User login
Pseudo-Pedicle Heterotopic Ossification From Use of Recombinant Human Bone Morphogenetic Protein 2 (rhBMP-2) in Transforaminal Lumbar Interbody Fusion Cages
ABSTRACT
We conducted a study to determine the common characteristics of patients who developed radiculopathy symptoms and corresponding heterotopic ossification (HO) from transforaminal lumbar interbody fusions (TLIF) using recombinant human bone morphogenetic protein 2 (rhBMP-2). HO can arise from a disk space with rhBMP-2 use in TLIF. Formation of bone around nerve roots or the thecal sac can cause a radiculopathy with a consistent pattern of symptoms.
We identified 38 patients (26 males, 12 females) with a mean (SD) age of 50.8 (7.5) years who developed radiculopathy symptoms and corresponding HO from TLIF with rhBMP-2 in the disk space between 2002 and 2015. To document this complication and improve its recognition, we recorded common patterns of symptom development and radiologic findings: specifically, time from implantation of rhBMP-2 to symptom development, consistency with side of TLIF placement, and radiologic findings.
Radicular pain generally developed a mean (SD) of 3.8 (1.0) months after TLIF with rhBMP-2. Development of radiculopathy symptoms corresponded to consistent “pseudo-pedicle”-like HO. In all 38 patients, HO arising from the annulotomy site showed a distinct pseudo-pedicle pattern encompassing nerve roots and the thecal sac. In addition, development of radiculopathy symptoms and corresponding HO appear to be independent of amount of rhBMP-2. HO resulting from TLIF with rhBMP-2 in the disk space is a pain generator and a recognizable complication that can be diagnosed by assessment of symptoms and computed tomography characteristics.
Continue to: Bone morphogenetic proteins...
Bone morphogenetic proteins (BMPs), first isolated by Urist in 19641, are a family of growth factors that stimulate the cascade of bone formation. Recombinant human BMP (rhBMP), specifically rhBMP-2 and rhBMP-7 (also known as osteogenic protein 1 [OP-1]), was developed in the 1990s after the advent of gene splicing. Then, in 2002, the US Food and Drug Administration (FDA) approved use of rhBMP to stimulate fusion in the human spine. Specifically, rhBMP-2 (Medtronic) was approved for use in combination with a specific brand of interbody cage in 1-level anterior lumbar interbody fusion.2 Over the past decade, off-label use of rhBMP-2 to achieve osseous union has increased dramatically, particularly in spinal surgery: transforaminal lumbar interbody fusion (TLIF), posterior lumbar interbody fusion, and posterolateral lumbar fusion.3-9 However, this widespread off-label use for posterior spinal fusion began despite FDA data indicating that specific complications were underreported in the peer-reviewed literature.10,11 Although rhBMP-2 is very effective in increasing osteoblast formation and improving osteogenesis and subsequent bone healing in spinal surgery,12,13 its use in TLIF resulted in significant adverse side effects, including radiculopathy with and without neuroforaminal heterotopic ossification (HO); 14-24 complications in the FDA studies; 14,22,25-27 and osteolysis causing intervertebral cage subsidence, inflammatory radiculitis, genitourinary complications, infections, possible systemic effects, and significant HO complications.10,28-30 Of these, HO complications involved rhBMP leakage through the annulotomy to the disk space that led to HO. Specifically, rhBMP leaked directly out of the disk space and formed a pillar of bone that encased the nerve roots and dura, which led to occlusion of the foramen and symptoms of radiculopathy.10,28-30
Despite this frequent finding of HO in the intervertebral space outside the target fusion area, use of rhBMP-2 with intervertebral cages increased so rapidly that rhBMP-2 was used more often than autologous bone.5,11,17,31 In this study, we reviewed the common characteristics of patients who developed HO and subsequent radiculopathy from TLIF with rhBMP.
METHODS
After this study received Institutional Review Board approval, we retrospectively reviewed cases of radiculopathy symptoms that developed after TLIF with rhBMP between January 2002 and January 2015. During this period, 38 patients (26 males, 12 females) with a mean (SD) age of 50.8 (7.5) years and radiculopathy symptoms arising from TLIF with rhBMP-2 were identified to determine commonalities and defining characteristics that will help facilitate diagnosis.
Inclusion criteria were computed tomography (CT)–documented HO arising from the TLIF annulotomy site in continuity with bone in the disk space or ectopic bone forming a distinctive shell with contouring around the thecal sac or nerve roots, as well as recurrence or initial occurrence of radiculopathy with signs and symptoms corresponding to the CT site of aberrant bone growth in terms of laterality and particular nerve root(s) involved. Exclusion criteria were malplacement of interbody cage or pedicle screws, disk herniation, systemic neuropathic disease, and new or unresolved radiculopathy immediately after index surgery.
To improve recognition of this complication, we also documented the amount of BMP used, common patterns of radiculopathy symptom development, and radiologic findings. Type and timing of radiculopathy symptom onset and consistency with side of TLIF placement were documented as well. Radiculopathy symptoms included shooting pain in the legs, incontinence, sexual dysfunction, and severe paralysis. Radiologic findings were specific to bone formation from the disk space (detected with CT).
Continue to: RESULTS
RESULTS
All 38 selected patients had radiculopathy symptoms from HO out of the intervertebral space. The Table lists the patients’ overall characteristics. The left side had the most radiculopathy symptoms (31/38 patients), followed by the right side (5/38) and both sides (2/38). Radiculopathy symptoms began a mean (SD) of 3.8 (1.0) months (range, 2-6 months) after index surgery. The 38 patients had 4 characteristics in common:
Table. Transforaminal Lumbar Interbody Fusion With Recombinant Human Bone Morphogenetic Protein 2: Onset Time for Radiculopathy Symptoms, Surgery Level, Side of Pseudo-Pedicle Bone Formation, and Subsequent Complications
| Pt | Sympton Onset, mo | Surgery Level(s) | Side(s) | Complication(s) |
| 1 | 3 | L3-L5 (2) | Both | Radiculopathy, pseudo-pedicle, urine |
| 2 | 3 | L4-L5 (2) | R | Radiculopathy, pseudo-pedicle |
| 3 | 4 | L5-S1 (1) | R | Radiculopathy, pseudo-pedicle |
| 4 | 5 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 5 | 4 | L4-S1 (2) | L | Radiculopathy, pseudo-pedicle, subsidence |
| 6 | 5 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 7 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 8 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 9 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 10 | 2 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 11 | 2 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, subsidence, neurologic |
| 12 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 13 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, neurologic |
| 14 | 2 | L2-L3 (1) | R | Radiculopathy, pseudo-pedicle |
| 15 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 16 | 3 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 17 | 3 | L2-L3, L4-L5 (2) | L | Radiculopathy, pseudo-pedicle |
| 18 | 3 | L4-L5, L2-L3 (1) | L | Radiculopathy, pseudo-pedicle, nonunion |
| 19 | 4 | L4-L5 (1) | R | Radiculopathy, pseudo-pedicle |
| 20 | 5 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 21 | 5 | L5-S1 (1) | R | Radiculopathy, pseudo-pedicle |
| 22 | 3 | L3-L4, L5-S1 (2) | Both | Radiculopathy, pseudo-pedicle |
| 23 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 24 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 25 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 26 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, urine, bowel |
| 27 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 28 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 29 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 30 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 31 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 32 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 33 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 34 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 35 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 36 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 37 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 38 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
1. Bone growing out of the annulotomy site for TLIF cage placement was present and in continuity with the disk space in 33 (87%) of the 38 cases. In the other 5 cases (13%), HO was present around the neural tissue, but not necessarily in continuity with the disk space. This bone appeared ectopic and not osteophytic and facet-related, as it formed a shell around either the nerve root or the thecal sac, contouring to the structure.
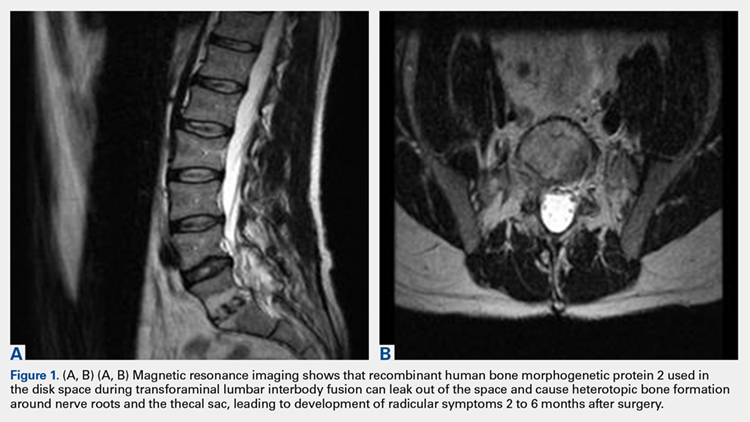
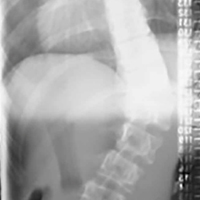
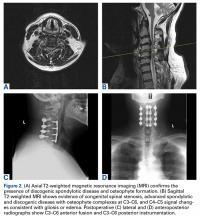
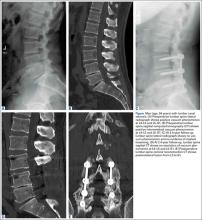
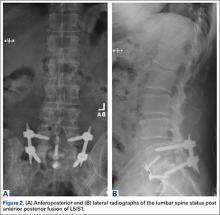
2. The common, novel finding on CT was a “pseudo-pedicle” (Figures 1A, 1B), which appeared as ectopic growth from the disk space—a solid piece of bone in the same direction as the anatomical pedicle. Confusing similarity to the anatomical pedicle is present on axial cuts and during surgery. The pseudo-pedicle varied in thickness and extent out of the disk space, but was always presented as a bar of bone arising from the annulotomy site. After arising from the disk space, the HO could disperse in any direction, further calcifying neural structures or the facet joints above or below. There was no apparent distinguishable repeating pattern, given the variable nature of arthritic facet changes, scoliotic deformities, size of annulotomies, amount of rhBMP used, and placement in cage and disk space or only in cage.
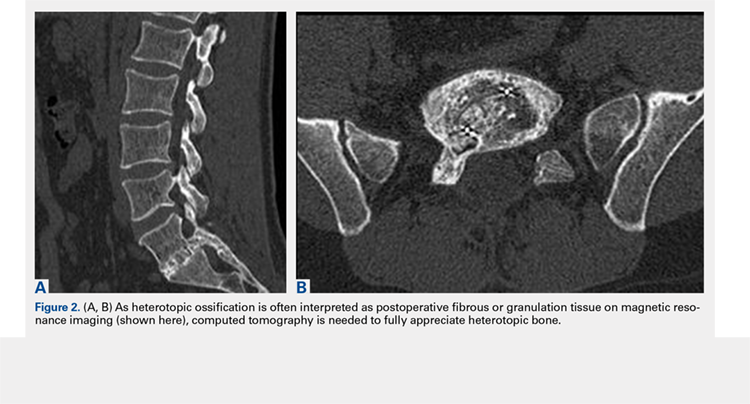
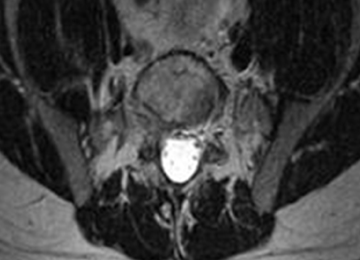
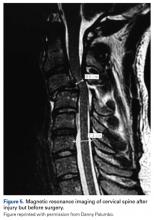
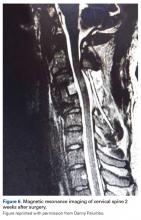
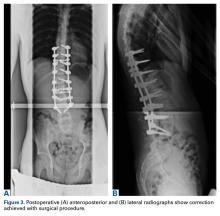
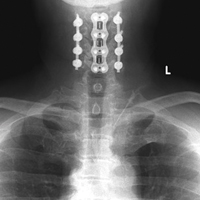
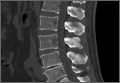
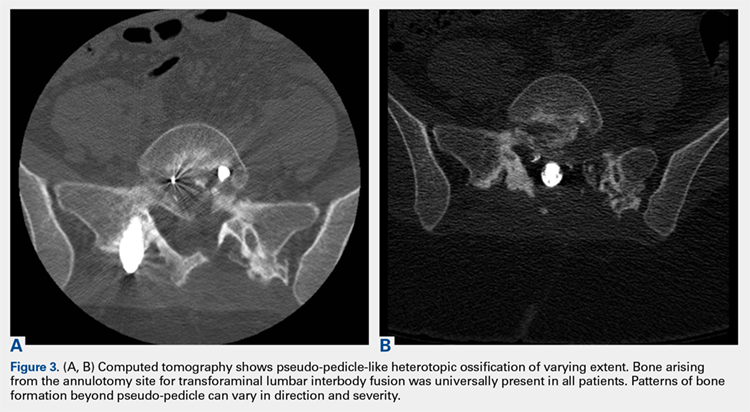
3. In 36 (95%) of the 38 cases, the initial interpretation of HO on magnetic resonance imaging (MRI) was of tissue other than bone, such as fibrous tissue, granulation tissue, recurrent disk herniation, or postoperative changes. However, this tissue was later determined to be bone from HO complications, which we confirmed with CT in all 38 cases. It is important to note that HO on MRI (Figures 2A, 2B) was initially interpreted by a radiologist as fibrous tissue, but same-level CT of the same case (Figures 3A, 3B) showed clear HO.
4. The radiculopathy symptoms caused by HO were independent of the amount of rhBMP-2 used in TLIF. Of the 38 patients, 19 had 1 rhBMP-2 sponge placed in the cage, 12 had a small kit sponge (1.05 mg), 5 had 1 sponge placed in the cage and 1 sponge placed directly in the disk space before cage placement (no notation of precise size or amount of rhBMP-2), and 2 had 1 sponge placed in the cage (no notation of rhBMP-2 amount). The data showed that HO can occur with even a small amount of rhBMP-2.
Continue to: Bone formation with rhBMP-2...
Bone formation with rhBMP-2 is robust and beneficial, but HO-related complications are significant, and identifiable on assessment of radiculopathy symptoms and CT characteristics.
DISCUSSION
We identified 38 patients with a recognizable and consistent pattern of complications of off-label use of rhBMP-2 in TLIF performed at our institution between 2002 and 2015. This pattern included consistent radiculopathy symptoms with corresponding HO at the annulotomy site in continuity with bone in the disk space or ectopic bone forming a distinctive shell around the thecal sac or nerve roots, as well as showing a distinct pseudo-pedicle pattern encompassing nerve roots and the thecal sac. Our finding differs from other findings of similar complication characteristics, but with much larger variations without consistency within the patient population.19,20,22,24 Specifically, previous studies found an association between off-label rhBMP-2 use in the posterior spine and radiculopathy with and without neuroforaminal HO. However, our study found consistent radiculopathy symptoms with pseudo-pedicle-like HO complications in all its 38 patients a mean (SD) of 3.8 (1.0) months after surgery.
In this study, consistent radiculopathy symptoms with pseudo-pedicle-like HO complications were independent of the amount of rhBMP-2 used, as some complications occurred with use of small pack rhBMP-2 with TLIF. It is well understood that high doses of rhBMP-2 may be required to improve fusion rates, but to our knowledge an optimal dosing strategy for TLIF has not been reported, particularly with respect to potential complications.8,20,31-33 For anterior lumbar interbody fusion surgery, the FDA-approved use of rhBMP-2 appears to have a significantly decreased risk of neuroforaminal HO complications. This may be attributable to the protective presence of the intact posterior annulus and longitudinal ligament for this procedure.20,33 For TLIF, it has been suggested that rhBMP-2 should be placed only along the anterior annulus with a posterior strut and morselized bone allograft barricade,33 and that fibrin glue should be used to limit BMP diffusion through the annulotomy site31 to prevent this complication.
Our study results suggest that radiculopathy symptoms with pseudo-pedicle-like HO complications appear to be caused by leakage of rhBMP-2 from the disk space through the annulotomy site. This was often initially interpreted incorrectly on MRI in the first year after surgery as being fibrous or granulation tissue, or even postoperative changes that the heterotopic tissue was bone was obvious only on CT. Even then the tissue may be incorrectly identified, as the encasing nerve roots in bone are similar to the scar tissue having no compressive effect. HO may compress, but it also has an inflammatory component that the scars lack. Additionally, the HO from the disk space, caused by leakage of the BMP placed in or around the fusion cage, can create a pseudo-pedicle of varying size and extent. This was present in all 38 of our cases.
This retrospective case series had its limitations. Its clinical and radiographic findings were not blinded. Confounding variables cannot be isolated for causal relationships, if any, to the complication in a case series such as this.
Bone formation with rhBMP-2 is robust and beneficial, but HO-related complications are significant, and identifiable on assessment of radiculopathy symptoms and CT characteristics.
1. Urist MR. Bone: formation by autoinduction. Science. 1965;150(3698):893-899.
2. Burkus JK, Gornet MF, Schuler TC, Kleeman TJ, Zdeblick TA. Six-year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphogenetic protein-2. J Bone Joint Surg Am. 2009;91(5):1181-1189.
3. Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo award in clinical studies. Spine. 2002;27(23):2662-2673.
4. Boden SD, Zdeblick TA, Sandhu HS, Heim SE. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000;25(3):376-381.
5. Haid RW Jr, Branch CL Jr, Alexander JT, Burkus JK. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004;4(5):527-538.
6. Meisel HJ, Schnöring M, Hohaus C, et al. Posterior lumbar interbody fusion using rhBMP-2. Eur Spine J. 2008;17(12):1735-1744.
7. Mummaneni PV, Pan J, Haid RW, Rodts GE. Contribution of recombinant human bone morphogenetic protein-2 to the rapid creation of interbody fusion when used in transforaminal lumbar interbody fusion: a preliminary report. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1(1):19-23.
8. Shimer AL, Oner FC, Vaccaro AR. Spinal reconstruction and bone morphogenetic proteins: open questions. Injury. 2009;40(suppl 3):S32-S38.
9. Slosar PJ, Josey R, Reynolds J. Accelerating lumbar fusions by combining rhBMP-2 with allograft bone: a prospective analysis of interbody fusion rates and clinical outcomes. Spine J. 2007;7(3):301-307.
10. Knox JB, Dai JM 3rd, Orchowski J. Osteolysis in transforaminal lumbar interbody fusion with bone morphogenetic protein-2. Spine. 2011;36(8):672-676.
11. Owens K, Glassman SD, Howard JM, Djurasovic M, Witten JL, Carreon LY. Perioperative complications with rhBMP-2 in transforaminal lumbar interbody fusion. Eur Spine J. 2011;20(4):612-617.
12. Mindea SA, Shih P, Song JK. Recombinant human bone morphogenetic protein-2-induced radiculitis in elective minimally invasive transforaminal lumbar interbody fusions: a series review. Spine. 2009;34(14):1480-1484.
13. Yoon ST, Park JS, Kim KS, et al. ISSLS prize winner: LMP-1 upregulates intervertebral disc cell production of proteoglycans and BMPs in vitro and in vivo. Spine. 2004;29(23):2603-2611.
14. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302(1):58-66.
15. Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471-491.
16. Chen NF, Smith ZA, Stiner E, Armin S, Sheikh H, Khoo LT. Symptomatic ectopic bone formation after off-label use of recombinant human bone morphogenetic protein-2 in transforaminal lumbar interbody fusion. J Neurosurg Spine. 2010;12(1):40-46.
17. Joseph V, Rampersaud YR. Heterotopic bone formation with the use of rhBMP2 in posterior minimal access interbody fusion: a CT analysis. Spine. 2007;32(25):2885-2890.
18. McClellan JW, Mulconrey DS, Forbes RJ, Fullmer N. Vertebral bone resorption after transforaminal lumbar interbody fusion with bone morphogenetic protein (rhBMP-2). J Spinal Disord Tech. 2006;19(7):483-486.
19. Mroz TE, Wang JC, Hashimoto R, Norvell DC. Complications related to osteobiologics use in spine surgery: a systematic review. Spine. 2010;35(9 suppl):S86-S104.
20. Muchow RD, Hsu WK, Anderson PA. Histopathologic inflammatory response induced by recombinant bone morphogenetic protein-2 causing radiculopathy after transforaminal lumbar interbody fusion. Spine J. 2010;10(9):e1-e6.
21. Ong KL, Villarraga ML, Lau E, Carreon LY, Kurtz SM, Glassman SD. Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine. 2010;35(19):1794-1800.
22. Rihn JA, Patel R, Makda J, et al. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J. 2009;9(8):623-629.
23. Vaidya R, Sethi A, Bartol S, Jacobson M, Coe C, Craig JG. Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech. 2008;21(8):557-562.
24. Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J. 2008;8(6):1011-1018.
25. Delawi D, Dhert WJ, Rillardon L, et al. A prospective, randomized, controlled, multicenter study of osteogenic protein-1 in instrumented posterolateral fusions: report on safety and feasibility. Spine. 2010;35(12):1185-1191.
26. Vaccaro AR, Patel T, Fischgrund J, et al. A pilot study evaluating the safety and efficacy of OP-1 putty (rhBMP-7) as a replacement for iliac crest autograft in posterolateral lumbar arthrodesis for degenerative spondylolisthesis. Spine. 2004;29(17):1885-1892.
27. Vaidya R, Weir R, Sethi A, Meisterling S, Hakeos W, Wybo CD. Interbody fusion with allograft and rhBMP-2 leads to consistent fusion but early subsidence. J Bone Joint Surg Br. 2007;89(3):342-345.
28. Glassman SD, Howard J, Dimar J, Sweet A, Wilson G, Carreon L. Complications with recombinant human bone morphogenic protein-2 in posterolateral spine fusion: a consecutive series of 1037 cases. Spine. 2011;36(22):1849-1854.
29. Helgeson MD, Lehman RA Jr, Patzkowski JC, Dmitriev AE, Rosner MK, Mack AW. Adjacent vertebral body osteolysis with bone morphogenetic protein use in transforaminal lumbar interbody fusion. Spine J. 2011;11(6):507-510.
30. Hoffmann MF, Jones CB, Sietsema DL. Adjuncts in posterior lumbar spine fusion: comparison of complications and efficacy. Arch Orthop Trauma Surg. 2012;132(8):1105-1110.
31. Villavicencio AT, Burneikiene S, Nelson EL, Bulsara KR, Favors M, Thramann J. Safety of transforaminal lumbar interbody fusion and intervertebral recombinant human bone morphogenetic protein-2. J Neurosurg Spine. 2005;3(6):436-443.
32. Patel VV, Zhao L, Wong P, et al. Controlling bone morphogenetic protein diffusion and bone morphogenetic protein-stimulated bone growth using fibrin glue. Spine. 2006;31(11):1201-1206.
33. Zhang H, Sucato DJ, Welch RD. Recombinant human bone morphogenic protein-2-enhanced anterior spine fusion without bone encroachment into the spinal canal: a histomorphometric study in a thoracoscopically instrumented porcine model. Spine. 2005;30(5):512-518.
ABSTRACT
We conducted a study to determine the common characteristics of patients who developed radiculopathy symptoms and corresponding heterotopic ossification (HO) from transforaminal lumbar interbody fusions (TLIF) using recombinant human bone morphogenetic protein 2 (rhBMP-2). HO can arise from a disk space with rhBMP-2 use in TLIF. Formation of bone around nerve roots or the thecal sac can cause a radiculopathy with a consistent pattern of symptoms.
We identified 38 patients (26 males, 12 females) with a mean (SD) age of 50.8 (7.5) years who developed radiculopathy symptoms and corresponding HO from TLIF with rhBMP-2 in the disk space between 2002 and 2015. To document this complication and improve its recognition, we recorded common patterns of symptom development and radiologic findings: specifically, time from implantation of rhBMP-2 to symptom development, consistency with side of TLIF placement, and radiologic findings.
Radicular pain generally developed a mean (SD) of 3.8 (1.0) months after TLIF with rhBMP-2. Development of radiculopathy symptoms corresponded to consistent “pseudo-pedicle”-like HO. In all 38 patients, HO arising from the annulotomy site showed a distinct pseudo-pedicle pattern encompassing nerve roots and the thecal sac. In addition, development of radiculopathy symptoms and corresponding HO appear to be independent of amount of rhBMP-2. HO resulting from TLIF with rhBMP-2 in the disk space is a pain generator and a recognizable complication that can be diagnosed by assessment of symptoms and computed tomography characteristics.
Continue to: Bone morphogenetic proteins...
Bone morphogenetic proteins (BMPs), first isolated by Urist in 19641, are a family of growth factors that stimulate the cascade of bone formation. Recombinant human BMP (rhBMP), specifically rhBMP-2 and rhBMP-7 (also known as osteogenic protein 1 [OP-1]), was developed in the 1990s after the advent of gene splicing. Then, in 2002, the US Food and Drug Administration (FDA) approved use of rhBMP to stimulate fusion in the human spine. Specifically, rhBMP-2 (Medtronic) was approved for use in combination with a specific brand of interbody cage in 1-level anterior lumbar interbody fusion.2 Over the past decade, off-label use of rhBMP-2 to achieve osseous union has increased dramatically, particularly in spinal surgery: transforaminal lumbar interbody fusion (TLIF), posterior lumbar interbody fusion, and posterolateral lumbar fusion.3-9 However, this widespread off-label use for posterior spinal fusion began despite FDA data indicating that specific complications were underreported in the peer-reviewed literature.10,11 Although rhBMP-2 is very effective in increasing osteoblast formation and improving osteogenesis and subsequent bone healing in spinal surgery,12,13 its use in TLIF resulted in significant adverse side effects, including radiculopathy with and without neuroforaminal heterotopic ossification (HO); 14-24 complications in the FDA studies; 14,22,25-27 and osteolysis causing intervertebral cage subsidence, inflammatory radiculitis, genitourinary complications, infections, possible systemic effects, and significant HO complications.10,28-30 Of these, HO complications involved rhBMP leakage through the annulotomy to the disk space that led to HO. Specifically, rhBMP leaked directly out of the disk space and formed a pillar of bone that encased the nerve roots and dura, which led to occlusion of the foramen and symptoms of radiculopathy.10,28-30
Despite this frequent finding of HO in the intervertebral space outside the target fusion area, use of rhBMP-2 with intervertebral cages increased so rapidly that rhBMP-2 was used more often than autologous bone.5,11,17,31 In this study, we reviewed the common characteristics of patients who developed HO and subsequent radiculopathy from TLIF with rhBMP.
METHODS
After this study received Institutional Review Board approval, we retrospectively reviewed cases of radiculopathy symptoms that developed after TLIF with rhBMP between January 2002 and January 2015. During this period, 38 patients (26 males, 12 females) with a mean (SD) age of 50.8 (7.5) years and radiculopathy symptoms arising from TLIF with rhBMP-2 were identified to determine commonalities and defining characteristics that will help facilitate diagnosis.
Inclusion criteria were computed tomography (CT)–documented HO arising from the TLIF annulotomy site in continuity with bone in the disk space or ectopic bone forming a distinctive shell with contouring around the thecal sac or nerve roots, as well as recurrence or initial occurrence of radiculopathy with signs and symptoms corresponding to the CT site of aberrant bone growth in terms of laterality and particular nerve root(s) involved. Exclusion criteria were malplacement of interbody cage or pedicle screws, disk herniation, systemic neuropathic disease, and new or unresolved radiculopathy immediately after index surgery.
To improve recognition of this complication, we also documented the amount of BMP used, common patterns of radiculopathy symptom development, and radiologic findings. Type and timing of radiculopathy symptom onset and consistency with side of TLIF placement were documented as well. Radiculopathy symptoms included shooting pain in the legs, incontinence, sexual dysfunction, and severe paralysis. Radiologic findings were specific to bone formation from the disk space (detected with CT).
Continue to: RESULTS
RESULTS
All 38 selected patients had radiculopathy symptoms from HO out of the intervertebral space. The Table lists the patients’ overall characteristics. The left side had the most radiculopathy symptoms (31/38 patients), followed by the right side (5/38) and both sides (2/38). Radiculopathy symptoms began a mean (SD) of 3.8 (1.0) months (range, 2-6 months) after index surgery. The 38 patients had 4 characteristics in common:
Table. Transforaminal Lumbar Interbody Fusion With Recombinant Human Bone Morphogenetic Protein 2: Onset Time for Radiculopathy Symptoms, Surgery Level, Side of Pseudo-Pedicle Bone Formation, and Subsequent Complications
| Pt | Sympton Onset, mo | Surgery Level(s) | Side(s) | Complication(s) |
| 1 | 3 | L3-L5 (2) | Both | Radiculopathy, pseudo-pedicle, urine |
| 2 | 3 | L4-L5 (2) | R | Radiculopathy, pseudo-pedicle |
| 3 | 4 | L5-S1 (1) | R | Radiculopathy, pseudo-pedicle |
| 4 | 5 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 5 | 4 | L4-S1 (2) | L | Radiculopathy, pseudo-pedicle, subsidence |
| 6 | 5 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 7 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 8 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 9 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 10 | 2 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 11 | 2 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, subsidence, neurologic |
| 12 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 13 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, neurologic |
| 14 | 2 | L2-L3 (1) | R | Radiculopathy, pseudo-pedicle |
| 15 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 16 | 3 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 17 | 3 | L2-L3, L4-L5 (2) | L | Radiculopathy, pseudo-pedicle |
| 18 | 3 | L4-L5, L2-L3 (1) | L | Radiculopathy, pseudo-pedicle, nonunion |
| 19 | 4 | L4-L5 (1) | R | Radiculopathy, pseudo-pedicle |
| 20 | 5 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 21 | 5 | L5-S1 (1) | R | Radiculopathy, pseudo-pedicle |
| 22 | 3 | L3-L4, L5-S1 (2) | Both | Radiculopathy, pseudo-pedicle |
| 23 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 24 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 25 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 26 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, urine, bowel |
| 27 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 28 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 29 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 30 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 31 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 32 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 33 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 34 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 35 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 36 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 37 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 38 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
1. Bone growing out of the annulotomy site for TLIF cage placement was present and in continuity with the disk space in 33 (87%) of the 38 cases. In the other 5 cases (13%), HO was present around the neural tissue, but not necessarily in continuity with the disk space. This bone appeared ectopic and not osteophytic and facet-related, as it formed a shell around either the nerve root or the thecal sac, contouring to the structure.

2. The common, novel finding on CT was a “pseudo-pedicle” (Figures 1A, 1B), which appeared as ectopic growth from the disk space—a solid piece of bone in the same direction as the anatomical pedicle. Confusing similarity to the anatomical pedicle is present on axial cuts and during surgery. The pseudo-pedicle varied in thickness and extent out of the disk space, but was always presented as a bar of bone arising from the annulotomy site. After arising from the disk space, the HO could disperse in any direction, further calcifying neural structures or the facet joints above or below. There was no apparent distinguishable repeating pattern, given the variable nature of arthritic facet changes, scoliotic deformities, size of annulotomies, amount of rhBMP used, and placement in cage and disk space or only in cage.

3. In 36 (95%) of the 38 cases, the initial interpretation of HO on magnetic resonance imaging (MRI) was of tissue other than bone, such as fibrous tissue, granulation tissue, recurrent disk herniation, or postoperative changes. However, this tissue was later determined to be bone from HO complications, which we confirmed with CT in all 38 cases. It is important to note that HO on MRI (Figures 2A, 2B) was initially interpreted by a radiologist as fibrous tissue, but same-level CT of the same case (Figures 3A, 3B) showed clear HO.

4. The radiculopathy symptoms caused by HO were independent of the amount of rhBMP-2 used in TLIF. Of the 38 patients, 19 had 1 rhBMP-2 sponge placed in the cage, 12 had a small kit sponge (1.05 mg), 5 had 1 sponge placed in the cage and 1 sponge placed directly in the disk space before cage placement (no notation of precise size or amount of rhBMP-2), and 2 had 1 sponge placed in the cage (no notation of rhBMP-2 amount). The data showed that HO can occur with even a small amount of rhBMP-2.
Continue to: Bone formation with rhBMP-2...
Bone formation with rhBMP-2 is robust and beneficial, but HO-related complications are significant, and identifiable on assessment of radiculopathy symptoms and CT characteristics.
DISCUSSION
We identified 38 patients with a recognizable and consistent pattern of complications of off-label use of rhBMP-2 in TLIF performed at our institution between 2002 and 2015. This pattern included consistent radiculopathy symptoms with corresponding HO at the annulotomy site in continuity with bone in the disk space or ectopic bone forming a distinctive shell around the thecal sac or nerve roots, as well as showing a distinct pseudo-pedicle pattern encompassing nerve roots and the thecal sac. Our finding differs from other findings of similar complication characteristics, but with much larger variations without consistency within the patient population.19,20,22,24 Specifically, previous studies found an association between off-label rhBMP-2 use in the posterior spine and radiculopathy with and without neuroforaminal HO. However, our study found consistent radiculopathy symptoms with pseudo-pedicle-like HO complications in all its 38 patients a mean (SD) of 3.8 (1.0) months after surgery.
In this study, consistent radiculopathy symptoms with pseudo-pedicle-like HO complications were independent of the amount of rhBMP-2 used, as some complications occurred with use of small pack rhBMP-2 with TLIF. It is well understood that high doses of rhBMP-2 may be required to improve fusion rates, but to our knowledge an optimal dosing strategy for TLIF has not been reported, particularly with respect to potential complications.8,20,31-33 For anterior lumbar interbody fusion surgery, the FDA-approved use of rhBMP-2 appears to have a significantly decreased risk of neuroforaminal HO complications. This may be attributable to the protective presence of the intact posterior annulus and longitudinal ligament for this procedure.20,33 For TLIF, it has been suggested that rhBMP-2 should be placed only along the anterior annulus with a posterior strut and morselized bone allograft barricade,33 and that fibrin glue should be used to limit BMP diffusion through the annulotomy site31 to prevent this complication.
Our study results suggest that radiculopathy symptoms with pseudo-pedicle-like HO complications appear to be caused by leakage of rhBMP-2 from the disk space through the annulotomy site. This was often initially interpreted incorrectly on MRI in the first year after surgery as being fibrous or granulation tissue, or even postoperative changes that the heterotopic tissue was bone was obvious only on CT. Even then the tissue may be incorrectly identified, as the encasing nerve roots in bone are similar to the scar tissue having no compressive effect. HO may compress, but it also has an inflammatory component that the scars lack. Additionally, the HO from the disk space, caused by leakage of the BMP placed in or around the fusion cage, can create a pseudo-pedicle of varying size and extent. This was present in all 38 of our cases.
This retrospective case series had its limitations. Its clinical and radiographic findings were not blinded. Confounding variables cannot be isolated for causal relationships, if any, to the complication in a case series such as this.
Bone formation with rhBMP-2 is robust and beneficial, but HO-related complications are significant, and identifiable on assessment of radiculopathy symptoms and CT characteristics.
ABSTRACT
We conducted a study to determine the common characteristics of patients who developed radiculopathy symptoms and corresponding heterotopic ossification (HO) from transforaminal lumbar interbody fusions (TLIF) using recombinant human bone morphogenetic protein 2 (rhBMP-2). HO can arise from a disk space with rhBMP-2 use in TLIF. Formation of bone around nerve roots or the thecal sac can cause a radiculopathy with a consistent pattern of symptoms.
We identified 38 patients (26 males, 12 females) with a mean (SD) age of 50.8 (7.5) years who developed radiculopathy symptoms and corresponding HO from TLIF with rhBMP-2 in the disk space between 2002 and 2015. To document this complication and improve its recognition, we recorded common patterns of symptom development and radiologic findings: specifically, time from implantation of rhBMP-2 to symptom development, consistency with side of TLIF placement, and radiologic findings.
Radicular pain generally developed a mean (SD) of 3.8 (1.0) months after TLIF with rhBMP-2. Development of radiculopathy symptoms corresponded to consistent “pseudo-pedicle”-like HO. In all 38 patients, HO arising from the annulotomy site showed a distinct pseudo-pedicle pattern encompassing nerve roots and the thecal sac. In addition, development of radiculopathy symptoms and corresponding HO appear to be independent of amount of rhBMP-2. HO resulting from TLIF with rhBMP-2 in the disk space is a pain generator and a recognizable complication that can be diagnosed by assessment of symptoms and computed tomography characteristics.
Continue to: Bone morphogenetic proteins...
Bone morphogenetic proteins (BMPs), first isolated by Urist in 19641, are a family of growth factors that stimulate the cascade of bone formation. Recombinant human BMP (rhBMP), specifically rhBMP-2 and rhBMP-7 (also known as osteogenic protein 1 [OP-1]), was developed in the 1990s after the advent of gene splicing. Then, in 2002, the US Food and Drug Administration (FDA) approved use of rhBMP to stimulate fusion in the human spine. Specifically, rhBMP-2 (Medtronic) was approved for use in combination with a specific brand of interbody cage in 1-level anterior lumbar interbody fusion.2 Over the past decade, off-label use of rhBMP-2 to achieve osseous union has increased dramatically, particularly in spinal surgery: transforaminal lumbar interbody fusion (TLIF), posterior lumbar interbody fusion, and posterolateral lumbar fusion.3-9 However, this widespread off-label use for posterior spinal fusion began despite FDA data indicating that specific complications were underreported in the peer-reviewed literature.10,11 Although rhBMP-2 is very effective in increasing osteoblast formation and improving osteogenesis and subsequent bone healing in spinal surgery,12,13 its use in TLIF resulted in significant adverse side effects, including radiculopathy with and without neuroforaminal heterotopic ossification (HO); 14-24 complications in the FDA studies; 14,22,25-27 and osteolysis causing intervertebral cage subsidence, inflammatory radiculitis, genitourinary complications, infections, possible systemic effects, and significant HO complications.10,28-30 Of these, HO complications involved rhBMP leakage through the annulotomy to the disk space that led to HO. Specifically, rhBMP leaked directly out of the disk space and formed a pillar of bone that encased the nerve roots and dura, which led to occlusion of the foramen and symptoms of radiculopathy.10,28-30
Despite this frequent finding of HO in the intervertebral space outside the target fusion area, use of rhBMP-2 with intervertebral cages increased so rapidly that rhBMP-2 was used more often than autologous bone.5,11,17,31 In this study, we reviewed the common characteristics of patients who developed HO and subsequent radiculopathy from TLIF with rhBMP.
METHODS
After this study received Institutional Review Board approval, we retrospectively reviewed cases of radiculopathy symptoms that developed after TLIF with rhBMP between January 2002 and January 2015. During this period, 38 patients (26 males, 12 females) with a mean (SD) age of 50.8 (7.5) years and radiculopathy symptoms arising from TLIF with rhBMP-2 were identified to determine commonalities and defining characteristics that will help facilitate diagnosis.
Inclusion criteria were computed tomography (CT)–documented HO arising from the TLIF annulotomy site in continuity with bone in the disk space or ectopic bone forming a distinctive shell with contouring around the thecal sac or nerve roots, as well as recurrence or initial occurrence of radiculopathy with signs and symptoms corresponding to the CT site of aberrant bone growth in terms of laterality and particular nerve root(s) involved. Exclusion criteria were malplacement of interbody cage or pedicle screws, disk herniation, systemic neuropathic disease, and new or unresolved radiculopathy immediately after index surgery.
To improve recognition of this complication, we also documented the amount of BMP used, common patterns of radiculopathy symptom development, and radiologic findings. Type and timing of radiculopathy symptom onset and consistency with side of TLIF placement were documented as well. Radiculopathy symptoms included shooting pain in the legs, incontinence, sexual dysfunction, and severe paralysis. Radiologic findings were specific to bone formation from the disk space (detected with CT).
Continue to: RESULTS
RESULTS
All 38 selected patients had radiculopathy symptoms from HO out of the intervertebral space. The Table lists the patients’ overall characteristics. The left side had the most radiculopathy symptoms (31/38 patients), followed by the right side (5/38) and both sides (2/38). Radiculopathy symptoms began a mean (SD) of 3.8 (1.0) months (range, 2-6 months) after index surgery. The 38 patients had 4 characteristics in common:
Table. Transforaminal Lumbar Interbody Fusion With Recombinant Human Bone Morphogenetic Protein 2: Onset Time for Radiculopathy Symptoms, Surgery Level, Side of Pseudo-Pedicle Bone Formation, and Subsequent Complications
| Pt | Sympton Onset, mo | Surgery Level(s) | Side(s) | Complication(s) |
| 1 | 3 | L3-L5 (2) | Both | Radiculopathy, pseudo-pedicle, urine |
| 2 | 3 | L4-L5 (2) | R | Radiculopathy, pseudo-pedicle |
| 3 | 4 | L5-S1 (1) | R | Radiculopathy, pseudo-pedicle |
| 4 | 5 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 5 | 4 | L4-S1 (2) | L | Radiculopathy, pseudo-pedicle, subsidence |
| 6 | 5 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 7 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 8 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 9 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 10 | 2 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 11 | 2 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, subsidence, neurologic |
| 12 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 13 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, neurologic |
| 14 | 2 | L2-L3 (1) | R | Radiculopathy, pseudo-pedicle |
| 15 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 16 | 3 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 17 | 3 | L2-L3, L4-L5 (2) | L | Radiculopathy, pseudo-pedicle |
| 18 | 3 | L4-L5, L2-L3 (1) | L | Radiculopathy, pseudo-pedicle, nonunion |
| 19 | 4 | L4-L5 (1) | R | Radiculopathy, pseudo-pedicle |
| 20 | 5 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 21 | 5 | L5-S1 (1) | R | Radiculopathy, pseudo-pedicle |
| 22 | 3 | L3-L4, L5-S1 (2) | Both | Radiculopathy, pseudo-pedicle |
| 23 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 24 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 25 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 26 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, urine, bowel |
| 27 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 28 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 29 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 30 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 31 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 32 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 33 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 34 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 35 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 36 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 37 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 38 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
1. Bone growing out of the annulotomy site for TLIF cage placement was present and in continuity with the disk space in 33 (87%) of the 38 cases. In the other 5 cases (13%), HO was present around the neural tissue, but not necessarily in continuity with the disk space. This bone appeared ectopic and not osteophytic and facet-related, as it formed a shell around either the nerve root or the thecal sac, contouring to the structure.

2. The common, novel finding on CT was a “pseudo-pedicle” (Figures 1A, 1B), which appeared as ectopic growth from the disk space—a solid piece of bone in the same direction as the anatomical pedicle. Confusing similarity to the anatomical pedicle is present on axial cuts and during surgery. The pseudo-pedicle varied in thickness and extent out of the disk space, but was always presented as a bar of bone arising from the annulotomy site. After arising from the disk space, the HO could disperse in any direction, further calcifying neural structures or the facet joints above or below. There was no apparent distinguishable repeating pattern, given the variable nature of arthritic facet changes, scoliotic deformities, size of annulotomies, amount of rhBMP used, and placement in cage and disk space or only in cage.

3. In 36 (95%) of the 38 cases, the initial interpretation of HO on magnetic resonance imaging (MRI) was of tissue other than bone, such as fibrous tissue, granulation tissue, recurrent disk herniation, or postoperative changes. However, this tissue was later determined to be bone from HO complications, which we confirmed with CT in all 38 cases. It is important to note that HO on MRI (Figures 2A, 2B) was initially interpreted by a radiologist as fibrous tissue, but same-level CT of the same case (Figures 3A, 3B) showed clear HO.

4. The radiculopathy symptoms caused by HO were independent of the amount of rhBMP-2 used in TLIF. Of the 38 patients, 19 had 1 rhBMP-2 sponge placed in the cage, 12 had a small kit sponge (1.05 mg), 5 had 1 sponge placed in the cage and 1 sponge placed directly in the disk space before cage placement (no notation of precise size or amount of rhBMP-2), and 2 had 1 sponge placed in the cage (no notation of rhBMP-2 amount). The data showed that HO can occur with even a small amount of rhBMP-2.
Continue to: Bone formation with rhBMP-2...
Bone formation with rhBMP-2 is robust and beneficial, but HO-related complications are significant, and identifiable on assessment of radiculopathy symptoms and CT characteristics.
DISCUSSION
We identified 38 patients with a recognizable and consistent pattern of complications of off-label use of rhBMP-2 in TLIF performed at our institution between 2002 and 2015. This pattern included consistent radiculopathy symptoms with corresponding HO at the annulotomy site in continuity with bone in the disk space or ectopic bone forming a distinctive shell around the thecal sac or nerve roots, as well as showing a distinct pseudo-pedicle pattern encompassing nerve roots and the thecal sac. Our finding differs from other findings of similar complication characteristics, but with much larger variations without consistency within the patient population.19,20,22,24 Specifically, previous studies found an association between off-label rhBMP-2 use in the posterior spine and radiculopathy with and without neuroforaminal HO. However, our study found consistent radiculopathy symptoms with pseudo-pedicle-like HO complications in all its 38 patients a mean (SD) of 3.8 (1.0) months after surgery.
In this study, consistent radiculopathy symptoms with pseudo-pedicle-like HO complications were independent of the amount of rhBMP-2 used, as some complications occurred with use of small pack rhBMP-2 with TLIF. It is well understood that high doses of rhBMP-2 may be required to improve fusion rates, but to our knowledge an optimal dosing strategy for TLIF has not been reported, particularly with respect to potential complications.8,20,31-33 For anterior lumbar interbody fusion surgery, the FDA-approved use of rhBMP-2 appears to have a significantly decreased risk of neuroforaminal HO complications. This may be attributable to the protective presence of the intact posterior annulus and longitudinal ligament for this procedure.20,33 For TLIF, it has been suggested that rhBMP-2 should be placed only along the anterior annulus with a posterior strut and morselized bone allograft barricade,33 and that fibrin glue should be used to limit BMP diffusion through the annulotomy site31 to prevent this complication.
Our study results suggest that radiculopathy symptoms with pseudo-pedicle-like HO complications appear to be caused by leakage of rhBMP-2 from the disk space through the annulotomy site. This was often initially interpreted incorrectly on MRI in the first year after surgery as being fibrous or granulation tissue, or even postoperative changes that the heterotopic tissue was bone was obvious only on CT. Even then the tissue may be incorrectly identified, as the encasing nerve roots in bone are similar to the scar tissue having no compressive effect. HO may compress, but it also has an inflammatory component that the scars lack. Additionally, the HO from the disk space, caused by leakage of the BMP placed in or around the fusion cage, can create a pseudo-pedicle of varying size and extent. This was present in all 38 of our cases.
This retrospective case series had its limitations. Its clinical and radiographic findings were not blinded. Confounding variables cannot be isolated for causal relationships, if any, to the complication in a case series such as this.
Bone formation with rhBMP-2 is robust and beneficial, but HO-related complications are significant, and identifiable on assessment of radiculopathy symptoms and CT characteristics.
1. Urist MR. Bone: formation by autoinduction. Science. 1965;150(3698):893-899.
2. Burkus JK, Gornet MF, Schuler TC, Kleeman TJ, Zdeblick TA. Six-year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphogenetic protein-2. J Bone Joint Surg Am. 2009;91(5):1181-1189.
3. Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo award in clinical studies. Spine. 2002;27(23):2662-2673.
4. Boden SD, Zdeblick TA, Sandhu HS, Heim SE. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000;25(3):376-381.
5. Haid RW Jr, Branch CL Jr, Alexander JT, Burkus JK. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004;4(5):527-538.
6. Meisel HJ, Schnöring M, Hohaus C, et al. Posterior lumbar interbody fusion using rhBMP-2. Eur Spine J. 2008;17(12):1735-1744.
7. Mummaneni PV, Pan J, Haid RW, Rodts GE. Contribution of recombinant human bone morphogenetic protein-2 to the rapid creation of interbody fusion when used in transforaminal lumbar interbody fusion: a preliminary report. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1(1):19-23.
8. Shimer AL, Oner FC, Vaccaro AR. Spinal reconstruction and bone morphogenetic proteins: open questions. Injury. 2009;40(suppl 3):S32-S38.
9. Slosar PJ, Josey R, Reynolds J. Accelerating lumbar fusions by combining rhBMP-2 with allograft bone: a prospective analysis of interbody fusion rates and clinical outcomes. Spine J. 2007;7(3):301-307.
10. Knox JB, Dai JM 3rd, Orchowski J. Osteolysis in transforaminal lumbar interbody fusion with bone morphogenetic protein-2. Spine. 2011;36(8):672-676.
11. Owens K, Glassman SD, Howard JM, Djurasovic M, Witten JL, Carreon LY. Perioperative complications with rhBMP-2 in transforaminal lumbar interbody fusion. Eur Spine J. 2011;20(4):612-617.
12. Mindea SA, Shih P, Song JK. Recombinant human bone morphogenetic protein-2-induced radiculitis in elective minimally invasive transforaminal lumbar interbody fusions: a series review. Spine. 2009;34(14):1480-1484.
13. Yoon ST, Park JS, Kim KS, et al. ISSLS prize winner: LMP-1 upregulates intervertebral disc cell production of proteoglycans and BMPs in vitro and in vivo. Spine. 2004;29(23):2603-2611.
14. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302(1):58-66.
15. Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471-491.
16. Chen NF, Smith ZA, Stiner E, Armin S, Sheikh H, Khoo LT. Symptomatic ectopic bone formation after off-label use of recombinant human bone morphogenetic protein-2 in transforaminal lumbar interbody fusion. J Neurosurg Spine. 2010;12(1):40-46.
17. Joseph V, Rampersaud YR. Heterotopic bone formation with the use of rhBMP2 in posterior minimal access interbody fusion: a CT analysis. Spine. 2007;32(25):2885-2890.
