User login
14-Year-Old Boy With Mild Antecedent Neck Pain in Setting of Acute Trauma: A Rare Case of Benign Fibrous Histiocytoma of the Spine
Benign fibrous histiocytoma (BFH) is a rare, well-recognized, primary skeletal tumor accounting for approximately 1% of all benign bone tumors. Spinal involvement is exceedingly rare with only 11 cases reported in the literature.1,2 We present a case of BFH located in the cervical spine of a pediatric patient that was successfully treated with curretage through an anterior surgical approach, along with a review of the literature and appropriate management concerning BFH of the spine.
Case Report
A 14-year-old boy was tackled while playing football and noticed immediate neck pain and subjective paresthesia in the upper extremities. Examination revealed a nontender spine (cervical, thoracic, lumbar) and normal strength and range of motion in all extremities. Sensation was diffusely intact, long tract signs were absent, and gait was normal. On questioning, the patient endorsed mild antecedent neck pain but denied prior history of any trauma. Neck pain did not radiate and was slightly worsened by activity but was mostly intermittent and random. As the neck pain was very mild and was not interfering with daily activities, the patient had not sought care before presenting to the emergency department. He had no pertinent past medical or surgical history.
The patient presented with a computed tomography (CT) scan of his head and cervical spine and a magnetic resonance imaging (MRI) scan of the cervical spine. A magnetic resonance angiography (MRA) scan of the neck was ordered after his arrival.
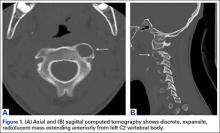
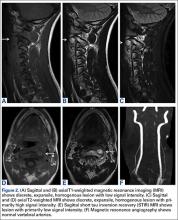
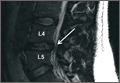
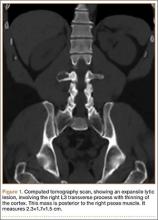
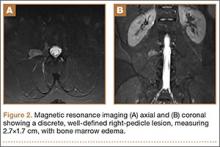
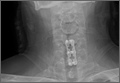
Axial and sagittal CT (Figures 1A, 1B) showed a 1×1.2-cm discrete, expansile, lytic, radiolucent mass extending anterior from the left C2 vertebral body. The mass appeared to abut the left vertebral artery foramen. The cortical bone surrounding the lesion was thin but uniform. Sagittal and axial T1-weighted MRI (Figures 2A, 2B) showed the discrete, expansile, homogenous lesion with the same intensity as normal bone marrow. Sagittal and axial T2-weighted MRI (Figures 2C, 2D) showed a discrete, expansile, homogenous lesion with primarily high signal intensity. Sagittal short tau inversion recovery (STIR) MRI (Figure 2E) again showed the lesion with primarily low intensity. Given the close proximity of the lesion to the vertebral foramen, MRA was ordered; it showed the lesion was not interfering with the vertebral artery (Figure 2F).
The tumor’s location, in the left anterior aspect of the C2 vertebral body, was not conducive to percutaneous biopsy for establishing tissue diagnosis, so the decision was made to surgically excise the lesion. A left-sided anterior incision was made 2 fingerbreadths inferior to the jaw line in a neck crease. A head and neck surgeon assisted with dissection. Dissection was carried down through the skin, subcutaneous tissue, and platysma on to the anterior part of the spine medial to the carotid sheath. Superior thyroid nerve and vessels and superior laryngeal nerve were identified and preserved. Fluoroscopy confirmed correct location at C2. The tumor was easily visualized, and the outer shell broke easily with palpation. Gentle curettage was necessary when removing the tumor off the vertebral artery. A portion of the specimen was sent during surgery for frozen section, which showed infrequent mitotic figures and no other findings concerning for malignancy. No instability was created after curettage and excision of the tumor, so no grafting or instrumentation was necessary.
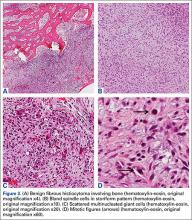
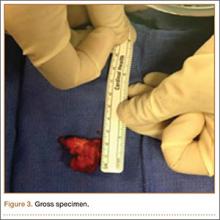
Grossly, the tumor was pale tan and firm. Histologic examination with hematoxylin-eosin staining revealed a bland spindle-cell neoplasm that focally involved bone. A storiform pattern was present. The cells had scant cytoplasm and oval to elongate nuclei with tapered ends. Significant nuclear pleomorphism was not seen. The stroma was loose, with focal myxoid change. Benign multinucleated giant cells were present. Mitotic activity was infrequent (Figures 3A–3D). Two attending pathologists reviewed the case material and the frozen and formalin-fixed specimens independently and concurred with the diagnosis of BFH. In addition, the case was reviewed at the surgical pathology consensus conference; the reviewers agreed on BFH, and additional studies were deemed unnecessary.
Given the patient’s complete clinical picture, the differential diagnosis included nonossifying fibroma (NOF), eosinophilic granuloma (EG), BFH, fibrous dysplasia, giant cell tumor (GCT), aneurysmal bone cyst (ABC), and osteoblastoma (OB).
Discussion
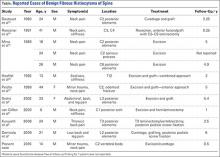
BFH is an extremely rare bone lesion, accounting for only 1% of all surgically managed bone tumors; not counting the present case, only 11 spine cases have been reported in the literature.1,2 BFH of the spine traditionally causes nonspecific, poorly localized pain. The Table lists the reported cases of spinal BFH and their presenting symptoms, location, and treatment. BFH usually occurs in young adults, but the age range is 5 to 75 years.2-4 Mean age of the 12 patients with spinal BFH in the literature (including ours) is 25 years.1 In addition, spinal BFH appears to have no predilection for sex.
Skeletal BFH presents as a discrete, well-defined, osteolytic lesion with sharp borders and potentially a sclerotic rim.4-6 Cortical expansion and even cortical disruption with invasion into adjacent tissue have occurred in flat bones.7 Histologically, BFHs contain spindle cells, multinucleated giant cells, and foam cells in storiform pattern.6
BFH shares many of its radiologic and histologic characteristics and clinical symptoms with other benign bone lesions (the tumors listed above). Therefore, accurate diagnosis of BFH requires appropriate correlation of clinical, radiographic, and histologic data.2,3,8 Below is a comparison of BFH with related bone lesions.
Spinal BFH causes a nonspecific, poorly localized pain similar to that of EG, ABC, GCT, and OB.3,9 NOF and fibrous dysplasia generally do not cause pain, unless these lesions are discovered secondary to a pathologic fracture.8,10,11 Our patient had minor antecedent neck pain, which was brought to light by his football accident. ABC and OB are more locally aggressive than BFH and can cause neurologic symptoms by mass effect and spinal cord or nerve root compression.1,8 In this case and in the 6 other cases of BFH of the cervical spine, there were no neurologic changes.4,10
Of the tumors mentioned, NOF and EG almost always occur in children. However, NOF usually occurs in the metaphyseal region of long bones, and EG is usually accompanied by systemic symptoms, such as lymphadenopathy, hepatomegaly, and increased inflammatory markers.1,8 Fibrous dysplasia usually presents in childhood but does not become symptomatic until adulthood. GCTs and OB predominantly occur in adulthood.12,13 Our patient’s age and lack of other systemic symptoms supported the diagnosis of BFH.
Appearance on MRI is reported less with BFH than with other tumors, but heterogenous signal intensity similar to that of skeletal muscle on T1-weighted images and high signal intensity on T2-weighted images is typically reported.8,14 NOF and fibrous dysplasia do not disrupt the bony cortex unless a pathologic fracture has occurred.4 GCTs are more aggressive lytic lesions with more aggressive radiologic features. GCTs generally cause cortical expansion/attenuation, and lack a sclerotic rim. GCTs also have a heterogenous appearance on MRI and give a low to intermediate signal on both T1- and T2-weighted images.12,15 The appearance of EG is similar to that of BFH as an osteolytic lesion with a sclerotic rim, though EGs typically break through the cortex and acquire a “punched-out” look.1,8 ABC typically is described as an expansile osteolytic lesion with a “soap-bubble” appearance on radiographs; periosteal elevation and cortical attenuation can also be visualized. MRI shows the typical multilobular appearance of the lesion with fluid levels.13
OB appears as a radiolucent lesion, with or without calcifications, surrounded by a thin margin of reactive bone.14,16 A distinguishing characteristic of OB was thought to be intense radioisotope uptake on bone scintigraphy, but recently a bony BFH demonstrated intense uptake.17 OBs typically demonstrate nonspecific MRI results similar to those of BFH: low to intermediate signal on T1-weighted images and intermediate to high signal on T2-weighted images.13 In our patient’s case, the radiographic appearance and lack of specific radiographic findings consistent with the other tumors supported the diagnosis of BFH.
Histologically, BFHs contain spindle cells, multinucleated giant cells, and foam cells in a storiform pattern6 which was demonstrated in our patient’s case. In addition, significant nuclear pleomorphism, mitotic activity, and necrosis were absent—a difference between BFH and malignant fibrous histiocytoma.4,15 The microscopic characteristics of BFH readily differentiate it from OB, ABC, EG, and GCT, but not from NOF on microscopic appearance alone. Clinical and radiographic findings must be consistent, as mentioned.7,18
Complete surgical excision is the reported treatment for BFH. Prognosis after resection or curettage is usually good, and recurrences have been rare.1,2 Depending on the intraspinous location of BFH, stabilization after resection or curettage may be necessary to prevent residual instability. Three of the 11 reported cases of spinal BFH required stabilization by anterior fusion or posterior pedicle screw fixation after resection.1,2 The other 8 cases underwent excision alone or excision and grafting. All 11 patients were disease-free at a mean follow-up of 3.5 years.1 In nonspinal BFH, however, both local recurrence and lung metastasis have been reported.2,5,9,19 Clarke and colleagues9 reported local recurrences in 3 of 8 cases. These recurrences involved BFH in long bones of the leg, which had been treated with curettage and grafting. There has been no reliable report of a malignant change in BFH.2,9 The only case of lung metastasis, reported by Unni and Dahlin6 in their study of 10 cases, occurred 2 years after local recurrence in the distal femur.Our patient was doing well at most recent follow-up, 6 months after surgery. He had no pain and had returned to normal activities. Although there are no reported cases of spinal BFH recurrence, we will follow this patient with imaging on an annual basis. His case is of particular interest to orthopedic surgeons because they encounter benign bone lesions every day, and many of these lesions are in difficult anatomical locations. Knowing the characteristics, differential diagnoses, and appropriate diagnostic workups for benign bone lesions is important for optimal and timely patient care.
1. Demiralp B, Kose O, Oguz E, Sanal T, Ozcan A, Sehirlioglu A. Benign fibrous histiocytoma of the lumbar vertebrae. Skeletal Radiol. 2009;38(2):187-191.
2. Kuruvath S, O’Donovan DG, Aspoas AR, David KM. Benign fibrous histiocytoma of the thoracic spine: case report and review of the literature. J Neurosurg Spine. 2006;4(3):260-264.
3. Ceroni D, Dayer R, De Coulon G, Kaelin A. Benign fibrous histiocytoma of bone in a paediatric population: a report of 6 cases. Musculoskelet Surg. 2011;95(2):107-114.
4. Dorfman HD, Czerniak B. Bone Tumors. St. Louis, MO: Mosby; 1998.
5. Grohs JG, Nicolakis M, Kainberger F, Lang S, Kotz R. Benign fibrous histiocytoma of bone: a report of ten cases and review of literature. Wien Klin Wochenschr. 2002;114(1-2):56-63.
6. Unni KK, Dahlin DC. Dahlin’s Bone Tumors. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
7. Balasubramanian C, Rajaraman G, Singh CS, Baliga DK. Benign fibrous histiocytoma of the sacrum—diagnostic difficulties facing this rare bone tumor. Pediatr Neurosurg. 2005;41(5):253-257.
8. van Giffen NH, van Rhijn LW, van Ooij A, et al. Benign fibrous histiocytoma of the posterior arch of C1 in a 6-year old boy: a case report. Spine. 2003;28(18):E359-E363.
9. Clarke BE, Xipell JM, Thomas DP. Benign fibrous histiocytoma of bone. Am J Surg Pathol. 1985;9(11):806-815.
10. Peicha G, Siebert FJ, Bratschitsch G, Fankhauser F, Grechenig W. Pathologic odontoid fracture and benign fibrous histiocytoma of bone. Eur Spine J. 1999;8(2):161-163.
11. Unni KK, Inwards CY, Bridge JA, Kindblom LG, Wold LE. Tumors of the Bones and Joints (AFIP Atlas of Tumor Pathology Series IV). Annapolis Junction, MD: American Registry of Pathology Press; 2005.
12. Dee R. Principles of Orthopaedic Practice. 2nd ed. New York, NY: McGraw-Hill; 1997.
13. Murphey M, Andrews C, Flemming D, Temple HT, Smith WS, Smirniotopoulos JG. Primary tumors of the spine: radiologic–pathologic correlation. Radiographics. 1996;16(5):1131-1158.
14. Hamada T, Ito H, Araki Y, Fujii K, Inoue M, Ishida O. Benign fibrous histiocytoma of the femur: review of three cases. Skeletal Radiol. 1996;25(1):25-29.
15. Mirra JM, Picci P, Gold RH. Bone Tumors: Clinical, Radiologic, and Pathologic Correlations. Vol 1. Philadelphia, PA: Lea & Febiger; 1989.
16. Theodorou DJ, Theodorou SJ, Sartoris DJ. An imaging overview of primary tumors of the spine: part 1. Benign tumors. Clin Imaging. 2008;32(3):196-203.
17. Li X, Meng Z, Li D, Tan J, Song X. Benign fibrous histiocytoma of a rib. Clin Nucl Med. 2014;39(9): 837-841.
18. Roessner A, Immenkamp M, Weidner A, Hobik HP, Grundmann E. Benign fibrous histiocytoma of bone. Light- and electron-microscopic observations. J Cancer Res Clin Oncol. 1981;101(2):191-202.
19. Destouet JM, Kyriakos M, Gilula LA. Fibrous histiocytoma (fibroxanthoma) of a cervical vertebra. A report with a review of the literature. Skeletal Radiol. 1980;5(4):241-246.
20. Hoeffel JC, Bomand-Ferrand F, Tachet F, Lascombes P, Czorny A, Bernard C. So-called benign fibrous histiocytoma: report of a case. J Pediatr Surg. 1992;27(5):672-674.
Benign fibrous histiocytoma (BFH) is a rare, well-recognized, primary skeletal tumor accounting for approximately 1% of all benign bone tumors. Spinal involvement is exceedingly rare with only 11 cases reported in the literature.1,2 We present a case of BFH located in the cervical spine of a pediatric patient that was successfully treated with curretage through an anterior surgical approach, along with a review of the literature and appropriate management concerning BFH of the spine.
Case Report
A 14-year-old boy was tackled while playing football and noticed immediate neck pain and subjective paresthesia in the upper extremities. Examination revealed a nontender spine (cervical, thoracic, lumbar) and normal strength and range of motion in all extremities. Sensation was diffusely intact, long tract signs were absent, and gait was normal. On questioning, the patient endorsed mild antecedent neck pain but denied prior history of any trauma. Neck pain did not radiate and was slightly worsened by activity but was mostly intermittent and random. As the neck pain was very mild and was not interfering with daily activities, the patient had not sought care before presenting to the emergency department. He had no pertinent past medical or surgical history.
The patient presented with a computed tomography (CT) scan of his head and cervical spine and a magnetic resonance imaging (MRI) scan of the cervical spine. A magnetic resonance angiography (MRA) scan of the neck was ordered after his arrival.
Axial and sagittal CT (Figures 1A, 1B) showed a 1×1.2-cm discrete, expansile, lytic, radiolucent mass extending anterior from the left C2 vertebral body. The mass appeared to abut the left vertebral artery foramen. The cortical bone surrounding the lesion was thin but uniform. Sagittal and axial T1-weighted MRI (Figures 2A, 2B) showed the discrete, expansile, homogenous lesion with the same intensity as normal bone marrow. Sagittal and axial T2-weighted MRI (Figures 2C, 2D) showed a discrete, expansile, homogenous lesion with primarily high signal intensity. Sagittal short tau inversion recovery (STIR) MRI (Figure 2E) again showed the lesion with primarily low intensity. Given the close proximity of the lesion to the vertebral foramen, MRA was ordered; it showed the lesion was not interfering with the vertebral artery (Figure 2F).
The tumor’s location, in the left anterior aspect of the C2 vertebral body, was not conducive to percutaneous biopsy for establishing tissue diagnosis, so the decision was made to surgically excise the lesion. A left-sided anterior incision was made 2 fingerbreadths inferior to the jaw line in a neck crease. A head and neck surgeon assisted with dissection. Dissection was carried down through the skin, subcutaneous tissue, and platysma on to the anterior part of the spine medial to the carotid sheath. Superior thyroid nerve and vessels and superior laryngeal nerve were identified and preserved. Fluoroscopy confirmed correct location at C2. The tumor was easily visualized, and the outer shell broke easily with palpation. Gentle curettage was necessary when removing the tumor off the vertebral artery. A portion of the specimen was sent during surgery for frozen section, which showed infrequent mitotic figures and no other findings concerning for malignancy. No instability was created after curettage and excision of the tumor, so no grafting or instrumentation was necessary.
Grossly, the tumor was pale tan and firm. Histologic examination with hematoxylin-eosin staining revealed a bland spindle-cell neoplasm that focally involved bone. A storiform pattern was present. The cells had scant cytoplasm and oval to elongate nuclei with tapered ends. Significant nuclear pleomorphism was not seen. The stroma was loose, with focal myxoid change. Benign multinucleated giant cells were present. Mitotic activity was infrequent (Figures 3A–3D). Two attending pathologists reviewed the case material and the frozen and formalin-fixed specimens independently and concurred with the diagnosis of BFH. In addition, the case was reviewed at the surgical pathology consensus conference; the reviewers agreed on BFH, and additional studies were deemed unnecessary.
Given the patient’s complete clinical picture, the differential diagnosis included nonossifying fibroma (NOF), eosinophilic granuloma (EG), BFH, fibrous dysplasia, giant cell tumor (GCT), aneurysmal bone cyst (ABC), and osteoblastoma (OB).
Discussion
BFH is an extremely rare bone lesion, accounting for only 1% of all surgically managed bone tumors; not counting the present case, only 11 spine cases have been reported in the literature.1,2 BFH of the spine traditionally causes nonspecific, poorly localized pain. The Table lists the reported cases of spinal BFH and their presenting symptoms, location, and treatment. BFH usually occurs in young adults, but the age range is 5 to 75 years.2-4 Mean age of the 12 patients with spinal BFH in the literature (including ours) is 25 years.1 In addition, spinal BFH appears to have no predilection for sex.
Skeletal BFH presents as a discrete, well-defined, osteolytic lesion with sharp borders and potentially a sclerotic rim.4-6 Cortical expansion and even cortical disruption with invasion into adjacent tissue have occurred in flat bones.7 Histologically, BFHs contain spindle cells, multinucleated giant cells, and foam cells in storiform pattern.6
BFH shares many of its radiologic and histologic characteristics and clinical symptoms with other benign bone lesions (the tumors listed above). Therefore, accurate diagnosis of BFH requires appropriate correlation of clinical, radiographic, and histologic data.2,3,8 Below is a comparison of BFH with related bone lesions.
Spinal BFH causes a nonspecific, poorly localized pain similar to that of EG, ABC, GCT, and OB.3,9 NOF and fibrous dysplasia generally do not cause pain, unless these lesions are discovered secondary to a pathologic fracture.8,10,11 Our patient had minor antecedent neck pain, which was brought to light by his football accident. ABC and OB are more locally aggressive than BFH and can cause neurologic symptoms by mass effect and spinal cord or nerve root compression.1,8 In this case and in the 6 other cases of BFH of the cervical spine, there were no neurologic changes.4,10
Of the tumors mentioned, NOF and EG almost always occur in children. However, NOF usually occurs in the metaphyseal region of long bones, and EG is usually accompanied by systemic symptoms, such as lymphadenopathy, hepatomegaly, and increased inflammatory markers.1,8 Fibrous dysplasia usually presents in childhood but does not become symptomatic until adulthood. GCTs and OB predominantly occur in adulthood.12,13 Our patient’s age and lack of other systemic symptoms supported the diagnosis of BFH.
Appearance on MRI is reported less with BFH than with other tumors, but heterogenous signal intensity similar to that of skeletal muscle on T1-weighted images and high signal intensity on T2-weighted images is typically reported.8,14 NOF and fibrous dysplasia do not disrupt the bony cortex unless a pathologic fracture has occurred.4 GCTs are more aggressive lytic lesions with more aggressive radiologic features. GCTs generally cause cortical expansion/attenuation, and lack a sclerotic rim. GCTs also have a heterogenous appearance on MRI and give a low to intermediate signal on both T1- and T2-weighted images.12,15 The appearance of EG is similar to that of BFH as an osteolytic lesion with a sclerotic rim, though EGs typically break through the cortex and acquire a “punched-out” look.1,8 ABC typically is described as an expansile osteolytic lesion with a “soap-bubble” appearance on radiographs; periosteal elevation and cortical attenuation can also be visualized. MRI shows the typical multilobular appearance of the lesion with fluid levels.13
OB appears as a radiolucent lesion, with or without calcifications, surrounded by a thin margin of reactive bone.14,16 A distinguishing characteristic of OB was thought to be intense radioisotope uptake on bone scintigraphy, but recently a bony BFH demonstrated intense uptake.17 OBs typically demonstrate nonspecific MRI results similar to those of BFH: low to intermediate signal on T1-weighted images and intermediate to high signal on T2-weighted images.13 In our patient’s case, the radiographic appearance and lack of specific radiographic findings consistent with the other tumors supported the diagnosis of BFH.
Histologically, BFHs contain spindle cells, multinucleated giant cells, and foam cells in a storiform pattern6 which was demonstrated in our patient’s case. In addition, significant nuclear pleomorphism, mitotic activity, and necrosis were absent—a difference between BFH and malignant fibrous histiocytoma.4,15 The microscopic characteristics of BFH readily differentiate it from OB, ABC, EG, and GCT, but not from NOF on microscopic appearance alone. Clinical and radiographic findings must be consistent, as mentioned.7,18
Complete surgical excision is the reported treatment for BFH. Prognosis after resection or curettage is usually good, and recurrences have been rare.1,2 Depending on the intraspinous location of BFH, stabilization after resection or curettage may be necessary to prevent residual instability. Three of the 11 reported cases of spinal BFH required stabilization by anterior fusion or posterior pedicle screw fixation after resection.1,2 The other 8 cases underwent excision alone or excision and grafting. All 11 patients were disease-free at a mean follow-up of 3.5 years.1 In nonspinal BFH, however, both local recurrence and lung metastasis have been reported.2,5,9,19 Clarke and colleagues9 reported local recurrences in 3 of 8 cases. These recurrences involved BFH in long bones of the leg, which had been treated with curettage and grafting. There has been no reliable report of a malignant change in BFH.2,9 The only case of lung metastasis, reported by Unni and Dahlin6 in their study of 10 cases, occurred 2 years after local recurrence in the distal femur.Our patient was doing well at most recent follow-up, 6 months after surgery. He had no pain and had returned to normal activities. Although there are no reported cases of spinal BFH recurrence, we will follow this patient with imaging on an annual basis. His case is of particular interest to orthopedic surgeons because they encounter benign bone lesions every day, and many of these lesions are in difficult anatomical locations. Knowing the characteristics, differential diagnoses, and appropriate diagnostic workups for benign bone lesions is important for optimal and timely patient care.
Benign fibrous histiocytoma (BFH) is a rare, well-recognized, primary skeletal tumor accounting for approximately 1% of all benign bone tumors. Spinal involvement is exceedingly rare with only 11 cases reported in the literature.1,2 We present a case of BFH located in the cervical spine of a pediatric patient that was successfully treated with curretage through an anterior surgical approach, along with a review of the literature and appropriate management concerning BFH of the spine.
Case Report
A 14-year-old boy was tackled while playing football and noticed immediate neck pain and subjective paresthesia in the upper extremities. Examination revealed a nontender spine (cervical, thoracic, lumbar) and normal strength and range of motion in all extremities. Sensation was diffusely intact, long tract signs were absent, and gait was normal. On questioning, the patient endorsed mild antecedent neck pain but denied prior history of any trauma. Neck pain did not radiate and was slightly worsened by activity but was mostly intermittent and random. As the neck pain was very mild and was not interfering with daily activities, the patient had not sought care before presenting to the emergency department. He had no pertinent past medical or surgical history.
The patient presented with a computed tomography (CT) scan of his head and cervical spine and a magnetic resonance imaging (MRI) scan of the cervical spine. A magnetic resonance angiography (MRA) scan of the neck was ordered after his arrival.
Axial and sagittal CT (Figures 1A, 1B) showed a 1×1.2-cm discrete, expansile, lytic, radiolucent mass extending anterior from the left C2 vertebral body. The mass appeared to abut the left vertebral artery foramen. The cortical bone surrounding the lesion was thin but uniform. Sagittal and axial T1-weighted MRI (Figures 2A, 2B) showed the discrete, expansile, homogenous lesion with the same intensity as normal bone marrow. Sagittal and axial T2-weighted MRI (Figures 2C, 2D) showed a discrete, expansile, homogenous lesion with primarily high signal intensity. Sagittal short tau inversion recovery (STIR) MRI (Figure 2E) again showed the lesion with primarily low intensity. Given the close proximity of the lesion to the vertebral foramen, MRA was ordered; it showed the lesion was not interfering with the vertebral artery (Figure 2F).
The tumor’s location, in the left anterior aspect of the C2 vertebral body, was not conducive to percutaneous biopsy for establishing tissue diagnosis, so the decision was made to surgically excise the lesion. A left-sided anterior incision was made 2 fingerbreadths inferior to the jaw line in a neck crease. A head and neck surgeon assisted with dissection. Dissection was carried down through the skin, subcutaneous tissue, and platysma on to the anterior part of the spine medial to the carotid sheath. Superior thyroid nerve and vessels and superior laryngeal nerve were identified and preserved. Fluoroscopy confirmed correct location at C2. The tumor was easily visualized, and the outer shell broke easily with palpation. Gentle curettage was necessary when removing the tumor off the vertebral artery. A portion of the specimen was sent during surgery for frozen section, which showed infrequent mitotic figures and no other findings concerning for malignancy. No instability was created after curettage and excision of the tumor, so no grafting or instrumentation was necessary.
Grossly, the tumor was pale tan and firm. Histologic examination with hematoxylin-eosin staining revealed a bland spindle-cell neoplasm that focally involved bone. A storiform pattern was present. The cells had scant cytoplasm and oval to elongate nuclei with tapered ends. Significant nuclear pleomorphism was not seen. The stroma was loose, with focal myxoid change. Benign multinucleated giant cells were present. Mitotic activity was infrequent (Figures 3A–3D). Two attending pathologists reviewed the case material and the frozen and formalin-fixed specimens independently and concurred with the diagnosis of BFH. In addition, the case was reviewed at the surgical pathology consensus conference; the reviewers agreed on BFH, and additional studies were deemed unnecessary.
Given the patient’s complete clinical picture, the differential diagnosis included nonossifying fibroma (NOF), eosinophilic granuloma (EG), BFH, fibrous dysplasia, giant cell tumor (GCT), aneurysmal bone cyst (ABC), and osteoblastoma (OB).
Discussion
BFH is an extremely rare bone lesion, accounting for only 1% of all surgically managed bone tumors; not counting the present case, only 11 spine cases have been reported in the literature.1,2 BFH of the spine traditionally causes nonspecific, poorly localized pain. The Table lists the reported cases of spinal BFH and their presenting symptoms, location, and treatment. BFH usually occurs in young adults, but the age range is 5 to 75 years.2-4 Mean age of the 12 patients with spinal BFH in the literature (including ours) is 25 years.1 In addition, spinal BFH appears to have no predilection for sex.
Skeletal BFH presents as a discrete, well-defined, osteolytic lesion with sharp borders and potentially a sclerotic rim.4-6 Cortical expansion and even cortical disruption with invasion into adjacent tissue have occurred in flat bones.7 Histologically, BFHs contain spindle cells, multinucleated giant cells, and foam cells in storiform pattern.6
BFH shares many of its radiologic and histologic characteristics and clinical symptoms with other benign bone lesions (the tumors listed above). Therefore, accurate diagnosis of BFH requires appropriate correlation of clinical, radiographic, and histologic data.2,3,8 Below is a comparison of BFH with related bone lesions.
Spinal BFH causes a nonspecific, poorly localized pain similar to that of EG, ABC, GCT, and OB.3,9 NOF and fibrous dysplasia generally do not cause pain, unless these lesions are discovered secondary to a pathologic fracture.8,10,11 Our patient had minor antecedent neck pain, which was brought to light by his football accident. ABC and OB are more locally aggressive than BFH and can cause neurologic symptoms by mass effect and spinal cord or nerve root compression.1,8 In this case and in the 6 other cases of BFH of the cervical spine, there were no neurologic changes.4,10
Of the tumors mentioned, NOF and EG almost always occur in children. However, NOF usually occurs in the metaphyseal region of long bones, and EG is usually accompanied by systemic symptoms, such as lymphadenopathy, hepatomegaly, and increased inflammatory markers.1,8 Fibrous dysplasia usually presents in childhood but does not become symptomatic until adulthood. GCTs and OB predominantly occur in adulthood.12,13 Our patient’s age and lack of other systemic symptoms supported the diagnosis of BFH.
Appearance on MRI is reported less with BFH than with other tumors, but heterogenous signal intensity similar to that of skeletal muscle on T1-weighted images and high signal intensity on T2-weighted images is typically reported.8,14 NOF and fibrous dysplasia do not disrupt the bony cortex unless a pathologic fracture has occurred.4 GCTs are more aggressive lytic lesions with more aggressive radiologic features. GCTs generally cause cortical expansion/attenuation, and lack a sclerotic rim. GCTs also have a heterogenous appearance on MRI and give a low to intermediate signal on both T1- and T2-weighted images.12,15 The appearance of EG is similar to that of BFH as an osteolytic lesion with a sclerotic rim, though EGs typically break through the cortex and acquire a “punched-out” look.1,8 ABC typically is described as an expansile osteolytic lesion with a “soap-bubble” appearance on radiographs; periosteal elevation and cortical attenuation can also be visualized. MRI shows the typical multilobular appearance of the lesion with fluid levels.13
OB appears as a radiolucent lesion, with or without calcifications, surrounded by a thin margin of reactive bone.14,16 A distinguishing characteristic of OB was thought to be intense radioisotope uptake on bone scintigraphy, but recently a bony BFH demonstrated intense uptake.17 OBs typically demonstrate nonspecific MRI results similar to those of BFH: low to intermediate signal on T1-weighted images and intermediate to high signal on T2-weighted images.13 In our patient’s case, the radiographic appearance and lack of specific radiographic findings consistent with the other tumors supported the diagnosis of BFH.
Histologically, BFHs contain spindle cells, multinucleated giant cells, and foam cells in a storiform pattern6 which was demonstrated in our patient’s case. In addition, significant nuclear pleomorphism, mitotic activity, and necrosis were absent—a difference between BFH and malignant fibrous histiocytoma.4,15 The microscopic characteristics of BFH readily differentiate it from OB, ABC, EG, and GCT, but not from NOF on microscopic appearance alone. Clinical and radiographic findings must be consistent, as mentioned.7,18
Complete surgical excision is the reported treatment for BFH. Prognosis after resection or curettage is usually good, and recurrences have been rare.1,2 Depending on the intraspinous location of BFH, stabilization after resection or curettage may be necessary to prevent residual instability. Three of the 11 reported cases of spinal BFH required stabilization by anterior fusion or posterior pedicle screw fixation after resection.1,2 The other 8 cases underwent excision alone or excision and grafting. All 11 patients were disease-free at a mean follow-up of 3.5 years.1 In nonspinal BFH, however, both local recurrence and lung metastasis have been reported.2,5,9,19 Clarke and colleagues9 reported local recurrences in 3 of 8 cases. These recurrences involved BFH in long bones of the leg, which had been treated with curettage and grafting. There has been no reliable report of a malignant change in BFH.2,9 The only case of lung metastasis, reported by Unni and Dahlin6 in their study of 10 cases, occurred 2 years after local recurrence in the distal femur.Our patient was doing well at most recent follow-up, 6 months after surgery. He had no pain and had returned to normal activities. Although there are no reported cases of spinal BFH recurrence, we will follow this patient with imaging on an annual basis. His case is of particular interest to orthopedic surgeons because they encounter benign bone lesions every day, and many of these lesions are in difficult anatomical locations. Knowing the characteristics, differential diagnoses, and appropriate diagnostic workups for benign bone lesions is important for optimal and timely patient care.
1. Demiralp B, Kose O, Oguz E, Sanal T, Ozcan A, Sehirlioglu A. Benign fibrous histiocytoma of the lumbar vertebrae. Skeletal Radiol. 2009;38(2):187-191.
2. Kuruvath S, O’Donovan DG, Aspoas AR, David KM. Benign fibrous histiocytoma of the thoracic spine: case report and review of the literature. J Neurosurg Spine. 2006;4(3):260-264.
3. Ceroni D, Dayer R, De Coulon G, Kaelin A. Benign fibrous histiocytoma of bone in a paediatric population: a report of 6 cases. Musculoskelet Surg. 2011;95(2):107-114.
4. Dorfman HD, Czerniak B. Bone Tumors. St. Louis, MO: Mosby; 1998.
5. Grohs JG, Nicolakis M, Kainberger F, Lang S, Kotz R. Benign fibrous histiocytoma of bone: a report of ten cases and review of literature. Wien Klin Wochenschr. 2002;114(1-2):56-63.
6. Unni KK, Dahlin DC. Dahlin’s Bone Tumors. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
7. Balasubramanian C, Rajaraman G, Singh CS, Baliga DK. Benign fibrous histiocytoma of the sacrum—diagnostic difficulties facing this rare bone tumor. Pediatr Neurosurg. 2005;41(5):253-257.
8. van Giffen NH, van Rhijn LW, van Ooij A, et al. Benign fibrous histiocytoma of the posterior arch of C1 in a 6-year old boy: a case report. Spine. 2003;28(18):E359-E363.
9. Clarke BE, Xipell JM, Thomas DP. Benign fibrous histiocytoma of bone. Am J Surg Pathol. 1985;9(11):806-815.
10. Peicha G, Siebert FJ, Bratschitsch G, Fankhauser F, Grechenig W. Pathologic odontoid fracture and benign fibrous histiocytoma of bone. Eur Spine J. 1999;8(2):161-163.
11. Unni KK, Inwards CY, Bridge JA, Kindblom LG, Wold LE. Tumors of the Bones and Joints (AFIP Atlas of Tumor Pathology Series IV). Annapolis Junction, MD: American Registry of Pathology Press; 2005.
12. Dee R. Principles of Orthopaedic Practice. 2nd ed. New York, NY: McGraw-Hill; 1997.
13. Murphey M, Andrews C, Flemming D, Temple HT, Smith WS, Smirniotopoulos JG. Primary tumors of the spine: radiologic–pathologic correlation. Radiographics. 1996;16(5):1131-1158.
