User login
Previous endemic coronavirus encounters linked with long COVID
People who develop long COVID may be responding more strongly to a non–SARS-CoV-2 virus they encountered in the past than to SARS-CoV-2, a study by researchers at Harvard Medical School suggests.
Long COVID, also called postacute sequelae of COVID-19 (PASC), causes various symptoms that persist at least 4 weeks after the initial SARS-CoV-2 infection, they write in the preprint server medRxiv. Four authors explained their research into possible mechanisms of long COVID in an interview.
“Immunity to non-COVID endemic coronaviruses may play a role in who develops PASC,” co–lead author Jonathan D. Herman, MD, PhD, said. “There’s still so much more we need to understand, but it is striking that back-boosting of immune responses to coronavirus OC43 was uniquely enriched in individuals with PASC.”
“In the study, individuals with PASC preferentially generated stronger responses to previously encountered cold-causing coronaviruses,” co–senior author Galit Alter, PhD, said.
“Instead of generating strong SARS-CoV-2 immunity, they bolstered a response to a different coronavirus, potentially making their response less effective in clearing SARS-CoV-2. Surprisingly, most of the individuals had been vaccinated – and they still maintained this unusual antibody response – pointing to new therapeutic pathways to treat PASC,” Dr. Alter said.
Humoral immunity offers a clue to long-COVID origins
One-fifth of COVID-19 patients progress to long COVID, but which patients develop PASC and why are not well understood, the authors write.
“Antibodies represent powerful biomarkers that have been used for decades to diagnose disease. However, antibodies also provide a powerful source of information on previous infections. The use of antibody profiling, here, pointed to the presence of incomplete antibody responses to SARS-CoV-2 in individuals with PASC,” Dr. Alter said.
The researchers reviewed the medical records of patients in the Mass General Brigham health care system in Boston, including referrals from rheumatologists of participants diagnosed with COVID-19 outside the MGB system, starting on March 1, 2020.
They focused on patients with systemic autoimmune rheumatic diseases (SARDs) because their tendency toward inflammation and autoantibody production may make them more susceptible to PASC and enrich for specific inflammatory-driven endotypes.
All 43 participants had COVID-19 without hospital admission and SARDs. Patients treated only for fibromyalgia, osteoarthritis, mechanical back pain, gout, or pseudogout without a SARD were excluded from the study.
Overall, 79% of participants were female, 35% had rheumatoid arthritis, 19% had psoriatic arthritis, and 95% had received a COVID-19 vaccine.
The researchers used systems serology to perform comprehensive antibody profiling against SARS-CoV-2 and a panel of endemic pathogens or routine vaccine antigens.
Long-COVID patients had a distinct immune response
Overall, 17 patients developed PASC and 26 did not, and in those with PASC, they found a distinct humoral immune response. Patients with PASC:
- harbored less inflamed and weaker Fc-gamma receptor–binding anti–SARS-CoV-2 antibodies;
- showed a significantly expanded and more inflamed antibody response against endemic coronavirus OC43; and
- mounted more avid IgM responses and developed expanded inflammatory OC43 S2–specific Fc-receptor–binding responses, which were linked to cross reactivity across SARS-CoV-2 and common coronaviruses.
“Strengths of the study include the detailed phenotypes of cases after COVID-19, particularly to classify PASC presence or absence, as well as the depth and breadth of antibody profiling. This allowed us to identify a humoral immune signature of PASC,” said co–senior author Jeffrey A. Sparks, MD, MMSc.
“However, the study was limited in its size to investigate different types of PASC, such as fatigue or lung symptoms, that may have biologic differences. Also, all patients in the study had a preexisting rheumatic disease,” he acknowledged.
“A substantial portion of patients with COVID-19 will develop PASC, which can have substantial impact on health and quality of life,” said co–senior author Zachary S. Wallace, MD, MS. “Given the higher risk of COVID-19 in many patients with rheumatic disease, it is important to understand the etiology of PASC in this vulnerable population, to enable future diagnostic and therapeutic advances.”
Davey Smith, MD, professor of medicine and head of infectious diseases and global public health at the University of California, San Diego, in La Jolla, who was not involved in the study, called the findings interesting even though the results will not immediately affect patient care.
“There may be a link between previous non–SARS-CoV-2 coronavirus infection and PASC,” he added. “Perhaps, by understanding why some people do and do not get PASC, we can develop treatments for the condition.
“This paper is a preprint and will need to go through peer review,” Dr. Smith said. “There are many elements that need to be scrutinized. For example, there is no definition of PASC that is universally accepted, so how did that play into this study?”
Mark Cameron, PhD, associate professor in the department of population and quantitative health sciences at Case Western Reserve University, Cleveland, called this a strong study from a strong group, although it is a preprint prior to peer review.
“In this initial study, the scientists focused on people who had rheumatic disease before getting COVID-19, knowing they are at higher risk for lasting complications and hopefully are more immunologically similar when diagnosed with long COVID – a single ‘endotype’ or group of patients with similar clinical symptoms and background,” he noted.
“Our immune system’s memory sometimes fails to effectively fight a new virus that looks too much like a virus it saw before. This ineffective immune response can set up various problems, including the poor recoveries we see in people with long COVID,” he said.
“OC43 probably emerged in the late 1800s and probably caused a pandemic of severe respiratory illness between 1889 and 1890, previously thought to be a flu,” Dr. Cameron recalled. “OC43 is still around as an endemic coronavirus, usually causing mild or moderate upper-respiratory infections.”
COVID-19 immunity is complex, and previous SARS-CoV-2 infection doesn’t guarantee we won't get COVID-19 again, especially as new variants emerge, added Dr. Cameron, who also was not involved in the study.
“This study may help us better understand the risks and possible mechanisms associated with COVID-19 and long COVID in the face of previous coronavirus infections,” he said. “It may also help guide future COVID-19 therapies and vaccines.”
The authors plan further related research.
The study received grant support and an anonymous donation. Dr. Alter, Dr. Sparks, and Dr. Wallace report financial relationships with the pharmaceutical industry. All other authors, and Dr. Davey and Dr. Cameron, report no conflicts of interest with the study. All experts commented by email.
* This story was updated 10/12/2022.
People who develop long COVID may be responding more strongly to a non–SARS-CoV-2 virus they encountered in the past than to SARS-CoV-2, a study by researchers at Harvard Medical School suggests.
Long COVID, also called postacute sequelae of COVID-19 (PASC), causes various symptoms that persist at least 4 weeks after the initial SARS-CoV-2 infection, they write in the preprint server medRxiv. Four authors explained their research into possible mechanisms of long COVID in an interview.
“Immunity to non-COVID endemic coronaviruses may play a role in who develops PASC,” co–lead author Jonathan D. Herman, MD, PhD, said. “There’s still so much more we need to understand, but it is striking that back-boosting of immune responses to coronavirus OC43 was uniquely enriched in individuals with PASC.”
“In the study, individuals with PASC preferentially generated stronger responses to previously encountered cold-causing coronaviruses,” co–senior author Galit Alter, PhD, said.
“Instead of generating strong SARS-CoV-2 immunity, they bolstered a response to a different coronavirus, potentially making their response less effective in clearing SARS-CoV-2. Surprisingly, most of the individuals had been vaccinated – and they still maintained this unusual antibody response – pointing to new therapeutic pathways to treat PASC,” Dr. Alter said.
Humoral immunity offers a clue to long-COVID origins
One-fifth of COVID-19 patients progress to long COVID, but which patients develop PASC and why are not well understood, the authors write.
“Antibodies represent powerful biomarkers that have been used for decades to diagnose disease. However, antibodies also provide a powerful source of information on previous infections. The use of antibody profiling, here, pointed to the presence of incomplete antibody responses to SARS-CoV-2 in individuals with PASC,” Dr. Alter said.
The researchers reviewed the medical records of patients in the Mass General Brigham health care system in Boston, including referrals from rheumatologists of participants diagnosed with COVID-19 outside the MGB system, starting on March 1, 2020.
They focused on patients with systemic autoimmune rheumatic diseases (SARDs) because their tendency toward inflammation and autoantibody production may make them more susceptible to PASC and enrich for specific inflammatory-driven endotypes.
All 43 participants had COVID-19 without hospital admission and SARDs. Patients treated only for fibromyalgia, osteoarthritis, mechanical back pain, gout, or pseudogout without a SARD were excluded from the study.
Overall, 79% of participants were female, 35% had rheumatoid arthritis, 19% had psoriatic arthritis, and 95% had received a COVID-19 vaccine.
The researchers used systems serology to perform comprehensive antibody profiling against SARS-CoV-2 and a panel of endemic pathogens or routine vaccine antigens.
Long-COVID patients had a distinct immune response
Overall, 17 patients developed PASC and 26 did not, and in those with PASC, they found a distinct humoral immune response. Patients with PASC:
- harbored less inflamed and weaker Fc-gamma receptor–binding anti–SARS-CoV-2 antibodies;
- showed a significantly expanded and more inflamed antibody response against endemic coronavirus OC43; and
- mounted more avid IgM responses and developed expanded inflammatory OC43 S2–specific Fc-receptor–binding responses, which were linked to cross reactivity across SARS-CoV-2 and common coronaviruses.
“Strengths of the study include the detailed phenotypes of cases after COVID-19, particularly to classify PASC presence or absence, as well as the depth and breadth of antibody profiling. This allowed us to identify a humoral immune signature of PASC,” said co–senior author Jeffrey A. Sparks, MD, MMSc.
“However, the study was limited in its size to investigate different types of PASC, such as fatigue or lung symptoms, that may have biologic differences. Also, all patients in the study had a preexisting rheumatic disease,” he acknowledged.
“A substantial portion of patients with COVID-19 will develop PASC, which can have substantial impact on health and quality of life,” said co–senior author Zachary S. Wallace, MD, MS. “Given the higher risk of COVID-19 in many patients with rheumatic disease, it is important to understand the etiology of PASC in this vulnerable population, to enable future diagnostic and therapeutic advances.”
Davey Smith, MD, professor of medicine and head of infectious diseases and global public health at the University of California, San Diego, in La Jolla, who was not involved in the study, called the findings interesting even though the results will not immediately affect patient care.
“There may be a link between previous non–SARS-CoV-2 coronavirus infection and PASC,” he added. “Perhaps, by understanding why some people do and do not get PASC, we can develop treatments for the condition.
“This paper is a preprint and will need to go through peer review,” Dr. Smith said. “There are many elements that need to be scrutinized. For example, there is no definition of PASC that is universally accepted, so how did that play into this study?”
Mark Cameron, PhD, associate professor in the department of population and quantitative health sciences at Case Western Reserve University, Cleveland, called this a strong study from a strong group, although it is a preprint prior to peer review.
“In this initial study, the scientists focused on people who had rheumatic disease before getting COVID-19, knowing they are at higher risk for lasting complications and hopefully are more immunologically similar when diagnosed with long COVID – a single ‘endotype’ or group of patients with similar clinical symptoms and background,” he noted.
“Our immune system’s memory sometimes fails to effectively fight a new virus that looks too much like a virus it saw before. This ineffective immune response can set up various problems, including the poor recoveries we see in people with long COVID,” he said.
“OC43 probably emerged in the late 1800s and probably caused a pandemic of severe respiratory illness between 1889 and 1890, previously thought to be a flu,” Dr. Cameron recalled. “OC43 is still around as an endemic coronavirus, usually causing mild or moderate upper-respiratory infections.”
COVID-19 immunity is complex, and previous SARS-CoV-2 infection doesn’t guarantee we won't get COVID-19 again, especially as new variants emerge, added Dr. Cameron, who also was not involved in the study.
“This study may help us better understand the risks and possible mechanisms associated with COVID-19 and long COVID in the face of previous coronavirus infections,” he said. “It may also help guide future COVID-19 therapies and vaccines.”
The authors plan further related research.
The study received grant support and an anonymous donation. Dr. Alter, Dr. Sparks, and Dr. Wallace report financial relationships with the pharmaceutical industry. All other authors, and Dr. Davey and Dr. Cameron, report no conflicts of interest with the study. All experts commented by email.
* This story was updated 10/12/2022.
People who develop long COVID may be responding more strongly to a non–SARS-CoV-2 virus they encountered in the past than to SARS-CoV-2, a study by researchers at Harvard Medical School suggests.
Long COVID, also called postacute sequelae of COVID-19 (PASC), causes various symptoms that persist at least 4 weeks after the initial SARS-CoV-2 infection, they write in the preprint server medRxiv. Four authors explained their research into possible mechanisms of long COVID in an interview.
“Immunity to non-COVID endemic coronaviruses may play a role in who develops PASC,” co–lead author Jonathan D. Herman, MD, PhD, said. “There’s still so much more we need to understand, but it is striking that back-boosting of immune responses to coronavirus OC43 was uniquely enriched in individuals with PASC.”
“In the study, individuals with PASC preferentially generated stronger responses to previously encountered cold-causing coronaviruses,” co–senior author Galit Alter, PhD, said.
“Instead of generating strong SARS-CoV-2 immunity, they bolstered a response to a different coronavirus, potentially making their response less effective in clearing SARS-CoV-2. Surprisingly, most of the individuals had been vaccinated – and they still maintained this unusual antibody response – pointing to new therapeutic pathways to treat PASC,” Dr. Alter said.
Humoral immunity offers a clue to long-COVID origins
One-fifth of COVID-19 patients progress to long COVID, but which patients develop PASC and why are not well understood, the authors write.
“Antibodies represent powerful biomarkers that have been used for decades to diagnose disease. However, antibodies also provide a powerful source of information on previous infections. The use of antibody profiling, here, pointed to the presence of incomplete antibody responses to SARS-CoV-2 in individuals with PASC,” Dr. Alter said.
The researchers reviewed the medical records of patients in the Mass General Brigham health care system in Boston, including referrals from rheumatologists of participants diagnosed with COVID-19 outside the MGB system, starting on March 1, 2020.
They focused on patients with systemic autoimmune rheumatic diseases (SARDs) because their tendency toward inflammation and autoantibody production may make them more susceptible to PASC and enrich for specific inflammatory-driven endotypes.
All 43 participants had COVID-19 without hospital admission and SARDs. Patients treated only for fibromyalgia, osteoarthritis, mechanical back pain, gout, or pseudogout without a SARD were excluded from the study.
Overall, 79% of participants were female, 35% had rheumatoid arthritis, 19% had psoriatic arthritis, and 95% had received a COVID-19 vaccine.
The researchers used systems serology to perform comprehensive antibody profiling against SARS-CoV-2 and a panel of endemic pathogens or routine vaccine antigens.
Long-COVID patients had a distinct immune response
Overall, 17 patients developed PASC and 26 did not, and in those with PASC, they found a distinct humoral immune response. Patients with PASC:
- harbored less inflamed and weaker Fc-gamma receptor–binding anti–SARS-CoV-2 antibodies;
- showed a significantly expanded and more inflamed antibody response against endemic coronavirus OC43; and
- mounted more avid IgM responses and developed expanded inflammatory OC43 S2–specific Fc-receptor–binding responses, which were linked to cross reactivity across SARS-CoV-2 and common coronaviruses.
“Strengths of the study include the detailed phenotypes of cases after COVID-19, particularly to classify PASC presence or absence, as well as the depth and breadth of antibody profiling. This allowed us to identify a humoral immune signature of PASC,” said co–senior author Jeffrey A. Sparks, MD, MMSc.
“However, the study was limited in its size to investigate different types of PASC, such as fatigue or lung symptoms, that may have biologic differences. Also, all patients in the study had a preexisting rheumatic disease,” he acknowledged.
“A substantial portion of patients with COVID-19 will develop PASC, which can have substantial impact on health and quality of life,” said co–senior author Zachary S. Wallace, MD, MS. “Given the higher risk of COVID-19 in many patients with rheumatic disease, it is important to understand the etiology of PASC in this vulnerable population, to enable future diagnostic and therapeutic advances.”
Davey Smith, MD, professor of medicine and head of infectious diseases and global public health at the University of California, San Diego, in La Jolla, who was not involved in the study, called the findings interesting even though the results will not immediately affect patient care.
“There may be a link between previous non–SARS-CoV-2 coronavirus infection and PASC,” he added. “Perhaps, by understanding why some people do and do not get PASC, we can develop treatments for the condition.
“This paper is a preprint and will need to go through peer review,” Dr. Smith said. “There are many elements that need to be scrutinized. For example, there is no definition of PASC that is universally accepted, so how did that play into this study?”
Mark Cameron, PhD, associate professor in the department of population and quantitative health sciences at Case Western Reserve University, Cleveland, called this a strong study from a strong group, although it is a preprint prior to peer review.
“In this initial study, the scientists focused on people who had rheumatic disease before getting COVID-19, knowing they are at higher risk for lasting complications and hopefully are more immunologically similar when diagnosed with long COVID – a single ‘endotype’ or group of patients with similar clinical symptoms and background,” he noted.
“Our immune system’s memory sometimes fails to effectively fight a new virus that looks too much like a virus it saw before. This ineffective immune response can set up various problems, including the poor recoveries we see in people with long COVID,” he said.
“OC43 probably emerged in the late 1800s and probably caused a pandemic of severe respiratory illness between 1889 and 1890, previously thought to be a flu,” Dr. Cameron recalled. “OC43 is still around as an endemic coronavirus, usually causing mild or moderate upper-respiratory infections.”
COVID-19 immunity is complex, and previous SARS-CoV-2 infection doesn’t guarantee we won't get COVID-19 again, especially as new variants emerge, added Dr. Cameron, who also was not involved in the study.
“This study may help us better understand the risks and possible mechanisms associated with COVID-19 and long COVID in the face of previous coronavirus infections,” he said. “It may also help guide future COVID-19 therapies and vaccines.”
The authors plan further related research.
The study received grant support and an anonymous donation. Dr. Alter, Dr. Sparks, and Dr. Wallace report financial relationships with the pharmaceutical industry. All other authors, and Dr. Davey and Dr. Cameron, report no conflicts of interest with the study. All experts commented by email.
* This story was updated 10/12/2022.
FROM MEDRXIV
Would your patient benefit from a monoclonal antibody?
Small-molecule drugs such as aspirin, albuterol, atorvastatin, and lisinopril are the backbone of disease management in family medicine.1 However, large-molecule biological drugs such as monoclonal antibodies (MAbs) are increasingly prescribed to treat common conditions. In the past decade, MAbs comprised 20% of all drug approvals by the US Food and Drug Administration (FDA), and today they represent more than half of drugs currently in development.2 Fifteen MAbs have been approved by the FDA over the past decade for asthma, atopic dermatitis (AD), hyperlipidemia, osteoporosis, and migraine prevention.3 This review details what makes MAbs unique and what you should know about them.
The uniqueness of monoclonal antibodies
MAbs are biologics, but not all biologics are MAbs—eg, adalimumab (Humira) is a MAb, but etanercept (Enbrel) is not. MAbs are therapeutic proteins made possible by hybridoma technology used to create an antibody with single specificity.4-6 Monoclonal antibodies differ from small-molecule drugs in structure, dosing, route of administration, manufacturing, metabolism, drug interactions, and elimination (TABLE 17-9).
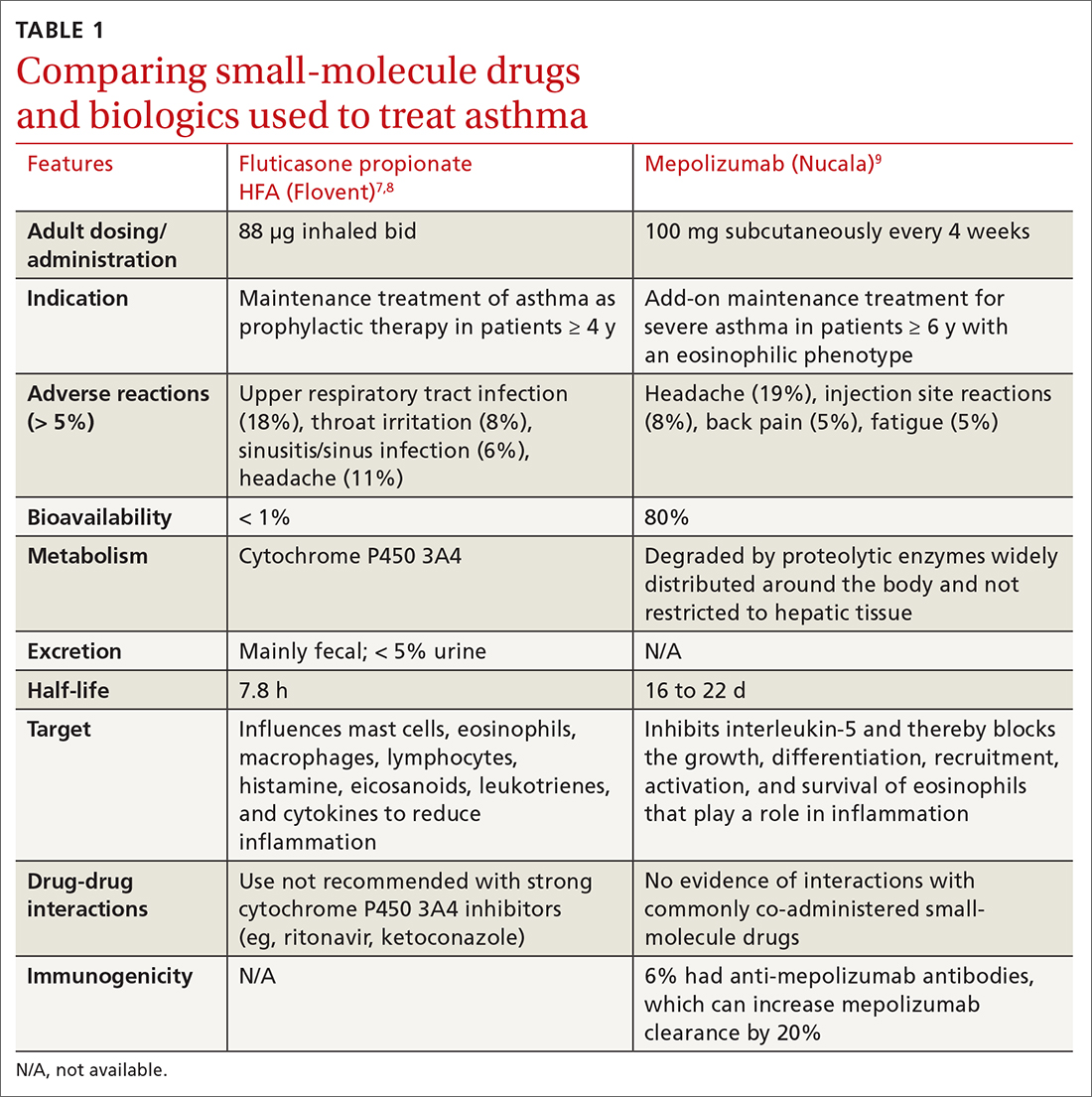
MAbs can be classified as naked, “without any drug or radioactive material attached to them,” or conjugated, “joined to a chemotherapy drug, radioactive isotope, or toxin.”10 MAbs work in several ways, including competitively inhibiting ligand-receptor binding, receptor blockade, or cell elimination from indirect immune system activities such as antibody-dependent cell-mediated cytotoxicity.11,12
Monoclonal antibody uses in family medicine
Asthma
Several MAbs have been approved for use in severe asthma, including but not limited to: omalizumab (Xolair),13 mepolizumab (Nucala),9,14 and dupilumab (Dupixent).15
Omalizumab is a humanized MAb that prevents IgE antibodies from binding to mast cells and basophils, thereby reducing inflammatory mediators.13 A systematic review found that, compared with placebo, omalizumab used in patients with inadequately controlled moderate-to-severe asthma led to significantly fewer asthma exacerbations (absolute risk reduction [ARR], 16% vs 26%; odds ratio [OR] = 0.55; 95% CI, 0.42-0.60; number needed to treat [NNT] = 10) and fewer hospitalizations (ARR, 0.5% vs 3%; OR = 0.16; 95% CI, 0.06-0.42; NNT = 40).13
Significantly more patients in the omalizumab group were able to withdraw from, or reduce, the dose of ICS. GINA recommends omalizumab for patients with positive skin sensitization, total serum IgE ≥ 30 IU/mL, weight within 30 kg to 150 kg, history of childhood asthma and recent exacerbations, and blood eosinophils ≥ 260/mcL.16 Omalizumab is also approved for use in chronic spontaneous urticaria and nasal polyps.
Mepolizumab
Continue to: Another trial found that...
Another trial found that mepolizumab reduced total OCS doses in patients with severe asthma by 50% without increasing exacerbations or worsening asthma control.18 All 3 anti-IL-5 drugs—including not only mepolizumab, but also benralizumab (Fasenra) and reslizumab (Cinqair)—appear to yield similar improvements. A 2017 systematic review found all anti-IL-5 treatments reduced rates of clinically significant asthma exacerbations (treatment with OCS for ≥ 3 days) by roughly 50% in patients with severe eosinophilic asthma and a history of ≥ 2 exacerbations in the past year.
Dupilumab is a humanized MAb that inhibits IL-4 and IL-13, which influence multiple cell types involved in inflammation (eg, mast cells, eosinophils) and inflammatory mediators (histamine, leukotrienes, cytokines).15 In a recent study of patients with uncontrolled asthma, dupilumab 200 mg every 2 weeks compared with placebo showed a modest reduction in the annualized rate of severe asthma exacerbations (0.46 exacerbations vs 0.87, respectively). Dupilumab was effective in patients with blood eosinophil counts ≥ 150/μL but was ineffective in patients with eosinophil counts < 150/μL.15
For patients ≥ 12 years old with severe eosinophilic asthma, GINA recommends using dupilumab as add-on therapy for an initial trial of 4 months at doses of 200 or 300 mg SC every 2 weeks, with preference for 300 mg SC every 2 weeks for OCS-dependent asthma. Dupilumab is approved for use in AD and chronic rhinosinusitis with nasal polyposis. If a biologic agent is not successful after a 4-month trial, consider a 6- to 12-month trial. If efficacy is still minimal, consider switching to an alternative biologic therapy approved for asthma.16
❯ Asthma: Test your skills
Subjective findings: A 19-year-old man presents to your clinic. He has a history of nasal polyps and allergic asthma. At age 18, he was given a diagnosis of severe persistent asthma. He has shortness of breath during waking hours 4 times per week, and treats each of these episodes with albuterol. He also wakes up about twice a week with shortness of breath and has some limitations in normal activities. He reports missing his prescribed fluticasone/salmeterol 500/50 μg, 1 inhalation bid, only once each month. In the last year, he has had 2 exacerbations requiring oral steroids.
Medications: Albuterol 90 μg, 1-2 inhalations, q6h prn; fluticasone/salmeterol 500/50 μg, 1 inhalation bid; tiotropium 1.25 μg, 2 puffs/d; montelukast 10 mg every morning; prednisone 10 mg/d.
Continue to: Objective data
Objective data: Patient is in no apparent distress and afebrile, and oxygen saturation on room air is 97%. Ht, 70 inches; wt, 75 kg. Labs: IgE, 15 IU/mL; serum eosinophils, 315/μL.
Which MAb would be appropriate for this patient? Given that the patient has a blood eosinophil level ≥ 300/μL and severe exacerbations, adult-onset asthma, nasal polyposis, and maintenance OCS at baseline, it would be reasonable to initiate mepolizumab 100 mg SC every 4 weeks, or dupilumab 600 mg once, then 300 mg SC every 2 weeks. Both agents can be self-administered.
