User login
No clear benefit from conservative oxygen in mechanical ventilation
More conservative oxygen therapy during mechanical ventilation in intensive care does not appear to increase the number of ventilator-free days or reduce mortality, according to a study published online in the New England Journal of Medicine.
Diane Mackle of the Medical Research Institute of New Zealand and her co-authors wrote that hyperoxemia in adults undergoing mechanical ventilation has been associated with increased mortality, as well as fewer days free of ventilation, but there was a lack of data to guide oxygen administration.
In a parallel-group trial, 1,000 adults who were expected to require mechanical ventilation – with an intention-to-treat population of 965 – were randomized either to conservative oxygen therapy or usual therapy. For the conservative therapy, the upper limit of the pulse oximetry alarm would sound when levels reached 97% and the F102 was decreased to 0.21 if the pulse oximetry was above the acceptable lower limit, while usual therapy involved no specific limiting measures. In both groups, the default lower limit for oxygen saturation was 90%.
At day 28 after ventilation, there was no significant difference between the conservative and usual care groups in the number of ventilator-free days (21.3 days vs. 22.1 days). The patients in the conservative oxygen group spent a median of 29 hours receiving an F102 level of 0.21, compared with 1 hour in the usual care group.
The mortality rate at day 180 was 35.7% in the conservative oxygen group, and 34.5% in the usual-oxygen group (HR 1.05, 95% CI 0.85 – 1.30). Researchers also saw no differences between the two groups in paid employment and cognitive function.
In patients with suspected hypoxic-ischemic encephalopathy between-group differences were apparent; At day 28, those in the conservative-oxygen group had a median of 21.1 ventilator-free days, compared with none in the usual-oxygen group. The usual-oxygen group also had a higher 180-day mortality rate than those in the conservative-oxygen group (43% vs. 59%).
“Our data are suggestive of a possible benefit of conservative oxygen therapy in patients with suspected hypoxic-ischemic encephalopathy,” the authors wrote. “It is biologically plausible that conservative oxygen therapy reduces the incidence of secondary brain damage after resuscitation from cardiac arrest, and observational data suggest that exposure to hyperoxemia in such patients may be harmful.”
The authors noted that their trial did not rule out the possibility of benefit or harm had they used a more liberal oxygen regimen in their usual-care group, and that different conservative regimens might also have achieved different outcomes.
The study was funded by the New Zealand Health Research Council. Six authors declared research support for the trial from the study funder, and two declared unrelated research grants from private industry. No other conflicts of interest were declared.
SOURCE: Mackle D et al. NJEM 2019, October 14. DOI:10.1056/NEJMoa1903297.
More conservative oxygen therapy during mechanical ventilation in intensive care does not appear to increase the number of ventilator-free days or reduce mortality, according to a study published online in the New England Journal of Medicine.
Diane Mackle of the Medical Research Institute of New Zealand and her co-authors wrote that hyperoxemia in adults undergoing mechanical ventilation has been associated with increased mortality, as well as fewer days free of ventilation, but there was a lack of data to guide oxygen administration.
In a parallel-group trial, 1,000 adults who were expected to require mechanical ventilation – with an intention-to-treat population of 965 – were randomized either to conservative oxygen therapy or usual therapy. For the conservative therapy, the upper limit of the pulse oximetry alarm would sound when levels reached 97% and the F102 was decreased to 0.21 if the pulse oximetry was above the acceptable lower limit, while usual therapy involved no specific limiting measures. In both groups, the default lower limit for oxygen saturation was 90%.
At day 28 after ventilation, there was no significant difference between the conservative and usual care groups in the number of ventilator-free days (21.3 days vs. 22.1 days). The patients in the conservative oxygen group spent a median of 29 hours receiving an F102 level of 0.21, compared with 1 hour in the usual care group.
The mortality rate at day 180 was 35.7% in the conservative oxygen group, and 34.5% in the usual-oxygen group (HR 1.05, 95% CI 0.85 – 1.30). Researchers also saw no differences between the two groups in paid employment and cognitive function.
In patients with suspected hypoxic-ischemic encephalopathy between-group differences were apparent; At day 28, those in the conservative-oxygen group had a median of 21.1 ventilator-free days, compared with none in the usual-oxygen group. The usual-oxygen group also had a higher 180-day mortality rate than those in the conservative-oxygen group (43% vs. 59%).
“Our data are suggestive of a possible benefit of conservative oxygen therapy in patients with suspected hypoxic-ischemic encephalopathy,” the authors wrote. “It is biologically plausible that conservative oxygen therapy reduces the incidence of secondary brain damage after resuscitation from cardiac arrest, and observational data suggest that exposure to hyperoxemia in such patients may be harmful.”
The authors noted that their trial did not rule out the possibility of benefit or harm had they used a more liberal oxygen regimen in their usual-care group, and that different conservative regimens might also have achieved different outcomes.
The study was funded by the New Zealand Health Research Council. Six authors declared research support for the trial from the study funder, and two declared unrelated research grants from private industry. No other conflicts of interest were declared.
SOURCE: Mackle D et al. NJEM 2019, October 14. DOI:10.1056/NEJMoa1903297.
More conservative oxygen therapy during mechanical ventilation in intensive care does not appear to increase the number of ventilator-free days or reduce mortality, according to a study published online in the New England Journal of Medicine.
Diane Mackle of the Medical Research Institute of New Zealand and her co-authors wrote that hyperoxemia in adults undergoing mechanical ventilation has been associated with increased mortality, as well as fewer days free of ventilation, but there was a lack of data to guide oxygen administration.
In a parallel-group trial, 1,000 adults who were expected to require mechanical ventilation – with an intention-to-treat population of 965 – were randomized either to conservative oxygen therapy or usual therapy. For the conservative therapy, the upper limit of the pulse oximetry alarm would sound when levels reached 97% and the F102 was decreased to 0.21 if the pulse oximetry was above the acceptable lower limit, while usual therapy involved no specific limiting measures. In both groups, the default lower limit for oxygen saturation was 90%.
At day 28 after ventilation, there was no significant difference between the conservative and usual care groups in the number of ventilator-free days (21.3 days vs. 22.1 days). The patients in the conservative oxygen group spent a median of 29 hours receiving an F102 level of 0.21, compared with 1 hour in the usual care group.
The mortality rate at day 180 was 35.7% in the conservative oxygen group, and 34.5% in the usual-oxygen group (HR 1.05, 95% CI 0.85 – 1.30). Researchers also saw no differences between the two groups in paid employment and cognitive function.
In patients with suspected hypoxic-ischemic encephalopathy between-group differences were apparent; At day 28, those in the conservative-oxygen group had a median of 21.1 ventilator-free days, compared with none in the usual-oxygen group. The usual-oxygen group also had a higher 180-day mortality rate than those in the conservative-oxygen group (43% vs. 59%).
“Our data are suggestive of a possible benefit of conservative oxygen therapy in patients with suspected hypoxic-ischemic encephalopathy,” the authors wrote. “It is biologically plausible that conservative oxygen therapy reduces the incidence of secondary brain damage after resuscitation from cardiac arrest, and observational data suggest that exposure to hyperoxemia in such patients may be harmful.”
The authors noted that their trial did not rule out the possibility of benefit or harm had they used a more liberal oxygen regimen in their usual-care group, and that different conservative regimens might also have achieved different outcomes.
The study was funded by the New Zealand Health Research Council. Six authors declared research support for the trial from the study funder, and two declared unrelated research grants from private industry. No other conflicts of interest were declared.
SOURCE: Mackle D et al. NJEM 2019, October 14. DOI:10.1056/NEJMoa1903297.
FROM NEJM
Key clinical point: Conservative oxygen therapy during mechanical ventilation does not increase ventilation-free days.
Major finding: The number of ventilation-free days was similar in adults on conservative oxygen therapy and those on usual care.
Study details: Parallel-group randomized controlled trial in 965 adults undergoing mechanical ventilation.
Disclosures: The study was funded by the New Zealand Health Research Council. Six authors declared research support for the trial from the study funder, and two declared unrelated research grants from private industry. No other conflicts of interest were declared.
Source: Mackle D et al. NJEM 2019, October 14. DOI: 10.1056/NEJMoa1903297.
Wildfire smoke impact, part 2: Resources, advice for patients
Wildfires are on the move in California and communities from the Bay Area to Los Angeles County are once again coping with evacuation, possible destruction of homes, and health concerns related to poor air quality and smoke.
What can doctors tell their patients with cardiovascular and pulmonary conditions about the risks of smoke from wildfires?
EPA resources online
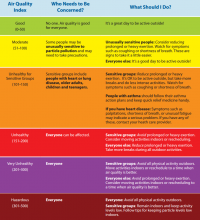
AirNow, a website managed by the Environmental Protection Agency, provides a variety of resources for the public and for health providers, including links to online tutorials, printable health fact sheets, and the newly updated document “Wildfire Smoke: Guide for Public Health Officials 2019.” When wildfire smoke generates an Air Quality Index (AQI) from 101-150, at-risk subgroups like people with asthma, chronic obstructive pulmonary disease (COPD), or heart disease should take precautions.
“An AQI of 151-200 is unhealthy for everyone, and an AQI above 200 is very unhealthy,” John R. Balmes, MD, a pulmonologist at the University of California, San Francisco, and an expert on the respiratory and cardiovascular effects of air pollutants, said in an interview. “That does not mean that everybody is going to die, though. You’re going to have some symptoms of scratchy throat, and you may cough once or twice an hour [from exposure to wildfire smoke], but people who don’t have any preexisting health problems are probably going to be fine and don’t necessarily have to wear an N95 mask. People should wear one if they need to feel comfortable.”
Masks – How much protection?
Wayne Cascio, MD, who directs the EPA’s National Health and Environmental Effects Research Laboratory, notes that some public health officials don’t recommend wearing N95 masks during wildfire smoke events. “There’s a lot of concern that people won’t use them correctly and will therefore feel like they’re protected and will spend more time outdoors than they should and still not get the benefit,” he said. “The masks also pose a challenge for children and for people with severe asthma and COPD.”
Masks also have to fit properly, which can be problematic for kids, added Dr. Balmes, one of the authors of “Wildfires Disaster Guidance: Tips for Staying Healthy During Wildfires” (Am J Respir Crit Care Med. 2019;199[2]:3-4).
“Even the small versions don’t necessarily fit kids well, so they’re not recommended for kids,” he said. “It doesn’t mean a kid couldn’t wear them, but it’s not officially recommended. The actual physiologic work of breathing isn’t much increased by using the N95 mask, but if you’re already struggling to get your breath, or experience dyspnea, then it might be hard to wear one. People with milder COPD can wear an N95 mask just like people with mild asthma if they have to go out.”
The EPA published a tip sheet about where and when to use an N95 or P100 mask, with warnings about the limited protection these devices offer, especially if not used correctly. Most masks do not protect the wearer from harmful gases that can be in wildfire smoke.
Ventilation systems
The EPA also recommends that people with more severe disease should stay indoors and avoid using air conditioning units that only draw in air from the outside or do not have a recirculating option. “If you have to bring in outside air because that’s how your system works, you should have a MERV 13 or better filter to filter out the fine particles,” Dr. Balmes said. “Not every ventilation system can handle it, but most can. That will help the house.”
Dr. Cascio pointed out that the instruction to close all windows and doors is a difficult proposition for people who live in states with moderate weather climates such as Montana and Colorado, where few homes have central air conditioning. “The treatment may be worse than the disease in this case, because it may exacerbate heat stress,” he said. “Try to find a place that has cleaner air. That might be a public building, a school, a fitness center, or a library. Yet we don’t know a lot about whether those areas are cleaner or not. That is currently the subject of some research on our part.”
Traveling away from an area affected by wildfire smoke to ride out the conditions is one option, but that can backfire. One weekend when smoke from the 2008 North Carolina peat fire was particularly troublesome, Dr. Cascio and his family drove about 60 miles west of Greenville to the town of Zebulon, where a minor league baseball game was being played and the air quality was good.
“My thought was to get the family to a better environment for at least a few hours,” Dr. Cascio recalled. “When we arrived in Zebulon the air quality was good as advertised. However, the direction of the wind shifted and the smoke started to move due west and within a short time you could barely see the players on the field. This experience also pointed out one of the lessons of wildfire smoke. That is, in the short term, it is sometimes difficult to predict where it will be present because of the nature of changes in weather and wind.”
Consumer tools to monitor air quality
Colleen E. Reid, PhD, of the department of geography at the University of Colorado, Boulder, an expert on the impact of wildfire smoke on human health, has observed in increase in consumer action to counter smoke inhalation during wildfire events. She said that consumers are buying personal laser particle counters, like the ones made by PurpleAir, to provide a real-time assessment of air quality.
“There is a lot of error with these smaller, cheaper monitors, but I think they give you a sense of trends over time,” Dr. Reid said. “People are trying to figure out how we can work with this sort of real-time data along with the high-quality EPA monitors. If everybody has their own monitor, or ways to better calibrate them to the high-quality data, that would be amazing. Researchers are trying to see how they can use that data to inform our understanding of the spatial and temporal patterning of air pollution.”
The EPA’s Smoke Sense app also holds promise. Characterized on its website as “a citizen science project,” the study uses a free mobile app to engage people living in affected communities to monitor their air quality and their cardiorespiratory symptoms. “Through engagement over time, you learn what the effects on your body are and what the expected effects are, so you can recognize the hazards and change the behavior when you’re experiencing it,” said Ana G. Rappold, PhD, who is the app’s principal investigator at the National Health and Environmental Effects Research Laboratory. One component of the app is time of last measurement of fine particulate matter and ozone based on the user’s location. Another is a module called Be Smoke Smart, which tests the user’s knowledge of wildfire smoke exposure. For example, one question is: “How likely are you to reduce your exposure on an Orange AQI alert day?” (which indicates that sensitive populations may experience health effects).
“Through gamification, they’re engaging with the issue,” Dr. Rappold said. “Then they learn about what others are reporting. In that part we also study how different messages change individuals’ perspective on how likely they are to make a change the next time they’re impacted by smoke.”
Wildfires are on the move in California and communities from the Bay Area to Los Angeles County are once again coping with evacuation, possible destruction of homes, and health concerns related to poor air quality and smoke.
What can doctors tell their patients with cardiovascular and pulmonary conditions about the risks of smoke from wildfires?
EPA resources online
AirNow, a website managed by the Environmental Protection Agency, provides a variety of resources for the public and for health providers, including links to online tutorials, printable health fact sheets, and the newly updated document “Wildfire Smoke: Guide for Public Health Officials 2019.” When wildfire smoke generates an Air Quality Index (AQI) from 101-150, at-risk subgroups like people with asthma, chronic obstructive pulmonary disease (COPD), or heart disease should take precautions.
“An AQI of 151-200 is unhealthy for everyone, and an AQI above 200 is very unhealthy,” John R. Balmes, MD, a pulmonologist at the University of California, San Francisco, and an expert on the respiratory and cardiovascular effects of air pollutants, said in an interview. “That does not mean that everybody is going to die, though. You’re going to have some symptoms of scratchy throat, and you may cough once or twice an hour [from exposure to wildfire smoke], but people who don’t have any preexisting health problems are probably going to be fine and don’t necessarily have to wear an N95 mask. People should wear one if they need to feel comfortable.”
Masks – How much protection?
Wayne Cascio, MD, who directs the EPA’s National Health and Environmental Effects Research Laboratory, notes that some public health officials don’t recommend wearing N95 masks during wildfire smoke events. “There’s a lot of concern that people won’t use them correctly and will therefore feel like they’re protected and will spend more time outdoors than they should and still not get the benefit,” he said. “The masks also pose a challenge for children and for people with severe asthma and COPD.”
Masks also have to fit properly, which can be problematic for kids, added Dr. Balmes, one of the authors of “Wildfires Disaster Guidance: Tips for Staying Healthy During Wildfires” (Am J Respir Crit Care Med. 2019;199[2]:3-4).
“Even the small versions don’t necessarily fit kids well, so they’re not recommended for kids,” he said. “It doesn’t mean a kid couldn’t wear them, but it’s not officially recommended. The actual physiologic work of breathing isn’t much increased by using the N95 mask, but if you’re already struggling to get your breath, or experience dyspnea, then it might be hard to wear one. People with milder COPD can wear an N95 mask just like people with mild asthma if they have to go out.”
The EPA published a tip sheet about where and when to use an N95 or P100 mask, with warnings about the limited protection these devices offer, especially if not used correctly. Most masks do not protect the wearer from harmful gases that can be in wildfire smoke.
Ventilation systems
The EPA also recommends that people with more severe disease should stay indoors and avoid using air conditioning units that only draw in air from the outside or do not have a recirculating option. “If you have to bring in outside air because that’s how your system works, you should have a MERV 13 or better filter to filter out the fine particles,” Dr. Balmes said. “Not every ventilation system can handle it, but most can. That will help the house.”