14. Hamada T, Ito H, Araki Y, Fujii K, Inoue M, Ishida O. Benign fibrous histiocytoma of the femur: review of three cases. Skeletal Radiol. 1996;25(1):25-29.
15. Mirra JM, Picci P, Gold RH. Bone Tumors: Clinical, Radiologic, and Pathologic Correlations. Vol 1. Philadelphia, PA: Lea & Febiger; 1989.
16. Theodorou DJ, Theodorou SJ, Sartoris DJ. An imaging overview of primary tumors of the spine: part 1. Benign tumors. Clin Imaging. 2008;32(3):196-203.
17. Li X, Meng Z, Li D, Tan J, Song X. Benign fibrous histiocytoma of a rib. Clin Nucl Med. 2014;39(9): 837-841.
18. Roessner A, Immenkamp M, Weidner A, Hobik HP, Grundmann E. Benign fibrous histiocytoma of bone. Light- and electron-microscopic observations. J Cancer Res Clin Oncol. 1981;101(2):191-202.
19. Destouet JM, Kyriakos M, Gilula LA. Fibrous histiocytoma (fibroxanthoma) of a cervical vertebra. A report with a review of the literature. Skeletal Radiol. 1980;5(4):241-246.
20. Hoeffel JC, Bomand-Ferrand F, Tachet F, Lascombes P, Czorny A, Bernard C. So-called benign fibrous histiocytoma: report of a case. J Pediatr Surg. 1992;27(5):672-674.
1. Demiralp B, Kose O, Oguz E, Sanal T, Ozcan A, Sehirlioglu A. Benign fibrous histiocytoma of the lumbar vertebrae. Skeletal Radiol. 2009;38(2):187-191.
2. Kuruvath S, O’Donovan DG, Aspoas AR, David KM. Benign fibrous histiocytoma of the thoracic spine: case report and review of the literature. J Neurosurg Spine. 2006;4(3):260-264.
3. Ceroni D, Dayer R, De Coulon G, Kaelin A. Benign fibrous histiocytoma of bone in a paediatric population: a report of 6 cases. Musculoskelet Surg. 2011;95(2):107-114.
4. Dorfman HD, Czerniak B. Bone Tumors. St. Louis, MO: Mosby; 1998.
5. Grohs JG, Nicolakis M, Kainberger F, Lang S, Kotz R. Benign fibrous histiocytoma of bone: a report of ten cases and review of literature. Wien Klin Wochenschr. 2002;114(1-2):56-63.
6. Unni KK, Dahlin DC. Dahlin’s Bone Tumors. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
7. Balasubramanian C, Rajaraman G, Singh CS, Baliga DK. Benign fibrous histiocytoma of the sacrum—diagnostic difficulties facing this rare bone tumor. Pediatr Neurosurg. 2005;41(5):253-257.
8. van Giffen NH, van Rhijn LW, van Ooij A, et al. Benign fibrous histiocytoma of the posterior arch of C1 in a 6-year old boy: a case report. Spine. 2003;28(18):E359-E363.
9. Clarke BE, Xipell JM, Thomas DP. Benign fibrous histiocytoma of bone. Am J Surg Pathol. 1985;9(11):806-815.
10. Peicha G, Siebert FJ, Bratschitsch G, Fankhauser F, Grechenig W. Pathologic odontoid fracture and benign fibrous histiocytoma of bone. Eur Spine J. 1999;8(2):161-163.
11. Unni KK, Inwards CY, Bridge JA, Kindblom LG, Wold LE. Tumors of the Bones and Joints (AFIP Atlas of Tumor Pathology Series IV). Annapolis Junction, MD: American Registry of Pathology Press; 2005.
12. Dee R. Principles of Orthopaedic Practice. 2nd ed. New York, NY: McGraw-Hill; 1997.
13. Murphey M, Andrews C, Flemming D, Temple HT, Smith WS, Smirniotopoulos JG. Primary tumors of the spine: radiologic–pathologic correlation. Radiographics. 1996;16(5):1131-1158.
14. Hamada T, Ito H, Araki Y, Fujii K, Inoue M, Ishida O. Benign fibrous histiocytoma of the femur: review of three cases. Skeletal Radiol. 1996;25(1):25-29.
15. Mirra JM, Picci P, Gold RH. Bone Tumors: Clinical, Radiologic, and Pathologic Correlations. Vol 1. Philadelphia, PA: Lea & Febiger; 1989.
16. Theodorou DJ, Theodorou SJ, Sartoris DJ. An imaging overview of primary tumors of the spine: part 1. Benign tumors. Clin Imaging. 2008;32(3):196-203.
17. Li X, Meng Z, Li D, Tan J, Song X. Benign fibrous histiocytoma of a rib. Clin Nucl Med. 2014;39(9): 837-841.
18. Roessner A, Immenkamp M, Weidner A, Hobik HP, Grundmann E. Benign fibrous histiocytoma of bone. Light- and electron-microscopic observations. J Cancer Res Clin Oncol. 1981;101(2):191-202.
19. Destouet JM, Kyriakos M, Gilula LA. Fibrous histiocytoma (fibroxanthoma) of a cervical vertebra. A report with a review of the literature. Skeletal Radiol. 1980;5(4):241-246.
20. Hoeffel JC, Bomand-Ferrand F, Tachet F, Lascombes P, Czorny A, Bernard C. So-called benign fibrous histiocytoma: report of a case. J Pediatr Surg. 1992;27(5):672-674.
Clinical Outcomes of Minimally Invasive Versus Open TLIF: A Propensity-Matched Cohort Study
Transforaminal lumbar interbody fusion (TLIF) has become an increasingly popular method of lumbar fusion, since its introduction by Harms and Rolinger in 1982.1 The procedure allows for a circumferential fusion through a posterior-only approach, with improved sagittal alignment2 and minimal risk for iatrogenic nerve injury. In the past decade, a minimally invasive surgical method of TLIF (MIS TLIF) has been introduced3-5 and involves neural decompression and interbody fusion through a tubular retractor, and percutaneous placement of pedicle-screw instrumentation. This technique uses muscle dilation rather than large-scale detachment of muscle. Proponents of the MIS technique have postulated that decreased muscle damage would lead to better short-term, and possibly long-term, clinical outcomes, because of less iatrogenic soft-tissue damage.
Studies that have compared results of MIS TLIF with open TLIF have shown improved perioperative outcomes, but most have shown similar intermediate-term clinical outcomes.6 In the short term, multiple studies demonstrate that MIS TLIF is associated with decreased blood loss, less postoperative pain and narcotic requirements, and shorter hospital length of stay.7-13 However, changes in pain score and disease-specific and generic health-related quality of life measures have been similar for the 2 procedures, beyond 6 months postoperatively.10,13-15 These studies have generally involved retrospective reviews of unmatched patient groups, with small sample sizes and significant heterogeneity in surgical indications and case complexity. In our study, we compared intermediate-term clinical outcomes of MIS TLIF with open TLIF, using propensity matching to optimize baseline similarity of the groups.
Methods
This retrospective study was conducted after receiving approval from the Institutional Review Board. Surgical and clinical databases of 2 centers from 2008 to 2012 were reviewed for eligible subjects. Cases in 2007 were excluded because this was the year that MIS was introduced as a new technique in the practice. Inclusion criteria consisted of patients who underwent 1- to 2-level MIS TLIF and had complete baseline, 1- and 2-year postoperative outcome measures. Patients who had surgery for trauma, tumor, or osteomyelitis were excluded. Outcome measures collected and reviewed in this study included the Oswestry Disability Index (ODI),16,17 the Medical Outcomes Study Short-Form 36 (SF-36),18 and numeric rating scales for back and leg pain (0-100 scale).19 The Physical Composite Summary (PCS) and Mental Composite Summary of the SF-36 were reviewed separately. We recorded the following patient demographic data: age, gender, American Society of Anesthesiologists (ASA) grade, body mass index, indication for surgery, workers’ compensation, and smoking status. Surgical data included number of levels fused, operative time, estimated blood loss, and length of hospital stay.
Propensity-scoring technique20,21 was used to match the MIS TLIF patients to a control group of patients who underwent TLIF using an open approach (open TLIF), matching for multiple characteristics to produce 2 similar comparison groups. Propensity matching was performed to control for bias. In controlling for known confounders or biases, propensity matching, in theory, should also control for unknown confounders. Gender, age, body mass index, smoking status, indication for fusion, as well as preoperative ODI, SF-36 PCS, SF-36 Mental Composite Summary, and pain scores were used to generate a control open TLIF group.
MIS TLIF Surgical Technique
Patients in the MIS TLIF group underwent neural decompression and interbody fusion through a tubular retractor system (METRx, Medtronic Inc.), followed by percutaneous pedicle-screw fixation under fluoroscopic guidance (Sextant, Medtronic Inc.). After successful induction of general endotracheal anesthesia, patients were positioned prone on a radiolucent table. Posteroanterior (PA) and lateral fluoroscopic images were used to localize 2 paramedian incisions, approximately 3-cm to 5-cm lateral to midline, over the pedicles of interest. Modified Jamshidi needles (Medtronic Inc.) were used to cannulate the pedicles under PA, posterior-oblique, PA, and lateral fluoroscopic guidance. The pedicles were tapped with a cannulated tap. Pedicle screws and rods were introduced on the side contralateral to the TLIF and were used as needed to maintain intradiscal distraction during the TLIF portion of the procedure.
Decompression and TLIF were carried out on the side of the patient’s radicular pain or bilaterally, according to the surgeon’s discretion. A K-wire was advanced to the facet joint complex, after which sequential dilators were used to dilate through the muscles to establish an intramuscular corridor to the facet. A 26-mm fixed tubular retractor was docked over the facet and locked in place, using a post attached to the operating room table. Neural decompression was obtained by removal of the entire facet-joint complex and lamina to the base of the spinous process, using a combination of high-speed drills and Kerrison rongeurs. The ligamentum flavum was completely resected. The superior articular process of the caudal vertebra was removed all the way to the pedicle below. Ball-tipped probes were used to confirm that traversing and exiting nerve roots were completely free. An annulotomy was performed, and all disc material was removed from the disc through a combination of rotating shavers, serrated curettes, endplate scrapers, and rasps. Bone graft was placed anterior and contralateral to the interbody cage. (Bone grafts included autogenous iliac crest, local bone obtained from the decompression, recombinant human bone morphogenetic protein 2, or allograft demineralized bone matrix at the surgeon’s discretion.) After placement of the interbody cage, the ipsilateral pedicle-screw instrumentation was put over the remaining guide wires and compression applied across the construct to lock the interbody cage and restore lordosis. Wounds were closed without drains.
Open TLIF Surgical Technique
In patients undergoing open TLIF, a midline incision was made over the vertebrae of interest, and paraspinal muscles were subperiosteally dissected to the tips of the transverse processes. The appropriate level was confirmed with intraoperative radiograph. Pedicle screws were placed free-hand using anatomic landmarks, and appropriate placement was confirmed with intraoperative radiograph and evoked electromyography stimulation. Laminectomy and facetectomy were performed, and the disc was entered on the side of the facetectomy. After thorough disc-space preparation, bone graft and an interbody cage were placed, rods inserted, and compression carried out. A supplemental posterolateral fusion was also performed after decortication of the transverse processes and cartilaginous surface of the contralateral facet. Layered wound closure was performed over drains.
Analysis
Statistical analysis was carried out using SPSS Statistics version 17.0 (IBM) with significance set at the P < .01 level. A small, conservative P-value threshold was used to minimize type II error that resulted from the multiple comparisons performed. Student t test was used to determine any significant differences between continuous demographic variables, and to compare preoperative and postoperative outcome measure scores within and between study groups. Fisher’s exact test was used to compare categorical variables between the 2 groups.
Results
The MIS TLIF group consisted of 64 patients (average age, 52 years), and included 22 patients with degenerative spondylolisthesis, 33 with disc pathology, 8 with postdecompression, and 1 non-union patient. The open TLIF group consisted of 64 patients (average age, 54 years), and included 39 degenerative spondylolisthesis, 15 disc pathology, 7 postdecompression, and 3 nonunion patients (Table 1). All 64 open and 19 MIS cases were from a spine practice with 6 surgeons, and 45 MIS cases came from a spine practice with 2 surgeons. There was also an unequal distribution of the specific levels fused between the open and MIS groups.
Although the operative time was similar in both groups, the MIS TLIF group had a statistically significantly lower blood loss compared with the open TLIF group (Table 2). Both MIS TLIF and open TLIF lead to significant improvements in pain, ODI, and SF-36 PCS (P < .01) (Table 3). At 1 year, both groups had similar improvements in pain (36.9 vs 30.8, P = .178) and SF-36 PCS (9.9 vs 7.5, P = .231), but the MIS TLIF group had a statistically significantly greater improvement in ODI compared with the open TLIF group (30.4 vs 15.1, P < .000). At 2 years, both groups had similar improvements in SF-36 PCS (12.1 vs 7.5, P = .033), but the MIS TLIF group had a statistically significantly greater improvement in pain (40.2 vs 27.0, P = .005) and ODI (33.1 vs 15.4, P < .000) compared with the open TLIF group (Table 4).
Discussion
The current study compared intermediate-term clinical outcomes of MIS TLIF to open TLIF. We used propensity matching to identify a control group of open TLIFs that were comparable to the MIS TLIF group across a variety of covariates that are known to influence the results of lumbar fusion. This created comparison groups that were as closely matched at baseline as possible. We found that, at 2-year follow-up, MIS TLIF patients had less pain and less low-back pain–related disability as measured by ODI. There was also a trend toward better generic health-related quality of life in the MIS TLIF group.
These data suggest that the decreased soft-tissue trauma of the minimally invasive surgical technique, which leads to improved perioperative parameters in the short term, may also lead to some advantages that translate to improved intermediate-term clinical outcomes. Traditional lumbar fusion procedures have shown excellent clinical results when used for accepted clinical indications.22 However, the procedure requires extensive dissection of the paraspinal muscles, which causes significant muscle damage as evidenced by muscle breakdown products that can be detected in the bloodstream postoperatively.23,24 The lateral dissection also transects the dorsal ramus of the segmental nerves, which innervate the paraspinal muscles, leading to significant scarring and atrophy on postoperative imaging studies.23 Some authors have used the term “fusion disease” to describe the constellation of soft-tissue degradation seen after open lumbar fusion.5
An MIS version of the TLIF procedure that was described in 20033 avoids much of this iatrogenic soft-tissue trauma. It involves intramuscular dilation to approach the spine and to carry out neural decompression and interbody fusion, in conjunction with percutaneous pedicle-screw instrumentation. Proponents of this technique point to diminished iatrogenic soft-tissue and muscle damage as an advantage. Multiple studies have, in fact, confirmed improved short-term perioperative parameters, such as less blood loss, lower narcotic requirements, and decreased length-of-hospital stay.25 Economic analyses have also shown lower direct and indirect costs with the MIS technique.26
Several studies have compared patient-reported outcome measures of MIS and open TLIF, and the results have been mixed. Most of these studies have shown similar improvement in clinical outcomes between the 2 procedures, but the MIS technique demonstrated short-term perioperative advantages, such as lower blood loss, less narcotic requirements, and shorter length of stay.7-15 The authors of these studies conclude that the MIS technique can provide similar long-term results with lower short-term morbidity when compared with open TLIF. In contrast, some studies have shown better short- and intermediate-term clinical outcomes with the MIS technique.23,27-29 As a whole, the literature comparing the 2 procedures consists of mostly small retrospective studies with nonrandomized patient samples, heterogeneous surgical indications, and differing surgical techniques, making it difficult to draw conclusions.
The current study suggests that MIS TLIF may lead to improved clinical results at 2-year follow-up, compared with open TLIF. Our study used propensity-score matching to minimize the effects of nonrandom assignment of subjects to MIS TLIF or open TLIF. A limitation of observational studies is that bias in assignment of subjects to treatment groups can lead to overestimation or underestimation of the effect of the treatment itself. Propensity-score matching attempts to reduce this bias by accounting for several covariates that predict whether a subject will receive a certain treatment. These covariates are used in a logistic regression to produce a propensity score, which can be used to match subjects to controls across multiple dimensions, thus ensuring groups are as comparable as possible at baseline.
Our study still has several limitations. Sample size is relatively small, and follow-up is still only intermediate, at 2 years. There was unequal distribution of specific levels of surgery. Because patients were not blinded to the treatment they received, it is possible that patient perception of receiving a newer, less-invasive treatment method may influence their subjective improvement. The study sample was drawn from 2 different centers, with one center providing mostly MIS cases and the other providing mostly open cases. Because of this, undetected differences in how patients were selected for surgery could also affect outcomes. Any latent confounding variables, which are not identified a priori, will not be accounted for in the matching process. Only a prospective, randomized study with large numbers can control for observed and unobserved confounding patient characteristics.
In summary, our study shows that MIS TLIF is associated with improved low back pain and low back–related disability at 2 years compared with open TLIF. Other studies comparing the 2 techniques have come to different conclusions regarding whether the short-term benefits of MIS TLIF translate into long-term differences in clinical outcome. This study adds to this evidence and suggests there may be longer term advantages to the MIS approach, but prospective randomized trials are needed to confirm this finding and determine the true magnitude of these differences.
1. Harms J, Rolinger H. A one-stager procedure in operative treatment of spondylolisthesis: dorsal traction-reposition and anterior fusion (author’s transl). Z Orthop Ihre Grenzgeb. 1982;120(3):343-347.
2. Jagannathan J, Sansur CA, Oskouian RJ Jr, Fu KM, Shaffrey CI. Radiographic restoration of lumbar alignment after transforaminal lumbar interbody fusion. Neurosurgery. 2009;64(5):955-963.
3. Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine. 2003;28(15 suppl):S26-S35.
4. Rouben D, Casnellie M, Ferguson M. Long-term durability of minimally invasive posterior transforaminal lumbar interbody fusion: a clinical and radiographic follow-up. J Spinal Disord Tech. 2011;24(5):288-296.
5. Schwender JD, Holly LT, Rouben DP, Foley KT. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech. 2005;18(suppl):S1-S6.
6. Goldstein CL, Macwan K, Sundararajan K, Rampersaud YR. Comparative outcomes of minimally invasive surgery for posterior lumbar fusion: a systematic review. Clin Orthop Relat Res. 2014;472(6):1727-1737.
7. Adogwa O, Parker SL, Bydon A, Cheng J, McGirt MJ. Comparative effectiveness of minimally invasive versus open transforaminal lumbar interbody fusion: 2-year assessment of narcotic use, return to work, disability, and quality of life. J Spinal Disord Tech. 2011;24(8):479-484.
8. Ghahreman A, Ferch RD, Rao PJ, Bogduk N. Minimal access versus open posterior lumbar interbody fusion in the treatment of spondylolisthesis. Neurosurgery. 2010;66(2):296-304.
9. Park Y, Ha JW. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine. 2007;32(5):537-543.
10. Saetia K, Phankhongsab A, Kuansongtham V, Paiboonsirijit S. Comparison between minimally invasive and open transforaminal lumbar interbody fusion. J Med Assoc Thai. 2013;96(1):41-46.
11. Schizas C, Tzinieris N, Tsiridis E, Kosmopoulos V. Minimally invasive versus open transforaminal lumbar interbody fusion: evaluating initial experience. Int Ortop. 2009;33(6):1683-1688.
12. Wang J, Zhou Y, Zhang ZF, Li CQ, Zheng WJ, Liu J. Comparison of one-level minimally invasive and open transforaminal lumbar interbody fusion in degenerative and isthmic spondylolisthesis grades 1 and 2. Eur Spine J. 2010;19(1):1780-1784.
13. Lee KH, Yue WM, Yeo W, Soeharno H, Tan SB. Clinical and radiological outcomes of open versus minimally invasive transforaminal lumbar interbody fusion. Eur Spine J. 2012;21(11):2265-2270.
14. Peng CW, Yue WM, Poh SY, Yeo W, Tan SB. Clinical and radiological outcomes of minimally invasive versus open transforaminal lumbar interbody fusion. Spine. 2009;34(13):1385-1389.
15. Seng C, Siddiqui MA, Wong KP, et al. Five-year outcomes of minimally invasive versus open transforaminal lumbar interbody fusion: a matched-pair comparison study. Spine. 2013;38(23):2049-2055.
16. Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25(22):2940-2953.
17. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271-273.
18. Ware JE, Kosinski M, Keller SK. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA: The Health Institute, 1994.
19. McCaffery M, Beebe A. Pain: Clinical Manual for Nursing Practice. Baltimore, MD: V.V. Mosby Company, 1993.
20. D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265-2281.
21. Rosenbaum PR. Model-based direct adjustment. J Am Stat Assn. 1987;82:387-394.
22. Glassman SD, Carreon LY, Djurasovic M, et al. Lumbar fusion outcomes stratified by specific diagnostic indication. Spine J. 2009;9(1):13-21.
23. Fan S, Hu Z, Zhao F, Zhao X, Huang Y, Fang X. Multifidus muscle changes and clinical effects of one-level posterior lumbar interbody fusion: minimally invasive procedure versus conventional open approach. Eur Spine J. 2010;19(2):316-324.
24. Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. A histologic and enzymatic analysis. Spine. 1996;21(8):941-944.
25. Sun ZJ, Li WJ, Zhao Y, Qui GX. Comparing minimally invasive and open transforaminal lumbar interbody fusion for treatment of degenerative lumbar disease: a meta-analysis. Chin Med J. 2013;126(2):3962-3971.
26. Parker SL, Mendenhall SK, Shau DN, et al. Minimally invasive versus open transforaminal lumbar interbody fusion for degenerative spondylolisthesis: comparative effectiveness and cost-utility analysis. World Neurosurg. 2014;82(1-2):230-238.
27. Kotani Y, Abumi K, Ito M, Sudo H, Abe Y, Minami A. Mid-term clinical results of minimally invasive decompression and posterolateral fusion with percutaneous pedicle screws versus conventional approach for degenerative spondylolisthesis with spinal stenosis. Eur Spine J. 2012;21(6):1171-1177.
28. Pelton MA, Phillips FM, Singh K. A comparison of perioperative costs and outcomes in patients with and without worker’s compensation claims treated with MIS or open TLIF. Spine. 2012;37(22):1914-1919.
29. Wong AP, Smith ZA, Stadler JA 3rd, et al. Minimally invasive transforaminal lumbar interbody fusion (MI-TLIF). Surgical technique, long-term 4 year prospective outcomes and complications compared with an open TLIF cohort. Neurosurg Clin N Am. 2014;25(2):279-304.
Transforaminal lumbar interbody fusion (TLIF) has become an increasingly popular method of lumbar fusion, since its introduction by Harms and Rolinger in 1982.1 The procedure allows for a circumferential fusion through a posterior-only approach, with improved sagittal alignment2 and minimal risk for iatrogenic nerve injury. In the past decade, a minimally invasive surgical method of TLIF (MIS TLIF) has been introduced3-5 and involves neural decompression and interbody fusion through a tubular retractor, and percutaneous placement of pedicle-screw instrumentation. This technique uses muscle dilation rather than large-scale detachment of muscle. Proponents of the MIS technique have postulated that decreased muscle damage would lead to better short-term, and possibly long-term, clinical outcomes, because of less iatrogenic soft-tissue damage.
Studies that have compared results of MIS TLIF with open TLIF have shown improved perioperative outcomes, but most have shown similar intermediate-term clinical outcomes.6 In the short term, multiple studies demonstrate that MIS TLIF is associated with decreased blood loss, less postoperative pain and narcotic requirements, and shorter hospital length of stay.7-13 However, changes in pain score and disease-specific and generic health-related quality of life measures have been similar for the 2 procedures, beyond 6 months postoperatively.10,13-15 These studies have generally involved retrospective reviews of unmatched patient groups, with small sample sizes and significant heterogeneity in surgical indications and case complexity. In our study, we compared intermediate-term clinical outcomes of MIS TLIF with open TLIF, using propensity matching to optimize baseline similarity of the groups.
Methods
This retrospective study was conducted after receiving approval from the Institutional Review Board. Surgical and clinical databases of 2 centers from 2008 to 2012 were reviewed for eligible subjects. Cases in 2007 were excluded because this was the year that MIS was introduced as a new technique in the practice. Inclusion criteria consisted of patients who underwent 1- to 2-level MIS TLIF and had complete baseline, 1- and 2-year postoperative outcome measures. Patients who had surgery for trauma, tumor, or osteomyelitis were excluded. Outcome measures collected and reviewed in this study included the Oswestry Disability Index (ODI),16,17 the Medical Outcomes Study Short-Form 36 (SF-36),18 and numeric rating scales for back and leg pain (0-100 scale).19 The Physical Composite Summary (PCS) and Mental Composite Summary of the SF-36 were reviewed separately. We recorded the following patient demographic data: age, gender, American Society of Anesthesiologists (ASA) grade, body mass index, indication for surgery, workers’ compensation, and smoking status. Surgical data included number of levels fused, operative time, estimated blood loss, and length of hospital stay.
Propensity-scoring technique20,21 was used to match the MIS TLIF patients to a control group of patients who underwent TLIF using an open approach (open TLIF), matching for multiple characteristics to produce 2 similar comparison groups. Propensity matching was performed to control for bias. In controlling for known confounders or biases, propensity matching, in theory, should also control for unknown confounders. Gender, age, body mass index, smoking status, indication for fusion, as well as preoperative ODI, SF-36 PCS, SF-36 Mental Composite Summary, and pain scores were used to generate a control open TLIF group.
MIS TLIF Surgical Technique
Patients in the MIS TLIF group underwent neural decompression and interbody fusion through a tubular retractor system (METRx, Medtronic Inc.), followed by percutaneous pedicle-screw fixation under fluoroscopic guidance (Sextant, Medtronic Inc.). After successful induction of general endotracheal anesthesia, patients were positioned prone on a radiolucent table. Posteroanterior (PA) and lateral fluoroscopic images were used to localize 2 paramedian incisions, approximately 3-cm to 5-cm lateral to midline, over the pedicles of interest. Modified Jamshidi needles (Medtronic Inc.) were used to cannulate the pedicles under PA, posterior-oblique, PA, and lateral fluoroscopic guidance. The pedicles were tapped with a cannulated tap. Pedicle screws and rods were introduced on the side contralateral to the TLIF and were used as needed to maintain intradiscal distraction during the TLIF portion of the procedure.
Decompression and TLIF were carried out on the side of the patient’s radicular pain or bilaterally, according to the surgeon’s discretion. A K-wire was advanced to the facet joint complex, after which sequential dilators were used to dilate through the muscles to establish an intramuscular corridor to the facet. A 26-mm fixed tubular retractor was docked over the facet and locked in place, using a post attached to the operating room table. Neural decompression was obtained by removal of the entire facet-joint complex and lamina to the base of the spinous process, using a combination of high-speed drills and Kerrison rongeurs. The ligamentum flavum was completely resected. The superior articular process of the caudal vertebra was removed all the way to the pedicle below. Ball-tipped probes were used to confirm that traversing and exiting nerve roots were completely free. An annulotomy was performed, and all disc material was removed from the disc through a combination of rotating shavers, serrated curettes, endplate scrapers, and rasps. Bone graft was placed anterior and contralateral to the interbody cage. (Bone grafts included autogenous iliac crest, local bone obtained from the decompression, recombinant human bone morphogenetic protein 2, or allograft demineralized bone matrix at the surgeon’s discretion.) After placement of the interbody cage, the ipsilateral pedicle-screw instrumentation was put over the remaining guide wires and compression applied across the construct to lock the interbody cage and restore lordosis. Wounds were closed without drains.
Open TLIF Surgical Technique
In patients undergoing open TLIF, a midline incision was made over the vertebrae of interest, and paraspinal muscles were subperiosteally dissected to the tips of the transverse processes. The appropriate level was confirmed with intraoperative radiograph. Pedicle screws were placed free-hand using anatomic landmarks, and appropriate placement was confirmed with intraoperative radiograph and evoked electromyography stimulation. Laminectomy and facetectomy were performed, and the disc was entered on the side of the facetectomy. After thorough disc-space preparation, bone graft and an interbody cage were placed, rods inserted, and compression carried out. A supplemental posterolateral fusion was also performed after decortication of the transverse processes and cartilaginous surface of the contralateral facet. Layered wound closure was performed over drains.
Analysis
Statistical analysis was carried out using SPSS Statistics version 17.0 (IBM) with significance set at the P < .01 level. A small, conservative P-value threshold was used to minimize type II error that resulted from the multiple comparisons performed. Student t test was used to determine any significant differences between continuous demographic variables, and to compare preoperative and postoperative outcome measure scores within and between study groups. Fisher’s exact test was used to compare categorical variables between the 2 groups.
Results
The MIS TLIF group consisted of 64 patients (average age, 52 years), and included 22 patients with degenerative spondylolisthesis, 33 with disc pathology, 8 with postdecompression, and 1 non-union patient. The open TLIF group consisted of 64 patients (average age, 54 years), and included 39 degenerative spondylolisthesis, 15 disc pathology, 7 postdecompression, and 3 nonunion patients (Table 1). All 64 open and 19 MIS cases were from a spine practice with 6 surgeons, and 45 MIS cases came from a spine practice with 2 surgeons. There was also an unequal distribution of the specific levels fused between the open and MIS groups.
Although the operative time was similar in both groups, the MIS TLIF group had a statistically significantly lower blood loss compared with the open TLIF group (Table 2). Both MIS TLIF and open TLIF lead to significant improvements in pain, ODI, and SF-36 PCS (P < .01) (Table 3). At 1 year, both groups had similar improvements in pain (36.9 vs 30.8, P = .178) and SF-36 PCS (9.9 vs 7.5, P = .231), but the MIS TLIF group had a statistically significantly greater improvement in ODI compared with the open TLIF group (30.4 vs 15.1, P < .000). At 2 years, both groups had similar improvements in SF-36 PCS (12.1 vs 7.5, P = .033), but the MIS TLIF group had a statistically significantly greater improvement in pain (40.2 vs 27.0, P = .005) and ODI (33.1 vs 15.4, P < .000) compared with the open TLIF group (Table 4).
Discussion
The current study compared intermediate-term clinical outcomes of MIS TLIF to open TLIF. We used propensity matching to identify a control group of open TLIFs that were comparable to the MIS TLIF group across a variety of covariates that are known to influence the results of lumbar fusion. This created comparison groups that were as closely matched at baseline as possible. We found that, at 2-year follow-up, MIS TLIF patients had less pain and less low-back pain–related disability as measured by ODI. There was also a trend toward better generic health-related quality of life in the MIS TLIF group.
These data suggest that the decreased soft-tissue trauma of the minimally invasive surgical technique, which leads to improved perioperative parameters in the short term, may also lead to some advantages that translate to improved intermediate-term clinical outcomes. Traditional lumbar fusion procedures have shown excellent clinical results when used for accepted clinical indications.22 However, the procedure requires extensive dissection of the paraspinal muscles, which causes significant muscle damage as evidenced by muscle breakdown products that can be detected in the bloodstream postoperatively.23,24 The lateral dissection also transects the dorsal ramus of the segmental nerves, which innervate the paraspinal muscles, leading to significant scarring and atrophy on postoperative imaging studies.23 Some authors have used the term “fusion disease” to describe the constellation of soft-tissue degradation seen after open lumbar fusion.5
An MIS version of the TLIF procedure that was described in 20033 avoids much of this iatrogenic soft-tissue trauma. It involves intramuscular dilation to approach the spine and to carry out neural decompression and interbody fusion, in conjunction with percutaneous pedicle-screw instrumentation. Proponents of this technique point to diminished iatrogenic soft-tissue and muscle damage as an advantage. Multiple studies have, in fact, confirmed improved short-term perioperative parameters, such as less blood loss, lower narcotic requirements, and decreased length-of-hospital stay.25 Economic analyses have also shown lower direct and indirect costs with the MIS technique.26
Several studies have compared patient-reported outcome measures of MIS and open TLIF, and the results have been mixed. Most of these studies have shown similar improvement in clinical outcomes between the 2 procedures, but the MIS technique demonstrated short-term perioperative advantages, such as lower blood loss, less narcotic requirements, and shorter length of stay.7-15 The authors of these studies conclude that the MIS technique can provide similar long-term results with lower short-term morbidity when compared with open TLIF. In contrast, some studies have shown better short- and intermediate-term clinical outcomes with the MIS technique.23,27-29 As a whole, the literature comparing the 2 procedures consists of mostly small retrospective studies with nonrandomized patient samples, heterogeneous surgical indications, and differing surgical techniques, making it difficult to draw conclusions.
The current study suggests that MIS TLIF may lead to improved clinical results at 2-year follow-up, compared with open TLIF. Our study used propensity-score matching to minimize the effects of nonrandom assignment of subjects to MIS TLIF or open TLIF. A limitation of observational studies is that bias in assignment of subjects to treatment groups can lead to overestimation or underestimation of the effect of the treatment itself. Propensity-score matching attempts to reduce this bias by accounting for several covariates that predict whether a subject will receive a certain treatment. These covariates are used in a logistic regression to produce a propensity score, which can be used to match subjects to controls across multiple dimensions, thus ensuring groups are as comparable as possible at baseline.
Our study still has several limitations. Sample size is relatively small, and follow-up is still only intermediate, at 2 years. There was unequal distribution of specific levels of surgery. Because patients were not blinded to the treatment they received, it is possible that patient perception of receiving a newer, less-invasive treatment method may influence their subjective improvement. The study sample was drawn from 2 different centers, with one center providing mostly MIS cases and the other providing mostly open cases. Because of this, undetected differences in how patients were selected for surgery could also affect outcomes. Any latent confounding variables, which are not identified a priori, will not be accounted for in the matching process. Only a prospective, randomized study with large numbers can control for observed and unobserved confounding patient characteristics.
In summary, our study shows that MIS TLIF is associated with improved low back pain and low back–related disability at 2 years compared with open TLIF. Other studies comparing the 2 techniques have come to different conclusions regarding whether the short-term benefits of MIS TLIF translate into long-term differences in clinical outcome. This study adds to this evidence and suggests there may be longer term advantages to the MIS approach, but prospective randomized trials are needed to confirm this finding and determine the true magnitude of these differences.
Transforaminal lumbar interbody fusion (TLIF) has become an increasingly popular method of lumbar fusion, since its introduction by Harms and Rolinger in 1982.1 The procedure allows for a circumferential fusion through a posterior-only approach, with improved sagittal alignment2 and minimal risk for iatrogenic nerve injury. In the past decade, a minimally invasive surgical method of TLIF (MIS TLIF) has been introduced3-5 and involves neural decompression and interbody fusion through a tubular retractor, and percutaneous placement of pedicle-screw instrumentation. This technique uses muscle dilation rather than large-scale detachment of muscle. Proponents of the MIS technique have postulated that decreased muscle damage would lead to better short-term, and possibly long-term, clinical outcomes, because of less iatrogenic soft-tissue damage.
Studies that have compared results of MIS TLIF with open TLIF have shown improved perioperative outcomes, but most have shown similar intermediate-term clinical outcomes.6 In the short term, multiple studies demonstrate that MIS TLIF is associated with decreased blood loss, less postoperative pain and narcotic requirements, and shorter hospital length of stay.7-13 However, changes in pain score and disease-specific and generic health-related quality of life measures have been similar for the 2 procedures, beyond 6 months postoperatively.10,13-15 These studies have generally involved retrospective reviews of unmatched patient groups, with small sample sizes and significant heterogeneity in surgical indications and case complexity. In our study, we compared intermediate-term clinical outcomes of MIS TLIF with open TLIF, using propensity matching to optimize baseline similarity of the groups.
