User login
Stroke, arterial dissection events reported with Lemtrada, FDA says
Instances of stroke and arterial dissection in the head and neck have been reported in some multiple sclerosis patients soon after an infusion of alemtuzumab (Lemtrada), according to a safety announcement issued by the Food and Drug Administration on Nov. 29.
Since the FDA approved alemtuzumab in 2014 for relapsing forms of MS, 13 cases of ischemic and hemorrhagic stroke or arterial dissection have been reported worldwide via the FDA Adverse Event Reporting System, but “additional cases we are unaware of may have occurred,” the FDA said in the announcement.
Most of the patients who developed stroke or arterial lining tears showed symptoms within a day of taking the medication, although one patient reported symptoms three days after treatment. The drug is given via intravenous infusion and is generally reserved for patients with relapsing MS who have not responded adequately to other approved MS medications, according to the FDA.
Symptoms include sudden onset of the following: severe headache or neck pain; numbness or weakness in the arms or legs, especially on only one side of the body; confusion or trouble speaking or understanding speech; vision problems in one or both eyes; and dizziness, loss of balance, or difficulty walking.
As a result of the reports, the FDA has updated the drug label prescribing information and the patient Medication Guide to reflect these risks, and added the risk of stroke to the medication’s existing boxed warning.
Health care providers should remind patients of the potential for stroke and arterial dissection at each treatment visit and advise them to seek immediate medical attention if they experience any of the symptoms reported in previous cases. “The diagnosis is often complicated because early symptoms such as headache and neck pain are not specific,” according to the agency, but patients complaining of such symptoms should be evaluated immediately.
Alemtuzumab was also approved in May 2001 for treating B-cell chronic lymphocytic leukemia (B-CLL) under the brand name Campath. The FDA will update the Campath label to reflect the new warnings and risks.
Instances of stroke and arterial dissection in the head and neck have been reported in some multiple sclerosis patients soon after an infusion of alemtuzumab (Lemtrada), according to a safety announcement issued by the Food and Drug Administration on Nov. 29.
Since the FDA approved alemtuzumab in 2014 for relapsing forms of MS, 13 cases of ischemic and hemorrhagic stroke or arterial dissection have been reported worldwide via the FDA Adverse Event Reporting System, but “additional cases we are unaware of may have occurred,” the FDA said in the announcement.
Most of the patients who developed stroke or arterial lining tears showed symptoms within a day of taking the medication, although one patient reported symptoms three days after treatment. The drug is given via intravenous infusion and is generally reserved for patients with relapsing MS who have not responded adequately to other approved MS medications, according to the FDA.
Symptoms include sudden onset of the following: severe headache or neck pain; numbness or weakness in the arms or legs, especially on only one side of the body; confusion or trouble speaking or understanding speech; vision problems in one or both eyes; and dizziness, loss of balance, or difficulty walking.
As a result of the reports, the FDA has updated the drug label prescribing information and the patient Medication Guide to reflect these risks, and added the risk of stroke to the medication’s existing boxed warning.
Health care providers should remind patients of the potential for stroke and arterial dissection at each treatment visit and advise them to seek immediate medical attention if they experience any of the symptoms reported in previous cases. “The diagnosis is often complicated because early symptoms such as headache and neck pain are not specific,” according to the agency, but patients complaining of such symptoms should be evaluated immediately.
Alemtuzumab was also approved in May 2001 for treating B-cell chronic lymphocytic leukemia (B-CLL) under the brand name Campath. The FDA will update the Campath label to reflect the new warnings and risks.
Instances of stroke and arterial dissection in the head and neck have been reported in some multiple sclerosis patients soon after an infusion of alemtuzumab (Lemtrada), according to a safety announcement issued by the Food and Drug Administration on Nov. 29.
Since the FDA approved alemtuzumab in 2014 for relapsing forms of MS, 13 cases of ischemic and hemorrhagic stroke or arterial dissection have been reported worldwide via the FDA Adverse Event Reporting System, but “additional cases we are unaware of may have occurred,” the FDA said in the announcement.
Most of the patients who developed stroke or arterial lining tears showed symptoms within a day of taking the medication, although one patient reported symptoms three days after treatment. The drug is given via intravenous infusion and is generally reserved for patients with relapsing MS who have not responded adequately to other approved MS medications, according to the FDA.
Symptoms include sudden onset of the following: severe headache or neck pain; numbness or weakness in the arms or legs, especially on only one side of the body; confusion or trouble speaking or understanding speech; vision problems in one or both eyes; and dizziness, loss of balance, or difficulty walking.
As a result of the reports, the FDA has updated the drug label prescribing information and the patient Medication Guide to reflect these risks, and added the risk of stroke to the medication’s existing boxed warning.
Health care providers should remind patients of the potential for stroke and arterial dissection at each treatment visit and advise them to seek immediate medical attention if they experience any of the symptoms reported in previous cases. “The diagnosis is often complicated because early symptoms such as headache and neck pain are not specific,” according to the agency, but patients complaining of such symptoms should be evaluated immediately.
Alemtuzumab was also approved in May 2001 for treating B-cell chronic lymphocytic leukemia (B-CLL) under the brand name Campath. The FDA will update the Campath label to reflect the new warnings and risks.
Heart disease remains the leading cause of death in U.S.
The 10 leading causes of death in the United States remained unchanged over the past year, according to a new report from the Centers for Disease Control (CDC). Though life expectancy at birth decreased to 78.6 years in 2017, down from 78.7 years in 2016, that change was driven primarily by suicide and drug overdose.
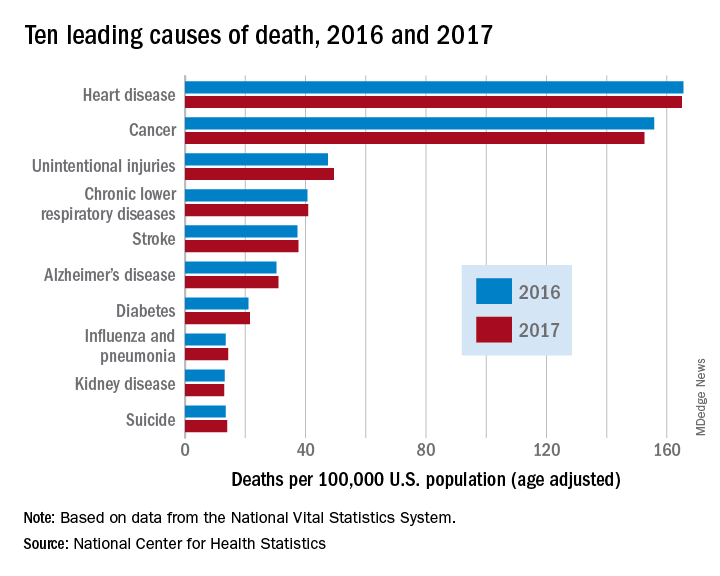
However, heart disease remains the leading cause of death in the United States, at 165 deaths per 100,000 individuals in 2017. This represents a slight, statistically nonsignificant, decrease from the 165.5 deaths per 100,000 caused by heart disease in the previous year.
Other diseases related to cardiometabolic health saw increases. Stroke and diabetes each caused a small but significant increase in deaths in 2017, which saw a 1-year increase to 37.6 from 37.3 stroke deaths per 100,000 people. Diabetes deaths increased to 21.5 from 21 per 100,000 the previous year. Stroke was the fifth and diabetes the seventh most common cause of death, according to the data brief published by the CDC’s National Center for Health Statistics (NCHS).
Alzheimer’s disease deaths also increased significantly, from 30.3 per 100,000 in 2016 to 31 per 100,000 in 2017. Although Alzheimer’s exact etiology remains under study, cardiovascular disease factors and Alzheimer’s disease share many risk factors and are often comorbid .
“With a slight decrease in deaths from heart disease in 2017 and a slight increase in deaths from stroke, this lack of any major movement in these areas has been a trend we’ve seen the last couple of years,” said Ivor Benjamin, MD, president of the American Heart Association, in a press release. “It is discouraging after experiencing decades when heart disease and stroke death rates both dropped more dramatically.”
Infant deaths from congenital malformations decreased from 2016 to 2017, from 122.1 to 118.8 deaths per 100,000 live births. “While the report doesn’t specify death rates for specific types of congenital malformations, this is heartening news as it could reflect fewer deaths from congenital heart defects,” said the AHA in its release.
According to the CDC, the 10 leading causes of death together account for about three quarters of United States deaths. Cancer caused nearly as many deaths as heart disease – 152.5 per 100,000. This represented a significant decrease from the 155.8 cancer deaths per 100,000 seen in 2016. The remaining top 10 causes of death, in decreasing order, were unintentional injuries, chronic lower respiratory diseases, influenza and pneumonia, kidney disease, and suicide.
The 10 leading causes of death in the United States remained unchanged over the past year, according to a new report from the Centers for Disease Control (CDC). Though life expectancy at birth decreased to 78.6 years in 2017, down from 78.7 years in 2016, that change was driven primarily by suicide and drug overdose.

However, heart disease remains the leading cause of death in the United States, at 165 deaths per 100,000 individuals in 2017. This represents a slight, statistically nonsignificant, decrease from the 165.5 deaths per 100,000 caused by heart disease in the previous year.
Other diseases related to cardiometabolic health saw increases. Stroke and diabetes each caused a small but significant increase in deaths in 2017, which saw a 1-year increase to 37.6 from 37.3 stroke deaths per 100,000 people. Diabetes deaths increased to 21.5 from 21 per 100,000 the previous year. Stroke was the fifth and diabetes the seventh most common cause of death, according to the data brief published by the CDC’s National Center for Health Statistics (NCHS).
Alzheimer’s disease deaths also increased significantly, from 30.3 per 100,000 in 2016 to 31 per 100,000 in 2017. Although Alzheimer’s exact etiology remains under study, cardiovascular disease factors and Alzheimer’s disease share many risk factors and are often comorbid .
“With a slight decrease in deaths from heart disease in 2017 and a slight increase in deaths from stroke, this lack of any major movement in these areas has been a trend we’ve seen the last couple of years,” said Ivor Benjamin, MD, president of the American Heart Association, in a press release. “It is discouraging after experiencing decades when heart disease and stroke death rates both dropped more dramatically.”
Infant deaths from congenital malformations decreased from 2016 to 2017, from 122.1 to 118.8 deaths per 100,000 live births. “While the report doesn’t specify death rates for specific types of congenital malformations, this is heartening news as it could reflect fewer deaths from congenital heart defects,” said the AHA in its release.
According to the CDC, the 10 leading causes of death together account for about three quarters of United States deaths. Cancer caused nearly as many deaths as heart disease – 152.5 per 100,000. This represented a significant decrease from the 155.8 cancer deaths per 100,000 seen in 2016. The remaining top 10 causes of death, in decreasing order, were unintentional injuries, chronic lower respiratory diseases, influenza and pneumonia, kidney disease, and suicide.
The 10 leading causes of death in the United States remained unchanged over the past year, according to a new report from the Centers for Disease Control (CDC). Though life expectancy at birth decreased to 78.6 years in 2017, down from 78.7 years in 2016, that change was driven primarily by suicide and drug overdose.

However, heart disease remains the leading cause of death in the United States, at 165 deaths per 100,000 individuals in 2017. This represents a slight, statistically nonsignificant, decrease from the 165.5 deaths per 100,000 caused by heart disease in the previous year.
Other diseases related to cardiometabolic health saw increases. Stroke and diabetes each caused a small but significant increase in deaths in 2017, which saw a 1-year increase to 37.6 from 37.3 stroke deaths per 100,000 people. Diabetes deaths increased to 21.5 from 21 per 100,000 the previous year. Stroke was the fifth and diabetes the seventh most common cause of death, according to the data brief published by the CDC’s National Center for Health Statistics (NCHS).
Alzheimer’s disease deaths also increased significantly, from 30.3 per 100,000 in 2016 to 31 per 100,000 in 2017. Although Alzheimer’s exact etiology remains under study, cardiovascular disease factors and Alzheimer’s disease share many risk factors and are often comorbid .
“With a slight decrease in deaths from heart disease in 2017 and a slight increase in deaths from stroke, this lack of any major movement in these areas has been a trend we’ve seen the last couple of years,” said Ivor Benjamin, MD, president of the American Heart Association, in a press release. “It is discouraging after experiencing decades when heart disease and stroke death rates both dropped more dramatically.”
Infant deaths from congenital malformations decreased from 2016 to 2017, from 122.1 to 118.8 deaths per 100,000 live births. “While the report doesn’t specify death rates for specific types of congenital malformations, this is heartening news as it could reflect fewer deaths from congenital heart defects,” said the AHA in its release.
According to the CDC, the 10 leading causes of death together account for about three quarters of United States deaths. Cancer caused nearly as many deaths as heart disease – 152.5 per 100,000. This represented a significant decrease from the 155.8 cancer deaths per 100,000 seen in 2016. The remaining top 10 causes of death, in decreasing order, were unintentional injuries, chronic lower respiratory diseases, influenza and pneumonia, kidney disease, and suicide.
FROM A CDC DATA BRIEF
Expert highlights rare causes of stroke to keep in mind
ATLANTA – According to Peter Berlit, MD, clinicians should .
Other factors include combination of ischemic and hemorrhagic stroke, exclusive involvement of intracranial vessels, systemic signs, and lab tests indicating inflammation.
At the annual meeting of the American Neurological Association, Dr. Berlit, secretary general of the German Society of Neurology in Berlin, discussed the diagnosis and management of rare causes of stroke.
Giant cell arteritis (GCA)
One of the rare causes of stroke, GCA can be diagnosed when three of five criteria are met: being 50 years of age or older, having a newly developed headache, tenderness of the superficial temporal artery, elevated sedimentation rate of at least 50 mm per hour, and GCA in a biopsy specimen from the temporal artery.
“What we fear most is sudden blindness due to involvement of arteries serving the eyes, which appears in up to 30% of GCA patients,” said Dr. Berlit, who formerly chaired the department of neurology at Alfried Krupp Hospital, Essen, Germany. “Stroke occurs in approximately 2% of GCA patients, so it’s a lot rarer.” GCA can also be diagnosed by ultrasound. One meta-analysis of 23 studies using halo, stenosis, and occlusion as ultrasound criteria found a sensitivity of 87% and a specificity of 96% (Ann Intern Med. 2005;142[5]:359-69). “You can also use 3-Tesla MRI with the use of contrast agent, which shows inflammation of the temporal artery, but also other large vessels including the aortic arch,” he said. “The treatment of GCA has changed since the end of 2017 and involves starting with prednisolone 1 mg/kg body weight.” After a dose of 30 mg for 4 weeks, reduce the dose by 2.5 mg every 2 weeks. After reaching the dose of 15 mg daily, reduce by 1 mg per month. “The recommended steroid-sparing treatment is subcutaneous tocilizumab at a dose of 162 mg weekly or every other week, combined with a prednisone taper for a minimum of 26 weeks,” he said. Supportive therapies include pantoprazole 20 mg, aspirin 100 mg, calcium, vitamin D, and bisphosphonates.
Primary angiitis of the central nervous system (PACNS)
Next, Dr. Berlit discussed diagnostic criteria for PACNS, an acquired neurological deficit unexplained after complete evaluation. “You should have a diagnostic cerebral angiogram or biopsy demonstrating vasculitis,” he said. “There should be no evidence of systemic vasculitis or any other conditions that could mimic the angiogram findings. Usually you have abnormal CSF findings, including pleocytosis and protein elevation, and a biopsy demonstrating vasculitis.”
MRI studies in suspected vasculitis include fluid-attenuated inversion recovery (FLAIR), diffusion imaging with apparent diffusion coefficient (ADC) maps, gradient ECHO, MR angiography, and contrast-enhanced imaging. “These usually show multifocal lesions of different ages, and hemorrhages occur in about 10% of lesions,” Dr. Berlit said. “Leptomeningeal enhancement is an indicator of good treatment response.”
