User login
Resident Participation Impact on Operative Time and Outcomes in Veterans Undergoing Total Laryngectomy
Resident Participation Impact on Operative Time and Outcomes in Veterans Undergoing Total Laryngectomy
The US Department of Veterans Affairs (VA) has been integral in resident training. Resident surgical training requires a balance of supervision and autonomy, along with procedure repetition and appropriate feedback.1-3 Non-VA research has found that resident participation across various otolaryngology procedures, including thyroidectomy, neck dissection, and laryngectomy, does not increase patient morbidity.4-7 However, resident involvement in private and academic settings that included nonhead and neck procedures was linked to increased operative time and reduced productivity, as determined by work relative value units (wRVUs).7-13 This has also been identified in other specialties, including general surgery, orthopedics, and ophthalmology.14-16
Unlike the private sector, surgeon compensation at the VA is not as closely linked to operative productivity, offering a unique setting for resident training. While VA integration in otolaryngology residency programs increases resident case numbers, particularly in head and neck cases, the impact on VA patient outcomes and productivity is unknown.17 The use of larynxpreserving treatment modalities for laryngeal cancer has led to a decline in the number of total laryngectomies performed, which could potentially impact resident operative training for laryngectomies.18-20
This study sought to determine the impact of resident participation on operative time, wRVUs, and patient outcomes in veterans who underwent a total laryngectomy. This study was reviewed and approved by the MedStar Georgetown University Hospital Institutional Review Board and Research and Development Committee (#1595672).
Methods
A retrospective cohort of veterans nationwide who underwent total laryngectomy between 2001 and 2021, with or without neck dissection, was identified from the Veterans Affairs Surgical Quality Improvement Program (VASQIP). Data were extracted via the VA Informatics and Computing Infrastructure and patients were included based on Current Procedural Terminology codes for total laryngectomy, with or without neck dissection (31320, 31360, 31365). Laryngopharyngectomies, partial laryngectomies, and minimally invasive laryngectomies were excluded. VASQIP nurse data managers reviewed patient data for operative data, postoperative outcomes (including 30- day morbidity and mortality), and preoperative risk factors (Appendix).21
The VASQIP data provide the highest resident or postgraduate year (PGY) per surgery. PGY 1, 2, and 3 were considered junior residents and PGY ≥4, surgical fellows, and individuals who took research years during residency were considered senior residents. Cases performed by attending physicians alone were compared with those involving junior or senior residents.
Patient demographic data included age, body mass index, smoking and alcohol use, weight loss, and functional status. Consumption of any tobacco products within 12 months of surgery was considered tobacco use. Drinking on average ≥2 alcoholic beverages daily was considered alcohol use. Weight loss was defined as a 10% reduction in body weight within the 6 months before surgery, excluding patients enrolled in a weight loss program. Functional status was categorized as independent, partially dependent, totally dependent, and unknown.
Primary outcomes included operative time, wRVUs generated, and wRVUs generated per hour of operative time. Postoperative complications were recorded both as a continuous variable and as a binary variable for presence or absence of a complication. Additional outcome variables included length of postoperative hospital stay, return to the operating room (OR), and death within 30 days of surgery.
Statistical Analysis
Data were summarized using frequency and percentage for categorical variables and median with IQR for continuous variables. Data were also summarized based on resident involvement in the surgery and the PGY level of the residents involved. The occurrence of total laryngectomy, rate of complications, and patient return to the OR were summarized by year.
Univariate associations between resident involvement and surgical outcomes were analyzed using the Kruskal-Wallis test for continuous variables and the ÷2 test for categorical variables. A Fisher exact test was used when the cell count in the contingency table was < 5. The univariate associations between surgical outcomes and demographic/preoperative variables were examined using 2-sided Wilcoxon ranksum tests or Kruskal-Wallis tests between continuous variables and categorical variables, X2 or Fisher exact test between 2 categorical variables, and 2-sided Spearman correlation test between 2 continuous variables. A false-discovery rate approach was used for simultaneous posthoc tests to determine the adjusted P values for wRVUs generated/operative time for attending physicians alone vs with junior residents and for attending physicians alone vs with senior residents. Models were used to evaluate the effects of resident involvement on surgical outcomes, adjusting for variables that showed significant univariate associations. Linear regression models were used for operative time, wRVUs generated, wRVUs generated/operative time, and length of postoperative stay. A logistic regression model was used for death within 30 days. Models were not built for postoperative complications or patient return to the OR, as these were only statistically significantly associated with the patient’s preoperative functional status. A finding was considered significant if P < .05. All analyses were performed using statistical software RStudio Version 2023.03.0.
Results
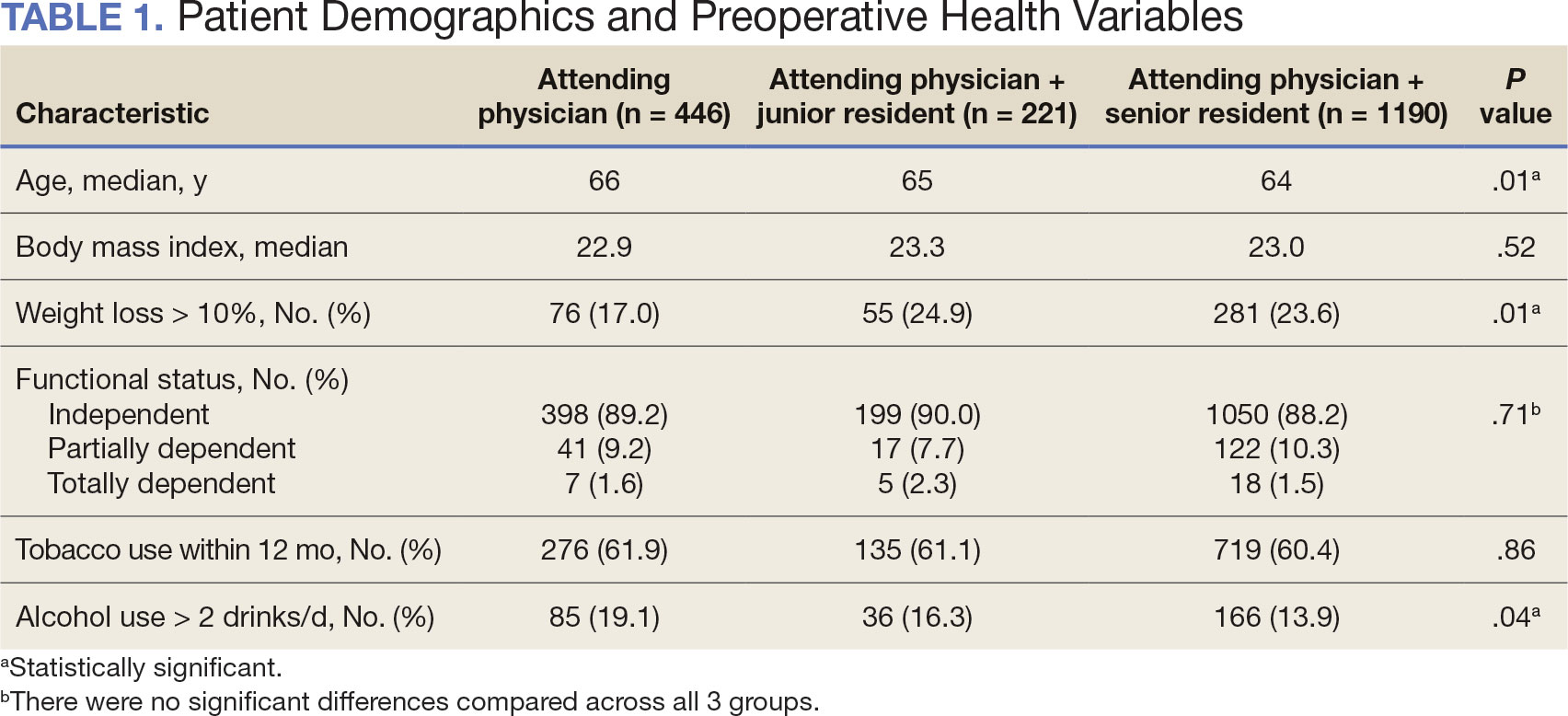
Between 2001 and 2021, 1857 patients who underwent total laryngectomy were identified from the VASQIP database nationwide. Most of the total laryngectomies were staffed by an attending physician with a senior resident (n = 1190, 64%), 446 (24%) were conducted by the attending physician alone, and 221 (12%) by an attending physician with a junior resident (Table 1). The mean operating time for an attending physician alone was 378 minutes, 384 minutes for an attending physician with a senior resident, and 432 minutes for an attending physician with a junior resident (Table 2). There was a statistically significant increase in operating time for laryngectomies with resident participation compared to attending physicians operating alone (P < .001).
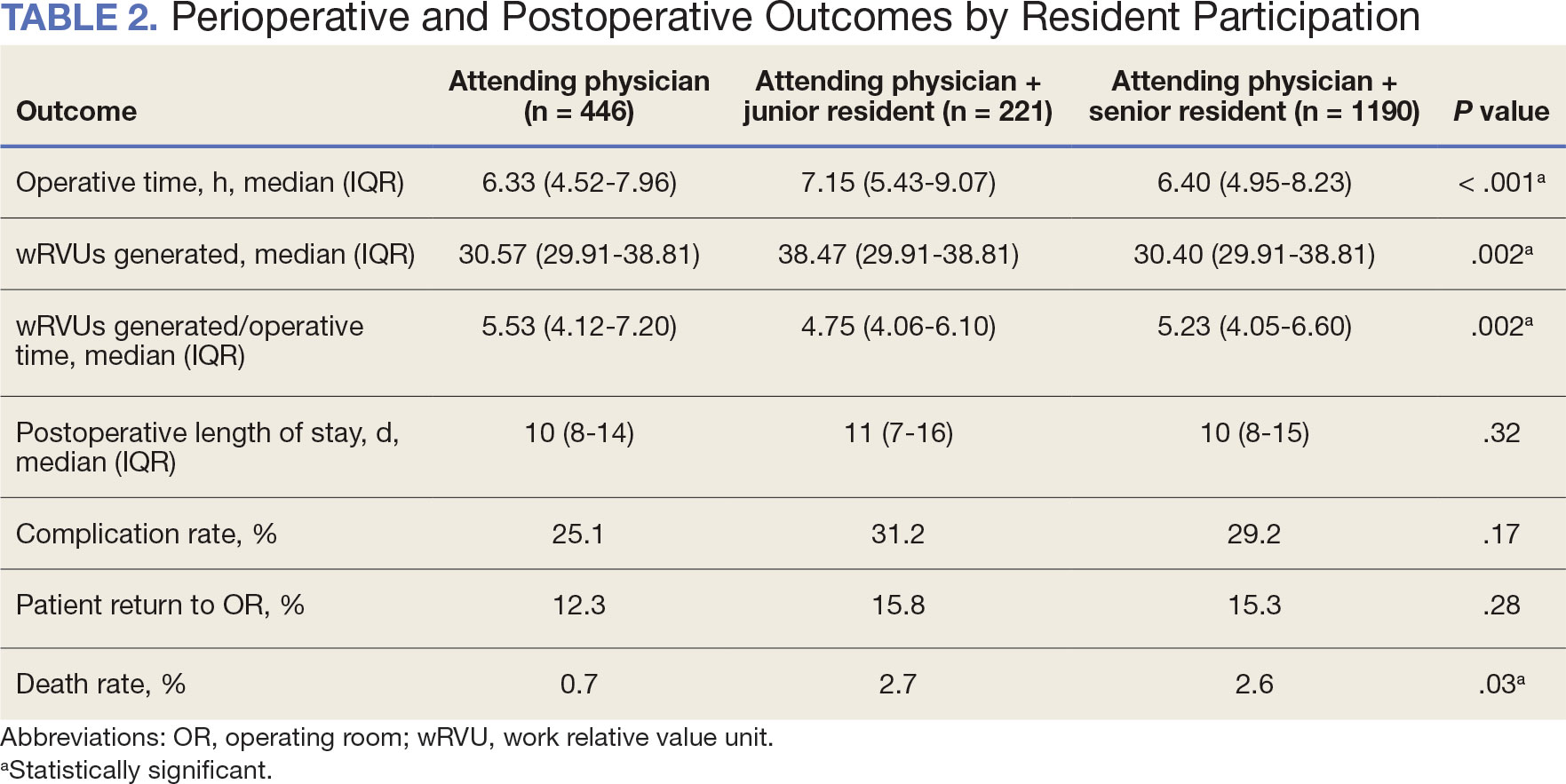
When the wRVUs generated/operative time was analyzed, there was a statistically significant difference between comparison groups. Total laryngectomies performed by attending physicians alone had the highest wRVUs generated/operative time (5.5), followed by laryngectomies performed by attending physicians with senior residents and laryngectomies performed by attending physicians with junior residents (5.2 and 4.8, respectively; P = .002). Table 3 describes adjusted P values for wRVUs generated/ operative time for total laryngectomies performed by attending physicians alone vs with junior residents (P = .003) and for attending physicians alone vs with senior residents (P = .02). Resident participation in total laryngectomies did not significantly impact the development or number of postoperative complications or the rate of return to the OR.
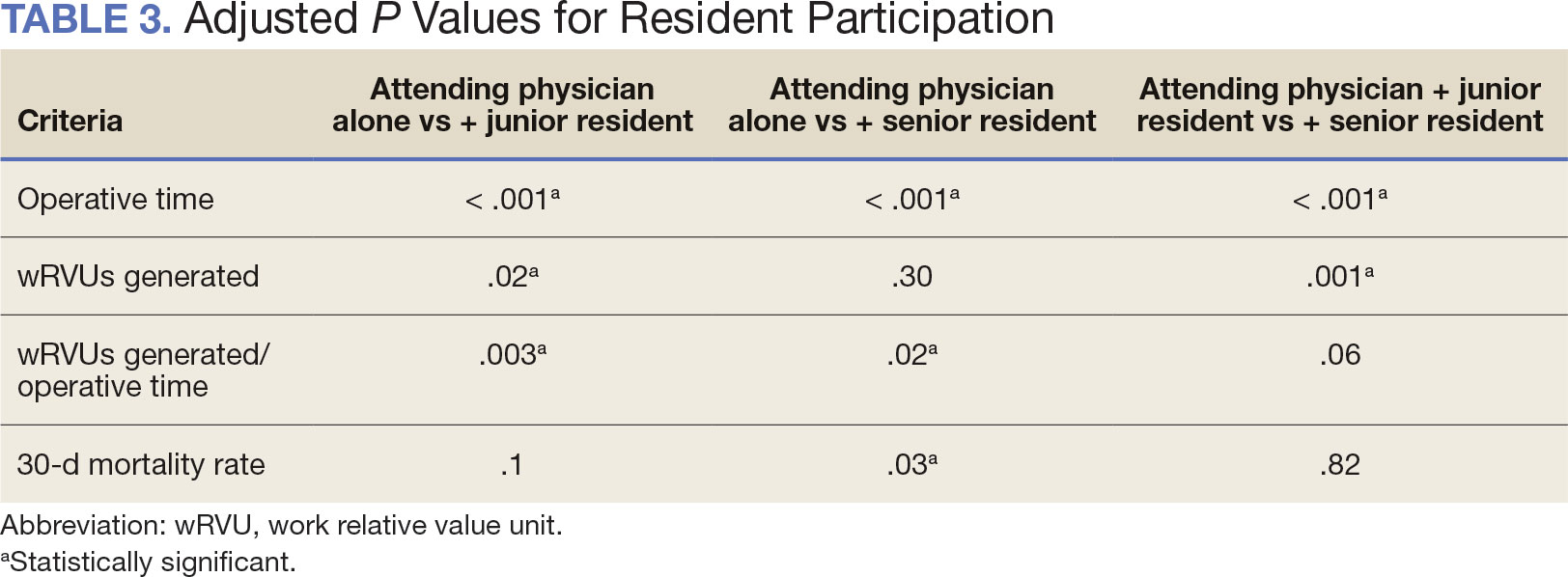
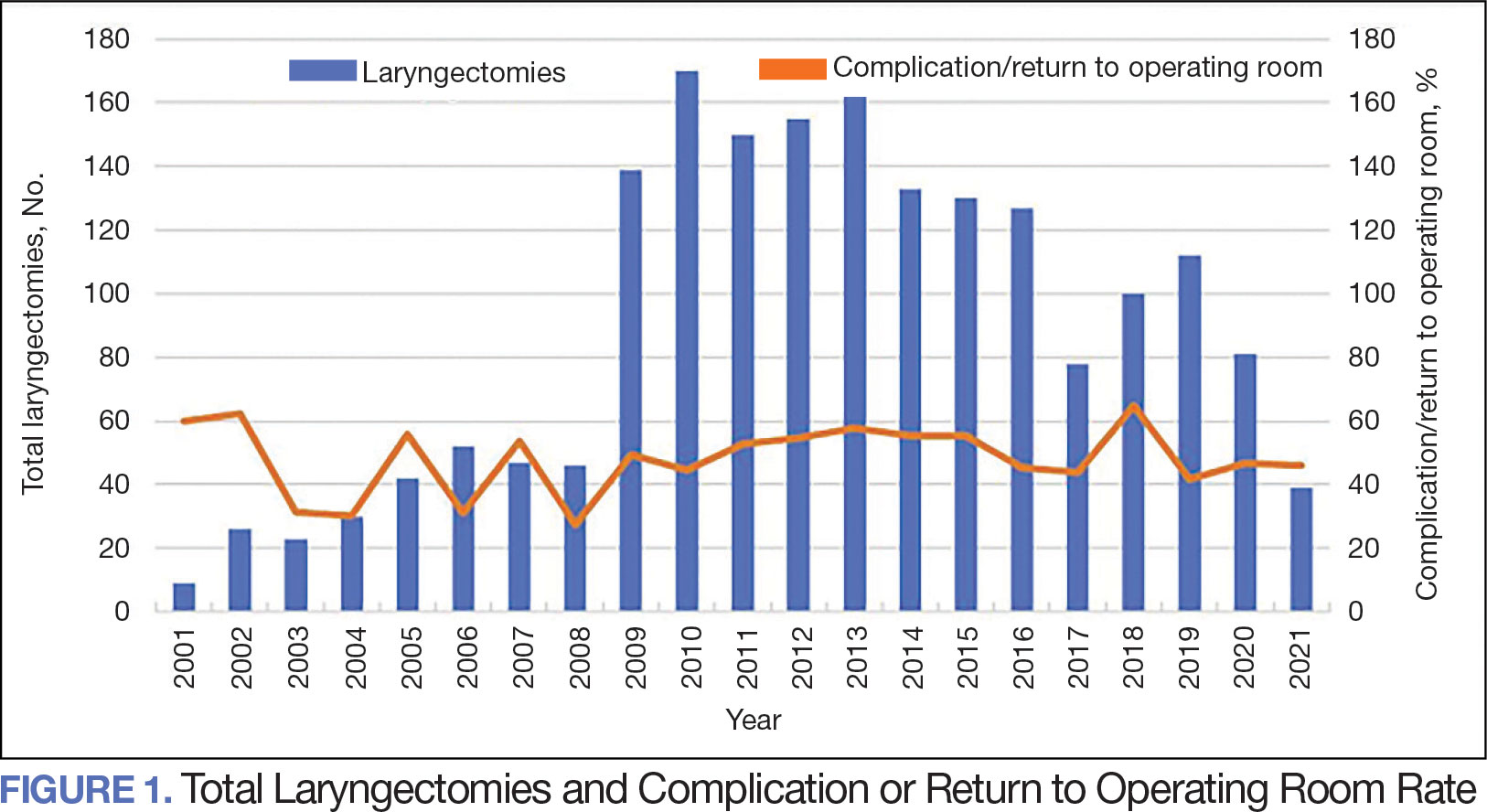
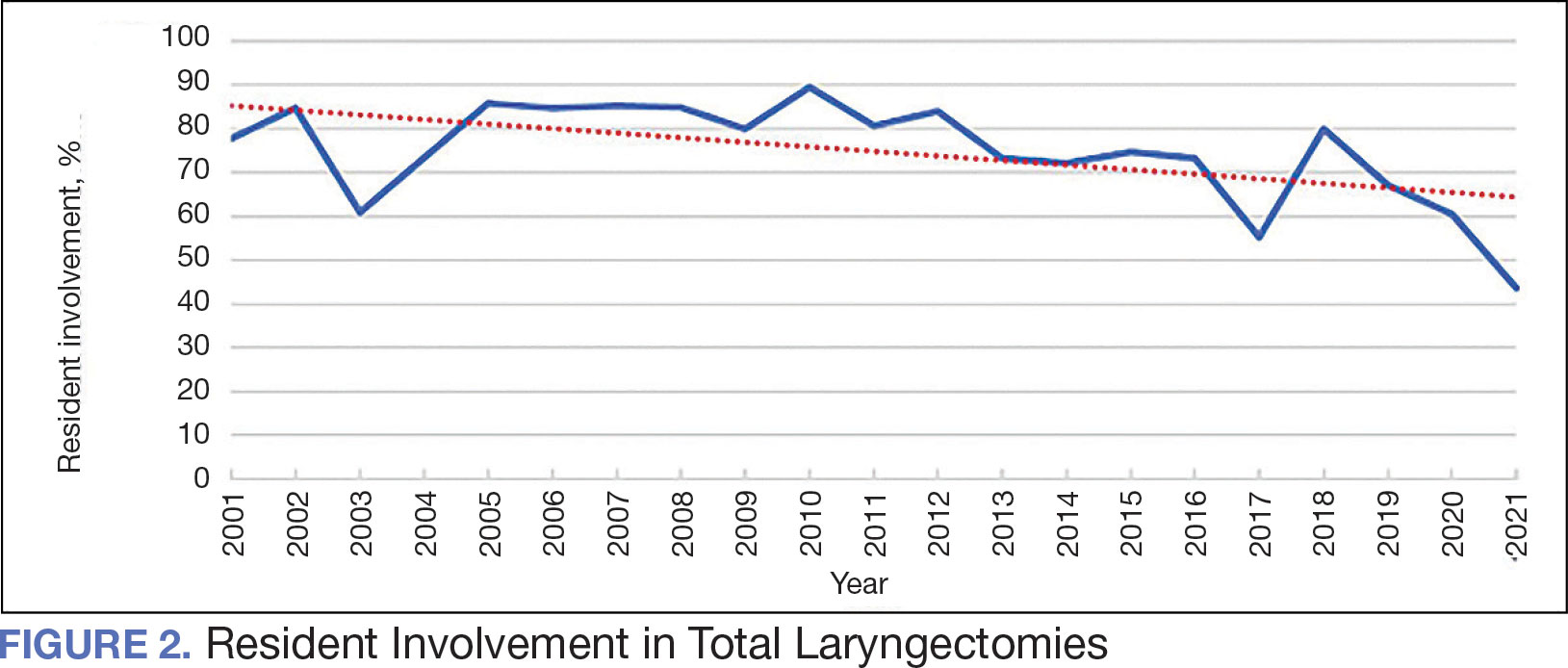
The number of laryngectomies performed in a single fiscal year peaked in 2010 at 170 cases (Figure 1). Between 2001 and 2021, the mean rates of postoperative complications (21.3%) and patient return to the OR (14.6%) did not significantly change. Resident participation in total laryngectomies also peaked in 2010 at 89.0% but has significantly declined, falling to a low of 43.6% in 2021 (Figure 2). From 2001 to 2011, the mean resident participation rate in total laryngectomies was 80.6%, compared with 68.3% from 2012 to 2021 (P < .001).