Methods
This retrospective study was conducted after receiving approval from the Institutional Review Board. Surgical and clinical databases of 2 centers from 2008 to 2012 were reviewed for eligible subjects. Cases in 2007 were excluded because this was the year that MIS was introduced as a new technique in the practice. Inclusion criteria consisted of patients who underwent 1- to 2-level MIS TLIF and had complete baseline, 1- and 2-year postoperative outcome measures. Patients who had surgery for trauma, tumor, or osteomyelitis were excluded. Outcome measures collected and reviewed in this study included the Oswestry Disability Index (ODI),16,17 the Medical Outcomes Study Short-Form 36 (SF-36),18 and numeric rating scales for back and leg pain (0-100 scale).19 The Physical Composite Summary (PCS) and Mental Composite Summary of the SF-36 were reviewed separately. We recorded the following patient demographic data: age, gender, American Society of Anesthesiologists (ASA) grade, body mass index, indication for surgery, workers’ compensation, and smoking status. Surgical data included number of levels fused, operative time, estimated blood loss, and length of hospital stay.
Propensity-scoring technique20,21 was used to match the MIS TLIF patients to a control group of patients who underwent TLIF using an open approach (open TLIF), matching for multiple characteristics to produce 2 similar comparison groups. Propensity matching was performed to control for bias. In controlling for known confounders or biases, propensity matching, in theory, should also control for unknown confounders. Gender, age, body mass index, smoking status, indication for fusion, as well as preoperative ODI, SF-36 PCS, SF-36 Mental Composite Summary, and pain scores were used to generate a control open TLIF group.
MIS TLIF Surgical Technique
Patients in the MIS TLIF group underwent neural decompression and interbody fusion through a tubular retractor system (METRx, Medtronic Inc.), followed by percutaneous pedicle-screw fixation under fluoroscopic guidance (Sextant, Medtronic Inc.). After successful induction of general endotracheal anesthesia, patients were positioned prone on a radiolucent table. Posteroanterior (PA) and lateral fluoroscopic images were used to localize 2 paramedian incisions, approximately 3-cm to 5-cm lateral to midline, over the pedicles of interest. Modified Jamshidi needles (Medtronic Inc.) were used to cannulate the pedicles under PA, posterior-oblique, PA, and lateral fluoroscopic guidance. The pedicles were tapped with a cannulated tap. Pedicle screws and rods were introduced on the side contralateral to the TLIF and were used as needed to maintain intradiscal distraction during the TLIF portion of the procedure.
Decompression and TLIF were carried out on the side of the patient’s radicular pain or bilaterally, according to the surgeon’s discretion. A K-wire was advanced to the facet joint complex, after which sequential dilators were used to dilate through the muscles to establish an intramuscular corridor to the facet. A 26-mm fixed tubular retractor was docked over the facet and locked in place, using a post attached to the operating room table. Neural decompression was obtained by removal of the entire facet-joint complex and lamina to the base of the spinous process, using a combination of high-speed drills and Kerrison rongeurs. The ligamentum flavum was completely resected. The superior articular process of the caudal vertebra was removed all the way to the pedicle below. Ball-tipped probes were used to confirm that traversing and exiting nerve roots were completely free. An annulotomy was performed, and all disc material was removed from the disc through a combination of rotating shavers, serrated curettes, endplate scrapers, and rasps. Bone graft was placed anterior and contralateral to the interbody cage. (Bone grafts included autogenous iliac crest, local bone obtained from the decompression, recombinant human bone morphogenetic protein 2, or allograft demineralized bone matrix at the surgeon’s discretion.) After placement of the interbody cage, the ipsilateral pedicle-screw instrumentation was put over the remaining guide wires and compression applied across the construct to lock the interbody cage and restore lordosis. Wounds were closed without drains.
Open TLIF Surgical Technique
In patients undergoing open TLIF, a midline incision was made over the vertebrae of interest, and paraspinal muscles were subperiosteally dissected to the tips of the transverse processes. The appropriate level was confirmed with intraoperative radiograph. Pedicle screws were placed free-hand using anatomic landmarks, and appropriate placement was confirmed with intraoperative radiograph and evoked electromyography stimulation. Laminectomy and facetectomy were performed, and the disc was entered on the side of the facetectomy. After thorough disc-space preparation, bone graft and an interbody cage were placed, rods inserted, and compression carried out. A supplemental posterolateral fusion was also performed after decortication of the transverse processes and cartilaginous surface of the contralateral facet. Layered wound closure was performed over drains.
Analysis
Statistical analysis was carried out using SPSS Statistics version 17.0 (IBM) with significance set at the P < .01 level. A small, conservative P-value threshold was used to minimize type II error that resulted from the multiple comparisons performed. Student t test was used to determine any significant differences between continuous demographic variables, and to compare preoperative and postoperative outcome measure scores within and between study groups. Fisher’s exact test was used to compare categorical variables between the 2 groups.
Results
The MIS TLIF group consisted of 64 patients (average age, 52 years), and included 22 patients with degenerative spondylolisthesis, 33 with disc pathology, 8 with postdecompression, and 1 non-union patient. The open TLIF group consisted of 64 patients (average age, 54 years), and included 39 degenerative spondylolisthesis, 15 disc pathology, 7 postdecompression, and 3 nonunion patients (Table 1). All 64 open and 19 MIS cases were from a spine practice with 6 surgeons, and 45 MIS cases came from a spine practice with 2 surgeons. There was also an unequal distribution of the specific levels fused between the open and MIS groups.
Although the operative time was similar in both groups, the MIS TLIF group had a statistically significantly lower blood loss compared with the open TLIF group (Table 2). Both MIS TLIF and open TLIF lead to significant improvements in pain, ODI, and SF-36 PCS (P < .01) (Table 3). At 1 year, both groups had similar improvements in pain (36.9 vs 30.8, P = .178) and SF-36 PCS (9.9 vs 7.5, P = .231), but the MIS TLIF group had a statistically significantly greater improvement in ODI compared with the open TLIF group (30.4 vs 15.1, P < .000). At 2 years, both groups had similar improvements in SF-36 PCS (12.1 vs 7.5, P = .033), but the MIS TLIF group had a statistically significantly greater improvement in pain (40.2 vs 27.0, P = .005) and ODI (33.1 vs 15.4, P < .000) compared with the open TLIF group (Table 4).
Discussion
The current study compared intermediate-term clinical outcomes of MIS TLIF to open TLIF. We used propensity matching to identify a control group of open TLIFs that were comparable to the MIS TLIF group across a variety of covariates that are known to influence the results of lumbar fusion. This created comparison groups that were as closely matched at baseline as possible. We found that, at 2-year follow-up, MIS TLIF patients had less pain and less low-back pain–related disability as measured by ODI. There was also a trend toward better generic health-related quality of life in the MIS TLIF group.
These data suggest that the decreased soft-tissue trauma of the minimally invasive surgical technique, which leads to improved perioperative parameters in the short term, may also lead to some advantages that translate to improved intermediate-term clinical outcomes. Traditional lumbar fusion procedures have shown excellent clinical results when used for accepted clinical indications.22 However, the procedure requires extensive dissection of the paraspinal muscles, which causes significant muscle damage as evidenced by muscle breakdown products that can be detected in the bloodstream postoperatively.23,24 The lateral dissection also transects the dorsal ramus of the segmental nerves, which innervate the paraspinal muscles, leading to significant scarring and atrophy on postoperative imaging studies.23 Some authors have used the term “fusion disease” to describe the constellation of soft-tissue degradation seen after open lumbar fusion.5
An MIS version of the TLIF procedure that was described in 20033 avoids much of this iatrogenic soft-tissue trauma. It involves intramuscular dilation to approach the spine and to carry out neural decompression and interbody fusion, in conjunction with percutaneous pedicle-screw instrumentation. Proponents of this technique point to diminished iatrogenic soft-tissue and muscle damage as an advantage. Multiple studies have, in fact, confirmed improved short-term perioperative parameters, such as less blood loss, lower narcotic requirements, and decreased length-of-hospital stay.25 Economic analyses have also shown lower direct and indirect costs with the MIS technique.26
Several studies have compared patient-reported outcome measures of MIS and open TLIF, and the results have been mixed. Most of these studies have shown similar improvement in clinical outcomes between the 2 procedures, but the MIS technique demonstrated short-term perioperative advantages, such as lower blood loss, less narcotic requirements, and shorter length of stay.7-15 The authors of these studies conclude that the MIS technique can provide similar long-term results with lower short-term morbidity when compared with open TLIF. In contrast, some studies have shown better short- and intermediate-term clinical outcomes with the MIS technique.23,27-29 As a whole, the literature comparing the 2 procedures consists of mostly small retrospective studies with nonrandomized patient samples, heterogeneous surgical indications, and differing surgical techniques, making it difficult to draw conclusions.
The current study suggests that MIS TLIF may lead to improved clinical results at 2-year follow-up, compared with open TLIF. Our study used propensity-score matching to minimize the effects of nonrandom assignment of subjects to MIS TLIF or open TLIF. A limitation of observational studies is that bias in assignment of subjects to treatment groups can lead to overestimation or underestimation of the effect of the treatment itself. Propensity-score matching attempts to reduce this bias by accounting for several covariates that predict whether a subject will receive a certain treatment. These covariates are used in a logistic regression to produce a propensity score, which can be used to match subjects to controls across multiple dimensions, thus ensuring groups are as comparable as possible at baseline.
Our study still has several limitations. Sample size is relatively small, and follow-up is still only intermediate, at 2 years. There was unequal distribution of specific levels of surgery. Because patients were not blinded to the treatment they received, it is possible that patient perception of receiving a newer, less-invasive treatment method may influence their subjective improvement. The study sample was drawn from 2 different centers, with one center providing mostly MIS cases and the other providing mostly open cases. Because of this, undetected differences in how patients were selected for surgery could also affect outcomes. Any latent confounding variables, which are not identified a priori, will not be accounted for in the matching process. Only a prospective, randomized study with large numbers can control for observed and unobserved confounding patient characteristics.
In summary, our study shows that MIS TLIF is associated with improved low back pain and low back–related disability at 2 years compared with open TLIF. Other studies comparing the 2 techniques have come to different conclusions regarding whether the short-term benefits of MIS TLIF translate into long-term differences in clinical outcome. This study adds to this evidence and suggests there may be longer term advantages to the MIS approach, but prospective randomized trials are needed to confirm this finding and determine the true magnitude of these differences.
1. Harms J, Rolinger H. A one-stager procedure in operative treatment of spondylolisthesis: dorsal traction-reposition and anterior fusion (author’s transl). Z Orthop Ihre Grenzgeb. 1982;120(3):343-347.
2. Jagannathan J, Sansur CA, Oskouian RJ Jr, Fu KM, Shaffrey CI. Radiographic restoration of lumbar alignment after transforaminal lumbar interbody fusion. Neurosurgery. 2009;64(5):955-963.
3. Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine. 2003;28(15 suppl):S26-S35.
4. Rouben D, Casnellie M, Ferguson M. Long-term durability of minimally invasive posterior transforaminal lumbar interbody fusion: a clinical and radiographic follow-up. J Spinal Disord Tech. 2011;24(5):288-296.
5. Schwender JD, Holly LT, Rouben DP, Foley KT. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech. 2005;18(suppl):S1-S6.
6. Goldstein CL, Macwan K, Sundararajan K, Rampersaud YR. Comparative outcomes of minimally invasive surgery for posterior lumbar fusion: a systematic review. Clin Orthop Relat Res. 2014;472(6):1727-1737.
7. Adogwa O, Parker SL, Bydon A, Cheng J, McGirt MJ. Comparative effectiveness of minimally invasive versus open transforaminal lumbar interbody fusion: 2-year assessment of narcotic use, return to work, disability, and quality of life. J Spinal Disord Tech. 2011;24(8):479-484.
8. Ghahreman A, Ferch RD, Rao PJ, Bogduk N. Minimal access versus open posterior lumbar interbody fusion in the treatment of spondylolisthesis. Neurosurgery. 2010;66(2):296-304.
9. Park Y, Ha JW. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine. 2007;32(5):537-543.
10. Saetia K, Phankhongsab A, Kuansongtham V, Paiboonsirijit S. Comparison between minimally invasive and open transforaminal lumbar interbody fusion. J Med Assoc Thai. 2013;96(1):41-46.
11. Schizas C, Tzinieris N, Tsiridis E, Kosmopoulos V. Minimally invasive versus open transforaminal lumbar interbody fusion: evaluating initial experience. Int Ortop. 2009;33(6):1683-1688.
12. Wang J, Zhou Y, Zhang ZF, Li CQ, Zheng WJ, Liu J. Comparison of one-level minimally invasive and open transforaminal lumbar interbody fusion in degenerative and isthmic spondylolisthesis grades 1 and 2. Eur Spine J. 2010;19(1):1780-1784.
13. Lee KH, Yue WM, Yeo W, Soeharno H, Tan SB. Clinical and radiological outcomes of open versus minimally invasive transforaminal lumbar interbody fusion. Eur Spine J. 2012;21(11):2265-2270.
14. Peng CW, Yue WM, Poh SY, Yeo W, Tan SB. Clinical and radiological outcomes of minimally invasive versus open transforaminal lumbar interbody fusion. Spine. 2009;34(13):1385-1389.
15. Seng C, Siddiqui MA, Wong KP, et al. Five-year outcomes of minimally invasive versus open transforaminal lumbar interbody fusion: a matched-pair comparison study. Spine. 2013;38(23):2049-2055.
16. Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25(22):2940-2953.
17. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271-273.
18. Ware JE, Kosinski M, Keller SK. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA: The Health Institute, 1994.
19. McCaffery M, Beebe A. Pain: Clinical Manual for Nursing Practice. Baltimore, MD: V.V. Mosby Company, 1993.
20. D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265-2281.
21. Rosenbaum PR. Model-based direct adjustment. J Am Stat Assn. 1987;82:387-394.
22. Glassman SD, Carreon LY, Djurasovic M, et al. Lumbar fusion outcomes stratified by specific diagnostic indication. Spine J. 2009;9(1):13-21.
23. Fan S, Hu Z, Zhao F, Zhao X, Huang Y, Fang X. Multifidus muscle changes and clinical effects of one-level posterior lumbar interbody fusion: minimally invasive procedure versus conventional open approach. Eur Spine J. 2010;19(2):316-324.
24. Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. A histologic and enzymatic analysis. Spine. 1996;21(8):941-944.
25. Sun ZJ, Li WJ, Zhao Y, Qui GX. Comparing minimally invasive and open transforaminal lumbar interbody fusion for treatment of degenerative lumbar disease: a meta-analysis. Chin Med J. 2013;126(2):3962-3971.
26. Parker SL, Mendenhall SK, Shau DN, et al. Minimally invasive versus open transforaminal lumbar interbody fusion for degenerative spondylolisthesis: comparative effectiveness and cost-utility analysis. World Neurosurg. 2014;82(1-2):230-238.
27. Kotani Y, Abumi K, Ito M, Sudo H, Abe Y, Minami A. Mid-term clinical results of minimally invasive decompression and posterolateral fusion with percutaneous pedicle screws versus conventional approach for degenerative spondylolisthesis with spinal stenosis. Eur Spine J. 2012;21(6):1171-1177.
28. Pelton MA, Phillips FM, Singh K. A comparison of perioperative costs and outcomes in patients with and without worker’s compensation claims treated with MIS or open TLIF. Spine. 2012;37(22):1914-1919.
29. Wong AP, Smith ZA, Stadler JA 3rd, et al. Minimally invasive transforaminal lumbar interbody fusion (MI-TLIF). Surgical technique, long-term 4 year prospective outcomes and complications compared with an open TLIF cohort. Neurosurg Clin N Am. 2014;25(2):279-304.
1. Harms J, Rolinger H. A one-stager procedure in operative treatment of spondylolisthesis: dorsal traction-reposition and anterior fusion (author’s transl). Z Orthop Ihre Grenzgeb. 1982;120(3):343-347.
2. Jagannathan J, Sansur CA, Oskouian RJ Jr, Fu KM, Shaffrey CI. Radiographic restoration of lumbar alignment after transforaminal lumbar interbody fusion. Neurosurgery. 2009;64(5):955-963.
3. Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine. 2003;28(15 suppl):S26-S35.
4. Rouben D, Casnellie M, Ferguson M. Long-term durability of minimally invasive posterior transforaminal lumbar interbody fusion: a clinical and radiographic follow-up. J Spinal Disord Tech. 2011;24(5):288-296.
5. Schwender JD, Holly LT, Rouben DP, Foley KT. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech. 2005;18(suppl):S1-S6.
6. Goldstein CL, Macwan K, Sundararajan K, Rampersaud YR. Comparative outcomes of minimally invasive surgery for posterior lumbar fusion: a systematic review. Clin Orthop Relat Res. 2014;472(6):1727-1737.
7. Adogwa O, Parker SL, Bydon A, Cheng J, McGirt MJ. Comparative effectiveness of minimally invasive versus open transforaminal lumbar interbody fusion: 2-year assessment of narcotic use, return to work, disability, and quality of life. J Spinal Disord Tech. 2011;24(8):479-484.
8. Ghahreman A, Ferch RD, Rao PJ, Bogduk N. Minimal access versus open posterior lumbar interbody fusion in the treatment of spondylolisthesis. Neurosurgery. 2010;66(2):296-304.
9. Park Y, Ha JW. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine. 2007;32(5):537-543.
10. Saetia K, Phankhongsab A, Kuansongtham V, Paiboonsirijit S. Comparison between minimally invasive and open transforaminal lumbar interbody fusion. J Med Assoc Thai. 2013;96(1):41-46.
11. Schizas C, Tzinieris N, Tsiridis E, Kosmopoulos V. Minimally invasive versus open transforaminal lumbar interbody fusion: evaluating initial experience. Int Ortop. 2009;33(6):1683-1688.
12. Wang J, Zhou Y, Zhang ZF, Li CQ, Zheng WJ, Liu J. Comparison of one-level minimally invasive and open transforaminal lumbar interbody fusion in degenerative and isthmic spondylolisthesis grades 1 and 2. Eur Spine J. 2010;19(1):1780-1784.
13. Lee KH, Yue WM, Yeo W, Soeharno H, Tan SB. Clinical and radiological outcomes of open versus minimally invasive transforaminal lumbar interbody fusion. Eur Spine J. 2012;21(11):2265-2270.
14. Peng CW, Yue WM, Poh SY, Yeo W, Tan SB. Clinical and radiological outcomes of minimally invasive versus open transforaminal lumbar interbody fusion. Spine. 2009;34(13):1385-1389.
15. Seng C, Siddiqui MA, Wong KP, et al. Five-year outcomes of minimally invasive versus open transforaminal lumbar interbody fusion: a matched-pair comparison study. Spine. 2013;38(23):2049-2055.
16. Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25(22):2940-2953.
17. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271-273.
18. Ware JE, Kosinski M, Keller SK. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA: The Health Institute, 1994.
19. McCaffery M, Beebe A. Pain: Clinical Manual for Nursing Practice. Baltimore, MD: V.V. Mosby Company, 1993.
20. D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265-2281.
21. Rosenbaum PR. Model-based direct adjustment. J Am Stat Assn. 1987;82:387-394.
22. Glassman SD, Carreon LY, Djurasovic M, et al. Lumbar fusion outcomes stratified by specific diagnostic indication. Spine J. 2009;9(1):13-21.
23. Fan S, Hu Z, Zhao F, Zhao X, Huang Y, Fang X. Multifidus muscle changes and clinical effects of one-level posterior lumbar interbody fusion: minimally invasive procedure versus conventional open approach. Eur Spine J. 2010;19(2):316-324.
24. Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. A histologic and enzymatic analysis. Spine. 1996;21(8):941-944.
25. Sun ZJ, Li WJ, Zhao Y, Qui GX. Comparing minimally invasive and open transforaminal lumbar interbody fusion for treatment of degenerative lumbar disease: a meta-analysis. Chin Med J. 2013;126(2):3962-3971.
26. Parker SL, Mendenhall SK, Shau DN, et al. Minimally invasive versus open transforaminal lumbar interbody fusion for degenerative spondylolisthesis: comparative effectiveness and cost-utility analysis. World Neurosurg. 2014;82(1-2):230-238.
27. Kotani Y, Abumi K, Ito M, Sudo H, Abe Y, Minami A. Mid-term clinical results of minimally invasive decompression and posterolateral fusion with percutaneous pedicle screws versus conventional approach for degenerative spondylolisthesis with spinal stenosis. Eur Spine J. 2012;21(6):1171-1177.
28. Pelton MA, Phillips FM, Singh K. A comparison of perioperative costs and outcomes in patients with and without worker’s compensation claims treated with MIS or open TLIF. Spine. 2012;37(22):1914-1919.
29. Wong AP, Smith ZA, Stadler JA 3rd, et al. Minimally invasive transforaminal lumbar interbody fusion (MI-TLIF). Surgical technique, long-term 4 year prospective outcomes and complications compared with an open TLIF cohort. Neurosurg Clin N Am. 2014;25(2):279-304.
Epidemiology, Treatment, and Prevention of Lumbar Spine Injuries in Major League Baseball Players
For the last 20 years, injuries resulting in time out of play have been on the rise in Major League Baseball (MLB), and those affecting the back are no exception.1,2 In the first comprehensive report on injuries in MLB players, back injuries resulted in a mean of 1016 disabled list days per season from 1995 to 1999.1 Similarly, core and back injuries were responsible for 359 disabled list designations from 2002 to 2008. This represented 11.7% of all injuries resulting in time out of play during that time span.2 During that time, back injury prevalence ranked 6th highest of all possible body regions (out of 17), and both position players and pitchers were similarly affected (7.8% and 7.4% of all injuries, respectively).2 These injuries often result in a significant time out of play and can have a tremendous impact on player health. A healthy, stable, and well-functioning lumbar spine is a prerequisite for nearly all baseball-related activities, including pitching, throwing, batting, and running. Accordingly, even minor lumbar spine injuries may profoundly influence baseball performance. Despite this, less is currently known about the true epidemiology and impact of back injuries in professional baseball compared to other professional sporting organizations.3
The most common causes of low back pain and injury in elite baseball players include muscle strains, stress fractures (spondylolysis), annular tears, disc herniation, stenosis, transverse process fractures, facetogenic pain, and sacroiliac (SI) joint arthropathy.4-8 These injuries present in a variety of ways with varying symptomatology. Accordingly, a thorough understanding and comprehensive approach to the diagnosis and treatment of these injuries is necessary. The purpose of this article is to discuss the current state of lumbar spine injuries in professional baseball players. Specifically, we will discuss the critical role of the spine in baseball activities, common causes of injury, tips for making the diagnosis, treatment options, outcomes, and injury prevention and rehabilitation strategies.
Role of the Spine in Baseball
The spine and core musculature are responsible for positioning the head, shoulders, and upper extremities in space over the hips and lower extremities. Proper maintenance of this relationship is required during all phases of throwing, pitching, running, and hitting. During these activities, the spine may dynamically flex, extend, rotate, and laterally bend as needed to keep the body balanced with the head centered over the trunk.
Pitching and Throwing
Whether pitching from the wind-up or the stretch, the head begins centered over the hips and pelvis. As the pitching motion progresses, the hips undergo rotation, flexion, extension, abduction, and circumduction. While this is occurring, the shoulders and upper truck must bend, rotate, and translate toward home plate with the body. Just prior to front foot contact, trunk rotation averages 55 ± 6° with a maximal mean angular acceleration of 11,600 ± 3100°/s2. 9 In order for the body to remain balanced, controlled, and synchronized throughout this delivery, the lumbar spine and core musculature must work diligently to stabilize the entire kinetic chain. Of all the trunk muscles (paraspinal, rectus abdominis, obliques, and glutei), the lumbar paraspinal muscles often work the hardest during the pitching motion, demonstrating activity increases ranging from 100% to 400%.10 Accordingly, it is not uncommon for pitchers to develop SI joint or lumbar facet joint pain due to this high degree of torsional strain exerted on the low back.4 Poor lumbopelvic control has been shown to be a predictor of subsequent injury, and the degree of lumbopelvic dysfunction is proportional to injury severity in MLB pitchers.5
Hitting
Similar to pitching, hitting involves a complex combination of movements from the upper and lower extremities that must be balanced by the core and spine. Although numerous movements occur simultaneously, rotational motion is primarily responsible for generating power. The trunk rotates an average of 46 ± 9° during the swing and reaches a maximal angular acceleration of 7200 ± 2800°/s2 just after contact.9 During this period of rapid torsion, the spine must rotate in conjunction with the hips and shoulders to create a stable cylinder and axis of rotation. The spine and core are responsible for synchronizing rotation to ensure that hip and shoulder parallelism is maintained from swing initiation to ball contact. If the body does not rotate as a unit, the position of the head is affected and the batter’s ability to see the ball may be compromised. Additionally, if delivery of the shoulders lags too far behind that of the hips, the position of the hands (and bat) in space is adversely affected. The entire kinetic chain must remain balanced, coordinated, precisely timed, and standardized throughout the entire swing from initial trigger to final follow-through. The lumbar spine plays a critical role in each of these steps. If lumbar spine mechanics are not sound, this can have significant adverse effects on batting performance and may predispose hitters to injury.4
Common Etiologies for Spinal Injury
The vast majority of baseball players who experience lumbar pain will have injuries that can be classified as mechanical back pain (ie, spondylolysis, annular tears, facetogenic pain, SI joint arthropathy, or muscle injuries) (Table). Although less likely to occur, nerve entrapment or impingement syndromes (ie, disc herniation, stenosis, and peripheral nerve entrapment) have been observed in professional baseball players. Finally, more concerning pathologies such as infection and tumor are extremely rare, but they must not be overlooked in this high-demand patient population.
Stress Fracture or Spondylolysis
In young athletic patients, up to one-third of those with low back pain may have evidence of a lumbar stress fracture on bone scan.11,12 This is particularly true for athletes who undergo repetitive lumbar extension and rotation, such as linemen, gymnasts, wrestlers, weight lifters, and baseball players.4,13 Although the majority of lumbar stress fractures occur at the pars interarticularis, they can occur in the pedicle or articular process (Figure 1). Most spondylolytic lesions do not progress to spondylolisthesis, especially once patients reach skeletal maturity. Because the fifth lumbar vertebra represents the transition from the lumbar to the sacral spine, most stress fractures occur at L5. These typically present as localized low back pain that worsens with flexion, extension, and rotation.
Muscle Injury
One of the most common causes of low back pain in athletes is muscle strains and spasms. Because the lumbar paraspinal muscles are extremely active during throwing and hitting,10 they are particularly susceptible to injury. This is particularly true in deconditioned athletes or those who report to spring training having not adequately maintained strength and flexibility through the off-season.4,5 These injuries typically present in an acute fashion with an obvious inciting incident. Players may have a history of similar muscle injuries in the past. On examination, they tend to have difficulty maintaining normal posture or ranging the spine through a full arc of motion. Localized, superficial tenderness to palpation in the injured muscle is a key component of the diagnosis.
Annular Tears and Disc Herniation
These injuries typically occur as the result of a combination of compressive and rotary forces on the lumbar spine that overcome the ability of the annulus fibrosus to resist hoop stresses. Patients with annular tears typically present with severe lower back pain that may be accompanied by spasm and pain radiation into the buttock or lower extremities. Pain is usually worsened by valsalva, coughing, sneezing, or bearing down.4 Although annular tears can occur in isolation, they can also lead to herniation of the nucleus pulposus into the spinal canal (Figure 2). Depending on the location and severity of the herniation, nerve entrapment or impingement can occur. This may initially present as pain that radiates into the lower extremities in a dermatomal fashion. As the herniation progresses, decreased sensation and weakness may develop.
Facet Joint Pain
Facetogenic pain can occur as the result of degenerative changes, trauma, or joint inflammation. Facet injury typically occurs during rotation while the back is extended.4 This results in localized pain and tenderness that can be reproduced by loading the facet joint (lumbar extension) during the examination, and patients will often demonstrate discomfort and altered motion when extending the flexed back.
Sacroiliac Joint Pain
Although pain in the region of the SI joint is very common, much of this may actually be referred from more centrally located neuromotion segments.4 SI joint pathology can occur as a result of trauma, degeneration, or inflammatory processes as is seen in ankylosing spondylitis (AS). Patients with AS typically present with a gradual onset of progressive stiffness and pain in the low back and hips that is worse in the morning or following periods of inactivity. It is most common in Caucasian males in their second to fourth decades.14 Although 80% to 95% of patients with AS will test positive for human leukocyte antigen B27 (HLA-B27), it is important to note that the vast majority of people with HLA-B27 do not go on to develop AS.14 Regardless of the cause, SI joint pain can be very debilitating and negatively impact all baseball-related activities.
Stenosis
Lumbar stenosis may develop from arthritic changes, disc protrusion, facet hypertrophy, or ligament ossification. In this young, athletic population, congenital stenosis should also be a consideration. Patients with congenital stenosis at baseline are at increased risk for developing neurologic symptoms from disc protrusion or other acquired spinal pathology. Lumbar stenosis generally manifests as a gradual onset of progressive low back pain with radicular symptoms or neurogenic claudication.4
Making the Diagnosis
History
When identifying the cause of any musculoskeletal complaint, the diagnosis begins with a thorough history. In addition to the standard components of the history, such as timing, severity, relation to activity, exacerbating factors, associated symptoms, and prior treatments, Watkins and colleagues4 have outlined a number of key factors that should be determined when specifically evaluating the athlete with low back pain.These include quantification of the morbidity, identification of contributing psychosocial factors, ruling out of urgent diagnoses (ie, neoplasm, infection, rapidly progressive neurologic deficits, cauda equina, and paralysis), determination of injury type and duration, identification of the clinical syndrome/etiology, pinpointing the location of the pathology (what nerve at what level?), and quantification of back versus leg symptoms. Answers to these questions will set the framework for an appropriately directed physical examination, imaging, and diagnostic tests.
Physical Examination
The physical examination begins by observing the patient or player walk across the playing field, training room, or examination room, paying attention to posture, gait, and overall body movement. Many patients with lumbar injuries will demonstrate adaptive patterns of motion in an attempt to accommodate their pain. This may be seen during baseball-related activities such as throwing, batting, or running. The spine should be visualized and palpated for malalignment while standing erect and during forward bending. If possible, motion should be assessed in rotation, lateral bending, and the flexion and extension planes. Special attention should be paid to any positions or maneuvers that reproduce pain or neurologic symptoms. Areas of tenderness and radiating pain should be fully palpated. A full neurologic examination consisting of manual muscle testing, sensory examination, and reflex evaluation of both the upper and lower extremities should be performed. Numerous special tests and neurologic stretch maneuvers that assess specific lumbar nerve roots have been described.15
Imaging and Diagnostic Tests
Depending on the history and physical examination, imaging of the lumbar spine is not always warranted in the acute setting. This is especially the case if muscle injury, herniation, or annular tears are suspected. In cases of persistent pain, trauma, or suspected neoplasia, imaging is generally warranted. When x-rays are negative and spondylolysis is suspected, bone scan with lumbar single photon emission computed tomography (SPECT) is the most sensitive test.16 SPECT scans are positive in active spondylolysis because the radio-nucleotide is taken up by active, bone-forming osteoblasts. Quiescent stress fractures that are not apparent on SPECT scans are generally chronic and painless.4 If the SPECT scan is positive, the injury can be further characterized by computed tomography (CT) (Figure 1), which can distinguish between spondylolysis, osteoid osteoma, osteoblastoma, acute fracture, or arthritic degeneration. When the SPECT is negative, or if neural impingement is suspected, magnetic resonance imaging (MRI) (Figure 2) is likely the best diagnostic imaging tool. MRI allows identification of bone edema, disc herniation, annular disruption, disc desiccation, stenosis, and nerve entrapment. Finally, when attempting to distinguish between central and peripheral nerve entrapment syndromes, an electromyogram (EMG) or nerve conduction study (NCS) is a reliable way to identify the location of injury.
Treatment and Outcomes
The approach to a patient with low back pain begins with identification of the etiology and discontinuation of the activities that reproduce pain.4 Trunk stabilization exercises and anti-inflammatory medications are the mainstays of treatment regardless of the cause of the lumbar spinal injury in the baseball player.4
Stress Fracture or Spondylolysis
Management of symptomatic spondylolysis or spondylolisthesis in the athlete initially consists of conservative treatment, which achieves good to excellent long-term outcomes and return to play in 70% to 90% of athletes, especially for acute injuries.17-19 After stopping the activity that causes the pain, trunk stabilization exercises should be started as soon as tolerated with the use of non-steroidal anti-inflammatory medications (NSAIDs), oral steroids, and spinal injections to control symptoms and permit initiation of the rehabilitation program.4 Although bracing is a commonly used adjunctive treatment, a recent meta-analysis did not demonstrate any difference in clinical outcomes between patients treated with a brace compared to non-braced controls.20
Surgical indications for the treatment of spondylolysis or spondylolisthesis are limited; however, failure of nonoperative treatment after 6 months is a reasonable time to consider surgery.17 The spondylolytic defect can often be repaired directly using hook screws, translaminar screws, wiring, pedicle screws, or image-guided lag screws across the lesion with grafting.4 Lumbar spinal fusion is less successful in professional athletes due to the high demands placed on adjacent levels as well as the time required for the fusion to heal.4 Bony union can be determined by a CT scan at 6 months postoperatively if the patient has met appropriate return to play criteria.4
Muscle Injury
Management of lumbar sprains and strains typically includes restricting painful postures and a rehabilitation program that focuses on core strengthening within a pain-free arc of motion.21 Because acute injuries typically resolve quickly and spontaneously, a short interval of decreased activity, icing, NSAIDs, and stretching followed by focused strength training is appropriate before return to sports activity.22
Annular Tears and Disc Herniation
Initial management of baseball players with acute lumbar disc herniation and/or annular tears consists of rest for up to 5 days followed by physical therapy and NSAIDs, Medrol Dose pak, or epidural injections.4 Professional baseball players return to play at high rates following a herniated lumbar disc.6 Earhart and colleagues6 found that 97.1% of players returned to play at an average time of 6.6 months from the time of injury. When stratified by position, all pitchers (29 of 29) returned to competitive play after operative or nonoperative management, while 38 of 40 hitters returned.6 The average career length after lumbar disc herniation in the professional baseball player is between 4.1 and 5.3 years or between 256 and 471 games.6,23 Other work has suggested that players undergoing operative treatment for lumbar herniation had shorter career lengths; however, patients in the operative group tended to be older at the time of injury.23
Emphasis should be placed on nonoperative management of baseball players with disc pathology except in cases of cauda equina syndrome.4 Hitters and pitchers who require surgery have demonstrated decreased 1-year and 3-year postoperative statistical performance compared to preinjury levels.6 No significant changes in any performance statistic were seen in baseball following nonoperative management.6 Consequently, indications for surgery in the baseball player with lumbar disc pathology includes cauda equina syndrome, progressive neurologic deficit, sufficient morbidity, failure of conservative care, a lesion that can be corrected safely with surgery, and the ability for the patient to comply with a comprehensive postoperative rehabilitation program.4 Operative treatment typically consists of a lumbar microdiscectomy and/or laminotomy. 4,6
Facet Joint Pain
The mainstay of therapy in patients with facet joint pain consists of analgesia and a trunk stabilization program.24 Lumbar zygapophysial joint injections and radiofrequency denervation can be considered if the patient fails 4 weeks of directed conservative treatment.24,25 Injections may be useful in select patients; however, the literature supporting the use of lumbar facet joint injections or radiofrequency denervation for facetogenic pain is limited.24,25
Sacroiliac Joint Pain
Acute injury of the SI joint can be treated with NSAIDs, icing, and relative rest.26 Mobilization of the SI joint in addition to correcting any asymmetries in muscle length or stiffness should be started and progressed as soon as tolerated within a pain-free range of motion.26 Rehabilitation should correct biomechanical deficits and maladaptation with a special focus on agonist and antagonist muscle groups across the sacrum and ilium.26 Treatment of AS in the athlete should emphasize symptom control, as there is no definite treatment. For patients with AS, other long-term therapeutic options include sulfasalazine, methotrexate, thalidomide, and anti-tumor necrosis factor therapies.14
Stenosis
Lumbar spinal stenosis, whether congenital or acquired, should initially be managed conservatively.27 Although they do not alter the progression of the disease, epidural steroids and local injections may temporarily decrease symptoms in approximately 40% of cases.27 Those who fail conservative therapy after 3 months may be candidates for surgical decompression and/or fusion.27,28 However, surgical treatment for lumbar spinal stenosis in elite baseball players has not been thoroughly studied, so the long-term prognosis is not well documented.27
Rehabilitation and Prevention of Injuries
After an appropriate diagnosis has been made, a structured rehabilitation process should commence. During rehabilitation, it is of primary importance that deep core stabilization is established. As an initial step in this process, athletes are trained to initiate deep core stabilization with breathing techniques in a static, supine position.29 Proper diaphragm activation with co-contractions of the transverse abdominis (TA) and pelvic floor has been shown to increase lumbar spine stability.30 This will allow for an increase in intra-abdominal pressure (IAP) and improved stabilization of the lumbar spine, creating a muscular cylinder between the bottom of the rib cage and top of the pelvis. These activities are initiated in the supine position but are soon advanced as upper and lower extremity movement against resistance is added. It is important to make sure IAP and contraction of the TA is maintained throughout this sequence of progression.
