User login
FDA approves benralizumab autoinjector for eosinophilic asthma
according to a press release from AstraZeneca. Benralizumab is already approved as add-on maintenance for this form of asthma, but not for other eosinophilic conditions or for acute bronchospasm or status asthmaticus.
The autoinjector “pen” was tested for usability and pharmacokinetic exposure in two studies, the phase 3 GRECO trial and the phase 1 AMES trial, respectively. The multicenter, open-label GRECO trial was designed to assess patient- or caregiver-reported functionality, and it found that 97% of at-home administrations were successful at week 12 and week 16. The multicenter, randomized, open-label, parallel-group AMES trial compared pharmacokinetic exposure with the subcutaneous administration using either prefilled syringe or prefilled autoinjector; it found that the eosinophils were rapidly depleted in patients with use of either device.
The safety profiles in both trials were comparable to those seen in previous trials. Hypersensitivity reactions have been sometimes observed in the hours following administration of benralizumab; discontinuation is advised in case of any hypersensitivity reaction. The therapy should not be used to treat acute asthma symptoms, such as exacerbations, or bronchospasm, and any reduction in corticosteroid therapy should be gradual and performed under careful supervision of a health care professional. Although benralizumab’s effects on helminth infections are currently unknown, care should be taken with preexisting or incident infections.
Full prescribing information can be found on the AstraZeneca website.
according to a press release from AstraZeneca. Benralizumab is already approved as add-on maintenance for this form of asthma, but not for other eosinophilic conditions or for acute bronchospasm or status asthmaticus.
The autoinjector “pen” was tested for usability and pharmacokinetic exposure in two studies, the phase 3 GRECO trial and the phase 1 AMES trial, respectively. The multicenter, open-label GRECO trial was designed to assess patient- or caregiver-reported functionality, and it found that 97% of at-home administrations were successful at week 12 and week 16. The multicenter, randomized, open-label, parallel-group AMES trial compared pharmacokinetic exposure with the subcutaneous administration using either prefilled syringe or prefilled autoinjector; it found that the eosinophils were rapidly depleted in patients with use of either device.
The safety profiles in both trials were comparable to those seen in previous trials. Hypersensitivity reactions have been sometimes observed in the hours following administration of benralizumab; discontinuation is advised in case of any hypersensitivity reaction. The therapy should not be used to treat acute asthma symptoms, such as exacerbations, or bronchospasm, and any reduction in corticosteroid therapy should be gradual and performed under careful supervision of a health care professional. Although benralizumab’s effects on helminth infections are currently unknown, care should be taken with preexisting or incident infections.
Full prescribing information can be found on the AstraZeneca website.
according to a press release from AstraZeneca. Benralizumab is already approved as add-on maintenance for this form of asthma, but not for other eosinophilic conditions or for acute bronchospasm or status asthmaticus.
The autoinjector “pen” was tested for usability and pharmacokinetic exposure in two studies, the phase 3 GRECO trial and the phase 1 AMES trial, respectively. The multicenter, open-label GRECO trial was designed to assess patient- or caregiver-reported functionality, and it found that 97% of at-home administrations were successful at week 12 and week 16. The multicenter, randomized, open-label, parallel-group AMES trial compared pharmacokinetic exposure with the subcutaneous administration using either prefilled syringe or prefilled autoinjector; it found that the eosinophils were rapidly depleted in patients with use of either device.
The safety profiles in both trials were comparable to those seen in previous trials. Hypersensitivity reactions have been sometimes observed in the hours following administration of benralizumab; discontinuation is advised in case of any hypersensitivity reaction. The therapy should not be used to treat acute asthma symptoms, such as exacerbations, or bronchospasm, and any reduction in corticosteroid therapy should be gradual and performed under careful supervision of a health care professional. Although benralizumab’s effects on helminth infections are currently unknown, care should be taken with preexisting or incident infections.
Full prescribing information can be found on the AstraZeneca website.
Histologic analysis of vaping-associated lung injury suggests chemical pneumonitis
Vaping-associated lung injury is likely a form of airway-centered chemical pneumonitis, not exogenous lipoid pneumonia, according to Yasmeen M. Butt, MD, of the University of Texas Southwestern Medical Center, Dallas, and associates.
Dr. Butt and associates performed a review of lung biopsies from 17 patients (13 men; median age, 35 years) with a history of vaping and either suspected or confirmed vaping-associated lung injury. All cases showed patterns of acute lung injury, including acute fibrinous pneumonitis, diffuse alveolar damage, or organizing pneumonia, the authors noted in a letter to the editor published in the New England Journal of Medicine.
While no histologic findings were specific, foamy macrophages and pneumocyte vacuolization were seen in all cases, the authors added. Pigmented macrophages were occasionally present but not dominant, neutrophils were often prominent, eosinophils were rare, and granulomas were not seen. Two patients eventually died, despite treatment with glucocorticoids and maximum supportive care.
“None of our cases showed histologic evidence of exogenous lipoid pneumonia and no radiologic evidence thereof has been found; this calls into question the diagnostic utility of identifying lipid-laden macrophages or performing oil red O staining on bronchioloalveolar lavage fluid as a marker of vaping-associated lung injury, as has been proposed,” Dr. Butt and associates wrote.
No conflicts of interest were reported.
SOURCE: Butt YM et al. N Engl J Med. 2019 Oct 2. doi: 10.1056/NEJMc1913069.
Vaping-associated lung injury is likely a form of airway-centered chemical pneumonitis, not exogenous lipoid pneumonia, according to Yasmeen M. Butt, MD, of the University of Texas Southwestern Medical Center, Dallas, and associates.
Dr. Butt and associates performed a review of lung biopsies from 17 patients (13 men; median age, 35 years) with a history of vaping and either suspected or confirmed vaping-associated lung injury. All cases showed patterns of acute lung injury, including acute fibrinous pneumonitis, diffuse alveolar damage, or organizing pneumonia, the authors noted in a letter to the editor published in the New England Journal of Medicine.
While no histologic findings were specific, foamy macrophages and pneumocyte vacuolization were seen in all cases, the authors added. Pigmented macrophages were occasionally present but not dominant, neutrophils were often prominent, eosinophils were rare, and granulomas were not seen. Two patients eventually died, despite treatment with glucocorticoids and maximum supportive care.
“None of our cases showed histologic evidence of exogenous lipoid pneumonia and no radiologic evidence thereof has been found; this calls into question the diagnostic utility of identifying lipid-laden macrophages or performing oil red O staining on bronchioloalveolar lavage fluid as a marker of vaping-associated lung injury, as has been proposed,” Dr. Butt and associates wrote.
No conflicts of interest were reported.
SOURCE: Butt YM et al. N Engl J Med. 2019 Oct 2. doi: 10.1056/NEJMc1913069.
Vaping-associated lung injury is likely a form of airway-centered chemical pneumonitis, not exogenous lipoid pneumonia, according to Yasmeen M. Butt, MD, of the University of Texas Southwestern Medical Center, Dallas, and associates.
Dr. Butt and associates performed a review of lung biopsies from 17 patients (13 men; median age, 35 years) with a history of vaping and either suspected or confirmed vaping-associated lung injury. All cases showed patterns of acute lung injury, including acute fibrinous pneumonitis, diffuse alveolar damage, or organizing pneumonia, the authors noted in a letter to the editor published in the New England Journal of Medicine.
While no histologic findings were specific, foamy macrophages and pneumocyte vacuolization were seen in all cases, the authors added. Pigmented macrophages were occasionally present but not dominant, neutrophils were often prominent, eosinophils were rare, and granulomas were not seen. Two patients eventually died, despite treatment with glucocorticoids and maximum supportive care.
“None of our cases showed histologic evidence of exogenous lipoid pneumonia and no radiologic evidence thereof has been found; this calls into question the diagnostic utility of identifying lipid-laden macrophages or performing oil red O staining on bronchioloalveolar lavage fluid as a marker of vaping-associated lung injury, as has been proposed,” Dr. Butt and associates wrote.
No conflicts of interest were reported.
SOURCE: Butt YM et al. N Engl J Med. 2019 Oct 2. doi: 10.1056/NEJMc1913069.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Oral drug cut viral respiratory tract infections in elderly
WASHINGTON – An investigational, oral, small molecule designed to boost innate antiviral immunity safely cut the incidence of various viral respiratory infections in elderly people during a winter season by nearly a third when administered once daily in a placebo-controlled, multicenter, phase 2 study of 952 patients. Based on these and other findings the drug, RTB101, is now undergoing testing in a phase 3 study, Joan Mannick, MD, said at an annual scientific meeting on infectious diseases.
At a dosage of 10 mg once daily, RTB101 was “well tolerated, upregulated innate antiviral gene expression, and reduced the incidence” of laboratory-confirmed respiratory tract infections caused by several different viruses, said Dr. Mannick, who disclosed that she is a cofounder and chief medical officer of resTORbio, a Boston-based company that’s developing the drug.
During 16 weeks of treatment during the winter virus season, once-daily dosing led to cuts in the rates of respiratory infections compared with placebo by rhinovirus and enterovirus, respiratory syncytial virus, coronavirus, influenza virus, metapneuomovirus, and parainfluenza virus, especially in patients whom the results identified as having the best drug responses: those who were at least 85 years old, and those who were at least 65 years old and also had asthma. Enrolled patients who were at least 65 years old and had other risk factors – current smoking, chronic obstructive pulmonary disease, or diabetes – had notably less robust responses to treatment, and the phase 3 study is not enrolling elderly people who currently smoke or have chronic obstructive pulmonary disease, Dr. Mannick said in an interview.
RTB101 inhibits the active site of the “mechanistic target of rapamycin” (mTOR) protein, the key player of the TORC1 protein complex that appears to downregulate innate antiviral immunity when active. Hence inhibiting mTOR and TORC1 activity should boost innate antiviral immunity. Once-daily dosing with 10 mg of RTB101 appears to mimic the normal daily cycle of high and low levels of TORC1 activity seen in younger adults but which is missing the elderly who generally have persistently elevated levels of TORC1 activity, Dr. Mannick explained.
The study she reported enrolled a total of 952 people at any of 10 sites in the Southern Hemisphere or 17 Northern Hemisphere study sites. The researchers randomized patients to receive either RTB101 or placebo at either of two once-daily dosages or either of two twice-daily regimens. The best drug performance was among the 356 patients treated with 10 mg once daily or placebo. Those who received the active drug at this level had a 19% incidence of any laboratory-confirmed respiratory tract infection, while those who received placebo had a 28% incidence, a 30.6% relative risk reduction with RTB101 treatment that was statistically significant.
The actively-treated patients showed upregulation for 19 of 20 “antiviral” genes assessed in the study compared with upregulation of just five of these genes in the those who received placebo. Two post hoc analyses showed that the people who received 10 mg once daily had about half the rate of all-cause hospitalizations compared with those on placebo, and among those who had respiratory infections treated patients had alleviation of their moderate or severe symptoms in about half the time compared with patients on placebo.
The 10-mg daily dosage of RTB101 is less than 1% of the maximum-tolerated dose in people, and the safety data collected in the current study showed adverse events occurring at similar rates in the patients who received the active drug and those who got placebo. Discontinuations because of adverse events occurred in 5% of people who received RTB101 and in 6% of those on placebo.
The researchers are planning to run a cost-effectiveness study to see whether the observed prevention of respiratory tract infections and their consequences can offset the cost of taking RTB101 daily for 16 weeks, Dr. Mannick said.
WASHINGTON – An investigational, oral, small molecule designed to boost innate antiviral immunity safely cut the incidence of various viral respiratory infections in elderly people during a winter season by nearly a third when administered once daily in a placebo-controlled, multicenter, phase 2 study of 952 patients. Based on these and other findings the drug, RTB101, is now undergoing testing in a phase 3 study, Joan Mannick, MD, said at an annual scientific meeting on infectious diseases.
At a dosage of 10 mg once daily, RTB101 was “well tolerated, upregulated innate antiviral gene expression, and reduced the incidence” of laboratory-confirmed respiratory tract infections caused by several different viruses, said Dr. Mannick, who disclosed that she is a cofounder and chief medical officer of resTORbio, a Boston-based company that’s developing the drug.
During 16 weeks of treatment during the winter virus season, once-daily dosing led to cuts in the rates of respiratory infections compared with placebo by rhinovirus and enterovirus, respiratory syncytial virus, coronavirus, influenza virus, metapneuomovirus, and parainfluenza virus, especially in patients whom the results identified as having the best drug responses: those who were at least 85 years old, and those who were at least 65 years old and also had asthma. Enrolled patients who were at least 65 years old and had other risk factors – current smoking, chronic obstructive pulmonary disease, or diabetes – had notably less robust responses to treatment, and the phase 3 study is not enrolling elderly people who currently smoke or have chronic obstructive pulmonary disease, Dr. Mannick said in an interview.