Dr. Cascio pointed out that the instruction to close all windows and doors is a difficult proposition for people who live in states with moderate weather climates such as Montana and Colorado, where few homes have central air conditioning. “The treatment may be worse than the disease in this case, because it may exacerbate heat stress,” he said. “Try to find a place that has cleaner air. That might be a public building, a school, a fitness center, or a library. Yet we don’t know a lot about whether those areas are cleaner or not. That is currently the subject of some research on our part.”
Traveling away from an area affected by wildfire smoke to ride out the conditions is one option, but that can backfire. One weekend when smoke from the 2008 North Carolina peat fire was particularly troublesome, Dr. Cascio and his family drove about 60 miles west of Greenville to the town of Zebulon, where a minor league baseball game was being played and the air quality was good.
“My thought was to get the family to a better environment for at least a few hours,” Dr. Cascio recalled. “When we arrived in Zebulon the air quality was good as advertised. However, the direction of the wind shifted and the smoke started to move due west and within a short time you could barely see the players on the field. This experience also pointed out one of the lessons of wildfire smoke. That is, in the short term, it is sometimes difficult to predict where it will be present because of the nature of changes in weather and wind.”
Consumer tools to monitor air quality
Colleen E. Reid, PhD, of the department of geography at the University of Colorado, Boulder, an expert on the impact of wildfire smoke on human health, has observed in increase in consumer action to counter smoke inhalation during wildfire events. She said that consumers are buying personal laser particle counters, like the ones made by PurpleAir, to provide a real-time assessment of air quality.
“There is a lot of error with these smaller, cheaper monitors, but I think they give you a sense of trends over time,” Dr. Reid said. “People are trying to figure out how we can work with this sort of real-time data along with the high-quality EPA monitors. If everybody has their own monitor, or ways to better calibrate them to the high-quality data, that would be amazing. Researchers are trying to see how they can use that data to inform our understanding of the spatial and temporal patterning of air pollution.”
The EPA’s Smoke Sense app also holds promise. Characterized on its website as “a citizen science project,” the study uses a free mobile app to engage people living in affected communities to monitor their air quality and their cardiorespiratory symptoms. “Through engagement over time, you learn what the effects on your body are and what the expected effects are, so you can recognize the hazards and change the behavior when you’re experiencing it,” said Ana G. Rappold, PhD, who is the app’s principal investigator at the National Health and Environmental Effects Research Laboratory. One component of the app is time of last measurement of fine particulate matter and ozone based on the user’s location. Another is a module called Be Smoke Smart, which tests the user’s knowledge of wildfire smoke exposure. For example, one question is: “How likely are you to reduce your exposure on an Orange AQI alert day?” (which indicates that sensitive populations may experience health effects).
“Through gamification, they’re engaging with the issue,” Dr. Rappold said. “Then they learn about what others are reporting. In that part we also study how different messages change individuals’ perspective on how likely they are to make a change the next time they’re impacted by smoke.”
Wildfires are on the move in California and communities from the Bay Area to Los Angeles County are once again coping with evacuation, possible destruction of homes, and health concerns related to poor air quality and smoke.
What can doctors tell their patients with cardiovascular and pulmonary conditions about the risks of smoke from wildfires?
EPA resources online
AirNow, a website managed by the Environmental Protection Agency, provides a variety of resources for the public and for health providers, including links to online tutorials, printable health fact sheets, and the newly updated document “Wildfire Smoke: Guide for Public Health Officials 2019.” When wildfire smoke generates an Air Quality Index (AQI) from 101-150, at-risk subgroups like people with asthma, chronic obstructive pulmonary disease (COPD), or heart disease should take precautions.
“An AQI of 151-200 is unhealthy for everyone, and an AQI above 200 is very unhealthy,” John R. Balmes, MD, a pulmonologist at the University of California, San Francisco, and an expert on the respiratory and cardiovascular effects of air pollutants, said in an interview. “That does not mean that everybody is going to die, though. You’re going to have some symptoms of scratchy throat, and you may cough once or twice an hour [from exposure to wildfire smoke], but people who don’t have any preexisting health problems are probably going to be fine and don’t necessarily have to wear an N95 mask. People should wear one if they need to feel comfortable.”
Masks – How much protection?
Wayne Cascio, MD, who directs the EPA’s National Health and Environmental Effects Research Laboratory, notes that some public health officials don’t recommend wearing N95 masks during wildfire smoke events. “There’s a lot of concern that people won’t use them correctly and will therefore feel like they’re protected and will spend more time outdoors than they should and still not get the benefit,” he said. “The masks also pose a challenge for children and for people with severe asthma and COPD.”
Masks also have to fit properly, which can be problematic for kids, added Dr. Balmes, one of the authors of “Wildfires Disaster Guidance: Tips for Staying Healthy During Wildfires” (Am J Respir Crit Care Med. 2019;199[2]:3-4).
“Even the small versions don’t necessarily fit kids well, so they’re not recommended for kids,” he said. “It doesn’t mean a kid couldn’t wear them, but it’s not officially recommended. The actual physiologic work of breathing isn’t much increased by using the N95 mask, but if you’re already struggling to get your breath, or experience dyspnea, then it might be hard to wear one. People with milder COPD can wear an N95 mask just like people with mild asthma if they have to go out.”
The EPA published a tip sheet about where and when to use an N95 or P100 mask, with warnings about the limited protection these devices offer, especially if not used correctly. Most masks do not protect the wearer from harmful gases that can be in wildfire smoke.
Ventilation systems
The EPA also recommends that people with more severe disease should stay indoors and avoid using air conditioning units that only draw in air from the outside or do not have a recirculating option. “If you have to bring in outside air because that’s how your system works, you should have a MERV 13 or better filter to filter out the fine particles,” Dr. Balmes said. “Not every ventilation system can handle it, but most can. That will help the house.”
Dr. Cascio pointed out that the instruction to close all windows and doors is a difficult proposition for people who live in states with moderate weather climates such as Montana and Colorado, where few homes have central air conditioning. “The treatment may be worse than the disease in this case, because it may exacerbate heat stress,” he said. “Try to find a place that has cleaner air. That might be a public building, a school, a fitness center, or a library. Yet we don’t know a lot about whether those areas are cleaner or not. That is currently the subject of some research on our part.”
Traveling away from an area affected by wildfire smoke to ride out the conditions is one option, but that can backfire. One weekend when smoke from the 2008 North Carolina peat fire was particularly troublesome, Dr. Cascio and his family drove about 60 miles west of Greenville to the town of Zebulon, where a minor league baseball game was being played and the air quality was good.
“My thought was to get the family to a better environment for at least a few hours,” Dr. Cascio recalled. “When we arrived in Zebulon the air quality was good as advertised. However, the direction of the wind shifted and the smoke started to move due west and within a short time you could barely see the players on the field. This experience also pointed out one of the lessons of wildfire smoke. That is, in the short term, it is sometimes difficult to predict where it will be present because of the nature of changes in weather and wind.”
Consumer tools to monitor air quality
Colleen E. Reid, PhD, of the department of geography at the University of Colorado, Boulder, an expert on the impact of wildfire smoke on human health, has observed in increase in consumer action to counter smoke inhalation during wildfire events. She said that consumers are buying personal laser particle counters, like the ones made by PurpleAir, to provide a real-time assessment of air quality.
“There is a lot of error with these smaller, cheaper monitors, but I think they give you a sense of trends over time,” Dr. Reid said. “People are trying to figure out how we can work with this sort of real-time data along with the high-quality EPA monitors. If everybody has their own monitor, or ways to better calibrate them to the high-quality data, that would be amazing. Researchers are trying to see how they can use that data to inform our understanding of the spatial and temporal patterning of air pollution.”
The EPA’s Smoke Sense app also holds promise. Characterized on its website as “a citizen science project,” the study uses a free mobile app to engage people living in affected communities to monitor their air quality and their cardiorespiratory symptoms. “Through engagement over time, you learn what the effects on your body are and what the expected effects are, so you can recognize the hazards and change the behavior when you’re experiencing it,” said Ana G. Rappold, PhD, who is the app’s principal investigator at the National Health and Environmental Effects Research Laboratory. One component of the app is time of last measurement of fine particulate matter and ozone based on the user’s location. Another is a module called Be Smoke Smart, which tests the user’s knowledge of wildfire smoke exposure. For example, one question is: “How likely are you to reduce your exposure on an Orange AQI alert day?” (which indicates that sensitive populations may experience health effects).
“Through gamification, they’re engaging with the issue,” Dr. Rappold said. “Then they learn about what others are reporting. In that part we also study how different messages change individuals’ perspective on how likely they are to make a change the next time they’re impacted by smoke.”
Azithromycin prevents airway complications of antibody deficiency
MADRID – Low-dose azithromycin prophylaxis significantly reduced exacerbations and hospitalizations in patients with primary antibody deficiency relative to placebo, according to a randomized multicenter phase 2 trial.
The study results support routine use of low-dose azithromycin in patients with primary antibody deficiency, according to Cinzia Milito, MD, PhD, department of molecular medicine, Sapienza University, Rome. Perhaps more importantly, the long-term benefits might be even greater.
“In patients with primary antibody deficiency, the respiratory tract is the major target of acute infections, leading to inflammation, increased airway reactivity, and over time to tissue remodeling and chronic lung disease,” Dr. Milito said at the annual congress of the European Respiratory Society. “Chronic lung disease is a major cause of death in this population.”
In this study 89 patients with primary antibody deficiency were randomized at seven centers in Italy to 250 mg per day of azithromycin or placebo administered on three consecutive days of each week for three years. Patients were maintained on other treatments, such as IgG replacement.
At the end of study, 33 of the 44 patients randomized to azithromycin and 34 of the 45 patients randomized to placebo remained on therapy. When compared for the primary endpoint of exacerbations, the median incidence rates were 3.6 episodes in the azithromycin group and 5.2 episodes in the placebo group, providing a 1.6 episode or 31% relative reduction (P=0.02).
The median number of hospitalizations for any cause, which was a secondary endpoint, was also significantly lower in the azithromycin arm (0.1 vs. 0.3 episodes).
In addition, the number of additional courses of antibiotics was significantly lower (2.3 vs. 3.6), and the time to the first course of antibiotic course was significantly longer (181.5 vs. 122.4 days) in the azithromycin group, reported Dr. Milito, whose study is now published (Milito C et al. J Allergy Clin Immunol 2019;144: 584-593).
“In a six-month washout at the end of the study, the relative advantages seen for azithromycin were lost,” Dr. Milito said.
Quality of life measured with the St. George’s Respiratory Questionnaire showed an association between low-dose azithromycin prophylaxis and significant improvement in the symptom domain when evaluated during and at the end of the study. Improvement on the Short-Form 36, which was observed one year into the study, was no longer significant at the end of the study.
Azithromycin was well tolerated with no significant differences in the rate of serious adverse events observed between the experimental and control arms of the study. Over the course of the study, however, azithromycin was associated with a significant protective effect against diarrhea (13% vs. 53%) and acute rhinosinusitus (4% vs. 27%).
There was no observed increase in macrolide resistance associated with azithromycin prophylaxis.
Macrolides have been evaluated for preventing progression of several chronic lung diseases, including chronic obstructive pulmonary disease, bronchiectasis, and cystic fibrosis. Like other drugs in its class, “azithromycin, in addition to its antimicrobial effect, has anti-inflammatory properties,” Dr. Milito said. This increases its potential to slow the time to airway damage in patients with primary antibiotic deficiency.
“Chronic lung disease is the result of a vicious cycle that begins with the inflammatory response to infection,” Dr. Milito explained. On the basis of these data, she believes azithromycin “should be considered a valuable addition to usual treatment” for primary antibody deficiencies.
SOURCE: EUROPEAN RESPIRATORY SOCIETY 2019 INTERNATIONAL CONGRESS
MADRID – Low-dose azithromycin prophylaxis significantly reduced exacerbations and hospitalizations in patients with primary antibody deficiency relative to placebo, according to a randomized multicenter phase 2 trial.
The study results support routine use of low-dose azithromycin in patients with primary antibody deficiency, according to Cinzia Milito, MD, PhD, department of molecular medicine, Sapienza University, Rome. Perhaps more importantly, the long-term benefits might be even greater.
“In patients with primary antibody deficiency, the respiratory tract is the major target of acute infections, leading to inflammation, increased airway reactivity, and over time to tissue remodeling and chronic lung disease,” Dr. Milito said at the annual congress of the European Respiratory Society. “Chronic lung disease is a major cause of death in this population.”
In this study 89 patients with primary antibody deficiency were randomized at seven centers in Italy to 250 mg per day of azithromycin or placebo administered on three consecutive days of each week for three years. Patients were maintained on other treatments, such as IgG replacement.
At the end of study, 33 of the 44 patients randomized to azithromycin and 34 of the 45 patients randomized to placebo remained on therapy. When compared for the primary endpoint of exacerbations, the median incidence rates were 3.6 episodes in the azithromycin group and 5.2 episodes in the placebo group, providing a 1.6 episode or 31% relative reduction (P=0.02).
The median number of hospitalizations for any cause, which was a secondary endpoint, was also significantly lower in the azithromycin arm (0.1 vs. 0.3 episodes).
In addition, the number of additional courses of antibiotics was significantly lower (2.3 vs. 3.6), and the time to the first course of antibiotic course was significantly longer (181.5 vs. 122.4 days) in the azithromycin group, reported Dr. Milito, whose study is now published (Milito C et al. J Allergy Clin Immunol 2019;144: 584-593).
“In a six-month washout at the end of the study, the relative advantages seen for azithromycin were lost,” Dr. Milito said.
Quality of life measured with the St. George’s Respiratory Questionnaire showed an association between low-dose azithromycin prophylaxis and significant improvement in the symptom domain when evaluated during and at the end of the study. Improvement on the Short-Form 36, which was observed one year into the study, was no longer significant at the end of the study.
Azithromycin was well tolerated with no significant differences in the rate of serious adverse events observed between the experimental and control arms of the study. Over the course of the study, however, azithromycin was associated with a significant protective effect against diarrhea (13% vs. 53%) and acute rhinosinusitus (4% vs. 27%).
There was no observed increase in macrolide resistance associated with azithromycin prophylaxis.
Macrolides have been evaluated for preventing progression of several chronic lung diseases, including chronic obstructive pulmonary disease, bronchiectasis, and cystic fibrosis. Like other drugs in its class, “azithromycin, in addition to its antimicrobial effect, has anti-inflammatory properties,” Dr. Milito said. This increases its potential to slow the time to airway damage in patients with primary antibiotic deficiency.
“Chronic lung disease is the result of a vicious cycle that begins with the inflammatory response to infection,” Dr. Milito explained. On the basis of these data, she believes azithromycin “should be considered a valuable addition to usual treatment” for primary antibody deficiencies.
SOURCE: EUROPEAN RESPIRATORY SOCIETY 2019 INTERNATIONAL CONGRESS
MADRID – Low-dose azithromycin prophylaxis significantly reduced exacerbations and hospitalizations in patients with primary antibody deficiency relative to placebo, according to a randomized multicenter phase 2 trial.
The study results support routine use of low-dose azithromycin in patients with primary antibody deficiency, according to Cinzia Milito, MD, PhD, department of molecular medicine, Sapienza University, Rome. Perhaps more importantly, the long-term benefits might be even greater.
“In patients with primary antibody deficiency, the respiratory tract is the major target of acute infections, leading to inflammation, increased airway reactivity, and over time to tissue remodeling and chronic lung disease,” Dr. Milito said at the annual congress of the European Respiratory Society. “Chronic lung disease is a major cause of death in this population.”
In this study 89 patients with primary antibody deficiency were randomized at seven centers in Italy to 250 mg per day of azithromycin or placebo administered on three consecutive days of each week for three years. Patients were maintained on other treatments, such as IgG replacement.
At the end of study, 33 of the 44 patients randomized to azithromycin and 34 of the 45 patients randomized to placebo remained on therapy. When compared for the primary endpoint of exacerbations, the median incidence rates were 3.6 episodes in the azithromycin group and 5.2 episodes in the placebo group, providing a 1.6 episode or 31% relative reduction (P=0.02).
The median number of hospitalizations for any cause, which was a secondary endpoint, was also significantly lower in the azithromycin arm (0.1 vs. 0.3 episodes).
In addition, the number of additional courses of antibiotics was significantly lower (2.3 vs. 3.6), and the time to the first course of antibiotic course was significantly longer (181.5 vs. 122.4 days) in the azithromycin group, reported Dr. Milito, whose study is now published (Milito C et al. J Allergy Clin Immunol 2019;144: 584-593).
“In a six-month washout at the end of the study, the relative advantages seen for azithromycin were lost,” Dr. Milito said.
Quality of life measured with the St. George’s Respiratory Questionnaire showed an association between low-dose azithromycin prophylaxis and significant improvement in the symptom domain when evaluated during and at the end of the study. Improvement on the Short-Form 36, which was observed one year into the study, was no longer significant at the end of the study.
Azithromycin was well tolerated with no significant differences in the rate of serious adverse events observed between the experimental and control arms of the study. Over the course of the study, however, azithromycin was associated with a significant protective effect against diarrhea (13% vs. 53%) and acute rhinosinusitus (4% vs. 27%).
