User login
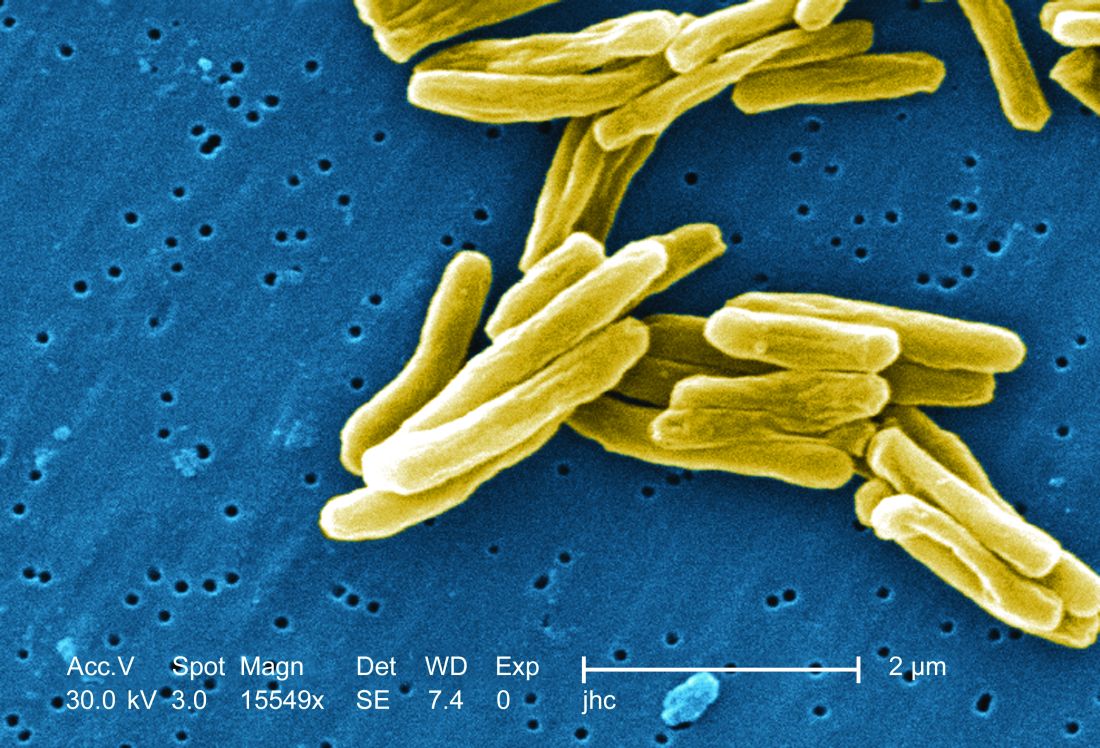
Non-TB mycobacteria infections rising in COPD patients
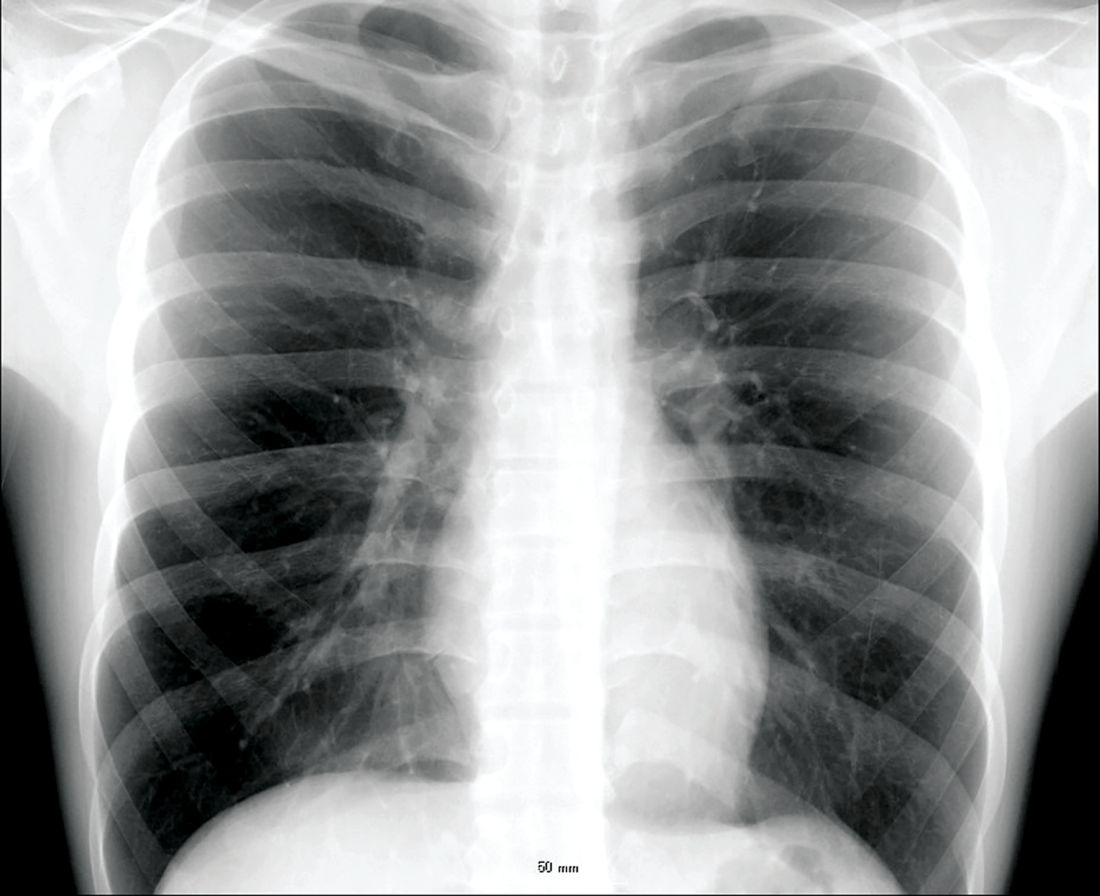
Veterans with chronic obstructive pulmonary disease (COPD) have seen a sharp increase since 2012 in rates of non-TB mycobacteria infections, which carry a significantly higher risk of death in COPD patients, according to findings from a nationwide study.
For their research, published in Frontiers of Medicine, Fahim Pyarali, MD, and colleagues at the University of Miami, reviewed data from Veterans Affairs hospitals to identify non-TB mycobacteria (NTM) infections among more than 2 million COPD patients seen between 2000 and 2015. Incidence of NTM infections was 34.2 per 100,000 COPD patients in 2001, a rate that remained steady until 2012, when it began climbing sharply through 2015 to reach 70.3 per 100,000 (P = .035). Dr. Pyarali and colleagues also found that, during the study period, prevalence of NTM climbed from 93.1 infections per 100,000 population in 2001 to 277.6 per 100,000 in 2015.
Hotspots for NTM infections included Puerto Rico, which had the highest prevalence seen in the study at 370 infections per 100,000 COPD population; Florida, with 351 per 100,000; and Washington, D.C., with 309 per 100,000. Additional hotspots were identified around Lake Michigan, in coastal Louisiana, and in parts of the Southwest.
Dr. Pyarali and colleagues noted that the geographical concentration of cases near oceans and lakes was “supported by previous findings that warmer temperatures, lower dissolved oxygen, and lower pH in the soils and waters provide a major environmental source for NTM organisms;” however, the study is the first to identify Puerto Rico as having exceptionally high prevalence. The reasons for this should be extensively investigated, the investigators argued.
The mortality risk was 43% higher among NTM-infected patients than in COPD patients without an NTM diagnosis (95% confidence interval, 1.31-1.58; P less than .001), independent of other comorbidities.
Though rates of NTM infection were seen rising steeply in men and women alike, Dr. Pyarali and colleagues noted as a limitation of their study its use of an overwhelmingly male population, writing that this may obscure “the true reach of NTM disease and mortality” in the general population. The average age of NTM diagnosis remained steady throughout the study period, suggesting that rising incidence is not attributable to earlier diagnosis.
Dr. Pyarali and colleagues reported no outside sources of funding or financial conflicts of interest.
SOURCE: Pyarali F et al. Front Med. 2018 Nov 6. doi: 10.3389/fmed2018.00311.
Veterans with chronic obstructive pulmonary disease (COPD) have seen a sharp increase since 2012 in rates of non-TB mycobacteria infections, which carry a significantly higher risk of death in COPD patients, according to findings from a nationwide study.
For their research, published in Frontiers of Medicine, Fahim Pyarali, MD, and colleagues at the University of Miami, reviewed data from Veterans Affairs hospitals to identify non-TB mycobacteria (NTM) infections among more than 2 million COPD patients seen between 2000 and 2015. Incidence of NTM infections was 34.2 per 100,000 COPD patients in 2001, a rate that remained steady until 2012, when it began climbing sharply through 2015 to reach 70.3 per 100,000 (P = .035). Dr. Pyarali and colleagues also found that, during the study period, prevalence of NTM climbed from 93.1 infections per 100,000 population in 2001 to 277.6 per 100,000 in 2015.
Hotspots for NTM infections included Puerto Rico, which had the highest prevalence seen in the study at 370 infections per 100,000 COPD population; Florida, with 351 per 100,000; and Washington, D.C., with 309 per 100,000. Additional hotspots were identified around Lake Michigan, in coastal Louisiana, and in parts of the Southwest.
Dr. Pyarali and colleagues noted that the geographical concentration of cases near oceans and lakes was “supported by previous findings that warmer temperatures, lower dissolved oxygen, and lower pH in the soils and waters provide a major environmental source for NTM organisms;” however, the study is the first to identify Puerto Rico as having exceptionally high prevalence. The reasons for this should be extensively investigated, the investigators argued.
The mortality risk was 43% higher among NTM-infected patients than in COPD patients without an NTM diagnosis (95% confidence interval, 1.31-1.58; P less than .001), independent of other comorbidities.
Though rates of NTM infection were seen rising steeply in men and women alike, Dr. Pyarali and colleagues noted as a limitation of their study its use of an overwhelmingly male population, writing that this may obscure “the true reach of NTM disease and mortality” in the general population. The average age of NTM diagnosis remained steady throughout the study period, suggesting that rising incidence is not attributable to earlier diagnosis.
Dr. Pyarali and colleagues reported no outside sources of funding or financial conflicts of interest.
SOURCE: Pyarali F et al. Front Med. 2018 Nov 6. doi: 10.3389/fmed2018.00311.
Veterans with chronic obstructive pulmonary disease (COPD) have seen a sharp increase since 2012 in rates of non-TB mycobacteria infections, which carry a significantly higher risk of death in COPD patients, according to findings from a nationwide study.
