User login
Resection of five-centimeter cesarean scar ectopic pregnancy and isthmocele repair using vascular clamps
A hypogastric nerve-focused approach to nerve-sparing endometriosis surgery
Radical resection of deep infiltrating endometriosis (DIE) or pelvic malignancies can lead to inadvertent damage to the pelvic autonomic nerve bundles, causing urinary dysfunction in up to 41% of cases, as well as anorectal and sexual dysfunction.1 Each of these sequelae can significantly affect the patient’s quality of life.
Nerve-sparing techniques have therefore been a trending topic in gynecologic surgery in the 21st century, starting with papers by Marc Possover, MD, of Switzerland, on the laparoscopic neuronavigation (LANN) technique. In an important 2005 publication, he described how the LANN technique can significantly reduce postoperative functional morbidity in laparoscopic radical pelvic surgery.2
The LANN method utilizes intraoperative neurostimulation to identify and dissect the intrapelvic nerve bundles away from surrounding tissue prior to dissection of the DIE or pelvic malignancies. The nerves are exposed and preserved under direct visualization in a fashion similar to that used to expose and preserve the ureters. Pelvic dissection using the LANN technique is extensive and occurs down to the level of the sacral nerve roots.
Dr. Possover’s 2005 paper and others like it spurred increased awareness of the intrapelvic part of the autonomic nervous system – in particular, the hypogastric nerves, the pelvic splanchnic nerves, and the inferior hypogastric plexus. Across additional published studies, nerve-sparing techniques were shown to be effective in preserving neurologic pelvic functions, with significantly less urinary retention and rectal/sexual dysfunction than seen with traditional laparoscopy techniques.
For example, in a single-center prospective clinical trial reported in 2012, 56 of 65 (86.2%) patients treated with a classical laparoscopic technique for excision of DIE reported neurologic pelvic dysfunctions, compared with 1 of 61 (1.6%) patients treated with a nerve-sparing approach.3
While research has confirmed the importance of nerve-sparing techniques, it also shone light on the reality that the LANN technique is extremely technically challenging and requires a high level of surgical expertise and advanced training. In my teaching of the technique, I also saw that few gynecologic surgeons were able to incorporate the advanced nerve-sparing technique into their practices.
A group consisting of myself and collaborators at the University of Bologna, Italy, and the University of Cambridge, England, recently developed an alternative to the LANN approach that uses the hypogastric nerves as landmarks. The technique requires less dissection and should be technically achievable when the pelvic neuroanatomy and anatomy of the presacral fascia are well understood. The hypogastric nerve is identified and used as a landmark to preserve the deeper autonomic nerve bundles in the pelvis without exposure and without more extensive dissection to the level of the sacral nerve roots.4,5
This hypogastric nerve-based technique will cover the vast majority of radical surgeries for DIE. When more advanced nerve sparing and more extensive dissection is needed for the very deepest levels of disease infiltration, patients can be referred to surgeons with advanced training, comfort, and experience with the LANN technique.
The pelvic neuroanatomy
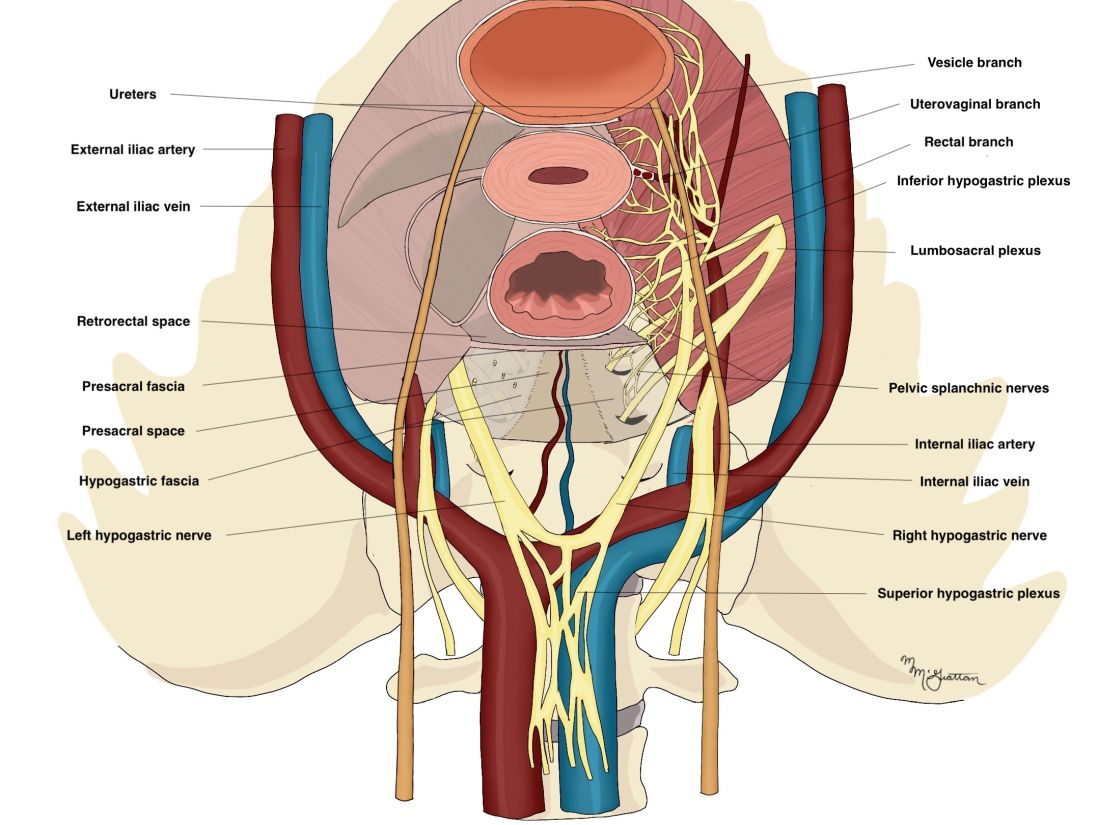
As described in our video articles published in 2015 in Fertility and Sterility6 and 2019 in the Journal of Minimally Invasive Gynecology,5 the left and right hypogastric nerves are the main sympathetic nerves of the autonomic nervous system in the pelvis. They originate from the superior hypogastric plexus and, at the level of the middle rectal vessels, they join the pelvic sacral splanchnic nerves to form the inferior hypogastric plexus. They are easily identifiable at their origin and are the most superficial and readily identifiable component of the inferior hypogastric plexus.
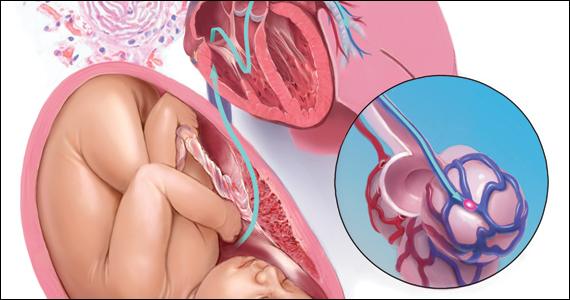
The sympathetic input from the hypogastric nerves causes the internal urethral and anal sphincters to contract, as well as detrusor relaxation and a reduction of peristalsis of the descending colon, sigmoid, and rectum; thus, hypogastric nerve input promotes continence.
The hypogastric nerves also carry afferent signals for pelvic visceral proprioception. Lesion to the hypogastric nerves will usually be subclinical and will put the patient at risk for unnoticeable bladder distension, which usually becomes symptomatic about 7 years after the procedure.7
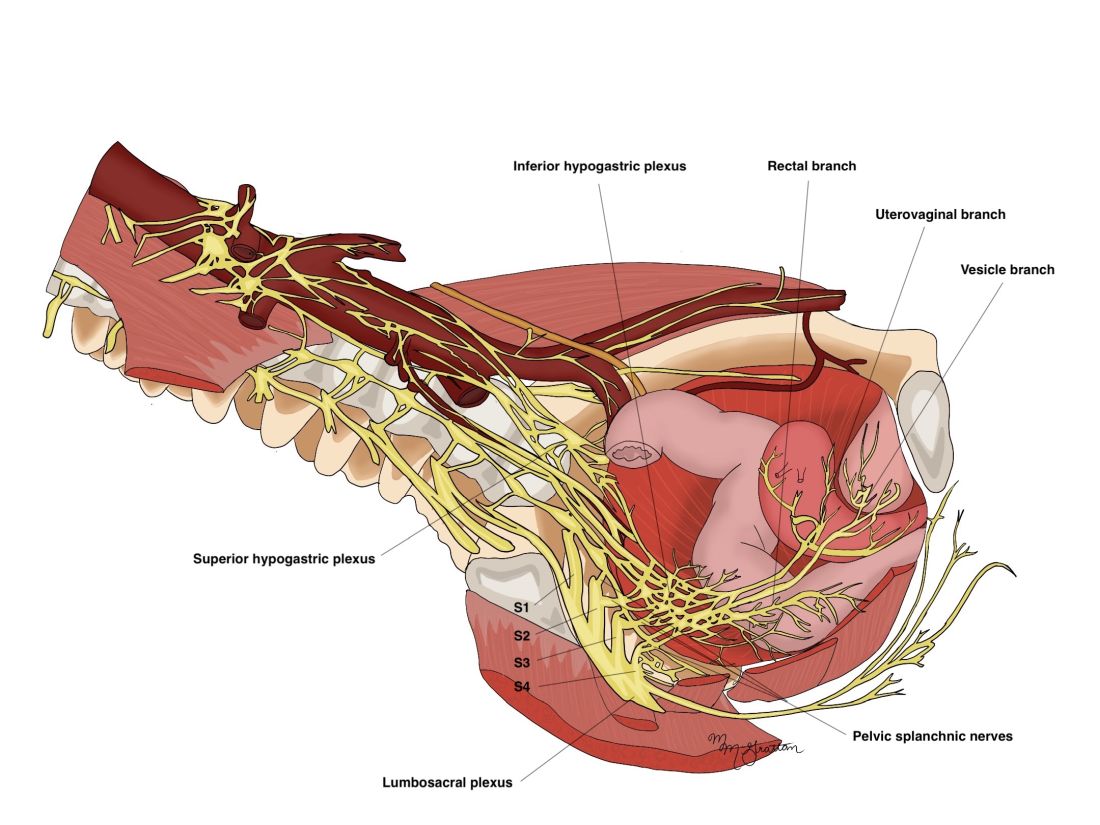
The thin pelvic splanchnic nerves – which merge with the hypogastric nerves into the pararectal fossae to form the inferior hypogastric plexus – arise from nerve roots S2 and S4 and carry all parasympathetic signals to the bladder, rectum, and the sigmoid and left colons. Lesions to these bundles are the main cause of neurogenic urinary retention.
The inferior hypogastric plexi split into the vesical, uterine, and rectal branches, which carry the sympathetic, parasympathetic, and sensory fibers from both the splanchnic and hypogastric nerves. Damage to the inferior hypogastric plexi and/or its branches may induce severe dysfunction to the target organs of the injured fibers.
A focus on the hypogastric nerve
Our approach was developed after we studied the anatomic reliability of the hypogastric nerves through a prospective observational study consisting of measurements during five cadaveric dissections and 10 in-vivo laparoscopic surgeries for rectosigmoid endometriosis.4 We took an interfascial approach to dissection.
Our goal was to clarify the distances between the hypogastric nerves and the ureters, the midsagittal plane, the midcervical plane, and the uterosacral ligaments in each hemipelvis, and in doing so, enable identification of the hypogastric nerves and establish recognizable limits for dissection.
We found quite a bit of variance in the anatomic position and appearance of the hypogastric nerves, but the variances were not very broad. Most notably, the right hypogastric nerve was significantly farther toward the ureter (mean, 14.5 mm; range, 10-25 mm) than the left one (mean, 8.6 mm; range, 7-12 mm).
The ureters were a good landmark for identification of the hypogastric nerves because the nerves were consistently found medially and posteriorly to the ureter at a mean distance of 11.6 mm. Overall, we demonstrated reproducibility in the identification and dissection of the hypogastric nerves using recognizable interfascial planes and anatomic landmarks.4
With good anatomic understanding, a stepwise approach can be taken to identify and preserve the hypogastric nerve and the deeper inferior hypogastric plexus without the need for more extensive dissection.
As shown in our 2019 video, the right hypogastric nerves can be identified transperitoneally in most cases.5 For confirmation, a gentle anterior pulling on the hypogastric nerve causes a caudal movement of the peritoneum overlying the superior hypogastric plexus. (Intermittent pulling on the nerve can also be helpful in localizing the left hypogastric nerve.)
To dissect a hypogastric nerve, the retroperitoneum is opened at the level of the pelvic brim, just inferomedially to the external iliac vessels, and the incision is extended anteriorly, with gentle dissection of the underlying tissue until the ureter is identified.
Once the ureter is identified and lateralized, dissection along the peritoneum is carried deeper and medially into the pelvis until the hypogastric nerve is identified. Lateral to this area are the internal iliac artery, the branching uterine artery, and the obliterated umbilical ligament. In the left hemipelvis, the hypogastric nerve can reliably be found at a mean distance of 8.6 mm from the ureter, while the right one will be found on average 14.5 mm away.
The hypogastric nerves form the posteromedial limit for a safe and simple nerve-sparing dissection. Any dissection posteriorly and laterally to these landmarks should start with the identification of sacral nerve roots and hypogastric nerves.
Dr. Lemos reported that he has no relevant disclosures.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto.
References
1. Imboden S et al. J Minim Invasive Gynecol. 2021 Aug;28(8):1544-51. doi: 10.1016/j.jmig.2021.01.009.
2. Possover M et al. J Am Coll Surg. 2005;201(6):913-7. doi: 10.1016/j.jamcollsurg.2005.07.006.
3. Ceccaroni M et al. Surg Endosc. 2012;26(7):2029-45. doi: 10.1007/s00464-012-2153-3.
4. Seracchioli R et al. J Minim Invasive Gynecol. 2019;26(7):1340-5. doi: 10.1016/j.jmig.2019.01.010.
5. Zakhari A et al. J Minim Invasive Gynecol. 2020;27(4):813-4. doi: 10.1016/j.jmig.2019.08.001
6. Lemos N et al. Fertil Steril. 2015 Nov;104(5):e11-2. doi: 10.1016/j.fertnstert.2015.07.1138.
7. Possover M. Fertil Steril. 2014 Mar;101(3):754-8. doi: 10.1016/j.fertnstert.2013.12.019.
Radical resection of deep infiltrating endometriosis (DIE) or pelvic malignancies can lead to inadvertent damage to the pelvic autonomic nerve bundles, causing urinary dysfunction in up to 41% of cases, as well as anorectal and sexual dysfunction.1 Each of these sequelae can significantly affect the patient’s quality of life.
Nerve-sparing techniques have therefore been a trending topic in gynecologic surgery in the 21st century, starting with papers by Marc Possover, MD, of Switzerland, on the laparoscopic neuronavigation (LANN) technique. In an important 2005 publication, he described how the LANN technique can significantly reduce postoperative functional morbidity in laparoscopic radical pelvic surgery.2
The LANN method utilizes intraoperative neurostimulation to identify and dissect the intrapelvic nerve bundles away from surrounding tissue prior to dissection of the DIE or pelvic malignancies. The nerves are exposed and preserved under direct visualization in a fashion similar to that used to expose and preserve the ureters. Pelvic dissection using the LANN technique is extensive and occurs down to the level of the sacral nerve roots.
Dr. Possover’s 2005 paper and others like it spurred increased awareness of the intrapelvic part of the autonomic nervous system – in particular, the hypogastric nerves, the pelvic splanchnic nerves, and the inferior hypogastric plexus. Across additional published studies, nerve-sparing techniques were shown to be effective in preserving neurologic pelvic functions, with significantly less urinary retention and rectal/sexual dysfunction than seen with traditional laparoscopy techniques.
For example, in a single-center prospective clinical trial reported in 2012, 56 of 65 (86.2%) patients treated with a classical laparoscopic technique for excision of DIE reported neurologic pelvic dysfunctions, compared with 1 of 61 (1.6%) patients treated with a nerve-sparing approach.3
While research has confirmed the importance of nerve-sparing techniques, it also shone light on the reality that the LANN technique is extremely technically challenging and requires a high level of surgical expertise and advanced training. In my teaching of the technique, I also saw that few gynecologic surgeons were able to incorporate the advanced nerve-sparing technique into their practices.
A group consisting of myself and collaborators at the University of Bologna, Italy, and the University of Cambridge, England, recently developed an alternative to the LANN approach that uses the hypogastric nerves as landmarks. The technique requires less dissection and should be technically achievable when the pelvic neuroanatomy and anatomy of the presacral fascia are well understood. The hypogastric nerve is identified and used as a landmark to preserve the deeper autonomic nerve bundles in the pelvis without exposure and without more extensive dissection to the level of the sacral nerve roots.4,5
This hypogastric nerve-based technique will cover the vast majority of radical surgeries for DIE. When more advanced nerve sparing and more extensive dissection is needed for the very deepest levels of disease infiltration, patients can be referred to surgeons with advanced training, comfort, and experience with the LANN technique.
The pelvic neuroanatomy
As described in our video articles published in 2015 in Fertility and Sterility6 and 2019 in the Journal of Minimally Invasive Gynecology,5 the left and right hypogastric nerves are the main sympathetic nerves of the autonomic nervous system in the pelvis. They originate from the superior hypogastric plexus and, at the level of the middle rectal vessels, they join the pelvic sacral splanchnic nerves to form the inferior hypogastric plexus. They are easily identifiable at their origin and are the most superficial and readily identifiable component of the inferior hypogastric plexus.

The sympathetic input from the hypogastric nerves causes the internal urethral and anal sphincters to contract, as well as detrusor relaxation and a reduction of peristalsis of the descending colon, sigmoid, and rectum; thus, hypogastric nerve input promotes continence.
The hypogastric nerves also carry afferent signals for pelvic visceral proprioception. Lesion to the hypogastric nerves will usually be subclinical and will put the patient at risk for unnoticeable bladder distension, which usually becomes symptomatic about 7 years after the procedure.7

The thin pelvic splanchnic nerves – which merge with the hypogastric nerves into the pararectal fossae to form the inferior hypogastric plexus – arise from nerve roots S2 and S4 and carry all parasympathetic signals to the bladder, rectum, and the sigmoid and left colons. Lesions to these bundles are the main cause of neurogenic urinary retention.
The inferior hypogastric plexi split into the vesical, uterine, and rectal branches, which carry the sympathetic, parasympathetic, and sensory fibers from both the splanchnic and hypogastric nerves. Damage to the inferior hypogastric plexi and/or its branches may induce severe dysfunction to the target organs of the injured fibers.
A focus on the hypogastric nerve
Our approach was developed after we studied the anatomic reliability of the hypogastric nerves through a prospective observational study consisting of measurements during five cadaveric dissections and 10 in-vivo laparoscopic surgeries for rectosigmoid endometriosis.4 We took an interfascial approach to dissection.
Our goal was to clarify the distances between the hypogastric nerves and the ureters, the midsagittal plane, the midcervical plane, and the uterosacral ligaments in each hemipelvis, and in doing so, enable identification of the hypogastric nerves and establish recognizable limits for dissection.
We found quite a bit of variance in the anatomic position and appearance of the hypogastric nerves, but the variances were not very broad. Most notably, the right hypogastric nerve was significantly farther toward the ureter (mean, 14.5 mm; range, 10-25 mm) than the left one (mean, 8.6 mm; range, 7-12 mm).
The ureters were a good landmark for identification of the hypogastric nerves because the nerves were consistently found medially and posteriorly to the ureter at a mean distance of 11.6 mm. Overall, we demonstrated reproducibility in the identification and dissection of the hypogastric nerves using recognizable interfascial planes and anatomic landmarks.4
With good anatomic understanding, a stepwise approach can be taken to identify and preserve the hypogastric nerve and the deeper inferior hypogastric plexus without the need for more extensive dissection.
As shown in our 2019 video, the right hypogastric nerves can be identified transperitoneally in most cases.5 For confirmation, a gentle anterior pulling on the hypogastric nerve causes a caudal movement of the peritoneum overlying the superior hypogastric plexus. (Intermittent pulling on the nerve can also be helpful in localizing the left hypogastric nerve.)
To dissect a hypogastric nerve, the retroperitoneum is opened at the level of the pelvic brim, just inferomedially to the external iliac vessels, and the incision is extended anteriorly, with gentle dissection of the underlying tissue until the ureter is identified.
Once the ureter is identified and lateralized, dissection along the peritoneum is carried deeper and medially into the pelvis until the hypogastric nerve is identified. Lateral to this area are the internal iliac artery, the branching uterine artery, and the obliterated umbilical ligament. In the left hemipelvis, the hypogastric nerve can reliably be found at a mean distance of 8.6 mm from the ureter, while the right one will be found on average 14.5 mm away.
The hypogastric nerves form the posteromedial limit for a safe and simple nerve-sparing dissection. Any dissection posteriorly and laterally to these landmarks should start with the identification of sacral nerve roots and hypogastric nerves.
Dr. Lemos reported that he has no relevant disclosures.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto.
References
1. Imboden S et al. J Minim Invasive Gynecol. 2021 Aug;28(8):1544-51. doi: 10.1016/j.jmig.2021.01.009.
2. Possover M et al. J Am Coll Surg. 2005;201(6):913-7. doi: 10.1016/j.jamcollsurg.2005.07.006.
3. Ceccaroni M et al. Surg Endosc. 2012;26(7):2029-45. doi: 10.1007/s00464-012-2153-3.
4. Seracchioli R et al. J Minim Invasive Gynecol. 2019;26(7):1340-5. doi: 10.1016/j.jmig.2019.01.010.
5. Zakhari A et al. J Minim Invasive Gynecol. 2020;27(4):813-4. doi: 10.1016/j.jmig.2019.08.001
6. Lemos N et al. Fertil Steril. 2015 Nov;104(5):e11-2. doi: 10.1016/j.fertnstert.2015.07.1138.
7. Possover M. Fertil Steril. 2014 Mar;101(3):754-8. doi: 10.1016/j.fertnstert.2013.12.019.
Radical resection of deep infiltrating endometriosis (DIE) or pelvic malignancies can lead to inadvertent damage to the pelvic autonomic nerve bundles, causing urinary dysfunction in up to 41% of cases, as well as anorectal and sexual dysfunction.1 Each of these sequelae can significantly affect the patient’s quality of life.