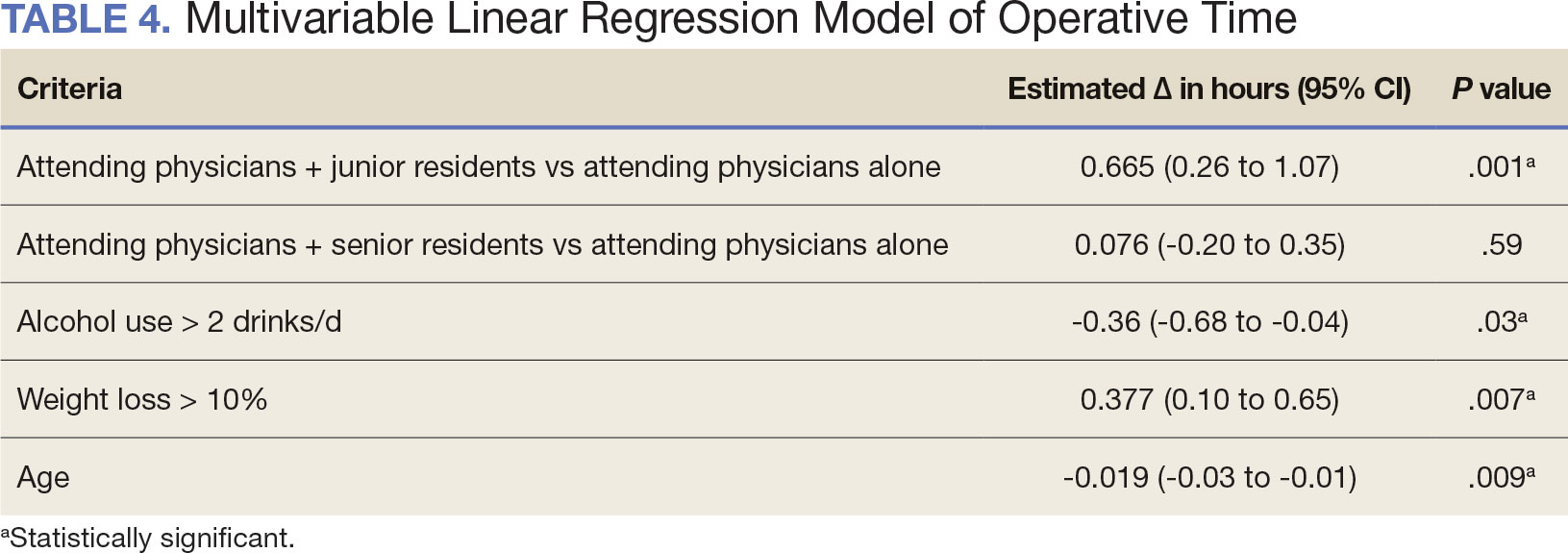
The effect of various demographic and preoperative characteristics on surgical outcomes was also analyzed. A linear regression model accounted for each variable significantly associated with operative time. On multivariable analysis, when all other variables were held constant, Table 4 shows the estimated change in operative time based on certain criteria. For instance, the operative time for attendings with junior residents surgeries was 40 minutes longer (95% CI, 16 to 64) than that of attending alone surgeries (P = .001). Furthermore, operative time decreased by 1.1 minutes (95% CI, 0.30 to 2.04) for each 1-year increase in patient age (P = .009).
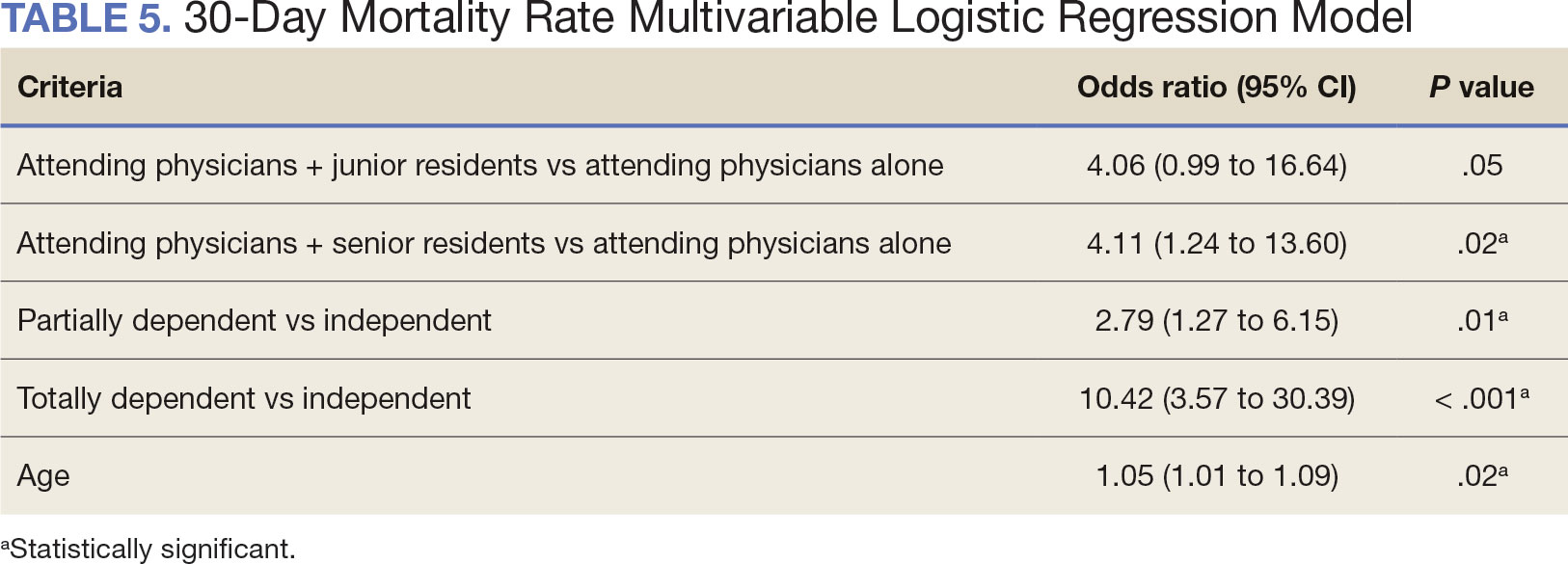
A multivariable logistic regression model evaluated the effect of resident involvement on 30-day mortality rates. Senior resident involvement (P = .02), partially dependent functional status (P = .01), totally dependent functional status (P < .001), and advanced age (P = .02) all were significantly associated with 30-day mortality (Table 5). When other variables remained constant, the odds of death for totally dependent patients were 10.4 times higher than that of patients with independent functional status. Thus, totally dependent functional status appeared to have a greater impact on this outcome than resident participation. The linear regression model for postoperative length of stay demonstrated that senior resident involvement (P = .04), functional status (partially dependent vs independent P < .001), and age (P = .03) were significantly associated with prolonged length of stay.
Discussion
Otolaryngology residency training is designed to educate future otolaryngologists through hands-on learning, adequate feedback, and supervision.1 Although this exposure is paramount for resident education, balancing appropriate supervision and autonomy while mitigating patient risk has been difficult. Numerous non-VA studies have reviewed the impact of resident participation on patient outcomes in various specialties, ranging from a single institution to the National Surgical Quality Improvement Program (NSQIP).4,5,7,22 This study is the first to describe the nationwide impact of resident participation on outcomes in veterans undergoing total laryngectomy.
This study found that resident participation increases operative time and decreases wRVUs generated/operative time without impacting complication rates or patient return to the OR. This reinforces the notion that under close supervision, resident participation does not negatively impact patient outcomes. Resident operative training requires time and dedication by the attending physician and surgical team, thereby increasing operative time. Because VA physician compensation is not linked with productivity as closely as it is in other private and academic settings, surgeons can dedicate more time to operative teaching. This study found that a total laryngectomy involving a junior resident took about 45 minutes longer than an attending physician working alone.
As expected, with longer operative times, the wRVUs generated/operative time ratio was lower in cases with resident participation. Even though resident participation leads to lower OR efficiency, their participation may not significantly impact ancillary costs.23 However, a recent study from NSQIP found an opportunity cost of $60.44 per hour for surgeons operating with a resident in head and neck cases.13
Postoperative complications and mortality are key measures of surgical outcomes in addition to operative time and efficiency. This study found that neither junior nor senior resident participation significantly increased complication rates or patient return to the OR. Despite declining resident involvement and the number of total laryngectomy surgeries in the VA, the complication rate has remained steady. The 30-day mortality rate was significantly higher in cases involving senior residents compared to cases with attending physicians alone. This could be a result of senior resident participation in more challenging cases, such as laryngectomies performed as salvage surgery following radiation. Residents are more often involved in cases with greater complexity at teaching institutions.24-26 Therefore, the higher mortality seen among laryngectomies with senior resident involvement is likely due to the higher complexity of those cases.
The proportion of resident involvement in laryngectomies at VA medical centers has been decreasing over time. Due to the single payer nature of the VA health care system and the number of complex and comorbid patients, the VA offers an invaluable space for resident education in the OR. The fact that less than half of laryngectomies in 2021 involved resident participation is noteworthy for residency training programs. As wRVU compensation models evolve, VA attending surgeons may face less pressure to move the case along, leading to a high potential for operative teaching. Therefore, complex cases, such as laryngectomies, are often ideal for resident participation in the VA.
The steady decline in total laryngectomies at the VA parallels the recent decrease seen in non-VA settings.20 This is due in part to the use of larynx-preserving treatment modalities for laryngeal cancer as well as decreases in the incidence of laryngeal cancer due to population level changes in smoking behaviors. 18,19 Although a laryngectomy is not a key indicator case as determined by the Accreditation Council for Graduate Medical Education, it is important for otolaryngology residents to be exposed to these cases and have a thorough understanding of the operative technique.27 Total laryngectomy was selected for this study because it is a complex and time-consuming surgery with somewhat standardized surgical steps. Unlike microvascular surgery that is very rarely performed by an attending physician alone, laryngectomies can be performed by attending physicians alone or with a resident.28
Limitations
Since this was a retrospective study, it was susceptible to errors in data entry and data extraction from the VASQIP database. Another limitation is the lack of preoperative treatment data on tumor stage and prior nonoperative treatment. For example, a salvage laryngectomy after treatment with radiation and/or chemoradiation is a higher risk procedure than an upfront laryngectomy. Senior resident involvement may be more common in patients undergoing salvage laryngectomy due to the high risk of postoperative fistula and other complications. This may have contributed to the association identified between senior resident participation and 30-day mortality.
Since we could not account for residents who took research years or were fellows, a senior resident may have been mislabeled as a junior resident or vice versa. However, because most research years occur following the third year of residency. We are confident that PGY-1, PGY-2, and PGY-3 is likely to capture junior residents. Other factors, such as coattending surgeon cases, medical student assistance, and fellow involvement may have also impacted the results of this study.
Conclusions
This study is the first to investigate the impact of resident participation on operative time, wRVUs generated, and complication rates in head and neck surgery at VA medical centers. It found that resident participation in total laryngectomies among veterans increased operative time and reduced wRVUs generated per hour but did not impact complication rate or patient return to the OR. The VA offers a unique and invaluable space for resident education and operative training, and the recent decline in resident participation among laryngectomies is important for residency programs to acknowledge and potentially address moving forward.
In contrast to oral cavity resections which can vary from partial glossectomies to composite resections, laryngectomy represents a homogenous procedure from which to draw meaningful conclusions about complication rates, operative time, and outcome. Future directions should include studying other types of head and neck surgery in the VA to determine whether the impact of resident participation mirrors the findings of this study.
- Chung RS. How much time do surgical residents need to learn operative surgery? Am J Surg. 2005;190(3):351-353. doi:10.1016/j.amjsurg.2005.06.035
- S, Darzi A. Defining quality in surgical training: perceptions of the profession. Am J Surg. 2014;207(4):628-636. doi:10.1016/j.amjsurg.2013.07.044
- Bhatti NI, Ahmed A, Choi SS. Identifying quality indicators of surgical of surgical training: a national survey. Laryngoscope. 2015;125(12):2685-2689. doi:10.1002/lary.25262
- Abt NB, Reh DD, Eisele DW, Francis HW, Gourin CG. Does resident participation influence otolaryngology-head and neck surgery morbidity and mortality? Laryngoscope. 2016;126(10):2263-2269. doi:10.1002/lary.25973
- Jubbal KT, Chang D, Izaddoost SA, Pederson W, Zavlin D, Echo A. Resident involvement in microsurgery: an American College of Surgeons national surgical quality improvement program analysis. J Surg Educ. 2017;74(6):1124-1132. doi:10.1016/j.jsurg.2017.05.017
- Kshirsagar RS, Chandy Z, Mahboubi H, Verma SP. Does resident involvement in thyroid surgery lead to increased postoperative complications? Laryngoscope. 2017;127(5):1242-1246. doi:10.1002/lary.26176
- Vieira BL, Hernandez DJ, Qin C, Smith SS, Kim JY, Dutra JC. The impact of resident involvement on otolaryngology surgical outcomes. Laryngoscope. 2016;126(3):602-607. doi:10.1002/lary.25046
- Advani V, Ahad S, Gonczy C, Markwell S, Hassan I. Does resident involvement effect surgical times and complication rates during laparoscopic appendectomy for uncomplicated appendicitis? An analysis of 16,849 cases from the ACS-NSQIP. Am J Surg. 2012;203(3):347-352. doi:10.1016/j.amjsurg.2011.08.015
- Quinn NA, Alt JA, Ashby S, Orlandi RR. Time, resident involvement, and supply drive cost variability in septoplasty with turbinate reduction. Otolaryngol Head Neck Surg. 2018;159(2):310-314. doi:10.1177/0194599818765099
- Leader BA, Wiebracht ND, Meinzen-Derr J, Ishman SL. The impact of resident involvement on tonsillectomy outcomes and surgical time. Laryngoscope. 2020;130(10):2481-2486. doi:10.1002/lary.28427
- Muelleman T, Shew M, Muelleman RJ, et al. Impact of resident participation on operative time and outcomes in otologic surgery. Otolaryngol Head Neck Surg. 2018;158(1):151-154. doi:10.1177/0194599817737270
- Puram SV, Kozin ED, Sethi R, et al. Impact of resident surgeons on procedure length based on common pediatric otolaryngology cases. Laryngoscope. 2015;125(4):991 -997. doi:10.1002/lary.24912
- Chow MS, Gordon AJ, Talwar A, Lydiatt WM, Yueh B, Givi B. The RVU compensation model and head and neck surgical education. Laryngoscope. 2024;134(1):113-119. doi:10.1002/lary.30807
- Papandria D, Rhee D, Ortega G, et al. Assessing trainee impact on operative time for common general surgical procedures in ACS-NSQIP. J Surg Educ. 2012;69(2):149-155. doi:10.1016/j.jsurg.2011.08.003
- Pugely AJ, Gao Y, Martin CT, Callagh JJ, Weinstein SL, Marsh JL. The effect of resident participation on short-term outcomes after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(7):2290-2300. doi:10.1007/s11999-014-3567-0
- Hosler MR, Scott IU, Kunselman AR, Wolford KR, Oltra EZ, Murray WB. Impact of resident participation in cataract surgery on operative time and cost. Ophthalmology. 2012;119(1):95-98. doi:10.1016/j.ophtha.2011.06.026
- Lanigan A, Spaw M, Donaghe C, Brennan J. The impact of the Veteran’s Affairs medical system on an otolaryngology residency training program. Mil Med. 2018;183(11-12):e671-e675. doi:10.1093/milmed/usy041
- American Society of Clinical Oncology, Pfister DG, Laurie SA, et al. American Society of Clinical Oncology clinical practice guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer. J Clin Oncol. 2006;24(22):3693-3704. doi:10.1200/JCO.2006.07.4559
- Forastiere AA, Ismaila N, Lewin JS, et al. Use of larynxpreservation strategies in the treatment of laryngeal cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(11):1143-1169. doi:10.1200/JCO.2017.75.7385
- Verma SP, Mahboubi H. The changing landscape of total laryngectomy surgery. Otolaryngol Head Neck Surg. 2014;150(3):413-418. doi:10.1177/0194599813514515
- Habermann EB, Harris AHS, Giori NJ. Large surgical databases with direct data abstraction: VASQIP and ACSNSQIP. J Bone Joint Surg Am. 2022;104(suppl 3):9-14. doi:10.2106/JBJS.22.00596
- Benito DA, Mamidi I, Pasick LJ, et al. Evaluating resident involvement and the ‘July effect’ in parotidectomy. J Laryngol Otol. 2021;135(5):452-457. doi:10.1017/S0022215121000578
- Hwang CS, Wichterman KA, Alfrey EJ. The cost of resident education. J Surg Res. 2010;163(1):18-23. doi:10.1016/j.jss.2010.03.013
- Saliba AN, Taher AT, Tamim H, et al. Impact of resident involvement in surgery (IRIS-NSQIP): looking at the bigger picture based on the American College of Surgeons- NSQIP database. J Am Coll Surg. 2016; 222(1):30-40. doi:10.1016/j.jamcollsurg.2015.10.011
- Khuri SF, Najjar SF, Daley J, et al. Comparison of surgical outcomes between teaching and nonteaching hospitals in the Department of Veterans Affairs. Ann Surg. 2001;234(3):370-383. doi:10.1097/00000658-200109000-00011
- Relles DM, Burkhart RA, Pucci MJ et al. Does resident experience affect outcomes in complex abdominal surgery? Pancreaticoduodenectomy as an example. J Gastrointest Surg. 2014;18(2):279-285. doi:10.1007/s11605-013-2372-5
- Accreditation Council for Graduate Medical Education. Required minimum number of key indicator procedures for graduating residents. June 2019. Accessed January 2, 2025. https://www.acgme.org/globalassets/pfassets/programresources/280_core_case_log_minimums.pdf
- Brady JS, Crippen MM, Filimonov A, et al. The effect of training level on complications after free flap surgery of the head and neck. Am J Otolaryngol. 2017;38(5):560-564. doi:10.1016/j.amjoto.2017.06.001
The US Department of Veterans Affairs (VA) has been integral in resident training. Resident surgical training requires a balance of supervision and autonomy, along with procedure repetition and appropriate feedback.1-3 Non-VA research has found that resident participation across various otolaryngology procedures, including thyroidectomy, neck dissection, and laryngectomy, does not increase patient morbidity.4-7 However, resident involvement in private and academic settings that included nonhead and neck procedures was linked to increased operative time and reduced productivity, as determined by work relative value units (wRVUs).7-13 This has also been identified in other specialties, including general surgery, orthopedics, and ophthalmology.14-16
Unlike the private sector, surgeon compensation at the VA is not as closely linked to operative productivity, offering a unique setting for resident training. While VA integration in otolaryngology residency programs increases resident case numbers, particularly in head and neck cases, the impact on VA patient outcomes and productivity is unknown.17 The use of larynxpreserving treatment modalities for laryngeal cancer has led to a decline in the number of total laryngectomies performed, which could potentially impact resident operative training for laryngectomies.18-20
This study sought to determine the impact of resident participation on operative time, wRVUs, and patient outcomes in veterans who underwent a total laryngectomy. This study was reviewed and approved by the MedStar Georgetown University Hospital Institutional Review Board and Research and Development Committee (#1595672).
Methods
A retrospective cohort of veterans nationwide who underwent total laryngectomy between 2001 and 2021, with or without neck dissection, was identified from the Veterans Affairs Surgical Quality Improvement Program (VASQIP). Data were extracted via the VA Informatics and Computing Infrastructure and patients were included based on Current Procedural Terminology codes for total laryngectomy, with or without neck dissection (31320, 31360, 31365). Laryngopharyngectomies, partial laryngectomies, and minimally invasive laryngectomies were excluded. VASQIP nurse data managers reviewed patient data for operative data, postoperative outcomes (including 30- day morbidity and mortality), and preoperative risk factors (Appendix).21
The VASQIP data provide the highest resident or postgraduate year (PGY) per surgery. PGY 1, 2, and 3 were considered junior residents and PGY ≥4, surgical fellows, and individuals who took research years during residency were considered senior residents. Cases performed by attending physicians alone were compared with those involving junior or senior residents.
Patient demographic data included age, body mass index, smoking and alcohol use, weight loss, and functional status. Consumption of any tobacco products within 12 months of surgery was considered tobacco use. Drinking on average ≥2 alcoholic beverages daily was considered alcohol use. Weight loss was defined as a 10% reduction in body weight within the 6 months before surgery, excluding patients enrolled in a weight loss program. Functional status was categorized as independent, partially dependent, totally dependent, and unknown.
Primary outcomes included operative time, wRVUs generated, and wRVUs generated per hour of operative time. Postoperative complications were recorded both as a continuous variable and as a binary variable for presence or absence of a complication. Additional outcome variables included length of postoperative hospital stay, return to the operating room (OR), and death within 30 days of surgery.
Statistical Analysis
Data were summarized using frequency and percentage for categorical variables and median with IQR for continuous variables. Data were also summarized based on resident involvement in the surgery and the PGY level of the residents involved. The occurrence of total laryngectomy, rate of complications, and patient return to the OR were summarized by year.