Once deep core stabilization has been established, athletes are progressed to global muscle training and kinetic linking in all 3 planes of movement. This is an important phase, as lumbar stability is a result of coordinated muscle activation involving many muscles.31 This program progresses from supine breathing exercises to a modified side bridge position to enhance core activation along with frontal plane stability. Next, athletes are progressed to a half kneeling position and then on to standing. Rotational activities are introduced starting with isometric holds progressing to chops/lifts and rotational medicine ball toss. During these tasks, focus should be on quality of movement and maintenance of core activation. Endurance of these muscles should be trained during this process. Appropriate pain-free and safe cardiovascular exercise, such as walking, biking, swimming, and jogging, should be performed throughout each stage in the rehabilitation process. Activities should be halted with any increase in pain. At the completion of the rehabilitation process, it is important to observe the athlete while performing sport-specific tasks. Spinal stabilization must be translational and monitored by observing maintenance of the “cylinder” from the training room to sports specific movements.
Since poor lumbar control has been associated with increased amount of time on the disabled list,5 it would be ideal to identify those at risk of injury before problems arise. Conte and colleagues32 have shown that core muscle strains could be a result of muscle imbalance or improper pitching or hitting technique. Other work has demonstrated that pitchers with poor lumbopelvic control did not perform as well as those with superior control.33 By assessing spinal stability and biomechanics at baseline, we may be able to identify those at risk. Pitchers with suboptimal spinal stabilization can present with an unstable balance phase, increased amounts of hyperextension of the lumbar spine from the moment of max cocking through ball release, as well as increased lateral trunk tilt at ball release. Correcting these flaws and increasing deep core stabilization can prevent injuries and improve performance.
Summary
A stable, well-functioning lumbar spine is vital to nearly every baseball-related activity, including pitching, throwing, batting, fielding, and running. The spine serves as a critical link in the kinetic chain between the upper and lower extremities. Due to the high demand on the lumbar spine, injuries to this area represent a significant amount of time out of play in MLB. Initial treatment typically consists of a comprehensive nonoperative rehabilitation process involving analgesics, rest, and therapy focusing on core stabilization. Because poor lumbopelvic control and mechanics have been demonstrated to increase injury risk, preemptive spinal and core stabilization is likely an appropriate step towards injury prevention.
1. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
2. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
3. Makhni EC, Buza JA, Byram I, Ahmad CS. Sports reporting: a comprehensive review of the medical literature regarding North American professional sports. Phys Sportsmed. 2014;42(2):154-162.
4. Watkins RG III, Watkins RG IV. Chapter 36: Lumbar injuries. In: Sports Medicine of Baseball. Dines JS, Altchek DW, Andrews JR, ElAttrache NS, Wilk KE, Yocum LA, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2012; 383-398.
5. Chaudhari AMW, McKenzie CS, Pan X, Oñate JA. Lumbopelvic control and days missed because of injury in professional baseball pitchers. Am J Sports Med. 2014;42(11):2734-2740.
6. Earhart JS, Roberts D, Roc G, Gryzlo S, Hsu W. Effects of lumbar disk herniation on the careers of professional baseball players. Orthopedics. 2012;35(1):43-49.
7. Hamid KS, Nwachukwu BU, Hsu E, Edgerton CA, Hobson DR, Lang JE. Orthopedic resident work-shift analysis: Are we making the best use of resident work hours? J Surg Educ. 2014;71(2):205-210.
8. Nair R, Kahlenberg CA, Hsu WK. Outcomes of lumbar discectomy in elite athletes: the need for high-level evidence. Clin Orthop Relat Res. 2015;473(6):1971-1977.
9. Fleisig GS, Hsu WK, Fortenbaugh D, Cordover A, Press JM. Trunk axial rotation in baseball pitching and batting. Sports Biomech. 2013;12(4):324-333.
10. Watkins RG, Dennis S, Dillin WH, et al. Dynamic EMG analysis of torque transfer in professional baseball pitchers. Spine (Phila Pa 1976). 1989;14(4):404-408.
11. Micheli LJ. Back injuries in gymnastics. Clin Sports Med. 1985;4(1):85-93.
12. Papanicolaou N, Wilkinson RH, Emans JB, Treves S, Micheli LJ. Bone scintigraphy and radiography in young athletes with low back pain. AJR Am J Roentgenol. 1985;145(5):1039-1044.
13. Elliott S, Hutson MA, Wastie ML. Bone scintigraphy in the assessment of spondylolysis in patients attending a sports injury clinic. Clin Radiol. 1988;39(3):269-272.
14. Kubiak EN, Moskovich R, Errico TJ, Di Cesare PE. Orthopaedic management of ankylosing spondylitis. J Am Acad Orthop Surg. 2005;13(4):267-278.
15. Miller KJ. Physical assessment of lower extremity radiculopathy and sciatica. J Chiropr Med. 2007;6(2):75-82.
16. Bellah RD, Summerville DA, Treves ST, Micheli LJ. Low-back pain in adolescent athletes: detection of stress injury to the pars interarticularis with SPECT. Radiology. 1991;180(2):509-512.
17. Radcliff KE, Kalantar SB, Reitman CA. Surgical management of spondylolysis and spondylolisthesis in athletes: indications and return to play. Curr Sports Med Rep. 8(1):35-40.
18. Morita T, Ikata T, Katoh S, Miyake R. Lumbar spondylolysis in children and adolescents. J Bone Joint Surg Br. 1995;77(4):620-625.
19. Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656-671.
20. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29(2):146-156.
21. Bono CM. Low-back pain in athletes. J Bone Joint Surg Am. 2004;86-A(2):382-396.
22. Dreisinger TE, Nelson B. Management of back pain in athletes. Sports Med. 1996;21(4):313-320.
23. Hsu WK, McCarthy KJ, Savage JW, et al. The Professional Athlete Spine Initiative: outcomes after lumbar disc herniation in 342 elite professional athletes. Spine J. 2011;11(3):180-186.
24. Dreyfuss PH, Dreyer SJ; NASS. Lumbar zygapophysial (facet) joint injections. Spine J. 2003;3(3 Suppl):50S-59S.
25. Slipman CW, Bhat AL, Gilchrist R V, Issac Z, Chou L, Lenrow DA. A critical review of the evidence for the use of zygapophysial injections and radiofrequency denervation in the treatment of low back pain. Spine J. 2003;3(4):310-316.
26. Prather H. Sacroiliac joint pain: practical management. Clin J Sport Med. 2003;13(4):252-255.
27. Graw BP, Wiesel SW. Low back pain in the aging athlete. Sports Med Arthrosc. 2008;16(1):39-46.
28. Melancia JL, Francisco AF, Antunes JL. Spinal stenosis. Handb Clin Neurol. 2014;119:541-549.
29. Frank C, Kobesova A, Kolar P. Dynamic neuromuscular stabilization & sports rehabilitation. Int J Sports Phys Ther. 2013;8(1):62-73.
30. Cholewicki J, Juluru K, McGill SM. Intra-abdominal pressure mechanism for stabilizing the lumbar spine. J Biomech. 1999;32(1):13-17.
31. McGill SM, Grenier S, Kavcic N, Cholewicki J. Coordination of muscle activity to assure stability of the lumbar spine. J Electromyogr Kinesiol. 2003;13(4):353-359.
32. Conte SA, Thompson MM, Marks MA, Dines JS. Abdominal muscle strains in professional baseball: 1991-2010. Am J Sports Med. 2012;40(3):650-656.
33. Chaudhari AMW, McKenzie CS, Borchers JR, Best TM. Lumbopelvic control and pitching performance of professional baseball pitchers. J Strength Cond Res. 2011;25(8):2127-2132.
For the last 20 years, injuries resulting in time out of play have been on the rise in Major League Baseball (MLB), and those affecting the back are no exception.1,2 In the first comprehensive report on injuries in MLB players, back injuries resulted in a mean of 1016 disabled list days per season from 1995 to 1999.1 Similarly, core and back injuries were responsible for 359 disabled list designations from 2002 to 2008. This represented 11.7% of all injuries resulting in time out of play during that time span.2 During that time, back injury prevalence ranked 6th highest of all possible body regions (out of 17), and both position players and pitchers were similarly affected (7.8% and 7.4% of all injuries, respectively).2 These injuries often result in a significant time out of play and can have a tremendous impact on player health. A healthy, stable, and well-functioning lumbar spine is a prerequisite for nearly all baseball-related activities, including pitching, throwing, batting, and running. Accordingly, even minor lumbar spine injuries may profoundly influence baseball performance. Despite this, less is currently known about the true epidemiology and impact of back injuries in professional baseball compared to other professional sporting organizations.3
The most common causes of low back pain and injury in elite baseball players include muscle strains, stress fractures (spondylolysis), annular tears, disc herniation, stenosis, transverse process fractures, facetogenic pain, and sacroiliac (SI) joint arthropathy.4-8 These injuries present in a variety of ways with varying symptomatology. Accordingly, a thorough understanding and comprehensive approach to the diagnosis and treatment of these injuries is necessary. The purpose of this article is to discuss the current state of lumbar spine injuries in professional baseball players. Specifically, we will discuss the critical role of the spine in baseball activities, common causes of injury, tips for making the diagnosis, treatment options, outcomes, and injury prevention and rehabilitation strategies.
Role of the Spine in Baseball
The spine and core musculature are responsible for positioning the head, shoulders, and upper extremities in space over the hips and lower extremities. Proper maintenance of this relationship is required during all phases of throwing, pitching, running, and hitting. During these activities, the spine may dynamically flex, extend, rotate, and laterally bend as needed to keep the body balanced with the head centered over the trunk.
Pitching and Throwing
Whether pitching from the wind-up or the stretch, the head begins centered over the hips and pelvis. As the pitching motion progresses, the hips undergo rotation, flexion, extension, abduction, and circumduction. While this is occurring, the shoulders and upper truck must bend, rotate, and translate toward home plate with the body. Just prior to front foot contact, trunk rotation averages 55 ± 6° with a maximal mean angular acceleration of 11,600 ± 3100°/s2. 9 In order for the body to remain balanced, controlled, and synchronized throughout this delivery, the lumbar spine and core musculature must work diligently to stabilize the entire kinetic chain. Of all the trunk muscles (paraspinal, rectus abdominis, obliques, and glutei), the lumbar paraspinal muscles often work the hardest during the pitching motion, demonstrating activity increases ranging from 100% to 400%.10 Accordingly, it is not uncommon for pitchers to develop SI joint or lumbar facet joint pain due to this high degree of torsional strain exerted on the low back.4 Poor lumbopelvic control has been shown to be a predictor of subsequent injury, and the degree of lumbopelvic dysfunction is proportional to injury severity in MLB pitchers.5
Hitting
Similar to pitching, hitting involves a complex combination of movements from the upper and lower extremities that must be balanced by the core and spine. Although numerous movements occur simultaneously, rotational motion is primarily responsible for generating power. The trunk rotates an average of 46 ± 9° during the swing and reaches a maximal angular acceleration of 7200 ± 2800°/s2 just after contact.9 During this period of rapid torsion, the spine must rotate in conjunction with the hips and shoulders to create a stable cylinder and axis of rotation. The spine and core are responsible for synchronizing rotation to ensure that hip and shoulder parallelism is maintained from swing initiation to ball contact. If the body does not rotate as a unit, the position of the head is affected and the batter’s ability to see the ball may be compromised. Additionally, if delivery of the shoulders lags too far behind that of the hips, the position of the hands (and bat) in space is adversely affected. The entire kinetic chain must remain balanced, coordinated, precisely timed, and standardized throughout the entire swing from initial trigger to final follow-through. The lumbar spine plays a critical role in each of these steps. If lumbar spine mechanics are not sound, this can have significant adverse effects on batting performance and may predispose hitters to injury.4
Common Etiologies for Spinal Injury
The vast majority of baseball players who experience lumbar pain will have injuries that can be classified as mechanical back pain (ie, spondylolysis, annular tears, facetogenic pain, SI joint arthropathy, or muscle injuries) (Table). Although less likely to occur, nerve entrapment or impingement syndromes (ie, disc herniation, stenosis, and peripheral nerve entrapment) have been observed in professional baseball players. Finally, more concerning pathologies such as infection and tumor are extremely rare, but they must not be overlooked in this high-demand patient population.
Stress Fracture or Spondylolysis
In young athletic patients, up to one-third of those with low back pain may have evidence of a lumbar stress fracture on bone scan.11,12 This is particularly true for athletes who undergo repetitive lumbar extension and rotation, such as linemen, gymnasts, wrestlers, weight lifters, and baseball players.4,13 Although the majority of lumbar stress fractures occur at the pars interarticularis, they can occur in the pedicle or articular process (Figure 1). Most spondylolytic lesions do not progress to spondylolisthesis, especially once patients reach skeletal maturity. Because the fifth lumbar vertebra represents the transition from the lumbar to the sacral spine, most stress fractures occur at L5. These typically present as localized low back pain that worsens with flexion, extension, and rotation.
Muscle Injury
One of the most common causes of low back pain in athletes is muscle strains and spasms. Because the lumbar paraspinal muscles are extremely active during throwing and hitting,10 they are particularly susceptible to injury. This is particularly true in deconditioned athletes or those who report to spring training having not adequately maintained strength and flexibility through the off-season.4,5 These injuries typically present in an acute fashion with an obvious inciting incident. Players may have a history of similar muscle injuries in the past. On examination, they tend to have difficulty maintaining normal posture or ranging the spine through a full arc of motion. Localized, superficial tenderness to palpation in the injured muscle is a key component of the diagnosis.
Annular Tears and Disc Herniation
These injuries typically occur as the result of a combination of compressive and rotary forces on the lumbar spine that overcome the ability of the annulus fibrosus to resist hoop stresses. Patients with annular tears typically present with severe lower back pain that may be accompanied by spasm and pain radiation into the buttock or lower extremities. Pain is usually worsened by valsalva, coughing, sneezing, or bearing down.4 Although annular tears can occur in isolation, they can also lead to herniation of the nucleus pulposus into the spinal canal (Figure 2). Depending on the location and severity of the herniation, nerve entrapment or impingement can occur. This may initially present as pain that radiates into the lower extremities in a dermatomal fashion. As the herniation progresses, decreased sensation and weakness may develop.
Facet Joint Pain
Facetogenic pain can occur as the result of degenerative changes, trauma, or joint inflammation. Facet injury typically occurs during rotation while the back is extended.4 This results in localized pain and tenderness that can be reproduced by loading the facet joint (lumbar extension) during the examination, and patients will often demonstrate discomfort and altered motion when extending the flexed back.
Sacroiliac Joint Pain
Although pain in the region of the SI joint is very common, much of this may actually be referred from more centrally located neuromotion segments.4 SI joint pathology can occur as a result of trauma, degeneration, or inflammatory processes as is seen in ankylosing spondylitis (AS). Patients with AS typically present with a gradual onset of progressive stiffness and pain in the low back and hips that is worse in the morning or following periods of inactivity. It is most common in Caucasian males in their second to fourth decades.14 Although 80% to 95% of patients with AS will test positive for human leukocyte antigen B27 (HLA-B27), it is important to note that the vast majority of people with HLA-B27 do not go on to develop AS.14 Regardless of the cause, SI joint pain can be very debilitating and negatively impact all baseball-related activities.
Stenosis
Lumbar stenosis may develop from arthritic changes, disc protrusion, facet hypertrophy, or ligament ossification. In this young, athletic population, congenital stenosis should also be a consideration. Patients with congenital stenosis at baseline are at increased risk for developing neurologic symptoms from disc protrusion or other acquired spinal pathology. Lumbar stenosis generally manifests as a gradual onset of progressive low back pain with radicular symptoms or neurogenic claudication.4
Making the Diagnosis
History
When identifying the cause of any musculoskeletal complaint, the diagnosis begins with a thorough history. In addition to the standard components of the history, such as timing, severity, relation to activity, exacerbating factors, associated symptoms, and prior treatments, Watkins and colleagues4 have outlined a number of key factors that should be determined when specifically evaluating the athlete with low back pain.These include quantification of the morbidity, identification of contributing psychosocial factors, ruling out of urgent diagnoses (ie, neoplasm, infection, rapidly progressive neurologic deficits, cauda equina, and paralysis), determination of injury type and duration, identification of the clinical syndrome/etiology, pinpointing the location of the pathology (what nerve at what level?), and quantification of back versus leg symptoms. Answers to these questions will set the framework for an appropriately directed physical examination, imaging, and diagnostic tests.
Physical Examination
The physical examination begins by observing the patient or player walk across the playing field, training room, or examination room, paying attention to posture, gait, and overall body movement. Many patients with lumbar injuries will demonstrate adaptive patterns of motion in an attempt to accommodate their pain. This may be seen during baseball-related activities such as throwing, batting, or running. The spine should be visualized and palpated for malalignment while standing erect and during forward bending. If possible, motion should be assessed in rotation, lateral bending, and the flexion and extension planes. Special attention should be paid to any positions or maneuvers that reproduce pain or neurologic symptoms. Areas of tenderness and radiating pain should be fully palpated. A full neurologic examination consisting of manual muscle testing, sensory examination, and reflex evaluation of both the upper and lower extremities should be performed. Numerous special tests and neurologic stretch maneuvers that assess specific lumbar nerve roots have been described.15
Imaging and Diagnostic Tests
Depending on the history and physical examination, imaging of the lumbar spine is not always warranted in the acute setting. This is especially the case if muscle injury, herniation, or annular tears are suspected. In cases of persistent pain, trauma, or suspected neoplasia, imaging is generally warranted. When x-rays are negative and spondylolysis is suspected, bone scan with lumbar single photon emission computed tomography (SPECT) is the most sensitive test.16 SPECT scans are positive in active spondylolysis because the radio-nucleotide is taken up by active, bone-forming osteoblasts. Quiescent stress fractures that are not apparent on SPECT scans are generally chronic and painless.4 If the SPECT scan is positive, the injury can be further characterized by computed tomography (CT) (Figure 1), which can distinguish between spondylolysis, osteoid osteoma, osteoblastoma, acute fracture, or arthritic degeneration. When the SPECT is negative, or if neural impingement is suspected, magnetic resonance imaging (MRI) (Figure 2) is likely the best diagnostic imaging tool. MRI allows identification of bone edema, disc herniation, annular disruption, disc desiccation, stenosis, and nerve entrapment. Finally, when attempting to distinguish between central and peripheral nerve entrapment syndromes, an electromyogram (EMG) or nerve conduction study (NCS) is a reliable way to identify the location of injury.
Treatment and Outcomes
The approach to a patient with low back pain begins with identification of the etiology and discontinuation of the activities that reproduce pain.4 Trunk stabilization exercises and anti-inflammatory medications are the mainstays of treatment regardless of the cause of the lumbar spinal injury in the baseball player.4
Stress Fracture or Spondylolysis
Management of symptomatic spondylolysis or spondylolisthesis in the athlete initially consists of conservative treatment, which achieves good to excellent long-term outcomes and return to play in 70% to 90% of athletes, especially for acute injuries.17-19 After stopping the activity that causes the pain, trunk stabilization exercises should be started as soon as tolerated with the use of non-steroidal anti-inflammatory medications (NSAIDs), oral steroids, and spinal injections to control symptoms and permit initiation of the rehabilitation program.4 Although bracing is a commonly used adjunctive treatment, a recent meta-analysis did not demonstrate any difference in clinical outcomes between patients treated with a brace compared to non-braced controls.20
Surgical indications for the treatment of spondylolysis or spondylolisthesis are limited; however, failure of nonoperative treatment after 6 months is a reasonable time to consider surgery.17 The spondylolytic defect can often be repaired directly using hook screws, translaminar screws, wiring, pedicle screws, or image-guided lag screws across the lesion with grafting.4 Lumbar spinal fusion is less successful in professional athletes due to the high demands placed on adjacent levels as well as the time required for the fusion to heal.4 Bony union can be determined by a CT scan at 6 months postoperatively if the patient has met appropriate return to play criteria.4
Muscle Injury
Management of lumbar sprains and strains typically includes restricting painful postures and a rehabilitation program that focuses on core strengthening within a pain-free arc of motion.21 Because acute injuries typically resolve quickly and spontaneously, a short interval of decreased activity, icing, NSAIDs, and stretching followed by focused strength training is appropriate before return to sports activity.22
Annular Tears and Disc Herniation
Initial management of baseball players with acute lumbar disc herniation and/or annular tears consists of rest for up to 5 days followed by physical therapy and NSAIDs, Medrol Dose pak, or epidural injections.4 Professional baseball players return to play at high rates following a herniated lumbar disc.6 Earhart and colleagues6 found that 97.1% of players returned to play at an average time of 6.6 months from the time of injury. When stratified by position, all pitchers (29 of 29) returned to competitive play after operative or nonoperative management, while 38 of 40 hitters returned.6 The average career length after lumbar disc herniation in the professional baseball player is between 4.1 and 5.3 years or between 256 and 471 games.6,23 Other work has suggested that players undergoing operative treatment for lumbar herniation had shorter career lengths; however, patients in the operative group tended to be older at the time of injury.23
Emphasis should be placed on nonoperative management of baseball players with disc pathology except in cases of cauda equina syndrome.4 Hitters and pitchers who require surgery have demonstrated decreased 1-year and 3-year postoperative statistical performance compared to preinjury levels.6 No significant changes in any performance statistic were seen in baseball following nonoperative management.6 Consequently, indications for surgery in the baseball player with lumbar disc pathology includes cauda equina syndrome, progressive neurologic deficit, sufficient morbidity, failure of conservative care, a lesion that can be corrected safely with surgery, and the ability for the patient to comply with a comprehensive postoperative rehabilitation program.4 Operative treatment typically consists of a lumbar microdiscectomy and/or laminotomy. 4,6
Facet Joint Pain
The mainstay of therapy in patients with facet joint pain consists of analgesia and a trunk stabilization program.24 Lumbar zygapophysial joint injections and radiofrequency denervation can be considered if the patient fails 4 weeks of directed conservative treatment.24,25 Injections may be useful in select patients; however, the literature supporting the use of lumbar facet joint injections or radiofrequency denervation for facetogenic pain is limited.24,25
Sacroiliac Joint Pain
Acute injury of the SI joint can be treated with NSAIDs, icing, and relative rest.26 Mobilization of the SI joint in addition to correcting any asymmetries in muscle length or stiffness should be started and progressed as soon as tolerated within a pain-free range of motion.26 Rehabilitation should correct biomechanical deficits and maladaptation with a special focus on agonist and antagonist muscle groups across the sacrum and ilium.26 Treatment of AS in the athlete should emphasize symptom control, as there is no definite treatment. For patients with AS, other long-term therapeutic options include sulfasalazine, methotrexate, thalidomide, and anti-tumor necrosis factor therapies.14
Stenosis
Lumbar spinal stenosis, whether congenital or acquired, should initially be managed conservatively.27 Although they do not alter the progression of the disease, epidural steroids and local injections may temporarily decrease symptoms in approximately 40% of cases.27 Those who fail conservative therapy after 3 months may be candidates for surgical decompression and/or fusion.27,28 However, surgical treatment for lumbar spinal stenosis in elite baseball players has not been thoroughly studied, so the long-term prognosis is not well documented.27
Rehabilitation and Prevention of Injuries
After an appropriate diagnosis has been made, a structured rehabilitation process should commence. During rehabilitation, it is of primary importance that deep core stabilization is established. As an initial step in this process, athletes are trained to initiate deep core stabilization with breathing techniques in a static, supine position.29 Proper diaphragm activation with co-contractions of the transverse abdominis (TA) and pelvic floor has been shown to increase lumbar spine stability.30 This will allow for an increase in intra-abdominal pressure (IAP) and improved stabilization of the lumbar spine, creating a muscular cylinder between the bottom of the rib cage and top of the pelvis. These activities are initiated in the supine position but are soon advanced as upper and lower extremity movement against resistance is added. It is important to make sure IAP and contraction of the TA is maintained throughout this sequence of progression.
Once deep core stabilization has been established, athletes are progressed to global muscle training and kinetic linking in all 3 planes of movement. This is an important phase, as lumbar stability is a result of coordinated muscle activation involving many muscles.31 This program progresses from supine breathing exercises to a modified side bridge position to enhance core activation along with frontal plane stability. Next, athletes are progressed to a half kneeling position and then on to standing. Rotational activities are introduced starting with isometric holds progressing to chops/lifts and rotational medicine ball toss. During these tasks, focus should be on quality of movement and maintenance of core activation. Endurance of these muscles should be trained during this process. Appropriate pain-free and safe cardiovascular exercise, such as walking, biking, swimming, and jogging, should be performed throughout each stage in the rehabilitation process. Activities should be halted with any increase in pain. At the completion of the rehabilitation process, it is important to observe the athlete while performing sport-specific tasks. Spinal stabilization must be translational and monitored by observing maintenance of the “cylinder” from the training room to sports specific movements.
Since poor lumbar control has been associated with increased amount of time on the disabled list,5 it would be ideal to identify those at risk of injury before problems arise. Conte and colleagues32 have shown that core muscle strains could be a result of muscle imbalance or improper pitching or hitting technique. Other work has demonstrated that pitchers with poor lumbopelvic control did not perform as well as those with superior control.33 By assessing spinal stability and biomechanics at baseline, we may be able to identify those at risk. Pitchers with suboptimal spinal stabilization can present with an unstable balance phase, increased amounts of hyperextension of the lumbar spine from the moment of max cocking through ball release, as well as increased lateral trunk tilt at ball release. Correcting these flaws and increasing deep core stabilization can prevent injuries and improve performance.
Summary
A stable, well-functioning lumbar spine is vital to nearly every baseball-related activity, including pitching, throwing, batting, fielding, and running. The spine serves as a critical link in the kinetic chain between the upper and lower extremities. Due to the high demand on the lumbar spine, injuries to this area represent a significant amount of time out of play in MLB. Initial treatment typically consists of a comprehensive nonoperative rehabilitation process involving analgesics, rest, and therapy focusing on core stabilization. Because poor lumbopelvic control and mechanics have been demonstrated to increase injury risk, preemptive spinal and core stabilization is likely an appropriate step towards injury prevention.
For the last 20 years, injuries resulting in time out of play have been on the rise in Major League Baseball (MLB), and those affecting the back are no exception.1,2 In the first comprehensive report on injuries in MLB players, back injuries resulted in a mean of 1016 disabled list days per season from 1995 to 1999.1 Similarly, core and back injuries were responsible for 359 disabled list designations from 2002 to 2008. This represented 11.7% of all injuries resulting in time out of play during that time span.2 During that time, back injury prevalence ranked 6th highest of all possible body regions (out of 17), and both position players and pitchers were similarly affected (7.8% and 7.4% of all injuries, respectively).2 These injuries often result in a significant time out of play and can have a tremendous impact on player health. A healthy, stable, and well-functioning lumbar spine is a prerequisite for nearly all baseball-related activities, including pitching, throwing, batting, and running. Accordingly, even minor lumbar spine injuries may profoundly influence baseball performance. Despite this, less is currently known about the true epidemiology and impact of back injuries in professional baseball compared to other professional sporting organizations.3
The most common causes of low back pain and injury in elite baseball players include muscle strains, stress fractures (spondylolysis), annular tears, disc herniation, stenosis, transverse process fractures, facetogenic pain, and sacroiliac (SI) joint arthropathy.4-8 These injuries present in a variety of ways with varying symptomatology. Accordingly, a thorough understanding and comprehensive approach to the diagnosis and treatment of these injuries is necessary. The purpose of this article is to discuss the current state of lumbar spine injuries in professional baseball players. Specifically, we will discuss the critical role of the spine in baseball activities, common causes of injury, tips for making the diagnosis, treatment options, outcomes, and injury prevention and rehabilitation strategies.
Role of the Spine in Baseball
The spine and core musculature are responsible for positioning the head, shoulders, and upper extremities in space over the hips and lower extremities. Proper maintenance of this relationship is required during all phases of throwing, pitching, running, and hitting. During these activities, the spine may dynamically flex, extend, rotate, and laterally bend as needed to keep the body balanced with the head centered over the trunk.
Pitching and Throwing
Whether pitching from the wind-up or the stretch, the head begins centered over the hips and pelvis. As the pitching motion progresses, the hips undergo rotation, flexion, extension, abduction, and circumduction. While this is occurring, the shoulders and upper truck must bend, rotate, and translate toward home plate with the body. Just prior to front foot contact, trunk rotation averages 55 ± 6° with a maximal mean angular acceleration of 11,600 ± 3100°/s2. 9 In order for the body to remain balanced, controlled, and synchronized throughout this delivery, the lumbar spine and core musculature must work diligently to stabilize the entire kinetic chain. Of all the trunk muscles (paraspinal, rectus abdominis, obliques, and glutei), the lumbar paraspinal muscles often work the hardest during the pitching motion, demonstrating activity increases ranging from 100% to 400%.10 Accordingly, it is not uncommon for pitchers to develop SI joint or lumbar facet joint pain due to this high degree of torsional strain exerted on the low back.4 Poor lumbopelvic control has been shown to be a predictor of subsequent injury, and the degree of lumbopelvic dysfunction is proportional to injury severity in MLB pitchers.5
Hitting
Similar to pitching, hitting involves a complex combination of movements from the upper and lower extremities that must be balanced by the core and spine. Although numerous movements occur simultaneously, rotational motion is primarily responsible for generating power. The trunk rotates an average of 46 ± 9° during the swing and reaches a maximal angular acceleration of 7200 ± 2800°/s2 just after contact.9 During this period of rapid torsion, the spine must rotate in conjunction with the hips and shoulders to create a stable cylinder and axis of rotation. The spine and core are responsible for synchronizing rotation to ensure that hip and shoulder parallelism is maintained from swing initiation to ball contact. If the body does not rotate as a unit, the position of the head is affected and the batter’s ability to see the ball may be compromised. Additionally, if delivery of the shoulders lags too far behind that of the hips, the position of the hands (and bat) in space is adversely affected. The entire kinetic chain must remain balanced, coordinated, precisely timed, and standardized throughout the entire swing from initial trigger to final follow-through. The lumbar spine plays a critical role in each of these steps. If lumbar spine mechanics are not sound, this can have significant adverse effects on batting performance and may predispose hitters to injury.4
Common Etiologies for Spinal Injury
The vast majority of baseball players who experience lumbar pain will have injuries that can be classified as mechanical back pain (ie, spondylolysis, annular tears, facetogenic pain, SI joint arthropathy, or muscle injuries) (Table). Although less likely to occur, nerve entrapment or impingement syndromes (ie, disc herniation, stenosis, and peripheral nerve entrapment) have been observed in professional baseball players. Finally, more concerning pathologies such as infection and tumor are extremely rare, but they must not be overlooked in this high-demand patient population.
Stress Fracture or Spondylolysis
In young athletic patients, up to one-third of those with low back pain may have evidence of a lumbar stress fracture on bone scan.11,12 This is particularly true for athletes who undergo repetitive lumbar extension and rotation, such as linemen, gymnasts, wrestlers, weight lifters, and baseball players.4,13 Although the majority of lumbar stress fractures occur at the pars interarticularis, they can occur in the pedicle or articular process (Figure 1). Most spondylolytic lesions do not progress to spondylolisthesis, especially once patients reach skeletal maturity. Because the fifth lumbar vertebra represents the transition from the lumbar to the sacral spine, most stress fractures occur at L5. These typically present as localized low back pain that worsens with flexion, extension, and rotation.
Muscle Injury
One of the most common causes of low back pain in athletes is muscle strains and spasms. Because the lumbar paraspinal muscles are extremely active during throwing and hitting,10 they are particularly susceptible to injury. This is particularly true in deconditioned athletes or those who report to spring training having not adequately maintained strength and flexibility through the off-season.4,5 These injuries typically present in an acute fashion with an obvious inciting incident. Players may have a history of similar muscle injuries in the past. On examination, they tend to have difficulty maintaining normal posture or ranging the spine through a full arc of motion. Localized, superficial tenderness to palpation in the injured muscle is a key component of the diagnosis.
Annular Tears and Disc Herniation
These injuries typically occur as the result of a combination of compressive and rotary forces on the lumbar spine that overcome the ability of the annulus fibrosus to resist hoop stresses. Patients with annular tears typically present with severe lower back pain that may be accompanied by spasm and pain radiation into the buttock or lower extremities. Pain is usually worsened by valsalva, coughing, sneezing, or bearing down.4 Although annular tears can occur in isolation, they can also lead to herniation of the nucleus pulposus into the spinal canal (Figure 2). Depending on the location and severity of the herniation, nerve entrapment or impingement can occur. This may initially present as pain that radiates into the lower extremities in a dermatomal fashion. As the herniation progresses, decreased sensation and weakness may develop.
