User login
Obesity doesn’t hamper flu vaccine response in pregnancy
LJUBLJANA, SLOVENIA – ; indeed, it might actually improve their seroconversion rate, Michelle Clarke reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
She presented a prospective cohort study of 90 women vaccinated against influenza during pregnancy, 24 of whom had a BMI of 30 kg/m2 or more. The impetus for the study was the investigators’ understanding that influenza in pregnancy carries an increased risk of severe complications, obesity is a known risk factor for more severe episodes of influenza, and vaccine responses could potentially be adversely affected by obesity, either because of the associated inflammatory state and altered cytokine profile or inadequate vaccine delivery via the intramuscular route. Yet the impact of obesity on vaccine responses in pregnancy has been unclear.
Blood samples obtained before and 1 month after vaccination showed similarly high-titer postvaccination seropositivity rates against influenza B, H3N2, and H1N1 regardless of the women’s weight status. Indeed, the seropositivity rate against all three influenza viruses was higher in the obese subgroup, by a margin of 92%-74%. Also, postvaccination geometric mean antibody titers were significantly higher in the obese group. Particularly impressive was the difference in H1N1 seroconversion, defined as a fourfold increase in titer 28 days after vaccination: 79% versus 55%, noted Ms. Clarke of the University of Adelaide.
Of note, influenza vaccination in the first trimester resulted in a significantly lower seropositive antibody rate than vaccination in the second or third trimesters. The implication is that gestational age at vaccination, regardless of BMI, may be an important determinant of optimal vaccine protection for mothers and their newborns. However, this tentative conclusion requires confirmation in an independent larger sample, because the patient numbers in the study were small: Seropositive antibodies to all three vaccine antigens were documented in just 7 of 12 women (58%) vaccinated in the first trimester, compared with 47 of 53 (89%) vaccinated in the second trimester and 18 of 25 (72%) in the third.
Ms. Clarke reported having no financial conflicts regarding the study, which was supported by the Women’s and Children’s Hospital Research Foundation.
LJUBLJANA, SLOVENIA – ; indeed, it might actually improve their seroconversion rate, Michelle Clarke reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
She presented a prospective cohort study of 90 women vaccinated against influenza during pregnancy, 24 of whom had a BMI of 30 kg/m2 or more. The impetus for the study was the investigators’ understanding that influenza in pregnancy carries an increased risk of severe complications, obesity is a known risk factor for more severe episodes of influenza, and vaccine responses could potentially be adversely affected by obesity, either because of the associated inflammatory state and altered cytokine profile or inadequate vaccine delivery via the intramuscular route. Yet the impact of obesity on vaccine responses in pregnancy has been unclear.
Blood samples obtained before and 1 month after vaccination showed similarly high-titer postvaccination seropositivity rates against influenza B, H3N2, and H1N1 regardless of the women’s weight status. Indeed, the seropositivity rate against all three influenza viruses was higher in the obese subgroup, by a margin of 92%-74%. Also, postvaccination geometric mean antibody titers were significantly higher in the obese group. Particularly impressive was the difference in H1N1 seroconversion, defined as a fourfold increase in titer 28 days after vaccination: 79% versus 55%, noted Ms. Clarke of the University of Adelaide.
Of note, influenza vaccination in the first trimester resulted in a significantly lower seropositive antibody rate than vaccination in the second or third trimesters. The implication is that gestational age at vaccination, regardless of BMI, may be an important determinant of optimal vaccine protection for mothers and their newborns. However, this tentative conclusion requires confirmation in an independent larger sample, because the patient numbers in the study were small: Seropositive antibodies to all three vaccine antigens were documented in just 7 of 12 women (58%) vaccinated in the first trimester, compared with 47 of 53 (89%) vaccinated in the second trimester and 18 of 25 (72%) in the third.
Ms. Clarke reported having no financial conflicts regarding the study, which was supported by the Women’s and Children’s Hospital Research Foundation.
LJUBLJANA, SLOVENIA – ; indeed, it might actually improve their seroconversion rate, Michelle Clarke reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
She presented a prospective cohort study of 90 women vaccinated against influenza during pregnancy, 24 of whom had a BMI of 30 kg/m2 or more. The impetus for the study was the investigators’ understanding that influenza in pregnancy carries an increased risk of severe complications, obesity is a known risk factor for more severe episodes of influenza, and vaccine responses could potentially be adversely affected by obesity, either because of the associated inflammatory state and altered cytokine profile or inadequate vaccine delivery via the intramuscular route. Yet the impact of obesity on vaccine responses in pregnancy has been unclear.
Blood samples obtained before and 1 month after vaccination showed similarly high-titer postvaccination seropositivity rates against influenza B, H3N2, and H1N1 regardless of the women’s weight status. Indeed, the seropositivity rate against all three influenza viruses was higher in the obese subgroup, by a margin of 92%-74%. Also, postvaccination geometric mean antibody titers were significantly higher in the obese group. Particularly impressive was the difference in H1N1 seroconversion, defined as a fourfold increase in titer 28 days after vaccination: 79% versus 55%, noted Ms. Clarke of the University of Adelaide.
Of note, influenza vaccination in the first trimester resulted in a significantly lower seropositive antibody rate than vaccination in the second or third trimesters. The implication is that gestational age at vaccination, regardless of BMI, may be an important determinant of optimal vaccine protection for mothers and their newborns. However, this tentative conclusion requires confirmation in an independent larger sample, because the patient numbers in the study were small: Seropositive antibodies to all three vaccine antigens were documented in just 7 of 12 women (58%) vaccinated in the first trimester, compared with 47 of 53 (89%) vaccinated in the second trimester and 18 of 25 (72%) in the third.
Ms. Clarke reported having no financial conflicts regarding the study, which was supported by the Women’s and Children’s Hospital Research Foundation.
REPORTING FROM ESPID 2019
Key clinical point: High BMI doesn’t impair influenza vaccine responses in pregnant women.
Major finding: Protective antibody levels against all three vaccine antigens were documented 1 month post vaccination in 92% of the obese and 74% of the nonobese mothers.
Study details: This was a prospective observational study of 90 women vaccinated against influenza during pregnancy, 24 of whom were obese.
Disclosures: The study was supported by the University of Adelaide Women’s and Children’s Hospital Research Foundation.
New recommendations on TB screening for health care workers
U.S. health care personnel no longer need to undergo routine tuberculosis testing in the absence of known exposure, according to new screening guidelines from the National Tuberculosis Controllers Association and CDC.
The revised guidelines on tuberculosis screening, testing, and treatment of U.S. health care personnel, published in Morbidity and Mortality Weekly Report, are the first update since 2005. The new recommendations reflect a reduction in concern about U.S. health care personnel’s risk of occupational exposure to latent and active tuberculosis infection.
Lynn E. Sosa, MD, from the Connecticut Department of Public Health and National Tuberculosis Controllers Association, and coauthors wrote that rates of tuberculosis infection in the United States have declined by 73% since 1991, from 10.4/100,000 population in 1991 to 2.8/100,000 in 2017. This has been matched by similar declines among health care workers, which the authors said raised questions about the cost-effectiveness of the previously recommended routine serial occupational testing.
“In addition, a recent retrospective cohort study of approximately 40,000 health care personnel at a tertiary U.S. medical center in a low TB-incidence state found an extremely low rate of TST conversion (0.3%) during 1998-2014, with a limited proportion attributable to occupational exposure,” they wrote.
The new guidelines recommend health care personnel undergo baseline or preplacement tuberculosis testing with an interferon-gamma release assay (IGRA) or a tuberculin skin test (TST), as well as individual risk assessment and symptom evaluation.
The individual risk assessment considers whether the person has lived in a country with a high tuberculosis rate, whether they are immunosuppressed, or whether they have had close contact with someone with infectious tuberculosis.
This risk assessment can help decide how to interpret an initial positive test result, the authors said.
“For example, health care personnel with a positive test who are asymptomatic, unlikely to be infected with M. [Mycobacterium] tuberculosis, and at low risk for progression on the basis of their risk assessment should have a second test (either an IGRA or a TST) as recommended in the 2017 TB diagnostic guidelines of the American Thoracic Society, Infectious Diseases Society of America, and CDC,” they wrote. “In this example, the health care personnel should be considered infected with M. tuberculosis only if both the first and second tests are positive.”
After that baseline testing, personnel do not need to undergo routine serial testing except in the case of known exposure or ongoing transmission. The guideline authors suggested serial screening might be considered for health care workers whose work puts them at greater risk – for example, pulmonologists or respiratory therapists – or for those working in settings in which transmission has happened in the past.
For personnel with latent tuberculosis infection, the guidelines recommend “encouragement of treatment” unless it is contraindicated, and annual symptom screening in those not undergoing treatment.
The guideline committee also advocated for annual tuberculosis education for all health care workers.
The new recommendations were based on a systematic review of 36 studies of tuberculosis screening and testing among health care personnel, 16 of which were performed in the United States, and all but two of which were conducted in a hospital setting.
The authors stressed that recommendations from the 2005 CDC guidelines – which do not pertain to health care personnel screening, testing, treatment and education – remain unchanged.
One author declared personal fees from the National Tuberculosis Controllers Association during the conduct of the study. Two others reported unrelated grants and personal fees from private industry. No other conflicts of interest were disclosed.
SOURCE: Sosa L et al. MMWR. 2019;68:439-43.
U.S. health care personnel no longer need to undergo routine tuberculosis testing in the absence of known exposure, according to new screening guidelines from the National Tuberculosis Controllers Association and CDC.
The revised guidelines on tuberculosis screening, testing, and treatment of U.S. health care personnel, published in Morbidity and Mortality Weekly Report, are the first update since 2005. The new recommendations reflect a reduction in concern about U.S. health care personnel’s risk of occupational exposure to latent and active tuberculosis infection.
Lynn E. Sosa, MD, from the Connecticut Department of Public Health and National Tuberculosis Controllers Association, and coauthors wrote that rates of tuberculosis infection in the United States have declined by 73% since 1991, from 10.4/100,000 population in 1991 to 2.8/100,000 in 2017. This has been matched by similar declines among health care workers, which the authors said raised questions about the cost-effectiveness of the previously recommended routine serial occupational testing.
“In addition, a recent retrospective cohort study of approximately 40,000 health care personnel at a tertiary U.S. medical center in a low TB-incidence state found an extremely low rate of TST conversion (0.3%) during 1998-2014, with a limited proportion attributable to occupational exposure,” they wrote.
The new guidelines recommend health care personnel undergo baseline or preplacement tuberculosis testing with an interferon-gamma release assay (IGRA) or a tuberculin skin test (TST), as well as individual risk assessment and symptom evaluation.
The individual risk assessment considers whether the person has lived in a country with a high tuberculosis rate, whether they are immunosuppressed, or whether they have had close contact with someone with infectious tuberculosis.
This risk assessment can help decide how to interpret an initial positive test result, the authors said.
“For example, health care personnel with a positive test who are asymptomatic, unlikely to be infected with M. [Mycobacterium] tuberculosis, and at low risk for progression on the basis of their risk assessment should have a second test (either an IGRA or a TST) as recommended in the 2017 TB diagnostic guidelines of the American Thoracic Society, Infectious Diseases Society of America, and CDC,” they wrote. “In this example, the health care personnel should be considered infected with M. tuberculosis only if both the first and second tests are positive.”
After that baseline testing, personnel do not need to undergo routine serial testing except in the case of known exposure or ongoing transmission. The guideline authors suggested serial screening might be considered for health care workers whose work puts them at greater risk – for example, pulmonologists or respiratory therapists – or for those working in settings in which transmission has happened in the past.
For personnel with latent tuberculosis infection, the guidelines recommend “encouragement of treatment” unless it is contraindicated, and annual symptom screening in those not undergoing treatment.
The guideline committee also advocated for annual tuberculosis education for all health care workers.
The new recommendations were based on a systematic review of 36 studies of tuberculosis screening and testing among health care personnel, 16 of which were performed in the United States, and all but two of which were conducted in a hospital setting.
The authors stressed that recommendations from the 2005 CDC guidelines – which do not pertain to health care personnel screening, testing, treatment and education – remain unchanged.
One author declared personal fees from the National Tuberculosis Controllers Association during the conduct of the study. Two others reported unrelated grants and personal fees from private industry. No other conflicts of interest were disclosed.
SOURCE: Sosa L et al. MMWR. 2019;68:439-43.
U.S. health care personnel no longer need to undergo routine tuberculosis testing in the absence of known exposure, according to new screening guidelines from the National Tuberculosis Controllers Association and CDC.
The revised guidelines on tuberculosis screening, testing, and treatment of U.S. health care personnel, published in Morbidity and Mortality Weekly Report, are the first update since 2005. The new recommendations reflect a reduction in concern about U.S. health care personnel’s risk of occupational exposure to latent and active tuberculosis infection.
Lynn E. Sosa, MD, from the Connecticut Department of Public Health and National Tuberculosis Controllers Association, and coauthors wrote that rates of tuberculosis infection in the United States have declined by 73% since 1991, from 10.4/100,000 population in 1991 to 2.8/100,000 in 2017. This has been matched by similar declines among health care workers, which the authors said raised questions about the cost-effectiveness of the previously recommended routine serial occupational testing.