A brain and leptomeningeal biopsy demonstrating the angiitis remains the preferred method for diagnosis of PACNS. “Open biopsies out of recent MRI lesions are especially diagnostic,” he said. “If there are no lesions accessible for surgery in noneloquent brain areas, a biopsy from the right frontal lobe is recommended.” The histologic findings of PACNS consist of granulomatous inflammation, fibrinoid necrosis of vessel walls, or exclusively lymphocytic cellular infiltrates. “The treatment of choice in PACNS is the combination of steroids and cyclophosphamide pulse therapy,” he said. “There are also data showing that rituximab or methotrexate might be treatment options. With a relapse rate of 25% and a reduced survival rate, a close follow-up of suspected PACNS is mandatory.”
Reversible cerebral vasoconstriction syndrome (RCVS)
Another rare cause of stroke is RCVS, which typically presents as thunderclap headaches with or without neurologic symptoms. MRI may be normal, but symmetric border zone infarctions and small subarachnoid hemorrhages are possible. Catheter, CT, or MR angiography show segmental arterial vasoconstriction. “You always have to exclude cerebral aneurysm,” Dr. Berlit said. “There is reversibility of RCVS within 3 months.” RCVS is often associated with a long list of drugs, including phenylpropanolamine, Methergine (methylergonovine), bromocriptine, lisuride, SSRIs, triptans, isometheptene, tacrolimus, cyclophosphamide, erythropoietin, intravenous immunoglobulins, erythrocyte concentrates, nasal sprays, cocaine, ecstasy, amphetamines, cannabis, and LSD. “After stopping responsible medications, treatment involves a course of nimodipine,” he said.
Moyamoya disease (MMD)
Dr. Berlit closed his presentation by discussing MMD, a rare occlusive cerebrovascular disorder characterized by progressive stenosis or occlusion of the intracranial portion of the internal carotid artery and proximal cerebral arteries with an extensive network of fine collaterals. “This is an idiopathic vasculopathy with remarkable regional and racial differences worldwide; it’s most frequently found in Asians, especially in Japan and Korea,” he said. “In Europe, there is about one-tenth the incidence, compared with that of Japan. In Asian MMD, about 15% of cases follow an autosomal dominant inheritance. The collaterals in MMD present histologically as a thin media, a fragmented elastic laminae, and the formation of microaneurysms. There is no inflammation.”
MMD diagnostic criteria include stenosis or occlusion of the terminal portion of the internal carotid artery and at the proximal portion of the anterior and middle cerebral arteries. Abnormal vascular networks are present in the basal ganglia and angiographic findings present bilaterally. Cases with unilateral angiographic findings are considered probable. Clinicians should exclude the following conditions: arteriosclerosis, autoimmune disease, brain neoplasm, history of cranial irradiation, Down syndrome, head trauma, neurofibromatosis, and meningitis. “If the angiographic pattern is resembled by one of these conditions, this is called moyamoya syndrome,” Dr. Berlit noted. “MMD is a progressive disorder. Within a few months you can see occlusion of the middle cerebral artery and the anterior cerebral artery, so you have to treat these patients.”
In patients who are white, MMD presents with lower rates of hemorrhage, but in Asians, microbleeds occur in up to 44% of patients and hemorrhages in up to 65% patients. “Both subarachnoidal and intracerebral hemorrhages occur, especially in connection with pregnancy and delivery,” he said. “The risk of both cerebral ischemia and hemorrhagic complications increases with stages of MMD.”
Direct or indirect intracranial bypass surgery is recommended in stages 3 or more, and has been shown to significantly reduce the 5-year stroke risk. To date, Dr. Berlit and his associates have treated 86 hemispheres in 56 patients. The average age of the patients was 42 years, 70% were female, and the average follow-up was 39 months. All intracranial bypasses were open on follow-up, and a decrease of the typical moyamoya vessels was observed in 81% of patients.
Dr. Berlit reported having no financial disclosures.
ATLANTA – According to Peter Berlit, MD, clinicians should .
Other factors include combination of ischemic and hemorrhagic stroke, exclusive involvement of intracranial vessels, systemic signs, and lab tests indicating inflammation.
At the annual meeting of the American Neurological Association, Dr. Berlit, secretary general of the German Society of Neurology in Berlin, discussed the diagnosis and management of rare causes of stroke.
Giant cell arteritis (GCA)
One of the rare causes of stroke, GCA can be diagnosed when three of five criteria are met: being 50 years of age or older, having a newly developed headache, tenderness of the superficial temporal artery, elevated sedimentation rate of at least 50 mm per hour, and GCA in a biopsy specimen from the temporal artery.
“What we fear most is sudden blindness due to involvement of arteries serving the eyes, which appears in up to 30% of GCA patients,” said Dr. Berlit, who formerly chaired the department of neurology at Alfried Krupp Hospital, Essen, Germany. “Stroke occurs in approximately 2% of GCA patients, so it’s a lot rarer.” GCA can also be diagnosed by ultrasound. One meta-analysis of 23 studies using halo, stenosis, and occlusion as ultrasound criteria found a sensitivity of 87% and a specificity of 96% (Ann Intern Med. 2005;142[5]:359-69). “You can also use 3-Tesla MRI with the use of contrast agent, which shows inflammation of the temporal artery, but also other large vessels including the aortic arch,” he said. “The treatment of GCA has changed since the end of 2017 and involves starting with prednisolone 1 mg/kg body weight.” After a dose of 30 mg for 4 weeks, reduce the dose by 2.5 mg every 2 weeks. After reaching the dose of 15 mg daily, reduce by 1 mg per month. “The recommended steroid-sparing treatment is subcutaneous tocilizumab at a dose of 162 mg weekly or every other week, combined with a prednisone taper for a minimum of 26 weeks,” he said. Supportive therapies include pantoprazole 20 mg, aspirin 100 mg, calcium, vitamin D, and bisphosphonates.
Primary angiitis of the central nervous system (PACNS)
Next, Dr. Berlit discussed diagnostic criteria for PACNS, an acquired neurological deficit unexplained after complete evaluation. “You should have a diagnostic cerebral angiogram or biopsy demonstrating vasculitis,” he said. “There should be no evidence of systemic vasculitis or any other conditions that could mimic the angiogram findings. Usually you have abnormal CSF findings, including pleocytosis and protein elevation, and a biopsy demonstrating vasculitis.”
MRI studies in suspected vasculitis include fluid-attenuated inversion recovery (FLAIR), diffusion imaging with apparent diffusion coefficient (ADC) maps, gradient ECHO, MR angiography, and contrast-enhanced imaging. “These usually show multifocal lesions of different ages, and hemorrhages occur in about 10% of lesions,” Dr. Berlit said. “Leptomeningeal enhancement is an indicator of good treatment response.”
A brain and leptomeningeal biopsy demonstrating the angiitis remains the preferred method for diagnosis of PACNS. “Open biopsies out of recent MRI lesions are especially diagnostic,” he said. “If there are no lesions accessible for surgery in noneloquent brain areas, a biopsy from the right frontal lobe is recommended.” The histologic findings of PACNS consist of granulomatous inflammation, fibrinoid necrosis of vessel walls, or exclusively lymphocytic cellular infiltrates. “The treatment of choice in PACNS is the combination of steroids and cyclophosphamide pulse therapy,” he said. “There are also data showing that rituximab or methotrexate might be treatment options. With a relapse rate of 25% and a reduced survival rate, a close follow-up of suspected PACNS is mandatory.”
Reversible cerebral vasoconstriction syndrome (RCVS)
Another rare cause of stroke is RCVS, which typically presents as thunderclap headaches with or without neurologic symptoms. MRI may be normal, but symmetric border zone infarctions and small subarachnoid hemorrhages are possible. Catheter, CT, or MR angiography show segmental arterial vasoconstriction. “You always have to exclude cerebral aneurysm,” Dr. Berlit said. “There is reversibility of RCVS within 3 months.” RCVS is often associated with a long list of drugs, including phenylpropanolamine, Methergine (methylergonovine), bromocriptine, lisuride, SSRIs, triptans, isometheptene, tacrolimus, cyclophosphamide, erythropoietin, intravenous immunoglobulins, erythrocyte concentrates, nasal sprays, cocaine, ecstasy, amphetamines, cannabis, and LSD. “After stopping responsible medications, treatment involves a course of nimodipine,” he said.
Moyamoya disease (MMD)
Dr. Berlit closed his presentation by discussing MMD, a rare occlusive cerebrovascular disorder characterized by progressive stenosis or occlusion of the intracranial portion of the internal carotid artery and proximal cerebral arteries with an extensive network of fine collaterals. “This is an idiopathic vasculopathy with remarkable regional and racial differences worldwide; it’s most frequently found in Asians, especially in Japan and Korea,” he said. “In Europe, there is about one-tenth the incidence, compared with that of Japan. In Asian MMD, about 15% of cases follow an autosomal dominant inheritance. The collaterals in MMD present histologically as a thin media, a fragmented elastic laminae, and the formation of microaneurysms. There is no inflammation.”
MMD diagnostic criteria include stenosis or occlusion of the terminal portion of the internal carotid artery and at the proximal portion of the anterior and middle cerebral arteries. Abnormal vascular networks are present in the basal ganglia and angiographic findings present bilaterally. Cases with unilateral angiographic findings are considered probable. Clinicians should exclude the following conditions: arteriosclerosis, autoimmune disease, brain neoplasm, history of cranial irradiation, Down syndrome, head trauma, neurofibromatosis, and meningitis. “If the angiographic pattern is resembled by one of these conditions, this is called moyamoya syndrome,” Dr. Berlit noted. “MMD is a progressive disorder. Within a few months you can see occlusion of the middle cerebral artery and the anterior cerebral artery, so you have to treat these patients.”
In patients who are white, MMD presents with lower rates of hemorrhage, but in Asians, microbleeds occur in up to 44% of patients and hemorrhages in up to 65% patients. “Both subarachnoidal and intracerebral hemorrhages occur, especially in connection with pregnancy and delivery,” he said. “The risk of both cerebral ischemia and hemorrhagic complications increases with stages of MMD.”
Direct or indirect intracranial bypass surgery is recommended in stages 3 or more, and has been shown to significantly reduce the 5-year stroke risk. To date, Dr. Berlit and his associates have treated 86 hemispheres in 56 patients. The average age of the patients was 42 years, 70% were female, and the average follow-up was 39 months. All intracranial bypasses were open on follow-up, and a decrease of the typical moyamoya vessels was observed in 81% of patients.
Dr. Berlit reported having no financial disclosures.
ATLANTA – According to Peter Berlit, MD, clinicians should .
Other factors include combination of ischemic and hemorrhagic stroke, exclusive involvement of intracranial vessels, systemic signs, and lab tests indicating inflammation.
At the annual meeting of the American Neurological Association, Dr. Berlit, secretary general of the German Society of Neurology in Berlin, discussed the diagnosis and management of rare causes of stroke.
Giant cell arteritis (GCA)
One of the rare causes of stroke, GCA can be diagnosed when three of five criteria are met: being 50 years of age or older, having a newly developed headache, tenderness of the superficial temporal artery, elevated sedimentation rate of at least 50 mm per hour, and GCA in a biopsy specimen from the temporal artery.
“What we fear most is sudden blindness due to involvement of arteries serving the eyes, which appears in up to 30% of GCA patients,” said Dr. Berlit, who formerly chaired the department of neurology at Alfried Krupp Hospital, Essen, Germany. “Stroke occurs in approximately 2% of GCA patients, so it’s a lot rarer.” GCA can also be diagnosed by ultrasound. One meta-analysis of 23 studies using halo, stenosis, and occlusion as ultrasound criteria found a sensitivity of 87% and a specificity of 96% (Ann Intern Med. 2005;142[5]:359-69). “You can also use 3-Tesla MRI with the use of contrast agent, which shows inflammation of the temporal artery, but also other large vessels including the aortic arch,” he said. “The treatment of GCA has changed since the end of 2017 and involves starting with prednisolone 1 mg/kg body weight.” After a dose of 30 mg for 4 weeks, reduce the dose by 2.5 mg every 2 weeks. After reaching the dose of 15 mg daily, reduce by 1 mg per month. “The recommended steroid-sparing treatment is subcutaneous tocilizumab at a dose of 162 mg weekly or every other week, combined with a prednisone taper for a minimum of 26 weeks,” he said. Supportive therapies include pantoprazole 20 mg, aspirin 100 mg, calcium, vitamin D, and bisphosphonates.
Primary angiitis of the central nervous system (PACNS)
Next, Dr. Berlit discussed diagnostic criteria for PACNS, an acquired neurological deficit unexplained after complete evaluation. “You should have a diagnostic cerebral angiogram or biopsy demonstrating vasculitis,” he said. “There should be no evidence of systemic vasculitis or any other conditions that could mimic the angiogram findings. Usually you have abnormal CSF findings, including pleocytosis and protein elevation, and a biopsy demonstrating vasculitis.”
MRI studies in suspected vasculitis include fluid-attenuated inversion recovery (FLAIR), diffusion imaging with apparent diffusion coefficient (ADC) maps, gradient ECHO, MR angiography, and contrast-enhanced imaging. “These usually show multifocal lesions of different ages, and hemorrhages occur in about 10% of lesions,” Dr. Berlit said. “Leptomeningeal enhancement is an indicator of good treatment response.”
A brain and leptomeningeal biopsy demonstrating the angiitis remains the preferred method for diagnosis of PACNS. “Open biopsies out of recent MRI lesions are especially diagnostic,” he said. “If there are no lesions accessible for surgery in noneloquent brain areas, a biopsy from the right frontal lobe is recommended.” The histologic findings of PACNS consist of granulomatous inflammation, fibrinoid necrosis of vessel walls, or exclusively lymphocytic cellular infiltrates. “The treatment of choice in PACNS is the combination of steroids and cyclophosphamide pulse therapy,” he said. “There are also data showing that rituximab or methotrexate might be treatment options. With a relapse rate of 25% and a reduced survival rate, a close follow-up of suspected PACNS is mandatory.”
Reversible cerebral vasoconstriction syndrome (RCVS)
Another rare cause of stroke is RCVS, which typically presents as thunderclap headaches with or without neurologic symptoms. MRI may be normal, but symmetric border zone infarctions and small subarachnoid hemorrhages are possible. Catheter, CT, or MR angiography show segmental arterial vasoconstriction. “You always have to exclude cerebral aneurysm,” Dr. Berlit said. “There is reversibility of RCVS within 3 months.” RCVS is often associated with a long list of drugs, including phenylpropanolamine, Methergine (methylergonovine), bromocriptine, lisuride, SSRIs, triptans, isometheptene, tacrolimus, cyclophosphamide, erythropoietin, intravenous immunoglobulins, erythrocyte concentrates, nasal sprays, cocaine, ecstasy, amphetamines, cannabis, and LSD. “After stopping responsible medications, treatment involves a course of nimodipine,” he said.
Moyamoya disease (MMD)
Dr. Berlit closed his presentation by discussing MMD, a rare occlusive cerebrovascular disorder characterized by progressive stenosis or occlusion of the intracranial portion of the internal carotid artery and proximal cerebral arteries with an extensive network of fine collaterals. “This is an idiopathic vasculopathy with remarkable regional and racial differences worldwide; it’s most frequently found in Asians, especially in Japan and Korea,” he said. “In Europe, there is about one-tenth the incidence, compared with that of Japan. In Asian MMD, about 15% of cases follow an autosomal dominant inheritance. The collaterals in MMD present histologically as a thin media, a fragmented elastic laminae, and the formation of microaneurysms. There is no inflammation.”
MMD diagnostic criteria include stenosis or occlusion of the terminal portion of the internal carotid artery and at the proximal portion of the anterior and middle cerebral arteries. Abnormal vascular networks are present in the basal ganglia and angiographic findings present bilaterally. Cases with unilateral angiographic findings are considered probable. Clinicians should exclude the following conditions: arteriosclerosis, autoimmune disease, brain neoplasm, history of cranial irradiation, Down syndrome, head trauma, neurofibromatosis, and meningitis. “If the angiographic pattern is resembled by one of these conditions, this is called moyamoya syndrome,” Dr. Berlit noted. “MMD is a progressive disorder. Within a few months you can see occlusion of the middle cerebral artery and the anterior cerebral artery, so you have to treat these patients.”