RTB101 inhibits the active site of the “mechanistic target of rapamycin” (mTOR) protein, the key player of the TORC1 protein complex that appears to downregulate innate antiviral immunity when active. Hence inhibiting mTOR and TORC1 activity should boost innate antiviral immunity. Once-daily dosing with 10 mg of RTB101 appears to mimic the normal daily cycle of high and low levels of TORC1 activity seen in younger adults but which is missing the elderly who generally have persistently elevated levels of TORC1 activity, Dr. Mannick explained.
The study she reported enrolled a total of 952 people at any of 10 sites in the Southern Hemisphere or 17 Northern Hemisphere study sites. The researchers randomized patients to receive either RTB101 or placebo at either of two once-daily dosages or either of two twice-daily regimens. The best drug performance was among the 356 patients treated with 10 mg once daily or placebo. Those who received the active drug at this level had a 19% incidence of any laboratory-confirmed respiratory tract infection, while those who received placebo had a 28% incidence, a 30.6% relative risk reduction with RTB101 treatment that was statistically significant.
The actively-treated patients showed upregulation for 19 of 20 “antiviral” genes assessed in the study compared with upregulation of just five of these genes in the those who received placebo. Two post hoc analyses showed that the people who received 10 mg once daily had about half the rate of all-cause hospitalizations compared with those on placebo, and among those who had respiratory infections treated patients had alleviation of their moderate or severe symptoms in about half the time compared with patients on placebo.
The 10-mg daily dosage of RTB101 is less than 1% of the maximum-tolerated dose in people, and the safety data collected in the current study showed adverse events occurring at similar rates in the patients who received the active drug and those who got placebo. Discontinuations because of adverse events occurred in 5% of people who received RTB101 and in 6% of those on placebo.
The researchers are planning to run a cost-effectiveness study to see whether the observed prevention of respiratory tract infections and their consequences can offset the cost of taking RTB101 daily for 16 weeks, Dr. Mannick said.
WASHINGTON – An investigational, oral, small molecule designed to boost innate antiviral immunity safely cut the incidence of various viral respiratory infections in elderly people during a winter season by nearly a third when administered once daily in a placebo-controlled, multicenter, phase 2 study of 952 patients. Based on these and other findings the drug, RTB101, is now undergoing testing in a phase 3 study, Joan Mannick, MD, said at an annual scientific meeting on infectious diseases.
At a dosage of 10 mg once daily, RTB101 was “well tolerated, upregulated innate antiviral gene expression, and reduced the incidence” of laboratory-confirmed respiratory tract infections caused by several different viruses, said Dr. Mannick, who disclosed that she is a cofounder and chief medical officer of resTORbio, a Boston-based company that’s developing the drug.
During 16 weeks of treatment during the winter virus season, once-daily dosing led to cuts in the rates of respiratory infections compared with placebo by rhinovirus and enterovirus, respiratory syncytial virus, coronavirus, influenza virus, metapneuomovirus, and parainfluenza virus, especially in patients whom the results identified as having the best drug responses: those who were at least 85 years old, and those who were at least 65 years old and also had asthma. Enrolled patients who were at least 65 years old and had other risk factors – current smoking, chronic obstructive pulmonary disease, or diabetes – had notably less robust responses to treatment, and the phase 3 study is not enrolling elderly people who currently smoke or have chronic obstructive pulmonary disease, Dr. Mannick said in an interview.
RTB101 inhibits the active site of the “mechanistic target of rapamycin” (mTOR) protein, the key player of the TORC1 protein complex that appears to downregulate innate antiviral immunity when active. Hence inhibiting mTOR and TORC1 activity should boost innate antiviral immunity. Once-daily dosing with 10 mg of RTB101 appears to mimic the normal daily cycle of high and low levels of TORC1 activity seen in younger adults but which is missing the elderly who generally have persistently elevated levels of TORC1 activity, Dr. Mannick explained.
The study she reported enrolled a total of 952 people at any of 10 sites in the Southern Hemisphere or 17 Northern Hemisphere study sites. The researchers randomized patients to receive either RTB101 or placebo at either of two once-daily dosages or either of two twice-daily regimens. The best drug performance was among the 356 patients treated with 10 mg once daily or placebo. Those who received the active drug at this level had a 19% incidence of any laboratory-confirmed respiratory tract infection, while those who received placebo had a 28% incidence, a 30.6% relative risk reduction with RTB101 treatment that was statistically significant.
The actively-treated patients showed upregulation for 19 of 20 “antiviral” genes assessed in the study compared with upregulation of just five of these genes in the those who received placebo. Two post hoc analyses showed that the people who received 10 mg once daily had about half the rate of all-cause hospitalizations compared with those on placebo, and among those who had respiratory infections treated patients had alleviation of their moderate or severe symptoms in about half the time compared with patients on placebo.
The 10-mg daily dosage of RTB101 is less than 1% of the maximum-tolerated dose in people, and the safety data collected in the current study showed adverse events occurring at similar rates in the patients who received the active drug and those who got placebo. Discontinuations because of adverse events occurred in 5% of people who received RTB101 and in 6% of those on placebo.
The researchers are planning to run a cost-effectiveness study to see whether the observed prevention of respiratory tract infections and their consequences can offset the cost of taking RTB101 daily for 16 weeks, Dr. Mannick said.
REPORTING FROM IDWEEK 2019
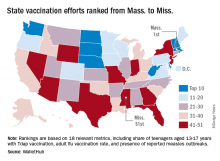
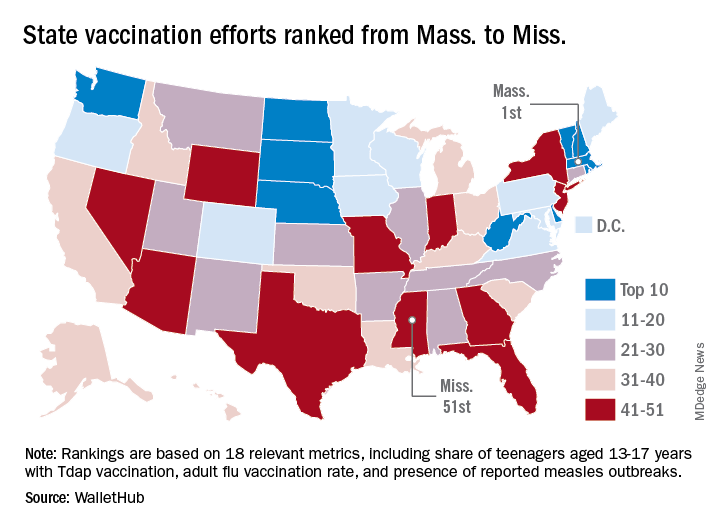
Massachusetts tops state vaccination rankings
according to a new analysis from personal finance website WalletHub.
The Bay State’s top finish in the “children and teenagers immunization rates” category moved it ahead of Vermont in the overall rankings, which had the highest score in each of the other two broad categories – “adult and elderly vaccination rates” and “immunization uptake disparities and influencing factors” – but only finished 15th in child/teen immunization, Wallethub reported.
The state that ranked 51st in child/teen immunization – Mississippi – also finished 51st overall, behind every other state and Washington, D.C. The rest of the bottom five consisted of Texas (50th); Florida (49th), which ranked last in the adult/elderly category; Georgia (48th); and Indiana (47th). New Mexico, however, managed to show that last is not always least by earning a mid-pack overall rank of 30 despite its last-place showing in the disparities/influencing factors category, the WalletHub analysis showed.
Scores for the three broad categories were determined using 18 relevant metrics, including influenza vaccination rate in children aged 6 months to 17 years (1st, Rhode Island; 51st, Wyoming), share of adults aged 60 years and older with zoster vaccination (1st, Vermont; 51st, Mississippi), and share of population without health insurance coverage (1st, Massachusetts; 51st, Texas), WalletHub said.
“Each state should tailor its vaccines policy to its need, with an understanding that those needs may change,” Dorit Rubinstein Reiss of the University of California Hastings College of the Law, San Francisco, told WalletHub. When parents refuse to have their children vaccinated, it’s important to remember that “the state is not denying these children schooling. It is requiring that they be protected from disease first.”
according to a new analysis from personal finance website WalletHub.
The Bay State’s top finish in the “children and teenagers immunization rates” category moved it ahead of Vermont in the overall rankings, which had the highest score in each of the other two broad categories – “adult and elderly vaccination rates” and “immunization uptake disparities and influencing factors” – but only finished 15th in child/teen immunization, Wallethub reported.
The state that ranked 51st in child/teen immunization – Mississippi – also finished 51st overall, behind every other state and Washington, D.C. The rest of the bottom five consisted of Texas (50th); Florida (49th), which ranked last in the adult/elderly category; Georgia (48th); and Indiana (47th). New Mexico, however, managed to show that last is not always least by earning a mid-pack overall rank of 30 despite its last-place showing in the disparities/influencing factors category, the WalletHub analysis showed.
Scores for the three broad categories were determined using 18 relevant metrics, including influenza vaccination rate in children aged 6 months to 17 years (1st, Rhode Island; 51st, Wyoming), share of adults aged 60 years and older with zoster vaccination (1st, Vermont; 51st, Mississippi), and share of population without health insurance coverage (1st, Massachusetts; 51st, Texas), WalletHub said.
“Each state should tailor its vaccines policy to its need, with an understanding that those needs may change,” Dorit Rubinstein Reiss of the University of California Hastings College of the Law, San Francisco, told WalletHub. When parents refuse to have their children vaccinated, it’s important to remember that “the state is not denying these children schooling. It is requiring that they be protected from disease first.”
according to a new analysis from personal finance website WalletHub.
The Bay State’s top finish in the “children and teenagers immunization rates” category moved it ahead of Vermont in the overall rankings, which had the highest score in each of the other two broad categories – “adult and elderly vaccination rates” and “immunization uptake disparities and influencing factors” – but only finished 15th in child/teen immunization, Wallethub reported.
The state that ranked 51st in child/teen immunization – Mississippi – also finished 51st overall, behind every other state and Washington, D.C. The rest of the bottom five consisted of Texas (50th); Florida (49th), which ranked last in the adult/elderly category; Georgia (48th); and Indiana (47th). New Mexico, however, managed to show that last is not always least by earning a mid-pack overall rank of 30 despite its last-place showing in the disparities/influencing factors category, the WalletHub analysis showed.
Scores for the three broad categories were determined using 18 relevant metrics, including influenza vaccination rate in children aged 6 months to 17 years (1st, Rhode Island; 51st, Wyoming), share of adults aged 60 years and older with zoster vaccination (1st, Vermont; 51st, Mississippi), and share of population without health insurance coverage (1st, Massachusetts; 51st, Texas), WalletHub said.
“Each state should tailor its vaccines policy to its need, with an understanding that those needs may change,” Dorit Rubinstein Reiss of the University of California Hastings College of the Law, San Francisco, told WalletHub. When parents refuse to have their children vaccinated, it’s important to remember that “the state is not denying these children schooling. It is requiring that they be protected from disease first.”
Vaping-associated lung injury cases exceed 1,000
More than 1,000 cases of vaping-associated lung injury have been reported in 48 states and the U.S. Virgin Islands, according to a telebriefing by the Centers for Disease Control and Prevention.
As of Oct. 1, there have been 1,080 confirmed and probable cases of lung injury associated with the use of e-cigarettes, or vaping, said Anne Schuchat, MD, principal deputy director of the CDC. The latest figures were also reported in a statement issued by the CDC.
Dr. Schuchat said 18 related deaths in 15 states have been confirmed, and additional deaths are under investigation.
“As we have continued to get data for additional cases, the trends we reported last week persist,” Dr. Schuchat said (MMWR. 2019 Sep 27;68[39];860-4).
“Most patients reported a history of using THC [tetrahydrocannabinol]-containing products, and most patients are male and young people.” Of the 1,080 cases identified, approximately 70% are male, roughly 80% are younger than 35 years of age, and 37% are under 21 years of age. The patients’ median age is 23 years (range, 13-75 years). Among patients who have died, the median age is 50 years (range, 27-71 years).
The CDC now has information from 578 patients on the substances used in vaping products in the 90 days before symptom onset. About 78% of these patients reported using THC-containing products, and 37% reported exclusive use of THC-containing products. Roughly 58% of patients reported using nicotine-containing products, and 17% reported exclusive use of nicotine-containing products.
“I wish we had more answers regarding the specific harmful products or components that are causing these illnesses,” Dr. Schuchat said. She noted that THC-containing products appear to be the most commonly used, but these products don’t appear to be the only culprit. Additionally, in a report released recently in the New England Journal of Medicine (2019 Sep 9. doi: 10.1056/NEJMoa1911614), THC-containing products bought “off the street” were commonly used by patients with lung injuries. However, the CDC can’t say for certain if it’s safer for consumers to buy THC-containing products from a licensed dispensary.