18. McClellan JW, Mulconrey DS, Forbes RJ, Fullmer N. Vertebral bone resorption after transforaminal lumbar interbody fusion with bone morphogenetic protein (rhBMP-2). J Spinal Disord Tech. 2006;19(7):483-486.
19. Mroz TE, Wang JC, Hashimoto R, Norvell DC. Complications related to osteobiologics use in spine surgery: a systematic review. Spine. 2010;35(9 suppl):S86-S104.
20. Muchow RD, Hsu WK, Anderson PA. Histopathologic inflammatory response induced by recombinant bone morphogenetic protein-2 causing radiculopathy after transforaminal lumbar interbody fusion. Spine J. 2010;10(9):e1-e6.
21. Ong KL, Villarraga ML, Lau E, Carreon LY, Kurtz SM, Glassman SD. Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine. 2010;35(19):1794-1800.
22. Rihn JA, Patel R, Makda J, et al. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J. 2009;9(8):623-629.
23. Vaidya R, Sethi A, Bartol S, Jacobson M, Coe C, Craig JG. Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech. 2008;21(8):557-562.
24. Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J. 2008;8(6):1011-1018.
25. Delawi D, Dhert WJ, Rillardon L, et al. A prospective, randomized, controlled, multicenter study of osteogenic protein-1 in instrumented posterolateral fusions: report on safety and feasibility. Spine. 2010;35(12):1185-1191.
26. Vaccaro AR, Patel T, Fischgrund J, et al. A pilot study evaluating the safety and efficacy of OP-1 putty (rhBMP-7) as a replacement for iliac crest autograft in posterolateral lumbar arthrodesis for degenerative spondylolisthesis. Spine. 2004;29(17):1885-1892.
27. Vaidya R, Weir R, Sethi A, Meisterling S, Hakeos W, Wybo CD. Interbody fusion with allograft and rhBMP-2 leads to consistent fusion but early subsidence. J Bone Joint Surg Br. 2007;89(3):342-345.
28. Glassman SD, Howard J, Dimar J, Sweet A, Wilson G, Carreon L. Complications with recombinant human bone morphogenic protein-2 in posterolateral spine fusion: a consecutive series of 1037 cases. Spine. 2011;36(22):1849-1854.
29. Helgeson MD, Lehman RA Jr, Patzkowski JC, Dmitriev AE, Rosner MK, Mack AW. Adjacent vertebral body osteolysis with bone morphogenetic protein use in transforaminal lumbar interbody fusion. Spine J. 2011;11(6):507-510.
30. Hoffmann MF, Jones CB, Sietsema DL. Adjuncts in posterior lumbar spine fusion: comparison of complications and efficacy. Arch Orthop Trauma Surg. 2012;132(8):1105-1110.
31. Villavicencio AT, Burneikiene S, Nelson EL, Bulsara KR, Favors M, Thramann J. Safety of transforaminal lumbar interbody fusion and intervertebral recombinant human bone morphogenetic protein-2. J Neurosurg Spine. 2005;3(6):436-443.
32. Patel VV, Zhao L, Wong P, et al. Controlling bone morphogenetic protein diffusion and bone morphogenetic protein-stimulated bone growth using fibrin glue. Spine. 2006;31(11):1201-1206.
33. Zhang H, Sucato DJ, Welch RD. Recombinant human bone morphogenic protein-2-enhanced anterior spine fusion without bone encroachment into the spinal canal: a histomorphometric study in a thoracoscopically instrumented porcine model. Spine. 2005;30(5):512-518.
1. Urist MR. Bone: formation by autoinduction. Science. 1965;150(3698):893-899.
2. Burkus JK, Gornet MF, Schuler TC, Kleeman TJ, Zdeblick TA. Six-year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphogenetic protein-2. J Bone Joint Surg Am. 2009;91(5):1181-1189.
3. Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo award in clinical studies. Spine. 2002;27(23):2662-2673.
4. Boden SD, Zdeblick TA, Sandhu HS, Heim SE. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000;25(3):376-381.
5. Haid RW Jr, Branch CL Jr, Alexander JT, Burkus JK. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004;4(5):527-538.
6. Meisel HJ, Schnöring M, Hohaus C, et al. Posterior lumbar interbody fusion using rhBMP-2. Eur Spine J. 2008;17(12):1735-1744.
7. Mummaneni PV, Pan J, Haid RW, Rodts GE. Contribution of recombinant human bone morphogenetic protein-2 to the rapid creation of interbody fusion when used in transforaminal lumbar interbody fusion: a preliminary report. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1(1):19-23.
8. Shimer AL, Oner FC, Vaccaro AR. Spinal reconstruction and bone morphogenetic proteins: open questions. Injury. 2009;40(suppl 3):S32-S38.
9. Slosar PJ, Josey R, Reynolds J. Accelerating lumbar fusions by combining rhBMP-2 with allograft bone: a prospective analysis of interbody fusion rates and clinical outcomes. Spine J. 2007;7(3):301-307.
10. Knox JB, Dai JM 3rd, Orchowski J. Osteolysis in transforaminal lumbar interbody fusion with bone morphogenetic protein-2. Spine. 2011;36(8):672-676.
11. Owens K, Glassman SD, Howard JM, Djurasovic M, Witten JL, Carreon LY. Perioperative complications with rhBMP-2 in transforaminal lumbar interbody fusion. Eur Spine J. 2011;20(4):612-617.
12. Mindea SA, Shih P, Song JK. Recombinant human bone morphogenetic protein-2-induced radiculitis in elective minimally invasive transforaminal lumbar interbody fusions: a series review. Spine. 2009;34(14):1480-1484.
13. Yoon ST, Park JS, Kim KS, et al. ISSLS prize winner: LMP-1 upregulates intervertebral disc cell production of proteoglycans and BMPs in vitro and in vivo. Spine. 2004;29(23):2603-2611.
14. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302(1):58-66.
15. Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471-491.
16. Chen NF, Smith ZA, Stiner E, Armin S, Sheikh H, Khoo LT. Symptomatic ectopic bone formation after off-label use of recombinant human bone morphogenetic protein-2 in transforaminal lumbar interbody fusion. J Neurosurg Spine. 2010;12(1):40-46.
17. Joseph V, Rampersaud YR. Heterotopic bone formation with the use of rhBMP2 in posterior minimal access interbody fusion: a CT analysis. Spine. 2007;32(25):2885-2890.
18. McClellan JW, Mulconrey DS, Forbes RJ, Fullmer N. Vertebral bone resorption after transforaminal lumbar interbody fusion with bone morphogenetic protein (rhBMP-2). J Spinal Disord Tech. 2006;19(7):483-486.
19. Mroz TE, Wang JC, Hashimoto R, Norvell DC. Complications related to osteobiologics use in spine surgery: a systematic review. Spine. 2010;35(9 suppl):S86-S104.
20. Muchow RD, Hsu WK, Anderson PA. Histopathologic inflammatory response induced by recombinant bone morphogenetic protein-2 causing radiculopathy after transforaminal lumbar interbody fusion. Spine J. 2010;10(9):e1-e6.
21. Ong KL, Villarraga ML, Lau E, Carreon LY, Kurtz SM, Glassman SD. Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine. 2010;35(19):1794-1800.
22. Rihn JA, Patel R, Makda J, et al. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J. 2009;9(8):623-629.
23. Vaidya R, Sethi A, Bartol S, Jacobson M, Coe C, Craig JG. Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech. 2008;21(8):557-562.
24. Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J. 2008;8(6):1011-1018.
25. Delawi D, Dhert WJ, Rillardon L, et al. A prospective, randomized, controlled, multicenter study of osteogenic protein-1 in instrumented posterolateral fusions: report on safety and feasibility. Spine. 2010;35(12):1185-1191.
26. Vaccaro AR, Patel T, Fischgrund J, et al. A pilot study evaluating the safety and efficacy of OP-1 putty (rhBMP-7) as a replacement for iliac crest autograft in posterolateral lumbar arthrodesis for degenerative spondylolisthesis. Spine. 2004;29(17):1885-1892.
27. Vaidya R, Weir R, Sethi A, Meisterling S, Hakeos W, Wybo CD. Interbody fusion with allograft and rhBMP-2 leads to consistent fusion but early subsidence. J Bone Joint Surg Br. 2007;89(3):342-345.
28. Glassman SD, Howard J, Dimar J, Sweet A, Wilson G, Carreon L. Complications with recombinant human bone morphogenic protein-2 in posterolateral spine fusion: a consecutive series of 1037 cases. Spine. 2011;36(22):1849-1854.
29. Helgeson MD, Lehman RA Jr, Patzkowski JC, Dmitriev AE, Rosner MK, Mack AW. Adjacent vertebral body osteolysis with bone morphogenetic protein use in transforaminal lumbar interbody fusion. Spine J. 2011;11(6):507-510.
30. Hoffmann MF, Jones CB, Sietsema DL. Adjuncts in posterior lumbar spine fusion: comparison of complications and efficacy. Arch Orthop Trauma Surg. 2012;132(8):1105-1110.
31. Villavicencio AT, Burneikiene S, Nelson EL, Bulsara KR, Favors M, Thramann J. Safety of transforaminal lumbar interbody fusion and intervertebral recombinant human bone morphogenetic protein-2. J Neurosurg Spine. 2005;3(6):436-443.
32. Patel VV, Zhao L, Wong P, et al. Controlling bone morphogenetic protein diffusion and bone morphogenetic protein-stimulated bone growth using fibrin glue. Spine. 2006;31(11):1201-1206.
33. Zhang H, Sucato DJ, Welch RD. Recombinant human bone morphogenic protein-2-enhanced anterior spine fusion without bone encroachment into the spinal canal: a histomorphometric study in a thoracoscopically instrumented porcine model. Spine. 2005;30(5):512-518.
TAKE-HOME POINTS
- Use of rhBMP-2 in TLIF cages can result in HO out of the cage into the spinal canal.
- HO from rhBMP-2 in TLIF cages can result in a radiculopathy from compression or inflammatory reaction.
- HO out of the cage into the spinal canal resulting from use of rhBMP-2 in TLIF cages can be adequately diagnosed only with CT.
- HO can appear as a pedicle or pseudo-pedicle.
- Consider potential HO when using rhBMP-2 in TLIF cages.
Severity Weighting of Postoperative Adverse Events in Orthopedic Surgery
Take-Home Points
- Studies of AEs after orthopedic surgery commonly use composite AE outcomes.
- These types of outcomes treat AEs with different clinical significance similarly.
- This study created a single severity-weighted outcome that can be used to characterize the overall severity of a given patient’s postoperative course.
- Future studies may benefit from using this new severity-weighted outcome score.
Recently there has been an increase in the use of national databases for orthopedic surgery research.1-4 Studies commonly compare rates of postoperative adverse events (AEs) across different demographic, comorbidity, and procedural characteristics.5-23 Their conclusions often highlight different modifiable and/or nonmodifiable risk factors associated with the occurrence of postoperative events.
The several dozen AEs that have been investigated range from very severe (eg, death, myocardial infarction, coma) to less severe (eg, urinary tract infection [UTI], anemia requiring blood transfusion). A common approach for these studies is to consider many AEs together in the same analysis, asking a question such as, “What are risk factors for the occurrence of ‘adverse events’ after spine surgery?” Such studies test for associations with the occurrence of “any adverse event,” the occurrence of any “serious adverse event,” or similar composite outcomes. How common this type of study has become is indicated by the fact that in 2013 and 2014, at least 12 such studies were published in Clinical Orthopaedics and Related Research and the Journal of Bone and Joint Surgery,5-14,21-23 and many more in other orthopedic journals.15-20 However, there is a problem in using this type of composite outcome to perform such analyses: AEs with highly varying degrees of severity have identical impacts on the outcome variable, changing it from negative (“no adverse event”) to positive (“at least one adverse event”). As a result, the system may treat a very severe AE such as death and a very minor AE such as UTI similarly. Even in studies that use the slightly more specific composite outcome of “serious adverse events,” death and a nonlethal thromboembolic event would be treated similarly. Failure to differentiate these AEs in terms of their clinical significance detracts from the clinical applicability of conclusions drawn from studies using these types of composite AE outcomes.
In one of many examples that can be considered, a retrospective cohort study compared general and spinal anesthesia used in total knee arthroplasty.10 The rate of any AEs was higher with general anesthesia than with spinal anesthesia (12.34% vs 10.72%; P = .003). However, the only 2 specific AEs that had statistically significant differences were anemia requiring blood transfusion (6.07% vs 5.02%; P = .009) and superficial surgical-site infection (SSI; 0.92% vs 0.68%; P < .001). These 2 AEs are of relatively low severity; nevertheless, because these AEs are common, their differences constituted the majority of the difference in the rate of any AEs. In contrast, differences in the more severe AEs, such as death (0.11% vs 0.22%; P > .05), septic shock (0.14% vs 0.12%; P > .05), and myocardial infarction (0.20% vs 0.20%; P > .05), were small and not statistically significant. Had more weight been given to these more severe events, the outcome of the study likely would have been “no difference.”
To address this shortcoming in orthopedic research methodology, we created a severity-weighted outcome score that can be used to determine the overall “severity” of any given patient’s postoperative course. We also tested this novel outcome score for correlation with procedure type and patient characteristics using orthopedic patients from the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP). Our intention is for database investigators to be able to use this outcome score in place of the composite outcomes that are dominating this type of research.
Methods
Generation of Severity Weights
Our method is described generally as utility weighting, assigning value weights reflective of overall impact to differing outcome states.24 Parallel methods have been used to generate the disability weights used to determine disability-adjusted life years for the Global Burden of Disease project25 and many other areas of health, economic, and policy research.
All orthopedic faculty members at 2 geographically disparate, large US academic institutions were invited to participate in a severity-weighting exercise. Each surgeon who agreed to participate performed the exercise independently.
- STEP 1: Please reorder the AE cards by your perception of “severity” for a patient experiencing that event after an orthopedic procedure.
- STEP 2: Once your cards are in order, please determine how many postoperative occurrences of each event you would “trade” for 1 patient experiencing postoperative death. Place this number of occurrences in the box in the upper right corner of each card.
- NOTES: As you consider each AE:
- Please consider an “average” occurrence of that AE, but note that in no case does the AE result in perioperative death.
- Please consider only the “severity” for the patient. (Do not consider the extent to which the event may be related to surgical error.)
- Please consider that the numbers you assign are relative to each other. Hence, if you would trade 20 of “event A” for 1 death, and if you would trade 40 of “event B” for 1 death, the implication is that you would trade 20 of “event A” for 40 of “event B.”
- You may readjust the order of your cards at any point.
Participants’ responses were recorded. For each number provided by each participant, the inverse (reciprocal) was taken and multiplied by 100%. This new number was taken to be the percentage severity of death that the given participant considered the given AE to embody. For example, as a hypothetical on one end of the spectrum, if a participant reported 1 (he/she would trade 1 AE X for 1 death), then the severity would be 1/1 × 100% = 100% of death, a very severe AE. Conversely, if a participant reported a very large number like 100,000 (he/she would trade 100,000 AEs X for 1 death), then the severity would be 1/100,000 × 100% = 0.001% of death, a very minor AE. More commonly, a participant will report a number like 25, which would translate to 4% of death (1/25 × 100% = 4%). For each AE, weights were then averaged across participants to derive a mean severity weight to be used to generate a novel composite outcome score.
Definition of Novel Composite Outcome Score
The novel composite outcome score would be expressed as a percentage to be interpreted as percentage severity of death, which we termed severity-weighted outcome relative to death (SWORD). For each patient, SWORD was defined as no AE (0%) or postoperative death (100%), with other AEs assigned mean severity weights based on faculty members’ survey responses. A patient with multiple AEs would be assigned the weight for the more severe AE. This method was chosen over summing the AE weights because in many cases the AEs were thought to overlap; hence, summing would be inappropriate. For example, generally a deep SSI would result in a return to the operating room, and one would not want to double-count this AE. Similarly, it would not make sense for a patient who died of a complication to have a SWORD of >100%, which would be the summing result.
Application to ACS-NSQIP Patients
ACS-NSQIP is a surgical registry that prospectively identifies patients undergoing major surgery at any of >500 institutions nationwide.26,27 Patients are characterized at baseline and are followed for AEs over the first 30 postoperative days.
First, mean SWORD was calculated and reported for patients undergoing each of the 8 procedures. Analysis of variance (ANOVA) was used to test for associations of mean SWORD with type of procedure both before and after multivariate adjustment for demographics (sex; age in years, <40, 40-49, 50-59, 60-69, 70-79, 80-89, ≥90) and comorbidities (diabetes, hypertension, chronic obstructive pulmonary disease, exertional dyspnea, end-stage renal disease, congestive heart failure).
Second, patients undergoing the procedure with the highest mean SWORD (hip fracture surgery) were examined in depth. Among only these patients, multivariate ANOVA was used to test for associations of mean SWORD with the same demographics and comorbidities.
All statistical tests were 2-tailed. Significance was set at α = 0.05 (P < .05).
All 23 institution A faculty members (100%) and 24 (89%) of the 27 institution B faculty members completed the exercise.
In the ACS-NSQIP database, 85,109 patients were identified on the basis of the initial inclusion criteria.
Results
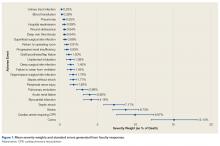
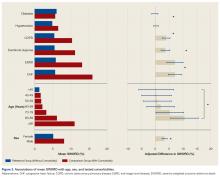
Figure 1 shows mean severity weights and standard errors generated from faculty responses. Mean (standard error) severity weight for UTI was 0.23% (0.08%); blood transfusion, 0.28% (0.09%); pneumonia, 0.55% (0.15%); hospital readmission, 0.59% (0.23%); wound dehiscence, 0.64% (0.17%); deep vein thrombosis, 0.64% (0.19%); superficial SSI, 0.68% (0.23%); return to operating room, 0.91% (0.29%); progressive renal insufficiency, 0.93% (0.27%); graft/prosthesis/flap failure, 1.20% (0.34%); unplanned intubation, 1.38% (0.53%); deep SSI, 1.45% (0.38%); failure to wean from ventilator, 1.45% (0.48%); organ/space SSI, 1.76% (0.46%); sepsis without shock, 1.77% (0.42%); peripheral nerve injury, 1.83% (0.47%); pulmonary embolism, 2.99% (0.76%); acute renal failure, 3.95% (0.85%); myocardial infarction, 4.16% (0.98%); septic shock, 7.17% (1.36%); stroke, 8.73% (1.74%); cardiac arrest requiring cardiopulmonary resuscitation, 9.97% (2.46%); and coma, 15.14% (3.04%).
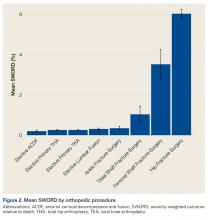
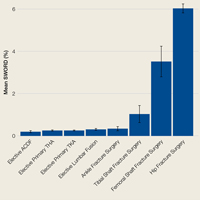
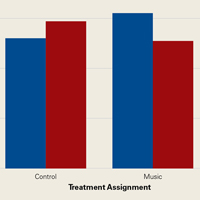
Among ACS-NSQIP patients, mean SWORD ranged from 0.2% (elective anterior cervical decompression and fusion) to 6.0% (hip fracture surgery) (Figure 2).
Discussion
The use of national databases in studies has become increasingly common in orthopedic surgery.1-4
The academic orthopedic surgeons who participated in our severity-weighting exercise thought the various AEs have markedly different severities. The least severe AE (UTI) was considered 0.23% as severe as postoperative death, with other events spanning the range up to 15.14% as severe as death. This wide range of severities demonstrates the problem with composite outcomes that implicitly consider all AEs similarly severe. Use of these markedly disparate weights in the development of SWORD enables this outcome to be more clinically applicable than outcomes such as “any adverse events.”
SWORD was highly associated with procedure type both before and after adjustment for demographics and comorbidities. Among patients undergoing the highest SWORD procedure (hip fracture surgery), SWORD was also associated with age, sex, and 4 of 6 tested comorbidities. Together, our findings show how SWORD is intended to be used in studies: to identify demographic, comorbidity, and procedural risk factors for an adverse postoperative course. We propose that researchers use our weighted outcome as their primary outcome—it is more meaningful than the simpler composite outcomes commonly used.
Outside orthopedic surgery, a small series of studies has addressed severity weighting of postoperative AEs.25,28-30 However, their approach was very different, as they were not designed to generate weights that could be transferred to future studies; rather, they simply compared severities of postoperative courses for patients within each individual study. In each study, a review of each original patient record was required, as the severity of each patient’s postoperative course was characterized according to the degree of any postoperative intervention—from no intervention to minor interventions such as placement of an intravenous catheter and major interventions such as endoscopic, radiologic, and surgical procedures. Only after the degree of intervention was defined could an outcome score be assigned to a given patient. However, databases do not depict the degree of intervention with nearly enough detail for this type of approach; they typically identify only occurrence or nonoccurrence of each event. Our work, which arose independently from this body of literature, enables an entirely different type of analysis. SWORD, which is not based on degree of intervention but on perceived severity of an “average” event, enables direct application of severity weights to large databases that store simple information on occurrence and nonoccurrence of specific AEs.
This study had several limitations. Most significantly, the generated severity weights were based on the surgeons’ subjective perceptions of severity, not on definitive assessments of the impacts of specific AEs on actual patients. We did not query the specialists who treat the complications or who present data on the costs and disabilities that may arise from these AEs. In addition, to develop our severity weighting scale, we queried faculty at only 2 institutions. A survey of surgeons throughout the United States would be more representative and would minimize selection bias. This is a potential research area. Another limitation is that scoring was subjective, based on surgeons’ perceptions of patients—in contrast to the Global Burden of Disease project, in which severity was based more objectively on epidemiologic data from >150 countries.
Orthopedic database research itself has often-noted limitations, including inability to sufficiently control for confounders, potential inaccuracies in data coding, limited follow-up, and lack of orthopedic-specific outcomes.1-4,31-33 However, this research also has much to offer, has increased tremendously over the past several years, and is expected to continue to expand. Many of the limitations of database studies cannot be entirely reversed. In providing a system for weighting postoperative AEs, our study fills a methodologic void. Future studies in orthopedics may benefit from using the severity-weighted outcome score presented here. Other fields with growth in database research may consider using similar methods to create severity-weighting systems of their own.
Am J Orthop. 2017;46(4):E235-E243. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680.
2. Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the Nationwide Inpatient Sample and National Surgical Quality Improvement Program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193.
3. Bohl DD, Grauer JN, Leopold SS. Editor’s spotlight/Take 5: Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1667-1671.
4. Levin PE. Apples, oranges, and national databases: commentary on an article by Daniel D. Bohl, MPH, et al.: “Variations in data collection methods between national databases affect study results: a comparison of the Nationwide Inpatient Sample and National Surgical Quality Improvement Program databases for lumbar spine fusion procedures.” J Bone Joint Surg Am. 2014;96(23):e198.
5. Duchman KR, Gao Y, Pugely AJ, Martin CT, Callaghan JJ. Differences in short-term complications between unicompartmental and total knee arthroplasty: a propensity score matched analysis. J Bone Joint Surg Am. 2014;96(16):1387-1394.
6. Edelstein AI, Lovecchio FC, Saha S, Hsu WK, Kim JY. Impact of resident involvement on orthopaedic surgery outcomes: an analysis of 30,628 patients from the American College of Surgeons National Surgical Quality Improvement Program database. J Bone Joint Surg Am. 2014;96(15):e131.
7. Belmont PJ Jr, Goodman GP, Waterman BR, Bader JO, Schoenfeld AJ. Thirty-day postoperative complications and mortality following total knee arthroplasty: incidence and risk factors among a national sample of 15,321 patients. J Bone Joint Surg Am. 2014;96(1):20-26.
8. Martin CT, Pugely AJ, Gao Y, Mendoza-Lattes S. Thirty-day morbidity after single-level anterior cervical discectomy and fusion: identification of risk factors and emphasis on the safety of outpatient procedures. J Bone Joint Surg Am. 2014;96(15):1288-1294.
9. Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the National Surgical Quality Improvement Program database. J Bone Joint Surg Am. 2013;95(14):e98 1-10.
10. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
11. Odum SM, Springer BD. In-hospital complication rates and associated factors after simultaneous bilateral versus unilateral total knee arthroplasty. J Bone Joint Surg Am. 2014;96(13):1058-1065.
12. Yoshihara H, Yoneoka D. Trends in the incidence and in-hospital outcomes of elective major orthopaedic surgery in patients eighty years of age and older in the United States from 2000 to 2009. J Bone Joint Surg Am. 2014;96(14):1185-1191.
13. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036.
14. Mednick RE, Alvi HM, Krishnan V, Lovecchio F, Manning DW. Factors affecting readmission rates following primary total hip arthroplasty. J Bone Joint Surg Am. 2014;96(14):1201-1209.
15. Pugely AJ, Martin CT, Gao Y, Ilgenfritz R, Weinstein SL. The incidence and risk factors for short-term morbidity and mortality in pediatric deformity spinal surgery: an analysis of the NSQIP pediatric database. Spine. 2014;39(15):1225-1234.
16. Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Resident involvement does not influence complication after total hip arthroplasty: an analysis of 13,109 cases. J Arthroplasty. 2014;29(10):1919-1924.
17. Belmont PJ Jr, Goodman GP, Hamilton W, Waterman BR, Bader JO, Schoenfeld AJ. Morbidity and mortality in the thirty-day period following total hip arthroplasty: risk factors and incidence. J Arthroplasty. 2014;29(10):2025-2030.
18. Bohl DD, Fu MC, Golinvaux NS, Basques BA, Gruskay JA, Grauer JN. The “July effect” in primary total hip and knee arthroplasty: analysis of 21,434 cases from the ACS-NSQIP database. J Arthroplasty. 2014;29(7):1332-1338.
19. Bohl DD, Fu MC, Gruskay JA, Basques BA, Golinvaux NS, Grauer JN. “July effect” in elective spine surgery: analysis of the American College of Surgeons National Surgical Quality Improvement Program database. Spine. 2014;39(7):603-611.
20. Babu R, Thomas S, Hazzard MA, et al. Morbidity, mortality, and health care costs for patients undergoing spine surgery following the ACGME resident duty-hour reform: clinical article. J Neurosurg Spine. 2014;21(4):502-515.
21. Lovecchio F, Beal M, Kwasny M, Manning D. Do patients with insulin-dependent and noninsulin-dependent diabetes have different risks for complications after arthroplasty? Clin Orthop Relat Res. 2014;472(11):3570-3575.
22. Pugely AJ, Gao Y, Martin CT, Callagh JJ, Weinstein SL, Marsh JL. The effect of resident participation on short-term outcomes after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(7):2290-2300.
23. Easterlin MC, Chang DG, Talamini M, Chang DC. Older age increases short-term surgical complications after primary knee arthroplasty. Clin Orthop Relat Res. 2013;471(8):2611-2620.
24. Morimoto T, Fukui T. Utilities measured by rating scale, time trade-off, and standard gamble: review and reference for health care professionals. J Epidemiology. 2002;12(2):160-178.
25. Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2129-2143.
26. American College of Surgeons National Surgical Quality Improvement Program. User Guide for the 2011 Participant Use Data File. https://www.facs.org/~/media/files/quality%20programs/nsqip/ug11.ashx. Published October 2012. Accessed December 1, 2013.
27. Molina CS, Thakore RV, Blumer A, Obremskey WT, Sethi MK. Use of the National Surgical Quality Improvement Program in orthopaedic surgery. Clin Orthop Relat Res. 2015;473(5):1574-1581.
28. Strasberg SM, Hall BL. Postoperative Morbidity Index: a quantitative measure of severity of postoperative complications. J Am Coll Surg. 2011;213(5):616-626.
29. Beilan J, Strakosha R, Palacios DA, Rosser CJ. The Postoperative Morbidity Index: a quantitative weighing of postoperative complications applied to urological procedures. BMC Urol. 2014;14:1.
30. Porembka MR, Hall BL, Hirbe M, Strasberg SM. Quantitative weighting of postoperative complications based on the Accordion Severity Grading System: demonstration of potential impact using the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(3):286-298.
31. Golinvaux NS, Bohl DD, Basques BA, Fu MC, Gardner EC, Grauer JN. Limitations of administrative databases in spine research: a study in obesity. Spine J. 2014;14(12):2923-2928.
32. Golinvaux NS, Bohl DD, Basques BA, Grauer JN. Administrative database concerns: accuracy of International Classification of Diseases, Ninth Revision coding is poor for preoperative anemia in patients undergoing spinal fusion. Spine. 2014;39(24):2019-2023.
33. Bekkers S, Bot AG, Makarawung D, Neuhaus V, Ring D. The National Hospital Discharge Survey and Nationwide Inpatient Sample: the databases used affect results in THA research. Clin Orthop Relat Res. 2014;472(11):3441-3449.
Take-Home Points
- Studies of AEs after orthopedic surgery commonly use composite AE outcomes.
- These types of outcomes treat AEs with different clinical significance similarly.
- This study created a single severity-weighted outcome that can be used to characterize the overall severity of a given patient’s postoperative course.
- Future studies may benefit from using this new severity-weighted outcome score.
Recently there has been an increase in the use of national databases for orthopedic surgery research.1-4 Studies commonly compare rates of postoperative adverse events (AEs) across different demographic, comorbidity, and procedural characteristics.5-23 Their conclusions often highlight different modifiable and/or nonmodifiable risk factors associated with the occurrence of postoperative events.
The several dozen AEs that have been investigated range from very severe (eg, death, myocardial infarction, coma) to less severe (eg, urinary tract infection [UTI], anemia requiring blood transfusion). A common approach for these studies is to consider many AEs together in the same analysis, asking a question such as, “What are risk factors for the occurrence of ‘adverse events’ after spine surgery?” Such studies test for associations with the occurrence of “any adverse event,” the occurrence of any “serious adverse event,” or similar composite outcomes. How common this type of study has become is indicated by the fact that in 2013 and 2014, at least 12 such studies were published in Clinical Orthopaedics and Related Research and the Journal of Bone and Joint Surgery,5-14,21-23 and many more in other orthopedic journals.15-20 However, there is a problem in using this type of composite outcome to perform such analyses: AEs with highly varying degrees of severity have identical impacts on the outcome variable, changing it from negative (“no adverse event”) to positive (“at least one adverse event”). As a result, the system may treat a very severe AE such as death and a very minor AE such as UTI similarly. Even in studies that use the slightly more specific composite outcome of “serious adverse events,” death and a nonlethal thromboembolic event would be treated similarly. Failure to differentiate these AEs in terms of their clinical significance detracts from the clinical applicability of conclusions drawn from studies using these types of composite AE outcomes.
In one of many examples that can be considered, a retrospective cohort study compared general and spinal anesthesia used in total knee arthroplasty.10 The rate of any AEs was higher with general anesthesia than with spinal anesthesia (12.34% vs 10.72%; P = .003). However, the only 2 specific AEs that had statistically significant differences were anemia requiring blood transfusion (6.07% vs 5.02%; P = .009) and superficial surgical-site infection (SSI; 0.92% vs 0.68%; P < .001). These 2 AEs are of relatively low severity; nevertheless, because these AEs are common, their differences constituted the majority of the difference in the rate of any AEs. In contrast, differences in the more severe AEs, such as death (0.11% vs 0.22%; P > .05), septic shock (0.14% vs 0.12%; P > .05), and myocardial infarction (0.20% vs 0.20%; P > .05), were small and not statistically significant. Had more weight been given to these more severe events, the outcome of the study likely would have been “no difference.”
To address this shortcoming in orthopedic research methodology, we created a severity-weighted outcome score that can be used to determine the overall “severity” of any given patient’s postoperative course. We also tested this novel outcome score for correlation with procedure type and patient characteristics using orthopedic patients from the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP). Our intention is for database investigators to be able to use this outcome score in place of the composite outcomes that are dominating this type of research.
Methods
Generation of Severity Weights
Our method is described generally as utility weighting, assigning value weights reflective of overall impact to differing outcome states.24 Parallel methods have been used to generate the disability weights used to determine disability-adjusted life years for the Global Burden of Disease project25 and many other areas of health, economic, and policy research.
All orthopedic faculty members at 2 geographically disparate, large US academic institutions were invited to participate in a severity-weighting exercise. Each surgeon who agreed to participate performed the exercise independently.
- STEP 1: Please reorder the AE cards by your perception of “severity” for a patient experiencing that event after an orthopedic procedure.
- STEP 2: Once your cards are in order, please determine how many postoperative occurrences of each event you would “trade” for 1 patient experiencing postoperative death. Place this number of occurrences in the box in the upper right corner of each card.
- NOTES: As you consider each AE:
- Please consider an “average” occurrence of that AE, but note that in no case does the AE result in perioperative death.
- Please consider only the “severity” for the patient. (Do not consider the extent to which the event may be related to surgical error.)
- Please consider that the numbers you assign are relative to each other. Hence, if you would trade 20 of “event A” for 1 death, and if you would trade 40 of “event B” for 1 death, the implication is that you would trade 20 of “event A” for 40 of “event B.”
- You may readjust the order of your cards at any point.
Participants’ responses were recorded. For each number provided by each participant, the inverse (reciprocal) was taken and multiplied by 100%. This new number was taken to be the percentage severity of death that the given participant considered the given AE to embody. For example, as a hypothetical on one end of the spectrum, if a participant reported 1 (he/she would trade 1 AE X for 1 death), then the severity would be 1/1 × 100% = 100% of death, a very severe AE. Conversely, if a participant reported a very large number like 100,000 (he/she would trade 100,000 AEs X for 1 death), then the severity would be 1/100,000 × 100% = 0.001% of death, a very minor AE. More commonly, a participant will report a number like 25, which would translate to 4% of death (1/25 × 100% = 4%). For each AE, weights were then averaged across participants to derive a mean severity weight to be used to generate a novel composite outcome score.
Definition of Novel Composite Outcome Score
The novel composite outcome score would be expressed as a percentage to be interpreted as percentage severity of death, which we termed severity-weighted outcome relative to death (SWORD). For each patient, SWORD was defined as no AE (0%) or postoperative death (100%), with other AEs assigned mean severity weights based on faculty members’ survey responses. A patient with multiple AEs would be assigned the weight for the more severe AE. This method was chosen over summing the AE weights because in many cases the AEs were thought to overlap; hence, summing would be inappropriate. For example, generally a deep SSI would result in a return to the operating room, and one would not want to double-count this AE. Similarly, it would not make sense for a patient who died of a complication to have a SWORD of >100%, which would be the summing result.
Application to ACS-NSQIP Patients
ACS-NSQIP is a surgical registry that prospectively identifies patients undergoing major surgery at any of >500 institutions nationwide.26,27 Patients are characterized at baseline and are followed for AEs over the first 30 postoperative days.
First, mean SWORD was calculated and reported for patients undergoing each of the 8 procedures. Analysis of variance (ANOVA) was used to test for associations of mean SWORD with type of procedure both before and after multivariate adjustment for demographics (sex; age in years, <40, 40-49, 50-59, 60-69, 70-79, 80-89, ≥90) and comorbidities (diabetes, hypertension, chronic obstructive pulmonary disease, exertional dyspnea, end-stage renal disease, congestive heart failure).
Second, patients undergoing the procedure with the highest mean SWORD (hip fracture surgery) were examined in depth. Among only these patients, multivariate ANOVA was used to test for associations of mean SWORD with the same demographics and comorbidities.
All statistical tests were 2-tailed. Significance was set at α = 0.05 (P < .05).
All 23 institution A faculty members (100%) and 24 (89%) of the 27 institution B faculty members completed the exercise.
In the ACS-NSQIP database, 85,109 patients were identified on the basis of the initial inclusion criteria.
Results
Figure 1 shows mean severity weights and standard errors generated from faculty responses. Mean (standard error) severity weight for UTI was 0.23% (0.08%); blood transfusion, 0.28% (0.09%); pneumonia, 0.55% (0.15%); hospital readmission, 0.59% (0.23%); wound dehiscence, 0.64% (0.17%); deep vein thrombosis, 0.64% (0.19%); superficial SSI, 0.68% (0.23%); return to operating room, 0.91% (0.29%); progressive renal insufficiency, 0.93% (0.27%); graft/prosthesis/flap failure, 1.20% (0.34%); unplanned intubation, 1.38% (0.53%); deep SSI, 1.45% (0.38%); failure to wean from ventilator, 1.45% (0.48%); organ/space SSI, 1.76% (0.46%); sepsis without shock, 1.77% (0.42%); peripheral nerve injury, 1.83% (0.47%); pulmonary embolism, 2.99% (0.76%); acute renal failure, 3.95% (0.85%); myocardial infarction, 4.16% (0.98%); septic shock, 7.17% (1.36%); stroke, 8.73% (1.74%); cardiac arrest requiring cardiopulmonary resuscitation, 9.97% (2.46%); and coma, 15.14% (3.04%).
Among ACS-NSQIP patients, mean SWORD ranged from 0.2% (elective anterior cervical decompression and fusion) to 6.0% (hip fracture surgery) (Figure 2).
Discussion
The use of national databases in studies has become increasingly common in orthopedic surgery.1-4
The academic orthopedic surgeons who participated in our severity-weighting exercise thought the various AEs have markedly different severities. The least severe AE (UTI) was considered 0.23% as severe as postoperative death, with other events spanning the range up to 15.14% as severe as death. This wide range of severities demonstrates the problem with composite outcomes that implicitly consider all AEs similarly severe. Use of these markedly disparate weights in the development of SWORD enables this outcome to be more clinically applicable than outcomes such as “any adverse events.”
SWORD was highly associated with procedure type both before and after adjustment for demographics and comorbidities. Among patients undergoing the highest SWORD procedure (hip fracture surgery), SWORD was also associated with age, sex, and 4 of 6 tested comorbidities. Together, our findings show how SWORD is intended to be used in studies: to identify demographic, comorbidity, and procedural risk factors for an adverse postoperative course. We propose that researchers use our weighted outcome as their primary outcome—it is more meaningful than the simpler composite outcomes commonly used.
Outside orthopedic surgery, a small series of studies has addressed severity weighting of postoperative AEs.25,28-30 However, their approach was very different, as they were not designed to generate weights that could be transferred to future studies; rather, they simply compared severities of postoperative courses for patients within each individual study. In each study, a review of each original patient record was required, as the severity of each patient’s postoperative course was characterized according to the degree of any postoperative intervention—from no intervention to minor interventions such as placement of an intravenous catheter and major interventions such as endoscopic, radiologic, and surgical procedures. Only after the degree of intervention was defined could an outcome score be assigned to a given patient. However, databases do not depict the degree of intervention with nearly enough detail for this type of approach; they typically identify only occurrence or nonoccurrence of each event. Our work, which arose independently from this body of literature, enables an entirely different type of analysis. SWORD, which is not based on degree of intervention but on perceived severity of an “average” event, enables direct application of severity weights to large databases that store simple information on occurrence and nonoccurrence of specific AEs.
This study had several limitations. Most significantly, the generated severity weights were based on the surgeons’ subjective perceptions of severity, not on definitive assessments of the impacts of specific AEs on actual patients. We did not query the specialists who treat the complications or who present data on the costs and disabilities that may arise from these AEs. In addition, to develop our severity weighting scale, we queried faculty at only 2 institutions. A survey of surgeons throughout the United States would be more representative and would minimize selection bias. This is a potential research area. Another limitation is that scoring was subjective, based on surgeons’ perceptions of patients—in contrast to the Global Burden of Disease project, in which severity was based more objectively on epidemiologic data from >150 countries.
Orthopedic database research itself has often-noted limitations, including inability to sufficiently control for confounders, potential inaccuracies in data coding, limited follow-up, and lack of orthopedic-specific outcomes.1-4,31-33 However, this research also has much to offer, has increased tremendously over the past several years, and is expected to continue to expand. Many of the limitations of database studies cannot be entirely reversed. In providing a system for weighting postoperative AEs, our study fills a methodologic void. Future studies in orthopedics may benefit from using the severity-weighted outcome score presented here. Other fields with growth in database research may consider using similar methods to create severity-weighting systems of their own.
Am J Orthop. 2017;46(4):E235-E243. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Studies of AEs after orthopedic surgery commonly use composite AE outcomes.
- These types of outcomes treat AEs with different clinical significance similarly.
- This study created a single severity-weighted outcome that can be used to characterize the overall severity of a given patient’s postoperative course.
- Future studies may benefit from using this new severity-weighted outcome score.
Recently there has been an increase in the use of national databases for orthopedic surgery research.1-4 Studies commonly compare rates of postoperative adverse events (AEs) across different demographic, comorbidity, and procedural characteristics.5-23 Their conclusions often highlight different modifiable and/or nonmodifiable risk factors associated with the occurrence of postoperative events.
The several dozen AEs that have been investigated range from very severe (eg, death, myocardial infarction, coma) to less severe (eg, urinary tract infection [UTI], anemia requiring blood transfusion). A common approach for these studies is to consider many AEs together in the same analysis, asking a question such as, “What are risk factors for the occurrence of ‘adverse events’ after spine surgery?” Such studies test for associations with the occurrence of “any adverse event,” the occurrence of any “serious adverse event,” or similar composite outcomes. How common this type of study has become is indicated by the fact that in 2013 and 2014, at least 12 such studies were published in Clinical Orthopaedics and Related Research and the Journal of Bone and Joint Surgery,5-14,21-23 and many more in other orthopedic journals.15-20 However, there is a problem in using this type of composite outcome to perform such analyses: AEs with highly varying degrees of severity have identical impacts on the outcome variable, changing it from negative (“no adverse event”) to positive (“at least one adverse event”). As a result, the system may treat a very severe AE such as death and a very minor AE such as UTI similarly. Even in studies that use the slightly more specific composite outcome of “serious adverse events,” death and a nonlethal thromboembolic event would be treated similarly. Failure to differentiate these AEs in terms of their clinical significance detracts from the clinical applicability of conclusions drawn from studies using these types of composite AE outcomes.
In one of many examples that can be considered, a retrospective cohort study compared general and spinal anesthesia used in total knee arthroplasty.10 The rate of any AEs was higher with general anesthesia than with spinal anesthesia (12.34% vs 10.72%; P = .003). However, the only 2 specific AEs that had statistically significant differences were anemia requiring blood transfusion (6.07% vs 5.02%; P = .009) and superficial surgical-site infection (SSI; 0.92% vs 0.68%; P < .001). These 2 AEs are of relatively low severity; nevertheless, because these AEs are common, their differences constituted the majority of the difference in the rate of any AEs. In contrast, differences in the more severe AEs, such as death (0.11% vs 0.22%; P > .05), septic shock (0.14% vs 0.12%; P > .05), and myocardial infarction (0.20% vs 0.20%; P > .05), were small and not statistically significant. Had more weight been given to these more severe events, the outcome of the study likely would have been “no difference.”
To address this shortcoming in orthopedic research methodology, we created a severity-weighted outcome score that can be used to determine the overall “severity” of any given patient’s postoperative course. We also tested this novel outcome score for correlation with procedure type and patient characteristics using orthopedic patients from the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP). Our intention is for database investigators to be able to use this outcome score in place of the composite outcomes that are dominating this type of research.
Methods
Generation of Severity Weights
Our method is described generally as utility weighting, assigning value weights reflective of overall impact to differing outcome states.24 Parallel methods have been used to generate the disability weights used to determine disability-adjusted life years for the Global Burden of Disease project25 and many other areas of health, economic, and policy research.
All orthopedic faculty members at 2 geographically disparate, large US academic institutions were invited to participate in a severity-weighting exercise. Each surgeon who agreed to participate performed the exercise independently.
- STEP 1: Please reorder the AE cards by your perception of “severity” for a patient experiencing that event after an orthopedic procedure.
- STEP 2: Once your cards are in order, please determine how many postoperative occurrences of each event you would “trade” for 1 patient experiencing postoperative death. Place this number of occurrences in the box in the upper right corner of each card.
- NOTES: As you consider each AE:
- Please consider an “average” occurrence of that AE, but note that in no case does the AE result in perioperative death.
- Please consider only the “severity” for the patient. (Do not consider the extent to which the event may be related to surgical error.)
- Please consider that the numbers you assign are relative to each other. Hence, if you would trade 20 of “event A” for 1 death, and if you would trade 40 of “event B” for 1 death, the implication is that you would trade 20 of “event A” for 40 of “event B.”
- You may readjust the order of your cards at any point.
Participants’ responses were recorded. For each number provided by each participant, the inverse (reciprocal) was taken and multiplied by 100%. This new number was taken to be the percentage severity of death that the given participant considered the given AE to embody. For example, as a hypothetical on one end of the spectrum, if a participant reported 1 (he/she would trade 1 AE X for 1 death), then the severity would be 1/1 × 100% = 100% of death, a very severe AE. Conversely, if a participant reported a very large number like 100,000 (he/she would trade 100,000 AEs X for 1 death), then the severity would be 1/100,000 × 100% = 0.001% of death, a very minor AE. More commonly, a participant will report a number like 25, which would translate to 4% of death (1/25 × 100% = 4%). For each AE, weights were then averaged across participants to derive a mean severity weight to be used to generate a novel composite outcome score.
Definition of Novel Composite Outcome Score
The novel composite outcome score would be expressed as a percentage to be interpreted as percentage severity of death, which we termed severity-weighted outcome relative to death (SWORD). For each patient, SWORD was defined as no AE (0%) or postoperative death (100%), with other AEs assigned mean severity weights based on faculty members’ survey responses. A patient with multiple AEs would be assigned the weight for the more severe AE. This method was chosen over summing the AE weights because in many cases the AEs were thought to overlap; hence, summing would be inappropriate. For example, generally a deep SSI would result in a return to the operating room, and one would not want to double-count this AE. Similarly, it would not make sense for a patient who died of a complication to have a SWORD of >100%, which would be the summing result.
Application to ACS-NSQIP Patients
ACS-NSQIP is a surgical registry that prospectively identifies patients undergoing major surgery at any of >500 institutions nationwide.26,27 Patients are characterized at baseline and are followed for AEs over the first 30 postoperative days.
First, mean SWORD was calculated and reported for patients undergoing each of the 8 procedures. Analysis of variance (ANOVA) was used to test for associations of mean SWORD with type of procedure both before and after multivariate adjustment for demographics (sex; age in years, <40, 40-49, 50-59, 60-69, 70-79, 80-89, ≥90) and comorbidities (diabetes, hypertension, chronic obstructive pulmonary disease, exertional dyspnea, end-stage renal disease, congestive heart failure).
Second, patients undergoing the procedure with the highest mean SWORD (hip fracture surgery) were examined in depth. Among only these patients, multivariate ANOVA was used to test for associations of mean SWORD with the same demographics and comorbidities.
All statistical tests were 2-tailed. Significance was set at α = 0.05 (P < .05).
All 23 institution A faculty members (100%) and 24 (89%) of the 27 institution B faculty members completed the exercise.
In the ACS-NSQIP database, 85,109 patients were identified on the basis of the initial inclusion criteria.
Results
Figure 1 shows mean severity weights and standard errors generated from faculty responses. Mean (standard error) severity weight for UTI was 0.23% (0.08%); blood transfusion, 0.28% (0.09%); pneumonia, 0.55% (0.15%); hospital readmission, 0.59% (0.23%); wound dehiscence, 0.64% (0.17%); deep vein thrombosis, 0.64% (0.19%); superficial SSI, 0.68% (0.23%); return to operating room, 0.91% (0.29%); progressive renal insufficiency, 0.93% (0.27%); graft/prosthesis/flap failure, 1.20% (0.34%); unplanned intubation, 1.38% (0.53%); deep SSI, 1.45% (0.38%); failure to wean from ventilator, 1.45% (0.48%); organ/space SSI, 1.76% (0.46%); sepsis without shock, 1.77% (0.42%); peripheral nerve injury, 1.83% (0.47%); pulmonary embolism, 2.99% (0.76%); acute renal failure, 3.95% (0.85%); myocardial infarction, 4.16% (0.98%); septic shock, 7.17% (1.36%); stroke, 8.73% (1.74%); cardiac arrest requiring cardiopulmonary resuscitation, 9.97% (2.46%); and coma, 15.14% (3.04%).
Among ACS-NSQIP patients, mean SWORD ranged from 0.2% (elective anterior cervical decompression and fusion) to 6.0% (hip fracture surgery) (Figure 2).
Discussion
The use of national databases in studies has become increasingly common in orthopedic surgery.1-4
The academic orthopedic surgeons who participated in our severity-weighting exercise thought the various AEs have markedly different severities. The least severe AE (UTI) was considered 0.23% as severe as postoperative death, with other events spanning the range up to 15.14% as severe as death. This wide range of severities demonstrates the problem with composite outcomes that implicitly consider all AEs similarly severe. Use of these markedly disparate weights in the development of SWORD enables this outcome to be more clinically applicable than outcomes such as “any adverse events.”
SWORD was highly associated with procedure type both before and after adjustment for demographics and comorbidities. Among patients undergoing the highest SWORD procedure (hip fracture surgery), SWORD was also associated with age, sex, and 4 of 6 tested comorbidities. Together, our findings show how SWORD is intended to be used in studies: to identify demographic, comorbidity, and procedural risk factors for an adverse postoperative course. We propose that researchers use our weighted outcome as their primary outcome—it is more meaningful than the simpler composite outcomes commonly used.