Nerve-sparing techniques have therefore been a trending topic in gynecologic surgery in the 21st century, starting with papers by Marc Possover, MD, of Switzerland, on the laparoscopic neuronavigation (LANN) technique. In an important 2005 publication, he described how the LANN technique can significantly reduce postoperative functional morbidity in laparoscopic radical pelvic surgery.2
The LANN method utilizes intraoperative neurostimulation to identify and dissect the intrapelvic nerve bundles away from surrounding tissue prior to dissection of the DIE or pelvic malignancies. The nerves are exposed and preserved under direct visualization in a fashion similar to that used to expose and preserve the ureters. Pelvic dissection using the LANN technique is extensive and occurs down to the level of the sacral nerve roots.
Dr. Possover’s 2005 paper and others like it spurred increased awareness of the intrapelvic part of the autonomic nervous system – in particular, the hypogastric nerves, the pelvic splanchnic nerves, and the inferior hypogastric plexus. Across additional published studies, nerve-sparing techniques were shown to be effective in preserving neurologic pelvic functions, with significantly less urinary retention and rectal/sexual dysfunction than seen with traditional laparoscopy techniques.
For example, in a single-center prospective clinical trial reported in 2012, 56 of 65 (86.2%) patients treated with a classical laparoscopic technique for excision of DIE reported neurologic pelvic dysfunctions, compared with 1 of 61 (1.6%) patients treated with a nerve-sparing approach.3
While research has confirmed the importance of nerve-sparing techniques, it also shone light on the reality that the LANN technique is extremely technically challenging and requires a high level of surgical expertise and advanced training. In my teaching of the technique, I also saw that few gynecologic surgeons were able to incorporate the advanced nerve-sparing technique into their practices.
A group consisting of myself and collaborators at the University of Bologna, Italy, and the University of Cambridge, England, recently developed an alternative to the LANN approach that uses the hypogastric nerves as landmarks. The technique requires less dissection and should be technically achievable when the pelvic neuroanatomy and anatomy of the presacral fascia are well understood. The hypogastric nerve is identified and used as a landmark to preserve the deeper autonomic nerve bundles in the pelvis without exposure and without more extensive dissection to the level of the sacral nerve roots.4,5
This hypogastric nerve-based technique will cover the vast majority of radical surgeries for DIE. When more advanced nerve sparing and more extensive dissection is needed for the very deepest levels of disease infiltration, patients can be referred to surgeons with advanced training, comfort, and experience with the LANN technique.
The pelvic neuroanatomy
As described in our video articles published in 2015 in Fertility and Sterility6 and 2019 in the Journal of Minimally Invasive Gynecology,5 the left and right hypogastric nerves are the main sympathetic nerves of the autonomic nervous system in the pelvis. They originate from the superior hypogastric plexus and, at the level of the middle rectal vessels, they join the pelvic sacral splanchnic nerves to form the inferior hypogastric plexus. They are easily identifiable at their origin and are the most superficial and readily identifiable component of the inferior hypogastric plexus.

The sympathetic input from the hypogastric nerves causes the internal urethral and anal sphincters to contract, as well as detrusor relaxation and a reduction of peristalsis of the descending colon, sigmoid, and rectum; thus, hypogastric nerve input promotes continence.
The hypogastric nerves also carry afferent signals for pelvic visceral proprioception. Lesion to the hypogastric nerves will usually be subclinical and will put the patient at risk for unnoticeable bladder distension, which usually becomes symptomatic about 7 years after the procedure.7

The thin pelvic splanchnic nerves – which merge with the hypogastric nerves into the pararectal fossae to form the inferior hypogastric plexus – arise from nerve roots S2 and S4 and carry all parasympathetic signals to the bladder, rectum, and the sigmoid and left colons. Lesions to these bundles are the main cause of neurogenic urinary retention.
The inferior hypogastric plexi split into the vesical, uterine, and rectal branches, which carry the sympathetic, parasympathetic, and sensory fibers from both the splanchnic and hypogastric nerves. Damage to the inferior hypogastric plexi and/or its branches may induce severe dysfunction to the target organs of the injured fibers.
A focus on the hypogastric nerve
Our approach was developed after we studied the anatomic reliability of the hypogastric nerves through a prospective observational study consisting of measurements during five cadaveric dissections and 10 in-vivo laparoscopic surgeries for rectosigmoid endometriosis.4 We took an interfascial approach to dissection.
Our goal was to clarify the distances between the hypogastric nerves and the ureters, the midsagittal plane, the midcervical plane, and the uterosacral ligaments in each hemipelvis, and in doing so, enable identification of the hypogastric nerves and establish recognizable limits for dissection.
We found quite a bit of variance in the anatomic position and appearance of the hypogastric nerves, but the variances were not very broad. Most notably, the right hypogastric nerve was significantly farther toward the ureter (mean, 14.5 mm; range, 10-25 mm) than the left one (mean, 8.6 mm; range, 7-12 mm).
The ureters were a good landmark for identification of the hypogastric nerves because the nerves were consistently found medially and posteriorly to the ureter at a mean distance of 11.6 mm. Overall, we demonstrated reproducibility in the identification and dissection of the hypogastric nerves using recognizable interfascial planes and anatomic landmarks.4
With good anatomic understanding, a stepwise approach can be taken to identify and preserve the hypogastric nerve and the deeper inferior hypogastric plexus without the need for more extensive dissection.
As shown in our 2019 video, the right hypogastric nerves can be identified transperitoneally in most cases.5 For confirmation, a gentle anterior pulling on the hypogastric nerve causes a caudal movement of the peritoneum overlying the superior hypogastric plexus. (Intermittent pulling on the nerve can also be helpful in localizing the left hypogastric nerve.)
To dissect a hypogastric nerve, the retroperitoneum is opened at the level of the pelvic brim, just inferomedially to the external iliac vessels, and the incision is extended anteriorly, with gentle dissection of the underlying tissue until the ureter is identified.
Once the ureter is identified and lateralized, dissection along the peritoneum is carried deeper and medially into the pelvis until the hypogastric nerve is identified. Lateral to this area are the internal iliac artery, the branching uterine artery, and the obliterated umbilical ligament. In the left hemipelvis, the hypogastric nerve can reliably be found at a mean distance of 8.6 mm from the ureter, while the right one will be found on average 14.5 mm away.
The hypogastric nerves form the posteromedial limit for a safe and simple nerve-sparing dissection. Any dissection posteriorly and laterally to these landmarks should start with the identification of sacral nerve roots and hypogastric nerves.
Dr. Lemos reported that he has no relevant disclosures.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto.
References
1. Imboden S et al. J Minim Invasive Gynecol. 2021 Aug;28(8):1544-51. doi: 10.1016/j.jmig.2021.01.009.
2. Possover M et al. J Am Coll Surg. 2005;201(6):913-7. doi: 10.1016/j.jamcollsurg.2005.07.006.
3. Ceccaroni M et al. Surg Endosc. 2012;26(7):2029-45. doi: 10.1007/s00464-012-2153-3.
4. Seracchioli R et al. J Minim Invasive Gynecol. 2019;26(7):1340-5. doi: 10.1016/j.jmig.2019.01.010.
5. Zakhari A et al. J Minim Invasive Gynecol. 2020;27(4):813-4. doi: 10.1016/j.jmig.2019.08.001
6. Lemos N et al. Fertil Steril. 2015 Nov;104(5):e11-2. doi: 10.1016/j.fertnstert.2015.07.1138.
7. Possover M. Fertil Steril. 2014 Mar;101(3):754-8. doi: 10.1016/j.fertnstert.2013.12.019.
Spare the nerves in deep infiltrative endometriosis surgery
The pelvic autonomic nerves are responsible for the neurogenic control of the rectum and bladder and for sexual arousal. Over the past 30 years, different nerve-sparing techniques have been recommended and adopted to minimize risk of urinary or rectal dysfunction and incontinence, as well as sexual dysfunction, in radical surgery for rectal and early cervical cancer without compromising surgical outcome.
As the treatment of deep infiltrative endometriosis has become more aggressive and radical, it is certainly feasible to consider nerve-sparing techniques at the time of dissection and endometriosis excision to minimize the known risk of urinary, rectal, and sexual dysfunction. Interestingly, because endometriosis generally follows an asymmetric distribution, effect on bladder function is not as problematic as it is in the case of cancer surgery.
Early innovators include Dr. Marc Possover from Switzerland and Dr. Marcello Ceccaroni from Italy. Both physicians are superior pelvic neuroanatomists. Both describe meticulous and extensive dissection of the nerves of the pelvis at the time of excision of deep infiltrative endometriosis. Unfortunately, their techniques would appear to be beyond the scope of even the most experienced excisional surgeons.
A simplified approach to nerve sparing at the time of excision of deep infiltrative endometriosis has been developed by our guest author, Dr. Nucelio Lemos, in collaboration with physicians at the University of Bologna and the University of Cambridge. By using the hypogastric nerves as the landmark, they have developed a more surgeon friendly and less radical approach to nerve sparing at the time of deep infiltrative endometriosis surgery.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of both Dr. Lemos and his fellow in advanced gynecologic surgery, Dr. Meghan McGrattan, from Mount Sinai and Women’s College Hospital in Toronto. Dr. McGrattan drew the anatomic illustrations that accompany Dr. Lemos’ description of the new technique.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto. He specializes in pelvic pain, pelvic floor dysfunction, pelvic organ prolapse, endometriosis, and neuropelveology. Dr. Lemos is a founding member and second vice president of the International Society of Neuropelveology. In addition, Dr. Lemos started the Pelvic Functional Surgery and Neuropelveology Clinic in the department of obstetrics and gynecology of Mount Sinai and Women’s College Hospitals, Toronto.
It is a pleasure and honor to welcome Dr. Lemos and Dr. McGrattan to this addition of the Master Class in Gynecologic Surgery.
Dr. Miller is a professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago, Ill. He has no conflicts of interest to report.
The pelvic autonomic nerves are responsible for the neurogenic control of the rectum and bladder and for sexual arousal. Over the past 30 years, different nerve-sparing techniques have been recommended and adopted to minimize risk of urinary or rectal dysfunction and incontinence, as well as sexual dysfunction, in radical surgery for rectal and early cervical cancer without compromising surgical outcome.
As the treatment of deep infiltrative endometriosis has become more aggressive and radical, it is certainly feasible to consider nerve-sparing techniques at the time of dissection and endometriosis excision to minimize the known risk of urinary, rectal, and sexual dysfunction. Interestingly, because endometriosis generally follows an asymmetric distribution, effect on bladder function is not as problematic as it is in the case of cancer surgery.
Early innovators include Dr. Marc Possover from Switzerland and Dr. Marcello Ceccaroni from Italy. Both physicians are superior pelvic neuroanatomists. Both describe meticulous and extensive dissection of the nerves of the pelvis at the time of excision of deep infiltrative endometriosis. Unfortunately, their techniques would appear to be beyond the scope of even the most experienced excisional surgeons.
A simplified approach to nerve sparing at the time of excision of deep infiltrative endometriosis has been developed by our guest author, Dr. Nucelio Lemos, in collaboration with physicians at the University of Bologna and the University of Cambridge. By using the hypogastric nerves as the landmark, they have developed a more surgeon friendly and less radical approach to nerve sparing at the time of deep infiltrative endometriosis surgery.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of both Dr. Lemos and his fellow in advanced gynecologic surgery, Dr. Meghan McGrattan, from Mount Sinai and Women’s College Hospital in Toronto. Dr. McGrattan drew the anatomic illustrations that accompany Dr. Lemos’ description of the new technique.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto. He specializes in pelvic pain, pelvic floor dysfunction, pelvic organ prolapse, endometriosis, and neuropelveology. Dr. Lemos is a founding member and second vice president of the International Society of Neuropelveology. In addition, Dr. Lemos started the Pelvic Functional Surgery and Neuropelveology Clinic in the department of obstetrics and gynecology of Mount Sinai and Women’s College Hospitals, Toronto.
It is a pleasure and honor to welcome Dr. Lemos and Dr. McGrattan to this addition of the Master Class in Gynecologic Surgery.
Dr. Miller is a professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago, Ill. He has no conflicts of interest to report.
The pelvic autonomic nerves are responsible for the neurogenic control of the rectum and bladder and for sexual arousal. Over the past 30 years, different nerve-sparing techniques have been recommended and adopted to minimize risk of urinary or rectal dysfunction and incontinence, as well as sexual dysfunction, in radical surgery for rectal and early cervical cancer without compromising surgical outcome.
As the treatment of deep infiltrative endometriosis has become more aggressive and radical, it is certainly feasible to consider nerve-sparing techniques at the time of dissection and endometriosis excision to minimize the known risk of urinary, rectal, and sexual dysfunction. Interestingly, because endometriosis generally follows an asymmetric distribution, effect on bladder function is not as problematic as it is in the case of cancer surgery.
Early innovators include Dr. Marc Possover from Switzerland and Dr. Marcello Ceccaroni from Italy. Both physicians are superior pelvic neuroanatomists. Both describe meticulous and extensive dissection of the nerves of the pelvis at the time of excision of deep infiltrative endometriosis. Unfortunately, their techniques would appear to be beyond the scope of even the most experienced excisional surgeons.
A simplified approach to nerve sparing at the time of excision of deep infiltrative endometriosis has been developed by our guest author, Dr. Nucelio Lemos, in collaboration with physicians at the University of Bologna and the University of Cambridge. By using the hypogastric nerves as the landmark, they have developed a more surgeon friendly and less radical approach to nerve sparing at the time of deep infiltrative endometriosis surgery.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of both Dr. Lemos and his fellow in advanced gynecologic surgery, Dr. Meghan McGrattan, from Mount Sinai and Women’s College Hospital in Toronto. Dr. McGrattan drew the anatomic illustrations that accompany Dr. Lemos’ description of the new technique.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto. He specializes in pelvic pain, pelvic floor dysfunction, pelvic organ prolapse, endometriosis, and neuropelveology. Dr. Lemos is a founding member and second vice president of the International Society of Neuropelveology. In addition, Dr. Lemos started the Pelvic Functional Surgery and Neuropelveology Clinic in the department of obstetrics and gynecology of Mount Sinai and Women’s College Hospitals, Toronto.
It is a pleasure and honor to welcome Dr. Lemos and Dr. McGrattan to this addition of the Master Class in Gynecologic Surgery.
Dr. Miller is a professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago, Ill. He has no conflicts of interest to report.
Pediatric obesity treatment options: Beyond lifestyle modification
Pediatric obesity is a serious problem, not only in the United States but worldwide. Unfortunately, the ongoing COVID-19 pandemic has worsened the epidemic of childhood obesity. Solutions for treating the millions of children and adolescents with obesity are desperately needed because prevention efforts over the past several decades have not been sufficient in slowing the steady rise in obesity prevalence.
Lifestyle modification, including dietary changes, increases in activity, and behavioral modification, are the cornerstone of any obesity treatment, but they alone are not powerful enough to treat obesity by itself in the vast majority of children and adolescents. This is because obesity is not a lifestyle choice; rather, it is a disease, and a disease that has a tremendous amount of biology driving individuals toward weight gain and the propensity toward weight regain if weight is lost.
Fortunately, the tools to treat the underlying biology driving obesity are becoming safer, more effective, and more widely used every year. The two most effective biology-based treatments for pediatric obesity are antiobesity medications and bariatric surgery. These two treatments, when accompanied by lifestyle modification, have the potential to reduce not only body weight but also treat many other risk factors, such as prediabetes, diabetes, high blood pressure, poor cholesterol, liver disease, and sleep apnea, as well as others.
Rise in antiobesity medications
Antiobesity medications are developing at a rapid pace. Seven medications have been approved by the Food and Drug Administration for adults, and three medications (phentermine, orlistat, and liraglutide) are now approved for children and adolescents.
The number of antiobesity medications for use in children and adolescents is expected to expand to five, with semaglutide and phentermine-topiramate (Qsymia) both completing trials in adolescents in 2022. Each of these medications works by treating the biology that drives weight gain, whether it is decreasing impulsivity, reducing hunger and appetite hormone pathways, or improving energy regulation pathways. Weight loss at 1 year for currently FDA-approved medications in adolescents ranges from 3% to 6% on average, depending on the medications. The newer medications already FDA approved in adults that will soon, hopefully, be available in pediatrics result in 10%-16% weight loss on average.
A common parent and patient question regarding antiobesity medications is: “If I start an antiobesity medication, how long will I need to be on it?” The simple answer is: “Probably for the rest of your life.”
This can be a shock to hear, but obesity treatment is very similar to that of hypertension or diabetes. Using high blood pressure as an example: If a patient has high blood pressure (for example, 160/90 mm Hg), they will be prescribed a medication to treat it. Once blood pressure comes down to near-normal levels (for example, 120/80 mm Hg), a dose will be maintained, not removed, because that is the biological mediator keeping the blood pressure low. Removal of the medication would result in blood pressure going back to homeostasis (160/90 mm Hg in our example) in a short period of time).
The same can be said for obesity. For example, if a 16-year-old girl is prescribed liraglutide, a glucagonlike peptide–1 receptor agonist, and loses 10% of her body weight at 1 year, that is great success. Why would we remove the medication that is treating the underlying biology causing successful weight loss?
In short, we would not want to do that. Even if our example patient only maintained that 10% initial weight loss, that would be very successful, just like someone maintaining their low blood pressure. As medications begin to develop at a rapid pace and become more available to pediatric patients, the messaging and conversation around anti-obesity medications must continue to focus on obesity being a biological disease and not a behavior for treatment to be effective and not stigmatized.
Bariatric surgery most effective treatment for pediatric obesity
Currently, the most effective treatment for pediatric obesity is bariatric surgery. The two most commonly used surgical procedures today are the sleeve gastrectomy and gastric bypass. Sleeve gastrectomy works by removing 75%-85% of the stomach and creating a new stomach, called a “sleeve.” Gastric bypass works by separating the stomach into two parts and connecting one part of the new stomach into the intestine.
Both surgeries are very effective at treating obesity in adolescents, with an average weight loss of 30%-35%. Surgery is not just a restrictive means of controlling body weight; it also changes key hormones for appetite and satiety that signal the brain. In fact, many of the same biological signals that are changed by surgery are the same signals being targeted by antiobesity medications. Long-term outcome of bariatric surgery in adolescents, provided by Teen-LABS, show it to be safe and maybe even more effective than in adults for treating diabetes and hypertension, with similar weight loss.
Does treatment outweigh the potential risks?
Although obesity surgery and antiobesity medications are more successful at treating obesity in children and adolescents than lifestyle medications, they do have some risks. Surgery, depending on the type of surgery, can cause nutritional deficiencies, reduce body mineral density, and is a life-changing medical procedure. Antiobesity medications, depending on the type, can cause nausea and vomiting and increase heart rate – and because they are relatively new, we do not fully understand the long-term impact of continued use past 1 year.
However, an important question to ask is: “Do the risks of obesity surgery and antiobesity medications outweigh the risk of having lifelong obesity?” The answer to me and many of my colleagues is: “Yes!” Although there are risks associated with the two best treatments for pediatric obesity, those risks under proper supervision of a medical professional far outweigh the risks of not properly treating obesity and allowing it to persist and get worse over many years to come. Obesity is a disease deeply rooted in biology, and we must use biology-based treatments to tackle this problem in children and adolescents, who deserve the best care and treatments possible.
Dr. Ryder is assistant professor of pediatrics and associate director of research, Center for Pediatric Obesity Medicine, at the University of Minnesota, Minneapolis. She reported receiving donations for clinical trials from Boehringer Ingelheim. A version of this article first appeared on Medscape.com.
Pediatric obesity is a serious problem, not only in the United States but worldwide. Unfortunately, the ongoing COVID-19 pandemic has worsened the epidemic of childhood obesity. Solutions for treating the millions of children and adolescents with obesity are desperately needed because prevention efforts over the past several decades have not been sufficient in slowing the steady rise in obesity prevalence.
Lifestyle modification, including dietary changes, increases in activity, and behavioral modification, are the cornerstone of any obesity treatment, but they alone are not powerful enough to treat obesity by itself in the vast majority of children and adolescents. This is because obesity is not a lifestyle choice; rather, it is a disease, and a disease that has a tremendous amount of biology driving individuals toward weight gain and the propensity toward weight regain if weight is lost.
Fortunately, the tools to treat the underlying biology driving obesity are becoming safer, more effective, and more widely used every year. The two most effective biology-based treatments for pediatric obesity are antiobesity medications and bariatric surgery. These two treatments, when accompanied by lifestyle modification, have the potential to reduce not only body weight but also treat many other risk factors, such as prediabetes, diabetes, high blood pressure, poor cholesterol, liver disease, and sleep apnea, as well as others.
Rise in antiobesity medications
Antiobesity medications are developing at a rapid pace. Seven medications have been approved by the Food and Drug Administration for adults, and three medications (phentermine, orlistat, and liraglutide) are now approved for children and adolescents.
The number of antiobesity medications for use in children and adolescents is expected to expand to five, with semaglutide and phentermine-topiramate (Qsymia) both completing trials in adolescents in 2022. Each of these medications works by treating the biology that drives weight gain, whether it is decreasing impulsivity, reducing hunger and appetite hormone pathways, or improving energy regulation pathways. Weight loss at 1 year for currently FDA-approved medications in adolescents ranges from 3% to 6% on average, depending on the medications. The newer medications already FDA approved in adults that will soon, hopefully, be available in pediatrics result in 10%-16% weight loss on average.