In patients who are white, MMD presents with lower rates of hemorrhage, but in Asians, microbleeds occur in up to 44% of patients and hemorrhages in up to 65% patients. “Both subarachnoidal and intracerebral hemorrhages occur, especially in connection with pregnancy and delivery,” he said. “The risk of both cerebral ischemia and hemorrhagic complications increases with stages of MMD.”
Direct or indirect intracranial bypass surgery is recommended in stages 3 or more, and has been shown to significantly reduce the 5-year stroke risk. To date, Dr. Berlit and his associates have treated 86 hemispheres in 56 patients. The average age of the patients was 42 years, 70% were female, and the average follow-up was 39 months. All intracranial bypasses were open on follow-up, and a decrease of the typical moyamoya vessels was observed in 81% of patients.
Dr. Berlit reported having no financial disclosures.
EXPERT ANALYSIS FROM ANA 2018
Ganglion stimulation boosts cerebral blood flow, improves stroke outcomes
MONTREAL – Stimulation of the sphenopalatine ganglion (SPG) using a small, implanted electrode for 5 days in patients who had just had an acute ischemic stroke led to statistically significant and clinically meaningful improvements in the subset of patients with confirmed cortical involvement in a pivotal, sham-controlled trial.
SPG stimulation started within 24 hours of an acute ischemic stroke “reduced poststroke disability over the entire outcome range and increased the proportion of patients who were alive and independent 3 months after their stroke” in the subgroup with a confirmed cortical infarction (CCI), Jeffrey L. Saver, MD, said at the World Stroke Congress. Five days of SPG stimulation, done once daily starting within 24 hours of stroke onset, “enhances ipsilateral collateral blood flow” and may also stabilize the blood brain barrier, explained Dr. Saver, professor of neurology and director of the stroke center at the University of California, Los Angeles. The study included a prespecified primary endpoint analysis that focused exclusively on the CCI subgroup, 52% of the total enrolled population.
If the reported data result in Food and Drug Administration marketing approval for the system, Dr. Saver said that he anticipated “substantial uptake” of the strategy, which he tested in patients who had not undergone thrombectomy or thrombolysis treatment. In current U.S. practice, there is “a large group of patients with a missed opportunity for recanalization” who would be candidates for treatment with SPG stimulation, a treatment that appeared to provide benefits beyond current standard care, he said in an interview.
Ongoing studies are also testing whether SPG stimulation can benefit acute ischemic stroke patients who have already undergone treatment with thrombectomy or thrombolysis, he added. The same SPG stimulation device is additionally undergoing U.S. testing as a treatment for headache and has regulatory approval in the European Union for treating headache and migraine.
The ImpACT-24B (Implant for Augmentation of Cerebral Blood Flow Trial, Effectiveness and Safety in a 24-Hour Window) trial involved 1,000 patients at 73 centers in 18 countries, including the United States. The investigators enrolled acute ischemic stroke patients 8-24 hours after stroke onset who had a National Institutes of Health Stroke Scale (NIHSS) score of 7-18.
Each patient received an implant of a short, thin metal electrode placed through the soft palate at the rear roof of the mouth, near the SPG. Neurologists primarily performed the implants in a procedure that had a “skin to skin” time of less than 5 minutes. Patients received either electrical stimulation or a sham stimulation through the electrode immediately after placement and then daily for the next 4 days. The investigators titrated the strength of the treatment stimulation in each patient to maximize its strength while maintaining patient comfort. Subsequent analysis of the results showed that the stronger the tolerated stimulation, the bigger the treatment effect in a clear dose-response pattern, Dr. Saver reported.
The study’s primary endpoint was improvement in the modified Rankin scale (mRS) score at 90 days after the index stroke when measured against historical expectations. By this measure, the overall study cohort showed a small, statistically insignificant improvement in actively treated patients, compared with sham-treated patients. However, in the prespecified, coprimary endpoint cohort of patients with a CCI, active treatment resulted in 50% of patients having a better-than-expected 90-day outcome, compared with 40% of controls, a 48% relative improvement in this measure that met the prespecified definition of statistical significance. The results also showed about a 50% relative improvement in each of three secondary outcomes in the CCI cohort: the percentage of patients with a mRS score of 0-2 after 90 days, the percentage with a mRS score of 0-3 after 90 days, and average stroke-related quality of life at 90 days.
Dr. Saver also reported results of a meta-analysis that combined the results he reported from 520 patients with CCI with results from 87 CCI patients enrolled in the preceding pilot study of this treatment strategy, ImpACT-1. The pilot findings were completely consistent and when combined with the current results strengthened the statistical significance of the primary and secondary endpoints.
“There is a compelling story” of efficacy based on the study results, the dose-response relationship, and the meta-analysis results, Dr. Saver said. “I think it’s a very strong case.”
He also reported “no safety concerns” raised in the new study, with no serious adverse effects seen in or experienced by the patients on active treatment.
“The data are compelling” for safety and efficacy, for this novel approach for treating acute ischemic stroke, commented Pooja Khatri, MD, professor of neurology and director of the acute stroke program at the University of Cincinnati.
The study was sponsored by BrainsGate, the company developing the tested device. Dr. Saver has been a consultant to BrainsGate. Dr. Khatri has been a consultant to Biogen, Greenwich, Lumosa, and PTC Therapeutics.
SOURCE: Saver J et al. Int J. Stroke. 2018 Oct;13(2S):28, Abstract 104.
MONTREAL – Stimulation of the sphenopalatine ganglion (SPG) using a small, implanted electrode for 5 days in patients who had just had an acute ischemic stroke led to statistically significant and clinically meaningful improvements in the subset of patients with confirmed cortical involvement in a pivotal, sham-controlled trial.
SPG stimulation started within 24 hours of an acute ischemic stroke “reduced poststroke disability over the entire outcome range and increased the proportion of patients who were alive and independent 3 months after their stroke” in the subgroup with a confirmed cortical infarction (CCI), Jeffrey L. Saver, MD, said at the World Stroke Congress. Five days of SPG stimulation, done once daily starting within 24 hours of stroke onset, “enhances ipsilateral collateral blood flow” and may also stabilize the blood brain barrier, explained Dr. Saver, professor of neurology and director of the stroke center at the University of California, Los Angeles. The study included a prespecified primary endpoint analysis that focused exclusively on the CCI subgroup, 52% of the total enrolled population.
If the reported data result in Food and Drug Administration marketing approval for the system, Dr. Saver said that he anticipated “substantial uptake” of the strategy, which he tested in patients who had not undergone thrombectomy or thrombolysis treatment. In current U.S. practice, there is “a large group of patients with a missed opportunity for recanalization” who would be candidates for treatment with SPG stimulation, a treatment that appeared to provide benefits beyond current standard care, he said in an interview.
Ongoing studies are also testing whether SPG stimulation can benefit acute ischemic stroke patients who have already undergone treatment with thrombectomy or thrombolysis, he added. The same SPG stimulation device is additionally undergoing U.S. testing as a treatment for headache and has regulatory approval in the European Union for treating headache and migraine.
The ImpACT-24B (Implant for Augmentation of Cerebral Blood Flow Trial, Effectiveness and Safety in a 24-Hour Window) trial involved 1,000 patients at 73 centers in 18 countries, including the United States. The investigators enrolled acute ischemic stroke patients 8-24 hours after stroke onset who had a National Institutes of Health Stroke Scale (NIHSS) score of 7-18.
Each patient received an implant of a short, thin metal electrode placed through the soft palate at the rear roof of the mouth, near the SPG. Neurologists primarily performed the implants in a procedure that had a “skin to skin” time of less than 5 minutes. Patients received either electrical stimulation or a sham stimulation through the electrode immediately after placement and then daily for the next 4 days. The investigators titrated the strength of the treatment stimulation in each patient to maximize its strength while maintaining patient comfort. Subsequent analysis of the results showed that the stronger the tolerated stimulation, the bigger the treatment effect in a clear dose-response pattern, Dr. Saver reported.
The study’s primary endpoint was improvement in the modified Rankin scale (mRS) score at 90 days after the index stroke when measured against historical expectations. By this measure, the overall study cohort showed a small, statistically insignificant improvement in actively treated patients, compared with sham-treated patients. However, in the prespecified, coprimary endpoint cohort of patients with a CCI, active treatment resulted in 50% of patients having a better-than-expected 90-day outcome, compared with 40% of controls, a 48% relative improvement in this measure that met the prespecified definition of statistical significance. The results also showed about a 50% relative improvement in each of three secondary outcomes in the CCI cohort: the percentage of patients with a mRS score of 0-2 after 90 days, the percentage with a mRS score of 0-3 after 90 days, and average stroke-related quality of life at 90 days.
Dr. Saver also reported results of a meta-analysis that combined the results he reported from 520 patients with CCI with results from 87 CCI patients enrolled in the preceding pilot study of this treatment strategy, ImpACT-1. The pilot findings were completely consistent and when combined with the current results strengthened the statistical significance of the primary and secondary endpoints.
“There is a compelling story” of efficacy based on the study results, the dose-response relationship, and the meta-analysis results, Dr. Saver said. “I think it’s a very strong case.”
He also reported “no safety concerns” raised in the new study, with no serious adverse effects seen in or experienced by the patients on active treatment.
“The data are compelling” for safety and efficacy, for this novel approach for treating acute ischemic stroke, commented Pooja Khatri, MD, professor of neurology and director of the acute stroke program at the University of Cincinnati.
The study was sponsored by BrainsGate, the company developing the tested device. Dr. Saver has been a consultant to BrainsGate. Dr. Khatri has been a consultant to Biogen, Greenwich, Lumosa, and PTC Therapeutics.
SOURCE: Saver J et al. Int J. Stroke. 2018 Oct;13(2S):28, Abstract 104.
MONTREAL – Stimulation of the sphenopalatine ganglion (SPG) using a small, implanted electrode for 5 days in patients who had just had an acute ischemic stroke led to statistically significant and clinically meaningful improvements in the subset of patients with confirmed cortical involvement in a pivotal, sham-controlled trial.
SPG stimulation started within 24 hours of an acute ischemic stroke “reduced poststroke disability over the entire outcome range and increased the proportion of patients who were alive and independent 3 months after their stroke” in the subgroup with a confirmed cortical infarction (CCI), Jeffrey L. Saver, MD, said at the World Stroke Congress. Five days of SPG stimulation, done once daily starting within 24 hours of stroke onset, “enhances ipsilateral collateral blood flow” and may also stabilize the blood brain barrier, explained Dr. Saver, professor of neurology and director of the stroke center at the University of California, Los Angeles. The study included a prespecified primary endpoint analysis that focused exclusively on the CCI subgroup, 52% of the total enrolled population.
If the reported data result in Food and Drug Administration marketing approval for the system, Dr. Saver said that he anticipated “substantial uptake” of the strategy, which he tested in patients who had not undergone thrombectomy or thrombolysis treatment. In current U.S. practice, there is “a large group of patients with a missed opportunity for recanalization” who would be candidates for treatment with SPG stimulation, a treatment that appeared to provide benefits beyond current standard care, he said in an interview.
Ongoing studies are also testing whether SPG stimulation can benefit acute ischemic stroke patients who have already undergone treatment with thrombectomy or thrombolysis, he added. The same SPG stimulation device is additionally undergoing U.S. testing as a treatment for headache and has regulatory approval in the European Union for treating headache and migraine.
The ImpACT-24B (Implant for Augmentation of Cerebral Blood Flow Trial, Effectiveness and Safety in a 24-Hour Window) trial involved 1,000 patients at 73 centers in 18 countries, including the United States. The investigators enrolled acute ischemic stroke patients 8-24 hours after stroke onset who had a National Institutes of Health Stroke Scale (NIHSS) score of 7-18.
Each patient received an implant of a short, thin metal electrode placed through the soft palate at the rear roof of the mouth, near the SPG. Neurologists primarily performed the implants in a procedure that had a “skin to skin” time of less than 5 minutes. Patients received either electrical stimulation or a sham stimulation through the electrode immediately after placement and then daily for the next 4 days. The investigators titrated the strength of the treatment stimulation in each patient to maximize its strength while maintaining patient comfort. Subsequent analysis of the results showed that the stronger the tolerated stimulation, the bigger the treatment effect in a clear dose-response pattern, Dr. Saver reported.
The study’s primary endpoint was improvement in the modified Rankin scale (mRS) score at 90 days after the index stroke when measured against historical expectations. By this measure, the overall study cohort showed a small, statistically insignificant improvement in actively treated patients, compared with sham-treated patients. However, in the prespecified, coprimary endpoint cohort of patients with a CCI, active treatment resulted in 50% of patients having a better-than-expected 90-day outcome, compared with 40% of controls, a 48% relative improvement in this measure that met the prespecified definition of statistical significance. The results also showed about a 50% relative improvement in each of three secondary outcomes in the CCI cohort: the percentage of patients with a mRS score of 0-2 after 90 days, the percentage with a mRS score of 0-3 after 90 days, and average stroke-related quality of life at 90 days.
Dr. Saver also reported results of a meta-analysis that combined the results he reported from 520 patients with CCI with results from 87 CCI patients enrolled in the preceding pilot study of this treatment strategy, ImpACT-1. The pilot findings were completely consistent and when combined with the current results strengthened the statistical significance of the primary and secondary endpoints.
“There is a compelling story” of efficacy based on the study results, the dose-response relationship, and the meta-analysis results, Dr. Saver said. “I think it’s a very strong case.”
He also reported “no safety concerns” raised in the new study, with no serious adverse effects seen in or experienced by the patients on active treatment.
“The data are compelling” for safety and efficacy, for this novel approach for treating acute ischemic stroke, commented Pooja Khatri, MD, professor of neurology and director of the acute stroke program at the University of Cincinnati.
The study was sponsored by BrainsGate, the company developing the tested device. Dr. Saver has been a consultant to BrainsGate. Dr. Khatri has been a consultant to Biogen, Greenwich, Lumosa, and PTC Therapeutics.
SOURCE: Saver J et al. Int J. Stroke. 2018 Oct;13(2S):28, Abstract 104.
REPORTING FROM THE WORLD STROKE CONGRESS
Key clinical point: Sphenopalatine ganglion stimulation of acute ischemic stroke patients boosted cerebral blood flow and improved 90-day outcomes in patients with confirmed cortical infarctions.
Major finding: For confirmed cortical infarctions ganglion stimulation led to a 48% higher rate of better-than-expected outcomes, compared with controls.
Study details: ImpACT-24B, a multicenter pivotal trial with 1,000 acute ischemic stroke patients.
Disclosures: The study was sponsored by BrainsGate, the company developing the tested device. Dr. Saver has been a consultant to BrainsGate. Dr. Khatri has been a consultant to Biogen, Greenwich, Lumosa, and PTC Therapeutics.
Source: Saver J et al. Int J. Stroke. 2018 Oct;13(2S):28, Abstract 104.
Apixaban is safest effective DOAC for stroke prevention in Afib, per AHRQ report
, according to results of an updated comparative effectiveness review.
Dabigatran (Pradaxa), by contrast, has shown reductions in stroke events but a similar rate of bleeding events compared to warfarin, according to the report from the Duke Evidence-based Practice Center, Durham, N.C.
Rivaroxaban (Xarelto), meanwhile, is “similar in both benefits and harms with warfarin” in evidence to date, investigators wrote in the report, which was prepared for the Agency for Healthcare Research and Quality (AHRQ) and the Patient-Centered Outcomes Research Institute (PCORI).
Finally, edoxaban (Savaysa) is “most likely similar” to warfarin with respect to preventing stroke or systemic embolism, with less risk for major bleeding and hemorrhagic stroke, investigators wrote in a summary of their findings on the AHRQ website.
“Effectiveness of these direct oral anticoagulants as compared to one another however is limited by the lack of randomized studies directly comparing their safety and effectiveness,” concluded investigators, led by Gillian D. Sanders, PhD, of Duke University.
The 612-page report details a systematic review based on 320 articles representing 185 unique studies. The review was designed to update a 2013 AHRQ report that evaluated evidence not only for treatment options to prevent stroke in patients with atrial fibrillation, but also for tools used to predict risk of stroke or bleeding.
