User login
FDA Removes Harmful Chemicals From Food Packaging
Issued on February 28, 2024, “this means the major source of dietary exposure to PFAS from food packaging like fast-food wrappers, microwave popcorn bags, take-out paperboard containers, and pet food bags is being eliminated,” the FDA said in a statement.
In 2020, the FDA had secured commitments from manufacturers to stop selling products containing PFAS used in the food packaging for grease-proofing. “Today’s announcement marks the fulfillment of these voluntary commitments,” according to the agency.
PFAS, a class of thousands of chemicals also called “forever chemicals” are widely used in consumer and industrial products. People may be exposed via contaminated food packaging (although perhaps no longer in the United States) or occupationally. Studies have found that some PFAS disrupt hormones including estrogen and testosterone, whereas others may impair thyroid function.
Endocrine Society Report Sounds the Alarm About PFAS and Others
The FDA’s announcement came just 2 days after the Endocrine Society issued a new alarm about the human health dangers from environmental EDCs including PFAS in a report covering the latest science.
“Endocrine disrupting chemicals” are individual substances or mixtures that can interfere with natural hormonal function, leading to disease or even death. Many are ubiquitous in the modern environment and contribute to a wide range of human diseases.
The new report Endocrine Disrupting Chemicals: Threats to Human Health was issued jointly with the International Pollutants Elimination Network (IPEN), a global advocacy organization. It’s an update to the Endocrine Society’s 2015 report, providing new data on the endocrine-disrupting substances previously covered and adding four EDCs not discussed in that document: Pesticides, plastics, PFAS, and children’s products containing arsenic.
At a briefing held during the United Nations Environment Assembly meeting in Nairobi, Kenya, last week, the new report’s lead author Andrea C. Gore, PhD, of the University of Texas at Austin, noted, “A well-established body of scientific research indicates that endocrine-disrupting chemicals that are part of our daily lives are making us more susceptible to reproductive disorders, cancer, diabetes, obesity, heart disease, and other serious health conditions.”
Added Dr. Gore, who is also a member of the Endocrine Society’s Board of Directors, “These chemicals pose particularly serious risks to pregnant women and children. Now is the time for the UN Environment Assembly and other global policymakers to take action to address this threat to public health.”
While the science has been emerging rapidly, global and national chemical control policies haven’t kept up, the authors said. Of particular concern is that EDCs behave differently from other chemicals in many ways, including that even very low-dose exposures can pose health threats, but policies thus far haven’t dealt with that aspect.
Moreover, “the effects of low doses cannot be predicted by the effects observed at high doses. This means there may be no safe dose for exposure to EDCs,” according to the report.
Exposures can come from household products, including furniture, toys, and food packages, as well as electronics building materials and cosmetics. These chemicals are also in the outdoor environment, via pesticides, air pollution, and industrial waste.
“IPEN and the Endocrine Society call for chemical regulations based on the most modern scientific understanding of how hormones act and how EDCs can perturb these actions. We work to educate policy makers in global, regional, and national government assemblies and help ensure that regulations correlate with current scientific understanding,” they said in the report.
New Data on Four Classes of EDCs
Chapters of the report summarized the latest information about the science of EDCs and their links to endocrine disease and real-world exposure. It included a special section about “EDCs throughout the plastics life cycle” and a summary of the links between EDCs and climate change.
The report reviewed three pesticides, including the world’s most heavily applied herbicide, glycophosphate. Exposures can occur directly from the air, water, dust, and food residues. Recent data linked glycophosphate to adverse reproductive health outcomes.
Two toxic plastic chemicals, phthalates and bisphenols, are present in personal care products, among others. Emerging evidence links them with impaired neurodevelopment, leading to impaired cognitive function, learning, attention, and impulsivity.
Arsenic has long been linked to human health conditions including cancer, but more recent evidence finds it can disrupt multiple endocrine systems and lead to metabolic conditions including diabetes, reproductive dysfunction, and cardiovascular and neurocognitive conditions.
The special section about plastics noted that they are made from fossil fuels and chemicals, including many toxic substances that are known or suspected EDCs. People who live near plastic production facilities or waste dumps may be at greatest risk, but anyone can be exposed using any plastic product. Plastic waste disposal is increasingly problematic and often foisted on lower- and middle-income countries.
‘Additional Education and Awareness-Raising Among Stakeholders Remain Necessary’
Policies aimed at reducing human health risks from EDCs have included the 2022 Plastics Treaty, a resolution adopted by 175 countries at the United Nations Environmental Assembly that “may be a significant step toward global control of plastics and elimination of threats from exposures to EDCs in plastics,” the report said.
The authors added, “While significant progress has been made in recent years connecting scientific advances on EDCs with health-protective policies, additional education and awareness-raising among stakeholders remain necessary to achieve a safer and more sustainable environment that minimizes exposure to these harmful chemicals.”
The document was produced with financial contributions from the Government of Sweden, the Tides Foundation, Passport Foundation, and other donors.
A version of this article appeared on Medscape.com.
Issued on February 28, 2024, “this means the major source of dietary exposure to PFAS from food packaging like fast-food wrappers, microwave popcorn bags, take-out paperboard containers, and pet food bags is being eliminated,” the FDA said in a statement.
In 2020, the FDA had secured commitments from manufacturers to stop selling products containing PFAS used in the food packaging for grease-proofing. “Today’s announcement marks the fulfillment of these voluntary commitments,” according to the agency.
PFAS, a class of thousands of chemicals also called “forever chemicals” are widely used in consumer and industrial products. People may be exposed via contaminated food packaging (although perhaps no longer in the United States) or occupationally. Studies have found that some PFAS disrupt hormones including estrogen and testosterone, whereas others may impair thyroid function.
Endocrine Society Report Sounds the Alarm About PFAS and Others
The FDA’s announcement came just 2 days after the Endocrine Society issued a new alarm about the human health dangers from environmental EDCs including PFAS in a report covering the latest science.
“Endocrine disrupting chemicals” are individual substances or mixtures that can interfere with natural hormonal function, leading to disease or even death. Many are ubiquitous in the modern environment and contribute to a wide range of human diseases.
The new report Endocrine Disrupting Chemicals: Threats to Human Health was issued jointly with the International Pollutants Elimination Network (IPEN), a global advocacy organization. It’s an update to the Endocrine Society’s 2015 report, providing new data on the endocrine-disrupting substances previously covered and adding four EDCs not discussed in that document: Pesticides, plastics, PFAS, and children’s products containing arsenic.
At a briefing held during the United Nations Environment Assembly meeting in Nairobi, Kenya, last week, the new report’s lead author Andrea C. Gore, PhD, of the University of Texas at Austin, noted, “A well-established body of scientific research indicates that endocrine-disrupting chemicals that are part of our daily lives are making us more susceptible to reproductive disorders, cancer, diabetes, obesity, heart disease, and other serious health conditions.”
Added Dr. Gore, who is also a member of the Endocrine Society’s Board of Directors, “These chemicals pose particularly serious risks to pregnant women and children. Now is the time for the UN Environment Assembly and other global policymakers to take action to address this threat to public health.”
While the science has been emerging rapidly, global and national chemical control policies haven’t kept up, the authors said. Of particular concern is that EDCs behave differently from other chemicals in many ways, including that even very low-dose exposures can pose health threats, but policies thus far haven’t dealt with that aspect.
Moreover, “the effects of low doses cannot be predicted by the effects observed at high doses. This means there may be no safe dose for exposure to EDCs,” according to the report.
Exposures can come from household products, including furniture, toys, and food packages, as well as electronics building materials and cosmetics. These chemicals are also in the outdoor environment, via pesticides, air pollution, and industrial waste.
“IPEN and the Endocrine Society call for chemical regulations based on the most modern scientific understanding of how hormones act and how EDCs can perturb these actions. We work to educate policy makers in global, regional, and national government assemblies and help ensure that regulations correlate with current scientific understanding,” they said in the report.
New Data on Four Classes of EDCs
Chapters of the report summarized the latest information about the science of EDCs and their links to endocrine disease and real-world exposure. It included a special section about “EDCs throughout the plastics life cycle” and a summary of the links between EDCs and climate change.
The report reviewed three pesticides, including the world’s most heavily applied herbicide, glycophosphate. Exposures can occur directly from the air, water, dust, and food residues. Recent data linked glycophosphate to adverse reproductive health outcomes.
Two toxic plastic chemicals, phthalates and bisphenols, are present in personal care products, among others. Emerging evidence links them with impaired neurodevelopment, leading to impaired cognitive function, learning, attention, and impulsivity.
Arsenic has long been linked to human health conditions including cancer, but more recent evidence finds it can disrupt multiple endocrine systems and lead to metabolic conditions including diabetes, reproductive dysfunction, and cardiovascular and neurocognitive conditions.
The special section about plastics noted that they are made from fossil fuels and chemicals, including many toxic substances that are known or suspected EDCs. People who live near plastic production facilities or waste dumps may be at greatest risk, but anyone can be exposed using any plastic product. Plastic waste disposal is increasingly problematic and often foisted on lower- and middle-income countries.
‘Additional Education and Awareness-Raising Among Stakeholders Remain Necessary’
Policies aimed at reducing human health risks from EDCs have included the 2022 Plastics Treaty, a resolution adopted by 175 countries at the United Nations Environmental Assembly that “may be a significant step toward global control of plastics and elimination of threats from exposures to EDCs in plastics,” the report said.
The authors added, “While significant progress has been made in recent years connecting scientific advances on EDCs with health-protective policies, additional education and awareness-raising among stakeholders remain necessary to achieve a safer and more sustainable environment that minimizes exposure to these harmful chemicals.”
The document was produced with financial contributions from the Government of Sweden, the Tides Foundation, Passport Foundation, and other donors.
A version of this article appeared on Medscape.com.
Issued on February 28, 2024, “this means the major source of dietary exposure to PFAS from food packaging like fast-food wrappers, microwave popcorn bags, take-out paperboard containers, and pet food bags is being eliminated,” the FDA said in a statement.
In 2020, the FDA had secured commitments from manufacturers to stop selling products containing PFAS used in the food packaging for grease-proofing. “Today’s announcement marks the fulfillment of these voluntary commitments,” according to the agency.
PFAS, a class of thousands of chemicals also called “forever chemicals” are widely used in consumer and industrial products. People may be exposed via contaminated food packaging (although perhaps no longer in the United States) or occupationally. Studies have found that some PFAS disrupt hormones including estrogen and testosterone, whereas others may impair thyroid function.
Endocrine Society Report Sounds the Alarm About PFAS and Others
The FDA’s announcement came just 2 days after the Endocrine Society issued a new alarm about the human health dangers from environmental EDCs including PFAS in a report covering the latest science.
“Endocrine disrupting chemicals” are individual substances or mixtures that can interfere with natural hormonal function, leading to disease or even death. Many are ubiquitous in the modern environment and contribute to a wide range of human diseases.
The new report Endocrine Disrupting Chemicals: Threats to Human Health was issued jointly with the International Pollutants Elimination Network (IPEN), a global advocacy organization. It’s an update to the Endocrine Society’s 2015 report, providing new data on the endocrine-disrupting substances previously covered and adding four EDCs not discussed in that document: Pesticides, plastics, PFAS, and children’s products containing arsenic.
At a briefing held during the United Nations Environment Assembly meeting in Nairobi, Kenya, last week, the new report’s lead author Andrea C. Gore, PhD, of the University of Texas at Austin, noted, “A well-established body of scientific research indicates that endocrine-disrupting chemicals that are part of our daily lives are making us more susceptible to reproductive disorders, cancer, diabetes, obesity, heart disease, and other serious health conditions.”
Added Dr. Gore, who is also a member of the Endocrine Society’s Board of Directors, “These chemicals pose particularly serious risks to pregnant women and children. Now is the time for the UN Environment Assembly and other global policymakers to take action to address this threat to public health.”
While the science has been emerging rapidly, global and national chemical control policies haven’t kept up, the authors said. Of particular concern is that EDCs behave differently from other chemicals in many ways, including that even very low-dose exposures can pose health threats, but policies thus far haven’t dealt with that aspect.
Moreover, “the effects of low doses cannot be predicted by the effects observed at high doses. This means there may be no safe dose for exposure to EDCs,” according to the report.
Exposures can come from household products, including furniture, toys, and food packages, as well as electronics building materials and cosmetics. These chemicals are also in the outdoor environment, via pesticides, air pollution, and industrial waste.
“IPEN and the Endocrine Society call for chemical regulations based on the most modern scientific understanding of how hormones act and how EDCs can perturb these actions. We work to educate policy makers in global, regional, and national government assemblies and help ensure that regulations correlate with current scientific understanding,” they said in the report.
New Data on Four Classes of EDCs
Chapters of the report summarized the latest information about the science of EDCs and their links to endocrine disease and real-world exposure. It included a special section about “EDCs throughout the plastics life cycle” and a summary of the links between EDCs and climate change.
The report reviewed three pesticides, including the world’s most heavily applied herbicide, glycophosphate. Exposures can occur directly from the air, water, dust, and food residues. Recent data linked glycophosphate to adverse reproductive health outcomes.
Two toxic plastic chemicals, phthalates and bisphenols, are present in personal care products, among others. Emerging evidence links them with impaired neurodevelopment, leading to impaired cognitive function, learning, attention, and impulsivity.
Arsenic has long been linked to human health conditions including cancer, but more recent evidence finds it can disrupt multiple endocrine systems and lead to metabolic conditions including diabetes, reproductive dysfunction, and cardiovascular and neurocognitive conditions.
The special section about plastics noted that they are made from fossil fuels and chemicals, including many toxic substances that are known or suspected EDCs. People who live near plastic production facilities or waste dumps may be at greatest risk, but anyone can be exposed using any plastic product. Plastic waste disposal is increasingly problematic and often foisted on lower- and middle-income countries.
‘Additional Education and Awareness-Raising Among Stakeholders Remain Necessary’
Policies aimed at reducing human health risks from EDCs have included the 2022 Plastics Treaty, a resolution adopted by 175 countries at the United Nations Environmental Assembly that “may be a significant step toward global control of plastics and elimination of threats from exposures to EDCs in plastics,” the report said.
The authors added, “While significant progress has been made in recent years connecting scientific advances on EDCs with health-protective policies, additional education and awareness-raising among stakeholders remain necessary to achieve a safer and more sustainable environment that minimizes exposure to these harmful chemicals.”
The document was produced with financial contributions from the Government of Sweden, the Tides Foundation, Passport Foundation, and other donors.
A version of this article appeared on Medscape.com.
Testosterone Replacement Shows No Benefit in Diabetes Prevention
Testosterone replacement therapy in the treatment of hypogonadism showed no benefit in slowing the progression of prediabetes or diabetes, contrary to previous evidence that suggested potential improvements in insulin sensitivity and metabolism.
“The findings of this study suggest that testosterone replacement therapy alone should not be used as a therapeutic intervention to prevent or treat diabetes in men with hypogonadism,” reported the authors of research published this month in JAMA Internal Medicine.
The suggestion that testosterone replacement could prevent or slow diabetes stems from numerous studies linking testosterone deficiency to a host of adverse effects that include increases in insulin resistance and an increased risk for prediabetes and type 2 diabetes.
Furthermore, one recent uncontrolled study showed a lower rate of progression from prediabetes to diabetes in testosterone-treated vs untreated men with hypogonadism.
