoclonal antibody that binds to the alpha subunit of the interleukin-4 receptor. This results in blocking the function of not only IL-4 but also IL-13.
Dr. Kirkham and colleagues think this might not only alter the balance of cytokines in the skin but also in the joints and entheses with IL-17, IL-23, or even tumor necrosis factor playing a possible role. Another thought is that many circulating T-cells in the skin move to the joints and entheses to trigger symptoms.
IL-13 inhibition does seem to be important, as another British research team, from the Centre for Epidemiology Versus Arthritis at the University of Manchester (England), has found.
At the recent annual meeting of the British Society for Rheumatology, Sizheng Steven Zhao, MBChB, PhD, and colleagues reported that among people who carried a genetic variant predisposing them to having low IL-13 function, there was a higher risk for inflammatory diseases such as psoriatic arthritis and other spondyloarthropathy-related diseases.
Indeed, when the single nucleotide polymorphism rs20541 was present, the odds for having psoriatic arthritis and psoriasis were higher than when it was not.
The findings are consistent with the idea that IL-4 and IL-13 may be acting as a restraint towards MSK diseases in some patients, Dr. Zhao and co-authors suggest.
“The genetic data supports what [Dr. Kirkham and team] have said from a mechanistic point of view,” Dr. Zhao said in an interview. “What you’re observing has a genetic basis.”
Dermatology perspective
Approved by the U.S. Food and Drug Administration in 2017, dupilumab has since been hailed as a “breakthrough” in atopic dermatitis treatment. Given as a subcutaneous injection every 2 weeks, it provides a much-needed option for people who have moderate-to-severe disease and have tried other available treatments, including corticosteroids.
Dupilumab has since also been approved for asthma, chronic sinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis and is used off-label for other skin conditions such as contact dermatitis, chronic spontaneous urticaria, and alopecia areata.
“Dupilumab, like a lot of medications for atopic dermatitis, is a relatively new drug, and we are still learning about its safety,” Joel M. Gelfand, MD, MSCE, of the University of Pennsylvania Perelman School of Medicine, Philadelphia, told this news organization.
“Inflammatory arthritis has been reported in patients treated with dupilumab, and this new study provides some useful estimates,” added Dr. Gelfand, who is a professor of dermatology and epidemiology and directs the Psoriasis and Phototherapy Treatment Center, Philadelphia.
“There was no control group,” Dr. Gelfand said, so “a causal relationship cannot be well established based on these data alone. The mechanism is not known but may result from a shifting of the immune system.”
Dr. Zhao observed: “We don’t know what the natural history of these adverse events is. We don’t know if stopping the drug early will prevent long-term adverse events. So, we don’t know if people will ultimately develop permanent psoriatic arthritis if we don’t intervene quick enough when we observe an adverse event.”
Being aware of the possibility of rheumatic side effects occurring with dupilumab and similar agents is key, Dr. Gelfand and Dr. Kirkham both said independently.
“I have personally seen this entity in my practice,” Dr. Gelfand said. “It is important to clinicians prescribing dupilumab to alert patients about this potential side effect and ask about joint symptoms in follow-up.”
Dr. Kirkham said: “Prescribers need to be aware of it, because up until now it’s been just very vaguely discussed as sort of aches and pains, arthralgias, and it’s a much more specific of a kind of syndrome of enthesitis, arthritis, tenosynovitis – a little like psoriatic arthritis.”
Not everyone has come across these side effects, however, as Steven Daveluy, MD, associate professor and dermatology program director at Wayne State University, Detroit, said in an interview.
“This article and the other case series both noted the musculoskeletal symptoms occurred in about 5% of patients, which surprised me since I haven’t seen it in my practice and have enough patients being treated with dupilumab that I would expect to see a case at that rate,” Dr. Daveluy said.
“The majority of cases are mild and respond to treatment with anti-inflammatories like naproxen, which is available over the counter. It’s likely that patients with a mild case could simply treat their pain with naproxen that’s already in their medicine cabinet until it resolves, never bringing it to the doctor’s attention,” he suggested.
“Dupilumab is still a safe and effective medication that can change the lives of patients suffering from atopic dermatitis,” he said.
“Awareness of this potential side effect can help dermatologists recognize it early and work together with patients to determine the best course of action.”
All research mentioned in this article was independently supported. Dr. Kirkham, Mr. Nathan, Dr. Zhao, and Dr. Daveluy report no relevant financial relationships. Dr. Gelfand has served as a consultant for numerous pharmaceutical companies and receives research grants from Amgen, Boehringer Ingelheim, and Pfizer. He is a co-patent holder of resiquimod for treatment of cutaneous T-cell lymphoma.
A version of this article first appeared on Medscape.com.
Around 5% of patients treated with dupilumab (Dupixent) for moderate-to-severe atopic dermatitis experience musculoskeletal (MSK) symptoms, according to the results of a descriptive study.
The main MSK symptom seen in the observational cohort was enthesitis, but some patients also experienced arthritis and tenosynovitis a median of 17 weeks after starting dupilumab treatment. Together these symptoms represent a new MSK syndrome, say researchers from the United Kingdom.
“The pattern of MSK symptoms and signs is characteristic of psoriatic arthritis/peripheral spondyloarthritis,” Bruce Kirkham, MD, and collaborators report in Arthritis & Rheumatology.
“We started a few years ago and have been following the patients for quite a long time,” Dr. Kirkham, a consultant rheumatologist at Guy’s and St. Thomas’ NHS Foundation Trust, London, told this news organization.
“We’re still seeing patients with the same type of syndrome presenting occasionally. It’s not a very common adverse event, but we think it continues,” he observed.
“Most of them don’t have very severe problems, and a lot of them can be treated with quite simple drugs or, alternatively, reducing the frequency of the injection,” Dr. Kirkham added.
Characterizing the MSK symptoms
Of 470 patients with atopic dermatitis who started treatment with dupilumab at Guy’s and St. Thomas’ NHS Foundation Trust between October 2018 and February 2021, 36 (7.65%) developed rheumatic symptoms and were referred to the rheumatology department. These individuals had their family history assessed and thorough MSK evaluations, which included antibody and inflammatory markers, ultrasound of the peripheral small joints, and MRI of the large joints and spine.