The CDC has deployed staff to several states to help investigate the lung injuries, reached out to the clinical community to increase awareness of the injuries, and worked with clinicians and medical examiners to review assessments of patients who have developed these injuries, including those who have died. The CDC has also convened clinical professional societies to “help strengthen the detection, reporting, and management of cases,” Dr. Schuchat said.
In addition, the CDC has joined with the Food and Drug Administration and other public health partners to develop a laboratory plan for “continued testing of products, aerosol testing of substances produced by the products, and clinical pathology lung specimens from patients,” Dr. Schuchat said.
The FDA is also working to gather more information about vaping-associated lung injuries. The FDA is trying to obtain “critical details” about the specific products or substances that may be involved, said Judy McMeekin, PharmD, deputy associate commissioner for regulatory affairs at the FDA.
“There does not currently appear to be one product or substance involved in all of the cases,” Dr. McMeekin said. “We are leaving no stone unturned and following all potential leads regarding any particular product, constituent, or compound that may be at issue.”
The FDA has collected more than 440 samples of vaping devices and products from 18 states. The agency is still analyzing these samples, but a preliminary analysis has shown that some products contain THC concentrations ranging from 14% to 76%, and some products contain a combination of THC and vitamin E acetate ranging from 31% to 88%.
For information about the collection of vaping products for possible testing by the FDA, email [email protected]. For information about collection and submission of clinical specimens for possible testing by the CDC, see the Healthcare Provider webpage.
Clinicians and health officials who have questions about this outbreak can email [email protected]. All others with questions about this outbreak can contact CDC-INFO at 800-232-4636 or submit information at the Contact CDC-INFO page.
More than 1,000 cases of vaping-associated lung injury have been reported in 48 states and the U.S. Virgin Islands, according to a telebriefing by the Centers for Disease Control and Prevention.
As of Oct. 1, there have been 1,080 confirmed and probable cases of lung injury associated with the use of e-cigarettes, or vaping, said Anne Schuchat, MD, principal deputy director of the CDC. The latest figures were also reported in a statement issued by the CDC.
Dr. Schuchat said 18 related deaths in 15 states have been confirmed, and additional deaths are under investigation.
“As we have continued to get data for additional cases, the trends we reported last week persist,” Dr. Schuchat said (MMWR. 2019 Sep 27;68[39];860-4).
“Most patients reported a history of using THC [tetrahydrocannabinol]-containing products, and most patients are male and young people.” Of the 1,080 cases identified, approximately 70% are male, roughly 80% are younger than 35 years of age, and 37% are under 21 years of age. The patients’ median age is 23 years (range, 13-75 years). Among patients who have died, the median age is 50 years (range, 27-71 years).
The CDC now has information from 578 patients on the substances used in vaping products in the 90 days before symptom onset. About 78% of these patients reported using THC-containing products, and 37% reported exclusive use of THC-containing products. Roughly 58% of patients reported using nicotine-containing products, and 17% reported exclusive use of nicotine-containing products.
“I wish we had more answers regarding the specific harmful products or components that are causing these illnesses,” Dr. Schuchat said. She noted that THC-containing products appear to be the most commonly used, but these products don’t appear to be the only culprit. Additionally, in a report released recently in the New England Journal of Medicine (2019 Sep 9. doi: 10.1056/NEJMoa1911614), THC-containing products bought “off the street” were commonly used by patients with lung injuries. However, the CDC can’t say for certain if it’s safer for consumers to buy THC-containing products from a licensed dispensary.
The CDC has deployed staff to several states to help investigate the lung injuries, reached out to the clinical community to increase awareness of the injuries, and worked with clinicians and medical examiners to review assessments of patients who have developed these injuries, including those who have died. The CDC has also convened clinical professional societies to “help strengthen the detection, reporting, and management of cases,” Dr. Schuchat said.
In addition, the CDC has joined with the Food and Drug Administration and other public health partners to develop a laboratory plan for “continued testing of products, aerosol testing of substances produced by the products, and clinical pathology lung specimens from patients,” Dr. Schuchat said.
The FDA is also working to gather more information about vaping-associated lung injuries. The FDA is trying to obtain “critical details” about the specific products or substances that may be involved, said Judy McMeekin, PharmD, deputy associate commissioner for regulatory affairs at the FDA.
“There does not currently appear to be one product or substance involved in all of the cases,” Dr. McMeekin said. “We are leaving no stone unturned and following all potential leads regarding any particular product, constituent, or compound that may be at issue.”
The FDA has collected more than 440 samples of vaping devices and products from 18 states. The agency is still analyzing these samples, but a preliminary analysis has shown that some products contain THC concentrations ranging from 14% to 76%, and some products contain a combination of THC and vitamin E acetate ranging from 31% to 88%.
For information about the collection of vaping products for possible testing by the FDA, email [email protected]. For information about collection and submission of clinical specimens for possible testing by the CDC, see the Healthcare Provider webpage.
Clinicians and health officials who have questions about this outbreak can email [email protected]. All others with questions about this outbreak can contact CDC-INFO at 800-232-4636 or submit information at the Contact CDC-INFO page.
More than 1,000 cases of vaping-associated lung injury have been reported in 48 states and the U.S. Virgin Islands, according to a telebriefing by the Centers for Disease Control and Prevention.
As of Oct. 1, there have been 1,080 confirmed and probable cases of lung injury associated with the use of e-cigarettes, or vaping, said Anne Schuchat, MD, principal deputy director of the CDC. The latest figures were also reported in a statement issued by the CDC.
Dr. Schuchat said 18 related deaths in 15 states have been confirmed, and additional deaths are under investigation.
“As we have continued to get data for additional cases, the trends we reported last week persist,” Dr. Schuchat said (MMWR. 2019 Sep 27;68[39];860-4).
“Most patients reported a history of using THC [tetrahydrocannabinol]-containing products, and most patients are male and young people.” Of the 1,080 cases identified, approximately 70% are male, roughly 80% are younger than 35 years of age, and 37% are under 21 years of age. The patients’ median age is 23 years (range, 13-75 years). Among patients who have died, the median age is 50 years (range, 27-71 years).
The CDC now has information from 578 patients on the substances used in vaping products in the 90 days before symptom onset. About 78% of these patients reported using THC-containing products, and 37% reported exclusive use of THC-containing products. Roughly 58% of patients reported using nicotine-containing products, and 17% reported exclusive use of nicotine-containing products.
“I wish we had more answers regarding the specific harmful products or components that are causing these illnesses,” Dr. Schuchat said. She noted that THC-containing products appear to be the most commonly used, but these products don’t appear to be the only culprit. Additionally, in a report released recently in the New England Journal of Medicine (2019 Sep 9. doi: 10.1056/NEJMoa1911614), THC-containing products bought “off the street” were commonly used by patients with lung injuries. However, the CDC can’t say for certain if it’s safer for consumers to buy THC-containing products from a licensed dispensary.
The CDC has deployed staff to several states to help investigate the lung injuries, reached out to the clinical community to increase awareness of the injuries, and worked with clinicians and medical examiners to review assessments of patients who have developed these injuries, including those who have died. The CDC has also convened clinical professional societies to “help strengthen the detection, reporting, and management of cases,” Dr. Schuchat said.
In addition, the CDC has joined with the Food and Drug Administration and other public health partners to develop a laboratory plan for “continued testing of products, aerosol testing of substances produced by the products, and clinical pathology lung specimens from patients,” Dr. Schuchat said.
The FDA is also working to gather more information about vaping-associated lung injuries. The FDA is trying to obtain “critical details” about the specific products or substances that may be involved, said Judy McMeekin, PharmD, deputy associate commissioner for regulatory affairs at the FDA.
“There does not currently appear to be one product or substance involved in all of the cases,” Dr. McMeekin said. “We are leaving no stone unturned and following all potential leads regarding any particular product, constituent, or compound that may be at issue.”
The FDA has collected more than 440 samples of vaping devices and products from 18 states. The agency is still analyzing these samples, but a preliminary analysis has shown that some products contain THC concentrations ranging from 14% to 76%, and some products contain a combination of THC and vitamin E acetate ranging from 31% to 88%.
For information about the collection of vaping products for possible testing by the FDA, email [email protected]. For information about collection and submission of clinical specimens for possible testing by the CDC, see the Healthcare Provider webpage.
Clinicians and health officials who have questions about this outbreak can email [email protected]. All others with questions about this outbreak can contact CDC-INFO at 800-232-4636 or submit information at the Contact CDC-INFO page.
One-third of patients with severe asthma are overusing corticosteroids
MADRID – if data from a Dutch study presented at the annual congress of the European Respiratory Society are representative of practice elsewhere.
“The main message from our study is that OCS overuse is common and unnecessary in the majority of asthma patients,” reported Katrien A.B. Eger, MD, Amsterdam University Medical Centre.
In this study, 5,002 patients on high doses of inhaled corticosteroids (ICS), defined as at least 500 mcg/day, were identified in a pharmacy database in the Netherlands. These patients were asked to complete a questionnaire to determine how many had severe asthma and had received rescue or maintenance OCS in the past year.
Drawing from the pharmacy database, it could be determined that 29% of the 2,312 patients who responded to the questionnaire were taking harmfully high doses of OCS as well as high doses of ICS. For this study, harmful exposure was defined as a cumulative intake of 420 mg of prednisone-equivalent OCS over a 1-year period. The median cumulative 1-year exposure, according to Dr. Eger, was 750 mg of prednisone equivalent.
In this population, the investigators then calculated ICS medication adherence based on prescription refills. In addition, a subset of this population was evaluated for inhaler technique.
On the basis of these calculations, 47.4% of patients with harmful OCS exposure were found not to be adherent to their prescribed ICS. Of those who were adherent, 53.9% were found not be taking their inhaled steroids appropriately,
When these numbers are put together, the data suggest “78.1% of high OCS users are either nonadherent or using poor inhalation techniques, which means there is a big potential for treatment optimization,” Dr. Eger said.
Yet even among the 21.9% who were adherent and using good inhaler technique, identifying a group who presumably require OCS for exacerbations, the study found that only 46.1% had been prescribed a biologic, which Dr. Eger considers an important steroid-sparing option. She conceded that many of those not on a biologic might not be candidates, but she believes this is another missed opportunity for reducing OCS exposure.
“In the Netherlands, we have very good access to health care, and biologics are available to anyone who needs them,” said Dr. Eger, explaining that access to these drugs is not a barrier.
The evidence overall is that not enough is being done to ensure that asthma patients are being protected from the risks of OCS, according to Dr. Eger. Citing evidence that adverse events associated with OCS begin with a cumulative lifetime prednisone-equivalent exposure of only 500 mg, she believes that clinicians should be more aggressive in intervening.
“We know that there are both acute and chronic complications associated with OCS that involve a range of organ systems,” Dr. Eger said. She listed osteoporosis, diabetes mellitus, hypertension, and adrenal insufficiency as examples. Rescue OCS, even if used sparingly, can drive risk of OCS complications attributable to the importance of cumulative exposure.
In the session where these data were presented, the moderator, Guy Brusselle, MD, professor of asthma and immunology, Ghent (Belgium) University, labeled them “important.” However, he quibbled with Dr. Eger’s assertion that biologics represent a major opportunity to reduce OCS exposure.
“By suggesting that biologics are not being used often enough, there is an assumption that all of these patients have type 2 inflammatory asthma,” Dr. Brusselle said. “I think it makes more sense to emphasize steroid-sparing strategies, not just biologics.”
Dr. Eger did not disagree, but she emphasized that steroid-sparing alternatives are just one strategy to reduce OCS exposure, and ensuring that patients are adherent to prescribed ICS therapies and are using them correctly might have an even greater impact.
Dr. Eger reports no potential conflicts of interest.
MADRID – if data from a Dutch study presented at the annual congress of the European Respiratory Society are representative of practice elsewhere.
“The main message from our study is that OCS overuse is common and unnecessary in the majority of asthma patients,” reported Katrien A.B. Eger, MD, Amsterdam University Medical Centre.
In this study, 5,002 patients on high doses of inhaled corticosteroids (ICS), defined as at least 500 mcg/day, were identified in a pharmacy database in the Netherlands. These patients were asked to complete a questionnaire to determine how many had severe asthma and had received rescue or maintenance OCS in the past year.
Drawing from the pharmacy database, it could be determined that 29% of the 2,312 patients who responded to the questionnaire were taking harmfully high doses of OCS as well as high doses of ICS. For this study, harmful exposure was defined as a cumulative intake of 420 mg of prednisone-equivalent OCS over a 1-year period. The median cumulative 1-year exposure, according to Dr. Eger, was 750 mg of prednisone equivalent.
In this population, the investigators then calculated ICS medication adherence based on prescription refills. In addition, a subset of this population was evaluated for inhaler technique.
On the basis of these calculations, 47.4% of patients with harmful OCS exposure were found not to be adherent to their prescribed ICS. Of those who were adherent, 53.9% were found not be taking their inhaled steroids appropriately,
When these numbers are put together, the data suggest “78.1% of high OCS users are either nonadherent or using poor inhalation techniques, which means there is a big potential for treatment optimization,” Dr. Eger said.