But with no known randomized clinical trials evaluating the effects of testosterone on diabetes in the absence of a concurrent lifestyle intervention, Shalender Bhasin, MB, of the Research Program in Men’s Health: Aging and Metabolism, at Brigham and Women’s Hospital, Harvard Medical School, Boston, and colleagues conducted a substudy of the randomized TRAVERSE trial, which was conducted at 316 sites in the United States.
“We hypothesized that testosterone replacement therapy for men with hypogonadism and prediabetes would be associated with a significantly lower rate of progression to diabetes,” they wrote.
In the study, named the TRAVERSE Diabetes Study, 5204 participants aged between 40 and 85 years with hypogonadism as well as prediabetes (n = 1175) or diabetes (n = 3880) were randomized 1:1 to receive treatment either with 1.62% testosterone gel or placebo gel.
The participants had a mean age of 63.2 years, and the mean A1c among those with prediabetes was 5.8%.
For the primary outcome, the risk for progression to diabetes did not differ significantly between the testosterone-treated and placebo groups at 6 months (0.7% vs 1.4%), 12 months (7.8% vs 10.7%), 24 months (10.1% vs 14.6%), 36 months (12.8% vs 15.8%), or 48 months (13.4% vs 15.7%; omnibus test P = .49).
There were also no significant differences in terms of glycemic remission and the changes in glucose and A1c levels between the testosterone- and placebo-treated men with prediabetes or diabetes, consistent with findings from previous smaller trials.
The authors pointed out that the participants in the TRAVERSE trial had mild to moderate testosterone deficiency, and “it is possible that greater improvements in insulin sensitivity may be observed in men with severe testosterone deficiency.”
However, they noted that most men with hypogonadism who are treated with testosterone replacement therapy have only mild testosterone deficiency.
The parent TRAVERSE study did show testosterone replacement therapy to be associated with higher incidences of venous thromboembolism, atrial fibrillation, and acute kidney injury; however, no additional between-group differences were observed based on diabetes or prediabetes status.
“The findings of this study do not support the use of testosterone replacement therapy alone to prevent or to treat diabetes in men with hypogonadism,” the authors concluded.
Study ‘Overcomes Limitations of Prior Studies’
In an editorial published concurrently with the study, Lona Mody, MD, of the Division of Geriatric and Palliative Care Medicine, University of Michigan Medical School, in Ann Arbor, and colleagues underscored that “the results of this study suggest that testosterone replacement therapy will not benefit glycemic control in men without hypogonadism despite the inappropriately high rates of use in this group.”
Further commenting, Dr. Mody elaborated on the high rates of use, noting that data have shown androgen use among men over 40 years increased more than threefold from 0.81% in 2001 to 2.91% in 2011.
“Based on sales data, testosterone prescribing has increased 100-fold from $18 million in the late 1980s to $1.8 billion over three decades,” Dr. Mody said.
She noted that while some previous research has shown a similar lack of benefits, “the current study overcomes some limitations of prior studies.”
Ultimately, the evidence indicated that “the only major indication for testosterone replacement therapy remains to treat bothersome symptoms of hypogonadism,” Dr. Mody said. “It does not appear to have metabolic benefits.”
This trial was funded by a consortium of testosterone manufacturers led by AbbVie Inc., with additional financial support provided by Endo Pharmaceuticals, Acerus Pharmaceuticals Corporation, and Upsher-Smith Laboratories, LLC.
A version of this article appeared on Medscape.com.
Testosterone replacement therapy in the treatment of hypogonadism showed no benefit in slowing the progression of prediabetes or diabetes, contrary to previous evidence that suggested potential improvements in insulin sensitivity and metabolism.
“The findings of this study suggest that testosterone replacement therapy alone should not be used as a therapeutic intervention to prevent or treat diabetes in men with hypogonadism,” reported the authors of research published this month in JAMA Internal Medicine.
The suggestion that testosterone replacement could prevent or slow diabetes stems from numerous studies linking testosterone deficiency to a host of adverse effects that include increases in insulin resistance and an increased risk for prediabetes and type 2 diabetes.
Furthermore, one recent uncontrolled study showed a lower rate of progression from prediabetes to diabetes in testosterone-treated vs untreated men with hypogonadism.
But with no known randomized clinical trials evaluating the effects of testosterone on diabetes in the absence of a concurrent lifestyle intervention, Shalender Bhasin, MB, of the Research Program in Men’s Health: Aging and Metabolism, at Brigham and Women’s Hospital, Harvard Medical School, Boston, and colleagues conducted a substudy of the randomized TRAVERSE trial, which was conducted at 316 sites in the United States.
“We hypothesized that testosterone replacement therapy for men with hypogonadism and prediabetes would be associated with a significantly lower rate of progression to diabetes,” they wrote.
In the study, named the TRAVERSE Diabetes Study, 5204 participants aged between 40 and 85 years with hypogonadism as well as prediabetes (n = 1175) or diabetes (n = 3880) were randomized 1:1 to receive treatment either with 1.62% testosterone gel or placebo gel.
The participants had a mean age of 63.2 years, and the mean A1c among those with prediabetes was 5.8%.
For the primary outcome, the risk for progression to diabetes did not differ significantly between the testosterone-treated and placebo groups at 6 months (0.7% vs 1.4%), 12 months (7.8% vs 10.7%), 24 months (10.1% vs 14.6%), 36 months (12.8% vs 15.8%), or 48 months (13.4% vs 15.7%; omnibus test P = .49).
There were also no significant differences in terms of glycemic remission and the changes in glucose and A1c levels between the testosterone- and placebo-treated men with prediabetes or diabetes, consistent with findings from previous smaller trials.
The authors pointed out that the participants in the TRAVERSE trial had mild to moderate testosterone deficiency, and “it is possible that greater improvements in insulin sensitivity may be observed in men with severe testosterone deficiency.”
However, they noted that most men with hypogonadism who are treated with testosterone replacement therapy have only mild testosterone deficiency.
The parent TRAVERSE study did show testosterone replacement therapy to be associated with higher incidences of venous thromboembolism, atrial fibrillation, and acute kidney injury; however, no additional between-group differences were observed based on diabetes or prediabetes status.
“The findings of this study do not support the use of testosterone replacement therapy alone to prevent or to treat diabetes in men with hypogonadism,” the authors concluded.
Study ‘Overcomes Limitations of Prior Studies’
In an editorial published concurrently with the study, Lona Mody, MD, of the Division of Geriatric and Palliative Care Medicine, University of Michigan Medical School, in Ann Arbor, and colleagues underscored that “the results of this study suggest that testosterone replacement therapy will not benefit glycemic control in men without hypogonadism despite the inappropriately high rates of use in this group.”
Further commenting, Dr. Mody elaborated on the high rates of use, noting that data have shown androgen use among men over 40 years increased more than threefold from 0.81% in 2001 to 2.91% in 2011.
“Based on sales data, testosterone prescribing has increased 100-fold from $18 million in the late 1980s to $1.8 billion over three decades,” Dr. Mody said.
She noted that while some previous research has shown a similar lack of benefits, “the current study overcomes some limitations of prior studies.”
Ultimately, the evidence indicated that “the only major indication for testosterone replacement therapy remains to treat bothersome symptoms of hypogonadism,” Dr. Mody said. “It does not appear to have metabolic benefits.”
This trial was funded by a consortium of testosterone manufacturers led by AbbVie Inc., with additional financial support provided by Endo Pharmaceuticals, Acerus Pharmaceuticals Corporation, and Upsher-Smith Laboratories, LLC.
A version of this article appeared on Medscape.com.
Testosterone replacement therapy in the treatment of hypogonadism showed no benefit in slowing the progression of prediabetes or diabetes, contrary to previous evidence that suggested potential improvements in insulin sensitivity and metabolism.
“The findings of this study suggest that testosterone replacement therapy alone should not be used as a therapeutic intervention to prevent or treat diabetes in men with hypogonadism,” reported the authors of research published this month in JAMA Internal Medicine.
The suggestion that testosterone replacement could prevent or slow diabetes stems from numerous studies linking testosterone deficiency to a host of adverse effects that include increases in insulin resistance and an increased risk for prediabetes and type 2 diabetes.
Furthermore, one recent uncontrolled study showed a lower rate of progression from prediabetes to diabetes in testosterone-treated vs untreated men with hypogonadism.
But with no known randomized clinical trials evaluating the effects of testosterone on diabetes in the absence of a concurrent lifestyle intervention, Shalender Bhasin, MB, of the Research Program in Men’s Health: Aging and Metabolism, at Brigham and Women’s Hospital, Harvard Medical School, Boston, and colleagues conducted a substudy of the randomized TRAVERSE trial, which was conducted at 316 sites in the United States.
“We hypothesized that testosterone replacement therapy for men with hypogonadism and prediabetes would be associated with a significantly lower rate of progression to diabetes,” they wrote.
In the study, named the TRAVERSE Diabetes Study, 5204 participants aged between 40 and 85 years with hypogonadism as well as prediabetes (n = 1175) or diabetes (n = 3880) were randomized 1:1 to receive treatment either with 1.62% testosterone gel or placebo gel.
The participants had a mean age of 63.2 years, and the mean A1c among those with prediabetes was 5.8%.
For the primary outcome, the risk for progression to diabetes did not differ significantly between the testosterone-treated and placebo groups at 6 months (0.7% vs 1.4%), 12 months (7.8% vs 10.7%), 24 months (10.1% vs 14.6%), 36 months (12.8% vs 15.8%), or 48 months (13.4% vs 15.7%; omnibus test P = .49).
There were also no significant differences in terms of glycemic remission and the changes in glucose and A1c levels between the testosterone- and placebo-treated men with prediabetes or diabetes, consistent with findings from previous smaller trials.
The authors pointed out that the participants in the TRAVERSE trial had mild to moderate testosterone deficiency, and “it is possible that greater improvements in insulin sensitivity may be observed in men with severe testosterone deficiency.”
However, they noted that most men with hypogonadism who are treated with testosterone replacement therapy have only mild testosterone deficiency.
The parent TRAVERSE study did show testosterone replacement therapy to be associated with higher incidences of venous thromboembolism, atrial fibrillation, and acute kidney injury; however, no additional between-group differences were observed based on diabetes or prediabetes status.
“The findings of this study do not support the use of testosterone replacement therapy alone to prevent or to treat diabetes in men with hypogonadism,” the authors concluded.
Study ‘Overcomes Limitations of Prior Studies’
In an editorial published concurrently with the study, Lona Mody, MD, of the Division of Geriatric and Palliative Care Medicine, University of Michigan Medical School, in Ann Arbor, and colleagues underscored that “the results of this study suggest that testosterone replacement therapy will not benefit glycemic control in men without hypogonadism despite the inappropriately high rates of use in this group.”
Further commenting, Dr. Mody elaborated on the high rates of use, noting that data have shown androgen use among men over 40 years increased more than threefold from 0.81% in 2001 to 2.91% in 2011.
“Based on sales data, testosterone prescribing has increased 100-fold from $18 million in the late 1980s to $1.8 billion over three decades,” Dr. Mody said.
She noted that while some previous research has shown a similar lack of benefits, “the current study overcomes some limitations of prior studies.”
Ultimately, the evidence indicated that “the only major indication for testosterone replacement therapy remains to treat bothersome symptoms of hypogonadism,” Dr. Mody said. “It does not appear to have metabolic benefits.”
This trial was funded by a consortium of testosterone manufacturers led by AbbVie Inc., with additional financial support provided by Endo Pharmaceuticals, Acerus Pharmaceuticals Corporation, and Upsher-Smith Laboratories, LLC.
A version of this article appeared on Medscape.com.
Surveillance for 21 Possible Effects of Endocrine Disruptors
Santé Publique France (SPF), the French national public health agency, has released the findings of the PEPS’PE study, which was launched in 2021. The study aims to prioritize, following extensive consultation, the health effects to be monitored for their potential link to endocrine disruptors (EDs). Out of 59 health effects suspected to be associated with exposure to EDs, 21 have been considered a priority for surveillance. Based on these results and others, SPF will expand the scope of the Agency’s surveillance by incorporating new pathologies.
As part of its environmental health program and the National Strategy on EDs, To incorporate new scientific knowledge, the PEPS’PE project aims to prioritize health effects related to EDs and identify health events to integrate into the agency’s current surveillance. The 59 health effects suspected to be associated with exposure to EDs were to be evaluated based on two criteria: The weight of evidence and the epidemiological and societal impact of the health effect. A diverse panel of international experts and French stakeholders in the field of EDs classified 21 health effects as a priority for surveillance.
Among these effects, six reproductive health effects are already monitored in the surveillance program: Cryptorchidism, hypospadias, early puberty, testicular cancer, alteration of sperm quality, and endometriosis. In addition, infertility and decreased fertility (which are not currently monitored for their link to EDs) have been included.
Metabolic effects (including overweight and obesity, cardiovascular diseases, type 2 diabetes, and metabolic syndrome), child neurodevelopmental disorders (including behavioral disorders, intellectual deficits, and attention-deficit disorders), cancers (including breast cancer, prostate cancer, lymphomas, and leukemias in children), and asthma have also been highlighted.
Furthermore, 22 effects were considered low priorities or deemed nonpriorities when, for example, they presented weak or moderate evidence with varying levels of interest in implementing surveillance. Finally, 16 health effects could not be prioritized because of a lack of scientific experts on these topics and a failure to achieve consensus (eg, bone disorders, adrenal disorders, and skin and eye disorders). Consensus was sought during this consultation using a Delphi method.
“These results indicate the need to expand the scope of the Agency’s surveillance beyond reproductive health, incorporating new pathologies when surveillance data are available,” SPF declared in a press release.
“With the initial decision elements obtained through this study, Santé Publique France will analyze the feasibility of implementing surveillance for effects classified as priorities.”
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Santé Publique France (SPF), the French national public health agency, has released the findings of the PEPS’PE study, which was launched in 2021. The study aims to prioritize, following extensive consultation, the health effects to be monitored for their potential link to endocrine disruptors (EDs). Out of 59 health effects suspected to be associated with exposure to EDs, 21 have been considered a priority for surveillance. Based on these results and others, SPF will expand the scope of the Agency’s surveillance by incorporating new pathologies.
As part of its environmental health program and the National Strategy on EDs, To incorporate new scientific knowledge, the PEPS’PE project aims to prioritize health effects related to EDs and identify health events to integrate into the agency’s current surveillance. The 59 health effects suspected to be associated with exposure to EDs were to be evaluated based on two criteria: The weight of evidence and the epidemiological and societal impact of the health effect. A diverse panel of international experts and French stakeholders in the field of EDs classified 21 health effects as a priority for surveillance.
Among these effects, six reproductive health effects are already monitored in the surveillance program: Cryptorchidism, hypospadias, early puberty, testicular cancer, alteration of sperm quality, and endometriosis. In addition, infertility and decreased fertility (which are not currently monitored for their link to EDs) have been included.
Metabolic effects (including overweight and obesity, cardiovascular diseases, type 2 diabetes, and metabolic syndrome), child neurodevelopmental disorders (including behavioral disorders, intellectual deficits, and attention-deficit disorders), cancers (including breast cancer, prostate cancer, lymphomas, and leukemias in children), and asthma have also been highlighted.
Furthermore, 22 effects were considered low priorities or deemed nonpriorities when, for example, they presented weak or moderate evidence with varying levels of interest in implementing surveillance. Finally, 16 health effects could not be prioritized because of a lack of scientific experts on these topics and a failure to achieve consensus (eg, bone disorders, adrenal disorders, and skin and eye disorders). Consensus was sought during this consultation using a Delphi method.