A total of 26 (5.5%) patients – 14 of whom were male – had inflammatory enthesitis, arthritis, and/or tenosynovitis. Of the others, seven had osteoarthritis and three had degenerative spine disease.
Enthesitis was the most common finding in those with rheumatic symptoms, occurring on its own in 11 patients, with arthritis in three patients, and tenosynovitis in two patients.
These symptoms appeared 2-48 weeks after starting dupilumab treatment and were categorized as mild in 16 (61%) cases, moderate in six cases, and severe in four cases.
No specific predictors of the MSK symptoms seen were noted. Patient age, sex, duration of their atopic dermatitis, or how their skin condition had been previously treated did not help identify those who might develop rheumatic problems.
Conservative management approach
All patients had “outstanding” responses to treatment, Dr. Kirkham noted: The mean Eczema Area and Severity Index score before dupilumab treatment was 21, falling to 4.2 with treatment, indicating a mean 80% improvement.
Co-author Joseph Nathan, MBChB, of London North West Healthcare NHS Trust, who collaborated on the research while working within Dr. Kirkham’s group, said separately: “The concern that patients have is that when they start a medication and develop a side effect is that the medication is going to be stopped.”
Clinicians treating the patients took a conservative approach, prescribing NSAIDs such as cyclooxygenase-2 inhibitors or altering the frequency with which dupilumab was given.
With this approach, MSK symptoms resolved in 15 patients who remained on treatment and in seven who had to stop dupilumab. There were four patients, however, who had unresolved symptoms even once dupilumab treatment had been stopped.
Altering the local cytokine balance
Dupilumab is a monoclonal antibody that binds to the alpha subunit of the interleukin-4 receptor. This results in blocking the function of not only IL-4 but also IL-13.
Dr. Kirkham and colleagues think this might not only alter the balance of cytokines in the skin but also in the joints and entheses with IL-17, IL-23, or even tumor necrosis factor playing a possible role. Another thought is that many circulating T-cells in the skin move to the joints and entheses to trigger symptoms.
IL-13 inhibition does seem to be important, as another British research team, from the Centre for Epidemiology Versus Arthritis at the University of Manchester (England), has found.
At the recent annual meeting of the British Society for Rheumatology, Sizheng Steven Zhao, MBChB, PhD, and colleagues reported that among people who carried a genetic variant predisposing them to having low IL-13 function, there was a higher risk for inflammatory diseases such as psoriatic arthritis and other spondyloarthropathy-related diseases.
Indeed, when the single nucleotide polymorphism rs20541 was present, the odds for having psoriatic arthritis and psoriasis were higher than when it was not.
The findings are consistent with the idea that IL-4 and IL-13 may be acting as a restraint towards MSK diseases in some patients, Dr. Zhao and co-authors suggest.
“The genetic data supports what [Dr. Kirkham and team] have said from a mechanistic point of view,” Dr. Zhao said in an interview. “What you’re observing has a genetic basis.”
Dermatology perspective
Approved by the U.S. Food and Drug Administration in 2017, dupilumab has since been hailed as a “breakthrough” in atopic dermatitis treatment. Given as a subcutaneous injection every 2 weeks, it provides a much-needed option for people who have moderate-to-severe disease and have tried other available treatments, including corticosteroids.
Dupilumab has since also been approved for asthma, chronic sinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis and is used off-label for other skin conditions such as contact dermatitis, chronic spontaneous urticaria, and alopecia areata.
“Dupilumab, like a lot of medications for atopic dermatitis, is a relatively new drug, and we are still learning about its safety,” Joel M. Gelfand, MD, MSCE, of the University of Pennsylvania Perelman School of Medicine, Philadelphia, told this news organization.
“Inflammatory arthritis has been reported in patients treated with dupilumab, and this new study provides some useful estimates,” added Dr. Gelfand, who is a professor of dermatology and epidemiology and directs the Psoriasis and Phototherapy Treatment Center, Philadelphia.
“There was no control group,” Dr. Gelfand said, so “a causal relationship cannot be well established based on these data alone. The mechanism is not known but may result from a shifting of the immune system.”
Dr. Zhao observed: “We don’t know what the natural history of these adverse events is. We don’t know if stopping the drug early will prevent long-term adverse events. So, we don’t know if people will ultimately develop permanent psoriatic arthritis if we don’t intervene quick enough when we observe an adverse event.”
Being aware of the possibility of rheumatic side effects occurring with dupilumab and similar agents is key, Dr. Gelfand and Dr. Kirkham both said independently.
“I have personally seen this entity in my practice,” Dr. Gelfand said. “It is important to clinicians prescribing dupilumab to alert patients about this potential side effect and ask about joint symptoms in follow-up.”
Dr. Kirkham said: “Prescribers need to be aware of it, because up until now it’s been just very vaguely discussed as sort of aches and pains, arthralgias, and it’s a much more specific of a kind of syndrome of enthesitis, arthritis, tenosynovitis – a little like psoriatic arthritis.”
Not everyone has come across these side effects, however, as Steven Daveluy, MD, associate professor and dermatology program director at Wayne State University, Detroit, said in an interview.
“This article and the other case series both noted the musculoskeletal symptoms occurred in about 5% of patients, which surprised me since I haven’t seen it in my practice and have enough patients being treated with dupilumab that I would expect to see a case at that rate,” Dr. Daveluy said.
“The majority of cases are mild and respond to treatment with anti-inflammatories like naproxen, which is available over the counter. It’s likely that patients with a mild case could simply treat their pain with naproxen that’s already in their medicine cabinet until it resolves, never bringing it to the doctor’s attention,” he suggested.
“Dupilumab is still a safe and effective medication that can change the lives of patients suffering from atopic dermatitis,” he said.
“Awareness of this potential side effect can help dermatologists recognize it early and work together with patients to determine the best course of action.”
