User login
Plasma microRNA assay differentiates colorectal neoplasia
CHICAGO – A novel plasma microRNA assay and prediction model appears to successfully differentiate colorectal neoplasia from other neoplasms and from controls.
The assay includes seven microRNAs that were selected, based on P value, area under the curve (AUC), fold change, and biological plausibility, from among 380 microRNAs screened using microfluidic array technology from a “training” cohort of 60 patients. The training cohort included groups of patients, 10 each, with colorectal cancer, advanced adenoma, breast cancer, pancreatic cancer, and lung cancer – cancers chosen because they frequently develop at similar ages as colon cancer – and 10 controls.
A panel of seven “uniquely dysregulated” microRNAs specific for colorectal neoplasia was evaluated using single assays in a “test” cohort of 120 patients. A mathematical model was developed to predict sample identity in a 150-patient blinded “validation” cohort using repeat-subsampling validation of the testing dataset with 1,000 iterations each to assess model detection accuracy, Dr. Jane V. Carter of the University of Louisville (Ky.) explained at the annual meeting of the American Surgical Association.
The area under the curve for test cohort comparisons with the assay was 0.91, 0.79, and 0.98 for comparison No. 1 (comparing any neoplasia vs. controls), comparison No. 2 (comparing colorectal neoplasia with other cancers) and comparison No. 3 (comparing colorectal cancer with colorectal adenomas) respectively, Dr. Carter reported.
“Our prediction model identified blinded sample identity with 69%-77% accuracy in comparison No. 1, 66%-76% accuracy in comparison No. 2, and 86%-90% accuracy in comparison No. 3,” she said, noting that the sensitivity and specificity of the assay compare very well with current clinical standards.
Colorectal neoplasms frequently develop in individuals at ages when other common cancers also occur. Current screening methods, including endoscopic and imaging studies and fecal testing have poor patient compliance. Fecal and blood tests lack sensitivity and specificity for the detection of adenomas, limiting their use as screening methods, she said.
But this novel assay, which builds on the earlier work identifying miR-21 as a potential marker for colorectal cancer, provides a useful tool for identifying colorectal neoplasms, she said.
Efforts are underway to confirm the findings in a larger study population. If the findings are confirmed, the assay may have other potential uses such as monitoring therapy by comparing microRNA expression before and after treatment, and also for predicting response to treatment such as following preoperative neoadjuvant chemoradiation, Dr. Carter suggested.
The current findings have significant implications for the development of a noninvasive, reliable, and reproducible screening test for detection of colorectal neoplasia.
“If we can improve early-stage detection, we can improve survival,” she said.
Dr. Carter reported having no relevant disclosures.
The complete manuscript of this presentation is anticipated to be published in the Annals of Surgery pending editorial review.
CHICAGO – A novel plasma microRNA assay and prediction model appears to successfully differentiate colorectal neoplasia from other neoplasms and from controls.
The assay includes seven microRNAs that were selected, based on P value, area under the curve (AUC), fold change, and biological plausibility, from among 380 microRNAs screened using microfluidic array technology from a “training” cohort of 60 patients. The training cohort included groups of patients, 10 each, with colorectal cancer, advanced adenoma, breast cancer, pancreatic cancer, and lung cancer – cancers chosen because they frequently develop at similar ages as colon cancer – and 10 controls.
A panel of seven “uniquely dysregulated” microRNAs specific for colorectal neoplasia was evaluated using single assays in a “test” cohort of 120 patients. A mathematical model was developed to predict sample identity in a 150-patient blinded “validation” cohort using repeat-subsampling validation of the testing dataset with 1,000 iterations each to assess model detection accuracy, Dr. Jane V. Carter of the University of Louisville (Ky.) explained at the annual meeting of the American Surgical Association.
The area under the curve for test cohort comparisons with the assay was 0.91, 0.79, and 0.98 for comparison No. 1 (comparing any neoplasia vs. controls), comparison No. 2 (comparing colorectal neoplasia with other cancers) and comparison No. 3 (comparing colorectal cancer with colorectal adenomas) respectively, Dr. Carter reported.
“Our prediction model identified blinded sample identity with 69%-77% accuracy in comparison No. 1, 66%-76% accuracy in comparison No. 2, and 86%-90% accuracy in comparison No. 3,” she said, noting that the sensitivity and specificity of the assay compare very well with current clinical standards.
Colorectal neoplasms frequently develop in individuals at ages when other common cancers also occur. Current screening methods, including endoscopic and imaging studies and fecal testing have poor patient compliance. Fecal and blood tests lack sensitivity and specificity for the detection of adenomas, limiting their use as screening methods, she said.
But this novel assay, which builds on the earlier work identifying miR-21 as a potential marker for colorectal cancer, provides a useful tool for identifying colorectal neoplasms, she said.
Efforts are underway to confirm the findings in a larger study population. If the findings are confirmed, the assay may have other potential uses such as monitoring therapy by comparing microRNA expression before and after treatment, and also for predicting response to treatment such as following preoperative neoadjuvant chemoradiation, Dr. Carter suggested.
The current findings have significant implications for the development of a noninvasive, reliable, and reproducible screening test for detection of colorectal neoplasia.
“If we can improve early-stage detection, we can improve survival,” she said.
Dr. Carter reported having no relevant disclosures.
The complete manuscript of this presentation is anticipated to be published in the Annals of Surgery pending editorial review.
CHICAGO – A novel plasma microRNA assay and prediction model appears to successfully differentiate colorectal neoplasia from other neoplasms and from controls.
The assay includes seven microRNAs that were selected, based on P value, area under the curve (AUC), fold change, and biological plausibility, from among 380 microRNAs screened using microfluidic array technology from a “training” cohort of 60 patients. The training cohort included groups of patients, 10 each, with colorectal cancer, advanced adenoma, breast cancer, pancreatic cancer, and lung cancer – cancers chosen because they frequently develop at similar ages as colon cancer – and 10 controls.
A panel of seven “uniquely dysregulated” microRNAs specific for colorectal neoplasia was evaluated using single assays in a “test” cohort of 120 patients. A mathematical model was developed to predict sample identity in a 150-patient blinded “validation” cohort using repeat-subsampling validation of the testing dataset with 1,000 iterations each to assess model detection accuracy, Dr. Jane V. Carter of the University of Louisville (Ky.) explained at the annual meeting of the American Surgical Association.
The area under the curve for test cohort comparisons with the assay was 0.91, 0.79, and 0.98 for comparison No. 1 (comparing any neoplasia vs. controls), comparison No. 2 (comparing colorectal neoplasia with other cancers) and comparison No. 3 (comparing colorectal cancer with colorectal adenomas) respectively, Dr. Carter reported.
“Our prediction model identified blinded sample identity with 69%-77% accuracy in comparison No. 1, 66%-76% accuracy in comparison No. 2, and 86%-90% accuracy in comparison No. 3,” she said, noting that the sensitivity and specificity of the assay compare very well with current clinical standards.
Colorectal neoplasms frequently develop in individuals at ages when other common cancers also occur. Current screening methods, including endoscopic and imaging studies and fecal testing have poor patient compliance. Fecal and blood tests lack sensitivity and specificity for the detection of adenomas, limiting their use as screening methods, she said.
But this novel assay, which builds on the earlier work identifying miR-21 as a potential marker for colorectal cancer, provides a useful tool for identifying colorectal neoplasms, she said.
Efforts are underway to confirm the findings in a larger study population. If the findings are confirmed, the assay may have other potential uses such as monitoring therapy by comparing microRNA expression before and after treatment, and also for predicting response to treatment such as following preoperative neoadjuvant chemoradiation, Dr. Carter suggested.
The current findings have significant implications for the development of a noninvasive, reliable, and reproducible screening test for detection of colorectal neoplasia.
“If we can improve early-stage detection, we can improve survival,” she said.
Dr. Carter reported having no relevant disclosures.
The complete manuscript of this presentation is anticipated to be published in the Annals of Surgery pending editorial review.
AT THE ASA ANNUAL MEETING
Key clinical point: A novel plasma microRNA assay and prediction model appears to successfully differentiate colorectal neoplasia from other neoplasms and from controls.
Major finding: The prediction model identified sample identity with 69%-77% accuracy when comparing any neoplasia vs. controls, 66%-76% accuracy when comparing colorectal neoplasia with other cancers, and 86%-90% accuracy when comparing colorectal cancer with colorectal adenomas.
Data source: A prediction model used in a 60-person training cohort, a 120-person testing cohort, and a 150-person validation cohort.
Disclosures: Dr. Carter reported having no relevant disclosures.
Racial disparities in colon cancer survival mainly driven by tumor stage at presentation
Although black patients with colon cancer received significantly less treatment than white patients, particularly for late stage disease, much of the overall survival disparity between black and white patients was explained by tumor presentation at diagnosis rather than treatment differences, according to an analysis of SEER data.
Among demographically matched black and white patients, the 5-year survival difference was 8.3% (P less than .0001). Presentation match reduced the difference to 5.0% (P less than .0001), which accounted for 39.8% of the overall disparity. Additional matching by treatment reduced the difference only slightly to 4.9% (P less than .0001), which accounted for 1.2% of the overall disparity. Black patients had lower rates for most treatments, including surgery, than presentation-matched white patients (88.5% vs. 91.4%), and these differences were most pronounced at advanced stages. For example, significant differences between black and white patients in the use of chemotherapy was observed for stage III (53.1% vs. 64.2%; P less than .0001) and stage IV (56.1% vs. 63.3%; P = .001).
“Our results indicate that tumor presentation, including tumor stage, is indeed one of the most important factors contributing to the racial disparity in colon cancer survival. We observed that, after controlling for demographic factors, black patients in comparison with white patients had a significantly higher proportion of stage IV and lower proportions of stages I and II disease. Adequately matching on tumor presentation variables (e.g., stage, grade, size, and comorbidity) significantly reduced survival disparities,” wrote Dr. Yinzhi Lai of the Department of Medical Oncology at Sidney Kimmel Cancer Center, Philadelphia, and colleagues (Gastroenterology. 2016 Apr 4. doi: 10.1053/j.gastro.2016.01.030).
Treatment differences in advanced-stage patients, compared with early-stage patients, explained a higher proportion of the demographic-matched survival disparity. For example, in stage II patients, treatment match resulted in modest reductions in 2-, 3-, and 5-year survival rate disparities (2.7%-2.8%, 4.1%-3.6%, and 4.6%-4.0%, respectively); by contrast, in stage III patients, treatment match resulted in more substantial reductions in 2-, 3-, and 5-year survival rate disparities (4.5%-2.2%, 3.1%-2.0%, and 4.3%-2.8%, respectively). A similar effect was observed in patients with stage IV disease. The results suggest that, “to control survival disparity, more efforts may need to be tailored to minimize treatment disparities (especially chemotherapy use) in patients with advanced-stage disease,” the investigators wrote.
The retrospective data analysis used patient information from 68,141 patients (6,190 black, 61,951 white) aged 66 years and older with colon cancer identified from the National Cancer Institute SEER-Medicare database. Using a novel minimum distance matching strategy, investigators drew from the pool of white patients to match three distinct comparison cohorts to the same 6,190 black patients. Close matches between black and white patients bypassed the need for model-based analysis.
The primary matching analysis was limited by the inability to control for substantial differences in socioeconomic status, marital status, and urban/rural residence. A subcohort analysis of 2,000 matched black and white patients showed that when socioeconomic status was added to the demographic match, survival differences were reduced, indicating the important role of socioeconomic status on racial survival disparities.
Significantly better survival was observed in all patients who were diagnosed in 2004 or later, the year the Food and Drug Administration approved the important chemotherapy medicines oxaliplatin and bevacizumab. Separating the cohorts into those who were diagnosed before and after 2004 revealed that the racial survival disparity was lower in the more recent group, indicating a favorable impact of oxaliplatin and/or bevacizumab in reducing the survival disparity.
Prior studies have documented racial disparities in the incidence and outcomes of colon cancer in the United States. Black men and women have a higher overall incidence and more advanced stage of disease at diagnosis than white men and women, while being less likely to receive guideline-concordant treatment.

|
| Dr. Jennifer Lund |
To extend this work, the authors evaluated treatment disparities between black and white colon cancer patients aged 66 years and older and examined the impact of a variety of patient characteristics on racial disparities in overall survival using a novel, sequential matching algorithm that minimized the overall distance between black and white patients based on demographic-, tumor specific–, and treatment-related variables. The authors found that differences in overall survival were mainly driven by tumor presentation; however, advanced-stage black colon cancer patients received less guideline concordant-treatment than white patients. While this minimum-distance algorithm provided close black-white matches on prespecified factors, it could not accommodate other factors (for example, socioeconomic, marital, and urban/rural status); therefore, methodologic improvements to this method and comparisons to other commonly used approaches (that is, propensity score matching and weighting) are warranted.
Finally, these results apply to older black and white colon cancer patients with Medicare fee-for-service coverage only. Additional research using similar methods in older Medicare Advantage populations or younger adults may uncover unique drivers of overall survival disparities by race, which may require tailored interventions.
Jennifer L. Lund, Ph.D., is an assistant professor, department of epidemiology, University of North Carolina at Chapel Hill. She receives research support from the UNC Oncology Clinical Translational Research Training Program (K12 CA120780), as well as through a Research Starter Award from the PhRMA Foundation to the UNC Department of Epidemiology.
Prior studies have documented racial disparities in the incidence and outcomes of colon cancer in the United States. Black men and women have a higher overall incidence and more advanced stage of disease at diagnosis than white men and women, while being less likely to receive guideline-concordant treatment.