For their research, published in Frontiers of Medicine, Fahim Pyarali, MD, and colleagues at the University of Miami, reviewed data from Veterans Affairs hospitals to identify non-TB mycobacteria (NTM) infections among more than 2 million COPD patients seen between 2000 and 2015. Incidence of NTM infections was 34.2 per 100,000 COPD patients in 2001, a rate that remained steady until 2012, when it began climbing sharply through 2015 to reach 70.3 per 100,000 (P = .035). Dr. Pyarali and colleagues also found that, during the study period, prevalence of NTM climbed from 93.1 infections per 100,000 population in 2001 to 277.6 per 100,000 in 2015.
Hotspots for NTM infections included Puerto Rico, which had the highest prevalence seen in the study at 370 infections per 100,000 COPD population; Florida, with 351 per 100,000; and Washington, D.C., with 309 per 100,000. Additional hotspots were identified around Lake Michigan, in coastal Louisiana, and in parts of the Southwest.
Dr. Pyarali and colleagues noted that the geographical concentration of cases near oceans and lakes was “supported by previous findings that warmer temperatures, lower dissolved oxygen, and lower pH in the soils and waters provide a major environmental source for NTM organisms;” however, the study is the first to identify Puerto Rico as having exceptionally high prevalence. The reasons for this should be extensively investigated, the investigators argued.
The mortality risk was 43% higher among NTM-infected patients than in COPD patients without an NTM diagnosis (95% confidence interval, 1.31-1.58; P less than .001), independent of other comorbidities.
Though rates of NTM infection were seen rising steeply in men and women alike, Dr. Pyarali and colleagues noted as a limitation of their study its use of an overwhelmingly male population, writing that this may obscure “the true reach of NTM disease and mortality” in the general population. The average age of NTM diagnosis remained steady throughout the study period, suggesting that rising incidence is not attributable to earlier diagnosis.
Dr. Pyarali and colleagues reported no outside sources of funding or financial conflicts of interest.
SOURCE: Pyarali F et al. Front Med. 2018 Nov 6. doi: 10.3389/fmed2018.00311.
FROM FRONTIERS IN MEDICINE
Key clinical point: Incidence and prevalence of non-TB mycobacteria infections rose sharply in a national veterans population with chronic obstructive pulmonary disease after 2012.
Major finding: Incidence of non-TB mycobacteria infections doubled in chronic obstructive pulmonary disease patients between 2001 and 2015, with most of the increase seen after 2012
Study details: A retrospective, cross-sectional study using records from over 2 million, mostly male chronic obstructive pulmonary disease patients in a Veterans Affairs database.
Disclosures: The study authors reported no outside sources of funding or financial conflicts of interest.
Source: Pyarali F et al. Front Med. 2018 Nov 6. doi: 10.3389/fmed2018.00311.
ACIP resuscitates pertussis working group
The recent rise in pertussis rates may have peaked, but the experts are responding by reinstating a working group.
The new working group for pertussis was announced at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices. The ACIP’s new group, led by Fiona Havers, MD, of the CDC, heard data on the currently available pertussis vaccines and solicited ideas from ACIP members about what other data they would like before the February meeting.
One question on the agenda is whether the current recommendation that nonpregnant adults receive a single lifetime dose of Tdap and then tetanus-diphtheria (Td) boosters every 10 years be expanded to allow either Tdap or Td as the booster. Reasons for considering the change include possible changes in the circulating pertussis strain, improved diagnosis and reporting, and the waning of protection under the current guidelines, as well as the potential economic impact, Dr. Havers said.
This change could make booster administration easier for many physicians who do not routinely stock Td, some committee members noted. In addition, the Food and Drug Administration has approved a label change for one Tdap manufacturer to remove “single use” language.
In a study presented by David P. Greenberg, MD, associate vice president of Sanofi Pasteur, seroprotection rates to tetanus and diphtheria were similar in a comparison between groups of adults aged 18 years and older, receiving either Tdap (Adacel) or Td as a booster. “Seroprotection was greater than 99% in both groups,” he said.
Pain was the most common injection site reaction in both groups, rates of serious adverse events were similarly low (0.8% and 0.3%, respectively), and no deaths occurred in patients given either vaccine.
The postvaccination antipertussis geometric mean concentrations were noninferior in the Tdap group, compared with the Td group, Dr. Greenberg said.
A phase III open label study presented by Leonard Silverstein, MD, of GlaxoSmithKline also showed similar seroprotection rates for adults revaccinated with Tdap after an initial vaccination with either of two different Tdap vaccines.
Also at the February meeting, the committee will address whether any vaccine that contained Td should be allowed for use as tetanus prophylaxis in the setting of wound management, said Dr. Havers.
The committee members expressed interest in more information on several topics including pregnancy and pertussis, whether manufacturers could discuss vaccines in the pipeline, data on responses to multiple doses and if there is a point of diminishing returns, and whether some states are covering Tdap for adults.
The committee members had no financial conflicts to disclose.
The recent rise in pertussis rates may have peaked, but the experts are responding by reinstating a working group.
The new working group for pertussis was announced at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices. The ACIP’s new group, led by Fiona Havers, MD, of the CDC, heard data on the currently available pertussis vaccines and solicited ideas from ACIP members about what other data they would like before the February meeting.
One question on the agenda is whether the current recommendation that nonpregnant adults receive a single lifetime dose of Tdap and then tetanus-diphtheria (Td) boosters every 10 years be expanded to allow either Tdap or Td as the booster. Reasons for considering the change include possible changes in the circulating pertussis strain, improved diagnosis and reporting, and the waning of protection under the current guidelines, as well as the potential economic impact, Dr. Havers said.
This change could make booster administration easier for many physicians who do not routinely stock Td, some committee members noted. In addition, the Food and Drug Administration has approved a label change for one Tdap manufacturer to remove “single use” language.
In a study presented by David P. Greenberg, MD, associate vice president of Sanofi Pasteur, seroprotection rates to tetanus and diphtheria were similar in a comparison between groups of adults aged 18 years and older, receiving either Tdap (Adacel) or Td as a booster. “Seroprotection was greater than 99% in both groups,” he said.
Pain was the most common injection site reaction in both groups, rates of serious adverse events were similarly low (0.8% and 0.3%, respectively), and no deaths occurred in patients given either vaccine.
The postvaccination antipertussis geometric mean concentrations were noninferior in the Tdap group, compared with the Td group, Dr. Greenberg said.
A phase III open label study presented by Leonard Silverstein, MD, of GlaxoSmithKline also showed similar seroprotection rates for adults revaccinated with Tdap after an initial vaccination with either of two different Tdap vaccines.
Also at the February meeting, the committee will address whether any vaccine that contained Td should be allowed for use as tetanus prophylaxis in the setting of wound management, said Dr. Havers.
The committee members expressed interest in more information on several topics including pregnancy and pertussis, whether manufacturers could discuss vaccines in the pipeline, data on responses to multiple doses and if there is a point of diminishing returns, and whether some states are covering Tdap for adults.
The committee members had no financial conflicts to disclose.
The recent rise in pertussis rates may have peaked, but the experts are responding by reinstating a working group.
The new working group for pertussis was announced at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices. The ACIP’s new group, led by Fiona Havers, MD, of the CDC, heard data on the currently available pertussis vaccines and solicited ideas from ACIP members about what other data they would like before the February meeting.
One question on the agenda is whether the current recommendation that nonpregnant adults receive a single lifetime dose of Tdap and then tetanus-diphtheria (Td) boosters every 10 years be expanded to allow either Tdap or Td as the booster. Reasons for considering the change include possible changes in the circulating pertussis strain, improved diagnosis and reporting, and the waning of protection under the current guidelines, as well as the potential economic impact, Dr. Havers said.
This change could make booster administration easier for many physicians who do not routinely stock Td, some committee members noted. In addition, the Food and Drug Administration has approved a label change for one Tdap manufacturer to remove “single use” language.
In a study presented by David P. Greenberg, MD, associate vice president of Sanofi Pasteur, seroprotection rates to tetanus and diphtheria were similar in a comparison between groups of adults aged 18 years and older, receiving either Tdap (Adacel) or Td as a booster. “Seroprotection was greater than 99% in both groups,” he said.
Pain was the most common injection site reaction in both groups, rates of serious adverse events were similarly low (0.8% and 0.3%, respectively), and no deaths occurred in patients given either vaccine.
The postvaccination antipertussis geometric mean concentrations were noninferior in the Tdap group, compared with the Td group, Dr. Greenberg said.
A phase III open label study presented by Leonard Silverstein, MD, of GlaxoSmithKline also showed similar seroprotection rates for adults revaccinated with Tdap after an initial vaccination with either of two different Tdap vaccines.
Also at the February meeting, the committee will address whether any vaccine that contained Td should be allowed for use as tetanus prophylaxis in the setting of wound management, said Dr. Havers.
The committee members expressed interest in more information on several topics including pregnancy and pertussis, whether manufacturers could discuss vaccines in the pipeline, data on responses to multiple doses and if there is a point of diminishing returns, and whether some states are covering Tdap for adults.
The committee members had no financial conflicts to disclose.
AT AN ACIP MEETING
FDA approves Xofluza for treatment of influenza
The Food and Drug Administration has approved Xofluza (baloxavir marboxil) for the treatment of acute uncomplicated influenza in people aged 12 years or older who have been symptomatic for 48 hours or less.
The FDA approval is based on results from two randomized, clinical trials. In both trials, patients who received Xofluza experienced a shorter duration until alleviation of symptoms, compared with patients who received a placebo. In the second trial, patients who received Xofluza and patients who received another approved antiviral influenza medication experienced similar durations until symptom alleviation.
“When treatment is started within 48 hours of becoming sick with flu symptoms, antiviral drugs can lessen symptoms and shorten the time patients feel sick. Having more treatment options that work in different ways to attack the virus is important because flu viruses can become resistant to antiviral drugs,” Debra Birnkrant, MD, director of the Division of Antiviral Products in the FDA’s Center for Drug Evaluation and Research, said in a press release.
The most common adverse events associated with Xofluza were diarrhea and bronchitis.
“This is the first new antiviral flu treatment with a novel mechanism of action approved by the FDA in nearly 20 years,” FDA Commissioner Scott Gottlieb, MD, added. “With thousands of people getting the flu every year, and many people becoming seriously ill, having safe and effective treatment alternatives is critical. This novel drug provides an important, additional treatment option.”
Find the full press release on the FDA website.
The Food and Drug Administration has approved Xofluza (baloxavir marboxil) for the treatment of acute uncomplicated influenza in people aged 12 years or older who have been symptomatic for 48 hours or less.
The FDA approval is based on results from two randomized, clinical trials. In both trials, patients who received Xofluza experienced a shorter duration until alleviation of symptoms, compared with patients who received a placebo. In the second trial, patients who received Xofluza and patients who received another approved antiviral influenza medication experienced similar durations until symptom alleviation.
“When treatment is started within 48 hours of becoming sick with flu symptoms, antiviral drugs can lessen symptoms and shorten the time patients feel sick. Having more treatment options that work in different ways to attack the virus is important because flu viruses can become resistant to antiviral drugs,” Debra Birnkrant, MD, director of the Division of Antiviral Products in the FDA’s Center for Drug Evaluation and Research, said in a press release.