All research mentioned in this article was independently supported. Dr. Kirkham, Mr. Nathan, Dr. Zhao, and Dr. Daveluy report no relevant financial relationships. Dr. Gelfand has served as a consultant for numerous pharmaceutical companies and receives research grants from Amgen, Boehringer Ingelheim, and Pfizer. He is a co-patent holder of resiquimod for treatment of cutaneous T-cell lymphoma.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS & RHEUMATOLOGY
ILD risk elevated in RA, PsA after starting biologic or targeted synthetic DMARDs
MILAN – Patients with psoriatic arthritis (PsA) who are using biologic and targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) have fivefold higher risk for interstitial lung disease (ILD) than does the general population, according to the first study to explore risk of ILD in this particular patient group.
The study also found 10-fold higher risk of ILD in patients with RA who were starting a b/tsDMARD, compared with the general population, while the addition of methotrexate did not appear to be associated with increased risk for ILD in either RA nor PsA.
Sella Aarrestad Provan, MD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases at Diakonhjemmet Hospital, Oslo, presented the results at the annual European Congress of Rheumatology.
Explaining the motivation for the study, Dr. Aarrestad Provan said that, in RA, methotrexate’s role in ILD development remained unclear, while some small studies linked b/tsDMARDs with risk for ILD. “In PsA, very few studies have explored the risk of ILD, and no systematic studies have looked at ILD risk factors in this disease.”
The researchers analyzed patient data from hospital and death registries across five Nordic countries (Denmark, Norway, Finland, Iceland, and Sweden) and compared them with general population controls. They calculated risk ratios for people who developed ILD within 5 years of starting a b/tsDMARD (with or without methotrexate).
A total of 37,010 patients with RA, 12,341 with PsA, and 569,451 members of the general population were included in the analysis, with respective disease durations of 10 and 8.9 years. Methotrexate was used along with b/tsDMARDs in 49% of patients with RA and 41% with PsA, and most patients were already on methotrexate when b/tsDMARDs were started. The tumor necrosis factor inhibitor etanercept (Enbrel) was the most commonly used b/tsDMARD in both RA and PsA, followed by infliximab (Remicade and biosimilars) and adalimumab (Humira and biosimilars).
The incidence of ILD within 5 years of starting a b/tsDMARD was 0.8% in patients with RA, 0.2% with PsA, and 0.1% in the general population, and these findings generated hazard ratios of 10.1 (95% confidence interval, 8.6-11.9) for RA and 5.0 (95% CI, 3.4-7.4) for PsA, compared with the general population as reference.
When the risk for ILD was explored according to methotrexate use in RA patients, “there was no signal of increased risk across patients using methotrexate,” Dr. Aarrestad Provan reported. When risk of ILD was explored according to b/tsDMARD use in RA patients, a signal of increased risk was observed with rituximab, she noted, “but upon adjusting for age, sex, and comorbidities, this association was no longer significant, but was still numerically increased.”
Iain McInnes, MD, PhD, vice principal, professor of rheumatology, and head of the College of Medical, Veterinary and Life Sciences at the University of Glasgow, remarked that he “loves results that are unexpected” and thanked the researcher for such an “important study.”
“For years, we’ve been interested in the potential for DMARDs to impact interstitial lung disease, with potential that drugs could make it worse, or better,” he said. “This study is wonderful and novel because first of all, there hasn’t, until now, been a direct comparison between RA and PsA in quite this way, and secondly, we haven’t really assessed whether there is a drug-related risk in PsA. Note that drug related does not necessarily imply causality.”
Regarding mechanisms, Dr. McInnes added that “epidemiologic studies suggest that PsA often coexists with the presence of cardiometabolic syndrome and obesity, which has a higher prevalence in PsA than in RA. Obesity is also related to ILD. As such, it begs the question of whether cardiometabolic, diabetes, or obesity-related features may give us a clue as to what is going on in these PsA patients.”
The research was supported by NordForsk and FOREUM. Dr. Aarrestad Provan reported serving as a consultant to Boehringer Ingelheim and Novartis and receiving grant/research support from Boehringer Ingelheim. Dr. McInnes declared no disclosures relevant to this study.
MILAN – Patients with psoriatic arthritis (PsA) who are using biologic and targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) have fivefold higher risk for interstitial lung disease (ILD) than does the general population, according to the first study to explore risk of ILD in this particular patient group.
The study also found 10-fold higher risk of ILD in patients with RA who were starting a b/tsDMARD, compared with the general population, while the addition of methotrexate did not appear to be associated with increased risk for ILD in either RA nor PsA.
Sella Aarrestad Provan, MD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases at Diakonhjemmet Hospital, Oslo, presented the results at the annual European Congress of Rheumatology.
Explaining the motivation for the study, Dr. Aarrestad Provan said that, in RA, methotrexate’s role in ILD development remained unclear, while some small studies linked b/tsDMARDs with risk for ILD. “In PsA, very few studies have explored the risk of ILD, and no systematic studies have looked at ILD risk factors in this disease.”
The researchers analyzed patient data from hospital and death registries across five Nordic countries (Denmark, Norway, Finland, Iceland, and Sweden) and compared them with general population controls. They calculated risk ratios for people who developed ILD within 5 years of starting a b/tsDMARD (with or without methotrexate).
A total of 37,010 patients with RA, 12,341 with PsA, and 569,451 members of the general population were included in the analysis, with respective disease durations of 10 and 8.9 years. Methotrexate was used along with b/tsDMARDs in 49% of patients with RA and 41% with PsA, and most patients were already on methotrexate when b/tsDMARDs were started. The tumor necrosis factor inhibitor etanercept (Enbrel) was the most commonly used b/tsDMARD in both RA and PsA, followed by infliximab (Remicade and biosimilars) and adalimumab (Humira and biosimilars).
The incidence of ILD within 5 years of starting a b/tsDMARD was 0.8% in patients with RA, 0.2% with PsA, and 0.1% in the general population, and these findings generated hazard ratios of 10.1 (95% confidence interval, 8.6-11.9) for RA and 5.0 (95% CI, 3.4-7.4) for PsA, compared with the general population as reference.
When the risk for ILD was explored according to methotrexate use in RA patients, “there was no signal of increased risk across patients using methotrexate,” Dr. Aarrestad Provan reported. When risk of ILD was explored according to b/tsDMARD use in RA patients, a signal of increased risk was observed with rituximab, she noted, “but upon adjusting for age, sex, and comorbidities, this association was no longer significant, but was still numerically increased.”