|
| Dr. Jennifer Lund |
To extend this work, the authors evaluated treatment disparities between black and white colon cancer patients aged 66 years and older and examined the impact of a variety of patient characteristics on racial disparities in overall survival using a novel, sequential matching algorithm that minimized the overall distance between black and white patients based on demographic-, tumor specific–, and treatment-related variables. The authors found that differences in overall survival were mainly driven by tumor presentation; however, advanced-stage black colon cancer patients received less guideline concordant-treatment than white patients. While this minimum-distance algorithm provided close black-white matches on prespecified factors, it could not accommodate other factors (for example, socioeconomic, marital, and urban/rural status); therefore, methodologic improvements to this method and comparisons to other commonly used approaches (that is, propensity score matching and weighting) are warranted.
Finally, these results apply to older black and white colon cancer patients with Medicare fee-for-service coverage only. Additional research using similar methods in older Medicare Advantage populations or younger adults may uncover unique drivers of overall survival disparities by race, which may require tailored interventions.
Jennifer L. Lund, Ph.D., is an assistant professor, department of epidemiology, University of North Carolina at Chapel Hill. She receives research support from the UNC Oncology Clinical Translational Research Training Program (K12 CA120780), as well as through a Research Starter Award from the PhRMA Foundation to the UNC Department of Epidemiology.
Prior studies have documented racial disparities in the incidence and outcomes of colon cancer in the United States. Black men and women have a higher overall incidence and more advanced stage of disease at diagnosis than white men and women, while being less likely to receive guideline-concordant treatment.

|
| Dr. Jennifer Lund |
To extend this work, the authors evaluated treatment disparities between black and white colon cancer patients aged 66 years and older and examined the impact of a variety of patient characteristics on racial disparities in overall survival using a novel, sequential matching algorithm that minimized the overall distance between black and white patients based on demographic-, tumor specific–, and treatment-related variables. The authors found that differences in overall survival were mainly driven by tumor presentation; however, advanced-stage black colon cancer patients received less guideline concordant-treatment than white patients. While this minimum-distance algorithm provided close black-white matches on prespecified factors, it could not accommodate other factors (for example, socioeconomic, marital, and urban/rural status); therefore, methodologic improvements to this method and comparisons to other commonly used approaches (that is, propensity score matching and weighting) are warranted.
Finally, these results apply to older black and white colon cancer patients with Medicare fee-for-service coverage only. Additional research using similar methods in older Medicare Advantage populations or younger adults may uncover unique drivers of overall survival disparities by race, which may require tailored interventions.
Jennifer L. Lund, Ph.D., is an assistant professor, department of epidemiology, University of North Carolina at Chapel Hill. She receives research support from the UNC Oncology Clinical Translational Research Training Program (K12 CA120780), as well as through a Research Starter Award from the PhRMA Foundation to the UNC Department of Epidemiology.
Although black patients with colon cancer received significantly less treatment than white patients, particularly for late stage disease, much of the overall survival disparity between black and white patients was explained by tumor presentation at diagnosis rather than treatment differences, according to an analysis of SEER data.
Among demographically matched black and white patients, the 5-year survival difference was 8.3% (P less than .0001). Presentation match reduced the difference to 5.0% (P less than .0001), which accounted for 39.8% of the overall disparity. Additional matching by treatment reduced the difference only slightly to 4.9% (P less than .0001), which accounted for 1.2% of the overall disparity. Black patients had lower rates for most treatments, including surgery, than presentation-matched white patients (88.5% vs. 91.4%), and these differences were most pronounced at advanced stages. For example, significant differences between black and white patients in the use of chemotherapy was observed for stage III (53.1% vs. 64.2%; P less than .0001) and stage IV (56.1% vs. 63.3%; P = .001).
“Our results indicate that tumor presentation, including tumor stage, is indeed one of the most important factors contributing to the racial disparity in colon cancer survival. We observed that, after controlling for demographic factors, black patients in comparison with white patients had a significantly higher proportion of stage IV and lower proportions of stages I and II disease. Adequately matching on tumor presentation variables (e.g., stage, grade, size, and comorbidity) significantly reduced survival disparities,” wrote Dr. Yinzhi Lai of the Department of Medical Oncology at Sidney Kimmel Cancer Center, Philadelphia, and colleagues (Gastroenterology. 2016 Apr 4. doi: 10.1053/j.gastro.2016.01.030).
Treatment differences in advanced-stage patients, compared with early-stage patients, explained a higher proportion of the demographic-matched survival disparity. For example, in stage II patients, treatment match resulted in modest reductions in 2-, 3-, and 5-year survival rate disparities (2.7%-2.8%, 4.1%-3.6%, and 4.6%-4.0%, respectively); by contrast, in stage III patients, treatment match resulted in more substantial reductions in 2-, 3-, and 5-year survival rate disparities (4.5%-2.2%, 3.1%-2.0%, and 4.3%-2.8%, respectively). A similar effect was observed in patients with stage IV disease. The results suggest that, “to control survival disparity, more efforts may need to be tailored to minimize treatment disparities (especially chemotherapy use) in patients with advanced-stage disease,” the investigators wrote.
The retrospective data analysis used patient information from 68,141 patients (6,190 black, 61,951 white) aged 66 years and older with colon cancer identified from the National Cancer Institute SEER-Medicare database. Using a novel minimum distance matching strategy, investigators drew from the pool of white patients to match three distinct comparison cohorts to the same 6,190 black patients. Close matches between black and white patients bypassed the need for model-based analysis.
The primary matching analysis was limited by the inability to control for substantial differences in socioeconomic status, marital status, and urban/rural residence. A subcohort analysis of 2,000 matched black and white patients showed that when socioeconomic status was added to the demographic match, survival differences were reduced, indicating the important role of socioeconomic status on racial survival disparities.
Significantly better survival was observed in all patients who were diagnosed in 2004 or later, the year the Food and Drug Administration approved the important chemotherapy medicines oxaliplatin and bevacizumab. Separating the cohorts into those who were diagnosed before and after 2004 revealed that the racial survival disparity was lower in the more recent group, indicating a favorable impact of oxaliplatin and/or bevacizumab in reducing the survival disparity.
Although black patients with colon cancer received significantly less treatment than white patients, particularly for late stage disease, much of the overall survival disparity between black and white patients was explained by tumor presentation at diagnosis rather than treatment differences, according to an analysis of SEER data.
Among demographically matched black and white patients, the 5-year survival difference was 8.3% (P less than .0001). Presentation match reduced the difference to 5.0% (P less than .0001), which accounted for 39.8% of the overall disparity. Additional matching by treatment reduced the difference only slightly to 4.9% (P less than .0001), which accounted for 1.2% of the overall disparity. Black patients had lower rates for most treatments, including surgery, than presentation-matched white patients (88.5% vs. 91.4%), and these differences were most pronounced at advanced stages. For example, significant differences between black and white patients in the use of chemotherapy was observed for stage III (53.1% vs. 64.2%; P less than .0001) and stage IV (56.1% vs. 63.3%; P = .001).
“Our results indicate that tumor presentation, including tumor stage, is indeed one of the most important factors contributing to the racial disparity in colon cancer survival. We observed that, after controlling for demographic factors, black patients in comparison with white patients had a significantly higher proportion of stage IV and lower proportions of stages I and II disease. Adequately matching on tumor presentation variables (e.g., stage, grade, size, and comorbidity) significantly reduced survival disparities,” wrote Dr. Yinzhi Lai of the Department of Medical Oncology at Sidney Kimmel Cancer Center, Philadelphia, and colleagues (Gastroenterology. 2016 Apr 4. doi: 10.1053/j.gastro.2016.01.030).
Treatment differences in advanced-stage patients, compared with early-stage patients, explained a higher proportion of the demographic-matched survival disparity. For example, in stage II patients, treatment match resulted in modest reductions in 2-, 3-, and 5-year survival rate disparities (2.7%-2.8%, 4.1%-3.6%, and 4.6%-4.0%, respectively); by contrast, in stage III patients, treatment match resulted in more substantial reductions in 2-, 3-, and 5-year survival rate disparities (4.5%-2.2%, 3.1%-2.0%, and 4.3%-2.8%, respectively). A similar effect was observed in patients with stage IV disease. The results suggest that, “to control survival disparity, more efforts may need to be tailored to minimize treatment disparities (especially chemotherapy use) in patients with advanced-stage disease,” the investigators wrote.
The retrospective data analysis used patient information from 68,141 patients (6,190 black, 61,951 white) aged 66 years and older with colon cancer identified from the National Cancer Institute SEER-Medicare database. Using a novel minimum distance matching strategy, investigators drew from the pool of white patients to match three distinct comparison cohorts to the same 6,190 black patients. Close matches between black and white patients bypassed the need for model-based analysis.
The primary matching analysis was limited by the inability to control for substantial differences in socioeconomic status, marital status, and urban/rural residence. A subcohort analysis of 2,000 matched black and white patients showed that when socioeconomic status was added to the demographic match, survival differences were reduced, indicating the important role of socioeconomic status on racial survival disparities.
Significantly better survival was observed in all patients who were diagnosed in 2004 or later, the year the Food and Drug Administration approved the important chemotherapy medicines oxaliplatin and bevacizumab. Separating the cohorts into those who were diagnosed before and after 2004 revealed that the racial survival disparity was lower in the more recent group, indicating a favorable impact of oxaliplatin and/or bevacizumab in reducing the survival disparity.
FROM GASTROENTEROLOGY
Key clinical point: Tumor stage at diagnosis had a greater effect on survival disparities between black and white patients with colon cancer than treatment differences.
Major finding: Among demographically matched black and white patients, the 5-year survival difference was 8.3% (P less than .0001); matching by presentation reduced the difference to 5.0% (P less than .0001), and additional matching by treatment reduced the difference only slightly to 4.9% (P less than .0001).
Data sources: In total, 68,141 patients (6,190 black, 61,951 white) aged 66 years and older with colon cancer were identified from the National Cancer Institute SEER-Medicare database. Three white comparison cohorts were assembled and matched to the same 6,190 black patients.
Disclosures: Dr. Lai and coauthors reported having no disclosures.
Search is on for cases of aggressive, ruxolitinib-associated skin cancers
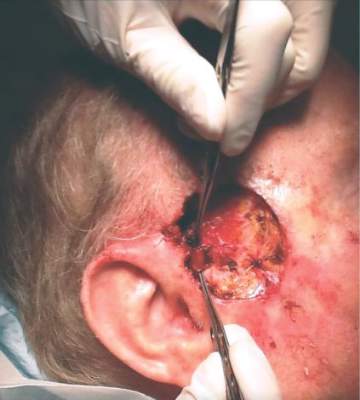
ORLANDO – The hematologic cancer drug ruxolitinib seems to be associated with cases of aggressive nonmelanoma skin cancer.
After treating a very aggressive squamous cell carcinoma in a 55-year-old man treated with ruxolitinib for polycythemia vera, and hearing firsthand of three other similar cases, Dr. Fiona Zwald is collecting additional data on the association. She intends to publish these cases in a monograph as a warning to dermatologists, hematologists, oncologists, and other physicians who manage patients with hematologic malignancies, she said at the annual meeting of the American College of Mohs Surgery.
The prescribing information for ruxolitinib (Jakafi, Incyte Pharmaceuticals; Jakavi, Novartis) was updated in 2014 to warn that patients taking the drug face an increased risk of nonmelanoma skin cancers. The label also recommends that physicians inspect the skin regularly and urge patients to be alert for and report any new or changing lesions.
Despite the warnings and recommendations, cases are occurring – and some are quite serious, said Dr. Zwald, a Mohs surgeon in Atlanta.
“People should know this is actually happening. If you have experience with this medication, please let us know so we can compile this report. We are trying to assess the number of skin cancers before and after initiating this medication,” she said.
Ruxolitinib is an inhibitor of Janus kinase with a special affinity for the JAK1 and JAK2 subtypes. Like other cytokine-signaling molecules, their function depends on cell context; it may inhibit cell growth in one setting, and, in another, stimulate it. Ruxolitinib was initially approved in 2011 for the treatment of intermediate- and high-risk myelofibrosis, including primary myelofibrosis, post–polycythemia vera myelofibrosis, and post–essential thrombocythemia myelofibrosis.
In 2014, indications for ruxolitinib were expanded to include treatment of patients with polycythemia vera who have had an inadequate response to or are intolerant of hydroxyurea.
Dr. Zwald’s patient had a 10-year history of polycythemia vera. He was initially well controlled on the standard hydroxyurea treatment. In the meantime, he began working as a caddy at a major U.S. golf club. He developed many facial squamous cell carcinomas that were treated with excision and radiation. A year before he presented to Dr. Zwald, he stopped responding to hydroxyurea and was placed on ruxolitinib.
The patient presented with a 4-cm ulcerated lesion over part of his right temple and to the right helical crus; the lesion had developed over 3 months. Dr. Zwald consulted with the patient’s medical oncologist; treatment with ruxolitinib continued, albeit at a reduced dosage in light of recent events.
She performed Mohs surgery on the patient. It was a challenging case, she said, not the least because adequate anesthesia could not be achieved with local anesthetic. Preoperative staging showed no nodal spread.
“He did, unfortunately demonstrate a large, indurated mass located over one branch of the superficial temporal artery. At the helical crus there was an area of bound-down, fixed tumor. Knowing that I would not be able to fully resect this, I passed him on to the operating room,” Dr. Zwald said. “This tumor was found to extend down to the parotid capsule, but margins were clear.” The surgical defect was successfully repaired with a split-thickness skin graft.
The tumor recurred about 3 months later, and the patient underwent another surgery.
“This time we could not get clear surgical margins, and the tumor was approaching the external auditory meatus. Surgery was abandoned due to fears of complications to that area,” she said.
