User login
Teamwork guides cardio-rheumatology clinics that care for unique patient population
Clinical cardiologist Heba Wassif, MD, MPH, knows the value of working with her fellow rheumatologists, surgeons, and other clinicians to establish a care plan for her patients with cardiac conditions and autoimmune diseases.
She is the cofounder of the Cleveland Clinic’s new cardio-rheumatology program, which places an emphasis on multidisciplinary care. In her role, Dr. Wassif closely follows her patients, and if she sees any inflammation or any other condition that requires the rheumatologist, she reaches out to her colleagues to adjust medications if needed.
Collaboration with a rheumatologist was important when a patient with valvular disease was prepping for surgery. The patient was on significant immunosuppressants and the surgery had to be timed appropriately, accounting for any decreases in her immunosuppression, explained Dr. Wassif, director of inpatient clinical cardiology at Cleveland Clinic in Ohio.
Cardio-rheumatology programs are “the newest child” in a series of cardiology offshoots focusing on different populations. Cardio-oncology and cardio-obstetrics took off about 6 years ago, with cardio-rheumatology clinics and interested physicians rising in number over the last several years, Dr. Wassif noted.
The relationship between cardiovascular diseases and rheumatologic conditions is certainly recognized more often, “which means more literature is being published to discuss the link,” according to Rekha Mankad, MD, a trailblazer of this model of care. She directs the Women’s Heart Clinic at Mayo Clinic in Rochester, Minn., which was one of the earliest adopters of a cardio-rheumatology clinic.
Ten years ago, “nobody was talking about the link between rheumatologic conditions and cardiovascular disease,” Dr. Mankad said. “I’ve been asked to speak on this topic, and programs have asked me to speak about establishing cardio-rheumatology practices. So, there’s been an evolution as far as a recognition that these two conditions overlap.”
Patients have come to her independent of internal referrals, which means they have done Google searches on cardiology and rheumatology. “I think that it has made a splash, at least in the world of cardiology,” Dr. Mankad observed in an interview.
Other institutions such as NYU-Langone, Yale, Stanford, Brigham and Women’s Hospital in Boston, and Women’s College Hospital in Toronto have formed similar clinics whose focus is to address the specific needs of rheumatology patients with cardiac conditions through a teamwork approach.
Challenges of treating cardiac, rheumatologic conditions
The rise in clinics addresses the longstanding connection between autoimmune disorders and cardiac conditions.
Cardiologists have known that there is an element of inflammation that contributes to atherosclerosis, said Dr. Wassif, who has researched this topic extensively. A recent study she led found a strong association between rheumatic immune-mediated inflammatory diseases (IMIDs) and high risk of acute coronary syndrome in Medicare patients.
“This particular population has a very clear increased risk for cardiovascular conditions, including valve disease and heart failure,” she emphasized.
Patients with rheumatoid arthritis and lupus have up to a twofold and eightfold higher risk of heart disease, respectively, noted Michael S. Garshick, MD, a cardiovascular disease specialist who directs the cardio-rheumatology program at NYU-Langone Health, in New York. Cardiologists “have really developed an understanding that the immune system can impact the heart, and that there’s a need for people to understand the nuance behind how the immune system can affect them and what to do about it,” Dr. Garshick said.
Caring for patients with both afflictions comes with specific challenges. Many physicians are not well trained on managing and treating patients with these dual conditions.
The “lipid paradox,” in which lipids are reduced with active inflammation in some rheumatologic conditions, can make treatment more nuanced. In addition, the traditional ASCVD (atherosclerotic cardiovascular disease) score often underestimates the cardiovascular risk of these patients, noted cardiologist Margaret Furman, MD, MPH, assistant professor and codirector of Yale’s Cardio-Rheumatology Program, New Haven, Conn.
Newer biologic medications used to treat rheumatologic diseases can alter a patient’s lipid profile, she said in an interview.
“It can be difficult to assess each individual patient’s cardiovascular risk as their disease state and treatment can vary throughout their lifetime based on their degree of inflammation. The importance of aggressive lipid management is often underestimated,” Dr. Furman added.
Cardiology and rheumatology partnerships can address gaps in care of this unique group of patients, said Vaidehi R. Chowdhary, MBBS, MD, clinical chief of the Yale Section of Rheumatology, Allergy, and Immunology at Yale University.
“The role of the rheumatologist in this dyad is to educate patients on this risk, work toward adequate control of inflammation, and minimize use of medications that contribute to increased cardiovascular risks,” said Dr. Chowdhary, who cofounded Yale’s cardio-rheumatology program with Dr. Furman.
Cardiologists in turn can assert their knowledge about medications and their impact on lipids and inflammation, Dr. Wassif said.
Many anti-inflammatory therapies are now within the cardiologist’s purview, Dr. Garshick noted. “For example, specifically with pericarditis, there’s [Food and Drug Administration]–approved anti-inflammatories or biologics. We’re the ones who feel the most comfortable giving them right now.” Cardiologists quite often are consulted about medications that are efficacious in rheumatologic conditions but could negatively impact the cardiovascular system, such as Janus kinase inhibitors, he added.
‘Reading the tea leaves’
Each program has its own unique story. For the Cleveland Clinic, the concept of a cardio-rheumatology program began during the COVID-19 pandemic in 2020. Developing such a concept and gaining institutional acceptance is always a work in process, Dr. Wassif said. “It’s not that you decide one day that you’re going to build a center, and that center is going to come into fruition overnight. You first gauge interest within your division. Who are the individuals that are interested in this area?”
Cleveland Clinic’s center is seeking to build relations between medical disciplines while spotlighting the concept of cardio-rheumatology, said Dr. Wassif, who has been providing education within the clinic and at other health institutions to ensure that patients receive appropriate attention early.
NYU-Langone launched its program amid this heightened awareness that the immune system could affect atherosclerosis, “kind of reading of the tea leaves, so to speak,” Dr. Garshick said.
Several clinical trials served as a catalyst for this movement. “A lot of clinical cardiologists were never 100% convinced that targeting the immune system reduced cardiovascular disease,” he said. Then the CANTOS clinical trial came along and showed for the first time that a therapeutic monoclonal antibody targeting interleukin-1beta, a cytokine central to inflammatory response, could in fact reduce cardiovascular disease.
Trials like this, along with epidemiologic literature connecting the rheumatologic and the autoimmune conditions with cardiovascular disease, pushed this concept to the forefront, Dr. Garshick said.
The notion that a clinic could successfully address cardiac problems in patients with rheumatic diseases yielded promising returns at Women’s College Hospital in Toronto, according to a report presented at the 2018 American College of Rheumatology annual meeting. Researchers reported that patients with rheumatologic conditions who attended a cardio-rheumatology clinic at this center saw improvements in care. The clinic identified increased cardiovascular risk and early atherosclerosis, and 53.8% of patients altered their medications after being seen in the clinic.
A total of 39.7% and 32.1% received lipid lowering and antiplatelet therapies, respectively, and 14% received antihypertensive therapy. A small percentage were treated for heart failure or placed on lifelong anticoagulation therapy for atrial fibrillation, and one patient received a percutaneous coronary stent.
Ins and outs of the referral process
Initially designed for preventive cardiac risk assessment, Yale’s program evolved into a multidisciplinary, patient-centered approach for the management of complex cardiovascular conditions in patients with autoimmune rheumatologic diseases.
The program is open to anyone who carries a diagnosis of rheumatologic disease or has elevated inflammatory markers. “Every patient, regardless of the reason for the referral, receives a cardiovascular risk assessment,” Dr. Furman said.
Most referrals come from rheumatologists, although cardiology colleagues and pulmonologists have also sent referrals. A pulmonologist, for example, may want to rule out a cardiac cause to shortness of breath. The patient’s workup, care, and follow-up are based on the reason for referral.
“We are currently referring patients with established cardiac disease, traditional risk factors, or for better risk assessment for primary prevention of coronary artery disease,” Dr. Chowdhary said. “We communicate very frequently about medication changes, and patients are aware of goals of care from both sides.”
Dr. Furman works closely with several of the rheumatology specialists taking care of patients with rheumatoid arthritis, systemic lupus erythematosus, and scleroderma.
Rheumatology follows patients every 3-6 months or more frequently based on their disease activity.
Dr. Mankad uses her sleuthing skills at Mayo Clinic to determine what the patients need. If they come in for a preventive assessment, she looks more closely at their cardiovascular risks and may order additional imaging to look for subclinical atherosclerosis. “We’re more aggressive with statin therapy in this population because of that,” she said.
If it’s valve disease, she pays extra attention to the patients’ valves in the echocardiograms and follows them a bit more regularly than someone without a rheumatologic condition and valve disease.
For patients with heart failure signs or symptoms, “it depends on how symptomatic they are,” Dr. Mankad said. In some instances, she may look for evidence of heart failure with preserved ejection fraction in patients who have rheumatoid arthritis who happen to be short of breath. “There’s so many different manifestations that patients with rheumatologic conditions can have as far as what could be affected in the heart,” she noted.
Quite frequently, Dr. Mankad identifies subclinical disease in her patients with rheumatoid arthritis. “I’ve seen many patients whose risk scores would not dictate statin therapy. But I went looking for subclinical disease by either doing coronary assessment or carotid assessment and have found atherosclerosis that would be enough to warrant statin therapy.”
A personalized assessment to reduce cardiac risk
NYU-Langone’s program offers opportunities to educate patients about the link between cardiac and rheumatologic disease.
“Their rheumatologist or their dermatologist will say, ‘Hey, have you heard about the connection between psoriasis, psoriatic or rheumatoid arthritis, and heart disease and the risk of heart attack or stroke?’ ” Dr. Garshick said.
The patients will often say they know nothing about these connections and want to learn more about how to treat it.
“We’ll say, ‘we have someone here that can help you.’ They’ll send them to myself or other colleagues like me across the country. We’ll assess blood pressure, weight, lipids, hemoglobin A1c, and other serologic and oftentimes imaging biomarkers of cardiovascular risk.” The patients will receive a personalized assessment, listing things they can do to lower their risk, whether it’s diet, exercise, or lifestyle. “Many times it can involve medications to reduce heart disease risk,” said Dr. Garshick.
In some instances, a rheumatologist or dermatologist may be concerned about starting a patient on a specific medication for the disease such as a JAK inhibitor. “We’ll help assess their risk because there’s been a lot of literature out in the rheumatology world about the risk of JAK inhibitors and heart disease and blood clots,” said Dr. Garshick.
Dr. Garshick also sees patients with rheumatologic conditions who have a specific cardiovascular concern or complaint such as shortness of breath or chest pain. “We’ll work that up with a specific knowledge of the underlying immune condition and how that may impact their heart,” he said.
Advances in research
As they continue to see patients and devise specific care plans, developers of cardio-rheumatology programs have been supplementing their work with ongoing research.
Yale’s clinic is expanding this year to include a new attending physician, Attila Feher, MD, PhD, who has conducted research in autoimmunity and microcirculation using molecular imaging and multimodality imaging techniques. Prevalence of coronary microvascular dysfunction appears to be increased in this patient population, Dr. Furman said.
Dr. Wassif recently coauthored a paper that examined patients with underlying rheumatologic conditions who undergo valvular and aortic valve replacement. “To our surprise, there was really no difference between patients with autoimmune conditions and others with nonautoimmune conditions,” she said, adding that the study had its limitations.
Other work includes data on Medicare patients with ST- and non-ST-elevation myocardial infarctions who have an underlying autoimmune disorder. Dr. Wassif and her colleagues found that their long-term outcomes are worse than those of patients without these conditions. “It’s unclear if worse outcomes are related to complications of autoimmunity versus the extent of their underlying disease. This is a work in progress and certainly an area that is ripe for research.”
Dr. Garshick and other collaborators at NYU have been focusing on the endothelium, specifically platelet biology in patients with psoriasis, psoriatic arthritis, and lupus. “We’re about to start the same research with gout as well,” he said.
“The process we’re most interested in is understanding how these diseases impact the early stages of cholesterol. And the way we’re doing that is evaluating the vasculature, specifically the endothelium,” he said.
He has finished two clinical trials that evaluate how standard heart disease medications such as aspirin and statins impact or can potentially benefit patients with psoriasis and/or psoriatic arthritis. “We have a whole list of other trials in the pipeline with other institutions across the country.”
Through a grant, Dr. Mankad is assessing whether a PET scan could detect inflammation in the hearts of rheumatoid arthritis patients. “We’re looking to see if the reason these patients have heart failure later in life is because their heart muscle actually shows evidence of inflammation, even when they have no symptoms,” she explained.
Other tests such as echocardiogram and CT scans will be used to evaluate coronary disease in about 40-50 patients. The goal of using these multiple imaging tools is to find markers indicating that the heart is affected by rheumatoid arthritis, which may indicate a higher likelihood of developing heart failure, she said.
Clinics are popping up
Through these new clinics, some collaborations have emerged. Dr. Garshick works closely with Brigham and Women’s Hospital, which has a similar cardio-rheumatology program, run by Brittany Weber, MD, to exchange ideas, discuss challenging cases, and collaborate.
“There are a lot of clinics like us popping up across the country,” he observed. Every so often, he hears from other institutions that are interested in starting their own cardio-rheumatology programs. “They ask us: How do you start, what should we look for?”
It’s an education process for both patients and providers, Dr. Garshick emphasized. “I also think it’s a bandwidth issue. Many of our rheumatology and dermatology colleagues are acutely aware of the connection, but there may not be enough time at a clinic visit to really go in depth” with these dual conditions, he said.
NYU-Langone Health for the past several years has been holding a symposium to educate people on the cardio-rheumatology connection and treating inflammation in cardiovascular disease. This year’s symposium, held in conjunction with Brigham and Women’s Hospital, is scheduled for April 28. For more information, visit the course website: nyulmc.org/cvinflammationcme.
“What we’re trying to do is help [other institutions] get that bandwidth” to adequately help and serve these patients, he said.
Dr. Garshick has received consultant fees from Abbvie and Horizon therapeutics and an unrestricted research grant from Pfizer. No other sources had relevant financial disclosures.
Clinical cardiologist Heba Wassif, MD, MPH, knows the value of working with her fellow rheumatologists, surgeons, and other clinicians to establish a care plan for her patients with cardiac conditions and autoimmune diseases.
She is the cofounder of the Cleveland Clinic’s new cardio-rheumatology program, which places an emphasis on multidisciplinary care. In her role, Dr. Wassif closely follows her patients, and if she sees any inflammation or any other condition that requires the rheumatologist, she reaches out to her colleagues to adjust medications if needed.
Collaboration with a rheumatologist was important when a patient with valvular disease was prepping for surgery. The patient was on significant immunosuppressants and the surgery had to be timed appropriately, accounting for any decreases in her immunosuppression, explained Dr. Wassif, director of inpatient clinical cardiology at Cleveland Clinic in Ohio.