The most common adverse events associated with Xofluza were diarrhea and bronchitis.
“This is the first new antiviral flu treatment with a novel mechanism of action approved by the FDA in nearly 20 years,” FDA Commissioner Scott Gottlieb, MD, added. “With thousands of people getting the flu every year, and many people becoming seriously ill, having safe and effective treatment alternatives is critical. This novel drug provides an important, additional treatment option.”
Find the full press release on the FDA website.
The Food and Drug Administration has approved Xofluza (baloxavir marboxil) for the treatment of acute uncomplicated influenza in people aged 12 years or older who have been symptomatic for 48 hours or less.
The FDA approval is based on results from two randomized, clinical trials. In both trials, patients who received Xofluza experienced a shorter duration until alleviation of symptoms, compared with patients who received a placebo. In the second trial, patients who received Xofluza and patients who received another approved antiviral influenza medication experienced similar durations until symptom alleviation.
“When treatment is started within 48 hours of becoming sick with flu symptoms, antiviral drugs can lessen symptoms and shorten the time patients feel sick. Having more treatment options that work in different ways to attack the virus is important because flu viruses can become resistant to antiviral drugs,” Debra Birnkrant, MD, director of the Division of Antiviral Products in the FDA’s Center for Drug Evaluation and Research, said in a press release.
The most common adverse events associated with Xofluza were diarrhea and bronchitis.
“This is the first new antiviral flu treatment with a novel mechanism of action approved by the FDA in nearly 20 years,” FDA Commissioner Scott Gottlieb, MD, added. “With thousands of people getting the flu every year, and many people becoming seriously ill, having safe and effective treatment alternatives is critical. This novel drug provides an important, additional treatment option.”
Find the full press release on the FDA website.
TB vaccine shows promise in previously infected
san francisco – A new The vaccine showed efficacy in young adults – an important finding because models suggest that inducing immunity in adolescents and young adults would be the fastest and most cost-effective approach to dealing with the global TB epidemic.
The study recruited adults who had previously been exposed to Mycobacterium tuberculosis, a population that receives no benefit from the long-standing bacillus Calmette-Guérin (BCG) vaccine. The overall efficacy of protection was 54%. “There isn’t any vaccine that’s been demonstrated to work in people who are already infected. It’s also the first vaccine to show this level of statistically significant protection in adults, and it’s adults who are the major transmitters of tuberculosis. The modeling has shown that even a vaccine that could protect infected adults at 20% vaccine efficacy would have a substantial impact on the epidemic and be cost effective,” said Ann Ginsberg, MD, PhD, chief medical officer at Aeras, which developed the vaccine and is now testing it in partnership with GlaxoSmithKline.
The results of the study were presented at ID Week 2018 and published in the New England Journal of Medicine (2018 Sep 25. doi: 10.1056/NEJMoa1803484).
The results address a major weakness of the BCG vaccine, which is that some studies have shown it offers little benefit to subjects who are already infected with the disease, which is the case for about a quarter of the world’s population, according to Dr. Ginsberg. The probable explanation is that previous infection with M. tuberculosis or a related bacteria is common in some populations and that this exposure grants some protection against progression to active disease.
The researchers tested the M72/AS01E vaccine, which includes two M. tuberculosis antigens that were identified from patients who had controlled their infection and also the AS01 adjuvant, which contains two immunostimulating agents and is a component of a developmental malaria vaccine and the recombinant zoster vaccine Shingrix.
In Kenya, South Africa, and Zambia, the researchers randomized 3,330 participants (mean age, 28.9 years; 43% female) to receive two doses 1 month apart of either vaccine or placebo. After a mean follow-up of 2.3 years, the protocol efficacy analysis showed that the vaccine had an efficacy rate of 54.0% (P = .04) for pulmonary tuberculosis.
The vaccine had greater efficacy in men (75.2%; P = .03) than it did in women (27.4%; P = .52) and among individuals aged 25 years or younger (84.4%; P = .01) than it did among older subjects (10.2%, P = .82).
The frequency of serious adverse events was similar between the vaccine (1.6%) and the placebo group (1.8%). Unsolicited reports of adverse events were more common in the vaccine group than the placebo group (67.4% vs. 45.4%, respectively), driven largely by more reports of injection site reactions and flu-like symptoms. Solicited reports of adverse events were highlighted by a greater frequency of injection site pain in the vaccine group (81.8% vs. 34.4%). A total of 24.3% of the vaccine recipients reported grade 3 pain, compared with 3.3% in the placebo arm. Rates of fatigue, headache, malaise, or myalgia were also higher in the vaccine group, as was fever.
All of the subjects in the vaccine group had seroconversion at month 2, and 99% remained seroconverted at 12 months.
Next, the researchers plan to conduct studies in HIV-infected individuals and to proceed with phase III trials.
The trial was funded by GlaxoSmithKline Biologicals and Aeras. Dr. Ginsberg is an employee of Aeras.
SOURCE: Ginsberg A et al. IDWeek 2018, Abstract 120
san francisco – A new The vaccine showed efficacy in young adults – an important finding because models suggest that inducing immunity in adolescents and young adults would be the fastest and most cost-effective approach to dealing with the global TB epidemic.
The study recruited adults who had previously been exposed to Mycobacterium tuberculosis, a population that receives no benefit from the long-standing bacillus Calmette-Guérin (BCG) vaccine. The overall efficacy of protection was 54%. “There isn’t any vaccine that’s been demonstrated to work in people who are already infected. It’s also the first vaccine to show this level of statistically significant protection in adults, and it’s adults who are the major transmitters of tuberculosis. The modeling has shown that even a vaccine that could protect infected adults at 20% vaccine efficacy would have a substantial impact on the epidemic and be cost effective,” said Ann Ginsberg, MD, PhD, chief medical officer at Aeras, which developed the vaccine and is now testing it in partnership with GlaxoSmithKline.
The results of the study were presented at ID Week 2018 and published in the New England Journal of Medicine (2018 Sep 25. doi: 10.1056/NEJMoa1803484).
The results address a major weakness of the BCG vaccine, which is that some studies have shown it offers little benefit to subjects who are already infected with the disease, which is the case for about a quarter of the world’s population, according to Dr. Ginsberg. The probable explanation is that previous infection with M. tuberculosis or a related bacteria is common in some populations and that this exposure grants some protection against progression to active disease.
The researchers tested the M72/AS01E vaccine, which includes two M. tuberculosis antigens that were identified from patients who had controlled their infection and also the AS01 adjuvant, which contains two immunostimulating agents and is a component of a developmental malaria vaccine and the recombinant zoster vaccine Shingrix.
In Kenya, South Africa, and Zambia, the researchers randomized 3,330 participants (mean age, 28.9 years; 43% female) to receive two doses 1 month apart of either vaccine or placebo. After a mean follow-up of 2.3 years, the protocol efficacy analysis showed that the vaccine had an efficacy rate of 54.0% (P = .04) for pulmonary tuberculosis.
The vaccine had greater efficacy in men (75.2%; P = .03) than it did in women (27.4%; P = .52) and among individuals aged 25 years or younger (84.4%; P = .01) than it did among older subjects (10.2%, P = .82).
The frequency of serious adverse events was similar between the vaccine (1.6%) and the placebo group (1.8%). Unsolicited reports of adverse events were more common in the vaccine group than the placebo group (67.4% vs. 45.4%, respectively), driven largely by more reports of injection site reactions and flu-like symptoms. Solicited reports of adverse events were highlighted by a greater frequency of injection site pain in the vaccine group (81.8% vs. 34.4%). A total of 24.3% of the vaccine recipients reported grade 3 pain, compared with 3.3% in the placebo arm. Rates of fatigue, headache, malaise, or myalgia were also higher in the vaccine group, as was fever.
All of the subjects in the vaccine group had seroconversion at month 2, and 99% remained seroconverted at 12 months.
Next, the researchers plan to conduct studies in HIV-infected individuals and to proceed with phase III trials.
The trial was funded by GlaxoSmithKline Biologicals and Aeras. Dr. Ginsberg is an employee of Aeras.
SOURCE: Ginsberg A et al. IDWeek 2018, Abstract 120
san francisco – A new The vaccine showed efficacy in young adults – an important finding because models suggest that inducing immunity in adolescents and young adults would be the fastest and most cost-effective approach to dealing with the global TB epidemic.
The study recruited adults who had previously been exposed to Mycobacterium tuberculosis, a population that receives no benefit from the long-standing bacillus Calmette-Guérin (BCG) vaccine. The overall efficacy of protection was 54%. “There isn’t any vaccine that’s been demonstrated to work in people who are already infected. It’s also the first vaccine to show this level of statistically significant protection in adults, and it’s adults who are the major transmitters of tuberculosis. The modeling has shown that even a vaccine that could protect infected adults at 20% vaccine efficacy would have a substantial impact on the epidemic and be cost effective,” said Ann Ginsberg, MD, PhD, chief medical officer at Aeras, which developed the vaccine and is now testing it in partnership with GlaxoSmithKline.
The results of the study were presented at ID Week 2018 and published in the New England Journal of Medicine (2018 Sep 25. doi: 10.1056/NEJMoa1803484).
The results address a major weakness of the BCG vaccine, which is that some studies have shown it offers little benefit to subjects who are already infected with the disease, which is the case for about a quarter of the world’s population, according to Dr. Ginsberg. The probable explanation is that previous infection with M. tuberculosis or a related bacteria is common in some populations and that this exposure grants some protection against progression to active disease.
The researchers tested the M72/AS01E vaccine, which includes two M. tuberculosis antigens that were identified from patients who had controlled their infection and also the AS01 adjuvant, which contains two immunostimulating agents and is a component of a developmental malaria vaccine and the recombinant zoster vaccine Shingrix.
In Kenya, South Africa, and Zambia, the researchers randomized 3,330 participants (mean age, 28.9 years; 43% female) to receive two doses 1 month apart of either vaccine or placebo. After a mean follow-up of 2.3 years, the protocol efficacy analysis showed that the vaccine had an efficacy rate of 54.0% (P = .04) for pulmonary tuberculosis.
The vaccine had greater efficacy in men (75.2%; P = .03) than it did in women (27.4%; P = .52) and among individuals aged 25 years or younger (84.4%; P = .01) than it did among older subjects (10.2%, P = .82).
The frequency of serious adverse events was similar between the vaccine (1.6%) and the placebo group (1.8%). Unsolicited reports of adverse events were more common in the vaccine group than the placebo group (67.4% vs. 45.4%, respectively), driven largely by more reports of injection site reactions and flu-like symptoms. Solicited reports of adverse events were highlighted by a greater frequency of injection site pain in the vaccine group (81.8% vs. 34.4%). A total of 24.3% of the vaccine recipients reported grade 3 pain, compared with 3.3% in the placebo arm. Rates of fatigue, headache, malaise, or myalgia were also higher in the vaccine group, as was fever.
