User login
No link seen between methotrexate, interstitial lung disease in RA
Patients with rheumatoid arthritis (RA) have an elevated risk of interstitial lung disease (ILD), but methotrexate does not accentuate that risk and may in fact be protective, new data show. These were among key findings of a pair of studies reported at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Although a guideline-recommended cornerstone in the management of RA, methotrexate has been associated with both hypersensitivity pneumonitis and diffuse lung disease. However, its involvement in the development of ILD among patients with RA is unclear.
A Danish study of more than 30,000 RA patients reported at the congress found that their risk of ILD was about three to five times that of the general population. However, risk did not differ significantly whether they had filled a methotrexate prescription or not.
In addition, a multinational case-control study of more than 1,000 RA patients also reported at the congress found that, compared with never-users of methotrexate, ever-users actually had a 59% lower likelihood of developing ILD.
However, both studies were limited by their retrospective design, Elizabeth R. Volkmann, MD, codirector of the connective tissue disease–related interstitial lung disease program at the University of California, Los Angeles, cautioned in an interview. Hence, there was likely systematic bias and confounding.
“I would interpret the conclusions of both studies with caution,” she maintained. “To understand how a particular intervention, such as methotrexate use, affects the outcome of ILD development, a prospective design is needed, which adequately adjusts for known ILD risk factors, such as male sex and smoking.”
As to whether the new findings are practice changing and how they might affect patient counseling, “the answers to these questions are not straightforward and depend on other patient-related factors,” according to Dr. Volkmann.
Danish nationwide study
René Cordtz, MD, a clinical assistant at the Center for Rheumatology and Spine Diseases, Rigshospitalet‐Gentofte, Copenhagen, and colleagues conducted a nationwide population-based cohort study using registry data from 1997 to 2015 to assess lung disease among patients with RA by prescriptions filled.
Results based on 30,512 RA patients showed that, compared with peers filling no methotrexate prescriptions, patients filling at least one did not have a significantly elevated risk of ILD at either 1 year of follow-up (hazard ratio, 1.03) or 5 years of follow-up (HR, 1.00). (Findings were similar for sulfasalazine, with respective nonsignificant HRs of 0.88 and 1.14.)
In addition, patients with RA had a similarly sharply elevated 5-year risk of ILD relative to the general population regardless of whether they had filled neither methotrexate nor sulfasalazine prescriptions (standardized incidence ratio, 3.38) or had filled prescriptions for methotrexate only (SIR, 3.63), sulfasalazine only (SIR, 4.12), or both (SIR, 5.45).
“RA patients have an increased risk of ILD, compared to the general population, which was not surprising, but very importantly, that risk was not further exacerbated in those treated with methotrexate,” Dr. Cordtz concluded. “We do acknowledge that purchasing your medicine is different from taking your medicine, which is why we found it extra reassuring that when requiring at least two methotrexate prescriptions to be considered exposed, it did not change our results.”
Multinational study
Pierre-Antoine Juge, MD, a rheumatologist at Bichat-Claude Bernard Hospital, Paris, and colleagues performed a case-control study among 482 RA patients with ILD and 741 RA patients without ILD in three cohorts: a French discovery cohort, a multinational (Brazilian, Italian, Mexican, United Kingdom, and United States) replication cohort, and a combined cohort. Those with methotrexate hypersensitivity pneumonitis were excluded.
Results showed that relative to peers without ILD, patients with ILD had a lower prevalence of ever having used methotrexate and had received a lower cumulative methotrexate dose, findings that were consistent across all three cohorts.
Methotrexate ever-use was associated with a significantly lower adjusted likelihood of ILD in the discovery cohort (odds ratio, 0.46), the replication cohort (OR, 0.38), and the combined cohort (OR, 0.41). Furthermore, ever-users were less commonly represented among patients with ILD regardless of chest high-resolution CT pattern (usual interstitial pneumonia pattern vs. not).
Finally, methotrexate use appeared to delay the adjusted time to onset of ILD by 3.5 years in the discovery cohort (P = .001), by 3.2 years in the replication cohort (P < .0001), and by 3.5 years in the combined cohort (P < .0001).
“Outside of methotrexate hypersensitivity pneumonitis, methotrexate was not a risk factor for RA-associated ILD in our study. We observed an inverse relationship that was similar whatever the high-resolution CT pattern,” Dr. Juge commented. “But this possible protective effect should be confirmed through a dedicated prospective, randomized, controlled trial.”
“Methotrexate should not be considered as a causal factor for RA-associated ILD, and its [discontinuation] should be discussed through a multidisciplinary discussion,” he recommended. In addition, “this study does not investigate the impact of methotrexate use on RA-associated ILD prognosis.”
The Danish study did not receive any specific funding, and none of its authors reported having any financial disclosures. The multinational study did not receive any specific funding. Dr. Juge disclosed that he had no relevant conflicts of interest, but many of his coauthors reported financial relationships with industry. Dr. Volkmann disclosed consulting for Boehringer Ingelheim and Forbius, and receiving grant support from Forbius and Corbus.
SOURCES: Cordtz R et al. Ann Rheum Dis. 2020;79[suppl 1]:147-8, Abstract OP0232; Juge P-A et al. Ann Rheum Dis. 2020;79[suppl 1]:25, Abstract OP0236.
Patients with rheumatoid arthritis (RA) have an elevated risk of interstitial lung disease (ILD), but methotrexate does not accentuate that risk and may in fact be protective, new data show. These were among key findings of a pair of studies reported at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Although a guideline-recommended cornerstone in the management of RA, methotrexate has been associated with both hypersensitivity pneumonitis and diffuse lung disease. However, its involvement in the development of ILD among patients with RA is unclear.
A Danish study of more than 30,000 RA patients reported at the congress found that their risk of ILD was about three to five times that of the general population. However, risk did not differ significantly whether they had filled a methotrexate prescription or not.
In addition, a multinational case-control study of more than 1,000 RA patients also reported at the congress found that, compared with never-users of methotrexate, ever-users actually had a 59% lower likelihood of developing ILD.
However, both studies were limited by their retrospective design, Elizabeth R. Volkmann, MD, codirector of the connective tissue disease–related interstitial lung disease program at the University of California, Los Angeles, cautioned in an interview. Hence, there was likely systematic bias and confounding.
“I would interpret the conclusions of both studies with caution,” she maintained. “To understand how a particular intervention, such as methotrexate use, affects the outcome of ILD development, a prospective design is needed, which adequately adjusts for known ILD risk factors, such as male sex and smoking.”
As to whether the new findings are practice changing and how they might affect patient counseling, “the answers to these questions are not straightforward and depend on other patient-related factors,” according to Dr. Volkmann.
Danish nationwide study
René Cordtz, MD, a clinical assistant at the Center for Rheumatology and Spine Diseases, Rigshospitalet‐Gentofte, Copenhagen, and colleagues conducted a nationwide population-based cohort study using registry data from 1997 to 2015 to assess lung disease among patients with RA by prescriptions filled.
Results based on 30,512 RA patients showed that, compared with peers filling no methotrexate prescriptions, patients filling at least one did not have a significantly elevated risk of ILD at either 1 year of follow-up (hazard ratio, 1.03) or 5 years of follow-up (HR, 1.00). (Findings were similar for sulfasalazine, with respective nonsignificant HRs of 0.88 and 1.14.)
In addition, patients with RA had a similarly sharply elevated 5-year risk of ILD relative to the general population regardless of whether they had filled neither methotrexate nor sulfasalazine prescriptions (standardized incidence ratio, 3.38) or had filled prescriptions for methotrexate only (SIR, 3.63), sulfasalazine only (SIR, 4.12), or both (SIR, 5.45).
“RA patients have an increased risk of ILD, compared to the general population, which was not surprising, but very importantly, that risk was not further exacerbated in those treated with methotrexate,” Dr. Cordtz concluded. “We do acknowledge that purchasing your medicine is different from taking your medicine, which is why we found it extra reassuring that when requiring at least two methotrexate prescriptions to be considered exposed, it did not change our results.”
Multinational study
Pierre-Antoine Juge, MD, a rheumatologist at Bichat-Claude Bernard Hospital, Paris, and colleagues performed a case-control study among 482 RA patients with ILD and 741 RA patients without ILD in three cohorts: a French discovery cohort, a multinational (Brazilian, Italian, Mexican, United Kingdom, and United States) replication cohort, and a combined cohort. Those with methotrexate hypersensitivity pneumonitis were excluded.
Results showed that relative to peers without ILD, patients with ILD had a lower prevalence of ever having used methotrexate and had received a lower cumulative methotrexate dose, findings that were consistent across all three cohorts.
Methotrexate ever-use was associated with a significantly lower adjusted likelihood of ILD in the discovery cohort (odds ratio, 0.46), the replication cohort (OR, 0.38), and the combined cohort (OR, 0.41). Furthermore, ever-users were less commonly represented among patients with ILD regardless of chest high-resolution CT pattern (usual interstitial pneumonia pattern vs. not).
Finally, methotrexate use appeared to delay the adjusted time to onset of ILD by 3.5 years in the discovery cohort (P = .001), by 3.2 years in the replication cohort (P < .0001), and by 3.5 years in the combined cohort (P < .0001).
“Outside of methotrexate hypersensitivity pneumonitis, methotrexate was not a risk factor for RA-associated ILD in our study. We observed an inverse relationship that was similar whatever the high-resolution CT pattern,” Dr. Juge commented. “But this possible protective effect should be confirmed through a dedicated prospective, randomized, controlled trial.”
“Methotrexate should not be considered as a causal factor for RA-associated ILD, and its [discontinuation] should be discussed through a multidisciplinary discussion,” he recommended. In addition, “this study does not investigate the impact of methotrexate use on RA-associated ILD prognosis.”
The Danish study did not receive any specific funding, and none of its authors reported having any financial disclosures. The multinational study did not receive any specific funding. Dr. Juge disclosed that he had no relevant conflicts of interest, but many of his coauthors reported financial relationships with industry. Dr. Volkmann disclosed consulting for Boehringer Ingelheim and Forbius, and receiving grant support from Forbius and Corbus.
SOURCES: Cordtz R et al. Ann Rheum Dis. 2020;79[suppl 1]:147-8, Abstract OP0232; Juge P-A et al. Ann Rheum Dis. 2020;79[suppl 1]:25, Abstract OP0236.
Patients with rheumatoid arthritis (RA) have an elevated risk of interstitial lung disease (ILD), but methotrexate does not accentuate that risk and may in fact be protective, new data show. These were among key findings of a pair of studies reported at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Although a guideline-recommended cornerstone in the management of RA, methotrexate has been associated with both hypersensitivity pneumonitis and diffuse lung disease. However, its involvement in the development of ILD among patients with RA is unclear.
A Danish study of more than 30,000 RA patients reported at the congress found that their risk of ILD was about three to five times that of the general population. However, risk did not differ significantly whether they had filled a methotrexate prescription or not.
In addition, a multinational case-control study of more than 1,000 RA patients also reported at the congress found that, compared with never-users of methotrexate, ever-users actually had a 59% lower likelihood of developing ILD.
However, both studies were limited by their retrospective design, Elizabeth R. Volkmann, MD, codirector of the connective tissue disease–related interstitial lung disease program at the University of California, Los Angeles, cautioned in an interview. Hence, there was likely systematic bias and confounding.
“I would interpret the conclusions of both studies with caution,” she maintained. “To understand how a particular intervention, such as methotrexate use, affects the outcome of ILD development, a prospective design is needed, which adequately adjusts for known ILD risk factors, such as male sex and smoking.”
As to whether the new findings are practice changing and how they might affect patient counseling, “the answers to these questions are not straightforward and depend on other patient-related factors,” according to Dr. Volkmann.
Danish nationwide study
René Cordtz, MD, a clinical assistant at the Center for Rheumatology and Spine Diseases, Rigshospitalet‐Gentofte, Copenhagen, and colleagues conducted a nationwide population-based cohort study using registry data from 1997 to 2015 to assess lung disease among patients with RA by prescriptions filled.
Results based on 30,512 RA patients showed that, compared with peers filling no methotrexate prescriptions, patients filling at least one did not have a significantly elevated risk of ILD at either 1 year of follow-up (hazard ratio, 1.03) or 5 years of follow-up (HR, 1.00). (Findings were similar for sulfasalazine, with respective nonsignificant HRs of 0.88 and 1.14.)
In addition, patients with RA had a similarly sharply elevated 5-year risk of ILD relative to the general population regardless of whether they had filled neither methotrexate nor sulfasalazine prescriptions (standardized incidence ratio, 3.38) or had filled prescriptions for methotrexate only (SIR, 3.63), sulfasalazine only (SIR, 4.12), or both (SIR, 5.45).
“RA patients have an increased risk of ILD, compared to the general population, which was not surprising, but very importantly, that risk was not further exacerbated in those treated with methotrexate,” Dr. Cordtz concluded. “We do acknowledge that purchasing your medicine is different from taking your medicine, which is why we found it extra reassuring that when requiring at least two methotrexate prescriptions to be considered exposed, it did not change our results.”
Multinational study
Pierre-Antoine Juge, MD, a rheumatologist at Bichat-Claude Bernard Hospital, Paris, and colleagues performed a case-control study among 482 RA patients with ILD and 741 RA patients without ILD in three cohorts: a French discovery cohort, a multinational (Brazilian, Italian, Mexican, United Kingdom, and United States) replication cohort, and a combined cohort. Those with methotrexate hypersensitivity pneumonitis were excluded.
Results showed that relative to peers without ILD, patients with ILD had a lower prevalence of ever having used methotrexate and had received a lower cumulative methotrexate dose, findings that were consistent across all three cohorts.
Methotrexate ever-use was associated with a significantly lower adjusted likelihood of ILD in the discovery cohort (odds ratio, 0.46), the replication cohort (OR, 0.38), and the combined cohort (OR, 0.41). Furthermore, ever-users were less commonly represented among patients with ILD regardless of chest high-resolution CT pattern (usual interstitial pneumonia pattern vs. not).
Finally, methotrexate use appeared to delay the adjusted time to onset of ILD by 3.5 years in the discovery cohort (P = .001), by 3.2 years in the replication cohort (P < .0001), and by 3.5 years in the combined cohort (P < .0001).
“Outside of methotrexate hypersensitivity pneumonitis, methotrexate was not a risk factor for RA-associated ILD in our study. We observed an inverse relationship that was similar whatever the high-resolution CT pattern,” Dr. Juge commented. “But this possible protective effect should be confirmed through a dedicated prospective, randomized, controlled trial.”
“Methotrexate should not be considered as a causal factor for RA-associated ILD, and its [discontinuation] should be discussed through a multidisciplinary discussion,” he recommended. In addition, “this study does not investigate the impact of methotrexate use on RA-associated ILD prognosis.”