Cardio-rheumatology programs are “the newest child” in a series of cardiology offshoots focusing on different populations. Cardio-oncology and cardio-obstetrics took off about 6 years ago, with cardio-rheumatology clinics and interested physicians rising in number over the last several years, Dr. Wassif noted.
The relationship between cardiovascular diseases and rheumatologic conditions is certainly recognized more often, “which means more literature is being published to discuss the link,” according to Rekha Mankad, MD, a trailblazer of this model of care. She directs the Women’s Heart Clinic at Mayo Clinic in Rochester, Minn., which was one of the earliest adopters of a cardio-rheumatology clinic.
Ten years ago, “nobody was talking about the link between rheumatologic conditions and cardiovascular disease,” Dr. Mankad said. “I’ve been asked to speak on this topic, and programs have asked me to speak about establishing cardio-rheumatology practices. So, there’s been an evolution as far as a recognition that these two conditions overlap.”
Patients have come to her independent of internal referrals, which means they have done Google searches on cardiology and rheumatology. “I think that it has made a splash, at least in the world of cardiology,” Dr. Mankad observed in an interview.
Other institutions such as NYU-Langone, Yale, Stanford, Brigham and Women’s Hospital in Boston, and Women’s College Hospital in Toronto have formed similar clinics whose focus is to address the specific needs of rheumatology patients with cardiac conditions through a teamwork approach.
Challenges of treating cardiac, rheumatologic conditions
The rise in clinics addresses the longstanding connection between autoimmune disorders and cardiac conditions.
Cardiologists have known that there is an element of inflammation that contributes to atherosclerosis, said Dr. Wassif, who has researched this topic extensively. A recent study she led found a strong association between rheumatic immune-mediated inflammatory diseases (IMIDs) and high risk of acute coronary syndrome in Medicare patients.
“This particular population has a very clear increased risk for cardiovascular conditions, including valve disease and heart failure,” she emphasized.
Patients with rheumatoid arthritis and lupus have up to a twofold and eightfold higher risk of heart disease, respectively, noted Michael S. Garshick, MD, a cardiovascular disease specialist who directs the cardio-rheumatology program at NYU-Langone Health, in New York. Cardiologists “have really developed an understanding that the immune system can impact the heart, and that there’s a need for people to understand the nuance behind how the immune system can affect them and what to do about it,” Dr. Garshick said.
Caring for patients with both afflictions comes with specific challenges. Many physicians are not well trained on managing and treating patients with these dual conditions.
The “lipid paradox,” in which lipids are reduced with active inflammation in some rheumatologic conditions, can make treatment more nuanced. In addition, the traditional ASCVD (atherosclerotic cardiovascular disease) score often underestimates the cardiovascular risk of these patients, noted cardiologist Margaret Furman, MD, MPH, assistant professor and codirector of Yale’s Cardio-Rheumatology Program, New Haven, Conn.
Newer biologic medications used to treat rheumatologic diseases can alter a patient’s lipid profile, she said in an interview.
“It can be difficult to assess each individual patient’s cardiovascular risk as their disease state and treatment can vary throughout their lifetime based on their degree of inflammation. The importance of aggressive lipid management is often underestimated,” Dr. Furman added.
Cardiology and rheumatology partnerships can address gaps in care of this unique group of patients, said Vaidehi R. Chowdhary, MBBS, MD, clinical chief of the Yale Section of Rheumatology, Allergy, and Immunology at Yale University.
“The role of the rheumatologist in this dyad is to educate patients on this risk, work toward adequate control of inflammation, and minimize use of medications that contribute to increased cardiovascular risks,” said Dr. Chowdhary, who cofounded Yale’s cardio-rheumatology program with Dr. Furman.
Cardiologists in turn can assert their knowledge about medications and their impact on lipids and inflammation, Dr. Wassif said.
Many anti-inflammatory therapies are now within the cardiologist’s purview, Dr. Garshick noted. “For example, specifically with pericarditis, there’s [Food and Drug Administration]–approved anti-inflammatories or biologics. We’re the ones who feel the most comfortable giving them right now.” Cardiologists quite often are consulted about medications that are efficacious in rheumatologic conditions but could negatively impact the cardiovascular system, such as Janus kinase inhibitors, he added.
‘Reading the tea leaves’
Each program has its own unique story. For the Cleveland Clinic, the concept of a cardio-rheumatology program began during the COVID-19 pandemic in 2020. Developing such a concept and gaining institutional acceptance is always a work in process, Dr. Wassif said. “It’s not that you decide one day that you’re going to build a center, and that center is going to come into fruition overnight. You first gauge interest within your division. Who are the individuals that are interested in this area?”
Cleveland Clinic’s center is seeking to build relations between medical disciplines while spotlighting the concept of cardio-rheumatology, said Dr. Wassif, who has been providing education within the clinic and at other health institutions to ensure that patients receive appropriate attention early.
NYU-Langone launched its program amid this heightened awareness that the immune system could affect atherosclerosis, “kind of reading of the tea leaves, so to speak,” Dr. Garshick said.
Several clinical trials served as a catalyst for this movement. “A lot of clinical cardiologists were never 100% convinced that targeting the immune system reduced cardiovascular disease,” he said. Then the CANTOS clinical trial came along and showed for the first time that a therapeutic monoclonal antibody targeting interleukin-1beta, a cytokine central to inflammatory response, could in fact reduce cardiovascular disease.
Trials like this, along with epidemiologic literature connecting the rheumatologic and the autoimmune conditions with cardiovascular disease, pushed this concept to the forefront, Dr. Garshick said.
The notion that a clinic could successfully address cardiac problems in patients with rheumatic diseases yielded promising returns at Women’s College Hospital in Toronto, according to a report presented at the 2018 American College of Rheumatology annual meeting. Researchers reported that patients with rheumatologic conditions who attended a cardio-rheumatology clinic at this center saw improvements in care. The clinic identified increased cardiovascular risk and early atherosclerosis, and 53.8% of patients altered their medications after being seen in the clinic.
A total of 39.7% and 32.1% received lipid lowering and antiplatelet therapies, respectively, and 14% received antihypertensive therapy. A small percentage were treated for heart failure or placed on lifelong anticoagulation therapy for atrial fibrillation, and one patient received a percutaneous coronary stent.
Ins and outs of the referral process
Initially designed for preventive cardiac risk assessment, Yale’s program evolved into a multidisciplinary, patient-centered approach for the management of complex cardiovascular conditions in patients with autoimmune rheumatologic diseases.
The program is open to anyone who carries a diagnosis of rheumatologic disease or has elevated inflammatory markers. “Every patient, regardless of the reason for the referral, receives a cardiovascular risk assessment,” Dr. Furman said.
Most referrals come from rheumatologists, although cardiology colleagues and pulmonologists have also sent referrals. A pulmonologist, for example, may want to rule out a cardiac cause to shortness of breath. The patient’s workup, care, and follow-up are based on the reason for referral.
“We are currently referring patients with established cardiac disease, traditional risk factors, or for better risk assessment for primary prevention of coronary artery disease,” Dr. Chowdhary said. “We communicate very frequently about medication changes, and patients are aware of goals of care from both sides.”
Dr. Furman works closely with several of the rheumatology specialists taking care of patients with rheumatoid arthritis, systemic lupus erythematosus, and scleroderma.
Rheumatology follows patients every 3-6 months or more frequently based on their disease activity.
Dr. Mankad uses her sleuthing skills at Mayo Clinic to determine what the patients need. If they come in for a preventive assessment, she looks more closely at their cardiovascular risks and may order additional imaging to look for subclinical atherosclerosis. “We’re more aggressive with statin therapy in this population because of that,” she said.
If it’s valve disease, she pays extra attention to the patients’ valves in the echocardiograms and follows them a bit more regularly than someone without a rheumatologic condition and valve disease.
For patients with heart failure signs or symptoms, “it depends on how symptomatic they are,” Dr. Mankad said. In some instances, she may look for evidence of heart failure with preserved ejection fraction in patients who have rheumatoid arthritis who happen to be short of breath. “There’s so many different manifestations that patients with rheumatologic conditions can have as far as what could be affected in the heart,” she noted.
Quite frequently, Dr. Mankad identifies subclinical disease in her patients with rheumatoid arthritis. “I’ve seen many patients whose risk scores would not dictate statin therapy. But I went looking for subclinical disease by either doing coronary assessment or carotid assessment and have found atherosclerosis that would be enough to warrant statin therapy.”
A personalized assessment to reduce cardiac risk
NYU-Langone’s program offers opportunities to educate patients about the link between cardiac and rheumatologic disease.
“Their rheumatologist or their dermatologist will say, ‘Hey, have you heard about the connection between psoriasis, psoriatic or rheumatoid arthritis, and heart disease and the risk of heart attack or stroke?’ ” Dr. Garshick said.
The patients will often say they know nothing about these connections and want to learn more about how to treat it.
“We’ll say, ‘we have someone here that can help you.’ They’ll send them to myself or other colleagues like me across the country. We’ll assess blood pressure, weight, lipids, hemoglobin A1c, and other serologic and oftentimes imaging biomarkers of cardiovascular risk.” The patients will receive a personalized assessment, listing things they can do to lower their risk, whether it’s diet, exercise, or lifestyle. “Many times it can involve medications to reduce heart disease risk,” said Dr. Garshick.
In some instances, a rheumatologist or dermatologist may be concerned about starting a patient on a specific medication for the disease such as a JAK inhibitor. “We’ll help assess their risk because there’s been a lot of literature out in the rheumatology world about the risk of JAK inhibitors and heart disease and blood clots,” said Dr. Garshick.
Dr. Garshick also sees patients with rheumatologic conditions who have a specific cardiovascular concern or complaint such as shortness of breath or chest pain. “We’ll work that up with a specific knowledge of the underlying immune condition and how that may impact their heart,” he said.
Advances in research
As they continue to see patients and devise specific care plans, developers of cardio-rheumatology programs have been supplementing their work with ongoing research.
Yale’s clinic is expanding this year to include a new attending physician, Attila Feher, MD, PhD, who has conducted research in autoimmunity and microcirculation using molecular imaging and multimodality imaging techniques. Prevalence of coronary microvascular dysfunction appears to be increased in this patient population, Dr. Furman said.
Dr. Wassif recently coauthored a paper that examined patients with underlying rheumatologic conditions who undergo valvular and aortic valve replacement. “To our surprise, there was really no difference between patients with autoimmune conditions and others with nonautoimmune conditions,” she said, adding that the study had its limitations.
Other work includes data on Medicare patients with ST- and non-ST-elevation myocardial infarctions who have an underlying autoimmune disorder. Dr. Wassif and her colleagues found that their long-term outcomes are worse than those of patients without these conditions. “It’s unclear if worse outcomes are related to complications of autoimmunity versus the extent of their underlying disease. This is a work in progress and certainly an area that is ripe for research.”
Dr. Garshick and other collaborators at NYU have been focusing on the endothelium, specifically platelet biology in patients with psoriasis, psoriatic arthritis, and lupus. “We’re about to start the same research with gout as well,” he said.
“The process we’re most interested in is understanding how these diseases impact the early stages of cholesterol. And the way we’re doing that is evaluating the vasculature, specifically the endothelium,” he said.
He has finished two clinical trials that evaluate how standard heart disease medications such as aspirin and statins impact or can potentially benefit patients with psoriasis and/or psoriatic arthritis. “We have a whole list of other trials in the pipeline with other institutions across the country.”
Through a grant, Dr. Mankad is assessing whether a PET scan could detect inflammation in the hearts of rheumatoid arthritis patients. “We’re looking to see if the reason these patients have heart failure later in life is because their heart muscle actually shows evidence of inflammation, even when they have no symptoms,” she explained.
Other tests such as echocardiogram and CT scans will be used to evaluate coronary disease in about 40-50 patients. The goal of using these multiple imaging tools is to find markers indicating that the heart is affected by rheumatoid arthritis, which may indicate a higher likelihood of developing heart failure, she said.
Clinics are popping up
Through these new clinics, some collaborations have emerged. Dr. Garshick works closely with Brigham and Women’s Hospital, which has a similar cardio-rheumatology program, run by Brittany Weber, MD, to exchange ideas, discuss challenging cases, and collaborate.
“There are a lot of clinics like us popping up across the country,” he observed. Every so often, he hears from other institutions that are interested in starting their own cardio-rheumatology programs. “They ask us: How do you start, what should we look for?”
It’s an education process for both patients and providers, Dr. Garshick emphasized. “I also think it’s a bandwidth issue. Many of our rheumatology and dermatology colleagues are acutely aware of the connection, but there may not be enough time at a clinic visit to really go in depth” with these dual conditions, he said.
NYU-Langone Health for the past several years has been holding a symposium to educate people on the cardio-rheumatology connection and treating inflammation in cardiovascular disease. This year’s symposium, held in conjunction with Brigham and Women’s Hospital, is scheduled for April 28. For more information, visit the course website: nyulmc.org/cvinflammationcme.
“What we’re trying to do is help [other institutions] get that bandwidth” to adequately help and serve these patients, he said.
Dr. Garshick has received consultant fees from Abbvie and Horizon therapeutics and an unrestricted research grant from Pfizer. No other sources had relevant financial disclosures.
Clinical cardiologist Heba Wassif, MD, MPH, knows the value of working with her fellow rheumatologists, surgeons, and other clinicians to establish a care plan for her patients with cardiac conditions and autoimmune diseases.
She is the cofounder of the Cleveland Clinic’s new cardio-rheumatology program, which places an emphasis on multidisciplinary care. In her role, Dr. Wassif closely follows her patients, and if she sees any inflammation or any other condition that requires the rheumatologist, she reaches out to her colleagues to adjust medications if needed.
Collaboration with a rheumatologist was important when a patient with valvular disease was prepping for surgery. The patient was on significant immunosuppressants and the surgery had to be timed appropriately, accounting for any decreases in her immunosuppression, explained Dr. Wassif, director of inpatient clinical cardiology at Cleveland Clinic in Ohio.
Cardio-rheumatology programs are “the newest child” in a series of cardiology offshoots focusing on different populations. Cardio-oncology and cardio-obstetrics took off about 6 years ago, with cardio-rheumatology clinics and interested physicians rising in number over the last several years, Dr. Wassif noted.
The relationship between cardiovascular diseases and rheumatologic conditions is certainly recognized more often, “which means more literature is being published to discuss the link,” according to Rekha Mankad, MD, a trailblazer of this model of care. She directs the Women’s Heart Clinic at Mayo Clinic in Rochester, Minn., which was one of the earliest adopters of a cardio-rheumatology clinic.