All of the subjects in the vaccine group had seroconversion at month 2, and 99% remained seroconverted at 12 months.
Next, the researchers plan to conduct studies in HIV-infected individuals and to proceed with phase III trials.
The trial was funded by GlaxoSmithKline Biologicals and Aeras. Dr. Ginsberg is an employee of Aeras.
SOURCE: Ginsberg A et al. IDWeek 2018, Abstract 120
REPORTING FROM IDWEEK 2018
Key clinical point: The vaccine is the first to show efficacy in patients previously exposed to the TB bacterium.
Major finding: The vaccine had a protective efficacy of 54%.
Study details: Randomized, controlled trial with 3,330 participants.
Disclosures: The trial was funded by GlaxoSmithKline Biologicals and Aeras. Dr. Ginsberg is an employee of Aeras.
Source: Ginsberg A et al. IDWeek 2018, Abstract 120.
Flu vaccination lags among patients with psoriasis
Psoriasis patients are more vulnerable to systemic infections, including influenza-related pneumonia, but a new study shows that they are less likely to receive the influenza vaccine than patients with RA.
Vaccination rates were higher in psoriasis patients aged over 50 years, those who were female, and those with other chronic medical conditions, however.
Megan H. Noe, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, and her coauthors referred to recent evidence suggesting that psoriasis involves systemic inflammation that increase the risk of comorbidities and that hospitalization rates for serious infections, including lower respiratory tract infections and pneumonia, are higher among adults with psoriasis than those who do not have psoriasis.
drawing from administrative and commercial claims data from OptumInsight Clinformatics Data Mart. They examined all adult patients with psoriasis, RA, or chronic hypertension who required oral antihypertensive medication. The study population included individuals tracked during the 2010-2011 flu season and 24 months prior (September 2008 to March 2011). This year was chosen because it was labeled as a “typical” season by the Centers for Disease Control and Prevention.
The primary outcome was a claim for an influenza vaccine, and covariates included age, length of residency, gender, and a clinical history of a range of conditions known to be associated with greater risk of influenza complications.
The population included 17,078 patients with psoriasis, 21,832 with RA, and 496,972 with chronic hypertension. After controlling for sex and age, the probability of getting a flu vaccine was similar between psoriasis and hypertension patients, but RA patients were more likely to be vaccinated than patients with psoriasis (odds ratio, 1.08; 95% confidence interval, 1.03-1.13). But the likelihood varied with age: 30-year-old patients with RA were more likely than a 30-year-old psoriasis patient to get a flu shot (OR, 1.30; 95% CI, 1.18-1.45), while a 70-year-old patient with RA was about as likely to get the flu vaccine as a 70-year-old patient with psoriasis.
Female psoriasis patients were more likely to get a flu shot than males (OR, 1.29; 95% CI, 1.20-1.38). Among the psoriasis patients, having some medical comorbidities were linked to a greater likelihood of being vaccinated, including asthma (OR, 1.58; 95% CI, 1.40-1.77), chronic liver disease (OR, 1.23; 95%, 1.03-1.47), diabetes (OR, 1.48; 95% CI, 1.36-1.63), HIV (OR, 3.68; 95% CI, 2.06-6.57), history of malignancy (OR, 1.21; 95% CI, 1.09-1.34), and psoriatic arthritis (OR, 1.40; 95% CI, 1.25-1.58).
There was no association between the use of an oral systemic therapy or biologic treatment and vaccination rates.
The authors suggested that psoriasis patients, especially younger ones, may not get adequate counseling on the value of the flu vaccine from their physicians. Studies have shown that, among the American public, health care providers are the most influential source of information about the flu vaccine. Among younger patients, the dermatologist may be a psoriasis patient’s primary health care provider, so it is important for dermatologists to counsel patients about the recommended vaccines, the authors wrote.
“Further research understanding why adults with psoriasis do not receive recommended vaccinations will help to create targeted interventions to improve vaccination rates and decrease hospitalizations in adults with psoriasis,” they concluded.
The study relied on administrative claims, so the results may not be generalizable to patients with insurance types other than those in the database or who are uninsured, the authors noted.
This study was funded by the National Psoriasis Foundation, the Dermatology Foundation, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Noe and three other authors did not report any disclosures, the fifth author reported multiple disclosures related to various pharmaceutical companies.
SOURCE: Noe MH et al. J Invest Dermatol. 2018 Oct 10. doi: 10.1016/j.jid.2018.09.012.
Psoriasis patients are more vulnerable to systemic infections, including influenza-related pneumonia, but a new study shows that they are less likely to receive the influenza vaccine than patients with RA.
Vaccination rates were higher in psoriasis patients aged over 50 years, those who were female, and those with other chronic medical conditions, however.
Megan H. Noe, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, and her coauthors referred to recent evidence suggesting that psoriasis involves systemic inflammation that increase the risk of comorbidities and that hospitalization rates for serious infections, including lower respiratory tract infections and pneumonia, are higher among adults with psoriasis than those who do not have psoriasis.
drawing from administrative and commercial claims data from OptumInsight Clinformatics Data Mart. They examined all adult patients with psoriasis, RA, or chronic hypertension who required oral antihypertensive medication. The study population included individuals tracked during the 2010-2011 flu season and 24 months prior (September 2008 to March 2011). This year was chosen because it was labeled as a “typical” season by the Centers for Disease Control and Prevention.
The primary outcome was a claim for an influenza vaccine, and covariates included age, length of residency, gender, and a clinical history of a range of conditions known to be associated with greater risk of influenza complications.
The population included 17,078 patients with psoriasis, 21,832 with RA, and 496,972 with chronic hypertension. After controlling for sex and age, the probability of getting a flu vaccine was similar between psoriasis and hypertension patients, but RA patients were more likely to be vaccinated than patients with psoriasis (odds ratio, 1.08; 95% confidence interval, 1.03-1.13). But the likelihood varied with age: 30-year-old patients with RA were more likely than a 30-year-old psoriasis patient to get a flu shot (OR, 1.30; 95% CI, 1.18-1.45), while a 70-year-old patient with RA was about as likely to get the flu vaccine as a 70-year-old patient with psoriasis.
Female psoriasis patients were more likely to get a flu shot than males (OR, 1.29; 95% CI, 1.20-1.38). Among the psoriasis patients, having some medical comorbidities were linked to a greater likelihood of being vaccinated, including asthma (OR, 1.58; 95% CI, 1.40-1.77), chronic liver disease (OR, 1.23; 95%, 1.03-1.47), diabetes (OR, 1.48; 95% CI, 1.36-1.63), HIV (OR, 3.68; 95% CI, 2.06-6.57), history of malignancy (OR, 1.21; 95% CI, 1.09-1.34), and psoriatic arthritis (OR, 1.40; 95% CI, 1.25-1.58).
There was no association between the use of an oral systemic therapy or biologic treatment and vaccination rates.
The authors suggested that psoriasis patients, especially younger ones, may not get adequate counseling on the value of the flu vaccine from their physicians. Studies have shown that, among the American public, health care providers are the most influential source of information about the flu vaccine. Among younger patients, the dermatologist may be a psoriasis patient’s primary health care provider, so it is important for dermatologists to counsel patients about the recommended vaccines, the authors wrote.
“Further research understanding why adults with psoriasis do not receive recommended vaccinations will help to create targeted interventions to improve vaccination rates and decrease hospitalizations in adults with psoriasis,” they concluded.
The study relied on administrative claims, so the results may not be generalizable to patients with insurance types other than those in the database or who are uninsured, the authors noted.
This study was funded by the National Psoriasis Foundation, the Dermatology Foundation, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Noe and three other authors did not report any disclosures, the fifth author reported multiple disclosures related to various pharmaceutical companies.
SOURCE: Noe MH et al. J Invest Dermatol. 2018 Oct 10. doi: 10.1016/j.jid.2018.09.012.
Psoriasis patients are more vulnerable to systemic infections, including influenza-related pneumonia, but a new study shows that they are less likely to receive the influenza vaccine than patients with RA.
Vaccination rates were higher in psoriasis patients aged over 50 years, those who were female, and those with other chronic medical conditions, however.
Megan H. Noe, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, and her coauthors referred to recent evidence suggesting that psoriasis involves systemic inflammation that increase the risk of comorbidities and that hospitalization rates for serious infections, including lower respiratory tract infections and pneumonia, are higher among adults with psoriasis than those who do not have psoriasis.
drawing from administrative and commercial claims data from OptumInsight Clinformatics Data Mart. They examined all adult patients with psoriasis, RA, or chronic hypertension who required oral antihypertensive medication. The study population included individuals tracked during the 2010-2011 flu season and 24 months prior (September 2008 to March 2011). This year was chosen because it was labeled as a “typical” season by the Centers for Disease Control and Prevention.
The primary outcome was a claim for an influenza vaccine, and covariates included age, length of residency, gender, and a clinical history of a range of conditions known to be associated with greater risk of influenza complications.
The population included 17,078 patients with psoriasis, 21,832 with RA, and 496,972 with chronic hypertension. After controlling for sex and age, the probability of getting a flu vaccine was similar between psoriasis and hypertension patients, but RA patients were more likely to be vaccinated than patients with psoriasis (odds ratio, 1.08; 95% confidence interval, 1.03-1.13). But the likelihood varied with age: 30-year-old patients with RA were more likely than a 30-year-old psoriasis patient to get a flu shot (OR, 1.30; 95% CI, 1.18-1.45), while a 70-year-old patient with RA was about as likely to get the flu vaccine as a 70-year-old patient with psoriasis.
Female psoriasis patients were more likely to get a flu shot than males (OR, 1.29; 95% CI, 1.20-1.38). Among the psoriasis patients, having some medical comorbidities were linked to a greater likelihood of being vaccinated, including asthma (OR, 1.58; 95% CI, 1.40-1.77), chronic liver disease (OR, 1.23; 95%, 1.03-1.47), diabetes (OR, 1.48; 95% CI, 1.36-1.63), HIV (OR, 3.68; 95% CI, 2.06-6.57), history of malignancy (OR, 1.21; 95% CI, 1.09-1.34), and psoriatic arthritis (OR, 1.40; 95% CI, 1.25-1.58).
There was no association between the use of an oral systemic therapy or biologic treatment and vaccination rates.
The authors suggested that psoriasis patients, especially younger ones, may not get adequate counseling on the value of the flu vaccine from their physicians. Studies have shown that, among the American public, health care providers are the most influential source of information about the flu vaccine. Among younger patients, the dermatologist may be a psoriasis patient’s primary health care provider, so it is important for dermatologists to counsel patients about the recommended vaccines, the authors wrote.
“Further research understanding why adults with psoriasis do not receive recommended vaccinations will help to create targeted interventions to improve vaccination rates and decrease hospitalizations in adults with psoriasis,” they concluded.