“In addition, a recent retrospective cohort study of approximately 40,000 health care personnel at a tertiary U.S. medical center in a low TB-incidence state found an extremely low rate of TST conversion (0.3%) during 1998-2014, with a limited proportion attributable to occupational exposure,” they wrote.
The new guidelines recommend health care personnel undergo baseline or preplacement tuberculosis testing with an interferon-gamma release assay (IGRA) or a tuberculin skin test (TST), as well as individual risk assessment and symptom evaluation.
The individual risk assessment considers whether the person has lived in a country with a high tuberculosis rate, whether they are immunosuppressed, or whether they have had close contact with someone with infectious tuberculosis.
This risk assessment can help decide how to interpret an initial positive test result, the authors said.
“For example, health care personnel with a positive test who are asymptomatic, unlikely to be infected with M. [Mycobacterium] tuberculosis, and at low risk for progression on the basis of their risk assessment should have a second test (either an IGRA or a TST) as recommended in the 2017 TB diagnostic guidelines of the American Thoracic Society, Infectious Diseases Society of America, and CDC,” they wrote. “In this example, the health care personnel should be considered infected with M. tuberculosis only if both the first and second tests are positive.”
After that baseline testing, personnel do not need to undergo routine serial testing except in the case of known exposure or ongoing transmission. The guideline authors suggested serial screening might be considered for health care workers whose work puts them at greater risk – for example, pulmonologists or respiratory therapists – or for those working in settings in which transmission has happened in the past.
For personnel with latent tuberculosis infection, the guidelines recommend “encouragement of treatment” unless it is contraindicated, and annual symptom screening in those not undergoing treatment.
The guideline committee also advocated for annual tuberculosis education for all health care workers.
The new recommendations were based on a systematic review of 36 studies of tuberculosis screening and testing among health care personnel, 16 of which were performed in the United States, and all but two of which were conducted in a hospital setting.
The authors stressed that recommendations from the 2005 CDC guidelines – which do not pertain to health care personnel screening, testing, treatment and education – remain unchanged.
One author declared personal fees from the National Tuberculosis Controllers Association during the conduct of the study. Two others reported unrelated grants and personal fees from private industry. No other conflicts of interest were disclosed.
SOURCE: Sosa L et al. MMWR. 2019;68:439-43.
FROM MMWR
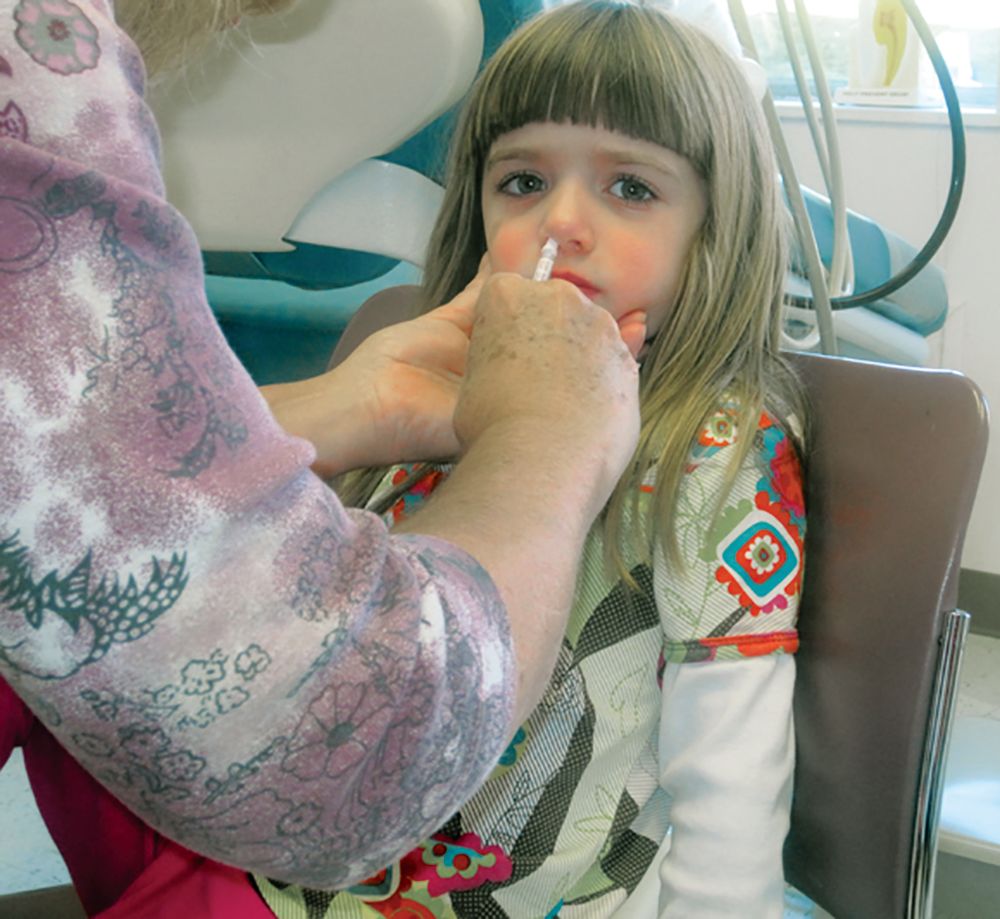
Young children with neuromuscular disease are vulnerable to respiratory viruses
This highlights the need for new vaccines
Influenza gets a lot of attention each winter, but respiratory syncytial virus (RSV) and other respiratory viruses have as much or more impact on pediatric populations, particularly certain high-risk groups. But currently there are no vaccines for noninfluenza respiratory viruses. That said, several are under development, for RSV and parainfluenza.
Which groups are likely to get the most benefit from these newer vaccines?
We all are aware of the extra vulnerability to respiratory viruses (RSV being the most frequent) in premature infants, those with chronic lung disease, or those with congenital heart syndromes; such vulnerable patients are not infrequently seen in routine practice. A recent report shined a brighter light on such a group.
Real-world data from a nationwide Canadian surveillance system (CARESS) was used to analyze relative risks of categories of young children who are thought to be vulnerable to respiratory viruses, with a particular focus on those with neuromuscular disease. The CARESS investigators analyzed 12 years’ data on respiratory hospitalizations from among palivizumab-prophylaxed patients (including specific data on RSV when patients were tested for RSV per standard of care).1 Unfortunately, RSV testing was not universal despite hospitalization, so the true incidence of RSV-specific hospitalizations was likely underestimated.
Nevertheless, more than 25,000 children from 2005 through 2017 were grouped into three categories of palivizumab-prophylaxed high-risk children: standard indications (SI), n = 20,335; chronic medical conditions (CMD), n = 4,063; and neuromuscular disease (NMD), n = 605. This study is notable for having a relatively large number of neuromuscular disease subjects. Two-thirds of each group were fully palivizumab adherent.
The SI group included the standard American Academy of Pediatrics–recommended groups, such as premature infants, congenital heart disease, etc.
The CMD group included conditions that lead clinicians to use palivizumab off label, such as cystic fibrosis, congenital airway anomalies, immunodeficiency, and pulmonary disorders.
The NMD participants were subdivided into two groups. Group 1 comprised general hypotonic neuromuscular diseases such as hypoxic-ischemic encephalopathy, Prader-Willi syndrome, chromosomal disorders, and migration/demyelinating diseases. Group 2 included more severe infantile neuromuscular disorders, such as spinal muscular atrophy, myotonic dystrophy, centronuclear and nemaline myopathy, mitochondrial and glycogen storage myopathies, or arthrogryposis.
Overall, 6.9% of CARESS RSV-prophylaxed subjects were hospitalized. About one in five hospitalized patients from each group was hospitalized more than once. Specific respiratory hospitalization rates for each group were 6% (n = 1,228) for SI subjects and 9.4% (n = 380) for CMD, compared with 19.2% (n = 116) for NMD subjects.
It is unclear what proportion underwent RSV testing, but a total of 334 were confirmed RSV positive: 261 were SI, 54 were CMD and 19 were NMD. The RSV-test-positive rate was 1.5% for SI, 1.6% for CMD and 3.3% for NMD; so while a higher number of SI children were RSV positive, the rate of RSV positivity was actually highest with NMD.
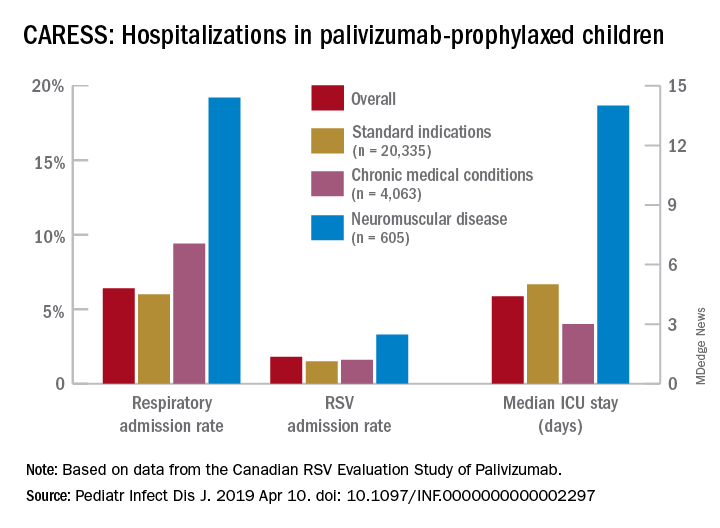
RSV-positive subjects needing ICU care among NMD patients also had longer ICU stays (median 14 days), compared with RSV-positive CMD or SI subjects (median 3 and 5 days, respectively). Further, hospitalized RSV-positive NMD subjects presented more frequently with pneumonia (42% vs. 30% for CMD and 20% for SI) while hospitalized RSV-positive SI subjects more often had apnea (17% vs. 10% for NMD and 5% for CMD, P less than .05).
These differences in the courses of NMD patients raise the question as to whether the NMD group was somehow different from the SI and CMD groups, other than muscular weakness that likely leads to less ability to clear secretions and a less efficient cough. It turns out that NMD children were older and had worse neonatal medical courses (longer hospital stays, more often ventilated, and used oxygen longer). It could be argued that these differences may have been in part due to the muscular weakness inherent in their underlying disease, but they appear to be predictors of worse respiratory infectious disease than other vulnerable populations as the NMD children get older.
Indeed, the overall risk of any respiratory admission among NMD subjects was nearly twice as high, compared with SI (hazard ratio, 1.90, P less than .0005); but the somewhat higher risk for NMD vs. CMD was not significant (HR, 1.33, P = .090). However, when looking specifically at RSV confirmed admissions, NMD had more than twice the hospitalization risk than either other group (HR, 2.26, P = .001 vs. SI; and HR, 2.74, P = .001 vs. CMD).
Further, an NMD subgroup analysis showed 1.69 times the overall respiratory hospitalization risk among the more severe vs. less severe NMD group, but a similar risk of RSV admission. The authors point out that one reason for this discrepancy may be a higher probability of aspiration causing hospitalization because of more dramatic acute events during respiratory infections in patients with more severe NMD. It also may be that palivizumab evened the playing field for RSV but not for other viruses such as parainfluenza, adenovirus, or even rhinovirus.
Nevertheless, these data tell us that risk of respiratory disease severe enough to need hospitalization continues to an older age in NMD than SI or CMD patients, well past 2 years of age. And the risk is not only from RSV. That said, RSV remains a player in some patients (particularly NMD patients) despite palivizumab prophylaxis, highlighting the need for RSV as well as parainfluenza vaccines. While these vaccines should help all young children, they seem likely to be even more beneficial for high-risk children including those with NMD, and particularly those with more severe NMD.
Eleven among 60 total candidate RSV vaccines (live attenuated, particle based, or vector based) are currently in clinical trials.2 Fewer parainfluenza vaccines are in the pipeline, but clinical trials also are underway.3-5 Approval of such vaccines is not expected until the mid-2020s, so at present we are left with providing palivizumab to our vulnerable patients while emphasizing nonmedical strategies that may help prevent respiratory viruses. These only partially successful preventive interventions include breastfeeding, avoiding secondhand smoke, and avoiding known high-risk exposures, such as large day care centers.
My hope is for quicker than projected progress on the vaccine front so that winter admissions for respiratory viruses might decrease in numbers similar to the decrease we have noted with another vaccine successful against a seasonally active pathogen – rotavirus.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Pediatr Infect Dis J. 2019 Apr 10. doi: 10.1097/INF.0000000000002297.
2. “Advances in RSV Vaccine Research and Development – A Global Agenda.”
3. J Pediatric Infect Dis Soc. 2015 Dec;4(4): e143-6.
4. J Virol. 2015 Oct;89(20):10319-32.
5. Vaccine. 2017 Dec 18;35(51):7139-46.
This highlights the need for new vaccines
This highlights the need for new vaccines
Influenza gets a lot of attention each winter, but respiratory syncytial virus (RSV) and other respiratory viruses have as much or more impact on pediatric populations, particularly certain high-risk groups. But currently there are no vaccines for noninfluenza respiratory viruses. That said, several are under development, for RSV and parainfluenza.
Which groups are likely to get the most benefit from these newer vaccines?
We all are aware of the extra vulnerability to respiratory viruses (RSV being the most frequent) in premature infants, those with chronic lung disease, or those with congenital heart syndromes; such vulnerable patients are not infrequently seen in routine practice. A recent report shined a brighter light on such a group.