“These results indicate the need to expand the scope of the Agency’s surveillance beyond reproductive health, incorporating new pathologies when surveillance data are available,” SPF declared in a press release.
“With the initial decision elements obtained through this study, Santé Publique France will analyze the feasibility of implementing surveillance for effects classified as priorities.”
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Santé Publique France (SPF), the French national public health agency, has released the findings of the PEPS’PE study, which was launched in 2021. The study aims to prioritize, following extensive consultation, the health effects to be monitored for their potential link to endocrine disruptors (EDs). Out of 59 health effects suspected to be associated with exposure to EDs, 21 have been considered a priority for surveillance. Based on these results and others, SPF will expand the scope of the Agency’s surveillance by incorporating new pathologies.
As part of its environmental health program and the National Strategy on EDs, To incorporate new scientific knowledge, the PEPS’PE project aims to prioritize health effects related to EDs and identify health events to integrate into the agency’s current surveillance. The 59 health effects suspected to be associated with exposure to EDs were to be evaluated based on two criteria: The weight of evidence and the epidemiological and societal impact of the health effect. A diverse panel of international experts and French stakeholders in the field of EDs classified 21 health effects as a priority for surveillance.
Among these effects, six reproductive health effects are already monitored in the surveillance program: Cryptorchidism, hypospadias, early puberty, testicular cancer, alteration of sperm quality, and endometriosis. In addition, infertility and decreased fertility (which are not currently monitored for their link to EDs) have been included.
Metabolic effects (including overweight and obesity, cardiovascular diseases, type 2 diabetes, and metabolic syndrome), child neurodevelopmental disorders (including behavioral disorders, intellectual deficits, and attention-deficit disorders), cancers (including breast cancer, prostate cancer, lymphomas, and leukemias in children), and asthma have also been highlighted.
Furthermore, 22 effects were considered low priorities or deemed nonpriorities when, for example, they presented weak or moderate evidence with varying levels of interest in implementing surveillance. Finally, 16 health effects could not be prioritized because of a lack of scientific experts on these topics and a failure to achieve consensus (eg, bone disorders, adrenal disorders, and skin and eye disorders). Consensus was sought during this consultation using a Delphi method.
“These results indicate the need to expand the scope of the Agency’s surveillance beyond reproductive health, incorporating new pathologies when surveillance data are available,” SPF declared in a press release.
“With the initial decision elements obtained through this study, Santé Publique France will analyze the feasibility of implementing surveillance for effects classified as priorities.”
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Testosterone Replacement May Cause ... Fracture?
This transcript has been edited for clarity.
I am showing you a graph without any labels.
What could this line represent? The stock price of some company that made a big splash but failed to live up to expectations? An outbreak curve charting the introduction of a new infectious agent to a population? The performance of a viral tweet?
I’ll tell you what it is in a moment, but I wanted you to recognize that there is something inherently wistful in this shape, something that speaks of past glory and inevitable declines. It’s a graph that induces a feeling of resistance — no, do not go gently into that good night.
The graph actually represents (roughly) the normal level of serum testosterone in otherwise-healthy men as they age.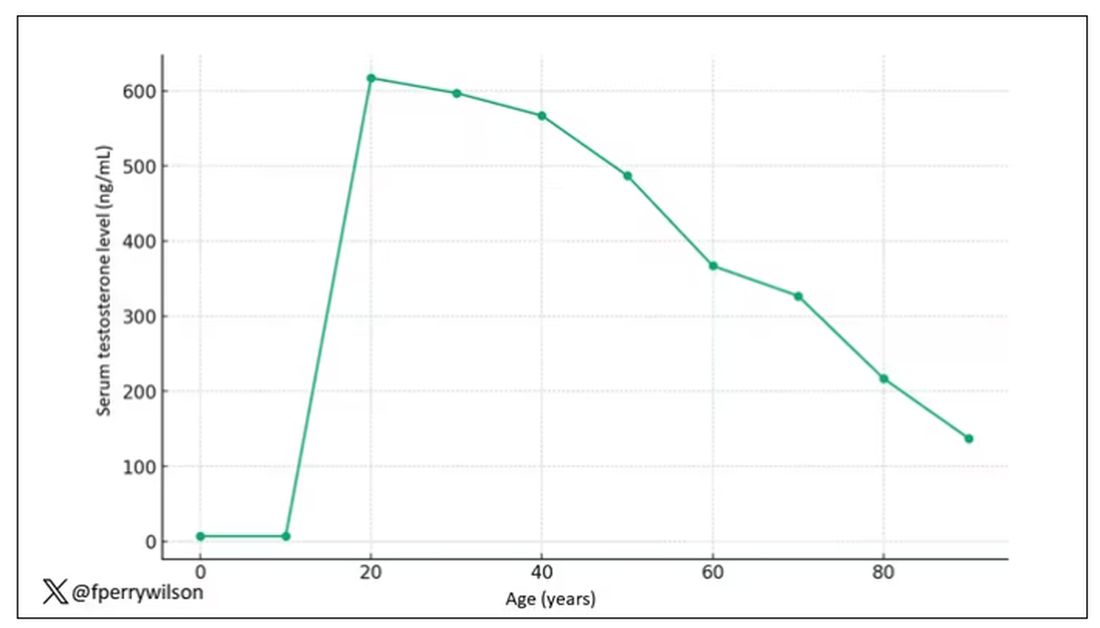
A caveat here: These numbers are not as well defined as I made them seem on this graph, particularly for those older than 65 years. But it is clear that testosterone levels decline with time, and the idea to supplement testosterone is hardly new. Like all treatments, testosterone supplementation has risks and benefits. Some risks are predictable, like exacerbating the symptoms of benign prostatic hyperplasia. Some risks seem to come completely out of left field. That’s what we have today, in a study suggesting that testosterone supplementation increases the risk for bone fractures.
Let me set the stage here by saying that nearly all prior research into the effects of testosterone supplementation has suggested that it is pretty good for bone health. It increases bone mineral density, bone strength, and improves bone architecture.
So if you were to do a randomized trial of testosterone supplementation and look at fracture risk in the testosterone group compared with the placebo group, you would expect the fracture risk would be much lower in those getting supplemented. Of course, this is why we actually do studies instead of assuming we know the answer already — because in this case, you’d be wrong.
I’m talking about this study, appearing in The New England Journal of Medicine.
It’s a prespecified secondary analysis of a randomized trial known as the TRAVERSE trial, which randomly assigned 5246 men with low testosterone levels to transdermal testosterone gel vs placebo. The primary goal of that trial was to assess the cardiovascular risk associated with testosterone supplementation, and the major take-home was that there was no difference in cardiovascular event rates between the testosterone and placebo groups.
This secondary analysis looked at fracture incidence. Researchers contacted participants multiple times in the first year of the study and yearly thereafter. Each time, they asked whether the participant had sustained a fracture. If they answered in the affirmative, a request for medical records was made and the researchers, still blinded to randomization status, adjudicated whether there was indeed a fracture or not, along with some details as to location, situation, and so on.
This was a big study, though, and that translates to just a 3.5% fracture rate in testosterone vs 2.5% in control, but the difference was statistically significant.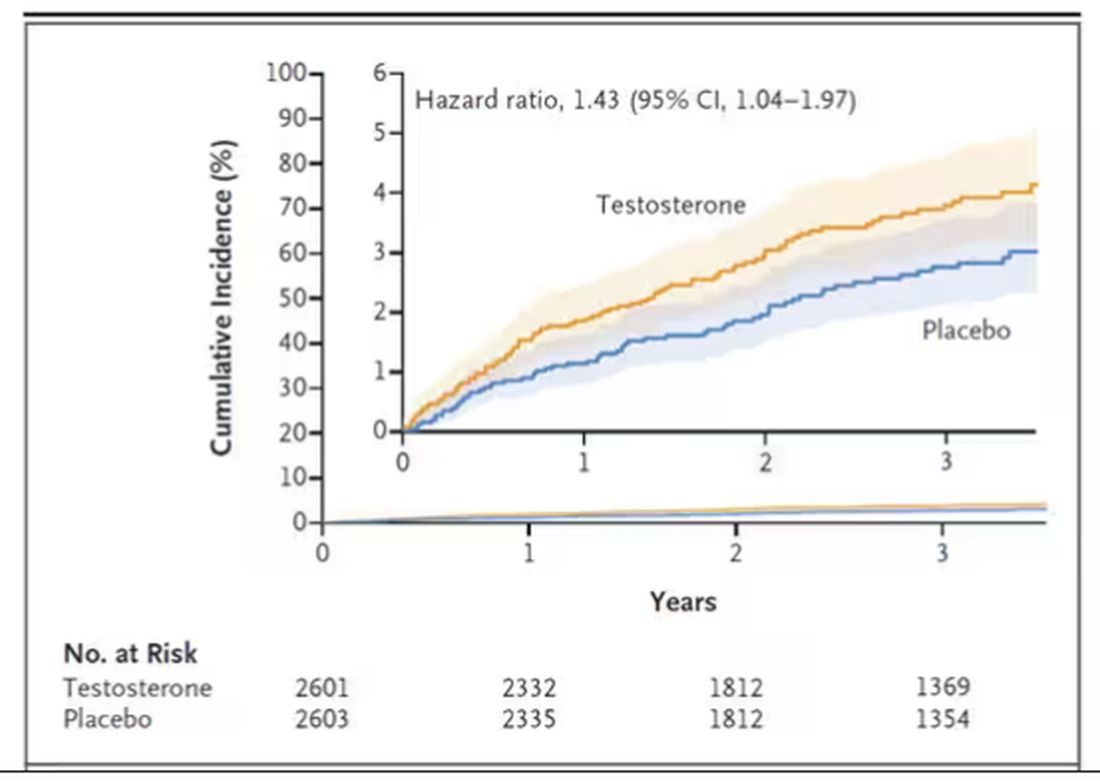
This difference persisted across various fracture types (non–high-impact fractures, for example) after excluding the small percentage of men taking osteoporosis medication.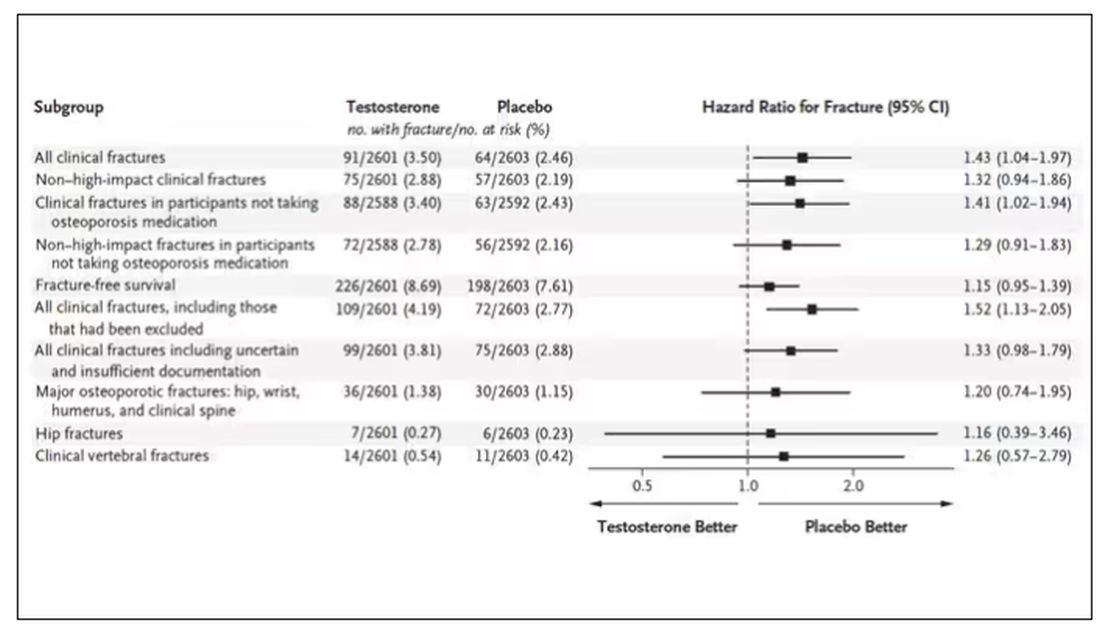
How does a drug that increases bone mineral density and bone strength increase the risk for fracture?
Well, one clue — and this was pointed out in a nice editorial by Matthis Grossman and Bradley Anawalt — is that the increased risk for fracture occurs quite soon after starting treatment, which is not consistent with direct bone effects. Rather, this might represent behavioral differences. Testosterone supplementation seems to increase energy levels; might it lead men to engage in activities that put them at higher risk for fracture?
Regardless of the cause, this adds to our knowledge about the rather complex mix of risks and benefits of testosterone supplementation and probably puts a bit more weight on the risks side. The truth is that testosterone levels do decline with age, as do many things, and it may not be appropriate to try to fight against that in all people. It’s worth noting that all of these studies use low levels of total serum testosterone as an entry criterion. But total testosterone is not what your body “sees.” It sees free testosterone, the portion not bound to sex hormone–binding globulin. And that binding protein is affected by lots of stuff — diabetes and obesity lower it, for example — making total testosterone levels seem low when free testosterone might be just fine.
In other words, testosterone supplementation is probably not terrible, but it is definitely not the cure for aging. In situations like this, we need better data to guide exactly who will benefit from the therapy and who will only be exposed to the risks.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I am showing you a graph without any labels.
What could this line represent? The stock price of some company that made a big splash but failed to live up to expectations? An outbreak curve charting the introduction of a new infectious agent to a population? The performance of a viral tweet?
I’ll tell you what it is in a moment, but I wanted you to recognize that there is something inherently wistful in this shape, something that speaks of past glory and inevitable declines. It’s a graph that induces a feeling of resistance — no, do not go gently into that good night.
The graph actually represents (roughly) the normal level of serum testosterone in otherwise-healthy men as they age.
A caveat here: These numbers are not as well defined as I made them seem on this graph, particularly for those older than 65 years. But it is clear that testosterone levels decline with time, and the idea to supplement testosterone is hardly new. Like all treatments, testosterone supplementation has risks and benefits. Some risks are predictable, like exacerbating the symptoms of benign prostatic hyperplasia. Some risks seem to come completely out of left field. That’s what we have today, in a study suggesting that testosterone supplementation increases the risk for bone fractures.
Let me set the stage here by saying that nearly all prior research into the effects of testosterone supplementation has suggested that it is pretty good for bone health. It increases bone mineral density, bone strength, and improves bone architecture.
So if you were to do a randomized trial of testosterone supplementation and look at fracture risk in the testosterone group compared with the placebo group, you would expect the fracture risk would be much lower in those getting supplemented. Of course, this is why we actually do studies instead of assuming we know the answer already — because in this case, you’d be wrong.
I’m talking about this study, appearing in The New England Journal of Medicine.
It’s a prespecified secondary analysis of a randomized trial known as the TRAVERSE trial, which randomly assigned 5246 men with low testosterone levels to transdermal testosterone gel vs placebo. The primary goal of that trial was to assess the cardiovascular risk associated with testosterone supplementation, and the major take-home was that there was no difference in cardiovascular event rates between the testosterone and placebo groups.
This secondary analysis looked at fracture incidence. Researchers contacted participants multiple times in the first year of the study and yearly thereafter. Each time, they asked whether the participant had sustained a fracture. If they answered in the affirmative, a request for medical records was made and the researchers, still blinded to randomization status, adjudicated whether there was indeed a fracture or not, along with some details as to location, situation, and so on.