Outside orthopedic surgery, a small series of studies has addressed severity weighting of postoperative AEs.25,28-30 However, their approach was very different, as they were not designed to generate weights that could be transferred to future studies; rather, they simply compared severities of postoperative courses for patients within each individual study. In each study, a review of each original patient record was required, as the severity of each patient’s postoperative course was characterized according to the degree of any postoperative intervention—from no intervention to minor interventions such as placement of an intravenous catheter and major interventions such as endoscopic, radiologic, and surgical procedures. Only after the degree of intervention was defined could an outcome score be assigned to a given patient. However, databases do not depict the degree of intervention with nearly enough detail for this type of approach; they typically identify only occurrence or nonoccurrence of each event. Our work, which arose independently from this body of literature, enables an entirely different type of analysis. SWORD, which is not based on degree of intervention but on perceived severity of an “average” event, enables direct application of severity weights to large databases that store simple information on occurrence and nonoccurrence of specific AEs.
This study had several limitations. Most significantly, the generated severity weights were based on the surgeons’ subjective perceptions of severity, not on definitive assessments of the impacts of specific AEs on actual patients. We did not query the specialists who treat the complications or who present data on the costs and disabilities that may arise from these AEs. In addition, to develop our severity weighting scale, we queried faculty at only 2 institutions. A survey of surgeons throughout the United States would be more representative and would minimize selection bias. This is a potential research area. Another limitation is that scoring was subjective, based on surgeons’ perceptions of patients—in contrast to the Global Burden of Disease project, in which severity was based more objectively on epidemiologic data from >150 countries.
Orthopedic database research itself has often-noted limitations, including inability to sufficiently control for confounders, potential inaccuracies in data coding, limited follow-up, and lack of orthopedic-specific outcomes.1-4,31-33 However, this research also has much to offer, has increased tremendously over the past several years, and is expected to continue to expand. Many of the limitations of database studies cannot be entirely reversed. In providing a system for weighting postoperative AEs, our study fills a methodologic void. Future studies in orthopedics may benefit from using the severity-weighted outcome score presented here. Other fields with growth in database research may consider using similar methods to create severity-weighting systems of their own.
Am J Orthop. 2017;46(4):E235-E243. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680.
2. Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the Nationwide Inpatient Sample and National Surgical Quality Improvement Program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193.
3. Bohl DD, Grauer JN, Leopold SS. Editor’s spotlight/Take 5: Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1667-1671.
4. Levin PE. Apples, oranges, and national databases: commentary on an article by Daniel D. Bohl, MPH, et al.: “Variations in data collection methods between national databases affect study results: a comparison of the Nationwide Inpatient Sample and National Surgical Quality Improvement Program databases for lumbar spine fusion procedures.” J Bone Joint Surg Am. 2014;96(23):e198.
5. Duchman KR, Gao Y, Pugely AJ, Martin CT, Callaghan JJ. Differences in short-term complications between unicompartmental and total knee arthroplasty: a propensity score matched analysis. J Bone Joint Surg Am. 2014;96(16):1387-1394.
6. Edelstein AI, Lovecchio FC, Saha S, Hsu WK, Kim JY. Impact of resident involvement on orthopaedic surgery outcomes: an analysis of 30,628 patients from the American College of Surgeons National Surgical Quality Improvement Program database. J Bone Joint Surg Am. 2014;96(15):e131.
7. Belmont PJ Jr, Goodman GP, Waterman BR, Bader JO, Schoenfeld AJ. Thirty-day postoperative complications and mortality following total knee arthroplasty: incidence and risk factors among a national sample of 15,321 patients. J Bone Joint Surg Am. 2014;96(1):20-26.
8. Martin CT, Pugely AJ, Gao Y, Mendoza-Lattes S. Thirty-day morbidity after single-level anterior cervical discectomy and fusion: identification of risk factors and emphasis on the safety of outpatient procedures. J Bone Joint Surg Am. 2014;96(15):1288-1294.
9. Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the National Surgical Quality Improvement Program database. J Bone Joint Surg Am. 2013;95(14):e98 1-10.
10. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
11. Odum SM, Springer BD. In-hospital complication rates and associated factors after simultaneous bilateral versus unilateral total knee arthroplasty. J Bone Joint Surg Am. 2014;96(13):1058-1065.
12. Yoshihara H, Yoneoka D. Trends in the incidence and in-hospital outcomes of elective major orthopaedic surgery in patients eighty years of age and older in the United States from 2000 to 2009. J Bone Joint Surg Am. 2014;96(14):1185-1191.
13. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036.
14. Mednick RE, Alvi HM, Krishnan V, Lovecchio F, Manning DW. Factors affecting readmission rates following primary total hip arthroplasty. J Bone Joint Surg Am. 2014;96(14):1201-1209.
15. Pugely AJ, Martin CT, Gao Y, Ilgenfritz R, Weinstein SL. The incidence and risk factors for short-term morbidity and mortality in pediatric deformity spinal surgery: an analysis of the NSQIP pediatric database. Spine. 2014;39(15):1225-1234.
16. Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Resident involvement does not influence complication after total hip arthroplasty: an analysis of 13,109 cases. J Arthroplasty. 2014;29(10):1919-1924.
17. Belmont PJ Jr, Goodman GP, Hamilton W, Waterman BR, Bader JO, Schoenfeld AJ. Morbidity and mortality in the thirty-day period following total hip arthroplasty: risk factors and incidence. J Arthroplasty. 2014;29(10):2025-2030.
18. Bohl DD, Fu MC, Golinvaux NS, Basques BA, Gruskay JA, Grauer JN. The “July effect” in primary total hip and knee arthroplasty: analysis of 21,434 cases from the ACS-NSQIP database. J Arthroplasty. 2014;29(7):1332-1338.
19. Bohl DD, Fu MC, Gruskay JA, Basques BA, Golinvaux NS, Grauer JN. “July effect” in elective spine surgery: analysis of the American College of Surgeons National Surgical Quality Improvement Program database. Spine. 2014;39(7):603-611.
20. Babu R, Thomas S, Hazzard MA, et al. Morbidity, mortality, and health care costs for patients undergoing spine surgery following the ACGME resident duty-hour reform: clinical article. J Neurosurg Spine. 2014;21(4):502-515.
21. Lovecchio F, Beal M, Kwasny M, Manning D. Do patients with insulin-dependent and noninsulin-dependent diabetes have different risks for complications after arthroplasty? Clin Orthop Relat Res. 2014;472(11):3570-3575.
22. Pugely AJ, Gao Y, Martin CT, Callagh JJ, Weinstein SL, Marsh JL. The effect of resident participation on short-term outcomes after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(7):2290-2300.
23. Easterlin MC, Chang DG, Talamini M, Chang DC. Older age increases short-term surgical complications after primary knee arthroplasty. Clin Orthop Relat Res. 2013;471(8):2611-2620.
24. Morimoto T, Fukui T. Utilities measured by rating scale, time trade-off, and standard gamble: review and reference for health care professionals. J Epidemiology. 2002;12(2):160-178.
25. Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2129-2143.
26. American College of Surgeons National Surgical Quality Improvement Program. User Guide for the 2011 Participant Use Data File. https://www.facs.org/~/media/files/quality%20programs/nsqip/ug11.ashx. Published October 2012. Accessed December 1, 2013.
27. Molina CS, Thakore RV, Blumer A, Obremskey WT, Sethi MK. Use of the National Surgical Quality Improvement Program in orthopaedic surgery. Clin Orthop Relat Res. 2015;473(5):1574-1581.
28. Strasberg SM, Hall BL. Postoperative Morbidity Index: a quantitative measure of severity of postoperative complications. J Am Coll Surg. 2011;213(5):616-626.
29. Beilan J, Strakosha R, Palacios DA, Rosser CJ. The Postoperative Morbidity Index: a quantitative weighing of postoperative complications applied to urological procedures. BMC Urol. 2014;14:1.
30. Porembka MR, Hall BL, Hirbe M, Strasberg SM. Quantitative weighting of postoperative complications based on the Accordion Severity Grading System: demonstration of potential impact using the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(3):286-298.
31. Golinvaux NS, Bohl DD, Basques BA, Fu MC, Gardner EC, Grauer JN. Limitations of administrative databases in spine research: a study in obesity. Spine J. 2014;14(12):2923-2928.
32. Golinvaux NS, Bohl DD, Basques BA, Grauer JN. Administrative database concerns: accuracy of International Classification of Diseases, Ninth Revision coding is poor for preoperative anemia in patients undergoing spinal fusion. Spine. 2014;39(24):2019-2023.
33. Bekkers S, Bot AG, Makarawung D, Neuhaus V, Ring D. The National Hospital Discharge Survey and Nationwide Inpatient Sample: the databases used affect results in THA research. Clin Orthop Relat Res. 2014;472(11):3441-3449.
1. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680.
2. Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the Nationwide Inpatient Sample and National Surgical Quality Improvement Program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193.
3. Bohl DD, Grauer JN, Leopold SS. Editor’s spotlight/Take 5: Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1667-1671.
4. Levin PE. Apples, oranges, and national databases: commentary on an article by Daniel D. Bohl, MPH, et al.: “Variations in data collection methods between national databases affect study results: a comparison of the Nationwide Inpatient Sample and National Surgical Quality Improvement Program databases for lumbar spine fusion procedures.” J Bone Joint Surg Am. 2014;96(23):e198.
5. Duchman KR, Gao Y, Pugely AJ, Martin CT, Callaghan JJ. Differences in short-term complications between unicompartmental and total knee arthroplasty: a propensity score matched analysis. J Bone Joint Surg Am. 2014;96(16):1387-1394.
6. Edelstein AI, Lovecchio FC, Saha S, Hsu WK, Kim JY. Impact of resident involvement on orthopaedic surgery outcomes: an analysis of 30,628 patients from the American College of Surgeons National Surgical Quality Improvement Program database. J Bone Joint Surg Am. 2014;96(15):e131.
7. Belmont PJ Jr, Goodman GP, Waterman BR, Bader JO, Schoenfeld AJ. Thirty-day postoperative complications and mortality following total knee arthroplasty: incidence and risk factors among a national sample of 15,321 patients. J Bone Joint Surg Am. 2014;96(1):20-26.
8. Martin CT, Pugely AJ, Gao Y, Mendoza-Lattes S. Thirty-day morbidity after single-level anterior cervical discectomy and fusion: identification of risk factors and emphasis on the safety of outpatient procedures. J Bone Joint Surg Am. 2014;96(15):1288-1294.
9. Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the National Surgical Quality Improvement Program database. J Bone Joint Surg Am. 2013;95(14):e98 1-10.
10. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
11. Odum SM, Springer BD. In-hospital complication rates and associated factors after simultaneous bilateral versus unilateral total knee arthroplasty. J Bone Joint Surg Am. 2014;96(13):1058-1065.
12. Yoshihara H, Yoneoka D. Trends in the incidence and in-hospital outcomes of elective major orthopaedic surgery in patients eighty years of age and older in the United States from 2000 to 2009. J Bone Joint Surg Am. 2014;96(14):1185-1191.
13. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036.
14. Mednick RE, Alvi HM, Krishnan V, Lovecchio F, Manning DW. Factors affecting readmission rates following primary total hip arthroplasty. J Bone Joint Surg Am. 2014;96(14):1201-1209.
15. Pugely AJ, Martin CT, Gao Y, Ilgenfritz R, Weinstein SL. The incidence and risk factors for short-term morbidity and mortality in pediatric deformity spinal surgery: an analysis of the NSQIP pediatric database. Spine. 2014;39(15):1225-1234.
16. Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Resident involvement does not influence complication after total hip arthroplasty: an analysis of 13,109 cases. J Arthroplasty. 2014;29(10):1919-1924.
17. Belmont PJ Jr, Goodman GP, Hamilton W, Waterman BR, Bader JO, Schoenfeld AJ. Morbidity and mortality in the thirty-day period following total hip arthroplasty: risk factors and incidence. J Arthroplasty. 2014;29(10):2025-2030.
18. Bohl DD, Fu MC, Golinvaux NS, Basques BA, Gruskay JA, Grauer JN. The “July effect” in primary total hip and knee arthroplasty: analysis of 21,434 cases from the ACS-NSQIP database. J Arthroplasty. 2014;29(7):1332-1338.
19. Bohl DD, Fu MC, Gruskay JA, Basques BA, Golinvaux NS, Grauer JN. “July effect” in elective spine surgery: analysis of the American College of Surgeons National Surgical Quality Improvement Program database. Spine. 2014;39(7):603-611.
20. Babu R, Thomas S, Hazzard MA, et al. Morbidity, mortality, and health care costs for patients undergoing spine surgery following the ACGME resident duty-hour reform: clinical article. J Neurosurg Spine. 2014;21(4):502-515.
21. Lovecchio F, Beal M, Kwasny M, Manning D. Do patients with insulin-dependent and noninsulin-dependent diabetes have different risks for complications after arthroplasty? Clin Orthop Relat Res. 2014;472(11):3570-3575.
22. Pugely AJ, Gao Y, Martin CT, Callagh JJ, Weinstein SL, Marsh JL. The effect of resident participation on short-term outcomes after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(7):2290-2300.
23. Easterlin MC, Chang DG, Talamini M, Chang DC. Older age increases short-term surgical complications after primary knee arthroplasty. Clin Orthop Relat Res. 2013;471(8):2611-2620.
24. Morimoto T, Fukui T. Utilities measured by rating scale, time trade-off, and standard gamble: review and reference for health care professionals. J Epidemiology. 2002;12(2):160-178.
25. Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2129-2143.
26. American College of Surgeons National Surgical Quality Improvement Program. User Guide for the 2011 Participant Use Data File. https://www.facs.org/~/media/files/quality%20programs/nsqip/ug11.ashx. Published October 2012. Accessed December 1, 2013.
27. Molina CS, Thakore RV, Blumer A, Obremskey WT, Sethi MK. Use of the National Surgical Quality Improvement Program in orthopaedic surgery. Clin Orthop Relat Res. 2015;473(5):1574-1581.
28. Strasberg SM, Hall BL. Postoperative Morbidity Index: a quantitative measure of severity of postoperative complications. J Am Coll Surg. 2011;213(5):616-626.
29. Beilan J, Strakosha R, Palacios DA, Rosser CJ. The Postoperative Morbidity Index: a quantitative weighing of postoperative complications applied to urological procedures. BMC Urol. 2014;14:1.
30. Porembka MR, Hall BL, Hirbe M, Strasberg SM. Quantitative weighting of postoperative complications based on the Accordion Severity Grading System: demonstration of potential impact using the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(3):286-298.
31. Golinvaux NS, Bohl DD, Basques BA, Fu MC, Gardner EC, Grauer JN. Limitations of administrative databases in spine research: a study in obesity. Spine J. 2014;14(12):2923-2928.
32. Golinvaux NS, Bohl DD, Basques BA, Grauer JN. Administrative database concerns: accuracy of International Classification of Diseases, Ninth Revision coding is poor for preoperative anemia in patients undergoing spinal fusion. Spine. 2014;39(24):2019-2023.
33. Bekkers S, Bot AG, Makarawung D, Neuhaus V, Ring D. The National Hospital Discharge Survey and Nationwide Inpatient Sample: the databases used affect results in THA research. Clin Orthop Relat Res. 2014;472(11):3441-3449.
For bone and joint infections, oral antibiotics match IV, cost less
VIENNA – Oral antibiotic therapy is just as effective as intravenous treatment in curing bone and joint infections, but costs about $3,500 less.
Treating these infections with oral agents also “improves patient autonomy, as it’s not necessary to have IV lines at home,” and represents a generally wiser use of powerful antibiotics, Matthew Scarborough, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
OVIVA (Oral vs. Intravenous Antibiotics for Bone and Joint Infection) was conducted at 26 sites in the United Kingdom. It randomized 1,054 adults with bone or joint infections to 6 weeks of either oral or intravenous treatment.
An important aspect of the trial was that both oral and IV treatment choices were made before randomization, Dr. Scarborough said. However, the decisions on what drug to use were left up to the treating physician and depended on the infection site and pathogen.
The primary outcome was definite treatment failure (bacteriologic, histologic, and clinical). Patients were followed for 1 year.
Patients were a median of 60 years old. All had surgical treatment before antibiotic therapy, including debridement and, in those with implants, removal of infected devices. The lower limb was involved in 81%, including hip, knee, and foot. The infection was in an upper limb in 10% and in the spine in 7%.
Staphylococcus aureus was present in 38% of cases, coagulase-negative staphylococci in 27%, and streptococci in 15%. Gram-negative bacteria were found in 22%.
For those patients randomized to IV therapy, glycopeptides and cephalosporins were most commonly employed (41% and 33%, respectively). For oral therapy, quinolones and penicillins were most common (37% and 16%). Most patients (74%) continued antibiotic treatment for more than 6 weeks. Forty patients were lost to follow-up.
In the primary intent-to-treat analysis, the failure rate was 13% for oral therapy and 14% for IV therapy, not a significant difference. Results were similar in the other analyses, including a modified intent to treat with only patients who had complete 1-year data, and a per-protocol analysis. All of the point prevalence numbers favored oral therapy, but crossed the null. Curves in the time-to-treatment-failure analysis were virtually superimposable, as were curves in time to discontinuation of therapy.
Another subgroup analysis examined treatment failure by infective organism; again, there were no significant treatment differences in any of the pathogen subgroups examined (S. aureus, coagulase-negative staph, streptococci species, and other gram-negative bacteria).
Nor did the type of antibiotic significantly affect failure rate, Dr. Scarborough noted. The median length of stay was 14 days for patients on IV treatment and 11 days for those taking oral medications. The incidence of serious adverse events was very similar – about 86% in each group.
On a visual analog scale that assessed health-related quality of life, patients taking oral treatment reported better mobility, self-care, and activity level, and less pain, discomfort, anxiety, and depression than those taking IV medications.
Cost represented the other significant difference between the groups. Over 1 year, the mean IV treatment cost was the equivalent of $17,152, and the mean oral treatment cost was $13,611 – a significant difference of $3,541.
“This represents a potential savings to the National Health Service of 16-25 million pounds sterling ($20.6 million-$32.3 million) per year,” Dr. Scarborough said. “All coming at no expense of good clinical outcomes.”
OVIVA was sponsored by the U.K. National Institute of Health Research. Dr. Scarborough had no financial disclosures.
[email protected]
On Twitter @alz_gal
VIENNA – Oral antibiotic therapy is just as effective as intravenous treatment in curing bone and joint infections, but costs about $3,500 less.
Treating these infections with oral agents also “improves patient autonomy, as it’s not necessary to have IV lines at home,” and represents a generally wiser use of powerful antibiotics, Matthew Scarborough, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
OVIVA (Oral vs. Intravenous Antibiotics for Bone and Joint Infection) was conducted at 26 sites in the United Kingdom. It randomized 1,054 adults with bone or joint infections to 6 weeks of either oral or intravenous treatment.
An important aspect of the trial was that both oral and IV treatment choices were made before randomization, Dr. Scarborough said. However, the decisions on what drug to use were left up to the treating physician and depended on the infection site and pathogen.
The primary outcome was definite treatment failure (bacteriologic, histologic, and clinical). Patients were followed for 1 year.
Patients were a median of 60 years old. All had surgical treatment before antibiotic therapy, including debridement and, in those with implants, removal of infected devices. The lower limb was involved in 81%, including hip, knee, and foot. The infection was in an upper limb in 10% and in the spine in 7%.
Staphylococcus aureus was present in 38% of cases, coagulase-negative staphylococci in 27%, and streptococci in 15%. Gram-negative bacteria were found in 22%.
For those patients randomized to IV therapy, glycopeptides and cephalosporins were most commonly employed (41% and 33%, respectively). For oral therapy, quinolones and penicillins were most common (37% and 16%). Most patients (74%) continued antibiotic treatment for more than 6 weeks. Forty patients were lost to follow-up.
In the primary intent-to-treat analysis, the failure rate was 13% for oral therapy and 14% for IV therapy, not a significant difference. Results were similar in the other analyses, including a modified intent to treat with only patients who had complete 1-year data, and a per-protocol analysis. All of the point prevalence numbers favored oral therapy, but crossed the null. Curves in the time-to-treatment-failure analysis were virtually superimposable, as were curves in time to discontinuation of therapy.
Another subgroup analysis examined treatment failure by infective organism; again, there were no significant treatment differences in any of the pathogen subgroups examined (S. aureus, coagulase-negative staph, streptococci species, and other gram-negative bacteria).
Nor did the type of antibiotic significantly affect failure rate, Dr. Scarborough noted. The median length of stay was 14 days for patients on IV treatment and 11 days for those taking oral medications. The incidence of serious adverse events was very similar – about 86% in each group.
On a visual analog scale that assessed health-related quality of life, patients taking oral treatment reported better mobility, self-care, and activity level, and less pain, discomfort, anxiety, and depression than those taking IV medications.
Cost represented the other significant difference between the groups. Over 1 year, the mean IV treatment cost was the equivalent of $17,152, and the mean oral treatment cost was $13,611 – a significant difference of $3,541.
“This represents a potential savings to the National Health Service of 16-25 million pounds sterling ($20.6 million-$32.3 million) per year,” Dr. Scarborough said. “All coming at no expense of good clinical outcomes.”
OVIVA was sponsored by the U.K. National Institute of Health Research. Dr. Scarborough had no financial disclosures.
[email protected]
On Twitter @alz_gal
VIENNA – Oral antibiotic therapy is just as effective as intravenous treatment in curing bone and joint infections, but costs about $3,500 less.
Treating these infections with oral agents also “improves patient autonomy, as it’s not necessary to have IV lines at home,” and represents a generally wiser use of powerful antibiotics, Matthew Scarborough, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
OVIVA (Oral vs. Intravenous Antibiotics for Bone and Joint Infection) was conducted at 26 sites in the United Kingdom. It randomized 1,054 adults with bone or joint infections to 6 weeks of either oral or intravenous treatment.
An important aspect of the trial was that both oral and IV treatment choices were made before randomization, Dr. Scarborough said. However, the decisions on what drug to use were left up to the treating physician and depended on the infection site and pathogen.
The primary outcome was definite treatment failure (bacteriologic, histologic, and clinical). Patients were followed for 1 year.
Patients were a median of 60 years old. All had surgical treatment before antibiotic therapy, including debridement and, in those with implants, removal of infected devices. The lower limb was involved in 81%, including hip, knee, and foot. The infection was in an upper limb in 10% and in the spine in 7%.
Staphylococcus aureus was present in 38% of cases, coagulase-negative staphylococci in 27%, and streptococci in 15%. Gram-negative bacteria were found in 22%.
For those patients randomized to IV therapy, glycopeptides and cephalosporins were most commonly employed (41% and 33%, respectively). For oral therapy, quinolones and penicillins were most common (37% and 16%). Most patients (74%) continued antibiotic treatment for more than 6 weeks. Forty patients were lost to follow-up.
In the primary intent-to-treat analysis, the failure rate was 13% for oral therapy and 14% for IV therapy, not a significant difference. Results were similar in the other analyses, including a modified intent to treat with only patients who had complete 1-year data, and a per-protocol analysis. All of the point prevalence numbers favored oral therapy, but crossed the null. Curves in the time-to-treatment-failure analysis were virtually superimposable, as were curves in time to discontinuation of therapy.
Another subgroup analysis examined treatment failure by infective organism; again, there were no significant treatment differences in any of the pathogen subgroups examined (S. aureus, coagulase-negative staph, streptococci species, and other gram-negative bacteria).
Nor did the type of antibiotic significantly affect failure rate, Dr. Scarborough noted. The median length of stay was 14 days for patients on IV treatment and 11 days for those taking oral medications. The incidence of serious adverse events was very similar – about 86% in each group.
On a visual analog scale that assessed health-related quality of life, patients taking oral treatment reported better mobility, self-care, and activity level, and less pain, discomfort, anxiety, and depression than those taking IV medications.
Cost represented the other significant difference between the groups. Over 1 year, the mean IV treatment cost was the equivalent of $17,152, and the mean oral treatment cost was $13,611 – a significant difference of $3,541.
“This represents a potential savings to the National Health Service of 16-25 million pounds sterling ($20.6 million-$32.3 million) per year,” Dr. Scarborough said. “All coming at no expense of good clinical outcomes.”
OVIVA was sponsored by the U.K. National Institute of Health Research. Dr. Scarborough had no financial disclosures.
[email protected]
On Twitter @alz_gal
AT ECCMID 2017
Key clinical point:
Major finding: At 1 year, cure rates were identical, but oral treatment cost about $3,500 less than IV treatment.
Data source: The study randomized 1,054 patients.
Disclosures: OVIVA was sponsored by the U.K. National Institute of Health Research. Dr. Scarborough had no financial disclosures.
Systemic Hypothermia as Treatment for an Acute Cervical Spinal Cord Injury in a Professional Football Player: 9-Year Follow-Up
Take-Home Points
- Importance of on-field management.
- Preseason drilling of spinal injury management.
- Early and rapid intervention.
- Possible benefit of moderate systemic hypothermia as treatment for acute cervical injury.
In 2010, we reported the case of a professional American football player who sustained a complete cervical spinal cord injury (SCI) while tackling an opposing player.1 He received prompt medical and surgical care based on then-current recommendations, but was also treated with systemic hypothermia soon after his injury. Although systemic hypothermia had been used in the management of other neurologic injuries at that time, it had not been used in humans with acute SCI, except as described in 2 case reports.2,3 However, Dietrich4 described early emerging animal data on the efficacy of systemic hypothermia for acute SCI. We now provide a clinical update on our patient, who provided written informed consent for print and electronic publication of this case report.
Case Report
During a National Football League game, the player sustained a C3–C4 fracture-dislocation after a helmet-to-helmet hit on an opposing player. He fell face down on the ground and did not move. The team’s physician and trainer rushed to the player’s side, immediately assessed him, and initiated the emergency spinal resuscitation protocol.
As per protocol, the assigned team leader took charge of managing the player’s head to maintain in-line traction with the helmet in place until the head was secured in place on a backboard designed to accommodate the helmet.
Complete motor paralysis and sensory loss (American Spinal Injury Association [ASIA] level A) were noted below the clavicles during physical examination by the head athletic trainer and 2 independent physicians, and by self-report.
On arrival at the hospital, the patient had a core temperature of 98°F, which is substantially lower than the average core temperature (≤101.7°F) of an active football player.6He had a normal level of consciousness and normal cranial nerve function but remained without any voluntary motor function in the extremities and still had no sensation below the clavicles, except crude pressure sensation in one hand while in the emergency department. After the helmet and shoulder pads were removed, per National Athletic Trainers’ Association (NATA) protocol7 (Figure 2), he was stabilized, and a hard cervical collar was placed. A lateral radiograph (Figure 4) showed a C3–C4 facet dislocation with about 46% anterior translation of C3 on C4 and obvious disruption of the facets.
About 3 hours after injury, the patient was taken to the operating room. Although closed reduction improved alignment dramatically, it failed to completely reduce the dislocated left C3–C4 facet. An hour later, anterior C3–C4 discectomy was performed from the front with instrumented anterior interbody fusion. This was immediately followed by posterior decompressive laminectomy, bilateral facet reduction, and fusion with instrumentation. Surgery was completed within about 4 hours, almost exactly 7 hours after injury. Anesthesia records indicated a core temperature range of 94.1°F to 95.3°F with passive cooling during surgery. CT and MRI performed within 4 hours after surgery showed excellent cord decompression.
The next morning, about 14.5 hours after injury, the patient demonstrated a flicker of the adductor muscles of the lower extremities. An examination an hour later revealed 1/5 quadriceps, 2/5 adductors, and 1/5 gastrocnemius/soleus. A nurse’s hourly examinations and the surgeon’s repeat examinations revealed no other motor function. Sensory function was more difficult to evaluate because of sedation, but rudimentary sensation was noted throughout the lower extremities, and proprioception and vibratory sensation were noted as well. With passive cooling, it was difficult to consistently maintain moderate hypothermia; the patient’s core temperature ranged from 94.8°F to 98.8°F by 6:00 a.m. Therefore, the decision was made to place a Cordis sheath in the left femoral vein and introduce an intra-vena cava cooling catheter through it. This catheter was highly effective in maintaining the patient’s temperature at about 92.5°F.
Over the next 36 hours, the patient demonstrated increased motor activity in the upper and lower extremities: 1/5 biceps, 2-3/5 triceps, 3/5 quadriceps. He was slowly rewarmed and, on postoperative day 3, extubated.
At 2 years, the patient underwent another anterior-only cervical procedure: The inferior adjacent segment (C4–C5) was fused because of neck pain and deformity.
With respect to the original injury and the evolution in cord appearance, the patient had solid arthrodesis from C3–C5 with instrumentation in good position. There was evidence of loss of lordosis at C5–C6 with disk dessication and broad-based bulging. The spinal cord had evidence of myelomalacia; this was noted when the patient was in rehabilitation, 1 month after injury. The 2-cm × 11-mm area of myelomalacia was directly posterior to the fused C3–C4 interval (original MRI, Figure 5; 2-week MRI, Figure 6).
Conclusion
At the time this player was injured, use of systemic hypothermia with standard therapy for acute SCI was unique and controversial. Since then, smaller randomized human studies have described the tolerable safety profile, efficacy, and potential benefits of this intervention in acute SCI in humans.8-10 Now, modest systemic hypothermia can be one of many tools considered in the treatment of acute SCI. Before it can become the standard of care, however, additional larger prospective randomized studies need to be completed.
Am J Orthop. 2017;46(2):E79-E82. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cappuccino A, Bisson LJ, Carpenter B, Marzo J, Dietrich WD 3rd, Cappuccino H. The use of systemic hypothermia for the treatment of an acute cervical spinal cord injury in a professional football player. Spine. 2010;35(2):E57-E62.
2. Goldstein J. Lowering body temp shows promise for trauma treatment. Spinal Cord Injury Information Pages news blog. http://www.sci-info-pages.com/2006/05/lowering-body-temp-shows-promise-for.html. Published May 3, 2006. Accessed March 19, 2009.
3. Hartemink KJ, Wisselink W, Rauwerda JA, Girbes AR, Polderman KH. Novel applications of therapeutic hypothermia: report of three cases. Crit Care. 2004;8(5):R343-R346.
4. Dietrich WD. Presidential address presented at: 34th Annual Meeting of the Cervical Spine Research Society; November 30, 2006; Palm Beach, FL.
5. Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322(20):1405-1411.
6. Horodyski MB, LuCante K, Escobar E, et al. Intermittent Cool, Dry Air Underneath Football Shoulder Pads Assists in Temperature Homeostasis. In: The American Orthopaedic Society for Sports Medicine Proceedings 2008; 87-88.
7. Kleiner DM, Almquist JL, Bailes J, et al; Inter-Association Task Force for Appropriate Care of the Spine-Injured Athlete. Prehospital Care of the Spine-Injured Athlete. Dallas, TX: National Athletic Trainers’ Association; 2001. http://www.msata.org/Resources/Documents/PreHospitalCare4SpineInjuredAthlete.pdf. Published March 2001. Accessed January 10, 2017.
8. Dididze M, Green BA, Dietrich WD, Vanni S, Wang MY, Levi AD. Systemic hypothermia in acute cervical spinal cord injury: a case-controlled study. Spinal Cord. 2013;51(5):395-400.
9. Levi AD, Casella G, Green BA, et al. Clinical outcomes using modest intravascular hypothermia after acute cervical spinal cord injury. Neurosurgery. 2010;66(4):670-677.
10. Levi AD, Green BA, Wang MY, et al. Clinical application of modest hypothermia after spinal cord injury. J Neurotrauma. 2009;26(3):407-415.
Take-Home Points
- Importance of on-field management.
- Preseason drilling of spinal injury management.
- Early and rapid intervention.
- Possible benefit of moderate systemic hypothermia as treatment for acute cervical injury.
In 2010, we reported the case of a professional American football player who sustained a complete cervical spinal cord injury (SCI) while tackling an opposing player.1 He received prompt medical and surgical care based on then-current recommendations, but was also treated with systemic hypothermia soon after his injury. Although systemic hypothermia had been used in the management of other neurologic injuries at that time, it had not been used in humans with acute SCI, except as described in 2 case reports.2,3 However, Dietrich4 described early emerging animal data on the efficacy of systemic hypothermia for acute SCI. We now provide a clinical update on our patient, who provided written informed consent for print and electronic publication of this case report.
Case Report
During a National Football League game, the player sustained a C3–C4 fracture-dislocation after a helmet-to-helmet hit on an opposing player. He fell face down on the ground and did not move. The team’s physician and trainer rushed to the player’s side, immediately assessed him, and initiated the emergency spinal resuscitation protocol.
As per protocol, the assigned team leader took charge of managing the player’s head to maintain in-line traction with the helmet in place until the head was secured in place on a backboard designed to accommodate the helmet.
Complete motor paralysis and sensory loss (American Spinal Injury Association [ASIA] level A) were noted below the clavicles during physical examination by the head athletic trainer and 2 independent physicians, and by self-report.
On arrival at the hospital, the patient had a core temperature of 98°F, which is substantially lower than the average core temperature (≤101.7°F) of an active football player.6He had a normal level of consciousness and normal cranial nerve function but remained without any voluntary motor function in the extremities and still had no sensation below the clavicles, except crude pressure sensation in one hand while in the emergency department. After the helmet and shoulder pads were removed, per National Athletic Trainers’ Association (NATA) protocol7 (Figure 2), he was stabilized, and a hard cervical collar was placed. A lateral radiograph (Figure 4) showed a C3–C4 facet dislocation with about 46% anterior translation of C3 on C4 and obvious disruption of the facets.
About 3 hours after injury, the patient was taken to the operating room. Although closed reduction improved alignment dramatically, it failed to completely reduce the dislocated left C3–C4 facet. An hour later, anterior C3–C4 discectomy was performed from the front with instrumented anterior interbody fusion. This was immediately followed by posterior decompressive laminectomy, bilateral facet reduction, and fusion with instrumentation. Surgery was completed within about 4 hours, almost exactly 7 hours after injury. Anesthesia records indicated a core temperature range of 94.1°F to 95.3°F with passive cooling during surgery. CT and MRI performed within 4 hours after surgery showed excellent cord decompression.
The next morning, about 14.5 hours after injury, the patient demonstrated a flicker of the adductor muscles of the lower extremities. An examination an hour later revealed 1/5 quadriceps, 2/5 adductors, and 1/5 gastrocnemius/soleus. A nurse’s hourly examinations and the surgeon’s repeat examinations revealed no other motor function. Sensory function was more difficult to evaluate because of sedation, but rudimentary sensation was noted throughout the lower extremities, and proprioception and vibratory sensation were noted as well. With passive cooling, it was difficult to consistently maintain moderate hypothermia; the patient’s core temperature ranged from 94.8°F to 98.8°F by 6:00 a.m. Therefore, the decision was made to place a Cordis sheath in the left femoral vein and introduce an intra-vena cava cooling catheter through it. This catheter was highly effective in maintaining the patient’s temperature at about 92.5°F.
Over the next 36 hours, the patient demonstrated increased motor activity in the upper and lower extremities: 1/5 biceps, 2-3/5 triceps, 3/5 quadriceps. He was slowly rewarmed and, on postoperative day 3, extubated.
At 2 years, the patient underwent another anterior-only cervical procedure: The inferior adjacent segment (C4–C5) was fused because of neck pain and deformity.
With respect to the original injury and the evolution in cord appearance, the patient had solid arthrodesis from C3–C5 with instrumentation in good position. There was evidence of loss of lordosis at C5–C6 with disk dessication and broad-based bulging. The spinal cord had evidence of myelomalacia; this was noted when the patient was in rehabilitation, 1 month after injury. The 2-cm × 11-mm area of myelomalacia was directly posterior to the fused C3–C4 interval (original MRI, Figure 5; 2-week MRI, Figure 6).
Conclusion
At the time this player was injured, use of systemic hypothermia with standard therapy for acute SCI was unique and controversial. Since then, smaller randomized human studies have described the tolerable safety profile, efficacy, and potential benefits of this intervention in acute SCI in humans.8-10 Now, modest systemic hypothermia can be one of many tools considered in the treatment of acute SCI. Before it can become the standard of care, however, additional larger prospective randomized studies need to be completed.
Am J Orthop. 2017;46(2):E79-E82. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Importance of on-field management.
- Preseason drilling of spinal injury management.
- Early and rapid intervention.
- Possible benefit of moderate systemic hypothermia as treatment for acute cervical injury.
In 2010, we reported the case of a professional American football player who sustained a complete cervical spinal cord injury (SCI) while tackling an opposing player.1 He received prompt medical and surgical care based on then-current recommendations, but was also treated with systemic hypothermia soon after his injury. Although systemic hypothermia had been used in the management of other neurologic injuries at that time, it had not been used in humans with acute SCI, except as described in 2 case reports.2,3 However, Dietrich4 described early emerging animal data on the efficacy of systemic hypothermia for acute SCI. We now provide a clinical update on our patient, who provided written informed consent for print and electronic publication of this case report.
Case Report
During a National Football League game, the player sustained a C3–C4 fracture-dislocation after a helmet-to-helmet hit on an opposing player. He fell face down on the ground and did not move. The team’s physician and trainer rushed to the player’s side, immediately assessed him, and initiated the emergency spinal resuscitation protocol.
As per protocol, the assigned team leader took charge of managing the player’s head to maintain in-line traction with the helmet in place until the head was secured in place on a backboard designed to accommodate the helmet.
Complete motor paralysis and sensory loss (American Spinal Injury Association [ASIA] level A) were noted below the clavicles during physical examination by the head athletic trainer and 2 independent physicians, and by self-report.
On arrival at the hospital, the patient had a core temperature of 98°F, which is substantially lower than the average core temperature (≤101.7°F) of an active football player.6He had a normal level of consciousness and normal cranial nerve function but remained without any voluntary motor function in the extremities and still had no sensation below the clavicles, except crude pressure sensation in one hand while in the emergency department. After the helmet and shoulder pads were removed, per National Athletic Trainers’ Association (NATA) protocol7 (Figure 2), he was stabilized, and a hard cervical collar was placed. A lateral radiograph (Figure 4) showed a C3–C4 facet dislocation with about 46% anterior translation of C3 on C4 and obvious disruption of the facets.
About 3 hours after injury, the patient was taken to the operating room. Although closed reduction improved alignment dramatically, it failed to completely reduce the dislocated left C3–C4 facet. An hour later, anterior C3–C4 discectomy was performed from the front with instrumented anterior interbody fusion. This was immediately followed by posterior decompressive laminectomy, bilateral facet reduction, and fusion with instrumentation. Surgery was completed within about 4 hours, almost exactly 7 hours after injury. Anesthesia records indicated a core temperature range of 94.1°F to 95.3°F with passive cooling during surgery. CT and MRI performed within 4 hours after surgery showed excellent cord decompression.
The next morning, about 14.5 hours after injury, the patient demonstrated a flicker of the adductor muscles of the lower extremities. An examination an hour later revealed 1/5 quadriceps, 2/5 adductors, and 1/5 gastrocnemius/soleus. A nurse’s hourly examinations and the surgeon’s repeat examinations revealed no other motor function. Sensory function was more difficult to evaluate because of sedation, but rudimentary sensation was noted throughout the lower extremities, and proprioception and vibratory sensation were noted as well. With passive cooling, it was difficult to consistently maintain moderate hypothermia; the patient’s core temperature ranged from 94.8°F to 98.8°F by 6:00 a.m. Therefore, the decision was made to place a Cordis sheath in the left femoral vein and introduce an intra-vena cava cooling catheter through it. This catheter was highly effective in maintaining the patient’s temperature at about 92.5°F.
Over the next 36 hours, the patient demonstrated increased motor activity in the upper and lower extremities: 1/5 biceps, 2-3/5 triceps, 3/5 quadriceps. He was slowly rewarmed and, on postoperative day 3, extubated.
At 2 years, the patient underwent another anterior-only cervical procedure: The inferior adjacent segment (C4–C5) was fused because of neck pain and deformity.
With respect to the original injury and the evolution in cord appearance, the patient had solid arthrodesis from C3–C5 with instrumentation in good position. There was evidence of loss of lordosis at C5–C6 with disk dessication and broad-based bulging. The spinal cord had evidence of myelomalacia; this was noted when the patient was in rehabilitation, 1 month after injury. The 2-cm × 11-mm area of myelomalacia was directly posterior to the fused C3–C4 interval (original MRI, Figure 5; 2-week MRI, Figure 6).
Conclusion
At the time this player was injured, use of systemic hypothermia with standard therapy for acute SCI was unique and controversial. Since then, smaller randomized human studies have described the tolerable safety profile, efficacy, and potential benefits of this intervention in acute SCI in humans.8-10 Now, modest systemic hypothermia can be one of many tools considered in the treatment of acute SCI. Before it can become the standard of care, however, additional larger prospective randomized studies need to be completed.
Am J Orthop. 2017;46(2):E79-E82. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cappuccino A, Bisson LJ, Carpenter B, Marzo J, Dietrich WD 3rd, Cappuccino H. The use of systemic hypothermia for the treatment of an acute cervical spinal cord injury in a professional football player. Spine. 2010;35(2):E57-E62.
2. Goldstein J. Lowering body temp shows promise for trauma treatment. Spinal Cord Injury Information Pages news blog. http://www.sci-info-pages.com/2006/05/lowering-body-temp-shows-promise-for.html. Published May 3, 2006. Accessed March 19, 2009.
3. Hartemink KJ, Wisselink W, Rauwerda JA, Girbes AR, Polderman KH. Novel applications of therapeutic hypothermia: report of three cases. Crit Care. 2004;8(5):R343-R346.
4. Dietrich WD. Presidential address presented at: 34th Annual Meeting of the Cervical Spine Research Society; November 30, 2006; Palm Beach, FL.
5. Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322(20):1405-1411.
6. Horodyski MB, LuCante K, Escobar E, et al. Intermittent Cool, Dry Air Underneath Football Shoulder Pads Assists in Temperature Homeostasis. In: The American Orthopaedic Society for Sports Medicine Proceedings 2008; 87-88.
7. Kleiner DM, Almquist JL, Bailes J, et al; Inter-Association Task Force for Appropriate Care of the Spine-Injured Athlete. Prehospital Care of the Spine-Injured Athlete. Dallas, TX: National Athletic Trainers’ Association; 2001. http://www.msata.org/Resources/Documents/PreHospitalCare4SpineInjuredAthlete.pdf. Published March 2001. Accessed January 10, 2017.
8. Dididze M, Green BA, Dietrich WD, Vanni S, Wang MY, Levi AD. Systemic hypothermia in acute cervical spinal cord injury: a case-controlled study. Spinal Cord. 2013;51(5):395-400.
9. Levi AD, Casella G, Green BA, et al. Clinical outcomes using modest intravascular hypothermia after acute cervical spinal cord injury. Neurosurgery. 2010;66(4):670-677.
10. Levi AD, Green BA, Wang MY, et al. Clinical application of modest hypothermia after spinal cord injury. J Neurotrauma. 2009;26(3):407-415.
1. Cappuccino A, Bisson LJ, Carpenter B, Marzo J, Dietrich WD 3rd, Cappuccino H. The use of systemic hypothermia for the treatment of an acute cervical spinal cord injury in a professional football player. Spine. 2010;35(2):E57-E62.
2. Goldstein J. Lowering body temp shows promise for trauma treatment. Spinal Cord Injury Information Pages news blog. http://www.sci-info-pages.com/2006/05/lowering-body-temp-shows-promise-for.html. Published May 3, 2006. Accessed March 19, 2009.
3. Hartemink KJ, Wisselink W, Rauwerda JA, Girbes AR, Polderman KH. Novel applications of therapeutic hypothermia: report of three cases. Crit Care. 2004;8(5):R343-R346.
4. Dietrich WD. Presidential address presented at: 34th Annual Meeting of the Cervical Spine Research Society; November 30, 2006; Palm Beach, FL.
5. Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322(20):1405-1411.
6. Horodyski MB, LuCante K, Escobar E, et al. Intermittent Cool, Dry Air Underneath Football Shoulder Pads Assists in Temperature Homeostasis. In: The American Orthopaedic Society for Sports Medicine Proceedings 2008; 87-88.
7. Kleiner DM, Almquist JL, Bailes J, et al; Inter-Association Task Force for Appropriate Care of the Spine-Injured Athlete. Prehospital Care of the Spine-Injured Athlete. Dallas, TX: National Athletic Trainers’ Association; 2001. http://www.msata.org/Resources/Documents/PreHospitalCare4SpineInjuredAthlete.pdf. Published March 2001. Accessed January 10, 2017.
8. Dididze M, Green BA, Dietrich WD, Vanni S, Wang MY, Levi AD. Systemic hypothermia in acute cervical spinal cord injury: a case-controlled study. Spinal Cord. 2013;51(5):395-400.
9. Levi AD, Casella G, Green BA, et al. Clinical outcomes using modest intravascular hypothermia after acute cervical spinal cord injury. Neurosurgery. 2010;66(4):670-677.
10. Levi AD, Green BA, Wang MY, et al. Clinical application of modest hypothermia after spinal cord injury. J Neurotrauma. 2009;26(3):407-415.
Superior Mesenteric Artery Syndrome as a Complication of Scoliosis Surgery
Take-Home Points
- Adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome following pediatric spine surgery.
- Must recognize nonspecific symptoms such as abdominal pain, tenderness, distention, bilious or projectile vomiting, hypoactive bowel sounds, and anorexia postoperatively.
- Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, or cardiovascular collapse and death.
Superior mesenteric artery (SMA) syndrome resulting from surgical treatment of scoliosis has been recognized in the medical literature since 1752.1 Throughout the literature, SMA syndrome variably has been referred to as cast syndrome, Wilkie syndrome, arteriomesenteric duodenal obstruction, and chronic duodenal ileus.2 We now recognize numerous etiologies of SMA syndrome, as several sources can externally compress the duodenum. Classic acute symptoms of bowel obstruction include bilious vomiting, nausea, and epigastric pain. Chronic manifestations of SMA syndrome may include weight loss and decreased appetite. Our literature review revealed that adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome after pediatric spine surgery.
We report the case of a 14-year-old boy who developed SMA syndrome after undergoing scoliosis surgery. The patient and his mother provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old boy with a history of idiopathic scoliosis presented to Cohen Children’s Hospital (Long Island Jewish Medical Center) with bilious vomiting that had persisted for 7 days after posterior T9–L4 fusion with instrumentation.
Discussion
SMA syndrome is attributed to the anatomical orientation of the third part of the duodenum, which passes between the aorta and the SMA (Figure 4).
Adolescents are particularly vulnerable to this condition. Faster adolescent bone growth relative to visceral growth is accompanied by a decrease in SMA angle.3 Occasionally, body casts are used after surgery to immobilize the vertebrae and augment healing. Cast syndrome occurs when pressure from a body cast causes a bowel obstruction secondary to spinal hyperextension and amplified spinal lordosis.2 This finding, dating to the 19th century, was reported by Willet4 when a patient died 48 hours after application of a body cast. In 1950, the term cast syndrome was coined after a motorcyclist’s injuries were treated with a hip spica cast and the patient died of cardiovascular collapse secondary to persistent vomiting.5
Table 1 summarizes various evaluation, diagnosis, and treatment algorithms designed to optimize nutrition and weight in patients developing signs and symptoms of SMA syndrome after posterior spinal instrumentation and fusion for adolescent idiopathic scoliosis (AIS).
The third unique feature in this case is electrocardiogram findings. Although some cases briefly discussed electrolyte abnormalities, none presented evidence that these abnormalities caused cardiac changes.6,16,18 The overall clinical significance of the QT prolongation in our patient’s case is unknown, as this finding was improved with correction of the electrolyte abnormalities and appropriate fluid replenishment.
Early recognition of nonspecific symptoms (eg, abdominal pain, tenderness, distension, bilious or projectile vomiting, hypoactive bowel sounds, anorexia) plays a key role in preventing severe morbidity and mortality from SMA syndrome after scoliosis surgery. Although many patients present in the semiclassic obstructed pattern, notable reasons for diagnostic delay include normal appetite and bowel sounds.3 For example, SMA syndrome may be misdiagnosed as stomach flu because of unfamiliarity with disease diagnosis and management.20 Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, and cardiovascular collapse and death.
Am J Orthop. 2017;46(2):E124-E130. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Evarts CM, Winter RB, Hall JE. Vascular compression of the duodenum associated with the treatment of scoliosis. Review of the literature and report of eighteen cases. J Bone Joint Surg Am. 1971;53(3):431-444.
2. Zhu ZZ, Qiu Y. Superior mesenteric artery syndrome following scoliosis surgery: its risk indicators and treatment strategy. World J Gastroenterol. 2005;11(21):3307-3310.
3. Hutchinson DT, Bassett GS. Superior mesenteric artery syndrome in pediatric orthopedic patients. Clin Orthop Relat Res. 1990;(250):250-257.
4. Willet A. Fatal vomiting following application of plaster-of-Paris bandage in case of spinal curvature. St Barth Hosp Rep. 1878;14:333-335.
5. Dorph MH. The cast syndrome; review of the literature and report of a case. N Engl J Med. 1950;243(12):440-442.
6. Lam DJ, Lee JZ, Chua JH, Lee YT, Lim KB. Superior mesenteric artery syndrome following surgery for adolescent idiopathic scoliosis: a case series, review of the literature, and an algorithm for management. J Pediatr Orthop B. 2014;23(4):312-318.
7. Tsirikos AI, Anakwe RE, Baker AD. Late presentation of superior mesenteric artery syndrome following scoliosis surgery: a case report. J Med Case Rep. 2008;2:9.
8. Akin JT Jr, Skandalakis JE, Gray SW. The anatomic basis of vascular compression of the duodenum. Surg Clin North Am. 1974;54(6):1361-1370.