Facet Joint Pain
Facetogenic pain can occur as the result of degenerative changes, trauma, or joint inflammation. Facet injury typically occurs during rotation while the back is extended.4 This results in localized pain and tenderness that can be reproduced by loading the facet joint (lumbar extension) during the examination, and patients will often demonstrate discomfort and altered motion when extending the flexed back.
Sacroiliac Joint Pain
Although pain in the region of the SI joint is very common, much of this may actually be referred from more centrally located neuromotion segments.4 SI joint pathology can occur as a result of trauma, degeneration, or inflammatory processes as is seen in ankylosing spondylitis (AS). Patients with AS typically present with a gradual onset of progressive stiffness and pain in the low back and hips that is worse in the morning or following periods of inactivity. It is most common in Caucasian males in their second to fourth decades.14 Although 80% to 95% of patients with AS will test positive for human leukocyte antigen B27 (HLA-B27), it is important to note that the vast majority of people with HLA-B27 do not go on to develop AS.14 Regardless of the cause, SI joint pain can be very debilitating and negatively impact all baseball-related activities.
Stenosis
Lumbar stenosis may develop from arthritic changes, disc protrusion, facet hypertrophy, or ligament ossification. In this young, athletic population, congenital stenosis should also be a consideration. Patients with congenital stenosis at baseline are at increased risk for developing neurologic symptoms from disc protrusion or other acquired spinal pathology. Lumbar stenosis generally manifests as a gradual onset of progressive low back pain with radicular symptoms or neurogenic claudication.4
Making the Diagnosis
History
When identifying the cause of any musculoskeletal complaint, the diagnosis begins with a thorough history. In addition to the standard components of the history, such as timing, severity, relation to activity, exacerbating factors, associated symptoms, and prior treatments, Watkins and colleagues4 have outlined a number of key factors that should be determined when specifically evaluating the athlete with low back pain.These include quantification of the morbidity, identification of contributing psychosocial factors, ruling out of urgent diagnoses (ie, neoplasm, infection, rapidly progressive neurologic deficits, cauda equina, and paralysis), determination of injury type and duration, identification of the clinical syndrome/etiology, pinpointing the location of the pathology (what nerve at what level?), and quantification of back versus leg symptoms. Answers to these questions will set the framework for an appropriately directed physical examination, imaging, and diagnostic tests.
Physical Examination
The physical examination begins by observing the patient or player walk across the playing field, training room, or examination room, paying attention to posture, gait, and overall body movement. Many patients with lumbar injuries will demonstrate adaptive patterns of motion in an attempt to accommodate their pain. This may be seen during baseball-related activities such as throwing, batting, or running. The spine should be visualized and palpated for malalignment while standing erect and during forward bending. If possible, motion should be assessed in rotation, lateral bending, and the flexion and extension planes. Special attention should be paid to any positions or maneuvers that reproduce pain or neurologic symptoms. Areas of tenderness and radiating pain should be fully palpated. A full neurologic examination consisting of manual muscle testing, sensory examination, and reflex evaluation of both the upper and lower extremities should be performed. Numerous special tests and neurologic stretch maneuvers that assess specific lumbar nerve roots have been described.15
Imaging and Diagnostic Tests
Depending on the history and physical examination, imaging of the lumbar spine is not always warranted in the acute setting. This is especially the case if muscle injury, herniation, or annular tears are suspected. In cases of persistent pain, trauma, or suspected neoplasia, imaging is generally warranted. When x-rays are negative and spondylolysis is suspected, bone scan with lumbar single photon emission computed tomography (SPECT) is the most sensitive test.16 SPECT scans are positive in active spondylolysis because the radio-nucleotide is taken up by active, bone-forming osteoblasts. Quiescent stress fractures that are not apparent on SPECT scans are generally chronic and painless.4 If the SPECT scan is positive, the injury can be further characterized by computed tomography (CT) (Figure 1), which can distinguish between spondylolysis, osteoid osteoma, osteoblastoma, acute fracture, or arthritic degeneration. When the SPECT is negative, or if neural impingement is suspected, magnetic resonance imaging (MRI) (Figure 2) is likely the best diagnostic imaging tool. MRI allows identification of bone edema, disc herniation, annular disruption, disc desiccation, stenosis, and nerve entrapment. Finally, when attempting to distinguish between central and peripheral nerve entrapment syndromes, an electromyogram (EMG) or nerve conduction study (NCS) is a reliable way to identify the location of injury.
Treatment and Outcomes
The approach to a patient with low back pain begins with identification of the etiology and discontinuation of the activities that reproduce pain.4 Trunk stabilization exercises and anti-inflammatory medications are the mainstays of treatment regardless of the cause of the lumbar spinal injury in the baseball player.4
Stress Fracture or Spondylolysis
Management of symptomatic spondylolysis or spondylolisthesis in the athlete initially consists of conservative treatment, which achieves good to excellent long-term outcomes and return to play in 70% to 90% of athletes, especially for acute injuries.17-19 After stopping the activity that causes the pain, trunk stabilization exercises should be started as soon as tolerated with the use of non-steroidal anti-inflammatory medications (NSAIDs), oral steroids, and spinal injections to control symptoms and permit initiation of the rehabilitation program.4 Although bracing is a commonly used adjunctive treatment, a recent meta-analysis did not demonstrate any difference in clinical outcomes between patients treated with a brace compared to non-braced controls.20
Surgical indications for the treatment of spondylolysis or spondylolisthesis are limited; however, failure of nonoperative treatment after 6 months is a reasonable time to consider surgery.17 The spondylolytic defect can often be repaired directly using hook screws, translaminar screws, wiring, pedicle screws, or image-guided lag screws across the lesion with grafting.4 Lumbar spinal fusion is less successful in professional athletes due to the high demands placed on adjacent levels as well as the time required for the fusion to heal.4 Bony union can be determined by a CT scan at 6 months postoperatively if the patient has met appropriate return to play criteria.4
Muscle Injury
Management of lumbar sprains and strains typically includes restricting painful postures and a rehabilitation program that focuses on core strengthening within a pain-free arc of motion.21 Because acute injuries typically resolve quickly and spontaneously, a short interval of decreased activity, icing, NSAIDs, and stretching followed by focused strength training is appropriate before return to sports activity.22
Annular Tears and Disc Herniation
Initial management of baseball players with acute lumbar disc herniation and/or annular tears consists of rest for up to 5 days followed by physical therapy and NSAIDs, Medrol Dose pak, or epidural injections.4 Professional baseball players return to play at high rates following a herniated lumbar disc.6 Earhart and colleagues6 found that 97.1% of players returned to play at an average time of 6.6 months from the time of injury. When stratified by position, all pitchers (29 of 29) returned to competitive play after operative or nonoperative management, while 38 of 40 hitters returned.6 The average career length after lumbar disc herniation in the professional baseball player is between 4.1 and 5.3 years or between 256 and 471 games.6,23 Other work has suggested that players undergoing operative treatment for lumbar herniation had shorter career lengths; however, patients in the operative group tended to be older at the time of injury.23
Emphasis should be placed on nonoperative management of baseball players with disc pathology except in cases of cauda equina syndrome.4 Hitters and pitchers who require surgery have demonstrated decreased 1-year and 3-year postoperative statistical performance compared to preinjury levels.6 No significant changes in any performance statistic were seen in baseball following nonoperative management.6 Consequently, indications for surgery in the baseball player with lumbar disc pathology includes cauda equina syndrome, progressive neurologic deficit, sufficient morbidity, failure of conservative care, a lesion that can be corrected safely with surgery, and the ability for the patient to comply with a comprehensive postoperative rehabilitation program.4 Operative treatment typically consists of a lumbar microdiscectomy and/or laminotomy. 4,6
Facet Joint Pain
The mainstay of therapy in patients with facet joint pain consists of analgesia and a trunk stabilization program.24 Lumbar zygapophysial joint injections and radiofrequency denervation can be considered if the patient fails 4 weeks of directed conservative treatment.24,25 Injections may be useful in select patients; however, the literature supporting the use of lumbar facet joint injections or radiofrequency denervation for facetogenic pain is limited.24,25
Sacroiliac Joint Pain
Acute injury of the SI joint can be treated with NSAIDs, icing, and relative rest.26 Mobilization of the SI joint in addition to correcting any asymmetries in muscle length or stiffness should be started and progressed as soon as tolerated within a pain-free range of motion.26 Rehabilitation should correct biomechanical deficits and maladaptation with a special focus on agonist and antagonist muscle groups across the sacrum and ilium.26 Treatment of AS in the athlete should emphasize symptom control, as there is no definite treatment. For patients with AS, other long-term therapeutic options include sulfasalazine, methotrexate, thalidomide, and anti-tumor necrosis factor therapies.14
Stenosis
Lumbar spinal stenosis, whether congenital or acquired, should initially be managed conservatively.27 Although they do not alter the progression of the disease, epidural steroids and local injections may temporarily decrease symptoms in approximately 40% of cases.27 Those who fail conservative therapy after 3 months may be candidates for surgical decompression and/or fusion.27,28 However, surgical treatment for lumbar spinal stenosis in elite baseball players has not been thoroughly studied, so the long-term prognosis is not well documented.27
Rehabilitation and Prevention of Injuries
After an appropriate diagnosis has been made, a structured rehabilitation process should commence. During rehabilitation, it is of primary importance that deep core stabilization is established. As an initial step in this process, athletes are trained to initiate deep core stabilization with breathing techniques in a static, supine position.29 Proper diaphragm activation with co-contractions of the transverse abdominis (TA) and pelvic floor has been shown to increase lumbar spine stability.30 This will allow for an increase in intra-abdominal pressure (IAP) and improved stabilization of the lumbar spine, creating a muscular cylinder between the bottom of the rib cage and top of the pelvis. These activities are initiated in the supine position but are soon advanced as upper and lower extremity movement against resistance is added. It is important to make sure IAP and contraction of the TA is maintained throughout this sequence of progression.
Once deep core stabilization has been established, athletes are progressed to global muscle training and kinetic linking in all 3 planes of movement. This is an important phase, as lumbar stability is a result of coordinated muscle activation involving many muscles.31 This program progresses from supine breathing exercises to a modified side bridge position to enhance core activation along with frontal plane stability. Next, athletes are progressed to a half kneeling position and then on to standing. Rotational activities are introduced starting with isometric holds progressing to chops/lifts and rotational medicine ball toss. During these tasks, focus should be on quality of movement and maintenance of core activation. Endurance of these muscles should be trained during this process. Appropriate pain-free and safe cardiovascular exercise, such as walking, biking, swimming, and jogging, should be performed throughout each stage in the rehabilitation process. Activities should be halted with any increase in pain. At the completion of the rehabilitation process, it is important to observe the athlete while performing sport-specific tasks. Spinal stabilization must be translational and monitored by observing maintenance of the “cylinder” from the training room to sports specific movements.
Since poor lumbar control has been associated with increased amount of time on the disabled list,5 it would be ideal to identify those at risk of injury before problems arise. Conte and colleagues32 have shown that core muscle strains could be a result of muscle imbalance or improper pitching or hitting technique. Other work has demonstrated that pitchers with poor lumbopelvic control did not perform as well as those with superior control.33 By assessing spinal stability and biomechanics at baseline, we may be able to identify those at risk. Pitchers with suboptimal spinal stabilization can present with an unstable balance phase, increased amounts of hyperextension of the lumbar spine from the moment of max cocking through ball release, as well as increased lateral trunk tilt at ball release. Correcting these flaws and increasing deep core stabilization can prevent injuries and improve performance.
Summary
A stable, well-functioning lumbar spine is vital to nearly every baseball-related activity, including pitching, throwing, batting, fielding, and running. The spine serves as a critical link in the kinetic chain between the upper and lower extremities. Due to the high demand on the lumbar spine, injuries to this area represent a significant amount of time out of play in MLB. Initial treatment typically consists of a comprehensive nonoperative rehabilitation process involving analgesics, rest, and therapy focusing on core stabilization. Because poor lumbopelvic control and mechanics have been demonstrated to increase injury risk, preemptive spinal and core stabilization is likely an appropriate step towards injury prevention.
1. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
2. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
3. Makhni EC, Buza JA, Byram I, Ahmad CS. Sports reporting: a comprehensive review of the medical literature regarding North American professional sports. Phys Sportsmed. 2014;42(2):154-162.
4. Watkins RG III, Watkins RG IV. Chapter 36: Lumbar injuries. In: Sports Medicine of Baseball. Dines JS, Altchek DW, Andrews JR, ElAttrache NS, Wilk KE, Yocum LA, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2012; 383-398.
5. Chaudhari AMW, McKenzie CS, Pan X, Oñate JA. Lumbopelvic control and days missed because of injury in professional baseball pitchers. Am J Sports Med. 2014;42(11):2734-2740.
6. Earhart JS, Roberts D, Roc G, Gryzlo S, Hsu W. Effects of lumbar disk herniation on the careers of professional baseball players. Orthopedics. 2012;35(1):43-49.
7. Hamid KS, Nwachukwu BU, Hsu E, Edgerton CA, Hobson DR, Lang JE. Orthopedic resident work-shift analysis: Are we making the best use of resident work hours? J Surg Educ. 2014;71(2):205-210.
8. Nair R, Kahlenberg CA, Hsu WK. Outcomes of lumbar discectomy in elite athletes: the need for high-level evidence. Clin Orthop Relat Res. 2015;473(6):1971-1977.
9. Fleisig GS, Hsu WK, Fortenbaugh D, Cordover A, Press JM. Trunk axial rotation in baseball pitching and batting. Sports Biomech. 2013;12(4):324-333.
10. Watkins RG, Dennis S, Dillin WH, et al. Dynamic EMG analysis of torque transfer in professional baseball pitchers. Spine (Phila Pa 1976). 1989;14(4):404-408.
11. Micheli LJ. Back injuries in gymnastics. Clin Sports Med. 1985;4(1):85-93.
12. Papanicolaou N, Wilkinson RH, Emans JB, Treves S, Micheli LJ. Bone scintigraphy and radiography in young athletes with low back pain. AJR Am J Roentgenol. 1985;145(5):1039-1044.
13. Elliott S, Hutson MA, Wastie ML. Bone scintigraphy in the assessment of spondylolysis in patients attending a sports injury clinic. Clin Radiol. 1988;39(3):269-272.
14. Kubiak EN, Moskovich R, Errico TJ, Di Cesare PE. Orthopaedic management of ankylosing spondylitis. J Am Acad Orthop Surg. 2005;13(4):267-278.
15. Miller KJ. Physical assessment of lower extremity radiculopathy and sciatica. J Chiropr Med. 2007;6(2):75-82.
16. Bellah RD, Summerville DA, Treves ST, Micheli LJ. Low-back pain in adolescent athletes: detection of stress injury to the pars interarticularis with SPECT. Radiology. 1991;180(2):509-512.
17. Radcliff KE, Kalantar SB, Reitman CA. Surgical management of spondylolysis and spondylolisthesis in athletes: indications and return to play. Curr Sports Med Rep. 8(1):35-40.
18. Morita T, Ikata T, Katoh S, Miyake R. Lumbar spondylolysis in children and adolescents. J Bone Joint Surg Br. 1995;77(4):620-625.
19. Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656-671.
20. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29(2):146-156.
21. Bono CM. Low-back pain in athletes. J Bone Joint Surg Am. 2004;86-A(2):382-396.
22. Dreisinger TE, Nelson B. Management of back pain in athletes. Sports Med. 1996;21(4):313-320.
23. Hsu WK, McCarthy KJ, Savage JW, et al. The Professional Athlete Spine Initiative: outcomes after lumbar disc herniation in 342 elite professional athletes. Spine J. 2011;11(3):180-186.
24. Dreyfuss PH, Dreyer SJ; NASS. Lumbar zygapophysial (facet) joint injections. Spine J. 2003;3(3 Suppl):50S-59S.
25. Slipman CW, Bhat AL, Gilchrist R V, Issac Z, Chou L, Lenrow DA. A critical review of the evidence for the use of zygapophysial injections and radiofrequency denervation in the treatment of low back pain. Spine J. 2003;3(4):310-316.
26. Prather H. Sacroiliac joint pain: practical management. Clin J Sport Med. 2003;13(4):252-255.
27. Graw BP, Wiesel SW. Low back pain in the aging athlete. Sports Med Arthrosc. 2008;16(1):39-46.
28. Melancia JL, Francisco AF, Antunes JL. Spinal stenosis. Handb Clin Neurol. 2014;119:541-549.
29. Frank C, Kobesova A, Kolar P. Dynamic neuromuscular stabilization & sports rehabilitation. Int J Sports Phys Ther. 2013;8(1):62-73.
30. Cholewicki J, Juluru K, McGill SM. Intra-abdominal pressure mechanism for stabilizing the lumbar spine. J Biomech. 1999;32(1):13-17.
31. McGill SM, Grenier S, Kavcic N, Cholewicki J. Coordination of muscle activity to assure stability of the lumbar spine. J Electromyogr Kinesiol. 2003;13(4):353-359.
32. Conte SA, Thompson MM, Marks MA, Dines JS. Abdominal muscle strains in professional baseball: 1991-2010. Am J Sports Med. 2012;40(3):650-656.
33. Chaudhari AMW, McKenzie CS, Borchers JR, Best TM. Lumbopelvic control and pitching performance of professional baseball pitchers. J Strength Cond Res. 2011;25(8):2127-2132.
1. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
2. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
3. Makhni EC, Buza JA, Byram I, Ahmad CS. Sports reporting: a comprehensive review of the medical literature regarding North American professional sports. Phys Sportsmed. 2014;42(2):154-162.
4. Watkins RG III, Watkins RG IV. Chapter 36: Lumbar injuries. In: Sports Medicine of Baseball. Dines JS, Altchek DW, Andrews JR, ElAttrache NS, Wilk KE, Yocum LA, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2012; 383-398.
5. Chaudhari AMW, McKenzie CS, Pan X, Oñate JA. Lumbopelvic control and days missed because of injury in professional baseball pitchers. Am J Sports Med. 2014;42(11):2734-2740.
6. Earhart JS, Roberts D, Roc G, Gryzlo S, Hsu W. Effects of lumbar disk herniation on the careers of professional baseball players. Orthopedics. 2012;35(1):43-49.
7. Hamid KS, Nwachukwu BU, Hsu E, Edgerton CA, Hobson DR, Lang JE. Orthopedic resident work-shift analysis: Are we making the best use of resident work hours? J Surg Educ. 2014;71(2):205-210.
8. Nair R, Kahlenberg CA, Hsu WK. Outcomes of lumbar discectomy in elite athletes: the need for high-level evidence. Clin Orthop Relat Res. 2015;473(6):1971-1977.
9. Fleisig GS, Hsu WK, Fortenbaugh D, Cordover A, Press JM. Trunk axial rotation in baseball pitching and batting. Sports Biomech. 2013;12(4):324-333.
10. Watkins RG, Dennis S, Dillin WH, et al. Dynamic EMG analysis of torque transfer in professional baseball pitchers. Spine (Phila Pa 1976). 1989;14(4):404-408.
11. Micheli LJ. Back injuries in gymnastics. Clin Sports Med. 1985;4(1):85-93.
12. Papanicolaou N, Wilkinson RH, Emans JB, Treves S, Micheli LJ. Bone scintigraphy and radiography in young athletes with low back pain. AJR Am J Roentgenol. 1985;145(5):1039-1044.
13. Elliott S, Hutson MA, Wastie ML. Bone scintigraphy in the assessment of spondylolysis in patients attending a sports injury clinic. Clin Radiol. 1988;39(3):269-272.
14. Kubiak EN, Moskovich R, Errico TJ, Di Cesare PE. Orthopaedic management of ankylosing spondylitis. J Am Acad Orthop Surg. 2005;13(4):267-278.
15. Miller KJ. Physical assessment of lower extremity radiculopathy and sciatica. J Chiropr Med. 2007;6(2):75-82.
16. Bellah RD, Summerville DA, Treves ST, Micheli LJ. Low-back pain in adolescent athletes: detection of stress injury to the pars interarticularis with SPECT. Radiology. 1991;180(2):509-512.
17. Radcliff KE, Kalantar SB, Reitman CA. Surgical management of spondylolysis and spondylolisthesis in athletes: indications and return to play. Curr Sports Med Rep. 8(1):35-40.
18. Morita T, Ikata T, Katoh S, Miyake R. Lumbar spondylolysis in children and adolescents. J Bone Joint Surg Br. 1995;77(4):620-625.
19. Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656-671.
20. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29(2):146-156.
21. Bono CM. Low-back pain in athletes. J Bone Joint Surg Am. 2004;86-A(2):382-396.
22. Dreisinger TE, Nelson B. Management of back pain in athletes. Sports Med. 1996;21(4):313-320.
23. Hsu WK, McCarthy KJ, Savage JW, et al. The Professional Athlete Spine Initiative: outcomes after lumbar disc herniation in 342 elite professional athletes. Spine J. 2011;11(3):180-186.
24. Dreyfuss PH, Dreyer SJ; NASS. Lumbar zygapophysial (facet) joint injections. Spine J. 2003;3(3 Suppl):50S-59S.
25. Slipman CW, Bhat AL, Gilchrist R V, Issac Z, Chou L, Lenrow DA. A critical review of the evidence for the use of zygapophysial injections and radiofrequency denervation in the treatment of low back pain. Spine J. 2003;3(4):310-316.
26. Prather H. Sacroiliac joint pain: practical management. Clin J Sport Med. 2003;13(4):252-255.
27. Graw BP, Wiesel SW. Low back pain in the aging athlete. Sports Med Arthrosc. 2008;16(1):39-46.
28. Melancia JL, Francisco AF, Antunes JL. Spinal stenosis. Handb Clin Neurol. 2014;119:541-549.
29. Frank C, Kobesova A, Kolar P. Dynamic neuromuscular stabilization & sports rehabilitation. Int J Sports Phys Ther. 2013;8(1):62-73.
30. Cholewicki J, Juluru K, McGill SM. Intra-abdominal pressure mechanism for stabilizing the lumbar spine. J Biomech. 1999;32(1):13-17.
31. McGill SM, Grenier S, Kavcic N, Cholewicki J. Coordination of muscle activity to assure stability of the lumbar spine. J Electromyogr Kinesiol. 2003;13(4):353-359.
32. Conte SA, Thompson MM, Marks MA, Dines JS. Abdominal muscle strains in professional baseball: 1991-2010. Am J Sports Med. 2012;40(3):650-656.
33. Chaudhari AMW, McKenzie CS, Borchers JR, Best TM. Lumbopelvic control and pitching performance of professional baseball pitchers. J Strength Cond Res. 2011;25(8):2127-2132.
Ureter and Nerve Root Compression Secondary to Expansile Fibrous Dysplasia of the Transverse Process
Fibrous dysplasia is a developmental abnormality caused by excessive proliferation of immature spindle-cell fibrous tissues in bones. It is characterized by benign bony growths, which can lead to local swelling, bony deformities, and lytic conversion, predisposing the bone to pathologic fractures. Although this process can occur in cortical bone, it primarily affects the trabecular bone, leading to enlargement and expansion from within the medullary space. Malignant transformation to osteosarcoma or fibrosarcoma can occur, although this is exceedingly rare (<0.5%).1,2
This case report describes a patient who presented with an expansile lytic mass in a lumbar transverse process that was postoperatively identified on pathology as monostotic fibrous dysplasia. Such lesions that involve the transverse processes are rare and have been associated with pain and significant discomfort.3-5 This is the first reported case of a transverse process fibrous dysplasia causing urinary retention and neurologic symptoms simultaneously. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
History
A 52-year-old black man presented to us with 6 to 8 months of increasing right flank pain, difficulty with urination, and right lower extremity pain in the area of his anterior thigh. He also complained of “buckling” of his thigh with ambulation. On review of systems, the patient denied any fevers, chills, headache, changes in weight or vision, or hearing problems. He had no systemic symptoms except for 6 months of frequent urinary tract infections and difficulty emptying his bladder, which resulted in urinary retention. He denied any significant medical history and denied any use of alcohol or tobacco.
Physical Examination
On physical examination, the patient was a well-appearing 52-year-old man in no apparent distress. No signs of gross deformity, erythema, ecchymosis, or infection were noted upon examination of his lower extremities. His motor examination was within normal limits from L2 to S1. However, both fine and gross sensation were decreased in the L3 distribution. Sensation was intact to the remaining nerve-root distributions. The Babinski sign was negative for both lower extremities, and clonus was within physiologic limits. Examination of his gait was notable for quadriceps buckling with ambulation.
Radiographic Examination
The patient initially presented to his primary care physician, who evaluated his symptoms with a computed tomography scan of his abdomen and pelvis. This showed a mass of the right L3 transverse process (Figure 1). The patient was referred to us for further management of this lesion. Dedicated magnetic resonance imaging of his lumbar spine was performed, showing an expansile, lytic, homogeneous mass in the patient’s right L3 transverse process. The mass showed a significant mass effect, compressing the exiting nerve roots and, presumably, his right ureter (Figure 2). A bone scan showed monostotic disease. The patient had failed conservative management, including physical therapy and anti-inflammatory medications. His right-sided radiculopathy was worsening, and he complained that the pain was affecting his quality of life and limiting his performance of his daily activities. A pain management specialist was requested to better manage his pain. Considering progression of his condition, surgical management was discussed, leading to a planned biopsy and resection of the mass.
Surgical Procedure
The patient was taken into the operating room and positioned prone on a Jackson table with a Wilson frame. Fluoroscopy was used to localize the right L3 transverse process. An incision was made over the right L3 transverse process and a Wiltse intramuscular approach was performed. After the right L3 transverse process was identified, the soft tissue from the transverse process was retracted in all directions, including medially up to the pedicle. The intertransverse ligament was detached from both the cephalad and caudal edges of the transverse process. We used a Woodson elevator to perform subperiosteal dissection to remove the soft tissue circumferentially. After dissection, we placed a Cobb elevator to protect the rostral and caudal soft tissue and used a high-speed burr to amputate the lytic transverse process at its base. The transverse process was removed en bloc (Figure 3) and sent for frozen pathologic evaluation (Figure 4). After the diagnosis of a benign lesion, the wound was closed in layers.
Complete resolution of both urinary and neurologic symptoms were immediately noted and up to 1 month postoperatively.
Discussion
Primary bone tumors of the spine are rare, with a reported incidence of 2.5 to 8.5 per 100,000 people per year.6 The estimated incidence of benign primary tumors involving the spine accounts for about 1% of all primary skeletal tumors and nearly 5% for malignant tumors.7-9 In contrast, secondary tumors involving the bony spinal column are relatively common. Postmortem studies indicate up to 70% of cancer patients demonstrate axial skeletal involvement.10,11 The most commonly encountered benign tumors affecting the spine include giant cell tumors, osteoid osteomas, osteoblastomas, and hemangiomas. Chordomas are frequently reported as the most common malignant primary spine neoplasms. Of all primary benign bone lesions, fibrous dysplasia accounts for approximately 1.4%.8
Primary and secondary malignant osseous tumors have a predilection for the anterior column, and primary benign lesions usually affect the posterior column.8,12-14 Because of the greater blood supply and more direct communication with the viscera via the Batson plexus, the anterior column is most likely to be seeded by metastatic disease. Similarly, hemangiomas and multiple myeloma are typically located in the anterior column, most likely because of the more abundant blood supply there. Chordomas are also found in this cancellous anterior column. Osteoid osteoma, osteoblastoma, and aneurysmal bone cysts are found within the more cortical architecture of posterior elements. The location of this patient’s lesion within the transverse process elevates confidence in the diagnosis of a benign lesion.
The conventional, isolated form of fibrous dysplasia was originally described in 1942 by Lichtenstein and Jaffe.2 They described 15 cases of benign “nonosteogenic fibromas” near the ends of long bones in young patients. Monostotic fibrous dysplasia constitutes the majority of these cases, approximately 80%.1,2,8,15 Fibrous dysplasia may also present as part of McCune-Albright syndrome, in which case it is associated with precocious puberty and café au lait spots. Less commonly, they are associated with intramuscular myxomas, as in Mazabraud syndrome. The lesions in these syndromes are typically polyostotic. In all forms, fibrous dysplasia develops from an activating mutation in the gene that encodes the alpha subunit of the G protein on chromosome 20q13, activating cyclic adenylate cyclase and slowing the differentiation of osteoblasts.3,8
With regard to presentation, fibrous dysplasia is usually asymptomatic and discovered incidentally. The literature reports that the most common presenting symptom for patients with monostotic fibrous dysplasia of the spine is back pain localized to the lesion.15 Meredith and Healey2 completed a comprehensive review of 54 cases of monostotic fibrous dysplasia involving the spine in which the majority of symptoms included back pain, neck pain, sacral region pain, pathologic fracture, painful torticollis, progressive myelopathy, paresthesias of the foot, and only 1 case of radiculopathy involving thoracic vertebra. In normal anatomy, the ureter lies within retroperitoneal fat anterior to the psoas muscle and L2-L5 transverse processes and is normally mobile.16-18 This becomes clinically significant in lean patients as the ureter becomes closer to the spine. There are several reports of iatrogenic ureter injury in lumbar disc surgery.16-18 Normal variants, including medialization towards the spine, may predispose the ureters to injury, iatrogenic, or otherwise. In fact, medialization of the ureters occurs commonly in black men and usually involves the right side, which may have occurred in this black patient.19
Fibrous dysplasia is most often diagnosed by its radiographic appearance or biopsy. However, recent data suggest that deoxyribonucleic acid (DNA) analysis may soon be able to diagnose this process.20 Imaging typically reveals expansile, central lytic lesions within the medullary cavity. Pathology shows dense fibroblasts around immature woven bone, commonly referred to as “Chinese lettering.” The treatment varies from observation to en bloc surgical resection. Clinical observation is warranted for asymptomatic or incidental findings of monostotic fibrous dysplasia, as long as the risk for pathologic fracture is low.11 Bisphosphonate therapy, both oral and intravenous, offers promising outcomes for the treatment of fibrous dysplasia, with improvement in pain and function as well as in the radiographic findings.11,21 Management of monostotic fibrous dysplasia presenting as an isolated expansile mass of the transverse process in lumbar spine has rarely been described.3-5 Troop and Herring5 reported a case of monostotic fibrous dysplasia in the lumbar spine, with involvement of the vertebral body and the posterior elements. Chow and coauthors3 and Harris and colleagues4 described the involvement of the transverse process of L4. Chow and coauthors’3 treatment consisted of excision that resulted in an asymptomatic patient at 8-year follow-up, while Harris and colleagues4 chose observation. In the latter study, the patient’s lower back pain persisted at 4-year follow-up.
Progressive enlargement, recurrence, and malignant transformation have all been described. Meredith and Healey2 reported the reappearance of monostotic fibrous dysplasia affecting C2, extending through the fusion mass to involve a previously unaffected vertebra 20 years after the original C2 posterior elements excision via posterior spinal fusion from C1 to C3. In the literature, the incidence of malignant transformation ranges from 0.4% to 4%.8 One case of malignant transformation in thoracic spine was reported by Fu and colleagues.22 Therefore, complete removal of all affected bone is recommended.1,2,4,5,15,22,23
Conclusion
We present an unusual condition with complete resolution of symptoms after surgical resection. Several points may be considered from this report. Fibrous dysplasia lesions have been found in all bones of the body, including the skull, face, and extremities; however, monostotic fibrous dysplasia involving the spine is rare.11,23,24 Furthermore, there are no other reports of these lesions causing simultaneous nerve compression and urologic symptoms. Considering anatomy, clinicians may consider lesions of the lumbar transverse process in patients presenting to orthopedic surgeons with urinary symptoms, especially when combined with neurologic symptoms. In these lesions, fibrous dysplasia should be within the differential diagnosis. Clinicians should also recognize that complete resolution of symptoms has been reported with wide resection of these lesions.
1. Leet AI, Magur E, Lee JS, Weintroub S, Robey PG, Collins MT. Fibrous dysplasia in the spine: prevalence of lesions and association with scoliosis. J Bone Joint Surg Am. 2004;86(3):531-537.
2. Meredith DS, Healey JH. Twenty-year follow-up of monostotic fibrous dysplasia of the second cervical vertebra: a case report and review of the literature. J Bone Joint Surg Am. 2011;93(13):e74.
3. Chow LT, Griffith J, Chow WH, Kumta SM. Monostotic fibrous dysplasia of the spine: report of a case involving the lumbar transverse process and review of the literature. Arch Orthop Trauma Surg. 2000;120(7-8):460-464.
4. Harris WH, Dudley HR Jr, Barry RJ. The natural history of fibrous dysplasia. An orthopaedic, pathologic, and roentgenographic study. J Bone Joint Surg Am. 1962;44(2):207-233.
5. Troop JK, Herring JA. Monostotic fibrous dysplasia of the lumbar spine: case report and review of the literature. J Pediatr Orthop. 1988;8(5):599-601.
6. Dreghorn CR, Newman RJ, Hardy GJ, Dickson RA. Primary tumors of the axial skeleton. Experience of the Leeds Regional Bone Tumor Registry. Spine. 1990;15(2):137-140.
7. Schuster JM, Grady MS. Medical management and adjuvant therapies in spinal metastatic disease. Neurosurg Focus. 2001;11(6):e3.
8. Unni K. Introduction and scope. In: Unni K, ed. Dahlin’s Bone Tumors—General Aspects and Data on 11,087 Cases. Philadelphia, PA: Lippincott-Raven; 1996:1-9.
9. Wong DA, Fornasier VL, MacNab I. Spinal metastases: the obvious, the occult, and the impostors. Spine. 1990;15(1):1-4.
10. Dagi TF, Schmidek HH. Vascular tumors of the spine. In: Sundaresan N, Schmidek HH, Schiller AL, eds. Tumors of the Spine: Diagnosis and Clinical Management. Philadelphia, PA: W.B. Saunders Co; 1990:181-191.
11. DiCaprio M, Enneking W. Fibrous dysplasia. Pathophysiology, evaluation, and treatment. J Bone Joint Surg Am. 2005;87(8):1848-1864.
12. Gasbarrini A, Cappuccio M, Mirabile L, et al. Spinal metastases: treatment evaluation algorithm. Eur Rev Med Pharmacol Sci. 2004;8(6):265-274.
13. Loblaw DA, Laperriere NJ, Mackillop WJ. A population-based study of malignant spinal cord compression in Ontario. Clin Oncol. 2003;15(4):211-217.
14. Ortiz Gómez JA. The incidence of vertebral body metastases. Int Orthop. 1995;19(5):309-311.
15. Avimadje AM, Goupille P, Zerkak D, Begnard G, Brunais-Besse J, Valat JP. Monostotic fibrous dysplasia of the lumbar spine. Joint Bone Spine. 2000;67(1):65-70.
16. Isiklar ZU, Lindsey RW, Coburn M. Ureteral injury after anterior lumbar interbody fusion. A case report. Spine. 1996;21(20):2379-2382.
17. Krone A, Heller V, Osterhage HR. Ureteral injury in lumbar disc surgery. Acta Neurochir (Wien). 1985;78(3-4):108–112.
18. Cho KT, Im SH, Hong SK. Ureteral injury after inadvertent violation of the intertransverse space during posterior lumbar diskectomy: a case report. Surg Neurol. 2008;69(2):135-137.