Atopic dermatitis
Two MAbs—dupilumab and tralokinumab (Adbry; inhibits IL-13)—are approved for treatment of AD in adults that is uncontrolled with conventional therapy.15,19 Dupilumab is also approved for children ≥ 6 months old.20 Both MAbs are dosed at 600 mg SC, followed by 300 mg every 2 weeks. Dupilumab was compared with placebo in adult patients who had moderate-to-severe AD inadequately controlled on topical corticosteroids (TCSs), to determine the proportion of patients in each group achieving improvement of either 0 or 1 points or ≥ 2 points in the 5-point Investigator Global Assessment (IGA) score from baseline to 16 weeks.21 Thirty-seven percent of patients receiving dupilumab 300 mg SC weekly and 38% of patients receiving dupilumab 300 mg SC every 2 weeks achieved the primary outcome, compared with 10% of those receiving placebo (P < .001).21 Similar IGA scores were reported when dupilumab was combined with TCS, compared with placebo.22
It would be reasonable to consider dupilumab or tralokinumab in patients with: cutaneous atrophy or hypothalamic-pituitary-adrenal axis suppression with TCS, concerns of malignancy with topical calcineurin inhibitors, or problems with the alternative systemic therapies (cyclosporine-induced hypertension, nephrotoxicity, or immunosuppression; azathioprine-induced malignancy; or methotrexate-induced bone marrow suppression, renal impairment, hepatotoxicity, pneumonitis, or gastrointestinal toxicity).23
A distinct advantage of MAbs over other systemic agents in the management of AD is that MAbs do not require frequent monitoring of blood pressure, renal or liver function, complete blood count with differential, electrolytes, or uric acid. Additionally, MAbs have fewer black box warnings and adverse reactions when compared with other systemic agents.
Continue to: Hyperlipidemia
Hyperlipidemia
Three MAbs are approved for use in hyperlipidemia: the angiopoietin-like protein 3 (ANGPTL3) inhibitor evinacumab (Evkeeza)24 and 2 proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, evolocumab (Repatha)25 and alirocumab (Praluent).26
ANGPTL3 inhibitors block ANGPTL3 and reduce endothelial lipase and lipoprotein lipase activity, which in turn decreases low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride formation. PCSK9 inhibitors prevent PCSK9 from binding to LDL receptors, thereby maintaining the number of active LDL receptors and increasing LDL-C removal.
Evinacumab is indicated for homozygous familial hypercholesterolemia and is administered intravenously every 4 weeks. Evinacumab has not been evaluated for effects on cardiovascular morbidity and mortality.
Evolocumab 140 mg SC every 2 weeks or 420 mg SC monthly has been studied in patients on statin therapy with LDL-C ≥ 70 mg/dL. Patients on evolocumab experienced significantly less of the composite endpoint of cardiovascular death, myocardial infarction (MI), stroke, hospitalization for unstable angina, or coronary revascularization compared with placebo (9.8% vs 11.3%; hazard ratio [HR] = 0.85; 95% CI, 0.79-0.92; NNT = 67.27
Alirocumab 75 mg SC every 2 weeks has also been studied in patients receiving statin therapy with LDL-C ≥ 70 mg/dL. Patients taking alirocumab experienced significantly less of the composite endpoint of death from coronary heart disease, nonfatal MI, ischemic stroke, or hospitalization for unstable angina compared with placebo (9.5% vs 11.1%; HR = 0.85; 95% CI, 0.78-0.93; NNT = 63).
Continue to: According to the 2018...
According to the 2018 AHA Cholesterol Guidelines, PCSK9 inhibitors are indicated for patients receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe) with LDL-C ≥ 70 mg/dL, if they have had multiple atherosclerotic cardiovascular disease (ASCVD) events or 1 major ASCVD event with multiple high-risk conditions (eg, heterozygous familial hypercholesterolemia, history of coronary artery bypass grafting or percutaneous coronary intervention, hypertension, estimated glomerular filtration rate of 15 to 59 mL/min/1.73m2).29 For patients without prior ASCVD events or high-risk conditions who are receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe), PCSK9 inhibitors are indicated if the LDL-C remains ≥ 100 mg/dL.
Osteoporosis
The 2 MAbs approved for use in osteoporosis are the receptor activator of nuclear factor kB ligand (RANKL) inhibitor denosumab (Prolia)30 and the sclerostin inhibitor romosozumab (Evenity).31
Denosumab prevents RANKL from binding to the RANK receptor, thereby inhibiting osteoclast formation and decreasing bone resorption. Denosumab is approved for use in women and men who are at high risk of osteoporotic fracture, including those taking OCSs, men receiving androgen deprivation therapy for prostate cancer, and women receiving adjuvant aromatase inhibitor therapy for breast cancer.
In a 3-year randomized trial, denosumab 60 mg SC every 6 months was compared with placebo in postmenopausal women with T-scores < –2.5, but not < –4.0 at the lumbar spine or total hip. Denosumab significantly reduced new radiographic vertebral fractures (2.3% vs 7.2%; risk ratio [RR] = 0.32; 95% CI, 0.26-0.41; NNT = 21), hip fracture (0.7% vs 1.2%), and nonvertebral fracture (6.5% vs 8.0%).32 Denosumab carries an increased risk of multiple vertebral fractures following discontinuation, skin infections, dermatologic reactions, and severe bone, joint, and muscle pain.
Romosozumab inhibits sclerostin, thereby increasing bone formation and, to a lesser degree, decreasing bone resorption. Romosozumab is approved for use in postmenopausal women at high risk for fracture (ie, those with a history of osteoporotic fracture or multiple risk factors for fracture) or in patients who have not benefited from or are intolerant of other therapies. In one study, postmenopausal women with a T-score of –2.5 to –3.5 at the total hip or femoral neck were randomly assigned to receive either romosozumab 210 mg SC or placebo for 12 months, then each group was switched to denosumab 60 mg SC for 12 months. After the first year, prior to initiating denosumab, patients taking romosozumab experienced significantly fewer new vertebral fractures than patients taking placebo (0.5% vs 1.8%; RR = 0.27; 95% CI, 0.16-0.47; NNT = 77); however, there was no significant difference between the 2 groups with nonvertebral fractures (HR = 0.75; 95% CI, 0.53-1.05).33
Continue to: In another study...
In another study, romosozumab 210 mg SC was compared with alendronate 70 mg weekly, followed by alendronate 70 mg weekly in both groups. Over the first 12 months, patients treated with romosozumab saw a significant reduction in the incidence of new vertebral fractures (4% vs 6.3%; RR = 0.63, P < .003; NNT = 44). Patients treated with romosozumab with alendronate added for another 12 months also saw a significant reduction in new incidence of vertebral fractures (6.2% vs 11.9%; RR = 0.52; P < .001; NNT = 18).34 There was a higher risk of cardiovascular events among patients receiving romosozumab compared with those treated with alendronate, so romosozumab should not be used in individuals who have had an MI or stroke within the previous year.34 Denosumab and romosozumab offer an advantage over some bisphosphonates in that they require less frequent dosing and can be used in patients with renal impairment (creatinine clearance < 35 mL/min, in which zoledronic acid is contraindicated and alendronate is not recommended; < 30 mL/min, in which risedronate and ibandronate are not recommended).
Migraine prevention
Four
Erenumab, fremanezumab, and galcanezumab are all available in subcutaneous autoinjectors (or syringe with fremanezumab). Eptinezumab is an intravenous (IV) infusion given every 3 months.
Erenumab is available in both 70-mg and 140-mg dosing options. Fremanezumab can be given as 225 mg monthly or 675 mg quarterly. Galcanezumab has an initial loading dose of 240 mg followed by 120 mg given monthly. Erenumab targets the CGRP receptor; the others target the CGRP ligand. Eptinezumab has 100% bioavailability and reaches maximum serum concentration sooner than the other antagonists (due to its route of administration), but it must be given in an infusion center. Few insurers approve the use of eptinezumab unless a trial of least 1 of the monthly injectables has failed.
There are no head-to-head studies of the medications in this class. Additionally, differing study designs, definitions, statistical analyses, endpoints, and responder-rate calculations make it challenging to compare them directly against one another. At the very least, all of the CGRP MAbs have efficacy comparable to conventional preventive migraine medications such as propranolol, amitriptyline, and topiramate.40
Continue to: The most commonly reported adverse...
The most commonly reported adverse effect for all 4 CGRPs is injection site reaction, which was highest with the quarterly fremanezumab dose (45%).37 Constipation was most notable with the 140-mg dose of erenumab (3%)35; with the other CGRP MAbs it is comparable to that seen with placebo (< 1%).
Erenumab-induced hypertension has been identified in 61 cases reported through the FDA Adverse Event Reporting System (FAERS) as of 2021.41 This was not reported during MAb development programs, nor was it noted during clinical trials. Blood pressure elevation was seen within 1 week of injection in nearly 50% of the cases, and nearly one-third had pre-existing hypertension.41 Due to these findings, the erenumab prescribing information was updated to include hypertension in its warnings and precautions. It is possible that hypertension could be a class effect, although trial data and posthoc studies have yet to bear that out. Since erenumab was the first CGRP antagonist brought to market (May 2018 vs September 2018 for fremanezumab and galcanezumab), it may have accumulated more FAERS reports. Nearly all studies exclude patients with older age, uncontrolled hypertension, and unstable cardiovascular disease, which could impact data.41
Overall, this class of medications is very well tolerated, easy to use (again, excluding eptinezumab), and maintains a low adverse effect profile, giving added value compared with conventional preventive migraine medications.
The American Headache Society recommends a preventive oral therapy for at least 3 months before trying an alternative medication. After treatment failure with at least 2 oral agents, CGRP MAbs are recommended.42 CGRP antagonists offer convenient dosing, bypass gastrointestinal metabolism (which is useful in patients with nausea/vomiting), and have fewer adverse effects than traditional oral medications.
Worth noting. Several newer oral agents have been recently approved for migraine prevention, including atogepant (Qulipta) and rimegepant (Nurtec), which are also CGRP antagonists. Rimegepant is approved for both acute migraine treatment and prevention.
Continue to: Migraine
❯ Migraine: Test your skills
Subjective findings: A 25-year-old woman presents to your clinic for management of episodic migraines with aura. Her baseline average migraine frequency is 9 headache days/month. Her migraines are becoming more frequent despite treatment. She fears IV medication use and avoids hospitals.
History: Hypertension, irritable bowel syndrome with constipation (IBS-C), and depression. The patient is not pregnant or trying to get pregnant.
Medications: Current medications (for previous 4 months) include propranolol 40 mg at bedtime, linaclotide 145 μg/d, citalopram 20 mg/d, and sumatriptan 50 mg prn. Past medications include venlafaxine 150 mg po bid for 5 months.
What would be appropriate for this patient? This patient meets the criteria for using a CGRP antagonist because she has tried 2 preventive treatments for more than 60 to 90 days. Erenumab is not the best option, given the patient’s history of hypertension and IBS-C. The patient fears hospitals and IV medications, making eptinezumab a less-than-ideal choice. Depending on her insurance, fremanezumab or galcanezumab would be good options at this time.
CGRP antagonists have not been studied or evaluated in pregnancy, but if this patient becomes pregnant, a first-line agent for prevention would be propranolol, and a second-line agent would be a tricyclic antidepressant, memantine, or verapamil. Avoid ergotamines and antiepileptics (topiramate or valproate) in pregnancy.43,44
Continue to: The challenges associated with MAbs
The challenges associated with MAbs
MAbs can be expensive (TABLE 2),45 some prohibitively so. On a population scale, biologics account for around 40% of prescription drug spending and may cost 22 times more than small-molecule drugs.46 Estimates in 2016 showed that MAbs comprise $90.2 billion (43%) of the biologic market.46
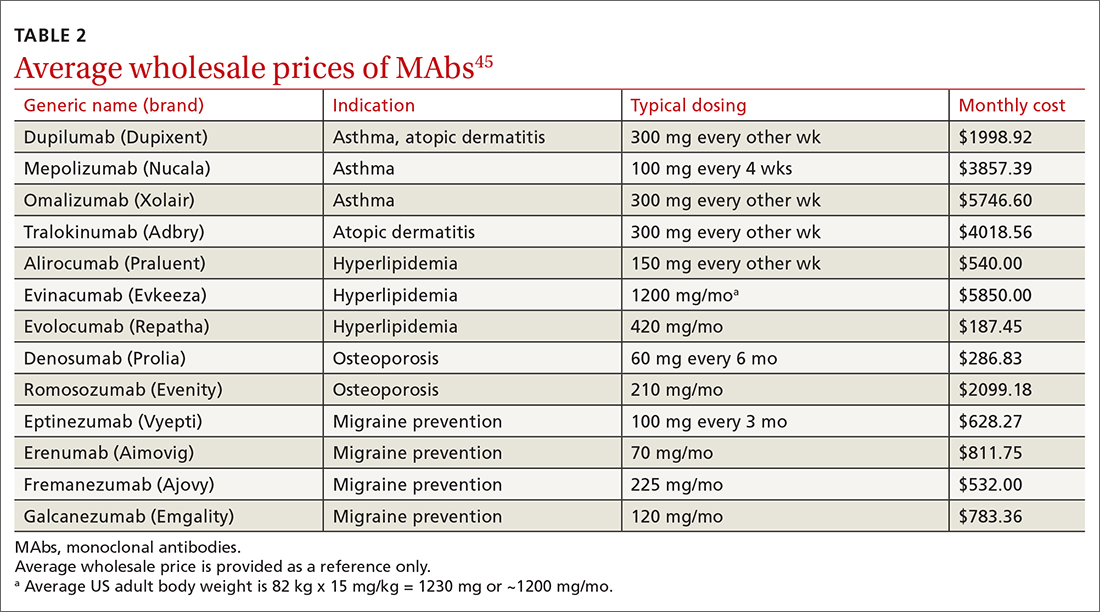
MAbs also require prior authorization forms to be submitted. Prior authorization criteria vary by state and by insurance plan. In my (ES) experience, submitting letters of medical necessity justifying the need for therapy or expertise in the disease states for which the MAb is being prescribed help your patient get the medication they need.
Expect to see additional MAbs approved in the future. If the costs come down, adoption of these agents into practice will likely increase.
CORRESPONDENCE
Evelyn Sbar, MD, Texas Tech University Health Sciences Center, 1400 South Coulter Street, Suite 5100, Amarillo, TX 79106; [email protected]
1. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. National Center for Health Statistics. Accessed June 15, 2022. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
2. IDBS. The future of biologics drug development is today. June 27, 2018. Accessed June 15, 2022. www.idbs.com/blog/2018/06/the-future-of-biologics-drug-development-is-today/
3. Antibody therapeutics approved or in regulatory review in the EU or US. Antibody Society. Accessed June 15, 2022. www.antibodysociety.org/resources/approved-antibodies/
4. FDA. Code of Federal Regulations, Title 21, Chapter I, Subchapter F biologics. March 29, 2022. Accessed June 15, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=600.3
5. Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495-497. doi: 10.1038/256495a0
6. Raejewsky K. The advent and rise of monoclonal antibodies. Nature. November 4, 2019. Accessed June 15, 2022. www.nature.com/articles/d41586-019-02840-w
7. Flovent. Prescribing information. GlaxoSmithKline; 2010. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2010/021433s015lbl.pdf
8. NLM. National Center for Biotechnology Information. PubChem. Method for the preparation of fluticasone and related 17beta-carbothioic esters using a novel carbothioic acid synthesis and novel purification methods. Accessed June 15, 2022. pubchem.ncbi.nlm.nih.gov/patent/WO-0162722-A2
9. Nucala. Prescribing information. GlaxoSmithKline; 2019. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761122s000lbl.pdf
10. Argyriou AA, Kalofonos HP. Recent advances relating to the clinical application of naked monoclonal antibodies in solid tumors. Mol Med. 2009;15:183-191. doi: 10.2119/molmed.2009.00007
11. Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548-558. doi: 10.1038/clpt.2008.170
12. Zahavi D, AlDeghaither D, O’Connell A, et al. Enhancing antibody-dependent cell-mediated cytotoxicity: a strategy for improving antibody-based immunotherapy. Antib Ther. 2018;1:7-12. doi: 10.1093/abt/tby002
13. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014:CD003559. doi: 10.1002/14651858.CD003559.pub4
14. Farne HA, Wilson A, Powell C, et al. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9:CD010834. doi: 10.1002/14651858.CD010834.pub3
15. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486-2496. doi: 10.1056/NEJMoa1804092
16. GINA. Global strategy for asthma management and prevention. 2022 Difficult-to-treat and severe asthma guide—slide set. Accessed June 23, 2022. https://ginasthma.org/severeasthma/
17. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207. doi: 10.1056/NEJMoa1403290
18. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197. doi: 10.1056/NEJMoa1403291
19. Adbry. Prescribing information. Leo Pharma Inc; 2021. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761180Orig1s000lbl.pdf
20. Dupixent. Prescribing information. Regeneron Pharmaceuticals; 2022. Accessed October 5, 2022. https://www.regeneron.com/downloads/dupixent_fpi.pdf
21. Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348. doi: 10.1056/NEJMoa1610020
22. Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303. doi: 10.1016/s0140-6736(17)31191-1
23. Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi: 10.1016/j.jaad.2014.03.030
24. Evkeeza. Prescribing information. Regeneron Pharmaceuticals; 2021. Accessed June 24, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761181s000lbl.pdf
25. Repatha. Prescribing information. Amgen; 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125522s014lbl.pdf
26. Praluent. Prescribing information. Sanofi Aventis and Regeneron Pharmaceuticals. 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125559s002lbl.pdf
27. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722. doi: 10.1056/NEJMoa1615664
28. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097-2107. doi:10.1056/NEJMoa1801174
29. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
30. Prolia. Prescribing information. Amgen; 2010. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2013/125320s094lbl.pdf
31. Evenity. Prescribing information. Amgen; 2019. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761062s000lbl.pdf
32. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
33. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543. doi: 10.1056/NEJMoa1607948
34. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427. doi: 10.1056/NEJMoa1708322
35. Aimovig. Prescribing information. Amgen; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761077s000lbl.pdf
36. Vyepti. Prescribing information. Lundbeck Seattle BioPharmaceuticals; 2020. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2020/761119s000lbl.pdf
37. Ajovy. Prescribing information. Teva Pharmaceuticals; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761089s000lbl.pdf
38. Emgality. Prescribing information. Eli Lilly and Co.; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761063s000lbl.pdf
39. Edvinsson L, Haanes KA, Warfvinge K, et al. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338-350. doi: 10.1038/s41582-018-0003-1
40. Vandervorst F. Van Deun L, Van Dycke A, et al. CGRP monoclonal antibodies in migraine: an efficacy and tolerability comparison with standard prophylactic drugs. J Headache Pain. 2021;22:128. doi: 10.1186/s10194-021-01335-2
41. Saely S, Croteau D, Jawidzik L, et al. Hypertension: a new safety risk for patients treated with erenumab. Headache. 2021;61:202-208. doi: 10.1111/head.14051
42. American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1-18. doi: 10.1111/head.13456
43. Burch R. Headache in pregnancy and the puerperium. Neurol Clin. 2019;37:31-51. doi: 10.1016/j.ncl.2018.09.004
44. Burch R. Epidemiology and treatment of menstrual migraine and migraine during pregnancy and lactation: a narrative review. Headache. 2020;60:200-216. doi: 10.1111/head.13665
45. Lexi-Comp. Lexi-drug database. Accessed April 4, 2022. https://online.lexi.com/lco/action/login
46. Walker N. Biologics: driving force in pharma. Pharma’s Almanac. June 5, 2017. Accessed June 15, 2020. www.pharmasalmanac.com/articles/biologics-driving-force-in-pharma
Small-molecule drugs such as aspirin, albuterol, atorvastatin, and lisinopril are the backbone of disease management in family medicine.1 However, large-molecule biological drugs such as monoclonal antibodies (MAbs) are increasingly prescribed to treat common conditions. In the past decade, MAbs comprised 20% of all drug approvals by the US Food and Drug Administration (FDA), and today they represent more than half of drugs currently in development.2 Fifteen MAbs have been approved by the FDA over the past decade for asthma, atopic dermatitis (AD), hyperlipidemia, osteoporosis, and migraine prevention.3 This review details what makes MAbs unique and what you should know about them.
The uniqueness of monoclonal antibodies
MAbs are biologics, but not all biologics are MAbs—eg, adalimumab (Humira) is a MAb, but etanercept (Enbrel) is not. MAbs are therapeutic proteins made possible by hybridoma technology used to create an antibody with single specificity.4-6 Monoclonal antibodies differ from small-molecule drugs in structure, dosing, route of administration, manufacturing, metabolism, drug interactions, and elimination (TABLE 17-9).

MAbs can be classified as naked, “without any drug or radioactive material attached to them,” or conjugated, “joined to a chemotherapy drug, radioactive isotope, or toxin.”10 MAbs work in several ways, including competitively inhibiting ligand-receptor binding, receptor blockade, or cell elimination from indirect immune system activities such as antibody-dependent cell-mediated cytotoxicity.11,12
Monoclonal antibody uses in family medicine
Asthma
Several MAbs have been approved for use in severe asthma, including but not limited to: omalizumab (Xolair),13 mepolizumab (Nucala),9,14 and dupilumab (Dupixent).15
Omalizumab is a humanized MAb that prevents IgE antibodies from binding to mast cells and basophils, thereby reducing inflammatory mediators.13 A systematic review found that, compared with placebo, omalizumab used in patients with inadequately controlled moderate-to-severe asthma led to significantly fewer asthma exacerbations (absolute risk reduction [ARR], 16% vs 26%; odds ratio [OR] = 0.55; 95% CI, 0.42-0.60; number needed to treat [NNT] = 10) and fewer hospitalizations (ARR, 0.5% vs 3%; OR = 0.16; 95% CI, 0.06-0.42; NNT = 40).13
Significantly more patients in the omalizumab group were able to withdraw from, or reduce, the dose of ICS. GINA recommends omalizumab for patients with positive skin sensitization, total serum IgE ≥ 30 IU/mL, weight within 30 kg to 150 kg, history of childhood asthma and recent exacerbations, and blood eosinophils ≥ 260/mcL.16 Omalizumab is also approved for use in chronic spontaneous urticaria and nasal polyps.
Mepolizumab
Continue to: Another trial found that...
Another trial found that mepolizumab reduced total OCS doses in patients with severe asthma by 50% without increasing exacerbations or worsening asthma control.18 All 3 anti-IL-5 drugs—including not only mepolizumab, but also benralizumab (Fasenra) and reslizumab (Cinqair)—appear to yield similar improvements. A 2017 systematic review found all anti-IL-5 treatments reduced rates of clinically significant asthma exacerbations (treatment with OCS for ≥ 3 days) by roughly 50% in patients with severe eosinophilic asthma and a history of ≥ 2 exacerbations in the past year.
Dupilumab is a humanized MAb that inhibits IL-4 and IL-13, which influence multiple cell types involved in inflammation (eg, mast cells, eosinophils) and inflammatory mediators (histamine, leukotrienes, cytokines).15 In a recent study of patients with uncontrolled asthma, dupilumab 200 mg every 2 weeks compared with placebo showed a modest reduction in the annualized rate of severe asthma exacerbations (0.46 exacerbations vs 0.87, respectively). Dupilumab was effective in patients with blood eosinophil counts ≥ 150/μL but was ineffective in patients with eosinophil counts < 150/μL.15
For patients ≥ 12 years old with severe eosinophilic asthma, GINA recommends using dupilumab as add-on therapy for an initial trial of 4 months at doses of 200 or 300 mg SC every 2 weeks, with preference for 300 mg SC every 2 weeks for OCS-dependent asthma. Dupilumab is approved for use in AD and chronic rhinosinusitis with nasal polyposis. If a biologic agent is not successful after a 4-month trial, consider a 6- to 12-month trial. If efficacy is still minimal, consider switching to an alternative biologic therapy approved for asthma.16
❯ Asthma: Test your skills
Subjective findings: A 19-year-old man presents to your clinic. He has a history of nasal polyps and allergic asthma. At age 18, he was given a diagnosis of severe persistent asthma. He has shortness of breath during waking hours 4 times per week, and treats each of these episodes with albuterol. He also wakes up about twice a week with shortness of breath and has some limitations in normal activities. He reports missing his prescribed fluticasone/salmeterol 500/50 μg, 1 inhalation bid, only once each month. In the last year, he has had 2 exacerbations requiring oral steroids.
Medications: Albuterol 90 μg, 1-2 inhalations, q6h prn; fluticasone/salmeterol 500/50 μg, 1 inhalation bid; tiotropium 1.25 μg, 2 puffs/d; montelukast 10 mg every morning; prednisone 10 mg/d.
Continue to: Objective data
Objective data: Patient is in no apparent distress and afebrile, and oxygen saturation on room air is 97%. Ht, 70 inches; wt, 75 kg. Labs: IgE, 15 IU/mL; serum eosinophils, 315/μL.
Which MAb would be appropriate for this patient? Given that the patient has a blood eosinophil level ≥ 300/μL and severe exacerbations, adult-onset asthma, nasal polyposis, and maintenance OCS at baseline, it would be reasonable to initiate mepolizumab 100 mg SC every 4 weeks, or dupilumab 600 mg once, then 300 mg SC every 2 weeks. Both agents can be self-administered.
Atopic dermatitis
Two MAbs—dupilumab and tralokinumab (Adbry; inhibits IL-13)—are approved for treatment of AD in adults that is uncontrolled with conventional therapy.15,19 Dupilumab is also approved for children ≥ 6 months old.20 Both MAbs are dosed at 600 mg SC, followed by 300 mg every 2 weeks. Dupilumab was compared with placebo in adult patients who had moderate-to-severe AD inadequately controlled on topical corticosteroids (TCSs), to determine the proportion of patients in each group achieving improvement of either 0 or 1 points or ≥ 2 points in the 5-point Investigator Global Assessment (IGA) score from baseline to 16 weeks.21 Thirty-seven percent of patients receiving dupilumab 300 mg SC weekly and 38% of patients receiving dupilumab 300 mg SC every 2 weeks achieved the primary outcome, compared with 10% of those receiving placebo (P < .001).21 Similar IGA scores were reported when dupilumab was combined with TCS, compared with placebo.22
It would be reasonable to consider dupilumab or tralokinumab in patients with: cutaneous atrophy or hypothalamic-pituitary-adrenal axis suppression with TCS, concerns of malignancy with topical calcineurin inhibitors, or problems with the alternative systemic therapies (cyclosporine-induced hypertension, nephrotoxicity, or immunosuppression; azathioprine-induced malignancy; or methotrexate-induced bone marrow suppression, renal impairment, hepatotoxicity, pneumonitis, or gastrointestinal toxicity).23
A distinct advantage of MAbs over other systemic agents in the management of AD is that MAbs do not require frequent monitoring of blood pressure, renal or liver function, complete blood count with differential, electrolytes, or uric acid. Additionally, MAbs have fewer black box warnings and adverse reactions when compared with other systemic agents.
Continue to: Hyperlipidemia
Hyperlipidemia
Three MAbs are approved for use in hyperlipidemia: the angiopoietin-like protein 3 (ANGPTL3) inhibitor evinacumab (Evkeeza)24 and 2 proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, evolocumab (Repatha)25 and alirocumab (Praluent).26
ANGPTL3 inhibitors block ANGPTL3 and reduce endothelial lipase and lipoprotein lipase activity, which in turn decreases low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride formation. PCSK9 inhibitors prevent PCSK9 from binding to LDL receptors, thereby maintaining the number of active LDL receptors and increasing LDL-C removal.
Evinacumab is indicated for homozygous familial hypercholesterolemia and is administered intravenously every 4 weeks. Evinacumab has not been evaluated for effects on cardiovascular morbidity and mortality.
Evolocumab 140 mg SC every 2 weeks or 420 mg SC monthly has been studied in patients on statin therapy with LDL-C ≥ 70 mg/dL. Patients on evolocumab experienced significantly less of the composite endpoint of cardiovascular death, myocardial infarction (MI), stroke, hospitalization for unstable angina, or coronary revascularization compared with placebo (9.8% vs 11.3%; hazard ratio [HR] = 0.85; 95% CI, 0.79-0.92; NNT = 67.27
Alirocumab 75 mg SC every 2 weeks has also been studied in patients receiving statin therapy with LDL-C ≥ 70 mg/dL. Patients taking alirocumab experienced significantly less of the composite endpoint of death from coronary heart disease, nonfatal MI, ischemic stroke, or hospitalization for unstable angina compared with placebo (9.5% vs 11.1%; HR = 0.85; 95% CI, 0.78-0.93; NNT = 63).
Continue to: According to the 2018...