Univariate associations between resident involvement and surgical outcomes were analyzed using the Kruskal-Wallis test for continuous variables and the ÷2 test for categorical variables. A Fisher exact test was used when the cell count in the contingency table was < 5. The univariate associations between surgical outcomes and demographic/preoperative variables were examined using 2-sided Wilcoxon ranksum tests or Kruskal-Wallis tests between continuous variables and categorical variables, X2 or Fisher exact test between 2 categorical variables, and 2-sided Spearman correlation test between 2 continuous variables. A false-discovery rate approach was used for simultaneous posthoc tests to determine the adjusted P values for wRVUs generated/operative time for attending physicians alone vs with junior residents and for attending physicians alone vs with senior residents. Models were used to evaluate the effects of resident involvement on surgical outcomes, adjusting for variables that showed significant univariate associations. Linear regression models were used for operative time, wRVUs generated, wRVUs generated/operative time, and length of postoperative stay. A logistic regression model was used for death within 30 days. Models were not built for postoperative complications or patient return to the OR, as these were only statistically significantly associated with the patient’s preoperative functional status. A finding was considered significant if P < .05. All analyses were performed using statistical software RStudio Version 2023.03.0.
Results
Between 2001 and 2021, 1857 patients who underwent total laryngectomy were identified from the VASQIP database nationwide. Most of the total laryngectomies were staffed by an attending physician with a senior resident (n = 1190, 64%), 446 (24%) were conducted by the attending physician alone, and 221 (12%) by an attending physician with a junior resident (Table 1). The mean operating time for an attending physician alone was 378 minutes, 384 minutes for an attending physician with a senior resident, and 432 minutes for an attending physician with a junior resident (Table 2). There was a statistically significant increase in operating time for laryngectomies with resident participation compared to attending physicians operating alone (P < .001).


When the wRVUs generated/operative time was analyzed, there was a statistically significant difference between comparison groups. Total laryngectomies performed by attending physicians alone had the highest wRVUs generated/operative time (5.5), followed by laryngectomies performed by attending physicians with senior residents and laryngectomies performed by attending physicians with junior residents (5.2 and 4.8, respectively; P = .002). Table 3 describes adjusted P values for wRVUs generated/ operative time for total laryngectomies performed by attending physicians alone vs with junior residents (P = .003) and for attending physicians alone vs with senior residents (P = .02). Resident participation in total laryngectomies did not significantly impact the development or number of postoperative complications or the rate of return to the OR.

The number of laryngectomies performed in a single fiscal year peaked in 2010 at 170 cases (Figure 1). Between 2001 and 2021, the mean rates of postoperative complications (21.3%) and patient return to the OR (14.6%) did not significantly change. Resident participation in total laryngectomies also peaked in 2010 at 89.0% but has significantly declined, falling to a low of 43.6% in 2021 (Figure 2). From 2001 to 2011, the mean resident participation rate in total laryngectomies was 80.6%, compared with 68.3% from 2012 to 2021 (P < .001).


The effect of various demographic and preoperative characteristics on surgical outcomes was also analyzed. A linear regression model accounted for each variable significantly associated with operative time. On multivariable analysis, when all other variables were held constant, Table 4 shows the estimated change in operative time based on certain criteria. For instance, the operative time for attendings with junior residents surgeries was 40 minutes longer (95% CI, 16 to 64) than that of attending alone surgeries (P = .001). Furthermore, operative time decreased by 1.1 minutes (95% CI, 0.30 to 2.04) for each 1-year increase in patient age (P = .009).

A multivariable logistic regression model evaluated the effect of resident involvement on 30-day mortality rates. Senior resident involvement (P = .02), partially dependent functional status (P = .01), totally dependent functional status (P < .001), and advanced age (P = .02) all were significantly associated with 30-day mortality (Table 5). When other variables remained constant, the odds of death for totally dependent patients were 10.4 times higher than that of patients with independent functional status. Thus, totally dependent functional status appeared to have a greater impact on this outcome than resident participation. The linear regression model for postoperative length of stay demonstrated that senior resident involvement (P = .04), functional status (partially dependent vs independent P < .001), and age (P = .03) were significantly associated with prolonged length of stay.

Discussion
Otolaryngology residency training is designed to educate future otolaryngologists through hands-on learning, adequate feedback, and supervision.1 Although this exposure is paramount for resident education, balancing appropriate supervision and autonomy while mitigating patient risk has been difficult. Numerous non-VA studies have reviewed the impact of resident participation on patient outcomes in various specialties, ranging from a single institution to the National Surgical Quality Improvement Program (NSQIP).4,5,7,22 This study is the first to describe the nationwide impact of resident participation on outcomes in veterans undergoing total laryngectomy.
This study found that resident participation increases operative time and decreases wRVUs generated/operative time without impacting complication rates or patient return to the OR. This reinforces the notion that under close supervision, resident participation does not negatively impact patient outcomes. Resident operative training requires time and dedication by the attending physician and surgical team, thereby increasing operative time. Because VA physician compensation is not linked with productivity as closely as it is in other private and academic settings, surgeons can dedicate more time to operative teaching. This study found that a total laryngectomy involving a junior resident took about 45 minutes longer than an attending physician working alone.
As expected, with longer operative times, the wRVUs generated/operative time ratio was lower in cases with resident participation. Even though resident participation leads to lower OR efficiency, their participation may not significantly impact ancillary costs.23 However, a recent study from NSQIP found an opportunity cost of $60.44 per hour for surgeons operating with a resident in head and neck cases.13
Postoperative complications and mortality are key measures of surgical outcomes in addition to operative time and efficiency. This study found that neither junior nor senior resident participation significantly increased complication rates or patient return to the OR. Despite declining resident involvement and the number of total laryngectomy surgeries in the VA, the complication rate has remained steady. The 30-day mortality rate was significantly higher in cases involving senior residents compared to cases with attending physicians alone. This could be a result of senior resident participation in more challenging cases, such as laryngectomies performed as salvage surgery following radiation. Residents are more often involved in cases with greater complexity at teaching institutions.24-26 Therefore, the higher mortality seen among laryngectomies with senior resident involvement is likely due to the higher complexity of those cases.
The proportion of resident involvement in laryngectomies at VA medical centers has been decreasing over time. Due to the single payer nature of the VA health care system and the number of complex and comorbid patients, the VA offers an invaluable space for resident education in the OR. The fact that less than half of laryngectomies in 2021 involved resident participation is noteworthy for residency training programs. As wRVU compensation models evolve, VA attending surgeons may face less pressure to move the case along, leading to a high potential for operative teaching. Therefore, complex cases, such as laryngectomies, are often ideal for resident participation in the VA.
The steady decline in total laryngectomies at the VA parallels the recent decrease seen in non-VA settings.20 This is due in part to the use of larynx-preserving treatment modalities for laryngeal cancer as well as decreases in the incidence of laryngeal cancer due to population level changes in smoking behaviors. 18,19 Although a laryngectomy is not a key indicator case as determined by the Accreditation Council for Graduate Medical Education, it is important for otolaryngology residents to be exposed to these cases and have a thorough understanding of the operative technique.27 Total laryngectomy was selected for this study because it is a complex and time-consuming surgery with somewhat standardized surgical steps. Unlike microvascular surgery that is very rarely performed by an attending physician alone, laryngectomies can be performed by attending physicians alone or with a resident.28
Limitations
Since this was a retrospective study, it was susceptible to errors in data entry and data extraction from the VASQIP database. Another limitation is the lack of preoperative treatment data on tumor stage and prior nonoperative treatment. For example, a salvage laryngectomy after treatment with radiation and/or chemoradiation is a higher risk procedure than an upfront laryngectomy. Senior resident involvement may be more common in patients undergoing salvage laryngectomy due to the high risk of postoperative fistula and other complications. This may have contributed to the association identified between senior resident participation and 30-day mortality.
Since we could not account for residents who took research years or were fellows, a senior resident may have been mislabeled as a junior resident or vice versa. However, because most research years occur following the third year of residency. We are confident that PGY-1, PGY-2, and PGY-3 is likely to capture junior residents. Other factors, such as coattending surgeon cases, medical student assistance, and fellow involvement may have also impacted the results of this study.
Conclusions
This study is the first to investigate the impact of resident participation on operative time, wRVUs generated, and complication rates in head and neck surgery at VA medical centers. It found that resident participation in total laryngectomies among veterans increased operative time and reduced wRVUs generated per hour but did not impact complication rate or patient return to the OR. The VA offers a unique and invaluable space for resident education and operative training, and the recent decline in resident participation among laryngectomies is important for residency programs to acknowledge and potentially address moving forward.
In contrast to oral cavity resections which can vary from partial glossectomies to composite resections, laryngectomy represents a homogenous procedure from which to draw meaningful conclusions about complication rates, operative time, and outcome. Future directions should include studying other types of head and neck surgery in the VA to determine whether the impact of resident participation mirrors the findings of this study.
The US Department of Veterans Affairs (VA) has been integral in resident training. Resident surgical training requires a balance of supervision and autonomy, along with procedure repetition and appropriate feedback.1-3 Non-VA research has found that resident participation across various otolaryngology procedures, including thyroidectomy, neck dissection, and laryngectomy, does not increase patient morbidity.4-7 However, resident involvement in private and academic settings that included nonhead and neck procedures was linked to increased operative time and reduced productivity, as determined by work relative value units (wRVUs).7-13 This has also been identified in other specialties, including general surgery, orthopedics, and ophthalmology.14-16
Unlike the private sector, surgeon compensation at the VA is not as closely linked to operative productivity, offering a unique setting for resident training. While VA integration in otolaryngology residency programs increases resident case numbers, particularly in head and neck cases, the impact on VA patient outcomes and productivity is unknown.17 The use of larynxpreserving treatment modalities for laryngeal cancer has led to a decline in the number of total laryngectomies performed, which could potentially impact resident operative training for laryngectomies.18-20
This study sought to determine the impact of resident participation on operative time, wRVUs, and patient outcomes in veterans who underwent a total laryngectomy. This study was reviewed and approved by the MedStar Georgetown University Hospital Institutional Review Board and Research and Development Committee (#1595672).
Methods
A retrospective cohort of veterans nationwide who underwent total laryngectomy between 2001 and 2021, with or without neck dissection, was identified from the Veterans Affairs Surgical Quality Improvement Program (VASQIP). Data were extracted via the VA Informatics and Computing Infrastructure and patients were included based on Current Procedural Terminology codes for total laryngectomy, with or without neck dissection (31320, 31360, 31365). Laryngopharyngectomies, partial laryngectomies, and minimally invasive laryngectomies were excluded. VASQIP nurse data managers reviewed patient data for operative data, postoperative outcomes (including 30- day morbidity and mortality), and preoperative risk factors (Appendix).21
The VASQIP data provide the highest resident or postgraduate year (PGY) per surgery. PGY 1, 2, and 3 were considered junior residents and PGY ≥4, surgical fellows, and individuals who took research years during residency were considered senior residents. Cases performed by attending physicians alone were compared with those involving junior or senior residents.
Patient demographic data included age, body mass index, smoking and alcohol use, weight loss, and functional status. Consumption of any tobacco products within 12 months of surgery was considered tobacco use. Drinking on average ≥2 alcoholic beverages daily was considered alcohol use. Weight loss was defined as a 10% reduction in body weight within the 6 months before surgery, excluding patients enrolled in a weight loss program. Functional status was categorized as independent, partially dependent, totally dependent, and unknown.
Primary outcomes included operative time, wRVUs generated, and wRVUs generated per hour of operative time. Postoperative complications were recorded both as a continuous variable and as a binary variable for presence or absence of a complication. Additional outcome variables included length of postoperative hospital stay, return to the operating room (OR), and death within 30 days of surgery.
Statistical Analysis
Data were summarized using frequency and percentage for categorical variables and median with IQR for continuous variables. Data were also summarized based on resident involvement in the surgery and the PGY level of the residents involved. The occurrence of total laryngectomy, rate of complications, and patient return to the OR were summarized by year.
Univariate associations between resident involvement and surgical outcomes were analyzed using the Kruskal-Wallis test for continuous variables and the ÷2 test for categorical variables. A Fisher exact test was used when the cell count in the contingency table was < 5. The univariate associations between surgical outcomes and demographic/preoperative variables were examined using 2-sided Wilcoxon ranksum tests or Kruskal-Wallis tests between continuous variables and categorical variables, X2 or Fisher exact test between 2 categorical variables, and 2-sided Spearman correlation test between 2 continuous variables. A false-discovery rate approach was used for simultaneous posthoc tests to determine the adjusted P values for wRVUs generated/operative time for attending physicians alone vs with junior residents and for attending physicians alone vs with senior residents. Models were used to evaluate the effects of resident involvement on surgical outcomes, adjusting for variables that showed significant univariate associations. Linear regression models were used for operative time, wRVUs generated, wRVUs generated/operative time, and length of postoperative stay. A logistic regression model was used for death within 30 days. Models were not built for postoperative complications or patient return to the OR, as these were only statistically significantly associated with the patient’s preoperative functional status. A finding was considered significant if P < .05. All analyses were performed using statistical software RStudio Version 2023.03.0.
Results
Between 2001 and 2021, 1857 patients who underwent total laryngectomy were identified from the VASQIP database nationwide. Most of the total laryngectomies were staffed by an attending physician with a senior resident (n = 1190, 64%), 446 (24%) were conducted by the attending physician alone, and 221 (12%) by an attending physician with a junior resident (Table 1). The mean operating time for an attending physician alone was 378 minutes, 384 minutes for an attending physician with a senior resident, and 432 minutes for an attending physician with a junior resident (Table 2). There was a statistically significant increase in operating time for laryngectomies with resident participation compared to attending physicians operating alone (P < .001).


When the wRVUs generated/operative time was analyzed, there was a statistically significant difference between comparison groups. Total laryngectomies performed by attending physicians alone had the highest wRVUs generated/operative time (5.5), followed by laryngectomies performed by attending physicians with senior residents and laryngectomies performed by attending physicians with junior residents (5.2 and 4.8, respectively; P = .002). Table 3 describes adjusted P values for wRVUs generated/ operative time for total laryngectomies performed by attending physicians alone vs with junior residents (P = .003) and for attending physicians alone vs with senior residents (P = .02). Resident participation in total laryngectomies did not significantly impact the development or number of postoperative complications or the rate of return to the OR.

The number of laryngectomies performed in a single fiscal year peaked in 2010 at 170 cases (Figure 1). Between 2001 and 2021, the mean rates of postoperative complications (21.3%) and patient return to the OR (14.6%) did not significantly change. Resident participation in total laryngectomies also peaked in 2010 at 89.0% but has significantly declined, falling to a low of 43.6% in 2021 (Figure 2). From 2001 to 2011, the mean resident participation rate in total laryngectomies was 80.6%, compared with 68.3% from 2012 to 2021 (P < .001).


The effect of various demographic and preoperative characteristics on surgical outcomes was also analyzed. A linear regression model accounted for each variable significantly associated with operative time. On multivariable analysis, when all other variables were held constant, Table 4 shows the estimated change in operative time based on certain criteria. For instance, the operative time for attendings with junior residents surgeries was 40 minutes longer (95% CI, 16 to 64) than that of attending alone surgeries (P = .001). Furthermore, operative time decreased by 1.1 minutes (95% CI, 0.30 to 2.04) for each 1-year increase in patient age (P = .009).

A multivariable logistic regression model evaluated the effect of resident involvement on 30-day mortality rates. Senior resident involvement (P = .02), partially dependent functional status (P = .01), totally dependent functional status (P < .001), and advanced age (P = .02) all were significantly associated with 30-day mortality (Table 5). When other variables remained constant, the odds of death for totally dependent patients were 10.4 times higher than that of patients with independent functional status. Thus, totally dependent functional status appeared to have a greater impact on this outcome than resident participation. The linear regression model for postoperative length of stay demonstrated that senior resident involvement (P = .04), functional status (partially dependent vs independent P < .001), and age (P = .03) were significantly associated with prolonged length of stay.

Discussion
Otolaryngology residency training is designed to educate future otolaryngologists through hands-on learning, adequate feedback, and supervision.1 Although this exposure is paramount for resident education, balancing appropriate supervision and autonomy while mitigating patient risk has been difficult. Numerous non-VA studies have reviewed the impact of resident participation on patient outcomes in various specialties, ranging from a single institution to the National Surgical Quality Improvement Program (NSQIP).4,5,7,22 This study is the first to describe the nationwide impact of resident participation on outcomes in veterans undergoing total laryngectomy.
This study found that resident participation increases operative time and decreases wRVUs generated/operative time without impacting complication rates or patient return to the OR. This reinforces the notion that under close supervision, resident participation does not negatively impact patient outcomes. Resident operative training requires time and dedication by the attending physician and surgical team, thereby increasing operative time. Because VA physician compensation is not linked with productivity as closely as it is in other private and academic settings, surgeons can dedicate more time to operative teaching. This study found that a total laryngectomy involving a junior resident took about 45 minutes longer than an attending physician working alone.
As expected, with longer operative times, the wRVUs generated/operative time ratio was lower in cases with resident participation. Even though resident participation leads to lower OR efficiency, their participation may not significantly impact ancillary costs.23 However, a recent study from NSQIP found an opportunity cost of $60.44 per hour for surgeons operating with a resident in head and neck cases.13
Postoperative complications and mortality are key measures of surgical outcomes in addition to operative time and efficiency. This study found that neither junior nor senior resident participation significantly increased complication rates or patient return to the OR. Despite declining resident involvement and the number of total laryngectomy surgeries in the VA, the complication rate has remained steady. The 30-day mortality rate was significantly higher in cases involving senior residents compared to cases with attending physicians alone. This could be a result of senior resident participation in more challenging cases, such as laryngectomies performed as salvage surgery following radiation. Residents are more often involved in cases with greater complexity at teaching institutions.24-26 Therefore, the higher mortality seen among laryngectomies with senior resident involvement is likely due to the higher complexity of those cases.
The proportion of resident involvement in laryngectomies at VA medical centers has been decreasing over time. Due to the single payer nature of the VA health care system and the number of complex and comorbid patients, the VA offers an invaluable space for resident education in the OR. The fact that less than half of laryngectomies in 2021 involved resident participation is noteworthy for residency training programs. As wRVU compensation models evolve, VA attending surgeons may face less pressure to move the case along, leading to a high potential for operative teaching. Therefore, complex cases, such as laryngectomies, are often ideal for resident participation in the VA.
The steady decline in total laryngectomies at the VA parallels the recent decrease seen in non-VA settings.20 This is due in part to the use of larynx-preserving treatment modalities for laryngeal cancer as well as decreases in the incidence of laryngeal cancer due to population level changes in smoking behaviors. 18,19 Although a laryngectomy is not a key indicator case as determined by the Accreditation Council for Graduate Medical Education, it is important for otolaryngology residents to be exposed to these cases and have a thorough understanding of the operative technique.27 Total laryngectomy was selected for this study because it is a complex and time-consuming surgery with somewhat standardized surgical steps. Unlike microvascular surgery that is very rarely performed by an attending physician alone, laryngectomies can be performed by attending physicians alone or with a resident.28
Limitations
Since this was a retrospective study, it was susceptible to errors in data entry and data extraction from the VASQIP database. Another limitation is the lack of preoperative treatment data on tumor stage and prior nonoperative treatment. For example, a salvage laryngectomy after treatment with radiation and/or chemoradiation is a higher risk procedure than an upfront laryngectomy. Senior resident involvement may be more common in patients undergoing salvage laryngectomy due to the high risk of postoperative fistula and other complications. This may have contributed to the association identified between senior resident participation and 30-day mortality.
Since we could not account for residents who took research years or were fellows, a senior resident may have been mislabeled as a junior resident or vice versa. However, because most research years occur following the third year of residency. We are confident that PGY-1, PGY-2, and PGY-3 is likely to capture junior residents. Other factors, such as coattending surgeon cases, medical student assistance, and fellow involvement may have also impacted the results of this study.
Conclusions
This study is the first to investigate the impact of resident participation on operative time, wRVUs generated, and complication rates in head and neck surgery at VA medical centers. It found that resident participation in total laryngectomies among veterans increased operative time and reduced wRVUs generated per hour but did not impact complication rate or patient return to the OR. The VA offers a unique and invaluable space for resident education and operative training, and the recent decline in resident participation among laryngectomies is important for residency programs to acknowledge and potentially address moving forward.
In contrast to oral cavity resections which can vary from partial glossectomies to composite resections, laryngectomy represents a homogenous procedure from which to draw meaningful conclusions about complication rates, operative time, and outcome. Future directions should include studying other types of head and neck surgery in the VA to determine whether the impact of resident participation mirrors the findings of this study.
