User login
Pseudo-Ludwig angina
An 83-year-old woman with hypertension, hypothyroidism, and a history of depression presented to the emergency department with acute shortness of breath and hypoxia. She was found to have submassive pulmonary embolism, and a heparin infusion was started immediately.
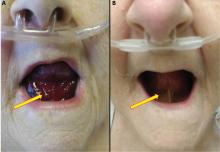
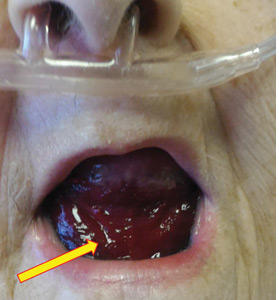
Urgent nasopharyngeal laryngoscopy revealed a hematoma at the base of her tongue that extended into the vallecula, piriform sinuses, and aryepiglottic fold, causing acute airway obstruction. These features combined with the supratherapeutic aPTT led to the diagnosis of pseudo-Ludwig angina.
DANGER OF RAPID AIRWAY COMPROMISE
Pseudo-Ludwig angina is a rare condition in which over-anticoagulation causes sublingual swelling leading to airway obstruction, whereas true Ludwig angina is an infectious regional suppuration of the neck.
Most reported cases of pseudo-Ludwig angina have resulted from overanticogulation with warfarin or warfarin-like substances (rodenticides), or from coagulopathy due to liver disease.1–3 Early recognition is essential to avoid airway compromise.
In our patient, all anticoagulation was discontinued, and she was intubated until the hematoma began to resolve, the aPTT returned to normal, and respiratory compromise improved. At follow-up 2 months later, the sublingual hematoma had completely resolved (Figure 1). And at a 6-month follow-up visit, the pulmonary embolism had resolved, and pulmonary pressures by 2-dimensional echocardiography were normal.
- Lovallo E, Patterson S, Erickson M, Chin C, Blanc P, Durrani TS. When is “pseudo-Ludwig’s angina” associated with coagulopathy also a “pseudo” hemorrhage? J Investig Med High Impact Case Rep 2013; 1(2):2324709613492503. doi:10.1177/2324709613492503
- Smith RG, Parker TJ, Anderson TA. Noninfectious acute upper airway obstruction (pseudo-Ludwig phenomenon): report of a case. J Oral Maxillofac Surg 1987; 45(8):701–704. pmid:3475442
- Zacharia GS, Kandiyil S, Thomas V. Pseudo-Ludwig's phenomenon: a rare clinical manifestation in liver cirrhosis. ACG Case Rep J 2014; 2(1):53–54. doi:10.14309/crj.2014.83
An 83-year-old woman with hypertension, hypothyroidism, and a history of depression presented to the emergency department with acute shortness of breath and hypoxia. She was found to have submassive pulmonary embolism, and a heparin infusion was started immediately.
Urgent nasopharyngeal laryngoscopy revealed a hematoma at the base of her tongue that extended into the vallecula, piriform sinuses, and aryepiglottic fold, causing acute airway obstruction. These features combined with the supratherapeutic aPTT led to the diagnosis of pseudo-Ludwig angina.
DANGER OF RAPID AIRWAY COMPROMISE
Pseudo-Ludwig angina is a rare condition in which over-anticoagulation causes sublingual swelling leading to airway obstruction, whereas true Ludwig angina is an infectious regional suppuration of the neck.
Most reported cases of pseudo-Ludwig angina have resulted from overanticogulation with warfarin or warfarin-like substances (rodenticides), or from coagulopathy due to liver disease.1–3 Early recognition is essential to avoid airway compromise.
In our patient, all anticoagulation was discontinued, and she was intubated until the hematoma began to resolve, the aPTT returned to normal, and respiratory compromise improved. At follow-up 2 months later, the sublingual hematoma had completely resolved (Figure 1). And at a 6-month follow-up visit, the pulmonary embolism had resolved, and pulmonary pressures by 2-dimensional echocardiography were normal.
An 83-year-old woman with hypertension, hypothyroidism, and a history of depression presented to the emergency department with acute shortness of breath and hypoxia. She was found to have submassive pulmonary embolism, and a heparin infusion was started immediately.
Urgent nasopharyngeal laryngoscopy revealed a hematoma at the base of her tongue that extended into the vallecula, piriform sinuses, and aryepiglottic fold, causing acute airway obstruction. These features combined with the supratherapeutic aPTT led to the diagnosis of pseudo-Ludwig angina.
DANGER OF RAPID AIRWAY COMPROMISE
Pseudo-Ludwig angina is a rare condition in which over-anticoagulation causes sublingual swelling leading to airway obstruction, whereas true Ludwig angina is an infectious regional suppuration of the neck.
Most reported cases of pseudo-Ludwig angina have resulted from overanticogulation with warfarin or warfarin-like substances (rodenticides), or from coagulopathy due to liver disease.1–3 Early recognition is essential to avoid airway compromise.
In our patient, all anticoagulation was discontinued, and she was intubated until the hematoma began to resolve, the aPTT returned to normal, and respiratory compromise improved. At follow-up 2 months later, the sublingual hematoma had completely resolved (Figure 1). And at a 6-month follow-up visit, the pulmonary embolism had resolved, and pulmonary pressures by 2-dimensional echocardiography were normal.
- Lovallo E, Patterson S, Erickson M, Chin C, Blanc P, Durrani TS. When is “pseudo-Ludwig’s angina” associated with coagulopathy also a “pseudo” hemorrhage? J Investig Med High Impact Case Rep 2013; 1(2):2324709613492503. doi:10.1177/2324709613492503
- Smith RG, Parker TJ, Anderson TA. Noninfectious acute upper airway obstruction (pseudo-Ludwig phenomenon): report of a case. J Oral Maxillofac Surg 1987; 45(8):701–704. pmid:3475442
- Zacharia GS, Kandiyil S, Thomas V. Pseudo-Ludwig's phenomenon: a rare clinical manifestation in liver cirrhosis. ACG Case Rep J 2014; 2(1):53–54. doi:10.14309/crj.2014.83
- Lovallo E, Patterson S, Erickson M, Chin C, Blanc P, Durrani TS. When is “pseudo-Ludwig’s angina” associated with coagulopathy also a “pseudo” hemorrhage? J Investig Med High Impact Case Rep 2013; 1(2):2324709613492503. doi:10.1177/2324709613492503
- Smith RG, Parker TJ, Anderson TA. Noninfectious acute upper airway obstruction (pseudo-Ludwig phenomenon): report of a case. J Oral Maxillofac Surg 1987; 45(8):701–704. pmid:3475442
- Zacharia GS, Kandiyil S, Thomas V. Pseudo-Ludwig's phenomenon: a rare clinical manifestation in liver cirrhosis. ACG Case Rep J 2014; 2(1):53–54. doi:10.14309/crj.2014.83
Mediastinal granuloma due to histoplasmosis in a patient on infliximab
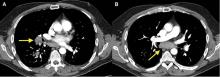
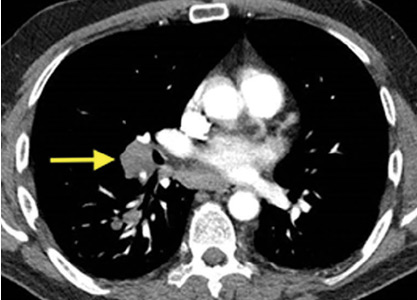
A 50-year-old man with Crohn disease and psoriatic arthritis treated with infliximab and methotrexate presented to a tertiary care hospital with fever, cough, and chest discomfort. The symptoms had first appeared 2 weeks earlier, and he had gone to an urgent care center, where he was prescribed a 5-day course of azithromycin and a corticosteroid, but this had not relieved his symptoms.
Bronchoscopy revealed edematous mucosa throughout, with minimal secretion. Specimens for bacterial, acid-fast bacillus, and fungal cultures were obtained from bronchoalveolar lavage. Endobronchial lymph node biopsy with ultrasonographic guidance revealed nonnecrotizing granuloma.
Bronchoalveolar lavage cultures showed no growth, but the patient’s serum histoplasma antigen was positive at 5.99 ng/dL (reference range: none detected), leading to the diagnosis of mediastinal granuloma due to histoplasmosis with possible dissemination. His immunosuppressant drugs were stopped, and oral itraconazole was started.
At a follow-up visit 2 months later, his serum antigen level had decreased to 0.68 ng/dL, and he had no symptoms whatsoever. At a visit 1 month after that, infliximab and methotrexate were restarted because of an exacerbation of Crohn disease. His oral itraconazole treatment was to be continued for at least 12 months, given the high suspicion for disseminated histoplasmosis while on immunosuppressant therapy.
DIFFERENTIAL DIAGNOSIS OF GRANULOMATOUS LUNG DISEASE AND LYMPHADENOPATHY
The differential diagnosis of granulomatous lung disease and lymphadenopathy is broad and includes noninfectious and infectious conditions.1
Noninfectious causes include lymphoma, sarcoidosis, inflammatory bowel disease, hypersensitivity pneumonia, side effects of drugs (eg, methotrexate, etanercept), rheumatoid nodules, vasculitis (eg, Churg-Strauss syndrome, granulomatosis with polyangiitis, primary amyloidosis, pneumoconiosis (eg, beryllium, cobalt), and Castleman disease.
There is concern that tumor necrosis factor antagonists may increase the risk of lymphoma, but a 2017 study found no evidence of this.2
Infectious conditions associated with granulomatous lung disease include tuberculosis, nontuberculous mycobacterial infection, fungal infection (eg, Cryptococcus, Coccidioides, Histoplasma, Blastomyces), brucellosis, tularemia (respiratory type B), parasitic infection (eg, Toxocara, Leishmania, Echinococcus, Schistosoma), and Whipple disease.
HISTOPLASMOSIS
Histoplasmosis, caused by infection with Histoplasma capsulatum, is the most prevalent endemic mycotic disease in the United States.3 The fungus is commonly found in the Ohio and Mississippi River valleys in the United States, and also in Central and South America and Asia.
Risk factors for histoplasmosis include living in or traveling to an endemic area, exposure to aerosolized soil that contains spores, and exposure to bats or birds and their droppings.4
Fewer than 5% of exposed individuals develop symptoms, which include fever, chills, headache, myalgia, anorexia, cough, and chest pain.5 Patients may experience symptoms shortly after exposure or may remain free of symptoms for years, with intermittent relapses of symptoms.6 Hilar or mediastinal lymphadenopathy is common in acute pulmonary histoplasmosis.7
The risk of disseminated histoplasmosis is greater in patients with reduced cell-mediated immunity, such as in human immunodeficiency virus infection, acquired immunodeficiency syndrome, solid-organ or bone marrow transplant, hematologic malignancies, immunosuppression (corticosteroids, disease-modifying antirheumatic drugs, and tumor necrosis factor antagonists), and congenital T-cell deficiencies.8
In a retrospective study, infliximab was the tumor necrosis factor antagonist most commonly associated with histoplasmosis.9 In a study of patients with rheumatoid arthritis, the disease-modifying drug most commonly associated was methotrexate.10
GOLD STANDARD FOR DIAGNOSIS
Isolation of H capsulatum from clinical specimens remains the gold standard for confirmation of histoplasmosis. The sensitivity of culture to detect H capsulatum depends on the clinical manifestations: it is 74% in patients with disseminated histoplasmosis, but only 42% in patients with acute pulmonary histoplasmosis.11 The serum histoplasma antigen test has a sensitivity of 91.8% in disseminated histoplasmosis, 87.5% in chronic pulmonary histoplasmosis, and 83% in acute pulmonary histoplasmosis.12
Urine testing for histoplasma antigen has generally proven to be slightly more sensitive than serum testing in all manifestations of histoplasmosis.13 Combining urine and serum testing increases the likelihood of antigen detection.
TREATMENT
Asymptomatic patients with mediastinal histoplasmosis do not require treatment. (Note: in some cases, lymphadenopathy is found incidentally, and biopsy is done to rule out malignancy.)
Standard treatment of symptomatic mediastinal histoplasmosis is oral itraconazole 200 mg, 3 times daily for 3 days, followed by 200 mg orally once or twice daily for 6 to 12 weeks.14
Although stopping immunosuppressant drugs is considered the standard of care in treating histoplasmosis in immunocompromised patients, there are no guidelines on when to resume them. However, a retrospective study of 98 cases of histoplasmosis in patients on tumor necrosis factor antagonists found that resuming immunosuppressants might be safe with close monitoring during the course of antifungal therapy.9 The role of long-term suppressive therapy with antifungal agents in patients on chronic immunosuppressive therapy is still unknown and needs further study.
TAKE-HOME MESSAGES
- Histoplasmosis is the most prevalent endemic mycotic disease in the United States, and mediastinal lymphadenopathy is commonly seen in acute pulmonary histoplasmosis.
- Histoplasmosis should be included in the differential diagnosis of granulomatous lung disease in patients from an endemic area or with a history of travel to an endemic area.
- Immunosuppressive agents such as tumor necrosis factor antagonists and disease-modifying antirheumatic drugs can predispose to invasive fungal infection, including histoplasmosis.
- While isolation of H capsulatum from culture remains the gold standard for the diagnosis of histoplasmosis, the histoplasma antigen tests (serum and urine) is more sensitive than culture.
- Ohshimo S, Guzman J, Costabel U, Bonella F. Differential diagnosis of granulomatous lung disease: clues and pitfalls: number 4 in the Series “Pathology for the clinician.” Edited by Peter Dorfmüller and Alberto Cavazza. Eur Respir Rev 2017; 26(145). doi:10.1183/16000617.0012-2017
- Mercer LK, Galloway JB, Lunt M, et al. Risk of lymphoma in patients exposed to antitumour necrosis factor therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis 2017; 76(3):497–503. doi:10.1136/annrheumdis-2016-209389
- Chu JH, Feudtner C, Heydon K, Walsh TJ, Zaoutis TE. Hospitalizations for endemic mycoses: a population-based national study. Clin Infect Dis 2006; 42(6):822–825. doi:10.1086/500405
- Benedict K, Mody RK. Epidemiology of histoplasmosis outbreaks, United States, 1938–2013. Emerg Infect Dis 2016; 22(3):370–378. doi:10.3201/eid2203.151117
- Wheat LJ. Diagnosis and management of histoplasmosis. Eur J Clin Microbiol Infect Dis 1989; 8(5):480–490. pmid:2502413
- Goodwin RA Jr, Shapiro JL, Thurman GH, Thurman SS, Des Prez RM. Disseminated histoplasmosis: clinical and pathologic correlations. Medicine (Baltimore) 1980; 59(1):1–33. pmid:7356773
- Wheat LJ, Conces D, Allen SD, Blue-Hnidy D, Loyd J. Pulmonary histoplasmosis syndromes: recognition, diagnosis, and management. Semin Respir Crit Care Med 2004; 25(2):129–144. doi:10.1055/s-2004-824898
- Assi MA, Sandid MS, Baddour LM, Roberts GD, Walker RC. Systemic histoplasmosis: a 15-year retrospective institutional review of 111 patients. Medicine (Baltimore) 2007; 86(3):162–169. doi:10.1097/md.0b013e3180679130
- Vergidis P, Avery RK, Wheat LJ, et al. Histoplasmosis complicating tumor necrosis factor-a blocker therapy: a retrospective analysis of 98 cases. Clin Infect Dis 2015; 61(3):409–417. doi:10.1093/cid/civ299
- Olson TC, Bongartz T, Crowson CS, Roberts GD, Orenstein R, Matteson EL. Histoplasmosis infection in patients with rheumatoid arthritis, 1998–2009. BMC Infect Dis 2011; 11:145. doi:10.1186/1471-2334-11-145
- Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis 2011; 53(5):448–454. doi:10.1093/cid/cir435
- Azar MM, Hage CA. Laboratory diagnostics for histoplasmosis. J Clin Microbiol 2017; 55(6):1612–1620. doi:10.1128/JCM.02430-16
- Swartzentruber S, Rhodes L, Kurkjian K, et al. Diagnosis of acute pulmonary histoplasmosis by antigen detection. Clin Infect Dis 2009; 49(12):1878–1882. doi:10.1086/648421
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45(7):807–825. doi:10.1086/521259
A 50-year-old man with Crohn disease and psoriatic arthritis treated with infliximab and methotrexate presented to a tertiary care hospital with fever, cough, and chest discomfort. The symptoms had first appeared 2 weeks earlier, and he had gone to an urgent care center, where he was prescribed a 5-day course of azithromycin and a corticosteroid, but this had not relieved his symptoms.
Bronchoscopy revealed edematous mucosa throughout, with minimal secretion. Specimens for bacterial, acid-fast bacillus, and fungal cultures were obtained from bronchoalveolar lavage. Endobronchial lymph node biopsy with ultrasonographic guidance revealed nonnecrotizing granuloma.
Bronchoalveolar lavage cultures showed no growth, but the patient’s serum histoplasma antigen was positive at 5.99 ng/dL (reference range: none detected), leading to the diagnosis of mediastinal granuloma due to histoplasmosis with possible dissemination. His immunosuppressant drugs were stopped, and oral itraconazole was started.
At a follow-up visit 2 months later, his serum antigen level had decreased to 0.68 ng/dL, and he had no symptoms whatsoever. At a visit 1 month after that, infliximab and methotrexate were restarted because of an exacerbation of Crohn disease. His oral itraconazole treatment was to be continued for at least 12 months, given the high suspicion for disseminated histoplasmosis while on immunosuppressant therapy.
DIFFERENTIAL DIAGNOSIS OF GRANULOMATOUS LUNG DISEASE AND LYMPHADENOPATHY
The differential diagnosis of granulomatous lung disease and lymphadenopathy is broad and includes noninfectious and infectious conditions.1
Noninfectious causes include lymphoma, sarcoidosis, inflammatory bowel disease, hypersensitivity pneumonia, side effects of drugs (eg, methotrexate, etanercept), rheumatoid nodules, vasculitis (eg, Churg-Strauss syndrome, granulomatosis with polyangiitis, primary amyloidosis, pneumoconiosis (eg, beryllium, cobalt), and Castleman disease.
There is concern that tumor necrosis factor antagonists may increase the risk of lymphoma, but a 2017 study found no evidence of this.2
Infectious conditions associated with granulomatous lung disease include tuberculosis, nontuberculous mycobacterial infection, fungal infection (eg, Cryptococcus, Coccidioides, Histoplasma, Blastomyces), brucellosis, tularemia (respiratory type B), parasitic infection (eg, Toxocara, Leishmania, Echinococcus, Schistosoma), and Whipple disease.
HISTOPLASMOSIS
Histoplasmosis, caused by infection with Histoplasma capsulatum, is the most prevalent endemic mycotic disease in the United States.3 The fungus is commonly found in the Ohio and Mississippi River valleys in the United States, and also in Central and South America and Asia.
Risk factors for histoplasmosis include living in or traveling to an endemic area, exposure to aerosolized soil that contains spores, and exposure to bats or birds and their droppings.4
Fewer than 5% of exposed individuals develop symptoms, which include fever, chills, headache, myalgia, anorexia, cough, and chest pain.5 Patients may experience symptoms shortly after exposure or may remain free of symptoms for years, with intermittent relapses of symptoms.6 Hilar or mediastinal lymphadenopathy is common in acute pulmonary histoplasmosis.7
The risk of disseminated histoplasmosis is greater in patients with reduced cell-mediated immunity, such as in human immunodeficiency virus infection, acquired immunodeficiency syndrome, solid-organ or bone marrow transplant, hematologic malignancies, immunosuppression (corticosteroids, disease-modifying antirheumatic drugs, and tumor necrosis factor antagonists), and congenital T-cell deficiencies.8
In a retrospective study, infliximab was the tumor necrosis factor antagonist most commonly associated with histoplasmosis.9 In a study of patients with rheumatoid arthritis, the disease-modifying drug most commonly associated was methotrexate.10
GOLD STANDARD FOR DIAGNOSIS
Isolation of H capsulatum from clinical specimens remains the gold standard for confirmation of histoplasmosis. The sensitivity of culture to detect H capsulatum depends on the clinical manifestations: it is 74% in patients with disseminated histoplasmosis, but only 42% in patients with acute pulmonary histoplasmosis.11 The serum histoplasma antigen test has a sensitivity of 91.8% in disseminated histoplasmosis, 87.5% in chronic pulmonary histoplasmosis, and 83% in acute pulmonary histoplasmosis.12
Urine testing for histoplasma antigen has generally proven to be slightly more sensitive than serum testing in all manifestations of histoplasmosis.13 Combining urine and serum testing increases the likelihood of antigen detection.
TREATMENT
Asymptomatic patients with mediastinal histoplasmosis do not require treatment. (Note: in some cases, lymphadenopathy is found incidentally, and biopsy is done to rule out malignancy.)
Standard treatment of symptomatic mediastinal histoplasmosis is oral itraconazole 200 mg, 3 times daily for 3 days, followed by 200 mg orally once or twice daily for 6 to 12 weeks.14
Although stopping immunosuppressant drugs is considered the standard of care in treating histoplasmosis in immunocompromised patients, there are no guidelines on when to resume them. However, a retrospective study of 98 cases of histoplasmosis in patients on tumor necrosis factor antagonists found that resuming immunosuppressants might be safe with close monitoring during the course of antifungal therapy.9 The role of long-term suppressive therapy with antifungal agents in patients on chronic immunosuppressive therapy is still unknown and needs further study.
TAKE-HOME MESSAGES
- Histoplasmosis is the most prevalent endemic mycotic disease in the United States, and mediastinal lymphadenopathy is commonly seen in acute pulmonary histoplasmosis.
- Histoplasmosis should be included in the differential diagnosis of granulomatous lung disease in patients from an endemic area or with a history of travel to an endemic area.
- Immunosuppressive agents such as tumor necrosis factor antagonists and disease-modifying antirheumatic drugs can predispose to invasive fungal infection, including histoplasmosis.
- While isolation of H capsulatum from culture remains the gold standard for the diagnosis of histoplasmosis, the histoplasma antigen tests (serum and urine) is more sensitive than culture.
A 50-year-old man with Crohn disease and psoriatic arthritis treated with infliximab and methotrexate presented to a tertiary care hospital with fever, cough, and chest discomfort. The symptoms had first appeared 2 weeks earlier, and he had gone to an urgent care center, where he was prescribed a 5-day course of azithromycin and a corticosteroid, but this had not relieved his symptoms.
Bronchoscopy revealed edematous mucosa throughout, with minimal secretion. Specimens for bacterial, acid-fast bacillus, and fungal cultures were obtained from bronchoalveolar lavage. Endobronchial lymph node biopsy with ultrasonographic guidance revealed nonnecrotizing granuloma.
Bronchoalveolar lavage cultures showed no growth, but the patient’s serum histoplasma antigen was positive at 5.99 ng/dL (reference range: none detected), leading to the diagnosis of mediastinal granuloma due to histoplasmosis with possible dissemination. His immunosuppressant drugs were stopped, and oral itraconazole was started.
At a follow-up visit 2 months later, his serum antigen level had decreased to 0.68 ng/dL, and he had no symptoms whatsoever. At a visit 1 month after that, infliximab and methotrexate were restarted because of an exacerbation of Crohn disease. His oral itraconazole treatment was to be continued for at least 12 months, given the high suspicion for disseminated histoplasmosis while on immunosuppressant therapy.
DIFFERENTIAL DIAGNOSIS OF GRANULOMATOUS LUNG DISEASE AND LYMPHADENOPATHY
The differential diagnosis of granulomatous lung disease and lymphadenopathy is broad and includes noninfectious and infectious conditions.1
Noninfectious causes include lymphoma, sarcoidosis, inflammatory bowel disease, hypersensitivity pneumonia, side effects of drugs (eg, methotrexate, etanercept), rheumatoid nodules, vasculitis (eg, Churg-Strauss syndrome, granulomatosis with polyangiitis, primary amyloidosis, pneumoconiosis (eg, beryllium, cobalt), and Castleman disease.
There is concern that tumor necrosis factor antagonists may increase the risk of lymphoma, but a 2017 study found no evidence of this.2
Infectious conditions associated with granulomatous lung disease include tuberculosis, nontuberculous mycobacterial infection, fungal infection (eg, Cryptococcus, Coccidioides, Histoplasma, Blastomyces), brucellosis, tularemia (respiratory type B), parasitic infection (eg, Toxocara, Leishmania, Echinococcus, Schistosoma), and Whipple disease.
HISTOPLASMOSIS
Histoplasmosis, caused by infection with Histoplasma capsulatum, is the most prevalent endemic mycotic disease in the United States.3 The fungus is commonly found in the Ohio and Mississippi River valleys in the United States, and also in Central and South America and Asia.
Risk factors for histoplasmosis include living in or traveling to an endemic area, exposure to aerosolized soil that contains spores, and exposure to bats or birds and their droppings.4
Fewer than 5% of exposed individuals develop symptoms, which include fever, chills, headache, myalgia, anorexia, cough, and chest pain.5 Patients may experience symptoms shortly after exposure or may remain free of symptoms for years, with intermittent relapses of symptoms.6 Hilar or mediastinal lymphadenopathy is common in acute pulmonary histoplasmosis.7
The risk of disseminated histoplasmosis is greater in patients with reduced cell-mediated immunity, such as in human immunodeficiency virus infection, acquired immunodeficiency syndrome, solid-organ or bone marrow transplant, hematologic malignancies, immunosuppression (corticosteroids, disease-modifying antirheumatic drugs, and tumor necrosis factor antagonists), and congenital T-cell deficiencies.8
In a retrospective study, infliximab was the tumor necrosis factor antagonist most commonly associated with histoplasmosis.9 In a study of patients with rheumatoid arthritis, the disease-modifying drug most commonly associated was methotrexate.10
GOLD STANDARD FOR DIAGNOSIS
Isolation of H capsulatum from clinical specimens remains the gold standard for confirmation of histoplasmosis. The sensitivity of culture to detect H capsulatum depends on the clinical manifestations: it is 74% in patients with disseminated histoplasmosis, but only 42% in patients with acute pulmonary histoplasmosis.11 The serum histoplasma antigen test has a sensitivity of 91.8% in disseminated histoplasmosis, 87.5% in chronic pulmonary histoplasmosis, and 83% in acute pulmonary histoplasmosis.12
Urine testing for histoplasma antigen has generally proven to be slightly more sensitive than serum testing in all manifestations of histoplasmosis.13 Combining urine and serum testing increases the likelihood of antigen detection.
TREATMENT
Asymptomatic patients with mediastinal histoplasmosis do not require treatment. (Note: in some cases, lymphadenopathy is found incidentally, and biopsy is done to rule out malignancy.)
Standard treatment of symptomatic mediastinal histoplasmosis is oral itraconazole 200 mg, 3 times daily for 3 days, followed by 200 mg orally once or twice daily for 6 to 12 weeks.14
Although stopping immunosuppressant drugs is considered the standard of care in treating histoplasmosis in immunocompromised patients, there are no guidelines on when to resume them. However, a retrospective study of 98 cases of histoplasmosis in patients on tumor necrosis factor antagonists found that resuming immunosuppressants might be safe with close monitoring during the course of antifungal therapy.9 The role of long-term suppressive therapy with antifungal agents in patients on chronic immunosuppressive therapy is still unknown and needs further study.
TAKE-HOME MESSAGES
- Histoplasmosis is the most prevalent endemic mycotic disease in the United States, and mediastinal lymphadenopathy is commonly seen in acute pulmonary histoplasmosis.
- Histoplasmosis should be included in the differential diagnosis of granulomatous lung disease in patients from an endemic area or with a history of travel to an endemic area.
- Immunosuppressive agents such as tumor necrosis factor antagonists and disease-modifying antirheumatic drugs can predispose to invasive fungal infection, including histoplasmosis.
- While isolation of H capsulatum from culture remains the gold standard for the diagnosis of histoplasmosis, the histoplasma antigen tests (serum and urine) is more sensitive than culture.
- Ohshimo S, Guzman J, Costabel U, Bonella F. Differential diagnosis of granulomatous lung disease: clues and pitfalls: number 4 in the Series “Pathology for the clinician.” Edited by Peter Dorfmüller and Alberto Cavazza. Eur Respir Rev 2017; 26(145). doi:10.1183/16000617.0012-2017
- Mercer LK, Galloway JB, Lunt M, et al. Risk of lymphoma in patients exposed to antitumour necrosis factor therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis 2017; 76(3):497–503. doi:10.1136/annrheumdis-2016-209389
- Chu JH, Feudtner C, Heydon K, Walsh TJ, Zaoutis TE. Hospitalizations for endemic mycoses: a population-based national study. Clin Infect Dis 2006; 42(6):822–825. doi:10.1086/500405
- Benedict K, Mody RK. Epidemiology of histoplasmosis outbreaks, United States, 1938–2013. Emerg Infect Dis 2016; 22(3):370–378. doi:10.3201/eid2203.151117
- Wheat LJ. Diagnosis and management of histoplasmosis. Eur J Clin Microbiol Infect Dis 1989; 8(5):480–490. pmid:2502413
- Goodwin RA Jr, Shapiro JL, Thurman GH, Thurman SS, Des Prez RM. Disseminated histoplasmosis: clinical and pathologic correlations. Medicine (Baltimore) 1980; 59(1):1–33. pmid:7356773
- Wheat LJ, Conces D, Allen SD, Blue-Hnidy D, Loyd J. Pulmonary histoplasmosis syndromes: recognition, diagnosis, and management. Semin Respir Crit Care Med 2004; 25(2):129–144. doi:10.1055/s-2004-824898
- Assi MA, Sandid MS, Baddour LM, Roberts GD, Walker RC. Systemic histoplasmosis: a 15-year retrospective institutional review of 111 patients. Medicine (Baltimore) 2007; 86(3):162–169. doi:10.1097/md.0b013e3180679130
- Vergidis P, Avery RK, Wheat LJ, et al. Histoplasmosis complicating tumor necrosis factor-a blocker therapy: a retrospective analysis of 98 cases. Clin Infect Dis 2015; 61(3):409–417. doi:10.1093/cid/civ299
- Olson TC, Bongartz T, Crowson CS, Roberts GD, Orenstein R, Matteson EL. Histoplasmosis infection in patients with rheumatoid arthritis, 1998–2009. BMC Infect Dis 2011; 11:145. doi:10.1186/1471-2334-11-145
- Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis 2011; 53(5):448–454. doi:10.1093/cid/cir435
- Azar MM, Hage CA. Laboratory diagnostics for histoplasmosis. J Clin Microbiol 2017; 55(6):1612–1620. doi:10.1128/JCM.02430-16
- Swartzentruber S, Rhodes L, Kurkjian K, et al. Diagnosis of acute pulmonary histoplasmosis by antigen detection. Clin Infect Dis 2009; 49(12):1878–1882. doi:10.1086/648421
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45(7):807–825. doi:10.1086/521259
- Ohshimo S, Guzman J, Costabel U, Bonella F. Differential diagnosis of granulomatous lung disease: clues and pitfalls: number 4 in the Series “Pathology for the clinician.” Edited by Peter Dorfmüller and Alberto Cavazza. Eur Respir Rev 2017; 26(145). doi:10.1183/16000617.0012-2017
- Mercer LK, Galloway JB, Lunt M, et al. Risk of lymphoma in patients exposed to antitumour necrosis factor therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis 2017; 76(3):497–503. doi:10.1136/annrheumdis-2016-209389
- Chu JH, Feudtner C, Heydon K, Walsh TJ, Zaoutis TE. Hospitalizations for endemic mycoses: a population-based national study. Clin Infect Dis 2006; 42(6):822–825. doi:10.1086/500405
- Benedict K, Mody RK. Epidemiology of histoplasmosis outbreaks, United States, 1938–2013. Emerg Infect Dis 2016; 22(3):370–378. doi:10.3201/eid2203.151117
- Wheat LJ. Diagnosis and management of histoplasmosis. Eur J Clin Microbiol Infect Dis 1989; 8(5):480–490. pmid:2502413
- Goodwin RA Jr, Shapiro JL, Thurman GH, Thurman SS, Des Prez RM. Disseminated histoplasmosis: clinical and pathologic correlations. Medicine (Baltimore) 1980; 59(1):1–33. pmid:7356773
- Wheat LJ, Conces D, Allen SD, Blue-Hnidy D, Loyd J. Pulmonary histoplasmosis syndromes: recognition, diagnosis, and management. Semin Respir Crit Care Med 2004; 25(2):129–144. doi:10.1055/s-2004-824898
- Assi MA, Sandid MS, Baddour LM, Roberts GD, Walker RC. Systemic histoplasmosis: a 15-year retrospective institutional review of 111 patients. Medicine (Baltimore) 2007; 86(3):162–169. doi:10.1097/md.0b013e3180679130
- Vergidis P, Avery RK, Wheat LJ, et al. Histoplasmosis complicating tumor necrosis factor-a blocker therapy: a retrospective analysis of 98 cases. Clin Infect Dis 2015; 61(3):409–417. doi:10.1093/cid/civ299
- Olson TC, Bongartz T, Crowson CS, Roberts GD, Orenstein R, Matteson EL. Histoplasmosis infection in patients with rheumatoid arthritis, 1998–2009. BMC Infect Dis 2011; 11:145. doi:10.1186/1471-2334-11-145
- Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis 2011; 53(5):448–454. doi:10.1093/cid/cir435
- Azar MM, Hage CA. Laboratory diagnostics for histoplasmosis. J Clin Microbiol 2017; 55(6):1612–1620. doi:10.1128/JCM.02430-16
- Swartzentruber S, Rhodes L, Kurkjian K, et al. Diagnosis of acute pulmonary histoplasmosis by antigen detection. Clin Infect Dis 2009; 49(12):1878–1882. doi:10.1086/648421
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45(7):807–825. doi:10.1086/521259
HFNC 12 L/min on floor cuts down on bronchiolitis ICU transfers
SEATTLE – ICU transfers for acute bronchiolitis dropped 63% at Johns Hopkins All Children’s Hospital in St. Petersburg, Fla., after the high-flow nasal cannula limit on the floor was raised from 6 L/min to 12 L/min, and treatment was started in the emergency department, according to a presentation at Pediatric Hospital Medicine.
A year before the change was made in April 2018, there were 17 transfers among 249 bronchiolitis patients treated on the floor, a transfer rate of 6.8%. In the year after the change, there were eight among 319 patients, a transfer rate of 2.5%. Raising the limit to 12 L/min prevented an estimated 14 transfers, for a total savings of almost $250,000, said pediatric hospitalist and assistant professor Shaila Siraj, MD.
The change was made after Dr. Siraj and her colleagues noticed that when children topped out at 6 L, they sometimes only needed a slightly higher flow rate in the ICU, maybe 8 L or 10 L, for a short while before they came back to the floor. Given the safety of high-flow nasal cannula (HFNC), the ICU transfer often seemed like a waste of time and resources.
“As hospitalists, we felt we could safely take care of these patients,” Dr. Siraj said.
So she and her colleague pediatric critical care specialist Anthony Sochet, MD, also an assistant professor of pediatrics, reviewed over a year’s worth of data at All Children’s. They found that 12 L/min – roughly 1.5 L/kg/min – was the cutoff that best discriminated between patients who needed intubation and those who did not, “so that’s what we chose,” Dr. Sochet said.
For simplicity, they broke limits down by age: A maximum flow rate of 8 L/min for children up to 6 months old; 10 L for children aged 6-12 months; and up to 12 L/min for children age 12-24 months. The fraction of inspired oxygen remained the same at 50%. Children were started at maximum flows, then weaned down as they improved. Respiratory assessments were made at least every 4 hours.
The changes were part of a larger revision of the hospital’s pathway for uncomplicated bronchiolitis in children up to 2 years old; it was a joint effort involving nurses, respiratory therapists, and pediatric hospitalists, and ED and ICU teams.