Yet even among the 21.9% who were adherent and using good inhaler technique, identifying a group who presumably require OCS for exacerbations, the study found that only 46.1% had been prescribed a biologic, which Dr. Eger considers an important steroid-sparing option. She conceded that many of those not on a biologic might not be candidates, but she believes this is another missed opportunity for reducing OCS exposure.
“In the Netherlands, we have very good access to health care, and biologics are available to anyone who needs them,” said Dr. Eger, explaining that access to these drugs is not a barrier.
The evidence overall is that not enough is being done to ensure that asthma patients are being protected from the risks of OCS, according to Dr. Eger. Citing evidence that adverse events associated with OCS begin with a cumulative lifetime prednisone-equivalent exposure of only 500 mg, she believes that clinicians should be more aggressive in intervening.
“We know that there are both acute and chronic complications associated with OCS that involve a range of organ systems,” Dr. Eger said. She listed osteoporosis, diabetes mellitus, hypertension, and adrenal insufficiency as examples. Rescue OCS, even if used sparingly, can drive risk of OCS complications attributable to the importance of cumulative exposure.
In the session where these data were presented, the moderator, Guy Brusselle, MD, professor of asthma and immunology, Ghent (Belgium) University, labeled them “important.” However, he quibbled with Dr. Eger’s assertion that biologics represent a major opportunity to reduce OCS exposure.
“By suggesting that biologics are not being used often enough, there is an assumption that all of these patients have type 2 inflammatory asthma,” Dr. Brusselle said. “I think it makes more sense to emphasize steroid-sparing strategies, not just biologics.”
Dr. Eger did not disagree, but she emphasized that steroid-sparing alternatives are just one strategy to reduce OCS exposure, and ensuring that patients are adherent to prescribed ICS therapies and are using them correctly might have an even greater impact.
Dr. Eger reports no potential conflicts of interest.
MADRID – if data from a Dutch study presented at the annual congress of the European Respiratory Society are representative of practice elsewhere.
“The main message from our study is that OCS overuse is common and unnecessary in the majority of asthma patients,” reported Katrien A.B. Eger, MD, Amsterdam University Medical Centre.
In this study, 5,002 patients on high doses of inhaled corticosteroids (ICS), defined as at least 500 mcg/day, were identified in a pharmacy database in the Netherlands. These patients were asked to complete a questionnaire to determine how many had severe asthma and had received rescue or maintenance OCS in the past year.
Drawing from the pharmacy database, it could be determined that 29% of the 2,312 patients who responded to the questionnaire were taking harmfully high doses of OCS as well as high doses of ICS. For this study, harmful exposure was defined as a cumulative intake of 420 mg of prednisone-equivalent OCS over a 1-year period. The median cumulative 1-year exposure, according to Dr. Eger, was 750 mg of prednisone equivalent.
In this population, the investigators then calculated ICS medication adherence based on prescription refills. In addition, a subset of this population was evaluated for inhaler technique.
On the basis of these calculations, 47.4% of patients with harmful OCS exposure were found not to be adherent to their prescribed ICS. Of those who were adherent, 53.9% were found not be taking their inhaled steroids appropriately,
When these numbers are put together, the data suggest “78.1% of high OCS users are either nonadherent or using poor inhalation techniques, which means there is a big potential for treatment optimization,” Dr. Eger said.
Yet even among the 21.9% who were adherent and using good inhaler technique, identifying a group who presumably require OCS for exacerbations, the study found that only 46.1% had been prescribed a biologic, which Dr. Eger considers an important steroid-sparing option. She conceded that many of those not on a biologic might not be candidates, but she believes this is another missed opportunity for reducing OCS exposure.
“In the Netherlands, we have very good access to health care, and biologics are available to anyone who needs them,” said Dr. Eger, explaining that access to these drugs is not a barrier.
The evidence overall is that not enough is being done to ensure that asthma patients are being protected from the risks of OCS, according to Dr. Eger. Citing evidence that adverse events associated with OCS begin with a cumulative lifetime prednisone-equivalent exposure of only 500 mg, she believes that clinicians should be more aggressive in intervening.
“We know that there are both acute and chronic complications associated with OCS that involve a range of organ systems,” Dr. Eger said. She listed osteoporosis, diabetes mellitus, hypertension, and adrenal insufficiency as examples. Rescue OCS, even if used sparingly, can drive risk of OCS complications attributable to the importance of cumulative exposure.
In the session where these data were presented, the moderator, Guy Brusselle, MD, professor of asthma and immunology, Ghent (Belgium) University, labeled them “important.” However, he quibbled with Dr. Eger’s assertion that biologics represent a major opportunity to reduce OCS exposure.
“By suggesting that biologics are not being used often enough, there is an assumption that all of these patients have type 2 inflammatory asthma,” Dr. Brusselle said. “I think it makes more sense to emphasize steroid-sparing strategies, not just biologics.”
Dr. Eger did not disagree, but she emphasized that steroid-sparing alternatives are just one strategy to reduce OCS exposure, and ensuring that patients are adherent to prescribed ICS therapies and are using them correctly might have an even greater impact.
Dr. Eger reports no potential conflicts of interest.
REPORTING FROM ERS 2019
IPD in children may be a signal of immunodeficiency
according to a systematic review published in JAMA Pediatrics.
Coen Butters, BMed, DCH, of the Royal Children’s Hospital in Melbourne, and coauthors wrote that, even with optimal vaccine coverage, there is still a group of children with increased susceptibility to invasive pneumococcal disease (IPD), and this could be a potential marker of primary immunodeficiency.
They conducted a systematic review of 17 studies of 6,002 children to examine the evidence on the incidence of primary immunodeficiency in children who presented with IPD but without any other risk factors or predisposing conditions.
Overall, the frequency of primary immunodeficiency in children presenting with IPD who did not have any other predisposing condition ranged from 1% to 26%.
One study of 162 children with IPD, which had an overall frequency of primary immunodeficiency of 10%, found that children older than 2 years were significantly more likely to have primary immunodeficiency than those aged under 2 years (26% vs. 3%; P less than .001).
Primary antibody deficiency was the most commonly diagnosed immunodeficiency in these children with IPD, accounting for 71% of cases. These deficiencies presented as hypogammaglobulinemia, specific pneumococcal antibody deficiency, X-linked agammaglobulinemia, and IgG2 deficiency.
The review also included four studies that looked at the frequency of mannose-binding lectin deficiency in 1,493 children with primary IPD. Two of these studies reported a prevalence of mannose-binding lectin deficiency ranging from 31% in children aged younger than 2 years to 41% in children younger than 1 year.
Five studies looked at the rate of primary immunodeficiency in children presenting with recurrent IPD. In addition to other predisposing conditions such as sickle cell disease, cancer, and anatomical breach in the blood-brain barrier, the three studies that screened for primary immunodeficiency found rates ranging from 10% to 67%. The most common conditions were complement deficiency, pneumococcal antibody deficiency, and a single case of TLR-signaling defect.
In a study of 162 children with primary IPD, screening for asplenia identified a single case of congenital asplenia. In another study of 2,498 cases of IPD, 22 patients had asplenia at presentation, half of whom died at presentation.
Dr. Butters and associates concluded that “this review’s findings suggests that existing data support the immune evaluation of children older than 2 years without a known predisposing condition who present with their first episode of Streptococcus pneumoniae meningitis, pneumonia, or recurrent IPD. Immune evaluation should include assessment for immunoglobulin deficiency, pneumococcal antibody deficiency, complement disorders, and asplenia.”
In an accompanying editorial, Stephen I. Pelton, MD, of the Maxwell Finland Laboratory for Infectious Diseases at Boston Medical Center, and coauthors wrote that in children with recurrent episodes of IPD caused by nonvaccine serotypes – particularly those aged over 5 years – evaluation for primary immunodeficiencies could uncover immune defects.
“Once identified, direct and indirect protection, penicillin prophylaxis, or a combination of these offers great potential for disease prevention and reduction of mortality and morbidity in children with [primary immunodeficiency],” they wrote.
No funding or conflicts of interest were declared for the study. Two of the editorialists declared research funding or honoraria from the pharmaceutical sector.
SOURCES: Butters C et al. JAMA Pediatr. 2019 Sep 30. doi: 10.1001/jamapediatrics.2019.3203; Pelton SI et al. JAMA Pediatr. 2019 Sep 30. doi: 10.1001/jamapediatrics.2019.3185.
according to a systematic review published in JAMA Pediatrics.
Coen Butters, BMed, DCH, of the Royal Children’s Hospital in Melbourne, and coauthors wrote that, even with optimal vaccine coverage, there is still a group of children with increased susceptibility to invasive pneumococcal disease (IPD), and this could be a potential marker of primary immunodeficiency.
They conducted a systematic review of 17 studies of 6,002 children to examine the evidence on the incidence of primary immunodeficiency in children who presented with IPD but without any other risk factors or predisposing conditions.
Overall, the frequency of primary immunodeficiency in children presenting with IPD who did not have any other predisposing condition ranged from 1% to 26%.
One study of 162 children with IPD, which had an overall frequency of primary immunodeficiency of 10%, found that children older than 2 years were significantly more likely to have primary immunodeficiency than those aged under 2 years (26% vs. 3%; P less than .001).
Primary antibody deficiency was the most commonly diagnosed immunodeficiency in these children with IPD, accounting for 71% of cases. These deficiencies presented as hypogammaglobulinemia, specific pneumococcal antibody deficiency, X-linked agammaglobulinemia, and IgG2 deficiency.
The review also included four studies that looked at the frequency of mannose-binding lectin deficiency in 1,493 children with primary IPD. Two of these studies reported a prevalence of mannose-binding lectin deficiency ranging from 31% in children aged younger than 2 years to 41% in children younger than 1 year.
Five studies looked at the rate of primary immunodeficiency in children presenting with recurrent IPD. In addition to other predisposing conditions such as sickle cell disease, cancer, and anatomical breach in the blood-brain barrier, the three studies that screened for primary immunodeficiency found rates ranging from 10% to 67%. The most common conditions were complement deficiency, pneumococcal antibody deficiency, and a single case of TLR-signaling defect.
In a study of 162 children with primary IPD, screening for asplenia identified a single case of congenital asplenia. In another study of 2,498 cases of IPD, 22 patients had asplenia at presentation, half of whom died at presentation.
Dr. Butters and associates concluded that “this review’s findings suggests that existing data support the immune evaluation of children older than 2 years without a known predisposing condition who present with their first episode of Streptococcus pneumoniae meningitis, pneumonia, or recurrent IPD. Immune evaluation should include assessment for immunoglobulin deficiency, pneumococcal antibody deficiency, complement disorders, and asplenia.”
In an accompanying editorial, Stephen I. Pelton, MD, of the Maxwell Finland Laboratory for Infectious Diseases at Boston Medical Center, and coauthors wrote that in children with recurrent episodes of IPD caused by nonvaccine serotypes – particularly those aged over 5 years – evaluation for primary immunodeficiencies could uncover immune defects.
“Once identified, direct and indirect protection, penicillin prophylaxis, or a combination of these offers great potential for disease prevention and reduction of mortality and morbidity in children with [primary immunodeficiency],” they wrote.
No funding or conflicts of interest were declared for the study. Two of the editorialists declared research funding or honoraria from the pharmaceutical sector.
SOURCES: Butters C et al. JAMA Pediatr. 2019 Sep 30. doi: 10.1001/jamapediatrics.2019.3203; Pelton SI et al. JAMA Pediatr. 2019 Sep 30. doi: 10.1001/jamapediatrics.2019.3185.
according to a systematic review published in JAMA Pediatrics.
Coen Butters, BMed, DCH, of the Royal Children’s Hospital in Melbourne, and coauthors wrote that, even with optimal vaccine coverage, there is still a group of children with increased susceptibility to invasive pneumococcal disease (IPD), and this could be a potential marker of primary immunodeficiency.
They conducted a systematic review of 17 studies of 6,002 children to examine the evidence on the incidence of primary immunodeficiency in children who presented with IPD but without any other risk factors or predisposing conditions.
Overall, the frequency of primary immunodeficiency in children presenting with IPD who did not have any other predisposing condition ranged from 1% to 26%.
One study of 162 children with IPD, which had an overall frequency of primary immunodeficiency of 10%, found that children older than 2 years were significantly more likely to have primary immunodeficiency than those aged under 2 years (26% vs. 3%; P less than .001).
Primary antibody deficiency was the most commonly diagnosed immunodeficiency in these children with IPD, accounting for 71% of cases. These deficiencies presented as hypogammaglobulinemia, specific pneumococcal antibody deficiency, X-linked agammaglobulinemia, and IgG2 deficiency.
