User login
Smartphone Versus Holter Monitoring for Poststroke Atrial Fibrillation Detection
Although guidelines recommend Holter monitoring for all patients with ischemic stroke or TIA, comparatively few receive it.
MONTREAL—In patients hospitalized for a recent acute ischemic stroke or transient ischemic attack (TIA), a smartphone-based method identified three times more patients with atrial fibrillation than did 24-hour Holter monitoring after discharge, according to a study presented at the 11th World Stroke Congress.
This high level of atrial fibrillation detection suggests that this relatively cheap and noninvasive device is a good complement to conventional monitoring by a 24-hour Holter recording or an implanted loop recorder in patients with recent stroke. The device may thus satisfy the requirements of current guidelines from the world’s cardiology societies.
In-Hospital and Postdischarge Monitoring
In the study, 294 of 1,079 patients with acute ischemic stroke or TIA underwent serial, 30-second monitoring with the AliveCor device while hospitalized. The device was designed for smartphone-enabled ECG measurement. After discharge, the same patients underwent Holter monitoring. The latter technique identified eight patients (3%) with atrial fibrillation, compared with 25 patients (9%) who were identified using the AliveCor device, said Bernard Yan, MD, a consultant neurologist and endovascular neurointerventionist in the Comprehensive Stroke Center of the Royal Melbourne Hospital. Seven of the eight patients identified with atrial fibrillation by Holter monitoring were also found to have atrial fibrillation by the AliveCor device.
Dr. Yan attributed the higher in-hospital detection rate for atrial fibrillation to the timing of screening, which occurred within days of the stroke or TIA, rather than after the patient had left the hospital. “The difference may be because we monitored patients [with the AliveCor device] much earlier, during their hot period right after their stroke.”
Practice Does Not Match Recommendations
The trial, which was called SPOT-AF, was conducted at several centers in Australia, China, and Hong Kong. All patients underwent AliveCor monitoring during their stay in the hospital, which lasted for a median of four days. Patients performed a 30-second heart rhythm check every time a nurse saw them for a routine vital-sign examination, which usually was three or four times per day. The current analysis focused on the 294 patients (27% of the 1,079 patients) who also underwent 24-hour Holter monitoring following hospital discharge when ordered by their personal physician.
This 27% rate of postdischarge Holter monitoring was consistent with that of a 2016 review of more than 17,000 patients with stroke or TIA in Canada. That study found that 31% of participants underwent 24-hour Holter monitoring for atrial fibrillation during the 30 days following their index event. Guidelines, however, call for atrial fibrillation screening in all patients with recent ischemic stroke and TIA.
Although screening for atrial fibrillation with a smartphone-based device is inexpensive and easy, Dr. Yan did not suggest that this approach could replace a Holter monitor or an implanted loop recorder, which is what current guidelines recommend. “To change the guidelines, we need a different study that compares these approaches head to head.”
SPOT-AF received partial funding from Boehringer Ingelheim. Dr. Yan has spoken on behalf of Bayer, Boehringer Ingelheim, Pfizer, and Stryker.
—Mitchel L. Zoler
Suggested Reading
Edwards JD, Kapral MK, Fang J, et al. Underutilization of ambulatory ECG monitoring after stroke and transient ischemic attack: missed opportunities for atrial fibrillation detection. Stroke. 2016;47(8):1982-1989.
Tu HT, Chen Z, Swift C, et al. Smartphone electrographic monitoring for atrial fibrillation in acute ischemic stroke and transient ischemic attack. Int J Stroke. 2017;12(7):786-789.
Although guidelines recommend Holter monitoring for all patients with ischemic stroke or TIA, comparatively few receive it.
Although guidelines recommend Holter monitoring for all patients with ischemic stroke or TIA, comparatively few receive it.
MONTREAL—In patients hospitalized for a recent acute ischemic stroke or transient ischemic attack (TIA), a smartphone-based method identified three times more patients with atrial fibrillation than did 24-hour Holter monitoring after discharge, according to a study presented at the 11th World Stroke Congress.
This high level of atrial fibrillation detection suggests that this relatively cheap and noninvasive device is a good complement to conventional monitoring by a 24-hour Holter recording or an implanted loop recorder in patients with recent stroke. The device may thus satisfy the requirements of current guidelines from the world’s cardiology societies.
In-Hospital and Postdischarge Monitoring
In the study, 294 of 1,079 patients with acute ischemic stroke or TIA underwent serial, 30-second monitoring with the AliveCor device while hospitalized. The device was designed for smartphone-enabled ECG measurement. After discharge, the same patients underwent Holter monitoring. The latter technique identified eight patients (3%) with atrial fibrillation, compared with 25 patients (9%) who were identified using the AliveCor device, said Bernard Yan, MD, a consultant neurologist and endovascular neurointerventionist in the Comprehensive Stroke Center of the Royal Melbourne Hospital. Seven of the eight patients identified with atrial fibrillation by Holter monitoring were also found to have atrial fibrillation by the AliveCor device.
Dr. Yan attributed the higher in-hospital detection rate for atrial fibrillation to the timing of screening, which occurred within days of the stroke or TIA, rather than after the patient had left the hospital. “The difference may be because we monitored patients [with the AliveCor device] much earlier, during their hot period right after their stroke.”
Practice Does Not Match Recommendations
The trial, which was called SPOT-AF, was conducted at several centers in Australia, China, and Hong Kong. All patients underwent AliveCor monitoring during their stay in the hospital, which lasted for a median of four days. Patients performed a 30-second heart rhythm check every time a nurse saw them for a routine vital-sign examination, which usually was three or four times per day. The current analysis focused on the 294 patients (27% of the 1,079 patients) who also underwent 24-hour Holter monitoring following hospital discharge when ordered by their personal physician.
This 27% rate of postdischarge Holter monitoring was consistent with that of a 2016 review of more than 17,000 patients with stroke or TIA in Canada. That study found that 31% of participants underwent 24-hour Holter monitoring for atrial fibrillation during the 30 days following their index event. Guidelines, however, call for atrial fibrillation screening in all patients with recent ischemic stroke and TIA.
Although screening for atrial fibrillation with a smartphone-based device is inexpensive and easy, Dr. Yan did not suggest that this approach could replace a Holter monitor or an implanted loop recorder, which is what current guidelines recommend. “To change the guidelines, we need a different study that compares these approaches head to head.”
SPOT-AF received partial funding from Boehringer Ingelheim. Dr. Yan has spoken on behalf of Bayer, Boehringer Ingelheim, Pfizer, and Stryker.
—Mitchel L. Zoler
Suggested Reading
Edwards JD, Kapral MK, Fang J, et al. Underutilization of ambulatory ECG monitoring after stroke and transient ischemic attack: missed opportunities for atrial fibrillation detection. Stroke. 2016;47(8):1982-1989.
Tu HT, Chen Z, Swift C, et al. Smartphone electrographic monitoring for atrial fibrillation in acute ischemic stroke and transient ischemic attack. Int J Stroke. 2017;12(7):786-789.
MONTREAL—In patients hospitalized for a recent acute ischemic stroke or transient ischemic attack (TIA), a smartphone-based method identified three times more patients with atrial fibrillation than did 24-hour Holter monitoring after discharge, according to a study presented at the 11th World Stroke Congress.
This high level of atrial fibrillation detection suggests that this relatively cheap and noninvasive device is a good complement to conventional monitoring by a 24-hour Holter recording or an implanted loop recorder in patients with recent stroke. The device may thus satisfy the requirements of current guidelines from the world’s cardiology societies.
In-Hospital and Postdischarge Monitoring
In the study, 294 of 1,079 patients with acute ischemic stroke or TIA underwent serial, 30-second monitoring with the AliveCor device while hospitalized. The device was designed for smartphone-enabled ECG measurement. After discharge, the same patients underwent Holter monitoring. The latter technique identified eight patients (3%) with atrial fibrillation, compared with 25 patients (9%) who were identified using the AliveCor device, said Bernard Yan, MD, a consultant neurologist and endovascular neurointerventionist in the Comprehensive Stroke Center of the Royal Melbourne Hospital. Seven of the eight patients identified with atrial fibrillation by Holter monitoring were also found to have atrial fibrillation by the AliveCor device.
Dr. Yan attributed the higher in-hospital detection rate for atrial fibrillation to the timing of screening, which occurred within days of the stroke or TIA, rather than after the patient had left the hospital. “The difference may be because we monitored patients [with the AliveCor device] much earlier, during their hot period right after their stroke.”
Practice Does Not Match Recommendations
The trial, which was called SPOT-AF, was conducted at several centers in Australia, China, and Hong Kong. All patients underwent AliveCor monitoring during their stay in the hospital, which lasted for a median of four days. Patients performed a 30-second heart rhythm check every time a nurse saw them for a routine vital-sign examination, which usually was three or four times per day. The current analysis focused on the 294 patients (27% of the 1,079 patients) who also underwent 24-hour Holter monitoring following hospital discharge when ordered by their personal physician.
This 27% rate of postdischarge Holter monitoring was consistent with that of a 2016 review of more than 17,000 patients with stroke or TIA in Canada. That study found that 31% of participants underwent 24-hour Holter monitoring for atrial fibrillation during the 30 days following their index event. Guidelines, however, call for atrial fibrillation screening in all patients with recent ischemic stroke and TIA.
Although screening for atrial fibrillation with a smartphone-based device is inexpensive and easy, Dr. Yan did not suggest that this approach could replace a Holter monitor or an implanted loop recorder, which is what current guidelines recommend. “To change the guidelines, we need a different study that compares these approaches head to head.”
SPOT-AF received partial funding from Boehringer Ingelheim. Dr. Yan has spoken on behalf of Bayer, Boehringer Ingelheim, Pfizer, and Stryker.
—Mitchel L. Zoler
Suggested Reading
Edwards JD, Kapral MK, Fang J, et al. Underutilization of ambulatory ECG monitoring after stroke and transient ischemic attack: missed opportunities for atrial fibrillation detection. Stroke. 2016;47(8):1982-1989.
Tu HT, Chen Z, Swift C, et al. Smartphone electrographic monitoring for atrial fibrillation in acute ischemic stroke and transient ischemic attack. Int J Stroke. 2017;12(7):786-789.
Data support revising ASCVD cardiovascular risk threshold
MUNICH – Revising the threshold for actionable high cardiovascular risk from the current 7.5% or greater risk of an event within 10 years as defined in American College of Cardiology/American Heart Association guidelines using the Atherosclerotic Cardiovascular Disease (ASCVD ) Risk Calculator to a 10% or greater 10-year risk would provide the optimal balance of sensitivity and specificity for discriminating future risk of cardiovascular events, according to Robert S. Rosenman, MD.
“I think this is very important from a public health policy perspective,” Dr. Rosenman, a cardiologist who is professor of medicine at Mount Sinai School of Medicine in New York, said at the annual congress of the European Society of Cardiology.
He elaborated: “This would eliminate 11.4 million people who are currently candidates for a statin but may not be getting the benefits of statin therapy. We feel that this information is actually quite important for the primary prevention population because there’s been a lot of pushback from our primary care physician colleagues about the overtreatment of low-risk individuals” under the current guidelines (Circulation. 2014 Jun 24;129[25 Suppl 2]:S49-73).
Dr. Rosenman and his coinvestigators conducted a secondary analysis of data on 21,343 adults in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study. All participants were free of a baseline history of heart disease or stroke. During a median 8.5 years of follow-up, 1,717 of them experienced adjudicated coronary heart disease or stroke events.