Ten years ago, “nobody was talking about the link between rheumatologic conditions and cardiovascular disease,” Dr. Mankad said. “I’ve been asked to speak on this topic, and programs have asked me to speak about establishing cardio-rheumatology practices. So, there’s been an evolution as far as a recognition that these two conditions overlap.”
Patients have come to her independent of internal referrals, which means they have done Google searches on cardiology and rheumatology. “I think that it has made a splash, at least in the world of cardiology,” Dr. Mankad observed in an interview.
Other institutions such as NYU-Langone, Yale, Stanford, Brigham and Women’s Hospital in Boston, and Women’s College Hospital in Toronto have formed similar clinics whose focus is to address the specific needs of rheumatology patients with cardiac conditions through a teamwork approach.
Challenges of treating cardiac, rheumatologic conditions
The rise in clinics addresses the longstanding connection between autoimmune disorders and cardiac conditions.
Cardiologists have known that there is an element of inflammation that contributes to atherosclerosis, said Dr. Wassif, who has researched this topic extensively. A recent study she led found a strong association between rheumatic immune-mediated inflammatory diseases (IMIDs) and high risk of acute coronary syndrome in Medicare patients.
“This particular population has a very clear increased risk for cardiovascular conditions, including valve disease and heart failure,” she emphasized.
Patients with rheumatoid arthritis and lupus have up to a twofold and eightfold higher risk of heart disease, respectively, noted Michael S. Garshick, MD, a cardiovascular disease specialist who directs the cardio-rheumatology program at NYU-Langone Health, in New York. Cardiologists “have really developed an understanding that the immune system can impact the heart, and that there’s a need for people to understand the nuance behind how the immune system can affect them and what to do about it,” Dr. Garshick said.
Caring for patients with both afflictions comes with specific challenges. Many physicians are not well trained on managing and treating patients with these dual conditions.
The “lipid paradox,” in which lipids are reduced with active inflammation in some rheumatologic conditions, can make treatment more nuanced. In addition, the traditional ASCVD (atherosclerotic cardiovascular disease) score often underestimates the cardiovascular risk of these patients, noted cardiologist Margaret Furman, MD, MPH, assistant professor and codirector of Yale’s Cardio-Rheumatology Program, New Haven, Conn.
Newer biologic medications used to treat rheumatologic diseases can alter a patient’s lipid profile, she said in an interview.
“It can be difficult to assess each individual patient’s cardiovascular risk as their disease state and treatment can vary throughout their lifetime based on their degree of inflammation. The importance of aggressive lipid management is often underestimated,” Dr. Furman added.
Cardiology and rheumatology partnerships can address gaps in care of this unique group of patients, said Vaidehi R. Chowdhary, MBBS, MD, clinical chief of the Yale Section of Rheumatology, Allergy, and Immunology at Yale University.
“The role of the rheumatologist in this dyad is to educate patients on this risk, work toward adequate control of inflammation, and minimize use of medications that contribute to increased cardiovascular risks,” said Dr. Chowdhary, who cofounded Yale’s cardio-rheumatology program with Dr. Furman.
Cardiologists in turn can assert their knowledge about medications and their impact on lipids and inflammation, Dr. Wassif said.
Many anti-inflammatory therapies are now within the cardiologist’s purview, Dr. Garshick noted. “For example, specifically with pericarditis, there’s [Food and Drug Administration]–approved anti-inflammatories or biologics. We’re the ones who feel the most comfortable giving them right now.” Cardiologists quite often are consulted about medications that are efficacious in rheumatologic conditions but could negatively impact the cardiovascular system, such as Janus kinase inhibitors, he added.
‘Reading the tea leaves’
Each program has its own unique story. For the Cleveland Clinic, the concept of a cardio-rheumatology program began during the COVID-19 pandemic in 2020. Developing such a concept and gaining institutional acceptance is always a work in process, Dr. Wassif said. “It’s not that you decide one day that you’re going to build a center, and that center is going to come into fruition overnight. You first gauge interest within your division. Who are the individuals that are interested in this area?”
Cleveland Clinic’s center is seeking to build relations between medical disciplines while spotlighting the concept of cardio-rheumatology, said Dr. Wassif, who has been providing education within the clinic and at other health institutions to ensure that patients receive appropriate attention early.
NYU-Langone launched its program amid this heightened awareness that the immune system could affect atherosclerosis, “kind of reading of the tea leaves, so to speak,” Dr. Garshick said.
Several clinical trials served as a catalyst for this movement. “A lot of clinical cardiologists were never 100% convinced that targeting the immune system reduced cardiovascular disease,” he said. Then the CANTOS clinical trial came along and showed for the first time that a therapeutic monoclonal antibody targeting interleukin-1beta, a cytokine central to inflammatory response, could in fact reduce cardiovascular disease.
Trials like this, along with epidemiologic literature connecting the rheumatologic and the autoimmune conditions with cardiovascular disease, pushed this concept to the forefront, Dr. Garshick said.
The notion that a clinic could successfully address cardiac problems in patients with rheumatic diseases yielded promising returns at Women’s College Hospital in Toronto, according to a report presented at the 2018 American College of Rheumatology annual meeting. Researchers reported that patients with rheumatologic conditions who attended a cardio-rheumatology clinic at this center saw improvements in care. The clinic identified increased cardiovascular risk and early atherosclerosis, and 53.8% of patients altered their medications after being seen in the clinic.
A total of 39.7% and 32.1% received lipid lowering and antiplatelet therapies, respectively, and 14% received antihypertensive therapy. A small percentage were treated for heart failure or placed on lifelong anticoagulation therapy for atrial fibrillation, and one patient received a percutaneous coronary stent.
Ins and outs of the referral process
Initially designed for preventive cardiac risk assessment, Yale’s program evolved into a multidisciplinary, patient-centered approach for the management of complex cardiovascular conditions in patients with autoimmune rheumatologic diseases.
The program is open to anyone who carries a diagnosis of rheumatologic disease or has elevated inflammatory markers. “Every patient, regardless of the reason for the referral, receives a cardiovascular risk assessment,” Dr. Furman said.
Most referrals come from rheumatologists, although cardiology colleagues and pulmonologists have also sent referrals. A pulmonologist, for example, may want to rule out a cardiac cause to shortness of breath. The patient’s workup, care, and follow-up are based on the reason for referral.
“We are currently referring patients with established cardiac disease, traditional risk factors, or for better risk assessment for primary prevention of coronary artery disease,” Dr. Chowdhary said. “We communicate very frequently about medication changes, and patients are aware of goals of care from both sides.”
Dr. Furman works closely with several of the rheumatology specialists taking care of patients with rheumatoid arthritis, systemic lupus erythematosus, and scleroderma.
Rheumatology follows patients every 3-6 months or more frequently based on their disease activity.
Dr. Mankad uses her sleuthing skills at Mayo Clinic to determine what the patients need. If they come in for a preventive assessment, she looks more closely at their cardiovascular risks and may order additional imaging to look for subclinical atherosclerosis. “We’re more aggressive with statin therapy in this population because of that,” she said.
If it’s valve disease, she pays extra attention to the patients’ valves in the echocardiograms and follows them a bit more regularly than someone without a rheumatologic condition and valve disease.
For patients with heart failure signs or symptoms, “it depends on how symptomatic they are,” Dr. Mankad said. In some instances, she may look for evidence of heart failure with preserved ejection fraction in patients who have rheumatoid arthritis who happen to be short of breath. “There’s so many different manifestations that patients with rheumatologic conditions can have as far as what could be affected in the heart,” she noted.
Quite frequently, Dr. Mankad identifies subclinical disease in her patients with rheumatoid arthritis. “I’ve seen many patients whose risk scores would not dictate statin therapy. But I went looking for subclinical disease by either doing coronary assessment or carotid assessment and have found atherosclerosis that would be enough to warrant statin therapy.”
A personalized assessment to reduce cardiac risk
NYU-Langone’s program offers opportunities to educate patients about the link between cardiac and rheumatologic disease.
“Their rheumatologist or their dermatologist will say, ‘Hey, have you heard about the connection between psoriasis, psoriatic or rheumatoid arthritis, and heart disease and the risk of heart attack or stroke?’ ” Dr. Garshick said.
The patients will often say they know nothing about these connections and want to learn more about how to treat it.
“We’ll say, ‘we have someone here that can help you.’ They’ll send them to myself or other colleagues like me across the country. We’ll assess blood pressure, weight, lipids, hemoglobin A1c, and other serologic and oftentimes imaging biomarkers of cardiovascular risk.” The patients will receive a personalized assessment, listing things they can do to lower their risk, whether it’s diet, exercise, or lifestyle. “Many times it can involve medications to reduce heart disease risk,” said Dr. Garshick.
In some instances, a rheumatologist or dermatologist may be concerned about starting a patient on a specific medication for the disease such as a JAK inhibitor. “We’ll help assess their risk because there’s been a lot of literature out in the rheumatology world about the risk of JAK inhibitors and heart disease and blood clots,” said Dr. Garshick.
Dr. Garshick also sees patients with rheumatologic conditions who have a specific cardiovascular concern or complaint such as shortness of breath or chest pain. “We’ll work that up with a specific knowledge of the underlying immune condition and how that may impact their heart,” he said.
Advances in research
As they continue to see patients and devise specific care plans, developers of cardio-rheumatology programs have been supplementing their work with ongoing research.
Yale’s clinic is expanding this year to include a new attending physician, Attila Feher, MD, PhD, who has conducted research in autoimmunity and microcirculation using molecular imaging and multimodality imaging techniques. Prevalence of coronary microvascular dysfunction appears to be increased in this patient population, Dr. Furman said.
Dr. Wassif recently coauthored a paper that examined patients with underlying rheumatologic conditions who undergo valvular and aortic valve replacement. “To our surprise, there was really no difference between patients with autoimmune conditions and others with nonautoimmune conditions,” she said, adding that the study had its limitations.
Other work includes data on Medicare patients with ST- and non-ST-elevation myocardial infarctions who have an underlying autoimmune disorder. Dr. Wassif and her colleagues found that their long-term outcomes are worse than those of patients without these conditions. “It’s unclear if worse outcomes are related to complications of autoimmunity versus the extent of their underlying disease. This is a work in progress and certainly an area that is ripe for research.”
Dr. Garshick and other collaborators at NYU have been focusing on the endothelium, specifically platelet biology in patients with psoriasis, psoriatic arthritis, and lupus. “We’re about to start the same research with gout as well,” he said.
“The process we’re most interested in is understanding how these diseases impact the early stages of cholesterol. And the way we’re doing that is evaluating the vasculature, specifically the endothelium,” he said.
He has finished two clinical trials that evaluate how standard heart disease medications such as aspirin and statins impact or can potentially benefit patients with psoriasis and/or psoriatic arthritis. “We have a whole list of other trials in the pipeline with other institutions across the country.”
Through a grant, Dr. Mankad is assessing whether a PET scan could detect inflammation in the hearts of rheumatoid arthritis patients. “We’re looking to see if the reason these patients have heart failure later in life is because their heart muscle actually shows evidence of inflammation, even when they have no symptoms,” she explained.
Other tests such as echocardiogram and CT scans will be used to evaluate coronary disease in about 40-50 patients. The goal of using these multiple imaging tools is to find markers indicating that the heart is affected by rheumatoid arthritis, which may indicate a higher likelihood of developing heart failure, she said.
Clinics are popping up
Through these new clinics, some collaborations have emerged. Dr. Garshick works closely with Brigham and Women’s Hospital, which has a similar cardio-rheumatology program, run by Brittany Weber, MD, to exchange ideas, discuss challenging cases, and collaborate.
“There are a lot of clinics like us popping up across the country,” he observed. Every so often, he hears from other institutions that are interested in starting their own cardio-rheumatology programs. “They ask us: How do you start, what should we look for?”
It’s an education process for both patients and providers, Dr. Garshick emphasized. “I also think it’s a bandwidth issue. Many of our rheumatology and dermatology colleagues are acutely aware of the connection, but there may not be enough time at a clinic visit to really go in depth” with these dual conditions, he said.
NYU-Langone Health for the past several years has been holding a symposium to educate people on the cardio-rheumatology connection and treating inflammation in cardiovascular disease. This year’s symposium, held in conjunction with Brigham and Women’s Hospital, is scheduled for April 28. For more information, visit the course website: nyulmc.org/cvinflammationcme.
“What we’re trying to do is help [other institutions] get that bandwidth” to adequately help and serve these patients, he said.
Dr. Garshick has received consultant fees from Abbvie and Horizon therapeutics and an unrestricted research grant from Pfizer. No other sources had relevant financial disclosures.
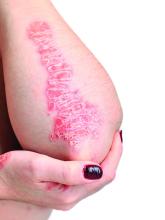
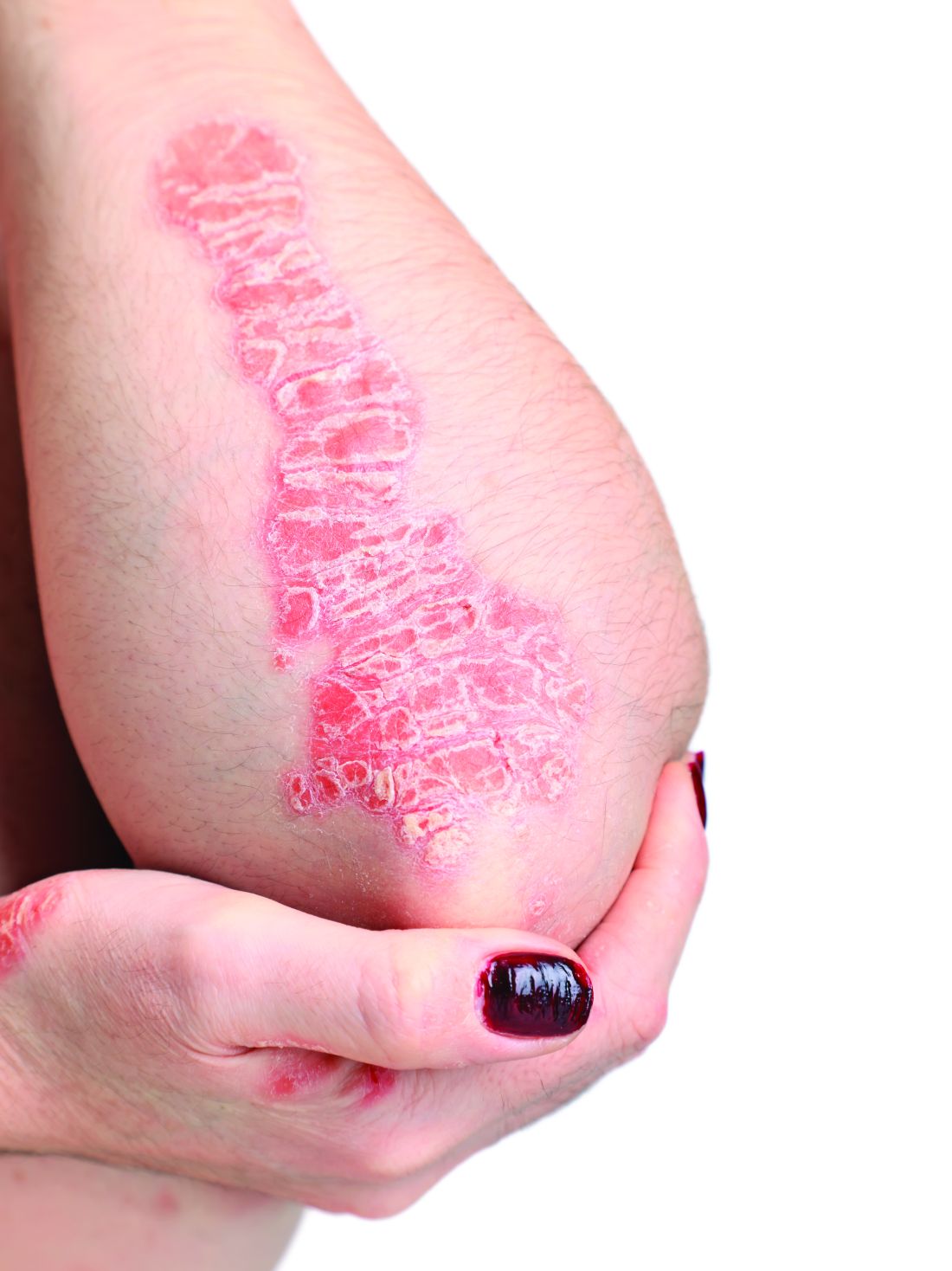
Topical psoriasis treatments
LAS VEGAS – ,” said Linda Stein Gold, MD, in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
However, when using topical treatments, combination therapy is generally more effective than monotherapy for psoriasis, especially for plaque psoriasis, said Dr. Stein Gold, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit.