The review also included four studies that looked at the frequency of mannose-binding lectin deficiency in 1,493 children with primary IPD. Two of these studies reported a prevalence of mannose-binding lectin deficiency ranging from 31% in children aged younger than 2 years to 41% in children younger than 1 year.
Five studies looked at the rate of primary immunodeficiency in children presenting with recurrent IPD. In addition to other predisposing conditions such as sickle cell disease, cancer, and anatomical breach in the blood-brain barrier, the three studies that screened for primary immunodeficiency found rates ranging from 10% to 67%. The most common conditions were complement deficiency, pneumococcal antibody deficiency, and a single case of TLR-signaling defect.
In a study of 162 children with primary IPD, screening for asplenia identified a single case of congenital asplenia. In another study of 2,498 cases of IPD, 22 patients had asplenia at presentation, half of whom died at presentation.
Dr. Butters and associates concluded that “this review’s findings suggests that existing data support the immune evaluation of children older than 2 years without a known predisposing condition who present with their first episode of Streptococcus pneumoniae meningitis, pneumonia, or recurrent IPD. Immune evaluation should include assessment for immunoglobulin deficiency, pneumococcal antibody deficiency, complement disorders, and asplenia.”
In an accompanying editorial, Stephen I. Pelton, MD, of the Maxwell Finland Laboratory for Infectious Diseases at Boston Medical Center, and coauthors wrote that in children with recurrent episodes of IPD caused by nonvaccine serotypes – particularly those aged over 5 years – evaluation for primary immunodeficiencies could uncover immune defects.
“Once identified, direct and indirect protection, penicillin prophylaxis, or a combination of these offers great potential for disease prevention and reduction of mortality and morbidity in children with [primary immunodeficiency],” they wrote.
No funding or conflicts of interest were declared for the study. Two of the editorialists declared research funding or honoraria from the pharmaceutical sector.
SOURCES: Butters C et al. JAMA Pediatr. 2019 Sep 30. doi: 10.1001/jamapediatrics.2019.3203; Pelton SI et al. JAMA Pediatr. 2019 Sep 30. doi: 10.1001/jamapediatrics.2019.3185.
FROM JAMA PEDIATRICS
Refractory Status Asthmaticus: Treatment With Sevoflurane
Asthma attacks account for 1.8 million emergency department (ED) visits each year in the US and for 10 deaths daily.1 Management of asthma attacks includes administration of inhaled ß2 adrenergic agonists, inhaled anticholinergic agents, IV magnesium sulfate, and corticosteroids.2 Status asthmaticus is an intense acute exacerbation of asthma that does not respond to repeated treatments of bronchodilators and corticosteroids.3 It is a medical emergency requiring immediate recognition and treatment. The decision to intubate a patient with status asthmaticus is a clinical decision based on work of breathing, respiratory acidosis, and failure to respond to medical interventions.
In refractory cases of status asthmaticus, intubation and mechanical ventilation are undertaken to provide oxygenation and ventilation until the bronchospasm resolves. However, mechanical ventilation is associated with significant risks, including high end-inspiratory pressures, barotrauma, and volutrauma.4 Rescue therapies include muscle relaxation, infusion of ketamine (central acting nonopioid analgesic with bronchodilatory properties), heliox, and general anesthesia.2,4 We report a case of a patient with life-threatening asthma and status asthmaticus treated with sevoflurane general anesthesia.
Case Presentation
A 55-year-old woman whose medical history was notable for asthma, psoriasis, hypothyroidism, tobacco, and alcohol abuse, and posttraumatic stress disorder (PTSD) presented to the ED. The patient had rarely sought medical attention and had no prior ED visits or hospitalizations in the electronic health record. Her home regimen included an albuterol inhaler used as needed. Her family reported that they had found her in distress in bed in a tripod position, unable to speak and struggling to breath.
Emergency medical services found the patient cyanotic, apneic, and pulseless. She received cardiopulmonary resuscitation for 30 seconds and 1-mg IV epinephrine, and spontaneous circulation returned. The patient arrived in the ED with an oral airway in place receiving bag valve mask ventilation. The patient expelled the oral airway. She was unable to speak due to dyspnea, exhibited persistent cyanosis, fatigue due to work of breathing, and failed to respond to nebulized albuterol/ipratropium bromide, IV methylprednisolone, and magnesium sulfate. The patient met criteria for acute severe asthma, or status asthmaticus. Thus, the patient received rapid sequence induction with rocuronium and ketamine and was intubated.
According to her family, the patient had no previous intensive care unit (ICU) admissions or prior intubations. Her only asthma medication was an albuterol inhaler as needed. The patient worked as a supervisor at a window blind manufacturing company. She lived alone, smoked 2 packs of cigarettes a day for more than 30 years, had no pets, drank unknown quantities of beer, wine, and hard liquor daily, and had smoked marijuana for several years.
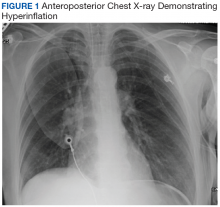
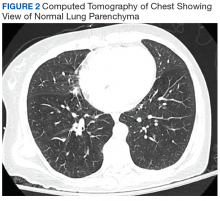
The patient’s physical examination was notable for diffuse expiratory wheezes. Laboratory analysis revealed white blood cell count of 13.7 k/mcL, sodium 140 mmol/L, potassium 4.9 mmol/L, chloride 105 mmol/L, CO2 17 mmol/L, creatinine 0.98 mg/dL, troponin 0.03 ng/mL, lactate 7.2 mmol/L. Her chest X-ray showed hyperinflation but no focal opacities, pneumothorax, or pulmonary edema. Her endotracheal tube was in good position (Figure 1). A computed tomography pulmonary angiogram showed no pulmonary embolus or emphysema. There were atelectatic changes in the dependent portion of the right lower lobe, central bronchial wall thickening, and no stigmata of air trapping (Figure 2). An echocardiogram revealed a left ventricular ejection fraction of 45%, normal right ventricle and right ventricular size and function with an estimated right ventricular systolic pressure of 40 mm Hg.
The patient was admitted to the ICU and started on continuous infusion cisatracurium for paralysis and deep sedation to improve ventilatory synchrony and decrease auto positive end-expiratory pressure (PEEP). Mechanical ventilation was initiated with volume-cycled assist control ventilation, 6 mL/kg/ideal body weight (IBW) at 5-cm H2O PEEP, and 1 minute ventilation of 10 liters. The patient had severe air trapping and high airway pressures. The dynamic PEEP was 22-cm H2O (normal PEEP of 5-cm H2O), peak airway pressure (PAP) 41-cm H2O, and plateau pressure 31-cm H2O. In addition, the arterial blood gas (ABG) showed severe hypercapnic respiratory acidosis without significant hypoxemia with pH 7.15, PaCO2 90 mm Hg, and PaO2 150 mm Hg.
Pressure controlled ventilation was attempted unsuccessfully due to high airway resistance. Ultimately, the patient was set on volume control with low tidal volume, 6 mL/kg/IBW, high flow 90 L/min, PEEP 0 cm of H2O, and a low respiratory rate of 10 to achieve an inspiratory to expiratory (I:E) ratio of 1:7. Managing the ventilator to avoid dynamic hyperinflation and auto-PEEP, she remained relatively stable and improved.
By day 4 the patient’s ventilator was set to volume assist control with respiratory rate of 16, tidal volume, 6 mL/kg/IBW, PEEP 5-cm H2O with auto PEEP of 3-cm H2O, and fraction of inspired ABG O2 (FiO2) 0.35 with PAP of 46-cm H2O and plateau pressure of 17-cm H2O. The ABG was pH 7.32, PaCO2 65 mm Hg, and PaO2 74 mm Hg. However, on hospital day 5, she developed worsening PAP 60 to 77-cm H2O, plateau pressures 17-cm H2O, and a dynamic PEEP 16-cm H2O and was unresponsive to ventilator maneuvers to lower airway pressures and improve ventilation.
The patient had been receiving continuous albuterol and ipratropium nebulizer treatments. Ketamine infusion was considered fraught with potential for a dissociative reaction due to the patient’s significant PTSD. The patient’s family requested avoidance of ketamine infusion since the patient was paralyzed and psychiatric effects could not be monitored. Heliox 80/20 mixture was considered; however, it is incompatible with the ventilator that was being used since it could not account for the density of the helium gas flow in the tidal volumes. Extracorporeal membrane oxygenation (ECMO) was not available at our facility, and the patient was not a candidate for the regional ECMO center.
On hospital day 8, the patient developed worsening respiratory acidosis. The patient’s PAP increased to > 77-cm H2O, and her ABG revealed pH 7.22, PaCO2 90 mm Hg, and PaO2 77 mm Hg with FiO2 0.4. A chest X-ray demonstrated a new left lower lobe infiltrate. Fiber optic bronchoscopy was notable for scattered thick secretions throughout both lungs without obstructing mucus plug. Removal of airway secretions did not improve airway pressures or dynamic hyperinflation.
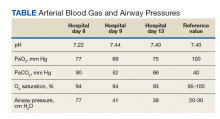
After consultation and discussion with the chief of anesthesia, the patient was placed on an anesthesia ventilator and started on sevoflurane 1.5% in the ICU. Anesthesiology was available 24 hours a day, and the anesthesiologist rounded with the intensivist frequently for this patient. The anesthesia technician worked closely with respiratory therapy regarding ventilator setting and changing the anesthesia gas scavenging charcoal canister. Within 4 hours, her gas exchange normalized (Table). The patient’s ABG was pH 7.44, PaCO2 52 mm Hg, and PaO2 69 mm Hg on FiO2 0.4. On volume cycled ventilation with a rate of 12, flow rate of 40 L/min, and tidal volume 6 mL/kg/IBW, the PAP decreased to 41-cm H2O.
Within 24 hours bronchospasm improved as evidenced by decreased airway pressures, resolution of wheezing, and decreased CO2 retention. The sevoflurane was easily weaned over the next 48 hours by decreasing the dose by 25% every 12-hour shift without rebound bronchospasm. Airway pressures and ABGs were frequently monitored during the weaning process. The patient resumed conventional mechanical ventilation, cisatracurium was discontinued, and she underwent a percutaneous tracheostomy for critical illness polymyopathy. Her respiratory muscle strength recovered more robustly than anticipated. Prior to discharge to a skilled nursing facility for continued rehabilitation, she was removed from mechanical ventilation and decannulated.
Discussion
This case illustrates the successful treatment of a patient with extreme status asthmaticus given inhalational anesthesia as supportive care while the bronchospasm and status asthmaticus abated. This is an unusual treatment in an ominous situation. Inhalational anesthetics are potent bronchodilators and have been successfully used in the management of status asthmaticus refractory to conventional therapy.4 Inhalational anesthetics have been shown to decrease airway resistance, dynamic hyperinflation, and intrinsic PEEP.5 These agents result in rapid bronchodilation by relaxing the smooth muscle and are associated with early liberation from mechanical ventilation.5,6 Although there are no guidelines regarding which inhalational agent is best, specific dosing, duration, or titration, case reports in the literature regarding the successful use of inhalational agents in life-threatening status asthmaticus exist.2,5,7
Caveats regarding the use of inhalational anesthetics in status asthmaticus include proarrhythmias, severe hepatic and renal toxicity. Although isoflurane is less likely to cause arrhythmia, both isoflurane and sevoflurane can cause dose-dependent hypotension by peripheral vasodilatation.7 Ourpatient did not manifest any adverse effects.
Additional challenges regarding the use of inhalational anesthetics for status asthmaticus include differences in ventilators and occupational hazards.8 Anesthesia or operating room ventilators differ from ICU ventilators in flow and pressure capabilities.7 The anesthesia ventilator is not capable of generating inspiratory pressures sufficient to ventilate patients with severely elevated airway resistance. Thus, the decrease inspiratory flow that occurs with increasing airway pressure limits the tidal volume delivered and consequently the minute volume. Although newer anesthesia ventilators have increased flow capabilities, they require a fully trained staff.8
Potential occupational exposure to these volatile anesthetic gases occurs as patients being treated may exhale considerable amounts of volatile anesthetics.8 An anesthesia gas scavenging device, such as a charcoal canister, must be attached to the ventilator to capture the exhaled anesthetic gases and should be changed every 12 hours.8 Finally, there is a potential for rebound bronchospasm as the anesthetic agent is tapered.6,7,9-11
Conclusion
Inhalational anesthetics are an option as rescue therapy for severe life-threatening asthma when all other therapies have failed. Use of inhalational anesthetics in status asthmaticus consists of case reports of which half are in children.2,5,7 Our patient contributes to the literature of case reports regarding using sevoflurane in refractory status asthmaticus. A decision to choose them must be a collaborative team approach with anesthesiology, pulmonary/critical care medicine, respiratory therapy, and ICU nurses, and the risks and benefits should be discussed with decision-making family members. Since there are no specific guidelines for the use of inhalational agents in status asthmaticus, close attention to inspiratory flows, gas scavenging devices, and clinical response is required. Additionally, the team must be comfortable with the plan to use an anesthesia ventilator and trained on its limitations.