According to the 2018 AHA Cholesterol Guidelines, PCSK9 inhibitors are indicated for patients receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe) with LDL-C ≥ 70 mg/dL, if they have had multiple atherosclerotic cardiovascular disease (ASCVD) events or 1 major ASCVD event with multiple high-risk conditions (eg, heterozygous familial hypercholesterolemia, history of coronary artery bypass grafting or percutaneous coronary intervention, hypertension, estimated glomerular filtration rate of 15 to 59 mL/min/1.73m2).29 For patients without prior ASCVD events or high-risk conditions who are receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe), PCSK9 inhibitors are indicated if the LDL-C remains ≥ 100 mg/dL.
Osteoporosis
The 2 MAbs approved for use in osteoporosis are the receptor activator of nuclear factor kB ligand (RANKL) inhibitor denosumab (Prolia)30 and the sclerostin inhibitor romosozumab (Evenity).31
Denosumab prevents RANKL from binding to the RANK receptor, thereby inhibiting osteoclast formation and decreasing bone resorption. Denosumab is approved for use in women and men who are at high risk of osteoporotic fracture, including those taking OCSs, men receiving androgen deprivation therapy for prostate cancer, and women receiving adjuvant aromatase inhibitor therapy for breast cancer.
In a 3-year randomized trial, denosumab 60 mg SC every 6 months was compared with placebo in postmenopausal women with T-scores < –2.5, but not < –4.0 at the lumbar spine or total hip. Denosumab significantly reduced new radiographic vertebral fractures (2.3% vs 7.2%; risk ratio [RR] = 0.32; 95% CI, 0.26-0.41; NNT = 21), hip fracture (0.7% vs 1.2%), and nonvertebral fracture (6.5% vs 8.0%).32 Denosumab carries an increased risk of multiple vertebral fractures following discontinuation, skin infections, dermatologic reactions, and severe bone, joint, and muscle pain.
Romosozumab inhibits sclerostin, thereby increasing bone formation and, to a lesser degree, decreasing bone resorption. Romosozumab is approved for use in postmenopausal women at high risk for fracture (ie, those with a history of osteoporotic fracture or multiple risk factors for fracture) or in patients who have not benefited from or are intolerant of other therapies. In one study, postmenopausal women with a T-score of –2.5 to –3.5 at the total hip or femoral neck were randomly assigned to receive either romosozumab 210 mg SC or placebo for 12 months, then each group was switched to denosumab 60 mg SC for 12 months. After the first year, prior to initiating denosumab, patients taking romosozumab experienced significantly fewer new vertebral fractures than patients taking placebo (0.5% vs 1.8%; RR = 0.27; 95% CI, 0.16-0.47; NNT = 77); however, there was no significant difference between the 2 groups with nonvertebral fractures (HR = 0.75; 95% CI, 0.53-1.05).33
Continue to: In another study...
In another study, romosozumab 210 mg SC was compared with alendronate 70 mg weekly, followed by alendronate 70 mg weekly in both groups. Over the first 12 months, patients treated with romosozumab saw a significant reduction in the incidence of new vertebral fractures (4% vs 6.3%; RR = 0.63, P < .003; NNT = 44). Patients treated with romosozumab with alendronate added for another 12 months also saw a significant reduction in new incidence of vertebral fractures (6.2% vs 11.9%; RR = 0.52; P < .001; NNT = 18).34 There was a higher risk of cardiovascular events among patients receiving romosozumab compared with those treated with alendronate, so romosozumab should not be used in individuals who have had an MI or stroke within the previous year.34 Denosumab and romosozumab offer an advantage over some bisphosphonates in that they require less frequent dosing and can be used in patients with renal impairment (creatinine clearance < 35 mL/min, in which zoledronic acid is contraindicated and alendronate is not recommended; < 30 mL/min, in which risedronate and ibandronate are not recommended).
Migraine prevention
Four
Erenumab, fremanezumab, and galcanezumab are all available in subcutaneous autoinjectors (or syringe with fremanezumab). Eptinezumab is an intravenous (IV) infusion given every 3 months.
Erenumab is available in both 70-mg and 140-mg dosing options. Fremanezumab can be given as 225 mg monthly or 675 mg quarterly. Galcanezumab has an initial loading dose of 240 mg followed by 120 mg given monthly. Erenumab targets the CGRP receptor; the others target the CGRP ligand. Eptinezumab has 100% bioavailability and reaches maximum serum concentration sooner than the other antagonists (due to its route of administration), but it must be given in an infusion center. Few insurers approve the use of eptinezumab unless a trial of least 1 of the monthly injectables has failed.
There are no head-to-head studies of the medications in this class. Additionally, differing study designs, definitions, statistical analyses, endpoints, and responder-rate calculations make it challenging to compare them directly against one another. At the very least, all of the CGRP MAbs have efficacy comparable to conventional preventive migraine medications such as propranolol, amitriptyline, and topiramate.40
Continue to: The most commonly reported adverse...
The most commonly reported adverse effect for all 4 CGRPs is injection site reaction, which was highest with the quarterly fremanezumab dose (45%).37 Constipation was most notable with the 140-mg dose of erenumab (3%)35; with the other CGRP MAbs it is comparable to that seen with placebo (< 1%).
Erenumab-induced hypertension has been identified in 61 cases reported through the FDA Adverse Event Reporting System (FAERS) as of 2021.41 This was not reported during MAb development programs, nor was it noted during clinical trials. Blood pressure elevation was seen within 1 week of injection in nearly 50% of the cases, and nearly one-third had pre-existing hypertension.41 Due to these findings, the erenumab prescribing information was updated to include hypertension in its warnings and precautions. It is possible that hypertension could be a class effect, although trial data and posthoc studies have yet to bear that out. Since erenumab was the first CGRP antagonist brought to market (May 2018 vs September 2018 for fremanezumab and galcanezumab), it may have accumulated more FAERS reports. Nearly all studies exclude patients with older age, uncontrolled hypertension, and unstable cardiovascular disease, which could impact data.41
Overall, this class of medications is very well tolerated, easy to use (again, excluding eptinezumab), and maintains a low adverse effect profile, giving added value compared with conventional preventive migraine medications.
The American Headache Society recommends a preventive oral therapy for at least 3 months before trying an alternative medication. After treatment failure with at least 2 oral agents, CGRP MAbs are recommended.42 CGRP antagonists offer convenient dosing, bypass gastrointestinal metabolism (which is useful in patients with nausea/vomiting), and have fewer adverse effects than traditional oral medications.
Worth noting. Several newer oral agents have been recently approved for migraine prevention, including atogepant (Qulipta) and rimegepant (Nurtec), which are also CGRP antagonists. Rimegepant is approved for both acute migraine treatment and prevention.
Continue to: Migraine
❯ Migraine: Test your skills
Subjective findings: A 25-year-old woman presents to your clinic for management of episodic migraines with aura. Her baseline average migraine frequency is 9 headache days/month. Her migraines are becoming more frequent despite treatment. She fears IV medication use and avoids hospitals.
History: Hypertension, irritable bowel syndrome with constipation (IBS-C), and depression. The patient is not pregnant or trying to get pregnant.
Medications: Current medications (for previous 4 months) include propranolol 40 mg at bedtime, linaclotide 145 μg/d, citalopram 20 mg/d, and sumatriptan 50 mg prn. Past medications include venlafaxine 150 mg po bid for 5 months.
What would be appropriate for this patient? This patient meets the criteria for using a CGRP antagonist because she has tried 2 preventive treatments for more than 60 to 90 days. Erenumab is not the best option, given the patient’s history of hypertension and IBS-C. The patient fears hospitals and IV medications, making eptinezumab a less-than-ideal choice. Depending on her insurance, fremanezumab or galcanezumab would be good options at this time.
CGRP antagonists have not been studied or evaluated in pregnancy, but if this patient becomes pregnant, a first-line agent for prevention would be propranolol, and a second-line agent would be a tricyclic antidepressant, memantine, or verapamil. Avoid ergotamines and antiepileptics (topiramate or valproate) in pregnancy.43,44
Continue to: The challenges associated with MAbs
The challenges associated with MAbs
MAbs can be expensive (TABLE 2),45 some prohibitively so. On a population scale, biologics account for around 40% of prescription drug spending and may cost 22 times more than small-molecule drugs.46 Estimates in 2016 showed that MAbs comprise $90.2 billion (43%) of the biologic market.46

MAbs also require prior authorization forms to be submitted. Prior authorization criteria vary by state and by insurance plan. In my (ES) experience, submitting letters of medical necessity justifying the need for therapy or expertise in the disease states for which the MAb is being prescribed help your patient get the medication they need.
Expect to see additional MAbs approved in the future. If the costs come down, adoption of these agents into practice will likely increase.
CORRESPONDENCE
Evelyn Sbar, MD, Texas Tech University Health Sciences Center, 1400 South Coulter Street, Suite 5100, Amarillo, TX 79106; [email protected]
Small-molecule drugs such as aspirin, albuterol, atorvastatin, and lisinopril are the backbone of disease management in family medicine.1 However, large-molecule biological drugs such as monoclonal antibodies (MAbs) are increasingly prescribed to treat common conditions. In the past decade, MAbs comprised 20% of all drug approvals by the US Food and Drug Administration (FDA), and today they represent more than half of drugs currently in development.2 Fifteen MAbs have been approved by the FDA over the past decade for asthma, atopic dermatitis (AD), hyperlipidemia, osteoporosis, and migraine prevention.3 This review details what makes MAbs unique and what you should know about them.
The uniqueness of monoclonal antibodies
MAbs are biologics, but not all biologics are MAbs—eg, adalimumab (Humira) is a MAb, but etanercept (Enbrel) is not. MAbs are therapeutic proteins made possible by hybridoma technology used to create an antibody with single specificity.4-6 Monoclonal antibodies differ from small-molecule drugs in structure, dosing, route of administration, manufacturing, metabolism, drug interactions, and elimination (TABLE 17-9).

MAbs can be classified as naked, “without any drug or radioactive material attached to them,” or conjugated, “joined to a chemotherapy drug, radioactive isotope, or toxin.”10 MAbs work in several ways, including competitively inhibiting ligand-receptor binding, receptor blockade, or cell elimination from indirect immune system activities such as antibody-dependent cell-mediated cytotoxicity.11,12
Monoclonal antibody uses in family medicine
Asthma
Several MAbs have been approved for use in severe asthma, including but not limited to: omalizumab (Xolair),13 mepolizumab (Nucala),9,14 and dupilumab (Dupixent).15
Omalizumab is a humanized MAb that prevents IgE antibodies from binding to mast cells and basophils, thereby reducing inflammatory mediators.13 A systematic review found that, compared with placebo, omalizumab used in patients with inadequately controlled moderate-to-severe asthma led to significantly fewer asthma exacerbations (absolute risk reduction [ARR], 16% vs 26%; odds ratio [OR] = 0.55; 95% CI, 0.42-0.60; number needed to treat [NNT] = 10) and fewer hospitalizations (ARR, 0.5% vs 3%; OR = 0.16; 95% CI, 0.06-0.42; NNT = 40).13
Significantly more patients in the omalizumab group were able to withdraw from, or reduce, the dose of ICS. GINA recommends omalizumab for patients with positive skin sensitization, total serum IgE ≥ 30 IU/mL, weight within 30 kg to 150 kg, history of childhood asthma and recent exacerbations, and blood eosinophils ≥ 260/mcL.16 Omalizumab is also approved for use in chronic spontaneous urticaria and nasal polyps.
Mepolizumab
Continue to: Another trial found that...
Another trial found that mepolizumab reduced total OCS doses in patients with severe asthma by 50% without increasing exacerbations or worsening asthma control.18 All 3 anti-IL-5 drugs—including not only mepolizumab, but also benralizumab (Fasenra) and reslizumab (Cinqair)—appear to yield similar improvements. A 2017 systematic review found all anti-IL-5 treatments reduced rates of clinically significant asthma exacerbations (treatment with OCS for ≥ 3 days) by roughly 50% in patients with severe eosinophilic asthma and a history of ≥ 2 exacerbations in the past year.
Dupilumab is a humanized MAb that inhibits IL-4 and IL-13, which influence multiple cell types involved in inflammation (eg, mast cells, eosinophils) and inflammatory mediators (histamine, leukotrienes, cytokines).15 In a recent study of patients with uncontrolled asthma, dupilumab 200 mg every 2 weeks compared with placebo showed a modest reduction in the annualized rate of severe asthma exacerbations (0.46 exacerbations vs 0.87, respectively). Dupilumab was effective in patients with blood eosinophil counts ≥ 150/μL but was ineffective in patients with eosinophil counts < 150/μL.15
For patients ≥ 12 years old with severe eosinophilic asthma, GINA recommends using dupilumab as add-on therapy for an initial trial of 4 months at doses of 200 or 300 mg SC every 2 weeks, with preference for 300 mg SC every 2 weeks for OCS-dependent asthma. Dupilumab is approved for use in AD and chronic rhinosinusitis with nasal polyposis. If a biologic agent is not successful after a 4-month trial, consider a 6- to 12-month trial. If efficacy is still minimal, consider switching to an alternative biologic therapy approved for asthma.16
❯ Asthma: Test your skills
Subjective findings: A 19-year-old man presents to your clinic. He has a history of nasal polyps and allergic asthma. At age 18, he was given a diagnosis of severe persistent asthma. He has shortness of breath during waking hours 4 times per week, and treats each of these episodes with albuterol. He also wakes up about twice a week with shortness of breath and has some limitations in normal activities. He reports missing his prescribed fluticasone/salmeterol 500/50 μg, 1 inhalation bid, only once each month. In the last year, he has had 2 exacerbations requiring oral steroids.
Medications: Albuterol 90 μg, 1-2 inhalations, q6h prn; fluticasone/salmeterol 500/50 μg, 1 inhalation bid; tiotropium 1.25 μg, 2 puffs/d; montelukast 10 mg every morning; prednisone 10 mg/d.
Continue to: Objective data
Objective data: Patient is in no apparent distress and afebrile, and oxygen saturation on room air is 97%. Ht, 70 inches; wt, 75 kg. Labs: IgE, 15 IU/mL; serum eosinophils, 315/μL.
Which MAb would be appropriate for this patient? Given that the patient has a blood eosinophil level ≥ 300/μL and severe exacerbations, adult-onset asthma, nasal polyposis, and maintenance OCS at baseline, it would be reasonable to initiate mepolizumab 100 mg SC every 4 weeks, or dupilumab 600 mg once, then 300 mg SC every 2 weeks. Both agents can be self-administered.
Atopic dermatitis
Two MAbs—dupilumab and tralokinumab (Adbry; inhibits IL-13)—are approved for treatment of AD in adults that is uncontrolled with conventional therapy.15,19 Dupilumab is also approved for children ≥ 6 months old.20 Both MAbs are dosed at 600 mg SC, followed by 300 mg every 2 weeks. Dupilumab was compared with placebo in adult patients who had moderate-to-severe AD inadequately controlled on topical corticosteroids (TCSs), to determine the proportion of patients in each group achieving improvement of either 0 or 1 points or ≥ 2 points in the 5-point Investigator Global Assessment (IGA) score from baseline to 16 weeks.21 Thirty-seven percent of patients receiving dupilumab 300 mg SC weekly and 38% of patients receiving dupilumab 300 mg SC every 2 weeks achieved the primary outcome, compared with 10% of those receiving placebo (P < .001).21 Similar IGA scores were reported when dupilumab was combined with TCS, compared with placebo.22
It would be reasonable to consider dupilumab or tralokinumab in patients with: cutaneous atrophy or hypothalamic-pituitary-adrenal axis suppression with TCS, concerns of malignancy with topical calcineurin inhibitors, or problems with the alternative systemic therapies (cyclosporine-induced hypertension, nephrotoxicity, or immunosuppression; azathioprine-induced malignancy; or methotrexate-induced bone marrow suppression, renal impairment, hepatotoxicity, pneumonitis, or gastrointestinal toxicity).23
A distinct advantage of MAbs over other systemic agents in the management of AD is that MAbs do not require frequent monitoring of blood pressure, renal or liver function, complete blood count with differential, electrolytes, or uric acid. Additionally, MAbs have fewer black box warnings and adverse reactions when compared with other systemic agents.
Continue to: Hyperlipidemia
Hyperlipidemia
Three MAbs are approved for use in hyperlipidemia: the angiopoietin-like protein 3 (ANGPTL3) inhibitor evinacumab (Evkeeza)24 and 2 proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, evolocumab (Repatha)25 and alirocumab (Praluent).26
ANGPTL3 inhibitors block ANGPTL3 and reduce endothelial lipase and lipoprotein lipase activity, which in turn decreases low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride formation. PCSK9 inhibitors prevent PCSK9 from binding to LDL receptors, thereby maintaining the number of active LDL receptors and increasing LDL-C removal.
Evinacumab is indicated for homozygous familial hypercholesterolemia and is administered intravenously every 4 weeks. Evinacumab has not been evaluated for effects on cardiovascular morbidity and mortality.
Evolocumab 140 mg SC every 2 weeks or 420 mg SC monthly has been studied in patients on statin therapy with LDL-C ≥ 70 mg/dL. Patients on evolocumab experienced significantly less of the composite endpoint of cardiovascular death, myocardial infarction (MI), stroke, hospitalization for unstable angina, or coronary revascularization compared with placebo (9.8% vs 11.3%; hazard ratio [HR] = 0.85; 95% CI, 0.79-0.92; NNT = 67.27
Alirocumab 75 mg SC every 2 weeks has also been studied in patients receiving statin therapy with LDL-C ≥ 70 mg/dL. Patients taking alirocumab experienced significantly less of the composite endpoint of death from coronary heart disease, nonfatal MI, ischemic stroke, or hospitalization for unstable angina compared with placebo (9.5% vs 11.1%; HR = 0.85; 95% CI, 0.78-0.93; NNT = 63).
Continue to: According to the 2018...
According to the 2018 AHA Cholesterol Guidelines, PCSK9 inhibitors are indicated for patients receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe) with LDL-C ≥ 70 mg/dL, if they have had multiple atherosclerotic cardiovascular disease (ASCVD) events or 1 major ASCVD event with multiple high-risk conditions (eg, heterozygous familial hypercholesterolemia, history of coronary artery bypass grafting or percutaneous coronary intervention, hypertension, estimated glomerular filtration rate of 15 to 59 mL/min/1.73m2).29 For patients without prior ASCVD events or high-risk conditions who are receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe), PCSK9 inhibitors are indicated if the LDL-C remains ≥ 100 mg/dL.
Osteoporosis
The 2 MAbs approved for use in osteoporosis are the receptor activator of nuclear factor kB ligand (RANKL) inhibitor denosumab (Prolia)30 and the sclerostin inhibitor romosozumab (Evenity).31
Denosumab prevents RANKL from binding to the RANK receptor, thereby inhibiting osteoclast formation and decreasing bone resorption. Denosumab is approved for use in women and men who are at high risk of osteoporotic fracture, including those taking OCSs, men receiving androgen deprivation therapy for prostate cancer, and women receiving adjuvant aromatase inhibitor therapy for breast cancer.
In a 3-year randomized trial, denosumab 60 mg SC every 6 months was compared with placebo in postmenopausal women with T-scores < –2.5, but not < –4.0 at the lumbar spine or total hip. Denosumab significantly reduced new radiographic vertebral fractures (2.3% vs 7.2%; risk ratio [RR] = 0.32; 95% CI, 0.26-0.41; NNT = 21), hip fracture (0.7% vs 1.2%), and nonvertebral fracture (6.5% vs 8.0%).32 Denosumab carries an increased risk of multiple vertebral fractures following discontinuation, skin infections, dermatologic reactions, and severe bone, joint, and muscle pain.
Romosozumab inhibits sclerostin, thereby increasing bone formation and, to a lesser degree, decreasing bone resorption. Romosozumab is approved for use in postmenopausal women at high risk for fracture (ie, those with a history of osteoporotic fracture or multiple risk factors for fracture) or in patients who have not benefited from or are intolerant of other therapies. In one study, postmenopausal women with a T-score of –2.5 to –3.5 at the total hip or femoral neck were randomly assigned to receive either romosozumab 210 mg SC or placebo for 12 months, then each group was switched to denosumab 60 mg SC for 12 months. After the first year, prior to initiating denosumab, patients taking romosozumab experienced significantly fewer new vertebral fractures than patients taking placebo (0.5% vs 1.8%; RR = 0.27; 95% CI, 0.16-0.47; NNT = 77); however, there was no significant difference between the 2 groups with nonvertebral fractures (HR = 0.75; 95% CI, 0.53-1.05).33
Continue to: In another study...
In another study, romosozumab 210 mg SC was compared with alendronate 70 mg weekly, followed by alendronate 70 mg weekly in both groups. Over the first 12 months, patients treated with romosozumab saw a significant reduction in the incidence of new vertebral fractures (4% vs 6.3%; RR = 0.63, P < .003; NNT = 44). Patients treated with romosozumab with alendronate added for another 12 months also saw a significant reduction in new incidence of vertebral fractures (6.2% vs 11.9%; RR = 0.52; P < .001; NNT = 18).34 There was a higher risk of cardiovascular events among patients receiving romosozumab compared with those treated with alendronate, so romosozumab should not be used in individuals who have had an MI or stroke within the previous year.34 Denosumab and romosozumab offer an advantage over some bisphosphonates in that they require less frequent dosing and can be used in patients with renal impairment (creatinine clearance < 35 mL/min, in which zoledronic acid is contraindicated and alendronate is not recommended; < 30 mL/min, in which risedronate and ibandronate are not recommended).
Migraine prevention
Four
Erenumab, fremanezumab, and galcanezumab are all available in subcutaneous autoinjectors (or syringe with fremanezumab). Eptinezumab is an intravenous (IV) infusion given every 3 months.
Erenumab is available in both 70-mg and 140-mg dosing options. Fremanezumab can be given as 225 mg monthly or 675 mg quarterly. Galcanezumab has an initial loading dose of 240 mg followed by 120 mg given monthly. Erenumab targets the CGRP receptor; the others target the CGRP ligand. Eptinezumab has 100% bioavailability and reaches maximum serum concentration sooner than the other antagonists (due to its route of administration), but it must be given in an infusion center. Few insurers approve the use of eptinezumab unless a trial of least 1 of the monthly injectables has failed.
There are no head-to-head studies of the medications in this class. Additionally, differing study designs, definitions, statistical analyses, endpoints, and responder-rate calculations make it challenging to compare them directly against one another. At the very least, all of the CGRP MAbs have efficacy comparable to conventional preventive migraine medications such as propranolol, amitriptyline, and topiramate.40
Continue to: The most commonly reported adverse...
The most commonly reported adverse effect for all 4 CGRPs is injection site reaction, which was highest with the quarterly fremanezumab dose (45%).37 Constipation was most notable with the 140-mg dose of erenumab (3%)35; with the other CGRP MAbs it is comparable to that seen with placebo (< 1%).
Erenumab-induced hypertension has been identified in 61 cases reported through the FDA Adverse Event Reporting System (FAERS) as of 2021.41 This was not reported during MAb development programs, nor was it noted during clinical trials. Blood pressure elevation was seen within 1 week of injection in nearly 50% of the cases, and nearly one-third had pre-existing hypertension.41 Due to these findings, the erenumab prescribing information was updated to include hypertension in its warnings and precautions. It is possible that hypertension could be a class effect, although trial data and posthoc studies have yet to bear that out. Since erenumab was the first CGRP antagonist brought to market (May 2018 vs September 2018 for fremanezumab and galcanezumab), it may have accumulated more FAERS reports. Nearly all studies exclude patients with older age, uncontrolled hypertension, and unstable cardiovascular disease, which could impact data.41
Overall, this class of medications is very well tolerated, easy to use (again, excluding eptinezumab), and maintains a low adverse effect profile, giving added value compared with conventional preventive migraine medications.
The American Headache Society recommends a preventive oral therapy for at least 3 months before trying an alternative medication. After treatment failure with at least 2 oral agents, CGRP MAbs are recommended.42 CGRP antagonists offer convenient dosing, bypass gastrointestinal metabolism (which is useful in patients with nausea/vomiting), and have fewer adverse effects than traditional oral medications.
Worth noting. Several newer oral agents have been recently approved for migraine prevention, including atogepant (Qulipta) and rimegepant (Nurtec), which are also CGRP antagonists. Rimegepant is approved for both acute migraine treatment and prevention.
Continue to: Migraine
❯ Migraine: Test your skills
Subjective findings: A 25-year-old woman presents to your clinic for management of episodic migraines with aura. Her baseline average migraine frequency is 9 headache days/month. Her migraines are becoming more frequent despite treatment. She fears IV medication use and avoids hospitals.
History: Hypertension, irritable bowel syndrome with constipation (IBS-C), and depression. The patient is not pregnant or trying to get pregnant.
Medications: Current medications (for previous 4 months) include propranolol 40 mg at bedtime, linaclotide 145 μg/d, citalopram 20 mg/d, and sumatriptan 50 mg prn. Past medications include venlafaxine 150 mg po bid for 5 months.
What would be appropriate for this patient? This patient meets the criteria for using a CGRP antagonist because she has tried 2 preventive treatments for more than 60 to 90 days. Erenumab is not the best option, given the patient’s history of hypertension and IBS-C. The patient fears hospitals and IV medications, making eptinezumab a less-than-ideal choice. Depending on her insurance, fremanezumab or galcanezumab would be good options at this time.
CGRP antagonists have not been studied or evaluated in pregnancy, but if this patient becomes pregnant, a first-line agent for prevention would be propranolol, and a second-line agent would be a tricyclic antidepressant, memantine, or verapamil. Avoid ergotamines and antiepileptics (topiramate or valproate) in pregnancy.43,44
Continue to: The challenges associated with MAbs
The challenges associated with MAbs
MAbs can be expensive (TABLE 2),45 some prohibitively so. On a population scale, biologics account for around 40% of prescription drug spending and may cost 22 times more than small-molecule drugs.46 Estimates in 2016 showed that MAbs comprise $90.2 billion (43%) of the biologic market.46

MAbs also require prior authorization forms to be submitted. Prior authorization criteria vary by state and by insurance plan. In my (ES) experience, submitting letters of medical necessity justifying the need for therapy or expertise in the disease states for which the MAb is being prescribed help your patient get the medication they need.
Expect to see additional MAbs approved in the future. If the costs come down, adoption of these agents into practice will likely increase.