The study relied on administrative claims, so the results may not be generalizable to patients with insurance types other than those in the database or who are uninsured, the authors noted.
This study was funded by the National Psoriasis Foundation, the Dermatology Foundation, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Noe and three other authors did not report any disclosures, the fifth author reported multiple disclosures related to various pharmaceutical companies.
SOURCE: Noe MH et al. J Invest Dermatol. 2018 Oct 10. doi: 10.1016/j.jid.2018.09.012.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Key clinical point: Despite vulnerability to complications, fewer psoriasis patients received the vaccine, compared with RA patients.
Major finding: Patients with RA were 8% more likely to receive a flu vaccine than patients with psoriasis.
Study details: A retrospective cohort study of 535,882 subjects with psoriasis, RA, or hypertension.
Disclosures: This study was funded by the National Psoriasis Foundation, the Dermatology Foundation, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Four authors did not report any disclosures; the fifth author reported multiple disclosures related to various pharmaceutical companies.
Source: Noe MH et al. J Invest Dermatol. 2018 Oct 10. doi: 10.1016/j.jid.2018.09.012.
Adjuvanted flu vaccine reduces hospitalizations in oldest old
SAN FRANCISCO – presented at an annual scientific meeting on infectious diseases.
“It’s one thing to say you have a more immunogenic vaccine, it’s another thing to be able to say it offers clinical benefit, especially in the oldest old and the frailest frail,” says Stefan Gravenstein, MD, professor of medicine and health services, policy and practice at the Brown University School of Public Health, Providence, R.I. Dr. Gravenstein presented a poster outlying a randomized, clinical trial of the Fluad vaccine in nursing homes.
The study randomized the nursing homes so that some facilities would offer Fluad as part of their standard of care. The design helped address the problem of consent. Any clinical trial that requires individual consent would likely exclude many of the frailest patients, leading to an unrepresentative sample. “So if you want to have a generalizable result, you’d like to have it applied to the population the way you would in the real world, so randomizing the nursing homes rather than the people makes a lot of sense,” said Dr. Gravenstein.
Dr. Gravenstein chose to test the vaccine in nursing home residents, hoping to see a signal in a population in which flu complications are more common. “If you can get a difference in a nursing home population, that’s clinically important, that gives you hope that you can see it in all the other populations, too,” he said.
SOURCE: Gravenstein S et al. IDWeek 2018, Abstract 996.
SAN FRANCISCO – presented at an annual scientific meeting on infectious diseases.
“It’s one thing to say you have a more immunogenic vaccine, it’s another thing to be able to say it offers clinical benefit, especially in the oldest old and the frailest frail,” says Stefan Gravenstein, MD, professor of medicine and health services, policy and practice at the Brown University School of Public Health, Providence, R.I. Dr. Gravenstein presented a poster outlying a randomized, clinical trial of the Fluad vaccine in nursing homes.
The study randomized the nursing homes so that some facilities would offer Fluad as part of their standard of care. The design helped address the problem of consent. Any clinical trial that requires individual consent would likely exclude many of the frailest patients, leading to an unrepresentative sample. “So if you want to have a generalizable result, you’d like to have it applied to the population the way you would in the real world, so randomizing the nursing homes rather than the people makes a lot of sense,” said Dr. Gravenstein.
Dr. Gravenstein chose to test the vaccine in nursing home residents, hoping to see a signal in a population in which flu complications are more common. “If you can get a difference in a nursing home population, that’s clinically important, that gives you hope that you can see it in all the other populations, too,” he said.
SOURCE: Gravenstein S et al. IDWeek 2018, Abstract 996.
SAN FRANCISCO – presented at an annual scientific meeting on infectious diseases.
“It’s one thing to say you have a more immunogenic vaccine, it’s another thing to be able to say it offers clinical benefit, especially in the oldest old and the frailest frail,” says Stefan Gravenstein, MD, professor of medicine and health services, policy and practice at the Brown University School of Public Health, Providence, R.I. Dr. Gravenstein presented a poster outlying a randomized, clinical trial of the Fluad vaccine in nursing homes.
The study randomized the nursing homes so that some facilities would offer Fluad as part of their standard of care. The design helped address the problem of consent. Any clinical trial that requires individual consent would likely exclude many of the frailest patients, leading to an unrepresentative sample. “So if you want to have a generalizable result, you’d like to have it applied to the population the way you would in the real world, so randomizing the nursing homes rather than the people makes a lot of sense,” said Dr. Gravenstein.
Dr. Gravenstein chose to test the vaccine in nursing home residents, hoping to see a signal in a population in which flu complications are more common. “If you can get a difference in a nursing home population, that’s clinically important, that gives you hope that you can see it in all the other populations, too,” he said.
SOURCE: Gravenstein S et al. IDWeek 2018, Abstract 996.
REPORTING FROM ID WEEK 2018
Encourage influenza vaccination in pregnant women
They are at greater risk for more severe illness, and influenza can lead to adverse outcomes in infants. The good news is that recent studies have shown that flu vaccines are safe and effective in pregnant women.
The bad news is that many women are hesitant to be vaccinated out of concerns over safety, in a trend that reflects broader societal worries over vaccination, said Dr. Chu, of the University of Washington, Seattle. In a video interview at an annual scientific meeting on infectious diseases, Dr. Chu advised steps to ensure that pregnant women are aware of the safety and efficacy of flu vaccines, and the benefits to the infant who acquires immunity through the mother. It’s also a good idea to have vaccine on hand to be able to offer it immediately during an office visit.
They are at greater risk for more severe illness, and influenza can lead to adverse outcomes in infants. The good news is that recent studies have shown that flu vaccines are safe and effective in pregnant women.
The bad news is that many women are hesitant to be vaccinated out of concerns over safety, in a trend that reflects broader societal worries over vaccination, said Dr. Chu, of the University of Washington, Seattle. In a video interview at an annual scientific meeting on infectious diseases, Dr. Chu advised steps to ensure that pregnant women are aware of the safety and efficacy of flu vaccines, and the benefits to the infant who acquires immunity through the mother. It’s also a good idea to have vaccine on hand to be able to offer it immediately during an office visit.
They are at greater risk for more severe illness, and influenza can lead to adverse outcomes in infants. The good news is that recent studies have shown that flu vaccines are safe and effective in pregnant women.
The bad news is that many women are hesitant to be vaccinated out of concerns over safety, in a trend that reflects broader societal worries over vaccination, said Dr. Chu, of the University of Washington, Seattle. In a video interview at an annual scientific meeting on infectious diseases, Dr. Chu advised steps to ensure that pregnant women are aware of the safety and efficacy of flu vaccines, and the benefits to the infant who acquires immunity through the mother. It’s also a good idea to have vaccine on hand to be able to offer it immediately during an office visit.
REPORTING FROM ID WEEK 2018
PCV13 moderately effective in older adults
ATLANTA – (IPD) caused by PCV13 vaccine serotypes in adults aged 65 years and older, according to a case-control study involving Medicare beneficiaries.
Conversely, the 23-valent pneumococcal polysaccharide vaccine (PPSV23) showed limited effectiveness against serotypes unique to that vaccine in the study, which included 699 cases and more than 10,000 controls, Olivia Almendares, an epidemiologist at the Centers for Disease Control and Prevention, Atlanta, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“Vaccine efficacy against PCV13 [plus 6C type, which has cross-reactivity with serotype 6A] was 47% in those who received PCV13 vaccine only,” Ms. Almendares said in an interview, noting that efficacy was 26% against serotype 3 and 67% against other PCV13 serotypes (plus 6C). “Vaccine efficacy against PPSV23-unique types was 36% for those who received only PPSV23.”
Neither vaccine showed effectiveness against serotypes not included in the respective vaccines, she said.
The findings are timely given that the Advisory Committee on Immunization Practices (ACIP) is reevaluating its PCV13 recommendation for adults aged 65 years and older, she added.
“Specifically, ACIP is addressing whether PCV13 should be recommended routinely for all immunocompetent adults aged 65 and older given sustained indirect effects,” she said, explaining that, in 2014 when ACIP recommended routine use of the vaccine in series with PPSV23 for adults aged 65 years and older, the committee recognized that herd immunity effects from PCV13 use in children might eventually limit the utility of this recommendation, and therefore it proposed reevaluation and revision as needed after 4 years.
For the current study, she and her colleagues linked IPD cases in persons aged 65 years and older, which were identified through Active Bacterial Core surveillance during 2015-2016, to records for Centers for Medicare & Medicaid Services (CMS) beneficiaries. Vaccination and medical histories were obtained through medical records, and vaccine effectiveness was estimated as one minus the odds ratio for vaccination with PCV13 only or PPSV23 only versus neither vaccine using conditional logistic regression, with adjustment for sex and underlying medical conditions.
Of 2,246 IPD cases, 1,017 (45%) were matched to Medicare beneficiaries, and 699 were included in the analysis after those with noncontinuous enrollment in Medicare, long-term care residence, and missing census tract data were excluded. The cases were matched based on age, census tract of residence, and length of Medicare enrollment to 10,152 matched controls identified through CMS.
IPD associated with PCV13 (plus type 6C) accounted for 164 (23% of cases), of which 88 (12% of cases) involved serotype 3, and invasive pneumococcal disease associated with PPSV23 accounted for 350 cases (50%), she said.
PCV13 vaccine was given alone in 14% and 18% of cases and controls, respectively; PPSV23 alone was given in 22% and 21% of case patients and controls, respectively; and both vaccines were given in 8% of cases and controls.
Compared with controls, case patients were more likely to be of nonwhite race (16% vs. 11%), to have more than one chronic medical condition (88% vs. 58%), and to have one or more immunocompromising conditions (54% vs. 32%), she and her colleagues reported.
“PCV13 showed moderate overall effectiveness in preventing IPD caused by PCV13 (including 6C), but effectiveness may be lower for serotype 3 than for other PCV13 types,” she said.
“These results are in agreement with those from CAPiTA – a large clinical trial conducted in the Netherlands, which showed PCV13 to be effective against IPD caused by vaccine serotypes among community-dwelling adults aged 65 and older,” she noted. “Additionally, data from CDC surveillance suggest that PCV13-serotype [invasive pneumococcal disease] among children and adults aged 65 and older has declined dramatically following PCV13 introduction for children in 2010, as predicted.”
In fact, among adults aged 65 years and older, PCV13-serotype invasive pneumococcal disease declined by 40% after the vaccine was introduced in children. This corresponds to a change in the annual PCV13-serotype incidence from 14 cases per 100,000 population in 2010 to five cases per 100,000 population in 2014, she said; she added that IPD incidence plateaued in 2014-2016 with vaccine serotypes contributing to a small proportion of overall IPD burden among adults aged 65 years and older.
ACIP’s reevaluation of the PCV13 recommendation is ongoing and will be addressed at upcoming meetings.