She presented the case at tumor board, during which she and her colleagues discussed adjuvant radiation. They initially abandoned this idea because he had already had so much radiation to his face. After the second surgery, they decide to proceed with radiation. “The next conversation we have will be whether to add another adjuvant therapy to treatment.”
She sent out the case and requests for feedback to the International Transplant Skin Cancer Collaborative, an 800-member consortium of dermatologists and Mohs surgeons who take care of transplant patients. She received information on three additional cases of aggressive squamous cell carcinoma (SCC) associated with ruxolitinib treatment:
• A patient with myelodysplastic syndrome with aggressive scalp SCC with cutaneous metastases.
• A patient with undifferentiated pleomorphic sarcoma of the scalp, several cutaneous SCCs.
• A patient with a myelodysplastic syndrome with in-transit metastases and explosive cutaneous SCCs. The patient has had the ruxolitinib dose reduced and may be switched to capecitabine.
Dr. Zwald noted that her patient was at risk for aggressive skin cancers for reasons in addition to ruxolitinib treatment.
“He was already immunosuppressed from his malignancy. He was on hydroxyurea, a drug that’s a cumulative phototoxin, and he’s out in the sun playing golf every day, and then was put on ruxolitinib. But the question we face now is how to try and stop this medication so we can get better treatment for him which will, of course, be very difficult.”
To contribute to Dr. Zwald’s case series, please email her at [email protected].
She had no relevant financial disclosures.
On Twitter @Alz_Gal
ORLANDO – The hematologic cancer drug ruxolitinib seems to be associated with cases of aggressive nonmelanoma skin cancer.
After treating a very aggressive squamous cell carcinoma in a 55-year-old man treated with ruxolitinib for polycythemia vera, and hearing firsthand of three other similar cases, Dr. Fiona Zwald is collecting additional data on the association. She intends to publish these cases in a monograph as a warning to dermatologists, hematologists, oncologists, and other physicians who manage patients with hematologic malignancies, she said at the annual meeting of the American College of Mohs Surgery.
The prescribing information for ruxolitinib (Jakafi, Incyte Pharmaceuticals; Jakavi, Novartis) was updated in 2014 to warn that patients taking the drug face an increased risk of nonmelanoma skin cancers. The label also recommends that physicians inspect the skin regularly and urge patients to be alert for and report any new or changing lesions.
Despite the warnings and recommendations, cases are occurring – and some are quite serious, said Dr. Zwald, a Mohs surgeon in Atlanta.
“People should know this is actually happening. If you have experience with this medication, please let us know so we can compile this report. We are trying to assess the number of skin cancers before and after initiating this medication,” she said.
Ruxolitinib is an inhibitor of Janus kinase with a special affinity for the JAK1 and JAK2 subtypes. Like other cytokine-signaling molecules, their function depends on cell context; it may inhibit cell growth in one setting, and, in another, stimulate it. Ruxolitinib was initially approved in 2011 for the treatment of intermediate- and high-risk myelofibrosis, including primary myelofibrosis, post–polycythemia vera myelofibrosis, and post–essential thrombocythemia myelofibrosis.
In 2014, indications for ruxolitinib were expanded to include treatment of patients with polycythemia vera who have had an inadequate response to or are intolerant of hydroxyurea.
Dr. Zwald’s patient had a 10-year history of polycythemia vera. He was initially well controlled on the standard hydroxyurea treatment. In the meantime, he began working as a caddy at a major U.S. golf club. He developed many facial squamous cell carcinomas that were treated with excision and radiation. A year before he presented to Dr. Zwald, he stopped responding to hydroxyurea and was placed on ruxolitinib.
The patient presented with a 4-cm ulcerated lesion over part of his right temple and to the right helical crus; the lesion had developed over 3 months. Dr. Zwald consulted with the patient’s medical oncologist; treatment with ruxolitinib continued, albeit at a reduced dosage in light of recent events.
She performed Mohs surgery on the patient. It was a challenging case, she said, not the least because adequate anesthesia could not be achieved with local anesthetic. Preoperative staging showed no nodal spread.
“He did, unfortunately demonstrate a large, indurated mass located over one branch of the superficial temporal artery. At the helical crus there was an area of bound-down, fixed tumor. Knowing that I would not be able to fully resect this, I passed him on to the operating room,” Dr. Zwald said. “This tumor was found to extend down to the parotid capsule, but margins were clear.” The surgical defect was successfully repaired with a split-thickness skin graft.
The tumor recurred about 3 months later, and the patient underwent another surgery.
“This time we could not get clear surgical margins, and the tumor was approaching the external auditory meatus. Surgery was abandoned due to fears of complications to that area,” she said.
She presented the case at tumor board, during which she and her colleagues discussed adjuvant radiation. They initially abandoned this idea because he had already had so much radiation to his face. After the second surgery, they decide to proceed with radiation. “The next conversation we have will be whether to add another adjuvant therapy to treatment.”
She sent out the case and requests for feedback to the International Transplant Skin Cancer Collaborative, an 800-member consortium of dermatologists and Mohs surgeons who take care of transplant patients. She received information on three additional cases of aggressive squamous cell carcinoma (SCC) associated with ruxolitinib treatment:
• A patient with myelodysplastic syndrome with aggressive scalp SCC with cutaneous metastases.
• A patient with undifferentiated pleomorphic sarcoma of the scalp, several cutaneous SCCs.
• A patient with a myelodysplastic syndrome with in-transit metastases and explosive cutaneous SCCs. The patient has had the ruxolitinib dose reduced and may be switched to capecitabine.
Dr. Zwald noted that her patient was at risk for aggressive skin cancers for reasons in addition to ruxolitinib treatment.
“He was already immunosuppressed from his malignancy. He was on hydroxyurea, a drug that’s a cumulative phototoxin, and he’s out in the sun playing golf every day, and then was put on ruxolitinib. But the question we face now is how to try and stop this medication so we can get better treatment for him which will, of course, be very difficult.”
To contribute to Dr. Zwald’s case series, please email her at [email protected].
She had no relevant financial disclosures.
On Twitter @Alz_Gal
ORLANDO – The hematologic cancer drug ruxolitinib seems to be associated with cases of aggressive nonmelanoma skin cancer.
After treating a very aggressive squamous cell carcinoma in a 55-year-old man treated with ruxolitinib for polycythemia vera, and hearing firsthand of three other similar cases, Dr. Fiona Zwald is collecting additional data on the association. She intends to publish these cases in a monograph as a warning to dermatologists, hematologists, oncologists, and other physicians who manage patients with hematologic malignancies, she said at the annual meeting of the American College of Mohs Surgery.
The prescribing information for ruxolitinib (Jakafi, Incyte Pharmaceuticals; Jakavi, Novartis) was updated in 2014 to warn that patients taking the drug face an increased risk of nonmelanoma skin cancers. The label also recommends that physicians inspect the skin regularly and urge patients to be alert for and report any new or changing lesions.
Despite the warnings and recommendations, cases are occurring – and some are quite serious, said Dr. Zwald, a Mohs surgeon in Atlanta.
“People should know this is actually happening. If you have experience with this medication, please let us know so we can compile this report. We are trying to assess the number of skin cancers before and after initiating this medication,” she said.
Ruxolitinib is an inhibitor of Janus kinase with a special affinity for the JAK1 and JAK2 subtypes. Like other cytokine-signaling molecules, their function depends on cell context; it may inhibit cell growth in one setting, and, in another, stimulate it. Ruxolitinib was initially approved in 2011 for the treatment of intermediate- and high-risk myelofibrosis, including primary myelofibrosis, post–polycythemia vera myelofibrosis, and post–essential thrombocythemia myelofibrosis.
In 2014, indications for ruxolitinib were expanded to include treatment of patients with polycythemia vera who have had an inadequate response to or are intolerant of hydroxyurea.
Dr. Zwald’s patient had a 10-year history of polycythemia vera. He was initially well controlled on the standard hydroxyurea treatment. In the meantime, he began working as a caddy at a major U.S. golf club. He developed many facial squamous cell carcinomas that were treated with excision and radiation. A year before he presented to Dr. Zwald, he stopped responding to hydroxyurea and was placed on ruxolitinib.
The patient presented with a 4-cm ulcerated lesion over part of his right temple and to the right helical crus; the lesion had developed over 3 months. Dr. Zwald consulted with the patient’s medical oncologist; treatment with ruxolitinib continued, albeit at a reduced dosage in light of recent events.
She performed Mohs surgery on the patient. It was a challenging case, she said, not the least because adequate anesthesia could not be achieved with local anesthetic. Preoperative staging showed no nodal spread.
“He did, unfortunately demonstrate a large, indurated mass located over one branch of the superficial temporal artery. At the helical crus there was an area of bound-down, fixed tumor. Knowing that I would not be able to fully resect this, I passed him on to the operating room,” Dr. Zwald said. “This tumor was found to extend down to the parotid capsule, but margins were clear.” The surgical defect was successfully repaired with a split-thickness skin graft.
The tumor recurred about 3 months later, and the patient underwent another surgery.
“This time we could not get clear surgical margins, and the tumor was approaching the external auditory meatus. Surgery was abandoned due to fears of complications to that area,” she said.
She presented the case at tumor board, during which she and her colleagues discussed adjuvant radiation. They initially abandoned this idea because he had already had so much radiation to his face. After the second surgery, they decide to proceed with radiation. “The next conversation we have will be whether to add another adjuvant therapy to treatment.”
She sent out the case and requests for feedback to the International Transplant Skin Cancer Collaborative, an 800-member consortium of dermatologists and Mohs surgeons who take care of transplant patients. She received information on three additional cases of aggressive squamous cell carcinoma (SCC) associated with ruxolitinib treatment:
• A patient with myelodysplastic syndrome with aggressive scalp SCC with cutaneous metastases.
• A patient with undifferentiated pleomorphic sarcoma of the scalp, several cutaneous SCCs.
• A patient with a myelodysplastic syndrome with in-transit metastases and explosive cutaneous SCCs. The patient has had the ruxolitinib dose reduced and may be switched to capecitabine.
Dr. Zwald noted that her patient was at risk for aggressive skin cancers for reasons in addition to ruxolitinib treatment.
“He was already immunosuppressed from his malignancy. He was on hydroxyurea, a drug that’s a cumulative phototoxin, and he’s out in the sun playing golf every day, and then was put on ruxolitinib. But the question we face now is how to try and stop this medication so we can get better treatment for him which will, of course, be very difficult.”
To contribute to Dr. Zwald’s case series, please email her at [email protected].
She had no relevant financial disclosures.
On Twitter @Alz_Gal
AT THE ACMS ANNUAL MEETING
Premenopausal age linked to lower sexual function after gynecologic cancer surgery
INDIAN WELLS, CALIF. – Premenopausal age was associated with a greater temporary decline in sexual desire 1 month after undergoing surgery for suspected gynecologic malignancies, results from an ancillary analysis showed.
“Sexual health is an important dimension of quality of life for women with gynecologic cancer,” Dr. C. Emi Bretschneider, lead study author, said at the annual scientific meeting of the Society of Gynecologic Surgeons. “Limited data exists on the impact of surgery for treatment of gynecologic cancer on patient-reported sexual desire and interest.”
In an effort to evaluate the impact on sexual function in women undergoing surgery for presumed or known gynecologic malignancies, the researchers performed an ancillary analysis of a cohort study analyzing quality-of-life and operative outcomes in 185 women who underwent gynecologic oncology procedures at the University of North Carolina, Chapel Hill, between October 2013 and October 2014.
Study participants completed the Patient-Reported Outcomes Measurement Information System Sexual Function and Satisfaction Questionnaire (PROMIS-SFQ) at baseline and at 1, 3, and 6 months postoperatively. The questionnaire evaluates four subdomains of sexual function: global satisfaction with sex life, interest in sexual activity, lubrication, and vaginal discomfort. The researchers used student t-test and linear regression to compare mean score changes between cancer types, surgical route, menopausal status, and postoperative complications, said Dr. Bretschneider of the university’s department of obstetrics and gynecology.
Of the 281 patients initially enrolled, 185 (66%) completed the PROMIS-SFQ at baseline and at 1 month postoperatively, forming the primary cohort from which the researchers performed the analysis. Of these 185 patients, 170 (92%) completed the PROMIS-SFQ at 3 months and 174 (94%) completed the survey at 6 months postoperatively.
The average age of patients at baseline was 56 years: most (77%) were white, mean body mass index was 32.9 kg/m2, 62% were partnered, and 63% underwent minimally invasive procedures. Following surgery, 131 of the patients (71%) were diagnosed with a malignancy, most commonly uterine cancer (84%), followed by ovarian (23%), cervical (17%), and vulvar cancer (3%).
Dr. Bretschneider reported that the mean baseline sexual interest score among all study participants was 44.8. At 1 month postoperatively, the mean scores decreased a mean of 3.8 points from baseline to 41. By 3 and 6 months postoperatively, the mean sexual interest scores increased from baseline by 1.9 and 2.7 points, respectively, to 46.7 and 47.5.
Women younger than age 55 years had a greater decrease in sexual interest between baseline and 1 month postoperatively, compared with their counterparts aged 55 and older (a mean of –5.5 vs. –2.3 points, respectively; P = .02).
On multivariate analysis adjusted for cancer diagnosis, minimally invasive surgery, and cancer site, women younger than age 55 continued to have a greater decrease in sexual interest between baseline and 1 month postoperatively, compared with their counterparts aged 55 and older (a mean of –4.59 points). Additionally, women who had cancer had a greater drop in sexual desire, compared with those with benign disease (a mean of –5.6 points).