The Danish study did not receive any specific funding, and none of its authors reported having any financial disclosures. The multinational study did not receive any specific funding. Dr. Juge disclosed that he had no relevant conflicts of interest, but many of his coauthors reported financial relationships with industry. Dr. Volkmann disclosed consulting for Boehringer Ingelheim and Forbius, and receiving grant support from Forbius and Corbus.
SOURCES: Cordtz R et al. Ann Rheum Dis. 2020;79[suppl 1]:147-8, Abstract OP0232; Juge P-A et al. Ann Rheum Dis. 2020;79[suppl 1]:25, Abstract OP0236.
FROM THE EULAR 2020 E-CONGRESS
Former smokers using e-cigarettes at risk for cigarette smoking relapse
The use of , results from a large longitudinal cohort study demonstrated.
“For the many clinicians treating former smokers who have successfully quit all nicotine products, the implications are that use of [electronic nicotine delivery systems] should be discouraged, just as use of all other tobacco products is discouraged,” researchers led by Colm D. Everard, PhD, reported in a study published in JAMA Network Open (2020 Jun 5. doi: 10.1001/jamanetworkopen.2020.4813).
Dr. Everard, of the National Institute on Drug Abuse, and colleagues based their comments on results from a survey of adult former smokers who participated in Waves 1-4 of the Population Assessment of Tobacco and Health (PATH) Study (2013-2018). They limited the analysis to 2,273 former cigarette smokers who self-reported reported no tobacco product use at Wave 1, and categorized them as recent former smokers (defined as having last smoked within the past 12 previous months) or as long-term former smokers (defined as having last smoked for longer ago than in the previous 12 months). The main outcome of interest was the self-reported current use of cigarettes at follow-up interviews, which was defined as every day or some days. Electronic nicotine delivery systems (ENDS) comprised e-cigarettes, e-cigars, e-pipes, and e-hookahs. Other tobacco products included cigars, pipe tobacco, hookahs, snus tobacco, other smokeless tobacco, and dissolvable tobacco.
Of the 2,273 adult former smokers, 52% were women, 60% were older than age 50, and 80% were non-Hispanic white. Adjusted hazard ratio (AHR) analysis revealed that the use of ENDS was associated with significant risk of cigarette smoking relapse among recent former smokers (AHR 1.63) and among long-term former smokers (AHR 3.79). The use of other tobacco products was associated with significant risk for cigarette smoking relapse among recent former smokers (AHR 1.97) and among long-term former smokers (AHR 3.82).
The authors acknowledged certain limitations of the study, including the fact that it did not assess different ENDS devices, different e-liquid nicotine levels, or frequency of ENDS use and their associations with cigarette smoking relapse. It also did not explore the mechanism by which ENDS use may lead to reestablishing or reinforcing nicotine-seeking behavior among former cigarette users. “Determining pharmacologic, behavioral, or some other explanation for these findings may require laboratory-based research,” they wrote.
The PATH Study is supported with federal funds from the National Institute on Drug Abuse, the National Institutes of Health, and the Food and Drug Administration and Department of Health and Human Services under a contract to Westat. One of the study authors, Wilson M. Compton, MD, reported having long-term stock holdings in General Electric, 3M, and Pfizer. The other authors reported having no financial disclosures.
The use of , results from a large longitudinal cohort study demonstrated.
“For the many clinicians treating former smokers who have successfully quit all nicotine products, the implications are that use of [electronic nicotine delivery systems] should be discouraged, just as use of all other tobacco products is discouraged,” researchers led by Colm D. Everard, PhD, reported in a study published in JAMA Network Open (2020 Jun 5. doi: 10.1001/jamanetworkopen.2020.4813).
Dr. Everard, of the National Institute on Drug Abuse, and colleagues based their comments on results from a survey of adult former smokers who participated in Waves 1-4 of the Population Assessment of Tobacco and Health (PATH) Study (2013-2018). They limited the analysis to 2,273 former cigarette smokers who self-reported reported no tobacco product use at Wave 1, and categorized them as recent former smokers (defined as having last smoked within the past 12 previous months) or as long-term former smokers (defined as having last smoked for longer ago than in the previous 12 months). The main outcome of interest was the self-reported current use of cigarettes at follow-up interviews, which was defined as every day or some days. Electronic nicotine delivery systems (ENDS) comprised e-cigarettes, e-cigars, e-pipes, and e-hookahs. Other tobacco products included cigars, pipe tobacco, hookahs, snus tobacco, other smokeless tobacco, and dissolvable tobacco.
Of the 2,273 adult former smokers, 52% were women, 60% were older than age 50, and 80% were non-Hispanic white. Adjusted hazard ratio (AHR) analysis revealed that the use of ENDS was associated with significant risk of cigarette smoking relapse among recent former smokers (AHR 1.63) and among long-term former smokers (AHR 3.79). The use of other tobacco products was associated with significant risk for cigarette smoking relapse among recent former smokers (AHR 1.97) and among long-term former smokers (AHR 3.82).
The authors acknowledged certain limitations of the study, including the fact that it did not assess different ENDS devices, different e-liquid nicotine levels, or frequency of ENDS use and their associations with cigarette smoking relapse. It also did not explore the mechanism by which ENDS use may lead to reestablishing or reinforcing nicotine-seeking behavior among former cigarette users. “Determining pharmacologic, behavioral, or some other explanation for these findings may require laboratory-based research,” they wrote.
The PATH Study is supported with federal funds from the National Institute on Drug Abuse, the National Institutes of Health, and the Food and Drug Administration and Department of Health and Human Services under a contract to Westat. One of the study authors, Wilson M. Compton, MD, reported having long-term stock holdings in General Electric, 3M, and Pfizer. The other authors reported having no financial disclosures.
The use of , results from a large longitudinal cohort study demonstrated.
“For the many clinicians treating former smokers who have successfully quit all nicotine products, the implications are that use of [electronic nicotine delivery systems] should be discouraged, just as use of all other tobacco products is discouraged,” researchers led by Colm D. Everard, PhD, reported in a study published in JAMA Network Open (2020 Jun 5. doi: 10.1001/jamanetworkopen.2020.4813).
Dr. Everard, of the National Institute on Drug Abuse, and colleagues based their comments on results from a survey of adult former smokers who participated in Waves 1-4 of the Population Assessment of Tobacco and Health (PATH) Study (2013-2018). They limited the analysis to 2,273 former cigarette smokers who self-reported reported no tobacco product use at Wave 1, and categorized them as recent former smokers (defined as having last smoked within the past 12 previous months) or as long-term former smokers (defined as having last smoked for longer ago than in the previous 12 months). The main outcome of interest was the self-reported current use of cigarettes at follow-up interviews, which was defined as every day or some days. Electronic nicotine delivery systems (ENDS) comprised e-cigarettes, e-cigars, e-pipes, and e-hookahs. Other tobacco products included cigars, pipe tobacco, hookahs, snus tobacco, other smokeless tobacco, and dissolvable tobacco.
Of the 2,273 adult former smokers, 52% were women, 60% were older than age 50, and 80% were non-Hispanic white. Adjusted hazard ratio (AHR) analysis revealed that the use of ENDS was associated with significant risk of cigarette smoking relapse among recent former smokers (AHR 1.63) and among long-term former smokers (AHR 3.79). The use of other tobacco products was associated with significant risk for cigarette smoking relapse among recent former smokers (AHR 1.97) and among long-term former smokers (AHR 3.82).
The authors acknowledged certain limitations of the study, including the fact that it did not assess different ENDS devices, different e-liquid nicotine levels, or frequency of ENDS use and their associations with cigarette smoking relapse. It also did not explore the mechanism by which ENDS use may lead to reestablishing or reinforcing nicotine-seeking behavior among former cigarette users. “Determining pharmacologic, behavioral, or some other explanation for these findings may require laboratory-based research,” they wrote.
The PATH Study is supported with federal funds from the National Institute on Drug Abuse, the National Institutes of Health, and the Food and Drug Administration and Department of Health and Human Services under a contract to Westat. One of the study authors, Wilson M. Compton, MD, reported having long-term stock holdings in General Electric, 3M, and Pfizer. The other authors reported having no financial disclosures.
FROM JAMA NETWORK OPEN
WHO clarifies comments on asymptomatic transmission of SARS-CoV-2
A World Health Organization (WHO) official is walking back her comments characterizing the spread of SARS-CoV-2 by asymptomatic individuals as “rare.”
Maria Van Kerkhove, PhD, WHO’s COVID-19 technical lead and an infectious disease epidemiologist, caused a stir June 8 when she said that countries are reporting that many of their asymptomatic cases develop into cases of mild disease. For patients with truly asymptomatic disease, countries are “not finding secondary transmission onward. It’s very rare,” she said.
Suppressing symptomatic cases, on the other hand, would result in a “drastic reduction” in transmission, she noted. “But from the data we have, it still seems to be rare that an asymptomatic person actually transmits onward to a secondary individual,” she said.
But on June 9 – following a day of confusion and criticism – Dr. Van Kerkhove sought to clarify her comments on asymptomatic transmission during a live social media Q&A. She noted that while “the majority of transmission that we know about” is through individuals with symptoms, “there are a subset of people who don’t develop symptoms, and to truly understand how many people don’t have symptoms – we don’t actually have that answer yet.”
Between 6% and 41% of individuals may be asymptomatic based on estimates, she acknowledged.“What we need to better understand is how many of the people in the population don’t have symptoms, and separately, how many of those individuals go on to transmit to others,” she said.
Dr. Van Kerkhove also emphasized that her initial comments were made in response to a question raised at the press conference, and called it a misunderstanding. “I wasn’t stating a policy of WHO or anything like that,” she said. “I was just trying to articulate what we know.”
The phrase “very rare” referred to a subset of studies and reports WHO had received from its member states following asymptomatic individuals with COVID-19. “I was referring to some detailed investigations, cluster investigations, case contact tracing, where we had reports from member states saying that, when we follow asymptomatic cases, it’s very rare – and I used the phrase very rare – that we found a secondary transmission,” she said.
Dr. Van Kerkhove’s initial comments drew criticism from medical and public health professionals, who said the statement was “confusing” and communicated poorly.
Eric J. Topol, MD, tweeted that WHO had “engendered considerable confusion” with the comments about asymptomatic individuals rarely transmitting SARS-CoV-2. Dr. Topol, the author of a recent analysis published in Annals of Internal Medicine that suggested as many as 40%-45% of COVID-19 cases may be asymptomatic, said that it was not possible to determine whether asymptomatic individuals in the cohorts he studied were capable of spread like pre-symptomatic individuals. “We only know the viral loads are similar from multiple reports. And we do know some spread occurs from [asymptomatic] people,” he said.
Andy Slavitt, former acting administrator of the Centers for Medicare and Medicaid Services, said in a tweet that he believed WHO made “an irresponsible statement even though it was based on legitimate observations.” Reports by Member States do not reach a “bar of rigor,” he said.
Natalie E. Dean, PhD, assistant professor of biostatistics at the University of Florida, tweeted that the initial comments by the WHO seemed to be trying to draw a distinction between asymptomatic individuals who never develop symptoms, and presymptomatic individuals who present as asymptomatic, but later develop symptoms. Finding that asymptomatic cases rarely transmit the virus could change how people exposed to those asymptomatic individuals are monitored, but “it seems more of scientific than practical interest,” she noted. “People without current symptoms could be infectious. Act accordingly.”
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, also weighed in on the controversial WHO comments, telling Good Morning America on June 10 that Dr. Van Kerkhove's initial statement that asymptomatic SARS-CoV-2 transmission is a rare event is "not correct."
This article was updated 6/10/20.
A World Health Organization (WHO) official is walking back her comments characterizing the spread of SARS-CoV-2 by asymptomatic individuals as “rare.”
Maria Van Kerkhove, PhD, WHO’s COVID-19 technical lead and an infectious disease epidemiologist, caused a stir June 8 when she said that countries are reporting that many of their asymptomatic cases develop into cases of mild disease. For patients with truly asymptomatic disease, countries are “not finding secondary transmission onward. It’s very rare,” she said.
Suppressing symptomatic cases, on the other hand, would result in a “drastic reduction” in transmission, she noted. “But from the data we have, it still seems to be rare that an asymptomatic person actually transmits onward to a secondary individual,” she said.
But on June 9 – following a day of confusion and criticism – Dr. Van Kerkhove sought to clarify her comments on asymptomatic transmission during a live social media Q&A. She noted that while “the majority of transmission that we know about” is through individuals with symptoms, “there are a subset of people who don’t develop symptoms, and to truly understand how many people don’t have symptoms – we don’t actually have that answer yet.”
Between 6% and 41% of individuals may be asymptomatic based on estimates, she acknowledged.“What we need to better understand is how many of the people in the population don’t have symptoms, and separately, how many of those individuals go on to transmit to others,” she said.
Dr. Van Kerkhove also emphasized that her initial comments were made in response to a question raised at the press conference, and called it a misunderstanding. “I wasn’t stating a policy of WHO or anything like that,” she said. “I was just trying to articulate what we know.”
The phrase “very rare” referred to a subset of studies and reports WHO had received from its member states following asymptomatic individuals with COVID-19. “I was referring to some detailed investigations, cluster investigations, case contact tracing, where we had reports from member states saying that, when we follow asymptomatic cases, it’s very rare – and I used the phrase very rare – that we found a secondary transmission,” she said.
Dr. Van Kerkhove’s initial comments drew criticism from medical and public health professionals, who said the statement was “confusing” and communicated poorly.
Eric J. Topol, MD, tweeted that WHO had “engendered considerable confusion” with the comments about asymptomatic individuals rarely transmitting SARS-CoV-2. Dr. Topol, the author of a recent analysis published in Annals of Internal Medicine that suggested as many as 40%-45% of COVID-19 cases may be asymptomatic, said that it was not possible to determine whether asymptomatic individuals in the cohorts he studied were capable of spread like pre-symptomatic individuals. “We only know the viral loads are similar from multiple reports. And we do know some spread occurs from [asymptomatic] people,” he said.
Andy Slavitt, former acting administrator of the Centers for Medicare and Medicaid Services, said in a tweet that he believed WHO made “an irresponsible statement even though it was based on legitimate observations.” Reports by Member States do not reach a “bar of rigor,” he said.
Natalie E. Dean, PhD, assistant professor of biostatistics at the University of Florida, tweeted that the initial comments by the WHO seemed to be trying to draw a distinction between asymptomatic individuals who never develop symptoms, and presymptomatic individuals who present as asymptomatic, but later develop symptoms. Finding that asymptomatic cases rarely transmit the virus could change how people exposed to those asymptomatic individuals are monitored, but “it seems more of scientific than practical interest,” she noted. “People without current symptoms could be infectious. Act accordingly.”
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, also weighed in on the controversial WHO comments, telling Good Morning America on June 10 that Dr. Van Kerkhove's initial statement that asymptomatic SARS-CoV-2 transmission is a rare event is "not correct."
This article was updated 6/10/20.