In multivariate analyses adjusted for standard cardiovascular risk factors, socioeconomic and demographic factors, and the use of statins and/or antihypertensive drugs, the higher the baseline 10-year predicted risk using the ACC/AHA ASCVD Risk Calculator based on the Pooled Cohort risk equations, the higher the incidence rate of cardiovascular events. No surprise there.
What was impressive, however, was that the optimal combination of sensitivity and specificity as captured in a statistic known as Youden’s index occurred at a 10-year predicted risk of 10%-12%. The biggest net improvement obtained through reclassification resulted from moving the threshold for elevated 10-year cardiovascular risk warranting statin therapy from 7.5% or greater to 10% or more, rather than using thresholds of 15% or 20%.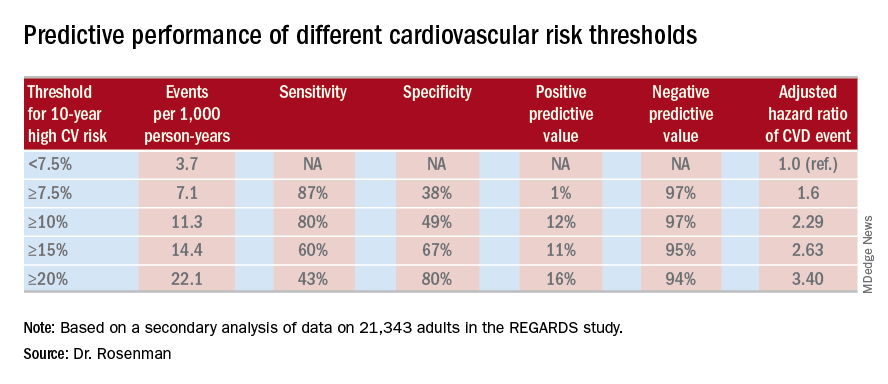
He cited data from the 2011-2014 National Health and Nutrition Examination Survey in support of his estimate that switching to a 10% threshold from the current 7.5% threshold would reduce the number of Americans deemed at high cardiovascular risk from 57.1 million to 45.8 million.
“This cutoff value of 10%, by the way, is the same cutoff value used in the recently published ACC/AHA guideline on hypertension. And it’s also the same cutoff value used for antiplatelet therapy in looking at the benefit/risk ratio. So this value of 10% is, I think, really the right number. Our study is the first effort that has been shown to validate that number, and it brings the cutoff values in the various guidelines in line,” the cardiologist observed.
Asked if these new findings are likely to result in a revision of the ACC/AHA cardiovascular risk assessment guidelines, Dr. Rosenman replied that the guidelines are under revision, with the draft update now circulating for comment. So the timing is dicey: His study is now in prepublication peer review, but hasn’t yet been published and thus may not carry persuasive weight.
“Hopefully, the guideline panel is going to make an adjustment to make the 10% figure in line with the blood pressure guidelines,” he said.
The new analysis of the REGARDS study was funded by a collaboration between Amgen, Mount Sinai School of Medicine, and the University of Alabama. Dr. Rosenman reported receiving research funding from and serving as an advisor to Amgen and a handful of other companies.
MUNICH – Revising the threshold for actionable high cardiovascular risk from the current 7.5% or greater risk of an event within 10 years as defined in American College of Cardiology/American Heart Association guidelines using the Atherosclerotic Cardiovascular Disease (ASCVD ) Risk Calculator to a 10% or greater 10-year risk would provide the optimal balance of sensitivity and specificity for discriminating future risk of cardiovascular events, according to Robert S. Rosenman, MD.
“I think this is very important from a public health policy perspective,” Dr. Rosenman, a cardiologist who is professor of medicine at Mount Sinai School of Medicine in New York, said at the annual congress of the European Society of Cardiology.
He elaborated: “This would eliminate 11.4 million people who are currently candidates for a statin but may not be getting the benefits of statin therapy. We feel that this information is actually quite important for the primary prevention population because there’s been a lot of pushback from our primary care physician colleagues about the overtreatment of low-risk individuals” under the current guidelines (Circulation. 2014 Jun 24;129[25 Suppl 2]:S49-73).
Dr. Rosenman and his coinvestigators conducted a secondary analysis of data on 21,343 adults in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study. All participants were free of a baseline history of heart disease or stroke. During a median 8.5 years of follow-up, 1,717 of them experienced adjudicated coronary heart disease or stroke events.
In multivariate analyses adjusted for standard cardiovascular risk factors, socioeconomic and demographic factors, and the use of statins and/or antihypertensive drugs, the higher the baseline 10-year predicted risk using the ACC/AHA ASCVD Risk Calculator based on the Pooled Cohort risk equations, the higher the incidence rate of cardiovascular events. No surprise there.
What was impressive, however, was that the optimal combination of sensitivity and specificity as captured in a statistic known as Youden’s index occurred at a 10-year predicted risk of 10%-12%. The biggest net improvement obtained through reclassification resulted from moving the threshold for elevated 10-year cardiovascular risk warranting statin therapy from 7.5% or greater to 10% or more, rather than using thresholds of 15% or 20%.
He cited data from the 2011-2014 National Health and Nutrition Examination Survey in support of his estimate that switching to a 10% threshold from the current 7.5% threshold would reduce the number of Americans deemed at high cardiovascular risk from 57.1 million to 45.8 million.
“This cutoff value of 10%, by the way, is the same cutoff value used in the recently published ACC/AHA guideline on hypertension. And it’s also the same cutoff value used for antiplatelet therapy in looking at the benefit/risk ratio. So this value of 10% is, I think, really the right number. Our study is the first effort that has been shown to validate that number, and it brings the cutoff values in the various guidelines in line,” the cardiologist observed.
Asked if these new findings are likely to result in a revision of the ACC/AHA cardiovascular risk assessment guidelines, Dr. Rosenman replied that the guidelines are under revision, with the draft update now circulating for comment. So the timing is dicey: His study is now in prepublication peer review, but hasn’t yet been published and thus may not carry persuasive weight.
“Hopefully, the guideline panel is going to make an adjustment to make the 10% figure in line with the blood pressure guidelines,” he said.
The new analysis of the REGARDS study was funded by a collaboration between Amgen, Mount Sinai School of Medicine, and the University of Alabama. Dr. Rosenman reported receiving research funding from and serving as an advisor to Amgen and a handful of other companies.
MUNICH – Revising the threshold for actionable high cardiovascular risk from the current 7.5% or greater risk of an event within 10 years as defined in American College of Cardiology/American Heart Association guidelines using the Atherosclerotic Cardiovascular Disease (ASCVD ) Risk Calculator to a 10% or greater 10-year risk would provide the optimal balance of sensitivity and specificity for discriminating future risk of cardiovascular events, according to Robert S. Rosenman, MD.
“I think this is very important from a public health policy perspective,” Dr. Rosenman, a cardiologist who is professor of medicine at Mount Sinai School of Medicine in New York, said at the annual congress of the European Society of Cardiology.
He elaborated: “This would eliminate 11.4 million people who are currently candidates for a statin but may not be getting the benefits of statin therapy. We feel that this information is actually quite important for the primary prevention population because there’s been a lot of pushback from our primary care physician colleagues about the overtreatment of low-risk individuals” under the current guidelines (Circulation. 2014 Jun 24;129[25 Suppl 2]:S49-73).
Dr. Rosenman and his coinvestigators conducted a secondary analysis of data on 21,343 adults in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study. All participants were free of a baseline history of heart disease or stroke. During a median 8.5 years of follow-up, 1,717 of them experienced adjudicated coronary heart disease or stroke events.
In multivariate analyses adjusted for standard cardiovascular risk factors, socioeconomic and demographic factors, and the use of statins and/or antihypertensive drugs, the higher the baseline 10-year predicted risk using the ACC/AHA ASCVD Risk Calculator based on the Pooled Cohort risk equations, the higher the incidence rate of cardiovascular events. No surprise there.
What was impressive, however, was that the optimal combination of sensitivity and specificity as captured in a statistic known as Youden’s index occurred at a 10-year predicted risk of 10%-12%. The biggest net improvement obtained through reclassification resulted from moving the threshold for elevated 10-year cardiovascular risk warranting statin therapy from 7.5% or greater to 10% or more, rather than using thresholds of 15% or 20%.
He cited data from the 2011-2014 National Health and Nutrition Examination Survey in support of his estimate that switching to a 10% threshold from the current 7.5% threshold would reduce the number of Americans deemed at high cardiovascular risk from 57.1 million to 45.8 million.
“This cutoff value of 10%, by the way, is the same cutoff value used in the recently published ACC/AHA guideline on hypertension. And it’s also the same cutoff value used for antiplatelet therapy in looking at the benefit/risk ratio. So this value of 10% is, I think, really the right number. Our study is the first effort that has been shown to validate that number, and it brings the cutoff values in the various guidelines in line,” the cardiologist observed.
Asked if these new findings are likely to result in a revision of the ACC/AHA cardiovascular risk assessment guidelines, Dr. Rosenman replied that the guidelines are under revision, with the draft update now circulating for comment. So the timing is dicey: His study is now in prepublication peer review, but hasn’t yet been published and thus may not carry persuasive weight.
“Hopefully, the guideline panel is going to make an adjustment to make the 10% figure in line with the blood pressure guidelines,” he said.
The new analysis of the REGARDS study was funded by a collaboration between Amgen, Mount Sinai School of Medicine, and the University of Alabama. Dr. Rosenman reported receiving research funding from and serving as an advisor to Amgen and a handful of other companies.
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point:
Major finding: Redefining the threshold for high 10-year cardiovascular risk from the current 7.5% to 10% would reduce the number of Americans warranting statin therapy by 11.4 million.
Study details: This was a secondary analysis of data on 21,343 adults in the REGARDS study, 1,717 of whom experienced coronary heart disease or stroke events during a median 8.5 years of prospective follow-up.
Disclosures: The new analysis of the REGARDS study was funded by a collaboration between Amgen, Mount Sinai School of Medicine, and the University of Alabama. The presenter reported ties to Amgen and a handful of other companies.
Canagliflozin approved for cardiovascular event risk reduction
The Food and Drug Administration has approved canagliflozin (Invokana) as a way to reduce the risk of major adverse cardiovascular events in patients with type 2 diabetes and cardiovascular disease, according to Janssen Pharmaceuticals.
The sodium–glucose cotransporter 2 inhibitor was first approved in 2013 to improve glycemic control in adults with type 2 diabetes.
FDA approval was based on results from the CANVAS (Canagliflozin Cardiovascular Assessment Study) trial, which included more than 10,000 adults with type 2 diabetes who either had cardiovascular disease or were at risk for cardiovascular disease. Overall, patients who received canagliflozin had a 14% lower risk of experiencing a major cardiovascular event over the control group, and patients with established cardiovascular disease had an 18% lower risk.
The most common adverse events associated with canagliflozin include female genital mycotic infections, urinary tract infection, and increased urination. Notably, canagliflozin also increases the risk of lower-extremity amputation, especially in those with a history of amputation.
“Americans living with type 2 diabetes are two to three times more likely to die from heart disease than adults without diabetes. With this approval, Invokana now plays an even more important role in the overall treatment mix with its demonstrated ability to reduce the risk of potentially devastating cardiovascular events,” Ralph A. DeFronzo, MD, professor and division chief of medicine and diabetes at the University of Texas, San Antonio, said in the press release.
The new indication applies to all formulations of canagliflozin.
Find the full press release on the Janssen website.