There was no observed increase in macrolide resistance associated with azithromycin prophylaxis.
Macrolides have been evaluated for preventing progression of several chronic lung diseases, including chronic obstructive pulmonary disease, bronchiectasis, and cystic fibrosis. Like other drugs in its class, “azithromycin, in addition to its antimicrobial effect, has anti-inflammatory properties,” Dr. Milito said. This increases its potential to slow the time to airway damage in patients with primary antibiotic deficiency.
“Chronic lung disease is the result of a vicious cycle that begins with the inflammatory response to infection,” Dr. Milito explained. On the basis of these data, she believes azithromycin “should be considered a valuable addition to usual treatment” for primary antibody deficiencies.
SOURCE: EUROPEAN RESPIRATORY SOCIETY 2019 INTERNATIONAL CONGRESS
REPORTING FROM ERS 2019
CDC updates guidance on vaping-associated lung injury
The Centers for Disease Control and Prevention has released an updated interim clinical guidance for health providers for evaluating and treating patients with lung injury associated with e-cigarette use or vaping.
In a telebriefing, Anne Schuchat, MD, CDC principal deputy director, and her colleagues answered questions about the current investigation into the source of this lung injury outbreak and the updated clinical guidance. Dr. Schuchat said, “I can’t stress enough the seriousness of these injuries.” She added, “We are not seeing a drop in cases” but a continuation of the trend of hospitalization and deaths that started in August 2019.
Investigation update
The investigation to date has yielded some information about current cases of lung injury related to vaping:
• The acronym EVALI has been developed to refer to e-cigarette, or vaping products use associated lung injury;
• 1,299 EVALI cases have been reported as of Oct. 8;
• No single compound or ingredient has emerged as the cause of these injuries, and more than one substance may be involved;
• Among the 573 patients for whom data are available on vaping products used in the previous 90 days, 76% reported using THC-containing products; 58% reported using nicotine-containing products; 32% reported exclusive use of THC-containing products, and 13% reported exclusive use of nicotine-containing products;
• Of the 700+ samples sent to the CDC for analysis, most had little or no liquid remaining in the device, limiting content analysis. In 28 THC-containing samples, THC concentrations were found to be 13% - 77% (mean 41%).
• A “handful” of cases of readmission have been reported and the CDC is currently investigating whether these cases included patients who took up vaping again or had some other possible contributing factor.
• The CDC is currently developing an ICD-10 code relevant to EVALI.
Clinical guidance update
The CDC provided detailed guidance on evaluating and caring for patients with EVALI. The recommendations focus on patient history, lab testing, criteria for hospitalization, and follow-up of these patients.
Detailed history of patients presenting with suspected EVALI is especially important for this patient population, given the many unknowns surrounding this condition. The updated guidance states, “All health care providers evaluating patients for EVALI should ask about the use of e-cigarette, or vaping, products and ideally should ask about types of substances used (e.g.,THC, cannabis [oil, dabs], nicotine, modified products or the addition of substances not intended by the manufacturer); product source, specific product brand and name; duration and frequency of use, time of last use; product delivery system, and method of use (aerosolization, dabbing, or dripping).” The approach recommended for soliciting accurate information is “empathetic, nonjudgmental” and, the guidelines say, patients should be questioned in private regarding sensitive information to assure confidentiality.
A respiratory virus panel is recommended for all suspected EVALI patients, although at this time, these tests cannot be used to distinguish EVALI from infectious etiologies. All patients should be considered for urine toxicology testing, including testing for THC.
Imaging guidance for suspected EVALI patients includes chest x-ray, with additional CT scan when the x-ray result does not correlate with clinical findings or to evaluate severe or worsening disease.
Recommended criteria for hospitalization of patients with suspected EVALI are those patients with decreased O2 saturation (less than 95%) on room air, are in respiratory distress, or have comorbidities that compromise pulmonary reserve. As of Oct. 8, 96% of patients with suspected EVALI reported to CDC have been hospitalized.
As for medical treatment of these patients, corticosteroids have been found helpful. The statement noted, “Among 140 cases reported nationally to CDC that received corticosteroids, 82% of patients improved
The natural progression of this injury is not known, however, and it is possible that patients might recover without corticosteroids. Given the unknown etiology of the disease and “because the diagnosis remains one of exclusion, aggressive empiric therapy with corticosteroids, antimicrobial, and antiviral therapy might be warranted for patients with severe illness. A range of corticosteroid doses, durations, and taper plans might be considered on a case-by-case basis.”
The report concludes with a strong recommendation that patients hospitalized with EVALI are followed closely with a visit 1-2 weeks after discharge and again with additional testing 1-2 months later. Health care providers are also advised to consult medical specialists, in particular pulmonologists, who can offer further evaluation, recommend empiric treatment, and review indications for bronchoscopy.
Mitch Zeller, JD, director, Center for Tobacco Products with the Food and Drug Administration emphasized the extraordinary complexity of the EVALI problem but noted that the FDA and CDC “will leave no stone unturned until we get to the bottom of it.”
The Centers for Disease Control and Prevention has released an updated interim clinical guidance for health providers for evaluating and treating patients with lung injury associated with e-cigarette use or vaping.
In a telebriefing, Anne Schuchat, MD, CDC principal deputy director, and her colleagues answered questions about the current investigation into the source of this lung injury outbreak and the updated clinical guidance. Dr. Schuchat said, “I can’t stress enough the seriousness of these injuries.” She added, “We are not seeing a drop in cases” but a continuation of the trend of hospitalization and deaths that started in August 2019.
Investigation update
The investigation to date has yielded some information about current cases of lung injury related to vaping:
• The acronym EVALI has been developed to refer to e-cigarette, or vaping products use associated lung injury;
• 1,299 EVALI cases have been reported as of Oct. 8;
• No single compound or ingredient has emerged as the cause of these injuries, and more than one substance may be involved;
• Among the 573 patients for whom data are available on vaping products used in the previous 90 days, 76% reported using THC-containing products; 58% reported using nicotine-containing products; 32% reported exclusive use of THC-containing products, and 13% reported exclusive use of nicotine-containing products;
• Of the 700+ samples sent to the CDC for analysis, most had little or no liquid remaining in the device, limiting content analysis. In 28 THC-containing samples, THC concentrations were found to be 13% - 77% (mean 41%).
• A “handful” of cases of readmission have been reported and the CDC is currently investigating whether these cases included patients who took up vaping again or had some other possible contributing factor.
• The CDC is currently developing an ICD-10 code relevant to EVALI.
Clinical guidance update
The CDC provided detailed guidance on evaluating and caring for patients with EVALI. The recommendations focus on patient history, lab testing, criteria for hospitalization, and follow-up of these patients.
Detailed history of patients presenting with suspected EVALI is especially important for this patient population, given the many unknowns surrounding this condition. The updated guidance states, “All health care providers evaluating patients for EVALI should ask about the use of e-cigarette, or vaping, products and ideally should ask about types of substances used (e.g.,THC, cannabis [oil, dabs], nicotine, modified products or the addition of substances not intended by the manufacturer); product source, specific product brand and name; duration and frequency of use, time of last use; product delivery system, and method of use (aerosolization, dabbing, or dripping).” The approach recommended for soliciting accurate information is “empathetic, nonjudgmental” and, the guidelines say, patients should be questioned in private regarding sensitive information to assure confidentiality.
A respiratory virus panel is recommended for all suspected EVALI patients, although at this time, these tests cannot be used to distinguish EVALI from infectious etiologies. All patients should be considered for urine toxicology testing, including testing for THC.
Imaging guidance for suspected EVALI patients includes chest x-ray, with additional CT scan when the x-ray result does not correlate with clinical findings or to evaluate severe or worsening disease.
Recommended criteria for hospitalization of patients with suspected EVALI are those patients with decreased O2 saturation (less than 95%) on room air, are in respiratory distress, or have comorbidities that compromise pulmonary reserve. As of Oct. 8, 96% of patients with suspected EVALI reported to CDC have been hospitalized.
As for medical treatment of these patients, corticosteroids have been found helpful. The statement noted, “Among 140 cases reported nationally to CDC that received corticosteroids, 82% of patients improved
The natural progression of this injury is not known, however, and it is possible that patients might recover without corticosteroids. Given the unknown etiology of the disease and “because the diagnosis remains one of exclusion, aggressive empiric therapy with corticosteroids, antimicrobial, and antiviral therapy might be warranted for patients with severe illness. A range of corticosteroid doses, durations, and taper plans might be considered on a case-by-case basis.”
The report concludes with a strong recommendation that patients hospitalized with EVALI are followed closely with a visit 1-2 weeks after discharge and again with additional testing 1-2 months later. Health care providers are also advised to consult medical specialists, in particular pulmonologists, who can offer further evaluation, recommend empiric treatment, and review indications for bronchoscopy.
Mitch Zeller, JD, director, Center for Tobacco Products with the Food and Drug Administration emphasized the extraordinary complexity of the EVALI problem but noted that the FDA and CDC “will leave no stone unturned until we get to the bottom of it.”
The Centers for Disease Control and Prevention has released an updated interim clinical guidance for health providers for evaluating and treating patients with lung injury associated with e-cigarette use or vaping.
In a telebriefing, Anne Schuchat, MD, CDC principal deputy director, and her colleagues answered questions about the current investigation into the source of this lung injury outbreak and the updated clinical guidance. Dr. Schuchat said, “I can’t stress enough the seriousness of these injuries.” She added, “We are not seeing a drop in cases” but a continuation of the trend of hospitalization and deaths that started in August 2019.
Investigation update
The investigation to date has yielded some information about current cases of lung injury related to vaping:
• The acronym EVALI has been developed to refer to e-cigarette, or vaping products use associated lung injury;
• 1,299 EVALI cases have been reported as of Oct. 8;
• No single compound or ingredient has emerged as the cause of these injuries, and more than one substance may be involved;
• Among the 573 patients for whom data are available on vaping products used in the previous 90 days, 76% reported using THC-containing products; 58% reported using nicotine-containing products; 32% reported exclusive use of THC-containing products, and 13% reported exclusive use of nicotine-containing products;
• Of the 700+ samples sent to the CDC for analysis, most had little or no liquid remaining in the device, limiting content analysis. In 28 THC-containing samples, THC concentrations were found to be 13% - 77% (mean 41%).
• A “handful” of cases of readmission have been reported and the CDC is currently investigating whether these cases included patients who took up vaping again or had some other possible contributing factor.
• The CDC is currently developing an ICD-10 code relevant to EVALI.
Clinical guidance update
The CDC provided detailed guidance on evaluating and caring for patients with EVALI. The recommendations focus on patient history, lab testing, criteria for hospitalization, and follow-up of these patients.
Detailed history of patients presenting with suspected EVALI is especially important for this patient population, given the many unknowns surrounding this condition. The updated guidance states, “All health care providers evaluating patients for EVALI should ask about the use of e-cigarette, or vaping, products and ideally should ask about types of substances used (e.g.,THC, cannabis [oil, dabs], nicotine, modified products or the addition of substances not intended by the manufacturer); product source, specific product brand and name; duration and frequency of use, time of last use; product delivery system, and method of use (aerosolization, dabbing, or dripping).” The approach recommended for soliciting accurate information is “empathetic, nonjudgmental” and, the guidelines say, patients should be questioned in private regarding sensitive information to assure confidentiality.
A respiratory virus panel is recommended for all suspected EVALI patients, although at this time, these tests cannot be used to distinguish EVALI from infectious etiologies. All patients should be considered for urine toxicology testing, including testing for THC.
Imaging guidance for suspected EVALI patients includes chest x-ray, with additional CT scan when the x-ray result does not correlate with clinical findings or to evaluate severe or worsening disease.
Recommended criteria for hospitalization of patients with suspected EVALI are those patients with decreased O2 saturation (less than 95%) on room air, are in respiratory distress, or have comorbidities that compromise pulmonary reserve. As of Oct. 8, 96% of patients with suspected EVALI reported to CDC have been hospitalized.
As for medical treatment of these patients, corticosteroids have been found helpful. The statement noted, “Among 140 cases reported nationally to CDC that received corticosteroids, 82% of patients improved
The natural progression of this injury is not known, however, and it is possible that patients might recover without corticosteroids. Given the unknown etiology of the disease and “because the diagnosis remains one of exclusion, aggressive empiric therapy with corticosteroids, antimicrobial, and antiviral therapy might be warranted for patients with severe illness. A range of corticosteroid doses, durations, and taper plans might be considered on a case-by-case basis.”
The report concludes with a strong recommendation that patients hospitalized with EVALI are followed closely with a visit 1-2 weeks after discharge and again with additional testing 1-2 months later. Health care providers are also advised to consult medical specialists, in particular pulmonologists, who can offer further evaluation, recommend empiric treatment, and review indications for bronchoscopy.
Mitch Zeller, JD, director, Center for Tobacco Products with the Food and Drug Administration emphasized the extraordinary complexity of the EVALI problem but noted that the FDA and CDC “will leave no stone unturned until we get to the bottom of it.”
REPORTING FROM A CDC TELEBRIEFING
Mesh nebulizer worked faster to control acute asthma
MADRID – Consistent with previous evidence of higher relative rates of drug delivery, mesh nebulizers offer several advantages over jet nebulizers for treatment of acute asthma in children presenting to an emergency department, according to results of a randomized trial presented at the annual congress of the European Respiratory Society.
For the primary outcome of hospital admission, the advantage of the mesh over the jet nebulizer only reached significance when used with a mask, rather than a valve, but trial results overall support the conclusion that the mesh device delivers drug more efficiently, according to Gerald Moody, RRT-NPS, clinical research coordinator at Children’s Medical Center, Dallas.
In this multicenter, single-blinded trial, 217 children presenting to an ED with acute asthma of moderate or greater severity were randomized to receive bronchodilator treatment delivered with a mesh device or a jet device. For drug delivery, aerosol masks or mouthpiece valves were permitted and selected at the discretion of the clinician administrating treatment. Masks were used in 80% of cases.
Patients remained in the study until either symptom control was achieved or a decision was reached to advise hospital admission. Patients with complex comorbidities or who had received oral corticosteroids within the previous 24 hours were excluded.
For the primary outcome of hospital discharge, the 31% reduction (P = .22) in hospitalization in favor of the mesh nebulizer failed to reach statistical significance. Although the study is likely to have been underpowered, Mr. Moody also pointed out an uneven distribution in severity of disease at baseline. In addition to a significantly higher median asthma score (9.0 vs. 8.0; P = .042) in the mesh nebulizer group, there was also a significantly higher percentage with severe disease (57% vs. 42%; P = .025).
“There were no significant differences in any of the other variables we evaluated, such as age, gender, race, or body mass index,” Mr. Moody reported.
Despite the higher disease burden in the mesh nebulizer group, there was a 48% reduction (P = .03) in hospital admissions among those randomized to the mesh nebulizer when both groups received treatment through a mask.
In addition, those treated with the mask required on average only two treatments before achieving symptom control whether they met criteria for moderate or severe asthma at baseline. The median numbers of treatments in the jet nebulizer group for moderate and severe asthma were 3 and 3.5, respectively.
In previous experimental studies, which ultimately provided the rationale for this trial, the estimated amount of drug reaching the airways with a mesh nebulizer was approximately twice as great as that estimated in the model when delivery was performed with a jet device, according to Mr. Moody.
This study appeared to corroborate that advantage. Both the median doses of albuterol (10 mg vs. 15 mg) and ipratropium (1,000 mcg vs. 1,500 mcg) were significantly lower (P less than .001 for both) among the patients randomized to the mesh nebulizer.
Although the jet nebulizers are widely employed “for their ease of use and low cost,” Mr. Moody characterized mesh nebulizers as an advance in technology. In this study, which Mr. Moody said is the first to evaluate whether the experimental evidence of greater drug delivery efficiency translates into a clinical advantage, the primary endpoint was missed, but Mr. Moody indicated that the overall findings support the potential for a difference.
The ERS-invited discussant on this study, Celeste Michala Porsbjerg, MD, Bispebjerg Hospital, Copenhagen University, expressed a concern that might deserve attention in a larger trial. Based on the premise that more efficient delivery increases drug exposure, she questioned whether it might not also increase risks.
There were no significant treatment-related adverse events reported in either arm of this study, Mr. Moody responded, but he conceded that this is an appropriate focus of attention for future studies.
Mr. Moody reported a financial relationship with Aerogen, which produces the mesh device tested in this trial.
MADRID – Consistent with previous evidence of higher relative rates of drug delivery, mesh nebulizers offer several advantages over jet nebulizers for treatment of acute asthma in children presenting to an emergency department, according to results of a randomized trial presented at the annual congress of the European Respiratory Society.
For the primary outcome of hospital admission, the advantage of the mesh over the jet nebulizer only reached significance when used with a mask, rather than a valve, but trial results overall support the conclusion that the mesh device delivers drug more efficiently, according to Gerald Moody, RRT-NPS, clinical research coordinator at Children’s Medical Center, Dallas.