9. Amy BW, Priebe CJ Jr, King A. Superior mesenteric artery syndrome associated with scoliosis treated by a modified Ladd procedure. J Pediatr Orthop. 1985;5(3):361-363.
10. Richardson WS, Surowiec WJ. Laparoscopic repair of superior mesenteric artery syndrome. Am J Surg. 2001;181(4):377-378.
11. Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83(8):1169-1181.
12. Braun SV, Hedden DM, Howard AW. Superior mesenteric artery syndrome following spinal deformity correction. J Bone Joint Surg Am. 2006;88(10):2252-2257.
13. Smith BG, Hakim-Zargar M, Thomson JD. Low body mass index: a risk factor for superior mesenteric artery syndrome in adolescents undergoing spinal fusion for scoliosis. J Spinal Disord Tech. 2009;22(2):144-148.
14. Pan CH, Tzeng ST, Chen CS, Chen PQ. Superior mesenteric artery syndrome complicating staged corrective surgery for scoliosis. J Formos Med Assoc. 2007;106(2 suppl):S37-S45.
15. Kennedy RH, Cooper MJ. An unusually severe case of the cast syndrome. Postgrad Med J. 1983;59(694):539-540.
16. Keskin M, Akgül T, Bayraktar A, Dikici F, Balik E. Superior mesenteric artery syndrome: an infrequent complication of scoliosis surgery. Case Rep Surg. 2014;2014:263431.
17. Amarawickrama H, Harikrishnan A, Krijgsman B. Superior mesenteric artery syndrome in a young girl following spinal surgery for scoliosis. Br J Hosp Med. 2005;66(12):700-701.
18. Crowther MA, Webb PJ, Eyre-Brook IA. Superior mesenteric artery syndrome following surgery for scoliosis. Spine. 2002;27(24):E528-E533.
19. Moskovich R, Cheong-Leen P. Vascular compression of the duodenum. J R Soc Med. 1986;79(8):465-467.
20. Shah MA, Albright MB, Vogt MT, Moreland MS. Superior mesenteric artery syndrome in scoliosis surgery: weight percentile for height as an indicator of risk. J Pediatr Orthop. 2003;23(5):665-668.
Take-Home Points
- Adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome following pediatric spine surgery.
- Must recognize nonspecific symptoms such as abdominal pain, tenderness, distention, bilious or projectile vomiting, hypoactive bowel sounds, and anorexia postoperatively.
- Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, or cardiovascular collapse and death.
Superior mesenteric artery (SMA) syndrome resulting from surgical treatment of scoliosis has been recognized in the medical literature since 1752.1 Throughout the literature, SMA syndrome variably has been referred to as cast syndrome, Wilkie syndrome, arteriomesenteric duodenal obstruction, and chronic duodenal ileus.2 We now recognize numerous etiologies of SMA syndrome, as several sources can externally compress the duodenum. Classic acute symptoms of bowel obstruction include bilious vomiting, nausea, and epigastric pain. Chronic manifestations of SMA syndrome may include weight loss and decreased appetite. Our literature review revealed that adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome after pediatric spine surgery.
We report the case of a 14-year-old boy who developed SMA syndrome after undergoing scoliosis surgery. The patient and his mother provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old boy with a history of idiopathic scoliosis presented to Cohen Children’s Hospital (Long Island Jewish Medical Center) with bilious vomiting that had persisted for 7 days after posterior T9–L4 fusion with instrumentation.
Discussion
SMA syndrome is attributed to the anatomical orientation of the third part of the duodenum, which passes between the aorta and the SMA (Figure 4).
Adolescents are particularly vulnerable to this condition. Faster adolescent bone growth relative to visceral growth is accompanied by a decrease in SMA angle.3 Occasionally, body casts are used after surgery to immobilize the vertebrae and augment healing. Cast syndrome occurs when pressure from a body cast causes a bowel obstruction secondary to spinal hyperextension and amplified spinal lordosis.2 This finding, dating to the 19th century, was reported by Willet4 when a patient died 48 hours after application of a body cast. In 1950, the term cast syndrome was coined after a motorcyclist’s injuries were treated with a hip spica cast and the patient died of cardiovascular collapse secondary to persistent vomiting.5
Table 1 summarizes various evaluation, diagnosis, and treatment algorithms designed to optimize nutrition and weight in patients developing signs and symptoms of SMA syndrome after posterior spinal instrumentation and fusion for adolescent idiopathic scoliosis (AIS).
The third unique feature in this case is electrocardiogram findings. Although some cases briefly discussed electrolyte abnormalities, none presented evidence that these abnormalities caused cardiac changes.6,16,18 The overall clinical significance of the QT prolongation in our patient’s case is unknown, as this finding was improved with correction of the electrolyte abnormalities and appropriate fluid replenishment.
Early recognition of nonspecific symptoms (eg, abdominal pain, tenderness, distension, bilious or projectile vomiting, hypoactive bowel sounds, anorexia) plays a key role in preventing severe morbidity and mortality from SMA syndrome after scoliosis surgery. Although many patients present in the semiclassic obstructed pattern, notable reasons for diagnostic delay include normal appetite and bowel sounds.3 For example, SMA syndrome may be misdiagnosed as stomach flu because of unfamiliarity with disease diagnosis and management.20 Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, and cardiovascular collapse and death.
Am J Orthop. 2017;46(2):E124-E130. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome following pediatric spine surgery.
- Must recognize nonspecific symptoms such as abdominal pain, tenderness, distention, bilious or projectile vomiting, hypoactive bowel sounds, and anorexia postoperatively.
- Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, or cardiovascular collapse and death.
Superior mesenteric artery (SMA) syndrome resulting from surgical treatment of scoliosis has been recognized in the medical literature since 1752.1 Throughout the literature, SMA syndrome variably has been referred to as cast syndrome, Wilkie syndrome, arteriomesenteric duodenal obstruction, and chronic duodenal ileus.2 We now recognize numerous etiologies of SMA syndrome, as several sources can externally compress the duodenum. Classic acute symptoms of bowel obstruction include bilious vomiting, nausea, and epigastric pain. Chronic manifestations of SMA syndrome may include weight loss and decreased appetite. Our literature review revealed that adolescent growth spurt, height-to-weight ratio, and perioperative weight loss are risk factors associated with SMA syndrome after pediatric spine surgery.
We report the case of a 14-year-old boy who developed SMA syndrome after undergoing scoliosis surgery. The patient and his mother provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old boy with a history of idiopathic scoliosis presented to Cohen Children’s Hospital (Long Island Jewish Medical Center) with bilious vomiting that had persisted for 7 days after posterior T9–L4 fusion with instrumentation.
Discussion
SMA syndrome is attributed to the anatomical orientation of the third part of the duodenum, which passes between the aorta and the SMA (Figure 4).
Adolescents are particularly vulnerable to this condition. Faster adolescent bone growth relative to visceral growth is accompanied by a decrease in SMA angle.3 Occasionally, body casts are used after surgery to immobilize the vertebrae and augment healing. Cast syndrome occurs when pressure from a body cast causes a bowel obstruction secondary to spinal hyperextension and amplified spinal lordosis.2 This finding, dating to the 19th century, was reported by Willet4 when a patient died 48 hours after application of a body cast. In 1950, the term cast syndrome was coined after a motorcyclist’s injuries were treated with a hip spica cast and the patient died of cardiovascular collapse secondary to persistent vomiting.5
Table 1 summarizes various evaluation, diagnosis, and treatment algorithms designed to optimize nutrition and weight in patients developing signs and symptoms of SMA syndrome after posterior spinal instrumentation and fusion for adolescent idiopathic scoliosis (AIS).
The third unique feature in this case is electrocardiogram findings. Although some cases briefly discussed electrolyte abnormalities, none presented evidence that these abnormalities caused cardiac changes.6,16,18 The overall clinical significance of the QT prolongation in our patient’s case is unknown, as this finding was improved with correction of the electrolyte abnormalities and appropriate fluid replenishment.
Early recognition of nonspecific symptoms (eg, abdominal pain, tenderness, distension, bilious or projectile vomiting, hypoactive bowel sounds, anorexia) plays a key role in preventing severe morbidity and mortality from SMA syndrome after scoliosis surgery. Although many patients present in the semiclassic obstructed pattern, notable reasons for diagnostic delay include normal appetite and bowel sounds.3 For example, SMA syndrome may be misdiagnosed as stomach flu because of unfamiliarity with disease diagnosis and management.20 Complications of SMA syndrome can potentially lead to aspiration pneumonia, acute gastric rupture, and cardiovascular collapse and death.
Am J Orthop. 2017;46(2):E124-E130. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Evarts CM, Winter RB, Hall JE. Vascular compression of the duodenum associated with the treatment of scoliosis. Review of the literature and report of eighteen cases. J Bone Joint Surg Am. 1971;53(3):431-444.
2. Zhu ZZ, Qiu Y. Superior mesenteric artery syndrome following scoliosis surgery: its risk indicators and treatment strategy. World J Gastroenterol. 2005;11(21):3307-3310.
3. Hutchinson DT, Bassett GS. Superior mesenteric artery syndrome in pediatric orthopedic patients. Clin Orthop Relat Res. 1990;(250):250-257.
4. Willet A. Fatal vomiting following application of plaster-of-Paris bandage in case of spinal curvature. St Barth Hosp Rep. 1878;14:333-335.
5. Dorph MH. The cast syndrome; review of the literature and report of a case. N Engl J Med. 1950;243(12):440-442.
6. Lam DJ, Lee JZ, Chua JH, Lee YT, Lim KB. Superior mesenteric artery syndrome following surgery for adolescent idiopathic scoliosis: a case series, review of the literature, and an algorithm for management. J Pediatr Orthop B. 2014;23(4):312-318.
7. Tsirikos AI, Anakwe RE, Baker AD. Late presentation of superior mesenteric artery syndrome following scoliosis surgery: a case report. J Med Case Rep. 2008;2:9.
8. Akin JT Jr, Skandalakis JE, Gray SW. The anatomic basis of vascular compression of the duodenum. Surg Clin North Am. 1974;54(6):1361-1370.
9. Amy BW, Priebe CJ Jr, King A. Superior mesenteric artery syndrome associated with scoliosis treated by a modified Ladd procedure. J Pediatr Orthop. 1985;5(3):361-363.
10. Richardson WS, Surowiec WJ. Laparoscopic repair of superior mesenteric artery syndrome. Am J Surg. 2001;181(4):377-378.
11. Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83(8):1169-1181.
12. Braun SV, Hedden DM, Howard AW. Superior mesenteric artery syndrome following spinal deformity correction. J Bone Joint Surg Am. 2006;88(10):2252-2257.
13. Smith BG, Hakim-Zargar M, Thomson JD. Low body mass index: a risk factor for superior mesenteric artery syndrome in adolescents undergoing spinal fusion for scoliosis. J Spinal Disord Tech. 2009;22(2):144-148.
14. Pan CH, Tzeng ST, Chen CS, Chen PQ. Superior mesenteric artery syndrome complicating staged corrective surgery for scoliosis. J Formos Med Assoc. 2007;106(2 suppl):S37-S45.
15. Kennedy RH, Cooper MJ. An unusually severe case of the cast syndrome. Postgrad Med J. 1983;59(694):539-540.
16. Keskin M, Akgül T, Bayraktar A, Dikici F, Balik E. Superior mesenteric artery syndrome: an infrequent complication of scoliosis surgery. Case Rep Surg. 2014;2014:263431.
17. Amarawickrama H, Harikrishnan A, Krijgsman B. Superior mesenteric artery syndrome in a young girl following spinal surgery for scoliosis. Br J Hosp Med. 2005;66(12):700-701.
18. Crowther MA, Webb PJ, Eyre-Brook IA. Superior mesenteric artery syndrome following surgery for scoliosis. Spine. 2002;27(24):E528-E533.
19. Moskovich R, Cheong-Leen P. Vascular compression of the duodenum. J R Soc Med. 1986;79(8):465-467.
20. Shah MA, Albright MB, Vogt MT, Moreland MS. Superior mesenteric artery syndrome in scoliosis surgery: weight percentile for height as an indicator of risk. J Pediatr Orthop. 2003;23(5):665-668.
1. Evarts CM, Winter RB, Hall JE. Vascular compression of the duodenum associated with the treatment of scoliosis. Review of the literature and report of eighteen cases. J Bone Joint Surg Am. 1971;53(3):431-444.
2. Zhu ZZ, Qiu Y. Superior mesenteric artery syndrome following scoliosis surgery: its risk indicators and treatment strategy. World J Gastroenterol. 2005;11(21):3307-3310.
3. Hutchinson DT, Bassett GS. Superior mesenteric artery syndrome in pediatric orthopedic patients. Clin Orthop Relat Res. 1990;(250):250-257.
4. Willet A. Fatal vomiting following application of plaster-of-Paris bandage in case of spinal curvature. St Barth Hosp Rep. 1878;14:333-335.
5. Dorph MH. The cast syndrome; review of the literature and report of a case. N Engl J Med. 1950;243(12):440-442.
6. Lam DJ, Lee JZ, Chua JH, Lee YT, Lim KB. Superior mesenteric artery syndrome following surgery for adolescent idiopathic scoliosis: a case series, review of the literature, and an algorithm for management. J Pediatr Orthop B. 2014;23(4):312-318.
7. Tsirikos AI, Anakwe RE, Baker AD. Late presentation of superior mesenteric artery syndrome following scoliosis surgery: a case report. J Med Case Rep. 2008;2:9.
8. Akin JT Jr, Skandalakis JE, Gray SW. The anatomic basis of vascular compression of the duodenum. Surg Clin North Am. 1974;54(6):1361-1370.
9. Amy BW, Priebe CJ Jr, King A. Superior mesenteric artery syndrome associated with scoliosis treated by a modified Ladd procedure. J Pediatr Orthop. 1985;5(3):361-363.
10. Richardson WS, Surowiec WJ. Laparoscopic repair of superior mesenteric artery syndrome. Am J Surg. 2001;181(4):377-378.
11. Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83(8):1169-1181.
12. Braun SV, Hedden DM, Howard AW. Superior mesenteric artery syndrome following spinal deformity correction. J Bone Joint Surg Am. 2006;88(10):2252-2257.
13. Smith BG, Hakim-Zargar M, Thomson JD. Low body mass index: a risk factor for superior mesenteric artery syndrome in adolescents undergoing spinal fusion for scoliosis. J Spinal Disord Tech. 2009;22(2):144-148.
14. Pan CH, Tzeng ST, Chen CS, Chen PQ. Superior mesenteric artery syndrome complicating staged corrective surgery for scoliosis. J Formos Med Assoc. 2007;106(2 suppl):S37-S45.
15. Kennedy RH, Cooper MJ. An unusually severe case of the cast syndrome. Postgrad Med J. 1983;59(694):539-540.
16. Keskin M, Akgül T, Bayraktar A, Dikici F, Balik E. Superior mesenteric artery syndrome: an infrequent complication of scoliosis surgery. Case Rep Surg. 2014;2014:263431.
17. Amarawickrama H, Harikrishnan A, Krijgsman B. Superior mesenteric artery syndrome in a young girl following spinal surgery for scoliosis. Br J Hosp Med. 2005;66(12):700-701.
18. Crowther MA, Webb PJ, Eyre-Brook IA. Superior mesenteric artery syndrome following surgery for scoliosis. Spine. 2002;27(24):E528-E533.
19. Moskovich R, Cheong-Leen P. Vascular compression of the duodenum. J R Soc Med. 1986;79(8):465-467.
20. Shah MA, Albright MB, Vogt MT, Moreland MS. Superior mesenteric artery syndrome in scoliosis surgery: weight percentile for height as an indicator of risk. J Pediatr Orthop. 2003;23(5):665-668.
Combined Anterior-Posterior Decompression and Fusion for Cervical Spondylotic Myelopathy
Take-Home Points
- Surgical intervention for cervical spondylosis and radiculopathy classically involves either an anterior or posterior approach for adequate decompression of the spinal cord and associated nerve roots.
- Combined anterior-posterior surgery for cervical spondylotic myelopathy is a relatively new technique that has previously been used for disorders of the thoracolumbar spine.
- Combined anterior-posterior cervical decompression and fusion for the treatment of cervical spondylotic myelopathy is associated with minor complications and excellent neurologic outcomes.
- Combined surgery can either be performed in a single day or in a staged manner, with current literature showing that same-day surgery is superior with respect to estimated blood loss and length of stay.
Cervical spondylotic myelopathy (CSM) is a degenerative disease characterized by progressive compression of the spinal cord. CSM has been found to be the most common cause of spinal impairment as well as the most frequently acquired cause of spinal dysfunction in people over 55 years of age.1,2 If left untreated, this condition can reduce manual dexterity and cause gait disturbances, dysesthesias, and weakness in the extremities. When conservative treatments fail, surgical intervention often becomes the preferred course of action for CSM and/or myeloradiculopathy.
The surgical approach for CSM and other advanced cervical spine (CS) deformities varies and is often a source of debate. Being a relatively safe and effective procedure, anterior decompression with fusion is optimal in treating discogenic lesions causing myelopathy but is less effective in multilevel disease.3,4 When pseudarthrosis, adjacent segment degeneration (ASD), and hardware failure are of concern, posterior decompressive laminectomy with instrumentation is a promising option.5 However, this method is less effective in restoring lordosis and can increase the risk for later clinical deterioration.6 There is a select subset of patients for whom a combined anterior-posterior approach is ideal.7-9In cases in which a combined anterior-posterior approach is identified as the best treatment option, whether to perform the operation in a sequential or staged manner must be decided, and this question is another source of debate. Single-day surgery is sometimes anecdotally criticized as posing a greater risk to the patient. On the other hand, some comparative studies have shown no statistically significant difference in major complication rates between the 2 options.10,11 More descriptive studies of combined anterior-posterior decompression and fusion (CAPDF) are needed to explore the efficacy of the procedure. In this article, we describe a study we conducted to characterize the operative data, perioperative complications, and short-term outcomes associated with CAPDF for the treatment of CSM in a select group of patients.
Methods
After receiving Institutional Review Board approval for this study (formal consent was not required), we retrospectively reviewed the charts of 21 patients who underwent CAPDF for CSM at our institution. All patients underwent surgery between February 2010 and March 2015. Criteria for inclusion in the study included same-day CAPDF for CSM. Staged procedures were excluded, as were combined procedures for the treatment of other diseases (eg, malignancies). All patients were operated on by the same primary surgeon (Dr. Davis) and co-surgeon (Dr. Labiak). The 1 patient who was lost to follow-up was excluded from the postoperative outcome analysis.
We reviewed the patients’ medical records for surgical consultations, operative reports, intraoperative reports, progress notes, and postoperative office visit reports. Demographic information included age, sex, body mass index, and preoperative risk factors, such as diabetes and tobacco use. All patients had been diagnosed with myelopathy. Clinical data included previous history of CS surgery, levels fused (and number of levels fused) anteriorly and posteriorly, operative time, estimated blood loss (EBL), length of stay (LOS), and perioperative complications. Short-term (3-month follow-up) neurologic improvement was determined both objectively, with the Nurick grading system,12 and subjectively, with determination of patient quality of life before and after surgery and with neurologic examination.
Operative Technique: Anterior Approach
All operations were performed with continuous somatosensory evoked potential monitoring of both upper and lower extremities. Each patient, positioned supine with the head in a neutral position, underwent standard endotracheal intubation. Intubation was followed by a transverse incision and dissection down to the deep cervical fascia with maintenance of the carotid sheath laterally and tracheoesophageal complex medially. Interspaces were identified and later were confirmed with lateral radiographs. Discectomy, osteophytectomy, and removal of hypertrophied or calcified ligament were then performed until decompression was satisfactory. Corpectomies were not performed. Polyetheretherketone interbody spacers (Stryker) were used with autograft harvested from vertebral body resection. Low-profile screw-plate systems were placed. After completion of the anterior procedure, the patient was placed prone, with the head fixed in a Mayfield clamping device in neutral position and with all pressure points carefully padded.
Operative Technique: Posterior Approach
A midline incision was made through the skin and subcutaneous tissue to the level of the deep cervical fascia. Then, dissection was performed to the tips of the lateral masses. Instrumentation and fusion preceded spinal decompression. This order, chosen to preserve bony landmarks for guidance during instrumentation, did not interfere with subsequent decompression. Segmental spinal instrumentation was placed using lateral mass screw-rod fixation. After the laminae and ligamenta flava were bilaterally mobilized, the entire bony ligamentous complex spanning the area of fusion was removed en masse (most commonly C3–C7) in order to decrease the number of instrument passes near the spinal cord. Next, a modest foraminotomy was performed to extend the opening laterally and ensure adequate decompression of the nerve roots. Autograft harvested from the spinous processes and laminae was used. The posterior portion of the operation contributed significantly to blood loss and postoperative pain during the perioperative period. We recommend performing a very meticulous dissection to minimize these consequences. No patient in this study required a halo orthosis.
Results
Twenty-one patients with CSM were treated with CAPDF between February 2010 and March 2015 (Table 1).
Table 2 summarizes the operative data. Mean number of levels fused was 2 (range, 1-3) anteriorly and 3 (range, 1-4) posteriorly.
Of the 21 patients, 9 (42.3%) had at least 1 complication during the perioperative period. Table 3 summarizes all encountered complications. Neither neurologic instability nor mortality was observed after surgery.
Patient 7 was lost to follow-up. For the other 20 patients, mean time to “3-month follow-up” was 96 days (range, 51-149 days). The most commonly noted improvements in quality of life included resolution of numbness, improvement in gait, and return to previous activities, such as walking and even exercising.
Representative Case
Patient 15, a 53-year-old man, presented with complaints of dysesthesias of the hands. Focused neurologic evaluation at the time revealed limited CS range of motion on extension. The patient (Figures 2A-2D) was diffusely hyperreflexic and had pathologic spread in the upper extremities.
Discussion
Cervical myelopathy is a common yet frequently underdiagnosed disease, owing to the fact that many patients remain asymptomatic even after experiencing degenerative changes in the spinal column.14-16 The additive effects of spondylosis, osteophyte formation, ligamentous hypertrophy, and listhesis lead to progressive canal and intervertebral foraminal compromise, ultimately producing the clinical syndromes of myelopathy and radiculopathy.17 The characteristic symptoms of CSM are known to have an insidious onset. In the early stages, patients note a subtle gait disturbance and later experience manual dexterity reductions and upper extremity dysesthesias.18 As the condition progresses and conservative management fails, surgical intervention is sought.
Nevertheless, the pursuit of surgical treatment for CSM remains somewhat controversial. Some authors have found no statistically significant difference between conservative and surgical management of mild to moderate CSM,19 whereas others have found that surgically treated patients had much better outcomes than their medically treated counterparts.20 In 2010, Scardino and colleagues21 reported that CSM patients who were bedridden and/or wheelchair-bound with seemingly irreversible myelopathy were capable of neurologic improvement after surgical intervention. At the very least, what remains clear is that untreated CSM is known to follow an unpredictable course, with the condition deteriorating faster for some patients than others.22Traditional anterior or posterior approaches, which can be used in the majority of cases of cervical spondylosis and/or radiculopathy, have been compared extensively.23,24 The inverse relationship concerning the integrity of an anterior construct and the number of levels fused is a well-established clinical finding.3,4,8,25-28 Laminectomy with fusion is not without its disadvantages: Cervical instability secondary to mechanical loss of posterior cervical support, and subsequent post-laminectomy kyphosis, is a common complication.23 In cases in which more stability is required, the combined anterior-posterior approach is more promising than either approach alone. This technique has its roots in the treatment of several thoracolumbar spine disorders, including infections, scoliosis, trauma, and tumors.29-31 More recently, the technique has been applied to CS disorders.
In 2008, Gok and colleagues32 retrospectively compared the results of anterior-only fusion and CAPDF for CSM. Forty-six patients underwent anterior surgery only, and 21 underwent CAPDF. The groups’ complication rates were similar: 28.6% (anterior only) and 24% (CAPDF); the incidence of ASD was lower in the combined group. Song and colleagues33 conducted a similar study in 2010. They compared anterior fusion alone and CAPDF in treating degenerative cervical kyphosis. Results were strongly in favor of the combined technique, as it led to “greater correction of sagittal alignment, a better maintenance of correction angle, a higher rate of fusion, a lower rate of subsidence and lower complications.” Both studies established that, in a select group of patients, the benefits of CAPDF outweighed the risks. These findings, combined with our study’s findings of no major complications and the transience of minor complications, suggest CAPDF should not be considered too invasive or risky.
The results of our study also mirror those of 3 other studies on the use of CAPDF for CS disorders. In 1995, McAfee and colleagues34 reported on a group of 100 patients with follow-up of 2 years or more. In most cases, the surgical indication was trauma, but neoplasm, infection, rheumatoid arthritis, and CSM were found as well. Outcomes were very favorable: improvement in a previous neurologic deficit (57/75 patients), ability to walk again (21/35 patients), no new neurologic deficits, and no hardware failures. In 2000, Schultz and colleagues35 retrospectively reviewed the cases of 72 patients who underwent CAPDF for a variety of complex CS disorders. Two of the 72 experienced transient neurologic deficits, and, though the immediate complication rate was relatively high (32%), the long-term complication rate was down to 5%. In 2009, Konya and colleagues36 retrospectively reviewed the cases of 40 patients who underwent CAPDF, primarily for CSM. Within 1 week after surgery, neurologic deficits were reduced in 36 patients; by 1 year after surgery, neurologic deficits were reduced in all 40 patients, and fusion was achieved in 39. These 3 studies34-36 helped establish CAPDF of the CS as a viable and effective procedure that can be performed within a single day.
Although many physicians have achieved favorable results with single-day surgery, the decision to operate in a sequential or staged manner remains controversial. Some anecdotally claim CAPDF poses a greater operative risk to the patient. In 1991, the continuous procedure was found to involve less blood loss and shorter LOS while providing for better correction of severe spinal deformity in patients with scoliosis and rigid kyphosis.37 Three more recent comparative studies examining the same issue in the treatment of CS diseases found staging did not reduce the complication rate and may in fact have been associated with higher complication rates, more blood loss, and longer total operative time and LOS.10,11,38 Our study’s lower blood loss, shorter LOS, and lower major complication rate relative to the combined groups in all 3 of those studies are most likely attributable to our operating on a lower mean number of spinal levels and our restricting the surgical indication to CSM. The positive short-term outcomes and low rate of long-term complications in our study, combined with the data from these 3 comparative studies, suggest that same-day surgery is superior to staged surgery. A staged operation should be considered only if the patient cannot tolerate long periods under general anesthesia.
Many have advocated extending fusion down to T1 to prevent ASD at the C7–T1 disk space.35,39,40 We decided against this approach for 2 reasons. First, at C7, lateral mass screws were always chosen over pedicle screws. When possible, shorter lateral mass screws were used at this level, making C7 much less rigid. Second, the C7–T1 facet capsule was maintained to preserve joint integrity. We suggest extending fusion down to T1 only if there is prior evidence of spinal disease and/or listhesis at C7–T1. Although long-term (many-year) follow-up is often desired, we specifically assessed short-term (3-month) outcomes. We have anecdotally found that degree of improvement often follows a predictable course after 3-month follow-up. If myelopathy resolves even to a small extent during the first 3 postoperative months, later improvement will likely follow an upward course. Conversely, if myelopathy does not improve during the first 3 months, further improvement is much less likely.
This trend in neurologic improvement likely is directly related to degree of myelopathy before surgery. Patients with CSM generally experience symptoms over an extended period and try conservative management before any surgical consultation. Although spinal ischemia is often resolved by decompression, permanent ischemic damage to the cord is not uncommon. In this setting, postoperative neurologic improvement is minimal or even nonexistent, and decompression is preventive rather than curative. In our study, 1 patient had no subjective improvement after surgery. At 3-month follow-up, magnetic resonance imaging showed notable myelomalacia without residual spinal cord compression. We attribute the failure of the ischemic changes to resolve to long-standing preoperative damage to the cord. Nevertheless, surgery stabilized the myelopathy and prevented further ischemic damage and clinical deterioration.
As is the case with any operation, patients must be carefully selected for CAPDF. Indications for CAPDF, as described by Kim and Alexander,7 include acute spinal trauma, post-laminectomy kyphosis, kyphotic deformity with intact posterior tension band, multilevel spondylosis and OPLL, and preexisting risk factors for pseudarthrosis. Clearly, the severity of each varies, and the pathologies are not mutually exclusive. We emphasize that these indications provide only a guideline for performing CAPDF, and patients must be selected on a case-by-case basis. All the patients in our study were symptomatic and exhibited significant compression of the spinal cord anteriorly and posteriorly at multiple levels. Several presented with concomitant pathologies, such as cervical kyphotic deformity, congenital spinal stenosis, and OPLL. In each case, the indication for surgical intervention was undoubted. We sought both to improve the patient’s baseline symptoms and to prevent further damage to the spinal cord.
This study had its limitations. First, its retrospective design predisposed it to a higher degree of bias. Second, because CAPDF is not commonly performed, the sample size was relatively small. Third, although it provided a descriptive analysis of CAPDF for CSM, the study did not use a direct comparison group to establish whether treatment within a single day or staged treatment was more beneficial for our cohort in particular. On the basis of prior experience and observation, we think performing the operation within a single day is much more beneficial for the patient. Our discussion of studies that have compared same-day and staged surgery supports this observation. Therefore, staged treatment was not recommended to our patients.
Conclusion
Few descriptive studies have explored CAPDF for CSM. Our study’s results showed the procedure was associated with minor complications and provided symptomatic relief for a majority of patients as early as 3 months after surgery. In addition, CAPDF can be successfully performed sequentially within a single day. As such, it represents an excellent option for treating multilevel symptomatic CSM cases that require more extensive spinal decompression and more stability.
Am J Orthop. 2017;46(2):E97-E104. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 suppl):190S-197S.
2. Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist. 2013;19(4):409-421.
3. Sasso RC, Ruggiero RA Jr, Reilly TM, Hall PV. Early reconstruction failures after multilevel cervical corpectomy. Spine. 2003;28(2):140-142.
4. Zdeblick TA, Hughes SS, Riew KD, Bohlman HH. Failed anterior cervical discectomy and arthrodesis. Analysis and treatment of thirty-five patients. J Bone Joint Surg Am. 1997;79(4):523-532.
5. Zhu B, Xu Y, Liu X, Liu Z, Dang G. Anterior approach versus posterior approach for the treatment of multilevel cervical spondylotic myelopathy: a systemic review and meta-analysis. Eur Spine J. 2013;22(7):1583-1593.
6. Cabraja M, Abbushi A, Koeppen D, Kroppenstedt S, Woiciechowsky C. Comparison between anterior and posterior decompression with instrumentation for cervical spondylotic myelopathy: sagittal alignment and clinical outcome. Neurosurg Focus. 2010;28(3):E15.
7. Kim PK, Alexander JT. Indications for circumferential surgery for cervical spondylotic myelopathy. Spine J. 2006;6(6 suppl):299S-307S.
8. König SA, Ranguis S, Spetzger U. Management of complex cervical instability. J Neurol Surg A Cent Eur Neurosurg. 2015;76(2):119-125.
9. König SA, Spetzger U. Surgical management of cervical spondylotic myelopathy—indications for anterior, posterior or combined procedures for decompression and stabilisation. Acta Neurochir. 2014;156(2):253-258.
10. Harel R, Hwang R, Fakhar M, et al. Circumferential cervical surgery: to stage or not to stage? J Spinal Disord Tech. 2013;26(4):183-188.
11. Siemionow K, Tyrakowski M, Patel K, Neckrysh S. Comparison of perioperative complications following staged versus one-day anterior and posterior cervical decompression and fusion crossing the cervico-thoracic junction. Neurol Neurochir Pol. 2014;48(6):403-409.
12. Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95(1):87-100.
13. Chen CJ, Saulle D, Fu KM, Smith JS, Shaffrey CI. Dysphagia following combined anterior-posterior cervical spine surgeries. J Neurosurg Spine. 2013;19(3):279-287.
14. Boden SD, McCowin PR, Davis DO, Dina TS, Mark AS, Wiesel S. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72(8):1178-1184.
15. Gore DR, Sepic SB, Gardner GM. Roentgenographic findings of the cervical spine in asymptomatic people. Spine. 1986;11(6):521-524.
16. Law MD Jr, Bernhardt M, White AA 3rd. Cervical spondylotic myelopathy: a review of surgical indications and decision making. Yale J Biol Med. 1993;66(3):165-177.
17. Kelly JC, Groarke PJ, Butler JS, Poynton AR, O’Byrne JM. The natural history and clinical syndromes of degenerative cervical spondylosis. Adv Orthop. 2012;(2012):393642.
18. Baron EM, Young WF. Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery. 2007;60(1 suppl 1):S35-S41.
19. Kadanka Z, Mares M, Bednarik J, et al. Approaches to spondylotic cervical myelopathy: conservative versus surgical results in a 3-year follow-up study. Spine. 2002;27(20):2205-2210.
20. Sampath P, Bendebba M, Davis JD, Ducker TB. Outcome of patients treated for cervical myelopathy. A prospective, multicenter study with independent clinical review. Spine. 2000;25(6):670-676.
21. Scardino FB, Rocha LP, Barcelos AC, Rotta JM, Botelho RV. Is there a benefit to operating on patients (bedridden or in wheelchairs) with advanced stage cervical spondylotic myelopathy? Eur Spine J. 2010;19(5):699-705.
22. Edwards CC 2nd, Riew KD, Anderson PA, Hilibrand AS, Vaccaro AF. Cervical myelopathy. Current diagnostic and treatment strategies. Spine J. 2003;3(1):68-81.
23. Herkowitz HN. A comparison of anterior cervical fusion, cervical laminectomy, and cervical laminoplasty for the surgical management of multiple level spondylotic radiculopathy. Spine. 1988;13(7):774-780.
24. Hukuda S, Mochizuki T, Ogata M, Shichikawa K, Shimomura Y. Operations for cervical spondylotic myelopathy. A comparison of the results of anterior and posterior procedures. J Bone Joint Surg Br. 1985;67(4):609-615.
25. Fernyhough JC, White JI, LaRocca H. Fusion rates in multilevel cervical spondylosis comparing allograft fibula with autograft fibula in 126 patients. Spine. 1991;16(10 suppl):S561-S564.
26. Macdonald RL, Fehlings MG, Tator CH, et al. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J Neurosurg. 1997;86(6):990-997.
27. Mayr MT, Subach BR, Comey CH, Rodts GE, Haid RW Jr. Cervical spinal stenosis: outcome after anterior corpectomy, allograft reconstruction, and instrumentation. J Neurosurg. 2002;96(1 suppl):10-16.
28. Swank ML, Lowery GL, Bhat AL, McDonough RF. Anterior cervical allograft arthrodesis and instrumentation: multilevel interbody grafting or strut graft reconstruction. Eur Spine J. 1997;6(2):138-143.
29. Böhm H, Harms J, Donk R, Zielke K. Correction and stabilization of angular kyphosis. Clin Orthop Relat Res. 1990;(258):56-61.
30. Spencer DL, DeWald RL. Simultaneous anterior and posterior surgical approach to the thoracic and lumbar spine. Spine. 1979;4(1):29-36.
31. Whitesides TE Jr, Shah SGA. On the management of unstable fractures of the thoracolumbar spine: rationale for use of anterior decompression and fusion and posterior stabilization. Spine. 1976;1(2):99-107.
32. Gok B, Sciubba DM, McLoughlin GS, et al. Surgical treatment of cervical spondylotic myelopathy with anterior compression: a review of 67 cases. J Neurosurg Spine. 2008;9(2):152-157.
33. Song KJ, Johnson JS, Choi BR, Wang JC, Lee KB. Anterior fusion alone compared with combined anterior and posterior fusion for the treatment of degenerative cervical kyphosis. J Bone Joint Surg Br. 2010;92(11):1548-1552.
34. McAfee PC, Bohlman HH, Ducker TB, Zeidman SM, Goldstein JA. One-stage anterior cervical decompression and posterior stabilization. A study of one hundred patients with a minimum of two years of follow-up. J Bone Joint Surg Am. 1995;77(12):1791-1800.
35. Schultz KD Jr, McLaughlin MR, Haid RW Jr, Comey CH, Rodts GE Jr, Alexander J. Single-stage anterior-posterior decompression and stabilization for complex cervical spine disorders. J Neurosurg. 2000;93(2 suppl):214-221.
36. Konya D, Ozgen S, Gercek A, Pamir MN. Outcomes for combined anterior and posterior surgical approaches for patients with multisegmental cervical spondylotic myelopathy. J Clin Neurosci. 2009;16(3):404-409.
37. Shufflebarger HL, Grimm JO, Bui V, Thomson JD. Anterior and posterior spinal fusion. Staged versus same-day surgery. Spine. 1991;16(8):930-933.
38. Ozturk C, Aydinli U, Vural R, Sehirlioglu A, Mutlu M. Simultaneous versus sequential one-stage combined anterior and posterior spinal surgery for spinal infections (outcomes and complications). Int Orthop. 2007;31(3):363-366.
39. Aryan HE, Sanchez-Mejia RO, Ben-Haim S, Ames CP. Successful treatment of cervical myelopathy with minimal morbidity by circumferential decompression and fusion. Eur Spine J. 2007;16(9):1401-1409.
40. Steinmetz MP, Miller J, Warbel A, Krishnaney AA, Bingaman W, Benzel EC. Regional instability following cervicothoracic junction surgery. J Neurosurg Spine. 2006;4(4):278-284.
Take-Home Points
- Surgical intervention for cervical spondylosis and radiculopathy classically involves either an anterior or posterior approach for adequate decompression of the spinal cord and associated nerve roots.
- Combined anterior-posterior surgery for cervical spondylotic myelopathy is a relatively new technique that has previously been used for disorders of the thoracolumbar spine.
- Combined anterior-posterior cervical decompression and fusion for the treatment of cervical spondylotic myelopathy is associated with minor complications and excellent neurologic outcomes.
- Combined surgery can either be performed in a single day or in a staged manner, with current literature showing that same-day surgery is superior with respect to estimated blood loss and length of stay.
Cervical spondylotic myelopathy (CSM) is a degenerative disease characterized by progressive compression of the spinal cord. CSM has been found to be the most common cause of spinal impairment as well as the most frequently acquired cause of spinal dysfunction in people over 55 years of age.1,2 If left untreated, this condition can reduce manual dexterity and cause gait disturbances, dysesthesias, and weakness in the extremities. When conservative treatments fail, surgical intervention often becomes the preferred course of action for CSM and/or myeloradiculopathy.
The surgical approach for CSM and other advanced cervical spine (CS) deformities varies and is often a source of debate. Being a relatively safe and effective procedure, anterior decompression with fusion is optimal in treating discogenic lesions causing myelopathy but is less effective in multilevel disease.3,4 When pseudarthrosis, adjacent segment degeneration (ASD), and hardware failure are of concern, posterior decompressive laminectomy with instrumentation is a promising option.5 However, this method is less effective in restoring lordosis and can increase the risk for later clinical deterioration.6 There is a select subset of patients for whom a combined anterior-posterior approach is ideal.7-9In cases in which a combined anterior-posterior approach is identified as the best treatment option, whether to perform the operation in a sequential or staged manner must be decided, and this question is another source of debate. Single-day surgery is sometimes anecdotally criticized as posing a greater risk to the patient. On the other hand, some comparative studies have shown no statistically significant difference in major complication rates between the 2 options.10,11 More descriptive studies of combined anterior-posterior decompression and fusion (CAPDF) are needed to explore the efficacy of the procedure. In this article, we describe a study we conducted to characterize the operative data, perioperative complications, and short-term outcomes associated with CAPDF for the treatment of CSM in a select group of patients.
Methods
After receiving Institutional Review Board approval for this study (formal consent was not required), we retrospectively reviewed the charts of 21 patients who underwent CAPDF for CSM at our institution. All patients underwent surgery between February 2010 and March 2015. Criteria for inclusion in the study included same-day CAPDF for CSM. Staged procedures were excluded, as were combined procedures for the treatment of other diseases (eg, malignancies). All patients were operated on by the same primary surgeon (Dr. Davis) and co-surgeon (Dr. Labiak). The 1 patient who was lost to follow-up was excluded from the postoperative outcome analysis.
We reviewed the patients’ medical records for surgical consultations, operative reports, intraoperative reports, progress notes, and postoperative office visit reports. Demographic information included age, sex, body mass index, and preoperative risk factors, such as diabetes and tobacco use. All patients had been diagnosed with myelopathy. Clinical data included previous history of CS surgery, levels fused (and number of levels fused) anteriorly and posteriorly, operative time, estimated blood loss (EBL), length of stay (LOS), and perioperative complications. Short-term (3-month follow-up) neurologic improvement was determined both objectively, with the Nurick grading system,12 and subjectively, with determination of patient quality of life before and after surgery and with neurologic examination.
Operative Technique: Anterior Approach
All operations were performed with continuous somatosensory evoked potential monitoring of both upper and lower extremities. Each patient, positioned supine with the head in a neutral position, underwent standard endotracheal intubation. Intubation was followed by a transverse incision and dissection down to the deep cervical fascia with maintenance of the carotid sheath laterally and tracheoesophageal complex medially. Interspaces were identified and later were confirmed with lateral radiographs. Discectomy, osteophytectomy, and removal of hypertrophied or calcified ligament were then performed until decompression was satisfactory. Corpectomies were not performed. Polyetheretherketone interbody spacers (Stryker) were used with autograft harvested from vertebral body resection. Low-profile screw-plate systems were placed. After completion of the anterior procedure, the patient was placed prone, with the head fixed in a Mayfield clamping device in neutral position and with all pressure points carefully padded.
Operative Technique: Posterior Approach
A midline incision was made through the skin and subcutaneous tissue to the level of the deep cervical fascia. Then, dissection was performed to the tips of the lateral masses. Instrumentation and fusion preceded spinal decompression. This order, chosen to preserve bony landmarks for guidance during instrumentation, did not interfere with subsequent decompression. Segmental spinal instrumentation was placed using lateral mass screw-rod fixation. After the laminae and ligamenta flava were bilaterally mobilized, the entire bony ligamentous complex spanning the area of fusion was removed en masse (most commonly C3–C7) in order to decrease the number of instrument passes near the spinal cord. Next, a modest foraminotomy was performed to extend the opening laterally and ensure adequate decompression of the nerve roots. Autograft harvested from the spinous processes and laminae was used. The posterior portion of the operation contributed significantly to blood loss and postoperative pain during the perioperative period. We recommend performing a very meticulous dissection to minimize these consequences. No patient in this study required a halo orthosis.
Results
Twenty-one patients with CSM were treated with CAPDF between February 2010 and March 2015 (Table 1).
Table 2 summarizes the operative data. Mean number of levels fused was 2 (range, 1-3) anteriorly and 3 (range, 1-4) posteriorly.
Of the 21 patients, 9 (42.3%) had at least 1 complication during the perioperative period. Table 3 summarizes all encountered complications. Neither neurologic instability nor mortality was observed after surgery.
Patient 7 was lost to follow-up. For the other 20 patients, mean time to “3-month follow-up” was 96 days (range, 51-149 days). The most commonly noted improvements in quality of life included resolution of numbness, improvement in gait, and return to previous activities, such as walking and even exercising.
Representative Case
Patient 15, a 53-year-old man, presented with complaints of dysesthesias of the hands. Focused neurologic evaluation at the time revealed limited CS range of motion on extension. The patient (Figures 2A-2D) was diffusely hyperreflexic and had pathologic spread in the upper extremities.
Discussion
Cervical myelopathy is a common yet frequently underdiagnosed disease, owing to the fact that many patients remain asymptomatic even after experiencing degenerative changes in the spinal column.14-16 The additive effects of spondylosis, osteophyte formation, ligamentous hypertrophy, and listhesis lead to progressive canal and intervertebral foraminal compromise, ultimately producing the clinical syndromes of myelopathy and radiculopathy.17 The characteristic symptoms of CSM are known to have an insidious onset. In the early stages, patients note a subtle gait disturbance and later experience manual dexterity reductions and upper extremity dysesthesias.18 As the condition progresses and conservative management fails, surgical intervention is sought.
Nevertheless, the pursuit of surgical treatment for CSM remains somewhat controversial. Some authors have found no statistically significant difference between conservative and surgical management of mild to moderate CSM,19 whereas others have found that surgically treated patients had much better outcomes than their medically treated counterparts.20 In 2010, Scardino and colleagues21 reported that CSM patients who were bedridden and/or wheelchair-bound with seemingly irreversible myelopathy were capable of neurologic improvement after surgical intervention. At the very least, what remains clear is that untreated CSM is known to follow an unpredictable course, with the condition deteriorating faster for some patients than others.22Traditional anterior or posterior approaches, which can be used in the majority of cases of cervical spondylosis and/or radiculopathy, have been compared extensively.23,24 The inverse relationship concerning the integrity of an anterior construct and the number of levels fused is a well-established clinical finding.3,4,8,25-28 Laminectomy with fusion is not without its disadvantages: Cervical instability secondary to mechanical loss of posterior cervical support, and subsequent post-laminectomy kyphosis, is a common complication.23 In cases in which more stability is required, the combined anterior-posterior approach is more promising than either approach alone. This technique has its roots in the treatment of several thoracolumbar spine disorders, including infections, scoliosis, trauma, and tumors.29-31 More recently, the technique has been applied to CS disorders.
In 2008, Gok and colleagues32 retrospectively compared the results of anterior-only fusion and CAPDF for CSM. Forty-six patients underwent anterior surgery only, and 21 underwent CAPDF. The groups’ complication rates were similar: 28.6% (anterior only) and 24% (CAPDF); the incidence of ASD was lower in the combined group. Song and colleagues33 conducted a similar study in 2010. They compared anterior fusion alone and CAPDF in treating degenerative cervical kyphosis. Results were strongly in favor of the combined technique, as it led to “greater correction of sagittal alignment, a better maintenance of correction angle, a higher rate of fusion, a lower rate of subsidence and lower complications.” Both studies established that, in a select group of patients, the benefits of CAPDF outweighed the risks. These findings, combined with our study’s findings of no major complications and the transience of minor complications, suggest CAPDF should not be considered too invasive or risky.
The results of our study also mirror those of 3 other studies on the use of CAPDF for CS disorders. In 1995, McAfee and colleagues34 reported on a group of 100 patients with follow-up of 2 years or more. In most cases, the surgical indication was trauma, but neoplasm, infection, rheumatoid arthritis, and CSM were found as well. Outcomes were very favorable: improvement in a previous neurologic deficit (57/75 patients), ability to walk again (21/35 patients), no new neurologic deficits, and no hardware failures. In 2000, Schultz and colleagues35 retrospectively reviewed the cases of 72 patients who underwent CAPDF for a variety of complex CS disorders. Two of the 72 experienced transient neurologic deficits, and, though the immediate complication rate was relatively high (32%), the long-term complication rate was down to 5%. In 2009, Konya and colleagues36 retrospectively reviewed the cases of 40 patients who underwent CAPDF, primarily for CSM. Within 1 week after surgery, neurologic deficits were reduced in 36 patients; by 1 year after surgery, neurologic deficits were reduced in all 40 patients, and fusion was achieved in 39. These 3 studies34-36 helped establish CAPDF of the CS as a viable and effective procedure that can be performed within a single day.
Although many physicians have achieved favorable results with single-day surgery, the decision to operate in a sequential or staged manner remains controversial. Some anecdotally claim CAPDF poses a greater operative risk to the patient. In 1991, the continuous procedure was found to involve less blood loss and shorter LOS while providing for better correction of severe spinal deformity in patients with scoliosis and rigid kyphosis.37 Three more recent comparative studies examining the same issue in the treatment of CS diseases found staging did not reduce the complication rate and may in fact have been associated with higher complication rates, more blood loss, and longer total operative time and LOS.10,11,38 Our study’s lower blood loss, shorter LOS, and lower major complication rate relative to the combined groups in all 3 of those studies are most likely attributable to our operating on a lower mean number of spinal levels and our restricting the surgical indication to CSM. The positive short-term outcomes and low rate of long-term complications in our study, combined with the data from these 3 comparative studies, suggest that same-day surgery is superior to staged surgery. A staged operation should be considered only if the patient cannot tolerate long periods under general anesthesia.
Many have advocated extending fusion down to T1 to prevent ASD at the C7–T1 disk space.35,39,40 We decided against this approach for 2 reasons. First, at C7, lateral mass screws were always chosen over pedicle screws. When possible, shorter lateral mass screws were used at this level, making C7 much less rigid. Second, the C7–T1 facet capsule was maintained to preserve joint integrity. We suggest extending fusion down to T1 only if there is prior evidence of spinal disease and/or listhesis at C7–T1. Although long-term (many-year) follow-up is often desired, we specifically assessed short-term (3-month) outcomes. We have anecdotally found that degree of improvement often follows a predictable course after 3-month follow-up. If myelopathy resolves even to a small extent during the first 3 postoperative months, later improvement will likely follow an upward course. Conversely, if myelopathy does not improve during the first 3 months, further improvement is much less likely.
This trend in neurologic improvement likely is directly related to degree of myelopathy before surgery. Patients with CSM generally experience symptoms over an extended period and try conservative management before any surgical consultation. Although spinal ischemia is often resolved by decompression, permanent ischemic damage to the cord is not uncommon. In this setting, postoperative neurologic improvement is minimal or even nonexistent, and decompression is preventive rather than curative. In our study, 1 patient had no subjective improvement after surgery. At 3-month follow-up, magnetic resonance imaging showed notable myelomalacia without residual spinal cord compression. We attribute the failure of the ischemic changes to resolve to long-standing preoperative damage to the cord. Nevertheless, surgery stabilized the myelopathy and prevented further ischemic damage and clinical deterioration.