CORRESPONDENCE
Evelyn Sbar, MD, Texas Tech University Health Sciences Center, 1400 South Coulter Street, Suite 5100, Amarillo, TX 79106; [email protected]
1. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. National Center for Health Statistics. Accessed June 15, 2022. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
2. IDBS. The future of biologics drug development is today. June 27, 2018. Accessed June 15, 2022. www.idbs.com/blog/2018/06/the-future-of-biologics-drug-development-is-today/
3. Antibody therapeutics approved or in regulatory review in the EU or US. Antibody Society. Accessed June 15, 2022. www.antibodysociety.org/resources/approved-antibodies/
4. FDA. Code of Federal Regulations, Title 21, Chapter I, Subchapter F biologics. March 29, 2022. Accessed June 15, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=600.3
5. Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495-497. doi: 10.1038/256495a0
6. Raejewsky K. The advent and rise of monoclonal antibodies. Nature. November 4, 2019. Accessed June 15, 2022. www.nature.com/articles/d41586-019-02840-w
7. Flovent. Prescribing information. GlaxoSmithKline; 2010. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2010/021433s015lbl.pdf
8. NLM. National Center for Biotechnology Information. PubChem. Method for the preparation of fluticasone and related 17beta-carbothioic esters using a novel carbothioic acid synthesis and novel purification methods. Accessed June 15, 2022. pubchem.ncbi.nlm.nih.gov/patent/WO-0162722-A2
9. Nucala. Prescribing information. GlaxoSmithKline; 2019. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761122s000lbl.pdf
10. Argyriou AA, Kalofonos HP. Recent advances relating to the clinical application of naked monoclonal antibodies in solid tumors. Mol Med. 2009;15:183-191. doi: 10.2119/molmed.2009.00007
11. Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548-558. doi: 10.1038/clpt.2008.170
12. Zahavi D, AlDeghaither D, O’Connell A, et al. Enhancing antibody-dependent cell-mediated cytotoxicity: a strategy for improving antibody-based immunotherapy. Antib Ther. 2018;1:7-12. doi: 10.1093/abt/tby002
13. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014:CD003559. doi: 10.1002/14651858.CD003559.pub4
14. Farne HA, Wilson A, Powell C, et al. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9:CD010834. doi: 10.1002/14651858.CD010834.pub3
15. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486-2496. doi: 10.1056/NEJMoa1804092
16. GINA. Global strategy for asthma management and prevention. 2022 Difficult-to-treat and severe asthma guide—slide set. Accessed June 23, 2022. https://ginasthma.org/severeasthma/
17. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207. doi: 10.1056/NEJMoa1403290
18. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197. doi: 10.1056/NEJMoa1403291
19. Adbry. Prescribing information. Leo Pharma Inc; 2021. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761180Orig1s000lbl.pdf
20. Dupixent. Prescribing information. Regeneron Pharmaceuticals; 2022. Accessed October 5, 2022. https://www.regeneron.com/downloads/dupixent_fpi.pdf
21. Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348. doi: 10.1056/NEJMoa1610020
22. Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303. doi: 10.1016/s0140-6736(17)31191-1
23. Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi: 10.1016/j.jaad.2014.03.030
24. Evkeeza. Prescribing information. Regeneron Pharmaceuticals; 2021. Accessed June 24, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761181s000lbl.pdf
25. Repatha. Prescribing information. Amgen; 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125522s014lbl.pdf
26. Praluent. Prescribing information. Sanofi Aventis and Regeneron Pharmaceuticals. 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125559s002lbl.pdf
27. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722. doi: 10.1056/NEJMoa1615664
28. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097-2107. doi:10.1056/NEJMoa1801174
29. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
30. Prolia. Prescribing information. Amgen; 2010. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2013/125320s094lbl.pdf
31. Evenity. Prescribing information. Amgen; 2019. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761062s000lbl.pdf
32. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
33. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543. doi: 10.1056/NEJMoa1607948
34. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427. doi: 10.1056/NEJMoa1708322
35. Aimovig. Prescribing information. Amgen; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761077s000lbl.pdf
36. Vyepti. Prescribing information. Lundbeck Seattle BioPharmaceuticals; 2020. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2020/761119s000lbl.pdf
37. Ajovy. Prescribing information. Teva Pharmaceuticals; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761089s000lbl.pdf
38. Emgality. Prescribing information. Eli Lilly and Co.; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761063s000lbl.pdf
39. Edvinsson L, Haanes KA, Warfvinge K, et al. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338-350. doi: 10.1038/s41582-018-0003-1
40. Vandervorst F. Van Deun L, Van Dycke A, et al. CGRP monoclonal antibodies in migraine: an efficacy and tolerability comparison with standard prophylactic drugs. J Headache Pain. 2021;22:128. doi: 10.1186/s10194-021-01335-2
41. Saely S, Croteau D, Jawidzik L, et al. Hypertension: a new safety risk for patients treated with erenumab. Headache. 2021;61:202-208. doi: 10.1111/head.14051
42. American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1-18. doi: 10.1111/head.13456
43. Burch R. Headache in pregnancy and the puerperium. Neurol Clin. 2019;37:31-51. doi: 10.1016/j.ncl.2018.09.004
44. Burch R. Epidemiology and treatment of menstrual migraine and migraine during pregnancy and lactation: a narrative review. Headache. 2020;60:200-216. doi: 10.1111/head.13665
45. Lexi-Comp. Lexi-drug database. Accessed April 4, 2022. https://online.lexi.com/lco/action/login
46. Walker N. Biologics: driving force in pharma. Pharma’s Almanac. June 5, 2017. Accessed June 15, 2020. www.pharmasalmanac.com/articles/biologics-driving-force-in-pharma
1. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. National Center for Health Statistics. Accessed June 15, 2022. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
2. IDBS. The future of biologics drug development is today. June 27, 2018. Accessed June 15, 2022. www.idbs.com/blog/2018/06/the-future-of-biologics-drug-development-is-today/
3. Antibody therapeutics approved or in regulatory review in the EU or US. Antibody Society. Accessed June 15, 2022. www.antibodysociety.org/resources/approved-antibodies/
4. FDA. Code of Federal Regulations, Title 21, Chapter I, Subchapter F biologics. March 29, 2022. Accessed June 15, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=600.3
5. Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495-497. doi: 10.1038/256495a0
6. Raejewsky K. The advent and rise of monoclonal antibodies. Nature. November 4, 2019. Accessed June 15, 2022. www.nature.com/articles/d41586-019-02840-w
7. Flovent. Prescribing information. GlaxoSmithKline; 2010. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2010/021433s015lbl.pdf
8. NLM. National Center for Biotechnology Information. PubChem. Method for the preparation of fluticasone and related 17beta-carbothioic esters using a novel carbothioic acid synthesis and novel purification methods. Accessed June 15, 2022. pubchem.ncbi.nlm.nih.gov/patent/WO-0162722-A2
9. Nucala. Prescribing information. GlaxoSmithKline; 2019. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761122s000lbl.pdf
10. Argyriou AA, Kalofonos HP. Recent advances relating to the clinical application of naked monoclonal antibodies in solid tumors. Mol Med. 2009;15:183-191. doi: 10.2119/molmed.2009.00007
11. Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548-558. doi: 10.1038/clpt.2008.170
12. Zahavi D, AlDeghaither D, O’Connell A, et al. Enhancing antibody-dependent cell-mediated cytotoxicity: a strategy for improving antibody-based immunotherapy. Antib Ther. 2018;1:7-12. doi: 10.1093/abt/tby002
13. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014:CD003559. doi: 10.1002/14651858.CD003559.pub4
14. Farne HA, Wilson A, Powell C, et al. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9:CD010834. doi: 10.1002/14651858.CD010834.pub3
15. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486-2496. doi: 10.1056/NEJMoa1804092
16. GINA. Global strategy for asthma management and prevention. 2022 Difficult-to-treat and severe asthma guide—slide set. Accessed June 23, 2022. https://ginasthma.org/severeasthma/
17. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207. doi: 10.1056/NEJMoa1403290
18. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197. doi: 10.1056/NEJMoa1403291
19. Adbry. Prescribing information. Leo Pharma Inc; 2021. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761180Orig1s000lbl.pdf
20. Dupixent. Prescribing information. Regeneron Pharmaceuticals; 2022. Accessed October 5, 2022. https://www.regeneron.com/downloads/dupixent_fpi.pdf
21. Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348. doi: 10.1056/NEJMoa1610020
22. Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303. doi: 10.1016/s0140-6736(17)31191-1
23. Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi: 10.1016/j.jaad.2014.03.030
24. Evkeeza. Prescribing information. Regeneron Pharmaceuticals; 2021. Accessed June 24, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761181s000lbl.pdf
25. Repatha. Prescribing information. Amgen; 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125522s014lbl.pdf
26. Praluent. Prescribing information. Sanofi Aventis and Regeneron Pharmaceuticals. 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125559s002lbl.pdf
27. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722. doi: 10.1056/NEJMoa1615664
28. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097-2107. doi:10.1056/NEJMoa1801174
29. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
30. Prolia. Prescribing information. Amgen; 2010. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2013/125320s094lbl.pdf
31. Evenity. Prescribing information. Amgen; 2019. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761062s000lbl.pdf
32. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
33. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543. doi: 10.1056/NEJMoa1607948
34. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427. doi: 10.1056/NEJMoa1708322
35. Aimovig. Prescribing information. Amgen; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761077s000lbl.pdf
36. Vyepti. Prescribing information. Lundbeck Seattle BioPharmaceuticals; 2020. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2020/761119s000lbl.pdf
37. Ajovy. Prescribing information. Teva Pharmaceuticals; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761089s000lbl.pdf
38. Emgality. Prescribing information. Eli Lilly and Co.; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761063s000lbl.pdf
39. Edvinsson L, Haanes KA, Warfvinge K, et al. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338-350. doi: 10.1038/s41582-018-0003-1
40. Vandervorst F. Van Deun L, Van Dycke A, et al. CGRP monoclonal antibodies in migraine: an efficacy and tolerability comparison with standard prophylactic drugs. J Headache Pain. 2021;22:128. doi: 10.1186/s10194-021-01335-2
41. Saely S, Croteau D, Jawidzik L, et al. Hypertension: a new safety risk for patients treated with erenumab. Headache. 2021;61:202-208. doi: 10.1111/head.14051
42. American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1-18. doi: 10.1111/head.13456
43. Burch R. Headache in pregnancy and the puerperium. Neurol Clin. 2019;37:31-51. doi: 10.1016/j.ncl.2018.09.004
44. Burch R. Epidemiology and treatment of menstrual migraine and migraine during pregnancy and lactation: a narrative review. Headache. 2020;60:200-216. doi: 10.1111/head.13665
45. Lexi-Comp. Lexi-drug database. Accessed April 4, 2022. https://online.lexi.com/lco/action/login
46. Walker N. Biologics: driving force in pharma. Pharma’s Almanac. June 5, 2017. Accessed June 15, 2020. www.pharmasalmanac.com/articles/biologics-driving-force-in-pharma
PRACTICE RECOMMENDATIONS
› Consider anti-immunoglobulin E, anti-interleukin 5, or anti-interleukin 4/interleukin 13 for patients with moderate-to-severe asthma and type 2 airway inflammation. B
› Consider dupilumab for patients with moderate-to-severe atopic dermatitis (with or without topical corticosteroids), or when traditional oral therapies are inadequate or contraindicated. B
› Consider proprotein convertase subtilisin/kexin type 9 inhibitors for patients with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease when maximally tolerated statins or ezetimibe have not lowered low-density lipoprotein cholesterol levels far enough. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
IVIG proves effective for dermatomyositis in phase 3 trial
With use of intravenous immunoglobulin for the treatment of adults with dermatomyositis, a significantly higher percentage of patients experienced at least minimal improvement in disease activity in comparison with placebo in the first-ever phase 3 trial of the blood-product therapy for the condition.
Until this trial, published in the New England Journal of Medicine, there had not been an extensive evaluation of IVIG for the treatment of dermatomyositis, the study’s authors noted.
Glucocorticoids are typically offered as first-line therapy, followed by various immunosuppressants. IVIG is composed of purified liquid IgG concentrates from human plasma. It has been prescribed off label as second- or third-line therapy for dermatomyositis, usually along with immunosuppressive drugs. In European guidelines, it has been recommended as a glucocorticoid-sparing agent for patients with this condition.
“The study provides support that IVIG is effective in treating the signs and symptoms of patients with dermatomyositis, at least in the short term,” said David Fiorentino, MD, PhD, professor of dermatology and associate residency program director at Stanford Health Care, Stanford, California, who was not involved in the study.
“IVIG appears to be effective for patients with any severity level and works relatively quickly [within 1 month of therapy],” he added. “IVIG is effective in treating both the muscle symptoms as well as the rash of dermatomyositis, which is important, as both organ systems can cause significant patient morbidity in this disease.”
Time to improvement was shorter with IVIG than with placebo (a median of 35 days vs. 115 days), said Kathryn H. Dao, MD, associate professor in the division of rheumatic diseases at the University of Texas Southwestern Medical Center, Dallas, who was not involved in the study.
The study’s greatest strengths are its international, multicenter, randomized, placebo-controlled design, Dr. Dao said. In addition, “these patients were permitted to be on background medicines that we typically use in real-world situations.”
Study methodology
Researchers led by Rohit Aggarwal, MD, of the division of rheumatology and clinical immunology at the University of Pittsburgh, recruited patients aged 18-80 years with active dermatomyositis. Individuals were randomly assigned in a 1:1 ratio to receive either IVIG at a dose of 2.0 g/kg of body weight or placebo (0.9% sodium chloride) every 4 weeks for 16 weeks.
Those who were administered placebo and those who did not experience confirmed clinical deterioration while receiving IVIG could participate in an open-label extension phase for another 24 weeks.
The primary endpoint was a response, defined as a Total Improvement Score (TIS) of at least 20 (indicating at least minimal improvement) at week 16 and no confirmed deterioration up to week 16. The TIS is a weighted composite score that reflects the change in a core set of six measures of myositis activity over time. Scores span from 0 to 100, with higher scores indicating more significant improvement.
Secondary endpoints
Key secondary endpoints included moderate improvement (TIS ≥ 40) and major improvement (TIS ≥ 60) and change in score on the Cutaneous Dermatomyositis Disease Area and Severity Index.
A total of 95 patients underwent randomization; 47 patients received IVIG and 48 received placebo. At 16 weeks, a TIS of at least 20 occurred in 37 of 47 (79%) patients who received IVIG and in 21 of 48 (44%) patients with placebo (difference, 35%; 95% confidence interval, 17%-53%; P < .001).
The results with respect to the secondary endpoints, including at least moderate improvement and major improvement, were generally in the same direction as the results of the primary endpoint analysis, except for change in creatine kinase (CK) level (an individual core measure of the TIS), which did not differ meaningfully between the two groups.
Adverse events
Over the course of 40 weeks, 282 treatment-related adverse events were documented among patients who received IVIG. Headache was experienced by 42%, pyrexia by 19%, and nausea by 16%. Nine serious adverse events occurred and were believed to be associated with IVIG, including six thromboembolic events.
Despite the favorable outcome observed with IVIG, in an editorial that accompanied the study, Anthony A. Amato, MD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, noted that “most of the core components of the TIS are subjective. Because of the high percentage of patients who had a response with placebo, large numbers of patients will be needed in future trials to show a significant difference between trial groups, or the primary endpoint would need to be set higher (e.g., a TIS of ≥40).”
Dr. Dao thought it was significant that the study proactively assessed patients for venous thrombotic events (VTEs) after each infusion. There were eight events in six patients who received IVIG. “Of interest and possibly practice changing is the finding that slowing the IVIG infusion rate from 0.12 to 0.04 mL/kg per minute reduced the incidence of VTEs from 1.54/100 patient-months to 0.54/100 patient-months,” she said. “This is important, as it informs clinicians that IVIG infusion rates should be slower for patients with active dermatomyositis to reduce the risk for blood clots.”
Study weaknesses
A considerable proportion of patients with dermatomyositis do not have clinical muscle involvement but do have rash and do not substantially differ in any other ways from those with classic dermatomyositis, Dr. Fiorentino said.
“These patients were not eligible to enter the trial, and so we have no data on the efficacy of IVIG in this population,” he said. “Unfortunately, these patients might now be denied insurance reimbursement for IVIG therapy, given that they are not part of the indicated patient population in the label.”
In addition, there is limited information about Black, Asian, or Hispanic patients because few of those patients participated in the study. That is also the case for patients younger than 18, which for this disease is relevant because incidence peaks in younger patients (juvenile dermatomyositis), Dr. Fiorentino noted.
Among the study’s weaknesses, Dr. Dao noted that more than 70% of participants were women. The study was short in duration, fewer than half of patients underwent muscle biopsy to confirm myositis, and only two thirds of patients underwent electromyography/nerve conduction studies to show evidence of myositis. There was a high placebo response (44%), the CK values were not high at the start of the trial, and they did not change with treatment.
No analysis was performed to evaluate the efficacy of IVIG across dermatomyositis subgroups – defined by autoantibodies – but the study likely was not powered to do so. These subgroups might respond differently to IVIG, yielding important information, Fiorentino said.
The study provided efficacy data for only one formulation of IVIG, Octagam 10%, which was approved for dermatomyositis by the Food and Drug Administration in 2021 on the basis of this trial. However, in the United States, patients with dermatomyositis are treated with multiple brands of IVIG. “The decision around IVIG brand is largely determined by third-party payers, and for the most part, the different brands are used interchangeably from the standpoint of the treating provider,” Dr. Fiorentino said. “This will likely continue to be the case, as the results of this study are generally being extrapolated to all brands of IVIG.”
Multiple IVIG brands that have been used for immune-mediated diseases differ in concentration, content of IgA, sugar concentration, additives, and preparations (for example, the need for reconstitution vs. being ready to use), Dr. Dao said. Octagam 10% is the only brand approved by the FDA for adult dermatomyositis; hence, cost can be an issue for patients if other brands are used off label. The typical cost of IVIG is $100-$400 per gram; a typical course of treatment is estimated to be $30,000-$40,000 per month. “However, if Octagam is not available or a patient has a reaction to it, clinicians may use other IVIG brands as deemed medically necessary to treat their patients,” she said.
Dr. Aggarwal has financial relationships with more than 15 pharmaceutical companies, including Octapharma, which provided financial support for this trial. Some of the coauthors were employees of Octapharma or had financial relationships with the company. Dr. Dao disclosed no relevant financial relationships. Dr. Fiorentino has conducted sponsored research for Pfizer and Argenyx, has received research funding from Serono, and is a paid adviser to Bristol-Myers Squibb, Janssen, Acelyrin, and Corbus.
A version of this article first appeared on Medscape.com.
With use of intravenous immunoglobulin for the treatment of adults with dermatomyositis, a significantly higher percentage of patients experienced at least minimal improvement in disease activity in comparison with placebo in the first-ever phase 3 trial of the blood-product therapy for the condition.
Until this trial, published in the New England Journal of Medicine, there had not been an extensive evaluation of IVIG for the treatment of dermatomyositis, the study’s authors noted.
Glucocorticoids are typically offered as first-line therapy, followed by various immunosuppressants. IVIG is composed of purified liquid IgG concentrates from human plasma. It has been prescribed off label as second- or third-line therapy for dermatomyositis, usually along with immunosuppressive drugs. In European guidelines, it has been recommended as a glucocorticoid-sparing agent for patients with this condition.
“The study provides support that IVIG is effective in treating the signs and symptoms of patients with dermatomyositis, at least in the short term,” said David Fiorentino, MD, PhD, professor of dermatology and associate residency program director at Stanford Health Care, Stanford, California, who was not involved in the study.
“IVIG appears to be effective for patients with any severity level and works relatively quickly [within 1 month of therapy],” he added. “IVIG is effective in treating both the muscle symptoms as well as the rash of dermatomyositis, which is important, as both organ systems can cause significant patient morbidity in this disease.”
Time to improvement was shorter with IVIG than with placebo (a median of 35 days vs. 115 days), said Kathryn H. Dao, MD, associate professor in the division of rheumatic diseases at the University of Texas Southwestern Medical Center, Dallas, who was not involved in the study.
The study’s greatest strengths are its international, multicenter, randomized, placebo-controlled design, Dr. Dao said. In addition, “these patients were permitted to be on background medicines that we typically use in real-world situations.”
Study methodology
Researchers led by Rohit Aggarwal, MD, of the division of rheumatology and clinical immunology at the University of Pittsburgh, recruited patients aged 18-80 years with active dermatomyositis. Individuals were randomly assigned in a 1:1 ratio to receive either IVIG at a dose of 2.0 g/kg of body weight or placebo (0.9% sodium chloride) every 4 weeks for 16 weeks.
Those who were administered placebo and those who did not experience confirmed clinical deterioration while receiving IVIG could participate in an open-label extension phase for another 24 weeks.
The primary endpoint was a response, defined as a Total Improvement Score (TIS) of at least 20 (indicating at least minimal improvement) at week 16 and no confirmed deterioration up to week 16. The TIS is a weighted composite score that reflects the change in a core set of six measures of myositis activity over time. Scores span from 0 to 100, with higher scores indicating more significant improvement.
Secondary endpoints
Key secondary endpoints included moderate improvement (TIS ≥ 40) and major improvement (TIS ≥ 60) and change in score on the Cutaneous Dermatomyositis Disease Area and Severity Index.
A total of 95 patients underwent randomization; 47 patients received IVIG and 48 received placebo. At 16 weeks, a TIS of at least 20 occurred in 37 of 47 (79%) patients who received IVIG and in 21 of 48 (44%) patients with placebo (difference, 35%; 95% confidence interval, 17%-53%; P < .001).
The results with respect to the secondary endpoints, including at least moderate improvement and major improvement, were generally in the same direction as the results of the primary endpoint analysis, except for change in creatine kinase (CK) level (an individual core measure of the TIS), which did not differ meaningfully between the two groups.
Adverse events
Over the course of 40 weeks, 282 treatment-related adverse events were documented among patients who received IVIG. Headache was experienced by 42%, pyrexia by 19%, and nausea by 16%. Nine serious adverse events occurred and were believed to be associated with IVIG, including six thromboembolic events.
Despite the favorable outcome observed with IVIG, in an editorial that accompanied the study, Anthony A. Amato, MD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, noted that “most of the core components of the TIS are subjective. Because of the high percentage of patients who had a response with placebo, large numbers of patients will be needed in future trials to show a significant difference between trial groups, or the primary endpoint would need to be set higher (e.g., a TIS of ≥40).”
Dr. Dao thought it was significant that the study proactively assessed patients for venous thrombotic events (VTEs) after each infusion. There were eight events in six patients who received IVIG. “Of interest and possibly practice changing is the finding that slowing the IVIG infusion rate from 0.12 to 0.04 mL/kg per minute reduced the incidence of VTEs from 1.54/100 patient-months to 0.54/100 patient-months,” she said. “This is important, as it informs clinicians that IVIG infusion rates should be slower for patients with active dermatomyositis to reduce the risk for blood clots.”
Study weaknesses
A considerable proportion of patients with dermatomyositis do not have clinical muscle involvement but do have rash and do not substantially differ in any other ways from those with classic dermatomyositis, Dr. Fiorentino said.
“These patients were not eligible to enter the trial, and so we have no data on the efficacy of IVIG in this population,” he said. “Unfortunately, these patients might now be denied insurance reimbursement for IVIG therapy, given that they are not part of the indicated patient population in the label.”
In addition, there is limited information about Black, Asian, or Hispanic patients because few of those patients participated in the study. That is also the case for patients younger than 18, which for this disease is relevant because incidence peaks in younger patients (juvenile dermatomyositis), Dr. Fiorentino noted.
Among the study’s weaknesses, Dr. Dao noted that more than 70% of participants were women. The study was short in duration, fewer than half of patients underwent muscle biopsy to confirm myositis, and only two thirds of patients underwent electromyography/nerve conduction studies to show evidence of myositis. There was a high placebo response (44%), the CK values were not high at the start of the trial, and they did not change with treatment.
No analysis was performed to evaluate the efficacy of IVIG across dermatomyositis subgroups – defined by autoantibodies – but the study likely was not powered to do so. These subgroups might respond differently to IVIG, yielding important information, Fiorentino said.
The study provided efficacy data for only one formulation of IVIG, Octagam 10%, which was approved for dermatomyositis by the Food and Drug Administration in 2021 on the basis of this trial. However, in the United States, patients with dermatomyositis are treated with multiple brands of IVIG. “The decision around IVIG brand is largely determined by third-party payers, and for the most part, the different brands are used interchangeably from the standpoint of the treating provider,” Dr. Fiorentino said. “This will likely continue to be the case, as the results of this study are generally being extrapolated to all brands of IVIG.”
Multiple IVIG brands that have been used for immune-mediated diseases differ in concentration, content of IgA, sugar concentration, additives, and preparations (for example, the need for reconstitution vs. being ready to use), Dr. Dao said. Octagam 10% is the only brand approved by the FDA for adult dermatomyositis; hence, cost can be an issue for patients if other brands are used off label. The typical cost of IVIG is $100-$400 per gram; a typical course of treatment is estimated to be $30,000-$40,000 per month. “However, if Octagam is not available or a patient has a reaction to it, clinicians may use other IVIG brands as deemed medically necessary to treat their patients,” she said.
Dr. Aggarwal has financial relationships with more than 15 pharmaceutical companies, including Octapharma, which provided financial support for this trial. Some of the coauthors were employees of Octapharma or had financial relationships with the company. Dr. Dao disclosed no relevant financial relationships. Dr. Fiorentino has conducted sponsored research for Pfizer and Argenyx, has received research funding from Serono, and is a paid adviser to Bristol-Myers Squibb, Janssen, Acelyrin, and Corbus.
A version of this article first appeared on Medscape.com.
With use of intravenous immunoglobulin for the treatment of adults with dermatomyositis, a significantly higher percentage of patients experienced at least minimal improvement in disease activity in comparison with placebo in the first-ever phase 3 trial of the blood-product therapy for the condition.
Until this trial, published in the New England Journal of Medicine, there had not been an extensive evaluation of IVIG for the treatment of dermatomyositis, the study’s authors noted.
Glucocorticoids are typically offered as first-line therapy, followed by various immunosuppressants. IVIG is composed of purified liquid IgG concentrates from human plasma. It has been prescribed off label as second- or third-line therapy for dermatomyositis, usually along with immunosuppressive drugs. In European guidelines, it has been recommended as a glucocorticoid-sparing agent for patients with this condition.
“The study provides support that IVIG is effective in treating the signs and symptoms of patients with dermatomyositis, at least in the short term,” said David Fiorentino, MD, PhD, professor of dermatology and associate residency program director at Stanford Health Care, Stanford, California, who was not involved in the study.
“IVIG appears to be effective for patients with any severity level and works relatively quickly [within 1 month of therapy],” he added. “IVIG is effective in treating both the muscle symptoms as well as the rash of dermatomyositis, which is important, as both organ systems can cause significant patient morbidity in this disease.”
Time to improvement was shorter with IVIG than with placebo (a median of 35 days vs. 115 days), said Kathryn H. Dao, MD, associate professor in the division of rheumatic diseases at the University of Texas Southwestern Medical Center, Dallas, who was not involved in the study.
The study’s greatest strengths are its international, multicenter, randomized, placebo-controlled design, Dr. Dao said. In addition, “these patients were permitted to be on background medicines that we typically use in real-world situations.”
Study methodology
Researchers led by Rohit Aggarwal, MD, of the division of rheumatology and clinical immunology at the University of Pittsburgh, recruited patients aged 18-80 years with active dermatomyositis. Individuals were randomly assigned in a 1:1 ratio to receive either IVIG at a dose of 2.0 g/kg of body weight or placebo (0.9% sodium chloride) every 4 weeks for 16 weeks.
Those who were administered placebo and those who did not experience confirmed clinical deterioration while receiving IVIG could participate in an open-label extension phase for another 24 weeks.
The primary endpoint was a response, defined as a Total Improvement Score (TIS) of at least 20 (indicating at least minimal improvement) at week 16 and no confirmed deterioration up to week 16. The TIS is a weighted composite score that reflects the change in a core set of six measures of myositis activity over time. Scores span from 0 to 100, with higher scores indicating more significant improvement.
Secondary endpoints
Key secondary endpoints included moderate improvement (TIS ≥ 40) and major improvement (TIS ≥ 60) and change in score on the Cutaneous Dermatomyositis Disease Area and Severity Index.
A total of 95 patients underwent randomization; 47 patients received IVIG and 48 received placebo. At 16 weeks, a TIS of at least 20 occurred in 37 of 47 (79%) patients who received IVIG and in 21 of 48 (44%) patients with placebo (difference, 35%; 95% confidence interval, 17%-53%; P < .001).
The results with respect to the secondary endpoints, including at least moderate improvement and major improvement, were generally in the same direction as the results of the primary endpoint analysis, except for change in creatine kinase (CK) level (an individual core measure of the TIS), which did not differ meaningfully between the two groups.
Adverse events
Over the course of 40 weeks, 282 treatment-related adverse events were documented among patients who received IVIG. Headache was experienced by 42%, pyrexia by 19%, and nausea by 16%. Nine serious adverse events occurred and were believed to be associated with IVIG, including six thromboembolic events.
Despite the favorable outcome observed with IVIG, in an editorial that accompanied the study, Anthony A. Amato, MD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, noted that “most of the core components of the TIS are subjective. Because of the high percentage of patients who had a response with placebo, large numbers of patients will be needed in future trials to show a significant difference between trial groups, or the primary endpoint would need to be set higher (e.g., a TIS of ≥40).”