“As part of the review process, we look at changes in disease incidence focusing primarily on invasive pneumococcal disease and noninvasive pneumonia, vaccine efficacy and effectiveness, and vaccine safety,” she said. She noted that ACIP currently has no plans to consider revising PCV13 recommendations for adults who have immunocompromising conditions, for whom PCV13 has been recommended since 2012.
Ms. Almendares reported having no disclosures.
SOURCE: Almendares O et al. ICEID 2018, Board 376.
ATLANTA – (IPD) caused by PCV13 vaccine serotypes in adults aged 65 years and older, according to a case-control study involving Medicare beneficiaries.
Conversely, the 23-valent pneumococcal polysaccharide vaccine (PPSV23) showed limited effectiveness against serotypes unique to that vaccine in the study, which included 699 cases and more than 10,000 controls, Olivia Almendares, an epidemiologist at the Centers for Disease Control and Prevention, Atlanta, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“Vaccine efficacy against PCV13 [plus 6C type, which has cross-reactivity with serotype 6A] was 47% in those who received PCV13 vaccine only,” Ms. Almendares said in an interview, noting that efficacy was 26% against serotype 3 and 67% against other PCV13 serotypes (plus 6C). “Vaccine efficacy against PPSV23-unique types was 36% for those who received only PPSV23.”
Neither vaccine showed effectiveness against serotypes not included in the respective vaccines, she said.
The findings are timely given that the Advisory Committee on Immunization Practices (ACIP) is reevaluating its PCV13 recommendation for adults aged 65 years and older, she added.
“Specifically, ACIP is addressing whether PCV13 should be recommended routinely for all immunocompetent adults aged 65 and older given sustained indirect effects,” she said, explaining that, in 2014 when ACIP recommended routine use of the vaccine in series with PPSV23 for adults aged 65 years and older, the committee recognized that herd immunity effects from PCV13 use in children might eventually limit the utility of this recommendation, and therefore it proposed reevaluation and revision as needed after 4 years.
For the current study, she and her colleagues linked IPD cases in persons aged 65 years and older, which were identified through Active Bacterial Core surveillance during 2015-2016, to records for Centers for Medicare & Medicaid Services (CMS) beneficiaries. Vaccination and medical histories were obtained through medical records, and vaccine effectiveness was estimated as one minus the odds ratio for vaccination with PCV13 only or PPSV23 only versus neither vaccine using conditional logistic regression, with adjustment for sex and underlying medical conditions.
Of 2,246 IPD cases, 1,017 (45%) were matched to Medicare beneficiaries, and 699 were included in the analysis after those with noncontinuous enrollment in Medicare, long-term care residence, and missing census tract data were excluded. The cases were matched based on age, census tract of residence, and length of Medicare enrollment to 10,152 matched controls identified through CMS.
IPD associated with PCV13 (plus type 6C) accounted for 164 (23% of cases), of which 88 (12% of cases) involved serotype 3, and invasive pneumococcal disease associated with PPSV23 accounted for 350 cases (50%), she said.
PCV13 vaccine was given alone in 14% and 18% of cases and controls, respectively; PPSV23 alone was given in 22% and 21% of case patients and controls, respectively; and both vaccines were given in 8% of cases and controls.
Compared with controls, case patients were more likely to be of nonwhite race (16% vs. 11%), to have more than one chronic medical condition (88% vs. 58%), and to have one or more immunocompromising conditions (54% vs. 32%), she and her colleagues reported.
“PCV13 showed moderate overall effectiveness in preventing IPD caused by PCV13 (including 6C), but effectiveness may be lower for serotype 3 than for other PCV13 types,” she said.
“These results are in agreement with those from CAPiTA – a large clinical trial conducted in the Netherlands, which showed PCV13 to be effective against IPD caused by vaccine serotypes among community-dwelling adults aged 65 and older,” she noted. “Additionally, data from CDC surveillance suggest that PCV13-serotype [invasive pneumococcal disease] among children and adults aged 65 and older has declined dramatically following PCV13 introduction for children in 2010, as predicted.”
In fact, among adults aged 65 years and older, PCV13-serotype invasive pneumococcal disease declined by 40% after the vaccine was introduced in children. This corresponds to a change in the annual PCV13-serotype incidence from 14 cases per 100,000 population in 2010 to five cases per 100,000 population in 2014, she said; she added that IPD incidence plateaued in 2014-2016 with vaccine serotypes contributing to a small proportion of overall IPD burden among adults aged 65 years and older.
ACIP’s reevaluation of the PCV13 recommendation is ongoing and will be addressed at upcoming meetings.
“As part of the review process, we look at changes in disease incidence focusing primarily on invasive pneumococcal disease and noninvasive pneumonia, vaccine efficacy and effectiveness, and vaccine safety,” she said. She noted that ACIP currently has no plans to consider revising PCV13 recommendations for adults who have immunocompromising conditions, for whom PCV13 has been recommended since 2012.
Ms. Almendares reported having no disclosures.
SOURCE: Almendares O et al. ICEID 2018, Board 376.
ATLANTA – (IPD) caused by PCV13 vaccine serotypes in adults aged 65 years and older, according to a case-control study involving Medicare beneficiaries.
Conversely, the 23-valent pneumococcal polysaccharide vaccine (PPSV23) showed limited effectiveness against serotypes unique to that vaccine in the study, which included 699 cases and more than 10,000 controls, Olivia Almendares, an epidemiologist at the Centers for Disease Control and Prevention, Atlanta, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“Vaccine efficacy against PCV13 [plus 6C type, which has cross-reactivity with serotype 6A] was 47% in those who received PCV13 vaccine only,” Ms. Almendares said in an interview, noting that efficacy was 26% against serotype 3 and 67% against other PCV13 serotypes (plus 6C). “Vaccine efficacy against PPSV23-unique types was 36% for those who received only PPSV23.”
Neither vaccine showed effectiveness against serotypes not included in the respective vaccines, she said.
The findings are timely given that the Advisory Committee on Immunization Practices (ACIP) is reevaluating its PCV13 recommendation for adults aged 65 years and older, she added.
“Specifically, ACIP is addressing whether PCV13 should be recommended routinely for all immunocompetent adults aged 65 and older given sustained indirect effects,” she said, explaining that, in 2014 when ACIP recommended routine use of the vaccine in series with PPSV23 for adults aged 65 years and older, the committee recognized that herd immunity effects from PCV13 use in children might eventually limit the utility of this recommendation, and therefore it proposed reevaluation and revision as needed after 4 years.
For the current study, she and her colleagues linked IPD cases in persons aged 65 years and older, which were identified through Active Bacterial Core surveillance during 2015-2016, to records for Centers for Medicare & Medicaid Services (CMS) beneficiaries. Vaccination and medical histories were obtained through medical records, and vaccine effectiveness was estimated as one minus the odds ratio for vaccination with PCV13 only or PPSV23 only versus neither vaccine using conditional logistic regression, with adjustment for sex and underlying medical conditions.
Of 2,246 IPD cases, 1,017 (45%) were matched to Medicare beneficiaries, and 699 were included in the analysis after those with noncontinuous enrollment in Medicare, long-term care residence, and missing census tract data were excluded. The cases were matched based on age, census tract of residence, and length of Medicare enrollment to 10,152 matched controls identified through CMS.
IPD associated with PCV13 (plus type 6C) accounted for 164 (23% of cases), of which 88 (12% of cases) involved serotype 3, and invasive pneumococcal disease associated with PPSV23 accounted for 350 cases (50%), she said.
PCV13 vaccine was given alone in 14% and 18% of cases and controls, respectively; PPSV23 alone was given in 22% and 21% of case patients and controls, respectively; and both vaccines were given in 8% of cases and controls.
Compared with controls, case patients were more likely to be of nonwhite race (16% vs. 11%), to have more than one chronic medical condition (88% vs. 58%), and to have one or more immunocompromising conditions (54% vs. 32%), she and her colleagues reported.
“PCV13 showed moderate overall effectiveness in preventing IPD caused by PCV13 (including 6C), but effectiveness may be lower for serotype 3 than for other PCV13 types,” she said.
“These results are in agreement with those from CAPiTA – a large clinical trial conducted in the Netherlands, which showed PCV13 to be effective against IPD caused by vaccine serotypes among community-dwelling adults aged 65 and older,” she noted. “Additionally, data from CDC surveillance suggest that PCV13-serotype [invasive pneumococcal disease] among children and adults aged 65 and older has declined dramatically following PCV13 introduction for children in 2010, as predicted.”
In fact, among adults aged 65 years and older, PCV13-serotype invasive pneumococcal disease declined by 40% after the vaccine was introduced in children. This corresponds to a change in the annual PCV13-serotype incidence from 14 cases per 100,000 population in 2010 to five cases per 100,000 population in 2014, she said; she added that IPD incidence plateaued in 2014-2016 with vaccine serotypes contributing to a small proportion of overall IPD burden among adults aged 65 years and older.
ACIP’s reevaluation of the PCV13 recommendation is ongoing and will be addressed at upcoming meetings.
“As part of the review process, we look at changes in disease incidence focusing primarily on invasive pneumococcal disease and noninvasive pneumonia, vaccine efficacy and effectiveness, and vaccine safety,” she said. She noted that ACIP currently has no plans to consider revising PCV13 recommendations for adults who have immunocompromising conditions, for whom PCV13 has been recommended since 2012.
Ms. Almendares reported having no disclosures.
SOURCE: Almendares O et al. ICEID 2018, Board 376.
REPORTING FROM ICEID 2018
Point-of-care test for respiratory viruses lowers antibiotic use
Routine testing in the ED is advocated
PARIS – Using a point-of-care test for viral pathogens, hospital admissions were avoided in about a third of emergency department patients with suspected respiratory infection when other clinical signs also suggested a low risk of a bacterial pathogen, according to a single-center experience presented at the annual congress of the European Respiratory Society.
“We found that when patients had point-of-care respiratory viral testing soon after they were admitted to the emergency department, we were able to reduce unnecessary admission and improve bed flow in our center,” reported Kay Roy, MBBS, consultant physician in respiratory medicine, West Hertfordshire (England) Hospital NHS Trust.
In a protocol that was launched at Dr. Kay’s institution in January 2018, the point-of-care viral test was combined with other clinical factors, particularly chest x-rays and elevated C-reactive protein (CRP), to determine whether patients had a viral pathogen and whether they could be discharged without antibiotics.
“Clinical judgment will always be required in individual patient decisions regarding antibiotic avoidance and early discharge,” Dr. Roy maintained. “But the point-of-care viral assay can be integrated into a strategy that permits more informed and rapid decision-making.”
This assertion is supported by the experience using a protocol anchored with the point-of-care viral test over a 4-month period. During this time, 901 patients with respiratory symptoms suspected of having a viral etiology were evaluated with the proprietary point-of-care device called FilmArray (bioMérieux).