Two combination products, calcipotriene/betamethasone (CAL/BDP) and tazarotene/halobetasol lotion, each offer a complimentary mechanism of action that minimizes side effects, with decreased irritation and less atrophy, she said. Calcipotriene/betamethasone (CAL/BDP) is available as a cream or foam, Dr. Stein Gold noted. The cream is engineered for rapid onset, as well as enhanced penetration, she said. CAL/BDP foam also is designed for enhanced penetration, and has been shown to have long-term maintenance efficacy, she said.
The currently available CAL/BDP cream is made using a patented technology known as “PAD,” in which the internal oil of the cream vehicle is stabilized by encapsulation in “a robust aqueous film,” Dr. Stein Gold said, noting that the greater solubility enhances skin penetration. The creation of “a robust oil droplet” addresses the problems associated with the surfactants present in many cream vehicles, namely irritation and impedance of skin penetration of the cream, she said.
In an 8-week study published in 2021, researchers compared CAL/BDP cream with PAD technology to CAL/BDP topical suspension in adults with mild to moderate psoriasis.
Patients randomized to treatment with CAL/BDP cream were significantly more likely to achieve the primary endpoint of Physician Global Assessment (PGA) treatment success than those randomized to the topical solution or vehicle (37.4%, 22.8%, and 3.7%, respectively).
Get proactive to maintain results
With topical psoriasis treatment, a proactive strategy helps maintain results over time, Dr. Stein Gold said. As an example, she cited a study published in 2021. In that study, known as PSO-LONG, which evaluated topical CAL/BDP foam, proactive management with the CAL/BDP foam formulation, “reduced the risk of experiencing relapse by 43%,” compared with reactive management (treatment with the vehicle foam), she said. Patients in the proactive-management group experienced an average of 41 more days in remission, compared with those in the reactive management group over a 1-year period.
Dr. Stein Gold also highlighted the value of tazarotene/halobetasol lotion for psoriasis, which she described as having synergistic efficacy,
She shared data presented at the 2021 Maui Dermatology meeting showing treatment success by 8 weeks with halobetasol/tazarotene with significantly reduced mean scores on measures of itching, dryness, and burning/stinging, compared with those on vehicle.
What’s new and approved
Joining the current topical treatment options for psoriasis is tapinarof, a small molecule that works by down-regulating Th17 cytokines, said Dr. Stein Gold. Tapinarof is Food and Drug Administration approved for treating psoriasis and is being studied in clinical trials for atopic dermatitis, she noted.
Dr. Stein Gold reviewed data from the PSOARING program published in the New England Journal of Medicine that served as a foundation for the FDA approval of tapinarof 1% cream. In the PSOARING 1 and 2 studies, patients with PSORIASIS showed significant improvement compared with vehicle over 12 weeks for the primary endpoint of Physicians’ Global Assessment scores of 0 or 1 (clear or almost clear). In the two studies, 60.7% and 56.9% of patients randomized to tapinarof met the patient-reported outcome of a minimum 4-point improvement in peak pruritus on the numerical rating scale (NRS) from baseline vs. 43.2% and 29.7% of placebo patients in the two studies, respectively.
In PSOARING 1 and 2, folliculitis (mostly mild or moderate), contact dermatitis, headache, pruritus, and dermatitis were the most common treatment-emergent adverse events, occurring in 1% or more of patients. Adverse event profiles for tapinarof are similar to those seen in previous studies, and a long-term extension showed a consistent safety profile, Dr. Stein Gold said.
Another recently approved topical treatment for psoriasis, a cream formulation of roflumilast, a phosphodiesterase (PDE)-4 inhibitor, has shown efficacy for treating plaque psoriasis, she said.
Patients with psoriasis in the DERMIS 1 and DERMIS 2 phase 3 studies randomized to 0.3% roflumilast cream showed significant improvement compared with those randomized to vehicle in terms of Investigator Global Assessment scores of clear or almost clear with an improvement of at least 2 grades from baseline.
Roflumilast foam also has shown success in improving scalp and body psoriasis, but this vehicle and indication has not yet been approved, Dr. Stein Gold said.
Dr. Stein Gold disclosed serving as a consultant or adviser for companies including AbbVie, Amgen, Arcutis, Bristol Myers Squibb, Dermavant, EPI Health, Galderma, Janssen, Incyte, Ortho Dermatologics, Pfizer, Regeneron, Sanofi; UCB, and serving as a speaker or member of speakers’ bureau for Amgen, AbbVie, Incyte, Pfizer, Regeneron, Sanofi, and Sun Research. She also disclosed receiving funding from AbbVie Amgen, Arcutis, Dermata, Dermavant, Galderma, Incyte, Ortho Dermatologics, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – ,” said Linda Stein Gold, MD, in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
However, when using topical treatments, combination therapy is generally more effective than monotherapy for psoriasis, especially for plaque psoriasis, said Dr. Stein Gold, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit.
Two combination products, calcipotriene/betamethasone (CAL/BDP) and tazarotene/halobetasol lotion, each offer a complimentary mechanism of action that minimizes side effects, with decreased irritation and less atrophy, she said. Calcipotriene/betamethasone (CAL/BDP) is available as a cream or foam, Dr. Stein Gold noted. The cream is engineered for rapid onset, as well as enhanced penetration, she said. CAL/BDP foam also is designed for enhanced penetration, and has been shown to have long-term maintenance efficacy, she said.
The currently available CAL/BDP cream is made using a patented technology known as “PAD,” in which the internal oil of the cream vehicle is stabilized by encapsulation in “a robust aqueous film,” Dr. Stein Gold said, noting that the greater solubility enhances skin penetration. The creation of “a robust oil droplet” addresses the problems associated with the surfactants present in many cream vehicles, namely irritation and impedance of skin penetration of the cream, she said.
In an 8-week study published in 2021, researchers compared CAL/BDP cream with PAD technology to CAL/BDP topical suspension in adults with mild to moderate psoriasis.
Patients randomized to treatment with CAL/BDP cream were significantly more likely to achieve the primary endpoint of Physician Global Assessment (PGA) treatment success than those randomized to the topical solution or vehicle (37.4%, 22.8%, and 3.7%, respectively).
Get proactive to maintain results
With topical psoriasis treatment, a proactive strategy helps maintain results over time, Dr. Stein Gold said. As an example, she cited a study published in 2021. In that study, known as PSO-LONG, which evaluated topical CAL/BDP foam, proactive management with the CAL/BDP foam formulation, “reduced the risk of experiencing relapse by 43%,” compared with reactive management (treatment with the vehicle foam), she said. Patients in the proactive-management group experienced an average of 41 more days in remission, compared with those in the reactive management group over a 1-year period.
Dr. Stein Gold also highlighted the value of tazarotene/halobetasol lotion for psoriasis, which she described as having synergistic efficacy,
She shared data presented at the 2021 Maui Dermatology meeting showing treatment success by 8 weeks with halobetasol/tazarotene with significantly reduced mean scores on measures of itching, dryness, and burning/stinging, compared with those on vehicle.
What’s new and approved
Joining the current topical treatment options for psoriasis is tapinarof, a small molecule that works by down-regulating Th17 cytokines, said Dr. Stein Gold. Tapinarof is Food and Drug Administration approved for treating psoriasis and is being studied in clinical trials for atopic dermatitis, she noted.
Dr. Stein Gold reviewed data from the PSOARING program published in the New England Journal of Medicine that served as a foundation for the FDA approval of tapinarof 1% cream. In the PSOARING 1 and 2 studies, patients with PSORIASIS showed significant improvement compared with vehicle over 12 weeks for the primary endpoint of Physicians’ Global Assessment scores of 0 or 1 (clear or almost clear). In the two studies, 60.7% and 56.9% of patients randomized to tapinarof met the patient-reported outcome of a minimum 4-point improvement in peak pruritus on the numerical rating scale (NRS) from baseline vs. 43.2% and 29.7% of placebo patients in the two studies, respectively.
In PSOARING 1 and 2, folliculitis (mostly mild or moderate), contact dermatitis, headache, pruritus, and dermatitis were the most common treatment-emergent adverse events, occurring in 1% or more of patients. Adverse event profiles for tapinarof are similar to those seen in previous studies, and a long-term extension showed a consistent safety profile, Dr. Stein Gold said.
Another recently approved topical treatment for psoriasis, a cream formulation of roflumilast, a phosphodiesterase (PDE)-4 inhibitor, has shown efficacy for treating plaque psoriasis, she said.
Patients with psoriasis in the DERMIS 1 and DERMIS 2 phase 3 studies randomized to 0.3% roflumilast cream showed significant improvement compared with those randomized to vehicle in terms of Investigator Global Assessment scores of clear or almost clear with an improvement of at least 2 grades from baseline.
Roflumilast foam also has shown success in improving scalp and body psoriasis, but this vehicle and indication has not yet been approved, Dr. Stein Gold said.
Dr. Stein Gold disclosed serving as a consultant or adviser for companies including AbbVie, Amgen, Arcutis, Bristol Myers Squibb, Dermavant, EPI Health, Galderma, Janssen, Incyte, Ortho Dermatologics, Pfizer, Regeneron, Sanofi; UCB, and serving as a speaker or member of speakers’ bureau for Amgen, AbbVie, Incyte, Pfizer, Regeneron, Sanofi, and Sun Research. She also disclosed receiving funding from AbbVie Amgen, Arcutis, Dermata, Dermavant, Galderma, Incyte, Ortho Dermatologics, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – ,” said Linda Stein Gold, MD, in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
However, when using topical treatments, combination therapy is generally more effective than monotherapy for psoriasis, especially for plaque psoriasis, said Dr. Stein Gold, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit.
Two combination products, calcipotriene/betamethasone (CAL/BDP) and tazarotene/halobetasol lotion, each offer a complimentary mechanism of action that minimizes side effects, with decreased irritation and less atrophy, she said. Calcipotriene/betamethasone (CAL/BDP) is available as a cream or foam, Dr. Stein Gold noted. The cream is engineered for rapid onset, as well as enhanced penetration, she said. CAL/BDP foam also is designed for enhanced penetration, and has been shown to have long-term maintenance efficacy, she said.
The currently available CAL/BDP cream is made using a patented technology known as “PAD,” in which the internal oil of the cream vehicle is stabilized by encapsulation in “a robust aqueous film,” Dr. Stein Gold said, noting that the greater solubility enhances skin penetration. The creation of “a robust oil droplet” addresses the problems associated with the surfactants present in many cream vehicles, namely irritation and impedance of skin penetration of the cream, she said.
In an 8-week study published in 2021, researchers compared CAL/BDP cream with PAD technology to CAL/BDP topical suspension in adults with mild to moderate psoriasis.
Patients randomized to treatment with CAL/BDP cream were significantly more likely to achieve the primary endpoint of Physician Global Assessment (PGA) treatment success than those randomized to the topical solution or vehicle (37.4%, 22.8%, and 3.7%, respectively).
Get proactive to maintain results
With topical psoriasis treatment, a proactive strategy helps maintain results over time, Dr. Stein Gold said. As an example, she cited a study published in 2021. In that study, known as PSO-LONG, which evaluated topical CAL/BDP foam, proactive management with the CAL/BDP foam formulation, “reduced the risk of experiencing relapse by 43%,” compared with reactive management (treatment with the vehicle foam), she said. Patients in the proactive-management group experienced an average of 41 more days in remission, compared with those in the reactive management group over a 1-year period.
Dr. Stein Gold also highlighted the value of tazarotene/halobetasol lotion for psoriasis, which she described as having synergistic efficacy,
She shared data presented at the 2021 Maui Dermatology meeting showing treatment success by 8 weeks with halobetasol/tazarotene with significantly reduced mean scores on measures of itching, dryness, and burning/stinging, compared with those on vehicle.
What’s new and approved
Joining the current topical treatment options for psoriasis is tapinarof, a small molecule that works by down-regulating Th17 cytokines, said Dr. Stein Gold. Tapinarof is Food and Drug Administration approved for treating psoriasis and is being studied in clinical trials for atopic dermatitis, she noted.
Dr. Stein Gold reviewed data from the PSOARING program published in the New England Journal of Medicine that served as a foundation for the FDA approval of tapinarof 1% cream. In the PSOARING 1 and 2 studies, patients with PSORIASIS showed significant improvement compared with vehicle over 12 weeks for the primary endpoint of Physicians’ Global Assessment scores of 0 or 1 (clear or almost clear). In the two studies, 60.7% and 56.9% of patients randomized to tapinarof met the patient-reported outcome of a minimum 4-point improvement in peak pruritus on the numerical rating scale (NRS) from baseline vs. 43.2% and 29.7% of placebo patients in the two studies, respectively.