A common parent and patient question regarding antiobesity medications is: “If I start an antiobesity medication, how long will I need to be on it?” The simple answer is: “Probably for the rest of your life.”
This can be a shock to hear, but obesity treatment is very similar to that of hypertension or diabetes. Using high blood pressure as an example: If a patient has high blood pressure (for example, 160/90 mm Hg), they will be prescribed a medication to treat it. Once blood pressure comes down to near-normal levels (for example, 120/80 mm Hg), a dose will be maintained, not removed, because that is the biological mediator keeping the blood pressure low. Removal of the medication would result in blood pressure going back to homeostasis (160/90 mm Hg in our example) in a short period of time).
The same can be said for obesity. For example, if a 16-year-old girl is prescribed liraglutide, a glucagonlike peptide–1 receptor agonist, and loses 10% of her body weight at 1 year, that is great success. Why would we remove the medication that is treating the underlying biology causing successful weight loss?
In short, we would not want to do that. Even if our example patient only maintained that 10% initial weight loss, that would be very successful, just like someone maintaining their low blood pressure. As medications begin to develop at a rapid pace and become more available to pediatric patients, the messaging and conversation around anti-obesity medications must continue to focus on obesity being a biological disease and not a behavior for treatment to be effective and not stigmatized.
Bariatric surgery most effective treatment for pediatric obesity
Currently, the most effective treatment for pediatric obesity is bariatric surgery. The two most commonly used surgical procedures today are the sleeve gastrectomy and gastric bypass. Sleeve gastrectomy works by removing 75%-85% of the stomach and creating a new stomach, called a “sleeve.” Gastric bypass works by separating the stomach into two parts and connecting one part of the new stomach into the intestine.
Both surgeries are very effective at treating obesity in adolescents, with an average weight loss of 30%-35%. Surgery is not just a restrictive means of controlling body weight; it also changes key hormones for appetite and satiety that signal the brain. In fact, many of the same biological signals that are changed by surgery are the same signals being targeted by antiobesity medications. Long-term outcome of bariatric surgery in adolescents, provided by Teen-LABS, show it to be safe and maybe even more effective than in adults for treating diabetes and hypertension, with similar weight loss.
Does treatment outweigh the potential risks?
Although obesity surgery and antiobesity medications are more successful at treating obesity in children and adolescents than lifestyle medications, they do have some risks. Surgery, depending on the type of surgery, can cause nutritional deficiencies, reduce body mineral density, and is a life-changing medical procedure. Antiobesity medications, depending on the type, can cause nausea and vomiting and increase heart rate – and because they are relatively new, we do not fully understand the long-term impact of continued use past 1 year.
However, an important question to ask is: “Do the risks of obesity surgery and antiobesity medications outweigh the risk of having lifelong obesity?” The answer to me and many of my colleagues is: “Yes!” Although there are risks associated with the two best treatments for pediatric obesity, those risks under proper supervision of a medical professional far outweigh the risks of not properly treating obesity and allowing it to persist and get worse over many years to come. Obesity is a disease deeply rooted in biology, and we must use biology-based treatments to tackle this problem in children and adolescents, who deserve the best care and treatments possible.
Dr. Ryder is assistant professor of pediatrics and associate director of research, Center for Pediatric Obesity Medicine, at the University of Minnesota, Minneapolis. She reported receiving donations for clinical trials from Boehringer Ingelheim. A version of this article first appeared on Medscape.com.
Pediatric obesity is a serious problem, not only in the United States but worldwide. Unfortunately, the ongoing COVID-19 pandemic has worsened the epidemic of childhood obesity. Solutions for treating the millions of children and adolescents with obesity are desperately needed because prevention efforts over the past several decades have not been sufficient in slowing the steady rise in obesity prevalence.
Lifestyle modification, including dietary changes, increases in activity, and behavioral modification, are the cornerstone of any obesity treatment, but they alone are not powerful enough to treat obesity by itself in the vast majority of children and adolescents. This is because obesity is not a lifestyle choice; rather, it is a disease, and a disease that has a tremendous amount of biology driving individuals toward weight gain and the propensity toward weight regain if weight is lost.
Fortunately, the tools to treat the underlying biology driving obesity are becoming safer, more effective, and more widely used every year. The two most effective biology-based treatments for pediatric obesity are antiobesity medications and bariatric surgery. These two treatments, when accompanied by lifestyle modification, have the potential to reduce not only body weight but also treat many other risk factors, such as prediabetes, diabetes, high blood pressure, poor cholesterol, liver disease, and sleep apnea, as well as others.
Rise in antiobesity medications
Antiobesity medications are developing at a rapid pace. Seven medications have been approved by the Food and Drug Administration for adults, and three medications (phentermine, orlistat, and liraglutide) are now approved for children and adolescents.
The number of antiobesity medications for use in children and adolescents is expected to expand to five, with semaglutide and phentermine-topiramate (Qsymia) both completing trials in adolescents in 2022. Each of these medications works by treating the biology that drives weight gain, whether it is decreasing impulsivity, reducing hunger and appetite hormone pathways, or improving energy regulation pathways. Weight loss at 1 year for currently FDA-approved medications in adolescents ranges from 3% to 6% on average, depending on the medications. The newer medications already FDA approved in adults that will soon, hopefully, be available in pediatrics result in 10%-16% weight loss on average.
A common parent and patient question regarding antiobesity medications is: “If I start an antiobesity medication, how long will I need to be on it?” The simple answer is: “Probably for the rest of your life.”
This can be a shock to hear, but obesity treatment is very similar to that of hypertension or diabetes. Using high blood pressure as an example: If a patient has high blood pressure (for example, 160/90 mm Hg), they will be prescribed a medication to treat it. Once blood pressure comes down to near-normal levels (for example, 120/80 mm Hg), a dose will be maintained, not removed, because that is the biological mediator keeping the blood pressure low. Removal of the medication would result in blood pressure going back to homeostasis (160/90 mm Hg in our example) in a short period of time).
The same can be said for obesity. For example, if a 16-year-old girl is prescribed liraglutide, a glucagonlike peptide–1 receptor agonist, and loses 10% of her body weight at 1 year, that is great success. Why would we remove the medication that is treating the underlying biology causing successful weight loss?
In short, we would not want to do that. Even if our example patient only maintained that 10% initial weight loss, that would be very successful, just like someone maintaining their low blood pressure. As medications begin to develop at a rapid pace and become more available to pediatric patients, the messaging and conversation around anti-obesity medications must continue to focus on obesity being a biological disease and not a behavior for treatment to be effective and not stigmatized.
Bariatric surgery most effective treatment for pediatric obesity
Currently, the most effective treatment for pediatric obesity is bariatric surgery. The two most commonly used surgical procedures today are the sleeve gastrectomy and gastric bypass. Sleeve gastrectomy works by removing 75%-85% of the stomach and creating a new stomach, called a “sleeve.” Gastric bypass works by separating the stomach into two parts and connecting one part of the new stomach into the intestine.
Both surgeries are very effective at treating obesity in adolescents, with an average weight loss of 30%-35%. Surgery is not just a restrictive means of controlling body weight; it also changes key hormones for appetite and satiety that signal the brain. In fact, many of the same biological signals that are changed by surgery are the same signals being targeted by antiobesity medications. Long-term outcome of bariatric surgery in adolescents, provided by Teen-LABS, show it to be safe and maybe even more effective than in adults for treating diabetes and hypertension, with similar weight loss.
Does treatment outweigh the potential risks?
Although obesity surgery and antiobesity medications are more successful at treating obesity in children and adolescents than lifestyle medications, they do have some risks. Surgery, depending on the type of surgery, can cause nutritional deficiencies, reduce body mineral density, and is a life-changing medical procedure. Antiobesity medications, depending on the type, can cause nausea and vomiting and increase heart rate – and because they are relatively new, we do not fully understand the long-term impact of continued use past 1 year.
However, an important question to ask is: “Do the risks of obesity surgery and antiobesity medications outweigh the risk of having lifelong obesity?” The answer to me and many of my colleagues is: “Yes!” Although there are risks associated with the two best treatments for pediatric obesity, those risks under proper supervision of a medical professional far outweigh the risks of not properly treating obesity and allowing it to persist and get worse over many years to come. Obesity is a disease deeply rooted in biology, and we must use biology-based treatments to tackle this problem in children and adolescents, who deserve the best care and treatments possible.
Dr. Ryder is assistant professor of pediatrics and associate director of research, Center for Pediatric Obesity Medicine, at the University of Minnesota, Minneapolis. She reported receiving donations for clinical trials from Boehringer Ingelheim. A version of this article first appeared on Medscape.com.
Two congressmen targeting ‘gender transition’ physicians
Two GOP congressmen have introduced legislation aimed at holding doctors who perform gender transition procedures on minors liable for their actions, says a story reported on KATV.com, among other news sites.
The two GOP lawmakers – Rep. Jim Banks (IN) and Sen. Tom Cotton (AR) – introduced the Protecting Minors from Medical Malpractice Act in their respective chambers.
If passed, the House and Senate bills would make doctors liable for any gender transition surgery on a minor that results in injury, whether physical, psychological, emotional, or physiological. Minors who believe they’ve been harmed would have up to 30 years from when they turn 18 to file a claim.
The House proposal would also strip federal funding from states that require health care professionals to provide gender transition procedures, including puberty blockers, cross-sex hormones, and gender reassignment surgeries.
A companion House bill, also sponsored by Banks, targets another issue related to gender transitioning for minors: parental consent.
If passed, the Empower Parents to Protect Their Kids Act of 2022 would deny federal funding to any elementary and secondary schools that initiate a minor’s gender transition without first securing parental consent. (Last October, Sen. Cotton released a similar bill in the Senate.)
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
Two GOP congressmen have introduced legislation aimed at holding doctors who perform gender transition procedures on minors liable for their actions, says a story reported on KATV.com, among other news sites.
The two GOP lawmakers – Rep. Jim Banks (IN) and Sen. Tom Cotton (AR) – introduced the Protecting Minors from Medical Malpractice Act in their respective chambers.
If passed, the House and Senate bills would make doctors liable for any gender transition surgery on a minor that results in injury, whether physical, psychological, emotional, or physiological. Minors who believe they’ve been harmed would have up to 30 years from when they turn 18 to file a claim.
The House proposal would also strip federal funding from states that require health care professionals to provide gender transition procedures, including puberty blockers, cross-sex hormones, and gender reassignment surgeries.
A companion House bill, also sponsored by Banks, targets another issue related to gender transitioning for minors: parental consent.
If passed, the Empower Parents to Protect Their Kids Act of 2022 would deny federal funding to any elementary and secondary schools that initiate a minor’s gender transition without first securing parental consent. (Last October, Sen. Cotton released a similar bill in the Senate.)
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
Two GOP congressmen have introduced legislation aimed at holding doctors who perform gender transition procedures on minors liable for their actions, says a story reported on KATV.com, among other news sites.
The two GOP lawmakers – Rep. Jim Banks (IN) and Sen. Tom Cotton (AR) – introduced the Protecting Minors from Medical Malpractice Act in their respective chambers.
If passed, the House and Senate bills would make doctors liable for any gender transition surgery on a minor that results in injury, whether physical, psychological, emotional, or physiological. Minors who believe they’ve been harmed would have up to 30 years from when they turn 18 to file a claim.
The House proposal would also strip federal funding from states that require health care professionals to provide gender transition procedures, including puberty blockers, cross-sex hormones, and gender reassignment surgeries.
A companion House bill, also sponsored by Banks, targets another issue related to gender transitioning for minors: parental consent.
If passed, the Empower Parents to Protect Their Kids Act of 2022 would deny federal funding to any elementary and secondary schools that initiate a minor’s gender transition without first securing parental consent. (Last October, Sen. Cotton released a similar bill in the Senate.)
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
Best practices for evaluating pelvic pain in patients with Essure tubal occlusion devices
The evaluation and management of chronic pelvic pain in patients with a history of Essure device (Bayer HealthCare Pharmaceuticals Inc, Whippany, New Jersey) insertion have posed many challenges for both clinicians and patients. The availability of high-quality, evidence-based clinical guidance has been limited. We have reviewed the currently available published data, and here provide an overview of takeaways, as well as share our perspective and approach on evaluating and managing chronic pelvic pain in this unique patient population.
The device
The Essure microinsert is a hysteroscopically placed device that facilitates permanent sterilization by occluding the bilateral proximal fallopian tubes. The microinsert has an inner and outer nitinol coil that attaches the device to the proximal fallopian tube to ensure retention. The inner coil releases polyethylene terephthalate fibers that cause tubal fiber proliferation to occlude the lumen of the fallopian tube and achieve sterilization.
The device was first approved by the US Food and Drug Administration (FDA) in 2002. In subsequent years, the device was well received and widely used, with approximately 750,000 women worldwide undergoing Essure placement.1,2 Shortly after approval, many adverse events (AEs), including pelvic pain and abnormal uterine bleeding, were reported, resulting in a public meeting of the FDA Obstetrics and Gynecology Devices Panel in September 2015. A postmarket surveillance study on the device ensued to assess complication rates including unplanned pregnancy, pelvic pain, and surgery for removal. In February 2016, the FDA issued a black box warning and a patient decision checklist.3,4 In December 2018, Bayer stopped selling and distributing Essure in the United States.5 A 4-year follow-up surveillance study on Essure was submitted to the FDA in March 2020.
Adverse outcomes
Common AEs related to the Essure device include heavy uterine bleeding, pelvic pain, and other quality-of-life symptoms such as fatigue and weight gain.6-8 The main safety endpoints for the mandated FDA postmarket 522 surveillance studies were chronic lower abdominal and pelvic pain; abnormal uterine bleeding; hypersensitivity; allergic reaction, as well as autoimmune disorders incorporating inflammatory markers and human leukocyte antigen; and gynecologic surgery for device removal.9 Postmarket surveillence has shown that most AEs are related to placement complications or pelvic pain after Essure insertion. However, there have been several reports of autoimmune diseases categorized as serious AEs, such as new-onset systemic lupus erythematosus, rheumatoid arthritis, and worsening ulcerative colitis, after Essure insertion.5
Evaluation of symptoms
Prevalence of pelvic pain following device placement
We conducted a PubMed and MEDLINE search from January 2000 to May 2020, which identified 43 studies citing AEs related to device placement, including pelvic or abdominal pain, abnormal uterine bleeding, hypersensitivity, and autoimmune disorders. A particularly debilitating and frequently cited AE was new-onset pelvic pain or worsening of preexisting pelvic pain. Perforation of the uterus or fallopian tube, resulting in displacement of the device into the peritoneal cavity, or fragmentation of the microinsert was reported as a serious AE that occurred after device placement. However, due to the complexity of chronic pelvic pain pathogenesis, the effect of the insert on patients with existing chronic pelvic pain remains unknown.
Authors of a large retrospective study found that approximately 2.7% of 1,430 patients developed new-onset or worsening pelvic pain after device placement. New-onset pelvic pain in 1% of patients was thought to be secondary to device placement, without a coexisting pathology or diagnosis.10
In a retrospective study by Clark and colleagues, 22 of 50 women (44%) with pelvic pain after microinsert placement were found to have at least one other cause of pelvic pain. The most common alternative diagnoses were endometriosis, adenomyosis, salpingitis, and adhesive disease. Nine of the 50 patients (18%) were found to have endometriosis upon surgical removal of the microinsert.7
Another case series examined outcomes in 29 patients undergoing laparoscopic device removal due to new-onset pelvic pain. Intraoperative findings included endometriosis in 5 patients (17.2%) and pelvic adhesions in 3 (10.3%).2 Chronic pelvic pain secondary to endometriosis may be exacerbated with Essure insertion due to discontinuation of hormonal birth control after device placement,7 and this diagnosis along with adenomyosis should be strongly considered in patients whose pelvic pain began when hormonal contraception was discontinued after placement of the device.
Continue to: Risk factors...
Risk factors
Authors of a retrospective cohort study found that patients with prior diagnosis of a chronic pain syndrome, low back pain, headaches, or fibromyalgia were 5 to 6 times more likely to report acute and chronic pain after hysteroscopic sterilization with Essure.11 Since chronic pain is often thought to be driven by a hyperalgesic state of the central nervous system, as previously shown in patients with conditions such as vulvodynia, interstitial cystitis, and fibromyalgia,12 a hyperalgesic state can potentially explain why some patients are more susceptible to developing worsening pain.
Van Limburg and colleagues conducted a retrospective cohort study with prospective follow-up on 284 women who underwent Essure sterilization. Among these patients, 48% reported negative AEs; risk factors included young age at placement, increasing gravidity, and no prior abdominal surgery.13
Onset of pain
The timing and onset of pelvic pain vary widely, suggesting there is no particular time frame for this AE after device placement.2,6,14-18 A case series by Arjona and colleagues analyzed the incidence of chronic pelvic pain in 4,274 patients after Essure sterilization. Seven patients (0.16%) reported chronic pelvic pain that necessitated device removal. In 6 of the women, the pelvic pain began within 1 week of device placement. In 3 of the 6 cases, the surgeon reported the removal procedures as “difficult.” In all 6 cases, the level of pelvic pain increased with time and was not alleviated with standard analgesic medications.6
In another case series of 26 patients, the authors evaluated patients undergoing laparoscopic removal of Essure secondary to pelvic pain and reported that the time range for symptom presentation was immediate to 85 months. Thirteen of 26 patients (50%) reported pain onset within less than 1 month of device placement, 5 of 26 patients (19.2%) reported pain between 1 and 12 months after device placement, and 8 of 26 patients (30.8%) reported pain onset more than 12 months after microinsert placement.2 In this study, 17.2% of operative reports indicated difficulty with device placement. It is unclear whether difficulty with placement was associated with development of subsequent abdominal or pelvic pain; however, the relevance of initial insertion difficulty diminished with longer follow-up.
Workup and evaluation
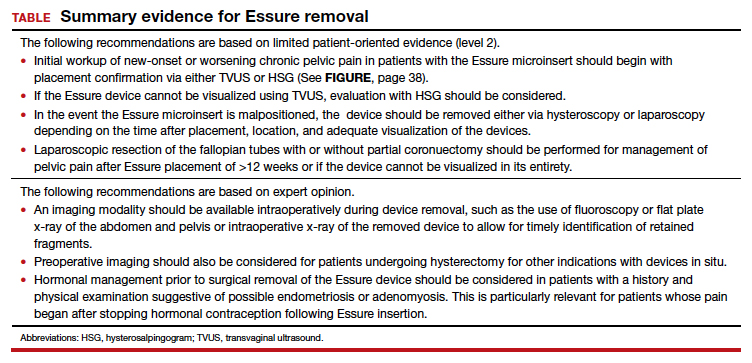
We found 5 studies that provided some framework for evaluating a patient with new-onset or worsening pelvic pain after microinsert placement. Overall, correct placement and functionality of the device should be confirmed by either hysterosalpingogram (HSG) or transvaginal ultrasonography (TVUS). The gold standard to determine tubal occlusion is the HSG. However, TVUS may be a dependable alternative, and either test can accurately demonstrate Essure location.19 Patients often prefer TVUS over HSG due to the low cost, minimal discomfort, and short examination time.1 TVUS is a noninvasive and reasonable test to start the initial assessment. The Essure devices are highly echogenic on pelvic ultrasound and easily identifiable by the proximity of the device to the uterotubal junction and its relationship with the surrounding soft tissue. If the device perforates the peritoneal cavity, then the echogenic bowel can impede adequate visualization of the Essure microinsert. If the Essure insert is not visualized on TVUS, an HSG will not only confirm placement but also test insert functionality. After confirming correct placement of the device, the provider can proceed with standard workup for chronic pelvic pain.
If one or more of the devices are malpositioned, the devices are generally presumed to be the etiology of the new pain. Multiple case reports demonstrate pain due to Essure misconfiguration or perforation with subsequent resolution of symptoms after device removal.18,20,21 A case study by Alcantara and colleagues described a patient with chronic pelvic pain and an Essure coil that was curved in an elliptical shape, not adhering to the anatomic course of the fallopian tube. The patient reported pain resolution after laparoscopic removal of the device.20 Another case report by Mahmoud et al described a subserosal malpositioned device that caused acute pelvic pain 4 months after sterilization. The patient reported resolution of pain after the microinsert was removed via laparoscopy.21 These reports highlight the importance of considering malpositioned devices as the etiology of new pelvic pain after Essure placement.
Continue to: Device removal and patient outcomes...
Device removal and patient outcomes
Removal
Several studies that we evaluated included a discussion on the methods for Essure removal. which are divided into 2 general categories: hysteroscopy and laparoscopy.
Hysteroscopic removal is generally used when the device was placed less than 12 weeks prior to removal.7,19 After 12 weeks, removal is more difficult due to fibrosis within the fallopian tubes. A risk with hysteroscopic removal is failure to remove all fibers, which allows inflammation and fibrosis to continue.7 This risk is mitigated via laparoscopic hysterectomy or mini-cornuectomy with bilateral salpingectomy, where the devices can be removed en bloc and without excessive traction.
Laparoscopic Essure removal procedures described in the literature include salpingostomy and traction on the device, salpingectomy, and salpingectomy with mini-cornuectomy. The incision and traction method is typically performed via a 2- to 3-cm incision on the antimesial edge of the fallopian tube along with a circumferential incision to surround the interstitial tubal area. The implant is carefully extracted from the fallopian tube and cornua, and a salpingectomy is then performed.22 The implant is removed prior to the salpingectomy to ensure that the Essure device is removed in its entirety prior to performing a salpingectomy.