A World Health Organization (WHO) official is walking back her comments characterizing the spread of SARS-CoV-2 by asymptomatic individuals as “rare.”
Maria Van Kerkhove, PhD, WHO’s COVID-19 technical lead and an infectious disease epidemiologist, caused a stir June 8 when she said that countries are reporting that many of their asymptomatic cases develop into cases of mild disease. For patients with truly asymptomatic disease, countries are “not finding secondary transmission onward. It’s very rare,” she said.
Suppressing symptomatic cases, on the other hand, would result in a “drastic reduction” in transmission, she noted. “But from the data we have, it still seems to be rare that an asymptomatic person actually transmits onward to a secondary individual,” she said.
But on June 9 – following a day of confusion and criticism – Dr. Van Kerkhove sought to clarify her comments on asymptomatic transmission during a live social media Q&A. She noted that while “the majority of transmission that we know about” is through individuals with symptoms, “there are a subset of people who don’t develop symptoms, and to truly understand how many people don’t have symptoms – we don’t actually have that answer yet.”
Between 6% and 41% of individuals may be asymptomatic based on estimates, she acknowledged.“What we need to better understand is how many of the people in the population don’t have symptoms, and separately, how many of those individuals go on to transmit to others,” she said.
Dr. Van Kerkhove also emphasized that her initial comments were made in response to a question raised at the press conference, and called it a misunderstanding. “I wasn’t stating a policy of WHO or anything like that,” she said. “I was just trying to articulate what we know.”
The phrase “very rare” referred to a subset of studies and reports WHO had received from its member states following asymptomatic individuals with COVID-19. “I was referring to some detailed investigations, cluster investigations, case contact tracing, where we had reports from member states saying that, when we follow asymptomatic cases, it’s very rare – and I used the phrase very rare – that we found a secondary transmission,” she said.
Dr. Van Kerkhove’s initial comments drew criticism from medical and public health professionals, who said the statement was “confusing” and communicated poorly.
Eric J. Topol, MD, tweeted that WHO had “engendered considerable confusion” with the comments about asymptomatic individuals rarely transmitting SARS-CoV-2. Dr. Topol, the author of a recent analysis published in Annals of Internal Medicine that suggested as many as 40%-45% of COVID-19 cases may be asymptomatic, said that it was not possible to determine whether asymptomatic individuals in the cohorts he studied were capable of spread like pre-symptomatic individuals. “We only know the viral loads are similar from multiple reports. And we do know some spread occurs from [asymptomatic] people,” he said.
Andy Slavitt, former acting administrator of the Centers for Medicare and Medicaid Services, said in a tweet that he believed WHO made “an irresponsible statement even though it was based on legitimate observations.” Reports by Member States do not reach a “bar of rigor,” he said.
Natalie E. Dean, PhD, assistant professor of biostatistics at the University of Florida, tweeted that the initial comments by the WHO seemed to be trying to draw a distinction between asymptomatic individuals who never develop symptoms, and presymptomatic individuals who present as asymptomatic, but later develop symptoms. Finding that asymptomatic cases rarely transmit the virus could change how people exposed to those asymptomatic individuals are monitored, but “it seems more of scientific than practical interest,” she noted. “People without current symptoms could be infectious. Act accordingly.”
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, also weighed in on the controversial WHO comments, telling Good Morning America on June 10 that Dr. Van Kerkhove's initial statement that asymptomatic SARS-CoV-2 transmission is a rare event is "not correct."
This article was updated 6/10/20.
Evolving treatment of cystic fibrosis: Path toward a normal lifespan
Cystic fibrosis (CF) is an autosomal recessive disorder affecting thousands of people worldwide. When this genetic disease was first discovered in the first half of the 20th century, the median survival was approximately 5 years of age. Since then, median survival for patients with CF has steadily improved. Today, it is 47.4 years based on Cystic Fibrosis Foundation registry data from 2018. Patients with CF are living longer and staying healthier; the discussion to follow is how patients, researchers, and the CF Foundation reached this point.
In 1938, pediatrician and pathologist Dorothy Anderson observed on the autopsies of children thought to have celiac disease pancreatic lesions she termed “cystic fibrosis of the pancreas.” In addition to the abnormal pancreas, she noted abnormal lungs filled with mucus that obstructed the airways.
Paul Di Sant’Agnese recognized during a heatwave in late 1948 that children with CF were routinely being diagnosed with heatstroke and dehydration. This helped lead to the discovery that these children had elevated salt content in their sweat, paving the way for the development of the sweat chloride test in 1959 by Gibson and Cooke. Not only did Dr. Di Sant’Agnese recognize excess salt in the sweat of patients with CF, but with the help of several concerned parents of children with CF, he established the Cystic Fibrosis Foundation in 1955. The Foundation helped organize the care center model over the next decades, increasing from 30 care centers in 1962 to over 100 in 1978. The care center model also developed a patient registry to track patient care longitudinally.
In June 1989, Francis Collins and Lap-Chee Tsui discovered the location of the CF transmembrane conductance regulator (CFTR) protein using a novel technique called chromosome jumping (Rommens JM, et al. Science. 1989;245[4922]1059). The discovery was a breakthrough in basic science research, but it would take 3 more decades before this discovery could be translated into a medication that could be used by most patients for everyday care.
In the early 1990s, when median survival for patients with CF was 29 years of age, the CF Foundation and Genentech, Inc., coordinated a 24-week multicenter double-blind randomized control trial (RCT) for a new inhaled medication that digested the extracellular DNA from the neutrophils that accumulated in the airways of patients with CF. Inhaled recombinant human DNase in these patients reduced the risk of pulmonary exacerbations and also had a small improvement in pulmonary function in the group compared with the placebo group (Fuchs H, et al. N Engl J Med. 1994;331:637). Five years later, another double-blind RCT demonstrated that inhaled tobramycin in patients with CF whose disease was colonized with Pseudomonas aeruginosa improved pulmonary function and reduced the risk of hospitalizations (Ramsey B, et al. N Engl J Med. 1999;340:23). In 2006, the use of hypertonic saline solution in patients with CF decreased the overall pulmonary exacerbation rate (Elkins MR, et al. N Engl J Med. 2006;354:229). The combination of these inhaled medications, along with inhaled aztreonam, formed the backbone of inhalation therapy for CF care today.
In 1998, even with the ongoing development and approval of new CF medications by the pharmaceutical industry, Robert Beall, CEO of the CF Foundation, realized that he needed to challenge the current drug development paradigm. Instead of trying to convince companies to develop CF medications, he started a concept called venture philanthropy. This concept entailed the CF Foundation financially investing in pharmaceutical companies’ development of new medications. The Foundation first invested in a small company named Aurora Biosciences (known today as Vertex Pharmaceuticals) in 2000. Aurora Biosciences specialized in high throughput screening. This process uses a unique technology allowing one to test the therapeutic reaction of airway cells to thousands of chemical compounds in a single day, instead of using the traditional process of tediously pipetting compounds one by one. Today, the CF Foundation has invested millions of dollars into bioscience research to advance CF care.
In 2011, the results of a study were published in which a small molecule altered defective CFTR protein in patients with CF with the CFTR mutation G551D, thus improving chloride transport at the airway surface. In the original study, after 24 weeks of therapy receiving the medication known as ivacaftor, predicted FEV1 in patients with CF improved 10.6%, and the patients were 55% less likely to have a pulmonary exacerbation compared with those receiving a placebo. This breakthrough provided patients with CF the first medication that could correct the CFTR at the source of the problem (Ramsey BW, et al. N Engl J Med. 2011;365:1663). Ivacaftor was approved by the US FDA in 2012.
Ivacaftor provided proof of concept that using small molecules could improve CFTR function. Ivacaftor was only beneficial to a small percentage of patients and was not effective in patients with CF who had either 1 or 2 F508del CFTR mutations. In 2015, patients with CF with F508del homozygous treated with a combination therapy of lumacaftor/ivacaftor had predicted FEV1% improved 2.6% to 4.0%. More importantly, there was a significant reduction in the number of pulmonary exacerbations per year compared with placebo. Unexpectedly, some of the patients experienced bronchoconstriction while receiving lumacaftor/ivacaftor (Wainwright CE, et al. N Engl J Med. 2015; 373:220). The problem was recognized, and a new small molecule to improve the processing and trafficking of CFTR called tezacaftor was developed. The combination of tezacaftor/ivacaftor in patients with CF who were F508del homozygous demonstrated a similar reduction in pulmonary exacerbations, an absolute improvement of predicted FEV1 of 4%, and no increased respiratory symptoms compared with the placebo arm (Taylor-Cousar JL, et al. N Engl J Med. 2017;377[21]2013).
CFTR modulators were a major breakthrough for patients with CF, but the efficacy of these therapies was dependent on the patients’ genotype and ranged from mildly to moderately effective. Unfortunately, these therapies were ineffective for the patients who were delta 508 heterozygotes. Starting in the summer of 2018, VX 445-tezacaftor-ivacaftor (ETI) was compared with placebo in patients with CF who were 1 copy of F508del and a second CFTR mutation that has minimal function. The study found an absolute improvement in predicted FEV1 of 14.3% and a 63% reduction in exacerbations at 24 weeks compared with placebo (Middleton PG, et al. N Engl J Med. 2019;381:1809). In late 2019, based on these data, ETI was approved by the FDA for all patients with CF who were F508del heterozygous. This innovation provided effective therapy to 90% of the CF population.
With the discovery of many highly effective therapies beneficial in most patients, the CF Foundation started a program called Path to a Cure to find therapies for the 10% of patients with CF who were not candidates for ETI or other CFTR modulators. This program looks to develop novel methods to restore CFTR protein function and repair or replace the CFTR protein via gene editing or gene transfer. This process creates many challenges that are quite complex, but patients, researchers, physicians, and CF Foundation will not stop working until CF stands for CURE FOUND.
Today, patients with CF are living longer, and many are eligible or have already started ETI therapy. This medication and the many others being developed will hopefully lead to patients with CF living a normal lifespan in the near future.
Dr. Finklea is Assistant Professor of Medicine, Division of Pulmonary and Critical Care, University of Texas Southwestern, Dallas, Texas. Dr. Finklea receives grant support from the Cystic Fibrosis Foundation.
Cystic fibrosis (CF) is an autosomal recessive disorder affecting thousands of people worldwide. When this genetic disease was first discovered in the first half of the 20th century, the median survival was approximately 5 years of age. Since then, median survival for patients with CF has steadily improved. Today, it is 47.4 years based on Cystic Fibrosis Foundation registry data from 2018. Patients with CF are living longer and staying healthier; the discussion to follow is how patients, researchers, and the CF Foundation reached this point.
In 1938, pediatrician and pathologist Dorothy Anderson observed on the autopsies of children thought to have celiac disease pancreatic lesions she termed “cystic fibrosis of the pancreas.” In addition to the abnormal pancreas, she noted abnormal lungs filled with mucus that obstructed the airways.
Paul Di Sant’Agnese recognized during a heatwave in late 1948 that children with CF were routinely being diagnosed with heatstroke and dehydration. This helped lead to the discovery that these children had elevated salt content in their sweat, paving the way for the development of the sweat chloride test in 1959 by Gibson and Cooke. Not only did Dr. Di Sant’Agnese recognize excess salt in the sweat of patients with CF, but with the help of several concerned parents of children with CF, he established the Cystic Fibrosis Foundation in 1955. The Foundation helped organize the care center model over the next decades, increasing from 30 care centers in 1962 to over 100 in 1978. The care center model also developed a patient registry to track patient care longitudinally.
In June 1989, Francis Collins and Lap-Chee Tsui discovered the location of the CF transmembrane conductance regulator (CFTR) protein using a novel technique called chromosome jumping (Rommens JM, et al. Science. 1989;245[4922]1059). The discovery was a breakthrough in basic science research, but it would take 3 more decades before this discovery could be translated into a medication that could be used by most patients for everyday care.
In the early 1990s, when median survival for patients with CF was 29 years of age, the CF Foundation and Genentech, Inc., coordinated a 24-week multicenter double-blind randomized control trial (RCT) for a new inhaled medication that digested the extracellular DNA from the neutrophils that accumulated in the airways of patients with CF. Inhaled recombinant human DNase in these patients reduced the risk of pulmonary exacerbations and also had a small improvement in pulmonary function in the group compared with the placebo group (Fuchs H, et al. N Engl J Med. 1994;331:637). Five years later, another double-blind RCT demonstrated that inhaled tobramycin in patients with CF whose disease was colonized with Pseudomonas aeruginosa improved pulmonary function and reduced the risk of hospitalizations (Ramsey B, et al. N Engl J Med. 1999;340:23). In 2006, the use of hypertonic saline solution in patients with CF decreased the overall pulmonary exacerbation rate (Elkins MR, et al. N Engl J Med. 2006;354:229). The combination of these inhaled medications, along with inhaled aztreonam, formed the backbone of inhalation therapy for CF care today.
In 1998, even with the ongoing development and approval of new CF medications by the pharmaceutical industry, Robert Beall, CEO of the CF Foundation, realized that he needed to challenge the current drug development paradigm. Instead of trying to convince companies to develop CF medications, he started a concept called venture philanthropy. This concept entailed the CF Foundation financially investing in pharmaceutical companies’ development of new medications. The Foundation first invested in a small company named Aurora Biosciences (known today as Vertex Pharmaceuticals) in 2000. Aurora Biosciences specialized in high throughput screening. This process uses a unique technology allowing one to test the therapeutic reaction of airway cells to thousands of chemical compounds in a single day, instead of using the traditional process of tediously pipetting compounds one by one. Today, the CF Foundation has invested millions of dollars into bioscience research to advance CF care.
In 2011, the results of a study were published in which a small molecule altered defective CFTR protein in patients with CF with the CFTR mutation G551D, thus improving chloride transport at the airway surface. In the original study, after 24 weeks of therapy receiving the medication known as ivacaftor, predicted FEV1 in patients with CF improved 10.6%, and the patients were 55% less likely to have a pulmonary exacerbation compared with those receiving a placebo. This breakthrough provided patients with CF the first medication that could correct the CFTR at the source of the problem (Ramsey BW, et al. N Engl J Med. 2011;365:1663). Ivacaftor was approved by the US FDA in 2012.
Ivacaftor provided proof of concept that using small molecules could improve CFTR function. Ivacaftor was only beneficial to a small percentage of patients and was not effective in patients with CF who had either 1 or 2 F508del CFTR mutations. In 2015, patients with CF with F508del homozygous treated with a combination therapy of lumacaftor/ivacaftor had predicted FEV1% improved 2.6% to 4.0%. More importantly, there was a significant reduction in the number of pulmonary exacerbations per year compared with placebo. Unexpectedly, some of the patients experienced bronchoconstriction while receiving lumacaftor/ivacaftor (Wainwright CE, et al. N Engl J Med. 2015; 373:220). The problem was recognized, and a new small molecule to improve the processing and trafficking of CFTR called tezacaftor was developed. The combination of tezacaftor/ivacaftor in patients with CF who were F508del homozygous demonstrated a similar reduction in pulmonary exacerbations, an absolute improvement of predicted FEV1 of 4%, and no increased respiratory symptoms compared with the placebo arm (Taylor-Cousar JL, et al. N Engl J Med. 2017;377[21]2013).