Early initiation in the ED was “probably one of the most important” changes; it kept children from wearing out as they struggled to breath. Kids often start to improve right away, but when then don’t after 30-60 minutes, it’s an indication that they should probably be triaged to the ICU for possible intubation, Dr. Siraj said.
Dr. Sochet was careful to note that institutions have to assess their own situations before taking similar steps. “Not everyone has a tertiary care ICU staffed 24 and 7,” he said.
“You have to ask what floor resources you have, what’s your ability to escalate when you need to. Use data from your own institution to guide where you pick your cutoffs. Adequate staffing is really about respiratory [therapist]/nursing ratios, not the physicians,” he said.
In addition, “in an otherwise healthy child that just has [HFNC] for bronchiolitis, there is absolutely no reason why you should be withholding feeds.” Fed children will feel better and do better, he said.
The presenters had no disclosures.
SEATTLE – ICU transfers for acute bronchiolitis dropped 63% at Johns Hopkins All Children’s Hospital in St. Petersburg, Fla., after the high-flow nasal cannula limit on the floor was raised from 6 L/min to 12 L/min, and treatment was started in the emergency department, according to a presentation at Pediatric Hospital Medicine.
A year before the change was made in April 2018, there were 17 transfers among 249 bronchiolitis patients treated on the floor, a transfer rate of 6.8%. In the year after the change, there were eight among 319 patients, a transfer rate of 2.5%. Raising the limit to 12 L/min prevented an estimated 14 transfers, for a total savings of almost $250,000, said pediatric hospitalist and assistant professor Shaila Siraj, MD.
The change was made after Dr. Siraj and her colleagues noticed that when children topped out at 6 L, they sometimes only needed a slightly higher flow rate in the ICU, maybe 8 L or 10 L, for a short while before they came back to the floor. Given the safety of high-flow nasal cannula (HFNC), the ICU transfer often seemed like a waste of time and resources.
“As hospitalists, we felt we could safely take care of these patients,” Dr. Siraj said.
So she and her colleague pediatric critical care specialist Anthony Sochet, MD, also an assistant professor of pediatrics, reviewed over a year’s worth of data at All Children’s. They found that 12 L/min – roughly 1.5 L/kg/min – was the cutoff that best discriminated between patients who needed intubation and those who did not, “so that’s what we chose,” Dr. Sochet said.
For simplicity, they broke limits down by age: A maximum flow rate of 8 L/min for children up to 6 months old; 10 L for children aged 6-12 months; and up to 12 L/min for children age 12-24 months. The fraction of inspired oxygen remained the same at 50%. Children were started at maximum flows, then weaned down as they improved. Respiratory assessments were made at least every 4 hours.
The changes were part of a larger revision of the hospital’s pathway for uncomplicated bronchiolitis in children up to 2 years old; it was a joint effort involving nurses, respiratory therapists, and pediatric hospitalists, and ED and ICU teams.
Early initiation in the ED was “probably one of the most important” changes; it kept children from wearing out as they struggled to breath. Kids often start to improve right away, but when then don’t after 30-60 minutes, it’s an indication that they should probably be triaged to the ICU for possible intubation, Dr. Siraj said.
Dr. Sochet was careful to note that institutions have to assess their own situations before taking similar steps. “Not everyone has a tertiary care ICU staffed 24 and 7,” he said.
“You have to ask what floor resources you have, what’s your ability to escalate when you need to. Use data from your own institution to guide where you pick your cutoffs. Adequate staffing is really about respiratory [therapist]/nursing ratios, not the physicians,” he said.
In addition, “in an otherwise healthy child that just has [HFNC] for bronchiolitis, there is absolutely no reason why you should be withholding feeds.” Fed children will feel better and do better, he said.
The presenters had no disclosures.
SEATTLE – ICU transfers for acute bronchiolitis dropped 63% at Johns Hopkins All Children’s Hospital in St. Petersburg, Fla., after the high-flow nasal cannula limit on the floor was raised from 6 L/min to 12 L/min, and treatment was started in the emergency department, according to a presentation at Pediatric Hospital Medicine.
A year before the change was made in April 2018, there were 17 transfers among 249 bronchiolitis patients treated on the floor, a transfer rate of 6.8%. In the year after the change, there were eight among 319 patients, a transfer rate of 2.5%. Raising the limit to 12 L/min prevented an estimated 14 transfers, for a total savings of almost $250,000, said pediatric hospitalist and assistant professor Shaila Siraj, MD.
The change was made after Dr. Siraj and her colleagues noticed that when children topped out at 6 L, they sometimes only needed a slightly higher flow rate in the ICU, maybe 8 L or 10 L, for a short while before they came back to the floor. Given the safety of high-flow nasal cannula (HFNC), the ICU transfer often seemed like a waste of time and resources.
“As hospitalists, we felt we could safely take care of these patients,” Dr. Siraj said.
So she and her colleague pediatric critical care specialist Anthony Sochet, MD, also an assistant professor of pediatrics, reviewed over a year’s worth of data at All Children’s. They found that 12 L/min – roughly 1.5 L/kg/min – was the cutoff that best discriminated between patients who needed intubation and those who did not, “so that’s what we chose,” Dr. Sochet said.
For simplicity, they broke limits down by age: A maximum flow rate of 8 L/min for children up to 6 months old; 10 L for children aged 6-12 months; and up to 12 L/min for children age 12-24 months. The fraction of inspired oxygen remained the same at 50%. Children were started at maximum flows, then weaned down as they improved. Respiratory assessments were made at least every 4 hours.
The changes were part of a larger revision of the hospital’s pathway for uncomplicated bronchiolitis in children up to 2 years old; it was a joint effort involving nurses, respiratory therapists, and pediatric hospitalists, and ED and ICU teams.
Early initiation in the ED was “probably one of the most important” changes; it kept children from wearing out as they struggled to breath. Kids often start to improve right away, but when then don’t after 30-60 minutes, it’s an indication that they should probably be triaged to the ICU for possible intubation, Dr. Siraj said.
Dr. Sochet was careful to note that institutions have to assess their own situations before taking similar steps. “Not everyone has a tertiary care ICU staffed 24 and 7,” he said.
“You have to ask what floor resources you have, what’s your ability to escalate when you need to. Use data from your own institution to guide where you pick your cutoffs. Adequate staffing is really about respiratory [therapist]/nursing ratios, not the physicians,” he said.
In addition, “in an otherwise healthy child that just has [HFNC] for bronchiolitis, there is absolutely no reason why you should be withholding feeds.” Fed children will feel better and do better, he said.
The presenters had no disclosures.
REPORTING FROM PHM 2019
Key clinical point:
Major finding: ICU transfers dropped 63% after the floor limit was raised from 6 L/min to 12 L/min.
Study details: Before/after quality improvement project
Disclosures: There was no external funding, and the presenters had no disclosures.
Community-Acquired Pneumonia: Treatment
Initial management decisions for patients with community-acquired pneumonia (CAP) will depend on severity of infection, with need for hospitalization being one of the first decisions. Because empiric antibiotics are the mainstay of treatment and the causative organisms are seldom identified, underlying medical conditions and epidemiologic risk factors are considered when selecting an empiric regimen. As with other infections, duration of therapy is not standardized, but rather is guided by clinical improvement. Prevention of pneumonia centers around vaccination and smoking cessation. This article, the second in a 2-part review of CAP in adults, focuses on site of care decision, empiric and directed therapies, length of treatment, and prevention strategies. Evaluation and diagnosis of CAP are discussed in a separate article.
Site of Care Decision
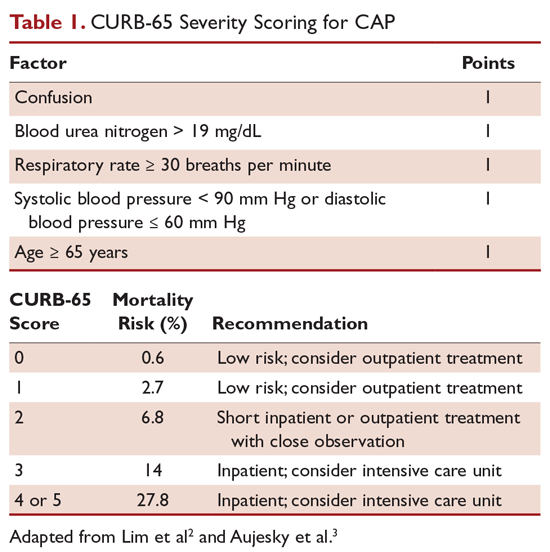
For patients diagnosed with CAP, the clinician must decide whether treatment will be done in an outpatient or inpatient setting, and for those in the inpatient setting, whether they can safely be treated on the general medical ward or in the intensive care unit (ICU). Two common scoring systems that can be used to aid the clinician in determining severity of the infection and guide site-of-care decisions are the Pneumonia Severity Index (PSI) and CURB-65 scores.
The PSI score uses 20 different parameters, including comorbidities, laboratory parameters, and radiographic findings, to stratify patients into 5 mortality risk classes.1 On the basis of associated mortality rates, it has been suggested that risk class I and II patients should be treated as outpatients, risk class III patients should be treated in an observation unit or with a short hospitalization, and risk class IV and V patients should be treated as inpatients.1
The CURB-65 method of risk stratification is based on 5 clinical parameters: confusion, urea level, respiratory rate, systolic blood pressure, and age ≥ 65 years (Table 1).2,3 A modification to the CURB-65 algorithm tool was CRB-65, which excludes urea nitrogen, making it optimal for making determinations in a clinic-based setting. It should be emphasized that these tools do not take into account other factors that should be used in determining location of treatment, such as stable home, mental illness, or concerns about compliance with medications. In many instances, it is these factors that preclude low-risk patients from being treated as outpatients.4,5 Similarly, these scoring systems have not been validated for immunocompromised patients or those who would qualify as having health care–associated pneumonia.
Patients with CURB-65 scores of 4 or 5 are considered to have severe pneumonia, and admission to the ICU should be considered for these patients. Aside from the CURB-65 score, anyone requiring vasopressor support or mechanical ventilation merits admission to the ICU.6 American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guidelines also recommend the use of “minor criteria” for making ICU admission decisions; these include respiratory rate ≥ 30 breaths/minute, PaO2 fraction ≤ 250 mm Hg, multilobar infiltrates, confusion, blood urea nitrogen ≥ 20 mg/dL, leukopenia, thrombocytopenia, hypothermia, and hypotension.6 These factors are associated with increased mortality due to CAP, and ICU admission is indicated if 3 of the minor criteria for severe CAP are present.
Another clinical calculator that can be used for assessing severity of CAP is SMART-COP (systolic blood pressure, multilobar chest radiography involvement, albumin level, respiratory rate, tachycardia, confusion, oxygenation and arterial pH).7 This scoring system uses 8 weighted criteria to predict which patients will require intensive respiratory or vasopressor support. SMART-COP has a sensitivity of 79% and a specificity of 64% in predicting ICU admission, whereas CURB-65 has a pooled sensitivity of 57.2% and specificity of 77.2%.8
Antibiotic Therapy
Antibiotics are the mainstay of treatment for CAP, with the majority of patients with CAP treated empirically taking into account the site of care, likely pathogen, and antimicrobial resistance issues. Patients with pneumonia who are treated as outpatients usually respond well to empiric antibiotic treatment, and a causative pathogen is not usually sought. Patients who are hospitalized for treatment of CAP usually receive empiric antibiotic on admission. Once the etiology has been determined by microbiologic or serologic means, antimicrobial therapy should be adjusted accordingly. A CDC study found that the burden of viral etiologies was higher than previously thought, with rhinovirus and influenza accounting for 15% of cases and Streptococcus pneumoniae for only 5%.9 This study highlighted the fact that despite advances in molecular techniques, no pathogen is identified for most patients with pneumonia.9 Given the lack of discernable pathogens in the majority of cases, patients should continue to be treated with antibiotics unless a nonbacterial etiology is found.
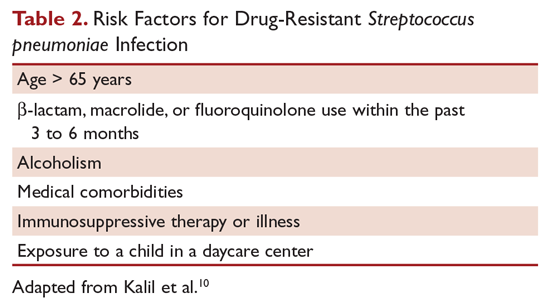
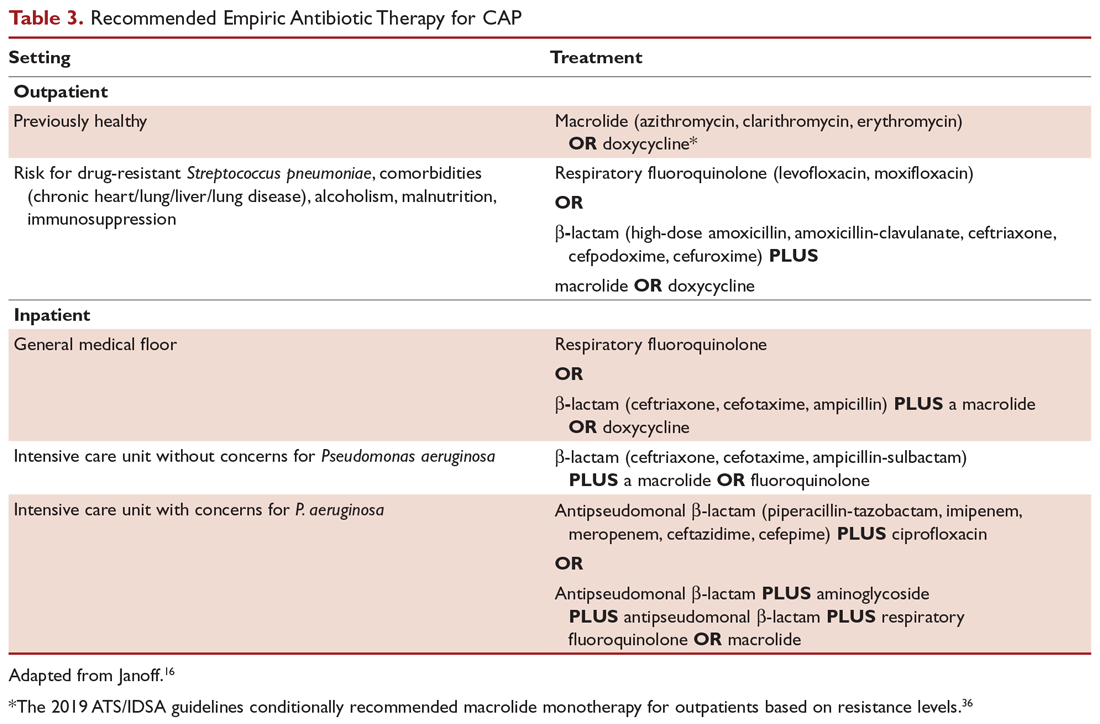
Outpatients without comorbidities or risk factors for drug-resistant S. pneumoniae (Table 2)10 can be treated with monotherapy. Hospitalized patients are usually treated with combination intravenous therapy, although non-ICU patients who receive a respiratory fluoroquinolone can be treated orally.
As previously mentioned, antibiotic therapy is typically empiric, since neither clinical features nor radiographic features are sufficient to include or exclude infectious etiologies. Epidemiologic risk factors should be considered and, in certain cases, antimicrobial coverage should be expanded to include those entities; for example, treatment of anaerobes in the setting of lung abscess and antipseudomonal antibiotics for patients with bronchiectasis.
Of concern in the treatment of CAP is the increased prevalence of antimicrobial resistance among S. pneumoniae. The IDSA guidelines report that drug-resistant S. pneumoniae is more common in persons aged < 2 or > 65 years, and those with β-lactam therapy within the previous 3 months, alcoholism, medical comorbidities, immunosuppressive illness or therapy, or exposure to a child who attends a day care center.6
Staphylococcus aureus should be considered during influenza outbreaks, with either vancomycin or linezolid being the recommended agents in the setting of methicillin-resistant S. aureus (MRSA). In a study comparing vancomycin versus linezolid for nosocomial pneumonia, the all-cause 60-day mortality was similar for both agents.11 Daptomycin, another agent used against MRSA, is not indicated in the setting of pneumonia because daptomycin binds to surfactant, yielding it ineffective in the treatment of pneumonia.12 Ceftaroline is a newer cephalosporin with activity against MRSA; its role in treatment of community-acquired MRSA pneumonia has not been fully elucidated, but it appears to be a useful agent for this indication.13,14 Similarly, other agents known to have antibacterial properties against MRSA, such as trimethoprim/sulfamethoxazole and doxycycline, have not been studied for this indication. Clindamycin has been used to treat MRSA in children, and IDSA guidelines on the treatment of MRSA list clindamycin as an alternative15 if MRSA is known to be sensitive.
A summary of recommended empiric antibiotic therapy is presented in Table 3.16
Three antibiotics were approved by the US Food and Drug Administration (FDA) for the treatment of CAP after the release of the IDSA/ATS guidelines in 2007. Ceftaroline fosamil is a fifth-generation cephalosporin that has coverage for MRSA and was approved in November 2010.17 It can only be administered intravenously and needs dose adjustment for renal function. Omadacycline is a new tetracycline that was approved by the FDA in October 2018.18 It is available in both intravenous injectable and oral forms. No dose adjustment is needed for renal function. Lefamulin is a first-in-class novel pleuromutilin antibiotic which was FDA-approved in August 2019. It can be administered intravenously or orally, with no dosage adjustment necessary in patients with renal impairment.19
Antibiotic Therapy for Selected Pathogens
Streptococcus pneumoniae
Patients with pneumococcal pneumonia who have penicillin-susceptible strains can be treated with intravenous penicillin (2 or 3 million units every 4 hours) or ceftriaxone. Once a patient meets criteria of stability, they can then be transitioned to oral penicillin, amoxicillin, or clarithromycin. Those with strains with reduced susceptibility can still be treated with penicillin, but at a higher dose (4 million units intravenously [IV] every 4 hours), or a third-generation cephalosporin. Those whose pneumococcal pneumonia is complicated by bacteremia will benefit from dual therapy if severely ill, requiring ICU monitoring. Those not severely ill can be treated with monotherapy.20
Staphylococcus aureus
Staphylococcus aureus is more commonly associated with hospital-acquired pneumonia, but it may also be seen during the influenza season and in those with severe necrotizing CAP. Both linezolid and vancomycin can be used to treat MRSA CAP. As noted, ceftaroline has activity against MRSA and is approved for treatment of CAP, but is not approved by the FDA for MRSA CAP treatment. Similarly, tigecycline is approved for CAP and has activity against MRSA, but is not approved for MRSA CAP. Moreover, the FDA has warned of increased risk of death with tigecycline and has a black box warning to that effect.21
Legionella
Legionellosis can be treated with tetra¬cyclines, macrolides, or fluoroquinolones. For non-immunocompromised patients with mild pneumonia, any of the listed antibiotics is considered appropriate. However, patients with severe infection or those with immunosuppression should be treated with either levofloxacin or azithromycin for 7 to 10 days.22
Chlamydophila pneumoniae
As with other atypical organisms, Chlamydophila pneumoniae can be treated with doxycycline, a macrolide, or respiratory fluoroquinolones. However, length of therapy varies by regimen used; treating with doxycycline 100 mg twice daily generally requires 14 to 21 days, whereas moxifloxacin 400 mg daily requires 10 days.23
Mycoplasma pneumoniae
As with C. pneumoniae, length of therapy of Mycoplasma pneumoniae varies by which antimicrobial regimen is used. Shortest courses are seen with the use of macrolides for 5 days, whereas 14 days is considered standard for doxycycline or a respiratory fluoroquinolone.24 It should be noted that there has been increasingly documented resistance to macrolides, with known resistance of 8.2% in the United States.25
Duration of Treatment
Most patients with CAP respond to appropriate therapy within 72 hours. IDSA/ATS guidelines recommend that patients with routine cases of CAP be treated for a minimum of 5 days. Despite this, many patients are treated for an excessive amount of time, with over 70% of patients reported to have received antibiotics for more than 10 days for uncomplicated CAP.26 There are instances that require longer courses of antibiotics, including cases caused by Pseudomonas aeruginosa, S. aureus, and Legionella species and patients with lung abscesses or necrotizing infections, among others.27
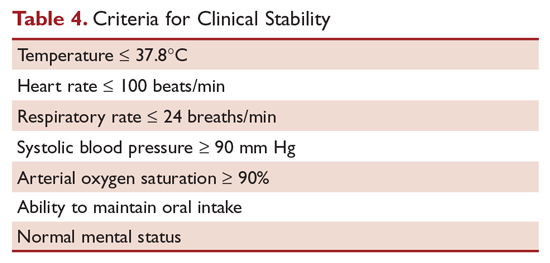
Hospitalized patients do not need to be monitored for an additional day once they have reached clinical stability (Table 4), are able to maintain oral intake, and have normal mentation, provided that other comorbidities are stable and social needs have been met.6 C-reactive protein (CRP) level has been postulated as an additional measure of stability, specifically monitoring for a greater than 50% reduction in CRP; however, this was validated only for those with complicated pneumonia.28 Patients discharged from the hospital with instability have higher risk of readmission or death.29
Transition to Oral Therapy
IDSA/ATS guidelines6 recommend that patients should be transitioned from intravenous to oral antibiotics when they are improving clinically, have stable vital signs, and are able to ingest food/fluids and medications.
Management of Nonresponders
Although the majority of patients respond to antibiotics within 72 hours, treatment failure occurs in up to 15% of patients.15 Nonresponding pneumonia is generally seen in 2 patterns: worsening of clinical status despite empiric antibiotics or delay in achieving clinical stability, as defined in Table 4, after 72 hours of treatment.30 Risk factors associated with nonresponding pneumonia31 are:
- Radiographic: multilobar infiltrates, pleural effusion, cavitation
- Bacteriologic: MRSA, gram-negative or Legionella pneumonia
- Severity index: PSI > 90
- Pharmacologic: incorrect antibiotic choice based on susceptibility
Patients with acute deterioration of clinical status require prompt transfer to a higher level of care and may require mechanical ventilator support. In those with delay in achieving clinical stability, a question centers on whether the same antibiotics can be continued while doing further radiographic/microbiologic work-up and/or changing antibiotics. History should be reviewed, with particular attention to exposures, travel history, and microbiologic and radiographic data. Clinicians should recall that viruses account for up to 20% of pneumonias and that there are also noninfectious causes that can mimic pyogenic infections.32 If adequate initial cultures were not obtained, they should be obtained; however, care must be taken in reviewing new sets of cultures while on antibiotics, as they may reveal colonization selected out by antibiotics and not a true pathogen. If repeat evaluation is unrevealing, then further evaluation with computed tomography (CT) scan and bronchoscopy with bronchoalveolar lavage and biopsy is warranted. CT scans can show pleural effusions, bronchial obstructions, or a pattern suggestive of cryptogenic pneumonia. A bronchoscopy might yield a microbiologic diagnosis and, when combined with biopsy, can also evaluate for noninfectious causes.
As with other infections, if escalation of antibiotics is undertaken, clinicians should try to determine the reason for nonresponse. To simply broaden antimicrobial therapy without attempts at establishing a microbiologic or radiographic cause for nonresponse may lead to inappropriate treatment and recurrence of infection. Aside from patients who have bacteremic pneumococcal pneumonia in an ICU setting, there are no published reports pointing to superiority of combination antibiotics.20
Other Treatment
Several agents have been evaluated as adjunctive treatment of pneumonia to decrease the inflammatory response associated with pneumonia; namely, steroids, macrolide antibiotics, and statins. To date, only the use of steroids (methylprednisolone 0.5 mg/kg every 12 hours for 5 days) in those with severe CAP and high initial anti-inflammatory response (CRP > 150) has been shown to decrease treatment failure, decrease risk of acute respiratory distress syndrome, and possibly reduce length of stay and duration of intravenous antibiotics, without effect on mortality or adverse side effects.33,34 However, a recent double-blind randomized study conducted in Australia in which patients admitted with CAP were prescribed prednisolone acetate (50 mg/day for 7 days) and de-escalated from parenteral to oral antibiotics according to standardized criteria revealed no difference in mortality, length of stay, or readmission rates between the corticosteroids group and the control group at 90-day follow-up.35 At this point, corticosteroid as an adjunctive treatment for CAP is still controversial and the new 2019 ATS/IDSA guidelines recommend not routinely using corticosteroids in all patients with CAP.36 Other adjunctive methods have not been found to have significant impact.6
Prevention of Pneumonia
Prevention of pneumococcal pneumonia involves vaccinations to prevent infection caused by S. pneumoniae and influenza viruses. As influenza is a risk factor for bacterial infection, specifically with S. pneumoniae, influenza vaccination can help prevent bacterial pneumonia.37 In their most recent recommendations, the CDC continues to recommend routine influenza vaccination for all persons older than age 6 months, unless otherwise contraindicated.38
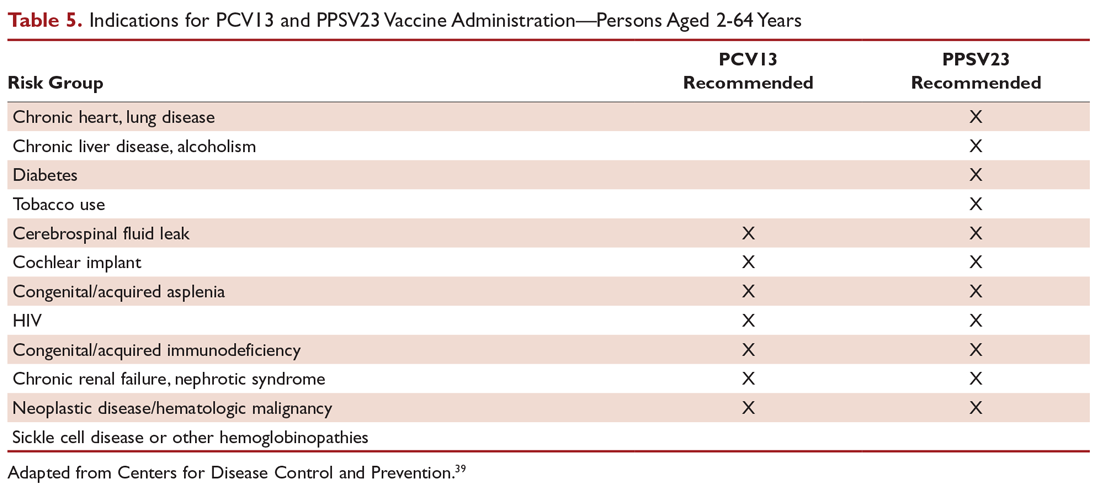
There are 2 vaccines for prevention of pneumococcal disease: the pneumococcal polysaccharide vaccine (PPSV23) and a conjugate vaccine (PCV13). Following vaccination with PPSV23, 80% of adults develop antibodies against at least 18 of the 23 serotypes.39 PPSV23 is reported to be protective against invasive pneumococcal infection, although there is no consensus regarding whether PPSV23 leads to decreased rates of pneumonia.40 On the other hand, PCV13 vaccination was associated with prevention of both invasive disease and CAP in adults aged 65 years or older.41 The CDC recommends that all children aged 2 years or younger receive PCV13, and those aged 65 or older receive PCV13 followed by a dose of PPSV23.42,43 The dose of PPSV23 should be given at least 1 year after the dose of PCV13 is administered.44 Persons younger than 65 years with immunocompromising and certain other conditions should also receive vaccination (Table 5).44 Full recommendations, many scenarios, and details on timing of vaccinations can be found at the CDC’s website.
Cigarette smoking increases the risk of respiratory infections, as evidenced by smokers accounting for almost half of all patients with invasive pneumococcal disease.11 As this is a modifiable risk factor, smoking cessation should be part of a comprehensive approach toward prevention of pneumonia.
Summary
Most patients with CAP are treated empirically with antibiotics, with therapy selection based on the site of care, likely pathogen, and antimicrobial resistance issues. Those treated as outpatients usually respond well to empiric antibiotic treatment, and a causative pathogen is not usually sought. Patients who are hospitalized for treatment usually receive empiric antibiotic on admission, and antimicrobial therapy is adjusted accordingly once the etiology has been determined by microbiologic or serologic means. At this time, the use of corticosteroid as an adjunctive treatment for CAP is still controversial, so not all patients with CAP should routinely receive corticosteroids. Because vaccination (PPSV23, PCV13, and influenza vaccine) remains the most effective tool in preventing the development of CAP, clinicians should strive for 100% vaccination rates in persons without contraindications.
1. Fine MJ, Auble TE, Yealy DM, et al A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med.1997;336:243-250.
2. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377-382.
3. Aujesky D, Auble TE, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med. 2005;118:384-392.
4. Arnold FW, Ramirez JA, McDonald LC, Xia EL. Hospitalization for community-acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest. 2003;124:121-124.
5. Aujesky D, McCausland JB, Whittle J, et al. Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumonia. Clin Infect Dis. 2009;49:e100-108.
6. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-72.
7. Charles PG, Wolfe R, Whitby M, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008;47:375-384.
8. Marti C, Garin N, Grosgurin O, et al. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Crit Care. 2012;16:R141.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415-427.
10. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
11. Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54:621-629.
12. Silverman JA, Mortin LI, Vanpraagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis. 2005;191:2149-2152.
13. El Hajj MS, Turgeon RD, Wilby KJ. Ceftaroline fosamil for community-acquired pneumonia and skin and skin structure infections: a systematic review. Int J Clin Pharm. 2017;39:26-32.
14. Taboada M, Melnick D, Iaconis JP, et al. Ceftaroline fosamil versus ceftriaxone for the treatment of community-acquired pneumonia: individual patient data meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2016;71:862-870.
15. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285-292.
16. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Sauders; 2015:2310-2327.
17. Teflaro (ceftaroline fosamil) [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2010.
18. Nuzyra (omadacycline) [package insert]. Boston, MA: Paratek Pharmaceuticals; 2018.
19. Xenleta (lefamulin) [package insert]. Dublin, Ireland: Nabriva Therapeutics; 2019.
20. Baddour LM, Yu VL, Klugman KP, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170:440-444.
21. FDA Drug Safety Communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new boxed warning. www.fda.gov/Drugs/DrugSafety/ucm369580.htm. Accessed 16 September 2019.
22. Edelstein PR, CR. Legionnaires’ disease and Pontiac fever. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2633.
23. Hammerschlag MR, Kohlhoff SA, Gaydos, CA. Chlamydia pneumoniae. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2174.
24. Holzman RS, MS. Mycoplasma pneumoniae and atypical pneumonia. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2183.
25. Yamada M, Buller R, Bledsoe S, Storch GA. Rising rates of macrolide-resistant Mycoplasma pneumoniae in the central United States. Pediatr Infect Dis J. 2012;31:409-410.
26. Yi SH, Hatfield KM, Baggs J, et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66:1333-1341.
27. Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis. 2011;52:1232-1240.
28. Akram AR, Chalmers JD, Taylor JK, et al. An evaluation of clinical stability criteria to predict hospital course in community-acquired pneumonia. Clin Microbiol Infect. 2013;19:1174-1180.
29. Halm EA, Fine MJ, Kapoor WN, et al. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med. 2002;162:1278-1284.
30. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Saunders; 2015:2310-2327.
31. Roson B, Carratala J, Fernandez-Sabe N, et al. Causes and factors associated with early failure in hospitalized patients with community-acquired pneumonia. Arch Intern Med. 2004;164:502-508.
32. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167:1650-1654.
33. Wan YD, Sun TW, Liu ZQ, et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest. 2016;149:209-219.
34. Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015;313:677-686.
35. Lloyd M, Karahalios, Janus E, et al. Effectiveness of a bundled intervention including adjunctive corticosteroids on outcomes of hospitalized patients with community-acquired pneumonia: a stepped-wedge randomized clinical trial. JAMA Intern Med. 2019;179:1052-1060.
36. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
37. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571-582.
38. Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2019-20 influenza season. MMWR Recomm Rep. 2019;68:1-21.
39. Rubins JB, Alter M, Loch J, Janoff EN. Determination of antibody responses of elderly adults to all 23 capsular polysaccharides after pneumococcal vaccination. Infect Immun. 1999;67:5979-5984.
40. Vaccines and preventable diseases. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html. Accessed 16 September 2019.
41. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114-1125.
42. Recommended adult immunization schedule -- United States -- 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed 16 September 2019.
43. Recommended child and adolescent immunization schedule for ages 18 years or younger – United States – 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html. Accessed 22 September 2019.
44. Pneumococcal vaccine timing for adults – United States – 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/vpd/pneumo/downloads/pneumo-vaccine-timing.pdf. Accessed 22 September 2019.
Initial management decisions for patients with community-acquired pneumonia (CAP) will depend on severity of infection, with need for hospitalization being one of the first decisions. Because empiric antibiotics are the mainstay of treatment and the causative organisms are seldom identified, underlying medical conditions and epidemiologic risk factors are considered when selecting an empiric regimen. As with other infections, duration of therapy is not standardized, but rather is guided by clinical improvement. Prevention of pneumonia centers around vaccination and smoking cessation. This article, the second in a 2-part review of CAP in adults, focuses on site of care decision, empiric and directed therapies, length of treatment, and prevention strategies. Evaluation and diagnosis of CAP are discussed in a separate article.
Site of Care Decision
For patients diagnosed with CAP, the clinician must decide whether treatment will be done in an outpatient or inpatient setting, and for those in the inpatient setting, whether they can safely be treated on the general medical ward or in the intensive care unit (ICU). Two common scoring systems that can be used to aid the clinician in determining severity of the infection and guide site-of-care decisions are the Pneumonia Severity Index (PSI) and CURB-65 scores.
The PSI score uses 20 different parameters, including comorbidities, laboratory parameters, and radiographic findings, to stratify patients into 5 mortality risk classes.1 On the basis of associated mortality rates, it has been suggested that risk class I and II patients should be treated as outpatients, risk class III patients should be treated in an observation unit or with a short hospitalization, and risk class IV and V patients should be treated as inpatients.1
The CURB-65 method of risk stratification is based on 5 clinical parameters: confusion, urea level, respiratory rate, systolic blood pressure, and age ≥ 65 years (Table 1).2,3 A modification to the CURB-65 algorithm tool was CRB-65, which excludes urea nitrogen, making it optimal for making determinations in a clinic-based setting. It should be emphasized that these tools do not take into account other factors that should be used in determining location of treatment, such as stable home, mental illness, or concerns about compliance with medications. In many instances, it is these factors that preclude low-risk patients from being treated as outpatients.4,5 Similarly, these scoring systems have not been validated for immunocompromised patients or those who would qualify as having health care–associated pneumonia.
Patients with CURB-65 scores of 4 or 5 are considered to have severe pneumonia, and admission to the ICU should be considered for these patients. Aside from the CURB-65 score, anyone requiring vasopressor support or mechanical ventilation merits admission to the ICU.6 American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guidelines also recommend the use of “minor criteria” for making ICU admission decisions; these include respiratory rate ≥ 30 breaths/minute, PaO2 fraction ≤ 250 mm Hg, multilobar infiltrates, confusion, blood urea nitrogen ≥ 20 mg/dL, leukopenia, thrombocytopenia, hypothermia, and hypotension.6 These factors are associated with increased mortality due to CAP, and ICU admission is indicated if 3 of the minor criteria for severe CAP are present.