The Food and Drug Administration has approved canagliflozin (Invokana) as a way to reduce the risk of major adverse cardiovascular events in patients with type 2 diabetes and cardiovascular disease, according to Janssen Pharmaceuticals.
The sodium–glucose cotransporter 2 inhibitor was first approved in 2013 to improve glycemic control in adults with type 2 diabetes.
FDA approval was based on results from the CANVAS (Canagliflozin Cardiovascular Assessment Study) trial, which included more than 10,000 adults with type 2 diabetes who either had cardiovascular disease or were at risk for cardiovascular disease. Overall, patients who received canagliflozin had a 14% lower risk of experiencing a major cardiovascular event over the control group, and patients with established cardiovascular disease had an 18% lower risk.
The most common adverse events associated with canagliflozin include female genital mycotic infections, urinary tract infection, and increased urination. Notably, canagliflozin also increases the risk of lower-extremity amputation, especially in those with a history of amputation.
“Americans living with type 2 diabetes are two to three times more likely to die from heart disease than adults without diabetes. With this approval, Invokana now plays an even more important role in the overall treatment mix with its demonstrated ability to reduce the risk of potentially devastating cardiovascular events,” Ralph A. DeFronzo, MD, professor and division chief of medicine and diabetes at the University of Texas, San Antonio, said in the press release.
The new indication applies to all formulations of canagliflozin.
Find the full press release on the Janssen website.
The Food and Drug Administration has approved canagliflozin (Invokana) as a way to reduce the risk of major adverse cardiovascular events in patients with type 2 diabetes and cardiovascular disease, according to Janssen Pharmaceuticals.
The sodium–glucose cotransporter 2 inhibitor was first approved in 2013 to improve glycemic control in adults with type 2 diabetes.
FDA approval was based on results from the CANVAS (Canagliflozin Cardiovascular Assessment Study) trial, which included more than 10,000 adults with type 2 diabetes who either had cardiovascular disease or were at risk for cardiovascular disease. Overall, patients who received canagliflozin had a 14% lower risk of experiencing a major cardiovascular event over the control group, and patients with established cardiovascular disease had an 18% lower risk.
The most common adverse events associated with canagliflozin include female genital mycotic infections, urinary tract infection, and increased urination. Notably, canagliflozin also increases the risk of lower-extremity amputation, especially in those with a history of amputation.
“Americans living with type 2 diabetes are two to three times more likely to die from heart disease than adults without diabetes. With this approval, Invokana now plays an even more important role in the overall treatment mix with its demonstrated ability to reduce the risk of potentially devastating cardiovascular events,” Ralph A. DeFronzo, MD, professor and division chief of medicine and diabetes at the University of Texas, San Antonio, said in the press release.
The new indication applies to all formulations of canagliflozin.
Find the full press release on the Janssen website.
Stroke risk in elderly following AMI extends to 12 weeks
ATLANTA – Acute myocardial infarction is associated with a risk of stroke that extends beyond the 1-month time window currently considered the at-risk period, according to an analysis of Medicare data.
“The results of our study may allow clinicians to more accurately counsel patients regarding their stroke etiology and may allow refinement of stroke etiology classification systems and clinical trial selection criteria,” lead study author Alexander E. Merkler, MD, said in an interview in advance of the annual meeting of the American Neurological Association.
In an effort to better understand the duration of heightened stroke risk after acute myocardial infarction, Dr. Merkler, a neurologist at New York–based Weill Cornell Medicine, and his colleagues conducted a retrospective cohort study using inpatient and outpatient claims during 2008-2015 from a nationally representative 5% sample of Medicare beneficiaries who were at least 66 years old. They used previously validated ICD-9-CM diagnosis codes to ascertain the exposure variable of acute MI and the outcome of ischemic stroke but excluded strokes that occurred during an acute MI hospitalization.
Patients were censored at the time of ischemic stroke, death, end of Medicare coverage, or by Sept. 30, 2015. The researchers fit Cox regression models separately for the groups with and without acute MI to examine its association with ischemic stroke after adjusting for demographics, stroke risk factors, and Charlson comorbidities. Next, they used the corresponding survival probabilities to compute the hazard ratio (HR) in each 4-week interval after discharge, up to week 12. They also conducted a subgroup analysis to evaluate the duration of heightened ischemic stroke risk by MI type: ST-segment elevation MI (STEMI) versus non-STEMI (NSTEMI).
Dr. Merkler and his colleagues drew from data on 1.7 million eligible beneficiaries. Of these, 46,182 were hospitalized for acute MI and 80,466 for ischemic stroke. After they adjusted for demographics, stroke risk factors, and Charlson comorbidities, the researchers found that the risk of ischemic stroke was highest in the first 4 weeks after discharge from the MI hospitalization (HR, 2.7), yet remained elevated during weeks 5-8 (HR, 2.0) and weeks 9-12 (HR, 1.6). It was no longer significantly elevated afterward. The prolonged period of heightened ischemic stroke risk was evident in patients with both STEMI and NSTEMI.
“We were surprised by how long the risk of stroke lasts after MI,” Dr. Merkler said. He acknowledged certain limitations of the analysis, including the fact that patients were all over the age of 65 years. “In addition, we lack granular detail such as severity of MI [and] the extent of stroke work-up,” he said.
Dr. Merkler disclosed that he is supported by a grant from the National Institutes of Health and by the Leon Levy Foundation in Neuroscience. Most of his coauthors are also supported by NIH grants.
[email protected]
Source: Ann Neurol. 2018;84[S22]:S146-7, Abstract M122.
ATLANTA – Acute myocardial infarction is associated with a risk of stroke that extends beyond the 1-month time window currently considered the at-risk period, according to an analysis of Medicare data.
“The results of our study may allow clinicians to more accurately counsel patients regarding their stroke etiology and may allow refinement of stroke etiology classification systems and clinical trial selection criteria,” lead study author Alexander E. Merkler, MD, said in an interview in advance of the annual meeting of the American Neurological Association.
In an effort to better understand the duration of heightened stroke risk after acute myocardial infarction, Dr. Merkler, a neurologist at New York–based Weill Cornell Medicine, and his colleagues conducted a retrospective cohort study using inpatient and outpatient claims during 2008-2015 from a nationally representative 5% sample of Medicare beneficiaries who were at least 66 years old. They used previously validated ICD-9-CM diagnosis codes to ascertain the exposure variable of acute MI and the outcome of ischemic stroke but excluded strokes that occurred during an acute MI hospitalization.
Patients were censored at the time of ischemic stroke, death, end of Medicare coverage, or by Sept. 30, 2015. The researchers fit Cox regression models separately for the groups with and without acute MI to examine its association with ischemic stroke after adjusting for demographics, stroke risk factors, and Charlson comorbidities. Next, they used the corresponding survival probabilities to compute the hazard ratio (HR) in each 4-week interval after discharge, up to week 12. They also conducted a subgroup analysis to evaluate the duration of heightened ischemic stroke risk by MI type: ST-segment elevation MI (STEMI) versus non-STEMI (NSTEMI).
Dr. Merkler and his colleagues drew from data on 1.7 million eligible beneficiaries. Of these, 46,182 were hospitalized for acute MI and 80,466 for ischemic stroke. After they adjusted for demographics, stroke risk factors, and Charlson comorbidities, the researchers found that the risk of ischemic stroke was highest in the first 4 weeks after discharge from the MI hospitalization (HR, 2.7), yet remained elevated during weeks 5-8 (HR, 2.0) and weeks 9-12 (HR, 1.6). It was no longer significantly elevated afterward. The prolonged period of heightened ischemic stroke risk was evident in patients with both STEMI and NSTEMI.
“We were surprised by how long the risk of stroke lasts after MI,” Dr. Merkler said. He acknowledged certain limitations of the analysis, including the fact that patients were all over the age of 65 years. “In addition, we lack granular detail such as severity of MI [and] the extent of stroke work-up,” he said.
Dr. Merkler disclosed that he is supported by a grant from the National Institutes of Health and by the Leon Levy Foundation in Neuroscience. Most of his coauthors are also supported by NIH grants.
[email protected]
Source: Ann Neurol. 2018;84[S22]:S146-7, Abstract M122.
ATLANTA – Acute myocardial infarction is associated with a risk of stroke that extends beyond the 1-month time window currently considered the at-risk period, according to an analysis of Medicare data.
“The results of our study may allow clinicians to more accurately counsel patients regarding their stroke etiology and may allow refinement of stroke etiology classification systems and clinical trial selection criteria,” lead study author Alexander E. Merkler, MD, said in an interview in advance of the annual meeting of the American Neurological Association.
In an effort to better understand the duration of heightened stroke risk after acute myocardial infarction, Dr. Merkler, a neurologist at New York–based Weill Cornell Medicine, and his colleagues conducted a retrospective cohort study using inpatient and outpatient claims during 2008-2015 from a nationally representative 5% sample of Medicare beneficiaries who were at least 66 years old. They used previously validated ICD-9-CM diagnosis codes to ascertain the exposure variable of acute MI and the outcome of ischemic stroke but excluded strokes that occurred during an acute MI hospitalization.
Patients were censored at the time of ischemic stroke, death, end of Medicare coverage, or by Sept. 30, 2015. The researchers fit Cox regression models separately for the groups with and without acute MI to examine its association with ischemic stroke after adjusting for demographics, stroke risk factors, and Charlson comorbidities. Next, they used the corresponding survival probabilities to compute the hazard ratio (HR) in each 4-week interval after discharge, up to week 12. They also conducted a subgroup analysis to evaluate the duration of heightened ischemic stroke risk by MI type: ST-segment elevation MI (STEMI) versus non-STEMI (NSTEMI).
Dr. Merkler and his colleagues drew from data on 1.7 million eligible beneficiaries. Of these, 46,182 were hospitalized for acute MI and 80,466 for ischemic stroke. After they adjusted for demographics, stroke risk factors, and Charlson comorbidities, the researchers found that the risk of ischemic stroke was highest in the first 4 weeks after discharge from the MI hospitalization (HR, 2.7), yet remained elevated during weeks 5-8 (HR, 2.0) and weeks 9-12 (HR, 1.6). It was no longer significantly elevated afterward. The prolonged period of heightened ischemic stroke risk was evident in patients with both STEMI and NSTEMI.
“We were surprised by how long the risk of stroke lasts after MI,” Dr. Merkler said. He acknowledged certain limitations of the analysis, including the fact that patients were all over the age of 65 years. “In addition, we lack granular detail such as severity of MI [and] the extent of stroke work-up,” he said.
Dr. Merkler disclosed that he is supported by a grant from the National Institutes of Health and by the Leon Levy Foundation in Neuroscience. Most of his coauthors are also supported by NIH grants.
[email protected]
Source: Ann Neurol. 2018;84[S22]:S146-7, Abstract M122.
AT ANA 2018
Key clinical point: .
Major finding: The risk of ischemic stroke was highest in the first 4 weeks after discharge from the MI hospitalization (HR, 2.7), yet remained elevated during weeks 5-8 (HR, 2.0) and weeks 9-12 (HR, 1.6).
Study details: An analysis of 46,182 Medicare beneficiaries who were hospitalized for acute MI and 80,466 who were hospitalized for ischemic stroke.
Disclosures: Dr. Merkler disclosed that he is supported by a grant from the National Institutes of Health and by the Leon Levy Foundation in Neuroscience. Most of his coauthors are also supported by NIH grants.
Source: Ann Neurol. 2018;84[S22]:S146-7, Abstract M122.