From a sample taken with a nasopharyngeal swab, the test can identify a broad array of viruses using polymerase chain reaction technology in less than 45 minutes. However, the ED protocol for considering discharge without antibiotics requires additional evidence that the pathogen is viral, including a normal chest x-ray and a CRP less than 50 mg/L.
Of the 901 patients tested, a substantial proportion of whom had chronic obstructive pulmonary disease (COPD) or asthma, 507 (56%) tested positive for at least one virus, including influenza, rhinoviruses, coronaviruses, and adenovirus. Of these, 239 had normal chest x-rays and CRPs less than 50 mg/L. Because of the severity of symptoms or other clinical considerations, 154 patients were admitted, but 85 (36% of those meeting protocol criteria) were discharged without an antibiotic prescription.
“Antibiotics were continued in 90% of the patients who had an abnormal chest x-ray and abnormal CRP,” Dr. Roy reported. However, an objective strategy that permits clinicians to discharge patients at very low risk of a bacterial infection has many advantages even if it applies to a relatively modest proportion of those tested, according to Dr. Roy.
“Each respiratory admission can cost around [2,000 pounds] at our center,” reported Dr. Kay, referring to a figure equivalent to more than $2,600. In addition, she said that avoiding hospitalization frees up hospital beds and facilitates improved antimicrobial stewardship, which is vital to stem resistance.
Avoiding antibiotic use in patients with viral respiratory infections also is relevant to improved antibiotic stewardship in the community. For this reason, a randomized trial with a similar protocol involving the point-of-care viral test is planned in the outpatient setting. According to Dr. Roy, this will involve a community hub to which patients can be referred for testing and clinical evaluation.
“We hope that the quality of care can be improved with the point-of-care test for respiratory viruses as well as helping to reduce antibiotic resistance,” Dr. Roy said.
This approach is promising, according to Tobias Welte, MD, of the department of respiratory medicine at Hannover (Germany) Medical School, but he cautioned that it is not a standard approach.
“The protocol described by Dr. Roy will have to be compared to guidelines and recommended best clinical practice to confirm its usefulness,” he said, while conceding that any strategy that reduces unnecessary hospitalizations deserves further evaluation.
Routine testing in the ED is advocated
Routine testing in the ED is advocated
PARIS – Using a point-of-care test for viral pathogens, hospital admissions were avoided in about a third of emergency department patients with suspected respiratory infection when other clinical signs also suggested a low risk of a bacterial pathogen, according to a single-center experience presented at the annual congress of the European Respiratory Society.
“We found that when patients had point-of-care respiratory viral testing soon after they were admitted to the emergency department, we were able to reduce unnecessary admission and improve bed flow in our center,” reported Kay Roy, MBBS, consultant physician in respiratory medicine, West Hertfordshire (England) Hospital NHS Trust.
In a protocol that was launched at Dr. Kay’s institution in January 2018, the point-of-care viral test was combined with other clinical factors, particularly chest x-rays and elevated C-reactive protein (CRP), to determine whether patients had a viral pathogen and whether they could be discharged without antibiotics.
“Clinical judgment will always be required in individual patient decisions regarding antibiotic avoidance and early discharge,” Dr. Roy maintained. “But the point-of-care viral assay can be integrated into a strategy that permits more informed and rapid decision-making.”
This assertion is supported by the experience using a protocol anchored with the point-of-care viral test over a 4-month period. During this time, 901 patients with respiratory symptoms suspected of having a viral etiology were evaluated with the proprietary point-of-care device called FilmArray (bioMérieux).
From a sample taken with a nasopharyngeal swab, the test can identify a broad array of viruses using polymerase chain reaction technology in less than 45 minutes. However, the ED protocol for considering discharge without antibiotics requires additional evidence that the pathogen is viral, including a normal chest x-ray and a CRP less than 50 mg/L.
Of the 901 patients tested, a substantial proportion of whom had chronic obstructive pulmonary disease (COPD) or asthma, 507 (56%) tested positive for at least one virus, including influenza, rhinoviruses, coronaviruses, and adenovirus. Of these, 239 had normal chest x-rays and CRPs less than 50 mg/L. Because of the severity of symptoms or other clinical considerations, 154 patients were admitted, but 85 (36% of those meeting protocol criteria) were discharged without an antibiotic prescription.
“Antibiotics were continued in 90% of the patients who had an abnormal chest x-ray and abnormal CRP,” Dr. Roy reported. However, an objective strategy that permits clinicians to discharge patients at very low risk of a bacterial infection has many advantages even if it applies to a relatively modest proportion of those tested, according to Dr. Roy.
“Each respiratory admission can cost around [2,000 pounds] at our center,” reported Dr. Kay, referring to a figure equivalent to more than $2,600. In addition, she said that avoiding hospitalization frees up hospital beds and facilitates improved antimicrobial stewardship, which is vital to stem resistance.
Avoiding antibiotic use in patients with viral respiratory infections also is relevant to improved antibiotic stewardship in the community. For this reason, a randomized trial with a similar protocol involving the point-of-care viral test is planned in the outpatient setting. According to Dr. Roy, this will involve a community hub to which patients can be referred for testing and clinical evaluation.
“We hope that the quality of care can be improved with the point-of-care test for respiratory viruses as well as helping to reduce antibiotic resistance,” Dr. Roy said.
This approach is promising, according to Tobias Welte, MD, of the department of respiratory medicine at Hannover (Germany) Medical School, but he cautioned that it is not a standard approach.
“The protocol described by Dr. Roy will have to be compared to guidelines and recommended best clinical practice to confirm its usefulness,” he said, while conceding that any strategy that reduces unnecessary hospitalizations deserves further evaluation.
PARIS – Using a point-of-care test for viral pathogens, hospital admissions were avoided in about a third of emergency department patients with suspected respiratory infection when other clinical signs also suggested a low risk of a bacterial pathogen, according to a single-center experience presented at the annual congress of the European Respiratory Society.
“We found that when patients had point-of-care respiratory viral testing soon after they were admitted to the emergency department, we were able to reduce unnecessary admission and improve bed flow in our center,” reported Kay Roy, MBBS, consultant physician in respiratory medicine, West Hertfordshire (England) Hospital NHS Trust.
In a protocol that was launched at Dr. Kay’s institution in January 2018, the point-of-care viral test was combined with other clinical factors, particularly chest x-rays and elevated C-reactive protein (CRP), to determine whether patients had a viral pathogen and whether they could be discharged without antibiotics.
“Clinical judgment will always be required in individual patient decisions regarding antibiotic avoidance and early discharge,” Dr. Roy maintained. “But the point-of-care viral assay can be integrated into a strategy that permits more informed and rapid decision-making.”
This assertion is supported by the experience using a protocol anchored with the point-of-care viral test over a 4-month period. During this time, 901 patients with respiratory symptoms suspected of having a viral etiology were evaluated with the proprietary point-of-care device called FilmArray (bioMérieux).
From a sample taken with a nasopharyngeal swab, the test can identify a broad array of viruses using polymerase chain reaction technology in less than 45 minutes. However, the ED protocol for considering discharge without antibiotics requires additional evidence that the pathogen is viral, including a normal chest x-ray and a CRP less than 50 mg/L.
Of the 901 patients tested, a substantial proportion of whom had chronic obstructive pulmonary disease (COPD) or asthma, 507 (56%) tested positive for at least one virus, including influenza, rhinoviruses, coronaviruses, and adenovirus. Of these, 239 had normal chest x-rays and CRPs less than 50 mg/L. Because of the severity of symptoms or other clinical considerations, 154 patients were admitted, but 85 (36% of those meeting protocol criteria) were discharged without an antibiotic prescription.
“Antibiotics were continued in 90% of the patients who had an abnormal chest x-ray and abnormal CRP,” Dr. Roy reported. However, an objective strategy that permits clinicians to discharge patients at very low risk of a bacterial infection has many advantages even if it applies to a relatively modest proportion of those tested, according to Dr. Roy.
“Each respiratory admission can cost around [2,000 pounds] at our center,” reported Dr. Kay, referring to a figure equivalent to more than $2,600. In addition, she said that avoiding hospitalization frees up hospital beds and facilitates improved antimicrobial stewardship, which is vital to stem resistance.
Avoiding antibiotic use in patients with viral respiratory infections also is relevant to improved antibiotic stewardship in the community. For this reason, a randomized trial with a similar protocol involving the point-of-care viral test is planned in the outpatient setting. According to Dr. Roy, this will involve a community hub to which patients can be referred for testing and clinical evaluation.
“We hope that the quality of care can be improved with the point-of-care test for respiratory viruses as well as helping to reduce antibiotic resistance,” Dr. Roy said.
This approach is promising, according to Tobias Welte, MD, of the department of respiratory medicine at Hannover (Germany) Medical School, but he cautioned that it is not a standard approach.
“The protocol described by Dr. Roy will have to be compared to guidelines and recommended best clinical practice to confirm its usefulness,” he said, while conceding that any strategy that reduces unnecessary hospitalizations deserves further evaluation.
REPORTING FROM THE ERS CONGRESS 2018
Key clinical point:
Major finding: Of patients with a negative chest x-ray and low CRP level, 36% avoided hospital admission due to a positive test for a virus.
Study details: A case series.
Disclosures: Dr. Roy reports no financial relationships relevant to this study.
Severe influenza increases risk of invasive pulmonary aspergillosis in the ICU
Severe influenza is an independent risk factor for invasive pulmonary aspergillosis with an accompanying increased mortality in the ICU, according to a multicenter retrospective cohort study at seven tertiary centers in Belgium and the Netherlands.
Data was collected from criteria-meeting adult patients admitted to the ICU for more than 24 hours with acute respiratory failure during the 2009-2016 influenza seasons. The included cohort of 432 patients was composed of 56% men and had a median age of 59 years; all participants were diagnosed as having severe type A or type B influenza infection according to positive airway RT-PCR results.
The full cohort was subcategorized into 117 immunocompromised and 315 as nonimmunocompromised individuals using criteria established by the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study group (EORTC/MSG) . To assess influenza as an independent variable in the development of invasive pulmonary aspergillosis, the 315 nonimmunocompromised influenza positive individuals were compared to an influenza-negative control group of 315 nonimmunocompromised patients admitted to the ICU that presented similar respiratory insufficiency symptoms with community-acquired pneumonia.
Determination of other independent risk factors for incidence of invasive pulmonary aspergillosis was achieved by multivariate analysis of factors such as sex, diabetes status, prednisone use, age, and acute physiology and chronic health evaluation (APACHE) II score. The mean APACHE II score was 22, with the majority of patients requiring intubation for mechanical ventilation for a median duration of 11 days.