Another clinical calculator that can be used for assessing severity of CAP is SMART-COP (systolic blood pressure, multilobar chest radiography involvement, albumin level, respiratory rate, tachycardia, confusion, oxygenation and arterial pH).7 This scoring system uses 8 weighted criteria to predict which patients will require intensive respiratory or vasopressor support. SMART-COP has a sensitivity of 79% and a specificity of 64% in predicting ICU admission, whereas CURB-65 has a pooled sensitivity of 57.2% and specificity of 77.2%.8
Antibiotic Therapy
Antibiotics are the mainstay of treatment for CAP, with the majority of patients with CAP treated empirically taking into account the site of care, likely pathogen, and antimicrobial resistance issues. Patients with pneumonia who are treated as outpatients usually respond well to empiric antibiotic treatment, and a causative pathogen is not usually sought. Patients who are hospitalized for treatment of CAP usually receive empiric antibiotic on admission. Once the etiology has been determined by microbiologic or serologic means, antimicrobial therapy should be adjusted accordingly. A CDC study found that the burden of viral etiologies was higher than previously thought, with rhinovirus and influenza accounting for 15% of cases and Streptococcus pneumoniae for only 5%.9 This study highlighted the fact that despite advances in molecular techniques, no pathogen is identified for most patients with pneumonia.9 Given the lack of discernable pathogens in the majority of cases, patients should continue to be treated with antibiotics unless a nonbacterial etiology is found.
Outpatients without comorbidities or risk factors for drug-resistant S. pneumoniae (Table 2)10 can be treated with monotherapy. Hospitalized patients are usually treated with combination intravenous therapy, although non-ICU patients who receive a respiratory fluoroquinolone can be treated orally.
As previously mentioned, antibiotic therapy is typically empiric, since neither clinical features nor radiographic features are sufficient to include or exclude infectious etiologies. Epidemiologic risk factors should be considered and, in certain cases, antimicrobial coverage should be expanded to include those entities; for example, treatment of anaerobes in the setting of lung abscess and antipseudomonal antibiotics for patients with bronchiectasis.
Of concern in the treatment of CAP is the increased prevalence of antimicrobial resistance among S. pneumoniae. The IDSA guidelines report that drug-resistant S. pneumoniae is more common in persons aged < 2 or > 65 years, and those with β-lactam therapy within the previous 3 months, alcoholism, medical comorbidities, immunosuppressive illness or therapy, or exposure to a child who attends a day care center.6
Staphylococcus aureus should be considered during influenza outbreaks, with either vancomycin or linezolid being the recommended agents in the setting of methicillin-resistant S. aureus (MRSA). In a study comparing vancomycin versus linezolid for nosocomial pneumonia, the all-cause 60-day mortality was similar for both agents.11 Daptomycin, another agent used against MRSA, is not indicated in the setting of pneumonia because daptomycin binds to surfactant, yielding it ineffective in the treatment of pneumonia.12 Ceftaroline is a newer cephalosporin with activity against MRSA; its role in treatment of community-acquired MRSA pneumonia has not been fully elucidated, but it appears to be a useful agent for this indication.13,14 Similarly, other agents known to have antibacterial properties against MRSA, such as trimethoprim/sulfamethoxazole and doxycycline, have not been studied for this indication. Clindamycin has been used to treat MRSA in children, and IDSA guidelines on the treatment of MRSA list clindamycin as an alternative15 if MRSA is known to be sensitive.
A summary of recommended empiric antibiotic therapy is presented in Table 3.16
Three antibiotics were approved by the US Food and Drug Administration (FDA) for the treatment of CAP after the release of the IDSA/ATS guidelines in 2007. Ceftaroline fosamil is a fifth-generation cephalosporin that has coverage for MRSA and was approved in November 2010.17 It can only be administered intravenously and needs dose adjustment for renal function. Omadacycline is a new tetracycline that was approved by the FDA in October 2018.18 It is available in both intravenous injectable and oral forms. No dose adjustment is needed for renal function. Lefamulin is a first-in-class novel pleuromutilin antibiotic which was FDA-approved in August 2019. It can be administered intravenously or orally, with no dosage adjustment necessary in patients with renal impairment.19
Antibiotic Therapy for Selected Pathogens
Streptococcus pneumoniae
Patients with pneumococcal pneumonia who have penicillin-susceptible strains can be treated with intravenous penicillin (2 or 3 million units every 4 hours) or ceftriaxone. Once a patient meets criteria of stability, they can then be transitioned to oral penicillin, amoxicillin, or clarithromycin. Those with strains with reduced susceptibility can still be treated with penicillin, but at a higher dose (4 million units intravenously [IV] every 4 hours), or a third-generation cephalosporin. Those whose pneumococcal pneumonia is complicated by bacteremia will benefit from dual therapy if severely ill, requiring ICU monitoring. Those not severely ill can be treated with monotherapy.20
Staphylococcus aureus
Staphylococcus aureus is more commonly associated with hospital-acquired pneumonia, but it may also be seen during the influenza season and in those with severe necrotizing CAP. Both linezolid and vancomycin can be used to treat MRSA CAP. As noted, ceftaroline has activity against MRSA and is approved for treatment of CAP, but is not approved by the FDA for MRSA CAP treatment. Similarly, tigecycline is approved for CAP and has activity against MRSA, but is not approved for MRSA CAP. Moreover, the FDA has warned of increased risk of death with tigecycline and has a black box warning to that effect.21
Legionella
Legionellosis can be treated with tetra¬cyclines, macrolides, or fluoroquinolones. For non-immunocompromised patients with mild pneumonia, any of the listed antibiotics is considered appropriate. However, patients with severe infection or those with immunosuppression should be treated with either levofloxacin or azithromycin for 7 to 10 days.22
Chlamydophila pneumoniae
As with other atypical organisms, Chlamydophila pneumoniae can be treated with doxycycline, a macrolide, or respiratory fluoroquinolones. However, length of therapy varies by regimen used; treating with doxycycline 100 mg twice daily generally requires 14 to 21 days, whereas moxifloxacin 400 mg daily requires 10 days.23
Mycoplasma pneumoniae
As with C. pneumoniae, length of therapy of Mycoplasma pneumoniae varies by which antimicrobial regimen is used. Shortest courses are seen with the use of macrolides for 5 days, whereas 14 days is considered standard for doxycycline or a respiratory fluoroquinolone.24 It should be noted that there has been increasingly documented resistance to macrolides, with known resistance of 8.2% in the United States.25
Duration of Treatment
Most patients with CAP respond to appropriate therapy within 72 hours. IDSA/ATS guidelines recommend that patients with routine cases of CAP be treated for a minimum of 5 days. Despite this, many patients are treated for an excessive amount of time, with over 70% of patients reported to have received antibiotics for more than 10 days for uncomplicated CAP.26 There are instances that require longer courses of antibiotics, including cases caused by Pseudomonas aeruginosa, S. aureus, and Legionella species and patients with lung abscesses or necrotizing infections, among others.27
Hospitalized patients do not need to be monitored for an additional day once they have reached clinical stability (Table 4), are able to maintain oral intake, and have normal mentation, provided that other comorbidities are stable and social needs have been met.6 C-reactive protein (CRP) level has been postulated as an additional measure of stability, specifically monitoring for a greater than 50% reduction in CRP; however, this was validated only for those with complicated pneumonia.28 Patients discharged from the hospital with instability have higher risk of readmission or death.29
Transition to Oral Therapy
IDSA/ATS guidelines6 recommend that patients should be transitioned from intravenous to oral antibiotics when they are improving clinically, have stable vital signs, and are able to ingest food/fluids and medications.
Management of Nonresponders
Although the majority of patients respond to antibiotics within 72 hours, treatment failure occurs in up to 15% of patients.15 Nonresponding pneumonia is generally seen in 2 patterns: worsening of clinical status despite empiric antibiotics or delay in achieving clinical stability, as defined in Table 4, after 72 hours of treatment.30 Risk factors associated with nonresponding pneumonia31 are:
- Radiographic: multilobar infiltrates, pleural effusion, cavitation
- Bacteriologic: MRSA, gram-negative or Legionella pneumonia
- Severity index: PSI > 90
- Pharmacologic: incorrect antibiotic choice based on susceptibility
Patients with acute deterioration of clinical status require prompt transfer to a higher level of care and may require mechanical ventilator support. In those with delay in achieving clinical stability, a question centers on whether the same antibiotics can be continued while doing further radiographic/microbiologic work-up and/or changing antibiotics. History should be reviewed, with particular attention to exposures, travel history, and microbiologic and radiographic data. Clinicians should recall that viruses account for up to 20% of pneumonias and that there are also noninfectious causes that can mimic pyogenic infections.32 If adequate initial cultures were not obtained, they should be obtained; however, care must be taken in reviewing new sets of cultures while on antibiotics, as they may reveal colonization selected out by antibiotics and not a true pathogen. If repeat evaluation is unrevealing, then further evaluation with computed tomography (CT) scan and bronchoscopy with bronchoalveolar lavage and biopsy is warranted. CT scans can show pleural effusions, bronchial obstructions, or a pattern suggestive of cryptogenic pneumonia. A bronchoscopy might yield a microbiologic diagnosis and, when combined with biopsy, can also evaluate for noninfectious causes.
As with other infections, if escalation of antibiotics is undertaken, clinicians should try to determine the reason for nonresponse. To simply broaden antimicrobial therapy without attempts at establishing a microbiologic or radiographic cause for nonresponse may lead to inappropriate treatment and recurrence of infection. Aside from patients who have bacteremic pneumococcal pneumonia in an ICU setting, there are no published reports pointing to superiority of combination antibiotics.20
Other Treatment
Several agents have been evaluated as adjunctive treatment of pneumonia to decrease the inflammatory response associated with pneumonia; namely, steroids, macrolide antibiotics, and statins. To date, only the use of steroids (methylprednisolone 0.5 mg/kg every 12 hours for 5 days) in those with severe CAP and high initial anti-inflammatory response (CRP > 150) has been shown to decrease treatment failure, decrease risk of acute respiratory distress syndrome, and possibly reduce length of stay and duration of intravenous antibiotics, without effect on mortality or adverse side effects.33,34 However, a recent double-blind randomized study conducted in Australia in which patients admitted with CAP were prescribed prednisolone acetate (50 mg/day for 7 days) and de-escalated from parenteral to oral antibiotics according to standardized criteria revealed no difference in mortality, length of stay, or readmission rates between the corticosteroids group and the control group at 90-day follow-up.35 At this point, corticosteroid as an adjunctive treatment for CAP is still controversial and the new 2019 ATS/IDSA guidelines recommend not routinely using corticosteroids in all patients with CAP.36 Other adjunctive methods have not been found to have significant impact.6
Prevention of Pneumonia
Prevention of pneumococcal pneumonia involves vaccinations to prevent infection caused by S. pneumoniae and influenza viruses. As influenza is a risk factor for bacterial infection, specifically with S. pneumoniae, influenza vaccination can help prevent bacterial pneumonia.37 In their most recent recommendations, the CDC continues to recommend routine influenza vaccination for all persons older than age 6 months, unless otherwise contraindicated.38
There are 2 vaccines for prevention of pneumococcal disease: the pneumococcal polysaccharide vaccine (PPSV23) and a conjugate vaccine (PCV13). Following vaccination with PPSV23, 80% of adults develop antibodies against at least 18 of the 23 serotypes.39 PPSV23 is reported to be protective against invasive pneumococcal infection, although there is no consensus regarding whether PPSV23 leads to decreased rates of pneumonia.40 On the other hand, PCV13 vaccination was associated with prevention of both invasive disease and CAP in adults aged 65 years or older.41 The CDC recommends that all children aged 2 years or younger receive PCV13, and those aged 65 or older receive PCV13 followed by a dose of PPSV23.42,43 The dose of PPSV23 should be given at least 1 year after the dose of PCV13 is administered.44 Persons younger than 65 years with immunocompromising and certain other conditions should also receive vaccination (Table 5).44 Full recommendations, many scenarios, and details on timing of vaccinations can be found at the CDC’s website.
Cigarette smoking increases the risk of respiratory infections, as evidenced by smokers accounting for almost half of all patients with invasive pneumococcal disease.11 As this is a modifiable risk factor, smoking cessation should be part of a comprehensive approach toward prevention of pneumonia.
Summary
Most patients with CAP are treated empirically with antibiotics, with therapy selection based on the site of care, likely pathogen, and antimicrobial resistance issues. Those treated as outpatients usually respond well to empiric antibiotic treatment, and a causative pathogen is not usually sought. Patients who are hospitalized for treatment usually receive empiric antibiotic on admission, and antimicrobial therapy is adjusted accordingly once the etiology has been determined by microbiologic or serologic means. At this time, the use of corticosteroid as an adjunctive treatment for CAP is still controversial, so not all patients with CAP should routinely receive corticosteroids. Because vaccination (PPSV23, PCV13, and influenza vaccine) remains the most effective tool in preventing the development of CAP, clinicians should strive for 100% vaccination rates in persons without contraindications.
Initial management decisions for patients with community-acquired pneumonia (CAP) will depend on severity of infection, with need for hospitalization being one of the first decisions. Because empiric antibiotics are the mainstay of treatment and the causative organisms are seldom identified, underlying medical conditions and epidemiologic risk factors are considered when selecting an empiric regimen. As with other infections, duration of therapy is not standardized, but rather is guided by clinical improvement. Prevention of pneumonia centers around vaccination and smoking cessation. This article, the second in a 2-part review of CAP in adults, focuses on site of care decision, empiric and directed therapies, length of treatment, and prevention strategies. Evaluation and diagnosis of CAP are discussed in a separate article.
Site of Care Decision
For patients diagnosed with CAP, the clinician must decide whether treatment will be done in an outpatient or inpatient setting, and for those in the inpatient setting, whether they can safely be treated on the general medical ward or in the intensive care unit (ICU). Two common scoring systems that can be used to aid the clinician in determining severity of the infection and guide site-of-care decisions are the Pneumonia Severity Index (PSI) and CURB-65 scores.
The PSI score uses 20 different parameters, including comorbidities, laboratory parameters, and radiographic findings, to stratify patients into 5 mortality risk classes.1 On the basis of associated mortality rates, it has been suggested that risk class I and II patients should be treated as outpatients, risk class III patients should be treated in an observation unit or with a short hospitalization, and risk class IV and V patients should be treated as inpatients.1
The CURB-65 method of risk stratification is based on 5 clinical parameters: confusion, urea level, respiratory rate, systolic blood pressure, and age ≥ 65 years (Table 1).2,3 A modification to the CURB-65 algorithm tool was CRB-65, which excludes urea nitrogen, making it optimal for making determinations in a clinic-based setting. It should be emphasized that these tools do not take into account other factors that should be used in determining location of treatment, such as stable home, mental illness, or concerns about compliance with medications. In many instances, it is these factors that preclude low-risk patients from being treated as outpatients.4,5 Similarly, these scoring systems have not been validated for immunocompromised patients or those who would qualify as having health care–associated pneumonia.
Patients with CURB-65 scores of 4 or 5 are considered to have severe pneumonia, and admission to the ICU should be considered for these patients. Aside from the CURB-65 score, anyone requiring vasopressor support or mechanical ventilation merits admission to the ICU.6 American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guidelines also recommend the use of “minor criteria” for making ICU admission decisions; these include respiratory rate ≥ 30 breaths/minute, PaO2 fraction ≤ 250 mm Hg, multilobar infiltrates, confusion, blood urea nitrogen ≥ 20 mg/dL, leukopenia, thrombocytopenia, hypothermia, and hypotension.6 These factors are associated with increased mortality due to CAP, and ICU admission is indicated if 3 of the minor criteria for severe CAP are present.
Another clinical calculator that can be used for assessing severity of CAP is SMART-COP (systolic blood pressure, multilobar chest radiography involvement, albumin level, respiratory rate, tachycardia, confusion, oxygenation and arterial pH).7 This scoring system uses 8 weighted criteria to predict which patients will require intensive respiratory or vasopressor support. SMART-COP has a sensitivity of 79% and a specificity of 64% in predicting ICU admission, whereas CURB-65 has a pooled sensitivity of 57.2% and specificity of 77.2%.8
Antibiotic Therapy
Antibiotics are the mainstay of treatment for CAP, with the majority of patients with CAP treated empirically taking into account the site of care, likely pathogen, and antimicrobial resistance issues. Patients with pneumonia who are treated as outpatients usually respond well to empiric antibiotic treatment, and a causative pathogen is not usually sought. Patients who are hospitalized for treatment of CAP usually receive empiric antibiotic on admission. Once the etiology has been determined by microbiologic or serologic means, antimicrobial therapy should be adjusted accordingly. A CDC study found that the burden of viral etiologies was higher than previously thought, with rhinovirus and influenza accounting for 15% of cases and Streptococcus pneumoniae for only 5%.9 This study highlighted the fact that despite advances in molecular techniques, no pathogen is identified for most patients with pneumonia.9 Given the lack of discernable pathogens in the majority of cases, patients should continue to be treated with antibiotics unless a nonbacterial etiology is found.
Outpatients without comorbidities or risk factors for drug-resistant S. pneumoniae (Table 2)10 can be treated with monotherapy. Hospitalized patients are usually treated with combination intravenous therapy, although non-ICU patients who receive a respiratory fluoroquinolone can be treated orally.
As previously mentioned, antibiotic therapy is typically empiric, since neither clinical features nor radiographic features are sufficient to include or exclude infectious etiologies. Epidemiologic risk factors should be considered and, in certain cases, antimicrobial coverage should be expanded to include those entities; for example, treatment of anaerobes in the setting of lung abscess and antipseudomonal antibiotics for patients with bronchiectasis.
Of concern in the treatment of CAP is the increased prevalence of antimicrobial resistance among S. pneumoniae. The IDSA guidelines report that drug-resistant S. pneumoniae is more common in persons aged < 2 or > 65 years, and those with β-lactam therapy within the previous 3 months, alcoholism, medical comorbidities, immunosuppressive illness or therapy, or exposure to a child who attends a day care center.6
Staphylococcus aureus should be considered during influenza outbreaks, with either vancomycin or linezolid being the recommended agents in the setting of methicillin-resistant S. aureus (MRSA). In a study comparing vancomycin versus linezolid for nosocomial pneumonia, the all-cause 60-day mortality was similar for both agents.11 Daptomycin, another agent used against MRSA, is not indicated in the setting of pneumonia because daptomycin binds to surfactant, yielding it ineffective in the treatment of pneumonia.12 Ceftaroline is a newer cephalosporin with activity against MRSA; its role in treatment of community-acquired MRSA pneumonia has not been fully elucidated, but it appears to be a useful agent for this indication.13,14 Similarly, other agents known to have antibacterial properties against MRSA, such as trimethoprim/sulfamethoxazole and doxycycline, have not been studied for this indication. Clindamycin has been used to treat MRSA in children, and IDSA guidelines on the treatment of MRSA list clindamycin as an alternative15 if MRSA is known to be sensitive.
A summary of recommended empiric antibiotic therapy is presented in Table 3.16
Three antibiotics were approved by the US Food and Drug Administration (FDA) for the treatment of CAP after the release of the IDSA/ATS guidelines in 2007. Ceftaroline fosamil is a fifth-generation cephalosporin that has coverage for MRSA and was approved in November 2010.17 It can only be administered intravenously and needs dose adjustment for renal function. Omadacycline is a new tetracycline that was approved by the FDA in October 2018.18 It is available in both intravenous injectable and oral forms. No dose adjustment is needed for renal function. Lefamulin is a first-in-class novel pleuromutilin antibiotic which was FDA-approved in August 2019. It can be administered intravenously or orally, with no dosage adjustment necessary in patients with renal impairment.19
Antibiotic Therapy for Selected Pathogens
Streptococcus pneumoniae
Patients with pneumococcal pneumonia who have penicillin-susceptible strains can be treated with intravenous penicillin (2 or 3 million units every 4 hours) or ceftriaxone. Once a patient meets criteria of stability, they can then be transitioned to oral penicillin, amoxicillin, or clarithromycin. Those with strains with reduced susceptibility can still be treated with penicillin, but at a higher dose (4 million units intravenously [IV] every 4 hours), or a third-generation cephalosporin. Those whose pneumococcal pneumonia is complicated by bacteremia will benefit from dual therapy if severely ill, requiring ICU monitoring. Those not severely ill can be treated with monotherapy.20
Staphylococcus aureus
Staphylococcus aureus is more commonly associated with hospital-acquired pneumonia, but it may also be seen during the influenza season and in those with severe necrotizing CAP. Both linezolid and vancomycin can be used to treat MRSA CAP. As noted, ceftaroline has activity against MRSA and is approved for treatment of CAP, but is not approved by the FDA for MRSA CAP treatment. Similarly, tigecycline is approved for CAP and has activity against MRSA, but is not approved for MRSA CAP. Moreover, the FDA has warned of increased risk of death with tigecycline and has a black box warning to that effect.21
Legionella
Legionellosis can be treated with tetra¬cyclines, macrolides, or fluoroquinolones. For non-immunocompromised patients with mild pneumonia, any of the listed antibiotics is considered appropriate. However, patients with severe infection or those with immunosuppression should be treated with either levofloxacin or azithromycin for 7 to 10 days.22
Chlamydophila pneumoniae
As with other atypical organisms, Chlamydophila pneumoniae can be treated with doxycycline, a macrolide, or respiratory fluoroquinolones. However, length of therapy varies by regimen used; treating with doxycycline 100 mg twice daily generally requires 14 to 21 days, whereas moxifloxacin 400 mg daily requires 10 days.23
Mycoplasma pneumoniae
As with C. pneumoniae, length of therapy of Mycoplasma pneumoniae varies by which antimicrobial regimen is used. Shortest courses are seen with the use of macrolides for 5 days, whereas 14 days is considered standard for doxycycline or a respiratory fluoroquinolone.24 It should be noted that there has been increasingly documented resistance to macrolides, with known resistance of 8.2% in the United States.25
Duration of Treatment
Most patients with CAP respond to appropriate therapy within 72 hours. IDSA/ATS guidelines recommend that patients with routine cases of CAP be treated for a minimum of 5 days. Despite this, many patients are treated for an excessive amount of time, with over 70% of patients reported to have received antibiotics for more than 10 days for uncomplicated CAP.26 There are instances that require longer courses of antibiotics, including cases caused by Pseudomonas aeruginosa, S. aureus, and Legionella species and patients with lung abscesses or necrotizing infections, among others.27
Hospitalized patients do not need to be monitored for an additional day once they have reached clinical stability (Table 4), are able to maintain oral intake, and have normal mentation, provided that other comorbidities are stable and social needs have been met.6 C-reactive protein (CRP) level has been postulated as an additional measure of stability, specifically monitoring for a greater than 50% reduction in CRP; however, this was validated only for those with complicated pneumonia.28 Patients discharged from the hospital with instability have higher risk of readmission or death.29
Transition to Oral Therapy
IDSA/ATS guidelines6 recommend that patients should be transitioned from intravenous to oral antibiotics when they are improving clinically, have stable vital signs, and are able to ingest food/fluids and medications.
Management of Nonresponders
Although the majority of patients respond to antibiotics within 72 hours, treatment failure occurs in up to 15% of patients.15 Nonresponding pneumonia is generally seen in 2 patterns: worsening of clinical status despite empiric antibiotics or delay in achieving clinical stability, as defined in Table 4, after 72 hours of treatment.30 Risk factors associated with nonresponding pneumonia31 are:
- Radiographic: multilobar infiltrates, pleural effusion, cavitation
- Bacteriologic: MRSA, gram-negative or Legionella pneumonia
- Severity index: PSI > 90
- Pharmacologic: incorrect antibiotic choice based on susceptibility
Patients with acute deterioration of clinical status require prompt transfer to a higher level of care and may require mechanical ventilator support. In those with delay in achieving clinical stability, a question centers on whether the same antibiotics can be continued while doing further radiographic/microbiologic work-up and/or changing antibiotics. History should be reviewed, with particular attention to exposures, travel history, and microbiologic and radiographic data. Clinicians should recall that viruses account for up to 20% of pneumonias and that there are also noninfectious causes that can mimic pyogenic infections.32 If adequate initial cultures were not obtained, they should be obtained; however, care must be taken in reviewing new sets of cultures while on antibiotics, as they may reveal colonization selected out by antibiotics and not a true pathogen. If repeat evaluation is unrevealing, then further evaluation with computed tomography (CT) scan and bronchoscopy with bronchoalveolar lavage and biopsy is warranted. CT scans can show pleural effusions, bronchial obstructions, or a pattern suggestive of cryptogenic pneumonia. A bronchoscopy might yield a microbiologic diagnosis and, when combined with biopsy, can also evaluate for noninfectious causes.
As with other infections, if escalation of antibiotics is undertaken, clinicians should try to determine the reason for nonresponse. To simply broaden antimicrobial therapy without attempts at establishing a microbiologic or radiographic cause for nonresponse may lead to inappropriate treatment and recurrence of infection. Aside from patients who have bacteremic pneumococcal pneumonia in an ICU setting, there are no published reports pointing to superiority of combination antibiotics.20
Other Treatment
Several agents have been evaluated as adjunctive treatment of pneumonia to decrease the inflammatory response associated with pneumonia; namely, steroids, macrolide antibiotics, and statins. To date, only the use of steroids (methylprednisolone 0.5 mg/kg every 12 hours for 5 days) in those with severe CAP and high initial anti-inflammatory response (CRP > 150) has been shown to decrease treatment failure, decrease risk of acute respiratory distress syndrome, and possibly reduce length of stay and duration of intravenous antibiotics, without effect on mortality or adverse side effects.33,34 However, a recent double-blind randomized study conducted in Australia in which patients admitted with CAP were prescribed prednisolone acetate (50 mg/day for 7 days) and de-escalated from parenteral to oral antibiotics according to standardized criteria revealed no difference in mortality, length of stay, or readmission rates between the corticosteroids group and the control group at 90-day follow-up.35 At this point, corticosteroid as an adjunctive treatment for CAP is still controversial and the new 2019 ATS/IDSA guidelines recommend not routinely using corticosteroids in all patients with CAP.36 Other adjunctive methods have not been found to have significant impact.6
Prevention of Pneumonia
Prevention of pneumococcal pneumonia involves vaccinations to prevent infection caused by S. pneumoniae and influenza viruses. As influenza is a risk factor for bacterial infection, specifically with S. pneumoniae, influenza vaccination can help prevent bacterial pneumonia.37 In their most recent recommendations, the CDC continues to recommend routine influenza vaccination for all persons older than age 6 months, unless otherwise contraindicated.38
There are 2 vaccines for prevention of pneumococcal disease: the pneumococcal polysaccharide vaccine (PPSV23) and a conjugate vaccine (PCV13). Following vaccination with PPSV23, 80% of adults develop antibodies against at least 18 of the 23 serotypes.39 PPSV23 is reported to be protective against invasive pneumococcal infection, although there is no consensus regarding whether PPSV23 leads to decreased rates of pneumonia.40 On the other hand, PCV13 vaccination was associated with prevention of both invasive disease and CAP in adults aged 65 years or older.41 The CDC recommends that all children aged 2 years or younger receive PCV13, and those aged 65 or older receive PCV13 followed by a dose of PPSV23.42,43 The dose of PPSV23 should be given at least 1 year after the dose of PCV13 is administered.44 Persons younger than 65 years with immunocompromising and certain other conditions should also receive vaccination (Table 5).44 Full recommendations, many scenarios, and details on timing of vaccinations can be found at the CDC’s website.
Cigarette smoking increases the risk of respiratory infections, as evidenced by smokers accounting for almost half of all patients with invasive pneumococcal disease.11 As this is a modifiable risk factor, smoking cessation should be part of a comprehensive approach toward prevention of pneumonia.
Summary
Most patients with CAP are treated empirically with antibiotics, with therapy selection based on the site of care, likely pathogen, and antimicrobial resistance issues. Those treated as outpatients usually respond well to empiric antibiotic treatment, and a causative pathogen is not usually sought. Patients who are hospitalized for treatment usually receive empiric antibiotic on admission, and antimicrobial therapy is adjusted accordingly once the etiology has been determined by microbiologic or serologic means. At this time, the use of corticosteroid as an adjunctive treatment for CAP is still controversial, so not all patients with CAP should routinely receive corticosteroids. Because vaccination (PPSV23, PCV13, and influenza vaccine) remains the most effective tool in preventing the development of CAP, clinicians should strive for 100% vaccination rates in persons without contraindications.
1. Fine MJ, Auble TE, Yealy DM, et al A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med.1997;336:243-250.
2. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377-382.
3. Aujesky D, Auble TE, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med. 2005;118:384-392.
4. Arnold FW, Ramirez JA, McDonald LC, Xia EL. Hospitalization for community-acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest. 2003;124:121-124.
5. Aujesky D, McCausland JB, Whittle J, et al. Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumonia. Clin Infect Dis. 2009;49:e100-108.
6. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-72.
7. Charles PG, Wolfe R, Whitby M, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008;47:375-384.
8. Marti C, Garin N, Grosgurin O, et al. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Crit Care. 2012;16:R141.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415-427.
10. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
11. Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54:621-629.
12. Silverman JA, Mortin LI, Vanpraagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis. 2005;191:2149-2152.
13. El Hajj MS, Turgeon RD, Wilby KJ. Ceftaroline fosamil for community-acquired pneumonia and skin and skin structure infections: a systematic review. Int J Clin Pharm. 2017;39:26-32.
14. Taboada M, Melnick D, Iaconis JP, et al. Ceftaroline fosamil versus ceftriaxone for the treatment of community-acquired pneumonia: individual patient data meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2016;71:862-870.
15. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285-292.
16. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Sauders; 2015:2310-2327.
17. Teflaro (ceftaroline fosamil) [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2010.
18. Nuzyra (omadacycline) [package insert]. Boston, MA: Paratek Pharmaceuticals; 2018.
19. Xenleta (lefamulin) [package insert]. Dublin, Ireland: Nabriva Therapeutics; 2019.
20. Baddour LM, Yu VL, Klugman KP, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170:440-444.
21. FDA Drug Safety Communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new boxed warning. www.fda.gov/Drugs/DrugSafety/ucm369580.htm. Accessed 16 September 2019.
22. Edelstein PR, CR. Legionnaires’ disease and Pontiac fever. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2633.
23. Hammerschlag MR, Kohlhoff SA, Gaydos, CA. Chlamydia pneumoniae. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2174.
24. Holzman RS, MS. Mycoplasma pneumoniae and atypical pneumonia. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2183.
25. Yamada M, Buller R, Bledsoe S, Storch GA. Rising rates of macrolide-resistant Mycoplasma pneumoniae in the central United States. Pediatr Infect Dis J. 2012;31:409-410.
26. Yi SH, Hatfield KM, Baggs J, et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66:1333-1341.
27. Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis. 2011;52:1232-1240.
28. Akram AR, Chalmers JD, Taylor JK, et al. An evaluation of clinical stability criteria to predict hospital course in community-acquired pneumonia. Clin Microbiol Infect. 2013;19:1174-1180.
29. Halm EA, Fine MJ, Kapoor WN, et al. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med. 2002;162:1278-1284.
30. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Saunders; 2015:2310-2327.
31. Roson B, Carratala J, Fernandez-Sabe N, et al. Causes and factors associated with early failure in hospitalized patients with community-acquired pneumonia. Arch Intern Med. 2004;164:502-508.
32. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167:1650-1654.
33. Wan YD, Sun TW, Liu ZQ, et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest. 2016;149:209-219.
34. Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015;313:677-686.
35. Lloyd M, Karahalios, Janus E, et al. Effectiveness of a bundled intervention including adjunctive corticosteroids on outcomes of hospitalized patients with community-acquired pneumonia: a stepped-wedge randomized clinical trial. JAMA Intern Med. 2019;179:1052-1060.
36. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
37. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571-582.
38. Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2019-20 influenza season. MMWR Recomm Rep. 2019;68:1-21.
39. Rubins JB, Alter M, Loch J, Janoff EN. Determination of antibody responses of elderly adults to all 23 capsular polysaccharides after pneumococcal vaccination. Infect Immun. 1999;67:5979-5984.
40. Vaccines and preventable diseases. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html. Accessed 16 September 2019.
41. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114-1125.
42. Recommended adult immunization schedule -- United States -- 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed 16 September 2019.
43. Recommended child and adolescent immunization schedule for ages 18 years or younger – United States – 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html. Accessed 22 September 2019.
44. Pneumococcal vaccine timing for adults – United States – 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/vpd/pneumo/downloads/pneumo-vaccine-timing.pdf. Accessed 22 September 2019.
1. Fine MJ, Auble TE, Yealy DM, et al A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med.1997;336:243-250.
2. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377-382.
3. Aujesky D, Auble TE, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med. 2005;118:384-392.
4. Arnold FW, Ramirez JA, McDonald LC, Xia EL. Hospitalization for community-acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest. 2003;124:121-124.
5. Aujesky D, McCausland JB, Whittle J, et al. Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumonia. Clin Infect Dis. 2009;49:e100-108.
6. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-72.
7. Charles PG, Wolfe R, Whitby M, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008;47:375-384.
8. Marti C, Garin N, Grosgurin O, et al. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Crit Care. 2012;16:R141.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415-427.
10. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
11. Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54:621-629.
12. Silverman JA, Mortin LI, Vanpraagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis. 2005;191:2149-2152.
13. El Hajj MS, Turgeon RD, Wilby KJ. Ceftaroline fosamil for community-acquired pneumonia and skin and skin structure infections: a systematic review. Int J Clin Pharm. 2017;39:26-32.
14. Taboada M, Melnick D, Iaconis JP, et al. Ceftaroline fosamil versus ceftriaxone for the treatment of community-acquired pneumonia: individual patient data meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2016;71:862-870.
15. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285-292.
16. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Sauders; 2015:2310-2327.
17. Teflaro (ceftaroline fosamil) [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2010.
18. Nuzyra (omadacycline) [package insert]. Boston, MA: Paratek Pharmaceuticals; 2018.
19. Xenleta (lefamulin) [package insert]. Dublin, Ireland: Nabriva Therapeutics; 2019.
20. Baddour LM, Yu VL, Klugman KP, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170:440-444.
21. FDA Drug Safety Communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new boxed warning. www.fda.gov/Drugs/DrugSafety/ucm369580.htm. Accessed 16 September 2019.
22. Edelstein PR, CR. Legionnaires’ disease and Pontiac fever. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2633.
23. Hammerschlag MR, Kohlhoff SA, Gaydos, CA. Chlamydia pneumoniae. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2174.
24. Holzman RS, MS. Mycoplasma pneumoniae and atypical pneumonia. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2183.
25. Yamada M, Buller R, Bledsoe S, Storch GA. Rising rates of macrolide-resistant Mycoplasma pneumoniae in the central United States. Pediatr Infect Dis J. 2012;31:409-410.
26. Yi SH, Hatfield KM, Baggs J, et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66:1333-1341.
27. Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis. 2011;52:1232-1240.
28. Akram AR, Chalmers JD, Taylor JK, et al. An evaluation of clinical stability criteria to predict hospital course in community-acquired pneumonia. Clin Microbiol Infect. 2013;19:1174-1180.
29. Halm EA, Fine MJ, Kapoor WN, et al. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med. 2002;162:1278-1284.
30. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Saunders; 2015:2310-2327.
31. Roson B, Carratala J, Fernandez-Sabe N, et al. Causes and factors associated with early failure in hospitalized patients with community-acquired pneumonia. Arch Intern Med. 2004;164:502-508.
32. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167:1650-1654.
33. Wan YD, Sun TW, Liu ZQ, et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest. 2016;149:209-219.
34. Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015;313:677-686.
35. Lloyd M, Karahalios, Janus E, et al. Effectiveness of a bundled intervention including adjunctive corticosteroids on outcomes of hospitalized patients with community-acquired pneumonia: a stepped-wedge randomized clinical trial. JAMA Intern Med. 2019;179:1052-1060.
36. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
37. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571-582.
38. Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2019-20 influenza season. MMWR Recomm Rep. 2019;68:1-21.
39. Rubins JB, Alter M, Loch J, Janoff EN. Determination of antibody responses of elderly adults to all 23 capsular polysaccharides after pneumococcal vaccination. Infect Immun. 1999;67:5979-5984.
40. Vaccines and preventable diseases. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html. Accessed 16 September 2019.
41. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114-1125.
42. Recommended adult immunization schedule -- United States -- 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed 16 September 2019.