A prospective observational study evaluated laparoscopic removal of Essure devices in 80 women with or without cornual excision. Results suggest that the incision and traction method poses more technical difficulties than the cornuectomy approach.23 Surgeons reported significant difficulty controlling the tensile pressure with traction, whereas use of the cornuectomy approach eliminated this risk and decreased the risk of fragmentation and incomplete removal.23,24
Charavil and colleagues demonstrated in a prospective observational study that a vaginal hysterectomy with bilateral salpingectomy is a feasible approach to Essure removal. Twenty-six vaginal hysterectomies with bilateral salpingectomy and Essure removal were performed without conversion to laparoscopy or laparotomy. The surgeons performed an en bloc removal of each hemiuterus along with the ipsilateral tube, which ensured complete removal of the Essure device. Each case was confirmed with an x-ray of the surgical specimen.25
If device fragmentation occurs, there are different methods recommended for locating fragments. A case report of bilateral uterine perforation after uncomplicated Essure placement used a preoperative computed tomography (CT) scan to locate the Essure fragments, but no intraoperative imaging was performed to confirm complete fragment removal.26 The patient continued reporting chronic pelvic pain and ultimately underwent exploratory laparotomy with intraoperative fluoroscopy. Using fluoroscopy, investigators identified omental fragments that were missed on preoperative CT imaging. Fluoroscopy is not commonly used intraoperatively, but it may have added benefit for localizing retained fragments.
A retrospective cohort study reviewed the use of intraoperative x-ray of the removed specimen to confirm complete Essure removal.27 If an x-ray of the removed specimen showed incomplete removal, an intraoperative pelvic x-ray was performed to locate missing fragments. X-ray of the removed devices confirmed complete removal in 63 of 72 patients (87.5%). Six of 9 women with an unsatisfactory specimen x-ray had no residual fragments identified during pelvic x-ray, and the device removal was deemed adequate. The remaining 3 women had radiologic evidence of incomplete device removal and required additional dissection for complete removal. Overall, use of x-ray or fluoroscopy is a relatively safe and accessible way to ensure complete removal of the Essure device and is worth consideration, especially when retained device fragments are suspected.
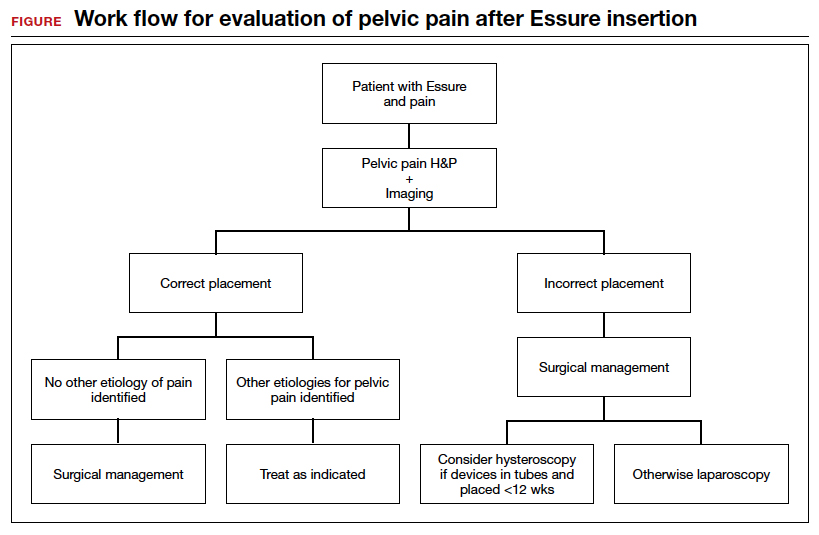
Symptom resolution
We reviewed 5 studies that examined pain outcomes after removal of the Essure devices. Casey et al found that 23 of 26 patients (88.5%) reported significant pain relief at the postoperative visit, while 3 of 26 (11.5%) reported persistent pelvic pain.2 Two of 3 case series examined other outcomes in addition to postoperative pelvic pain, including sexual function and activities of daily living.7,14 In the first case series by Brito and colleagues, 8 of 11 patients (72.7%) reported an improvement in pelvic pain, ability to perform daily activities, sexual life, and overall quality of life after Essure removal. For the remaining 3 patients with persistent pelvic pain after surgical removal of the device, 2 patients reported worsening pain symptoms and dyspareunia.14 In this study, 5 of 11 patients reported a history of chronic pelvic pain at baseline. In a retrospective case series by Clark et al, 28 of 32 women (87.5%) reported some improvement in all domains, with 24 of 32 patients (75%) reporting almost total or complete improvement in quality of life, sexual life, pelvic pain, and scores related to activities of daily living. Pain and quality-of-life scores were similar for women who underwent uterine-preserving surgery and for those who underwent hysterectomy. Ten of 32 women (31.3%) reported persistent or worsening symptoms after the Essure removal surgery. In these patients, the authors recommended consideration of other autoimmune and hypersensitivity etiologies.7
In a retrospective cohort study by Kamencic et al from 2002 to 2013 of 1,430 patients who underwent Essure placement with postplacement imaging, 62 patients (4.3%) required a second surgery after Essure placement due to pelvic pain.10 This study also found that 4 of 62 patients (0.3%) had no other obvious cause for the pelvic pain. All 4 of these women had complete resolution of their pain with removal of the Essure microinsert device. A prospective observational study by Chene e
Summary
Although Essure products were withdrawn from the market in the United States in 2018, many patients still experience significant AEs associated with the device. The goal of the perspectives and data presented here is to assist clinicians in addressing and managing the pain experienced by patients after device insertion. ●
- Connor VF. Essure: a review six years later. J Minim Invasive Gynecol. 2009;16:282-290. doi:10.1016/j.jmig.2009.02.009.
- Casey J, Aguirre F, Yunker A. Outcomes of laparoscopic removal of the Essure sterilization device for pelvic pain: a case series. Contraception. 2016;94:190-192. doi:10.1016/j.contraception.2016.03.017.
- Jackson I. Essure device removed entirely from market, with 99% of unused birth control implants retrieved: FDA. AboutLawsuits.com. January 13, 2020. https://www.aboutlawsuits.com/Essure-removal-update-166509. Accessed June 7, 2022.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization. October 31, 2016. https://www.fda.gov/media/96315/download. Accessed June 7, 2022.
- US Food and Drug Administration. FDA activities related to Essure. March 14, 2022. https://www.fda.gov/medical-devices/essure-permanent-birth-control/fda-activities-related-essure. Accessed June 8, 2022.
- Arjona Berral JE, Rodríguez Jiménez B, Velasco Sánchez E, et al. Essure and chronic pelvic pain: a population-based cohort. J Obstet Gynaecol. 2014;34:712-713. doi:10.3109/01443615.2014.92075.
- Clark NV, Rademaker D, Mushinski AA, et al. Essure removal for the treatment of device-attributed symptoms: an expanded case series and follow-up survey. J Minim Invasive Gynecol. 2017;24:971-976. doi:10.1016/j.jmig.2017.05.015.
- Sills ES, Rickers NS, Li X. Surgical management after hysteroscopic sterilization: minimally invasive approach incorporating intraoperative fluoroscopy for symptomatic patients with >2 Essure devices. Surg Technol Int. 2018;32:156-161.
- Administration USF and D. 522 Postmarket Surveillance Studies. Center for Devices and Radiological Health; 2020.
- Kamencic H, Thiel L, Karreman E, et al. Does Essure cause significant de novo pain? A retrospective review of indications for second surgeries after Essure placement. J Minim Invasive Gynecol. 2016;23:1158-1162. doi:10.1016/j.jmig.2016.08.823.
- Yunker AC, Ritch JM, Robinson EF, et al. Incidence and risk factors for chronic pelvic pain after hysteroscopic sterilization. J Minim Invasive Gynecol. 2015;22:390-994. doi:10.1016/j.jmig.2014.06.007.
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141-154. doi:10.1016/j.berh.2011.02.005.
- van Limburg Stirum EVJ, Clark NV, Lindsey A, et al. Factors associated with negative patient experiences with Essure sterilization. JSLS. 2020;24(1):e2019.00065. doi:10.4293/JSLS.2019.00065.
- Brito LG, Cohen SL, Goggins ER, et al. Essure surgical removal and subsequent symptom resolution: case series and follow-up survey. J Minim Invasive Gynecol. 2015;22:910-913. doi:10.1016/j.jmig.2015.03.018.
- Maassen LW, van Gastel DM, Haveman I, et al. Removal of Essure sterilization devices: a retrospective cohort study in the Netherlands. J Minim Invasive Gynecol. 2019;26:1056-1062. doi:10.1016/j.jmig.2018.10.009.
- Sills ES, Palermo GD. Surgical excision of Essure devices with ESHRE class IIb uterine malformation: sequential hysteroscopic-laparoscopic approach to the septate uterus. Facts Views Vis Obgyn. 2016;8:49-52.
- Ricci G, Restaino S, Di Lorenzo G, et al. Risk of Essure microinsert abdominal migration: case report and review of literature. Ther Clin Risk Manag. 2014;10:963-968. doi:10.2147/TCRM.S65634.
- Borley J, Shabajee N, Tan TL. A kink is not always a perforation: assessing Essure hysteroscopic sterilization placement. Fertil Steril. 2011;95:2429.e15-7. doi:10.1016/j.fertnstert.2011.02.006.
- Djeffal H, Blouet M, Pizzoferato AC, et al. Imaging findings in Essure-related complications: a pictorial review.7Br J Radiol. 2018;91(1090):20170686. doi:10.1259/bjr.20170686.
- Lora Alcantara I, Rezai S, Kirby C, et al. Essure surgical removal and subsequent resolution of chronic pelvic pain: a case report and review of the literature. Case Rep Obstet Gynecol. 2016;2016:6961202. doi:10.1155/2016/6961202.
- Mahmoud MS, Fridman D, Merhi ZO. Subserosal misplacement of Essure device manifested by late-onset acute pelvic pain. Fertil Steril. 2009;92:2038.e1-3. doi:10.1016/j.fertnstert.2009.07.1677.
- Tissot M, Petry S, Lecointre L, et al. Two surgical techniques for Essure device ablation: the hysteroscopic way and the laparoscopic way by salpingectomy with tubal interstitial resection. J Minim Invasive Gynecol. 2019;26(4):603. doi:10.1016/j.jmig.2018.07.017.
- Chene G, Cerruto E, Moret S, et al. Quality of life after laparoscopic removal of Essure sterilization devices. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100054. doi:10.1016/j.eurox.2019.100054.
- Thiel L, Rattray D, Thiel J. Laparoscopic cornuectomy as a technique for removal of Essure microinserts. J Minim Invasive Gynecol. 2017;24(1):10. doi:10.1016/j.jmig.2016.07.004.
- Charavil A, Agostini A, Rambeaud C, et al. Vaginal hysterectomy with salpingectomy for Essure insert removal. J Minim Invasive Gynecol. 2019;2:695-701. doi:10.1016/j.jmig.2018.07.019.
- Howard DL, Christenson PJ, Strickland JL. Use of intraoperative fluoroscopy during laparotomy to identify fragments of retained Essure microinserts: case report. J Minim Invasive Gynecol. 2012;19:667-670. doi:10.1016/j.jmig.2012.04.007.
- Miquel L, Crochet P, Francini S, et al. Laparoscopic Essure device removal by en bloc salpingectomy-cornuectomy with intraoperative x-ray checking: a retrospective cohort study. J Minim Invasive Gynecol. 2020;27:697-703. doi:10.1016/j. jmig.2019.06.006.
The evaluation and management of chronic pelvic pain in patients with a history of Essure device (Bayer HealthCare Pharmaceuticals Inc, Whippany, New Jersey) insertion have posed many challenges for both clinicians and patients. The availability of high-quality, evidence-based clinical guidance has been limited. We have reviewed the currently available published data, and here provide an overview of takeaways, as well as share our perspective and approach on evaluating and managing chronic pelvic pain in this unique patient population.
The device
The Essure microinsert is a hysteroscopically placed device that facilitates permanent sterilization by occluding the bilateral proximal fallopian tubes. The microinsert has an inner and outer nitinol coil that attaches the device to the proximal fallopian tube to ensure retention. The inner coil releases polyethylene terephthalate fibers that cause tubal fiber proliferation to occlude the lumen of the fallopian tube and achieve sterilization.
The device was first approved by the US Food and Drug Administration (FDA) in 2002. In subsequent years, the device was well received and widely used, with approximately 750,000 women worldwide undergoing Essure placement.1,2 Shortly after approval, many adverse events (AEs), including pelvic pain and abnormal uterine bleeding, were reported, resulting in a public meeting of the FDA Obstetrics and Gynecology Devices Panel in September 2015. A postmarket surveillance study on the device ensued to assess complication rates including unplanned pregnancy, pelvic pain, and surgery for removal. In February 2016, the FDA issued a black box warning and a patient decision checklist.3,4 In December 2018, Bayer stopped selling and distributing Essure in the United States.5 A 4-year follow-up surveillance study on Essure was submitted to the FDA in March 2020.
Adverse outcomes
Common AEs related to the Essure device include heavy uterine bleeding, pelvic pain, and other quality-of-life symptoms such as fatigue and weight gain.6-8 The main safety endpoints for the mandated FDA postmarket 522 surveillance studies were chronic lower abdominal and pelvic pain; abnormal uterine bleeding; hypersensitivity; allergic reaction, as well as autoimmune disorders incorporating inflammatory markers and human leukocyte antigen; and gynecologic surgery for device removal.9 Postmarket surveillence has shown that most AEs are related to placement complications or pelvic pain after Essure insertion. However, there have been several reports of autoimmune diseases categorized as serious AEs, such as new-onset systemic lupus erythematosus, rheumatoid arthritis, and worsening ulcerative colitis, after Essure insertion.5
Evaluation of symptoms
Prevalence of pelvic pain following device placement
We conducted a PubMed and MEDLINE search from January 2000 to May 2020, which identified 43 studies citing AEs related to device placement, including pelvic or abdominal pain, abnormal uterine bleeding, hypersensitivity, and autoimmune disorders. A particularly debilitating and frequently cited AE was new-onset pelvic pain or worsening of preexisting pelvic pain. Perforation of the uterus or fallopian tube, resulting in displacement of the device into the peritoneal cavity, or fragmentation of the microinsert was reported as a serious AE that occurred after device placement. However, due to the complexity of chronic pelvic pain pathogenesis, the effect of the insert on patients with existing chronic pelvic pain remains unknown.
Authors of a large retrospective study found that approximately 2.7% of 1,430 patients developed new-onset or worsening pelvic pain after device placement. New-onset pelvic pain in 1% of patients was thought to be secondary to device placement, without a coexisting pathology or diagnosis.10
In a retrospective study by Clark and colleagues, 22 of 50 women (44%) with pelvic pain after microinsert placement were found to have at least one other cause of pelvic pain. The most common alternative diagnoses were endometriosis, adenomyosis, salpingitis, and adhesive disease. Nine of the 50 patients (18%) were found to have endometriosis upon surgical removal of the microinsert.7
Another case series examined outcomes in 29 patients undergoing laparoscopic device removal due to new-onset pelvic pain. Intraoperative findings included endometriosis in 5 patients (17.2%) and pelvic adhesions in 3 (10.3%).2 Chronic pelvic pain secondary to endometriosis may be exacerbated with Essure insertion due to discontinuation of hormonal birth control after device placement,7 and this diagnosis along with adenomyosis should be strongly considered in patients whose pelvic pain began when hormonal contraception was discontinued after placement of the device.
Continue to: Risk factors...
Risk factors
Authors of a retrospective cohort study found that patients with prior diagnosis of a chronic pain syndrome, low back pain, headaches, or fibromyalgia were 5 to 6 times more likely to report acute and chronic pain after hysteroscopic sterilization with Essure.11 Since chronic pain is often thought to be driven by a hyperalgesic state of the central nervous system, as previously shown in patients with conditions such as vulvodynia, interstitial cystitis, and fibromyalgia,12 a hyperalgesic state can potentially explain why some patients are more susceptible to developing worsening pain.
Van Limburg and colleagues conducted a retrospective cohort study with prospective follow-up on 284 women who underwent Essure sterilization. Among these patients, 48% reported negative AEs; risk factors included young age at placement, increasing gravidity, and no prior abdominal surgery.13
Onset of pain
The timing and onset of pelvic pain vary widely, suggesting there is no particular time frame for this AE after device placement.2,6,14-18 A case series by Arjona and colleagues analyzed the incidence of chronic pelvic pain in 4,274 patients after Essure sterilization. Seven patients (0.16%) reported chronic pelvic pain that necessitated device removal. In 6 of the women, the pelvic pain began within 1 week of device placement. In 3 of the 6 cases, the surgeon reported the removal procedures as “difficult.” In all 6 cases, the level of pelvic pain increased with time and was not alleviated with standard analgesic medications.6
In another case series of 26 patients, the authors evaluated patients undergoing laparoscopic removal of Essure secondary to pelvic pain and reported that the time range for symptom presentation was immediate to 85 months. Thirteen of 26 patients (50%) reported pain onset within less than 1 month of device placement, 5 of 26 patients (19.2%) reported pain between 1 and 12 months after device placement, and 8 of 26 patients (30.8%) reported pain onset more than 12 months after microinsert placement.2 In this study, 17.2% of operative reports indicated difficulty with device placement. It is unclear whether difficulty with placement was associated with development of subsequent abdominal or pelvic pain; however, the relevance of initial insertion difficulty diminished with longer follow-up.
Workup and evaluation
We found 5 studies that provided some framework for evaluating a patient with new-onset or worsening pelvic pain after microinsert placement. Overall, correct placement and functionality of the device should be confirmed by either hysterosalpingogram (HSG) or transvaginal ultrasonography (TVUS). The gold standard to determine tubal occlusion is the HSG. However, TVUS may be a dependable alternative, and either test can accurately demonstrate Essure location.19 Patients often prefer TVUS over HSG due to the low cost, minimal discomfort, and short examination time.1 TVUS is a noninvasive and reasonable test to start the initial assessment. The Essure devices are highly echogenic on pelvic ultrasound and easily identifiable by the proximity of the device to the uterotubal junction and its relationship with the surrounding soft tissue. If the device perforates the peritoneal cavity, then the echogenic bowel can impede adequate visualization of the Essure microinsert. If the Essure insert is not visualized on TVUS, an HSG will not only confirm placement but also test insert functionality. After confirming correct placement of the device, the provider can proceed with standard workup for chronic pelvic pain.
If one or more of the devices are malpositioned, the devices are generally presumed to be the etiology of the new pain. Multiple case reports demonstrate pain due to Essure misconfiguration or perforation with subsequent resolution of symptoms after device removal.18,20,21 A case study by Alcantara and colleagues described a patient with chronic pelvic pain and an Essure coil that was curved in an elliptical shape, not adhering to the anatomic course of the fallopian tube. The patient reported pain resolution after laparoscopic removal of the device.20 Another case report by Mahmoud et al described a subserosal malpositioned device that caused acute pelvic pain 4 months after sterilization. The patient reported resolution of pain after the microinsert was removed via laparoscopy.21 These reports highlight the importance of considering malpositioned devices as the etiology of new pelvic pain after Essure placement.

Continue to: Device removal and patient outcomes...
Device removal and patient outcomes
Removal
Several studies that we evaluated included a discussion on the methods for Essure removal. which are divided into 2 general categories: hysteroscopy and laparoscopy.
Hysteroscopic removal is generally used when the device was placed less than 12 weeks prior to removal.7,19 After 12 weeks, removal is more difficult due to fibrosis within the fallopian tubes. A risk with hysteroscopic removal is failure to remove all fibers, which allows inflammation and fibrosis to continue.7 This risk is mitigated via laparoscopic hysterectomy or mini-cornuectomy with bilateral salpingectomy, where the devices can be removed en bloc and without excessive traction.
Laparoscopic Essure removal procedures described in the literature include salpingostomy and traction on the device, salpingectomy, and salpingectomy with mini-cornuectomy. The incision and traction method is typically performed via a 2- to 3-cm incision on the antimesial edge of the fallopian tube along with a circumferential incision to surround the interstitial tubal area. The implant is carefully extracted from the fallopian tube and cornua, and a salpingectomy is then performed.22 The implant is removed prior to the salpingectomy to ensure that the Essure device is removed in its entirety prior to performing a salpingectomy.
A prospective observational study evaluated laparoscopic removal of Essure devices in 80 women with or without cornual excision. Results suggest that the incision and traction method poses more technical difficulties than the cornuectomy approach.23 Surgeons reported significant difficulty controlling the tensile pressure with traction, whereas use of the cornuectomy approach eliminated this risk and decreased the risk of fragmentation and incomplete removal.23,24
Charavil and colleagues demonstrated in a prospective observational study that a vaginal hysterectomy with bilateral salpingectomy is a feasible approach to Essure removal. Twenty-six vaginal hysterectomies with bilateral salpingectomy and Essure removal were performed without conversion to laparoscopy or laparotomy. The surgeons performed an en bloc removal of each hemiuterus along with the ipsilateral tube, which ensured complete removal of the Essure device. Each case was confirmed with an x-ray of the surgical specimen.25
If device fragmentation occurs, there are different methods recommended for locating fragments. A case report of bilateral uterine perforation after uncomplicated Essure placement used a preoperative computed tomography (CT) scan to locate the Essure fragments, but no intraoperative imaging was performed to confirm complete fragment removal.26 The patient continued reporting chronic pelvic pain and ultimately underwent exploratory laparotomy with intraoperative fluoroscopy. Using fluoroscopy, investigators identified omental fragments that were missed on preoperative CT imaging. Fluoroscopy is not commonly used intraoperatively, but it may have added benefit for localizing retained fragments.