Dr. Dao thought it was significant that the study proactively assessed patients for venous thrombotic events (VTEs) after each infusion. There were eight events in six patients who received IVIG. “Of interest and possibly practice changing is the finding that slowing the IVIG infusion rate from 0.12 to 0.04 mL/kg per minute reduced the incidence of VTEs from 1.54/100 patient-months to 0.54/100 patient-months,” she said. “This is important, as it informs clinicians that IVIG infusion rates should be slower for patients with active dermatomyositis to reduce the risk for blood clots.”
Study weaknesses
A considerable proportion of patients with dermatomyositis do not have clinical muscle involvement but do have rash and do not substantially differ in any other ways from those with classic dermatomyositis, Dr. Fiorentino said.
“These patients were not eligible to enter the trial, and so we have no data on the efficacy of IVIG in this population,” he said. “Unfortunately, these patients might now be denied insurance reimbursement for IVIG therapy, given that they are not part of the indicated patient population in the label.”
In addition, there is limited information about Black, Asian, or Hispanic patients because few of those patients participated in the study. That is also the case for patients younger than 18, which for this disease is relevant because incidence peaks in younger patients (juvenile dermatomyositis), Dr. Fiorentino noted.
Among the study’s weaknesses, Dr. Dao noted that more than 70% of participants were women. The study was short in duration, fewer than half of patients underwent muscle biopsy to confirm myositis, and only two thirds of patients underwent electromyography/nerve conduction studies to show evidence of myositis. There was a high placebo response (44%), the CK values were not high at the start of the trial, and they did not change with treatment.
No analysis was performed to evaluate the efficacy of IVIG across dermatomyositis subgroups – defined by autoantibodies – but the study likely was not powered to do so. These subgroups might respond differently to IVIG, yielding important information, Fiorentino said.
The study provided efficacy data for only one formulation of IVIG, Octagam 10%, which was approved for dermatomyositis by the Food and Drug Administration in 2021 on the basis of this trial. However, in the United States, patients with dermatomyositis are treated with multiple brands of IVIG. “The decision around IVIG brand is largely determined by third-party payers, and for the most part, the different brands are used interchangeably from the standpoint of the treating provider,” Dr. Fiorentino said. “This will likely continue to be the case, as the results of this study are generally being extrapolated to all brands of IVIG.”
Multiple IVIG brands that have been used for immune-mediated diseases differ in concentration, content of IgA, sugar concentration, additives, and preparations (for example, the need for reconstitution vs. being ready to use), Dr. Dao said. Octagam 10% is the only brand approved by the FDA for adult dermatomyositis; hence, cost can be an issue for patients if other brands are used off label. The typical cost of IVIG is $100-$400 per gram; a typical course of treatment is estimated to be $30,000-$40,000 per month. “However, if Octagam is not available or a patient has a reaction to it, clinicians may use other IVIG brands as deemed medically necessary to treat their patients,” she said.
Dr. Aggarwal has financial relationships with more than 15 pharmaceutical companies, including Octapharma, which provided financial support for this trial. Some of the coauthors were employees of Octapharma or had financial relationships with the company. Dr. Dao disclosed no relevant financial relationships. Dr. Fiorentino has conducted sponsored research for Pfizer and Argenyx, has received research funding from Serono, and is a paid adviser to Bristol-Myers Squibb, Janssen, Acelyrin, and Corbus.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Evusheld PrEP may protect immunocompromised patients from severe COVID-19
Tixagevimab copackaged with cilgavimab (Evusheld) is a safe and effective preexposure prophylaxis (PrEP) in patients undergoing B-cell-depleting therapies who have poor immune response to COVID-19 vaccination and are at high risk for serious COVID-19 illness, a small, single-site study suggests.
Evusheld, the only COVID-19 PrEP option available, has Emergency Use Authorization (EUA) from the Food and Drug Administration for treatment of immunocompromised patients who may not respond sufficiently to COVID-19 vaccination and patients who’ve had a severe adverse reaction to COVID-19 vaccination.
“We report the largest real-world experience of Evusheld in this population, and our findings are encouraging,” lead study author Cassandra Calabrese, DO, rheumatologist and infectious disease specialist at Cleveland Clinic, said in an interview.
“Of 412 patients who received Evusheld, 12 [2.9%] developed breakthrough COVID-19, with 11 having mild courses and 1 who required hospitalization but recovered,” she added.
“Our data suggest that Evusheld PrEP, in combination with aggressive outpatient treatment of COVID-19, is likely effective in lowering risk of severe COVID in this vulnerable group.
“Practitioners who care for patients with immune-mediated inflammatory diseases should triage high-risk patients for Evusheld as well as rapid diagnosis and aggressive outpatient therapy if infected,” Dr. Calabrese advised.
For the study, Dr. Calabrese and colleagues at Cleveland Clinic searched the health care system pharmacy records for patients with immune‐mediated inflammatory diseases (IMIDs) or inborn errors of humoral immunity (IEI) who met the criteria to receive Evusheld. The researchers included patients on B-cell-depleting therapies or with humoral IEI who had received at least one dose of Evusheld and were later diagnosed with COVID-19, and they excluded those treated with B-cell-depleting therapies for cancer.
EVUSHELD was well tolerated
After extracting data on COVID-19 infection, vaccination status, and outcomes, they found that, between Jan. 18 and May 28, 2022, 412 patients with IMIDs or humoral IEI received Evusheld. No deaths occurred among these patients and, overall, they tolerated the medication well.
All 12 patients who experienced breakthrough COVID-19 infection were treated with B-cell-depleting therapies. Among the 12 patients:
- Six patients developed infection 13-84 (median 19) days after receiving 150 mg/150 mg tixagevimab/cilgavimab.
- Six patients developed infection 19-72 (median of 38.5) days after either a single dose of 300 mg/300 mg or a second dose of 150 mg/150 mg.
- Eleven patients had mild illness and recovered at home; one patient was hospitalized and treated with high-flow oxygen. All cases had been vaccinated against COVID-19 (five received two vaccinations, six received three, and one received four).
- One possible serious adverse event involved a patient with COVID-19 and immune-mediated thrombocytopenia (ITP) who was hospitalized soon after receiving Evusheld with ITP flare that resolved with intravenous immunoglobulin.
Dr. Calabrese acknowledged limitations to the study, including few patients, lack of a comparator group, and the study period falling during the Omicron wave.
“Also, nine of the breakthrough cases received additional COVID-19 therapy (oral antiviral or monoclonal antibody), which falls within standard of care for this high-risk group but prevents ascribing effectiveness to individual components of the regimen,” she added.
“Evusheld is authorized for PrEP against COVID-19 in patients at high risk for severe COVID due to suboptimal vaccine responses. This includes patients receiving B-cell-depleting drugs like rituximab, and patients with inborn errors of humoral immunity,” Dr. Calabrese explained.
“It is well known that this group of patients is at very high risk for severe COVID and death, even when fully vaccinated, and it has become clear that more strategies are needed to protect this vulnerable group, including use of Evusheld as well as aggressive treatment if infected,” she added.
Evusheld not always easy to obtain
Although the medication has been available in the United States since January 2022, Dr. Calabrese said, patients may not receive it because of barriers including lack of both awareness and access.
Davey Smith, MD, professor of medicine and head of infectious diseases and global public health at the University of California San Diego, in La Jolla, said in an interview that he was not surprised by the results, but added that the study was conducted in too few patients to draw any strong conclusions or affect patient care.
“This small study that showed that breakthrough infections occurred but were generally mild, provides a small glimpse of real-world use of tixagevimab/cilgavimab as PrEP for immunocompromised persons,” said Dr. Smith, who was not involved in the study.
“In the setting of Omicron and vaccination, I would expect the same outcomes reported even without the treatment,” he added.
Dr. Smith recommends larger related randomized, controlled trials to provide clinicians with sufficient data to guide them in their patient care.
Graham Snyder, MD, associate professor in the division of infectious diseases at the University of Pittsburgh and medical director of infection prevention and hospital epidemiology at the University of Pittsburgh Medical Center, noted that the study “adds to a quickly growing literature on the real-world benefits of tixagevimab/cilgavimab to protect vulnerable individuals with weakened immune systems from the complications of COVID-19.
“This study provides a modest addition to our understanding of the role and benefit of Evusheld,” Dr. Snyder said in an interview. “By characterizing only patients who have received Evusheld without an untreated comparison group, we can’t draw any inference about the extent of benefit the agent provided to these patients.
“Substantial data already show that this agent is effective in preventing complications of COVID-19 infection in immunocompromised individuals,” added Dr. Snyder, who was not involved in the study.
“ ‘Immunocompromised’ represents a very diverse set of clinical conditions,” he said. “The research agenda should therefore focus on a more refined description of the effect in specific populations and a continued understanding of the effect of Evusheld in the context of updated vaccination strategies and changing virus ecology.”
Dr. Calabrese and her colleagues wrote that larger, controlled trials are underway.
FDA: Evusheld may not neutralize certain SARS-CoV-2 variants
“The biggest unanswered question is how Evusheld will hold up against new variants,” Dr. Calabrese said.
In an Oct. 3, 2022, update, the Food and Drug Administration released a statement about the risk of developing COVID-19 from SARS-CoV-2 variants that are not neutralized by Evusheld. The statement mentions an updated fact sheet that describes reduced protection from Evusheld against the Omicron subvariant BA.4.6, which accounted for nearly 13% of all new COVID-19 cases in the United States in the week ending Oct. 1.
There was no outside funding for the study. Dr. Smith reported no relevant financial conflicts of interest. Dr. Snyder said he is an unpaid adviser to an AstraZeneca observational study that’s assessing the real-world effectiveness of Evusheld.
Tixagevimab copackaged with cilgavimab (Evusheld) is a safe and effective preexposure prophylaxis (PrEP) in patients undergoing B-cell-depleting therapies who have poor immune response to COVID-19 vaccination and are at high risk for serious COVID-19 illness, a small, single-site study suggests.
Evusheld, the only COVID-19 PrEP option available, has Emergency Use Authorization (EUA) from the Food and Drug Administration for treatment of immunocompromised patients who may not respond sufficiently to COVID-19 vaccination and patients who’ve had a severe adverse reaction to COVID-19 vaccination.
“We report the largest real-world experience of Evusheld in this population, and our findings are encouraging,” lead study author Cassandra Calabrese, DO, rheumatologist and infectious disease specialist at Cleveland Clinic, said in an interview.
“Of 412 patients who received Evusheld, 12 [2.9%] developed breakthrough COVID-19, with 11 having mild courses and 1 who required hospitalization but recovered,” she added.
“Our data suggest that Evusheld PrEP, in combination with aggressive outpatient treatment of COVID-19, is likely effective in lowering risk of severe COVID in this vulnerable group.
“Practitioners who care for patients with immune-mediated inflammatory diseases should triage high-risk patients for Evusheld as well as rapid diagnosis and aggressive outpatient therapy if infected,” Dr. Calabrese advised.
For the study, Dr. Calabrese and colleagues at Cleveland Clinic searched the health care system pharmacy records for patients with immune‐mediated inflammatory diseases (IMIDs) or inborn errors of humoral immunity (IEI) who met the criteria to receive Evusheld. The researchers included patients on B-cell-depleting therapies or with humoral IEI who had received at least one dose of Evusheld and were later diagnosed with COVID-19, and they excluded those treated with B-cell-depleting therapies for cancer.
EVUSHELD was well tolerated
After extracting data on COVID-19 infection, vaccination status, and outcomes, they found that, between Jan. 18 and May 28, 2022, 412 patients with IMIDs or humoral IEI received Evusheld. No deaths occurred among these patients and, overall, they tolerated the medication well.
All 12 patients who experienced breakthrough COVID-19 infection were treated with B-cell-depleting therapies. Among the 12 patients:
- Six patients developed infection 13-84 (median 19) days after receiving 150 mg/150 mg tixagevimab/cilgavimab.
- Six patients developed infection 19-72 (median of 38.5) days after either a single dose of 300 mg/300 mg or a second dose of 150 mg/150 mg.
- Eleven patients had mild illness and recovered at home; one patient was hospitalized and treated with high-flow oxygen. All cases had been vaccinated against COVID-19 (five received two vaccinations, six received three, and one received four).
- One possible serious adverse event involved a patient with COVID-19 and immune-mediated thrombocytopenia (ITP) who was hospitalized soon after receiving Evusheld with ITP flare that resolved with intravenous immunoglobulin.
Dr. Calabrese acknowledged limitations to the study, including few patients, lack of a comparator group, and the study period falling during the Omicron wave.
“Also, nine of the breakthrough cases received additional COVID-19 therapy (oral antiviral or monoclonal antibody), which falls within standard of care for this high-risk group but prevents ascribing effectiveness to individual components of the regimen,” she added.
“Evusheld is authorized for PrEP against COVID-19 in patients at high risk for severe COVID due to suboptimal vaccine responses. This includes patients receiving B-cell-depleting drugs like rituximab, and patients with inborn errors of humoral immunity,” Dr. Calabrese explained.
“It is well known that this group of patients is at very high risk for severe COVID and death, even when fully vaccinated, and it has become clear that more strategies are needed to protect this vulnerable group, including use of Evusheld as well as aggressive treatment if infected,” she added.
Evusheld not always easy to obtain
Although the medication has been available in the United States since January 2022, Dr. Calabrese said, patients may not receive it because of barriers including lack of both awareness and access.
Davey Smith, MD, professor of medicine and head of infectious diseases and global public health at the University of California San Diego, in La Jolla, said in an interview that he was not surprised by the results, but added that the study was conducted in too few patients to draw any strong conclusions or affect patient care.
“This small study that showed that breakthrough infections occurred but were generally mild, provides a small glimpse of real-world use of tixagevimab/cilgavimab as PrEP for immunocompromised persons,” said Dr. Smith, who was not involved in the study.
“In the setting of Omicron and vaccination, I would expect the same outcomes reported even without the treatment,” he added.
Dr. Smith recommends larger related randomized, controlled trials to provide clinicians with sufficient data to guide them in their patient care.
Graham Snyder, MD, associate professor in the division of infectious diseases at the University of Pittsburgh and medical director of infection prevention and hospital epidemiology at the University of Pittsburgh Medical Center, noted that the study “adds to a quickly growing literature on the real-world benefits of tixagevimab/cilgavimab to protect vulnerable individuals with weakened immune systems from the complications of COVID-19.
“This study provides a modest addition to our understanding of the role and benefit of Evusheld,” Dr. Snyder said in an interview. “By characterizing only patients who have received Evusheld without an untreated comparison group, we can’t draw any inference about the extent of benefit the agent provided to these patients.
“Substantial data already show that this agent is effective in preventing complications of COVID-19 infection in immunocompromised individuals,” added Dr. Snyder, who was not involved in the study.
“ ‘Immunocompromised’ represents a very diverse set of clinical conditions,” he said. “The research agenda should therefore focus on a more refined description of the effect in specific populations and a continued understanding of the effect of Evusheld in the context of updated vaccination strategies and changing virus ecology.”
Dr. Calabrese and her colleagues wrote that larger, controlled trials are underway.
FDA: Evusheld may not neutralize certain SARS-CoV-2 variants
“The biggest unanswered question is how Evusheld will hold up against new variants,” Dr. Calabrese said.
In an Oct. 3, 2022, update, the Food and Drug Administration released a statement about the risk of developing COVID-19 from SARS-CoV-2 variants that are not neutralized by Evusheld. The statement mentions an updated fact sheet that describes reduced protection from Evusheld against the Omicron subvariant BA.4.6, which accounted for nearly 13% of all new COVID-19 cases in the United States in the week ending Oct. 1.
There was no outside funding for the study. Dr. Smith reported no relevant financial conflicts of interest. Dr. Snyder said he is an unpaid adviser to an AstraZeneca observational study that’s assessing the real-world effectiveness of Evusheld.
Tixagevimab copackaged with cilgavimab (Evusheld) is a safe and effective preexposure prophylaxis (PrEP) in patients undergoing B-cell-depleting therapies who have poor immune response to COVID-19 vaccination and are at high risk for serious COVID-19 illness, a small, single-site study suggests.
Evusheld, the only COVID-19 PrEP option available, has Emergency Use Authorization (EUA) from the Food and Drug Administration for treatment of immunocompromised patients who may not respond sufficiently to COVID-19 vaccination and patients who’ve had a severe adverse reaction to COVID-19 vaccination.
“We report the largest real-world experience of Evusheld in this population, and our findings are encouraging,” lead study author Cassandra Calabrese, DO, rheumatologist and infectious disease specialist at Cleveland Clinic, said in an interview.
“Of 412 patients who received Evusheld, 12 [2.9%] developed breakthrough COVID-19, with 11 having mild courses and 1 who required hospitalization but recovered,” she added.
“Our data suggest that Evusheld PrEP, in combination with aggressive outpatient treatment of COVID-19, is likely effective in lowering risk of severe COVID in this vulnerable group.
“Practitioners who care for patients with immune-mediated inflammatory diseases should triage high-risk patients for Evusheld as well as rapid diagnosis and aggressive outpatient therapy if infected,” Dr. Calabrese advised.
For the study, Dr. Calabrese and colleagues at Cleveland Clinic searched the health care system pharmacy records for patients with immune‐mediated inflammatory diseases (IMIDs) or inborn errors of humoral immunity (IEI) who met the criteria to receive Evusheld. The researchers included patients on B-cell-depleting therapies or with humoral IEI who had received at least one dose of Evusheld and were later diagnosed with COVID-19, and they excluded those treated with B-cell-depleting therapies for cancer.
EVUSHELD was well tolerated
After extracting data on COVID-19 infection, vaccination status, and outcomes, they found that, between Jan. 18 and May 28, 2022, 412 patients with IMIDs or humoral IEI received Evusheld. No deaths occurred among these patients and, overall, they tolerated the medication well.
All 12 patients who experienced breakthrough COVID-19 infection were treated with B-cell-depleting therapies. Among the 12 patients:
- Six patients developed infection 13-84 (median 19) days after receiving 150 mg/150 mg tixagevimab/cilgavimab.
- Six patients developed infection 19-72 (median of 38.5) days after either a single dose of 300 mg/300 mg or a second dose of 150 mg/150 mg.
- Eleven patients had mild illness and recovered at home; one patient was hospitalized and treated with high-flow oxygen. All cases had been vaccinated against COVID-19 (five received two vaccinations, six received three, and one received four).
- One possible serious adverse event involved a patient with COVID-19 and immune-mediated thrombocytopenia (ITP) who was hospitalized soon after receiving Evusheld with ITP flare that resolved with intravenous immunoglobulin.
Dr. Calabrese acknowledged limitations to the study, including few patients, lack of a comparator group, and the study period falling during the Omicron wave.
“Also, nine of the breakthrough cases received additional COVID-19 therapy (oral antiviral or monoclonal antibody), which falls within standard of care for this high-risk group but prevents ascribing effectiveness to individual components of the regimen,” she added.
“Evusheld is authorized for PrEP against COVID-19 in patients at high risk for severe COVID due to suboptimal vaccine responses. This includes patients receiving B-cell-depleting drugs like rituximab, and patients with inborn errors of humoral immunity,” Dr. Calabrese explained.
“It is well known that this group of patients is at very high risk for severe COVID and death, even when fully vaccinated, and it has become clear that more strategies are needed to protect this vulnerable group, including use of Evusheld as well as aggressive treatment if infected,” she added.
Evusheld not always easy to obtain
Although the medication has been available in the United States since January 2022, Dr. Calabrese said, patients may not receive it because of barriers including lack of both awareness and access.
Davey Smith, MD, professor of medicine and head of infectious diseases and global public health at the University of California San Diego, in La Jolla, said in an interview that he was not surprised by the results, but added that the study was conducted in too few patients to draw any strong conclusions or affect patient care.
“This small study that showed that breakthrough infections occurred but were generally mild, provides a small glimpse of real-world use of tixagevimab/cilgavimab as PrEP for immunocompromised persons,” said Dr. Smith, who was not involved in the study.
“In the setting of Omicron and vaccination, I would expect the same outcomes reported even without the treatment,” he added.
Dr. Smith recommends larger related randomized, controlled trials to provide clinicians with sufficient data to guide them in their patient care.
Graham Snyder, MD, associate professor in the division of infectious diseases at the University of Pittsburgh and medical director of infection prevention and hospital epidemiology at the University of Pittsburgh Medical Center, noted that the study “adds to a quickly growing literature on the real-world benefits of tixagevimab/cilgavimab to protect vulnerable individuals with weakened immune systems from the complications of COVID-19.
“This study provides a modest addition to our understanding of the role and benefit of Evusheld,” Dr. Snyder said in an interview. “By characterizing only patients who have received Evusheld without an untreated comparison group, we can’t draw any inference about the extent of benefit the agent provided to these patients.
“Substantial data already show that this agent is effective in preventing complications of COVID-19 infection in immunocompromised individuals,” added Dr. Snyder, who was not involved in the study.
“ ‘Immunocompromised’ represents a very diverse set of clinical conditions,” he said. “The research agenda should therefore focus on a more refined description of the effect in specific populations and a continued understanding of the effect of Evusheld in the context of updated vaccination strategies and changing virus ecology.”
Dr. Calabrese and her colleagues wrote that larger, controlled trials are underway.
FDA: Evusheld may not neutralize certain SARS-CoV-2 variants
“The biggest unanswered question is how Evusheld will hold up against new variants,” Dr. Calabrese said.
In an Oct. 3, 2022, update, the Food and Drug Administration released a statement about the risk of developing COVID-19 from SARS-CoV-2 variants that are not neutralized by Evusheld. The statement mentions an updated fact sheet that describes reduced protection from Evusheld against the Omicron subvariant BA.4.6, which accounted for nearly 13% of all new COVID-19 cases in the United States in the week ending Oct. 1.
There was no outside funding for the study. Dr. Smith reported no relevant financial conflicts of interest. Dr. Snyder said he is an unpaid adviser to an AstraZeneca observational study that’s assessing the real-world effectiveness of Evusheld.
FROM RMD OPEN
Could vitamin C help reduce gout?
Could taking vitamin C help reduce the chances of developing gout? A new study sheds light on this possibility.
Gout is a form of inflammatory arthritis that has been on the rise in the United States in recent decades. Considered a lifestyle disease, some research has shown that instances of the condition have more than doubled in recent years as rates of obesity have skyrocketed. It’s caused by uric acid in the blood that builds up and crystallizes in the joints. Flare-ups are so intense that the joints can turn a cherry red and vibrate with intense – and sometimes seemingly intolerable – pain.
While there are effective treatments, many people fail to take their medications when they’re not in pain, and if the condition goes unchecked, it can get much worse and cause permanent damage to the joints.
“Gout can cause flare-ups that vary in frequency and severity; but sometimes when people aren’t experiencing them, they’re less likely to stay on top of their medications,” said Stephen Juraschek, MD, an assistant professor of medicine at Harvard Medical School, Boston.
That’s why lifestyle interventions are seen as particularly relevant to a disease like gout. Vitamin C, for example, has few side effects, and for those with higher levels of uric acid in the blood, it could reduce the likelihood of getting the condition. A recent study published in The American Journal of Clinical Nutrition found that people who were given 500 mg of vitamin C versus a placebo had a 12% reduced risk of getting gout. The study of over 14,000 male doctors showed that men who weren’t overweight had the most significant reduction in the risk of getting the condition. (Excess weight has been shown to increase the risk of gout.)
As part of the study, participants responded to a questionnaire that asked whether they had ever been diagnosed with gout. Other studies have shown that vitamin C reduced the levels of urate in people without gout and broke down uric crystals in the blood, but this study took it a step further to show that the supplement actually reduced the risk of getting the condition.
“In addition to lowering levels of uric acid in the body, it’s thought that vitamin C may also minimize the inflammatory response to urate crystals,” said Dr. Juraschek. That’s because when flare-ups develop in joints throughout the body, much of the painful irritation is caused by the immune system’s response as it fights to break down the crystals.
Dr. Juraschek said this likely wouldn’t change recommendations for patients with serious gout, but it could still have an impact.
“For individuals who were told that they have gout but have had fewer flare-ups, they might be more open to taking vitamin C,” he said.
Will Settle, 42, of Hilton Head, S.C., was not involved in the study, but he said he would be inclined to try most any safe preventive method. Gout runs in his family. His father and grandfather had it, and now, so does he. His flare-ups have slowed in recent years, which he said has a lot to do with his diet and lifestyle. He stopped eating seafood, started drinking more water, and stopped drinking as much alcohol – all of which he thinks has had a huge impact on the severity of his condition. (Both seafood and beer contain high levels of purines, which have been shown to increase the buildup of uric acid in the blood.) Mr. Settle said that other simple lifestyle changes like vitamin C would be an easy addition to his routine with few downsides. Plus, he hates having to take colchicine, a medication that’s meant to relieve pain but causes him intense diarrhea when he takes it.
“Anything to reduce my flare-ups without having to take colchicine,” he said.
But the jury is still out as to whether vitamin C will have any real benefits. Study coauthor Robert H. Shmerling, MD, is the former clinical chief of the division of rheumatology at Beth Israel Deaconess Medical Center, New York. He said the study shows that the effect of vitamin C in those undiagnosed with gout was rather modest. Also, vitamin C did not show a reduction in gout flare-ups in those who were already diagnosed with the condition. Not to mention that the study lacked diversity, as the people in it were all male and mostly white. Still, there’s little downside risk to taking vitamin C, and it might end up being worthwhile.
“Maybe it will turn out to be an effective treatment in those who are at high risk, but we’re not there yet,” he said.
Robert Terkeltaub, MD, chief of rheumatology at the Veterans Administration Medical Center in San Diego and a professor of medicine at the University of California, San Diego, said there’s an unmet need when it comes to tools for gout prevention.
“The disease impacts some 10 million Americans, and we need to better identify these individuals so we can intervene earlier,” he said.
While vitamin C had a small but significant association with fewer new cases of gout, it did not lower it in those who already had the disease, said Dr. Terkeltaub. What’s more, researchers didn’t measure the levels of uric acid in the blood, which would have painted a more accurate picture of whether vitamin C actually reduced it in the body.
“There remains no clarity on the potential role of vitamin C in either prevention or treatment of gout. That said, future research would be of interest,” he said.
Still, gout patients like Mr. Settle aren’t ruling it out. Anything to avoid the pain that, at times, makes it difficult for him to get out of bed. He’s seen the benefit that simple lifestyle changes can make, and he’s willing to try just about anything to live a normal, arthritis-free life.
“I’m always looking for simple ways to keep my flare-ups at bay,” he said.
A version of this article first appeared on WebMD.com.
Could taking vitamin C help reduce the chances of developing gout? A new study sheds light on this possibility.
Gout is a form of inflammatory arthritis that has been on the rise in the United States in recent decades. Considered a lifestyle disease, some research has shown that instances of the condition have more than doubled in recent years as rates of obesity have skyrocketed. It’s caused by uric acid in the blood that builds up and crystallizes in the joints. Flare-ups are so intense that the joints can turn a cherry red and vibrate with intense – and sometimes seemingly intolerable – pain.
While there are effective treatments, many people fail to take their medications when they’re not in pain, and if the condition goes unchecked, it can get much worse and cause permanent damage to the joints.
“Gout can cause flare-ups that vary in frequency and severity; but sometimes when people aren’t experiencing them, they’re less likely to stay on top of their medications,” said Stephen Juraschek, MD, an assistant professor of medicine at Harvard Medical School, Boston.
That’s why lifestyle interventions are seen as particularly relevant to a disease like gout. Vitamin C, for example, has few side effects, and for those with higher levels of uric acid in the blood, it could reduce the likelihood of getting the condition. A recent study published in The American Journal of Clinical Nutrition found that people who were given 500 mg of vitamin C versus a placebo had a 12% reduced risk of getting gout. The study of over 14,000 male doctors showed that men who weren’t overweight had the most significant reduction in the risk of getting the condition. (Excess weight has been shown to increase the risk of gout.)
As part of the study, participants responded to a questionnaire that asked whether they had ever been diagnosed with gout. Other studies have shown that vitamin C reduced the levels of urate in people without gout and broke down uric crystals in the blood, but this study took it a step further to show that the supplement actually reduced the risk of getting the condition.
