User login
Mysterious vaping lung injuries may have flown under regulatory radar
It was the arrival of the second man in his early 20s gasping for air that alarmed Dixie Harris, MD. Young patients rarely get so sick, so fast, with a severe lung illness, and this was her second case in a matter of days.
Then she saw three more patients at her Utah telehealth clinic with similar symptoms. They did not have infections, but all had been vaping. When Dr. Harris heard several teenagers in Wisconsin had been hospitalized in similar cases, she quickly alerted her state health department.
As patients in hospitals across the country combat a mysterious illness linked to e-cigarettes, federal and state investigators are frantically trying to trace the outbreaks to specific vaping products that, until recently, were virtually unregulated.
As of Aug. 22, 2019, 193 potential vaping-related illnesses in 22 states had been reported to the Centers for Disease Control and Prevention. Wisconsin, which first put out an alert in July, has at least 16 confirmed and 15 suspected cases. Illinois has reported 34 patients, 1 of whom has died. Indiana is investigating 24 cases.
Lung doctors said they had seen warning signs for years that vaping could be hazardous as they treated patients. Medically it seemed problematic since it often involved inhaling chemicals not normally inhaled into the lungs. Despite that, assessing the safety of a new product storming the market fell between regulatory cracks, leaving doctors unsure where to register concerns before the outbreak. The Food and Drug Administration took years to regulate e-cigarettes once a court determined it had the authority to do so.
“You don’t know what you’re putting into your lungs when you vape,” said Dr. Harris, a critical care pulmonologist. “It’s purported to be safe, but how do you know if it’s safe? To me, it’s a very dangerous thing.”
Off the radar
When e-cigarettes came to market about a decade ago, they fell into a regulatory no man’s land. They are not a food, not a drug, and not a medical device, any of which would have put them immediately in the FDA’s purview. And, until a few years ago, they weren’t even lumped in with tobacco products.
As a result, billions of dollars of vaping products have been sold online, at big-box retailers, and in corner stores without going through the FDA’s rigorous review process to assess their safety. Companies like Juul, Blu, and NJoy quickly established their brands of devices and cartridges, or pods. And thousands of related products are sold, sometimes on the black market, over the Internet, or beyond.
“It makes it really tough because we don’t know what we’re looking for,” said Ruth Lynfield, MD, the state epidemiologist for Minnesota, where several patients were admitted to the ICU as a result of the illness. She added that, if it turns out that the products in question were sold by unregistered retailers and manufacturers “on the street,” outbreak sleuths will have a harder time figuring out exactly what is in them.
With e-cigarettes, people can vape – or smoke – nicotine products, selecting flavorings like mint, mango, blueberry crème brûlée, or cookies and milk. They can also inhale cannabis products. Many are hopeful that e-cigarettes might be useful smoking cessation tools, but some research has called that into question.
The mysterious pulmonary disease cases have been linked to vaping, but it’s unclear whether there is a common device or chemical. In some states, including California and Utah, all of the patients had vaped cannabis products. One or more substances could be involved, health officials have said. The products used by several victims are being tested to see what they contained.
And this has apparently been the case for years.
Multiple doctors described seeing earlier cases of severe lung problems linked to vaping that were not officially reported or included in the current CDC count.
Laura Crotty Alexander, MD, a pulmonologist and researcher with the University of California, San Diego, said she saw her first case about 2 years ago. A young man had been vaping for months with the same device but developed acute lung injury when he switched flavors. She strongly suspected a link, but did not report the illness anywhere.
“It wasn’t that I didn’t want to report it, it’s that there’s no pathway” to do so, Dr. Alexander said.
She said she’s concerned that many physicians haven’t been asking patients about e-cigarette use and that there’s no way to document a case like this in the medical coding system.
John E. Parker, MD, of West Virginia University, Morgantown, said he saw his first patient with pneumonia tied to vaping in 2015. Doctors there were intrigued enough to report on the case at the annual meeting of the American College of Chest Physicians. Dr. Parker and his team didn’t contact a federal agency, and Dr. Parker said it was unclear whom to call.
Numerous other cases have been reported in medical journals and at professional conferences in the years since. The FDA’s voluntary system for reporting tobacco-related health problems included 96 seizures and only 1 lung ailment tied to e-cigarettes between April and June 2019. The system appears to be utilized most by concerned citizens, rather than manufacturers or health care professionals.
But several lung specialists said that due to the patchwork nature of regulatory oversight over the years, the true scope of the problem is yet to be identified.
“We do know that e-cigarettes do not emit a harmless aerosol,” said Brian King, PhD, MPH, a deputy director in the Office on Smoking and Health at the CDC in a call with media on Aug. 23 about the outbreak. “It is possible that some of these cases were already occurring but we were not picking them up.”
Regulatory limits
The FDA has had limited authority to regulate e-cigarettes over the years.
In 2009, Congress passed the Family Smoking Prevention and Tobacco Control Act, empowering the FDA to oversee the safety and sale of tobacco products. But e-cigarettes, still new, were not top of mind.
Later that year, the FDA tried to block imports of e-cigarettes, saying the combination drug-device products were unapproved and therefore illegal for sale in the United States. Two vaping companies, Smoking Everywhere and NJoy, sued, and a federal judge ruled in 2010 that the FDA should regulate e-cigarettes as tobacco products.
It took the agency 6 years to finalize what’s become known as the “deeming rule,” in which it formally began regulating e-cigarettes and e-liquids.
By then, it was May 2016, and the e-cigarette market had swelled to an estimated $4.1 billion, Wells Fargo Securities analyst Bonnie Herzog said at the time. Market researchers now project that the global industry could reach $48 billion by 2023.
Critics say the FDA took too long to act.
“I think the fact that FDA has been dillydallying [has made] figuring out what’s going on [with this outbreak] much harder,” said Stanton Glantz, PhD, a University of California, San Francisco, professor in its Center for Tobacco Control Research and Education. “No question.”
The agency began by banning e-cigarette sales to minors and requiring all new vaping products to submit applications for authorization before they could come to market. Companies and retailers with thousands of products already on the market were granted 2 years to submit applications, and the FDA would get an additional year to evaluate the applications. Meanwhile, existing products could still be sold.
But when Scott Gottlieb, MD, arrived as the new FDA commissioner in 2017, the rule hadn’t been implemented and there was no formal guidance for companies to file applications, he said. As a result, he pushed the deadline back to 2022, drawing ire from public health advocates, who called foul over his previous ties to an e-cigarette retailer called Kure.
“I thought e-cigarettes at the time – and I still believe – that they represent an opportunity for currently addicted adult smokers to transition off of combustible tobacco,” he said in an interview, adding that other parts of the deeming rule went into effect as planned. “All I did was delay the application deadline.”
Dr. Gottlieb’s thinking changed the following year, when a national survey showed a sharp rise in teen vaping, which he called an “epidemic.” He announced that the agency would rethink the extended deadline and weigh whether to take flavors that appeal to kids off the market.
A judge ruled last month that e-cigarette makers would have only 10 more months to submit applications to the FDA. They’re now due in May 2020.
Asked about the lung injuries appearing now, Dr. Gottlieb, who left the FDA in April 2019, said he suspected counterfeit pods are to blame, given the geographic clustering of cases and the fact that, overall, the FDA is inspecting registered e-cigarette makers and retailers to make sure they’re complying with existing regulations.
“I think the manufacturers are culpable if their products are being used, whether the liquids are counterfeit or real,” he said. “Ultimately, they’re responsible for keeping their products out of the hands of kids.”
Juul, the leading e-cigarette maker, agreed that children shouldn’t be able to vape its products, and said curtailing access should be done “through significant regulation” and “enforcement.”
“When people say ‘Why aren’t these being regulated?’ They actually are all being regulated,” Dr. Gottlieb said.
For example, companies are required to label their products as potentially addictive, sell only to adults and comply with manufacturing standards. The agency has conducted thousands of inspections of e-cigarette manufacturers and retailers and taken enforcement actions against companies selling e-cigarettes that look like juice boxes, and against a company that was putting the ingredients found in erectile dysfunction drugs into its vape liquid.
Health departments investigating the outbreak told Kaiser Health News that e-cigarettes’ niche as a tobacco product instead of a drug has presented challenges. Most weren’t aware that adverse events could be reported to a database that tracks problems with tobacco products. And, because e-cigarettes never went through the FDA’s “gold-standard” approval process for drugs, doctors can’t readily look up a detailed list of known side effects.
But like other arms of the FDA, the tobacco office has tools and a team to investigate a public health threat just as the teams for drugs and devices do, Dr. Gottlieb said. It may even be better equipped because of its funding.
“I don’t think FDA is operating in any way with hands tied behind its back because of the way that the statute is set up,” he said.
Teen vaping has exploded during this regulatory tussle. In 2011, 1.5% of high school students reported vaping. By 2018, it was 20.8%, according to a CDC report.
Unknown components
Still, doctors and researchers are concerned about the ingredients in e-cigarettes and how little the public knows about the risks of vaping.
In Juul’s terms and conditions, posted on its website, it says, “We encourage consumers to do their own research regarding vapor products and what is right for them.” Many ingredients in e-cigarette products, however, are protected as trade secrets.
Since at least 2013, the flavor industry has expressed concern about the use of flavoring chemicals in vaping products.
The vast majority of the chemicals have been tested only by ingesting them in small quantities because they’re encountered in foods. For most of these chemicals, there have been no tests to determine whether it is safe to inhale them, as happens daily by millions when they use e-cigarettes.
“Many of the ingredients of vaping products, including flavoring substances, have not been tested for … the exposure one would get from using a vaping device,” said John Hallagan, a senior adviser to the Flavor and Extract Manufacturers Association. The group has sent cease-and-desist letters to e-cigarette companies in previous years for using the food safety certification of the flavor industry to imply that the chemicals are also safe in e-cigarettes.
Some flavor chemicals are thought to be harmful when inhaled in high doses. Research suggests that cinnamaldehyde, the main component of many cinnamon flavors, may impair lung function when inhaled. Sven-Eric Jordt, PhD, a professor at Duke University, Durham, N.C., says he presented evidence of its dangers at an FDA meeting in 2015 — and its relative abundance in many e-cigarette vaping liquids. In response, one major e-cigarette liquid seller, Tasty Vapor, voluntarily took its cinnamon-flavored liquid off the shelves.
In 2017, when Dr. Gottlieb delayed the FDA application deadline, the product was back. A company email to its customers put it this way: “Two years ago, Tasty Vapor allowed itself to be intimidated by scaremongering tactics. … We lost a lot of sales as well as a good number of long-time customers. We no long see reason to disappoint our customers hostage for these shady tactics.”
At the time of publication, Tasty Vapor’s owner did not reply to a request for comment.
Dr. Jordt said he is frustrated by the delays in the regulatory approval process.
“As a parent, I would say that the government has not acted on this,” he said. “You’re basically left to act alone with your addicted kid. It’s kind of terrifying that this was allowed to happen. The industry needs to be held to account.”
Kaiser Health News correspondents Cara Anthony, Markian Hawryluk, and Lauren Weber, as well as reporter Victoria Knight contributed to this report. This story first published on California Healthline, a service of the California Health Care Foundation.
Kaiser Health News is a national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation which is not affiliated with Kaiser Permanente.
It was the arrival of the second man in his early 20s gasping for air that alarmed Dixie Harris, MD. Young patients rarely get so sick, so fast, with a severe lung illness, and this was her second case in a matter of days.
Then she saw three more patients at her Utah telehealth clinic with similar symptoms. They did not have infections, but all had been vaping. When Dr. Harris heard several teenagers in Wisconsin had been hospitalized in similar cases, she quickly alerted her state health department.
As patients in hospitals across the country combat a mysterious illness linked to e-cigarettes, federal and state investigators are frantically trying to trace the outbreaks to specific vaping products that, until recently, were virtually unregulated.
As of Aug. 22, 2019, 193 potential vaping-related illnesses in 22 states had been reported to the Centers for Disease Control and Prevention. Wisconsin, which first put out an alert in July, has at least 16 confirmed and 15 suspected cases. Illinois has reported 34 patients, 1 of whom has died. Indiana is investigating 24 cases.
Lung doctors said they had seen warning signs for years that vaping could be hazardous as they treated patients. Medically it seemed problematic since it often involved inhaling chemicals not normally inhaled into the lungs. Despite that, assessing the safety of a new product storming the market fell between regulatory cracks, leaving doctors unsure where to register concerns before the outbreak. The Food and Drug Administration took years to regulate e-cigarettes once a court determined it had the authority to do so.
“You don’t know what you’re putting into your lungs when you vape,” said Dr. Harris, a critical care pulmonologist. “It’s purported to be safe, but how do you know if it’s safe? To me, it’s a very dangerous thing.”
Off the radar
When e-cigarettes came to market about a decade ago, they fell into a regulatory no man’s land. They are not a food, not a drug, and not a medical device, any of which would have put them immediately in the FDA’s purview. And, until a few years ago, they weren’t even lumped in with tobacco products.
As a result, billions of dollars of vaping products have been sold online, at big-box retailers, and in corner stores without going through the FDA’s rigorous review process to assess their safety. Companies like Juul, Blu, and NJoy quickly established their brands of devices and cartridges, or pods. And thousands of related products are sold, sometimes on the black market, over the Internet, or beyond.
“It makes it really tough because we don’t know what we’re looking for,” said Ruth Lynfield, MD, the state epidemiologist for Minnesota, where several patients were admitted to the ICU as a result of the illness. She added that, if it turns out that the products in question were sold by unregistered retailers and manufacturers “on the street,” outbreak sleuths will have a harder time figuring out exactly what is in them.
With e-cigarettes, people can vape – or smoke – nicotine products, selecting flavorings like mint, mango, blueberry crème brûlée, or cookies and milk. They can also inhale cannabis products. Many are hopeful that e-cigarettes might be useful smoking cessation tools, but some research has called that into question.
The mysterious pulmonary disease cases have been linked to vaping, but it’s unclear whether there is a common device or chemical. In some states, including California and Utah, all of the patients had vaped cannabis products. One or more substances could be involved, health officials have said. The products used by several victims are being tested to see what they contained.
And this has apparently been the case for years.
Multiple doctors described seeing earlier cases of severe lung problems linked to vaping that were not officially reported or included in the current CDC count.
Laura Crotty Alexander, MD, a pulmonologist and researcher with the University of California, San Diego, said she saw her first case about 2 years ago. A young man had been vaping for months with the same device but developed acute lung injury when he switched flavors. She strongly suspected a link, but did not report the illness anywhere.
“It wasn’t that I didn’t want to report it, it’s that there’s no pathway” to do so, Dr. Alexander said.
She said she’s concerned that many physicians haven’t been asking patients about e-cigarette use and that there’s no way to document a case like this in the medical coding system.
John E. Parker, MD, of West Virginia University, Morgantown, said he saw his first patient with pneumonia tied to vaping in 2015. Doctors there were intrigued enough to report on the case at the annual meeting of the American College of Chest Physicians. Dr. Parker and his team didn’t contact a federal agency, and Dr. Parker said it was unclear whom to call.
Numerous other cases have been reported in medical journals and at professional conferences in the years since. The FDA’s voluntary system for reporting tobacco-related health problems included 96 seizures and only 1 lung ailment tied to e-cigarettes between April and June 2019. The system appears to be utilized most by concerned citizens, rather than manufacturers or health care professionals.
But several lung specialists said that due to the patchwork nature of regulatory oversight over the years, the true scope of the problem is yet to be identified.
“We do know that e-cigarettes do not emit a harmless aerosol,” said Brian King, PhD, MPH, a deputy director in the Office on Smoking and Health at the CDC in a call with media on Aug. 23 about the outbreak. “It is possible that some of these cases were already occurring but we were not picking them up.”
Regulatory limits
The FDA has had limited authority to regulate e-cigarettes over the years.
In 2009, Congress passed the Family Smoking Prevention and Tobacco Control Act, empowering the FDA to oversee the safety and sale of tobacco products. But e-cigarettes, still new, were not top of mind.
Later that year, the FDA tried to block imports of e-cigarettes, saying the combination drug-device products were unapproved and therefore illegal for sale in the United States. Two vaping companies, Smoking Everywhere and NJoy, sued, and a federal judge ruled in 2010 that the FDA should regulate e-cigarettes as tobacco products.
It took the agency 6 years to finalize what’s become known as the “deeming rule,” in which it formally began regulating e-cigarettes and e-liquids.
By then, it was May 2016, and the e-cigarette market had swelled to an estimated $4.1 billion, Wells Fargo Securities analyst Bonnie Herzog said at the time. Market researchers now project that the global industry could reach $48 billion by 2023.
Critics say the FDA took too long to act.
“I think the fact that FDA has been dillydallying [has made] figuring out what’s going on [with this outbreak] much harder,” said Stanton Glantz, PhD, a University of California, San Francisco, professor in its Center for Tobacco Control Research and Education. “No question.”
The agency began by banning e-cigarette sales to minors and requiring all new vaping products to submit applications for authorization before they could come to market. Companies and retailers with thousands of products already on the market were granted 2 years to submit applications, and the FDA would get an additional year to evaluate the applications. Meanwhile, existing products could still be sold.
But when Scott Gottlieb, MD, arrived as the new FDA commissioner in 2017, the rule hadn’t been implemented and there was no formal guidance for companies to file applications, he said. As a result, he pushed the deadline back to 2022, drawing ire from public health advocates, who called foul over his previous ties to an e-cigarette retailer called Kure.
“I thought e-cigarettes at the time – and I still believe – that they represent an opportunity for currently addicted adult smokers to transition off of combustible tobacco,” he said in an interview, adding that other parts of the deeming rule went into effect as planned. “All I did was delay the application deadline.”
Dr. Gottlieb’s thinking changed the following year, when a national survey showed a sharp rise in teen vaping, which he called an “epidemic.” He announced that the agency would rethink the extended deadline and weigh whether to take flavors that appeal to kids off the market.
A judge ruled last month that e-cigarette makers would have only 10 more months to submit applications to the FDA. They’re now due in May 2020.
Asked about the lung injuries appearing now, Dr. Gottlieb, who left the FDA in April 2019, said he suspected counterfeit pods are to blame, given the geographic clustering of cases and the fact that, overall, the FDA is inspecting registered e-cigarette makers and retailers to make sure they’re complying with existing regulations.
“I think the manufacturers are culpable if their products are being used, whether the liquids are counterfeit or real,” he said. “Ultimately, they’re responsible for keeping their products out of the hands of kids.”
Juul, the leading e-cigarette maker, agreed that children shouldn’t be able to vape its products, and said curtailing access should be done “through significant regulation” and “enforcement.”
“When people say ‘Why aren’t these being regulated?’ They actually are all being regulated,” Dr. Gottlieb said.
For example, companies are required to label their products as potentially addictive, sell only to adults and comply with manufacturing standards. The agency has conducted thousands of inspections of e-cigarette manufacturers and retailers and taken enforcement actions against companies selling e-cigarettes that look like juice boxes, and against a company that was putting the ingredients found in erectile dysfunction drugs into its vape liquid.
Health departments investigating the outbreak told Kaiser Health News that e-cigarettes’ niche as a tobacco product instead of a drug has presented challenges. Most weren’t aware that adverse events could be reported to a database that tracks problems with tobacco products. And, because e-cigarettes never went through the FDA’s “gold-standard” approval process for drugs, doctors can’t readily look up a detailed list of known side effects.
But like other arms of the FDA, the tobacco office has tools and a team to investigate a public health threat just as the teams for drugs and devices do, Dr. Gottlieb said. It may even be better equipped because of its funding.
“I don’t think FDA is operating in any way with hands tied behind its back because of the way that the statute is set up,” he said.
Teen vaping has exploded during this regulatory tussle. In 2011, 1.5% of high school students reported vaping. By 2018, it was 20.8%, according to a CDC report.
Unknown components
Still, doctors and researchers are concerned about the ingredients in e-cigarettes and how little the public knows about the risks of vaping.
In Juul’s terms and conditions, posted on its website, it says, “We encourage consumers to do their own research regarding vapor products and what is right for them.” Many ingredients in e-cigarette products, however, are protected as trade secrets.
Since at least 2013, the flavor industry has expressed concern about the use of flavoring chemicals in vaping products.
The vast majority of the chemicals have been tested only by ingesting them in small quantities because they’re encountered in foods. For most of these chemicals, there have been no tests to determine whether it is safe to inhale them, as happens daily by millions when they use e-cigarettes.
“Many of the ingredients of vaping products, including flavoring substances, have not been tested for … the exposure one would get from using a vaping device,” said John Hallagan, a senior adviser to the Flavor and Extract Manufacturers Association. The group has sent cease-and-desist letters to e-cigarette companies in previous years for using the food safety certification of the flavor industry to imply that the chemicals are also safe in e-cigarettes.
Some flavor chemicals are thought to be harmful when inhaled in high doses. Research suggests that cinnamaldehyde, the main component of many cinnamon flavors, may impair lung function when inhaled. Sven-Eric Jordt, PhD, a professor at Duke University, Durham, N.C., says he presented evidence of its dangers at an FDA meeting in 2015 — and its relative abundance in many e-cigarette vaping liquids. In response, one major e-cigarette liquid seller, Tasty Vapor, voluntarily took its cinnamon-flavored liquid off the shelves.
In 2017, when Dr. Gottlieb delayed the FDA application deadline, the product was back. A company email to its customers put it this way: “Two years ago, Tasty Vapor allowed itself to be intimidated by scaremongering tactics. … We lost a lot of sales as well as a good number of long-time customers. We no long see reason to disappoint our customers hostage for these shady tactics.”
At the time of publication, Tasty Vapor’s owner did not reply to a request for comment.
Dr. Jordt said he is frustrated by the delays in the regulatory approval process.
“As a parent, I would say that the government has not acted on this,” he said. “You’re basically left to act alone with your addicted kid. It’s kind of terrifying that this was allowed to happen. The industry needs to be held to account.”
Kaiser Health News correspondents Cara Anthony, Markian Hawryluk, and Lauren Weber, as well as reporter Victoria Knight contributed to this report. This story first published on California Healthline, a service of the California Health Care Foundation.
Kaiser Health News is a national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation which is not affiliated with Kaiser Permanente.
It was the arrival of the second man in his early 20s gasping for air that alarmed Dixie Harris, MD. Young patients rarely get so sick, so fast, with a severe lung illness, and this was her second case in a matter of days.
Then she saw three more patients at her Utah telehealth clinic with similar symptoms. They did not have infections, but all had been vaping. When Dr. Harris heard several teenagers in Wisconsin had been hospitalized in similar cases, she quickly alerted her state health department.
As patients in hospitals across the country combat a mysterious illness linked to e-cigarettes, federal and state investigators are frantically trying to trace the outbreaks to specific vaping products that, until recently, were virtually unregulated.
As of Aug. 22, 2019, 193 potential vaping-related illnesses in 22 states had been reported to the Centers for Disease Control and Prevention. Wisconsin, which first put out an alert in July, has at least 16 confirmed and 15 suspected cases. Illinois has reported 34 patients, 1 of whom has died. Indiana is investigating 24 cases.
Lung doctors said they had seen warning signs for years that vaping could be hazardous as they treated patients. Medically it seemed problematic since it often involved inhaling chemicals not normally inhaled into the lungs. Despite that, assessing the safety of a new product storming the market fell between regulatory cracks, leaving doctors unsure where to register concerns before the outbreak. The Food and Drug Administration took years to regulate e-cigarettes once a court determined it had the authority to do so.
“You don’t know what you’re putting into your lungs when you vape,” said Dr. Harris, a critical care pulmonologist. “It’s purported to be safe, but how do you know if it’s safe? To me, it’s a very dangerous thing.”
Off the radar
When e-cigarettes came to market about a decade ago, they fell into a regulatory no man’s land. They are not a food, not a drug, and not a medical device, any of which would have put them immediately in the FDA’s purview. And, until a few years ago, they weren’t even lumped in with tobacco products.
As a result, billions of dollars of vaping products have been sold online, at big-box retailers, and in corner stores without going through the FDA’s rigorous review process to assess their safety. Companies like Juul, Blu, and NJoy quickly established their brands of devices and cartridges, or pods. And thousands of related products are sold, sometimes on the black market, over the Internet, or beyond.
“It makes it really tough because we don’t know what we’re looking for,” said Ruth Lynfield, MD, the state epidemiologist for Minnesota, where several patients were admitted to the ICU as a result of the illness. She added that, if it turns out that the products in question were sold by unregistered retailers and manufacturers “on the street,” outbreak sleuths will have a harder time figuring out exactly what is in them.
With e-cigarettes, people can vape – or smoke – nicotine products, selecting flavorings like mint, mango, blueberry crème brûlée, or cookies and milk. They can also inhale cannabis products. Many are hopeful that e-cigarettes might be useful smoking cessation tools, but some research has called that into question.
The mysterious pulmonary disease cases have been linked to vaping, but it’s unclear whether there is a common device or chemical. In some states, including California and Utah, all of the patients had vaped cannabis products. One or more substances could be involved, health officials have said. The products used by several victims are being tested to see what they contained.
And this has apparently been the case for years.
Multiple doctors described seeing earlier cases of severe lung problems linked to vaping that were not officially reported or included in the current CDC count.
Laura Crotty Alexander, MD, a pulmonologist and researcher with the University of California, San Diego, said she saw her first case about 2 years ago. A young man had been vaping for months with the same device but developed acute lung injury when he switched flavors. She strongly suspected a link, but did not report the illness anywhere.
“It wasn’t that I didn’t want to report it, it’s that there’s no pathway” to do so, Dr. Alexander said.
She said she’s concerned that many physicians haven’t been asking patients about e-cigarette use and that there’s no way to document a case like this in the medical coding system.
John E. Parker, MD, of West Virginia University, Morgantown, said he saw his first patient with pneumonia tied to vaping in 2015. Doctors there were intrigued enough to report on the case at the annual meeting of the American College of Chest Physicians. Dr. Parker and his team didn’t contact a federal agency, and Dr. Parker said it was unclear whom to call.
Numerous other cases have been reported in medical journals and at professional conferences in the years since. The FDA’s voluntary system for reporting tobacco-related health problems included 96 seizures and only 1 lung ailment tied to e-cigarettes between April and June 2019. The system appears to be utilized most by concerned citizens, rather than manufacturers or health care professionals.
But several lung specialists said that due to the patchwork nature of regulatory oversight over the years, the true scope of the problem is yet to be identified.
“We do know that e-cigarettes do not emit a harmless aerosol,” said Brian King, PhD, MPH, a deputy director in the Office on Smoking and Health at the CDC in a call with media on Aug. 23 about the outbreak. “It is possible that some of these cases were already occurring but we were not picking them up.”
Regulatory limits
The FDA has had limited authority to regulate e-cigarettes over the years.
In 2009, Congress passed the Family Smoking Prevention and Tobacco Control Act, empowering the FDA to oversee the safety and sale of tobacco products. But e-cigarettes, still new, were not top of mind.
Later that year, the FDA tried to block imports of e-cigarettes, saying the combination drug-device products were unapproved and therefore illegal for sale in the United States. Two vaping companies, Smoking Everywhere and NJoy, sued, and a federal judge ruled in 2010 that the FDA should regulate e-cigarettes as tobacco products.
It took the agency 6 years to finalize what’s become known as the “deeming rule,” in which it formally began regulating e-cigarettes and e-liquids.
By then, it was May 2016, and the e-cigarette market had swelled to an estimated $4.1 billion, Wells Fargo Securities analyst Bonnie Herzog said at the time. Market researchers now project that the global industry could reach $48 billion by 2023.
Critics say the FDA took too long to act.
“I think the fact that FDA has been dillydallying [has made] figuring out what’s going on [with this outbreak] much harder,” said Stanton Glantz, PhD, a University of California, San Francisco, professor in its Center for Tobacco Control Research and Education. “No question.”
The agency began by banning e-cigarette sales to minors and requiring all new vaping products to submit applications for authorization before they could come to market. Companies and retailers with thousands of products already on the market were granted 2 years to submit applications, and the FDA would get an additional year to evaluate the applications. Meanwhile, existing products could still be sold.
But when Scott Gottlieb, MD, arrived as the new FDA commissioner in 2017, the rule hadn’t been implemented and there was no formal guidance for companies to file applications, he said. As a result, he pushed the deadline back to 2022, drawing ire from public health advocates, who called foul over his previous ties to an e-cigarette retailer called Kure.
“I thought e-cigarettes at the time – and I still believe – that they represent an opportunity for currently addicted adult smokers to transition off of combustible tobacco,” he said in an interview, adding that other parts of the deeming rule went into effect as planned. “All I did was delay the application deadline.”
Dr. Gottlieb’s thinking changed the following year, when a national survey showed a sharp rise in teen vaping, which he called an “epidemic.” He announced that the agency would rethink the extended deadline and weigh whether to take flavors that appeal to kids off the market.
A judge ruled last month that e-cigarette makers would have only 10 more months to submit applications to the FDA. They’re now due in May 2020.
Asked about the lung injuries appearing now, Dr. Gottlieb, who left the FDA in April 2019, said he suspected counterfeit pods are to blame, given the geographic clustering of cases and the fact that, overall, the FDA is inspecting registered e-cigarette makers and retailers to make sure they’re complying with existing regulations.
“I think the manufacturers are culpable if their products are being used, whether the liquids are counterfeit or real,” he said. “Ultimately, they’re responsible for keeping their products out of the hands of kids.”
Juul, the leading e-cigarette maker, agreed that children shouldn’t be able to vape its products, and said curtailing access should be done “through significant regulation” and “enforcement.”
“When people say ‘Why aren’t these being regulated?’ They actually are all being regulated,” Dr. Gottlieb said.
For example, companies are required to label their products as potentially addictive, sell only to adults and comply with manufacturing standards. The agency has conducted thousands of inspections of e-cigarette manufacturers and retailers and taken enforcement actions against companies selling e-cigarettes that look like juice boxes, and against a company that was putting the ingredients found in erectile dysfunction drugs into its vape liquid.
Health departments investigating the outbreak told Kaiser Health News that e-cigarettes’ niche as a tobacco product instead of a drug has presented challenges. Most weren’t aware that adverse events could be reported to a database that tracks problems with tobacco products. And, because e-cigarettes never went through the FDA’s “gold-standard” approval process for drugs, doctors can’t readily look up a detailed list of known side effects.
But like other arms of the FDA, the tobacco office has tools and a team to investigate a public health threat just as the teams for drugs and devices do, Dr. Gottlieb said. It may even be better equipped because of its funding.
“I don’t think FDA is operating in any way with hands tied behind its back because of the way that the statute is set up,” he said.
Teen vaping has exploded during this regulatory tussle. In 2011, 1.5% of high school students reported vaping. By 2018, it was 20.8%, according to a CDC report.
Unknown components
Still, doctors and researchers are concerned about the ingredients in e-cigarettes and how little the public knows about the risks of vaping.
In Juul’s terms and conditions, posted on its website, it says, “We encourage consumers to do their own research regarding vapor products and what is right for them.” Many ingredients in e-cigarette products, however, are protected as trade secrets.