Readmission to non-index hospital following acute stroke linked to worse outcomes
ATLANTA – Following an acute stroke, optimizing stroke secondary prevention measures, medical complications, and transitions of care is essential to reducing 30-day readmissions and improving patient outcomes, a large analysis of national data showed.
“Care that is fragmented with readmissions to other hospitals results not only in more expensive care and longer length of stay but also increased mortality for our acute stroke patients,” lead study author Laura K. Stein, MD, said in an interview in advance of the annual meeting of the American Neurological Association.
In 2017, a study of the Nationwide Readmissions Database demonstrated that 12.1% of patients with acute ischemic stroke were readmitted within 30 days (Stroke 2017;48:1386-8). It cited that 89.6% were unplanned and 12.9% were preventable. “However, this study did not examine whether patients were admitted to the discharging hospital or a different hospital,” said Dr. Stein, a neurologist at the Icahn School of Medicine at Mount Sinai, New York. “Furthermore, it did not include metrics such as cost, length of stay, and mortality with 30-day readmissions. Hospitals are increasingly held accountable and penalized for metrics such as length of stay and 30-day readmissions.”
In 2010, the Centers for Medicare & Medicaid Services introduced the Hospital Readmissions Reduction Program in an attempt to decrease readmissions following hospitalizations for acute myocardial infarction, heart failure, and pneumonia. “In 2012, CMS started reducing Medicare payments for hospitals with excess readmissions,” said Dr. Stein, who is a fellowship-trained stroke specialist. “While readmission to the same hospital has great implications for hospital systems, any readmission has great implications for patients.”
In what is believed to be the first study of its kind, Dr. Stein and her colleagues drew from the 2013 Nationwide Readmissions Database to examine in-hospital outcomes associated with 30-day readmission to a different hospital for acute ischemic stroke. They used ICD-9 codes to identify index stroke admissions and all-cause readmissions. Outcomes of interest were length of stay, total charges, and in-hospital mortality during the 30-day readmission. The main predictor was readmission to another hospital, compared with readmission to the same hospital as the index acute stroke admission. The researchers used linear regression for the outcomes of length of stay and charges, and logistic regression for in-hospital mortality. They adjusted for several variables during the index admission, including age, sex, vascular risk factors, hospital bed size, teaching hospital status, insurance status, discharge destination, National Center for Health Statistics urban-rural location classification, length of stay, and total charges.
Of 24,545 acute stroke patients readmitted within 30 days, 7,274 (30%) were readmitted to a different hospital. The top three reasons for readmission were acute cerebrovascular disease, septicemia, and renal failure. In fully adjusted models, readmission to a different hospital was associated with an increased length of stay of 0.97 days (P less than .0001) and a mean of $7,677.28 greater total charges, compared with readmission to the same hospital (P less than .0001). The fully adjusted odds ratio for in-hospital mortality during readmission was 1.17 for readmission to another hospital vs. readmission to the same hospital (P = .0079).
“While it is conceivable that cost and length of stay could be higher with readmission to a different hospital because of a need for additional testing with a lack of familiarity with the patient, it is concerning that mortality is higher,” Dr. Stein said. “These findings emphasize the importance of optimizing secondary stroke prevention and medical complications following acute stroke before discharge. Additionally, they emphasize the importance of good transitions of care from the inpatient to outpatient setting (whether that’s to a rehabilitation facility, skilled nursing facility, or home) and accessibility of the discharging stroke team after discharge.”
She acknowledged certain limitations of the analysis, including its reliance of administrative data, which could include misclassification of diagnoses and comorbidities based on ICD-9 codes. “However, we have chosen ICD-9 codes for stroke that have been previously validated in the literature,” Dr. Stein said. “For instance, the validated codes for stroke as the primary discharge diagnosis have a sensitivity of 74%, specificity of 95%, and positive predictive value of 88%. Second, we do not know stroke subtype or severity of stroke. Third, we do not know what the transitions of care plan were when the patients left the hospital following index acute ischemic stroke admission and why these patients ended up being readmitted to a different hospital rather than the one that treated them for their acute stroke.”
The researchers reported having no financial disclosures.
SOURCE: Stein L et al. Ann Neurol. 2018;84[S22]:S149. Abstract M127.
ATLANTA – Following an acute stroke, optimizing stroke secondary prevention measures, medical complications, and transitions of care is essential to reducing 30-day readmissions and improving patient outcomes, a large analysis of national data showed.
“Care that is fragmented with readmissions to other hospitals results not only in more expensive care and longer length of stay but also increased mortality for our acute stroke patients,” lead study author Laura K. Stein, MD, said in an interview in advance of the annual meeting of the American Neurological Association.
In 2017, a study of the Nationwide Readmissions Database demonstrated that 12.1% of patients with acute ischemic stroke were readmitted within 30 days (Stroke 2017;48:1386-8). It cited that 89.6% were unplanned and 12.9% were preventable. “However, this study did not examine whether patients were admitted to the discharging hospital or a different hospital,” said Dr. Stein, a neurologist at the Icahn School of Medicine at Mount Sinai, New York. “Furthermore, it did not include metrics such as cost, length of stay, and mortality with 30-day readmissions. Hospitals are increasingly held accountable and penalized for metrics such as length of stay and 30-day readmissions.”
In 2010, the Centers for Medicare & Medicaid Services introduced the Hospital Readmissions Reduction Program in an attempt to decrease readmissions following hospitalizations for acute myocardial infarction, heart failure, and pneumonia. “In 2012, CMS started reducing Medicare payments for hospitals with excess readmissions,” said Dr. Stein, who is a fellowship-trained stroke specialist. “While readmission to the same hospital has great implications for hospital systems, any readmission has great implications for patients.”
In what is believed to be the first study of its kind, Dr. Stein and her colleagues drew from the 2013 Nationwide Readmissions Database to examine in-hospital outcomes associated with 30-day readmission to a different hospital for acute ischemic stroke. They used ICD-9 codes to identify index stroke admissions and all-cause readmissions. Outcomes of interest were length of stay, total charges, and in-hospital mortality during the 30-day readmission. The main predictor was readmission to another hospital, compared with readmission to the same hospital as the index acute stroke admission. The researchers used linear regression for the outcomes of length of stay and charges, and logistic regression for in-hospital mortality. They adjusted for several variables during the index admission, including age, sex, vascular risk factors, hospital bed size, teaching hospital status, insurance status, discharge destination, National Center for Health Statistics urban-rural location classification, length of stay, and total charges.
Of 24,545 acute stroke patients readmitted within 30 days, 7,274 (30%) were readmitted to a different hospital. The top three reasons for readmission were acute cerebrovascular disease, septicemia, and renal failure. In fully adjusted models, readmission to a different hospital was associated with an increased length of stay of 0.97 days (P less than .0001) and a mean of $7,677.28 greater total charges, compared with readmission to the same hospital (P less than .0001). The fully adjusted odds ratio for in-hospital mortality during readmission was 1.17 for readmission to another hospital vs. readmission to the same hospital (P = .0079).
“While it is conceivable that cost and length of stay could be higher with readmission to a different hospital because of a need for additional testing with a lack of familiarity with the patient, it is concerning that mortality is higher,” Dr. Stein said. “These findings emphasize the importance of optimizing secondary stroke prevention and medical complications following acute stroke before discharge. Additionally, they emphasize the importance of good transitions of care from the inpatient to outpatient setting (whether that’s to a rehabilitation facility, skilled nursing facility, or home) and accessibility of the discharging stroke team after discharge.”
She acknowledged certain limitations of the analysis, including its reliance of administrative data, which could include misclassification of diagnoses and comorbidities based on ICD-9 codes. “However, we have chosen ICD-9 codes for stroke that have been previously validated in the literature,” Dr. Stein said. “For instance, the validated codes for stroke as the primary discharge diagnosis have a sensitivity of 74%, specificity of 95%, and positive predictive value of 88%. Second, we do not know stroke subtype or severity of stroke. Third, we do not know what the transitions of care plan were when the patients left the hospital following index acute ischemic stroke admission and why these patients ended up being readmitted to a different hospital rather than the one that treated them for their acute stroke.”
The researchers reported having no financial disclosures.
SOURCE: Stein L et al. Ann Neurol. 2018;84[S22]:S149. Abstract M127.
ATLANTA – Following an acute stroke, optimizing stroke secondary prevention measures, medical complications, and transitions of care is essential to reducing 30-day readmissions and improving patient outcomes, a large analysis of national data showed.
“Care that is fragmented with readmissions to other hospitals results not only in more expensive care and longer length of stay but also increased mortality for our acute stroke patients,” lead study author Laura K. Stein, MD, said in an interview in advance of the annual meeting of the American Neurological Association.
In 2017, a study of the Nationwide Readmissions Database demonstrated that 12.1% of patients with acute ischemic stroke were readmitted within 30 days (Stroke 2017;48:1386-8). It cited that 89.6% were unplanned and 12.9% were preventable. “However, this study did not examine whether patients were admitted to the discharging hospital or a different hospital,” said Dr. Stein, a neurologist at the Icahn School of Medicine at Mount Sinai, New York. “Furthermore, it did not include metrics such as cost, length of stay, and mortality with 30-day readmissions. Hospitals are increasingly held accountable and penalized for metrics such as length of stay and 30-day readmissions.”
In 2010, the Centers for Medicare & Medicaid Services introduced the Hospital Readmissions Reduction Program in an attempt to decrease readmissions following hospitalizations for acute myocardial infarction, heart failure, and pneumonia. “In 2012, CMS started reducing Medicare payments for hospitals with excess readmissions,” said Dr. Stein, who is a fellowship-trained stroke specialist. “While readmission to the same hospital has great implications for hospital systems, any readmission has great implications for patients.”
In what is believed to be the first study of its kind, Dr. Stein and her colleagues drew from the 2013 Nationwide Readmissions Database to examine in-hospital outcomes associated with 30-day readmission to a different hospital for acute ischemic stroke. They used ICD-9 codes to identify index stroke admissions and all-cause readmissions. Outcomes of interest were length of stay, total charges, and in-hospital mortality during the 30-day readmission. The main predictor was readmission to another hospital, compared with readmission to the same hospital as the index acute stroke admission. The researchers used linear regression for the outcomes of length of stay and charges, and logistic regression for in-hospital mortality. They adjusted for several variables during the index admission, including age, sex, vascular risk factors, hospital bed size, teaching hospital status, insurance status, discharge destination, National Center for Health Statistics urban-rural location classification, length of stay, and total charges.
Of 24,545 acute stroke patients readmitted within 30 days, 7,274 (30%) were readmitted to a different hospital. The top three reasons for readmission were acute cerebrovascular disease, septicemia, and renal failure. In fully adjusted models, readmission to a different hospital was associated with an increased length of stay of 0.97 days (P less than .0001) and a mean of $7,677.28 greater total charges, compared with readmission to the same hospital (P less than .0001). The fully adjusted odds ratio for in-hospital mortality during readmission was 1.17 for readmission to another hospital vs. readmission to the same hospital (P = .0079).
“While it is conceivable that cost and length of stay could be higher with readmission to a different hospital because of a need for additional testing with a lack of familiarity with the patient, it is concerning that mortality is higher,” Dr. Stein said. “These findings emphasize the importance of optimizing secondary stroke prevention and medical complications following acute stroke before discharge. Additionally, they emphasize the importance of good transitions of care from the inpatient to outpatient setting (whether that’s to a rehabilitation facility, skilled nursing facility, or home) and accessibility of the discharging stroke team after discharge.”