“This study offers new information on the impact of surgery on sexual function for women with gynecologic cancer,” Dr. Bretschneider said at the meeting, which was jointly sponsored by the American College of Surgeons. “The study was further strengthened by its prospective design and well-characterized, large cohort of women.” Weaknesses, she continued, include its generalizability, “which may be limited, as the study cohort was recruited from a single academic institution. Also, the small sample size for some cancer sites reduced our ability to sense cancer site as a causal agent for sexual dysfunction.”
Dr. Bretschneider reported having no financial disclosures.
INDIAN WELLS, CALIF. – Premenopausal age was associated with a greater temporary decline in sexual desire 1 month after undergoing surgery for suspected gynecologic malignancies, results from an ancillary analysis showed.
“Sexual health is an important dimension of quality of life for women with gynecologic cancer,” Dr. C. Emi Bretschneider, lead study author, said at the annual scientific meeting of the Society of Gynecologic Surgeons. “Limited data exists on the impact of surgery for treatment of gynecologic cancer on patient-reported sexual desire and interest.”
In an effort to evaluate the impact on sexual function in women undergoing surgery for presumed or known gynecologic malignancies, the researchers performed an ancillary analysis of a cohort study analyzing quality-of-life and operative outcomes in 185 women who underwent gynecologic oncology procedures at the University of North Carolina, Chapel Hill, between October 2013 and October 2014.
Study participants completed the Patient-Reported Outcomes Measurement Information System Sexual Function and Satisfaction Questionnaire (PROMIS-SFQ) at baseline and at 1, 3, and 6 months postoperatively. The questionnaire evaluates four subdomains of sexual function: global satisfaction with sex life, interest in sexual activity, lubrication, and vaginal discomfort. The researchers used student t-test and linear regression to compare mean score changes between cancer types, surgical route, menopausal status, and postoperative complications, said Dr. Bretschneider of the university’s department of obstetrics and gynecology.
Of the 281 patients initially enrolled, 185 (66%) completed the PROMIS-SFQ at baseline and at 1 month postoperatively, forming the primary cohort from which the researchers performed the analysis. Of these 185 patients, 170 (92%) completed the PROMIS-SFQ at 3 months and 174 (94%) completed the survey at 6 months postoperatively.
The average age of patients at baseline was 56 years: most (77%) were white, mean body mass index was 32.9 kg/m2, 62% were partnered, and 63% underwent minimally invasive procedures. Following surgery, 131 of the patients (71%) were diagnosed with a malignancy, most commonly uterine cancer (84%), followed by ovarian (23%), cervical (17%), and vulvar cancer (3%).
Dr. Bretschneider reported that the mean baseline sexual interest score among all study participants was 44.8. At 1 month postoperatively, the mean scores decreased a mean of 3.8 points from baseline to 41. By 3 and 6 months postoperatively, the mean sexual interest scores increased from baseline by 1.9 and 2.7 points, respectively, to 46.7 and 47.5.
Women younger than age 55 years had a greater decrease in sexual interest between baseline and 1 month postoperatively, compared with their counterparts aged 55 and older (a mean of –5.5 vs. –2.3 points, respectively; P = .02).
On multivariate analysis adjusted for cancer diagnosis, minimally invasive surgery, and cancer site, women younger than age 55 continued to have a greater decrease in sexual interest between baseline and 1 month postoperatively, compared with their counterparts aged 55 and older (a mean of –4.59 points). Additionally, women who had cancer had a greater drop in sexual desire, compared with those with benign disease (a mean of –5.6 points).
“This study offers new information on the impact of surgery on sexual function for women with gynecologic cancer,” Dr. Bretschneider said at the meeting, which was jointly sponsored by the American College of Surgeons. “The study was further strengthened by its prospective design and well-characterized, large cohort of women.” Weaknesses, she continued, include its generalizability, “which may be limited, as the study cohort was recruited from a single academic institution. Also, the small sample size for some cancer sites reduced our ability to sense cancer site as a causal agent for sexual dysfunction.”
Dr. Bretschneider reported having no financial disclosures.
INDIAN WELLS, CALIF. – Premenopausal age was associated with a greater temporary decline in sexual desire 1 month after undergoing surgery for suspected gynecologic malignancies, results from an ancillary analysis showed.
“Sexual health is an important dimension of quality of life for women with gynecologic cancer,” Dr. C. Emi Bretschneider, lead study author, said at the annual scientific meeting of the Society of Gynecologic Surgeons. “Limited data exists on the impact of surgery for treatment of gynecologic cancer on patient-reported sexual desire and interest.”
In an effort to evaluate the impact on sexual function in women undergoing surgery for presumed or known gynecologic malignancies, the researchers performed an ancillary analysis of a cohort study analyzing quality-of-life and operative outcomes in 185 women who underwent gynecologic oncology procedures at the University of North Carolina, Chapel Hill, between October 2013 and October 2014.
Study participants completed the Patient-Reported Outcomes Measurement Information System Sexual Function and Satisfaction Questionnaire (PROMIS-SFQ) at baseline and at 1, 3, and 6 months postoperatively. The questionnaire evaluates four subdomains of sexual function: global satisfaction with sex life, interest in sexual activity, lubrication, and vaginal discomfort. The researchers used student t-test and linear regression to compare mean score changes between cancer types, surgical route, menopausal status, and postoperative complications, said Dr. Bretschneider of the university’s department of obstetrics and gynecology.
Of the 281 patients initially enrolled, 185 (66%) completed the PROMIS-SFQ at baseline and at 1 month postoperatively, forming the primary cohort from which the researchers performed the analysis. Of these 185 patients, 170 (92%) completed the PROMIS-SFQ at 3 months and 174 (94%) completed the survey at 6 months postoperatively.
The average age of patients at baseline was 56 years: most (77%) were white, mean body mass index was 32.9 kg/m2, 62% were partnered, and 63% underwent minimally invasive procedures. Following surgery, 131 of the patients (71%) were diagnosed with a malignancy, most commonly uterine cancer (84%), followed by ovarian (23%), cervical (17%), and vulvar cancer (3%).
Dr. Bretschneider reported that the mean baseline sexual interest score among all study participants was 44.8. At 1 month postoperatively, the mean scores decreased a mean of 3.8 points from baseline to 41. By 3 and 6 months postoperatively, the mean sexual interest scores increased from baseline by 1.9 and 2.7 points, respectively, to 46.7 and 47.5.
Women younger than age 55 years had a greater decrease in sexual interest between baseline and 1 month postoperatively, compared with their counterparts aged 55 and older (a mean of –5.5 vs. –2.3 points, respectively; P = .02).
On multivariate analysis adjusted for cancer diagnosis, minimally invasive surgery, and cancer site, women younger than age 55 continued to have a greater decrease in sexual interest between baseline and 1 month postoperatively, compared with their counterparts aged 55 and older (a mean of –4.59 points). Additionally, women who had cancer had a greater drop in sexual desire, compared with those with benign disease (a mean of –5.6 points).
“This study offers new information on the impact of surgery on sexual function for women with gynecologic cancer,” Dr. Bretschneider said at the meeting, which was jointly sponsored by the American College of Surgeons. “The study was further strengthened by its prospective design and well-characterized, large cohort of women.” Weaknesses, she continued, include its generalizability, “which may be limited, as the study cohort was recruited from a single academic institution. Also, the small sample size for some cancer sites reduced our ability to sense cancer site as a causal agent for sexual dysfunction.”
Dr. Bretschneider reported having no financial disclosures.
AT SGS 2016
Key clinical point: Premenopausal age was associated with a greater temporary decline in sexual function following gynecologic oncology procedures.
Major finding: Women younger than age 55 years had a greater decrease in sexual interest between baseline and 1 month postoperatively, compared with their counterparts aged 55 and older (a mean of –5.5 vs. –2.3 points on the PROMIS-SFQ, respectively; P = .02).
Data source: An ancillary analysis of a cohort study analyzing quality-of-life and operative outcomes in 185 women who underwent gynecologic oncology procedures between October 2013 and October 2014.
Disclosures: Dr. Bretschneider reported having no financial disclosures.
ACOSOG Z0011: Good long-term results with SLND without ALND
CHICAGO – Sentinel lymph node dissection without axillary lymph node dissection offers excellent regional control in select patients with early metastatic breast cancer who are treated using breast-conserving therapy and adjuvant systemic therapy, according to 10-year results from the American College of Surgeons Oncology Group (ACOSOG) Z0011 Randomized Trial. ACOSOG is now part of Alliance for Clinical Trials in Oncology.
The findings confirm the previously reported 5-year outcomes, which demonstrated no significant difference in locoregional recurrence for patients with positive sentinel nodes who were randomized to undergo axillary lymph node dissection (ALND) or no further axillary treatment, Dr. Armando E. Giuliano of Cedars-Sinai Medical Center, Los Angeles, reported at the annual meeting of the American Surgical Association.
“In fact, the [5-year] results were highly significant showing noninferiority of sentinel lymph node dissection,” he said.
At a median follow-up of 9.25 years, there still was no statistically significant difference between 446 sentinel lymph node dissection (SLND)–only patients and 445 completion ALND patients with respect to the rate of locoregional recurrence, Dr. Giuliano said.
“The 10-year locoregional recurrence incidence after axillary lymph node dissection is 6.2%, compared to 5.3% after sentinel lymph node dissection alone,” he said, noting that most recurrences were seen in the first 5 years.
Of the ALND patients, 27% had additional positive nodes removed beyond the sentinel nodes.
“Therefore, about 27% of patients who underwent sentinel node dissection alone had residual disease remaining in the axilla undissected. Despite this high possibility of residual disease, very few regional recurrences were seen in either arm,” he noted.
Local recurrences occurred in 19 (5.6%) of patients in the ALND group and 12 (3.8%) in the SLND group, and regional recurrence was seen in 2 (0.5%) patients in the ALND group and 5 (1.5%) in the SLND group. The differences were not statistically significant.
Only hormone receptor status, Bloom-Richardson score, and tumor size were associated with locoregional recurrence. Omission of radiation increased local but not regional recurrence, but numbers were too few to draw further conclusions, he said.
“We can conclude, however, that sentinel lymph node dissection provides excellent locoregional control comparable to completion axillary lymph node dissection in these selected patients,” he said.
ACOSOG Z0011 subjects were patients with hematoxylin-eosin (H&E)–detected sentinel lymph node metastases undergoing breast-conserving therapy. The groups randomized to undergo ALND or to receive no further axillary treatment were similar with respect to age, Bloom-Richardson score, estrogen-receptor status, adjuvant systemic therapy, histology, and tumor size.
Nearly all patients had adjuvant systemic therapy (96% and 97% in the ALND and SLND groups, respectively), and about 60% in each group received chemotherapy.
Dr. Giuliano concluded that, despite the potential for residual axillary disease after SLND, SLND without ALND offers excellent regional control for selected patients with early metastatic breast cancer treated with breast-conserving therapy and adjuvant systemic therapy.
“Axillary lymph node dissection is not necessary for patients with early metastatic breast cancer and should be abandoned,” he said.
Dr. Giuliano had no disclosures.
The complete manuscript of this presentation is anticipated to be published in the Annals of Surgery pending editorial review
CHICAGO – Sentinel lymph node dissection without axillary lymph node dissection offers excellent regional control in select patients with early metastatic breast cancer who are treated using breast-conserving therapy and adjuvant systemic therapy, according to 10-year results from the American College of Surgeons Oncology Group (ACOSOG) Z0011 Randomized Trial. ACOSOG is now part of Alliance for Clinical Trials in Oncology.
The findings confirm the previously reported 5-year outcomes, which demonstrated no significant difference in locoregional recurrence for patients with positive sentinel nodes who were randomized to undergo axillary lymph node dissection (ALND) or no further axillary treatment, Dr. Armando E. Giuliano of Cedars-Sinai Medical Center, Los Angeles, reported at the annual meeting of the American Surgical Association.
“In fact, the [5-year] results were highly significant showing noninferiority of sentinel lymph node dissection,” he said.
At a median follow-up of 9.25 years, there still was no statistically significant difference between 446 sentinel lymph node dissection (SLND)–only patients and 445 completion ALND patients with respect to the rate of locoregional recurrence, Dr. Giuliano said.
“The 10-year locoregional recurrence incidence after axillary lymph node dissection is 6.2%, compared to 5.3% after sentinel lymph node dissection alone,” he said, noting that most recurrences were seen in the first 5 years.
Of the ALND patients, 27% had additional positive nodes removed beyond the sentinel nodes.
“Therefore, about 27% of patients who underwent sentinel node dissection alone had residual disease remaining in the axilla undissected. Despite this high possibility of residual disease, very few regional recurrences were seen in either arm,” he noted.
Local recurrences occurred in 19 (5.6%) of patients in the ALND group and 12 (3.8%) in the SLND group, and regional recurrence was seen in 2 (0.5%) patients in the ALND group and 5 (1.5%) in the SLND group. The differences were not statistically significant.
Only hormone receptor status, Bloom-Richardson score, and tumor size were associated with locoregional recurrence. Omission of radiation increased local but not regional recurrence, but numbers were too few to draw further conclusions, he said.
“We can conclude, however, that sentinel lymph node dissection provides excellent locoregional control comparable to completion axillary lymph node dissection in these selected patients,” he said.
ACOSOG Z0011 subjects were patients with hematoxylin-eosin (H&E)–detected sentinel lymph node metastases undergoing breast-conserving therapy. The groups randomized to undergo ALND or to receive no further axillary treatment were similar with respect to age, Bloom-Richardson score, estrogen-receptor status, adjuvant systemic therapy, histology, and tumor size.
Nearly all patients had adjuvant systemic therapy (96% and 97% in the ALND and SLND groups, respectively), and about 60% in each group received chemotherapy.