1. Centers for Disease Control and Prevention. Most recent national asthma data. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Updated May 2019. Accessed September 5, 2019.
2. Lazarus SC. Emergency treatment of asthma. N Engl J Med. 2010;363(8):755-764.
3. Shah R, Saltoun CA. Acute severe asthma (status asthmaticus). Allergy Asthma Proc. 2012;33(suppl 1):47-50.
4. Mutlu GM, Factor P, Schwartz DE, Snajder JI. Severe status asthmaticus: management with permissive hypercapnia and inhalation anesthesia Crit Care Med. 2002;30(2):477-480.
5. Maltais, F, Sovilj M, Goldber P, Gottfried SB. Respiratory mechanism in status asthmaticus. Effects of inhalational anesthesia. Chest. 1994;106(5):1401-1406.
6. Parnass SM, Feld JM, Chamberlin WH, Segil LJ. Status asthmaticus treated with isoflurane and enflurane. Anesth Analg. 1987;66(2):193-195.
7. Johnston RG, Noseworthy TW, Friesen EG, Yule HA, Shustack A. Isoflurane therapy for status asthmaticus in children and adults. Chest. 1990;97(3):698-701.
8. Meiser A, Laubenthal H. Inhalational anesthetics in the ICU: theory and practice of inhalational sedation in the ICU economics, risk-benefit. Best Pract Res Clin Anesthesiol. 2005;19(3):523-538.
9. Miller RD. Miller’s Anesthesia. 7th ed. Philadelphia, PA: Churchill Livingstone; 2010.
10. Nakao S, Hatano K, Sumi C, et al. Sevoflurane causes greater QTc interval prolongation in elderly patients than in younger patients. Anesth Analg. 2010;110(3):775-779.
11. Stachnik J. Inhaled anesthetic agents. Am J Health-Syst Pharm. 2006;63(7):623-634.
Asthma attacks account for 1.8 million emergency department (ED) visits each year in the US and for 10 deaths daily.1 Management of asthma attacks includes administration of inhaled ß2 adrenergic agonists, inhaled anticholinergic agents, IV magnesium sulfate, and corticosteroids.2 Status asthmaticus is an intense acute exacerbation of asthma that does not respond to repeated treatments of bronchodilators and corticosteroids.3 It is a medical emergency requiring immediate recognition and treatment. The decision to intubate a patient with status asthmaticus is a clinical decision based on work of breathing, respiratory acidosis, and failure to respond to medical interventions.
In refractory cases of status asthmaticus, intubation and mechanical ventilation are undertaken to provide oxygenation and ventilation until the bronchospasm resolves. However, mechanical ventilation is associated with significant risks, including high end-inspiratory pressures, barotrauma, and volutrauma.4 Rescue therapies include muscle relaxation, infusion of ketamine (central acting nonopioid analgesic with bronchodilatory properties), heliox, and general anesthesia.2,4 We report a case of a patient with life-threatening asthma and status asthmaticus treated with sevoflurane general anesthesia.
Case Presentation
A 55-year-old woman whose medical history was notable for asthma, psoriasis, hypothyroidism, tobacco, and alcohol abuse, and posttraumatic stress disorder (PTSD) presented to the ED. The patient had rarely sought medical attention and had no prior ED visits or hospitalizations in the electronic health record. Her home regimen included an albuterol inhaler used as needed. Her family reported that they had found her in distress in bed in a tripod position, unable to speak and struggling to breath.
Emergency medical services found the patient cyanotic, apneic, and pulseless. She received cardiopulmonary resuscitation for 30 seconds and 1-mg IV epinephrine, and spontaneous circulation returned. The patient arrived in the ED with an oral airway in place receiving bag valve mask ventilation. The patient expelled the oral airway. She was unable to speak due to dyspnea, exhibited persistent cyanosis, fatigue due to work of breathing, and failed to respond to nebulized albuterol/ipratropium bromide, IV methylprednisolone, and magnesium sulfate. The patient met criteria for acute severe asthma, or status asthmaticus. Thus, the patient received rapid sequence induction with rocuronium and ketamine and was intubated.
According to her family, the patient had no previous intensive care unit (ICU) admissions or prior intubations. Her only asthma medication was an albuterol inhaler as needed. The patient worked as a supervisor at a window blind manufacturing company. She lived alone, smoked 2 packs of cigarettes a day for more than 30 years, had no pets, drank unknown quantities of beer, wine, and hard liquor daily, and had smoked marijuana for several years.
The patient’s physical examination was notable for diffuse expiratory wheezes. Laboratory analysis revealed white blood cell count of 13.7 k/mcL, sodium 140 mmol/L, potassium 4.9 mmol/L, chloride 105 mmol/L, CO2 17 mmol/L, creatinine 0.98 mg/dL, troponin 0.03 ng/mL, lactate 7.2 mmol/L. Her chest X-ray showed hyperinflation but no focal opacities, pneumothorax, or pulmonary edema. Her endotracheal tube was in good position (Figure 1). A computed tomography pulmonary angiogram showed no pulmonary embolus or emphysema. There were atelectatic changes in the dependent portion of the right lower lobe, central bronchial wall thickening, and no stigmata of air trapping (Figure 2). An echocardiogram revealed a left ventricular ejection fraction of 45%, normal right ventricle and right ventricular size and function with an estimated right ventricular systolic pressure of 40 mm Hg.
The patient was admitted to the ICU and started on continuous infusion cisatracurium for paralysis and deep sedation to improve ventilatory synchrony and decrease auto positive end-expiratory pressure (PEEP). Mechanical ventilation was initiated with volume-cycled assist control ventilation, 6 mL/kg/ideal body weight (IBW) at 5-cm H2O PEEP, and 1 minute ventilation of 10 liters. The patient had severe air trapping and high airway pressures. The dynamic PEEP was 22-cm H2O (normal PEEP of 5-cm H2O), peak airway pressure (PAP) 41-cm H2O, and plateau pressure 31-cm H2O. In addition, the arterial blood gas (ABG) showed severe hypercapnic respiratory acidosis without significant hypoxemia with pH 7.15, PaCO2 90 mm Hg, and PaO2 150 mm Hg.
Pressure controlled ventilation was attempted unsuccessfully due to high airway resistance. Ultimately, the patient was set on volume control with low tidal volume, 6 mL/kg/IBW, high flow 90 L/min, PEEP 0 cm of H2O, and a low respiratory rate of 10 to achieve an inspiratory to expiratory (I:E) ratio of 1:7. Managing the ventilator to avoid dynamic hyperinflation and auto-PEEP, she remained relatively stable and improved.
By day 4 the patient’s ventilator was set to volume assist control with respiratory rate of 16, tidal volume, 6 mL/kg/IBW, PEEP 5-cm H2O with auto PEEP of 3-cm H2O, and fraction of inspired ABG O2 (FiO2) 0.35 with PAP of 46-cm H2O and plateau pressure of 17-cm H2O. The ABG was pH 7.32, PaCO2 65 mm Hg, and PaO2 74 mm Hg. However, on hospital day 5, she developed worsening PAP 60 to 77-cm H2O, plateau pressures 17-cm H2O, and a dynamic PEEP 16-cm H2O and was unresponsive to ventilator maneuvers to lower airway pressures and improve ventilation.
The patient had been receiving continuous albuterol and ipratropium nebulizer treatments. Ketamine infusion was considered fraught with potential for a dissociative reaction due to the patient’s significant PTSD. The patient’s family requested avoidance of ketamine infusion since the patient was paralyzed and psychiatric effects could not be monitored. Heliox 80/20 mixture was considered; however, it is incompatible with the ventilator that was being used since it could not account for the density of the helium gas flow in the tidal volumes. Extracorporeal membrane oxygenation (ECMO) was not available at our facility, and the patient was not a candidate for the regional ECMO center.
On hospital day 8, the patient developed worsening respiratory acidosis. The patient’s PAP increased to > 77-cm H2O, and her ABG revealed pH 7.22, PaCO2 90 mm Hg, and PaO2 77 mm Hg with FiO2 0.4. A chest X-ray demonstrated a new left lower lobe infiltrate. Fiber optic bronchoscopy was notable for scattered thick secretions throughout both lungs without obstructing mucus plug. Removal of airway secretions did not improve airway pressures or dynamic hyperinflation.
After consultation and discussion with the chief of anesthesia, the patient was placed on an anesthesia ventilator and started on sevoflurane 1.5% in the ICU. Anesthesiology was available 24 hours a day, and the anesthesiologist rounded with the intensivist frequently for this patient. The anesthesia technician worked closely with respiratory therapy regarding ventilator setting and changing the anesthesia gas scavenging charcoal canister. Within 4 hours, her gas exchange normalized (Table). The patient’s ABG was pH 7.44, PaCO2 52 mm Hg, and PaO2 69 mm Hg on FiO2 0.4. On volume cycled ventilation with a rate of 12, flow rate of 40 L/min, and tidal volume 6 mL/kg/IBW, the PAP decreased to 41-cm H2O.
Within 24 hours bronchospasm improved as evidenced by decreased airway pressures, resolution of wheezing, and decreased CO2 retention. The sevoflurane was easily weaned over the next 48 hours by decreasing the dose by 25% every 12-hour shift without rebound bronchospasm. Airway pressures and ABGs were frequently monitored during the weaning process. The patient resumed conventional mechanical ventilation, cisatracurium was discontinued, and she underwent a percutaneous tracheostomy for critical illness polymyopathy. Her respiratory muscle strength recovered more robustly than anticipated. Prior to discharge to a skilled nursing facility for continued rehabilitation, she was removed from mechanical ventilation and decannulated.
Discussion
This case illustrates the successful treatment of a patient with extreme status asthmaticus given inhalational anesthesia as supportive care while the bronchospasm and status asthmaticus abated. This is an unusual treatment in an ominous situation. Inhalational anesthetics are potent bronchodilators and have been successfully used in the management of status asthmaticus refractory to conventional therapy.4 Inhalational anesthetics have been shown to decrease airway resistance, dynamic hyperinflation, and intrinsic PEEP.5 These agents result in rapid bronchodilation by relaxing the smooth muscle and are associated with early liberation from mechanical ventilation.5,6 Although there are no guidelines regarding which inhalational agent is best, specific dosing, duration, or titration, case reports in the literature regarding the successful use of inhalational agents in life-threatening status asthmaticus exist.2,5,7
Caveats regarding the use of inhalational anesthetics in status asthmaticus include proarrhythmias, severe hepatic and renal toxicity. Although isoflurane is less likely to cause arrhythmia, both isoflurane and sevoflurane can cause dose-dependent hypotension by peripheral vasodilatation.7 Ourpatient did not manifest any adverse effects.
Additional challenges regarding the use of inhalational anesthetics for status asthmaticus include differences in ventilators and occupational hazards.8 Anesthesia or operating room ventilators differ from ICU ventilators in flow and pressure capabilities.7 The anesthesia ventilator is not capable of generating inspiratory pressures sufficient to ventilate patients with severely elevated airway resistance. Thus, the decrease inspiratory flow that occurs with increasing airway pressure limits the tidal volume delivered and consequently the minute volume. Although newer anesthesia ventilators have increased flow capabilities, they require a fully trained staff.8
Potential occupational exposure to these volatile anesthetic gases occurs as patients being treated may exhale considerable amounts of volatile anesthetics.8 An anesthesia gas scavenging device, such as a charcoal canister, must be attached to the ventilator to capture the exhaled anesthetic gases and should be changed every 12 hours.8 Finally, there is a potential for rebound bronchospasm as the anesthetic agent is tapered.6,7,9-11
Conclusion
Inhalational anesthetics are an option as rescue therapy for severe life-threatening asthma when all other therapies have failed. Use of inhalational anesthetics in status asthmaticus consists of case reports of which half are in children.2,5,7 Our patient contributes to the literature of case reports regarding using sevoflurane in refractory status asthmaticus. A decision to choose them must be a collaborative team approach with anesthesiology, pulmonary/critical care medicine, respiratory therapy, and ICU nurses, and the risks and benefits should be discussed with decision-making family members. Since there are no specific guidelines for the use of inhalational agents in status asthmaticus, close attention to inspiratory flows, gas scavenging devices, and clinical response is required. Additionally, the team must be comfortable with the plan to use an anesthesia ventilator and trained on its limitations.
Asthma attacks account for 1.8 million emergency department (ED) visits each year in the US and for 10 deaths daily.1 Management of asthma attacks includes administration of inhaled ß2 adrenergic agonists, inhaled anticholinergic agents, IV magnesium sulfate, and corticosteroids.2 Status asthmaticus is an intense acute exacerbation of asthma that does not respond to repeated treatments of bronchodilators and corticosteroids.3 It is a medical emergency requiring immediate recognition and treatment. The decision to intubate a patient with status asthmaticus is a clinical decision based on work of breathing, respiratory acidosis, and failure to respond to medical interventions.