Real-world data from a nationwide Canadian surveillance system (CARESS) was used to analyze relative risks of categories of young children who are thought to be vulnerable to respiratory viruses, with a particular focus on those with neuromuscular disease. The CARESS investigators analyzed 12 years’ data on respiratory hospitalizations from among palivizumab-prophylaxed patients (including specific data on RSV when patients were tested for RSV per standard of care).1 Unfortunately, RSV testing was not universal despite hospitalization, so the true incidence of RSV-specific hospitalizations was likely underestimated.
Nevertheless, more than 25,000 children from 2005 through 2017 were grouped into three categories of palivizumab-prophylaxed high-risk children: standard indications (SI), n = 20,335; chronic medical conditions (CMD), n = 4,063; and neuromuscular disease (NMD), n = 605. This study is notable for having a relatively large number of neuromuscular disease subjects. Two-thirds of each group were fully palivizumab adherent.
The SI group included the standard American Academy of Pediatrics–recommended groups, such as premature infants, congenital heart disease, etc.
The CMD group included conditions that lead clinicians to use palivizumab off label, such as cystic fibrosis, congenital airway anomalies, immunodeficiency, and pulmonary disorders.
The NMD participants were subdivided into two groups. Group 1 comprised general hypotonic neuromuscular diseases such as hypoxic-ischemic encephalopathy, Prader-Willi syndrome, chromosomal disorders, and migration/demyelinating diseases. Group 2 included more severe infantile neuromuscular disorders, such as spinal muscular atrophy, myotonic dystrophy, centronuclear and nemaline myopathy, mitochondrial and glycogen storage myopathies, or arthrogryposis.
Overall, 6.9% of CARESS RSV-prophylaxed subjects were hospitalized. About one in five hospitalized patients from each group was hospitalized more than once. Specific respiratory hospitalization rates for each group were 6% (n = 1,228) for SI subjects and 9.4% (n = 380) for CMD, compared with 19.2% (n = 116) for NMD subjects.
It is unclear what proportion underwent RSV testing, but a total of 334 were confirmed RSV positive: 261 were SI, 54 were CMD and 19 were NMD. The RSV-test-positive rate was 1.5% for SI, 1.6% for CMD and 3.3% for NMD; so while a higher number of SI children were RSV positive, the rate of RSV positivity was actually highest with NMD.

RSV-positive subjects needing ICU care among NMD patients also had longer ICU stays (median 14 days), compared with RSV-positive CMD or SI subjects (median 3 and 5 days, respectively). Further, hospitalized RSV-positive NMD subjects presented more frequently with pneumonia (42% vs. 30% for CMD and 20% for SI) while hospitalized RSV-positive SI subjects more often had apnea (17% vs. 10% for NMD and 5% for CMD, P less than .05).
These differences in the courses of NMD patients raise the question as to whether the NMD group was somehow different from the SI and CMD groups, other than muscular weakness that likely leads to less ability to clear secretions and a less efficient cough. It turns out that NMD children were older and had worse neonatal medical courses (longer hospital stays, more often ventilated, and used oxygen longer). It could be argued that these differences may have been in part due to the muscular weakness inherent in their underlying disease, but they appear to be predictors of worse respiratory infectious disease than other vulnerable populations as the NMD children get older.
Indeed, the overall risk of any respiratory admission among NMD subjects was nearly twice as high, compared with SI (hazard ratio, 1.90, P less than .0005); but the somewhat higher risk for NMD vs. CMD was not significant (HR, 1.33, P = .090). However, when looking specifically at RSV confirmed admissions, NMD had more than twice the hospitalization risk than either other group (HR, 2.26, P = .001 vs. SI; and HR, 2.74, P = .001 vs. CMD).
Further, an NMD subgroup analysis showed 1.69 times the overall respiratory hospitalization risk among the more severe vs. less severe NMD group, but a similar risk of RSV admission. The authors point out that one reason for this discrepancy may be a higher probability of aspiration causing hospitalization because of more dramatic acute events during respiratory infections in patients with more severe NMD. It also may be that palivizumab evened the playing field for RSV but not for other viruses such as parainfluenza, adenovirus, or even rhinovirus.
Nevertheless, these data tell us that risk of respiratory disease severe enough to need hospitalization continues to an older age in NMD than SI or CMD patients, well past 2 years of age. And the risk is not only from RSV. That said, RSV remains a player in some patients (particularly NMD patients) despite palivizumab prophylaxis, highlighting the need for RSV as well as parainfluenza vaccines. While these vaccines should help all young children, they seem likely to be even more beneficial for high-risk children including those with NMD, and particularly those with more severe NMD.
Eleven among 60 total candidate RSV vaccines (live attenuated, particle based, or vector based) are currently in clinical trials.2 Fewer parainfluenza vaccines are in the pipeline, but clinical trials also are underway.3-5 Approval of such vaccines is not expected until the mid-2020s, so at present we are left with providing palivizumab to our vulnerable patients while emphasizing nonmedical strategies that may help prevent respiratory viruses. These only partially successful preventive interventions include breastfeeding, avoiding secondhand smoke, and avoiding known high-risk exposures, such as large day care centers.
My hope is for quicker than projected progress on the vaccine front so that winter admissions for respiratory viruses might decrease in numbers similar to the decrease we have noted with another vaccine successful against a seasonally active pathogen – rotavirus.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Pediatr Infect Dis J. 2019 Apr 10. doi: 10.1097/INF.0000000000002297.
2. “Advances in RSV Vaccine Research and Development – A Global Agenda.”
3. J Pediatric Infect Dis Soc. 2015 Dec;4(4): e143-6.
4. J Virol. 2015 Oct;89(20):10319-32.
5. Vaccine. 2017 Dec 18;35(51):7139-46.
Influenza gets a lot of attention each winter, but respiratory syncytial virus (RSV) and other respiratory viruses have as much or more impact on pediatric populations, particularly certain high-risk groups. But currently there are no vaccines for noninfluenza respiratory viruses. That said, several are under development, for RSV and parainfluenza.
Which groups are likely to get the most benefit from these newer vaccines?
We all are aware of the extra vulnerability to respiratory viruses (RSV being the most frequent) in premature infants, those with chronic lung disease, or those with congenital heart syndromes; such vulnerable patients are not infrequently seen in routine practice. A recent report shined a brighter light on such a group.
Real-world data from a nationwide Canadian surveillance system (CARESS) was used to analyze relative risks of categories of young children who are thought to be vulnerable to respiratory viruses, with a particular focus on those with neuromuscular disease. The CARESS investigators analyzed 12 years’ data on respiratory hospitalizations from among palivizumab-prophylaxed patients (including specific data on RSV when patients were tested for RSV per standard of care).1 Unfortunately, RSV testing was not universal despite hospitalization, so the true incidence of RSV-specific hospitalizations was likely underestimated.
Nevertheless, more than 25,000 children from 2005 through 2017 were grouped into three categories of palivizumab-prophylaxed high-risk children: standard indications (SI), n = 20,335; chronic medical conditions (CMD), n = 4,063; and neuromuscular disease (NMD), n = 605. This study is notable for having a relatively large number of neuromuscular disease subjects. Two-thirds of each group were fully palivizumab adherent.
The SI group included the standard American Academy of Pediatrics–recommended groups, such as premature infants, congenital heart disease, etc.
The CMD group included conditions that lead clinicians to use palivizumab off label, such as cystic fibrosis, congenital airway anomalies, immunodeficiency, and pulmonary disorders.
The NMD participants were subdivided into two groups. Group 1 comprised general hypotonic neuromuscular diseases such as hypoxic-ischemic encephalopathy, Prader-Willi syndrome, chromosomal disorders, and migration/demyelinating diseases. Group 2 included more severe infantile neuromuscular disorders, such as spinal muscular atrophy, myotonic dystrophy, centronuclear and nemaline myopathy, mitochondrial and glycogen storage myopathies, or arthrogryposis.
Overall, 6.9% of CARESS RSV-prophylaxed subjects were hospitalized. About one in five hospitalized patients from each group was hospitalized more than once. Specific respiratory hospitalization rates for each group were 6% (n = 1,228) for SI subjects and 9.4% (n = 380) for CMD, compared with 19.2% (n = 116) for NMD subjects.
It is unclear what proportion underwent RSV testing, but a total of 334 were confirmed RSV positive: 261 were SI, 54 were CMD and 19 were NMD. The RSV-test-positive rate was 1.5% for SI, 1.6% for CMD and 3.3% for NMD; so while a higher number of SI children were RSV positive, the rate of RSV positivity was actually highest with NMD.

RSV-positive subjects needing ICU care among NMD patients also had longer ICU stays (median 14 days), compared with RSV-positive CMD or SI subjects (median 3 and 5 days, respectively). Further, hospitalized RSV-positive NMD subjects presented more frequently with pneumonia (42% vs. 30% for CMD and 20% for SI) while hospitalized RSV-positive SI subjects more often had apnea (17% vs. 10% for NMD and 5% for CMD, P less than .05).
These differences in the courses of NMD patients raise the question as to whether the NMD group was somehow different from the SI and CMD groups, other than muscular weakness that likely leads to less ability to clear secretions and a less efficient cough. It turns out that NMD children were older and had worse neonatal medical courses (longer hospital stays, more often ventilated, and used oxygen longer). It could be argued that these differences may have been in part due to the muscular weakness inherent in their underlying disease, but they appear to be predictors of worse respiratory infectious disease than other vulnerable populations as the NMD children get older.
Indeed, the overall risk of any respiratory admission among NMD subjects was nearly twice as high, compared with SI (hazard ratio, 1.90, P less than .0005); but the somewhat higher risk for NMD vs. CMD was not significant (HR, 1.33, P = .090). However, when looking specifically at RSV confirmed admissions, NMD had more than twice the hospitalization risk than either other group (HR, 2.26, P = .001 vs. SI; and HR, 2.74, P = .001 vs. CMD).
Further, an NMD subgroup analysis showed 1.69 times the overall respiratory hospitalization risk among the more severe vs. less severe NMD group, but a similar risk of RSV admission. The authors point out that one reason for this discrepancy may be a higher probability of aspiration causing hospitalization because of more dramatic acute events during respiratory infections in patients with more severe NMD. It also may be that palivizumab evened the playing field for RSV but not for other viruses such as parainfluenza, adenovirus, or even rhinovirus.
Nevertheless, these data tell us that risk of respiratory disease severe enough to need hospitalization continues to an older age in NMD than SI or CMD patients, well past 2 years of age. And the risk is not only from RSV. That said, RSV remains a player in some patients (particularly NMD patients) despite palivizumab prophylaxis, highlighting the need for RSV as well as parainfluenza vaccines. While these vaccines should help all young children, they seem likely to be even more beneficial for high-risk children including those with NMD, and particularly those with more severe NMD.
Eleven among 60 total candidate RSV vaccines (live attenuated, particle based, or vector based) are currently in clinical trials.2 Fewer parainfluenza vaccines are in the pipeline, but clinical trials also are underway.3-5 Approval of such vaccines is not expected until the mid-2020s, so at present we are left with providing palivizumab to our vulnerable patients while emphasizing nonmedical strategies that may help prevent respiratory viruses. These only partially successful preventive interventions include breastfeeding, avoiding secondhand smoke, and avoiding known high-risk exposures, such as large day care centers.
My hope is for quicker than projected progress on the vaccine front so that winter admissions for respiratory viruses might decrease in numbers similar to the decrease we have noted with another vaccine successful against a seasonally active pathogen – rotavirus.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Pediatr Infect Dis J. 2019 Apr 10. doi: 10.1097/INF.0000000000002297.
2. “Advances in RSV Vaccine Research and Development – A Global Agenda.”
3. J Pediatric Infect Dis Soc. 2015 Dec;4(4): e143-6.
4. J Virol. 2015 Oct;89(20):10319-32.
5. Vaccine. 2017 Dec 18;35(51):7139-46.
Most measles cases in 25 years prompts government pleas to vaccinate
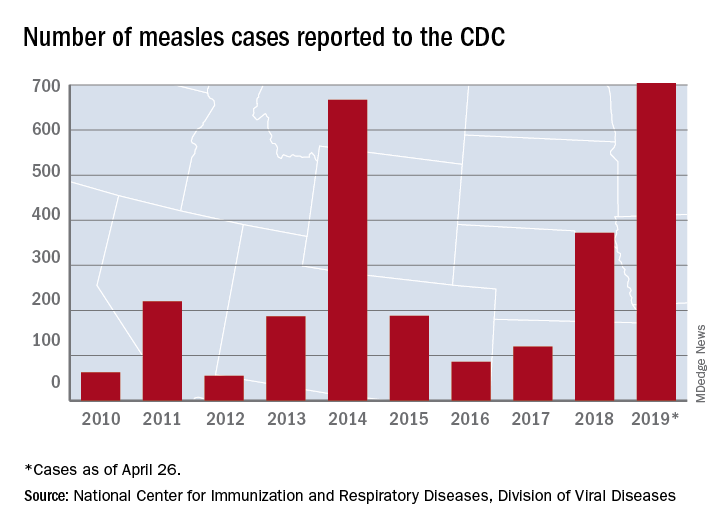
The updated figure adds 9 cases to the previous tally of 695 cases as of April 24, when the CDC announced that the number of cases in 2019 had surpassed the total for any year since the disease was considered effectively eliminated from the country in 2000.