She acknowledged certain limitations of the analysis, including its reliance of administrative data, which could include misclassification of diagnoses and comorbidities based on ICD-9 codes. “However, we have chosen ICD-9 codes for stroke that have been previously validated in the literature,” Dr. Stein said. “For instance, the validated codes for stroke as the primary discharge diagnosis have a sensitivity of 74%, specificity of 95%, and positive predictive value of 88%. Second, we do not know stroke subtype or severity of stroke. Third, we do not know what the transitions of care plan were when the patients left the hospital following index acute ischemic stroke admission and why these patients ended up being readmitted to a different hospital rather than the one that treated them for their acute stroke.”
The researchers reported having no financial disclosures.
SOURCE: Stein L et al. Ann Neurol. 2018;84[S22]:S149. Abstract M127.
REPORTING FROM ANA 2018
Key clinical point:
Major finding: The adjusted odds ratio for in-hospital mortality during readmission was 1.17 for readmission to another hospital vs. readmission to the same hospital (P = .0079).
Study details: A review of 24,545 acute stroke patients 2013 from the Nationwide Readmissions Database.
Disclosures: The researchers reported having no financial disclosures.
Source: Stein L et al. Ann Neurol. 2018;84[S22]:S149. Abstract M127.
Prior TIA proves less risky than stroke in AF patients
MONTREAL – Stroke and transient ischemic attack, usually joined at the hip as related histories that each flag a similar need for anticoagulation may in fact not be nearly as equivalent as conventional wisdom says.
Analysis of 2-year follow-up data from the GARFIELD-AF registry of more than 52,000 patients with newly diagnosed atrial fibrillation (AF) showed that, while patients with a history of stroke had significantly elevated rates of both all-cause mortality and stroke, those with just a history of a transient ischemic attack (TIA) had mortality and stroke rates virtually identical to AF patients with no history of a cerebrovascular event.
“A history of TIA only is not a reliable predictor of an increased risk for events,” Werner Hacke, MD, said at the World Stroke Congress. “A history of TIA should be removed from scores estimating the risk for stroke and systemic embolism in AF patients,” said Dr. Hacke, professor and chairman of the department of neurology at the University of Heidelberg (Germany).
“The weak predictive power of a history of TIA is probably caused by the relatively low reliability of establishing the diagnosis of TIA,” especially when the diagnosis is made by someone who’s not a neurologist. “It’s a very fuzzy diagnosis,” even for a neurologist, and it consistently confounds other clinicians, he said in an interview. “I’d be really careful of deciding to anticoagulate a patient [with AF] based on a history of TIA.” In the GARFIELD-AF registry, “I’m convinced that most people with a history of TIA actually never had a TIA.”
Dr. Hacke has been unable to find a good explanation of why, years ago, TIAs began to get routinely lumped with stroke. “I asked all the old AF guys when did TIA start coming in and why, and none of them could remember,” he said. “At first, they talked about a history of ‘cerebrovascular events,’ but then that became stroke and TIA, and it was as if it was one word,” always said in the same breath. Both the CHADS2 score (JAMA. 2001 Jun 13;285[22]:2864-70) and the CHA2DS2-VASc score (Chest. 2010 Feb;137[2]:263-72) make a history of stroke or TIA, as well as thromboembolism, coequal risk factors that count for 2 points when calculating the thrombotic risk score for a patient with AF.
To test whether this really made sense, Dr. Hacke and his associates decided to look at the separate consequences of a history of stroke only, compared with a history of a TIA only. They used data collected in GARFIELD-AF (Global Anticoagulant Registry in the Field), a multinational registry with 51,670 patients newly diagnosed with AF, followed for 2 years, and with complete information on their stroke and TIA history. This included 5,617 patients with a history of at least one diagnosed cerebrovascular event, including 3,362 diagnosed with stroke only, 1,788 diagnosed with TIA only, and the remaining patients diagnosed with both types of events.
When compared with AF patients without a history of any type of cerebrovascular event, those with a history of a stroke only had a statistically significant 29% increased rate of all-cause death and a 2.3-fold higher rate of stroke after adjustment for baseline demographic and clinical differences. In contrast, the patients with a history of TIA only had mortality and stroke rates during follow-up that did not differ significantly from the comparator group.
Source: Hacke W et al. World Stroke Congress, Abstract.
MONTREAL – Stroke and transient ischemic attack, usually joined at the hip as related histories that each flag a similar need for anticoagulation may in fact not be nearly as equivalent as conventional wisdom says.
Analysis of 2-year follow-up data from the GARFIELD-AF registry of more than 52,000 patients with newly diagnosed atrial fibrillation (AF) showed that, while patients with a history of stroke had significantly elevated rates of both all-cause mortality and stroke, those with just a history of a transient ischemic attack (TIA) had mortality and stroke rates virtually identical to AF patients with no history of a cerebrovascular event.
“A history of TIA only is not a reliable predictor of an increased risk for events,” Werner Hacke, MD, said at the World Stroke Congress. “A history of TIA should be removed from scores estimating the risk for stroke and systemic embolism in AF patients,” said Dr. Hacke, professor and chairman of the department of neurology at the University of Heidelberg (Germany).
“The weak predictive power of a history of TIA is probably caused by the relatively low reliability of establishing the diagnosis of TIA,” especially when the diagnosis is made by someone who’s not a neurologist. “It’s a very fuzzy diagnosis,” even for a neurologist, and it consistently confounds other clinicians, he said in an interview. “I’d be really careful of deciding to anticoagulate a patient [with AF] based on a history of TIA.” In the GARFIELD-AF registry, “I’m convinced that most people with a history of TIA actually never had a TIA.”
Dr. Hacke has been unable to find a good explanation of why, years ago, TIAs began to get routinely lumped with stroke. “I asked all the old AF guys when did TIA start coming in and why, and none of them could remember,” he said. “At first, they talked about a history of ‘cerebrovascular events,’ but then that became stroke and TIA, and it was as if it was one word,” always said in the same breath. Both the CHADS2 score (JAMA. 2001 Jun 13;285[22]:2864-70) and the CHA2DS2-VASc score (Chest. 2010 Feb;137[2]:263-72) make a history of stroke or TIA, as well as thromboembolism, coequal risk factors that count for 2 points when calculating the thrombotic risk score for a patient with AF.
To test whether this really made sense, Dr. Hacke and his associates decided to look at the separate consequences of a history of stroke only, compared with a history of a TIA only. They used data collected in GARFIELD-AF (Global Anticoagulant Registry in the Field), a multinational registry with 51,670 patients newly diagnosed with AF, followed for 2 years, and with complete information on their stroke and TIA history. This included 5,617 patients with a history of at least one diagnosed cerebrovascular event, including 3,362 diagnosed with stroke only, 1,788 diagnosed with TIA only, and the remaining patients diagnosed with both types of events.
When compared with AF patients without a history of any type of cerebrovascular event, those with a history of a stroke only had a statistically significant 29% increased rate of all-cause death and a 2.3-fold higher rate of stroke after adjustment for baseline demographic and clinical differences. In contrast, the patients with a history of TIA only had mortality and stroke rates during follow-up that did not differ significantly from the comparator group.
Source: Hacke W et al. World Stroke Congress, Abstract.
MONTREAL – Stroke and transient ischemic attack, usually joined at the hip as related histories that each flag a similar need for anticoagulation may in fact not be nearly as equivalent as conventional wisdom says.
Analysis of 2-year follow-up data from the GARFIELD-AF registry of more than 52,000 patients with newly diagnosed atrial fibrillation (AF) showed that, while patients with a history of stroke had significantly elevated rates of both all-cause mortality and stroke, those with just a history of a transient ischemic attack (TIA) had mortality and stroke rates virtually identical to AF patients with no history of a cerebrovascular event.
“A history of TIA only is not a reliable predictor of an increased risk for events,” Werner Hacke, MD, said at the World Stroke Congress. “A history of TIA should be removed from scores estimating the risk for stroke and systemic embolism in AF patients,” said Dr. Hacke, professor and chairman of the department of neurology at the University of Heidelberg (Germany).
“The weak predictive power of a history of TIA is probably caused by the relatively low reliability of establishing the diagnosis of TIA,” especially when the diagnosis is made by someone who’s not a neurologist. “It’s a very fuzzy diagnosis,” even for a neurologist, and it consistently confounds other clinicians, he said in an interview. “I’d be really careful of deciding to anticoagulate a patient [with AF] based on a history of TIA.” In the GARFIELD-AF registry, “I’m convinced that most people with a history of TIA actually never had a TIA.”
Dr. Hacke has been unable to find a good explanation of why, years ago, TIAs began to get routinely lumped with stroke. “I asked all the old AF guys when did TIA start coming in and why, and none of them could remember,” he said. “At first, they talked about a history of ‘cerebrovascular events,’ but then that became stroke and TIA, and it was as if it was one word,” always said in the same breath. Both the CHADS2 score (JAMA. 2001 Jun 13;285[22]:2864-70) and the CHA2DS2-VASc score (Chest. 2010 Feb;137[2]:263-72) make a history of stroke or TIA, as well as thromboembolism, coequal risk factors that count for 2 points when calculating the thrombotic risk score for a patient with AF.
To test whether this really made sense, Dr. Hacke and his associates decided to look at the separate consequences of a history of stroke only, compared with a history of a TIA only. They used data collected in GARFIELD-AF (Global Anticoagulant Registry in the Field), a multinational registry with 51,670 patients newly diagnosed with AF, followed for 2 years, and with complete information on their stroke and TIA history. This included 5,617 patients with a history of at least one diagnosed cerebrovascular event, including 3,362 diagnosed with stroke only, 1,788 diagnosed with TIA only, and the remaining patients diagnosed with both types of events.
When compared with AF patients without a history of any type of cerebrovascular event, those with a history of a stroke only had a statistically significant 29% increased rate of all-cause death and a 2.3-fold higher rate of stroke after adjustment for baseline demographic and clinical differences. In contrast, the patients with a history of TIA only had mortality and stroke rates during follow-up that did not differ significantly from the comparator group.
Source: Hacke W et al. World Stroke Congress, Abstract.
REPORTING FROM THE WORLD STROKE CONGRESS
Key clinical point:
Major finding: The adjusted rate of stroke in patients with a TIA history was not significantly different from patients with no history.
Study details: GARFIELD-AF, a multinational registry of 52,014 patients newly diagnosed with atrial fibrillation.
Disclosures: GARFIELD-AF is funded by Bayer. Dr. Hacke has been an adviser to Boehringer Ingelheim and Cerenovus and he has received research funding from Boehringer Ingelheim.
Source: Hacke W et al. World Stroke Congress, Abstract.
DAPT’s benefit after stroke or TIA clusters in first 21 days
MONTREAL – The optimal length for dual antiplatelet therapy in patients who have just had a mild stroke or transient ischemic attack is 21 days, a duration of combined treatment that maximized protection against major ischemic events while minimizing the extra risk for a major hemorrhage, according to a prespecified analysis of data from the POINT trial.
The POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) trial randomized 4,881 patients with a very recent mild stroke or transient ischemic attack and without atrial fibrillation to treatment with either clopidogrel plus aspirin or aspirin alone for 90 days. Compared with aspirin alone, dual antiplatelet therapy (DAPT) cut the incidence of a major ischemic event by a relative 25% but also more than doubled the rate of major hemorrhage (New Engl J Med. 2018 Jul 19;377[3]:215-25).