In PSOARING 1 and 2, folliculitis (mostly mild or moderate), contact dermatitis, headache, pruritus, and dermatitis were the most common treatment-emergent adverse events, occurring in 1% or more of patients. Adverse event profiles for tapinarof are similar to those seen in previous studies, and a long-term extension showed a consistent safety profile, Dr. Stein Gold said.
Another recently approved topical treatment for psoriasis, a cream formulation of roflumilast, a phosphodiesterase (PDE)-4 inhibitor, has shown efficacy for treating plaque psoriasis, she said.
Patients with psoriasis in the DERMIS 1 and DERMIS 2 phase 3 studies randomized to 0.3% roflumilast cream showed significant improvement compared with those randomized to vehicle in terms of Investigator Global Assessment scores of clear or almost clear with an improvement of at least 2 grades from baseline.
Roflumilast foam also has shown success in improving scalp and body psoriasis, but this vehicle and indication has not yet been approved, Dr. Stein Gold said.
Dr. Stein Gold disclosed serving as a consultant or adviser for companies including AbbVie, Amgen, Arcutis, Bristol Myers Squibb, Dermavant, EPI Health, Galderma, Janssen, Incyte, Ortho Dermatologics, Pfizer, Regeneron, Sanofi; UCB, and serving as a speaker or member of speakers’ bureau for Amgen, AbbVie, Incyte, Pfizer, Regeneron, Sanofi, and Sun Research. She also disclosed receiving funding from AbbVie Amgen, Arcutis, Dermata, Dermavant, Galderma, Incyte, Ortho Dermatologics, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
Commentary: Bimekizumab, and PsA's Relationships With AS and Crohn's Disease, January 2023
Two new papers published recently provide evidence of the efficacy and safety of bimekizumab in psoriatic arthritis (PsA). Bimekizumab is a novel bispecific monoclonal antibody targeting interleukin (IL)-17A and IL-17F and is already approved for the treatment of chronic plaque psoriasis. McInnes and colleagues reported the results of the phase 3 BE OPTIMAL study, which included 852 patients with active PsA who were naive to biologic disease-modifying antirheumatic drugs (bDMARD) and were randomly assigned to receive bimekizumab, placebo, or adalimumab. At week 16, a significantly higher proportion of patients receiving bimekizumab vs placebo achieved ≥ 50% improvement in American College of Rheumatology response (ACR50; 44% vs 10%; odds ratio [OR] 7.1; P < .0001). Compared with placebo, significant improvements were also noted in psoriasis, enthesitis, and dactylitis in the bimekizumab group and there was less progression of radiographic damage.
Bimekizumab was also demonstrated to be beneficial in PsA patients with inadequate response or intolerance to tumor necrosis factor inhibitors (TNFi). In the phase 3 BE COMPLETE study, which included 400 patients with active PsA and previous inadequate response or intolerance to TNFi, patients were randomly assigned to receive 160 mg subcutaneous bimekizumab every 4 weeks or placebo. Merola and colleagues reported that at week 16, a significantly higher proportion of patients receiving bimekizumab vs placebo achieved ACR50 response (43% vs 7%; OR 11.1; P < .0001). Thus, bimekizumab is a welcome addition to the treatment portfolio we have for PsA. In regard to side effects of special concern when inhibiting IL-17, bimekizumab was associated with higher risk for oral and genital candidiasis, occurring in 4% of the treated patients within 16 weeks in the two studies; however, no cases of systemic fungal infections occurred. The incidence of inflammatory bowel disease was also very low, but head-to-head studies against other available agents would be required to help rheumatologists decide the place of bimekizumab in PsA management.
A common clinical question is whether axial PsA is similar to ankylosing spondylitis (AS) with psoriasis. Assuming that it is, clinicians have used treatments approved for AS for managing axial PsA. Recent studies have questioned this assumption, however. Michelena and colleagues conducted a cross-sectional study that included 109 patients with axial PsA and 127 patients with AS and psoriasis from the REGISPONSER registry. Compared with patients with AS and psoriasis, patients with human leukocyte antigen (HLA)-B27–negative axial PsA had less inflammatory pain (P = .002), anterior uveitis (P = .014), and structural damage (P < .001), along with a higher prevalence of nail disease (P = .009). Patients with HLA-B27–positive axial PsA vs AS and psoriasis were similar but had less structural damage to the spine (P < .001). Thus, there seem to be significant clinical and genetic differences between these two diseases that require further investigation. Lack of an accepted definition of axial PsA, however, is a hindrance to multiple high-quality genetic, clinical, and interventional studies comparing axial PsA and AS with psoriasis.
Observational studies have recognized the clinical and familial association between psoriatic disease and Crohn's disease (CD), but such cross-sectional or retrospective studies cannot identify causal relationships. Mendelian randomization is a method used to identify causal relationships. Using this method, Sun and colleagues demonstrated that PsA was associated with a 31.9% increased risk for CD (P < .001) and genetically predicted CD was linked to a 44.8% higher risk for PsA (P = .001). No such association was found with ulcerative colitis. Thus, there is a bidirectional causal relationship between the two diseases. Patients with PsA should be evaluated for symptoms of CD, and those with CD for psoriatic disease, to facilitate early diagnosis and better long-term outcomes.
Two new papers published recently provide evidence of the efficacy and safety of bimekizumab in psoriatic arthritis (PsA). Bimekizumab is a novel bispecific monoclonal antibody targeting interleukin (IL)-17A and IL-17F and is already approved for the treatment of chronic plaque psoriasis. McInnes and colleagues reported the results of the phase 3 BE OPTIMAL study, which included 852 patients with active PsA who were naive to biologic disease-modifying antirheumatic drugs (bDMARD) and were randomly assigned to receive bimekizumab, placebo, or adalimumab. At week 16, a significantly higher proportion of patients receiving bimekizumab vs placebo achieved ≥ 50% improvement in American College of Rheumatology response (ACR50; 44% vs 10%; odds ratio [OR] 7.1; P < .0001). Compared with placebo, significant improvements were also noted in psoriasis, enthesitis, and dactylitis in the bimekizumab group and there was less progression of radiographic damage.
Bimekizumab was also demonstrated to be beneficial in PsA patients with inadequate response or intolerance to tumor necrosis factor inhibitors (TNFi). In the phase 3 BE COMPLETE study, which included 400 patients with active PsA and previous inadequate response or intolerance to TNFi, patients were randomly assigned to receive 160 mg subcutaneous bimekizumab every 4 weeks or placebo. Merola and colleagues reported that at week 16, a significantly higher proportion of patients receiving bimekizumab vs placebo achieved ACR50 response (43% vs 7%; OR 11.1; P < .0001). Thus, bimekizumab is a welcome addition to the treatment portfolio we have for PsA. In regard to side effects of special concern when inhibiting IL-17, bimekizumab was associated with higher risk for oral and genital candidiasis, occurring in 4% of the treated patients within 16 weeks in the two studies; however, no cases of systemic fungal infections occurred. The incidence of inflammatory bowel disease was also very low, but head-to-head studies against other available agents would be required to help rheumatologists decide the place of bimekizumab in PsA management.
A common clinical question is whether axial PsA is similar to ankylosing spondylitis (AS) with psoriasis. Assuming that it is, clinicians have used treatments approved for AS for managing axial PsA. Recent studies have questioned this assumption, however. Michelena and colleagues conducted a cross-sectional study that included 109 patients with axial PsA and 127 patients with AS and psoriasis from the REGISPONSER registry. Compared with patients with AS and psoriasis, patients with human leukocyte antigen (HLA)-B27–negative axial PsA had less inflammatory pain (P = .002), anterior uveitis (P = .014), and structural damage (P < .001), along with a higher prevalence of nail disease (P = .009). Patients with HLA-B27–positive axial PsA vs AS and psoriasis were similar but had less structural damage to the spine (P < .001). Thus, there seem to be significant clinical and genetic differences between these two diseases that require further investigation. Lack of an accepted definition of axial PsA, however, is a hindrance to multiple high-quality genetic, clinical, and interventional studies comparing axial PsA and AS with psoriasis.
Observational studies have recognized the clinical and familial association between psoriatic disease and Crohn's disease (CD), but such cross-sectional or retrospective studies cannot identify causal relationships. Mendelian randomization is a method used to identify causal relationships. Using this method, Sun and colleagues demonstrated that PsA was associated with a 31.9% increased risk for CD (P < .001) and genetically predicted CD was linked to a 44.8% higher risk for PsA (P = .001). No such association was found with ulcerative colitis. Thus, there is a bidirectional causal relationship between the two diseases. Patients with PsA should be evaluated for symptoms of CD, and those with CD for psoriatic disease, to facilitate early diagnosis and better long-term outcomes.
Two new papers published recently provide evidence of the efficacy and safety of bimekizumab in psoriatic arthritis (PsA). Bimekizumab is a novel bispecific monoclonal antibody targeting interleukin (IL)-17A and IL-17F and is already approved for the treatment of chronic plaque psoriasis. McInnes and colleagues reported the results of the phase 3 BE OPTIMAL study, which included 852 patients with active PsA who were naive to biologic disease-modifying antirheumatic drugs (bDMARD) and were randomly assigned to receive bimekizumab, placebo, or adalimumab. At week 16, a significantly higher proportion of patients receiving bimekizumab vs placebo achieved ≥ 50% improvement in American College of Rheumatology response (ACR50; 44% vs 10%; odds ratio [OR] 7.1; P < .0001). Compared with placebo, significant improvements were also noted in psoriasis, enthesitis, and dactylitis in the bimekizumab group and there was less progression of radiographic damage.
Bimekizumab was also demonstrated to be beneficial in PsA patients with inadequate response or intolerance to tumor necrosis factor inhibitors (TNFi). In the phase 3 BE COMPLETE study, which included 400 patients with active PsA and previous inadequate response or intolerance to TNFi, patients were randomly assigned to receive 160 mg subcutaneous bimekizumab every 4 weeks or placebo. Merola and colleagues reported that at week 16, a significantly higher proportion of patients receiving bimekizumab vs placebo achieved ACR50 response (43% vs 7%; OR 11.1; P < .0001). Thus, bimekizumab is a welcome addition to the treatment portfolio we have for PsA. In regard to side effects of special concern when inhibiting IL-17, bimekizumab was associated with higher risk for oral and genital candidiasis, occurring in 4% of the treated patients within 16 weeks in the two studies; however, no cases of systemic fungal infections occurred. The incidence of inflammatory bowel disease was also very low, but head-to-head studies against other available agents would be required to help rheumatologists decide the place of bimekizumab in PsA management.
A common clinical question is whether axial PsA is similar to ankylosing spondylitis (AS) with psoriasis. Assuming that it is, clinicians have used treatments approved for AS for managing axial PsA. Recent studies have questioned this assumption, however. Michelena and colleagues conducted a cross-sectional study that included 109 patients with axial PsA and 127 patients with AS and psoriasis from the REGISPONSER registry. Compared with patients with AS and psoriasis, patients with human leukocyte antigen (HLA)-B27–negative axial PsA had less inflammatory pain (P = .002), anterior uveitis (P = .014), and structural damage (P < .001), along with a higher prevalence of nail disease (P = .009). Patients with HLA-B27–positive axial PsA vs AS and psoriasis were similar but had less structural damage to the spine (P < .001). Thus, there seem to be significant clinical and genetic differences between these two diseases that require further investigation. Lack of an accepted definition of axial PsA, however, is a hindrance to multiple high-quality genetic, clinical, and interventional studies comparing axial PsA and AS with psoriasis.
Observational studies have recognized the clinical and familial association between psoriatic disease and Crohn's disease (CD), but such cross-sectional or retrospective studies cannot identify causal relationships. Mendelian randomization is a method used to identify causal relationships. Using this method, Sun and colleagues demonstrated that PsA was associated with a 31.9% increased risk for CD (P < .001) and genetically predicted CD was linked to a 44.8% higher risk for PsA (P = .001). No such association was found with ulcerative colitis. Thus, there is a bidirectional causal relationship between the two diseases. Patients with PsA should be evaluated for symptoms of CD, and those with CD for psoriatic disease, to facilitate early diagnosis and better long-term outcomes.
Cardiovascular risk score multipliers suggested for rheumatic diseases
A re-evaluation of cardiovascular risk management guidelines intended for use by rheumatologists may be warranted based on findings from a recently published population-based study of the risks for 12 different cardiovascular disease outcomes in patients with autoimmune diseases.
“The notion that patients with rheumatic diseases are at increased risk of developing cardiovascular diseases has been ongoing for many years,” Nathalie Conrad, PhD, and coauthors wrote in a viewpoint article in Annals of the Rheumatic Diseases.
This has “sparked much debate concerning whether and when to initiate cardiovascular prevention therapies,” they said.
Dr. Conrad was first author on the population-based study published in The Lancet in August 2022 that used linked primary and secondary care records from datasets in the U.K. Clinical Practice Research Datalink involving individuals who were recently diagnosed with any of 19 different autoimmune diseases during an 18-year period stretching from 2000 to 2017 but free of cardiovascular disease until at least 12 months after incident autoimmune disease. “Every single autoimmune disorder we looked at was associated with increased cardiovascular risk,” Dr. Conrad, of the department of public health and primary care at Catholic University Leuven (Belgium), said in an interview.
Not only was the risk for cardiovascular disease increased for people with rheumatic diseases by an average of 68%, compared with people without rheumatic diseases, but also the whole spectrum of cardiovascular disorders was seen.
“We saw increases in thromboembolic diseases, degenerative heart diseases, and heart inflammation,” Dr. Conrad said.
Large datasets examined
The idea for the epidemiologic study came from mounting evidence for cardiovascular disease risk among people with autoimmune diseases but not enough to support the design of specific prevention measures.
Dr. Conrad’s Lancet study examined electronic health records of 446,449 individuals with autoimmune diseases and matched them to 2,102,830 individuals without autoimmune disease. This included 160,217 individuals with seven rheumatic diseases: rheumatoid arthritis, polymyalgia rheumatica, vasculitis, systemic lupus erythematosus, Sjögren’s syndrome, ankylosing spondylitis, and systemic sclerosis.
In addition to looking for any evidence of cardiovascular disease, Dr. Conrad and coauthors looked at 12 specific outcomes: atherosclerotic diseases, peripheral arterial disease, stroke or transient ischemic attack, heart failure, valve disorders, thromboembolic disease, atrial fibrillation or flutter, conduction system disease, supraventricular arrhythmias, aortic aneurysm, myocarditis and pericarditis, and infective endocarditis.
CV risk in rheumatic diseases
As might be expected, “greater magnitudes of risk” were seen for individuals with systemic lupus erythematosus and systemic sclerosis than for people in the general population, with the chances of cardiovascular disease being two to four times higher. But what perhaps wasn’t expected was that all rheumatic diseases carried an increased risk for heart or vascular-related problems.