Cases have been reported in 22 states, with the largest outbreaks in Washington and New York. The outbreak in Washington, which included 72 cases, was declared over last week. Two outbreaks in New York, however, are the largest and longest-lasting measles outbreaks since the disease was considered eliminated, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases. The longer they continue, the “greater the chance that measles will again gain a foothold in the United States,” she said at CDC telebriefing on measles.
The outbreaks are linked to travelers who are exposed to measles abroad and bring it to the United States. The disease then may spread, especially in communities with high rates of unvaccinated people. “A significant factor contributing to the outbreaks in New York is misinformation in the communities about the safety of the measles/mumps/rubella vaccine,” according to the CDC.
National Infant Immunization Week
Until last week, 2014 – with 667 measles cases – had been the year with the most cases since the disease was effectively eliminated. The last time the United States had more measles cases was in 1994, when there were 963 cases for the year.
Health and Human Services Secretary Alex Azar, also at the telebriefing, pointed out that 1994 also was the year that the United States first observed National Infant Immunization Week, which is April 27–May 4 this year. The CDC is marking the 25th anniversary of the annual observance, which highlights “the importance of protecting infants from vaccine-preventable diseases” and celebrates “the achievements of immunization programs in promoting healthy communities,” Secretary Azar said.
Message to health care providers
CDC director Robert Redfield Jr., MD, noted that measles has “no treatment, no cure, and no way to predict how bad a case will be.”
Some patients may have mild symptoms, whereas others may have serious complications such as pneumonia or encephalitis. In 2019, 3% of the patients with measles have developed pneumonia, he said. No patients have died.
Dr. Redfield, a virologist, noted that the CDC is recommending that children aged 6-12 months receive 1 dose of the measles vaccine if traveling abroad.
“As CDC director and as a physician, I have and continue to wholeheartedly advocate for infant immunization,” he said in a statement. “More importantly, as a father and grandfather I have ensured all of my children and grandchildren are vaccinated on the recommended schedule. Vaccines are safe. Vaccines do not cause autism. Vaccine-preventable diseases are dangerous.”
More than 94% of parents vaccinate their children, Dr. Redfield added. “CDC is working to reach the small percentage of vaccine-hesitant individuals so they too understand the importance of vaccines. It is imperative that we correct misinformation and reassure fearful parents so they protect their children from illnesses with long-lasting health impacts.”
About 1.3%, or 100,000 children, in the United States under 2 years old have not been vaccinated, he said.
“I call upon health care providers to encourage parents and expectant parents to vaccinate their children for their own protection and to avoid the spread of vaccine-preventable diseases within their families and communities,” he said. “We must join together as a nation to once again eliminate measles and prevent future disease outbreaks.”
The CDC has a complete list of clinical recommendations for health care providers on its website.
The president weighs in
President Donald Trump said that children should receive vaccinations – his first public comment about vaccines since his inauguration. Previously, he had questioned the safety of vaccines.
Asked by reporters about the measles outbreaks and his message for parents about having their kids vaccinated, he said: “They have to get the shot. The vaccinations are so important. This is really going around now. They have to get their shots.”

The updated figure adds 9 cases to the previous tally of 695 cases as of April 24, when the CDC announced that the number of cases in 2019 had surpassed the total for any year since the disease was considered effectively eliminated from the country in 2000.
Cases have been reported in 22 states, with the largest outbreaks in Washington and New York. The outbreak in Washington, which included 72 cases, was declared over last week. Two outbreaks in New York, however, are the largest and longest-lasting measles outbreaks since the disease was considered eliminated, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases. The longer they continue, the “greater the chance that measles will again gain a foothold in the United States,” she said at CDC telebriefing on measles.
The outbreaks are linked to travelers who are exposed to measles abroad and bring it to the United States. The disease then may spread, especially in communities with high rates of unvaccinated people. “A significant factor contributing to the outbreaks in New York is misinformation in the communities about the safety of the measles/mumps/rubella vaccine,” according to the CDC.
National Infant Immunization Week
Until last week, 2014 – with 667 measles cases – had been the year with the most cases since the disease was effectively eliminated. The last time the United States had more measles cases was in 1994, when there were 963 cases for the year.
Health and Human Services Secretary Alex Azar, also at the telebriefing, pointed out that 1994 also was the year that the United States first observed National Infant Immunization Week, which is April 27–May 4 this year. The CDC is marking the 25th anniversary of the annual observance, which highlights “the importance of protecting infants from vaccine-preventable diseases” and celebrates “the achievements of immunization programs in promoting healthy communities,” Secretary Azar said.
Message to health care providers
CDC director Robert Redfield Jr., MD, noted that measles has “no treatment, no cure, and no way to predict how bad a case will be.”
Some patients may have mild symptoms, whereas others may have serious complications such as pneumonia or encephalitis. In 2019, 3% of the patients with measles have developed pneumonia, he said. No patients have died.
Dr. Redfield, a virologist, noted that the CDC is recommending that children aged 6-12 months receive 1 dose of the measles vaccine if traveling abroad.
“As CDC director and as a physician, I have and continue to wholeheartedly advocate for infant immunization,” he said in a statement. “More importantly, as a father and grandfather I have ensured all of my children and grandchildren are vaccinated on the recommended schedule. Vaccines are safe. Vaccines do not cause autism. Vaccine-preventable diseases are dangerous.”
More than 94% of parents vaccinate their children, Dr. Redfield added. “CDC is working to reach the small percentage of vaccine-hesitant individuals so they too understand the importance of vaccines. It is imperative that we correct misinformation and reassure fearful parents so they protect their children from illnesses with long-lasting health impacts.”
About 1.3%, or 100,000 children, in the United States under 2 years old have not been vaccinated, he said.
“I call upon health care providers to encourage parents and expectant parents to vaccinate their children for their own protection and to avoid the spread of vaccine-preventable diseases within their families and communities,” he said. “We must join together as a nation to once again eliminate measles and prevent future disease outbreaks.”
The CDC has a complete list of clinical recommendations for health care providers on its website.
The president weighs in
President Donald Trump said that children should receive vaccinations – his first public comment about vaccines since his inauguration. Previously, he had questioned the safety of vaccines.
Asked by reporters about the measles outbreaks and his message for parents about having their kids vaccinated, he said: “They have to get the shot. The vaccinations are so important. This is really going around now. They have to get their shots.”

The updated figure adds 9 cases to the previous tally of 695 cases as of April 24, when the CDC announced that the number of cases in 2019 had surpassed the total for any year since the disease was considered effectively eliminated from the country in 2000.
Cases have been reported in 22 states, with the largest outbreaks in Washington and New York. The outbreak in Washington, which included 72 cases, was declared over last week. Two outbreaks in New York, however, are the largest and longest-lasting measles outbreaks since the disease was considered eliminated, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases. The longer they continue, the “greater the chance that measles will again gain a foothold in the United States,” she said at CDC telebriefing on measles.
The outbreaks are linked to travelers who are exposed to measles abroad and bring it to the United States. The disease then may spread, especially in communities with high rates of unvaccinated people. “A significant factor contributing to the outbreaks in New York is misinformation in the communities about the safety of the measles/mumps/rubella vaccine,” according to the CDC.
National Infant Immunization Week
Until last week, 2014 – with 667 measles cases – had been the year with the most cases since the disease was effectively eliminated. The last time the United States had more measles cases was in 1994, when there were 963 cases for the year.
Health and Human Services Secretary Alex Azar, also at the telebriefing, pointed out that 1994 also was the year that the United States first observed National Infant Immunization Week, which is April 27–May 4 this year. The CDC is marking the 25th anniversary of the annual observance, which highlights “the importance of protecting infants from vaccine-preventable diseases” and celebrates “the achievements of immunization programs in promoting healthy communities,” Secretary Azar said.
Message to health care providers
CDC director Robert Redfield Jr., MD, noted that measles has “no treatment, no cure, and no way to predict how bad a case will be.”
Some patients may have mild symptoms, whereas others may have serious complications such as pneumonia or encephalitis. In 2019, 3% of the patients with measles have developed pneumonia, he said. No patients have died.
Dr. Redfield, a virologist, noted that the CDC is recommending that children aged 6-12 months receive 1 dose of the measles vaccine if traveling abroad.
“As CDC director and as a physician, I have and continue to wholeheartedly advocate for infant immunization,” he said in a statement. “More importantly, as a father and grandfather I have ensured all of my children and grandchildren are vaccinated on the recommended schedule. Vaccines are safe. Vaccines do not cause autism. Vaccine-preventable diseases are dangerous.”
More than 94% of parents vaccinate their children, Dr. Redfield added. “CDC is working to reach the small percentage of vaccine-hesitant individuals so they too understand the importance of vaccines. It is imperative that we correct misinformation and reassure fearful parents so they protect their children from illnesses with long-lasting health impacts.”
About 1.3%, or 100,000 children, in the United States under 2 years old have not been vaccinated, he said.
“I call upon health care providers to encourage parents and expectant parents to vaccinate their children for their own protection and to avoid the spread of vaccine-preventable diseases within their families and communities,” he said. “We must join together as a nation to once again eliminate measles and prevent future disease outbreaks.”
The CDC has a complete list of clinical recommendations for health care providers on its website.
The president weighs in
President Donald Trump said that children should receive vaccinations – his first public comment about vaccines since his inauguration. Previously, he had questioned the safety of vaccines.
Asked by reporters about the measles outbreaks and his message for parents about having their kids vaccinated, he said: “They have to get the shot. The vaccinations are so important. This is really going around now. They have to get their shots.”
FROM A CDC TELEBRIEFING
Combo respiratory pathogen tests miss pertussis
BALTIMORE – Ann Arbor.
Respiratory pathogen panels are popular because they test for many things at once, but providers have to know their limits, said lead investigator Colleen Mayhew, MD, a pediatric emergency medicine fellow at the University of Michigan.
“Should RPAN be used to diagnosis pertussis? No,” she said at the Pediatric Academic Societies annual meeting. RPAN was negative for confirmed pertussis 44% of the time in the study.
“In our cohort, [it] was no better than a coin flip for detecting pertussis,” she said. Also, even when it missed pertussis, it still detected other pathogens, which raises the risk that symptoms might be attributed to a different infection. “This has serious public health implications.”
“The bottom line is, if you are concerned about pertussis, it’s important to use a dedicated pertussis PCR [polymerase chain reaction] assay, and to use comprehensive respiratory pathogen testing only if there are other, specific targets that will change your clinical management,” such as mycoplasma or the flu, Dr. Mayhew said.
In the study, 102 nasopharyngeal swabs positive for pertussis on standalone PCR testing – the university uses an assay from Focus Diagnostics – were thawed and tested with RPAN.
RPAN was negative for pertussis on 45 swabs (44%). “These are the potential missed pertussis cases if RPAN is used alone,” Dr. Mayhew said. RPAN detected other pathogens, such as coronavirus, about half the time, whether or not it tested positive for pertussis. “Those additional pathogens might represent coinfection, but might also represent asymptomatic carriage.” It’s impossible to differentiate between the two, she noted.
In short, “neither positive testing for other respiratory pathogens, nor negative testing for pertussis by RPAN, is reliable for excluding the diagnosis of pertussis. Dedicated pertussis PCR testing should be used for diagnosis,” she and her team concluded.
RPAN also is a PCR test, but with a different, perhaps less robust, genetic target.
The 102 positive swabs were from patients aged 1 month to 73 years, so “it’s important for all of us to keep pertussis on our differential diagnose” no matter how old patients are, Dr. Mayhew said.
Freezing and thawing the swabs shouldn’t have degraded the genetic material, but it might have; that was one of the limits of the study.
The team hopes to run a quality improvement project to encourage the use of standalone pertussis PCR in Ann Arbor.
There was no industry funding. Dr. Mayhew didn’t report any disclosures.
BALTIMORE – Ann Arbor.
Respiratory pathogen panels are popular because they test for many things at once, but providers have to know their limits, said lead investigator Colleen Mayhew, MD, a pediatric emergency medicine fellow at the University of Michigan.
“Should RPAN be used to diagnosis pertussis? No,” she said at the Pediatric Academic Societies annual meeting. RPAN was negative for confirmed pertussis 44% of the time in the study.
“In our cohort, [it] was no better than a coin flip for detecting pertussis,” she said. Also, even when it missed pertussis, it still detected other pathogens, which raises the risk that symptoms might be attributed to a different infection. “This has serious public health implications.”
“The bottom line is, if you are concerned about pertussis, it’s important to use a dedicated pertussis PCR [polymerase chain reaction] assay, and to use comprehensive respiratory pathogen testing only if there are other, specific targets that will change your clinical management,” such as mycoplasma or the flu, Dr. Mayhew said.
In the study, 102 nasopharyngeal swabs positive for pertussis on standalone PCR testing – the university uses an assay from Focus Diagnostics – were thawed and tested with RPAN.
RPAN was negative for pertussis on 45 swabs (44%). “These are the potential missed pertussis cases if RPAN is used alone,” Dr. Mayhew said. RPAN detected other pathogens, such as coronavirus, about half the time, whether or not it tested positive for pertussis. “Those additional pathogens might represent coinfection, but might also represent asymptomatic carriage.” It’s impossible to differentiate between the two, she noted.