CFTR modulators were a major breakthrough for patients with CF, but the efficacy of these therapies was dependent on the patients’ genotype and ranged from mildly to moderately effective. Unfortunately, these therapies were ineffective for the patients who were delta 508 heterozygotes. Starting in the summer of 2018, VX 445-tezacaftor-ivacaftor (ETI) was compared with placebo in patients with CF who were 1 copy of F508del and a second CFTR mutation that has minimal function. The study found an absolute improvement in predicted FEV1 of 14.3% and a 63% reduction in exacerbations at 24 weeks compared with placebo (Middleton PG, et al. N Engl J Med. 2019;381:1809). In late 2019, based on these data, ETI was approved by the FDA for all patients with CF who were F508del heterozygous. This innovation provided effective therapy to 90% of the CF population.
With the discovery of many highly effective therapies beneficial in most patients, the CF Foundation started a program called Path to a Cure to find therapies for the 10% of patients with CF who were not candidates for ETI or other CFTR modulators. This program looks to develop novel methods to restore CFTR protein function and repair or replace the CFTR protein via gene editing or gene transfer. This process creates many challenges that are quite complex, but patients, researchers, physicians, and CF Foundation will not stop working until CF stands for CURE FOUND.
Today, patients with CF are living longer, and many are eligible or have already started ETI therapy. This medication and the many others being developed will hopefully lead to patients with CF living a normal lifespan in the near future.
Dr. Finklea is Assistant Professor of Medicine, Division of Pulmonary and Critical Care, University of Texas Southwestern, Dallas, Texas. Dr. Finklea receives grant support from the Cystic Fibrosis Foundation.
Cystic fibrosis (CF) is an autosomal recessive disorder affecting thousands of people worldwide. When this genetic disease was first discovered in the first half of the 20th century, the median survival was approximately 5 years of age. Since then, median survival for patients with CF has steadily improved. Today, it is 47.4 years based on Cystic Fibrosis Foundation registry data from 2018. Patients with CF are living longer and staying healthier; the discussion to follow is how patients, researchers, and the CF Foundation reached this point.
In 1938, pediatrician and pathologist Dorothy Anderson observed on the autopsies of children thought to have celiac disease pancreatic lesions she termed “cystic fibrosis of the pancreas.” In addition to the abnormal pancreas, she noted abnormal lungs filled with mucus that obstructed the airways.
Paul Di Sant’Agnese recognized during a heatwave in late 1948 that children with CF were routinely being diagnosed with heatstroke and dehydration. This helped lead to the discovery that these children had elevated salt content in their sweat, paving the way for the development of the sweat chloride test in 1959 by Gibson and Cooke. Not only did Dr. Di Sant’Agnese recognize excess salt in the sweat of patients with CF, but with the help of several concerned parents of children with CF, he established the Cystic Fibrosis Foundation in 1955. The Foundation helped organize the care center model over the next decades, increasing from 30 care centers in 1962 to over 100 in 1978. The care center model also developed a patient registry to track patient care longitudinally.
In June 1989, Francis Collins and Lap-Chee Tsui discovered the location of the CF transmembrane conductance regulator (CFTR) protein using a novel technique called chromosome jumping (Rommens JM, et al. Science. 1989;245[4922]1059). The discovery was a breakthrough in basic science research, but it would take 3 more decades before this discovery could be translated into a medication that could be used by most patients for everyday care.
In the early 1990s, when median survival for patients with CF was 29 years of age, the CF Foundation and Genentech, Inc., coordinated a 24-week multicenter double-blind randomized control trial (RCT) for a new inhaled medication that digested the extracellular DNA from the neutrophils that accumulated in the airways of patients with CF. Inhaled recombinant human DNase in these patients reduced the risk of pulmonary exacerbations and also had a small improvement in pulmonary function in the group compared with the placebo group (Fuchs H, et al. N Engl J Med. 1994;331:637). Five years later, another double-blind RCT demonstrated that inhaled tobramycin in patients with CF whose disease was colonized with Pseudomonas aeruginosa improved pulmonary function and reduced the risk of hospitalizations (Ramsey B, et al. N Engl J Med. 1999;340:23). In 2006, the use of hypertonic saline solution in patients with CF decreased the overall pulmonary exacerbation rate (Elkins MR, et al. N Engl J Med. 2006;354:229). The combination of these inhaled medications, along with inhaled aztreonam, formed the backbone of inhalation therapy for CF care today.
In 1998, even with the ongoing development and approval of new CF medications by the pharmaceutical industry, Robert Beall, CEO of the CF Foundation, realized that he needed to challenge the current drug development paradigm. Instead of trying to convince companies to develop CF medications, he started a concept called venture philanthropy. This concept entailed the CF Foundation financially investing in pharmaceutical companies’ development of new medications. The Foundation first invested in a small company named Aurora Biosciences (known today as Vertex Pharmaceuticals) in 2000. Aurora Biosciences specialized in high throughput screening. This process uses a unique technology allowing one to test the therapeutic reaction of airway cells to thousands of chemical compounds in a single day, instead of using the traditional process of tediously pipetting compounds one by one. Today, the CF Foundation has invested millions of dollars into bioscience research to advance CF care.
In 2011, the results of a study were published in which a small molecule altered defective CFTR protein in patients with CF with the CFTR mutation G551D, thus improving chloride transport at the airway surface. In the original study, after 24 weeks of therapy receiving the medication known as ivacaftor, predicted FEV1 in patients with CF improved 10.6%, and the patients were 55% less likely to have a pulmonary exacerbation compared with those receiving a placebo. This breakthrough provided patients with CF the first medication that could correct the CFTR at the source of the problem (Ramsey BW, et al. N Engl J Med. 2011;365:1663). Ivacaftor was approved by the US FDA in 2012.
Ivacaftor provided proof of concept that using small molecules could improve CFTR function. Ivacaftor was only beneficial to a small percentage of patients and was not effective in patients with CF who had either 1 or 2 F508del CFTR mutations. In 2015, patients with CF with F508del homozygous treated with a combination therapy of lumacaftor/ivacaftor had predicted FEV1% improved 2.6% to 4.0%. More importantly, there was a significant reduction in the number of pulmonary exacerbations per year compared with placebo. Unexpectedly, some of the patients experienced bronchoconstriction while receiving lumacaftor/ivacaftor (Wainwright CE, et al. N Engl J Med. 2015; 373:220). The problem was recognized, and a new small molecule to improve the processing and trafficking of CFTR called tezacaftor was developed. The combination of tezacaftor/ivacaftor in patients with CF who were F508del homozygous demonstrated a similar reduction in pulmonary exacerbations, an absolute improvement of predicted FEV1 of 4%, and no increased respiratory symptoms compared with the placebo arm (Taylor-Cousar JL, et al. N Engl J Med. 2017;377[21]2013).
CFTR modulators were a major breakthrough for patients with CF, but the efficacy of these therapies was dependent on the patients’ genotype and ranged from mildly to moderately effective. Unfortunately, these therapies were ineffective for the patients who were delta 508 heterozygotes. Starting in the summer of 2018, VX 445-tezacaftor-ivacaftor (ETI) was compared with placebo in patients with CF who were 1 copy of F508del and a second CFTR mutation that has minimal function. The study found an absolute improvement in predicted FEV1 of 14.3% and a 63% reduction in exacerbations at 24 weeks compared with placebo (Middleton PG, et al. N Engl J Med. 2019;381:1809). In late 2019, based on these data, ETI was approved by the FDA for all patients with CF who were F508del heterozygous. This innovation provided effective therapy to 90% of the CF population.
With the discovery of many highly effective therapies beneficial in most patients, the CF Foundation started a program called Path to a Cure to find therapies for the 10% of patients with CF who were not candidates for ETI or other CFTR modulators. This program looks to develop novel methods to restore CFTR protein function and repair or replace the CFTR protein via gene editing or gene transfer. This process creates many challenges that are quite complex, but patients, researchers, physicians, and CF Foundation will not stop working until CF stands for CURE FOUND.
Today, patients with CF are living longer, and many are eligible or have already started ETI therapy. This medication and the many others being developed will hopefully lead to patients with CF living a normal lifespan in the near future.
Dr. Finklea is Assistant Professor of Medicine, Division of Pulmonary and Critical Care, University of Texas Southwestern, Dallas, Texas. Dr. Finklea receives grant support from the Cystic Fibrosis Foundation.
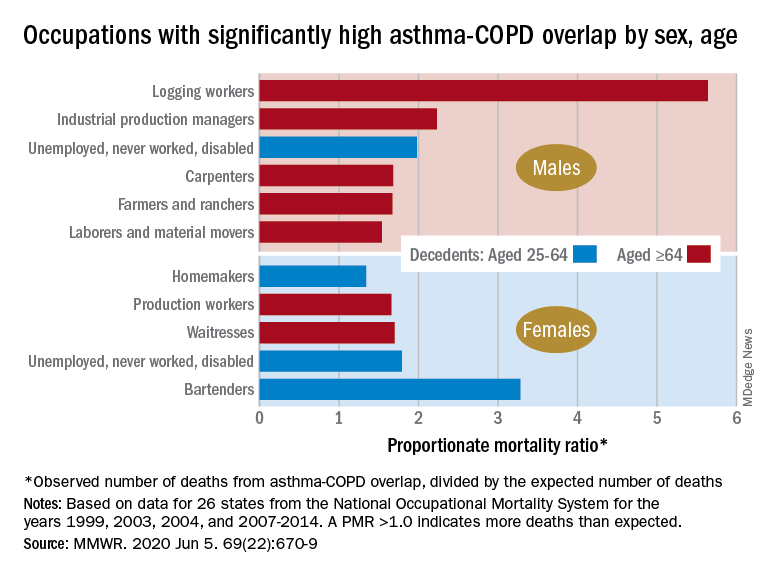
By the numbers: Asthma-COPD overlap deaths
Death rates for combined asthma and chronic obstructive pulmonary disease declined during 1999-2016, but the risk remains higher among women, compared with men, and in certain occupations, according to a recent report from the Centers for Disease Control and Prevention.
There is also an association between mortality and nonworking status among adults aged 25-64 years, which “suggests that asthma-COPD overlap might be associated with substantial morbidity,” Katelynn E. Dodd, MPH, and associates at the CDC’s National Institute for Occupational Safety and Health said in the Morbidity and Mortality Weekly Report. “These patients have been reported to have worse health outcomes than do those with asthma or COPD alone.”
For females with asthma-COPD overlap, the age-adjusted death rate among adults aged 25 years and older dropped from 7.71 per million in 1999 to 4.01 in 2016, with corresponding rates of 6.70 and 3.01 per million for males, they reported.
In 1999-2016, a total of 18,766 U.S. decedents aged ≥25 years had both asthma and COPD assigned as the underlying or contributing cause of death (12,028 women and 6,738 men), for an overall death rate of 5.03 per million persons (women, 5.59; men, 4.30), data from the National Vital Statistics System show.
Additional analysis, based on the calculation of proportionate mortality ratios (PMRs), also showed that mortality varied by occupational status and age for both males and females, the investigators said, noting that workplace exposures, such as dusts and secondhand smoke, are known to cause both asthma and COPD.
The PMR represents the observed number of deaths from asthma-COPD overlap in a specified industry or occupation, divided by the expected number of deaths, so a value over 1.0 indicates that there were more deaths associated with the condition than expected, Ms. Dodd and her associates explained.
Among female decedents, the occupation with the highest PMR that was statistically significant was bartending at 3.28. For men, the highest significant PMR, 5.64, occurred in logging workers. Those rates, however, only applied to one of the two age groups: 25-64 years in women and ≥65 in men, based on data from the National Occupational Mortality Surveillance, which included information from 26 states for the years 1999, 2003, 2004, and 2007-2014.
Occupationally speaking, the one area of common ground between males and females was lack of occupation. PMRs for those aged 25-64 years “were significantly elevated among men (1.98) and women (1.79) who were unemployed, never worked, or were disabled workers,” they said. PMRs were elevated for nonworking older males and females but were not significant.
The elevated PMRs suggest “that asthma-COPD overlap might be associated with substantial morbidity resulting in loss of employment [because] retired and unemployed persons might have left the workforce because of severe asthma or COPD,” the investigators wrote.
SOURCE: Dodd KE et al. MMWR. 2020 Jun 5. 69(22):670-9.
Death rates for combined asthma and chronic obstructive pulmonary disease declined during 1999-2016, but the risk remains higher among women, compared with men, and in certain occupations, according to a recent report from the Centers for Disease Control and Prevention.

There is also an association between mortality and nonworking status among adults aged 25-64 years, which “suggests that asthma-COPD overlap might be associated with substantial morbidity,” Katelynn E. Dodd, MPH, and associates at the CDC’s National Institute for Occupational Safety and Health said in the Morbidity and Mortality Weekly Report. “These patients have been reported to have worse health outcomes than do those with asthma or COPD alone.”
For females with asthma-COPD overlap, the age-adjusted death rate among adults aged 25 years and older dropped from 7.71 per million in 1999 to 4.01 in 2016, with corresponding rates of 6.70 and 3.01 per million for males, they reported.
In 1999-2016, a total of 18,766 U.S. decedents aged ≥25 years had both asthma and COPD assigned as the underlying or contributing cause of death (12,028 women and 6,738 men), for an overall death rate of 5.03 per million persons (women, 5.59; men, 4.30), data from the National Vital Statistics System show.
Additional analysis, based on the calculation of proportionate mortality ratios (PMRs), also showed that mortality varied by occupational status and age for both males and females, the investigators said, noting that workplace exposures, such as dusts and secondhand smoke, are known to cause both asthma and COPD.
The PMR represents the observed number of deaths from asthma-COPD overlap in a specified industry or occupation, divided by the expected number of deaths, so a value over 1.0 indicates that there were more deaths associated with the condition than expected, Ms. Dodd and her associates explained.
Among female decedents, the occupation with the highest PMR that was statistically significant was bartending at 3.28. For men, the highest significant PMR, 5.64, occurred in logging workers. Those rates, however, only applied to one of the two age groups: 25-64 years in women and ≥65 in men, based on data from the National Occupational Mortality Surveillance, which included information from 26 states for the years 1999, 2003, 2004, and 2007-2014.
Occupationally speaking, the one area of common ground between males and females was lack of occupation. PMRs for those aged 25-64 years “were significantly elevated among men (1.98) and women (1.79) who were unemployed, never worked, or were disabled workers,” they said. PMRs were elevated for nonworking older males and females but were not significant.