Iain McInnes, MD, PhD, vice principal, professor of rheumatology, and head of the College of Medical, Veterinary and Life Sciences at the University of Glasgow, remarked that he “loves results that are unexpected” and thanked the researcher for such an “important study.”
“For years, we’ve been interested in the potential for DMARDs to impact interstitial lung disease, with potential that drugs could make it worse, or better,” he said. “This study is wonderful and novel because first of all, there hasn’t, until now, been a direct comparison between RA and PsA in quite this way, and secondly, we haven’t really assessed whether there is a drug-related risk in PsA. Note that drug related does not necessarily imply causality.”
Regarding mechanisms, Dr. McInnes added that “epidemiologic studies suggest that PsA often coexists with the presence of cardiometabolic syndrome and obesity, which has a higher prevalence in PsA than in RA. Obesity is also related to ILD. As such, it begs the question of whether cardiometabolic, diabetes, or obesity-related features may give us a clue as to what is going on in these PsA patients.”
The research was supported by NordForsk and FOREUM. Dr. Aarrestad Provan reported serving as a consultant to Boehringer Ingelheim and Novartis and receiving grant/research support from Boehringer Ingelheim. Dr. McInnes declared no disclosures relevant to this study.
MILAN – Patients with psoriatic arthritis (PsA) who are using biologic and targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) have fivefold higher risk for interstitial lung disease (ILD) than does the general population, according to the first study to explore risk of ILD in this particular patient group.
The study also found 10-fold higher risk of ILD in patients with RA who were starting a b/tsDMARD, compared with the general population, while the addition of methotrexate did not appear to be associated with increased risk for ILD in either RA nor PsA.
Sella Aarrestad Provan, MD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases at Diakonhjemmet Hospital, Oslo, presented the results at the annual European Congress of Rheumatology.
Explaining the motivation for the study, Dr. Aarrestad Provan said that, in RA, methotrexate’s role in ILD development remained unclear, while some small studies linked b/tsDMARDs with risk for ILD. “In PsA, very few studies have explored the risk of ILD, and no systematic studies have looked at ILD risk factors in this disease.”
The researchers analyzed patient data from hospital and death registries across five Nordic countries (Denmark, Norway, Finland, Iceland, and Sweden) and compared them with general population controls. They calculated risk ratios for people who developed ILD within 5 years of starting a b/tsDMARD (with or without methotrexate).
A total of 37,010 patients with RA, 12,341 with PsA, and 569,451 members of the general population were included in the analysis, with respective disease durations of 10 and 8.9 years. Methotrexate was used along with b/tsDMARDs in 49% of patients with RA and 41% with PsA, and most patients were already on methotrexate when b/tsDMARDs were started. The tumor necrosis factor inhibitor etanercept (Enbrel) was the most commonly used b/tsDMARD in both RA and PsA, followed by infliximab (Remicade and biosimilars) and adalimumab (Humira and biosimilars).
The incidence of ILD within 5 years of starting a b/tsDMARD was 0.8% in patients with RA, 0.2% with PsA, and 0.1% in the general population, and these findings generated hazard ratios of 10.1 (95% confidence interval, 8.6-11.9) for RA and 5.0 (95% CI, 3.4-7.4) for PsA, compared with the general population as reference.
When the risk for ILD was explored according to methotrexate use in RA patients, “there was no signal of increased risk across patients using methotrexate,” Dr. Aarrestad Provan reported. When risk of ILD was explored according to b/tsDMARD use in RA patients, a signal of increased risk was observed with rituximab, she noted, “but upon adjusting for age, sex, and comorbidities, this association was no longer significant, but was still numerically increased.”
Iain McInnes, MD, PhD, vice principal, professor of rheumatology, and head of the College of Medical, Veterinary and Life Sciences at the University of Glasgow, remarked that he “loves results that are unexpected” and thanked the researcher for such an “important study.”
“For years, we’ve been interested in the potential for DMARDs to impact interstitial lung disease, with potential that drugs could make it worse, or better,” he said. “This study is wonderful and novel because first of all, there hasn’t, until now, been a direct comparison between RA and PsA in quite this way, and secondly, we haven’t really assessed whether there is a drug-related risk in PsA. Note that drug related does not necessarily imply causality.”
Regarding mechanisms, Dr. McInnes added that “epidemiologic studies suggest that PsA often coexists with the presence of cardiometabolic syndrome and obesity, which has a higher prevalence in PsA than in RA. Obesity is also related to ILD. As such, it begs the question of whether cardiometabolic, diabetes, or obesity-related features may give us a clue as to what is going on in these PsA patients.”
The research was supported by NordForsk and FOREUM. Dr. Aarrestad Provan reported serving as a consultant to Boehringer Ingelheim and Novartis and receiving grant/research support from Boehringer Ingelheim. Dr. McInnes declared no disclosures relevant to this study.
AT EULAR 2023
FDA approves Yuflyma as ninth adalimumab biosimilar
The U.S. Food and Drug Administration has approved the biosimilar adalimumab-aaty (Yuflyma) in a citrate-free, high-concentration formulation, the manufacturer, Celltrion USA, announced today. It is the ninth biosimilar of adalimumab (Humira) to be approved in the United States.
Yuflyma is approved for the treatment of adult patients with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, plaque psoriasis, and hidradenitis suppurativa. It is also approved for polyarticular juvenile idiopathic arthritis for patients aged 2 years or older, as well as for Crohn’s disease in adults and in pediatric patients aged 6 years or older.
The formulation was approved on the basis of a comprehensive data package of analytic, preclinical, and clinical studies, according to Celltrion USA, “demonstrating that Yuflyma is comparable to the reference product Humira in terms of efficacy, safety, pharmacokinetics, and immunogenicity up to 24 weeks and 1 year following treatment.”
The company conducted a double-blind, randomized phase 3 trial that compared switching from reference adalimumab to Yuflyma with continuing either reference adalimumab or Yuflyma for patients with active rheumatoid arthritis. In that trial, the efficacy, pharmacokinetics, safety, and immunogenicity of Yuflyma and reference adalimumab were comparable after 1 year of treatment, including after switching from reference adalimumab to Yuflyma.