In short, “neither positive testing for other respiratory pathogens, nor negative testing for pertussis by RPAN, is reliable for excluding the diagnosis of pertussis. Dedicated pertussis PCR testing should be used for diagnosis,” she and her team concluded.
RPAN also is a PCR test, but with a different, perhaps less robust, genetic target.
The 102 positive swabs were from patients aged 1 month to 73 years, so “it’s important for all of us to keep pertussis on our differential diagnose” no matter how old patients are, Dr. Mayhew said.
Freezing and thawing the swabs shouldn’t have degraded the genetic material, but it might have; that was one of the limits of the study.
The team hopes to run a quality improvement project to encourage the use of standalone pertussis PCR in Ann Arbor.
There was no industry funding. Dr. Mayhew didn’t report any disclosures.
BALTIMORE – Ann Arbor.
Respiratory pathogen panels are popular because they test for many things at once, but providers have to know their limits, said lead investigator Colleen Mayhew, MD, a pediatric emergency medicine fellow at the University of Michigan.
“Should RPAN be used to diagnosis pertussis? No,” she said at the Pediatric Academic Societies annual meeting. RPAN was negative for confirmed pertussis 44% of the time in the study.
“In our cohort, [it] was no better than a coin flip for detecting pertussis,” she said. Also, even when it missed pertussis, it still detected other pathogens, which raises the risk that symptoms might be attributed to a different infection. “This has serious public health implications.”
“The bottom line is, if you are concerned about pertussis, it’s important to use a dedicated pertussis PCR [polymerase chain reaction] assay, and to use comprehensive respiratory pathogen testing only if there are other, specific targets that will change your clinical management,” such as mycoplasma or the flu, Dr. Mayhew said.
In the study, 102 nasopharyngeal swabs positive for pertussis on standalone PCR testing – the university uses an assay from Focus Diagnostics – were thawed and tested with RPAN.
RPAN was negative for pertussis on 45 swabs (44%). “These are the potential missed pertussis cases if RPAN is used alone,” Dr. Mayhew said. RPAN detected other pathogens, such as coronavirus, about half the time, whether or not it tested positive for pertussis. “Those additional pathogens might represent coinfection, but might also represent asymptomatic carriage.” It’s impossible to differentiate between the two, she noted.
In short, “neither positive testing for other respiratory pathogens, nor negative testing for pertussis by RPAN, is reliable for excluding the diagnosis of pertussis. Dedicated pertussis PCR testing should be used for diagnosis,” she and her team concluded.
RPAN also is a PCR test, but with a different, perhaps less robust, genetic target.
The 102 positive swabs were from patients aged 1 month to 73 years, so “it’s important for all of us to keep pertussis on our differential diagnose” no matter how old patients are, Dr. Mayhew said.
Freezing and thawing the swabs shouldn’t have degraded the genetic material, but it might have; that was one of the limits of the study.
The team hopes to run a quality improvement project to encourage the use of standalone pertussis PCR in Ann Arbor.
There was no industry funding. Dr. Mayhew didn’t report any disclosures.
REPORTING FROM PAS 2019
Filamentous bacteriophage linked to lung infections in patients with cystic fibrosis
according to a study of the prevalence and clinical relevance of Pf phage in two patient cohorts.
“Our data from both the Stanford and Danish CF cohorts together suggest that either patients with CF acquire Pf phage–producing strains of P. aeruginosa or the Pf phage–negative P. aeruginosa become infected with Pf phage as patients age and their disease progresses,” wrote Elizabeth B. Burgener, MD, of Stanford (Calif.) University, and her coauthors. The study was published in Science Translational Medicine.The study analyzed a previous Danish longitudinal cohort of 34 patients and a prospective cross-sectional cohort of 76 patients at Stanford, 58 of which had P. aeruginosa. The researchers also reviewed a collection of genetic sequences called the Pseudomonas Genome Database to determine the prevalence of Pf phage, finding evidence in 1,159 of 2,226 P. aeruginosa sequences (52.1%).
In the Danish cohort, 21 of the 34 CF patients (61.8%; 95% confidence interval, 43.6%-77.8%) had at least one P. aeruginosa isolate containing Pf phage; 9 (26.5%) of the patients were found to be consistently positive for Pf phage. Those who were consistently positive were also older than those who never had Pf phage detected (19.1 years vs. 13.9 years; P = .046), suggesting that “there may be a tendency for P. aeruginosa strains that produce Pf phage to dominate in the sputum of individual patients with CF over time.”
In the Stanford cohort, the prevalence of Pf phage was 36.2% (21 of 58; 95% CI, 24.0%-49.9%) in patients with P. aeruginosa infection and 27.6% (21 of 76; 95% CI, 18.0%-39.1%) in all patients. No Pf phage was detected in any P. aeruginosa–negative samples. Patients positive for Pf phage in this cohort were also older than patients who were negative.
The authors acknowledged their study’s limitations, describing the methods used to collect and sequence the analyzed samples as “highly heterogeneous.” In addition, the two cohorts were CF specific while the Genome Database is not. Finally, the CF cohorts only had a single dominant strain sampled, though multiple P. aeruginosa lineages are often present in CF patients.
One author reported receiving grants from the Cystic Fibrosis Foundation, clinical trial support, and consulting fees from industry. Two other authors are inventors on a patent application that covers the development of a vaccine that targets Pf phage. The others reported no conflicts of interest.
SOURCE: Burgener EB et al. Sci Transl Med. 2019 Apr 17. doi: 10.1126/scitranslmed.aau9748.
according to a study of the prevalence and clinical relevance of Pf phage in two patient cohorts.
“Our data from both the Stanford and Danish CF cohorts together suggest that either patients with CF acquire Pf phage–producing strains of P. aeruginosa or the Pf phage–negative P. aeruginosa become infected with Pf phage as patients age and their disease progresses,” wrote Elizabeth B. Burgener, MD, of Stanford (Calif.) University, and her coauthors. The study was published in Science Translational Medicine.The study analyzed a previous Danish longitudinal cohort of 34 patients and a prospective cross-sectional cohort of 76 patients at Stanford, 58 of which had P. aeruginosa. The researchers also reviewed a collection of genetic sequences called the Pseudomonas Genome Database to determine the prevalence of Pf phage, finding evidence in 1,159 of 2,226 P. aeruginosa sequences (52.1%).
In the Danish cohort, 21 of the 34 CF patients (61.8%; 95% confidence interval, 43.6%-77.8%) had at least one P. aeruginosa isolate containing Pf phage; 9 (26.5%) of the patients were found to be consistently positive for Pf phage. Those who were consistently positive were also older than those who never had Pf phage detected (19.1 years vs. 13.9 years; P = .046), suggesting that “there may be a tendency for P. aeruginosa strains that produce Pf phage to dominate in the sputum of individual patients with CF over time.”
In the Stanford cohort, the prevalence of Pf phage was 36.2% (21 of 58; 95% CI, 24.0%-49.9%) in patients with P. aeruginosa infection and 27.6% (21 of 76; 95% CI, 18.0%-39.1%) in all patients. No Pf phage was detected in any P. aeruginosa–negative samples. Patients positive for Pf phage in this cohort were also older than patients who were negative.
The authors acknowledged their study’s limitations, describing the methods used to collect and sequence the analyzed samples as “highly heterogeneous.” In addition, the two cohorts were CF specific while the Genome Database is not. Finally, the CF cohorts only had a single dominant strain sampled, though multiple P. aeruginosa lineages are often present in CF patients.
One author reported receiving grants from the Cystic Fibrosis Foundation, clinical trial support, and consulting fees from industry. Two other authors are inventors on a patent application that covers the development of a vaccine that targets Pf phage. The others reported no conflicts of interest.
SOURCE: Burgener EB et al. Sci Transl Med. 2019 Apr 17. doi: 10.1126/scitranslmed.aau9748.
according to a study of the prevalence and clinical relevance of Pf phage in two patient cohorts.
“Our data from both the Stanford and Danish CF cohorts together suggest that either patients with CF acquire Pf phage–producing strains of P. aeruginosa or the Pf phage–negative P. aeruginosa become infected with Pf phage as patients age and their disease progresses,” wrote Elizabeth B. Burgener, MD, of Stanford (Calif.) University, and her coauthors. The study was published in Science Translational Medicine.The study analyzed a previous Danish longitudinal cohort of 34 patients and a prospective cross-sectional cohort of 76 patients at Stanford, 58 of which had P. aeruginosa. The researchers also reviewed a collection of genetic sequences called the Pseudomonas Genome Database to determine the prevalence of Pf phage, finding evidence in 1,159 of 2,226 P. aeruginosa sequences (52.1%).
In the Danish cohort, 21 of the 34 CF patients (61.8%; 95% confidence interval, 43.6%-77.8%) had at least one P. aeruginosa isolate containing Pf phage; 9 (26.5%) of the patients were found to be consistently positive for Pf phage. Those who were consistently positive were also older than those who never had Pf phage detected (19.1 years vs. 13.9 years; P = .046), suggesting that “there may be a tendency for P. aeruginosa strains that produce Pf phage to dominate in the sputum of individual patients with CF over time.”
In the Stanford cohort, the prevalence of Pf phage was 36.2% (21 of 58; 95% CI, 24.0%-49.9%) in patients with P. aeruginosa infection and 27.6% (21 of 76; 95% CI, 18.0%-39.1%) in all patients. No Pf phage was detected in any P. aeruginosa–negative samples. Patients positive for Pf phage in this cohort were also older than patients who were negative.
The authors acknowledged their study’s limitations, describing the methods used to collect and sequence the analyzed samples as “highly heterogeneous.” In addition, the two cohorts were CF specific while the Genome Database is not. Finally, the CF cohorts only had a single dominant strain sampled, though multiple P. aeruginosa lineages are often present in CF patients.
One author reported receiving grants from the Cystic Fibrosis Foundation, clinical trial support, and consulting fees from industry. Two other authors are inventors on a patent application that covers the development of a vaccine that targets Pf phage. The others reported no conflicts of interest.
SOURCE: Burgener EB et al. Sci Transl Med. 2019 Apr 17. doi: 10.1126/scitranslmed.aau9748.
FROM SCIENCE TRANSLATIONAL MEDICINE
Ibrexafungerp effective against C. auris in two early case reports
A novel antifungal successfully eradicated Candida auris in two critically ill patients with fungemia, according to data presented in a poster session at the European Congress of Clinical Microbiology & Infectious Diseases.
The case reports, drawn from the phase 3 CARES study of the oral formulation of ibrexafungerp, demonstrated complete response to the glucan synthase inhibitor, according to Deven Juneja, MD, and his coauthors of the Max Super Specialty Hospital, New Delhi.
The first patient was an Asian male, aged 58 years, who had a previous history of diabetes and experienced a protracted ICU stay after acute ischemic stroke. He developed septic shock after aspiration pneumonia, and also experienced a popliteal thrombosis and liver, spleen, and kidney infarcts.
The patient had received empiric antibiotics with the addition of fluconazole; the antifungal was later switched to micafungin after C. auris was identified from blood cultures. Despite clinical improvement on micafungin, blood cultures remained positive for C. auris, so ibrexafungerp was started and continued for 17 days. Blood cultures became negative by day 3 of ibrexafungerp and remained negative for the follow-up period. The patient later developed Klebsiella pneumonia and died.
The second patient, an Asian female, aged 64 years, presented with a lower respiratory tract infection accompanied by fever and hypotension. She had a previous history of diabetes, hypertension, and chronic kidney disease with maintenance hemodialysis. Her fever also persisted despite antibiotics, and C. auris was isolated from her blood cultures with the subsequent initiation of ibrexafungerp. Her blood cultures were still positive at day 3 of ibrexafungerp, but negative at day 9 and 21. She completed 22 days of ibrexafungerp therapy and was asymptomatic with no evidence of C. auris recurrence at a 6-week follow-up visit.
The male patient experienced 2 days of loose stools soon after initiating ibrexafungerp; the female patient had no adverse events.
“These cases provide initial evidence of efficacy and safety of ibrexafungerp in the treatment of candidemia caused by C. auris, including in patients who failed previous therapies,” wrote Dr. Juneja and his coauthors in the late-breaking poster.
Ibrexafungerp belongs to a novel class of glucan synthase inhibitors called triterpenoids. Scynexis funded the CARES study and also is evaluating it alone or in combination with other antifungals for treatment of vulvovaginal candidiasis, invasive pulmonary aspergillosis, and refractory invasive and/or severe fungal disease.
SOURCE: Juneja D et al. ECCMID 2019, Poster L0028.
A novel antifungal successfully eradicated Candida auris in two critically ill patients with fungemia, according to data presented in a poster session at the European Congress of Clinical Microbiology & Infectious Diseases.
The case reports, drawn from the phase 3 CARES study of the oral formulation of ibrexafungerp, demonstrated complete response to the glucan synthase inhibitor, according to Deven Juneja, MD, and his coauthors of the Max Super Specialty Hospital, New Delhi.
The first patient was an Asian male, aged 58 years, who had a previous history of diabetes and experienced a protracted ICU stay after acute ischemic stroke. He developed septic shock after aspiration pneumonia, and also experienced a popliteal thrombosis and liver, spleen, and kidney infarcts.