Since at least 2013, the flavor industry has expressed concern about the use of flavoring chemicals in vaping products.
The vast majority of the chemicals have been tested only by ingesting them in small quantities because they’re encountered in foods. For most of these chemicals, there have been no tests to determine whether it is safe to inhale them, as happens daily by millions when they use e-cigarettes.
“Many of the ingredients of vaping products, including flavoring substances, have not been tested for … the exposure one would get from using a vaping device,” said John Hallagan, a senior adviser to the Flavor and Extract Manufacturers Association. The group has sent cease-and-desist letters to e-cigarette companies in previous years for using the food safety certification of the flavor industry to imply that the chemicals are also safe in e-cigarettes.
Some flavor chemicals are thought to be harmful when inhaled in high doses. Research suggests that cinnamaldehyde, the main component of many cinnamon flavors, may impair lung function when inhaled. Sven-Eric Jordt, PhD, a professor at Duke University, Durham, N.C., says he presented evidence of its dangers at an FDA meeting in 2015 — and its relative abundance in many e-cigarette vaping liquids. In response, one major e-cigarette liquid seller, Tasty Vapor, voluntarily took its cinnamon-flavored liquid off the shelves.
In 2017, when Dr. Gottlieb delayed the FDA application deadline, the product was back. A company email to its customers put it this way: “Two years ago, Tasty Vapor allowed itself to be intimidated by scaremongering tactics. … We lost a lot of sales as well as a good number of long-time customers. We no long see reason to disappoint our customers hostage for these shady tactics.”
At the time of publication, Tasty Vapor’s owner did not reply to a request for comment.
Dr. Jordt said he is frustrated by the delays in the regulatory approval process.
“As a parent, I would say that the government has not acted on this,” he said. “You’re basically left to act alone with your addicted kid. It’s kind of terrifying that this was allowed to happen. The industry needs to be held to account.”
Kaiser Health News correspondents Cara Anthony, Markian Hawryluk, and Lauren Weber, as well as reporter Victoria Knight contributed to this report. This story first published on California Healthline, a service of the California Health Care Foundation.
Kaiser Health News is a national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation which is not affiliated with Kaiser Permanente.
Malignant Pleural Effusion: Therapeutic Options and Strategies
Malignant pleural effusion (MPE) is a common clinical problem in patients with advanced stage cancer. Each year in the United States, more than 150,000 individuals are diagnosed with MPE, and there are approximately 126,000 admissions for MPE.1-3 Providing effective therapeutic management remains a challenge, and currently available therapeutic interventions are palliative rather than curative. This article, the second in a 2-part review of MPE, focuses on the available management options.
Therapeutic Thoracentesis
Evaluation of pleural fluid cytology is a crucial step in the diagnosis and staging of disease. As a result, large-volume fluid removal is often the first therapeutic intervention for patients who present with symptomatic effusions. A patient’s clinical response to therapeutic thoracentesis dictates which additional therapeutic options are appropriate for palliation. Lack of symptom relief suggests that other comorbid conditions or trapped lung physiology may be the primary cause of the patient’s symptoms and discourages more invasive interventions. Radiographic evidence of lung re-expansion after fluid removal is also an important predictor of success for potential pleurodesis.4,5
There are no absolute contraindications to thoracentesis. However, caution should be used for patients with risk factors that may predispose to complications of pneumothorax and bleeding, such as coagulopathy, treatment with anticoagulation medications, thrombocytopenia, platelet dysfunction (eg, antiplatelet medications, uremia), positive pressure ventilation, and small effusion size. These factors are only relative contraindications, however, as thoracentesis can still be safely performed by experienced operators using guidance technology such as ultrasonography.
A retrospective review of 1009 ultrasound-guided thoracenteses with risk factors of an international normalized ratio (INR) greater than 1.6, platelet values less than 50,000/μL, or both, reported an overall rate of hemorrhagic complication of 0.4%, with no difference between procedures performed with (n = 303) or without (n = 706) transfusion correction of coagulopathy or thrombocytopenia.6 A similar retrospective evaluation of 1076 ultrasound-guided thoracenteses, including 267 patients with an INR greater than 1.5 and 58 patients with a platelet count less than 50,000/μL, reported a 0% complication rate.7 Small case series have also demonstrated low hemorrhagic complication rates for thoracentesis in patients treated with clopidogrel8,9 and with increased bleeding risk from elevated INR (liver disease or warfarin therapy) and renal disease.10
Complications from pneumothorax can similarly be affected by patient- and operator-dependent risk factors. Meta-analysis of 24 studies including 6605 thoracenteses demonstrated an overall pneumothorax rate of 6.0%, with 34.1% requiring chest tube insertion.11 Lower pneumothorax rates were associated with the use of ultrasound guidance (odds ratio, 0.3; 95% confidence interval, 0.2-0.7). Experienced operators also had fewer pneumothorax complications, though this factor was not significant in the studies directly comparing this variable. Therapeutic thoracentesis and use of a larger-bore needle were also significantly correlated with pneumothorax, while mechanical ventilation had a nonsignificant trend towards increased risk.
Although there is no consensus on the volume of pleural fluid that may be safely removed, it is recommended not to remove more than 1.5 L during a procedure in order to avoid precipitating re-expansion pulmonary edema.2,12 However, re-expansion pulmonary edema rates remain low even when larger volumes are removed if the patient remains symptom-free during the procedure and pleural manometry pressure does not exceed –20 cm H2O.13 Patient symptoms alone, however, are neither a sensitive nor specific indicator that pleural pressures exceed –20 cm H2O.14 Use of excessive negative pressure during drainage, such as from a vacuum bottle, should also be avoided. Comparison of suction generated manually with a syringe versus a vacuum bottle suggests decreased complications with manual drainage, though the sample size in the supporting study was small relative to the infrequency of the complications being evaluated.15
Given the low morbidity and noninvasive nature of the procedure, serial large-volume thoracentesis remains a viable therapeutic intervention for patients who are unable or unwilling to undergo more invasive interventions, especially for patients with a slow fluid re-accumulation rate or who are anticipated to have limited survival. Unfortunately, many symptomatic effusions will recur within a short interval time span, which necessitates repeat procedures.16,17 Therefore, factors such as poor symptom control, patient inconvenience, recurrent procedural risk, and utilization of medical resources need to be considered as well.
Tunneled Pleural Catheter
Tunneled pleural catheters (TPCs) are a potentially permanent and minimally invasive therapy which allow intermittent drainage of pleural fluid (Figure 1). The catheter is tunneled under the skin to prevent infection. A polyester cuff attached to the catheter is positioned within the tunnel and induces fibrosis around the catheter, thereby securing the catheter in place. Placement can be performed under local anesthesia at the patient’s bedside or in an outpatient procedure space. Fluid can then be drained via specialized drainage bottles or bags by the patient, a family member, or visiting home nurse. The catheter can also be removed in the event of a complication or the development of spontaneous pleurodesis.

TPCs are an effective palliative management strategy for patients with recurrent effusions and are an efficacious alternative to pleurodesis.18-20 TPCs may be used in patients with poor prognosis or trapped lung or in those in whom prior pleurodesis has failed.21-23 Meta-analysis of 19 studies showed symptomatic improvement in 95.6% of patients, with development of spontaneous pleurodesis in 45.6% of patients (range, 11.8% to 76.4%) after an average of 52 days.24 However, most of the studies included in this analysis were retrospective case series. Development of spontaneous pleurodesis from TPC drainage in prospective, controlled trials has been considerably more modest, supporting a range of approximately 20% to 30% with routine drainage strategies.20,25-27 Spontaneous pleurodesis develops greater rapidity and frequency in patients undergoing daily drainage compared to every-other-day or symptom-directed drainage strategies.25,26 However, there is no appreciable improvement in quality of life scores with a specific drainage strategy. Small case series also demonstrate that TPC drainage may induce spontaneous pleurodesis in some patients initially presenting with trapped lung physiology.22
Catheter placement can be performed successfully in the vast majority of patients.28 Increased bleeding risk, significant malignancy-related involvement of the skin and chest wall, and pleural loculations can complicate TPC placement. TPC-related complications are relatively uncommon, but include pneumothorax, catheter malfunction and obstruction, and infections including soft tissue and pleural space infections.24 In a multicenter retrospective series of 1021 patients, only 4.9% developed a TPC-related pleural infection.29 Over 94% were successfully managed with antibiotic therapy, and the TPC was able to be preserved in 54%. Staphylococcus aureus was the most common causative organism and was identified in 48% of cases. Of note, spontaneous pleurodesis occurred in 62% of cases following a pleural space infection, which likely occurred as sequelae of the inflammatory nature of the infection. Retrospective analysis suggests that the risk of TPC-related infections is not substantially higher for patients with higher risks of immunosuppression from chemotherapy or hematologic malignancies.30,31 Tumor metastasis along the catheter tract is a rare occurrence (< 1%), but is most notable with mesothelioma, which has an incidence as high as 10%.24,32 In addition, development of pleural loculations can impede fluid drainage and relief of dyspnea. Intrapleural instillation of fibrinolytics can be used to improve drainage and improve symptom palliation.33
Pleurodesis
Pleurodesis obliterates the potential pleural space by inducing inflammation and fibrosis, resulting in adherence of the visceral and parietal pleura together. This process can be induced through mechanical abrasion of the pleural surface, introduction of chemical sclerosants, or from prolonged use of a chest tube. Chemical sclerosants are the most commonly used method for MPEs and are introduced through a chest tube or under visual guidance such as medical thoracoscopy or video-assisted thoracoscopic surgery (VATS). The pleurodesis process is thought to occur by induction of a systemic inflammatory response with localized deposition of fibrin.34 Activation of fibroblasts and successful pleurodesis have been correlated with higher basic fibroblast growth factor (bFGF) levels in pleural fluid.35 Increased tumor burden is associated with lower bFGF levels, suggesting a possible mechanism for reduced pleurodesis success in these cases. Corticosteroids may reduce the likelihood of pleurodesis due to a reduction of inflammation, as demonstrated in a rabbit model using talc and doxycycline.36,37 Animal data also suggests that use of nonsteroidal anti-inflammatory drugs may hinder the likelihood of successful pleurodesis, but this has not been observed in humans.38,39
Patients selected for pleurodesis should have significant symptom relief from large-volume removal of pleural fluid, good functional status, and evidence of full lung re-expansion after thoracentesis. Lack of visceral and parietal pleural apposition will prevent pleural adhesion from developing. As a result, trapped lung is associated with chemical pleurodesis failure and is an absolute contraindication to the procedure.4,5,12 The pleurodesis process typically requires 5 to 7 days, during which time the patient is hospitalized for chest tube drainage and pain control. When pleural fluid output diminishes, the chest tube is removed and the patient can be discharged. Modified protocols are now emerging which may shorten the required hospitalization associated with pleurodesis procedures.
Pleurodesis Agents
A variety of chemical sclerosants have been used for pleurodesis, including talc, bleomycin, tetracycline, doxycycline, iodopovidone, and mepacrine. Published data regarding these agents are heterogenous, with significant variability in reported outcomes. However, systematic review and meta-analysis suggests that talc is likely to have higher success rates compared to other agents or chest tube drainage alone for treatment of MPE.40,41
Additional factors have been shown to be associated with pleurodesis outcomes. Pleurodesis success is negatively associated with low pleural pH, with receiver operating curve thresholds of 7.28 to 7.34.42,43 Trapped lung, large bulky tumor lining the pleural surfaces, and elevated adenosine deaminase levels are also associated with poor pleurodesis outcomes.4,5,12,35,43 In contrast, pleural fluid output less than 200 mL per day and the presence of EGFR (epidermal growth factor receptor) mutation treated with targeted tyrosine kinase inhibitors are associated with successful pleurodesis.44,45
The most common complications associated with chemical pleurodesis are fever and pain. Other potential complications include soft tissue infections at the chest tube site and of the pleural space, arrhythmias, cardiac arrest, myocardial infarction, and hypotension. Doxycycline is commonly associated with greater pleuritic pain than talc. Acute respiratory distress syndrome (ARDS), acute pneumonitis, and respiratory failure have been described with talc pleurodesis. ARDS secondary to talc pleurodesis occurs in 1% to 9% of cases, though this may be related to the use of ungraded talc. A prospective description of 558 patients treated with large particle talc (> 5 μm) reported no occurrences of ARDS, suggesting the safety of graded large particle talc.46
Pleurodesis Methods
Chest tube thoracostomy is an inpatient procedure performed under local anesthesia or conscious sedation. It can be used for measured, intermittent drainage of large effusions for immediate symptom relief, as well as to demonstrate complete lung re-expansion prior to instillation of a chemical sclerosant. Pleurodesis using a chest tube is performed by instillation of a slurry created by mixing the sclerosing agent of choice with 50 to 100 mL of sterile saline. This slurry is instilled into the pleural cavity through the chest tube. The chest tube is clamped for 1 to 2 hours before being reconnected to suction. Intermittent rotation of the patient has not been shown to improve distribution of the sclerosant or result in better procedural outcomes.47,48 Typically, a 24F to 32F chest tube is used because of the concern about obstruction of smaller bore tubes by fibrin plugs. A noninferiority study comparing 12F to 24F chest tubes for talc pleurodesis demonstrated a higher procedure failure rate with the 12F tube (30% versus 24%) and failed to meet noninferiority criteria.39 However, larger caliber tubes are also associated with greater patient discomfort compared to smaller bore tubes.
Medical thoracoscopy and VATS are minimally invasive means to visualize the pleural space, obtain visually guided biopsy of the parietal pleura, perform lysis of adhesions, and introduce chemical sclerosants for pleurodesis (Figure 2). Medical thoracoscopy can be performed under local anesthesia with procedural sedation in an endoscopy suite or procedure room. 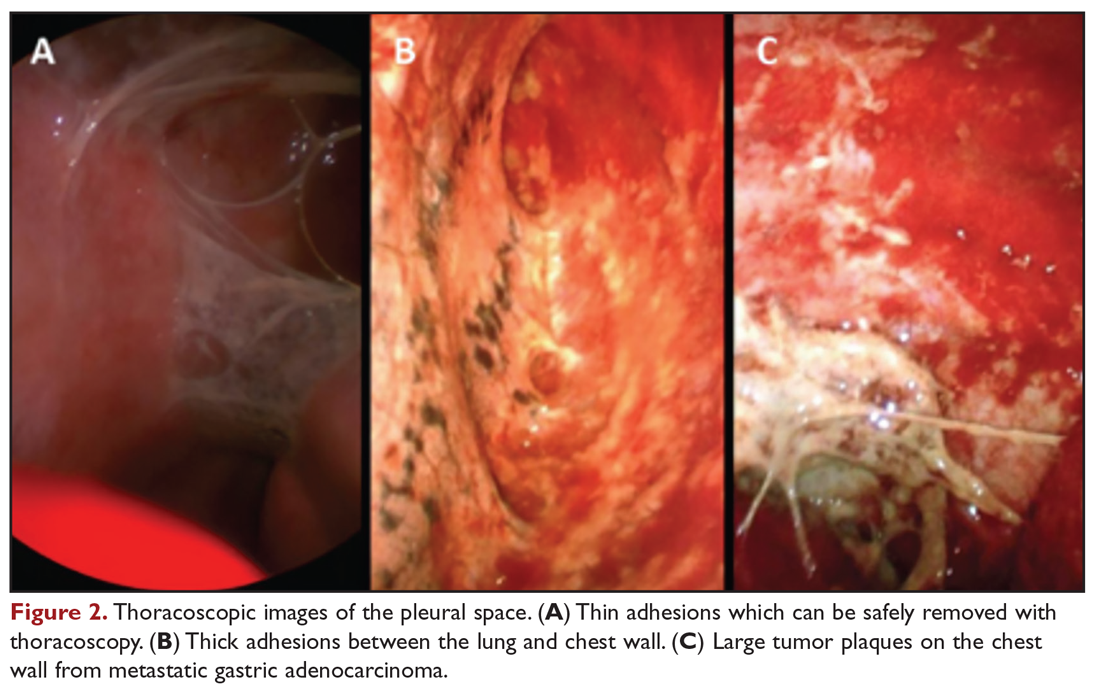
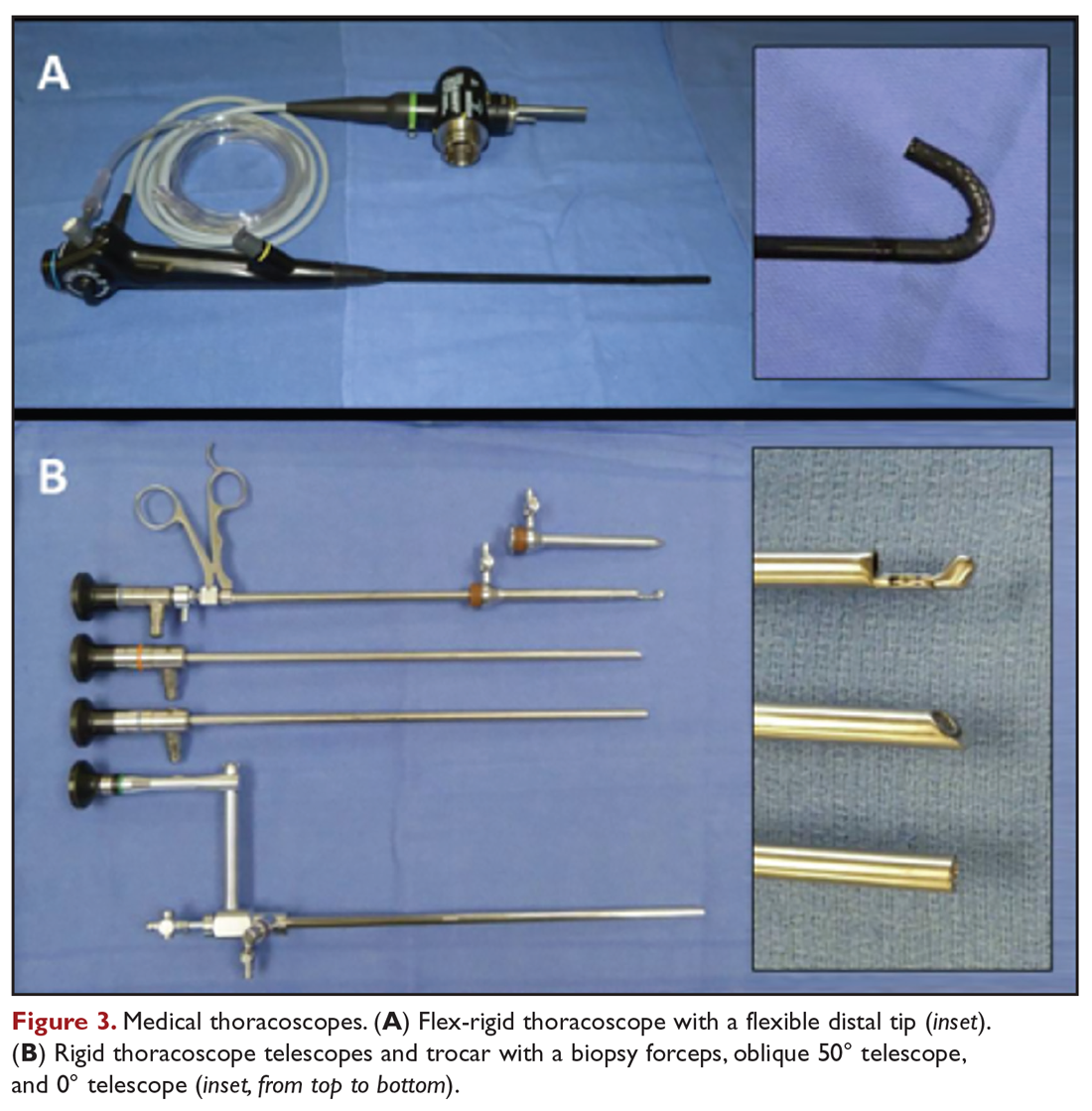
VATS has several distinct and clinically important differences. The equipment is slightly larger but otherwise similar in concept to rigid medical thoracoscopes. A greater number of diagnostic and therapeutic options, such as diagnostic biopsy of lung parenchyma and select hilar lymph nodes, are also possible. However, VATS requires surgical training and is performed in an operating room setting, which necessitates additional ancillary and logistical support. VATS also uses at least 2 trocar insertion sites, requires general anesthesia, and utilizes single-lung ventilation through a double-lumen endotracheal tube. Procedure-related complications for medical thoracoscopy and VATS include pneumothorax, subcutaneous emphysema, fever, and pain.49
Data comparing talc slurry versus talc poudrage are heterogenous, without clear advantage for either method. Reported rates of successful pleurodesis are generally in the range of 70% to 80% for both methods.19,20,40,50 There is a possible suggestion of benefit with talc poudrage compared to slurry, but data is lacking to support either as a definitive choice in current guidelines.12,51 An advantage of medical thoracoscopy or VATS is that pleural biopsy can be performed simultaneously, if necessary, thereby allowing both diagnostic and therapeutic interventions.52 Visualizing the thoracic cavity may also permit creation of optimal conditions for pleurodesis in select individuals by allowing access to loculated spaces and providing visual confirmation of complete drainage of pleural fluid and uniform distribution of the chemical sclerosant.
Other Surgical Interventions
Thoracotomy with decortication is rarely used as treatment of malignant effusions complicated by loculations or trapped lung due to the significantly increased procedural morbidity and mortality. Therefore, it is reserved for the limited population of patients in whom other therapeutic interventions have failed but who otherwise have significant symptoms with a long life expectancy. Mesothelioma is a specific situation in which variations of pleurectomy, such as radical pleurectomy with decortication, lung-sparing total pleurectomy, and extrapleural pneumonectomy (EPP), have been used as front-line therapy. The Mesothelioma and Radical Surgery (MARS) trial, the only randomized, controlled evaluation of EPP, demonstrated decreased median survival in patients treated by EPP compared to controls (14.4 months versus 19.5 months).53 EPP is also associated with high procedure-related morbidity and mortality rates of approximately 50% and 4% to 15%, respectively.54 While successful at achieving pleurodesis, use of EPP as a treatment for mesothelioma is now discouraged.53,55 Less invasive surgical approaches, such as pleurectomy with decortication, are able to palliate symptoms with significantly less operative risk.56
Management Considerations
Selection of Therapeutic Interventions
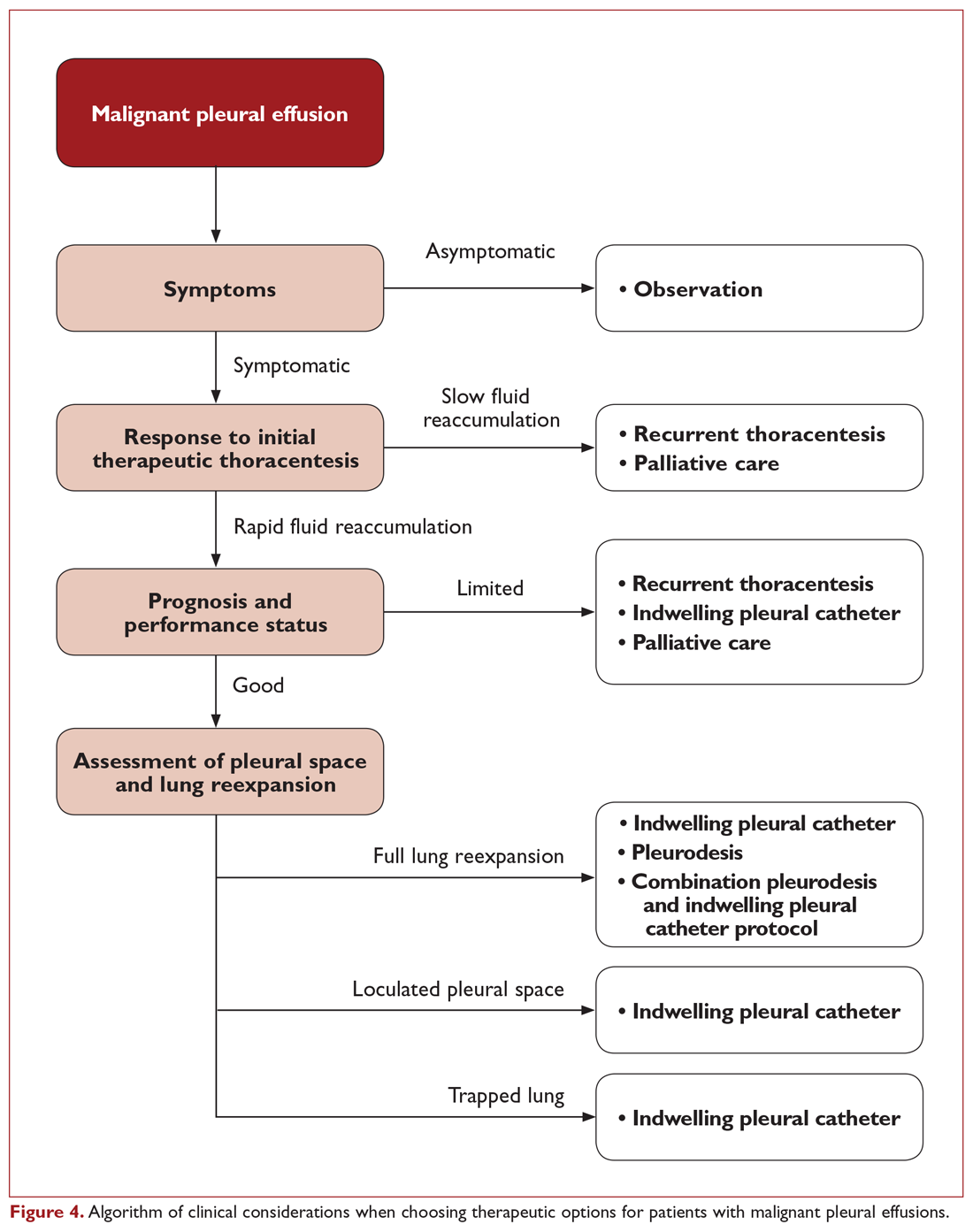
The ideal management strategy provides both immediate and long-term symptom palliation, has minimal associated morbidity and side effects, minimizes hospitalization time and clinic visits, avoids the risks and inconvenience of recurring procedures, is inexpensive, and minimizes utilization of medical resources. Unfortunately, no single palliation methodology fits these needs for all patients. When evaluating therapeutic options for patients with MPE, it is important to consider factors such as the severity of symptoms, fluid quantity, fluid re-accumulation rate, pleural physiology, functional status, overall prognosis, and anticipated response of the malignancy to therapy. One example management algorithm (Figure 4) demonstrates the impact of these variables on the appropriate treatment options. However, this is a simplified algorithm and the selected palliation strategy should be decided upon after shared decision-making between the patient and physician and should fit within the context of the patient’s desired goals of care. It is also crucial for patients to understand that these therapeutic interventions are palliative rather than curative.
When compared directly with pleurodesis, TPC provides similar control of symptoms but with a reduction in hospital length of stay by a median of 3.5 to 5.5 days.19,57 In a nonrandomized trial where patients chose palliation by TPC or talc pleurodesis, more TPC patients had a significant immediate improvement in quality of life and dyspnea after the first 7 days of therapy.58 This is reasonably attributed to the differences between the immediate relief from fluid drainage after TPC placement compared to the time required for pleural symphysis to occur with pleurodesis. However, control of dyspnea symptoms is similar between the 2 strategies after 6 weeks.19 Therefore, both TPC and pleurodesis strategies are viewed as first-line options for patients with expandable lung and no prior palliative interventions for MPE.59
Pleural adhesions and trapped lung also pose specific dilemmas. Pleural adhesions can create loculated fluid pockets, thereby complicating drainage by thoracentesis or TPC and hindering dispersal of pleurodesis agents. Adhesiolysis by medical thoracoscopy or VATS may be useful in these patients to free up the pleural space and improve efficacy of long-term drainage options or facilitate pleurodesis. Intrapleural administration of fibrinolytics, such as streptokinase and urokinase, has also been used for treatment of loculated effusions and may improve drainage of pleural fluid and lung re-expansion.60-63 However routine use of intrapleural fibrinolytics with pleurodesis has not been shown to be beneficial. In a randomized comparison using intrapleural urokinase prior to pleurodesis for patients with septated malignant pleural effusions, no difference in pleurodesis outcomes were identified.63 As a result, TPC is the preferred palliation approach for patients with trapped lung physiology.51,59
Combination Strategies
Combinations of different therapeutic interventions are being evaluated as a means for patients to achieve long-term benefits from pleurodesis while minimizing hospitalization time. One strategy using simultaneous treatment with thoracoscopic talc poudrage and insertion of a large-bore chest tube and TPC has been shown to permit early removal of the chest tube and discharge home using the TPC for continued daily pleural drainage. This “rapid pleurodesis” strategy has an 80% to 90% successful pleurodesis rate, permitting removal of the TPC at a median of 7 to 10 days.64,65 With this approach, median hospitalization length of stay was approximately 2 days. While there was no control arm in these early reports with limited sample sizes, the pleurodesis success rate and length of hospitalization compare favorably to other published studies. A prospective, randomized trial of TPC versus an outpatient regimen of talc slurry via TPC has also shown promise, with successful pleurodesis after 35 days in 43% of those treated with the combination of talc slurry and TPC compared to only 23% in those treated by TPC alone.27
Another novel approach to obtain the benefits of both TPC and pleurodesis strategies is the use of drug-eluting TPC to induce inflammation and promote adhesion of the visceral and parietal pleura. An early report of slow-release silver nitrate (AgNO3) –coated TPC demonstrated an encouraging 89% spontaneous pleurodesis rate after a median of 4 days in the small subgroup of patients with fully expandable lung.66 Device-related adverse events were relatively high at 24.6%, though only one was deemed a serious adverse event. Additional studies of these novel and combination strategies are ongoing at this time.
Costs
While cost of care is not a consideration in the decision-making for individual patients, it is important from a systems-based perspective. Upfront costs for pleurodesis are generally higher due to the facility and hospitalization costs, whereas TPC have ongoing costs for drainage bottles and supplies. In a prospective, randomized trial of TPC versus talc pleurodesis, there was no appreciable difference in overall costs between the 2 approaches.67 The cost of TPC was significantly less, however, for patients with a shorter survival of less than 14 weeks.
Readmissions
Subsequent hospitalization requirements beyond just the initial treatment for a MPE remains another significant consideration for this patient population. A prospective, randomized trial comparing TPC to talc pleurodesis demonstrated a reduction in total all-cause hospital stay for TPC, with a median all-cause hospitalization time of 10 days for patients treated with TPC compared to 12 days for the talc pleurodesis group.20 The primary difference in the number of hospitalization days was due to a difference in effusion-related hospital days (median 1 versus 4 days, respectively), which was primarily comprised of the initial hospitalization. In addition, fewer patients treated with TPC required subsequent ipsilateral invasive procedures (4.1% versus 22.5%, respectively). However, it is important to note that the majority of all-cause hospital days were not effusion-related, demonstrating that this population has a high utilization of acute inpatient services for other reasons related to their advanced malignancy. In a study of regional hospitals in the United States, 38.3% of patients admitted for a primary diagnosis of MPE were readmitted within 30 days.68 There was remarkably little variability in readmission rates among hospitals, despite differences in factors such as institution size, location, patient distribution, and potential practice differences. This suggests that utilization of palliation strategies for MPE are only one component related to hospitalization in this population. Even at the best performing hospitals, there are significant common drivers for readmission that are not addressed. Therefore, additional effort should be focused on addressing aspects of care beyond just the palliation of MPE that predispose this population to requiring frequent treatment in an acute care setting.