43. Recommended child and adolescent immunization schedule for ages 18 years or younger – United States – 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html. Accessed 22 September 2019.
44. Pneumococcal vaccine timing for adults – United States – 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/vpd/pneumo/downloads/pneumo-vaccine-timing.pdf. Accessed 22 September 2019.
Community-Acquired Pneumonia: Evaluation and Diagnosis
Despite advances in medical science, pneumonia remains a major cause of morbidity and mortality. In 2017, 49,157 patients in the United States died from the disease.1 Pneumonia can be classified as community-acquired, hospital-acquired, or ventilator-associated. Another category, healthcare-associated pneumonia, was included in an earlier Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) guideline but was removed from the 2016 guideline because there was no clear evidence that patients diagnosed with healthcare-associated pneumonia were at higher risk for harboring multidrug-resistant pathogens.2 This review is the first of 2 articles focusing on the management of community-acquired pneumonia (CAP). Here, we review CAP epidemiology, microbiology, predisposing factors, and diagnosis; current treatment and prevention of CAP are reviewed in a separate article.
Definition and Epidemiology
CAP is defined as an acute infection of the lungs that develops in patients who have not been hospitalized recently and have not had regular exposure to the health care system.3 A previously ambulatory patient who is diagnosed with pneumonia within 48 hours after admission also meets the criteria for CAP. Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually.4 About 25% of CAP patients require hospitalization, and about 5% to 10% of these patients are admitted to the intensive care unit (ICU).5 In-hospital mortality is considerable (~10% in population-based studies),6 and 30-day mortality was found to be as high as 23% in a review by File and Marrie.7 CAP also confers a high risk of long-term morbidity and mortality compared with the general population who have never had CAP, irrespective of age.8
Causative Organisms
Numerous microorganisms can cause CAP. Common causes and less common causes are delineated in Table 1. Until recently, many studies had demonstrated that pneumococcus was the most common cause of CAP. However, in the CDC Etiology of Pneumonia in the Community (EPIC) study team’s 2015 prospective, multicenter, population-based study, no pathogen was detected in the majority of patients diagnosed with CAP requiring hospitalization. The most common pathogens they detected were rhinovirus (9%), followed by influenza virus (6%) and pneumococcus (5%).9 Factors considered to be contributing to the decrease in the percentage of pneumococcus in patients diagnosed with CAP are the widespread use of pneumococcal vaccine and reduced rates of smoking.10,11
Predisposing Factors
Most people diagnosed with CAP have 1 or more predisposing factors (Table 2).12,13 Patients who develop CAP typically have a combination of these predisposing factors rather than a single factor. Aging, in combination with other risk factors, increases the susceptibility of a person to pneumonia.
Clinical Signs and Symptoms
Symptoms of CAP include fever, chills, rigors, fatigue, anorexia, diaphoresis, dyspnea, cough (with or without sputum production), and pleuritic chest pain. There is no individual symptom or cluster of symptoms that can absolutely differentiate pneumonia from other acute respiratory diseases, including upper and lower respiratory infections. However, patients presenting with the constellation of symptoms of fever ≥ 100°F (37.8°C), productive cough, and tachycardia is more suggestive of pneumonia.14 Abnormal vital signs include fever, hypothermia, tachypnea, tachycardia, and oxygen desaturation. Auscultation of the chest reveals crackles or other adventitious breath sounds. Elderly patients with pneumonia report a significantly lower number of both respiratory and nonrespiratory symptoms compared with younger patients. Clinicians should be aware of this phenomenon to avoid delayed diagnosis and treatment.15
Imaging Evaluation
The presence of a pulmonary consolidation or an infiltrate on chest radiograph is required to diagnose CAP, and a chest radiograph should be obtained when CAP is suspected.16 However, there is no pattern of radiographic abnormalities reliable enough to differentiate infectious pneumonia from noninfectious causes.17
There are case reports and case series demonstrating false-negative plain chest radiographs in dehydrated patients18 or in patients in a neutropenic state. However, animal studies have shown that dogs challenged with pneumococcus showed abnormal pulmonary shadow, suggestive of pneumonia, regardless of hydration status.19 There is also no reliable scientific evidence to support the notion that severe neutropenia can cause false-negative radiographs because of the inability to develop an acute inflammatory reaction in the lungs.20
A chest computed tomography (CT) scan is more sensitive than a plain chest radiograph in detecting pneumonia. Therefore, a chest CT should be performed in a patient with negative plain chest radiograph when pneumonia is still highly suspected.21 A chest CT scan is also more sensitive in detecting cavitation, adenopathy, interstitial disease, and empyema. It also has the advantage of better defining anatomical changes than plain films.22
Because improvement of pulmonary opacities in patients with CAP lags behind clinical improvement, repeating chest imaging studies is not recommended in patients who demonstrate clinical improvement. Clearing of pulmonary infiltrate or consolidation sometimes can take 6 weeks or longer.23
Laboratory Evaluation
Generally, the etiologic agent of CAP cannot be determined solely on the basis of clinical signs and symptoms or imaging studies. Although routine microbiological testing for patients suspicious for CAP is not necessary for empirical treatment, determining the etiologic agent of the pneumonia allows the clinician to narrow the antibiotics from a broad-spectrum empirical regimen to specific pathogen-directed therapy. Determination of certain etiologic agents causing the pneumonia can have important public health implications (eg, Mycobacterium tuberculosis and influenza virus).24
Sputum Gram Stain and Culture
Sputum Gram stain is an inexpensive test that may identify pathogens that cause CAP (eg, Streptococcus pneumoniae and Haemophilus influenzae). A quality specimen is required. A sputum sample must contain more than 25 neutrophils and less than 10 squamous epithelial cells/low power field on Gram stain to be considered suitable for culture. The sensitivity and specificity of sputum Gram stain and culture are highly variable in different clinical settings (eg, outpatient setting, nursing home, ICU). Reed et al’s meta-analysis of patients diagnosed with CAP in the United States showed the sensitivity and specificity of sputum Gram stain (compared with sputum culture) ranged from 15% to 100% and 11% to 100%, respectively.24 In cases of proven bacteremic pneumococcal pneumonia, positive cultures from sputum samples were positive less than 50% of the time.25
For patients who cannot provide sputum samples or are intubated, deep-suction aspirate or bronchoalveolar lavage through a bronchoscopic procedure may be necessary to obtain pulmonary secretion for Gram stain and culture. Besides bacterial culture, sputum samples can also be sent for fungal and mycobacterial cultures and acid-fast stain, if deemed clinically necessary.
The 2019 ATS/IDSA guidelines for diagnosis and treatment of adults with CAP recommend sputum culture in patients with severe disease and in all inpatients empirically treated for MRSA or Pseudomonas aeruginosa.26
Blood Culture
Because the positivity rate of blood culture in patients who are suspected to have pneumonia but not exposed to antimicrobial agents is low (5%–14%), blood cultures are not recommended for all patients with CAP. Another reason for not recommending blood culture is positive culture rarely leads to changes in antibiotic regimen in patients without underlying diseases.27 However, the 2019 ATS/IDSA guidelines recommend blood culture in patients with severe disease and in all inpatients treated empirically for MRSA or P. aeruginosa.26
A multinational study published in 2008 examined 125 patients with pneumococcal bacteremic CAP versus 1847 patients with non-bacteremic CAP.28 Analysis of the data demonstrated no association between pneumococcal bacteremic CAP and time to clinical stability, length of hospital stay, all-cause mortality, or CAP-related mortality. The authors concluded that pneumococcal bacteremia does not increase the risk of poor outcomes in patients with CAP compared to non-bacteremic patients, and the presence of pneumococcal bacteremia should not deter de-escalation of therapy in clinically stable patients.
Urinary Antigen Tests
Urinary antigen tests may assist clinicians in narrowing antibiotic therapy when test results are positive. There are 2 US Food and Drug Administration–approved tests available to clinicians for detecting pneumococcal and Legionella antigen in urine. The test for Legionella pneumophila detects disease due to serogroup 1 only, which accounts for 80% of community-acquired Legionnaires’ disease. The sensitivity and specificity of the Legionella urine antigen test are 90% and 99%, respectively. The pneumococcal urine antigen test is less sensitive and specific than the Legionella urine antigen test (sensitivity 80% and specificity > 90%).29,30
Advantages of the urinary antigen tests are that they are easily performed, results are available in less than an hour if done in-house, and results are not affected by prior exposure to antibiotics. However, the tests do not meet Clinical Laboratory Improvements Amendments criteria for waiver and must be performed by a technician in the laboratory. A multicenter, prospective surveillance study of hospitalized patients with CAP showed that the 2007 IDSA/ATS guidelines’ recommended indications for S. pneumoniae and L. pneumophila urinary antigen tests do not have sufficient sensitivity and specificity to identify patients with positive tests.31
Polymerase Chain Reaction
There are several FDA-approved polymerase chain reaction (PCR) tests commercially available to assist clinicians in diagnosing pneumonia. PCR testing of nasopharyngeal swabs for diagnosis of influenza has become standard in many US medical facilities. The great advantages of using PCR to diagnose influenza are its high sensitivity and specificity and rapid turnaround time. PCR can also be used to detect Legionella species, S. pneumonia, Mycoplasma pneumoniae, Chlamydophila pneumonia, and mycobacterial species.24
One limitation of using PCR tests on respiratory specimens is that specimens can be contaminated with oral or upper airway flora, so the results must be interpreted with caution, bearing in mind that some of the pathogens isolated may be colonizers of the oral or upper airway flora.32
Biologic Markers
Two biologic markers—procalcitonin and C-reactive protein (CRP)—can be used in conjunction with history, physical examination, laboratory tests, and imaging studies to assist in the diagnosis and treatment of CAP.24 Procalcitonin is a peptide precursor of the hormone calcitonin that is released by parenchymal cells into the bloodstream, resulting in increased serum level in patients with bacterial infections. In contrast, there is no remarkable procalcitonin level increase with viral or noninfectious inflammation. The reference value of procalcitonin in the blood of an adult individual without infection or inflammation is < 0.15 ng/mL. In the blood, procalcitonin has a half-life of 25 to 30 hours. The quantitative immunoluminometric method (LUMI test, Brahms PCT, Berlin, Germany) is the preferred test to use because of its high sensitivity.33 A meta-analysis of 12 studies involving more than 2400 patients with CAP demonstrated that serum procalcitonin does not have sufficient sensitivity or specificity to distinguish between bacterial and nonbacterial pneumonia. The authors concluded that procalcitonin level cannot be used to decide whether an antibiotic should be administered.34
A 2012 Cochrane meta-analysis that involved 4221 patients with acute respiratory infections (with half of the patients diagnosed with CAP) from 14 prospective trials found the use of procalcitonin test for antibiotic use significantly decreased median antibiotic exposure from 8 to 4 days without an increase in treatment failure, mortality rates in any clinical setting (eg, outpatient clinic, emergency room), or length of hospitalization.35 An update of the 2012 Cochrane review that examined the safety and efficacy of using procalcitonin for starting or stopping antibiotics again demonstrated procalcitonin use was associated with a reduction of antibiotic use (2.4 days).36 A prospective study conducted in France on 100 ICU patients showed that increased procalcitonin from day 1 to day 3 has a poor prognosis factor for severe CAP, whereas decreasing procalcitonin levels is associated with a favorable outcome.37
Because of conflicting data, the 2019 ATS/IDSA guidelines do not recommend using procalcitonin to determine need for initial antibacterial therapy.26
CRP is an acute phase protein produced by the liver. CRP level in the blood increases in response to acute infection or inflammation. Use of CRP in assisting diagnosis and guiding treatment of CAP is more limited in part due to its poor specificity. A prospective study conducted on 168 consecutive patients who presented with cough showed that a CRP level > 40 mg/L had a sensitivity and specificity of 70% and 90%, respectively.38
Summary
CAP remains a leading cause of hospitalization and death in the 21st century. Traditionally, pneumococcus has been considered the major pathogen causing CAP; however, the 2015 EPIC study found that S. pneumoniae was detected in only 5% of patients diagnosed with CAP. Despite the new findings, it is still recommended that empiric treatment for CAP target common typical bacteria (pneumococcus, H. influenzae, Moraxella catarrhalis) and atypical bacteria (M. pneumonia, C. pneumoniae, L. pneumophila).
Because diagnosing pneumonia through history and clinical examination is less than 50% sensitive, a chest imaging study (a plain chest radiograph or a chest CT scan) is usually required to make the diagnosis. Laboratory tests, such as sputum Gram stain/culture, blood culture, urinary antigen tests, PCR test, procalcitonin, and CRP are important adjunctive diagnostic modalities to assist in the diagnosis and management of CAP. However, because no single test is sensitive and specific enough to be a stand-alone test, they should be used in conjunction with history, physical examination, and imaging studies.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. FastStats - Pneumonia. www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed 16 September 2019.
2. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
3. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med. 2014;371:1619-1628.
4. Mandell LA. Epidemiology and etiology of community-acquired pneumonia. Infect Dis Clin North Am. 2004;18:761-776.
5. Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ. 2006;332:1077-1079.
6. Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med. 2007;167:1938-1943.
7. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122:130-141.
8. Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-year mortality after community-acquired pneumonia. a prospective cohort. Am J Respir Crit Care Med. 2015;192:597-604.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415-427.
10. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155-163.
11. Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med. 2000;342:681-689.
12. Almirall J, Serra-Prat M, Bolíbar I, Balasso V. Risk factors for community-acquired pneumonia in adults: a systemic review of observational studies. Respiration. 2017;94:299-311.
13. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Saunders; 2015:2310-2327.
14. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough--a statistical approach. J Chronic Dis. 1984;37:215-225.
15. Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med. 1997;157:1453-1459.
16. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-72.
17. Jartti A, Rauvala E, Kauma H, et al. Chest imaging findings in hospitalized patients with H1N1 influenza. Acta Radiol. 2011;52:297-304.
18. Basi SK, Marrie TJ, Huang JQ, Majumdar SR. Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117:305-311.
19. Caldwell A, Glauser FL, Smith WR, et al. The effects of dehydration on the radiologic and pathologic appearance of experimental canine segmental pneumonia. Am Rev Respir Dis. 1975;112:651-656.
20. Bartlett JG. Pneumonia. In: Barlett JG, editor. Management of Respiratory Tract Infections. Philadelphia: Lippincott, Williams & Wilkins; 2001:1-122.
21. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192:974-982.
22. Wheeler JH, Fishman EK. Computed tomography in the management of chest infections: current status. Clin Infect Dis. 1996;23:232-240.
23. Chesnutt MP. Pulmonary disorders. In: Papadakis MM, editor. Current Medical Diagnosis and Treatment. New York: McGraw-Hill; 2016:242-320.
24. Mandell LW. Pneumonia. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:188-201.
25. Reed WW, Byrd GS, Gates RH Jr, et al. Sputum gram’s stain in community-acquired pneumococcal pneumonia. A meta-analysis. West J Med. 1996;165:197-204.
26. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
27. Chalasani NP, Valdecanas MA, Gopal AK, et al. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest. 1995;108:932-936.
28. Bordon J, Peyrani P, Brock GN, et al. The presence of pneumococcal bacteremia does not influence clinical outcomes in patients with community-acquired pneumonia: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort study. Chest. 2008;133:618-624.
29. Helbig JH, Uldum SA, Bernander S, et al. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires’ disease. J Clin Microbiol. 2003;41:838-840.
30. Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol. 2003;41:2810-2813.
31. Bellew S, Grijalva CG, Williams DJ, et al. Pneumococcal and Legionella urinary antigen tests in community-acquired pneumonia: Prospective evaluation of indications for testing. Clin Infect Dis. 2019;68:2026-2033.
32. Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50:202-209.
33. Gilbert DN. Procalcitonin as a biomarker in respiratory tract infection. Clin Infect Dis. 2011;52 Suppl 4:S346-350.
34. Kamat IS Ramachandran V, Eswaran H, et al. Procalcitonin to distinguish viral from bacterial pneumonia: A systematic review and meta-analysis. Clin Infect Dis. 2019 Jun 25. [Epub ahead of print]
35. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;(9):CD007498.
36. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
37. Boussekey N, Leroy O, Alfandari S, et al. Procalcitonin kinetics in the prognosis of severe community-acquired pneumonia. Intensive Care Med. 2006;32:469-472.
38. Flanders SA, Stein J, Shochat G, et al. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am J Med. 2004;116:529-535.
Despite advances in medical science, pneumonia remains a major cause of morbidity and mortality. In 2017, 49,157 patients in the United States died from the disease.1 Pneumonia can be classified as community-acquired, hospital-acquired, or ventilator-associated. Another category, healthcare-associated pneumonia, was included in an earlier Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) guideline but was removed from the 2016 guideline because there was no clear evidence that patients diagnosed with healthcare-associated pneumonia were at higher risk for harboring multidrug-resistant pathogens.2 This review is the first of 2 articles focusing on the management of community-acquired pneumonia (CAP). Here, we review CAP epidemiology, microbiology, predisposing factors, and diagnosis; current treatment and prevention of CAP are reviewed in a separate article.
Definition and Epidemiology
CAP is defined as an acute infection of the lungs that develops in patients who have not been hospitalized recently and have not had regular exposure to the health care system.3 A previously ambulatory patient who is diagnosed with pneumonia within 48 hours after admission also meets the criteria for CAP. Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually.4 About 25% of CAP patients require hospitalization, and about 5% to 10% of these patients are admitted to the intensive care unit (ICU).5 In-hospital mortality is considerable (~10% in population-based studies),6 and 30-day mortality was found to be as high as 23% in a review by File and Marrie.7 CAP also confers a high risk of long-term morbidity and mortality compared with the general population who have never had CAP, irrespective of age.8
Causative Organisms
Numerous microorganisms can cause CAP. Common causes and less common causes are delineated in Table 1. Until recently, many studies had demonstrated that pneumococcus was the most common cause of CAP. However, in the CDC Etiology of Pneumonia in the Community (EPIC) study team’s 2015 prospective, multicenter, population-based study, no pathogen was detected in the majority of patients diagnosed with CAP requiring hospitalization. The most common pathogens they detected were rhinovirus (9%), followed by influenza virus (6%) and pneumococcus (5%).9 Factors considered to be contributing to the decrease in the percentage of pneumococcus in patients diagnosed with CAP are the widespread use of pneumococcal vaccine and reduced rates of smoking.10,11
Predisposing Factors
Most people diagnosed with CAP have 1 or more predisposing factors (Table 2).12,13 Patients who develop CAP typically have a combination of these predisposing factors rather than a single factor. Aging, in combination with other risk factors, increases the susceptibility of a person to pneumonia.
Clinical Signs and Symptoms
Symptoms of CAP include fever, chills, rigors, fatigue, anorexia, diaphoresis, dyspnea, cough (with or without sputum production), and pleuritic chest pain. There is no individual symptom or cluster of symptoms that can absolutely differentiate pneumonia from other acute respiratory diseases, including upper and lower respiratory infections. However, patients presenting with the constellation of symptoms of fever ≥ 100°F (37.8°C), productive cough, and tachycardia is more suggestive of pneumonia.14 Abnormal vital signs include fever, hypothermia, tachypnea, tachycardia, and oxygen desaturation. Auscultation of the chest reveals crackles or other adventitious breath sounds. Elderly patients with pneumonia report a significantly lower number of both respiratory and nonrespiratory symptoms compared with younger patients. Clinicians should be aware of this phenomenon to avoid delayed diagnosis and treatment.15
Imaging Evaluation
The presence of a pulmonary consolidation or an infiltrate on chest radiograph is required to diagnose CAP, and a chest radiograph should be obtained when CAP is suspected.16 However, there is no pattern of radiographic abnormalities reliable enough to differentiate infectious pneumonia from noninfectious causes.17
There are case reports and case series demonstrating false-negative plain chest radiographs in dehydrated patients18 or in patients in a neutropenic state. However, animal studies have shown that dogs challenged with pneumococcus showed abnormal pulmonary shadow, suggestive of pneumonia, regardless of hydration status.19 There is also no reliable scientific evidence to support the notion that severe neutropenia can cause false-negative radiographs because of the inability to develop an acute inflammatory reaction in the lungs.20
A chest computed tomography (CT) scan is more sensitive than a plain chest radiograph in detecting pneumonia. Therefore, a chest CT should be performed in a patient with negative plain chest radiograph when pneumonia is still highly suspected.21 A chest CT scan is also more sensitive in detecting cavitation, adenopathy, interstitial disease, and empyema. It also has the advantage of better defining anatomical changes than plain films.22
Because improvement of pulmonary opacities in patients with CAP lags behind clinical improvement, repeating chest imaging studies is not recommended in patients who demonstrate clinical improvement. Clearing of pulmonary infiltrate or consolidation sometimes can take 6 weeks or longer.23
Laboratory Evaluation
Generally, the etiologic agent of CAP cannot be determined solely on the basis of clinical signs and symptoms or imaging studies. Although routine microbiological testing for patients suspicious for CAP is not necessary for empirical treatment, determining the etiologic agent of the pneumonia allows the clinician to narrow the antibiotics from a broad-spectrum empirical regimen to specific pathogen-directed therapy. Determination of certain etiologic agents causing the pneumonia can have important public health implications (eg, Mycobacterium tuberculosis and influenza virus).24
Sputum Gram Stain and Culture
Sputum Gram stain is an inexpensive test that may identify pathogens that cause CAP (eg, Streptococcus pneumoniae and Haemophilus influenzae). A quality specimen is required. A sputum sample must contain more than 25 neutrophils and less than 10 squamous epithelial cells/low power field on Gram stain to be considered suitable for culture. The sensitivity and specificity of sputum Gram stain and culture are highly variable in different clinical settings (eg, outpatient setting, nursing home, ICU). Reed et al’s meta-analysis of patients diagnosed with CAP in the United States showed the sensitivity and specificity of sputum Gram stain (compared with sputum culture) ranged from 15% to 100% and 11% to 100%, respectively.24 In cases of proven bacteremic pneumococcal pneumonia, positive cultures from sputum samples were positive less than 50% of the time.25
For patients who cannot provide sputum samples or are intubated, deep-suction aspirate or bronchoalveolar lavage through a bronchoscopic procedure may be necessary to obtain pulmonary secretion for Gram stain and culture. Besides bacterial culture, sputum samples can also be sent for fungal and mycobacterial cultures and acid-fast stain, if deemed clinically necessary.
The 2019 ATS/IDSA guidelines for diagnosis and treatment of adults with CAP recommend sputum culture in patients with severe disease and in all inpatients empirically treated for MRSA or Pseudomonas aeruginosa.26
Blood Culture
Because the positivity rate of blood culture in patients who are suspected to have pneumonia but not exposed to antimicrobial agents is low (5%–14%), blood cultures are not recommended for all patients with CAP. Another reason for not recommending blood culture is positive culture rarely leads to changes in antibiotic regimen in patients without underlying diseases.27 However, the 2019 ATS/IDSA guidelines recommend blood culture in patients with severe disease and in all inpatients treated empirically for MRSA or P. aeruginosa.26
A multinational study published in 2008 examined 125 patients with pneumococcal bacteremic CAP versus 1847 patients with non-bacteremic CAP.28 Analysis of the data demonstrated no association between pneumococcal bacteremic CAP and time to clinical stability, length of hospital stay, all-cause mortality, or CAP-related mortality. The authors concluded that pneumococcal bacteremia does not increase the risk of poor outcomes in patients with CAP compared to non-bacteremic patients, and the presence of pneumococcal bacteremia should not deter de-escalation of therapy in clinically stable patients.
Urinary Antigen Tests
Urinary antigen tests may assist clinicians in narrowing antibiotic therapy when test results are positive. There are 2 US Food and Drug Administration–approved tests available to clinicians for detecting pneumococcal and Legionella antigen in urine. The test for Legionella pneumophila detects disease due to serogroup 1 only, which accounts for 80% of community-acquired Legionnaires’ disease. The sensitivity and specificity of the Legionella urine antigen test are 90% and 99%, respectively. The pneumococcal urine antigen test is less sensitive and specific than the Legionella urine antigen test (sensitivity 80% and specificity > 90%).29,30
Advantages of the urinary antigen tests are that they are easily performed, results are available in less than an hour if done in-house, and results are not affected by prior exposure to antibiotics. However, the tests do not meet Clinical Laboratory Improvements Amendments criteria for waiver and must be performed by a technician in the laboratory. A multicenter, prospective surveillance study of hospitalized patients with CAP showed that the 2007 IDSA/ATS guidelines’ recommended indications for S. pneumoniae and L. pneumophila urinary antigen tests do not have sufficient sensitivity and specificity to identify patients with positive tests.31
Polymerase Chain Reaction
There are several FDA-approved polymerase chain reaction (PCR) tests commercially available to assist clinicians in diagnosing pneumonia. PCR testing of nasopharyngeal swabs for diagnosis of influenza has become standard in many US medical facilities. The great advantages of using PCR to diagnose influenza are its high sensitivity and specificity and rapid turnaround time. PCR can also be used to detect Legionella species, S. pneumonia, Mycoplasma pneumoniae, Chlamydophila pneumonia, and mycobacterial species.24
One limitation of using PCR tests on respiratory specimens is that specimens can be contaminated with oral or upper airway flora, so the results must be interpreted with caution, bearing in mind that some of the pathogens isolated may be colonizers of the oral or upper airway flora.32
Biologic Markers
Two biologic markers—procalcitonin and C-reactive protein (CRP)—can be used in conjunction with history, physical examination, laboratory tests, and imaging studies to assist in the diagnosis and treatment of CAP.24 Procalcitonin is a peptide precursor of the hormone calcitonin that is released by parenchymal cells into the bloodstream, resulting in increased serum level in patients with bacterial infections. In contrast, there is no remarkable procalcitonin level increase with viral or noninfectious inflammation. The reference value of procalcitonin in the blood of an adult individual without infection or inflammation is < 0.15 ng/mL. In the blood, procalcitonin has a half-life of 25 to 30 hours. The quantitative immunoluminometric method (LUMI test, Brahms PCT, Berlin, Germany) is the preferred test to use because of its high sensitivity.33 A meta-analysis of 12 studies involving more than 2400 patients with CAP demonstrated that serum procalcitonin does not have sufficient sensitivity or specificity to distinguish between bacterial and nonbacterial pneumonia. The authors concluded that procalcitonin level cannot be used to decide whether an antibiotic should be administered.34
A 2012 Cochrane meta-analysis that involved 4221 patients with acute respiratory infections (with half of the patients diagnosed with CAP) from 14 prospective trials found the use of procalcitonin test for antibiotic use significantly decreased median antibiotic exposure from 8 to 4 days without an increase in treatment failure, mortality rates in any clinical setting (eg, outpatient clinic, emergency room), or length of hospitalization.35 An update of the 2012 Cochrane review that examined the safety and efficacy of using procalcitonin for starting or stopping antibiotics again demonstrated procalcitonin use was associated with a reduction of antibiotic use (2.4 days).36 A prospective study conducted in France on 100 ICU patients showed that increased procalcitonin from day 1 to day 3 has a poor prognosis factor for severe CAP, whereas decreasing procalcitonin levels is associated with a favorable outcome.37
Because of conflicting data, the 2019 ATS/IDSA guidelines do not recommend using procalcitonin to determine need for initial antibacterial therapy.26
CRP is an acute phase protein produced by the liver. CRP level in the blood increases in response to acute infection or inflammation. Use of CRP in assisting diagnosis and guiding treatment of CAP is more limited in part due to its poor specificity. A prospective study conducted on 168 consecutive patients who presented with cough showed that a CRP level > 40 mg/L had a sensitivity and specificity of 70% and 90%, respectively.38
Summary
CAP remains a leading cause of hospitalization and death in the 21st century. Traditionally, pneumococcus has been considered the major pathogen causing CAP; however, the 2015 EPIC study found that S. pneumoniae was detected in only 5% of patients diagnosed with CAP. Despite the new findings, it is still recommended that empiric treatment for CAP target common typical bacteria (pneumococcus, H. influenzae, Moraxella catarrhalis) and atypical bacteria (M. pneumonia, C. pneumoniae, L. pneumophila).
Because diagnosing pneumonia through history and clinical examination is less than 50% sensitive, a chest imaging study (a plain chest radiograph or a chest CT scan) is usually required to make the diagnosis. Laboratory tests, such as sputum Gram stain/culture, blood culture, urinary antigen tests, PCR test, procalcitonin, and CRP are important adjunctive diagnostic modalities to assist in the diagnosis and management of CAP. However, because no single test is sensitive and specific enough to be a stand-alone test, they should be used in conjunction with history, physical examination, and imaging studies.
Despite advances in medical science, pneumonia remains a major cause of morbidity and mortality. In 2017, 49,157 patients in the United States died from the disease.1 Pneumonia can be classified as community-acquired, hospital-acquired, or ventilator-associated. Another category, healthcare-associated pneumonia, was included in an earlier Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) guideline but was removed from the 2016 guideline because there was no clear evidence that patients diagnosed with healthcare-associated pneumonia were at higher risk for harboring multidrug-resistant pathogens.2 This review is the first of 2 articles focusing on the management of community-acquired pneumonia (CAP). Here, we review CAP epidemiology, microbiology, predisposing factors, and diagnosis; current treatment and prevention of CAP are reviewed in a separate article.
Definition and Epidemiology
CAP is defined as an acute infection of the lungs that develops in patients who have not been hospitalized recently and have not had regular exposure to the health care system.3 A previously ambulatory patient who is diagnosed with pneumonia within 48 hours after admission also meets the criteria for CAP. Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually.4 About 25% of CAP patients require hospitalization, and about 5% to 10% of these patients are admitted to the intensive care unit (ICU).5 In-hospital mortality is considerable (~10% in population-based studies),6 and 30-day mortality was found to be as high as 23% in a review by File and Marrie.7 CAP also confers a high risk of long-term morbidity and mortality compared with the general population who have never had CAP, irrespective of age.8
Causative Organisms
Numerous microorganisms can cause CAP. Common causes and less common causes are delineated in Table 1. Until recently, many studies had demonstrated that pneumococcus was the most common cause of CAP. However, in the CDC Etiology of Pneumonia in the Community (EPIC) study team’s 2015 prospective, multicenter, population-based study, no pathogen was detected in the majority of patients diagnosed with CAP requiring hospitalization. The most common pathogens they detected were rhinovirus (9%), followed by influenza virus (6%) and pneumococcus (5%).9 Factors considered to be contributing to the decrease in the percentage of pneumococcus in patients diagnosed with CAP are the widespread use of pneumococcal vaccine and reduced rates of smoking.10,11
Predisposing Factors
Most people diagnosed with CAP have 1 or more predisposing factors (Table 2).12,13 Patients who develop CAP typically have a combination of these predisposing factors rather than a single factor. Aging, in combination with other risk factors, increases the susceptibility of a person to pneumonia.
Clinical Signs and Symptoms
Symptoms of CAP include fever, chills, rigors, fatigue, anorexia, diaphoresis, dyspnea, cough (with or without sputum production), and pleuritic chest pain. There is no individual symptom or cluster of symptoms that can absolutely differentiate pneumonia from other acute respiratory diseases, including upper and lower respiratory infections. However, patients presenting with the constellation of symptoms of fever ≥ 100°F (37.8°C), productive cough, and tachycardia is more suggestive of pneumonia.14 Abnormal vital signs include fever, hypothermia, tachypnea, tachycardia, and oxygen desaturation. Auscultation of the chest reveals crackles or other adventitious breath sounds. Elderly patients with pneumonia report a significantly lower number of both respiratory and nonrespiratory symptoms compared with younger patients. Clinicians should be aware of this phenomenon to avoid delayed diagnosis and treatment.15
Imaging Evaluation
The presence of a pulmonary consolidation or an infiltrate on chest radiograph is required to diagnose CAP, and a chest radiograph should be obtained when CAP is suspected.16 However, there is no pattern of radiographic abnormalities reliable enough to differentiate infectious pneumonia from noninfectious causes.17
There are case reports and case series demonstrating false-negative plain chest radiographs in dehydrated patients18 or in patients in a neutropenic state. However, animal studies have shown that dogs challenged with pneumococcus showed abnormal pulmonary shadow, suggestive of pneumonia, regardless of hydration status.19 There is also no reliable scientific evidence to support the notion that severe neutropenia can cause false-negative radiographs because of the inability to develop an acute inflammatory reaction in the lungs.20
A chest computed tomography (CT) scan is more sensitive than a plain chest radiograph in detecting pneumonia. Therefore, a chest CT should be performed in a patient with negative plain chest radiograph when pneumonia is still highly suspected.21 A chest CT scan is also more sensitive in detecting cavitation, adenopathy, interstitial disease, and empyema. It also has the advantage of better defining anatomical changes than plain films.22
Because improvement of pulmonary opacities in patients with CAP lags behind clinical improvement, repeating chest imaging studies is not recommended in patients who demonstrate clinical improvement. Clearing of pulmonary infiltrate or consolidation sometimes can take 6 weeks or longer.23
Laboratory Evaluation
Generally, the etiologic agent of CAP cannot be determined solely on the basis of clinical signs and symptoms or imaging studies. Although routine microbiological testing for patients suspicious for CAP is not necessary for empirical treatment, determining the etiologic agent of the pneumonia allows the clinician to narrow the antibiotics from a broad-spectrum empirical regimen to specific pathogen-directed therapy. Determination of certain etiologic agents causing the pneumonia can have important public health implications (eg, Mycobacterium tuberculosis and influenza virus).24
Sputum Gram Stain and Culture
Sputum Gram stain is an inexpensive test that may identify pathogens that cause CAP (eg, Streptococcus pneumoniae and Haemophilus influenzae). A quality specimen is required. A sputum sample must contain more than 25 neutrophils and less than 10 squamous epithelial cells/low power field on Gram stain to be considered suitable for culture. The sensitivity and specificity of sputum Gram stain and culture are highly variable in different clinical settings (eg, outpatient setting, nursing home, ICU). Reed et al’s meta-analysis of patients diagnosed with CAP in the United States showed the sensitivity and specificity of sputum Gram stain (compared with sputum culture) ranged from 15% to 100% and 11% to 100%, respectively.24 In cases of proven bacteremic pneumococcal pneumonia, positive cultures from sputum samples were positive less than 50% of the time.25
For patients who cannot provide sputum samples or are intubated, deep-suction aspirate or bronchoalveolar lavage through a bronchoscopic procedure may be necessary to obtain pulmonary secretion for Gram stain and culture. Besides bacterial culture, sputum samples can also be sent for fungal and mycobacterial cultures and acid-fast stain, if deemed clinically necessary.
The 2019 ATS/IDSA guidelines for diagnosis and treatment of adults with CAP recommend sputum culture in patients with severe disease and in all inpatients empirically treated for MRSA or Pseudomonas aeruginosa.26
Blood Culture
Because the positivity rate of blood culture in patients who are suspected to have pneumonia but not exposed to antimicrobial agents is low (5%–14%), blood cultures are not recommended for all patients with CAP. Another reason for not recommending blood culture is positive culture rarely leads to changes in antibiotic regimen in patients without underlying diseases.27 However, the 2019 ATS/IDSA guidelines recommend blood culture in patients with severe disease and in all inpatients treated empirically for MRSA or P. aeruginosa.26
A multinational study published in 2008 examined 125 patients with pneumococcal bacteremic CAP versus 1847 patients with non-bacteremic CAP.28 Analysis of the data demonstrated no association between pneumococcal bacteremic CAP and time to clinical stability, length of hospital stay, all-cause mortality, or CAP-related mortality. The authors concluded that pneumococcal bacteremia does not increase the risk of poor outcomes in patients with CAP compared to non-bacteremic patients, and the presence of pneumococcal bacteremia should not deter de-escalation of therapy in clinically stable patients.