The patient had received empiric antibiotics with the addition of fluconazole; the antifungal was later switched to micafungin after C. auris was identified from blood cultures. Despite clinical improvement on micafungin, blood cultures remained positive for C. auris, so ibrexafungerp was started and continued for 17 days. Blood cultures became negative by day 3 of ibrexafungerp and remained negative for the follow-up period. The patient later developed Klebsiella pneumonia and died.
The second patient, an Asian female, aged 64 years, presented with a lower respiratory tract infection accompanied by fever and hypotension. She had a previous history of diabetes, hypertension, and chronic kidney disease with maintenance hemodialysis. Her fever also persisted despite antibiotics, and C. auris was isolated from her blood cultures with the subsequent initiation of ibrexafungerp. Her blood cultures were still positive at day 3 of ibrexafungerp, but negative at day 9 and 21. She completed 22 days of ibrexafungerp therapy and was asymptomatic with no evidence of C. auris recurrence at a 6-week follow-up visit.
The male patient experienced 2 days of loose stools soon after initiating ibrexafungerp; the female patient had no adverse events.
“These cases provide initial evidence of efficacy and safety of ibrexafungerp in the treatment of candidemia caused by C. auris, including in patients who failed previous therapies,” wrote Dr. Juneja and his coauthors in the late-breaking poster.
Ibrexafungerp belongs to a novel class of glucan synthase inhibitors called triterpenoids. Scynexis funded the CARES study and also is evaluating it alone or in combination with other antifungals for treatment of vulvovaginal candidiasis, invasive pulmonary aspergillosis, and refractory invasive and/or severe fungal disease.
SOURCE: Juneja D et al. ECCMID 2019, Poster L0028.
A novel antifungal successfully eradicated Candida auris in two critically ill patients with fungemia, according to data presented in a poster session at the European Congress of Clinical Microbiology & Infectious Diseases.
The case reports, drawn from the phase 3 CARES study of the oral formulation of ibrexafungerp, demonstrated complete response to the glucan synthase inhibitor, according to Deven Juneja, MD, and his coauthors of the Max Super Specialty Hospital, New Delhi.
The first patient was an Asian male, aged 58 years, who had a previous history of diabetes and experienced a protracted ICU stay after acute ischemic stroke. He developed septic shock after aspiration pneumonia, and also experienced a popliteal thrombosis and liver, spleen, and kidney infarcts.
The patient had received empiric antibiotics with the addition of fluconazole; the antifungal was later switched to micafungin after C. auris was identified from blood cultures. Despite clinical improvement on micafungin, blood cultures remained positive for C. auris, so ibrexafungerp was started and continued for 17 days. Blood cultures became negative by day 3 of ibrexafungerp and remained negative for the follow-up period. The patient later developed Klebsiella pneumonia and died.
The second patient, an Asian female, aged 64 years, presented with a lower respiratory tract infection accompanied by fever and hypotension. She had a previous history of diabetes, hypertension, and chronic kidney disease with maintenance hemodialysis. Her fever also persisted despite antibiotics, and C. auris was isolated from her blood cultures with the subsequent initiation of ibrexafungerp. Her blood cultures were still positive at day 3 of ibrexafungerp, but negative at day 9 and 21. She completed 22 days of ibrexafungerp therapy and was asymptomatic with no evidence of C. auris recurrence at a 6-week follow-up visit.
The male patient experienced 2 days of loose stools soon after initiating ibrexafungerp; the female patient had no adverse events.
“These cases provide initial evidence of efficacy and safety of ibrexafungerp in the treatment of candidemia caused by C. auris, including in patients who failed previous therapies,” wrote Dr. Juneja and his coauthors in the late-breaking poster.
Ibrexafungerp belongs to a novel class of glucan synthase inhibitors called triterpenoids. Scynexis funded the CARES study and also is evaluating it alone or in combination with other antifungals for treatment of vulvovaginal candidiasis, invasive pulmonary aspergillosis, and refractory invasive and/or severe fungal disease.
SOURCE: Juneja D et al. ECCMID 2019, Poster L0028.
FROM ECCMID 2019
Rounding team boosts ICU liberation efforts
SAN DIEGO – A rounding team formed to oversee implementation of a bundle of ICU interventions reduced the incidence of ventilator-associated pneumonia (VAP) and the number of ventilation days, as well as the ICU and hospital length of stay, according to a new study conducted at a level 1 trauma center in California. The rounding team worked toward optimal implementation of the Society of Critical Care Medicine’s ABCDEF bundle, part of the society’s ICU liberation initiative.
ABCDEF stands for: Assessment, prevention, and management of pain; Both spontaneous awakening and breathing trials; Choice of analgesia and sedation; Delirium assessment, prevention, and management; Early mobility and exercise; and Family engagement and empowerment.
The Community Regional Medical Center in Fresno, Calif., where the study was conducted, was chosen in 2015 to participate in the ICU liberation initiative. The facility serves a population of 3.2 million and sees just under 4,000 trauma patients per year.
After a 6-month retrospective analysis, the team members at the medical center realized they needed to improve ABCDEF implementation with respect to evaluating sedation practices and improving delirium assessment.
Before the start of the 17-month collaborative period, they formed an ICU liberation team called SMART, short for Sedation, Mobilization, Assessment Rounding Team, which included representatives from ICU nursing, pharmacy, respiratory therapy, physical therapy, physicians, and administration. They developed a daily rounding tool to help the team implement procedures, with the goal of reducing the continuous infusion of benzodiazepines and increasing intermittent dosing, the use of short-acting medications, and conducting spontaneous awakening and breathing trials. The SMART team made daily rounds to ensure that the ABCDEF bundle was being implemented.
The researchers then continued the analysis for another 12 months after the end of the initiative. During this last phase, the benefits of the SMART team became evident.
“Stick with it. Don’t let up. Don’t quit,” Wade Veneman, a respiratory therapist at the medical center, said in an interview. He presented the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine. “It can be particularly difficult in the face of critical care providers who may be skeptical of new initiatives. They think it’s something new, and they hope that it goes away. But this is something we feel we’re going to keep for a long time,” he added.
Mr. Veneman hopes to implement the SMART program in the neurological critical care ICU. The medical director of that unit did not participate in the initial collaborative, but Mr. Veneman hopes to change that. “The data is going to show that his VAP and ventilator days are going up, and everywhere else they’re going down,” he said.
The researchers analyzed data on 1,127 mechanically ventilated patients in the ICU. At total of 197 patients were treated 6 months before the implementation of the collaborative, 519 during 17 months of collaborative implementation, and 411 in the 12 months after implementation. There were some differences between the populations: The before group was slightly younger than the after-implementation group (mean 41 vs. 44, P = .04), and the mean Injury Severity Score score was 24 in the before group, 22 during, and 20 after (P = .002). The researchers noted that the differences were clinically significant.
Benzodiazepine use declined, but the effect was statistically significant only in the after population. Continuous use declined from 87% before implementation to 83% during (P = .21) and 53% after (P less than .001). Intermittent use was 57% before implementation, increased to 61% during (P = .44), and fell to 44% after (P less than .001). Delirium assessment performance improved throughout, from 9% before implementation to 42% during (P less than .001) to 73% after implementation (P less than .001).
The VAP rate increased from 3.4% before the SMART program to 4.5% during implementation (P = .53), and then dropped to 0.9% afterward (P = .001). Ventilation days started at a mean of 10.5, then dropped to 9.5 during implementation (P = .30), and 8.2 after implementation (P = .027).
ICU length of stay improved from 10.7 before implementation to 9.3 afterward (P = .021), and overall hospital length of stay went from 17.3 days to 16.3 (P = .005).
The study was not funded. Mr. Veneman has no relevant financial disclosures.
SOURCE: Veneman W et al. CCC48, Abstract 63.
SAN DIEGO – A rounding team formed to oversee implementation of a bundle of ICU interventions reduced the incidence of ventilator-associated pneumonia (VAP) and the number of ventilation days, as well as the ICU and hospital length of stay, according to a new study conducted at a level 1 trauma center in California. The rounding team worked toward optimal implementation of the Society of Critical Care Medicine’s ABCDEF bundle, part of the society’s ICU liberation initiative.
ABCDEF stands for: Assessment, prevention, and management of pain; Both spontaneous awakening and breathing trials; Choice of analgesia and sedation; Delirium assessment, prevention, and management; Early mobility and exercise; and Family engagement and empowerment.
The Community Regional Medical Center in Fresno, Calif., where the study was conducted, was chosen in 2015 to participate in the ICU liberation initiative. The facility serves a population of 3.2 million and sees just under 4,000 trauma patients per year.
After a 6-month retrospective analysis, the team members at the medical center realized they needed to improve ABCDEF implementation with respect to evaluating sedation practices and improving delirium assessment.
Before the start of the 17-month collaborative period, they formed an ICU liberation team called SMART, short for Sedation, Mobilization, Assessment Rounding Team, which included representatives from ICU nursing, pharmacy, respiratory therapy, physical therapy, physicians, and administration. They developed a daily rounding tool to help the team implement procedures, with the goal of reducing the continuous infusion of benzodiazepines and increasing intermittent dosing, the use of short-acting medications, and conducting spontaneous awakening and breathing trials. The SMART team made daily rounds to ensure that the ABCDEF bundle was being implemented.
The researchers then continued the analysis for another 12 months after the end of the initiative. During this last phase, the benefits of the SMART team became evident.
“Stick with it. Don’t let up. Don’t quit,” Wade Veneman, a respiratory therapist at the medical center, said in an interview. He presented the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine. “It can be particularly difficult in the face of critical care providers who may be skeptical of new initiatives. They think it’s something new, and they hope that it goes away. But this is something we feel we’re going to keep for a long time,” he added.
Mr. Veneman hopes to implement the SMART program in the neurological critical care ICU. The medical director of that unit did not participate in the initial collaborative, but Mr. Veneman hopes to change that. “The data is going to show that his VAP and ventilator days are going up, and everywhere else they’re going down,” he said.
The researchers analyzed data on 1,127 mechanically ventilated patients in the ICU. At total of 197 patients were treated 6 months before the implementation of the collaborative, 519 during 17 months of collaborative implementation, and 411 in the 12 months after implementation. There were some differences between the populations: The before group was slightly younger than the after-implementation group (mean 41 vs. 44, P = .04), and the mean Injury Severity Score score was 24 in the before group, 22 during, and 20 after (P = .002). The researchers noted that the differences were clinically significant.
Benzodiazepine use declined, but the effect was statistically significant only in the after population. Continuous use declined from 87% before implementation to 83% during (P = .21) and 53% after (P less than .001). Intermittent use was 57% before implementation, increased to 61% during (P = .44), and fell to 44% after (P less than .001). Delirium assessment performance improved throughout, from 9% before implementation to 42% during (P less than .001) to 73% after implementation (P less than .001).
The VAP rate increased from 3.4% before the SMART program to 4.5% during implementation (P = .53), and then dropped to 0.9% afterward (P = .001). Ventilation days started at a mean of 10.5, then dropped to 9.5 during implementation (P = .30), and 8.2 after implementation (P = .027).
ICU length of stay improved from 10.7 before implementation to 9.3 afterward (P = .021), and overall hospital length of stay went from 17.3 days to 16.3 (P = .005).
The study was not funded. Mr. Veneman has no relevant financial disclosures.
SOURCE: Veneman W et al. CCC48, Abstract 63.
SAN DIEGO – A rounding team formed to oversee implementation of a bundle of ICU interventions reduced the incidence of ventilator-associated pneumonia (VAP) and the number of ventilation days, as well as the ICU and hospital length of stay, according to a new study conducted at a level 1 trauma center in California. The rounding team worked toward optimal implementation of the Society of Critical Care Medicine’s ABCDEF bundle, part of the society’s ICU liberation initiative.
ABCDEF stands for: Assessment, prevention, and management of pain; Both spontaneous awakening and breathing trials; Choice of analgesia and sedation; Delirium assessment, prevention, and management; Early mobility and exercise; and Family engagement and empowerment.
The Community Regional Medical Center in Fresno, Calif., where the study was conducted, was chosen in 2015 to participate in the ICU liberation initiative. The facility serves a population of 3.2 million and sees just under 4,000 trauma patients per year.
After a 6-month retrospective analysis, the team members at the medical center realized they needed to improve ABCDEF implementation with respect to evaluating sedation practices and improving delirium assessment.
Before the start of the 17-month collaborative period, they formed an ICU liberation team called SMART, short for Sedation, Mobilization, Assessment Rounding Team, which included representatives from ICU nursing, pharmacy, respiratory therapy, physical therapy, physicians, and administration. They developed a daily rounding tool to help the team implement procedures, with the goal of reducing the continuous infusion of benzodiazepines and increasing intermittent dosing, the use of short-acting medications, and conducting spontaneous awakening and breathing trials. The SMART team made daily rounds to ensure that the ABCDEF bundle was being implemented.
The researchers then continued the analysis for another 12 months after the end of the initiative. During this last phase, the benefits of the SMART team became evident.
“Stick with it. Don’t let up. Don’t quit,” Wade Veneman, a respiratory therapist at the medical center, said in an interview. He presented the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine. “It can be particularly difficult in the face of critical care providers who may be skeptical of new initiatives. They think it’s something new, and they hope that it goes away. But this is something we feel we’re going to keep for a long time,” he added.
Mr. Veneman hopes to implement the SMART program in the neurological critical care ICU. The medical director of that unit did not participate in the initial collaborative, but Mr. Veneman hopes to change that. “The data is going to show that his VAP and ventilator days are going up, and everywhere else they’re going down,” he said.