In this multicenter, single-blinded trial, 217 children presenting to an ED with acute asthma of moderate or greater severity were randomized to receive bronchodilator treatment delivered with a mesh device or a jet device. For drug delivery, aerosol masks or mouthpiece valves were permitted and selected at the discretion of the clinician administrating treatment. Masks were used in 80% of cases.
Patients remained in the study until either symptom control was achieved or a decision was reached to advise hospital admission. Patients with complex comorbidities or who had received oral corticosteroids within the previous 24 hours were excluded.
For the primary outcome of hospital discharge, the 31% reduction (P = .22) in hospitalization in favor of the mesh nebulizer failed to reach statistical significance. Although the study is likely to have been underpowered, Mr. Moody also pointed out an uneven distribution in severity of disease at baseline. In addition to a significantly higher median asthma score (9.0 vs. 8.0; P = .042) in the mesh nebulizer group, there was also a significantly higher percentage with severe disease (57% vs. 42%; P = .025).
“There were no significant differences in any of the other variables we evaluated, such as age, gender, race, or body mass index,” Mr. Moody reported.
Despite the higher disease burden in the mesh nebulizer group, there was a 48% reduction (P = .03) in hospital admissions among those randomized to the mesh nebulizer when both groups received treatment through a mask.
In addition, those treated with the mask required on average only two treatments before achieving symptom control whether they met criteria for moderate or severe asthma at baseline. The median numbers of treatments in the jet nebulizer group for moderate and severe asthma were 3 and 3.5, respectively.
In previous experimental studies, which ultimately provided the rationale for this trial, the estimated amount of drug reaching the airways with a mesh nebulizer was approximately twice as great as that estimated in the model when delivery was performed with a jet device, according to Mr. Moody.
This study appeared to corroborate that advantage. Both the median doses of albuterol (10 mg vs. 15 mg) and ipratropium (1,000 mcg vs. 1,500 mcg) were significantly lower (P less than .001 for both) among the patients randomized to the mesh nebulizer.
Although the jet nebulizers are widely employed “for their ease of use and low cost,” Mr. Moody characterized mesh nebulizers as an advance in technology. In this study, which Mr. Moody said is the first to evaluate whether the experimental evidence of greater drug delivery efficiency translates into a clinical advantage, the primary endpoint was missed, but Mr. Moody indicated that the overall findings support the potential for a difference.
The ERS-invited discussant on this study, Celeste Michala Porsbjerg, MD, Bispebjerg Hospital, Copenhagen University, expressed a concern that might deserve attention in a larger trial. Based on the premise that more efficient delivery increases drug exposure, she questioned whether it might not also increase risks.
There were no significant treatment-related adverse events reported in either arm of this study, Mr. Moody responded, but he conceded that this is an appropriate focus of attention for future studies.
Mr. Moody reported a financial relationship with Aerogen, which produces the mesh device tested in this trial.
MADRID – Consistent with previous evidence of higher relative rates of drug delivery, mesh nebulizers offer several advantages over jet nebulizers for treatment of acute asthma in children presenting to an emergency department, according to results of a randomized trial presented at the annual congress of the European Respiratory Society.
For the primary outcome of hospital admission, the advantage of the mesh over the jet nebulizer only reached significance when used with a mask, rather than a valve, but trial results overall support the conclusion that the mesh device delivers drug more efficiently, according to Gerald Moody, RRT-NPS, clinical research coordinator at Children’s Medical Center, Dallas.
In this multicenter, single-blinded trial, 217 children presenting to an ED with acute asthma of moderate or greater severity were randomized to receive bronchodilator treatment delivered with a mesh device or a jet device. For drug delivery, aerosol masks or mouthpiece valves were permitted and selected at the discretion of the clinician administrating treatment. Masks were used in 80% of cases.
Patients remained in the study until either symptom control was achieved or a decision was reached to advise hospital admission. Patients with complex comorbidities or who had received oral corticosteroids within the previous 24 hours were excluded.
For the primary outcome of hospital discharge, the 31% reduction (P = .22) in hospitalization in favor of the mesh nebulizer failed to reach statistical significance. Although the study is likely to have been underpowered, Mr. Moody also pointed out an uneven distribution in severity of disease at baseline. In addition to a significantly higher median asthma score (9.0 vs. 8.0; P = .042) in the mesh nebulizer group, there was also a significantly higher percentage with severe disease (57% vs. 42%; P = .025).
“There were no significant differences in any of the other variables we evaluated, such as age, gender, race, or body mass index,” Mr. Moody reported.
Despite the higher disease burden in the mesh nebulizer group, there was a 48% reduction (P = .03) in hospital admissions among those randomized to the mesh nebulizer when both groups received treatment through a mask.
In addition, those treated with the mask required on average only two treatments before achieving symptom control whether they met criteria for moderate or severe asthma at baseline. The median numbers of treatments in the jet nebulizer group for moderate and severe asthma were 3 and 3.5, respectively.
In previous experimental studies, which ultimately provided the rationale for this trial, the estimated amount of drug reaching the airways with a mesh nebulizer was approximately twice as great as that estimated in the model when delivery was performed with a jet device, according to Mr. Moody.
This study appeared to corroborate that advantage. Both the median doses of albuterol (10 mg vs. 15 mg) and ipratropium (1,000 mcg vs. 1,500 mcg) were significantly lower (P less than .001 for both) among the patients randomized to the mesh nebulizer.
Although the jet nebulizers are widely employed “for their ease of use and low cost,” Mr. Moody characterized mesh nebulizers as an advance in technology. In this study, which Mr. Moody said is the first to evaluate whether the experimental evidence of greater drug delivery efficiency translates into a clinical advantage, the primary endpoint was missed, but Mr. Moody indicated that the overall findings support the potential for a difference.
The ERS-invited discussant on this study, Celeste Michala Porsbjerg, MD, Bispebjerg Hospital, Copenhagen University, expressed a concern that might deserve attention in a larger trial. Based on the premise that more efficient delivery increases drug exposure, she questioned whether it might not also increase risks.
There were no significant treatment-related adverse events reported in either arm of this study, Mr. Moody responded, but he conceded that this is an appropriate focus of attention for future studies.
Mr. Moody reported a financial relationship with Aerogen, which produces the mesh device tested in this trial.
REPORTING FROM ERS 2019
Vaping-associated lung injury cases nears 1,300
according to a statement released by the Centers for Disease Control and Prevention.
These cases have been reported to the CDC from 49 states, the District of Columbia, and the U.S. Virgin Islands. The increase in lung injury cases from Oct. 1 (reported to be 1,080) represents both new patients and recent reporting of patients previously identified to the CDC.
Twenty-six deaths have been confirmed in 21 states and more deaths are currently being reviewed.
The causes of the injuries are still under investigation. The CDC stated, “The latest findings from the investigation into lung injuries associated with e-cigarette use, or vaping, suggest products containing THC play a role in the outbreak. All patients have a reported history of e-cigarette product use, or vaping, and no consistent evidence of an infectious cause has been discovered. Therefore, the suspected cause is a chemical exposure.” The specific chemical causing the lung injuries associated with vaping remains unknown at this time.
The CDC has created information hubs and resources for the public, for health care providers, and for state and local health department officials. The CDC has also provided additional resources to address the outbreak of vaping-associated lung injuries.
according to a statement released by the Centers for Disease Control and Prevention.
These cases have been reported to the CDC from 49 states, the District of Columbia, and the U.S. Virgin Islands. The increase in lung injury cases from Oct. 1 (reported to be 1,080) represents both new patients and recent reporting of patients previously identified to the CDC.
Twenty-six deaths have been confirmed in 21 states and more deaths are currently being reviewed.
The causes of the injuries are still under investigation. The CDC stated, “The latest findings from the investigation into lung injuries associated with e-cigarette use, or vaping, suggest products containing THC play a role in the outbreak. All patients have a reported history of e-cigarette product use, or vaping, and no consistent evidence of an infectious cause has been discovered. Therefore, the suspected cause is a chemical exposure.” The specific chemical causing the lung injuries associated with vaping remains unknown at this time.
The CDC has created information hubs and resources for the public, for health care providers, and for state and local health department officials. The CDC has also provided additional resources to address the outbreak of vaping-associated lung injuries.
according to a statement released by the Centers for Disease Control and Prevention.
These cases have been reported to the CDC from 49 states, the District of Columbia, and the U.S. Virgin Islands. The increase in lung injury cases from Oct. 1 (reported to be 1,080) represents both new patients and recent reporting of patients previously identified to the CDC.
Twenty-six deaths have been confirmed in 21 states and more deaths are currently being reviewed.
The causes of the injuries are still under investigation. The CDC stated, “The latest findings from the investigation into lung injuries associated with e-cigarette use, or vaping, suggest products containing THC play a role in the outbreak. All patients have a reported history of e-cigarette product use, or vaping, and no consistent evidence of an infectious cause has been discovered. Therefore, the suspected cause is a chemical exposure.” The specific chemical causing the lung injuries associated with vaping remains unknown at this time.
The CDC has created information hubs and resources for the public, for health care providers, and for state and local health department officials. The CDC has also provided additional resources to address the outbreak of vaping-associated lung injuries.
REPORTING FROM CDC
Newly described lung disorder strikes children with systemic juvenile idiopathic arthritis
An uncommon but potentially deadly inflammatory lung disease is emerging among children with systemic juvenile idiopathic arthritis, and its history appears to coincide with the rise of powerful biologics as first-line therapy for children with the disease.
Most confirmed cases of systemic juvenile idiopathic arthritis with lung disease (sJIA-LD) are in the United States. But it’s popping up in other places that have adopted early biologic treatment for sJIA – including Canada, South America, Europe, and the Middle East.
The respiratory symptoms are relatively subtle, so by the time of lung disease detection, the amount of affected lung can be extensive, said Elizabeth Mellins, MD, a Stanford (Calif.) University researcher who, along with first author Vivian Saper, MD, recently published the largest case series comprising reports from 37 institutions (Ann Rheum Dis. 2019 Sep 27. doi: 10.1136/annrheumdis-2019-216040). By the end of follow-up, 22 of the 61 children in her cohort had died, including all 12 patients who demonstrated excessively high neutrophil levels in bronchoalveolar lavage samples.
Another recent report, authored by Grant Schulert, MD, PhD, and colleagues of the Cincinnati Children’s Hospital Medical Center, described 18 patients, 9 of whom were also included in the Stanford cohort (Arthritis Rheumatol. 2019 Aug 5. doi: 10.1002/art.41073).
Both investigators have now identified new patients.
“We are aware of 60 additional cases beyond what were included in our series,” Dr. Mellins said in an interview, bringing her entire cohort to 121. Dr. Schulert also continues to expand his group, detailing nine new cases at a recent private meeting.
“We are up to 27 now,” he said. “The features of these new patients are all very similar: The children are very young, all have had macrophage activation syndrome in the past and very-difficult-to-control JIA. Reactions to tocilizumab [Actemra] were also not uncommon in this group.”
Dr. Mellins also saw this association with allergic-type tocilizumab reactions, severe delayed hypersensitivity reactions to anakinra (Kineret) or canakinumab (Ilaris). Although serious lung disease in sJIA patients is not unheard of, this phenotype was virtually unknown until about a decade ago. Both investigators said that it’s been rising steadily since 2010 – just about the time that powerful cytokine-inhibiting biologics were changing these patients’ world for the better. After decades of relying almost solely on steroids and methotrexate, with rather poor results and significant long-term side effects, children were not only improving, but thriving. Gone was the life-changing glucocorticoid-related growth inhibition. Biologics could halt fevers, rash, and joint destruction in their tracks.
“For the first time in history, these kids could look forward to a more or less normal life,” Dr. Schulert said.
But the emergence of this particular type of lung disease could throw a pall over that success story, he said. If sJIA-LD is temporally associated with increasing reliance on long-term interleukin-1/IL-6 inhibition in children with early-onset disease, could these drugs actually be the causative agent? The picture remains unclear.
Some of the 18 in his initial series have improved, while 36% of those in the Stanford series died. Most who do recover stay on their IL-1 or IL-6 blocking therapy with good disease control without further lung problems. Both investigators found compelling genetic hints, but nothing conclusive. Children with trisomy 21 appear especially vulnerable. Most patients are very young – around 2 years old – but others are school aged. Some had a history of macrophage activation syndrome. Some had hard-to-control disease and some were clinically well controlled when the lung disease presented.
There are simply no answers yet.
With so many potential links, all unproven, clinicians may question the wisdom of embarking on long-term biologic therapy for their children with sJIA. Peter Nigrovic, MD, of Boston Children’s Hospital, addressed this in an accompanying editorial (Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41071).
“My take on this is that it’s a very worrisome trend,” he said in an interview. “We’ve been going full bore toward early biologic therapy in sJIA and at the same time we are seeing more of this lung disease. Is it guilt by association? Or is there something more? The challenge for us is not to jump too soon to that conclusion.”
Although the association is there, he said, association does not equal causation. And there’s no doubt that biologics have vastly improved the lives of sJIA patients. “The drugs might be causal, and I worry about that and think we need to study it. But we absolutely need stronger evidence before we change practice.”
“This is a new manifestation of the disease, and it’s coming at the same time we are changing the treatment paradigm,” Dr. Nigrovic continued. “It could be because of interleukin-1 or interleukin-6 blockade. There is biological plausibility for such a link. It could also be related to the fact that we are using less steroids and methotrexate, which might have been preventing this. The appearance of sJIA lung disease could also be that a distinct secular trend unrelated to treatment, just as we saw amyloid come and go in this population in Europe. These other therapies were actually preventing this. We just don’t know.”
Clinical characteristics
Children presented with similar symptoms. Respiratory symptoms are usually subtle and mild. These can include tachypnea, hypoxia (43% in the Stanford series), and pulmonary hypertension (30% in the Stanford series).
Digital clubbing, often with erythema, was a common finding. Some children showed pruritic, nonevanescent rashes. Eosinophilia occurred in 37% of the Stanford series and severe abdominal pain in 16%, although Dr. Mellins noted that belly pain may be underestimated, as it was only volunteered, not queried, information.
“There are some red flags that should raise suspicion even without obvious respiratory symptoms,” Dr. Mellins said. These include lymphopenia, unexplained abdominal pain, eosinophilia, an unusual rash, and finger clubbing with or without erythema.
Findings on imaging were consistent in both series. Several key clinic features emerged: pleural thickening, septal thickening, bronchial wall or peribronchovascular thickening, “tree-in-bud” opacities, “ground-glass” opacities, peripheral consolidation, and lymphadenopathy.
“The imaging findings correspond to two things,” Dr. Schulert said. “The first is inflammation in the interstitium, which is evidence of chronic and ongoing inflammation. The other thing is that the alveoli are filled with a lipoproteinaceous material which is actually surfactant that’s not being normally recycled by the lung macrophages. You can see these features in other conditions where there’s a problem with lung macrophages, like pulmonary alveolar proteinosis, genetic and autoimmune disorders, infections, or inhalants.”
Pathology showed alveolar filling – a location in the lung that hides usual symptoms until the lung disease is advanced. Prior drug reactions were common. Tocilizumab anaphylaxis occurred in close to 40% of the Stanford series – a surprising finding given the 0.6% reaction incidence in the drug’s sJIA trials. Dr. Schulert saw a similar story.
“In our cohort we also observed a striking number of adverse events to cytokine-targeted biologics exposure,” Dr. Schulert said. “Most of these reactions were to tocilizumab, and were described variously from pain and feeling unwell, to difficulty breathing, to anaphylaxis.”
In a risk analysis, Dr. Schulert determined that adverse events to cytokine-targeting biologics increased the likelihood of lung disease more than 13 times (odds ratio, 13.6).
“We also identified a statistically significant association with history of macrophage activation syndrome when compared to controls (OR, 14.5),” Dr. Schulert and associates wrote.
Genetics
Both the Cincinnati and Stanford teams conducted genetic analyses on some of their patients.
Among eight lung biopsy samples, Dr. Schulert found 37 differentially expressed genes: 36 with increased expression and 1 with decreased expression. Many of the up-regulated genes are involved in interferon-gamma response. Two (CXCL10 and CXCL9) are interferon-induced chemokines associated with macrophage activation syndrome. The down-regulated gene, PADI4, modulates immune response in lupus, and has been associated with the risk of interstitial lung disease in RA.
Dr. Mellins and her team analyzed whole-exome sequencing data from 20 patients and found some rare protein-altering gene variants in genes related to pulmonary alveolar proteinosis, all of which were heterozygous and shared with a healthy parent. But none of them could be directly tied to the disorder.
Another genetic puzzle demands attention, she said. About 10% of the children had trisomy 21 – a stark contrast to the typical 0.2% prevalence among a control group of sJIA patients without any known lung disease in the Childhood Arthritis and Rheumatology Research Alliance Registry cohort, similar to the background population rate. There were suggestions of more aggressive lung disease in all six of these children. Four presented with hypoxia, and two showed advanced interstitial fibrosis. Children with trisomy 21 also seemed more susceptible to infections; 83% had a viral or fungal lung infection at diagnosis, compared with 29% of those without trisomy 21.