Conclusion
MPEs represent advanced stage disease and frequently adversely affect a patient’s quality of life. The treating clinician has access to a variety of therapeutic options, though no single intervention strategy is universally superior in all circumstances. Initial thoracentesis is important in evaluating whether removal of a large volume of fluid provides significant symptom relief and restores functional status. Both talc pleurodesis and TPC provide similar control of symptoms and are first-line approaches for symptomatic patients with MPE and fully expandable lungs. Pleurodesis is associated with greater procedure-related risk and length of hospitalization and is contraindicated in patients with trapped lung, but does not require long-term catheter care or disposable resources. Determination of the appropriate therapeutic management strategy requires careful evaluation of the patient’s clinical situation and informed discussion with the patient to make sure that the treatment plan fits within the context of their goals of medical care.
1. Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J. 2001;18:402-419.
2. Society AT. Management of malignant pleural effusions. Am J Respir Crit Care Med. 2000;162:1987-2001.
3. Taghizadeh N, Fortin M, Tremblay A. US hospitalizations for malignant pleural effusions: data from the 2012 National Inpatient Sample. Chest. 2017;151:845-854.
4. Adler RH, Sayek I. Treatment of malignant pleural effusion: a method using tube thoracostomy and talc. Ann Thorac Surg. 1976;22:8-15.
5. Villanueva AG, Gray AW, Shahian DM, et al. Efficacy of short term versus long term tube thoracostomy drainage before tetracycline pleurodesis in the treatment of malignant pleural effusions. Thorax. 1994;49:23-25.
6. Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest. 2013;144:456-463.
7. Patel MD, Joshi SD. Abnormal preprocedural international normalized ratio and platelet counts are not associated with increased bleeding complications after ultrasound-guided thoracentesis. AJR Am J Roentgenol. 2011;197:W164-168.
8. Zalt MB, Bechara RI, Parks C, Berkowitz DM. Effect of routine clopidogrel use on bleeding complications after ultrasound-guided thoracentesis. J Bronchology Interv Pulmonol. 2012;19:284-287.
9. Mahmood K, Shofer SL, Moser BK, et al. Hemorrhagic complications of thoracentesis and small-bore chest tube placement in patients taking clopidogrel. Ann Am Thorac Soc. 2014;11:73-79.
10. Puchalski JT, Argento AC, Murphy TE, et al. The safety of thoracentesis in patients with uncorrected bleeding risk. Ann Am Thorac Soc. 2013;10:336-341.
11. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170:332-339.
12. Roberts ME, Neville E, Berrisford RG, et al; Group BPDG. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii32-40.
13. Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84:1656-1661.
14. Feller-Kopman D, Walkey A, Berkowitz D, Ernst A. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest. 2006;129:1556-1560.
15. Senitko M, Ray AS, Murphy TE, et al. Safety and tolerability of vacuum versus manual drainage during thoracentesis: a randomized trial. J Bronchology Interv Pulmonol. 2019;26:166-171.
16. Ost DE, Niu J, Zhao H, et al. Quality gaps and comparative effectiveness of management strategies for recurrent malignant pleural effusions. Chest. 2018;153:438-452.
17. Grosu HB, Molina S, Casal R, et al. Risk factors for pleural effusion recurrence in patients with malignancy. Respirology. 2019;24:76-82.
18. Musani AI, Haas AR, Seijo L, et al. Outpatient management of malignant pleural effusions with small-bore, tunneled pleural catheters. Respiration. 2004;71:559-566.
19. Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307:2383-2389.
20. Thomas R, Fysh ETH, Smith NA, et al. Effect of an indwelling pleural catheter vs talc pleurodesis on hospitalization days in patients with malignant pleural effusion: the AMPLE randomized clinical trial. JAMA. 2017;318:1903-1912.
21. Qureshi RA, Collinson SL, Powell RJ, et al. Management of malignant pleural effusion associated with trapped lung syndrome. Asian Cardiovasc Thorac Ann. 2008;16:120-123.
22. Efthymiou CA, Masudi T, Thorpe JA, Papagiannopoulos K. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg. 2009;9:961-964.
23. Sioris T, Sihvo E, Salo J, et al. Long-term indwelling pleural catheter (PleurX) for malignant pleural effusion unsuitable for talc pleurodesis. Eur J Surg Oncol. 2009;35:546-551.
24. Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med. 2011;26:70-76.
25. Wahidi MM, Reddy C, Yarmus L, et al. Randomized trial of pleural fluid drainage frequency in patients with malignant pleural effusions. the ASAP trial. Am J Respir Crit Care Med. 2017;195:1050-1057.
26. Muruganandan S, Azzopardi M, Fitzgerald DB, et al. Aggressive versus symptom-guided drainage of malignant pleural effusion via indwelling pleural catheters (AMPLE-2): an open-label randomised trial. Lancet Respir Med. 2018;6:671-680.
27. Bhatnagar R, Keenan EK, Morley AJ, et al. Outpatient talc administration by indwelling pleural catheter for malignant effusion. N Engl J Med. 2018;378:1313-1322.
28. Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest. 2006;129:362-368.
29. Fysh ETH, Tremblay A, Feller-Kopman D, et al. Clinical outcomes of indwelling pleural catheter-related pleural infections: an international multicenter study. Chest. 2013;144:1597-1602.
30. Morel A, Mishra E, Medley L, et al. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax. 2011;66:448-449.
31. Gilbert CR, Lee HJ, Skalski JH, et al. The use of indwelling tunneled pleural catheters for recurrent pleural effusions in patients with hematologic malignancies: a multicenter study. Chest. 2015;148:752-758.
32. Thomas R, Budgeon CA, Kuok YJ, et al. Catheter tract metastasis associated with indwelling pleural catheters. Chest. 2014;146:557-562.
33. Thomas R, Piccolo F, Miller D, et al. Intrapleural fibrinolysis for the treatment of indwelling pleural catheter-related symptomatic loculations: a multicenter observational study. Chest. 2015;148:746-751.
34. Antony VB. Pathogenesis of malignant pleural effusions and talc pleurodesis. Pneumologie. 1999;53:493-498.
35. Antony VB, Nasreen N, Mohammed KA, et al. Talc pleurodesis: basic fibroblast growth factor mediates pleural fibrosis. Chest. 2004;126:1522-1528.
36. Xie C, Teixeira LR, McGovern JP, Light RW. Systemic corticosteroids decrease the effectiveness of talc pleurodesis. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1441-1444.
37. Teixeira LR, Wu W, Chang DS, Light RW. The effect of corticosteroids on pleurodesis induced by doxycycline in rabbits. Chest. 2002;121:216-219.
38. Hunt I, Teh E, Southon R, Treasure T. Using non-steroidal anti-inflammatory drugs (NSAIDs) following pleurodesis. Interact Cardiovasc Thorac Surg. 2007;6:102-104.
39. Rahman NM, Pepperell J, Rehal S, et al. Effect of opioids vs NSAIDs and larger vs smaller chest tube size on pain control and pleurodesis efficacy among patients with malignant pleural effusion: the TIME1 randomized clinical trial. JAMA. 2015;314:2641-2653.
40. Clive AO, Jones HE, Bhatnagar R, Preston NJ, Maskell N. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev. 2016(5):CD010529.
41. Tan C, Sedrakyan A, Browne J, et al. The evidence on the effectiveness of management for malignant pleural effusion: a systematic review. Eur J Cardiothorac Surg. 2006;29:829-838.
42. Heffner JE, Nietert PJ, Barbieri C. Pleural fluid pH as a predictor of pleurodesis failure: analysis of primary data. Chest. 2000;117:87-95.
43. Yildirim H, Metintas M, Ak G, et al. Predictors of talc pleurodesis outcome in patients with malignant pleural effusions. Lung Cancer. 2008;62:139-144.
44. Aydogmus U, Ozdemir S, Cansever L, et al. Bedside talc pleurodesis for malignant pleural effusion: factors affecting success. Ann Surg Oncol. 2009;16:745-750.
45. Guo H, Wan Y, Tian G, et al. EGFR mutations predict a favorable outcome for malignant pleural effusion of lung adenocarcinoma with Tarceva therapy. Oncol Rep. 2012;27:880-890.
46. Janssen JP, Collier G, Astoul P, et al. Safety of pleurodesis with talc poudrage in malignant pleural effusion: a prospective cohort study. Lancet. 2007;369(9572):1535-1539.
47. Dryzer SR, Allen ML, Strange C, Sahn SA. A comparison of rotation and nonrotation in tetracycline pleurodesis. Chest. 1993;104:1763-1766.
48. Mager HJ, Maesen B, Verzijlbergen F, Schramel F. Distribution of talc suspension during treatment of malignant pleural effusion with talc pleurodesis. Lung Cancer. 2002;36:77-81.
49. Hsia D, Musani AI. Interventional pulmonology. Med Clin North Am. 2011;95:1095-1114.
50. Dresler CM, Olak J, Herndon JE, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest. 2005;127:909-915.
51. Bibby AC, Dorn P, Psallidas I, et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur Respir J. 2018;52(1).
52. Sakuraba M, Masuda K, Hebisawa A, et al. Diagnostic value of thoracoscopic pleural biopsy for pleurisy under local anaesthesia. ANZ J Surg. 2006;76:722-724.
53. Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011;12:763-772.
54. Zellos L, Jaklitsch MT, Al-Mourgi MA, Sugarbaker DJ. Complications of extrapleural pneumonectomy. Semin Thorac Cardiovasc Surg. 2007;19:355-359.
55. Zahid I, Sharif S, Routledge T, Scarci M. Is pleurectomy and decortication superior to palliative care in the treatment of malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg. 2011;12:812-817.
56. Soysal O, Karaoğlanoğlu N, Demiracan S, et al. Pleurectomy/decortication for palliation in malignant pleural mesothelioma: results of surgery. Eur J Cardiothorac Surg. 1997;11:210-213.
57. Putnam JB, Light RW, Rodriguez RM, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer. 1999;86:1992-1999.
58. Fysh ETH, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest. 2012;142:394-400.
59. Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med. 2018;198:839-849.
60. Davies CW, Traill ZC, Gleeson FV, Davies RJ. Intrapleural streptokinase in the management of malignant multiloculated pleural effusions. Chest. 1999;115:729-733.
61. Hsu LH, Soong TC, Feng AC, Liu MC. Intrapleural urokinase for the treatment of loculated malignant pleural effusions and trapped lungs in medically inoperable cancer patients. J Thorac Oncol. 2006;1:460-467.
62. Okur E, Baysungur V, Tezel C, et al. Streptokinase for malignant pleural effusions: a randomized controlled study. Asian Cardiovasc Thorac Ann. 2011;19:238-243.
63. Mishra EK, Clive AO, Wills GH, et al. Randomized controlled trial of urokinase versus placebo for nondraining malignant pleural effusion. Am J Respir Crit Care Med. 2018;197:502-508.
64. Reddy C, Ernst A, Lamb C, Feller-Kopman D. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest. 2011;139:1419-1423.
65. Krochmal R, Reddy C, Yarmus L, et al. Patient evaluation for rapid pleurodesis of malignant pleural effusions. J Thorac Dis. 2016;8:2538-2543.
66. Bhatnagar R, Zahan-Evans N, Kearney C, et al. A novel drug-eluting indwelling pleural catheter for the management of malignant effusions. Am J Respir Crit Care Med. 2018;197:136-138.
67. Penz ED, Mishra EK, Davies HE, Manns BJ, Miller RF, Rahman NM. Comparing cost of indwelling pleural catheter vs talc pleurodesis for malignant pleural effusion. Chest. 2014;146:991-1000.
68. Yang TS, Hsia DW, Chang DW. Patient- and hospital-level factors associated with readmission for malignant pleural effusion. J Oncol Pract. 2018;14:e547-e556.
Malignant pleural effusion (MPE) is a common clinical problem in patients with advanced stage cancer. Each year in the United States, more than 150,000 individuals are diagnosed with MPE, and there are approximately 126,000 admissions for MPE.1-3 Providing effective therapeutic management remains a challenge, and currently available therapeutic interventions are palliative rather than curative. This article, the second in a 2-part review of MPE, focuses on the available management options.
Therapeutic Thoracentesis
Evaluation of pleural fluid cytology is a crucial step in the diagnosis and staging of disease. As a result, large-volume fluid removal is often the first therapeutic intervention for patients who present with symptomatic effusions. A patient’s clinical response to therapeutic thoracentesis dictates which additional therapeutic options are appropriate for palliation. Lack of symptom relief suggests that other comorbid conditions or trapped lung physiology may be the primary cause of the patient’s symptoms and discourages more invasive interventions. Radiographic evidence of lung re-expansion after fluid removal is also an important predictor of success for potential pleurodesis.4,5
There are no absolute contraindications to thoracentesis. However, caution should be used for patients with risk factors that may predispose to complications of pneumothorax and bleeding, such as coagulopathy, treatment with anticoagulation medications, thrombocytopenia, platelet dysfunction (eg, antiplatelet medications, uremia), positive pressure ventilation, and small effusion size. These factors are only relative contraindications, however, as thoracentesis can still be safely performed by experienced operators using guidance technology such as ultrasonography.
A retrospective review of 1009 ultrasound-guided thoracenteses with risk factors of an international normalized ratio (INR) greater than 1.6, platelet values less than 50,000/μL, or both, reported an overall rate of hemorrhagic complication of 0.4%, with no difference between procedures performed with (n = 303) or without (n = 706) transfusion correction of coagulopathy or thrombocytopenia.6 A similar retrospective evaluation of 1076 ultrasound-guided thoracenteses, including 267 patients with an INR greater than 1.5 and 58 patients with a platelet count less than 50,000/μL, reported a 0% complication rate.7 Small case series have also demonstrated low hemorrhagic complication rates for thoracentesis in patients treated with clopidogrel8,9 and with increased bleeding risk from elevated INR (liver disease or warfarin therapy) and renal disease.10
Complications from pneumothorax can similarly be affected by patient- and operator-dependent risk factors. Meta-analysis of 24 studies including 6605 thoracenteses demonstrated an overall pneumothorax rate of 6.0%, with 34.1% requiring chest tube insertion.11 Lower pneumothorax rates were associated with the use of ultrasound guidance (odds ratio, 0.3; 95% confidence interval, 0.2-0.7). Experienced operators also had fewer pneumothorax complications, though this factor was not significant in the studies directly comparing this variable. Therapeutic thoracentesis and use of a larger-bore needle were also significantly correlated with pneumothorax, while mechanical ventilation had a nonsignificant trend towards increased risk.
Although there is no consensus on the volume of pleural fluid that may be safely removed, it is recommended not to remove more than 1.5 L during a procedure in order to avoid precipitating re-expansion pulmonary edema.2,12 However, re-expansion pulmonary edema rates remain low even when larger volumes are removed if the patient remains symptom-free during the procedure and pleural manometry pressure does not exceed –20 cm H2O.13 Patient symptoms alone, however, are neither a sensitive nor specific indicator that pleural pressures exceed –20 cm H2O.14 Use of excessive negative pressure during drainage, such as from a vacuum bottle, should also be avoided. Comparison of suction generated manually with a syringe versus a vacuum bottle suggests decreased complications with manual drainage, though the sample size in the supporting study was small relative to the infrequency of the complications being evaluated.15
Given the low morbidity and noninvasive nature of the procedure, serial large-volume thoracentesis remains a viable therapeutic intervention for patients who are unable or unwilling to undergo more invasive interventions, especially for patients with a slow fluid re-accumulation rate or who are anticipated to have limited survival. Unfortunately, many symptomatic effusions will recur within a short interval time span, which necessitates repeat procedures.16,17 Therefore, factors such as poor symptom control, patient inconvenience, recurrent procedural risk, and utilization of medical resources need to be considered as well.
Tunneled Pleural Catheter
Tunneled pleural catheters (TPCs) are a potentially permanent and minimally invasive therapy which allow intermittent drainage of pleural fluid (Figure 1). The catheter is tunneled under the skin to prevent infection. A polyester cuff attached to the catheter is positioned within the tunnel and induces fibrosis around the catheter, thereby securing the catheter in place. Placement can be performed under local anesthesia at the patient’s bedside or in an outpatient procedure space. Fluid can then be drained via specialized drainage bottles or bags by the patient, a family member, or visiting home nurse. The catheter can also be removed in the event of a complication or the development of spontaneous pleurodesis.

TPCs are an effective palliative management strategy for patients with recurrent effusions and are an efficacious alternative to pleurodesis.18-20 TPCs may be used in patients with poor prognosis or trapped lung or in those in whom prior pleurodesis has failed.21-23 Meta-analysis of 19 studies showed symptomatic improvement in 95.6% of patients, with development of spontaneous pleurodesis in 45.6% of patients (range, 11.8% to 76.4%) after an average of 52 days.24 However, most of the studies included in this analysis were retrospective case series. Development of spontaneous pleurodesis from TPC drainage in prospective, controlled trials has been considerably more modest, supporting a range of approximately 20% to 30% with routine drainage strategies.20,25-27 Spontaneous pleurodesis develops greater rapidity and frequency in patients undergoing daily drainage compared to every-other-day or symptom-directed drainage strategies.25,26 However, there is no appreciable improvement in quality of life scores with a specific drainage strategy. Small case series also demonstrate that TPC drainage may induce spontaneous pleurodesis in some patients initially presenting with trapped lung physiology.22
Catheter placement can be performed successfully in the vast majority of patients.28 Increased bleeding risk, significant malignancy-related involvement of the skin and chest wall, and pleural loculations can complicate TPC placement. TPC-related complications are relatively uncommon, but include pneumothorax, catheter malfunction and obstruction, and infections including soft tissue and pleural space infections.24 In a multicenter retrospective series of 1021 patients, only 4.9% developed a TPC-related pleural infection.29 Over 94% were successfully managed with antibiotic therapy, and the TPC was able to be preserved in 54%. Staphylococcus aureus was the most common causative organism and was identified in 48% of cases. Of note, spontaneous pleurodesis occurred in 62% of cases following a pleural space infection, which likely occurred as sequelae of the inflammatory nature of the infection. Retrospective analysis suggests that the risk of TPC-related infections is not substantially higher for patients with higher risks of immunosuppression from chemotherapy or hematologic malignancies.30,31 Tumor metastasis along the catheter tract is a rare occurrence (< 1%), but is most notable with mesothelioma, which has an incidence as high as 10%.24,32 In addition, development of pleural loculations can impede fluid drainage and relief of dyspnea. Intrapleural instillation of fibrinolytics can be used to improve drainage and improve symptom palliation.33
Pleurodesis
Pleurodesis obliterates the potential pleural space by inducing inflammation and fibrosis, resulting in adherence of the visceral and parietal pleura together. This process can be induced through mechanical abrasion of the pleural surface, introduction of chemical sclerosants, or from prolonged use of a chest tube. Chemical sclerosants are the most commonly used method for MPEs and are introduced through a chest tube or under visual guidance such as medical thoracoscopy or video-assisted thoracoscopic surgery (VATS). The pleurodesis process is thought to occur by induction of a systemic inflammatory response with localized deposition of fibrin.34 Activation of fibroblasts and successful pleurodesis have been correlated with higher basic fibroblast growth factor (bFGF) levels in pleural fluid.35 Increased tumor burden is associated with lower bFGF levels, suggesting a possible mechanism for reduced pleurodesis success in these cases. Corticosteroids may reduce the likelihood of pleurodesis due to a reduction of inflammation, as demonstrated in a rabbit model using talc and doxycycline.36,37 Animal data also suggests that use of nonsteroidal anti-inflammatory drugs may hinder the likelihood of successful pleurodesis, but this has not been observed in humans.38,39
Patients selected for pleurodesis should have significant symptom relief from large-volume removal of pleural fluid, good functional status, and evidence of full lung re-expansion after thoracentesis. Lack of visceral and parietal pleural apposition will prevent pleural adhesion from developing. As a result, trapped lung is associated with chemical pleurodesis failure and is an absolute contraindication to the procedure.4,5,12 The pleurodesis process typically requires 5 to 7 days, during which time the patient is hospitalized for chest tube drainage and pain control. When pleural fluid output diminishes, the chest tube is removed and the patient can be discharged. Modified protocols are now emerging which may shorten the required hospitalization associated with pleurodesis procedures.
Pleurodesis Agents
A variety of chemical sclerosants have been used for pleurodesis, including talc, bleomycin, tetracycline, doxycycline, iodopovidone, and mepacrine. Published data regarding these agents are heterogenous, with significant variability in reported outcomes. However, systematic review and meta-analysis suggests that talc is likely to have higher success rates compared to other agents or chest tube drainage alone for treatment of MPE.40,41
Additional factors have been shown to be associated with pleurodesis outcomes. Pleurodesis success is negatively associated with low pleural pH, with receiver operating curve thresholds of 7.28 to 7.34.42,43 Trapped lung, large bulky tumor lining the pleural surfaces, and elevated adenosine deaminase levels are also associated with poor pleurodesis outcomes.4,5,12,35,43 In contrast, pleural fluid output less than 200 mL per day and the presence of EGFR (epidermal growth factor receptor) mutation treated with targeted tyrosine kinase inhibitors are associated with successful pleurodesis.44,45
The most common complications associated with chemical pleurodesis are fever and pain. Other potential complications include soft tissue infections at the chest tube site and of the pleural space, arrhythmias, cardiac arrest, myocardial infarction, and hypotension. Doxycycline is commonly associated with greater pleuritic pain than talc. Acute respiratory distress syndrome (ARDS), acute pneumonitis, and respiratory failure have been described with talc pleurodesis. ARDS secondary to talc pleurodesis occurs in 1% to 9% of cases, though this may be related to the use of ungraded talc. A prospective description of 558 patients treated with large particle talc (> 5 μm) reported no occurrences of ARDS, suggesting the safety of graded large particle talc.46
Pleurodesis Methods
Chest tube thoracostomy is an inpatient procedure performed under local anesthesia or conscious sedation. It can be used for measured, intermittent drainage of large effusions for immediate symptom relief, as well as to demonstrate complete lung re-expansion prior to instillation of a chemical sclerosant. Pleurodesis using a chest tube is performed by instillation of a slurry created by mixing the sclerosing agent of choice with 50 to 100 mL of sterile saline. This slurry is instilled into the pleural cavity through the chest tube. The chest tube is clamped for 1 to 2 hours before being reconnected to suction. Intermittent rotation of the patient has not been shown to improve distribution of the sclerosant or result in better procedural outcomes.47,48 Typically, a 24F to 32F chest tube is used because of the concern about obstruction of smaller bore tubes by fibrin plugs. A noninferiority study comparing 12F to 24F chest tubes for talc pleurodesis demonstrated a higher procedure failure rate with the 12F tube (30% versus 24%) and failed to meet noninferiority criteria.39 However, larger caliber tubes are also associated with greater patient discomfort compared to smaller bore tubes.
Medical thoracoscopy and VATS are minimally invasive means to visualize the pleural space, obtain visually guided biopsy of the parietal pleura, perform lysis of adhesions, and introduce chemical sclerosants for pleurodesis (Figure 2). Medical thoracoscopy can be performed under local anesthesia with procedural sedation in an endoscopy suite or procedure room. 

VATS has several distinct and clinically important differences. The equipment is slightly larger but otherwise similar in concept to rigid medical thoracoscopes. A greater number of diagnostic and therapeutic options, such as diagnostic biopsy of lung parenchyma and select hilar lymph nodes, are also possible. However, VATS requires surgical training and is performed in an operating room setting, which necessitates additional ancillary and logistical support. VATS also uses at least 2 trocar insertion sites, requires general anesthesia, and utilizes single-lung ventilation through a double-lumen endotracheal tube. Procedure-related complications for medical thoracoscopy and VATS include pneumothorax, subcutaneous emphysema, fever, and pain.49
Data comparing talc slurry versus talc poudrage are heterogenous, without clear advantage for either method. Reported rates of successful pleurodesis are generally in the range of 70% to 80% for both methods.19,20,40,50 There is a possible suggestion of benefit with talc poudrage compared to slurry, but data is lacking to support either as a definitive choice in current guidelines.12,51 An advantage of medical thoracoscopy or VATS is that pleural biopsy can be performed simultaneously, if necessary, thereby allowing both diagnostic and therapeutic interventions.52 Visualizing the thoracic cavity may also permit creation of optimal conditions for pleurodesis in select individuals by allowing access to loculated spaces and providing visual confirmation of complete drainage of pleural fluid and uniform distribution of the chemical sclerosant.
Other Surgical Interventions
Thoracotomy with decortication is rarely used as treatment of malignant effusions complicated by loculations or trapped lung due to the significantly increased procedural morbidity and mortality. Therefore, it is reserved for the limited population of patients in whom other therapeutic interventions have failed but who otherwise have significant symptoms with a long life expectancy. Mesothelioma is a specific situation in which variations of pleurectomy, such as radical pleurectomy with decortication, lung-sparing total pleurectomy, and extrapleural pneumonectomy (EPP), have been used as front-line therapy. The Mesothelioma and Radical Surgery (MARS) trial, the only randomized, controlled evaluation of EPP, demonstrated decreased median survival in patients treated by EPP compared to controls (14.4 months versus 19.5 months).53 EPP is also associated with high procedure-related morbidity and mortality rates of approximately 50% and 4% to 15%, respectively.54 While successful at achieving pleurodesis, use of EPP as a treatment for mesothelioma is now discouraged.53,55 Less invasive surgical approaches, such as pleurectomy with decortication, are able to palliate symptoms with significantly less operative risk.56
Management Considerations
Selection of Therapeutic Interventions
The ideal management strategy provides both immediate and long-term symptom palliation, has minimal associated morbidity and side effects, minimizes hospitalization time and clinic visits, avoids the risks and inconvenience of recurring procedures, is inexpensive, and minimizes utilization of medical resources. Unfortunately, no single palliation methodology fits these needs for all patients. When evaluating therapeutic options for patients with MPE, it is important to consider factors such as the severity of symptoms, fluid quantity, fluid re-accumulation rate, pleural physiology, functional status, overall prognosis, and anticipated response of the malignancy to therapy. One example management algorithm (Figure 4) demonstrates the impact of these variables on the appropriate treatment options. However, this is a simplified algorithm and the selected palliation strategy should be decided upon after shared decision-making between the patient and physician and should fit within the context of the patient’s desired goals of care. It is also crucial for patients to understand that these therapeutic interventions are palliative rather than curative.

When compared directly with pleurodesis, TPC provides similar control of symptoms but with a reduction in hospital length of stay by a median of 3.5 to 5.5 days.19,57 In a nonrandomized trial where patients chose palliation by TPC or talc pleurodesis, more TPC patients had a significant immediate improvement in quality of life and dyspnea after the first 7 days of therapy.58 This is reasonably attributed to the differences between the immediate relief from fluid drainage after TPC placement compared to the time required for pleural symphysis to occur with pleurodesis. However, control of dyspnea symptoms is similar between the 2 strategies after 6 weeks.19 Therefore, both TPC and pleurodesis strategies are viewed as first-line options for patients with expandable lung and no prior palliative interventions for MPE.59
Pleural adhesions and trapped lung also pose specific dilemmas. Pleural adhesions can create loculated fluid pockets, thereby complicating drainage by thoracentesis or TPC and hindering dispersal of pleurodesis agents. Adhesiolysis by medical thoracoscopy or VATS may be useful in these patients to free up the pleural space and improve efficacy of long-term drainage options or facilitate pleurodesis. Intrapleural administration of fibrinolytics, such as streptokinase and urokinase, has also been used for treatment of loculated effusions and may improve drainage of pleural fluid and lung re-expansion.60-63 However routine use of intrapleural fibrinolytics with pleurodesis has not been shown to be beneficial. In a randomized comparison using intrapleural urokinase prior to pleurodesis for patients with septated malignant pleural effusions, no difference in pleurodesis outcomes were identified.63 As a result, TPC is the preferred palliation approach for patients with trapped lung physiology.51,59
Combination Strategies
Combinations of different therapeutic interventions are being evaluated as a means for patients to achieve long-term benefits from pleurodesis while minimizing hospitalization time. One strategy using simultaneous treatment with thoracoscopic talc poudrage and insertion of a large-bore chest tube and TPC has been shown to permit early removal of the chest tube and discharge home using the TPC for continued daily pleural drainage. This “rapid pleurodesis” strategy has an 80% to 90% successful pleurodesis rate, permitting removal of the TPC at a median of 7 to 10 days.64,65 With this approach, median hospitalization length of stay was approximately 2 days. While there was no control arm in these early reports with limited sample sizes, the pleurodesis success rate and length of hospitalization compare favorably to other published studies. A prospective, randomized trial of TPC versus an outpatient regimen of talc slurry via TPC has also shown promise, with successful pleurodesis after 35 days in 43% of those treated with the combination of talc slurry and TPC compared to only 23% in those treated by TPC alone.27
Another novel approach to obtain the benefits of both TPC and pleurodesis strategies is the use of drug-eluting TPC to induce inflammation and promote adhesion of the visceral and parietal pleura. An early report of slow-release silver nitrate (AgNO3) –coated TPC demonstrated an encouraging 89% spontaneous pleurodesis rate after a median of 4 days in the small subgroup of patients with fully expandable lung.66 Device-related adverse events were relatively high at 24.6%, though only one was deemed a serious adverse event. Additional studies of these novel and combination strategies are ongoing at this time.
Costs
While cost of care is not a consideration in the decision-making for individual patients, it is important from a systems-based perspective. Upfront costs for pleurodesis are generally higher due to the facility and hospitalization costs, whereas TPC have ongoing costs for drainage bottles and supplies. In a prospective, randomized trial of TPC versus talc pleurodesis, there was no appreciable difference in overall costs between the 2 approaches.67 The cost of TPC was significantly less, however, for patients with a shorter survival of less than 14 weeks.