In the 2013 report, investigators concluded that the newer anticoagulants showed “early promise” in reducing stroke and bleeding events compared with warfarin.
That earlier report said that CHA2 and CHA2DS2-VASc had the best evidence to support prediction of stroke events, while HAS-BLED provided the best discrimination of bleeding risk.
The updated report adds the ABC stroke risk score as a tool that, along with CHADS2 and CHA2DS2-VASc, has the “best evidence” predicting thromboembolic risk, authors said.
Imaging tools, on the other hand, still need more evidence supporting their use to predict thromboembolic risk, Dr. Sanders and colleagues said in their report.
The literature review, which covered the January 2000 through February 2018, turned up 61 studies relevant to predicting thromboembolic risk, 38 on bleeding risk, and 117 on preventing thromboembolic events with anticoagulation therapies, antiplatelet therapies, or procedures.
Direct oral anticoagulants were evaluated in randomized clinical trials that were “often very large, of good quality, and considered definitive in the field,” Dr. Sanders and colleagues wrote in their report.
However, these trials were constrained to comparing direct oral anticoagulants with warfarin or aspirin, and have not involved head-to-head comparison among the newer agents, they added.
“Based on these trials though, clinical leaders and professional societies have determined that these newer agents are better than the prior lone treatment of warfarin in terms of stroke prevention, side effects, and risk of bleeding,” they said in the published report.
SOURCE: Sanders GD, et al. 2018 Oct 30. AHRQ Publication No. 18(19)-EHC018-EF.
, according to results of an updated comparative effectiveness review.
Dabigatran (Pradaxa), by contrast, has shown reductions in stroke events but a similar rate of bleeding events compared to warfarin, according to the report from the Duke Evidence-based Practice Center, Durham, N.C.
Rivaroxaban (Xarelto), meanwhile, is “similar in both benefits and harms with warfarin” in evidence to date, investigators wrote in the report, which was prepared for the Agency for Healthcare Research and Quality (AHRQ) and the Patient-Centered Outcomes Research Institute (PCORI).
Finally, edoxaban (Savaysa) is “most likely similar” to warfarin with respect to preventing stroke or systemic embolism, with less risk for major bleeding and hemorrhagic stroke, investigators wrote in a summary of their findings on the AHRQ website.
“Effectiveness of these direct oral anticoagulants as compared to one another however is limited by the lack of randomized studies directly comparing their safety and effectiveness,” concluded investigators, led by Gillian D. Sanders, PhD, of Duke University.
The 612-page report details a systematic review based on 320 articles representing 185 unique studies. The review was designed to update a 2013 AHRQ report that evaluated evidence not only for treatment options to prevent stroke in patients with atrial fibrillation, but also for tools used to predict risk of stroke or bleeding.
In the 2013 report, investigators concluded that the newer anticoagulants showed “early promise” in reducing stroke and bleeding events compared with warfarin.
That earlier report said that CHA2 and CHA2DS2-VASc had the best evidence to support prediction of stroke events, while HAS-BLED provided the best discrimination of bleeding risk.
The updated report adds the ABC stroke risk score as a tool that, along with CHADS2 and CHA2DS2-VASc, has the “best evidence” predicting thromboembolic risk, authors said.
Imaging tools, on the other hand, still need more evidence supporting their use to predict thromboembolic risk, Dr. Sanders and colleagues said in their report.
The literature review, which covered the January 2000 through February 2018, turned up 61 studies relevant to predicting thromboembolic risk, 38 on bleeding risk, and 117 on preventing thromboembolic events with anticoagulation therapies, antiplatelet therapies, or procedures.
Direct oral anticoagulants were evaluated in randomized clinical trials that were “often very large, of good quality, and considered definitive in the field,” Dr. Sanders and colleagues wrote in their report.
However, these trials were constrained to comparing direct oral anticoagulants with warfarin or aspirin, and have not involved head-to-head comparison among the newer agents, they added.
“Based on these trials though, clinical leaders and professional societies have determined that these newer agents are better than the prior lone treatment of warfarin in terms of stroke prevention, side effects, and risk of bleeding,” they said in the published report.
SOURCE: Sanders GD, et al. 2018 Oct 30. AHRQ Publication No. 18(19)-EHC018-EF.
, according to results of an updated comparative effectiveness review.
Dabigatran (Pradaxa), by contrast, has shown reductions in stroke events but a similar rate of bleeding events compared to warfarin, according to the report from the Duke Evidence-based Practice Center, Durham, N.C.
Rivaroxaban (Xarelto), meanwhile, is “similar in both benefits and harms with warfarin” in evidence to date, investigators wrote in the report, which was prepared for the Agency for Healthcare Research and Quality (AHRQ) and the Patient-Centered Outcomes Research Institute (PCORI).
Finally, edoxaban (Savaysa) is “most likely similar” to warfarin with respect to preventing stroke or systemic embolism, with less risk for major bleeding and hemorrhagic stroke, investigators wrote in a summary of their findings on the AHRQ website.
“Effectiveness of these direct oral anticoagulants as compared to one another however is limited by the lack of randomized studies directly comparing their safety and effectiveness,” concluded investigators, led by Gillian D. Sanders, PhD, of Duke University.
The 612-page report details a systematic review based on 320 articles representing 185 unique studies. The review was designed to update a 2013 AHRQ report that evaluated evidence not only for treatment options to prevent stroke in patients with atrial fibrillation, but also for tools used to predict risk of stroke or bleeding.
In the 2013 report, investigators concluded that the newer anticoagulants showed “early promise” in reducing stroke and bleeding events compared with warfarin.
That earlier report said that CHA2 and CHA2DS2-VASc had the best evidence to support prediction of stroke events, while HAS-BLED provided the best discrimination of bleeding risk.
The updated report adds the ABC stroke risk score as a tool that, along with CHADS2 and CHA2DS2-VASc, has the “best evidence” predicting thromboembolic risk, authors said.
Imaging tools, on the other hand, still need more evidence supporting their use to predict thromboembolic risk, Dr. Sanders and colleagues said in their report.
The literature review, which covered the January 2000 through February 2018, turned up 61 studies relevant to predicting thromboembolic risk, 38 on bleeding risk, and 117 on preventing thromboembolic events with anticoagulation therapies, antiplatelet therapies, or procedures.
Direct oral anticoagulants were evaluated in randomized clinical trials that were “often very large, of good quality, and considered definitive in the field,” Dr. Sanders and colleagues wrote in their report.
However, these trials were constrained to comparing direct oral anticoagulants with warfarin or aspirin, and have not involved head-to-head comparison among the newer agents, they added.
“Based on these trials though, clinical leaders and professional societies have determined that these newer agents are better than the prior lone treatment of warfarin in terms of stroke prevention, side effects, and risk of bleeding,” they said in the published report.
SOURCE: Sanders GD, et al. 2018 Oct 30. AHRQ Publication No. 18(19)-EHC018-EF.
Migraine Elevates the Risk of Perioperative Stroke
Migraineurs are more likely to have an ischemic stroke in the 30 days after surgery, compared with patients without a history of migraine.
ASHEVILLE, NC—The 30 days after surgery are a period of exceptionally high risk for ischemic stroke, and the risk is greater for patients with migraine, compared with patients without migraine, according to a lecture at the Eighth Annual Scienti
“When we send individuals with migraine to surgery who do not have classical risk factors [for stroke], they may, in fact, still be at risk for stroke during the perioperative period,” said Timothy T. Houle, PhD, Associate Professor of Anesthesia at Massachusetts General Hospital and Harvard Medical School in Boston.
Dr. Houle described a hospital-based registry study that he and his colleagues published in BMJ. They found that the rate of ischemic stroke within 30 days of surgery was about 240 strokes per 100,000 patients without migraine, whereas among migraineurs, the rate was 430 strokes per 100,000 patients. Among patients with migraine without aura, the rate was 390 strokes per 100,000 patients, and among patients with migraine with aura, the rate was 630 strokes per 100,000 patients. “If you have migraine with aura, your risk of having a stroke is appreciably elevated and not trivial,” Dr. Houle said. “This is something to take seriously.”
The Migraine–Stroke Connection
Researchers consistently have found that migraine is associated with an increased risk of ischemic stroke in the general population, but the relationship has been challenging to study.
Possible mechanisms underlying the relationship between migraine and stroke include comorbidities (eg, higher BMI and increased cardiovascular risk factors) and medication use. In addition, research suggests that cortical spreading depression might make migraineurs’ brains more susceptible to stroke, Dr. Houle said. Patent foramen ovale, arterial dissection, coagulation dysfunction, endothelial dysfunction, or a genotype that increases the risk of migraine and stroke are other potential pathways.
Spector et al conducted a meta-analysis of 21 studies and concluded that migraine appears to be independently associated with a twofold increased risk of ischemic stroke. A meta-analysis by Schürks et al found that the relative risk of stroke was about 1.73 for patients with migraine and 2.16 for patients with migraine with aura. Migraine without aura was associated with a relative risk of 1.23, but this result was not statistically significant.
Dr. Houle and colleagues hypothesized that focusing on the perioperative period, when stroke is more prevalent, “could yield unique insights into the migraine–stroke connection,” he said.
Ischemic stroke in the perioperative period occurs at a rate of about 100 strokes per 100,000 individuals for the lowest-risk surgeries. After major cardiac and vascular surgery, the risk may be between 600 and 7,400 strokes per 100,000 individuals. “We have … a risk period that is intensely elevated for patients about to receive surgical insult,” Dr. Houle said. The increased risk may result from the indication for the surgery, as well as factors related to surgery itself, such as surgical stress, inflammatory responses, and intraoperative hypotension.
A Retrospective Cohort Study
Based on the increased risk of stroke in the general population, the investigators hypothesized that individuals with migraine also would have an elevated risk of stroke in the 30 days after surgery. They analyzed data from 124,558 patients (mean age, 52.6; 54.5% women) who underwent surgery between 2007 and 2014 at Massachusetts General Hospital and two satellite campuses. They included patients who had surgery under general anesthesia with mechanical ventilation and were successfully extubated. They used ICD-9 codes to identify patients with migraine. The primary outcome was ischemic stroke within 30 days. They identified strokes using ICD-9 codes and confirmed strokes by reviewing brain scans and medical records.
The investigators adjusted for confounders, including sex, age, BMI, emergent versus nonemergent surgery, prescriptions of antiplatelet drugs or beta blockers within four weeks before surgery, minutes of intraoperative hypotension, diabetes, hypertension, atrial fibrillation, Charleston Comorbidity Index, and work relative value units (ie, a surrogate for surgical complexity).
The cohort included 10,179 individuals with migraine (8.2%). Of the patients with migraine, 12.6% had migraine with aura.
Patients with migraine generally were younger, had higher BMI, and were more likely to be women. They were less likely to have diabetes or hypertension and to be taking antiplatelet drugs or beta blockers. Patients with migraine “were a little healthier” than the patients without migraine, Dr. Houle said.
In all, 771 patients had perioperative stroke, of whom 89 (11.5%) had migraine. About 0.6% of patients without migraine had perioperative stroke versus 0.9% of patients with migraine. The unadjusted odds ratio for stroke among migraineurs was 1.47, and the adjusted odds ratio was 1.75. “Individuals in this sample who had any migraine were at greater risk for stroke during the period after surgery, just like in the regular population,” said Dr. Houle. Although migraine without aura was not a statistically significant risk factor for stroke in the general population, it was after surgery.
Prediction Models
In one sensitivity analysis, the researchers determined each patient’s stroke risk based on known risk factors excluding migraine, such as age and cardiovascular disorders, and grouped patients by low, intermediate, and high levels of risk. Among patients in the low-risk group, the relative risk of stroke for patients with migraine versus patients without migraine was 3.5-fold higher. “These are people you would not have identified as having risk,” said Dr. Houle.
Future studies should try to identify the mechanisms involved in this relationship and assess interventions to mitigate the risk of stroke in patients with migraine who undergo surgery, Dr. Houle said.
Dr. Houle and colleagues have created a stroke prediction model that includes migraine and will “give surgeons a risk model to predict the risk of stroke for their patients,” he said. The model will “realize the risks that we uncovered in this study.”
—Jake Remaly
Suggested Reading
Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914.
Spector JT, Kahn SR, Jones MR, et al. Migraine headache and ischemic stroke risk: an updated meta-analysis. Am J Med. 2010;123(7):612-624.
Timm FP, Houle TT, Grabitz SD, et al. Migraine and risk of perioperative ischemic stroke and hospital readmission: hospital based registry study. BMJ. 2017;356:i6635.
Migraineurs are more likely to have an ischemic stroke in the 30 days after surgery, compared with patients without a history of migraine.
Migraineurs are more likely to have an ischemic stroke in the 30 days after surgery, compared with patients without a history of migraine.
ASHEVILLE, NC—The 30 days after surgery are a period of exceptionally high risk for ischemic stroke, and the risk is greater for patients with migraine, compared with patients without migraine, according to a lecture at the Eighth Annual Scienti
“When we send individuals with migraine to surgery who do not have classical risk factors [for stroke], they may, in fact, still be at risk for stroke during the perioperative period,” said Timothy T. Houle, PhD, Associate Professor of Anesthesia at Massachusetts General Hospital and Harvard Medical School in Boston.
Dr. Houle described a hospital-based registry study that he and his colleagues published in BMJ. They found that the rate of ischemic stroke within 30 days of surgery was about 240 strokes per 100,000 patients without migraine, whereas among migraineurs, the rate was 430 strokes per 100,000 patients. Among patients with migraine without aura, the rate was 390 strokes per 100,000 patients, and among patients with migraine with aura, the rate was 630 strokes per 100,000 patients. “If you have migraine with aura, your risk of having a stroke is appreciably elevated and not trivial,” Dr. Houle said. “This is something to take seriously.”
The Migraine–Stroke Connection
Researchers consistently have found that migraine is associated with an increased risk of ischemic stroke in the general population, but the relationship has been challenging to study.
Possible mechanisms underlying the relationship between migraine and stroke include comorbidities (eg, higher BMI and increased cardiovascular risk factors) and medication use. In addition, research suggests that cortical spreading depression might make migraineurs’ brains more susceptible to stroke, Dr. Houle said. Patent foramen ovale, arterial dissection, coagulation dysfunction, endothelial dysfunction, or a genotype that increases the risk of migraine and stroke are other potential pathways.
Spector et al conducted a meta-analysis of 21 studies and concluded that migraine appears to be independently associated with a twofold increased risk of ischemic stroke. A meta-analysis by Schürks et al found that the relative risk of stroke was about 1.73 for patients with migraine and 2.16 for patients with migraine with aura. Migraine without aura was associated with a relative risk of 1.23, but this result was not statistically significant.
Dr. Houle and colleagues hypothesized that focusing on the perioperative period, when stroke is more prevalent, “could yield unique insights into the migraine–stroke connection,” he said.
Ischemic stroke in the perioperative period occurs at a rate of about 100 strokes per 100,000 individuals for the lowest-risk surgeries. After major cardiac and vascular surgery, the risk may be between 600 and 7,400 strokes per 100,000 individuals. “We have … a risk period that is intensely elevated for patients about to receive surgical insult,” Dr. Houle said. The increased risk may result from the indication for the surgery, as well as factors related to surgery itself, such as surgical stress, inflammatory responses, and intraoperative hypotension.
A Retrospective Cohort Study
Based on the increased risk of stroke in the general population, the investigators hypothesized that individuals with migraine also would have an elevated risk of stroke in the 30 days after surgery. They analyzed data from 124,558 patients (mean age, 52.6; 54.5% women) who underwent surgery between 2007 and 2014 at Massachusetts General Hospital and two satellite campuses. They included patients who had surgery under general anesthesia with mechanical ventilation and were successfully extubated. They used ICD-9 codes to identify patients with migraine. The primary outcome was ischemic stroke within 30 days. They identified strokes using ICD-9 codes and confirmed strokes by reviewing brain scans and medical records.