“Currently, more than 80% of patients treated with Humira in the United States rely on a high-concentration and citrate-free formulation of this medication. The availability of a high-concentration and citrate-free formulation adalimumab biosimilar provides an important treatment option for patients with inflammatory diseases who benefit from this effective therapy,” said Jonathan Kay, MD, of the University of Massachusetts, Worcester, in the press release.
The citrate-free formulation is thought to lead to less pain on injection.
Yuflyma will be available in prefilled syringe and autoinjector administration options.
Celltrion USA plans to market the drug in the United States in July 2023. Following the initial launch of 40 mg/0.4 mL, the company plans to launch dose forms of 80 mg/0.8 mL and 20 mg/0.2 mL.
Celltrion USA is also seeking an interchangeability designation from the FDA following the completion of an interchangeability trial of 366 patients with chronic plaque psoriasis. The interchangeability designation would mean that patients successfully switched from Humira to Yuflyma multiple times in the trial. The interchangeability designation would allow pharmacists to autosubstitute Humira with Yuflyma. In these cases, individual state laws control how and whether physicians will be notified of this switch.
If interchangeability is approved for Yuflyma, which the company tentatively expects in the fourth quarter of 2024, it would be just the third interchangeable biosimilar approved by the FDA overall and the second adalimumab biosimilar to be designated as such, after adalimumab-adbm (Cyltezo) in October 2021.
Yuflyma was approved in Canada in December 2021 for 10 indications: rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, adult ulcerative colitis, hidradenitis suppurativa, plaque psoriasis, adult uveitis, and pediatric uveitis.
In February 2022, the European Commission granted marketing authorization for Yuflyma across those 10 indications, as well as for nonradiographic axial spondyloarthritis, pediatric plaque psoriasis, and pediatric Crohn’s disease.
In April 2022, Celltrion USA signed a licensing agreement with AbbVie, the manufacturer of Humira. Under that agreement, Celltrion will pay royalties to AbbVie on sales of their individual biosimilars, and AbbVie agreed to drop all patent litigation.
The full prescribing information for Yuflyma is available here.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved the biosimilar adalimumab-aaty (Yuflyma) in a citrate-free, high-concentration formulation, the manufacturer, Celltrion USA, announced today. It is the ninth biosimilar of adalimumab (Humira) to be approved in the United States.
Yuflyma is approved for the treatment of adult patients with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, plaque psoriasis, and hidradenitis suppurativa. It is also approved for polyarticular juvenile idiopathic arthritis for patients aged 2 years or older, as well as for Crohn’s disease in adults and in pediatric patients aged 6 years or older.
The formulation was approved on the basis of a comprehensive data package of analytic, preclinical, and clinical studies, according to Celltrion USA, “demonstrating that Yuflyma is comparable to the reference product Humira in terms of efficacy, safety, pharmacokinetics, and immunogenicity up to 24 weeks and 1 year following treatment.”
The company conducted a double-blind, randomized phase 3 trial that compared switching from reference adalimumab to Yuflyma with continuing either reference adalimumab or Yuflyma for patients with active rheumatoid arthritis. In that trial, the efficacy, pharmacokinetics, safety, and immunogenicity of Yuflyma and reference adalimumab were comparable after 1 year of treatment, including after switching from reference adalimumab to Yuflyma.
“Currently, more than 80% of patients treated with Humira in the United States rely on a high-concentration and citrate-free formulation of this medication. The availability of a high-concentration and citrate-free formulation adalimumab biosimilar provides an important treatment option for patients with inflammatory diseases who benefit from this effective therapy,” said Jonathan Kay, MD, of the University of Massachusetts, Worcester, in the press release.
The citrate-free formulation is thought to lead to less pain on injection.
Yuflyma will be available in prefilled syringe and autoinjector administration options.
Celltrion USA plans to market the drug in the United States in July 2023. Following the initial launch of 40 mg/0.4 mL, the company plans to launch dose forms of 80 mg/0.8 mL and 20 mg/0.2 mL.
Celltrion USA is also seeking an interchangeability designation from the FDA following the completion of an interchangeability trial of 366 patients with chronic plaque psoriasis. The interchangeability designation would mean that patients successfully switched from Humira to Yuflyma multiple times in the trial. The interchangeability designation would allow pharmacists to autosubstitute Humira with Yuflyma. In these cases, individual state laws control how and whether physicians will be notified of this switch.
If interchangeability is approved for Yuflyma, which the company tentatively expects in the fourth quarter of 2024, it would be just the third interchangeable biosimilar approved by the FDA overall and the second adalimumab biosimilar to be designated as such, after adalimumab-adbm (Cyltezo) in October 2021.
Yuflyma was approved in Canada in December 2021 for 10 indications: rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, adult ulcerative colitis, hidradenitis suppurativa, plaque psoriasis, adult uveitis, and pediatric uveitis.
In February 2022, the European Commission granted marketing authorization for Yuflyma across those 10 indications, as well as for nonradiographic axial spondyloarthritis, pediatric plaque psoriasis, and pediatric Crohn’s disease.
In April 2022, Celltrion USA signed a licensing agreement with AbbVie, the manufacturer of Humira. Under that agreement, Celltrion will pay royalties to AbbVie on sales of their individual biosimilars, and AbbVie agreed to drop all patent litigation.
The full prescribing information for Yuflyma is available here.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved the biosimilar adalimumab-aaty (Yuflyma) in a citrate-free, high-concentration formulation, the manufacturer, Celltrion USA, announced today. It is the ninth biosimilar of adalimumab (Humira) to be approved in the United States.
Yuflyma is approved for the treatment of adult patients with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, plaque psoriasis, and hidradenitis suppurativa. It is also approved for polyarticular juvenile idiopathic arthritis for patients aged 2 years or older, as well as for Crohn’s disease in adults and in pediatric patients aged 6 years or older.
The formulation was approved on the basis of a comprehensive data package of analytic, preclinical, and clinical studies, according to Celltrion USA, “demonstrating that Yuflyma is comparable to the reference product Humira in terms of efficacy, safety, pharmacokinetics, and immunogenicity up to 24 weeks and 1 year following treatment.”