The researchers analyzed data on 1,127 mechanically ventilated patients in the ICU. At total of 197 patients were treated 6 months before the implementation of the collaborative, 519 during 17 months of collaborative implementation, and 411 in the 12 months after implementation. There were some differences between the populations: The before group was slightly younger than the after-implementation group (mean 41 vs. 44, P = .04), and the mean Injury Severity Score score was 24 in the before group, 22 during, and 20 after (P = .002). The researchers noted that the differences were clinically significant.
Benzodiazepine use declined, but the effect was statistically significant only in the after population. Continuous use declined from 87% before implementation to 83% during (P = .21) and 53% after (P less than .001). Intermittent use was 57% before implementation, increased to 61% during (P = .44), and fell to 44% after (P less than .001). Delirium assessment performance improved throughout, from 9% before implementation to 42% during (P less than .001) to 73% after implementation (P less than .001).
The VAP rate increased from 3.4% before the SMART program to 4.5% during implementation (P = .53), and then dropped to 0.9% afterward (P = .001). Ventilation days started at a mean of 10.5, then dropped to 9.5 during implementation (P = .30), and 8.2 after implementation (P = .027).
ICU length of stay improved from 10.7 before implementation to 9.3 afterward (P = .021), and overall hospital length of stay went from 17.3 days to 16.3 (P = .005).
The study was not funded. Mr. Veneman has no relevant financial disclosures.
SOURCE: Veneman W et al. CCC48, Abstract 63.
REPORTING FROM CCC48
LAIV4 was less effective for children than IIV against influenza A/H1N1pdm09
The live attenuated influenza vaccine was less effective against the influenza A/H1N1pdm09 virus in children and adolescents across multiple influenza seasons between 2013 and 2016, compared with the inactivated influenza vaccine, according to research published in the journal Pediatrics.
Jessie R. Chung, MPH, from the influenza division at the Centers for Disease Control and Prevention in Atlanta, and her colleagues performed an analysis of five different studies where vaccine effectiveness (VE) was examined for quadrivalent live attenuated vaccine (LAIV4) and inactivated influenza vaccine (IIV) in children and adolescents aged 2-17 years from 42 states.
The analysis included data from the U.S. Influenza Vaccine Effectiveness Network (6,793 patients), a study from the Louisiana State University Health Sciences Center (3,822 patients), the Influenza Clinical Investigation for Children (3,521 patients), Department of Defense Global, Laboratory-based, Influenza Surveillance Program (1,935 patients), and the Influenza Incidence Surveillance Project (1,102 patients) between the periods of 2013-2014 and 2015-2016. The researchers sourced current and previous season vaccination history from electronic medical records and immunization registries.
Of patients who were vaccinated across all seasons, there was 67% effectiveness against influenza A/H1N1pdm09 (95% confidence interval, 62%-72%) for those who received the IIV and 20% (95% CI, −6%-39%) for LAIV4. Among patients who received the LAIV4 vaccination, there was a significantly higher likelihood of developing influenza A/H1N1pdm09 (odds ratio, 2.66; 95% CI, 2.06-3.44) compared with patients who received the IIV vaccination.
With regard to other strains, there was similar effectiveness against influenza A/H3N2 and influenza B with LAIV4 and IIV vaccinations.
“In contrast to findings of reduced LAIV4 effectiveness against influenza A/H1N1pdm09 viruses, our results suggest a possible but nonsignificant benefit of LAIV4 over IIV against influenza B viruses, which has been described previously,” the investigators wrote.
Limitations of the study included having data only one season prior to enrollment and little available demographic information beyond age, gender, and geographic location.
The Influenza Clinical Investigation for Children was funded by MedImmune, a member of the AstraZeneca Group. Two of the researchers are employees of AstraZeneca. The other authors reported having no conflicts of interest. The U.S. Influenza Vaccine Effectiveness Network was supported by the CDC through cooperative agreements with the University of Michigan, Kaiser Permanente Washington Health Research Institute, Marshfield Clinic Research Institute, University of Pittsburgh, and Baylor Scott & White Health. At the University of Pittsburgh, the project also was supported by the National Institutes of Health.
SOURCE: Chung JR et al. Pediatrics. 2018. doi: 10.1542/peds.2018-2094.
There are many explanations for the decline in effectiveness of the live attenuated influenza vaccine (LAIV4), but the data are complicated by conflicting information from studies outside the United States indicating “reasonable protection” against influenza A/H1N1pdm09, A/H3N2, and influenza B, compared with the inactivated influenza virus (IIV), Pedro A. Piedra, MD, wrote in an accompanying editorial.
In 2016, the World Health Organization met to discuss LAIV effectiveness and highlighted factors such as methodological study differences, inadequate vaccine handling at distribution centers, intrinsic virological differences of the A/H1N1pdm09 virus, and increased preexisting population immunity in the United States since 2010 as potential explanations. During the transition from LAIV3 to LAIV4 for the 2013-2014 influenza season, viral interference may have also occurred when the influenza B strain was introduced into the vaccine, he added.
According to the CDC’s Advisory Committee on Immunization Practices (ACIP), viral growth properties of A/H1N1pdm09 has improved in LAIV4, and viral shedding also has improved for children between 2 years and 4 years of age. Although effectiveness numbers were not available for the ACIP recommendation, an interim analysis from Public Health England for the 2017-2018 influenza season found a vaccine effectiveness of 90.3% (95% confidence interval, 16.4%-98.9%).
“This early result is encouraging and supports the reintroduction of LAIV4 in the United States as an option for the control of seasonal influenza,” he said. “It also highlights the need for annual influenza vaccine effectiveness estimates and the importance of the U.S. Influenza Vaccine Effectiveness Network in providing updated information for ACIP recommendations.”
Dr. Piedra is from the departments of molecular virology and microbiology and pediatrics, Baylor College of Medicine, Houston. He reports being a consultant for AstraZeneca, Sanofi Pasteur, GlaxoSmithKline, and Merck Sharp and Dohme, and he has received travel support to present at an influenza seminar supported by Seqirus. His comments are from an editorial accompanying the article by Chung and colleagues ( Pediatrics. 2019. doi: 10.1542/peds.2018- 3290 ).
There are many explanations for the decline in effectiveness of the live attenuated influenza vaccine (LAIV4), but the data are complicated by conflicting information from studies outside the United States indicating “reasonable protection” against influenza A/H1N1pdm09, A/H3N2, and influenza B, compared with the inactivated influenza virus (IIV), Pedro A. Piedra, MD, wrote in an accompanying editorial.
In 2016, the World Health Organization met to discuss LAIV effectiveness and highlighted factors such as methodological study differences, inadequate vaccine handling at distribution centers, intrinsic virological differences of the A/H1N1pdm09 virus, and increased preexisting population immunity in the United States since 2010 as potential explanations. During the transition from LAIV3 to LAIV4 for the 2013-2014 influenza season, viral interference may have also occurred when the influenza B strain was introduced into the vaccine, he added.
According to the CDC’s Advisory Committee on Immunization Practices (ACIP), viral growth properties of A/H1N1pdm09 has improved in LAIV4, and viral shedding also has improved for children between 2 years and 4 years of age. Although effectiveness numbers were not available for the ACIP recommendation, an interim analysis from Public Health England for the 2017-2018 influenza season found a vaccine effectiveness of 90.3% (95% confidence interval, 16.4%-98.9%).
“This early result is encouraging and supports the reintroduction of LAIV4 in the United States as an option for the control of seasonal influenza,” he said. “It also highlights the need for annual influenza vaccine effectiveness estimates and the importance of the U.S. Influenza Vaccine Effectiveness Network in providing updated information for ACIP recommendations.”
Dr. Piedra is from the departments of molecular virology and microbiology and pediatrics, Baylor College of Medicine, Houston. He reports being a consultant for AstraZeneca, Sanofi Pasteur, GlaxoSmithKline, and Merck Sharp and Dohme, and he has received travel support to present at an influenza seminar supported by Seqirus. His comments are from an editorial accompanying the article by Chung and colleagues ( Pediatrics. 2019. doi: 10.1542/peds.2018- 3290 ).
There are many explanations for the decline in effectiveness of the live attenuated influenza vaccine (LAIV4), but the data are complicated by conflicting information from studies outside the United States indicating “reasonable protection” against influenza A/H1N1pdm09, A/H3N2, and influenza B, compared with the inactivated influenza virus (IIV), Pedro A. Piedra, MD, wrote in an accompanying editorial.
In 2016, the World Health Organization met to discuss LAIV effectiveness and highlighted factors such as methodological study differences, inadequate vaccine handling at distribution centers, intrinsic virological differences of the A/H1N1pdm09 virus, and increased preexisting population immunity in the United States since 2010 as potential explanations. During the transition from LAIV3 to LAIV4 for the 2013-2014 influenza season, viral interference may have also occurred when the influenza B strain was introduced into the vaccine, he added.
According to the CDC’s Advisory Committee on Immunization Practices (ACIP), viral growth properties of A/H1N1pdm09 has improved in LAIV4, and viral shedding also has improved for children between 2 years and 4 years of age. Although effectiveness numbers were not available for the ACIP recommendation, an interim analysis from Public Health England for the 2017-2018 influenza season found a vaccine effectiveness of 90.3% (95% confidence interval, 16.4%-98.9%).
“This early result is encouraging and supports the reintroduction of LAIV4 in the United States as an option for the control of seasonal influenza,” he said. “It also highlights the need for annual influenza vaccine effectiveness estimates and the importance of the U.S. Influenza Vaccine Effectiveness Network in providing updated information for ACIP recommendations.”
Dr. Piedra is from the departments of molecular virology and microbiology and pediatrics, Baylor College of Medicine, Houston. He reports being a consultant for AstraZeneca, Sanofi Pasteur, GlaxoSmithKline, and Merck Sharp and Dohme, and he has received travel support to present at an influenza seminar supported by Seqirus. His comments are from an editorial accompanying the article by Chung and colleagues ( Pediatrics. 2019. doi: 10.1542/peds.2018- 3290 ).
The live attenuated influenza vaccine was less effective against the influenza A/H1N1pdm09 virus in children and adolescents across multiple influenza seasons between 2013 and 2016, compared with the inactivated influenza vaccine, according to research published in the journal Pediatrics.
Jessie R. Chung, MPH, from the influenza division at the Centers for Disease Control and Prevention in Atlanta, and her colleagues performed an analysis of five different studies where vaccine effectiveness (VE) was examined for quadrivalent live attenuated vaccine (LAIV4) and inactivated influenza vaccine (IIV) in children and adolescents aged 2-17 years from 42 states.
The analysis included data from the U.S. Influenza Vaccine Effectiveness Network (6,793 patients), a study from the Louisiana State University Health Sciences Center (3,822 patients), the Influenza Clinical Investigation for Children (3,521 patients), Department of Defense Global, Laboratory-based, Influenza Surveillance Program (1,935 patients), and the Influenza Incidence Surveillance Project (1,102 patients) between the periods of 2013-2014 and 2015-2016. The researchers sourced current and previous season vaccination history from electronic medical records and immunization registries.
Of patients who were vaccinated across all seasons, there was 67% effectiveness against influenza A/H1N1pdm09 (95% confidence interval, 62%-72%) for those who received the IIV and 20% (95% CI, −6%-39%) for LAIV4. Among patients who received the LAIV4 vaccination, there was a significantly higher likelihood of developing influenza A/H1N1pdm09 (odds ratio, 2.66; 95% CI, 2.06-3.44) compared with patients who received the IIV vaccination.
With regard to other strains, there was similar effectiveness against influenza A/H3N2 and influenza B with LAIV4 and IIV vaccinations.
“In contrast to findings of reduced LAIV4 effectiveness against influenza A/H1N1pdm09 viruses, our results suggest a possible but nonsignificant benefit of LAIV4 over IIV against influenza B viruses, which has been described previously,” the investigators wrote.
Limitations of the study included having data only one season prior to enrollment and little available demographic information beyond age, gender, and geographic location.
The Influenza Clinical Investigation for Children was funded by MedImmune, a member of the AstraZeneca Group. Two of the researchers are employees of AstraZeneca. The other authors reported having no conflicts of interest. The U.S. Influenza Vaccine Effectiveness Network was supported by the CDC through cooperative agreements with the University of Michigan, Kaiser Permanente Washington Health Research Institute, Marshfield Clinic Research Institute, University of Pittsburgh, and Baylor Scott & White Health. At the University of Pittsburgh, the project also was supported by the National Institutes of Health.
SOURCE: Chung JR et al. Pediatrics. 2018. doi: 10.1542/peds.2018-2094.
The live attenuated influenza vaccine was less effective against the influenza A/H1N1pdm09 virus in children and adolescents across multiple influenza seasons between 2013 and 2016, compared with the inactivated influenza vaccine, according to research published in the journal Pediatrics.
Jessie R. Chung, MPH, from the influenza division at the Centers for Disease Control and Prevention in Atlanta, and her colleagues performed an analysis of five different studies where vaccine effectiveness (VE) was examined for quadrivalent live attenuated vaccine (LAIV4) and inactivated influenza vaccine (IIV) in children and adolescents aged 2-17 years from 42 states.