The investigators adjusted for confounders, including sex, age, BMI, emergent versus nonemergent surgery, prescriptions of antiplatelet drugs or beta blockers within four weeks before surgery, minutes of intraoperative hypotension, diabetes, hypertension, atrial fibrillation, Charleston Comorbidity Index, and work relative value units (ie, a surrogate for surgical complexity).
The cohort included 10,179 individuals with migraine (8.2%). Of the patients with migraine, 12.6% had migraine with aura.
Patients with migraine generally were younger, had higher BMI, and were more likely to be women. They were less likely to have diabetes or hypertension and to be taking antiplatelet drugs or beta blockers. Patients with migraine “were a little healthier” than the patients without migraine, Dr. Houle said.
In all, 771 patients had perioperative stroke, of whom 89 (11.5%) had migraine. About 0.6% of patients without migraine had perioperative stroke versus 0.9% of patients with migraine. The unadjusted odds ratio for stroke among migraineurs was 1.47, and the adjusted odds ratio was 1.75. “Individuals in this sample who had any migraine were at greater risk for stroke during the period after surgery, just like in the regular population,” said Dr. Houle. Although migraine without aura was not a statistically significant risk factor for stroke in the general population, it was after surgery.
Prediction Models
In one sensitivity analysis, the researchers determined each patient’s stroke risk based on known risk factors excluding migraine, such as age and cardiovascular disorders, and grouped patients by low, intermediate, and high levels of risk. Among patients in the low-risk group, the relative risk of stroke for patients with migraine versus patients without migraine was 3.5-fold higher. “These are people you would not have identified as having risk,” said Dr. Houle.
Future studies should try to identify the mechanisms involved in this relationship and assess interventions to mitigate the risk of stroke in patients with migraine who undergo surgery, Dr. Houle said.
Dr. Houle and colleagues have created a stroke prediction model that includes migraine and will “give surgeons a risk model to predict the risk of stroke for their patients,” he said. The model will “realize the risks that we uncovered in this study.”
—Jake Remaly
Suggested Reading
Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914.
Spector JT, Kahn SR, Jones MR, et al. Migraine headache and ischemic stroke risk: an updated meta-analysis. Am J Med. 2010;123(7):612-624.
Timm FP, Houle TT, Grabitz SD, et al. Migraine and risk of perioperative ischemic stroke and hospital readmission: hospital based registry study. BMJ. 2017;356:i6635.
ASHEVILLE, NC—The 30 days after surgery are a period of exceptionally high risk for ischemic stroke, and the risk is greater for patients with migraine, compared with patients without migraine, according to a lecture at the Eighth Annual Scienti
“When we send individuals with migraine to surgery who do not have classical risk factors [for stroke], they may, in fact, still be at risk for stroke during the perioperative period,” said Timothy T. Houle, PhD, Associate Professor of Anesthesia at Massachusetts General Hospital and Harvard Medical School in Boston.
Dr. Houle described a hospital-based registry study that he and his colleagues published in BMJ. They found that the rate of ischemic stroke within 30 days of surgery was about 240 strokes per 100,000 patients without migraine, whereas among migraineurs, the rate was 430 strokes per 100,000 patients. Among patients with migraine without aura, the rate was 390 strokes per 100,000 patients, and among patients with migraine with aura, the rate was 630 strokes per 100,000 patients. “If you have migraine with aura, your risk of having a stroke is appreciably elevated and not trivial,” Dr. Houle said. “This is something to take seriously.”
The Migraine–Stroke Connection
Researchers consistently have found that migraine is associated with an increased risk of ischemic stroke in the general population, but the relationship has been challenging to study.
Possible mechanisms underlying the relationship between migraine and stroke include comorbidities (eg, higher BMI and increased cardiovascular risk factors) and medication use. In addition, research suggests that cortical spreading depression might make migraineurs’ brains more susceptible to stroke, Dr. Houle said. Patent foramen ovale, arterial dissection, coagulation dysfunction, endothelial dysfunction, or a genotype that increases the risk of migraine and stroke are other potential pathways.
Spector et al conducted a meta-analysis of 21 studies and concluded that migraine appears to be independently associated with a twofold increased risk of ischemic stroke. A meta-analysis by Schürks et al found that the relative risk of stroke was about 1.73 for patients with migraine and 2.16 for patients with migraine with aura. Migraine without aura was associated with a relative risk of 1.23, but this result was not statistically significant.
Dr. Houle and colleagues hypothesized that focusing on the perioperative period, when stroke is more prevalent, “could yield unique insights into the migraine–stroke connection,” he said.
Ischemic stroke in the perioperative period occurs at a rate of about 100 strokes per 100,000 individuals for the lowest-risk surgeries. After major cardiac and vascular surgery, the risk may be between 600 and 7,400 strokes per 100,000 individuals. “We have … a risk period that is intensely elevated for patients about to receive surgical insult,” Dr. Houle said. The increased risk may result from the indication for the surgery, as well as factors related to surgery itself, such as surgical stress, inflammatory responses, and intraoperative hypotension.
A Retrospective Cohort Study
Based on the increased risk of stroke in the general population, the investigators hypothesized that individuals with migraine also would have an elevated risk of stroke in the 30 days after surgery. They analyzed data from 124,558 patients (mean age, 52.6; 54.5% women) who underwent surgery between 2007 and 2014 at Massachusetts General Hospital and two satellite campuses. They included patients who had surgery under general anesthesia with mechanical ventilation and were successfully extubated. They used ICD-9 codes to identify patients with migraine. The primary outcome was ischemic stroke within 30 days. They identified strokes using ICD-9 codes and confirmed strokes by reviewing brain scans and medical records.
The investigators adjusted for confounders, including sex, age, BMI, emergent versus nonemergent surgery, prescriptions of antiplatelet drugs or beta blockers within four weeks before surgery, minutes of intraoperative hypotension, diabetes, hypertension, atrial fibrillation, Charleston Comorbidity Index, and work relative value units (ie, a surrogate for surgical complexity).
The cohort included 10,179 individuals with migraine (8.2%). Of the patients with migraine, 12.6% had migraine with aura.
Patients with migraine generally were younger, had higher BMI, and were more likely to be women. They were less likely to have diabetes or hypertension and to be taking antiplatelet drugs or beta blockers. Patients with migraine “were a little healthier” than the patients without migraine, Dr. Houle said.
In all, 771 patients had perioperative stroke, of whom 89 (11.5%) had migraine. About 0.6% of patients without migraine had perioperative stroke versus 0.9% of patients with migraine. The unadjusted odds ratio for stroke among migraineurs was 1.47, and the adjusted odds ratio was 1.75. “Individuals in this sample who had any migraine were at greater risk for stroke during the period after surgery, just like in the regular population,” said Dr. Houle. Although migraine without aura was not a statistically significant risk factor for stroke in the general population, it was after surgery.
Prediction Models
In one sensitivity analysis, the researchers determined each patient’s stroke risk based on known risk factors excluding migraine, such as age and cardiovascular disorders, and grouped patients by low, intermediate, and high levels of risk. Among patients in the low-risk group, the relative risk of stroke for patients with migraine versus patients without migraine was 3.5-fold higher. “These are people you would not have identified as having risk,” said Dr. Houle.
Future studies should try to identify the mechanisms involved in this relationship and assess interventions to mitigate the risk of stroke in patients with migraine who undergo surgery, Dr. Houle said.
Dr. Houle and colleagues have created a stroke prediction model that includes migraine and will “give surgeons a risk model to predict the risk of stroke for their patients,” he said. The model will “realize the risks that we uncovered in this study.”
—Jake Remaly
Suggested Reading
Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914.
Spector JT, Kahn SR, Jones MR, et al. Migraine headache and ischemic stroke risk: an updated meta-analysis. Am J Med. 2010;123(7):612-624.
Timm FP, Houle TT, Grabitz SD, et al. Migraine and risk of perioperative ischemic stroke and hospital readmission: hospital based registry study. BMJ. 2017;356:i6635.
How Long Should Dual Antiplatelet Therapy Last After Stroke or TIA?
A 21-day period could maximize the therapy’s benefits and minimize the risk of major hemorrhage.
MONTREAL—The optimal length for dual antiplatelet therapy (DAPT) in patients with mild stroke or transient ischemic attack (TIA) is 21 days, according to a prespecified analysis of data from the Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT) trial that was presented at the 11th World Stroke Congress. This duration of combined treatment maximizes protection against major ischemic events while minimizing the extra risk of a major hemorrhage, the researchers said.
Clopidogrel Plus Aspirin or Aspirin Alone
The POINT trial randomized 4,881 patients with a recent mild stroke or TIA and without atrial fibrillation to treatment with either clopidogrel plus aspirin or aspirin alone for 90 days. Compared with aspirin alone, DAPT decreased the incidence of a major ischemic event by 25% and more than doubled the rate of major hemorrhage.
The new prespecified analysis looked at outcomes on a weekly basis during 90 days of treatment. During the first 21 days, the rate of major hemorrhage events was 5.6% among those patients on aspirin alone and 3.6% among those on DAPT. Thus, DAPT was associated with a statistically significant 35% decrease in these adverse outcomes, said Jordan J. Elm, PhD, Associate Professor of Biostatistics at the Medical University of South Carolina in Charleston. During the subsequent 69 days of treatment, the incidence of major ischemic events was approximately 1% in both arms of the study, showing that after three weeks, the incremental benefit of DAPT disappeared, said Dr. Elm.
In contrast, the doubled rate of major hemorrhages (which mostly were reversible gastrointestinal bleeds) with DAPT, compared with aspirin alone, occurred at a relatively uniform rate throughout the 90 days of treatment. This suggests that limiting DAPT to 21 days could prevent many of the excess hemorrhages, maximize benefit, and reduce risk, said Dr. Elm. The findings of the Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events (CHANCE) trial confirmed the efficacy of 21 days of DAPT following a minor stroke or TIA.
Although the new finding from the POINT study resulted from a secondary analysis, it should be taken into account when writing treatment guidelines, said Dr. Elm. “This is an important analysis that is not just hypothesis-generating.”
Early Treatment
Another finding from the new analysis was that many major ischemic events, hence many of the events prevented by DAPT, occurred during the first two days following the index event. The POINT investigators were able to observe this finding because they enrolled patients and started treatment within 12 hours of the qualifying events.
“It is better to start treatment early,” said Dr. Elm. Major ischemic events continued to accumulate during Days 3 through 21, suggesting that patients could still benefit from DAPT if treatment did not start until 24 or 48 hours after the index event.
—Mitchel L. Zoler
Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379(3):215-225.
Tsivgoulis G, Safouris A, Kim DE, Alexandrov AV. Recent advances in primary and secondary prevention of atherosclerotic stroke. J Stroke. 2018;20(2):145-166.
Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369(1):11-19.
A 21-day period could maximize the therapy’s benefits and minimize the risk of major hemorrhage.
A 21-day period could maximize the therapy’s benefits and minimize the risk of major hemorrhage.
MONTREAL—The optimal length for dual antiplatelet therapy (DAPT) in patients with mild stroke or transient ischemic attack (TIA) is 21 days, according to a prespecified analysis of data from the Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT) trial that was presented at the 11th World Stroke Congress. This duration of combined treatment maximizes protection against major ischemic events while minimizing the extra risk of a major hemorrhage, the researchers said.
Clopidogrel Plus Aspirin or Aspirin Alone
The POINT trial randomized 4,881 patients with a recent mild stroke or TIA and without atrial fibrillation to treatment with either clopidogrel plus aspirin or aspirin alone for 90 days. Compared with aspirin alone, DAPT decreased the incidence of a major ischemic event by 25% and more than doubled the rate of major hemorrhage.
The new prespecified analysis looked at outcomes on a weekly basis during 90 days of treatment. During the first 21 days, the rate of major hemorrhage events was 5.6% among those patients on aspirin alone and 3.6% among those on DAPT. Thus, DAPT was associated with a statistically significant 35% decrease in these adverse outcomes, said Jordan J. Elm, PhD, Associate Professor of Biostatistics at the Medical University of South Carolina in Charleston. During the subsequent 69 days of treatment, the incidence of major ischemic events was approximately 1% in both arms of the study, showing that after three weeks, the incremental benefit of DAPT disappeared, said Dr. Elm.
In contrast, the doubled rate of major hemorrhages (which mostly were reversible gastrointestinal bleeds) with DAPT, compared with aspirin alone, occurred at a relatively uniform rate throughout the 90 days of treatment. This suggests that limiting DAPT to 21 days could prevent many of the excess hemorrhages, maximize benefit, and reduce risk, said Dr. Elm. The findings of the Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events (CHANCE) trial confirmed the efficacy of 21 days of DAPT following a minor stroke or TIA.
Although the new finding from the POINT study resulted from a secondary analysis, it should be taken into account when writing treatment guidelines, said Dr. Elm. “This is an important analysis that is not just hypothesis-generating.”
Early Treatment
Another finding from the new analysis was that many major ischemic events, hence many of the events prevented by DAPT, occurred during the first two days following the index event. The POINT investigators were able to observe this finding because they enrolled patients and started treatment within 12 hours of the qualifying events.
“It is better to start treatment early,” said Dr. Elm. Major ischemic events continued to accumulate during Days 3 through 21, suggesting that patients could still benefit from DAPT if treatment did not start until 24 or 48 hours after the index event.
—Mitchel L. Zoler
Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379(3):215-225.
Tsivgoulis G, Safouris A, Kim DE, Alexandrov AV. Recent advances in primary and secondary prevention of atherosclerotic stroke. J Stroke. 2018;20(2):145-166.
Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369(1):11-19.
MONTREAL—The optimal length for dual antiplatelet therapy (DAPT) in patients with mild stroke or transient ischemic attack (TIA) is 21 days, according to a prespecified analysis of data from the Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT) trial that was presented at the 11th World Stroke Congress. This duration of combined treatment maximizes protection against major ischemic events while minimizing the extra risk of a major hemorrhage, the researchers said.
Clopidogrel Plus Aspirin or Aspirin Alone
The POINT trial randomized 4,881 patients with a recent mild stroke or TIA and without atrial fibrillation to treatment with either clopidogrel plus aspirin or aspirin alone for 90 days. Compared with aspirin alone, DAPT decreased the incidence of a major ischemic event by 25% and more than doubled the rate of major hemorrhage.
The new prespecified analysis looked at outcomes on a weekly basis during 90 days of treatment. During the first 21 days, the rate of major hemorrhage events was 5.6% among those patients on aspirin alone and 3.6% among those on DAPT. Thus, DAPT was associated with a statistically significant 35% decrease in these adverse outcomes, said Jordan J. Elm, PhD, Associate Professor of Biostatistics at the Medical University of South Carolina in Charleston. During the subsequent 69 days of treatment, the incidence of major ischemic events was approximately 1% in both arms of the study, showing that after three weeks, the incremental benefit of DAPT disappeared, said Dr. Elm.
In contrast, the doubled rate of major hemorrhages (which mostly were reversible gastrointestinal bleeds) with DAPT, compared with aspirin alone, occurred at a relatively uniform rate throughout the 90 days of treatment. This suggests that limiting DAPT to 21 days could prevent many of the excess hemorrhages, maximize benefit, and reduce risk, said Dr. Elm. The findings of the Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events (CHANCE) trial confirmed the efficacy of 21 days of DAPT following a minor stroke or TIA.
Although the new finding from the POINT study resulted from a secondary analysis, it should be taken into account when writing treatment guidelines, said Dr. Elm. “This is an important analysis that is not just hypothesis-generating.”
Early Treatment
Another finding from the new analysis was that many major ischemic events, hence many of the events prevented by DAPT, occurred during the first two days following the index event. The POINT investigators were able to observe this finding because they enrolled patients and started treatment within 12 hours of the qualifying events.
“It is better to start treatment early,” said Dr. Elm. Major ischemic events continued to accumulate during Days 3 through 21, suggesting that patients could still benefit from DAPT if treatment did not start until 24 or 48 hours after the index event.
—Mitchel L. Zoler
Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379(3):215-225.
Tsivgoulis G, Safouris A, Kim DE, Alexandrov AV. Recent advances in primary and secondary prevention of atherosclerotic stroke. J Stroke. 2018;20(2):145-166.
Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369(1):11-19.