Dr. Giuliano concluded that, despite the potential for residual axillary disease after SLND, SLND without ALND offers excellent regional control for selected patients with early metastatic breast cancer treated with breast-conserving therapy and adjuvant systemic therapy.
“Axillary lymph node dissection is not necessary for patients with early metastatic breast cancer and should be abandoned,” he said.
Dr. Giuliano had no disclosures.
The complete manuscript of this presentation is anticipated to be published in the Annals of Surgery pending editorial review
CHICAGO – Sentinel lymph node dissection without axillary lymph node dissection offers excellent regional control in select patients with early metastatic breast cancer who are treated using breast-conserving therapy and adjuvant systemic therapy, according to 10-year results from the American College of Surgeons Oncology Group (ACOSOG) Z0011 Randomized Trial. ACOSOG is now part of Alliance for Clinical Trials in Oncology.
The findings confirm the previously reported 5-year outcomes, which demonstrated no significant difference in locoregional recurrence for patients with positive sentinel nodes who were randomized to undergo axillary lymph node dissection (ALND) or no further axillary treatment, Dr. Armando E. Giuliano of Cedars-Sinai Medical Center, Los Angeles, reported at the annual meeting of the American Surgical Association.
“In fact, the [5-year] results were highly significant showing noninferiority of sentinel lymph node dissection,” he said.
At a median follow-up of 9.25 years, there still was no statistically significant difference between 446 sentinel lymph node dissection (SLND)–only patients and 445 completion ALND patients with respect to the rate of locoregional recurrence, Dr. Giuliano said.
“The 10-year locoregional recurrence incidence after axillary lymph node dissection is 6.2%, compared to 5.3% after sentinel lymph node dissection alone,” he said, noting that most recurrences were seen in the first 5 years.
Of the ALND patients, 27% had additional positive nodes removed beyond the sentinel nodes.
“Therefore, about 27% of patients who underwent sentinel node dissection alone had residual disease remaining in the axilla undissected. Despite this high possibility of residual disease, very few regional recurrences were seen in either arm,” he noted.
Local recurrences occurred in 19 (5.6%) of patients in the ALND group and 12 (3.8%) in the SLND group, and regional recurrence was seen in 2 (0.5%) patients in the ALND group and 5 (1.5%) in the SLND group. The differences were not statistically significant.
Only hormone receptor status, Bloom-Richardson score, and tumor size were associated with locoregional recurrence. Omission of radiation increased local but not regional recurrence, but numbers were too few to draw further conclusions, he said.
“We can conclude, however, that sentinel lymph node dissection provides excellent locoregional control comparable to completion axillary lymph node dissection in these selected patients,” he said.
ACOSOG Z0011 subjects were patients with hematoxylin-eosin (H&E)–detected sentinel lymph node metastases undergoing breast-conserving therapy. The groups randomized to undergo ALND or to receive no further axillary treatment were similar with respect to age, Bloom-Richardson score, estrogen-receptor status, adjuvant systemic therapy, histology, and tumor size.
Nearly all patients had adjuvant systemic therapy (96% and 97% in the ALND and SLND groups, respectively), and about 60% in each group received chemotherapy.
Dr. Giuliano concluded that, despite the potential for residual axillary disease after SLND, SLND without ALND offers excellent regional control for selected patients with early metastatic breast cancer treated with breast-conserving therapy and adjuvant systemic therapy.
“Axillary lymph node dissection is not necessary for patients with early metastatic breast cancer and should be abandoned,” he said.
Dr. Giuliano had no disclosures.
The complete manuscript of this presentation is anticipated to be published in the Annals of Surgery pending editorial review
AT THE ASA ANNUAL MEETING
Key clinical point: Sentinel lymph node dissection without axillary lymph node dissection offers excellent long-term regional control in select patients with early metastatic breast cancer who are treated using breast-conserving therapy and adjuvant systemic therapy.
Major finding: The 10-year locoregional recurrence after axillary lymph node dissection was 6.2%, compared with 5.3% after SLND alone.
Data source: The American College of Surgeons Oncology Group (ACOSOG) Z0011 Randomized Trial involving 891 patients.
Disclosures: Dr. Giuliano had no disclosures.
Surveillance finds pancreatic ductal carcinoma in situ at resectable stage
Surveillance of CDNK2A mutation carriers detected most pancreatic ductal carcinoma in situ (PDAC) at a resectable stage, while the surveillance benefit was lower for those with familial prostate cancer.
Among 178 CDKN2A mutation carriers, PDAC was detected in 13 (7.3%), 9 of whom underwent surgery. Compared with previously reported rates of 15%-20% for symptomatic PDAC, this 70% resection rate represents a substantial increase. The 5-year survival rate of 24% for screen-detected PDAC was higher than 4%-7% reported for symptomatic sporadic PDAC. Among individuals with familial prostate cancer (FPC), 13 of 214 individuals (6.1%) underwent surgery, but with a higher proportion of precursor lesions detected, just four high-risk lesions (1.9% of screened FPC patients) were removed.
Whether surveillance improved prognosis for FPC families was difficult to determine, according to the investigators. The yield of PDAC was low at 0.9%, as was the yield of relevant precursor lesions (grade 3 PanIN and high-grade IPMN) at 1.9%.
“However, if surgical removal of multifocal grade 2 PanIN and multifocal BD-IPMNs is regarded as beneficial, the diagnostic yield increases to 3.7% (eight of 214 patients), and surveillance of FPC might also be considered effective,” wrote Dr. Hans Vasen, professor in the department of gastroenterology and hepatology at the Leiden University Medical Center, the Netherlands, and colleagues. “The value of surveillance of FPC is still not clear, and the main effect seems to be prevention of PDAC by removal of” precursor lesions, they added (J Clin Oncol. 2016 Apr 25. doi: 10.1200/JCO.2015.64.0730).
The retrospective evaluation of an ongoing prospective follow-up study included 411 high-risk individuals: 178 with CDKN2A mutations, 214 with familial pancreatic cancer, and 19 with BRCA1/2 or PALB2 mutations. The study was conducted at three expert centers in Marburg, Germany; Leiden, the Netherlands; and Madrid.
In the BRCA1/2 and PALB2 mutation cohort, one individual (3.8%) with a BRCA2 mutation developed PDAC and underwent surgery; 17 months after the surgery this patient died of liver metastasis. Two others underwent surgery for cystic lesions and are in good health at 10 and 21 months after surgery.
In the cohort of CDKN2A mutation carriers, the mean age at the start of surveillance was 56 years (range, 37-75) and the mean follow-up time was 53 months (range, 0-169): in total, 866 MRIs and 106 endoscopic ultrasounds were conducted. In the FPC group, the mean age was 48 years (range, 27-81), and the mean follow up was 2.8 years (range, 0-10.8): 618 MRIs and 402 endoscopic ultrasounds were conducted. Among BRCA1/2 and PALB2 mutation carriers, the mean age was 52.6 years (range, 25-70), and the mean follow up was 32.7 months (range, 1-119).
Given the difficulty of detecting precursor lesions and distinguishing incipient neoplasia from lower grade or nonneoplastic cystic lesions, the authors of the accompanying study achieved impressive results in improving cancer outcomes among high-risk individuals.
Several strategies for earlier cancer detection can be gleaned from the study. Improved outcomes may depend on expert centers running the surveillance. The detection rate of 2%-7%, depending on the cohort studied and the surveillance protocol, may have room for improvement with better risk stratification and refined protocols for cost effectiveness. The age at the start of surveillance may be one place to start: the mean age of pancreatic ductal carcinoma in situ detection was 53-68 years, depending on the center, and it may be possible to shift the starting age upward to improve yield.
The type of mutation conferring susceptibility may aid in risk stratification. For example, CDKN2A mutation carriers had a higher cancer rate (16%) than BRCA/PALB2 mutation carriers (5%). Other factors that could mitigate risk upward include diabetes, family history, and smoking history. A composite risk assessment could aid in identifying the highest-risk patients. Lastly, future studies are needed to determine which surveillance protocols are best. To make valid comparisons, several surveillance protocols must be tested.
These results impact not only high-risk individuals, but the general population as well. The data support that early detection improves outcomes and highlights the need for developing better biomarkers and tests for early detection of PDAC.
Dr. Teresa A. Brentnall is professor in the department of medicine, division of gastroenterology, University of Washington, Seattle. These remarks were part of an accompanying editorial (J Clin Oncol. 2016 Apr 25. doi: 10.1200/JCO.2015.64.0730).
Given the difficulty of detecting precursor lesions and distinguishing incipient neoplasia from lower grade or nonneoplastic cystic lesions, the authors of the accompanying study achieved impressive results in improving cancer outcomes among high-risk individuals.
Several strategies for earlier cancer detection can be gleaned from the study. Improved outcomes may depend on expert centers running the surveillance. The detection rate of 2%-7%, depending on the cohort studied and the surveillance protocol, may have room for improvement with better risk stratification and refined protocols for cost effectiveness. The age at the start of surveillance may be one place to start: the mean age of pancreatic ductal carcinoma in situ detection was 53-68 years, depending on the center, and it may be possible to shift the starting age upward to improve yield.
The type of mutation conferring susceptibility may aid in risk stratification. For example, CDKN2A mutation carriers had a higher cancer rate (16%) than BRCA/PALB2 mutation carriers (5%). Other factors that could mitigate risk upward include diabetes, family history, and smoking history. A composite risk assessment could aid in identifying the highest-risk patients. Lastly, future studies are needed to determine which surveillance protocols are best. To make valid comparisons, several surveillance protocols must be tested.
These results impact not only high-risk individuals, but the general population as well. The data support that early detection improves outcomes and highlights the need for developing better biomarkers and tests for early detection of PDAC.
Dr. Teresa A. Brentnall is professor in the department of medicine, division of gastroenterology, University of Washington, Seattle. These remarks were part of an accompanying editorial (J Clin Oncol. 2016 Apr 25. doi: 10.1200/JCO.2015.64.0730).
Given the difficulty of detecting precursor lesions and distinguishing incipient neoplasia from lower grade or nonneoplastic cystic lesions, the authors of the accompanying study achieved impressive results in improving cancer outcomes among high-risk individuals.
Several strategies for earlier cancer detection can be gleaned from the study. Improved outcomes may depend on expert centers running the surveillance. The detection rate of 2%-7%, depending on the cohort studied and the surveillance protocol, may have room for improvement with better risk stratification and refined protocols for cost effectiveness. The age at the start of surveillance may be one place to start: the mean age of pancreatic ductal carcinoma in situ detection was 53-68 years, depending on the center, and it may be possible to shift the starting age upward to improve yield.
The type of mutation conferring susceptibility may aid in risk stratification. For example, CDKN2A mutation carriers had a higher cancer rate (16%) than BRCA/PALB2 mutation carriers (5%). Other factors that could mitigate risk upward include diabetes, family history, and smoking history. A composite risk assessment could aid in identifying the highest-risk patients. Lastly, future studies are needed to determine which surveillance protocols are best. To make valid comparisons, several surveillance protocols must be tested.
These results impact not only high-risk individuals, but the general population as well. The data support that early detection improves outcomes and highlights the need for developing better biomarkers and tests for early detection of PDAC.
Dr. Teresa A. Brentnall is professor in the department of medicine, division of gastroenterology, University of Washington, Seattle. These remarks were part of an accompanying editorial (J Clin Oncol. 2016 Apr 25. doi: 10.1200/JCO.2015.64.0730).
Surveillance of CDNK2A mutation carriers detected most pancreatic ductal carcinoma in situ (PDAC) at a resectable stage, while the surveillance benefit was lower for those with familial prostate cancer.
Among 178 CDKN2A mutation carriers, PDAC was detected in 13 (7.3%), 9 of whom underwent surgery. Compared with previously reported rates of 15%-20% for symptomatic PDAC, this 70% resection rate represents a substantial increase. The 5-year survival rate of 24% for screen-detected PDAC was higher than 4%-7% reported for symptomatic sporadic PDAC. Among individuals with familial prostate cancer (FPC), 13 of 214 individuals (6.1%) underwent surgery, but with a higher proportion of precursor lesions detected, just four high-risk lesions (1.9% of screened FPC patients) were removed.
Whether surveillance improved prognosis for FPC families was difficult to determine, according to the investigators. The yield of PDAC was low at 0.9%, as was the yield of relevant precursor lesions (grade 3 PanIN and high-grade IPMN) at 1.9%.
“However, if surgical removal of multifocal grade 2 PanIN and multifocal BD-IPMNs is regarded as beneficial, the diagnostic yield increases to 3.7% (eight of 214 patients), and surveillance of FPC might also be considered effective,” wrote Dr. Hans Vasen, professor in the department of gastroenterology and hepatology at the Leiden University Medical Center, the Netherlands, and colleagues. “The value of surveillance of FPC is still not clear, and the main effect seems to be prevention of PDAC by removal of” precursor lesions, they added (J Clin Oncol. 2016 Apr 25. doi: 10.1200/JCO.2015.64.0730).
The retrospective evaluation of an ongoing prospective follow-up study included 411 high-risk individuals: 178 with CDKN2A mutations, 214 with familial pancreatic cancer, and 19 with BRCA1/2 or PALB2 mutations. The study was conducted at three expert centers in Marburg, Germany; Leiden, the Netherlands; and Madrid.
In the BRCA1/2 and PALB2 mutation cohort, one individual (3.8%) with a BRCA2 mutation developed PDAC and underwent surgery; 17 months after the surgery this patient died of liver metastasis. Two others underwent surgery for cystic lesions and are in good health at 10 and 21 months after surgery.