The company conducted a double-blind, randomized phase 3 trial that compared switching from reference adalimumab to Yuflyma with continuing either reference adalimumab or Yuflyma for patients with active rheumatoid arthritis. In that trial, the efficacy, pharmacokinetics, safety, and immunogenicity of Yuflyma and reference adalimumab were comparable after 1 year of treatment, including after switching from reference adalimumab to Yuflyma.
“Currently, more than 80% of patients treated with Humira in the United States rely on a high-concentration and citrate-free formulation of this medication. The availability of a high-concentration and citrate-free formulation adalimumab biosimilar provides an important treatment option for patients with inflammatory diseases who benefit from this effective therapy,” said Jonathan Kay, MD, of the University of Massachusetts, Worcester, in the press release.
The citrate-free formulation is thought to lead to less pain on injection.
Yuflyma will be available in prefilled syringe and autoinjector administration options.
Celltrion USA plans to market the drug in the United States in July 2023. Following the initial launch of 40 mg/0.4 mL, the company plans to launch dose forms of 80 mg/0.8 mL and 20 mg/0.2 mL.
Celltrion USA is also seeking an interchangeability designation from the FDA following the completion of an interchangeability trial of 366 patients with chronic plaque psoriasis. The interchangeability designation would mean that patients successfully switched from Humira to Yuflyma multiple times in the trial. The interchangeability designation would allow pharmacists to autosubstitute Humira with Yuflyma. In these cases, individual state laws control how and whether physicians will be notified of this switch.
If interchangeability is approved for Yuflyma, which the company tentatively expects in the fourth quarter of 2024, it would be just the third interchangeable biosimilar approved by the FDA overall and the second adalimumab biosimilar to be designated as such, after adalimumab-adbm (Cyltezo) in October 2021.
Yuflyma was approved in Canada in December 2021 for 10 indications: rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, adult ulcerative colitis, hidradenitis suppurativa, plaque psoriasis, adult uveitis, and pediatric uveitis.
In February 2022, the European Commission granted marketing authorization for Yuflyma across those 10 indications, as well as for nonradiographic axial spondyloarthritis, pediatric plaque psoriasis, and pediatric Crohn’s disease.
In April 2022, Celltrion USA signed a licensing agreement with AbbVie, the manufacturer of Humira. Under that agreement, Celltrion will pay royalties to AbbVie on sales of their individual biosimilars, and AbbVie agreed to drop all patent litigation.
The full prescribing information for Yuflyma is available here.
A version of this article first appeared on Medscape.com.
Commentary: Enthesitis, synovitis, spondyloarthritis, and PsA, June 2023
The relationship between enthesitis and synovitis is of considerable interest to both clinicians and researchers. This relationship is best evaluated using imaging, particularly ultrasonography, and could provide pathophysiologic insights. Balulu and colleagues recruited 158 patients with PsA who underwent sonographic assessment of 52 joints, 40 tendons, and 14 entheses as well as clinical evaluation. Overall, total sonographic enthesitis scores were significantly associated with total sonographic synovitis and sonographic tenosynovitis scores and also with older age, male sex, swollen joint count, C-reactive protein, physical occupation, and patient-reported outcomes. The association between enthesitis and synovitis was also demonstrated at the elbows, knees, and ankles. This study demonstrates that psoriatic enthesitis and synovitis are closely related and thus may share pathophysiologic mechanisms. Longitudinal studies in very early PsA using ultrasound might provide clues to confirm the hypothesis that psoriatic synovitis is secondary to enthesitis.
Another important domain that is increasingly studied is axial PsA. Currently, the evidence for treatment of axial PsA is extrapolated from that for axial spondyloarthritis (SpA), in the belief that the two diseases are pathophysiologically similar. However, there is increasing evidence for differences between axial PsA and axial SpA that might influence the choice of treatment. In a recent study, de Hooge and colleagues demonstrated that patients with axial PsA have lower severity of damage to the spine compared with those with axial SpA. Using data from 312 patients with PsA and 213 patients with SpA who underwent radiographic imaging assessment in the Belgian Epidemiological Psoriatic Arthritis Study (BEPAS) and the Ghent and Belgian Inflammatory Arthritis and Spondylitis (Be-GIANT) study, respectively, they show that the proportion of patients with PsA vs SpA having spinal damage was comparable. Patients with SpA and spinal damage had higher modified Stoke Ankylosing Spondylitis Spine Scores, indicating more severe damage. These results are consistent with other published studies and indicate that patients with PsA have less severe spinal disease compared with other patients with axial SpA. Randomized controlled trials (RCTs) specifically investigating the treatment of axial PsA are currently underway. Nevertheless, post hoc analyses of data from PsA RCTs indicate that most drugs efficacious for PsA overall also provide benefit in axial disease.
In a recent report, Baraliakos and colleagues analyzed data from the SELECT-PsA 1 and SELECT-PsA 2 trials that evaluated the efficacy of upadacitinib in PsA. They show that, compared with placebo, 15 mg upadacitinib led to a greater improvement in axial symptoms. The improvement in overall Bath Ankylosing Spondylitis Disease Activity Index score at week 24 was significantly higher with 15 mg upadacitinib compared with placebo in both trials. However, these results are not definitive because there is yet no consensus on the definition of and outcome measures for axial PsA.
The relationship between enthesitis and synovitis is of considerable interest to both clinicians and researchers. This relationship is best evaluated using imaging, particularly ultrasonography, and could provide pathophysiologic insights. Balulu and colleagues recruited 158 patients with PsA who underwent sonographic assessment of 52 joints, 40 tendons, and 14 entheses as well as clinical evaluation. Overall, total sonographic enthesitis scores were significantly associated with total sonographic synovitis and sonographic tenosynovitis scores and also with older age, male sex, swollen joint count, C-reactive protein, physical occupation, and patient-reported outcomes. The association between enthesitis and synovitis was also demonstrated at the elbows, knees, and ankles. This study demonstrates that psoriatic enthesitis and synovitis are closely related and thus may share pathophysiologic mechanisms. Longitudinal studies in very early PsA using ultrasound might provide clues to confirm the hypothesis that psoriatic synovitis is secondary to enthesitis.