Influenza is not considered a host factor for invasive pulmonary aspergillosis and will often miss being diagnosed when using strict interpretation of the current EORTC/MSG or AspICU algorithm criteria, according to the researchers. Consequently for patients with influenza and the noninfluenza control group with community-acquired pneumonia, the definition of invasive pulmonary aspergillosis was modified from the AspICU algorithm. Stringent mycological criteria, including bronchoaveolar lavage (BAL) culture, a positive Aspergillus culture, positive galactomannan test, and/or positive serum galactomannan tests, provided supporting diagnostics for an invasive pulmonary aspergillosis determination.
At a median of 3 days following admission to the ICU, a diagnosis of invasive pulmonary aspergillosis was determined for 19% of the 432 influenza patients. Similar incident percentages of invasive pulmonary aspergillosis occurring for type A and type B, 71/355 (20%) and 12/77 (16%) patients respectively, showed that there was no clear association of the disease development with influenza subtypes that occurred during different annual seasons.
AspICU or EORTC/MSG criteria characterized only 43% and 58% of cases as proven or possible aspergillosis, respectively. On the other hand, stringent mycological tests yielded better invasive pulmonary aspergillosis classification, with 63% of BAL cultures being positive for Aspergillus, 88% of BAL galactomannan tests being positive, and 65% of serum galactomannan tests being positive in the 81/83 patients tested.
The study found that, for influenza patients, being immunocompromised more than doubled the incidence of invasive pulmonary aspergillosis, at 32% versus the 14% of those patients who were nonimmunocompromised. In contrast only 5% in the control group developed invasive pulmonary aspergillosis.
Influenza patients who developed invasive pulmonary aspergillosis in the ICU tended to have their stays significantly lengthened from 9 days (interquartile range, 5-20 days) for those without it to 19 days (IQR, 12-38 days) for those infected (P less than .0001). Likewise, 90-day mortality significantly rose from 28% for those influenza patients without invasive pulmonary aspergillosis to 51% for those with it (P = .0001).
The authors concluded that influenza was “independently associated with invasive pulmonary aspergillosis (adjusted odds ratio, 5.19; P less than.0001) along with a higher APACHE II score, male sex, and use of corticosteroids.”
Furthermore, as influenza appears to be an independent risk factor for invasive pulmonary aspergillosis and its associated high mortality, the authors suggested that “future studies should assess whether a faster diagnosis or antifungal prophylaxis could improve the outcome of influenza-associated aspergillosis.”
The authors reported that they had no conflicts of interest.
SOURCE: Schauwvlieghe AFAD et al. Lancet Respir Med. 2018 Jul 31. doi: 10.1016/S2213-2600(18)30274-1
Severe influenza is an independent risk factor for invasive pulmonary aspergillosis with an accompanying increased mortality in the ICU, according to a multicenter retrospective cohort study at seven tertiary centers in Belgium and the Netherlands.
Data was collected from criteria-meeting adult patients admitted to the ICU for more than 24 hours with acute respiratory failure during the 2009-2016 influenza seasons. The included cohort of 432 patients was composed of 56% men and had a median age of 59 years; all participants were diagnosed as having severe type A or type B influenza infection according to positive airway RT-PCR results.
The full cohort was subcategorized into 117 immunocompromised and 315 as nonimmunocompromised individuals using criteria established by the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study group (EORTC/MSG) . To assess influenza as an independent variable in the development of invasive pulmonary aspergillosis, the 315 nonimmunocompromised influenza positive individuals were compared to an influenza-negative control group of 315 nonimmunocompromised patients admitted to the ICU that presented similar respiratory insufficiency symptoms with community-acquired pneumonia.
Determination of other independent risk factors for incidence of invasive pulmonary aspergillosis was achieved by multivariate analysis of factors such as sex, diabetes status, prednisone use, age, and acute physiology and chronic health evaluation (APACHE) II score. The mean APACHE II score was 22, with the majority of patients requiring intubation for mechanical ventilation for a median duration of 11 days.
Influenza is not considered a host factor for invasive pulmonary aspergillosis and will often miss being diagnosed when using strict interpretation of the current EORTC/MSG or AspICU algorithm criteria, according to the researchers. Consequently for patients with influenza and the noninfluenza control group with community-acquired pneumonia, the definition of invasive pulmonary aspergillosis was modified from the AspICU algorithm. Stringent mycological criteria, including bronchoaveolar lavage (BAL) culture, a positive Aspergillus culture, positive galactomannan test, and/or positive serum galactomannan tests, provided supporting diagnostics for an invasive pulmonary aspergillosis determination.
At a median of 3 days following admission to the ICU, a diagnosis of invasive pulmonary aspergillosis was determined for 19% of the 432 influenza patients. Similar incident percentages of invasive pulmonary aspergillosis occurring for type A and type B, 71/355 (20%) and 12/77 (16%) patients respectively, showed that there was no clear association of the disease development with influenza subtypes that occurred during different annual seasons.
AspICU or EORTC/MSG criteria characterized only 43% and 58% of cases as proven or possible aspergillosis, respectively. On the other hand, stringent mycological tests yielded better invasive pulmonary aspergillosis classification, with 63% of BAL cultures being positive for Aspergillus, 88% of BAL galactomannan tests being positive, and 65% of serum galactomannan tests being positive in the 81/83 patients tested.
The study found that, for influenza patients, being immunocompromised more than doubled the incidence of invasive pulmonary aspergillosis, at 32% versus the 14% of those patients who were nonimmunocompromised. In contrast only 5% in the control group developed invasive pulmonary aspergillosis.
Influenza patients who developed invasive pulmonary aspergillosis in the ICU tended to have their stays significantly lengthened from 9 days (interquartile range, 5-20 days) for those without it to 19 days (IQR, 12-38 days) for those infected (P less than .0001). Likewise, 90-day mortality significantly rose from 28% for those influenza patients without invasive pulmonary aspergillosis to 51% for those with it (P = .0001).
The authors concluded that influenza was “independently associated with invasive pulmonary aspergillosis (adjusted odds ratio, 5.19; P less than.0001) along with a higher APACHE II score, male sex, and use of corticosteroids.”
Furthermore, as influenza appears to be an independent risk factor for invasive pulmonary aspergillosis and its associated high mortality, the authors suggested that “future studies should assess whether a faster diagnosis or antifungal prophylaxis could improve the outcome of influenza-associated aspergillosis.”
The authors reported that they had no conflicts of interest.
SOURCE: Schauwvlieghe AFAD et al. Lancet Respir Med. 2018 Jul 31. doi: 10.1016/S2213-2600(18)30274-1
Severe influenza is an independent risk factor for invasive pulmonary aspergillosis with an accompanying increased mortality in the ICU, according to a multicenter retrospective cohort study at seven tertiary centers in Belgium and the Netherlands.
Data was collected from criteria-meeting adult patients admitted to the ICU for more than 24 hours with acute respiratory failure during the 2009-2016 influenza seasons. The included cohort of 432 patients was composed of 56% men and had a median age of 59 years; all participants were diagnosed as having severe type A or type B influenza infection according to positive airway RT-PCR results.
The full cohort was subcategorized into 117 immunocompromised and 315 as nonimmunocompromised individuals using criteria established by the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study group (EORTC/MSG) . To assess influenza as an independent variable in the development of invasive pulmonary aspergillosis, the 315 nonimmunocompromised influenza positive individuals were compared to an influenza-negative control group of 315 nonimmunocompromised patients admitted to the ICU that presented similar respiratory insufficiency symptoms with community-acquired pneumonia.
Determination of other independent risk factors for incidence of invasive pulmonary aspergillosis was achieved by multivariate analysis of factors such as sex, diabetes status, prednisone use, age, and acute physiology and chronic health evaluation (APACHE) II score. The mean APACHE II score was 22, with the majority of patients requiring intubation for mechanical ventilation for a median duration of 11 days.
Influenza is not considered a host factor for invasive pulmonary aspergillosis and will often miss being diagnosed when using strict interpretation of the current EORTC/MSG or AspICU algorithm criteria, according to the researchers. Consequently for patients with influenza and the noninfluenza control group with community-acquired pneumonia, the definition of invasive pulmonary aspergillosis was modified from the AspICU algorithm. Stringent mycological criteria, including bronchoaveolar lavage (BAL) culture, a positive Aspergillus culture, positive galactomannan test, and/or positive serum galactomannan tests, provided supporting diagnostics for an invasive pulmonary aspergillosis determination.
At a median of 3 days following admission to the ICU, a diagnosis of invasive pulmonary aspergillosis was determined for 19% of the 432 influenza patients. Similar incident percentages of invasive pulmonary aspergillosis occurring for type A and type B, 71/355 (20%) and 12/77 (16%) patients respectively, showed that there was no clear association of the disease development with influenza subtypes that occurred during different annual seasons.
AspICU or EORTC/MSG criteria characterized only 43% and 58% of cases as proven or possible aspergillosis, respectively. On the other hand, stringent mycological tests yielded better invasive pulmonary aspergillosis classification, with 63% of BAL cultures being positive for Aspergillus, 88% of BAL galactomannan tests being positive, and 65% of serum galactomannan tests being positive in the 81/83 patients tested.
The study found that, for influenza patients, being immunocompromised more than doubled the incidence of invasive pulmonary aspergillosis, at 32% versus the 14% of those patients who were nonimmunocompromised. In contrast only 5% in the control group developed invasive pulmonary aspergillosis.
Influenza patients who developed invasive pulmonary aspergillosis in the ICU tended to have their stays significantly lengthened from 9 days (interquartile range, 5-20 days) for those without it to 19 days (IQR, 12-38 days) for those infected (P less than .0001). Likewise, 90-day mortality significantly rose from 28% for those influenza patients without invasive pulmonary aspergillosis to 51% for those with it (P = .0001).
The authors concluded that influenza was “independently associated with invasive pulmonary aspergillosis (adjusted odds ratio, 5.19; P less than.0001) along with a higher APACHE II score, male sex, and use of corticosteroids.”
Furthermore, as influenza appears to be an independent risk factor for invasive pulmonary aspergillosis and its associated high mortality, the authors suggested that “future studies should assess whether a faster diagnosis or antifungal prophylaxis could improve the outcome of influenza-associated aspergillosis.”
The authors reported that they had no conflicts of interest.
SOURCE: Schauwvlieghe AFAD et al. Lancet Respir Med. 2018 Jul 31. doi: 10.1016/S2213-2600(18)30274-1
FROM THE LANCET RESPIRATORY MEDICINE
Key clinical point: ICU admission for severe influenza as significant a risk factor should be included in the existing diagnostic criteria for predicting incidence of invasive pulmonary aspergillosis.
Major finding: Influenza is an independent risk factor associated with invasive pulmonary aspergillosis, with 90-day mortality rising from 28% to 51% when this fungal infection occurs.
Study details: Multicenter retrospective study of 432 adult patients with confirmed severe influenza admitted to the ICU with acute respiratory failure.
Disclosures: The authors reported that they had no conflicts of interest.
Source: Schauwvlieghe AFAD et al. Lancet Respir Med. 2018 Jul 31. doi: 10.1016/S2213-2600(18)30274-1.