In the cohort of CDKN2A mutation carriers, the mean age at the start of surveillance was 56 years (range, 37-75) and the mean follow-up time was 53 months (range, 0-169): in total, 866 MRIs and 106 endoscopic ultrasounds were conducted. In the FPC group, the mean age was 48 years (range, 27-81), and the mean follow up was 2.8 years (range, 0-10.8): 618 MRIs and 402 endoscopic ultrasounds were conducted. Among BRCA1/2 and PALB2 mutation carriers, the mean age was 52.6 years (range, 25-70), and the mean follow up was 32.7 months (range, 1-119).
Surveillance of CDNK2A mutation carriers detected most pancreatic ductal carcinoma in situ (PDAC) at a resectable stage, while the surveillance benefit was lower for those with familial prostate cancer.
Among 178 CDKN2A mutation carriers, PDAC was detected in 13 (7.3%), 9 of whom underwent surgery. Compared with previously reported rates of 15%-20% for symptomatic PDAC, this 70% resection rate represents a substantial increase. The 5-year survival rate of 24% for screen-detected PDAC was higher than 4%-7% reported for symptomatic sporadic PDAC. Among individuals with familial prostate cancer (FPC), 13 of 214 individuals (6.1%) underwent surgery, but with a higher proportion of precursor lesions detected, just four high-risk lesions (1.9% of screened FPC patients) were removed.
Whether surveillance improved prognosis for FPC families was difficult to determine, according to the investigators. The yield of PDAC was low at 0.9%, as was the yield of relevant precursor lesions (grade 3 PanIN and high-grade IPMN) at 1.9%.
“However, if surgical removal of multifocal grade 2 PanIN and multifocal BD-IPMNs is regarded as beneficial, the diagnostic yield increases to 3.7% (eight of 214 patients), and surveillance of FPC might also be considered effective,” wrote Dr. Hans Vasen, professor in the department of gastroenterology and hepatology at the Leiden University Medical Center, the Netherlands, and colleagues. “The value of surveillance of FPC is still not clear, and the main effect seems to be prevention of PDAC by removal of” precursor lesions, they added (J Clin Oncol. 2016 Apr 25. doi: 10.1200/JCO.2015.64.0730).
The retrospective evaluation of an ongoing prospective follow-up study included 411 high-risk individuals: 178 with CDKN2A mutations, 214 with familial pancreatic cancer, and 19 with BRCA1/2 or PALB2 mutations. The study was conducted at three expert centers in Marburg, Germany; Leiden, the Netherlands; and Madrid.
In the BRCA1/2 and PALB2 mutation cohort, one individual (3.8%) with a BRCA2 mutation developed PDAC and underwent surgery; 17 months after the surgery this patient died of liver metastasis. Two others underwent surgery for cystic lesions and are in good health at 10 and 21 months after surgery.
In the cohort of CDKN2A mutation carriers, the mean age at the start of surveillance was 56 years (range, 37-75) and the mean follow-up time was 53 months (range, 0-169): in total, 866 MRIs and 106 endoscopic ultrasounds were conducted. In the FPC group, the mean age was 48 years (range, 27-81), and the mean follow up was 2.8 years (range, 0-10.8): 618 MRIs and 402 endoscopic ultrasounds were conducted. Among BRCA1/2 and PALB2 mutation carriers, the mean age was 52.6 years (range, 25-70), and the mean follow up was 32.7 months (range, 1-119).
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Surveillance of high-risk individuals was relatively successful in detecting pancreatic ductal carcinoma in situ (PDAC) at a resectable stage.
Major finding: The detection rate in CDKN2A mutation carriers was 7.3% and the resection rate for screen-detected PDAC was 75%, compared with previous reports of 15%-20% for symptomatic PDAC; the PDAC detection rate in individuals with familial prostate cancer was much lower at 0.9%.
Data source: Evaluation of an ongoing prospective follow-up study at three European centers included 411 individuals: 178 with CDKN2A mutations, 214 with familial pancreatic cancer, and 19 with BRCA1/2 or PALB2 mutations.
Disclosures: Dr. Vasen and most coauthors reported having no disclosures. Five coauthors reported financial ties to industry sources.
Surgery has edge over surveillance for micropapillary thyroid cancer
BALTIMORE – Hemithyroidectomy for low-risk micropapillary thyroid cancer can have advantages over active surveillance, according to findings from a study that examined outcomes by cost and quality of life data.
Endocrinologists and surgeons need to have in-depth conversations with their patients to determine their level of anxiety about cancer, surgery, and about their quality of life, to determine the best course of treatment, researchers at the University of California, San Francisco (UCSF) reported at the annual meeting of the American Association of Endocrine Surgeons.
“Our study found that hemithyroidectomy is cost effective in the majority of scenarios,” presenter Shriya Venkatesh said. “However, patient perception of micropapillary thyroid cancer as well as [the patient’s] life expectancy can play a major role in deciding which therapeutic option to choose.”
The study involved a cost-effectiveness analysis of the surgery vs. active surveillance, “which is especially relevant in our current times,” Ms. Venkatesh said in an interview. “What we wanted to do is give physicians information for when they approach their patients, not only in assessing the tumor from the medical aspect but also when looking at it from quality-of-life and cost-benefit perspectives.”
Both courses of management were modeled over a 20-year period with Medicare data and literature review to calculate costs and health utilities. The UCSF researchers used Markov statistical models for both approaches in which the reference case was a 40-year-old, otherwise healthy patient with a recent diagnosis of micropapillary thyroid cancer without high-risk factors. Either hemithyroidectomy or surveillance would be reasonable treatment options.
“We found that hemithyroidectomy was about $8,000 more costly than active surveillance, but it also afforded an increase in about 1.09 quality-adjusted life years,” Ms. Venkatesh said. Hemithyroidectomy is most cost effective for patients with a life expectancy of 3 years or more and who perceive that living with low-grade thyroid cancer would have even a modest detriment on their quality of life, she said.
“Unfortunately there is no current published quality-of-life assessment of active surveillance for thyroid cancer,” Ms. Venkatesh said. “We believe that estimating active surveillance to the equivalent of surgery underestimates the anxiety some patients may feel upon receiving their diagnosis.”
The paucity of literature on active surveillance for thyroid cancer prompted the UCSF researchers to turn to the prostate cancer literature, which has more data on active surveillance, to try to determine the disutility of active surveillance for micropapillary thyroid cancer. “Our extrapolation from the literature yields a mean disutility of 0.11,” she said.
However, the utility estimates the researchers came up with were variable, Ms. Venkatesh said. “This really pushes physicians to have that conversation with their patients, not only about the physical aspects of how they’re doing but also the mental aspects,” she said.
But quality of life is difficult to quantify, senior author Dr. Insoo Suh said in an interview. “What we found is that no matter how one measures quality of life, the qualitative degree of quality of life decrease that people associate with ‘living with cancer’ need not be that significant in order for surgery to be a potentially cost-effective treatment for them,” said Dr. Suh, an endocrine surgeon at UCSF and an ACS Fellow.
During the discussion, Dr. Peter Angelos of the University of Chicago and an ACS Fellow, said, “I’m curious how this information should impact the individual decision-making and informed consent for a specific patient, because I’m not sure that an individual patient would care if active surveillance is more cost effective or not.”
“When speaking to your patients, obviously discussing the rates of progression of the disease is important and then [so is] talking to them about different therapeutic options,” Ms. Venkatesh said. “The physician should also make an assessment about the patient’s quality of life to see if there are likely to be any changes due to the diagnosis.”
The limitations of the study include the extrapolation of data from the prostate cancer literature to define a utility scale and also the reference case used in the Markov model. Other utility measures showed variability as well.
Ms. Venkatesh, Dr. Suh and their coauthors had no financial relationships to disclose.
BALTIMORE – Hemithyroidectomy for low-risk micropapillary thyroid cancer can have advantages over active surveillance, according to findings from a study that examined outcomes by cost and quality of life data.
Endocrinologists and surgeons need to have in-depth conversations with their patients to determine their level of anxiety about cancer, surgery, and about their quality of life, to determine the best course of treatment, researchers at the University of California, San Francisco (UCSF) reported at the annual meeting of the American Association of Endocrine Surgeons.
“Our study found that hemithyroidectomy is cost effective in the majority of scenarios,” presenter Shriya Venkatesh said. “However, patient perception of micropapillary thyroid cancer as well as [the patient’s] life expectancy can play a major role in deciding which therapeutic option to choose.”
The study involved a cost-effectiveness analysis of the surgery vs. active surveillance, “which is especially relevant in our current times,” Ms. Venkatesh said in an interview. “What we wanted to do is give physicians information for when they approach their patients, not only in assessing the tumor from the medical aspect but also when looking at it from quality-of-life and cost-benefit perspectives.”
Both courses of management were modeled over a 20-year period with Medicare data and literature review to calculate costs and health utilities. The UCSF researchers used Markov statistical models for both approaches in which the reference case was a 40-year-old, otherwise healthy patient with a recent diagnosis of micropapillary thyroid cancer without high-risk factors. Either hemithyroidectomy or surveillance would be reasonable treatment options.
“We found that hemithyroidectomy was about $8,000 more costly than active surveillance, but it also afforded an increase in about 1.09 quality-adjusted life years,” Ms. Venkatesh said. Hemithyroidectomy is most cost effective for patients with a life expectancy of 3 years or more and who perceive that living with low-grade thyroid cancer would have even a modest detriment on their quality of life, she said.
“Unfortunately there is no current published quality-of-life assessment of active surveillance for thyroid cancer,” Ms. Venkatesh said. “We believe that estimating active surveillance to the equivalent of surgery underestimates the anxiety some patients may feel upon receiving their diagnosis.”
The paucity of literature on active surveillance for thyroid cancer prompted the UCSF researchers to turn to the prostate cancer literature, which has more data on active surveillance, to try to determine the disutility of active surveillance for micropapillary thyroid cancer. “Our extrapolation from the literature yields a mean disutility of 0.11,” she said.
However, the utility estimates the researchers came up with were variable, Ms. Venkatesh said. “This really pushes physicians to have that conversation with their patients, not only about the physical aspects of how they’re doing but also the mental aspects,” she said.
But quality of life is difficult to quantify, senior author Dr. Insoo Suh said in an interview. “What we found is that no matter how one measures quality of life, the qualitative degree of quality of life decrease that people associate with ‘living with cancer’ need not be that significant in order for surgery to be a potentially cost-effective treatment for them,” said Dr. Suh, an endocrine surgeon at UCSF and an ACS Fellow.
During the discussion, Dr. Peter Angelos of the University of Chicago and an ACS Fellow, said, “I’m curious how this information should impact the individual decision-making and informed consent for a specific patient, because I’m not sure that an individual patient would care if active surveillance is more cost effective or not.”
“When speaking to your patients, obviously discussing the rates of progression of the disease is important and then [so is] talking to them about different therapeutic options,” Ms. Venkatesh said. “The physician should also make an assessment about the patient’s quality of life to see if there are likely to be any changes due to the diagnosis.”
The limitations of the study include the extrapolation of data from the prostate cancer literature to define a utility scale and also the reference case used in the Markov model. Other utility measures showed variability as well.
Ms. Venkatesh, Dr. Suh and their coauthors had no financial relationships to disclose.
BALTIMORE – Hemithyroidectomy for low-risk micropapillary thyroid cancer can have advantages over active surveillance, according to findings from a study that examined outcomes by cost and quality of life data.
Endocrinologists and surgeons need to have in-depth conversations with their patients to determine their level of anxiety about cancer, surgery, and about their quality of life, to determine the best course of treatment, researchers at the University of California, San Francisco (UCSF) reported at the annual meeting of the American Association of Endocrine Surgeons.
“Our study found that hemithyroidectomy is cost effective in the majority of scenarios,” presenter Shriya Venkatesh said. “However, patient perception of micropapillary thyroid cancer as well as [the patient’s] life expectancy can play a major role in deciding which therapeutic option to choose.”
The study involved a cost-effectiveness analysis of the surgery vs. active surveillance, “which is especially relevant in our current times,” Ms. Venkatesh said in an interview. “What we wanted to do is give physicians information for when they approach their patients, not only in assessing the tumor from the medical aspect but also when looking at it from quality-of-life and cost-benefit perspectives.”
Both courses of management were modeled over a 20-year period with Medicare data and literature review to calculate costs and health utilities. The UCSF researchers used Markov statistical models for both approaches in which the reference case was a 40-year-old, otherwise healthy patient with a recent diagnosis of micropapillary thyroid cancer without high-risk factors. Either hemithyroidectomy or surveillance would be reasonable treatment options.
“We found that hemithyroidectomy was about $8,000 more costly than active surveillance, but it also afforded an increase in about 1.09 quality-adjusted life years,” Ms. Venkatesh said. Hemithyroidectomy is most cost effective for patients with a life expectancy of 3 years or more and who perceive that living with low-grade thyroid cancer would have even a modest detriment on their quality of life, she said.
“Unfortunately there is no current published quality-of-life assessment of active surveillance for thyroid cancer,” Ms. Venkatesh said. “We believe that estimating active surveillance to the equivalent of surgery underestimates the anxiety some patients may feel upon receiving their diagnosis.”
The paucity of literature on active surveillance for thyroid cancer prompted the UCSF researchers to turn to the prostate cancer literature, which has more data on active surveillance, to try to determine the disutility of active surveillance for micropapillary thyroid cancer. “Our extrapolation from the literature yields a mean disutility of 0.11,” she said.
However, the utility estimates the researchers came up with were variable, Ms. Venkatesh said. “This really pushes physicians to have that conversation with their patients, not only about the physical aspects of how they’re doing but also the mental aspects,” she said.