A retrospective cohort study reviewed the use of intraoperative x-ray of the removed specimen to confirm complete Essure removal.27 If an x-ray of the removed specimen showed incomplete removal, an intraoperative pelvic x-ray was performed to locate missing fragments. X-ray of the removed devices confirmed complete removal in 63 of 72 patients (87.5%). Six of 9 women with an unsatisfactory specimen x-ray had no residual fragments identified during pelvic x-ray, and the device removal was deemed adequate. The remaining 3 women had radiologic evidence of incomplete device removal and required additional dissection for complete removal. Overall, use of x-ray or fluoroscopy is a relatively safe and accessible way to ensure complete removal of the Essure device and is worth consideration, especially when retained device fragments are suspected.

Symptom resolution
We reviewed 5 studies that examined pain outcomes after removal of the Essure devices. Casey et al found that 23 of 26 patients (88.5%) reported significant pain relief at the postoperative visit, while 3 of 26 (11.5%) reported persistent pelvic pain.2 Two of 3 case series examined other outcomes in addition to postoperative pelvic pain, including sexual function and activities of daily living.7,14 In the first case series by Brito and colleagues, 8 of 11 patients (72.7%) reported an improvement in pelvic pain, ability to perform daily activities, sexual life, and overall quality of life after Essure removal. For the remaining 3 patients with persistent pelvic pain after surgical removal of the device, 2 patients reported worsening pain symptoms and dyspareunia.14 In this study, 5 of 11 patients reported a history of chronic pelvic pain at baseline. In a retrospective case series by Clark et al, 28 of 32 women (87.5%) reported some improvement in all domains, with 24 of 32 patients (75%) reporting almost total or complete improvement in quality of life, sexual life, pelvic pain, and scores related to activities of daily living. Pain and quality-of-life scores were similar for women who underwent uterine-preserving surgery and for those who underwent hysterectomy. Ten of 32 women (31.3%) reported persistent or worsening symptoms after the Essure removal surgery. In these patients, the authors recommended consideration of other autoimmune and hypersensitivity etiologies.7
In a retrospective cohort study by Kamencic et al from 2002 to 2013 of 1,430 patients who underwent Essure placement with postplacement imaging, 62 patients (4.3%) required a second surgery after Essure placement due to pelvic pain.10 This study also found that 4 of 62 patients (0.3%) had no other obvious cause for the pelvic pain. All 4 of these women had complete resolution of their pain with removal of the Essure microinsert device. A prospective observational study by Chene e
Summary
Although Essure products were withdrawn from the market in the United States in 2018, many patients still experience significant AEs associated with the device. The goal of the perspectives and data presented here is to assist clinicians in addressing and managing the pain experienced by patients after device insertion. ●
The evaluation and management of chronic pelvic pain in patients with a history of Essure device (Bayer HealthCare Pharmaceuticals Inc, Whippany, New Jersey) insertion have posed many challenges for both clinicians and patients. The availability of high-quality, evidence-based clinical guidance has been limited. We have reviewed the currently available published data, and here provide an overview of takeaways, as well as share our perspective and approach on evaluating and managing chronic pelvic pain in this unique patient population.
The device
The Essure microinsert is a hysteroscopically placed device that facilitates permanent sterilization by occluding the bilateral proximal fallopian tubes. The microinsert has an inner and outer nitinol coil that attaches the device to the proximal fallopian tube to ensure retention. The inner coil releases polyethylene terephthalate fibers that cause tubal fiber proliferation to occlude the lumen of the fallopian tube and achieve sterilization.
The device was first approved by the US Food and Drug Administration (FDA) in 2002. In subsequent years, the device was well received and widely used, with approximately 750,000 women worldwide undergoing Essure placement.1,2 Shortly after approval, many adverse events (AEs), including pelvic pain and abnormal uterine bleeding, were reported, resulting in a public meeting of the FDA Obstetrics and Gynecology Devices Panel in September 2015. A postmarket surveillance study on the device ensued to assess complication rates including unplanned pregnancy, pelvic pain, and surgery for removal. In February 2016, the FDA issued a black box warning and a patient decision checklist.3,4 In December 2018, Bayer stopped selling and distributing Essure in the United States.5 A 4-year follow-up surveillance study on Essure was submitted to the FDA in March 2020.
Adverse outcomes
Common AEs related to the Essure device include heavy uterine bleeding, pelvic pain, and other quality-of-life symptoms such as fatigue and weight gain.6-8 The main safety endpoints for the mandated FDA postmarket 522 surveillance studies were chronic lower abdominal and pelvic pain; abnormal uterine bleeding; hypersensitivity; allergic reaction, as well as autoimmune disorders incorporating inflammatory markers and human leukocyte antigen; and gynecologic surgery for device removal.9 Postmarket surveillence has shown that most AEs are related to placement complications or pelvic pain after Essure insertion. However, there have been several reports of autoimmune diseases categorized as serious AEs, such as new-onset systemic lupus erythematosus, rheumatoid arthritis, and worsening ulcerative colitis, after Essure insertion.5
Evaluation of symptoms
Prevalence of pelvic pain following device placement
We conducted a PubMed and MEDLINE search from January 2000 to May 2020, which identified 43 studies citing AEs related to device placement, including pelvic or abdominal pain, abnormal uterine bleeding, hypersensitivity, and autoimmune disorders. A particularly debilitating and frequently cited AE was new-onset pelvic pain or worsening of preexisting pelvic pain. Perforation of the uterus or fallopian tube, resulting in displacement of the device into the peritoneal cavity, or fragmentation of the microinsert was reported as a serious AE that occurred after device placement. However, due to the complexity of chronic pelvic pain pathogenesis, the effect of the insert on patients with existing chronic pelvic pain remains unknown.
Authors of a large retrospective study found that approximately 2.7% of 1,430 patients developed new-onset or worsening pelvic pain after device placement. New-onset pelvic pain in 1% of patients was thought to be secondary to device placement, without a coexisting pathology or diagnosis.10
In a retrospective study by Clark and colleagues, 22 of 50 women (44%) with pelvic pain after microinsert placement were found to have at least one other cause of pelvic pain. The most common alternative diagnoses were endometriosis, adenomyosis, salpingitis, and adhesive disease. Nine of the 50 patients (18%) were found to have endometriosis upon surgical removal of the microinsert.7
Another case series examined outcomes in 29 patients undergoing laparoscopic device removal due to new-onset pelvic pain. Intraoperative findings included endometriosis in 5 patients (17.2%) and pelvic adhesions in 3 (10.3%).2 Chronic pelvic pain secondary to endometriosis may be exacerbated with Essure insertion due to discontinuation of hormonal birth control after device placement,7 and this diagnosis along with adenomyosis should be strongly considered in patients whose pelvic pain began when hormonal contraception was discontinued after placement of the device.
Continue to: Risk factors...
Risk factors
Authors of a retrospective cohort study found that patients with prior diagnosis of a chronic pain syndrome, low back pain, headaches, or fibromyalgia were 5 to 6 times more likely to report acute and chronic pain after hysteroscopic sterilization with Essure.11 Since chronic pain is often thought to be driven by a hyperalgesic state of the central nervous system, as previously shown in patients with conditions such as vulvodynia, interstitial cystitis, and fibromyalgia,12 a hyperalgesic state can potentially explain why some patients are more susceptible to developing worsening pain.
Van Limburg and colleagues conducted a retrospective cohort study with prospective follow-up on 284 women who underwent Essure sterilization. Among these patients, 48% reported negative AEs; risk factors included young age at placement, increasing gravidity, and no prior abdominal surgery.13
Onset of pain
The timing and onset of pelvic pain vary widely, suggesting there is no particular time frame for this AE after device placement.2,6,14-18 A case series by Arjona and colleagues analyzed the incidence of chronic pelvic pain in 4,274 patients after Essure sterilization. Seven patients (0.16%) reported chronic pelvic pain that necessitated device removal. In 6 of the women, the pelvic pain began within 1 week of device placement. In 3 of the 6 cases, the surgeon reported the removal procedures as “difficult.” In all 6 cases, the level of pelvic pain increased with time and was not alleviated with standard analgesic medications.6
In another case series of 26 patients, the authors evaluated patients undergoing laparoscopic removal of Essure secondary to pelvic pain and reported that the time range for symptom presentation was immediate to 85 months. Thirteen of 26 patients (50%) reported pain onset within less than 1 month of device placement, 5 of 26 patients (19.2%) reported pain between 1 and 12 months after device placement, and 8 of 26 patients (30.8%) reported pain onset more than 12 months after microinsert placement.2 In this study, 17.2% of operative reports indicated difficulty with device placement. It is unclear whether difficulty with placement was associated with development of subsequent abdominal or pelvic pain; however, the relevance of initial insertion difficulty diminished with longer follow-up.
Workup and evaluation
We found 5 studies that provided some framework for evaluating a patient with new-onset or worsening pelvic pain after microinsert placement. Overall, correct placement and functionality of the device should be confirmed by either hysterosalpingogram (HSG) or transvaginal ultrasonography (TVUS). The gold standard to determine tubal occlusion is the HSG. However, TVUS may be a dependable alternative, and either test can accurately demonstrate Essure location.19 Patients often prefer TVUS over HSG due to the low cost, minimal discomfort, and short examination time.1 TVUS is a noninvasive and reasonable test to start the initial assessment. The Essure devices are highly echogenic on pelvic ultrasound and easily identifiable by the proximity of the device to the uterotubal junction and its relationship with the surrounding soft tissue. If the device perforates the peritoneal cavity, then the echogenic bowel can impede adequate visualization of the Essure microinsert. If the Essure insert is not visualized on TVUS, an HSG will not only confirm placement but also test insert functionality. After confirming correct placement of the device, the provider can proceed with standard workup for chronic pelvic pain.
If one or more of the devices are malpositioned, the devices are generally presumed to be the etiology of the new pain. Multiple case reports demonstrate pain due to Essure misconfiguration or perforation with subsequent resolution of symptoms after device removal.18,20,21 A case study by Alcantara and colleagues described a patient with chronic pelvic pain and an Essure coil that was curved in an elliptical shape, not adhering to the anatomic course of the fallopian tube. The patient reported pain resolution after laparoscopic removal of the device.20 Another case report by Mahmoud et al described a subserosal malpositioned device that caused acute pelvic pain 4 months after sterilization. The patient reported resolution of pain after the microinsert was removed via laparoscopy.21 These reports highlight the importance of considering malpositioned devices as the etiology of new pelvic pain after Essure placement.

Continue to: Device removal and patient outcomes...
Device removal and patient outcomes
Removal
Several studies that we evaluated included a discussion on the methods for Essure removal. which are divided into 2 general categories: hysteroscopy and laparoscopy.
Hysteroscopic removal is generally used when the device was placed less than 12 weeks prior to removal.7,19 After 12 weeks, removal is more difficult due to fibrosis within the fallopian tubes. A risk with hysteroscopic removal is failure to remove all fibers, which allows inflammation and fibrosis to continue.7 This risk is mitigated via laparoscopic hysterectomy or mini-cornuectomy with bilateral salpingectomy, where the devices can be removed en bloc and without excessive traction.
Laparoscopic Essure removal procedures described in the literature include salpingostomy and traction on the device, salpingectomy, and salpingectomy with mini-cornuectomy. The incision and traction method is typically performed via a 2- to 3-cm incision on the antimesial edge of the fallopian tube along with a circumferential incision to surround the interstitial tubal area. The implant is carefully extracted from the fallopian tube and cornua, and a salpingectomy is then performed.22 The implant is removed prior to the salpingectomy to ensure that the Essure device is removed in its entirety prior to performing a salpingectomy.
A prospective observational study evaluated laparoscopic removal of Essure devices in 80 women with or without cornual excision. Results suggest that the incision and traction method poses more technical difficulties than the cornuectomy approach.23 Surgeons reported significant difficulty controlling the tensile pressure with traction, whereas use of the cornuectomy approach eliminated this risk and decreased the risk of fragmentation and incomplete removal.23,24
Charavil and colleagues demonstrated in a prospective observational study that a vaginal hysterectomy with bilateral salpingectomy is a feasible approach to Essure removal. Twenty-six vaginal hysterectomies with bilateral salpingectomy and Essure removal were performed without conversion to laparoscopy or laparotomy. The surgeons performed an en bloc removal of each hemiuterus along with the ipsilateral tube, which ensured complete removal of the Essure device. Each case was confirmed with an x-ray of the surgical specimen.25
If device fragmentation occurs, there are different methods recommended for locating fragments. A case report of bilateral uterine perforation after uncomplicated Essure placement used a preoperative computed tomography (CT) scan to locate the Essure fragments, but no intraoperative imaging was performed to confirm complete fragment removal.26 The patient continued reporting chronic pelvic pain and ultimately underwent exploratory laparotomy with intraoperative fluoroscopy. Using fluoroscopy, investigators identified omental fragments that were missed on preoperative CT imaging. Fluoroscopy is not commonly used intraoperatively, but it may have added benefit for localizing retained fragments.
A retrospective cohort study reviewed the use of intraoperative x-ray of the removed specimen to confirm complete Essure removal.27 If an x-ray of the removed specimen showed incomplete removal, an intraoperative pelvic x-ray was performed to locate missing fragments. X-ray of the removed devices confirmed complete removal in 63 of 72 patients (87.5%). Six of 9 women with an unsatisfactory specimen x-ray had no residual fragments identified during pelvic x-ray, and the device removal was deemed adequate. The remaining 3 women had radiologic evidence of incomplete device removal and required additional dissection for complete removal. Overall, use of x-ray or fluoroscopy is a relatively safe and accessible way to ensure complete removal of the Essure device and is worth consideration, especially when retained device fragments are suspected.

Symptom resolution
We reviewed 5 studies that examined pain outcomes after removal of the Essure devices. Casey et al found that 23 of 26 patients (88.5%) reported significant pain relief at the postoperative visit, while 3 of 26 (11.5%) reported persistent pelvic pain.2 Two of 3 case series examined other outcomes in addition to postoperative pelvic pain, including sexual function and activities of daily living.7,14 In the first case series by Brito and colleagues, 8 of 11 patients (72.7%) reported an improvement in pelvic pain, ability to perform daily activities, sexual life, and overall quality of life after Essure removal. For the remaining 3 patients with persistent pelvic pain after surgical removal of the device, 2 patients reported worsening pain symptoms and dyspareunia.14 In this study, 5 of 11 patients reported a history of chronic pelvic pain at baseline. In a retrospective case series by Clark et al, 28 of 32 women (87.5%) reported some improvement in all domains, with 24 of 32 patients (75%) reporting almost total or complete improvement in quality of life, sexual life, pelvic pain, and scores related to activities of daily living. Pain and quality-of-life scores were similar for women who underwent uterine-preserving surgery and for those who underwent hysterectomy. Ten of 32 women (31.3%) reported persistent or worsening symptoms after the Essure removal surgery. In these patients, the authors recommended consideration of other autoimmune and hypersensitivity etiologies.7
In a retrospective cohort study by Kamencic et al from 2002 to 2013 of 1,430 patients who underwent Essure placement with postplacement imaging, 62 patients (4.3%) required a second surgery after Essure placement due to pelvic pain.10 This study also found that 4 of 62 patients (0.3%) had no other obvious cause for the pelvic pain. All 4 of these women had complete resolution of their pain with removal of the Essure microinsert device. A prospective observational study by Chene e
Summary
Although Essure products were withdrawn from the market in the United States in 2018, many patients still experience significant AEs associated with the device. The goal of the perspectives and data presented here is to assist clinicians in addressing and managing the pain experienced by patients after device insertion. ●
- Connor VF. Essure: a review six years later. J Minim Invasive Gynecol. 2009;16:282-290. doi:10.1016/j.jmig.2009.02.009.
- Casey J, Aguirre F, Yunker A. Outcomes of laparoscopic removal of the Essure sterilization device for pelvic pain: a case series. Contraception. 2016;94:190-192. doi:10.1016/j.contraception.2016.03.017.
- Jackson I. Essure device removed entirely from market, with 99% of unused birth control implants retrieved: FDA. AboutLawsuits.com. January 13, 2020. https://www.aboutlawsuits.com/Essure-removal-update-166509. Accessed June 7, 2022.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization. October 31, 2016. https://www.fda.gov/media/96315/download. Accessed June 7, 2022.
- US Food and Drug Administration. FDA activities related to Essure. March 14, 2022. https://www.fda.gov/medical-devices/essure-permanent-birth-control/fda-activities-related-essure. Accessed June 8, 2022.
- Arjona Berral JE, Rodríguez Jiménez B, Velasco Sánchez E, et al. Essure and chronic pelvic pain: a population-based cohort. J Obstet Gynaecol. 2014;34:712-713. doi:10.3109/01443615.2014.92075.
- Clark NV, Rademaker D, Mushinski AA, et al. Essure removal for the treatment of device-attributed symptoms: an expanded case series and follow-up survey. J Minim Invasive Gynecol. 2017;24:971-976. doi:10.1016/j.jmig.2017.05.015.
- Sills ES, Rickers NS, Li X. Surgical management after hysteroscopic sterilization: minimally invasive approach incorporating intraoperative fluoroscopy for symptomatic patients with >2 Essure devices. Surg Technol Int. 2018;32:156-161.
- Administration USF and D. 522 Postmarket Surveillance Studies. Center for Devices and Radiological Health; 2020.
- Kamencic H, Thiel L, Karreman E, et al. Does Essure cause significant de novo pain? A retrospective review of indications for second surgeries after Essure placement. J Minim Invasive Gynecol. 2016;23:1158-1162. doi:10.1016/j.jmig.2016.08.823.
- Yunker AC, Ritch JM, Robinson EF, et al. Incidence and risk factors for chronic pelvic pain after hysteroscopic sterilization. J Minim Invasive Gynecol. 2015;22:390-994. doi:10.1016/j.jmig.2014.06.007.
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141-154. doi:10.1016/j.berh.2011.02.005.
- van Limburg Stirum EVJ, Clark NV, Lindsey A, et al. Factors associated with negative patient experiences with Essure sterilization. JSLS. 2020;24(1):e2019.00065. doi:10.4293/JSLS.2019.00065.
- Brito LG, Cohen SL, Goggins ER, et al. Essure surgical removal and subsequent symptom resolution: case series and follow-up survey. J Minim Invasive Gynecol. 2015;22:910-913. doi:10.1016/j.jmig.2015.03.018.
- Maassen LW, van Gastel DM, Haveman I, et al. Removal of Essure sterilization devices: a retrospective cohort study in the Netherlands. J Minim Invasive Gynecol. 2019;26:1056-1062. doi:10.1016/j.jmig.2018.10.009.
- Sills ES, Palermo GD. Surgical excision of Essure devices with ESHRE class IIb uterine malformation: sequential hysteroscopic-laparoscopic approach to the septate uterus. Facts Views Vis Obgyn. 2016;8:49-52.
- Ricci G, Restaino S, Di Lorenzo G, et al. Risk of Essure microinsert abdominal migration: case report and review of literature. Ther Clin Risk Manag. 2014;10:963-968. doi:10.2147/TCRM.S65634.
- Borley J, Shabajee N, Tan TL. A kink is not always a perforation: assessing Essure hysteroscopic sterilization placement. Fertil Steril. 2011;95:2429.e15-7. doi:10.1016/j.fertnstert.2011.02.006.
- Djeffal H, Blouet M, Pizzoferato AC, et al. Imaging findings in Essure-related complications: a pictorial review.7Br J Radiol. 2018;91(1090):20170686. doi:10.1259/bjr.20170686.
- Lora Alcantara I, Rezai S, Kirby C, et al. Essure surgical removal and subsequent resolution of chronic pelvic pain: a case report and review of the literature. Case Rep Obstet Gynecol. 2016;2016:6961202. doi:10.1155/2016/6961202.
- Mahmoud MS, Fridman D, Merhi ZO. Subserosal misplacement of Essure device manifested by late-onset acute pelvic pain. Fertil Steril. 2009;92:2038.e1-3. doi:10.1016/j.fertnstert.2009.07.1677.
- Tissot M, Petry S, Lecointre L, et al. Two surgical techniques for Essure device ablation: the hysteroscopic way and the laparoscopic way by salpingectomy with tubal interstitial resection. J Minim Invasive Gynecol. 2019;26(4):603. doi:10.1016/j.jmig.2018.07.017.
- Chene G, Cerruto E, Moret S, et al. Quality of life after laparoscopic removal of Essure sterilization devices. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100054. doi:10.1016/j.eurox.2019.100054.
- Thiel L, Rattray D, Thiel J. Laparoscopic cornuectomy as a technique for removal of Essure microinserts. J Minim Invasive Gynecol. 2017;24(1):10. doi:10.1016/j.jmig.2016.07.004.
- Charavil A, Agostini A, Rambeaud C, et al. Vaginal hysterectomy with salpingectomy for Essure insert removal. J Minim Invasive Gynecol. 2019;2:695-701. doi:10.1016/j.jmig.2018.07.019.
- Howard DL, Christenson PJ, Strickland JL. Use of intraoperative fluoroscopy during laparotomy to identify fragments of retained Essure microinserts: case report. J Minim Invasive Gynecol. 2012;19:667-670. doi:10.1016/j.jmig.2012.04.007.
- Miquel L, Crochet P, Francini S, et al. Laparoscopic Essure device removal by en bloc salpingectomy-cornuectomy with intraoperative x-ray checking: a retrospective cohort study. J Minim Invasive Gynecol. 2020;27:697-703. doi:10.1016/j. jmig.2019.06.006.
- Connor VF. Essure: a review six years later. J Minim Invasive Gynecol. 2009;16:282-290. doi:10.1016/j.jmig.2009.02.009.
- Casey J, Aguirre F, Yunker A. Outcomes of laparoscopic removal of the Essure sterilization device for pelvic pain: a case series. Contraception. 2016;94:190-192. doi:10.1016/j.contraception.2016.03.017.
- Jackson I. Essure device removed entirely from market, with 99% of unused birth control implants retrieved: FDA. AboutLawsuits.com. January 13, 2020. https://www.aboutlawsuits.com/Essure-removal-update-166509. Accessed June 7, 2022.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization. October 31, 2016. https://www.fda.gov/media/96315/download. Accessed June 7, 2022.