Urinary Antigen Tests
Urinary antigen tests may assist clinicians in narrowing antibiotic therapy when test results are positive. There are 2 US Food and Drug Administration–approved tests available to clinicians for detecting pneumococcal and Legionella antigen in urine. The test for Legionella pneumophila detects disease due to serogroup 1 only, which accounts for 80% of community-acquired Legionnaires’ disease. The sensitivity and specificity of the Legionella urine antigen test are 90% and 99%, respectively. The pneumococcal urine antigen test is less sensitive and specific than the Legionella urine antigen test (sensitivity 80% and specificity > 90%).29,30
Advantages of the urinary antigen tests are that they are easily performed, results are available in less than an hour if done in-house, and results are not affected by prior exposure to antibiotics. However, the tests do not meet Clinical Laboratory Improvements Amendments criteria for waiver and must be performed by a technician in the laboratory. A multicenter, prospective surveillance study of hospitalized patients with CAP showed that the 2007 IDSA/ATS guidelines’ recommended indications for S. pneumoniae and L. pneumophila urinary antigen tests do not have sufficient sensitivity and specificity to identify patients with positive tests.31
Polymerase Chain Reaction
There are several FDA-approved polymerase chain reaction (PCR) tests commercially available to assist clinicians in diagnosing pneumonia. PCR testing of nasopharyngeal swabs for diagnosis of influenza has become standard in many US medical facilities. The great advantages of using PCR to diagnose influenza are its high sensitivity and specificity and rapid turnaround time. PCR can also be used to detect Legionella species, S. pneumonia, Mycoplasma pneumoniae, Chlamydophila pneumonia, and mycobacterial species.24
One limitation of using PCR tests on respiratory specimens is that specimens can be contaminated with oral or upper airway flora, so the results must be interpreted with caution, bearing in mind that some of the pathogens isolated may be colonizers of the oral or upper airway flora.32
Biologic Markers
Two biologic markers—procalcitonin and C-reactive protein (CRP)—can be used in conjunction with history, physical examination, laboratory tests, and imaging studies to assist in the diagnosis and treatment of CAP.24 Procalcitonin is a peptide precursor of the hormone calcitonin that is released by parenchymal cells into the bloodstream, resulting in increased serum level in patients with bacterial infections. In contrast, there is no remarkable procalcitonin level increase with viral or noninfectious inflammation. The reference value of procalcitonin in the blood of an adult individual without infection or inflammation is < 0.15 ng/mL. In the blood, procalcitonin has a half-life of 25 to 30 hours. The quantitative immunoluminometric method (LUMI test, Brahms PCT, Berlin, Germany) is the preferred test to use because of its high sensitivity.33 A meta-analysis of 12 studies involving more than 2400 patients with CAP demonstrated that serum procalcitonin does not have sufficient sensitivity or specificity to distinguish between bacterial and nonbacterial pneumonia. The authors concluded that procalcitonin level cannot be used to decide whether an antibiotic should be administered.34
A 2012 Cochrane meta-analysis that involved 4221 patients with acute respiratory infections (with half of the patients diagnosed with CAP) from 14 prospective trials found the use of procalcitonin test for antibiotic use significantly decreased median antibiotic exposure from 8 to 4 days without an increase in treatment failure, mortality rates in any clinical setting (eg, outpatient clinic, emergency room), or length of hospitalization.35 An update of the 2012 Cochrane review that examined the safety and efficacy of using procalcitonin for starting or stopping antibiotics again demonstrated procalcitonin use was associated with a reduction of antibiotic use (2.4 days).36 A prospective study conducted in France on 100 ICU patients showed that increased procalcitonin from day 1 to day 3 has a poor prognosis factor for severe CAP, whereas decreasing procalcitonin levels is associated with a favorable outcome.37
Because of conflicting data, the 2019 ATS/IDSA guidelines do not recommend using procalcitonin to determine need for initial antibacterial therapy.26
CRP is an acute phase protein produced by the liver. CRP level in the blood increases in response to acute infection or inflammation. Use of CRP in assisting diagnosis and guiding treatment of CAP is more limited in part due to its poor specificity. A prospective study conducted on 168 consecutive patients who presented with cough showed that a CRP level > 40 mg/L had a sensitivity and specificity of 70% and 90%, respectively.38
Summary
CAP remains a leading cause of hospitalization and death in the 21st century. Traditionally, pneumococcus has been considered the major pathogen causing CAP; however, the 2015 EPIC study found that S. pneumoniae was detected in only 5% of patients diagnosed with CAP. Despite the new findings, it is still recommended that empiric treatment for CAP target common typical bacteria (pneumococcus, H. influenzae, Moraxella catarrhalis) and atypical bacteria (M. pneumonia, C. pneumoniae, L. pneumophila).
Because diagnosing pneumonia through history and clinical examination is less than 50% sensitive, a chest imaging study (a plain chest radiograph or a chest CT scan) is usually required to make the diagnosis. Laboratory tests, such as sputum Gram stain/culture, blood culture, urinary antigen tests, PCR test, procalcitonin, and CRP are important adjunctive diagnostic modalities to assist in the diagnosis and management of CAP. However, because no single test is sensitive and specific enough to be a stand-alone test, they should be used in conjunction with history, physical examination, and imaging studies.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. FastStats - Pneumonia. www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed 16 September 2019.
2. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
3. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med. 2014;371:1619-1628.
4. Mandell LA. Epidemiology and etiology of community-acquired pneumonia. Infect Dis Clin North Am. 2004;18:761-776.
5. Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ. 2006;332:1077-1079.
6. Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med. 2007;167:1938-1943.
7. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122:130-141.
8. Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-year mortality after community-acquired pneumonia. a prospective cohort. Am J Respir Crit Care Med. 2015;192:597-604.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415-427.
10. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155-163.
11. Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med. 2000;342:681-689.
12. Almirall J, Serra-Prat M, Bolíbar I, Balasso V. Risk factors for community-acquired pneumonia in adults: a systemic review of observational studies. Respiration. 2017;94:299-311.
13. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Saunders; 2015:2310-2327.
14. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough--a statistical approach. J Chronic Dis. 1984;37:215-225.
15. Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med. 1997;157:1453-1459.
16. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-72.
17. Jartti A, Rauvala E, Kauma H, et al. Chest imaging findings in hospitalized patients with H1N1 influenza. Acta Radiol. 2011;52:297-304.
18. Basi SK, Marrie TJ, Huang JQ, Majumdar SR. Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117:305-311.
19. Caldwell A, Glauser FL, Smith WR, et al. The effects of dehydration on the radiologic and pathologic appearance of experimental canine segmental pneumonia. Am Rev Respir Dis. 1975;112:651-656.
20. Bartlett JG. Pneumonia. In: Barlett JG, editor. Management of Respiratory Tract Infections. Philadelphia: Lippincott, Williams & Wilkins; 2001:1-122.
21. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192:974-982.
22. Wheeler JH, Fishman EK. Computed tomography in the management of chest infections: current status. Clin Infect Dis. 1996;23:232-240.
23. Chesnutt MP. Pulmonary disorders. In: Papadakis MM, editor. Current Medical Diagnosis and Treatment. New York: McGraw-Hill; 2016:242-320.
24. Mandell LW. Pneumonia. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:188-201.
25. Reed WW, Byrd GS, Gates RH Jr, et al. Sputum gram’s stain in community-acquired pneumococcal pneumonia. A meta-analysis. West J Med. 1996;165:197-204.
26. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
27. Chalasani NP, Valdecanas MA, Gopal AK, et al. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest. 1995;108:932-936.
28. Bordon J, Peyrani P, Brock GN, et al. The presence of pneumococcal bacteremia does not influence clinical outcomes in patients with community-acquired pneumonia: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort study. Chest. 2008;133:618-624.
29. Helbig JH, Uldum SA, Bernander S, et al. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires’ disease. J Clin Microbiol. 2003;41:838-840.
30. Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol. 2003;41:2810-2813.
31. Bellew S, Grijalva CG, Williams DJ, et al. Pneumococcal and Legionella urinary antigen tests in community-acquired pneumonia: Prospective evaluation of indications for testing. Clin Infect Dis. 2019;68:2026-2033.
32. Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50:202-209.
33. Gilbert DN. Procalcitonin as a biomarker in respiratory tract infection. Clin Infect Dis. 2011;52 Suppl 4:S346-350.
34. Kamat IS Ramachandran V, Eswaran H, et al. Procalcitonin to distinguish viral from bacterial pneumonia: A systematic review and meta-analysis. Clin Infect Dis. 2019 Jun 25. [Epub ahead of print]
35. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;(9):CD007498.
36. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
37. Boussekey N, Leroy O, Alfandari S, et al. Procalcitonin kinetics in the prognosis of severe community-acquired pneumonia. Intensive Care Med. 2006;32:469-472.
38. Flanders SA, Stein J, Shochat G, et al. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am J Med. 2004;116:529-535.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. FastStats - Pneumonia. www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed 16 September 2019.
2. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
3. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med. 2014;371:1619-1628.
4. Mandell LA. Epidemiology and etiology of community-acquired pneumonia. Infect Dis Clin North Am. 2004;18:761-776.
5. Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ. 2006;332:1077-1079.
6. Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med. 2007;167:1938-1943.
7. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122:130-141.
8. Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-year mortality after community-acquired pneumonia. a prospective cohort. Am J Respir Crit Care Med. 2015;192:597-604.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415-427.
10. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155-163.
11. Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med. 2000;342:681-689.
12. Almirall J, Serra-Prat M, Bolíbar I, Balasso V. Risk factors for community-acquired pneumonia in adults: a systemic review of observational studies. Respiration. 2017;94:299-311.
13. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Saunders; 2015:2310-2327.
14. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough--a statistical approach. J Chronic Dis. 1984;37:215-225.
15. Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med. 1997;157:1453-1459.
16. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-72.
17. Jartti A, Rauvala E, Kauma H, et al. Chest imaging findings in hospitalized patients with H1N1 influenza. Acta Radiol. 2011;52:297-304.
18. Basi SK, Marrie TJ, Huang JQ, Majumdar SR. Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117:305-311.
19. Caldwell A, Glauser FL, Smith WR, et al. The effects of dehydration on the radiologic and pathologic appearance of experimental canine segmental pneumonia. Am Rev Respir Dis. 1975;112:651-656.
20. Bartlett JG. Pneumonia. In: Barlett JG, editor. Management of Respiratory Tract Infections. Philadelphia: Lippincott, Williams & Wilkins; 2001:1-122.
21. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192:974-982.
22. Wheeler JH, Fishman EK. Computed tomography in the management of chest infections: current status. Clin Infect Dis. 1996;23:232-240.
23. Chesnutt MP. Pulmonary disorders. In: Papadakis MM, editor. Current Medical Diagnosis and Treatment. New York: McGraw-Hill; 2016:242-320.
24. Mandell LW. Pneumonia. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:188-201.
25. Reed WW, Byrd GS, Gates RH Jr, et al. Sputum gram’s stain in community-acquired pneumococcal pneumonia. A meta-analysis. West J Med. 1996;165:197-204.
26. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
27. Chalasani NP, Valdecanas MA, Gopal AK, et al. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest. 1995;108:932-936.
28. Bordon J, Peyrani P, Brock GN, et al. The presence of pneumococcal bacteremia does not influence clinical outcomes in patients with community-acquired pneumonia: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort study. Chest. 2008;133:618-624.
29. Helbig JH, Uldum SA, Bernander S, et al. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires’ disease. J Clin Microbiol. 2003;41:838-840.
30. Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol. 2003;41:2810-2813.
31. Bellew S, Grijalva CG, Williams DJ, et al. Pneumococcal and Legionella urinary antigen tests in community-acquired pneumonia: Prospective evaluation of indications for testing. Clin Infect Dis. 2019;68:2026-2033.
32. Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50:202-209.
33. Gilbert DN. Procalcitonin as a biomarker in respiratory tract infection. Clin Infect Dis. 2011;52 Suppl 4:S346-350.
34. Kamat IS Ramachandran V, Eswaran H, et al. Procalcitonin to distinguish viral from bacterial pneumonia: A systematic review and meta-analysis. Clin Infect Dis. 2019 Jun 25. [Epub ahead of print]
35. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;(9):CD007498.
36. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
37. Boussekey N, Leroy O, Alfandari S, et al. Procalcitonin kinetics in the prognosis of severe community-acquired pneumonia. Intensive Care Med. 2006;32:469-472.
38. Flanders SA, Stein J, Shochat G, et al. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am J Med. 2004;116:529-535.
Vaping-related lung disease cases rise, case reporting standardized
The number of possible cases of vaping-related pulmonary illness has risen to 215, reported from 25 states, as of Aug. 27, 2019, according to the Centers for Disease Control and Prevention, Atlanta. Additional reports of pulmonary illness are under investigation.
The CDC has released a standardized case definition that states are using to complete their own investigations and verifications of cases. It appears that all cases are linked to e-cigarette product use, but the cause of the respiratory illnesses is still unconfirmed.
In many cases, patients reported a gradual start of symptoms, including breathing difficulty, shortness of breath, and/or chest pain before hospitalization. Some cases reported mild to moderate gastrointestinal illness including vomiting and diarrhea, or other symptoms such as fevers or fatigue. In many cases, patients have also acknowledged recent use of tetrahydrocannabinol (THC)-containing e-cigarette products while speaking to health care personnel or in follow-up interviews by health department staff, according to a statement from the CDC and the Food and Drug Administration.
The agencies are working with state health departments to standardize information collection at the state level to help build a more comprehensive picture of these incidents, including the brand and types of e-cigarette products, whether any of them would fall within the FDA’s regulatory authority, where they were obtained, and whether there is a link to specific devices, ingredients, or contaminants in the devices or substances associated with e-cigarette product use.
CDC staff have been deployed to Illinois and Wisconsin to assist their state health departments. The agencies have released a Clinician Outreach and Communication Activity (COCA) Clinical Action Alert describing this investigation and asking providers to report possible cases to their state health departments. In addition to a standardized case definition, the agencies have issued a medical chart abstraction form and case interview questionnaire, are reviewing and providing feedback on data collection and health messaging tools for states, and are facilitating information sharing between states with possible cases.
More information on the cases and reporting are available from the CDC.
The number of possible cases of vaping-related pulmonary illness has risen to 215, reported from 25 states, as of Aug. 27, 2019, according to the Centers for Disease Control and Prevention, Atlanta. Additional reports of pulmonary illness are under investigation.
The CDC has released a standardized case definition that states are using to complete their own investigations and verifications of cases. It appears that all cases are linked to e-cigarette product use, but the cause of the respiratory illnesses is still unconfirmed.
In many cases, patients reported a gradual start of symptoms, including breathing difficulty, shortness of breath, and/or chest pain before hospitalization. Some cases reported mild to moderate gastrointestinal illness including vomiting and diarrhea, or other symptoms such as fevers or fatigue. In many cases, patients have also acknowledged recent use of tetrahydrocannabinol (THC)-containing e-cigarette products while speaking to health care personnel or in follow-up interviews by health department staff, according to a statement from the CDC and the Food and Drug Administration.
The agencies are working with state health departments to standardize information collection at the state level to help build a more comprehensive picture of these incidents, including the brand and types of e-cigarette products, whether any of them would fall within the FDA’s regulatory authority, where they were obtained, and whether there is a link to specific devices, ingredients, or contaminants in the devices or substances associated with e-cigarette product use.
CDC staff have been deployed to Illinois and Wisconsin to assist their state health departments. The agencies have released a Clinician Outreach and Communication Activity (COCA) Clinical Action Alert describing this investigation and asking providers to report possible cases to their state health departments. In addition to a standardized case definition, the agencies have issued a medical chart abstraction form and case interview questionnaire, are reviewing and providing feedback on data collection and health messaging tools for states, and are facilitating information sharing between states with possible cases.
More information on the cases and reporting are available from the CDC.
The number of possible cases of vaping-related pulmonary illness has risen to 215, reported from 25 states, as of Aug. 27, 2019, according to the Centers for Disease Control and Prevention, Atlanta. Additional reports of pulmonary illness are under investigation.
The CDC has released a standardized case definition that states are using to complete their own investigations and verifications of cases. It appears that all cases are linked to e-cigarette product use, but the cause of the respiratory illnesses is still unconfirmed.
In many cases, patients reported a gradual start of symptoms, including breathing difficulty, shortness of breath, and/or chest pain before hospitalization. Some cases reported mild to moderate gastrointestinal illness including vomiting and diarrhea, or other symptoms such as fevers or fatigue. In many cases, patients have also acknowledged recent use of tetrahydrocannabinol (THC)-containing e-cigarette products while speaking to health care personnel or in follow-up interviews by health department staff, according to a statement from the CDC and the Food and Drug Administration.
The agencies are working with state health departments to standardize information collection at the state level to help build a more comprehensive picture of these incidents, including the brand and types of e-cigarette products, whether any of them would fall within the FDA’s regulatory authority, where they were obtained, and whether there is a link to specific devices, ingredients, or contaminants in the devices or substances associated with e-cigarette product use.
CDC staff have been deployed to Illinois and Wisconsin to assist their state health departments. The agencies have released a Clinician Outreach and Communication Activity (COCA) Clinical Action Alert describing this investigation and asking providers to report possible cases to their state health departments. In addition to a standardized case definition, the agencies have issued a medical chart abstraction form and case interview questionnaire, are reviewing and providing feedback on data collection and health messaging tools for states, and are facilitating information sharing between states with possible cases.
More information on the cases and reporting are available from the CDC.
Mysterious vaping lung injuries may have flown under regulatory radar
It was the arrival of the second man in his early 20s gasping for air that alarmed Dixie Harris, MD. Young patients rarely get so sick, so fast, with a severe lung illness, and this was her second case in a matter of days.
Then she saw three more patients at her Utah telehealth clinic with similar symptoms. They did not have infections, but all had been vaping. When Dr. Harris heard several teenagers in Wisconsin had been hospitalized in similar cases, she quickly alerted her state health department.
As patients in hospitals across the country combat a mysterious illness linked to e-cigarettes, federal and state investigators are frantically trying to trace the outbreaks to specific vaping products that, until recently, were virtually unregulated.
As of Aug. 22, 2019, 193 potential vaping-related illnesses in 22 states had been reported to the Centers for Disease Control and Prevention. Wisconsin, which first put out an alert in July, has at least 16 confirmed and 15 suspected cases. Illinois has reported 34 patients, 1 of whom has died. Indiana is investigating 24 cases.
Lung doctors said they had seen warning signs for years that vaping could be hazardous as they treated patients. Medically it seemed problematic since it often involved inhaling chemicals not normally inhaled into the lungs. Despite that, assessing the safety of a new product storming the market fell between regulatory cracks, leaving doctors unsure where to register concerns before the outbreak. The Food and Drug Administration took years to regulate e-cigarettes once a court determined it had the authority to do so.
“You don’t know what you’re putting into your lungs when you vape,” said Dr. Harris, a critical care pulmonologist. “It’s purported to be safe, but how do you know if it’s safe? To me, it’s a very dangerous thing.”
Off the radar
When e-cigarettes came to market about a decade ago, they fell into a regulatory no man’s land. They are not a food, not a drug, and not a medical device, any of which would have put them immediately in the FDA’s purview. And, until a few years ago, they weren’t even lumped in with tobacco products.
As a result, billions of dollars of vaping products have been sold online, at big-box retailers, and in corner stores without going through the FDA’s rigorous review process to assess their safety. Companies like Juul, Blu, and NJoy quickly established their brands of devices and cartridges, or pods. And thousands of related products are sold, sometimes on the black market, over the Internet, or beyond.
“It makes it really tough because we don’t know what we’re looking for,” said Ruth Lynfield, MD, the state epidemiologist for Minnesota, where several patients were admitted to the ICU as a result of the illness. She added that, if it turns out that the products in question were sold by unregistered retailers and manufacturers “on the street,” outbreak sleuths will have a harder time figuring out exactly what is in them.
With e-cigarettes, people can vape – or smoke – nicotine products, selecting flavorings like mint, mango, blueberry crème brûlée, or cookies and milk. They can also inhale cannabis products. Many are hopeful that e-cigarettes might be useful smoking cessation tools, but some research has called that into question.
The mysterious pulmonary disease cases have been linked to vaping, but it’s unclear whether there is a common device or chemical. In some states, including California and Utah, all of the patients had vaped cannabis products. One or more substances could be involved, health officials have said. The products used by several victims are being tested to see what they contained.
And this has apparently been the case for years.
Multiple doctors described seeing earlier cases of severe lung problems linked to vaping that were not officially reported or included in the current CDC count.
Laura Crotty Alexander, MD, a pulmonologist and researcher with the University of California, San Diego, said she saw her first case about 2 years ago. A young man had been vaping for months with the same device but developed acute lung injury when he switched flavors. She strongly suspected a link, but did not report the illness anywhere.
“It wasn’t that I didn’t want to report it, it’s that there’s no pathway” to do so, Dr. Alexander said.
She said she’s concerned that many physicians haven’t been asking patients about e-cigarette use and that there’s no way to document a case like this in the medical coding system.
John E. Parker, MD, of West Virginia University, Morgantown, said he saw his first patient with pneumonia tied to vaping in 2015. Doctors there were intrigued enough to report on the case at the annual meeting of the American College of Chest Physicians. Dr. Parker and his team didn’t contact a federal agency, and Dr. Parker said it was unclear whom to call.
Numerous other cases have been reported in medical journals and at professional conferences in the years since. The FDA’s voluntary system for reporting tobacco-related health problems included 96 seizures and only 1 lung ailment tied to e-cigarettes between April and June 2019. The system appears to be utilized most by concerned citizens, rather than manufacturers or health care professionals.
But several lung specialists said that due to the patchwork nature of regulatory oversight over the years, the true scope of the problem is yet to be identified.
“We do know that e-cigarettes do not emit a harmless aerosol,” said Brian King, PhD, MPH, a deputy director in the Office on Smoking and Health at the CDC in a call with media on Aug. 23 about the outbreak. “It is possible that some of these cases were already occurring but we were not picking them up.”
Regulatory limits
The FDA has had limited authority to regulate e-cigarettes over the years.
In 2009, Congress passed the Family Smoking Prevention and Tobacco Control Act, empowering the FDA to oversee the safety and sale of tobacco products. But e-cigarettes, still new, were not top of mind.
Later that year, the FDA tried to block imports of e-cigarettes, saying the combination drug-device products were unapproved and therefore illegal for sale in the United States. Two vaping companies, Smoking Everywhere and NJoy, sued, and a federal judge ruled in 2010 that the FDA should regulate e-cigarettes as tobacco products.
It took the agency 6 years to finalize what’s become known as the “deeming rule,” in which it formally began regulating e-cigarettes and e-liquids.
By then, it was May 2016, and the e-cigarette market had swelled to an estimated $4.1 billion, Wells Fargo Securities analyst Bonnie Herzog said at the time. Market researchers now project that the global industry could reach $48 billion by 2023.
Critics say the FDA took too long to act.
“I think the fact that FDA has been dillydallying [has made] figuring out what’s going on [with this outbreak] much harder,” said Stanton Glantz, PhD, a University of California, San Francisco, professor in its Center for Tobacco Control Research and Education. “No question.”
The agency began by banning e-cigarette sales to minors and requiring all new vaping products to submit applications for authorization before they could come to market. Companies and retailers with thousands of products already on the market were granted 2 years to submit applications, and the FDA would get an additional year to evaluate the applications. Meanwhile, existing products could still be sold.
But when Scott Gottlieb, MD, arrived as the new FDA commissioner in 2017, the rule hadn’t been implemented and there was no formal guidance for companies to file applications, he said. As a result, he pushed the deadline back to 2022, drawing ire from public health advocates, who called foul over his previous ties to an e-cigarette retailer called Kure.
“I thought e-cigarettes at the time – and I still believe – that they represent an opportunity for currently addicted adult smokers to transition off of combustible tobacco,” he said in an interview, adding that other parts of the deeming rule went into effect as planned. “All I did was delay the application deadline.”
Dr. Gottlieb’s thinking changed the following year, when a national survey showed a sharp rise in teen vaping, which he called an “epidemic.” He announced that the agency would rethink the extended deadline and weigh whether to take flavors that appeal to kids off the market.
A judge ruled last month that e-cigarette makers would have only 10 more months to submit applications to the FDA. They’re now due in May 2020.
Asked about the lung injuries appearing now, Dr. Gottlieb, who left the FDA in April 2019, said he suspected counterfeit pods are to blame, given the geographic clustering of cases and the fact that, overall, the FDA is inspecting registered e-cigarette makers and retailers to make sure they’re complying with existing regulations.
“I think the manufacturers are culpable if their products are being used, whether the liquids are counterfeit or real,” he said. “Ultimately, they’re responsible for keeping their products out of the hands of kids.”
Juul, the leading e-cigarette maker, agreed that children shouldn’t be able to vape its products, and said curtailing access should be done “through significant regulation” and “enforcement.”
“When people say ‘Why aren’t these being regulated?’ They actually are all being regulated,” Dr. Gottlieb said.
For example, companies are required to label their products as potentially addictive, sell only to adults and comply with manufacturing standards. The agency has conducted thousands of inspections of e-cigarette manufacturers and retailers and taken enforcement actions against companies selling e-cigarettes that look like juice boxes, and against a company that was putting the ingredients found in erectile dysfunction drugs into its vape liquid.
Health departments investigating the outbreak told Kaiser Health News that e-cigarettes’ niche as a tobacco product instead of a drug has presented challenges. Most weren’t aware that adverse events could be reported to a database that tracks problems with tobacco products. And, because e-cigarettes never went through the FDA’s “gold-standard” approval process for drugs, doctors can’t readily look up a detailed list of known side effects.
But like other arms of the FDA, the tobacco office has tools and a team to investigate a public health threat just as the teams for drugs and devices do, Dr. Gottlieb said. It may even be better equipped because of its funding.
“I don’t think FDA is operating in any way with hands tied behind its back because of the way that the statute is set up,” he said.
Teen vaping has exploded during this regulatory tussle. In 2011, 1.5% of high school students reported vaping. By 2018, it was 20.8%, according to a CDC report.
Unknown components
Still, doctors and researchers are concerned about the ingredients in e-cigarettes and how little the public knows about the risks of vaping.
In Juul’s terms and conditions, posted on its website, it says, “We encourage consumers to do their own research regarding vapor products and what is right for them.” Many ingredients in e-cigarette products, however, are protected as trade secrets.
Since at least 2013, the flavor industry has expressed concern about the use of flavoring chemicals in vaping products.
The vast majority of the chemicals have been tested only by ingesting them in small quantities because they’re encountered in foods. For most of these chemicals, there have been no tests to determine whether it is safe to inhale them, as happens daily by millions when they use e-cigarettes.
“Many of the ingredients of vaping products, including flavoring substances, have not been tested for … the exposure one would get from using a vaping device,” said John Hallagan, a senior adviser to the Flavor and Extract Manufacturers Association. The group has sent cease-and-desist letters to e-cigarette companies in previous years for using the food safety certification of the flavor industry to imply that the chemicals are also safe in e-cigarettes.
Some flavor chemicals are thought to be harmful when inhaled in high doses. Research suggests that cinnamaldehyde, the main component of many cinnamon flavors, may impair lung function when inhaled. Sven-Eric Jordt, PhD, a professor at Duke University, Durham, N.C., says he presented evidence of its dangers at an FDA meeting in 2015 — and its relative abundance in many e-cigarette vaping liquids. In response, one major e-cigarette liquid seller, Tasty Vapor, voluntarily took its cinnamon-flavored liquid off the shelves.
In 2017, when Dr. Gottlieb delayed the FDA application deadline, the product was back. A company email to its customers put it this way: “Two years ago, Tasty Vapor allowed itself to be intimidated by scaremongering tactics. … We lost a lot of sales as well as a good number of long-time customers. We no long see reason to disappoint our customers hostage for these shady tactics.”
At the time of publication, Tasty Vapor’s owner did not reply to a request for comment.
Dr. Jordt said he is frustrated by the delays in the regulatory approval process.
“As a parent, I would say that the government has not acted on this,” he said. “You’re basically left to act alone with your addicted kid. It’s kind of terrifying that this was allowed to happen. The industry needs to be held to account.”
Kaiser Health News correspondents Cara Anthony, Markian Hawryluk, and Lauren Weber, as well as reporter Victoria Knight contributed to this report. This story first published on California Healthline, a service of the California Health Care Foundation.
Kaiser Health News is a national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation which is not affiliated with Kaiser Permanente.
It was the arrival of the second man in his early 20s gasping for air that alarmed Dixie Harris, MD. Young patients rarely get so sick, so fast, with a severe lung illness, and this was her second case in a matter of days.
Then she saw three more patients at her Utah telehealth clinic with similar symptoms. They did not have infections, but all had been vaping. When Dr. Harris heard several teenagers in Wisconsin had been hospitalized in similar cases, she quickly alerted her state health department.
As patients in hospitals across the country combat a mysterious illness linked to e-cigarettes, federal and state investigators are frantically trying to trace the outbreaks to specific vaping products that, until recently, were virtually unregulated.
As of Aug. 22, 2019, 193 potential vaping-related illnesses in 22 states had been reported to the Centers for Disease Control and Prevention. Wisconsin, which first put out an alert in July, has at least 16 confirmed and 15 suspected cases. Illinois has reported 34 patients, 1 of whom has died. Indiana is investigating 24 cases.
Lung doctors said they had seen warning signs for years that vaping could be hazardous as they treated patients. Medically it seemed problematic since it often involved inhaling chemicals not normally inhaled into the lungs. Despite that, assessing the safety of a new product storming the market fell between regulatory cracks, leaving doctors unsure where to register concerns before the outbreak. The Food and Drug Administration took years to regulate e-cigarettes once a court determined it had the authority to do so.
“You don’t know what you’re putting into your lungs when you vape,” said Dr. Harris, a critical care pulmonologist. “It’s purported to be safe, but how do you know if it’s safe? To me, it’s a very dangerous thing.”
Off the radar
When e-cigarettes came to market about a decade ago, they fell into a regulatory no man’s land. They are not a food, not a drug, and not a medical device, any of which would have put them immediately in the FDA’s purview. And, until a few years ago, they weren’t even lumped in with tobacco products.
As a result, billions of dollars of vaping products have been sold online, at big-box retailers, and in corner stores without going through the FDA’s rigorous review process to assess their safety. Companies like Juul, Blu, and NJoy quickly established their brands of devices and cartridges, or pods. And thousands of related products are sold, sometimes on the black market, over the Internet, or beyond.
“It makes it really tough because we don’t know what we’re looking for,” said Ruth Lynfield, MD, the state epidemiologist for Minnesota, where several patients were admitted to the ICU as a result of the illness. She added that, if it turns out that the products in question were sold by unregistered retailers and manufacturers “on the street,” outbreak sleuths will have a harder time figuring out exactly what is in them.
With e-cigarettes, people can vape – or smoke – nicotine products, selecting flavorings like mint, mango, blueberry crème brûlée, or cookies and milk. They can also inhale cannabis products. Many are hopeful that e-cigarettes might be useful smoking cessation tools, but some research has called that into question.
The mysterious pulmonary disease cases have been linked to vaping, but it’s unclear whether there is a common device or chemical. In some states, including California and Utah, all of the patients had vaped cannabis products. One or more substances could be involved, health officials have said. The products used by several victims are being tested to see what they contained.
And this has apparently been the case for years.
Multiple doctors described seeing earlier cases of severe lung problems linked to vaping that were not officially reported or included in the current CDC count.
Laura Crotty Alexander, MD, a pulmonologist and researcher with the University of California, San Diego, said she saw her first case about 2 years ago. A young man had been vaping for months with the same device but developed acute lung injury when he switched flavors. She strongly suspected a link, but did not report the illness anywhere.
“It wasn’t that I didn’t want to report it, it’s that there’s no pathway” to do so, Dr. Alexander said.
She said she’s concerned that many physicians haven’t been asking patients about e-cigarette use and that there’s no way to document a case like this in the medical coding system.
John E. Parker, MD, of West Virginia University, Morgantown, said he saw his first patient with pneumonia tied to vaping in 2015. Doctors there were intrigued enough to report on the case at the annual meeting of the American College of Chest Physicians. Dr. Parker and his team didn’t contact a federal agency, and Dr. Parker said it was unclear whom to call.
Numerous other cases have been reported in medical journals and at professional conferences in the years since. The FDA’s voluntary system for reporting tobacco-related health problems included 96 seizures and only 1 lung ailment tied to e-cigarettes between April and June 2019. The system appears to be utilized most by concerned citizens, rather than manufacturers or health care professionals.
But several lung specialists said that due to the patchwork nature of regulatory oversight over the years, the true scope of the problem is yet to be identified.
“We do know that e-cigarettes do not emit a harmless aerosol,” said Brian King, PhD, MPH, a deputy director in the Office on Smoking and Health at the CDC in a call with media on Aug. 23 about the outbreak. “It is possible that some of these cases were already occurring but we were not picking them up.”
Regulatory limits
The FDA has had limited authority to regulate e-cigarettes over the years.
In 2009, Congress passed the Family Smoking Prevention and Tobacco Control Act, empowering the FDA to oversee the safety and sale of tobacco products. But e-cigarettes, still new, were not top of mind.
Later that year, the FDA tried to block imports of e-cigarettes, saying the combination drug-device products were unapproved and therefore illegal for sale in the United States. Two vaping companies, Smoking Everywhere and NJoy, sued, and a federal judge ruled in 2010 that the FDA should regulate e-cigarettes as tobacco products.
It took the agency 6 years to finalize what’s become known as the “deeming rule,” in which it formally began regulating e-cigarettes and e-liquids.
By then, it was May 2016, and the e-cigarette market had swelled to an estimated $4.1 billion, Wells Fargo Securities analyst Bonnie Herzog said at the time. Market researchers now project that the global industry could reach $48 billion by 2023.
Critics say the FDA took too long to act.
“I think the fact that FDA has been dillydallying [has made] figuring out what’s going on [with this outbreak] much harder,” said Stanton Glantz, PhD, a University of California, San Francisco, professor in its Center for Tobacco Control Research and Education. “No question.”
The agency began by banning e-cigarette sales to minors and requiring all new vaping products to submit applications for authorization before they could come to market. Companies and retailers with thousands of products already on the market were granted 2 years to submit applications, and the FDA would get an additional year to evaluate the applications. Meanwhile, existing products could still be sold.
But when Scott Gottlieb, MD, arrived as the new FDA commissioner in 2017, the rule hadn’t been implemented and there was no formal guidance for companies to file applications, he said. As a result, he pushed the deadline back to 2022, drawing ire from public health advocates, who called foul over his previous ties to an e-cigarette retailer called Kure.
“I thought e-cigarettes at the time – and I still believe – that they represent an opportunity for currently addicted adult smokers to transition off of combustible tobacco,” he said in an interview, adding that other parts of the deeming rule went into effect as planned. “All I did was delay the application deadline.”
Dr. Gottlieb’s thinking changed the following year, when a national survey showed a sharp rise in teen vaping, which he called an “epidemic.” He announced that the agency would rethink the extended deadline and weigh whether to take flavors that appeal to kids off the market.
A judge ruled last month that e-cigarette makers would have only 10 more months to submit applications to the FDA. They’re now due in May 2020.
Asked about the lung injuries appearing now, Dr. Gottlieb, who left the FDA in April 2019, said he suspected counterfeit pods are to blame, given the geographic clustering of cases and the fact that, overall, the FDA is inspecting registered e-cigarette makers and retailers to make sure they’re complying with existing regulations.
“I think the manufacturers are culpable if their products are being used, whether the liquids are counterfeit or real,” he said. “Ultimately, they’re responsible for keeping their products out of the hands of kids.”
Juul, the leading e-cigarette maker, agreed that children shouldn’t be able to vape its products, and said curtailing access should be done “through significant regulation” and “enforcement.”
“When people say ‘Why aren’t these being regulated?’ They actually are all being regulated,” Dr. Gottlieb said.