As is the case with any operation, patients must be carefully selected for CAPDF. Indications for CAPDF, as described by Kim and Alexander,7 include acute spinal trauma, post-laminectomy kyphosis, kyphotic deformity with intact posterior tension band, multilevel spondylosis and OPLL, and preexisting risk factors for pseudarthrosis. Clearly, the severity of each varies, and the pathologies are not mutually exclusive. We emphasize that these indications provide only a guideline for performing CAPDF, and patients must be selected on a case-by-case basis. All the patients in our study were symptomatic and exhibited significant compression of the spinal cord anteriorly and posteriorly at multiple levels. Several presented with concomitant pathologies, such as cervical kyphotic deformity, congenital spinal stenosis, and OPLL. In each case, the indication for surgical intervention was undoubted. We sought both to improve the patient’s baseline symptoms and to prevent further damage to the spinal cord.
This study had its limitations. First, its retrospective design predisposed it to a higher degree of bias. Second, because CAPDF is not commonly performed, the sample size was relatively small. Third, although it provided a descriptive analysis of CAPDF for CSM, the study did not use a direct comparison group to establish whether treatment within a single day or staged treatment was more beneficial for our cohort in particular. On the basis of prior experience and observation, we think performing the operation within a single day is much more beneficial for the patient. Our discussion of studies that have compared same-day and staged surgery supports this observation. Therefore, staged treatment was not recommended to our patients.
Conclusion
Few descriptive studies have explored CAPDF for CSM. Our study’s results showed the procedure was associated with minor complications and provided symptomatic relief for a majority of patients as early as 3 months after surgery. In addition, CAPDF can be successfully performed sequentially within a single day. As such, it represents an excellent option for treating multilevel symptomatic CSM cases that require more extensive spinal decompression and more stability.
Am J Orthop. 2017;46(2):E97-E104. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Surgical intervention for cervical spondylosis and radiculopathy classically involves either an anterior or posterior approach for adequate decompression of the spinal cord and associated nerve roots.
- Combined anterior-posterior surgery for cervical spondylotic myelopathy is a relatively new technique that has previously been used for disorders of the thoracolumbar spine.
- Combined anterior-posterior cervical decompression and fusion for the treatment of cervical spondylotic myelopathy is associated with minor complications and excellent neurologic outcomes.
- Combined surgery can either be performed in a single day or in a staged manner, with current literature showing that same-day surgery is superior with respect to estimated blood loss and length of stay.
Cervical spondylotic myelopathy (CSM) is a degenerative disease characterized by progressive compression of the spinal cord. CSM has been found to be the most common cause of spinal impairment as well as the most frequently acquired cause of spinal dysfunction in people over 55 years of age.1,2 If left untreated, this condition can reduce manual dexterity and cause gait disturbances, dysesthesias, and weakness in the extremities. When conservative treatments fail, surgical intervention often becomes the preferred course of action for CSM and/or myeloradiculopathy.
The surgical approach for CSM and other advanced cervical spine (CS) deformities varies and is often a source of debate. Being a relatively safe and effective procedure, anterior decompression with fusion is optimal in treating discogenic lesions causing myelopathy but is less effective in multilevel disease.3,4 When pseudarthrosis, adjacent segment degeneration (ASD), and hardware failure are of concern, posterior decompressive laminectomy with instrumentation is a promising option.5 However, this method is less effective in restoring lordosis and can increase the risk for later clinical deterioration.6 There is a select subset of patients for whom a combined anterior-posterior approach is ideal.7-9In cases in which a combined anterior-posterior approach is identified as the best treatment option, whether to perform the operation in a sequential or staged manner must be decided, and this question is another source of debate. Single-day surgery is sometimes anecdotally criticized as posing a greater risk to the patient. On the other hand, some comparative studies have shown no statistically significant difference in major complication rates between the 2 options.10,11 More descriptive studies of combined anterior-posterior decompression and fusion (CAPDF) are needed to explore the efficacy of the procedure. In this article, we describe a study we conducted to characterize the operative data, perioperative complications, and short-term outcomes associated with CAPDF for the treatment of CSM in a select group of patients.
Methods
After receiving Institutional Review Board approval for this study (formal consent was not required), we retrospectively reviewed the charts of 21 patients who underwent CAPDF for CSM at our institution. All patients underwent surgery between February 2010 and March 2015. Criteria for inclusion in the study included same-day CAPDF for CSM. Staged procedures were excluded, as were combined procedures for the treatment of other diseases (eg, malignancies). All patients were operated on by the same primary surgeon (Dr. Davis) and co-surgeon (Dr. Labiak). The 1 patient who was lost to follow-up was excluded from the postoperative outcome analysis.
We reviewed the patients’ medical records for surgical consultations, operative reports, intraoperative reports, progress notes, and postoperative office visit reports. Demographic information included age, sex, body mass index, and preoperative risk factors, such as diabetes and tobacco use. All patients had been diagnosed with myelopathy. Clinical data included previous history of CS surgery, levels fused (and number of levels fused) anteriorly and posteriorly, operative time, estimated blood loss (EBL), length of stay (LOS), and perioperative complications. Short-term (3-month follow-up) neurologic improvement was determined both objectively, with the Nurick grading system,12 and subjectively, with determination of patient quality of life before and after surgery and with neurologic examination.
Operative Technique: Anterior Approach
All operations were performed with continuous somatosensory evoked potential monitoring of both upper and lower extremities. Each patient, positioned supine with the head in a neutral position, underwent standard endotracheal intubation. Intubation was followed by a transverse incision and dissection down to the deep cervical fascia with maintenance of the carotid sheath laterally and tracheoesophageal complex medially. Interspaces were identified and later were confirmed with lateral radiographs. Discectomy, osteophytectomy, and removal of hypertrophied or calcified ligament were then performed until decompression was satisfactory. Corpectomies were not performed. Polyetheretherketone interbody spacers (Stryker) were used with autograft harvested from vertebral body resection. Low-profile screw-plate systems were placed. After completion of the anterior procedure, the patient was placed prone, with the head fixed in a Mayfield clamping device in neutral position and with all pressure points carefully padded.
Operative Technique: Posterior Approach
A midline incision was made through the skin and subcutaneous tissue to the level of the deep cervical fascia. Then, dissection was performed to the tips of the lateral masses. Instrumentation and fusion preceded spinal decompression. This order, chosen to preserve bony landmarks for guidance during instrumentation, did not interfere with subsequent decompression. Segmental spinal instrumentation was placed using lateral mass screw-rod fixation. After the laminae and ligamenta flava were bilaterally mobilized, the entire bony ligamentous complex spanning the area of fusion was removed en masse (most commonly C3–C7) in order to decrease the number of instrument passes near the spinal cord. Next, a modest foraminotomy was performed to extend the opening laterally and ensure adequate decompression of the nerve roots. Autograft harvested from the spinous processes and laminae was used. The posterior portion of the operation contributed significantly to blood loss and postoperative pain during the perioperative period. We recommend performing a very meticulous dissection to minimize these consequences. No patient in this study required a halo orthosis.
Results
Twenty-one patients with CSM were treated with CAPDF between February 2010 and March 2015 (Table 1).
Table 2 summarizes the operative data. Mean number of levels fused was 2 (range, 1-3) anteriorly and 3 (range, 1-4) posteriorly.
Of the 21 patients, 9 (42.3%) had at least 1 complication during the perioperative period. Table 3 summarizes all encountered complications. Neither neurologic instability nor mortality was observed after surgery.
Patient 7 was lost to follow-up. For the other 20 patients, mean time to “3-month follow-up” was 96 days (range, 51-149 days). The most commonly noted improvements in quality of life included resolution of numbness, improvement in gait, and return to previous activities, such as walking and even exercising.
Representative Case
Patient 15, a 53-year-old man, presented with complaints of dysesthesias of the hands. Focused neurologic evaluation at the time revealed limited CS range of motion on extension. The patient (Figures 2A-2D) was diffusely hyperreflexic and had pathologic spread in the upper extremities.
Discussion
Cervical myelopathy is a common yet frequently underdiagnosed disease, owing to the fact that many patients remain asymptomatic even after experiencing degenerative changes in the spinal column.14-16 The additive effects of spondylosis, osteophyte formation, ligamentous hypertrophy, and listhesis lead to progressive canal and intervertebral foraminal compromise, ultimately producing the clinical syndromes of myelopathy and radiculopathy.17 The characteristic symptoms of CSM are known to have an insidious onset. In the early stages, patients note a subtle gait disturbance and later experience manual dexterity reductions and upper extremity dysesthesias.18 As the condition progresses and conservative management fails, surgical intervention is sought.
Nevertheless, the pursuit of surgical treatment for CSM remains somewhat controversial. Some authors have found no statistically significant difference between conservative and surgical management of mild to moderate CSM,19 whereas others have found that surgically treated patients had much better outcomes than their medically treated counterparts.20 In 2010, Scardino and colleagues21 reported that CSM patients who were bedridden and/or wheelchair-bound with seemingly irreversible myelopathy were capable of neurologic improvement after surgical intervention. At the very least, what remains clear is that untreated CSM is known to follow an unpredictable course, with the condition deteriorating faster for some patients than others.22Traditional anterior or posterior approaches, which can be used in the majority of cases of cervical spondylosis and/or radiculopathy, have been compared extensively.23,24 The inverse relationship concerning the integrity of an anterior construct and the number of levels fused is a well-established clinical finding.3,4,8,25-28 Laminectomy with fusion is not without its disadvantages: Cervical instability secondary to mechanical loss of posterior cervical support, and subsequent post-laminectomy kyphosis, is a common complication.23 In cases in which more stability is required, the combined anterior-posterior approach is more promising than either approach alone. This technique has its roots in the treatment of several thoracolumbar spine disorders, including infections, scoliosis, trauma, and tumors.29-31 More recently, the technique has been applied to CS disorders.
In 2008, Gok and colleagues32 retrospectively compared the results of anterior-only fusion and CAPDF for CSM. Forty-six patients underwent anterior surgery only, and 21 underwent CAPDF. The groups’ complication rates were similar: 28.6% (anterior only) and 24% (CAPDF); the incidence of ASD was lower in the combined group. Song and colleagues33 conducted a similar study in 2010. They compared anterior fusion alone and CAPDF in treating degenerative cervical kyphosis. Results were strongly in favor of the combined technique, as it led to “greater correction of sagittal alignment, a better maintenance of correction angle, a higher rate of fusion, a lower rate of subsidence and lower complications.” Both studies established that, in a select group of patients, the benefits of CAPDF outweighed the risks. These findings, combined with our study’s findings of no major complications and the transience of minor complications, suggest CAPDF should not be considered too invasive or risky.
The results of our study also mirror those of 3 other studies on the use of CAPDF for CS disorders. In 1995, McAfee and colleagues34 reported on a group of 100 patients with follow-up of 2 years or more. In most cases, the surgical indication was trauma, but neoplasm, infection, rheumatoid arthritis, and CSM were found as well. Outcomes were very favorable: improvement in a previous neurologic deficit (57/75 patients), ability to walk again (21/35 patients), no new neurologic deficits, and no hardware failures. In 2000, Schultz and colleagues35 retrospectively reviewed the cases of 72 patients who underwent CAPDF for a variety of complex CS disorders. Two of the 72 experienced transient neurologic deficits, and, though the immediate complication rate was relatively high (32%), the long-term complication rate was down to 5%. In 2009, Konya and colleagues36 retrospectively reviewed the cases of 40 patients who underwent CAPDF, primarily for CSM. Within 1 week after surgery, neurologic deficits were reduced in 36 patients; by 1 year after surgery, neurologic deficits were reduced in all 40 patients, and fusion was achieved in 39. These 3 studies34-36 helped establish CAPDF of the CS as a viable and effective procedure that can be performed within a single day.
Although many physicians have achieved favorable results with single-day surgery, the decision to operate in a sequential or staged manner remains controversial. Some anecdotally claim CAPDF poses a greater operative risk to the patient. In 1991, the continuous procedure was found to involve less blood loss and shorter LOS while providing for better correction of severe spinal deformity in patients with scoliosis and rigid kyphosis.37 Three more recent comparative studies examining the same issue in the treatment of CS diseases found staging did not reduce the complication rate and may in fact have been associated with higher complication rates, more blood loss, and longer total operative time and LOS.10,11,38 Our study’s lower blood loss, shorter LOS, and lower major complication rate relative to the combined groups in all 3 of those studies are most likely attributable to our operating on a lower mean number of spinal levels and our restricting the surgical indication to CSM. The positive short-term outcomes and low rate of long-term complications in our study, combined with the data from these 3 comparative studies, suggest that same-day surgery is superior to staged surgery. A staged operation should be considered only if the patient cannot tolerate long periods under general anesthesia.
Many have advocated extending fusion down to T1 to prevent ASD at the C7–T1 disk space.35,39,40 We decided against this approach for 2 reasons. First, at C7, lateral mass screws were always chosen over pedicle screws. When possible, shorter lateral mass screws were used at this level, making C7 much less rigid. Second, the C7–T1 facet capsule was maintained to preserve joint integrity. We suggest extending fusion down to T1 only if there is prior evidence of spinal disease and/or listhesis at C7–T1. Although long-term (many-year) follow-up is often desired, we specifically assessed short-term (3-month) outcomes. We have anecdotally found that degree of improvement often follows a predictable course after 3-month follow-up. If myelopathy resolves even to a small extent during the first 3 postoperative months, later improvement will likely follow an upward course. Conversely, if myelopathy does not improve during the first 3 months, further improvement is much less likely.
This trend in neurologic improvement likely is directly related to degree of myelopathy before surgery. Patients with CSM generally experience symptoms over an extended period and try conservative management before any surgical consultation. Although spinal ischemia is often resolved by decompression, permanent ischemic damage to the cord is not uncommon. In this setting, postoperative neurologic improvement is minimal or even nonexistent, and decompression is preventive rather than curative. In our study, 1 patient had no subjective improvement after surgery. At 3-month follow-up, magnetic resonance imaging showed notable myelomalacia without residual spinal cord compression. We attribute the failure of the ischemic changes to resolve to long-standing preoperative damage to the cord. Nevertheless, surgery stabilized the myelopathy and prevented further ischemic damage and clinical deterioration.
As is the case with any operation, patients must be carefully selected for CAPDF. Indications for CAPDF, as described by Kim and Alexander,7 include acute spinal trauma, post-laminectomy kyphosis, kyphotic deformity with intact posterior tension band, multilevel spondylosis and OPLL, and preexisting risk factors for pseudarthrosis. Clearly, the severity of each varies, and the pathologies are not mutually exclusive. We emphasize that these indications provide only a guideline for performing CAPDF, and patients must be selected on a case-by-case basis. All the patients in our study were symptomatic and exhibited significant compression of the spinal cord anteriorly and posteriorly at multiple levels. Several presented with concomitant pathologies, such as cervical kyphotic deformity, congenital spinal stenosis, and OPLL. In each case, the indication for surgical intervention was undoubted. We sought both to improve the patient’s baseline symptoms and to prevent further damage to the spinal cord.
This study had its limitations. First, its retrospective design predisposed it to a higher degree of bias. Second, because CAPDF is not commonly performed, the sample size was relatively small. Third, although it provided a descriptive analysis of CAPDF for CSM, the study did not use a direct comparison group to establish whether treatment within a single day or staged treatment was more beneficial for our cohort in particular. On the basis of prior experience and observation, we think performing the operation within a single day is much more beneficial for the patient. Our discussion of studies that have compared same-day and staged surgery supports this observation. Therefore, staged treatment was not recommended to our patients.
Conclusion
Few descriptive studies have explored CAPDF for CSM. Our study’s results showed the procedure was associated with minor complications and provided symptomatic relief for a majority of patients as early as 3 months after surgery. In addition, CAPDF can be successfully performed sequentially within a single day. As such, it represents an excellent option for treating multilevel symptomatic CSM cases that require more extensive spinal decompression and more stability.
Am J Orthop. 2017;46(2):E97-E104. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 suppl):190S-197S.
2. Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist. 2013;19(4):409-421.
3. Sasso RC, Ruggiero RA Jr, Reilly TM, Hall PV. Early reconstruction failures after multilevel cervical corpectomy. Spine. 2003;28(2):140-142.
4. Zdeblick TA, Hughes SS, Riew KD, Bohlman HH. Failed anterior cervical discectomy and arthrodesis. Analysis and treatment of thirty-five patients. J Bone Joint Surg Am. 1997;79(4):523-532.
5. Zhu B, Xu Y, Liu X, Liu Z, Dang G. Anterior approach versus posterior approach for the treatment of multilevel cervical spondylotic myelopathy: a systemic review and meta-analysis. Eur Spine J. 2013;22(7):1583-1593.
6. Cabraja M, Abbushi A, Koeppen D, Kroppenstedt S, Woiciechowsky C. Comparison between anterior and posterior decompression with instrumentation for cervical spondylotic myelopathy: sagittal alignment and clinical outcome. Neurosurg Focus. 2010;28(3):E15.
7. Kim PK, Alexander JT. Indications for circumferential surgery for cervical spondylotic myelopathy. Spine J. 2006;6(6 suppl):299S-307S.
8. König SA, Ranguis S, Spetzger U. Management of complex cervical instability. J Neurol Surg A Cent Eur Neurosurg. 2015;76(2):119-125.
9. König SA, Spetzger U. Surgical management of cervical spondylotic myelopathy—indications for anterior, posterior or combined procedures for decompression and stabilisation. Acta Neurochir. 2014;156(2):253-258.
10. Harel R, Hwang R, Fakhar M, et al. Circumferential cervical surgery: to stage or not to stage? J Spinal Disord Tech. 2013;26(4):183-188.
11. Siemionow K, Tyrakowski M, Patel K, Neckrysh S. Comparison of perioperative complications following staged versus one-day anterior and posterior cervical decompression and fusion crossing the cervico-thoracic junction. Neurol Neurochir Pol. 2014;48(6):403-409.
12. Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95(1):87-100.
13. Chen CJ, Saulle D, Fu KM, Smith JS, Shaffrey CI. Dysphagia following combined anterior-posterior cervical spine surgeries. J Neurosurg Spine. 2013;19(3):279-287.
14. Boden SD, McCowin PR, Davis DO, Dina TS, Mark AS, Wiesel S. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72(8):1178-1184.
15. Gore DR, Sepic SB, Gardner GM. Roentgenographic findings of the cervical spine in asymptomatic people. Spine. 1986;11(6):521-524.
16. Law MD Jr, Bernhardt M, White AA 3rd. Cervical spondylotic myelopathy: a review of surgical indications and decision making. Yale J Biol Med. 1993;66(3):165-177.
17. Kelly JC, Groarke PJ, Butler JS, Poynton AR, O’Byrne JM. The natural history and clinical syndromes of degenerative cervical spondylosis. Adv Orthop. 2012;(2012):393642.
18. Baron EM, Young WF. Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery. 2007;60(1 suppl 1):S35-S41.
19. Kadanka Z, Mares M, Bednarik J, et al. Approaches to spondylotic cervical myelopathy: conservative versus surgical results in a 3-year follow-up study. Spine. 2002;27(20):2205-2210.
20. Sampath P, Bendebba M, Davis JD, Ducker TB. Outcome of patients treated for cervical myelopathy. A prospective, multicenter study with independent clinical review. Spine. 2000;25(6):670-676.
21. Scardino FB, Rocha LP, Barcelos AC, Rotta JM, Botelho RV. Is there a benefit to operating on patients (bedridden or in wheelchairs) with advanced stage cervical spondylotic myelopathy? Eur Spine J. 2010;19(5):699-705.
22. Edwards CC 2nd, Riew KD, Anderson PA, Hilibrand AS, Vaccaro AF. Cervical myelopathy. Current diagnostic and treatment strategies. Spine J. 2003;3(1):68-81.
23. Herkowitz HN. A comparison of anterior cervical fusion, cervical laminectomy, and cervical laminoplasty for the surgical management of multiple level spondylotic radiculopathy. Spine. 1988;13(7):774-780.
24. Hukuda S, Mochizuki T, Ogata M, Shichikawa K, Shimomura Y. Operations for cervical spondylotic myelopathy. A comparison of the results of anterior and posterior procedures. J Bone Joint Surg Br. 1985;67(4):609-615.
25. Fernyhough JC, White JI, LaRocca H. Fusion rates in multilevel cervical spondylosis comparing allograft fibula with autograft fibula in 126 patients. Spine. 1991;16(10 suppl):S561-S564.
26. Macdonald RL, Fehlings MG, Tator CH, et al. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J Neurosurg. 1997;86(6):990-997.
27. Mayr MT, Subach BR, Comey CH, Rodts GE, Haid RW Jr. Cervical spinal stenosis: outcome after anterior corpectomy, allograft reconstruction, and instrumentation. J Neurosurg. 2002;96(1 suppl):10-16.
28. Swank ML, Lowery GL, Bhat AL, McDonough RF. Anterior cervical allograft arthrodesis and instrumentation: multilevel interbody grafting or strut graft reconstruction. Eur Spine J. 1997;6(2):138-143.
29. Böhm H, Harms J, Donk R, Zielke K. Correction and stabilization of angular kyphosis. Clin Orthop Relat Res. 1990;(258):56-61.
30. Spencer DL, DeWald RL. Simultaneous anterior and posterior surgical approach to the thoracic and lumbar spine. Spine. 1979;4(1):29-36.
31. Whitesides TE Jr, Shah SGA. On the management of unstable fractures of the thoracolumbar spine: rationale for use of anterior decompression and fusion and posterior stabilization. Spine. 1976;1(2):99-107.
32. Gok B, Sciubba DM, McLoughlin GS, et al. Surgical treatment of cervical spondylotic myelopathy with anterior compression: a review of 67 cases. J Neurosurg Spine. 2008;9(2):152-157.
33. Song KJ, Johnson JS, Choi BR, Wang JC, Lee KB. Anterior fusion alone compared with combined anterior and posterior fusion for the treatment of degenerative cervical kyphosis. J Bone Joint Surg Br. 2010;92(11):1548-1552.
34. McAfee PC, Bohlman HH, Ducker TB, Zeidman SM, Goldstein JA. One-stage anterior cervical decompression and posterior stabilization. A study of one hundred patients with a minimum of two years of follow-up. J Bone Joint Surg Am. 1995;77(12):1791-1800.
35. Schultz KD Jr, McLaughlin MR, Haid RW Jr, Comey CH, Rodts GE Jr, Alexander J. Single-stage anterior-posterior decompression and stabilization for complex cervical spine disorders. J Neurosurg. 2000;93(2 suppl):214-221.
36. Konya D, Ozgen S, Gercek A, Pamir MN. Outcomes for combined anterior and posterior surgical approaches for patients with multisegmental cervical spondylotic myelopathy. J Clin Neurosci. 2009;16(3):404-409.
37. Shufflebarger HL, Grimm JO, Bui V, Thomson JD. Anterior and posterior spinal fusion. Staged versus same-day surgery. Spine. 1991;16(8):930-933.
38. Ozturk C, Aydinli U, Vural R, Sehirlioglu A, Mutlu M. Simultaneous versus sequential one-stage combined anterior and posterior spinal surgery for spinal infections (outcomes and complications). Int Orthop. 2007;31(3):363-366.
39. Aryan HE, Sanchez-Mejia RO, Ben-Haim S, Ames CP. Successful treatment of cervical myelopathy with minimal morbidity by circumferential decompression and fusion. Eur Spine J. 2007;16(9):1401-1409.
40. Steinmetz MP, Miller J, Warbel A, Krishnaney AA, Bingaman W, Benzel EC. Regional instability following cervicothoracic junction surgery. J Neurosurg Spine. 2006;4(4):278-284.
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 suppl):190S-197S.
2. Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist. 2013;19(4):409-421.
3. Sasso RC, Ruggiero RA Jr, Reilly TM, Hall PV. Early reconstruction failures after multilevel cervical corpectomy. Spine. 2003;28(2):140-142.
4. Zdeblick TA, Hughes SS, Riew KD, Bohlman HH. Failed anterior cervical discectomy and arthrodesis. Analysis and treatment of thirty-five patients. J Bone Joint Surg Am. 1997;79(4):523-532.
5. Zhu B, Xu Y, Liu X, Liu Z, Dang G. Anterior approach versus posterior approach for the treatment of multilevel cervical spondylotic myelopathy: a systemic review and meta-analysis. Eur Spine J. 2013;22(7):1583-1593.
6. Cabraja M, Abbushi A, Koeppen D, Kroppenstedt S, Woiciechowsky C. Comparison between anterior and posterior decompression with instrumentation for cervical spondylotic myelopathy: sagittal alignment and clinical outcome. Neurosurg Focus. 2010;28(3):E15.
7. Kim PK, Alexander JT. Indications for circumferential surgery for cervical spondylotic myelopathy. Spine J. 2006;6(6 suppl):299S-307S.
8. König SA, Ranguis S, Spetzger U. Management of complex cervical instability. J Neurol Surg A Cent Eur Neurosurg. 2015;76(2):119-125.
9. König SA, Spetzger U. Surgical management of cervical spondylotic myelopathy—indications for anterior, posterior or combined procedures for decompression and stabilisation. Acta Neurochir. 2014;156(2):253-258.
10. Harel R, Hwang R, Fakhar M, et al. Circumferential cervical surgery: to stage or not to stage? J Spinal Disord Tech. 2013;26(4):183-188.
11. Siemionow K, Tyrakowski M, Patel K, Neckrysh S. Comparison of perioperative complications following staged versus one-day anterior and posterior cervical decompression and fusion crossing the cervico-thoracic junction. Neurol Neurochir Pol. 2014;48(6):403-409.
12. Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95(1):87-100.
13. Chen CJ, Saulle D, Fu KM, Smith JS, Shaffrey CI. Dysphagia following combined anterior-posterior cervical spine surgeries. J Neurosurg Spine. 2013;19(3):279-287.
14. Boden SD, McCowin PR, Davis DO, Dina TS, Mark AS, Wiesel S. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72(8):1178-1184.
15. Gore DR, Sepic SB, Gardner GM. Roentgenographic findings of the cervical spine in asymptomatic people. Spine. 1986;11(6):521-524.
16. Law MD Jr, Bernhardt M, White AA 3rd. Cervical spondylotic myelopathy: a review of surgical indications and decision making. Yale J Biol Med. 1993;66(3):165-177.
17. Kelly JC, Groarke PJ, Butler JS, Poynton AR, O’Byrne JM. The natural history and clinical syndromes of degenerative cervical spondylosis. Adv Orthop. 2012;(2012):393642.
18. Baron EM, Young WF. Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery. 2007;60(1 suppl 1):S35-S41.
19. Kadanka Z, Mares M, Bednarik J, et al. Approaches to spondylotic cervical myelopathy: conservative versus surgical results in a 3-year follow-up study. Spine. 2002;27(20):2205-2210.
20. Sampath P, Bendebba M, Davis JD, Ducker TB. Outcome of patients treated for cervical myelopathy. A prospective, multicenter study with independent clinical review. Spine. 2000;25(6):670-676.
21. Scardino FB, Rocha LP, Barcelos AC, Rotta JM, Botelho RV. Is there a benefit to operating on patients (bedridden or in wheelchairs) with advanced stage cervical spondylotic myelopathy? Eur Spine J. 2010;19(5):699-705.
22. Edwards CC 2nd, Riew KD, Anderson PA, Hilibrand AS, Vaccaro AF. Cervical myelopathy. Current diagnostic and treatment strategies. Spine J. 2003;3(1):68-81.
23. Herkowitz HN. A comparison of anterior cervical fusion, cervical laminectomy, and cervical laminoplasty for the surgical management of multiple level spondylotic radiculopathy. Spine. 1988;13(7):774-780.
24. Hukuda S, Mochizuki T, Ogata M, Shichikawa K, Shimomura Y. Operations for cervical spondylotic myelopathy. A comparison of the results of anterior and posterior procedures. J Bone Joint Surg Br. 1985;67(4):609-615.
25. Fernyhough JC, White JI, LaRocca H. Fusion rates in multilevel cervical spondylosis comparing allograft fibula with autograft fibula in 126 patients. Spine. 1991;16(10 suppl):S561-S564.
26. Macdonald RL, Fehlings MG, Tator CH, et al. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J Neurosurg. 1997;86(6):990-997.
27. Mayr MT, Subach BR, Comey CH, Rodts GE, Haid RW Jr. Cervical spinal stenosis: outcome after anterior corpectomy, allograft reconstruction, and instrumentation. J Neurosurg. 2002;96(1 suppl):10-16.
28. Swank ML, Lowery GL, Bhat AL, McDonough RF. Anterior cervical allograft arthrodesis and instrumentation: multilevel interbody grafting or strut graft reconstruction. Eur Spine J. 1997;6(2):138-143.
29. Böhm H, Harms J, Donk R, Zielke K. Correction and stabilization of angular kyphosis. Clin Orthop Relat Res. 1990;(258):56-61.
30. Spencer DL, DeWald RL. Simultaneous anterior and posterior surgical approach to the thoracic and lumbar spine. Spine. 1979;4(1):29-36.
31. Whitesides TE Jr, Shah SGA. On the management of unstable fractures of the thoracolumbar spine: rationale for use of anterior decompression and fusion and posterior stabilization. Spine. 1976;1(2):99-107.
32. Gok B, Sciubba DM, McLoughlin GS, et al. Surgical treatment of cervical spondylotic myelopathy with anterior compression: a review of 67 cases. J Neurosurg Spine. 2008;9(2):152-157.
33. Song KJ, Johnson JS, Choi BR, Wang JC, Lee KB. Anterior fusion alone compared with combined anterior and posterior fusion for the treatment of degenerative cervical kyphosis. J Bone Joint Surg Br. 2010;92(11):1548-1552.
34. McAfee PC, Bohlman HH, Ducker TB, Zeidman SM, Goldstein JA. One-stage anterior cervical decompression and posterior stabilization. A study of one hundred patients with a minimum of two years of follow-up. J Bone Joint Surg Am. 1995;77(12):1791-1800.
35. Schultz KD Jr, McLaughlin MR, Haid RW Jr, Comey CH, Rodts GE Jr, Alexander J. Single-stage anterior-posterior decompression and stabilization for complex cervical spine disorders. J Neurosurg. 2000;93(2 suppl):214-221.
36. Konya D, Ozgen S, Gercek A, Pamir MN. Outcomes for combined anterior and posterior surgical approaches for patients with multisegmental cervical spondylotic myelopathy. J Clin Neurosci. 2009;16(3):404-409.
37. Shufflebarger HL, Grimm JO, Bui V, Thomson JD. Anterior and posterior spinal fusion. Staged versus same-day surgery. Spine. 1991;16(8):930-933.
38. Ozturk C, Aydinli U, Vural R, Sehirlioglu A, Mutlu M. Simultaneous versus sequential one-stage combined anterior and posterior spinal surgery for spinal infections (outcomes and complications). Int Orthop. 2007;31(3):363-366.
39. Aryan HE, Sanchez-Mejia RO, Ben-Haim S, Ames CP. Successful treatment of cervical myelopathy with minimal morbidity by circumferential decompression and fusion. Eur Spine J. 2007;16(9):1401-1409.
40. Steinmetz MP, Miller J, Warbel A, Krishnaney AA, Bingaman W, Benzel EC. Regional instability following cervicothoracic junction surgery. J Neurosurg Spine. 2006;4(4):278-284.
Music Therapy Increases Comfort and Reduces Pain in Patients Recovering From Spine Surgery
Take-Home Points
- Music therapists use patient-preferred live music, increasing neurologic cues that enhance movement—a seminal recovery function in postoperative spine patients.
- Music therapy is an evidence-based, integrative treatment addressing body, mind, and spirit.
- Tension release through music therapy can serve as a critical mechanism for building resilience related to pain management.
- Music therapy and music medicine are distinct forms of clinical practice that focus on mind-body integration in the healing process.
- Music therapists, board-certified and licensed by the state as recognized healthcare professionals, address pain management, which is an increasing subspecialty in postoperative care.
About 70% of people in the United States experience at least 1 episode of back pain in their lifetime,1 and more than 5 million are temporarily or permanently disabled by spinal disorders.2-4 Some require surgery, which may rectify injury, but pain during recovery is often inevitable, and the road to recovery is not guaranteed to be smooth.5-20
Postoperative spine patients are at major risk for pain management challenges.14,15,18,20 Treatment is primarily pharmacologic and based on the surgical team’s pain management orders. Nursing care consists of monitoring the airway, vital signs, and neurovascular status and having patients rate their pain on a visual analog scale (VAS; 0 = no pain, 10 = worst pain imaginable). Nurses have the challenge of monitoring and continually assessing to make sure patients are achieving the optimal outcomes, particularly during the immediate postoperative period, when pain and anxiety are prominently increased.
Variability in spine surgery outcomes can be explained at least partly on the basis of prognostic psychological factors, including hypochondriasis, hysteria, depression, and poor pain coping strategies (eg, catastrophizing).21 In spine surgery patients, kinesiophobia (fear of moving) is a common component of distress that can impede recuperation.21-23
Rationale for Live Music
Pain is subjective and personal, and warrants an individualized approach to care. There is a body of music medicine research on the use of recorded music in modulating psychological and physiological factors in pain perception.30,32,34-54 This research supports the unique relationship of music to well-being, and the understanding that controlling any of these factors affects the duration, intensity, and quality of that experience.41,43,52
These findings provide incentive for breathing-entrained music therapy interventions, which enhance the relaxation response and release of pain-related tension;32,55-58 empower patients to unlock physical and emotional tension;32,57,58 provide a channel for expression and body movement; and enhance blood flow and/or alleviate pain by activating neurologic areas involved in the experience of pain.59-62Studies have found that physical endurance may be enhanced when movement is rhythmically coordinated with a musical stimulus.63-66 Music may prolong physical endurance by inhibiting psychological feedback associated with physical exertion related to fatigue, which may translate into accelerated recovery periods. When we listen to a rhythmic sound, our brains tend to automatically synchronize, or entrain, to external rhythmic cues that can stimulate increased motor control and coordination.63 Sound can arouse and raise the excitability of spinal motor neurons mediated by auditory-motor neuronal connections on the brain stem and spinal cord level.64-66 Rhythmically organized sounds serve as a neurological function in our capacity to organize predictable timing cues that are apparent in music, and may result in an effective treatment intervention in recovery.63,64
Music Therapy in Recovery From Spine Surgery
In music therapy, music is used within a therapeutic relationship to support or affect change in the patient and the treatment regimen.32,33,56-58 Research on music therapy with patients who are recovering from spine surgery is scant.67-69 Kleiber and Adamek67 studied perceptions of music therapy in 8 adolescents after spinal fusion surgery. In their study, a music therapist provided patients with a postoperative music therapy session focusing on the use of patient-preferred live music for relaxation and expression. Although their qualitative query was based on a therapeutic approach similar to that used in the present study, only 1 session was offered during the recovery period, and follow-up was conducted by survey invitation and telephone. In addition, the number of participants was small, and there was no quantitative measure of pain or other symptoms.
Another study focused on the effects of listening to music on pain intensity and distress after spine surgery.68 Patients in the study’s music group made their selections from prerecorded classical music and domestic and international popular songs from various genres and listened to their chosen recordings 30 minutes a day. Although the study was not a music therapy study per se, it showed a positive impact of listening to music on anxiety and pain perception in 60 adults who were randomly assigned to the music group or to a non-music control group (n = 30 in each). Differences between the music and control groups’ VAS ratings of anxiety (Ps = .018-.001) and pain (P = .001) were statistically significant.
Different from our study, the aforementioned studies did not include tension release–focused live music offered within a therapeutic relationship. Our 1.5-year pilot study, conducted prior to the present study indicated that music therapy led to increased resilience and recovery mechanisms.58
Methods
Our mixed-methods study design combined standard medical treatment with integrative music therapy interventions based on pain assessments to better understand the effects of music therapy on the recovery of patients after spine surgery.
The Spine Institute of New York within the Department of Orthopedic Surgery at Mount Sinai Beth Israel provides surgical treatment of common spinal cord conditions. Prioritizing patient satisfaction and positive outcomes,27,28 the institute integrates music therapy through the Louis Armstrong Center for Music and Medicine to enhance treatment of pain symptoms.
Patients were recruited by the research team as per the daily surgical schedule, or through referral by the medical team or patient care navigator. Sixty patients (35 female, 25 male) ranging in age from 40 to 55 years underwent anterior, posterior, or anterior-posterior spinal fusion and were enrolled in the study after signing a participation consent form. Minorities, women, and patients with Medicaid and Medicare were included. Patients who received a diagnosis of clinical psychosis or depression prior to spine injury were excluded.
The experimental group received music therapy plus standard care (medical and nursing care with scheduled pharmacologic pain intervention), and a wait-listed control group received standard care only. A randomization chart created by a blinded statistician who did not have access to the patient census determined the intervention–nonintervention schedule. Patients in the music therapy group received one 30-minute music therapy session during an 8-hour period within 72 hours after surgery.
For both groups, measurements were completed before and after the study window. Control patients were offered music therapy after completion of the post-intervention surveys in order to minimize the ethical dilemma of denying potentially helpful pain intervention. For this same reason, both groups were given the option of receiving follow-up music therapy sessions for the duration of their hospitalization.
The research team consisted of 2 licensed, board-certified music therapists. In addition, Master’s-level music therapy interns completing clinical hours as part of the trajectory for board certification served on the research team over the 5-year period 2009 to 2014, and 13 blinded research assistants helped with enrolling and collecting data on patients.
Intervention
Each music therapy session included a warm-up phase of verbal or musical discourse. Next was the treatment phase, which was based on patient need as assessed during warm-up. Treatment options included use of patient-preferred live music that supported tension release/relaxation through incentive-based clinical improvisation, singing, and/or rhythmic drumming or through breathwork and visualization. Psychoeducation about mind–body awareness through the use of breath and imagery was introduced and explained by the therapist at this time.
The improvised music intervention was focused on making salient the natural harmonic tension-resolution cycles that occur in music and that were entrained to the patient’s presentation (respiratory rate, verbal report, clinical presentation). When patient-preferred precomposed songs were used, tension resolution was achieved by sustaining cadence and resolution, also entrained to the patient’s respiratory cycles.32,57,58
After the music therapy intervention, a period of closure or integration was facilitated by the therapist contingent on the patient’s degree of alertness. If awake, the patient was supported in a reflexive process of thoughts, impressions, or issues that may have contributed to the overall experience. If the patient was asleep, the researcher returned within 30 minutes for post-intervention interviewing. Interview information was recorded in a qualitative post-participation survey. To prevent bias, researchers who were not the treating clinicians conducted the surveys.
Outcome Measures
Both primary and secondary outcome measures were collected before and after the intervention. The primary outcome measure was VAS pain ratings, and the secondary outcome measures were scores on the Hospital Anxiety and Depression Scale (HADS), the Tampa Scale for Kinesiophobia (TSK), and the Color Analysis Scale (CAS).
VAS. With the VAS, images are used to rate pain. The scale has points labeled 0 to 10 and corresponding faces representing progression in pain intensity. The scale is quickly rendered and can be interpreted according to the patient’s recovery phase at time of rendering.
HADS. The HADS70 provides a specific baseline for anxiety and depression as an indicator of how the patient might fare during hospitalization (admission through recovery and discharge).
TSK. The TSK71 provides insight into the patient’s perception of fear-related movement, which is an important factor in this study because of the movement required for rehabilitation. We used a shortened version of the TSK to accommodate the sensitive threshold for pain tolerance and pharmacologic side effects commonly experienced by spine patients.
CAS. The CAS was developed at the Louis Armstrong Center for Music and Medicine to assess comorbidities and dynamic aspects of pain. Through a coloring exercise, patients illustrate their pain experience, which gives tangible form to the abstract experience of pain.
Coding
We collected patients’ demographic data, including age, sex, and diagnoses. Clinical indicators of the preoperative baseline included lifestyle, surgical history, and prior experience with music or other mind–body strategies for self-regulation.
As fundamental to qualitative methodology,72,73 the reported responses to questions were grouped into themes that were peer-tested with members of the research team before and during the coding process.
VAS, HADS, and TSK data were tabulated by blinded research assistants and analyzed by a statistician. Patients were identified by number assignment, and their data and personal information were kept confidentially stored.
Statistical Methods
Means and standard deviations were used for continuous variables, and frequencies (percentages) for categorical variables. All outcomes were analyzed on an intent-to-treat basis. Repeated-measures analysis of variance was used to compare changes in outcomes from before to after intervention for the music and control groups. In particular, a statistically significant Group (music vs control) × Time (before vs after intervention) interaction would support the hypothesis that there would be more benefit (less pain) in the music group as a result of the music therapy. For all tests, significance was set at P < .05. SPSS Version 20 (IBM) was used for all statistical analyses. Based on previously found differences in heart rate and mobility,31 we assumed an effect size of 0.71 for the difference between music and control (no music), which would require 32 patients per group to achieve a power of 0.8 with an α of 0.05.
Results
Of the 136 patients who were asked to participate in the study, 76 were not enrolled; the other 60 were equally assigned to either the control group or the music therapy group (n = 30 in each) according to randomization indicated by a blinded statistician (Figure 1).
Table 2 lists the pre-intervention and post-intervention comparisons of the main outcomes between groups.
The emerging themes of the responses are listed in Tables 3 and 4 and are explained here:
Relationship with music was coded for significance and included reports of music as a resource accessed for stimulation and/or relaxation through listening; direct involvement with instrument playing; and history of music training.
Perceptions of surgical outcome in patients’ responses were coded across 3 themes: (1) optimistic (belief and hope in returning to original baseline of functionality), (2) indifferent (neither hopeful nor cynical about results of surgery), and (3) pessimistic (belief that nothing will restore the quality of life that existed before the spinal condition).
The CAS helped us better understand the diversity and complexity of the pain experience.
Discussion
Our hospital has the unique capability of providing music therapy to postoperative and other hospitalized patients. In this study, we compared the impact of a structured postoperative music therapy program on spine patients relative to control patients who did not receive music therapy after spine surgery.
We found a significant benefit in VAS pain levels (>1 point) but no statistically significant differences in HADS Anxiety, HADS Depression, or TSK scores. Although a 2-point difference is usually considered clinically significant, the degree of change in the music group is notable for having been achieved by nonpharmacologic means with scant chance of adverse effects. We suspect the lack of significant change in HADS Anxiety, HADS Depression, and TSK scores is attributable to the narrow study window. Given the observational data from our pilot study58 and ongoing results with spine patients,32 it seems clear that both mood state and resilience in coping are enhanced through an ongoing relationship with music therapy.
The study of a population as vulnerable as patients recovering from spine surgery raises many issues for providers and researchers. Although it is worthwhile to determine the efficacy of integrative modalities in serving these patients, the request for participation in a protocol at such a vulnerable time was often resisted. During our pilot work, it became clear that the ability of potential subjects to comprehend and complete protocol surveys was impacted by adverse effects, including sedation drowsiness; respiratory depression; nausea and vomiting; pruritus; and urinary retention caused by the medications used for postoperative pain management. Consequently, after piloting 5 cases before the main study, we extended the enrollment window to 72 hours.
Other unforeseen intrinsic or external obstacles were identified: Patient-related issues—including availability, level of interest in participation, and inability to participate because of the medication adverse effects mentioned.
Staff investment/education—addressed over the first 3 study years with several in-services, starting with the surgical team and continuing with nursing and support staff in various combinations. These meetings led to the creation of an Institutional Review Board (IRB) approved educational sheet for inclusion in the information packet given to surgical patients on registration.
Programming interruptions—caused by the convergence of several unanticipated factors, including a delay in expedited review of the IRB renewal during the year of Hurricane Sandy and an interruption in the spine team’s service for administrative and program modification.
Conclusion
Music therapy interventions (eg, use of patient-preferred live music) offered within a therapeutic relationship favorably affected pain perceptions in patients recovering from spine surgery. This effect was achieved through several therapeutic entry points, including support of expression and opportunities for emotional catharsis.
At the core of music therapy’s efficacy is individualized treatment, through which patients are supported in their recovery of “self.” Measurable benefits—including increased comfort; reduced pain; improved gait; increased range of motion, endurance, and ability to relax; and empowerment to actively participate in one’s own care through daily activities imbued with an enhanced sense of agency—are of cardinal importance, as they may lead to quicker recovery perceptions and enhanced quality of life.
Am J Orthop. 2017;46(1):E13-E22. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Miller B, Gatchel RJ, Lou L, Stowell A, Robinson R, Polatin PB. Interdisciplinary treatment of failed back surgery syndrome (FBSS): a comparison of FBSS and non-FBSS patients. Pain Pract. 2005;5(3):190-202.
2. Aebi M. The adult scoliosis. Eur Spine J. 2005;14(10):925-948.
3. Engstrom JW, Deyo, RA. Back and neck pain. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison’s Principles of Internal Medicine, 19th edition. New York, NY: McGraw-Hill; 2007:207-214.
4. Cavanaugh JM, Lu Y, Chen C, Kallakuri S. Pain generation in lumbar and cervical facet joints. J Bone Joint Surg Am. 2006;88(suppl 2):63-67.
5. Hart RA, Prendergast MA. Spine surgery for lumbar degenerative disease in elderly and osteoporotic patients. Instr Course Lect. 2007;56:257-272.
6. Boswell MV, Trescot AM, Datta S, et al; American Society of Interventional Pain Physicians. Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician. 2007;10(1):7-111.
7. Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356(22):2257-2270.
8. Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT): A randomized trial. JAMA. 2006;296(20):2441-2450.
9. Malmivaara A, Slätis P, Heliövaara M, et al; Finnish Lumbar Spinal Research Group. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine. 2007;32(1):1-8.
10. Chang Y, Singer DE, Wu YA, Keller RB, Atlas SJ. The effect of surgical and nonsurgical treatment on longitudinal outcomes of lumbar spinal stenosis over 10 years. J Am Geriatr Soc. 2005;53(5):785-792.
11. Cowan JA Jr, Dimick JB, Wainess R, Upchurch GR Jr, Chandler WF, La Marca F. Changes in the utilization of spinal fusion in the United States. Neurosurgery. 2006;59(1):15-20.
12. Lonner BS, Scharf CS, Antonacci D, Goldstein Y, Panagopoulos G. The learning curve associated with thoracoscopic spinal instrumentation. Spine. 2005;30(24):2835-2840.
13. Lonner BS, Kondrachov D, Siddiqi F, Hayes V, Scharf C. Thoracoscopic spinal fusion compared with posterior spinal fusion for the treatment of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2006;88(5):1022-1034.
14. Boakye M, Patil CG, Santarelli J, Ho C, Tian W, Lad SP. Cervical spondylotic myelopathy: complications and outcomes after spinal fusion. Neurosurgery. 2008;62(2):455-461.
15. Boakye M, Patil CG, Santarelli J, Ho C, Tian W, Lad SP. Laminectomy and fusion after spinal cord injury: national inpatient complications and outcomes. J Neurotrauma. 2008;25(3):173-183.
16. Dekutoski MB, Norvell DC, Dettori JR, Fehlings MG, Chapman JR. Surgeon perceptions and reported complications in spine surgery. Spine. 2010;35(9 suppl):S9-S21.
17. Nasser R, Yadla S, Maltenfort MG, et al. Complications in spine surgery. J Neurosurg Spine. 2010;13(2):144-157.
18. Patil CG, Santarelli J, Lad SP, Ho C, Tian W, Boakye M. Inpatient complications, mortality, and discharge disposition after surgical correction of idiopathic scoliosis: a national perspective. Spine J. 2008;8(6):904-910.
19. Rampersaud YR, Moro ER, Neary MA, et al. Intraoperative adverse events and related postoperative complications in spine surgery: implications for enhancing patient safety founded on evidence-based protocols. Spine. 2006;31(13):1503-1510.
20. Shen Y, Silverstein JC, Roth S. In-hospital complications and mortality after elective spinal fusion surgery in the United States: a study of the Nationwide Inpatient Sample from 2001 to 2005. J Neurosurg Anesthesiol. 2009;21(1):21-30.
21. Picavet HSJ, Vlaeyen JWS, Schouten JSAG. Pain catastrophizing and kinesiophobia: predictors of chronic low back pain. Am J Epidemiol. 2002;156(11):1028-1034.
22. French DJ, France CR, Vigneau F, French JA, Evans RT. Fear of movement/(re)injury in chronic pain: a psychometric assessment of the original English version of the Tampa Scale for Kinesiophobia (TSK). Pain. 2007;127(1-2):42-51.
23. Goubert L, Crombez G, Van Damme S, Vlaeyen JW, Bijttebier P, Roelofs J. Confirmatory factor analysis of the Tampa Scale for Kinesiophobia: invariant two-factor model across low back pain patients and fibromyalgia patients. Clin J Pain. 2004;20(2):103-110.
24. Selimen D, Andsoy II. The importance of a holistic approach during the perioperative period. AORN J. 2011;93(4):482-487.
25. Zheng Z. Xue CC. Pain research in complementary and alternative medicine in Australia: a critical review. J Altern Complement Med. 2013;19(2):81-91.
26. Wright J, Adams D, Vohra S. Complementary, holistic, and integrative medicine: music for procedural pain. Pediatr Rev. 2013;34(11):e42-e46.