The new, prespecified analysis looked at outcomes on a week-by-week basis over the course of 90 days of treatment, and showed that during the first 21 days the rate of major ischemic events was 5.6% among patients on aspirin only and 3.6% among those on DAPT, a statistically significant 35% relative cut in these adverse outcomes by using DAPT, Jordan J. Elm, PhD, reported at the World Stroke Congress. During the subsequent 69 days on treatment, the incidence of major ischemic events was roughly 1% in both arms of the study, showing that after 3 weeks the incremental benefit from DAPT disappeared, said Dr. Elm, a biostatistician at the Medical University of South Carolina, Charleston.
In contrast, the doubled rate of major hemorrhages (mostly reversible gastrointestinal bleeds) with DAPT, compared with aspirin alone, occurred at a relatively uniform rate throughout the 90 days of treatment, meaning that limiting DAPT to just 21 days could prevent many of the excess hemorrhages.
“These results suggest that limiting clopidogrel plus aspirin use to 21 days may maximize benefit and reduce risk,” Dr. Elm said, especially in light of the findings confirming the efficacy of 21 days of DAPT following a minor stroke or TIA that had been reported several years ago in the CHANCE (Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events) trial (New Engl J Med. 2013 Jul 4;369[1]:11-9).
Although the new finding from the POINT results came in a secondary analysis, it’s statistically legitimate and should be taken into account when writing treatment guidelines, she said, emphasizing that “this is a very important analysis that is not just hypothesis generating.”
Another finding from the new analysis was that a large number of major ischemic events, and hence a large number of the events prevented by DAPT, occurred in the first 2 days following the index event, a finding made possible because the POINT investigators enrolled patients and started treatment within 12 hours of the qualifying events.
“It’s better to start treatment early,” Dr. Elm noted, but she also highlighted that major ischemic events continued to accumulate during days 3-21, suggesting that patients could still benefit from DAPT even if treatment did not start until 24 or 48 hours after their index event.
POINT received no commercial funding aside from study drugs supplied by Sanofi. Dr. Elm reported no disclosures.
SOURCE: Elm JJ et al. World Stroke Congress, Late-breaking session.
The new model using data from the POINT trial confirms what had been previously shown in the CHANCE trial – that 21 days is a sensible cutoff for dual antiplatelet treatment for patients immediately following a mild stroke or transient ischemic attack. Treatment with dual antiplatelet therapy for 21 days provides the same added benefit as 90 days of treatment but with less excess bleeding. The new findings confirm that the CHANCE results were not specific to a Chinese population.

For the time being, clopidogrel is the evidence-based antiplatelet drug to pair with aspirin for this indication. Clopidogrel has the advantages of being generic, cheap, available, and familiar. It’s possible that another P2Y12 inhibitor, such as ticagrelor (Brilinta), might work even better, but that needs to be proven to justify the added expense of a brand-name antiplatelet drug.
Mike Sharma, MD , is a stroke neurologist at McMaster University, Hamilton, Ont. He has been an advisor to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Janssen Pharmaceuticals, and Pfizer. He made these comments in an interview.
The new model using data from the POINT trial confirms what had been previously shown in the CHANCE trial – that 21 days is a sensible cutoff for dual antiplatelet treatment for patients immediately following a mild stroke or transient ischemic attack. Treatment with dual antiplatelet therapy for 21 days provides the same added benefit as 90 days of treatment but with less excess bleeding. The new findings confirm that the CHANCE results were not specific to a Chinese population.

For the time being, clopidogrel is the evidence-based antiplatelet drug to pair with aspirin for this indication. Clopidogrel has the advantages of being generic, cheap, available, and familiar. It’s possible that another P2Y12 inhibitor, such as ticagrelor (Brilinta), might work even better, but that needs to be proven to justify the added expense of a brand-name antiplatelet drug.
Mike Sharma, MD , is a stroke neurologist at McMaster University, Hamilton, Ont. He has been an advisor to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Janssen Pharmaceuticals, and Pfizer. He made these comments in an interview.
The new model using data from the POINT trial confirms what had been previously shown in the CHANCE trial – that 21 days is a sensible cutoff for dual antiplatelet treatment for patients immediately following a mild stroke or transient ischemic attack. Treatment with dual antiplatelet therapy for 21 days provides the same added benefit as 90 days of treatment but with less excess bleeding. The new findings confirm that the CHANCE results were not specific to a Chinese population.

For the time being, clopidogrel is the evidence-based antiplatelet drug to pair with aspirin for this indication. Clopidogrel has the advantages of being generic, cheap, available, and familiar. It’s possible that another P2Y12 inhibitor, such as ticagrelor (Brilinta), might work even better, but that needs to be proven to justify the added expense of a brand-name antiplatelet drug.
Mike Sharma, MD , is a stroke neurologist at McMaster University, Hamilton, Ont. He has been an advisor to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Janssen Pharmaceuticals, and Pfizer. He made these comments in an interview.
MONTREAL – The optimal length for dual antiplatelet therapy in patients who have just had a mild stroke or transient ischemic attack is 21 days, a duration of combined treatment that maximized protection against major ischemic events while minimizing the extra risk for a major hemorrhage, according to a prespecified analysis of data from the POINT trial.
The POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) trial randomized 4,881 patients with a very recent mild stroke or transient ischemic attack and without atrial fibrillation to treatment with either clopidogrel plus aspirin or aspirin alone for 90 days. Compared with aspirin alone, dual antiplatelet therapy (DAPT) cut the incidence of a major ischemic event by a relative 25% but also more than doubled the rate of major hemorrhage (New Engl J Med. 2018 Jul 19;377[3]:215-25).
The new, prespecified analysis looked at outcomes on a week-by-week basis over the course of 90 days of treatment, and showed that during the first 21 days the rate of major ischemic events was 5.6% among patients on aspirin only and 3.6% among those on DAPT, a statistically significant 35% relative cut in these adverse outcomes by using DAPT, Jordan J. Elm, PhD, reported at the World Stroke Congress. During the subsequent 69 days on treatment, the incidence of major ischemic events was roughly 1% in both arms of the study, showing that after 3 weeks the incremental benefit from DAPT disappeared, said Dr. Elm, a biostatistician at the Medical University of South Carolina, Charleston.
In contrast, the doubled rate of major hemorrhages (mostly reversible gastrointestinal bleeds) with DAPT, compared with aspirin alone, occurred at a relatively uniform rate throughout the 90 days of treatment, meaning that limiting DAPT to just 21 days could prevent many of the excess hemorrhages.
“These results suggest that limiting clopidogrel plus aspirin use to 21 days may maximize benefit and reduce risk,” Dr. Elm said, especially in light of the findings confirming the efficacy of 21 days of DAPT following a minor stroke or TIA that had been reported several years ago in the CHANCE (Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events) trial (New Engl J Med. 2013 Jul 4;369[1]:11-9).
Although the new finding from the POINT results came in a secondary analysis, it’s statistically legitimate and should be taken into account when writing treatment guidelines, she said, emphasizing that “this is a very important analysis that is not just hypothesis generating.”
Another finding from the new analysis was that a large number of major ischemic events, and hence a large number of the events prevented by DAPT, occurred in the first 2 days following the index event, a finding made possible because the POINT investigators enrolled patients and started treatment within 12 hours of the qualifying events.
“It’s better to start treatment early,” Dr. Elm noted, but she also highlighted that major ischemic events continued to accumulate during days 3-21, suggesting that patients could still benefit from DAPT even if treatment did not start until 24 or 48 hours after their index event.
POINT received no commercial funding aside from study drugs supplied by Sanofi. Dr. Elm reported no disclosures.
SOURCE: Elm JJ et al. World Stroke Congress, Late-breaking session.
MONTREAL – The optimal length for dual antiplatelet therapy in patients who have just had a mild stroke or transient ischemic attack is 21 days, a duration of combined treatment that maximized protection against major ischemic events while minimizing the extra risk for a major hemorrhage, according to a prespecified analysis of data from the POINT trial.
The POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) trial randomized 4,881 patients with a very recent mild stroke or transient ischemic attack and without atrial fibrillation to treatment with either clopidogrel plus aspirin or aspirin alone for 90 days. Compared with aspirin alone, dual antiplatelet therapy (DAPT) cut the incidence of a major ischemic event by a relative 25% but also more than doubled the rate of major hemorrhage (New Engl J Med. 2018 Jul 19;377[3]:215-25).
The new, prespecified analysis looked at outcomes on a week-by-week basis over the course of 90 days of treatment, and showed that during the first 21 days the rate of major ischemic events was 5.6% among patients on aspirin only and 3.6% among those on DAPT, a statistically significant 35% relative cut in these adverse outcomes by using DAPT, Jordan J. Elm, PhD, reported at the World Stroke Congress. During the subsequent 69 days on treatment, the incidence of major ischemic events was roughly 1% in both arms of the study, showing that after 3 weeks the incremental benefit from DAPT disappeared, said Dr. Elm, a biostatistician at the Medical University of South Carolina, Charleston.
In contrast, the doubled rate of major hemorrhages (mostly reversible gastrointestinal bleeds) with DAPT, compared with aspirin alone, occurred at a relatively uniform rate throughout the 90 days of treatment, meaning that limiting DAPT to just 21 days could prevent many of the excess hemorrhages.
“These results suggest that limiting clopidogrel plus aspirin use to 21 days may maximize benefit and reduce risk,” Dr. Elm said, especially in light of the findings confirming the efficacy of 21 days of DAPT following a minor stroke or TIA that had been reported several years ago in the CHANCE (Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events) trial (New Engl J Med. 2013 Jul 4;369[1]:11-9).
Although the new finding from the POINT results came in a secondary analysis, it’s statistically legitimate and should be taken into account when writing treatment guidelines, she said, emphasizing that “this is a very important analysis that is not just hypothesis generating.”
Another finding from the new analysis was that a large number of major ischemic events, and hence a large number of the events prevented by DAPT, occurred in the first 2 days following the index event, a finding made possible because the POINT investigators enrolled patients and started treatment within 12 hours of the qualifying events.
“It’s better to start treatment early,” Dr. Elm noted, but she also highlighted that major ischemic events continued to accumulate during days 3-21, suggesting that patients could still benefit from DAPT even if treatment did not start until 24 or 48 hours after their index event.
POINT received no commercial funding aside from study drugs supplied by Sanofi. Dr. Elm reported no disclosures.
SOURCE: Elm JJ et al. World Stroke Congress, Late-breaking session.
REPORTING FROM THE WORLD STROKE CONGRESS
Key clinical point: All of DAPT’s extra benefit over aspirin alone in recent stroke or transient ischemic attack patients happened during the first 21 days.
Major finding: During the first 21 days, DAPT cut major ischemic events by 35%, compared with aspirin only.
Study details: A prespecified, secondary analysis from POINT, a multicenter, randomized trial with 4,881 patients.
Disclosures: POINT received no commercial funding aside from study drugs supplied by Sanofi. Dr. Elm had no disclosures.
Source: Elm JJ et al. World Stroke Congress, Late-breaking session.
Smartphone device beat Holter for post-stroke AF detection
MONTREAL – A smartphone-based method for quick and inexpensive monitoring for atrial fibrillation in patients hospitalized for a recent acute ischemic stroke or transient ischemic attack identified three times more patients with the arrhythmia than did 24-hour Holter monitoring of the same patients after their hospital discharge.
This high level of atrial fibrillation (AF) detection using a relatively cheap and noninvasive device suggests that this method is a good “complement” to conventional monitoring by a 24-hour Holter recording or an implanted loop recorder in recent stroke patients, as called for in current guidelines of the world’s cardiology societies.