“In addition to lowering levels of uric acid in the body, it’s thought that vitamin C may also minimize the inflammatory response to urate crystals,” said Dr. Juraschek. That’s because when flare-ups develop in joints throughout the body, much of the painful irritation is caused by the immune system’s response as it fights to break down the crystals.
Dr. Juraschek said this likely wouldn’t change recommendations for patients with serious gout, but it could still have an impact.
“For individuals who were told that they have gout but have had fewer flare-ups, they might be more open to taking vitamin C,” he said.
Will Settle, 42, of Hilton Head, S.C., was not involved in the study, but he said he would be inclined to try most any safe preventive method. Gout runs in his family. His father and grandfather had it, and now, so does he. His flare-ups have slowed in recent years, which he said has a lot to do with his diet and lifestyle. He stopped eating seafood, started drinking more water, and stopped drinking as much alcohol – all of which he thinks has had a huge impact on the severity of his condition. (Both seafood and beer contain high levels of purines, which have been shown to increase the buildup of uric acid in the blood.) Mr. Settle said that other simple lifestyle changes like vitamin C would be an easy addition to his routine with few downsides. Plus, he hates having to take colchicine, a medication that’s meant to relieve pain but causes him intense diarrhea when he takes it.
“Anything to reduce my flare-ups without having to take colchicine,” he said.
But the jury is still out as to whether vitamin C will have any real benefits. Study coauthor Robert H. Shmerling, MD, is the former clinical chief of the division of rheumatology at Beth Israel Deaconess Medical Center, New York. He said the study shows that the effect of vitamin C in those undiagnosed with gout was rather modest. Also, vitamin C did not show a reduction in gout flare-ups in those who were already diagnosed with the condition. Not to mention that the study lacked diversity, as the people in it were all male and mostly white. Still, there’s little downside risk to taking vitamin C, and it might end up being worthwhile.
“Maybe it will turn out to be an effective treatment in those who are at high risk, but we’re not there yet,” he said.
Robert Terkeltaub, MD, chief of rheumatology at the Veterans Administration Medical Center in San Diego and a professor of medicine at the University of California, San Diego, said there’s an unmet need when it comes to tools for gout prevention.
“The disease impacts some 10 million Americans, and we need to better identify these individuals so we can intervene earlier,” he said.
While vitamin C had a small but significant association with fewer new cases of gout, it did not lower it in those who already had the disease, said Dr. Terkeltaub. What’s more, researchers didn’t measure the levels of uric acid in the blood, which would have painted a more accurate picture of whether vitamin C actually reduced it in the body.
“There remains no clarity on the potential role of vitamin C in either prevention or treatment of gout. That said, future research would be of interest,” he said.
Still, gout patients like Mr. Settle aren’t ruling it out. Anything to avoid the pain that, at times, makes it difficult for him to get out of bed. He’s seen the benefit that simple lifestyle changes can make, and he’s willing to try just about anything to live a normal, arthritis-free life.
“I’m always looking for simple ways to keep my flare-ups at bay,” he said.
A version of this article first appeared on WebMD.com.
Could taking vitamin C help reduce the chances of developing gout? A new study sheds light on this possibility.
Gout is a form of inflammatory arthritis that has been on the rise in the United States in recent decades. Considered a lifestyle disease, some research has shown that instances of the condition have more than doubled in recent years as rates of obesity have skyrocketed. It’s caused by uric acid in the blood that builds up and crystallizes in the joints. Flare-ups are so intense that the joints can turn a cherry red and vibrate with intense – and sometimes seemingly intolerable – pain.
While there are effective treatments, many people fail to take their medications when they’re not in pain, and if the condition goes unchecked, it can get much worse and cause permanent damage to the joints.
“Gout can cause flare-ups that vary in frequency and severity; but sometimes when people aren’t experiencing them, they’re less likely to stay on top of their medications,” said Stephen Juraschek, MD, an assistant professor of medicine at Harvard Medical School, Boston.
That’s why lifestyle interventions are seen as particularly relevant to a disease like gout. Vitamin C, for example, has few side effects, and for those with higher levels of uric acid in the blood, it could reduce the likelihood of getting the condition. A recent study published in The American Journal of Clinical Nutrition found that people who were given 500 mg of vitamin C versus a placebo had a 12% reduced risk of getting gout. The study of over 14,000 male doctors showed that men who weren’t overweight had the most significant reduction in the risk of getting the condition. (Excess weight has been shown to increase the risk of gout.)
As part of the study, participants responded to a questionnaire that asked whether they had ever been diagnosed with gout. Other studies have shown that vitamin C reduced the levels of urate in people without gout and broke down uric crystals in the blood, but this study took it a step further to show that the supplement actually reduced the risk of getting the condition.
“In addition to lowering levels of uric acid in the body, it’s thought that vitamin C may also minimize the inflammatory response to urate crystals,” said Dr. Juraschek. That’s because when flare-ups develop in joints throughout the body, much of the painful irritation is caused by the immune system’s response as it fights to break down the crystals.
Dr. Juraschek said this likely wouldn’t change recommendations for patients with serious gout, but it could still have an impact.
“For individuals who were told that they have gout but have had fewer flare-ups, they might be more open to taking vitamin C,” he said.
Will Settle, 42, of Hilton Head, S.C., was not involved in the study, but he said he would be inclined to try most any safe preventive method. Gout runs in his family. His father and grandfather had it, and now, so does he. His flare-ups have slowed in recent years, which he said has a lot to do with his diet and lifestyle. He stopped eating seafood, started drinking more water, and stopped drinking as much alcohol – all of which he thinks has had a huge impact on the severity of his condition. (Both seafood and beer contain high levels of purines, which have been shown to increase the buildup of uric acid in the blood.) Mr. Settle said that other simple lifestyle changes like vitamin C would be an easy addition to his routine with few downsides. Plus, he hates having to take colchicine, a medication that’s meant to relieve pain but causes him intense diarrhea when he takes it.
“Anything to reduce my flare-ups without having to take colchicine,” he said.
But the jury is still out as to whether vitamin C will have any real benefits. Study coauthor Robert H. Shmerling, MD, is the former clinical chief of the division of rheumatology at Beth Israel Deaconess Medical Center, New York. He said the study shows that the effect of vitamin C in those undiagnosed with gout was rather modest. Also, vitamin C did not show a reduction in gout flare-ups in those who were already diagnosed with the condition. Not to mention that the study lacked diversity, as the people in it were all male and mostly white. Still, there’s little downside risk to taking vitamin C, and it might end up being worthwhile.
“Maybe it will turn out to be an effective treatment in those who are at high risk, but we’re not there yet,” he said.
Robert Terkeltaub, MD, chief of rheumatology at the Veterans Administration Medical Center in San Diego and a professor of medicine at the University of California, San Diego, said there’s an unmet need when it comes to tools for gout prevention.
“The disease impacts some 10 million Americans, and we need to better identify these individuals so we can intervene earlier,” he said.
While vitamin C had a small but significant association with fewer new cases of gout, it did not lower it in those who already had the disease, said Dr. Terkeltaub. What’s more, researchers didn’t measure the levels of uric acid in the blood, which would have painted a more accurate picture of whether vitamin C actually reduced it in the body.
“There remains no clarity on the potential role of vitamin C in either prevention or treatment of gout. That said, future research would be of interest,” he said.
Still, gout patients like Mr. Settle aren’t ruling it out. Anything to avoid the pain that, at times, makes it difficult for him to get out of bed. He’s seen the benefit that simple lifestyle changes can make, and he’s willing to try just about anything to live a normal, arthritis-free life.
“I’m always looking for simple ways to keep my flare-ups at bay,” he said.
A version of this article first appeared on WebMD.com.
FROM THE AMERICAN JOURNAL OF CLINICAL NUTRITION
Analysis of PsA guidelines reveals much room for improvement on conflicts of interest
, according to a retrospective analysis of all authors on the most recent guidelines issued by the American College of Rheumatology (ACR) and the Japanese Dermatological Association (JDA).
In addition to finding that the majority of the authors of psoriatic arthritis (PsA) clinical practice guidelines (CPGs) issued by the JDA and ACR received substantial personal payments from pharmaceutical companies before and during CPG development, researchers led by Hanano Mamada and Anju Murayama of the Medical Governance Research Institute, Tokyo, wrote in Arthritis Care & Research that “several CPG authors self-cited their articles without the disclosure of NFCOI [nonfinancial conflicts of interest], and most of the recommendations were based on low or very low quality of evidence. Although the COI policies used by JDA and ACR are clearly inadequate, no significant revisions have been made for the last 3 years.”
Based on their findings, which were made using payment data from major Japanese pharmaceutical companies and the U.S. Open Payments Database from 2016 to 2018, the researchers suggested that the medical societies should:
- Adopt global standard COI policies from organizations such as the National Academy of Medicine and Guidelines International Network, including a 3-year lookback period for COI declaration.
- Consider a comprehensive definition and rigorous management with full disclosure of NFCOI.
- Publish a list of authors making each recommendation to grasp the implications of COI in clinical practice guidelines.
- Mention the detailed date of the COI disclosure, which should be close to the publication date as much as possible.
Financial conflicts of interest
The researchers used payment data published between 2016 and 2018 for all 83 companies belonging to the Japan Pharmaceutical Manufacturers Association, focusing on personal payments (for lecturing, writing, and consultancy) and excluding research payments, “since in Japan, the name, institution, and position of the author or researcher who received the research payment is not disclosed, which makes assessing research payments difficult.” To evaluate authors’ FCOI in the ACR’s CPG, the researchers analyzed the U.S. Open Payments Database “for all categories of general payments such as speaking, consulting, meals, and travel expenses 3 years from before the guideline’s first online publication on November 30, 2018.”
The 2018 ACR/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis had 36 authors and the JDA’s Clinical Practice Guideline for the Treatment of Psoriatic Arthritis 2019 had 23. Overall, 61% of JDA authors and half of ACR authors voluntarily declared FCOI with pharmaceutical companies; 25 of the ACR authors were U.S. physicians and could be included in the Open Payments Database search.
A total of 21 (91.3%) JDA authors and 21 (84.0%) ACR authors received at least one payment, with the combined total of $3,335,413 and $4,081,629 payments, respectively, over the 3 years. The average and median personal payments were $145,018 and $123,876 for JDA authors and $162,825 and $58,826 for ACR authors. When the payments to ACR authors were limited to lecturing, writing, and consulting fees that are required under the ACR’s COI policy, the mean was $130,102 and median was $39,375. The corresponding payments for JDA authors were $123,876 and $8,170, respectively,
The researchers found undisclosed payments for more than three-quarters of physician authors of the Japanese guideline, and nearly half of the doctors authoring the American guideline had undisclosed payments. These added up to $474,000 for the JDA, which amounted to 38% of the total for personal payments that must be reported to the JDA based on its COI policy for clinical practice guidelines, and $218,000 for the ACR, amounting to 18% of the total for personal payments that must be reported to the society based on its COI policy.
Of the 11 ACR authors who were not eligible for the U.S. Open Payments Database search, 5 declared FCOI with pharmaceutical companies in the guideline, meaning that 26 (72%) of the 36 authors had FCOI with pharmaceutical companies.
The ACR only required authors to declare FCOI covering 1 year before and during guideline development, and although the JDA required authors to declare their FCOI for the past 3 years of guideline development, the study authors noted that the JDA guideline disclosed them for only 2 years (between Jan. 1, 2017, and Dec. 31, 2018).
“It is true that influential doctors such as clinical practice guideline authors tend to receive various types of payments from pharmaceutical companies and that it is difficult to conduct research without funding from pharmaceutical companies. However, our current research mainly focuses on personal payments from pharmaceutical companies such as lecture fees and consulting fees. These payments are recognized as pocket money and are not used for research. Thus, it is questionable that the observed relationships are something evitable,” the researchers wrote.
Nonfinancial conflicts of interest
Many authors of the ACR’s CPG and the JDA’s CPG also had NFCOI, defined objectively in this study as self-citation rate. NFCOI have been more broadly defined by the International Committee of Medical Journal Editors (ICMJE) as “conflicts, such as personal relationships or rivalries, academic competition, and intellectual beliefs”; the ICMJE recommends reporting NFCOI on its COI form.
The JDA guideline included self-citations by 78% of its authors, compared with 32% of the ACR guideline authors, but this weighed differently among the two guidelines in that only 12 of the 354 (3.4%) citations in the JDA guideline were self-cited, compared with 46 of 137 (34%) citations in the ACR guideline.
The researchers noted that while the self-citation rates between JDA and ACR authors “differed remarkably,” the impact of ACR authors on CPG recommendations was much more direct. Three-quarters of JDA authors’ self-cited articles were about observational studies, whereas 52% of the ACR authors’ self-cited articles were clinical trials, most of which were randomized, controlled studies, and these NFCOI were not disclosed in the guideline.
Half of the strong recommendations in the JDA guideline were based on low or very low quality of evidence, whereas the ACR guideline had no strong recommendations based on low or very low quality of evidence.
This study was supported by the nonprofit Medical Governance Research Institute, which receives donations from Ain Pharmacies Inc., other organizations, and private individuals. The study also received support from the Tansa (formerly known as the Waseda Chronicle), an independent nonprofit news organization dedicated to investigative journalism. Three authors reported receiving personal fees from several pharmaceutical companies for work outside of the scope of this study.
, according to a retrospective analysis of all authors on the most recent guidelines issued by the American College of Rheumatology (ACR) and the Japanese Dermatological Association (JDA).
In addition to finding that the majority of the authors of psoriatic arthritis (PsA) clinical practice guidelines (CPGs) issued by the JDA and ACR received substantial personal payments from pharmaceutical companies before and during CPG development, researchers led by Hanano Mamada and Anju Murayama of the Medical Governance Research Institute, Tokyo, wrote in Arthritis Care & Research that “several CPG authors self-cited their articles without the disclosure of NFCOI [nonfinancial conflicts of interest], and most of the recommendations were based on low or very low quality of evidence. Although the COI policies used by JDA and ACR are clearly inadequate, no significant revisions have been made for the last 3 years.”
Based on their findings, which were made using payment data from major Japanese pharmaceutical companies and the U.S. Open Payments Database from 2016 to 2018, the researchers suggested that the medical societies should:
- Adopt global standard COI policies from organizations such as the National Academy of Medicine and Guidelines International Network, including a 3-year lookback period for COI declaration.
- Consider a comprehensive definition and rigorous management with full disclosure of NFCOI.
- Publish a list of authors making each recommendation to grasp the implications of COI in clinical practice guidelines.
- Mention the detailed date of the COI disclosure, which should be close to the publication date as much as possible.
Financial conflicts of interest
The researchers used payment data published between 2016 and 2018 for all 83 companies belonging to the Japan Pharmaceutical Manufacturers Association, focusing on personal payments (for lecturing, writing, and consultancy) and excluding research payments, “since in Japan, the name, institution, and position of the author or researcher who received the research payment is not disclosed, which makes assessing research payments difficult.” To evaluate authors’ FCOI in the ACR’s CPG, the researchers analyzed the U.S. Open Payments Database “for all categories of general payments such as speaking, consulting, meals, and travel expenses 3 years from before the guideline’s first online publication on November 30, 2018.”
The 2018 ACR/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis had 36 authors and the JDA’s Clinical Practice Guideline for the Treatment of Psoriatic Arthritis 2019 had 23. Overall, 61% of JDA authors and half of ACR authors voluntarily declared FCOI with pharmaceutical companies; 25 of the ACR authors were U.S. physicians and could be included in the Open Payments Database search.
A total of 21 (91.3%) JDA authors and 21 (84.0%) ACR authors received at least one payment, with the combined total of $3,335,413 and $4,081,629 payments, respectively, over the 3 years. The average and median personal payments were $145,018 and $123,876 for JDA authors and $162,825 and $58,826 for ACR authors. When the payments to ACR authors were limited to lecturing, writing, and consulting fees that are required under the ACR’s COI policy, the mean was $130,102 and median was $39,375. The corresponding payments for JDA authors were $123,876 and $8,170, respectively,
The researchers found undisclosed payments for more than three-quarters of physician authors of the Japanese guideline, and nearly half of the doctors authoring the American guideline had undisclosed payments. These added up to $474,000 for the JDA, which amounted to 38% of the total for personal payments that must be reported to the JDA based on its COI policy for clinical practice guidelines, and $218,000 for the ACR, amounting to 18% of the total for personal payments that must be reported to the society based on its COI policy.
Of the 11 ACR authors who were not eligible for the U.S. Open Payments Database search, 5 declared FCOI with pharmaceutical companies in the guideline, meaning that 26 (72%) of the 36 authors had FCOI with pharmaceutical companies.
The ACR only required authors to declare FCOI covering 1 year before and during guideline development, and although the JDA required authors to declare their FCOI for the past 3 years of guideline development, the study authors noted that the JDA guideline disclosed them for only 2 years (between Jan. 1, 2017, and Dec. 31, 2018).
“It is true that influential doctors such as clinical practice guideline authors tend to receive various types of payments from pharmaceutical companies and that it is difficult to conduct research without funding from pharmaceutical companies. However, our current research mainly focuses on personal payments from pharmaceutical companies such as lecture fees and consulting fees. These payments are recognized as pocket money and are not used for research. Thus, it is questionable that the observed relationships are something evitable,” the researchers wrote.
Nonfinancial conflicts of interest
Many authors of the ACR’s CPG and the JDA’s CPG also had NFCOI, defined objectively in this study as self-citation rate. NFCOI have been more broadly defined by the International Committee of Medical Journal Editors (ICMJE) as “conflicts, such as personal relationships or rivalries, academic competition, and intellectual beliefs”; the ICMJE recommends reporting NFCOI on its COI form.
The JDA guideline included self-citations by 78% of its authors, compared with 32% of the ACR guideline authors, but this weighed differently among the two guidelines in that only 12 of the 354 (3.4%) citations in the JDA guideline were self-cited, compared with 46 of 137 (34%) citations in the ACR guideline.
The researchers noted that while the self-citation rates between JDA and ACR authors “differed remarkably,” the impact of ACR authors on CPG recommendations was much more direct. Three-quarters of JDA authors’ self-cited articles were about observational studies, whereas 52% of the ACR authors’ self-cited articles were clinical trials, most of which were randomized, controlled studies, and these NFCOI were not disclosed in the guideline.
Half of the strong recommendations in the JDA guideline were based on low or very low quality of evidence, whereas the ACR guideline had no strong recommendations based on low or very low quality of evidence.
This study was supported by the nonprofit Medical Governance Research Institute, which receives donations from Ain Pharmacies Inc., other organizations, and private individuals. The study also received support from the Tansa (formerly known as the Waseda Chronicle), an independent nonprofit news organization dedicated to investigative journalism. Three authors reported receiving personal fees from several pharmaceutical companies for work outside of the scope of this study.
, according to a retrospective analysis of all authors on the most recent guidelines issued by the American College of Rheumatology (ACR) and the Japanese Dermatological Association (JDA).
In addition to finding that the majority of the authors of psoriatic arthritis (PsA) clinical practice guidelines (CPGs) issued by the JDA and ACR received substantial personal payments from pharmaceutical companies before and during CPG development, researchers led by Hanano Mamada and Anju Murayama of the Medical Governance Research Institute, Tokyo, wrote in Arthritis Care & Research that “several CPG authors self-cited their articles without the disclosure of NFCOI [nonfinancial conflicts of interest], and most of the recommendations were based on low or very low quality of evidence. Although the COI policies used by JDA and ACR are clearly inadequate, no significant revisions have been made for the last 3 years.”
Based on their findings, which were made using payment data from major Japanese pharmaceutical companies and the U.S. Open Payments Database from 2016 to 2018, the researchers suggested that the medical societies should:
- Adopt global standard COI policies from organizations such as the National Academy of Medicine and Guidelines International Network, including a 3-year lookback period for COI declaration.
- Consider a comprehensive definition and rigorous management with full disclosure of NFCOI.
- Publish a list of authors making each recommendation to grasp the implications of COI in clinical practice guidelines.
- Mention the detailed date of the COI disclosure, which should be close to the publication date as much as possible.
Financial conflicts of interest
The researchers used payment data published between 2016 and 2018 for all 83 companies belonging to the Japan Pharmaceutical Manufacturers Association, focusing on personal payments (for lecturing, writing, and consultancy) and excluding research payments, “since in Japan, the name, institution, and position of the author or researcher who received the research payment is not disclosed, which makes assessing research payments difficult.” To evaluate authors’ FCOI in the ACR’s CPG, the researchers analyzed the U.S. Open Payments Database “for all categories of general payments such as speaking, consulting, meals, and travel expenses 3 years from before the guideline’s first online publication on November 30, 2018.”
The 2018 ACR/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis had 36 authors and the JDA’s Clinical Practice Guideline for the Treatment of Psoriatic Arthritis 2019 had 23. Overall, 61% of JDA authors and half of ACR authors voluntarily declared FCOI with pharmaceutical companies; 25 of the ACR authors were U.S. physicians and could be included in the Open Payments Database search.
A total of 21 (91.3%) JDA authors and 21 (84.0%) ACR authors received at least one payment, with the combined total of $3,335,413 and $4,081,629 payments, respectively, over the 3 years. The average and median personal payments were $145,018 and $123,876 for JDA authors and $162,825 and $58,826 for ACR authors. When the payments to ACR authors were limited to lecturing, writing, and consulting fees that are required under the ACR’s COI policy, the mean was $130,102 and median was $39,375. The corresponding payments for JDA authors were $123,876 and $8,170, respectively,
The researchers found undisclosed payments for more than three-quarters of physician authors of the Japanese guideline, and nearly half of the doctors authoring the American guideline had undisclosed payments. These added up to $474,000 for the JDA, which amounted to 38% of the total for personal payments that must be reported to the JDA based on its COI policy for clinical practice guidelines, and $218,000 for the ACR, amounting to 18% of the total for personal payments that must be reported to the society based on its COI policy.
Of the 11 ACR authors who were not eligible for the U.S. Open Payments Database search, 5 declared FCOI with pharmaceutical companies in the guideline, meaning that 26 (72%) of the 36 authors had FCOI with pharmaceutical companies.
The ACR only required authors to declare FCOI covering 1 year before and during guideline development, and although the JDA required authors to declare their FCOI for the past 3 years of guideline development, the study authors noted that the JDA guideline disclosed them for only 2 years (between Jan. 1, 2017, and Dec. 31, 2018).
“It is true that influential doctors such as clinical practice guideline authors tend to receive various types of payments from pharmaceutical companies and that it is difficult to conduct research without funding from pharmaceutical companies. However, our current research mainly focuses on personal payments from pharmaceutical companies such as lecture fees and consulting fees. These payments are recognized as pocket money and are not used for research. Thus, it is questionable that the observed relationships are something evitable,” the researchers wrote.
Nonfinancial conflicts of interest
Many authors of the ACR’s CPG and the JDA’s CPG also had NFCOI, defined objectively in this study as self-citation rate. NFCOI have been more broadly defined by the International Committee of Medical Journal Editors (ICMJE) as “conflicts, such as personal relationships or rivalries, academic competition, and intellectual beliefs”; the ICMJE recommends reporting NFCOI on its COI form.
The JDA guideline included self-citations by 78% of its authors, compared with 32% of the ACR guideline authors, but this weighed differently among the two guidelines in that only 12 of the 354 (3.4%) citations in the JDA guideline were self-cited, compared with 46 of 137 (34%) citations in the ACR guideline.
The researchers noted that while the self-citation rates between JDA and ACR authors “differed remarkably,” the impact of ACR authors on CPG recommendations was much more direct. Three-quarters of JDA authors’ self-cited articles were about observational studies, whereas 52% of the ACR authors’ self-cited articles were clinical trials, most of which were randomized, controlled studies, and these NFCOI were not disclosed in the guideline.
Half of the strong recommendations in the JDA guideline were based on low or very low quality of evidence, whereas the ACR guideline had no strong recommendations based on low or very low quality of evidence.
This study was supported by the nonprofit Medical Governance Research Institute, which receives donations from Ain Pharmacies Inc., other organizations, and private individuals. The study also received support from the Tansa (formerly known as the Waseda Chronicle), an independent nonprofit news organization dedicated to investigative journalism. Three authors reported receiving personal fees from several pharmaceutical companies for work outside of the scope of this study.
FROM ARTHRITIS CARE & RESEARCH
Breakthrough COVID studies lend support to use of new boosters in immunosuppressed patients
People with immune-mediated inflammatory diseases who are taking immunosuppressants don’t mount as strong of an immune defense against the Omicron variant as they did against the original SARS-CoV-2 wild-type virus, according to two studies published in Annals of the Rheumatic Diseases. One of the studies further showed that vaccinated individuals taking immunosuppressants have poorer cross-neutralizing responses to Omicron than do healthy vaccinated individuals, even after three doses of the COVID-19 mRNA vaccines.
“We carefully suggest that if Omicron-specific vaccination can be administered, it may be an effective way to reduce the risk of breakthrough infections in patients with autoimmune rheumatic disease,” Sang Tae Choi, MD, PhD, of the University College of Medicine, Seoul, Korea, and one of the authors of the study on cross-neutralizing protection, told this news organization. “However, further research is needed on Omicron-specific vaccine effectiveness in patients with immune dysfunctions. We believe that these study results can be of great benefit in determining the strategy of vaccination in the future.”
The earlier study, published in July, examined the ability of COVID-19 vaccines to induce cross-reactive antibody responses against Omicron infections in patients with autoimmune rheumatic diseases (ARDs). The observational study involved 149 patients with ARDs and 94 health care workers as controls, all of whom provided blood samples a median 15 weeks after their second COVID vaccine dose or a median 8 weeks after their third dose. A little more than two-thirds of the patients (68.5%) had received a third mRNA vaccine dose. None of the participants previously had COVID-19.
The researchers compared the rate of breakthrough infections with the Omicron variant to the neutralizing responses in patients’ blood, specifically the cross-neutralizing antibody responses because the original mRNA vaccines targeted a different variant than Omicron. Breakthrough infections were assessed by survey questions.
“Our findings suggested that neither primary series vaccinations nor booster doses are sufficient to induce Omicron-neutralizing responses above the threshold in patients with ARDs, although responses were noticeably increased following the third dose of an mRNA vaccine,” write Woo-Joong Kim, of the Chung-Ang University College of Medicine, Seoul, Korea, and his colleagues. “This impairment of cross-neutralization responses across most of our patients contrasts starkly with a potent elicitation of the Omicron-neutralizing responses after the third vaccination in healthy recipients.”
The average neutralizing responses against the original SARS-CoV-2 strain were similar in both groups: 76% in patients with ARDs and 72% in health care workers after the second dose. The mean response after a third dose was 97% in health care workers and 88% in patients.
The average cross-neutralizing response against the Omicron variant was far lower, particularly in those with rheumatic disease: only 11.5%, which rose to 27% after the third dose. Only 39% of the patient sera showed neutralization of Omicron, even after the third dose. Meanwhile, the mean cross-neutralizing response in health care workers was 18% after the second dose and 50% after the third.
When the researchers compared seropositivity rates against the original virus to neutralizing responses against Omicron, the association between these was stronger in health care workers than in those with ARDs. In fact, among patients with ARDs who seroconverted, only 41% showed any response against Omicron. Among all the patients, most of those who didn’t respond to Omicron (93.5%) had initially seroconverted.
The researchers also looked at the ability to neutralize Omicron on the basis of disease in those who received three doses of the vaccine. About half of those with lupus (52%) showed any neutralization against Omicron, compared with 25% of those with rheumatoid arthritis, 37.5% of those with ankylosing spondylitis, 33% of those with Behçet snydrome, and all of those with adult-onset Still’s disease.
The rate of breakthrough infections was lower in patients (19%) than in health care workers (33%). A similar pattern was seen in the more recent study published Sept. 5. Researchers used data from a prospective cohort study in the Netherlands to examine incidence and severity of Omicron breakthrough infections in patients with immune-mediated inflammatory diseases. The researchers compared infection rates and severity among 1,593 vaccinated patients with inflammatory disease who were taking immunosuppressants and 579 vaccinated controls (418 patients with inflammatory disease not on immunosuppressants and 161 healthy controls).