Furthermore, the increased risk could not solely be accounted for by the presence of traditional risk factors, such as blood pressure, smoking, or obesity.
“The background here is that any context of systemic inflammation would be predicted to lead to an increased vascular risk,” Iain McInnes, MD, PhD, professor of medicine and rheumatology at the University of Glasgow, said in an interview. Dr. McInnes was a coauthor of the viewpoint article in Annals of the Rheumatic Diseases.
“The implication is that there may well be increased vascular risk across the whole range of immune-mediated inflammatory diseases,” he added. “We should not, however, infer the magnitude of risk will be the same for each disease.”
What is more intriguing, Dr. McInnes said, is that “we don’t know yet whether there’s one final common pathway that leads to the blood vessel being damaged or whether different diseases might contribute different pathways.”
He added: “A question for the future is to see what are those mechanisms that drive risk across different diseases? And the reason that matters, of course, is that we might want to think about the effectiveness of different therapeutic interventions.”
Determining cardiovascular risk
Dr. Conrad and associates in their viewpoint article suggested that an update to the European Alliance of Associations for Rheumatology guidelines for cardiovascular risk management of rheumatic and musculoskeletal diseases (RMDs) could tailor cardiovascular risk scores to certain diseases.
They suggested that the guidelines could consider a risk multiplier of 2.5 for systemic sclerosis, 2.0 for lupus, and 1.5 for any other rheumatic disease.
“We argue that [EULAR] recommendations should consider this new evidence of poorer cardiovascular health in numerous RMDs and envisage cardiovascular screening and associated prevention measures,” Dr. Conrad said.
While they recognize that risk multipliers aren’t perfect, “they are the best available option until personalized risk prediction tools are developed specifically for patients with RMDs.”
Addressing cardiovascular risk
As a former president of EULAR, Dr. McInnes was keen to point out that “EULAR’s recommendations are evidence based and are rigorously built on [standard operating procedures] that work and have stood the test of time. I’m quite sure that the members of relevant EULAR task forces will be looking at these data, but they’ll be looking at the whole range of literature to see whether change is necessary.”
Good-quality inflammatory disease control will certainly contribute to reducing vascular risk, “but we should not make the assumption that it will be sufficient,” he cautioned. “We still have to be very careful in addressing so called conventional risk factors, but in particular thinking about obesity and cardiometabolic syndrome to be sure that when those are present, that we detect them and we treat them appropriately.”
As to who is best placed to manage a patient’s cardiovascular risk profile, Dr. McInnes said: “I think the rheumatologist has a responsibility to make sure that as much of the patient’s disease spectrum is being treated as possible.”
“As a rheumatologist, I would like to know that those elements of a patient’s disease presentation are being addressed,” whether that is by a primary care physician, cardiologist, diabetologist, or other specialist involved in the optimal management of the patient.
Dr. Conrad acknowledged receiving support from the European Union’s Horizon 2020 Program, the European Society for Cardiology, and grant funding paid to her institution from the Belgian-based Research Foundation Flounders. She also acknowledged receipt of royalties in regard to the intellectual property of a home-monitoring system for heart failure paid to Oxford University Innovation. Dr. McInnes acknowledged financial relationships with many pharmaceutical companies.
*This article was updated 12/30/2022.
A re-evaluation of cardiovascular risk management guidelines intended for use by rheumatologists may be warranted based on findings from a recently published population-based study of the risks for 12 different cardiovascular disease outcomes in patients with autoimmune diseases.
“The notion that patients with rheumatic diseases are at increased risk of developing cardiovascular diseases has been ongoing for many years,” Nathalie Conrad, PhD, and coauthors wrote in a viewpoint article in Annals of the Rheumatic Diseases.
This has “sparked much debate concerning whether and when to initiate cardiovascular prevention therapies,” they said.
Dr. Conrad was first author on the population-based study published in The Lancet in August 2022 that used linked primary and secondary care records from datasets in the U.K. Clinical Practice Research Datalink involving individuals who were recently diagnosed with any of 19 different autoimmune diseases during an 18-year period stretching from 2000 to 2017 but free of cardiovascular disease until at least 12 months after incident autoimmune disease. “Every single autoimmune disorder we looked at was associated with increased cardiovascular risk,” Dr. Conrad, of the department of public health and primary care at Catholic University Leuven (Belgium), said in an interview.
Not only was the risk for cardiovascular disease increased for people with rheumatic diseases by an average of 68%, compared with people without rheumatic diseases, but also the whole spectrum of cardiovascular disorders was seen.
“We saw increases in thromboembolic diseases, degenerative heart diseases, and heart inflammation,” Dr. Conrad said.
Large datasets examined
The idea for the epidemiologic study came from mounting evidence for cardiovascular disease risk among people with autoimmune diseases but not enough to support the design of specific prevention measures.
Dr. Conrad’s Lancet study examined electronic health records of 446,449 individuals with autoimmune diseases and matched them to 2,102,830 individuals without autoimmune disease. This included 160,217 individuals with seven rheumatic diseases: rheumatoid arthritis, polymyalgia rheumatica, vasculitis, systemic lupus erythematosus, Sjögren’s syndrome, ankylosing spondylitis, and systemic sclerosis.
In addition to looking for any evidence of cardiovascular disease, Dr. Conrad and coauthors looked at 12 specific outcomes: atherosclerotic diseases, peripheral arterial disease, stroke or transient ischemic attack, heart failure, valve disorders, thromboembolic disease, atrial fibrillation or flutter, conduction system disease, supraventricular arrhythmias, aortic aneurysm, myocarditis and pericarditis, and infective endocarditis.
CV risk in rheumatic diseases
As might be expected, “greater magnitudes of risk” were seen for individuals with systemic lupus erythematosus and systemic sclerosis than for people in the general population, with the chances of cardiovascular disease being two to four times higher. But what perhaps wasn’t expected was that all rheumatic diseases carried an increased risk for heart or vascular-related problems.
Furthermore, the increased risk could not solely be accounted for by the presence of traditional risk factors, such as blood pressure, smoking, or obesity.
“The background here is that any context of systemic inflammation would be predicted to lead to an increased vascular risk,” Iain McInnes, MD, PhD, professor of medicine and rheumatology at the University of Glasgow, said in an interview. Dr. McInnes was a coauthor of the viewpoint article in Annals of the Rheumatic Diseases.
“The implication is that there may well be increased vascular risk across the whole range of immune-mediated inflammatory diseases,” he added. “We should not, however, infer the magnitude of risk will be the same for each disease.”
What is more intriguing, Dr. McInnes said, is that “we don’t know yet whether there’s one final common pathway that leads to the blood vessel being damaged or whether different diseases might contribute different pathways.”
He added: “A question for the future is to see what are those mechanisms that drive risk across different diseases? And the reason that matters, of course, is that we might want to think about the effectiveness of different therapeutic interventions.”
Determining cardiovascular risk
Dr. Conrad and associates in their viewpoint article suggested that an update to the European Alliance of Associations for Rheumatology guidelines for cardiovascular risk management of rheumatic and musculoskeletal diseases (RMDs) could tailor cardiovascular risk scores to certain diseases.
They suggested that the guidelines could consider a risk multiplier of 2.5 for systemic sclerosis, 2.0 for lupus, and 1.5 for any other rheumatic disease.
“We argue that [EULAR] recommendations should consider this new evidence of poorer cardiovascular health in numerous RMDs and envisage cardiovascular screening and associated prevention measures,” Dr. Conrad said.
While they recognize that risk multipliers aren’t perfect, “they are the best available option until personalized risk prediction tools are developed specifically for patients with RMDs.”
Addressing cardiovascular risk
As a former president of EULAR, Dr. McInnes was keen to point out that “EULAR’s recommendations are evidence based and are rigorously built on [standard operating procedures] that work and have stood the test of time. I’m quite sure that the members of relevant EULAR task forces will be looking at these data, but they’ll be looking at the whole range of literature to see whether change is necessary.”
Good-quality inflammatory disease control will certainly contribute to reducing vascular risk, “but we should not make the assumption that it will be sufficient,” he cautioned. “We still have to be very careful in addressing so called conventional risk factors, but in particular thinking about obesity and cardiometabolic syndrome to be sure that when those are present, that we detect them and we treat them appropriately.”
As to who is best placed to manage a patient’s cardiovascular risk profile, Dr. McInnes said: “I think the rheumatologist has a responsibility to make sure that as much of the patient’s disease spectrum is being treated as possible.”
“As a rheumatologist, I would like to know that those elements of a patient’s disease presentation are being addressed,” whether that is by a primary care physician, cardiologist, diabetologist, or other specialist involved in the optimal management of the patient.
Dr. Conrad acknowledged receiving support from the European Union’s Horizon 2020 Program, the European Society for Cardiology, and grant funding paid to her institution from the Belgian-based Research Foundation Flounders. She also acknowledged receipt of royalties in regard to the intellectual property of a home-monitoring system for heart failure paid to Oxford University Innovation. Dr. McInnes acknowledged financial relationships with many pharmaceutical companies.
*This article was updated 12/30/2022.
A re-evaluation of cardiovascular risk management guidelines intended for use by rheumatologists may be warranted based on findings from a recently published population-based study of the risks for 12 different cardiovascular disease outcomes in patients with autoimmune diseases.
“The notion that patients with rheumatic diseases are at increased risk of developing cardiovascular diseases has been ongoing for many years,” Nathalie Conrad, PhD, and coauthors wrote in a viewpoint article in Annals of the Rheumatic Diseases.
This has “sparked much debate concerning whether and when to initiate cardiovascular prevention therapies,” they said.
Dr. Conrad was first author on the population-based study published in The Lancet in August 2022 that used linked primary and secondary care records from datasets in the U.K. Clinical Practice Research Datalink involving individuals who were recently diagnosed with any of 19 different autoimmune diseases during an 18-year period stretching from 2000 to 2017 but free of cardiovascular disease until at least 12 months after incident autoimmune disease. “Every single autoimmune disorder we looked at was associated with increased cardiovascular risk,” Dr. Conrad, of the department of public health and primary care at Catholic University Leuven (Belgium), said in an interview.
Not only was the risk for cardiovascular disease increased for people with rheumatic diseases by an average of 68%, compared with people without rheumatic diseases, but also the whole spectrum of cardiovascular disorders was seen.
“We saw increases in thromboembolic diseases, degenerative heart diseases, and heart inflammation,” Dr. Conrad said.
Large datasets examined
The idea for the epidemiologic study came from mounting evidence for cardiovascular disease risk among people with autoimmune diseases but not enough to support the design of specific prevention measures.
Dr. Conrad’s Lancet study examined electronic health records of 446,449 individuals with autoimmune diseases and matched them to 2,102,830 individuals without autoimmune disease. This included 160,217 individuals with seven rheumatic diseases: rheumatoid arthritis, polymyalgia rheumatica, vasculitis, systemic lupus erythematosus, Sjögren’s syndrome, ankylosing spondylitis, and systemic sclerosis.
In addition to looking for any evidence of cardiovascular disease, Dr. Conrad and coauthors looked at 12 specific outcomes: atherosclerotic diseases, peripheral arterial disease, stroke or transient ischemic attack, heart failure, valve disorders, thromboembolic disease, atrial fibrillation or flutter, conduction system disease, supraventricular arrhythmias, aortic aneurysm, myocarditis and pericarditis, and infective endocarditis.
CV risk in rheumatic diseases
As might be expected, “greater magnitudes of risk” were seen for individuals with systemic lupus erythematosus and systemic sclerosis than for people in the general population, with the chances of cardiovascular disease being two to four times higher. But what perhaps wasn’t expected was that all rheumatic diseases carried an increased risk for heart or vascular-related problems.
Furthermore, the increased risk could not solely be accounted for by the presence of traditional risk factors, such as blood pressure, smoking, or obesity.
“The background here is that any context of systemic inflammation would be predicted to lead to an increased vascular risk,” Iain McInnes, MD, PhD, professor of medicine and rheumatology at the University of Glasgow, said in an interview. Dr. McInnes was a coauthor of the viewpoint article in Annals of the Rheumatic Diseases.
“The implication is that there may well be increased vascular risk across the whole range of immune-mediated inflammatory diseases,” he added. “We should not, however, infer the magnitude of risk will be the same for each disease.”
What is more intriguing, Dr. McInnes said, is that “we don’t know yet whether there’s one final common pathway that leads to the blood vessel being damaged or whether different diseases might contribute different pathways.”
He added: “A question for the future is to see what are those mechanisms that drive risk across different diseases? And the reason that matters, of course, is that we might want to think about the effectiveness of different therapeutic interventions.”
Determining cardiovascular risk
Dr. Conrad and associates in their viewpoint article suggested that an update to the European Alliance of Associations for Rheumatology guidelines for cardiovascular risk management of rheumatic and musculoskeletal diseases (RMDs) could tailor cardiovascular risk scores to certain diseases.
They suggested that the guidelines could consider a risk multiplier of 2.5 for systemic sclerosis, 2.0 for lupus, and 1.5 for any other rheumatic disease.
“We argue that [EULAR] recommendations should consider this new evidence of poorer cardiovascular health in numerous RMDs and envisage cardiovascular screening and associated prevention measures,” Dr. Conrad said.
While they recognize that risk multipliers aren’t perfect, “they are the best available option until personalized risk prediction tools are developed specifically for patients with RMDs.”
Addressing cardiovascular risk
As a former president of EULAR, Dr. McInnes was keen to point out that “EULAR’s recommendations are evidence based and are rigorously built on [standard operating procedures] that work and have stood the test of time. I’m quite sure that the members of relevant EULAR task forces will be looking at these data, but they’ll be looking at the whole range of literature to see whether change is necessary.”
Good-quality inflammatory disease control will certainly contribute to reducing vascular risk, “but we should not make the assumption that it will be sufficient,” he cautioned. “We still have to be very careful in addressing so called conventional risk factors, but in particular thinking about obesity and cardiometabolic syndrome to be sure that when those are present, that we detect them and we treat them appropriately.”
As to who is best placed to manage a patient’s cardiovascular risk profile, Dr. McInnes said: “I think the rheumatologist has a responsibility to make sure that as much of the patient’s disease spectrum is being treated as possible.”
“As a rheumatologist, I would like to know that those elements of a patient’s disease presentation are being addressed,” whether that is by a primary care physician, cardiologist, diabetologist, or other specialist involved in the optimal management of the patient.
Dr. Conrad acknowledged receiving support from the European Union’s Horizon 2020 Program, the European Society for Cardiology, and grant funding paid to her institution from the Belgian-based Research Foundation Flounders. She also acknowledged receipt of royalties in regard to the intellectual property of a home-monitoring system for heart failure paid to Oxford University Innovation. Dr. McInnes acknowledged financial relationships with many pharmaceutical companies.