19. Adam EJ, Desai SC, Lawton G. Racial variations in normal ureteric course. Clin Radiol. 1985;36(4):373-375.
20. Stathopoulos IP, Balanika AP, Baltas CS, et al. Fibrous dysplasia; confirmation of clinical diagnosis by DNA tests instead of biopsy. J Musculoskelet Neuronal Interact. 2013;13(1):120-123.
21. Lane JM, Khan SN, O’Connor WJ, et al. Bisphosphonate therapy in fibrous dysplasia. Clin Orthop Relat Res. 2001;382:6-12.
22. Fu CJ, Hsu CY, Shih TT, Wu MZ. Monostotic fibrous dysplasia of the thoracic spine with malignant transformation. J Formos Med Assoc. 2004;103(9):711-714.
23. McCarthy EF. Fibro-osseous lesions of the maxillofacial bones. Head Neck Pathol. 2013;7(1):5-10.
24. Manjila S, Zender CA, Weaver J, Rodgers M, Cohen AR. Aneurysmal bone cyst within fibrous dysplasia of the anterior skull base: continued intracranial extension after endoscopic resections requiring craniofacial approach with free tissue transfer reconstruction [published online ahead of print February 26, 2013]. Childs Nerv Syst. 2013;29(7).
Fibrous dysplasia is a developmental abnormality caused by excessive proliferation of immature spindle-cell fibrous tissues in bones. It is characterized by benign bony growths, which can lead to local swelling, bony deformities, and lytic conversion, predisposing the bone to pathologic fractures. Although this process can occur in cortical bone, it primarily affects the trabecular bone, leading to enlargement and expansion from within the medullary space. Malignant transformation to osteosarcoma or fibrosarcoma can occur, although this is exceedingly rare (<0.5%).1,2
This case report describes a patient who presented with an expansile lytic mass in a lumbar transverse process that was postoperatively identified on pathology as monostotic fibrous dysplasia. Such lesions that involve the transverse processes are rare and have been associated with pain and significant discomfort.3-5 This is the first reported case of a transverse process fibrous dysplasia causing urinary retention and neurologic symptoms simultaneously. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
History
A 52-year-old black man presented to us with 6 to 8 months of increasing right flank pain, difficulty with urination, and right lower extremity pain in the area of his anterior thigh. He also complained of “buckling” of his thigh with ambulation. On review of systems, the patient denied any fevers, chills, headache, changes in weight or vision, or hearing problems. He had no systemic symptoms except for 6 months of frequent urinary tract infections and difficulty emptying his bladder, which resulted in urinary retention. He denied any significant medical history and denied any use of alcohol or tobacco.
Physical Examination
On physical examination, the patient was a well-appearing 52-year-old man in no apparent distress. No signs of gross deformity, erythema, ecchymosis, or infection were noted upon examination of his lower extremities. His motor examination was within normal limits from L2 to S1. However, both fine and gross sensation were decreased in the L3 distribution. Sensation was intact to the remaining nerve-root distributions. The Babinski sign was negative for both lower extremities, and clonus was within physiologic limits. Examination of his gait was notable for quadriceps buckling with ambulation.
Radiographic Examination
The patient initially presented to his primary care physician, who evaluated his symptoms with a computed tomography scan of his abdomen and pelvis. This showed a mass of the right L3 transverse process (Figure 1). The patient was referred to us for further management of this lesion. Dedicated magnetic resonance imaging of his lumbar spine was performed, showing an expansile, lytic, homogeneous mass in the patient’s right L3 transverse process. The mass showed a significant mass effect, compressing the exiting nerve roots and, presumably, his right ureter (Figure 2). A bone scan showed monostotic disease. The patient had failed conservative management, including physical therapy and anti-inflammatory medications. His right-sided radiculopathy was worsening, and he complained that the pain was affecting his quality of life and limiting his performance of his daily activities. A pain management specialist was requested to better manage his pain. Considering progression of his condition, surgical management was discussed, leading to a planned biopsy and resection of the mass.
Surgical Procedure
The patient was taken into the operating room and positioned prone on a Jackson table with a Wilson frame. Fluoroscopy was used to localize the right L3 transverse process. An incision was made over the right L3 transverse process and a Wiltse intramuscular approach was performed. After the right L3 transverse process was identified, the soft tissue from the transverse process was retracted in all directions, including medially up to the pedicle. The intertransverse ligament was detached from both the cephalad and caudal edges of the transverse process. We used a Woodson elevator to perform subperiosteal dissection to remove the soft tissue circumferentially. After dissection, we placed a Cobb elevator to protect the rostral and caudal soft tissue and used a high-speed burr to amputate the lytic transverse process at its base. The transverse process was removed en bloc (Figure 3) and sent for frozen pathologic evaluation (Figure 4). After the diagnosis of a benign lesion, the wound was closed in layers.
Complete resolution of both urinary and neurologic symptoms were immediately noted and up to 1 month postoperatively.
Discussion
Primary bone tumors of the spine are rare, with a reported incidence of 2.5 to 8.5 per 100,000 people per year.6 The estimated incidence of benign primary tumors involving the spine accounts for about 1% of all primary skeletal tumors and nearly 5% for malignant tumors.7-9 In contrast, secondary tumors involving the bony spinal column are relatively common. Postmortem studies indicate up to 70% of cancer patients demonstrate axial skeletal involvement.10,11 The most commonly encountered benign tumors affecting the spine include giant cell tumors, osteoid osteomas, osteoblastomas, and hemangiomas. Chordomas are frequently reported as the most common malignant primary spine neoplasms. Of all primary benign bone lesions, fibrous dysplasia accounts for approximately 1.4%.8
Primary and secondary malignant osseous tumors have a predilection for the anterior column, and primary benign lesions usually affect the posterior column.8,12-14 Because of the greater blood supply and more direct communication with the viscera via the Batson plexus, the anterior column is most likely to be seeded by metastatic disease. Similarly, hemangiomas and multiple myeloma are typically located in the anterior column, most likely because of the more abundant blood supply there. Chordomas are also found in this cancellous anterior column. Osteoid osteoma, osteoblastoma, and aneurysmal bone cysts are found within the more cortical architecture of posterior elements. The location of this patient’s lesion within the transverse process elevates confidence in the diagnosis of a benign lesion.
The conventional, isolated form of fibrous dysplasia was originally described in 1942 by Lichtenstein and Jaffe.2 They described 15 cases of benign “nonosteogenic fibromas” near the ends of long bones in young patients. Monostotic fibrous dysplasia constitutes the majority of these cases, approximately 80%.1,2,8,15 Fibrous dysplasia may also present as part of McCune-Albright syndrome, in which case it is associated with precocious puberty and café au lait spots. Less commonly, they are associated with intramuscular myxomas, as in Mazabraud syndrome. The lesions in these syndromes are typically polyostotic. In all forms, fibrous dysplasia develops from an activating mutation in the gene that encodes the alpha subunit of the G protein on chromosome 20q13, activating cyclic adenylate cyclase and slowing the differentiation of osteoblasts.3,8
With regard to presentation, fibrous dysplasia is usually asymptomatic and discovered incidentally. The literature reports that the most common presenting symptom for patients with monostotic fibrous dysplasia of the spine is back pain localized to the lesion.15 Meredith and Healey2 completed a comprehensive review of 54 cases of monostotic fibrous dysplasia involving the spine in which the majority of symptoms included back pain, neck pain, sacral region pain, pathologic fracture, painful torticollis, progressive myelopathy, paresthesias of the foot, and only 1 case of radiculopathy involving thoracic vertebra. In normal anatomy, the ureter lies within retroperitoneal fat anterior to the psoas muscle and L2-L5 transverse processes and is normally mobile.16-18 This becomes clinically significant in lean patients as the ureter becomes closer to the spine. There are several reports of iatrogenic ureter injury in lumbar disc surgery.16-18 Normal variants, including medialization towards the spine, may predispose the ureters to injury, iatrogenic, or otherwise. In fact, medialization of the ureters occurs commonly in black men and usually involves the right side, which may have occurred in this black patient.19
Fibrous dysplasia is most often diagnosed by its radiographic appearance or biopsy. However, recent data suggest that deoxyribonucleic acid (DNA) analysis may soon be able to diagnose this process.20 Imaging typically reveals expansile, central lytic lesions within the medullary cavity. Pathology shows dense fibroblasts around immature woven bone, commonly referred to as “Chinese lettering.” The treatment varies from observation to en bloc surgical resection. Clinical observation is warranted for asymptomatic or incidental findings of monostotic fibrous dysplasia, as long as the risk for pathologic fracture is low.11 Bisphosphonate therapy, both oral and intravenous, offers promising outcomes for the treatment of fibrous dysplasia, with improvement in pain and function as well as in the radiographic findings.11,21 Management of monostotic fibrous dysplasia presenting as an isolated expansile mass of the transverse process in lumbar spine has rarely been described.3-5 Troop and Herring5 reported a case of monostotic fibrous dysplasia in the lumbar spine, with involvement of the vertebral body and the posterior elements. Chow and coauthors3 and Harris and colleagues4 described the involvement of the transverse process of L4. Chow and coauthors’3 treatment consisted of excision that resulted in an asymptomatic patient at 8-year follow-up, while Harris and colleagues4 chose observation. In the latter study, the patient’s lower back pain persisted at 4-year follow-up.
Progressive enlargement, recurrence, and malignant transformation have all been described. Meredith and Healey2 reported the reappearance of monostotic fibrous dysplasia affecting C2, extending through the fusion mass to involve a previously unaffected vertebra 20 years after the original C2 posterior elements excision via posterior spinal fusion from C1 to C3. In the literature, the incidence of malignant transformation ranges from 0.4% to 4%.8 One case of malignant transformation in thoracic spine was reported by Fu and colleagues.22 Therefore, complete removal of all affected bone is recommended.1,2,4,5,15,22,23
Conclusion
We present an unusual condition with complete resolution of symptoms after surgical resection. Several points may be considered from this report. Fibrous dysplasia lesions have been found in all bones of the body, including the skull, face, and extremities; however, monostotic fibrous dysplasia involving the spine is rare.11,23,24 Furthermore, there are no other reports of these lesions causing simultaneous nerve compression and urologic symptoms. Considering anatomy, clinicians may consider lesions of the lumbar transverse process in patients presenting to orthopedic surgeons with urinary symptoms, especially when combined with neurologic symptoms. In these lesions, fibrous dysplasia should be within the differential diagnosis. Clinicians should also recognize that complete resolution of symptoms has been reported with wide resection of these lesions.
Fibrous dysplasia is a developmental abnormality caused by excessive proliferation of immature spindle-cell fibrous tissues in bones. It is characterized by benign bony growths, which can lead to local swelling, bony deformities, and lytic conversion, predisposing the bone to pathologic fractures. Although this process can occur in cortical bone, it primarily affects the trabecular bone, leading to enlargement and expansion from within the medullary space. Malignant transformation to osteosarcoma or fibrosarcoma can occur, although this is exceedingly rare (<0.5%).1,2
This case report describes a patient who presented with an expansile lytic mass in a lumbar transverse process that was postoperatively identified on pathology as monostotic fibrous dysplasia. Such lesions that involve the transverse processes are rare and have been associated with pain and significant discomfort.3-5 This is the first reported case of a transverse process fibrous dysplasia causing urinary retention and neurologic symptoms simultaneously. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
History
A 52-year-old black man presented to us with 6 to 8 months of increasing right flank pain, difficulty with urination, and right lower extremity pain in the area of his anterior thigh. He also complained of “buckling” of his thigh with ambulation. On review of systems, the patient denied any fevers, chills, headache, changes in weight or vision, or hearing problems. He had no systemic symptoms except for 6 months of frequent urinary tract infections and difficulty emptying his bladder, which resulted in urinary retention. He denied any significant medical history and denied any use of alcohol or tobacco.
Physical Examination
On physical examination, the patient was a well-appearing 52-year-old man in no apparent distress. No signs of gross deformity, erythema, ecchymosis, or infection were noted upon examination of his lower extremities. His motor examination was within normal limits from L2 to S1. However, both fine and gross sensation were decreased in the L3 distribution. Sensation was intact to the remaining nerve-root distributions. The Babinski sign was negative for both lower extremities, and clonus was within physiologic limits. Examination of his gait was notable for quadriceps buckling with ambulation.
Radiographic Examination
The patient initially presented to his primary care physician, who evaluated his symptoms with a computed tomography scan of his abdomen and pelvis. This showed a mass of the right L3 transverse process (Figure 1). The patient was referred to us for further management of this lesion. Dedicated magnetic resonance imaging of his lumbar spine was performed, showing an expansile, lytic, homogeneous mass in the patient’s right L3 transverse process. The mass showed a significant mass effect, compressing the exiting nerve roots and, presumably, his right ureter (Figure 2). A bone scan showed monostotic disease. The patient had failed conservative management, including physical therapy and anti-inflammatory medications. His right-sided radiculopathy was worsening, and he complained that the pain was affecting his quality of life and limiting his performance of his daily activities. A pain management specialist was requested to better manage his pain. Considering progression of his condition, surgical management was discussed, leading to a planned biopsy and resection of the mass.
Surgical Procedure
The patient was taken into the operating room and positioned prone on a Jackson table with a Wilson frame. Fluoroscopy was used to localize the right L3 transverse process. An incision was made over the right L3 transverse process and a Wiltse intramuscular approach was performed. After the right L3 transverse process was identified, the soft tissue from the transverse process was retracted in all directions, including medially up to the pedicle. The intertransverse ligament was detached from both the cephalad and caudal edges of the transverse process. We used a Woodson elevator to perform subperiosteal dissection to remove the soft tissue circumferentially. After dissection, we placed a Cobb elevator to protect the rostral and caudal soft tissue and used a high-speed burr to amputate the lytic transverse process at its base. The transverse process was removed en bloc (Figure 3) and sent for frozen pathologic evaluation (Figure 4). After the diagnosis of a benign lesion, the wound was closed in layers.
Complete resolution of both urinary and neurologic symptoms were immediately noted and up to 1 month postoperatively.
Discussion
Primary bone tumors of the spine are rare, with a reported incidence of 2.5 to 8.5 per 100,000 people per year.6 The estimated incidence of benign primary tumors involving the spine accounts for about 1% of all primary skeletal tumors and nearly 5% for malignant tumors.7-9 In contrast, secondary tumors involving the bony spinal column are relatively common. Postmortem studies indicate up to 70% of cancer patients demonstrate axial skeletal involvement.10,11 The most commonly encountered benign tumors affecting the spine include giant cell tumors, osteoid osteomas, osteoblastomas, and hemangiomas. Chordomas are frequently reported as the most common malignant primary spine neoplasms. Of all primary benign bone lesions, fibrous dysplasia accounts for approximately 1.4%.8
Primary and secondary malignant osseous tumors have a predilection for the anterior column, and primary benign lesions usually affect the posterior column.8,12-14 Because of the greater blood supply and more direct communication with the viscera via the Batson plexus, the anterior column is most likely to be seeded by metastatic disease. Similarly, hemangiomas and multiple myeloma are typically located in the anterior column, most likely because of the more abundant blood supply there. Chordomas are also found in this cancellous anterior column. Osteoid osteoma, osteoblastoma, and aneurysmal bone cysts are found within the more cortical architecture of posterior elements. The location of this patient’s lesion within the transverse process elevates confidence in the diagnosis of a benign lesion.
The conventional, isolated form of fibrous dysplasia was originally described in 1942 by Lichtenstein and Jaffe.2 They described 15 cases of benign “nonosteogenic fibromas” near the ends of long bones in young patients. Monostotic fibrous dysplasia constitutes the majority of these cases, approximately 80%.1,2,8,15 Fibrous dysplasia may also present as part of McCune-Albright syndrome, in which case it is associated with precocious puberty and café au lait spots. Less commonly, they are associated with intramuscular myxomas, as in Mazabraud syndrome. The lesions in these syndromes are typically polyostotic. In all forms, fibrous dysplasia develops from an activating mutation in the gene that encodes the alpha subunit of the G protein on chromosome 20q13, activating cyclic adenylate cyclase and slowing the differentiation of osteoblasts.3,8
With regard to presentation, fibrous dysplasia is usually asymptomatic and discovered incidentally. The literature reports that the most common presenting symptom for patients with monostotic fibrous dysplasia of the spine is back pain localized to the lesion.15 Meredith and Healey2 completed a comprehensive review of 54 cases of monostotic fibrous dysplasia involving the spine in which the majority of symptoms included back pain, neck pain, sacral region pain, pathologic fracture, painful torticollis, progressive myelopathy, paresthesias of the foot, and only 1 case of radiculopathy involving thoracic vertebra. In normal anatomy, the ureter lies within retroperitoneal fat anterior to the psoas muscle and L2-L5 transverse processes and is normally mobile.16-18 This becomes clinically significant in lean patients as the ureter becomes closer to the spine. There are several reports of iatrogenic ureter injury in lumbar disc surgery.16-18 Normal variants, including medialization towards the spine, may predispose the ureters to injury, iatrogenic, or otherwise. In fact, medialization of the ureters occurs commonly in black men and usually involves the right side, which may have occurred in this black patient.19
Fibrous dysplasia is most often diagnosed by its radiographic appearance or biopsy. However, recent data suggest that deoxyribonucleic acid (DNA) analysis may soon be able to diagnose this process.20 Imaging typically reveals expansile, central lytic lesions within the medullary cavity. Pathology shows dense fibroblasts around immature woven bone, commonly referred to as “Chinese lettering.” The treatment varies from observation to en bloc surgical resection. Clinical observation is warranted for asymptomatic or incidental findings of monostotic fibrous dysplasia, as long as the risk for pathologic fracture is low.11 Bisphosphonate therapy, both oral and intravenous, offers promising outcomes for the treatment of fibrous dysplasia, with improvement in pain and function as well as in the radiographic findings.11,21 Management of monostotic fibrous dysplasia presenting as an isolated expansile mass of the transverse process in lumbar spine has rarely been described.3-5 Troop and Herring5 reported a case of monostotic fibrous dysplasia in the lumbar spine, with involvement of the vertebral body and the posterior elements. Chow and coauthors3 and Harris and colleagues4 described the involvement of the transverse process of L4. Chow and coauthors’3 treatment consisted of excision that resulted in an asymptomatic patient at 8-year follow-up, while Harris and colleagues4 chose observation. In the latter study, the patient’s lower back pain persisted at 4-year follow-up.
Progressive enlargement, recurrence, and malignant transformation have all been described. Meredith and Healey2 reported the reappearance of monostotic fibrous dysplasia affecting C2, extending through the fusion mass to involve a previously unaffected vertebra 20 years after the original C2 posterior elements excision via posterior spinal fusion from C1 to C3. In the literature, the incidence of malignant transformation ranges from 0.4% to 4%.8 One case of malignant transformation in thoracic spine was reported by Fu and colleagues.22 Therefore, complete removal of all affected bone is recommended.1,2,4,5,15,22,23
Conclusion
We present an unusual condition with complete resolution of symptoms after surgical resection. Several points may be considered from this report. Fibrous dysplasia lesions have been found in all bones of the body, including the skull, face, and extremities; however, monostotic fibrous dysplasia involving the spine is rare.11,23,24 Furthermore, there are no other reports of these lesions causing simultaneous nerve compression and urologic symptoms. Considering anatomy, clinicians may consider lesions of the lumbar transverse process in patients presenting to orthopedic surgeons with urinary symptoms, especially when combined with neurologic symptoms. In these lesions, fibrous dysplasia should be within the differential diagnosis. Clinicians should also recognize that complete resolution of symptoms has been reported with wide resection of these lesions.
1. Leet AI, Magur E, Lee JS, Weintroub S, Robey PG, Collins MT. Fibrous dysplasia in the spine: prevalence of lesions and association with scoliosis. J Bone Joint Surg Am. 2004;86(3):531-537.
2. Meredith DS, Healey JH. Twenty-year follow-up of monostotic fibrous dysplasia of the second cervical vertebra: a case report and review of the literature. J Bone Joint Surg Am. 2011;93(13):e74.
3. Chow LT, Griffith J, Chow WH, Kumta SM. Monostotic fibrous dysplasia of the spine: report of a case involving the lumbar transverse process and review of the literature. Arch Orthop Trauma Surg. 2000;120(7-8):460-464.
4. Harris WH, Dudley HR Jr, Barry RJ. The natural history of fibrous dysplasia. An orthopaedic, pathologic, and roentgenographic study. J Bone Joint Surg Am. 1962;44(2):207-233.
5. Troop JK, Herring JA. Monostotic fibrous dysplasia of the lumbar spine: case report and review of the literature. J Pediatr Orthop. 1988;8(5):599-601.
6. Dreghorn CR, Newman RJ, Hardy GJ, Dickson RA. Primary tumors of the axial skeleton. Experience of the Leeds Regional Bone Tumor Registry. Spine. 1990;15(2):137-140.
7. Schuster JM, Grady MS. Medical management and adjuvant therapies in spinal metastatic disease. Neurosurg Focus. 2001;11(6):e3.
8. Unni K. Introduction and scope. In: Unni K, ed. Dahlin’s Bone Tumors—General Aspects and Data on 11,087 Cases. Philadelphia, PA: Lippincott-Raven; 1996:1-9.
9. Wong DA, Fornasier VL, MacNab I. Spinal metastases: the obvious, the occult, and the impostors. Spine. 1990;15(1):1-4.
10. Dagi TF, Schmidek HH. Vascular tumors of the spine. In: Sundaresan N, Schmidek HH, Schiller AL, eds. Tumors of the Spine: Diagnosis and Clinical Management. Philadelphia, PA: W.B. Saunders Co; 1990:181-191.
11. DiCaprio M, Enneking W. Fibrous dysplasia. Pathophysiology, evaluation, and treatment. J Bone Joint Surg Am. 2005;87(8):1848-1864.
12. Gasbarrini A, Cappuccio M, Mirabile L, et al. Spinal metastases: treatment evaluation algorithm. Eur Rev Med Pharmacol Sci. 2004;8(6):265-274.
13. Loblaw DA, Laperriere NJ, Mackillop WJ. A population-based study of malignant spinal cord compression in Ontario. Clin Oncol. 2003;15(4):211-217.
14. Ortiz Gómez JA. The incidence of vertebral body metastases. Int Orthop. 1995;19(5):309-311.
15. Avimadje AM, Goupille P, Zerkak D, Begnard G, Brunais-Besse J, Valat JP. Monostotic fibrous dysplasia of the lumbar spine. Joint Bone Spine. 2000;67(1):65-70.
16. Isiklar ZU, Lindsey RW, Coburn M. Ureteral injury after anterior lumbar interbody fusion. A case report. Spine. 1996;21(20):2379-2382.
17. Krone A, Heller V, Osterhage HR. Ureteral injury in lumbar disc surgery. Acta Neurochir (Wien). 1985;78(3-4):108–112.
18. Cho KT, Im SH, Hong SK. Ureteral injury after inadvertent violation of the intertransverse space during posterior lumbar diskectomy: a case report. Surg Neurol. 2008;69(2):135-137.
19. Adam EJ, Desai SC, Lawton G. Racial variations in normal ureteric course. Clin Radiol. 1985;36(4):373-375.
20. Stathopoulos IP, Balanika AP, Baltas CS, et al. Fibrous dysplasia; confirmation of clinical diagnosis by DNA tests instead of biopsy. J Musculoskelet Neuronal Interact. 2013;13(1):120-123.
21. Lane JM, Khan SN, O’Connor WJ, et al. Bisphosphonate therapy in fibrous dysplasia. Clin Orthop Relat Res. 2001;382:6-12.
22. Fu CJ, Hsu CY, Shih TT, Wu MZ. Monostotic fibrous dysplasia of the thoracic spine with malignant transformation. J Formos Med Assoc. 2004;103(9):711-714.
23. McCarthy EF. Fibro-osseous lesions of the maxillofacial bones. Head Neck Pathol. 2013;7(1):5-10.
24. Manjila S, Zender CA, Weaver J, Rodgers M, Cohen AR. Aneurysmal bone cyst within fibrous dysplasia of the anterior skull base: continued intracranial extension after endoscopic resections requiring craniofacial approach with free tissue transfer reconstruction [published online ahead of print February 26, 2013]. Childs Nerv Syst. 2013;29(7).
1. Leet AI, Magur E, Lee JS, Weintroub S, Robey PG, Collins MT. Fibrous dysplasia in the spine: prevalence of lesions and association with scoliosis. J Bone Joint Surg Am. 2004;86(3):531-537.
2. Meredith DS, Healey JH. Twenty-year follow-up of monostotic fibrous dysplasia of the second cervical vertebra: a case report and review of the literature. J Bone Joint Surg Am. 2011;93(13):e74.
3. Chow LT, Griffith J, Chow WH, Kumta SM. Monostotic fibrous dysplasia of the spine: report of a case involving the lumbar transverse process and review of the literature. Arch Orthop Trauma Surg. 2000;120(7-8):460-464.
4. Harris WH, Dudley HR Jr, Barry RJ. The natural history of fibrous dysplasia. An orthopaedic, pathologic, and roentgenographic study. J Bone Joint Surg Am. 1962;44(2):207-233.
5. Troop JK, Herring JA. Monostotic fibrous dysplasia of the lumbar spine: case report and review of the literature. J Pediatr Orthop. 1988;8(5):599-601.
6. Dreghorn CR, Newman RJ, Hardy GJ, Dickson RA. Primary tumors of the axial skeleton. Experience of the Leeds Regional Bone Tumor Registry. Spine. 1990;15(2):137-140.
7. Schuster JM, Grady MS. Medical management and adjuvant therapies in spinal metastatic disease. Neurosurg Focus. 2001;11(6):e3.
8. Unni K. Introduction and scope. In: Unni K, ed. Dahlin’s Bone Tumors—General Aspects and Data on 11,087 Cases. Philadelphia, PA: Lippincott-Raven; 1996:1-9.
9. Wong DA, Fornasier VL, MacNab I. Spinal metastases: the obvious, the occult, and the impostors. Spine. 1990;15(1):1-4.
10. Dagi TF, Schmidek HH. Vascular tumors of the spine. In: Sundaresan N, Schmidek HH, Schiller AL, eds. Tumors of the Spine: Diagnosis and Clinical Management. Philadelphia, PA: W.B. Saunders Co; 1990:181-191.
11. DiCaprio M, Enneking W. Fibrous dysplasia. Pathophysiology, evaluation, and treatment. J Bone Joint Surg Am. 2005;87(8):1848-1864.
12. Gasbarrini A, Cappuccio M, Mirabile L, et al. Spinal metastases: treatment evaluation algorithm. Eur Rev Med Pharmacol Sci. 2004;8(6):265-274.
13. Loblaw DA, Laperriere NJ, Mackillop WJ. A population-based study of malignant spinal cord compression in Ontario. Clin Oncol. 2003;15(4):211-217.
14. Ortiz Gómez JA. The incidence of vertebral body metastases. Int Orthop. 1995;19(5):309-311.
15. Avimadje AM, Goupille P, Zerkak D, Begnard G, Brunais-Besse J, Valat JP. Monostotic fibrous dysplasia of the lumbar spine. Joint Bone Spine. 2000;67(1):65-70.
16. Isiklar ZU, Lindsey RW, Coburn M. Ureteral injury after anterior lumbar interbody fusion. A case report. Spine. 1996;21(20):2379-2382.
17. Krone A, Heller V, Osterhage HR. Ureteral injury in lumbar disc surgery. Acta Neurochir (Wien). 1985;78(3-4):108–112.
18. Cho KT, Im SH, Hong SK. Ureteral injury after inadvertent violation of the intertransverse space during posterior lumbar diskectomy: a case report. Surg Neurol. 2008;69(2):135-137.
19. Adam EJ, Desai SC, Lawton G. Racial variations in normal ureteric course. Clin Radiol. 1985;36(4):373-375.
20. Stathopoulos IP, Balanika AP, Baltas CS, et al. Fibrous dysplasia; confirmation of clinical diagnosis by DNA tests instead of biopsy. J Musculoskelet Neuronal Interact. 2013;13(1):120-123.
21. Lane JM, Khan SN, O’Connor WJ, et al. Bisphosphonate therapy in fibrous dysplasia. Clin Orthop Relat Res. 2001;382:6-12.
22. Fu CJ, Hsu CY, Shih TT, Wu MZ. Monostotic fibrous dysplasia of the thoracic spine with malignant transformation. J Formos Med Assoc. 2004;103(9):711-714.
23. McCarthy EF. Fibro-osseous lesions of the maxillofacial bones. Head Neck Pathol. 2013;7(1):5-10.
24. Manjila S, Zender CA, Weaver J, Rodgers M, Cohen AR. Aneurysmal bone cyst within fibrous dysplasia of the anterior skull base: continued intracranial extension after endoscopic resections requiring craniofacial approach with free tissue transfer reconstruction [published online ahead of print February 26, 2013]. Childs Nerv Syst. 2013;29(7).
Novel Intraoperative Technique to Visualize the Lower Cervical Spine: A Case Series
Two adequate views of the lower cervical vertebrae are necessary to confirm the 3-dimensional location of any hardware placed during cervical spine fusion. Visualizing the lower cervical vertebrae in 2 planes intraoperatively is often a challenge because the shoulders obstruct the lateral view.1 Techniques have been described to improve lateral visualization, including gentle traction of the arms via wrist restraints or taping the shoulders down inferiorly.2,3 These techniques have their inadequacies, including an association with peripheral nerve injury and brachial plexopathy.4 In patients with stout necks, these methods may still be insufficient to achieve adequate visualization of the lower cervical vertebrae.
Invasive techniques to improve visualization have also been described. In 1 study, exposure had to be extended cephalad to allow for manual counting of cervical vertebrae when the mid- to lower cervical vertebrae had to be identified in a morbidly obese patient.5 More invasive spine procedures are associated with higher rates of complications, increased blood loss, more soft-tissue trauma, and longer hospital stays.6 We present a view 30º oblique from horizontal and 30º cephalad from neutral as a variation of the lateral radiograph that improves visualization of the mid- to lower cervical vertebrae. The authors have obtained the patients’ informed written consent for print and electronic publication of these case reports.
Technique
We used either the Smith-Robinson or Cloward approach to the anterior spine. Both techniques use the avascular plane between the medially located esophagus and trachea and the lateral sternocleidomastoid and carotid sheath to approach the anterior cervical spine. Once adequate exposure was achieved, standard anteroposterior and lateral radiographs were obtained to confirm the correct vertebral level. Gentle caudal traction was applied to the patient’s wrist straps, and when visualization continued to be compromised, a view 30º oblique from horizontal and 30º cephalad from neutral was obtained (Figure 1).
Case Series
Case 1
A 54-year-old man with a body mass index (BMI) of 50 presented with neck and bilateral arm pain, with left greater than right radicular symptoms in the C6 and C7 distribution. Magnetic resonance imaging (MRI) showed disc herniations at C5-C6 and C6-C7 with spinal cord signal changes, and he underwent a C5-C6 and C6-C7 anterior cervical discectomy and fusion. Initial localization was determined using a lateral radiograph and vertebral needle. During hardware placement, anteroposterior and lateral fluoroscopic radiographs confirmed adequate placement of the superior screw, but visualization of the inferior portion of the plate and inferior screw was challenging (Figure 2). Our oblique 30º–30º view provided better visualization of the plate and screws in the lower cervical vertebrae than lateral imaging, and allowed confirmation that the hardware was positioned correctly (Figure 3). It took 1 attempt to achieve adequate visualization with the 30º–30º view.
Postoperatively, the patient’s radiculopathy and motor weakness improved. Radiographs confirmed adequate hardware placement, and he was discharged on postoperative day 1 (Figure 4). Imaging at the patient’s 6-week follow-up confirmed adequate fusion from C5-C7, anatomically aligned facet joints, and no hardware failure. The patient’s Neck Disability Index was 31/50 preoperatively and 26/50 at this visit.
Case 2
A 51-year-old man with a BMI of 29 presented with a long-standing history of neck pain and bilateral arm pain left greater than right in the C6 and C7 dermomyotome. MRI showed a broad-based disc herniation with foraminal narrowing at C5-C6 and C6-C7, and the patient underwent a 2-level anterior cervical discectomy and fusion. This patient had pronounced neck musculature, and a deeper than normal incision was required.
Intraoperative lateral fluoroscopy was obtained to confirm the C5-C6 and C6-C7 level prior to discectomy. The musculature of the patient’s neck and shoulder made visualization of the C6-C7 disc space difficult on the lateral radiograph (Figure 5). One attempt was required to obtain the 30º–30º oblique view, which was used to ensure correct placement of the screws and plate (Figure 6).
Postoperatively, the patient’s pain had improved, and radiographs confirmed adequate hardware placement. He was discharged 1 day after surgery (Figure 7). Imaging at the patient’s 6-week follow-up confirmed adequate fusion from C5-C7, stable disc spaces, and anatomically aligned facet joints. His Neck Disability Index was 34/50 preoperatively and 32/50 at 2-week follow-up.
Discussion
The aim of this study was to describe an alternative to the lateral radiograph for imaging the cervical spine in patients with challenging anatomy or in procedures involving hardware placement at the lower cervical vertebrae. Techniques have been developed to assist with improved lateral visualization, including gentle traction of the arms via wrist restraints or taping the shoulders down inferiorly.2,3 However, visualization in 2 planes continues to be a challenge in a subset of patients. It is particularly difficult to obtain adequate lateral radiographs of the cervical spine in patients with stout necks.3 In patients with stout necks, there is more obstruction of the radiography path through the cervical spine. This leads to imaging that is unclear or may fail to show the mid- to lower cervical spine. The extent to which one should rely on the 30º–30º oblique technique for adequate visualization of the cervical spine depends on the anatomy of a particular patient. Historically, it is more challenging to obtain satisfactory lateral radiographs in patients with stout necks,3 and these patients have benefited the most from using the 30º–30º degree oblique view.
Lack of visualization can lead to aborted surgeries or, potentially, surgery at the wrong level.3 A 2008 American Academy of Neurological Surgeons survey indicated that 50% of spine surgeons had performed a wrong-level surgery at least once in their career, and the cervical spine accounted for 21% of all incorrect-level spine surgeries.7 Intraoperative factors reported during cases of wrong-level spinal surgeries included misinterpretation of intraoperative imaging, no intraoperative imaging, and unusual anatomy or physical characteristics.8 Such complications can lead to revision surgery and other significant morbidities for the patient.
In most patients, fluoroscopy allows confirmation of the correct level before disc incision.3 However, operating at a lower cervical level in a patient with a short neck or prominent shoulders poses a significant problem.3 A case report from Singh and colleagues9 described a modified intraoperative fluoroscopic view for spinal level localization at cervicothoracic levels. Their method focuses on identifying the bony lamina and using them as landmarks to count spinal levels, whereas our 30º–30º oblique image is useful for confirmation of adequate hardware placement during anterior cervical spinal fusions. Often, the initial localization of cervical vertebral levels can be achieved with a standard lateral radiograph. We recognized the utility of the 30º–30º oblique view when we were attempting to visualize the inferior aspect of the plate and inferior screw placement.
In patients with stout necks, a lateral radiograph may show only visualization down to C4 or C5.3 Even with applying traction to the arms or taping the shoulders down, it can be impossible to visualize C6, C7, or T1 because the shoulder bones and muscles obstruct the image.3 Using a 30º–30º oblique view, we were able to obtain adequate visualization and assess the accurate placement of hardware.