This was a big study, though, and that translates to just a 3.5% fracture rate in testosterone vs 2.5% in control, but the difference was statistically significant.
This difference persisted across various fracture types (non–high-impact fractures, for example) after excluding the small percentage of men taking osteoporosis medication.
How does a drug that increases bone mineral density and bone strength increase the risk for fracture?
Well, one clue — and this was pointed out in a nice editorial by Matthis Grossman and Bradley Anawalt — is that the increased risk for fracture occurs quite soon after starting treatment, which is not consistent with direct bone effects. Rather, this might represent behavioral differences. Testosterone supplementation seems to increase energy levels; might it lead men to engage in activities that put them at higher risk for fracture?
Regardless of the cause, this adds to our knowledge about the rather complex mix of risks and benefits of testosterone supplementation and probably puts a bit more weight on the risks side. The truth is that testosterone levels do decline with age, as do many things, and it may not be appropriate to try to fight against that in all people. It’s worth noting that all of these studies use low levels of total serum testosterone as an entry criterion. But total testosterone is not what your body “sees.” It sees free testosterone, the portion not bound to sex hormone–binding globulin. And that binding protein is affected by lots of stuff — diabetes and obesity lower it, for example — making total testosterone levels seem low when free testosterone might be just fine.
In other words, testosterone supplementation is probably not terrible, but it is definitely not the cure for aging. In situations like this, we need better data to guide exactly who will benefit from the therapy and who will only be exposed to the risks.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I am showing you a graph without any labels.
What could this line represent? The stock price of some company that made a big splash but failed to live up to expectations? An outbreak curve charting the introduction of a new infectious agent to a population? The performance of a viral tweet?
I’ll tell you what it is in a moment, but I wanted you to recognize that there is something inherently wistful in this shape, something that speaks of past glory and inevitable declines. It’s a graph that induces a feeling of resistance — no, do not go gently into that good night.
The graph actually represents (roughly) the normal level of serum testosterone in otherwise-healthy men as they age.
A caveat here: These numbers are not as well defined as I made them seem on this graph, particularly for those older than 65 years. But it is clear that testosterone levels decline with time, and the idea to supplement testosterone is hardly new. Like all treatments, testosterone supplementation has risks and benefits. Some risks are predictable, like exacerbating the symptoms of benign prostatic hyperplasia. Some risks seem to come completely out of left field. That’s what we have today, in a study suggesting that testosterone supplementation increases the risk for bone fractures.
Let me set the stage here by saying that nearly all prior research into the effects of testosterone supplementation has suggested that it is pretty good for bone health. It increases bone mineral density, bone strength, and improves bone architecture.
So if you were to do a randomized trial of testosterone supplementation and look at fracture risk in the testosterone group compared with the placebo group, you would expect the fracture risk would be much lower in those getting supplemented. Of course, this is why we actually do studies instead of assuming we know the answer already — because in this case, you’d be wrong.
I’m talking about this study, appearing in The New England Journal of Medicine.
It’s a prespecified secondary analysis of a randomized trial known as the TRAVERSE trial, which randomly assigned 5246 men with low testosterone levels to transdermal testosterone gel vs placebo. The primary goal of that trial was to assess the cardiovascular risk associated with testosterone supplementation, and the major take-home was that there was no difference in cardiovascular event rates between the testosterone and placebo groups.
This secondary analysis looked at fracture incidence. Researchers contacted participants multiple times in the first year of the study and yearly thereafter. Each time, they asked whether the participant had sustained a fracture. If they answered in the affirmative, a request for medical records was made and the researchers, still blinded to randomization status, adjudicated whether there was indeed a fracture or not, along with some details as to location, situation, and so on.
This was a big study, though, and that translates to just a 3.5% fracture rate in testosterone vs 2.5% in control, but the difference was statistically significant.
This difference persisted across various fracture types (non–high-impact fractures, for example) after excluding the small percentage of men taking osteoporosis medication.
How does a drug that increases bone mineral density and bone strength increase the risk for fracture?
Well, one clue — and this was pointed out in a nice editorial by Matthis Grossman and Bradley Anawalt — is that the increased risk for fracture occurs quite soon after starting treatment, which is not consistent with direct bone effects. Rather, this might represent behavioral differences. Testosterone supplementation seems to increase energy levels; might it lead men to engage in activities that put them at higher risk for fracture?
Regardless of the cause, this adds to our knowledge about the rather complex mix of risks and benefits of testosterone supplementation and probably puts a bit more weight on the risks side. The truth is that testosterone levels do decline with age, as do many things, and it may not be appropriate to try to fight against that in all people. It’s worth noting that all of these studies use low levels of total serum testosterone as an entry criterion. But total testosterone is not what your body “sees.” It sees free testosterone, the portion not bound to sex hormone–binding globulin. And that binding protein is affected by lots of stuff — diabetes and obesity lower it, for example — making total testosterone levels seem low when free testosterone might be just fine.
In other words, testosterone supplementation is probably not terrible, but it is definitely not the cure for aging. In situations like this, we need better data to guide exactly who will benefit from the therapy and who will only be exposed to the risks.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Testosterone Supplements: Overcoming Current Misconceptions
Underdiagnosis, reluctant doctors, patient preconceptions: Treating low testosterone levels is a tricky business in France despite the proven benefits of replacement therapy. About 20% of patients with symptomatic low testosterone levels are treated for the deficiency, said Eric Huygue, MD, PhD, urologic surgeon at Toulouse University Hospital in France, at the 117th annual conference of the French Urology Association (AFU).
, said Dr. Huygue, who was involved in drawing up the first French recommendations on treating low testosterone in 2021.
“We must keep up communication efforts to make patients and doctors aware” of the benefits of supplementation, he said.
Testosterone Levels
Testosterone deficiency mostly affects men older than 40 years. A drop in androgen levels, which varies by individual, can lead to sexual problems (such as erectile dysfunction and low libido), physical symptoms (fatigue, hot flashes, loss of muscle mass, and osteoporosis), and mental disorders (anxiety, irritability, and depression).
There are an estimated 340,000 men with symptomatic testosterone deficiency in France. Just 70,000 of these are receiving replacement therapy (see box), which accounts for only 20% of those affected. For Dr. Huygue, this low treatment rate is due to underdiagnosis, as well as reluctance on the part of doctors and patients.
Although routine screening of low testosterone in the general population is not recommended, some individuals are particularly at risk, noted the urologist.
This is especially true for patients with metabolic disorders associated with insulin resistance (such as obesity and type 2 diabetes), cardiovascular diseases (hypertension, heart failure, and atrial fibrillation), or other chronic conditions (chronic obstructive pulmonary disease, cancer, and depression). Some medications (corticosteroids, antipsychotics, chemotherapy drugs, and antiretroviral therapies) can also lead to low testosterone.
Per the French recommendations for managing low testosterone, diagnosis must be based on free or bioavailable testosterone and not total testosterone levels, which can give a skewed result. Levels must be tested twice, 1 month apart, in the morning and while fasting. The reference range is determined by taking the lower threshold level of young men as measured in the laboratory.
Threshold Values
The current practice of using the reference range associated with the patient’s age group undoubtedly contributes to the underdiagnosis of low testosterone, said Dr. Huygue. According to a survey of AFU members in 2021, the year in which the recommendations were published, 77% of urologists interviewed reported referring to reference ranges for patients of the same age.
In their defense, “this method has long been in use, but it has eventually become apparent that symptomatic patients with an undiagnosed deficiency could be in the reference patients’ group,” Dr. Huygue explained.
Once a deficiency has been diagnosed, doctors may be reluctant to prescribe replacement therapy due to the perceived risk of developing prostate cancer. Several international studies have shown that “the risk of prostate cancer is the single biggest reason for doctors refusing to prescribe testosterone,” said Dr. Huygue.
Despite this reluctance, numerous studies have clearly shown that there is no link between a high testosterone level and the risk of developing prostate cancer. It even seems that a low testosterone level might expose a person to an increased risk for an aggressive form of cancer.
“This is a time of many surprising discoveries concerning the link between the prostate and testosterone, which go against what we have thought up to now. It has been observed that men with low testosterone develop more serious types of cancer,” said Dr. Huygue at a previous meeting of the AFU, during which he announced the publication of the French recommendations.
Prostate Cancer Recurrence
Urologists are also wary of testosterone supplementation in patients with a previous history of prostate cancer. According to the AFU’s survey, 40% of urologists questioned think that testosterone is contraindicated in this population. One in two urologists prescribe testosterone after radical prostatectomy for low or intermediate risk and most commonly after 3 years of undetectable prostate-specific antigen (PSA) levels.
Nevertheless, “several retrospective studies show the safety of testosterone replacement therapy in men who have undergone radical prostatectomy or radiotherapy or who are under active monitoring,” said Dr. Huygue. Testosterone “does not appear to increase the risk of relapse” after treatment of prostate cancer.
Dr. Huygue invited prescribing physicians to refer to the French recommendations, which specify that 1 year of undetectable PSA after prostatectomy is sufficient before prescribing replacement therapy. “This is clearly indicated in the recommendations for patients with a previous history of prostate cancer.”
Neither prostate cancer nor benign prostatic hyperplasia is a contraindication. According to the recommendations, the only contraindications to testosterone prescription are the following:
- Hematocrit > 54%
- Current breast or prostate cancer
- Cardiovascular event less than 3-6 months prior
- Trying to conceive
Cardiovascular Benefits
Another more commonly used argument by general practitioners and endocrinologists to justify their reluctance to prescribe testosterone is the risk to cardiovascular health. In early 2010, a series of American studies alerted clinicians to this risk when taking testosterone. Since then, other studies have had reassuring findings.
In response to the alert issued by the United States, the European Medicines Agency specified that “the data are not sufficient for a warning,” before the American Heart Association colleagues concluded that testosterone should only be avoided in the first 6 months following a severe cardiovascular event.
Conversely, in 2021, the European Society of Cardiology put forward the benefits of testosterone in an article in favor of replacement therapy to prevent cardiovascular risk. In particular, the hormone is thought to have a beneficial effect on arterial stiffness, the appearance of calcified plaques, and coronary artery dilatation.
The final hurdle to overcome before a testosterone prescription is filled relates to patients themselves, who often regard such treatment unfavorably. Many wrongly believe that androgens are hormones that “increase the risk of cancer, make you aggressive, cause weight gain, lead to hair loss, and cause body hair growth,” said Dr. Huygue.
Finally, breaks in the supply chain for Androtardyl, the only injectable form available for reimbursement by French social security schemes, were reported in the country in 2023, said Dr. Huygue. This situation only complicates further the prescription and use of testosterone replacement therapy.
Which Supplement?
Testosterone replacement therapies are available on the market in the following formulations:
Via transcutaneous administration: Testosterone-based gels, not covered by the French social security system (Androgel and Fortigel), to be applied daily. Users must be careful to avoid any potential transfer of the product to women or children in case of contact with the site after application.
Via an injection: Androtardyl (testosterone enanthate), covered by French social security, to be administered intramuscularly once a month. Nebido (testosterone undecanoate), not covered by French social security, with a more beneficial bioavailability profile, to be administered once every 3 months.
Pantestone (testosterone undecanoate), administered orally, is not marketed since 2021. It had the major disadvantage of requiring a high-fat diet to ensure optimal absorption.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Underdiagnosis, reluctant doctors, patient preconceptions: Treating low testosterone levels is a tricky business in France despite the proven benefits of replacement therapy. About 20% of patients with symptomatic low testosterone levels are treated for the deficiency, said Eric Huygue, MD, PhD, urologic surgeon at Toulouse University Hospital in France, at the 117th annual conference of the French Urology Association (AFU).
, said Dr. Huygue, who was involved in drawing up the first French recommendations on treating low testosterone in 2021.
“We must keep up communication efforts to make patients and doctors aware” of the benefits of supplementation, he said.
Testosterone Levels
Testosterone deficiency mostly affects men older than 40 years. A drop in androgen levels, which varies by individual, can lead to sexual problems (such as erectile dysfunction and low libido), physical symptoms (fatigue, hot flashes, loss of muscle mass, and osteoporosis), and mental disorders (anxiety, irritability, and depression).
There are an estimated 340,000 men with symptomatic testosterone deficiency in France. Just 70,000 of these are receiving replacement therapy (see box), which accounts for only 20% of those affected. For Dr. Huygue, this low treatment rate is due to underdiagnosis, as well as reluctance on the part of doctors and patients.
Although routine screening of low testosterone in the general population is not recommended, some individuals are particularly at risk, noted the urologist.
This is especially true for patients with metabolic disorders associated with insulin resistance (such as obesity and type 2 diabetes), cardiovascular diseases (hypertension, heart failure, and atrial fibrillation), or other chronic conditions (chronic obstructive pulmonary disease, cancer, and depression). Some medications (corticosteroids, antipsychotics, chemotherapy drugs, and antiretroviral therapies) can also lead to low testosterone.
Per the French recommendations for managing low testosterone, diagnosis must be based on free or bioavailable testosterone and not total testosterone levels, which can give a skewed result. Levels must be tested twice, 1 month apart, in the morning and while fasting. The reference range is determined by taking the lower threshold level of young men as measured in the laboratory.
Threshold Values
The current practice of using the reference range associated with the patient’s age group undoubtedly contributes to the underdiagnosis of low testosterone, said Dr. Huygue. According to a survey of AFU members in 2021, the year in which the recommendations were published, 77% of urologists interviewed reported referring to reference ranges for patients of the same age.
In their defense, “this method has long been in use, but it has eventually become apparent that symptomatic patients with an undiagnosed deficiency could be in the reference patients’ group,” Dr. Huygue explained.
Once a deficiency has been diagnosed, doctors may be reluctant to prescribe replacement therapy due to the perceived risk of developing prostate cancer. Several international studies have shown that “the risk of prostate cancer is the single biggest reason for doctors refusing to prescribe testosterone,” said Dr. Huygue.
Despite this reluctance, numerous studies have clearly shown that there is no link between a high testosterone level and the risk of developing prostate cancer. It even seems that a low testosterone level might expose a person to an increased risk for an aggressive form of cancer.
“This is a time of many surprising discoveries concerning the link between the prostate and testosterone, which go against what we have thought up to now. It has been observed that men with low testosterone develop more serious types of cancer,” said Dr. Huygue at a previous meeting of the AFU, during which he announced the publication of the French recommendations.
Prostate Cancer Recurrence
Urologists are also wary of testosterone supplementation in patients with a previous history of prostate cancer. According to the AFU’s survey, 40% of urologists questioned think that testosterone is contraindicated in this population. One in two urologists prescribe testosterone after radical prostatectomy for low or intermediate risk and most commonly after 3 years of undetectable prostate-specific antigen (PSA) levels.
Nevertheless, “several retrospective studies show the safety of testosterone replacement therapy in men who have undergone radical prostatectomy or radiotherapy or who are under active monitoring,” said Dr. Huygue. Testosterone “does not appear to increase the risk of relapse” after treatment of prostate cancer.
Dr. Huygue invited prescribing physicians to refer to the French recommendations, which specify that 1 year of undetectable PSA after prostatectomy is sufficient before prescribing replacement therapy. “This is clearly indicated in the recommendations for patients with a previous history of prostate cancer.”