For example, companies are required to label their products as potentially addictive, sell only to adults and comply with manufacturing standards. The agency has conducted thousands of inspections of e-cigarette manufacturers and retailers and taken enforcement actions against companies selling e-cigarettes that look like juice boxes, and against a company that was putting the ingredients found in erectile dysfunction drugs into its vape liquid.
Health departments investigating the outbreak told Kaiser Health News that e-cigarettes’ niche as a tobacco product instead of a drug has presented challenges. Most weren’t aware that adverse events could be reported to a database that tracks problems with tobacco products. And, because e-cigarettes never went through the FDA’s “gold-standard” approval process for drugs, doctors can’t readily look up a detailed list of known side effects.
But like other arms of the FDA, the tobacco office has tools and a team to investigate a public health threat just as the teams for drugs and devices do, Dr. Gottlieb said. It may even be better equipped because of its funding.
“I don’t think FDA is operating in any way with hands tied behind its back because of the way that the statute is set up,” he said.
Teen vaping has exploded during this regulatory tussle. In 2011, 1.5% of high school students reported vaping. By 2018, it was 20.8%, according to a CDC report.
Unknown components
Still, doctors and researchers are concerned about the ingredients in e-cigarettes and how little the public knows about the risks of vaping.
In Juul’s terms and conditions, posted on its website, it says, “We encourage consumers to do their own research regarding vapor products and what is right for them.” Many ingredients in e-cigarette products, however, are protected as trade secrets.
Since at least 2013, the flavor industry has expressed concern about the use of flavoring chemicals in vaping products.
The vast majority of the chemicals have been tested only by ingesting them in small quantities because they’re encountered in foods. For most of these chemicals, there have been no tests to determine whether it is safe to inhale them, as happens daily by millions when they use e-cigarettes.
“Many of the ingredients of vaping products, including flavoring substances, have not been tested for … the exposure one would get from using a vaping device,” said John Hallagan, a senior adviser to the Flavor and Extract Manufacturers Association. The group has sent cease-and-desist letters to e-cigarette companies in previous years for using the food safety certification of the flavor industry to imply that the chemicals are also safe in e-cigarettes.
Some flavor chemicals are thought to be harmful when inhaled in high doses. Research suggests that cinnamaldehyde, the main component of many cinnamon flavors, may impair lung function when inhaled. Sven-Eric Jordt, PhD, a professor at Duke University, Durham, N.C., says he presented evidence of its dangers at an FDA meeting in 2015 — and its relative abundance in many e-cigarette vaping liquids. In response, one major e-cigarette liquid seller, Tasty Vapor, voluntarily took its cinnamon-flavored liquid off the shelves.
In 2017, when Dr. Gottlieb delayed the FDA application deadline, the product was back. A company email to its customers put it this way: “Two years ago, Tasty Vapor allowed itself to be intimidated by scaremongering tactics. … We lost a lot of sales as well as a good number of long-time customers. We no long see reason to disappoint our customers hostage for these shady tactics.”
At the time of publication, Tasty Vapor’s owner did not reply to a request for comment.
Dr. Jordt said he is frustrated by the delays in the regulatory approval process.
“As a parent, I would say that the government has not acted on this,” he said. “You’re basically left to act alone with your addicted kid. It’s kind of terrifying that this was allowed to happen. The industry needs to be held to account.”
Kaiser Health News correspondents Cara Anthony, Markian Hawryluk, and Lauren Weber, as well as reporter Victoria Knight contributed to this report. This story first published on California Healthline, a service of the California Health Care Foundation.
Kaiser Health News is a national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation which is not affiliated with Kaiser Permanente.
It was the arrival of the second man in his early 20s gasping for air that alarmed Dixie Harris, MD. Young patients rarely get so sick, so fast, with a severe lung illness, and this was her second case in a matter of days.
Then she saw three more patients at her Utah telehealth clinic with similar symptoms. They did not have infections, but all had been vaping. When Dr. Harris heard several teenagers in Wisconsin had been hospitalized in similar cases, she quickly alerted her state health department.
As patients in hospitals across the country combat a mysterious illness linked to e-cigarettes, federal and state investigators are frantically trying to trace the outbreaks to specific vaping products that, until recently, were virtually unregulated.
As of Aug. 22, 2019, 193 potential vaping-related illnesses in 22 states had been reported to the Centers for Disease Control and Prevention. Wisconsin, which first put out an alert in July, has at least 16 confirmed and 15 suspected cases. Illinois has reported 34 patients, 1 of whom has died. Indiana is investigating 24 cases.
Lung doctors said they had seen warning signs for years that vaping could be hazardous as they treated patients. Medically it seemed problematic since it often involved inhaling chemicals not normally inhaled into the lungs. Despite that, assessing the safety of a new product storming the market fell between regulatory cracks, leaving doctors unsure where to register concerns before the outbreak. The Food and Drug Administration took years to regulate e-cigarettes once a court determined it had the authority to do so.
“You don’t know what you’re putting into your lungs when you vape,” said Dr. Harris, a critical care pulmonologist. “It’s purported to be safe, but how do you know if it’s safe? To me, it’s a very dangerous thing.”
Off the radar
When e-cigarettes came to market about a decade ago, they fell into a regulatory no man’s land. They are not a food, not a drug, and not a medical device, any of which would have put them immediately in the FDA’s purview. And, until a few years ago, they weren’t even lumped in with tobacco products.
As a result, billions of dollars of vaping products have been sold online, at big-box retailers, and in corner stores without going through the FDA’s rigorous review process to assess their safety. Companies like Juul, Blu, and NJoy quickly established their brands of devices and cartridges, or pods. And thousands of related products are sold, sometimes on the black market, over the Internet, or beyond.
“It makes it really tough because we don’t know what we’re looking for,” said Ruth Lynfield, MD, the state epidemiologist for Minnesota, where several patients were admitted to the ICU as a result of the illness. She added that, if it turns out that the products in question were sold by unregistered retailers and manufacturers “on the street,” outbreak sleuths will have a harder time figuring out exactly what is in them.
With e-cigarettes, people can vape – or smoke – nicotine products, selecting flavorings like mint, mango, blueberry crème brûlée, or cookies and milk. They can also inhale cannabis products. Many are hopeful that e-cigarettes might be useful smoking cessation tools, but some research has called that into question.
The mysterious pulmonary disease cases have been linked to vaping, but it’s unclear whether there is a common device or chemical. In some states, including California and Utah, all of the patients had vaped cannabis products. One or more substances could be involved, health officials have said. The products used by several victims are being tested to see what they contained.
And this has apparently been the case for years.
Multiple doctors described seeing earlier cases of severe lung problems linked to vaping that were not officially reported or included in the current CDC count.
Laura Crotty Alexander, MD, a pulmonologist and researcher with the University of California, San Diego, said she saw her first case about 2 years ago. A young man had been vaping for months with the same device but developed acute lung injury when he switched flavors. She strongly suspected a link, but did not report the illness anywhere.
“It wasn’t that I didn’t want to report it, it’s that there’s no pathway” to do so, Dr. Alexander said.
She said she’s concerned that many physicians haven’t been asking patients about e-cigarette use and that there’s no way to document a case like this in the medical coding system.
John E. Parker, MD, of West Virginia University, Morgantown, said he saw his first patient with pneumonia tied to vaping in 2015. Doctors there were intrigued enough to report on the case at the annual meeting of the American College of Chest Physicians. Dr. Parker and his team didn’t contact a federal agency, and Dr. Parker said it was unclear whom to call.
Numerous other cases have been reported in medical journals and at professional conferences in the years since. The FDA’s voluntary system for reporting tobacco-related health problems included 96 seizures and only 1 lung ailment tied to e-cigarettes between April and June 2019. The system appears to be utilized most by concerned citizens, rather than manufacturers or health care professionals.
But several lung specialists said that due to the patchwork nature of regulatory oversight over the years, the true scope of the problem is yet to be identified.
“We do know that e-cigarettes do not emit a harmless aerosol,” said Brian King, PhD, MPH, a deputy director in the Office on Smoking and Health at the CDC in a call with media on Aug. 23 about the outbreak. “It is possible that some of these cases were already occurring but we were not picking them up.”
Regulatory limits
The FDA has had limited authority to regulate e-cigarettes over the years.
In 2009, Congress passed the Family Smoking Prevention and Tobacco Control Act, empowering the FDA to oversee the safety and sale of tobacco products. But e-cigarettes, still new, were not top of mind.
Later that year, the FDA tried to block imports of e-cigarettes, saying the combination drug-device products were unapproved and therefore illegal for sale in the United States. Two vaping companies, Smoking Everywhere and NJoy, sued, and a federal judge ruled in 2010 that the FDA should regulate e-cigarettes as tobacco products.
It took the agency 6 years to finalize what’s become known as the “deeming rule,” in which it formally began regulating e-cigarettes and e-liquids.
By then, it was May 2016, and the e-cigarette market had swelled to an estimated $4.1 billion, Wells Fargo Securities analyst Bonnie Herzog said at the time. Market researchers now project that the global industry could reach $48 billion by 2023.
Critics say the FDA took too long to act.
“I think the fact that FDA has been dillydallying [has made] figuring out what’s going on [with this outbreak] much harder,” said Stanton Glantz, PhD, a University of California, San Francisco, professor in its Center for Tobacco Control Research and Education. “No question.”
The agency began by banning e-cigarette sales to minors and requiring all new vaping products to submit applications for authorization before they could come to market. Companies and retailers with thousands of products already on the market were granted 2 years to submit applications, and the FDA would get an additional year to evaluate the applications. Meanwhile, existing products could still be sold.
But when Scott Gottlieb, MD, arrived as the new FDA commissioner in 2017, the rule hadn’t been implemented and there was no formal guidance for companies to file applications, he said. As a result, he pushed the deadline back to 2022, drawing ire from public health advocates, who called foul over his previous ties to an e-cigarette retailer called Kure.
“I thought e-cigarettes at the time – and I still believe – that they represent an opportunity for currently addicted adult smokers to transition off of combustible tobacco,” he said in an interview, adding that other parts of the deeming rule went into effect as planned. “All I did was delay the application deadline.”
Dr. Gottlieb’s thinking changed the following year, when a national survey showed a sharp rise in teen vaping, which he called an “epidemic.” He announced that the agency would rethink the extended deadline and weigh whether to take flavors that appeal to kids off the market.
A judge ruled last month that e-cigarette makers would have only 10 more months to submit applications to the FDA. They’re now due in May 2020.
Asked about the lung injuries appearing now, Dr. Gottlieb, who left the FDA in April 2019, said he suspected counterfeit pods are to blame, given the geographic clustering of cases and the fact that, overall, the FDA is inspecting registered e-cigarette makers and retailers to make sure they’re complying with existing regulations.
“I think the manufacturers are culpable if their products are being used, whether the liquids are counterfeit or real,” he said. “Ultimately, they’re responsible for keeping their products out of the hands of kids.”
Juul, the leading e-cigarette maker, agreed that children shouldn’t be able to vape its products, and said curtailing access should be done “through significant regulation” and “enforcement.”
“When people say ‘Why aren’t these being regulated?’ They actually are all being regulated,” Dr. Gottlieb said.
For example, companies are required to label their products as potentially addictive, sell only to adults and comply with manufacturing standards. The agency has conducted thousands of inspections of e-cigarette manufacturers and retailers and taken enforcement actions against companies selling e-cigarettes that look like juice boxes, and against a company that was putting the ingredients found in erectile dysfunction drugs into its vape liquid.
Health departments investigating the outbreak told Kaiser Health News that e-cigarettes’ niche as a tobacco product instead of a drug has presented challenges. Most weren’t aware that adverse events could be reported to a database that tracks problems with tobacco products. And, because e-cigarettes never went through the FDA’s “gold-standard” approval process for drugs, doctors can’t readily look up a detailed list of known side effects.
But like other arms of the FDA, the tobacco office has tools and a team to investigate a public health threat just as the teams for drugs and devices do, Dr. Gottlieb said. It may even be better equipped because of its funding.
“I don’t think FDA is operating in any way with hands tied behind its back because of the way that the statute is set up,” he said.
Teen vaping has exploded during this regulatory tussle. In 2011, 1.5% of high school students reported vaping. By 2018, it was 20.8%, according to a CDC report.
Unknown components
Still, doctors and researchers are concerned about the ingredients in e-cigarettes and how little the public knows about the risks of vaping.
In Juul’s terms and conditions, posted on its website, it says, “We encourage consumers to do their own research regarding vapor products and what is right for them.” Many ingredients in e-cigarette products, however, are protected as trade secrets.
Since at least 2013, the flavor industry has expressed concern about the use of flavoring chemicals in vaping products.
The vast majority of the chemicals have been tested only by ingesting them in small quantities because they’re encountered in foods. For most of these chemicals, there have been no tests to determine whether it is safe to inhale them, as happens daily by millions when they use e-cigarettes.
“Many of the ingredients of vaping products, including flavoring substances, have not been tested for … the exposure one would get from using a vaping device,” said John Hallagan, a senior adviser to the Flavor and Extract Manufacturers Association. The group has sent cease-and-desist letters to e-cigarette companies in previous years for using the food safety certification of the flavor industry to imply that the chemicals are also safe in e-cigarettes.
Some flavor chemicals are thought to be harmful when inhaled in high doses. Research suggests that cinnamaldehyde, the main component of many cinnamon flavors, may impair lung function when inhaled. Sven-Eric Jordt, PhD, a professor at Duke University, Durham, N.C., says he presented evidence of its dangers at an FDA meeting in 2015 — and its relative abundance in many e-cigarette vaping liquids. In response, one major e-cigarette liquid seller, Tasty Vapor, voluntarily took its cinnamon-flavored liquid off the shelves.
In 2017, when Dr. Gottlieb delayed the FDA application deadline, the product was back. A company email to its customers put it this way: “Two years ago, Tasty Vapor allowed itself to be intimidated by scaremongering tactics. … We lost a lot of sales as well as a good number of long-time customers. We no long see reason to disappoint our customers hostage for these shady tactics.”
At the time of publication, Tasty Vapor’s owner did not reply to a request for comment.
Dr. Jordt said he is frustrated by the delays in the regulatory approval process.
“As a parent, I would say that the government has not acted on this,” he said. “You’re basically left to act alone with your addicted kid. It’s kind of terrifying that this was allowed to happen. The industry needs to be held to account.”
Kaiser Health News correspondents Cara Anthony, Markian Hawryluk, and Lauren Weber, as well as reporter Victoria Knight contributed to this report. This story first published on California Healthline, a service of the California Health Care Foundation.
Kaiser Health News is a national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation which is not affiliated with Kaiser Permanente.
Malignant Pleural Effusion: Therapeutic Options and Strategies
Malignant pleural effusion (MPE) is a common clinical problem in patients with advanced stage cancer. Each year in the United States, more than 150,000 individuals are diagnosed with MPE, and there are approximately 126,000 admissions for MPE.1-3 Providing effective therapeutic management remains a challenge, and currently available therapeutic interventions are palliative rather than curative. This article, the second in a 2-part review of MPE, focuses on the available management options.
Therapeutic Thoracentesis
Evaluation of pleural fluid cytology is a crucial step in the diagnosis and staging of disease. As a result, large-volume fluid removal is often the first therapeutic intervention for patients who present with symptomatic effusions. A patient’s clinical response to therapeutic thoracentesis dictates which additional therapeutic options are appropriate for palliation. Lack of symptom relief suggests that other comorbid conditions or trapped lung physiology may be the primary cause of the patient’s symptoms and discourages more invasive interventions. Radiographic evidence of lung re-expansion after fluid removal is also an important predictor of success for potential pleurodesis.4,5
There are no absolute contraindications to thoracentesis. However, caution should be used for patients with risk factors that may predispose to complications of pneumothorax and bleeding, such as coagulopathy, treatment with anticoagulation medications, thrombocytopenia, platelet dysfunction (eg, antiplatelet medications, uremia), positive pressure ventilation, and small effusion size. These factors are only relative contraindications, however, as thoracentesis can still be safely performed by experienced operators using guidance technology such as ultrasonography.
A retrospective review of 1009 ultrasound-guided thoracenteses with risk factors of an international normalized ratio (INR) greater than 1.6, platelet values less than 50,000/μL, or both, reported an overall rate of hemorrhagic complication of 0.4%, with no difference between procedures performed with (n = 303) or without (n = 706) transfusion correction of coagulopathy or thrombocytopenia.6 A similar retrospective evaluation of 1076 ultrasound-guided thoracenteses, including 267 patients with an INR greater than 1.5 and 58 patients with a platelet count less than 50,000/μL, reported a 0% complication rate.7 Small case series have also demonstrated low hemorrhagic complication rates for thoracentesis in patients treated with clopidogrel8,9 and with increased bleeding risk from elevated INR (liver disease or warfarin therapy) and renal disease.10
Complications from pneumothorax can similarly be affected by patient- and operator-dependent risk factors. Meta-analysis of 24 studies including 6605 thoracenteses demonstrated an overall pneumothorax rate of 6.0%, with 34.1% requiring chest tube insertion.11 Lower pneumothorax rates were associated with the use of ultrasound guidance (odds ratio, 0.3; 95% confidence interval, 0.2-0.7). Experienced operators also had fewer pneumothorax complications, though this factor was not significant in the studies directly comparing this variable. Therapeutic thoracentesis and use of a larger-bore needle were also significantly correlated with pneumothorax, while mechanical ventilation had a nonsignificant trend towards increased risk.
Although there is no consensus on the volume of pleural fluid that may be safely removed, it is recommended not to remove more than 1.5 L during a procedure in order to avoid precipitating re-expansion pulmonary edema.2,12 However, re-expansion pulmonary edema rates remain low even when larger volumes are removed if the patient remains symptom-free during the procedure and pleural manometry pressure does not exceed –20 cm H2O.13 Patient symptoms alone, however, are neither a sensitive nor specific indicator that pleural pressures exceed –20 cm H2O.14 Use of excessive negative pressure during drainage, such as from a vacuum bottle, should also be avoided. Comparison of suction generated manually with a syringe versus a vacuum bottle suggests decreased complications with manual drainage, though the sample size in the supporting study was small relative to the infrequency of the complications being evaluated.15
Given the low morbidity and noninvasive nature of the procedure, serial large-volume thoracentesis remains a viable therapeutic intervention for patients who are unable or unwilling to undergo more invasive interventions, especially for patients with a slow fluid re-accumulation rate or who are anticipated to have limited survival. Unfortunately, many symptomatic effusions will recur within a short interval time span, which necessitates repeat procedures.16,17 Therefore, factors such as poor symptom control, patient inconvenience, recurrent procedural risk, and utilization of medical resources need to be considered as well.
Tunneled Pleural Catheter
Tunneled pleural catheters (TPCs) are a potentially permanent and minimally invasive therapy which allow intermittent drainage of pleural fluid (Figure 1). The catheter is tunneled under the skin to prevent infection. A polyester cuff attached to the catheter is positioned within the tunnel and induces fibrosis around the catheter, thereby securing the catheter in place. Placement can be performed under local anesthesia at the patient’s bedside or in an outpatient procedure space. Fluid can then be drained via specialized drainage bottles or bags by the patient, a family member, or visiting home nurse. The catheter can also be removed in the event of a complication or the development of spontaneous pleurodesis.
TPCs are an effective palliative management strategy for patients with recurrent effusions and are an efficacious alternative to pleurodesis.18-20 TPCs may be used in patients with poor prognosis or trapped lung or in those in whom prior pleurodesis has failed.21-23 Meta-analysis of 19 studies showed symptomatic improvement in 95.6% of patients, with development of spontaneous pleurodesis in 45.6% of patients (range, 11.8% to 76.4%) after an average of 52 days.24 However, most of the studies included in this analysis were retrospective case series. Development of spontaneous pleurodesis from TPC drainage in prospective, controlled trials has been considerably more modest, supporting a range of approximately 20% to 30% with routine drainage strategies.20,25-27 Spontaneous pleurodesis develops greater rapidity and frequency in patients undergoing daily drainage compared to every-other-day or symptom-directed drainage strategies.25,26 However, there is no appreciable improvement in quality of life scores with a specific drainage strategy. Small case series also demonstrate that TPC drainage may induce spontaneous pleurodesis in some patients initially presenting with trapped lung physiology.22
Catheter placement can be performed successfully in the vast majority of patients.28 Increased bleeding risk, significant malignancy-related involvement of the skin and chest wall, and pleural loculations can complicate TPC placement. TPC-related complications are relatively uncommon, but include pneumothorax, catheter malfunction and obstruction, and infections including soft tissue and pleural space infections.24 In a multicenter retrospective series of 1021 patients, only 4.9% developed a TPC-related pleural infection.29 Over 94% were successfully managed with antibiotic therapy, and the TPC was able to be preserved in 54%. Staphylococcus aureus was the most common causative organism and was identified in 48% of cases. Of note, spontaneous pleurodesis occurred in 62% of cases following a pleural space infection, which likely occurred as sequelae of the inflammatory nature of the infection. Retrospective analysis suggests that the risk of TPC-related infections is not substantially higher for patients with higher risks of immunosuppression from chemotherapy or hematologic malignancies.30,31 Tumor metastasis along the catheter tract is a rare occurrence (< 1%), but is most notable with mesothelioma, which has an incidence as high as 10%.24,32 In addition, development of pleural loculations can impede fluid drainage and relief of dyspnea. Intrapleural instillation of fibrinolytics can be used to improve drainage and improve symptom palliation.33
Pleurodesis
Pleurodesis obliterates the potential pleural space by inducing inflammation and fibrosis, resulting in adherence of the visceral and parietal pleura together. This process can be induced through mechanical abrasion of the pleural surface, introduction of chemical sclerosants, or from prolonged use of a chest tube. Chemical sclerosants are the most commonly used method for MPEs and are introduced through a chest tube or under visual guidance such as medical thoracoscopy or video-assisted thoracoscopic surgery (VATS). The pleurodesis process is thought to occur by induction of a systemic inflammatory response with localized deposition of fibrin.34 Activation of fibroblasts and successful pleurodesis have been correlated with higher basic fibroblast growth factor (bFGF) levels in pleural fluid.35 Increased tumor burden is associated with lower bFGF levels, suggesting a possible mechanism for reduced pleurodesis success in these cases. Corticosteroids may reduce the likelihood of pleurodesis due to a reduction of inflammation, as demonstrated in a rabbit model using talc and doxycycline.36,37 Animal data also suggests that use of nonsteroidal anti-inflammatory drugs may hinder the likelihood of successful pleurodesis, but this has not been observed in humans.38,39
Patients selected for pleurodesis should have significant symptom relief from large-volume removal of pleural fluid, good functional status, and evidence of full lung re-expansion after thoracentesis. Lack of visceral and parietal pleural apposition will prevent pleural adhesion from developing. As a result, trapped lung is associated with chemical pleurodesis failure and is an absolute contraindication to the procedure.4,5,12 The pleurodesis process typically requires 5 to 7 days, during which time the patient is hospitalized for chest tube drainage and pain control. When pleural fluid output diminishes, the chest tube is removed and the patient can be discharged. Modified protocols are now emerging which may shorten the required hospitalization associated with pleurodesis procedures.
Pleurodesis Agents
A variety of chemical sclerosants have been used for pleurodesis, including talc, bleomycin, tetracycline, doxycycline, iodopovidone, and mepacrine. Published data regarding these agents are heterogenous, with significant variability in reported outcomes. However, systematic review and meta-analysis suggests that talc is likely to have higher success rates compared to other agents or chest tube drainage alone for treatment of MPE.40,41
Additional factors have been shown to be associated with pleurodesis outcomes. Pleurodesis success is negatively associated with low pleural pH, with receiver operating curve thresholds of 7.28 to 7.34.42,43 Trapped lung, large bulky tumor lining the pleural surfaces, and elevated adenosine deaminase levels are also associated with poor pleurodesis outcomes.4,5,12,35,43 In contrast, pleural fluid output less than 200 mL per day and the presence of EGFR (epidermal growth factor receptor) mutation treated with targeted tyrosine kinase inhibitors are associated with successful pleurodesis.44,45
The most common complications associated with chemical pleurodesis are fever and pain. Other potential complications include soft tissue infections at the chest tube site and of the pleural space, arrhythmias, cardiac arrest, myocardial infarction, and hypotension. Doxycycline is commonly associated with greater pleuritic pain than talc. Acute respiratory distress syndrome (ARDS), acute pneumonitis, and respiratory failure have been described with talc pleurodesis. ARDS secondary to talc pleurodesis occurs in 1% to 9% of cases, though this may be related to the use of ungraded talc. A prospective description of 558 patients treated with large particle talc (> 5 μm) reported no occurrences of ARDS, suggesting the safety of graded large particle talc.46
Pleurodesis Methods
Chest tube thoracostomy is an inpatient procedure performed under local anesthesia or conscious sedation. It can be used for measured, intermittent drainage of large effusions for immediate symptom relief, as well as to demonstrate complete lung re-expansion prior to instillation of a chemical sclerosant. Pleurodesis using a chest tube is performed by instillation of a slurry created by mixing the sclerosing agent of choice with 50 to 100 mL of sterile saline. This slurry is instilled into the pleural cavity through the chest tube. The chest tube is clamped for 1 to 2 hours before being reconnected to suction. Intermittent rotation of the patient has not been shown to improve distribution of the sclerosant or result in better procedural outcomes.47,48 Typically, a 24F to 32F chest tube is used because of the concern about obstruction of smaller bore tubes by fibrin plugs. A noninferiority study comparing 12F to 24F chest tubes for talc pleurodesis demonstrated a higher procedure failure rate with the 12F tube (30% versus 24%) and failed to meet noninferiority criteria.39 However, larger caliber tubes are also associated with greater patient discomfort compared to smaller bore tubes.
Medical thoracoscopy and VATS are minimally invasive means to visualize the pleural space, obtain visually guided biopsy of the parietal pleura, perform lysis of adhesions, and introduce chemical sclerosants for pleurodesis (Figure 2). Medical thoracoscopy can be performed under local anesthesia with procedural sedation in an endoscopy suite or procedure room.
VATS has several distinct and clinically important differences. The equipment is slightly larger but otherwise similar in concept to rigid medical thoracoscopes. A greater number of diagnostic and therapeutic options, such as diagnostic biopsy of lung parenchyma and select hilar lymph nodes, are also possible. However, VATS requires surgical training and is performed in an operating room setting, which necessitates additional ancillary and logistical support. VATS also uses at least 2 trocar insertion sites, requires general anesthesia, and utilizes single-lung ventilation through a double-lumen endotracheal tube. Procedure-related complications for medical thoracoscopy and VATS include pneumothorax, subcutaneous emphysema, fever, and pain.49
Data comparing talc slurry versus talc poudrage are heterogenous, without clear advantage for either method. Reported rates of successful pleurodesis are generally in the range of 70% to 80% for both methods.19,20,40,50 There is a possible suggestion of benefit with talc poudrage compared to slurry, but data is lacking to support either as a definitive choice in current guidelines.12,51 An advantage of medical thoracoscopy or VATS is that pleural biopsy can be performed simultaneously, if necessary, thereby allowing both diagnostic and therapeutic interventions.52 Visualizing the thoracic cavity may also permit creation of optimal conditions for pleurodesis in select individuals by allowing access to loculated spaces and providing visual confirmation of complete drainage of pleural fluid and uniform distribution of the chemical sclerosant.
Other Surgical Interventions
Thoracotomy with decortication is rarely used as treatment of malignant effusions complicated by loculations or trapped lung due to the significantly increased procedural morbidity and mortality. Therefore, it is reserved for the limited population of patients in whom other therapeutic interventions have failed but who otherwise have significant symptoms with a long life expectancy. Mesothelioma is a specific situation in which variations of pleurectomy, such as radical pleurectomy with decortication, lung-sparing total pleurectomy, and extrapleural pneumonectomy (EPP), have been used as front-line therapy. The Mesothelioma and Radical Surgery (MARS) trial, the only randomized, controlled evaluation of EPP, demonstrated decreased median survival in patients treated by EPP compared to controls (14.4 months versus 19.5 months).53 EPP is also associated with high procedure-related morbidity and mortality rates of approximately 50% and 4% to 15%, respectively.54 While successful at achieving pleurodesis, use of EPP as a treatment for mesothelioma is now discouraged.53,55 Less invasive surgical approaches, such as pleurectomy with decortication, are able to palliate symptoms with significantly less operative risk.56
Management Considerations
Selection of Therapeutic Interventions
The ideal management strategy provides both immediate and long-term symptom palliation, has minimal associated morbidity and side effects, minimizes hospitalization time and clinic visits, avoids the risks and inconvenience of recurring procedures, is inexpensive, and minimizes utilization of medical resources. Unfortunately, no single palliation methodology fits these needs for all patients. When evaluating therapeutic options for patients with MPE, it is important to consider factors such as the severity of symptoms, fluid quantity, fluid re-accumulation rate, pleural physiology, functional status, overall prognosis, and anticipated response of the malignancy to therapy. One example management algorithm (Figure 4) demonstrates the impact of these variables on the appropriate treatment options. However, this is a simplified algorithm and the selected palliation strategy should be decided upon after shared decision-making between the patient and physician and should fit within the context of the patient’s desired goals of care. It is also crucial for patients to understand that these therapeutic interventions are palliative rather than curative.
When compared directly with pleurodesis, TPC provides similar control of symptoms but with a reduction in hospital length of stay by a median of 3.5 to 5.5 days.19,57 In a nonrandomized trial where patients chose palliation by TPC or talc pleurodesis, more TPC patients had a significant immediate improvement in quality of life and dyspnea after the first 7 days of therapy.58 This is reasonably attributed to the differences between the immediate relief from fluid drainage after TPC placement compared to the time required for pleural symphysis to occur with pleurodesis. However, control of dyspnea symptoms is similar between the 2 strategies after 6 weeks.19 Therefore, both TPC and pleurodesis strategies are viewed as first-line options for patients with expandable lung and no prior palliative interventions for MPE.59
Pleural adhesions and trapped lung also pose specific dilemmas. Pleural adhesions can create loculated fluid pockets, thereby complicating drainage by thoracentesis or TPC and hindering dispersal of pleurodesis agents. Adhesiolysis by medical thoracoscopy or VATS may be useful in these patients to free up the pleural space and improve efficacy of long-term drainage options or facilitate pleurodesis. Intrapleural administration of fibrinolytics, such as streptokinase and urokinase, has also been used for treatment of loculated effusions and may improve drainage of pleural fluid and lung re-expansion.60-63 However routine use of intrapleural fibrinolytics with pleurodesis has not been shown to be beneficial. In a randomized comparison using intrapleural urokinase prior to pleurodesis for patients with septated malignant pleural effusions, no difference in pleurodesis outcomes were identified.63 As a result, TPC is the preferred palliation approach for patients with trapped lung physiology.51,59
Combination Strategies
Combinations of different therapeutic interventions are being evaluated as a means for patients to achieve long-term benefits from pleurodesis while minimizing hospitalization time. One strategy using simultaneous treatment with thoracoscopic talc poudrage and insertion of a large-bore chest tube and TPC has been shown to permit early removal of the chest tube and discharge home using the TPC for continued daily pleural drainage. This “rapid pleurodesis” strategy has an 80% to 90% successful pleurodesis rate, permitting removal of the TPC at a median of 7 to 10 days.64,65 With this approach, median hospitalization length of stay was approximately 2 days. While there was no control arm in these early reports with limited sample sizes, the pleurodesis success rate and length of hospitalization compare favorably to other published studies. A prospective, randomized trial of TPC versus an outpatient regimen of talc slurry via TPC has also shown promise, with successful pleurodesis after 35 days in 43% of those treated with the combination of talc slurry and TPC compared to only 23% in those treated by TPC alone.27
Another novel approach to obtain the benefits of both TPC and pleurodesis strategies is the use of drug-eluting TPC to induce inflammation and promote adhesion of the visceral and parietal pleura. An early report of slow-release silver nitrate (AgNO3) –coated TPC demonstrated an encouraging 89% spontaneous pleurodesis rate after a median of 4 days in the small subgroup of patients with fully expandable lung.66 Device-related adverse events were relatively high at 24.6%, though only one was deemed a serious adverse event. Additional studies of these novel and combination strategies are ongoing at this time.
Costs
While cost of care is not a consideration in the decision-making for individual patients, it is important from a systems-based perspective. Upfront costs for pleurodesis are generally higher due to the facility and hospitalization costs, whereas TPC have ongoing costs for drainage bottles and supplies. In a prospective, randomized trial of TPC versus talc pleurodesis, there was no appreciable difference in overall costs between the 2 approaches.67 The cost of TPC was significantly less, however, for patients with a shorter survival of less than 14 weeks.
Readmissions
Subsequent hospitalization requirements beyond just the initial treatment for a MPE remains another significant consideration for this patient population. A prospective, randomized trial comparing TPC to talc pleurodesis demonstrated a reduction in total all-cause hospital stay for TPC, with a median all-cause hospitalization time of 10 days for patients treated with TPC compared to 12 days for the talc pleurodesis group.20 The primary difference in the number of hospitalization days was due to a difference in effusion-related hospital days (median 1 versus 4 days, respectively), which was primarily comprised of the initial hospitalization. In addition, fewer patients treated with TPC required subsequent ipsilateral invasive procedures (4.1% versus 22.5%, respectively). However, it is important to note that the majority of all-cause hospital days were not effusion-related, demonstrating that this population has a high utilization of acute inpatient services for other reasons related to their advanced malignancy. In a study of regional hospitals in the United States, 38.3% of patients admitted for a primary diagnosis of MPE were readmitted within 30 days.68 There was remarkably little variability in readmission rates among hospitals, despite differences in factors such as institution size, location, patient distribution, and potential practice differences. This suggests that utilization of palliation strategies for MPE are only one component related to hospitalization in this population. Even at the best performing hospitals, there are significant common drivers for readmission that are not addressed. Therefore, additional effort should be focused on addressing aspects of care beyond just the palliation of MPE that predispose this population to requiring frequent treatment in an acute care setting.
Conclusion
MPEs represent advanced stage disease and frequently adversely affect a patient’s quality of life. The treating clinician has access to a variety of therapeutic options, though no single intervention strategy is universally superior in all circumstances. Initial thoracentesis is important in evaluating whether removal of a large volume of fluid provides significant symptom relief and restores functional status. Both talc pleurodesis and TPC provide similar control of symptoms and are first-line approaches for symptomatic patients with MPE and fully expandable lungs. Pleurodesis is associated with greater procedure-related risk and length of hospitalization and is contraindicated in patients with trapped lung, but does not require long-term catheter care or disposable resources. Determination of the appropriate therapeutic management strategy requires careful evaluation of the patient’s clinical situation and informed discussion with the patient to make sure that the treatment plan fits within the context of their goals of medical care.
1. Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J. 2001;18:402-419.
2. Society AT. Management of malignant pleural effusions. Am J Respir Crit Care Med. 2000;162:1987-2001.
3. Taghizadeh N, Fortin M, Tremblay A. US hospitalizations for malignant pleural effusions: data from the 2012 National Inpatient Sample. Chest. 2017;151:845-854.
4. Adler RH, Sayek I. Treatment of malignant pleural effusion: a method using tube thoracostomy and talc. Ann Thorac Surg. 1976;22:8-15.
5. Villanueva AG, Gray AW, Shahian DM, et al. Efficacy of short term versus long term tube thoracostomy drainage before tetracycline pleurodesis in the treatment of malignant pleural effusions. Thorax. 1994;49:23-25.
6. Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest. 2013;144:456-463.
7. Patel MD, Joshi SD. Abnormal preprocedural international normalized ratio and platelet counts are not associated with increased bleeding complications after ultrasound-guided thoracentesis. AJR Am J Roentgenol. 2011;197:W164-168.
8. Zalt MB, Bechara RI, Parks C, Berkowitz DM. Effect of routine clopidogrel use on bleeding complications after ultrasound-guided thoracentesis. J Bronchology Interv Pulmonol. 2012;19:284-287.