Another important domain that is increasingly studied is axial PsA. Currently, the evidence for treatment of axial PsA is extrapolated from that for axial spondyloarthritis (SpA), in the belief that the two diseases are pathophysiologically similar. However, there is increasing evidence for differences between axial PsA and axial SpA that might influence the choice of treatment. In a recent study, de Hooge and colleagues demonstrated that patients with axial PsA have lower severity of damage to the spine compared with those with axial SpA. Using data from 312 patients with PsA and 213 patients with SpA who underwent radiographic imaging assessment in the Belgian Epidemiological Psoriatic Arthritis Study (BEPAS) and the Ghent and Belgian Inflammatory Arthritis and Spondylitis (Be-GIANT) study, respectively, they show that the proportion of patients with PsA vs SpA having spinal damage was comparable. Patients with SpA and spinal damage had higher modified Stoke Ankylosing Spondylitis Spine Scores, indicating more severe damage. These results are consistent with other published studies and indicate that patients with PsA have less severe spinal disease compared with other patients with axial SpA. Randomized controlled trials (RCTs) specifically investigating the treatment of axial PsA are currently underway. Nevertheless, post hoc analyses of data from PsA RCTs indicate that most drugs efficacious for PsA overall also provide benefit in axial disease.
In a recent report, Baraliakos and colleagues analyzed data from the SELECT-PsA 1 and SELECT-PsA 2 trials that evaluated the efficacy of upadacitinib in PsA. They show that, compared with placebo, 15 mg upadacitinib led to a greater improvement in axial symptoms. The improvement in overall Bath Ankylosing Spondylitis Disease Activity Index score at week 24 was significantly higher with 15 mg upadacitinib compared with placebo in both trials. However, these results are not definitive because there is yet no consensus on the definition of and outcome measures for axial PsA.
The relationship between enthesitis and synovitis is of considerable interest to both clinicians and researchers. This relationship is best evaluated using imaging, particularly ultrasonography, and could provide pathophysiologic insights. Balulu and colleagues recruited 158 patients with PsA who underwent sonographic assessment of 52 joints, 40 tendons, and 14 entheses as well as clinical evaluation. Overall, total sonographic enthesitis scores were significantly associated with total sonographic synovitis and sonographic tenosynovitis scores and also with older age, male sex, swollen joint count, C-reactive protein, physical occupation, and patient-reported outcomes. The association between enthesitis and synovitis was also demonstrated at the elbows, knees, and ankles. This study demonstrates that psoriatic enthesitis and synovitis are closely related and thus may share pathophysiologic mechanisms. Longitudinal studies in very early PsA using ultrasound might provide clues to confirm the hypothesis that psoriatic synovitis is secondary to enthesitis.
Another important domain that is increasingly studied is axial PsA. Currently, the evidence for treatment of axial PsA is extrapolated from that for axial spondyloarthritis (SpA), in the belief that the two diseases are pathophysiologically similar. However, there is increasing evidence for differences between axial PsA and axial SpA that might influence the choice of treatment. In a recent study, de Hooge and colleagues demonstrated that patients with axial PsA have lower severity of damage to the spine compared with those with axial SpA. Using data from 312 patients with PsA and 213 patients with SpA who underwent radiographic imaging assessment in the Belgian Epidemiological Psoriatic Arthritis Study (BEPAS) and the Ghent and Belgian Inflammatory Arthritis and Spondylitis (Be-GIANT) study, respectively, they show that the proportion of patients with PsA vs SpA having spinal damage was comparable. Patients with SpA and spinal damage had higher modified Stoke Ankylosing Spondylitis Spine Scores, indicating more severe damage. These results are consistent with other published studies and indicate that patients with PsA have less severe spinal disease compared with other patients with axial SpA. Randomized controlled trials (RCTs) specifically investigating the treatment of axial PsA are currently underway. Nevertheless, post hoc analyses of data from PsA RCTs indicate that most drugs efficacious for PsA overall also provide benefit in axial disease.
In a recent report, Baraliakos and colleagues analyzed data from the SELECT-PsA 1 and SELECT-PsA 2 trials that evaluated the efficacy of upadacitinib in PsA. They show that, compared with placebo, 15 mg upadacitinib led to a greater improvement in axial symptoms. The improvement in overall Bath Ankylosing Spondylitis Disease Activity Index score at week 24 was significantly higher with 15 mg upadacitinib compared with placebo in both trials. However, these results are not definitive because there is yet no consensus on the definition of and outcome measures for axial PsA.
Commentary: Enthesitis, synovitis, spondyloarthritis, and PsA, June 2023
The relationship between enthesitis and synovitis is of considerable interest to both clinicians and researchers. This relationship is best evaluated using imaging, particularly ultrasonography, and could provide pathophysiologic insights. Balulu and colleagues recruited 158 patients with PsA who underwent sonographic assessment of 52 joints, 40 tendons, and 14 entheses as well as clinical evaluation. Overall, total sonographic enthesitis scores were significantly associated with total sonographic synovitis and sonographic tenosynovitis scores and also with older age, male sex, swollen joint count, C-reactive protein, physical occupation, and patient-reported outcomes. The association between enthesitis and synovitis was also demonstrated at the elbows, knees, and ankles. This study demonstrates that psoriatic enthesitis and synovitis are closely related and thus may share pathophysiologic mechanisms. Longitudinal studies in very early PsA using ultrasound might provide clues to confirm the hypothesis that psoriatic synovitis is secondary to enthesitis.
Another important domain that is increasingly studied is axial PsA. Currently, the evidence for treatment of axial PsA is extrapolated from that for axial spondyloarthritis (SpA), in the belief that the two diseases are pathophysiologically similar. However, there is increasing evidence for differences between axial PsA and axial SpA that might influence the choice of treatment. In a recent study, de Hooge and colleagues demonstrated that patients with axial PsA have lower severity of damage to the spine compared with those with axial SpA. Using data from 312 patients with PsA and 213 patients with SpA who underwent radiographic imaging assessment in the Belgian Epidemiological Psoriatic Arthritis Study (BEPAS) and the Ghent and Belgian Inflammatory Arthritis and Spondylitis (Be-GIANT) study, respectively, they show that the proportion of patients with PsA vs SpA having spinal damage was comparable. Patients with SpA and spinal damage had higher modified Stoke Ankylosing Spondylitis Spine Scores, indicating more severe damage. These results are consistent with other published studies and indicate that patients with PsA have less severe spinal disease compared with other patients with axial SpA. Randomized controlled trials (RCTs) specifically investigating the treatment of axial PsA are currently underway. Nevertheless, post hoc analyses of data from PsA RCTs indicate that most drugs efficacious for PsA overall also provide benefit in axial disease.