The elevated PMRs suggest “that asthma-COPD overlap might be associated with substantial morbidity resulting in loss of employment [because] retired and unemployed persons might have left the workforce because of severe asthma or COPD,” the investigators wrote.
SOURCE: Dodd KE et al. MMWR. 2020 Jun 5. 69(22):670-9.
Death rates for combined asthma and chronic obstructive pulmonary disease declined during 1999-2016, but the risk remains higher among women, compared with men, and in certain occupations, according to a recent report from the Centers for Disease Control and Prevention.

There is also an association between mortality and nonworking status among adults aged 25-64 years, which “suggests that asthma-COPD overlap might be associated with substantial morbidity,” Katelynn E. Dodd, MPH, and associates at the CDC’s National Institute for Occupational Safety and Health said in the Morbidity and Mortality Weekly Report. “These patients have been reported to have worse health outcomes than do those with asthma or COPD alone.”
For females with asthma-COPD overlap, the age-adjusted death rate among adults aged 25 years and older dropped from 7.71 per million in 1999 to 4.01 in 2016, with corresponding rates of 6.70 and 3.01 per million for males, they reported.
In 1999-2016, a total of 18,766 U.S. decedents aged ≥25 years had both asthma and COPD assigned as the underlying or contributing cause of death (12,028 women and 6,738 men), for an overall death rate of 5.03 per million persons (women, 5.59; men, 4.30), data from the National Vital Statistics System show.
Additional analysis, based on the calculation of proportionate mortality ratios (PMRs), also showed that mortality varied by occupational status and age for both males and females, the investigators said, noting that workplace exposures, such as dusts and secondhand smoke, are known to cause both asthma and COPD.
The PMR represents the observed number of deaths from asthma-COPD overlap in a specified industry or occupation, divided by the expected number of deaths, so a value over 1.0 indicates that there were more deaths associated with the condition than expected, Ms. Dodd and her associates explained.
Among female decedents, the occupation with the highest PMR that was statistically significant was bartending at 3.28. For men, the highest significant PMR, 5.64, occurred in logging workers. Those rates, however, only applied to one of the two age groups: 25-64 years in women and ≥65 in men, based on data from the National Occupational Mortality Surveillance, which included information from 26 states for the years 1999, 2003, 2004, and 2007-2014.
Occupationally speaking, the one area of common ground between males and females was lack of occupation. PMRs for those aged 25-64 years “were significantly elevated among men (1.98) and women (1.79) who were unemployed, never worked, or were disabled workers,” they said. PMRs were elevated for nonworking older males and females but were not significant.
The elevated PMRs suggest “that asthma-COPD overlap might be associated with substantial morbidity resulting in loss of employment [because] retired and unemployed persons might have left the workforce because of severe asthma or COPD,” the investigators wrote.
SOURCE: Dodd KE et al. MMWR. 2020 Jun 5. 69(22):670-9.
FROM MMWR
Pandemic conditions can complicate care of patients with PAH
in these patients, according to a research article published in Pulmonary Circulation.
“The impetus for this manuscript was a recent discussion within the Pulmonary Hypertension Association (PHA) and [its] Scientific Leadership Council who expressed a need for guidelines from experts in the field,” wrote John J. Ryan, MD, of the University of Utah, Salt Lake City, and colleagues.
The authors highlight some of the unique challenges in caring for patients with pulmonary hypertension (PH), particularly pulmonary arterial hypertension (PAH), in the context of the COVID-19 pandemic.
Telemedicine and temporary visit schedules for new and returning PAH patients can help reduce risk of virus transmission, if patient accessibility to telemedicine is feasible. Protocols to reduce the risk of virus exposure or transmission in the office setting included less frequent echocardiography and 6-Minute Walk Tests (6MWTs) for patients in stable condition. In stable patients, “avoid pulmonary function of V/Q tests when possible,” the authors wrote.
New patients who have been referred for PAH present a challenge in conducting a thorough evaluation that would normally include measurement of invasive hemodynamics in keeping with current diagnostic guidelines. Clinicians will need to balance the potential risks of COVID-19 exposure during elective procedures against the benefits of full evaluations to plan PAH treatment, the authors noted.
For established patients who are clinically stable, remote visits may be an option, with a risk/benefit assessment of the need for in-person diagnostic tests at the current time, they said. However, telemedicine’s limitations include not only patient accessibility and understanding of audio and video technology, but also inability to accurately measure vital signs, they said.
As for routine testing such as echocardiograms, 6MWTs, and other laboratory testing, “it is important to consider the additive value of these sometimes comprehensive tests in the context of the risks associated with visiting the hospital or clinic to obtain them,” the authors said.
Patients who are unstable and experience worsening right heart failure (RHF) at home may have contracted a COVID-19 infection, but the differential diagnosis includes sepsis, ischemia, and PAH disease progression. “During the current pandemic, fever at home in a PAH patient should be assumed to represent a COVID-19 infection,” and patients with worsening respiratory symptoms that require hospitalization should be tested for COVID-19, the authors emphasized.
Use of ECMO or other intensive interventions should be considered in the context of risk assessment, the authors said. “As a general recommendation, practitioners should consider utilizing an established PAH-specific risk assessment tool to help identify patients who are more likely to survive heroic interventions during the COVID-19 outbreak,” they wrote.
Training and education of PH providers will continue to be limited by the pandemic, and many clinical trials and research programs have been suspended and will need to be restructured to minimize risk of transmission of the COVID-19 virus, the authors said. However, health care providers must continue to provide PAH patients and families with advice and updates in best practices, while “acknowledging that the situation changes rapidly,” they concluded.
Dr. Ryan disclosed participating on the speakers bureau, and provides consulting services for, Actelion and Bayer, as well as research support from the Reagan Corporation, the Gordon Family, and the Cushman Family.
SOURCE: Ryan JJ et al. Pulm Circ. 2020 Apr 29. doi: 10.1177/2045894020920153.
in these patients, according to a research article published in Pulmonary Circulation.
“The impetus for this manuscript was a recent discussion within the Pulmonary Hypertension Association (PHA) and [its] Scientific Leadership Council who expressed a need for guidelines from experts in the field,” wrote John J. Ryan, MD, of the University of Utah, Salt Lake City, and colleagues.
The authors highlight some of the unique challenges in caring for patients with pulmonary hypertension (PH), particularly pulmonary arterial hypertension (PAH), in the context of the COVID-19 pandemic.
Telemedicine and temporary visit schedules for new and returning PAH patients can help reduce risk of virus transmission, if patient accessibility to telemedicine is feasible. Protocols to reduce the risk of virus exposure or transmission in the office setting included less frequent echocardiography and 6-Minute Walk Tests (6MWTs) for patients in stable condition. In stable patients, “avoid pulmonary function of V/Q tests when possible,” the authors wrote.
New patients who have been referred for PAH present a challenge in conducting a thorough evaluation that would normally include measurement of invasive hemodynamics in keeping with current diagnostic guidelines. Clinicians will need to balance the potential risks of COVID-19 exposure during elective procedures against the benefits of full evaluations to plan PAH treatment, the authors noted.
For established patients who are clinically stable, remote visits may be an option, with a risk/benefit assessment of the need for in-person diagnostic tests at the current time, they said. However, telemedicine’s limitations include not only patient accessibility and understanding of audio and video technology, but also inability to accurately measure vital signs, they said.
As for routine testing such as echocardiograms, 6MWTs, and other laboratory testing, “it is important to consider the additive value of these sometimes comprehensive tests in the context of the risks associated with visiting the hospital or clinic to obtain them,” the authors said.
Patients who are unstable and experience worsening right heart failure (RHF) at home may have contracted a COVID-19 infection, but the differential diagnosis includes sepsis, ischemia, and PAH disease progression. “During the current pandemic, fever at home in a PAH patient should be assumed to represent a COVID-19 infection,” and patients with worsening respiratory symptoms that require hospitalization should be tested for COVID-19, the authors emphasized.
Use of ECMO or other intensive interventions should be considered in the context of risk assessment, the authors said. “As a general recommendation, practitioners should consider utilizing an established PAH-specific risk assessment tool to help identify patients who are more likely to survive heroic interventions during the COVID-19 outbreak,” they wrote.
Training and education of PH providers will continue to be limited by the pandemic, and many clinical trials and research programs have been suspended and will need to be restructured to minimize risk of transmission of the COVID-19 virus, the authors said. However, health care providers must continue to provide PAH patients and families with advice and updates in best practices, while “acknowledging that the situation changes rapidly,” they concluded.
Dr. Ryan disclosed participating on the speakers bureau, and provides consulting services for, Actelion and Bayer, as well as research support from the Reagan Corporation, the Gordon Family, and the Cushman Family.
SOURCE: Ryan JJ et al. Pulm Circ. 2020 Apr 29. doi: 10.1177/2045894020920153.
in these patients, according to a research article published in Pulmonary Circulation.
“The impetus for this manuscript was a recent discussion within the Pulmonary Hypertension Association (PHA) and [its] Scientific Leadership Council who expressed a need for guidelines from experts in the field,” wrote John J. Ryan, MD, of the University of Utah, Salt Lake City, and colleagues.
The authors highlight some of the unique challenges in caring for patients with pulmonary hypertension (PH), particularly pulmonary arterial hypertension (PAH), in the context of the COVID-19 pandemic.
Telemedicine and temporary visit schedules for new and returning PAH patients can help reduce risk of virus transmission, if patient accessibility to telemedicine is feasible. Protocols to reduce the risk of virus exposure or transmission in the office setting included less frequent echocardiography and 6-Minute Walk Tests (6MWTs) for patients in stable condition. In stable patients, “avoid pulmonary function of V/Q tests when possible,” the authors wrote.
New patients who have been referred for PAH present a challenge in conducting a thorough evaluation that would normally include measurement of invasive hemodynamics in keeping with current diagnostic guidelines. Clinicians will need to balance the potential risks of COVID-19 exposure during elective procedures against the benefits of full evaluations to plan PAH treatment, the authors noted.
For established patients who are clinically stable, remote visits may be an option, with a risk/benefit assessment of the need for in-person diagnostic tests at the current time, they said. However, telemedicine’s limitations include not only patient accessibility and understanding of audio and video technology, but also inability to accurately measure vital signs, they said.
As for routine testing such as echocardiograms, 6MWTs, and other laboratory testing, “it is important to consider the additive value of these sometimes comprehensive tests in the context of the risks associated with visiting the hospital or clinic to obtain them,” the authors said.
Patients who are unstable and experience worsening right heart failure (RHF) at home may have contracted a COVID-19 infection, but the differential diagnosis includes sepsis, ischemia, and PAH disease progression. “During the current pandemic, fever at home in a PAH patient should be assumed to represent a COVID-19 infection,” and patients with worsening respiratory symptoms that require hospitalization should be tested for COVID-19, the authors emphasized.
Use of ECMO or other intensive interventions should be considered in the context of risk assessment, the authors said. “As a general recommendation, practitioners should consider utilizing an established PAH-specific risk assessment tool to help identify patients who are more likely to survive heroic interventions during the COVID-19 outbreak,” they wrote.
Training and education of PH providers will continue to be limited by the pandemic, and many clinical trials and research programs have been suspended and will need to be restructured to minimize risk of transmission of the COVID-19 virus, the authors said. However, health care providers must continue to provide PAH patients and families with advice and updates in best practices, while “acknowledging that the situation changes rapidly,” they concluded.
Dr. Ryan disclosed participating on the speakers bureau, and provides consulting services for, Actelion and Bayer, as well as research support from the Reagan Corporation, the Gordon Family, and the Cushman Family.
SOURCE: Ryan JJ et al. Pulm Circ. 2020 Apr 29. doi: 10.1177/2045894020920153.
FROM PULMONARY CIRCULATION
Prolonged azithromycin Tx for asthma?
In “Asthma: Newer Tx options mean more targeted therapy” (J Fam Pract. 2020;65:135-144), Rali et al recommend azithromycin as an add-on therapy to ICS-LABA for a select group of patients with uncontrolled persistent asthma (neutrophilic phenotype)—a Grade C recommendation. However, the best available evidence demonstrates that azithromycin is equally efficacious for uncontrolled persistent eosinophilic asthma.1,2 Thus, family physicians need not refer patients for bronchoscopy to identify the inflammatory “phenotype.”
An important unanswered question is whether azithromycin needs to be administered continuously. Emerging evidence indicates that some patients may experience prolonged benefit after time-limited azithromycin treatment. This suggests that the mechanism of action, which has been described as anti-inflammatory, is (at least in part) antimicrobial.3
For azithromycin-treated asthma patients who experience a significant clinical response after 3 to 6 months of treatment, I recommend that the prescribing clinician try taking the patient off azithromycin to assess whether clinical improvement persists or wanes. Nothing is lost, and much is gained, by this approach; patients who relapse can resume azithromycin, and patients who remain improved are spared exposure to an unnecessary and prolonged treatment.
David L. Hahn, MD, MS
Madison, WI
1. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390: 659-668.
2. Gibson PG, Yang IA, Upham JW, et al. Efficacy of azithromycin in severe asthma from the AMAZES randomised trial. ERJ Open Res. 2019;5.
3. Hahn D. When guideline treatment of asthma fails, consider a macrolide antibiotic. J Fam Pract. 2019;68:536-545.
In “Asthma: Newer Tx options mean more targeted therapy” (J Fam Pract. 2020;65:135-144), Rali et al recommend azithromycin as an add-on therapy to ICS-LABA for a select group of patients with uncontrolled persistent asthma (neutrophilic phenotype)—a Grade C recommendation. However, the best available evidence demonstrates that azithromycin is equally efficacious for uncontrolled persistent eosinophilic asthma.1,2 Thus, family physicians need not refer patients for bronchoscopy to identify the inflammatory “phenotype.”
An important unanswered question is whether azithromycin needs to be administered continuously. Emerging evidence indicates that some patients may experience prolonged benefit after time-limited azithromycin treatment. This suggests that the mechanism of action, which has been described as anti-inflammatory, is (at least in part) antimicrobial.3
For azithromycin-treated asthma patients who experience a significant clinical response after 3 to 6 months of treatment, I recommend that the prescribing clinician try taking the patient off azithromycin to assess whether clinical improvement persists or wanes. Nothing is lost, and much is gained, by this approach; patients who relapse can resume azithromycin, and patients who remain improved are spared exposure to an unnecessary and prolonged treatment.
David L. Hahn, MD, MS
Madison, WI
In “Asthma: Newer Tx options mean more targeted therapy” (J Fam Pract. 2020;65:135-144), Rali et al recommend azithromycin as an add-on therapy to ICS-LABA for a select group of patients with uncontrolled persistent asthma (neutrophilic phenotype)—a Grade C recommendation. However, the best available evidence demonstrates that azithromycin is equally efficacious for uncontrolled persistent eosinophilic asthma.1,2 Thus, family physicians need not refer patients for bronchoscopy to identify the inflammatory “phenotype.”