Prior exposure to cytokine inhibitors
Parenchymal lung disease and pulmonary hypertension complicating sJIA was first highlighted in a series of 25 cases reported by Kimura et al. in 2013. These authors raised the question of the possible relationship of this and the increasing use of anti–IL-1 and anti–IL-6 biologics in sJIA treatment.
Following this lead, Dr. Mellins started looking into this new clinical entity in 2015. By then, she was identifying some past cases by autopsy records and current cases by clinical presentation. She saw a dramatic shift over time. From 2002 to 2011, she identified four cases, half of which had been exposed to IL-1/IL-6 inhibitors. From 2012 to 2014, eight new cases came to light, and seven had been exposed to those drugs. The crescendo continued from 2015 to 2017. During those years, Dr. Mellins and associates identified 10 new patients, 7 of whom had taken interleukin-inhibiting biologics. The mean time from initial drug exposure to diagnosis was a little more than 1 year.
An adjusted analysis comparing sJIA-LD patients and sJIA patients without lung disease didn’t find any significant difference in drug exposure. However, children with lung disease were more likely to have taken anakinra before the symptoms developed. Additionally, the symptoms of clubbing, abdominal pain, eosinophilia, hyperenhancing lymph nodes, and pulmonary alveolar proteinosis were much more common in children who’d taken the drugs.
The authors pointed out that this association does not prove causality and is confounded by the concomitant reduction in glucocorticoids with IL-1/IL-6 inhibitor use. And the vast majority of children with sJIA take cytokine inhibitors with no problems.
“Possibly, drug exposure may promote lung disease in a subset of children with sJIA, among the substantially larger group of patients who derive striking benefit from these drugs,” Dr. Mellins said, “Importantly, our results argue strongly for more investigation into a possible connection.”
Survival
After a mean follow-up of 1.7 years, the Stanford group saw high mortality. The 5-year survival rate translated to a mortality incidence of 159 deaths per 1,000 person-years, compared with 3.9 per 1,000 person-years in a historical cohort of sJIA patients who required biologic therapy.
Diffuse lung disease was the cause of 12 deaths; 5 of these patients also had macrophage activation syndrome at the time of death. Factors significantly associated with shortened survival included male sex, hypoxia at presentation, and neutrophilic bronchoalveolar lavage with more than 10 times the normal count. In an adjusted analysis, all of these variables fell out. However, none of the children with excessively high neutrophilic bronchoalveolar lavage survived.
Does it affect adults?
Could adults be experiencing the same disorder? There is some evidence to support it: The Food and Drug Administration adverse event website shows alveolar disease or pulmonary hypertension in 39 adults who have been exposed to IL-1 or IL-6 inhibition. Of these, 23 had RA, 11 adult-onset Still’s disease, and 5 unclassified rheumatic disorders.
The research groups were supported by grants from the sJIA Foundation, the Lucile Packard Foundation for Children’s Health, Stanford graduate fellowships, the Life Sciences Research Foundation, the Bill & Melinda Gates Foundation, Cincinnati Children’s Research Foundation, the Childhood Arthritis and Rheumatology Research Alliance, the Arthritis Foundation, and the National Institutes of Health. Many authors on both papers reported financial ties to Genentech, which markets tocilizumab, and other pharmaceutical companies*. Dr. Nigrovic reported receiving consulting fees and research support from Novartis and other companies.
SOURCES: Saper V et al. Ann Rheum Dis. 2019 Sep 27. doi: 10.1136/annrheumdis-2019-216040; Schulert GS et al. Arthritis Rheumatol. 2019 Aug 5. doi: 10.1002/art.41073; Nigrovic PA. Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41071.
*Correction, 10/12/19: An earlier version of this article misstated the manufacturer of Actemra (tocilizumab).
This article was updated 10/14/19.
An uncommon but potentially deadly inflammatory lung disease is emerging among children with systemic juvenile idiopathic arthritis, and its history appears to coincide with the rise of powerful biologics as first-line therapy for children with the disease.
Most confirmed cases of systemic juvenile idiopathic arthritis with lung disease (sJIA-LD) are in the United States. But it’s popping up in other places that have adopted early biologic treatment for sJIA – including Canada, South America, Europe, and the Middle East.
The respiratory symptoms are relatively subtle, so by the time of lung disease detection, the amount of affected lung can be extensive, said Elizabeth Mellins, MD, a Stanford (Calif.) University researcher who, along with first author Vivian Saper, MD, recently published the largest case series comprising reports from 37 institutions (Ann Rheum Dis. 2019 Sep 27. doi: 10.1136/annrheumdis-2019-216040). By the end of follow-up, 22 of the 61 children in her cohort had died, including all 12 patients who demonstrated excessively high neutrophil levels in bronchoalveolar lavage samples.
Another recent report, authored by Grant Schulert, MD, PhD, and colleagues of the Cincinnati Children’s Hospital Medical Center, described 18 patients, 9 of whom were also included in the Stanford cohort (Arthritis Rheumatol. 2019 Aug 5. doi: 10.1002/art.41073).
Both investigators have now identified new patients.
“We are aware of 60 additional cases beyond what were included in our series,” Dr. Mellins said in an interview, bringing her entire cohort to 121. Dr. Schulert also continues to expand his group, detailing nine new cases at a recent private meeting.
“We are up to 27 now,” he said. “The features of these new patients are all very similar: The children are very young, all have had macrophage activation syndrome in the past and very-difficult-to-control JIA. Reactions to tocilizumab [Actemra] were also not uncommon in this group.”
Dr. Mellins also saw this association with allergic-type tocilizumab reactions, severe delayed hypersensitivity reactions to anakinra (Kineret) or canakinumab (Ilaris). Although serious lung disease in sJIA patients is not unheard of, this phenotype was virtually unknown until about a decade ago. Both investigators said that it’s been rising steadily since 2010 – just about the time that powerful cytokine-inhibiting biologics were changing these patients’ world for the better. After decades of relying almost solely on steroids and methotrexate, with rather poor results and significant long-term side effects, children were not only improving, but thriving. Gone was the life-changing glucocorticoid-related growth inhibition. Biologics could halt fevers, rash, and joint destruction in their tracks.
“For the first time in history, these kids could look forward to a more or less normal life,” Dr. Schulert said.
But the emergence of this particular type of lung disease could throw a pall over that success story, he said. If sJIA-LD is temporally associated with increasing reliance on long-term interleukin-1/IL-6 inhibition in children with early-onset disease, could these drugs actually be the causative agent? The picture remains unclear.
Some of the 18 in his initial series have improved, while 36% of those in the Stanford series died. Most who do recover stay on their IL-1 or IL-6 blocking therapy with good disease control without further lung problems. Both investigators found compelling genetic hints, but nothing conclusive. Children with trisomy 21 appear especially vulnerable. Most patients are very young – around 2 years old – but others are school aged. Some had a history of macrophage activation syndrome. Some had hard-to-control disease and some were clinically well controlled when the lung disease presented.
There are simply no answers yet.
With so many potential links, all unproven, clinicians may question the wisdom of embarking on long-term biologic therapy for their children with sJIA. Peter Nigrovic, MD, of Boston Children’s Hospital, addressed this in an accompanying editorial (Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41071).
“My take on this is that it’s a very worrisome trend,” he said in an interview. “We’ve been going full bore toward early biologic therapy in sJIA and at the same time we are seeing more of this lung disease. Is it guilt by association? Or is there something more? The challenge for us is not to jump too soon to that conclusion.”
Although the association is there, he said, association does not equal causation. And there’s no doubt that biologics have vastly improved the lives of sJIA patients. “The drugs might be causal, and I worry about that and think we need to study it. But we absolutely need stronger evidence before we change practice.”
“This is a new manifestation of the disease, and it’s coming at the same time we are changing the treatment paradigm,” Dr. Nigrovic continued. “It could be because of interleukin-1 or interleukin-6 blockade. There is biological plausibility for such a link. It could also be related to the fact that we are using less steroids and methotrexate, which might have been preventing this. The appearance of sJIA lung disease could also be that a distinct secular trend unrelated to treatment, just as we saw amyloid come and go in this population in Europe. These other therapies were actually preventing this. We just don’t know.”
Clinical characteristics
Children presented with similar symptoms. Respiratory symptoms are usually subtle and mild. These can include tachypnea, hypoxia (43% in the Stanford series), and pulmonary hypertension (30% in the Stanford series).
Digital clubbing, often with erythema, was a common finding. Some children showed pruritic, nonevanescent rashes. Eosinophilia occurred in 37% of the Stanford series and severe abdominal pain in 16%, although Dr. Mellins noted that belly pain may be underestimated, as it was only volunteered, not queried, information.
“There are some red flags that should raise suspicion even without obvious respiratory symptoms,” Dr. Mellins said. These include lymphopenia, unexplained abdominal pain, eosinophilia, an unusual rash, and finger clubbing with or without erythema.
Findings on imaging were consistent in both series. Several key clinic features emerged: pleural thickening, septal thickening, bronchial wall or peribronchovascular thickening, “tree-in-bud” opacities, “ground-glass” opacities, peripheral consolidation, and lymphadenopathy.
“The imaging findings correspond to two things,” Dr. Schulert said. “The first is inflammation in the interstitium, which is evidence of chronic and ongoing inflammation. The other thing is that the alveoli are filled with a lipoproteinaceous material which is actually surfactant that’s not being normally recycled by the lung macrophages. You can see these features in other conditions where there’s a problem with lung macrophages, like pulmonary alveolar proteinosis, genetic and autoimmune disorders, infections, or inhalants.”
Pathology showed alveolar filling – a location in the lung that hides usual symptoms until the lung disease is advanced. Prior drug reactions were common. Tocilizumab anaphylaxis occurred in close to 40% of the Stanford series – a surprising finding given the 0.6% reaction incidence in the drug’s sJIA trials. Dr. Schulert saw a similar story.
“In our cohort we also observed a striking number of adverse events to cytokine-targeted biologics exposure,” Dr. Schulert said. “Most of these reactions were to tocilizumab, and were described variously from pain and feeling unwell, to difficulty breathing, to anaphylaxis.”
In a risk analysis, Dr. Schulert determined that adverse events to cytokine-targeting biologics increased the likelihood of lung disease more than 13 times (odds ratio, 13.6).
“We also identified a statistically significant association with history of macrophage activation syndrome when compared to controls (OR, 14.5),” Dr. Schulert and associates wrote.
Genetics
Both the Cincinnati and Stanford teams conducted genetic analyses on some of their patients.
Among eight lung biopsy samples, Dr. Schulert found 37 differentially expressed genes: 36 with increased expression and 1 with decreased expression. Many of the up-regulated genes are involved in interferon-gamma response. Two (CXCL10 and CXCL9) are interferon-induced chemokines associated with macrophage activation syndrome. The down-regulated gene, PADI4, modulates immune response in lupus, and has been associated with the risk of interstitial lung disease in RA.
Dr. Mellins and her team analyzed whole-exome sequencing data from 20 patients and found some rare protein-altering gene variants in genes related to pulmonary alveolar proteinosis, all of which were heterozygous and shared with a healthy parent. But none of them could be directly tied to the disorder.
Another genetic puzzle demands attention, she said. About 10% of the children had trisomy 21 – a stark contrast to the typical 0.2% prevalence among a control group of sJIA patients without any known lung disease in the Childhood Arthritis and Rheumatology Research Alliance Registry cohort, similar to the background population rate. There were suggestions of more aggressive lung disease in all six of these children. Four presented with hypoxia, and two showed advanced interstitial fibrosis. Children with trisomy 21 also seemed more susceptible to infections; 83% had a viral or fungal lung infection at diagnosis, compared with 29% of those without trisomy 21.
Prior exposure to cytokine inhibitors
Parenchymal lung disease and pulmonary hypertension complicating sJIA was first highlighted in a series of 25 cases reported by Kimura et al. in 2013. These authors raised the question of the possible relationship of this and the increasing use of anti–IL-1 and anti–IL-6 biologics in sJIA treatment.
Following this lead, Dr. Mellins started looking into this new clinical entity in 2015. By then, she was identifying some past cases by autopsy records and current cases by clinical presentation. She saw a dramatic shift over time. From 2002 to 2011, she identified four cases, half of which had been exposed to IL-1/IL-6 inhibitors. From 2012 to 2014, eight new cases came to light, and seven had been exposed to those drugs. The crescendo continued from 2015 to 2017. During those years, Dr. Mellins and associates identified 10 new patients, 7 of whom had taken interleukin-inhibiting biologics. The mean time from initial drug exposure to diagnosis was a little more than 1 year.
An adjusted analysis comparing sJIA-LD patients and sJIA patients without lung disease didn’t find any significant difference in drug exposure. However, children with lung disease were more likely to have taken anakinra before the symptoms developed. Additionally, the symptoms of clubbing, abdominal pain, eosinophilia, hyperenhancing lymph nodes, and pulmonary alveolar proteinosis were much more common in children who’d taken the drugs.
The authors pointed out that this association does not prove causality and is confounded by the concomitant reduction in glucocorticoids with IL-1/IL-6 inhibitor use. And the vast majority of children with sJIA take cytokine inhibitors with no problems.
“Possibly, drug exposure may promote lung disease in a subset of children with sJIA, among the substantially larger group of patients who derive striking benefit from these drugs,” Dr. Mellins said, “Importantly, our results argue strongly for more investigation into a possible connection.”
Survival
After a mean follow-up of 1.7 years, the Stanford group saw high mortality. The 5-year survival rate translated to a mortality incidence of 159 deaths per 1,000 person-years, compared with 3.9 per 1,000 person-years in a historical cohort of sJIA patients who required biologic therapy.
Diffuse lung disease was the cause of 12 deaths; 5 of these patients also had macrophage activation syndrome at the time of death. Factors significantly associated with shortened survival included male sex, hypoxia at presentation, and neutrophilic bronchoalveolar lavage with more than 10 times the normal count. In an adjusted analysis, all of these variables fell out. However, none of the children with excessively high neutrophilic bronchoalveolar lavage survived.
Does it affect adults?
Could adults be experiencing the same disorder? There is some evidence to support it: The Food and Drug Administration adverse event website shows alveolar disease or pulmonary hypertension in 39 adults who have been exposed to IL-1 or IL-6 inhibition. Of these, 23 had RA, 11 adult-onset Still’s disease, and 5 unclassified rheumatic disorders.
The research groups were supported by grants from the sJIA Foundation, the Lucile Packard Foundation for Children’s Health, Stanford graduate fellowships, the Life Sciences Research Foundation, the Bill & Melinda Gates Foundation, Cincinnati Children’s Research Foundation, the Childhood Arthritis and Rheumatology Research Alliance, the Arthritis Foundation, and the National Institutes of Health. Many authors on both papers reported financial ties to Genentech, which markets tocilizumab, and other pharmaceutical companies*. Dr. Nigrovic reported receiving consulting fees and research support from Novartis and other companies.
SOURCES: Saper V et al. Ann Rheum Dis. 2019 Sep 27. doi: 10.1136/annrheumdis-2019-216040; Schulert GS et al. Arthritis Rheumatol. 2019 Aug 5. doi: 10.1002/art.41073; Nigrovic PA. Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41071.
*Correction, 10/12/19: An earlier version of this article misstated the manufacturer of Actemra (tocilizumab).
This article was updated 10/14/19.
An uncommon but potentially deadly inflammatory lung disease is emerging among children with systemic juvenile idiopathic arthritis, and its history appears to coincide with the rise of powerful biologics as first-line therapy for children with the disease.
Most confirmed cases of systemic juvenile idiopathic arthritis with lung disease (sJIA-LD) are in the United States. But it’s popping up in other places that have adopted early biologic treatment for sJIA – including Canada, South America, Europe, and the Middle East.
The respiratory symptoms are relatively subtle, so by the time of lung disease detection, the amount of affected lung can be extensive, said Elizabeth Mellins, MD, a Stanford (Calif.) University researcher who, along with first author Vivian Saper, MD, recently published the largest case series comprising reports from 37 institutions (Ann Rheum Dis. 2019 Sep 27. doi: 10.1136/annrheumdis-2019-216040). By the end of follow-up, 22 of the 61 children in her cohort had died, including all 12 patients who demonstrated excessively high neutrophil levels in bronchoalveolar lavage samples.
Another recent report, authored by Grant Schulert, MD, PhD, and colleagues of the Cincinnati Children’s Hospital Medical Center, described 18 patients, 9 of whom were also included in the Stanford cohort (Arthritis Rheumatol. 2019 Aug 5. doi: 10.1002/art.41073).
Both investigators have now identified new patients.
“We are aware of 60 additional cases beyond what were included in our series,” Dr. Mellins said in an interview, bringing her entire cohort to 121. Dr. Schulert also continues to expand his group, detailing nine new cases at a recent private meeting.