Heart drug spotlights troubling trends in drug marketing
At the end of September, Amarin Corp. teased some early findings for Vascepa, its preventive medicine for people at risk of heart disease. The claim was astounding: a 25% relative risk reduction for deaths related to heart attacks, strokes, and other conditions. Headlines proclaimed a potential game changer in treating cardiovascular disease. And company shares quickly soared, from $3 a share to about $20.
Vascepa is Amarin’s only product. The company hopes to turn its pill made of purified fish oil into a cash cow, allowing it to staff up both in the United States and abroad so it can sell doctors and millions of consumers on its medical benefits. Although the product has been on the market for more than 5 years, its first TV ad campaign rolled out this summer in anticipation of the study findings.
Except there is one problem. The particulars of the scientific study on which this claim was based remain a mystery.
Amarin’s preliminary announcement came via a news release on Sept. 24. The company plans to release detailed findings in November at the national American Heart Association conference. Then early next year, it plans to seek Food and Drug Administration approval to use the drug as a preventive for a range of heart conditions, beyond its current role targeting high triglyceride levels.
In the interim, a battle is brewing among physicians, cardiovascular experts, and pharma watchers who say Vascepa brings to the foreground troubling trends in the marketing and advertising of new drugs. Companies sometimes promote new products, but withhold the detailed findings until much later. The consequences for both consumers and the health system are vast.
“Until all the data is available for review by the public and medical community, it’s really premature to see some of the cheerleading that’s being done,” said Eric Strong, MD, a hospitalist and clinical assistant professor at Stanford (Calif.) University. “It’s harder to change people’s minds once you have these rosy pictures.”
John Thero, Amarin’s CEO, argued that the imminent release of the drug’s complete picture should alleviate those concerns.
In unveiling topline findings in a news release, he said, the company’s playbook doesn’t diverge from that of other pharmaceutical makers and provides a necessary level of disclosure for shareholders.
But it’s the specifics in the data – for instance, which patients benefited, by how much, their absolute risk reduction and which precise conditions saw improvement – that illustrate whether a product is cost effective, said medical and drug experts.
That’s especially true in the case of Vascepa, whose manufacturer is working hard to convince people the product is clinically superior to ordinary fish oil supplements. Fish oil, which can retail for a few dollars a bottle, has long been promoted as a preventive for heart disease. But the substance has never held up in clinical trials as a way to systematically lower disease risk, said experts.
That’s where Amarin’s product is superior, Mr. Thero said.
The manufacturer has tried to limit competition by seeking to block other fish oil products, arguing to the U.S. International Trade Commission that omega-3 supplements aren’t equivalents, and calling on the FDA to block a chemical component of fish oil, known as EPA or eicosapentaenoic acid, and marketed by a number of supplement companies, from being sold as a dietary supplement. Amarin hasn’t yet prevailed.
Preston Mason, PhD, a biologist and faculty member in the division of cardiology at Brigham and Women’s Hospital, Boston, who consults for Amarin and has advocated on its behalf, argued that ordinary fish oil supplements carry risks because they are not regulated or approved by the FDA, which does oversee prescription drugs like Vascepa.
How Vascepa performs against regular fish oil remains unknown. Amarin’s trial compared the drug against a placebo, not over-the-counter supplements.
Vascepa itself isn’t new. It was approved in 2012 as a remedy for extremely high triglyceride levels, which can put patients at risk for pancreatic problems. But reducing that fat hadn’t been conclusively tied to, say, lowering the risk of heart attacks, or other major cardiac problems.
That link, ostensibly, is what Amarin is trying now to assert. And there’s plenty of money to be made if it succeeds.
As of last December, Vascepa retailed for about $280 for a month-long supply, a list price increase of 43% over 5 years, though the company says its net sale price has stayed the same. (That difference would come if Amarin increased the size of rebates, or discounts it provides, commensurate with price hikes.)
Now, citing the drug’s potentially increased value, Amarin has declined to say whether it will change the price again – though Mr. Thero said he sees greater profit potential if the company increases sales volume rather than price.
This gets at the crux of this debate. If a company makes available the technical details of a product, but only after hyping the findings, and if the details undercut some of that buzz – is it too late?
Khurram Nasir, MD, a cardiologist at Yale University in New Haven, Conn., acknowledged that it’s unclear how effective Vascepa really is, but maintained those ambiguities will be cleared up soon enough.
“As the findings reveal themselves, there will be a lot of discussion around cost effectiveness, and whether this is worth the spend,” Dr. Nasir said.
Dr. Mason, the Amarin scientist, said FDA scrutiny also can alleviate concerns about overhype.
But others worry the perception of Vascepa’s effectiveness is now set.
“People are weighing in with really strong language, without enough information,” said Lisa Schwartz, MD, MS, who codirects the Center for Medicine and Media at Dartmouth Institute in Hanover, N.H., and studies effective scientific communication.
That has both clinical and financial consequences, she added. Doctors are more likely to prescribe a product that’s been heavily promoted, even if subsequent discussion indicates the drug isn’t as powerful as initially implied. And manufacturers can cash in, whether through increased company stock market value or by charging higher list prices.
For Vascepa, the central question is which specific heart conditions saw risk reduction, she and others said. In its news release, Amarin noted a “composite outcome” – that is, the 25% relative improvement encompassed all conditions for which the researchers tested.
“People are saying, Wow, it reduced heart attack, stroke and blah, blah, blah – when it may just reduce the least important one,” said Steven Woloshin, MD, MS, who also codirects the Center for Medicine and Media at Dartmouth.
Another issue: The Vascepa trial focused on a specific population — patients with high triglyceride levels plus elevated risk of cardiovascular disease or diabetes who were already taking a daily statin. That means any proof of benefit is limited to that group.
Dr. Woloshin and Dr. Schwartz both suggested that nuance could get lost in translation. “It is this much narrower, high-risk population,” Dr. Schwartz said.
Dr. Woloshin added, “The fear is [the message] would generalize to anyone with high triglycerides.”
This concern is amplified by a 2016 court settlement in which the FDA permitted Amarin to market Vascepa to audiences for whom it hasn’t been specifically approved – so long as the company doesn’t say anything untrue about the drug.
Mr. Thero said Amarin’s marketing of Vascepa has stayed, and will remain, consistent with what is factual and relevant.
“We are proceeding consistently with what the FDA has guided,” he said.
But, some experts said, the 2016 settlement could unlock the door to wider marketing of Vascepa’s off-label use, implying the pill benefits more people than it actually does.
“They’ll take pains to show how different this is from everything out there ... and its results in these populations,” said Ameet Sarpatwari, MD, JD, an epidemiologist and lawyer at Harvard Medical School, Boston, who studies the pharmaceutical industry. “What they can’t do is say it will be beneficial to these other populations. But they can hint at that.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
At the end of September, Amarin Corp. teased some early findings for Vascepa, its preventive medicine for people at risk of heart disease. The claim was astounding: a 25% relative risk reduction for deaths related to heart attacks, strokes, and other conditions. Headlines proclaimed a potential game changer in treating cardiovascular disease. And company shares quickly soared, from $3 a share to about $20.
Vascepa is Amarin’s only product. The company hopes to turn its pill made of purified fish oil into a cash cow, allowing it to staff up both in the United States and abroad so it can sell doctors and millions of consumers on its medical benefits. Although the product has been on the market for more than 5 years, its first TV ad campaign rolled out this summer in anticipation of the study findings.
Except there is one problem. The particulars of the scientific study on which this claim was based remain a mystery.
Amarin’s preliminary announcement came via a news release on Sept. 24. The company plans to release detailed findings in November at the national American Heart Association conference. Then early next year, it plans to seek Food and Drug Administration approval to use the drug as a preventive for a range of heart conditions, beyond its current role targeting high triglyceride levels.
In the interim, a battle is brewing among physicians, cardiovascular experts, and pharma watchers who say Vascepa brings to the foreground troubling trends in the marketing and advertising of new drugs. Companies sometimes promote new products, but withhold the detailed findings until much later. The consequences for both consumers and the health system are vast.
“Until all the data is available for review by the public and medical community, it’s really premature to see some of the cheerleading that’s being done,” said Eric Strong, MD, a hospitalist and clinical assistant professor at Stanford (Calif.) University. “It’s harder to change people’s minds once you have these rosy pictures.”
John Thero, Amarin’s CEO, argued that the imminent release of the drug’s complete picture should alleviate those concerns.
In unveiling topline findings in a news release, he said, the company’s playbook doesn’t diverge from that of other pharmaceutical makers and provides a necessary level of disclosure for shareholders.
But it’s the specifics in the data – for instance, which patients benefited, by how much, their absolute risk reduction and which precise conditions saw improvement – that illustrate whether a product is cost effective, said medical and drug experts.
That’s especially true in the case of Vascepa, whose manufacturer is working hard to convince people the product is clinically superior to ordinary fish oil supplements. Fish oil, which can retail for a few dollars a bottle, has long been promoted as a preventive for heart disease. But the substance has never held up in clinical trials as a way to systematically lower disease risk, said experts.
That’s where Amarin’s product is superior, Mr. Thero said.
The manufacturer has tried to limit competition by seeking to block other fish oil products, arguing to the U.S. International Trade Commission that omega-3 supplements aren’t equivalents, and calling on the FDA to block a chemical component of fish oil, known as EPA or eicosapentaenoic acid, and marketed by a number of supplement companies, from being sold as a dietary supplement. Amarin hasn’t yet prevailed.
Preston Mason, PhD, a biologist and faculty member in the division of cardiology at Brigham and Women’s Hospital, Boston, who consults for Amarin and has advocated on its behalf, argued that ordinary fish oil supplements carry risks because they are not regulated or approved by the FDA, which does oversee prescription drugs like Vascepa.
How Vascepa performs against regular fish oil remains unknown. Amarin’s trial compared the drug against a placebo, not over-the-counter supplements.
Vascepa itself isn’t new. It was approved in 2012 as a remedy for extremely high triglyceride levels, which can put patients at risk for pancreatic problems. But reducing that fat hadn’t been conclusively tied to, say, lowering the risk of heart attacks, or other major cardiac problems.
That link, ostensibly, is what Amarin is trying now to assert. And there’s plenty of money to be made if it succeeds.
As of last December, Vascepa retailed for about $280 for a month-long supply, a list price increase of 43% over 5 years, though the company says its net sale price has stayed the same. (That difference would come if Amarin increased the size of rebates, or discounts it provides, commensurate with price hikes.)
Now, citing the drug’s potentially increased value, Amarin has declined to say whether it will change the price again – though Mr. Thero said he sees greater profit potential if the company increases sales volume rather than price.
This gets at the crux of this debate. If a company makes available the technical details of a product, but only after hyping the findings, and if the details undercut some of that buzz – is it too late?
Khurram Nasir, MD, a cardiologist at Yale University in New Haven, Conn., acknowledged that it’s unclear how effective Vascepa really is, but maintained those ambiguities will be cleared up soon enough.
“As the findings reveal themselves, there will be a lot of discussion around cost effectiveness, and whether this is worth the spend,” Dr. Nasir said.
Dr. Mason, the Amarin scientist, said FDA scrutiny also can alleviate concerns about overhype.
But others worry the perception of Vascepa’s effectiveness is now set.
“People are weighing in with really strong language, without enough information,” said Lisa Schwartz, MD, MS, who codirects the Center for Medicine and Media at Dartmouth Institute in Hanover, N.H., and studies effective scientific communication.
That has both clinical and financial consequences, she added. Doctors are more likely to prescribe a product that’s been heavily promoted, even if subsequent discussion indicates the drug isn’t as powerful as initially implied. And manufacturers can cash in, whether through increased company stock market value or by charging higher list prices.
For Vascepa, the central question is which specific heart conditions saw risk reduction, she and others said. In its news release, Amarin noted a “composite outcome” – that is, the 25% relative improvement encompassed all conditions for which the researchers tested.
“People are saying, Wow, it reduced heart attack, stroke and blah, blah, blah – when it may just reduce the least important one,” said Steven Woloshin, MD, MS, who also codirects the Center for Medicine and Media at Dartmouth.
Another issue: The Vascepa trial focused on a specific population — patients with high triglyceride levels plus elevated risk of cardiovascular disease or diabetes who were already taking a daily statin. That means any proof of benefit is limited to that group.
Dr. Woloshin and Dr. Schwartz both suggested that nuance could get lost in translation. “It is this much narrower, high-risk population,” Dr. Schwartz said.
Dr. Woloshin added, “The fear is [the message] would generalize to anyone with high triglycerides.”
This concern is amplified by a 2016 court settlement in which the FDA permitted Amarin to market Vascepa to audiences for whom it hasn’t been specifically approved – so long as the company doesn’t say anything untrue about the drug.
Mr. Thero said Amarin’s marketing of Vascepa has stayed, and will remain, consistent with what is factual and relevant.
“We are proceeding consistently with what the FDA has guided,” he said.
But, some experts said, the 2016 settlement could unlock the door to wider marketing of Vascepa’s off-label use, implying the pill benefits more people than it actually does.
“They’ll take pains to show how different this is from everything out there ... and its results in these populations,” said Ameet Sarpatwari, MD, JD, an epidemiologist and lawyer at Harvard Medical School, Boston, who studies the pharmaceutical industry. “What they can’t do is say it will be beneficial to these other populations. But they can hint at that.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
At the end of September, Amarin Corp. teased some early findings for Vascepa, its preventive medicine for people at risk of heart disease. The claim was astounding: a 25% relative risk reduction for deaths related to heart attacks, strokes, and other conditions. Headlines proclaimed a potential game changer in treating cardiovascular disease. And company shares quickly soared, from $3 a share to about $20.
Vascepa is Amarin’s only product. The company hopes to turn its pill made of purified fish oil into a cash cow, allowing it to staff up both in the United States and abroad so it can sell doctors and millions of consumers on its medical benefits. Although the product has been on the market for more than 5 years, its first TV ad campaign rolled out this summer in anticipation of the study findings.
Except there is one problem. The particulars of the scientific study on which this claim was based remain a mystery.
Amarin’s preliminary announcement came via a news release on Sept. 24. The company plans to release detailed findings in November at the national American Heart Association conference. Then early next year, it plans to seek Food and Drug Administration approval to use the drug as a preventive for a range of heart conditions, beyond its current role targeting high triglyceride levels.
In the interim, a battle is brewing among physicians, cardiovascular experts, and pharma watchers who say Vascepa brings to the foreground troubling trends in the marketing and advertising of new drugs. Companies sometimes promote new products, but withhold the detailed findings until much later. The consequences for both consumers and the health system are vast.
“Until all the data is available for review by the public and medical community, it’s really premature to see some of the cheerleading that’s being done,” said Eric Strong, MD, a hospitalist and clinical assistant professor at Stanford (Calif.) University. “It’s harder to change people’s minds once you have these rosy pictures.”
John Thero, Amarin’s CEO, argued that the imminent release of the drug’s complete picture should alleviate those concerns.
In unveiling topline findings in a news release, he said, the company’s playbook doesn’t diverge from that of other pharmaceutical makers and provides a necessary level of disclosure for shareholders.
But it’s the specifics in the data – for instance, which patients benefited, by how much, their absolute risk reduction and which precise conditions saw improvement – that illustrate whether a product is cost effective, said medical and drug experts.
That’s especially true in the case of Vascepa, whose manufacturer is working hard to convince people the product is clinically superior to ordinary fish oil supplements. Fish oil, which can retail for a few dollars a bottle, has long been promoted as a preventive for heart disease. But the substance has never held up in clinical trials as a way to systematically lower disease risk, said experts.
That’s where Amarin’s product is superior, Mr. Thero said.
The manufacturer has tried to limit competition by seeking to block other fish oil products, arguing to the U.S. International Trade Commission that omega-3 supplements aren’t equivalents, and calling on the FDA to block a chemical component of fish oil, known as EPA or eicosapentaenoic acid, and marketed by a number of supplement companies, from being sold as a dietary supplement. Amarin hasn’t yet prevailed.