Conclusion
A 30º oblique view from horizontal and 30º cephalad from neutral radiograph can be used intraoperatively in patients with challenging anatomy to identify placement of hardware at the correct vertebral level in the lower cervical spine. It is a noninvasive technique that can help reduce the risk of wrong-site surgeries without prolonging operation time. This technique describes an alternative to the lateral radiograph and provides a solution to the difficult problem of intraoperative imaging of the mid- to lower cervical spine in 2 adequate planes.
1. Bebawy JF, Koht A, Mirkovic S. Anterior cervical spine surgery. In: Khot A, Sloan TB, Toleikis JR, eds. Monitoring the Nervous System for Anesthesiologists and Other Health Care Professionals. New York, NY: Springer; 2012:539-554.
2. Abumi K, Shono Y, Ito M, Taneichi H, Kotani Y, Kaneda K. Complications of pedicle screw fixation in reconstructive surgery of the cervical spine. Spine. 2000;25(8):962-969.
3. Irace C. Intraoperative imaging for verification of the correct level during spinal surgery. In: Fountas KN, ed. Novel Frontiers of Advanced Neuroimaging. Rijeka, Croatia: Intech; 2013:175-188.
4. Schwartz DM, Sestokas AK, Hilibrand AS, et al. Neurophysiological identification of position-induced neurologic injury during anterior cervical spine surgery. J Clin Monit Comput. 2006;20(6):437-444.
5. Telfeian AE, Reiter GT, Durham SR, Marcotte P. Spine surgery in morbidly obese patients. J Neurosurg Spine. 2002;97(1):20-24.
6. Oppenheimer JH, DeCastro I, McDonnell DE. Minimally invasive spine technology and minimally invasive spine surgery: a historical review. Neurosurg Focus. 2009;27(3):E9.
7. Mody MG, Nourbakhsh A, Stahl DL, Gibbs M, Alfawareh M, Garges KJ. The prevalence of wrong level surgery among spine surgeons. Spine. 2008;33(2):194.
8. Jhawar BS, Mitsis D, Duggal N. Wrong-sided and wrong-level neurosurgery: A national survey. J Neurosurg Spine. 2007;7(5):467-472.
9. Singh H, Meyer SA, Hecht AC, Jenkins AL 3rd. Novel fluoroscopic technique for localization at cervicothoracic levels. J Spinal Disord Tech. 2009;22(8):615-618.
Two adequate views of the lower cervical vertebrae are necessary to confirm the 3-dimensional location of any hardware placed during cervical spine fusion. Visualizing the lower cervical vertebrae in 2 planes intraoperatively is often a challenge because the shoulders obstruct the lateral view.1 Techniques have been described to improve lateral visualization, including gentle traction of the arms via wrist restraints or taping the shoulders down inferiorly.2,3 These techniques have their inadequacies, including an association with peripheral nerve injury and brachial plexopathy.4 In patients with stout necks, these methods may still be insufficient to achieve adequate visualization of the lower cervical vertebrae.
Invasive techniques to improve visualization have also been described. In 1 study, exposure had to be extended cephalad to allow for manual counting of cervical vertebrae when the mid- to lower cervical vertebrae had to be identified in a morbidly obese patient.5 More invasive spine procedures are associated with higher rates of complications, increased blood loss, more soft-tissue trauma, and longer hospital stays.6 We present a view 30º oblique from horizontal and 30º cephalad from neutral as a variation of the lateral radiograph that improves visualization of the mid- to lower cervical vertebrae. The authors have obtained the patients’ informed written consent for print and electronic publication of these case reports.
Technique
We used either the Smith-Robinson or Cloward approach to the anterior spine. Both techniques use the avascular plane between the medially located esophagus and trachea and the lateral sternocleidomastoid and carotid sheath to approach the anterior cervical spine. Once adequate exposure was achieved, standard anteroposterior and lateral radiographs were obtained to confirm the correct vertebral level. Gentle caudal traction was applied to the patient’s wrist straps, and when visualization continued to be compromised, a view 30º oblique from horizontal and 30º cephalad from neutral was obtained (Figure 1).
Case Series
Case 1
A 54-year-old man with a body mass index (BMI) of 50 presented with neck and bilateral arm pain, with left greater than right radicular symptoms in the C6 and C7 distribution. Magnetic resonance imaging (MRI) showed disc herniations at C5-C6 and C6-C7 with spinal cord signal changes, and he underwent a C5-C6 and C6-C7 anterior cervical discectomy and fusion. Initial localization was determined using a lateral radiograph and vertebral needle. During hardware placement, anteroposterior and lateral fluoroscopic radiographs confirmed adequate placement of the superior screw, but visualization of the inferior portion of the plate and inferior screw was challenging (Figure 2). Our oblique 30º–30º view provided better visualization of the plate and screws in the lower cervical vertebrae than lateral imaging, and allowed confirmation that the hardware was positioned correctly (Figure 3). It took 1 attempt to achieve adequate visualization with the 30º–30º view.
Postoperatively, the patient’s radiculopathy and motor weakness improved. Radiographs confirmed adequate hardware placement, and he was discharged on postoperative day 1 (Figure 4). Imaging at the patient’s 6-week follow-up confirmed adequate fusion from C5-C7, anatomically aligned facet joints, and no hardware failure. The patient’s Neck Disability Index was 31/50 preoperatively and 26/50 at this visit.
Case 2
A 51-year-old man with a BMI of 29 presented with a long-standing history of neck pain and bilateral arm pain left greater than right in the C6 and C7 dermomyotome. MRI showed a broad-based disc herniation with foraminal narrowing at C5-C6 and C6-C7, and the patient underwent a 2-level anterior cervical discectomy and fusion. This patient had pronounced neck musculature, and a deeper than normal incision was required.
Intraoperative lateral fluoroscopy was obtained to confirm the C5-C6 and C6-C7 level prior to discectomy. The musculature of the patient’s neck and shoulder made visualization of the C6-C7 disc space difficult on the lateral radiograph (Figure 5). One attempt was required to obtain the 30º–30º oblique view, which was used to ensure correct placement of the screws and plate (Figure 6).
Postoperatively, the patient’s pain had improved, and radiographs confirmed adequate hardware placement. He was discharged 1 day after surgery (Figure 7). Imaging at the patient’s 6-week follow-up confirmed adequate fusion from C5-C7, stable disc spaces, and anatomically aligned facet joints. His Neck Disability Index was 34/50 preoperatively and 32/50 at 2-week follow-up.
Discussion
The aim of this study was to describe an alternative to the lateral radiograph for imaging the cervical spine in patients with challenging anatomy or in procedures involving hardware placement at the lower cervical vertebrae. Techniques have been developed to assist with improved lateral visualization, including gentle traction of the arms via wrist restraints or taping the shoulders down inferiorly.2,3 However, visualization in 2 planes continues to be a challenge in a subset of patients. It is particularly difficult to obtain adequate lateral radiographs of the cervical spine in patients with stout necks.3 In patients with stout necks, there is more obstruction of the radiography path through the cervical spine. This leads to imaging that is unclear or may fail to show the mid- to lower cervical spine. The extent to which one should rely on the 30º–30º oblique technique for adequate visualization of the cervical spine depends on the anatomy of a particular patient. Historically, it is more challenging to obtain satisfactory lateral radiographs in patients with stout necks,3 and these patients have benefited the most from using the 30º–30º degree oblique view.
Lack of visualization can lead to aborted surgeries or, potentially, surgery at the wrong level.3 A 2008 American Academy of Neurological Surgeons survey indicated that 50% of spine surgeons had performed a wrong-level surgery at least once in their career, and the cervical spine accounted for 21% of all incorrect-level spine surgeries.7 Intraoperative factors reported during cases of wrong-level spinal surgeries included misinterpretation of intraoperative imaging, no intraoperative imaging, and unusual anatomy or physical characteristics.8 Such complications can lead to revision surgery and other significant morbidities for the patient.
In most patients, fluoroscopy allows confirmation of the correct level before disc incision.3 However, operating at a lower cervical level in a patient with a short neck or prominent shoulders poses a significant problem.3 A case report from Singh and colleagues9 described a modified intraoperative fluoroscopic view for spinal level localization at cervicothoracic levels. Their method focuses on identifying the bony lamina and using them as landmarks to count spinal levels, whereas our 30º–30º oblique image is useful for confirmation of adequate hardware placement during anterior cervical spinal fusions. Often, the initial localization of cervical vertebral levels can be achieved with a standard lateral radiograph. We recognized the utility of the 30º–30º oblique view when we were attempting to visualize the inferior aspect of the plate and inferior screw placement.
In patients with stout necks, a lateral radiograph may show only visualization down to C4 or C5.3 Even with applying traction to the arms or taping the shoulders down, it can be impossible to visualize C6, C7, or T1 because the shoulder bones and muscles obstruct the image.3 Using a 30º–30º oblique view, we were able to obtain adequate visualization and assess the accurate placement of hardware.
Conclusion
A 30º oblique view from horizontal and 30º cephalad from neutral radiograph can be used intraoperatively in patients with challenging anatomy to identify placement of hardware at the correct vertebral level in the lower cervical spine. It is a noninvasive technique that can help reduce the risk of wrong-site surgeries without prolonging operation time. This technique describes an alternative to the lateral radiograph and provides a solution to the difficult problem of intraoperative imaging of the mid- to lower cervical spine in 2 adequate planes.
Two adequate views of the lower cervical vertebrae are necessary to confirm the 3-dimensional location of any hardware placed during cervical spine fusion. Visualizing the lower cervical vertebrae in 2 planes intraoperatively is often a challenge because the shoulders obstruct the lateral view.1 Techniques have been described to improve lateral visualization, including gentle traction of the arms via wrist restraints or taping the shoulders down inferiorly.2,3 These techniques have their inadequacies, including an association with peripheral nerve injury and brachial plexopathy.4 In patients with stout necks, these methods may still be insufficient to achieve adequate visualization of the lower cervical vertebrae.
Invasive techniques to improve visualization have also been described. In 1 study, exposure had to be extended cephalad to allow for manual counting of cervical vertebrae when the mid- to lower cervical vertebrae had to be identified in a morbidly obese patient.5 More invasive spine procedures are associated with higher rates of complications, increased blood loss, more soft-tissue trauma, and longer hospital stays.6 We present a view 30º oblique from horizontal and 30º cephalad from neutral as a variation of the lateral radiograph that improves visualization of the mid- to lower cervical vertebrae. The authors have obtained the patients’ informed written consent for print and electronic publication of these case reports.
Technique
We used either the Smith-Robinson or Cloward approach to the anterior spine. Both techniques use the avascular plane between the medially located esophagus and trachea and the lateral sternocleidomastoid and carotid sheath to approach the anterior cervical spine. Once adequate exposure was achieved, standard anteroposterior and lateral radiographs were obtained to confirm the correct vertebral level. Gentle caudal traction was applied to the patient’s wrist straps, and when visualization continued to be compromised, a view 30º oblique from horizontal and 30º cephalad from neutral was obtained (Figure 1).
Case Series
Case 1
A 54-year-old man with a body mass index (BMI) of 50 presented with neck and bilateral arm pain, with left greater than right radicular symptoms in the C6 and C7 distribution. Magnetic resonance imaging (MRI) showed disc herniations at C5-C6 and C6-C7 with spinal cord signal changes, and he underwent a C5-C6 and C6-C7 anterior cervical discectomy and fusion. Initial localization was determined using a lateral radiograph and vertebral needle. During hardware placement, anteroposterior and lateral fluoroscopic radiographs confirmed adequate placement of the superior screw, but visualization of the inferior portion of the plate and inferior screw was challenging (Figure 2). Our oblique 30º–30º view provided better visualization of the plate and screws in the lower cervical vertebrae than lateral imaging, and allowed confirmation that the hardware was positioned correctly (Figure 3). It took 1 attempt to achieve adequate visualization with the 30º–30º view.
Postoperatively, the patient’s radiculopathy and motor weakness improved. Radiographs confirmed adequate hardware placement, and he was discharged on postoperative day 1 (Figure 4). Imaging at the patient’s 6-week follow-up confirmed adequate fusion from C5-C7, anatomically aligned facet joints, and no hardware failure. The patient’s Neck Disability Index was 31/50 preoperatively and 26/50 at this visit.
Case 2
A 51-year-old man with a BMI of 29 presented with a long-standing history of neck pain and bilateral arm pain left greater than right in the C6 and C7 dermomyotome. MRI showed a broad-based disc herniation with foraminal narrowing at C5-C6 and C6-C7, and the patient underwent a 2-level anterior cervical discectomy and fusion. This patient had pronounced neck musculature, and a deeper than normal incision was required.
Intraoperative lateral fluoroscopy was obtained to confirm the C5-C6 and C6-C7 level prior to discectomy. The musculature of the patient’s neck and shoulder made visualization of the C6-C7 disc space difficult on the lateral radiograph (Figure 5). One attempt was required to obtain the 30º–30º oblique view, which was used to ensure correct placement of the screws and plate (Figure 6).
Postoperatively, the patient’s pain had improved, and radiographs confirmed adequate hardware placement. He was discharged 1 day after surgery (Figure 7). Imaging at the patient’s 6-week follow-up confirmed adequate fusion from C5-C7, stable disc spaces, and anatomically aligned facet joints. His Neck Disability Index was 34/50 preoperatively and 32/50 at 2-week follow-up.
Discussion
The aim of this study was to describe an alternative to the lateral radiograph for imaging the cervical spine in patients with challenging anatomy or in procedures involving hardware placement at the lower cervical vertebrae. Techniques have been developed to assist with improved lateral visualization, including gentle traction of the arms via wrist restraints or taping the shoulders down inferiorly.2,3 However, visualization in 2 planes continues to be a challenge in a subset of patients. It is particularly difficult to obtain adequate lateral radiographs of the cervical spine in patients with stout necks.3 In patients with stout necks, there is more obstruction of the radiography path through the cervical spine. This leads to imaging that is unclear or may fail to show the mid- to lower cervical spine. The extent to which one should rely on the 30º–30º oblique technique for adequate visualization of the cervical spine depends on the anatomy of a particular patient. Historically, it is more challenging to obtain satisfactory lateral radiographs in patients with stout necks,3 and these patients have benefited the most from using the 30º–30º degree oblique view.
Lack of visualization can lead to aborted surgeries or, potentially, surgery at the wrong level.3 A 2008 American Academy of Neurological Surgeons survey indicated that 50% of spine surgeons had performed a wrong-level surgery at least once in their career, and the cervical spine accounted for 21% of all incorrect-level spine surgeries.7 Intraoperative factors reported during cases of wrong-level spinal surgeries included misinterpretation of intraoperative imaging, no intraoperative imaging, and unusual anatomy or physical characteristics.8 Such complications can lead to revision surgery and other significant morbidities for the patient.
In most patients, fluoroscopy allows confirmation of the correct level before disc incision.3 However, operating at a lower cervical level in a patient with a short neck or prominent shoulders poses a significant problem.3 A case report from Singh and colleagues9 described a modified intraoperative fluoroscopic view for spinal level localization at cervicothoracic levels. Their method focuses on identifying the bony lamina and using them as landmarks to count spinal levels, whereas our 30º–30º oblique image is useful for confirmation of adequate hardware placement during anterior cervical spinal fusions. Often, the initial localization of cervical vertebral levels can be achieved with a standard lateral radiograph. We recognized the utility of the 30º–30º oblique view when we were attempting to visualize the inferior aspect of the plate and inferior screw placement.
In patients with stout necks, a lateral radiograph may show only visualization down to C4 or C5.3 Even with applying traction to the arms or taping the shoulders down, it can be impossible to visualize C6, C7, or T1 because the shoulder bones and muscles obstruct the image.3 Using a 30º–30º oblique view, we were able to obtain adequate visualization and assess the accurate placement of hardware.
Conclusion
A 30º oblique view from horizontal and 30º cephalad from neutral radiograph can be used intraoperatively in patients with challenging anatomy to identify placement of hardware at the correct vertebral level in the lower cervical spine. It is a noninvasive technique that can help reduce the risk of wrong-site surgeries without prolonging operation time. This technique describes an alternative to the lateral radiograph and provides a solution to the difficult problem of intraoperative imaging of the mid- to lower cervical spine in 2 adequate planes.
1. Bebawy JF, Koht A, Mirkovic S. Anterior cervical spine surgery. In: Khot A, Sloan TB, Toleikis JR, eds. Monitoring the Nervous System for Anesthesiologists and Other Health Care Professionals. New York, NY: Springer; 2012:539-554.
2. Abumi K, Shono Y, Ito M, Taneichi H, Kotani Y, Kaneda K. Complications of pedicle screw fixation in reconstructive surgery of the cervical spine. Spine. 2000;25(8):962-969.
3. Irace C. Intraoperative imaging for verification of the correct level during spinal surgery. In: Fountas KN, ed. Novel Frontiers of Advanced Neuroimaging. Rijeka, Croatia: Intech; 2013:175-188.
4. Schwartz DM, Sestokas AK, Hilibrand AS, et al. Neurophysiological identification of position-induced neurologic injury during anterior cervical spine surgery. J Clin Monit Comput. 2006;20(6):437-444.
5. Telfeian AE, Reiter GT, Durham SR, Marcotte P. Spine surgery in morbidly obese patients. J Neurosurg Spine. 2002;97(1):20-24.
6. Oppenheimer JH, DeCastro I, McDonnell DE. Minimally invasive spine technology and minimally invasive spine surgery: a historical review. Neurosurg Focus. 2009;27(3):E9.
7. Mody MG, Nourbakhsh A, Stahl DL, Gibbs M, Alfawareh M, Garges KJ. The prevalence of wrong level surgery among spine surgeons. Spine. 2008;33(2):194.
8. Jhawar BS, Mitsis D, Duggal N. Wrong-sided and wrong-level neurosurgery: A national survey. J Neurosurg Spine. 2007;7(5):467-472.
9. Singh H, Meyer SA, Hecht AC, Jenkins AL 3rd. Novel fluoroscopic technique for localization at cervicothoracic levels. J Spinal Disord Tech. 2009;22(8):615-618.
1. Bebawy JF, Koht A, Mirkovic S. Anterior cervical spine surgery. In: Khot A, Sloan TB, Toleikis JR, eds. Monitoring the Nervous System for Anesthesiologists and Other Health Care Professionals. New York, NY: Springer; 2012:539-554.
2. Abumi K, Shono Y, Ito M, Taneichi H, Kotani Y, Kaneda K. Complications of pedicle screw fixation in reconstructive surgery of the cervical spine. Spine. 2000;25(8):962-969.
3. Irace C. Intraoperative imaging for verification of the correct level during spinal surgery. In: Fountas KN, ed. Novel Frontiers of Advanced Neuroimaging. Rijeka, Croatia: Intech; 2013:175-188.
4. Schwartz DM, Sestokas AK, Hilibrand AS, et al. Neurophysiological identification of position-induced neurologic injury during anterior cervical spine surgery. J Clin Monit Comput. 2006;20(6):437-444.
5. Telfeian AE, Reiter GT, Durham SR, Marcotte P. Spine surgery in morbidly obese patients. J Neurosurg Spine. 2002;97(1):20-24.
6. Oppenheimer JH, DeCastro I, McDonnell DE. Minimally invasive spine technology and minimally invasive spine surgery: a historical review. Neurosurg Focus. 2009;27(3):E9.
7. Mody MG, Nourbakhsh A, Stahl DL, Gibbs M, Alfawareh M, Garges KJ. The prevalence of wrong level surgery among spine surgeons. Spine. 2008;33(2):194.
8. Jhawar BS, Mitsis D, Duggal N. Wrong-sided and wrong-level neurosurgery: A national survey. J Neurosurg Spine. 2007;7(5):467-472.
9. Singh H, Meyer SA, Hecht AC, Jenkins AL 3rd. Novel fluoroscopic technique for localization at cervicothoracic levels. J Spinal Disord Tech. 2009;22(8):615-618.
Long spine fusions can give patients improved quality of life
SAN DIEGO – When necessary, long fusions that extend from the C-spine to the pelvis can result in health-related quality of life improvements, results from a multicenter study suggest.
“Patients with spinal deformities will sometimes require long fusion constructs that extend into the cervical spine,” lead study author Dr. Han-Jo Kim said at the annual meeting of the Cervical Spine Research Society. “The prevalence of these cases is increasing, especially as revision surgery for conditions such as proximal junctional kyphosis increase. They are also indicated for other diagnoses, such a progressive cervical deformity, cervical myelopathy as well as neuromuscular disorders.”
Prior investigations that have examined outcomes for these long constructs usually focus on patients who have had fusions from the upper thoracic spine to the pelvis, added Dr. Kim, an orthopedic spine surgeon at the Hospital for Special Surgery, New York. “To my knowledge, there are no studies in the literature that report on the subset of patients who have had fusions from the cervical spine to the pelvis,” he said. “The question is, even though these revisions may be necessary, does surgical intervention result in improved outcomes for these patients despite the extent of these long fusions?”
In an effort to determine the outcomes and rates of complications in patients who had fusions from the cervical spine to the pelvis, Dr. Kim and his associates conducted a retrospective review of patients who underwent fusions from the cervical spine to the pelvis at four institutions during 2003-2014. The researchers administered outcome scores utilizing the Scoliosis Research Society 22 (SRS-22r) questionnaire; the Oswestry Disability Index (ODI); and the Neck Disability Index (NDI); and collected demographic data including age, body mass index, and follow-up time; medical history including comorbidity data, operative details, radiographic and articular outcomes data; and postoperative complications.
Of 55 patients initially included in the study, complete data were available for 46 (84%). Their average age was 42 years, nearly one-third (30%) were classified as ASA III, 4.2% were smokers, and the average follow-up time was 2.7 years. “The majority of these cases were revision operations, and osteotomies were performed in close to 60% of these patients,” Dr. Kim said. “The average operating time was over 300 minutes, and there was an average of over 2 L of blood loss for these cases.”
The researchers observed improvements in the activity, pain, and mental health domains of the SRS, as well as an improvement in the SRS total score, which improved from an average of 3.0 preoperatively to 3.5 postoperatively (P less than .01). This was greater than the minimally clinically important difference for the SRS-22r. “At least one [minimally clinically important difference] was met in all of the SRS domains, as well as in the NDI,” Dr. Kim said. “There was no change in the ODI, as we would expect for this patient subset.”
Radiographic outcomes improved significantly, he continued, with an average 31-degree correction in maximum kyphosis and a 3.3-cm improvement in sagittal vertical axis. The overall rate of complications was 71%, with major complications comprising about 39% of these cases. Medical complications were high as well (a rate of 61%), as was the rate of surgical complications (43%). More than half of the patients (54%) required reoperation during the follow-up period, and the rate of pseudarthrosis was 29%.
“These results demonstrate improved outcomes following cervical to pelvic fusions, despite the magnitude of their operations and extent of fusion,” Dr. Kim concluded. “In addition, despite the high rate of complications and reoperations, we noted a significant improvement in radiographic and clinical outcomes.”
Dr. Kim disclosed that he is a consultant for Zimmer Biomet and K2M.
SAN DIEGO – When necessary, long fusions that extend from the C-spine to the pelvis can result in health-related quality of life improvements, results from a multicenter study suggest.
“Patients with spinal deformities will sometimes require long fusion constructs that extend into the cervical spine,” lead study author Dr. Han-Jo Kim said at the annual meeting of the Cervical Spine Research Society. “The prevalence of these cases is increasing, especially as revision surgery for conditions such as proximal junctional kyphosis increase. They are also indicated for other diagnoses, such a progressive cervical deformity, cervical myelopathy as well as neuromuscular disorders.”
Prior investigations that have examined outcomes for these long constructs usually focus on patients who have had fusions from the upper thoracic spine to the pelvis, added Dr. Kim, an orthopedic spine surgeon at the Hospital for Special Surgery, New York. “To my knowledge, there are no studies in the literature that report on the subset of patients who have had fusions from the cervical spine to the pelvis,” he said. “The question is, even though these revisions may be necessary, does surgical intervention result in improved outcomes for these patients despite the extent of these long fusions?”
In an effort to determine the outcomes and rates of complications in patients who had fusions from the cervical spine to the pelvis, Dr. Kim and his associates conducted a retrospective review of patients who underwent fusions from the cervical spine to the pelvis at four institutions during 2003-2014. The researchers administered outcome scores utilizing the Scoliosis Research Society 22 (SRS-22r) questionnaire; the Oswestry Disability Index (ODI); and the Neck Disability Index (NDI); and collected demographic data including age, body mass index, and follow-up time; medical history including comorbidity data, operative details, radiographic and articular outcomes data; and postoperative complications.
Of 55 patients initially included in the study, complete data were available for 46 (84%). Their average age was 42 years, nearly one-third (30%) were classified as ASA III, 4.2% were smokers, and the average follow-up time was 2.7 years. “The majority of these cases were revision operations, and osteotomies were performed in close to 60% of these patients,” Dr. Kim said. “The average operating time was over 300 minutes, and there was an average of over 2 L of blood loss for these cases.”
The researchers observed improvements in the activity, pain, and mental health domains of the SRS, as well as an improvement in the SRS total score, which improved from an average of 3.0 preoperatively to 3.5 postoperatively (P less than .01). This was greater than the minimally clinically important difference for the SRS-22r. “At least one [minimally clinically important difference] was met in all of the SRS domains, as well as in the NDI,” Dr. Kim said. “There was no change in the ODI, as we would expect for this patient subset.”
Radiographic outcomes improved significantly, he continued, with an average 31-degree correction in maximum kyphosis and a 3.3-cm improvement in sagittal vertical axis. The overall rate of complications was 71%, with major complications comprising about 39% of these cases. Medical complications were high as well (a rate of 61%), as was the rate of surgical complications (43%). More than half of the patients (54%) required reoperation during the follow-up period, and the rate of pseudarthrosis was 29%.
“These results demonstrate improved outcomes following cervical to pelvic fusions, despite the magnitude of their operations and extent of fusion,” Dr. Kim concluded. “In addition, despite the high rate of complications and reoperations, we noted a significant improvement in radiographic and clinical outcomes.”
Dr. Kim disclosed that he is a consultant for Zimmer Biomet and K2M.
SAN DIEGO – When necessary, long fusions that extend from the C-spine to the pelvis can result in health-related quality of life improvements, results from a multicenter study suggest.
“Patients with spinal deformities will sometimes require long fusion constructs that extend into the cervical spine,” lead study author Dr. Han-Jo Kim said at the annual meeting of the Cervical Spine Research Society. “The prevalence of these cases is increasing, especially as revision surgery for conditions such as proximal junctional kyphosis increase. They are also indicated for other diagnoses, such a progressive cervical deformity, cervical myelopathy as well as neuromuscular disorders.”
Prior investigations that have examined outcomes for these long constructs usually focus on patients who have had fusions from the upper thoracic spine to the pelvis, added Dr. Kim, an orthopedic spine surgeon at the Hospital for Special Surgery, New York. “To my knowledge, there are no studies in the literature that report on the subset of patients who have had fusions from the cervical spine to the pelvis,” he said. “The question is, even though these revisions may be necessary, does surgical intervention result in improved outcomes for these patients despite the extent of these long fusions?”
In an effort to determine the outcomes and rates of complications in patients who had fusions from the cervical spine to the pelvis, Dr. Kim and his associates conducted a retrospective review of patients who underwent fusions from the cervical spine to the pelvis at four institutions during 2003-2014. The researchers administered outcome scores utilizing the Scoliosis Research Society 22 (SRS-22r) questionnaire; the Oswestry Disability Index (ODI); and the Neck Disability Index (NDI); and collected demographic data including age, body mass index, and follow-up time; medical history including comorbidity data, operative details, radiographic and articular outcomes data; and postoperative complications.
Of 55 patients initially included in the study, complete data were available for 46 (84%). Their average age was 42 years, nearly one-third (30%) were classified as ASA III, 4.2% were smokers, and the average follow-up time was 2.7 years. “The majority of these cases were revision operations, and osteotomies were performed in close to 60% of these patients,” Dr. Kim said. “The average operating time was over 300 minutes, and there was an average of over 2 L of blood loss for these cases.”
The researchers observed improvements in the activity, pain, and mental health domains of the SRS, as well as an improvement in the SRS total score, which improved from an average of 3.0 preoperatively to 3.5 postoperatively (P less than .01). This was greater than the minimally clinically important difference for the SRS-22r. “At least one [minimally clinically important difference] was met in all of the SRS domains, as well as in the NDI,” Dr. Kim said. “There was no change in the ODI, as we would expect for this patient subset.”
Radiographic outcomes improved significantly, he continued, with an average 31-degree correction in maximum kyphosis and a 3.3-cm improvement in sagittal vertical axis. The overall rate of complications was 71%, with major complications comprising about 39% of these cases. Medical complications were high as well (a rate of 61%), as was the rate of surgical complications (43%). More than half of the patients (54%) required reoperation during the follow-up period, and the rate of pseudarthrosis was 29%.
“These results demonstrate improved outcomes following cervical to pelvic fusions, despite the magnitude of their operations and extent of fusion,” Dr. Kim concluded. “In addition, despite the high rate of complications and reoperations, we noted a significant improvement in radiographic and clinical outcomes.”
Dr. Kim disclosed that he is a consultant for Zimmer Biomet and K2M.
AT CSRS 2015
Key clinical point: Following cervical to pelvic fusions, patients can achieve improved clinical and quality of life outcomes.
Major finding: The Scoliosis Research Society total score improved from an average of 3.0 preoperatively to 3.5 postoperatively (P less than .01).
Data source: A retrospective review of 55 patients who underwent fusions from the cervical spine to the pelvis at four institutions during 2003-2014.
Disclosures: Dr. Kim disclosed that he is a consultant for Zimmer Biomet and K2M.
SF-6D best quality of life measure in cervical spine patients
SAN DIEGO – Among patients undergoing elective surgical spine procedures, the Short Form–6D derived from the Neck Disability Index was more valid and a better responsive measure of general health and quality of life, compared with the Short Form–6D derived from the Short Form–12 or the EuroQol-5D, results from a single-center study showed.
For such quality of life measures to be useful and meaningful, they “should be reproducible, responsive, economical, easy to use, and sensitive to responder burden,” Dr. John A. Sielatycki said at the annual meeting of the Cervical Spine Research Society.
“The EQ-5D is well established and commonly used in many of these studies, as is SF-6D, which in some cases has been shown to be more sensitive in certain disease states,” explained Dr. Sielatycki, a resident in the department of orthopedics at Vanderbilt University, Nashville, Tenn. “The differences between SF-6D and EQ-5D have been studied in a wide variety of disease conditions, but to our knowledge few have looked at this specifically in the setting of cervical spine operations.”
To analyze the validity and responsiveness of the SF-6D (derived from both the SF-12 and the NDI) and the EQ-5D in determining overall health and quality of life following elective cervical spine procedures, Dr. Sielatycki and his associates compared the three tools in 420 consecutive patients who presented over the course of 2 years. Trauma and workers’ compensation cases were excluded from the study, as were patients who had a tumor or an infection.
The researchers collected outcome measures at baseline, 3 months, 6 months, 12 months, and yearly thereafter, and defined meaningful improvement as having a North American Spine Society patient satisfaction score of 1, indicating the procedure “met the patient’s expectations.” Next, they generated receiver operating characteristic curves to discriminate between meaningful and nonmeaningful improvement.
The SF-6D (NDI) was a more valid discriminator of meaningful improvement, compared with the SF-6D (SF-12) or the EQ-5D (area under the curve of .69, .65, and .62, respectively). It was also a more responsive measure, compared with the SF-6D (SF-12) and the EQ-5D (standardized response means difference of .66, .48, and .44, respectively).
“Surgeons, outcomes researchers, and payers should use health metrics that are most responsive to changes in the particular disease in question,” Dr. Sielatycki said. “Based on this analysis, SF-6D derived from NDI may be a more valid and responsive measure of improvement in patients undergoing cervical procedures. We suggest that this metric be used in cost-effectiveness analysis and in calculating quality-adjusted life years for cervical spine patients.”
Dr. Sielatycki acknowledged certain limitations of the study, including the fact that it “should have some external validation done to further corroborate our findings. Our gold standard of meaningful improvement has not been established.”
Dr. Sielatycki reported having no financial disclosures.
SAN DIEGO – Among patients undergoing elective surgical spine procedures, the Short Form–6D derived from the Neck Disability Index was more valid and a better responsive measure of general health and quality of life, compared with the Short Form–6D derived from the Short Form–12 or the EuroQol-5D, results from a single-center study showed.
For such quality of life measures to be useful and meaningful, they “should be reproducible, responsive, economical, easy to use, and sensitive to responder burden,” Dr. John A. Sielatycki said at the annual meeting of the Cervical Spine Research Society.
“The EQ-5D is well established and commonly used in many of these studies, as is SF-6D, which in some cases has been shown to be more sensitive in certain disease states,” explained Dr. Sielatycki, a resident in the department of orthopedics at Vanderbilt University, Nashville, Tenn. “The differences between SF-6D and EQ-5D have been studied in a wide variety of disease conditions, but to our knowledge few have looked at this specifically in the setting of cervical spine operations.”
To analyze the validity and responsiveness of the SF-6D (derived from both the SF-12 and the NDI) and the EQ-5D in determining overall health and quality of life following elective cervical spine procedures, Dr. Sielatycki and his associates compared the three tools in 420 consecutive patients who presented over the course of 2 years. Trauma and workers’ compensation cases were excluded from the study, as were patients who had a tumor or an infection.
The researchers collected outcome measures at baseline, 3 months, 6 months, 12 months, and yearly thereafter, and defined meaningful improvement as having a North American Spine Society patient satisfaction score of 1, indicating the procedure “met the patient’s expectations.” Next, they generated receiver operating characteristic curves to discriminate between meaningful and nonmeaningful improvement.
The SF-6D (NDI) was a more valid discriminator of meaningful improvement, compared with the SF-6D (SF-12) or the EQ-5D (area under the curve of .69, .65, and .62, respectively). It was also a more responsive measure, compared with the SF-6D (SF-12) and the EQ-5D (standardized response means difference of .66, .48, and .44, respectively).
“Surgeons, outcomes researchers, and payers should use health metrics that are most responsive to changes in the particular disease in question,” Dr. Sielatycki said. “Based on this analysis, SF-6D derived from NDI may be a more valid and responsive measure of improvement in patients undergoing cervical procedures. We suggest that this metric be used in cost-effectiveness analysis and in calculating quality-adjusted life years for cervical spine patients.”
Dr. Sielatycki acknowledged certain limitations of the study, including the fact that it “should have some external validation done to further corroborate our findings. Our gold standard of meaningful improvement has not been established.”
Dr. Sielatycki reported having no financial disclosures.
SAN DIEGO – Among patients undergoing elective surgical spine procedures, the Short Form–6D derived from the Neck Disability Index was more valid and a better responsive measure of general health and quality of life, compared with the Short Form–6D derived from the Short Form–12 or the EuroQol-5D, results from a single-center study showed.
For such quality of life measures to be useful and meaningful, they “should be reproducible, responsive, economical, easy to use, and sensitive to responder burden,” Dr. John A. Sielatycki said at the annual meeting of the Cervical Spine Research Society.