But quality of life is difficult to quantify, senior author Dr. Insoo Suh said in an interview. “What we found is that no matter how one measures quality of life, the qualitative degree of quality of life decrease that people associate with ‘living with cancer’ need not be that significant in order for surgery to be a potentially cost-effective treatment for them,” said Dr. Suh, an endocrine surgeon at UCSF and an ACS Fellow.
During the discussion, Dr. Peter Angelos of the University of Chicago and an ACS Fellow, said, “I’m curious how this information should impact the individual decision-making and informed consent for a specific patient, because I’m not sure that an individual patient would care if active surveillance is more cost effective or not.”
“When speaking to your patients, obviously discussing the rates of progression of the disease is important and then [so is] talking to them about different therapeutic options,” Ms. Venkatesh said. “The physician should also make an assessment about the patient’s quality of life to see if there are likely to be any changes due to the diagnosis.”
The limitations of the study include the extrapolation of data from the prostate cancer literature to define a utility scale and also the reference case used in the Markov model. Other utility measures showed variability as well.
Ms. Venkatesh, Dr. Suh and their coauthors had no financial relationships to disclose.
AT AAES 2016
Key clinical point: Patient psychological factors are key determinants in choosing a course of management for low-risk micropapillary thyroid cancer.
Major finding: Hemithyroidectomy typically costs about $8,000 more than active surveillance but also accounts for improved quality of life in these patients.
Data source: Markov models for both courses of management over a 20-year period with Medicare data and literature review to calculate costs and health utilities.
Disclosures: Ms. Venkatesh and her coauthors reported having no financial disclosures.
Neoadjuvant chemotherapy improves survival for some with pancreatic cancer
MONTREAL – For individuals with stage III pancreatic cancer, neoadjuvant therapy may improve survival, compared with surgery-first treatment. “This study supports, however does not prove, the hypothesis that early and continued control of occult metastatic disease prolongs survival in surgical patients,” said Dr. Christopher Shubert.
Dr. Shubert and his colleagues used an intention-to-treat (ITT) analysis to compare overall survival (OS) for 377 patients who were to receive neoadjuvant chemotherapy with 216 patients who received surgery first. Median OS for the neoadjuvant group was 20.7 months, compared with 13.7 months for the surgery-first group (log-rank P less than .0001).
This study was the first to utilize national data to look at how patients who received neoadjuvant chemotherapy for stage III pancreatic cancer fared, when compared with those treated with a surgery-first approach, Dr. Shubert, a general surgery resident at the Mayo Clinic in Rochester, Minn., said at the annual meeting of the Central Surgical Association.
Stage III pancreatic cancer (T4, any N, M0) means that the cancer has invaded the celiac trunk, or there is superior mesenteric artery involvement, he noted.
Using data from the National Cancer Database from 2002 to 2011, the investigators identified patients with clinical stage III pancreatic adenocarcinoma of the head or body of the pancreas. The ITT neoadjuvant therapy cohort included all patients whose treatment recommendations included curative-intent surgery and neoadjuvant chemotherapy, regardless of what therapies the patients received. The surgery-first cohort included those who were recommended to receive adjuvant therapy.
A total of 593 patients were identified, of whom 377 (63.5%) were in the neoadjuvant group. Of these, 104 (27.6%) were lost to presurgical attrition. The surgery-first group included 216 patients (36.3%), 30 (13.9%) of whom did not receive the intended adjuvant chemotherapy. Comparing the two ITT groups yielded an adjusted hazard ratio of 0.68 (P = .001).
A secondary aim of the study was to see which aspects of therapy, and which pathologic features, were associated with longer OS. The addition of postsurgical therapy was associated with additional survival benefit (31.6 vs. 22.6 months for no postsurgical therapy; HR, 0.60; P = .002). Node-negative and R0 status were both also significantly increased among those receiving neoadjuvant chemotherapy, and both disease characteristics were associated with increased OS, he reported.
Dr. Shubert said that study limitations included its review of a prospective database. Also, investigators could not determine the type or duration of chemotherapy; they also were unable to tell when systemic chemotherapy plus chemoradiation or just chemoradiation alone had been used. No recurrence data were available, and all cases were grouped under one procedure code, limiting information about vascular resections.
“Neoadjuvant therapy may offer survival advantages compared to a surgery-first approach,” said Dr. Shubert, and control of small occult metastases may be the mechanism behind this advantage. Still to be determined, however, are the optimal type, duration, and sequencing of neoadjuvant chemotherapy and chemoradiation, he said.
Dr. Shubert reported no relevant disclosures.
On Twitter @karioakes
MONTREAL – For individuals with stage III pancreatic cancer, neoadjuvant therapy may improve survival, compared with surgery-first treatment. “This study supports, however does not prove, the hypothesis that early and continued control of occult metastatic disease prolongs survival in surgical patients,” said Dr. Christopher Shubert.
Dr. Shubert and his colleagues used an intention-to-treat (ITT) analysis to compare overall survival (OS) for 377 patients who were to receive neoadjuvant chemotherapy with 216 patients who received surgery first. Median OS for the neoadjuvant group was 20.7 months, compared with 13.7 months for the surgery-first group (log-rank P less than .0001).
This study was the first to utilize national data to look at how patients who received neoadjuvant chemotherapy for stage III pancreatic cancer fared, when compared with those treated with a surgery-first approach, Dr. Shubert, a general surgery resident at the Mayo Clinic in Rochester, Minn., said at the annual meeting of the Central Surgical Association.
Stage III pancreatic cancer (T4, any N, M0) means that the cancer has invaded the celiac trunk, or there is superior mesenteric artery involvement, he noted.
Using data from the National Cancer Database from 2002 to 2011, the investigators identified patients with clinical stage III pancreatic adenocarcinoma of the head or body of the pancreas. The ITT neoadjuvant therapy cohort included all patients whose treatment recommendations included curative-intent surgery and neoadjuvant chemotherapy, regardless of what therapies the patients received. The surgery-first cohort included those who were recommended to receive adjuvant therapy.
A total of 593 patients were identified, of whom 377 (63.5%) were in the neoadjuvant group. Of these, 104 (27.6%) were lost to presurgical attrition. The surgery-first group included 216 patients (36.3%), 30 (13.9%) of whom did not receive the intended adjuvant chemotherapy. Comparing the two ITT groups yielded an adjusted hazard ratio of 0.68 (P = .001).
A secondary aim of the study was to see which aspects of therapy, and which pathologic features, were associated with longer OS. The addition of postsurgical therapy was associated with additional survival benefit (31.6 vs. 22.6 months for no postsurgical therapy; HR, 0.60; P = .002). Node-negative and R0 status were both also significantly increased among those receiving neoadjuvant chemotherapy, and both disease characteristics were associated with increased OS, he reported.
Dr. Shubert said that study limitations included its review of a prospective database. Also, investigators could not determine the type or duration of chemotherapy; they also were unable to tell when systemic chemotherapy plus chemoradiation or just chemoradiation alone had been used. No recurrence data were available, and all cases were grouped under one procedure code, limiting information about vascular resections.
“Neoadjuvant therapy may offer survival advantages compared to a surgery-first approach,” said Dr. Shubert, and control of small occult metastases may be the mechanism behind this advantage. Still to be determined, however, are the optimal type, duration, and sequencing of neoadjuvant chemotherapy and chemoradiation, he said.
Dr. Shubert reported no relevant disclosures.
On Twitter @karioakes
MONTREAL – For individuals with stage III pancreatic cancer, neoadjuvant therapy may improve survival, compared with surgery-first treatment. “This study supports, however does not prove, the hypothesis that early and continued control of occult metastatic disease prolongs survival in surgical patients,” said Dr. Christopher Shubert.
Dr. Shubert and his colleagues used an intention-to-treat (ITT) analysis to compare overall survival (OS) for 377 patients who were to receive neoadjuvant chemotherapy with 216 patients who received surgery first. Median OS for the neoadjuvant group was 20.7 months, compared with 13.7 months for the surgery-first group (log-rank P less than .0001).
This study was the first to utilize national data to look at how patients who received neoadjuvant chemotherapy for stage III pancreatic cancer fared, when compared with those treated with a surgery-first approach, Dr. Shubert, a general surgery resident at the Mayo Clinic in Rochester, Minn., said at the annual meeting of the Central Surgical Association.
Stage III pancreatic cancer (T4, any N, M0) means that the cancer has invaded the celiac trunk, or there is superior mesenteric artery involvement, he noted.
Using data from the National Cancer Database from 2002 to 2011, the investigators identified patients with clinical stage III pancreatic adenocarcinoma of the head or body of the pancreas. The ITT neoadjuvant therapy cohort included all patients whose treatment recommendations included curative-intent surgery and neoadjuvant chemotherapy, regardless of what therapies the patients received. The surgery-first cohort included those who were recommended to receive adjuvant therapy.
A total of 593 patients were identified, of whom 377 (63.5%) were in the neoadjuvant group. Of these, 104 (27.6%) were lost to presurgical attrition. The surgery-first group included 216 patients (36.3%), 30 (13.9%) of whom did not receive the intended adjuvant chemotherapy. Comparing the two ITT groups yielded an adjusted hazard ratio of 0.68 (P = .001).
A secondary aim of the study was to see which aspects of therapy, and which pathologic features, were associated with longer OS. The addition of postsurgical therapy was associated with additional survival benefit (31.6 vs. 22.6 months for no postsurgical therapy; HR, 0.60; P = .002). Node-negative and R0 status were both also significantly increased among those receiving neoadjuvant chemotherapy, and both disease characteristics were associated with increased OS, he reported.
Dr. Shubert said that study limitations included its review of a prospective database. Also, investigators could not determine the type or duration of chemotherapy; they also were unable to tell when systemic chemotherapy plus chemoradiation or just chemoradiation alone had been used. No recurrence data were available, and all cases were grouped under one procedure code, limiting information about vascular resections.
“Neoadjuvant therapy may offer survival advantages compared to a surgery-first approach,” said Dr. Shubert, and control of small occult metastases may be the mechanism behind this advantage. Still to be determined, however, are the optimal type, duration, and sequencing of neoadjuvant chemotherapy and chemoradiation, he said.
Dr. Shubert reported no relevant disclosures.
On Twitter @karioakes
AT THE ANNUAL MEETING OF THE CENTRAL SURGICAL ASSOCIATION
Key clinical point: Overall survival in stage III pancreatic cancer was better with neoadjuvant chemotherapy.
Major finding: Median survival with neoadjuvant chemotherapy was 20.7 months, compared with 13.7 months for surgery-first patients.
Data source: Review of stage III pancreatic cancer patients in the prospective National Cancer Database, comparing 377 patients recommended to receive neoadjuvant chemotherapy with 216 patients who were to receive surgery first.
Disclosures: Dr. Shubert reported no relevant disclosures.
VIDEO: Stenting to improve quality of life in esophageal cancer
PHILADELPHIA – An esophageal stent can improve quality of life for patients with advanced esophageal cancer, according to Dr. Sushil Ahlawat, director of endoscopy at Rutgers University, New Brunswick, N.J.
“An esophageal stent can be an important modality for palliating patients’ dysphagia, [which] can happen because the tumor is obstructing the esophagus,” says Dr. Ahlawat in this video. He also discusses how this minimally invasive procedure can support those undergoing chemoradiation therapy or surgery for esophageal cancer.
The video was recorded at this year’s meeting, held by Global Academy for Medical Education and Rutgers, the State University of New Jersey. Global Academy for Medical Education and this news organization are owned by the same company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
PHILADELPHIA – An esophageal stent can improve quality of life for patients with advanced esophageal cancer, according to Dr. Sushil Ahlawat, director of endoscopy at Rutgers University, New Brunswick, N.J.
“An esophageal stent can be an important modality for palliating patients’ dysphagia, [which] can happen because the tumor is obstructing the esophagus,” says Dr. Ahlawat in this video. He also discusses how this minimally invasive procedure can support those undergoing chemoradiation therapy or surgery for esophageal cancer.
The video was recorded at this year’s meeting, held by Global Academy for Medical Education and Rutgers, the State University of New Jersey. Global Academy for Medical Education and this news organization are owned by the same company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
PHILADELPHIA – An esophageal stent can improve quality of life for patients with advanced esophageal cancer, according to Dr. Sushil Ahlawat, director of endoscopy at Rutgers University, New Brunswick, N.J.
“An esophageal stent can be an important modality for palliating patients’ dysphagia, [which] can happen because the tumor is obstructing the esophagus,” says Dr. Ahlawat in this video. He also discusses how this minimally invasive procedure can support those undergoing chemoradiation therapy or surgery for esophageal cancer.
The video was recorded at this year’s meeting, held by Global Academy for Medical Education and Rutgers, the State University of New Jersey. Global Academy for Medical Education and this news organization are owned by the same company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM DIGESTIVE DISEASES: NEW ADVANCES
Prostate cancer’s future seen in molecular tests
HOLLYWOOD, FLA. – Current evidence suggests that molecular tests for prostate cancer are prognostic and can help clinicians and patients with difficult treatment decisions. In the not-too-distant future, gene tests could also guide choice of therapies.
“I think that the largest impact is going to come in areas of both the greatest treatment uncertainty and areas where we can be predictive about the response to treatment,” said Dr. Ashley Ross, a urologic oncologist and pathologist at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore.
Multigene panels may soon be able help identify which patients might benefit more from radical prostatectomy or radiation therapy, whether radiation therapy effects could be enhanced with the addition of androgen-deprivation therapy, and whether early use of docetaxel might add therapeutic benefit, he said at the annual conference of the National Comprehensive Cancer Network.