Neither prostate cancer nor benign prostatic hyperplasia is a contraindication. According to the recommendations, the only contraindications to testosterone prescription are the following:
- Hematocrit > 54%
- Current breast or prostate cancer
- Cardiovascular event less than 3-6 months prior
- Trying to conceive
Cardiovascular Benefits
Another more commonly used argument by general practitioners and endocrinologists to justify their reluctance to prescribe testosterone is the risk to cardiovascular health. In early 2010, a series of American studies alerted clinicians to this risk when taking testosterone. Since then, other studies have had reassuring findings.
In response to the alert issued by the United States, the European Medicines Agency specified that “the data are not sufficient for a warning,” before the American Heart Association colleagues concluded that testosterone should only be avoided in the first 6 months following a severe cardiovascular event.
Conversely, in 2021, the European Society of Cardiology put forward the benefits of testosterone in an article in favor of replacement therapy to prevent cardiovascular risk. In particular, the hormone is thought to have a beneficial effect on arterial stiffness, the appearance of calcified plaques, and coronary artery dilatation.
The final hurdle to overcome before a testosterone prescription is filled relates to patients themselves, who often regard such treatment unfavorably. Many wrongly believe that androgens are hormones that “increase the risk of cancer, make you aggressive, cause weight gain, lead to hair loss, and cause body hair growth,” said Dr. Huygue.
Finally, breaks in the supply chain for Androtardyl, the only injectable form available for reimbursement by French social security schemes, were reported in the country in 2023, said Dr. Huygue. This situation only complicates further the prescription and use of testosterone replacement therapy.
Which Supplement?
Testosterone replacement therapies are available on the market in the following formulations:
Via transcutaneous administration: Testosterone-based gels, not covered by the French social security system (Androgel and Fortigel), to be applied daily. Users must be careful to avoid any potential transfer of the product to women or children in case of contact with the site after application.
Via an injection: Androtardyl (testosterone enanthate), covered by French social security, to be administered intramuscularly once a month. Nebido (testosterone undecanoate), not covered by French social security, with a more beneficial bioavailability profile, to be administered once every 3 months.
Pantestone (testosterone undecanoate), administered orally, is not marketed since 2021. It had the major disadvantage of requiring a high-fat diet to ensure optimal absorption.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Underdiagnosis, reluctant doctors, patient preconceptions: Treating low testosterone levels is a tricky business in France despite the proven benefits of replacement therapy. About 20% of patients with symptomatic low testosterone levels are treated for the deficiency, said Eric Huygue, MD, PhD, urologic surgeon at Toulouse University Hospital in France, at the 117th annual conference of the French Urology Association (AFU).
, said Dr. Huygue, who was involved in drawing up the first French recommendations on treating low testosterone in 2021.
“We must keep up communication efforts to make patients and doctors aware” of the benefits of supplementation, he said.
Testosterone Levels
Testosterone deficiency mostly affects men older than 40 years. A drop in androgen levels, which varies by individual, can lead to sexual problems (such as erectile dysfunction and low libido), physical symptoms (fatigue, hot flashes, loss of muscle mass, and osteoporosis), and mental disorders (anxiety, irritability, and depression).
There are an estimated 340,000 men with symptomatic testosterone deficiency in France. Just 70,000 of these are receiving replacement therapy (see box), which accounts for only 20% of those affected. For Dr. Huygue, this low treatment rate is due to underdiagnosis, as well as reluctance on the part of doctors and patients.
Although routine screening of low testosterone in the general population is not recommended, some individuals are particularly at risk, noted the urologist.
This is especially true for patients with metabolic disorders associated with insulin resistance (such as obesity and type 2 diabetes), cardiovascular diseases (hypertension, heart failure, and atrial fibrillation), or other chronic conditions (chronic obstructive pulmonary disease, cancer, and depression). Some medications (corticosteroids, antipsychotics, chemotherapy drugs, and antiretroviral therapies) can also lead to low testosterone.
Per the French recommendations for managing low testosterone, diagnosis must be based on free or bioavailable testosterone and not total testosterone levels, which can give a skewed result. Levels must be tested twice, 1 month apart, in the morning and while fasting. The reference range is determined by taking the lower threshold level of young men as measured in the laboratory.
Threshold Values
The current practice of using the reference range associated with the patient’s age group undoubtedly contributes to the underdiagnosis of low testosterone, said Dr. Huygue. According to a survey of AFU members in 2021, the year in which the recommendations were published, 77% of urologists interviewed reported referring to reference ranges for patients of the same age.
In their defense, “this method has long been in use, but it has eventually become apparent that symptomatic patients with an undiagnosed deficiency could be in the reference patients’ group,” Dr. Huygue explained.
Once a deficiency has been diagnosed, doctors may be reluctant to prescribe replacement therapy due to the perceived risk of developing prostate cancer. Several international studies have shown that “the risk of prostate cancer is the single biggest reason for doctors refusing to prescribe testosterone,” said Dr. Huygue.
Despite this reluctance, numerous studies have clearly shown that there is no link between a high testosterone level and the risk of developing prostate cancer. It even seems that a low testosterone level might expose a person to an increased risk for an aggressive form of cancer.
“This is a time of many surprising discoveries concerning the link between the prostate and testosterone, which go against what we have thought up to now. It has been observed that men with low testosterone develop more serious types of cancer,” said Dr. Huygue at a previous meeting of the AFU, during which he announced the publication of the French recommendations.
Prostate Cancer Recurrence
Urologists are also wary of testosterone supplementation in patients with a previous history of prostate cancer. According to the AFU’s survey, 40% of urologists questioned think that testosterone is contraindicated in this population. One in two urologists prescribe testosterone after radical prostatectomy for low or intermediate risk and most commonly after 3 years of undetectable prostate-specific antigen (PSA) levels.
Nevertheless, “several retrospective studies show the safety of testosterone replacement therapy in men who have undergone radical prostatectomy or radiotherapy or who are under active monitoring,” said Dr. Huygue. Testosterone “does not appear to increase the risk of relapse” after treatment of prostate cancer.
Dr. Huygue invited prescribing physicians to refer to the French recommendations, which specify that 1 year of undetectable PSA after prostatectomy is sufficient before prescribing replacement therapy. “This is clearly indicated in the recommendations for patients with a previous history of prostate cancer.”
Neither prostate cancer nor benign prostatic hyperplasia is a contraindication. According to the recommendations, the only contraindications to testosterone prescription are the following:
- Hematocrit > 54%
- Current breast or prostate cancer
- Cardiovascular event less than 3-6 months prior
- Trying to conceive
Cardiovascular Benefits
Another more commonly used argument by general practitioners and endocrinologists to justify their reluctance to prescribe testosterone is the risk to cardiovascular health. In early 2010, a series of American studies alerted clinicians to this risk when taking testosterone. Since then, other studies have had reassuring findings.
In response to the alert issued by the United States, the European Medicines Agency specified that “the data are not sufficient for a warning,” before the American Heart Association colleagues concluded that testosterone should only be avoided in the first 6 months following a severe cardiovascular event.
Conversely, in 2021, the European Society of Cardiology put forward the benefits of testosterone in an article in favor of replacement therapy to prevent cardiovascular risk. In particular, the hormone is thought to have a beneficial effect on arterial stiffness, the appearance of calcified plaques, and coronary artery dilatation.
The final hurdle to overcome before a testosterone prescription is filled relates to patients themselves, who often regard such treatment unfavorably. Many wrongly believe that androgens are hormones that “increase the risk of cancer, make you aggressive, cause weight gain, lead to hair loss, and cause body hair growth,” said Dr. Huygue.
Finally, breaks in the supply chain for Androtardyl, the only injectable form available for reimbursement by French social security schemes, were reported in the country in 2023, said Dr. Huygue. This situation only complicates further the prescription and use of testosterone replacement therapy.
Which Supplement?
Testosterone replacement therapies are available on the market in the following formulations:
Via transcutaneous administration: Testosterone-based gels, not covered by the French social security system (Androgel and Fortigel), to be applied daily. Users must be careful to avoid any potential transfer of the product to women or children in case of contact with the site after application.
Via an injection: Androtardyl (testosterone enanthate), covered by French social security, to be administered intramuscularly once a month. Nebido (testosterone undecanoate), not covered by French social security, with a more beneficial bioavailability profile, to be administered once every 3 months.
Pantestone (testosterone undecanoate), administered orally, is not marketed since 2021. It had the major disadvantage of requiring a high-fat diet to ensure optimal absorption.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Testosterone Replacement Therapy and Prostate Cancer Risk
TOPLINE:
Testosterone replacement therapy in middle-aged and older men with hypogonadism does not increase the risk for high-grade or any prostate cancer, new data confirmed.
METHODOLOGY:
- Epidemiologic studies have shown inconsistent findings, and clinical trials have not examined prostate safety. As a result, guidelines generally advise against testosterone replacement therapy in men with a history of or increased risk for prostate cancer.
- The current placebo-controlled, double-blind, parallel-group randomized study included 5204 men, ages 45-80, who had two fasting testosterone concentrations < 300 ng/dL, one or more hypogonadal symptoms, and a history of cardiovascular disease or increased . Patients were randomly assigned 1:1 to receive either testosterone replacement therapy or placebo.
- The primary prostate safety endpoint was incident high-grade prostate cancer (Gleason score 4 + 3 or higher).
- Secondary endpoints included incidence of any prostate cancer, acute urinary retention, invasive procedure for , , and new pharmacologic treatment for lower urinary tract symptoms.
TAKEAWAY:
- The incidence of high-grade prostate cancer did not differ significantly between groups. Over a mean follow-up of 33 months, only 0.19% (5 of 2596 participants) in the testosterone replacement therapy group and 0.12% (3 of 2602) in the placebo group were diagnosed with high-grade disease (hazard ratio [HR], 1.62; P = .51).
- The rate of any prostate cancer also did not differ significantly between the testosterone vs placebo groups (0.46% vs 0.42%; HR, 1.07; P = .87).
- The rates of acute urinary retention (0.77% vs 0.61%; HR, 1.25; P = .50), invasive procedures for benign prostatic hyperplasia (0.89% vs 0.46%; HR, 1.91; P = .07), prostate biopsy (0.62% vs 0.54%; HR, 1.13; P = .74), or new treatment for lower urinary tract symptoms (3.89% vs 3.34%; HR, 1.16; P = .32) did not differ significantly between the testosterone vs placebo groups.
- Compared with placebo, testosterone therapy did increase prostate-specific antigen (PSA) levels, but the differences were small and did not increase after 12 months.
IN PRACTICE:
In a population of middle-aged and older men with hypogonadism, “the incidences of high-grade or any prostate cancer and other prostate events were low and did not differ significantly between testosterone- and placebo-treated men,” the authors concluded. “The study’s findings will facilitate a more informed appraisal of the potential prostate risks of testosterone replacement therapy.”
SOURCE:
This study, led by Shalender Bhasin, MB, BS, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, was published online in JAMA Network Open.
LIMITATIONS:
These study findings do not apply to men with known prostate cancer, higher PSA values, or those without confirmed hypogonadism. The study design did not include prostate imaging or other biomarker tests after PSA testing, which may have affected the decision to perform a biopsy. Also, the rates of treatment discontinuation and loss to follow-up were high.
DISCLOSURES:
This study was funded by a consortium of testosterone manufacturers led by AbbVie Inc., with additional financial support from Endo Pharmaceuticals, Acerus Pharmaceuticals Corporation, and Upsher-Smith Laboratories. Bhasin, Lincoff, and Khera reported receiving grants and consulting and personal fees from various sources. The remaining authors disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Testosterone replacement therapy in middle-aged and older men with hypogonadism does not increase the risk for high-grade or any prostate cancer, new data confirmed.
METHODOLOGY:
- Epidemiologic studies have shown inconsistent findings, and clinical trials have not examined prostate safety. As a result, guidelines generally advise against testosterone replacement therapy in men with a history of or increased risk for prostate cancer.
- The current placebo-controlled, double-blind, parallel-group randomized study included 5204 men, ages 45-80, who had two fasting testosterone concentrations < 300 ng/dL, one or more hypogonadal symptoms, and a history of cardiovascular disease or increased . Patients were randomly assigned 1:1 to receive either testosterone replacement therapy or placebo.
- The primary prostate safety endpoint was incident high-grade prostate cancer (Gleason score 4 + 3 or higher).
- Secondary endpoints included incidence of any prostate cancer, acute urinary retention, invasive procedure for , , and new pharmacologic treatment for lower urinary tract symptoms.
TAKEAWAY:
- The incidence of high-grade prostate cancer did not differ significantly between groups. Over a mean follow-up of 33 months, only 0.19% (5 of 2596 participants) in the testosterone replacement therapy group and 0.12% (3 of 2602) in the placebo group were diagnosed with high-grade disease (hazard ratio [HR], 1.62; P = .51).
- The rate of any prostate cancer also did not differ significantly between the testosterone vs placebo groups (0.46% vs 0.42%; HR, 1.07; P = .87).
- The rates of acute urinary retention (0.77% vs 0.61%; HR, 1.25; P = .50), invasive procedures for benign prostatic hyperplasia (0.89% vs 0.46%; HR, 1.91; P = .07), prostate biopsy (0.62% vs 0.54%; HR, 1.13; P = .74), or new treatment for lower urinary tract symptoms (3.89% vs 3.34%; HR, 1.16; P = .32) did not differ significantly between the testosterone vs placebo groups.
- Compared with placebo, testosterone therapy did increase prostate-specific antigen (PSA) levels, but the differences were small and did not increase after 12 months.
IN PRACTICE:
In a population of middle-aged and older men with hypogonadism, “the incidences of high-grade or any prostate cancer and other prostate events were low and did not differ significantly between testosterone- and placebo-treated men,” the authors concluded. “The study’s findings will facilitate a more informed appraisal of the potential prostate risks of testosterone replacement therapy.”
SOURCE:
This study, led by Shalender Bhasin, MB, BS, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, was published online in JAMA Network Open.
LIMITATIONS:
These study findings do not apply to men with known prostate cancer, higher PSA values, or those without confirmed hypogonadism. The study design did not include prostate imaging or other biomarker tests after PSA testing, which may have affected the decision to perform a biopsy. Also, the rates of treatment discontinuation and loss to follow-up were high.
DISCLOSURES:
This study was funded by a consortium of testosterone manufacturers led by AbbVie Inc., with additional financial support from Endo Pharmaceuticals, Acerus Pharmaceuticals Corporation, and Upsher-Smith Laboratories. Bhasin, Lincoff, and Khera reported receiving grants and consulting and personal fees from various sources. The remaining authors disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Testosterone replacement therapy in middle-aged and older men with hypogonadism does not increase the risk for high-grade or any prostate cancer, new data confirmed.
METHODOLOGY:
- Epidemiologic studies have shown inconsistent findings, and clinical trials have not examined prostate safety. As a result, guidelines generally advise against testosterone replacement therapy in men with a history of or increased risk for prostate cancer.
- The current placebo-controlled, double-blind, parallel-group randomized study included 5204 men, ages 45-80, who had two fasting testosterone concentrations < 300 ng/dL, one or more hypogonadal symptoms, and a history of cardiovascular disease or increased . Patients were randomly assigned 1:1 to receive either testosterone replacement therapy or placebo.
- The primary prostate safety endpoint was incident high-grade prostate cancer (Gleason score 4 + 3 or higher).
- Secondary endpoints included incidence of any prostate cancer, acute urinary retention, invasive procedure for , , and new pharmacologic treatment for lower urinary tract symptoms.