An important unanswered question is whether azithromycin needs to be administered continuously. Emerging evidence indicates that some patients may experience prolonged benefit after time-limited azithromycin treatment. This suggests that the mechanism of action, which has been described as anti-inflammatory, is (at least in part) antimicrobial.3
For azithromycin-treated asthma patients who experience a significant clinical response after 3 to 6 months of treatment, I recommend that the prescribing clinician try taking the patient off azithromycin to assess whether clinical improvement persists or wanes. Nothing is lost, and much is gained, by this approach; patients who relapse can resume azithromycin, and patients who remain improved are spared exposure to an unnecessary and prolonged treatment.
David L. Hahn, MD, MS
Madison, WI
1. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390: 659-668.
2. Gibson PG, Yang IA, Upham JW, et al. Efficacy of azithromycin in severe asthma from the AMAZES randomised trial. ERJ Open Res. 2019;5.
3. Hahn D. When guideline treatment of asthma fails, consider a macrolide antibiotic. J Fam Pract. 2019;68:536-545.
1. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390: 659-668.
2. Gibson PG, Yang IA, Upham JW, et al. Efficacy of azithromycin in severe asthma from the AMAZES randomised trial. ERJ Open Res. 2019;5.
3. Hahn D. When guideline treatment of asthma fails, consider a macrolide antibiotic. J Fam Pract. 2019;68:536-545.
36-year-old man • persistent dry cough • frequent sinus congestion • hemoptysis
THE CASE
A 36-year-old nonsmoking white man presented with an episodic 3-month history of dry cough and nasal allergy symptoms. He reported a past history of sinus allergies but no history of asthma. His illness began with a flu-like syndrome, and he had been treated with antibiotics (amoxicillin and azithromycin) and oral steroids (methylprednisolone) by 2 other physicians for “viral syndrome” and “bronchitis.”
The patient reported some tactile fever initially but none thereafter. Symptoms included episodic wheezing but no overt shortness of breath. In addition to the persistent dry cough, he complained of frequent sinus congestion, post-nasal drip, and sneezing. He became concerned when he noticed a fleck of blood in his phlegm.
Physical exam was unimpressive, except for nasal congestion. His breath sounds were clear. Chest x-ray showed a benign-appearing granuloma in the right lower lobe (no previous films available for comparison). Peak-flow measurements taken in the office were persistently low (58%-70%) but improved with steroids and inhaled albuterol.
Over the following 7 weeks, the patient experienced waxing and waning symptoms. At his follow-up visit, he appeared well; chest auscultation revealed normal breath sounds. He was treated with an additional round of antibiotics (levofloxacin), oral steroids, nasal steroids, and inhaled albuterol.
At 13 weeks from his initial presentation, he developed frank hemoptysis and was diagnosed with a right lower-lobe pneumonia in the emergency department. While hospitalized, his clinical status deteriorated, requiring chest tube placement for a large pleural effusion.
Shortly thereafter, he underwent right middle and lower lobectomies and decortication. Multiple organisms were cultured from the pleural fluid. Tuberculosis testing and acid-fast bacilli stains were negative. No malignant cells were identified. Pathologic examination of the resected lung tissue confirmed the chest x-ray finding of a benign calcified granuloma. Additional testing, including a thin barium esophagram, was performed.
THE DIAGNOSIS
Results of the esophagram revealed a congenital bronchoesophageal fistula (C-BEF) between the patient’s esophagus and right mainstem bronchus, located 15 cm distal to his trachea.
Continue to: DISCUSSION
DISCUSSION
Fistulous connections between the esophagus and bronchi are rare but may arise in the setting of malignancy, trauma, inflammation, or congenital malformation.1 While the precise etiology of C-BEF remains unknown, it is believed to be a consequence of failed tracheoesophageal separation during the early stages of embryonic development.
Prevalence and epidemiology. C-BEF has been reported to occur in 1 in 3000 to 4000 live births, often with concomitant esophageal atresia.2 Infants with esophageal atresia demonstrate clinically significant respiratory symptoms and failure to thrive. However, C-BEF without esophageal atresia may be asymptomatic for years to decades.
Age at diagnosis ranges from 9 days to 83 years.3 Several explanations exist for the prolonged asymptomatic phase of this disease: (1) presence of a membrane overlying the fistula during childhood that subsequently ruptures; (2) presence of a proximal fold of esophageal mucosa overlapping the orifice; (3) antigravitational or upward extension of the fistulous tract from the esophagus; and (4) spasm of the smooth muscle of the fistula.4
Four subtypes. Type I fistulas are associated with a wide-necked congenital diverticulum of the esophagus, which may become inflamed and allow perforation into the nearby lung. Type II fistulas (most common) consist of a short tract running directly from the esophagus to a nearby lobar or segmental bronchus. Type III fistulas involve a communication between the esophagus and a cystic structure within the lung parenchyma. Type IV fistulas run from the esophagus into a sequestered pulmonary segment.1 Our patient had a type II fistula.
Is there a nonspecific cough? The most common signs and symptoms of C-BEF are nonspecific cough, cough after ingestion of fluids or meals, and hemoptysis.5,6 Symptoms may persist for decades prior to diagnosis, and the indolent course of C-BEF may lead to fatal complications such as recalcitrant pneumonia, bronchiectasis, and abscess formation.
Continue to: One test bests others for diagnosis
One test bests others for diagnosis. Plain chest x-ray may indicate enlarged lymph nodes or surrounding airspace disease but will not be able to identify a C-BEF. Computed tomography (CT) of the chest may detect the presence of a C-BEF but does not rule it out. Barium esophagram is the most sensitive test for BEF. Esophagoscopy and bronchoscopy may be helpful once the BEF has been identified, but neither has demonstrated reliability as a first-line test. In this particular case, the C-BEF was not seen on chest CT but was later
To confirm the congenital nature of the fistula, histopathology should be examined. C-BEFs will have a mucosal layer and definitive muscularis layer within the fistulous tract.3,5
Treatment. The preferred method of treatment for C-BEF is thoracotomy with resection of the fistula and insertion of a pleural or muscular flap graft to close the defects in the bronchus and esophagus.7 Alternatively, obliteration of the esophageal defect can be performed using biological glue or silver nitrate. Prognosis after surgical repair is excellent.
Our patient
Two weeks after hospital discharge, the patient was re-admitted for hydropneumothorax and underwent additional surgeries. Unfortunately, he died in the ICU due to a tension pneumothorax while intubated.
THE TAKEAWAY
C-BEF is a rare, insidious condition that may remain asymptomatic into adulthood. After common causes are ruled out, patients with adult-onset nonspecific cough, episodes of coughing after eating/drinking, and hemoptysis should be evaluated for BEF. The most useful diagnostic investigation is barium esophagram. Once C-BEF is identified, prompt surgical management is warranted. Because C-BEF persisting into adulthood is so rare, recommendations regarding diagnosis and treatment are based on expert opinion.
CORRESPONDENCE
Rade N. Pejic, MD, MMM, Department of Family Medicine, Tulane University School of Medicine, 1430 Tulane Avenue, Mailbox #8033, New Orleans, LA 70112; [email protected].
1. Braimbridge MV, Keith HI. Oesophago-bronchial fistula in the adult. Thorax. 1965;20:226-233.
2. Taira N, Kawasaki H, Atsumi E, et al. A rare case of congenital bronchoesophageal fistula in an adult. Int J Surg Case Rep. 2017;36:182-184.
3. Risher WH, Arensman RM, Ochsner JL. Congenital bronchoesophageal fistula. Ann Thorac Surg. 1990;49:500-505.
4. Paul M, John S, Ashton R. Recurrent pneumonia in a 51-year-old woman due to congenital bronchoesophageal fistula. Respir Care. 2011;56:1203-1205.
5. Rämö OJ, Salo JA, Mattila SP. Congenital bronchoesophageal fistula in the adult. Ann Thorac Surg. 1995;59:887-889.
6. Zhang B-S, Zhou N-K, Yu C-H. Congenital bronchoesophageal fistula in adults. World J Gastroenterol. 2011;17:1358-1361.
7. Su L, Wei X-Q, Zhi X-Y, et al. Congenital bronchoesophageal fistula in an adult: a case report. World J Gastroenterol. 2007;13:3776–3777.
THE CASE
A 36-year-old nonsmoking white man presented with an episodic 3-month history of dry cough and nasal allergy symptoms. He reported a past history of sinus allergies but no history of asthma. His illness began with a flu-like syndrome, and he had been treated with antibiotics (amoxicillin and azithromycin) and oral steroids (methylprednisolone) by 2 other physicians for “viral syndrome” and “bronchitis.”
The patient reported some tactile fever initially but none thereafter. Symptoms included episodic wheezing but no overt shortness of breath. In addition to the persistent dry cough, he complained of frequent sinus congestion, post-nasal drip, and sneezing. He became concerned when he noticed a fleck of blood in his phlegm.
Physical exam was unimpressive, except for nasal congestion. His breath sounds were clear. Chest x-ray showed a benign-appearing granuloma in the right lower lobe (no previous films available for comparison). Peak-flow measurements taken in the office were persistently low (58%-70%) but improved with steroids and inhaled albuterol.
Over the following 7 weeks, the patient experienced waxing and waning symptoms. At his follow-up visit, he appeared well; chest auscultation revealed normal breath sounds. He was treated with an additional round of antibiotics (levofloxacin), oral steroids, nasal steroids, and inhaled albuterol.
At 13 weeks from his initial presentation, he developed frank hemoptysis and was diagnosed with a right lower-lobe pneumonia in the emergency department. While hospitalized, his clinical status deteriorated, requiring chest tube placement for a large pleural effusion.
Shortly thereafter, he underwent right middle and lower lobectomies and decortication. Multiple organisms were cultured from the pleural fluid. Tuberculosis testing and acid-fast bacilli stains were negative. No malignant cells were identified. Pathologic examination of the resected lung tissue confirmed the chest x-ray finding of a benign calcified granuloma. Additional testing, including a thin barium esophagram, was performed.
THE DIAGNOSIS
Results of the esophagram revealed a congenital bronchoesophageal fistula (C-BEF) between the patient’s esophagus and right mainstem bronchus, located 15 cm distal to his trachea.
Continue to: DISCUSSION
DISCUSSION
Fistulous connections between the esophagus and bronchi are rare but may arise in the setting of malignancy, trauma, inflammation, or congenital malformation.1 While the precise etiology of C-BEF remains unknown, it is believed to be a consequence of failed tracheoesophageal separation during the early stages of embryonic development.
Prevalence and epidemiology. C-BEF has been reported to occur in 1 in 3000 to 4000 live births, often with concomitant esophageal atresia.2 Infants with esophageal atresia demonstrate clinically significant respiratory symptoms and failure to thrive. However, C-BEF without esophageal atresia may be asymptomatic for years to decades.
Age at diagnosis ranges from 9 days to 83 years.3 Several explanations exist for the prolonged asymptomatic phase of this disease: (1) presence of a membrane overlying the fistula during childhood that subsequently ruptures; (2) presence of a proximal fold of esophageal mucosa overlapping the orifice; (3) antigravitational or upward extension of the fistulous tract from the esophagus; and (4) spasm of the smooth muscle of the fistula.4
Four subtypes. Type I fistulas are associated with a wide-necked congenital diverticulum of the esophagus, which may become inflamed and allow perforation into the nearby lung. Type II fistulas (most common) consist of a short tract running directly from the esophagus to a nearby lobar or segmental bronchus. Type III fistulas involve a communication between the esophagus and a cystic structure within the lung parenchyma. Type IV fistulas run from the esophagus into a sequestered pulmonary segment.1 Our patient had a type II fistula.
Is there a nonspecific cough? The most common signs and symptoms of C-BEF are nonspecific cough, cough after ingestion of fluids or meals, and hemoptysis.5,6 Symptoms may persist for decades prior to diagnosis, and the indolent course of C-BEF may lead to fatal complications such as recalcitrant pneumonia, bronchiectasis, and abscess formation.
Continue to: One test bests others for diagnosis
One test bests others for diagnosis. Plain chest x-ray may indicate enlarged lymph nodes or surrounding airspace disease but will not be able to identify a C-BEF. Computed tomography (CT) of the chest may detect the presence of a C-BEF but does not rule it out. Barium esophagram is the most sensitive test for BEF. Esophagoscopy and bronchoscopy may be helpful once the BEF has been identified, but neither has demonstrated reliability as a first-line test. In this particular case, the C-BEF was not seen on chest CT but was later
To confirm the congenital nature of the fistula, histopathology should be examined. C-BEFs will have a mucosal layer and definitive muscularis layer within the fistulous tract.3,5
Treatment. The preferred method of treatment for C-BEF is thoracotomy with resection of the fistula and insertion of a pleural or muscular flap graft to close the defects in the bronchus and esophagus.7 Alternatively, obliteration of the esophageal defect can be performed using biological glue or silver nitrate. Prognosis after surgical repair is excellent.
Our patient
Two weeks after hospital discharge, the patient was re-admitted for hydropneumothorax and underwent additional surgeries. Unfortunately, he died in the ICU due to a tension pneumothorax while intubated.
THE TAKEAWAY
C-BEF is a rare, insidious condition that may remain asymptomatic into adulthood. After common causes are ruled out, patients with adult-onset nonspecific cough, episodes of coughing after eating/drinking, and hemoptysis should be evaluated for BEF. The most useful diagnostic investigation is barium esophagram. Once C-BEF is identified, prompt surgical management is warranted. Because C-BEF persisting into adulthood is so rare, recommendations regarding diagnosis and treatment are based on expert opinion.
CORRESPONDENCE
Rade N. Pejic, MD, MMM, Department of Family Medicine, Tulane University School of Medicine, 1430 Tulane Avenue, Mailbox #8033, New Orleans, LA 70112; [email protected].
THE CASE
A 36-year-old nonsmoking white man presented with an episodic 3-month history of dry cough and nasal allergy symptoms. He reported a past history of sinus allergies but no history of asthma. His illness began with a flu-like syndrome, and he had been treated with antibiotics (amoxicillin and azithromycin) and oral steroids (methylprednisolone) by 2 other physicians for “viral syndrome” and “bronchitis.”
The patient reported some tactile fever initially but none thereafter. Symptoms included episodic wheezing but no overt shortness of breath. In addition to the persistent dry cough, he complained of frequent sinus congestion, post-nasal drip, and sneezing. He became concerned when he noticed a fleck of blood in his phlegm.
Physical exam was unimpressive, except for nasal congestion. His breath sounds were clear. Chest x-ray showed a benign-appearing granuloma in the right lower lobe (no previous films available for comparison). Peak-flow measurements taken in the office were persistently low (58%-70%) but improved with steroids and inhaled albuterol.