- Chung RS. How much time do surgical residents need to learn operative surgery? Am J Surg. 2005;190(3):351-353. doi:10.1016/j.amjsurg.2005.06.035
- S, Darzi A. Defining quality in surgical training: perceptions of the profession. Am J Surg. 2014;207(4):628-636. doi:10.1016/j.amjsurg.2013.07.044
- Bhatti NI, Ahmed A, Choi SS. Identifying quality indicators of surgical of surgical training: a national survey. Laryngoscope. 2015;125(12):2685-2689. doi:10.1002/lary.25262
- Abt NB, Reh DD, Eisele DW, Francis HW, Gourin CG. Does resident participation influence otolaryngology-head and neck surgery morbidity and mortality? Laryngoscope. 2016;126(10):2263-2269. doi:10.1002/lary.25973
- Jubbal KT, Chang D, Izaddoost SA, Pederson W, Zavlin D, Echo A. Resident involvement in microsurgery: an American College of Surgeons national surgical quality improvement program analysis. J Surg Educ. 2017;74(6):1124-1132. doi:10.1016/j.jsurg.2017.05.017
- Kshirsagar RS, Chandy Z, Mahboubi H, Verma SP. Does resident involvement in thyroid surgery lead to increased postoperative complications? Laryngoscope. 2017;127(5):1242-1246. doi:10.1002/lary.26176
- Vieira BL, Hernandez DJ, Qin C, Smith SS, Kim JY, Dutra JC. The impact of resident involvement on otolaryngology surgical outcomes. Laryngoscope. 2016;126(3):602-607. doi:10.1002/lary.25046
- Advani V, Ahad S, Gonczy C, Markwell S, Hassan I. Does resident involvement effect surgical times and complication rates during laparoscopic appendectomy for uncomplicated appendicitis? An analysis of 16,849 cases from the ACS-NSQIP. Am J Surg. 2012;203(3):347-352. doi:10.1016/j.amjsurg.2011.08.015
- Quinn NA, Alt JA, Ashby S, Orlandi RR. Time, resident involvement, and supply drive cost variability in septoplasty with turbinate reduction. Otolaryngol Head Neck Surg. 2018;159(2):310-314. doi:10.1177/0194599818765099
- Leader BA, Wiebracht ND, Meinzen-Derr J, Ishman SL. The impact of resident involvement on tonsillectomy outcomes and surgical time. Laryngoscope. 2020;130(10):2481-2486. doi:10.1002/lary.28427
- Muelleman T, Shew M, Muelleman RJ, et al. Impact of resident participation on operative time and outcomes in otologic surgery. Otolaryngol Head Neck Surg. 2018;158(1):151-154. doi:10.1177/0194599817737270
- Puram SV, Kozin ED, Sethi R, et al. Impact of resident surgeons on procedure length based on common pediatric otolaryngology cases. Laryngoscope. 2015;125(4):991 -997. doi:10.1002/lary.24912
- Chow MS, Gordon AJ, Talwar A, Lydiatt WM, Yueh B, Givi B. The RVU compensation model and head and neck surgical education. Laryngoscope. 2024;134(1):113-119. doi:10.1002/lary.30807
- Papandria D, Rhee D, Ortega G, et al. Assessing trainee impact on operative time for common general surgical procedures in ACS-NSQIP. J Surg Educ. 2012;69(2):149-155. doi:10.1016/j.jsurg.2011.08.003
- Pugely AJ, Gao Y, Martin CT, Callagh JJ, Weinstein SL, Marsh JL. The effect of resident participation on short-term outcomes after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(7):2290-2300. doi:10.1007/s11999-014-3567-0
- Hosler MR, Scott IU, Kunselman AR, Wolford KR, Oltra EZ, Murray WB. Impact of resident participation in cataract surgery on operative time and cost. Ophthalmology. 2012;119(1):95-98. doi:10.1016/j.ophtha.2011.06.026
- Lanigan A, Spaw M, Donaghe C, Brennan J. The impact of the Veteran’s Affairs medical system on an otolaryngology residency training program. Mil Med. 2018;183(11-12):e671-e675. doi:10.1093/milmed/usy041
- American Society of Clinical Oncology, Pfister DG, Laurie SA, et al. American Society of Clinical Oncology clinical practice guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer. J Clin Oncol. 2006;24(22):3693-3704. doi:10.1200/JCO.2006.07.4559
- Forastiere AA, Ismaila N, Lewin JS, et al. Use of larynxpreservation strategies in the treatment of laryngeal cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(11):1143-1169. doi:10.1200/JCO.2017.75.7385
- Verma SP, Mahboubi H. The changing landscape of total laryngectomy surgery. Otolaryngol Head Neck Surg. 2014;150(3):413-418. doi:10.1177/0194599813514515
- Habermann EB, Harris AHS, Giori NJ. Large surgical databases with direct data abstraction: VASQIP and ACSNSQIP. J Bone Joint Surg Am. 2022;104(suppl 3):9-14. doi:10.2106/JBJS.22.00596
- Benito DA, Mamidi I, Pasick LJ, et al. Evaluating resident involvement and the ‘July effect’ in parotidectomy. J Laryngol Otol. 2021;135(5):452-457. doi:10.1017/S0022215121000578
- Hwang CS, Wichterman KA, Alfrey EJ. The cost of resident education. J Surg Res. 2010;163(1):18-23. doi:10.1016/j.jss.2010.03.013
- Saliba AN, Taher AT, Tamim H, et al. Impact of resident involvement in surgery (IRIS-NSQIP): looking at the bigger picture based on the American College of Surgeons- NSQIP database. J Am Coll Surg. 2016; 222(1):30-40. doi:10.1016/j.jamcollsurg.2015.10.011
- Khuri SF, Najjar SF, Daley J, et al. Comparison of surgical outcomes between teaching and nonteaching hospitals in the Department of Veterans Affairs. Ann Surg. 2001;234(3):370-383. doi:10.1097/00000658-200109000-00011
- Relles DM, Burkhart RA, Pucci MJ et al. Does resident experience affect outcomes in complex abdominal surgery? Pancreaticoduodenectomy as an example. J Gastrointest Surg. 2014;18(2):279-285. doi:10.1007/s11605-013-2372-5
- Accreditation Council for Graduate Medical Education. Required minimum number of key indicator procedures for graduating residents. June 2019. Accessed January 2, 2025. https://www.acgme.org/globalassets/pfassets/programresources/280_core_case_log_minimums.pdf
- Brady JS, Crippen MM, Filimonov A, et al. The effect of training level on complications after free flap surgery of the head and neck. Am J Otolaryngol. 2017;38(5):560-564. doi:10.1016/j.amjoto.2017.06.001
- Chung RS. How much time do surgical residents need to learn operative surgery? Am J Surg. 2005;190(3):351-353. doi:10.1016/j.amjsurg.2005.06.035
- S, Darzi A. Defining quality in surgical training: perceptions of the profession. Am J Surg. 2014;207(4):628-636. doi:10.1016/j.amjsurg.2013.07.044
- Bhatti NI, Ahmed A, Choi SS. Identifying quality indicators of surgical of surgical training: a national survey. Laryngoscope. 2015;125(12):2685-2689. doi:10.1002/lary.25262
- Abt NB, Reh DD, Eisele DW, Francis HW, Gourin CG. Does resident participation influence otolaryngology-head and neck surgery morbidity and mortality? Laryngoscope. 2016;126(10):2263-2269. doi:10.1002/lary.25973
- Jubbal KT, Chang D, Izaddoost SA, Pederson W, Zavlin D, Echo A. Resident involvement in microsurgery: an American College of Surgeons national surgical quality improvement program analysis. J Surg Educ. 2017;74(6):1124-1132. doi:10.1016/j.jsurg.2017.05.017
- Kshirsagar RS, Chandy Z, Mahboubi H, Verma SP. Does resident involvement in thyroid surgery lead to increased postoperative complications? Laryngoscope. 2017;127(5):1242-1246. doi:10.1002/lary.26176
- Vieira BL, Hernandez DJ, Qin C, Smith SS, Kim JY, Dutra JC. The impact of resident involvement on otolaryngology surgical outcomes. Laryngoscope. 2016;126(3):602-607. doi:10.1002/lary.25046
- Advani V, Ahad S, Gonczy C, Markwell S, Hassan I. Does resident involvement effect surgical times and complication rates during laparoscopic appendectomy for uncomplicated appendicitis? An analysis of 16,849 cases from the ACS-NSQIP. Am J Surg. 2012;203(3):347-352. doi:10.1016/j.amjsurg.2011.08.015
- Quinn NA, Alt JA, Ashby S, Orlandi RR. Time, resident involvement, and supply drive cost variability in septoplasty with turbinate reduction. Otolaryngol Head Neck Surg. 2018;159(2):310-314. doi:10.1177/0194599818765099
- Leader BA, Wiebracht ND, Meinzen-Derr J, Ishman SL. The impact of resident involvement on tonsillectomy outcomes and surgical time. Laryngoscope. 2020;130(10):2481-2486. doi:10.1002/lary.28427
- Muelleman T, Shew M, Muelleman RJ, et al. Impact of resident participation on operative time and outcomes in otologic surgery. Otolaryngol Head Neck Surg. 2018;158(1):151-154. doi:10.1177/0194599817737270
- Puram SV, Kozin ED, Sethi R, et al. Impact of resident surgeons on procedure length based on common pediatric otolaryngology cases. Laryngoscope. 2015;125(4):991 -997. doi:10.1002/lary.24912
- Chow MS, Gordon AJ, Talwar A, Lydiatt WM, Yueh B, Givi B. The RVU compensation model and head and neck surgical education. Laryngoscope. 2024;134(1):113-119. doi:10.1002/lary.30807
- Papandria D, Rhee D, Ortega G, et al. Assessing trainee impact on operative time for common general surgical procedures in ACS-NSQIP. J Surg Educ. 2012;69(2):149-155. doi:10.1016/j.jsurg.2011.08.003
- Pugely AJ, Gao Y, Martin CT, Callagh JJ, Weinstein SL, Marsh JL. The effect of resident participation on short-term outcomes after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(7):2290-2300. doi:10.1007/s11999-014-3567-0
- Hosler MR, Scott IU, Kunselman AR, Wolford KR, Oltra EZ, Murray WB. Impact of resident participation in cataract surgery on operative time and cost. Ophthalmology. 2012;119(1):95-98. doi:10.1016/j.ophtha.2011.06.026
- Lanigan A, Spaw M, Donaghe C, Brennan J. The impact of the Veteran’s Affairs medical system on an otolaryngology residency training program. Mil Med. 2018;183(11-12):e671-e675. doi:10.1093/milmed/usy041
- American Society of Clinical Oncology, Pfister DG, Laurie SA, et al. American Society of Clinical Oncology clinical practice guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer. J Clin Oncol. 2006;24(22):3693-3704. doi:10.1200/JCO.2006.07.4559
- Forastiere AA, Ismaila N, Lewin JS, et al. Use of larynxpreservation strategies in the treatment of laryngeal cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(11):1143-1169. doi:10.1200/JCO.2017.75.7385
- Verma SP, Mahboubi H. The changing landscape of total laryngectomy surgery. Otolaryngol Head Neck Surg. 2014;150(3):413-418. doi:10.1177/0194599813514515
- Habermann EB, Harris AHS, Giori NJ. Large surgical databases with direct data abstraction: VASQIP and ACSNSQIP. J Bone Joint Surg Am. 2022;104(suppl 3):9-14. doi:10.2106/JBJS.22.00596
- Benito DA, Mamidi I, Pasick LJ, et al. Evaluating resident involvement and the ‘July effect’ in parotidectomy. J Laryngol Otol. 2021;135(5):452-457. doi:10.1017/S0022215121000578
- Hwang CS, Wichterman KA, Alfrey EJ. The cost of resident education. J Surg Res. 2010;163(1):18-23. doi:10.1016/j.jss.2010.03.013
- Saliba AN, Taher AT, Tamim H, et al. Impact of resident involvement in surgery (IRIS-NSQIP): looking at the bigger picture based on the American College of Surgeons- NSQIP database. J Am Coll Surg. 2016; 222(1):30-40. doi:10.1016/j.jamcollsurg.2015.10.011
- Khuri SF, Najjar SF, Daley J, et al. Comparison of surgical outcomes between teaching and nonteaching hospitals in the Department of Veterans Affairs. Ann Surg. 2001;234(3):370-383. doi:10.1097/00000658-200109000-00011
- Relles DM, Burkhart RA, Pucci MJ et al. Does resident experience affect outcomes in complex abdominal surgery? Pancreaticoduodenectomy as an example. J Gastrointest Surg. 2014;18(2):279-285. doi:10.1007/s11605-013-2372-5
- Accreditation Council for Graduate Medical Education. Required minimum number of key indicator procedures for graduating residents. June 2019. Accessed January 2, 2025. https://www.acgme.org/globalassets/pfassets/programresources/280_core_case_log_minimums.pdf
- Brady JS, Crippen MM, Filimonov A, et al. The effect of training level on complications after free flap surgery of the head and neck. Am J Otolaryngol. 2017;38(5):560-564. doi:10.1016/j.amjoto.2017.06.001
Resident Participation Impact on Operative Time and Outcomes in Veterans Undergoing Total Laryngectomy
Resident Participation Impact on Operative Time and Outcomes in Veterans Undergoing Total Laryngectomy
Total Intravenous Anesthesia Enables Earlier Facial Nerve Monitoring Than Sevoflurane in Ear Surgery
TOPLINE:
Total intravenous anesthesia (TIVA) enables earlier intraoperative monitoring of facial nerve activity than sevoflurane anesthesia during ear surgery, with reduced patient-ventilator dyssynchrony and fewer requirements for postoperative antiemetics.
METHODOLOGY:
- Researchers evaluated the difference in the timeliness of intraoperative monitoring of facial nerve activity during ear surgery with TIVA vs sevoflurane anesthesia.
- They included 98 patients aged 18-74 years undergoing ear surgery between November 2021 and November 2022; patients were randomly assigned to receive either TIVA or sevoflurane during the procedure. Of these, 92 were included in the final analysis.
- Neuromuscular function was monitored quantitatively throughout anesthesia with train-of-four counts and train-of-four ratios.
- The time from the administration of rocuronium to the start of facial nerve monitoring was recorded.
- The primary outcome measure focused on the recovery index, defined as the time interval between a train-of-four ratio of 0.25 and 0.75; the key secondary outcome was the time to reach a train-of-four ratio of 0.25 from rocuronium administration.
TAKEAWAY:
- The time to reach a train-of-four ratio of 0.25 was achieved earlier with TIVA than with sevoflurane (34 minutes vs 51 minutes; P < .001).
- Patient-ventilator dyssynchrony occurred less frequently in the TIVA group than in the sevoflurane group (15% vs 39%; P = .01).
- Postoperative requests for antiemetics were less frequent in the TIVA group than in the sevoflurane group (2% vs 17%; P = .03).
IN PRACTICE:
“We suggest that TIVA may be a better choice than sevoflurane anesthesia to meet an earlier request” for intraoperative facial nerve monitoring by surgeons, the study authors wrote.
SOURCE:
The study was led by Yu Jeong Bang, MD, of the Department of Anesthesiology and Pain Medicine at Sungkyunkwan University School of Medicine, in Seoul, Republic of Korea. It was published online on November 27, 2024, in The Canadian Journal of Anesthesia.
LIMITATIONS:
A careful interpretation of results may be necessary when clinicians use balanced anesthesia, such as sevoflurane with adjuvants like opioids or nonopioids. The feasibility of intraoperative facial nerve monitoring was decided by the surgeon during surgery, and the lowest stimulation intensity threshold for electromyography amplitude was not detected, as it was not the focus of this study. Although patients requiring intraoperative facial nerve monitoring during ear surgery were enrolled, some did not undergo the procedure based on the surgeon’s judgment.
DISCLOSURES:
This study did not receive any funding. The authors disclosed no relevant conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Total intravenous anesthesia (TIVA) enables earlier intraoperative monitoring of facial nerve activity than sevoflurane anesthesia during ear surgery, with reduced patient-ventilator dyssynchrony and fewer requirements for postoperative antiemetics.
METHODOLOGY:
- Researchers evaluated the difference in the timeliness of intraoperative monitoring of facial nerve activity during ear surgery with TIVA vs sevoflurane anesthesia.
- They included 98 patients aged 18-74 years undergoing ear surgery between November 2021 and November 2022; patients were randomly assigned to receive either TIVA or sevoflurane during the procedure. Of these, 92 were included in the final analysis.
- Neuromuscular function was monitored quantitatively throughout anesthesia with train-of-four counts and train-of-four ratios.
- The time from the administration of rocuronium to the start of facial nerve monitoring was recorded.
- The primary outcome measure focused on the recovery index, defined as the time interval between a train-of-four ratio of 0.25 and 0.75; the key secondary outcome was the time to reach a train-of-four ratio of 0.25 from rocuronium administration.
TAKEAWAY:
- The time to reach a train-of-four ratio of 0.25 was achieved earlier with TIVA than with sevoflurane (34 minutes vs 51 minutes; P < .001).
- Patient-ventilator dyssynchrony occurred less frequently in the TIVA group than in the sevoflurane group (15% vs 39%; P = .01).
- Postoperative requests for antiemetics were less frequent in the TIVA group than in the sevoflurane group (2% vs 17%; P = .03).
IN PRACTICE:
“We suggest that TIVA may be a better choice than sevoflurane anesthesia to meet an earlier request” for intraoperative facial nerve monitoring by surgeons, the study authors wrote.
SOURCE:
The study was led by Yu Jeong Bang, MD, of the Department of Anesthesiology and Pain Medicine at Sungkyunkwan University School of Medicine, in Seoul, Republic of Korea. It was published online on November 27, 2024, in The Canadian Journal of Anesthesia.
LIMITATIONS:
A careful interpretation of results may be necessary when clinicians use balanced anesthesia, such as sevoflurane with adjuvants like opioids or nonopioids. The feasibility of intraoperative facial nerve monitoring was decided by the surgeon during surgery, and the lowest stimulation intensity threshold for electromyography amplitude was not detected, as it was not the focus of this study. Although patients requiring intraoperative facial nerve monitoring during ear surgery were enrolled, some did not undergo the procedure based on the surgeon’s judgment.
DISCLOSURES:
This study did not receive any funding. The authors disclosed no relevant conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Total intravenous anesthesia (TIVA) enables earlier intraoperative monitoring of facial nerve activity than sevoflurane anesthesia during ear surgery, with reduced patient-ventilator dyssynchrony and fewer requirements for postoperative antiemetics.
METHODOLOGY:
- Researchers evaluated the difference in the timeliness of intraoperative monitoring of facial nerve activity during ear surgery with TIVA vs sevoflurane anesthesia.
- They included 98 patients aged 18-74 years undergoing ear surgery between November 2021 and November 2022; patients were randomly assigned to receive either TIVA or sevoflurane during the procedure. Of these, 92 were included in the final analysis.
- Neuromuscular function was monitored quantitatively throughout anesthesia with train-of-four counts and train-of-four ratios.
- The time from the administration of rocuronium to the start of facial nerve monitoring was recorded.
- The primary outcome measure focused on the recovery index, defined as the time interval between a train-of-four ratio of 0.25 and 0.75; the key secondary outcome was the time to reach a train-of-four ratio of 0.25 from rocuronium administration.
TAKEAWAY:
- The time to reach a train-of-four ratio of 0.25 was achieved earlier with TIVA than with sevoflurane (34 minutes vs 51 minutes; P < .001).
- Patient-ventilator dyssynchrony occurred less frequently in the TIVA group than in the sevoflurane group (15% vs 39%; P = .01).
- Postoperative requests for antiemetics were less frequent in the TIVA group than in the sevoflurane group (2% vs 17%; P = .03).
IN PRACTICE:
“We suggest that TIVA may be a better choice than sevoflurane anesthesia to meet an earlier request” for intraoperative facial nerve monitoring by surgeons, the study authors wrote.
SOURCE:
The study was led by Yu Jeong Bang, MD, of the Department of Anesthesiology and Pain Medicine at Sungkyunkwan University School of Medicine, in Seoul, Republic of Korea. It was published online on November 27, 2024, in The Canadian Journal of Anesthesia.
LIMITATIONS:
A careful interpretation of results may be necessary when clinicians use balanced anesthesia, such as sevoflurane with adjuvants like opioids or nonopioids. The feasibility of intraoperative facial nerve monitoring was decided by the surgeon during surgery, and the lowest stimulation intensity threshold for electromyography amplitude was not detected, as it was not the focus of this study. Although patients requiring intraoperative facial nerve monitoring during ear surgery were enrolled, some did not undergo the procedure based on the surgeon’s judgment.
DISCLOSURES:
This study did not receive any funding. The authors disclosed no relevant conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Thyroidectomy Beneficial but Risky for Hashimoto Disease
TOPLINE:
In patients with Hashimoto disease and persistent symptoms despite adequate medical treatment, total thyroidectomy had a beneficial effect up to 5 years but with a substantially higher risk for complications than initially anticipated.
METHODOLOGY:
- The 5-year follow-up of 65 participants in a randomized, open-label trial of thyroidectomy plus medical management vs medical management alone aimed at testing the hypothesis that persistent symptoms despite adequate thyroxine replacement may be related to extrathyroidal autoimmune reactions and that complete removal of thyroid tissues may attenuate autoimmune responses and relieve symptoms.
- Patients in the control group were given the option of having surgery 18 months after enrollment, depending on trial results.
- The primary outcome was patient-reported health-related quality of life measured by the dimensional general health score in the generic Short Form-36 Health Survey questionnaire.
TAKEAWAY:
- The positive treatment effect seen after 18 months was maintained throughout the 3-year follow-up.
- In the intervention group, the improved general health score remained at the same level during the 5-year follow-up.
- Results were similar for the other Short Form-36 Health Survey domains and for total fatigue and chronic fatigue.
- Short-term (<12 months) or longer-lasting complications occurred in 23 patients, including 6 with recurrent laryngeal nerve paralysis (4 were long-term) and 12 with hypoparathyroidism (6 long-term, including 3 permanent).
- Five patients had postoperative hematoma and/or infection requiring intervention.
IN PRACTICE:
“The improvements in patient-reported outcome measures reported at 18 months after surgery were maintained at 5 years after surgery in the intervention group. In contrast, no spontaneous improvement was seen during 3 years in the control group.”
“Long-term complications in 10 of 73 (14%) patients despite use of meticulous dissection to achieve total thyroidectomy is unacceptably high. Medication and compensatory mechanisms for hypoparathyroidism and unilateral recurrent nerve injury, respectively, did alleviate symptoms.”
SOURCE:
This study was published in Annals of Internal Medicine, by Geir Hoff, MD, PhD, of the Department of Research, Telemark Hospital, Skien, and the Institute of Clinical Medicine, University of Oslo, Oslo, Norway, and colleagues.
LIMITATIONS:
None listed.
DISCLOSURES:
None.
TOPLINE:
In patients with Hashimoto disease and persistent symptoms despite adequate medical treatment, total thyroidectomy had a beneficial effect up to 5 years but with a substantially higher risk for complications than initially anticipated.
METHODOLOGY:
- The 5-year follow-up of 65 participants in a randomized, open-label trial of thyroidectomy plus medical management vs medical management alone aimed at testing the hypothesis that persistent symptoms despite adequate thyroxine replacement may be related to extrathyroidal autoimmune reactions and that complete removal of thyroid tissues may attenuate autoimmune responses and relieve symptoms.
- Patients in the control group were given the option of having surgery 18 months after enrollment, depending on trial results.
- The primary outcome was patient-reported health-related quality of life measured by the dimensional general health score in the generic Short Form-36 Health Survey questionnaire.
TAKEAWAY:
- The positive treatment effect seen after 18 months was maintained throughout the 3-year follow-up.
- In the intervention group, the improved general health score remained at the same level during the 5-year follow-up.
- Results were similar for the other Short Form-36 Health Survey domains and for total fatigue and chronic fatigue.
- Short-term (<12 months) or longer-lasting complications occurred in 23 patients, including 6 with recurrent laryngeal nerve paralysis (4 were long-term) and 12 with hypoparathyroidism (6 long-term, including 3 permanent).
- Five patients had postoperative hematoma and/or infection requiring intervention.