One in five patients with inflammatory disease (21%) were taking immunosuppressants that substantially impair antibodies, such as anti-CD20 therapy, S1P modulators, or mycophenolate mofetil combination therapy, and 48% of these patients seroconverted after primary vaccination, compared with 96% of patients taking other immunosuppressants and 98% of controls.
Breakthrough infection rates were similar between the control group (31%) and those taking immunosuppressants (30%). Only three participants had severe disease requiring hospitalization: one control and two patients taking immunosuppressants.
“In both studies, the controls had similar or higher rates of breakthrough infections, compared with the immunosuppressed,” noted Alfred Kim, MD, an assistant professor of medicine at Washington University, St Louis, but he added, “one has to consider differences in mitigation strategies, such as masking, that may explain these findings.” That is, patients taking immunosuppressants may be taking fewer risks in the community or have fewer potential exposures, especially in the Korean study, wherein the controls were health care workers.
A greater disparity in infections occurred when considering seroconversion rates. Breakthrough incidence was 38% among those taking immunosuppressants who did not seroconvert, compared with 29% among those who did. A similar trend was seen in breakthrough incidence between those taking strongly antibody-impairing immunosuppressants (36% breakthrough rate) and those taking other immunosuppressants (28%).
Among those taking immunosuppressants who seroconverted, a primary series of vaccination reduced the risk of a breakthrough infection by 29%. Protection became more robust with a booster or prior infection, both of which reduced breakthrough infection risk by 39% in those taking immunosuppressants who seroconverted.
“We demonstrate in patients with immune-mediated inflammatory diseases on immunosuppressants that additional vaccinations are associated with decreased risk of SARS-CoV-2 Omicron breakthrough infections,” wrote Eileen W. Stalman, MD, PhD, of Amsterdam UMC in the Netherlands, and her colleagues.
Though neither study broke down immune response or breakthrough infection based on individual medications, Kim said that previous research allows one to extrapolate “that prior culprits of poor vaccine responses [such as B-cell depleting drugs, mycophenolate, and TNF [tumor necrosis factor] inhibitors will continue to bear the greatest burden in breakthrough infection, including Omicron.”
Overall, he found the data from both studies relatively consistent with one another.
“Those on immunosuppression, particularly mechanisms that have been established as risk factors for poor vaccine responses, are at risk of breakthrough infection during the era of Omicron,” Dr. Kim said.
The earlier study from Korea also found that “the median time between the third-dose vaccination and the date of confirmed breakthrough infection in patients with ARDs was significantly shorter, compared with that in health care workers” at just 93 days in patients versus 122 days in health care workers. They postulated that this population’s limited neutralization of Omicron explained this short-lived protection.
Most of the patients with breakthrough infections (74%) in that study showed no neutralization against Omicron, including the only two hospitalized patients, both of whom had strong responses against the original SARS-CoV-2 strain. The significant decline over time of neutralization against Omicron suggested “the potential for a substantial loss of the protection from breakthrough infection,” the authors write.
“The third dose of an mRNA vaccine could improve the cross-neutralization of the SARS-CoV-2 Omicron variant in patients with autoimmune rheumatic disease [although] more than half of the patients failed to generate Omicron-neutralizing antibodies,” Tae Choi said in an interview. “Our study sheds light on the relative deficiency of the Omicron-specific neutralizing responses in patients with autoimmune rheumatic disease and their anticipated vulnerability to breakthrough infection.”
The message for clinicians, Dr. Kim said, is to “continue to urge our patients to maintain additional and boosting doses per guidance, use pre-exposure prophylaxis such as Evusheld, and continue other mitigation strategies as they have done.”
The Dutch study was funded by The Netherlands Organization for Health Research and Development; the Korean study used no external funding.
The authors of the Korean study had no disclosures. The Dutch study’s authors reported a wide range of disclosures involving more than a dozen pharmaceutical companies but not including Pfizer or Moderna. Dr. Kim’s industry disclosures include Alexion, ANI, AstraZeneca, Aurinia, Exagen, Foghorn Therapeutics, GlaxoSmithKline, Kypha, and Pfizer.
A version of this article first appeared on Medscape.com.
People with immune-mediated inflammatory diseases who are taking immunosuppressants don’t mount as strong of an immune defense against the Omicron variant as they did against the original SARS-CoV-2 wild-type virus, according to two studies published in Annals of the Rheumatic Diseases. One of the studies further showed that vaccinated individuals taking immunosuppressants have poorer cross-neutralizing responses to Omicron than do healthy vaccinated individuals, even after three doses of the COVID-19 mRNA vaccines.
“We carefully suggest that if Omicron-specific vaccination can be administered, it may be an effective way to reduce the risk of breakthrough infections in patients with autoimmune rheumatic disease,” Sang Tae Choi, MD, PhD, of the University College of Medicine, Seoul, Korea, and one of the authors of the study on cross-neutralizing protection, told this news organization. “However, further research is needed on Omicron-specific vaccine effectiveness in patients with immune dysfunctions. We believe that these study results can be of great benefit in determining the strategy of vaccination in the future.”
The earlier study, published in July, examined the ability of COVID-19 vaccines to induce cross-reactive antibody responses against Omicron infections in patients with autoimmune rheumatic diseases (ARDs). The observational study involved 149 patients with ARDs and 94 health care workers as controls, all of whom provided blood samples a median 15 weeks after their second COVID vaccine dose or a median 8 weeks after their third dose. A little more than two-thirds of the patients (68.5%) had received a third mRNA vaccine dose. None of the participants previously had COVID-19.
The researchers compared the rate of breakthrough infections with the Omicron variant to the neutralizing responses in patients’ blood, specifically the cross-neutralizing antibody responses because the original mRNA vaccines targeted a different variant than Omicron. Breakthrough infections were assessed by survey questions.
“Our findings suggested that neither primary series vaccinations nor booster doses are sufficient to induce Omicron-neutralizing responses above the threshold in patients with ARDs, although responses were noticeably increased following the third dose of an mRNA vaccine,” write Woo-Joong Kim, of the Chung-Ang University College of Medicine, Seoul, Korea, and his colleagues. “This impairment of cross-neutralization responses across most of our patients contrasts starkly with a potent elicitation of the Omicron-neutralizing responses after the third vaccination in healthy recipients.”
The average neutralizing responses against the original SARS-CoV-2 strain were similar in both groups: 76% in patients with ARDs and 72% in health care workers after the second dose. The mean response after a third dose was 97% in health care workers and 88% in patients.
The average cross-neutralizing response against the Omicron variant was far lower, particularly in those with rheumatic disease: only 11.5%, which rose to 27% after the third dose. Only 39% of the patient sera showed neutralization of Omicron, even after the third dose. Meanwhile, the mean cross-neutralizing response in health care workers was 18% after the second dose and 50% after the third.
When the researchers compared seropositivity rates against the original virus to neutralizing responses against Omicron, the association between these was stronger in health care workers than in those with ARDs. In fact, among patients with ARDs who seroconverted, only 41% showed any response against Omicron. Among all the patients, most of those who didn’t respond to Omicron (93.5%) had initially seroconverted.
The researchers also looked at the ability to neutralize Omicron on the basis of disease in those who received three doses of the vaccine. About half of those with lupus (52%) showed any neutralization against Omicron, compared with 25% of those with rheumatoid arthritis, 37.5% of those with ankylosing spondylitis, 33% of those with Behçet snydrome, and all of those with adult-onset Still’s disease.
The rate of breakthrough infections was lower in patients (19%) than in health care workers (33%). A similar pattern was seen in the more recent study published Sept. 5. Researchers used data from a prospective cohort study in the Netherlands to examine incidence and severity of Omicron breakthrough infections in patients with immune-mediated inflammatory diseases. The researchers compared infection rates and severity among 1,593 vaccinated patients with inflammatory disease who were taking immunosuppressants and 579 vaccinated controls (418 patients with inflammatory disease not on immunosuppressants and 161 healthy controls).
One in five patients with inflammatory disease (21%) were taking immunosuppressants that substantially impair antibodies, such as anti-CD20 therapy, S1P modulators, or mycophenolate mofetil combination therapy, and 48% of these patients seroconverted after primary vaccination, compared with 96% of patients taking other immunosuppressants and 98% of controls.
Breakthrough infection rates were similar between the control group (31%) and those taking immunosuppressants (30%). Only three participants had severe disease requiring hospitalization: one control and two patients taking immunosuppressants.
“In both studies, the controls had similar or higher rates of breakthrough infections, compared with the immunosuppressed,” noted Alfred Kim, MD, an assistant professor of medicine at Washington University, St Louis, but he added, “one has to consider differences in mitigation strategies, such as masking, that may explain these findings.” That is, patients taking immunosuppressants may be taking fewer risks in the community or have fewer potential exposures, especially in the Korean study, wherein the controls were health care workers.
A greater disparity in infections occurred when considering seroconversion rates. Breakthrough incidence was 38% among those taking immunosuppressants who did not seroconvert, compared with 29% among those who did. A similar trend was seen in breakthrough incidence between those taking strongly antibody-impairing immunosuppressants (36% breakthrough rate) and those taking other immunosuppressants (28%).
Among those taking immunosuppressants who seroconverted, a primary series of vaccination reduced the risk of a breakthrough infection by 29%. Protection became more robust with a booster or prior infection, both of which reduced breakthrough infection risk by 39% in those taking immunosuppressants who seroconverted.
“We demonstrate in patients with immune-mediated inflammatory diseases on immunosuppressants that additional vaccinations are associated with decreased risk of SARS-CoV-2 Omicron breakthrough infections,” wrote Eileen W. Stalman, MD, PhD, of Amsterdam UMC in the Netherlands, and her colleagues.
Though neither study broke down immune response or breakthrough infection based on individual medications, Kim said that previous research allows one to extrapolate “that prior culprits of poor vaccine responses [such as B-cell depleting drugs, mycophenolate, and TNF [tumor necrosis factor] inhibitors will continue to bear the greatest burden in breakthrough infection, including Omicron.”
Overall, he found the data from both studies relatively consistent with one another.
“Those on immunosuppression, particularly mechanisms that have been established as risk factors for poor vaccine responses, are at risk of breakthrough infection during the era of Omicron,” Dr. Kim said.
The earlier study from Korea also found that “the median time between the third-dose vaccination and the date of confirmed breakthrough infection in patients with ARDs was significantly shorter, compared with that in health care workers” at just 93 days in patients versus 122 days in health care workers. They postulated that this population’s limited neutralization of Omicron explained this short-lived protection.
Most of the patients with breakthrough infections (74%) in that study showed no neutralization against Omicron, including the only two hospitalized patients, both of whom had strong responses against the original SARS-CoV-2 strain. The significant decline over time of neutralization against Omicron suggested “the potential for a substantial loss of the protection from breakthrough infection,” the authors write.
“The third dose of an mRNA vaccine could improve the cross-neutralization of the SARS-CoV-2 Omicron variant in patients with autoimmune rheumatic disease [although] more than half of the patients failed to generate Omicron-neutralizing antibodies,” Tae Choi said in an interview. “Our study sheds light on the relative deficiency of the Omicron-specific neutralizing responses in patients with autoimmune rheumatic disease and their anticipated vulnerability to breakthrough infection.”
The message for clinicians, Dr. Kim said, is to “continue to urge our patients to maintain additional and boosting doses per guidance, use pre-exposure prophylaxis such as Evusheld, and continue other mitigation strategies as they have done.”
The Dutch study was funded by The Netherlands Organization for Health Research and Development; the Korean study used no external funding.
The authors of the Korean study had no disclosures. The Dutch study’s authors reported a wide range of disclosures involving more than a dozen pharmaceutical companies but not including Pfizer or Moderna. Dr. Kim’s industry disclosures include Alexion, ANI, AstraZeneca, Aurinia, Exagen, Foghorn Therapeutics, GlaxoSmithKline, Kypha, and Pfizer.
A version of this article first appeared on Medscape.com.
People with immune-mediated inflammatory diseases who are taking immunosuppressants don’t mount as strong of an immune defense against the Omicron variant as they did against the original SARS-CoV-2 wild-type virus, according to two studies published in Annals of the Rheumatic Diseases. One of the studies further showed that vaccinated individuals taking immunosuppressants have poorer cross-neutralizing responses to Omicron than do healthy vaccinated individuals, even after three doses of the COVID-19 mRNA vaccines.
“We carefully suggest that if Omicron-specific vaccination can be administered, it may be an effective way to reduce the risk of breakthrough infections in patients with autoimmune rheumatic disease,” Sang Tae Choi, MD, PhD, of the University College of Medicine, Seoul, Korea, and one of the authors of the study on cross-neutralizing protection, told this news organization. “However, further research is needed on Omicron-specific vaccine effectiveness in patients with immune dysfunctions. We believe that these study results can be of great benefit in determining the strategy of vaccination in the future.”
The earlier study, published in July, examined the ability of COVID-19 vaccines to induce cross-reactive antibody responses against Omicron infections in patients with autoimmune rheumatic diseases (ARDs). The observational study involved 149 patients with ARDs and 94 health care workers as controls, all of whom provided blood samples a median 15 weeks after their second COVID vaccine dose or a median 8 weeks after their third dose. A little more than two-thirds of the patients (68.5%) had received a third mRNA vaccine dose. None of the participants previously had COVID-19.
The researchers compared the rate of breakthrough infections with the Omicron variant to the neutralizing responses in patients’ blood, specifically the cross-neutralizing antibody responses because the original mRNA vaccines targeted a different variant than Omicron. Breakthrough infections were assessed by survey questions.
“Our findings suggested that neither primary series vaccinations nor booster doses are sufficient to induce Omicron-neutralizing responses above the threshold in patients with ARDs, although responses were noticeably increased following the third dose of an mRNA vaccine,” write Woo-Joong Kim, of the Chung-Ang University College of Medicine, Seoul, Korea, and his colleagues. “This impairment of cross-neutralization responses across most of our patients contrasts starkly with a potent elicitation of the Omicron-neutralizing responses after the third vaccination in healthy recipients.”
The average neutralizing responses against the original SARS-CoV-2 strain were similar in both groups: 76% in patients with ARDs and 72% in health care workers after the second dose. The mean response after a third dose was 97% in health care workers and 88% in patients.
The average cross-neutralizing response against the Omicron variant was far lower, particularly in those with rheumatic disease: only 11.5%, which rose to 27% after the third dose. Only 39% of the patient sera showed neutralization of Omicron, even after the third dose. Meanwhile, the mean cross-neutralizing response in health care workers was 18% after the second dose and 50% after the third.
When the researchers compared seropositivity rates against the original virus to neutralizing responses against Omicron, the association between these was stronger in health care workers than in those with ARDs. In fact, among patients with ARDs who seroconverted, only 41% showed any response against Omicron. Among all the patients, most of those who didn’t respond to Omicron (93.5%) had initially seroconverted.
The researchers also looked at the ability to neutralize Omicron on the basis of disease in those who received three doses of the vaccine. About half of those with lupus (52%) showed any neutralization against Omicron, compared with 25% of those with rheumatoid arthritis, 37.5% of those with ankylosing spondylitis, 33% of those with Behçet snydrome, and all of those with adult-onset Still’s disease.
The rate of breakthrough infections was lower in patients (19%) than in health care workers (33%). A similar pattern was seen in the more recent study published Sept. 5. Researchers used data from a prospective cohort study in the Netherlands to examine incidence and severity of Omicron breakthrough infections in patients with immune-mediated inflammatory diseases. The researchers compared infection rates and severity among 1,593 vaccinated patients with inflammatory disease who were taking immunosuppressants and 579 vaccinated controls (418 patients with inflammatory disease not on immunosuppressants and 161 healthy controls).
One in five patients with inflammatory disease (21%) were taking immunosuppressants that substantially impair antibodies, such as anti-CD20 therapy, S1P modulators, or mycophenolate mofetil combination therapy, and 48% of these patients seroconverted after primary vaccination, compared with 96% of patients taking other immunosuppressants and 98% of controls.
Breakthrough infection rates were similar between the control group (31%) and those taking immunosuppressants (30%). Only three participants had severe disease requiring hospitalization: one control and two patients taking immunosuppressants.
“In both studies, the controls had similar or higher rates of breakthrough infections, compared with the immunosuppressed,” noted Alfred Kim, MD, an assistant professor of medicine at Washington University, St Louis, but he added, “one has to consider differences in mitigation strategies, such as masking, that may explain these findings.” That is, patients taking immunosuppressants may be taking fewer risks in the community or have fewer potential exposures, especially in the Korean study, wherein the controls were health care workers.
A greater disparity in infections occurred when considering seroconversion rates. Breakthrough incidence was 38% among those taking immunosuppressants who did not seroconvert, compared with 29% among those who did. A similar trend was seen in breakthrough incidence between those taking strongly antibody-impairing immunosuppressants (36% breakthrough rate) and those taking other immunosuppressants (28%).
Among those taking immunosuppressants who seroconverted, a primary series of vaccination reduced the risk of a breakthrough infection by 29%. Protection became more robust with a booster or prior infection, both of which reduced breakthrough infection risk by 39% in those taking immunosuppressants who seroconverted.
“We demonstrate in patients with immune-mediated inflammatory diseases on immunosuppressants that additional vaccinations are associated with decreased risk of SARS-CoV-2 Omicron breakthrough infections,” wrote Eileen W. Stalman, MD, PhD, of Amsterdam UMC in the Netherlands, and her colleagues.
Though neither study broke down immune response or breakthrough infection based on individual medications, Kim said that previous research allows one to extrapolate “that prior culprits of poor vaccine responses [such as B-cell depleting drugs, mycophenolate, and TNF [tumor necrosis factor] inhibitors will continue to bear the greatest burden in breakthrough infection, including Omicron.”
Overall, he found the data from both studies relatively consistent with one another.
“Those on immunosuppression, particularly mechanisms that have been established as risk factors for poor vaccine responses, are at risk of breakthrough infection during the era of Omicron,” Dr. Kim said.
The earlier study from Korea also found that “the median time between the third-dose vaccination and the date of confirmed breakthrough infection in patients with ARDs was significantly shorter, compared with that in health care workers” at just 93 days in patients versus 122 days in health care workers. They postulated that this population’s limited neutralization of Omicron explained this short-lived protection.
Most of the patients with breakthrough infections (74%) in that study showed no neutralization against Omicron, including the only two hospitalized patients, both of whom had strong responses against the original SARS-CoV-2 strain. The significant decline over time of neutralization against Omicron suggested “the potential for a substantial loss of the protection from breakthrough infection,” the authors write.
“The third dose of an mRNA vaccine could improve the cross-neutralization of the SARS-CoV-2 Omicron variant in patients with autoimmune rheumatic disease [although] more than half of the patients failed to generate Omicron-neutralizing antibodies,” Tae Choi said in an interview. “Our study sheds light on the relative deficiency of the Omicron-specific neutralizing responses in patients with autoimmune rheumatic disease and their anticipated vulnerability to breakthrough infection.”
The message for clinicians, Dr. Kim said, is to “continue to urge our patients to maintain additional and boosting doses per guidance, use pre-exposure prophylaxis such as Evusheld, and continue other mitigation strategies as they have done.”
The Dutch study was funded by The Netherlands Organization for Health Research and Development; the Korean study used no external funding.
The authors of the Korean study had no disclosures. The Dutch study’s authors reported a wide range of disclosures involving more than a dozen pharmaceutical companies but not including Pfizer or Moderna. Dr. Kim’s industry disclosures include Alexion, ANI, AstraZeneca, Aurinia, Exagen, Foghorn Therapeutics, GlaxoSmithKline, Kypha, and Pfizer.
A version of this article first appeared on Medscape.com.
Sex differences seen in inflammatory arthritis health care use
Women with inflammatory arthritis (IA) are more likely to use healthcare services than men, a Canadian study found. The results suggest there are biological differences in disease course and sociocultural differences in health care access and patient behavior among the sexes, Sanjana Tarannum said in a presentation at the Lancet Summit on Sex and Gender in Rheumatology.
Ms. Tarannum and colleagues also recently published the study in Annals of the Rheumatic Diseases.
Effectively managing IA patients calls for timely access to and appropriate use of health care resources, said Ms. Tarannum, of the Women’s College Research Institute in Toronto.
Sex and gender are often used interchangeably but they refer to different things. “Sex is the biological characteristic of being male or female. It relates to disease inheritance patterns, pain processing mechanisms, and immune dysregulation in the context of inflammatory arthritis,” Ms. Tarannum said during her presentation.
Gender is a sociocultural construct associated with masculine or feminine traits. In the context of IA, gender relates to coping strategies, pain perception and reporting, and health care–seeking behavior of patients and interaction with care providers.
A patient’s sex relates to healthcare encounters, time to diagnosis, and prescription patterns. These all affect disease outcomes. Previous studies have yielded inconsistent results and mainly focused on rheumatoid arthritis rather than other IA types such as ankylosing spondylitis (AS).
Ms. Tarannum and colleagues sought to compare health care usage between male and female patients for musculoskeletal-related issues before and after IA diagnosis. They used Ontario administrative health data to create three cohorts of patients with RA, AS, and psoriatic arthritis (PsA), the three most common types of IA. The patients were diagnosed during 2010-2017, and outcomes were assessed in each year for 3 years before and after diagnosis.
Health care use indicators included visits to physicians, musculoskeletal imaging, laboratory tests, and dispensation of drugs. Regression models adjusting for sociodemographic factors and comorbidities were used to compare male and female patients.
Sex-related differences emerge in all IA groups
The investigators assessed 41,277 patients with RA (69% female), 8,150 patients with AS (51% female), and 6,446 patients with PsA (54% female). Male patients had more cardiovascular disease, whereas female patients had higher incidences of depression and osteoporosis.
Similar trends of sex-related differences emerged in all three cohorts. Before diagnosis, female patients were more likely to visit rheumatologists or family physicians for musculoskeletal reasons or use musculoskeletal imaging and laboratory tests. Women were also more likely to remain in rheumatology care after diagnosis.
Men were more likely to visit the ED for musculoskeletal reasons immediately before diagnosis.
No sex- or gender-related differences were observed in medication use, although older females with RA or AS were more likely to get prescriptions for NSAIDs and opioids and conventional disease-modifying antirheumatic drugs, respectively.
The findings show that overall musculoskeletal health care use was higher in female patients with IA. “Sex differences were more pronounced the earlier the encounter was from the time of diagnosis and tended to diminish with time,” Ms. Tarannum observed. Sex differences were also more prominent in the RA and AS cohorts.
Women seek out care, do repeat visits
Several reasons may explain why utilization was higher in females. Women with IA have a higher overall risk of musculoskeletal conditions such as osteoarthritis, which could have driven the health care encounters. Numerous studies have also reported that female patients have a lower threshold for pain as well as a greater tendency to seek out health care.
Additionally, female patients often present with pain and fatigue, which are often misdiagnosed as fibromyalgia or depression. Therefore, they often require repeated health care encounters to arrive at an IA diagnosis, Ms. Tarannum said.
An early prodromal phase in females could have triggered a health care encounter as well.
Men, by comparison, are more likely to have acute-onset or severe disease. Objective signs and radiologic features can facilitate diagnosis in men, she said. Male patients also show more reluctance in seeking care, have a higher threshold for pain, and are less likely to have a usual source of care such as a family physician.
Higher confidence in hospital-based emergency services also could have resulted in more ED visits and lower health care use in men. Better response to treatments could also have resulted in fewer episodes of rheumatology care after diagnosis.
The results aren’t surprising, said Scott Zashin, MD, a rheumatologist in Dallas who wasn’t a part of the study.
“At least in terms of musculoskeletal disorders, my clinical experience suggests that women are more compliant with their follow-up than male patients. Especially with gout, a common type of arthritis in men, male patients may wait until their symptoms are severe before seeking medical attention,” Dr. Zashin said.
The Enid Walker Graduate Student Award for Research in Women’s Health provided funding for this study.
A version of this article first appeared on Medscape.com.
Women with inflammatory arthritis (IA) are more likely to use healthcare services than men, a Canadian study found. The results suggest there are biological differences in disease course and sociocultural differences in health care access and patient behavior among the sexes, Sanjana Tarannum said in a presentation at the Lancet Summit on Sex and Gender in Rheumatology.
Ms. Tarannum and colleagues also recently published the study in Annals of the Rheumatic Diseases.
Effectively managing IA patients calls for timely access to and appropriate use of health care resources, said Ms. Tarannum, of the Women’s College Research Institute in Toronto.
Sex and gender are often used interchangeably but they refer to different things. “Sex is the biological characteristic of being male or female. It relates to disease inheritance patterns, pain processing mechanisms, and immune dysregulation in the context of inflammatory arthritis,” Ms. Tarannum said during her presentation.
Gender is a sociocultural construct associated with masculine or feminine traits. In the context of IA, gender relates to coping strategies, pain perception and reporting, and health care–seeking behavior of patients and interaction with care providers.
A patient’s sex relates to healthcare encounters, time to diagnosis, and prescription patterns. These all affect disease outcomes. Previous studies have yielded inconsistent results and mainly focused on rheumatoid arthritis rather than other IA types such as ankylosing spondylitis (AS).
Ms. Tarannum and colleagues sought to compare health care usage between male and female patients for musculoskeletal-related issues before and after IA diagnosis. They used Ontario administrative health data to create three cohorts of patients with RA, AS, and psoriatic arthritis (PsA), the three most common types of IA. The patients were diagnosed during 2010-2017, and outcomes were assessed in each year for 3 years before and after diagnosis.
Health care use indicators included visits to physicians, musculoskeletal imaging, laboratory tests, and dispensation of drugs. Regression models adjusting for sociodemographic factors and comorbidities were used to compare male and female patients.
Sex-related differences emerge in all IA groups
The investigators assessed 41,277 patients with RA (69% female), 8,150 patients with AS (51% female), and 6,446 patients with PsA (54% female). Male patients had more cardiovascular disease, whereas female patients had higher incidences of depression and osteoporosis.
Similar trends of sex-related differences emerged in all three cohorts. Before diagnosis, female patients were more likely to visit rheumatologists or family physicians for musculoskeletal reasons or use musculoskeletal imaging and laboratory tests. Women were also more likely to remain in rheumatology care after diagnosis.
Men were more likely to visit the ED for musculoskeletal reasons immediately before diagnosis.
No sex- or gender-related differences were observed in medication use, although older females with RA or AS were more likely to get prescriptions for NSAIDs and opioids and conventional disease-modifying antirheumatic drugs, respectively.
The findings show that overall musculoskeletal health care use was higher in female patients with IA. “Sex differences were more pronounced the earlier the encounter was from the time of diagnosis and tended to diminish with time,” Ms. Tarannum observed. Sex differences were also more prominent in the RA and AS cohorts.
Women seek out care, do repeat visits
Several reasons may explain why utilization was higher in females. Women with IA have a higher overall risk of musculoskeletal conditions such as osteoarthritis, which could have driven the health care encounters. Numerous studies have also reported that female patients have a lower threshold for pain as well as a greater tendency to seek out health care.
Additionally, female patients often present with pain and fatigue, which are often misdiagnosed as fibromyalgia or depression. Therefore, they often require repeated health care encounters to arrive at an IA diagnosis, Ms. Tarannum said.
An early prodromal phase in females could have triggered a health care encounter as well.
Men, by comparison, are more likely to have acute-onset or severe disease. Objective signs and radiologic features can facilitate diagnosis in men, she said. Male patients also show more reluctance in seeking care, have a higher threshold for pain, and are less likely to have a usual source of care such as a family physician.
Higher confidence in hospital-based emergency services also could have resulted in more ED visits and lower health care use in men. Better response to treatments could also have resulted in fewer episodes of rheumatology care after diagnosis.
The results aren’t surprising, said Scott Zashin, MD, a rheumatologist in Dallas who wasn’t a part of the study.
“At least in terms of musculoskeletal disorders, my clinical experience suggests that women are more compliant with their follow-up than male patients. Especially with gout, a common type of arthritis in men, male patients may wait until their symptoms are severe before seeking medical attention,” Dr. Zashin said.
The Enid Walker Graduate Student Award for Research in Women’s Health provided funding for this study.