- US Food and Drug Administration. FDA activities related to Essure. March 14, 2022. https://www.fda.gov/medical-devices/essure-permanent-birth-control/fda-activities-related-essure. Accessed June 8, 2022.
- Arjona Berral JE, Rodríguez Jiménez B, Velasco Sánchez E, et al. Essure and chronic pelvic pain: a population-based cohort. J Obstet Gynaecol. 2014;34:712-713. doi:10.3109/01443615.2014.92075.
- Clark NV, Rademaker D, Mushinski AA, et al. Essure removal for the treatment of device-attributed symptoms: an expanded case series and follow-up survey. J Minim Invasive Gynecol. 2017;24:971-976. doi:10.1016/j.jmig.2017.05.015.
- Sills ES, Rickers NS, Li X. Surgical management after hysteroscopic sterilization: minimally invasive approach incorporating intraoperative fluoroscopy for symptomatic patients with >2 Essure devices. Surg Technol Int. 2018;32:156-161.
- Administration USF and D. 522 Postmarket Surveillance Studies. Center for Devices and Radiological Health; 2020.
- Kamencic H, Thiel L, Karreman E, et al. Does Essure cause significant de novo pain? A retrospective review of indications for second surgeries after Essure placement. J Minim Invasive Gynecol. 2016;23:1158-1162. doi:10.1016/j.jmig.2016.08.823.
- Yunker AC, Ritch JM, Robinson EF, et al. Incidence and risk factors for chronic pelvic pain after hysteroscopic sterilization. J Minim Invasive Gynecol. 2015;22:390-994. doi:10.1016/j.jmig.2014.06.007.
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141-154. doi:10.1016/j.berh.2011.02.005.
- van Limburg Stirum EVJ, Clark NV, Lindsey A, et al. Factors associated with negative patient experiences with Essure sterilization. JSLS. 2020;24(1):e2019.00065. doi:10.4293/JSLS.2019.00065.
- Brito LG, Cohen SL, Goggins ER, et al. Essure surgical removal and subsequent symptom resolution: case series and follow-up survey. J Minim Invasive Gynecol. 2015;22:910-913. doi:10.1016/j.jmig.2015.03.018.
- Maassen LW, van Gastel DM, Haveman I, et al. Removal of Essure sterilization devices: a retrospective cohort study in the Netherlands. J Minim Invasive Gynecol. 2019;26:1056-1062. doi:10.1016/j.jmig.2018.10.009.
- Sills ES, Palermo GD. Surgical excision of Essure devices with ESHRE class IIb uterine malformation: sequential hysteroscopic-laparoscopic approach to the septate uterus. Facts Views Vis Obgyn. 2016;8:49-52.
- Ricci G, Restaino S, Di Lorenzo G, et al. Risk of Essure microinsert abdominal migration: case report and review of literature. Ther Clin Risk Manag. 2014;10:963-968. doi:10.2147/TCRM.S65634.
- Borley J, Shabajee N, Tan TL. A kink is not always a perforation: assessing Essure hysteroscopic sterilization placement. Fertil Steril. 2011;95:2429.e15-7. doi:10.1016/j.fertnstert.2011.02.006.
- Djeffal H, Blouet M, Pizzoferato AC, et al. Imaging findings in Essure-related complications: a pictorial review.7Br J Radiol. 2018;91(1090):20170686. doi:10.1259/bjr.20170686.
- Lora Alcantara I, Rezai S, Kirby C, et al. Essure surgical removal and subsequent resolution of chronic pelvic pain: a case report and review of the literature. Case Rep Obstet Gynecol. 2016;2016:6961202. doi:10.1155/2016/6961202.
- Mahmoud MS, Fridman D, Merhi ZO. Subserosal misplacement of Essure device manifested by late-onset acute pelvic pain. Fertil Steril. 2009;92:2038.e1-3. doi:10.1016/j.fertnstert.2009.07.1677.
- Tissot M, Petry S, Lecointre L, et al. Two surgical techniques for Essure device ablation: the hysteroscopic way and the laparoscopic way by salpingectomy with tubal interstitial resection. J Minim Invasive Gynecol. 2019;26(4):603. doi:10.1016/j.jmig.2018.07.017.
- Chene G, Cerruto E, Moret S, et al. Quality of life after laparoscopic removal of Essure sterilization devices. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100054. doi:10.1016/j.eurox.2019.100054.
- Thiel L, Rattray D, Thiel J. Laparoscopic cornuectomy as a technique for removal of Essure microinserts. J Minim Invasive Gynecol. 2017;24(1):10. doi:10.1016/j.jmig.2016.07.004.
- Charavil A, Agostini A, Rambeaud C, et al. Vaginal hysterectomy with salpingectomy for Essure insert removal. J Minim Invasive Gynecol. 2019;2:695-701. doi:10.1016/j.jmig.2018.07.019.
- Howard DL, Christenson PJ, Strickland JL. Use of intraoperative fluoroscopy during laparotomy to identify fragments of retained Essure microinserts: case report. J Minim Invasive Gynecol. 2012;19:667-670. doi:10.1016/j.jmig.2012.04.007.
- Miquel L, Crochet P, Francini S, et al. Laparoscopic Essure device removal by en bloc salpingectomy-cornuectomy with intraoperative x-ray checking: a retrospective cohort study. J Minim Invasive Gynecol. 2020;27:697-703. doi:10.1016/j. jmig.2019.06.006.
Amniotic fluid embolism: Management using a checklist
CASE Part 1: CPR initiated during induction of labor
A 32-year-old gravida 4 para 3-0-0-3 is undergoing induction of labor with intravenous (IV) oxytocin at 39 weeks of gestation. She has no significant medical or obstetric history. Fifteen minutes after reaching complete cervical dilation, she says “I don’t feel right,” then suddenly loses consciousness. The nurse finds no detectable pulse, calls a “code blue,” and initiates cardiopulmonary resuscitation (CPR). The obstetrician is notified, appears promptly, assesses the situation, and delivers a 3.6-kg baby via vacuum extraction. Apgar score is 2/10 at 1 minute and 6/10 at 5 minutes. After delivery of the placenta, there is uterine atony and brisk hemorrhage with 2 L of blood loss.
Management of AFE: A rare complication
This case demonstrates a classic presentation of amniotic fluid embolism (AFE) syndrome—a patient in labor or within 30 minutes after delivery has sudden onset of cardiorespiratory collapse followed by disseminated intravascular coagulation (DIC). AFE is rare, affecting only about 2 to 6 per 100,000 births, but classic cases have a reported maternal mortality rate that exceeds 50%.1 It is thought to reflect a complex, systemic proinflammatory response to maternal intravasation of pregnancy material, such as trophoblast, thromboplastins, fetal cells, or amniotic fluid. Because the syndrome is not necessarily directly caused by emboli or by amniotic fluid per se,2 it has been proposed that AFE be called “anaphylactoid syndrome of pregnancy,” but this terminology has not yet been widely adopted.3
Guidelines from the Society for Maternal-Fetal Medicine (SMFM) recommend several time-critical steps for the initial stabilization and management of patients with AFE.4 However, because AFE is rare, most obstetric providers may not encounter a case for many years or even decades after they have received training, so it is unrealistic to expect that they will remember these guidelines when they are needed. For this reason, when AFE occurs, it is important to have a readily accessible cognitive aid, such as a checklist that summarizes the key management steps. The SMFM provides a checklist for initial management of AFE that can be used at your institution; it is presented in the FIGURE and provides the outline for this discussion.5

Provide CPR immediately
Most AFE cases are accompanied by cardiorespiratory arrest. If the patient has no pulse, call a “code” to mobilize additional help and immediately start CPR. Use a backboard to make cardiac compressions most effective and manually displace the uterus or tilt the patient to avoid supine hypotension. Designate a timekeeper to call out 1-minute intervals and record critical data, such as medication administration and laboratory orders/results.
Expedite delivery
Immediate delivery is needed if maternal cardiac activity is not restored within 4 minutes of starting CPR, with a target to have delivery completed within 5 minutes. Operative vaginal delivery may be an option if delivery is imminent, as in the case presented, but cesarean delivery (CD) will be needed in most cases. This was previously called “perimortem cesarean” delivery, but the term “resuscitative hysterotomy” has been proposed because the primary goal is to improve the effectiveness of CPR6 and prevent both maternal and perinatal death. CPR is less effective in pregnant women because the pregnant uterus takes a substantial fraction of the maternal cardiac output, as well as compresses the vena cava. Some experts suggest that, rather than waiting 4 minutes, CD should be started as soon as an obstetrician or other surgeon is present, unless there is an immediate response to electrical cardioversion.6,7
In most cases, immediate CD should be performed wherever the patient is located rather than using precious minutes to move the patient to an operating room. Antiseptic preparation is expedited by simply pouring povidone-iodine or chlorhexidine over the lower abdomen if readily available; if not available, skip this step. Enter the abdomen and uterus as rapidly as possible using only a scalpel to make generous midline incisions.
If CPR is not required, proceed with cesarean or operative vaginal delivery as soon as the mother has been stabilized. These procedures should be performed using standard safety precautions outlined in the SMFM patient safety checklists for cesarean or operative vaginal delivery.8,9
Continue to: Anticipate hemorrhage...
Anticipate hemorrhage
Be prepared for uterine atony, coagulopathy, and catastrophic hemorrhage. Initiate IV oxytocin prophylaxis as soon as the infant is delivered. Have a low threshold for giving other uterotonic agents such as methylergonovine, carboprost, or misoprostol. If hemorrhage or DIC occurs, give tranexamic acid. Have the anesthesiologist or trauma team (if available) insert an intraosseous line for fluid resuscitation if peripheral IV access is inadequate.
Massive transfusion is often needed to treat DIC, which occurs in most AFE cases. Anticipate—do not wait—for DIC to occur. We propose activating your hospital’s massive transfusion protocol (MTP) as soon as you diagnose AFE so that blood products will be available as soon as possible. A typical MTP provides several units of red blood cells, a pheresis pack of platelets, and fresh/frozen plasma (FFP). If clinically indicated, administer cryoprecipitate instead of FFP to minimize volume overload, which may occur with FFP.
CASE Part 2: MTP initiated to treat DIC
The MTP is initiated. Laboratory results immediately pre-transfusion include hemoglobin 11.3 g/dL, platelet count 46,000 per mm3, fibrinogen 87 mg/dL, and an elevated prothrombin time international normalized ratio.
Expect heart failure
The initial hemodynamic picture in AFE is right heart failure, which should optimally be managed by a specialist from anesthesiology, cardiology, or critical care as soon as they are available. An emergency department physician may manage the hemodynamics until a specialist arrives. Avoidance of fluid overload is one important principle. If fluid challenges are needed for hypovolemic shock, boluses should be restricted to 500 mL rather than the traditional 1000 mL.
Pharmacologic treatment may include vasopressors, inotropic agents, and pulmonary vasodilators. Example medications and dosages recommended by SMFM are summarized in the checklist (FIGURE).5
After the initial phase of recovery, the hemodynamic picture often changes from right heart failure to left heart failure. Management of left heart failure is not covered in the SMFM checklist because, by the time it appears, the patient will usually be in the intensive care unit, managed by the critical care team. Management of left heart failure generally includes diuresis as needed for cardiogenic pulmonary edema, optimization of cardiac preload, and inotropic agents or vasopressors if needed to maintain cardiac output or perfusion pressure.4
Debrief, learning opportunities
Complex emergencies such as AFE are rarely handled 100% perfectly, even those with a good outcome, so they present opportunities for team learning and improvement. The team should conduct a 10- to 15-minute debrief soon after the patient is stabilized. Make an explicit statement that the main goal of the debrief is to gather suggestions as to how systems and processes could be improved for next time, not to find fault or lay blame on individuals. Encourage all personnel involved in the initial management to attend and discuss what went well and what did not. Another goal is to provide support for individuals who may feel traumatized by the dramatic, frightening events surrounding an AFE and by the poor patient outcome or guarded prognosis that frequently follows. Another goal is to discuss the plan for providing support and disclosure to the patient and family.
The vast majority of AFE cases meet criteria to be designated as “sentinel events,” because of patient transfer to the intensive care unit, multi-unit blood transfusion, other severe maternal morbidities, or maternal death. Therefore, most AFE cases will trigger a root cause analysis (RCA) or other formal sentinel event analysis conducted by the hospital’s Safety or Quality Department. As with the immediate post-event debrief, the first goal of the RCA is to identify systems issues that may have resulted in suboptimal care and that can be modified to improve future care. Specific issues regarding the checklist should also be addressed:
- Was the checklist used?
- Was the checklist available?
- Are there items on the checklist that need to be modified, added, or deleted?
The RCA concludes with the development of a performance improvement plan.
Ultimately, we encourage all AFE cases be reported to the registry maintained by the Amniotic Fluid Embolism Foundation at https://www.afesupport.org/, regardless of whether the outcome was favorable for the mother and newborn. The registry includes over 130 AFE cases since 2013 from around the world. Researchers periodically report on the registry findings.10 If providers report cases with both good and bad outcomes, the registry may provide future insights regarding which adjunctive or empiric treatments may or may not be promising.
Continue to: Empiric treatments...
Empiric treatments
From time-to-time, new regimens for empiric treatment of AFE are reported. It is important to recognize that these reports are generally uncontrolled case reports of favorable outcomes and that, without a control group, it is impossible to determine to what extent the treatment contributed to the outcome or was merely incidental. Given the rarity of AFE, it seems unlikely that there will ever be a randomized clinical trial or even a controlled prospective study comparing treatment regimens.
The “A-OK” regimen is an empiric treatment that has garnered some interest after an initial case report.11 It consists of an anticholinergic agent (atropine 0.2 mg IV), a selective 5-HT3 receptor antagonist (ondansetron 8 mg IV), and a nonsteroidal anti-inflammatory drug (ketorolac 15 mg IV). We have some reservations about this regimen, however, because atropine is relatively contraindicated if the patient has tachycardia (which is common in patients with hemorrhage) and ketorolac may suppress platelet function, which might be harmful for patients with DIC or thrombocytopenia.
Another empiric treatment is the “50-50-500” regimen, which includes an H1 antihistamine (diphenhydramine 50 mg IV), an H2 antihistamine (famotidine 50 mg IV), and a corticosteroid (hydrocortisone 500 mg IV). This regimen aims to suppress histamine-mediated and cell-mediated inflammatory responses, based on the notion that proinflammatory responses likely mediate much of the underlying pathophysiology of the AFE syndrome.
We would emphasize that these empiric regimens are not clinically validated, US Food and Drug Administration approved for treatment of AFE, or considered standard of care. Future reports of these and other regimens will be needed to evaluate their efficacy, limitations, and risks. Again, we encourage providers to report all AFE cases to the AFE Foundation registry, regardless of whether the treatments are successful.
CASE Conclusion
The hemorrhage stops after administration of oxytocin, carboprost, 6 units of cryoprecipitate, and a 6-unit platelet pheresis pack. The patient is transferred to the intensive care unit where she eventually requires a total of 10 units of red cells, 8 more units of cryoprecipitate, and another platelet pheresis pack. She is discharged to home in stable condition on postpartum day 4.
Be prepared, have the checklist ready
Because AFE is rare, most members of the health care team will have no prior experience managing a real case. It may have been years or decades since they had any education on AFE or they last read a review article such as this one. It is even possible the anesthesiologist, cardiologist, or critical care specialist has never heard of AFE. Thus if they rely on memory alone, there is substantial risk of forgetting items, getting dosages wrong, or other errors. With this in mind, what is the best way to prepare the team to expeditiously employ the management steps outlined here?
Use of a checklist that summarizes these key steps for early management, such as the SMFM checklist in the FIGURE, will help ensure that all relevant steps are performed in every AFE case. It is designed to be printed on a single sheet of letter-sized paper, and we propose that every labor and delivery (L&D) unit keep laminated copies of this checklist in several places where they will be immediately available should an AFE occur. Copies can be kept on the anesthesia carts in the L&D operating rooms, in an emergency procedures binder on the unit, and on the “crash carts” and hemorrhage supply carts in the L&D unit. Effective implementation of an AFE checklist requires all personnel know where to readily find it and have some familiarity with its contents.
An interdisciplinary team comprising representatives from nursing, obstetrics, and anesthesia should meet to discuss whether the checklist needs to be modified to fit the local hospital formulary or other unique local circumstances. The team should develop an implementation plan that includes where to keep checklist copies, a process to periodically ensure that the copies are still present and readable, a roll-out plan to inform all personnel about the checklist process, and most importantly a training plan that includes incorporating AFE cases into the schedule of multidisciplinary simulations and drills for obstetric emergencies. Other implementation strategies are outlined in the SMFM document.5
Ultimately an organized, systematic approach is recommended for management of AFE. There is no single best treatment of AFE; it is supportive and directed toward the underlying pathophysiology, which may vary from patient to patient. Therefore, although a checklist, in conjunction with regular education and simulation activities, may help optimize care and improve outcomes, there is still a high risk of maternal morbidity and mortality from AFE. ●
- Clark SL. Amniotic fluid embolism. Obstet Gynecol. 2014;123(2 Pt 1):337-348. doi:10.1097/AOG.0000000000000107.
- Funk M, Damron A, Bandi V, et al. Pulmonary vascular obstruction by squamous cells is not involved in amniotic fluid embolism. Am J Obstet Gynecol. 2018;218:460-461. doi:10.1016/j.ajog.2017.12.225.
- Gilmore DA, Wakim J, Secrest J, et al. Anaphylactoid syndrome of pregnancy: a review of the literature with latest management and outcome data. AANA J. 2003;71:120-126.
- Society for Maternal-Fetal Medicine, Pacheco LD, Saade G, et al. Amniotic fluid embolism: diagnosis and management. Am J Obstet Gynecol. 2016;215:B16-24. doi:10.1016/j.ajog.2016.03.012.
- Patient Safety and Quality Committee, Society for Maternal-Fetal Medicine; Combs CA, Montgomery DM, et al. Society for Maternal-Fetal Medicine Special Statement: checklist for initial management of amniotic fluid embolism. Am J Obstet Gynecol. 2021;224:B29-B32. doi:10.1016/j.ajog.2021.01.001.
- Rose CH, Faksh A, Traynor KD, et al. Challenging the 4- to 5-minute rule: from perimortem cesarean to resuscitative hysterotomy. Am J Obstet Gynecol. 2015;213:653-6, 653.e1. doi:10.1016/j.ajog.2015.07.019.
- Pacheco LD, Clark SL, Klassen M, et al. Amniotic fluid embolism: principles of early clinical management. Am J Obstet Gynecol. 2020;222:48-52. doi:10.1016/j.ajog.2019.07.036.
- Combs CA, Einerson BD, Toner LE, SMFM Patient Safety and Quality Committee. SMFM Special Statement: surgical safety checklists for cesarean delivery. Am J Obstet Gynecol. 2021;225:B43-B49. doi:10.1016/j.ajog.2021.07.011.
- SMFM Patient Safety and Quality Committee, Staat B, Combs CA. SMFM Special Statement: operative vaginal delivery: checklists for performance and documentation. Am J Obstet Gynecol. 2020;222:B15-B21. doi:10.1016/j.ajog.2020.02.011.
- Stafford IA, Moaddab A, Dildy GA, et al. Amniotic fluid embolism syndrome: analysis of the United States international registry. Am J Obstet Gynecol MFM. 2020;2:100083. doi:10.1016/j.ajogmf.2019.100083.
- Rezai S, Hughes AZC, Larsen TB, et al. Atypical amniotic f luid embolism managed with a novel therapeutic regimen. Case Rep Obstet Gynecol. 2017; 2017:8458375. doi:10.1155/2017/8458375.

CASE Part 1: CPR initiated during induction of labor
A 32-year-old gravida 4 para 3-0-0-3 is undergoing induction of labor with intravenous (IV) oxytocin at 39 weeks of gestation. She has no significant medical or obstetric history. Fifteen minutes after reaching complete cervical dilation, she says “I don’t feel right,” then suddenly loses consciousness. The nurse finds no detectable pulse, calls a “code blue,” and initiates cardiopulmonary resuscitation (CPR). The obstetrician is notified, appears promptly, assesses the situation, and delivers a 3.6-kg baby via vacuum extraction. Apgar score is 2/10 at 1 minute and 6/10 at 5 minutes. After delivery of the placenta, there is uterine atony and brisk hemorrhage with 2 L of blood loss.
Management of AFE: A rare complication
This case demonstrates a classic presentation of amniotic fluid embolism (AFE) syndrome—a patient in labor or within 30 minutes after delivery has sudden onset of cardiorespiratory collapse followed by disseminated intravascular coagulation (DIC). AFE is rare, affecting only about 2 to 6 per 100,000 births, but classic cases have a reported maternal mortality rate that exceeds 50%.1 It is thought to reflect a complex, systemic proinflammatory response to maternal intravasation of pregnancy material, such as trophoblast, thromboplastins, fetal cells, or amniotic fluid. Because the syndrome is not necessarily directly caused by emboli or by amniotic fluid per se,2 it has been proposed that AFE be called “anaphylactoid syndrome of pregnancy,” but this terminology has not yet been widely adopted.3
Guidelines from the Society for Maternal-Fetal Medicine (SMFM) recommend several time-critical steps for the initial stabilization and management of patients with AFE.4 However, because AFE is rare, most obstetric providers may not encounter a case for many years or even decades after they have received training, so it is unrealistic to expect that they will remember these guidelines when they are needed. For this reason, when AFE occurs, it is important to have a readily accessible cognitive aid, such as a checklist that summarizes the key management steps. The SMFM provides a checklist for initial management of AFE that can be used at your institution; it is presented in the FIGURE and provides the outline for this discussion.5

Provide CPR immediately
Most AFE cases are accompanied by cardiorespiratory arrest. If the patient has no pulse, call a “code” to mobilize additional help and immediately start CPR. Use a backboard to make cardiac compressions most effective and manually displace the uterus or tilt the patient to avoid supine hypotension. Designate a timekeeper to call out 1-minute intervals and record critical data, such as medication administration and laboratory orders/results.