9. Mahmood K, Shofer SL, Moser BK, et al. Hemorrhagic complications of thoracentesis and small-bore chest tube placement in patients taking clopidogrel. Ann Am Thorac Soc. 2014;11:73-79.
10. Puchalski JT, Argento AC, Murphy TE, et al. The safety of thoracentesis in patients with uncorrected bleeding risk. Ann Am Thorac Soc. 2013;10:336-341.
11. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170:332-339.
12. Roberts ME, Neville E, Berrisford RG, et al; Group BPDG. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii32-40.
13. Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84:1656-1661.
14. Feller-Kopman D, Walkey A, Berkowitz D, Ernst A. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest. 2006;129:1556-1560.
15. Senitko M, Ray AS, Murphy TE, et al. Safety and tolerability of vacuum versus manual drainage during thoracentesis: a randomized trial. J Bronchology Interv Pulmonol. 2019;26:166-171.
16. Ost DE, Niu J, Zhao H, et al. Quality gaps and comparative effectiveness of management strategies for recurrent malignant pleural effusions. Chest. 2018;153:438-452.
17. Grosu HB, Molina S, Casal R, et al. Risk factors for pleural effusion recurrence in patients with malignancy. Respirology. 2019;24:76-82.
18. Musani AI, Haas AR, Seijo L, et al. Outpatient management of malignant pleural effusions with small-bore, tunneled pleural catheters. Respiration. 2004;71:559-566.
19. Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307:2383-2389.
20. Thomas R, Fysh ETH, Smith NA, et al. Effect of an indwelling pleural catheter vs talc pleurodesis on hospitalization days in patients with malignant pleural effusion: the AMPLE randomized clinical trial. JAMA. 2017;318:1903-1912.
21. Qureshi RA, Collinson SL, Powell RJ, et al. Management of malignant pleural effusion associated with trapped lung syndrome. Asian Cardiovasc Thorac Ann. 2008;16:120-123.
22. Efthymiou CA, Masudi T, Thorpe JA, Papagiannopoulos K. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg. 2009;9:961-964.
23. Sioris T, Sihvo E, Salo J, et al. Long-term indwelling pleural catheter (PleurX) for malignant pleural effusion unsuitable for talc pleurodesis. Eur J Surg Oncol. 2009;35:546-551.
24. Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med. 2011;26:70-76.
25. Wahidi MM, Reddy C, Yarmus L, et al. Randomized trial of pleural fluid drainage frequency in patients with malignant pleural effusions. the ASAP trial. Am J Respir Crit Care Med. 2017;195:1050-1057.
26. Muruganandan S, Azzopardi M, Fitzgerald DB, et al. Aggressive versus symptom-guided drainage of malignant pleural effusion via indwelling pleural catheters (AMPLE-2): an open-label randomised trial. Lancet Respir Med. 2018;6:671-680.
27. Bhatnagar R, Keenan EK, Morley AJ, et al. Outpatient talc administration by indwelling pleural catheter for malignant effusion. N Engl J Med. 2018;378:1313-1322.
28. Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest. 2006;129:362-368.
29. Fysh ETH, Tremblay A, Feller-Kopman D, et al. Clinical outcomes of indwelling pleural catheter-related pleural infections: an international multicenter study. Chest. 2013;144:1597-1602.
30. Morel A, Mishra E, Medley L, et al. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax. 2011;66:448-449.
31. Gilbert CR, Lee HJ, Skalski JH, et al. The use of indwelling tunneled pleural catheters for recurrent pleural effusions in patients with hematologic malignancies: a multicenter study. Chest. 2015;148:752-758.
32. Thomas R, Budgeon CA, Kuok YJ, et al. Catheter tract metastasis associated with indwelling pleural catheters. Chest. 2014;146:557-562.
33. Thomas R, Piccolo F, Miller D, et al. Intrapleural fibrinolysis for the treatment of indwelling pleural catheter-related symptomatic loculations: a multicenter observational study. Chest. 2015;148:746-751.
34. Antony VB. Pathogenesis of malignant pleural effusions and talc pleurodesis. Pneumologie. 1999;53:493-498.
35. Antony VB, Nasreen N, Mohammed KA, et al. Talc pleurodesis: basic fibroblast growth factor mediates pleural fibrosis. Chest. 2004;126:1522-1528.
36. Xie C, Teixeira LR, McGovern JP, Light RW. Systemic corticosteroids decrease the effectiveness of talc pleurodesis. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1441-1444.
37. Teixeira LR, Wu W, Chang DS, Light RW. The effect of corticosteroids on pleurodesis induced by doxycycline in rabbits. Chest. 2002;121:216-219.
38. Hunt I, Teh E, Southon R, Treasure T. Using non-steroidal anti-inflammatory drugs (NSAIDs) following pleurodesis. Interact Cardiovasc Thorac Surg. 2007;6:102-104.
39. Rahman NM, Pepperell J, Rehal S, et al. Effect of opioids vs NSAIDs and larger vs smaller chest tube size on pain control and pleurodesis efficacy among patients with malignant pleural effusion: the TIME1 randomized clinical trial. JAMA. 2015;314:2641-2653.
40. Clive AO, Jones HE, Bhatnagar R, Preston NJ, Maskell N. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev. 2016(5):CD010529.
41. Tan C, Sedrakyan A, Browne J, et al. The evidence on the effectiveness of management for malignant pleural effusion: a systematic review. Eur J Cardiothorac Surg. 2006;29:829-838.
42. Heffner JE, Nietert PJ, Barbieri C. Pleural fluid pH as a predictor of pleurodesis failure: analysis of primary data. Chest. 2000;117:87-95.
43. Yildirim H, Metintas M, Ak G, et al. Predictors of talc pleurodesis outcome in patients with malignant pleural effusions. Lung Cancer. 2008;62:139-144.
44. Aydogmus U, Ozdemir S, Cansever L, et al. Bedside talc pleurodesis for malignant pleural effusion: factors affecting success. Ann Surg Oncol. 2009;16:745-750.
45. Guo H, Wan Y, Tian G, et al. EGFR mutations predict a favorable outcome for malignant pleural effusion of lung adenocarcinoma with Tarceva therapy. Oncol Rep. 2012;27:880-890.
46. Janssen JP, Collier G, Astoul P, et al. Safety of pleurodesis with talc poudrage in malignant pleural effusion: a prospective cohort study. Lancet. 2007;369(9572):1535-1539.
47. Dryzer SR, Allen ML, Strange C, Sahn SA. A comparison of rotation and nonrotation in tetracycline pleurodesis. Chest. 1993;104:1763-1766.
48. Mager HJ, Maesen B, Verzijlbergen F, Schramel F. Distribution of talc suspension during treatment of malignant pleural effusion with talc pleurodesis. Lung Cancer. 2002;36:77-81.
49. Hsia D, Musani AI. Interventional pulmonology. Med Clin North Am. 2011;95:1095-1114.
50. Dresler CM, Olak J, Herndon JE, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest. 2005;127:909-915.
51. Bibby AC, Dorn P, Psallidas I, et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur Respir J. 2018;52(1).
52. Sakuraba M, Masuda K, Hebisawa A, et al. Diagnostic value of thoracoscopic pleural biopsy for pleurisy under local anaesthesia. ANZ J Surg. 2006;76:722-724.
53. Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011;12:763-772.
54. Zellos L, Jaklitsch MT, Al-Mourgi MA, Sugarbaker DJ. Complications of extrapleural pneumonectomy. Semin Thorac Cardiovasc Surg. 2007;19:355-359.
55. Zahid I, Sharif S, Routledge T, Scarci M. Is pleurectomy and decortication superior to palliative care in the treatment of malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg. 2011;12:812-817.
56. Soysal O, Karaoğlanoğlu N, Demiracan S, et al. Pleurectomy/decortication for palliation in malignant pleural mesothelioma: results of surgery. Eur J Cardiothorac Surg. 1997;11:210-213.
57. Putnam JB, Light RW, Rodriguez RM, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer. 1999;86:1992-1999.
58. Fysh ETH, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest. 2012;142:394-400.
59. Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med. 2018;198:839-849.
60. Davies CW, Traill ZC, Gleeson FV, Davies RJ. Intrapleural streptokinase in the management of malignant multiloculated pleural effusions. Chest. 1999;115:729-733.
61. Hsu LH, Soong TC, Feng AC, Liu MC. Intrapleural urokinase for the treatment of loculated malignant pleural effusions and trapped lungs in medically inoperable cancer patients. J Thorac Oncol. 2006;1:460-467.
62. Okur E, Baysungur V, Tezel C, et al. Streptokinase for malignant pleural effusions: a randomized controlled study. Asian Cardiovasc Thorac Ann. 2011;19:238-243.
63. Mishra EK, Clive AO, Wills GH, et al. Randomized controlled trial of urokinase versus placebo for nondraining malignant pleural effusion. Am J Respir Crit Care Med. 2018;197:502-508.
64. Reddy C, Ernst A, Lamb C, Feller-Kopman D. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest. 2011;139:1419-1423.
65. Krochmal R, Reddy C, Yarmus L, et al. Patient evaluation for rapid pleurodesis of malignant pleural effusions. J Thorac Dis. 2016;8:2538-2543.
66. Bhatnagar R, Zahan-Evans N, Kearney C, et al. A novel drug-eluting indwelling pleural catheter for the management of malignant effusions. Am J Respir Crit Care Med. 2018;197:136-138.
67. Penz ED, Mishra EK, Davies HE, Manns BJ, Miller RF, Rahman NM. Comparing cost of indwelling pleural catheter vs talc pleurodesis for malignant pleural effusion. Chest. 2014;146:991-1000.
68. Yang TS, Hsia DW, Chang DW. Patient- and hospital-level factors associated with readmission for malignant pleural effusion. J Oncol Pract. 2018;14:e547-e556.
Malignant pleural effusion (MPE) is a common clinical problem in patients with advanced stage cancer. Each year in the United States, more than 150,000 individuals are diagnosed with MPE, and there are approximately 126,000 admissions for MPE.1-3 Providing effective therapeutic management remains a challenge, and currently available therapeutic interventions are palliative rather than curative. This article, the second in a 2-part review of MPE, focuses on the available management options.
Therapeutic Thoracentesis
Evaluation of pleural fluid cytology is a crucial step in the diagnosis and staging of disease. As a result, large-volume fluid removal is often the first therapeutic intervention for patients who present with symptomatic effusions. A patient’s clinical response to therapeutic thoracentesis dictates which additional therapeutic options are appropriate for palliation. Lack of symptom relief suggests that other comorbid conditions or trapped lung physiology may be the primary cause of the patient’s symptoms and discourages more invasive interventions. Radiographic evidence of lung re-expansion after fluid removal is also an important predictor of success for potential pleurodesis.4,5
There are no absolute contraindications to thoracentesis. However, caution should be used for patients with risk factors that may predispose to complications of pneumothorax and bleeding, such as coagulopathy, treatment with anticoagulation medications, thrombocytopenia, platelet dysfunction (eg, antiplatelet medications, uremia), positive pressure ventilation, and small effusion size. These factors are only relative contraindications, however, as thoracentesis can still be safely performed by experienced operators using guidance technology such as ultrasonography.
A retrospective review of 1009 ultrasound-guided thoracenteses with risk factors of an international normalized ratio (INR) greater than 1.6, platelet values less than 50,000/μL, or both, reported an overall rate of hemorrhagic complication of 0.4%, with no difference between procedures performed with (n = 303) or without (n = 706) transfusion correction of coagulopathy or thrombocytopenia.6 A similar retrospective evaluation of 1076 ultrasound-guided thoracenteses, including 267 patients with an INR greater than 1.5 and 58 patients with a platelet count less than 50,000/μL, reported a 0% complication rate.7 Small case series have also demonstrated low hemorrhagic complication rates for thoracentesis in patients treated with clopidogrel8,9 and with increased bleeding risk from elevated INR (liver disease or warfarin therapy) and renal disease.10
Complications from pneumothorax can similarly be affected by patient- and operator-dependent risk factors. Meta-analysis of 24 studies including 6605 thoracenteses demonstrated an overall pneumothorax rate of 6.0%, with 34.1% requiring chest tube insertion.11 Lower pneumothorax rates were associated with the use of ultrasound guidance (odds ratio, 0.3; 95% confidence interval, 0.2-0.7). Experienced operators also had fewer pneumothorax complications, though this factor was not significant in the studies directly comparing this variable. Therapeutic thoracentesis and use of a larger-bore needle were also significantly correlated with pneumothorax, while mechanical ventilation had a nonsignificant trend towards increased risk.
Although there is no consensus on the volume of pleural fluid that may be safely removed, it is recommended not to remove more than 1.5 L during a procedure in order to avoid precipitating re-expansion pulmonary edema.2,12 However, re-expansion pulmonary edema rates remain low even when larger volumes are removed if the patient remains symptom-free during the procedure and pleural manometry pressure does not exceed –20 cm H2O.13 Patient symptoms alone, however, are neither a sensitive nor specific indicator that pleural pressures exceed –20 cm H2O.14 Use of excessive negative pressure during drainage, such as from a vacuum bottle, should also be avoided. Comparison of suction generated manually with a syringe versus a vacuum bottle suggests decreased complications with manual drainage, though the sample size in the supporting study was small relative to the infrequency of the complications being evaluated.15
Given the low morbidity and noninvasive nature of the procedure, serial large-volume thoracentesis remains a viable therapeutic intervention for patients who are unable or unwilling to undergo more invasive interventions, especially for patients with a slow fluid re-accumulation rate or who are anticipated to have limited survival. Unfortunately, many symptomatic effusions will recur within a short interval time span, which necessitates repeat procedures.16,17 Therefore, factors such as poor symptom control, patient inconvenience, recurrent procedural risk, and utilization of medical resources need to be considered as well.
Tunneled Pleural Catheter
Tunneled pleural catheters (TPCs) are a potentially permanent and minimally invasive therapy which allow intermittent drainage of pleural fluid (Figure 1). The catheter is tunneled under the skin to prevent infection. A polyester cuff attached to the catheter is positioned within the tunnel and induces fibrosis around the catheter, thereby securing the catheter in place. Placement can be performed under local anesthesia at the patient’s bedside or in an outpatient procedure space. Fluid can then be drained via specialized drainage bottles or bags by the patient, a family member, or visiting home nurse. The catheter can also be removed in the event of a complication or the development of spontaneous pleurodesis.
TPCs are an effective palliative management strategy for patients with recurrent effusions and are an efficacious alternative to pleurodesis.18-20 TPCs may be used in patients with poor prognosis or trapped lung or in those in whom prior pleurodesis has failed.21-23 Meta-analysis of 19 studies showed symptomatic improvement in 95.6% of patients, with development of spontaneous pleurodesis in 45.6% of patients (range, 11.8% to 76.4%) after an average of 52 days.24 However, most of the studies included in this analysis were retrospective case series. Development of spontaneous pleurodesis from TPC drainage in prospective, controlled trials has been considerably more modest, supporting a range of approximately 20% to 30% with routine drainage strategies.20,25-27 Spontaneous pleurodesis develops greater rapidity and frequency in patients undergoing daily drainage compared to every-other-day or symptom-directed drainage strategies.25,26 However, there is no appreciable improvement in quality of life scores with a specific drainage strategy. Small case series also demonstrate that TPC drainage may induce spontaneous pleurodesis in some patients initially presenting with trapped lung physiology.22
Catheter placement can be performed successfully in the vast majority of patients.28 Increased bleeding risk, significant malignancy-related involvement of the skin and chest wall, and pleural loculations can complicate TPC placement. TPC-related complications are relatively uncommon, but include pneumothorax, catheter malfunction and obstruction, and infections including soft tissue and pleural space infections.24 In a multicenter retrospective series of 1021 patients, only 4.9% developed a TPC-related pleural infection.29 Over 94% were successfully managed with antibiotic therapy, and the TPC was able to be preserved in 54%. Staphylococcus aureus was the most common causative organism and was identified in 48% of cases. Of note, spontaneous pleurodesis occurred in 62% of cases following a pleural space infection, which likely occurred as sequelae of the inflammatory nature of the infection. Retrospective analysis suggests that the risk of TPC-related infections is not substantially higher for patients with higher risks of immunosuppression from chemotherapy or hematologic malignancies.30,31 Tumor metastasis along the catheter tract is a rare occurrence (< 1%), but is most notable with mesothelioma, which has an incidence as high as 10%.24,32 In addition, development of pleural loculations can impede fluid drainage and relief of dyspnea. Intrapleural instillation of fibrinolytics can be used to improve drainage and improve symptom palliation.33
Pleurodesis
Pleurodesis obliterates the potential pleural space by inducing inflammation and fibrosis, resulting in adherence of the visceral and parietal pleura together. This process can be induced through mechanical abrasion of the pleural surface, introduction of chemical sclerosants, or from prolonged use of a chest tube. Chemical sclerosants are the most commonly used method for MPEs and are introduced through a chest tube or under visual guidance such as medical thoracoscopy or video-assisted thoracoscopic surgery (VATS). The pleurodesis process is thought to occur by induction of a systemic inflammatory response with localized deposition of fibrin.34 Activation of fibroblasts and successful pleurodesis have been correlated with higher basic fibroblast growth factor (bFGF) levels in pleural fluid.35 Increased tumor burden is associated with lower bFGF levels, suggesting a possible mechanism for reduced pleurodesis success in these cases. Corticosteroids may reduce the likelihood of pleurodesis due to a reduction of inflammation, as demonstrated in a rabbit model using talc and doxycycline.36,37 Animal data also suggests that use of nonsteroidal anti-inflammatory drugs may hinder the likelihood of successful pleurodesis, but this has not been observed in humans.38,39
Patients selected for pleurodesis should have significant symptom relief from large-volume removal of pleural fluid, good functional status, and evidence of full lung re-expansion after thoracentesis. Lack of visceral and parietal pleural apposition will prevent pleural adhesion from developing. As a result, trapped lung is associated with chemical pleurodesis failure and is an absolute contraindication to the procedure.4,5,12 The pleurodesis process typically requires 5 to 7 days, during which time the patient is hospitalized for chest tube drainage and pain control. When pleural fluid output diminishes, the chest tube is removed and the patient can be discharged. Modified protocols are now emerging which may shorten the required hospitalization associated with pleurodesis procedures.
Pleurodesis Agents
A variety of chemical sclerosants have been used for pleurodesis, including talc, bleomycin, tetracycline, doxycycline, iodopovidone, and mepacrine. Published data regarding these agents are heterogenous, with significant variability in reported outcomes. However, systematic review and meta-analysis suggests that talc is likely to have higher success rates compared to other agents or chest tube drainage alone for treatment of MPE.40,41
Additional factors have been shown to be associated with pleurodesis outcomes. Pleurodesis success is negatively associated with low pleural pH, with receiver operating curve thresholds of 7.28 to 7.34.42,43 Trapped lung, large bulky tumor lining the pleural surfaces, and elevated adenosine deaminase levels are also associated with poor pleurodesis outcomes.4,5,12,35,43 In contrast, pleural fluid output less than 200 mL per day and the presence of EGFR (epidermal growth factor receptor) mutation treated with targeted tyrosine kinase inhibitors are associated with successful pleurodesis.44,45
The most common complications associated with chemical pleurodesis are fever and pain. Other potential complications include soft tissue infections at the chest tube site and of the pleural space, arrhythmias, cardiac arrest, myocardial infarction, and hypotension. Doxycycline is commonly associated with greater pleuritic pain than talc. Acute respiratory distress syndrome (ARDS), acute pneumonitis, and respiratory failure have been described with talc pleurodesis. ARDS secondary to talc pleurodesis occurs in 1% to 9% of cases, though this may be related to the use of ungraded talc. A prospective description of 558 patients treated with large particle talc (> 5 μm) reported no occurrences of ARDS, suggesting the safety of graded large particle talc.46
Pleurodesis Methods
Chest tube thoracostomy is an inpatient procedure performed under local anesthesia or conscious sedation. It can be used for measured, intermittent drainage of large effusions for immediate symptom relief, as well as to demonstrate complete lung re-expansion prior to instillation of a chemical sclerosant. Pleurodesis using a chest tube is performed by instillation of a slurry created by mixing the sclerosing agent of choice with 50 to 100 mL of sterile saline. This slurry is instilled into the pleural cavity through the chest tube. The chest tube is clamped for 1 to 2 hours before being reconnected to suction. Intermittent rotation of the patient has not been shown to improve distribution of the sclerosant or result in better procedural outcomes.47,48 Typically, a 24F to 32F chest tube is used because of the concern about obstruction of smaller bore tubes by fibrin plugs. A noninferiority study comparing 12F to 24F chest tubes for talc pleurodesis demonstrated a higher procedure failure rate with the 12F tube (30% versus 24%) and failed to meet noninferiority criteria.39 However, larger caliber tubes are also associated with greater patient discomfort compared to smaller bore tubes.
Medical thoracoscopy and VATS are minimally invasive means to visualize the pleural space, obtain visually guided biopsy of the parietal pleura, perform lysis of adhesions, and introduce chemical sclerosants for pleurodesis (Figure 2). Medical thoracoscopy can be performed under local anesthesia with procedural sedation in an endoscopy suite or procedure room.
VATS has several distinct and clinically important differences. The equipment is slightly larger but otherwise similar in concept to rigid medical thoracoscopes. A greater number of diagnostic and therapeutic options, such as diagnostic biopsy of lung parenchyma and select hilar lymph nodes, are also possible. However, VATS requires surgical training and is performed in an operating room setting, which necessitates additional ancillary and logistical support. VATS also uses at least 2 trocar insertion sites, requires general anesthesia, and utilizes single-lung ventilation through a double-lumen endotracheal tube. Procedure-related complications for medical thoracoscopy and VATS include pneumothorax, subcutaneous emphysema, fever, and pain.49
Data comparing talc slurry versus talc poudrage are heterogenous, without clear advantage for either method. Reported rates of successful pleurodesis are generally in the range of 70% to 80% for both methods.19,20,40,50 There is a possible suggestion of benefit with talc poudrage compared to slurry, but data is lacking to support either as a definitive choice in current guidelines.12,51 An advantage of medical thoracoscopy or VATS is that pleural biopsy can be performed simultaneously, if necessary, thereby allowing both diagnostic and therapeutic interventions.52 Visualizing the thoracic cavity may also permit creation of optimal conditions for pleurodesis in select individuals by allowing access to loculated spaces and providing visual confirmation of complete drainage of pleural fluid and uniform distribution of the chemical sclerosant.
Other Surgical Interventions
Thoracotomy with decortication is rarely used as treatment of malignant effusions complicated by loculations or trapped lung due to the significantly increased procedural morbidity and mortality. Therefore, it is reserved for the limited population of patients in whom other therapeutic interventions have failed but who otherwise have significant symptoms with a long life expectancy. Mesothelioma is a specific situation in which variations of pleurectomy, such as radical pleurectomy with decortication, lung-sparing total pleurectomy, and extrapleural pneumonectomy (EPP), have been used as front-line therapy. The Mesothelioma and Radical Surgery (MARS) trial, the only randomized, controlled evaluation of EPP, demonstrated decreased median survival in patients treated by EPP compared to controls (14.4 months versus 19.5 months).53 EPP is also associated with high procedure-related morbidity and mortality rates of approximately 50% and 4% to 15%, respectively.54 While successful at achieving pleurodesis, use of EPP as a treatment for mesothelioma is now discouraged.53,55 Less invasive surgical approaches, such as pleurectomy with decortication, are able to palliate symptoms with significantly less operative risk.56
Management Considerations
Selection of Therapeutic Interventions
The ideal management strategy provides both immediate and long-term symptom palliation, has minimal associated morbidity and side effects, minimizes hospitalization time and clinic visits, avoids the risks and inconvenience of recurring procedures, is inexpensive, and minimizes utilization of medical resources. Unfortunately, no single palliation methodology fits these needs for all patients. When evaluating therapeutic options for patients with MPE, it is important to consider factors such as the severity of symptoms, fluid quantity, fluid re-accumulation rate, pleural physiology, functional status, overall prognosis, and anticipated response of the malignancy to therapy. One example management algorithm (Figure 4) demonstrates the impact of these variables on the appropriate treatment options. However, this is a simplified algorithm and the selected palliation strategy should be decided upon after shared decision-making between the patient and physician and should fit within the context of the patient’s desired goals of care. It is also crucial for patients to understand that these therapeutic interventions are palliative rather than curative.
When compared directly with pleurodesis, TPC provides similar control of symptoms but with a reduction in hospital length of stay by a median of 3.5 to 5.5 days.19,57 In a nonrandomized trial where patients chose palliation by TPC or talc pleurodesis, more TPC patients had a significant immediate improvement in quality of life and dyspnea after the first 7 days of therapy.58 This is reasonably attributed to the differences between the immediate relief from fluid drainage after TPC placement compared to the time required for pleural symphysis to occur with pleurodesis. However, control of dyspnea symptoms is similar between the 2 strategies after 6 weeks.19 Therefore, both TPC and pleurodesis strategies are viewed as first-line options for patients with expandable lung and no prior palliative interventions for MPE.59
Pleural adhesions and trapped lung also pose specific dilemmas. Pleural adhesions can create loculated fluid pockets, thereby complicating drainage by thoracentesis or TPC and hindering dispersal of pleurodesis agents. Adhesiolysis by medical thoracoscopy or VATS may be useful in these patients to free up the pleural space and improve efficacy of long-term drainage options or facilitate pleurodesis. Intrapleural administration of fibrinolytics, such as streptokinase and urokinase, has also been used for treatment of loculated effusions and may improve drainage of pleural fluid and lung re-expansion.60-63 However routine use of intrapleural fibrinolytics with pleurodesis has not been shown to be beneficial. In a randomized comparison using intrapleural urokinase prior to pleurodesis for patients with septated malignant pleural effusions, no difference in pleurodesis outcomes were identified.63 As a result, TPC is the preferred palliation approach for patients with trapped lung physiology.51,59
Combination Strategies
Combinations of different therapeutic interventions are being evaluated as a means for patients to achieve long-term benefits from pleurodesis while minimizing hospitalization time. One strategy using simultaneous treatment with thoracoscopic talc poudrage and insertion of a large-bore chest tube and TPC has been shown to permit early removal of the chest tube and discharge home using the TPC for continued daily pleural drainage. This “rapid pleurodesis” strategy has an 80% to 90% successful pleurodesis rate, permitting removal of the TPC at a median of 7 to 10 days.64,65 With this approach, median hospitalization length of stay was approximately 2 days. While there was no control arm in these early reports with limited sample sizes, the pleurodesis success rate and length of hospitalization compare favorably to other published studies. A prospective, randomized trial of TPC versus an outpatient regimen of talc slurry via TPC has also shown promise, with successful pleurodesis after 35 days in 43% of those treated with the combination of talc slurry and TPC compared to only 23% in those treated by TPC alone.27
Another novel approach to obtain the benefits of both TPC and pleurodesis strategies is the use of drug-eluting TPC to induce inflammation and promote adhesion of the visceral and parietal pleura. An early report of slow-release silver nitrate (AgNO3) –coated TPC demonstrated an encouraging 89% spontaneous pleurodesis rate after a median of 4 days in the small subgroup of patients with fully expandable lung.66 Device-related adverse events were relatively high at 24.6%, though only one was deemed a serious adverse event. Additional studies of these novel and combination strategies are ongoing at this time.
Costs
While cost of care is not a consideration in the decision-making for individual patients, it is important from a systems-based perspective. Upfront costs for pleurodesis are generally higher due to the facility and hospitalization costs, whereas TPC have ongoing costs for drainage bottles and supplies. In a prospective, randomized trial of TPC versus talc pleurodesis, there was no appreciable difference in overall costs between the 2 approaches.67 The cost of TPC was significantly less, however, for patients with a shorter survival of less than 14 weeks.
Readmissions
Subsequent hospitalization requirements beyond just the initial treatment for a MPE remains another significant consideration for this patient population. A prospective, randomized trial comparing TPC to talc pleurodesis demonstrated a reduction in total all-cause hospital stay for TPC, with a median all-cause hospitalization time of 10 days for patients treated with TPC compared to 12 days for the talc pleurodesis group.20 The primary difference in the number of hospitalization days was due to a difference in effusion-related hospital days (median 1 versus 4 days, respectively), which was primarily comprised of the initial hospitalization. In addition, fewer patients treated with TPC required subsequent ipsilateral invasive procedures (4.1% versus 22.5%, respectively). However, it is important to note that the majority of all-cause hospital days were not effusion-related, demonstrating that this population has a high utilization of acute inpatient services for other reasons related to their advanced malignancy. In a study of regional hospitals in the United States, 38.3% of patients admitted for a primary diagnosis of MPE were readmitted within 30 days.68 There was remarkably little variability in readmission rates among hospitals, despite differences in factors such as institution size, location, patient distribution, and potential practice differences. This suggests that utilization of palliation strategies for MPE are only one component related to hospitalization in this population. Even at the best performing hospitals, there are significant common drivers for readmission that are not addressed. Therefore, additional effort should be focused on addressing aspects of care beyond just the palliation of MPE that predispose this population to requiring frequent treatment in an acute care setting.
Conclusion
MPEs represent advanced stage disease and frequently adversely affect a patient’s quality of life. The treating clinician has access to a variety of therapeutic options, though no single intervention strategy is universally superior in all circumstances. Initial thoracentesis is important in evaluating whether removal of a large volume of fluid provides significant symptom relief and restores functional status. Both talc pleurodesis and TPC provide similar control of symptoms and are first-line approaches for symptomatic patients with MPE and fully expandable lungs. Pleurodesis is associated with greater procedure-related risk and length of hospitalization and is contraindicated in patients with trapped lung, but does not require long-term catheter care or disposable resources. Determination of the appropriate therapeutic management strategy requires careful evaluation of the patient’s clinical situation and informed discussion with the patient to make sure that the treatment plan fits within the context of their goals of medical care.
Malignant pleural effusion (MPE) is a common clinical problem in patients with advanced stage cancer. Each year in the United States, more than 150,000 individuals are diagnosed with MPE, and there are approximately 126,000 admissions for MPE.1-3 Providing effective therapeutic management remains a challenge, and currently available therapeutic interventions are palliative rather than curative. This article, the second in a 2-part review of MPE, focuses on the available management options.
Therapeutic Thoracentesis
Evaluation of pleural fluid cytology is a crucial step in the diagnosis and staging of disease. As a result, large-volume fluid removal is often the first therapeutic intervention for patients who present with symptomatic effusions. A patient’s clinical response to therapeutic thoracentesis dictates which additional therapeutic options are appropriate for palliation. Lack of symptom relief suggests that other comorbid conditions or trapped lung physiology may be the primary cause of the patient’s symptoms and discourages more invasive interventions. Radiographic evidence of lung re-expansion after fluid removal is also an important predictor of success for potential pleurodesis.4,5
There are no absolute contraindications to thoracentesis. However, caution should be used for patients with risk factors that may predispose to complications of pneumothorax and bleeding, such as coagulopathy, treatment with anticoagulation medications, thrombocytopenia, platelet dysfunction (eg, antiplatelet medications, uremia), positive pressure ventilation, and small effusion size. These factors are only relative contraindications, however, as thoracentesis can still be safely performed by experienced operators using guidance technology such as ultrasonography.
A retrospective review of 1009 ultrasound-guided thoracenteses with risk factors of an international normalized ratio (INR) greater than 1.6, platelet values less than 50,000/μL, or both, reported an overall rate of hemorrhagic complication of 0.4%, with no difference between procedures performed with (n = 303) or without (n = 706) transfusion correction of coagulopathy or thrombocytopenia.6 A similar retrospective evaluation of 1076 ultrasound-guided thoracenteses, including 267 patients with an INR greater than 1.5 and 58 patients with a platelet count less than 50,000/μL, reported a 0% complication rate.7 Small case series have also demonstrated low hemorrhagic complication rates for thoracentesis in patients treated with clopidogrel8,9 and with increased bleeding risk from elevated INR (liver disease or warfarin therapy) and renal disease.10
Complications from pneumothorax can similarly be affected by patient- and operator-dependent risk factors. Meta-analysis of 24 studies including 6605 thoracenteses demonstrated an overall pneumothorax rate of 6.0%, with 34.1% requiring chest tube insertion.11 Lower pneumothorax rates were associated with the use of ultrasound guidance (odds ratio, 0.3; 95% confidence interval, 0.2-0.7). Experienced operators also had fewer pneumothorax complications, though this factor was not significant in the studies directly comparing this variable. Therapeutic thoracentesis and use of a larger-bore needle were also significantly correlated with pneumothorax, while mechanical ventilation had a nonsignificant trend towards increased risk.
Although there is no consensus on the volume of pleural fluid that may be safely removed, it is recommended not to remove more than 1.5 L during a procedure in order to avoid precipitating re-expansion pulmonary edema.2,12 However, re-expansion pulmonary edema rates remain low even when larger volumes are removed if the patient remains symptom-free during the procedure and pleural manometry pressure does not exceed –20 cm H2O.13 Patient symptoms alone, however, are neither a sensitive nor specific indicator that pleural pressures exceed –20 cm H2O.14 Use of excessive negative pressure during drainage, such as from a vacuum bottle, should also be avoided. Comparison of suction generated manually with a syringe versus a vacuum bottle suggests decreased complications with manual drainage, though the sample size in the supporting study was small relative to the infrequency of the complications being evaluated.15
Given the low morbidity and noninvasive nature of the procedure, serial large-volume thoracentesis remains a viable therapeutic intervention for patients who are unable or unwilling to undergo more invasive interventions, especially for patients with a slow fluid re-accumulation rate or who are anticipated to have limited survival. Unfortunately, many symptomatic effusions will recur within a short interval time span, which necessitates repeat procedures.16,17 Therefore, factors such as poor symptom control, patient inconvenience, recurrent procedural risk, and utilization of medical resources need to be considered as well.
Tunneled Pleural Catheter
Tunneled pleural catheters (TPCs) are a potentially permanent and minimally invasive therapy which allow intermittent drainage of pleural fluid (Figure 1). The catheter is tunneled under the skin to prevent infection. A polyester cuff attached to the catheter is positioned within the tunnel and induces fibrosis around the catheter, thereby securing the catheter in place. Placement can be performed under local anesthesia at the patient’s bedside or in an outpatient procedure space. Fluid can then be drained via specialized drainage bottles or bags by the patient, a family member, or visiting home nurse. The catheter can also be removed in the event of a complication or the development of spontaneous pleurodesis.