IN PRACTICE:
“The improvements in patient-reported outcome measures reported at 18 months after surgery were maintained at 5 years after surgery in the intervention group. In contrast, no spontaneous improvement was seen during 3 years in the control group.”
“Long-term complications in 10 of 73 (14%) patients despite use of meticulous dissection to achieve total thyroidectomy is unacceptably high. Medication and compensatory mechanisms for hypoparathyroidism and unilateral recurrent nerve injury, respectively, did alleviate symptoms.”
SOURCE:
This study was published in Annals of Internal Medicine, by Geir Hoff, MD, PhD, of the Department of Research, Telemark Hospital, Skien, and the Institute of Clinical Medicine, University of Oslo, Oslo, Norway, and colleagues.
LIMITATIONS:
None listed.
DISCLOSURES:
None.
TOPLINE:
In patients with Hashimoto disease and persistent symptoms despite adequate medical treatment, total thyroidectomy had a beneficial effect up to 5 years but with a substantially higher risk for complications than initially anticipated.
METHODOLOGY:
- The 5-year follow-up of 65 participants in a randomized, open-label trial of thyroidectomy plus medical management vs medical management alone aimed at testing the hypothesis that persistent symptoms despite adequate thyroxine replacement may be related to extrathyroidal autoimmune reactions and that complete removal of thyroid tissues may attenuate autoimmune responses and relieve symptoms.
- Patients in the control group were given the option of having surgery 18 months after enrollment, depending on trial results.
- The primary outcome was patient-reported health-related quality of life measured by the dimensional general health score in the generic Short Form-36 Health Survey questionnaire.
TAKEAWAY:
- The positive treatment effect seen after 18 months was maintained throughout the 3-year follow-up.
- In the intervention group, the improved general health score remained at the same level during the 5-year follow-up.
- Results were similar for the other Short Form-36 Health Survey domains and for total fatigue and chronic fatigue.
- Short-term (<12 months) or longer-lasting complications occurred in 23 patients, including 6 with recurrent laryngeal nerve paralysis (4 were long-term) and 12 with hypoparathyroidism (6 long-term, including 3 permanent).
- Five patients had postoperative hematoma and/or infection requiring intervention.
IN PRACTICE:
“The improvements in patient-reported outcome measures reported at 18 months after surgery were maintained at 5 years after surgery in the intervention group. In contrast, no spontaneous improvement was seen during 3 years in the control group.”
“Long-term complications in 10 of 73 (14%) patients despite use of meticulous dissection to achieve total thyroidectomy is unacceptably high. Medication and compensatory mechanisms for hypoparathyroidism and unilateral recurrent nerve injury, respectively, did alleviate symptoms.”
SOURCE:
This study was published in Annals of Internal Medicine, by Geir Hoff, MD, PhD, of the Department of Research, Telemark Hospital, Skien, and the Institute of Clinical Medicine, University of Oslo, Oslo, Norway, and colleagues.
LIMITATIONS:
None listed.
DISCLOSURES:
None.
Study Suggests Inappropriate Use of Thyroid Ultrasounds
“The number of thyroid ultrasounds performed in the United States has increased fivefold since 2002. This substantial increase produces a significant strain on healthcare resources and leads to over-detection and overtreatment of benign thyroid nodules and small, indolent cancers with questionable clinical relevance,” wrote Elena Kennedy, MD, then a medical student in the department of surgery at the University of Wisconsin School of Medicine and Public Health, Madison, and colleagues.
The data, published online in Thyroid, come from a retrospective chart analysis of more than 1700 people who underwent dedicated (ie, specifically to look for a nodule) thyroid ultrasounds at a tertiary academic center. The rates of detecting both nodules and biopsy-recommended nodules were highest when the indication was a nodule seen incidentally on other imaging (aka “incidentaloma”) and lowest when the ultrasound was ordered because the patient had either metabolic or compressive symptoms.
And for the most commonly listed indication, a suspected palpable nodule, nearly half of the ultrasounds found no nodule, and only one in five detected a nodule that warranted a biopsy.
The principal investigator of the study David O. Francis, MD, an otolaryngologist at the University of Wisconsin, Madison, said in an interview, “Thyroid cancer has grown in incidence three to four times over the last 30 years without a good explanation for why…It seems to be that we’re detecting smaller and smaller nodules…Why are people being referred for all these ultrasounds? We looked for the upstream factors.”
One clear clinical implication of the new data, Dr. Francis noted, is that “if someone has compressive symptoms including dysphagia, swallowing problems, voice change, or globus sensation, ultrasound should not be the first way to work them up…It would be smarter to have someone evaluate their voice or their swallowing to see if there’s another reason besides the thyroid. The thyroid would have to get pretty big to cause dysphagia or swallowing problems.”
No Current Guidelines Advise When not to Order a Thyroid Ultrasound
Problematically, while there are professional society guidelines for what to do when a thyroid “incidentaloma” is found and other specific situations, there are no overall guidelines addressing when it’s appropriate to order a thyroid ultrasound, Dr. Kennedy, now an otolaryngology resident at the Indiana University of Indianapolis, and colleagues, point out.
According to Dr. Francis, “Ultrasounds are low cost and low risk. Those two factors result in people ordering more tests…The problem with that is we find things, and then we have to figure out what to do with them. That leads to incidentalomas, the surveillance, worry and anxiety, and costs…It’s tricky. We don’t want to discourage people from ever ordering ultrasounds, but there need to be some guidelines around when it’s appropriate to order.”
Asked to comment, Trevor E. Angell, MD, associate medical director of Thyroid Center at Keck School of Medicine of the University of Southern California, Los Angeles, said that the study is “clinically very important.”
Dr. Angell pointed out that the current American Thyroid Association (ATA) guidelines on thyroid nodule management, of which he is an author, recommend ultrasound for a known or suspected nodule. But he added, “there certainly should be a message that obtaining ultrasound for these other reasons are less likely to identify a nodule or anything causative. Whether it’s gastroesophageal reflux or allergic rhinitis or vocal cord dysfunction, an ultrasound isn’t a good test for those either.”
Dr. Angell said that the next ATA thyroid nodule guidelines, expected out in 2024, will address this topic more fully, but he couldn’t provide more specific information because the document is still in development. He did say, however, “Addressing when not to do an ultrasound will be an important consideration in the next guidelines.”
Low Detection Rates for Most Indications
The retrospective observational cohort study included 1739 adults (76% women; mean age, 53 years) who underwent dedicated thyroid ultrasounds between 2017 and 2019. In most cases, the recommendation for biopsy was determined using the American College of Radiology TI-RADS system, based on nodule size and TI-RADS category.
The most common indication for thyroid ultrasound, suspected palpable nodule, accounted for 40% of those performed. Follow-up for an “incidentaloma” was the indication in 28% of patients, and referral for compressive and metabolic symptoms accounted for 13% and 6% of ultrasounds, respectively.
Among all ultrasounds performed, 62% identified a thyroid nodule. Patients referred for incidental findings had the highest percentage of ultrasounds with thyroid nodules present at 94%. By contrast, in those referred for suspected palpable nodule on exam and for compressive symptoms, nodules were identified on 55% and 39% of ultrasounds, respectively. Patients with metabolic symptoms had a nodule identified on ultrasound 43% of the time. Among those referred for high risk factors, 57% had a nodule present.
Overall, only 27% of ultrasounds identified a thyroid nodule that was recommended for a biopsy. Again, those referred because of an incidental imaging finding had the highest percentage (55%), followed by those referred for a suspected palpable nodule (21%), high risk factors (20%), combined indications (16%), metabolic symptoms (10%), and compressive symptoms (6%).
Mean nodule size was largest among the patients referred for incidentalomas (2.4 cm), whereas all the other groups had mean nodule sizes between 1.2 cm and 1.8 cm, a significant difference (P < .05). The median size of nodules among those referred to ultrasound for a suspected palpable nodule was 1.4 cm.
“That’s pretty small. It would have had to be in the front of the thyroid where they could actually touch it. I would argue that the number of clinicians who actually palpated something was smaller. We’ve done several projects looking at how small a nodule a clinician can actually feel in the thyroid gland from the neck. It turns out we’re pretty bad at physical examination of the thyroid. This paper kind of reinforces that,” Dr. Francis said in an interview.
Patients with incidental nodules were over 10 times more likely to have a nodule found on an ultrasound than those referred for a suspected palpable nodule on exam (odds ratio [OR], 10.6). Conversely, those referred for compressive symptoms were half as likely to have an identifiable nodule compared with those referred for physical exam findings (OR, 0.5).
The odds of finding a nodule increased with age, especially for those aged ≥ 65 years compared with those younger than 45 years (OR, 3.6). Women were twice as likely to have a nodule found on thyroid ultrasound (OR, 2.0). Results were similar for the biopsy-recommended nodules, except that there was no difference between sexes (female vs male OR, 1.2).
Dr. Angell called the study “a very robust comprehensive evaluation,” but also noted that the single center source is a limitation. “It would be nice to have those big databases of national healthcare settings, but getting that granular level of information about why something was done is nearly impossible in that context.”
Dr. Kennedy, Dr. Francis, and Dr. Angell have no disclosures.
A version of this article appeared on Medscape.com.
“The number of thyroid ultrasounds performed in the United States has increased fivefold since 2002. This substantial increase produces a significant strain on healthcare resources and leads to over-detection and overtreatment of benign thyroid nodules and small, indolent cancers with questionable clinical relevance,” wrote Elena Kennedy, MD, then a medical student in the department of surgery at the University of Wisconsin School of Medicine and Public Health, Madison, and colleagues.
The data, published online in Thyroid, come from a retrospective chart analysis of more than 1700 people who underwent dedicated (ie, specifically to look for a nodule) thyroid ultrasounds at a tertiary academic center. The rates of detecting both nodules and biopsy-recommended nodules were highest when the indication was a nodule seen incidentally on other imaging (aka “incidentaloma”) and lowest when the ultrasound was ordered because the patient had either metabolic or compressive symptoms.
And for the most commonly listed indication, a suspected palpable nodule, nearly half of the ultrasounds found no nodule, and only one in five detected a nodule that warranted a biopsy.
The principal investigator of the study David O. Francis, MD, an otolaryngologist at the University of Wisconsin, Madison, said in an interview, “Thyroid cancer has grown in incidence three to four times over the last 30 years without a good explanation for why…It seems to be that we’re detecting smaller and smaller nodules…Why are people being referred for all these ultrasounds? We looked for the upstream factors.”
One clear clinical implication of the new data, Dr. Francis noted, is that “if someone has compressive symptoms including dysphagia, swallowing problems, voice change, or globus sensation, ultrasound should not be the first way to work them up…It would be smarter to have someone evaluate their voice or their swallowing to see if there’s another reason besides the thyroid. The thyroid would have to get pretty big to cause dysphagia or swallowing problems.”
No Current Guidelines Advise When not to Order a Thyroid Ultrasound
Problematically, while there are professional society guidelines for what to do when a thyroid “incidentaloma” is found and other specific situations, there are no overall guidelines addressing when it’s appropriate to order a thyroid ultrasound, Dr. Kennedy, now an otolaryngology resident at the Indiana University of Indianapolis, and colleagues, point out.
According to Dr. Francis, “Ultrasounds are low cost and low risk. Those two factors result in people ordering more tests…The problem with that is we find things, and then we have to figure out what to do with them. That leads to incidentalomas, the surveillance, worry and anxiety, and costs…It’s tricky. We don’t want to discourage people from ever ordering ultrasounds, but there need to be some guidelines around when it’s appropriate to order.”
Asked to comment, Trevor E. Angell, MD, associate medical director of Thyroid Center at Keck School of Medicine of the University of Southern California, Los Angeles, said that the study is “clinically very important.”
Dr. Angell pointed out that the current American Thyroid Association (ATA) guidelines on thyroid nodule management, of which he is an author, recommend ultrasound for a known or suspected nodule. But he added, “there certainly should be a message that obtaining ultrasound for these other reasons are less likely to identify a nodule or anything causative. Whether it’s gastroesophageal reflux or allergic rhinitis or vocal cord dysfunction, an ultrasound isn’t a good test for those either.”
Dr. Angell said that the next ATA thyroid nodule guidelines, expected out in 2024, will address this topic more fully, but he couldn’t provide more specific information because the document is still in development. He did say, however, “Addressing when not to do an ultrasound will be an important consideration in the next guidelines.”
Low Detection Rates for Most Indications
The retrospective observational cohort study included 1739 adults (76% women; mean age, 53 years) who underwent dedicated thyroid ultrasounds between 2017 and 2019. In most cases, the recommendation for biopsy was determined using the American College of Radiology TI-RADS system, based on nodule size and TI-RADS category.
The most common indication for thyroid ultrasound, suspected palpable nodule, accounted for 40% of those performed. Follow-up for an “incidentaloma” was the indication in 28% of patients, and referral for compressive and metabolic symptoms accounted for 13% and 6% of ultrasounds, respectively.
Among all ultrasounds performed, 62% identified a thyroid nodule. Patients referred for incidental findings had the highest percentage of ultrasounds with thyroid nodules present at 94%. By contrast, in those referred for suspected palpable nodule on exam and for compressive symptoms, nodules were identified on 55% and 39% of ultrasounds, respectively. Patients with metabolic symptoms had a nodule identified on ultrasound 43% of the time. Among those referred for high risk factors, 57% had a nodule present.
Overall, only 27% of ultrasounds identified a thyroid nodule that was recommended for a biopsy. Again, those referred because of an incidental imaging finding had the highest percentage (55%), followed by those referred for a suspected palpable nodule (21%), high risk factors (20%), combined indications (16%), metabolic symptoms (10%), and compressive symptoms (6%).
Mean nodule size was largest among the patients referred for incidentalomas (2.4 cm), whereas all the other groups had mean nodule sizes between 1.2 cm and 1.8 cm, a significant difference (P < .05). The median size of nodules among those referred to ultrasound for a suspected palpable nodule was 1.4 cm.
“That’s pretty small. It would have had to be in the front of the thyroid where they could actually touch it. I would argue that the number of clinicians who actually palpated something was smaller. We’ve done several projects looking at how small a nodule a clinician can actually feel in the thyroid gland from the neck. It turns out we’re pretty bad at physical examination of the thyroid. This paper kind of reinforces that,” Dr. Francis said in an interview.
Patients with incidental nodules were over 10 times more likely to have a nodule found on an ultrasound than those referred for a suspected palpable nodule on exam (odds ratio [OR], 10.6). Conversely, those referred for compressive symptoms were half as likely to have an identifiable nodule compared with those referred for physical exam findings (OR, 0.5).
The odds of finding a nodule increased with age, especially for those aged ≥ 65 years compared with those younger than 45 years (OR, 3.6). Women were twice as likely to have a nodule found on thyroid ultrasound (OR, 2.0). Results were similar for the biopsy-recommended nodules, except that there was no difference between sexes (female vs male OR, 1.2).
Dr. Angell called the study “a very robust comprehensive evaluation,” but also noted that the single center source is a limitation. “It would be nice to have those big databases of national healthcare settings, but getting that granular level of information about why something was done is nearly impossible in that context.”
Dr. Kennedy, Dr. Francis, and Dr. Angell have no disclosures.
A version of this article appeared on Medscape.com.
“The number of thyroid ultrasounds performed in the United States has increased fivefold since 2002. This substantial increase produces a significant strain on healthcare resources and leads to over-detection and overtreatment of benign thyroid nodules and small, indolent cancers with questionable clinical relevance,” wrote Elena Kennedy, MD, then a medical student in the department of surgery at the University of Wisconsin School of Medicine and Public Health, Madison, and colleagues.
The data, published online in Thyroid, come from a retrospective chart analysis of more than 1700 people who underwent dedicated (ie, specifically to look for a nodule) thyroid ultrasounds at a tertiary academic center. The rates of detecting both nodules and biopsy-recommended nodules were highest when the indication was a nodule seen incidentally on other imaging (aka “incidentaloma”) and lowest when the ultrasound was ordered because the patient had either metabolic or compressive symptoms.
And for the most commonly listed indication, a suspected palpable nodule, nearly half of the ultrasounds found no nodule, and only one in five detected a nodule that warranted a biopsy.
The principal investigator of the study David O. Francis, MD, an otolaryngologist at the University of Wisconsin, Madison, said in an interview, “Thyroid cancer has grown in incidence three to four times over the last 30 years without a good explanation for why…It seems to be that we’re detecting smaller and smaller nodules…Why are people being referred for all these ultrasounds? We looked for the upstream factors.”
One clear clinical implication of the new data, Dr. Francis noted, is that “if someone has compressive symptoms including dysphagia, swallowing problems, voice change, or globus sensation, ultrasound should not be the first way to work them up…It would be smarter to have someone evaluate their voice or their swallowing to see if there’s another reason besides the thyroid. The thyroid would have to get pretty big to cause dysphagia or swallowing problems.”
No Current Guidelines Advise When not to Order a Thyroid Ultrasound
Problematically, while there are professional society guidelines for what to do when a thyroid “incidentaloma” is found and other specific situations, there are no overall guidelines addressing when it’s appropriate to order a thyroid ultrasound, Dr. Kennedy, now an otolaryngology resident at the Indiana University of Indianapolis, and colleagues, point out.
According to Dr. Francis, “Ultrasounds are low cost and low risk. Those two factors result in people ordering more tests…The problem with that is we find things, and then we have to figure out what to do with them. That leads to incidentalomas, the surveillance, worry and anxiety, and costs…It’s tricky. We don’t want to discourage people from ever ordering ultrasounds, but there need to be some guidelines around when it’s appropriate to order.”
Asked to comment, Trevor E. Angell, MD, associate medical director of Thyroid Center at Keck School of Medicine of the University of Southern California, Los Angeles, said that the study is “clinically very important.”
Dr. Angell pointed out that the current American Thyroid Association (ATA) guidelines on thyroid nodule management, of which he is an author, recommend ultrasound for a known or suspected nodule. But he added, “there certainly should be a message that obtaining ultrasound for these other reasons are less likely to identify a nodule or anything causative. Whether it’s gastroesophageal reflux or allergic rhinitis or vocal cord dysfunction, an ultrasound isn’t a good test for those either.”
Dr. Angell said that the next ATA thyroid nodule guidelines, expected out in 2024, will address this topic more fully, but he couldn’t provide more specific information because the document is still in development. He did say, however, “Addressing when not to do an ultrasound will be an important consideration in the next guidelines.”
Low Detection Rates for Most Indications
The retrospective observational cohort study included 1739 adults (76% women; mean age, 53 years) who underwent dedicated thyroid ultrasounds between 2017 and 2019. In most cases, the recommendation for biopsy was determined using the American College of Radiology TI-RADS system, based on nodule size and TI-RADS category.
The most common indication for thyroid ultrasound, suspected palpable nodule, accounted for 40% of those performed. Follow-up for an “incidentaloma” was the indication in 28% of patients, and referral for compressive and metabolic symptoms accounted for 13% and 6% of ultrasounds, respectively.
Among all ultrasounds performed, 62% identified a thyroid nodule. Patients referred for incidental findings had the highest percentage of ultrasounds with thyroid nodules present at 94%. By contrast, in those referred for suspected palpable nodule on exam and for compressive symptoms, nodules were identified on 55% and 39% of ultrasounds, respectively. Patients with metabolic symptoms had a nodule identified on ultrasound 43% of the time. Among those referred for high risk factors, 57% had a nodule present.
Overall, only 27% of ultrasounds identified a thyroid nodule that was recommended for a biopsy. Again, those referred because of an incidental imaging finding had the highest percentage (55%), followed by those referred for a suspected palpable nodule (21%), high risk factors (20%), combined indications (16%), metabolic symptoms (10%), and compressive symptoms (6%).
Mean nodule size was largest among the patients referred for incidentalomas (2.4 cm), whereas all the other groups had mean nodule sizes between 1.2 cm and 1.8 cm, a significant difference (P < .05). The median size of nodules among those referred to ultrasound for a suspected palpable nodule was 1.4 cm.
“That’s pretty small. It would have had to be in the front of the thyroid where they could actually touch it. I would argue that the number of clinicians who actually palpated something was smaller. We’ve done several projects looking at how small a nodule a clinician can actually feel in the thyroid gland from the neck. It turns out we’re pretty bad at physical examination of the thyroid. This paper kind of reinforces that,” Dr. Francis said in an interview.
Patients with incidental nodules were over 10 times more likely to have a nodule found on an ultrasound than those referred for a suspected palpable nodule on exam (odds ratio [OR], 10.6). Conversely, those referred for compressive symptoms were half as likely to have an identifiable nodule compared with those referred for physical exam findings (OR, 0.5).
The odds of finding a nodule increased with age, especially for those aged ≥ 65 years compared with those younger than 45 years (OR, 3.6). Women were twice as likely to have a nodule found on thyroid ultrasound (OR, 2.0). Results were similar for the biopsy-recommended nodules, except that there was no difference between sexes (female vs male OR, 1.2).
Dr. Angell called the study “a very robust comprehensive evaluation,” but also noted that the single center source is a limitation. “It would be nice to have those big databases of national healthcare settings, but getting that granular level of information about why something was done is nearly impossible in that context.”
Dr. Kennedy, Dr. Francis, and Dr. Angell have no disclosures.
A version of this article appeared on Medscape.com.
FROM THYROID
Endoscopic sinus surgery for chronic rhinosinusitis has no impact on comorbid asthma
Endoscopic sinus surgery (ESS) has no significant impact on asthma symptoms for patients with chronic rhinosinusitis up to a year after the procedure, a study of 64 patients shows.
, Anyull Dayanna Bohórquez Caballero said in a presentation at the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS) 2023 annual meeting.
The study “offers a unique approach to explore the effects of endoscopic sinus surgery in a real-world context, with valuable insights that differ from previous research,” Dr. Bohórquez Caballero, an international medical graduate and research fellow of the Mayo Clinic, Jacksonville, Fla., said in an interview.
Under the leadership of senior author Angela Donaldson, MD, Dr. Bohórquez Caballero and colleagues at the Mayo Clinic in Jacksonville analyzed data from 185 adults with both asthma and chronic rhinosinusitis who underwent ESS at the clinic between 2013 and 2023. Asthma severity was evaluated up to 3 months before and 1 year after surgery. Patients’ asthma severity was classified as mild, moderate, or severe on the basis of current Global Initiative for Asthma guidelines using medication requirements.
The final study population included 64 patients; 42 of these (66.7%) had chronic rhinosinusitis with nasal polyps. Outcomes included differences in asthma severity, asthma medication doses, and the number of medications.