“The EQ-5D is well established and commonly used in many of these studies, as is SF-6D, which in some cases has been shown to be more sensitive in certain disease states,” explained Dr. Sielatycki, a resident in the department of orthopedics at Vanderbilt University, Nashville, Tenn. “The differences between SF-6D and EQ-5D have been studied in a wide variety of disease conditions, but to our knowledge few have looked at this specifically in the setting of cervical spine operations.”
To analyze the validity and responsiveness of the SF-6D (derived from both the SF-12 and the NDI) and the EQ-5D in determining overall health and quality of life following elective cervical spine procedures, Dr. Sielatycki and his associates compared the three tools in 420 consecutive patients who presented over the course of 2 years. Trauma and workers’ compensation cases were excluded from the study, as were patients who had a tumor or an infection.
The researchers collected outcome measures at baseline, 3 months, 6 months, 12 months, and yearly thereafter, and defined meaningful improvement as having a North American Spine Society patient satisfaction score of 1, indicating the procedure “met the patient’s expectations.” Next, they generated receiver operating characteristic curves to discriminate between meaningful and nonmeaningful improvement.
The SF-6D (NDI) was a more valid discriminator of meaningful improvement, compared with the SF-6D (SF-12) or the EQ-5D (area under the curve of .69, .65, and .62, respectively). It was also a more responsive measure, compared with the SF-6D (SF-12) and the EQ-5D (standardized response means difference of .66, .48, and .44, respectively).
“Surgeons, outcomes researchers, and payers should use health metrics that are most responsive to changes in the particular disease in question,” Dr. Sielatycki said. “Based on this analysis, SF-6D derived from NDI may be a more valid and responsive measure of improvement in patients undergoing cervical procedures. We suggest that this metric be used in cost-effectiveness analysis and in calculating quality-adjusted life years for cervical spine patients.”
Dr. Sielatycki acknowledged certain limitations of the study, including the fact that it “should have some external validation done to further corroborate our findings. Our gold standard of meaningful improvement has not been established.”
Dr. Sielatycki reported having no financial disclosures.
AT CSRS 2015
Key clinical point: The Short Form–6D derived from the Neck Disability Index is an effective measure of outcomes in cervical spine patients.
Major finding: The Short Form–6D derived from the Neck Disability Index was a more valid discriminator of meaningful improvement, compared with the Short Form–6D derived from the Short Form–12 or the EuroQol-5D (AUC of .69, .65, and .62, respectively).
Data source: A single-center study that compared three quality of life measures in 420 patients presenting for elective surgical spine procedures.
Disclosures: Dr. Sielatycki reported having no financial disclosures.
Functional dependence linked to risk of complications after spine surgery
SAN DIEGO – Functional dependence following elective cervical spine procedures was associated with a significantly increased risk of almost all 30-day complications analyzed, including mortality, a large retrospective analysis of national data demonstrated.
The findings suggest that physicians should “include the patient’s level of functional independence, in addition to more traditional medical comorbidities, in the risk-benefit analysis of surgical decision making,” Dr. Alpesh A. Patel said in an interview in advance of the annual meeting of the Cervical Spine Research Society. “Those individuals with dependence need to be counseled appropriately about their increased risk of complications including mortality.”
Dr. Patel, professor and director of orthopedic spine surgery at Northwestern University Feinberg School of Medicine, Chicago, and his associates retrospectively reviewed the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data files from 2006 to 2013 and limited their analysis to patients undergoing elective anterior cervical fusions, posterior cervical fusions, cervical laminectomy, cervical laminotomy, cervical discectomy, or corpectomy. They divided patients into one of three groups based on the following preoperative functional status parameters: independent, comprising those not requiring assistance or any equipment for activities of daily living (ADLs); partially dependent, including those with equipment such as prosthetics, equipment, or devices and requiring some assistance from another person for ADLs; and totally dependent, in which patients require total assistance for all ADLs. The researchers used univariate analysis to compare patient demographics, comorbidities, and 30-day postoperative complications among the three groups, followed by multivariate logistic regression to analyze the independent association of functional dependence on 30-day complications when controlling for procedure and comorbidity variances.
Dr. Patel reported findings from 24,357 patients: 23,620 (97.0%) functionally independent, 664 (2.7%) partially dependent, and 73 (0.3%) totally dependent. Dependent patients were significantly older and had higher rates of all comorbidities (P less than .001), with the exception of obesity (P = .214). In addition, 30-day complication rates were higher for all complications (P less than .001) other than neurological (P =.060) and surgical site complications (P =.668). When the researchers controlled for type of procedure and for disparities in patient preoperative variables, multivariate analyses demonstrated that functional dependence was independently associated with sepsis (odds ratio 6.40; P less than .001), pulmonary (OR 4.13; P less than .001), venous thromboembolism (OR 4.27, P less than .001), renal (OR 3.32; P less than .001), and cardiac complications (OR 4.68; P =.001), along with mortality (OR 8.31; P less than .001).
“The very strong association between functional dependence and mortality was quite surprising,” Dr. Patel said. “It was, to the contrary, also surprising to see that, despite wide variance in medical comorbidities and functional status, surgical complications such as infection and neurological injury were similar in all groups.” He characterized the study as “the first large-scale assessment of functional status as a predictor of patient outcomes after cervical spine surgery. It fits in line with other studies utilizing large databases. Big data analysis of outcomes can be used to identify risk factors for complications including death after surgery. Identifying these factors is important if we are going to improve the care we provide. Accurately quantifying the impact of these risk factors is also critical when we risk stratify and compare hospitals and physicians.”
He acknowledged certain limitations of the study, including the fact that it is a retrospective study “with a heterogeneous population of patients, surgeons, hospitals, and procedures. This adds uncertainty to the analysis at the level of the individual patient but does provide generalizability to a broader patient population.”
Dr. Patel reported having no conflicts of interest.
SAN DIEGO – Functional dependence following elective cervical spine procedures was associated with a significantly increased risk of almost all 30-day complications analyzed, including mortality, a large retrospective analysis of national data demonstrated.
The findings suggest that physicians should “include the patient’s level of functional independence, in addition to more traditional medical comorbidities, in the risk-benefit analysis of surgical decision making,” Dr. Alpesh A. Patel said in an interview in advance of the annual meeting of the Cervical Spine Research Society. “Those individuals with dependence need to be counseled appropriately about their increased risk of complications including mortality.”
Dr. Patel, professor and director of orthopedic spine surgery at Northwestern University Feinberg School of Medicine, Chicago, and his associates retrospectively reviewed the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data files from 2006 to 2013 and limited their analysis to patients undergoing elective anterior cervical fusions, posterior cervical fusions, cervical laminectomy, cervical laminotomy, cervical discectomy, or corpectomy. They divided patients into one of three groups based on the following preoperative functional status parameters: independent, comprising those not requiring assistance or any equipment for activities of daily living (ADLs); partially dependent, including those with equipment such as prosthetics, equipment, or devices and requiring some assistance from another person for ADLs; and totally dependent, in which patients require total assistance for all ADLs. The researchers used univariate analysis to compare patient demographics, comorbidities, and 30-day postoperative complications among the three groups, followed by multivariate logistic regression to analyze the independent association of functional dependence on 30-day complications when controlling for procedure and comorbidity variances.
Dr. Patel reported findings from 24,357 patients: 23,620 (97.0%) functionally independent, 664 (2.7%) partially dependent, and 73 (0.3%) totally dependent. Dependent patients were significantly older and had higher rates of all comorbidities (P less than .001), with the exception of obesity (P = .214). In addition, 30-day complication rates were higher for all complications (P less than .001) other than neurological (P =.060) and surgical site complications (P =.668). When the researchers controlled for type of procedure and for disparities in patient preoperative variables, multivariate analyses demonstrated that functional dependence was independently associated with sepsis (odds ratio 6.40; P less than .001), pulmonary (OR 4.13; P less than .001), venous thromboembolism (OR 4.27, P less than .001), renal (OR 3.32; P less than .001), and cardiac complications (OR 4.68; P =.001), along with mortality (OR 8.31; P less than .001).
“The very strong association between functional dependence and mortality was quite surprising,” Dr. Patel said. “It was, to the contrary, also surprising to see that, despite wide variance in medical comorbidities and functional status, surgical complications such as infection and neurological injury were similar in all groups.” He characterized the study as “the first large-scale assessment of functional status as a predictor of patient outcomes after cervical spine surgery. It fits in line with other studies utilizing large databases. Big data analysis of outcomes can be used to identify risk factors for complications including death after surgery. Identifying these factors is important if we are going to improve the care we provide. Accurately quantifying the impact of these risk factors is also critical when we risk stratify and compare hospitals and physicians.”
He acknowledged certain limitations of the study, including the fact that it is a retrospective study “with a heterogeneous population of patients, surgeons, hospitals, and procedures. This adds uncertainty to the analysis at the level of the individual patient but does provide generalizability to a broader patient population.”
Dr. Patel reported having no conflicts of interest.
SAN DIEGO – Functional dependence following elective cervical spine procedures was associated with a significantly increased risk of almost all 30-day complications analyzed, including mortality, a large retrospective analysis of national data demonstrated.
The findings suggest that physicians should “include the patient’s level of functional independence, in addition to more traditional medical comorbidities, in the risk-benefit analysis of surgical decision making,” Dr. Alpesh A. Patel said in an interview in advance of the annual meeting of the Cervical Spine Research Society. “Those individuals with dependence need to be counseled appropriately about their increased risk of complications including mortality.”
Dr. Patel, professor and director of orthopedic spine surgery at Northwestern University Feinberg School of Medicine, Chicago, and his associates retrospectively reviewed the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data files from 2006 to 2013 and limited their analysis to patients undergoing elective anterior cervical fusions, posterior cervical fusions, cervical laminectomy, cervical laminotomy, cervical discectomy, or corpectomy. They divided patients into one of three groups based on the following preoperative functional status parameters: independent, comprising those not requiring assistance or any equipment for activities of daily living (ADLs); partially dependent, including those with equipment such as prosthetics, equipment, or devices and requiring some assistance from another person for ADLs; and totally dependent, in which patients require total assistance for all ADLs. The researchers used univariate analysis to compare patient demographics, comorbidities, and 30-day postoperative complications among the three groups, followed by multivariate logistic regression to analyze the independent association of functional dependence on 30-day complications when controlling for procedure and comorbidity variances.
Dr. Patel reported findings from 24,357 patients: 23,620 (97.0%) functionally independent, 664 (2.7%) partially dependent, and 73 (0.3%) totally dependent. Dependent patients were significantly older and had higher rates of all comorbidities (P less than .001), with the exception of obesity (P = .214). In addition, 30-day complication rates were higher for all complications (P less than .001) other than neurological (P =.060) and surgical site complications (P =.668). When the researchers controlled for type of procedure and for disparities in patient preoperative variables, multivariate analyses demonstrated that functional dependence was independently associated with sepsis (odds ratio 6.40; P less than .001), pulmonary (OR 4.13; P less than .001), venous thromboembolism (OR 4.27, P less than .001), renal (OR 3.32; P less than .001), and cardiac complications (OR 4.68; P =.001), along with mortality (OR 8.31; P less than .001).
“The very strong association between functional dependence and mortality was quite surprising,” Dr. Patel said. “It was, to the contrary, also surprising to see that, despite wide variance in medical comorbidities and functional status, surgical complications such as infection and neurological injury were similar in all groups.” He characterized the study as “the first large-scale assessment of functional status as a predictor of patient outcomes after cervical spine surgery. It fits in line with other studies utilizing large databases. Big data analysis of outcomes can be used to identify risk factors for complications including death after surgery. Identifying these factors is important if we are going to improve the care we provide. Accurately quantifying the impact of these risk factors is also critical when we risk stratify and compare hospitals and physicians.”
He acknowledged certain limitations of the study, including the fact that it is a retrospective study “with a heterogeneous population of patients, surgeons, hospitals, and procedures. This adds uncertainty to the analysis at the level of the individual patient but does provide generalizability to a broader patient population.”
Dr. Patel reported having no conflicts of interest.
AT CSRS 2015
Key clinical point: Preoperative functional status is predictive of morbidity and mortality following elective cervical spine surgery.
Major finding: Patients who were dependent from a functional standpoint were significantly older and had higher rates of all comorbidities, compared with their counterparts who were partially dependent or functionally independent (P less than .001).
Data source: A retrospective analysis of 24,357 patient files from the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP).
Disclosures: Dr. Patel reported having no conflicts of interest.
Study characterizes injury risk in cervical myelopathy patients
SAN DIEGO – Compared with age-matched controls, patients with cervical spondylotic myelopathy had a significantly increased incidence of falls, hip fractures, and other injuries, preliminary results from a study of Medicare data suggest.
“Cervical myelopathy is the most common cause of spinal cord dysfunction in patients over age 55,” Dr. Daniel J. Blizzard said at the annual meeting of the Cervical Spine Research Society. “In general, it’s cord compression secondary to their ossification of posterior latitudinal ligament, congenital stenosis, and/or degenerative changes to vertebral bodies, discs, and facet joints. These create an upper motor neuron lesion, which causes gait disturbances, imbalance, loss of manual dexterity and coordination, and sensory changes and weakness.”
Dr. Blizzard, an orthopedic surgery resident at Duke University, Durham, N.C., noted that myelopathy gait is the most common presenting symptom in cervical spondylotic myelopathy (CSM), affecting almost 30% of patients. “It’s present in three-quarters of CSM patients undergoing decompression,” he said. “Cord compression can lead to impaired proprioception, spasticity, and stiffness. We know that this gait dysfunction is multifactorial. Imbalance and unsteadiness lead to compensatory broad-based arrhythmic shuffling and clumsy-appearing gait to maintain balance.”
An estimated one-third of people over age 65 fall at least once per year and this may lead to significant morbidity, including institutionalization, loss of independence, and mortality, Dr. Blizzard continued. “We know that gait dysfunction is a significant risk factor for falls,” he said. “This can be CSM, lower extremity osteoarthritis, deconditioning, or poor vision. The primary cause of a gait disturbance may not be accurately identified, especially if a more obvious cause is already known.”
The researchers set out to determine the fall and injury risk of patients with CSM, “with the goal of guiding attention to what we thought might be a potentially underestimated disease with regard to morbidity, and to provide data to consider when determining the type and timing of CSM treatment,” Dr. Blizzard said. They used the PearlDiver database to search the Medicare sample during 2005-2012, and used ICD-9 codes to identify patients with CSM. They also identified a subpopulation of CSM patients that underwent decompression, “not for the purpose of comparing the effect of decompression, but to identify a population with more severe disease,” he explained. They included a control population with no CSM, vestibular disease, or Parkinson’s disease.
Dr. Blizzard reported preliminary results from a total of 601,390 patients with CSM, 77,346 patients with CSM plus decompression, and 49,550,651 controls. They looked at the incidence of falls, head injuries, skull fractures, subdural hematomas, and other orthopedic injuries including fractures of the hip, femur, leg, ankle, pelvis, and lower extremity sprains. The researchers found that when compared with controls, patients with CSM had a statistically significant increased incidence of all injuries, including hip fracture (risk ratio, 2.62), head injury (RR, 7.34), and fall (RR, 8.08). The incidence of hip fracture, head injury, and fall was also increased among the subset of CSM patients who had undergone decompression (RR of 2.25, 8.34, and 9.62, respectively).
Dr. Blizzard acknowledged certain limitations of the study, including its retrospective design. “Statistical and clinical significance are two very different things,” he emphasized. “When we get numbers this big, everything will become statistically significant, but whether things are clinically significant is up to interpretation. The presence of disease and complications is contingent upon proper coding and recognition by providers. We have no measures of severity, extent, or chronicity of disease.”
Despite such limitations, he concluded that the findings suggest that impact of CSM on morbidity “is probably underestimated by many. Symptoms of CSM can be insidious or masked. Patients can often attribute these to normal effects of aging, and often primary care physicians will not recognize these initial symptoms, especially if there is another confounding presenting complaint.”
Conservative interventions for CSM patients, he said, include gait training/physical therapy, assistive aids, hip pads, exercise programs with balance training, and an assessment of hazards in the home environment. From a surgical standpoint, the findings raise the possibility that surgeons may want to “be more aggressive” in their decision to operate on patients with CSM. “This dataset is in no way able to address this question, but I think it provides interesting information regarding the true morbidity of the disease,” Dr. Blizzard said. “There is clear risk and morbidity with cervical compression. Studies show improvement in patients regardless of age, severity, and chronicity.”
Dr. Blizzard reported having no financial disclosures.
SAN DIEGO – Compared with age-matched controls, patients with cervical spondylotic myelopathy had a significantly increased incidence of falls, hip fractures, and other injuries, preliminary results from a study of Medicare data suggest.
“Cervical myelopathy is the most common cause of spinal cord dysfunction in patients over age 55,” Dr. Daniel J. Blizzard said at the annual meeting of the Cervical Spine Research Society. “In general, it’s cord compression secondary to their ossification of posterior latitudinal ligament, congenital stenosis, and/or degenerative changes to vertebral bodies, discs, and facet joints. These create an upper motor neuron lesion, which causes gait disturbances, imbalance, loss of manual dexterity and coordination, and sensory changes and weakness.”
Dr. Blizzard, an orthopedic surgery resident at Duke University, Durham, N.C., noted that myelopathy gait is the most common presenting symptom in cervical spondylotic myelopathy (CSM), affecting almost 30% of patients. “It’s present in three-quarters of CSM patients undergoing decompression,” he said. “Cord compression can lead to impaired proprioception, spasticity, and stiffness. We know that this gait dysfunction is multifactorial. Imbalance and unsteadiness lead to compensatory broad-based arrhythmic shuffling and clumsy-appearing gait to maintain balance.”
An estimated one-third of people over age 65 fall at least once per year and this may lead to significant morbidity, including institutionalization, loss of independence, and mortality, Dr. Blizzard continued. “We know that gait dysfunction is a significant risk factor for falls,” he said. “This can be CSM, lower extremity osteoarthritis, deconditioning, or poor vision. The primary cause of a gait disturbance may not be accurately identified, especially if a more obvious cause is already known.”
The researchers set out to determine the fall and injury risk of patients with CSM, “with the goal of guiding attention to what we thought might be a potentially underestimated disease with regard to morbidity, and to provide data to consider when determining the type and timing of CSM treatment,” Dr. Blizzard said. They used the PearlDiver database to search the Medicare sample during 2005-2012, and used ICD-9 codes to identify patients with CSM. They also identified a subpopulation of CSM patients that underwent decompression, “not for the purpose of comparing the effect of decompression, but to identify a population with more severe disease,” he explained. They included a control population with no CSM, vestibular disease, or Parkinson’s disease.
Dr. Blizzard reported preliminary results from a total of 601,390 patients with CSM, 77,346 patients with CSM plus decompression, and 49,550,651 controls. They looked at the incidence of falls, head injuries, skull fractures, subdural hematomas, and other orthopedic injuries including fractures of the hip, femur, leg, ankle, pelvis, and lower extremity sprains. The researchers found that when compared with controls, patients with CSM had a statistically significant increased incidence of all injuries, including hip fracture (risk ratio, 2.62), head injury (RR, 7.34), and fall (RR, 8.08). The incidence of hip fracture, head injury, and fall was also increased among the subset of CSM patients who had undergone decompression (RR of 2.25, 8.34, and 9.62, respectively).
Dr. Blizzard acknowledged certain limitations of the study, including its retrospective design. “Statistical and clinical significance are two very different things,” he emphasized. “When we get numbers this big, everything will become statistically significant, but whether things are clinically significant is up to interpretation. The presence of disease and complications is contingent upon proper coding and recognition by providers. We have no measures of severity, extent, or chronicity of disease.”
Despite such limitations, he concluded that the findings suggest that impact of CSM on morbidity “is probably underestimated by many. Symptoms of CSM can be insidious or masked. Patients can often attribute these to normal effects of aging, and often primary care physicians will not recognize these initial symptoms, especially if there is another confounding presenting complaint.”
Conservative interventions for CSM patients, he said, include gait training/physical therapy, assistive aids, hip pads, exercise programs with balance training, and an assessment of hazards in the home environment. From a surgical standpoint, the findings raise the possibility that surgeons may want to “be more aggressive” in their decision to operate on patients with CSM. “This dataset is in no way able to address this question, but I think it provides interesting information regarding the true morbidity of the disease,” Dr. Blizzard said. “There is clear risk and morbidity with cervical compression. Studies show improvement in patients regardless of age, severity, and chronicity.”
Dr. Blizzard reported having no financial disclosures.
SAN DIEGO – Compared with age-matched controls, patients with cervical spondylotic myelopathy had a significantly increased incidence of falls, hip fractures, and other injuries, preliminary results from a study of Medicare data suggest.
“Cervical myelopathy is the most common cause of spinal cord dysfunction in patients over age 55,” Dr. Daniel J. Blizzard said at the annual meeting of the Cervical Spine Research Society. “In general, it’s cord compression secondary to their ossification of posterior latitudinal ligament, congenital stenosis, and/or degenerative changes to vertebral bodies, discs, and facet joints. These create an upper motor neuron lesion, which causes gait disturbances, imbalance, loss of manual dexterity and coordination, and sensory changes and weakness.”
Dr. Blizzard, an orthopedic surgery resident at Duke University, Durham, N.C., noted that myelopathy gait is the most common presenting symptom in cervical spondylotic myelopathy (CSM), affecting almost 30% of patients. “It’s present in three-quarters of CSM patients undergoing decompression,” he said. “Cord compression can lead to impaired proprioception, spasticity, and stiffness. We know that this gait dysfunction is multifactorial. Imbalance and unsteadiness lead to compensatory broad-based arrhythmic shuffling and clumsy-appearing gait to maintain balance.”
An estimated one-third of people over age 65 fall at least once per year and this may lead to significant morbidity, including institutionalization, loss of independence, and mortality, Dr. Blizzard continued. “We know that gait dysfunction is a significant risk factor for falls,” he said. “This can be CSM, lower extremity osteoarthritis, deconditioning, or poor vision. The primary cause of a gait disturbance may not be accurately identified, especially if a more obvious cause is already known.”
The researchers set out to determine the fall and injury risk of patients with CSM, “with the goal of guiding attention to what we thought might be a potentially underestimated disease with regard to morbidity, and to provide data to consider when determining the type and timing of CSM treatment,” Dr. Blizzard said. They used the PearlDiver database to search the Medicare sample during 2005-2012, and used ICD-9 codes to identify patients with CSM. They also identified a subpopulation of CSM patients that underwent decompression, “not for the purpose of comparing the effect of decompression, but to identify a population with more severe disease,” he explained. They included a control population with no CSM, vestibular disease, or Parkinson’s disease.
Dr. Blizzard reported preliminary results from a total of 601,390 patients with CSM, 77,346 patients with CSM plus decompression, and 49,550,651 controls. They looked at the incidence of falls, head injuries, skull fractures, subdural hematomas, and other orthopedic injuries including fractures of the hip, femur, leg, ankle, pelvis, and lower extremity sprains. The researchers found that when compared with controls, patients with CSM had a statistically significant increased incidence of all injuries, including hip fracture (risk ratio, 2.62), head injury (RR, 7.34), and fall (RR, 8.08). The incidence of hip fracture, head injury, and fall was also increased among the subset of CSM patients who had undergone decompression (RR of 2.25, 8.34, and 9.62, respectively).
Dr. Blizzard acknowledged certain limitations of the study, including its retrospective design. “Statistical and clinical significance are two very different things,” he emphasized. “When we get numbers this big, everything will become statistically significant, but whether things are clinically significant is up to interpretation. The presence of disease and complications is contingent upon proper coding and recognition by providers. We have no measures of severity, extent, or chronicity of disease.”
Despite such limitations, he concluded that the findings suggest that impact of CSM on morbidity “is probably underestimated by many. Symptoms of CSM can be insidious or masked. Patients can often attribute these to normal effects of aging, and often primary care physicians will not recognize these initial symptoms, especially if there is another confounding presenting complaint.”
Conservative interventions for CSM patients, he said, include gait training/physical therapy, assistive aids, hip pads, exercise programs with balance training, and an assessment of hazards in the home environment. From a surgical standpoint, the findings raise the possibility that surgeons may want to “be more aggressive” in their decision to operate on patients with CSM. “This dataset is in no way able to address this question, but I think it provides interesting information regarding the true morbidity of the disease,” Dr. Blizzard said. “There is clear risk and morbidity with cervical compression. Studies show improvement in patients regardless of age, severity, and chronicity.”
Dr. Blizzard reported having no financial disclosures.
AT CSRS 2015
Key clinical point: Medicare patients with cervical spondylotic myelopathy face an increased risk of falls and fractures.
Major finding: Compared with controls, patients with CSM had a statistically significant increased incidence of all injuries, including hip fracture (risk ratio, 2.62), head injury (RR, 7.34), and fall (RR, 8.08).
Data source: A retrospective analysis of Medicare patients during 2005-2012, including 601,390 patients with CSM, 77,346 patients with CSM plus decompression, and 49,550,651 controls.
Disclosures: Dr. Blizzard reported having no financial disclosures.
PROMIS physical function domain outperforms in cervical spine patients
SAN DIEGO – The Neck Disability Index–10 did not perform as well as the Neck Disability Index–5 in assessing patient-reported outcomes in cervical spine patients – and neither was as good as the PROMIS physical function domain delivered by computerized adaptive testing.
Those are the key findings from an analysis of data from more than 500 cervical spine patients treated at University of Utah Health Care in Salt Lake City.
“Previous studies by us and others have shown problems with the NDI [Neck Disability Index] as it is commonly administered” in 10 questions, lead study author Dr. Darrel S. Brodke said in an interview in advance of the annual meeting of the Cervical Spine Research Society. “It has a very poor floor effect, meaning that it does not differentiate between minimally disabled patients, and the scores cannot be appropriately handled with the kinds of statistics that we normally use – though because few of us know this, we still use it as a standard parametric measure.”
In what he said is the first study of its kind, Dr. Brodke, professor of orthopedics at the University of Utah, and his associates set out to compare the psychometric performance of the National Institutes of Health–funded PROMIS (Patient Reported Outcomes Measurement Information System) physical function (PF) domain, administered by computerized adaptive testing, with the standard NDI-10, the NDI-5, and the 36-Item Short Form physical function domain (SF-36 PFD).
In all, 566 patients completed the NDI and PROMIS PF computerized adaptive testing assessments, while 490 also completed the SF-36 PFD.
On average, the NDI-10 took the longest to complete (10 questions in a mean of 183 seconds), followed by the SF-36 PFD (5 questions in a mean of 123 seconds), the NDI-5 (5 questions in a mean of 99 seconds), and the PROMIS PF computerized adaptive testing (between 4 and 12 questions in a mean of 62 seconds).
The psychometric properties of the PROMIS PF computerized adaptive testing were superior to the other outcome measurement tools studied, Dr. Brodke reported. Specifically, the ceiling and floor effects were “excellent” for the PROMIS PF computerized adaptive testing (1.94% and 4.06%, respectively), while the ceiling effects were “fine” for the NDI-10 (4.77%), NDI-5 (7.60%), and SF-36 PFD (11.84%), he said.
However, the floor effects of these three instruments were poor (45.58%, 48.59% and 21.55%, respectively). “The NDI-10 also has the additional challenge of extremely poor raw score to measure correlation,” the researchers noted in their abstract.
“The legacy scale scores significantly predicted the PROMIS PF CAT scores (P less than .0001), with fair correlation for the PF CAT and NDI-10 (0.53) and good correlation of PF CAT and SF-36 PFD (0.62), allowing use of conversion equations to predict scores, which were generated,” the investigators explained.
PROMIS PF computerized adaptive testing “does much better than the NDI or the SF-36 physical function domain at characterizing patients’ physical function, with much better coverage,” Dr. Brodke said. “Not only this, but it is also much faster to fill out, so less burdensome to the patient and the clinic.”
One limitation of the study is that the researchers did not measure the responsiveness aspect of PROMIS performance. “We did not have enough pre- and posttreatment scores to do this measurement yet,” Dr. Brodke said. “The other thing is that minimum clinically important difference [MCID] is not yet worked out for PROMIS in this patient population, though we can infer an MCID as one-half of a standard deviation. More to come in future studies.”
Dr. Brodke reported having no financial disclosures.
SAN DIEGO – The Neck Disability Index–10 did not perform as well as the Neck Disability Index–5 in assessing patient-reported outcomes in cervical spine patients – and neither was as good as the PROMIS physical function domain delivered by computerized adaptive testing.
Those are the key findings from an analysis of data from more than 500 cervical spine patients treated at University of Utah Health Care in Salt Lake City.
“Previous studies by us and others have shown problems with the NDI [Neck Disability Index] as it is commonly administered” in 10 questions, lead study author Dr. Darrel S. Brodke said in an interview in advance of the annual meeting of the Cervical Spine Research Society. “It has a very poor floor effect, meaning that it does not differentiate between minimally disabled patients, and the scores cannot be appropriately handled with the kinds of statistics that we normally use – though because few of us know this, we still use it as a standard parametric measure.”
In what he said is the first study of its kind, Dr. Brodke, professor of orthopedics at the University of Utah, and his associates set out to compare the psychometric performance of the National Institutes of Health–funded PROMIS (Patient Reported Outcomes Measurement Information System) physical function (PF) domain, administered by computerized adaptive testing, with the standard NDI-10, the NDI-5, and the 36-Item Short Form physical function domain (SF-36 PFD).
In all, 566 patients completed the NDI and PROMIS PF computerized adaptive testing assessments, while 490 also completed the SF-36 PFD.
On average, the NDI-10 took the longest to complete (10 questions in a mean of 183 seconds), followed by the SF-36 PFD (5 questions in a mean of 123 seconds), the NDI-5 (5 questions in a mean of 99 seconds), and the PROMIS PF computerized adaptive testing (between 4 and 12 questions in a mean of 62 seconds).
The psychometric properties of the PROMIS PF computerized adaptive testing were superior to the other outcome measurement tools studied, Dr. Brodke reported. Specifically, the ceiling and floor effects were “excellent” for the PROMIS PF computerized adaptive testing (1.94% and 4.06%, respectively), while the ceiling effects were “fine” for the NDI-10 (4.77%), NDI-5 (7.60%), and SF-36 PFD (11.84%), he said.
However, the floor effects of these three instruments were poor (45.58%, 48.59% and 21.55%, respectively). “The NDI-10 also has the additional challenge of extremely poor raw score to measure correlation,” the researchers noted in their abstract.
“The legacy scale scores significantly predicted the PROMIS PF CAT scores (P less than .0001), with fair correlation for the PF CAT and NDI-10 (0.53) and good correlation of PF CAT and SF-36 PFD (0.62), allowing use of conversion equations to predict scores, which were generated,” the investigators explained.
PROMIS PF computerized adaptive testing “does much better than the NDI or the SF-36 physical function domain at characterizing patients’ physical function, with much better coverage,” Dr. Brodke said. “Not only this, but it is also much faster to fill out, so less burdensome to the patient and the clinic.”
One limitation of the study is that the researchers did not measure the responsiveness aspect of PROMIS performance. “We did not have enough pre- and posttreatment scores to do this measurement yet,” Dr. Brodke said. “The other thing is that minimum clinically important difference [MCID] is not yet worked out for PROMIS in this patient population, though we can infer an MCID as one-half of a standard deviation. More to come in future studies.”
Dr. Brodke reported having no financial disclosures.
SAN DIEGO – The Neck Disability Index–10 did not perform as well as the Neck Disability Index–5 in assessing patient-reported outcomes in cervical spine patients – and neither was as good as the PROMIS physical function domain delivered by computerized adaptive testing.
Those are the key findings from an analysis of data from more than 500 cervical spine patients treated at University of Utah Health Care in Salt Lake City.
“Previous studies by us and others have shown problems with the NDI [Neck Disability Index] as it is commonly administered” in 10 questions, lead study author Dr. Darrel S. Brodke said in an interview in advance of the annual meeting of the Cervical Spine Research Society. “It has a very poor floor effect, meaning that it does not differentiate between minimally disabled patients, and the scores cannot be appropriately handled with the kinds of statistics that we normally use – though because few of us know this, we still use it as a standard parametric measure.”
In what he said is the first study of its kind, Dr. Brodke, professor of orthopedics at the University of Utah, and his associates set out to compare the psychometric performance of the National Institutes of Health–funded PROMIS (Patient Reported Outcomes Measurement Information System) physical function (PF) domain, administered by computerized adaptive testing, with the standard NDI-10, the NDI-5, and the 36-Item Short Form physical function domain (SF-36 PFD).
In all, 566 patients completed the NDI and PROMIS PF computerized adaptive testing assessments, while 490 also completed the SF-36 PFD.
On average, the NDI-10 took the longest to complete (10 questions in a mean of 183 seconds), followed by the SF-36 PFD (5 questions in a mean of 123 seconds), the NDI-5 (5 questions in a mean of 99 seconds), and the PROMIS PF computerized adaptive testing (between 4 and 12 questions in a mean of 62 seconds).
The psychometric properties of the PROMIS PF computerized adaptive testing were superior to the other outcome measurement tools studied, Dr. Brodke reported. Specifically, the ceiling and floor effects were “excellent” for the PROMIS PF computerized adaptive testing (1.94% and 4.06%, respectively), while the ceiling effects were “fine” for the NDI-10 (4.77%), NDI-5 (7.60%), and SF-36 PFD (11.84%), he said.
However, the floor effects of these three instruments were poor (45.58%, 48.59% and 21.55%, respectively). “The NDI-10 also has the additional challenge of extremely poor raw score to measure correlation,” the researchers noted in their abstract.
“The legacy scale scores significantly predicted the PROMIS PF CAT scores (P less than .0001), with fair correlation for the PF CAT and NDI-10 (0.53) and good correlation of PF CAT and SF-36 PFD (0.62), allowing use of conversion equations to predict scores, which were generated,” the investigators explained.
PROMIS PF computerized adaptive testing “does much better than the NDI or the SF-36 physical function domain at characterizing patients’ physical function, with much better coverage,” Dr. Brodke said. “Not only this, but it is also much faster to fill out, so less burdensome to the patient and the clinic.”
One limitation of the study is that the researchers did not measure the responsiveness aspect of PROMIS performance. “We did not have enough pre- and posttreatment scores to do this measurement yet,” Dr. Brodke said. “The other thing is that minimum clinically important difference [MCID] is not yet worked out for PROMIS in this patient population, though we can infer an MCID as one-half of a standard deviation. More to come in future studies.”
Dr. Brodke reported having no financial disclosures.
AT CSRS 2015
Key clinical point: In the elective cervical spine surgery population, the PROMIS physical function domain as delivered by computerized adaptive testing outperforms other commonly used tools to measure patient-reported outcomes.
Major finding: The ceiling and floor effects were “excellent” for the PROMIS PF (1.94% and 4.06%, respectively), while the ceiling effects were “fine” for the Neck Disability Index–10 (4.77%), the Neck Disability Index–5 (7.60%), and the 36-Item Short Form physical function domain (11.84%). However, the floor effects of these three instruments were poor (45.58%, 48.59%, and 21.55%, respectively).
Data source: A study of the psychometric performance of the PROMIS physical function domain, administered by computerized adaptive testing, comparing the standard NDI-10, NDI-5, and SF-36 physical function domain.
Disclosures: Dr. Brodke reported having no financial disclosures.