The 2016 iteration of the NCCN guidelines for the treatment of prostate cancer include a note stating that “men with clinically localized disease may consider the use of tumor-based molecular assays. Retrospective case cohort studies have shown that molecular assays performed on biopsy or prostatectomy specimens provide prognostic information independent of NCCN risk groups.”
The use of molecular assays may inform treatment decisions by helping to predict the likelihood of death if a patient is managed conservatively, risks for biochemical progression after radical prostatectomy or external beam therapy, and the likelihood that a patient could develop metastatic disease after radical prostatectomy or salvage radiotherapy, the guidelines say.
Dr. Ross reviewed the molecular biology of localized prostate cancer and the benefits and risks of currently available molecular tests.
“We’ve had an increased ability to get molecular information or genomic information from very limited amounts of routinely-stored pathologic tissue, and that’s resulted in the generation of many molecular-based tissue tests in prostate cancer. With the emergence of those tests and a lot of aggressive marketing, there has been a lot of confusion for patients and providers about whether we should use them or not and in what context,” he said.
Prostate cancer is genomically complex, even in the localized stage, with copy number alterations, deletions, and amplifications; chromosomal rearrangements; and point mutations, he said.
One of the best characterized genomic events is the early loss of the tumor suppressor gene PTEN (phosphatase and tensin homolog). This gene works within the PI3 kinase (PI3K)/AKT pathway. PI3K pathway mutations have been identified in up to 40% of all primary prostate cancers and 100% of mutations, Dr. Ross explained.
Loss of PTEN itself has been detected in about 15%-40% of primary prostate cancers and 50% of metastases, and the loss correlates with disease stage and tumor grade.
The NCCN guidelines list six available tissue-based tests for prostate cancer prognosis, including tests based on general cancer features such as cell-cycle proliferation, and those based on specific molecular features of cancer.
An example of the general type of test is the Ki-67 immunohistochemistry (IHC) test, which looks for a cellular marker of proliferation, and has been shown to have independent prognostic significance after radiation therapy or radical prostatectomy. This test is not currently recommended by the Medicare Molecular Diagnostic Services (MolDx) program, however.
Another general-type test is the Polaris quantitative reverse-transcriptase polymerase chain reaction (RT-PCR) panel, which tests for 31 cell-cycle-related genes and 15 “housekeeping” controls. This test is recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy. It has been shown to independently predict prostate cancer–specific mortality, biochemical failure/recurrence, and metastasis, the guidelines say.
Tests based on molecular features include:
• PTEN/ERG, an IHC or fluorescent in situ hybridization test that has been shown to predict prostate cancer–specific mortality, upgrading to Gleason pattern 4 on radical prostatectomy, and biochemical recurrence (not MolDx recommended).
• Decipher, a whole-transcriptome 1.4M RNA expression oligonucleotide microarray shown to predict biochemical failure, metastasis, and prostate cancer–specific mortality (recommended for postradical prostatectomy for patients with pT2 tumors with positive margins, and pT3 disease, and rising PSA above nadir);
• Oncotype DX, an RT-PCR assay for 12 prostate cancer genes and five housekeeping controls (recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy).
• ProMark, multiplex immunofluorescent staining of eight proteins, which has been shown to independently predict non–organ-confined pT3 disease or Gleason pattern 4 disease on radical prostatectomy (not reviewed).
Dr. Ross said that in his practice, he generally does not order molecular testing for surveillance of men older than 65 who have very-low-risk disease. For men with low-risk disease, however, molecular testing may help in clinical decision making to predict upgrading or disease progression.
“There’s limited data from surveillance populations, but these tests can be used in this context with retrospective data available, realizing that in most cases the tests will be confirmative, or another way of thinking about it is ‘noninformative,’ so there are some considerations about cost in that context,” he said. For men with intermediate or high-risk disease, however, currently available tests are not good at predicting what an individual patient’s response would be to a specific type of therapy, whether surgery, radiation, androgen deprivation, chemotherapy, or a combination.
“This is an area where predictive biomarkers would be very informative. There is ongoing research, and I think this is an area of potentially large advancement in how we risk-stratify our patients,” Dr. Ross said.
HOLLYWOOD, FLA. – Current evidence suggests that molecular tests for prostate cancer are prognostic and can help clinicians and patients with difficult treatment decisions. In the not-too-distant future, gene tests could also guide choice of therapies.
“I think that the largest impact is going to come in areas of both the greatest treatment uncertainty and areas where we can be predictive about the response to treatment,” said Dr. Ashley Ross, a urologic oncologist and pathologist at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore.
Multigene panels may soon be able help identify which patients might benefit more from radical prostatectomy or radiation therapy, whether radiation therapy effects could be enhanced with the addition of androgen-deprivation therapy, and whether early use of docetaxel might add therapeutic benefit, he said at the annual conference of the National Comprehensive Cancer Network.
The 2016 iteration of the NCCN guidelines for the treatment of prostate cancer include a note stating that “men with clinically localized disease may consider the use of tumor-based molecular assays. Retrospective case cohort studies have shown that molecular assays performed on biopsy or prostatectomy specimens provide prognostic information independent of NCCN risk groups.”
The use of molecular assays may inform treatment decisions by helping to predict the likelihood of death if a patient is managed conservatively, risks for biochemical progression after radical prostatectomy or external beam therapy, and the likelihood that a patient could develop metastatic disease after radical prostatectomy or salvage radiotherapy, the guidelines say.
Dr. Ross reviewed the molecular biology of localized prostate cancer and the benefits and risks of currently available molecular tests.
“We’ve had an increased ability to get molecular information or genomic information from very limited amounts of routinely-stored pathologic tissue, and that’s resulted in the generation of many molecular-based tissue tests in prostate cancer. With the emergence of those tests and a lot of aggressive marketing, there has been a lot of confusion for patients and providers about whether we should use them or not and in what context,” he said.
Prostate cancer is genomically complex, even in the localized stage, with copy number alterations, deletions, and amplifications; chromosomal rearrangements; and point mutations, he said.
One of the best characterized genomic events is the early loss of the tumor suppressor gene PTEN (phosphatase and tensin homolog). This gene works within the PI3 kinase (PI3K)/AKT pathway. PI3K pathway mutations have been identified in up to 40% of all primary prostate cancers and 100% of mutations, Dr. Ross explained.
Loss of PTEN itself has been detected in about 15%-40% of primary prostate cancers and 50% of metastases, and the loss correlates with disease stage and tumor grade.
The NCCN guidelines list six available tissue-based tests for prostate cancer prognosis, including tests based on general cancer features such as cell-cycle proliferation, and those based on specific molecular features of cancer.
An example of the general type of test is the Ki-67 immunohistochemistry (IHC) test, which looks for a cellular marker of proliferation, and has been shown to have independent prognostic significance after radiation therapy or radical prostatectomy. This test is not currently recommended by the Medicare Molecular Diagnostic Services (MolDx) program, however.
Another general-type test is the Polaris quantitative reverse-transcriptase polymerase chain reaction (RT-PCR) panel, which tests for 31 cell-cycle-related genes and 15 “housekeeping” controls. This test is recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy. It has been shown to independently predict prostate cancer–specific mortality, biochemical failure/recurrence, and metastasis, the guidelines say.
Tests based on molecular features include:
• PTEN/ERG, an IHC or fluorescent in situ hybridization test that has been shown to predict prostate cancer–specific mortality, upgrading to Gleason pattern 4 on radical prostatectomy, and biochemical recurrence (not MolDx recommended).
• Decipher, a whole-transcriptome 1.4M RNA expression oligonucleotide microarray shown to predict biochemical failure, metastasis, and prostate cancer–specific mortality (recommended for postradical prostatectomy for patients with pT2 tumors with positive margins, and pT3 disease, and rising PSA above nadir);
• Oncotype DX, an RT-PCR assay for 12 prostate cancer genes and five housekeeping controls (recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy).
• ProMark, multiplex immunofluorescent staining of eight proteins, which has been shown to independently predict non–organ-confined pT3 disease or Gleason pattern 4 disease on radical prostatectomy (not reviewed).
Dr. Ross said that in his practice, he generally does not order molecular testing for surveillance of men older than 65 who have very-low-risk disease. For men with low-risk disease, however, molecular testing may help in clinical decision making to predict upgrading or disease progression.
“There’s limited data from surveillance populations, but these tests can be used in this context with retrospective data available, realizing that in most cases the tests will be confirmative, or another way of thinking about it is ‘noninformative,’ so there are some considerations about cost in that context,” he said. For men with intermediate or high-risk disease, however, currently available tests are not good at predicting what an individual patient’s response would be to a specific type of therapy, whether surgery, radiation, androgen deprivation, chemotherapy, or a combination.
“This is an area where predictive biomarkers would be very informative. There is ongoing research, and I think this is an area of potentially large advancement in how we risk-stratify our patients,” Dr. Ross said.
HOLLYWOOD, FLA. – Current evidence suggests that molecular tests for prostate cancer are prognostic and can help clinicians and patients with difficult treatment decisions. In the not-too-distant future, gene tests could also guide choice of therapies.
“I think that the largest impact is going to come in areas of both the greatest treatment uncertainty and areas where we can be predictive about the response to treatment,” said Dr. Ashley Ross, a urologic oncologist and pathologist at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore.
Multigene panels may soon be able help identify which patients might benefit more from radical prostatectomy or radiation therapy, whether radiation therapy effects could be enhanced with the addition of androgen-deprivation therapy, and whether early use of docetaxel might add therapeutic benefit, he said at the annual conference of the National Comprehensive Cancer Network.
The 2016 iteration of the NCCN guidelines for the treatment of prostate cancer include a note stating that “men with clinically localized disease may consider the use of tumor-based molecular assays. Retrospective case cohort studies have shown that molecular assays performed on biopsy or prostatectomy specimens provide prognostic information independent of NCCN risk groups.”
The use of molecular assays may inform treatment decisions by helping to predict the likelihood of death if a patient is managed conservatively, risks for biochemical progression after radical prostatectomy or external beam therapy, and the likelihood that a patient could develop metastatic disease after radical prostatectomy or salvage radiotherapy, the guidelines say.
Dr. Ross reviewed the molecular biology of localized prostate cancer and the benefits and risks of currently available molecular tests.
“We’ve had an increased ability to get molecular information or genomic information from very limited amounts of routinely-stored pathologic tissue, and that’s resulted in the generation of many molecular-based tissue tests in prostate cancer. With the emergence of those tests and a lot of aggressive marketing, there has been a lot of confusion for patients and providers about whether we should use them or not and in what context,” he said.
Prostate cancer is genomically complex, even in the localized stage, with copy number alterations, deletions, and amplifications; chromosomal rearrangements; and point mutations, he said.
One of the best characterized genomic events is the early loss of the tumor suppressor gene PTEN (phosphatase and tensin homolog). This gene works within the PI3 kinase (PI3K)/AKT pathway. PI3K pathway mutations have been identified in up to 40% of all primary prostate cancers and 100% of mutations, Dr. Ross explained.
Loss of PTEN itself has been detected in about 15%-40% of primary prostate cancers and 50% of metastases, and the loss correlates with disease stage and tumor grade.
The NCCN guidelines list six available tissue-based tests for prostate cancer prognosis, including tests based on general cancer features such as cell-cycle proliferation, and those based on specific molecular features of cancer.
An example of the general type of test is the Ki-67 immunohistochemistry (IHC) test, which looks for a cellular marker of proliferation, and has been shown to have independent prognostic significance after radiation therapy or radical prostatectomy. This test is not currently recommended by the Medicare Molecular Diagnostic Services (MolDx) program, however.
Another general-type test is the Polaris quantitative reverse-transcriptase polymerase chain reaction (RT-PCR) panel, which tests for 31 cell-cycle-related genes and 15 “housekeeping” controls. This test is recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy. It has been shown to independently predict prostate cancer–specific mortality, biochemical failure/recurrence, and metastasis, the guidelines say.
Tests based on molecular features include:
• PTEN/ERG, an IHC or fluorescent in situ hybridization test that has been shown to predict prostate cancer–specific mortality, upgrading to Gleason pattern 4 on radical prostatectomy, and biochemical recurrence (not MolDx recommended).
• Decipher, a whole-transcriptome 1.4M RNA expression oligonucleotide microarray shown to predict biochemical failure, metastasis, and prostate cancer–specific mortality (recommended for postradical prostatectomy for patients with pT2 tumors with positive margins, and pT3 disease, and rising PSA above nadir);
• Oncotype DX, an RT-PCR assay for 12 prostate cancer genes and five housekeeping controls (recommended for post-biopsy evaluation of men with very-low-risk or low-risk prostate cancer who at the time of diagnosis have at least 10 years of life expectancy).
• ProMark, multiplex immunofluorescent staining of eight proteins, which has been shown to independently predict non–organ-confined pT3 disease or Gleason pattern 4 disease on radical prostatectomy (not reviewed).
Dr. Ross said that in his practice, he generally does not order molecular testing for surveillance of men older than 65 who have very-low-risk disease. For men with low-risk disease, however, molecular testing may help in clinical decision making to predict upgrading or disease progression.
“There’s limited data from surveillance populations, but these tests can be used in this context with retrospective data available, realizing that in most cases the tests will be confirmative, or another way of thinking about it is ‘noninformative,’ so there are some considerations about cost in that context,” he said. For men with intermediate or high-risk disease, however, currently available tests are not good at predicting what an individual patient’s response would be to a specific type of therapy, whether surgery, radiation, androgen deprivation, chemotherapy, or a combination.
“This is an area where predictive biomarkers would be very informative. There is ongoing research, and I think this is an area of potentially large advancement in how we risk-stratify our patients,” Dr. Ross said.
EXPERT ANALYSIS FROM THE NCCN ANNUAL CONFERENCE