Overall, there was no significant difference in measures of mild, moderate, or severe asthma before and after ESS in a McNemar paired test (P values: .130, .999, and .288, respectively). Similarly, no difference was found before and after ESS in terms of total inhaled corticosteroid dose (P = .999), number of medications prescribed (P = .157), or control of the disease (P = .078).
The findings were limited by the relatively small number of patients. The study is the first known to assess the real-world impact of ESS on asthma severity, said Bohórquez Caballero.
Expected reduction in asthma severity not seen
Past studies have suggested that ESS improves parameters such as pulmonary function test results or sinonasal outcomes, Dr. Bohórquez Caballero told this news organization. “Our findings indicate that ESS does not significantly impact asthma severity or trends in treatment, including the number and/or dose of medications, in everyday practice.
Our study also identified crucial opportunities to reinforce interdisciplinary follow-up after ESS,” she noted, and it provides a comprehensive depiction of the outcomes experienced by patients with chronic rhinosinusitis and asthma who undergo ESS.
“We were expecting a reduction in severity or a decrease in the dose of inhaled corticosteroid therapies, and we expected to see a translation from previous evidence into clinical practice; however, we did not,” said Dr. Bohórquez Caballero.
“The take-home message is that while there is a strong correlation between CRS and asthma, it does not appear that ESS alone improves real-world treatment based on asthma severity,” she said. “However, our findings have shown that patients may experience a longer period without the need for a reliever medication in the early postoperative period.”
Looking ahead, “We want to explore what happens 5 or 6 months after sinus surgery that would explain the sudden need for a reliever medication,” she added. “Future studies are warranted to investigate the long-term effects of ESS on asthma severity as it relates to modifications of asthma regimens.”
Data important for patient discussions
The current study is important because of the frequency of comorbid asthma among patients with chronic rhinosinusitis, Megan Durr, MD, of the University of California, San Francisco, said in an interview.
“When we are considering functional endoscopy sinus surgery with patients, we are often asked if the surgery will impact the severity of their asthma symptoms,” said Dr. Durr, who served as a moderator for the session in which the study was presented.
“I am surprised the study did not see any difference in asthma severity after sinus surgery, as we often talk to patients about the unified airway that refers to the shared epidemiologic and pathophysiologic relationship between the upper and lower airways,” she told this news organization.
“This study will allow us to have a more informed evidenced-based discussion with patients and their primary care providers and/or pulmonologists” about what to expect for asthma outcomes following surgery, she said.
The study received no outside funding. Dr. Durr has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Endoscopic sinus surgery (ESS) has no significant impact on asthma symptoms for patients with chronic rhinosinusitis up to a year after the procedure, a study of 64 patients shows.
, Anyull Dayanna Bohórquez Caballero said in a presentation at the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS) 2023 annual meeting.
The study “offers a unique approach to explore the effects of endoscopic sinus surgery in a real-world context, with valuable insights that differ from previous research,” Dr. Bohórquez Caballero, an international medical graduate and research fellow of the Mayo Clinic, Jacksonville, Fla., said in an interview.
Under the leadership of senior author Angela Donaldson, MD, Dr. Bohórquez Caballero and colleagues at the Mayo Clinic in Jacksonville analyzed data from 185 adults with both asthma and chronic rhinosinusitis who underwent ESS at the clinic between 2013 and 2023. Asthma severity was evaluated up to 3 months before and 1 year after surgery. Patients’ asthma severity was classified as mild, moderate, or severe on the basis of current Global Initiative for Asthma guidelines using medication requirements.
The final study population included 64 patients; 42 of these (66.7%) had chronic rhinosinusitis with nasal polyps. Outcomes included differences in asthma severity, asthma medication doses, and the number of medications.
Overall, there was no significant difference in measures of mild, moderate, or severe asthma before and after ESS in a McNemar paired test (P values: .130, .999, and .288, respectively). Similarly, no difference was found before and after ESS in terms of total inhaled corticosteroid dose (P = .999), number of medications prescribed (P = .157), or control of the disease (P = .078).
The findings were limited by the relatively small number of patients. The study is the first known to assess the real-world impact of ESS on asthma severity, said Bohórquez Caballero.
Expected reduction in asthma severity not seen
Past studies have suggested that ESS improves parameters such as pulmonary function test results or sinonasal outcomes, Dr. Bohórquez Caballero told this news organization. “Our findings indicate that ESS does not significantly impact asthma severity or trends in treatment, including the number and/or dose of medications, in everyday practice.
Our study also identified crucial opportunities to reinforce interdisciplinary follow-up after ESS,” she noted, and it provides a comprehensive depiction of the outcomes experienced by patients with chronic rhinosinusitis and asthma who undergo ESS.
“We were expecting a reduction in severity or a decrease in the dose of inhaled corticosteroid therapies, and we expected to see a translation from previous evidence into clinical practice; however, we did not,” said Dr. Bohórquez Caballero.
“The take-home message is that while there is a strong correlation between CRS and asthma, it does not appear that ESS alone improves real-world treatment based on asthma severity,” she said. “However, our findings have shown that patients may experience a longer period without the need for a reliever medication in the early postoperative period.”
Looking ahead, “We want to explore what happens 5 or 6 months after sinus surgery that would explain the sudden need for a reliever medication,” she added. “Future studies are warranted to investigate the long-term effects of ESS on asthma severity as it relates to modifications of asthma regimens.”
Data important for patient discussions
The current study is important because of the frequency of comorbid asthma among patients with chronic rhinosinusitis, Megan Durr, MD, of the University of California, San Francisco, said in an interview.
“When we are considering functional endoscopy sinus surgery with patients, we are often asked if the surgery will impact the severity of their asthma symptoms,” said Dr. Durr, who served as a moderator for the session in which the study was presented.
“I am surprised the study did not see any difference in asthma severity after sinus surgery, as we often talk to patients about the unified airway that refers to the shared epidemiologic and pathophysiologic relationship between the upper and lower airways,” she told this news organization.
“This study will allow us to have a more informed evidenced-based discussion with patients and their primary care providers and/or pulmonologists” about what to expect for asthma outcomes following surgery, she said.
The study received no outside funding. Dr. Durr has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Endoscopic sinus surgery (ESS) has no significant impact on asthma symptoms for patients with chronic rhinosinusitis up to a year after the procedure, a study of 64 patients shows.
, Anyull Dayanna Bohórquez Caballero said in a presentation at the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS) 2023 annual meeting.
The study “offers a unique approach to explore the effects of endoscopic sinus surgery in a real-world context, with valuable insights that differ from previous research,” Dr. Bohórquez Caballero, an international medical graduate and research fellow of the Mayo Clinic, Jacksonville, Fla., said in an interview.
Under the leadership of senior author Angela Donaldson, MD, Dr. Bohórquez Caballero and colleagues at the Mayo Clinic in Jacksonville analyzed data from 185 adults with both asthma and chronic rhinosinusitis who underwent ESS at the clinic between 2013 and 2023. Asthma severity was evaluated up to 3 months before and 1 year after surgery. Patients’ asthma severity was classified as mild, moderate, or severe on the basis of current Global Initiative for Asthma guidelines using medication requirements.
The final study population included 64 patients; 42 of these (66.7%) had chronic rhinosinusitis with nasal polyps. Outcomes included differences in asthma severity, asthma medication doses, and the number of medications.
Overall, there was no significant difference in measures of mild, moderate, or severe asthma before and after ESS in a McNemar paired test (P values: .130, .999, and .288, respectively). Similarly, no difference was found before and after ESS in terms of total inhaled corticosteroid dose (P = .999), number of medications prescribed (P = .157), or control of the disease (P = .078).
The findings were limited by the relatively small number of patients. The study is the first known to assess the real-world impact of ESS on asthma severity, said Bohórquez Caballero.
Expected reduction in asthma severity not seen
Past studies have suggested that ESS improves parameters such as pulmonary function test results or sinonasal outcomes, Dr. Bohórquez Caballero told this news organization. “Our findings indicate that ESS does not significantly impact asthma severity or trends in treatment, including the number and/or dose of medications, in everyday practice.
Our study also identified crucial opportunities to reinforce interdisciplinary follow-up after ESS,” she noted, and it provides a comprehensive depiction of the outcomes experienced by patients with chronic rhinosinusitis and asthma who undergo ESS.
“We were expecting a reduction in severity or a decrease in the dose of inhaled corticosteroid therapies, and we expected to see a translation from previous evidence into clinical practice; however, we did not,” said Dr. Bohórquez Caballero.
“The take-home message is that while there is a strong correlation between CRS and asthma, it does not appear that ESS alone improves real-world treatment based on asthma severity,” she said. “However, our findings have shown that patients may experience a longer period without the need for a reliever medication in the early postoperative period.”
Looking ahead, “We want to explore what happens 5 or 6 months after sinus surgery that would explain the sudden need for a reliever medication,” she added. “Future studies are warranted to investigate the long-term effects of ESS on asthma severity as it relates to modifications of asthma regimens.”
Data important for patient discussions
The current study is important because of the frequency of comorbid asthma among patients with chronic rhinosinusitis, Megan Durr, MD, of the University of California, San Francisco, said in an interview.
“When we are considering functional endoscopy sinus surgery with patients, we are often asked if the surgery will impact the severity of their asthma symptoms,” said Dr. Durr, who served as a moderator for the session in which the study was presented.
“I am surprised the study did not see any difference in asthma severity after sinus surgery, as we often talk to patients about the unified airway that refers to the shared epidemiologic and pathophysiologic relationship between the upper and lower airways,” she told this news organization.
“This study will allow us to have a more informed evidenced-based discussion with patients and their primary care providers and/or pulmonologists” about what to expect for asthma outcomes following surgery, she said.
The study received no outside funding. Dr. Durr has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAO-HNSF 2023
Sentinel central events prevalent during DISE for obstructive sleep apnea
DISE has become the top choice for surgical selection in patients with OSA, but it has a variable effect on surgical outcomes, Julianna G. Rodin, MD, of the University of Pennsylvania, Philadelphia, and colleagues explained.
The University of Pennsylvania sleep surgery team developed a comprehensive DISE platform that includes simultaneous collection of respiratory airflow and effort measurements, airway collapsibility, and videoendoscopy.
“This home sleep study-style setup has allowed us to better characterize the upper airway during DISE, and even helped our team diagnose a patient with Cheyne-Stokes breathing/central sleep apnea,” Dr. Rodin said in an interview.
“With it, we also began to notice relatively frequent central and/or mixed sleep disordered breathing events during DISE after propofol dosing initiation,” she said.
In a study presented at the annual meeting of the American Academy of Otolaryngology–Head and Neck Surgery, Dr. Rodin and colleagues measured both the frequency and timing of sentinel central and/or mixed events (SCent) in adults undergoing DISE to assess the prevalence and impact on DISE.
The researchers also assessed differences in VOTE classification (velum, oropharynx, tongue base, and epiglottis) in sentinel central events, compared with obstructive events. VOTE scores were calculated using a grade of 0 for no obstruction, 1 for partial obstruction, and 2 for total obstruction.
The study population included 103 adults with OSA who underwent DISE with propofol sedation at a single tertiary academic medical center between June 2020 and November 2022. The mean age of the participants was 53.5 years, the mean body mass index (BMI) was 29.7 kg/m2, and 67% were male. The average apnea-hypopnea index (AHI) was 30.7 events per hour. The researchers used a polysomnography platform to capture data on nasal airflow, thoraco-abdominal effort belt signals, and videoendoscopy.
A total of 47 patients (46%) had at least one SCent. The average time to the first SCent was just under 6 minutes, and average transition to obstructive pathology in these patients occurred between 7 and 8 minutes. Using the one-sided prediction interval, at least 95% of patients were expected to transition to obstructive pathology within 12-13 minutes, Dr. Rodin said.
In addition, 29 of the 46 patients with SCent (63%) showed significant variability between central/mixed VOTE scores and obstructive VOTE scores.
No statistically significant differences were noted between patients with and without SCent in terms of demographics or AHI.
Surprising prevalence of SCents
“We anecdotally noted that SCents seemed to be somewhat common during the initial period of DISE, but were surprised that we saw at least one SCent in almost 50% of our DISE population,” Dr. Rodin said. “We also saw that the majority of these SCents eventually transitioned to obstructive events after approximately 12 minutes, which is often past the average duration of normal DISE exams.”
The high frequency of differing VOTE scores between SCents and obstructive events also was unexpected, she added. Within the changes in VOTE scores as defined in the study, “there was a higher tendency for SCents to have more complete tongue base collapse compared to no or partial collapse in obstructive events, and to transition from anterior-posterior velum to concentric velum collapse during the obstructive event.”
This outcome could potentially affect a patient’s candidacy for hypoglossal nerve stimulator therapy, she explained.
The takeaway from the current study is an increased awareness of the prevalence and timing of SCents in OSA patients, said Dr. Rodin. Clinicians who offer DISE and PAP alternatives also should be mindful of clinical signs of effort, by monitoring the chest and abdomen during DISE in the absence of respiratory effort belts.
The study findings also suggest that clinicians consider extending the minimum DISE duration to 10 minutes to ensure that the majority of SCents have passed, and delay VOTE scoring until patients transition to obstructive events, she added.
As for additional research, Dr. Rodin said: “If we could repeat the study with a standardized protocol of target-controlled infusion (TCI) of propofol, that would further bolster the data.” However, TCI is not approved in the United States.
“Our propofol dosing technique was not standardized across all patients, which in theory could account for more SCents if patients were more sedated,” Dr. Rodin noted. “However, we did not see a difference in average bispectral index levels across all patients.”
Other limitations of the current study included an inability to visualize the entire upper airway to achieve a complete VOTE score for every patient, which could have led to underestimation of the VOTE difference frequency, she added.
Data inform team approaches to DISE
As DISE procedures become more widespread, “it is paramount that we understand the risks associated with these procedures to increase safety, improve shared decision-making, and encourage a team-based approach in the operating room with our anesthesia colleagues,” said Daniel M. Zeitler, MD, from the University of Washington and Virgina Mason Medical Center, both in Seattle, who served as a moderator for the session in which the study was presented.
“I was surprised by these data for two reasons,” Dr. Zeitler said in an interview. “We typically don’t wait more than a few minutes between induction of anesthesia and the initiation of the airway procedure. This study calls that practice into question, and the duration of time before the onset of a sentinel event was much longer than I would have expected,” he said.
Second, “I was quite surprised that there were no differences in the demographics or AHI between the two groups; this reminds us that AHI and BMI alone may not be themselves predictive of risk and all patients should be assessed similarly.”
“Otolaryngologists performing DISE need to be aware of these data, communicate them to the involved teams, including anesthesia, nursing, and postanesthesia care units, and remember to delay the manipulation of the airway long enough to minimize the risk of a sentinel event,” Dr. Zeitler said. “Perhaps this also means we need improved intraoperative monitoring for these patients, including respiratory airflow and effort monitoring.”
For further research, “we need to increase the number of patients, perform a multicenter study, and expand the study to a wider range of ages, BMI, and AHI,” he added. A recommended algorithm for these cases in order to standardize the practice would be useful.
The study received no outside funding. Dr. Rodin and Dr. Zeitler reported no relevant financial relationships. Several coauthors disclosed funding and relationships with multiple companies unrelated to the current study.
A version of this article appeared on Medscape.com.
DISE has become the top choice for surgical selection in patients with OSA, but it has a variable effect on surgical outcomes, Julianna G. Rodin, MD, of the University of Pennsylvania, Philadelphia, and colleagues explained.
The University of Pennsylvania sleep surgery team developed a comprehensive DISE platform that includes simultaneous collection of respiratory airflow and effort measurements, airway collapsibility, and videoendoscopy.
“This home sleep study-style setup has allowed us to better characterize the upper airway during DISE, and even helped our team diagnose a patient with Cheyne-Stokes breathing/central sleep apnea,” Dr. Rodin said in an interview.
“With it, we also began to notice relatively frequent central and/or mixed sleep disordered breathing events during DISE after propofol dosing initiation,” she said.
In a study presented at the annual meeting of the American Academy of Otolaryngology–Head and Neck Surgery, Dr. Rodin and colleagues measured both the frequency and timing of sentinel central and/or mixed events (SCent) in adults undergoing DISE to assess the prevalence and impact on DISE.
The researchers also assessed differences in VOTE classification (velum, oropharynx, tongue base, and epiglottis) in sentinel central events, compared with obstructive events. VOTE scores were calculated using a grade of 0 for no obstruction, 1 for partial obstruction, and 2 for total obstruction.
The study population included 103 adults with OSA who underwent DISE with propofol sedation at a single tertiary academic medical center between June 2020 and November 2022. The mean age of the participants was 53.5 years, the mean body mass index (BMI) was 29.7 kg/m2, and 67% were male. The average apnea-hypopnea index (AHI) was 30.7 events per hour. The researchers used a polysomnography platform to capture data on nasal airflow, thoraco-abdominal effort belt signals, and videoendoscopy.
A total of 47 patients (46%) had at least one SCent. The average time to the first SCent was just under 6 minutes, and average transition to obstructive pathology in these patients occurred between 7 and 8 minutes. Using the one-sided prediction interval, at least 95% of patients were expected to transition to obstructive pathology within 12-13 minutes, Dr. Rodin said.
In addition, 29 of the 46 patients with SCent (63%) showed significant variability between central/mixed VOTE scores and obstructive VOTE scores.
No statistically significant differences were noted between patients with and without SCent in terms of demographics or AHI.
Surprising prevalence of SCents
“We anecdotally noted that SCents seemed to be somewhat common during the initial period of DISE, but were surprised that we saw at least one SCent in almost 50% of our DISE population,” Dr. Rodin said. “We also saw that the majority of these SCents eventually transitioned to obstructive events after approximately 12 minutes, which is often past the average duration of normal DISE exams.”
The high frequency of differing VOTE scores between SCents and obstructive events also was unexpected, she added. Within the changes in VOTE scores as defined in the study, “there was a higher tendency for SCents to have more complete tongue base collapse compared to no or partial collapse in obstructive events, and to transition from anterior-posterior velum to concentric velum collapse during the obstructive event.”
This outcome could potentially affect a patient’s candidacy for hypoglossal nerve stimulator therapy, she explained.
The takeaway from the current study is an increased awareness of the prevalence and timing of SCents in OSA patients, said Dr. Rodin. Clinicians who offer DISE and PAP alternatives also should be mindful of clinical signs of effort, by monitoring the chest and abdomen during DISE in the absence of respiratory effort belts.
The study findings also suggest that clinicians consider extending the minimum DISE duration to 10 minutes to ensure that the majority of SCents have passed, and delay VOTE scoring until patients transition to obstructive events, she added.
As for additional research, Dr. Rodin said: “If we could repeat the study with a standardized protocol of target-controlled infusion (TCI) of propofol, that would further bolster the data.” However, TCI is not approved in the United States.
“Our propofol dosing technique was not standardized across all patients, which in theory could account for more SCents if patients were more sedated,” Dr. Rodin noted. “However, we did not see a difference in average bispectral index levels across all patients.”
Other limitations of the current study included an inability to visualize the entire upper airway to achieve a complete VOTE score for every patient, which could have led to underestimation of the VOTE difference frequency, she added.
Data inform team approaches to DISE
As DISE procedures become more widespread, “it is paramount that we understand the risks associated with these procedures to increase safety, improve shared decision-making, and encourage a team-based approach in the operating room with our anesthesia colleagues,” said Daniel M. Zeitler, MD, from the University of Washington and Virgina Mason Medical Center, both in Seattle, who served as a moderator for the session in which the study was presented.
“I was surprised by these data for two reasons,” Dr. Zeitler said in an interview. “We typically don’t wait more than a few minutes between induction of anesthesia and the initiation of the airway procedure. This study calls that practice into question, and the duration of time before the onset of a sentinel event was much longer than I would have expected,” he said.
Second, “I was quite surprised that there were no differences in the demographics or AHI between the two groups; this reminds us that AHI and BMI alone may not be themselves predictive of risk and all patients should be assessed similarly.”
“Otolaryngologists performing DISE need to be aware of these data, communicate them to the involved teams, including anesthesia, nursing, and postanesthesia care units, and remember to delay the manipulation of the airway long enough to minimize the risk of a sentinel event,” Dr. Zeitler said. “Perhaps this also means we need improved intraoperative monitoring for these patients, including respiratory airflow and effort monitoring.”
For further research, “we need to increase the number of patients, perform a multicenter study, and expand the study to a wider range of ages, BMI, and AHI,” he added. A recommended algorithm for these cases in order to standardize the practice would be useful.
The study received no outside funding. Dr. Rodin and Dr. Zeitler reported no relevant financial relationships. Several coauthors disclosed funding and relationships with multiple companies unrelated to the current study.
A version of this article appeared on Medscape.com.
DISE has become the top choice for surgical selection in patients with OSA, but it has a variable effect on surgical outcomes, Julianna G. Rodin, MD, of the University of Pennsylvania, Philadelphia, and colleagues explained.
The University of Pennsylvania sleep surgery team developed a comprehensive DISE platform that includes simultaneous collection of respiratory airflow and effort measurements, airway collapsibility, and videoendoscopy.
“This home sleep study-style setup has allowed us to better characterize the upper airway during DISE, and even helped our team diagnose a patient with Cheyne-Stokes breathing/central sleep apnea,” Dr. Rodin said in an interview.
“With it, we also began to notice relatively frequent central and/or mixed sleep disordered breathing events during DISE after propofol dosing initiation,” she said.
In a study presented at the annual meeting of the American Academy of Otolaryngology–Head and Neck Surgery, Dr. Rodin and colleagues measured both the frequency and timing of sentinel central and/or mixed events (SCent) in adults undergoing DISE to assess the prevalence and impact on DISE.
The researchers also assessed differences in VOTE classification (velum, oropharynx, tongue base, and epiglottis) in sentinel central events, compared with obstructive events. VOTE scores were calculated using a grade of 0 for no obstruction, 1 for partial obstruction, and 2 for total obstruction.
The study population included 103 adults with OSA who underwent DISE with propofol sedation at a single tertiary academic medical center between June 2020 and November 2022. The mean age of the participants was 53.5 years, the mean body mass index (BMI) was 29.7 kg/m2, and 67% were male. The average apnea-hypopnea index (AHI) was 30.7 events per hour. The researchers used a polysomnography platform to capture data on nasal airflow, thoraco-abdominal effort belt signals, and videoendoscopy.