Readmissions
Subsequent hospitalization requirements beyond just the initial treatment for a MPE remains another significant consideration for this patient population. A prospective, randomized trial comparing TPC to talc pleurodesis demonstrated a reduction in total all-cause hospital stay for TPC, with a median all-cause hospitalization time of 10 days for patients treated with TPC compared to 12 days for the talc pleurodesis group.20 The primary difference in the number of hospitalization days was due to a difference in effusion-related hospital days (median 1 versus 4 days, respectively), which was primarily comprised of the initial hospitalization. In addition, fewer patients treated with TPC required subsequent ipsilateral invasive procedures (4.1% versus 22.5%, respectively). However, it is important to note that the majority of all-cause hospital days were not effusion-related, demonstrating that this population has a high utilization of acute inpatient services for other reasons related to their advanced malignancy. In a study of regional hospitals in the United States, 38.3% of patients admitted for a primary diagnosis of MPE were readmitted within 30 days.68 There was remarkably little variability in readmission rates among hospitals, despite differences in factors such as institution size, location, patient distribution, and potential practice differences. This suggests that utilization of palliation strategies for MPE are only one component related to hospitalization in this population. Even at the best performing hospitals, there are significant common drivers for readmission that are not addressed. Therefore, additional effort should be focused on addressing aspects of care beyond just the palliation of MPE that predispose this population to requiring frequent treatment in an acute care setting.
Conclusion
MPEs represent advanced stage disease and frequently adversely affect a patient’s quality of life. The treating clinician has access to a variety of therapeutic options, though no single intervention strategy is universally superior in all circumstances. Initial thoracentesis is important in evaluating whether removal of a large volume of fluid provides significant symptom relief and restores functional status. Both talc pleurodesis and TPC provide similar control of symptoms and are first-line approaches for symptomatic patients with MPE and fully expandable lungs. Pleurodesis is associated with greater procedure-related risk and length of hospitalization and is contraindicated in patients with trapped lung, but does not require long-term catheter care or disposable resources. Determination of the appropriate therapeutic management strategy requires careful evaluation of the patient’s clinical situation and informed discussion with the patient to make sure that the treatment plan fits within the context of their goals of medical care.
Malignant pleural effusion (MPE) is a common clinical problem in patients with advanced stage cancer. Each year in the United States, more than 150,000 individuals are diagnosed with MPE, and there are approximately 126,000 admissions for MPE.1-3 Providing effective therapeutic management remains a challenge, and currently available therapeutic interventions are palliative rather than curative. This article, the second in a 2-part review of MPE, focuses on the available management options.
Therapeutic Thoracentesis
Evaluation of pleural fluid cytology is a crucial step in the diagnosis and staging of disease. As a result, large-volume fluid removal is often the first therapeutic intervention for patients who present with symptomatic effusions. A patient’s clinical response to therapeutic thoracentesis dictates which additional therapeutic options are appropriate for palliation. Lack of symptom relief suggests that other comorbid conditions or trapped lung physiology may be the primary cause of the patient’s symptoms and discourages more invasive interventions. Radiographic evidence of lung re-expansion after fluid removal is also an important predictor of success for potential pleurodesis.4,5
There are no absolute contraindications to thoracentesis. However, caution should be used for patients with risk factors that may predispose to complications of pneumothorax and bleeding, such as coagulopathy, treatment with anticoagulation medications, thrombocytopenia, platelet dysfunction (eg, antiplatelet medications, uremia), positive pressure ventilation, and small effusion size. These factors are only relative contraindications, however, as thoracentesis can still be safely performed by experienced operators using guidance technology such as ultrasonography.
A retrospective review of 1009 ultrasound-guided thoracenteses with risk factors of an international normalized ratio (INR) greater than 1.6, platelet values less than 50,000/μL, or both, reported an overall rate of hemorrhagic complication of 0.4%, with no difference between procedures performed with (n = 303) or without (n = 706) transfusion correction of coagulopathy or thrombocytopenia.6 A similar retrospective evaluation of 1076 ultrasound-guided thoracenteses, including 267 patients with an INR greater than 1.5 and 58 patients with a platelet count less than 50,000/μL, reported a 0% complication rate.7 Small case series have also demonstrated low hemorrhagic complication rates for thoracentesis in patients treated with clopidogrel8,9 and with increased bleeding risk from elevated INR (liver disease or warfarin therapy) and renal disease.10
Complications from pneumothorax can similarly be affected by patient- and operator-dependent risk factors. Meta-analysis of 24 studies including 6605 thoracenteses demonstrated an overall pneumothorax rate of 6.0%, with 34.1% requiring chest tube insertion.11 Lower pneumothorax rates were associated with the use of ultrasound guidance (odds ratio, 0.3; 95% confidence interval, 0.2-0.7). Experienced operators also had fewer pneumothorax complications, though this factor was not significant in the studies directly comparing this variable. Therapeutic thoracentesis and use of a larger-bore needle were also significantly correlated with pneumothorax, while mechanical ventilation had a nonsignificant trend towards increased risk.
Although there is no consensus on the volume of pleural fluid that may be safely removed, it is recommended not to remove more than 1.5 L during a procedure in order to avoid precipitating re-expansion pulmonary edema.2,12 However, re-expansion pulmonary edema rates remain low even when larger volumes are removed if the patient remains symptom-free during the procedure and pleural manometry pressure does not exceed –20 cm H2O.13 Patient symptoms alone, however, are neither a sensitive nor specific indicator that pleural pressures exceed –20 cm H2O.14 Use of excessive negative pressure during drainage, such as from a vacuum bottle, should also be avoided. Comparison of suction generated manually with a syringe versus a vacuum bottle suggests decreased complications with manual drainage, though the sample size in the supporting study was small relative to the infrequency of the complications being evaluated.15
Given the low morbidity and noninvasive nature of the procedure, serial large-volume thoracentesis remains a viable therapeutic intervention for patients who are unable or unwilling to undergo more invasive interventions, especially for patients with a slow fluid re-accumulation rate or who are anticipated to have limited survival. Unfortunately, many symptomatic effusions will recur within a short interval time span, which necessitates repeat procedures.16,17 Therefore, factors such as poor symptom control, patient inconvenience, recurrent procedural risk, and utilization of medical resources need to be considered as well.
Tunneled Pleural Catheter
Tunneled pleural catheters (TPCs) are a potentially permanent and minimally invasive therapy which allow intermittent drainage of pleural fluid (Figure 1). The catheter is tunneled under the skin to prevent infection. A polyester cuff attached to the catheter is positioned within the tunnel and induces fibrosis around the catheter, thereby securing the catheter in place. Placement can be performed under local anesthesia at the patient’s bedside or in an outpatient procedure space. Fluid can then be drained via specialized drainage bottles or bags by the patient, a family member, or visiting home nurse. The catheter can also be removed in the event of a complication or the development of spontaneous pleurodesis.

TPCs are an effective palliative management strategy for patients with recurrent effusions and are an efficacious alternative to pleurodesis.18-20 TPCs may be used in patients with poor prognosis or trapped lung or in those in whom prior pleurodesis has failed.21-23 Meta-analysis of 19 studies showed symptomatic improvement in 95.6% of patients, with development of spontaneous pleurodesis in 45.6% of patients (range, 11.8% to 76.4%) after an average of 52 days.24 However, most of the studies included in this analysis were retrospective case series. Development of spontaneous pleurodesis from TPC drainage in prospective, controlled trials has been considerably more modest, supporting a range of approximately 20% to 30% with routine drainage strategies.20,25-27 Spontaneous pleurodesis develops greater rapidity and frequency in patients undergoing daily drainage compared to every-other-day or symptom-directed drainage strategies.25,26 However, there is no appreciable improvement in quality of life scores with a specific drainage strategy. Small case series also demonstrate that TPC drainage may induce spontaneous pleurodesis in some patients initially presenting with trapped lung physiology.22
Catheter placement can be performed successfully in the vast majority of patients.28 Increased bleeding risk, significant malignancy-related involvement of the skin and chest wall, and pleural loculations can complicate TPC placement. TPC-related complications are relatively uncommon, but include pneumothorax, catheter malfunction and obstruction, and infections including soft tissue and pleural space infections.24 In a multicenter retrospective series of 1021 patients, only 4.9% developed a TPC-related pleural infection.29 Over 94% were successfully managed with antibiotic therapy, and the TPC was able to be preserved in 54%. Staphylococcus aureus was the most common causative organism and was identified in 48% of cases. Of note, spontaneous pleurodesis occurred in 62% of cases following a pleural space infection, which likely occurred as sequelae of the inflammatory nature of the infection. Retrospective analysis suggests that the risk of TPC-related infections is not substantially higher for patients with higher risks of immunosuppression from chemotherapy or hematologic malignancies.30,31 Tumor metastasis along the catheter tract is a rare occurrence (< 1%), but is most notable with mesothelioma, which has an incidence as high as 10%.24,32 In addition, development of pleural loculations can impede fluid drainage and relief of dyspnea. Intrapleural instillation of fibrinolytics can be used to improve drainage and improve symptom palliation.33
Pleurodesis
Pleurodesis obliterates the potential pleural space by inducing inflammation and fibrosis, resulting in adherence of the visceral and parietal pleura together. This process can be induced through mechanical abrasion of the pleural surface, introduction of chemical sclerosants, or from prolonged use of a chest tube. Chemical sclerosants are the most commonly used method for MPEs and are introduced through a chest tube or under visual guidance such as medical thoracoscopy or video-assisted thoracoscopic surgery (VATS). The pleurodesis process is thought to occur by induction of a systemic inflammatory response with localized deposition of fibrin.34 Activation of fibroblasts and successful pleurodesis have been correlated with higher basic fibroblast growth factor (bFGF) levels in pleural fluid.35 Increased tumor burden is associated with lower bFGF levels, suggesting a possible mechanism for reduced pleurodesis success in these cases. Corticosteroids may reduce the likelihood of pleurodesis due to a reduction of inflammation, as demonstrated in a rabbit model using talc and doxycycline.36,37 Animal data also suggests that use of nonsteroidal anti-inflammatory drugs may hinder the likelihood of successful pleurodesis, but this has not been observed in humans.38,39
Patients selected for pleurodesis should have significant symptom relief from large-volume removal of pleural fluid, good functional status, and evidence of full lung re-expansion after thoracentesis. Lack of visceral and parietal pleural apposition will prevent pleural adhesion from developing. As a result, trapped lung is associated with chemical pleurodesis failure and is an absolute contraindication to the procedure.4,5,12 The pleurodesis process typically requires 5 to 7 days, during which time the patient is hospitalized for chest tube drainage and pain control. When pleural fluid output diminishes, the chest tube is removed and the patient can be discharged. Modified protocols are now emerging which may shorten the required hospitalization associated with pleurodesis procedures.
Pleurodesis Agents
A variety of chemical sclerosants have been used for pleurodesis, including talc, bleomycin, tetracycline, doxycycline, iodopovidone, and mepacrine. Published data regarding these agents are heterogenous, with significant variability in reported outcomes. However, systematic review and meta-analysis suggests that talc is likely to have higher success rates compared to other agents or chest tube drainage alone for treatment of MPE.40,41
Additional factors have been shown to be associated with pleurodesis outcomes. Pleurodesis success is negatively associated with low pleural pH, with receiver operating curve thresholds of 7.28 to 7.34.42,43 Trapped lung, large bulky tumor lining the pleural surfaces, and elevated adenosine deaminase levels are also associated with poor pleurodesis outcomes.4,5,12,35,43 In contrast, pleural fluid output less than 200 mL per day and the presence of EGFR (epidermal growth factor receptor) mutation treated with targeted tyrosine kinase inhibitors are associated with successful pleurodesis.44,45
The most common complications associated with chemical pleurodesis are fever and pain. Other potential complications include soft tissue infections at the chest tube site and of the pleural space, arrhythmias, cardiac arrest, myocardial infarction, and hypotension. Doxycycline is commonly associated with greater pleuritic pain than talc. Acute respiratory distress syndrome (ARDS), acute pneumonitis, and respiratory failure have been described with talc pleurodesis. ARDS secondary to talc pleurodesis occurs in 1% to 9% of cases, though this may be related to the use of ungraded talc. A prospective description of 558 patients treated with large particle talc (> 5 μm) reported no occurrences of ARDS, suggesting the safety of graded large particle talc.46
Pleurodesis Methods
Chest tube thoracostomy is an inpatient procedure performed under local anesthesia or conscious sedation. It can be used for measured, intermittent drainage of large effusions for immediate symptom relief, as well as to demonstrate complete lung re-expansion prior to instillation of a chemical sclerosant. Pleurodesis using a chest tube is performed by instillation of a slurry created by mixing the sclerosing agent of choice with 50 to 100 mL of sterile saline. This slurry is instilled into the pleural cavity through the chest tube. The chest tube is clamped for 1 to 2 hours before being reconnected to suction. Intermittent rotation of the patient has not been shown to improve distribution of the sclerosant or result in better procedural outcomes.47,48 Typically, a 24F to 32F chest tube is used because of the concern about obstruction of smaller bore tubes by fibrin plugs. A noninferiority study comparing 12F to 24F chest tubes for talc pleurodesis demonstrated a higher procedure failure rate with the 12F tube (30% versus 24%) and failed to meet noninferiority criteria.39 However, larger caliber tubes are also associated with greater patient discomfort compared to smaller bore tubes.
Medical thoracoscopy and VATS are minimally invasive means to visualize the pleural space, obtain visually guided biopsy of the parietal pleura, perform lysis of adhesions, and introduce chemical sclerosants for pleurodesis (Figure 2). Medical thoracoscopy can be performed under local anesthesia with procedural sedation in an endoscopy suite or procedure room. 

VATS has several distinct and clinically important differences. The equipment is slightly larger but otherwise similar in concept to rigid medical thoracoscopes. A greater number of diagnostic and therapeutic options, such as diagnostic biopsy of lung parenchyma and select hilar lymph nodes, are also possible. However, VATS requires surgical training and is performed in an operating room setting, which necessitates additional ancillary and logistical support. VATS also uses at least 2 trocar insertion sites, requires general anesthesia, and utilizes single-lung ventilation through a double-lumen endotracheal tube. Procedure-related complications for medical thoracoscopy and VATS include pneumothorax, subcutaneous emphysema, fever, and pain.49
Data comparing talc slurry versus talc poudrage are heterogenous, without clear advantage for either method. Reported rates of successful pleurodesis are generally in the range of 70% to 80% for both methods.19,20,40,50 There is a possible suggestion of benefit with talc poudrage compared to slurry, but data is lacking to support either as a definitive choice in current guidelines.12,51 An advantage of medical thoracoscopy or VATS is that pleural biopsy can be performed simultaneously, if necessary, thereby allowing both diagnostic and therapeutic interventions.52 Visualizing the thoracic cavity may also permit creation of optimal conditions for pleurodesis in select individuals by allowing access to loculated spaces and providing visual confirmation of complete drainage of pleural fluid and uniform distribution of the chemical sclerosant.
Other Surgical Interventions
Thoracotomy with decortication is rarely used as treatment of malignant effusions complicated by loculations or trapped lung due to the significantly increased procedural morbidity and mortality. Therefore, it is reserved for the limited population of patients in whom other therapeutic interventions have failed but who otherwise have significant symptoms with a long life expectancy. Mesothelioma is a specific situation in which variations of pleurectomy, such as radical pleurectomy with decortication, lung-sparing total pleurectomy, and extrapleural pneumonectomy (EPP), have been used as front-line therapy. The Mesothelioma and Radical Surgery (MARS) trial, the only randomized, controlled evaluation of EPP, demonstrated decreased median survival in patients treated by EPP compared to controls (14.4 months versus 19.5 months).53 EPP is also associated with high procedure-related morbidity and mortality rates of approximately 50% and 4% to 15%, respectively.54 While successful at achieving pleurodesis, use of EPP as a treatment for mesothelioma is now discouraged.53,55 Less invasive surgical approaches, such as pleurectomy with decortication, are able to palliate symptoms with significantly less operative risk.56
Management Considerations
Selection of Therapeutic Interventions
The ideal management strategy provides both immediate and long-term symptom palliation, has minimal associated morbidity and side effects, minimizes hospitalization time and clinic visits, avoids the risks and inconvenience of recurring procedures, is inexpensive, and minimizes utilization of medical resources. Unfortunately, no single palliation methodology fits these needs for all patients. When evaluating therapeutic options for patients with MPE, it is important to consider factors such as the severity of symptoms, fluid quantity, fluid re-accumulation rate, pleural physiology, functional status, overall prognosis, and anticipated response of the malignancy to therapy. One example management algorithm (Figure 4) demonstrates the impact of these variables on the appropriate treatment options. However, this is a simplified algorithm and the selected palliation strategy should be decided upon after shared decision-making between the patient and physician and should fit within the context of the patient’s desired goals of care. It is also crucial for patients to understand that these therapeutic interventions are palliative rather than curative.

When compared directly with pleurodesis, TPC provides similar control of symptoms but with a reduction in hospital length of stay by a median of 3.5 to 5.5 days.19,57 In a nonrandomized trial where patients chose palliation by TPC or talc pleurodesis, more TPC patients had a significant immediate improvement in quality of life and dyspnea after the first 7 days of therapy.58 This is reasonably attributed to the differences between the immediate relief from fluid drainage after TPC placement compared to the time required for pleural symphysis to occur with pleurodesis. However, control of dyspnea symptoms is similar between the 2 strategies after 6 weeks.19 Therefore, both TPC and pleurodesis strategies are viewed as first-line options for patients with expandable lung and no prior palliative interventions for MPE.59
Pleural adhesions and trapped lung also pose specific dilemmas. Pleural adhesions can create loculated fluid pockets, thereby complicating drainage by thoracentesis or TPC and hindering dispersal of pleurodesis agents. Adhesiolysis by medical thoracoscopy or VATS may be useful in these patients to free up the pleural space and improve efficacy of long-term drainage options or facilitate pleurodesis. Intrapleural administration of fibrinolytics, such as streptokinase and urokinase, has also been used for treatment of loculated effusions and may improve drainage of pleural fluid and lung re-expansion.60-63 However routine use of intrapleural fibrinolytics with pleurodesis has not been shown to be beneficial. In a randomized comparison using intrapleural urokinase prior to pleurodesis for patients with septated malignant pleural effusions, no difference in pleurodesis outcomes were identified.63 As a result, TPC is the preferred palliation approach for patients with trapped lung physiology.51,59
Combination Strategies
Combinations of different therapeutic interventions are being evaluated as a means for patients to achieve long-term benefits from pleurodesis while minimizing hospitalization time. One strategy using simultaneous treatment with thoracoscopic talc poudrage and insertion of a large-bore chest tube and TPC has been shown to permit early removal of the chest tube and discharge home using the TPC for continued daily pleural drainage. This “rapid pleurodesis” strategy has an 80% to 90% successful pleurodesis rate, permitting removal of the TPC at a median of 7 to 10 days.64,65 With this approach, median hospitalization length of stay was approximately 2 days. While there was no control arm in these early reports with limited sample sizes, the pleurodesis success rate and length of hospitalization compare favorably to other published studies. A prospective, randomized trial of TPC versus an outpatient regimen of talc slurry via TPC has also shown promise, with successful pleurodesis after 35 days in 43% of those treated with the combination of talc slurry and TPC compared to only 23% in those treated by TPC alone.27
Another novel approach to obtain the benefits of both TPC and pleurodesis strategies is the use of drug-eluting TPC to induce inflammation and promote adhesion of the visceral and parietal pleura. An early report of slow-release silver nitrate (AgNO3) –coated TPC demonstrated an encouraging 89% spontaneous pleurodesis rate after a median of 4 days in the small subgroup of patients with fully expandable lung.66 Device-related adverse events were relatively high at 24.6%, though only one was deemed a serious adverse event. Additional studies of these novel and combination strategies are ongoing at this time.
Costs
While cost of care is not a consideration in the decision-making for individual patients, it is important from a systems-based perspective. Upfront costs for pleurodesis are generally higher due to the facility and hospitalization costs, whereas TPC have ongoing costs for drainage bottles and supplies. In a prospective, randomized trial of TPC versus talc pleurodesis, there was no appreciable difference in overall costs between the 2 approaches.67 The cost of TPC was significantly less, however, for patients with a shorter survival of less than 14 weeks.
Readmissions
Subsequent hospitalization requirements beyond just the initial treatment for a MPE remains another significant consideration for this patient population. A prospective, randomized trial comparing TPC to talc pleurodesis demonstrated a reduction in total all-cause hospital stay for TPC, with a median all-cause hospitalization time of 10 days for patients treated with TPC compared to 12 days for the talc pleurodesis group.20 The primary difference in the number of hospitalization days was due to a difference in effusion-related hospital days (median 1 versus 4 days, respectively), which was primarily comprised of the initial hospitalization. In addition, fewer patients treated with TPC required subsequent ipsilateral invasive procedures (4.1% versus 22.5%, respectively). However, it is important to note that the majority of all-cause hospital days were not effusion-related, demonstrating that this population has a high utilization of acute inpatient services for other reasons related to their advanced malignancy. In a study of regional hospitals in the United States, 38.3% of patients admitted for a primary diagnosis of MPE were readmitted within 30 days.68 There was remarkably little variability in readmission rates among hospitals, despite differences in factors such as institution size, location, patient distribution, and potential practice differences. This suggests that utilization of palliation strategies for MPE are only one component related to hospitalization in this population. Even at the best performing hospitals, there are significant common drivers for readmission that are not addressed. Therefore, additional effort should be focused on addressing aspects of care beyond just the palliation of MPE that predispose this population to requiring frequent treatment in an acute care setting.
Conclusion
MPEs represent advanced stage disease and frequently adversely affect a patient’s quality of life. The treating clinician has access to a variety of therapeutic options, though no single intervention strategy is universally superior in all circumstances. Initial thoracentesis is important in evaluating whether removal of a large volume of fluid provides significant symptom relief and restores functional status. Both talc pleurodesis and TPC provide similar control of symptoms and are first-line approaches for symptomatic patients with MPE and fully expandable lungs. Pleurodesis is associated with greater procedure-related risk and length of hospitalization and is contraindicated in patients with trapped lung, but does not require long-term catheter care or disposable resources. Determination of the appropriate therapeutic management strategy requires careful evaluation of the patient’s clinical situation and informed discussion with the patient to make sure that the treatment plan fits within the context of their goals of medical care.
1. Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J. 2001;18:402-419.
2. Society AT. Management of malignant pleural effusions. Am J Respir Crit Care Med. 2000;162:1987-2001.
3. Taghizadeh N, Fortin M, Tremblay A. US hospitalizations for malignant pleural effusions: data from the 2012 National Inpatient Sample. Chest. 2017;151:845-854.
4. Adler RH, Sayek I. Treatment of malignant pleural effusion: a method using tube thoracostomy and talc. Ann Thorac Surg. 1976;22:8-15.
5. Villanueva AG, Gray AW, Shahian DM, et al. Efficacy of short term versus long term tube thoracostomy drainage before tetracycline pleurodesis in the treatment of malignant pleural effusions. Thorax. 1994;49:23-25.
6. Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest. 2013;144:456-463.
7. Patel MD, Joshi SD. Abnormal preprocedural international normalized ratio and platelet counts are not associated with increased bleeding complications after ultrasound-guided thoracentesis. AJR Am J Roentgenol. 2011;197:W164-168.
8. Zalt MB, Bechara RI, Parks C, Berkowitz DM. Effect of routine clopidogrel use on bleeding complications after ultrasound-guided thoracentesis. J Bronchology Interv Pulmonol. 2012;19:284-287.
9. Mahmood K, Shofer SL, Moser BK, et al. Hemorrhagic complications of thoracentesis and small-bore chest tube placement in patients taking clopidogrel. Ann Am Thorac Soc. 2014;11:73-79.
10. Puchalski JT, Argento AC, Murphy TE, et al. The safety of thoracentesis in patients with uncorrected bleeding risk. Ann Am Thorac Soc. 2013;10:336-341.
11. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170:332-339.
12. Roberts ME, Neville E, Berrisford RG, et al; Group BPDG. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii32-40.
13. Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84:1656-1661.
14. Feller-Kopman D, Walkey A, Berkowitz D, Ernst A. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest. 2006;129:1556-1560.
15. Senitko M, Ray AS, Murphy TE, et al. Safety and tolerability of vacuum versus manual drainage during thoracentesis: a randomized trial. J Bronchology Interv Pulmonol. 2019;26:166-171.
16. Ost DE, Niu J, Zhao H, et al. Quality gaps and comparative effectiveness of management strategies for recurrent malignant pleural effusions. Chest. 2018;153:438-452.
17. Grosu HB, Molina S, Casal R, et al. Risk factors for pleural effusion recurrence in patients with malignancy. Respirology. 2019;24:76-82.
18. Musani AI, Haas AR, Seijo L, et al. Outpatient management of malignant pleural effusions with small-bore, tunneled pleural catheters. Respiration. 2004;71:559-566.
19. Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307:2383-2389.
20. Thomas R, Fysh ETH, Smith NA, et al. Effect of an indwelling pleural catheter vs talc pleurodesis on hospitalization days in patients with malignant pleural effusion: the AMPLE randomized clinical trial. JAMA. 2017;318:1903-1912.
21. Qureshi RA, Collinson SL, Powell RJ, et al. Management of malignant pleural effusion associated with trapped lung syndrome. Asian Cardiovasc Thorac Ann. 2008;16:120-123.
22. Efthymiou CA, Masudi T, Thorpe JA, Papagiannopoulos K. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg. 2009;9:961-964.
23. Sioris T, Sihvo E, Salo J, et al. Long-term indwelling pleural catheter (PleurX) for malignant pleural effusion unsuitable for talc pleurodesis. Eur J Surg Oncol. 2009;35:546-551.
24. Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med. 2011;26:70-76.
25. Wahidi MM, Reddy C, Yarmus L, et al. Randomized trial of pleural fluid drainage frequency in patients with malignant pleural effusions. the ASAP trial. Am J Respir Crit Care Med. 2017;195:1050-1057.
26. Muruganandan S, Azzopardi M, Fitzgerald DB, et al. Aggressive versus symptom-guided drainage of malignant pleural effusion via indwelling pleural catheters (AMPLE-2): an open-label randomised trial. Lancet Respir Med. 2018;6:671-680.
27. Bhatnagar R, Keenan EK, Morley AJ, et al. Outpatient talc administration by indwelling pleural catheter for malignant effusion. N Engl J Med. 2018;378:1313-1322.
28. Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest. 2006;129:362-368.
29. Fysh ETH, Tremblay A, Feller-Kopman D, et al. Clinical outcomes of indwelling pleural catheter-related pleural infections: an international multicenter study. Chest. 2013;144:1597-1602.
30. Morel A, Mishra E, Medley L, et al. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax. 2011;66:448-449.
31. Gilbert CR, Lee HJ, Skalski JH, et al. The use of indwelling tunneled pleural catheters for recurrent pleural effusions in patients with hematologic malignancies: a multicenter study. Chest. 2015;148:752-758.
32. Thomas R, Budgeon CA, Kuok YJ, et al. Catheter tract metastasis associated with indwelling pleural catheters. Chest. 2014;146:557-562.
33. Thomas R, Piccolo F, Miller D, et al. Intrapleural fibrinolysis for the treatment of indwelling pleural catheter-related symptomatic loculations: a multicenter observational study. Chest. 2015;148:746-751.
34. Antony VB. Pathogenesis of malignant pleural effusions and talc pleurodesis. Pneumologie. 1999;53:493-498.
35. Antony VB, Nasreen N, Mohammed KA, et al. Talc pleurodesis: basic fibroblast growth factor mediates pleural fibrosis. Chest. 2004;126:1522-1528.
36. Xie C, Teixeira LR, McGovern JP, Light RW. Systemic corticosteroids decrease the effectiveness of talc pleurodesis. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1441-1444.
37. Teixeira LR, Wu W, Chang DS, Light RW. The effect of corticosteroids on pleurodesis induced by doxycycline in rabbits. Chest. 2002;121:216-219.
38. Hunt I, Teh E, Southon R, Treasure T. Using non-steroidal anti-inflammatory drugs (NSAIDs) following pleurodesis. Interact Cardiovasc Thorac Surg. 2007;6:102-104.
39. Rahman NM, Pepperell J, Rehal S, et al. Effect of opioids vs NSAIDs and larger vs smaller chest tube size on pain control and pleurodesis efficacy among patients with malignant pleural effusion: the TIME1 randomized clinical trial. JAMA. 2015;314:2641-2653.
40. Clive AO, Jones HE, Bhatnagar R, Preston NJ, Maskell N. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev. 2016(5):CD010529.
41. Tan C, Sedrakyan A, Browne J, et al. The evidence on the effectiveness of management for malignant pleural effusion: a systematic review. Eur J Cardiothorac Surg. 2006;29:829-838.
42. Heffner JE, Nietert PJ, Barbieri C. Pleural fluid pH as a predictor of pleurodesis failure: analysis of primary data. Chest. 2000;117:87-95.
43. Yildirim H, Metintas M, Ak G, et al. Predictors of talc pleurodesis outcome in patients with malignant pleural effusions. Lung Cancer. 2008;62:139-144.
44. Aydogmus U, Ozdemir S, Cansever L, et al. Bedside talc pleurodesis for malignant pleural effusion: factors affecting success. Ann Surg Oncol. 2009;16:745-750.
45. Guo H, Wan Y, Tian G, et al. EGFR mutations predict a favorable outcome for malignant pleural effusion of lung adenocarcinoma with Tarceva therapy. Oncol Rep. 2012;27:880-890.
46. Janssen JP, Collier G, Astoul P, et al. Safety of pleurodesis with talc poudrage in malignant pleural effusion: a prospective cohort study. Lancet. 2007;369(9572):1535-1539.
47. Dryzer SR, Allen ML, Strange C, Sahn SA. A comparison of rotation and nonrotation in tetracycline pleurodesis. Chest. 1993;104:1763-1766.
48. Mager HJ, Maesen B, Verzijlbergen F, Schramel F. Distribution of talc suspension during treatment of malignant pleural effusion with talc pleurodesis. Lung Cancer. 2002;36:77-81.
49. Hsia D, Musani AI. Interventional pulmonology. Med Clin North Am. 2011;95:1095-1114.
50. Dresler CM, Olak J, Herndon JE, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest. 2005;127:909-915.
51. Bibby AC, Dorn P, Psallidas I, et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur Respir J. 2018;52(1).
52. Sakuraba M, Masuda K, Hebisawa A, et al. Diagnostic value of thoracoscopic pleural biopsy for pleurisy under local anaesthesia. ANZ J Surg. 2006;76:722-724.
53. Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011;12:763-772.
54. Zellos L, Jaklitsch MT, Al-Mourgi MA, Sugarbaker DJ. Complications of extrapleural pneumonectomy. Semin Thorac Cardiovasc Surg. 2007;19:355-359.
55. Zahid I, Sharif S, Routledge T, Scarci M. Is pleurectomy and decortication superior to palliative care in the treatment of malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg. 2011;12:812-817.
56. Soysal O, Karaoğlanoğlu N, Demiracan S, et al. Pleurectomy/decortication for palliation in malignant pleural mesothelioma: results of surgery. Eur J Cardiothorac Surg. 1997;11:210-213.
57. Putnam JB, Light RW, Rodriguez RM, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer. 1999;86:1992-1999.
58. Fysh ETH, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest. 2012;142:394-400.
59. Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med. 2018;198:839-849.
60. Davies CW, Traill ZC, Gleeson FV, Davies RJ. Intrapleural streptokinase in the management of malignant multiloculated pleural effusions. Chest. 1999;115:729-733.