27. McCann PD. Orthopedic surgery and integrative medicine—strange bedfellows. Am J Orthop. 2009;38(2):66, 71.
28. McCann PD. Customer satisfaction: are hospitals “hospitable”? Am J Orthop. 2006;35(2):59.
29. Joanna Briggs Institute. The Joanna Briggs Institute best practice information sheet: music as an intervention in hospitals. Nurs Health Sci. 2011;13(1):99-102.
30. Spintge R. Thirty-five years of anxiolytic music (AAM) in pain and aversive clinical settings. In: Mondanaro J, Sara G, eds. Music and Medicine: Integrative Models in the Treatment of Pain. New York, NY: Satchnote Press; 2013:29-42.
31. Cepeda MS, Carr DB, Lau J, Alvarez H. Music for pain relief. Cochrane Database Syst Rev. 2006;(2):CD004843.
32. Mondanaro J. Music therapy based release strategies in the treatment of acute and chronic pain: an individualized approach. In: Mondanaro J, Sara G, eds. Music and Medicine: Integrative Models in the Treatment of Pain. New York, NY: Satchnote Press; 2013:133-148.
33. Quentzel S. Music has charms to soothe a savage breast. In: Mondanaro J, Sara G, eds. Music and Medicine: Integrative Models in the Treatment of Pain. New York, NY: Satchnote Press; 2013:11-28.
34. Ko YL. Lin PC. The effect of using a relaxation tape on pulse, respiration, blood pressure and anxiety levels of surgical patients. J Clin Nurs. 2012;21(5-6):689-697.
35. Roy M, Lebuis A, Hugueville L, Peretz I, Rainville P. Spinal modulation of nociception by music. Eur J Pain. 2012;16(6):870-877.
36. Roy M, Peretz I, Rainville P. Emotional valence contributes to music-induced analgesia. Pain. 2008;134(1-2):140-147.
37. Schröter T. Medicine needs music! Music therapy for chronic pain [in German]. Rev Med Suisse. 2014;10(415):286.
38. Bellieni CV, Cioncoloni D, Mazzanti S, et al. Music provided through a portable media player (iPod) blunts pain during physical therapy. Pain Manag Nurs. 2013;14(4):e151-e155.
39. Bernatzky G, Presch M, Anderson M, Panksepp J. Emotional foundations of music as a non-pharmacological pain management tool in modern medicine. Neurosci Biobehav Rev. 2011;35(9):1989-1999.
40. Bradshaw DH, Chapman CR, Jacobson RC, Donaldson GW. Effects of music engagement on response to painful stimulation. Clin J Pain. 2012;28(5):418-427.
41. Bradshaw DH, Donaldson GW, Jacobson RC, Nakamura Y, Chapman CR. Individual differences in the effects of music engagement on responses to painful stimulation. J Pain. 2011;12(12):1262-1273.
42. Chlan L, Halm MA. Does music ease pain and anxiety in the critically ill? Am J Crit Care. 2013;22(6):528-532.
43. Guétin S, Giniès P, Siou DK, et al. The effects of music intervention in the management of chronic pain: a single-blind, randomized, controlled trial. Clin J Pain. 2012;28(4):329-337.
44. Matsota P, Christodoulopoulou T, Smyrnioti ME, et al. Music’s use for anesthesia and analgesia. J Altern Complement Med. 2013;19(4):298-307.
45. Gooding L, Swezey S, Zwischenberger JB. Using music interventions in perioperative care. South Med J. 2012;105(9):486-490.
46. Graversen M, Sommer T. Perioperative music may reduce pain and fatigue in patients undergoing laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 2013;57(8):1010-1016.
47. Ni CH, Tsai WH, Lee LM, Kao CC, Chen YC. Minimising preoperative anxiety with music for day surgery patients—a randomised clinical trial. J Clin Nurs. 2012;21(5-6):620-625.
48. Good M, Albert JM, Anderson GC, et al. Supplementing relaxation and music for pain after surgery. Nurs Res. 2010;59(4):259-269.
49. Moris DN, Linos D. Music meets surgery: two sides to the art of “healing.” Surg Endosc. 2013;27(3):719-723.
50. Nilsson U, Rawal N, Unosson M. A comparison of intra-operative or postoperative exposure to music—a controlled trial of the effects on postoperative pain. Anaesthesia. 2003;58(7):699-703.
51. Özer N, Karaman Özlü Z, Arslan S, Günes N. Effect of music on postoperative pain and physiologic parameters of patients after open heart surgery. Pain Manag Nurs. 2013;14(1):20-28.
52. Sen H, Yanarateş O, Sızlan A, Kılıç E, Ozkan S, Dağlı G. The efficiency and duration of the analgesic effects of musical therapy on postoperative pain. Agri. 2010;22(4):145-150.
53. Vaajoki A, Pietilä AM, Kankkunen P, Vehviläinen-Julkunen K. Music intervention study in abdominal surgery patients: challenges of an intervention study in clinical practice. Int J Nurs Pract. 2013;19(2):206-213.
54. Vaajoki A, Pietilä AM, Kankkunen P, Vehviläinen-Julkunen K. Effects of listening to music on pain intensity and pain distress after surgery: an intervention. J Clin Nurs. 2012;21(5-6):708-717.
55. Whitaker MH. Sounds soothing: music therapy for postoperative pain. Nursing. 2010;40(12):53-54.
56. Edwards J. Developing pain management approaches in music therapy with hospitalized children. In: Loewy J, Dileo C, eds. Music Therapy at the End of Life. Cherry Hill, NJ: Jeffrey Books; 2005:57-76.
57. Loewy J. The quiet soldier: pain and sickle cell anemia. In: Hibben J, ed. Inside Music Therapy: Client Experiences. Gilsum, NH: Barcelona; 1999:69-76.
58. Lichtensztejn M. The clinical use of piano with patients suffering from breathing distress related to pain. In: Azoulay R, Loewy JV, eds. Music, the Breath and Health: Advances in Integrative Music Therapy. New York, NY: Satchnote Press; 2009:213-222.
59. Kwon IS, Kim J, Park KM. Effects of music therapy on pain, discomfort, and depression for patients with leg fractures. Taehan Kanho Hakhoe Chi. 2006;36(4):630-636.
60. Zengin S, Kabul S, Al B, Sarcan E, Doğan M, Yildirim C. Effects of music therapy on pain and anxiety in patients undergoing port catheter placement procedure. Complement Ther Med. 2013;21(6):689-696.
61. Boso M, Politi P, Barale F, Emanuele E. Neurophysiology and neurobiology of the musical experience. Funct Neurol. 2006;21(4):187-191.
62. Salimpoor VN, Benovoy M, Larcher K, Dagher A, Zatorre RJ. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat Neurosci. 2011;14(2):257-262.
63. Tomaino CM. Using rhythm for rehabilitation. Institute for Music and Neurologic Function website. http://musictherapy.imnf.org/images/uploads/rhythm.pdf. Published 2006. Accessed August 21, 2007.
64. Molinari M, Leggio MG, De Martin M, Cerasa A, Thaut M. Neurobiology of rhythmic motor entrainment. Ann N Y Acad Sci. 2003;999:313-321.
65. Thaut M. Neuropsychological processes in music perception. In: Unkefer R, ed. Music Therapy in the Treatment of Adults With Mental Disorders: Theoretical Bases and Clinical Interventions. Toronto, Canada: Schirmer Books; 2002:2-32.
66. Thaut M. Physiological and motor responses to music stimuli. In: Unkefer R, ed. Music Therapy in the Treatment of Adults With Mental Disorders: Theoretical Bases and Clinical Interventions. Toronto, Canada: Schimer Books; 2002:33-41.
67. Kleiber C, Adamek MS. Adolescents’ perceptions of music therapy following spinal fusion surgery. J Clin Nurs. 2013;22(3-4):414-422.
68. Lin PC, Lin ML, Huang LC, Hsu HC, Lin CC. Music therapy for patients receiving spine surgery. J Clin Nurs. 2011;20(7-8):960-968.
69. Maeyama A, Kodaka M, Miyao H. Effect of the music-therapy under spinal anesthesia [in Japanese]. Masui. 2009;58(6):684-691.
70. Golden J, Conroy RM, O’Dwyer AM. Reliability and validity of the Hospital Anxiety and Depression Scale and the Beck Depression Inventory (Full and FastScreen scales) in detecting depression in persons with hepatitis C. J Affect Disord. 2006;100(1-3):265-269.
71. Woby SR, Roach NK, Urmston M, Watson PJ. Psychometric properties of the TSK-11: a shortened version of the Tampa Scale for Kinesiophobia. Pain. 2005;117(1-2):137-144.
72. Humrichouse J, Chmielewski M, McDade-Montez EA, Watson D. Affect assessment through self-report methods. In: Rottenberg J, Johnson SL, eds. Emotion and Psychopathology: Bridging Affective and Clinical Science. Washington, DC: American Psychological Association; 2007:13-34.
73. Lincoln YS, Guba EG. Naturalistic Inquiry. Beverly Hills, CA: Sage; 1985.
Take-Home Points
- Music therapists use patient-preferred live music, increasing neurologic cues that enhance movement—a seminal recovery function in postoperative spine patients.
- Music therapy is an evidence-based, integrative treatment addressing body, mind, and spirit.
- Tension release through music therapy can serve as a critical mechanism for building resilience related to pain management.
- Music therapy and music medicine are distinct forms of clinical practice that focus on mind-body integration in the healing process.
- Music therapists, board-certified and licensed by the state as recognized healthcare professionals, address pain management, which is an increasing subspecialty in postoperative care.
About 70% of people in the United States experience at least 1 episode of back pain in their lifetime,1 and more than 5 million are temporarily or permanently disabled by spinal disorders.2-4 Some require surgery, which may rectify injury, but pain during recovery is often inevitable, and the road to recovery is not guaranteed to be smooth.5-20
Postoperative spine patients are at major risk for pain management challenges.14,15,18,20 Treatment is primarily pharmacologic and based on the surgical team’s pain management orders. Nursing care consists of monitoring the airway, vital signs, and neurovascular status and having patients rate their pain on a visual analog scale (VAS; 0 = no pain, 10 = worst pain imaginable). Nurses have the challenge of monitoring and continually assessing to make sure patients are achieving the optimal outcomes, particularly during the immediate postoperative period, when pain and anxiety are prominently increased.
Variability in spine surgery outcomes can be explained at least partly on the basis of prognostic psychological factors, including hypochondriasis, hysteria, depression, and poor pain coping strategies (eg, catastrophizing).21 In spine surgery patients, kinesiophobia (fear of moving) is a common component of distress that can impede recuperation.21-23
Rationale for Live Music
Pain is subjective and personal, and warrants an individualized approach to care. There is a body of music medicine research on the use of recorded music in modulating psychological and physiological factors in pain perception.30,32,34-54 This research supports the unique relationship of music to well-being, and the understanding that controlling any of these factors affects the duration, intensity, and quality of that experience.41,43,52
These findings provide incentive for breathing-entrained music therapy interventions, which enhance the relaxation response and release of pain-related tension;32,55-58 empower patients to unlock physical and emotional tension;32,57,58 provide a channel for expression and body movement; and enhance blood flow and/or alleviate pain by activating neurologic areas involved in the experience of pain.59-62Studies have found that physical endurance may be enhanced when movement is rhythmically coordinated with a musical stimulus.63-66 Music may prolong physical endurance by inhibiting psychological feedback associated with physical exertion related to fatigue, which may translate into accelerated recovery periods. When we listen to a rhythmic sound, our brains tend to automatically synchronize, or entrain, to external rhythmic cues that can stimulate increased motor control and coordination.63 Sound can arouse and raise the excitability of spinal motor neurons mediated by auditory-motor neuronal connections on the brain stem and spinal cord level.64-66 Rhythmically organized sounds serve as a neurological function in our capacity to organize predictable timing cues that are apparent in music, and may result in an effective treatment intervention in recovery.63,64
Music Therapy in Recovery From Spine Surgery
In music therapy, music is used within a therapeutic relationship to support or affect change in the patient and the treatment regimen.32,33,56-58 Research on music therapy with patients who are recovering from spine surgery is scant.67-69 Kleiber and Adamek67 studied perceptions of music therapy in 8 adolescents after spinal fusion surgery. In their study, a music therapist provided patients with a postoperative music therapy session focusing on the use of patient-preferred live music for relaxation and expression. Although their qualitative query was based on a therapeutic approach similar to that used in the present study, only 1 session was offered during the recovery period, and follow-up was conducted by survey invitation and telephone. In addition, the number of participants was small, and there was no quantitative measure of pain or other symptoms.
Another study focused on the effects of listening to music on pain intensity and distress after spine surgery.68 Patients in the study’s music group made their selections from prerecorded classical music and domestic and international popular songs from various genres and listened to their chosen recordings 30 minutes a day. Although the study was not a music therapy study per se, it showed a positive impact of listening to music on anxiety and pain perception in 60 adults who were randomly assigned to the music group or to a non-music control group (n = 30 in each). Differences between the music and control groups’ VAS ratings of anxiety (Ps = .018-.001) and pain (P = .001) were statistically significant.
Different from our study, the aforementioned studies did not include tension release–focused live music offered within a therapeutic relationship. Our 1.5-year pilot study, conducted prior to the present study indicated that music therapy led to increased resilience and recovery mechanisms.58
Methods
Our mixed-methods study design combined standard medical treatment with integrative music therapy interventions based on pain assessments to better understand the effects of music therapy on the recovery of patients after spine surgery.
The Spine Institute of New York within the Department of Orthopedic Surgery at Mount Sinai Beth Israel provides surgical treatment of common spinal cord conditions. Prioritizing patient satisfaction and positive outcomes,27,28 the institute integrates music therapy through the Louis Armstrong Center for Music and Medicine to enhance treatment of pain symptoms.
Patients were recruited by the research team as per the daily surgical schedule, or through referral by the medical team or patient care navigator. Sixty patients (35 female, 25 male) ranging in age from 40 to 55 years underwent anterior, posterior, or anterior-posterior spinal fusion and were enrolled in the study after signing a participation consent form. Minorities, women, and patients with Medicaid and Medicare were included. Patients who received a diagnosis of clinical psychosis or depression prior to spine injury were excluded.
The experimental group received music therapy plus standard care (medical and nursing care with scheduled pharmacologic pain intervention), and a wait-listed control group received standard care only. A randomization chart created by a blinded statistician who did not have access to the patient census determined the intervention–nonintervention schedule. Patients in the music therapy group received one 30-minute music therapy session during an 8-hour period within 72 hours after surgery.
For both groups, measurements were completed before and after the study window. Control patients were offered music therapy after completion of the post-intervention surveys in order to minimize the ethical dilemma of denying potentially helpful pain intervention. For this same reason, both groups were given the option of receiving follow-up music therapy sessions for the duration of their hospitalization.
The research team consisted of 2 licensed, board-certified music therapists. In addition, Master’s-level music therapy interns completing clinical hours as part of the trajectory for board certification served on the research team over the 5-year period 2009 to 2014, and 13 blinded research assistants helped with enrolling and collecting data on patients.
Intervention
Each music therapy session included a warm-up phase of verbal or musical discourse. Next was the treatment phase, which was based on patient need as assessed during warm-up. Treatment options included use of patient-preferred live music that supported tension release/relaxation through incentive-based clinical improvisation, singing, and/or rhythmic drumming or through breathwork and visualization. Psychoeducation about mind–body awareness through the use of breath and imagery was introduced and explained by the therapist at this time.
The improvised music intervention was focused on making salient the natural harmonic tension-resolution cycles that occur in music and that were entrained to the patient’s presentation (respiratory rate, verbal report, clinical presentation). When patient-preferred precomposed songs were used, tension resolution was achieved by sustaining cadence and resolution, also entrained to the patient’s respiratory cycles.32,57,58
After the music therapy intervention, a period of closure or integration was facilitated by the therapist contingent on the patient’s degree of alertness. If awake, the patient was supported in a reflexive process of thoughts, impressions, or issues that may have contributed to the overall experience. If the patient was asleep, the researcher returned within 30 minutes for post-intervention interviewing. Interview information was recorded in a qualitative post-participation survey. To prevent bias, researchers who were not the treating clinicians conducted the surveys.
Outcome Measures
Both primary and secondary outcome measures were collected before and after the intervention. The primary outcome measure was VAS pain ratings, and the secondary outcome measures were scores on the Hospital Anxiety and Depression Scale (HADS), the Tampa Scale for Kinesiophobia (TSK), and the Color Analysis Scale (CAS).
VAS. With the VAS, images are used to rate pain. The scale has points labeled 0 to 10 and corresponding faces representing progression in pain intensity. The scale is quickly rendered and can be interpreted according to the patient’s recovery phase at time of rendering.
HADS. The HADS70 provides a specific baseline for anxiety and depression as an indicator of how the patient might fare during hospitalization (admission through recovery and discharge).
TSK. The TSK71 provides insight into the patient’s perception of fear-related movement, which is an important factor in this study because of the movement required for rehabilitation. We used a shortened version of the TSK to accommodate the sensitive threshold for pain tolerance and pharmacologic side effects commonly experienced by spine patients.
CAS. The CAS was developed at the Louis Armstrong Center for Music and Medicine to assess comorbidities and dynamic aspects of pain. Through a coloring exercise, patients illustrate their pain experience, which gives tangible form to the abstract experience of pain.
Coding
We collected patients’ demographic data, including age, sex, and diagnoses. Clinical indicators of the preoperative baseline included lifestyle, surgical history, and prior experience with music or other mind–body strategies for self-regulation.
As fundamental to qualitative methodology,72,73 the reported responses to questions were grouped into themes that were peer-tested with members of the research team before and during the coding process.
VAS, HADS, and TSK data were tabulated by blinded research assistants and analyzed by a statistician. Patients were identified by number assignment, and their data and personal information were kept confidentially stored.
Statistical Methods
Means and standard deviations were used for continuous variables, and frequencies (percentages) for categorical variables. All outcomes were analyzed on an intent-to-treat basis. Repeated-measures analysis of variance was used to compare changes in outcomes from before to after intervention for the music and control groups. In particular, a statistically significant Group (music vs control) × Time (before vs after intervention) interaction would support the hypothesis that there would be more benefit (less pain) in the music group as a result of the music therapy. For all tests, significance was set at P < .05. SPSS Version 20 (IBM) was used for all statistical analyses. Based on previously found differences in heart rate and mobility,31 we assumed an effect size of 0.71 for the difference between music and control (no music), which would require 32 patients per group to achieve a power of 0.8 with an α of 0.05.
Results
Of the 136 patients who were asked to participate in the study, 76 were not enrolled; the other 60 were equally assigned to either the control group or the music therapy group (n = 30 in each) according to randomization indicated by a blinded statistician (Figure 1).
Table 2 lists the pre-intervention and post-intervention comparisons of the main outcomes between groups.
The emerging themes of the responses are listed in Tables 3 and 4 and are explained here:
Relationship with music was coded for significance and included reports of music as a resource accessed for stimulation and/or relaxation through listening; direct involvement with instrument playing; and history of music training.
Perceptions of surgical outcome in patients’ responses were coded across 3 themes: (1) optimistic (belief and hope in returning to original baseline of functionality), (2) indifferent (neither hopeful nor cynical about results of surgery), and (3) pessimistic (belief that nothing will restore the quality of life that existed before the spinal condition).
The CAS helped us better understand the diversity and complexity of the pain experience.
Discussion
Our hospital has the unique capability of providing music therapy to postoperative and other hospitalized patients. In this study, we compared the impact of a structured postoperative music therapy program on spine patients relative to control patients who did not receive music therapy after spine surgery.
We found a significant benefit in VAS pain levels (>1 point) but no statistically significant differences in HADS Anxiety, HADS Depression, or TSK scores. Although a 2-point difference is usually considered clinically significant, the degree of change in the music group is notable for having been achieved by nonpharmacologic means with scant chance of adverse effects. We suspect the lack of significant change in HADS Anxiety, HADS Depression, and TSK scores is attributable to the narrow study window. Given the observational data from our pilot study58 and ongoing results with spine patients,32 it seems clear that both mood state and resilience in coping are enhanced through an ongoing relationship with music therapy.
The study of a population as vulnerable as patients recovering from spine surgery raises many issues for providers and researchers. Although it is worthwhile to determine the efficacy of integrative modalities in serving these patients, the request for participation in a protocol at such a vulnerable time was often resisted. During our pilot work, it became clear that the ability of potential subjects to comprehend and complete protocol surveys was impacted by adverse effects, including sedation drowsiness; respiratory depression; nausea and vomiting; pruritus; and urinary retention caused by the medications used for postoperative pain management. Consequently, after piloting 5 cases before the main study, we extended the enrollment window to 72 hours.
Other unforeseen intrinsic or external obstacles were identified: Patient-related issues—including availability, level of interest in participation, and inability to participate because of the medication adverse effects mentioned.
Staff investment/education—addressed over the first 3 study years with several in-services, starting with the surgical team and continuing with nursing and support staff in various combinations. These meetings led to the creation of an Institutional Review Board (IRB) approved educational sheet for inclusion in the information packet given to surgical patients on registration.
Programming interruptions—caused by the convergence of several unanticipated factors, including a delay in expedited review of the IRB renewal during the year of Hurricane Sandy and an interruption in the spine team’s service for administrative and program modification.
Conclusion
Music therapy interventions (eg, use of patient-preferred live music) offered within a therapeutic relationship favorably affected pain perceptions in patients recovering from spine surgery. This effect was achieved through several therapeutic entry points, including support of expression and opportunities for emotional catharsis.
At the core of music therapy’s efficacy is individualized treatment, through which patients are supported in their recovery of “self.” Measurable benefits—including increased comfort; reduced pain; improved gait; increased range of motion, endurance, and ability to relax; and empowerment to actively participate in one’s own care through daily activities imbued with an enhanced sense of agency—are of cardinal importance, as they may lead to quicker recovery perceptions and enhanced quality of life.
Am J Orthop. 2017;46(1):E13-E22. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Music therapists use patient-preferred live music, increasing neurologic cues that enhance movement—a seminal recovery function in postoperative spine patients.
- Music therapy is an evidence-based, integrative treatment addressing body, mind, and spirit.
- Tension release through music therapy can serve as a critical mechanism for building resilience related to pain management.
- Music therapy and music medicine are distinct forms of clinical practice that focus on mind-body integration in the healing process.
- Music therapists, board-certified and licensed by the state as recognized healthcare professionals, address pain management, which is an increasing subspecialty in postoperative care.
About 70% of people in the United States experience at least 1 episode of back pain in their lifetime,1 and more than 5 million are temporarily or permanently disabled by spinal disorders.2-4 Some require surgery, which may rectify injury, but pain during recovery is often inevitable, and the road to recovery is not guaranteed to be smooth.5-20
Postoperative spine patients are at major risk for pain management challenges.14,15,18,20 Treatment is primarily pharmacologic and based on the surgical team’s pain management orders. Nursing care consists of monitoring the airway, vital signs, and neurovascular status and having patients rate their pain on a visual analog scale (VAS; 0 = no pain, 10 = worst pain imaginable). Nurses have the challenge of monitoring and continually assessing to make sure patients are achieving the optimal outcomes, particularly during the immediate postoperative period, when pain and anxiety are prominently increased.
Variability in spine surgery outcomes can be explained at least partly on the basis of prognostic psychological factors, including hypochondriasis, hysteria, depression, and poor pain coping strategies (eg, catastrophizing).21 In spine surgery patients, kinesiophobia (fear of moving) is a common component of distress that can impede recuperation.21-23
Rationale for Live Music
Pain is subjective and personal, and warrants an individualized approach to care. There is a body of music medicine research on the use of recorded music in modulating psychological and physiological factors in pain perception.30,32,34-54 This research supports the unique relationship of music to well-being, and the understanding that controlling any of these factors affects the duration, intensity, and quality of that experience.41,43,52
These findings provide incentive for breathing-entrained music therapy interventions, which enhance the relaxation response and release of pain-related tension;32,55-58 empower patients to unlock physical and emotional tension;32,57,58 provide a channel for expression and body movement; and enhance blood flow and/or alleviate pain by activating neurologic areas involved in the experience of pain.59-62Studies have found that physical endurance may be enhanced when movement is rhythmically coordinated with a musical stimulus.63-66 Music may prolong physical endurance by inhibiting psychological feedback associated with physical exertion related to fatigue, which may translate into accelerated recovery periods. When we listen to a rhythmic sound, our brains tend to automatically synchronize, or entrain, to external rhythmic cues that can stimulate increased motor control and coordination.63 Sound can arouse and raise the excitability of spinal motor neurons mediated by auditory-motor neuronal connections on the brain stem and spinal cord level.64-66 Rhythmically organized sounds serve as a neurological function in our capacity to organize predictable timing cues that are apparent in music, and may result in an effective treatment intervention in recovery.63,64
Music Therapy in Recovery From Spine Surgery
In music therapy, music is used within a therapeutic relationship to support or affect change in the patient and the treatment regimen.32,33,56-58 Research on music therapy with patients who are recovering from spine surgery is scant.67-69 Kleiber and Adamek67 studied perceptions of music therapy in 8 adolescents after spinal fusion surgery. In their study, a music therapist provided patients with a postoperative music therapy session focusing on the use of patient-preferred live music for relaxation and expression. Although their qualitative query was based on a therapeutic approach similar to that used in the present study, only 1 session was offered during the recovery period, and follow-up was conducted by survey invitation and telephone. In addition, the number of participants was small, and there was no quantitative measure of pain or other symptoms.
Another study focused on the effects of listening to music on pain intensity and distress after spine surgery.68 Patients in the study’s music group made their selections from prerecorded classical music and domestic and international popular songs from various genres and listened to their chosen recordings 30 minutes a day. Although the study was not a music therapy study per se, it showed a positive impact of listening to music on anxiety and pain perception in 60 adults who were randomly assigned to the music group or to a non-music control group (n = 30 in each). Differences between the music and control groups’ VAS ratings of anxiety (Ps = .018-.001) and pain (P = .001) were statistically significant.
Different from our study, the aforementioned studies did not include tension release–focused live music offered within a therapeutic relationship. Our 1.5-year pilot study, conducted prior to the present study indicated that music therapy led to increased resilience and recovery mechanisms.58
Methods
Our mixed-methods study design combined standard medical treatment with integrative music therapy interventions based on pain assessments to better understand the effects of music therapy on the recovery of patients after spine surgery.
The Spine Institute of New York within the Department of Orthopedic Surgery at Mount Sinai Beth Israel provides surgical treatment of common spinal cord conditions. Prioritizing patient satisfaction and positive outcomes,27,28 the institute integrates music therapy through the Louis Armstrong Center for Music and Medicine to enhance treatment of pain symptoms.
Patients were recruited by the research team as per the daily surgical schedule, or through referral by the medical team or patient care navigator. Sixty patients (35 female, 25 male) ranging in age from 40 to 55 years underwent anterior, posterior, or anterior-posterior spinal fusion and were enrolled in the study after signing a participation consent form. Minorities, women, and patients with Medicaid and Medicare were included. Patients who received a diagnosis of clinical psychosis or depression prior to spine injury were excluded.
The experimental group received music therapy plus standard care (medical and nursing care with scheduled pharmacologic pain intervention), and a wait-listed control group received standard care only. A randomization chart created by a blinded statistician who did not have access to the patient census determined the intervention–nonintervention schedule. Patients in the music therapy group received one 30-minute music therapy session during an 8-hour period within 72 hours after surgery.
For both groups, measurements were completed before and after the study window. Control patients were offered music therapy after completion of the post-intervention surveys in order to minimize the ethical dilemma of denying potentially helpful pain intervention. For this same reason, both groups were given the option of receiving follow-up music therapy sessions for the duration of their hospitalization.
The research team consisted of 2 licensed, board-certified music therapists. In addition, Master’s-level music therapy interns completing clinical hours as part of the trajectory for board certification served on the research team over the 5-year period 2009 to 2014, and 13 blinded research assistants helped with enrolling and collecting data on patients.
Intervention
Each music therapy session included a warm-up phase of verbal or musical discourse. Next was the treatment phase, which was based on patient need as assessed during warm-up. Treatment options included use of patient-preferred live music that supported tension release/relaxation through incentive-based clinical improvisation, singing, and/or rhythmic drumming or through breathwork and visualization. Psychoeducation about mind–body awareness through the use of breath and imagery was introduced and explained by the therapist at this time.
The improvised music intervention was focused on making salient the natural harmonic tension-resolution cycles that occur in music and that were entrained to the patient’s presentation (respiratory rate, verbal report, clinical presentation). When patient-preferred precomposed songs were used, tension resolution was achieved by sustaining cadence and resolution, also entrained to the patient’s respiratory cycles.32,57,58
After the music therapy intervention, a period of closure or integration was facilitated by the therapist contingent on the patient’s degree of alertness. If awake, the patient was supported in a reflexive process of thoughts, impressions, or issues that may have contributed to the overall experience. If the patient was asleep, the researcher returned within 30 minutes for post-intervention interviewing. Interview information was recorded in a qualitative post-participation survey. To prevent bias, researchers who were not the treating clinicians conducted the surveys.
Outcome Measures
Both primary and secondary outcome measures were collected before and after the intervention. The primary outcome measure was VAS pain ratings, and the secondary outcome measures were scores on the Hospital Anxiety and Depression Scale (HADS), the Tampa Scale for Kinesiophobia (TSK), and the Color Analysis Scale (CAS).
VAS. With the VAS, images are used to rate pain. The scale has points labeled 0 to 10 and corresponding faces representing progression in pain intensity. The scale is quickly rendered and can be interpreted according to the patient’s recovery phase at time of rendering.
HADS. The HADS70 provides a specific baseline for anxiety and depression as an indicator of how the patient might fare during hospitalization (admission through recovery and discharge).
TSK. The TSK71 provides insight into the patient’s perception of fear-related movement, which is an important factor in this study because of the movement required for rehabilitation. We used a shortened version of the TSK to accommodate the sensitive threshold for pain tolerance and pharmacologic side effects commonly experienced by spine patients.
CAS. The CAS was developed at the Louis Armstrong Center for Music and Medicine to assess comorbidities and dynamic aspects of pain. Through a coloring exercise, patients illustrate their pain experience, which gives tangible form to the abstract experience of pain.
Coding
We collected patients’ demographic data, including age, sex, and diagnoses. Clinical indicators of the preoperative baseline included lifestyle, surgical history, and prior experience with music or other mind–body strategies for self-regulation.
As fundamental to qualitative methodology,72,73 the reported responses to questions were grouped into themes that were peer-tested with members of the research team before and during the coding process.
VAS, HADS, and TSK data were tabulated by blinded research assistants and analyzed by a statistician. Patients were identified by number assignment, and their data and personal information were kept confidentially stored.
Statistical Methods
Means and standard deviations were used for continuous variables, and frequencies (percentages) for categorical variables. All outcomes were analyzed on an intent-to-treat basis. Repeated-measures analysis of variance was used to compare changes in outcomes from before to after intervention for the music and control groups. In particular, a statistically significant Group (music vs control) × Time (before vs after intervention) interaction would support the hypothesis that there would be more benefit (less pain) in the music group as a result of the music therapy. For all tests, significance was set at P < .05. SPSS Version 20 (IBM) was used for all statistical analyses. Based on previously found differences in heart rate and mobility,31 we assumed an effect size of 0.71 for the difference between music and control (no music), which would require 32 patients per group to achieve a power of 0.8 with an α of 0.05.
Results
Of the 136 patients who were asked to participate in the study, 76 were not enrolled; the other 60 were equally assigned to either the control group or the music therapy group (n = 30 in each) according to randomization indicated by a blinded statistician (Figure 1).
Table 2 lists the pre-intervention and post-intervention comparisons of the main outcomes between groups.
The emerging themes of the responses are listed in Tables 3 and 4 and are explained here:
Relationship with music was coded for significance and included reports of music as a resource accessed for stimulation and/or relaxation through listening; direct involvement with instrument playing; and history of music training.
Perceptions of surgical outcome in patients’ responses were coded across 3 themes: (1) optimistic (belief and hope in returning to original baseline of functionality), (2) indifferent (neither hopeful nor cynical about results of surgery), and (3) pessimistic (belief that nothing will restore the quality of life that existed before the spinal condition).
The CAS helped us better understand the diversity and complexity of the pain experience.
Discussion
Our hospital has the unique capability of providing music therapy to postoperative and other hospitalized patients. In this study, we compared the impact of a structured postoperative music therapy program on spine patients relative to control patients who did not receive music therapy after spine surgery.
We found a significant benefit in VAS pain levels (>1 point) but no statistically significant differences in HADS Anxiety, HADS Depression, or TSK scores. Although a 2-point difference is usually considered clinically significant, the degree of change in the music group is notable for having been achieved by nonpharmacologic means with scant chance of adverse effects. We suspect the lack of significant change in HADS Anxiety, HADS Depression, and TSK scores is attributable to the narrow study window. Given the observational data from our pilot study58 and ongoing results with spine patients,32 it seems clear that both mood state and resilience in coping are enhanced through an ongoing relationship with music therapy.
The study of a population as vulnerable as patients recovering from spine surgery raises many issues for providers and researchers. Although it is worthwhile to determine the efficacy of integrative modalities in serving these patients, the request for participation in a protocol at such a vulnerable time was often resisted. During our pilot work, it became clear that the ability of potential subjects to comprehend and complete protocol surveys was impacted by adverse effects, including sedation drowsiness; respiratory depression; nausea and vomiting; pruritus; and urinary retention caused by the medications used for postoperative pain management. Consequently, after piloting 5 cases before the main study, we extended the enrollment window to 72 hours.
Other unforeseen intrinsic or external obstacles were identified: Patient-related issues—including availability, level of interest in participation, and inability to participate because of the medication adverse effects mentioned.
Staff investment/education—addressed over the first 3 study years with several in-services, starting with the surgical team and continuing with nursing and support staff in various combinations. These meetings led to the creation of an Institutional Review Board (IRB) approved educational sheet for inclusion in the information packet given to surgical patients on registration.
Programming interruptions—caused by the convergence of several unanticipated factors, including a delay in expedited review of the IRB renewal during the year of Hurricane Sandy and an interruption in the spine team’s service for administrative and program modification.
Conclusion
Music therapy interventions (eg, use of patient-preferred live music) offered within a therapeutic relationship favorably affected pain perceptions in patients recovering from spine surgery. This effect was achieved through several therapeutic entry points, including support of expression and opportunities for emotional catharsis.
At the core of music therapy’s efficacy is individualized treatment, through which patients are supported in their recovery of “self.” Measurable benefits—including increased comfort; reduced pain; improved gait; increased range of motion, endurance, and ability to relax; and empowerment to actively participate in one’s own care through daily activities imbued with an enhanced sense of agency—are of cardinal importance, as they may lead to quicker recovery perceptions and enhanced quality of life.
Am J Orthop. 2017;46(1):E13-E22. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Miller B, Gatchel RJ, Lou L, Stowell A, Robinson R, Polatin PB. Interdisciplinary treatment of failed back surgery syndrome (FBSS): a comparison of FBSS and non-FBSS patients. Pain Pract. 2005;5(3):190-202.
2. Aebi M. The adult scoliosis. Eur Spine J. 2005;14(10):925-948.
3. Engstrom JW, Deyo, RA. Back and neck pain. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison’s Principles of Internal Medicine, 19th edition. New York, NY: McGraw-Hill; 2007:207-214.
4. Cavanaugh JM, Lu Y, Chen C, Kallakuri S. Pain generation in lumbar and cervical facet joints. J Bone Joint Surg Am. 2006;88(suppl 2):63-67.
5. Hart RA, Prendergast MA. Spine surgery for lumbar degenerative disease in elderly and osteoporotic patients. Instr Course Lect. 2007;56:257-272.
6. Boswell MV, Trescot AM, Datta S, et al; American Society of Interventional Pain Physicians. Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician. 2007;10(1):7-111.
7. Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356(22):2257-2270.
8. Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT): A randomized trial. JAMA. 2006;296(20):2441-2450.
9. Malmivaara A, Slätis P, Heliövaara M, et al; Finnish Lumbar Spinal Research Group. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine. 2007;32(1):1-8.
10. Chang Y, Singer DE, Wu YA, Keller RB, Atlas SJ. The effect of surgical and nonsurgical treatment on longitudinal outcomes of lumbar spinal stenosis over 10 years. J Am Geriatr Soc. 2005;53(5):785-792.
11. Cowan JA Jr, Dimick JB, Wainess R, Upchurch GR Jr, Chandler WF, La Marca F. Changes in the utilization of spinal fusion in the United States. Neurosurgery. 2006;59(1):15-20.
12. Lonner BS, Scharf CS, Antonacci D, Goldstein Y, Panagopoulos G. The learning curve associated with thoracoscopic spinal instrumentation. Spine. 2005;30(24):2835-2840.
13. Lonner BS, Kondrachov D, Siddiqi F, Hayes V, Scharf C. Thoracoscopic spinal fusion compared with posterior spinal fusion for the treatment of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2006;88(5):1022-1034.
14. Boakye M, Patil CG, Santarelli J, Ho C, Tian W, Lad SP. Cervical spondylotic myelopathy: complications and outcomes after spinal fusion. Neurosurgery. 2008;62(2):455-461.
15. Boakye M, Patil CG, Santarelli J, Ho C, Tian W, Lad SP. Laminectomy and fusion after spinal cord injury: national inpatient complications and outcomes. J Neurotrauma. 2008;25(3):173-183.
16. Dekutoski MB, Norvell DC, Dettori JR, Fehlings MG, Chapman JR. Surgeon perceptions and reported complications in spine surgery. Spine. 2010;35(9 suppl):S9-S21.
17. Nasser R, Yadla S, Maltenfort MG, et al. Complications in spine surgery. J Neurosurg Spine. 2010;13(2):144-157.
18. Patil CG, Santarelli J, Lad SP, Ho C, Tian W, Boakye M. Inpatient complications, mortality, and discharge disposition after surgical correction of idiopathic scoliosis: a national perspective. Spine J. 2008;8(6):904-910.
19. Rampersaud YR, Moro ER, Neary MA, et al. Intraoperative adverse events and related postoperative complications in spine surgery: implications for enhancing patient safety founded on evidence-based protocols. Spine. 2006;31(13):1503-1510.
20. Shen Y, Silverstein JC, Roth S. In-hospital complications and mortality after elective spinal fusion surgery in the United States: a study of the Nationwide Inpatient Sample from 2001 to 2005. J Neurosurg Anesthesiol. 2009;21(1):21-30.
21. Picavet HSJ, Vlaeyen JWS, Schouten JSAG. Pain catastrophizing and kinesiophobia: predictors of chronic low back pain. Am J Epidemiol. 2002;156(11):1028-1034.
22. French DJ, France CR, Vigneau F, French JA, Evans RT. Fear of movement/(re)injury in chronic pain: a psychometric assessment of the original English version of the Tampa Scale for Kinesiophobia (TSK). Pain. 2007;127(1-2):42-51.
23. Goubert L, Crombez G, Van Damme S, Vlaeyen JW, Bijttebier P, Roelofs J. Confirmatory factor analysis of the Tampa Scale for Kinesiophobia: invariant two-factor model across low back pain patients and fibromyalgia patients. Clin J Pain. 2004;20(2):103-110.
24. Selimen D, Andsoy II. The importance of a holistic approach during the perioperative period. AORN J. 2011;93(4):482-487.
25. Zheng Z. Xue CC. Pain research in complementary and alternative medicine in Australia: a critical review. J Altern Complement Med. 2013;19(2):81-91.
26. Wright J, Adams D, Vohra S. Complementary, holistic, and integrative medicine: music for procedural pain. Pediatr Rev. 2013;34(11):e42-e46.
27. McCann PD. Orthopedic surgery and integrative medicine—strange bedfellows. Am J Orthop. 2009;38(2):66, 71.
28. McCann PD. Customer satisfaction: are hospitals “hospitable”? Am J Orthop. 2006;35(2):59.
29. Joanna Briggs Institute. The Joanna Briggs Institute best practice information sheet: music as an intervention in hospitals. Nurs Health Sci. 2011;13(1):99-102.
30. Spintge R. Thirty-five years of anxiolytic music (AAM) in pain and aversive clinical settings. In: Mondanaro J, Sara G, eds. Music and Medicine: Integrative Models in the Treatment of Pain. New York, NY: Satchnote Press; 2013:29-42.
31. Cepeda MS, Carr DB, Lau J, Alvarez H. Music for pain relief. Cochrane Database Syst Rev. 2006;(2):CD004843.
32. Mondanaro J. Music therapy based release strategies in the treatment of acute and chronic pain: an individualized approach. In: Mondanaro J, Sara G, eds. Music and Medicine: Integrative Models in the Treatment of Pain. New York, NY: Satchnote Press; 2013:133-148.
33. Quentzel S. Music has charms to soothe a savage breast. In: Mondanaro J, Sara G, eds. Music and Medicine: Integrative Models in the Treatment of Pain. New York, NY: Satchnote Press; 2013:11-28.
34. Ko YL. Lin PC. The effect of using a relaxation tape on pulse, respiration, blood pressure and anxiety levels of surgical patients. J Clin Nurs. 2012;21(5-6):689-697.
35. Roy M, Lebuis A, Hugueville L, Peretz I, Rainville P. Spinal modulation of nociception by music. Eur J Pain. 2012;16(6):870-877.
36. Roy M, Peretz I, Rainville P. Emotional valence contributes to music-induced analgesia. Pain. 2008;134(1-2):140-147.
37. Schröter T. Medicine needs music! Music therapy for chronic pain [in German]. Rev Med Suisse. 2014;10(415):286.
38. Bellieni CV, Cioncoloni D, Mazzanti S, et al. Music provided through a portable media player (iPod) blunts pain during physical therapy. Pain Manag Nurs. 2013;14(4):e151-e155.
39. Bernatzky G, Presch M, Anderson M, Panksepp J. Emotional foundations of music as a non-pharmacological pain management tool in modern medicine. Neurosci Biobehav Rev. 2011;35(9):1989-1999.
40. Bradshaw DH, Chapman CR, Jacobson RC, Donaldson GW. Effects of music engagement on response to painful stimulation. Clin J Pain. 2012;28(5):418-427.
41. Bradshaw DH, Donaldson GW, Jacobson RC, Nakamura Y, Chapman CR. Individual differences in the effects of music engagement on responses to painful stimulation. J Pain. 2011;12(12):1262-1273.
42. Chlan L, Halm MA. Does music ease pain and anxiety in the critically ill? Am J Crit Care. 2013;22(6):528-532.
43. Guétin S, Giniès P, Siou DK, et al. The effects of music intervention in the management of chronic pain: a single-blind, randomized, controlled trial. Clin J Pain. 2012;28(4):329-337.
44. Matsota P, Christodoulopoulou T, Smyrnioti ME, et al. Music’s use for anesthesia and analgesia. J Altern Complement Med. 2013;19(4):298-307.
45. Gooding L, Swezey S, Zwischenberger JB. Using music interventions in perioperative care. South Med J. 2012;105(9):486-490.
46. Graversen M, Sommer T. Perioperative music may reduce pain and fatigue in patients undergoing laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 2013;57(8):1010-1016.
47. Ni CH, Tsai WH, Lee LM, Kao CC, Chen YC. Minimising preoperative anxiety with music for day surgery patients—a randomised clinical trial. J Clin Nurs. 2012;21(5-6):620-625.
48. Good M, Albert JM, Anderson GC, et al. Supplementing relaxation and music for pain after surgery. Nurs Res. 2010;59(4):259-269.
49. Moris DN, Linos D. Music meets surgery: two sides to the art of “healing.” Surg Endosc. 2013;27(3):719-723.
50. Nilsson U, Rawal N, Unosson M. A comparison of intra-operative or postoperative exposure to music—a controlled trial of the effects on postoperative pain. Anaesthesia. 2003;58(7):699-703.
51. Özer N, Karaman Özlü Z, Arslan S, Günes N. Effect of music on postoperative pain and physiologic parameters of patients after open heart surgery. Pain Manag Nurs. 2013;14(1):20-28.
52. Sen H, Yanarateş O, Sızlan A, Kılıç E, Ozkan S, Dağlı G. The efficiency and duration of the analgesic effects of musical therapy on postoperative pain. Agri. 2010;22(4):145-150.
53. Vaajoki A, Pietilä AM, Kankkunen P, Vehviläinen-Julkunen K. Music intervention study in abdominal surgery patients: challenges of an intervention study in clinical practice. Int J Nurs Pract. 2013;19(2):206-213.
54. Vaajoki A, Pietilä AM, Kankkunen P, Vehviläinen-Julkunen K. Effects of listening to music on pain intensity and pain distress after surgery: an intervention. J Clin Nurs. 2012;21(5-6):708-717.
55. Whitaker MH. Sounds soothing: music therapy for postoperative pain. Nursing. 2010;40(12):53-54.
56. Edwards J. Developing pain management approaches in music therapy with hospitalized children. In: Loewy J, Dileo C, eds. Music Therapy at the End of Life. Cherry Hill, NJ: Jeffrey Books; 2005:57-76.
57. Loewy J. The quiet soldier: pain and sickle cell anemia. In: Hibben J, ed. Inside Music Therapy: Client Experiences. Gilsum, NH: Barcelona; 1999:69-76.
58. Lichtensztejn M. The clinical use of piano with patients suffering from breathing distress related to pain. In: Azoulay R, Loewy JV, eds. Music, the Breath and Health: Advances in Integrative Music Therapy. New York, NY: Satchnote Press; 2009:213-222.
59. Kwon IS, Kim J, Park KM. Effects of music therapy on pain, discomfort, and depression for patients with leg fractures. Taehan Kanho Hakhoe Chi. 2006;36(4):630-636.
60. Zengin S, Kabul S, Al B, Sarcan E, Doğan M, Yildirim C. Effects of music therapy on pain and anxiety in patients undergoing port catheter placement procedure. Complement Ther Med. 2013;21(6):689-696.
61. Boso M, Politi P, Barale F, Emanuele E. Neurophysiology and neurobiology of the musical experience. Funct Neurol. 2006;21(4):187-191.
62. Salimpoor VN, Benovoy M, Larcher K, Dagher A, Zatorre RJ. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat Neurosci. 2011;14(2):257-262.
63. Tomaino CM. Using rhythm for rehabilitation. Institute for Music and Neurologic Function website. http://musictherapy.imnf.org/images/uploads/rhythm.pdf. Published 2006. Accessed August 21, 2007.
64. Molinari M, Leggio MG, De Martin M, Cerasa A, Thaut M. Neurobiology of rhythmic motor entrainment. Ann N Y Acad Sci. 2003;999:313-321.
65. Thaut M. Neuropsychological processes in music perception. In: Unkefer R, ed. Music Therapy in the Treatment of Adults With Mental Disorders: Theoretical Bases and Clinical Interventions. Toronto, Canada: Schirmer Books; 2002:2-32.
66. Thaut M. Physiological and motor responses to music stimuli. In: Unkefer R, ed. Music Therapy in the Treatment of Adults With Mental Disorders: Theoretical Bases and Clinical Interventions. Toronto, Canada: Schimer Books; 2002:33-41.
67. Kleiber C, Adamek MS. Adolescents’ perceptions of music therapy following spinal fusion surgery. J Clin Nurs. 2013;22(3-4):414-422.
68. Lin PC, Lin ML, Huang LC, Hsu HC, Lin CC. Music therapy for patients receiving spine surgery. J Clin Nurs. 2011;20(7-8):960-968.
69. Maeyama A, Kodaka M, Miyao H. Effect of the music-therapy under spinal anesthesia [in Japanese]. Masui. 2009;58(6):684-691.
70. Golden J, Conroy RM, O’Dwyer AM. Reliability and validity of the Hospital Anxiety and Depression Scale and the Beck Depression Inventory (Full and FastScreen scales) in detecting depression in persons with hepatitis C. J Affect Disord. 2006;100(1-3):265-269.
71. Woby SR, Roach NK, Urmston M, Watson PJ. Psychometric properties of the TSK-11: a shortened version of the Tampa Scale for Kinesiophobia. Pain. 2005;117(1-2):137-144.
72. Humrichouse J, Chmielewski M, McDade-Montez EA, Watson D. Affect assessment through self-report methods. In: Rottenberg J, Johnson SL, eds. Emotion and Psychopathology: Bridging Affective and Clinical Science. Washington, DC: American Psychological Association; 2007:13-34.
73. Lincoln YS, Guba EG. Naturalistic Inquiry. Beverly Hills, CA: Sage; 1985.
1. Miller B, Gatchel RJ, Lou L, Stowell A, Robinson R, Polatin PB. Interdisciplinary treatment of failed back surgery syndrome (FBSS): a comparison of FBSS and non-FBSS patients. Pain Pract. 2005;5(3):190-202.
2. Aebi M. The adult scoliosis. Eur Spine J. 2005;14(10):925-948.
3. Engstrom JW, Deyo, RA. Back and neck pain. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison’s Principles of Internal Medicine, 19th edition. New York, NY: McGraw-Hill; 2007:207-214.
4. Cavanaugh JM, Lu Y, Chen C, Kallakuri S. Pain generation in lumbar and cervical facet joints. J Bone Joint Surg Am. 2006;88(suppl 2):63-67.
5. Hart RA, Prendergast MA. Spine surgery for lumbar degenerative disease in elderly and osteoporotic patients. Instr Course Lect. 2007;56:257-272.
6. Boswell MV, Trescot AM, Datta S, et al; American Society of Interventional Pain Physicians. Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician. 2007;10(1):7-111.
7. Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356(22):2257-2270.
8. Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT): A randomized trial. JAMA. 2006;296(20):2441-2450.
9. Malmivaara A, Slätis P, Heliövaara M, et al; Finnish Lumbar Spinal Research Group. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine. 2007;32(1):1-8.