In a recent report, Baraliakos and colleagues analyzed data from the SELECT-PsA 1 and SELECT-PsA 2 trials that evaluated the efficacy of upadacitinib in PsA. They show that, compared with placebo, 15 mg upadacitinib led to a greater improvement in axial symptoms. The improvement in overall Bath Ankylosing Spondylitis Disease Activity Index score at week 24 was significantly higher with 15 mg upadacitinib compared with placebo in both trials. However, these results are not definitive because there is yet no consensus on the definition of and outcome measures for axial PsA.
The relationship between enthesitis and synovitis is of considerable interest to both clinicians and researchers. This relationship is best evaluated using imaging, particularly ultrasonography, and could provide pathophysiologic insights. Balulu and colleagues recruited 158 patients with PsA who underwent sonographic assessment of 52 joints, 40 tendons, and 14 entheses as well as clinical evaluation. Overall, total sonographic enthesitis scores were significantly associated with total sonographic synovitis and sonographic tenosynovitis scores and also with older age, male sex, swollen joint count, C-reactive protein, physical occupation, and patient-reported outcomes. The association between enthesitis and synovitis was also demonstrated at the elbows, knees, and ankles. This study demonstrates that psoriatic enthesitis and synovitis are closely related and thus may share pathophysiologic mechanisms. Longitudinal studies in very early PsA using ultrasound might provide clues to confirm the hypothesis that psoriatic synovitis is secondary to enthesitis.
Another important domain that is increasingly studied is axial PsA. Currently, the evidence for treatment of axial PsA is extrapolated from that for axial spondyloarthritis (SpA), in the belief that the two diseases are pathophysiologically similar. However, there is increasing evidence for differences between axial PsA and axial SpA that might influence the choice of treatment. In a recent study, de Hooge and colleagues demonstrated that patients with axial PsA have lower severity of damage to the spine compared with those with axial SpA. Using data from 312 patients with PsA and 213 patients with SpA who underwent radiographic imaging assessment in the Belgian Epidemiological Psoriatic Arthritis Study (BEPAS) and the Ghent and Belgian Inflammatory Arthritis and Spondylitis (Be-GIANT) study, respectively, they show that the proportion of patients with PsA vs SpA having spinal damage was comparable. Patients with SpA and spinal damage had higher modified Stoke Ankylosing Spondylitis Spine Scores, indicating more severe damage. These results are consistent with other published studies and indicate that patients with PsA have less severe spinal disease compared with other patients with axial SpA. Randomized controlled trials (RCTs) specifically investigating the treatment of axial PsA are currently underway. Nevertheless, post hoc analyses of data from PsA RCTs indicate that most drugs efficacious for PsA overall also provide benefit in axial disease.
In a recent report, Baraliakos and colleagues analyzed data from the SELECT-PsA 1 and SELECT-PsA 2 trials that evaluated the efficacy of upadacitinib in PsA. They show that, compared with placebo, 15 mg upadacitinib led to a greater improvement in axial symptoms. The improvement in overall Bath Ankylosing Spondylitis Disease Activity Index score at week 24 was significantly higher with 15 mg upadacitinib compared with placebo in both trials. However, these results are not definitive because there is yet no consensus on the definition of and outcome measures for axial PsA.
The relationship between enthesitis and synovitis is of considerable interest to both clinicians and researchers. This relationship is best evaluated using imaging, particularly ultrasonography, and could provide pathophysiologic insights. Balulu and colleagues recruited 158 patients with PsA who underwent sonographic assessment of 52 joints, 40 tendons, and 14 entheses as well as clinical evaluation. Overall, total sonographic enthesitis scores were significantly associated with total sonographic synovitis and sonographic tenosynovitis scores and also with older age, male sex, swollen joint count, C-reactive protein, physical occupation, and patient-reported outcomes. The association between enthesitis and synovitis was also demonstrated at the elbows, knees, and ankles. This study demonstrates that psoriatic enthesitis and synovitis are closely related and thus may share pathophysiologic mechanisms. Longitudinal studies in very early PsA using ultrasound might provide clues to confirm the hypothesis that psoriatic synovitis is secondary to enthesitis.
Another important domain that is increasingly studied is axial PsA. Currently, the evidence for treatment of axial PsA is extrapolated from that for axial spondyloarthritis (SpA), in the belief that the two diseases are pathophysiologically similar. However, there is increasing evidence for differences between axial PsA and axial SpA that might influence the choice of treatment. In a recent study, de Hooge and colleagues demonstrated that patients with axial PsA have lower severity of damage to the spine compared with those with axial SpA. Using data from 312 patients with PsA and 213 patients with SpA who underwent radiographic imaging assessment in the Belgian Epidemiological Psoriatic Arthritis Study (BEPAS) and the Ghent and Belgian Inflammatory Arthritis and Spondylitis (Be-GIANT) study, respectively, they show that the proportion of patients with PsA vs SpA having spinal damage was comparable. Patients with SpA and spinal damage had higher modified Stoke Ankylosing Spondylitis Spine Scores, indicating more severe damage. These results are consistent with other published studies and indicate that patients with PsA have less severe spinal disease compared with other patients with axial SpA. Randomized controlled trials (RCTs) specifically investigating the treatment of axial PsA are currently underway. Nevertheless, post hoc analyses of data from PsA RCTs indicate that most drugs efficacious for PsA overall also provide benefit in axial disease.
In a recent report, Baraliakos and colleagues analyzed data from the SELECT-PsA 1 and SELECT-PsA 2 trials that evaluated the efficacy of upadacitinib in PsA. They show that, compared with placebo, 15 mg upadacitinib led to a greater improvement in axial symptoms. The improvement in overall Bath Ankylosing Spondylitis Disease Activity Index score at week 24 was significantly higher with 15 mg upadacitinib compared with placebo in both trials. However, these results are not definitive because there is yet no consensus on the definition of and outcome measures for axial PsA.