61. Hsu LH, Soong TC, Feng AC, Liu MC. Intrapleural urokinase for the treatment of loculated malignant pleural effusions and trapped lungs in medically inoperable cancer patients. J Thorac Oncol. 2006;1:460-467.
62. Okur E, Baysungur V, Tezel C, et al. Streptokinase for malignant pleural effusions: a randomized controlled study. Asian Cardiovasc Thorac Ann. 2011;19:238-243.
63. Mishra EK, Clive AO, Wills GH, et al. Randomized controlled trial of urokinase versus placebo for nondraining malignant pleural effusion. Am J Respir Crit Care Med. 2018;197:502-508.
64. Reddy C, Ernst A, Lamb C, Feller-Kopman D. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest. 2011;139:1419-1423.
65. Krochmal R, Reddy C, Yarmus L, et al. Patient evaluation for rapid pleurodesis of malignant pleural effusions. J Thorac Dis. 2016;8:2538-2543.
66. Bhatnagar R, Zahan-Evans N, Kearney C, et al. A novel drug-eluting indwelling pleural catheter for the management of malignant effusions. Am J Respir Crit Care Med. 2018;197:136-138.
67. Penz ED, Mishra EK, Davies HE, Manns BJ, Miller RF, Rahman NM. Comparing cost of indwelling pleural catheter vs talc pleurodesis for malignant pleural effusion. Chest. 2014;146:991-1000.
68. Yang TS, Hsia DW, Chang DW. Patient- and hospital-level factors associated with readmission for malignant pleural effusion. J Oncol Pract. 2018;14:e547-e556.
1. Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J. 2001;18:402-419.
2. Society AT. Management of malignant pleural effusions. Am J Respir Crit Care Med. 2000;162:1987-2001.
3. Taghizadeh N, Fortin M, Tremblay A. US hospitalizations for malignant pleural effusions: data from the 2012 National Inpatient Sample. Chest. 2017;151:845-854.
4. Adler RH, Sayek I. Treatment of malignant pleural effusion: a method using tube thoracostomy and talc. Ann Thorac Surg. 1976;22:8-15.
5. Villanueva AG, Gray AW, Shahian DM, et al. Efficacy of short term versus long term tube thoracostomy drainage before tetracycline pleurodesis in the treatment of malignant pleural effusions. Thorax. 1994;49:23-25.
6. Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest. 2013;144:456-463.
7. Patel MD, Joshi SD. Abnormal preprocedural international normalized ratio and platelet counts are not associated with increased bleeding complications after ultrasound-guided thoracentesis. AJR Am J Roentgenol. 2011;197:W164-168.
8. Zalt MB, Bechara RI, Parks C, Berkowitz DM. Effect of routine clopidogrel use on bleeding complications after ultrasound-guided thoracentesis. J Bronchology Interv Pulmonol. 2012;19:284-287.
9. Mahmood K, Shofer SL, Moser BK, et al. Hemorrhagic complications of thoracentesis and small-bore chest tube placement in patients taking clopidogrel. Ann Am Thorac Soc. 2014;11:73-79.
10. Puchalski JT, Argento AC, Murphy TE, et al. The safety of thoracentesis in patients with uncorrected bleeding risk. Ann Am Thorac Soc. 2013;10:336-341.
11. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170:332-339.
12. Roberts ME, Neville E, Berrisford RG, et al; Group BPDG. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii32-40.
13. Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84:1656-1661.
14. Feller-Kopman D, Walkey A, Berkowitz D, Ernst A. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest. 2006;129:1556-1560.
15. Senitko M, Ray AS, Murphy TE, et al. Safety and tolerability of vacuum versus manual drainage during thoracentesis: a randomized trial. J Bronchology Interv Pulmonol. 2019;26:166-171.
16. Ost DE, Niu J, Zhao H, et al. Quality gaps and comparative effectiveness of management strategies for recurrent malignant pleural effusions. Chest. 2018;153:438-452.
17. Grosu HB, Molina S, Casal R, et al. Risk factors for pleural effusion recurrence in patients with malignancy. Respirology. 2019;24:76-82.
18. Musani AI, Haas AR, Seijo L, et al. Outpatient management of malignant pleural effusions with small-bore, tunneled pleural catheters. Respiration. 2004;71:559-566.
19. Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307:2383-2389.
20. Thomas R, Fysh ETH, Smith NA, et al. Effect of an indwelling pleural catheter vs talc pleurodesis on hospitalization days in patients with malignant pleural effusion: the AMPLE randomized clinical trial. JAMA. 2017;318:1903-1912.
21. Qureshi RA, Collinson SL, Powell RJ, et al. Management of malignant pleural effusion associated with trapped lung syndrome. Asian Cardiovasc Thorac Ann. 2008;16:120-123.
22. Efthymiou CA, Masudi T, Thorpe JA, Papagiannopoulos K. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg. 2009;9:961-964.
23. Sioris T, Sihvo E, Salo J, et al. Long-term indwelling pleural catheter (PleurX) for malignant pleural effusion unsuitable for talc pleurodesis. Eur J Surg Oncol. 2009;35:546-551.
24. Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med. 2011;26:70-76.
25. Wahidi MM, Reddy C, Yarmus L, et al. Randomized trial of pleural fluid drainage frequency in patients with malignant pleural effusions. the ASAP trial. Am J Respir Crit Care Med. 2017;195:1050-1057.
26. Muruganandan S, Azzopardi M, Fitzgerald DB, et al. Aggressive versus symptom-guided drainage of malignant pleural effusion via indwelling pleural catheters (AMPLE-2): an open-label randomised trial. Lancet Respir Med. 2018;6:671-680.
27. Bhatnagar R, Keenan EK, Morley AJ, et al. Outpatient talc administration by indwelling pleural catheter for malignant effusion. N Engl J Med. 2018;378:1313-1322.
28. Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest. 2006;129:362-368.
29. Fysh ETH, Tremblay A, Feller-Kopman D, et al. Clinical outcomes of indwelling pleural catheter-related pleural infections: an international multicenter study. Chest. 2013;144:1597-1602.
30. Morel A, Mishra E, Medley L, et al. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax. 2011;66:448-449.
31. Gilbert CR, Lee HJ, Skalski JH, et al. The use of indwelling tunneled pleural catheters for recurrent pleural effusions in patients with hematologic malignancies: a multicenter study. Chest. 2015;148:752-758.
32. Thomas R, Budgeon CA, Kuok YJ, et al. Catheter tract metastasis associated with indwelling pleural catheters. Chest. 2014;146:557-562.
33. Thomas R, Piccolo F, Miller D, et al. Intrapleural fibrinolysis for the treatment of indwelling pleural catheter-related symptomatic loculations: a multicenter observational study. Chest. 2015;148:746-751.
34. Antony VB. Pathogenesis of malignant pleural effusions and talc pleurodesis. Pneumologie. 1999;53:493-498.
35. Antony VB, Nasreen N, Mohammed KA, et al. Talc pleurodesis: basic fibroblast growth factor mediates pleural fibrosis. Chest. 2004;126:1522-1528.
36. Xie C, Teixeira LR, McGovern JP, Light RW. Systemic corticosteroids decrease the effectiveness of talc pleurodesis. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1441-1444.
37. Teixeira LR, Wu W, Chang DS, Light RW. The effect of corticosteroids on pleurodesis induced by doxycycline in rabbits. Chest. 2002;121:216-219.
38. Hunt I, Teh E, Southon R, Treasure T. Using non-steroidal anti-inflammatory drugs (NSAIDs) following pleurodesis. Interact Cardiovasc Thorac Surg. 2007;6:102-104.
39. Rahman NM, Pepperell J, Rehal S, et al. Effect of opioids vs NSAIDs and larger vs smaller chest tube size on pain control and pleurodesis efficacy among patients with malignant pleural effusion: the TIME1 randomized clinical trial. JAMA. 2015;314:2641-2653.
40. Clive AO, Jones HE, Bhatnagar R, Preston NJ, Maskell N. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev. 2016(5):CD010529.
41. Tan C, Sedrakyan A, Browne J, et al. The evidence on the effectiveness of management for malignant pleural effusion: a systematic review. Eur J Cardiothorac Surg. 2006;29:829-838.
42. Heffner JE, Nietert PJ, Barbieri C. Pleural fluid pH as a predictor of pleurodesis failure: analysis of primary data. Chest. 2000;117:87-95.
43. Yildirim H, Metintas M, Ak G, et al. Predictors of talc pleurodesis outcome in patients with malignant pleural effusions. Lung Cancer. 2008;62:139-144.
44. Aydogmus U, Ozdemir S, Cansever L, et al. Bedside talc pleurodesis for malignant pleural effusion: factors affecting success. Ann Surg Oncol. 2009;16:745-750.
45. Guo H, Wan Y, Tian G, et al. EGFR mutations predict a favorable outcome for malignant pleural effusion of lung adenocarcinoma with Tarceva therapy. Oncol Rep. 2012;27:880-890.
46. Janssen JP, Collier G, Astoul P, et al. Safety of pleurodesis with talc poudrage in malignant pleural effusion: a prospective cohort study. Lancet. 2007;369(9572):1535-1539.
47. Dryzer SR, Allen ML, Strange C, Sahn SA. A comparison of rotation and nonrotation in tetracycline pleurodesis. Chest. 1993;104:1763-1766.
48. Mager HJ, Maesen B, Verzijlbergen F, Schramel F. Distribution of talc suspension during treatment of malignant pleural effusion with talc pleurodesis. Lung Cancer. 2002;36:77-81.
49. Hsia D, Musani AI. Interventional pulmonology. Med Clin North Am. 2011;95:1095-1114.
50. Dresler CM, Olak J, Herndon JE, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest. 2005;127:909-915.
51. Bibby AC, Dorn P, Psallidas I, et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur Respir J. 2018;52(1).
52. Sakuraba M, Masuda K, Hebisawa A, et al. Diagnostic value of thoracoscopic pleural biopsy for pleurisy under local anaesthesia. ANZ J Surg. 2006;76:722-724.
53. Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011;12:763-772.
54. Zellos L, Jaklitsch MT, Al-Mourgi MA, Sugarbaker DJ. Complications of extrapleural pneumonectomy. Semin Thorac Cardiovasc Surg. 2007;19:355-359.
55. Zahid I, Sharif S, Routledge T, Scarci M. Is pleurectomy and decortication superior to palliative care in the treatment of malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg. 2011;12:812-817.
56. Soysal O, Karaoğlanoğlu N, Demiracan S, et al. Pleurectomy/decortication for palliation in malignant pleural mesothelioma: results of surgery. Eur J Cardiothorac Surg. 1997;11:210-213.
57. Putnam JB, Light RW, Rodriguez RM, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer. 1999;86:1992-1999.
58. Fysh ETH, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest. 2012;142:394-400.
59. Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med. 2018;198:839-849.
60. Davies CW, Traill ZC, Gleeson FV, Davies RJ. Intrapleural streptokinase in the management of malignant multiloculated pleural effusions. Chest. 1999;115:729-733.
61. Hsu LH, Soong TC, Feng AC, Liu MC. Intrapleural urokinase for the treatment of loculated malignant pleural effusions and trapped lungs in medically inoperable cancer patients. J Thorac Oncol. 2006;1:460-467.
62. Okur E, Baysungur V, Tezel C, et al. Streptokinase for malignant pleural effusions: a randomized controlled study. Asian Cardiovasc Thorac Ann. 2011;19:238-243.
63. Mishra EK, Clive AO, Wills GH, et al. Randomized controlled trial of urokinase versus placebo for nondraining malignant pleural effusion. Am J Respir Crit Care Med. 2018;197:502-508.
64. Reddy C, Ernst A, Lamb C, Feller-Kopman D. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest. 2011;139:1419-1423.
65. Krochmal R, Reddy C, Yarmus L, et al. Patient evaluation for rapid pleurodesis of malignant pleural effusions. J Thorac Dis. 2016;8:2538-2543.
66. Bhatnagar R, Zahan-Evans N, Kearney C, et al. A novel drug-eluting indwelling pleural catheter for the management of malignant effusions. Am J Respir Crit Care Med. 2018;197:136-138.
67. Penz ED, Mishra EK, Davies HE, Manns BJ, Miller RF, Rahman NM. Comparing cost of indwelling pleural catheter vs talc pleurodesis for malignant pleural effusion. Chest. 2014;146:991-1000.
68. Yang TS, Hsia DW, Chang DW. Patient- and hospital-level factors associated with readmission for malignant pleural effusion. J Oncol Pract. 2018;14:e547-e556.
Malignant Pleural Effusion: Evaluation and Diagnosis
Accumulation of pleural fluid is a common clinical problem associated with malignancy. Malignant pleural effusions (MPEs) are the second most common cause of a pleural exudate, with more than 150,000 patients diagnosed annually in the United States alone.1,2 MPEs represent advanced disease and are generally a poor prognostic indicator. Median survival for patients with MPE ranges from 3 to 12 months and depends on the tumor origin.3 In addition, MPEs are a frequent cause of dyspnea and discomfort, which adversely affect a patient’s quality of life. This group of patients requires substantial medical support to manage the burden of their disease, and providing effective therapeutic management remains a challenge. In the United States, there are approximately 126,000 admissions for MPE annually, with a median length of stay of 5.5 days.4 Thirty-day readmission rates are almost 40%, which is approximately 1.5 times higher than for acute myocardial infarction and 2 times higher than for congestive heart failure.5 In addition, palliative measures for patients with MPE are probably underutilized.6
This review is the first of 2 articles focusing on the management of MPE. Here, we discuss the pathophysiology of this disease process and provide an overview of the evaluation and diagnosis of MPE; available therapeutic options for the management of MPE are reviewed in a separate article.
Pathogenesis and Etiology
Normally, the thoracic cavity contains less than 15 mL of pleural fluid. Therefore, the visceral and parietal pleura are usually in close proximity to each other and the space between them is a potential space. Negative intrapleural pressures generated during regular breathing create a gradient for fluid movement into the pleural space from the parietal pleura dictated by Starling forces. Pleural fluid normally has low protein content and is primarily drained back into lymphatics through stomata lining the parietal pleura.7 This system’s ability to remove pleural fluid exceeds normal fluid production by 20- to 30-fold, suggesting that accumulation of excess pleural fluid requires a combination of increased fluid production and/or impaired fluid removal.8
Several mechanisms have been associated with the development of MPE. Pleural involvement by malignancy may occur from direct invasion of the pleural cavity by tumor (eg, lung cancer, breast cancer, chest wall neoplasms) or hematogenous spread of tumor to the pleura (eg, metastasis, non-Hodgkin lymphoma).9,10 Pleural malignancies can produce cytokine and inflammatory mediators, which may directly increase fluid production or indirectly alter vascular permeability.11,12 Tumor cells can also disrupt lymphatic drainage by occluding either pleural stomata or downstream lymphatic drainage. However, tumor involvement of the pleura does not always result in the development of an effusion and is only associated with fluid accumulation in approximately 60% of cases.13,14 MPE have also been strongly associated with mediastinal metastases, likely resulting from obstruction of mediastinal lymphatics.13,15,16
Pleural effusions with negative fluid cytology and pleural biopsies may result from secondary effects of tumor burden without direct pleural involvement and are referred to as paramalignant effusions. Common causes include thoracic duct obstruction (eg, Hodgkin lymphoma), bronchial obstruction, pneumonia, atelectasis, pulmonary embolism, trapped lung, and effects related to radiation or chemotherapy.15
Lung cancer is the most frequent cause of MPE and accounts for approximately one-third of cases. Other common primary tumor sites include breast, lymphoma, ovary, and gastrointestinal. Combined, these etiologies comprise about 75% of cases (Table 1).4,5 Females comprise a greater percentage of patients with MPE mainly due to the prevalence of ovarian and breast cancer. Mesothelioma-related effusions may be more prevalent in certain parts of the world due to associated exposure to asbestos.17 The primary tumor origin remains unknown in approximately 10% of cases.4
Clinical Presentation and Response to Therapeutic Drainage
More than 75% of patients with MPE are symptomatic. Dyspnea is the most common symptom and is present in more than half of patients.15 The mechanism of dyspnea caused by large effusions may not be solely due to impaired lung volumes or gas exchange. Other associated factors include decreased chest wall compliance, mediastinal shift causing decreased volume of the contralateral lung, paradoxical motion of the diaphragm, inefficient muscle length-tension relationships resulting from the stretch of respiratory muscles, and reflex stimulation from the lungs and chest wall.18-20 Other common presenting symptoms include cough, orthopnea, and chest pain. Hemoptysis suggests endobronchial involvement of the large airways. And, given the advanced nature of most MPEs, patients may also present with weight loss and cachexia.
A patient’s degree of symptom palliation and physiologic improvement in response to large-volume fluid removal is important to assess as these are important clinical factors that will influence management decision-making. Upwards of 50% of patients will not have significant palliation because they may be symptom-limited by other comorbid conditions, generalized deconditioning, or incomplete lung re-expansion. Presence of impaired lung compliance during fluid removal is also important to recognize. A trapped lung refers to a lung that cannot expand completely after removal of pleural fluid. Trapped lung may result from pleural-based malignancies or metastases, loculations and adhesions, or bronchial obstruction. Trapped lung is associated with high elastance (Pel) affecting pleural pressure-volume relationships (Figure 1). While clinically often considered together, some authors differentiate the category of incomplete lung expansion into 2 subgroups. In this context, the term trapped lung is used specifically to describe a mature, fibrous membrane that prevents lung re-expansion and is caused by a prior inflammatory pleural condition.21 Entrapped lung describes incomplete lung expansion resulting from an active disease process, such as malignancy, ongoing infection, or rheumatologic pleurisy. Differences in pleural manometry can be seen in the 2 subgroups. Pleural manometry can be helpful to monitor for the generation of high negative intrapleural pressures during fluid removal, with negative pressures in excess of –19 cm H2O being suggestive of trapped lung physiology.22 However routine use of pleural manometry has not been shown to avoid the development of procedure-related chest discomfort that develops when the lung is unable to expand in response to the removal of fluid.23
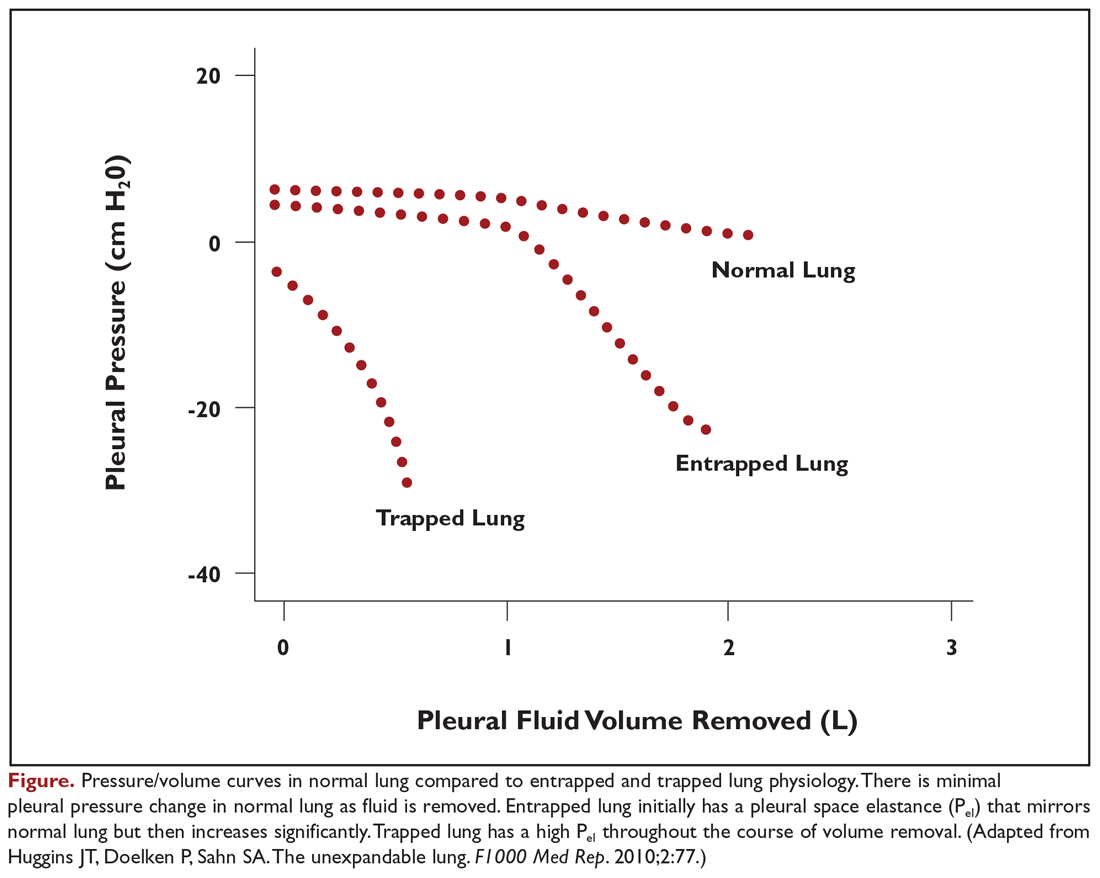
Pleural Fluid Analysis and Pleural Biopsy
While most MPEs are protein-rich exudates, approximately 2% to 5% may be transudates.24,25 MPEs often appear hemorrhagic, so a ratio of pleural fluid to blood serum hematocrit greater than 0.5 is used to distinguish a true hemothorax from bloody-appearing pleural fluid.26 The cell count may be lymphocyte-predominant, but other cell types, such as eosinophils, do not exclude malignancy.27 Fluid may have a low glucose concentration and pH as well.
Thoracentesis with pleural fluid cytology evaluation is the most common method of diagnosis. The diagnostic sensitivity of fluid cytology ranges from 62% to 90%, with variability resulting from the extent of disease and etiology of the primary malignancy.1 If the initial pleural fluid analysis is not diagnostic, repeat thoracentesis can improve the diagnostic yield, but subsequent sampling has diminishing utility. In one series, diagnosis of malignancy was made by fluid cytology analysis in 65% of patients from the initial thoracentesis, 27% from a second procedure, but only 5% from a third procedure.28 At least 50 to 60 mL of pleural fluid should be obtained for pleural fluid cytology, but analysis of significantly larger volumes may not appreciably improve diagnostic yield.29,30
In addition to diagnostic yield, adequate sample cellularity to test for genetic driver mutations has become increasingly important given the rapid development of targeted therapies that are now available. The relative paucity of malignant cells in pleural fluid compared to other types of biopsies can make MPEs difficult to analyze for molecular markers. Newer generation assays have increased sensitivity, with one series reporting that pleural fluid was sufficient in 71.4% of cases to analyze for a panel comprised of EGFR, KRAS, BRAF, ALK, and ROS1 mutations.31 Similarly, fluid analysis from patients with MPEs demonstrated that 71.3% had at least 100 tumor cells, which permitted evaluation for PD-L1, with a concordance of 0.78 when compared to matched parenchymal lung biopsies from the same patient.32
In contrast, pleural biopsy methods may be useful to increase the diagnostic yield when pleural fluid analysis is insufficient. Closed needle biopsy may marginally improve diagnostic yields for malignancy over pleural fluid analysis alone. Diagnostic sensitivity may improve with the use of point-of-care ultrasonography to guide needle placement.33,34 The true value of closed needle biopsy is seen in situations in which there is a high pretest probability to diagnose an alternative disseminated pleural process, such as in tuberculosis, where the diagnostic yield increases substantially with closed needle biopsy of the pleura.33 Otherwise, the diagnosis of lung cancer and mesothelioma is superior with visually guided pleural biopsies, such as medical thoracoscopy or video-assisted thoracoscopic surgery (VATS), with diagnostic yields over 90%.33,35 Testing for genetic driver mutations in pleural biopsies is also substantially improved, with sample adequacy of 90% to 95% for most molecular markers.36,37 Despite the advantages, pleural biopsies are generally reserved for cases when pleural fluid analysis is insufficient or when performed in conjunction with palliative therapeutic interventions due to the increased invasive nature of the procedure.
Predictors of Recurrence and Prognosis
Not all MPEs will progress in size or become symptomatic, and predicting which patients will develop symptoms from their effusions is difficult. Pleural effusions will develop in only a minority of patients with lung cancer, and only a small subset will progress and require therapeutic intervention.38,39 Therefore, management guidelines for malignant pleural effusions discourage empiric intervention for patients with small, asymptomatic effusions.40 However, patients with larger, symptomatic effusions are more likely to have significant and rapid fluid recurrence. In a series of 988 symptomatic patients undergoing drainage, 30% had fluid recurrence within 15 days, 40% within 30 days, 45% within 60 days, and 48% within 90 days.41 Factors associated with fluid recurrence included radiographic size of the effusion, requirement for a larger amount of fluid to be initially drained, and higher pleural fluid lactate dehydrogenase (LDH) level. Negative cytology was associated with lower likelihood for recurrence.
Prognostication of life expectancy is another important clinical assessment which impacts medical decision-making when weighing the risk and benefits of different palliation options. Patient performance status, pleural fluid LDH, serum neutrophil-to-lymphocyte ratio, and tumor origin are independently associated with prognosis in a validated scoring system (Table 2).3 In this study, the overall median survival of patients with MPE was approximately 4.5 months, while the median survival for patients with mesothelioma was 11.3 months, 6.6 months for breast cancer, and 2.5 months for lung cancer and other malignancies. When stratified based on the combination of these 4 variables, patients in the high-risk group had a median survival of just 44 days compared to 130 days for the moderate-risk group and 319 days for the low-risk group. Additional, more complex prediction systems for survival and response to MPE therapies are now emerging and may provide clinicians and patients with additional information useful in medical decision-making.42
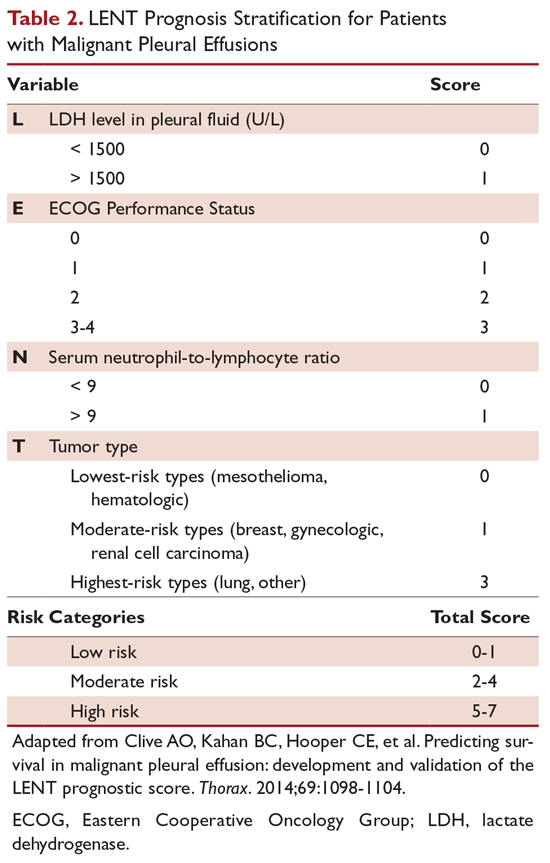
Conclusion
MPEs represent advanced stage disease and frequently adversely affect a patient’s quality of life. Ideal therapeutic options, discussed in the second part of this review, should effectively palliate symptoms, provide long-term relief, be minimally invasive with few side effects, minimize hospitalization and reliance on medical assistance, and be cost-effective.
1. Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J. 2001;18:402-419.
2. Society AT. Management of malignant pleural effusions. Am J Respir Crit Care Med. 2000;162:1987-2001.
3. Clive AO, Kahan BC, Hooper CE, et al. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax. 2014;69:1098-1104.
4. Taghizadeh N, Fortin M, Tremblay A. US hospitalizations for malignant pleural effusions: data from the 2012 National Inpatient Sample. Chest. 2017;151:845-854.
5. Yang TS, Hsia DW, Chang DW. Patient- and hospital-level factors associated with readmission for malignant pleural effusion. J Oncol Pract. 2018;14:e547-e556.
6. Ost DE, Niu J, Zhao H, et al. Quality gaps and comparative effectiveness of management strategies for recurrent malignant pleural effusions. Chest. 2018;153:438-452.
7. Miserocchi G. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J. 1997;10:219-225.
8. Sahn SA. State of the art. The pleura. Am Rev Respir Dis. 1988;138:184-234.
9. Khaleeq G, Musani AI. Emerging paradigms in the management of malignant pleural effusions. Respir Med. 2008;102:939-948.
10. Das DK. Serous effusions in malignant lymphomas: a review. Diagn Cytopathol. 2006;34:335-347.
11. Qian Q, Zhan P, Sun WK, et al. Vascular endothelial growth factor and soluble intercellular adhesion molecule-1 in lung adenocarcinoma with malignant pleural effusion: correlations with patient survival and pleural effusion control. Neoplasma. 2012;59:433-439.
12. Kraft A, Weindel K, Ochs A, et al. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer. 1999;85:178-187.
13. Meyer PC. Metastatic carcinoma of the pleura. Thorax. 1966;21:437-443.
14. Light RW, Hamm H. Malignant pleural effusion: would the real cause please stand up? Eur Respir J. 1997;10:1701-1702.
15. Chernow B, Sahn SA. Carcinomatous involvement of the pleura: an analysis of 96 patients. Am J Med. 1977;63:695-702.
16. Musani AI, Haas AR, Seijo L, et al. Outpatient management of malignant pleural effusions with small-bore, tunneled pleural catheters. Respiration. 2004;71:559-566.
17. Roberts ME, Neville E, Berrisford RG, et al; Group BPDG. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii32-40.
18. Estenne M, Yernault JC, De Troyer A. Mechanism of relief of dyspnea after thoracocentesis in patients with large pleural effusions. Am J Med. 1983;74:813-819.
19. Brown NE, Zamel N, Aberman A. Changes in pulmonary mechanics and gas exchange following thoracocentesis. Chest. 1978;74:540-542.
20. Wang LM, Cherng JM, Wang JS. Improved lung function after thoracocentesis in patients with paradoxical movement of a hemidiaphragm secondary to a large pleural effusion. Respirology. 2007;12:719-723.
21. Huggins JT, Doelken P, Sahn SA. The unexpandable lung. F1000 Med Rep. 2010;2:77.
22. Lan RS, Lo SK, Chuang ML, Yang CT, Tsao TC, Lee CH. Elastance of the pleural space: a predictor for the outcome of pleurodesis in patients with malignant pleural effusion. Ann Intern Med. 1997;126:768-774.
23. Lentz RJ, Lerner AD, Pannu JK, et al. Routine monitoring with pleural manometry during therapeutic large-volume thoracentesis to prevent pleural-pressure-related complications: a multicentre, single-blind randomised controlled trial. Lancet Respir Med. 2019;7:447-455.
24. Porcel JM, Alvarez M, Salud A, Vives M. Should a cytologic study be ordered in transudative pleural effusions? Chest. 1999;116:1836-1837.