The analysis included data from the U.S. Influenza Vaccine Effectiveness Network (6,793 patients), a study from the Louisiana State University Health Sciences Center (3,822 patients), the Influenza Clinical Investigation for Children (3,521 patients), Department of Defense Global, Laboratory-based, Influenza Surveillance Program (1,935 patients), and the Influenza Incidence Surveillance Project (1,102 patients) between the periods of 2013-2014 and 2015-2016. The researchers sourced current and previous season vaccination history from electronic medical records and immunization registries.
Of patients who were vaccinated across all seasons, there was 67% effectiveness against influenza A/H1N1pdm09 (95% confidence interval, 62%-72%) for those who received the IIV and 20% (95% CI, −6%-39%) for LAIV4. Among patients who received the LAIV4 vaccination, there was a significantly higher likelihood of developing influenza A/H1N1pdm09 (odds ratio, 2.66; 95% CI, 2.06-3.44) compared with patients who received the IIV vaccination.
With regard to other strains, there was similar effectiveness against influenza A/H3N2 and influenza B with LAIV4 and IIV vaccinations.
“In contrast to findings of reduced LAIV4 effectiveness against influenza A/H1N1pdm09 viruses, our results suggest a possible but nonsignificant benefit of LAIV4 over IIV against influenza B viruses, which has been described previously,” the investigators wrote.
Limitations of the study included having data only one season prior to enrollment and little available demographic information beyond age, gender, and geographic location.
The Influenza Clinical Investigation for Children was funded by MedImmune, a member of the AstraZeneca Group. Two of the researchers are employees of AstraZeneca. The other authors reported having no conflicts of interest. The U.S. Influenza Vaccine Effectiveness Network was supported by the CDC through cooperative agreements with the University of Michigan, Kaiser Permanente Washington Health Research Institute, Marshfield Clinic Research Institute, University of Pittsburgh, and Baylor Scott & White Health. At the University of Pittsburgh, the project also was supported by the National Institutes of Health.
SOURCE: Chung JR et al. Pediatrics. 2018. doi: 10.1542/peds.2018-2094.
FROM PEDIATRICS
Key clinical point: The live attenuated influenza vaccine (LAIV4) was significantly less effective than was the inactivated influenza vaccine (IIV) for children against the influenza A/H1N1pdm09 virus across multiple flu seasons.
Major finding:
Study details: A combined analysis of five studies in the United States between the periods of 2013-2014 and 2015-2016 from the U.S. Influenza Vaccine Effectiveness Network.
Disclosures: The Influenza Clinical Investigation for Children was funded by MedImmune, a member of the AstraZeneca Group. Two of the researchers are employees of AstraZeneca. The other authors reported having no conflicts of interest. The U.S. Influenza Vaccine Effectiveness Network was supported by the CDC through cooperative agreements with the University of Michigan, Kaiser Permanente Washington Health Research Institute, Marshfield Clinic Research Institute, University of Pittsburgh, and Baylor Scott & White Health. At the University of Pittsburgh, the project also was supported by the National Institutes of Health.
Source: Chung JR et al. Pediatrics. 2018. doi: 10.1542/peds.2018-2094.
Hospital Readmissions Reduction Program may be doing more harm than good
A Medicare program aimed at lowering readmissions to hospitals could be having an adverse effect on mortality.
Results from a retrospective cohort study of hospitalizations for heart failure, acute myocardial infarction, and pneumonia among Medicare beneficiaries aged 65 years and older between April 1, 2005 and March 31, 2015 (covering the period before and after the Medicare Hospital Readmissions Reduction Program was announced in April 2010 and implemented in October 2012) found a significant increase in 30-day post discharge mortality among heart failure and pneumonia patients.
“Most concerning, however, is the possibility that the relationship between the HRRP and postdischarge mortality for heart failure and pneumonia is causal, indicating that the HRRP led to changes in quality of care that adversely affected patients,” Rishi Wadhera, MD, Harvard Medical School, Boston, and his colleagues wrote in a report published Dec. 25, 2018, in JAMA.
They looked at 8.3 million hospitalizations for heart failure, acute MI, and pneumonia, among whom 7.9 million were alive at the time of discharge. There were roughly 270,000 deaths within 30 days of discharge for heart failure; 128,000 for acute MI; and 246,000 for pneumonia.
To examine trends, the timing was divided into four periods: two prior to the announcement of the HRRP (April 2005–September 2007 and October 2007–March 2010); a third covering the time when the HRRP was announced (April 2010–September 2012); and the fourth when HRRP was implemented (October 2012–March 2015).
They found that among patients discharged with heart failure, 30-day mortality was rising even before the announcement of the HRRP, by 0.27% from the first period to the second period. That baseline trend continued when the HRRP was announced, by 0.49%, from second period to third. The difference in change between those periods was 0.22%. After implementation, 30-day mortality increased by 0.52%, with a difference in change from the third period of 0.25%. Both changes were statistically significant.
Among pneumonia patients, postdischarge mortality was stable before HRRP, but significantly increased after HRRP announcement, by 0.26%, with a difference in change from the second period to the third period of 0.22%. After implementation, the 30-day postdischarge mortality was 0.44%, with a significant difference in change of 0.40%.
Acute MI was a different story. Postdischarge mortality decreased significantly after the implementation of the HRRP, by 0.22%. The difference in change was –0.26%.
The authors suggested that “some hospitals may have focused more resources and efforts on reducing or avoiding readmissions than on prioritizing survival.” They add that the increases in heart failure morbidity could be related to patients with more severe heart conditions.
They noted that “although hospitals that reduce readmissions also appear to reduce mortality, this hospital-level concordance does not reflect the change in readmissions and mortality at the level of the patient population, which is arguably of greater importance to individual patients and to public health.”
Further research is needed to understand whether the increase in 30-day postdischarge mortality is a result of the HRRP, the authors concluded.
SOURCE: Wadhera R et al. JAMA. 2018 Dec 25. doi: 10.1001/jama.2018.19232.
Evidence in this study shows that while the Hospital Readmissions Reduction Program my be succeeding in reducing hospital admissions, little evidence is available to show that it is having a positive effect on patient outcomes.
The Centers for Medicare & Medicaid Services needs to reexamine the program and find alternative methods that are both effective at reducing hospital readmissions while at the same time protect patients from unintentional harm, including death.
Gregg C. Fonarow, MD , University of California Medical Center, Los Angeles, in an editorial published in JAMA, Dec. 25, 2018. doi:10.1001/jama.2018.19325 .
Evidence in this study shows that while the Hospital Readmissions Reduction Program my be succeeding in reducing hospital admissions, little evidence is available to show that it is having a positive effect on patient outcomes.
The Centers for Medicare & Medicaid Services needs to reexamine the program and find alternative methods that are both effective at reducing hospital readmissions while at the same time protect patients from unintentional harm, including death.
Gregg C. Fonarow, MD , University of California Medical Center, Los Angeles, in an editorial published in JAMA, Dec. 25, 2018. doi:10.1001/jama.2018.19325 .
Evidence in this study shows that while the Hospital Readmissions Reduction Program my be succeeding in reducing hospital admissions, little evidence is available to show that it is having a positive effect on patient outcomes.
The Centers for Medicare & Medicaid Services needs to reexamine the program and find alternative methods that are both effective at reducing hospital readmissions while at the same time protect patients from unintentional harm, including death.
Gregg C. Fonarow, MD , University of California Medical Center, Los Angeles, in an editorial published in JAMA, Dec. 25, 2018. doi:10.1001/jama.2018.19325 .
A Medicare program aimed at lowering readmissions to hospitals could be having an adverse effect on mortality.
Results from a retrospective cohort study of hospitalizations for heart failure, acute myocardial infarction, and pneumonia among Medicare beneficiaries aged 65 years and older between April 1, 2005 and March 31, 2015 (covering the period before and after the Medicare Hospital Readmissions Reduction Program was announced in April 2010 and implemented in October 2012) found a significant increase in 30-day post discharge mortality among heart failure and pneumonia patients.
“Most concerning, however, is the possibility that the relationship between the HRRP and postdischarge mortality for heart failure and pneumonia is causal, indicating that the HRRP led to changes in quality of care that adversely affected patients,” Rishi Wadhera, MD, Harvard Medical School, Boston, and his colleagues wrote in a report published Dec. 25, 2018, in JAMA.
They looked at 8.3 million hospitalizations for heart failure, acute MI, and pneumonia, among whom 7.9 million were alive at the time of discharge. There were roughly 270,000 deaths within 30 days of discharge for heart failure; 128,000 for acute MI; and 246,000 for pneumonia.
To examine trends, the timing was divided into four periods: two prior to the announcement of the HRRP (April 2005–September 2007 and October 2007–March 2010); a third covering the time when the HRRP was announced (April 2010–September 2012); and the fourth when HRRP was implemented (October 2012–March 2015).
They found that among patients discharged with heart failure, 30-day mortality was rising even before the announcement of the HRRP, by 0.27% from the first period to the second period. That baseline trend continued when the HRRP was announced, by 0.49%, from second period to third. The difference in change between those periods was 0.22%. After implementation, 30-day mortality increased by 0.52%, with a difference in change from the third period of 0.25%. Both changes were statistically significant.
Among pneumonia patients, postdischarge mortality was stable before HRRP, but significantly increased after HRRP announcement, by 0.26%, with a difference in change from the second period to the third period of 0.22%. After implementation, the 30-day postdischarge mortality was 0.44%, with a significant difference in change of 0.40%.
Acute MI was a different story. Postdischarge mortality decreased significantly after the implementation of the HRRP, by 0.22%. The difference in change was –0.26%.
The authors suggested that “some hospitals may have focused more resources and efforts on reducing or avoiding readmissions than on prioritizing survival.” They add that the increases in heart failure morbidity could be related to patients with more severe heart conditions.
They noted that “although hospitals that reduce readmissions also appear to reduce mortality, this hospital-level concordance does not reflect the change in readmissions and mortality at the level of the patient population, which is arguably of greater importance to individual patients and to public health.”
Further research is needed to understand whether the increase in 30-day postdischarge mortality is a result of the HRRP, the authors concluded.
SOURCE: Wadhera R et al. JAMA. 2018 Dec 25. doi: 10.1001/jama.2018.19232.
A Medicare program aimed at lowering readmissions to hospitals could be having an adverse effect on mortality.
Results from a retrospective cohort study of hospitalizations for heart failure, acute myocardial infarction, and pneumonia among Medicare beneficiaries aged 65 years and older between April 1, 2005 and March 31, 2015 (covering the period before and after the Medicare Hospital Readmissions Reduction Program was announced in April 2010 and implemented in October 2012) found a significant increase in 30-day post discharge mortality among heart failure and pneumonia patients.
“Most concerning, however, is the possibility that the relationship between the HRRP and postdischarge mortality for heart failure and pneumonia is causal, indicating that the HRRP led to changes in quality of care that adversely affected patients,” Rishi Wadhera, MD, Harvard Medical School, Boston, and his colleagues wrote in a report published Dec. 25, 2018, in JAMA.
They looked at 8.3 million hospitalizations for heart failure, acute MI, and pneumonia, among whom 7.9 million were alive at the time of discharge. There were roughly 270,000 deaths within 30 days of discharge for heart failure; 128,000 for acute MI; and 246,000 for pneumonia.
To examine trends, the timing was divided into four periods: two prior to the announcement of the HRRP (April 2005–September 2007 and October 2007–March 2010); a third covering the time when the HRRP was announced (April 2010–September 2012); and the fourth when HRRP was implemented (October 2012–March 2015).
They found that among patients discharged with heart failure, 30-day mortality was rising even before the announcement of the HRRP, by 0.27% from the first period to the second period. That baseline trend continued when the HRRP was announced, by 0.49%, from second period to third. The difference in change between those periods was 0.22%. After implementation, 30-day mortality increased by 0.52%, with a difference in change from the third period of 0.25%. Both changes were statistically significant.
Among pneumonia patients, postdischarge mortality was stable before HRRP, but significantly increased after HRRP announcement, by 0.26%, with a difference in change from the second period to the third period of 0.22%. After implementation, the 30-day postdischarge mortality was 0.44%, with a significant difference in change of 0.40%.
Acute MI was a different story. Postdischarge mortality decreased significantly after the implementation of the HRRP, by 0.22%. The difference in change was –0.26%.
The authors suggested that “some hospitals may have focused more resources and efforts on reducing or avoiding readmissions than on prioritizing survival.” They add that the increases in heart failure morbidity could be related to patients with more severe heart conditions.
They noted that “although hospitals that reduce readmissions also appear to reduce mortality, this hospital-level concordance does not reflect the change in readmissions and mortality at the level of the patient population, which is arguably of greater importance to individual patients and to public health.”
Further research is needed to understand whether the increase in 30-day postdischarge mortality is a result of the HRRP, the authors concluded.
SOURCE: Wadhera R et al. JAMA. 2018 Dec 25. doi: 10.1001/jama.2018.19232.
FROM JAMA
Key clinical point:
Major finding: Heart failure patients saw mortality increase 0.52% after HRRP launched.
Study details: A retrospective cohort study across 10 years, including time before and after the implementation of the HRRP.
Disclosures: The Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology funded the study. No relevant conflicts of interest were disclosed.
Source: Wadhera R et al. JAMA 2018 Dec 25. doi: 10.1001/jama.2018.19232.