10. Chang Y, Singer DE, Wu YA, Keller RB, Atlas SJ. The effect of surgical and nonsurgical treatment on longitudinal outcomes of lumbar spinal stenosis over 10 years. J Am Geriatr Soc. 2005;53(5):785-792.
11. Cowan JA Jr, Dimick JB, Wainess R, Upchurch GR Jr, Chandler WF, La Marca F. Changes in the utilization of spinal fusion in the United States. Neurosurgery. 2006;59(1):15-20.
12. Lonner BS, Scharf CS, Antonacci D, Goldstein Y, Panagopoulos G. The learning curve associated with thoracoscopic spinal instrumentation. Spine. 2005;30(24):2835-2840.
13. Lonner BS, Kondrachov D, Siddiqi F, Hayes V, Scharf C. Thoracoscopic spinal fusion compared with posterior spinal fusion for the treatment of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2006;88(5):1022-1034.
14. Boakye M, Patil CG, Santarelli J, Ho C, Tian W, Lad SP. Cervical spondylotic myelopathy: complications and outcomes after spinal fusion. Neurosurgery. 2008;62(2):455-461.
15. Boakye M, Patil CG, Santarelli J, Ho C, Tian W, Lad SP. Laminectomy and fusion after spinal cord injury: national inpatient complications and outcomes. J Neurotrauma. 2008;25(3):173-183.
16. Dekutoski MB, Norvell DC, Dettori JR, Fehlings MG, Chapman JR. Surgeon perceptions and reported complications in spine surgery. Spine. 2010;35(9 suppl):S9-S21.
17. Nasser R, Yadla S, Maltenfort MG, et al. Complications in spine surgery. J Neurosurg Spine. 2010;13(2):144-157.
18. Patil CG, Santarelli J, Lad SP, Ho C, Tian W, Boakye M. Inpatient complications, mortality, and discharge disposition after surgical correction of idiopathic scoliosis: a national perspective. Spine J. 2008;8(6):904-910.
19. Rampersaud YR, Moro ER, Neary MA, et al. Intraoperative adverse events and related postoperative complications in spine surgery: implications for enhancing patient safety founded on evidence-based protocols. Spine. 2006;31(13):1503-1510.
20. Shen Y, Silverstein JC, Roth S. In-hospital complications and mortality after elective spinal fusion surgery in the United States: a study of the Nationwide Inpatient Sample from 2001 to 2005. J Neurosurg Anesthesiol. 2009;21(1):21-30.
21. Picavet HSJ, Vlaeyen JWS, Schouten JSAG. Pain catastrophizing and kinesiophobia: predictors of chronic low back pain. Am J Epidemiol. 2002;156(11):1028-1034.
22. French DJ, France CR, Vigneau F, French JA, Evans RT. Fear of movement/(re)injury in chronic pain: a psychometric assessment of the original English version of the Tampa Scale for Kinesiophobia (TSK). Pain. 2007;127(1-2):42-51.
23. Goubert L, Crombez G, Van Damme S, Vlaeyen JW, Bijttebier P, Roelofs J. Confirmatory factor analysis of the Tampa Scale for Kinesiophobia: invariant two-factor model across low back pain patients and fibromyalgia patients. Clin J Pain. 2004;20(2):103-110.
24. Selimen D, Andsoy II. The importance of a holistic approach during the perioperative period. AORN J. 2011;93(4):482-487.
25. Zheng Z. Xue CC. Pain research in complementary and alternative medicine in Australia: a critical review. J Altern Complement Med. 2013;19(2):81-91.
26. Wright J, Adams D, Vohra S. Complementary, holistic, and integrative medicine: music for procedural pain. Pediatr Rev. 2013;34(11):e42-e46.
27. McCann PD. Orthopedic surgery and integrative medicine—strange bedfellows. Am J Orthop. 2009;38(2):66, 71.
28. McCann PD. Customer satisfaction: are hospitals “hospitable”? Am J Orthop. 2006;35(2):59.
29. Joanna Briggs Institute. The Joanna Briggs Institute best practice information sheet: music as an intervention in hospitals. Nurs Health Sci. 2011;13(1):99-102.
30. Spintge R. Thirty-five years of anxiolytic music (AAM) in pain and aversive clinical settings. In: Mondanaro J, Sara G, eds. Music and Medicine: Integrative Models in the Treatment of Pain. New York, NY: Satchnote Press; 2013:29-42.
31. Cepeda MS, Carr DB, Lau J, Alvarez H. Music for pain relief. Cochrane Database Syst Rev. 2006;(2):CD004843.
32. Mondanaro J. Music therapy based release strategies in the treatment of acute and chronic pain: an individualized approach. In: Mondanaro J, Sara G, eds. Music and Medicine: Integrative Models in the Treatment of Pain. New York, NY: Satchnote Press; 2013:133-148.
33. Quentzel S. Music has charms to soothe a savage breast. In: Mondanaro J, Sara G, eds. Music and Medicine: Integrative Models in the Treatment of Pain. New York, NY: Satchnote Press; 2013:11-28.
34. Ko YL. Lin PC. The effect of using a relaxation tape on pulse, respiration, blood pressure and anxiety levels of surgical patients. J Clin Nurs. 2012;21(5-6):689-697.
35. Roy M, Lebuis A, Hugueville L, Peretz I, Rainville P. Spinal modulation of nociception by music. Eur J Pain. 2012;16(6):870-877.
36. Roy M, Peretz I, Rainville P. Emotional valence contributes to music-induced analgesia. Pain. 2008;134(1-2):140-147.
37. Schröter T. Medicine needs music! Music therapy for chronic pain [in German]. Rev Med Suisse. 2014;10(415):286.
38. Bellieni CV, Cioncoloni D, Mazzanti S, et al. Music provided through a portable media player (iPod) blunts pain during physical therapy. Pain Manag Nurs. 2013;14(4):e151-e155.
39. Bernatzky G, Presch M, Anderson M, Panksepp J. Emotional foundations of music as a non-pharmacological pain management tool in modern medicine. Neurosci Biobehav Rev. 2011;35(9):1989-1999.
40. Bradshaw DH, Chapman CR, Jacobson RC, Donaldson GW. Effects of music engagement on response to painful stimulation. Clin J Pain. 2012;28(5):418-427.
41. Bradshaw DH, Donaldson GW, Jacobson RC, Nakamura Y, Chapman CR. Individual differences in the effects of music engagement on responses to painful stimulation. J Pain. 2011;12(12):1262-1273.
42. Chlan L, Halm MA. Does music ease pain and anxiety in the critically ill? Am J Crit Care. 2013;22(6):528-532.
43. Guétin S, Giniès P, Siou DK, et al. The effects of music intervention in the management of chronic pain: a single-blind, randomized, controlled trial. Clin J Pain. 2012;28(4):329-337.
44. Matsota P, Christodoulopoulou T, Smyrnioti ME, et al. Music’s use for anesthesia and analgesia. J Altern Complement Med. 2013;19(4):298-307.
45. Gooding L, Swezey S, Zwischenberger JB. Using music interventions in perioperative care. South Med J. 2012;105(9):486-490.
46. Graversen M, Sommer T. Perioperative music may reduce pain and fatigue in patients undergoing laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 2013;57(8):1010-1016.
47. Ni CH, Tsai WH, Lee LM, Kao CC, Chen YC. Minimising preoperative anxiety with music for day surgery patients—a randomised clinical trial. J Clin Nurs. 2012;21(5-6):620-625.
48. Good M, Albert JM, Anderson GC, et al. Supplementing relaxation and music for pain after surgery. Nurs Res. 2010;59(4):259-269.
49. Moris DN, Linos D. Music meets surgery: two sides to the art of “healing.” Surg Endosc. 2013;27(3):719-723.
50. Nilsson U, Rawal N, Unosson M. A comparison of intra-operative or postoperative exposure to music—a controlled trial of the effects on postoperative pain. Anaesthesia. 2003;58(7):699-703.
51. Özer N, Karaman Özlü Z, Arslan S, Günes N. Effect of music on postoperative pain and physiologic parameters of patients after open heart surgery. Pain Manag Nurs. 2013;14(1):20-28.
52. Sen H, Yanarateş O, Sızlan A, Kılıç E, Ozkan S, Dağlı G. The efficiency and duration of the analgesic effects of musical therapy on postoperative pain. Agri. 2010;22(4):145-150.
53. Vaajoki A, Pietilä AM, Kankkunen P, Vehviläinen-Julkunen K. Music intervention study in abdominal surgery patients: challenges of an intervention study in clinical practice. Int J Nurs Pract. 2013;19(2):206-213.
54. Vaajoki A, Pietilä AM, Kankkunen P, Vehviläinen-Julkunen K. Effects of listening to music on pain intensity and pain distress after surgery: an intervention. J Clin Nurs. 2012;21(5-6):708-717.
55. Whitaker MH. Sounds soothing: music therapy for postoperative pain. Nursing. 2010;40(12):53-54.
56. Edwards J. Developing pain management approaches in music therapy with hospitalized children. In: Loewy J, Dileo C, eds. Music Therapy at the End of Life. Cherry Hill, NJ: Jeffrey Books; 2005:57-76.
57. Loewy J. The quiet soldier: pain and sickle cell anemia. In: Hibben J, ed. Inside Music Therapy: Client Experiences. Gilsum, NH: Barcelona; 1999:69-76.
58. Lichtensztejn M. The clinical use of piano with patients suffering from breathing distress related to pain. In: Azoulay R, Loewy JV, eds. Music, the Breath and Health: Advances in Integrative Music Therapy. New York, NY: Satchnote Press; 2009:213-222.
59. Kwon IS, Kim J, Park KM. Effects of music therapy on pain, discomfort, and depression for patients with leg fractures. Taehan Kanho Hakhoe Chi. 2006;36(4):630-636.
60. Zengin S, Kabul S, Al B, Sarcan E, Doğan M, Yildirim C. Effects of music therapy on pain and anxiety in patients undergoing port catheter placement procedure. Complement Ther Med. 2013;21(6):689-696.
61. Boso M, Politi P, Barale F, Emanuele E. Neurophysiology and neurobiology of the musical experience. Funct Neurol. 2006;21(4):187-191.
62. Salimpoor VN, Benovoy M, Larcher K, Dagher A, Zatorre RJ. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat Neurosci. 2011;14(2):257-262.
63. Tomaino CM. Using rhythm for rehabilitation. Institute for Music and Neurologic Function website. http://musictherapy.imnf.org/images/uploads/rhythm.pdf. Published 2006. Accessed August 21, 2007.
64. Molinari M, Leggio MG, De Martin M, Cerasa A, Thaut M. Neurobiology of rhythmic motor entrainment. Ann N Y Acad Sci. 2003;999:313-321.
65. Thaut M. Neuropsychological processes in music perception. In: Unkefer R, ed. Music Therapy in the Treatment of Adults With Mental Disorders: Theoretical Bases and Clinical Interventions. Toronto, Canada: Schirmer Books; 2002:2-32.
66. Thaut M. Physiological and motor responses to music stimuli. In: Unkefer R, ed. Music Therapy in the Treatment of Adults With Mental Disorders: Theoretical Bases and Clinical Interventions. Toronto, Canada: Schimer Books; 2002:33-41.
67. Kleiber C, Adamek MS. Adolescents’ perceptions of music therapy following spinal fusion surgery. J Clin Nurs. 2013;22(3-4):414-422.
68. Lin PC, Lin ML, Huang LC, Hsu HC, Lin CC. Music therapy for patients receiving spine surgery. J Clin Nurs. 2011;20(7-8):960-968.
69. Maeyama A, Kodaka M, Miyao H. Effect of the music-therapy under spinal anesthesia [in Japanese]. Masui. 2009;58(6):684-691.
70. Golden J, Conroy RM, O’Dwyer AM. Reliability and validity of the Hospital Anxiety and Depression Scale and the Beck Depression Inventory (Full and FastScreen scales) in detecting depression in persons with hepatitis C. J Affect Disord. 2006;100(1-3):265-269.
71. Woby SR, Roach NK, Urmston M, Watson PJ. Psychometric properties of the TSK-11: a shortened version of the Tampa Scale for Kinesiophobia. Pain. 2005;117(1-2):137-144.
72. Humrichouse J, Chmielewski M, McDade-Montez EA, Watson D. Affect assessment through self-report methods. In: Rottenberg J, Johnson SL, eds. Emotion and Psychopathology: Bridging Affective and Clinical Science. Washington, DC: American Psychological Association; 2007:13-34.
73. Lincoln YS, Guba EG. Naturalistic Inquiry. Beverly Hills, CA: Sage; 1985.
Is a Persistent Vacuum Phenomenon a Sign of Pseudarthrosis After Posterolateral Spinal Fusion?
The spinal vacuum sign or vacuum phenomenon (VP) is the radiographic finding of an air-density linear radiolucency in the intervertebral disc or vertebral body. The result of a gaseous accumulation, it is often a diagnostic sign of disc degeneration as well as a rare sign of infection, Schmorl node formation, or osteonecrosis.1,2 Although the VP was first described on plain radiographs, it is better seen on computed tomography (CT).3 Multiple studies have found a possible association between the VP and nonunion in diaphyseal fractures,4 ankylosing spondylitis,5,6 and lumbar spinal fusion.7
To our knowledge, no one has studied whether the intervertebral VP resolves after posterolateral lumbar spinal fusion in adults with degenerative spinal pathology, and no one has investigated the association between the persistence of the intervertebral VP and pseudarthrosis after posterolateral spinal fusion.
We conducted a study to determine whether the VP resolves after posterolateral lumbar spinal fusion procedures and whether persistence of the VP after fusion surgery is indicative of pseudarthrosis.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the medical records of patients who had degenerative spinal stenosis with instability and the intervertebral vacuum sign on preoperative digital lumbar spine CT scans and who underwent posterolateral lumbar spinal fusion with or without instrumentation. Study inclusion criteria were lumbar spine CT at minimum 6-month follow-up after spinal fusion and preoperative and postoperative lumbar spine radiographs. Exclusion criteria were any type of interbody fusion procedure (anterior, posterior, transforaminal, lateral) at a level with the VP, age under 21 years, follow-up of less than 6 months, and incomplete radiographic records. As this was a retrospective study, patient consent was not required.
CT was performed with a 16-, 64-, or 128-slice multidetector CT scanner with effective tube current set at 250 to 320 mA, voltage set at 120 to 140 kV, and pitch set at 0.75 to 0.9. After axial acquisition of 3×3-mm isometric voxels, sagittal and coronal multiplanar images were reconstructed with a slice thickness of 2 mm. Patient demographics, diagnoses, and surgical details were recorded. All digital lumbar spine CT scans and radiographs were initially screened on PACS (picture archiving and communication system) by the orthopedic spine surgery fellow at an academic medical institution; then they were reviewed on a radiology reading room monitor by 3 observers (senior radiologist, senior orthopedic spine surgeon, orthopedic spine surgery fellow). Axial images and sagittal and coronal reconstructed images of the preoperative and postoperative follow-up lumbar CT scans—together with the lateral and anteroposterior lumbar spine radiographs—were evaluated for the intervertebral VP. Mean (SD) follow-up (with CT to assess fusion) was 1.6 (0.86) years (range, 0.75-3.38 years). Fusion at each level was evaluated on the postoperative follow-up CT on axial images and sagittal and coronal reconstructed images; criteria for fusion were continuous bridging bone across posterolateral gutters and facets on one or both sides at each intervertebral level.8 Pseudarthrosis was recorded if there was no continuity of bridging bone across both posterolateral gutters and facets, a complete radiolucent line on both sides across a level, or lysis or loosening around screws. All recordings were made by consensus, or by majority decision in case of disagreement.
Presence of the VP at the lumbar levels not included in the fusion was also recorded on the preoperative and follow-up CT scan and radiographs.
Descriptive and inferential statistical tests were performed as applicable. Pearson χ2 test and Fischer exact test were used to evaluate if there was a significant association between the groups where the VP disappeared and persisted and fusion and pseudarthrosis. Significance was set at P < .05. Statistical analysis was performed with Stata Version 10.0.
Results
Using the preoperative lumbar spine CT scans of 18 patients (10 men, 8 women), we identified 36 cases of intervertebral levels exhibiting the VP (median positive vacuum sign levels per patient, 2; minimum, 1; maximum, 5) at the levels included in the fusion (Table 1). Mean (SD) age at surgery was 67.6 (9.4) years (range, 46.5-79.6 years). Mean (SD) radiologic follow-up was 1.6 (0.86) years (range, 0.75-3.38 years). All patients underwent lumbar fusion with local autograft, allograft, and recombinant human bone morphogenetic protein 2. Spinal instrumentation was used in 16 of the 18 patients.
On preoperative CT, positive VP was diagnosed in the 36 cases as follows: L5–S1 (11 cases), L4–L5 (9 cases), L3–L4 (4 cases), L2–L3 (6 cases), L1–L2 (4 cases), and T12–L1 (2 cases). On follow-up CT, 15 cases showed persistence of the VP, and 21 cases showed disappearance of the VP (Table 1).
Evidence of spinal fusion was identified on follow-up CT in 32 (88.9%) of the 36 cases. In 3 of the 18 patients, nonunion was diagnosed. Of the 15 intervertebral cases in which the VP persisted, 13 (86.7%) showed evidence of fusion on CT, and 2 (13.3%) showed evidence of pseudarthrosis. Of the 21 intervertebral cases in which the VP disappeared, 19 (90.5%) showed evidence of fusion on CT, and 2 (9.5%) showed evidence of pseudarthrosis (Table 2). There was no significant difference in fusion rate or pseudarthrosis rate in the groups in which the VP persisted or disappeared (Fischer exact test, P = .99). There was no significant association between VP persistence or disappearance and sex, primary or revision surgery, or intervertebral level (Fischer exact test, P > .05). A case example is shown in the Figure.
At levels not included in spinal fusion, CT identified the VP at 6 lumbar intervertebral levels before surgery and 11 levels at follow-up. The VP did not disappear at any level not included in the fusion. At follow-up, no new VP was identified in a segment included in fusion. Results are summarized in Table 3.
Discussion
The association of radiologic intervertebral VP and disc degeneration, first recognized by Knutsson1 in 1942, refers to the presence of gas, mainly containing nitrogen, in the crevices between or within vertebrae.2 The VP is more often seen in patients older than 50 years, on plain radiographs in hyperextension.9 CT is more sensitive than radiography in detecting the VP; Lardé and colleagues3 found it in about 50% of 50 patients on CT scans but in only 12% of patients on radiographs. The VP is visible because of the nitrogen gas that accumulates when there is a negative pressure within the disc space. Nitrogen emerges from the blood and moves into the disc space; perhaps the disc space opens, causing the negative pressure.1-3 On T1- or T2-weighted magnetic resonance imaging (MRI), the VP is visible as a signal void. MRI, however, is less accurate than CT.10 In a study of 10 patients who had low back pain and more than 1 level of intradiscal VP, and who underwent supine MRI examinations at 0, 1, and 2 hours, Wang and colleagues11 found that, after prolonged supine positioning, the signal intensity of the vacuum was replaced by hyperintense fluid contents. D’Anastasi and colleagues,12 in a study of 20 patients who had lumbar vacuum phenomenon on CT and underwent MRI examinations, found a significant correlation between presence of intradiscal fluid and amount of bone marrow edema on MRI and degenerative endplate abnormalities on CT. In the present study, we found that, after the spinal fusion vacuum phenomenon disappeared in 58.3% of the lumbar levels and persisted in 41.7% on follow-up CT at the levels included in posterolateral fusion, there were 5 new levels, adjacent to the lumbar fusion, where the VP was seen on the follow-up CT.
We studied whether evidence of a persistent vacuum sign on CT is indicative of pseudarthrosis. Other authors have reported an association between the VP and nonunion in fractures4 and ankylosing spondylitis.5,6 In a study of 19 patients with diaphyseal fractures, Stallenberg and colleagues4 found that, in 7 of the 10 patients with nonunion, the VP was detected on CT at the nonunion site. Martel5 first reported on the intervertebral VP in a case of ankylosing spondylitis with spinal pseudarthrosis. Ten years later, in a study of 18 patients with advanced ankylosing spondylitis with spinal pseudarthrosis, Chan and colleagues6 identified the intervertebral VP on CT in 7 patients. Edwards and colleagues7 studied 15 patients with prior lumbar fusion with 17 positive intervertebral VP levels on CT and found that the vacuum disc sign was a strong predictor of lumbar nonunion as determined by surgical exploration. Mirovsky and colleagues13 identified the intravertebral vacuum cleft in 26 patients with an osteoporotic vertebral fracture treated with vertebroplasty and concluded that nonunion of the vertebral fracture could be identified by presence of the intravertebral vacuum cleft on radiography. In the present study, there was radiologic evidence of lumbar spinal fusion in 89% of disc levels with a preoperative positive intervertebral VP and pseudarthrosis in 11% of disc levels. The rate of fusion at levels with the VP was comparable to the rate at intervertebral levels without the phenomenon. These findings indicate that persistence of the VP after spinal fusion is not an indication that fusion has not been achieved. Preoperative VP also did not predispose to failure of fusion. That there is a persistent vacuum disc might imply that, even after successful fusion as seen on CT, some motion may be occurring at the disc level to cause a negative pressure phenomenon. Even in cases of facet fusion with bridging bone, there may still be motion at the disc level, as fusions can plastically deform (even with screws in), particularly in elderly osteopenic bone. We found no association between a persistent vacuum sign and pseudarthrosis. Our study findings are clinically useful even if the benefits are limited. These findings may help surgeons avoid misinterpreting this sign as an indication for additional surgery.
This study had some limitations. First, radiographs were used to determine presence or absence of fusion. Although CT is widely considered the gold standard for noninvasive assessment of fusion,14 even when both posterolateral gutters and facets have been found to be fused on CT, the probability of a solid fusion on exploration ranges from 69% to 96%.8,15 Second, detection of the VP on radiographs and CT may be affected by patient position.11 Third, this was a retrospective series with a small number of patients and limited follow-up with CT. Arthrodesis and the VP may take years to fully evolve. It is possible that fusion rates could be higher on longer follow-up, and resolution of the VP may occur with longer follow-up. Fourth, clinical outcomes were not evaluated, as there are other confounding factors, apart from successful fusion, that could affect clinical outcomes. A larger prospective controlled study would be helpful.
Conclusion
The radiologic intervertebral VP may persist after posterolateral lumbar spinal fusion. We did not find an association between the VP and pseudarthrosis. In addition, VP persistence on follow-up CT was not indicative of pseudarthrosis, and VP disappearance was not indicative of fusion. The vacuum sign should not be misinterpreted as an indication for additional surgery.
1. Knutsson F. The vacuum phenomenon in the intervertebral discs. Acta Radiol. 1942;23:173-179.
2. Resnick D, Niwayama G, Guerra J Jr, Vint V, Usselman J. Spinal vacuum phenomenon: anatomical study and review. Radiology. 1981;139(2):341-348.
3. Lardé D, Mathieu D, Frija J, Gaston A, Vasile N. Spinal vacuum phenomenon: CT diagnosis and significance. J Comput Assist Tomogr. 1982;6(4):671-676.
4. Stallenberg B, Madani A, Burny F, Gevenois PA. The vacuum phenomenon: a CT sign of nonunited fracture. AJR Am J Roentgenol. 2001;176(5):1161-1164.
5. Martel W. Spinal pseudarthrosis: a complication of ankylosing spondylitis. Arthritis Rheum. 1978;21(4):485-490.
6. Chan FL, Ho EK, Chau EM. Spinal pseudarthrosis complicating ankylosing spondylitis: comparison of CT and conventional tomography. AJR Am J Roentgenol. 1988;150(3):611-614.
7. Edwards CE, Antonoiades SB, Ford L, Crabster E. CT vacuum disc sign: a highly specific predictor of lumbar nonunion. Poster presented at: 41st Annual Meeting of the Scoliosis Research Society; September 2006; Monterey, CA.
8. Carreon LY, Djurasovic M, Glassman SD, Sailer P. Diagnostic accuracy and reliability of fine-cut CT scans with reconstructions to determine the status of an instrumented posterolateral fusion with surgical exploration as reference standard. Spine. 2007;32(8):892-895.
9. Goobar JE, Pate D, Resnick D, Sartoris DJ. Radiography of the hyperextended lumbar spine: an effective technique for the demonstration of discal vacuum phenomena. Can Assoc Radiol J. 1987;38(4):271-274.
10. Grenier N, Grossman RI, Schiebler ML, Yeager BA, Goldberg HI, Kressel HY. Degenerative lumbar disk disease: pitfalls and usefulness of MR imaging in detection of vacuum phenomenon. Radiology. 1987;164(3):861-865.
11. Wang HJ, Chen BB, Yu CW, Hsu CY, Shih TT. Alteration of disc vacuum contents during prolonged supine positioning: evaluation with MR Image. Spine. 2007;32(23):2610-2615.
12. D’Anastasi M, Birkenmaier C, Schmidt GP, Wegener B, Reiser MF, Baur-Melnyk A. Correlation between vacuum phenomenon on CT and fluid on MRI in degenerative disks. AJR Am J Roentgenol. 2011;197(5):1182-1189.
13. Mirovsky Y, Anekstein Y, Shalmon E, Peer A. Vacuum clefts of the vertebral bodies. AJNR Am J Neuroradiol. 2005;26(7):1634-1640.
14. Selby MD, Clark SR, Hall DJ, Freeman BJ. Radiologic assessment of spinal fusion. J Am Acad Orthop Surg. 2012;20(11):694-703.
15. Kanayama M, Hashimoto T, Shigenobu K, Yamane S, Bauer TW, Togawa D. A prospective randomized study of posterolateral lumbar fusion using osteogenic protein-1 (OP-1) versus local autograft with ceramic bone substitute: emphasis of surgical exploration and histologic assessment. Spine. 2006;31(10):1067-1074.
The spinal vacuum sign or vacuum phenomenon (VP) is the radiographic finding of an air-density linear radiolucency in the intervertebral disc or vertebral body. The result of a gaseous accumulation, it is often a diagnostic sign of disc degeneration as well as a rare sign of infection, Schmorl node formation, or osteonecrosis.1,2 Although the VP was first described on plain radiographs, it is better seen on computed tomography (CT).3 Multiple studies have found a possible association between the VP and nonunion in diaphyseal fractures,4 ankylosing spondylitis,5,6 and lumbar spinal fusion.7
To our knowledge, no one has studied whether the intervertebral VP resolves after posterolateral lumbar spinal fusion in adults with degenerative spinal pathology, and no one has investigated the association between the persistence of the intervertebral VP and pseudarthrosis after posterolateral spinal fusion.
We conducted a study to determine whether the VP resolves after posterolateral lumbar spinal fusion procedures and whether persistence of the VP after fusion surgery is indicative of pseudarthrosis.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the medical records of patients who had degenerative spinal stenosis with instability and the intervertebral vacuum sign on preoperative digital lumbar spine CT scans and who underwent posterolateral lumbar spinal fusion with or without instrumentation. Study inclusion criteria were lumbar spine CT at minimum 6-month follow-up after spinal fusion and preoperative and postoperative lumbar spine radiographs. Exclusion criteria were any type of interbody fusion procedure (anterior, posterior, transforaminal, lateral) at a level with the VP, age under 21 years, follow-up of less than 6 months, and incomplete radiographic records. As this was a retrospective study, patient consent was not required.
CT was performed with a 16-, 64-, or 128-slice multidetector CT scanner with effective tube current set at 250 to 320 mA, voltage set at 120 to 140 kV, and pitch set at 0.75 to 0.9. After axial acquisition of 3×3-mm isometric voxels, sagittal and coronal multiplanar images were reconstructed with a slice thickness of 2 mm. Patient demographics, diagnoses, and surgical details were recorded. All digital lumbar spine CT scans and radiographs were initially screened on PACS (picture archiving and communication system) by the orthopedic spine surgery fellow at an academic medical institution; then they were reviewed on a radiology reading room monitor by 3 observers (senior radiologist, senior orthopedic spine surgeon, orthopedic spine surgery fellow). Axial images and sagittal and coronal reconstructed images of the preoperative and postoperative follow-up lumbar CT scans—together with the lateral and anteroposterior lumbar spine radiographs—were evaluated for the intervertebral VP. Mean (SD) follow-up (with CT to assess fusion) was 1.6 (0.86) years (range, 0.75-3.38 years). Fusion at each level was evaluated on the postoperative follow-up CT on axial images and sagittal and coronal reconstructed images; criteria for fusion were continuous bridging bone across posterolateral gutters and facets on one or both sides at each intervertebral level.8 Pseudarthrosis was recorded if there was no continuity of bridging bone across both posterolateral gutters and facets, a complete radiolucent line on both sides across a level, or lysis or loosening around screws. All recordings were made by consensus, or by majority decision in case of disagreement.
Presence of the VP at the lumbar levels not included in the fusion was also recorded on the preoperative and follow-up CT scan and radiographs.
Descriptive and inferential statistical tests were performed as applicable. Pearson χ2 test and Fischer exact test were used to evaluate if there was a significant association between the groups where the VP disappeared and persisted and fusion and pseudarthrosis. Significance was set at P < .05. Statistical analysis was performed with Stata Version 10.0.
Results
Using the preoperative lumbar spine CT scans of 18 patients (10 men, 8 women), we identified 36 cases of intervertebral levels exhibiting the VP (median positive vacuum sign levels per patient, 2; minimum, 1; maximum, 5) at the levels included in the fusion (Table 1). Mean (SD) age at surgery was 67.6 (9.4) years (range, 46.5-79.6 years). Mean (SD) radiologic follow-up was 1.6 (0.86) years (range, 0.75-3.38 years). All patients underwent lumbar fusion with local autograft, allograft, and recombinant human bone morphogenetic protein 2. Spinal instrumentation was used in 16 of the 18 patients.
On preoperative CT, positive VP was diagnosed in the 36 cases as follows: L5–S1 (11 cases), L4–L5 (9 cases), L3–L4 (4 cases), L2–L3 (6 cases), L1–L2 (4 cases), and T12–L1 (2 cases). On follow-up CT, 15 cases showed persistence of the VP, and 21 cases showed disappearance of the VP (Table 1).
Evidence of spinal fusion was identified on follow-up CT in 32 (88.9%) of the 36 cases. In 3 of the 18 patients, nonunion was diagnosed. Of the 15 intervertebral cases in which the VP persisted, 13 (86.7%) showed evidence of fusion on CT, and 2 (13.3%) showed evidence of pseudarthrosis. Of the 21 intervertebral cases in which the VP disappeared, 19 (90.5%) showed evidence of fusion on CT, and 2 (9.5%) showed evidence of pseudarthrosis (Table 2). There was no significant difference in fusion rate or pseudarthrosis rate in the groups in which the VP persisted or disappeared (Fischer exact test, P = .99). There was no significant association between VP persistence or disappearance and sex, primary or revision surgery, or intervertebral level (Fischer exact test, P > .05). A case example is shown in the Figure.
At levels not included in spinal fusion, CT identified the VP at 6 lumbar intervertebral levels before surgery and 11 levels at follow-up. The VP did not disappear at any level not included in the fusion. At follow-up, no new VP was identified in a segment included in fusion. Results are summarized in Table 3.
Discussion
The association of radiologic intervertebral VP and disc degeneration, first recognized by Knutsson1 in 1942, refers to the presence of gas, mainly containing nitrogen, in the crevices between or within vertebrae.2 The VP is more often seen in patients older than 50 years, on plain radiographs in hyperextension.9 CT is more sensitive than radiography in detecting the VP; Lardé and colleagues3 found it in about 50% of 50 patients on CT scans but in only 12% of patients on radiographs. The VP is visible because of the nitrogen gas that accumulates when there is a negative pressure within the disc space. Nitrogen emerges from the blood and moves into the disc space; perhaps the disc space opens, causing the negative pressure.1-3 On T1- or T2-weighted magnetic resonance imaging (MRI), the VP is visible as a signal void. MRI, however, is less accurate than CT.10 In a study of 10 patients who had low back pain and more than 1 level of intradiscal VP, and who underwent supine MRI examinations at 0, 1, and 2 hours, Wang and colleagues11 found that, after prolonged supine positioning, the signal intensity of the vacuum was replaced by hyperintense fluid contents. D’Anastasi and colleagues,12 in a study of 20 patients who had lumbar vacuum phenomenon on CT and underwent MRI examinations, found a significant correlation between presence of intradiscal fluid and amount of bone marrow edema on MRI and degenerative endplate abnormalities on CT. In the present study, we found that, after the spinal fusion vacuum phenomenon disappeared in 58.3% of the lumbar levels and persisted in 41.7% on follow-up CT at the levels included in posterolateral fusion, there were 5 new levels, adjacent to the lumbar fusion, where the VP was seen on the follow-up CT.
We studied whether evidence of a persistent vacuum sign on CT is indicative of pseudarthrosis. Other authors have reported an association between the VP and nonunion in fractures4 and ankylosing spondylitis.5,6 In a study of 19 patients with diaphyseal fractures, Stallenberg and colleagues4 found that, in 7 of the 10 patients with nonunion, the VP was detected on CT at the nonunion site. Martel5 first reported on the intervertebral VP in a case of ankylosing spondylitis with spinal pseudarthrosis. Ten years later, in a study of 18 patients with advanced ankylosing spondylitis with spinal pseudarthrosis, Chan and colleagues6 identified the intervertebral VP on CT in 7 patients. Edwards and colleagues7 studied 15 patients with prior lumbar fusion with 17 positive intervertebral VP levels on CT and found that the vacuum disc sign was a strong predictor of lumbar nonunion as determined by surgical exploration. Mirovsky and colleagues13 identified the intravertebral vacuum cleft in 26 patients with an osteoporotic vertebral fracture treated with vertebroplasty and concluded that nonunion of the vertebral fracture could be identified by presence of the intravertebral vacuum cleft on radiography. In the present study, there was radiologic evidence of lumbar spinal fusion in 89% of disc levels with a preoperative positive intervertebral VP and pseudarthrosis in 11% of disc levels. The rate of fusion at levels with the VP was comparable to the rate at intervertebral levels without the phenomenon. These findings indicate that persistence of the VP after spinal fusion is not an indication that fusion has not been achieved. Preoperative VP also did not predispose to failure of fusion. That there is a persistent vacuum disc might imply that, even after successful fusion as seen on CT, some motion may be occurring at the disc level to cause a negative pressure phenomenon. Even in cases of facet fusion with bridging bone, there may still be motion at the disc level, as fusions can plastically deform (even with screws in), particularly in elderly osteopenic bone. We found no association between a persistent vacuum sign and pseudarthrosis. Our study findings are clinically useful even if the benefits are limited. These findings may help surgeons avoid misinterpreting this sign as an indication for additional surgery.
This study had some limitations. First, radiographs were used to determine presence or absence of fusion. Although CT is widely considered the gold standard for noninvasive assessment of fusion,14 even when both posterolateral gutters and facets have been found to be fused on CT, the probability of a solid fusion on exploration ranges from 69% to 96%.8,15 Second, detection of the VP on radiographs and CT may be affected by patient position.11 Third, this was a retrospective series with a small number of patients and limited follow-up with CT. Arthrodesis and the VP may take years to fully evolve. It is possible that fusion rates could be higher on longer follow-up, and resolution of the VP may occur with longer follow-up. Fourth, clinical outcomes were not evaluated, as there are other confounding factors, apart from successful fusion, that could affect clinical outcomes. A larger prospective controlled study would be helpful.
Conclusion
The radiologic intervertebral VP may persist after posterolateral lumbar spinal fusion. We did not find an association between the VP and pseudarthrosis. In addition, VP persistence on follow-up CT was not indicative of pseudarthrosis, and VP disappearance was not indicative of fusion. The vacuum sign should not be misinterpreted as an indication for additional surgery.
The spinal vacuum sign or vacuum phenomenon (VP) is the radiographic finding of an air-density linear radiolucency in the intervertebral disc or vertebral body. The result of a gaseous accumulation, it is often a diagnostic sign of disc degeneration as well as a rare sign of infection, Schmorl node formation, or osteonecrosis.1,2 Although the VP was first described on plain radiographs, it is better seen on computed tomography (CT).3 Multiple studies have found a possible association between the VP and nonunion in diaphyseal fractures,4 ankylosing spondylitis,5,6 and lumbar spinal fusion.7
To our knowledge, no one has studied whether the intervertebral VP resolves after posterolateral lumbar spinal fusion in adults with degenerative spinal pathology, and no one has investigated the association between the persistence of the intervertebral VP and pseudarthrosis after posterolateral spinal fusion.
We conducted a study to determine whether the VP resolves after posterolateral lumbar spinal fusion procedures and whether persistence of the VP after fusion surgery is indicative of pseudarthrosis.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the medical records of patients who had degenerative spinal stenosis with instability and the intervertebral vacuum sign on preoperative digital lumbar spine CT scans and who underwent posterolateral lumbar spinal fusion with or without instrumentation. Study inclusion criteria were lumbar spine CT at minimum 6-month follow-up after spinal fusion and preoperative and postoperative lumbar spine radiographs. Exclusion criteria were any type of interbody fusion procedure (anterior, posterior, transforaminal, lateral) at a level with the VP, age under 21 years, follow-up of less than 6 months, and incomplete radiographic records. As this was a retrospective study, patient consent was not required.
CT was performed with a 16-, 64-, or 128-slice multidetector CT scanner with effective tube current set at 250 to 320 mA, voltage set at 120 to 140 kV, and pitch set at 0.75 to 0.9. After axial acquisition of 3×3-mm isometric voxels, sagittal and coronal multiplanar images were reconstructed with a slice thickness of 2 mm. Patient demographics, diagnoses, and surgical details were recorded. All digital lumbar spine CT scans and radiographs were initially screened on PACS (picture archiving and communication system) by the orthopedic spine surgery fellow at an academic medical institution; then they were reviewed on a radiology reading room monitor by 3 observers (senior radiologist, senior orthopedic spine surgeon, orthopedic spine surgery fellow). Axial images and sagittal and coronal reconstructed images of the preoperative and postoperative follow-up lumbar CT scans—together with the lateral and anteroposterior lumbar spine radiographs—were evaluated for the intervertebral VP. Mean (SD) follow-up (with CT to assess fusion) was 1.6 (0.86) years (range, 0.75-3.38 years). Fusion at each level was evaluated on the postoperative follow-up CT on axial images and sagittal and coronal reconstructed images; criteria for fusion were continuous bridging bone across posterolateral gutters and facets on one or both sides at each intervertebral level.8 Pseudarthrosis was recorded if there was no continuity of bridging bone across both posterolateral gutters and facets, a complete radiolucent line on both sides across a level, or lysis or loosening around screws. All recordings were made by consensus, or by majority decision in case of disagreement.
Presence of the VP at the lumbar levels not included in the fusion was also recorded on the preoperative and follow-up CT scan and radiographs.
Descriptive and inferential statistical tests were performed as applicable. Pearson χ2 test and Fischer exact test were used to evaluate if there was a significant association between the groups where the VP disappeared and persisted and fusion and pseudarthrosis. Significance was set at P < .05. Statistical analysis was performed with Stata Version 10.0.
Results
Using the preoperative lumbar spine CT scans of 18 patients (10 men, 8 women), we identified 36 cases of intervertebral levels exhibiting the VP (median positive vacuum sign levels per patient, 2; minimum, 1; maximum, 5) at the levels included in the fusion (Table 1). Mean (SD) age at surgery was 67.6 (9.4) years (range, 46.5-79.6 years). Mean (SD) radiologic follow-up was 1.6 (0.86) years (range, 0.75-3.38 years). All patients underwent lumbar fusion with local autograft, allograft, and recombinant human bone morphogenetic protein 2. Spinal instrumentation was used in 16 of the 18 patients.
On preoperative CT, positive VP was diagnosed in the 36 cases as follows: L5–S1 (11 cases), L4–L5 (9 cases), L3–L4 (4 cases), L2–L3 (6 cases), L1–L2 (4 cases), and T12–L1 (2 cases). On follow-up CT, 15 cases showed persistence of the VP, and 21 cases showed disappearance of the VP (Table 1).
Evidence of spinal fusion was identified on follow-up CT in 32 (88.9%) of the 36 cases. In 3 of the 18 patients, nonunion was diagnosed. Of the 15 intervertebral cases in which the VP persisted, 13 (86.7%) showed evidence of fusion on CT, and 2 (13.3%) showed evidence of pseudarthrosis. Of the 21 intervertebral cases in which the VP disappeared, 19 (90.5%) showed evidence of fusion on CT, and 2 (9.5%) showed evidence of pseudarthrosis (Table 2). There was no significant difference in fusion rate or pseudarthrosis rate in the groups in which the VP persisted or disappeared (Fischer exact test, P = .99). There was no significant association between VP persistence or disappearance and sex, primary or revision surgery, or intervertebral level (Fischer exact test, P > .05). A case example is shown in the Figure.
At levels not included in spinal fusion, CT identified the VP at 6 lumbar intervertebral levels before surgery and 11 levels at follow-up. The VP did not disappear at any level not included in the fusion. At follow-up, no new VP was identified in a segment included in fusion. Results are summarized in Table 3.
Discussion
The association of radiologic intervertebral VP and disc degeneration, first recognized by Knutsson1 in 1942, refers to the presence of gas, mainly containing nitrogen, in the crevices between or within vertebrae.2 The VP is more often seen in patients older than 50 years, on plain radiographs in hyperextension.9 CT is more sensitive than radiography in detecting the VP; Lardé and colleagues3 found it in about 50% of 50 patients on CT scans but in only 12% of patients on radiographs. The VP is visible because of the nitrogen gas that accumulates when there is a negative pressure within the disc space. Nitrogen emerges from the blood and moves into the disc space; perhaps the disc space opens, causing the negative pressure.1-3 On T1- or T2-weighted magnetic resonance imaging (MRI), the VP is visible as a signal void. MRI, however, is less accurate than CT.10 In a study of 10 patients who had low back pain and more than 1 level of intradiscal VP, and who underwent supine MRI examinations at 0, 1, and 2 hours, Wang and colleagues11 found that, after prolonged supine positioning, the signal intensity of the vacuum was replaced by hyperintense fluid contents. D’Anastasi and colleagues,12 in a study of 20 patients who had lumbar vacuum phenomenon on CT and underwent MRI examinations, found a significant correlation between presence of intradiscal fluid and amount of bone marrow edema on MRI and degenerative endplate abnormalities on CT. In the present study, we found that, after the spinal fusion vacuum phenomenon disappeared in 58.3% of the lumbar levels and persisted in 41.7% on follow-up CT at the levels included in posterolateral fusion, there were 5 new levels, adjacent to the lumbar fusion, where the VP was seen on the follow-up CT.
We studied whether evidence of a persistent vacuum sign on CT is indicative of pseudarthrosis. Other authors have reported an association between the VP and nonunion in fractures4 and ankylosing spondylitis.5,6 In a study of 19 patients with diaphyseal fractures, Stallenberg and colleagues4 found that, in 7 of the 10 patients with nonunion, the VP was detected on CT at the nonunion site. Martel5 first reported on the intervertebral VP in a case of ankylosing spondylitis with spinal pseudarthrosis. Ten years later, in a study of 18 patients with advanced ankylosing spondylitis with spinal pseudarthrosis, Chan and colleagues6 identified the intervertebral VP on CT in 7 patients. Edwards and colleagues7 studied 15 patients with prior lumbar fusion with 17 positive intervertebral VP levels on CT and found that the vacuum disc sign was a strong predictor of lumbar nonunion as determined by surgical exploration. Mirovsky and colleagues13 identified the intravertebral vacuum cleft in 26 patients with an osteoporotic vertebral fracture treated with vertebroplasty and concluded that nonunion of the vertebral fracture could be identified by presence of the intravertebral vacuum cleft on radiography. In the present study, there was radiologic evidence of lumbar spinal fusion in 89% of disc levels with a preoperative positive intervertebral VP and pseudarthrosis in 11% of disc levels. The rate of fusion at levels with the VP was comparable to the rate at intervertebral levels without the phenomenon. These findings indicate that persistence of the VP after spinal fusion is not an indication that fusion has not been achieved. Preoperative VP also did not predispose to failure of fusion. That there is a persistent vacuum disc might imply that, even after successful fusion as seen on CT, some motion may be occurring at the disc level to cause a negative pressure phenomenon. Even in cases of facet fusion with bridging bone, there may still be motion at the disc level, as fusions can plastically deform (even with screws in), particularly in elderly osteopenic bone. We found no association between a persistent vacuum sign and pseudarthrosis. Our study findings are clinically useful even if the benefits are limited. These findings may help surgeons avoid misinterpreting this sign as an indication for additional surgery.
This study had some limitations. First, radiographs were used to determine presence or absence of fusion. Although CT is widely considered the gold standard for noninvasive assessment of fusion,14 even when both posterolateral gutters and facets have been found to be fused on CT, the probability of a solid fusion on exploration ranges from 69% to 96%.8,15 Second, detection of the VP on radiographs and CT may be affected by patient position.11 Third, this was a retrospective series with a small number of patients and limited follow-up with CT. Arthrodesis and the VP may take years to fully evolve. It is possible that fusion rates could be higher on longer follow-up, and resolution of the VP may occur with longer follow-up. Fourth, clinical outcomes were not evaluated, as there are other confounding factors, apart from successful fusion, that could affect clinical outcomes. A larger prospective controlled study would be helpful.
Conclusion
The radiologic intervertebral VP may persist after posterolateral lumbar spinal fusion. We did not find an association between the VP and pseudarthrosis. In addition, VP persistence on follow-up CT was not indicative of pseudarthrosis, and VP disappearance was not indicative of fusion. The vacuum sign should not be misinterpreted as an indication for additional surgery.
1. Knutsson F. The vacuum phenomenon in the intervertebral discs. Acta Radiol. 1942;23:173-179.
2. Resnick D, Niwayama G, Guerra J Jr, Vint V, Usselman J. Spinal vacuum phenomenon: anatomical study and review. Radiology. 1981;139(2):341-348.
3. Lardé D, Mathieu D, Frija J, Gaston A, Vasile N. Spinal vacuum phenomenon: CT diagnosis and significance. J Comput Assist Tomogr. 1982;6(4):671-676.
4. Stallenberg B, Madani A, Burny F, Gevenois PA. The vacuum phenomenon: a CT sign of nonunited fracture. AJR Am J Roentgenol. 2001;176(5):1161-1164.
5. Martel W. Spinal pseudarthrosis: a complication of ankylosing spondylitis. Arthritis Rheum. 1978;21(4):485-490.
6. Chan FL, Ho EK, Chau EM. Spinal pseudarthrosis complicating ankylosing spondylitis: comparison of CT and conventional tomography. AJR Am J Roentgenol. 1988;150(3):611-614.
7. Edwards CE, Antonoiades SB, Ford L, Crabster E. CT vacuum disc sign: a highly specific predictor of lumbar nonunion. Poster presented at: 41st Annual Meeting of the Scoliosis Research Society; September 2006; Monterey, CA.
8. Carreon LY, Djurasovic M, Glassman SD, Sailer P. Diagnostic accuracy and reliability of fine-cut CT scans with reconstructions to determine the status of an instrumented posterolateral fusion with surgical exploration as reference standard. Spine. 2007;32(8):892-895.
9. Goobar JE, Pate D, Resnick D, Sartoris DJ. Radiography of the hyperextended lumbar spine: an effective technique for the demonstration of discal vacuum phenomena. Can Assoc Radiol J. 1987;38(4):271-274.
10. Grenier N, Grossman RI, Schiebler ML, Yeager BA, Goldberg HI, Kressel HY. Degenerative lumbar disk disease: pitfalls and usefulness of MR imaging in detection of vacuum phenomenon. Radiology. 1987;164(3):861-865.
11. Wang HJ, Chen BB, Yu CW, Hsu CY, Shih TT. Alteration of disc vacuum contents during prolonged supine positioning: evaluation with MR Image. Spine. 2007;32(23):2610-2615.
12. D’Anastasi M, Birkenmaier C, Schmidt GP, Wegener B, Reiser MF, Baur-Melnyk A. Correlation between vacuum phenomenon on CT and fluid on MRI in degenerative disks. AJR Am J Roentgenol. 2011;197(5):1182-1189.
13. Mirovsky Y, Anekstein Y, Shalmon E, Peer A. Vacuum clefts of the vertebral bodies. AJNR Am J Neuroradiol. 2005;26(7):1634-1640.
14. Selby MD, Clark SR, Hall DJ, Freeman BJ. Radiologic assessment of spinal fusion. J Am Acad Orthop Surg. 2012;20(11):694-703.
15. Kanayama M, Hashimoto T, Shigenobu K, Yamane S, Bauer TW, Togawa D. A prospective randomized study of posterolateral lumbar fusion using osteogenic protein-1 (OP-1) versus local autograft with ceramic bone substitute: emphasis of surgical exploration and histologic assessment. Spine. 2006;31(10):1067-1074.
1. Knutsson F. The vacuum phenomenon in the intervertebral discs. Acta Radiol. 1942;23:173-179.
2. Resnick D, Niwayama G, Guerra J Jr, Vint V, Usselman J. Spinal vacuum phenomenon: anatomical study and review. Radiology. 1981;139(2):341-348.
3. Lardé D, Mathieu D, Frija J, Gaston A, Vasile N. Spinal vacuum phenomenon: CT diagnosis and significance. J Comput Assist Tomogr. 1982;6(4):671-676.