In refractory cases of status asthmaticus, intubation and mechanical ventilation are undertaken to provide oxygenation and ventilation until the bronchospasm resolves. However, mechanical ventilation is associated with significant risks, including high end-inspiratory pressures, barotrauma, and volutrauma.4 Rescue therapies include muscle relaxation, infusion of ketamine (central acting nonopioid analgesic with bronchodilatory properties), heliox, and general anesthesia.2,4 We report a case of a patient with life-threatening asthma and status asthmaticus treated with sevoflurane general anesthesia.
Case Presentation
A 55-year-old woman whose medical history was notable for asthma, psoriasis, hypothyroidism, tobacco, and alcohol abuse, and posttraumatic stress disorder (PTSD) presented to the ED. The patient had rarely sought medical attention and had no prior ED visits or hospitalizations in the electronic health record. Her home regimen included an albuterol inhaler used as needed. Her family reported that they had found her in distress in bed in a tripod position, unable to speak and struggling to breath.
Emergency medical services found the patient cyanotic, apneic, and pulseless. She received cardiopulmonary resuscitation for 30 seconds and 1-mg IV epinephrine, and spontaneous circulation returned. The patient arrived in the ED with an oral airway in place receiving bag valve mask ventilation. The patient expelled the oral airway. She was unable to speak due to dyspnea, exhibited persistent cyanosis, fatigue due to work of breathing, and failed to respond to nebulized albuterol/ipratropium bromide, IV methylprednisolone, and magnesium sulfate. The patient met criteria for acute severe asthma, or status asthmaticus. Thus, the patient received rapid sequence induction with rocuronium and ketamine and was intubated.
According to her family, the patient had no previous intensive care unit (ICU) admissions or prior intubations. Her only asthma medication was an albuterol inhaler as needed. The patient worked as a supervisor at a window blind manufacturing company. She lived alone, smoked 2 packs of cigarettes a day for more than 30 years, had no pets, drank unknown quantities of beer, wine, and hard liquor daily, and had smoked marijuana for several years.
The patient’s physical examination was notable for diffuse expiratory wheezes. Laboratory analysis revealed white blood cell count of 13.7 k/mcL, sodium 140 mmol/L, potassium 4.9 mmol/L, chloride 105 mmol/L, CO2 17 mmol/L, creatinine 0.98 mg/dL, troponin 0.03 ng/mL, lactate 7.2 mmol/L. Her chest X-ray showed hyperinflation but no focal opacities, pneumothorax, or pulmonary edema. Her endotracheal tube was in good position (Figure 1). A computed tomography pulmonary angiogram showed no pulmonary embolus or emphysema. There were atelectatic changes in the dependent portion of the right lower lobe, central bronchial wall thickening, and no stigmata of air trapping (Figure 2). An echocardiogram revealed a left ventricular ejection fraction of 45%, normal right ventricle and right ventricular size and function with an estimated right ventricular systolic pressure of 40 mm Hg.
The patient was admitted to the ICU and started on continuous infusion cisatracurium for paralysis and deep sedation to improve ventilatory synchrony and decrease auto positive end-expiratory pressure (PEEP). Mechanical ventilation was initiated with volume-cycled assist control ventilation, 6 mL/kg/ideal body weight (IBW) at 5-cm H2O PEEP, and 1 minute ventilation of 10 liters. The patient had severe air trapping and high airway pressures. The dynamic PEEP was 22-cm H2O (normal PEEP of 5-cm H2O), peak airway pressure (PAP) 41-cm H2O, and plateau pressure 31-cm H2O. In addition, the arterial blood gas (ABG) showed severe hypercapnic respiratory acidosis without significant hypoxemia with pH 7.15, PaCO2 90 mm Hg, and PaO2 150 mm Hg.
Pressure controlled ventilation was attempted unsuccessfully due to high airway resistance. Ultimately, the patient was set on volume control with low tidal volume, 6 mL/kg/IBW, high flow 90 L/min, PEEP 0 cm of H2O, and a low respiratory rate of 10 to achieve an inspiratory to expiratory (I:E) ratio of 1:7. Managing the ventilator to avoid dynamic hyperinflation and auto-PEEP, she remained relatively stable and improved.
By day 4 the patient’s ventilator was set to volume assist control with respiratory rate of 16, tidal volume, 6 mL/kg/IBW, PEEP 5-cm H2O with auto PEEP of 3-cm H2O, and fraction of inspired ABG O2 (FiO2) 0.35 with PAP of 46-cm H2O and plateau pressure of 17-cm H2O. The ABG was pH 7.32, PaCO2 65 mm Hg, and PaO2 74 mm Hg. However, on hospital day 5, she developed worsening PAP 60 to 77-cm H2O, plateau pressures 17-cm H2O, and a dynamic PEEP 16-cm H2O and was unresponsive to ventilator maneuvers to lower airway pressures and improve ventilation.
The patient had been receiving continuous albuterol and ipratropium nebulizer treatments. Ketamine infusion was considered fraught with potential for a dissociative reaction due to the patient’s significant PTSD. The patient’s family requested avoidance of ketamine infusion since the patient was paralyzed and psychiatric effects could not be monitored. Heliox 80/20 mixture was considered; however, it is incompatible with the ventilator that was being used since it could not account for the density of the helium gas flow in the tidal volumes. Extracorporeal membrane oxygenation (ECMO) was not available at our facility, and the patient was not a candidate for the regional ECMO center.
On hospital day 8, the patient developed worsening respiratory acidosis. The patient’s PAP increased to > 77-cm H2O, and her ABG revealed pH 7.22, PaCO2 90 mm Hg, and PaO2 77 mm Hg with FiO2 0.4. A chest X-ray demonstrated a new left lower lobe infiltrate. Fiber optic bronchoscopy was notable for scattered thick secretions throughout both lungs without obstructing mucus plug. Removal of airway secretions did not improve airway pressures or dynamic hyperinflation.
After consultation and discussion with the chief of anesthesia, the patient was placed on an anesthesia ventilator and started on sevoflurane 1.5% in the ICU. Anesthesiology was available 24 hours a day, and the anesthesiologist rounded with the intensivist frequently for this patient. The anesthesia technician worked closely with respiratory therapy regarding ventilator setting and changing the anesthesia gas scavenging charcoal canister. Within 4 hours, her gas exchange normalized (Table). The patient’s ABG was pH 7.44, PaCO2 52 mm Hg, and PaO2 69 mm Hg on FiO2 0.4. On volume cycled ventilation with a rate of 12, flow rate of 40 L/min, and tidal volume 6 mL/kg/IBW, the PAP decreased to 41-cm H2O.
Within 24 hours bronchospasm improved as evidenced by decreased airway pressures, resolution of wheezing, and decreased CO2 retention. The sevoflurane was easily weaned over the next 48 hours by decreasing the dose by 25% every 12-hour shift without rebound bronchospasm. Airway pressures and ABGs were frequently monitored during the weaning process. The patient resumed conventional mechanical ventilation, cisatracurium was discontinued, and she underwent a percutaneous tracheostomy for critical illness polymyopathy. Her respiratory muscle strength recovered more robustly than anticipated. Prior to discharge to a skilled nursing facility for continued rehabilitation, she was removed from mechanical ventilation and decannulated.
Discussion
This case illustrates the successful treatment of a patient with extreme status asthmaticus given inhalational anesthesia as supportive care while the bronchospasm and status asthmaticus abated. This is an unusual treatment in an ominous situation. Inhalational anesthetics are potent bronchodilators and have been successfully used in the management of status asthmaticus refractory to conventional therapy.4 Inhalational anesthetics have been shown to decrease airway resistance, dynamic hyperinflation, and intrinsic PEEP.5 These agents result in rapid bronchodilation by relaxing the smooth muscle and are associated with early liberation from mechanical ventilation.5,6 Although there are no guidelines regarding which inhalational agent is best, specific dosing, duration, or titration, case reports in the literature regarding the successful use of inhalational agents in life-threatening status asthmaticus exist.2,5,7
Caveats regarding the use of inhalational anesthetics in status asthmaticus include proarrhythmias, severe hepatic and renal toxicity. Although isoflurane is less likely to cause arrhythmia, both isoflurane and sevoflurane can cause dose-dependent hypotension by peripheral vasodilatation.7 Ourpatient did not manifest any adverse effects.
Additional challenges regarding the use of inhalational anesthetics for status asthmaticus include differences in ventilators and occupational hazards.8 Anesthesia or operating room ventilators differ from ICU ventilators in flow and pressure capabilities.7 The anesthesia ventilator is not capable of generating inspiratory pressures sufficient to ventilate patients with severely elevated airway resistance. Thus, the decrease inspiratory flow that occurs with increasing airway pressure limits the tidal volume delivered and consequently the minute volume. Although newer anesthesia ventilators have increased flow capabilities, they require a fully trained staff.8
Potential occupational exposure to these volatile anesthetic gases occurs as patients being treated may exhale considerable amounts of volatile anesthetics.8 An anesthesia gas scavenging device, such as a charcoal canister, must be attached to the ventilator to capture the exhaled anesthetic gases and should be changed every 12 hours.8 Finally, there is a potential for rebound bronchospasm as the anesthetic agent is tapered.6,7,9-11
Conclusion
Inhalational anesthetics are an option as rescue therapy for severe life-threatening asthma when all other therapies have failed. Use of inhalational anesthetics in status asthmaticus consists of case reports of which half are in children.2,5,7 Our patient contributes to the literature of case reports regarding using sevoflurane in refractory status asthmaticus. A decision to choose them must be a collaborative team approach with anesthesiology, pulmonary/critical care medicine, respiratory therapy, and ICU nurses, and the risks and benefits should be discussed with decision-making family members. Since there are no specific guidelines for the use of inhalational agents in status asthmaticus, close attention to inspiratory flows, gas scavenging devices, and clinical response is required. Additionally, the team must be comfortable with the plan to use an anesthesia ventilator and trained on its limitations.
1. Centers for Disease Control and Prevention. Most recent national asthma data. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Updated May 2019. Accessed September 5, 2019.
2. Lazarus SC. Emergency treatment of asthma. N Engl J Med. 2010;363(8):755-764.
3. Shah R, Saltoun CA. Acute severe asthma (status asthmaticus). Allergy Asthma Proc. 2012;33(suppl 1):47-50.
4. Mutlu GM, Factor P, Schwartz DE, Snajder JI. Severe status asthmaticus: management with permissive hypercapnia and inhalation anesthesia Crit Care Med. 2002;30(2):477-480.
5. Maltais, F, Sovilj M, Goldber P, Gottfried SB. Respiratory mechanism in status asthmaticus. Effects of inhalational anesthesia. Chest. 1994;106(5):1401-1406.
6. Parnass SM, Feld JM, Chamberlin WH, Segil LJ. Status asthmaticus treated with isoflurane and enflurane. Anesth Analg. 1987;66(2):193-195.
7. Johnston RG, Noseworthy TW, Friesen EG, Yule HA, Shustack A. Isoflurane therapy for status asthmaticus in children and adults. Chest. 1990;97(3):698-701.
8. Meiser A, Laubenthal H. Inhalational anesthetics in the ICU: theory and practice of inhalational sedation in the ICU economics, risk-benefit. Best Pract Res Clin Anesthesiol. 2005;19(3):523-538.
9. Miller RD. Miller’s Anesthesia. 7th ed. Philadelphia, PA: Churchill Livingstone; 2010.
10. Nakao S, Hatano K, Sumi C, et al. Sevoflurane causes greater QTc interval prolongation in elderly patients than in younger patients. Anesth Analg. 2010;110(3):775-779.
11. Stachnik J. Inhaled anesthetic agents. Am J Health-Syst Pharm. 2006;63(7):623-634.
1. Centers for Disease Control and Prevention. Most recent national asthma data. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Updated May 2019. Accessed September 5, 2019.
2. Lazarus SC. Emergency treatment of asthma. N Engl J Med. 2010;363(8):755-764.
3. Shah R, Saltoun CA. Acute severe asthma (status asthmaticus). Allergy Asthma Proc. 2012;33(suppl 1):47-50.
4. Mutlu GM, Factor P, Schwartz DE, Snajder JI. Severe status asthmaticus: management with permissive hypercapnia and inhalation anesthesia Crit Care Med. 2002;30(2):477-480.
5. Maltais, F, Sovilj M, Goldber P, Gottfried SB. Respiratory mechanism in status asthmaticus. Effects of inhalational anesthesia. Chest. 1994;106(5):1401-1406.
6. Parnass SM, Feld JM, Chamberlin WH, Segil LJ. Status asthmaticus treated with isoflurane and enflurane. Anesth Analg. 1987;66(2):193-195.
7. Johnston RG, Noseworthy TW, Friesen EG, Yule HA, Shustack A. Isoflurane therapy for status asthmaticus in children and adults. Chest. 1990;97(3):698-701.