A total of 47 patients (46%) had at least one SCent. The average time to the first SCent was just under 6 minutes, and average transition to obstructive pathology in these patients occurred between 7 and 8 minutes. Using the one-sided prediction interval, at least 95% of patients were expected to transition to obstructive pathology within 12-13 minutes, Dr. Rodin said.
In addition, 29 of the 46 patients with SCent (63%) showed significant variability between central/mixed VOTE scores and obstructive VOTE scores.
No statistically significant differences were noted between patients with and without SCent in terms of demographics or AHI.
Surprising prevalence of SCents
“We anecdotally noted that SCents seemed to be somewhat common during the initial period of DISE, but were surprised that we saw at least one SCent in almost 50% of our DISE population,” Dr. Rodin said. “We also saw that the majority of these SCents eventually transitioned to obstructive events after approximately 12 minutes, which is often past the average duration of normal DISE exams.”
The high frequency of differing VOTE scores between SCents and obstructive events also was unexpected, she added. Within the changes in VOTE scores as defined in the study, “there was a higher tendency for SCents to have more complete tongue base collapse compared to no or partial collapse in obstructive events, and to transition from anterior-posterior velum to concentric velum collapse during the obstructive event.”
This outcome could potentially affect a patient’s candidacy for hypoglossal nerve stimulator therapy, she explained.
The takeaway from the current study is an increased awareness of the prevalence and timing of SCents in OSA patients, said Dr. Rodin. Clinicians who offer DISE and PAP alternatives also should be mindful of clinical signs of effort, by monitoring the chest and abdomen during DISE in the absence of respiratory effort belts.
The study findings also suggest that clinicians consider extending the minimum DISE duration to 10 minutes to ensure that the majority of SCents have passed, and delay VOTE scoring until patients transition to obstructive events, she added.
As for additional research, Dr. Rodin said: “If we could repeat the study with a standardized protocol of target-controlled infusion (TCI) of propofol, that would further bolster the data.” However, TCI is not approved in the United States.
“Our propofol dosing technique was not standardized across all patients, which in theory could account for more SCents if patients were more sedated,” Dr. Rodin noted. “However, we did not see a difference in average bispectral index levels across all patients.”
Other limitations of the current study included an inability to visualize the entire upper airway to achieve a complete VOTE score for every patient, which could have led to underestimation of the VOTE difference frequency, she added.
Data inform team approaches to DISE
As DISE procedures become more widespread, “it is paramount that we understand the risks associated with these procedures to increase safety, improve shared decision-making, and encourage a team-based approach in the operating room with our anesthesia colleagues,” said Daniel M. Zeitler, MD, from the University of Washington and Virgina Mason Medical Center, both in Seattle, who served as a moderator for the session in which the study was presented.
“I was surprised by these data for two reasons,” Dr. Zeitler said in an interview. “We typically don’t wait more than a few minutes between induction of anesthesia and the initiation of the airway procedure. This study calls that practice into question, and the duration of time before the onset of a sentinel event was much longer than I would have expected,” he said.
Second, “I was quite surprised that there were no differences in the demographics or AHI between the two groups; this reminds us that AHI and BMI alone may not be themselves predictive of risk and all patients should be assessed similarly.”
“Otolaryngologists performing DISE need to be aware of these data, communicate them to the involved teams, including anesthesia, nursing, and postanesthesia care units, and remember to delay the manipulation of the airway long enough to minimize the risk of a sentinel event,” Dr. Zeitler said. “Perhaps this also means we need improved intraoperative monitoring for these patients, including respiratory airflow and effort monitoring.”
For further research, “we need to increase the number of patients, perform a multicenter study, and expand the study to a wider range of ages, BMI, and AHI,” he added. A recommended algorithm for these cases in order to standardize the practice would be useful.
The study received no outside funding. Dr. Rodin and Dr. Zeitler reported no relevant financial relationships. Several coauthors disclosed funding and relationships with multiple companies unrelated to the current study.
A version of this article appeared on Medscape.com.
FROM THE AAOH-HNS MEETING
Measures of PTH predict postthyroidectomy hypocalcemia
according to the results of a prospective study of 60 patients.
Postthyroidectomy hypocalcemia remains a major complication in patients who have undergone total thyroidectomy, and early identification can reduce disease burden and improve outcomes, according to Ahmed Sobhy Youssef, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, and colleagues.
In a presentation at the annual meeting of the American Academy of Otolaryngology-Head and Neck Surgery, Dr. Youssef presented results of the study, which looked at early postoperative parathyroid hormone as a predictor of postthyroidectomy hypocalcemia.
During his fellowship in Oklahoma in the wake of the COVID-19 pandemic, Dr. Youssef observed a wide variation in follow-up for calcium levels after thyroidectomy. “Some surgeons will order PTH and ionized calcium 4 hours after surgery, others would order later, at 6-8 hours,” he said in an interview. However, “all patients would be admitted for 1-2 nights [before being] discharged home, which meant more restrictions on the number of beds allowed for our head and neck cancer service.”
Discussion with his department chair led to a literature review seeking strategies to discharge patients earlier, and Dr. Youssef developed the idea for early PTH testing.
The study population included 60 adults who underwent thyroidectomy for benign or malignant disease at a single center between January 2022 and January 2023. The researchers measured PTH at 1 hour after surgery and compared it to results of a standard postoperative measure at 4 hours after surgery.
The researchers found a significant positive correlation between PTH measured 1 hour after surgery and ionized calcium (Ca) at 4 hours. The sensitivity of the early PTH assay, defined as “measured below 14 pg/ml,” was 100% to detect hypocalcemia, with an area under the curve of 0.797.
“The results were amazing,” said Dr. Youssef. “We found that when we measure PTH as early as 1 hour after total thyroidectomy, while patients are still in recovery, PTH was very sensitive to predict hypocalcemia.” The correlation was strong with measures at 4 hours.
“Our takeaway message is the 1-hour level PTH is very reliable in predicting hypocalcemia,” he added. This measure can serve as a guide for discharging patients the same day, with instructions to return if they develop any symptoms of hypocalcemia.
The use of early PTH also helped to reduce hospital admissions and identified patients who were eligible for same-day discharge with no need for additional replacement medications, Dr. Youssef said.
So far, “we have had no readmissions for thyroidectomy patients since we started to follow this protocol at our institution,” he noted.
The findings were limited by the relatively small sample size, and more research is needed. However, the results suggest that early measurement of PTH at 1 hour after surgery is an accurate predictor of hypocalcemia in total thyroidectomy patients.
“I strongly recommend high thyroidectomy volume institutions apply the same protocol and publish their data about that so we can come up with a consensus/guideline for management of calcium following thyroidectomy,” Dr. Youssef said.
More proof of PTH’s predictive power
“The utility of postoperative PTH for predicting symptomatic hypocalcemia is beneficial for guiding postoperative management of patients following total thyroidectomy,” said Larissa Sweeny, MD, of the University of Miami, who served as a moderator for the session in which the study was presented.
“Proper identification of patients that require supplemental medications following surgery reduces administration of medications to patients that do not require supplemental medications,” Dr. Sweeny said in an interview.
In addition, better identification not only ensures that the patients who do require supplemental medications receive them but also reduces postoperative complications and readmissions, she said.
For clinical practice, the current study “reinforces the utility of postoperative PTH lab values for guiding medication administration following total thyroidectomy,” said Dr. Sweeny. “I have been using postoperative PTH lab values following total thyroidectomy to guide my postoperative management of these patients for over 6 years.”
However, looking ahead to additional research, “Correlation with dosage of supplemental calcium and duration to return of normal PTH would be helpful information,” Dr. Sweeny said.
The study received no outside funding. The researchers and Dr. Sweeny report no relevant financial relationships.
A version of this article appeared on Medscape.com.
according to the results of a prospective study of 60 patients.
Postthyroidectomy hypocalcemia remains a major complication in patients who have undergone total thyroidectomy, and early identification can reduce disease burden and improve outcomes, according to Ahmed Sobhy Youssef, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, and colleagues.
In a presentation at the annual meeting of the American Academy of Otolaryngology-Head and Neck Surgery, Dr. Youssef presented results of the study, which looked at early postoperative parathyroid hormone as a predictor of postthyroidectomy hypocalcemia.
During his fellowship in Oklahoma in the wake of the COVID-19 pandemic, Dr. Youssef observed a wide variation in follow-up for calcium levels after thyroidectomy. “Some surgeons will order PTH and ionized calcium 4 hours after surgery, others would order later, at 6-8 hours,” he said in an interview. However, “all patients would be admitted for 1-2 nights [before being] discharged home, which meant more restrictions on the number of beds allowed for our head and neck cancer service.”
Discussion with his department chair led to a literature review seeking strategies to discharge patients earlier, and Dr. Youssef developed the idea for early PTH testing.
The study population included 60 adults who underwent thyroidectomy for benign or malignant disease at a single center between January 2022 and January 2023. The researchers measured PTH at 1 hour after surgery and compared it to results of a standard postoperative measure at 4 hours after surgery.
The researchers found a significant positive correlation between PTH measured 1 hour after surgery and ionized calcium (Ca) at 4 hours. The sensitivity of the early PTH assay, defined as “measured below 14 pg/ml,” was 100% to detect hypocalcemia, with an area under the curve of 0.797.
“The results were amazing,” said Dr. Youssef. “We found that when we measure PTH as early as 1 hour after total thyroidectomy, while patients are still in recovery, PTH was very sensitive to predict hypocalcemia.” The correlation was strong with measures at 4 hours.
“Our takeaway message is the 1-hour level PTH is very reliable in predicting hypocalcemia,” he added. This measure can serve as a guide for discharging patients the same day, with instructions to return if they develop any symptoms of hypocalcemia.
The use of early PTH also helped to reduce hospital admissions and identified patients who were eligible for same-day discharge with no need for additional replacement medications, Dr. Youssef said.
So far, “we have had no readmissions for thyroidectomy patients since we started to follow this protocol at our institution,” he noted.
The findings were limited by the relatively small sample size, and more research is needed. However, the results suggest that early measurement of PTH at 1 hour after surgery is an accurate predictor of hypocalcemia in total thyroidectomy patients.
“I strongly recommend high thyroidectomy volume institutions apply the same protocol and publish their data about that so we can come up with a consensus/guideline for management of calcium following thyroidectomy,” Dr. Youssef said.
More proof of PTH’s predictive power
“The utility of postoperative PTH for predicting symptomatic hypocalcemia is beneficial for guiding postoperative management of patients following total thyroidectomy,” said Larissa Sweeny, MD, of the University of Miami, who served as a moderator for the session in which the study was presented.
“Proper identification of patients that require supplemental medications following surgery reduces administration of medications to patients that do not require supplemental medications,” Dr. Sweeny said in an interview.
In addition, better identification not only ensures that the patients who do require supplemental medications receive them but also reduces postoperative complications and readmissions, she said.
For clinical practice, the current study “reinforces the utility of postoperative PTH lab values for guiding medication administration following total thyroidectomy,” said Dr. Sweeny. “I have been using postoperative PTH lab values following total thyroidectomy to guide my postoperative management of these patients for over 6 years.”
However, looking ahead to additional research, “Correlation with dosage of supplemental calcium and duration to return of normal PTH would be helpful information,” Dr. Sweeny said.
The study received no outside funding. The researchers and Dr. Sweeny report no relevant financial relationships.
A version of this article appeared on Medscape.com.
according to the results of a prospective study of 60 patients.
Postthyroidectomy hypocalcemia remains a major complication in patients who have undergone total thyroidectomy, and early identification can reduce disease burden and improve outcomes, according to Ahmed Sobhy Youssef, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, and colleagues.
In a presentation at the annual meeting of the American Academy of Otolaryngology-Head and Neck Surgery, Dr. Youssef presented results of the study, which looked at early postoperative parathyroid hormone as a predictor of postthyroidectomy hypocalcemia.
During his fellowship in Oklahoma in the wake of the COVID-19 pandemic, Dr. Youssef observed a wide variation in follow-up for calcium levels after thyroidectomy. “Some surgeons will order PTH and ionized calcium 4 hours after surgery, others would order later, at 6-8 hours,” he said in an interview. However, “all patients would be admitted for 1-2 nights [before being] discharged home, which meant more restrictions on the number of beds allowed for our head and neck cancer service.”
Discussion with his department chair led to a literature review seeking strategies to discharge patients earlier, and Dr. Youssef developed the idea for early PTH testing.
The study population included 60 adults who underwent thyroidectomy for benign or malignant disease at a single center between January 2022 and January 2023. The researchers measured PTH at 1 hour after surgery and compared it to results of a standard postoperative measure at 4 hours after surgery.
The researchers found a significant positive correlation between PTH measured 1 hour after surgery and ionized calcium (Ca) at 4 hours. The sensitivity of the early PTH assay, defined as “measured below 14 pg/ml,” was 100% to detect hypocalcemia, with an area under the curve of 0.797.
“The results were amazing,” said Dr. Youssef. “We found that when we measure PTH as early as 1 hour after total thyroidectomy, while patients are still in recovery, PTH was very sensitive to predict hypocalcemia.” The correlation was strong with measures at 4 hours.
“Our takeaway message is the 1-hour level PTH is very reliable in predicting hypocalcemia,” he added. This measure can serve as a guide for discharging patients the same day, with instructions to return if they develop any symptoms of hypocalcemia.
The use of early PTH also helped to reduce hospital admissions and identified patients who were eligible for same-day discharge with no need for additional replacement medications, Dr. Youssef said.
So far, “we have had no readmissions for thyroidectomy patients since we started to follow this protocol at our institution,” he noted.
The findings were limited by the relatively small sample size, and more research is needed. However, the results suggest that early measurement of PTH at 1 hour after surgery is an accurate predictor of hypocalcemia in total thyroidectomy patients.
“I strongly recommend high thyroidectomy volume institutions apply the same protocol and publish their data about that so we can come up with a consensus/guideline for management of calcium following thyroidectomy,” Dr. Youssef said.
More proof of PTH’s predictive power
“The utility of postoperative PTH for predicting symptomatic hypocalcemia is beneficial for guiding postoperative management of patients following total thyroidectomy,” said Larissa Sweeny, MD, of the University of Miami, who served as a moderator for the session in which the study was presented.
“Proper identification of patients that require supplemental medications following surgery reduces administration of medications to patients that do not require supplemental medications,” Dr. Sweeny said in an interview.
In addition, better identification not only ensures that the patients who do require supplemental medications receive them but also reduces postoperative complications and readmissions, she said.
For clinical practice, the current study “reinforces the utility of postoperative PTH lab values for guiding medication administration following total thyroidectomy,” said Dr. Sweeny. “I have been using postoperative PTH lab values following total thyroidectomy to guide my postoperative management of these patients for over 6 years.”
However, looking ahead to additional research, “Correlation with dosage of supplemental calcium and duration to return of normal PTH would be helpful information,” Dr. Sweeny said.
The study received no outside funding. The researchers and Dr. Sweeny report no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM AAO-HNS ANNUAL MEETING
New cancer survival calculator focuses on oral cancer
This represents the first cancer survival calculator that provides “personalized estimates of the likelihood of surviving or dying from oral cancer or other causes,” according to the experts who developed the tool.
An analysis evaluating the new calculator revealed that people with oral cancer are more likely to die from other causes, compared with their peers without oral cancer, and that noncancer survival worsens with cancer stage.
With its unique design, the calculator “represents perhaps one of the most sophisticated and comprehensive tools to date by integrating multiple population-level data sources to account for general health status [and] disease exposures,” such as alcohol and tobacco, socioeconomic status, and coexisting conditions, the authors of an accompanying commentary wrote.
This calculator may just be the beginning. The broader aim of developing the tool, the study authors explained, is for this new calculator approach to be “applicable for developing future prognostic models of cancer and noncancer aspects of a person’s health in other cancers.”
The analysis was published in JAMA Otolaryngology–Head and Neck Surgery.
When assessing survival, factors such as cancer stage and tumor size are key, but comorbidities also play a crucial role. For oral cancer in particular, where alcohol and tobacco use are notorious risk factors, comorbidities occur frequently and are often serious.
To create a model that provides more “holistic and personalized” estimates and includes a host of factors that can affect the risk of death, the authors tapped into data from the Surveillance, Epidemiology, and End Results database to develop the SEER Oral Cancer Survival Calculator.
Alongside data from the SEER database, the calculator used data from the National Health Interview Survey’s Longitudinal Mortality Files to obtain estimates of general health status, life expectancy without cancer, and the probability of dying from the cancer or from other causes within 1-10 years among people with newly diagnosed oral cancer.
Overall, the data included 22,392 patients, aged 20-94, with oral squamous cell carcinoma, 60.5% of whom were male and 78% White, as well as 402,626 interviewees from the survey. The calculator did not include patients with tonsil- or tongue-based cancers, which were not considered anatomically part of the oral cavity.
The most common conditions coexisting with oral cancer were diabetes and chronic obstructive pulmonary disease among older patients. Among those with oral cancer, more than half (52.8%) had none of the major coexisting conditions, which also included peripheral and cerebrovascular disease, compared with 80% of the Medicare population.
The researchers described and validated four models – one that estimated the probability of death due to oral cancer, and then three others that estimated the probability of death from other causes, with variations based on the specific data and covariates included.
Overall, the models in the calculator estimated that patients with oral cancer have a higher risk of death from other causes, compared with the general population, and survival estimates for noncancer causes got worse with more advanced cancer stage.
For instance, for a patient diagnosed with stage 3 oral cancer after age 50, the chances of being alive at age 70 were 60% for females and 44% for males in the absence of cancer, whereas the corresponding survival estimates in the general U.S. population were 86% for females and 79% for males – an absolute difference of 26 and 35 percentage points.
One key reason for this trend is that patients with later-stage cancers likely also have more coexisting health conditions, first author Louise Davies, MD, from the Geisel School of Medicine at Dartmouth, Lebanon, N.H., explained.
Another reason: For cancers with low enough mortality rates, people might be more likely to die from causes other than their cancer. This can also occur in ductal carcinoma in situ breast cancer or papillary thyroid cancer, noted Dr. Davies, also from the Department of Veterans Affairs Medical Center, White River Junction, Vt.
Commenting on the study, Eric Moore, MD, a head and neck surgeon with the Mayo Clinic in Rochester, Minn., said that while such prediction tools are important, they also come with caveats.
“I think these calculators are helpful and certainly having them widely available to people gives them another piece of knowledge that can be powerful,” he told this news organization. “But you want to make sure you don’t interpret them as the end-all, be-all message, because there are an infinite number of variables that could influence survival that aren’t available in some of these datasets.”
Neil D. Gross, MD, a professor of head and neck surgery at the University of Texas MD Anderson Cancer Center, Houston, agreed. Although this new calculator uses a large dataset, such tools “can be imperfect” and some factors simply can’t be calculated, such as a person’s priorities, Dr. Gross said.
That’s why there’s no substitute for having a “very personal discussion between a patient and a physician to decide what’s best.” And this calculator is just one tool to help with that process, Dr. Gross said.
The commentary authors echoed these sentiments. “This calculator can potentially bridge the gaps between the survival estimates in the literature, life tables, clinical gestalt, and physician attempts to contextualize the inherent limitations of applying survival curves and averages to the one patient with the diagnosis,” wrote Leila J. Mady, MD, PhD, MPH, Wayne M. Koch, MD, and Carole Fakhry, MD, MPH, all from Johns Hopkins School of Medicine, Baltimore.
But a caveat in providing such predictions is the possible psychological effect the news can have.
“Potential risks of revealing personalized prognostic survival estimates to patients include increased anxiety and distress surrounding competing causes of death [and] misinterpretation of data,” the commentary authors cautioned, adding that “we must present such information with grace and sensitivity.”
Dr. Davies recommends that clinicians ask patients what they want to know because that will vary by patient and potentially over time for the same patient.
“People are more than their cancer diagnosis,” said Dr. Davies. “Giving them the opportunity to consider their life as a whole is the aim.”
The oral cancer calculator can be publicly accessed through the National Cancer Institute. The study was supported by the Department of Veterans Affairs and the National Cancer Institute as part of an interagency agreement. The authors report no relevant financial relationships.
A version of this article appeared on Medscape.com.
This represents the first cancer survival calculator that provides “personalized estimates of the likelihood of surviving or dying from oral cancer or other causes,” according to the experts who developed the tool.
An analysis evaluating the new calculator revealed that people with oral cancer are more likely to die from other causes, compared with their peers without oral cancer, and that noncancer survival worsens with cancer stage.
With its unique design, the calculator “represents perhaps one of the most sophisticated and comprehensive tools to date by integrating multiple population-level data sources to account for general health status [and] disease exposures,” such as alcohol and tobacco, socioeconomic status, and coexisting conditions, the authors of an accompanying commentary wrote.
This calculator may just be the beginning. The broader aim of developing the tool, the study authors explained, is for this new calculator approach to be “applicable for developing future prognostic models of cancer and noncancer aspects of a person’s health in other cancers.”
The analysis was published in JAMA Otolaryngology–Head and Neck Surgery.
When assessing survival, factors such as cancer stage and tumor size are key, but comorbidities also play a crucial role. For oral cancer in particular, where alcohol and tobacco use are notorious risk factors, comorbidities occur frequently and are often serious.
To create a model that provides more “holistic and personalized” estimates and includes a host of factors that can affect the risk of death, the authors tapped into data from the Surveillance, Epidemiology, and End Results database to develop the SEER Oral Cancer Survival Calculator.
Alongside data from the SEER database, the calculator used data from the National Health Interview Survey’s Longitudinal Mortality Files to obtain estimates of general health status, life expectancy without cancer, and the probability of dying from the cancer or from other causes within 1-10 years among people with newly diagnosed oral cancer.
Overall, the data included 22,392 patients, aged 20-94, with oral squamous cell carcinoma, 60.5% of whom were male and 78% White, as well as 402,626 interviewees from the survey. The calculator did not include patients with tonsil- or tongue-based cancers, which were not considered anatomically part of the oral cavity.
The most common conditions coexisting with oral cancer were diabetes and chronic obstructive pulmonary disease among older patients. Among those with oral cancer, more than half (52.8%) had none of the major coexisting conditions, which also included peripheral and cerebrovascular disease, compared with 80% of the Medicare population.
The researchers described and validated four models – one that estimated the probability of death due to oral cancer, and then three others that estimated the probability of death from other causes, with variations based on the specific data and covariates included.