4. Stallenberg B, Madani A, Burny F, Gevenois PA. The vacuum phenomenon: a CT sign of nonunited fracture. AJR Am J Roentgenol. 2001;176(5):1161-1164.
5. Martel W. Spinal pseudarthrosis: a complication of ankylosing spondylitis. Arthritis Rheum. 1978;21(4):485-490.
6. Chan FL, Ho EK, Chau EM. Spinal pseudarthrosis complicating ankylosing spondylitis: comparison of CT and conventional tomography. AJR Am J Roentgenol. 1988;150(3):611-614.
7. Edwards CE, Antonoiades SB, Ford L, Crabster E. CT vacuum disc sign: a highly specific predictor of lumbar nonunion. Poster presented at: 41st Annual Meeting of the Scoliosis Research Society; September 2006; Monterey, CA.
8. Carreon LY, Djurasovic M, Glassman SD, Sailer P. Diagnostic accuracy and reliability of fine-cut CT scans with reconstructions to determine the status of an instrumented posterolateral fusion with surgical exploration as reference standard. Spine. 2007;32(8):892-895.
9. Goobar JE, Pate D, Resnick D, Sartoris DJ. Radiography of the hyperextended lumbar spine: an effective technique for the demonstration of discal vacuum phenomena. Can Assoc Radiol J. 1987;38(4):271-274.
10. Grenier N, Grossman RI, Schiebler ML, Yeager BA, Goldberg HI, Kressel HY. Degenerative lumbar disk disease: pitfalls and usefulness of MR imaging in detection of vacuum phenomenon. Radiology. 1987;164(3):861-865.
11. Wang HJ, Chen BB, Yu CW, Hsu CY, Shih TT. Alteration of disc vacuum contents during prolonged supine positioning: evaluation with MR Image. Spine. 2007;32(23):2610-2615.
12. D’Anastasi M, Birkenmaier C, Schmidt GP, Wegener B, Reiser MF, Baur-Melnyk A. Correlation between vacuum phenomenon on CT and fluid on MRI in degenerative disks. AJR Am J Roentgenol. 2011;197(5):1182-1189.
13. Mirovsky Y, Anekstein Y, Shalmon E, Peer A. Vacuum clefts of the vertebral bodies. AJNR Am J Neuroradiol. 2005;26(7):1634-1640.
14. Selby MD, Clark SR, Hall DJ, Freeman BJ. Radiologic assessment of spinal fusion. J Am Acad Orthop Surg. 2012;20(11):694-703.
15. Kanayama M, Hashimoto T, Shigenobu K, Yamane S, Bauer TW, Togawa D. A prospective randomized study of posterolateral lumbar fusion using osteogenic protein-1 (OP-1) versus local autograft with ceramic bone substitute: emphasis of surgical exploration and histologic assessment. Spine. 2006;31(10):1067-1074.
Don’t Forget the Pulses! Aortoiliac Peripheral Artery Disease Masquerading as Lumbar Radiculopathy—A Report of 3 Cases
Lumbar radiculopathy is a common problem encountered by orthopedic surgeons, and typically presents with lower back or buttock pain radiating down the leg.1 While the most common causes of lumbar radiculopathy are lumbar disc herniation and spinal stenosis, the differential diagnosis for lower extremity pain is broad and can be musculoskeletal, vascular, neurologic, or inflammatory in nature.1,2 Differentiating between orthopedic, neurologic, and vascular causes of leg pain, such as peripheral artery disease (PAD), can sometimes be challenging. This is especially true in aortoiliac PAD, which can present with hip, buttock, and thigh pain. Dorsalis pedis pulses can be palpable due to collateral circulation. A careful history and physical examination is crucial to the correct diagnosis. The history should clearly document the nature of the pain, details of walking impairment, and the alleviating effects of standing still or positional changes. A complete neurovascular examination should include observations regarding the skin, hair, and nails, examination of dorsal pedis, popliteal, and femoral pulses in comparison to the contralateral side, and documentation of dural tension signs. Misdiagnoses can send the patient down a path of unnecessary tests, unindicated procedures, and ultimately, a delay in definitive diagnosis and treatment.1
To our knowledge, this is the first report on a series of patients with thigh pain initially diagnosed as radiculopathy who underwent unproductive diagnostic tests and procedures, and ultimately were given delayed diagnoses of aortoiliac PAD. The patients provided written informed consent for print and electronic publication of these case reports.
Case 1
An 81-year-old woman with a medical history notable for hypertension, hyperlipidemia, and stroke initially presented to an outside orthopedic institution with complaints of several months of lower back and right hip, thigh, and leg pain when walking. She did not report any history of night pain, weakness, or numbness. Examination at the time was notable for painful back extension, 4/5 hip flexion strength on the right compared to 5/5 on the left, but symmetric reflexes and negative dural tension signs. X-rays showed multilevel degenerative disc disease of the lumbar spine, and magnetic resonance imaging (MRI) showed a small L3/4 disc protrusion causing impingement of the L4 nerve root.
A transforaminal epidural steroid injection at the L4 level was performed with minimal resolution of symptoms. Several months later, right-sided intra-articular facet injections were performed at the L4/5 and L5/S1 levels, again with minimal relief of symptoms. At this point, the patient was sent for further physical therapy.
Over a year after symptom onset, the patient presented to our institution and was evaluated by a vascular surgeon. Physical examination was notable for 1+ femoral artery and dorsal pedis pulses on the right side, compared to 2+ on the left. An aortoiliac duplex ultrasound showed severe significant stenosis of the right common iliac artery (>75%).
The patient underwent a right common iliac artery angioplasty and stenting (Figures 1A, 1B), which resolved her symptoms.
Case 2
A 65-year-old man, who is a former smoker with a medical history notable for hyperlipidemia and coronary artery disease status post myocardial infarction, presented with a long history of right leg pain. He underwent a L5/S1 anterior posterior fusion at an outside institution and did well for about 5 years after the procedure (Figures 2A, 2B). The pain returned and he underwent several years of physical therapy, epidural steroid injections, and implantation of a spinal cord stimulator with no improvement. He reported right leg pain with minimal back pain, primarily in the thigh and not radiating to the feet and toes. The pain limited him from walking more than 1 block. On examination, strength was 5/5 bilaterally. Pulse examination was notable for lack of dorsalis pedis/posterior tibial pulses bilaterally. He had no bowel or bladder dysfunction.
Computed tomography myelogram showed a moderate amount of stenosis at L3/4 and L4/5. He was sent for evaluation by a vascular surgeon. Arterial duplex ultrasound showed significant stenosis of the right common iliac artery.
Angioplasty was attempted but vascular surgery was unable to cross the lesion (Figures 3A, 3B), and the patient ultimately had a femoral-femoral bypass, which resolved his leg pain.
Case 3
A 78-year-old woman, nonsmoker, presented with a 1-year history of left buttock and thigh pain exacerbated by ambulation. Ambulation was limited to 2 blocks. The patient was being worked up for spinal and hip etiologies of pain at an outside hospital. MRI revealed a mild posterior disc herniation at L3/4 and L4/5 and moderate narrowing of the spinal canal. She underwent 2 epidural steroid injections with no improvement. The patient’s relative, a physician, suggested that the patient receive a vascular surgery consultation, and the patient ultimately presented to our institution for evaluation by vascular surgery.
The physical examination was significant for a 1+ dorsal pedis pulse on the left compared to 2+ on the right. Moreover, the patient only demonstrated trace L femoral pulse compared to the right. Strength was 5/5 bilaterally.
The patient was taken to the operating room for angioplasty and stenting of the left common iliac artery (Figures 4A, 4B). This provided immediate symptom relief, and she has remained asymptomatic.
Discussion
Lumbar radiculopathy is a common diagnosis encountered by orthopedic surgeons. Although the diagnosis can appear to be straightforward in a patient presenting with lower back and leg pain, the etiology of lower back and leg pain can be extremely varied, and can be musculoskeletal, neurologic, vascular, rheumatologic, or oncologic in origin.1 In particular, differentiating between radiculopathy and vascular claudication can sometimes be challenging.
The 2 most common causes of lumbar radiculopathy are lumbar disc herniation and spinal stenosis.1 Lumbar disc herniation results from tear in the annulus of the intervertebral disc, resulting in herniation of disc material into the spinal canal causing compression and irritation of spinal nerve roots.1 Spinal stenosis is narrowing of the spinal canal that produces compression of neural elements before they exit the neural foramen.3 Adult degenerative spinal stenosis is most often caused by osteophytes from the facet joints or hypertrophy of the ligamentum flavum, and can be broadly categorized into central spinal stenosis or lateral spinal stenosis.
PAD is defined as progressive stenosis or occlusion, or aneurysmal dilation of noncoronary arteries.2 When PAD affects the vessels of the lower extremities, the symptoms typically manifest as intermittent claudication, which is exercise-induced ischemic pain in the lower extremity that is relieved by rest.2 As the disease progresses, symptoms can progress to rest pain, ulceration, and, eventually, gangrene. The most common cause of PAD is atherosclerosis, and the risk factors include smoking, hypertension, diabetes, and hyperlipidemia. The prevalence of PAD rises sharply with age, starting from <3% in ages less than 60 years to >20% in ages 75 years and older.4
A detailed and pertinent history from the patient provides important information for differentiating radiculopathy and neurogenic claudication from vascular claudication. Patients with lumbar radiculopathy typically report pain in the lower back radiating down the leg past the knee in a dermatomal distribution. The pain often begins soon if not immediately after activity, but often takes time for relief onset after rest. Positional changes in the back such as flexion can provide relief.2 Patients with neurogenic claudication from central spinal stenosis can present with bilateral thigh pain from prolonged standing and activity that is alleviated with flexion or stooping.3 Patients may admit to a positive “shopping cart sign,” with increased walking comfort stooped forward with hands on a shopping cart.
In contrast, patients with vascular claudication often report pain in the calf, thigh, or hip, but rarely in the foot. The location of pain varies with area of stenosis; generally, patients with superficial femoral artery occlusion present with calf claudication, while patients with aortoiliac disease present with buttock and thigh pain. The pain typically occurs after a very reproducible length of walking, and is relieved by cessation of walking, often even if the patient remains standing. Back positioning should have no effect on the pain.2-5
Physical examination should begin with observation of the patient’s gait and posture, which may be hunched over in the setting of spinal stenosis. Examination of the patient’s skin may show loss of hair, shiny skin, or atrophic changes suggestive of vascular disease (Figure 5).1 Prior to proceeding to a spine examination, palpating the trochanteric bursa and testing for hip range of motion is important to rule out intra-articular hip pathology and trochanteric bursitis as common causes of pain in the area. Patients with radiculopathy may show sensory disturbances in a dermatomal distribution, muscular weakness at the corresponding spinal level, and decreased deep tendon reflexes. The straight leg raise test can elicit signs of nerve root tension. A careful examination of bilateral lower extremity pulses at the dorsal pedis, popliteal, and femoral levels can help identify any asymmetric or decreased pulses that would indicate peripheral vascular disease. With chronic aortoiliac disease, it is important to check for femoral pulses, given the dorsal pedis pulse can be present due to collateral circulation. And finally, the ankle brachial index (ABI), measured as the ratio of the systolic pressure at the ankle divided by the systolic pressure at the arm, is a good screening test for PAD.6 A normal ABI is >1.
A thorough history and physical examination can elicit important information that is helpful in evaluating orthopedic patients, especially to differentiate between spinal and vascular causes of leg pain. This can help avoid misdiagnoses, which result in unnecessary tests, procedures, and wasted time. Don’t forget the pulses!
1. Grimm BD, Blessinger BJ, Darden BV, Brigham CD, Kneisl JS, Laxer EB. Mimickers of lumbar radiculopathy. J Am Acad Orthop Surg. 2015;23(1):7-17.
2. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)--summary of recommendations. J Vasc Interv Radiol. 2006;17(9):1383-1397.
3. Spivak JM. Degenerative lumbar spinal stenosis. J Bone Joint Surg Am. 1998;80(7):1053-1066.
4. Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71(3):510-515.
5. Ouriel K. Peripheral arterial disease. Lancet. 2001;358(9289):1257-1264.
6. Jeon CH, Han SH, Chung NS, Hyun HS. The validity of ankle-brachial index for the differential diagnosis of peripheral arterial disease and lumbar spinal stenosis in patients with atypical claudication. Eur Spine J. 2012;21(6):1165-1170.
Lumbar radiculopathy is a common problem encountered by orthopedic surgeons, and typically presents with lower back or buttock pain radiating down the leg.1 While the most common causes of lumbar radiculopathy are lumbar disc herniation and spinal stenosis, the differential diagnosis for lower extremity pain is broad and can be musculoskeletal, vascular, neurologic, or inflammatory in nature.1,2 Differentiating between orthopedic, neurologic, and vascular causes of leg pain, such as peripheral artery disease (PAD), can sometimes be challenging. This is especially true in aortoiliac PAD, which can present with hip, buttock, and thigh pain. Dorsalis pedis pulses can be palpable due to collateral circulation. A careful history and physical examination is crucial to the correct diagnosis. The history should clearly document the nature of the pain, details of walking impairment, and the alleviating effects of standing still or positional changes. A complete neurovascular examination should include observations regarding the skin, hair, and nails, examination of dorsal pedis, popliteal, and femoral pulses in comparison to the contralateral side, and documentation of dural tension signs. Misdiagnoses can send the patient down a path of unnecessary tests, unindicated procedures, and ultimately, a delay in definitive diagnosis and treatment.1
To our knowledge, this is the first report on a series of patients with thigh pain initially diagnosed as radiculopathy who underwent unproductive diagnostic tests and procedures, and ultimately were given delayed diagnoses of aortoiliac PAD. The patients provided written informed consent for print and electronic publication of these case reports.
Case 1
An 81-year-old woman with a medical history notable for hypertension, hyperlipidemia, and stroke initially presented to an outside orthopedic institution with complaints of several months of lower back and right hip, thigh, and leg pain when walking. She did not report any history of night pain, weakness, or numbness. Examination at the time was notable for painful back extension, 4/5 hip flexion strength on the right compared to 5/5 on the left, but symmetric reflexes and negative dural tension signs. X-rays showed multilevel degenerative disc disease of the lumbar spine, and magnetic resonance imaging (MRI) showed a small L3/4 disc protrusion causing impingement of the L4 nerve root.
A transforaminal epidural steroid injection at the L4 level was performed with minimal resolution of symptoms. Several months later, right-sided intra-articular facet injections were performed at the L4/5 and L5/S1 levels, again with minimal relief of symptoms. At this point, the patient was sent for further physical therapy.
Over a year after symptom onset, the patient presented to our institution and was evaluated by a vascular surgeon. Physical examination was notable for 1+ femoral artery and dorsal pedis pulses on the right side, compared to 2+ on the left. An aortoiliac duplex ultrasound showed severe significant stenosis of the right common iliac artery (>75%).
The patient underwent a right common iliac artery angioplasty and stenting (Figures 1A, 1B), which resolved her symptoms.
Case 2
A 65-year-old man, who is a former smoker with a medical history notable for hyperlipidemia and coronary artery disease status post myocardial infarction, presented with a long history of right leg pain. He underwent a L5/S1 anterior posterior fusion at an outside institution and did well for about 5 years after the procedure (Figures 2A, 2B). The pain returned and he underwent several years of physical therapy, epidural steroid injections, and implantation of a spinal cord stimulator with no improvement. He reported right leg pain with minimal back pain, primarily in the thigh and not radiating to the feet and toes. The pain limited him from walking more than 1 block. On examination, strength was 5/5 bilaterally. Pulse examination was notable for lack of dorsalis pedis/posterior tibial pulses bilaterally. He had no bowel or bladder dysfunction.
Computed tomography myelogram showed a moderate amount of stenosis at L3/4 and L4/5. He was sent for evaluation by a vascular surgeon. Arterial duplex ultrasound showed significant stenosis of the right common iliac artery.
Angioplasty was attempted but vascular surgery was unable to cross the lesion (Figures 3A, 3B), and the patient ultimately had a femoral-femoral bypass, which resolved his leg pain.
Case 3
A 78-year-old woman, nonsmoker, presented with a 1-year history of left buttock and thigh pain exacerbated by ambulation. Ambulation was limited to 2 blocks. The patient was being worked up for spinal and hip etiologies of pain at an outside hospital. MRI revealed a mild posterior disc herniation at L3/4 and L4/5 and moderate narrowing of the spinal canal. She underwent 2 epidural steroid injections with no improvement. The patient’s relative, a physician, suggested that the patient receive a vascular surgery consultation, and the patient ultimately presented to our institution for evaluation by vascular surgery.
The physical examination was significant for a 1+ dorsal pedis pulse on the left compared to 2+ on the right. Moreover, the patient only demonstrated trace L femoral pulse compared to the right. Strength was 5/5 bilaterally.
The patient was taken to the operating room for angioplasty and stenting of the left common iliac artery (Figures 4A, 4B). This provided immediate symptom relief, and she has remained asymptomatic.
Discussion
Lumbar radiculopathy is a common diagnosis encountered by orthopedic surgeons. Although the diagnosis can appear to be straightforward in a patient presenting with lower back and leg pain, the etiology of lower back and leg pain can be extremely varied, and can be musculoskeletal, neurologic, vascular, rheumatologic, or oncologic in origin.1 In particular, differentiating between radiculopathy and vascular claudication can sometimes be challenging.
The 2 most common causes of lumbar radiculopathy are lumbar disc herniation and spinal stenosis.1 Lumbar disc herniation results from tear in the annulus of the intervertebral disc, resulting in herniation of disc material into the spinal canal causing compression and irritation of spinal nerve roots.1 Spinal stenosis is narrowing of the spinal canal that produces compression of neural elements before they exit the neural foramen.3 Adult degenerative spinal stenosis is most often caused by osteophytes from the facet joints or hypertrophy of the ligamentum flavum, and can be broadly categorized into central spinal stenosis or lateral spinal stenosis.
PAD is defined as progressive stenosis or occlusion, or aneurysmal dilation of noncoronary arteries.2 When PAD affects the vessels of the lower extremities, the symptoms typically manifest as intermittent claudication, which is exercise-induced ischemic pain in the lower extremity that is relieved by rest.2 As the disease progresses, symptoms can progress to rest pain, ulceration, and, eventually, gangrene. The most common cause of PAD is atherosclerosis, and the risk factors include smoking, hypertension, diabetes, and hyperlipidemia. The prevalence of PAD rises sharply with age, starting from <3% in ages less than 60 years to >20% in ages 75 years and older.4
A detailed and pertinent history from the patient provides important information for differentiating radiculopathy and neurogenic claudication from vascular claudication. Patients with lumbar radiculopathy typically report pain in the lower back radiating down the leg past the knee in a dermatomal distribution. The pain often begins soon if not immediately after activity, but often takes time for relief onset after rest. Positional changes in the back such as flexion can provide relief.2 Patients with neurogenic claudication from central spinal stenosis can present with bilateral thigh pain from prolonged standing and activity that is alleviated with flexion or stooping.3 Patients may admit to a positive “shopping cart sign,” with increased walking comfort stooped forward with hands on a shopping cart.
In contrast, patients with vascular claudication often report pain in the calf, thigh, or hip, but rarely in the foot. The location of pain varies with area of stenosis; generally, patients with superficial femoral artery occlusion present with calf claudication, while patients with aortoiliac disease present with buttock and thigh pain. The pain typically occurs after a very reproducible length of walking, and is relieved by cessation of walking, often even if the patient remains standing. Back positioning should have no effect on the pain.2-5
Physical examination should begin with observation of the patient’s gait and posture, which may be hunched over in the setting of spinal stenosis. Examination of the patient’s skin may show loss of hair, shiny skin, or atrophic changes suggestive of vascular disease (Figure 5).1 Prior to proceeding to a spine examination, palpating the trochanteric bursa and testing for hip range of motion is important to rule out intra-articular hip pathology and trochanteric bursitis as common causes of pain in the area. Patients with radiculopathy may show sensory disturbances in a dermatomal distribution, muscular weakness at the corresponding spinal level, and decreased deep tendon reflexes. The straight leg raise test can elicit signs of nerve root tension. A careful examination of bilateral lower extremity pulses at the dorsal pedis, popliteal, and femoral levels can help identify any asymmetric or decreased pulses that would indicate peripheral vascular disease. With chronic aortoiliac disease, it is important to check for femoral pulses, given the dorsal pedis pulse can be present due to collateral circulation. And finally, the ankle brachial index (ABI), measured as the ratio of the systolic pressure at the ankle divided by the systolic pressure at the arm, is a good screening test for PAD.6 A normal ABI is >1.
A thorough history and physical examination can elicit important information that is helpful in evaluating orthopedic patients, especially to differentiate between spinal and vascular causes of leg pain. This can help avoid misdiagnoses, which result in unnecessary tests, procedures, and wasted time. Don’t forget the pulses!
Lumbar radiculopathy is a common problem encountered by orthopedic surgeons, and typically presents with lower back or buttock pain radiating down the leg.1 While the most common causes of lumbar radiculopathy are lumbar disc herniation and spinal stenosis, the differential diagnosis for lower extremity pain is broad and can be musculoskeletal, vascular, neurologic, or inflammatory in nature.1,2 Differentiating between orthopedic, neurologic, and vascular causes of leg pain, such as peripheral artery disease (PAD), can sometimes be challenging. This is especially true in aortoiliac PAD, which can present with hip, buttock, and thigh pain. Dorsalis pedis pulses can be palpable due to collateral circulation. A careful history and physical examination is crucial to the correct diagnosis. The history should clearly document the nature of the pain, details of walking impairment, and the alleviating effects of standing still or positional changes. A complete neurovascular examination should include observations regarding the skin, hair, and nails, examination of dorsal pedis, popliteal, and femoral pulses in comparison to the contralateral side, and documentation of dural tension signs. Misdiagnoses can send the patient down a path of unnecessary tests, unindicated procedures, and ultimately, a delay in definitive diagnosis and treatment.1
To our knowledge, this is the first report on a series of patients with thigh pain initially diagnosed as radiculopathy who underwent unproductive diagnostic tests and procedures, and ultimately were given delayed diagnoses of aortoiliac PAD. The patients provided written informed consent for print and electronic publication of these case reports.
Case 1
An 81-year-old woman with a medical history notable for hypertension, hyperlipidemia, and stroke initially presented to an outside orthopedic institution with complaints of several months of lower back and right hip, thigh, and leg pain when walking. She did not report any history of night pain, weakness, or numbness. Examination at the time was notable for painful back extension, 4/5 hip flexion strength on the right compared to 5/5 on the left, but symmetric reflexes and negative dural tension signs. X-rays showed multilevel degenerative disc disease of the lumbar spine, and magnetic resonance imaging (MRI) showed a small L3/4 disc protrusion causing impingement of the L4 nerve root.
A transforaminal epidural steroid injection at the L4 level was performed with minimal resolution of symptoms. Several months later, right-sided intra-articular facet injections were performed at the L4/5 and L5/S1 levels, again with minimal relief of symptoms. At this point, the patient was sent for further physical therapy.
Over a year after symptom onset, the patient presented to our institution and was evaluated by a vascular surgeon. Physical examination was notable for 1+ femoral artery and dorsal pedis pulses on the right side, compared to 2+ on the left. An aortoiliac duplex ultrasound showed severe significant stenosis of the right common iliac artery (>75%).
The patient underwent a right common iliac artery angioplasty and stenting (Figures 1A, 1B), which resolved her symptoms.
Case 2
A 65-year-old man, who is a former smoker with a medical history notable for hyperlipidemia and coronary artery disease status post myocardial infarction, presented with a long history of right leg pain. He underwent a L5/S1 anterior posterior fusion at an outside institution and did well for about 5 years after the procedure (Figures 2A, 2B). The pain returned and he underwent several years of physical therapy, epidural steroid injections, and implantation of a spinal cord stimulator with no improvement. He reported right leg pain with minimal back pain, primarily in the thigh and not radiating to the feet and toes. The pain limited him from walking more than 1 block. On examination, strength was 5/5 bilaterally. Pulse examination was notable for lack of dorsalis pedis/posterior tibial pulses bilaterally. He had no bowel or bladder dysfunction.
Computed tomography myelogram showed a moderate amount of stenosis at L3/4 and L4/5. He was sent for evaluation by a vascular surgeon. Arterial duplex ultrasound showed significant stenosis of the right common iliac artery.
Angioplasty was attempted but vascular surgery was unable to cross the lesion (Figures 3A, 3B), and the patient ultimately had a femoral-femoral bypass, which resolved his leg pain.
Case 3
A 78-year-old woman, nonsmoker, presented with a 1-year history of left buttock and thigh pain exacerbated by ambulation. Ambulation was limited to 2 blocks. The patient was being worked up for spinal and hip etiologies of pain at an outside hospital. MRI revealed a mild posterior disc herniation at L3/4 and L4/5 and moderate narrowing of the spinal canal. She underwent 2 epidural steroid injections with no improvement. The patient’s relative, a physician, suggested that the patient receive a vascular surgery consultation, and the patient ultimately presented to our institution for evaluation by vascular surgery.
The physical examination was significant for a 1+ dorsal pedis pulse on the left compared to 2+ on the right. Moreover, the patient only demonstrated trace L femoral pulse compared to the right. Strength was 5/5 bilaterally.
The patient was taken to the operating room for angioplasty and stenting of the left common iliac artery (Figures 4A, 4B). This provided immediate symptom relief, and she has remained asymptomatic.
Discussion
Lumbar radiculopathy is a common diagnosis encountered by orthopedic surgeons. Although the diagnosis can appear to be straightforward in a patient presenting with lower back and leg pain, the etiology of lower back and leg pain can be extremely varied, and can be musculoskeletal, neurologic, vascular, rheumatologic, or oncologic in origin.1 In particular, differentiating between radiculopathy and vascular claudication can sometimes be challenging.
The 2 most common causes of lumbar radiculopathy are lumbar disc herniation and spinal stenosis.1 Lumbar disc herniation results from tear in the annulus of the intervertebral disc, resulting in herniation of disc material into the spinal canal causing compression and irritation of spinal nerve roots.1 Spinal stenosis is narrowing of the spinal canal that produces compression of neural elements before they exit the neural foramen.3 Adult degenerative spinal stenosis is most often caused by osteophytes from the facet joints or hypertrophy of the ligamentum flavum, and can be broadly categorized into central spinal stenosis or lateral spinal stenosis.
PAD is defined as progressive stenosis or occlusion, or aneurysmal dilation of noncoronary arteries.2 When PAD affects the vessels of the lower extremities, the symptoms typically manifest as intermittent claudication, which is exercise-induced ischemic pain in the lower extremity that is relieved by rest.2 As the disease progresses, symptoms can progress to rest pain, ulceration, and, eventually, gangrene. The most common cause of PAD is atherosclerosis, and the risk factors include smoking, hypertension, diabetes, and hyperlipidemia. The prevalence of PAD rises sharply with age, starting from <3% in ages less than 60 years to >20% in ages 75 years and older.4
A detailed and pertinent history from the patient provides important information for differentiating radiculopathy and neurogenic claudication from vascular claudication. Patients with lumbar radiculopathy typically report pain in the lower back radiating down the leg past the knee in a dermatomal distribution. The pain often begins soon if not immediately after activity, but often takes time for relief onset after rest. Positional changes in the back such as flexion can provide relief.2 Patients with neurogenic claudication from central spinal stenosis can present with bilateral thigh pain from prolonged standing and activity that is alleviated with flexion or stooping.3 Patients may admit to a positive “shopping cart sign,” with increased walking comfort stooped forward with hands on a shopping cart.
In contrast, patients with vascular claudication often report pain in the calf, thigh, or hip, but rarely in the foot. The location of pain varies with area of stenosis; generally, patients with superficial femoral artery occlusion present with calf claudication, while patients with aortoiliac disease present with buttock and thigh pain. The pain typically occurs after a very reproducible length of walking, and is relieved by cessation of walking, often even if the patient remains standing. Back positioning should have no effect on the pain.2-5
Physical examination should begin with observation of the patient’s gait and posture, which may be hunched over in the setting of spinal stenosis. Examination of the patient’s skin may show loss of hair, shiny skin, or atrophic changes suggestive of vascular disease (Figure 5).1 Prior to proceeding to a spine examination, palpating the trochanteric bursa and testing for hip range of motion is important to rule out intra-articular hip pathology and trochanteric bursitis as common causes of pain in the area. Patients with radiculopathy may show sensory disturbances in a dermatomal distribution, muscular weakness at the corresponding spinal level, and decreased deep tendon reflexes. The straight leg raise test can elicit signs of nerve root tension. A careful examination of bilateral lower extremity pulses at the dorsal pedis, popliteal, and femoral levels can help identify any asymmetric or decreased pulses that would indicate peripheral vascular disease. With chronic aortoiliac disease, it is important to check for femoral pulses, given the dorsal pedis pulse can be present due to collateral circulation. And finally, the ankle brachial index (ABI), measured as the ratio of the systolic pressure at the ankle divided by the systolic pressure at the arm, is a good screening test for PAD.6 A normal ABI is >1.
A thorough history and physical examination can elicit important information that is helpful in evaluating orthopedic patients, especially to differentiate between spinal and vascular causes of leg pain. This can help avoid misdiagnoses, which result in unnecessary tests, procedures, and wasted time. Don’t forget the pulses!
1. Grimm BD, Blessinger BJ, Darden BV, Brigham CD, Kneisl JS, Laxer EB. Mimickers of lumbar radiculopathy. J Am Acad Orthop Surg. 2015;23(1):7-17.
2. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)--summary of recommendations. J Vasc Interv Radiol. 2006;17(9):1383-1397.
3. Spivak JM. Degenerative lumbar spinal stenosis. J Bone Joint Surg Am. 1998;80(7):1053-1066.
4. Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71(3):510-515.
5. Ouriel K. Peripheral arterial disease. Lancet. 2001;358(9289):1257-1264.
6. Jeon CH, Han SH, Chung NS, Hyun HS. The validity of ankle-brachial index for the differential diagnosis of peripheral arterial disease and lumbar spinal stenosis in patients with atypical claudication. Eur Spine J. 2012;21(6):1165-1170.
1. Grimm BD, Blessinger BJ, Darden BV, Brigham CD, Kneisl JS, Laxer EB. Mimickers of lumbar radiculopathy. J Am Acad Orthop Surg. 2015;23(1):7-17.
2. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)--summary of recommendations. J Vasc Interv Radiol. 2006;17(9):1383-1397.
3. Spivak JM. Degenerative lumbar spinal stenosis. J Bone Joint Surg Am. 1998;80(7):1053-1066.
4. Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71(3):510-515.
5. Ouriel K. Peripheral arterial disease. Lancet. 2001;358(9289):1257-1264.
6. Jeon CH, Han SH, Chung NS, Hyun HS. The validity of ankle-brachial index for the differential diagnosis of peripheral arterial disease and lumbar spinal stenosis in patients with atypical claudication. Eur Spine J. 2012;21(6):1165-1170.
Benefit of lumbar fusion for spinal stenosis found to be small to nonexistent
The benefit of adding lumbar fusion surgery to decompression surgery for spinal stenosis was nonexistent in one large clinical trial and very modest in another, according to separate reports published online April 13 in the New England Journal of Medicine.
Both studies indicated that, given the considerable cost and the potential complications associated with lumbar fusion, it may not be worthwhile to add it to decompression surgery for spinal stenosis. “The goal of surgery in lumbar spinal stenosis is to improve walking distance and to relieve pain by decompression of the nerve roots. The addition of instrumented fusion – ‘just to be sure’ – for the treatment of the most frequent forms of lumbar spinal stenosis does not create any added value for patients and might be regarded as an overcautious and unnecessary treatment,” Dr. Wilco C. Peul and Dr. Wouter A. Moojen said in an editorial accompanying the two reports.
Surgical decompression of spinal stenosis using laminectomy is increasingly being supplemented with lumbar fusion, which is thought to firm up spinal instability and minimize the risk of future deformity. In the United States, approximately half of patients who have surgery for spinal stenosis undergo fusion procedures. Of those who also show degenerative spondylolisthesis on preoperative imaging studies, 96% undergo fusion procedures because many spine surgeons see this as a sign of instability and a mandatory indication for fusion. However, the evidence supporting the use of fusion plus decompression, as opposed to decompression alone, is weak, according to the investigators who conducted the Swedish Spinal Stenosis Study. The other study in the New England Journal of Medicine, the Spinal Laminectomy Versus Instrumented Pedicle Screw (SLIP) trial, was conducted in the United States.
Both of those clinical trials were performed to shed further light on the issue.
In the Swedish Spinal Stenosis Study, the investigators assessed outcomes in 247 patients aged 50-80 years who were treated at seven Swedish hospitals over the course of 6 years. This open-label, superiority trial randomly assigned 124 patients to decompression surgery alone and 123 to decompression plus fusion. The primary outcome measure was score on the Oswestry Disability Index (ODI), which measures disability and quality of life in patients with low-back pain, 2 years after surgery. The ODI scale runs from 0 to 100, with higher scores indicating more severe disability, said Dr. Peter Försth of the department of surgical sciences at Uppsala (Sweden) University and the Stockholm Spine Center and his associates.
At 2 years, there was no significant difference between the two study groups; the decompression-only group had a mean ODI score of 24, and the fusion group had a mean score of 27. The ODI scores in both groups had improved from baseline to a similar degree: by 17 points with decompression alone and by 15 points with fusion. In addition, fusion surgery was not superior to decompression alone regardless of whether patients had any degree of spondylolisthesis and regardless of whether they had severe spondylolisthesis involving a vertebral slip of 7.4 mm or more, the investigators reported (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMoa1513721).The two study groups also showed no significant differences in secondary outcome measures, including performance on the 6-minute walk test and subjective patient assessment of improvement in walking ability. Moreover, these results persisted in the 144 patients who were assessed at 5-year follow-up.
In contrast, decompression alone was associated with fewer complications than decompression plus fusion. Postoperative wound infection developed in only 4% of the decompression-only group, compared with 10% of the fusion group. Although this study wasn’t adequately powered to draw firm conclusions regarding complications, a previous analysis of registry data reported that adding fusion surgery to decompression surgery doubles the risk of severe adverse events in older patients, Dr. Försth and his associates said.
Decompression alone also was markedly less expensive than fusion surgery. Mean direct costs were $6,800 higher for fusion than for decompression alone, because of the additional operating time needed, the extended hospital stay, and the cost of the implant.
In the SLIP trial, the researchers compared outcomes in 66 patients aged 50-80 years who all had spinal stenosis with grade 1 degenerative spondylolisthesis. The participants were randomly assigned to undergo decompression alone (35 patients) or decompression plus fusion (31 patients) at five U.S. medical centers, said Dr. Zoher Ghogawala of the Alan and Jacqueline B. Stuart Spine Research Center in the department of neurosurgery at Lahey Hospital and Medical Center, Burlington, Mass., and his associates.
The primary outcome measure was the physical-component summary score on the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) 2 years after surgery. This scale also runs from 0 to 100, but higher scores indicate better physical health. Five points was prespecified as the minimal clinically important difference on the SF-36.
At 2 years, patients in the fusion group showed a small but significant advantage of 5.7 points on the SF-36, with a mean score of 15.2, compared with patients in the decompression-only group (mean score, 9.5). However, the ODI scores, a secondary outcome measure in this study, were not significantly different between the two study groups, Dr. Ghogawala and his associates reported (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMoa1508788).Surgical complications, blood loss, and length of stay all were significantly greater with fusion than with decompression alone.
Dr. Försth’s study was supported by Uppsala University, Uppsala County Council, the Stockholm Spine Center, and Johnson & Johnson. Two of his associates reported ties to Medtronic and Quantify Research. Dr. Ghogawala’s study was supported by the Jean and David Wallace Foundation, the Greenwich Lumbar Stenosis SLIP Study Fund. His associates reported ties to numerous industry sources.
Both of these studies clearly demonstrated that for most patients, stenosis surgery should be limited to decompression when no overt instability is present. Dr. Ghogawala and his colleagues correctly concluded that the modest difference in SF-36 score in favor of fusion doesn’t justify that procedure’s higher cost and complication rate.
Fusion surgery is no longer best practice and should be restricted to patients who have proven spinal instability; vertebral destruction caused by trauma, tumors, infections, or spinal deformities; or possibly neuroforamen stenosis with compressed exiting nerves due to postsurgical disk collapse.
Dr. Wilco C. Peul is at Leiden (the Netherlands) University Medical Center and at Medical Center Haaglanden, the Hague. Dr. Wouter A. Moojen is at Medical Center Haaglanden. Dr. Peul reported receiving grants from ZonMW, Paradigm Spine, Medtronic, Eurospine Foundation, and CVZ. Dr. Moojen reported having no relevant financial disclosures. Dr. Peul and Dr. Moojen made these remarks in an editorial accompanying the reports on the Swedish Spinal Stenosis Study and the Spinal Laminectomy Versus Instrumented Pedicle Screw trial (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMe1600955).
Both of these studies clearly demonstrated that for most patients, stenosis surgery should be limited to decompression when no overt instability is present. Dr. Ghogawala and his colleagues correctly concluded that the modest difference in SF-36 score in favor of fusion doesn’t justify that procedure’s higher cost and complication rate.
Fusion surgery is no longer best practice and should be restricted to patients who have proven spinal instability; vertebral destruction caused by trauma, tumors, infections, or spinal deformities; or possibly neuroforamen stenosis with compressed exiting nerves due to postsurgical disk collapse.
Dr. Wilco C. Peul is at Leiden (the Netherlands) University Medical Center and at Medical Center Haaglanden, the Hague. Dr. Wouter A. Moojen is at Medical Center Haaglanden. Dr. Peul reported receiving grants from ZonMW, Paradigm Spine, Medtronic, Eurospine Foundation, and CVZ. Dr. Moojen reported having no relevant financial disclosures. Dr. Peul and Dr. Moojen made these remarks in an editorial accompanying the reports on the Swedish Spinal Stenosis Study and the Spinal Laminectomy Versus Instrumented Pedicle Screw trial (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMe1600955).
Both of these studies clearly demonstrated that for most patients, stenosis surgery should be limited to decompression when no overt instability is present. Dr. Ghogawala and his colleagues correctly concluded that the modest difference in SF-36 score in favor of fusion doesn’t justify that procedure’s higher cost and complication rate.
Fusion surgery is no longer best practice and should be restricted to patients who have proven spinal instability; vertebral destruction caused by trauma, tumors, infections, or spinal deformities; or possibly neuroforamen stenosis with compressed exiting nerves due to postsurgical disk collapse.
Dr. Wilco C. Peul is at Leiden (the Netherlands) University Medical Center and at Medical Center Haaglanden, the Hague. Dr. Wouter A. Moojen is at Medical Center Haaglanden. Dr. Peul reported receiving grants from ZonMW, Paradigm Spine, Medtronic, Eurospine Foundation, and CVZ. Dr. Moojen reported having no relevant financial disclosures. Dr. Peul and Dr. Moojen made these remarks in an editorial accompanying the reports on the Swedish Spinal Stenosis Study and the Spinal Laminectomy Versus Instrumented Pedicle Screw trial (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMe1600955).
The benefit of adding lumbar fusion surgery to decompression surgery for spinal stenosis was nonexistent in one large clinical trial and very modest in another, according to separate reports published online April 13 in the New England Journal of Medicine.
Both studies indicated that, given the considerable cost and the potential complications associated with lumbar fusion, it may not be worthwhile to add it to decompression surgery for spinal stenosis. “The goal of surgery in lumbar spinal stenosis is to improve walking distance and to relieve pain by decompression of the nerve roots. The addition of instrumented fusion – ‘just to be sure’ – for the treatment of the most frequent forms of lumbar spinal stenosis does not create any added value for patients and might be regarded as an overcautious and unnecessary treatment,” Dr. Wilco C. Peul and Dr. Wouter A. Moojen said in an editorial accompanying the two reports.
Surgical decompression of spinal stenosis using laminectomy is increasingly being supplemented with lumbar fusion, which is thought to firm up spinal instability and minimize the risk of future deformity. In the United States, approximately half of patients who have surgery for spinal stenosis undergo fusion procedures. Of those who also show degenerative spondylolisthesis on preoperative imaging studies, 96% undergo fusion procedures because many spine surgeons see this as a sign of instability and a mandatory indication for fusion. However, the evidence supporting the use of fusion plus decompression, as opposed to decompression alone, is weak, according to the investigators who conducted the Swedish Spinal Stenosis Study. The other study in the New England Journal of Medicine, the Spinal Laminectomy Versus Instrumented Pedicle Screw (SLIP) trial, was conducted in the United States.
Both of those clinical trials were performed to shed further light on the issue.
In the Swedish Spinal Stenosis Study, the investigators assessed outcomes in 247 patients aged 50-80 years who were treated at seven Swedish hospitals over the course of 6 years. This open-label, superiority trial randomly assigned 124 patients to decompression surgery alone and 123 to decompression plus fusion. The primary outcome measure was score on the Oswestry Disability Index (ODI), which measures disability and quality of life in patients with low-back pain, 2 years after surgery. The ODI scale runs from 0 to 100, with higher scores indicating more severe disability, said Dr. Peter Försth of the department of surgical sciences at Uppsala (Sweden) University and the Stockholm Spine Center and his associates.
At 2 years, there was no significant difference between the two study groups; the decompression-only group had a mean ODI score of 24, and the fusion group had a mean score of 27. The ODI scores in both groups had improved from baseline to a similar degree: by 17 points with decompression alone and by 15 points with fusion. In addition, fusion surgery was not superior to decompression alone regardless of whether patients had any degree of spondylolisthesis and regardless of whether they had severe spondylolisthesis involving a vertebral slip of 7.4 mm or more, the investigators reported (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMoa1513721).The two study groups also showed no significant differences in secondary outcome measures, including performance on the 6-minute walk test and subjective patient assessment of improvement in walking ability. Moreover, these results persisted in the 144 patients who were assessed at 5-year follow-up.
In contrast, decompression alone was associated with fewer complications than decompression plus fusion. Postoperative wound infection developed in only 4% of the decompression-only group, compared with 10% of the fusion group. Although this study wasn’t adequately powered to draw firm conclusions regarding complications, a previous analysis of registry data reported that adding fusion surgery to decompression surgery doubles the risk of severe adverse events in older patients, Dr. Försth and his associates said.
Decompression alone also was markedly less expensive than fusion surgery. Mean direct costs were $6,800 higher for fusion than for decompression alone, because of the additional operating time needed, the extended hospital stay, and the cost of the implant.
In the SLIP trial, the researchers compared outcomes in 66 patients aged 50-80 years who all had spinal stenosis with grade 1 degenerative spondylolisthesis. The participants were randomly assigned to undergo decompression alone (35 patients) or decompression plus fusion (31 patients) at five U.S. medical centers, said Dr. Zoher Ghogawala of the Alan and Jacqueline B. Stuart Spine Research Center in the department of neurosurgery at Lahey Hospital and Medical Center, Burlington, Mass., and his associates.
The primary outcome measure was the physical-component summary score on the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) 2 years after surgery. This scale also runs from 0 to 100, but higher scores indicate better physical health. Five points was prespecified as the minimal clinically important difference on the SF-36.
At 2 years, patients in the fusion group showed a small but significant advantage of 5.7 points on the SF-36, with a mean score of 15.2, compared with patients in the decompression-only group (mean score, 9.5). However, the ODI scores, a secondary outcome measure in this study, were not significantly different between the two study groups, Dr. Ghogawala and his associates reported (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMoa1508788).Surgical complications, blood loss, and length of stay all were significantly greater with fusion than with decompression alone.
Dr. Försth’s study was supported by Uppsala University, Uppsala County Council, the Stockholm Spine Center, and Johnson & Johnson. Two of his associates reported ties to Medtronic and Quantify Research. Dr. Ghogawala’s study was supported by the Jean and David Wallace Foundation, the Greenwich Lumbar Stenosis SLIP Study Fund. His associates reported ties to numerous industry sources.
The benefit of adding lumbar fusion surgery to decompression surgery for spinal stenosis was nonexistent in one large clinical trial and very modest in another, according to separate reports published online April 13 in the New England Journal of Medicine.
Both studies indicated that, given the considerable cost and the potential complications associated with lumbar fusion, it may not be worthwhile to add it to decompression surgery for spinal stenosis. “The goal of surgery in lumbar spinal stenosis is to improve walking distance and to relieve pain by decompression of the nerve roots. The addition of instrumented fusion – ‘just to be sure’ – for the treatment of the most frequent forms of lumbar spinal stenosis does not create any added value for patients and might be regarded as an overcautious and unnecessary treatment,” Dr. Wilco C. Peul and Dr. Wouter A. Moojen said in an editorial accompanying the two reports.
Surgical decompression of spinal stenosis using laminectomy is increasingly being supplemented with lumbar fusion, which is thought to firm up spinal instability and minimize the risk of future deformity. In the United States, approximately half of patients who have surgery for spinal stenosis undergo fusion procedures. Of those who also show degenerative spondylolisthesis on preoperative imaging studies, 96% undergo fusion procedures because many spine surgeons see this as a sign of instability and a mandatory indication for fusion. However, the evidence supporting the use of fusion plus decompression, as opposed to decompression alone, is weak, according to the investigators who conducted the Swedish Spinal Stenosis Study. The other study in the New England Journal of Medicine, the Spinal Laminectomy Versus Instrumented Pedicle Screw (SLIP) trial, was conducted in the United States.
Both of those clinical trials were performed to shed further light on the issue.
In the Swedish Spinal Stenosis Study, the investigators assessed outcomes in 247 patients aged 50-80 years who were treated at seven Swedish hospitals over the course of 6 years. This open-label, superiority trial randomly assigned 124 patients to decompression surgery alone and 123 to decompression plus fusion. The primary outcome measure was score on the Oswestry Disability Index (ODI), which measures disability and quality of life in patients with low-back pain, 2 years after surgery. The ODI scale runs from 0 to 100, with higher scores indicating more severe disability, said Dr. Peter Försth of the department of surgical sciences at Uppsala (Sweden) University and the Stockholm Spine Center and his associates.
At 2 years, there was no significant difference between the two study groups; the decompression-only group had a mean ODI score of 24, and the fusion group had a mean score of 27. The ODI scores in both groups had improved from baseline to a similar degree: by 17 points with decompression alone and by 15 points with fusion. In addition, fusion surgery was not superior to decompression alone regardless of whether patients had any degree of spondylolisthesis and regardless of whether they had severe spondylolisthesis involving a vertebral slip of 7.4 mm or more, the investigators reported (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMoa1513721).The two study groups also showed no significant differences in secondary outcome measures, including performance on the 6-minute walk test and subjective patient assessment of improvement in walking ability. Moreover, these results persisted in the 144 patients who were assessed at 5-year follow-up.
In contrast, decompression alone was associated with fewer complications than decompression plus fusion. Postoperative wound infection developed in only 4% of the decompression-only group, compared with 10% of the fusion group. Although this study wasn’t adequately powered to draw firm conclusions regarding complications, a previous analysis of registry data reported that adding fusion surgery to decompression surgery doubles the risk of severe adverse events in older patients, Dr. Försth and his associates said.
Decompression alone also was markedly less expensive than fusion surgery. Mean direct costs were $6,800 higher for fusion than for decompression alone, because of the additional operating time needed, the extended hospital stay, and the cost of the implant.
In the SLIP trial, the researchers compared outcomes in 66 patients aged 50-80 years who all had spinal stenosis with grade 1 degenerative spondylolisthesis. The participants were randomly assigned to undergo decompression alone (35 patients) or decompression plus fusion (31 patients) at five U.S. medical centers, said Dr. Zoher Ghogawala of the Alan and Jacqueline B. Stuart Spine Research Center in the department of neurosurgery at Lahey Hospital and Medical Center, Burlington, Mass., and his associates.
The primary outcome measure was the physical-component summary score on the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) 2 years after surgery. This scale also runs from 0 to 100, but higher scores indicate better physical health. Five points was prespecified as the minimal clinically important difference on the SF-36.
At 2 years, patients in the fusion group showed a small but significant advantage of 5.7 points on the SF-36, with a mean score of 15.2, compared with patients in the decompression-only group (mean score, 9.5). However, the ODI scores, a secondary outcome measure in this study, were not significantly different between the two study groups, Dr. Ghogawala and his associates reported (N Engl J Med. 2016 April 13. doi: 10.1056/NEJMoa1508788).Surgical complications, blood loss, and length of stay all were significantly greater with fusion than with decompression alone.
Dr. Försth’s study was supported by Uppsala University, Uppsala County Council, the Stockholm Spine Center, and Johnson & Johnson. Two of his associates reported ties to Medtronic and Quantify Research. Dr. Ghogawala’s study was supported by the Jean and David Wallace Foundation, the Greenwich Lumbar Stenosis SLIP Study Fund. His associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: The benefit of adding lumbar fusion surgery to decompression surgery for spinal stenosis was nonexistent in one large trial and very modest in another.
Major finding: At 2 years in the Swedish Spinal Stenosis Study, there was no significant difference between the two study groups; the decompression-only group had a mean Oswestry Disability Index score of 24, and the fusion group had a mean score of 27.
Data source: Two multicenter, randomized trials involving 247 patients and 66 patients, comparing decompression surgery alone with decompression plus fusion.
Disclosures: Dr. Försth’s study was supported by Uppsala University, Uppsala County Council, Stockholm Spine Center, and Johnson & Johnson. Two of his associates reported ties to Medtronic and Quantify Research. Dr. Ghogawala’s study was supported by the Jean and David Wallace Foundation, the Greenwich Lumbar Stenosis SLIP Study Fund. His associates reported ties to numerous industry sources.