TAKEAWAY:
- The incidence of high-grade prostate cancer did not differ significantly between groups. Over a mean follow-up of 33 months, only 0.19% (5 of 2596 participants) in the testosterone replacement therapy group and 0.12% (3 of 2602) in the placebo group were diagnosed with high-grade disease (hazard ratio [HR], 1.62; P = .51).
- The rate of any prostate cancer also did not differ significantly between the testosterone vs placebo groups (0.46% vs 0.42%; HR, 1.07; P = .87).
- The rates of acute urinary retention (0.77% vs 0.61%; HR, 1.25; P = .50), invasive procedures for benign prostatic hyperplasia (0.89% vs 0.46%; HR, 1.91; P = .07), prostate biopsy (0.62% vs 0.54%; HR, 1.13; P = .74), or new treatment for lower urinary tract symptoms (3.89% vs 3.34%; HR, 1.16; P = .32) did not differ significantly between the testosterone vs placebo groups.
- Compared with placebo, testosterone therapy did increase prostate-specific antigen (PSA) levels, but the differences were small and did not increase after 12 months.
IN PRACTICE:
In a population of middle-aged and older men with hypogonadism, “the incidences of high-grade or any prostate cancer and other prostate events were low and did not differ significantly between testosterone- and placebo-treated men,” the authors concluded. “The study’s findings will facilitate a more informed appraisal of the potential prostate risks of testosterone replacement therapy.”
SOURCE:
This study, led by Shalender Bhasin, MB, BS, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, was published online in JAMA Network Open.
LIMITATIONS:
These study findings do not apply to men with known prostate cancer, higher PSA values, or those without confirmed hypogonadism. The study design did not include prostate imaging or other biomarker tests after PSA testing, which may have affected the decision to perform a biopsy. Also, the rates of treatment discontinuation and loss to follow-up were high.
DISCLOSURES:
This study was funded by a consortium of testosterone manufacturers led by AbbVie Inc., with additional financial support from Endo Pharmaceuticals, Acerus Pharmaceuticals Corporation, and Upsher-Smith Laboratories. Bhasin, Lincoff, and Khera reported receiving grants and consulting and personal fees from various sources. The remaining authors disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
What’s the Disease Burden From Plastic Exposure?
Exposure to endocrine-disrupting chemicals (EDCs) via daily use of plastics is a major contributor to the overall disease burden in the United States and the associated costs to society amount to more than 1% of the gross domestic product, revealed a large-scale analysis.
The research, published in the Journal of the Endocrine Society, indicated that taken together, the disease burden attributable to EDCs used in the manufacture of plastics added up to almost $250 billion in 2018 alone.
“The diseases due to plastics run the entire life course from preterm birth to obesity, heart disease, and cancers,” commented lead author Leonardo Trasande, MD, MPP, Jim G. Hendrick, MD Professor of Pediatrics, Department of Pediatrics, NYU Langone Medical Center, New York, in a release.
“Our study drives home the need to address chemicals used in plastic materials” through global treaties and other policy initiatives, he said, so as to “reduce these costs” in line with reductions in exposure to the chemicals.
Co-author Michael Belliveau, Executive Director at Defend Our Health in Portland, ME, agreed, saying: “We can reduce these health costs and the prevalence of chronic endocrine diseases such as diabetes and obesity if governments and companies enact policies that minimize exposure to EDCs to protect public health and the environment.”
Plastics may contain any one of a number of EDCs, such as polybrominated diphenylethers in flame retardant additives, phthalates in food packaging, bisphenols in can linings, and perfluoroalkyl and polyfluoroalkyl substances (PFAS) in nonstick cooking utensils.
in developing fetuses and children, and even death.
In March 2022, the United Nations Environment Assembly committed to a global plastics treaty to “end plastic pollution and forge an international legally binding agreement by 2024” that “addresses the full life cycle of plastic, including its production, design and disposal.”
Minimizing EDC Exposure
But what can doctors tell their patients today to help them reduce their exposure to EDCs?
“There are safe and simple steps that people can take to limit their exposure to the chemicals of greatest concern,” Dr. Trasande told this news organization.
This can be partly achieved by reducing plastic use down to its essentials. “To use an example, when you are flying, fill up a stainless steel container after clearing security. At home, use glass or stainless steel” rather than plastic bottles or containers.
In particular, “avoiding microwaving plastic is important,” Dr. Trasande said, “even if a container says it’s microwave-safe.”
He warned that “many chemicals used in plastic are not covalently bound, and heat facilitates leaching into food. Microscopic contaminants can also get into food when you microwave plastic.”
Dr. Trasande also suggests limiting canned food consumption and avoiding cleaning plastic food containers in machine dishwashers.
Calculating the Disease Burden
To accurately assess the “the tradeoffs involved in the ongoing reliance on plastic production as a source of economic productivity,” the current researchers calculated the attributable disease burden and cost related to EDCs used in plastic materials in the United States in 2018.
Building on previously published analyses, they used industry reports, publications by national and international governing bodies, and peer-reviewed publications to determine the usage of each type of EDC and its attributable disease and disability burden.
This plastic-related fraction (PRF) of disease burden was then used to calculate an updated cost estimate for each EDC, based on the assumption that the disease burden is directly proportional to its exposure.
They found that for bisphenol A, 97.5% of its use, and therefore its estimated PRF of disease burden, was related to the manufacture of plastics, while this figure was 98%-100% for phthalates. For PDBE, 98% of its use was in plastics vs 93% for PFAS.
The researchers then estimated that the total plastic-attributable disease burden in the United States in 2018 cost the nation $249 billion, or 1.22% of the gross domestic product. Of this, $159 billion was linked to PDBE exposure, which is associated with diseases such as cancer.
Moreover, $1.02 billion plastic-attributable disease burden was associated with bisphenol A exposure, which can have potentially harmful health effects on the immune system; followed by $66.7 billion due to phthalates, which are linked to preterm birth, reduced sperm count, and childhood obesity; and $22.4 billion due to PFAS, which are associated with kidney failure and gestational diabetes.
The study was supported by the National Institutes of Health and the Passport Foundation.
Dr. Trasande declared relationships with Audible, Houghton Mifflin, Paidos, and Kobunsha, none of which relate to the present manuscript.
No other financial relationships were declared.
A version of this article appeared on Medscape.com.
Exposure to endocrine-disrupting chemicals (EDCs) via daily use of plastics is a major contributor to the overall disease burden in the United States and the associated costs to society amount to more than 1% of the gross domestic product, revealed a large-scale analysis.
The research, published in the Journal of the Endocrine Society, indicated that taken together, the disease burden attributable to EDCs used in the manufacture of plastics added up to almost $250 billion in 2018 alone.
“The diseases due to plastics run the entire life course from preterm birth to obesity, heart disease, and cancers,” commented lead author Leonardo Trasande, MD, MPP, Jim G. Hendrick, MD Professor of Pediatrics, Department of Pediatrics, NYU Langone Medical Center, New York, in a release.
“Our study drives home the need to address chemicals used in plastic materials” through global treaties and other policy initiatives, he said, so as to “reduce these costs” in line with reductions in exposure to the chemicals.
Co-author Michael Belliveau, Executive Director at Defend Our Health in Portland, ME, agreed, saying: “We can reduce these health costs and the prevalence of chronic endocrine diseases such as diabetes and obesity if governments and companies enact policies that minimize exposure to EDCs to protect public health and the environment.”
Plastics may contain any one of a number of EDCs, such as polybrominated diphenylethers in flame retardant additives, phthalates in food packaging, bisphenols in can linings, and perfluoroalkyl and polyfluoroalkyl substances (PFAS) in nonstick cooking utensils.
in developing fetuses and children, and even death.
In March 2022, the United Nations Environment Assembly committed to a global plastics treaty to “end plastic pollution and forge an international legally binding agreement by 2024” that “addresses the full life cycle of plastic, including its production, design and disposal.”
Minimizing EDC Exposure
But what can doctors tell their patients today to help them reduce their exposure to EDCs?
“There are safe and simple steps that people can take to limit their exposure to the chemicals of greatest concern,” Dr. Trasande told this news organization.
This can be partly achieved by reducing plastic use down to its essentials. “To use an example, when you are flying, fill up a stainless steel container after clearing security. At home, use glass or stainless steel” rather than plastic bottles or containers.
In particular, “avoiding microwaving plastic is important,” Dr. Trasande said, “even if a container says it’s microwave-safe.”
He warned that “many chemicals used in plastic are not covalently bound, and heat facilitates leaching into food. Microscopic contaminants can also get into food when you microwave plastic.”
Dr. Trasande also suggests limiting canned food consumption and avoiding cleaning plastic food containers in machine dishwashers.
Calculating the Disease Burden
To accurately assess the “the tradeoffs involved in the ongoing reliance on plastic production as a source of economic productivity,” the current researchers calculated the attributable disease burden and cost related to EDCs used in plastic materials in the United States in 2018.
Building on previously published analyses, they used industry reports, publications by national and international governing bodies, and peer-reviewed publications to determine the usage of each type of EDC and its attributable disease and disability burden.
This plastic-related fraction (PRF) of disease burden was then used to calculate an updated cost estimate for each EDC, based on the assumption that the disease burden is directly proportional to its exposure.
They found that for bisphenol A, 97.5% of its use, and therefore its estimated PRF of disease burden, was related to the manufacture of plastics, while this figure was 98%-100% for phthalates. For PDBE, 98% of its use was in plastics vs 93% for PFAS.
The researchers then estimated that the total plastic-attributable disease burden in the United States in 2018 cost the nation $249 billion, or 1.22% of the gross domestic product. Of this, $159 billion was linked to PDBE exposure, which is associated with diseases such as cancer.
Moreover, $1.02 billion plastic-attributable disease burden was associated with bisphenol A exposure, which can have potentially harmful health effects on the immune system; followed by $66.7 billion due to phthalates, which are linked to preterm birth, reduced sperm count, and childhood obesity; and $22.4 billion due to PFAS, which are associated with kidney failure and gestational diabetes.
The study was supported by the National Institutes of Health and the Passport Foundation.
Dr. Trasande declared relationships with Audible, Houghton Mifflin, Paidos, and Kobunsha, none of which relate to the present manuscript.
No other financial relationships were declared.
A version of this article appeared on Medscape.com.
Exposure to endocrine-disrupting chemicals (EDCs) via daily use of plastics is a major contributor to the overall disease burden in the United States and the associated costs to society amount to more than 1% of the gross domestic product, revealed a large-scale analysis.
The research, published in the Journal of the Endocrine Society, indicated that taken together, the disease burden attributable to EDCs used in the manufacture of plastics added up to almost $250 billion in 2018 alone.
“The diseases due to plastics run the entire life course from preterm birth to obesity, heart disease, and cancers,” commented lead author Leonardo Trasande, MD, MPP, Jim G. Hendrick, MD Professor of Pediatrics, Department of Pediatrics, NYU Langone Medical Center, New York, in a release.
“Our study drives home the need to address chemicals used in plastic materials” through global treaties and other policy initiatives, he said, so as to “reduce these costs” in line with reductions in exposure to the chemicals.
Co-author Michael Belliveau, Executive Director at Defend Our Health in Portland, ME, agreed, saying: “We can reduce these health costs and the prevalence of chronic endocrine diseases such as diabetes and obesity if governments and companies enact policies that minimize exposure to EDCs to protect public health and the environment.”
Plastics may contain any one of a number of EDCs, such as polybrominated diphenylethers in flame retardant additives, phthalates in food packaging, bisphenols in can linings, and perfluoroalkyl and polyfluoroalkyl substances (PFAS) in nonstick cooking utensils.
in developing fetuses and children, and even death.
In March 2022, the United Nations Environment Assembly committed to a global plastics treaty to “end plastic pollution and forge an international legally binding agreement by 2024” that “addresses the full life cycle of plastic, including its production, design and disposal.”
Minimizing EDC Exposure
But what can doctors tell their patients today to help them reduce their exposure to EDCs?
“There are safe and simple steps that people can take to limit their exposure to the chemicals of greatest concern,” Dr. Trasande told this news organization.
This can be partly achieved by reducing plastic use down to its essentials. “To use an example, when you are flying, fill up a stainless steel container after clearing security. At home, use glass or stainless steel” rather than plastic bottles or containers.
In particular, “avoiding microwaving plastic is important,” Dr. Trasande said, “even if a container says it’s microwave-safe.”
He warned that “many chemicals used in plastic are not covalently bound, and heat facilitates leaching into food. Microscopic contaminants can also get into food when you microwave plastic.”
Dr. Trasande also suggests limiting canned food consumption and avoiding cleaning plastic food containers in machine dishwashers.
Calculating the Disease Burden
To accurately assess the “the tradeoffs involved in the ongoing reliance on plastic production as a source of economic productivity,” the current researchers calculated the attributable disease burden and cost related to EDCs used in plastic materials in the United States in 2018.
Building on previously published analyses, they used industry reports, publications by national and international governing bodies, and peer-reviewed publications to determine the usage of each type of EDC and its attributable disease and disability burden.
This plastic-related fraction (PRF) of disease burden was then used to calculate an updated cost estimate for each EDC, based on the assumption that the disease burden is directly proportional to its exposure.
They found that for bisphenol A, 97.5% of its use, and therefore its estimated PRF of disease burden, was related to the manufacture of plastics, while this figure was 98%-100% for phthalates. For PDBE, 98% of its use was in plastics vs 93% for PFAS.
The researchers then estimated that the total plastic-attributable disease burden in the United States in 2018 cost the nation $249 billion, or 1.22% of the gross domestic product. Of this, $159 billion was linked to PDBE exposure, which is associated with diseases such as cancer.
Moreover, $1.02 billion plastic-attributable disease burden was associated with bisphenol A exposure, which can have potentially harmful health effects on the immune system; followed by $66.7 billion due to phthalates, which are linked to preterm birth, reduced sperm count, and childhood obesity; and $22.4 billion due to PFAS, which are associated with kidney failure and gestational diabetes.
The study was supported by the National Institutes of Health and the Passport Foundation.
Dr. Trasande declared relationships with Audible, Houghton Mifflin, Paidos, and Kobunsha, none of which relate to the present manuscript.
No other financial relationships were declared.
A version of this article appeared on Medscape.com.
FROM THE JOURNAL OF THE ENDOCRINE SOCIETY
Optimal Follow-up After Fertility-Sparing Cervical Cancer Surgery
TOPLINE:
METHODOLOGY:
- Among patients with early-stage cervical cancer, the optimal follow-up strategy to detect recurrence after fertility-sparing surgery remains unclear. The authors wanted to find out if follow-up could be tailored to the patient’s risk for recurrence instead of using the current inefficient one-size-fits-all approach.
- The retrospective cohort study, which used data from the Netherlands Cancer Registry and the Dutch Nationwide Pathology Databank, included 1462 patients aged 18-40 years with early-stage cervical cancer who received fertility-sparing surgery (large loop excision of the transformation zone, conization, or trachelectomy) between 2000 and 2020.
- The primary endpoint was the cumulative incidence of recurrent cervical intraepithelial neoplasia grade 2 or worse (CIN2+), including recurrent cervical cancer.
- The authors stratified the likelihood of recurrence by cytology and high-risk HPV results at the first follow-up visit within 12 months of fertility-sparing surgery; they also compared the cumulative incidence of recurrence — the number of new cases divided by all at-risk individuals over a specific interval — at four timepoints in 2 years (6, 12, 18, and 24 months).