In the study, 294 of 1,079 patients hospitalized for an acute ischemic stroke or transient ischemic attack (TIA) underwent Holter monitoring, which identified 8 patients (3%) with AF, compared with 25 of these 294 patients (9%) identified with AF while they were hospitalized using serial, 30-second monitoring with the AliveCor device for smartphone assessment of ECG measurement, Bernard Yan, MD, said at the World Stroke Congress. Seven of the eight patients identified with AF by Holter monitoring were also found to have AF by the AliveCor device.
Dr. Yan, an interventional neurologist at the Comprehensive Stroke Center at the Royal Melbourne Hospital, attributed the higher pick-up rate for AF by monitoring during hospitalization to the timing of screening, which was within days of the stroke or TIA, rather than waiting to run a Holter sometime after the patient left the hospital.
“I suspect the difference in timing explains the difference” in detection, he said in an interview. “The difference may be because we monitored patients [with the AliveCor device] much earlier, during their ‘hot’ period, right after their stroke.”
The SPOT-AF trial ran at several centers in Australia, China, and Hong Kong, and enrolled 1,079 patients hospitalized for acute ischemic stroke or TIA who all underwent AliveCor monitoring during their median 4-day stay in the hospital. Patients performed a 30-second heart rhythm check every time a nurse saw them for a routine vital-sign examination, usually three or four times a day. The current analysis focused on the 294 patients (27% of the 1,079 patients) who also underwent 24-hour Holter monitoring following hospital discharge when ordered by their personal physician. This 27% incidence of postdischarge Holter monitoring despite guidelines that call for AF screening in all recent ischemic stroke and TIA patients was consistent with a 2016 review of more than 17,000 stroke or TIA patients in Canada that showed 31% underwent 24-hour Holter monitoring for AF during the 30 days following their index event (Stroke. 2016 Aug;47[8]:1982-9).
Although AF screening with a smartphone-based device is inexpensive and easy, Dr. Yan stopped short of suggesting that it is time for this approach to replace a Holter monitor or an implanted loop recorder because that is what current guidelines call for. “To change the guidelines, we need a different study that compares these approaches head to head.”
SPOT-AF received partial funding from Boehringer Ingelheim. Dr. Yan has been a speaker on behalf of Bayer, Boehringer Ingelheim, Pfizer, and Stryker.
MONTREAL – A smartphone-based method for quick and inexpensive monitoring for atrial fibrillation in patients hospitalized for a recent acute ischemic stroke or transient ischemic attack identified three times more patients with the arrhythmia than did 24-hour Holter monitoring of the same patients after their hospital discharge.
This high level of atrial fibrillation (AF) detection using a relatively cheap and noninvasive device suggests that this method is a good “complement” to conventional monitoring by a 24-hour Holter recording or an implanted loop recorder in recent stroke patients, as called for in current guidelines of the world’s cardiology societies.
In the study, 294 of 1,079 patients hospitalized for an acute ischemic stroke or transient ischemic attack (TIA) underwent Holter monitoring, which identified 8 patients (3%) with AF, compared with 25 of these 294 patients (9%) identified with AF while they were hospitalized using serial, 30-second monitoring with the AliveCor device for smartphone assessment of ECG measurement, Bernard Yan, MD, said at the World Stroke Congress. Seven of the eight patients identified with AF by Holter monitoring were also found to have AF by the AliveCor device.
Dr. Yan, an interventional neurologist at the Comprehensive Stroke Center at the Royal Melbourne Hospital, attributed the higher pick-up rate for AF by monitoring during hospitalization to the timing of screening, which was within days of the stroke or TIA, rather than waiting to run a Holter sometime after the patient left the hospital.
“I suspect the difference in timing explains the difference” in detection, he said in an interview. “The difference may be because we monitored patients [with the AliveCor device] much earlier, during their ‘hot’ period, right after their stroke.”
The SPOT-AF trial ran at several centers in Australia, China, and Hong Kong, and enrolled 1,079 patients hospitalized for acute ischemic stroke or TIA who all underwent AliveCor monitoring during their median 4-day stay in the hospital. Patients performed a 30-second heart rhythm check every time a nurse saw them for a routine vital-sign examination, usually three or four times a day. The current analysis focused on the 294 patients (27% of the 1,079 patients) who also underwent 24-hour Holter monitoring following hospital discharge when ordered by their personal physician. This 27% incidence of postdischarge Holter monitoring despite guidelines that call for AF screening in all recent ischemic stroke and TIA patients was consistent with a 2016 review of more than 17,000 stroke or TIA patients in Canada that showed 31% underwent 24-hour Holter monitoring for AF during the 30 days following their index event (Stroke. 2016 Aug;47[8]:1982-9).
Although AF screening with a smartphone-based device is inexpensive and easy, Dr. Yan stopped short of suggesting that it is time for this approach to replace a Holter monitor or an implanted loop recorder because that is what current guidelines call for. “To change the guidelines, we need a different study that compares these approaches head to head.”
SPOT-AF received partial funding from Boehringer Ingelheim. Dr. Yan has been a speaker on behalf of Bayer, Boehringer Ingelheim, Pfizer, and Stryker.
MONTREAL – A smartphone-based method for quick and inexpensive monitoring for atrial fibrillation in patients hospitalized for a recent acute ischemic stroke or transient ischemic attack identified three times more patients with the arrhythmia than did 24-hour Holter monitoring of the same patients after their hospital discharge.
This high level of atrial fibrillation (AF) detection using a relatively cheap and noninvasive device suggests that this method is a good “complement” to conventional monitoring by a 24-hour Holter recording or an implanted loop recorder in recent stroke patients, as called for in current guidelines of the world’s cardiology societies.
In the study, 294 of 1,079 patients hospitalized for an acute ischemic stroke or transient ischemic attack (TIA) underwent Holter monitoring, which identified 8 patients (3%) with AF, compared with 25 of these 294 patients (9%) identified with AF while they were hospitalized using serial, 30-second monitoring with the AliveCor device for smartphone assessment of ECG measurement, Bernard Yan, MD, said at the World Stroke Congress. Seven of the eight patients identified with AF by Holter monitoring were also found to have AF by the AliveCor device.
Dr. Yan, an interventional neurologist at the Comprehensive Stroke Center at the Royal Melbourne Hospital, attributed the higher pick-up rate for AF by monitoring during hospitalization to the timing of screening, which was within days of the stroke or TIA, rather than waiting to run a Holter sometime after the patient left the hospital.
“I suspect the difference in timing explains the difference” in detection, he said in an interview. “The difference may be because we monitored patients [with the AliveCor device] much earlier, during their ‘hot’ period, right after their stroke.”
The SPOT-AF trial ran at several centers in Australia, China, and Hong Kong, and enrolled 1,079 patients hospitalized for acute ischemic stroke or TIA who all underwent AliveCor monitoring during their median 4-day stay in the hospital. Patients performed a 30-second heart rhythm check every time a nurse saw them for a routine vital-sign examination, usually three or four times a day. The current analysis focused on the 294 patients (27% of the 1,079 patients) who also underwent 24-hour Holter monitoring following hospital discharge when ordered by their personal physician. This 27% incidence of postdischarge Holter monitoring despite guidelines that call for AF screening in all recent ischemic stroke and TIA patients was consistent with a 2016 review of more than 17,000 stroke or TIA patients in Canada that showed 31% underwent 24-hour Holter monitoring for AF during the 30 days following their index event (Stroke. 2016 Aug;47[8]:1982-9).
Although AF screening with a smartphone-based device is inexpensive and easy, Dr. Yan stopped short of suggesting that it is time for this approach to replace a Holter monitor or an implanted loop recorder because that is what current guidelines call for. “To change the guidelines, we need a different study that compares these approaches head to head.”
SPOT-AF received partial funding from Boehringer Ingelheim. Dr. Yan has been a speaker on behalf of Bayer, Boehringer Ingelheim, Pfizer, and Stryker.
REPORTING FROM THE WORLD STROKE CONGRESS
Key clinical point:
Major finding: Holter monitoring detected atrial fibrillation in 8 of 294 patients, while smartphone monitoring identified 25 with the arrhythmia.
Study details: SPOT-AF, a multicenter study with 1,079 total patients, including 294 who underwent Holter monitoring.
Disclosures: SPOT-AF received partial funding from Boehringer Ingelheim. Dr. Yan has been a speaker on behalf of Bayer, Boehringer Ingelheim, Pfizer, and Stryker.
Rivaroxaban gains indication for prevention of major cardiovascular events in CAD/PAD
when taken with aspirin, Janssen Pharmaceuticals announced on October 11.
The Food and Drug Administration’s approval was based on a review of the 27,000-patient COMPASS trial, which showed last year that a low dosage of rivaroxaban (Xarelto) plus aspirin reduced the combined rate of cardiovascular disease events by 24% in patients with coronary artery disease and by 28% in participants with peripheral artery disease, compared with aspirin alone. (N Engl J Med. 2017 Oct 5;377[14]:1319-30)
The flip side to the reduction in COMPASS’s combined primary endpoint was a 51% increase in major bleeding. However, that bump did not translate to increases in fatal bleeds, intracerebral bleeds, or bleeding in other critical organs.
COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) studied two dosages of rivaroxaban, 2.5 mg and 5 mg twice daily, and it was the lower dosage that did the trick. Until this approval, that formulation wasn’t available; Janssen announced the coming of the 2.5-mg pill in its release.
The new prescribing information states specifically that Xarelto 2.5 mg is indicated, in combination with aspirin, to reduce the risk of major cardiovascular events, cardiovascular death, MI, and stroke in patients with chronic coronary artery disease or peripheral artery disease.
This is the sixth indication for rivaroxaban, a factor Xa inhibitor that was first approved in 2011. It is also the first indication for cardiovascular prevention for any factor Xa inhibitor. Others on the U.S. market are apixaban (Eliquis), edoxaban (Savaysa), and betrixaban (Bevyxxa).
COMPASS was presented at the 2017 annual congress of the European Society of Cardiology. At that time, Eugene Braunwald, MD, of Harvard Medical School and Brigham and Women’s Hospital in Boston, commented that the trial produced “unambiguous results that should change guidelines and the management of stable coronary artery disease.” He added that the results are “an important step for thrombocardiology.”
when taken with aspirin, Janssen Pharmaceuticals announced on October 11.
The Food and Drug Administration’s approval was based on a review of the 27,000-patient COMPASS trial, which showed last year that a low dosage of rivaroxaban (Xarelto) plus aspirin reduced the combined rate of cardiovascular disease events by 24% in patients with coronary artery disease and by 28% in participants with peripheral artery disease, compared with aspirin alone. (N Engl J Med. 2017 Oct 5;377[14]:1319-30)
The flip side to the reduction in COMPASS’s combined primary endpoint was a 51% increase in major bleeding. However, that bump did not translate to increases in fatal bleeds, intracerebral bleeds, or bleeding in other critical organs.
COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) studied two dosages of rivaroxaban, 2.5 mg and 5 mg twice daily, and it was the lower dosage that did the trick. Until this approval, that formulation wasn’t available; Janssen announced the coming of the 2.5-mg pill in its release.
The new prescribing information states specifically that Xarelto 2.5 mg is indicated, in combination with aspirin, to reduce the risk of major cardiovascular events, cardiovascular death, MI, and stroke in patients with chronic coronary artery disease or peripheral artery disease.
This is the sixth indication for rivaroxaban, a factor Xa inhibitor that was first approved in 2011. It is also the first indication for cardiovascular prevention for any factor Xa inhibitor. Others on the U.S. market are apixaban (Eliquis), edoxaban (Savaysa), and betrixaban (Bevyxxa).