Expedite delivery
Immediate delivery is needed if maternal cardiac activity is not restored within 4 minutes of starting CPR, with a target to have delivery completed within 5 minutes. Operative vaginal delivery may be an option if delivery is imminent, as in the case presented, but cesarean delivery (CD) will be needed in most cases. This was previously called “perimortem cesarean” delivery, but the term “resuscitative hysterotomy” has been proposed because the primary goal is to improve the effectiveness of CPR6 and prevent both maternal and perinatal death. CPR is less effective in pregnant women because the pregnant uterus takes a substantial fraction of the maternal cardiac output, as well as compresses the vena cava. Some experts suggest that, rather than waiting 4 minutes, CD should be started as soon as an obstetrician or other surgeon is present, unless there is an immediate response to electrical cardioversion.6,7
In most cases, immediate CD should be performed wherever the patient is located rather than using precious minutes to move the patient to an operating room. Antiseptic preparation is expedited by simply pouring povidone-iodine or chlorhexidine over the lower abdomen if readily available; if not available, skip this step. Enter the abdomen and uterus as rapidly as possible using only a scalpel to make generous midline incisions.
If CPR is not required, proceed with cesarean or operative vaginal delivery as soon as the mother has been stabilized. These procedures should be performed using standard safety precautions outlined in the SMFM patient safety checklists for cesarean or operative vaginal delivery.8,9
Continue to: Anticipate hemorrhage...
Anticipate hemorrhage
Be prepared for uterine atony, coagulopathy, and catastrophic hemorrhage. Initiate IV oxytocin prophylaxis as soon as the infant is delivered. Have a low threshold for giving other uterotonic agents such as methylergonovine, carboprost, or misoprostol. If hemorrhage or DIC occurs, give tranexamic acid. Have the anesthesiologist or trauma team (if available) insert an intraosseous line for fluid resuscitation if peripheral IV access is inadequate.
Massive transfusion is often needed to treat DIC, which occurs in most AFE cases. Anticipate—do not wait—for DIC to occur. We propose activating your hospital’s massive transfusion protocol (MTP) as soon as you diagnose AFE so that blood products will be available as soon as possible. A typical MTP provides several units of red blood cells, a pheresis pack of platelets, and fresh/frozen plasma (FFP). If clinically indicated, administer cryoprecipitate instead of FFP to minimize volume overload, which may occur with FFP.
CASE Part 2: MTP initiated to treat DIC
The MTP is initiated. Laboratory results immediately pre-transfusion include hemoglobin 11.3 g/dL, platelet count 46,000 per mm3, fibrinogen 87 mg/dL, and an elevated prothrombin time international normalized ratio.
Expect heart failure
The initial hemodynamic picture in AFE is right heart failure, which should optimally be managed by a specialist from anesthesiology, cardiology, or critical care as soon as they are available. An emergency department physician may manage the hemodynamics until a specialist arrives. Avoidance of fluid overload is one important principle. If fluid challenges are needed for hypovolemic shock, boluses should be restricted to 500 mL rather than the traditional 1000 mL.
Pharmacologic treatment may include vasopressors, inotropic agents, and pulmonary vasodilators. Example medications and dosages recommended by SMFM are summarized in the checklist (FIGURE).5
After the initial phase of recovery, the hemodynamic picture often changes from right heart failure to left heart failure. Management of left heart failure is not covered in the SMFM checklist because, by the time it appears, the patient will usually be in the intensive care unit, managed by the critical care team. Management of left heart failure generally includes diuresis as needed for cardiogenic pulmonary edema, optimization of cardiac preload, and inotropic agents or vasopressors if needed to maintain cardiac output or perfusion pressure.4
Debrief, learning opportunities
Complex emergencies such as AFE are rarely handled 100% perfectly, even those with a good outcome, so they present opportunities for team learning and improvement. The team should conduct a 10- to 15-minute debrief soon after the patient is stabilized. Make an explicit statement that the main goal of the debrief is to gather suggestions as to how systems and processes could be improved for next time, not to find fault or lay blame on individuals. Encourage all personnel involved in the initial management to attend and discuss what went well and what did not. Another goal is to provide support for individuals who may feel traumatized by the dramatic, frightening events surrounding an AFE and by the poor patient outcome or guarded prognosis that frequently follows. Another goal is to discuss the plan for providing support and disclosure to the patient and family.
The vast majority of AFE cases meet criteria to be designated as “sentinel events,” because of patient transfer to the intensive care unit, multi-unit blood transfusion, other severe maternal morbidities, or maternal death. Therefore, most AFE cases will trigger a root cause analysis (RCA) or other formal sentinel event analysis conducted by the hospital’s Safety or Quality Department. As with the immediate post-event debrief, the first goal of the RCA is to identify systems issues that may have resulted in suboptimal care and that can be modified to improve future care. Specific issues regarding the checklist should also be addressed:
- Was the checklist used?
- Was the checklist available?
- Are there items on the checklist that need to be modified, added, or deleted?
The RCA concludes with the development of a performance improvement plan.
Ultimately, we encourage all AFE cases be reported to the registry maintained by the Amniotic Fluid Embolism Foundation at https://www.afesupport.org/, regardless of whether the outcome was favorable for the mother and newborn. The registry includes over 130 AFE cases since 2013 from around the world. Researchers periodically report on the registry findings.10 If providers report cases with both good and bad outcomes, the registry may provide future insights regarding which adjunctive or empiric treatments may or may not be promising.
Continue to: Empiric treatments...
Empiric treatments
From time-to-time, new regimens for empiric treatment of AFE are reported. It is important to recognize that these reports are generally uncontrolled case reports of favorable outcomes and that, without a control group, it is impossible to determine to what extent the treatment contributed to the outcome or was merely incidental. Given the rarity of AFE, it seems unlikely that there will ever be a randomized clinical trial or even a controlled prospective study comparing treatment regimens.
The “A-OK” regimen is an empiric treatment that has garnered some interest after an initial case report.11 It consists of an anticholinergic agent (atropine 0.2 mg IV), a selective 5-HT3 receptor antagonist (ondansetron 8 mg IV), and a nonsteroidal anti-inflammatory drug (ketorolac 15 mg IV). We have some reservations about this regimen, however, because atropine is relatively contraindicated if the patient has tachycardia (which is common in patients with hemorrhage) and ketorolac may suppress platelet function, which might be harmful for patients with DIC or thrombocytopenia.
Another empiric treatment is the “50-50-500” regimen, which includes an H1 antihistamine (diphenhydramine 50 mg IV), an H2 antihistamine (famotidine 50 mg IV), and a corticosteroid (hydrocortisone 500 mg IV). This regimen aims to suppress histamine-mediated and cell-mediated inflammatory responses, based on the notion that proinflammatory responses likely mediate much of the underlying pathophysiology of the AFE syndrome.
We would emphasize that these empiric regimens are not clinically validated, US Food and Drug Administration approved for treatment of AFE, or considered standard of care. Future reports of these and other regimens will be needed to evaluate their efficacy, limitations, and risks. Again, we encourage providers to report all AFE cases to the AFE Foundation registry, regardless of whether the treatments are successful.
CASE Conclusion
The hemorrhage stops after administration of oxytocin, carboprost, 6 units of cryoprecipitate, and a 6-unit platelet pheresis pack. The patient is transferred to the intensive care unit where she eventually requires a total of 10 units of red cells, 8 more units of cryoprecipitate, and another platelet pheresis pack. She is discharged to home in stable condition on postpartum day 4.
Be prepared, have the checklist ready
Because AFE is rare, most members of the health care team will have no prior experience managing a real case. It may have been years or decades since they had any education on AFE or they last read a review article such as this one. It is even possible the anesthesiologist, cardiologist, or critical care specialist has never heard of AFE. Thus if they rely on memory alone, there is substantial risk of forgetting items, getting dosages wrong, or other errors. With this in mind, what is the best way to prepare the team to expeditiously employ the management steps outlined here?
Use of a checklist that summarizes these key steps for early management, such as the SMFM checklist in the FIGURE, will help ensure that all relevant steps are performed in every AFE case. It is designed to be printed on a single sheet of letter-sized paper, and we propose that every labor and delivery (L&D) unit keep laminated copies of this checklist in several places where they will be immediately available should an AFE occur. Copies can be kept on the anesthesia carts in the L&D operating rooms, in an emergency procedures binder on the unit, and on the “crash carts” and hemorrhage supply carts in the L&D unit. Effective implementation of an AFE checklist requires all personnel know where to readily find it and have some familiarity with its contents.
An interdisciplinary team comprising representatives from nursing, obstetrics, and anesthesia should meet to discuss whether the checklist needs to be modified to fit the local hospital formulary or other unique local circumstances. The team should develop an implementation plan that includes where to keep checklist copies, a process to periodically ensure that the copies are still present and readable, a roll-out plan to inform all personnel about the checklist process, and most importantly a training plan that includes incorporating AFE cases into the schedule of multidisciplinary simulations and drills for obstetric emergencies. Other implementation strategies are outlined in the SMFM document.5
Ultimately an organized, systematic approach is recommended for management of AFE. There is no single best treatment of AFE; it is supportive and directed toward the underlying pathophysiology, which may vary from patient to patient. Therefore, although a checklist, in conjunction with regular education and simulation activities, may help optimize care and improve outcomes, there is still a high risk of maternal morbidity and mortality from AFE. ●

CASE Part 1: CPR initiated during induction of labor
A 32-year-old gravida 4 para 3-0-0-3 is undergoing induction of labor with intravenous (IV) oxytocin at 39 weeks of gestation. She has no significant medical or obstetric history. Fifteen minutes after reaching complete cervical dilation, she says “I don’t feel right,” then suddenly loses consciousness. The nurse finds no detectable pulse, calls a “code blue,” and initiates cardiopulmonary resuscitation (CPR). The obstetrician is notified, appears promptly, assesses the situation, and delivers a 3.6-kg baby via vacuum extraction. Apgar score is 2/10 at 1 minute and 6/10 at 5 minutes. After delivery of the placenta, there is uterine atony and brisk hemorrhage with 2 L of blood loss.
Management of AFE: A rare complication
This case demonstrates a classic presentation of amniotic fluid embolism (AFE) syndrome—a patient in labor or within 30 minutes after delivery has sudden onset of cardiorespiratory collapse followed by disseminated intravascular coagulation (DIC). AFE is rare, affecting only about 2 to 6 per 100,000 births, but classic cases have a reported maternal mortality rate that exceeds 50%.1 It is thought to reflect a complex, systemic proinflammatory response to maternal intravasation of pregnancy material, such as trophoblast, thromboplastins, fetal cells, or amniotic fluid. Because the syndrome is not necessarily directly caused by emboli or by amniotic fluid per se,2 it has been proposed that AFE be called “anaphylactoid syndrome of pregnancy,” but this terminology has not yet been widely adopted.3
Guidelines from the Society for Maternal-Fetal Medicine (SMFM) recommend several time-critical steps for the initial stabilization and management of patients with AFE.4 However, because AFE is rare, most obstetric providers may not encounter a case for many years or even decades after they have received training, so it is unrealistic to expect that they will remember these guidelines when they are needed. For this reason, when AFE occurs, it is important to have a readily accessible cognitive aid, such as a checklist that summarizes the key management steps. The SMFM provides a checklist for initial management of AFE that can be used at your institution; it is presented in the FIGURE and provides the outline for this discussion.5

Provide CPR immediately
Most AFE cases are accompanied by cardiorespiratory arrest. If the patient has no pulse, call a “code” to mobilize additional help and immediately start CPR. Use a backboard to make cardiac compressions most effective and manually displace the uterus or tilt the patient to avoid supine hypotension. Designate a timekeeper to call out 1-minute intervals and record critical data, such as medication administration and laboratory orders/results.
Expedite delivery
Immediate delivery is needed if maternal cardiac activity is not restored within 4 minutes of starting CPR, with a target to have delivery completed within 5 minutes. Operative vaginal delivery may be an option if delivery is imminent, as in the case presented, but cesarean delivery (CD) will be needed in most cases. This was previously called “perimortem cesarean” delivery, but the term “resuscitative hysterotomy” has been proposed because the primary goal is to improve the effectiveness of CPR6 and prevent both maternal and perinatal death. CPR is less effective in pregnant women because the pregnant uterus takes a substantial fraction of the maternal cardiac output, as well as compresses the vena cava. Some experts suggest that, rather than waiting 4 minutes, CD should be started as soon as an obstetrician or other surgeon is present, unless there is an immediate response to electrical cardioversion.6,7
In most cases, immediate CD should be performed wherever the patient is located rather than using precious minutes to move the patient to an operating room. Antiseptic preparation is expedited by simply pouring povidone-iodine or chlorhexidine over the lower abdomen if readily available; if not available, skip this step. Enter the abdomen and uterus as rapidly as possible using only a scalpel to make generous midline incisions.
If CPR is not required, proceed with cesarean or operative vaginal delivery as soon as the mother has been stabilized. These procedures should be performed using standard safety precautions outlined in the SMFM patient safety checklists for cesarean or operative vaginal delivery.8,9
Continue to: Anticipate hemorrhage...
Anticipate hemorrhage
Be prepared for uterine atony, coagulopathy, and catastrophic hemorrhage. Initiate IV oxytocin prophylaxis as soon as the infant is delivered. Have a low threshold for giving other uterotonic agents such as methylergonovine, carboprost, or misoprostol. If hemorrhage or DIC occurs, give tranexamic acid. Have the anesthesiologist or trauma team (if available) insert an intraosseous line for fluid resuscitation if peripheral IV access is inadequate.
Massive transfusion is often needed to treat DIC, which occurs in most AFE cases. Anticipate—do not wait—for DIC to occur. We propose activating your hospital’s massive transfusion protocol (MTP) as soon as you diagnose AFE so that blood products will be available as soon as possible. A typical MTP provides several units of red blood cells, a pheresis pack of platelets, and fresh/frozen plasma (FFP). If clinically indicated, administer cryoprecipitate instead of FFP to minimize volume overload, which may occur with FFP.
CASE Part 2: MTP initiated to treat DIC
The MTP is initiated. Laboratory results immediately pre-transfusion include hemoglobin 11.3 g/dL, platelet count 46,000 per mm3, fibrinogen 87 mg/dL, and an elevated prothrombin time international normalized ratio.
Expect heart failure
The initial hemodynamic picture in AFE is right heart failure, which should optimally be managed by a specialist from anesthesiology, cardiology, or critical care as soon as they are available. An emergency department physician may manage the hemodynamics until a specialist arrives. Avoidance of fluid overload is one important principle. If fluid challenges are needed for hypovolemic shock, boluses should be restricted to 500 mL rather than the traditional 1000 mL.
Pharmacologic treatment may include vasopressors, inotropic agents, and pulmonary vasodilators. Example medications and dosages recommended by SMFM are summarized in the checklist (FIGURE).5
After the initial phase of recovery, the hemodynamic picture often changes from right heart failure to left heart failure. Management of left heart failure is not covered in the SMFM checklist because, by the time it appears, the patient will usually be in the intensive care unit, managed by the critical care team. Management of left heart failure generally includes diuresis as needed for cardiogenic pulmonary edema, optimization of cardiac preload, and inotropic agents or vasopressors if needed to maintain cardiac output or perfusion pressure.4
Debrief, learning opportunities
Complex emergencies such as AFE are rarely handled 100% perfectly, even those with a good outcome, so they present opportunities for team learning and improvement. The team should conduct a 10- to 15-minute debrief soon after the patient is stabilized. Make an explicit statement that the main goal of the debrief is to gather suggestions as to how systems and processes could be improved for next time, not to find fault or lay blame on individuals. Encourage all personnel involved in the initial management to attend and discuss what went well and what did not. Another goal is to provide support for individuals who may feel traumatized by the dramatic, frightening events surrounding an AFE and by the poor patient outcome or guarded prognosis that frequently follows. Another goal is to discuss the plan for providing support and disclosure to the patient and family.
The vast majority of AFE cases meet criteria to be designated as “sentinel events,” because of patient transfer to the intensive care unit, multi-unit blood transfusion, other severe maternal morbidities, or maternal death. Therefore, most AFE cases will trigger a root cause analysis (RCA) or other formal sentinel event analysis conducted by the hospital’s Safety or Quality Department. As with the immediate post-event debrief, the first goal of the RCA is to identify systems issues that may have resulted in suboptimal care and that can be modified to improve future care. Specific issues regarding the checklist should also be addressed:
- Was the checklist used?
- Was the checklist available?
- Are there items on the checklist that need to be modified, added, or deleted?
The RCA concludes with the development of a performance improvement plan.
Ultimately, we encourage all AFE cases be reported to the registry maintained by the Amniotic Fluid Embolism Foundation at https://www.afesupport.org/, regardless of whether the outcome was favorable for the mother and newborn. The registry includes over 130 AFE cases since 2013 from around the world. Researchers periodically report on the registry findings.10 If providers report cases with both good and bad outcomes, the registry may provide future insights regarding which adjunctive or empiric treatments may or may not be promising.
Continue to: Empiric treatments...
Empiric treatments
From time-to-time, new regimens for empiric treatment of AFE are reported. It is important to recognize that these reports are generally uncontrolled case reports of favorable outcomes and that, without a control group, it is impossible to determine to what extent the treatment contributed to the outcome or was merely incidental. Given the rarity of AFE, it seems unlikely that there will ever be a randomized clinical trial or even a controlled prospective study comparing treatment regimens.
The “A-OK” regimen is an empiric treatment that has garnered some interest after an initial case report.11 It consists of an anticholinergic agent (atropine 0.2 mg IV), a selective 5-HT3 receptor antagonist (ondansetron 8 mg IV), and a nonsteroidal anti-inflammatory drug (ketorolac 15 mg IV). We have some reservations about this regimen, however, because atropine is relatively contraindicated if the patient has tachycardia (which is common in patients with hemorrhage) and ketorolac may suppress platelet function, which might be harmful for patients with DIC or thrombocytopenia.
Another empiric treatment is the “50-50-500” regimen, which includes an H1 antihistamine (diphenhydramine 50 mg IV), an H2 antihistamine (famotidine 50 mg IV), and a corticosteroid (hydrocortisone 500 mg IV). This regimen aims to suppress histamine-mediated and cell-mediated inflammatory responses, based on the notion that proinflammatory responses likely mediate much of the underlying pathophysiology of the AFE syndrome.
We would emphasize that these empiric regimens are not clinically validated, US Food and Drug Administration approved for treatment of AFE, or considered standard of care. Future reports of these and other regimens will be needed to evaluate their efficacy, limitations, and risks. Again, we encourage providers to report all AFE cases to the AFE Foundation registry, regardless of whether the treatments are successful.
CASE Conclusion
The hemorrhage stops after administration of oxytocin, carboprost, 6 units of cryoprecipitate, and a 6-unit platelet pheresis pack. The patient is transferred to the intensive care unit where she eventually requires a total of 10 units of red cells, 8 more units of cryoprecipitate, and another platelet pheresis pack. She is discharged to home in stable condition on postpartum day 4.
Be prepared, have the checklist ready
Because AFE is rare, most members of the health care team will have no prior experience managing a real case. It may have been years or decades since they had any education on AFE or they last read a review article such as this one. It is even possible the anesthesiologist, cardiologist, or critical care specialist has never heard of AFE. Thus if they rely on memory alone, there is substantial risk of forgetting items, getting dosages wrong, or other errors. With this in mind, what is the best way to prepare the team to expeditiously employ the management steps outlined here?
Use of a checklist that summarizes these key steps for early management, such as the SMFM checklist in the FIGURE, will help ensure that all relevant steps are performed in every AFE case. It is designed to be printed on a single sheet of letter-sized paper, and we propose that every labor and delivery (L&D) unit keep laminated copies of this checklist in several places where they will be immediately available should an AFE occur. Copies can be kept on the anesthesia carts in the L&D operating rooms, in an emergency procedures binder on the unit, and on the “crash carts” and hemorrhage supply carts in the L&D unit. Effective implementation of an AFE checklist requires all personnel know where to readily find it and have some familiarity with its contents.
An interdisciplinary team comprising representatives from nursing, obstetrics, and anesthesia should meet to discuss whether the checklist needs to be modified to fit the local hospital formulary or other unique local circumstances. The team should develop an implementation plan that includes where to keep checklist copies, a process to periodically ensure that the copies are still present and readable, a roll-out plan to inform all personnel about the checklist process, and most importantly a training plan that includes incorporating AFE cases into the schedule of multidisciplinary simulations and drills for obstetric emergencies. Other implementation strategies are outlined in the SMFM document.5
Ultimately an organized, systematic approach is recommended for management of AFE. There is no single best treatment of AFE; it is supportive and directed toward the underlying pathophysiology, which may vary from patient to patient. Therefore, although a checklist, in conjunction with regular education and simulation activities, may help optimize care and improve outcomes, there is still a high risk of maternal morbidity and mortality from AFE. ●
- Clark SL. Amniotic fluid embolism. Obstet Gynecol. 2014;123(2 Pt 1):337-348. doi:10.1097/AOG.0000000000000107.
- Funk M, Damron A, Bandi V, et al. Pulmonary vascular obstruction by squamous cells is not involved in amniotic fluid embolism. Am J Obstet Gynecol. 2018;218:460-461. doi:10.1016/j.ajog.2017.12.225.
- Gilmore DA, Wakim J, Secrest J, et al. Anaphylactoid syndrome of pregnancy: a review of the literature with latest management and outcome data. AANA J. 2003;71:120-126.