TAKEAWAY:
- Overall, the 10-year recurrence-free survival for CIN2+ was 89.3%. Patients with high-grade cytology at the first follow-up had worse 10-year recurrence-free survival for CIN2+ (43.1%) than those who had normal (92.1%) and low-grade cytology (84.6%). Similarly for HPV status, patients positive for high-risk HPV at the first follow-up had worse 10-year recurrence-free survival rates for CIN2+ (73.6%) than those negative for high-risk HPV (91.1%).
- Patients negative for both high-risk HPV and high-grade cytology 6-24 months after fertility-sparing surgery had a cumulative incidence of recurrence of 0.0%-0.7% within 6 months of follow-up compared with 0.0%-33.3% among patients negative for high-risk HPV but who had high-grade cytology.
- By contrast, patients positive for high-risk HPV but not high-grade cytology had a cumulative incidence of recurrence of 0.0%-15.4% within 6 months of any follow-up visit compared with 50.0%-100.0% among those with both high-risk HPV and high-grade cytology.
- Patients who remained free of high-risk HPV and high-grade cytology at their 6-month and 12-month follow-ups had no disease recurrence over the next 6 months.
IN PRACTICE:
“Patients who are negative for high-risk HPV with normal or low-grade cytology at 6-24 months after fertility-sparing surgery could be offered a prolonged follow-up interval of 6 months,” the authors concluded, adding that this “group comprises 80% of all patients receiving fertility-sparing surgery.”
“Reducing the number of follow-up visits, and subsequently the number of follow-up tests, in patients with low risk for recurrence on the basis of co-testing has the potential to substantially reduce healthcare costs,” the authors explained.
SOURCE:
The study, led by Teska N. Schuurman, MD, of the Netherlands Cancer Institute, Amsterdam, was published in the December 2023 issue of The Lancet Oncology.
LIMITATIONS:
The retrospective design of the study meant that analysis was limited to available records, so data on patients’ symptoms, physical examinations, or colposcopic findings were not available. Follow-up biopsies, considered the gold standard for diagnosing recurrence, are not routine in the Netherlands, so recurrence could have been underreported.
DISCLOSURES:
The authors declared no competing interests.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Among patients with early-stage cervical cancer, the optimal follow-up strategy to detect recurrence after fertility-sparing surgery remains unclear. The authors wanted to find out if follow-up could be tailored to the patient’s risk for recurrence instead of using the current inefficient one-size-fits-all approach.
- The retrospective cohort study, which used data from the Netherlands Cancer Registry and the Dutch Nationwide Pathology Databank, included 1462 patients aged 18-40 years with early-stage cervical cancer who received fertility-sparing surgery (large loop excision of the transformation zone, conization, or trachelectomy) between 2000 and 2020.
- The primary endpoint was the cumulative incidence of recurrent cervical intraepithelial neoplasia grade 2 or worse (CIN2+), including recurrent cervical cancer.
- The authors stratified the likelihood of recurrence by cytology and high-risk HPV results at the first follow-up visit within 12 months of fertility-sparing surgery; they also compared the cumulative incidence of recurrence — the number of new cases divided by all at-risk individuals over a specific interval — at four timepoints in 2 years (6, 12, 18, and 24 months).
TAKEAWAY:
- Overall, the 10-year recurrence-free survival for CIN2+ was 89.3%. Patients with high-grade cytology at the first follow-up had worse 10-year recurrence-free survival for CIN2+ (43.1%) than those who had normal (92.1%) and low-grade cytology (84.6%). Similarly for HPV status, patients positive for high-risk HPV at the first follow-up had worse 10-year recurrence-free survival rates for CIN2+ (73.6%) than those negative for high-risk HPV (91.1%).
- Patients negative for both high-risk HPV and high-grade cytology 6-24 months after fertility-sparing surgery had a cumulative incidence of recurrence of 0.0%-0.7% within 6 months of follow-up compared with 0.0%-33.3% among patients negative for high-risk HPV but who had high-grade cytology.
- By contrast, patients positive for high-risk HPV but not high-grade cytology had a cumulative incidence of recurrence of 0.0%-15.4% within 6 months of any follow-up visit compared with 50.0%-100.0% among those with both high-risk HPV and high-grade cytology.
- Patients who remained free of high-risk HPV and high-grade cytology at their 6-month and 12-month follow-ups had no disease recurrence over the next 6 months.
IN PRACTICE:
“Patients who are negative for high-risk HPV with normal or low-grade cytology at 6-24 months after fertility-sparing surgery could be offered a prolonged follow-up interval of 6 months,” the authors concluded, adding that this “group comprises 80% of all patients receiving fertility-sparing surgery.”
“Reducing the number of follow-up visits, and subsequently the number of follow-up tests, in patients with low risk for recurrence on the basis of co-testing has the potential to substantially reduce healthcare costs,” the authors explained.
SOURCE:
The study, led by Teska N. Schuurman, MD, of the Netherlands Cancer Institute, Amsterdam, was published in the December 2023 issue of The Lancet Oncology.
LIMITATIONS:
The retrospective design of the study meant that analysis was limited to available records, so data on patients’ symptoms, physical examinations, or colposcopic findings were not available. Follow-up biopsies, considered the gold standard for diagnosing recurrence, are not routine in the Netherlands, so recurrence could have been underreported.
DISCLOSURES:
The authors declared no competing interests.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Among patients with early-stage cervical cancer, the optimal follow-up strategy to detect recurrence after fertility-sparing surgery remains unclear. The authors wanted to find out if follow-up could be tailored to the patient’s risk for recurrence instead of using the current inefficient one-size-fits-all approach.
- The retrospective cohort study, which used data from the Netherlands Cancer Registry and the Dutch Nationwide Pathology Databank, included 1462 patients aged 18-40 years with early-stage cervical cancer who received fertility-sparing surgery (large loop excision of the transformation zone, conization, or trachelectomy) between 2000 and 2020.
- The primary endpoint was the cumulative incidence of recurrent cervical intraepithelial neoplasia grade 2 or worse (CIN2+), including recurrent cervical cancer.
- The authors stratified the likelihood of recurrence by cytology and high-risk HPV results at the first follow-up visit within 12 months of fertility-sparing surgery; they also compared the cumulative incidence of recurrence — the number of new cases divided by all at-risk individuals over a specific interval — at four timepoints in 2 years (6, 12, 18, and 24 months).
TAKEAWAY:
- Overall, the 10-year recurrence-free survival for CIN2+ was 89.3%. Patients with high-grade cytology at the first follow-up had worse 10-year recurrence-free survival for CIN2+ (43.1%) than those who had normal (92.1%) and low-grade cytology (84.6%). Similarly for HPV status, patients positive for high-risk HPV at the first follow-up had worse 10-year recurrence-free survival rates for CIN2+ (73.6%) than those negative for high-risk HPV (91.1%).
- Patients negative for both high-risk HPV and high-grade cytology 6-24 months after fertility-sparing surgery had a cumulative incidence of recurrence of 0.0%-0.7% within 6 months of follow-up compared with 0.0%-33.3% among patients negative for high-risk HPV but who had high-grade cytology.
- By contrast, patients positive for high-risk HPV but not high-grade cytology had a cumulative incidence of recurrence of 0.0%-15.4% within 6 months of any follow-up visit compared with 50.0%-100.0% among those with both high-risk HPV and high-grade cytology.
- Patients who remained free of high-risk HPV and high-grade cytology at their 6-month and 12-month follow-ups had no disease recurrence over the next 6 months.
IN PRACTICE:
“Patients who are negative for high-risk HPV with normal or low-grade cytology at 6-24 months after fertility-sparing surgery could be offered a prolonged follow-up interval of 6 months,” the authors concluded, adding that this “group comprises 80% of all patients receiving fertility-sparing surgery.”
“Reducing the number of follow-up visits, and subsequently the number of follow-up tests, in patients with low risk for recurrence on the basis of co-testing has the potential to substantially reduce healthcare costs,” the authors explained.
SOURCE:
The study, led by Teska N. Schuurman, MD, of the Netherlands Cancer Institute, Amsterdam, was published in the December 2023 issue of The Lancet Oncology.
LIMITATIONS:
The retrospective design of the study meant that analysis was limited to available records, so data on patients’ symptoms, physical examinations, or colposcopic findings were not available. Follow-up biopsies, considered the gold standard for diagnosing recurrence, are not routine in the Netherlands, so recurrence could have been underreported.
DISCLOSURES:
The authors declared no competing interests.
A version of this article appeared on Medscape.com.
Patients with HR-positive breast cancer can safely use ART
SAN ANTONIO — who pause endocrine therapy to conceive, according to new data from the POSITIVE trial.
“We believe these data are of vital importance for the oncofertility counseling of young breast cancer patients,” Hatem A. Azim Jr., MD, PhD, adjunct professor, School of Medicine and Breast Cancer Center, Monterrey Institute of Technology, Mexico, said in a presentation at the San Antonio Breast Cancer Symposium.
As reported previously by this news organization, the primary results of the POSITIVE trial showed that interrupting endocrine therapy to allow pregnancy does not increase the risk of recurrence at 41 months follow-up.
Yet, there is concern that use of fertility preservation or assisted reproductive technology methods — especially those that entail the use of hormones — could have harmful effects on patients with HR-positive breast cancers, Dr. Azim explained.
To investigate, Dr. Azim and colleagues did a secondary analysis of outcomes from the POSITIVE trial, focusing on resumption of menstruation and use of fertility preservation and assisted reproductive technologies.
Among 516 women evaluated for the menstruation analysis, two thirds were aged 35 and older and a little more than half (53%) reported amenorrhea at enrollment, “which is not surprising,” Dr. Azim said.
“What is encouraging,” he said, is that 85% of women recovered menses within 6 months and 94% within 12 months of pausing endocrine therapy.
Among 497 evaluable participants who paused endocrine therapy to attempt pregnancy, 368 (74%) became pregnant.
Looking at time to pregnancy, there was a clear association between younger age at enrollment and shorter time to pregnancy. The cumulative incidence of pregnancy at 12 months was 64% in women younger than age 35 years, 54% in those aged 35-39, and 38% in those age 40-42. In a multivariable model, age < 35 was the only factor independently associated with a shorter time to pregnancy.
No Harmful Impact on Breast Cancer Outcomes
Turning to fertility preservation and use of assisted reproductive technologies, roughly half of the women (51%) underwent some form of fertility preservation at breast cancer diagnosis and before trial enrollment, most commonly ovarian stimulation for embryo or oocyte cryopreservation.
After enrollment, 43% of women underwent some form of assisted reproductive technology to attempt pregnancy, most commonly ovarian stimulation for in vitro fertilization (IVF) and cryopreserved embryo transfer.
In the multivariable model, cryopreserved embryo transfer was the only assisted reproductive technology significantly associated with a greater chance of becoming pregnant, more than doubling patients’ odds (odds ratio, 2.4).
“This means that at breast cancer diagnosis, we should consider cryopreservation of embryos for future use if desired,” Dr. Azim said.
Again, age mattered. Women younger than 35 undergoing assisted reproductive technologies had a 50% higher chance of becoming pregnant compared with peers aged 35-39, and an 84% higher chance than women aged 40-42.
Importantly, there was no apparent short-term detrimental impact of fertility preservation and/or assisted reproductive technologies on breast cancer outcomes, Dr. Azim reported. At 3 years, the breast cancer-free interval was almost identical between women who underwent ovarian stimulation for cryopreservation and those who did not (9.7% vs 8.7%).
“POSITIVE showed positive results that emphasize the importance of active oncofertility counseling with the patient starting at diagnosis,” said Hee Jeong Kim, MD, PhD, professor, Division of Breast Surgery, Asan Medical Center, Seoul, Republic of Korea, and discussant for the study.
“These data are reassuring for our young patients with a diagnosis of breast cancer and shows that assisted reproductive technology is an option and is probably safe to do with the caveat that it needs longer follow-up,” added SABCS codirector Carlos Arteaga, MD, director, Simmons Comprehensive Cancer Center, UT Southwestern Medical Center, Dallas.
Dr. Azim has no relevant disclosures. Dr. Arteaga is a scientific adviser to Novartis, Lilly, Merck, AstraZeneca, Daiichi Sankyo, OrigiMed, Immunomedics, PUMA Biotechnology, TAIHO Oncology, Sanofi, and the Susan G. Komen Foundation. He has received grant support from Pfizer, Lilly, and Takeda. Dr. Kim reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
SAN ANTONIO — who pause endocrine therapy to conceive, according to new data from the POSITIVE trial.
“We believe these data are of vital importance for the oncofertility counseling of young breast cancer patients,” Hatem A. Azim Jr., MD, PhD, adjunct professor, School of Medicine and Breast Cancer Center, Monterrey Institute of Technology, Mexico, said in a presentation at the San Antonio Breast Cancer Symposium.
As reported previously by this news organization, the primary results of the POSITIVE trial showed that interrupting endocrine therapy to allow pregnancy does not increase the risk of recurrence at 41 months follow-up.
Yet, there is concern that use of fertility preservation or assisted reproductive technology methods — especially those that entail the use of hormones — could have harmful effects on patients with HR-positive breast cancers, Dr. Azim explained.
To investigate, Dr. Azim and colleagues did a secondary analysis of outcomes from the POSITIVE trial, focusing on resumption of menstruation and use of fertility preservation and assisted reproductive technologies.
Among 516 women evaluated for the menstruation analysis, two thirds were aged 35 and older and a little more than half (53%) reported amenorrhea at enrollment, “which is not surprising,” Dr. Azim said.
“What is encouraging,” he said, is that 85% of women recovered menses within 6 months and 94% within 12 months of pausing endocrine therapy.
Among 497 evaluable participants who paused endocrine therapy to attempt pregnancy, 368 (74%) became pregnant.
Looking at time to pregnancy, there was a clear association between younger age at enrollment and shorter time to pregnancy. The cumulative incidence of pregnancy at 12 months was 64% in women younger than age 35 years, 54% in those aged 35-39, and 38% in those age 40-42. In a multivariable model, age < 35 was the only factor independently associated with a shorter time to pregnancy.
No Harmful Impact on Breast Cancer Outcomes
Turning to fertility preservation and use of assisted reproductive technologies, roughly half of the women (51%) underwent some form of fertility preservation at breast cancer diagnosis and before trial enrollment, most commonly ovarian stimulation for embryo or oocyte cryopreservation.
After enrollment, 43% of women underwent some form of assisted reproductive technology to attempt pregnancy, most commonly ovarian stimulation for in vitro fertilization (IVF) and cryopreserved embryo transfer.
In the multivariable model, cryopreserved embryo transfer was the only assisted reproductive technology significantly associated with a greater chance of becoming pregnant, more than doubling patients’ odds (odds ratio, 2.4).
“This means that at breast cancer diagnosis, we should consider cryopreservation of embryos for future use if desired,” Dr. Azim said.
Again, age mattered. Women younger than 35 undergoing assisted reproductive technologies had a 50% higher chance of becoming pregnant compared with peers aged 35-39, and an 84% higher chance than women aged 40-42.
Importantly, there was no apparent short-term detrimental impact of fertility preservation and/or assisted reproductive technologies on breast cancer outcomes, Dr. Azim reported. At 3 years, the breast cancer-free interval was almost identical between women who underwent ovarian stimulation for cryopreservation and those who did not (9.7% vs 8.7%).
“POSITIVE showed positive results that emphasize the importance of active oncofertility counseling with the patient starting at diagnosis,” said Hee Jeong Kim, MD, PhD, professor, Division of Breast Surgery, Asan Medical Center, Seoul, Republic of Korea, and discussant for the study.