A version of this article first appeared on Medscape.com.
Women with inflammatory arthritis (IA) are more likely to use healthcare services than men, a Canadian study found. The results suggest there are biological differences in disease course and sociocultural differences in health care access and patient behavior among the sexes, Sanjana Tarannum said in a presentation at the Lancet Summit on Sex and Gender in Rheumatology.
Ms. Tarannum and colleagues also recently published the study in Annals of the Rheumatic Diseases.
Effectively managing IA patients calls for timely access to and appropriate use of health care resources, said Ms. Tarannum, of the Women’s College Research Institute in Toronto.
Sex and gender are often used interchangeably but they refer to different things. “Sex is the biological characteristic of being male or female. It relates to disease inheritance patterns, pain processing mechanisms, and immune dysregulation in the context of inflammatory arthritis,” Ms. Tarannum said during her presentation.
Gender is a sociocultural construct associated with masculine or feminine traits. In the context of IA, gender relates to coping strategies, pain perception and reporting, and health care–seeking behavior of patients and interaction with care providers.
A patient’s sex relates to healthcare encounters, time to diagnosis, and prescription patterns. These all affect disease outcomes. Previous studies have yielded inconsistent results and mainly focused on rheumatoid arthritis rather than other IA types such as ankylosing spondylitis (AS).
Ms. Tarannum and colleagues sought to compare health care usage between male and female patients for musculoskeletal-related issues before and after IA diagnosis. They used Ontario administrative health data to create three cohorts of patients with RA, AS, and psoriatic arthritis (PsA), the three most common types of IA. The patients were diagnosed during 2010-2017, and outcomes were assessed in each year for 3 years before and after diagnosis.
Health care use indicators included visits to physicians, musculoskeletal imaging, laboratory tests, and dispensation of drugs. Regression models adjusting for sociodemographic factors and comorbidities were used to compare male and female patients.
Sex-related differences emerge in all IA groups
The investigators assessed 41,277 patients with RA (69% female), 8,150 patients with AS (51% female), and 6,446 patients with PsA (54% female). Male patients had more cardiovascular disease, whereas female patients had higher incidences of depression and osteoporosis.
Similar trends of sex-related differences emerged in all three cohorts. Before diagnosis, female patients were more likely to visit rheumatologists or family physicians for musculoskeletal reasons or use musculoskeletal imaging and laboratory tests. Women were also more likely to remain in rheumatology care after diagnosis.
Men were more likely to visit the ED for musculoskeletal reasons immediately before diagnosis.
No sex- or gender-related differences were observed in medication use, although older females with RA or AS were more likely to get prescriptions for NSAIDs and opioids and conventional disease-modifying antirheumatic drugs, respectively.
The findings show that overall musculoskeletal health care use was higher in female patients with IA. “Sex differences were more pronounced the earlier the encounter was from the time of diagnosis and tended to diminish with time,” Ms. Tarannum observed. Sex differences were also more prominent in the RA and AS cohorts.
Women seek out care, do repeat visits
Several reasons may explain why utilization was higher in females. Women with IA have a higher overall risk of musculoskeletal conditions such as osteoarthritis, which could have driven the health care encounters. Numerous studies have also reported that female patients have a lower threshold for pain as well as a greater tendency to seek out health care.
Additionally, female patients often present with pain and fatigue, which are often misdiagnosed as fibromyalgia or depression. Therefore, they often require repeated health care encounters to arrive at an IA diagnosis, Ms. Tarannum said.
An early prodromal phase in females could have triggered a health care encounter as well.
Men, by comparison, are more likely to have acute-onset or severe disease. Objective signs and radiologic features can facilitate diagnosis in men, she said. Male patients also show more reluctance in seeking care, have a higher threshold for pain, and are less likely to have a usual source of care such as a family physician.
Higher confidence in hospital-based emergency services also could have resulted in more ED visits and lower health care use in men. Better response to treatments could also have resulted in fewer episodes of rheumatology care after diagnosis.
The results aren’t surprising, said Scott Zashin, MD, a rheumatologist in Dallas who wasn’t a part of the study.
“At least in terms of musculoskeletal disorders, my clinical experience suggests that women are more compliant with their follow-up than male patients. Especially with gout, a common type of arthritis in men, male patients may wait until their symptoms are severe before seeking medical attention,” Dr. Zashin said.
The Enid Walker Graduate Student Award for Research in Women’s Health provided funding for this study.
A version of this article first appeared on Medscape.com.
FROM THE LANCET SUMMIT ON SEX AND GENDER IN RHEUMATOLOGY
Hormones’ impact described in transgender rheumatology patients
Gender-affirming hormone therapy’s effect on transgender patients with rheumatic disease is unclear but does not appear to modulate its course and does not need to be strictly contraindicated in most patients, according to a case series and systematic literature review.
More doctors are practicing transgender medicine, yet a limited amount of information is available on rheumatic disease in transgender and gender diverse (TGGD) individuals, Kristen Mathias, MD, a rheumatology fellow at Johns Hopkins University, Baltimore, said in her presentation of the study at the Lancet Summit on Sex and Gender in Rheumatology.
“This is important, as it is well known that sex hormones affect the pathogenesis and expression of autoimmune diseases,” Dr. Mathias said. Knowing more about the effects of gender-affirming hormone therapy (GAHT) and gender-affirming surgery on disease activity in TGGD individuals could better inform decisions about care in this population.
Dr. Mathias and colleagues identified 7 transgender patients with rheumatic diseases from a pool of 1,053 patients seen at the Los Angeles County and University of Southern California Medical Center, Los Angeles, from June 2019 to June 2021. This included five transgender males and two transgender females. They ranged in age from 13 to 52 years.
All seven were on GAHT, and its impact on disease activity was considered “possible” in two of the seven patients.
In a systematic literature review, investigators found 11 studies that included 11 transgender women and 2 transgender men, ranging in age from 22 to 49 years. All the patients were on GAHT. In 12 of 13 patients, the hormones were considered possibly related to their rheumatic disease activity.
The 20 patients had diagnoses of rheumatoid arthritis, cutaneous and systemic lupus erythematosus, adult-onset Still disease, spondyloarthritis, myositis, and systemic sclerosis.
GAHT should not be a strict contraindication in these patients, based on these findings, Dr. Mathias noted. Information to clarify the effect of GAHT on rheumatic disease is sparse, however. Physicians should adopt a personalized, shared decision-making approach when consulting patients.
“During patient encounters, they should be screened for psychosocial barriers when appropriate,” Dr. Mathias recommended.
Findings could pave way for larger studies, more data
Studies on the impact and consequences of rheumatic disease in TGGD individuals are sorely lacking, said Vagishwari Murugesan, MBBS, a clinical fellow in rheumatology at the University of Toronto.
“While this is a small study of only seven patients and no conclusive results can be drawn, studies like these can help pave the way for larger multicentric studies, which can give us more definitive data on gender-affirming hormone therapy and its consequences on rheumatic diseases,” said Dr. Murugesan, who was not involved in the study.
A registry would be a great way to collaborate with other stakeholders interested in the same topic and conduct larger studies, she said. “I would recommend that not only do we screen for psychosocial barriers but also actively engage as a health care community in addressing how we can overcome the barriers for patients to access effective health care.”
No external funding was obtained for the study.
A version of this article first appeared on Medscape.com.
Gender-affirming hormone therapy’s effect on transgender patients with rheumatic disease is unclear but does not appear to modulate its course and does not need to be strictly contraindicated in most patients, according to a case series and systematic literature review.
More doctors are practicing transgender medicine, yet a limited amount of information is available on rheumatic disease in transgender and gender diverse (TGGD) individuals, Kristen Mathias, MD, a rheumatology fellow at Johns Hopkins University, Baltimore, said in her presentation of the study at the Lancet Summit on Sex and Gender in Rheumatology.
“This is important, as it is well known that sex hormones affect the pathogenesis and expression of autoimmune diseases,” Dr. Mathias said. Knowing more about the effects of gender-affirming hormone therapy (GAHT) and gender-affirming surgery on disease activity in TGGD individuals could better inform decisions about care in this population.
Dr. Mathias and colleagues identified 7 transgender patients with rheumatic diseases from a pool of 1,053 patients seen at the Los Angeles County and University of Southern California Medical Center, Los Angeles, from June 2019 to June 2021. This included five transgender males and two transgender females. They ranged in age from 13 to 52 years.
All seven were on GAHT, and its impact on disease activity was considered “possible” in two of the seven patients.
In a systematic literature review, investigators found 11 studies that included 11 transgender women and 2 transgender men, ranging in age from 22 to 49 years. All the patients were on GAHT. In 12 of 13 patients, the hormones were considered possibly related to their rheumatic disease activity.
The 20 patients had diagnoses of rheumatoid arthritis, cutaneous and systemic lupus erythematosus, adult-onset Still disease, spondyloarthritis, myositis, and systemic sclerosis.
GAHT should not be a strict contraindication in these patients, based on these findings, Dr. Mathias noted. Information to clarify the effect of GAHT on rheumatic disease is sparse, however. Physicians should adopt a personalized, shared decision-making approach when consulting patients.
“During patient encounters, they should be screened for psychosocial barriers when appropriate,” Dr. Mathias recommended.
Findings could pave way for larger studies, more data
Studies on the impact and consequences of rheumatic disease in TGGD individuals are sorely lacking, said Vagishwari Murugesan, MBBS, a clinical fellow in rheumatology at the University of Toronto.
“While this is a small study of only seven patients and no conclusive results can be drawn, studies like these can help pave the way for larger multicentric studies, which can give us more definitive data on gender-affirming hormone therapy and its consequences on rheumatic diseases,” said Dr. Murugesan, who was not involved in the study.
A registry would be a great way to collaborate with other stakeholders interested in the same topic and conduct larger studies, she said. “I would recommend that not only do we screen for psychosocial barriers but also actively engage as a health care community in addressing how we can overcome the barriers for patients to access effective health care.”
No external funding was obtained for the study.
A version of this article first appeared on Medscape.com.
Gender-affirming hormone therapy’s effect on transgender patients with rheumatic disease is unclear but does not appear to modulate its course and does not need to be strictly contraindicated in most patients, according to a case series and systematic literature review.
More doctors are practicing transgender medicine, yet a limited amount of information is available on rheumatic disease in transgender and gender diverse (TGGD) individuals, Kristen Mathias, MD, a rheumatology fellow at Johns Hopkins University, Baltimore, said in her presentation of the study at the Lancet Summit on Sex and Gender in Rheumatology.
“This is important, as it is well known that sex hormones affect the pathogenesis and expression of autoimmune diseases,” Dr. Mathias said. Knowing more about the effects of gender-affirming hormone therapy (GAHT) and gender-affirming surgery on disease activity in TGGD individuals could better inform decisions about care in this population.
Dr. Mathias and colleagues identified 7 transgender patients with rheumatic diseases from a pool of 1,053 patients seen at the Los Angeles County and University of Southern California Medical Center, Los Angeles, from June 2019 to June 2021. This included five transgender males and two transgender females. They ranged in age from 13 to 52 years.
All seven were on GAHT, and its impact on disease activity was considered “possible” in two of the seven patients.
In a systematic literature review, investigators found 11 studies that included 11 transgender women and 2 transgender men, ranging in age from 22 to 49 years. All the patients were on GAHT. In 12 of 13 patients, the hormones were considered possibly related to their rheumatic disease activity.
The 20 patients had diagnoses of rheumatoid arthritis, cutaneous and systemic lupus erythematosus, adult-onset Still disease, spondyloarthritis, myositis, and systemic sclerosis.
GAHT should not be a strict contraindication in these patients, based on these findings, Dr. Mathias noted. Information to clarify the effect of GAHT on rheumatic disease is sparse, however. Physicians should adopt a personalized, shared decision-making approach when consulting patients.
“During patient encounters, they should be screened for psychosocial barriers when appropriate,” Dr. Mathias recommended.
Findings could pave way for larger studies, more data
Studies on the impact and consequences of rheumatic disease in TGGD individuals are sorely lacking, said Vagishwari Murugesan, MBBS, a clinical fellow in rheumatology at the University of Toronto.
“While this is a small study of only seven patients and no conclusive results can be drawn, studies like these can help pave the way for larger multicentric studies, which can give us more definitive data on gender-affirming hormone therapy and its consequences on rheumatic diseases,” said Dr. Murugesan, who was not involved in the study.
A registry would be a great way to collaborate with other stakeholders interested in the same topic and conduct larger studies, she said. “I would recommend that not only do we screen for psychosocial barriers but also actively engage as a health care community in addressing how we can overcome the barriers for patients to access effective health care.”
No external funding was obtained for the study.
A version of this article first appeared on Medscape.com.
FROM THE LANCET SUMMIT ON SEX AND GENDER IN RHEUMATOLOGY
Severe COVID-19–related outcomes found worse in men with RA
A retrospective study that analyzed sex disparities in patients with COVID-19 and rheumatoid arthritis found that men had more baseline comorbidities and increased risk of COVID-19–related outcomes, compared with women.
“Differences in genetics between sex and sex steroid hormones may play a role in predisposition to COVID-19 infection as well as modulating the disease progression,” according to Xiaofeng Zhou, PhD, senior director at Pfizer, New York, and the study’s lead author.
Dr. Zhou presented her findings at The Lancet Summit on Sex and Gender in Rheumatology.
Patients with chronic rheumatic diseases treated with immunomodulatory therapies may be at higher risk for more severe COVID-19 outcomes, including hospitalization, complications, and death. Research on sex-based disparities in RA patients with COVID-19 in the United States is limited, said Dr. Zhou, who embarked on a retrospective cohort study to examine the demographic and clinical characteristics of RA patients with COVID-19 and estimate the risk of possible COVID-19 outcomes by sex.
Dr. Zhou and colleagues used U.S. COVID-19 data collected through electronic health records by Optum during 2020 to June 2021. The study included adult patients with RA and a COVID-19 diagnosis (≥ 1 diagnosis code or positive SARS-CoV-2 laboratory test) and greater than or equal to 183 days of database enrollment who received treatment with immunomodulatory therapies prior to the diagnosis date. They were stratified by sex.
Investigators used logistic regression to estimate the risk of 11 possible COVID-19–related outcomes within 30 days of the COVID-19 diagnosis (hospitalization, ICU admission, pneumonia, kidney failure, thrombotic event, heart failure, acute respiratory distress syndrome [ARDS], sepsis/septic shock, mechanical ventilation/extracorporeal membrane oxygenation [ECMO], in-hospital death, and all-cause mortality), adjusting for demographics and baseline clinical covariates.
A total of 4,476 COVID-19 patients with RA (78% female) took part in the study. Male patients trended older (64 vs. 60 years) and had lower African American representation and Medicaid enrollment than female patients, but they had more baseline comorbidities such as hypertension (55% vs. 45%), hyperlipidemia (45% vs. 33%), diabetes (25% vs. 20%), coronary artery disease (28% vs. 12%), and chronic kidney disease (20% vs. 15%).
Eight of the eleven COVID-19 outcomes were significantly more likely to occur in men than women (hospitalization: odds ratio, 1.32 [95% confidence interval (CI), 1.11-1.56]; ICU admission: OR, 1.80 [95% CI, 1.36-2.40]; mechanical ventilation/ECMO: OR, 1.48 [95% CI, 1.04-2.11]; in-hospital death: OR, 1.53 [95% CI, 1.13-2.07]; all-cause mortality: OR, 1.42 [95% CI, 1.09-1.86]; sepsis: OR, 1.55 [95% CI, 1.20-2.02]; kidney failure: OR, 1.46 [95% CI, 1.15-1.85]; ARDS: OR, 1.39 [95% CI, 1.15-1.69]).
Sex hormones factor into risk
The data illustrated that men with RA had more baseline comorbidities and increased risk of COVID-19 outcomes than women.
Sex hormones regulate virus entry into host cells, respiratory function, immune response, the cardiovascular system, and coagulation, explained Dr. Zhou.
Estrogen and progesterone in women could help develop stronger and efficient immune responses to viruses and reduce virus entry into the host cells. Also, “[the] larger number of copies of ACE2 genes in women, [which] is linked with protection in the lungs against edema, permeability, and pulmonary damage, could be associated with lower incidence of severe COVID-19 outcomes, such as respiratory-related mortality and mortality,” Dr. Zhou said.
By comparison, androgens in men may increase virus entry into the host cells and promote unfavorable immune response through the induction of cytokine production and reducing the antibody response to the virus. This could lead to severe infection, Dr. Zhou said.
Sex-based differences in steroid hormones may also explain the higher incidence of morbidity and fatality that’s been observed in other studies of male patients with other infectious diseases, such as severe acute respiratory syndrome and Middle East respiratory syndrome.
Study bolsters evidence on sex disparities
The results add real-world evidence to the limited literature on sex disparities in COVID-19 outcomes among patients with RA in the United States, Dr. Zhou said. “The differential role in sex steroid hormones among women and men may shed light on clinical management of COVID-19 patients and the need to consider sex-specific approaches in clinical trials in preventing and treating COVID-19 patients,” she said.
Considering that all patients are recommended to get COVID-19 vaccinations, “it is difficult to say how this impacts clinical practice,” said Janet Pope, MD, MPH, professor of medicine in the division of rheumatology at the University of Western Ontario, London, who was not involved with the study.
Sharing results with some patients may help to encourage vaccination, thus reducing risk of poor COVID-19 outcomes, Dr. Pope said.
In future studies, Dr. Zhou suggests using multiple databases and considering other geographies beyond the United States to further understand the etiology of sexual dimorphism in COVID-19 and expand generalizability. “In addition, future research will seek to provide insights into health equity gaps in the management of COVID-19. This may inform development of precision medicines and vaccines, especially among patients on immunosuppressive treatments,” she said.
The study was sponsored by Pfizer. Dr. Zhou and other study authors are Pfizer employees and hold Pfizer stock.
A version of this article first appeared on Medscape.com.
A retrospective study that analyzed sex disparities in patients with COVID-19 and rheumatoid arthritis found that men had more baseline comorbidities and increased risk of COVID-19–related outcomes, compared with women.
“Differences in genetics between sex and sex steroid hormones may play a role in predisposition to COVID-19 infection as well as modulating the disease progression,” according to Xiaofeng Zhou, PhD, senior director at Pfizer, New York, and the study’s lead author.
Dr. Zhou presented her findings at The Lancet Summit on Sex and Gender in Rheumatology.
Patients with chronic rheumatic diseases treated with immunomodulatory therapies may be at higher risk for more severe COVID-19 outcomes, including hospitalization, complications, and death. Research on sex-based disparities in RA patients with COVID-19 in the United States is limited, said Dr. Zhou, who embarked on a retrospective cohort study to examine the demographic and clinical characteristics of RA patients with COVID-19 and estimate the risk of possible COVID-19 outcomes by sex.
Dr. Zhou and colleagues used U.S. COVID-19 data collected through electronic health records by Optum during 2020 to June 2021. The study included adult patients with RA and a COVID-19 diagnosis (≥ 1 diagnosis code or positive SARS-CoV-2 laboratory test) and greater than or equal to 183 days of database enrollment who received treatment with immunomodulatory therapies prior to the diagnosis date. They were stratified by sex.
Investigators used logistic regression to estimate the risk of 11 possible COVID-19–related outcomes within 30 days of the COVID-19 diagnosis (hospitalization, ICU admission, pneumonia, kidney failure, thrombotic event, heart failure, acute respiratory distress syndrome [ARDS], sepsis/septic shock, mechanical ventilation/extracorporeal membrane oxygenation [ECMO], in-hospital death, and all-cause mortality), adjusting for demographics and baseline clinical covariates.
A total of 4,476 COVID-19 patients with RA (78% female) took part in the study. Male patients trended older (64 vs. 60 years) and had lower African American representation and Medicaid enrollment than female patients, but they had more baseline comorbidities such as hypertension (55% vs. 45%), hyperlipidemia (45% vs. 33%), diabetes (25% vs. 20%), coronary artery disease (28% vs. 12%), and chronic kidney disease (20% vs. 15%).
Eight of the eleven COVID-19 outcomes were significantly more likely to occur in men than women (hospitalization: odds ratio, 1.32 [95% confidence interval (CI), 1.11-1.56]; ICU admission: OR, 1.80 [95% CI, 1.36-2.40]; mechanical ventilation/ECMO: OR, 1.48 [95% CI, 1.04-2.11]; in-hospital death: OR, 1.53 [95% CI, 1.13-2.07]; all-cause mortality: OR, 1.42 [95% CI, 1.09-1.86]; sepsis: OR, 1.55 [95% CI, 1.20-2.02]; kidney failure: OR, 1.46 [95% CI, 1.15-1.85]; ARDS: OR, 1.39 [95% CI, 1.15-1.69]).
Sex hormones factor into risk
The data illustrated that men with RA had more baseline comorbidities and increased risk of COVID-19 outcomes than women.
Sex hormones regulate virus entry into host cells, respiratory function, immune response, the cardiovascular system, and coagulation, explained Dr. Zhou.
Estrogen and progesterone in women could help develop stronger and efficient immune responses to viruses and reduce virus entry into the host cells. Also, “[the] larger number of copies of ACE2 genes in women, [which] is linked with protection in the lungs against edema, permeability, and pulmonary damage, could be associated with lower incidence of severe COVID-19 outcomes, such as respiratory-related mortality and mortality,” Dr. Zhou said.
By comparison, androgens in men may increase virus entry into the host cells and promote unfavorable immune response through the induction of cytokine production and reducing the antibody response to the virus. This could lead to severe infection, Dr. Zhou said.
Sex-based differences in steroid hormones may also explain the higher incidence of morbidity and fatality that’s been observed in other studies of male patients with other infectious diseases, such as severe acute respiratory syndrome and Middle East respiratory syndrome.
Study bolsters evidence on sex disparities
The results add real-world evidence to the limited literature on sex disparities in COVID-19 outcomes among patients with RA in the United States, Dr. Zhou said. “The differential role in sex steroid hormones among women and men may shed light on clinical management of COVID-19 patients and the need to consider sex-specific approaches in clinical trials in preventing and treating COVID-19 patients,” she said.
Considering that all patients are recommended to get COVID-19 vaccinations, “it is difficult to say how this impacts clinical practice,” said Janet Pope, MD, MPH, professor of medicine in the division of rheumatology at the University of Western Ontario, London, who was not involved with the study.
Sharing results with some patients may help to encourage vaccination, thus reducing risk of poor COVID-19 outcomes, Dr. Pope said.
In future studies, Dr. Zhou suggests using multiple databases and considering other geographies beyond the United States to further understand the etiology of sexual dimorphism in COVID-19 and expand generalizability. “In addition, future research will seek to provide insights into health equity gaps in the management of COVID-19. This may inform development of precision medicines and vaccines, especially among patients on immunosuppressive treatments,” she said.
The study was sponsored by Pfizer. Dr. Zhou and other study authors are Pfizer employees and hold Pfizer stock.
A version of this article first appeared on Medscape.com.
A retrospective study that analyzed sex disparities in patients with COVID-19 and rheumatoid arthritis found that men had more baseline comorbidities and increased risk of COVID-19–related outcomes, compared with women.
“Differences in genetics between sex and sex steroid hormones may play a role in predisposition to COVID-19 infection as well as modulating the disease progression,” according to Xiaofeng Zhou, PhD, senior director at Pfizer, New York, and the study’s lead author.
Dr. Zhou presented her findings at The Lancet Summit on Sex and Gender in Rheumatology.
Patients with chronic rheumatic diseases treated with immunomodulatory therapies may be at higher risk for more severe COVID-19 outcomes, including hospitalization, complications, and death. Research on sex-based disparities in RA patients with COVID-19 in the United States is limited, said Dr. Zhou, who embarked on a retrospective cohort study to examine the demographic and clinical characteristics of RA patients with COVID-19 and estimate the risk of possible COVID-19 outcomes by sex.
Dr. Zhou and colleagues used U.S. COVID-19 data collected through electronic health records by Optum during 2020 to June 2021. The study included adult patients with RA and a COVID-19 diagnosis (≥ 1 diagnosis code or positive SARS-CoV-2 laboratory test) and greater than or equal to 183 days of database enrollment who received treatment with immunomodulatory therapies prior to the diagnosis date. They were stratified by sex.
Investigators used logistic regression to estimate the risk of 11 possible COVID-19–related outcomes within 30 days of the COVID-19 diagnosis (hospitalization, ICU admission, pneumonia, kidney failure, thrombotic event, heart failure, acute respiratory distress syndrome [ARDS], sepsis/septic shock, mechanical ventilation/extracorporeal membrane oxygenation [ECMO], in-hospital death, and all-cause mortality), adjusting for demographics and baseline clinical covariates.
A total of 4,476 COVID-19 patients with RA (78% female) took part in the study. Male patients trended older (64 vs. 60 years) and had lower African American representation and Medicaid enrollment than female patients, but they had more baseline comorbidities such as hypertension (55% vs. 45%), hyperlipidemia (45% vs. 33%), diabetes (25% vs. 20%), coronary artery disease (28% vs. 12%), and chronic kidney disease (20% vs. 15%).
Eight of the eleven COVID-19 outcomes were significantly more likely to occur in men than women (hospitalization: odds ratio, 1.32 [95% confidence interval (CI), 1.11-1.56]; ICU admission: OR, 1.80 [95% CI, 1.36-2.40]; mechanical ventilation/ECMO: OR, 1.48 [95% CI, 1.04-2.11]; in-hospital death: OR, 1.53 [95% CI, 1.13-2.07]; all-cause mortality: OR, 1.42 [95% CI, 1.09-1.86]; sepsis: OR, 1.55 [95% CI, 1.20-2.02]; kidney failure: OR, 1.46 [95% CI, 1.15-1.85]; ARDS: OR, 1.39 [95% CI, 1.15-1.69]).
Sex hormones factor into risk
The data illustrated that men with RA had more baseline comorbidities and increased risk of COVID-19 outcomes than women.
Sex hormones regulate virus entry into host cells, respiratory function, immune response, the cardiovascular system, and coagulation, explained Dr. Zhou.
Estrogen and progesterone in women could help develop stronger and efficient immune responses to viruses and reduce virus entry into the host cells. Also, “[the] larger number of copies of ACE2 genes in women, [which] is linked with protection in the lungs against edema, permeability, and pulmonary damage, could be associated with lower incidence of severe COVID-19 outcomes, such as respiratory-related mortality and mortality,” Dr. Zhou said.
By comparison, androgens in men may increase virus entry into the host cells and promote unfavorable immune response through the induction of cytokine production and reducing the antibody response to the virus. This could lead to severe infection, Dr. Zhou said.
Sex-based differences in steroid hormones may also explain the higher incidence of morbidity and fatality that’s been observed in other studies of male patients with other infectious diseases, such as severe acute respiratory syndrome and Middle East respiratory syndrome.
Study bolsters evidence on sex disparities
The results add real-world evidence to the limited literature on sex disparities in COVID-19 outcomes among patients with RA in the United States, Dr. Zhou said. “The differential role in sex steroid hormones among women and men may shed light on clinical management of COVID-19 patients and the need to consider sex-specific approaches in clinical trials in preventing and treating COVID-19 patients,” she said.
Considering that all patients are recommended to get COVID-19 vaccinations, “it is difficult to say how this impacts clinical practice,” said Janet Pope, MD, MPH, professor of medicine in the division of rheumatology at the University of Western Ontario, London, who was not involved with the study.
Sharing results with some patients may help to encourage vaccination, thus reducing risk of poor COVID-19 outcomes, Dr. Pope said.
In future studies, Dr. Zhou suggests using multiple databases and considering other geographies beyond the United States to further understand the etiology of sexual dimorphism in COVID-19 and expand generalizability. “In addition, future research will seek to provide insights into health equity gaps in the management of COVID-19. This may inform development of precision medicines and vaccines, especially among patients on immunosuppressive treatments,” she said.
The study was sponsored by Pfizer. Dr. Zhou and other study authors are Pfizer employees and hold Pfizer stock.
A version of this article first appeared on Medscape.com.
FROM THE LANCET SUMMIT ON SEX AND GENDER IN RHEUMATOLOGY