“We are up to 27 now,” he said. “The features of these new patients are all very similar: The children are very young, all have had macrophage activation syndrome in the past and very-difficult-to-control JIA. Reactions to tocilizumab [Actemra] were also not uncommon in this group.”
Dr. Mellins also saw this association with allergic-type tocilizumab reactions, severe delayed hypersensitivity reactions to anakinra (Kineret) or canakinumab (Ilaris). Although serious lung disease in sJIA patients is not unheard of, this phenotype was virtually unknown until about a decade ago. Both investigators said that it’s been rising steadily since 2010 – just about the time that powerful cytokine-inhibiting biologics were changing these patients’ world for the better. After decades of relying almost solely on steroids and methotrexate, with rather poor results and significant long-term side effects, children were not only improving, but thriving. Gone was the life-changing glucocorticoid-related growth inhibition. Biologics could halt fevers, rash, and joint destruction in their tracks.
“For the first time in history, these kids could look forward to a more or less normal life,” Dr. Schulert said.
But the emergence of this particular type of lung disease could throw a pall over that success story, he said. If sJIA-LD is temporally associated with increasing reliance on long-term interleukin-1/IL-6 inhibition in children with early-onset disease, could these drugs actually be the causative agent? The picture remains unclear.
Some of the 18 in his initial series have improved, while 36% of those in the Stanford series died. Most who do recover stay on their IL-1 or IL-6 blocking therapy with good disease control without further lung problems. Both investigators found compelling genetic hints, but nothing conclusive. Children with trisomy 21 appear especially vulnerable. Most patients are very young – around 2 years old – but others are school aged. Some had a history of macrophage activation syndrome. Some had hard-to-control disease and some were clinically well controlled when the lung disease presented.
There are simply no answers yet.
With so many potential links, all unproven, clinicians may question the wisdom of embarking on long-term biologic therapy for their children with sJIA. Peter Nigrovic, MD, of Boston Children’s Hospital, addressed this in an accompanying editorial (Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41071).
“My take on this is that it’s a very worrisome trend,” he said in an interview. “We’ve been going full bore toward early biologic therapy in sJIA and at the same time we are seeing more of this lung disease. Is it guilt by association? Or is there something more? The challenge for us is not to jump too soon to that conclusion.”
Although the association is there, he said, association does not equal causation. And there’s no doubt that biologics have vastly improved the lives of sJIA patients. “The drugs might be causal, and I worry about that and think we need to study it. But we absolutely need stronger evidence before we change practice.”
“This is a new manifestation of the disease, and it’s coming at the same time we are changing the treatment paradigm,” Dr. Nigrovic continued. “It could be because of interleukin-1 or interleukin-6 blockade. There is biological plausibility for such a link. It could also be related to the fact that we are using less steroids and methotrexate, which might have been preventing this. The appearance of sJIA lung disease could also be that a distinct secular trend unrelated to treatment, just as we saw amyloid come and go in this population in Europe. These other therapies were actually preventing this. We just don’t know.”
Clinical characteristics
Children presented with similar symptoms. Respiratory symptoms are usually subtle and mild. These can include tachypnea, hypoxia (43% in the Stanford series), and pulmonary hypertension (30% in the Stanford series).
Digital clubbing, often with erythema, was a common finding. Some children showed pruritic, nonevanescent rashes. Eosinophilia occurred in 37% of the Stanford series and severe abdominal pain in 16%, although Dr. Mellins noted that belly pain may be underestimated, as it was only volunteered, not queried, information.
“There are some red flags that should raise suspicion even without obvious respiratory symptoms,” Dr. Mellins said. These include lymphopenia, unexplained abdominal pain, eosinophilia, an unusual rash, and finger clubbing with or without erythema.
Findings on imaging were consistent in both series. Several key clinic features emerged: pleural thickening, septal thickening, bronchial wall or peribronchovascular thickening, “tree-in-bud” opacities, “ground-glass” opacities, peripheral consolidation, and lymphadenopathy.
“The imaging findings correspond to two things,” Dr. Schulert said. “The first is inflammation in the interstitium, which is evidence of chronic and ongoing inflammation. The other thing is that the alveoli are filled with a lipoproteinaceous material which is actually surfactant that’s not being normally recycled by the lung macrophages. You can see these features in other conditions where there’s a problem with lung macrophages, like pulmonary alveolar proteinosis, genetic and autoimmune disorders, infections, or inhalants.”
Pathology showed alveolar filling – a location in the lung that hides usual symptoms until the lung disease is advanced. Prior drug reactions were common. Tocilizumab anaphylaxis occurred in close to 40% of the Stanford series – a surprising finding given the 0.6% reaction incidence in the drug’s sJIA trials. Dr. Schulert saw a similar story.
“In our cohort we also observed a striking number of adverse events to cytokine-targeted biologics exposure,” Dr. Schulert said. “Most of these reactions were to tocilizumab, and were described variously from pain and feeling unwell, to difficulty breathing, to anaphylaxis.”
In a risk analysis, Dr. Schulert determined that adverse events to cytokine-targeting biologics increased the likelihood of lung disease more than 13 times (odds ratio, 13.6).
“We also identified a statistically significant association with history of macrophage activation syndrome when compared to controls (OR, 14.5),” Dr. Schulert and associates wrote.
Genetics
Both the Cincinnati and Stanford teams conducted genetic analyses on some of their patients.
Among eight lung biopsy samples, Dr. Schulert found 37 differentially expressed genes: 36 with increased expression and 1 with decreased expression. Many of the up-regulated genes are involved in interferon-gamma response. Two (CXCL10 and CXCL9) are interferon-induced chemokines associated with macrophage activation syndrome. The down-regulated gene, PADI4, modulates immune response in lupus, and has been associated with the risk of interstitial lung disease in RA.
Dr. Mellins and her team analyzed whole-exome sequencing data from 20 patients and found some rare protein-altering gene variants in genes related to pulmonary alveolar proteinosis, all of which were heterozygous and shared with a healthy parent. But none of them could be directly tied to the disorder.
Another genetic puzzle demands attention, she said. About 10% of the children had trisomy 21 – a stark contrast to the typical 0.2% prevalence among a control group of sJIA patients without any known lung disease in the Childhood Arthritis and Rheumatology Research Alliance Registry cohort, similar to the background population rate. There were suggestions of more aggressive lung disease in all six of these children. Four presented with hypoxia, and two showed advanced interstitial fibrosis. Children with trisomy 21 also seemed more susceptible to infections; 83% had a viral or fungal lung infection at diagnosis, compared with 29% of those without trisomy 21.
Prior exposure to cytokine inhibitors
Parenchymal lung disease and pulmonary hypertension complicating sJIA was first highlighted in a series of 25 cases reported by Kimura et al. in 2013. These authors raised the question of the possible relationship of this and the increasing use of anti–IL-1 and anti–IL-6 biologics in sJIA treatment.
Following this lead, Dr. Mellins started looking into this new clinical entity in 2015. By then, she was identifying some past cases by autopsy records and current cases by clinical presentation. She saw a dramatic shift over time. From 2002 to 2011, she identified four cases, half of which had been exposed to IL-1/IL-6 inhibitors. From 2012 to 2014, eight new cases came to light, and seven had been exposed to those drugs. The crescendo continued from 2015 to 2017. During those years, Dr. Mellins and associates identified 10 new patients, 7 of whom had taken interleukin-inhibiting biologics. The mean time from initial drug exposure to diagnosis was a little more than 1 year.
An adjusted analysis comparing sJIA-LD patients and sJIA patients without lung disease didn’t find any significant difference in drug exposure. However, children with lung disease were more likely to have taken anakinra before the symptoms developed. Additionally, the symptoms of clubbing, abdominal pain, eosinophilia, hyperenhancing lymph nodes, and pulmonary alveolar proteinosis were much more common in children who’d taken the drugs.
The authors pointed out that this association does not prove causality and is confounded by the concomitant reduction in glucocorticoids with IL-1/IL-6 inhibitor use. And the vast majority of children with sJIA take cytokine inhibitors with no problems.
“Possibly, drug exposure may promote lung disease in a subset of children with sJIA, among the substantially larger group of patients who derive striking benefit from these drugs,” Dr. Mellins said, “Importantly, our results argue strongly for more investigation into a possible connection.”
Survival
After a mean follow-up of 1.7 years, the Stanford group saw high mortality. The 5-year survival rate translated to a mortality incidence of 159 deaths per 1,000 person-years, compared with 3.9 per 1,000 person-years in a historical cohort of sJIA patients who required biologic therapy.
Diffuse lung disease was the cause of 12 deaths; 5 of these patients also had macrophage activation syndrome at the time of death. Factors significantly associated with shortened survival included male sex, hypoxia at presentation, and neutrophilic bronchoalveolar lavage with more than 10 times the normal count. In an adjusted analysis, all of these variables fell out. However, none of the children with excessively high neutrophilic bronchoalveolar lavage survived.
Does it affect adults?
Could adults be experiencing the same disorder? There is some evidence to support it: The Food and Drug Administration adverse event website shows alveolar disease or pulmonary hypertension in 39 adults who have been exposed to IL-1 or IL-6 inhibition. Of these, 23 had RA, 11 adult-onset Still’s disease, and 5 unclassified rheumatic disorders.
The research groups were supported by grants from the sJIA Foundation, the Lucile Packard Foundation for Children’s Health, Stanford graduate fellowships, the Life Sciences Research Foundation, the Bill & Melinda Gates Foundation, Cincinnati Children’s Research Foundation, the Childhood Arthritis and Rheumatology Research Alliance, the Arthritis Foundation, and the National Institutes of Health. Many authors on both papers reported financial ties to Genentech, which markets tocilizumab, and other pharmaceutical companies*. Dr. Nigrovic reported receiving consulting fees and research support from Novartis and other companies.
SOURCES: Saper V et al. Ann Rheum Dis. 2019 Sep 27. doi: 10.1136/annrheumdis-2019-216040; Schulert GS et al. Arthritis Rheumatol. 2019 Aug 5. doi: 10.1002/art.41073; Nigrovic PA. Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41071.
*Correction, 10/12/19: An earlier version of this article misstated the manufacturer of Actemra (tocilizumab).
This article was updated 10/14/19.
Influenza vaccination modestly reduces risk of hospitalizations in patients with COPD
(COPD), according to data published in the Journal of Infectious Diseases.
“To the best of our knowledge, this is the first large, real-world population study to examine vaccine effectiveness in people with COPD using the test-negative design and influenza-specific study outcomes,” wrote Andrea S. Gershon, MD, of Sunnybrook Health Sciences Center in Toronto and colleagues. “These findings emphasize the need for more effective influenza vaccines for older COPD patients and other preventive strategies.”
A test-negative study design
Data suggest that 70% of COPD exacerbations are caused by infection, and influenza often is identified as the cause. Although all major COPD practice guidelines recommend seasonal influenza vaccination, the evidence indicating that vaccination reduces hospitalizations and death is limited. The inherent or corticosteroid-induced decrease in immune response to vaccination and respiratory infection among patients with COPD may reduce the effectiveness of influenza vaccination, wrote Dr. Gershon and colleagues.
The investigators used a test-negative design to evaluate how effectively influenza vaccination prevents laboratory-confirmed influenza–associated hospitalizations in community-dwelling older patients with COPD. They chose this design because it attenuates biases resulting from misclassification of infection and from differences in health care–seeking behavior between vaccinated and unvaccinated patients.
Dr. Gershon and colleagues examined health care administrative data and respiratory specimens collected from patients who had been tested for influenza during the 2010-2011 to 2015-2016 influenza seasons. Eligible patients were aged 66 years or older, had physician-diagnosed COPD, and had been tested for influenza within 3 days before and during an acute care hospitalization. The researchers determined influenza vaccination status using physician and pharmacist billing claims. They obtained demographic information through linkage with the provincial health insurance database. Multivariable logistic regression allowed Dr. Gershon and colleagues to estimate the adjusted odds ratio of influenza vaccination in people with laboratory-confirmed influenza, compared with those without.
Effectiveness did not vary by demographic factors
The investigators included 21,748 patients in their analysis. Of this population, 3,636 (16.7%) patients tested positive for influenza. Vaccinated patients were less likely than unvaccinated patients to test positive for influenza (15.3% vs. 18.6%). Vaccinated patients also were more likely to be older; live in an urban area; live in a higher income neighborhood; have had more outpatient visits with a physician in the previous year; have received a prescription for a COPD medication in the previous 6 months; have diabetes, asthma, or immunocompromising conditions; have a longer duration of COPD; and have had an outpatient COPD exacerbation in the previous year.
The overall unadjusted estimate of vaccine effectiveness against laboratory-confirmed influenza–associated hospitalizations was 21%. Multivariable adjustment yielded an effectiveness of 22%. When Dr. Gershon and colleagues corrected for misclassification of vaccination status among people with COPD, the effectiveness was estimated to be 43%. Vaccine effectiveness did not vary significantly according to influenza season, nor did it vary significantly by patient-specific factors such as age, sex, influenza subtype, codiagnosis of asthma, duration of COPD, previous outpatient COPD exacerbations, previous COPD hospitalization, previous receipt of inhaled corticosteroids, and previous pneumonia.
One limitation of the study was the possibility that COPD was misclassified because not all participants underwent pulmonary function testing. In addition, the estimates of vaccine effectiveness in the present study are specific to the outcome of influenza hospitalization and may not be generalizable to vaccine effectiveness estimates of outpatient outcomes, said the investigators. Finally, Dr. Gershon and colleagues could not identify the type of vaccine received.
“Given that a large pragmatic randomized controlled trial evaluating influenza vaccination would be unethical, this is likely the most robust estimate of vaccine effectiveness for hospitalizations in the COPD population to guide influenza vaccine recommendations for patients with COPD,” wrote Dr. Gershon and colleagues.
An Ontario Ministry of Health and Long-Term Care Health Systems Research Fund Capacity Grant and a Canadian Institutes of Health Research operating grant funded this research. One investigator received grants from the Canadian Institutes of Health Research during the study, and others received grants from pharmaceutical companies that were unrelated to this study.
SOURCE: Gershon AS et al. J Infect Dis. 2019 Sep 24. doi: 10.1093/infdis/jiz419.
(COPD), according to data published in the Journal of Infectious Diseases.
“To the best of our knowledge, this is the first large, real-world population study to examine vaccine effectiveness in people with COPD using the test-negative design and influenza-specific study outcomes,” wrote Andrea S. Gershon, MD, of Sunnybrook Health Sciences Center in Toronto and colleagues. “These findings emphasize the need for more effective influenza vaccines for older COPD patients and other preventive strategies.”
A test-negative study design
Data suggest that 70% of COPD exacerbations are caused by infection, and influenza often is identified as the cause. Although all major COPD practice guidelines recommend seasonal influenza vaccination, the evidence indicating that vaccination reduces hospitalizations and death is limited. The inherent or corticosteroid-induced decrease in immune response to vaccination and respiratory infection among patients with COPD may reduce the effectiveness of influenza vaccination, wrote Dr. Gershon and colleagues.
The investigators used a test-negative design to evaluate how effectively influenza vaccination prevents laboratory-confirmed influenza–associated hospitalizations in community-dwelling older patients with COPD. They chose this design because it attenuates biases resulting from misclassification of infection and from differences in health care–seeking behavior between vaccinated and unvaccinated patients.
Dr. Gershon and colleagues examined health care administrative data and respiratory specimens collected from patients who had been tested for influenza during the 2010-2011 to 2015-2016 influenza seasons. Eligible patients were aged 66 years or older, had physician-diagnosed COPD, and had been tested for influenza within 3 days before and during an acute care hospitalization. The researchers determined influenza vaccination status using physician and pharmacist billing claims. They obtained demographic information through linkage with the provincial health insurance database. Multivariable logistic regression allowed Dr. Gershon and colleagues to estimate the adjusted odds ratio of influenza vaccination in people with laboratory-confirmed influenza, compared with those without.
Effectiveness did not vary by demographic factors
The investigators included 21,748 patients in their analysis. Of this population, 3,636 (16.7%) patients tested positive for influenza. Vaccinated patients were less likely than unvaccinated patients to test positive for influenza (15.3% vs. 18.6%). Vaccinated patients also were more likely to be older; live in an urban area; live in a higher income neighborhood; have had more outpatient visits with a physician in the previous year; have received a prescription for a COPD medication in the previous 6 months; have diabetes, asthma, or immunocompromising conditions; have a longer duration of COPD; and have had an outpatient COPD exacerbation in the previous year.
The overall unadjusted estimate of vaccine effectiveness against laboratory-confirmed influenza–associated hospitalizations was 21%. Multivariable adjustment yielded an effectiveness of 22%. When Dr. Gershon and colleagues corrected for misclassification of vaccination status among people with COPD, the effectiveness was estimated to be 43%. Vaccine effectiveness did not vary significantly according to influenza season, nor did it vary significantly by patient-specific factors such as age, sex, influenza subtype, codiagnosis of asthma, duration of COPD, previous outpatient COPD exacerbations, previous COPD hospitalization, previous receipt of inhaled corticosteroids, and previous pneumonia.