“These data are reassuring for our young patients with a diagnosis of breast cancer and shows that assisted reproductive technology is an option and is probably safe to do with the caveat that it needs longer follow-up,” added SABCS codirector Carlos Arteaga, MD, director, Simmons Comprehensive Cancer Center, UT Southwestern Medical Center, Dallas.
Dr. Azim has no relevant disclosures. Dr. Arteaga is a scientific adviser to Novartis, Lilly, Merck, AstraZeneca, Daiichi Sankyo, OrigiMed, Immunomedics, PUMA Biotechnology, TAIHO Oncology, Sanofi, and the Susan G. Komen Foundation. He has received grant support from Pfizer, Lilly, and Takeda. Dr. Kim reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
SAN ANTONIO — who pause endocrine therapy to conceive, according to new data from the POSITIVE trial.
“We believe these data are of vital importance for the oncofertility counseling of young breast cancer patients,” Hatem A. Azim Jr., MD, PhD, adjunct professor, School of Medicine and Breast Cancer Center, Monterrey Institute of Technology, Mexico, said in a presentation at the San Antonio Breast Cancer Symposium.
As reported previously by this news organization, the primary results of the POSITIVE trial showed that interrupting endocrine therapy to allow pregnancy does not increase the risk of recurrence at 41 months follow-up.
Yet, there is concern that use of fertility preservation or assisted reproductive technology methods — especially those that entail the use of hormones — could have harmful effects on patients with HR-positive breast cancers, Dr. Azim explained.
To investigate, Dr. Azim and colleagues did a secondary analysis of outcomes from the POSITIVE trial, focusing on resumption of menstruation and use of fertility preservation and assisted reproductive technologies.
Among 516 women evaluated for the menstruation analysis, two thirds were aged 35 and older and a little more than half (53%) reported amenorrhea at enrollment, “which is not surprising,” Dr. Azim said.
“What is encouraging,” he said, is that 85% of women recovered menses within 6 months and 94% within 12 months of pausing endocrine therapy.
Among 497 evaluable participants who paused endocrine therapy to attempt pregnancy, 368 (74%) became pregnant.
Looking at time to pregnancy, there was a clear association between younger age at enrollment and shorter time to pregnancy. The cumulative incidence of pregnancy at 12 months was 64% in women younger than age 35 years, 54% in those aged 35-39, and 38% in those age 40-42. In a multivariable model, age < 35 was the only factor independently associated with a shorter time to pregnancy.
No Harmful Impact on Breast Cancer Outcomes
Turning to fertility preservation and use of assisted reproductive technologies, roughly half of the women (51%) underwent some form of fertility preservation at breast cancer diagnosis and before trial enrollment, most commonly ovarian stimulation for embryo or oocyte cryopreservation.
After enrollment, 43% of women underwent some form of assisted reproductive technology to attempt pregnancy, most commonly ovarian stimulation for in vitro fertilization (IVF) and cryopreserved embryo transfer.
In the multivariable model, cryopreserved embryo transfer was the only assisted reproductive technology significantly associated with a greater chance of becoming pregnant, more than doubling patients’ odds (odds ratio, 2.4).
“This means that at breast cancer diagnosis, we should consider cryopreservation of embryos for future use if desired,” Dr. Azim said.
Again, age mattered. Women younger than 35 undergoing assisted reproductive technologies had a 50% higher chance of becoming pregnant compared with peers aged 35-39, and an 84% higher chance than women aged 40-42.
Importantly, there was no apparent short-term detrimental impact of fertility preservation and/or assisted reproductive technologies on breast cancer outcomes, Dr. Azim reported. At 3 years, the breast cancer-free interval was almost identical between women who underwent ovarian stimulation for cryopreservation and those who did not (9.7% vs 8.7%).
“POSITIVE showed positive results that emphasize the importance of active oncofertility counseling with the patient starting at diagnosis,” said Hee Jeong Kim, MD, PhD, professor, Division of Breast Surgery, Asan Medical Center, Seoul, Republic of Korea, and discussant for the study.
“These data are reassuring for our young patients with a diagnosis of breast cancer and shows that assisted reproductive technology is an option and is probably safe to do with the caveat that it needs longer follow-up,” added SABCS codirector Carlos Arteaga, MD, director, Simmons Comprehensive Cancer Center, UT Southwestern Medical Center, Dallas.
Dr. Azim has no relevant disclosures. Dr. Arteaga is a scientific adviser to Novartis, Lilly, Merck, AstraZeneca, Daiichi Sankyo, OrigiMed, Immunomedics, PUMA Biotechnology, TAIHO Oncology, Sanofi, and the Susan G. Komen Foundation. He has received grant support from Pfizer, Lilly, and Takeda. Dr. Kim reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM SABCS 2023
What is the link between cellphones and male fertility?
Infertility affects approximately one in six couples worldwide. More than half the time, it is the man’s low sperm quality that is to blame. Over the last three decades, sperm quality seems to have declined for no clearly identifiable reason. Theories are running rampant without anyone having the proof to back them up.
Potential Causes
The environment, lifestyle, excess weight or obesity, smoking, alcohol consumption, and psychological stress have all been alternately offered up as potential causes, following low-quality epidemiological studies. Cellphones are not exempt from this list, due to their emission of high-frequency (800-2200 MHz) electromagnetic waves that can be absorbed by the body.
Clinical trials conducted in rats or mice suggest that these waves can affect sperm quality and lead to histological changes to the testicles, bearing in mind that the conditions met in these trials are very far from our day-to-day exposure to electromagnetic waves, mostly via our cellphones.
The same observation can be made about experiments conducted on human sperm in vitro, but changes to the latter caused by electromagnetic waves leave doubts. Observational studies are rare, carried out in small cohorts, and marred by largely conflicting results. Publication bias plays a major role, just as much as the abundance of potential confounding factors does.
Swiss Observational Study
An observational study carried out in Switzerland had the benefit of involving a large cohort of 2886 young men who were representative of the general population. The participants completed an online questionnaire describing their relationship with their cellphone in detail and in qualitative and quantitative terms.
The study was launched in 2005, before cellphone use became so widespread, and this timeline was considered when looking for a link between cellphone exposure and sperm quality. In addition, multiple adjustments were made in the multivariate analyses to account for as many potential confounding factors as possible.
The participants, aged between 18 and 22 years, were recruited during a 3-day period to assess their suitability for military service. Each year, this cohort makes up 97% of the male population in Switzerland in this age range, with the remaining 3% being excluded from the selection process due to disability or chronic illness.
Regardless of the review board’s decision, subjects wishing to take part in the study were given a detailed description of what it involved, a consent form, and two questionnaires. The first focused on the individual directly, asking questions about his health and lifestyle. The second, intended for his parents, dealt with the period before conception.
This recruitment, which took place between September 2005 and November 2018, involved the researchers contacting 106,924 men. Ultimately, only 5.3% of subjects contacted returned the completed documentation. In the end, the study involved 2886 participants (3.1%) who provided all the necessary information, especially the laboratory testing (including a sperm analysis) needed to meet the study objectives. The number of hours spent on a smartphone and how it was used were routinely considered, as was sperm quality (volume, concentration, and total sperm count, as well as sperm mobility and morphology).
Significant Associations
A data analysis using an adjusted linear model revealed a significant association between frequent phone use (> 20 times per day) and lower sperm concentration (in mL) (adjusted β: -0.152, 95% CI -0.316 to 0.011). The same was found for their total concentration in ejaculate (adjusted β: -0.271, 95% CI -0.515 to -0.027).
An adjusted logistic regression analysis estimated that the risk for subnormal male fertility levels, as determined by the World Health Organization (WHO), was increased by at most 30%, when referring to the concentration of sperm per mL (21% in terms of total concentration). This inverse link was shown to be more pronounced during the first phase of the study (2005-2007), compared with the other two phases (2008-2011 and 2012-2018). Yet no links involving sperm mobility or morphology were found, and carrying a cellphone in a trouser pocket had no impact on the results.
This study certainly involves a large cohort of nearly 3000 young men. It is, nonetheless, retrospective, and its methodology, despite being better than that of previous studies, is still open to criticism. Its results can only fuel hypotheses, nothing more. Only prospective cohort studies will allow conclusions to be drawn and, in the meantime,
This article was translated from JIM, which is part of the Medscape professional network. A version of this article appeared on Medscape.com.
Infertility affects approximately one in six couples worldwide. More than half the time, it is the man’s low sperm quality that is to blame. Over the last three decades, sperm quality seems to have declined for no clearly identifiable reason. Theories are running rampant without anyone having the proof to back them up.
Potential Causes
The environment, lifestyle, excess weight or obesity, smoking, alcohol consumption, and psychological stress have all been alternately offered up as potential causes, following low-quality epidemiological studies. Cellphones are not exempt from this list, due to their emission of high-frequency (800-2200 MHz) electromagnetic waves that can be absorbed by the body.
Clinical trials conducted in rats or mice suggest that these waves can affect sperm quality and lead to histological changes to the testicles, bearing in mind that the conditions met in these trials are very far from our day-to-day exposure to electromagnetic waves, mostly via our cellphones.
The same observation can be made about experiments conducted on human sperm in vitro, but changes to the latter caused by electromagnetic waves leave doubts. Observational studies are rare, carried out in small cohorts, and marred by largely conflicting results. Publication bias plays a major role, just as much as the abundance of potential confounding factors does.
Swiss Observational Study
An observational study carried out in Switzerland had the benefit of involving a large cohort of 2886 young men who were representative of the general population. The participants completed an online questionnaire describing their relationship with their cellphone in detail and in qualitative and quantitative terms.
The study was launched in 2005, before cellphone use became so widespread, and this timeline was considered when looking for a link between cellphone exposure and sperm quality. In addition, multiple adjustments were made in the multivariate analyses to account for as many potential confounding factors as possible.
The participants, aged between 18 and 22 years, were recruited during a 3-day period to assess their suitability for military service. Each year, this cohort makes up 97% of the male population in Switzerland in this age range, with the remaining 3% being excluded from the selection process due to disability or chronic illness.
Regardless of the review board’s decision, subjects wishing to take part in the study were given a detailed description of what it involved, a consent form, and two questionnaires. The first focused on the individual directly, asking questions about his health and lifestyle. The second, intended for his parents, dealt with the period before conception.
This recruitment, which took place between September 2005 and November 2018, involved the researchers contacting 106,924 men. Ultimately, only 5.3% of subjects contacted returned the completed documentation. In the end, the study involved 2886 participants (3.1%) who provided all the necessary information, especially the laboratory testing (including a sperm analysis) needed to meet the study objectives. The number of hours spent on a smartphone and how it was used were routinely considered, as was sperm quality (volume, concentration, and total sperm count, as well as sperm mobility and morphology).
Significant Associations
A data analysis using an adjusted linear model revealed a significant association between frequent phone use (> 20 times per day) and lower sperm concentration (in mL) (adjusted β: -0.152, 95% CI -0.316 to 0.011). The same was found for their total concentration in ejaculate (adjusted β: -0.271, 95% CI -0.515 to -0.027).
An adjusted logistic regression analysis estimated that the risk for subnormal male fertility levels, as determined by the World Health Organization (WHO), was increased by at most 30%, when referring to the concentration of sperm per mL (21% in terms of total concentration). This inverse link was shown to be more pronounced during the first phase of the study (2005-2007), compared with the other two phases (2008-2011 and 2012-2018). Yet no links involving sperm mobility or morphology were found, and carrying a cellphone in a trouser pocket had no impact on the results.
This study certainly involves a large cohort of nearly 3000 young men. It is, nonetheless, retrospective, and its methodology, despite being better than that of previous studies, is still open to criticism. Its results can only fuel hypotheses, nothing more. Only prospective cohort studies will allow conclusions to be drawn and, in the meantime,
This article was translated from JIM, which is part of the Medscape professional network. A version of this article appeared on Medscape.com.
Infertility affects approximately one in six couples worldwide. More than half the time, it is the man’s low sperm quality that is to blame. Over the last three decades, sperm quality seems to have declined for no clearly identifiable reason. Theories are running rampant without anyone having the proof to back them up.
Potential Causes
The environment, lifestyle, excess weight or obesity, smoking, alcohol consumption, and psychological stress have all been alternately offered up as potential causes, following low-quality epidemiological studies. Cellphones are not exempt from this list, due to their emission of high-frequency (800-2200 MHz) electromagnetic waves that can be absorbed by the body.
Clinical trials conducted in rats or mice suggest that these waves can affect sperm quality and lead to histological changes to the testicles, bearing in mind that the conditions met in these trials are very far from our day-to-day exposure to electromagnetic waves, mostly via our cellphones.
The same observation can be made about experiments conducted on human sperm in vitro, but changes to the latter caused by electromagnetic waves leave doubts. Observational studies are rare, carried out in small cohorts, and marred by largely conflicting results. Publication bias plays a major role, just as much as the abundance of potential confounding factors does.
Swiss Observational Study
An observational study carried out in Switzerland had the benefit of involving a large cohort of 2886 young men who were representative of the general population. The participants completed an online questionnaire describing their relationship with their cellphone in detail and in qualitative and quantitative terms.
The study was launched in 2005, before cellphone use became so widespread, and this timeline was considered when looking for a link between cellphone exposure and sperm quality. In addition, multiple adjustments were made in the multivariate analyses to account for as many potential confounding factors as possible.
The participants, aged between 18 and 22 years, were recruited during a 3-day period to assess their suitability for military service. Each year, this cohort makes up 97% of the male population in Switzerland in this age range, with the remaining 3% being excluded from the selection process due to disability or chronic illness.
Regardless of the review board’s decision, subjects wishing to take part in the study were given a detailed description of what it involved, a consent form, and two questionnaires. The first focused on the individual directly, asking questions about his health and lifestyle. The second, intended for his parents, dealt with the period before conception.
This recruitment, which took place between September 2005 and November 2018, involved the researchers contacting 106,924 men. Ultimately, only 5.3% of subjects contacted returned the completed documentation. In the end, the study involved 2886 participants (3.1%) who provided all the necessary information, especially the laboratory testing (including a sperm analysis) needed to meet the study objectives. The number of hours spent on a smartphone and how it was used were routinely considered, as was sperm quality (volume, concentration, and total sperm count, as well as sperm mobility and morphology).
Significant Associations
A data analysis using an adjusted linear model revealed a significant association between frequent phone use (> 20 times per day) and lower sperm concentration (in mL) (adjusted β: -0.152, 95% CI -0.316 to 0.011). The same was found for their total concentration in ejaculate (adjusted β: -0.271, 95% CI -0.515 to -0.027).
An adjusted logistic regression analysis estimated that the risk for subnormal male fertility levels, as determined by the World Health Organization (WHO), was increased by at most 30%, when referring to the concentration of sperm per mL (21% in terms of total concentration). This inverse link was shown to be more pronounced during the first phase of the study (2005-2007), compared with the other two phases (2008-2011 and 2012-2018). Yet no links involving sperm mobility or morphology were found, and carrying a cellphone in a trouser pocket had no impact on the results.
This study certainly involves a large cohort of nearly 3000 young men. It is, nonetheless, retrospective, and its methodology, despite being better than that of previous studies, is still open to criticism. Its results can only fuel hypotheses, nothing more. Only prospective cohort studies will allow conclusions to be drawn and, in the meantime,
This article was translated from JIM, which is part of the Medscape professional network. A version of this article appeared on Medscape.com.