25. Ryu JS, Ryu ST, Kim YS, et al. What is the clinical significance of transudative malignant pleural effusion? Korean J Intern Med. 2003;18:230-233.
26. Boersma WG, Stigt JA, Smit HJ. Treatment of haemothorax. Respir Med. 2010;104:1583-1587.
27. Light RW, Erozan YS, Ball WC. Cells in pleural fluid. Their value in differential diagnosis. Arch Intern Med. 1973;132:854-860.
28. Garcia LW, Ducatman BS, Wang HH. The value of multiple fluid specimens in the cytological diagnosis of malignancy. Mod Pathol. 1994;7:665-668.
29. Swiderek J, Morcos S, Donthireddy V, et al. Prospective study to determine the volume of pleural fluid required to diagnose malignancy. Chest. 2010;137:68-73.
30. Abouzgheib W, Bartter T, Dagher H, Pratter M, Klump W. A prospective study of the volume of pleural fluid required for accurate diagnosis of malignant pleural effusion. Chest. 2009;135:999-1001.
31. DeMaio A, Clarke JM, Dash R, et al. Yield of malignant pleural effusion for detection of oncogenic driver mutations in lung adenocarcinoma. J Bronchology Interv Pulmonol. 2019;26:96-101.
32. Grosu HB, Arriola A, Stewart J, et al. PD-L1 detection in histology specimens and matched pleural fluid cell blocks of patients with NSCLC. Respirology. 2019 Jun 17. doi: 10.1111/resp.13614.
33. Koegelenberg CF, Diacon AH. Pleural controversy: close needle pleural biopsy or thoracoscopy-which first? Respirology. 2011;16:738-746.
34. McLaughlin KM, Kerr KM, Currie GP. Closed pleural biopsy to diagnose mesothelioma: dead or alive? Lung Cancer. 2009;65:388-389.
35. Miyoshi S, Sasada S, Izumo T, et al. Diagnostic utility of pleural fluid cell block versus pleural biopsy collected by flex-rigid pleuroscopy for malignant pleural disease: a single center retrospective analysis. PLoS One. 2016;11:e0167186.
36. Vanderlaan PA, Yamaguchi N, Folch E, et al. Success and failure rates of tumor genotyping techniques in routine pathological samples with non-small-cell lung cancer. Lung Cancer. 2014;84:39-44.
37. Albanna AS, Kasymjanova G, Robitaille C, et al. Comparison of the yield of different diagnostic procedures for cellular differentiation and genetic profiling of non-small-cell lung cancer. J Thorac Oncol. 2014;9:1120-1125.
38. Tremblay A RS, Berthiaume L, and Michaud G. Natural history of asymptomatic pleural effusions in lung cancer patients. J Bronchol. 2007;14:98-100.
39. Porcel JM, Gasol A, Bielsa S, et al. Clinical features and survival of lung cancer patients with pleural effusions. Respirology. 2015;20:654-659.
40. Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med. 2018;198:839-849.
41. Grosu HB, Molina S, Casal R, et al. Risk factors for pleural effusion recurrence in patients with malignancy. Respirology. 2019;24:76-82.
42. Psallidas I, Kanellakis NI, Gerry S, et al. Development and validation of response markers to predict survival and pleurodesis success in patients with malignant pleural effusion (PROMISE): a multicohort analysis. Lancet Oncol. 2018;19:930-939.
Accumulation of pleural fluid is a common clinical problem associated with malignancy. Malignant pleural effusions (MPEs) are the second most common cause of a pleural exudate, with more than 150,000 patients diagnosed annually in the United States alone.1,2 MPEs represent advanced disease and are generally a poor prognostic indicator. Median survival for patients with MPE ranges from 3 to 12 months and depends on the tumor origin.3 In addition, MPEs are a frequent cause of dyspnea and discomfort, which adversely affect a patient’s quality of life. This group of patients requires substantial medical support to manage the burden of their disease, and providing effective therapeutic management remains a challenge. In the United States, there are approximately 126,000 admissions for MPE annually, with a median length of stay of 5.5 days.4 Thirty-day readmission rates are almost 40%, which is approximately 1.5 times higher than for acute myocardial infarction and 2 times higher than for congestive heart failure.5 In addition, palliative measures for patients with MPE are probably underutilized.6
This review is the first of 2 articles focusing on the management of MPE. Here, we discuss the pathophysiology of this disease process and provide an overview of the evaluation and diagnosis of MPE; available therapeutic options for the management of MPE are reviewed in a separate article.
Pathogenesis and Etiology
Normally, the thoracic cavity contains less than 15 mL of pleural fluid. Therefore, the visceral and parietal pleura are usually in close proximity to each other and the space between them is a potential space. Negative intrapleural pressures generated during regular breathing create a gradient for fluid movement into the pleural space from the parietal pleura dictated by Starling forces. Pleural fluid normally has low protein content and is primarily drained back into lymphatics through stomata lining the parietal pleura.7 This system’s ability to remove pleural fluid exceeds normal fluid production by 20- to 30-fold, suggesting that accumulation of excess pleural fluid requires a combination of increased fluid production and/or impaired fluid removal.8
Several mechanisms have been associated with the development of MPE. Pleural involvement by malignancy may occur from direct invasion of the pleural cavity by tumor (eg, lung cancer, breast cancer, chest wall neoplasms) or hematogenous spread of tumor to the pleura (eg, metastasis, non-Hodgkin lymphoma).9,10 Pleural malignancies can produce cytokine and inflammatory mediators, which may directly increase fluid production or indirectly alter vascular permeability.11,12 Tumor cells can also disrupt lymphatic drainage by occluding either pleural stomata or downstream lymphatic drainage. However, tumor involvement of the pleura does not always result in the development of an effusion and is only associated with fluid accumulation in approximately 60% of cases.13,14 MPE have also been strongly associated with mediastinal metastases, likely resulting from obstruction of mediastinal lymphatics.13,15,16
Pleural effusions with negative fluid cytology and pleural biopsies may result from secondary effects of tumor burden without direct pleural involvement and are referred to as paramalignant effusions. Common causes include thoracic duct obstruction (eg, Hodgkin lymphoma), bronchial obstruction, pneumonia, atelectasis, pulmonary embolism, trapped lung, and effects related to radiation or chemotherapy.15
Lung cancer is the most frequent cause of MPE and accounts for approximately one-third of cases. Other common primary tumor sites include breast, lymphoma, ovary, and gastrointestinal. Combined, these etiologies comprise about 75% of cases (Table 1).4,5 Females comprise a greater percentage of patients with MPE mainly due to the prevalence of ovarian and breast cancer. Mesothelioma-related effusions may be more prevalent in certain parts of the world due to associated exposure to asbestos.17 The primary tumor origin remains unknown in approximately 10% of cases.4

Clinical Presentation and Response to Therapeutic Drainage
More than 75% of patients with MPE are symptomatic. Dyspnea is the most common symptom and is present in more than half of patients.15 The mechanism of dyspnea caused by large effusions may not be solely due to impaired lung volumes or gas exchange. Other associated factors include decreased chest wall compliance, mediastinal shift causing decreased volume of the contralateral lung, paradoxical motion of the diaphragm, inefficient muscle length-tension relationships resulting from the stretch of respiratory muscles, and reflex stimulation from the lungs and chest wall.18-20 Other common presenting symptoms include cough, orthopnea, and chest pain. Hemoptysis suggests endobronchial involvement of the large airways. And, given the advanced nature of most MPEs, patients may also present with weight loss and cachexia.
A patient’s degree of symptom palliation and physiologic improvement in response to large-volume fluid removal is important to assess as these are important clinical factors that will influence management decision-making. Upwards of 50% of patients will not have significant palliation because they may be symptom-limited by other comorbid conditions, generalized deconditioning, or incomplete lung re-expansion. Presence of impaired lung compliance during fluid removal is also important to recognize. A trapped lung refers to a lung that cannot expand completely after removal of pleural fluid. Trapped lung may result from pleural-based malignancies or metastases, loculations and adhesions, or bronchial obstruction. Trapped lung is associated with high elastance (Pel) affecting pleural pressure-volume relationships (Figure 1). While clinically often considered together, some authors differentiate the category of incomplete lung expansion into 2 subgroups. In this context, the term trapped lung is used specifically to describe a mature, fibrous membrane that prevents lung re-expansion and is caused by a prior inflammatory pleural condition.21 Entrapped lung describes incomplete lung expansion resulting from an active disease process, such as malignancy, ongoing infection, or rheumatologic pleurisy. Differences in pleural manometry can be seen in the 2 subgroups. Pleural manometry can be helpful to monitor for the generation of high negative intrapleural pressures during fluid removal, with negative pressures in excess of –19 cm H2O being suggestive of trapped lung physiology.22 However routine use of pleural manometry has not been shown to avoid the development of procedure-related chest discomfort that develops when the lung is unable to expand in response to the removal of fluid.23

Pleural Fluid Analysis and Pleural Biopsy
While most MPEs are protein-rich exudates, approximately 2% to 5% may be transudates.24,25 MPEs often appear hemorrhagic, so a ratio of pleural fluid to blood serum hematocrit greater than 0.5 is used to distinguish a true hemothorax from bloody-appearing pleural fluid.26 The cell count may be lymphocyte-predominant, but other cell types, such as eosinophils, do not exclude malignancy.27 Fluid may have a low glucose concentration and pH as well.
Thoracentesis with pleural fluid cytology evaluation is the most common method of diagnosis. The diagnostic sensitivity of fluid cytology ranges from 62% to 90%, with variability resulting from the extent of disease and etiology of the primary malignancy.1 If the initial pleural fluid analysis is not diagnostic, repeat thoracentesis can improve the diagnostic yield, but subsequent sampling has diminishing utility. In one series, diagnosis of malignancy was made by fluid cytology analysis in 65% of patients from the initial thoracentesis, 27% from a second procedure, but only 5% from a third procedure.28 At least 50 to 60 mL of pleural fluid should be obtained for pleural fluid cytology, but analysis of significantly larger volumes may not appreciably improve diagnostic yield.29,30
In addition to diagnostic yield, adequate sample cellularity to test for genetic driver mutations has become increasingly important given the rapid development of targeted therapies that are now available. The relative paucity of malignant cells in pleural fluid compared to other types of biopsies can make MPEs difficult to analyze for molecular markers. Newer generation assays have increased sensitivity, with one series reporting that pleural fluid was sufficient in 71.4% of cases to analyze for a panel comprised of EGFR, KRAS, BRAF, ALK, and ROS1 mutations.31 Similarly, fluid analysis from patients with MPEs demonstrated that 71.3% had at least 100 tumor cells, which permitted evaluation for PD-L1, with a concordance of 0.78 when compared to matched parenchymal lung biopsies from the same patient.32
In contrast, pleural biopsy methods may be useful to increase the diagnostic yield when pleural fluid analysis is insufficient. Closed needle biopsy may marginally improve diagnostic yields for malignancy over pleural fluid analysis alone. Diagnostic sensitivity may improve with the use of point-of-care ultrasonography to guide needle placement.33,34 The true value of closed needle biopsy is seen in situations in which there is a high pretest probability to diagnose an alternative disseminated pleural process, such as in tuberculosis, where the diagnostic yield increases substantially with closed needle biopsy of the pleura.33 Otherwise, the diagnosis of lung cancer and mesothelioma is superior with visually guided pleural biopsies, such as medical thoracoscopy or video-assisted thoracoscopic surgery (VATS), with diagnostic yields over 90%.33,35 Testing for genetic driver mutations in pleural biopsies is also substantially improved, with sample adequacy of 90% to 95% for most molecular markers.36,37 Despite the advantages, pleural biopsies are generally reserved for cases when pleural fluid analysis is insufficient or when performed in conjunction with palliative therapeutic interventions due to the increased invasive nature of the procedure.
Predictors of Recurrence and Prognosis
Not all MPEs will progress in size or become symptomatic, and predicting which patients will develop symptoms from their effusions is difficult. Pleural effusions will develop in only a minority of patients with lung cancer, and only a small subset will progress and require therapeutic intervention.38,39 Therefore, management guidelines for malignant pleural effusions discourage empiric intervention for patients with small, asymptomatic effusions.40 However, patients with larger, symptomatic effusions are more likely to have significant and rapid fluid recurrence. In a series of 988 symptomatic patients undergoing drainage, 30% had fluid recurrence within 15 days, 40% within 30 days, 45% within 60 days, and 48% within 90 days.41 Factors associated with fluid recurrence included radiographic size of the effusion, requirement for a larger amount of fluid to be initially drained, and higher pleural fluid lactate dehydrogenase (LDH) level. Negative cytology was associated with lower likelihood for recurrence.
Prognostication of life expectancy is another important clinical assessment which impacts medical decision-making when weighing the risk and benefits of different palliation options. Patient performance status, pleural fluid LDH, serum neutrophil-to-lymphocyte ratio, and tumor origin are independently associated with prognosis in a validated scoring system (Table 2).3 In this study, the overall median survival of patients with MPE was approximately 4.5 months, while the median survival for patients with mesothelioma was 11.3 months, 6.6 months for breast cancer, and 2.5 months for lung cancer and other malignancies. When stratified based on the combination of these 4 variables, patients in the high-risk group had a median survival of just 44 days compared to 130 days for the moderate-risk group and 319 days for the low-risk group. Additional, more complex prediction systems for survival and response to MPE therapies are now emerging and may provide clinicians and patients with additional information useful in medical decision-making.42

Conclusion
MPEs represent advanced stage disease and frequently adversely affect a patient’s quality of life. Ideal therapeutic options, discussed in the second part of this review, should effectively palliate symptoms, provide long-term relief, be minimally invasive with few side effects, minimize hospitalization and reliance on medical assistance, and be cost-effective.
Accumulation of pleural fluid is a common clinical problem associated with malignancy. Malignant pleural effusions (MPEs) are the second most common cause of a pleural exudate, with more than 150,000 patients diagnosed annually in the United States alone.1,2 MPEs represent advanced disease and are generally a poor prognostic indicator. Median survival for patients with MPE ranges from 3 to 12 months and depends on the tumor origin.3 In addition, MPEs are a frequent cause of dyspnea and discomfort, which adversely affect a patient’s quality of life. This group of patients requires substantial medical support to manage the burden of their disease, and providing effective therapeutic management remains a challenge. In the United States, there are approximately 126,000 admissions for MPE annually, with a median length of stay of 5.5 days.4 Thirty-day readmission rates are almost 40%, which is approximately 1.5 times higher than for acute myocardial infarction and 2 times higher than for congestive heart failure.5 In addition, palliative measures for patients with MPE are probably underutilized.6
This review is the first of 2 articles focusing on the management of MPE. Here, we discuss the pathophysiology of this disease process and provide an overview of the evaluation and diagnosis of MPE; available therapeutic options for the management of MPE are reviewed in a separate article.
Pathogenesis and Etiology
Normally, the thoracic cavity contains less than 15 mL of pleural fluid. Therefore, the visceral and parietal pleura are usually in close proximity to each other and the space between them is a potential space. Negative intrapleural pressures generated during regular breathing create a gradient for fluid movement into the pleural space from the parietal pleura dictated by Starling forces. Pleural fluid normally has low protein content and is primarily drained back into lymphatics through stomata lining the parietal pleura.7 This system’s ability to remove pleural fluid exceeds normal fluid production by 20- to 30-fold, suggesting that accumulation of excess pleural fluid requires a combination of increased fluid production and/or impaired fluid removal.8
Several mechanisms have been associated with the development of MPE. Pleural involvement by malignancy may occur from direct invasion of the pleural cavity by tumor (eg, lung cancer, breast cancer, chest wall neoplasms) or hematogenous spread of tumor to the pleura (eg, metastasis, non-Hodgkin lymphoma).9,10 Pleural malignancies can produce cytokine and inflammatory mediators, which may directly increase fluid production or indirectly alter vascular permeability.11,12 Tumor cells can also disrupt lymphatic drainage by occluding either pleural stomata or downstream lymphatic drainage. However, tumor involvement of the pleura does not always result in the development of an effusion and is only associated with fluid accumulation in approximately 60% of cases.13,14 MPE have also been strongly associated with mediastinal metastases, likely resulting from obstruction of mediastinal lymphatics.13,15,16
Pleural effusions with negative fluid cytology and pleural biopsies may result from secondary effects of tumor burden without direct pleural involvement and are referred to as paramalignant effusions. Common causes include thoracic duct obstruction (eg, Hodgkin lymphoma), bronchial obstruction, pneumonia, atelectasis, pulmonary embolism, trapped lung, and effects related to radiation or chemotherapy.15
Lung cancer is the most frequent cause of MPE and accounts for approximately one-third of cases. Other common primary tumor sites include breast, lymphoma, ovary, and gastrointestinal. Combined, these etiologies comprise about 75% of cases (Table 1).4,5 Females comprise a greater percentage of patients with MPE mainly due to the prevalence of ovarian and breast cancer. Mesothelioma-related effusions may be more prevalent in certain parts of the world due to associated exposure to asbestos.17 The primary tumor origin remains unknown in approximately 10% of cases.4

Clinical Presentation and Response to Therapeutic Drainage
More than 75% of patients with MPE are symptomatic. Dyspnea is the most common symptom and is present in more than half of patients.15 The mechanism of dyspnea caused by large effusions may not be solely due to impaired lung volumes or gas exchange. Other associated factors include decreased chest wall compliance, mediastinal shift causing decreased volume of the contralateral lung, paradoxical motion of the diaphragm, inefficient muscle length-tension relationships resulting from the stretch of respiratory muscles, and reflex stimulation from the lungs and chest wall.18-20 Other common presenting symptoms include cough, orthopnea, and chest pain. Hemoptysis suggests endobronchial involvement of the large airways. And, given the advanced nature of most MPEs, patients may also present with weight loss and cachexia.
A patient’s degree of symptom palliation and physiologic improvement in response to large-volume fluid removal is important to assess as these are important clinical factors that will influence management decision-making. Upwards of 50% of patients will not have significant palliation because they may be symptom-limited by other comorbid conditions, generalized deconditioning, or incomplete lung re-expansion. Presence of impaired lung compliance during fluid removal is also important to recognize. A trapped lung refers to a lung that cannot expand completely after removal of pleural fluid. Trapped lung may result from pleural-based malignancies or metastases, loculations and adhesions, or bronchial obstruction. Trapped lung is associated with high elastance (Pel) affecting pleural pressure-volume relationships (Figure 1). While clinically often considered together, some authors differentiate the category of incomplete lung expansion into 2 subgroups. In this context, the term trapped lung is used specifically to describe a mature, fibrous membrane that prevents lung re-expansion and is caused by a prior inflammatory pleural condition.21 Entrapped lung describes incomplete lung expansion resulting from an active disease process, such as malignancy, ongoing infection, or rheumatologic pleurisy. Differences in pleural manometry can be seen in the 2 subgroups. Pleural manometry can be helpful to monitor for the generation of high negative intrapleural pressures during fluid removal, with negative pressures in excess of –19 cm H2O being suggestive of trapped lung physiology.22 However routine use of pleural manometry has not been shown to avoid the development of procedure-related chest discomfort that develops when the lung is unable to expand in response to the removal of fluid.23

Pleural Fluid Analysis and Pleural Biopsy
While most MPEs are protein-rich exudates, approximately 2% to 5% may be transudates.24,25 MPEs often appear hemorrhagic, so a ratio of pleural fluid to blood serum hematocrit greater than 0.5 is used to distinguish a true hemothorax from bloody-appearing pleural fluid.26 The cell count may be lymphocyte-predominant, but other cell types, such as eosinophils, do not exclude malignancy.27 Fluid may have a low glucose concentration and pH as well.
Thoracentesis with pleural fluid cytology evaluation is the most common method of diagnosis. The diagnostic sensitivity of fluid cytology ranges from 62% to 90%, with variability resulting from the extent of disease and etiology of the primary malignancy.1 If the initial pleural fluid analysis is not diagnostic, repeat thoracentesis can improve the diagnostic yield, but subsequent sampling has diminishing utility. In one series, diagnosis of malignancy was made by fluid cytology analysis in 65% of patients from the initial thoracentesis, 27% from a second procedure, but only 5% from a third procedure.28 At least 50 to 60 mL of pleural fluid should be obtained for pleural fluid cytology, but analysis of significantly larger volumes may not appreciably improve diagnostic yield.29,30
In addition to diagnostic yield, adequate sample cellularity to test for genetic driver mutations has become increasingly important given the rapid development of targeted therapies that are now available. The relative paucity of malignant cells in pleural fluid compared to other types of biopsies can make MPEs difficult to analyze for molecular markers. Newer generation assays have increased sensitivity, with one series reporting that pleural fluid was sufficient in 71.4% of cases to analyze for a panel comprised of EGFR, KRAS, BRAF, ALK, and ROS1 mutations.31 Similarly, fluid analysis from patients with MPEs demonstrated that 71.3% had at least 100 tumor cells, which permitted evaluation for PD-L1, with a concordance of 0.78 when compared to matched parenchymal lung biopsies from the same patient.32
In contrast, pleural biopsy methods may be useful to increase the diagnostic yield when pleural fluid analysis is insufficient. Closed needle biopsy may marginally improve diagnostic yields for malignancy over pleural fluid analysis alone. Diagnostic sensitivity may improve with the use of point-of-care ultrasonography to guide needle placement.33,34 The true value of closed needle biopsy is seen in situations in which there is a high pretest probability to diagnose an alternative disseminated pleural process, such as in tuberculosis, where the diagnostic yield increases substantially with closed needle biopsy of the pleura.33 Otherwise, the diagnosis of lung cancer and mesothelioma is superior with visually guided pleural biopsies, such as medical thoracoscopy or video-assisted thoracoscopic surgery (VATS), with diagnostic yields over 90%.33,35 Testing for genetic driver mutations in pleural biopsies is also substantially improved, with sample adequacy of 90% to 95% for most molecular markers.36,37 Despite the advantages, pleural biopsies are generally reserved for cases when pleural fluid analysis is insufficient or when performed in conjunction with palliative therapeutic interventions due to the increased invasive nature of the procedure.
Predictors of Recurrence and Prognosis
Not all MPEs will progress in size or become symptomatic, and predicting which patients will develop symptoms from their effusions is difficult. Pleural effusions will develop in only a minority of patients with lung cancer, and only a small subset will progress and require therapeutic intervention.38,39 Therefore, management guidelines for malignant pleural effusions discourage empiric intervention for patients with small, asymptomatic effusions.40 However, patients with larger, symptomatic effusions are more likely to have significant and rapid fluid recurrence. In a series of 988 symptomatic patients undergoing drainage, 30% had fluid recurrence within 15 days, 40% within 30 days, 45% within 60 days, and 48% within 90 days.41 Factors associated with fluid recurrence included radiographic size of the effusion, requirement for a larger amount of fluid to be initially drained, and higher pleural fluid lactate dehydrogenase (LDH) level. Negative cytology was associated with lower likelihood for recurrence.
Prognostication of life expectancy is another important clinical assessment which impacts medical decision-making when weighing the risk and benefits of different palliation options. Patient performance status, pleural fluid LDH, serum neutrophil-to-lymphocyte ratio, and tumor origin are independently associated with prognosis in a validated scoring system (Table 2).3 In this study, the overall median survival of patients with MPE was approximately 4.5 months, while the median survival for patients with mesothelioma was 11.3 months, 6.6 months for breast cancer, and 2.5 months for lung cancer and other malignancies. When stratified based on the combination of these 4 variables, patients in the high-risk group had a median survival of just 44 days compared to 130 days for the moderate-risk group and 319 days for the low-risk group. Additional, more complex prediction systems for survival and response to MPE therapies are now emerging and may provide clinicians and patients with additional information useful in medical decision-making.42

Conclusion
MPEs represent advanced stage disease and frequently adversely affect a patient’s quality of life. Ideal therapeutic options, discussed in the second part of this review, should effectively palliate symptoms, provide long-term relief, be minimally invasive with few side effects, minimize hospitalization and reliance on medical assistance, and be cost-effective.
1. Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J. 2001;18:402-419.
2. Society AT. Management of malignant pleural effusions. Am J Respir Crit Care Med. 2000;162:1987-2001.
3. Clive AO, Kahan BC, Hooper CE, et al. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax. 2014;69:1098-1104.
4. Taghizadeh N, Fortin M, Tremblay A. US hospitalizations for malignant pleural effusions: data from the 2012 National Inpatient Sample. Chest. 2017;151:845-854.
5. Yang TS, Hsia DW, Chang DW. Patient- and hospital-level factors associated with readmission for malignant pleural effusion. J Oncol Pract. 2018;14:e547-e556.
6. Ost DE, Niu J, Zhao H, et al. Quality gaps and comparative effectiveness of management strategies for recurrent malignant pleural effusions. Chest. 2018;153:438-452.
7. Miserocchi G. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J. 1997;10:219-225.
8. Sahn SA. State of the art. The pleura. Am Rev Respir Dis. 1988;138:184-234.
9. Khaleeq G, Musani AI. Emerging paradigms in the management of malignant pleural effusions. Respir Med. 2008;102:939-948.
10. Das DK. Serous effusions in malignant lymphomas: a review. Diagn Cytopathol. 2006;34:335-347.
11. Qian Q, Zhan P, Sun WK, et al. Vascular endothelial growth factor and soluble intercellular adhesion molecule-1 in lung adenocarcinoma with malignant pleural effusion: correlations with patient survival and pleural effusion control. Neoplasma. 2012;59:433-439.
12. Kraft A, Weindel K, Ochs A, et al. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer. 1999;85:178-187.
13. Meyer PC. Metastatic carcinoma of the pleura. Thorax. 1966;21:437-443.
14. Light RW, Hamm H. Malignant pleural effusion: would the real cause please stand up? Eur Respir J. 1997;10:1701-1702.
15. Chernow B, Sahn SA. Carcinomatous involvement of the pleura: an analysis of 96 patients. Am J Med. 1977;63:695-702.
16. Musani AI, Haas AR, Seijo L, et al. Outpatient management of malignant pleural effusions with small-bore, tunneled pleural catheters. Respiration. 2004;71:559-566.
17. Roberts ME, Neville E, Berrisford RG, et al; Group BPDG. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii32-40.
18. Estenne M, Yernault JC, De Troyer A. Mechanism of relief of dyspnea after thoracocentesis in patients with large pleural effusions. Am J Med. 1983;74:813-819.
19. Brown NE, Zamel N, Aberman A. Changes in pulmonary mechanics and gas exchange following thoracocentesis. Chest. 1978;74:540-542.
20. Wang LM, Cherng JM, Wang JS. Improved lung function after thoracocentesis in patients with paradoxical movement of a hemidiaphragm secondary to a large pleural effusion. Respirology. 2007;12:719-723.
21. Huggins JT, Doelken P, Sahn SA. The unexpandable lung. F1000 Med Rep. 2010;2:77.
22. Lan RS, Lo SK, Chuang ML, Yang CT, Tsao TC, Lee CH. Elastance of the pleural space: a predictor for the outcome of pleurodesis in patients with malignant pleural effusion. Ann Intern Med. 1997;126:768-774.
23. Lentz RJ, Lerner AD, Pannu JK, et al. Routine monitoring with pleural manometry during therapeutic large-volume thoracentesis to prevent pleural-pressure-related complications: a multicentre, single-blind randomised controlled trial. Lancet Respir Med. 2019;7:447-455.
24. Porcel JM, Alvarez M, Salud A, Vives M. Should a cytologic study be ordered in transudative pleural effusions? Chest. 1999;116:1836-1837.
25. Ryu JS, Ryu ST, Kim YS, et al. What is the clinical significance of transudative malignant pleural effusion? Korean J Intern Med. 2003;18:230-233.
26. Boersma WG, Stigt JA, Smit HJ. Treatment of haemothorax. Respir Med. 2010;104:1583-1587.
27. Light RW, Erozan YS, Ball WC. Cells in pleural fluid. Their value in differential diagnosis. Arch Intern Med. 1973;132:854-860.
28. Garcia LW, Ducatman BS, Wang HH. The value of multiple fluid specimens in the cytological diagnosis of malignancy. Mod Pathol. 1994;7:665-668.
29. Swiderek J, Morcos S, Donthireddy V, et al. Prospective study to determine the volume of pleural fluid required to diagnose malignancy. Chest. 2010;137:68-73.
30. Abouzgheib W, Bartter T, Dagher H, Pratter M, Klump W. A prospective study of the volume of pleural fluid required for accurate diagnosis of malignant pleural effusion. Chest. 2009;135:999-1001.
31. DeMaio A, Clarke JM, Dash R, et al. Yield of malignant pleural effusion for detection of oncogenic driver mutations in lung adenocarcinoma. J Bronchology Interv Pulmonol. 2019;26:96-101.
32. Grosu HB, Arriola A, Stewart J, et al. PD-L1 detection in histology specimens and matched pleural fluid cell blocks of patients with NSCLC. Respirology. 2019 Jun 17. doi: 10.1111/resp.13614.
33. Koegelenberg CF, Diacon AH. Pleural controversy: close needle pleural biopsy or thoracoscopy-which first? Respirology. 2011;16:738-746.
34. McLaughlin KM, Kerr KM, Currie GP. Closed pleural biopsy to diagnose mesothelioma: dead or alive? Lung Cancer. 2009;65:388-389.
35. Miyoshi S, Sasada S, Izumo T, et al. Diagnostic utility of pleural fluid cell block versus pleural biopsy collected by flex-rigid pleuroscopy for malignant pleural disease: a single center retrospective analysis. PLoS One. 2016;11:e0167186.
36. Vanderlaan PA, Yamaguchi N, Folch E, et al. Success and failure rates of tumor genotyping techniques in routine pathological samples with non-small-cell lung cancer. Lung Cancer. 2014;84:39-44.
37. Albanna AS, Kasymjanova G, Robitaille C, et al. Comparison of the yield of different diagnostic procedures for cellular differentiation and genetic profiling of non-small-cell lung cancer. J Thorac Oncol. 2014;9:1120-1125.
38. Tremblay A RS, Berthiaume L, and Michaud G. Natural history of asymptomatic pleural effusions in lung cancer patients. J Bronchol. 2007;14:98-100.
39. Porcel JM, Gasol A, Bielsa S, et al. Clinical features and survival of lung cancer patients with pleural effusions. Respirology. 2015;20:654-659.
40. Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med. 2018;198:839-849.
41. Grosu HB, Molina S, Casal R, et al. Risk factors for pleural effusion recurrence in patients with malignancy. Respirology. 2019;24:76-82.
42. Psallidas I, Kanellakis NI, Gerry S, et al. Development and validation of response markers to predict survival and pleurodesis success in patients with malignant pleural effusion (PROMISE): a multicohort analysis. Lancet Oncol. 2018;19:930-939.
1. Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J. 2001;18:402-419.