One limitation of the study was the possibility that COPD was misclassified because not all participants underwent pulmonary function testing. In addition, the estimates of vaccine effectiveness in the present study are specific to the outcome of influenza hospitalization and may not be generalizable to vaccine effectiveness estimates of outpatient outcomes, said the investigators. Finally, Dr. Gershon and colleagues could not identify the type of vaccine received.
“Given that a large pragmatic randomized controlled trial evaluating influenza vaccination would be unethical, this is likely the most robust estimate of vaccine effectiveness for hospitalizations in the COPD population to guide influenza vaccine recommendations for patients with COPD,” wrote Dr. Gershon and colleagues.
An Ontario Ministry of Health and Long-Term Care Health Systems Research Fund Capacity Grant and a Canadian Institutes of Health Research operating grant funded this research. One investigator received grants from the Canadian Institutes of Health Research during the study, and others received grants from pharmaceutical companies that were unrelated to this study.
SOURCE: Gershon AS et al. J Infect Dis. 2019 Sep 24. doi: 10.1093/infdis/jiz419.
(COPD), according to data published in the Journal of Infectious Diseases.
“To the best of our knowledge, this is the first large, real-world population study to examine vaccine effectiveness in people with COPD using the test-negative design and influenza-specific study outcomes,” wrote Andrea S. Gershon, MD, of Sunnybrook Health Sciences Center in Toronto and colleagues. “These findings emphasize the need for more effective influenza vaccines for older COPD patients and other preventive strategies.”
A test-negative study design
Data suggest that 70% of COPD exacerbations are caused by infection, and influenza often is identified as the cause. Although all major COPD practice guidelines recommend seasonal influenza vaccination, the evidence indicating that vaccination reduces hospitalizations and death is limited. The inherent or corticosteroid-induced decrease in immune response to vaccination and respiratory infection among patients with COPD may reduce the effectiveness of influenza vaccination, wrote Dr. Gershon and colleagues.
The investigators used a test-negative design to evaluate how effectively influenza vaccination prevents laboratory-confirmed influenza–associated hospitalizations in community-dwelling older patients with COPD. They chose this design because it attenuates biases resulting from misclassification of infection and from differences in health care–seeking behavior between vaccinated and unvaccinated patients.
Dr. Gershon and colleagues examined health care administrative data and respiratory specimens collected from patients who had been tested for influenza during the 2010-2011 to 2015-2016 influenza seasons. Eligible patients were aged 66 years or older, had physician-diagnosed COPD, and had been tested for influenza within 3 days before and during an acute care hospitalization. The researchers determined influenza vaccination status using physician and pharmacist billing claims. They obtained demographic information through linkage with the provincial health insurance database. Multivariable logistic regression allowed Dr. Gershon and colleagues to estimate the adjusted odds ratio of influenza vaccination in people with laboratory-confirmed influenza, compared with those without.
Effectiveness did not vary by demographic factors
The investigators included 21,748 patients in their analysis. Of this population, 3,636 (16.7%) patients tested positive for influenza. Vaccinated patients were less likely than unvaccinated patients to test positive for influenza (15.3% vs. 18.6%). Vaccinated patients also were more likely to be older; live in an urban area; live in a higher income neighborhood; have had more outpatient visits with a physician in the previous year; have received a prescription for a COPD medication in the previous 6 months; have diabetes, asthma, or immunocompromising conditions; have a longer duration of COPD; and have had an outpatient COPD exacerbation in the previous year.
The overall unadjusted estimate of vaccine effectiveness against laboratory-confirmed influenza–associated hospitalizations was 21%. Multivariable adjustment yielded an effectiveness of 22%. When Dr. Gershon and colleagues corrected for misclassification of vaccination status among people with COPD, the effectiveness was estimated to be 43%. Vaccine effectiveness did not vary significantly according to influenza season, nor did it vary significantly by patient-specific factors such as age, sex, influenza subtype, codiagnosis of asthma, duration of COPD, previous outpatient COPD exacerbations, previous COPD hospitalization, previous receipt of inhaled corticosteroids, and previous pneumonia.
One limitation of the study was the possibility that COPD was misclassified because not all participants underwent pulmonary function testing. In addition, the estimates of vaccine effectiveness in the present study are specific to the outcome of influenza hospitalization and may not be generalizable to vaccine effectiveness estimates of outpatient outcomes, said the investigators. Finally, Dr. Gershon and colleagues could not identify the type of vaccine received.
“Given that a large pragmatic randomized controlled trial evaluating influenza vaccination would be unethical, this is likely the most robust estimate of vaccine effectiveness for hospitalizations in the COPD population to guide influenza vaccine recommendations for patients with COPD,” wrote Dr. Gershon and colleagues.
An Ontario Ministry of Health and Long-Term Care Health Systems Research Fund Capacity Grant and a Canadian Institutes of Health Research operating grant funded this research. One investigator received grants from the Canadian Institutes of Health Research during the study, and others received grants from pharmaceutical companies that were unrelated to this study.
SOURCE: Gershon AS et al. J Infect Dis. 2019 Sep 24. doi: 10.1093/infdis/jiz419.
FROM JOURNAL OF INFECTIOUS DISEASES
The emerging role of quantitative CT scans in ILD terms
The role of imaging for interstitial lung disease (ILD) is of paramount importance. With the growth of high resolution chest computed tomography (HRCT) imaging techniques, we are able to visualize nuances between individual ILDs more critically. HRCT is an essential component of an initial ILD evaluation and also has become part of the armamentarium of tools used for routine management of these patients. The technology of HRCT scans has evolved over the years, most recently with the advent of quantitative HRCT (qCT). The technology employs texture-based classification, which identifies and quantifies different radiographic findings. The arrival of qCT scanning has been slowly emerging as a new player in the ILD world. What exactly is qCT, and what role can, and will it serve for our ILD patients?
Quantitative CT scanning has been introduced since the 1980s, but only within the last 15 years has its use for ILD taken form. Human interpretation of CTs is fraught with subjectivity, based on the interpreting radiologist’s training, experience, and individual visual perception of images. This can result in significant variability in radiographic interpretations and, ultimately, affects a patient’s diagnosis, disease monitoring, treatment, and prognosis. Semiquantitative visual scoring by radiologists is highly variable, especially in areas with limited availability of chest radiologists. qCT employs an automated histogram signature technique that utilizes density and texture-based analysis of the lung parenchyma. Utilizing machine learning from pathologically confirmed datasets, computer programs were trained with specialized thoracic radiologists to distinguish some commonly found radiographic abnormalities into four major groups: ground glass, reticular, honeycombing, and emphysema. In addition, these categories are quantified and spatially depicted on an analysis (Bartholmai, et al. J Thorac Imaging. 2013;28[5]:298). Various computer programs have been built to streamline the process and expedite the interpretation of an individual’s HRCT scan. The more commonly familiar program, CALIPER (Computer-Aided Lung Informatics for Pathology Evaluation and Ratings), has been used in multiple research studies of qCT in ILD and IPF. Each patient’s CT scan is uploaded to the program, and a breakdown of the patient’s lungs into each category is presented. Not only is each abnormality quantified and precisely defined, it is also color-coded by segments to help with visual interpretation by the physician.
The benefit of qCT lies not only in the automated, objective evaluation of interstitial lung disease, but also in its possible use in prognostication and mortality prediction. Neither use has been fully validated as of yet. However, growing evidence shows a promising role in both realms. Thus far, there have been some studies correlating PFT data with qCT findings. A follow-up study of the Scleroderma Lung Study II examined qCT changes over 24 months and correlated those findings with PFTs and patient-reported outcomes. Patients in this study were either treated with cyclophosphamide (CYC) for 1 year/placebo 1 year vs mycophenolate mofetil (MMF) for 2 years. A large portion of patients receiving CYC or MMF had a significant correlation between improved or stable qCT scores and their FVC and TLC. Neither CYC nor MMF was superior in qCT scores, aligning with the findings of the study, which showed noninferiority of MMF compared with CYC (Goldin, et al. Ann Am Thorac Soc. 2018 Nov;15[11]:1286). Interestingly, the improvement of ground glass is often viewed by physicians as positive, since this finding is typically thought of as active inflammation. However, if qCT determines that the fibrosis score actually increases over time, despite an improvement in ground glass, this may more accurately reflect the development of subtle fibrosis that is not easily appreciated by the human eye (Goldin, et al. Ann Am Thorac Soc. 2018 Nov;15[11]:1286). In this context, it is feasible that parenchymal changes occur prior to deterioration on PFTs. Diffusing capacity for carbon monoxide (DLCO) correlates largely with the extent of lung involvement on qCT, but DLCO is not a specific biomarker in predicting severity of ILD (ie, because pHTN or anemia can confound DLCO). Forced vital capacity (FVC) in certain diseases may also confound CT correlation (ie, muscle weakness or extrathoracic restriction from skin disease in systemic sclerosis). The usefulness of PFT data as a clinical endpoint in research studies may be replaced by qCTs more consistent and precise detection of disease modification.
IPF has been an interesting area of exploration for the role of qCT in disease monitoring and possible prognostication. It is known that the presence of honeycombing on HRCT is associated with increased mortality. Patients with a progressive fibrotic ILD have similar mortality rates to those with IPF (Adegunsoye, et al. Ann Am Thorac Soc. 2019 May;16[5]:580). The ability to correlate radiographic findings with mortality could potentially become an important marker of clinical deterioration, especially in those patients who are unable to perform PFTs. In addition, it can also be beneficial in those with co-existent emphysema, since PFTs may be confounded by this overlap. Nakagawa and colleagues proposed a computer-aided method for qCT analysis of honeycombing in patients with IPF. The algorithm for the qCT analysis also has specific parameters to exclude emphysematous lesions on imaging. The %honeycomb area (HA) was correlated with a composite physiologic index derived from PFTs (calculated from FEV1, FVC and DLCO). This tool can accurately quantify the percentage of honeycombing and aid in monitoring IPF. Using this protocol, Nakagawa was able to demonstrate a significant correlation with 3-year mortality, with a marked difference found when using a cutoff value of 4.8% (Nakagawa, et al. Plos One. 2019 Mar; 14[3]:e0214278). Furthermore, patient survival in IPF has been compared against the CALIPER program and PFTs. Mortality for patients was significantly associated with pulmonary vessel volume (PVV), an innovative tool that quantified the volume of the pulmonary artery and veins, which may become a new parameter used for disease monitoring. Using qCT in addition to PFTs provides more tangible evidence to help monitor patients with IPF, guide treatment decisions, and plan for transplant or palliative care. The growing use of PVV in qCT has yet to be fully elucidated, but it does have a promising role (Jacob, et al. Eur Respir J. 2017;49[1]. doi: 10.1183/13993003.01011-2016).
Despite the positive outlook for qCT, there are major issues that limit its widespread use. During the image acquisition process, there is a lack of consistency and quality control, stemming from multiple different manufacturers of CT scan machines, reconstitution methods, radiation doses, and noise or inspiratory efforts of patients. The Radiologic Society of North America (RSNA) is attempting to fix this issue by creating a standardized protocol for collecting images used for qCT (Castillo-Saldana, et al. J Thorac Imaging. 2019 Aug 7. doi: 10.1097/RTI.0000000000000440). In order to move forward with adaptation of qCT, a standardized approach and handling of images needs to be created.
Quantitative CT is an exciting new prospect for the care of patients with ILD. As these patients, and their management, becomes more complex, expanding the toolbox for physicians is much needed. It will be fascinating to see how the role of qCT takes shape over the coming years.
Dr. D’Annunzio is with Westmed Medical Group, Rye, N.Y.; Dr. Nayar is a Pulmonary/Critical Care Fellow at NYU School of Medicine; and Dr. Patel is with Columbia University Medical Center.
The role of imaging for interstitial lung disease (ILD) is of paramount importance. With the growth of high resolution chest computed tomography (HRCT) imaging techniques, we are able to visualize nuances between individual ILDs more critically. HRCT is an essential component of an initial ILD evaluation and also has become part of the armamentarium of tools used for routine management of these patients. The technology of HRCT scans has evolved over the years, most recently with the advent of quantitative HRCT (qCT). The technology employs texture-based classification, which identifies and quantifies different radiographic findings. The arrival of qCT scanning has been slowly emerging as a new player in the ILD world. What exactly is qCT, and what role can, and will it serve for our ILD patients?
Quantitative CT scanning has been introduced since the 1980s, but only within the last 15 years has its use for ILD taken form. Human interpretation of CTs is fraught with subjectivity, based on the interpreting radiologist’s training, experience, and individual visual perception of images. This can result in significant variability in radiographic interpretations and, ultimately, affects a patient’s diagnosis, disease monitoring, treatment, and prognosis. Semiquantitative visual scoring by radiologists is highly variable, especially in areas with limited availability of chest radiologists. qCT employs an automated histogram signature technique that utilizes density and texture-based analysis of the lung parenchyma. Utilizing machine learning from pathologically confirmed datasets, computer programs were trained with specialized thoracic radiologists to distinguish some commonly found radiographic abnormalities into four major groups: ground glass, reticular, honeycombing, and emphysema. In addition, these categories are quantified and spatially depicted on an analysis (Bartholmai, et al. J Thorac Imaging. 2013;28[5]:298). Various computer programs have been built to streamline the process and expedite the interpretation of an individual’s HRCT scan. The more commonly familiar program, CALIPER (Computer-Aided Lung Informatics for Pathology Evaluation and Ratings), has been used in multiple research studies of qCT in ILD and IPF. Each patient’s CT scan is uploaded to the program, and a breakdown of the patient’s lungs into each category is presented. Not only is each abnormality quantified and precisely defined, it is also color-coded by segments to help with visual interpretation by the physician.
The benefit of qCT lies not only in the automated, objective evaluation of interstitial lung disease, but also in its possible use in prognostication and mortality prediction. Neither use has been fully validated as of yet. However, growing evidence shows a promising role in both realms. Thus far, there have been some studies correlating PFT data with qCT findings. A follow-up study of the Scleroderma Lung Study II examined qCT changes over 24 months and correlated those findings with PFTs and patient-reported outcomes. Patients in this study were either treated with cyclophosphamide (CYC) for 1 year/placebo 1 year vs mycophenolate mofetil (MMF) for 2 years. A large portion of patients receiving CYC or MMF had a significant correlation between improved or stable qCT scores and their FVC and TLC. Neither CYC nor MMF was superior in qCT scores, aligning with the findings of the study, which showed noninferiority of MMF compared with CYC (Goldin, et al. Ann Am Thorac Soc. 2018 Nov;15[11]:1286). Interestingly, the improvement of ground glass is often viewed by physicians as positive, since this finding is typically thought of as active inflammation. However, if qCT determines that the fibrosis score actually increases over time, despite an improvement in ground glass, this may more accurately reflect the development of subtle fibrosis that is not easily appreciated by the human eye (Goldin, et al. Ann Am Thorac Soc. 2018 Nov;15[11]:1286). In this context, it is feasible that parenchymal changes occur prior to deterioration on PFTs. Diffusing capacity for carbon monoxide (DLCO) correlates largely with the extent of lung involvement on qCT, but DLCO is not a specific biomarker in predicting severity of ILD (ie, because pHTN or anemia can confound DLCO). Forced vital capacity (FVC) in certain diseases may also confound CT correlation (ie, muscle weakness or extrathoracic restriction from skin disease in systemic sclerosis). The usefulness of PFT data as a clinical endpoint in research studies may be replaced by qCTs more consistent and precise detection of disease modification.
IPF has been an interesting area of exploration for the role of qCT in disease monitoring and possible prognostication. It is known that the presence of honeycombing on HRCT is associated with increased mortality. Patients with a progressive fibrotic ILD have similar mortality rates to those with IPF (Adegunsoye, et al. Ann Am Thorac Soc. 2019 May;16[5]:580). The ability to correlate radiographic findings with mortality could potentially become an important marker of clinical deterioration, especially in those patients who are unable to perform PFTs. In addition, it can also be beneficial in those with co-existent emphysema, since PFTs may be confounded by this overlap. Nakagawa and colleagues proposed a computer-aided method for qCT analysis of honeycombing in patients with IPF. The algorithm for the qCT analysis also has specific parameters to exclude emphysematous lesions on imaging. The %honeycomb area (HA) was correlated with a composite physiologic index derived from PFTs (calculated from FEV1, FVC and DLCO). This tool can accurately quantify the percentage of honeycombing and aid in monitoring IPF. Using this protocol, Nakagawa was able to demonstrate a significant correlation with 3-year mortality, with a marked difference found when using a cutoff value of 4.8% (Nakagawa, et al. Plos One. 2019 Mar; 14[3]:e0214278). Furthermore, patient survival in IPF has been compared against the CALIPER program and PFTs. Mortality for patients was significantly associated with pulmonary vessel volume (PVV), an innovative tool that quantified the volume of the pulmonary artery and veins, which may become a new parameter used for disease monitoring. Using qCT in addition to PFTs provides more tangible evidence to help monitor patients with IPF, guide treatment decisions, and plan for transplant or palliative care. The growing use of PVV in qCT has yet to be fully elucidated, but it does have a promising role (Jacob, et al. Eur Respir J. 2017;49[1]. doi: 10.1183/13993003.01011-2016).