Over the following 7 weeks, the patient experienced waxing and waning symptoms. At his follow-up visit, he appeared well; chest auscultation revealed normal breath sounds. He was treated with an additional round of antibiotics (levofloxacin), oral steroids, nasal steroids, and inhaled albuterol.
At 13 weeks from his initial presentation, he developed frank hemoptysis and was diagnosed with a right lower-lobe pneumonia in the emergency department. While hospitalized, his clinical status deteriorated, requiring chest tube placement for a large pleural effusion.
Shortly thereafter, he underwent right middle and lower lobectomies and decortication. Multiple organisms were cultured from the pleural fluid. Tuberculosis testing and acid-fast bacilli stains were negative. No malignant cells were identified. Pathologic examination of the resected lung tissue confirmed the chest x-ray finding of a benign calcified granuloma. Additional testing, including a thin barium esophagram, was performed.
THE DIAGNOSIS
Results of the esophagram revealed a congenital bronchoesophageal fistula (C-BEF) between the patient’s esophagus and right mainstem bronchus, located 15 cm distal to his trachea.
Continue to: DISCUSSION
DISCUSSION
Fistulous connections between the esophagus and bronchi are rare but may arise in the setting of malignancy, trauma, inflammation, or congenital malformation.1 While the precise etiology of C-BEF remains unknown, it is believed to be a consequence of failed tracheoesophageal separation during the early stages of embryonic development.
Prevalence and epidemiology. C-BEF has been reported to occur in 1 in 3000 to 4000 live births, often with concomitant esophageal atresia.2 Infants with esophageal atresia demonstrate clinically significant respiratory symptoms and failure to thrive. However, C-BEF without esophageal atresia may be asymptomatic for years to decades.
Age at diagnosis ranges from 9 days to 83 years.3 Several explanations exist for the prolonged asymptomatic phase of this disease: (1) presence of a membrane overlying the fistula during childhood that subsequently ruptures; (2) presence of a proximal fold of esophageal mucosa overlapping the orifice; (3) antigravitational or upward extension of the fistulous tract from the esophagus; and (4) spasm of the smooth muscle of the fistula.4
Four subtypes. Type I fistulas are associated with a wide-necked congenital diverticulum of the esophagus, which may become inflamed and allow perforation into the nearby lung. Type II fistulas (most common) consist of a short tract running directly from the esophagus to a nearby lobar or segmental bronchus. Type III fistulas involve a communication between the esophagus and a cystic structure within the lung parenchyma. Type IV fistulas run from the esophagus into a sequestered pulmonary segment.1 Our patient had a type II fistula.
Is there a nonspecific cough? The most common signs and symptoms of C-BEF are nonspecific cough, cough after ingestion of fluids or meals, and hemoptysis.5,6 Symptoms may persist for decades prior to diagnosis, and the indolent course of C-BEF may lead to fatal complications such as recalcitrant pneumonia, bronchiectasis, and abscess formation.
Continue to: One test bests others for diagnosis
One test bests others for diagnosis. Plain chest x-ray may indicate enlarged lymph nodes or surrounding airspace disease but will not be able to identify a C-BEF. Computed tomography (CT) of the chest may detect the presence of a C-BEF but does not rule it out. Barium esophagram is the most sensitive test for BEF. Esophagoscopy and bronchoscopy may be helpful once the BEF has been identified, but neither has demonstrated reliability as a first-line test. In this particular case, the C-BEF was not seen on chest CT but was later
To confirm the congenital nature of the fistula, histopathology should be examined. C-BEFs will have a mucosal layer and definitive muscularis layer within the fistulous tract.3,5
Treatment. The preferred method of treatment for C-BEF is thoracotomy with resection of the fistula and insertion of a pleural or muscular flap graft to close the defects in the bronchus and esophagus.7 Alternatively, obliteration of the esophageal defect can be performed using biological glue or silver nitrate. Prognosis after surgical repair is excellent.
Our patient
Two weeks after hospital discharge, the patient was re-admitted for hydropneumothorax and underwent additional surgeries. Unfortunately, he died in the ICU due to a tension pneumothorax while intubated.
THE TAKEAWAY
C-BEF is a rare, insidious condition that may remain asymptomatic into adulthood. After common causes are ruled out, patients with adult-onset nonspecific cough, episodes of coughing after eating/drinking, and hemoptysis should be evaluated for BEF. The most useful diagnostic investigation is barium esophagram. Once C-BEF is identified, prompt surgical management is warranted. Because C-BEF persisting into adulthood is so rare, recommendations regarding diagnosis and treatment are based on expert opinion.
CORRESPONDENCE
Rade N. Pejic, MD, MMM, Department of Family Medicine, Tulane University School of Medicine, 1430 Tulane Avenue, Mailbox #8033, New Orleans, LA 70112; [email protected].
1. Braimbridge MV, Keith HI. Oesophago-bronchial fistula in the adult. Thorax. 1965;20:226-233.
2. Taira N, Kawasaki H, Atsumi E, et al. A rare case of congenital bronchoesophageal fistula in an adult. Int J Surg Case Rep. 2017;36:182-184.
3. Risher WH, Arensman RM, Ochsner JL. Congenital bronchoesophageal fistula. Ann Thorac Surg. 1990;49:500-505.
4. Paul M, John S, Ashton R. Recurrent pneumonia in a 51-year-old woman due to congenital bronchoesophageal fistula. Respir Care. 2011;56:1203-1205.
5. Rämö OJ, Salo JA, Mattila SP. Congenital bronchoesophageal fistula in the adult. Ann Thorac Surg. 1995;59:887-889.
6. Zhang B-S, Zhou N-K, Yu C-H. Congenital bronchoesophageal fistula in adults. World J Gastroenterol. 2011;17:1358-1361.
7. Su L, Wei X-Q, Zhi X-Y, et al. Congenital bronchoesophageal fistula in an adult: a case report. World J Gastroenterol. 2007;13:3776–3777.
1. Braimbridge MV, Keith HI. Oesophago-bronchial fistula in the adult. Thorax. 1965;20:226-233.
2. Taira N, Kawasaki H, Atsumi E, et al. A rare case of congenital bronchoesophageal fistula in an adult. Int J Surg Case Rep. 2017;36:182-184.
3. Risher WH, Arensman RM, Ochsner JL. Congenital bronchoesophageal fistula. Ann Thorac Surg. 1990;49:500-505.
4. Paul M, John S, Ashton R. Recurrent pneumonia in a 51-year-old woman due to congenital bronchoesophageal fistula. Respir Care. 2011;56:1203-1205.
5. Rämö OJ, Salo JA, Mattila SP. Congenital bronchoesophageal fistula in the adult. Ann Thorac Surg. 1995;59:887-889.
6. Zhang B-S, Zhou N-K, Yu C-H. Congenital bronchoesophageal fistula in adults. World J Gastroenterol. 2011;17:1358-1361.
7. Su L, Wei X-Q, Zhi X-Y, et al. Congenital bronchoesophageal fistula in an adult: a case report. World J Gastroenterol. 2007;13:3776–3777.
WHO: Asymptomatic COVID-19 spread deemed ‘rare’
An official with the World Health Organization (WHO) has stated that it appears to be “rare” that an asymptomatic individual can pass SARS-CoV-2 to someone else.
“From the data we have, it still seems to be rare that an asymptomatic person actually transmits onward to a secondary individual,” Maria Van Kerkhove, PhD, WHO’s COVID-19 technical lead and an infectious disease epidemiologist, said June 8 at a news briefing from the agency’s Geneva headquarters.
This announcement came on the heels of the publication of an analysis in the Annals of Internal Medicine, which suggested that as many as 40-45% of COVID-19 cases may be asymptomatic. In this paper, the authors, Daniel P. Oran, AM, and Eric J. Topol, MD, of the Scripps Research Translational Institute in La Jolla, Calif stated: “The likelihood that approximately 40%-45% of those infected with SARS-CoV-2 will remain asymptomatic suggests that the virus might have greater potential than previously estimated to spread silently and deeply through human populations.”
"The early data that we have assembled on the prevalence of asymptomatic SARS-CoV-2 infection suggest that this is a significant factor in the rapid progression of the COVID-19 pandemic," the authors concluded.
Dr. Van Kerkhove also made comments suggesting otherwise on Twitter, citing a new summary by WHO: “@WHO recently published a summary of transmission of #COVID19, incl. symptomatic, pre-symptomatic and asymptomatic transmission.”
She also tweeted the following lines from the WHO summary: “Comprehensive studies on transmission from asymptomatic individuals are difficult to conduct, but the available evidence from contact tracing reported by Member States suggests that asymptomatically-infected individuals are much less likely to transmit the virus than those who develop symptoms.”
In an additional post, Dr. Van Kerkhove added: “In these data, it is important to breakdown truly asymptomatic vs pre-symptomatic vs mildly symptomatic... also to note that the [percentage] reported or estimated to be ‘asymptomatic’ is not the same as the [percentage] that are asymptomatic that actually transmit.”
In the paper published in the Annals of Internal Medicine, Mr. Oran and Dr. Topol analyzed data of asymptomatic individuals from 16 cohorts between April 19 and May 26, 2020 – a wide-ranging group consisting of residents of cities, health care workers, individuals in homeless shelters, obstetric patients, residents of a nursing home, crew members of aircraft carriers, passengers on cruise ships, and inmates in correctional facilities. Each cohort had varying rates of asymptomatic or presymptomatic cases..
When residents of Iceland were tested, 43 of 100 individuals who tested positive for SARS-CoV-2 did not show symptoms. In Vo’, Italy, 30 of 73 people (41.1%) with positive SARS-CoV-2 test results did not have symptoms in a first round of testing, and 13 of 29 (44.8%) had no symptoms in a second round of testing. Over half of residents of San Francisco’s Mission District who received testing (39 of 74; 52.7%) did not have symptoms, while slightly less than half of Indiana residents tested showed no symptoms (35 of 78; 44.8%).
A majority of 41 individuals (65.9%) who were mostly health care workers at Rutgers University reported no symptoms of COVID-19 at the time of testing. Data from homeless shelters in Boston (129 of 147; 87.7%) and Los Angeles (27 of 43; 62.7%) also showed a high rate of individuals without symptoms. Among 33 obstetric patients in New York City who tested positive for SARS-CoV-2, 29 women (87.9%) were asymptomatic during a median 2-day length of stay. In a Washington state nursing facility, 12 of 23 individuals (52.1%) were positive for SARS-CoV-2 without showing symptoms in a first round of testing, with another 15 of 24 residents (62.5%) not showing symptoms in a second round of testing. Of these residents, 24 individuals (88.9%) later went on to show symptoms of COVID-19.
Most of the 783 Greek citizens who tested positive for SARS-CoV-2 after being evacuated from Spain, Turkey, and the United Kingdom showed no symptoms of COVID-19 (35 of 40; 87.5%). A group of 565 Japanese citizens evacuated from Wuhan, China, had a lower number of cases without initial symptoms – 13 people were positive for SARS-CoV-2, and 4 of 13 (30.8%) had no symptoms.
In closed cohorts, there appeared to also be a high rate of COVID-19 cases without initial symptoms. Of 3,277 inmates from correctional facilities in Arkansas, North Carolina, Ohio, and Virginia, 3,146 individuals (96%) had no symptoms at the time of testing. There was also a large percentage of passengers and crew of the Diamond Princess cruise ship (331 of 712; 46.5%) and an Argentine cruise ship (104 of 128; 81.3%) who were positive for SARS-CoV-2 without symptoms. On the aircraft carrier U.S.S. Theodore Roosevelt, 60% of 856 individuals, while on the French aircraft carrier Charles de Gaulle, nearly 50% of individuals were asymptomatic.
It is difficult to tell the difference between people who are presymptomatic and will later go on to develop symptoms of COVID-19 and those who will remain asymptomatic. “The simple solution to this conundrum is longitudinal testing – that is, repeated observations of the individual over time,” but only 5 of 16 cohorts studied had longitudinal data on individuals, Mr. Oran and Dr. Topol said.
Seth Trueger, MD, an emergency physician and assistant professor of emergency medicine at Northwestern University, Chicago, who was not involved in the study, said it was important to see this information all in one place, even if the data isn’t new.
“I think we’ve certainly kind of seen from the beginning there’s some level of asymptomatic and presymptomatic spread,” Dr. Trueger said. “In health care, we’ve been lucky to get those lessons early on and start to think of things like universal masking in hospitals, and unfortunate things like limiting visitors.”
A more nuanced understanding of how SARS-CoV-2 spreads has been difficult to capture, in part because of operating under a shortened time frame and handicapped testing capacity, he noted. “[Even] in the best of possible circumstances, trying to figure out epidemiology in people who don’t have symptoms is really tough,” Dr. Truegar said.
“Even the best studies are still relatively decent samples, and not totally representative,” he added.
Another limitation to capturing accurate data is method of testing. Real-time reverse transcriptase polymerase chain reaction using nasopharyngeal swabs can detect RNA fragments from SARS-CoV-2, which could potentially affect the results. “It’s really hard to know what is actually infected virus versus just fragments of RNA that make the test positive,” Dr. Trueger said.
If the rate of asymptomatic cases is higher than previously thought, it’s a “double-edged sword,” he noted. It may mean the infection fatality rate is lower than predicted, but “even at high levels of what we think community levels might be, we’re far from herd immunity.”
The study authors and Dr. Trueger reported no relevant conflicts of interest.
SOURCE: Oran DP, Topol EJ. Ann Intern Med. 2020 Jun 3. doi: 10.7326/M20-3012.
This article was updated 6/8/20.
An official with the World Health Organization (WHO) has stated that it appears to be “rare” that an asymptomatic individual can pass SARS-CoV-2 to someone else.
“From the data we have, it still seems to be rare that an asymptomatic person actually transmits onward to a secondary individual,” Maria Van Kerkhove, PhD, WHO’s COVID-19 technical lead and an infectious disease epidemiologist, said June 8 at a news briefing from the agency’s Geneva headquarters.
This announcement came on the heels of the publication of an analysis in the Annals of Internal Medicine, which suggested that as many as 40-45% of COVID-19 cases may be asymptomatic. In this paper, the authors, Daniel P. Oran, AM, and Eric J. Topol, MD, of the Scripps Research Translational Institute in La Jolla, Calif stated: “The likelihood that approximately 40%-45% of those infected with SARS-CoV-2 will remain asymptomatic suggests that the virus might have greater potential than previously estimated to spread silently and deeply through human populations.”
"The early data that we have assembled on the prevalence of asymptomatic SARS-CoV-2 infection suggest that this is a significant factor in the rapid progression of the COVID-19 pandemic," the authors concluded.
Dr. Van Kerkhove also made comments suggesting otherwise on Twitter, citing a new summary by WHO: “@WHO recently published a summary of transmission of #COVID19, incl. symptomatic, pre-symptomatic and asymptomatic transmission.”