2. Society AT. Management of malignant pleural effusions. Am J Respir Crit Care Med. 2000;162:1987-2001.
3. Clive AO, Kahan BC, Hooper CE, et al. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax. 2014;69:1098-1104.
4. Taghizadeh N, Fortin M, Tremblay A. US hospitalizations for malignant pleural effusions: data from the 2012 National Inpatient Sample. Chest. 2017;151:845-854.
5. Yang TS, Hsia DW, Chang DW. Patient- and hospital-level factors associated with readmission for malignant pleural effusion. J Oncol Pract. 2018;14:e547-e556.
6. Ost DE, Niu J, Zhao H, et al. Quality gaps and comparative effectiveness of management strategies for recurrent malignant pleural effusions. Chest. 2018;153:438-452.
7. Miserocchi G. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J. 1997;10:219-225.
8. Sahn SA. State of the art. The pleura. Am Rev Respir Dis. 1988;138:184-234.
9. Khaleeq G, Musani AI. Emerging paradigms in the management of malignant pleural effusions. Respir Med. 2008;102:939-948.
10. Das DK. Serous effusions in malignant lymphomas: a review. Diagn Cytopathol. 2006;34:335-347.
11. Qian Q, Zhan P, Sun WK, et al. Vascular endothelial growth factor and soluble intercellular adhesion molecule-1 in lung adenocarcinoma with malignant pleural effusion: correlations with patient survival and pleural effusion control. Neoplasma. 2012;59:433-439.
12. Kraft A, Weindel K, Ochs A, et al. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer. 1999;85:178-187.
13. Meyer PC. Metastatic carcinoma of the pleura. Thorax. 1966;21:437-443.
14. Light RW, Hamm H. Malignant pleural effusion: would the real cause please stand up? Eur Respir J. 1997;10:1701-1702.
15. Chernow B, Sahn SA. Carcinomatous involvement of the pleura: an analysis of 96 patients. Am J Med. 1977;63:695-702.
16. Musani AI, Haas AR, Seijo L, et al. Outpatient management of malignant pleural effusions with small-bore, tunneled pleural catheters. Respiration. 2004;71:559-566.
17. Roberts ME, Neville E, Berrisford RG, et al; Group BPDG. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii32-40.
18. Estenne M, Yernault JC, De Troyer A. Mechanism of relief of dyspnea after thoracocentesis in patients with large pleural effusions. Am J Med. 1983;74:813-819.
19. Brown NE, Zamel N, Aberman A. Changes in pulmonary mechanics and gas exchange following thoracocentesis. Chest. 1978;74:540-542.
20. Wang LM, Cherng JM, Wang JS. Improved lung function after thoracocentesis in patients with paradoxical movement of a hemidiaphragm secondary to a large pleural effusion. Respirology. 2007;12:719-723.
21. Huggins JT, Doelken P, Sahn SA. The unexpandable lung. F1000 Med Rep. 2010;2:77.
22. Lan RS, Lo SK, Chuang ML, Yang CT, Tsao TC, Lee CH. Elastance of the pleural space: a predictor for the outcome of pleurodesis in patients with malignant pleural effusion. Ann Intern Med. 1997;126:768-774.
23. Lentz RJ, Lerner AD, Pannu JK, et al. Routine monitoring with pleural manometry during therapeutic large-volume thoracentesis to prevent pleural-pressure-related complications: a multicentre, single-blind randomised controlled trial. Lancet Respir Med. 2019;7:447-455.
24. Porcel JM, Alvarez M, Salud A, Vives M. Should a cytologic study be ordered in transudative pleural effusions? Chest. 1999;116:1836-1837.
25. Ryu JS, Ryu ST, Kim YS, et al. What is the clinical significance of transudative malignant pleural effusion? Korean J Intern Med. 2003;18:230-233.
26. Boersma WG, Stigt JA, Smit HJ. Treatment of haemothorax. Respir Med. 2010;104:1583-1587.
27. Light RW, Erozan YS, Ball WC. Cells in pleural fluid. Their value in differential diagnosis. Arch Intern Med. 1973;132:854-860.
28. Garcia LW, Ducatman BS, Wang HH. The value of multiple fluid specimens in the cytological diagnosis of malignancy. Mod Pathol. 1994;7:665-668.
29. Swiderek J, Morcos S, Donthireddy V, et al. Prospective study to determine the volume of pleural fluid required to diagnose malignancy. Chest. 2010;137:68-73.
30. Abouzgheib W, Bartter T, Dagher H, Pratter M, Klump W. A prospective study of the volume of pleural fluid required for accurate diagnosis of malignant pleural effusion. Chest. 2009;135:999-1001.
31. DeMaio A, Clarke JM, Dash R, et al. Yield of malignant pleural effusion for detection of oncogenic driver mutations in lung adenocarcinoma. J Bronchology Interv Pulmonol. 2019;26:96-101.
32. Grosu HB, Arriola A, Stewart J, et al. PD-L1 detection in histology specimens and matched pleural fluid cell blocks of patients with NSCLC. Respirology. 2019 Jun 17. doi: 10.1111/resp.13614.
33. Koegelenberg CF, Diacon AH. Pleural controversy: close needle pleural biopsy or thoracoscopy-which first? Respirology. 2011;16:738-746.
34. McLaughlin KM, Kerr KM, Currie GP. Closed pleural biopsy to diagnose mesothelioma: dead or alive? Lung Cancer. 2009;65:388-389.
35. Miyoshi S, Sasada S, Izumo T, et al. Diagnostic utility of pleural fluid cell block versus pleural biopsy collected by flex-rigid pleuroscopy for malignant pleural disease: a single center retrospective analysis. PLoS One. 2016;11:e0167186.
36. Vanderlaan PA, Yamaguchi N, Folch E, et al. Success and failure rates of tumor genotyping techniques in routine pathological samples with non-small-cell lung cancer. Lung Cancer. 2014;84:39-44.
37. Albanna AS, Kasymjanova G, Robitaille C, et al. Comparison of the yield of different diagnostic procedures for cellular differentiation and genetic profiling of non-small-cell lung cancer. J Thorac Oncol. 2014;9:1120-1125.
38. Tremblay A RS, Berthiaume L, and Michaud G. Natural history of asymptomatic pleural effusions in lung cancer patients. J Bronchol. 2007;14:98-100.
39. Porcel JM, Gasol A, Bielsa S, et al. Clinical features and survival of lung cancer patients with pleural effusions. Respirology. 2015;20:654-659.
40. Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med. 2018;198:839-849.
41. Grosu HB, Molina S, Casal R, et al. Risk factors for pleural effusion recurrence in patients with malignancy. Respirology. 2019;24:76-82.
42. Psallidas I, Kanellakis NI, Gerry S, et al. Development and validation of response markers to predict survival and pleurodesis success in patients with malignant pleural effusion (PROMISE): a multicohort analysis. Lancet Oncol. 2018;19:930-939.
First death from severe lung illness associated with vaping reported in Illinois
The first death to occur in a patient with severe lung illness associated with e-cigarette product use has been reported in Illinois, officials announced at a Centers for Disease Control and Prevention telebriefing.
The cause for the mysterious lung illnesses has not been determined, but an infectious disease does not appear to be implicated. As of yesterday, 193 potential cases have been identified in 22 states since June 28.
No specific product has been implicated in all cases, and it is unclear if there is a common cause or if these are several diseases with a similar presentation.
Wisconsin and Illinois have asked the CDC to directly assist them in their investigations of cases. Other states are handling their own investigations. Further information is available from the CDC at cdc.gov/e-cigarettes.
There have been 22 cases of the illness in Illinois and an additional 12 individuals are being evaluated as possible cases, according to Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist, Illinois Department of Public Health.
Illinois is working with the CDC and the Food and Drug Administration to investigate devices that affected patients have used. No specific product has been implicated across all cases; all patients have reported vaping in recent months Several patients in Illinois have reported using tetrahydrocannabinol (THC) product oils, but Dr. Layden reiterated the investigations are reliant on information reported by affected patients only.
Mitch Zeller, JD, director, Center for Tobacco Products at the FDA, said product samples from a number of states are being evaluated to determine their contents. The FDA is examining samples sent and trying to identify product contents.
The cases reported to date have been in adults aged 17-38 years and have occurred primarily men. The investigation is in a relatively early stage and is working with incomplete case reports. These will become standardized to include more specific information, such as the name of the product, where it was purchased, and whether it was used as intended or whether other products were added, he said.
As e-cigarettes are not a new product, it’s possible that cases of this illness has been occurring but that the link was not recognized, and the cases were neither captured nor reported, said Brian King, PhD, MPH, deputy director, Research Translation, Office on Smoking and Health, CDC. He noted that e-cigarettes may contain “a variety of constituents that could be problematic in terms of pulmonary illness,” such as ingredients in certain flavorings and ultrafine particulates.
The agencies are now trying to harmonize reporting across all states so cases can be evaluated in a more standardized way. Information on standardized reporting on a national level will be issued in the next few days, according to the CDC.
The CDC notified U.S. health care systems and clinicians about the illnesses and what to watch for via a Clinician Outreach and Communication Activity Clinical Action Message.
In general, patients have reported a gradual onset of symptoms including shortness of breath or chest pain that increased over days or weeks before hospital admission. Gastrointestinal symptoms including vomiting, diarrhea, and fatigue have been reported by some.
The first death to occur in a patient with severe lung illness associated with e-cigarette product use has been reported in Illinois, officials announced at a Centers for Disease Control and Prevention telebriefing.
The cause for the mysterious lung illnesses has not been determined, but an infectious disease does not appear to be implicated. As of yesterday, 193 potential cases have been identified in 22 states since June 28.
No specific product has been implicated in all cases, and it is unclear if there is a common cause or if these are several diseases with a similar presentation.
Wisconsin and Illinois have asked the CDC to directly assist them in their investigations of cases. Other states are handling their own investigations. Further information is available from the CDC at cdc.gov/e-cigarettes.
There have been 22 cases of the illness in Illinois and an additional 12 individuals are being evaluated as possible cases, according to Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist, Illinois Department of Public Health.
Illinois is working with the CDC and the Food and Drug Administration to investigate devices that affected patients have used. No specific product has been implicated across all cases; all patients have reported vaping in recent months Several patients in Illinois have reported using tetrahydrocannabinol (THC) product oils, but Dr. Layden reiterated the investigations are reliant on information reported by affected patients only.
Mitch Zeller, JD, director, Center for Tobacco Products at the FDA, said product samples from a number of states are being evaluated to determine their contents. The FDA is examining samples sent and trying to identify product contents.
The cases reported to date have been in adults aged 17-38 years and have occurred primarily men. The investigation is in a relatively early stage and is working with incomplete case reports. These will become standardized to include more specific information, such as the name of the product, where it was purchased, and whether it was used as intended or whether other products were added, he said.
As e-cigarettes are not a new product, it’s possible that cases of this illness has been occurring but that the link was not recognized, and the cases were neither captured nor reported, said Brian King, PhD, MPH, deputy director, Research Translation, Office on Smoking and Health, CDC. He noted that e-cigarettes may contain “a variety of constituents that could be problematic in terms of pulmonary illness,” such as ingredients in certain flavorings and ultrafine particulates.
The agencies are now trying to harmonize reporting across all states so cases can be evaluated in a more standardized way. Information on standardized reporting on a national level will be issued in the next few days, according to the CDC.
The CDC notified U.S. health care systems and clinicians about the illnesses and what to watch for via a Clinician Outreach and Communication Activity Clinical Action Message.
In general, patients have reported a gradual onset of symptoms including shortness of breath or chest pain that increased over days or weeks before hospital admission. Gastrointestinal symptoms including vomiting, diarrhea, and fatigue have been reported by some.
The first death to occur in a patient with severe lung illness associated with e-cigarette product use has been reported in Illinois, officials announced at a Centers for Disease Control and Prevention telebriefing.
The cause for the mysterious lung illnesses has not been determined, but an infectious disease does not appear to be implicated. As of yesterday, 193 potential cases have been identified in 22 states since June 28.
No specific product has been implicated in all cases, and it is unclear if there is a common cause or if these are several diseases with a similar presentation.
Wisconsin and Illinois have asked the CDC to directly assist them in their investigations of cases. Other states are handling their own investigations. Further information is available from the CDC at cdc.gov/e-cigarettes.
There have been 22 cases of the illness in Illinois and an additional 12 individuals are being evaluated as possible cases, according to Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist, Illinois Department of Public Health.
Illinois is working with the CDC and the Food and Drug Administration to investigate devices that affected patients have used. No specific product has been implicated across all cases; all patients have reported vaping in recent months Several patients in Illinois have reported using tetrahydrocannabinol (THC) product oils, but Dr. Layden reiterated the investigations are reliant on information reported by affected patients only.
Mitch Zeller, JD, director, Center for Tobacco Products at the FDA, said product samples from a number of states are being evaluated to determine their contents. The FDA is examining samples sent and trying to identify product contents.
The cases reported to date have been in adults aged 17-38 years and have occurred primarily men. The investigation is in a relatively early stage and is working with incomplete case reports. These will become standardized to include more specific information, such as the name of the product, where it was purchased, and whether it was used as intended or whether other products were added, he said.
As e-cigarettes are not a new product, it’s possible that cases of this illness has been occurring but that the link was not recognized, and the cases were neither captured nor reported, said Brian King, PhD, MPH, deputy director, Research Translation, Office on Smoking and Health, CDC. He noted that e-cigarettes may contain “a variety of constituents that could be problematic in terms of pulmonary illness,” such as ingredients in certain flavorings and ultrafine particulates.
The agencies are now trying to harmonize reporting across all states so cases can be evaluated in a more standardized way. Information on standardized reporting on a national level will be issued in the next few days, according to the CDC.
The CDC notified U.S. health care systems and clinicians about the illnesses and what to watch for via a Clinician Outreach and Communication Activity Clinical Action Message.
In general, patients have reported a gradual onset of symptoms including shortness of breath or chest pain that increased over days or weeks before hospital admission. Gastrointestinal symptoms including vomiting, diarrhea, and fatigue have been reported by some.
Years ago, this doctor linked a mysterious lung disease to vaping
John E. Parker, MD, was working at a West Virginia hospital in 2015 when a 31-year-old female patient was admitted with acute respiratory problems. A team of doctors ultimately suspected that her mysterious case of lipoid pneumonia might be related to vaping and weren’t sure they had seen anything like it before. They were intrigued enough to submit the case for presentation at the CHEST Annual Meeting that year (Chest. 2015;148:382A. doi: 10.1378/chest.2274860).
Now, almost 4 years later, federal officials have begun investigating a national outbreak of severe lung illnesses linked to vaping that has struck more than 150 patients in 16 states. In an interview, Dr. Parker, a professor of pulmonary critical care and sleep medicine at West Virginia University, Morgantown, described what happened.
Q: Can you describe what the patient’s symptoms were when she arrived?
We would view them as classic for what is getting to be called vaping-associated lung disease. She was very, very short of breath and had a cough, and we were, of course, very worried that she might have pneumonia or some other acute respiratory illness. And then she was so sick she needed to be intubated.
Q: What happens next in cases like this?
We look for things like a [hemorrhage] or an active infection. And then for lipid-containing macrophages. And then we usually start some antibiotics [and a] low-dose steroid and then support the patient with a ventilator and oxygen and nutrition. And then just kind of wait and see if any other cultures come back to prove anything different than what you might be thinking.
Early on, we just felt like it was an unusual case and may not be a common viral or bacterial infection.
Q: How did you figure out the cause of her lipoid pneumonia was e-cigarettes?
It’s a diagnosis of exclusion. We excluded other [options], and it became the most likely cause.
We were convinced enough that the case was submitted for [presentation at the CHEST annual meeting] and was accepted.
Q: Once you figured out the cause could be e-cigarettes, did you contact the Centers for Disease Control and Prevention or the Food and Drug Administration or any other regulatory agency to tell them about this?
We did not. We felt at the time that putting it in the medical literature was appropriate. And if other case reports from other parts of the country came forward, then we’d have more of a clustering of findings that might then warrant research agencies [getting a] better understanding [about] the cause of the disease.
Q: Which federal agency would you report it to, if you did?
In 2015, the FDA, of course, was still regulating cigarettes, but I don’t think the government had yet decided who would regulate vaping products. So I’m sure it was unclear who we should call.
Q: So did you or your team think this was a one-off event when you witnessed it?
We really felt that it wasn’t going to be a one-off event and that it was what we usually called in public health a “sentinel” health event … that it was an example of a respiratory illness that can be caused by this exposure and that it probably wasn’t the first case ever seen nor would it be the last.
Q: Was it the first case that you had seen at your institution?
To our knowledge it was our first case, but we are humble enough clinicians to realize we may have missed some other cases that we interpreted [as] viral pneumonia or bacterial pneumonia.
Q: Have you seen more cases since then?
I know we’ve seen a case [of alveolar hemorrhage syndrome] that we published, and in polling some colleagues, we think we’ve probably also seen [cases of] cryptogenic organizing pneumonia as well as lipoid pneumonia and acute eosinophilic pneumonia. Yeah, we’ve certainly seen at least probably four forms of lung disease from vaping.
Q: If your team was seeing this back in 2015, is it possible that it’s been happening in the four years since then and people just don’t know about it?
I really have every reason to think we were not the first ones to see it, by any means.
And I don’t think we were even the first ones to report it. I think that there were some clusters in Wisconsin and some other places in the United States. I also know that the Japanese have been very interested. They’ve probably got four or five papers at least in the medical literature about vaping-related lung injury.
Q: Do you have a theory of what might be causing the lipoid pneumonia cases? Do you think there may be certain chemicals that are irritants?
We need a strong multidisciplinary team to understand the real etiology and cause of lung injury from inhalation. I think it could be any number of components in the mixtures. Lungs don’t like oil, in general, and probably the most specific agent that’s been studied recently is diacetyl, which was studied in popcorn-flavoring lung disease.
Q: Have these kinds of cases changed the way you approach patients?
Yeah, we search very carefully for a history of vaping. … I think it’s quite important to understand if they might be using inhaled agents or vaping that might present new toxicities to the lung.
Q: Will these illnesses have long-term health effects?
An inhalational injury may cause an acute lung injury that’s life-threatening and that someone may survive from and have no long-term sequelae [condition]. But there also is the possibility that long-term [e-cigarette] use may cause more insidious or chronic diseases from which there may not be a full recovery.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
John E. Parker, MD, was working at a West Virginia hospital in 2015 when a 31-year-old female patient was admitted with acute respiratory problems. A team of doctors ultimately suspected that her mysterious case of lipoid pneumonia might be related to vaping and weren’t sure they had seen anything like it before. They were intrigued enough to submit the case for presentation at the CHEST Annual Meeting that year (Chest. 2015;148:382A. doi: 10.1378/chest.2274860).
Now, almost 4 years later, federal officials have begun investigating a national outbreak of severe lung illnesses linked to vaping that has struck more than 150 patients in 16 states. In an interview, Dr. Parker, a professor of pulmonary critical care and sleep medicine at West Virginia University, Morgantown, described what happened.
Q: Can you describe what the patient’s symptoms were when she arrived?
We would view them as classic for what is getting to be called vaping-associated lung disease. She was very, very short of breath and had a cough, and we were, of course, very worried that she might have pneumonia or some other acute respiratory illness. And then she was so sick she needed to be intubated.
Q: What happens next in cases like this?
We look for things like a [hemorrhage] or an active infection. And then for lipid-containing macrophages. And then we usually start some antibiotics [and a] low-dose steroid and then support the patient with a ventilator and oxygen and nutrition. And then just kind of wait and see if any other cultures come back to prove anything different than what you might be thinking.
Early on, we just felt like it was an unusual case and may not be a common viral or bacterial infection.
Q: How did you figure out the cause of her lipoid pneumonia was e-cigarettes?
It’s a diagnosis of exclusion. We excluded other [options], and it became the most likely cause.
We were convinced enough that the case was submitted for [presentation at the CHEST annual meeting] and was accepted.
Q: Once you figured out the cause could be e-cigarettes, did you contact the Centers for Disease Control and Prevention or the Food and Drug Administration or any other regulatory agency to tell them about this?
We did not. We felt at the time that putting it in the medical literature was appropriate. And if other case reports from other parts of the country came forward, then we’d have more of a clustering of findings that might then warrant research agencies [getting a] better understanding [about] the cause of the disease.
Q: Which federal agency would you report it to, if you did?
In 2015, the FDA, of course, was still regulating cigarettes, but I don’t think the government had yet decided who would regulate vaping products. So I’m sure it was unclear who we should call.
Q: So did you or your team think this was a one-off event when you witnessed it?
We really felt that it wasn’t going to be a one-off event and that it was what we usually called in public health a “sentinel” health event … that it was an example of a respiratory illness that can be caused by this exposure and that it probably wasn’t the first case ever seen nor would it be the last.
Q: Was it the first case that you had seen at your institution?
To our knowledge it was our first case, but we are humble enough clinicians to realize we may have missed some other cases that we interpreted [as] viral pneumonia or bacterial pneumonia.
Q: Have you seen more cases since then?
I know we’ve seen a case [of alveolar hemorrhage syndrome] that we published, and in polling some colleagues, we think we’ve probably also seen [cases of] cryptogenic organizing pneumonia as well as lipoid pneumonia and acute eosinophilic pneumonia. Yeah, we’ve certainly seen at least probably four forms of lung disease from vaping.
Q: If your team was seeing this back in 2015, is it possible that it’s been happening in the four years since then and people just don’t know about it?
I really have every reason to think we were not the first ones to see it, by any means.
And I don’t think we were even the first ones to report it. I think that there were some clusters in Wisconsin and some other places in the United States. I also know that the Japanese have been very interested. They’ve probably got four or five papers at least in the medical literature about vaping-related lung injury.
Q: Do you have a theory of what might be causing the lipoid pneumonia cases? Do you think there may be certain chemicals that are irritants?
We need a strong multidisciplinary team to understand the real etiology and cause of lung injury from inhalation. I think it could be any number of components in the mixtures. Lungs don’t like oil, in general, and probably the most specific agent that’s been studied recently is diacetyl, which was studied in popcorn-flavoring lung disease.
Q: Have these kinds of cases changed the way you approach patients?
Yeah, we search very carefully for a history of vaping. … I think it’s quite important to understand if they might be using inhaled agents or vaping that might present new toxicities to the lung.
Q: Will these illnesses have long-term health effects?
An inhalational injury may cause an acute lung injury that’s life-threatening and that someone may survive from and have no long-term sequelae [condition]. But there also is the possibility that long-term [e-cigarette] use may cause more insidious or chronic diseases from which there may not be a full recovery.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
John E. Parker, MD, was working at a West Virginia hospital in 2015 when a 31-year-old female patient was admitted with acute respiratory problems. A team of doctors ultimately suspected that her mysterious case of lipoid pneumonia might be related to vaping and weren’t sure they had seen anything like it before. They were intrigued enough to submit the case for presentation at the CHEST Annual Meeting that year (Chest. 2015;148:382A. doi: 10.1378/chest.2274860).
Now, almost 4 years later, federal officials have begun investigating a national outbreak of severe lung illnesses linked to vaping that has struck more than 150 patients in 16 states. In an interview, Dr. Parker, a professor of pulmonary critical care and sleep medicine at West Virginia University, Morgantown, described what happened.
Q: Can you describe what the patient’s symptoms were when she arrived?
We would view them as classic for what is getting to be called vaping-associated lung disease. She was very, very short of breath and had a cough, and we were, of course, very worried that she might have pneumonia or some other acute respiratory illness. And then she was so sick she needed to be intubated.
Q: What happens next in cases like this?
We look for things like a [hemorrhage] or an active infection. And then for lipid-containing macrophages. And then we usually start some antibiotics [and a] low-dose steroid and then support the patient with a ventilator and oxygen and nutrition. And then just kind of wait and see if any other cultures come back to prove anything different than what you might be thinking.
Early on, we just felt like it was an unusual case and may not be a common viral or bacterial infection.
Q: How did you figure out the cause of her lipoid pneumonia was e-cigarettes?
It’s a diagnosis of exclusion. We excluded other [options], and it became the most likely cause.
We were convinced enough that the case was submitted for [presentation at the CHEST annual meeting] and was accepted.
Q: Once you figured out the cause could be e-cigarettes, did you contact the Centers for Disease Control and Prevention or the Food and Drug Administration or any other regulatory agency to tell them about this?
We did not. We felt at the time that putting it in the medical literature was appropriate. And if other case reports from other parts of the country came forward, then we’d have more of a clustering of findings that might then warrant research agencies [getting a] better understanding [about] the cause of the disease.
Q: Which federal agency would you report it to, if you did?
In 2015, the FDA, of course, was still regulating cigarettes, but I don’t think the government had yet decided who would regulate vaping products. So I’m sure it was unclear who we should call.
Q: So did you or your team think this was a one-off event when you witnessed it?
We really felt that it wasn’t going to be a one-off event and that it was what we usually called in public health a “sentinel” health event … that it was an example of a respiratory illness that can be caused by this exposure and that it probably wasn’t the first case ever seen nor would it be the last.
Q: Was it the first case that you had seen at your institution?
To our knowledge it was our first case, but we are humble enough clinicians to realize we may have missed some other cases that we interpreted [as] viral pneumonia or bacterial pneumonia.
Q: Have you seen more cases since then?
I know we’ve seen a case [of alveolar hemorrhage syndrome] that we published, and in polling some colleagues, we think we’ve probably also seen [cases of] cryptogenic organizing pneumonia as well as lipoid pneumonia and acute eosinophilic pneumonia. Yeah, we’ve certainly seen at least probably four forms of lung disease from vaping.
Q: If your team was seeing this back in 2015, is it possible that it’s been happening in the four years since then and people just don’t know about it?
I really have every reason to think we were not the first ones to see it, by any means.
And I don’t think we were even the first ones to report it. I think that there were some clusters in Wisconsin and some other places in the United States. I also know that the Japanese have been very interested. They’ve probably got four or five papers at least in the medical literature about vaping-related lung injury.
Q: Do you have a theory of what might be causing the lipoid pneumonia cases? Do you think there may be certain chemicals that are irritants?
We need a strong multidisciplinary team to understand the real etiology and cause of lung injury from inhalation. I think it could be any number of components in the mixtures. Lungs don’t like oil, in general, and probably the most specific agent that’s been studied recently is diacetyl, which was studied in popcorn-flavoring lung disease.
Q: Have these kinds of cases changed the way you approach patients?
Yeah, we search very carefully for a history of vaping. … I think it’s quite important to understand if they might be using inhaled agents or vaping that might present new toxicities to the lung.
Q: Will these illnesses have long-term health effects?
An inhalational injury may cause an acute lung injury that’s life-threatening and that someone may survive from and have no long-term sequelae [condition]. But there also is the possibility that long-term [e-cigarette] use may cause more insidious or chronic diseases from which there may not be a full recovery.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
FUO, pneumonia often distinguishes influenza from RSV in hospitalized young children
LJUBLJANA, SLOVENIA – as the cause of hospitalization in infants and young children, Cihan Papan, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Dr. Papan, a pediatrician at University Children’s Hospital Mannheim (Germany) and Heidelberg (Germany) University, presented a retrospective single-center study of all 573 children aged under 2 years hospitalized over the course of several seasons for respiratory syncytial virus (RSV) or influenza as confirmed by rapid antigen testing. Even though these are two of the leading causes of hospitalization among young children, there is surprisingly sparse data comparing the two in terms of disease severity and hospital resource utilization, including antibiotic consumption. That information gap provided the basis for this study.
There were 476 children with confirmed RSV, 96 with influenza, and 1 RSV/influenza coinfection. Notably, even though the RSV group had lower temperatures and C-reactive protein levels, they were nevertheless more likely to be treated with antibiotics, by a margin of 29% to 23%.
“These findings open new possibilities for antimicrobial stewardship in these groups of virally infected children,” observed Dr. Papan.
Fever of unknown origin was present in 68.8% of the influenza-positive patients, compared with just 0.2% of the RSV-positive children. In contrast, 50.2% of the RSV group had pneumonia and 49.6% had bronchitis or bronchiolitis, versus just 22.9% and 6.3% of the influenza patients, respectively. A larger proportion of the young children with RSV infection presented in a severely ill–looking condition. Children with RSV infection also were significantly younger.
Dr. Papan reported having no financial conflicts regarding his study.
LJUBLJANA, SLOVENIA – as the cause of hospitalization in infants and young children, Cihan Papan, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Dr. Papan, a pediatrician at University Children’s Hospital Mannheim (Germany) and Heidelberg (Germany) University, presented a retrospective single-center study of all 573 children aged under 2 years hospitalized over the course of several seasons for respiratory syncytial virus (RSV) or influenza as confirmed by rapid antigen testing. Even though these are two of the leading causes of hospitalization among young children, there is surprisingly sparse data comparing the two in terms of disease severity and hospital resource utilization, including antibiotic consumption. That information gap provided the basis for this study.
There were 476 children with confirmed RSV, 96 with influenza, and 1 RSV/influenza coinfection. Notably, even though the RSV group had lower temperatures and C-reactive protein levels, they were nevertheless more likely to be treated with antibiotics, by a margin of 29% to 23%.
“These findings open new possibilities for antimicrobial stewardship in these groups of virally infected children,” observed Dr. Papan.
Fever of unknown origin was present in 68.8% of the influenza-positive patients, compared with just 0.2% of the RSV-positive children. In contrast, 50.2% of the RSV group had pneumonia and 49.6% had bronchitis or bronchiolitis, versus just 22.9% and 6.3% of the influenza patients, respectively. A larger proportion of the young children with RSV infection presented in a severely ill–looking condition. Children with RSV infection also were significantly younger.
Dr. Papan reported having no financial conflicts regarding his study.
LJUBLJANA, SLOVENIA – as the cause of hospitalization in infants and young children, Cihan Papan, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
Dr. Papan, a pediatrician at University Children’s Hospital Mannheim (Germany) and Heidelberg (Germany) University, presented a retrospective single-center study of all 573 children aged under 2 years hospitalized over the course of several seasons for respiratory syncytial virus (RSV) or influenza as confirmed by rapid antigen testing. Even though these are two of the leading causes of hospitalization among young children, there is surprisingly sparse data comparing the two in terms of disease severity and hospital resource utilization, including antibiotic consumption. That information gap provided the basis for this study.
There were 476 children with confirmed RSV, 96 with influenza, and 1 RSV/influenza coinfection. Notably, even though the RSV group had lower temperatures and C-reactive protein levels, they were nevertheless more likely to be treated with antibiotics, by a margin of 29% to 23%.
“These findings open new possibilities for antimicrobial stewardship in these groups of virally infected children,” observed Dr. Papan.
Fever of unknown origin was present in 68.8% of the influenza-positive patients, compared with just 0.2% of the RSV-positive children. In contrast, 50.2% of the RSV group had pneumonia and 49.6% had bronchitis or bronchiolitis, versus just 22.9% and 6.3% of the influenza patients, respectively. A larger proportion of the young children with RSV infection presented in a severely ill–looking condition. Children with RSV infection also were significantly younger.
Dr. Papan reported having no financial conflicts regarding his study.