Preston Mason, PhD, a biologist and faculty member in the division of cardiology at Brigham and Women’s Hospital, Boston, who consults for Amarin and has advocated on its behalf, argued that ordinary fish oil supplements carry risks because they are not regulated or approved by the FDA, which does oversee prescription drugs like Vascepa.
How Vascepa performs against regular fish oil remains unknown. Amarin’s trial compared the drug against a placebo, not over-the-counter supplements.
Vascepa itself isn’t new. It was approved in 2012 as a remedy for extremely high triglyceride levels, which can put patients at risk for pancreatic problems. But reducing that fat hadn’t been conclusively tied to, say, lowering the risk of heart attacks, or other major cardiac problems.
That link, ostensibly, is what Amarin is trying now to assert. And there’s plenty of money to be made if it succeeds.
As of last December, Vascepa retailed for about $280 for a month-long supply, a list price increase of 43% over 5 years, though the company says its net sale price has stayed the same. (That difference would come if Amarin increased the size of rebates, or discounts it provides, commensurate with price hikes.)
Now, citing the drug’s potentially increased value, Amarin has declined to say whether it will change the price again – though Mr. Thero said he sees greater profit potential if the company increases sales volume rather than price.
This gets at the crux of this debate. If a company makes available the technical details of a product, but only after hyping the findings, and if the details undercut some of that buzz – is it too late?
Khurram Nasir, MD, a cardiologist at Yale University in New Haven, Conn., acknowledged that it’s unclear how effective Vascepa really is, but maintained those ambiguities will be cleared up soon enough.
“As the findings reveal themselves, there will be a lot of discussion around cost effectiveness, and whether this is worth the spend,” Dr. Nasir said.
Dr. Mason, the Amarin scientist, said FDA scrutiny also can alleviate concerns about overhype.
But others worry the perception of Vascepa’s effectiveness is now set.
“People are weighing in with really strong language, without enough information,” said Lisa Schwartz, MD, MS, who codirects the Center for Medicine and Media at Dartmouth Institute in Hanover, N.H., and studies effective scientific communication.
That has both clinical and financial consequences, she added. Doctors are more likely to prescribe a product that’s been heavily promoted, even if subsequent discussion indicates the drug isn’t as powerful as initially implied. And manufacturers can cash in, whether through increased company stock market value or by charging higher list prices.
For Vascepa, the central question is which specific heart conditions saw risk reduction, she and others said. In its news release, Amarin noted a “composite outcome” – that is, the 25% relative improvement encompassed all conditions for which the researchers tested.
“People are saying, Wow, it reduced heart attack, stroke and blah, blah, blah – when it may just reduce the least important one,” said Steven Woloshin, MD, MS, who also codirects the Center for Medicine and Media at Dartmouth.
Another issue: The Vascepa trial focused on a specific population — patients with high triglyceride levels plus elevated risk of cardiovascular disease or diabetes who were already taking a daily statin. That means any proof of benefit is limited to that group.
Dr. Woloshin and Dr. Schwartz both suggested that nuance could get lost in translation. “It is this much narrower, high-risk population,” Dr. Schwartz said.
Dr. Woloshin added, “The fear is [the message] would generalize to anyone with high triglycerides.”
This concern is amplified by a 2016 court settlement in which the FDA permitted Amarin to market Vascepa to audiences for whom it hasn’t been specifically approved – so long as the company doesn’t say anything untrue about the drug.
Mr. Thero said Amarin’s marketing of Vascepa has stayed, and will remain, consistent with what is factual and relevant.
“We are proceeding consistently with what the FDA has guided,” he said.
But, some experts said, the 2016 settlement could unlock the door to wider marketing of Vascepa’s off-label use, implying the pill benefits more people than it actually does.
“They’ll take pains to show how different this is from everything out there ... and its results in these populations,” said Ameet Sarpatwari, MD, JD, an epidemiologist and lawyer at Harvard Medical School, Boston, who studies the pharmaceutical industry. “What they can’t do is say it will be beneficial to these other populations. But they can hint at that.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
TIA and Stroke May Affect Risk of Subsequent Events Differently in Patients With Atrial Fibrillation
A history of TIA appears not to increase the risks of stroke and mortality significantly in this population.
MONTREAL—Neurologists usually consider stroke and transient ischemic attack (TIA) to indicate a similar need for anticoagulation, but these events may not entail equivalent risks, according to a presentation given at the 11th World Stroke Congress.
An analysis of two-year follow-up data from the Global Anticoagulant Registry in the Field (GARFIELD-AF), which included more than 52,000 patients with newly diagnosed atrial fibrillation, showed that while patients with a history of stroke had significantly elevated rates of all-cause mortality and stroke, those with a history of TIA alone had rates of mortality and stroke that were virtually identical to those of patients with atrial fibrillation with no history of a cerebrovascular event.
Should Risk Calculators Be Revised?
“A history of TIA [alone] is not a reliable predictor of an increased risk for events,” said Werner Hacke, MD, Professor and Chair of Neurology at the University of Heidelberg in Germany. “A history of TIA should be removed from scores estimating the risk for stroke and systemic embolism in patients with atrial fibrillation.
“The weak predictive power of a history of TIA is probably caused by the relatively low reliability of establishing the diagnosis of TIA,” especially when the diagnosis is made by someone who is not a neurologist. “It is a fuzzy diagnosis,” even for a neurologist, and it consistently confounds other clinicians, he said. “I would be careful about deciding to anticoagulate a patient [with atrial fibrillation] based on a history of TIA. I am convinced that most people with a history of TIA [in the GARFIELD-AF registry] actually never had a TIA.”
Dr. Hacke has been unable to find the reason that TIA began to be considered to entail similar risks as stroke. “I asked all the old atrial fibrillation guys, ‘When did TIA start coming in and why?’ And none of them could remember,” he said. “At first, they talked about a history of cerebrovascular events, but then that became stroke and TIA, and it was as if it was one word” always said in the same breath. The CHADS2 score and the CHA2DS2-VASc score make a history of stroke or TIA, as well as thromboembolism, coequal risk factors that count for 2 points when calculating the thrombotic risk score for a patient with atrial fibrillation.
An Examination of Registry Data
To test whether this convention was appropriate, Dr. Hacke and his associates examined the consequences of a history of stroke alone, compared with those of a history of a TIA alone. They used data collected in GARFIELD-AF, a multinational registry with 51,670 patients newly diagnosed with atrial fibrillation who were followed for two years. All participants had complete information on their stroke and TIA history. This information included 5,617 patients with a history of at least one diagnosed cerebrovascular event, including 3,362 diagnosed with stroke alone, 1,788 diagnosed with TIA alone, and the remaining patients diagnosed with both events.
When compared with patients with atrial fibrillation without a history of any type of cerebrovascular event, those with a history of a stroke alone had a statistically significant 29% increased rate of all-cause death and a 2.3-fold higher rate of stroke after adjustment for baseline demographic and clinical differences. In contrast, the patients with a history of TIA alone had mortality and stroke rates during follow-up that did not differ significantly from the comparator group.
—Mitchel L. Zoler
A history of TIA appears not to increase the risks of stroke and mortality significantly in this population.
A history of TIA appears not to increase the risks of stroke and mortality significantly in this population.
MONTREAL—Neurologists usually consider stroke and transient ischemic attack (TIA) to indicate a similar need for anticoagulation, but these events may not entail equivalent risks, according to a presentation given at the 11th World Stroke Congress.
An analysis of two-year follow-up data from the Global Anticoagulant Registry in the Field (GARFIELD-AF), which included more than 52,000 patients with newly diagnosed atrial fibrillation, showed that while patients with a history of stroke had significantly elevated rates of all-cause mortality and stroke, those with a history of TIA alone had rates of mortality and stroke that were virtually identical to those of patients with atrial fibrillation with no history of a cerebrovascular event.
Should Risk Calculators Be Revised?
“A history of TIA [alone] is not a reliable predictor of an increased risk for events,” said Werner Hacke, MD, Professor and Chair of Neurology at the University of Heidelberg in Germany. “A history of TIA should be removed from scores estimating the risk for stroke and systemic embolism in patients with atrial fibrillation.
“The weak predictive power of a history of TIA is probably caused by the relatively low reliability of establishing the diagnosis of TIA,” especially when the diagnosis is made by someone who is not a neurologist. “It is a fuzzy diagnosis,” even for a neurologist, and it consistently confounds other clinicians, he said. “I would be careful about deciding to anticoagulate a patient [with atrial fibrillation] based on a history of TIA. I am convinced that most people with a history of TIA [in the GARFIELD-AF registry] actually never had a TIA.”
Dr. Hacke has been unable to find the reason that TIA began to be considered to entail similar risks as stroke. “I asked all the old atrial fibrillation guys, ‘When did TIA start coming in and why?’ And none of them could remember,” he said. “At first, they talked about a history of cerebrovascular events, but then that became stroke and TIA, and it was as if it was one word” always said in the same breath. The CHADS2 score and the CHA2DS2-VASc score make a history of stroke or TIA, as well as thromboembolism, coequal risk factors that count for 2 points when calculating the thrombotic risk score for a patient with atrial fibrillation.
An Examination of Registry Data
To test whether this convention was appropriate, Dr. Hacke and his associates examined the consequences of a history of stroke alone, compared with those of a history of a TIA alone. They used data collected in GARFIELD-AF, a multinational registry with 51,670 patients newly diagnosed with atrial fibrillation who were followed for two years. All participants had complete information on their stroke and TIA history. This information included 5,617 patients with a history of at least one diagnosed cerebrovascular event, including 3,362 diagnosed with stroke alone, 1,788 diagnosed with TIA alone, and the remaining patients diagnosed with both events.
When compared with patients with atrial fibrillation without a history of any type of cerebrovascular event, those with a history of a stroke alone had a statistically significant 29% increased rate of all-cause death and a 2.3-fold higher rate of stroke after adjustment for baseline demographic and clinical differences. In contrast, the patients with a history of TIA alone had mortality and stroke rates during follow-up that did not differ significantly from the comparator group.
—Mitchel L. Zoler
MONTREAL—Neurologists usually consider stroke and transient ischemic attack (TIA) to indicate a similar need for anticoagulation, but these events may not entail equivalent risks, according to a presentation given at the 11th World Stroke Congress.
An analysis of two-year follow-up data from the Global Anticoagulant Registry in the Field (GARFIELD-AF), which included more than 52,000 patients with newly diagnosed atrial fibrillation, showed that while patients with a history of stroke had significantly elevated rates of all-cause mortality and stroke, those with a history of TIA alone had rates of mortality and stroke that were virtually identical to those of patients with atrial fibrillation with no history of a cerebrovascular event.
Should Risk Calculators Be Revised?
“A history of TIA [alone] is not a reliable predictor of an increased risk for events,” said Werner Hacke, MD, Professor and Chair of Neurology at the University of Heidelberg in Germany. “A history of TIA should be removed from scores estimating the risk for stroke and systemic embolism in patients with atrial fibrillation.
“The weak predictive power of a history of TIA is probably caused by the relatively low reliability of establishing the diagnosis of TIA,” especially when the diagnosis is made by someone who is not a neurologist. “It is a fuzzy diagnosis,” even for a neurologist, and it consistently confounds other clinicians, he said. “I would be careful about deciding to anticoagulate a patient [with atrial fibrillation] based on a history of TIA. I am convinced that most people with a history of TIA [in the GARFIELD-AF registry] actually never had a TIA.”
Dr. Hacke has been unable to find the reason that TIA began to be considered to entail similar risks as stroke. “I asked all the old atrial fibrillation guys, ‘When did TIA start coming in and why?’ And none of them could remember,” he said. “At first, they talked about a history of cerebrovascular events, but then that became stroke and TIA, and it was as if it was one word” always said in the same breath. The CHADS2 score and the CHA2DS2-VASc score make a history of stroke or TIA, as well as thromboembolism, coequal risk factors that count for 2 points when calculating the thrombotic risk score for a patient with atrial fibrillation.
An Examination of Registry Data
To test whether this convention was appropriate, Dr. Hacke and his associates examined the consequences of a history of stroke alone, compared with those of a history of a TIA alone. They used data collected in GARFIELD-AF, a multinational registry with 51,670 patients newly diagnosed with atrial fibrillation who were followed for two years. All participants had complete information on their stroke and TIA history. This information included 5,617 patients with a history of at least one diagnosed cerebrovascular event, including 3,362 diagnosed with stroke alone, 1,788 diagnosed with TIA alone, and the remaining patients diagnosed with both events.
When compared with patients with atrial fibrillation without a history of any type of cerebrovascular event, those with a history of a stroke alone had a statistically significant 29% increased rate of all-cause death and a 2.3-fold higher rate of stroke after adjustment for baseline demographic and clinical differences. In contrast, the patients with a history of TIA alone had mortality and stroke rates during follow-up that did not differ significantly from the comparator group.
—Mitchel L. Zoler
CT opens extended window for stroke thrombolysis
MONTREAL – An extended time window for thrombolytic treatment of acute ischemic stroke patients using tissue plasminogen activator out to 9 hours from stroke onset was safe and effective using CT perfusion imaging and automated imaging processing software to select suitable patients in the EXTEND trial. This result matches the groundbreaking finding reported earlier in 2018 that used MRI to select patients for extended thrombolysis.
“To reproduce our results you need to set up CT perfusion” as well as the RAPID software for automated image processing to identify patients with a small infarct core and a large area of salvageable brain, said Henry Ma, MD, a stroke neurologist at Monash University, Melbourne, as he reported results from the trial at the World Stroke Congress. “EXTEND is the first positive thrombolysis trial in an extended time window using automated penumbral imaging.”
The new finding, from a trial with 225 randomized stroke patients, was especially notable because, by showing the validity of CT imaging for patient selection, it makes applying the extended time window for thrombolytic therapy more feasible for U.S. and Canadian stroke centers where CT imaging is much more common than MRI. A report from European investigators published in August 2018 from the WAKE-UP trial showed that thrombolysis with tissue plasminogen activator (tPA) was safe and effective when administered to patients who woke up with an acute ischemic stroke that had occurred more than 4.5 hours before treatment, but this study exclusively used MRI for patient selection (N Engl J Med. 2018 Aug 16;379[7]:611-22).
“In North America, our systems are more equipped for using CT,” commented Ashfaq Shuaib, MD, a professor of medicine and neurologist at the University of Alberta, Edmonton. Based on the WAKE-UP results, “MR would be preferred, but what we’ve been doing [since the WAKE-UP report] is if we see a CT scan that’s good we go ahead” with thrombolysis.
“Biologically, it doesn’t matter whether you use MR or CT; they both index the same underlying pathology. We’ve been hesitant to go beyond the MR finding from WAKE-UP, where there were data, but the findings from EXTEND were right in line with the WAKE-UP results, and that’s all we need to be reassured” that CT perfusion imaging also works for patient selection, commented Jeffrey L. Saver, MD, professor of medicine and director of the Comprehensive Stroke Center at the University of California, Los Angeles.
CT perfusion imaging and automated image processing “worked to select stroke patients” for an extended time window for treatment with mechanical thrombectomy in the DAWN (N Engl J Med. 2018 Jan 4;378[1]:11-21) and DEFUSE 3 (N Engl J Med. 2018 Feb 22;378[8]:308-18) trials, a history that makes the new finding of successfully using CT imaging to select patients who qualify for extended use of thrombolysis “a convincing result,” Dr. Saver said in an interview. The new EXTEND findings “will have a major impact” on using an extended time window for thrombolysis in U.S. practice, he predicted.
The EXTEND trial (Int J Stroke. 2012 Jan 1;7[1]:74-80) ran at 22 sites in Australia, 11 sites in Taiwan, and 1 center in New Zealand. Recruitment of patients into the study stopped early, after enrolling 225 patients, in June 2018, when results from WAKE-UP came out.
The EXTEND investigators enrolled patients who were either 4.5-9 hours out from the onset of their stroke or patients with a wake-up stroke with an uncertain onset. Participating centers could use either CT perfusion or MRI to identify candidates for treatment, and all used the RAPID software for image processing to identify patients with a perfusion lesion of at least 10 mL and an ischemic core volume no greater than 70 mL. Dr. Ma did not report what percentage of patients underwent imaging with each of these methods, but hinted that clinicians had used CT for a majority of the cases. The study randomized patients to receive either 0.9 mg/kg tPA or placebo, and by the trial protocol none of the enrolled patients received treatment with mechanical thrombectomy.