She also tweeted the following lines from the WHO summary: “Comprehensive studies on transmission from asymptomatic individuals are difficult to conduct, but the available evidence from contact tracing reported by Member States suggests that asymptomatically-infected individuals are much less likely to transmit the virus than those who develop symptoms.”
In an additional post, Dr. Van Kerkhove added: “In these data, it is important to breakdown truly asymptomatic vs pre-symptomatic vs mildly symptomatic... also to note that the [percentage] reported or estimated to be ‘asymptomatic’ is not the same as the [percentage] that are asymptomatic that actually transmit.”
In the paper published in the Annals of Internal Medicine, Mr. Oran and Dr. Topol analyzed data of asymptomatic individuals from 16 cohorts between April 19 and May 26, 2020 – a wide-ranging group consisting of residents of cities, health care workers, individuals in homeless shelters, obstetric patients, residents of a nursing home, crew members of aircraft carriers, passengers on cruise ships, and inmates in correctional facilities. Each cohort had varying rates of asymptomatic or presymptomatic cases..
When residents of Iceland were tested, 43 of 100 individuals who tested positive for SARS-CoV-2 did not show symptoms. In Vo’, Italy, 30 of 73 people (41.1%) with positive SARS-CoV-2 test results did not have symptoms in a first round of testing, and 13 of 29 (44.8%) had no symptoms in a second round of testing. Over half of residents of San Francisco’s Mission District who received testing (39 of 74; 52.7%) did not have symptoms, while slightly less than half of Indiana residents tested showed no symptoms (35 of 78; 44.8%).
A majority of 41 individuals (65.9%) who were mostly health care workers at Rutgers University reported no symptoms of COVID-19 at the time of testing. Data from homeless shelters in Boston (129 of 147; 87.7%) and Los Angeles (27 of 43; 62.7%) also showed a high rate of individuals without symptoms. Among 33 obstetric patients in New York City who tested positive for SARS-CoV-2, 29 women (87.9%) were asymptomatic during a median 2-day length of stay. In a Washington state nursing facility, 12 of 23 individuals (52.1%) were positive for SARS-CoV-2 without showing symptoms in a first round of testing, with another 15 of 24 residents (62.5%) not showing symptoms in a second round of testing. Of these residents, 24 individuals (88.9%) later went on to show symptoms of COVID-19.
Most of the 783 Greek citizens who tested positive for SARS-CoV-2 after being evacuated from Spain, Turkey, and the United Kingdom showed no symptoms of COVID-19 (35 of 40; 87.5%). A group of 565 Japanese citizens evacuated from Wuhan, China, had a lower number of cases without initial symptoms – 13 people were positive for SARS-CoV-2, and 4 of 13 (30.8%) had no symptoms.
In closed cohorts, there appeared to also be a high rate of COVID-19 cases without initial symptoms. Of 3,277 inmates from correctional facilities in Arkansas, North Carolina, Ohio, and Virginia, 3,146 individuals (96%) had no symptoms at the time of testing. There was also a large percentage of passengers and crew of the Diamond Princess cruise ship (331 of 712; 46.5%) and an Argentine cruise ship (104 of 128; 81.3%) who were positive for SARS-CoV-2 without symptoms. On the aircraft carrier U.S.S. Theodore Roosevelt, 60% of 856 individuals, while on the French aircraft carrier Charles de Gaulle, nearly 50% of individuals were asymptomatic.
It is difficult to tell the difference between people who are presymptomatic and will later go on to develop symptoms of COVID-19 and those who will remain asymptomatic. “The simple solution to this conundrum is longitudinal testing – that is, repeated observations of the individual over time,” but only 5 of 16 cohorts studied had longitudinal data on individuals, Mr. Oran and Dr. Topol said.
Seth Trueger, MD, an emergency physician and assistant professor of emergency medicine at Northwestern University, Chicago, who was not involved in the study, said it was important to see this information all in one place, even if the data isn’t new.
“I think we’ve certainly kind of seen from the beginning there’s some level of asymptomatic and presymptomatic spread,” Dr. Trueger said. “In health care, we’ve been lucky to get those lessons early on and start to think of things like universal masking in hospitals, and unfortunate things like limiting visitors.”
A more nuanced understanding of how SARS-CoV-2 spreads has been difficult to capture, in part because of operating under a shortened time frame and handicapped testing capacity, he noted. “[Even] in the best of possible circumstances, trying to figure out epidemiology in people who don’t have symptoms is really tough,” Dr. Truegar said.
“Even the best studies are still relatively decent samples, and not totally representative,” he added.
Another limitation to capturing accurate data is method of testing. Real-time reverse transcriptase polymerase chain reaction using nasopharyngeal swabs can detect RNA fragments from SARS-CoV-2, which could potentially affect the results. “It’s really hard to know what is actually infected virus versus just fragments of RNA that make the test positive,” Dr. Trueger said.
If the rate of asymptomatic cases is higher than previously thought, it’s a “double-edged sword,” he noted. It may mean the infection fatality rate is lower than predicted, but “even at high levels of what we think community levels might be, we’re far from herd immunity.”
The study authors and Dr. Trueger reported no relevant conflicts of interest.
SOURCE: Oran DP, Topol EJ. Ann Intern Med. 2020 Jun 3. doi: 10.7326/M20-3012.
This article was updated 6/8/20.
An official with the World Health Organization (WHO) has stated that it appears to be “rare” that an asymptomatic individual can pass SARS-CoV-2 to someone else.
“From the data we have, it still seems to be rare that an asymptomatic person actually transmits onward to a secondary individual,” Maria Van Kerkhove, PhD, WHO’s COVID-19 technical lead and an infectious disease epidemiologist, said June 8 at a news briefing from the agency’s Geneva headquarters.
This announcement came on the heels of the publication of an analysis in the Annals of Internal Medicine, which suggested that as many as 40-45% of COVID-19 cases may be asymptomatic. In this paper, the authors, Daniel P. Oran, AM, and Eric J. Topol, MD, of the Scripps Research Translational Institute in La Jolla, Calif stated: “The likelihood that approximately 40%-45% of those infected with SARS-CoV-2 will remain asymptomatic suggests that the virus might have greater potential than previously estimated to spread silently and deeply through human populations.”
"The early data that we have assembled on the prevalence of asymptomatic SARS-CoV-2 infection suggest that this is a significant factor in the rapid progression of the COVID-19 pandemic," the authors concluded.
Dr. Van Kerkhove also made comments suggesting otherwise on Twitter, citing a new summary by WHO: “@WHO recently published a summary of transmission of #COVID19, incl. symptomatic, pre-symptomatic and asymptomatic transmission.”
She also tweeted the following lines from the WHO summary: “Comprehensive studies on transmission from asymptomatic individuals are difficult to conduct, but the available evidence from contact tracing reported by Member States suggests that asymptomatically-infected individuals are much less likely to transmit the virus than those who develop symptoms.”
In an additional post, Dr. Van Kerkhove added: “In these data, it is important to breakdown truly asymptomatic vs pre-symptomatic vs mildly symptomatic... also to note that the [percentage] reported or estimated to be ‘asymptomatic’ is not the same as the [percentage] that are asymptomatic that actually transmit.”
In the paper published in the Annals of Internal Medicine, Mr. Oran and Dr. Topol analyzed data of asymptomatic individuals from 16 cohorts between April 19 and May 26, 2020 – a wide-ranging group consisting of residents of cities, health care workers, individuals in homeless shelters, obstetric patients, residents of a nursing home, crew members of aircraft carriers, passengers on cruise ships, and inmates in correctional facilities. Each cohort had varying rates of asymptomatic or presymptomatic cases..
When residents of Iceland were tested, 43 of 100 individuals who tested positive for SARS-CoV-2 did not show symptoms. In Vo’, Italy, 30 of 73 people (41.1%) with positive SARS-CoV-2 test results did not have symptoms in a first round of testing, and 13 of 29 (44.8%) had no symptoms in a second round of testing. Over half of residents of San Francisco’s Mission District who received testing (39 of 74; 52.7%) did not have symptoms, while slightly less than half of Indiana residents tested showed no symptoms (35 of 78; 44.8%).
A majority of 41 individuals (65.9%) who were mostly health care workers at Rutgers University reported no symptoms of COVID-19 at the time of testing. Data from homeless shelters in Boston (129 of 147; 87.7%) and Los Angeles (27 of 43; 62.7%) also showed a high rate of individuals without symptoms. Among 33 obstetric patients in New York City who tested positive for SARS-CoV-2, 29 women (87.9%) were asymptomatic during a median 2-day length of stay. In a Washington state nursing facility, 12 of 23 individuals (52.1%) were positive for SARS-CoV-2 without showing symptoms in a first round of testing, with another 15 of 24 residents (62.5%) not showing symptoms in a second round of testing. Of these residents, 24 individuals (88.9%) later went on to show symptoms of COVID-19.
Most of the 783 Greek citizens who tested positive for SARS-CoV-2 after being evacuated from Spain, Turkey, and the United Kingdom showed no symptoms of COVID-19 (35 of 40; 87.5%). A group of 565 Japanese citizens evacuated from Wuhan, China, had a lower number of cases without initial symptoms – 13 people were positive for SARS-CoV-2, and 4 of 13 (30.8%) had no symptoms.
In closed cohorts, there appeared to also be a high rate of COVID-19 cases without initial symptoms. Of 3,277 inmates from correctional facilities in Arkansas, North Carolina, Ohio, and Virginia, 3,146 individuals (96%) had no symptoms at the time of testing. There was also a large percentage of passengers and crew of the Diamond Princess cruise ship (331 of 712; 46.5%) and an Argentine cruise ship (104 of 128; 81.3%) who were positive for SARS-CoV-2 without symptoms. On the aircraft carrier U.S.S. Theodore Roosevelt, 60% of 856 individuals, while on the French aircraft carrier Charles de Gaulle, nearly 50% of individuals were asymptomatic.
It is difficult to tell the difference between people who are presymptomatic and will later go on to develop symptoms of COVID-19 and those who will remain asymptomatic. “The simple solution to this conundrum is longitudinal testing – that is, repeated observations of the individual over time,” but only 5 of 16 cohorts studied had longitudinal data on individuals, Mr. Oran and Dr. Topol said.
Seth Trueger, MD, an emergency physician and assistant professor of emergency medicine at Northwestern University, Chicago, who was not involved in the study, said it was important to see this information all in one place, even if the data isn’t new.
“I think we’ve certainly kind of seen from the beginning there’s some level of asymptomatic and presymptomatic spread,” Dr. Trueger said. “In health care, we’ve been lucky to get those lessons early on and start to think of things like universal masking in hospitals, and unfortunate things like limiting visitors.”
A more nuanced understanding of how SARS-CoV-2 spreads has been difficult to capture, in part because of operating under a shortened time frame and handicapped testing capacity, he noted. “[Even] in the best of possible circumstances, trying to figure out epidemiology in people who don’t have symptoms is really tough,” Dr. Truegar said.
“Even the best studies are still relatively decent samples, and not totally representative,” he added.
Another limitation to capturing accurate data is method of testing. Real-time reverse transcriptase polymerase chain reaction using nasopharyngeal swabs can detect RNA fragments from SARS-CoV-2, which could potentially affect the results. “It’s really hard to know what is actually infected virus versus just fragments of RNA that make the test positive,” Dr. Trueger said.
If the rate of asymptomatic cases is higher than previously thought, it’s a “double-edged sword,” he noted. It may mean the infection fatality rate is lower than predicted, but “even at high levels of what we think community levels might be, we’re far from herd immunity.”
The study authors and Dr. Trueger reported no relevant conflicts of interest.
SOURCE: Oran DP, Topol EJ. Ann Intern Med. 2020 Jun 3. doi: 10.7326/M20-3012.
This article was updated 6/8/20.
FROM ANNALS OF INTERNAL MEDICINE
FDA approves new antibiotic for HABP/VABP treatment
in people aged 18 years and older.
Approval for Recarbrio was based on results of a randomized, controlled clinical trial of 535 hospitalized adults with hospital-acquired and ventilator-associated bacterial pneumonia who received either Recarbrio or piperacillin-tazobactam. After 28 days, 16% of patients who received Recarbrio and 21% of patients who received piperacillin-tazobactam had died.
The most common adverse events associated with Recarbrio are increased alanine aminotransferase/ aspartate aminotransferase, anemia, diarrhea, hypokalemia, and hyponatremia. Recarbrio was previously approved by the FDA to treat patients with complicated urinary tract infections and complicated intra-abdominal infections who have limited or no alternative treatment options, according to an FDA press release.
“As a public health agency, the FDA addresses the threat of antimicrobial-resistant infections by facilitating the development of safe and effective new treatments. These efforts provide more options to fight serious bacterial infections and get new, safe and effective therapies to patients as soon as possible,” said Sumathi Nambiar, MD, MPH, director of the division of anti-infectives within the office of infectious disease at the Center for Drug Evaluation and Research.
in people aged 18 years and older.
Approval for Recarbrio was based on results of a randomized, controlled clinical trial of 535 hospitalized adults with hospital-acquired and ventilator-associated bacterial pneumonia who received either Recarbrio or piperacillin-tazobactam. After 28 days, 16% of patients who received Recarbrio and 21% of patients who received piperacillin-tazobactam had died.
The most common adverse events associated with Recarbrio are increased alanine aminotransferase/ aspartate aminotransferase, anemia, diarrhea, hypokalemia, and hyponatremia. Recarbrio was previously approved by the FDA to treat patients with complicated urinary tract infections and complicated intra-abdominal infections who have limited or no alternative treatment options, according to an FDA press release.
“As a public health agency, the FDA addresses the threat of antimicrobial-resistant infections by facilitating the development of safe and effective new treatments. These efforts provide more options to fight serious bacterial infections and get new, safe and effective therapies to patients as soon as possible,” said Sumathi Nambiar, MD, MPH, director of the division of anti-infectives within the office of infectious disease at the Center for Drug Evaluation and Research.
in people aged 18 years and older.
Approval for Recarbrio was based on results of a randomized, controlled clinical trial of 535 hospitalized adults with hospital-acquired and ventilator-associated bacterial pneumonia who received either Recarbrio or piperacillin-tazobactam. After 28 days, 16% of patients who received Recarbrio and 21% of patients who received piperacillin-tazobactam had died.
The most common adverse events associated with Recarbrio are increased alanine aminotransferase/ aspartate aminotransferase, anemia, diarrhea, hypokalemia, and hyponatremia. Recarbrio was previously approved by the FDA to treat patients with complicated urinary tract infections and complicated intra-abdominal infections who have limited or no alternative treatment options, according to an FDA press release.
“As a public health agency, the FDA addresses the threat of antimicrobial-resistant infections by facilitating the development of safe and effective new treatments. These efforts provide more options to fight serious bacterial infections and get new, safe and effective therapies to patients as soon as possible,” said Sumathi Nambiar, MD, MPH, director of the division of anti-infectives within the office of infectious disease at the Center for Drug Evaluation and Research.