Overall, the models in the calculator estimated that patients with oral cancer have a higher risk of death from other causes, compared with the general population, and survival estimates for noncancer causes got worse with more advanced cancer stage.
For instance, for a patient diagnosed with stage 3 oral cancer after age 50, the chances of being alive at age 70 were 60% for females and 44% for males in the absence of cancer, whereas the corresponding survival estimates in the general U.S. population were 86% for females and 79% for males – an absolute difference of 26 and 35 percentage points.
One key reason for this trend is that patients with later-stage cancers likely also have more coexisting health conditions, first author Louise Davies, MD, from the Geisel School of Medicine at Dartmouth, Lebanon, N.H., explained.
Another reason: For cancers with low enough mortality rates, people might be more likely to die from causes other than their cancer. This can also occur in ductal carcinoma in situ breast cancer or papillary thyroid cancer, noted Dr. Davies, also from the Department of Veterans Affairs Medical Center, White River Junction, Vt.
Commenting on the study, Eric Moore, MD, a head and neck surgeon with the Mayo Clinic in Rochester, Minn., said that while such prediction tools are important, they also come with caveats.
“I think these calculators are helpful and certainly having them widely available to people gives them another piece of knowledge that can be powerful,” he told this news organization. “But you want to make sure you don’t interpret them as the end-all, be-all message, because there are an infinite number of variables that could influence survival that aren’t available in some of these datasets.”
Neil D. Gross, MD, a professor of head and neck surgery at the University of Texas MD Anderson Cancer Center, Houston, agreed. Although this new calculator uses a large dataset, such tools “can be imperfect” and some factors simply can’t be calculated, such as a person’s priorities, Dr. Gross said.
That’s why there’s no substitute for having a “very personal discussion between a patient and a physician to decide what’s best.” And this calculator is just one tool to help with that process, Dr. Gross said.
The commentary authors echoed these sentiments. “This calculator can potentially bridge the gaps between the survival estimates in the literature, life tables, clinical gestalt, and physician attempts to contextualize the inherent limitations of applying survival curves and averages to the one patient with the diagnosis,” wrote Leila J. Mady, MD, PhD, MPH, Wayne M. Koch, MD, and Carole Fakhry, MD, MPH, all from Johns Hopkins School of Medicine, Baltimore.
But a caveat in providing such predictions is the possible psychological effect the news can have.
“Potential risks of revealing personalized prognostic survival estimates to patients include increased anxiety and distress surrounding competing causes of death [and] misinterpretation of data,” the commentary authors cautioned, adding that “we must present such information with grace and sensitivity.”
Dr. Davies recommends that clinicians ask patients what they want to know because that will vary by patient and potentially over time for the same patient.
“People are more than their cancer diagnosis,” said Dr. Davies. “Giving them the opportunity to consider their life as a whole is the aim.”
The oral cancer calculator can be publicly accessed through the National Cancer Institute. The study was supported by the Department of Veterans Affairs and the National Cancer Institute as part of an interagency agreement. The authors report no relevant financial relationships.
A version of this article appeared on Medscape.com.
This represents the first cancer survival calculator that provides “personalized estimates of the likelihood of surviving or dying from oral cancer or other causes,” according to the experts who developed the tool.
An analysis evaluating the new calculator revealed that people with oral cancer are more likely to die from other causes, compared with their peers without oral cancer, and that noncancer survival worsens with cancer stage.
With its unique design, the calculator “represents perhaps one of the most sophisticated and comprehensive tools to date by integrating multiple population-level data sources to account for general health status [and] disease exposures,” such as alcohol and tobacco, socioeconomic status, and coexisting conditions, the authors of an accompanying commentary wrote.
This calculator may just be the beginning. The broader aim of developing the tool, the study authors explained, is for this new calculator approach to be “applicable for developing future prognostic models of cancer and noncancer aspects of a person’s health in other cancers.”
The analysis was published in JAMA Otolaryngology–Head and Neck Surgery.
When assessing survival, factors such as cancer stage and tumor size are key, but comorbidities also play a crucial role. For oral cancer in particular, where alcohol and tobacco use are notorious risk factors, comorbidities occur frequently and are often serious.
To create a model that provides more “holistic and personalized” estimates and includes a host of factors that can affect the risk of death, the authors tapped into data from the Surveillance, Epidemiology, and End Results database to develop the SEER Oral Cancer Survival Calculator.
Alongside data from the SEER database, the calculator used data from the National Health Interview Survey’s Longitudinal Mortality Files to obtain estimates of general health status, life expectancy without cancer, and the probability of dying from the cancer or from other causes within 1-10 years among people with newly diagnosed oral cancer.
Overall, the data included 22,392 patients, aged 20-94, with oral squamous cell carcinoma, 60.5% of whom were male and 78% White, as well as 402,626 interviewees from the survey. The calculator did not include patients with tonsil- or tongue-based cancers, which were not considered anatomically part of the oral cavity.
The most common conditions coexisting with oral cancer were diabetes and chronic obstructive pulmonary disease among older patients. Among those with oral cancer, more than half (52.8%) had none of the major coexisting conditions, which also included peripheral and cerebrovascular disease, compared with 80% of the Medicare population.
The researchers described and validated four models – one that estimated the probability of death due to oral cancer, and then three others that estimated the probability of death from other causes, with variations based on the specific data and covariates included.
Overall, the models in the calculator estimated that patients with oral cancer have a higher risk of death from other causes, compared with the general population, and survival estimates for noncancer causes got worse with more advanced cancer stage.
For instance, for a patient diagnosed with stage 3 oral cancer after age 50, the chances of being alive at age 70 were 60% for females and 44% for males in the absence of cancer, whereas the corresponding survival estimates in the general U.S. population were 86% for females and 79% for males – an absolute difference of 26 and 35 percentage points.
One key reason for this trend is that patients with later-stage cancers likely also have more coexisting health conditions, first author Louise Davies, MD, from the Geisel School of Medicine at Dartmouth, Lebanon, N.H., explained.
Another reason: For cancers with low enough mortality rates, people might be more likely to die from causes other than their cancer. This can also occur in ductal carcinoma in situ breast cancer or papillary thyroid cancer, noted Dr. Davies, also from the Department of Veterans Affairs Medical Center, White River Junction, Vt.
Commenting on the study, Eric Moore, MD, a head and neck surgeon with the Mayo Clinic in Rochester, Minn., said that while such prediction tools are important, they also come with caveats.
“I think these calculators are helpful and certainly having them widely available to people gives them another piece of knowledge that can be powerful,” he told this news organization. “But you want to make sure you don’t interpret them as the end-all, be-all message, because there are an infinite number of variables that could influence survival that aren’t available in some of these datasets.”
Neil D. Gross, MD, a professor of head and neck surgery at the University of Texas MD Anderson Cancer Center, Houston, agreed. Although this new calculator uses a large dataset, such tools “can be imperfect” and some factors simply can’t be calculated, such as a person’s priorities, Dr. Gross said.
That’s why there’s no substitute for having a “very personal discussion between a patient and a physician to decide what’s best.” And this calculator is just one tool to help with that process, Dr. Gross said.
The commentary authors echoed these sentiments. “This calculator can potentially bridge the gaps between the survival estimates in the literature, life tables, clinical gestalt, and physician attempts to contextualize the inherent limitations of applying survival curves and averages to the one patient with the diagnosis,” wrote Leila J. Mady, MD, PhD, MPH, Wayne M. Koch, MD, and Carole Fakhry, MD, MPH, all from Johns Hopkins School of Medicine, Baltimore.
But a caveat in providing such predictions is the possible psychological effect the news can have.
“Potential risks of revealing personalized prognostic survival estimates to patients include increased anxiety and distress surrounding competing causes of death [and] misinterpretation of data,” the commentary authors cautioned, adding that “we must present such information with grace and sensitivity.”
Dr. Davies recommends that clinicians ask patients what they want to know because that will vary by patient and potentially over time for the same patient.
“People are more than their cancer diagnosis,” said Dr. Davies. “Giving them the opportunity to consider their life as a whole is the aim.”
The oral cancer calculator can be publicly accessed through the National Cancer Institute. The study was supported by the Department of Veterans Affairs and the National Cancer Institute as part of an interagency agreement. The authors report no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM JAMA OTOLARYNGOLOGY–HEAD AND NECK SURGERY
Parathyroidectomy does not preserve kidney function in seniors
Early parathyroidectomy within 1 year of diagnosis of primary hyperparathyroidism (PHPT) did not reduce the risk of a sustained decline in kidney function, measured by a decline in estimated glomerular filtration rate (eGFR) of at least 50%, compared with observation (no surgery) in adults aged 60 and older.
Early parathyroidectomy was, however, associated with a reduced adjusted risk of this decline in kidney function in patients with newly diagnosed PHPT who were younger than age 60.
The findings, based on data from close to 43,000 veterans, were published online in Annals of Internal Medicine.
“The important takeaway from our study is that for older adults [age 60 or older] with primary hyperparathyroidism, preservation of kidney function should not be a primary consideration when making decisions about whether to undergo parathyroidectomy,” lead author Carolyn D. Seib, MD, told this news organization.
“It is important that physicians also discuss with their patients the potential long-term benefits of parathyroidectomy related to a reduced risk of fractures, kidney stones, and cardiovascular disease, and improved quality of life, in addition to the need for lifelong surveillance if surgery is declined, weighing these against an individual patient’s risk of surgery,” said Dr. Seib, a surgeon at Palo Alto (Calif.) VA Medical Center.
“However, in patients younger than 60, early parathyroidectomy may prevent progression to chronic kidney disease (CKD) and should be more strongly considered,” she noted.
Parathyroidectomy, she observed, is a low-risk outpatient surgery for most adults.
“Potential complications of surgery include temporary or permanent hoarseness, hypoparathyroidism (low postoperative parathyroid function), bleeding requiring return to the operating room, and complications related to general anesthesia, all of which are rare,” said Dr. Seib.
“Surgery by a high-volume surgeon is associated with a reduced risk of complications, so patients should seek out an experienced parathyroid surgeon,” she emphasized.
Moreover, parathyroidectomy is the only treatment for primary hyperparathyroidism.
Does parathyroidectomy slow loss of kidney function?
Multidisciplinary guidelines recommend parathyroidectomy, at least in part to mitigate the risk for, and effects related to, the progression of CKD in patients with PHPT and an eGFR below 60 mL/min per 1.73 m2, the researchers wrote.
However, whether parathyroidectomy slows the loss of kidney function in adults with PHPT is not clear.
Guidelines also state that “observation for PHPT disease progression can be considered when patients have no obvious end organ damage (i.e., eGFR > 60 mL/min per 1.73 m2, normal bone mineral density, and no history of kidney stones or fractures),” Dr. Seib noted.
To address the evidence gap, the researchers emulated a randomized target trial using observational data.
In this type of study, Dr. Seib explained, “although patients aren’t randomly assigned to a treatment, complex statistical methods are used to adjust for baseline confounders in an attempt to emulate random treatment assignment and account for bias that may affect the timing of when patients receive treatment.”
Using national Veterans Health Administration data, researchers identified 43,697 veterans with a new biochemical diagnosis of PHPT, defined as elevated parathyroid hormone (> 65 ng/mL) within 6 months of an elevated serum calcium level (> 32.55 mmol/L or >10.2 mg/dL), from 2000 to 2019.
Of these patients, 3,804 underwent parathyroidectomy within 1 year of diagnosis of PHPT, and 39,893 did not, and instead, a watchful waiting approach was adopted.
To be included in the analysis, patients had to have an eGFR above 30 mL/min per 1.73 m2 for 12 months before PHPT diagnosis to exclude secondary or tertiary hyperparathyroidism.
The primary outcome was a sustained decline in eGFR of at least 50% from baseline.
In the overall cohort, patients had a mean pretreatment eGFR of 71.8 mL/min per 1.73 m2. The mean age of patients was 67, 88% were men, and 68% were White.
After a median follow-up of 4.9 years, 6.7% of the patients had a decline in eGFR of at least 50%.
The cumulative incidence of this decline in eGFR was 5.1% at 5 years and 10.8% at 10 years in patients who had had early parathyroidectomy compared with 5.1% and 12.0%, respectively, in patients who did not undergo surgery.
In the overall population, the risk of at least a 50% decline in eGFR was similar in the early parathyroidectomy group versus the observation group (adjusted hazard ratio [HR], 0.98, 95% confidence interval [CI], 0.82-1.16).
However, diving deeper showed that parathyroidectomy was associated with a reduced risk of the primary outcome among patients younger than 60 years (adjusted HR, 0.75, 95% CI, 0.59-0.93) but not among those aged 60 or older (adjusted HR, 1.08, 95% CI, 0.87-1.34).
“When participating in shared decision-making for older adults [age 60 and older] with PHPT, clinicians should not consider parathyroidectomy for potential benefits of preservation of kidney function,” the researchers reiterated.
“For younger patients, clinicians should discuss the potential benefit of parathyroidectomy to reduce the risk for CKD and associated complications in adults with PHPT,” they concluded.
The study was funded by the National Institute on Aging. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Early parathyroidectomy within 1 year of diagnosis of primary hyperparathyroidism (PHPT) did not reduce the risk of a sustained decline in kidney function, measured by a decline in estimated glomerular filtration rate (eGFR) of at least 50%, compared with observation (no surgery) in adults aged 60 and older.
Early parathyroidectomy was, however, associated with a reduced adjusted risk of this decline in kidney function in patients with newly diagnosed PHPT who were younger than age 60.
The findings, based on data from close to 43,000 veterans, were published online in Annals of Internal Medicine.
“The important takeaway from our study is that for older adults [age 60 or older] with primary hyperparathyroidism, preservation of kidney function should not be a primary consideration when making decisions about whether to undergo parathyroidectomy,” lead author Carolyn D. Seib, MD, told this news organization.
“It is important that physicians also discuss with their patients the potential long-term benefits of parathyroidectomy related to a reduced risk of fractures, kidney stones, and cardiovascular disease, and improved quality of life, in addition to the need for lifelong surveillance if surgery is declined, weighing these against an individual patient’s risk of surgery,” said Dr. Seib, a surgeon at Palo Alto (Calif.) VA Medical Center.
“However, in patients younger than 60, early parathyroidectomy may prevent progression to chronic kidney disease (CKD) and should be more strongly considered,” she noted.
Parathyroidectomy, she observed, is a low-risk outpatient surgery for most adults.
“Potential complications of surgery include temporary or permanent hoarseness, hypoparathyroidism (low postoperative parathyroid function), bleeding requiring return to the operating room, and complications related to general anesthesia, all of which are rare,” said Dr. Seib.
“Surgery by a high-volume surgeon is associated with a reduced risk of complications, so patients should seek out an experienced parathyroid surgeon,” she emphasized.
Moreover, parathyroidectomy is the only treatment for primary hyperparathyroidism.
Does parathyroidectomy slow loss of kidney function?
Multidisciplinary guidelines recommend parathyroidectomy, at least in part to mitigate the risk for, and effects related to, the progression of CKD in patients with PHPT and an eGFR below 60 mL/min per 1.73 m2, the researchers wrote.
However, whether parathyroidectomy slows the loss of kidney function in adults with PHPT is not clear.
Guidelines also state that “observation for PHPT disease progression can be considered when patients have no obvious end organ damage (i.e., eGFR > 60 mL/min per 1.73 m2, normal bone mineral density, and no history of kidney stones or fractures),” Dr. Seib noted.
To address the evidence gap, the researchers emulated a randomized target trial using observational data.
In this type of study, Dr. Seib explained, “although patients aren’t randomly assigned to a treatment, complex statistical methods are used to adjust for baseline confounders in an attempt to emulate random treatment assignment and account for bias that may affect the timing of when patients receive treatment.”
Using national Veterans Health Administration data, researchers identified 43,697 veterans with a new biochemical diagnosis of PHPT, defined as elevated parathyroid hormone (> 65 ng/mL) within 6 months of an elevated serum calcium level (> 32.55 mmol/L or >10.2 mg/dL), from 2000 to 2019.
Of these patients, 3,804 underwent parathyroidectomy within 1 year of diagnosis of PHPT, and 39,893 did not, and instead, a watchful waiting approach was adopted.
To be included in the analysis, patients had to have an eGFR above 30 mL/min per 1.73 m2 for 12 months before PHPT diagnosis to exclude secondary or tertiary hyperparathyroidism.
The primary outcome was a sustained decline in eGFR of at least 50% from baseline.
In the overall cohort, patients had a mean pretreatment eGFR of 71.8 mL/min per 1.73 m2. The mean age of patients was 67, 88% were men, and 68% were White.
After a median follow-up of 4.9 years, 6.7% of the patients had a decline in eGFR of at least 50%.
The cumulative incidence of this decline in eGFR was 5.1% at 5 years and 10.8% at 10 years in patients who had had early parathyroidectomy compared with 5.1% and 12.0%, respectively, in patients who did not undergo surgery.
In the overall population, the risk of at least a 50% decline in eGFR was similar in the early parathyroidectomy group versus the observation group (adjusted hazard ratio [HR], 0.98, 95% confidence interval [CI], 0.82-1.16).
However, diving deeper showed that parathyroidectomy was associated with a reduced risk of the primary outcome among patients younger than 60 years (adjusted HR, 0.75, 95% CI, 0.59-0.93) but not among those aged 60 or older (adjusted HR, 1.08, 95% CI, 0.87-1.34).
“When participating in shared decision-making for older adults [age 60 and older] with PHPT, clinicians should not consider parathyroidectomy for potential benefits of preservation of kidney function,” the researchers reiterated.
“For younger patients, clinicians should discuss the potential benefit of parathyroidectomy to reduce the risk for CKD and associated complications in adults with PHPT,” they concluded.
The study was funded by the National Institute on Aging. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Early parathyroidectomy within 1 year of diagnosis of primary hyperparathyroidism (PHPT) did not reduce the risk of a sustained decline in kidney function, measured by a decline in estimated glomerular filtration rate (eGFR) of at least 50%, compared with observation (no surgery) in adults aged 60 and older.
Early parathyroidectomy was, however, associated with a reduced adjusted risk of this decline in kidney function in patients with newly diagnosed PHPT who were younger than age 60.
The findings, based on data from close to 43,000 veterans, were published online in Annals of Internal Medicine.
“The important takeaway from our study is that for older adults [age 60 or older] with primary hyperparathyroidism, preservation of kidney function should not be a primary consideration when making decisions about whether to undergo parathyroidectomy,” lead author Carolyn D. Seib, MD, told this news organization.
“It is important that physicians also discuss with their patients the potential long-term benefits of parathyroidectomy related to a reduced risk of fractures, kidney stones, and cardiovascular disease, and improved quality of life, in addition to the need for lifelong surveillance if surgery is declined, weighing these against an individual patient’s risk of surgery,” said Dr. Seib, a surgeon at Palo Alto (Calif.) VA Medical Center.
“However, in patients younger than 60, early parathyroidectomy may prevent progression to chronic kidney disease (CKD) and should be more strongly considered,” she noted.
Parathyroidectomy, she observed, is a low-risk outpatient surgery for most adults.
“Potential complications of surgery include temporary or permanent hoarseness, hypoparathyroidism (low postoperative parathyroid function), bleeding requiring return to the operating room, and complications related to general anesthesia, all of which are rare,” said Dr. Seib.
“Surgery by a high-volume surgeon is associated with a reduced risk of complications, so patients should seek out an experienced parathyroid surgeon,” she emphasized.
Moreover, parathyroidectomy is the only treatment for primary hyperparathyroidism.
Does parathyroidectomy slow loss of kidney function?
Multidisciplinary guidelines recommend parathyroidectomy, at least in part to mitigate the risk for, and effects related to, the progression of CKD in patients with PHPT and an eGFR below 60 mL/min per 1.73 m2, the researchers wrote.
However, whether parathyroidectomy slows the loss of kidney function in adults with PHPT is not clear.
Guidelines also state that “observation for PHPT disease progression can be considered when patients have no obvious end organ damage (i.e., eGFR > 60 mL/min per 1.73 m2, normal bone mineral density, and no history of kidney stones or fractures),” Dr. Seib noted.
To address the evidence gap, the researchers emulated a randomized target trial using observational data.
In this type of study, Dr. Seib explained, “although patients aren’t randomly assigned to a treatment, complex statistical methods are used to adjust for baseline confounders in an attempt to emulate random treatment assignment and account for bias that may affect the timing of when patients receive treatment.”
Using national Veterans Health Administration data, researchers identified 43,697 veterans with a new biochemical diagnosis of PHPT, defined as elevated parathyroid hormone (> 65 ng/mL) within 6 months of an elevated serum calcium level (> 32.55 mmol/L or >10.2 mg/dL), from 2000 to 2019.
Of these patients, 3,804 underwent parathyroidectomy within 1 year of diagnosis of PHPT, and 39,893 did not, and instead, a watchful waiting approach was adopted.
To be included in the analysis, patients had to have an eGFR above 30 mL/min per 1.73 m2 for 12 months before PHPT diagnosis to exclude secondary or tertiary hyperparathyroidism.
The primary outcome was a sustained decline in eGFR of at least 50% from baseline.
In the overall cohort, patients had a mean pretreatment eGFR of 71.8 mL/min per 1.73 m2. The mean age of patients was 67, 88% were men, and 68% were White.
After a median follow-up of 4.9 years, 6.7% of the patients had a decline in eGFR of at least 50%.
The cumulative incidence of this decline in eGFR was 5.1% at 5 years and 10.8% at 10 years in patients who had had early parathyroidectomy compared with 5.1% and 12.0%, respectively, in patients who did not undergo surgery.
In the overall population, the risk of at least a 50% decline in eGFR was similar in the early parathyroidectomy group versus the observation group (adjusted hazard ratio [HR], 0.98, 95% confidence interval [CI], 0.82-1.16).
However, diving deeper showed that parathyroidectomy was associated with a reduced risk of the primary outcome among patients younger than 60 years (adjusted HR, 0.75, 95% CI, 0.59-0.93) but not among those aged 60 or older (adjusted HR, 1.08, 95% CI, 0.87-1.34).
“When participating in shared decision-making for older adults [age 60 and older] with PHPT, clinicians should not consider parathyroidectomy for potential benefits of preservation of kidney function,” the researchers reiterated.
“For younger patients, clinicians should discuss the potential benefit of parathyroidectomy to reduce the risk for CKD and associated complications in adults with PHPT,” they concluded.
The study was funded by the National Institute on Aging. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
New ESC guidelines for cutting CV risk in noncardiac surgery
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022