*This article was updated 12/30/2022.
FROM ANNALS OF THE RHEUMATIC DISEASES
FDA will review pediatric indication for roflumilast cream
, according to a press release from the manufacturer.
The company, Arcutis Biotherapeutics, announced the submission of a supplemental new drug application for approval of roflumilast cream (Zoryve), a topical phosphodiesterase-4 (PDE-4) inhibitor, to treat psoriasis in children aged 2-11 years. If approved, this would be the first such product for young children with plaque psoriasis, according to the press release. In July 2022, the FDA approved roflumilast cream 0.3% for the treatment of plaque psoriasis in people 12 years of age and older, including in intertriginous areas, based on data from the phase 3 DERMIS-1 and DERMIS-2 trials.
The new submission is supported by data from two 4-week Maximal Usage Systemic Exposure (MUSE) studies in children ages 2-11 years with plaque psoriasis. In these phase 2, open-label studies, one study of children aged 2-5 years and another study of children aged 6-11 years, participants were treated with roflumilast cream 0.3% once daily for 4 weeks. The MUSE studies are also intended to fulfill postmarketing requirements for roflumilast, according to the company. The MUSE results were consistent with those from DERMIS-1 and DERMIS-2, according to the company press release. In DERMIS-1 and DERMIS-2, significantly more patients randomized to roflumilast met criteria for Investigators Global Success (IGA) scores after 8 weeks of daily treatment compared with placebo patients, and significantly more achieved a 75% reduction in Psoriasis Area and Severity Index (PASI) scores compared with those on placebo.
Common adverse events associated with roflumilast include diarrhea, headache, insomnia, nausea, application site pain, upper respiratory tract infection, and urinary tract infection. None of these have been reported in more than 3% of patients, the press release noted.
, according to a press release from the manufacturer.
The company, Arcutis Biotherapeutics, announced the submission of a supplemental new drug application for approval of roflumilast cream (Zoryve), a topical phosphodiesterase-4 (PDE-4) inhibitor, to treat psoriasis in children aged 2-11 years. If approved, this would be the first such product for young children with plaque psoriasis, according to the press release. In July 2022, the FDA approved roflumilast cream 0.3% for the treatment of plaque psoriasis in people 12 years of age and older, including in intertriginous areas, based on data from the phase 3 DERMIS-1 and DERMIS-2 trials.
The new submission is supported by data from two 4-week Maximal Usage Systemic Exposure (MUSE) studies in children ages 2-11 years with plaque psoriasis. In these phase 2, open-label studies, one study of children aged 2-5 years and another study of children aged 6-11 years, participants were treated with roflumilast cream 0.3% once daily for 4 weeks. The MUSE studies are also intended to fulfill postmarketing requirements for roflumilast, according to the company. The MUSE results were consistent with those from DERMIS-1 and DERMIS-2, according to the company press release. In DERMIS-1 and DERMIS-2, significantly more patients randomized to roflumilast met criteria for Investigators Global Success (IGA) scores after 8 weeks of daily treatment compared with placebo patients, and significantly more achieved a 75% reduction in Psoriasis Area and Severity Index (PASI) scores compared with those on placebo.
Common adverse events associated with roflumilast include diarrhea, headache, insomnia, nausea, application site pain, upper respiratory tract infection, and urinary tract infection. None of these have been reported in more than 3% of patients, the press release noted.
, according to a press release from the manufacturer.
The company, Arcutis Biotherapeutics, announced the submission of a supplemental new drug application for approval of roflumilast cream (Zoryve), a topical phosphodiesterase-4 (PDE-4) inhibitor, to treat psoriasis in children aged 2-11 years. If approved, this would be the first such product for young children with plaque psoriasis, according to the press release. In July 2022, the FDA approved roflumilast cream 0.3% for the treatment of plaque psoriasis in people 12 years of age and older, including in intertriginous areas, based on data from the phase 3 DERMIS-1 and DERMIS-2 trials.
The new submission is supported by data from two 4-week Maximal Usage Systemic Exposure (MUSE) studies in children ages 2-11 years with plaque psoriasis. In these phase 2, open-label studies, one study of children aged 2-5 years and another study of children aged 6-11 years, participants were treated with roflumilast cream 0.3% once daily for 4 weeks. The MUSE studies are also intended to fulfill postmarketing requirements for roflumilast, according to the company. The MUSE results were consistent with those from DERMIS-1 and DERMIS-2, according to the company press release. In DERMIS-1 and DERMIS-2, significantly more patients randomized to roflumilast met criteria for Investigators Global Success (IGA) scores after 8 weeks of daily treatment compared with placebo patients, and significantly more achieved a 75% reduction in Psoriasis Area and Severity Index (PASI) scores compared with those on placebo.
Common adverse events associated with roflumilast include diarrhea, headache, insomnia, nausea, application site pain, upper respiratory tract infection, and urinary tract infection. None of these have been reported in more than 3% of patients, the press release noted.
FDA approves Idacio as eighth adalimumab biosimilar in U.S.
A biosimilar drug to the tumor necrosis factor inhibitor adalimumab, marketed as Idacio (adalimumab-aacf), has been approved by the Food and Drug Administration for use in the United States, according to a press release from manufacturer Fresenius Kabi.
Idacio is a citrate-free, low-concentration formulation of adalimumab and is now approved for use for all but three of the indications that currently apply to the reference adalimumab product (Humira): rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis in adults, ankylosing spondylitis, Crohn’s disease in adults and children aged 6 years or older, ulcerative colitis in adults, and plaque psoriasis in adults. It does not apply to Humira’s indications for hidradenitis suppurativa, uveitis, or ulcerative colitis in pediatric patients aged 5 years and older.
Idacio is the eighth adalimumab biosimilar to be approved in the United States. Its approval was based on evidence of a similar profile of pharmacokinetics, safety, efficacy, and immunogenicity to Humira.
Idacio was first launched in 2019 and has been marketed in more than 37 countries worldwide, according to Fresenius Kabi. The U.S. launch is scheduled for July, and Idacio will be available as a self-administered prefilled syringe or prefilled pen.
A version of this article first appeared on Medscape.com.
A biosimilar drug to the tumor necrosis factor inhibitor adalimumab, marketed as Idacio (adalimumab-aacf), has been approved by the Food and Drug Administration for use in the United States, according to a press release from manufacturer Fresenius Kabi.
Idacio is a citrate-free, low-concentration formulation of adalimumab and is now approved for use for all but three of the indications that currently apply to the reference adalimumab product (Humira): rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis in adults, ankylosing spondylitis, Crohn’s disease in adults and children aged 6 years or older, ulcerative colitis in adults, and plaque psoriasis in adults. It does not apply to Humira’s indications for hidradenitis suppurativa, uveitis, or ulcerative colitis in pediatric patients aged 5 years and older.
Idacio is the eighth adalimumab biosimilar to be approved in the United States. Its approval was based on evidence of a similar profile of pharmacokinetics, safety, efficacy, and immunogenicity to Humira.
Idacio was first launched in 2019 and has been marketed in more than 37 countries worldwide, according to Fresenius Kabi. The U.S. launch is scheduled for July, and Idacio will be available as a self-administered prefilled syringe or prefilled pen.
A version of this article first appeared on Medscape.com.
A biosimilar drug to the tumor necrosis factor inhibitor adalimumab, marketed as Idacio (adalimumab-aacf), has been approved by the Food and Drug Administration for use in the United States, according to a press release from manufacturer Fresenius Kabi.
Idacio is a citrate-free, low-concentration formulation of adalimumab and is now approved for use for all but three of the indications that currently apply to the reference adalimumab product (Humira): rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis in adults, ankylosing spondylitis, Crohn’s disease in adults and children aged 6 years or older, ulcerative colitis in adults, and plaque psoriasis in adults. It does not apply to Humira’s indications for hidradenitis suppurativa, uveitis, or ulcerative colitis in pediatric patients aged 5 years and older.
Idacio is the eighth adalimumab biosimilar to be approved in the United States. Its approval was based on evidence of a similar profile of pharmacokinetics, safety, efficacy, and immunogenicity to Humira.
Idacio was first launched in 2019 and has been marketed in more than 37 countries worldwide, according to Fresenius Kabi. The U.S. launch is scheduled for July, and Idacio will be available as a self-administered prefilled syringe or prefilled pen.
A version of this article first appeared on Medscape.com.
Ustekinumab matches TNF inhibitors for psoriatic arthritis in 3-year, real-world study
The interleukin-12/23 inhibitor ustekinumab (Stelara) is nearly as effective as a tumor necrosis factor (TNF) inhibitor for psoriatic arthritis, and patients are slightly more likely to persist with it and have a lower rate of adverse events, a 3-year, real-world study has found.
In a paper published online in Annals of the Rheumatic Diseases, researchers presented the outcomes of the prospective, observational PsABio study of 895 adults with psoriatic arthritis, who were starting treatment for the first time with either ustekinumab or a TNF inhibitor as first-, second-, or third-line treatment.
At 3 years after starting therapy, 49.9% of the 439 patients prescribed ustekinumab were still on that treatment, compared with 47.8% of the 456 patients prescribed a TNF inhibitor. However, there were differences in persistence based on clinical presentation. Patients who had severe skin involvement who were treated with ustekinumab stayed on the drug for longer than did those with severe skin involvement treated with a TNF inhibitor, and they were more likely to persist with their treatment for the 3 years of the study. However, there were numerically more patients with mild or moderate skin involvement taking a TNF inhibitor who stayed persistent with the treatment, compared with those taking ustekinumab, although the differences were not statistically significant.
“In the ustekinumab group, skin response was an important reason for prolonged persistence, with more patients in the ustekinumab group stopping/switching due to lack of effectiveness,” wrote Laure Gossec, MD, of Pitié-Salpêtrière Hospital and Sorbonne University, Paris, and coauthors. “This is expected, as psoriasis can significantly affect morbidity, and successfully treating skin symptoms improves patients’ health-related quality of life.”
The authors also noted that patients on ustekinumab monotherapy had the highest rate of persistence and stayed on treatment longer than did those on TNF inhibitor monotherapy, or on dual therapy with either drug combined with methotrexate. They suggested this could be because patients on TNF inhibitor monotherapy may be more likely to develop antidrug antibodies than those on ustekinumab monotherapy. It could also be because adding methotrexate may increase the risk of adverse events, but without necessarily increasing the effectiveness of ustekinumab on skin involvement.
In terms of efficacy, researchers saw that 69.8% of patients in the TNF inhibitor group had achieved low disease activity and 45% had achieved remission, compared with 58.6% of patients in the ustekinumab group who achieved low disease activity and 31.4% who achieved remission.
A similar pattern was seen for minimal disease activity and very low disease activity, which were achieved by 54.2% and 26.9% respectively of those in the TNF inhibitor group, and 41.4% and 19.2% respectively of those in the ustekinumab group.
Because the study was observational and real-world, the choice of therapy was made by the treating rheumatologist rather than patients being randomized. There were some baseline differences between the ustekinumab and TNF inhibitor groups; for example, patients in ustekinumab group were generally older and with more comorbidities, and were more likely to have previous been treated with biologics. However, they were also less likely to be concurrently treated with methotrexate and NSAIDs, and more likely to have severe skin involvement.
The study saw a higher rate of adverse events in the TNF inhibitor group, compared with the ustekinumab, with 39.7% of patients treated with TNF inhibitor and 34.6% of those treated with ustekinumab reporting at least one adverse event. The rates of serious adverse events and malignancies were similar for the two groups, but overall the ustekinumab group had a lower rate of clinically-relevant adverse events including infections.
The study was sponsored by Janssen, which markets ustekinumab. Ten authors declared personal fees, grants, and nonfinancial support from the pharmaceutical sector, including Janssen. One author was an employee of Janssen, one an employee of Johnson & Johnson, and two are editorial board members of Annals of the Rheumatic Diseases.
The interleukin-12/23 inhibitor ustekinumab (Stelara) is nearly as effective as a tumor necrosis factor (TNF) inhibitor for psoriatic arthritis, and patients are slightly more likely to persist with it and have a lower rate of adverse events, a 3-year, real-world study has found.
In a paper published online in Annals of the Rheumatic Diseases, researchers presented the outcomes of the prospective, observational PsABio study of 895 adults with psoriatic arthritis, who were starting treatment for the first time with either ustekinumab or a TNF inhibitor as first-, second-, or third-line treatment.
At 3 years after starting therapy, 49.9% of the 439 patients prescribed ustekinumab were still on that treatment, compared with 47.8% of the 456 patients prescribed a TNF inhibitor. However, there were differences in persistence based on clinical presentation. Patients who had severe skin involvement who were treated with ustekinumab stayed on the drug for longer than did those with severe skin involvement treated with a TNF inhibitor, and they were more likely to persist with their treatment for the 3 years of the study. However, there were numerically more patients with mild or moderate skin involvement taking a TNF inhibitor who stayed persistent with the treatment, compared with those taking ustekinumab, although the differences were not statistically significant.
“In the ustekinumab group, skin response was an important reason for prolonged persistence, with more patients in the ustekinumab group stopping/switching due to lack of effectiveness,” wrote Laure Gossec, MD, of Pitié-Salpêtrière Hospital and Sorbonne University, Paris, and coauthors. “This is expected, as psoriasis can significantly affect morbidity, and successfully treating skin symptoms improves patients’ health-related quality of life.”
The authors also noted that patients on ustekinumab monotherapy had the highest rate of persistence and stayed on treatment longer than did those on TNF inhibitor monotherapy, or on dual therapy with either drug combined with methotrexate. They suggested this could be because patients on TNF inhibitor monotherapy may be more likely to develop antidrug antibodies than those on ustekinumab monotherapy. It could also be because adding methotrexate may increase the risk of adverse events, but without necessarily increasing the effectiveness of ustekinumab on skin involvement.
In terms of efficacy, researchers saw that 69.8% of patients in the TNF inhibitor group had achieved low disease activity and 45% had achieved remission, compared with 58.6% of patients in the ustekinumab group who achieved low disease activity and 31.4% who achieved remission.
A similar pattern was seen for minimal disease activity and very low disease activity, which were achieved by 54.2% and 26.9% respectively of those in the TNF inhibitor group, and 41.4% and 19.2% respectively of those in the ustekinumab group.
Because the study was observational and real-world, the choice of therapy was made by the treating rheumatologist rather than patients being randomized. There were some baseline differences between the ustekinumab and TNF inhibitor groups; for example, patients in ustekinumab group were generally older and with more comorbidities, and were more likely to have previous been treated with biologics. However, they were also less likely to be concurrently treated with methotrexate and NSAIDs, and more likely to have severe skin involvement.