8. Meiser A, Laubenthal H. Inhalational anesthetics in the ICU: theory and practice of inhalational sedation in the ICU economics, risk-benefit. Best Pract Res Clin Anesthesiol. 2005;19(3):523-538.
9. Miller RD. Miller’s Anesthesia. 7th ed. Philadelphia, PA: Churchill Livingstone; 2010.
10. Nakao S, Hatano K, Sumi C, et al. Sevoflurane causes greater QTc interval prolongation in elderly patients than in younger patients. Anesth Analg. 2010;110(3):775-779.
11. Stachnik J. Inhaled anesthetic agents. Am J Health-Syst Pharm. 2006;63(7):623-634.
Six factors predicted benefit from asthma triple therapy
MADRID – Two newly published but previously reported phase 3 trials associated triple therapy in a single inhaler with a 23% reduction (P = .008) in asthma exacerbations relative to a two-drug inhaler, but fresh data from a prespecified analysis presented at the annual congress of the European Respiratory Society has identified those patients most likely to benefit.
“Six easily identifiable factors appear to be associated with the most prominent response to treatment and may help in the treatment step-up decision at the point of care,” reported Dave Singh, MD, professor in the division of infection, immunity, and respiratory medicine, University of Manchester (England).
The primary results of these trials were presented several months ago at the 2019 American Thoracic Society (ATS) meeting, but the full data were published on the day that Dr. Singh spoke at the ERS.
To identify predictors of response, the pooled analysis of TRIMARIN and TRIGGER was prespecified. Both of these trials, which were similarly designed, compared a single inhaler of inhaled corticosteroids (ICS), long-acting beta agonist (LABA), and long-acting muscarinic antagonist (LAMA) to a single ICS/LABA inhaler.
On the basis of risk for severe exacerbations, greater protection from triple therapy relative to a conventional ICS/LABA inhaler was identified for those with a high degree of reversibility (defined as greater than 400 mL) relative to those with a lower degree (RR, 0.729; P = .024), those with a body mass index less than 25 kg/m2 relative to a higher BMI (RR, 0.570; P = .005), those with only one exacerbation in the previous 12 months relative to those with more (RR, 0.731; P = .009), never-smokers relative to those with smoking history (RR, 0.764; P = .013), those younger than age 65 years relative to older (RR, 0.770; P = .17), and males relative to females (RR, 0.651; P = .009).
“This gives us six factors to consider when you are thinking about stepping up to triple therapy and are trying to determine which patients would benefit the most,” Dr. Singh said.
Both the TRIMARIN and the TRIGGER trials were double blind and placebo controlled. In both, the experimental arm was a single inhaler triple therapy of the ICS beclomethasone, the LABA formoterol, and the LAMA glycopyrronium. The control arm was a single inhaler combination of beclomethasone and formoterol. All inhalers were used twice daily.
TRIMARIN, with 171 participating sites in 16 countries, randomized 1,155 patients to the triple-drug inhaler with a moderate dose of ICS (100 mcg) or to the ICS/LABA inhaler. In TRIGGER, with 221 sites in 17 countries, 1,437 patients were randomized to one of three arms. Both the triple-drug inhaler arm and the ICS/LABA arm contained a higher dose of ICS (200 mcg) than in TRIMARIN. In an open-label third arm, patients also received the higher dose of ICS plus LABA and a second inhaler with tiotropium. The formoterol dose in all arms of both studies was 6 mcg.
As reported at the ATS and now published in the Lancet, the reduction in exacerbations on single inhaler triple therapy relative to ICS/LABA was significant when the data were pooled (even though the reduction in the TRIGGER study fell short of statistical significance). The median improvement in lung function for single inhaler triple therapy relative to ICS/LABA was significant in both TRIMARIN (57 mL; P = .008) and TRIGGER (73 mL; P = .0025).
In discussing the new pooled analysis of response predictors in TRIMARIN/TRIGGER, the ERS-invited discussant, Celeste M. Porsbjerg, MD, Bispebjerg Hospital, Copenhagen, expressed particular interest in reversibility. A positive reversibility test to salbutamol was an entry criterion for both trials, but Dr. Porsbjerg pointed out that a greater response in those with the highest reversibility suggests these patients have a phenotype in which bronchodilation is a more important driver of disease than is inflammation.
While conceding that this was possible, Dr. Singh cautioned that he considers these predictors of response to be “exploratory.” He believes that the TRIMARIN/TRIGGER studies were not designed to tease out the relative importance of mechanisms of asthma in response to the assigned therapies. However, he believes the response predictor analysis is a step in this direction, which might be valuable for better individualizing therapy.
The studies were funded by Chiesi Farmaceutici. Dr. Singh reports no potential conflicts of interest.
Virchow JC et al. Lancet. 2019 Sep 30. doi. org/10.1016/S0140-6736(19)32215-9.
MADRID – Two newly published but previously reported phase 3 trials associated triple therapy in a single inhaler with a 23% reduction (P = .008) in asthma exacerbations relative to a two-drug inhaler, but fresh data from a prespecified analysis presented at the annual congress of the European Respiratory Society has identified those patients most likely to benefit.
“Six easily identifiable factors appear to be associated with the most prominent response to treatment and may help in the treatment step-up decision at the point of care,” reported Dave Singh, MD, professor in the division of infection, immunity, and respiratory medicine, University of Manchester (England).
The primary results of these trials were presented several months ago at the 2019 American Thoracic Society (ATS) meeting, but the full data were published on the day that Dr. Singh spoke at the ERS.
To identify predictors of response, the pooled analysis of TRIMARIN and TRIGGER was prespecified. Both of these trials, which were similarly designed, compared a single inhaler of inhaled corticosteroids (ICS), long-acting beta agonist (LABA), and long-acting muscarinic antagonist (LAMA) to a single ICS/LABA inhaler.
On the basis of risk for severe exacerbations, greater protection from triple therapy relative to a conventional ICS/LABA inhaler was identified for those with a high degree of reversibility (defined as greater than 400 mL) relative to those with a lower degree (RR, 0.729; P = .024), those with a body mass index less than 25 kg/m2 relative to a higher BMI (RR, 0.570; P = .005), those with only one exacerbation in the previous 12 months relative to those with more (RR, 0.731; P = .009), never-smokers relative to those with smoking history (RR, 0.764; P = .013), those younger than age 65 years relative to older (RR, 0.770; P = .17), and males relative to females (RR, 0.651; P = .009).
“This gives us six factors to consider when you are thinking about stepping up to triple therapy and are trying to determine which patients would benefit the most,” Dr. Singh said.
Both the TRIMARIN and the TRIGGER trials were double blind and placebo controlled. In both, the experimental arm was a single inhaler triple therapy of the ICS beclomethasone, the LABA formoterol, and the LAMA glycopyrronium. The control arm was a single inhaler combination of beclomethasone and formoterol. All inhalers were used twice daily.
TRIMARIN, with 171 participating sites in 16 countries, randomized 1,155 patients to the triple-drug inhaler with a moderate dose of ICS (100 mcg) or to the ICS/LABA inhaler. In TRIGGER, with 221 sites in 17 countries, 1,437 patients were randomized to one of three arms. Both the triple-drug inhaler arm and the ICS/LABA arm contained a higher dose of ICS (200 mcg) than in TRIMARIN. In an open-label third arm, patients also received the higher dose of ICS plus LABA and a second inhaler with tiotropium. The formoterol dose in all arms of both studies was 6 mcg.
As reported at the ATS and now published in the Lancet, the reduction in exacerbations on single inhaler triple therapy relative to ICS/LABA was significant when the data were pooled (even though the reduction in the TRIGGER study fell short of statistical significance). The median improvement in lung function for single inhaler triple therapy relative to ICS/LABA was significant in both TRIMARIN (57 mL; P = .008) and TRIGGER (73 mL; P = .0025).
In discussing the new pooled analysis of response predictors in TRIMARIN/TRIGGER, the ERS-invited discussant, Celeste M. Porsbjerg, MD, Bispebjerg Hospital, Copenhagen, expressed particular interest in reversibility. A positive reversibility test to salbutamol was an entry criterion for both trials, but Dr. Porsbjerg pointed out that a greater response in those with the highest reversibility suggests these patients have a phenotype in which bronchodilation is a more important driver of disease than is inflammation.
While conceding that this was possible, Dr. Singh cautioned that he considers these predictors of response to be “exploratory.” He believes that the TRIMARIN/TRIGGER studies were not designed to tease out the relative importance of mechanisms of asthma in response to the assigned therapies. However, he believes the response predictor analysis is a step in this direction, which might be valuable for better individualizing therapy.
The studies were funded by Chiesi Farmaceutici. Dr. Singh reports no potential conflicts of interest.
Virchow JC et al. Lancet. 2019 Sep 30. doi. org/10.1016/S0140-6736(19)32215-9.
MADRID – Two newly published but previously reported phase 3 trials associated triple therapy in a single inhaler with a 23% reduction (P = .008) in asthma exacerbations relative to a two-drug inhaler, but fresh data from a prespecified analysis presented at the annual congress of the European Respiratory Society has identified those patients most likely to benefit.
“Six easily identifiable factors appear to be associated with the most prominent response to treatment and may help in the treatment step-up decision at the point of care,” reported Dave Singh, MD, professor in the division of infection, immunity, and respiratory medicine, University of Manchester (England).
The primary results of these trials were presented several months ago at the 2019 American Thoracic Society (ATS) meeting, but the full data were published on the day that Dr. Singh spoke at the ERS.
To identify predictors of response, the pooled analysis of TRIMARIN and TRIGGER was prespecified. Both of these trials, which were similarly designed, compared a single inhaler of inhaled corticosteroids (ICS), long-acting beta agonist (LABA), and long-acting muscarinic antagonist (LAMA) to a single ICS/LABA inhaler.
On the basis of risk for severe exacerbations, greater protection from triple therapy relative to a conventional ICS/LABA inhaler was identified for those with a high degree of reversibility (defined as greater than 400 mL) relative to those with a lower degree (RR, 0.729; P = .024), those with a body mass index less than 25 kg/m2 relative to a higher BMI (RR, 0.570; P = .005), those with only one exacerbation in the previous 12 months relative to those with more (RR, 0.731; P = .009), never-smokers relative to those with smoking history (RR, 0.764; P = .013), those younger than age 65 years relative to older (RR, 0.770; P = .17), and males relative to females (RR, 0.651; P = .009).
“This gives us six factors to consider when you are thinking about stepping up to triple therapy and are trying to determine which patients would benefit the most,” Dr. Singh said.
Both the TRIMARIN and the TRIGGER trials were double blind and placebo controlled. In both, the experimental arm was a single inhaler triple therapy of the ICS beclomethasone, the LABA formoterol, and the LAMA glycopyrronium. The control arm was a single inhaler combination of beclomethasone and formoterol. All inhalers were used twice daily.
TRIMARIN, with 171 participating sites in 16 countries, randomized 1,155 patients to the triple-drug inhaler with a moderate dose of ICS (100 mcg) or to the ICS/LABA inhaler. In TRIGGER, with 221 sites in 17 countries, 1,437 patients were randomized to one of three arms. Both the triple-drug inhaler arm and the ICS/LABA arm contained a higher dose of ICS (200 mcg) than in TRIMARIN. In an open-label third arm, patients also received the higher dose of ICS plus LABA and a second inhaler with tiotropium. The formoterol dose in all arms of both studies was 6 mcg.
As reported at the ATS and now published in the Lancet, the reduction in exacerbations on single inhaler triple therapy relative to ICS/LABA was significant when the data were pooled (even though the reduction in the TRIGGER study fell short of statistical significance). The median improvement in lung function for single inhaler triple therapy relative to ICS/LABA was significant in both TRIMARIN (57 mL; P = .008) and TRIGGER (73 mL; P = .0025).
In discussing the new pooled analysis of response predictors in TRIMARIN/TRIGGER, the ERS-invited discussant, Celeste M. Porsbjerg, MD, Bispebjerg Hospital, Copenhagen, expressed particular interest in reversibility. A positive reversibility test to salbutamol was an entry criterion for both trials, but Dr. Porsbjerg pointed out that a greater response in those with the highest reversibility suggests these patients have a phenotype in which bronchodilation is a more important driver of disease than is inflammation.
While conceding that this was possible, Dr. Singh cautioned that he considers these predictors of response to be “exploratory.” He believes that the TRIMARIN/TRIGGER studies were not designed to tease out the relative importance of mechanisms of asthma in response to the assigned therapies. However, he believes the response predictor analysis is a step in this direction, which might be valuable for better individualizing therapy.
The studies were funded by Chiesi Farmaceutici. Dr. Singh reports no potential conflicts of interest.
Virchow JC et al. Lancet. 2019 Sep 30. doi. org/10.1016/S0140-6736(19)32215-9.
REPORTING FROM ERS 2019