COMPASS was presented at the 2017 annual congress of the European Society of Cardiology. At that time, Eugene Braunwald, MD, of Harvard Medical School and Brigham and Women’s Hospital in Boston, commented that the trial produced “unambiguous results that should change guidelines and the management of stable coronary artery disease.” He added that the results are “an important step for thrombocardiology.”
when taken with aspirin, Janssen Pharmaceuticals announced on October 11.
The Food and Drug Administration’s approval was based on a review of the 27,000-patient COMPASS trial, which showed last year that a low dosage of rivaroxaban (Xarelto) plus aspirin reduced the combined rate of cardiovascular disease events by 24% in patients with coronary artery disease and by 28% in participants with peripheral artery disease, compared with aspirin alone. (N Engl J Med. 2017 Oct 5;377[14]:1319-30)
The flip side to the reduction in COMPASS’s combined primary endpoint was a 51% increase in major bleeding. However, that bump did not translate to increases in fatal bleeds, intracerebral bleeds, or bleeding in other critical organs.
COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) studied two dosages of rivaroxaban, 2.5 mg and 5 mg twice daily, and it was the lower dosage that did the trick. Until this approval, that formulation wasn’t available; Janssen announced the coming of the 2.5-mg pill in its release.
The new prescribing information states specifically that Xarelto 2.5 mg is indicated, in combination with aspirin, to reduce the risk of major cardiovascular events, cardiovascular death, MI, and stroke in patients with chronic coronary artery disease or peripheral artery disease.
This is the sixth indication for rivaroxaban, a factor Xa inhibitor that was first approved in 2011. It is also the first indication for cardiovascular prevention for any factor Xa inhibitor. Others on the U.S. market are apixaban (Eliquis), edoxaban (Savaysa), and betrixaban (Bevyxxa).
COMPASS was presented at the 2017 annual congress of the European Society of Cardiology. At that time, Eugene Braunwald, MD, of Harvard Medical School and Brigham and Women’s Hospital in Boston, commented that the trial produced “unambiguous results that should change guidelines and the management of stable coronary artery disease.” He added that the results are “an important step for thrombocardiology.”
GARFIELD-AF registry: DOACs cut mortality 19%
MUNICH – Treatment of real-world patients newly diagnosed with atrial fibrillation using a direct oral anticoagulant led to benefits that tracked the advantages previously seen in randomized, controlled trials of these drugs, based on findings from more than 26,000 patients enrolled in a global registry.
Atrial fibrillation patients enrolled in the GARFIELD-AF(Global Anticoagulant Registry in the Field) study who started treatment with a direct oral anticoagulant (DOAC) had a 19% relative risk reduction in all-cause mortality during 2 years of follow-up, compared with patients on an oral vitamin K antagonist (VKA) regimen (such as warfarin), a statistically significant difference after adjustment for 30 demographic, clinical, and registry variables, A. John Camm, MD, said at the annual congress of the European Society of Cardiology. The analysis also showed trends toward lower rates of stroke or systemic thrombosis as well as major bleeding events when patients received a DOAC, compared with those on VKA, but these differences were not statistically significant, reported Dr. Camm, a professor of clinical cardiology at St. George’s University of London.
The analyses run by Dr. Camm and his associates also confirmed the superiority of oral anticoagulation. There was an adjusted 17% relative risk reduction in all-cause mortality during 2-year follow-up in patients on any form of oral anticoagulation, compared with patients who did not receive anticoagulation, a statistically significant difference. The comparison of patients on any oral anticoagulant with those not on treatment also showed a significant lowering of stroke or systemic embolism, as well as a 36% relative increase in the risk for a major bleeding episode that was close to statistical significance.
These findings in a registry of patients undergoing routine care “suggest that the effectiveness of oral anticoagulants in randomized clinical trials can be translated to the broad cross section of patients treated in everyday practice,” Dr. Camm said. However, he highlighted two important qualifications to the findings.
First, the analysis focused on the type of anticoagulation patients received at the time they entered the GARFIELD-AF registry and did not account for possible changes in treatment after that. Second, the analysis did not adjust for additional potential confounding variables, which Dr. Camm was certain existed and affected the findings.
“I’m concerned that a confounder we have not been able to account for is the quality of medical care that patients received,” he noted. “The substantial reduction in mortality [using a DOAC, compared with a VKA] is not simply due to reductions in stroke or major bleeding. We must look at other explanations, such as differences in quality of care and access to care.”
The analyses have also not yet looked at outcomes based on the specific DOAC a patient received – apixaban, dabigatran, edoxaban, or rivaroxaban – something that Dr. Camm said is in the works.
GARFIELD-AF enrolled nearly 35,000 patients with newly diagnosed atrial fibrillation and at least one stroke risk factor in 35 countries from April 2013 to September 2016. The analysis winnowed this down to 26,742 patients who also had a CHA2DS2-VASc score of at least 2 (which identifies patients with a high thrombotic risk) and had complete enrollment and follow-up data.
GARFIELD-AF was funded in part by Bayer. Dr. Camm reported being an adviser to Bayer, Boehringer Ingelheim, Daiichi Sankyo, and Pfizer/Bristol-Myers Squibb.
MUNICH – Treatment of real-world patients newly diagnosed with atrial fibrillation using a direct oral anticoagulant led to benefits that tracked the advantages previously seen in randomized, controlled trials of these drugs, based on findings from more than 26,000 patients enrolled in a global registry.
Atrial fibrillation patients enrolled in the GARFIELD-AF(Global Anticoagulant Registry in the Field) study who started treatment with a direct oral anticoagulant (DOAC) had a 19% relative risk reduction in all-cause mortality during 2 years of follow-up, compared with patients on an oral vitamin K antagonist (VKA) regimen (such as warfarin), a statistically significant difference after adjustment for 30 demographic, clinical, and registry variables, A. John Camm, MD, said at the annual congress of the European Society of Cardiology. The analysis also showed trends toward lower rates of stroke or systemic thrombosis as well as major bleeding events when patients received a DOAC, compared with those on VKA, but these differences were not statistically significant, reported Dr. Camm, a professor of clinical cardiology at St. George’s University of London.
The analyses run by Dr. Camm and his associates also confirmed the superiority of oral anticoagulation. There was an adjusted 17% relative risk reduction in all-cause mortality during 2-year follow-up in patients on any form of oral anticoagulation, compared with patients who did not receive anticoagulation, a statistically significant difference. The comparison of patients on any oral anticoagulant with those not on treatment also showed a significant lowering of stroke or systemic embolism, as well as a 36% relative increase in the risk for a major bleeding episode that was close to statistical significance.
These findings in a registry of patients undergoing routine care “suggest that the effectiveness of oral anticoagulants in randomized clinical trials can be translated to the broad cross section of patients treated in everyday practice,” Dr. Camm said. However, he highlighted two important qualifications to the findings.
First, the analysis focused on the type of anticoagulation patients received at the time they entered the GARFIELD-AF registry and did not account for possible changes in treatment after that. Second, the analysis did not adjust for additional potential confounding variables, which Dr. Camm was certain existed and affected the findings.
“I’m concerned that a confounder we have not been able to account for is the quality of medical care that patients received,” he noted. “The substantial reduction in mortality [using a DOAC, compared with a VKA] is not simply due to reductions in stroke or major bleeding. We must look at other explanations, such as differences in quality of care and access to care.”
The analyses have also not yet looked at outcomes based on the specific DOAC a patient received – apixaban, dabigatran, edoxaban, or rivaroxaban – something that Dr. Camm said is in the works.
GARFIELD-AF enrolled nearly 35,000 patients with newly diagnosed atrial fibrillation and at least one stroke risk factor in 35 countries from April 2013 to September 2016. The analysis winnowed this down to 26,742 patients who also had a CHA2DS2-VASc score of at least 2 (which identifies patients with a high thrombotic risk) and had complete enrollment and follow-up data.
GARFIELD-AF was funded in part by Bayer. Dr. Camm reported being an adviser to Bayer, Boehringer Ingelheim, Daiichi Sankyo, and Pfizer/Bristol-Myers Squibb.
MUNICH – Treatment of real-world patients newly diagnosed with atrial fibrillation using a direct oral anticoagulant led to benefits that tracked the advantages previously seen in randomized, controlled trials of these drugs, based on findings from more than 26,000 patients enrolled in a global registry.
Atrial fibrillation patients enrolled in the GARFIELD-AF(Global Anticoagulant Registry in the Field) study who started treatment with a direct oral anticoagulant (DOAC) had a 19% relative risk reduction in all-cause mortality during 2 years of follow-up, compared with patients on an oral vitamin K antagonist (VKA) regimen (such as warfarin), a statistically significant difference after adjustment for 30 demographic, clinical, and registry variables, A. John Camm, MD, said at the annual congress of the European Society of Cardiology. The analysis also showed trends toward lower rates of stroke or systemic thrombosis as well as major bleeding events when patients received a DOAC, compared with those on VKA, but these differences were not statistically significant, reported Dr. Camm, a professor of clinical cardiology at St. George’s University of London.
The analyses run by Dr. Camm and his associates also confirmed the superiority of oral anticoagulation. There was an adjusted 17% relative risk reduction in all-cause mortality during 2-year follow-up in patients on any form of oral anticoagulation, compared with patients who did not receive anticoagulation, a statistically significant difference. The comparison of patients on any oral anticoagulant with those not on treatment also showed a significant lowering of stroke or systemic embolism, as well as a 36% relative increase in the risk for a major bleeding episode that was close to statistical significance.
These findings in a registry of patients undergoing routine care “suggest that the effectiveness of oral anticoagulants in randomized clinical trials can be translated to the broad cross section of patients treated in everyday practice,” Dr. Camm said. However, he highlighted two important qualifications to the findings.
First, the analysis focused on the type of anticoagulation patients received at the time they entered the GARFIELD-AF registry and did not account for possible changes in treatment after that. Second, the analysis did not adjust for additional potential confounding variables, which Dr. Camm was certain existed and affected the findings.
“I’m concerned that a confounder we have not been able to account for is the quality of medical care that patients received,” he noted. “The substantial reduction in mortality [using a DOAC, compared with a VKA] is not simply due to reductions in stroke or major bleeding. We must look at other explanations, such as differences in quality of care and access to care.”
The analyses have also not yet looked at outcomes based on the specific DOAC a patient received – apixaban, dabigatran, edoxaban, or rivaroxaban – something that Dr. Camm said is in the works.
GARFIELD-AF enrolled nearly 35,000 patients with newly diagnosed atrial fibrillation and at least one stroke risk factor in 35 countries from April 2013 to September 2016. The analysis winnowed this down to 26,742 patients who also had a CHA2DS2-VASc score of at least 2 (which identifies patients with a high thrombotic risk) and had complete enrollment and follow-up data.
GARFIELD-AF was funded in part by Bayer. Dr. Camm reported being an adviser to Bayer, Boehringer Ingelheim, Daiichi Sankyo, and Pfizer/Bristol-Myers Squibb.
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point:
Major finding: Direct oral anticoagulant–treated patients had a 19% relative reduction in all-cause death, compared with patients on a vitamin K antagonist.
Study details: The GARFIELD-AF registry, which included 26,742 patients with newly diagnosed atrial fibrillation.
Disclosures: GARFIELD-AF was funded in part by Bayer. Dr. Camm has been an adviser to Bayer, Boehringer Ingelheim, Daiichi Sankyo, and Pfizer/Bristol-Myers Squibb.