- Society for Maternal-Fetal Medicine, Pacheco LD, Saade G, et al. Amniotic fluid embolism: diagnosis and management. Am J Obstet Gynecol. 2016;215:B16-24. doi:10.1016/j.ajog.2016.03.012.
- Patient Safety and Quality Committee, Society for Maternal-Fetal Medicine; Combs CA, Montgomery DM, et al. Society for Maternal-Fetal Medicine Special Statement: checklist for initial management of amniotic fluid embolism. Am J Obstet Gynecol. 2021;224:B29-B32. doi:10.1016/j.ajog.2021.01.001.
- Rose CH, Faksh A, Traynor KD, et al. Challenging the 4- to 5-minute rule: from perimortem cesarean to resuscitative hysterotomy. Am J Obstet Gynecol. 2015;213:653-6, 653.e1. doi:10.1016/j.ajog.2015.07.019.
- Pacheco LD, Clark SL, Klassen M, et al. Amniotic fluid embolism: principles of early clinical management. Am J Obstet Gynecol. 2020;222:48-52. doi:10.1016/j.ajog.2019.07.036.
- Combs CA, Einerson BD, Toner LE, SMFM Patient Safety and Quality Committee. SMFM Special Statement: surgical safety checklists for cesarean delivery. Am J Obstet Gynecol. 2021;225:B43-B49. doi:10.1016/j.ajog.2021.07.011.
- SMFM Patient Safety and Quality Committee, Staat B, Combs CA. SMFM Special Statement: operative vaginal delivery: checklists for performance and documentation. Am J Obstet Gynecol. 2020;222:B15-B21. doi:10.1016/j.ajog.2020.02.011.
- Stafford IA, Moaddab A, Dildy GA, et al. Amniotic fluid embolism syndrome: analysis of the United States international registry. Am J Obstet Gynecol MFM. 2020;2:100083. doi:10.1016/j.ajogmf.2019.100083.
- Rezai S, Hughes AZC, Larsen TB, et al. Atypical amniotic f luid embolism managed with a novel therapeutic regimen. Case Rep Obstet Gynecol. 2017; 2017:8458375. doi:10.1155/2017/8458375.
- Clark SL. Amniotic fluid embolism. Obstet Gynecol. 2014;123(2 Pt 1):337-348. doi:10.1097/AOG.0000000000000107.
- Funk M, Damron A, Bandi V, et al. Pulmonary vascular obstruction by squamous cells is not involved in amniotic fluid embolism. Am J Obstet Gynecol. 2018;218:460-461. doi:10.1016/j.ajog.2017.12.225.
- Gilmore DA, Wakim J, Secrest J, et al. Anaphylactoid syndrome of pregnancy: a review of the literature with latest management and outcome data. AANA J. 2003;71:120-126.
- Society for Maternal-Fetal Medicine, Pacheco LD, Saade G, et al. Amniotic fluid embolism: diagnosis and management. Am J Obstet Gynecol. 2016;215:B16-24. doi:10.1016/j.ajog.2016.03.012.
- Patient Safety and Quality Committee, Society for Maternal-Fetal Medicine; Combs CA, Montgomery DM, et al. Society for Maternal-Fetal Medicine Special Statement: checklist for initial management of amniotic fluid embolism. Am J Obstet Gynecol. 2021;224:B29-B32. doi:10.1016/j.ajog.2021.01.001.
- Rose CH, Faksh A, Traynor KD, et al. Challenging the 4- to 5-minute rule: from perimortem cesarean to resuscitative hysterotomy. Am J Obstet Gynecol. 2015;213:653-6, 653.e1. doi:10.1016/j.ajog.2015.07.019.
- Pacheco LD, Clark SL, Klassen M, et al. Amniotic fluid embolism: principles of early clinical management. Am J Obstet Gynecol. 2020;222:48-52. doi:10.1016/j.ajog.2019.07.036.
- Combs CA, Einerson BD, Toner LE, SMFM Patient Safety and Quality Committee. SMFM Special Statement: surgical safety checklists for cesarean delivery. Am J Obstet Gynecol. 2021;225:B43-B49. doi:10.1016/j.ajog.2021.07.011.
- SMFM Patient Safety and Quality Committee, Staat B, Combs CA. SMFM Special Statement: operative vaginal delivery: checklists for performance and documentation. Am J Obstet Gynecol. 2020;222:B15-B21. doi:10.1016/j.ajog.2020.02.011.
- Stafford IA, Moaddab A, Dildy GA, et al. Amniotic fluid embolism syndrome: analysis of the United States international registry. Am J Obstet Gynecol MFM. 2020;2:100083. doi:10.1016/j.ajogmf.2019.100083.
- Rezai S, Hughes AZC, Larsen TB, et al. Atypical amniotic f luid embolism managed with a novel therapeutic regimen. Case Rep Obstet Gynecol. 2017; 2017:8458375. doi:10.1155/2017/8458375.
A guide to understanding your anatomy
LGBTQ students would get new protections under Biden plan
On the 50th anniversary of Title IX’s inception, the Biden administration has proposed changes to the law that would protect transgender students and assault survivors on college and university campuses.
With these changes, the protections provided by Title IX – a civil rights law that prohibits sex-based discrimination in schools that receive federal funding – would now be extended to students who identify as trans. The update would ensure that government-funded schools make proper accommodations for a trans student population, such as allowing students to use bathrooms and other facilities that align with their gender identity, and enforcing the use of students’ correct pronouns.
The revisions also seek to undo amendments made to the law by Betsy DeVos, who was secretary of education during the Trump presidency, which strengthened due process protections for students accused of sexual assault and narrowed the definition of sexual harassment. These rules “weakened protections for survivors of sexual assault and diminished the promise of an education free from discrimination,” the Biden administration said.
“Our proposed changes will allow us to continue that progress and ensure all our nation’s students – no matter where they live, who they are, or whom they love – can learn, grow, and thrive in school,” Education Secretary Miguel Cardona, PhD, said in a news release. “We welcome public comment on these critical regulations so we can further the Biden-Harris Administration’s mission of creating educational environments free from sex discrimination and sexual violence.”
The revisions will go through a long period of public comment before they are set into law. Still, the proposed changes mark a way forward for trans students who are not explicitly protected under Title IX, and they offer solace to assault survivors who may have felt discouraged to come forward and report under Ms. DeVos’s rules.
“The proposed regulations reflect the [Education] Department’s commitment to give full effect to Title IX, ensuring that no person experiences sex discrimination in education, and that school procedures for addressing complaints of sex discrimination, including sexual violence and other forms of sex-based harassment, are clear, effective, and fair to all involved,” said Catherine Lhamon, JD, assistant secretary for the Education Department’s Office Of Civil Rights.
More specific rules about transgender students’ participation in school sports are still to come.
A version of this article first appeared on WebMD.com.
On the 50th anniversary of Title IX’s inception, the Biden administration has proposed changes to the law that would protect transgender students and assault survivors on college and university campuses.
With these changes, the protections provided by Title IX – a civil rights law that prohibits sex-based discrimination in schools that receive federal funding – would now be extended to students who identify as trans. The update would ensure that government-funded schools make proper accommodations for a trans student population, such as allowing students to use bathrooms and other facilities that align with their gender identity, and enforcing the use of students’ correct pronouns.
The revisions also seek to undo amendments made to the law by Betsy DeVos, who was secretary of education during the Trump presidency, which strengthened due process protections for students accused of sexual assault and narrowed the definition of sexual harassment. These rules “weakened protections for survivors of sexual assault and diminished the promise of an education free from discrimination,” the Biden administration said.
“Our proposed changes will allow us to continue that progress and ensure all our nation’s students – no matter where they live, who they are, or whom they love – can learn, grow, and thrive in school,” Education Secretary Miguel Cardona, PhD, said in a news release. “We welcome public comment on these critical regulations so we can further the Biden-Harris Administration’s mission of creating educational environments free from sex discrimination and sexual violence.”
The revisions will go through a long period of public comment before they are set into law. Still, the proposed changes mark a way forward for trans students who are not explicitly protected under Title IX, and they offer solace to assault survivors who may have felt discouraged to come forward and report under Ms. DeVos’s rules.
“The proposed regulations reflect the [Education] Department’s commitment to give full effect to Title IX, ensuring that no person experiences sex discrimination in education, and that school procedures for addressing complaints of sex discrimination, including sexual violence and other forms of sex-based harassment, are clear, effective, and fair to all involved,” said Catherine Lhamon, JD, assistant secretary for the Education Department’s Office Of Civil Rights.
More specific rules about transgender students’ participation in school sports are still to come.
A version of this article first appeared on WebMD.com.
On the 50th anniversary of Title IX’s inception, the Biden administration has proposed changes to the law that would protect transgender students and assault survivors on college and university campuses.
With these changes, the protections provided by Title IX – a civil rights law that prohibits sex-based discrimination in schools that receive federal funding – would now be extended to students who identify as trans. The update would ensure that government-funded schools make proper accommodations for a trans student population, such as allowing students to use bathrooms and other facilities that align with their gender identity, and enforcing the use of students’ correct pronouns.
The revisions also seek to undo amendments made to the law by Betsy DeVos, who was secretary of education during the Trump presidency, which strengthened due process protections for students accused of sexual assault and narrowed the definition of sexual harassment. These rules “weakened protections for survivors of sexual assault and diminished the promise of an education free from discrimination,” the Biden administration said.
“Our proposed changes will allow us to continue that progress and ensure all our nation’s students – no matter where they live, who they are, or whom they love – can learn, grow, and thrive in school,” Education Secretary Miguel Cardona, PhD, said in a news release. “We welcome public comment on these critical regulations so we can further the Biden-Harris Administration’s mission of creating educational environments free from sex discrimination and sexual violence.”
The revisions will go through a long period of public comment before they are set into law. Still, the proposed changes mark a way forward for trans students who are not explicitly protected under Title IX, and they offer solace to assault survivors who may have felt discouraged to come forward and report under Ms. DeVos’s rules.
“The proposed regulations reflect the [Education] Department’s commitment to give full effect to Title IX, ensuring that no person experiences sex discrimination in education, and that school procedures for addressing complaints of sex discrimination, including sexual violence and other forms of sex-based harassment, are clear, effective, and fair to all involved,” said Catherine Lhamon, JD, assistant secretary for the Education Department’s Office Of Civil Rights.
More specific rules about transgender students’ participation in school sports are still to come.
A version of this article first appeared on WebMD.com.
Deaths rare in tonsillectomy, but some children at more risk
It’s rare for a child to die after a tonsillectomy, but children who die are more likely to have a complex chronic condition such as cerebral palsy or Down syndrome, according to a retrospective cohort study published in JAMA.
“Among children undergoing tonsillectomy, the rate of postoperative death was 7 per 100,000 operations overall, [but] among children with complex chronic conditions, the rate of postoperative death was 117 per 100,000 operations, representing 44% of overall deaths,” write researchers at the University of Wisconsin–Madison. “These findings may inform decisionmaking for pediatric tonsillectomy.”
The rate of death in children after tonsillectomy has been uncertain, the authors write. Specific mortality rates for children at increased risk for complications, including those under 3 years old and those with sleep-disordered breathing or complex chronic conditions, have not been available.
To learn how likely children undergoing tonsillectomy are to die after their surgery, as well as which children are most at risk, lead study author M. Bruce Edmonson, MD, MPH, department of pediatrics, University of Wisconsin School of Medicine and Public Health, Madison, and his colleagues drew data from five states, including ambulatory surgery, inpatient, and emergency department discharge data sets provided by the Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality for California, Florida, Maryland, New York, and Wisconsin.
Participants included 504,262 patients under 21 years of age whose discharge records linked their inpatient or outpatient tonsillectomy, with or without adenoidectomy, with at least 90 days of follow-up.
In a longitudinal analysis, the research team investigated postoperative death within 30 days or during a surgical stay lasting over 30 days. They calculated postoperative mortality per 100,000 operations, both overall and classified by age group, sleep-disordered breathing, and complex chronic conditions.
The 504,262 children ranged in age from 0 to 20 years and underwent a total of 505,182 tonsillectomies. Of these, 10.1% were performed in children aged under 3 years, 28.9% in children with sleep-disordered breathing, and 2.8% in those with complex chronic conditions.
The 36 linked postoperative deaths occurred between 2 and 20.5 days after surgical admission, and 19 (53%) of the deaths occurred after surgical discharge.
The unadjusted mortality rate was 7.04 (95% confidence interval, 4.97-9.98) deaths per 100,000 procedures. In multivariable models, children younger than 3 years and children with sleep-disordered breathing were not significantly more likely to die.
But children with complex chronic conditions were significantly more likely to die than were children without those conditions (117.22 vs. 3.87 deaths per 100,000 procedures, respectively).
Children with complex chronic conditions underwent only 2.8% of all tonsillectomies, but they accounted for 44% of postoperative deaths. Most deaths linked with complex chronic conditions occurred among children with neurologic, neuromuscular, congenital, or genetic disorders.
Findings can help providers advise patients and their families about tonsillectomy risks
Kavita Dedhia, MD, MSHP, attending otolaryngologist, Division of Otolaryngology, Children’s Hospital of Philadelphia, Pennsylvania, told this news organization that she was not surprised by the findings.
“This study suggests that mortality is an extremely rare complication of tonsillectomy, and that children with complex medical conditions are at highest risk,” Dr. Dedhia, who was not involved in the study, said in an email.
“Due to their underlying comorbidities, medically fragile children are considered to be at higher risk while undergoing anesthesia and surgical procedures,” she added.
Dr. Dedhia noted that nonpatient factors the study did not explore may have affected the mortality rates, including each hospital’s experience with managing children with complex medical conditions, as well as whether the hospitals were tertiary care facilities, and pediatric or adult hospitals.
She would like to know what hospital or practice characteristics may have contributed to the mortality risk and whether increased mortality in these patients is limited to tonsillectomy or is also found with other surgical procedures.
“The strength of this study is that it is large and multi-regional and that it informs providers about patient factors impacting mortality in pediatric tonsillectomy,” Dr. Dedhia said. “This study arms surgeons with data to discuss mortality risk with the families of medically complex children undergoing tonsillectomy.”
The study authors and Dr. Dedhia report no relevant financial relationships. Funding information was not provided.
A version of this article first appeared on Medscape.com.
It’s rare for a child to die after a tonsillectomy, but children who die are more likely to have a complex chronic condition such as cerebral palsy or Down syndrome, according to a retrospective cohort study published in JAMA.
“Among children undergoing tonsillectomy, the rate of postoperative death was 7 per 100,000 operations overall, [but] among children with complex chronic conditions, the rate of postoperative death was 117 per 100,000 operations, representing 44% of overall deaths,” write researchers at the University of Wisconsin–Madison. “These findings may inform decisionmaking for pediatric tonsillectomy.”
The rate of death in children after tonsillectomy has been uncertain, the authors write. Specific mortality rates for children at increased risk for complications, including those under 3 years old and those with sleep-disordered breathing or complex chronic conditions, have not been available.
To learn how likely children undergoing tonsillectomy are to die after their surgery, as well as which children are most at risk, lead study author M. Bruce Edmonson, MD, MPH, department of pediatrics, University of Wisconsin School of Medicine and Public Health, Madison, and his colleagues drew data from five states, including ambulatory surgery, inpatient, and emergency department discharge data sets provided by the Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality for California, Florida, Maryland, New York, and Wisconsin.
Participants included 504,262 patients under 21 years of age whose discharge records linked their inpatient or outpatient tonsillectomy, with or without adenoidectomy, with at least 90 days of follow-up.
In a longitudinal analysis, the research team investigated postoperative death within 30 days or during a surgical stay lasting over 30 days. They calculated postoperative mortality per 100,000 operations, both overall and classified by age group, sleep-disordered breathing, and complex chronic conditions.
The 504,262 children ranged in age from 0 to 20 years and underwent a total of 505,182 tonsillectomies. Of these, 10.1% were performed in children aged under 3 years, 28.9% in children with sleep-disordered breathing, and 2.8% in those with complex chronic conditions.
The 36 linked postoperative deaths occurred between 2 and 20.5 days after surgical admission, and 19 (53%) of the deaths occurred after surgical discharge.
The unadjusted mortality rate was 7.04 (95% confidence interval, 4.97-9.98) deaths per 100,000 procedures. In multivariable models, children younger than 3 years and children with sleep-disordered breathing were not significantly more likely to die.
But children with complex chronic conditions were significantly more likely to die than were children without those conditions (117.22 vs. 3.87 deaths per 100,000 procedures, respectively).
Children with complex chronic conditions underwent only 2.8% of all tonsillectomies, but they accounted for 44% of postoperative deaths. Most deaths linked with complex chronic conditions occurred among children with neurologic, neuromuscular, congenital, or genetic disorders.
Findings can help providers advise patients and their families about tonsillectomy risks
Kavita Dedhia, MD, MSHP, attending otolaryngologist, Division of Otolaryngology, Children’s Hospital of Philadelphia, Pennsylvania, told this news organization that she was not surprised by the findings.
“This study suggests that mortality is an extremely rare complication of tonsillectomy, and that children with complex medical conditions are at highest risk,” Dr. Dedhia, who was not involved in the study, said in an email.
“Due to their underlying comorbidities, medically fragile children are considered to be at higher risk while undergoing anesthesia and surgical procedures,” she added.
Dr. Dedhia noted that nonpatient factors the study did not explore may have affected the mortality rates, including each hospital’s experience with managing children with complex medical conditions, as well as whether the hospitals were tertiary care facilities, and pediatric or adult hospitals.
She would like to know what hospital or practice characteristics may have contributed to the mortality risk and whether increased mortality in these patients is limited to tonsillectomy or is also found with other surgical procedures.
“The strength of this study is that it is large and multi-regional and that it informs providers about patient factors impacting mortality in pediatric tonsillectomy,” Dr. Dedhia said. “This study arms surgeons with data to discuss mortality risk with the families of medically complex children undergoing tonsillectomy.”
The study authors and Dr. Dedhia report no relevant financial relationships. Funding information was not provided.
A version of this article first appeared on Medscape.com.
It’s rare for a child to die after a tonsillectomy, but children who die are more likely to have a complex chronic condition such as cerebral palsy or Down syndrome, according to a retrospective cohort study published in JAMA.
“Among children undergoing tonsillectomy, the rate of postoperative death was 7 per 100,000 operations overall, [but] among children with complex chronic conditions, the rate of postoperative death was 117 per 100,000 operations, representing 44% of overall deaths,” write researchers at the University of Wisconsin–Madison. “These findings may inform decisionmaking for pediatric tonsillectomy.”
The rate of death in children after tonsillectomy has been uncertain, the authors write. Specific mortality rates for children at increased risk for complications, including those under 3 years old and those with sleep-disordered breathing or complex chronic conditions, have not been available.
To learn how likely children undergoing tonsillectomy are to die after their surgery, as well as which children are most at risk, lead study author M. Bruce Edmonson, MD, MPH, department of pediatrics, University of Wisconsin School of Medicine and Public Health, Madison, and his colleagues drew data from five states, including ambulatory surgery, inpatient, and emergency department discharge data sets provided by the Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality for California, Florida, Maryland, New York, and Wisconsin.
Participants included 504,262 patients under 21 years of age whose discharge records linked their inpatient or outpatient tonsillectomy, with or without adenoidectomy, with at least 90 days of follow-up.
In a longitudinal analysis, the research team investigated postoperative death within 30 days or during a surgical stay lasting over 30 days. They calculated postoperative mortality per 100,000 operations, both overall and classified by age group, sleep-disordered breathing, and complex chronic conditions.
The 504,262 children ranged in age from 0 to 20 years and underwent a total of 505,182 tonsillectomies. Of these, 10.1% were performed in children aged under 3 years, 28.9% in children with sleep-disordered breathing, and 2.8% in those with complex chronic conditions.
The 36 linked postoperative deaths occurred between 2 and 20.5 days after surgical admission, and 19 (53%) of the deaths occurred after surgical discharge.
The unadjusted mortality rate was 7.04 (95% confidence interval, 4.97-9.98) deaths per 100,000 procedures. In multivariable models, children younger than 3 years and children with sleep-disordered breathing were not significantly more likely to die.
But children with complex chronic conditions were significantly more likely to die than were children without those conditions (117.22 vs. 3.87 deaths per 100,000 procedures, respectively).
Children with complex chronic conditions underwent only 2.8% of all tonsillectomies, but they accounted for 44% of postoperative deaths. Most deaths linked with complex chronic conditions occurred among children with neurologic, neuromuscular, congenital, or genetic disorders.
Findings can help providers advise patients and their families about tonsillectomy risks
Kavita Dedhia, MD, MSHP, attending otolaryngologist, Division of Otolaryngology, Children’s Hospital of Philadelphia, Pennsylvania, told this news organization that she was not surprised by the findings.
“This study suggests that mortality is an extremely rare complication of tonsillectomy, and that children with complex medical conditions are at highest risk,” Dr. Dedhia, who was not involved in the study, said in an email.
“Due to their underlying comorbidities, medically fragile children are considered to be at higher risk while undergoing anesthesia and surgical procedures,” she added.
Dr. Dedhia noted that nonpatient factors the study did not explore may have affected the mortality rates, including each hospital’s experience with managing children with complex medical conditions, as well as whether the hospitals were tertiary care facilities, and pediatric or adult hospitals.
She would like to know what hospital or practice characteristics may have contributed to the mortality risk and whether increased mortality in these patients is limited to tonsillectomy or is also found with other surgical procedures.
“The strength of this study is that it is large and multi-regional and that it informs providers about patient factors impacting mortality in pediatric tonsillectomy,” Dr. Dedhia said. “This study arms surgeons with data to discuss mortality risk with the families of medically complex children undergoing tonsillectomy.”
The study authors and Dr. Dedhia report no relevant financial relationships. Funding information was not provided.
A version of this article first appeared on Medscape.com.
FROM JAMA