REPORTING FROM ESPID 2019
Vaping illness cases now over 150, CDC says
Officials from the CDC and the Food and Drug Administration are working with state health officials to gather information on the cases as well as any products or substances that might be involved.
A total of 153 potential cases were reported between June 28 and Aug. 20 in 16 states – California, Connecticut, Florida, Illinois, Indiana, Iowa, Michigan, Minnesota, New Jersey, New Mexico, New York, North Carolina, Pennsylvania, Texas, Utah, and Wisconsin.
Health officials have yet to find a cause for these illnesses; however, all patients have reported e-cigarette use or vaping, according to a CDC statement. Evidence to date does not seem to indicate that an infectious agent is the cause.
In general, patients have reported a gradual onset of symptoms including shortness of breath and/or chest pain that increased over days or weeks before hospital admission. Gastrointestinal symptoms including vomiting, diarrhea, and fatigue have been reported by some.
Many patients reported using products containing tetrahydrocannabinol, though no specific or consistent product has been linked definitively.
While cases reported across the country seem to be similar, there is no evidence currently indicating they have a common cause, according to the CDC statement.
The CDC is urging health care professionals to report possible cases to their state or local health department and the FDA is urging the public to provide detailed reports of any unusual or unexpected health concerns related to tobacco use or e-cigarette use through its Safety Reporting Portal.
Officials from the CDC and the Food and Drug Administration are working with state health officials to gather information on the cases as well as any products or substances that might be involved.
A total of 153 potential cases were reported between June 28 and Aug. 20 in 16 states – California, Connecticut, Florida, Illinois, Indiana, Iowa, Michigan, Minnesota, New Jersey, New Mexico, New York, North Carolina, Pennsylvania, Texas, Utah, and Wisconsin.
Health officials have yet to find a cause for these illnesses; however, all patients have reported e-cigarette use or vaping, according to a CDC statement. Evidence to date does not seem to indicate that an infectious agent is the cause.
In general, patients have reported a gradual onset of symptoms including shortness of breath and/or chest pain that increased over days or weeks before hospital admission. Gastrointestinal symptoms including vomiting, diarrhea, and fatigue have been reported by some.
Many patients reported using products containing tetrahydrocannabinol, though no specific or consistent product has been linked definitively.
While cases reported across the country seem to be similar, there is no evidence currently indicating they have a common cause, according to the CDC statement.
The CDC is urging health care professionals to report possible cases to their state or local health department and the FDA is urging the public to provide detailed reports of any unusual or unexpected health concerns related to tobacco use or e-cigarette use through its Safety Reporting Portal.
Officials from the CDC and the Food and Drug Administration are working with state health officials to gather information on the cases as well as any products or substances that might be involved.
A total of 153 potential cases were reported between June 28 and Aug. 20 in 16 states – California, Connecticut, Florida, Illinois, Indiana, Iowa, Michigan, Minnesota, New Jersey, New Mexico, New York, North Carolina, Pennsylvania, Texas, Utah, and Wisconsin.
Health officials have yet to find a cause for these illnesses; however, all patients have reported e-cigarette use or vaping, according to a CDC statement. Evidence to date does not seem to indicate that an infectious agent is the cause.
In general, patients have reported a gradual onset of symptoms including shortness of breath and/or chest pain that increased over days or weeks before hospital admission. Gastrointestinal symptoms including vomiting, diarrhea, and fatigue have been reported by some.
Many patients reported using products containing tetrahydrocannabinol, though no specific or consistent product has been linked definitively.
While cases reported across the country seem to be similar, there is no evidence currently indicating they have a common cause, according to the CDC statement.
The CDC is urging health care professionals to report possible cases to their state or local health department and the FDA is urging the public to provide detailed reports of any unusual or unexpected health concerns related to tobacco use or e-cigarette use through its Safety Reporting Portal.
Impact of climate change on mortality underlined by global study
Regardless of where people live in the world, air pollution is linked to increased rates of cardiovascular disease, respiratory problems, and all-cause mortality, according to one of the largest studies ever to assess the effects of inhalable particulate matter (PM), published Aug. 21 in the New England Journal of Medicine.
“These data reinforce the evidence of a link between mortality and PM concentration established in regional and local studies,” reported Cong Liu of the Huazhong University of Science and Technology in Wuhan, China, and an international team of researchers.
“Many people are experiencing worse allergy and asthma symptoms in the setting of increased heat and worse air quality,” Caren G. Solomon, MD, of Harvard Medical School, Boston, said in an interview. “It is often not appreciated that these are complications of climate change.”
Other such complications include heat-related illnesses and severe weather events, as well as the less visible manifestations, such as shifts in the epidemiology of vector-borne infectious disease, Dr. Solomon and colleagues wrote in an editorial accompanying Mr. Liu’s study.
“The stark reality is that high levels of greenhouse gases caused by the combustion of fossil fuels – and the resulting rise in temperature and sea levels and intensification of extreme weather – are having profound consequences for human health and health systems,” Dr. Solomon and colleagues wrote (N Engl J Med. 2019;381:773-4.).
In the new air pollution study, Mr. Liu and colleagues analyzed 59.6 million deaths from 652 cities across 24 countries, “thereby greatly increasing the generalizability of the association and decreasing the likelihood that the reported associations are subject to confounding bias,” wrote John R. Balmes, MD, of the University of California, San Francisco, and the University of California, Berkeley, in an editorial about the study (N Engl J Med. 2019;381:774-6).
The researchers compared air pollution data from 1986-2015 from the Multi-City Multi-Country (MCC) Collaborative Research Network to mortality data reported from individual countries. They assessed PM with an aerodynamic diameter of 10 mcg or less (PM10; n = 598 cities) and PM with an aerodynamic diameter of 2.5 mcg or less (PM2.5; n=499 cities).
Mr. Liu’s team used a time-series analysis – a standard upon which the majority of air pollution research relies. These studies “include daily measures of health events (e.g., daily mortality), regressed against concentrations of PM (e.g., 24-hour average PM2.5) and weather variables (e.g., daily average temperature) for a given geographic area,” Dr. Balmes wrote. “The population serves as its own control, and confounding by population characteristics is negligible because these are stable over short time frames.”
The researchers found a 0.44% increase in daily all-cause mortality for each 10-mcg/m3 increase in the 2-day moving average (current and previous day) of PM10. The same increase was linked to a 0.36% increase in daily cardiovascular mortality and a 0.47% increase in daily respiratory mortality. Similarly, a 10-mcg/m3 increase in the PM2.5 average was linked to 0.68% increase in all-cause mortality, a 0.55% increase in cardiovascular mortality, and 0.74% increase in respiratory mortality.
Locations with higher annual mean temperatures showed stronger associations, and all these associations remained statistically significant after the researchers adjusted for gaseous pollutants.
Although the majority of countries and cities included in the study came from the northern hemisphere, the researchers noted that the magnitude of effect they found, particularly for PM10 concentrations, matched up with that seen in previous studies of multiple cities or countries.
Still, they found “significant evidence of spatial heterogeneity in the associations between PM concentration and daily mortality across countries and regions.” Among the factors that could contribute to those variations are “different PM components, long-term air pollution levels, population susceptibility, and different lengths of study periods,” they speculated.
What makes this study remarkable – despite decades of previous similar studies – is its size and the implications of a curvilinear shape in its concentration-response relation, according to Dr. Balmes.
“The current study of PM data from many regions around the world provides the strongest evidence to date that higher levels of exposure may be associated with a lower per-unit risk,” Dr. Balmes wrote. “Regions that have lower exposures had a higher per-unit risk. This finding has profound policy implications, especially given that no threshold of effect was found. Even high-income countries, such as the United States, with relatively good air quality could still see public health benefits from further reduction of ambient PM concentrations.”
The policy implications, however, extend well beyond clean air regulations because the findings represent just one aspect of climate change’s negative effects on health, which are “frighteningly broad,” Dr. Solomon and colleagues wrote.
“As climate change continues to alter disease patterns and disrupt health systems, its effects on human health will become harder to ignore,” they wrote. “We, as a medical community, have the responsibility and the opportunity to mobilize the urgent, large-scale climate action required to protect health – as well as the ingenuity to develop novel and bold interventions to avert the most catastrophic outcomes.”
The new research and associated commentary marked the introduction of a new NEJM topic on climate change effects on health and health systems.
SOURCE: Liu C et al. N Engl J Med. 2019;381:705-15.
This article was updated 8/22/19.
The negative effects of climate change on global public health are already playing out around us, but scientific research shows that they will only get worse – unless we begin addressing the issue in earnest now.
At the macro level nationally, effective policy is actually being stripped away right now. “[While] scientists tell us we have little time to wait if we hope to avoid the most devastating effects of climate change, leaders in Washington, D.C., are attacking science and rolling back Obama-era rules from the Environmental Protection Agency,” such as working to weaken vehicle fuel-efficiency standards, relaxing methane emissions rules, ending mercury emissions regulation and taking other actions that will only increase air pollution.
“If these EPA rollbacks are successful, they will diminish our ability to mitigate health effects and diseases related to the burning of fossil fuels and the immense toll they take on our families. ... If we stop supporting and listening to the best available science, if we allow more pollution to be emitted, and if we start limiting the EPA’s ability to monitor and enforce pollution standards, then we put at risk everyone’s health – and especially the health and future of our children.”
Engaging in advocacy and communicating to our representatives that we want stronger regulations is one way people can personally take action, but we can take immediate actions in our everyday lives too. Rather than dwelling on the despair of helplessness and hopelessness that grips many people when it comes to climate change, this moment can be reframed as an opportunity for people to make decisions that immediately begin improving their health — and also happen to be good for the planet.
“To me, the most urgent challenge when it comes to health and climate change is the reality that, when climate change comes up, in the U.S. audience, the first thing that should come into people’s minds is that we need to do this now because we need to protect our children’s health. ... Too many people either don’t get that it matters to health at all, or they don’t get that the actions we need to take are exactly what we need to do to address the health problems that have been nearly impossible to deal with.”
For example, problems like rising child obesity and type 2 diabetes rates have plagued public health, yet people can make changes that reduce obesity and diabetes risk that also decrease their carbon footprints, he said. “One of the best ways to deal with obesity is to eat more plants, and it turns out that’s really good for the climate” Additionally, getting people out of cars and walking and cycling can reduce individuals’ risk of diabetes – while simultaneously decreasing air pollution. “We need to be doing these things regardless of climate change, and if parents and children understood that the pathway to a healthier future was through tackling climate change, we would see a transformation.”
The value of local policy actions should be emphasized, such as ones that call for a reduction in a city’s use of concrete – which increases localized heat – and constructing more efficient buildings. Healthcare providers have an opportunity – and responsibility – not only to recognize this reality but to help their patients recognize it too.
“We can also use our roles as trusted advisers to inform and motivate actions that are increasingly necessary to protect the health of the communities we serve.” They also need to be vigilant about conditions that will worsen as the planet heats up: For example, medications such as diuretics carry more risks in higher temperatures, and patients taking them need to know that.
The need to address climate change matters because we face the challenge of protecting the world’s most vulnerable people.
“One of the great things about climate change is if it causes us to rethink about what we need to do to protect the future, it’s going to help our health today. ... If we can use that as the motivator, then maybe we can stop arguing and start thinking about climate as a positive issue, as a more personal issue we can all participate in and be willing to invest in.”
Gina McCarthy, MS, was administrator of the Environmental Protection Agency during 2013-2017, and Aaron Bernstein, MD, MPH, is a pediatrician at Boston Children’s Hospital. Both are from the Center for Climate, Health, and the Global Environment (Harvard C-CHANGE) at the Harvard T.H. Chan School of Public Health in Boston. Their comments came from their perspective (N Engl J Med. 2019 Aug 22. doi: 10.1056/NEJMp1909643) published in NEJM along with this article and editorial and a phone interview. They reported not having any disclosures.
The negative effects of climate change on global public health are already playing out around us, but scientific research shows that they will only get worse – unless we begin addressing the issue in earnest now.
At the macro level nationally, effective policy is actually being stripped away right now. “[While] scientists tell us we have little time to wait if we hope to avoid the most devastating effects of climate change, leaders in Washington, D.C., are attacking science and rolling back Obama-era rules from the Environmental Protection Agency,” such as working to weaken vehicle fuel-efficiency standards, relaxing methane emissions rules, ending mercury emissions regulation and taking other actions that will only increase air pollution.
“If these EPA rollbacks are successful, they will diminish our ability to mitigate health effects and diseases related to the burning of fossil fuels and the immense toll they take on our families. ... If we stop supporting and listening to the best available science, if we allow more pollution to be emitted, and if we start limiting the EPA’s ability to monitor and enforce pollution standards, then we put at risk everyone’s health – and especially the health and future of our children.”
Engaging in advocacy and communicating to our representatives that we want stronger regulations is one way people can personally take action, but we can take immediate actions in our everyday lives too. Rather than dwelling on the despair of helplessness and hopelessness that grips many people when it comes to climate change, this moment can be reframed as an opportunity for people to make decisions that immediately begin improving their health — and also happen to be good for the planet.
“To me, the most urgent challenge when it comes to health and climate change is the reality that, when climate change comes up, in the U.S. audience, the first thing that should come into people’s minds is that we need to do this now because we need to protect our children’s health. ... Too many people either don’t get that it matters to health at all, or they don’t get that the actions we need to take are exactly what we need to do to address the health problems that have been nearly impossible to deal with.”
For example, problems like rising child obesity and type 2 diabetes rates have plagued public health, yet people can make changes that reduce obesity and diabetes risk that also decrease their carbon footprints, he said. “One of the best ways to deal with obesity is to eat more plants, and it turns out that’s really good for the climate” Additionally, getting people out of cars and walking and cycling can reduce individuals’ risk of diabetes – while simultaneously decreasing air pollution. “We need to be doing these things regardless of climate change, and if parents and children understood that the pathway to a healthier future was through tackling climate change, we would see a transformation.”
The value of local policy actions should be emphasized, such as ones that call for a reduction in a city’s use of concrete – which increases localized heat – and constructing more efficient buildings. Healthcare providers have an opportunity – and responsibility – not only to recognize this reality but to help their patients recognize it too.
“We can also use our roles as trusted advisers to inform and motivate actions that are increasingly necessary to protect the health of the communities we serve.” They also need to be vigilant about conditions that will worsen as the planet heats up: For example, medications such as diuretics carry more risks in higher temperatures, and patients taking them need to know that.
The need to address climate change matters because we face the challenge of protecting the world’s most vulnerable people.
“One of the great things about climate change is if it causes us to rethink about what we need to do to protect the future, it’s going to help our health today. ... If we can use that as the motivator, then maybe we can stop arguing and start thinking about climate as a positive issue, as a more personal issue we can all participate in and be willing to invest in.”
Gina McCarthy, MS, was administrator of the Environmental Protection Agency during 2013-2017, and Aaron Bernstein, MD, MPH, is a pediatrician at Boston Children’s Hospital. Both are from the Center for Climate, Health, and the Global Environment (Harvard C-CHANGE) at the Harvard T.H. Chan School of Public Health in Boston. Their comments came from their perspective (N Engl J Med. 2019 Aug 22. doi: 10.1056/NEJMp1909643) published in NEJM along with this article and editorial and a phone interview. They reported not having any disclosures.
The negative effects of climate change on global public health are already playing out around us, but scientific research shows that they will only get worse – unless we begin addressing the issue in earnest now.
At the macro level nationally, effective policy is actually being stripped away right now. “[While] scientists tell us we have little time to wait if we hope to avoid the most devastating effects of climate change, leaders in Washington, D.C., are attacking science and rolling back Obama-era rules from the Environmental Protection Agency,” such as working to weaken vehicle fuel-efficiency standards, relaxing methane emissions rules, ending mercury emissions regulation and taking other actions that will only increase air pollution.
“If these EPA rollbacks are successful, they will diminish our ability to mitigate health effects and diseases related to the burning of fossil fuels and the immense toll they take on our families. ... If we stop supporting and listening to the best available science, if we allow more pollution to be emitted, and if we start limiting the EPA’s ability to monitor and enforce pollution standards, then we put at risk everyone’s health – and especially the health and future of our children.”
Engaging in advocacy and communicating to our representatives that we want stronger regulations is one way people can personally take action, but we can take immediate actions in our everyday lives too. Rather than dwelling on the despair of helplessness and hopelessness that grips many people when it comes to climate change, this moment can be reframed as an opportunity for people to make decisions that immediately begin improving their health — and also happen to be good for the planet.
“To me, the most urgent challenge when it comes to health and climate change is the reality that, when climate change comes up, in the U.S. audience, the first thing that should come into people’s minds is that we need to do this now because we need to protect our children’s health. ... Too many people either don’t get that it matters to health at all, or they don’t get that the actions we need to take are exactly what we need to do to address the health problems that have been nearly impossible to deal with.”
For example, problems like rising child obesity and type 2 diabetes rates have plagued public health, yet people can make changes that reduce obesity and diabetes risk that also decrease their carbon footprints, he said. “One of the best ways to deal with obesity is to eat more plants, and it turns out that’s really good for the climate” Additionally, getting people out of cars and walking and cycling can reduce individuals’ risk of diabetes – while simultaneously decreasing air pollution. “We need to be doing these things regardless of climate change, and if parents and children understood that the pathway to a healthier future was through tackling climate change, we would see a transformation.”
The value of local policy actions should be emphasized, such as ones that call for a reduction in a city’s use of concrete – which increases localized heat – and constructing more efficient buildings. Healthcare providers have an opportunity – and responsibility – not only to recognize this reality but to help their patients recognize it too.
“We can also use our roles as trusted advisers to inform and motivate actions that are increasingly necessary to protect the health of the communities we serve.” They also need to be vigilant about conditions that will worsen as the planet heats up: For example, medications such as diuretics carry more risks in higher temperatures, and patients taking them need to know that.
The need to address climate change matters because we face the challenge of protecting the world’s most vulnerable people.
“One of the great things about climate change is if it causes us to rethink about what we need to do to protect the future, it’s going to help our health today. ... If we can use that as the motivator, then maybe we can stop arguing and start thinking about climate as a positive issue, as a more personal issue we can all participate in and be willing to invest in.”
Gina McCarthy, MS, was administrator of the Environmental Protection Agency during 2013-2017, and Aaron Bernstein, MD, MPH, is a pediatrician at Boston Children’s Hospital. Both are from the Center for Climate, Health, and the Global Environment (Harvard C-CHANGE) at the Harvard T.H. Chan School of Public Health in Boston. Their comments came from their perspective (N Engl J Med. 2019 Aug 22. doi: 10.1056/NEJMp1909643) published in NEJM along with this article and editorial and a phone interview. They reported not having any disclosures.
Regardless of where people live in the world, air pollution is linked to increased rates of cardiovascular disease, respiratory problems, and all-cause mortality, according to one of the largest studies ever to assess the effects of inhalable particulate matter (PM), published Aug. 21 in the New England Journal of Medicine.
“These data reinforce the evidence of a link between mortality and PM concentration established in regional and local studies,” reported Cong Liu of the Huazhong University of Science and Technology in Wuhan, China, and an international team of researchers.
“Many people are experiencing worse allergy and asthma symptoms in the setting of increased heat and worse air quality,” Caren G. Solomon, MD, of Harvard Medical School, Boston, said in an interview. “It is often not appreciated that these are complications of climate change.”
Other such complications include heat-related illnesses and severe weather events, as well as the less visible manifestations, such as shifts in the epidemiology of vector-borne infectious disease, Dr. Solomon and colleagues wrote in an editorial accompanying Mr. Liu’s study.
“The stark reality is that high levels of greenhouse gases caused by the combustion of fossil fuels – and the resulting rise in temperature and sea levels and intensification of extreme weather – are having profound consequences for human health and health systems,” Dr. Solomon and colleagues wrote (N Engl J Med. 2019;381:773-4.).
In the new air pollution study, Mr. Liu and colleagues analyzed 59.6 million deaths from 652 cities across 24 countries, “thereby greatly increasing the generalizability of the association and decreasing the likelihood that the reported associations are subject to confounding bias,” wrote John R. Balmes, MD, of the University of California, San Francisco, and the University of California, Berkeley, in an editorial about the study (N Engl J Med. 2019;381:774-6).
The researchers compared air pollution data from 1986-2015 from the Multi-City Multi-Country (MCC) Collaborative Research Network to mortality data reported from individual countries. They assessed PM with an aerodynamic diameter of 10 mcg or less (PM10; n = 598 cities) and PM with an aerodynamic diameter of 2.5 mcg or less (PM2.5; n=499 cities).
Mr. Liu’s team used a time-series analysis – a standard upon which the majority of air pollution research relies. These studies “include daily measures of health events (e.g., daily mortality), regressed against concentrations of PM (e.g., 24-hour average PM2.5) and weather variables (e.g., daily average temperature) for a given geographic area,” Dr. Balmes wrote. “The population serves as its own control, and confounding by population characteristics is negligible because these are stable over short time frames.”
The researchers found a 0.44% increase in daily all-cause mortality for each 10-mcg/m3 increase in the 2-day moving average (current and previous day) of PM10. The same increase was linked to a 0.36% increase in daily cardiovascular mortality and a 0.47% increase in daily respiratory mortality. Similarly, a 10-mcg/m3 increase in the PM2.5 average was linked to 0.68% increase in all-cause mortality, a 0.55% increase in cardiovascular mortality, and 0.74% increase in respiratory mortality.
Locations with higher annual mean temperatures showed stronger associations, and all these associations remained statistically significant after the researchers adjusted for gaseous pollutants.
Although the majority of countries and cities included in the study came from the northern hemisphere, the researchers noted that the magnitude of effect they found, particularly for PM10 concentrations, matched up with that seen in previous studies of multiple cities or countries.
Still, they found “significant evidence of spatial heterogeneity in the associations between PM concentration and daily mortality across countries and regions.” Among the factors that could contribute to those variations are “different PM components, long-term air pollution levels, population susceptibility, and different lengths of study periods,” they speculated.
What makes this study remarkable – despite decades of previous similar studies – is its size and the implications of a curvilinear shape in its concentration-response relation, according to Dr. Balmes.
“The current study of PM data from many regions around the world provides the strongest evidence to date that higher levels of exposure may be associated with a lower per-unit risk,” Dr. Balmes wrote. “Regions that have lower exposures had a higher per-unit risk. This finding has profound policy implications, especially given that no threshold of effect was found. Even high-income countries, such as the United States, with relatively good air quality could still see public health benefits from further reduction of ambient PM concentrations.”
The policy implications, however, extend well beyond clean air regulations because the findings represent just one aspect of climate change’s negative effects on health, which are “frighteningly broad,” Dr. Solomon and colleagues wrote.
“As climate change continues to alter disease patterns and disrupt health systems, its effects on human health will become harder to ignore,” they wrote. “We, as a medical community, have the responsibility and the opportunity to mobilize the urgent, large-scale climate action required to protect health – as well as the ingenuity to develop novel and bold interventions to avert the most catastrophic outcomes.”
The new research and associated commentary marked the introduction of a new NEJM topic on climate change effects on health and health systems.
SOURCE: Liu C et al. N Engl J Med. 2019;381:705-15.
This article was updated 8/22/19.
Regardless of where people live in the world, air pollution is linked to increased rates of cardiovascular disease, respiratory problems, and all-cause mortality, according to one of the largest studies ever to assess the effects of inhalable particulate matter (PM), published Aug. 21 in the New England Journal of Medicine.
“These data reinforce the evidence of a link between mortality and PM concentration established in regional and local studies,” reported Cong Liu of the Huazhong University of Science and Technology in Wuhan, China, and an international team of researchers.
“Many people are experiencing worse allergy and asthma symptoms in the setting of increased heat and worse air quality,” Caren G. Solomon, MD, of Harvard Medical School, Boston, said in an interview. “It is often not appreciated that these are complications of climate change.”
Other such complications include heat-related illnesses and severe weather events, as well as the less visible manifestations, such as shifts in the epidemiology of vector-borne infectious disease, Dr. Solomon and colleagues wrote in an editorial accompanying Mr. Liu’s study.
“The stark reality is that high levels of greenhouse gases caused by the combustion of fossil fuels – and the resulting rise in temperature and sea levels and intensification of extreme weather – are having profound consequences for human health and health systems,” Dr. Solomon and colleagues wrote (N Engl J Med. 2019;381:773-4.).
In the new air pollution study, Mr. Liu and colleagues analyzed 59.6 million deaths from 652 cities across 24 countries, “thereby greatly increasing the generalizability of the association and decreasing the likelihood that the reported associations are subject to confounding bias,” wrote John R. Balmes, MD, of the University of California, San Francisco, and the University of California, Berkeley, in an editorial about the study (N Engl J Med. 2019;381:774-6).
The researchers compared air pollution data from 1986-2015 from the Multi-City Multi-Country (MCC) Collaborative Research Network to mortality data reported from individual countries. They assessed PM with an aerodynamic diameter of 10 mcg or less (PM10; n = 598 cities) and PM with an aerodynamic diameter of 2.5 mcg or less (PM2.5; n=499 cities).
Mr. Liu’s team used a time-series analysis – a standard upon which the majority of air pollution research relies. These studies “include daily measures of health events (e.g., daily mortality), regressed against concentrations of PM (e.g., 24-hour average PM2.5) and weather variables (e.g., daily average temperature) for a given geographic area,” Dr. Balmes wrote. “The population serves as its own control, and confounding by population characteristics is negligible because these are stable over short time frames.”
The researchers found a 0.44% increase in daily all-cause mortality for each 10-mcg/m3 increase in the 2-day moving average (current and previous day) of PM10. The same increase was linked to a 0.36% increase in daily cardiovascular mortality and a 0.47% increase in daily respiratory mortality. Similarly, a 10-mcg/m3 increase in the PM2.5 average was linked to 0.68% increase in all-cause mortality, a 0.55% increase in cardiovascular mortality, and 0.74% increase in respiratory mortality.
Locations with higher annual mean temperatures showed stronger associations, and all these associations remained statistically significant after the researchers adjusted for gaseous pollutants.
Although the majority of countries and cities included in the study came from the northern hemisphere, the researchers noted that the magnitude of effect they found, particularly for PM10 concentrations, matched up with that seen in previous studies of multiple cities or countries.
Still, they found “significant evidence of spatial heterogeneity in the associations between PM concentration and daily mortality across countries and regions.” Among the factors that could contribute to those variations are “different PM components, long-term air pollution levels, population susceptibility, and different lengths of study periods,” they speculated.
What makes this study remarkable – despite decades of previous similar studies – is its size and the implications of a curvilinear shape in its concentration-response relation, according to Dr. Balmes.
“The current study of PM data from many regions around the world provides the strongest evidence to date that higher levels of exposure may be associated with a lower per-unit risk,” Dr. Balmes wrote. “Regions that have lower exposures had a higher per-unit risk. This finding has profound policy implications, especially given that no threshold of effect was found. Even high-income countries, such as the United States, with relatively good air quality could still see public health benefits from further reduction of ambient PM concentrations.”
The policy implications, however, extend well beyond clean air regulations because the findings represent just one aspect of climate change’s negative effects on health, which are “frighteningly broad,” Dr. Solomon and colleagues wrote.
“As climate change continues to alter disease patterns and disrupt health systems, its effects on human health will become harder to ignore,” they wrote. “We, as a medical community, have the responsibility and the opportunity to mobilize the urgent, large-scale climate action required to protect health – as well as the ingenuity to develop novel and bold interventions to avert the most catastrophic outcomes.”
The new research and associated commentary marked the introduction of a new NEJM topic on climate change effects on health and health systems.
SOURCE: Liu C et al. N Engl J Med. 2019;381:705-15.
This article was updated 8/22/19.
FROM NEJM
FDA approves lefamulin for community-acquired bacterial pneumonia treatment
The Food and Drug Administration has announced its approval of lefamulin (Xenleta) for the treatment of community-acquired bacterial pneumonia in adults.
Approval was based on results of two clinical trials assessing a total of 1,289 people with community-acquired bacterial pneumonia. In these trials, lefamulin was compared with moxifloxacin with and without linezolid. Patients who received lefamulin had similar rates of treatment success as those taking moxifloxacin alone or moxifloxacin plus linezolid.
The most common adverse reactions associated with lefamulin include diarrhea, nausea, reactions at the injection site, elevated liver enzymes, and vomiting. Patients with prolonged QT interval, patients with arrhythmias, patients receiving treatment with antiarrhythmic agents, and patients receiving other drugs that prolong the QT interval are contraindicated. In addition, because of evidence of fetal harm in animal studies, pregnant women should be advised of potential risks before receiving lefamulin.
“This new drug provides another option for the treatment of patients with community-acquired bacterial pneumonia, a serious disease. For managing this serious disease, it is important for physicians and patients to have treatment options,” Ed Cox, MD, MPH, director of the FDA’s Office of Antimicrobial Products, said in the press release.
The Food and Drug Administration has announced its approval of lefamulin (Xenleta) for the treatment of community-acquired bacterial pneumonia in adults.
Approval was based on results of two clinical trials assessing a total of 1,289 people with community-acquired bacterial pneumonia. In these trials, lefamulin was compared with moxifloxacin with and without linezolid. Patients who received lefamulin had similar rates of treatment success as those taking moxifloxacin alone or moxifloxacin plus linezolid.
The most common adverse reactions associated with lefamulin include diarrhea, nausea, reactions at the injection site, elevated liver enzymes, and vomiting. Patients with prolonged QT interval, patients with arrhythmias, patients receiving treatment with antiarrhythmic agents, and patients receiving other drugs that prolong the QT interval are contraindicated. In addition, because of evidence of fetal harm in animal studies, pregnant women should be advised of potential risks before receiving lefamulin.
“This new drug provides another option for the treatment of patients with community-acquired bacterial pneumonia, a serious disease. For managing this serious disease, it is important for physicians and patients to have treatment options,” Ed Cox, MD, MPH, director of the FDA’s Office of Antimicrobial Products, said in the press release.
The Food and Drug Administration has announced its approval of lefamulin (Xenleta) for the treatment of community-acquired bacterial pneumonia in adults.
Approval was based on results of two clinical trials assessing a total of 1,289 people with community-acquired bacterial pneumonia. In these trials, lefamulin was compared with moxifloxacin with and without linezolid. Patients who received lefamulin had similar rates of treatment success as those taking moxifloxacin alone or moxifloxacin plus linezolid.
The most common adverse reactions associated with lefamulin include diarrhea, nausea, reactions at the injection site, elevated liver enzymes, and vomiting. Patients with prolonged QT interval, patients with arrhythmias, patients receiving treatment with antiarrhythmic agents, and patients receiving other drugs that prolong the QT interval are contraindicated. In addition, because of evidence of fetal harm in animal studies, pregnant women should be advised of potential risks before receiving lefamulin.
“This new drug provides another option for the treatment of patients with community-acquired bacterial pneumonia, a serious disease. For managing this serious disease, it is important for physicians and patients to have treatment options,” Ed Cox, MD, MPH, director of the FDA’s Office of Antimicrobial Products, said in the press release.