Despite the positive outlook for qCT, there are major issues that limit its widespread use. During the image acquisition process, there is a lack of consistency and quality control, stemming from multiple different manufacturers of CT scan machines, reconstitution methods, radiation doses, and noise or inspiratory efforts of patients. The Radiologic Society of North America (RSNA) is attempting to fix this issue by creating a standardized protocol for collecting images used for qCT (Castillo-Saldana, et al. J Thorac Imaging. 2019 Aug 7. doi: 10.1097/RTI.0000000000000440). In order to move forward with adaptation of qCT, a standardized approach and handling of images needs to be created.
Quantitative CT is an exciting new prospect for the care of patients with ILD. As these patients, and their management, becomes more complex, expanding the toolbox for physicians is much needed. It will be fascinating to see how the role of qCT takes shape over the coming years.
Dr. D’Annunzio is with Westmed Medical Group, Rye, N.Y.; Dr. Nayar is a Pulmonary/Critical Care Fellow at NYU School of Medicine; and Dr. Patel is with Columbia University Medical Center.
The role of imaging for interstitial lung disease (ILD) is of paramount importance. With the growth of high resolution chest computed tomography (HRCT) imaging techniques, we are able to visualize nuances between individual ILDs more critically. HRCT is an essential component of an initial ILD evaluation and also has become part of the armamentarium of tools used for routine management of these patients. The technology of HRCT scans has evolved over the years, most recently with the advent of quantitative HRCT (qCT). The technology employs texture-based classification, which identifies and quantifies different radiographic findings. The arrival of qCT scanning has been slowly emerging as a new player in the ILD world. What exactly is qCT, and what role can, and will it serve for our ILD patients?
Quantitative CT scanning has been introduced since the 1980s, but only within the last 15 years has its use for ILD taken form. Human interpretation of CTs is fraught with subjectivity, based on the interpreting radiologist’s training, experience, and individual visual perception of images. This can result in significant variability in radiographic interpretations and, ultimately, affects a patient’s diagnosis, disease monitoring, treatment, and prognosis. Semiquantitative visual scoring by radiologists is highly variable, especially in areas with limited availability of chest radiologists. qCT employs an automated histogram signature technique that utilizes density and texture-based analysis of the lung parenchyma. Utilizing machine learning from pathologically confirmed datasets, computer programs were trained with specialized thoracic radiologists to distinguish some commonly found radiographic abnormalities into four major groups: ground glass, reticular, honeycombing, and emphysema. In addition, these categories are quantified and spatially depicted on an analysis (Bartholmai, et al. J Thorac Imaging. 2013;28[5]:298). Various computer programs have been built to streamline the process and expedite the interpretation of an individual’s HRCT scan. The more commonly familiar program, CALIPER (Computer-Aided Lung Informatics for Pathology Evaluation and Ratings), has been used in multiple research studies of qCT in ILD and IPF. Each patient’s CT scan is uploaded to the program, and a breakdown of the patient’s lungs into each category is presented. Not only is each abnormality quantified and precisely defined, it is also color-coded by segments to help with visual interpretation by the physician.
The benefit of qCT lies not only in the automated, objective evaluation of interstitial lung disease, but also in its possible use in prognostication and mortality prediction. Neither use has been fully validated as of yet. However, growing evidence shows a promising role in both realms. Thus far, there have been some studies correlating PFT data with qCT findings. A follow-up study of the Scleroderma Lung Study II examined qCT changes over 24 months and correlated those findings with PFTs and patient-reported outcomes. Patients in this study were either treated with cyclophosphamide (CYC) for 1 year/placebo 1 year vs mycophenolate mofetil (MMF) for 2 years. A large portion of patients receiving CYC or MMF had a significant correlation between improved or stable qCT scores and their FVC and TLC. Neither CYC nor MMF was superior in qCT scores, aligning with the findings of the study, which showed noninferiority of MMF compared with CYC (Goldin, et al. Ann Am Thorac Soc. 2018 Nov;15[11]:1286). Interestingly, the improvement of ground glass is often viewed by physicians as positive, since this finding is typically thought of as active inflammation. However, if qCT determines that the fibrosis score actually increases over time, despite an improvement in ground glass, this may more accurately reflect the development of subtle fibrosis that is not easily appreciated by the human eye (Goldin, et al. Ann Am Thorac Soc. 2018 Nov;15[11]:1286). In this context, it is feasible that parenchymal changes occur prior to deterioration on PFTs. Diffusing capacity for carbon monoxide (DLCO) correlates largely with the extent of lung involvement on qCT, but DLCO is not a specific biomarker in predicting severity of ILD (ie, because pHTN or anemia can confound DLCO). Forced vital capacity (FVC) in certain diseases may also confound CT correlation (ie, muscle weakness or extrathoracic restriction from skin disease in systemic sclerosis). The usefulness of PFT data as a clinical endpoint in research studies may be replaced by qCTs more consistent and precise detection of disease modification.
IPF has been an interesting area of exploration for the role of qCT in disease monitoring and possible prognostication. It is known that the presence of honeycombing on HRCT is associated with increased mortality. Patients with a progressive fibrotic ILD have similar mortality rates to those with IPF (Adegunsoye, et al. Ann Am Thorac Soc. 2019 May;16[5]:580). The ability to correlate radiographic findings with mortality could potentially become an important marker of clinical deterioration, especially in those patients who are unable to perform PFTs. In addition, it can also be beneficial in those with co-existent emphysema, since PFTs may be confounded by this overlap. Nakagawa and colleagues proposed a computer-aided method for qCT analysis of honeycombing in patients with IPF. The algorithm for the qCT analysis also has specific parameters to exclude emphysematous lesions on imaging. The %honeycomb area (HA) was correlated with a composite physiologic index derived from PFTs (calculated from FEV1, FVC and DLCO). This tool can accurately quantify the percentage of honeycombing and aid in monitoring IPF. Using this protocol, Nakagawa was able to demonstrate a significant correlation with 3-year mortality, with a marked difference found when using a cutoff value of 4.8% (Nakagawa, et al. Plos One. 2019 Mar; 14[3]:e0214278). Furthermore, patient survival in IPF has been compared against the CALIPER program and PFTs. Mortality for patients was significantly associated with pulmonary vessel volume (PVV), an innovative tool that quantified the volume of the pulmonary artery and veins, which may become a new parameter used for disease monitoring. Using qCT in addition to PFTs provides more tangible evidence to help monitor patients with IPF, guide treatment decisions, and plan for transplant or palliative care. The growing use of PVV in qCT has yet to be fully elucidated, but it does have a promising role (Jacob, et al. Eur Respir J. 2017;49[1]. doi: 10.1183/13993003.01011-2016).
Despite the positive outlook for qCT, there are major issues that limit its widespread use. During the image acquisition process, there is a lack of consistency and quality control, stemming from multiple different manufacturers of CT scan machines, reconstitution methods, radiation doses, and noise or inspiratory efforts of patients. The Radiologic Society of North America (RSNA) is attempting to fix this issue by creating a standardized protocol for collecting images used for qCT (Castillo-Saldana, et al. J Thorac Imaging. 2019 Aug 7. doi: 10.1097/RTI.0000000000000440). In order to move forward with adaptation of qCT, a standardized approach and handling of images needs to be created.
Quantitative CT is an exciting new prospect for the care of patients with ILD. As these patients, and their management, becomes more complex, expanding the toolbox for physicians is much needed. It will be fascinating to see how the role of qCT takes shape over the coming years.
Dr. D’Annunzio is with Westmed Medical Group, Rye, N.Y.; Dr. Nayar is a Pulmonary/Critical Care Fellow at NYU School of Medicine; and Dr. Patel is with Columbia University Medical Center.
Dysregulated sleep is common in children with eosinophilic esophagitis
, Rasintra Siriwat, MD, and colleagues have ascertained.
Children with eosinophilic esophagitis (EoE) also were found to have a high prevalence of atopic diseases, including allergic rhinitis and eczema – findings that could be driving the breathing problems, said Dr. Siriwat, a neurology fellow at the Cleveland Clinic, and coauthors.
The retrospective study comprised 81 children with a diagnosis of EoE who were referred to sleep clinics. In this group, 46 of the children had active EoE (having gastrointestinal symptoms, including feeding difficulties, dysphagia, reflux, nausea/vomiting, or epigastric pain at presentation). The other 35 had an EoE diagnosis but no symptoms on presentation and were categorized as having inactive EoE. Most were male (71.6%) and white (92.5%). The mean age in the cohort was 10 years and the mean body mass index for all subjects was 22 kg/m2. A control group of 192 children without an EoE diagnosis who had overnight polysomnography were included in the analysis.
Allergic-type comorbidities were common among those with active EoE, including allergic rhinitis (55.5%), food allergy (39.5%), and eczema (26%). In addition, a quarter had attention-deficit/hyperactivity disorder, 22% an autism spectrum disorder, 21% a neurological disease, and 29% a psychiatric disorder.
Several sleep complaints were common in the entire EoE cohort, including snoring (76.5 %), restless sleep (66.6%), legs jerking or leg discomfort (43.2%), and daytime sleepiness (58%).
All children underwent an overnight polysomnography. Compared with controls, the children with EoE had significantly higher non-REM2 sleep, significantly lower non-REM3 sleep, lower REM, increased periodic leg movement disorder, and increased arousal index.
“Of note, we found a much higher percentage of [periodic leg movement disorder] in active EoE compared to inactive EoE,” the authors said.
The most common sleep diagnosis for the children with EoE was sleep-disordered breathing. Of 62 children with EoE and sleep disordered breathing, 37% had obstructive sleep apnea (OSA). Two patients had central sleep apnea and five had nocturnal hypoventilation. Children with EoE also reported parasomnia symptoms such as sleep talking (35.8%), sleepwalking (16%), bruxism (23.4%), night terrors (28.4%), and nocturnal enuresis (21.2%).
Of the 59 children with leg movement, 20 had periodic limb movement disorder and 5 were diagnosed with restless leg syndrome. Two were diagnosed with narcolepsy and three with hypersomnia. Four children had a circadian rhythm disorder.
“Notably, the majority of children with EoE had symptoms of sleep-disordered breathing, and more than one-third of total subjects were diagnosed with OSA,” the authors noted. “However, most of them were mild-moderate OSA. It should be noted that the prevalence of OSA in the pediatric population is 1%-5% mostly between the ages of 2-8 years, while the mean age of our subjects was 10 years old. The high prevalence of mild-moderate OSA in the EoE population might be explained by the relationship between EoE and atopic disease.”
Dr. Siriwat had no financial disclosures. The study was supported by Cincinnati Children’s Hospital Research Fund.
SOURCE: Siriwat R et al. Sleep Med. 2019 Sep 11. doi: 10.1016/j.sleep.2019.08.018.
, Rasintra Siriwat, MD, and colleagues have ascertained.
Children with eosinophilic esophagitis (EoE) also were found to have a high prevalence of atopic diseases, including allergic rhinitis and eczema – findings that could be driving the breathing problems, said Dr. Siriwat, a neurology fellow at the Cleveland Clinic, and coauthors.
The retrospective study comprised 81 children with a diagnosis of EoE who were referred to sleep clinics. In this group, 46 of the children had active EoE (having gastrointestinal symptoms, including feeding difficulties, dysphagia, reflux, nausea/vomiting, or epigastric pain at presentation). The other 35 had an EoE diagnosis but no symptoms on presentation and were categorized as having inactive EoE. Most were male (71.6%) and white (92.5%). The mean age in the cohort was 10 years and the mean body mass index for all subjects was 22 kg/m2. A control group of 192 children without an EoE diagnosis who had overnight polysomnography were included in the analysis.
Allergic-type comorbidities were common among those with active EoE, including allergic rhinitis (55.5%), food allergy (39.5%), and eczema (26%). In addition, a quarter had attention-deficit/hyperactivity disorder, 22% an autism spectrum disorder, 21% a neurological disease, and 29% a psychiatric disorder.
Several sleep complaints were common in the entire EoE cohort, including snoring (76.5 %), restless sleep (66.6%), legs jerking or leg discomfort (43.2%), and daytime sleepiness (58%).
All children underwent an overnight polysomnography. Compared with controls, the children with EoE had significantly higher non-REM2 sleep, significantly lower non-REM3 sleep, lower REM, increased periodic leg movement disorder, and increased arousal index.
“Of note, we found a much higher percentage of [periodic leg movement disorder] in active EoE compared to inactive EoE,” the authors said.
The most common sleep diagnosis for the children with EoE was sleep-disordered breathing. Of 62 children with EoE and sleep disordered breathing, 37% had obstructive sleep apnea (OSA). Two patients had central sleep apnea and five had nocturnal hypoventilation. Children with EoE also reported parasomnia symptoms such as sleep talking (35.8%), sleepwalking (16%), bruxism (23.4%), night terrors (28.4%), and nocturnal enuresis (21.2%).
Of the 59 children with leg movement, 20 had periodic limb movement disorder and 5 were diagnosed with restless leg syndrome. Two were diagnosed with narcolepsy and three with hypersomnia. Four children had a circadian rhythm disorder.
“Notably, the majority of children with EoE had symptoms of sleep-disordered breathing, and more than one-third of total subjects were diagnosed with OSA,” the authors noted. “However, most of them were mild-moderate OSA. It should be noted that the prevalence of OSA in the pediatric population is 1%-5% mostly between the ages of 2-8 years, while the mean age of our subjects was 10 years old. The high prevalence of mild-moderate OSA in the EoE population might be explained by the relationship between EoE and atopic disease.”
Dr. Siriwat had no financial disclosures. The study was supported by Cincinnati Children’s Hospital Research Fund.
SOURCE: Siriwat R et al. Sleep Med. 2019 Sep 11. doi: 10.1016/j.sleep.2019.08.018.
, Rasintra Siriwat, MD, and colleagues have ascertained.
Children with eosinophilic esophagitis (EoE) also were found to have a high prevalence of atopic diseases, including allergic rhinitis and eczema – findings that could be driving the breathing problems, said Dr. Siriwat, a neurology fellow at the Cleveland Clinic, and coauthors.
The retrospective study comprised 81 children with a diagnosis of EoE who were referred to sleep clinics. In this group, 46 of the children had active EoE (having gastrointestinal symptoms, including feeding difficulties, dysphagia, reflux, nausea/vomiting, or epigastric pain at presentation). The other 35 had an EoE diagnosis but no symptoms on presentation and were categorized as having inactive EoE. Most were male (71.6%) and white (92.5%). The mean age in the cohort was 10 years and the mean body mass index for all subjects was 22 kg/m2. A control group of 192 children without an EoE diagnosis who had overnight polysomnography were included in the analysis.
Allergic-type comorbidities were common among those with active EoE, including allergic rhinitis (55.5%), food allergy (39.5%), and eczema (26%). In addition, a quarter had attention-deficit/hyperactivity disorder, 22% an autism spectrum disorder, 21% a neurological disease, and 29% a psychiatric disorder.
Several sleep complaints were common in the entire EoE cohort, including snoring (76.5 %), restless sleep (66.6%), legs jerking or leg discomfort (43.2%), and daytime sleepiness (58%).
All children underwent an overnight polysomnography. Compared with controls, the children with EoE had significantly higher non-REM2 sleep, significantly lower non-REM3 sleep, lower REM, increased periodic leg movement disorder, and increased arousal index.
“Of note, we found a much higher percentage of [periodic leg movement disorder] in active EoE compared to inactive EoE,” the authors said.
The most common sleep diagnosis for the children with EoE was sleep-disordered breathing. Of 62 children with EoE and sleep disordered breathing, 37% had obstructive sleep apnea (OSA). Two patients had central sleep apnea and five had nocturnal hypoventilation. Children with EoE also reported parasomnia symptoms such as sleep talking (35.8%), sleepwalking (16%), bruxism (23.4%), night terrors (28.4%), and nocturnal enuresis (21.2%).
Of the 59 children with leg movement, 20 had periodic limb movement disorder and 5 were diagnosed with restless leg syndrome. Two were diagnosed with narcolepsy and three with hypersomnia. Four children had a circadian rhythm disorder.
“Notably, the majority of children with EoE had symptoms of sleep-disordered breathing, and more than one-third of total subjects were diagnosed with OSA,” the authors noted. “However, most of them were mild-moderate OSA. It should be noted that the prevalence of OSA in the pediatric population is 1%-5% mostly between the ages of 2-8 years, while the mean age of our subjects was 10 years old. The high prevalence of mild-moderate OSA in the EoE population might be explained by the relationship between EoE and atopic disease.”
Dr. Siriwat had no financial disclosures. The study was supported by Cincinnati Children’s Hospital Research Fund.
SOURCE: Siriwat R et al. Sleep Med. 2019 Sep 11. doi: 10.1016/j.sleep.2019.08.018.
FROM SLEEP MEDICINE