The study saw a higher rate of adverse events in the TNF inhibitor group, compared with the ustekinumab, with 39.7% of patients treated with TNF inhibitor and 34.6% of those treated with ustekinumab reporting at least one adverse event. The rates of serious adverse events and malignancies were similar for the two groups, but overall the ustekinumab group had a lower rate of clinically-relevant adverse events including infections.
The study was sponsored by Janssen, which markets ustekinumab. Ten authors declared personal fees, grants, and nonfinancial support from the pharmaceutical sector, including Janssen. One author was an employee of Janssen, one an employee of Johnson & Johnson, and two are editorial board members of Annals of the Rheumatic Diseases.
The interleukin-12/23 inhibitor ustekinumab (Stelara) is nearly as effective as a tumor necrosis factor (TNF) inhibitor for psoriatic arthritis, and patients are slightly more likely to persist with it and have a lower rate of adverse events, a 3-year, real-world study has found.
In a paper published online in Annals of the Rheumatic Diseases, researchers presented the outcomes of the prospective, observational PsABio study of 895 adults with psoriatic arthritis, who were starting treatment for the first time with either ustekinumab or a TNF inhibitor as first-, second-, or third-line treatment.
At 3 years after starting therapy, 49.9% of the 439 patients prescribed ustekinumab were still on that treatment, compared with 47.8% of the 456 patients prescribed a TNF inhibitor. However, there were differences in persistence based on clinical presentation. Patients who had severe skin involvement who were treated with ustekinumab stayed on the drug for longer than did those with severe skin involvement treated with a TNF inhibitor, and they were more likely to persist with their treatment for the 3 years of the study. However, there were numerically more patients with mild or moderate skin involvement taking a TNF inhibitor who stayed persistent with the treatment, compared with those taking ustekinumab, although the differences were not statistically significant.
“In the ustekinumab group, skin response was an important reason for prolonged persistence, with more patients in the ustekinumab group stopping/switching due to lack of effectiveness,” wrote Laure Gossec, MD, of Pitié-Salpêtrière Hospital and Sorbonne University, Paris, and coauthors. “This is expected, as psoriasis can significantly affect morbidity, and successfully treating skin symptoms improves patients’ health-related quality of life.”
The authors also noted that patients on ustekinumab monotherapy had the highest rate of persistence and stayed on treatment longer than did those on TNF inhibitor monotherapy, or on dual therapy with either drug combined with methotrexate. They suggested this could be because patients on TNF inhibitor monotherapy may be more likely to develop antidrug antibodies than those on ustekinumab monotherapy. It could also be because adding methotrexate may increase the risk of adverse events, but without necessarily increasing the effectiveness of ustekinumab on skin involvement.
In terms of efficacy, researchers saw that 69.8% of patients in the TNF inhibitor group had achieved low disease activity and 45% had achieved remission, compared with 58.6% of patients in the ustekinumab group who achieved low disease activity and 31.4% who achieved remission.
A similar pattern was seen for minimal disease activity and very low disease activity, which were achieved by 54.2% and 26.9% respectively of those in the TNF inhibitor group, and 41.4% and 19.2% respectively of those in the ustekinumab group.
Because the study was observational and real-world, the choice of therapy was made by the treating rheumatologist rather than patients being randomized. There were some baseline differences between the ustekinumab and TNF inhibitor groups; for example, patients in ustekinumab group were generally older and with more comorbidities, and were more likely to have previous been treated with biologics. However, they were also less likely to be concurrently treated with methotrexate and NSAIDs, and more likely to have severe skin involvement.
The study saw a higher rate of adverse events in the TNF inhibitor group, compared with the ustekinumab, with 39.7% of patients treated with TNF inhibitor and 34.6% of those treated with ustekinumab reporting at least one adverse event. The rates of serious adverse events and malignancies were similar for the two groups, but overall the ustekinumab group had a lower rate of clinically-relevant adverse events including infections.
The study was sponsored by Janssen, which markets ustekinumab. Ten authors declared personal fees, grants, and nonfinancial support from the pharmaceutical sector, including Janssen. One author was an employee of Janssen, one an employee of Johnson & Johnson, and two are editorial board members of Annals of the Rheumatic Diseases.
FROM ANNALS OF THE RHEUMATIC DISEASES
Meta-analysis fails to identify specific diagnostic biomarker for PsA
Key clinical point: Although a few biomarkers can assist in distinguishing psoriatic arthritis (PsA) from psoriasis or osteoarthritis, a precise diagnostic biomarker that can distinguish PsA from osteoarthritis and most other chronic inflammatory diseases has not yet been identified.
Major finding: Serum cartilage oligometrix metalloproteinase levels were significantly increased in patients with PsA compared with control individuals without chronic inflammatory diseases (standardized mean difference [SMD] 2.305; P = .003) and patients with osteoarthritis (SMD 0.783; P = .046). Serum matrix metalloproteinase-3 levels were significantly higher in patients with PsA vs psoriasis (SMD 0.419; P = .006) but could not distinguish patients with PsA from control individuals.
Study details: Findings are from a meta-analysis of 124 studies including patients with PsA.
Disclosures: This study did not report the source of funding. The authors declared no conflicts of interest.
Source: Wirth T et al. Biomarkers in psoriatic arthritis: A meta-analysis and systematic review. Front Immunol. 2022;13:1054539 (Nov 30). Doi: 10.3389/fimmu.2022.1054539
Key clinical point: Although a few biomarkers can assist in distinguishing psoriatic arthritis (PsA) from psoriasis or osteoarthritis, a precise diagnostic biomarker that can distinguish PsA from osteoarthritis and most other chronic inflammatory diseases has not yet been identified.
Major finding: Serum cartilage oligometrix metalloproteinase levels were significantly increased in patients with PsA compared with control individuals without chronic inflammatory diseases (standardized mean difference [SMD] 2.305; P = .003) and patients with osteoarthritis (SMD 0.783; P = .046). Serum matrix metalloproteinase-3 levels were significantly higher in patients with PsA vs psoriasis (SMD 0.419; P = .006) but could not distinguish patients with PsA from control individuals.
Study details: Findings are from a meta-analysis of 124 studies including patients with PsA.
Disclosures: This study did not report the source of funding. The authors declared no conflicts of interest.
Source: Wirth T et al. Biomarkers in psoriatic arthritis: A meta-analysis and systematic review. Front Immunol. 2022;13:1054539 (Nov 30). Doi: 10.3389/fimmu.2022.1054539
Key clinical point: Although a few biomarkers can assist in distinguishing psoriatic arthritis (PsA) from psoriasis or osteoarthritis, a precise diagnostic biomarker that can distinguish PsA from osteoarthritis and most other chronic inflammatory diseases has not yet been identified.
Major finding: Serum cartilage oligometrix metalloproteinase levels were significantly increased in patients with PsA compared with control individuals without chronic inflammatory diseases (standardized mean difference [SMD] 2.305; P = .003) and patients with osteoarthritis (SMD 0.783; P = .046). Serum matrix metalloproteinase-3 levels were significantly higher in patients with PsA vs psoriasis (SMD 0.419; P = .006) but could not distinguish patients with PsA from control individuals.
Study details: Findings are from a meta-analysis of 124 studies including patients with PsA.
Disclosures: This study did not report the source of funding. The authors declared no conflicts of interest.
Source: Wirth T et al. Biomarkers in psoriatic arthritis: A meta-analysis and systematic review. Front Immunol. 2022;13:1054539 (Nov 30). Doi: 10.3389/fimmu.2022.1054539
Axial PsA: A distinct phenotype not to be confused with ankylosing spondylitis+psoriasis
Key clinical point: Axial psoriatic arthritis (PsA) can be categorized as a distinct subtype of PsA because it exhibits clinical and radiological symptoms that are different from those of ankylosing spondylitis (AS) with psoriasis.
Major finding: Compared with patients with AS and psoriasis, patients with human leukocyte antigen (HLA)-B27-negative axial PsA had lesser inflammatory pain (P = .002), anterior uveitis (P = .014), and structural damage (P < .001) along with a higher prevalence of nail disease (P = .009) and were more likely to present with psoriasis before spondyloarthritis onset (P = .020). However, patients with HLA-B27-positive axial PsA vs AS and psoriasis reported lesser structural damage as revealed by Bath Ankylosing Spondylitis Radiology Index scores (P < .001).
Study details: This cross-sectional study included 109 patients with axial PsA and 127 patients with AS and current presentation or a history of skin psoriasis from the REGISPONSER registry.
Disclosures: The REGISPONSER registry is funded by the Spanish Society for Rheumatology. The authors declared no conflicts of interest.
Source: Michelena X et al. Characterising the axial phenotype of psoriatic arthritis: a study comparing axial psoriatic arthritis and ankylosing spondylitis with psoriasis from the REGISPONSER registry. RMD Open. 2022;8:e002513 (Dec 5). Doi: 10.1136/rmdopen-2022-002513
Key clinical point: Axial psoriatic arthritis (PsA) can be categorized as a distinct subtype of PsA because it exhibits clinical and radiological symptoms that are different from those of ankylosing spondylitis (AS) with psoriasis.
Major finding: Compared with patients with AS and psoriasis, patients with human leukocyte antigen (HLA)-B27-negative axial PsA had lesser inflammatory pain (P = .002), anterior uveitis (P = .014), and structural damage (P < .001) along with a higher prevalence of nail disease (P = .009) and were more likely to present with psoriasis before spondyloarthritis onset (P = .020). However, patients with HLA-B27-positive axial PsA vs AS and psoriasis reported lesser structural damage as revealed by Bath Ankylosing Spondylitis Radiology Index scores (P < .001).
Study details: This cross-sectional study included 109 patients with axial PsA and 127 patients with AS and current presentation or a history of skin psoriasis from the REGISPONSER registry.
Disclosures: The REGISPONSER registry is funded by the Spanish Society for Rheumatology. The authors declared no conflicts of interest.
Source: Michelena X et al. Characterising the axial phenotype of psoriatic arthritis: a study comparing axial psoriatic arthritis and ankylosing spondylitis with psoriasis from the REGISPONSER registry. RMD Open. 2022;8:e002513 (Dec 5). Doi: 10.1136/rmdopen-2022-002513
Key clinical point: Axial psoriatic arthritis (PsA) can be categorized as a distinct subtype of PsA because it exhibits clinical and radiological symptoms that are different from those of ankylosing spondylitis (AS) with psoriasis.
Major finding: Compared with patients with AS and psoriasis, patients with human leukocyte antigen (HLA)-B27-negative axial PsA had lesser inflammatory pain (P = .002), anterior uveitis (P = .014), and structural damage (P < .001) along with a higher prevalence of nail disease (P = .009) and were more likely to present with psoriasis before spondyloarthritis onset (P = .020). However, patients with HLA-B27-positive axial PsA vs AS and psoriasis reported lesser structural damage as revealed by Bath Ankylosing Spondylitis Radiology Index scores (P < .001).
Study details: This cross-sectional study included 109 patients with axial PsA and 127 patients with AS and current presentation or a history of skin psoriasis from the REGISPONSER registry.
Disclosures: The REGISPONSER registry is funded by the Spanish Society for Rheumatology. The authors declared no conflicts of interest.
Source: Michelena X et al. Characterising the axial phenotype of psoriatic arthritis: a study comparing axial psoriatic arthritis and ankylosing spondylitis with psoriasis from the REGISPONSER registry. RMD Open. 2022;8:e002513 (Dec 5). Doi: 10.1136/rmdopen-2022-002513
Effect of alcohol consumption and smoking on PsA manifestations
Key clinical point: Smoking and alcohol consumption were associated with a lower prevalence of arthritis and peripheral manifestations in patients with psoriatic arthritis (PsA).
Major finding: Smoking was associated with a lower prevalence of arthritis ever (odds ratio [OR] 0.63; 95% CI 0.41-0.95), and current alcohol consumption was associated with a lower prevalence of current arthritis or enthesitis (OR 0.61; 95% CI 0.47-0.79), current arthritis alone (OR 0.69; 95% CI 0.53-0.90), and current enthesitis alone (OR 0.49; 95% CI, 0.34-0.71).
Study details: Findings are from a multinational, cross-sectional study including patients with axial spondyloarthritis (n = 2717), peripheral spondyloarthritis (n = 432), and PsA (n = 1032).
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Ladehesa-Pineda ML et al. Smoking and alcohol consumption are associated with peripheral musculoskeletal involvement in patients with spondyloarthritis (including psoriatic arthritis). Results from the ASAS-PerSpA study. Semin Arthritis Rheum. 2022;58:152146 (Nov 30). Doi: 10.1016/j.semarthrit.2022.152146
Key clinical point: Smoking and alcohol consumption were associated with a lower prevalence of arthritis and peripheral manifestations in patients with psoriatic arthritis (PsA).
Major finding: Smoking was associated with a lower prevalence of arthritis ever (odds ratio [OR] 0.63; 95% CI 0.41-0.95), and current alcohol consumption was associated with a lower prevalence of current arthritis or enthesitis (OR 0.61; 95% CI 0.47-0.79), current arthritis alone (OR 0.69; 95% CI 0.53-0.90), and current enthesitis alone (OR 0.49; 95% CI, 0.34-0.71).
Study details: Findings are from a multinational, cross-sectional study including patients with axial spondyloarthritis (n = 2717), peripheral spondyloarthritis (n = 432), and PsA (n = 1032).
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Ladehesa-Pineda ML et al. Smoking and alcohol consumption are associated with peripheral musculoskeletal involvement in patients with spondyloarthritis (including psoriatic arthritis). Results from the ASAS-PerSpA study. Semin Arthritis Rheum. 2022;58:152146 (Nov 30). Doi: 10.1016/j.semarthrit.2022.152146
Key clinical point: Smoking and alcohol consumption were associated with a lower prevalence of arthritis and peripheral manifestations in patients with psoriatic arthritis (PsA).
Major finding: Smoking was associated with a lower prevalence of arthritis ever (odds ratio [OR] 0.63; 95% CI 0.41-0.95), and current alcohol consumption was associated with a lower prevalence of current arthritis or enthesitis (OR 0.61; 95% CI 0.47-0.79), current arthritis alone (OR 0.69; 95% CI 0.53-0.90), and current enthesitis alone (OR 0.49; 95% CI, 0.34-0.71).
Study details: Findings are from a multinational, cross-sectional study including patients with axial spondyloarthritis (n = 2717), peripheral spondyloarthritis (n = 432), and PsA (n = 1032).
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Ladehesa-Pineda ML et al. Smoking and alcohol consumption are associated with peripheral musculoskeletal involvement in patients with spondyloarthritis (including psoriatic arthritis). Results from the ASAS-PerSpA study. Semin Arthritis Rheum. 2022;58:152146 (Nov 30). Doi: 10.1016/j.semarthrit.2022.152146