TPCs are an effective palliative management strategy for patients with recurrent effusions and are an efficacious alternative to pleurodesis.18-20 TPCs may be used in patients with poor prognosis or trapped lung or in those in whom prior pleurodesis has failed.21-23 Meta-analysis of 19 studies showed symptomatic improvement in 95.6% of patients, with development of spontaneous pleurodesis in 45.6% of patients (range, 11.8% to 76.4%) after an average of 52 days.24 However, most of the studies included in this analysis were retrospective case series. Development of spontaneous pleurodesis from TPC drainage in prospective, controlled trials has been considerably more modest, supporting a range of approximately 20% to 30% with routine drainage strategies.20,25-27 Spontaneous pleurodesis develops greater rapidity and frequency in patients undergoing daily drainage compared to every-other-day or symptom-directed drainage strategies.25,26 However, there is no appreciable improvement in quality of life scores with a specific drainage strategy. Small case series also demonstrate that TPC drainage may induce spontaneous pleurodesis in some patients initially presenting with trapped lung physiology.22
Catheter placement can be performed successfully in the vast majority of patients.28 Increased bleeding risk, significant malignancy-related involvement of the skin and chest wall, and pleural loculations can complicate TPC placement. TPC-related complications are relatively uncommon, but include pneumothorax, catheter malfunction and obstruction, and infections including soft tissue and pleural space infections.24 In a multicenter retrospective series of 1021 patients, only 4.9% developed a TPC-related pleural infection.29 Over 94% were successfully managed with antibiotic therapy, and the TPC was able to be preserved in 54%. Staphylococcus aureus was the most common causative organism and was identified in 48% of cases. Of note, spontaneous pleurodesis occurred in 62% of cases following a pleural space infection, which likely occurred as sequelae of the inflammatory nature of the infection. Retrospective analysis suggests that the risk of TPC-related infections is not substantially higher for patients with higher risks of immunosuppression from chemotherapy or hematologic malignancies.30,31 Tumor metastasis along the catheter tract is a rare occurrence (< 1%), but is most notable with mesothelioma, which has an incidence as high as 10%.24,32 In addition, development of pleural loculations can impede fluid drainage and relief of dyspnea. Intrapleural instillation of fibrinolytics can be used to improve drainage and improve symptom palliation.33
Pleurodesis
Pleurodesis obliterates the potential pleural space by inducing inflammation and fibrosis, resulting in adherence of the visceral and parietal pleura together. This process can be induced through mechanical abrasion of the pleural surface, introduction of chemical sclerosants, or from prolonged use of a chest tube. Chemical sclerosants are the most commonly used method for MPEs and are introduced through a chest tube or under visual guidance such as medical thoracoscopy or video-assisted thoracoscopic surgery (VATS). The pleurodesis process is thought to occur by induction of a systemic inflammatory response with localized deposition of fibrin.34 Activation of fibroblasts and successful pleurodesis have been correlated with higher basic fibroblast growth factor (bFGF) levels in pleural fluid.35 Increased tumor burden is associated with lower bFGF levels, suggesting a possible mechanism for reduced pleurodesis success in these cases. Corticosteroids may reduce the likelihood of pleurodesis due to a reduction of inflammation, as demonstrated in a rabbit model using talc and doxycycline.36,37 Animal data also suggests that use of nonsteroidal anti-inflammatory drugs may hinder the likelihood of successful pleurodesis, but this has not been observed in humans.38,39
Patients selected for pleurodesis should have significant symptom relief from large-volume removal of pleural fluid, good functional status, and evidence of full lung re-expansion after thoracentesis. Lack of visceral and parietal pleural apposition will prevent pleural adhesion from developing. As a result, trapped lung is associated with chemical pleurodesis failure and is an absolute contraindication to the procedure.4,5,12 The pleurodesis process typically requires 5 to 7 days, during which time the patient is hospitalized for chest tube drainage and pain control. When pleural fluid output diminishes, the chest tube is removed and the patient can be discharged. Modified protocols are now emerging which may shorten the required hospitalization associated with pleurodesis procedures.
Pleurodesis Agents
A variety of chemical sclerosants have been used for pleurodesis, including talc, bleomycin, tetracycline, doxycycline, iodopovidone, and mepacrine. Published data regarding these agents are heterogenous, with significant variability in reported outcomes. However, systematic review and meta-analysis suggests that talc is likely to have higher success rates compared to other agents or chest tube drainage alone for treatment of MPE.40,41
Additional factors have been shown to be associated with pleurodesis outcomes. Pleurodesis success is negatively associated with low pleural pH, with receiver operating curve thresholds of 7.28 to 7.34.42,43 Trapped lung, large bulky tumor lining the pleural surfaces, and elevated adenosine deaminase levels are also associated with poor pleurodesis outcomes.4,5,12,35,43 In contrast, pleural fluid output less than 200 mL per day and the presence of EGFR (epidermal growth factor receptor) mutation treated with targeted tyrosine kinase inhibitors are associated with successful pleurodesis.44,45
The most common complications associated with chemical pleurodesis are fever and pain. Other potential complications include soft tissue infections at the chest tube site and of the pleural space, arrhythmias, cardiac arrest, myocardial infarction, and hypotension. Doxycycline is commonly associated with greater pleuritic pain than talc. Acute respiratory distress syndrome (ARDS), acute pneumonitis, and respiratory failure have been described with talc pleurodesis. ARDS secondary to talc pleurodesis occurs in 1% to 9% of cases, though this may be related to the use of ungraded talc. A prospective description of 558 patients treated with large particle talc (> 5 μm) reported no occurrences of ARDS, suggesting the safety of graded large particle talc.46
Pleurodesis Methods
Chest tube thoracostomy is an inpatient procedure performed under local anesthesia or conscious sedation. It can be used for measured, intermittent drainage of large effusions for immediate symptom relief, as well as to demonstrate complete lung re-expansion prior to instillation of a chemical sclerosant. Pleurodesis using a chest tube is performed by instillation of a slurry created by mixing the sclerosing agent of choice with 50 to 100 mL of sterile saline. This slurry is instilled into the pleural cavity through the chest tube. The chest tube is clamped for 1 to 2 hours before being reconnected to suction. Intermittent rotation of the patient has not been shown to improve distribution of the sclerosant or result in better procedural outcomes.47,48 Typically, a 24F to 32F chest tube is used because of the concern about obstruction of smaller bore tubes by fibrin plugs. A noninferiority study comparing 12F to 24F chest tubes for talc pleurodesis demonstrated a higher procedure failure rate with the 12F tube (30% versus 24%) and failed to meet noninferiority criteria.39 However, larger caliber tubes are also associated with greater patient discomfort compared to smaller bore tubes.
Medical thoracoscopy and VATS are minimally invasive means to visualize the pleural space, obtain visually guided biopsy of the parietal pleura, perform lysis of adhesions, and introduce chemical sclerosants for pleurodesis (Figure 2). Medical thoracoscopy can be performed under local anesthesia with procedural sedation in an endoscopy suite or procedure room.
VATS has several distinct and clinically important differences. The equipment is slightly larger but otherwise similar in concept to rigid medical thoracoscopes. A greater number of diagnostic and therapeutic options, such as diagnostic biopsy of lung parenchyma and select hilar lymph nodes, are also possible. However, VATS requires surgical training and is performed in an operating room setting, which necessitates additional ancillary and logistical support. VATS also uses at least 2 trocar insertion sites, requires general anesthesia, and utilizes single-lung ventilation through a double-lumen endotracheal tube. Procedure-related complications for medical thoracoscopy and VATS include pneumothorax, subcutaneous emphysema, fever, and pain.49
Data comparing talc slurry versus talc poudrage are heterogenous, without clear advantage for either method. Reported rates of successful pleurodesis are generally in the range of 70% to 80% for both methods.19,20,40,50 There is a possible suggestion of benefit with talc poudrage compared to slurry, but data is lacking to support either as a definitive choice in current guidelines.12,51 An advantage of medical thoracoscopy or VATS is that pleural biopsy can be performed simultaneously, if necessary, thereby allowing both diagnostic and therapeutic interventions.52 Visualizing the thoracic cavity may also permit creation of optimal conditions for pleurodesis in select individuals by allowing access to loculated spaces and providing visual confirmation of complete drainage of pleural fluid and uniform distribution of the chemical sclerosant.
Other Surgical Interventions
Thoracotomy with decortication is rarely used as treatment of malignant effusions complicated by loculations or trapped lung due to the significantly increased procedural morbidity and mortality. Therefore, it is reserved for the limited population of patients in whom other therapeutic interventions have failed but who otherwise have significant symptoms with a long life expectancy. Mesothelioma is a specific situation in which variations of pleurectomy, such as radical pleurectomy with decortication, lung-sparing total pleurectomy, and extrapleural pneumonectomy (EPP), have been used as front-line therapy. The Mesothelioma and Radical Surgery (MARS) trial, the only randomized, controlled evaluation of EPP, demonstrated decreased median survival in patients treated by EPP compared to controls (14.4 months versus 19.5 months).53 EPP is also associated with high procedure-related morbidity and mortality rates of approximately 50% and 4% to 15%, respectively.54 While successful at achieving pleurodesis, use of EPP as a treatment for mesothelioma is now discouraged.53,55 Less invasive surgical approaches, such as pleurectomy with decortication, are able to palliate symptoms with significantly less operative risk.56
Management Considerations
Selection of Therapeutic Interventions
The ideal management strategy provides both immediate and long-term symptom palliation, has minimal associated morbidity and side effects, minimizes hospitalization time and clinic visits, avoids the risks and inconvenience of recurring procedures, is inexpensive, and minimizes utilization of medical resources. Unfortunately, no single palliation methodology fits these needs for all patients. When evaluating therapeutic options for patients with MPE, it is important to consider factors such as the severity of symptoms, fluid quantity, fluid re-accumulation rate, pleural physiology, functional status, overall prognosis, and anticipated response of the malignancy to therapy. One example management algorithm (Figure 4) demonstrates the impact of these variables on the appropriate treatment options. However, this is a simplified algorithm and the selected palliation strategy should be decided upon after shared decision-making between the patient and physician and should fit within the context of the patient’s desired goals of care. It is also crucial for patients to understand that these therapeutic interventions are palliative rather than curative.
When compared directly with pleurodesis, TPC provides similar control of symptoms but with a reduction in hospital length of stay by a median of 3.5 to 5.5 days.19,57 In a nonrandomized trial where patients chose palliation by TPC or talc pleurodesis, more TPC patients had a significant immediate improvement in quality of life and dyspnea after the first 7 days of therapy.58 This is reasonably attributed to the differences between the immediate relief from fluid drainage after TPC placement compared to the time required for pleural symphysis to occur with pleurodesis. However, control of dyspnea symptoms is similar between the 2 strategies after 6 weeks.19 Therefore, both TPC and pleurodesis strategies are viewed as first-line options for patients with expandable lung and no prior palliative interventions for MPE.59
Pleural adhesions and trapped lung also pose specific dilemmas. Pleural adhesions can create loculated fluid pockets, thereby complicating drainage by thoracentesis or TPC and hindering dispersal of pleurodesis agents. Adhesiolysis by medical thoracoscopy or VATS may be useful in these patients to free up the pleural space and improve efficacy of long-term drainage options or facilitate pleurodesis. Intrapleural administration of fibrinolytics, such as streptokinase and urokinase, has also been used for treatment of loculated effusions and may improve drainage of pleural fluid and lung re-expansion.60-63 However routine use of intrapleural fibrinolytics with pleurodesis has not been shown to be beneficial. In a randomized comparison using intrapleural urokinase prior to pleurodesis for patients with septated malignant pleural effusions, no difference in pleurodesis outcomes were identified.63 As a result, TPC is the preferred palliation approach for patients with trapped lung physiology.51,59
Combination Strategies
Combinations of different therapeutic interventions are being evaluated as a means for patients to achieve long-term benefits from pleurodesis while minimizing hospitalization time. One strategy using simultaneous treatment with thoracoscopic talc poudrage and insertion of a large-bore chest tube and TPC has been shown to permit early removal of the chest tube and discharge home using the TPC for continued daily pleural drainage. This “rapid pleurodesis” strategy has an 80% to 90% successful pleurodesis rate, permitting removal of the TPC at a median of 7 to 10 days.64,65 With this approach, median hospitalization length of stay was approximately 2 days. While there was no control arm in these early reports with limited sample sizes, the pleurodesis success rate and length of hospitalization compare favorably to other published studies. A prospective, randomized trial of TPC versus an outpatient regimen of talc slurry via TPC has also shown promise, with successful pleurodesis after 35 days in 43% of those treated with the combination of talc slurry and TPC compared to only 23% in those treated by TPC alone.27
Another novel approach to obtain the benefits of both TPC and pleurodesis strategies is the use of drug-eluting TPC to induce inflammation and promote adhesion of the visceral and parietal pleura. An early report of slow-release silver nitrate (AgNO3) –coated TPC demonstrated an encouraging 89% spontaneous pleurodesis rate after a median of 4 days in the small subgroup of patients with fully expandable lung.66 Device-related adverse events were relatively high at 24.6%, though only one was deemed a serious adverse event. Additional studies of these novel and combination strategies are ongoing at this time.
Costs
While cost of care is not a consideration in the decision-making for individual patients, it is important from a systems-based perspective. Upfront costs for pleurodesis are generally higher due to the facility and hospitalization costs, whereas TPC have ongoing costs for drainage bottles and supplies. In a prospective, randomized trial of TPC versus talc pleurodesis, there was no appreciable difference in overall costs between the 2 approaches.67 The cost of TPC was significantly less, however, for patients with a shorter survival of less than 14 weeks.
Readmissions
Subsequent hospitalization requirements beyond just the initial treatment for a MPE remains another significant consideration for this patient population. A prospective, randomized trial comparing TPC to talc pleurodesis demonstrated a reduction in total all-cause hospital stay for TPC, with a median all-cause hospitalization time of 10 days for patients treated with TPC compared to 12 days for the talc pleurodesis group.20 The primary difference in the number of hospitalization days was due to a difference in effusion-related hospital days (median 1 versus 4 days, respectively), which was primarily comprised of the initial hospitalization. In addition, fewer patients treated with TPC required subsequent ipsilateral invasive procedures (4.1% versus 22.5%, respectively). However, it is important to note that the majority of all-cause hospital days were not effusion-related, demonstrating that this population has a high utilization of acute inpatient services for other reasons related to their advanced malignancy. In a study of regional hospitals in the United States, 38.3% of patients admitted for a primary diagnosis of MPE were readmitted within 30 days.68 There was remarkably little variability in readmission rates among hospitals, despite differences in factors such as institution size, location, patient distribution, and potential practice differences. This suggests that utilization of palliation strategies for MPE are only one component related to hospitalization in this population. Even at the best performing hospitals, there are significant common drivers for readmission that are not addressed. Therefore, additional effort should be focused on addressing aspects of care beyond just the palliation of MPE that predispose this population to requiring frequent treatment in an acute care setting.
Conclusion
MPEs represent advanced stage disease and frequently adversely affect a patient’s quality of life. The treating clinician has access to a variety of therapeutic options, though no single intervention strategy is universally superior in all circumstances. Initial thoracentesis is important in evaluating whether removal of a large volume of fluid provides significant symptom relief and restores functional status. Both talc pleurodesis and TPC provide similar control of symptoms and are first-line approaches for symptomatic patients with MPE and fully expandable lungs. Pleurodesis is associated with greater procedure-related risk and length of hospitalization and is contraindicated in patients with trapped lung, but does not require long-term catheter care or disposable resources. Determination of the appropriate therapeutic management strategy requires careful evaluation of the patient’s clinical situation and informed discussion with the patient to make sure that the treatment plan fits within the context of their goals of medical care.
1. Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J. 2001;18:402-419.
2. Society AT. Management of malignant pleural effusions. Am J Respir Crit Care Med. 2000;162:1987-2001.
3. Taghizadeh N, Fortin M, Tremblay A. US hospitalizations for malignant pleural effusions: data from the 2012 National Inpatient Sample. Chest. 2017;151:845-854.
4. Adler RH, Sayek I. Treatment of malignant pleural effusion: a method using tube thoracostomy and talc. Ann Thorac Surg. 1976;22:8-15.
5. Villanueva AG, Gray AW, Shahian DM, et al. Efficacy of short term versus long term tube thoracostomy drainage before tetracycline pleurodesis in the treatment of malignant pleural effusions. Thorax. 1994;49:23-25.
6. Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest. 2013;144:456-463.
7. Patel MD, Joshi SD. Abnormal preprocedural international normalized ratio and platelet counts are not associated with increased bleeding complications after ultrasound-guided thoracentesis. AJR Am J Roentgenol. 2011;197:W164-168.
8. Zalt MB, Bechara RI, Parks C, Berkowitz DM. Effect of routine clopidogrel use on bleeding complications after ultrasound-guided thoracentesis. J Bronchology Interv Pulmonol. 2012;19:284-287.
9. Mahmood K, Shofer SL, Moser BK, et al. Hemorrhagic complications of thoracentesis and small-bore chest tube placement in patients taking clopidogrel. Ann Am Thorac Soc. 2014;11:73-79.
10. Puchalski JT, Argento AC, Murphy TE, et al. The safety of thoracentesis in patients with uncorrected bleeding risk. Ann Am Thorac Soc. 2013;10:336-341.
11. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170:332-339.
12. Roberts ME, Neville E, Berrisford RG, et al; Group BPDG. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii32-40.
13. Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84:1656-1661.
14. Feller-Kopman D, Walkey A, Berkowitz D, Ernst A. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest. 2006;129:1556-1560.
15. Senitko M, Ray AS, Murphy TE, et al. Safety and tolerability of vacuum versus manual drainage during thoracentesis: a randomized trial. J Bronchology Interv Pulmonol. 2019;26:166-171.
16. Ost DE, Niu J, Zhao H, et al. Quality gaps and comparative effectiveness of management strategies for recurrent malignant pleural effusions. Chest. 2018;153:438-452.
17. Grosu HB, Molina S, Casal R, et al. Risk factors for pleural effusion recurrence in patients with malignancy. Respirology. 2019;24:76-82.
18. Musani AI, Haas AR, Seijo L, et al. Outpatient management of malignant pleural effusions with small-bore, tunneled pleural catheters. Respiration. 2004;71:559-566.
19. Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307:2383-2389.
20. Thomas R, Fysh ETH, Smith NA, et al. Effect of an indwelling pleural catheter vs talc pleurodesis on hospitalization days in patients with malignant pleural effusion: the AMPLE randomized clinical trial. JAMA. 2017;318:1903-1912.
21. Qureshi RA, Collinson SL, Powell RJ, et al. Management of malignant pleural effusion associated with trapped lung syndrome. Asian Cardiovasc Thorac Ann. 2008;16:120-123.
22. Efthymiou CA, Masudi T, Thorpe JA, Papagiannopoulos K. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg. 2009;9:961-964.
23. Sioris T, Sihvo E, Salo J, et al. Long-term indwelling pleural catheter (PleurX) for malignant pleural effusion unsuitable for talc pleurodesis. Eur J Surg Oncol. 2009;35:546-551.
24. Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med. 2011;26:70-76.
25. Wahidi MM, Reddy C, Yarmus L, et al. Randomized trial of pleural fluid drainage frequency in patients with malignant pleural effusions. the ASAP trial. Am J Respir Crit Care Med. 2017;195:1050-1057.
26. Muruganandan S, Azzopardi M, Fitzgerald DB, et al. Aggressive versus symptom-guided drainage of malignant pleural effusion via indwelling pleural catheters (AMPLE-2): an open-label randomised trial. Lancet Respir Med. 2018;6:671-680.
27. Bhatnagar R, Keenan EK, Morley AJ, et al. Outpatient talc administration by indwelling pleural catheter for malignant effusion. N Engl J Med. 2018;378:1313-1322.
28. Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest. 2006;129:362-368.
29. Fysh ETH, Tremblay A, Feller-Kopman D, et al. Clinical outcomes of indwelling pleural catheter-related pleural infections: an international multicenter study. Chest. 2013;144:1597-1602.
30. Morel A, Mishra E, Medley L, et al. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax. 2011;66:448-449.
31. Gilbert CR, Lee HJ, Skalski JH, et al. The use of indwelling tunneled pleural catheters for recurrent pleural effusions in patients with hematologic malignancies: a multicenter study. Chest. 2015;148:752-758.
32. Thomas R, Budgeon CA, Kuok YJ, et al. Catheter tract metastasis associated with indwelling pleural catheters. Chest. 2014;146:557-562.
33. Thomas R, Piccolo F, Miller D, et al. Intrapleural fibrinolysis for the treatment of indwelling pleural catheter-related symptomatic loculations: a multicenter observational study. Chest. 2015;148:746-751.
34. Antony VB. Pathogenesis of malignant pleural effusions and talc pleurodesis. Pneumologie. 1999;53:493-498.
35. Antony VB, Nasreen N, Mohammed KA, et al. Talc pleurodesis: basic fibroblast growth factor mediates pleural fibrosis. Chest. 2004;126:1522-1528.
36. Xie C, Teixeira LR, McGovern JP, Light RW. Systemic corticosteroids decrease the effectiveness of talc pleurodesis. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1441-1444.
37. Teixeira LR, Wu W, Chang DS, Light RW. The effect of corticosteroids on pleurodesis induced by doxycycline in rabbits. Chest. 2002;121:216-219.
38. Hunt I, Teh E, Southon R, Treasure T. Using non-steroidal anti-inflammatory drugs (NSAIDs) following pleurodesis. Interact Cardiovasc Thorac Surg. 2007;6:102-104.
39. Rahman NM, Pepperell J, Rehal S, et al. Effect of opioids vs NSAIDs and larger vs smaller chest tube size on pain control and pleurodesis efficacy among patients with malignant pleural effusion: the TIME1 randomized clinical trial. JAMA. 2015;314:2641-2653.
40. Clive AO, Jones HE, Bhatnagar R, Preston NJ, Maskell N. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev. 2016(5):CD010529.
41. Tan C, Sedrakyan A, Browne J, et al. The evidence on the effectiveness of management for malignant pleural effusion: a systematic review. Eur J Cardiothorac Surg. 2006;29:829-838.
42. Heffner JE, Nietert PJ, Barbieri C. Pleural fluid pH as a predictor of pleurodesis failure: analysis of primary data. Chest. 2000;117:87-95.
43. Yildirim H, Metintas M, Ak G, et al. Predictors of talc pleurodesis outcome in patients with malignant pleural effusions. Lung Cancer. 2008;62:139-144.
44. Aydogmus U, Ozdemir S, Cansever L, et al. Bedside talc pleurodesis for malignant pleural effusion: factors affecting success. Ann Surg Oncol. 2009;16:745-750.
45. Guo H, Wan Y, Tian G, et al. EGFR mutations predict a favorable outcome for malignant pleural effusion of lung adenocarcinoma with Tarceva therapy. Oncol Rep. 2012;27:880-890.
46. Janssen JP, Collier G, Astoul P, et al. Safety of pleurodesis with talc poudrage in malignant pleural effusion: a prospective cohort study. Lancet. 2007;369(9572):1535-1539.
47. Dryzer SR, Allen ML, Strange C, Sahn SA. A comparison of rotation and nonrotation in tetracycline pleurodesis. Chest. 1993;104:1763-1766.
48. Mager HJ, Maesen B, Verzijlbergen F, Schramel F. Distribution of talc suspension during treatment of malignant pleural effusion with talc pleurodesis. Lung Cancer. 2002;36:77-81.
49. Hsia D, Musani AI. Interventional pulmonology. Med Clin North Am. 2011;95:1095-1114.
50. Dresler CM, Olak J, Herndon JE, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest. 2005;127:909-915.
51. Bibby AC, Dorn P, Psallidas I, et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur Respir J. 2018;52(1).
52. Sakuraba M, Masuda K, Hebisawa A, et al. Diagnostic value of thoracoscopic pleural biopsy for pleurisy under local anaesthesia. ANZ J Surg. 2006;76:722-724.
53. Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011;12:763-772.
54. Zellos L, Jaklitsch MT, Al-Mourgi MA, Sugarbaker DJ. Complications of extrapleural pneumonectomy. Semin Thorac Cardiovasc Surg. 2007;19:355-359.
55. Zahid I, Sharif S, Routledge T, Scarci M. Is pleurectomy and decortication superior to palliative care in the treatment of malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg. 2011;12:812-817.
56. Soysal O, Karaoğlanoğlu N, Demiracan S, et al. Pleurectomy/decortication for palliation in malignant pleural mesothelioma: results of surgery. Eur J Cardiothorac Surg. 1997;11:210-213.
57. Putnam JB, Light RW, Rodriguez RM, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer. 1999;86:1992-1999.
58. Fysh ETH, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest. 2012;142:394-400.
59. Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med. 2018;198:839-849.
60. Davies CW, Traill ZC, Gleeson FV, Davies RJ. Intrapleural streptokinase in the management of malignant multiloculated pleural effusions. Chest. 1999;115:729-733.
61. Hsu LH, Soong TC, Feng AC, Liu MC. Intrapleural urokinase for the treatment of loculated malignant pleural effusions and trapped lungs in medically inoperable cancer patients. J Thorac Oncol. 2006;1:460-467.
62. Okur E, Baysungur V, Tezel C, et al. Streptokinase for malignant pleural effusions: a randomized controlled study. Asian Cardiovasc Thorac Ann. 2011;19:238-243.
63. Mishra EK, Clive AO, Wills GH, et al. Randomized controlled trial of urokinase versus placebo for nondraining malignant pleural effusion. Am J Respir Crit Care Med. 2018;197:502-508.
64. Reddy C, Ernst A, Lamb C, Feller-Kopman D. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest. 2011;139:1419-1423.
65. Krochmal R, Reddy C, Yarmus L, et al. Patient evaluation for rapid pleurodesis of malignant pleural effusions. J Thorac Dis. 2016;8:2538-2543.
66. Bhatnagar R, Zahan-Evans N, Kearney C, et al. A novel drug-eluting indwelling pleural catheter for the management of malignant effusions. Am J Respir Crit Care Med. 2018;197:136-138.
67. Penz ED, Mishra EK, Davies HE, Manns BJ, Miller RF, Rahman NM. Comparing cost of indwelling pleural catheter vs talc pleurodesis for malignant pleural effusion. Chest. 2014;146:991-1000.
68. Yang TS, Hsia DW, Chang DW. Patient- and hospital-level factors associated with readmission for malignant pleural effusion. J Oncol Pract. 2018;14:e547-e556.
1. Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J. 2001;18:402-419.
2. Society AT. Management of malignant pleural effusions. Am J Respir Crit Care Med. 2000;162:1987-2001.
3. Taghizadeh N, Fortin M, Tremblay A. US hospitalizations for malignant pleural effusions: data from the 2012 National Inpatient Sample. Chest. 2017;151:845-854.
4. Adler RH, Sayek I. Treatment of malignant pleural effusion: a method using tube thoracostomy and talc. Ann Thorac Surg. 1976;22:8-15.
5. Villanueva AG, Gray AW, Shahian DM, et al. Efficacy of short term versus long term tube thoracostomy drainage before tetracycline pleurodesis in the treatment of malignant pleural effusions. Thorax. 1994;49:23-25.
6. Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest. 2013;144:456-463.
7. Patel MD, Joshi SD. Abnormal preprocedural international normalized ratio and platelet counts are not associated with increased bleeding complications after ultrasound-guided thoracentesis. AJR Am J Roentgenol. 2011;197:W164-168.
8. Zalt MB, Bechara RI, Parks C, Berkowitz DM. Effect of routine clopidogrel use on bleeding complications after ultrasound-guided thoracentesis. J Bronchology Interv Pulmonol. 2012;19:284-287.
9. Mahmood K, Shofer SL, Moser BK, et al. Hemorrhagic complications of thoracentesis and small-bore chest tube placement in patients taking clopidogrel. Ann Am Thorac Soc. 2014;11:73-79.
10. Puchalski JT, Argento AC, Murphy TE, et al. The safety of thoracentesis in patients with uncorrected bleeding risk. Ann Am Thorac Soc. 2013;10:336-341.
11. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170:332-339.
12. Roberts ME, Neville E, Berrisford RG, et al; Group BPDG. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii32-40.
13. Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84:1656-1661.
14. Feller-Kopman D, Walkey A, Berkowitz D, Ernst A. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest. 2006;129:1556-1560.
15. Senitko M, Ray AS, Murphy TE, et al. Safety and tolerability of vacuum versus manual drainage during thoracentesis: a randomized trial. J Bronchology Interv Pulmonol. 2019;26:166-171.
16. Ost DE, Niu J, Zhao H, et al. Quality gaps and comparative effectiveness of management strategies for recurrent malignant pleural effusions. Chest. 2018;153:438-452.
17. Grosu HB, Molina S, Casal R, et al. Risk factors for pleural effusion recurrence in patients with malignancy. Respirology. 2019;24:76-82.
18. Musani AI, Haas AR, Seijo L, et al. Outpatient management of malignant pleural effusions with small-bore, tunneled pleural catheters. Respiration. 2004;71:559-566.
19. Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307:2383-2389.
20. Thomas R, Fysh ETH, Smith NA, et al. Effect of an indwelling pleural catheter vs talc pleurodesis on hospitalization days in patients with malignant pleural effusion: the AMPLE randomized clinical trial. JAMA. 2017;318:1903-1912.
21. Qureshi RA, Collinson SL, Powell RJ, et al. Management of malignant pleural effusion associated with trapped lung syndrome. Asian Cardiovasc Thorac Ann. 2008;16:120-123.
22. Efthymiou CA, Masudi T, Thorpe JA, Papagiannopoulos K. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg. 2009;9:961-964.
23. Sioris T, Sihvo E, Salo J, et al. Long-term indwelling pleural catheter (PleurX) for malignant pleural effusion unsuitable for talc pleurodesis. Eur J Surg Oncol. 2009;35:546-551.
24. Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med. 2011;26:70-76.
25. Wahidi MM, Reddy C, Yarmus L, et al. Randomized trial of pleural fluid drainage frequency in patients with malignant pleural effusions. the ASAP trial. Am J Respir Crit Care Med. 2017;195:1050-1057.
26. Muruganandan S, Azzopardi M, Fitzgerald DB, et al. Aggressive versus symptom-guided drainage of malignant pleural effusion via indwelling pleural catheters (AMPLE-2): an open-label randomised trial. Lancet Respir Med. 2018;6:671-680.
27. Bhatnagar R, Keenan EK, Morley AJ, et al. Outpatient talc administration by indwelling pleural catheter for malignant effusion. N Engl J Med. 2018;378:1313-1322.
28. Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest. 2006;129:362-368.
29. Fysh ETH, Tremblay A, Feller-Kopman D, et al. Clinical outcomes of indwelling pleural catheter-related pleural infections: an international multicenter study. Chest. 2013;144:1597-1602.
30. Morel A, Mishra E, Medley L, et al. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax. 2011;66:448-449.
31. Gilbert CR, Lee HJ, Skalski JH, et al. The use of indwelling tunneled pleural catheters for recurrent pleural effusions in patients with hematologic malignancies: a multicenter study. Chest. 2015;148:752-758.
32. Thomas R, Budgeon CA, Kuok YJ, et al. Catheter tract metastasis associated with indwelling pleural catheters. Chest. 2014;146:557-562.
33. Thomas R, Piccolo F, Miller D, et al. Intrapleural fibrinolysis for the treatment of indwelling pleural catheter-related symptomatic loculations: a multicenter observational study. Chest. 2015;148:746-751.
34. Antony VB. Pathogenesis of malignant pleural effusions and talc pleurodesis. Pneumologie. 1999;53:493-498.
35. Antony VB, Nasreen N, Mohammed KA, et al. Talc pleurodesis: basic fibroblast growth factor mediates pleural fibrosis. Chest. 2004;126:1522-1528.
36. Xie C, Teixeira LR, McGovern JP, Light RW. Systemic corticosteroids decrease the effectiveness of talc pleurodesis. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1441-1444.
37. Teixeira LR, Wu W, Chang DS, Light RW. The effect of corticosteroids on pleurodesis induced by doxycycline in rabbits. Chest. 2002;121:216-219.
38. Hunt I, Teh E, Southon R, Treasure T. Using non-steroidal anti-inflammatory drugs (NSAIDs) following pleurodesis. Interact Cardiovasc Thorac Surg. 2007;6:102-104.
39. Rahman NM, Pepperell J, Rehal S, et al. Effect of opioids vs NSAIDs and larger vs smaller chest tube size on pain control and pleurodesis efficacy among patients with malignant pleural effusion: the TIME1 randomized clinical trial. JAMA. 2015;314:2641-2653.
40. Clive AO, Jones HE, Bhatnagar R, Preston NJ, Maskell N. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev. 2016(5):CD010529.
41. Tan C, Sedrakyan A, Browne J, et al. The evidence on the effectiveness of management for malignant pleural effusion: a systematic review. Eur J Cardiothorac Surg. 2006;29:829-838.
42. Heffner JE, Nietert PJ, Barbieri C. Pleural fluid pH as a predictor of pleurodesis failure: analysis of primary data. Chest. 2000;117:87-95.
43. Yildirim H, Metintas M, Ak G, et al. Predictors of talc pleurodesis outcome in patients with malignant pleural effusions. Lung Cancer. 2008;62:139-144.
44. Aydogmus U, Ozdemir S, Cansever L, et al. Bedside talc pleurodesis for malignant pleural effusion: factors affecting success. Ann Surg Oncol. 2009;16:745-750.
45. Guo H, Wan Y, Tian G, et al. EGFR mutations predict a favorable outcome for malignant pleural effusion of lung adenocarcinoma with Tarceva therapy. Oncol Rep. 2012;27:880-890.
46. Janssen JP, Collier G, Astoul P, et al. Safety of pleurodesis with talc poudrage in malignant pleural effusion: a prospective cohort study. Lancet. 2007;369(9572):1535-1539.
47. Dryzer SR, Allen ML, Strange C, Sahn SA. A comparison of rotation and nonrotation in tetracycline pleurodesis. Chest. 1993;104:1763-1766.
48. Mager HJ, Maesen B, Verzijlbergen F, Schramel F. Distribution of talc suspension during treatment of malignant pleural effusion with talc pleurodesis. Lung Cancer. 2002;36:77-81.
49. Hsia D, Musani AI. Interventional pulmonology. Med Clin North Am. 2011;95:1095-1114.
50. Dresler CM, Olak J, Herndon JE, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest. 2005;127:909-915.
51. Bibby AC, Dorn P, Psallidas I, et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur Respir J. 2018;52(1).
52. Sakuraba M, Masuda K, Hebisawa A, et al. Diagnostic value of thoracoscopic pleural biopsy for pleurisy under local anaesthesia. ANZ J Surg. 2006;76:722-724.
53. Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011;12:763-772.
54. Zellos L, Jaklitsch MT, Al-Mourgi MA, Sugarbaker DJ. Complications of extrapleural pneumonectomy. Semin Thorac Cardiovasc Surg. 2007;19:355-359.
55. Zahid I, Sharif S, Routledge T, Scarci M. Is pleurectomy and decortication superior to palliative care in the treatment of malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg. 2011;12:812-817.
56. Soysal O, Karaoğlanoğlu N, Demiracan S, et al. Pleurectomy/decortication for palliation in malignant pleural mesothelioma: results of surgery. Eur J Cardiothorac Surg. 1997;11:210-213.
57. Putnam JB, Light RW, Rodriguez RM, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer. 1999;86:1992-1999.
58. Fysh ETH, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest. 2012;142:394-400.
59. Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med. 2018;198:839-849.
60. Davies CW, Traill ZC, Gleeson FV, Davies RJ. Intrapleural streptokinase in the management of malignant multiloculated pleural effusions. Chest. 1999;115:729-733.
61. Hsu LH, Soong TC, Feng AC, Liu MC. Intrapleural urokinase for the treatment of loculated malignant pleural effusions and trapped lungs in medically inoperable cancer patients. J Thorac Oncol. 2006;1:460-467.
62. Okur E, Baysungur V, Tezel C, et al. Streptokinase for malignant pleural effusions: a randomized controlled study. Asian Cardiovasc Thorac Ann. 2011;19:238-243.
63. Mishra EK, Clive AO, Wills GH, et al. Randomized controlled trial of urokinase versus placebo for nondraining malignant pleural effusion. Am J Respir Crit Care Med. 2018;197:502-508.
64. Reddy C, Ernst A, Lamb C, Feller-Kopman D. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest. 2011;139:1419-1423.
65. Krochmal R, Reddy C, Yarmus L, et al. Patient evaluation for rapid pleurodesis of malignant pleural effusions. J Thorac Dis. 2016;8:2538-2543.
66. Bhatnagar R, Zahan-Evans N, Kearney C, et al. A novel drug-eluting indwelling pleural catheter for the management of malignant effusions. Am J Respir Crit Care Med. 2018;197:136-138.
67. Penz ED, Mishra EK, Davies HE, Manns BJ, Miller RF, Rahman NM. Comparing cost of indwelling pleural catheter vs talc pleurodesis for malignant pleural effusion. Chest. 2014;146:991-1000.
68. Yang TS, Hsia DW, Chang DW. Patient- and hospital-level factors associated with readmission for malignant pleural effusion. J Oncol Pract. 2018;14:e547-e556.