The trial’s primary endpoint was the percentage of patients with a modified Rankin Scale score of 0 or 1 at 90 days after their stroke, which was achieved by 44% more patients in the tPA group relative to the placebo arm after adjustment for age and baseline stroke severity, a statistically significant difference. The results were also positive for several secondary endpoints, such as recanalization 24 hours after treatment, which occurred in 67% of patients treated with tPA and 37% of the control patients, a statistically significant 68% relative improvement with thrombolysis.
Mortality at 90 days was similar in the two arms – 9% among the placebo patients and 12% among those who received tPA. The rate of symptomatic intracranial hemorrhage 36 hours after treatment was significantly higher among patients treated with tPA at 6%, compared with 1% in the placebo group, but the magnitude of this adverse effect was consistent with rates of intracranial hemorrhages previously reported in other studies of thrombolytic treatment for acute ischemic stroke, Dr. Ma said. The small number of increased intracranial hemorrhages “was not associated with increased mortality, and did not negate the positive result of an improved rate of excellent functional outcomes.”
These findings will likely spur further adoption of imaging processing software of the type used in EXTEND by U.S. stroke centers, Dr. Saver predicted.
“More and more centers have been getting this [software], and now they have two reasons to have it: to identify patients for an extended window for mechanical thrombectomy and to identify patients for an extended window for thrombolysis. It is a compelling case to have the imaging software as widely disseminated as possible. Centers that want to do the best for patients should have this imaging-processing software,” Dr. Saver said.
Dr. Ma and Dr. Shuaib reported no disclosures. Dr. Saver has received research funding and personal fees from Medtronic-Abbott and Neuravia.
SOURCE: Ma H et al. Int J. Stroke. 2018 Oct;13(2S):235, Abstract 1014.
Ever since results from the WAKE-UP trial came out earlier in 2018, we at the University of Cincinnati have been imaging acute ischemic stroke patients who presented outside the standard 4.5-hour time limit for thrombolysis with MRI to see if they qualify for an extended window for thrombolysis. But this has been a cumbersome and redundant process because our default imaging method is CT, so we have been imaging potential candidates for an extended thrombolytic window twice, first with CT and then later with MRI.
The EXTEND findings also provide a further reason for U.S. stroke centers to purchase and use some type of imaging processing software if they don’t already have it, either the RAPID software that was used in EXTEND or one of the several similar software packages that are now available. Several primary stroke centers in my area still do not currently use this software, although its use has been quickly spreading and it will now be increasingly hard for these centers to hold off acquiring it. Fortunately the increased competition among software vendors who sell this type of software has meant that the price has been dropping.
Pooja Khatri, MD , is a professor of neurology and director of acute stroke at the University of Cincinnati. She has been a consultant to Biogen, Greenwich, and PTC Therapeutics. She made these comments in an interview.
Ever since results from the WAKE-UP trial came out earlier in 2018, we at the University of Cincinnati have been imaging acute ischemic stroke patients who presented outside the standard 4.5-hour time limit for thrombolysis with MRI to see if they qualify for an extended window for thrombolysis. But this has been a cumbersome and redundant process because our default imaging method is CT, so we have been imaging potential candidates for an extended thrombolytic window twice, first with CT and then later with MRI.
The EXTEND findings also provide a further reason for U.S. stroke centers to purchase and use some type of imaging processing software if they don’t already have it, either the RAPID software that was used in EXTEND or one of the several similar software packages that are now available. Several primary stroke centers in my area still do not currently use this software, although its use has been quickly spreading and it will now be increasingly hard for these centers to hold off acquiring it. Fortunately the increased competition among software vendors who sell this type of software has meant that the price has been dropping.
Pooja Khatri, MD , is a professor of neurology and director of acute stroke at the University of Cincinnati. She has been a consultant to Biogen, Greenwich, and PTC Therapeutics. She made these comments in an interview.
Ever since results from the WAKE-UP trial came out earlier in 2018, we at the University of Cincinnati have been imaging acute ischemic stroke patients who presented outside the standard 4.5-hour time limit for thrombolysis with MRI to see if they qualify for an extended window for thrombolysis. But this has been a cumbersome and redundant process because our default imaging method is CT, so we have been imaging potential candidates for an extended thrombolytic window twice, first with CT and then later with MRI.
The EXTEND findings also provide a further reason for U.S. stroke centers to purchase and use some type of imaging processing software if they don’t already have it, either the RAPID software that was used in EXTEND or one of the several similar software packages that are now available. Several primary stroke centers in my area still do not currently use this software, although its use has been quickly spreading and it will now be increasingly hard for these centers to hold off acquiring it. Fortunately the increased competition among software vendors who sell this type of software has meant that the price has been dropping.
Pooja Khatri, MD , is a professor of neurology and director of acute stroke at the University of Cincinnati. She has been a consultant to Biogen, Greenwich, and PTC Therapeutics. She made these comments in an interview.
MONTREAL – An extended time window for thrombolytic treatment of acute ischemic stroke patients using tissue plasminogen activator out to 9 hours from stroke onset was safe and effective using CT perfusion imaging and automated imaging processing software to select suitable patients in the EXTEND trial. This result matches the groundbreaking finding reported earlier in 2018 that used MRI to select patients for extended thrombolysis.
“To reproduce our results you need to set up CT perfusion” as well as the RAPID software for automated image processing to identify patients with a small infarct core and a large area of salvageable brain, said Henry Ma, MD, a stroke neurologist at Monash University, Melbourne, as he reported results from the trial at the World Stroke Congress. “EXTEND is the first positive thrombolysis trial in an extended time window using automated penumbral imaging.”
The new finding, from a trial with 225 randomized stroke patients, was especially notable because, by showing the validity of CT imaging for patient selection, it makes applying the extended time window for thrombolytic therapy more feasible for U.S. and Canadian stroke centers where CT imaging is much more common than MRI. A report from European investigators published in August 2018 from the WAKE-UP trial showed that thrombolysis with tissue plasminogen activator (tPA) was safe and effective when administered to patients who woke up with an acute ischemic stroke that had occurred more than 4.5 hours before treatment, but this study exclusively used MRI for patient selection (N Engl J Med. 2018 Aug 16;379[7]:611-22).
“In North America, our systems are more equipped for using CT,” commented Ashfaq Shuaib, MD, a professor of medicine and neurologist at the University of Alberta, Edmonton. Based on the WAKE-UP results, “MR would be preferred, but what we’ve been doing [since the WAKE-UP report] is if we see a CT scan that’s good we go ahead” with thrombolysis.
“Biologically, it doesn’t matter whether you use MR or CT; they both index the same underlying pathology. We’ve been hesitant to go beyond the MR finding from WAKE-UP, where there were data, but the findings from EXTEND were right in line with the WAKE-UP results, and that’s all we need to be reassured” that CT perfusion imaging also works for patient selection, commented Jeffrey L. Saver, MD, professor of medicine and director of the Comprehensive Stroke Center at the University of California, Los Angeles.
CT perfusion imaging and automated image processing “worked to select stroke patients” for an extended time window for treatment with mechanical thrombectomy in the DAWN (N Engl J Med. 2018 Jan 4;378[1]:11-21) and DEFUSE 3 (N Engl J Med. 2018 Feb 22;378[8]:308-18) trials, a history that makes the new finding of successfully using CT imaging to select patients who qualify for extended use of thrombolysis “a convincing result,” Dr. Saver said in an interview. The new EXTEND findings “will have a major impact” on using an extended time window for thrombolysis in U.S. practice, he predicted.
The EXTEND trial (Int J Stroke. 2012 Jan 1;7[1]:74-80) ran at 22 sites in Australia, 11 sites in Taiwan, and 1 center in New Zealand. Recruitment of patients into the study stopped early, after enrolling 225 patients, in June 2018, when results from WAKE-UP came out.
The EXTEND investigators enrolled patients who were either 4.5-9 hours out from the onset of their stroke or patients with a wake-up stroke with an uncertain onset. Participating centers could use either CT perfusion or MRI to identify candidates for treatment, and all used the RAPID software for image processing to identify patients with a perfusion lesion of at least 10 mL and an ischemic core volume no greater than 70 mL. Dr. Ma did not report what percentage of patients underwent imaging with each of these methods, but hinted that clinicians had used CT for a majority of the cases. The study randomized patients to receive either 0.9 mg/kg tPA or placebo, and by the trial protocol none of the enrolled patients received treatment with mechanical thrombectomy.
The trial’s primary endpoint was the percentage of patients with a modified Rankin Scale score of 0 or 1 at 90 days after their stroke, which was achieved by 44% more patients in the tPA group relative to the placebo arm after adjustment for age and baseline stroke severity, a statistically significant difference. The results were also positive for several secondary endpoints, such as recanalization 24 hours after treatment, which occurred in 67% of patients treated with tPA and 37% of the control patients, a statistically significant 68% relative improvement with thrombolysis.
Mortality at 90 days was similar in the two arms – 9% among the placebo patients and 12% among those who received tPA. The rate of symptomatic intracranial hemorrhage 36 hours after treatment was significantly higher among patients treated with tPA at 6%, compared with 1% in the placebo group, but the magnitude of this adverse effect was consistent with rates of intracranial hemorrhages previously reported in other studies of thrombolytic treatment for acute ischemic stroke, Dr. Ma said. The small number of increased intracranial hemorrhages “was not associated with increased mortality, and did not negate the positive result of an improved rate of excellent functional outcomes.”
These findings will likely spur further adoption of imaging processing software of the type used in EXTEND by U.S. stroke centers, Dr. Saver predicted.
“More and more centers have been getting this [software], and now they have two reasons to have it: to identify patients for an extended window for mechanical thrombectomy and to identify patients for an extended window for thrombolysis. It is a compelling case to have the imaging software as widely disseminated as possible. Centers that want to do the best for patients should have this imaging-processing software,” Dr. Saver said.
Dr. Ma and Dr. Shuaib reported no disclosures. Dr. Saver has received research funding and personal fees from Medtronic-Abbott and Neuravia.
SOURCE: Ma H et al. Int J. Stroke. 2018 Oct;13(2S):235, Abstract 1014.
MONTREAL – An extended time window for thrombolytic treatment of acute ischemic stroke patients using tissue plasminogen activator out to 9 hours from stroke onset was safe and effective using CT perfusion imaging and automated imaging processing software to select suitable patients in the EXTEND trial. This result matches the groundbreaking finding reported earlier in 2018 that used MRI to select patients for extended thrombolysis.
“To reproduce our results you need to set up CT perfusion” as well as the RAPID software for automated image processing to identify patients with a small infarct core and a large area of salvageable brain, said Henry Ma, MD, a stroke neurologist at Monash University, Melbourne, as he reported results from the trial at the World Stroke Congress. “EXTEND is the first positive thrombolysis trial in an extended time window using automated penumbral imaging.”
The new finding, from a trial with 225 randomized stroke patients, was especially notable because, by showing the validity of CT imaging for patient selection, it makes applying the extended time window for thrombolytic therapy more feasible for U.S. and Canadian stroke centers where CT imaging is much more common than MRI. A report from European investigators published in August 2018 from the WAKE-UP trial showed that thrombolysis with tissue plasminogen activator (tPA) was safe and effective when administered to patients who woke up with an acute ischemic stroke that had occurred more than 4.5 hours before treatment, but this study exclusively used MRI for patient selection (N Engl J Med. 2018 Aug 16;379[7]:611-22).
“In North America, our systems are more equipped for using CT,” commented Ashfaq Shuaib, MD, a professor of medicine and neurologist at the University of Alberta, Edmonton. Based on the WAKE-UP results, “MR would be preferred, but what we’ve been doing [since the WAKE-UP report] is if we see a CT scan that’s good we go ahead” with thrombolysis.
“Biologically, it doesn’t matter whether you use MR or CT; they both index the same underlying pathology. We’ve been hesitant to go beyond the MR finding from WAKE-UP, where there were data, but the findings from EXTEND were right in line with the WAKE-UP results, and that’s all we need to be reassured” that CT perfusion imaging also works for patient selection, commented Jeffrey L. Saver, MD, professor of medicine and director of the Comprehensive Stroke Center at the University of California, Los Angeles.
CT perfusion imaging and automated image processing “worked to select stroke patients” for an extended time window for treatment with mechanical thrombectomy in the DAWN (N Engl J Med. 2018 Jan 4;378[1]:11-21) and DEFUSE 3 (N Engl J Med. 2018 Feb 22;378[8]:308-18) trials, a history that makes the new finding of successfully using CT imaging to select patients who qualify for extended use of thrombolysis “a convincing result,” Dr. Saver said in an interview. The new EXTEND findings “will have a major impact” on using an extended time window for thrombolysis in U.S. practice, he predicted.
The EXTEND trial (Int J Stroke. 2012 Jan 1;7[1]:74-80) ran at 22 sites in Australia, 11 sites in Taiwan, and 1 center in New Zealand. Recruitment of patients into the study stopped early, after enrolling 225 patients, in June 2018, when results from WAKE-UP came out.
The EXTEND investigators enrolled patients who were either 4.5-9 hours out from the onset of their stroke or patients with a wake-up stroke with an uncertain onset. Participating centers could use either CT perfusion or MRI to identify candidates for treatment, and all used the RAPID software for image processing to identify patients with a perfusion lesion of at least 10 mL and an ischemic core volume no greater than 70 mL. Dr. Ma did not report what percentage of patients underwent imaging with each of these methods, but hinted that clinicians had used CT for a majority of the cases. The study randomized patients to receive either 0.9 mg/kg tPA or placebo, and by the trial protocol none of the enrolled patients received treatment with mechanical thrombectomy.
The trial’s primary endpoint was the percentage of patients with a modified Rankin Scale score of 0 or 1 at 90 days after their stroke, which was achieved by 44% more patients in the tPA group relative to the placebo arm after adjustment for age and baseline stroke severity, a statistically significant difference. The results were also positive for several secondary endpoints, such as recanalization 24 hours after treatment, which occurred in 67% of patients treated with tPA and 37% of the control patients, a statistically significant 68% relative improvement with thrombolysis.
Mortality at 90 days was similar in the two arms – 9% among the placebo patients and 12% among those who received tPA. The rate of symptomatic intracranial hemorrhage 36 hours after treatment was significantly higher among patients treated with tPA at 6%, compared with 1% in the placebo group, but the magnitude of this adverse effect was consistent with rates of intracranial hemorrhages previously reported in other studies of thrombolytic treatment for acute ischemic stroke, Dr. Ma said. The small number of increased intracranial hemorrhages “was not associated with increased mortality, and did not negate the positive result of an improved rate of excellent functional outcomes.”
These findings will likely spur further adoption of imaging processing software of the type used in EXTEND by U.S. stroke centers, Dr. Saver predicted.
“More and more centers have been getting this [software], and now they have two reasons to have it: to identify patients for an extended window for mechanical thrombectomy and to identify patients for an extended window for thrombolysis. It is a compelling case to have the imaging software as widely disseminated as possible. Centers that want to do the best for patients should have this imaging-processing software,” Dr. Saver said.
Dr. Ma and Dr. Shuaib reported no disclosures. Dr. Saver has received research funding and personal fees from Medtronic-Abbott and Neuravia.
SOURCE: Ma H et al. Int J. Stroke. 2018 Oct;13(2S):235, Abstract 1014.
REPORTING FROM THE WORLD STROKE CONGRESS
Key clinical point:
Major finding: Patients who received thrombolysis 4.5-9 hours after stroke onset had a 44% increased rate of good outcomes, compared with controls.
Study details: EXTEND, a multicenter, controlled trial with 225 patients.
Disclosures: Dr. Ma and Dr. Shuaib had no disclosures. Dr. Saver has received research funding and personal fees from Medtronic-Abbott and Neuravia.
Source: Ma H et al. Int J. Stroke. 2018 Oct;13(2S):235, Abstract 1014.













